User login
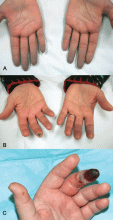
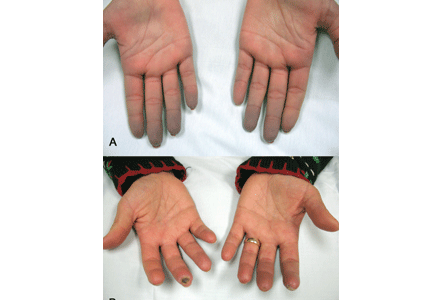
Indications that Raynaud phenomenon may be the presenting manifestation of a systemic autoimmune rheumatic disease are older age at onset (ie, over age 30), male sex, asymmetric involvement, and prolonged and painful attacks that can be severe enough to cause ischemic digital ulceration or gangrene (Figure 1).
Hence, chronic and severe digital ischemia causing ulceration or infarction differentiates secondary from primary Raynaud phenomenon and should prompt an investigation for an autoimmune rheumatic process. When taking the history, the clinician should seek clues to an underlying autoimmune condition, such as arthralgia, heartburn, dysphagia, shortness of breath, cough, and should examine the patient for telltale signs such as puffy hands and fingers, sclerodactyly, digital pitting scars, loss of fingertip pulp tissue, telangiectasias, and calcinosis.
CLUES TO PRIMARY VS SECONDARY RAYNAUD PHENOMENON
A diagnostic algorithm of digital ischemia (Figure 2) illustrates the range of presentations and possible causes. In Raynaud phenomenon, cold temperature and emotional stress provoke reversible color changes of the fingers and toes. Intense vasospasm of the digital arteries produces three well-defined phases1: white (pallor resulting from vasospasm), blue (dusky cyanosis due to deoxygenation of static venous blood) (Figure 1), and red (reactive hyperemia after the restoration of blood flow). However, only about 60% of patients have all three color changes. The attacks are associated with paresthesias, an uncomfortable feeling of coldness in the fingers, and ischemic pain.
Primary Raynaud phenomenon
Primary or idiopathic Raynaud phenomenon is seen in 5% to 10% of the general population. It more commonly affects women ages 15 to 30, is generally mild, involves the digits symmetrically, and is sometimes familial. An increase in alpha-2 adrenergic responses in the digital vessels leads to arterial vasospasm, an exaggerated physiologic response to cold temperatures.2 Geographic variability in prevalence likely represents differences in mean outdoor temperatures,3 which is in part why attacks of primary Raynaud phenomenon tend to be worse in the winter months.4
Secondary Raynaud phenomenon
Raynaud phenomenon also often occurs in certain autoimmune rheumatic diseases (secondary Raynaud phenomenon): for example, it is seen in scleroderma (90% to 95% of patients), mixed connective tissue disease (85%), systemic lupus erythematosus (40%), antisynthetase syndrome (40%), and sometimes in patients with other autoimmune rheumatic diseases. It may also be seen in hematologic disorders (cryoglobulinemia, cryofibrinogenemia, paraproteinemias, cold agglutinin disease, and polycythemia rubra vera), and it can also result from environmental and occupational exposures (frostbite, use of vibrating tools) and from exposure to certain drugs and toxins, such as polyvinyl chloride (Figure 2).
Acrocyanosis, a benign neurohormonal condition, should be included in the differential diagnosis for Raynaud phenomenon. Raynaud phenomenon is episodic, whereas acrocyanosis leads to persistent cyanosis of the acral body parts (fingers, toes) that is exacerbated by cold temperatures. However, the trophic skin changes, localized pain, and ulceration are not seen in acrocyanosis.
NAILFOLD CAPILLAROSCOPY: A KEY PART OF THE WORKUP
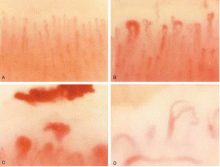
Nailfold capillaroscopy should be part of the evaluation of patients with Raynaud phenomenon (Figure 3), as it is one of the most reliable tests for distinguishing between primary and secondary Raynaud phenomenon.5 The sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis increases significantly with the addition of nailfold capillary abnormalities.6,7
A stereomicroscope or videocapillaroscope is usually recommended to evaluate nailfold capillary morphology,5 but if such equipment is not available, a regular ophthalmoscope (with the lens set at 20 diopters or higher for better resolution) can serve the purpose at the bedside.8 A drop of mineral oil is placed on the nailfold to improve the image resolution, as it makes the horny layer of the cuticle transparent.
Abnormal patterns include dilated and enlarged capillary loops, disorganized capillaries, “dropouts” (avascular areas), microhemorrhages, and arborized capillaries (Figure 3).5 At no additional cost, the presence of these microvascular changes would add to the suspicion of secondary Raynaud phenomenon (negative predictive value of 93%).9 In addition, evolving capillaroscopic changes can be seen during follow-up visits, indicating the progressive nature of the microvasculopathy seen in these autoimmune rheumatic diseases.10
ADDITIONAL TESTING
If an underlying autoimmune rheumatic disease is suspected, laboratory testing should include a complete blood cell count, an erythrocyte sedimentation rate, and an antinuclear antibody (ANA) assay. If the ANA assay is negative, no further testing is usually necessary; however, a positive test should alert the clinician to consider an underlying autoimmune rheumatic process (negative predictive value of 93%).9 In a patient presenting with Raynaud phenomenon, a positive ANA test (even in the absence of other symptoms) warrants more frequent follow-up, urinalysis, and perhaps referral to a rheumatologist.
In the case of a positive ANA test, before ordering additional autoantibody tests, it is useful to consider the relevant non-Raynaud clinical manifestations. Indiscriminate ordering of a battery of autoantibodies should be avoided because of significant added cost and because it is not likely to provide additional information to guide management.
On the other hand, these more specific antibody tests may be of value in confirming the diagnosis suggested by the clinical profile of specific autoimmune rheumatic diseases, eg, anti-double-stranded DNA11 and anti-Smith12 antibodies for lupus, anti-topoisomerase I (Scl-70) and anti-centromere antibodies for scleroderma, 13 and anti-synthetase (eg, anti-Jo-1) antibodies for autoimmune myositis.14,15
- Raynaud M. On local asphyxia and symmetrical gangrene of the extremities (1862), and new research on the nature and treatment of local asphyxia of the extremities (1872).Barlow T, trans. Selected monographs (121). London: New Sydenham Society, 1988.
- Boin F, Wigley FM. Understanding, assessing and treating Raynaud’s phenomenon. Curr Opin Rheumatol 2005; 17:752–760.
- Maricq HR, Carpentier PH, Weinrich MC, et al. Geographic variation in the prevalence of Raynaud’s phenomenon: a 5-region comparison. J Rheumatol 1997; 24:879–889.
- Wigley FM. Clinical practice. Raynaud’s phenomenon. N Engl J Med 2002; 347:1001–1018.
- Cutolo M, Pizzorni C, Sulli A. Capillaroscopy. Best Pract Res Clin Rheumatol 2005; 19:437–452.
- Lonzetti LS, Joyal F, Raynauld JP, et al. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum 2001; 44:735–736.
- Hudson M, Taillefer S, Steele R, et al. Improving the sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis. Clin Exp Rheumatol 2007; 25:754–757.
- Anders HJ, Sigl T, Schattenkirchner M. Differentiation between primary and secondary Raynaud’s phenomenon: a prospective study comparing nailfold capillaroscopy using an ophthalmoscope or stereomicroscope. Ann Rheum Dis 2001; 60:407–409.
- Spencer-Green G. Outcomes in primary Raynaud phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med 1998; 158:595–600.
- Wong ML, Highton J, Palmer DG. Sequential nailfold capillary microscopy in scleroderma and related disorders. Ann Rheum Dis 1988; 47:53–61.
- Weinstein A, Bordwell B, Stone B, Tibbetts C, Rothfield NF. Antibodies to native DNA and serum complement (C3) levels. Application to diagnosis and classification of systemic lupus erythematosus. Am J Med 1983; 74:206–216.
- Craft J. Antibodies to snRNPs in systemic lupus erythematosus. Rheum Dis Clin North Am 1992; 18:311–335.
- Weiner ES, Hildebrandt S, Senécal JL, et al. Prognostic significance of anticentromere antibodies and anti-topoisomerase I antibodies in Raynaud’s disease. A prospective study. Arthritis Rheum 1991; 34:68–77.
- Miller FW, Twitty SA, Biswas T, Plotz PH. Origin and regulation of a disease-specific autoantibody response. Antigenic epitopes, spectrotype stability, and isotype restriction of anti-Jo-1 autoantibodies. J Clin Invest 1990; 85:468–475.
- Ghirardello A, Zampieri S, Tarricone E, et al. Clinical implications of autoantibody screening in patients with autoimmune myositis. Autoimmunity 2006; 39:217–221.
Indications that Raynaud phenomenon may be the presenting manifestation of a systemic autoimmune rheumatic disease are older age at onset (ie, over age 30), male sex, asymmetric involvement, and prolonged and painful attacks that can be severe enough to cause ischemic digital ulceration or gangrene (Figure 1).
Hence, chronic and severe digital ischemia causing ulceration or infarction differentiates secondary from primary Raynaud phenomenon and should prompt an investigation for an autoimmune rheumatic process. When taking the history, the clinician should seek clues to an underlying autoimmune condition, such as arthralgia, heartburn, dysphagia, shortness of breath, cough, and should examine the patient for telltale signs such as puffy hands and fingers, sclerodactyly, digital pitting scars, loss of fingertip pulp tissue, telangiectasias, and calcinosis.
CLUES TO PRIMARY VS SECONDARY RAYNAUD PHENOMENON
A diagnostic algorithm of digital ischemia (Figure 2) illustrates the range of presentations and possible causes. In Raynaud phenomenon, cold temperature and emotional stress provoke reversible color changes of the fingers and toes. Intense vasospasm of the digital arteries produces three well-defined phases1: white (pallor resulting from vasospasm), blue (dusky cyanosis due to deoxygenation of static venous blood) (Figure 1), and red (reactive hyperemia after the restoration of blood flow). However, only about 60% of patients have all three color changes. The attacks are associated with paresthesias, an uncomfortable feeling of coldness in the fingers, and ischemic pain.
Primary Raynaud phenomenon
Primary or idiopathic Raynaud phenomenon is seen in 5% to 10% of the general population. It more commonly affects women ages 15 to 30, is generally mild, involves the digits symmetrically, and is sometimes familial. An increase in alpha-2 adrenergic responses in the digital vessels leads to arterial vasospasm, an exaggerated physiologic response to cold temperatures.2 Geographic variability in prevalence likely represents differences in mean outdoor temperatures,3 which is in part why attacks of primary Raynaud phenomenon tend to be worse in the winter months.4
Secondary Raynaud phenomenon
Raynaud phenomenon also often occurs in certain autoimmune rheumatic diseases (secondary Raynaud phenomenon): for example, it is seen in scleroderma (90% to 95% of patients), mixed connective tissue disease (85%), systemic lupus erythematosus (40%), antisynthetase syndrome (40%), and sometimes in patients with other autoimmune rheumatic diseases. It may also be seen in hematologic disorders (cryoglobulinemia, cryofibrinogenemia, paraproteinemias, cold agglutinin disease, and polycythemia rubra vera), and it can also result from environmental and occupational exposures (frostbite, use of vibrating tools) and from exposure to certain drugs and toxins, such as polyvinyl chloride (Figure 2).
Acrocyanosis, a benign neurohormonal condition, should be included in the differential diagnosis for Raynaud phenomenon. Raynaud phenomenon is episodic, whereas acrocyanosis leads to persistent cyanosis of the acral body parts (fingers, toes) that is exacerbated by cold temperatures. However, the trophic skin changes, localized pain, and ulceration are not seen in acrocyanosis.
NAILFOLD CAPILLAROSCOPY: A KEY PART OF THE WORKUP
Nailfold capillaroscopy should be part of the evaluation of patients with Raynaud phenomenon (Figure 3), as it is one of the most reliable tests for distinguishing between primary and secondary Raynaud phenomenon.5 The sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis increases significantly with the addition of nailfold capillary abnormalities.6,7
A stereomicroscope or videocapillaroscope is usually recommended to evaluate nailfold capillary morphology,5 but if such equipment is not available, a regular ophthalmoscope (with the lens set at 20 diopters or higher for better resolution) can serve the purpose at the bedside.8 A drop of mineral oil is placed on the nailfold to improve the image resolution, as it makes the horny layer of the cuticle transparent.
Abnormal patterns include dilated and enlarged capillary loops, disorganized capillaries, “dropouts” (avascular areas), microhemorrhages, and arborized capillaries (Figure 3).5 At no additional cost, the presence of these microvascular changes would add to the suspicion of secondary Raynaud phenomenon (negative predictive value of 93%).9 In addition, evolving capillaroscopic changes can be seen during follow-up visits, indicating the progressive nature of the microvasculopathy seen in these autoimmune rheumatic diseases.10
ADDITIONAL TESTING
If an underlying autoimmune rheumatic disease is suspected, laboratory testing should include a complete blood cell count, an erythrocyte sedimentation rate, and an antinuclear antibody (ANA) assay. If the ANA assay is negative, no further testing is usually necessary; however, a positive test should alert the clinician to consider an underlying autoimmune rheumatic process (negative predictive value of 93%).9 In a patient presenting with Raynaud phenomenon, a positive ANA test (even in the absence of other symptoms) warrants more frequent follow-up, urinalysis, and perhaps referral to a rheumatologist.
In the case of a positive ANA test, before ordering additional autoantibody tests, it is useful to consider the relevant non-Raynaud clinical manifestations. Indiscriminate ordering of a battery of autoantibodies should be avoided because of significant added cost and because it is not likely to provide additional information to guide management.
On the other hand, these more specific antibody tests may be of value in confirming the diagnosis suggested by the clinical profile of specific autoimmune rheumatic diseases, eg, anti-double-stranded DNA11 and anti-Smith12 antibodies for lupus, anti-topoisomerase I (Scl-70) and anti-centromere antibodies for scleroderma, 13 and anti-synthetase (eg, anti-Jo-1) antibodies for autoimmune myositis.14,15
Indications that Raynaud phenomenon may be the presenting manifestation of a systemic autoimmune rheumatic disease are older age at onset (ie, over age 30), male sex, asymmetric involvement, and prolonged and painful attacks that can be severe enough to cause ischemic digital ulceration or gangrene (Figure 1).
Hence, chronic and severe digital ischemia causing ulceration or infarction differentiates secondary from primary Raynaud phenomenon and should prompt an investigation for an autoimmune rheumatic process. When taking the history, the clinician should seek clues to an underlying autoimmune condition, such as arthralgia, heartburn, dysphagia, shortness of breath, cough, and should examine the patient for telltale signs such as puffy hands and fingers, sclerodactyly, digital pitting scars, loss of fingertip pulp tissue, telangiectasias, and calcinosis.
CLUES TO PRIMARY VS SECONDARY RAYNAUD PHENOMENON
A diagnostic algorithm of digital ischemia (Figure 2) illustrates the range of presentations and possible causes. In Raynaud phenomenon, cold temperature and emotional stress provoke reversible color changes of the fingers and toes. Intense vasospasm of the digital arteries produces three well-defined phases1: white (pallor resulting from vasospasm), blue (dusky cyanosis due to deoxygenation of static venous blood) (Figure 1), and red (reactive hyperemia after the restoration of blood flow). However, only about 60% of patients have all three color changes. The attacks are associated with paresthesias, an uncomfortable feeling of coldness in the fingers, and ischemic pain.
Primary Raynaud phenomenon
Primary or idiopathic Raynaud phenomenon is seen in 5% to 10% of the general population. It more commonly affects women ages 15 to 30, is generally mild, involves the digits symmetrically, and is sometimes familial. An increase in alpha-2 adrenergic responses in the digital vessels leads to arterial vasospasm, an exaggerated physiologic response to cold temperatures.2 Geographic variability in prevalence likely represents differences in mean outdoor temperatures,3 which is in part why attacks of primary Raynaud phenomenon tend to be worse in the winter months.4
Secondary Raynaud phenomenon
Raynaud phenomenon also often occurs in certain autoimmune rheumatic diseases (secondary Raynaud phenomenon): for example, it is seen in scleroderma (90% to 95% of patients), mixed connective tissue disease (85%), systemic lupus erythematosus (40%), antisynthetase syndrome (40%), and sometimes in patients with other autoimmune rheumatic diseases. It may also be seen in hematologic disorders (cryoglobulinemia, cryofibrinogenemia, paraproteinemias, cold agglutinin disease, and polycythemia rubra vera), and it can also result from environmental and occupational exposures (frostbite, use of vibrating tools) and from exposure to certain drugs and toxins, such as polyvinyl chloride (Figure 2).
Acrocyanosis, a benign neurohormonal condition, should be included in the differential diagnosis for Raynaud phenomenon. Raynaud phenomenon is episodic, whereas acrocyanosis leads to persistent cyanosis of the acral body parts (fingers, toes) that is exacerbated by cold temperatures. However, the trophic skin changes, localized pain, and ulceration are not seen in acrocyanosis.
NAILFOLD CAPILLAROSCOPY: A KEY PART OF THE WORKUP
Nailfold capillaroscopy should be part of the evaluation of patients with Raynaud phenomenon (Figure 3), as it is one of the most reliable tests for distinguishing between primary and secondary Raynaud phenomenon.5 The sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis increases significantly with the addition of nailfold capillary abnormalities.6,7
A stereomicroscope or videocapillaroscope is usually recommended to evaluate nailfold capillary morphology,5 but if such equipment is not available, a regular ophthalmoscope (with the lens set at 20 diopters or higher for better resolution) can serve the purpose at the bedside.8 A drop of mineral oil is placed on the nailfold to improve the image resolution, as it makes the horny layer of the cuticle transparent.
Abnormal patterns include dilated and enlarged capillary loops, disorganized capillaries, “dropouts” (avascular areas), microhemorrhages, and arborized capillaries (Figure 3).5 At no additional cost, the presence of these microvascular changes would add to the suspicion of secondary Raynaud phenomenon (negative predictive value of 93%).9 In addition, evolving capillaroscopic changes can be seen during follow-up visits, indicating the progressive nature of the microvasculopathy seen in these autoimmune rheumatic diseases.10
ADDITIONAL TESTING
If an underlying autoimmune rheumatic disease is suspected, laboratory testing should include a complete blood cell count, an erythrocyte sedimentation rate, and an antinuclear antibody (ANA) assay. If the ANA assay is negative, no further testing is usually necessary; however, a positive test should alert the clinician to consider an underlying autoimmune rheumatic process (negative predictive value of 93%).9 In a patient presenting with Raynaud phenomenon, a positive ANA test (even in the absence of other symptoms) warrants more frequent follow-up, urinalysis, and perhaps referral to a rheumatologist.
In the case of a positive ANA test, before ordering additional autoantibody tests, it is useful to consider the relevant non-Raynaud clinical manifestations. Indiscriminate ordering of a battery of autoantibodies should be avoided because of significant added cost and because it is not likely to provide additional information to guide management.
On the other hand, these more specific antibody tests may be of value in confirming the diagnosis suggested by the clinical profile of specific autoimmune rheumatic diseases, eg, anti-double-stranded DNA11 and anti-Smith12 antibodies for lupus, anti-topoisomerase I (Scl-70) and anti-centromere antibodies for scleroderma, 13 and anti-synthetase (eg, anti-Jo-1) antibodies for autoimmune myositis.14,15
- Raynaud M. On local asphyxia and symmetrical gangrene of the extremities (1862), and new research on the nature and treatment of local asphyxia of the extremities (1872).Barlow T, trans. Selected monographs (121). London: New Sydenham Society, 1988.
- Boin F, Wigley FM. Understanding, assessing and treating Raynaud’s phenomenon. Curr Opin Rheumatol 2005; 17:752–760.
- Maricq HR, Carpentier PH, Weinrich MC, et al. Geographic variation in the prevalence of Raynaud’s phenomenon: a 5-region comparison. J Rheumatol 1997; 24:879–889.
- Wigley FM. Clinical practice. Raynaud’s phenomenon. N Engl J Med 2002; 347:1001–1018.
- Cutolo M, Pizzorni C, Sulli A. Capillaroscopy. Best Pract Res Clin Rheumatol 2005; 19:437–452.
- Lonzetti LS, Joyal F, Raynauld JP, et al. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum 2001; 44:735–736.
- Hudson M, Taillefer S, Steele R, et al. Improving the sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis. Clin Exp Rheumatol 2007; 25:754–757.
- Anders HJ, Sigl T, Schattenkirchner M. Differentiation between primary and secondary Raynaud’s phenomenon: a prospective study comparing nailfold capillaroscopy using an ophthalmoscope or stereomicroscope. Ann Rheum Dis 2001; 60:407–409.
- Spencer-Green G. Outcomes in primary Raynaud phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med 1998; 158:595–600.
- Wong ML, Highton J, Palmer DG. Sequential nailfold capillary microscopy in scleroderma and related disorders. Ann Rheum Dis 1988; 47:53–61.
- Weinstein A, Bordwell B, Stone B, Tibbetts C, Rothfield NF. Antibodies to native DNA and serum complement (C3) levels. Application to diagnosis and classification of systemic lupus erythematosus. Am J Med 1983; 74:206–216.
- Craft J. Antibodies to snRNPs in systemic lupus erythematosus. Rheum Dis Clin North Am 1992; 18:311–335.
- Weiner ES, Hildebrandt S, Senécal JL, et al. Prognostic significance of anticentromere antibodies and anti-topoisomerase I antibodies in Raynaud’s disease. A prospective study. Arthritis Rheum 1991; 34:68–77.
- Miller FW, Twitty SA, Biswas T, Plotz PH. Origin and regulation of a disease-specific autoantibody response. Antigenic epitopes, spectrotype stability, and isotype restriction of anti-Jo-1 autoantibodies. J Clin Invest 1990; 85:468–475.
- Ghirardello A, Zampieri S, Tarricone E, et al. Clinical implications of autoantibody screening in patients with autoimmune myositis. Autoimmunity 2006; 39:217–221.
- Raynaud M. On local asphyxia and symmetrical gangrene of the extremities (1862), and new research on the nature and treatment of local asphyxia of the extremities (1872).Barlow T, trans. Selected monographs (121). London: New Sydenham Society, 1988.
- Boin F, Wigley FM. Understanding, assessing and treating Raynaud’s phenomenon. Curr Opin Rheumatol 2005; 17:752–760.
- Maricq HR, Carpentier PH, Weinrich MC, et al. Geographic variation in the prevalence of Raynaud’s phenomenon: a 5-region comparison. J Rheumatol 1997; 24:879–889.
- Wigley FM. Clinical practice. Raynaud’s phenomenon. N Engl J Med 2002; 347:1001–1018.
- Cutolo M, Pizzorni C, Sulli A. Capillaroscopy. Best Pract Res Clin Rheumatol 2005; 19:437–452.
- Lonzetti LS, Joyal F, Raynauld JP, et al. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum 2001; 44:735–736.
- Hudson M, Taillefer S, Steele R, et al. Improving the sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis. Clin Exp Rheumatol 2007; 25:754–757.
- Anders HJ, Sigl T, Schattenkirchner M. Differentiation between primary and secondary Raynaud’s phenomenon: a prospective study comparing nailfold capillaroscopy using an ophthalmoscope or stereomicroscope. Ann Rheum Dis 2001; 60:407–409.
- Spencer-Green G. Outcomes in primary Raynaud phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med 1998; 158:595–600.
- Wong ML, Highton J, Palmer DG. Sequential nailfold capillary microscopy in scleroderma and related disorders. Ann Rheum Dis 1988; 47:53–61.
- Weinstein A, Bordwell B, Stone B, Tibbetts C, Rothfield NF. Antibodies to native DNA and serum complement (C3) levels. Application to diagnosis and classification of systemic lupus erythematosus. Am J Med 1983; 74:206–216.
- Craft J. Antibodies to snRNPs in systemic lupus erythematosus. Rheum Dis Clin North Am 1992; 18:311–335.
- Weiner ES, Hildebrandt S, Senécal JL, et al. Prognostic significance of anticentromere antibodies and anti-topoisomerase I antibodies in Raynaud’s disease. A prospective study. Arthritis Rheum 1991; 34:68–77.
- Miller FW, Twitty SA, Biswas T, Plotz PH. Origin and regulation of a disease-specific autoantibody response. Antigenic epitopes, spectrotype stability, and isotype restriction of anti-Jo-1 autoantibodies. J Clin Invest 1990; 85:468–475.
- Ghirardello A, Zampieri S, Tarricone E, et al. Clinical implications of autoantibody screening in patients with autoimmune myositis. Autoimmunity 2006; 39:217–221.