User login
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
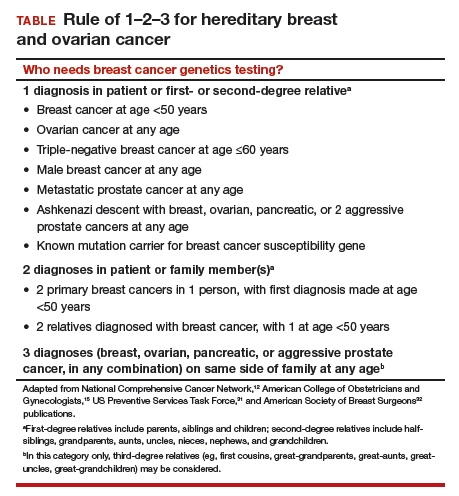
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13
Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13

Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13

Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
Take-home points
- The best genetics test is a good family history, updated annually
- Each year, 35,000 breast cancers are attributable to hereditary risk
- It is crucial to identify families at risk for hereditary breast cancer early, as cancers may begin in a woman's 30s; screening begins at age 25
- Multigene panel testing is efficient and cost-effective
- For patients who have highly penetrant pathogenic variants and are of childbearing age, preimplantation genetics diagnosis is an option