User login
Who needs breast cancer genetics testing?
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
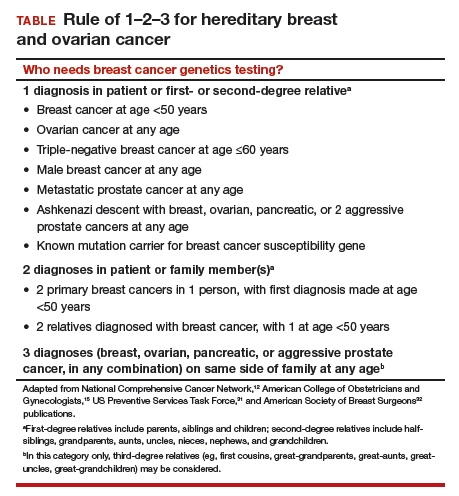
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13
Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13

Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13

Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
Take-home points
- The best genetics test is a good family history, updated annually
- Each year, 35,000 breast cancers are attributable to hereditary risk
- It is crucial to identify families at risk for hereditary breast cancer early, as cancers may begin in a woman's 30s; screening begins at age 25
- Multigene panel testing is efficient and cost-effective
- For patients who have highly penetrant pathogenic variants and are of childbearing age, preimplantation genetics diagnosis is an option
Managing patients at genetic risk of breast cancer
While most cases of breast cancer are sporadic (ie, not inherited), up to 10% are attributable to single-gene hereditary cancer syndromes.1–4 People with these syndromes have a lifetime risk of breast cancer much higher than in the general population, and the cancers often occur at a much earlier age.
With genetic testing becoming more common, primary care physicians need to be familiar with the known syndromes, associated risks, and evidence-based recommendations for management. Here, we review the management of cancer risk in the most common hereditary breast cancer syndromes, ie:
- Hereditary breast and ovarian cancer syndrome5
- Hereditary diffuse gastric cancer
- Cowden syndrome (PTEN hamartoma tumor syndrome)
- Peutz-Jeghers syndrome
- Li-Fraumeni syndrome.
IT TAKES A TEAM, BUT PRIMARY CARE PHYSICIANS ARE CENTRAL
Women who have a hereditary predisposition to breast cancer face complex and emotional decisions about the best ways to manage and reduce their risks. Their management includes close clinical surveillance, chemoprevention, and surgical risk reduction.1,4
Referral to multiple subspecialists is an important component of these patients’ preventive care. They may need referrals to a cancer genetic counselor, a high-risk breast clinic, a gynecologic oncologist, and counseling services. They may also require referrals to gastroenterologists, colorectal surgeons, endocrinologists, and endocrine surgeons, depending on the syndrome identified.
Consultation with a certified genetic counselor is critical for patients harboring mutations associated with cancer risk. The National Society of Genetic Counselors maintains a directory of genetic counselors by location and practice specialty at www.nsgc.org. The counselor’s evaluation will provide patients with a detailed explanation of the cancer risks and management guidelines for their particular condition, along with offering diagnostic genetic testing if appropriate. Women with germline mutations who plan to have children should be informed about preimplantation genetic diagnosis and about fertility specialists who can perform this service if they are interested in pursuing it.6
Screening and management guidelines for hereditary breast cancer syndromes are evolving. While subspecialists may be involved in enhanced surveillance and preventive care, the primary care physician is the central player, with both a broader perspective and knowledge of the patient’s competing medical issues, risks, and preferences.
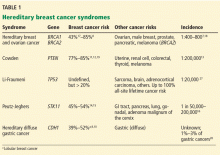
In addition to breast cancer, the risk of other malignancies is also higher, with the pattern varying by syndrome (Table 1).7–20 The management of these additional risks is beyond the scope of this review; however, primary care physicians need to be familiar with these risks to provide adequate referrals.
WHO IS AT INCREASED RISK OF BREAST CANCER?
In considering recommendations to reduce the risk of breast cancer, it is useful to think of a patient as being at either high risk or average risk.
The risk of breast cancer in women in the general population is about 12%, and most cases of breast cancer occur in patients who have no known risk factors for it. “High risk” of breast cancer generally means having more than a 20% lifetime risk (ie, before age 70) of developing the condition.
Even without a hereditary cancer syndrome, a combination of reproductive, environmental, personal, and family history factors can confer a 20% lifetime risk. But for women with hereditary syndromes, the risk far exceeds 20% regardless of such risk factors. It is likely that interactions with reproductive, environmental, and personal risk factors likely affect the individual risk of a woman with a known genetic mutation, and evidence is emerging with regard to further risk stratification.
In an earlier article in this journal, Smith and colleagues21 reviewed how to recognize heritable breast cancer syndromes. In general, referral for genetic counseling should be considered for patients and their families who have:
- Early-onset breast cancers (before age 50)
- Bilateral breast cancers at any age
- Ovarian cancers at any age
- “Triple-negative” breast cancers (ie, estrogen receptor-negative, progesterone receptor-negative, and human epidermal growth factor receptor 2-nonamplified (HER2-negative)
- Male breast cancer at any age
- Cancers affecting multiple individuals and in multiple generations.
- Breast, ovarian, pancreatic or prostate cancer in families with Ashkenazi Jewish ancestry
HEREDITARY BREAST CANCER SYNDROMES
Hereditary breast and ovarian cancer syndrome
The most common of these syndromes is hereditary breast and ovarian cancer syndrome, caused by germline mutations in the tumor-suppressor genes BRCA1 or BRCA2.7 The estimated prevalence of BRCA1 mutations is 1 in 250 to 300, and the prevalence of BRCA2 mutations is 1 in 800.1,4 However, in families of Ashkenazi Jewish ancestry, the population frequency of either a BRCA1 or BRCA2 mutation is approximately 1 in 40.1,4,6
Women with BRCA1 or BRCA2 mutations have a lifetime risk of breast cancer of up to 87%, or 5 to 7 times higher than in the general population, with the risk rising steeply beginning at age 30.1,5,8 In addition, the lifetime risk of ovarian cancer is nearly 59% in BRCA1 mutation carriers and 17% in BRCA2 mutation carriers.22
A meta-analysis found that BRCA1 mutation carriers diagnosed with cancer in one breast have a 5-year risk of developing cancer in the other breast of 15%, and BRCA2 mutation carriers have a risk of 9%.23 Overall, the risk of contralateral breast cancer is about 3% per year.3,4,24
BRCA1 mutations are strongly associated with triple-negative breast cancers.1,3,4
Hereditary diffuse gastric cancer
Hereditary diffuse gastric cancer is an autosomal-dominant syndrome associated with mutations in the CDH1 gene, although up to 75% of patients with this syndrome do not have an identifiable CDH1 mutation.9,25,26 In cases in which there is no identifiable CDH1 mutation, the diagnosis is made on the basis of the patient’s medical and family history.
Hereditary diffuse gastric cancer is associated with an increased risk of the lobular subtype of breast cancer as well as diffuse gastric cancer. The cumulative lifetime risk of breast cancer in women with CDH1 mutations is 39% to 52%,6,9–11,25 and their lifetime risk of diffuse gastric cancer is 83%.9 The combined risk of breast cancer and gastric cancer in women with this syndrome is 90% by age 80.9
Cowden syndrome (PTEN hamartoma tumor syndrome)
Cowden syndrome (PTEN hamartoma tumor syndrome) is caused by mutations in PTEN, another tumor-suppressor gene.11 The primary clinical concerns are melanoma and breast, endometrial, thyroid (follicular or papillary), colon, and renal cell cancers. Women with a PTEN mutation have a twofold greater risk of developing any type of cancer than men with a PTEN mutation.12 The cumulative lifetime risk of invasive breast cancer in women with this syndrome is 70% to 85%.11–13
Peutz-Jeghers syndrome
Peutz-Jeghers syndrome is an autosomal dominant polyposis disorder caused, in most patients, by a mutation in the serine/threonine kinase tumor-suppressor gene STK11.14
Patients with Peutz-Jeghers syndrome have higher risks of gastrointestinal, breast, gynecologic (uterine, ovarian, and cervical), pancreatic, and lung cancers. In women, the lifetime risk of breast cancer is 44% to 50% by age 70, regardless of the type of mutation.6,14,15 Breast cancers associated with Peutz-Jeghers syndrome are usually ductal, and the mean age at diagnosis is 37 years.16
Li-Fraumeni syndrome
Li-Fraumeni syndrome is an autosomal-dominant disorder caused by germline mutations in the TP53 gene, which codes for a transcription factor associated with cell proliferation and apoptosis.27
These mutations confer a lifetime cancer risk of 93% in women (mainly breast cancer) and 68% in men.1,27 Other cancers associated with TP53 mutations include sarcomas, brain cancer, leukemia, and adrenocortical tumors. Germline TP53 mutations are responsible for approximately 1% of all breast cancers.1,4
Breast cancers can occur at a young age in patients with a TP53 mutation. Women with TP53 mutations are 18 times more likely to develop breast cancer before age 45 compared with the general population.4
It is important to consider a TP53 mutation in premenopausal women or women less than 30 years of age with breast cancer who have no mutations in BRCA1 and BRCA2.1
MANAGING PATIENTS WITH GENETIC PREDISPOSITION TO BREAST CANCER
Management for patients at high risk fall into three broad categories: clinical surveillance, chemoprevention, and surgical risk reduction. The utility and benefit of each depend to a large degree on the patient’s specific mutation, family history, and comorbidities. Decisions must be shared with the patient.
CLOSE CLINICAL SURVEILLANCE
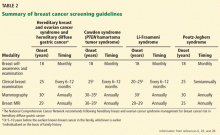
Consensus guidelines for cancer screening in the syndromes described here are available from the National Comprehensive Cancer Network at www.nccn.org and are summarized in Table 2.26,28 While the guidelines are broadly applicable to all women with these conditions, some individualization is required based on personal and family medical history.
In general, screening begins at the ages listed in Table 2 or 10 years earlier than the age at which cancer developed in the first affected relative, whichever is earlier. However, screening decisions are shared with the patient and are sometimes affected by significant out-of-pocket costs for the patient and anxiety resulting from the test or subsequent test findings, which must all be considered.
Breast self-awareness and clinical breast examination
Although controversial in the general population, breast self-examination is recommended for patients carrying mutations that increase risk.6
A discussion about breast self-awareness is recommended for all women at the age of 18. It should include the signs and symptoms of breast cancer, what feels “normal” to the patient, and what is known about modifiable risk factors for breast cancer. The patient should also be told to report any changes in her personal or family history.
Clinical breast examinations should be done every 6 months, as some cancers are found clinically, particularly in young women with dense tissue, and confirmed by diagnostic imaging and targeted ultrasonography.
Radiographic surveillance
Mammography and magnetic resonance imaging (MRI) are also important components of a breast cancer surveillance regimen in women at high risk. Adherence to a well-formulated plan of clinical and radiographic examinations increases early detection in patients who have a hereditary predisposition to breast cancer.
MRI is more sensitive than mammography and reduces the likelihood of finding advanced cancers by up to 70% compared with mammography in women at high risk of breast cancer.29–31 The sensitivity of breast MRI alone ranges from 71% to 100%, and the sensitivity increases to 89% to 100% when combined with mammography. In contrast, the sensitivity of mammography alone is 25% to 59%.29 MRI has also been shown to be cost-effective when added to mammography and physical examination in women at high risk.5,32
Adding MRI to the breast cancer screening regimen has been under discussion and has been endorsed by the American Cancer Society in formal recommendations set forth in 2007 for patients with known hereditary cancer syndromes, in untested first-degree relatives of identified genetic mutation carriers, or in women who have an estimated lifetime risk of breast cancer of 20% or more, as determined by models largely dependent on family history.33
But MRI has a downside—it is less specific than mammography.29,33 Its lower specificity (77% to 90% vs 95% with mammography alone) leads to additional radiographic studies and tissue samplings for the “suspicious” lesions discovered. From 3% to 15% of screening breast MRIs result in a biopsy, and the proportion of biopsies that reveal cancer is 13% to 40%.33 Furthermore, by itself, MRI has not been shown to reduce mortality in any high-risk group.
Mammography remains useful in conjunction with MRI due to its ability to detect breast calcifications, which may be the earliest sign of breast cancer, and ability to detect changes in breast architecture. A typical screening program (Table 2) should incorporate both modalities, commonly offset by 6 months (eg, mammography at baseline, then MRI 6 months later, then mammography again 6 months after that, and so on) to increase the detection of interval cancer development.
Chemoprevention
Chemoprevention means taking medications to reduce the risk. Certain selective estrogen receptor modulators and aromatase inhibitors decrease the risk of invasive breast cancer in healthy women at high risk. These drugs include tamoxifen, which can be used before menopause, and raloxifene, anastrozole, and exemestane, which must be used only after menopause.
Because data are limited, we cannot make any generalized recommendations about chemoprevention in patients with hereditary breast cancer syndromes. Decisions about chemoprevention should take into account the patient’s personal and family histories. Often, a medical oncologist or medical breast specialist can help by discussing the risks and benefits for the individual patient.
Tamoxifen has been the most studied, mainly in BRCA mutation carriers.6,34–37 As in the general population, tamoxifen reduces the incidence of estrogen receptor-positive breast cancers by 50%.36–38 It has not been shown to significantly reduce breast cancer risk in premenopausal women with BRCA1 mutations,37 most likely because most cancers that occur in this group are estrogen receptor-negative. In patients with a history of breast cancer, however, tamoxifen has been shown to reduce the risk of developing contralateral breast cancer by 45% to 60% in both BRCA1 and BRCA2 mutation carriers.6,35
There is also little evidence that giving a chemopreventive agent after bilateral salpingo-oophorectomy reduces the risk further in premenopausal BRCA mutation carriers.35 These patients often receive hormonal therapy with estrogen, which currently would preclude the use of tamoxifen. Tamoxifen in postmenopausal women is associated with a small increased risk of venous thromboembolic disease and endometrial cancer.38
Oral contraceptives reduce the risk of ovarian cancer by up to 50% in BRCA1 mutation carriers and up to 60% in BRCA2 mutation carriers.6 However, data conflict on their effect on the risk of breast cancer in BRCA1 and BRCA2 mutation carriers.39
Decisions about chemoprevention with agents other than tamoxifen and in syndromes other than hereditary breast and ovarian cancer syndrome must take into consideration the existing lack of data in this area.
SURGICAL PROPHYLAXIS
Surgical prophylactic options for patients at genetic risk of breast cancer are bilateral mastectomy and bilateral salpingo-oophorectomy.
Prophylactic mastectomy
Bilateral risk-reducing mastectomy reduces the risk of breast cancer by at least 90%24,39,40 and greatly reduces the need for complex surveillance. Patients are often followed annually clinically, with single-view mammography if they have tissue flap reconstruction.
Nipple-sparing and skin-sparing mastectomies, which facilitate reconstruction and cosmetic outcomes, are an option in the risk-reduction setting and have been shown thus far to be safe.41–43 In patients with breast cancer, the overall breast cancer recurrence rates with nipple-sparing mastectomy are similar to those of traditional mastectomy and breast conservation treatment.41
In patients at very high risk of breast cancer, risk-reducing operations also reduce the risk of ultimately needing chemotherapy and radiation to treat breast cancer, as the risk of developing breast cancer is significantly lowered.
The timing of risk-reducing mastectomy depends largely on personal and family medical history and personal choice. Bilateral mastectomy at age 25 results in the greatest survival gain for patients with hereditary breast and ovarian cancer syndrome.5 Such precise data are not available for other hereditary cancer syndromes, but it is reasonable to consider bilateral mastectomy as an option for any woman with a highly penetrant genetic mutation that predisposes her to breast cancer. Special consideration in the timing of risk-reducing mastectomy must be given to women with Li-Fraumeni syndrome, as this condition is often associated with an earlier age at breast cancer diagnosis (before age 30).1
Family planning, sexuality, self-image, and the anxiety associated with both cancer risk and surveillance are all factors women consider when deciding whether and when to undergo mastectomy. A survey of 12 high-risk women who elected prophylactic mastectomy elicited feelings of some regret in 3 of them, while all expressed a sense of relief and reduced anxiety related to both cancer risk and screenings.24 Another group of 14 women surveyed after the surgery reported initial distress related to physical appearance, self-image, and intimacy but also reported a significant decrease in anxiety related to breast cancer risk and were largely satisfied with their decision.44
Prophylactic salpingo-oophorectomy
In patients who have pathogenic mutations in BRCA1 or 2, prophylactic salpingo-oophorectomy before age 40 decreases the risk of ovarian cancer by up to 96% and breast cancer by 50%.1,37,45 This operation, in fact, is the only intervention that has been shown to reduce the mortality rate in patients with a hereditary predisposition to cancer.46
We recommend that women with hereditary breast and ovarian cancer syndrome strongly consider prophylactic salpingo-oophorectomy by age 40 or when childbearing is complete for the greatest reduction in risk.1,5 In 2006, Domchek et al46 reported an overall decrease in the mortality rate in BRCA1/2-positive patients who underwent this surgery, but not in breast cancer-specific or ovarian cancer-specific mortality.
On the other hand, removing the ovaries before menopause places women at risk of serious complications associated with premature loss of gonadal hormones, including cardiovascular disease, decreased bone density, reduced sexual satisfaction, dyspareunia, hot flashes, and night sweats.47 Therefore, it is generally reserved for women who are also at risk of ovarian cancer.
Hormonal therapy, ie, estrogen therapy for patients who choose complete hysterectomy, and estrogen-progesterone therapy for patients who choose to keep their uterus, reduces menopausal symptoms and symptoms of sexual dissatisfaction and has not thus far been shown to increase breast cancer risk.1,34 However, this information is from nonrandomized studies, which are inherently limited.
It is important to address and modify risk factors for heart disease and osteoporosis in women with premature surgical menopause, as they may be particularly vulnerable to these conditions.
HEREDITARY BREAST CANCER IN MEN
Fewer than 1% of cases of breast cancer arise in men, and fewer than 1% of cases of cancer in men are breast cancer.
Male breast cancer is more likely than female breast cancer to be estrogen receptor- and progesterone receptor-positive. In an analysis of the Surveillance, Epidemiology, and End Results registry between 1973 and 2005, triple-negative breast cancer was found in 23% of female patients but only 7.6% of male patients.2
Male breast cancer is most common in families with BRCA2, and to a lesser degree, BRCA1 mutations. Other genetic disorders including Li-Fraumeni syndrome, hereditary nonpolyposis colorectal cancer, and Klinefelter syndrome also increase the risk of male breast cancer. A genetic predisposition for breast cancer is present in approximately 10% of male breast cancer patients.2 Any man with breast cancer, therefore, should be referred for genetic counseling.
In men, a BRCA2 mutation confers a lifetime risk of breast cancer of 5% to 10%.2 This is similar to the lifetime risk of breast cancer for the average woman but it is still significant, as the lifetime risk of breast cancer for the average man is 0.1%.1,2
Five-year survival rates in male breast cancer range from only 36% to 66%, most likely because it is usually diagnosed in later stages, as men are not routinely screened for breast cancer. In men with known hereditary susceptibility, National Comprehensive Cancer Network guidelines recommend that they be educated about and begin breast self-examination at the age of 35 and be clinically examined every 12 months starting at age 35.48 There are limited data to support breast imaging in men. High-risk surveillance with MRI screening in this group is not recommended. Prostate cancer screening is recommended for men with BRCA2 mutations starting at age 40, and should be considered for men with BRCA1 mutations starting at age 40.
No specific guidelines exist for pancreatic cancer and melanoma, but screening may be individualized based on cancers observed in the family.
- Daly MB, Axilbund JE, Buys S, et al; National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw 2010; 8:562–594.
- Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol 2010; 28:2114–2122.
- Foulkes WD. Inherited susceptibility to common cancers. N Engl J Med 2008; 359:2143–2153.
- Schwartz GF, Hughes KS, Lynch HT, et al. Proceedings of the international consensus conference on breast cancer risk, genetics, and risk management, April 2007. Breast J 2009; 15:4–16.
- Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol 2010; 28:222–231.
- National Comprehensive Cancer Network Guidelines Version 2.2014. Genetic/familial high risk assessment: breast and ovarian. www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed January 22, 2016.
- Ford D, Easton DF, Peto J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am J Hum Genet 1995; 57:1457–1462.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Pharoah PD, Guilford P, Caldas C; International Gastric Cancer Linkage Consortium. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001; 121:1348–1353.
- Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007; 297:2360–2372.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Bubien V, Bonnet F, Brouste V, et al; French Cowden Disease Network. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J Med Genet 2013; 50:255–263.
- Nelen MR, Kremer H, Konings IB, et al. Novel PTEN mutations in patients with Cowden disease: absence of clear genotype-phenotype correlations. Eur J Hum Genet 1999; 7:267–273.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000; 119:1447–1453.
- Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut 2010; 59:975–986.
- Chen S, Iversen ES, Friebel T, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol 2006; 24:863–871.
- Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer 1996; 77:2318–2324.
- Riegert-Johnson DL, Gleeson FC, Roberts M, et al. Cancer and Lhermitte-Duclos disease are common in Cowden syndrome patients. Hered Cancer Clin Pract 2010; 8:6.
- Stone J, Bevan S, Cunningham D, et al. Low frequency of germline E-cadherin mutations in familial and nonfamilial gastric cancer. Br J Cancer 1999; 79:1935–1937.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med 2014; 81:31–40.
- Mavaddat N, Peock S, Frost D, et al; EMBRACE. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst 2013; 105:812–822.
- Molina-Montes E, Pérez-Nevot B, Pollán M, Sánchez-Cantalejo E, Espín J, Sánchez MJ. Cumulative risk of second primary contralateral breast cancer in BRCA1/BRCA2 mutation carriers with a first breast cancer: a systematic review and meta-analysis. Breast 2014; 23:721–742.
- Kwong A, Chu AT. What made her give up her breasts: a qualitative study on decisional considerations for contralateral prophylactic mastectomy among breast cancer survivors undergoing BRCA1/2 genetic testing. Asian Pac J Cancer Prev 2012; 13:2241–2247.
- Dixon M, Seevaratnam R, Wirtzfeld D, et al. A RAND/UCLA appropriateness study of the management of familial gastric cancer. Ann Surg Oncol 2013; 20:533–541.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- National Comprehensive Cancer Network Guidelines Version 1. 2015. Gastric Cancer. www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Accessed January 22, 2016.
- Warner, E. Impact of MRI surveillance and breast cancer detection in young women with BRCA mutations. Ann Oncol 2011; 22(suppl 1):i44–i49.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004; 351:427–437.
- Pederson HJ, O’Rourke C, Lyons J, Patrick RJ, Crowe JP Jr, Grobmyer SR. Time-related changes in yield and harms of screening breast magnetic resonance imaging. Clin Breast Cancer 2015 Jan 21: S1526-8209(15)00024–00025. Epub ahead of print.
- Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat 2011; 125:837–847.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75–89.
- Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE study group. J Clin Oncol 2005; 23:7804–7610.
- Narod SA, Brunet JS, Ghadirian P, et al; Hereditary Breast Cancer Clinical Study Group. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case control study. Lancet 2000; 356:1876–1881.
- Njiaju UO, Olopade OI. Genetic determinants of breast cancer risk: a review of the current literature and issues pertaining to clinical application. Breast J 2012; 18:436–442.
- King MC, Wieand S, Hale K, et al; National Surgical Adjuvant Breast and Bowel Project. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention trial. JAMA 2001; 286:2251–2256.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388.
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004; 22:1055–1062.
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999; 340:77–84.
- Mallon P, Feron JG, Couturaud B, et al. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg 2013; 131:969–984.
- Stanec Z, Žic R, Budi S, et al. Skin and nipple-areola complex sparing mastectomy in breast cancer patients: 15-year experience. Ann Plast Surg 2014; 73:485–491.
- Eisenberg RE, Chan JS, Swistel AJ, Hoda SA. Pathological evaluation of nipple-sparing mastectomies with emphasis on occult nipple involvement: the Weill-Cornell experience with 325 cases. Breast J 2014; 20:15–21.
- Lodder LN, Frets PG, Trijsburg RW, et al. One year follow-up of women opting for presymptomatic testing for BRCA1 and BRCA2: emotional impact of the test outcome and decisions on risk management (surveillance or prophylactic surgery). Breast Cancer Res Treat 2002; 73:97–112.
- Rebbeck TR, Lynch HT, Neuhausen SL, et al; Prevention and Observation of Surgical End Points Study Group. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 2002; 346:1616–1622.
- Domchek SM, Friebel TM, Neuhausen SL, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol 2006; 7:223–229.
- Finch A, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Womens Health (Lond Engl) 2012; 8:543–555.
- National Comprehensive Cancer Network Guidelines. Version 2.2015. Genetic/Familial High-Risk Assessment Breast and Ovarian. www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed February 8, 2016.
While most cases of breast cancer are sporadic (ie, not inherited), up to 10% are attributable to single-gene hereditary cancer syndromes.1–4 People with these syndromes have a lifetime risk of breast cancer much higher than in the general population, and the cancers often occur at a much earlier age.
With genetic testing becoming more common, primary care physicians need to be familiar with the known syndromes, associated risks, and evidence-based recommendations for management. Here, we review the management of cancer risk in the most common hereditary breast cancer syndromes, ie:
- Hereditary breast and ovarian cancer syndrome5
- Hereditary diffuse gastric cancer
- Cowden syndrome (PTEN hamartoma tumor syndrome)
- Peutz-Jeghers syndrome
- Li-Fraumeni syndrome.
IT TAKES A TEAM, BUT PRIMARY CARE PHYSICIANS ARE CENTRAL
Women who have a hereditary predisposition to breast cancer face complex and emotional decisions about the best ways to manage and reduce their risks. Their management includes close clinical surveillance, chemoprevention, and surgical risk reduction.1,4
Referral to multiple subspecialists is an important component of these patients’ preventive care. They may need referrals to a cancer genetic counselor, a high-risk breast clinic, a gynecologic oncologist, and counseling services. They may also require referrals to gastroenterologists, colorectal surgeons, endocrinologists, and endocrine surgeons, depending on the syndrome identified.
Consultation with a certified genetic counselor is critical for patients harboring mutations associated with cancer risk. The National Society of Genetic Counselors maintains a directory of genetic counselors by location and practice specialty at www.nsgc.org. The counselor’s evaluation will provide patients with a detailed explanation of the cancer risks and management guidelines for their particular condition, along with offering diagnostic genetic testing if appropriate. Women with germline mutations who plan to have children should be informed about preimplantation genetic diagnosis and about fertility specialists who can perform this service if they are interested in pursuing it.6
Screening and management guidelines for hereditary breast cancer syndromes are evolving. While subspecialists may be involved in enhanced surveillance and preventive care, the primary care physician is the central player, with both a broader perspective and knowledge of the patient’s competing medical issues, risks, and preferences.
In addition to breast cancer, the risk of other malignancies is also higher, with the pattern varying by syndrome (Table 1).7–20 The management of these additional risks is beyond the scope of this review; however, primary care physicians need to be familiar with these risks to provide adequate referrals.
WHO IS AT INCREASED RISK OF BREAST CANCER?
In considering recommendations to reduce the risk of breast cancer, it is useful to think of a patient as being at either high risk or average risk.
The risk of breast cancer in women in the general population is about 12%, and most cases of breast cancer occur in patients who have no known risk factors for it. “High risk” of breast cancer generally means having more than a 20% lifetime risk (ie, before age 70) of developing the condition.
Even without a hereditary cancer syndrome, a combination of reproductive, environmental, personal, and family history factors can confer a 20% lifetime risk. But for women with hereditary syndromes, the risk far exceeds 20% regardless of such risk factors. It is likely that interactions with reproductive, environmental, and personal risk factors likely affect the individual risk of a woman with a known genetic mutation, and evidence is emerging with regard to further risk stratification.
In an earlier article in this journal, Smith and colleagues21 reviewed how to recognize heritable breast cancer syndromes. In general, referral for genetic counseling should be considered for patients and their families who have:
- Early-onset breast cancers (before age 50)
- Bilateral breast cancers at any age
- Ovarian cancers at any age
- “Triple-negative” breast cancers (ie, estrogen receptor-negative, progesterone receptor-negative, and human epidermal growth factor receptor 2-nonamplified (HER2-negative)
- Male breast cancer at any age
- Cancers affecting multiple individuals and in multiple generations.
- Breast, ovarian, pancreatic or prostate cancer in families with Ashkenazi Jewish ancestry
HEREDITARY BREAST CANCER SYNDROMES
Hereditary breast and ovarian cancer syndrome
The most common of these syndromes is hereditary breast and ovarian cancer syndrome, caused by germline mutations in the tumor-suppressor genes BRCA1 or BRCA2.7 The estimated prevalence of BRCA1 mutations is 1 in 250 to 300, and the prevalence of BRCA2 mutations is 1 in 800.1,4 However, in families of Ashkenazi Jewish ancestry, the population frequency of either a BRCA1 or BRCA2 mutation is approximately 1 in 40.1,4,6
Women with BRCA1 or BRCA2 mutations have a lifetime risk of breast cancer of up to 87%, or 5 to 7 times higher than in the general population, with the risk rising steeply beginning at age 30.1,5,8 In addition, the lifetime risk of ovarian cancer is nearly 59% in BRCA1 mutation carriers and 17% in BRCA2 mutation carriers.22
A meta-analysis found that BRCA1 mutation carriers diagnosed with cancer in one breast have a 5-year risk of developing cancer in the other breast of 15%, and BRCA2 mutation carriers have a risk of 9%.23 Overall, the risk of contralateral breast cancer is about 3% per year.3,4,24
BRCA1 mutations are strongly associated with triple-negative breast cancers.1,3,4
Hereditary diffuse gastric cancer
Hereditary diffuse gastric cancer is an autosomal-dominant syndrome associated with mutations in the CDH1 gene, although up to 75% of patients with this syndrome do not have an identifiable CDH1 mutation.9,25,26 In cases in which there is no identifiable CDH1 mutation, the diagnosis is made on the basis of the patient’s medical and family history.
Hereditary diffuse gastric cancer is associated with an increased risk of the lobular subtype of breast cancer as well as diffuse gastric cancer. The cumulative lifetime risk of breast cancer in women with CDH1 mutations is 39% to 52%,6,9–11,25 and their lifetime risk of diffuse gastric cancer is 83%.9 The combined risk of breast cancer and gastric cancer in women with this syndrome is 90% by age 80.9
Cowden syndrome (PTEN hamartoma tumor syndrome)
Cowden syndrome (PTEN hamartoma tumor syndrome) is caused by mutations in PTEN, another tumor-suppressor gene.11 The primary clinical concerns are melanoma and breast, endometrial, thyroid (follicular or papillary), colon, and renal cell cancers. Women with a PTEN mutation have a twofold greater risk of developing any type of cancer than men with a PTEN mutation.12 The cumulative lifetime risk of invasive breast cancer in women with this syndrome is 70% to 85%.11–13
Peutz-Jeghers syndrome
Peutz-Jeghers syndrome is an autosomal dominant polyposis disorder caused, in most patients, by a mutation in the serine/threonine kinase tumor-suppressor gene STK11.14
Patients with Peutz-Jeghers syndrome have higher risks of gastrointestinal, breast, gynecologic (uterine, ovarian, and cervical), pancreatic, and lung cancers. In women, the lifetime risk of breast cancer is 44% to 50% by age 70, regardless of the type of mutation.6,14,15 Breast cancers associated with Peutz-Jeghers syndrome are usually ductal, and the mean age at diagnosis is 37 years.16
Li-Fraumeni syndrome
Li-Fraumeni syndrome is an autosomal-dominant disorder caused by germline mutations in the TP53 gene, which codes for a transcription factor associated with cell proliferation and apoptosis.27
These mutations confer a lifetime cancer risk of 93% in women (mainly breast cancer) and 68% in men.1,27 Other cancers associated with TP53 mutations include sarcomas, brain cancer, leukemia, and adrenocortical tumors. Germline TP53 mutations are responsible for approximately 1% of all breast cancers.1,4
Breast cancers can occur at a young age in patients with a TP53 mutation. Women with TP53 mutations are 18 times more likely to develop breast cancer before age 45 compared with the general population.4
It is important to consider a TP53 mutation in premenopausal women or women less than 30 years of age with breast cancer who have no mutations in BRCA1 and BRCA2.1
MANAGING PATIENTS WITH GENETIC PREDISPOSITION TO BREAST CANCER
Management for patients at high risk fall into three broad categories: clinical surveillance, chemoprevention, and surgical risk reduction. The utility and benefit of each depend to a large degree on the patient’s specific mutation, family history, and comorbidities. Decisions must be shared with the patient.
CLOSE CLINICAL SURVEILLANCE
Consensus guidelines for cancer screening in the syndromes described here are available from the National Comprehensive Cancer Network at www.nccn.org and are summarized in Table 2.26,28 While the guidelines are broadly applicable to all women with these conditions, some individualization is required based on personal and family medical history.
In general, screening begins at the ages listed in Table 2 or 10 years earlier than the age at which cancer developed in the first affected relative, whichever is earlier. However, screening decisions are shared with the patient and are sometimes affected by significant out-of-pocket costs for the patient and anxiety resulting from the test or subsequent test findings, which must all be considered.
Breast self-awareness and clinical breast examination
Although controversial in the general population, breast self-examination is recommended for patients carrying mutations that increase risk.6
A discussion about breast self-awareness is recommended for all women at the age of 18. It should include the signs and symptoms of breast cancer, what feels “normal” to the patient, and what is known about modifiable risk factors for breast cancer. The patient should also be told to report any changes in her personal or family history.
Clinical breast examinations should be done every 6 months, as some cancers are found clinically, particularly in young women with dense tissue, and confirmed by diagnostic imaging and targeted ultrasonography.
Radiographic surveillance
Mammography and magnetic resonance imaging (MRI) are also important components of a breast cancer surveillance regimen in women at high risk. Adherence to a well-formulated plan of clinical and radiographic examinations increases early detection in patients who have a hereditary predisposition to breast cancer.
MRI is more sensitive than mammography and reduces the likelihood of finding advanced cancers by up to 70% compared with mammography in women at high risk of breast cancer.29–31 The sensitivity of breast MRI alone ranges from 71% to 100%, and the sensitivity increases to 89% to 100% when combined with mammography. In contrast, the sensitivity of mammography alone is 25% to 59%.29 MRI has also been shown to be cost-effective when added to mammography and physical examination in women at high risk.5,32
Adding MRI to the breast cancer screening regimen has been under discussion and has been endorsed by the American Cancer Society in formal recommendations set forth in 2007 for patients with known hereditary cancer syndromes, in untested first-degree relatives of identified genetic mutation carriers, or in women who have an estimated lifetime risk of breast cancer of 20% or more, as determined by models largely dependent on family history.33
But MRI has a downside—it is less specific than mammography.29,33 Its lower specificity (77% to 90% vs 95% with mammography alone) leads to additional radiographic studies and tissue samplings for the “suspicious” lesions discovered. From 3% to 15% of screening breast MRIs result in a biopsy, and the proportion of biopsies that reveal cancer is 13% to 40%.33 Furthermore, by itself, MRI has not been shown to reduce mortality in any high-risk group.
Mammography remains useful in conjunction with MRI due to its ability to detect breast calcifications, which may be the earliest sign of breast cancer, and ability to detect changes in breast architecture. A typical screening program (Table 2) should incorporate both modalities, commonly offset by 6 months (eg, mammography at baseline, then MRI 6 months later, then mammography again 6 months after that, and so on) to increase the detection of interval cancer development.
Chemoprevention
Chemoprevention means taking medications to reduce the risk. Certain selective estrogen receptor modulators and aromatase inhibitors decrease the risk of invasive breast cancer in healthy women at high risk. These drugs include tamoxifen, which can be used before menopause, and raloxifene, anastrozole, and exemestane, which must be used only after menopause.
Because data are limited, we cannot make any generalized recommendations about chemoprevention in patients with hereditary breast cancer syndromes. Decisions about chemoprevention should take into account the patient’s personal and family histories. Often, a medical oncologist or medical breast specialist can help by discussing the risks and benefits for the individual patient.
Tamoxifen has been the most studied, mainly in BRCA mutation carriers.6,34–37 As in the general population, tamoxifen reduces the incidence of estrogen receptor-positive breast cancers by 50%.36–38 It has not been shown to significantly reduce breast cancer risk in premenopausal women with BRCA1 mutations,37 most likely because most cancers that occur in this group are estrogen receptor-negative. In patients with a history of breast cancer, however, tamoxifen has been shown to reduce the risk of developing contralateral breast cancer by 45% to 60% in both BRCA1 and BRCA2 mutation carriers.6,35
There is also little evidence that giving a chemopreventive agent after bilateral salpingo-oophorectomy reduces the risk further in premenopausal BRCA mutation carriers.35 These patients often receive hormonal therapy with estrogen, which currently would preclude the use of tamoxifen. Tamoxifen in postmenopausal women is associated with a small increased risk of venous thromboembolic disease and endometrial cancer.38
Oral contraceptives reduce the risk of ovarian cancer by up to 50% in BRCA1 mutation carriers and up to 60% in BRCA2 mutation carriers.6 However, data conflict on their effect on the risk of breast cancer in BRCA1 and BRCA2 mutation carriers.39
Decisions about chemoprevention with agents other than tamoxifen and in syndromes other than hereditary breast and ovarian cancer syndrome must take into consideration the existing lack of data in this area.
SURGICAL PROPHYLAXIS
Surgical prophylactic options for patients at genetic risk of breast cancer are bilateral mastectomy and bilateral salpingo-oophorectomy.
Prophylactic mastectomy
Bilateral risk-reducing mastectomy reduces the risk of breast cancer by at least 90%24,39,40 and greatly reduces the need for complex surveillance. Patients are often followed annually clinically, with single-view mammography if they have tissue flap reconstruction.
Nipple-sparing and skin-sparing mastectomies, which facilitate reconstruction and cosmetic outcomes, are an option in the risk-reduction setting and have been shown thus far to be safe.41–43 In patients with breast cancer, the overall breast cancer recurrence rates with nipple-sparing mastectomy are similar to those of traditional mastectomy and breast conservation treatment.41
In patients at very high risk of breast cancer, risk-reducing operations also reduce the risk of ultimately needing chemotherapy and radiation to treat breast cancer, as the risk of developing breast cancer is significantly lowered.
The timing of risk-reducing mastectomy depends largely on personal and family medical history and personal choice. Bilateral mastectomy at age 25 results in the greatest survival gain for patients with hereditary breast and ovarian cancer syndrome.5 Such precise data are not available for other hereditary cancer syndromes, but it is reasonable to consider bilateral mastectomy as an option for any woman with a highly penetrant genetic mutation that predisposes her to breast cancer. Special consideration in the timing of risk-reducing mastectomy must be given to women with Li-Fraumeni syndrome, as this condition is often associated with an earlier age at breast cancer diagnosis (before age 30).1
Family planning, sexuality, self-image, and the anxiety associated with both cancer risk and surveillance are all factors women consider when deciding whether and when to undergo mastectomy. A survey of 12 high-risk women who elected prophylactic mastectomy elicited feelings of some regret in 3 of them, while all expressed a sense of relief and reduced anxiety related to both cancer risk and screenings.24 Another group of 14 women surveyed after the surgery reported initial distress related to physical appearance, self-image, and intimacy but also reported a significant decrease in anxiety related to breast cancer risk and were largely satisfied with their decision.44
Prophylactic salpingo-oophorectomy
In patients who have pathogenic mutations in BRCA1 or 2, prophylactic salpingo-oophorectomy before age 40 decreases the risk of ovarian cancer by up to 96% and breast cancer by 50%.1,37,45 This operation, in fact, is the only intervention that has been shown to reduce the mortality rate in patients with a hereditary predisposition to cancer.46
We recommend that women with hereditary breast and ovarian cancer syndrome strongly consider prophylactic salpingo-oophorectomy by age 40 or when childbearing is complete for the greatest reduction in risk.1,5 In 2006, Domchek et al46 reported an overall decrease in the mortality rate in BRCA1/2-positive patients who underwent this surgery, but not in breast cancer-specific or ovarian cancer-specific mortality.
On the other hand, removing the ovaries before menopause places women at risk of serious complications associated with premature loss of gonadal hormones, including cardiovascular disease, decreased bone density, reduced sexual satisfaction, dyspareunia, hot flashes, and night sweats.47 Therefore, it is generally reserved for women who are also at risk of ovarian cancer.
Hormonal therapy, ie, estrogen therapy for patients who choose complete hysterectomy, and estrogen-progesterone therapy for patients who choose to keep their uterus, reduces menopausal symptoms and symptoms of sexual dissatisfaction and has not thus far been shown to increase breast cancer risk.1,34 However, this information is from nonrandomized studies, which are inherently limited.
It is important to address and modify risk factors for heart disease and osteoporosis in women with premature surgical menopause, as they may be particularly vulnerable to these conditions.
HEREDITARY BREAST CANCER IN MEN
Fewer than 1% of cases of breast cancer arise in men, and fewer than 1% of cases of cancer in men are breast cancer.
Male breast cancer is more likely than female breast cancer to be estrogen receptor- and progesterone receptor-positive. In an analysis of the Surveillance, Epidemiology, and End Results registry between 1973 and 2005, triple-negative breast cancer was found in 23% of female patients but only 7.6% of male patients.2
Male breast cancer is most common in families with BRCA2, and to a lesser degree, BRCA1 mutations. Other genetic disorders including Li-Fraumeni syndrome, hereditary nonpolyposis colorectal cancer, and Klinefelter syndrome also increase the risk of male breast cancer. A genetic predisposition for breast cancer is present in approximately 10% of male breast cancer patients.2 Any man with breast cancer, therefore, should be referred for genetic counseling.
In men, a BRCA2 mutation confers a lifetime risk of breast cancer of 5% to 10%.2 This is similar to the lifetime risk of breast cancer for the average woman but it is still significant, as the lifetime risk of breast cancer for the average man is 0.1%.1,2
Five-year survival rates in male breast cancer range from only 36% to 66%, most likely because it is usually diagnosed in later stages, as men are not routinely screened for breast cancer. In men with known hereditary susceptibility, National Comprehensive Cancer Network guidelines recommend that they be educated about and begin breast self-examination at the age of 35 and be clinically examined every 12 months starting at age 35.48 There are limited data to support breast imaging in men. High-risk surveillance with MRI screening in this group is not recommended. Prostate cancer screening is recommended for men with BRCA2 mutations starting at age 40, and should be considered for men with BRCA1 mutations starting at age 40.
No specific guidelines exist for pancreatic cancer and melanoma, but screening may be individualized based on cancers observed in the family.
While most cases of breast cancer are sporadic (ie, not inherited), up to 10% are attributable to single-gene hereditary cancer syndromes.1–4 People with these syndromes have a lifetime risk of breast cancer much higher than in the general population, and the cancers often occur at a much earlier age.
With genetic testing becoming more common, primary care physicians need to be familiar with the known syndromes, associated risks, and evidence-based recommendations for management. Here, we review the management of cancer risk in the most common hereditary breast cancer syndromes, ie:
- Hereditary breast and ovarian cancer syndrome5
- Hereditary diffuse gastric cancer
- Cowden syndrome (PTEN hamartoma tumor syndrome)
- Peutz-Jeghers syndrome
- Li-Fraumeni syndrome.
IT TAKES A TEAM, BUT PRIMARY CARE PHYSICIANS ARE CENTRAL
Women who have a hereditary predisposition to breast cancer face complex and emotional decisions about the best ways to manage and reduce their risks. Their management includes close clinical surveillance, chemoprevention, and surgical risk reduction.1,4
Referral to multiple subspecialists is an important component of these patients’ preventive care. They may need referrals to a cancer genetic counselor, a high-risk breast clinic, a gynecologic oncologist, and counseling services. They may also require referrals to gastroenterologists, colorectal surgeons, endocrinologists, and endocrine surgeons, depending on the syndrome identified.
Consultation with a certified genetic counselor is critical for patients harboring mutations associated with cancer risk. The National Society of Genetic Counselors maintains a directory of genetic counselors by location and practice specialty at www.nsgc.org. The counselor’s evaluation will provide patients with a detailed explanation of the cancer risks and management guidelines for their particular condition, along with offering diagnostic genetic testing if appropriate. Women with germline mutations who plan to have children should be informed about preimplantation genetic diagnosis and about fertility specialists who can perform this service if they are interested in pursuing it.6
Screening and management guidelines for hereditary breast cancer syndromes are evolving. While subspecialists may be involved in enhanced surveillance and preventive care, the primary care physician is the central player, with both a broader perspective and knowledge of the patient’s competing medical issues, risks, and preferences.
In addition to breast cancer, the risk of other malignancies is also higher, with the pattern varying by syndrome (Table 1).7–20 The management of these additional risks is beyond the scope of this review; however, primary care physicians need to be familiar with these risks to provide adequate referrals.
WHO IS AT INCREASED RISK OF BREAST CANCER?
In considering recommendations to reduce the risk of breast cancer, it is useful to think of a patient as being at either high risk or average risk.
The risk of breast cancer in women in the general population is about 12%, and most cases of breast cancer occur in patients who have no known risk factors for it. “High risk” of breast cancer generally means having more than a 20% lifetime risk (ie, before age 70) of developing the condition.
Even without a hereditary cancer syndrome, a combination of reproductive, environmental, personal, and family history factors can confer a 20% lifetime risk. But for women with hereditary syndromes, the risk far exceeds 20% regardless of such risk factors. It is likely that interactions with reproductive, environmental, and personal risk factors likely affect the individual risk of a woman with a known genetic mutation, and evidence is emerging with regard to further risk stratification.
In an earlier article in this journal, Smith and colleagues21 reviewed how to recognize heritable breast cancer syndromes. In general, referral for genetic counseling should be considered for patients and their families who have:
- Early-onset breast cancers (before age 50)
- Bilateral breast cancers at any age
- Ovarian cancers at any age
- “Triple-negative” breast cancers (ie, estrogen receptor-negative, progesterone receptor-negative, and human epidermal growth factor receptor 2-nonamplified (HER2-negative)
- Male breast cancer at any age
- Cancers affecting multiple individuals and in multiple generations.
- Breast, ovarian, pancreatic or prostate cancer in families with Ashkenazi Jewish ancestry
HEREDITARY BREAST CANCER SYNDROMES
Hereditary breast and ovarian cancer syndrome
The most common of these syndromes is hereditary breast and ovarian cancer syndrome, caused by germline mutations in the tumor-suppressor genes BRCA1 or BRCA2.7 The estimated prevalence of BRCA1 mutations is 1 in 250 to 300, and the prevalence of BRCA2 mutations is 1 in 800.1,4 However, in families of Ashkenazi Jewish ancestry, the population frequency of either a BRCA1 or BRCA2 mutation is approximately 1 in 40.1,4,6
Women with BRCA1 or BRCA2 mutations have a lifetime risk of breast cancer of up to 87%, or 5 to 7 times higher than in the general population, with the risk rising steeply beginning at age 30.1,5,8 In addition, the lifetime risk of ovarian cancer is nearly 59% in BRCA1 mutation carriers and 17% in BRCA2 mutation carriers.22
A meta-analysis found that BRCA1 mutation carriers diagnosed with cancer in one breast have a 5-year risk of developing cancer in the other breast of 15%, and BRCA2 mutation carriers have a risk of 9%.23 Overall, the risk of contralateral breast cancer is about 3% per year.3,4,24
BRCA1 mutations are strongly associated with triple-negative breast cancers.1,3,4
Hereditary diffuse gastric cancer
Hereditary diffuse gastric cancer is an autosomal-dominant syndrome associated with mutations in the CDH1 gene, although up to 75% of patients with this syndrome do not have an identifiable CDH1 mutation.9,25,26 In cases in which there is no identifiable CDH1 mutation, the diagnosis is made on the basis of the patient’s medical and family history.
Hereditary diffuse gastric cancer is associated with an increased risk of the lobular subtype of breast cancer as well as diffuse gastric cancer. The cumulative lifetime risk of breast cancer in women with CDH1 mutations is 39% to 52%,6,9–11,25 and their lifetime risk of diffuse gastric cancer is 83%.9 The combined risk of breast cancer and gastric cancer in women with this syndrome is 90% by age 80.9
Cowden syndrome (PTEN hamartoma tumor syndrome)
Cowden syndrome (PTEN hamartoma tumor syndrome) is caused by mutations in PTEN, another tumor-suppressor gene.11 The primary clinical concerns are melanoma and breast, endometrial, thyroid (follicular or papillary), colon, and renal cell cancers. Women with a PTEN mutation have a twofold greater risk of developing any type of cancer than men with a PTEN mutation.12 The cumulative lifetime risk of invasive breast cancer in women with this syndrome is 70% to 85%.11–13
Peutz-Jeghers syndrome
Peutz-Jeghers syndrome is an autosomal dominant polyposis disorder caused, in most patients, by a mutation in the serine/threonine kinase tumor-suppressor gene STK11.14
Patients with Peutz-Jeghers syndrome have higher risks of gastrointestinal, breast, gynecologic (uterine, ovarian, and cervical), pancreatic, and lung cancers. In women, the lifetime risk of breast cancer is 44% to 50% by age 70, regardless of the type of mutation.6,14,15 Breast cancers associated with Peutz-Jeghers syndrome are usually ductal, and the mean age at diagnosis is 37 years.16
Li-Fraumeni syndrome
Li-Fraumeni syndrome is an autosomal-dominant disorder caused by germline mutations in the TP53 gene, which codes for a transcription factor associated with cell proliferation and apoptosis.27
These mutations confer a lifetime cancer risk of 93% in women (mainly breast cancer) and 68% in men.1,27 Other cancers associated with TP53 mutations include sarcomas, brain cancer, leukemia, and adrenocortical tumors. Germline TP53 mutations are responsible for approximately 1% of all breast cancers.1,4
Breast cancers can occur at a young age in patients with a TP53 mutation. Women with TP53 mutations are 18 times more likely to develop breast cancer before age 45 compared with the general population.4
It is important to consider a TP53 mutation in premenopausal women or women less than 30 years of age with breast cancer who have no mutations in BRCA1 and BRCA2.1
MANAGING PATIENTS WITH GENETIC PREDISPOSITION TO BREAST CANCER
Management for patients at high risk fall into three broad categories: clinical surveillance, chemoprevention, and surgical risk reduction. The utility and benefit of each depend to a large degree on the patient’s specific mutation, family history, and comorbidities. Decisions must be shared with the patient.
CLOSE CLINICAL SURVEILLANCE
Consensus guidelines for cancer screening in the syndromes described here are available from the National Comprehensive Cancer Network at www.nccn.org and are summarized in Table 2.26,28 While the guidelines are broadly applicable to all women with these conditions, some individualization is required based on personal and family medical history.
In general, screening begins at the ages listed in Table 2 or 10 years earlier than the age at which cancer developed in the first affected relative, whichever is earlier. However, screening decisions are shared with the patient and are sometimes affected by significant out-of-pocket costs for the patient and anxiety resulting from the test or subsequent test findings, which must all be considered.
Breast self-awareness and clinical breast examination
Although controversial in the general population, breast self-examination is recommended for patients carrying mutations that increase risk.6
A discussion about breast self-awareness is recommended for all women at the age of 18. It should include the signs and symptoms of breast cancer, what feels “normal” to the patient, and what is known about modifiable risk factors for breast cancer. The patient should also be told to report any changes in her personal or family history.
Clinical breast examinations should be done every 6 months, as some cancers are found clinically, particularly in young women with dense tissue, and confirmed by diagnostic imaging and targeted ultrasonography.
Radiographic surveillance
Mammography and magnetic resonance imaging (MRI) are also important components of a breast cancer surveillance regimen in women at high risk. Adherence to a well-formulated plan of clinical and radiographic examinations increases early detection in patients who have a hereditary predisposition to breast cancer.
MRI is more sensitive than mammography and reduces the likelihood of finding advanced cancers by up to 70% compared with mammography in women at high risk of breast cancer.29–31 The sensitivity of breast MRI alone ranges from 71% to 100%, and the sensitivity increases to 89% to 100% when combined with mammography. In contrast, the sensitivity of mammography alone is 25% to 59%.29 MRI has also been shown to be cost-effective when added to mammography and physical examination in women at high risk.5,32
Adding MRI to the breast cancer screening regimen has been under discussion and has been endorsed by the American Cancer Society in formal recommendations set forth in 2007 for patients with known hereditary cancer syndromes, in untested first-degree relatives of identified genetic mutation carriers, or in women who have an estimated lifetime risk of breast cancer of 20% or more, as determined by models largely dependent on family history.33
But MRI has a downside—it is less specific than mammography.29,33 Its lower specificity (77% to 90% vs 95% with mammography alone) leads to additional radiographic studies and tissue samplings for the “suspicious” lesions discovered. From 3% to 15% of screening breast MRIs result in a biopsy, and the proportion of biopsies that reveal cancer is 13% to 40%.33 Furthermore, by itself, MRI has not been shown to reduce mortality in any high-risk group.
Mammography remains useful in conjunction with MRI due to its ability to detect breast calcifications, which may be the earliest sign of breast cancer, and ability to detect changes in breast architecture. A typical screening program (Table 2) should incorporate both modalities, commonly offset by 6 months (eg, mammography at baseline, then MRI 6 months later, then mammography again 6 months after that, and so on) to increase the detection of interval cancer development.
Chemoprevention
Chemoprevention means taking medications to reduce the risk. Certain selective estrogen receptor modulators and aromatase inhibitors decrease the risk of invasive breast cancer in healthy women at high risk. These drugs include tamoxifen, which can be used before menopause, and raloxifene, anastrozole, and exemestane, which must be used only after menopause.
Because data are limited, we cannot make any generalized recommendations about chemoprevention in patients with hereditary breast cancer syndromes. Decisions about chemoprevention should take into account the patient’s personal and family histories. Often, a medical oncologist or medical breast specialist can help by discussing the risks and benefits for the individual patient.
Tamoxifen has been the most studied, mainly in BRCA mutation carriers.6,34–37 As in the general population, tamoxifen reduces the incidence of estrogen receptor-positive breast cancers by 50%.36–38 It has not been shown to significantly reduce breast cancer risk in premenopausal women with BRCA1 mutations,37 most likely because most cancers that occur in this group are estrogen receptor-negative. In patients with a history of breast cancer, however, tamoxifen has been shown to reduce the risk of developing contralateral breast cancer by 45% to 60% in both BRCA1 and BRCA2 mutation carriers.6,35
There is also little evidence that giving a chemopreventive agent after bilateral salpingo-oophorectomy reduces the risk further in premenopausal BRCA mutation carriers.35 These patients often receive hormonal therapy with estrogen, which currently would preclude the use of tamoxifen. Tamoxifen in postmenopausal women is associated with a small increased risk of venous thromboembolic disease and endometrial cancer.38
Oral contraceptives reduce the risk of ovarian cancer by up to 50% in BRCA1 mutation carriers and up to 60% in BRCA2 mutation carriers.6 However, data conflict on their effect on the risk of breast cancer in BRCA1 and BRCA2 mutation carriers.39
Decisions about chemoprevention with agents other than tamoxifen and in syndromes other than hereditary breast and ovarian cancer syndrome must take into consideration the existing lack of data in this area.
SURGICAL PROPHYLAXIS
Surgical prophylactic options for patients at genetic risk of breast cancer are bilateral mastectomy and bilateral salpingo-oophorectomy.
Prophylactic mastectomy
Bilateral risk-reducing mastectomy reduces the risk of breast cancer by at least 90%24,39,40 and greatly reduces the need for complex surveillance. Patients are often followed annually clinically, with single-view mammography if they have tissue flap reconstruction.
Nipple-sparing and skin-sparing mastectomies, which facilitate reconstruction and cosmetic outcomes, are an option in the risk-reduction setting and have been shown thus far to be safe.41–43 In patients with breast cancer, the overall breast cancer recurrence rates with nipple-sparing mastectomy are similar to those of traditional mastectomy and breast conservation treatment.41
In patients at very high risk of breast cancer, risk-reducing operations also reduce the risk of ultimately needing chemotherapy and radiation to treat breast cancer, as the risk of developing breast cancer is significantly lowered.
The timing of risk-reducing mastectomy depends largely on personal and family medical history and personal choice. Bilateral mastectomy at age 25 results in the greatest survival gain for patients with hereditary breast and ovarian cancer syndrome.5 Such precise data are not available for other hereditary cancer syndromes, but it is reasonable to consider bilateral mastectomy as an option for any woman with a highly penetrant genetic mutation that predisposes her to breast cancer. Special consideration in the timing of risk-reducing mastectomy must be given to women with Li-Fraumeni syndrome, as this condition is often associated with an earlier age at breast cancer diagnosis (before age 30).1
Family planning, sexuality, self-image, and the anxiety associated with both cancer risk and surveillance are all factors women consider when deciding whether and when to undergo mastectomy. A survey of 12 high-risk women who elected prophylactic mastectomy elicited feelings of some regret in 3 of them, while all expressed a sense of relief and reduced anxiety related to both cancer risk and screenings.24 Another group of 14 women surveyed after the surgery reported initial distress related to physical appearance, self-image, and intimacy but also reported a significant decrease in anxiety related to breast cancer risk and were largely satisfied with their decision.44
Prophylactic salpingo-oophorectomy
In patients who have pathogenic mutations in BRCA1 or 2, prophylactic salpingo-oophorectomy before age 40 decreases the risk of ovarian cancer by up to 96% and breast cancer by 50%.1,37,45 This operation, in fact, is the only intervention that has been shown to reduce the mortality rate in patients with a hereditary predisposition to cancer.46
We recommend that women with hereditary breast and ovarian cancer syndrome strongly consider prophylactic salpingo-oophorectomy by age 40 or when childbearing is complete for the greatest reduction in risk.1,5 In 2006, Domchek et al46 reported an overall decrease in the mortality rate in BRCA1/2-positive patients who underwent this surgery, but not in breast cancer-specific or ovarian cancer-specific mortality.
On the other hand, removing the ovaries before menopause places women at risk of serious complications associated with premature loss of gonadal hormones, including cardiovascular disease, decreased bone density, reduced sexual satisfaction, dyspareunia, hot flashes, and night sweats.47 Therefore, it is generally reserved for women who are also at risk of ovarian cancer.
Hormonal therapy, ie, estrogen therapy for patients who choose complete hysterectomy, and estrogen-progesterone therapy for patients who choose to keep their uterus, reduces menopausal symptoms and symptoms of sexual dissatisfaction and has not thus far been shown to increase breast cancer risk.1,34 However, this information is from nonrandomized studies, which are inherently limited.
It is important to address and modify risk factors for heart disease and osteoporosis in women with premature surgical menopause, as they may be particularly vulnerable to these conditions.
HEREDITARY BREAST CANCER IN MEN
Fewer than 1% of cases of breast cancer arise in men, and fewer than 1% of cases of cancer in men are breast cancer.
Male breast cancer is more likely than female breast cancer to be estrogen receptor- and progesterone receptor-positive. In an analysis of the Surveillance, Epidemiology, and End Results registry between 1973 and 2005, triple-negative breast cancer was found in 23% of female patients but only 7.6% of male patients.2
Male breast cancer is most common in families with BRCA2, and to a lesser degree, BRCA1 mutations. Other genetic disorders including Li-Fraumeni syndrome, hereditary nonpolyposis colorectal cancer, and Klinefelter syndrome also increase the risk of male breast cancer. A genetic predisposition for breast cancer is present in approximately 10% of male breast cancer patients.2 Any man with breast cancer, therefore, should be referred for genetic counseling.
In men, a BRCA2 mutation confers a lifetime risk of breast cancer of 5% to 10%.2 This is similar to the lifetime risk of breast cancer for the average woman but it is still significant, as the lifetime risk of breast cancer for the average man is 0.1%.1,2
Five-year survival rates in male breast cancer range from only 36% to 66%, most likely because it is usually diagnosed in later stages, as men are not routinely screened for breast cancer. In men with known hereditary susceptibility, National Comprehensive Cancer Network guidelines recommend that they be educated about and begin breast self-examination at the age of 35 and be clinically examined every 12 months starting at age 35.48 There are limited data to support breast imaging in men. High-risk surveillance with MRI screening in this group is not recommended. Prostate cancer screening is recommended for men with BRCA2 mutations starting at age 40, and should be considered for men with BRCA1 mutations starting at age 40.
No specific guidelines exist for pancreatic cancer and melanoma, but screening may be individualized based on cancers observed in the family.
- Daly MB, Axilbund JE, Buys S, et al; National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw 2010; 8:562–594.
- Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol 2010; 28:2114–2122.
- Foulkes WD. Inherited susceptibility to common cancers. N Engl J Med 2008; 359:2143–2153.
- Schwartz GF, Hughes KS, Lynch HT, et al. Proceedings of the international consensus conference on breast cancer risk, genetics, and risk management, April 2007. Breast J 2009; 15:4–16.
- Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol 2010; 28:222–231.
- National Comprehensive Cancer Network Guidelines Version 2.2014. Genetic/familial high risk assessment: breast and ovarian. www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed January 22, 2016.
- Ford D, Easton DF, Peto J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am J Hum Genet 1995; 57:1457–1462.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Pharoah PD, Guilford P, Caldas C; International Gastric Cancer Linkage Consortium. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001; 121:1348–1353.
- Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007; 297:2360–2372.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Bubien V, Bonnet F, Brouste V, et al; French Cowden Disease Network. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J Med Genet 2013; 50:255–263.
- Nelen MR, Kremer H, Konings IB, et al. Novel PTEN mutations in patients with Cowden disease: absence of clear genotype-phenotype correlations. Eur J Hum Genet 1999; 7:267–273.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000; 119:1447–1453.
- Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut 2010; 59:975–986.
- Chen S, Iversen ES, Friebel T, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol 2006; 24:863–871.
- Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer 1996; 77:2318–2324.
- Riegert-Johnson DL, Gleeson FC, Roberts M, et al. Cancer and Lhermitte-Duclos disease are common in Cowden syndrome patients. Hered Cancer Clin Pract 2010; 8:6.
- Stone J, Bevan S, Cunningham D, et al. Low frequency of germline E-cadherin mutations in familial and nonfamilial gastric cancer. Br J Cancer 1999; 79:1935–1937.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med 2014; 81:31–40.
- Mavaddat N, Peock S, Frost D, et al; EMBRACE. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst 2013; 105:812–822.
- Molina-Montes E, Pérez-Nevot B, Pollán M, Sánchez-Cantalejo E, Espín J, Sánchez MJ. Cumulative risk of second primary contralateral breast cancer in BRCA1/BRCA2 mutation carriers with a first breast cancer: a systematic review and meta-analysis. Breast 2014; 23:721–742.
- Kwong A, Chu AT. What made her give up her breasts: a qualitative study on decisional considerations for contralateral prophylactic mastectomy among breast cancer survivors undergoing BRCA1/2 genetic testing. Asian Pac J Cancer Prev 2012; 13:2241–2247.
- Dixon M, Seevaratnam R, Wirtzfeld D, et al. A RAND/UCLA appropriateness study of the management of familial gastric cancer. Ann Surg Oncol 2013; 20:533–541.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- National Comprehensive Cancer Network Guidelines Version 1. 2015. Gastric Cancer. www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Accessed January 22, 2016.
- Warner, E. Impact of MRI surveillance and breast cancer detection in young women with BRCA mutations. Ann Oncol 2011; 22(suppl 1):i44–i49.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004; 351:427–437.
- Pederson HJ, O’Rourke C, Lyons J, Patrick RJ, Crowe JP Jr, Grobmyer SR. Time-related changes in yield and harms of screening breast magnetic resonance imaging. Clin Breast Cancer 2015 Jan 21: S1526-8209(15)00024–00025. Epub ahead of print.
- Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat 2011; 125:837–847.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75–89.
- Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE study group. J Clin Oncol 2005; 23:7804–7610.
- Narod SA, Brunet JS, Ghadirian P, et al; Hereditary Breast Cancer Clinical Study Group. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case control study. Lancet 2000; 356:1876–1881.
- Njiaju UO, Olopade OI. Genetic determinants of breast cancer risk: a review of the current literature and issues pertaining to clinical application. Breast J 2012; 18:436–442.
- King MC, Wieand S, Hale K, et al; National Surgical Adjuvant Breast and Bowel Project. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention trial. JAMA 2001; 286:2251–2256.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388.
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004; 22:1055–1062.
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999; 340:77–84.
- Mallon P, Feron JG, Couturaud B, et al. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg 2013; 131:969–984.
- Stanec Z, Žic R, Budi S, et al. Skin and nipple-areola complex sparing mastectomy in breast cancer patients: 15-year experience. Ann Plast Surg 2014; 73:485–491.
- Eisenberg RE, Chan JS, Swistel AJ, Hoda SA. Pathological evaluation of nipple-sparing mastectomies with emphasis on occult nipple involvement: the Weill-Cornell experience with 325 cases. Breast J 2014; 20:15–21.
- Lodder LN, Frets PG, Trijsburg RW, et al. One year follow-up of women opting for presymptomatic testing for BRCA1 and BRCA2: emotional impact of the test outcome and decisions on risk management (surveillance or prophylactic surgery). Breast Cancer Res Treat 2002; 73:97–112.
- Rebbeck TR, Lynch HT, Neuhausen SL, et al; Prevention and Observation of Surgical End Points Study Group. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 2002; 346:1616–1622.
- Domchek SM, Friebel TM, Neuhausen SL, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol 2006; 7:223–229.
- Finch A, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Womens Health (Lond Engl) 2012; 8:543–555.
- National Comprehensive Cancer Network Guidelines. Version 2.2015. Genetic/Familial High-Risk Assessment Breast and Ovarian. www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed February 8, 2016.
- Daly MB, Axilbund JE, Buys S, et al; National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw 2010; 8:562–594.
- Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol 2010; 28:2114–2122.
- Foulkes WD. Inherited susceptibility to common cancers. N Engl J Med 2008; 359:2143–2153.
- Schwartz GF, Hughes KS, Lynch HT, et al. Proceedings of the international consensus conference on breast cancer risk, genetics, and risk management, April 2007. Breast J 2009; 15:4–16.
- Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol 2010; 28:222–231.
- National Comprehensive Cancer Network Guidelines Version 2.2014. Genetic/familial high risk assessment: breast and ovarian. www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed January 22, 2016.
- Ford D, Easton DF, Peto J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am J Hum Genet 1995; 57:1457–1462.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Pharoah PD, Guilford P, Caldas C; International Gastric Cancer Linkage Consortium. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001; 121:1348–1353.
- Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007; 297:2360–2372.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Bubien V, Bonnet F, Brouste V, et al; French Cowden Disease Network. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J Med Genet 2013; 50:255–263.
- Nelen MR, Kremer H, Konings IB, et al. Novel PTEN mutations in patients with Cowden disease: absence of clear genotype-phenotype correlations. Eur J Hum Genet 1999; 7:267–273.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000; 119:1447–1453.
- Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut 2010; 59:975–986.
- Chen S, Iversen ES, Friebel T, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol 2006; 24:863–871.
- Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer 1996; 77:2318–2324.
- Riegert-Johnson DL, Gleeson FC, Roberts M, et al. Cancer and Lhermitte-Duclos disease are common in Cowden syndrome patients. Hered Cancer Clin Pract 2010; 8:6.
- Stone J, Bevan S, Cunningham D, et al. Low frequency of germline E-cadherin mutations in familial and nonfamilial gastric cancer. Br J Cancer 1999; 79:1935–1937.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med 2014; 81:31–40.
- Mavaddat N, Peock S, Frost D, et al; EMBRACE. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst 2013; 105:812–822.
- Molina-Montes E, Pérez-Nevot B, Pollán M, Sánchez-Cantalejo E, Espín J, Sánchez MJ. Cumulative risk of second primary contralateral breast cancer in BRCA1/BRCA2 mutation carriers with a first breast cancer: a systematic review and meta-analysis. Breast 2014; 23:721–742.
- Kwong A, Chu AT. What made her give up her breasts: a qualitative study on decisional considerations for contralateral prophylactic mastectomy among breast cancer survivors undergoing BRCA1/2 genetic testing. Asian Pac J Cancer Prev 2012; 13:2241–2247.
- Dixon M, Seevaratnam R, Wirtzfeld D, et al. A RAND/UCLA appropriateness study of the management of familial gastric cancer. Ann Surg Oncol 2013; 20:533–541.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- National Comprehensive Cancer Network Guidelines Version 1. 2015. Gastric Cancer. www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Accessed January 22, 2016.
- Warner, E. Impact of MRI surveillance and breast cancer detection in young women with BRCA mutations. Ann Oncol 2011; 22(suppl 1):i44–i49.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004; 351:427–437.
- Pederson HJ, O’Rourke C, Lyons J, Patrick RJ, Crowe JP Jr, Grobmyer SR. Time-related changes in yield and harms of screening breast magnetic resonance imaging. Clin Breast Cancer 2015 Jan 21: S1526-8209(15)00024–00025. Epub ahead of print.
- Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat 2011; 125:837–847.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75–89.
- Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE study group. J Clin Oncol 2005; 23:7804–7610.
- Narod SA, Brunet JS, Ghadirian P, et al; Hereditary Breast Cancer Clinical Study Group. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case control study. Lancet 2000; 356:1876–1881.
- Njiaju UO, Olopade OI. Genetic determinants of breast cancer risk: a review of the current literature and issues pertaining to clinical application. Breast J 2012; 18:436–442.
- King MC, Wieand S, Hale K, et al; National Surgical Adjuvant Breast and Bowel Project. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention trial. JAMA 2001; 286:2251–2256.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388.
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004; 22:1055–1062.
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999; 340:77–84.
- Mallon P, Feron JG, Couturaud B, et al. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg 2013; 131:969–984.
- Stanec Z, Žic R, Budi S, et al. Skin and nipple-areola complex sparing mastectomy in breast cancer patients: 15-year experience. Ann Plast Surg 2014; 73:485–491.
- Eisenberg RE, Chan JS, Swistel AJ, Hoda SA. Pathological evaluation of nipple-sparing mastectomies with emphasis on occult nipple involvement: the Weill-Cornell experience with 325 cases. Breast J 2014; 20:15–21.
- Lodder LN, Frets PG, Trijsburg RW, et al. One year follow-up of women opting for presymptomatic testing for BRCA1 and BRCA2: emotional impact of the test outcome and decisions on risk management (surveillance or prophylactic surgery). Breast Cancer Res Treat 2002; 73:97–112.
- Rebbeck TR, Lynch HT, Neuhausen SL, et al; Prevention and Observation of Surgical End Points Study Group. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 2002; 346:1616–1622.
- Domchek SM, Friebel TM, Neuhausen SL, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol 2006; 7:223–229.
- Finch A, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Womens Health (Lond Engl) 2012; 8:543–555.
- National Comprehensive Cancer Network Guidelines. Version 2.2015. Genetic/Familial High-Risk Assessment Breast and Ovarian. www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed February 8, 2016.
KEY POINTS
- In addition to breast cancer, hereditary cancer syndromes increase the risk of other malignancies, with the patterns of malignancy varying by causative genetic mutation.
- Genetic counselors, medical breast specialists, surgical breast specialists, gynecologic oncologists, and others can help, but the primary care provider is the nucleus of the multidisciplinary team.
- Management of these patients often includes surveillance, chemoprevention, and prophylactic surgery.
- All decisions about surveillance, chemoprevention, and surgical risk reduction should be shared with the patient.