User login
A 66-year-old Latin American woman presented to the emergency department (ED) with persistent abdominal and back pain of about one month’s duration. She had visited another ED eight days earlier for similar symptoms and was discharged home with a mild opioid pain medication and a proton pump inhibitor. However, she said that she had received neither a diagnosis nor an explanation for her symptoms.
Medical history, obtained with the assistance of an interpreter because the patient was not fluent in English, included hypertension, coronary artery disease, and hyperlipidemia; these had gone untreated for at least two years. She denied any personal or family history of cancer or endocrine disorders. Surgical history included a cholecystectomy and a percutaneous coronary intervention for an unknown coronary artery lesion.
She had a 14-pack-year history of cigarette smoking. Her medications included only ibuprofen and hydrocodone, and she had no known drug allergies. The patient denied use of herbal preparations or vitamin supplements and unusual dietary practices.
Review of systems revealed occasional dizziness, constipation, decreased appetite, and some mild confusion noted by family members, but no fever, chills, palpitations, chest pain, shortness of breath, muscle spasm, or weakness. Vital signs were normal. Physical examination was remarkable for tenderness of the upper quadrants of the abdomen with deep palpation, without guarding or rebound. Bony tenderness at the right anterior costal margin of the rib cage was also noted.
Laboratory work-up revealed marked hypercalcemia (15.4 mg/dL), electrolyte abnormalities, anemia, impaired renal function, and elevated alkaline phosphatase and globulin levels (see Table 1). In addition, a plain abdominal x-ray series was negative for acute findings, but x-rays of the right ribs revealed a fracture of the sixth rib and osteopenia.
Continued >>
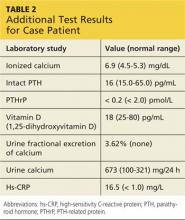
The patient was admitted to the hospital for treatment of hypercalcemia and hypokalemia and for work-up of elevated alkaline phosphatase and abdominal pain. Upon admission, serum ionized calcium measurement confirmed true hypercalcemia. Additional diagnostic tests were then ordered to help differentiate between parathyroid hormone (PTH)–mediated and non-PTH–mediated causes for the hypercalcemia (see Table 2).
The patient’s PTH level was normal and the urine fractional excretion of calcium level was high, ruling out familial hypocalciuric hypercalcemia (FHH), in which urine calcium level is low. A measurement of PTH-related protein (PTHrP), secreted by some cancers, was normal, suggesting exclusion of solid tumor malignancy. Vitamin D toxicity was ruled out because the patient’s 1,25-dihydroxyvitamin D level was low.
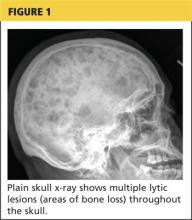
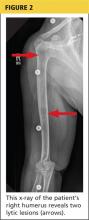
The patient continued to experience vague abdominal, back, and rib pain that seemed to migrate daily and worsened with movement. A skeletal x-ray was performed and revealed numerous lytic lesions of the skull (Figure 1), midright humerus (Figure 2), and distal left radius.
Continue for discussion >>
DISCUSSION
Hypercalcemia is a relatively common presentation in primary care. The most frequent causes are primary hyperparathyroidism and malignancy.1 One in 500 patients will be diagnosed incidentally with asymptomatic hypercalcemia caused by underlying hyperparathyroidism.1
Clinical manifestations of hypercalcemia can range from no symptoms to multisystem disease. Fatigue, nausea, vomiting, constipation, bone pain, osteoporosis, nephrolithiasis, mental status changes, hypertension, anemia, elevated creatinine, and cardiac arrhythmias are among the more common clinical conditions associated with hypercalcemia (hence the mnemonic “stones, bones, abdominal moans, and psychic groans” for its signs and symptoms).1
Diagnostic overview
Causes of hypercalcemia are numerous and can be broken down into two categories: PTH-mediated and non-PTH–mediated. PTH-mediated causes include primary and secondary hyperparathyroidism and FHH. Non-PTH–mediated causes include vitamin D toxicity, solid tumor malignancy with or without metastasis, multiple myeloma (MM) and other plasma cell dyscrasias, granulomatous disease such as sarcoid, and some medications.1
The differential diagnosis for hypercalcemia begins with measurement of the patient’s intact PTH level. An elevated or high-normal result indicates a PTH-mediated cause, so 24-hour measurement of excretion of urinary calcium is the next step. A low or low-normal PTH level (< 20 pg/mL), however, suggests the cause is non-PTH-mediated.2 The diagnostic approach in this situation is more challenging because testing to exclude or confirm various potential causes can be expensive and time-consuming. The degree of hypercalcemia, however, can aid in the diagnosis: Primary hyperparathyroidism is often associated with borderline or mild hypercalcemia, while calcium values > 13 mg/dL are more common in patients with malignancies.2
PTH elevates calcium levels in the blood when ionized (free) calcium levels are low by increasing gastrointestinal absorption, decreasing urinary excretion, and increasing bone resorption.3 With malignant tumors such as lung, breast, and renal cell, osteolytic metastases can destroy the bone, resulting in release of calcium. In other cases, solid-tumor cancers produce PTHrP, which increases serum calcium. This latter situation is referred to as humoral hypercalcemia of malignancy.3 Lymphoma and granulomatous disease, such as sarcoid, can be associated with excess production of 1,25-dihydroxyvitamin D.2 If vitamin D is elevated but PTH and PTHrP are normal, a chest x-ray should be obtained to evaluate the patient for sarcoid or lymphoma.
Hypercalcemia work-up
The work-up for suspected hypercalcemia begins with measurement of the patient’s calcium level. Because calcium is bound to albumin in the blood, the standard serum calcium test may not reflect the true calcium level. (If albumin is high, the calcium level will be high, and vice versa.)1 The true (serum ionized) calcium level (also known as corrected calcium level) should always be calculated to confirm true hypercalcemia. A formula commonly used to calculate the corrected calcium level is
Corrected calcium (mg/dL) = (measured calcium [mg/dL]) + 0.8 (4.0 – serum albumin [mg/dL])
Direct measurement of the serum ionized calcium level is not affected by the albumin level and can also confirm true hypercalcemia.4
Once hypercalcemia is confirmed, the next step is to measure the patient’s intact PTH level.
If intact PTH is elevated or high normal, consider primary hyperparathyroidism or FHH and confirm by obtaining the urine calcium level.
• If urine calcium level is high (> 200 mg/24 h), the diagnosis is primary hyperparathyroidism.
• If urine calcium level is low (< 100 mg/24 h), the diagnosis is FHH.
If intact PTH is low, consider non–PTH-mediated causes and confirm by obtaining PTHrP and vitamin D levels.
• If PTHrP level is elevated, scan for malignancy.
• If 1,25-dihydroxyvitamin D level is elevated, order a chest x-ray to rule out sarcoid or lymphoma.
• If both PTHrP and vitamin D levels are normal, order both serum protein electrophoresis (SPEP) and urine protein electrophoresis (UPEP) with immunofixation to rule out MM.
• If vitamin D level is elevated, check vitamin and herbal supplement use for excessive vitamin D intake.
Treatment of symptomatic hypercalcemia
The goals of treatment of symptomatic hypercalcemia are to reduce the serum calcium level to the normal range and to treat the underlying cause.1 Mild hypercalcemia (calcium level, 10-12 mg/dL) is typically asymptomatic and does not need to be treated. Moderate hypercalcemia (calcium level, 12-14 mg/dL) may not require treatment unless the patient is symptomatic and/or has had an acute rise in calcium level.1 In mild to moderate hypercalcemia, the serum calcium level should be monitored to establish a trend.
Treatment for symptomatic moderate and severe hypercalcemia (calcium level, > 14 mg/dL) typically involves a similar regimen:
• Volume expansion with isotonic saline at an initial rate of 2 to 4 L/d, which is then adjusted to achieve 200 mL/h of continuous urine output. IV furosemide can be used with caution (10-20 mg IV as needed) to promote diuresis if volume overload is a concern (furosemide promotes renal excretion of calcium).
• Administration of subcutaneous calcitonin (4-8 IU/kg, repeated every 6 h for 24 h). Calcitonin works rapidly to lower calcium levels in 4 to 6 h.
• Concurrent administration of IV bisphosphonate (zoledronic acid [4 mg over 15 min] or pamidronate [60-90 mg over 4 h]). Pamidronate is superior for reversal of malignancy-related hypercalcemia.1
Hypercalcemia and multiple myeloma
MM is a malignant neoplasm of plasma cells that accounts for approximately 1% of all cancers and about 10% of hematologic malignancies in the United States, with a median patient age of 70 at diagnosis.5-7 In MM, myeloma cells induce the secretion of cytokines and growth factors that alter plasma cells, activate osteoclasts, suppress osteoblasts, cause abnormal interactions between plasma cells and bone marrow, and stimulate aberrant angiogenesis.8 Osteoclastic bone resorption produces hypercalcemia as well as the lytic lesions seen on x-ray.7
Approximately 74% of patients present with typical MM symptoms of calcium elevation in the blood, renal insufficiency, anemia, and bone lesions, known as CRAB symptoms, but other myeloma-related manifestations may be present.9
Diagnostic criteria for MM include the following (all three must be present):10
• Monoclonal bone marrow plasma cells ≥ 10% and/or a biopsy-proven plasmacytoma
• Monoclonal protein in the serum and/or urine (if none is detected, disease is nonsecretory and diagnosis requires ≥ 30% bone marrow plasma cells and/or biopsy-proven plasmacytoma)
• Myeloma-related organ dysfunction, indicated by at least one of the CRAB symptoms.
In the absence of CRAB symptoms, an asymptomatic patient may have an MM precursor syndrome: monoclonal gammopathy of undetermined significance or smoldering (or indolent) MM.10
Treatment of multiple myeloma
In recent years, the use of induction therapy followed by autologous stem cell transplantation and the development of novel therapeutic agents have extended overall survival for patients with MM. These agents include proteasome inhibitors (bortezomib and the second-generation carfilzomib)11 and immunomodulators (thalidomide and the second-generation lenalidomide). Early diagnosis and treatment can improve progression-free survival as well as overall survival, including recovery of renal function for patients with renal failure.12 With survival ranging from one year or less—with aggressive disease—to 10 years or more for patients with responsive disease,7 there remains no cure for MM.
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
Further work-up included SPEP and UPEP with immunofixation, which revealed marked IgG free λ light chains with an M (monoclonal) component, making MM a very likely diagnosis. Confirmation by means of bone marrow biopsy was indicated, but the patient refused the procedure.
The patient’s hypercalcemia was treated by IV administration of calcitonin with isotonic saline. This reduced the serum calcium level from 15.4 mg/dL to 10.6 mg/dL within 48 hours. One dose of ergocalciferol (vitamin D2) was then administered to promote intestinal absorption of calcium and support bone mineralization, further lowering the patient’s serum calcium level to a normal 8.9 mg/dL. Hypokalemia was treated with oral potassium supplementation.
The patient, now stable, was referred to the hematology/oncology and bone mineral metabolism clinics and was discharged from the hospital. She did not keep those appointments and was lost to follow-up.
CONCLUSION
The most common causes of hypercalcemia are hyperparathyroidism and malignancy. Most cases do not require treatment unless the calcium level is >14 mg/dL and/or the patient is symptomatic. Red flag symptoms include weakness, abdominal pain, mental status changes, and coma.4 Primary care clinicians should suspect MM in older patients with laboratory findings of hypercalcemia, anemia, and renal dysfunction, with lytic lesions on x-ray.
REFERENCES
1. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67(9):1959-1966.
2. Endres DB. Investigation of hypercalcemia. Clin Biochem. 2012;45(12):
954-963.
3. Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care. 2008;35(2):215-237.
4. Sharma B, Misicko NE. How should you evaluate elevated calcium in an asymptomatic patient? J Fam Pract. 2008;57(4):267-269.
5. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
6. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
7. Shaheen SP, Talwalkar SS, Medeiros LJ. Multiple myeloma and immunosecretory disorders: an update. Adv Anat Pathol. 2008;15(4):196-210.
8. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11): 1046-1060.
9. Talamo G, Farooq U, Zangari M, et al. Beyond the CRAB symptoms: a study of presenting clinical manifestations of multiple myeloma. Clin Lymphoma Myeloma Leuk. 2010;10(6):464-468.
10. Palumbo A, Sezer O, Kyle R, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transportation. Leukemia. 2009;23(10):1716-1730.
11. Kyprolis [package insert]. South San Francisco, CA: Onyx Pharmaceuticals, Inc; 2012.
12. Suyani E, Sucak GT, Erten Y, et al. Evaluation of multiple myeloma patients presenting with renal failure in a university hospital in the year 2010. Ren Fail. 2012;34(2):257-262.
A 66-year-old Latin American woman presented to the emergency department (ED) with persistent abdominal and back pain of about one month’s duration. She had visited another ED eight days earlier for similar symptoms and was discharged home with a mild opioid pain medication and a proton pump inhibitor. However, she said that she had received neither a diagnosis nor an explanation for her symptoms.
Medical history, obtained with the assistance of an interpreter because the patient was not fluent in English, included hypertension, coronary artery disease, and hyperlipidemia; these had gone untreated for at least two years. She denied any personal or family history of cancer or endocrine disorders. Surgical history included a cholecystectomy and a percutaneous coronary intervention for an unknown coronary artery lesion.
She had a 14-pack-year history of cigarette smoking. Her medications included only ibuprofen and hydrocodone, and she had no known drug allergies. The patient denied use of herbal preparations or vitamin supplements and unusual dietary practices.
Review of systems revealed occasional dizziness, constipation, decreased appetite, and some mild confusion noted by family members, but no fever, chills, palpitations, chest pain, shortness of breath, muscle spasm, or weakness. Vital signs were normal. Physical examination was remarkable for tenderness of the upper quadrants of the abdomen with deep palpation, without guarding or rebound. Bony tenderness at the right anterior costal margin of the rib cage was also noted.
Laboratory work-up revealed marked hypercalcemia (15.4 mg/dL), electrolyte abnormalities, anemia, impaired renal function, and elevated alkaline phosphatase and globulin levels (see Table 1). In addition, a plain abdominal x-ray series was negative for acute findings, but x-rays of the right ribs revealed a fracture of the sixth rib and osteopenia.
Continued >>
The patient was admitted to the hospital for treatment of hypercalcemia and hypokalemia and for work-up of elevated alkaline phosphatase and abdominal pain. Upon admission, serum ionized calcium measurement confirmed true hypercalcemia. Additional diagnostic tests were then ordered to help differentiate between parathyroid hormone (PTH)–mediated and non-PTH–mediated causes for the hypercalcemia (see Table 2).
The patient’s PTH level was normal and the urine fractional excretion of calcium level was high, ruling out familial hypocalciuric hypercalcemia (FHH), in which urine calcium level is low. A measurement of PTH-related protein (PTHrP), secreted by some cancers, was normal, suggesting exclusion of solid tumor malignancy. Vitamin D toxicity was ruled out because the patient’s 1,25-dihydroxyvitamin D level was low.
The patient continued to experience vague abdominal, back, and rib pain that seemed to migrate daily and worsened with movement. A skeletal x-ray was performed and revealed numerous lytic lesions of the skull (Figure 1), midright humerus (Figure 2), and distal left radius.
Continue for discussion >>
DISCUSSION
Hypercalcemia is a relatively common presentation in primary care. The most frequent causes are primary hyperparathyroidism and malignancy.1 One in 500 patients will be diagnosed incidentally with asymptomatic hypercalcemia caused by underlying hyperparathyroidism.1
Clinical manifestations of hypercalcemia can range from no symptoms to multisystem disease. Fatigue, nausea, vomiting, constipation, bone pain, osteoporosis, nephrolithiasis, mental status changes, hypertension, anemia, elevated creatinine, and cardiac arrhythmias are among the more common clinical conditions associated with hypercalcemia (hence the mnemonic “stones, bones, abdominal moans, and psychic groans” for its signs and symptoms).1
Diagnostic overview
Causes of hypercalcemia are numerous and can be broken down into two categories: PTH-mediated and non-PTH–mediated. PTH-mediated causes include primary and secondary hyperparathyroidism and FHH. Non-PTH–mediated causes include vitamin D toxicity, solid tumor malignancy with or without metastasis, multiple myeloma (MM) and other plasma cell dyscrasias, granulomatous disease such as sarcoid, and some medications.1
The differential diagnosis for hypercalcemia begins with measurement of the patient’s intact PTH level. An elevated or high-normal result indicates a PTH-mediated cause, so 24-hour measurement of excretion of urinary calcium is the next step. A low or low-normal PTH level (< 20 pg/mL), however, suggests the cause is non-PTH-mediated.2 The diagnostic approach in this situation is more challenging because testing to exclude or confirm various potential causes can be expensive and time-consuming. The degree of hypercalcemia, however, can aid in the diagnosis: Primary hyperparathyroidism is often associated with borderline or mild hypercalcemia, while calcium values > 13 mg/dL are more common in patients with malignancies.2
PTH elevates calcium levels in the blood when ionized (free) calcium levels are low by increasing gastrointestinal absorption, decreasing urinary excretion, and increasing bone resorption.3 With malignant tumors such as lung, breast, and renal cell, osteolytic metastases can destroy the bone, resulting in release of calcium. In other cases, solid-tumor cancers produce PTHrP, which increases serum calcium. This latter situation is referred to as humoral hypercalcemia of malignancy.3 Lymphoma and granulomatous disease, such as sarcoid, can be associated with excess production of 1,25-dihydroxyvitamin D.2 If vitamin D is elevated but PTH and PTHrP are normal, a chest x-ray should be obtained to evaluate the patient for sarcoid or lymphoma.
Hypercalcemia work-up
The work-up for suspected hypercalcemia begins with measurement of the patient’s calcium level. Because calcium is bound to albumin in the blood, the standard serum calcium test may not reflect the true calcium level. (If albumin is high, the calcium level will be high, and vice versa.)1 The true (serum ionized) calcium level (also known as corrected calcium level) should always be calculated to confirm true hypercalcemia. A formula commonly used to calculate the corrected calcium level is
Corrected calcium (mg/dL) = (measured calcium [mg/dL]) + 0.8 (4.0 – serum albumin [mg/dL])
Direct measurement of the serum ionized calcium level is not affected by the albumin level and can also confirm true hypercalcemia.4
Once hypercalcemia is confirmed, the next step is to measure the patient’s intact PTH level.
If intact PTH is elevated or high normal, consider primary hyperparathyroidism or FHH and confirm by obtaining the urine calcium level.
• If urine calcium level is high (> 200 mg/24 h), the diagnosis is primary hyperparathyroidism.
• If urine calcium level is low (< 100 mg/24 h), the diagnosis is FHH.
If intact PTH is low, consider non–PTH-mediated causes and confirm by obtaining PTHrP and vitamin D levels.
• If PTHrP level is elevated, scan for malignancy.
• If 1,25-dihydroxyvitamin D level is elevated, order a chest x-ray to rule out sarcoid or lymphoma.
• If both PTHrP and vitamin D levels are normal, order both serum protein electrophoresis (SPEP) and urine protein electrophoresis (UPEP) with immunofixation to rule out MM.
• If vitamin D level is elevated, check vitamin and herbal supplement use for excessive vitamin D intake.
Treatment of symptomatic hypercalcemia
The goals of treatment of symptomatic hypercalcemia are to reduce the serum calcium level to the normal range and to treat the underlying cause.1 Mild hypercalcemia (calcium level, 10-12 mg/dL) is typically asymptomatic and does not need to be treated. Moderate hypercalcemia (calcium level, 12-14 mg/dL) may not require treatment unless the patient is symptomatic and/or has had an acute rise in calcium level.1 In mild to moderate hypercalcemia, the serum calcium level should be monitored to establish a trend.
Treatment for symptomatic moderate and severe hypercalcemia (calcium level, > 14 mg/dL) typically involves a similar regimen:
• Volume expansion with isotonic saline at an initial rate of 2 to 4 L/d, which is then adjusted to achieve 200 mL/h of continuous urine output. IV furosemide can be used with caution (10-20 mg IV as needed) to promote diuresis if volume overload is a concern (furosemide promotes renal excretion of calcium).
• Administration of subcutaneous calcitonin (4-8 IU/kg, repeated every 6 h for 24 h). Calcitonin works rapidly to lower calcium levels in 4 to 6 h.
• Concurrent administration of IV bisphosphonate (zoledronic acid [4 mg over 15 min] or pamidronate [60-90 mg over 4 h]). Pamidronate is superior for reversal of malignancy-related hypercalcemia.1
Hypercalcemia and multiple myeloma
MM is a malignant neoplasm of plasma cells that accounts for approximately 1% of all cancers and about 10% of hematologic malignancies in the United States, with a median patient age of 70 at diagnosis.5-7 In MM, myeloma cells induce the secretion of cytokines and growth factors that alter plasma cells, activate osteoclasts, suppress osteoblasts, cause abnormal interactions between plasma cells and bone marrow, and stimulate aberrant angiogenesis.8 Osteoclastic bone resorption produces hypercalcemia as well as the lytic lesions seen on x-ray.7
Approximately 74% of patients present with typical MM symptoms of calcium elevation in the blood, renal insufficiency, anemia, and bone lesions, known as CRAB symptoms, but other myeloma-related manifestations may be present.9
Diagnostic criteria for MM include the following (all three must be present):10
• Monoclonal bone marrow plasma cells ≥ 10% and/or a biopsy-proven plasmacytoma
• Monoclonal protein in the serum and/or urine (if none is detected, disease is nonsecretory and diagnosis requires ≥ 30% bone marrow plasma cells and/or biopsy-proven plasmacytoma)
• Myeloma-related organ dysfunction, indicated by at least one of the CRAB symptoms.
In the absence of CRAB symptoms, an asymptomatic patient may have an MM precursor syndrome: monoclonal gammopathy of undetermined significance or smoldering (or indolent) MM.10
Treatment of multiple myeloma
In recent years, the use of induction therapy followed by autologous stem cell transplantation and the development of novel therapeutic agents have extended overall survival for patients with MM. These agents include proteasome inhibitors (bortezomib and the second-generation carfilzomib)11 and immunomodulators (thalidomide and the second-generation lenalidomide). Early diagnosis and treatment can improve progression-free survival as well as overall survival, including recovery of renal function for patients with renal failure.12 With survival ranging from one year or less—with aggressive disease—to 10 years or more for patients with responsive disease,7 there remains no cure for MM.
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
Further work-up included SPEP and UPEP with immunofixation, which revealed marked IgG free λ light chains with an M (monoclonal) component, making MM a very likely diagnosis. Confirmation by means of bone marrow biopsy was indicated, but the patient refused the procedure.
The patient’s hypercalcemia was treated by IV administration of calcitonin with isotonic saline. This reduced the serum calcium level from 15.4 mg/dL to 10.6 mg/dL within 48 hours. One dose of ergocalciferol (vitamin D2) was then administered to promote intestinal absorption of calcium and support bone mineralization, further lowering the patient’s serum calcium level to a normal 8.9 mg/dL. Hypokalemia was treated with oral potassium supplementation.
The patient, now stable, was referred to the hematology/oncology and bone mineral metabolism clinics and was discharged from the hospital. She did not keep those appointments and was lost to follow-up.
CONCLUSION
The most common causes of hypercalcemia are hyperparathyroidism and malignancy. Most cases do not require treatment unless the calcium level is >14 mg/dL and/or the patient is symptomatic. Red flag symptoms include weakness, abdominal pain, mental status changes, and coma.4 Primary care clinicians should suspect MM in older patients with laboratory findings of hypercalcemia, anemia, and renal dysfunction, with lytic lesions on x-ray.
REFERENCES
1. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67(9):1959-1966.
2. Endres DB. Investigation of hypercalcemia. Clin Biochem. 2012;45(12):
954-963.
3. Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care. 2008;35(2):215-237.
4. Sharma B, Misicko NE. How should you evaluate elevated calcium in an asymptomatic patient? J Fam Pract. 2008;57(4):267-269.
5. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
6. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
7. Shaheen SP, Talwalkar SS, Medeiros LJ. Multiple myeloma and immunosecretory disorders: an update. Adv Anat Pathol. 2008;15(4):196-210.
8. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11): 1046-1060.
9. Talamo G, Farooq U, Zangari M, et al. Beyond the CRAB symptoms: a study of presenting clinical manifestations of multiple myeloma. Clin Lymphoma Myeloma Leuk. 2010;10(6):464-468.
10. Palumbo A, Sezer O, Kyle R, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transportation. Leukemia. 2009;23(10):1716-1730.
11. Kyprolis [package insert]. South San Francisco, CA: Onyx Pharmaceuticals, Inc; 2012.
12. Suyani E, Sucak GT, Erten Y, et al. Evaluation of multiple myeloma patients presenting with renal failure in a university hospital in the year 2010. Ren Fail. 2012;34(2):257-262.
A 66-year-old Latin American woman presented to the emergency department (ED) with persistent abdominal and back pain of about one month’s duration. She had visited another ED eight days earlier for similar symptoms and was discharged home with a mild opioid pain medication and a proton pump inhibitor. However, she said that she had received neither a diagnosis nor an explanation for her symptoms.
Medical history, obtained with the assistance of an interpreter because the patient was not fluent in English, included hypertension, coronary artery disease, and hyperlipidemia; these had gone untreated for at least two years. She denied any personal or family history of cancer or endocrine disorders. Surgical history included a cholecystectomy and a percutaneous coronary intervention for an unknown coronary artery lesion.
She had a 14-pack-year history of cigarette smoking. Her medications included only ibuprofen and hydrocodone, and she had no known drug allergies. The patient denied use of herbal preparations or vitamin supplements and unusual dietary practices.
Review of systems revealed occasional dizziness, constipation, decreased appetite, and some mild confusion noted by family members, but no fever, chills, palpitations, chest pain, shortness of breath, muscle spasm, or weakness. Vital signs were normal. Physical examination was remarkable for tenderness of the upper quadrants of the abdomen with deep palpation, without guarding or rebound. Bony tenderness at the right anterior costal margin of the rib cage was also noted.
Laboratory work-up revealed marked hypercalcemia (15.4 mg/dL), electrolyte abnormalities, anemia, impaired renal function, and elevated alkaline phosphatase and globulin levels (see Table 1). In addition, a plain abdominal x-ray series was negative for acute findings, but x-rays of the right ribs revealed a fracture of the sixth rib and osteopenia.
Continued >>
The patient was admitted to the hospital for treatment of hypercalcemia and hypokalemia and for work-up of elevated alkaline phosphatase and abdominal pain. Upon admission, serum ionized calcium measurement confirmed true hypercalcemia. Additional diagnostic tests were then ordered to help differentiate between parathyroid hormone (PTH)–mediated and non-PTH–mediated causes for the hypercalcemia (see Table 2).
The patient’s PTH level was normal and the urine fractional excretion of calcium level was high, ruling out familial hypocalciuric hypercalcemia (FHH), in which urine calcium level is low. A measurement of PTH-related protein (PTHrP), secreted by some cancers, was normal, suggesting exclusion of solid tumor malignancy. Vitamin D toxicity was ruled out because the patient’s 1,25-dihydroxyvitamin D level was low.
The patient continued to experience vague abdominal, back, and rib pain that seemed to migrate daily and worsened with movement. A skeletal x-ray was performed and revealed numerous lytic lesions of the skull (Figure 1), midright humerus (Figure 2), and distal left radius.
Continue for discussion >>
DISCUSSION
Hypercalcemia is a relatively common presentation in primary care. The most frequent causes are primary hyperparathyroidism and malignancy.1 One in 500 patients will be diagnosed incidentally with asymptomatic hypercalcemia caused by underlying hyperparathyroidism.1
Clinical manifestations of hypercalcemia can range from no symptoms to multisystem disease. Fatigue, nausea, vomiting, constipation, bone pain, osteoporosis, nephrolithiasis, mental status changes, hypertension, anemia, elevated creatinine, and cardiac arrhythmias are among the more common clinical conditions associated with hypercalcemia (hence the mnemonic “stones, bones, abdominal moans, and psychic groans” for its signs and symptoms).1
Diagnostic overview
Causes of hypercalcemia are numerous and can be broken down into two categories: PTH-mediated and non-PTH–mediated. PTH-mediated causes include primary and secondary hyperparathyroidism and FHH. Non-PTH–mediated causes include vitamin D toxicity, solid tumor malignancy with or without metastasis, multiple myeloma (MM) and other plasma cell dyscrasias, granulomatous disease such as sarcoid, and some medications.1
The differential diagnosis for hypercalcemia begins with measurement of the patient’s intact PTH level. An elevated or high-normal result indicates a PTH-mediated cause, so 24-hour measurement of excretion of urinary calcium is the next step. A low or low-normal PTH level (< 20 pg/mL), however, suggests the cause is non-PTH-mediated.2 The diagnostic approach in this situation is more challenging because testing to exclude or confirm various potential causes can be expensive and time-consuming. The degree of hypercalcemia, however, can aid in the diagnosis: Primary hyperparathyroidism is often associated with borderline or mild hypercalcemia, while calcium values > 13 mg/dL are more common in patients with malignancies.2
PTH elevates calcium levels in the blood when ionized (free) calcium levels are low by increasing gastrointestinal absorption, decreasing urinary excretion, and increasing bone resorption.3 With malignant tumors such as lung, breast, and renal cell, osteolytic metastases can destroy the bone, resulting in release of calcium. In other cases, solid-tumor cancers produce PTHrP, which increases serum calcium. This latter situation is referred to as humoral hypercalcemia of malignancy.3 Lymphoma and granulomatous disease, such as sarcoid, can be associated with excess production of 1,25-dihydroxyvitamin D.2 If vitamin D is elevated but PTH and PTHrP are normal, a chest x-ray should be obtained to evaluate the patient for sarcoid or lymphoma.
Hypercalcemia work-up
The work-up for suspected hypercalcemia begins with measurement of the patient’s calcium level. Because calcium is bound to albumin in the blood, the standard serum calcium test may not reflect the true calcium level. (If albumin is high, the calcium level will be high, and vice versa.)1 The true (serum ionized) calcium level (also known as corrected calcium level) should always be calculated to confirm true hypercalcemia. A formula commonly used to calculate the corrected calcium level is
Corrected calcium (mg/dL) = (measured calcium [mg/dL]) + 0.8 (4.0 – serum albumin [mg/dL])
Direct measurement of the serum ionized calcium level is not affected by the albumin level and can also confirm true hypercalcemia.4
Once hypercalcemia is confirmed, the next step is to measure the patient’s intact PTH level.
If intact PTH is elevated or high normal, consider primary hyperparathyroidism or FHH and confirm by obtaining the urine calcium level.
• If urine calcium level is high (> 200 mg/24 h), the diagnosis is primary hyperparathyroidism.
• If urine calcium level is low (< 100 mg/24 h), the diagnosis is FHH.
If intact PTH is low, consider non–PTH-mediated causes and confirm by obtaining PTHrP and vitamin D levels.
• If PTHrP level is elevated, scan for malignancy.
• If 1,25-dihydroxyvitamin D level is elevated, order a chest x-ray to rule out sarcoid or lymphoma.
• If both PTHrP and vitamin D levels are normal, order both serum protein electrophoresis (SPEP) and urine protein electrophoresis (UPEP) with immunofixation to rule out MM.
• If vitamin D level is elevated, check vitamin and herbal supplement use for excessive vitamin D intake.
Treatment of symptomatic hypercalcemia
The goals of treatment of symptomatic hypercalcemia are to reduce the serum calcium level to the normal range and to treat the underlying cause.1 Mild hypercalcemia (calcium level, 10-12 mg/dL) is typically asymptomatic and does not need to be treated. Moderate hypercalcemia (calcium level, 12-14 mg/dL) may not require treatment unless the patient is symptomatic and/or has had an acute rise in calcium level.1 In mild to moderate hypercalcemia, the serum calcium level should be monitored to establish a trend.
Treatment for symptomatic moderate and severe hypercalcemia (calcium level, > 14 mg/dL) typically involves a similar regimen:
• Volume expansion with isotonic saline at an initial rate of 2 to 4 L/d, which is then adjusted to achieve 200 mL/h of continuous urine output. IV furosemide can be used with caution (10-20 mg IV as needed) to promote diuresis if volume overload is a concern (furosemide promotes renal excretion of calcium).
• Administration of subcutaneous calcitonin (4-8 IU/kg, repeated every 6 h for 24 h). Calcitonin works rapidly to lower calcium levels in 4 to 6 h.
• Concurrent administration of IV bisphosphonate (zoledronic acid [4 mg over 15 min] or pamidronate [60-90 mg over 4 h]). Pamidronate is superior for reversal of malignancy-related hypercalcemia.1
Hypercalcemia and multiple myeloma
MM is a malignant neoplasm of plasma cells that accounts for approximately 1% of all cancers and about 10% of hematologic malignancies in the United States, with a median patient age of 70 at diagnosis.5-7 In MM, myeloma cells induce the secretion of cytokines and growth factors that alter plasma cells, activate osteoclasts, suppress osteoblasts, cause abnormal interactions between plasma cells and bone marrow, and stimulate aberrant angiogenesis.8 Osteoclastic bone resorption produces hypercalcemia as well as the lytic lesions seen on x-ray.7
Approximately 74% of patients present with typical MM symptoms of calcium elevation in the blood, renal insufficiency, anemia, and bone lesions, known as CRAB symptoms, but other myeloma-related manifestations may be present.9
Diagnostic criteria for MM include the following (all three must be present):10
• Monoclonal bone marrow plasma cells ≥ 10% and/or a biopsy-proven plasmacytoma
• Monoclonal protein in the serum and/or urine (if none is detected, disease is nonsecretory and diagnosis requires ≥ 30% bone marrow plasma cells and/or biopsy-proven plasmacytoma)
• Myeloma-related organ dysfunction, indicated by at least one of the CRAB symptoms.
In the absence of CRAB symptoms, an asymptomatic patient may have an MM precursor syndrome: monoclonal gammopathy of undetermined significance or smoldering (or indolent) MM.10
Treatment of multiple myeloma
In recent years, the use of induction therapy followed by autologous stem cell transplantation and the development of novel therapeutic agents have extended overall survival for patients with MM. These agents include proteasome inhibitors (bortezomib and the second-generation carfilzomib)11 and immunomodulators (thalidomide and the second-generation lenalidomide). Early diagnosis and treatment can improve progression-free survival as well as overall survival, including recovery of renal function for patients with renal failure.12 With survival ranging from one year or less—with aggressive disease—to 10 years or more for patients with responsive disease,7 there remains no cure for MM.
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
Further work-up included SPEP and UPEP with immunofixation, which revealed marked IgG free λ light chains with an M (monoclonal) component, making MM a very likely diagnosis. Confirmation by means of bone marrow biopsy was indicated, but the patient refused the procedure.
The patient’s hypercalcemia was treated by IV administration of calcitonin with isotonic saline. This reduced the serum calcium level from 15.4 mg/dL to 10.6 mg/dL within 48 hours. One dose of ergocalciferol (vitamin D2) was then administered to promote intestinal absorption of calcium and support bone mineralization, further lowering the patient’s serum calcium level to a normal 8.9 mg/dL. Hypokalemia was treated with oral potassium supplementation.
The patient, now stable, was referred to the hematology/oncology and bone mineral metabolism clinics and was discharged from the hospital. She did not keep those appointments and was lost to follow-up.
CONCLUSION
The most common causes of hypercalcemia are hyperparathyroidism and malignancy. Most cases do not require treatment unless the calcium level is >14 mg/dL and/or the patient is symptomatic. Red flag symptoms include weakness, abdominal pain, mental status changes, and coma.4 Primary care clinicians should suspect MM in older patients with laboratory findings of hypercalcemia, anemia, and renal dysfunction, with lytic lesions on x-ray.
REFERENCES
1. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67(9):1959-1966.
2. Endres DB. Investigation of hypercalcemia. Clin Biochem. 2012;45(12):
954-963.
3. Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care. 2008;35(2):215-237.
4. Sharma B, Misicko NE. How should you evaluate elevated calcium in an asymptomatic patient? J Fam Pract. 2008;57(4):267-269.
5. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
6. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
7. Shaheen SP, Talwalkar SS, Medeiros LJ. Multiple myeloma and immunosecretory disorders: an update. Adv Anat Pathol. 2008;15(4):196-210.
8. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11): 1046-1060.
9. Talamo G, Farooq U, Zangari M, et al. Beyond the CRAB symptoms: a study of presenting clinical manifestations of multiple myeloma. Clin Lymphoma Myeloma Leuk. 2010;10(6):464-468.
10. Palumbo A, Sezer O, Kyle R, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transportation. Leukemia. 2009;23(10):1716-1730.
11. Kyprolis [package insert]. South San Francisco, CA: Onyx Pharmaceuticals, Inc; 2012.
12. Suyani E, Sucak GT, Erten Y, et al. Evaluation of multiple myeloma patients presenting with renal failure in a university hospital in the year 2010. Ren Fail. 2012;34(2):257-262.