User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Should lithium and ECT be used concurrently in geriatric patients?
Delirium has been described as a potential complication of concurrent lithium and electroconvulsive therapy (ECT) for depression, in association with a range of serum lithium levels. Although debate persists about the safety of continuing previously established lithium therapy during a course of ECT for mood symptoms, withholding lithium for 24 hours before administering ECT and measuring the serum lithium level before ECT were found to decrease the risk of post-ECT neurocognitive effects.1
We have found that the conventional practice of holding lithium for 24 hours before ECT might need to be re-evaluated in geriatric patients, as the following case demonstrates. Only 24 hours of holding lithium therapy might result in a lithium level sufficient to contribute to delirium after ECT.
CASE REPORT
An older woman with recurrent unipolar psychotic depression
Mrs. A, age 81, was admitted to the hospital with a 1-week history of depressed mood, anhedonia, insomnia, anergia, anorexia, and nihilistic somatic delusions that her organs were “rotting and shutting down.” Treatment included nortriptyline, 40 mg/d; lithium, 150 mg/d; and haloperidol, 0.5 mg/d. Her serum lithium level was 0.3 mEq/L (reference range, 0.6 to 1.2 mEq/L); the serum nortriptyline level was 68 ng/mL (reference range, 50 to 150 ng/mL). CT of the head and an electrocardiogram were unremarkable.
A twice-weekly course of ECT was initiated.
The day before Treatment 1 of ECT, the serum lithium level (drawn 12 hours after the last dose) was 0.4 mEq/L. Lithium was withheld 24 hours before ECT; nortriptyline and haloperidol were continued at prescribed dosages.
Right unilateral stimulation was used at 50%/mC energy (Thymatron DG, with methohexital anesthesia, and succinylcholine for muscle relaxation). Seizure duration, measured by EEG, was 57 seconds.
Mrs. A developed postictal delirium after the first 2 ECT sessions. The serum lithium level was unchanged. Subsequently, lithium treatment was discontinued and ECT was continued; once lithium was stopped, delirium resolved. ECT sessions 3 and 4 were uneventful, with no post-treatment delirium. Seizure duration for Treatment 4 was 58 seconds. She started breathing easily after all ECT sessions.
After Treatment 4, Mrs. A experienced full remission of depressive and psychotic symptoms. Repeat CT of head, after Treatment 4, was unchanged from baseline.
What is the role of lithium?
Mrs. A did not exhibit typical signs of lithium intoxication (diarrhea, vomiting, tremor). Notably, lithium has an intrinsic anticholinergic activity2; concurrent nortriptyline, a secondary amine tricyclic antidepressant with fewer anticholinergic side effects than other tricyclics,2 could precipitate delirium in a vulnerable patient secondary to excessive cumulative anticholinergic exposure.
No prolonged time-to-respiration or time-to-awakening occurred during treatments in which concurrent lithium and ECT were used; seizure duration with and without concurrent lithium was relatively similar.
There are potential complications of concurrent use of lithium and ECT:
• prolongation of the duration of muscle paralysis and apnea induced by commonly used neuromuscular-blocking agents (eg, succinylcholine)
• post-ECT cognitive disturbance.1,3,4
There is debate about the safety of continuing lithium during, or in close proximity to, ECT. In a case series of 12 patients who underwent combined lithium therapy and ECT, the authors concluded that this combination can be safe, regardless of age, as long as appropriate clinical monitoring is provided.4 In Mrs. A’s case, once post-ECT delirium was noted, lithium was discontinued for subsequent ECT sessions.
Because further ECT was uneventful without lithium, and no other clear acute cause of delirium could be identified, we concluded that lithium likely played a role in Mrs. A’s delirium. Notably, nortriptyline had been continued, suggesting that the degree of anticholinergic blockade provided by nortriptyline was insufficient to provoke delirium post-ECT in the absence of potentiation of this effect, as it had been when lithium also was used initially.
Guidelines for dosing and serum lithium concentrations in geriatric patients are not well-established; the current traditional range of 0.6 to 1.2 mEq/L, is too high for geriatric patients and can result in episodes of lithium toxicity, including delirium.5 Although our patient’s lithium level was below the reference range for all patients, a level of 0.3 mEq/L can be considered at the low end of the reference range for geriatric patients.5 Inasmuch as the lithium-assisted post-ECT delirium could represent a clinical sign of lithium toxicity, perhaps even a subtherapeutic level in a certain patient could be paradoxically “toxic.”
Although the serum lithium level in our patient remained below the toxic level for the general population (>1.5 mEq/L), delirium in a geriatric patient could result from:
• age-related changes in the pharmacokinetics of lithium, a water-soluble drug; these changes reduce renal clearance of the drug and extend plasma elimination half-life of a single dose to 36 hours, with the result that lithium remains in the body longer and necessitating a lower dosage (ie, a dosage that yields a serum level of approximately 0.5 mEq/L)
• the CNS tissue concentration of lithium, which can be high even though the serum level is not toxic
• an age-related increase in blood-brain barrier permeability, making the barrier more porous for drugs
• changes in blood-brain barrier permeability by post-ECT biochemical induction, with subsequent increased drug availability in the CNS.5,6
What we recommend
Possible interactions between lithium and ECT that lead to ECT-associated delirium need further elucidation, but discontinuing lithium during the course of ECT in a geriatric patient warrants your consideration. Following a safe interval after the last ECT session, lithium likely can be safely re-introduced 1) if there is clinical need and 2) as long as clinical surveillance for cognitive side effects is provided— especially if ECT will need to be reconsidered in the future.
Two additional considerations:
• Actively reassess lithium dosing in all geriatric psychiatric patients, especially those with renal insufficiency and other systemic metabolic considerations.
• Actively examine the use of all other anticholinergic agents in the course of evaluating a patient’s candidacy for ECT.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. A task force report of the American Psychiatric Association. 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
2. Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333-1341.
3. Hill GE, Wong KC, Hodges MR. Potentiation of succinylcholine neuromuscular blockade by lithium carbonate. Anesthesiology. 1976;44(5):439-442.
4. Dolenc TJ, Rasmussen KG. The safety of electroconvulsive therapy and lithium in combination: a case series and review of the literature. J ECT. 2005;21(3):165-170.
5. Shulman KI. Lithium for older adults with bipolar disorder: should it still be considered a first line agent? Drugs Aging. 2010;27(8):607-615.
6. Grandjean EM, Aubry JM. Lithium: updated human knowledge using an evidence-based approach. Part II: clinical pharmacology and therapeutic monitoring. CNS Drugs. 2009;23(4):331-349.
Delirium has been described as a potential complication of concurrent lithium and electroconvulsive therapy (ECT) for depression, in association with a range of serum lithium levels. Although debate persists about the safety of continuing previously established lithium therapy during a course of ECT for mood symptoms, withholding lithium for 24 hours before administering ECT and measuring the serum lithium level before ECT were found to decrease the risk of post-ECT neurocognitive effects.1
We have found that the conventional practice of holding lithium for 24 hours before ECT might need to be re-evaluated in geriatric patients, as the following case demonstrates. Only 24 hours of holding lithium therapy might result in a lithium level sufficient to contribute to delirium after ECT.
CASE REPORT
An older woman with recurrent unipolar psychotic depression
Mrs. A, age 81, was admitted to the hospital with a 1-week history of depressed mood, anhedonia, insomnia, anergia, anorexia, and nihilistic somatic delusions that her organs were “rotting and shutting down.” Treatment included nortriptyline, 40 mg/d; lithium, 150 mg/d; and haloperidol, 0.5 mg/d. Her serum lithium level was 0.3 mEq/L (reference range, 0.6 to 1.2 mEq/L); the serum nortriptyline level was 68 ng/mL (reference range, 50 to 150 ng/mL). CT of the head and an electrocardiogram were unremarkable.
A twice-weekly course of ECT was initiated.
The day before Treatment 1 of ECT, the serum lithium level (drawn 12 hours after the last dose) was 0.4 mEq/L. Lithium was withheld 24 hours before ECT; nortriptyline and haloperidol were continued at prescribed dosages.
Right unilateral stimulation was used at 50%/mC energy (Thymatron DG, with methohexital anesthesia, and succinylcholine for muscle relaxation). Seizure duration, measured by EEG, was 57 seconds.
Mrs. A developed postictal delirium after the first 2 ECT sessions. The serum lithium level was unchanged. Subsequently, lithium treatment was discontinued and ECT was continued; once lithium was stopped, delirium resolved. ECT sessions 3 and 4 were uneventful, with no post-treatment delirium. Seizure duration for Treatment 4 was 58 seconds. She started breathing easily after all ECT sessions.
After Treatment 4, Mrs. A experienced full remission of depressive and psychotic symptoms. Repeat CT of head, after Treatment 4, was unchanged from baseline.
What is the role of lithium?
Mrs. A did not exhibit typical signs of lithium intoxication (diarrhea, vomiting, tremor). Notably, lithium has an intrinsic anticholinergic activity2; concurrent nortriptyline, a secondary amine tricyclic antidepressant with fewer anticholinergic side effects than other tricyclics,2 could precipitate delirium in a vulnerable patient secondary to excessive cumulative anticholinergic exposure.
No prolonged time-to-respiration or time-to-awakening occurred during treatments in which concurrent lithium and ECT were used; seizure duration with and without concurrent lithium was relatively similar.
There are potential complications of concurrent use of lithium and ECT:
• prolongation of the duration of muscle paralysis and apnea induced by commonly used neuromuscular-blocking agents (eg, succinylcholine)
• post-ECT cognitive disturbance.1,3,4
There is debate about the safety of continuing lithium during, or in close proximity to, ECT. In a case series of 12 patients who underwent combined lithium therapy and ECT, the authors concluded that this combination can be safe, regardless of age, as long as appropriate clinical monitoring is provided.4 In Mrs. A’s case, once post-ECT delirium was noted, lithium was discontinued for subsequent ECT sessions.
Because further ECT was uneventful without lithium, and no other clear acute cause of delirium could be identified, we concluded that lithium likely played a role in Mrs. A’s delirium. Notably, nortriptyline had been continued, suggesting that the degree of anticholinergic blockade provided by nortriptyline was insufficient to provoke delirium post-ECT in the absence of potentiation of this effect, as it had been when lithium also was used initially.
Guidelines for dosing and serum lithium concentrations in geriatric patients are not well-established; the current traditional range of 0.6 to 1.2 mEq/L, is too high for geriatric patients and can result in episodes of lithium toxicity, including delirium.5 Although our patient’s lithium level was below the reference range for all patients, a level of 0.3 mEq/L can be considered at the low end of the reference range for geriatric patients.5 Inasmuch as the lithium-assisted post-ECT delirium could represent a clinical sign of lithium toxicity, perhaps even a subtherapeutic level in a certain patient could be paradoxically “toxic.”
Although the serum lithium level in our patient remained below the toxic level for the general population (>1.5 mEq/L), delirium in a geriatric patient could result from:
• age-related changes in the pharmacokinetics of lithium, a water-soluble drug; these changes reduce renal clearance of the drug and extend plasma elimination half-life of a single dose to 36 hours, with the result that lithium remains in the body longer and necessitating a lower dosage (ie, a dosage that yields a serum level of approximately 0.5 mEq/L)
• the CNS tissue concentration of lithium, which can be high even though the serum level is not toxic
• an age-related increase in blood-brain barrier permeability, making the barrier more porous for drugs
• changes in blood-brain barrier permeability by post-ECT biochemical induction, with subsequent increased drug availability in the CNS.5,6
What we recommend
Possible interactions between lithium and ECT that lead to ECT-associated delirium need further elucidation, but discontinuing lithium during the course of ECT in a geriatric patient warrants your consideration. Following a safe interval after the last ECT session, lithium likely can be safely re-introduced 1) if there is clinical need and 2) as long as clinical surveillance for cognitive side effects is provided— especially if ECT will need to be reconsidered in the future.
Two additional considerations:
• Actively reassess lithium dosing in all geriatric psychiatric patients, especially those with renal insufficiency and other systemic metabolic considerations.
• Actively examine the use of all other anticholinergic agents in the course of evaluating a patient’s candidacy for ECT.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Delirium has been described as a potential complication of concurrent lithium and electroconvulsive therapy (ECT) for depression, in association with a range of serum lithium levels. Although debate persists about the safety of continuing previously established lithium therapy during a course of ECT for mood symptoms, withholding lithium for 24 hours before administering ECT and measuring the serum lithium level before ECT were found to decrease the risk of post-ECT neurocognitive effects.1
We have found that the conventional practice of holding lithium for 24 hours before ECT might need to be re-evaluated in geriatric patients, as the following case demonstrates. Only 24 hours of holding lithium therapy might result in a lithium level sufficient to contribute to delirium after ECT.
CASE REPORT
An older woman with recurrent unipolar psychotic depression
Mrs. A, age 81, was admitted to the hospital with a 1-week history of depressed mood, anhedonia, insomnia, anergia, anorexia, and nihilistic somatic delusions that her organs were “rotting and shutting down.” Treatment included nortriptyline, 40 mg/d; lithium, 150 mg/d; and haloperidol, 0.5 mg/d. Her serum lithium level was 0.3 mEq/L (reference range, 0.6 to 1.2 mEq/L); the serum nortriptyline level was 68 ng/mL (reference range, 50 to 150 ng/mL). CT of the head and an electrocardiogram were unremarkable.
A twice-weekly course of ECT was initiated.
The day before Treatment 1 of ECT, the serum lithium level (drawn 12 hours after the last dose) was 0.4 mEq/L. Lithium was withheld 24 hours before ECT; nortriptyline and haloperidol were continued at prescribed dosages.
Right unilateral stimulation was used at 50%/mC energy (Thymatron DG, with methohexital anesthesia, and succinylcholine for muscle relaxation). Seizure duration, measured by EEG, was 57 seconds.
Mrs. A developed postictal delirium after the first 2 ECT sessions. The serum lithium level was unchanged. Subsequently, lithium treatment was discontinued and ECT was continued; once lithium was stopped, delirium resolved. ECT sessions 3 and 4 were uneventful, with no post-treatment delirium. Seizure duration for Treatment 4 was 58 seconds. She started breathing easily after all ECT sessions.
After Treatment 4, Mrs. A experienced full remission of depressive and psychotic symptoms. Repeat CT of head, after Treatment 4, was unchanged from baseline.
What is the role of lithium?
Mrs. A did not exhibit typical signs of lithium intoxication (diarrhea, vomiting, tremor). Notably, lithium has an intrinsic anticholinergic activity2; concurrent nortriptyline, a secondary amine tricyclic antidepressant with fewer anticholinergic side effects than other tricyclics,2 could precipitate delirium in a vulnerable patient secondary to excessive cumulative anticholinergic exposure.
No prolonged time-to-respiration or time-to-awakening occurred during treatments in which concurrent lithium and ECT were used; seizure duration with and without concurrent lithium was relatively similar.
There are potential complications of concurrent use of lithium and ECT:
• prolongation of the duration of muscle paralysis and apnea induced by commonly used neuromuscular-blocking agents (eg, succinylcholine)
• post-ECT cognitive disturbance.1,3,4
There is debate about the safety of continuing lithium during, or in close proximity to, ECT. In a case series of 12 patients who underwent combined lithium therapy and ECT, the authors concluded that this combination can be safe, regardless of age, as long as appropriate clinical monitoring is provided.4 In Mrs. A’s case, once post-ECT delirium was noted, lithium was discontinued for subsequent ECT sessions.
Because further ECT was uneventful without lithium, and no other clear acute cause of delirium could be identified, we concluded that lithium likely played a role in Mrs. A’s delirium. Notably, nortriptyline had been continued, suggesting that the degree of anticholinergic blockade provided by nortriptyline was insufficient to provoke delirium post-ECT in the absence of potentiation of this effect, as it had been when lithium also was used initially.
Guidelines for dosing and serum lithium concentrations in geriatric patients are not well-established; the current traditional range of 0.6 to 1.2 mEq/L, is too high for geriatric patients and can result in episodes of lithium toxicity, including delirium.5 Although our patient’s lithium level was below the reference range for all patients, a level of 0.3 mEq/L can be considered at the low end of the reference range for geriatric patients.5 Inasmuch as the lithium-assisted post-ECT delirium could represent a clinical sign of lithium toxicity, perhaps even a subtherapeutic level in a certain patient could be paradoxically “toxic.”
Although the serum lithium level in our patient remained below the toxic level for the general population (>1.5 mEq/L), delirium in a geriatric patient could result from:
• age-related changes in the pharmacokinetics of lithium, a water-soluble drug; these changes reduce renal clearance of the drug and extend plasma elimination half-life of a single dose to 36 hours, with the result that lithium remains in the body longer and necessitating a lower dosage (ie, a dosage that yields a serum level of approximately 0.5 mEq/L)
• the CNS tissue concentration of lithium, which can be high even though the serum level is not toxic
• an age-related increase in blood-brain barrier permeability, making the barrier more porous for drugs
• changes in blood-brain barrier permeability by post-ECT biochemical induction, with subsequent increased drug availability in the CNS.5,6
What we recommend
Possible interactions between lithium and ECT that lead to ECT-associated delirium need further elucidation, but discontinuing lithium during the course of ECT in a geriatric patient warrants your consideration. Following a safe interval after the last ECT session, lithium likely can be safely re-introduced 1) if there is clinical need and 2) as long as clinical surveillance for cognitive side effects is provided— especially if ECT will need to be reconsidered in the future.
Two additional considerations:
• Actively reassess lithium dosing in all geriatric psychiatric patients, especially those with renal insufficiency and other systemic metabolic considerations.
• Actively examine the use of all other anticholinergic agents in the course of evaluating a patient’s candidacy for ECT.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. A task force report of the American Psychiatric Association. 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
2. Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333-1341.
3. Hill GE, Wong KC, Hodges MR. Potentiation of succinylcholine neuromuscular blockade by lithium carbonate. Anesthesiology. 1976;44(5):439-442.
4. Dolenc TJ, Rasmussen KG. The safety of electroconvulsive therapy and lithium in combination: a case series and review of the literature. J ECT. 2005;21(3):165-170.
5. Shulman KI. Lithium for older adults with bipolar disorder: should it still be considered a first line agent? Drugs Aging. 2010;27(8):607-615.
6. Grandjean EM, Aubry JM. Lithium: updated human knowledge using an evidence-based approach. Part II: clinical pharmacology and therapeutic monitoring. CNS Drugs. 2009;23(4):331-349.
1. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. A task force report of the American Psychiatric Association. 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
2. Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333-1341.
3. Hill GE, Wong KC, Hodges MR. Potentiation of succinylcholine neuromuscular blockade by lithium carbonate. Anesthesiology. 1976;44(5):439-442.
4. Dolenc TJ, Rasmussen KG. The safety of electroconvulsive therapy and lithium in combination: a case series and review of the literature. J ECT. 2005;21(3):165-170.
5. Shulman KI. Lithium for older adults with bipolar disorder: should it still be considered a first line agent? Drugs Aging. 2010;27(8):607-615.
6. Grandjean EM, Aubry JM. Lithium: updated human knowledge using an evidence-based approach. Part II: clinical pharmacology and therapeutic monitoring. CNS Drugs. 2009;23(4):331-349.
Personalized medicine for schizophrenia
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Personalized medicine for schizophrenia
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Guidelines for treating survivors of suicide
Delirium in the hospital: Emphasis on the management of geriatric patients
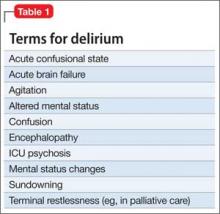
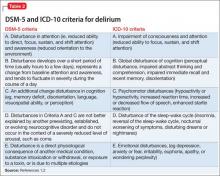
Although delirium has many descriptive terms (Table 1), a common unifying term is “acute global cognitive dysfunction,” now recognized as delirium; a consensus supported by DSM-51 and ICD-102 (Table 2). According to DSM-5, the essential feature is a disturbance of attention or awareness that is accompanied by a change in baseline cognition that cannot be explained by another preexisting, established, or evolving neurocognitive disorder (the newly named DSM-5 entity for dementia syndromes).1 Because delirium affects the cortex diffusely, psychiatric symptoms can include cognitive, mood, anxiety, or psychotic symptoms. Because many systemic illnesses can induce delirium, the differential diagnosis spans all organ systems.
Three subtypes
Delirium can be classified, based on symptoms,3,4 into 3 subtypes: hyperactive-hyperalert, hypoactive-hypoalert, and mixed delirium. Hyperactive patients present with restlessness and agitation. Hypoactive patients are lethargic, confused, slow to respond to questions, and often appear depressed. The differential prognostic significance of these subtypes has been examined in the literature, with conflicting results. Rabinowitz5 reported that hypoactive delirium has the worst prognosis, while Marcantonio et al6 indicated that the hyperactive subtype is associated with the highest mortality rate. Mixed delirium, with periods of both hyperactivity and hypoactivity, is the most common type of delirium.7
A prodromal phase, characterized by anxiety, frequent requests for nursing and medical assistance, decreased attention, restlessness, vivid dreams, disorientation immediately after awakening, and hallucinations, can occur before an episode of full-spectrum delirium; this prodromal state often is identified retrospectively —after the patient is in an episode of delirium.8,9
Evidence-based guidelines aim to improve recognition and clinical management.10-13 Disruptive behavior is the main reason for psychiatric referral in delirium.14,15 Delayed psychiatric consultation because of non-recognition of delirium is related to variables such as older age; history of a pre-existing, comorbid neurocognitive disorder; and the clinical appearance of hypoactive delirium.14
The case of Mr. D (Box),16 illustrates how the emergence of antipsychotic-associated neuroleptic malignant syndrome (NMS) can complicate antipsychotic treatment of delirium in a geriatric medical patient, although delirium also is a common presentation in NMS.17 Delirium developed after an increase in carbidopa/levodopa, which has central dopaminergic effects that can precipitate delirium, particularly in a geriatric patient with preexisting comorbid neurocognitive disorder. Further complicating Mr. D’s delirium presentation was the development of NMS, which had a multifactorial causation, such as the use of dopamine antagonists (ie, quetiapine, metoclopramide), and an abrupt decrease of a dopaminergic agent (ie, carbidopa/levodopa), all inducing a central dopamine relative hypoactivity.
Epidemiology
Delirium is more common in older patients,15 and is seen in 30% to 40% of hospitalized geriatric patients.18 Delirium in older patients, compared with other adults, is associated with more severe cognitive impairment.19 It is common among geriatric surgical patients (15% to 62%)20 with a peak 2 to 5 days postoperatively for hip fracture,21 and often is seen in ICU patients (70% to 87%).20 However, Spronk et al22 found that delirium is significantly under-recognized in the ICU. Nearly 90% of terminally ill patients become delirious before death.23 Terminal delirium often is unrecognized and can interfere with assessment of other clinical problems.24 A preexisting history of comorbid neurocognitive disorder was evident in as many as two-thirds of delirium cases.25
Pathophysiology and risk factors
The pathophysiology of delirium has been characterized as an imbalance of CNS metabolism, including decreased blood flow in various regions of the brain that may normalize once delirium resolves.26 Studies describe the simultaneous decrease of cholinergic transmission and dopaminergic excess.27,28 Predisposing and precipitating factors for delirium that are of particular importance in geriatric patients include:
• advanced age
• CNS disease
• infection
• cognitive impairment
• male sex
• poor nutrition
• dehydration and other metabolic abnormalities
• cardiovascular events
• substance use
• medication
• sensory deprivation (eg, impaired vision or hearing)
• sleep deprivation
• low level of physical activity.27,29,30
Table 3 lists the most common delirium-provocative medications.27
Evaluation and psychometric scales
The EEG can be useful in evaluating delirium, especially in clinically ambiguous cases. EEG findings may indicate generalized slowing or dropout of the posterior dominant rhythm, and generalized slow theta and delta waves, findings that are more common in delirium than in other neurocognitive disorders and other psychiatric illnesses. The EEG must be interpreted in the context of the delirium diagnostic workup, because abnormalities seen in other neurocognitive disorders can overlap with those of delirium.31
The EEG referral should specify the clinical suspicion of delirium to help interpret the results. Delirium cases in which the patient’s previous cognitive status is unknown may benefit from EEG evaluation, such as:
• in possible status epilepticus
• when delirium improvement has reached a plateau at a lower level of cognitive function than before onset of delirium
• when the patient is unable or unwilling to complete a psychiatric interview.27
Assessment instruments are available to diagnose and monitor delirium (Table 4). Typically, delirium assessment includes examining levels of arousal, psychomotor activity, cognition (ie, orientation, attention, and memory), and perceptual disturbances.
Psychometrically, a review of Table 4 suggests that validity appeared stable with adequate specificity (64% to 99%) but more variable sensitivity (36% to 100%). These reliability parameters also will be affected by the classification system (ie, DSM vs ICD) and the cut-off score employed.32 Most measures (eg, Confusion Assessment Method [CAM], CAM-ICU) provide an adequate sample of behavioral (ie, level of alertness), motor (ie, psychomotor activity), and cognitive (ie, orientation, attention, memory, and receptive language) function, with the exception of the Global Attentiveness Rating, which is a 2-minute open conversation protocol between physician and patient.
Some measures are stand-alone instruments, such as the Memorial Delirium Assessment Scale, whereas the CAM requires administration of separate cognitive screens, including the Mini-Mental State Examination (MMSE) and Digit Span.33 Instruments to detect delirium in critically ill patients are a more recent development. Wong et al34 reported that the most widely studied tool was the CAM. Obtaining collateral information from family, caregivers, and hospital staff is essential, particularly given the fluctuating nature of delirium.
Management
Prevention. Identify patients at high risk of delirium so that preventive strategies can be employed. Multi-component, nonpharmacotherapeutic interventions are used in clinical settings but few randomized trials have been conducted. The contributing effectiveness of individual components is not well-studied, but most include staff education to increase awareness of delirium. Of 3 multi-component intervention randomized trials, 2 reported a significantly lower incidence of delirium in the intervention group.35-37 Implementation of a multi-component protocol in medical/ surgical units was associated with a significant reduction in use of restraints.38
As in Mr. D’s case, complex drug regimens, particularly for CNS illness, can increase the risk of delirium. Considering the medication profile for patients with complex systemic illness—in particular, minimizing the use anticholinergics and dopamine agonists— may be crucial in preventing delirium.
Prophylactic administration of antipsychotics may reduce the risk of developing postoperative delirium.39 Studies of the use of these agents were characterized by small sample sizes and selected groups of patient populations. Of the 4 randomized studies evaluating prophylactic antipsychotics (vs placebo), 3 found a lower incidence of delirium in the intervention groups.39-41
A study of haloperidol in post-GI surgery patients showed a reduced occurrence of delirium,40 whereas its prophylactic use in patients undergoing hip surgery42 did not reduce the incidence of delirium compared with placebo, but did decrease severity when delirium occurred.42
Risperidone39 in post-cardiac surgery and olanzapine41 perioperatively in patients undergoing total knee or hip replacement have been shown to decrease delirium severity and duration. Targeted prophylaxis with risperidone43 in post-cardiac surgery patients who showed disturbed cognition but did not meet criteria for delirium reduced the number of patients requiring medication, compared with placebo.43
Dexmedetomidine, an α-2 adrenergic receptor agonist, compared with propofol or midazolam in post-cardiac valve surgery patients, resulted in a decreased incidence of delirium but no difference in delirium duration, hospital length of stay, or use of other medications.44 However, other studies have shown that dexmedetomidine reduces ICU length of stay and duration of mechanical ventilation.45
Treatment. Management of hospitalized medically ill geriatric patients with delirium is challenging and requires a comprehensive approach. The first step in delirium management is prompt identification and management of systemic medical disturbances associated with the delirium episode. First-line, nonpharmacotherapeutic strategies for patients with delirium include:
• reorientation
• behavioral interventions (eg, use of clear instructions and frequent eye contact with patients)
• environmental interventions (eg, minimal noise, adequate lighting, and limited room and staff changes)
• avoidance of physical restraints.46
Consider employing family members or hospital staff sitters to stay with the patient and to reassure, reorient, and watch for agitation and other unsafe behaviors (eg, attempted elopement). Psychoeducation for the patient and family on the phenomenology of delirium can be helpful.
The use of drug treatment strategies should be integrated into a comprehensive approach that includes the routine use of nondrug measures.46 Using medications for treating hypoactive delirium, formerly controversial, now has wider acceptance.47,48 A few high-quality randomized trials have been performed.25,49,50
Pharmacotherapy, especially in frail patients, should be initiated at the lowest starting dosage and titrated cautiously to clinical effect and for the shortest period of time necessary. Antipsychotics are preferred agents for treating all subtypes of delirium; haloperidol is widely used.46,51,52 However, antipsychotics, including haloperidol, can be associated with adverse neurologic effects such as extrapyramidal symptoms (EPS) and NMS.
Although reported less frequently than with haloperidol, other agents have been implicated in development of EPS and NMS, including atypical antipsychotics and antiemetic dopamine antagonists, particularly in parkinsonism-prone patients.53 Strategies that can minimize such risks in geriatric inpatients with delirium include oral, rather than parenteral, use of antipsychotics—preferential use of atypical over typical antipsychotics— and lowest effective dosages.54
In controlled trials, atypical antipsychotics for delirium showed efficacy compared with haloperidol.52,55 However, there is no research that demonstrates any advantage of one atypical over another.25
In Mr. D’s case, the most important intervention for managing delirium caused by NMS is to discontinue all dopamine antagonists and treat agitation with judicious doses of a benzodiazepine, with supportive care.17 In cases of sudden discontinuation or a dosage decrease of dopamine agonists, these medications should be resumed or optimized to minimize the risk of NMS-associated rhabdomyolysis and subsequent renal failure.17 Antipsychotics carry an increased risk of stroke and mortality in older patients with established or evolving neurocognitive disorders56,57 and can cause prolongation of the QTc interval.57
Other medications that could be used for delirium include cholinesterase inhibitors58,59 (although larger trials and a systematic review did not support this use60), and 5-HT receptor antagonists,61 such as trazodone. Benzodiazepines, such as lorazepam, are first-line treatment for delirium associated with seizures or withdrawal from alcohol, sedatives, hypnotics, and anxiolytics and for delirium caused by NMS. Be cautious about using benzodiazepines in geriatric patients because of a risk of respiratory depression, falls, sedation, and amnesia.
Geriatric patients with alcoholism and those with malnutrition are prone to thiamine and vitamin B12 deficiencies, which can induce delirium. Laboratory assessment and consideration of supplementation is recommended. Despite high occurrence of delirium in hospitalized older adults with preexisting comorbid neurocognitive disorders, there is no standard care for delirium comorbid with another neurocognitive disorder.62 Clinical practice guidelines for older patients receiving palliative care have been developed63; the goal is to minimize suffering and discomfort in patients in palliative care.64
Post-delirium prophylaxis. Medications for delirium usually can be tapered and discontinued once the episode has resolved and the patient is stable; it is common to discontinue medications when the patient has been symptom-free for 1 week.65 Some patients (eg, with end-stage liver disease, disseminated cancer) are prone to recurrent or to prolonged or chronic delirium. A period of post-recovery treatment with antipsychotics—even indefinite treatment in some cases—should be considered.
Post-delirium debriefing and aftercare. The psychological complications of delirium are distressing for the patient and his (her) caregivers. Psychiatric complications associated with delirium, including acute stress disorder—which might predict posttraumatic stress disorder—have been explored; early recognition and treatment may improve long-term outcomes.66 After recovery from acute delirium, cognitive assessment (eg, MMSE67 or Montreal Cognitive Assessment68) is recommended to validate current cognitive status because patients may have persistent decrement in cognitive function compared with pre-delirium condition, even after recovery from the acute episode.
Post-delirium debriefing may help patients who have recovered from a delirium episode. Patients may fear that their brief period of hallucinations might represent the onset of a chronic-relapsing psychotic disorder. Allow patients to communicate their distress about the delirium episode and give them the opportunity to talk through the experience. Brief them on the possibility that delirium will recur and advise them to seek emergency medical care in case of recurrence. Advise patients to monitor and maintain a normal sleep-wake cycle.
Family members can watch for syndromal recurrence of delirium. They should be encouraged to discuss their reaction to having seen their relative in a delirious state.
Health care systems with integrated electronic medical records should list “delirium, resolved” on the patient’s illness profile or problem list and alert the patient’s primary care provider to the delirium history to avoid future exposure to delirium-provocative medications, and to prompt the provider to assume an active role in post-delirium care, including delirium recurrence surveillance, medication adjustment, risk factor management, and post-recovery cognitive assessment.
Bottom Line
Evaluation of delirium in geriatric patients includes clinical vigilance and screening, differentiating delirium from other neurocognitive disorders, and identifying and treating underlying causes. Perioperative use of antipsychotics may reduce the incidence of delirium, although hospital length of stay generally has not been reduced with prophylaxis. Management interventions include staff education, systematic screening, use of multicomponent interventions, and pharmacologic interventions.
Related Resources
• Downing LJ, Caprio TV, Lyness JM. Geriatric psychiatry review: differential diagnosis and treatment of the 3 D’s - delirium, dementia, and depression. Curr Psychiatry Rep. 2013;15(6):365.
• Brooks PB. Postoperative delirium in elderly patients. Am J Nurs. 2012;112(9):38-49.
Drug Brand Names
Carbidopa/levodopa • Sinemet Midazolam • Versed
Dexmedetomidine • Precedex Olanzapine • Zyprexa
Haloperidol • Haldol Propofol • Diprivan
Lithium • Eskalith, Lithobid Quetiapine • Seroquel
Lorazepam • Ativan Risperidone • Risperdal
Metoclopramide • Reglan Trazodone • Desyrel
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioural disorders. Diagnostic criteria for research. Geneva, Switzerland: WHO; 1993.
3. Lipowski ZJ. Delirium in the elderly patient. N Engl J Med. 1989;320(9):578-582.
4. Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5(2):75-85.
5. Rabinowitz T. Delirium: an important (but often unrecognized) clinical syndrome. Curr Psychiatry Rep. 2002;4(3):202-208.
6. Marcantonio ER, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. Am J Geriatr Soc. 2002;50(5):850-857.
7. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710.
8. Duppils GS, Wikblad K. Delirium: behavioural changes before and during the prodromal phase. J Clin Nurs. 2004;13(5):609-616.
9. de Jonghe JF, Kalisvaart KJ, Dijkstra M, et al. Early symptoms in the prodromal phase of delirium: a prospective cohort study in elderly patients undergoing hip surgery. Am J Geriatr Psychiatry. 2007;15(2):112-121.
10. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
11. Hogan D, Gage L, Bruto V, et al. National guidelines for seniors’ mental health: the assessment and treatment of delirium. Canadian Journal of Geriatrics. 2006;9(suppl 2):S42-51.
12. Leentjens AF, Diefenbacher A. A survey of delirium guidelines in Europe. J Psychosom Res. 2006;61(1):123-128.
13. Tropea J, Slee JA, Brand CA, et al. Clinical practice guidelines for the management of delirium in older people in Australia. Australas J Ageing. 2008;27(3):150-156.
14. Mittal D, Majithia D, Kennedy R, et al. Differences in characteristics and outcome of delirium as based on referral patterns. Psychosomatics. 2006;47(5):367-375.
15. Grover S, Subodh BN, Avasthi A, et al. Prevalence and clinical profile of delirium: a study from a tertiary-care hospital in north India. Gen Hosp Psychiatry. 2009;31(1): 25-29.
16. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12): 941-948.
17. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
18. Dobmejer K. Delirium in elderly medical patients. Clinical Geriatrics. 1996;4:43-68.
19. Leentjens AF, Maclullich AM, Meagher DJ. Delirium, Cinderella no more...? J Psychosom Res. 2008;65(3):205.
20. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
21. Streubel PN, Ricci WM, Gardner MJ. Fragility fractures: preoperative, perioperative, and postoperative management. Current Orthopaedic Practice. 2009;20(5):482-489.
22. Spronk PE, Riekerk B, Hofhuis J, et al. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276-1280.
23. Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000;160(6):786-794.
24. Ganzini L. Care of patients with delirium at the end of life. Annals of Long-Term Care. 2007;15(3):35-40.
25. Bourne RS, Tahir TA, Borthwick M, et al. Drug treatment of delirium: past, present and future. J Psychosom Res. 2008;65(3):273-282.
26. Yokota H, Ogawa S, Kurokawa A, et al. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci. 2003;57(3):337-339.
27. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
28. Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5(2):132-148.
29. Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. 1994;97(3):278-288.
30. Laurila JV, Laakkonen ML, Tilvis RS, et al. Predisposing and precipitating factors for delirium in a frail geriatric population. J Psychosom Res. 2008;65(3):249-254.
31. Morandi A, McCurley J, Vasilevskis EE, et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005-2013.
32. Kazmierski J, Kowman M, Banach M, et al. The use of DSM-IV and ICD-10 criteria and diagnostic scales for delirium among cardiac surgery patients: results from the IPDACS study. J Neuropsychiatry Clin Neurosci. 2010; 22(4):426-432.
33. Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Rating Scale. J Pain Symptom Manage. 1997;13(3):128-137.
34. Wong CL, Holroyd-Leduc J, Simel DL, et al. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304(7):779-786.
35. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2011;49(5):516-522.
36. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628.
37. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007; 19(3):178-186.
38. Kratz A. Use of the acute confusion protocol: a research utilization project. J Nurs Care Qual. 2008;23(4):331-337.
39. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35(5):714-719.
40. Kaneko T, Cai J, Ishikura T, et al. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta Medica. 1999;42:179-184.
41. Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010;51(5):409-418.
42. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666.
43. Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116(5):987-997.
44. Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50(3): 206-217.
45. Short J. Use of dexmedetomidine for primary sedation in a general intensive care unit. Crit Care Nurse. 2010;30(1): 29-38; quiz 39.
46. Practice guideline for the treatment of patients with delirium. American Psychiatric Association [Comment in: Treatment of patients with delirium. Am J Psychiatry. 2000.]. Am J Psychiatry. 1999;156(suppl 5):1-20.
47. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis, and treatment. Crit Care Clin. 2008;24(4):657-722, vii.
48. Platt MM, Breitbart W, Smith M, et al. Efficacy of neuroleptics for hypoactive delirium. J Neuropsychiatry Clin Neurosci. 1994;6(1):66-67.
49. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594.
50. Seitz DP, Gill SS, van Zyl LT. Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68(1):11-21.
51. Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153(2):231-237.
52. Hu H, Deng W, Yang H, et al. Olanzapine and haloperidol for senile delirium: a randomized controlled observation. Chinese Journal of Clinical Rehabilitation. 2006;10(42): 188-190.
53. Friedman JH, Fernandez HH. Atypical antipsychotics in Parkinson-sensitive populations. J Geriatr Psychiatry Neurol. 2002;15(3):156-170.
54. Seitz DP, Gill SS. Neuroleptic malignant syndrome complicating antipsychotic treatment of delirium or agitation in medical and surgical patients: case reports and a review of the literature. Psychosomatics. 2009; 50(1):8-15.
55. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301.
56. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
57. Hermann N, Lanctôt KL. Atypical antipsychotics for neuropsychiatric symptoms of dementia: malignant or maligned? Drug Saf. 2006;29(10):833-843.
58. Noyan MA, Elbi H, Aksu H. Donepezil for anticholinergic drug intoxication: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(5):885-887.
59. Gleason OC. Donepezil for postoperative delirium. Psychosomatics. 2003;44(5):437-438.
60. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1): CD005317.
61. Davis MP. Does trazodone have a role in palliating symptoms? Support Care Cancer. 2007;15(2):221-224.
62. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002; 50(10):1723-1732.
63. Brajtman S, Wright D, Hogan D, et al. Developing guidelines for the assessment and treatment of delirium in older adults at the end of life. Can Geriatr J. 2011;14(2):40-50.
64. Caraceni A, Simonetti F. Palliating delirium in patients with cancer. Lancet Oncol. 2009;10(2):164-172.
65. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
66. Granja C, Gomes E, Amaro A, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med. 2008;36(10):2801-2809.
67. Ringdal GI, Ringdal K, Juliebø V, et al. Using the Mini- Mental State Examination to screen for delirium in elderly patients with hip fracture. Dement Geriatr Cogn Disord. 2011;32(6):394-400.
68. Olson RA, Chhanabhai T, McKenzie M. Feasibility study of the Montreal Cognitive Assessment (MoCA) in patients with brain metastases. Support Care Cancer. 2008;16(11):1273-1278.
Although delirium has many descriptive terms (Table 1), a common unifying term is “acute global cognitive dysfunction,” now recognized as delirium; a consensus supported by DSM-51 and ICD-102 (Table 2). According to DSM-5, the essential feature is a disturbance of attention or awareness that is accompanied by a change in baseline cognition that cannot be explained by another preexisting, established, or evolving neurocognitive disorder (the newly named DSM-5 entity for dementia syndromes).1 Because delirium affects the cortex diffusely, psychiatric symptoms can include cognitive, mood, anxiety, or psychotic symptoms. Because many systemic illnesses can induce delirium, the differential diagnosis spans all organ systems.
Three subtypes
Delirium can be classified, based on symptoms,3,4 into 3 subtypes: hyperactive-hyperalert, hypoactive-hypoalert, and mixed delirium. Hyperactive patients present with restlessness and agitation. Hypoactive patients are lethargic, confused, slow to respond to questions, and often appear depressed. The differential prognostic significance of these subtypes has been examined in the literature, with conflicting results. Rabinowitz5 reported that hypoactive delirium has the worst prognosis, while Marcantonio et al6 indicated that the hyperactive subtype is associated with the highest mortality rate. Mixed delirium, with periods of both hyperactivity and hypoactivity, is the most common type of delirium.7
A prodromal phase, characterized by anxiety, frequent requests for nursing and medical assistance, decreased attention, restlessness, vivid dreams, disorientation immediately after awakening, and hallucinations, can occur before an episode of full-spectrum delirium; this prodromal state often is identified retrospectively —after the patient is in an episode of delirium.8,9
Evidence-based guidelines aim to improve recognition and clinical management.10-13 Disruptive behavior is the main reason for psychiatric referral in delirium.14,15 Delayed psychiatric consultation because of non-recognition of delirium is related to variables such as older age; history of a pre-existing, comorbid neurocognitive disorder; and the clinical appearance of hypoactive delirium.14
The case of Mr. D (Box),16 illustrates how the emergence of antipsychotic-associated neuroleptic malignant syndrome (NMS) can complicate antipsychotic treatment of delirium in a geriatric medical patient, although delirium also is a common presentation in NMS.17 Delirium developed after an increase in carbidopa/levodopa, which has central dopaminergic effects that can precipitate delirium, particularly in a geriatric patient with preexisting comorbid neurocognitive disorder. Further complicating Mr. D’s delirium presentation was the development of NMS, which had a multifactorial causation, such as the use of dopamine antagonists (ie, quetiapine, metoclopramide), and an abrupt decrease of a dopaminergic agent (ie, carbidopa/levodopa), all inducing a central dopamine relative hypoactivity.
Epidemiology
Delirium is more common in older patients,15 and is seen in 30% to 40% of hospitalized geriatric patients.18 Delirium in older patients, compared with other adults, is associated with more severe cognitive impairment.19 It is common among geriatric surgical patients (15% to 62%)20 with a peak 2 to 5 days postoperatively for hip fracture,21 and often is seen in ICU patients (70% to 87%).20 However, Spronk et al22 found that delirium is significantly under-recognized in the ICU. Nearly 90% of terminally ill patients become delirious before death.23 Terminal delirium often is unrecognized and can interfere with assessment of other clinical problems.24 A preexisting history of comorbid neurocognitive disorder was evident in as many as two-thirds of delirium cases.25
Pathophysiology and risk factors
The pathophysiology of delirium has been characterized as an imbalance of CNS metabolism, including decreased blood flow in various regions of the brain that may normalize once delirium resolves.26 Studies describe the simultaneous decrease of cholinergic transmission and dopaminergic excess.27,28 Predisposing and precipitating factors for delirium that are of particular importance in geriatric patients include:
• advanced age
• CNS disease
• infection
• cognitive impairment
• male sex
• poor nutrition
• dehydration and other metabolic abnormalities
• cardiovascular events
• substance use
• medication
• sensory deprivation (eg, impaired vision or hearing)
• sleep deprivation
• low level of physical activity.27,29,30
Table 3 lists the most common delirium-provocative medications.27
Evaluation and psychometric scales
The EEG can be useful in evaluating delirium, especially in clinically ambiguous cases. EEG findings may indicate generalized slowing or dropout of the posterior dominant rhythm, and generalized slow theta and delta waves, findings that are more common in delirium than in other neurocognitive disorders and other psychiatric illnesses. The EEG must be interpreted in the context of the delirium diagnostic workup, because abnormalities seen in other neurocognitive disorders can overlap with those of delirium.31
The EEG referral should specify the clinical suspicion of delirium to help interpret the results. Delirium cases in which the patient’s previous cognitive status is unknown may benefit from EEG evaluation, such as:
• in possible status epilepticus
• when delirium improvement has reached a plateau at a lower level of cognitive function than before onset of delirium
• when the patient is unable or unwilling to complete a psychiatric interview.27
Assessment instruments are available to diagnose and monitor delirium (Table 4). Typically, delirium assessment includes examining levels of arousal, psychomotor activity, cognition (ie, orientation, attention, and memory), and perceptual disturbances.
Psychometrically, a review of Table 4 suggests that validity appeared stable with adequate specificity (64% to 99%) but more variable sensitivity (36% to 100%). These reliability parameters also will be affected by the classification system (ie, DSM vs ICD) and the cut-off score employed.32 Most measures (eg, Confusion Assessment Method [CAM], CAM-ICU) provide an adequate sample of behavioral (ie, level of alertness), motor (ie, psychomotor activity), and cognitive (ie, orientation, attention, memory, and receptive language) function, with the exception of the Global Attentiveness Rating, which is a 2-minute open conversation protocol between physician and patient.
Some measures are stand-alone instruments, such as the Memorial Delirium Assessment Scale, whereas the CAM requires administration of separate cognitive screens, including the Mini-Mental State Examination (MMSE) and Digit Span.33 Instruments to detect delirium in critically ill patients are a more recent development. Wong et al34 reported that the most widely studied tool was the CAM. Obtaining collateral information from family, caregivers, and hospital staff is essential, particularly given the fluctuating nature of delirium.
Management
Prevention. Identify patients at high risk of delirium so that preventive strategies can be employed. Multi-component, nonpharmacotherapeutic interventions are used in clinical settings but few randomized trials have been conducted. The contributing effectiveness of individual components is not well-studied, but most include staff education to increase awareness of delirium. Of 3 multi-component intervention randomized trials, 2 reported a significantly lower incidence of delirium in the intervention group.35-37 Implementation of a multi-component protocol in medical/ surgical units was associated with a significant reduction in use of restraints.38
As in Mr. D’s case, complex drug regimens, particularly for CNS illness, can increase the risk of delirium. Considering the medication profile for patients with complex systemic illness—in particular, minimizing the use anticholinergics and dopamine agonists— may be crucial in preventing delirium.
Prophylactic administration of antipsychotics may reduce the risk of developing postoperative delirium.39 Studies of the use of these agents were characterized by small sample sizes and selected groups of patient populations. Of the 4 randomized studies evaluating prophylactic antipsychotics (vs placebo), 3 found a lower incidence of delirium in the intervention groups.39-41
A study of haloperidol in post-GI surgery patients showed a reduced occurrence of delirium,40 whereas its prophylactic use in patients undergoing hip surgery42 did not reduce the incidence of delirium compared with placebo, but did decrease severity when delirium occurred.42
Risperidone39 in post-cardiac surgery and olanzapine41 perioperatively in patients undergoing total knee or hip replacement have been shown to decrease delirium severity and duration. Targeted prophylaxis with risperidone43 in post-cardiac surgery patients who showed disturbed cognition but did not meet criteria for delirium reduced the number of patients requiring medication, compared with placebo.43
Dexmedetomidine, an α-2 adrenergic receptor agonist, compared with propofol or midazolam in post-cardiac valve surgery patients, resulted in a decreased incidence of delirium but no difference in delirium duration, hospital length of stay, or use of other medications.44 However, other studies have shown that dexmedetomidine reduces ICU length of stay and duration of mechanical ventilation.45
Treatment. Management of hospitalized medically ill geriatric patients with delirium is challenging and requires a comprehensive approach. The first step in delirium management is prompt identification and management of systemic medical disturbances associated with the delirium episode. First-line, nonpharmacotherapeutic strategies for patients with delirium include:
• reorientation
• behavioral interventions (eg, use of clear instructions and frequent eye contact with patients)
• environmental interventions (eg, minimal noise, adequate lighting, and limited room and staff changes)
• avoidance of physical restraints.46
Consider employing family members or hospital staff sitters to stay with the patient and to reassure, reorient, and watch for agitation and other unsafe behaviors (eg, attempted elopement). Psychoeducation for the patient and family on the phenomenology of delirium can be helpful.
The use of drug treatment strategies should be integrated into a comprehensive approach that includes the routine use of nondrug measures.46 Using medications for treating hypoactive delirium, formerly controversial, now has wider acceptance.47,48 A few high-quality randomized trials have been performed.25,49,50
Pharmacotherapy, especially in frail patients, should be initiated at the lowest starting dosage and titrated cautiously to clinical effect and for the shortest period of time necessary. Antipsychotics are preferred agents for treating all subtypes of delirium; haloperidol is widely used.46,51,52 However, antipsychotics, including haloperidol, can be associated with adverse neurologic effects such as extrapyramidal symptoms (EPS) and NMS.
Although reported less frequently than with haloperidol, other agents have been implicated in development of EPS and NMS, including atypical antipsychotics and antiemetic dopamine antagonists, particularly in parkinsonism-prone patients.53 Strategies that can minimize such risks in geriatric inpatients with delirium include oral, rather than parenteral, use of antipsychotics—preferential use of atypical over typical antipsychotics— and lowest effective dosages.54
In controlled trials, atypical antipsychotics for delirium showed efficacy compared with haloperidol.52,55 However, there is no research that demonstrates any advantage of one atypical over another.25
In Mr. D’s case, the most important intervention for managing delirium caused by NMS is to discontinue all dopamine antagonists and treat agitation with judicious doses of a benzodiazepine, with supportive care.17 In cases of sudden discontinuation or a dosage decrease of dopamine agonists, these medications should be resumed or optimized to minimize the risk of NMS-associated rhabdomyolysis and subsequent renal failure.17 Antipsychotics carry an increased risk of stroke and mortality in older patients with established or evolving neurocognitive disorders56,57 and can cause prolongation of the QTc interval.57
Other medications that could be used for delirium include cholinesterase inhibitors58,59 (although larger trials and a systematic review did not support this use60), and 5-HT receptor antagonists,61 such as trazodone. Benzodiazepines, such as lorazepam, are first-line treatment for delirium associated with seizures or withdrawal from alcohol, sedatives, hypnotics, and anxiolytics and for delirium caused by NMS. Be cautious about using benzodiazepines in geriatric patients because of a risk of respiratory depression, falls, sedation, and amnesia.
Geriatric patients with alcoholism and those with malnutrition are prone to thiamine and vitamin B12 deficiencies, which can induce delirium. Laboratory assessment and consideration of supplementation is recommended. Despite high occurrence of delirium in hospitalized older adults with preexisting comorbid neurocognitive disorders, there is no standard care for delirium comorbid with another neurocognitive disorder.62 Clinical practice guidelines for older patients receiving palliative care have been developed63; the goal is to minimize suffering and discomfort in patients in palliative care.64
Post-delirium prophylaxis. Medications for delirium usually can be tapered and discontinued once the episode has resolved and the patient is stable; it is common to discontinue medications when the patient has been symptom-free for 1 week.65 Some patients (eg, with end-stage liver disease, disseminated cancer) are prone to recurrent or to prolonged or chronic delirium. A period of post-recovery treatment with antipsychotics—even indefinite treatment in some cases—should be considered.
Post-delirium debriefing and aftercare. The psychological complications of delirium are distressing for the patient and his (her) caregivers. Psychiatric complications associated with delirium, including acute stress disorder—which might predict posttraumatic stress disorder—have been explored; early recognition and treatment may improve long-term outcomes.66 After recovery from acute delirium, cognitive assessment (eg, MMSE67 or Montreal Cognitive Assessment68) is recommended to validate current cognitive status because patients may have persistent decrement in cognitive function compared with pre-delirium condition, even after recovery from the acute episode.
Post-delirium debriefing may help patients who have recovered from a delirium episode. Patients may fear that their brief period of hallucinations might represent the onset of a chronic-relapsing psychotic disorder. Allow patients to communicate their distress about the delirium episode and give them the opportunity to talk through the experience. Brief them on the possibility that delirium will recur and advise them to seek emergency medical care in case of recurrence. Advise patients to monitor and maintain a normal sleep-wake cycle.
Family members can watch for syndromal recurrence of delirium. They should be encouraged to discuss their reaction to having seen their relative in a delirious state.
Health care systems with integrated electronic medical records should list “delirium, resolved” on the patient’s illness profile or problem list and alert the patient’s primary care provider to the delirium history to avoid future exposure to delirium-provocative medications, and to prompt the provider to assume an active role in post-delirium care, including delirium recurrence surveillance, medication adjustment, risk factor management, and post-recovery cognitive assessment.
Bottom Line
Evaluation of delirium in geriatric patients includes clinical vigilance and screening, differentiating delirium from other neurocognitive disorders, and identifying and treating underlying causes. Perioperative use of antipsychotics may reduce the incidence of delirium, although hospital length of stay generally has not been reduced with prophylaxis. Management interventions include staff education, systematic screening, use of multicomponent interventions, and pharmacologic interventions.
Related Resources
• Downing LJ, Caprio TV, Lyness JM. Geriatric psychiatry review: differential diagnosis and treatment of the 3 D’s - delirium, dementia, and depression. Curr Psychiatry Rep. 2013;15(6):365.
• Brooks PB. Postoperative delirium in elderly patients. Am J Nurs. 2012;112(9):38-49.
Drug Brand Names
Carbidopa/levodopa • Sinemet Midazolam • Versed
Dexmedetomidine • Precedex Olanzapine • Zyprexa
Haloperidol • Haldol Propofol • Diprivan
Lithium • Eskalith, Lithobid Quetiapine • Seroquel
Lorazepam • Ativan Risperidone • Risperdal
Metoclopramide • Reglan Trazodone • Desyrel
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Although delirium has many descriptive terms (Table 1), a common unifying term is “acute global cognitive dysfunction,” now recognized as delirium; a consensus supported by DSM-51 and ICD-102 (Table 2). According to DSM-5, the essential feature is a disturbance of attention or awareness that is accompanied by a change in baseline cognition that cannot be explained by another preexisting, established, or evolving neurocognitive disorder (the newly named DSM-5 entity for dementia syndromes).1 Because delirium affects the cortex diffusely, psychiatric symptoms can include cognitive, mood, anxiety, or psychotic symptoms. Because many systemic illnesses can induce delirium, the differential diagnosis spans all organ systems.
Three subtypes
Delirium can be classified, based on symptoms,3,4 into 3 subtypes: hyperactive-hyperalert, hypoactive-hypoalert, and mixed delirium. Hyperactive patients present with restlessness and agitation. Hypoactive patients are lethargic, confused, slow to respond to questions, and often appear depressed. The differential prognostic significance of these subtypes has been examined in the literature, with conflicting results. Rabinowitz5 reported that hypoactive delirium has the worst prognosis, while Marcantonio et al6 indicated that the hyperactive subtype is associated with the highest mortality rate. Mixed delirium, with periods of both hyperactivity and hypoactivity, is the most common type of delirium.7
A prodromal phase, characterized by anxiety, frequent requests for nursing and medical assistance, decreased attention, restlessness, vivid dreams, disorientation immediately after awakening, and hallucinations, can occur before an episode of full-spectrum delirium; this prodromal state often is identified retrospectively —after the patient is in an episode of delirium.8,9
Evidence-based guidelines aim to improve recognition and clinical management.10-13 Disruptive behavior is the main reason for psychiatric referral in delirium.14,15 Delayed psychiatric consultation because of non-recognition of delirium is related to variables such as older age; history of a pre-existing, comorbid neurocognitive disorder; and the clinical appearance of hypoactive delirium.14
The case of Mr. D (Box),16 illustrates how the emergence of antipsychotic-associated neuroleptic malignant syndrome (NMS) can complicate antipsychotic treatment of delirium in a geriatric medical patient, although delirium also is a common presentation in NMS.17 Delirium developed after an increase in carbidopa/levodopa, which has central dopaminergic effects that can precipitate delirium, particularly in a geriatric patient with preexisting comorbid neurocognitive disorder. Further complicating Mr. D’s delirium presentation was the development of NMS, which had a multifactorial causation, such as the use of dopamine antagonists (ie, quetiapine, metoclopramide), and an abrupt decrease of a dopaminergic agent (ie, carbidopa/levodopa), all inducing a central dopamine relative hypoactivity.
Epidemiology
Delirium is more common in older patients,15 and is seen in 30% to 40% of hospitalized geriatric patients.18 Delirium in older patients, compared with other adults, is associated with more severe cognitive impairment.19 It is common among geriatric surgical patients (15% to 62%)20 with a peak 2 to 5 days postoperatively for hip fracture,21 and often is seen in ICU patients (70% to 87%).20 However, Spronk et al22 found that delirium is significantly under-recognized in the ICU. Nearly 90% of terminally ill patients become delirious before death.23 Terminal delirium often is unrecognized and can interfere with assessment of other clinical problems.24 A preexisting history of comorbid neurocognitive disorder was evident in as many as two-thirds of delirium cases.25
Pathophysiology and risk factors
The pathophysiology of delirium has been characterized as an imbalance of CNS metabolism, including decreased blood flow in various regions of the brain that may normalize once delirium resolves.26 Studies describe the simultaneous decrease of cholinergic transmission and dopaminergic excess.27,28 Predisposing and precipitating factors for delirium that are of particular importance in geriatric patients include:
• advanced age
• CNS disease
• infection
• cognitive impairment
• male sex
• poor nutrition
• dehydration and other metabolic abnormalities
• cardiovascular events
• substance use
• medication
• sensory deprivation (eg, impaired vision or hearing)
• sleep deprivation
• low level of physical activity.27,29,30
Table 3 lists the most common delirium-provocative medications.27
Evaluation and psychometric scales
The EEG can be useful in evaluating delirium, especially in clinically ambiguous cases. EEG findings may indicate generalized slowing or dropout of the posterior dominant rhythm, and generalized slow theta and delta waves, findings that are more common in delirium than in other neurocognitive disorders and other psychiatric illnesses. The EEG must be interpreted in the context of the delirium diagnostic workup, because abnormalities seen in other neurocognitive disorders can overlap with those of delirium.31
The EEG referral should specify the clinical suspicion of delirium to help interpret the results. Delirium cases in which the patient’s previous cognitive status is unknown may benefit from EEG evaluation, such as:
• in possible status epilepticus
• when delirium improvement has reached a plateau at a lower level of cognitive function than before onset of delirium
• when the patient is unable or unwilling to complete a psychiatric interview.27
Assessment instruments are available to diagnose and monitor delirium (Table 4). Typically, delirium assessment includes examining levels of arousal, psychomotor activity, cognition (ie, orientation, attention, and memory), and perceptual disturbances.
Psychometrically, a review of Table 4 suggests that validity appeared stable with adequate specificity (64% to 99%) but more variable sensitivity (36% to 100%). These reliability parameters also will be affected by the classification system (ie, DSM vs ICD) and the cut-off score employed.32 Most measures (eg, Confusion Assessment Method [CAM], CAM-ICU) provide an adequate sample of behavioral (ie, level of alertness), motor (ie, psychomotor activity), and cognitive (ie, orientation, attention, memory, and receptive language) function, with the exception of the Global Attentiveness Rating, which is a 2-minute open conversation protocol between physician and patient.
Some measures are stand-alone instruments, such as the Memorial Delirium Assessment Scale, whereas the CAM requires administration of separate cognitive screens, including the Mini-Mental State Examination (MMSE) and Digit Span.33 Instruments to detect delirium in critically ill patients are a more recent development. Wong et al34 reported that the most widely studied tool was the CAM. Obtaining collateral information from family, caregivers, and hospital staff is essential, particularly given the fluctuating nature of delirium.
Management
Prevention. Identify patients at high risk of delirium so that preventive strategies can be employed. Multi-component, nonpharmacotherapeutic interventions are used in clinical settings but few randomized trials have been conducted. The contributing effectiveness of individual components is not well-studied, but most include staff education to increase awareness of delirium. Of 3 multi-component intervention randomized trials, 2 reported a significantly lower incidence of delirium in the intervention group.35-37 Implementation of a multi-component protocol in medical/ surgical units was associated with a significant reduction in use of restraints.38
As in Mr. D’s case, complex drug regimens, particularly for CNS illness, can increase the risk of delirium. Considering the medication profile for patients with complex systemic illness—in particular, minimizing the use anticholinergics and dopamine agonists— may be crucial in preventing delirium.
Prophylactic administration of antipsychotics may reduce the risk of developing postoperative delirium.39 Studies of the use of these agents were characterized by small sample sizes and selected groups of patient populations. Of the 4 randomized studies evaluating prophylactic antipsychotics (vs placebo), 3 found a lower incidence of delirium in the intervention groups.39-41
A study of haloperidol in post-GI surgery patients showed a reduced occurrence of delirium,40 whereas its prophylactic use in patients undergoing hip surgery42 did not reduce the incidence of delirium compared with placebo, but did decrease severity when delirium occurred.42
Risperidone39 in post-cardiac surgery and olanzapine41 perioperatively in patients undergoing total knee or hip replacement have been shown to decrease delirium severity and duration. Targeted prophylaxis with risperidone43 in post-cardiac surgery patients who showed disturbed cognition but did not meet criteria for delirium reduced the number of patients requiring medication, compared with placebo.43
Dexmedetomidine, an α-2 adrenergic receptor agonist, compared with propofol or midazolam in post-cardiac valve surgery patients, resulted in a decreased incidence of delirium but no difference in delirium duration, hospital length of stay, or use of other medications.44 However, other studies have shown that dexmedetomidine reduces ICU length of stay and duration of mechanical ventilation.45
Treatment. Management of hospitalized medically ill geriatric patients with delirium is challenging and requires a comprehensive approach. The first step in delirium management is prompt identification and management of systemic medical disturbances associated with the delirium episode. First-line, nonpharmacotherapeutic strategies for patients with delirium include:
• reorientation
• behavioral interventions (eg, use of clear instructions and frequent eye contact with patients)
• environmental interventions (eg, minimal noise, adequate lighting, and limited room and staff changes)
• avoidance of physical restraints.46
Consider employing family members or hospital staff sitters to stay with the patient and to reassure, reorient, and watch for agitation and other unsafe behaviors (eg, attempted elopement). Psychoeducation for the patient and family on the phenomenology of delirium can be helpful.
The use of drug treatment strategies should be integrated into a comprehensive approach that includes the routine use of nondrug measures.46 Using medications for treating hypoactive delirium, formerly controversial, now has wider acceptance.47,48 A few high-quality randomized trials have been performed.25,49,50
Pharmacotherapy, especially in frail patients, should be initiated at the lowest starting dosage and titrated cautiously to clinical effect and for the shortest period of time necessary. Antipsychotics are preferred agents for treating all subtypes of delirium; haloperidol is widely used.46,51,52 However, antipsychotics, including haloperidol, can be associated with adverse neurologic effects such as extrapyramidal symptoms (EPS) and NMS.
Although reported less frequently than with haloperidol, other agents have been implicated in development of EPS and NMS, including atypical antipsychotics and antiemetic dopamine antagonists, particularly in parkinsonism-prone patients.53 Strategies that can minimize such risks in geriatric inpatients with delirium include oral, rather than parenteral, use of antipsychotics—preferential use of atypical over typical antipsychotics— and lowest effective dosages.54
In controlled trials, atypical antipsychotics for delirium showed efficacy compared with haloperidol.52,55 However, there is no research that demonstrates any advantage of one atypical over another.25
In Mr. D’s case, the most important intervention for managing delirium caused by NMS is to discontinue all dopamine antagonists and treat agitation with judicious doses of a benzodiazepine, with supportive care.17 In cases of sudden discontinuation or a dosage decrease of dopamine agonists, these medications should be resumed or optimized to minimize the risk of NMS-associated rhabdomyolysis and subsequent renal failure.17 Antipsychotics carry an increased risk of stroke and mortality in older patients with established or evolving neurocognitive disorders56,57 and can cause prolongation of the QTc interval.57
Other medications that could be used for delirium include cholinesterase inhibitors58,59 (although larger trials and a systematic review did not support this use60), and 5-HT receptor antagonists,61 such as trazodone. Benzodiazepines, such as lorazepam, are first-line treatment for delirium associated with seizures or withdrawal from alcohol, sedatives, hypnotics, and anxiolytics and for delirium caused by NMS. Be cautious about using benzodiazepines in geriatric patients because of a risk of respiratory depression, falls, sedation, and amnesia.
Geriatric patients with alcoholism and those with malnutrition are prone to thiamine and vitamin B12 deficiencies, which can induce delirium. Laboratory assessment and consideration of supplementation is recommended. Despite high occurrence of delirium in hospitalized older adults with preexisting comorbid neurocognitive disorders, there is no standard care for delirium comorbid with another neurocognitive disorder.62 Clinical practice guidelines for older patients receiving palliative care have been developed63; the goal is to minimize suffering and discomfort in patients in palliative care.64
Post-delirium prophylaxis. Medications for delirium usually can be tapered and discontinued once the episode has resolved and the patient is stable; it is common to discontinue medications when the patient has been symptom-free for 1 week.65 Some patients (eg, with end-stage liver disease, disseminated cancer) are prone to recurrent or to prolonged or chronic delirium. A period of post-recovery treatment with antipsychotics—even indefinite treatment in some cases—should be considered.
Post-delirium debriefing and aftercare. The psychological complications of delirium are distressing for the patient and his (her) caregivers. Psychiatric complications associated with delirium, including acute stress disorder—which might predict posttraumatic stress disorder—have been explored; early recognition and treatment may improve long-term outcomes.66 After recovery from acute delirium, cognitive assessment (eg, MMSE67 or Montreal Cognitive Assessment68) is recommended to validate current cognitive status because patients may have persistent decrement in cognitive function compared with pre-delirium condition, even after recovery from the acute episode.
Post-delirium debriefing may help patients who have recovered from a delirium episode. Patients may fear that their brief period of hallucinations might represent the onset of a chronic-relapsing psychotic disorder. Allow patients to communicate their distress about the delirium episode and give them the opportunity to talk through the experience. Brief them on the possibility that delirium will recur and advise them to seek emergency medical care in case of recurrence. Advise patients to monitor and maintain a normal sleep-wake cycle.
Family members can watch for syndromal recurrence of delirium. They should be encouraged to discuss their reaction to having seen their relative in a delirious state.
Health care systems with integrated electronic medical records should list “delirium, resolved” on the patient’s illness profile or problem list and alert the patient’s primary care provider to the delirium history to avoid future exposure to delirium-provocative medications, and to prompt the provider to assume an active role in post-delirium care, including delirium recurrence surveillance, medication adjustment, risk factor management, and post-recovery cognitive assessment.
Bottom Line
Evaluation of delirium in geriatric patients includes clinical vigilance and screening, differentiating delirium from other neurocognitive disorders, and identifying and treating underlying causes. Perioperative use of antipsychotics may reduce the incidence of delirium, although hospital length of stay generally has not been reduced with prophylaxis. Management interventions include staff education, systematic screening, use of multicomponent interventions, and pharmacologic interventions.
Related Resources
• Downing LJ, Caprio TV, Lyness JM. Geriatric psychiatry review: differential diagnosis and treatment of the 3 D’s - delirium, dementia, and depression. Curr Psychiatry Rep. 2013;15(6):365.
• Brooks PB. Postoperative delirium in elderly patients. Am J Nurs. 2012;112(9):38-49.
Drug Brand Names
Carbidopa/levodopa • Sinemet Midazolam • Versed
Dexmedetomidine • Precedex Olanzapine • Zyprexa
Haloperidol • Haldol Propofol • Diprivan
Lithium • Eskalith, Lithobid Quetiapine • Seroquel
Lorazepam • Ativan Risperidone • Risperdal
Metoclopramide • Reglan Trazodone • Desyrel
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioural disorders. Diagnostic criteria for research. Geneva, Switzerland: WHO; 1993.
3. Lipowski ZJ. Delirium in the elderly patient. N Engl J Med. 1989;320(9):578-582.
4. Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5(2):75-85.
5. Rabinowitz T. Delirium: an important (but often unrecognized) clinical syndrome. Curr Psychiatry Rep. 2002;4(3):202-208.
6. Marcantonio ER, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. Am J Geriatr Soc. 2002;50(5):850-857.
7. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710.
8. Duppils GS, Wikblad K. Delirium: behavioural changes before and during the prodromal phase. J Clin Nurs. 2004;13(5):609-616.
9. de Jonghe JF, Kalisvaart KJ, Dijkstra M, et al. Early symptoms in the prodromal phase of delirium: a prospective cohort study in elderly patients undergoing hip surgery. Am J Geriatr Psychiatry. 2007;15(2):112-121.
10. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
11. Hogan D, Gage L, Bruto V, et al. National guidelines for seniors’ mental health: the assessment and treatment of delirium. Canadian Journal of Geriatrics. 2006;9(suppl 2):S42-51.
12. Leentjens AF, Diefenbacher A. A survey of delirium guidelines in Europe. J Psychosom Res. 2006;61(1):123-128.
13. Tropea J, Slee JA, Brand CA, et al. Clinical practice guidelines for the management of delirium in older people in Australia. Australas J Ageing. 2008;27(3):150-156.
14. Mittal D, Majithia D, Kennedy R, et al. Differences in characteristics and outcome of delirium as based on referral patterns. Psychosomatics. 2006;47(5):367-375.
15. Grover S, Subodh BN, Avasthi A, et al. Prevalence and clinical profile of delirium: a study from a tertiary-care hospital in north India. Gen Hosp Psychiatry. 2009;31(1): 25-29.
16. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12): 941-948.
17. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
18. Dobmejer K. Delirium in elderly medical patients. Clinical Geriatrics. 1996;4:43-68.
19. Leentjens AF, Maclullich AM, Meagher DJ. Delirium, Cinderella no more...? J Psychosom Res. 2008;65(3):205.
20. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
21. Streubel PN, Ricci WM, Gardner MJ. Fragility fractures: preoperative, perioperative, and postoperative management. Current Orthopaedic Practice. 2009;20(5):482-489.
22. Spronk PE, Riekerk B, Hofhuis J, et al. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276-1280.
23. Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000;160(6):786-794.
24. Ganzini L. Care of patients with delirium at the end of life. Annals of Long-Term Care. 2007;15(3):35-40.
25. Bourne RS, Tahir TA, Borthwick M, et al. Drug treatment of delirium: past, present and future. J Psychosom Res. 2008;65(3):273-282.
26. Yokota H, Ogawa S, Kurokawa A, et al. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci. 2003;57(3):337-339.
27. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
28. Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5(2):132-148.
29. Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. 1994;97(3):278-288.
30. Laurila JV, Laakkonen ML, Tilvis RS, et al. Predisposing and precipitating factors for delirium in a frail geriatric population. J Psychosom Res. 2008;65(3):249-254.
31. Morandi A, McCurley J, Vasilevskis EE, et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005-2013.
32. Kazmierski J, Kowman M, Banach M, et al. The use of DSM-IV and ICD-10 criteria and diagnostic scales for delirium among cardiac surgery patients: results from the IPDACS study. J Neuropsychiatry Clin Neurosci. 2010; 22(4):426-432.
33. Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Rating Scale. J Pain Symptom Manage. 1997;13(3):128-137.
34. Wong CL, Holroyd-Leduc J, Simel DL, et al. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304(7):779-786.
35. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2011;49(5):516-522.
36. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628.
37. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007; 19(3):178-186.
38. Kratz A. Use of the acute confusion protocol: a research utilization project. J Nurs Care Qual. 2008;23(4):331-337.
39. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35(5):714-719.
40. Kaneko T, Cai J, Ishikura T, et al. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta Medica. 1999;42:179-184.
41. Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010;51(5):409-418.
42. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666.
43. Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116(5):987-997.
44. Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50(3): 206-217.
45. Short J. Use of dexmedetomidine for primary sedation in a general intensive care unit. Crit Care Nurse. 2010;30(1): 29-38; quiz 39.
46. Practice guideline for the treatment of patients with delirium. American Psychiatric Association [Comment in: Treatment of patients with delirium. Am J Psychiatry. 2000.]. Am J Psychiatry. 1999;156(suppl 5):1-20.
47. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis, and treatment. Crit Care Clin. 2008;24(4):657-722, vii.
48. Platt MM, Breitbart W, Smith M, et al. Efficacy of neuroleptics for hypoactive delirium. J Neuropsychiatry Clin Neurosci. 1994;6(1):66-67.
49. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594.
50. Seitz DP, Gill SS, van Zyl LT. Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68(1):11-21.
51. Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153(2):231-237.
52. Hu H, Deng W, Yang H, et al. Olanzapine and haloperidol for senile delirium: a randomized controlled observation. Chinese Journal of Clinical Rehabilitation. 2006;10(42): 188-190.
53. Friedman JH, Fernandez HH. Atypical antipsychotics in Parkinson-sensitive populations. J Geriatr Psychiatry Neurol. 2002;15(3):156-170.
54. Seitz DP, Gill SS. Neuroleptic malignant syndrome complicating antipsychotic treatment of delirium or agitation in medical and surgical patients: case reports and a review of the literature. Psychosomatics. 2009; 50(1):8-15.
55. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301.
56. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
57. Hermann N, Lanctôt KL. Atypical antipsychotics for neuropsychiatric symptoms of dementia: malignant or maligned? Drug Saf. 2006;29(10):833-843.
58. Noyan MA, Elbi H, Aksu H. Donepezil for anticholinergic drug intoxication: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(5):885-887.
59. Gleason OC. Donepezil for postoperative delirium. Psychosomatics. 2003;44(5):437-438.
60. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1): CD005317.
61. Davis MP. Does trazodone have a role in palliating symptoms? Support Care Cancer. 2007;15(2):221-224.
62. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002; 50(10):1723-1732.
63. Brajtman S, Wright D, Hogan D, et al. Developing guidelines for the assessment and treatment of delirium in older adults at the end of life. Can Geriatr J. 2011;14(2):40-50.
64. Caraceni A, Simonetti F. Palliating delirium in patients with cancer. Lancet Oncol. 2009;10(2):164-172.
65. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
66. Granja C, Gomes E, Amaro A, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med. 2008;36(10):2801-2809.
67. Ringdal GI, Ringdal K, Juliebø V, et al. Using the Mini- Mental State Examination to screen for delirium in elderly patients with hip fracture. Dement Geriatr Cogn Disord. 2011;32(6):394-400.
68. Olson RA, Chhanabhai T, McKenzie M. Feasibility study of the Montreal Cognitive Assessment (MoCA) in patients with brain metastases. Support Care Cancer. 2008;16(11):1273-1278.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioural disorders. Diagnostic criteria for research. Geneva, Switzerland: WHO; 1993.
3. Lipowski ZJ. Delirium in the elderly patient. N Engl J Med. 1989;320(9):578-582.
4. Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5(2):75-85.
5. Rabinowitz T. Delirium: an important (but often unrecognized) clinical syndrome. Curr Psychiatry Rep. 2002;4(3):202-208.
6. Marcantonio ER, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. Am J Geriatr Soc. 2002;50(5):850-857.
7. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710.
8. Duppils GS, Wikblad K. Delirium: behavioural changes before and during the prodromal phase. J Clin Nurs. 2004;13(5):609-616.
9. de Jonghe JF, Kalisvaart KJ, Dijkstra M, et al. Early symptoms in the prodromal phase of delirium: a prospective cohort study in elderly patients undergoing hip surgery. Am J Geriatr Psychiatry. 2007;15(2):112-121.
10. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
11. Hogan D, Gage L, Bruto V, et al. National guidelines for seniors’ mental health: the assessment and treatment of delirium. Canadian Journal of Geriatrics. 2006;9(suppl 2):S42-51.
12. Leentjens AF, Diefenbacher A. A survey of delirium guidelines in Europe. J Psychosom Res. 2006;61(1):123-128.
13. Tropea J, Slee JA, Brand CA, et al. Clinical practice guidelines for the management of delirium in older people in Australia. Australas J Ageing. 2008;27(3):150-156.
14. Mittal D, Majithia D, Kennedy R, et al. Differences in characteristics and outcome of delirium as based on referral patterns. Psychosomatics. 2006;47(5):367-375.
15. Grover S, Subodh BN, Avasthi A, et al. Prevalence and clinical profile of delirium: a study from a tertiary-care hospital in north India. Gen Hosp Psychiatry. 2009;31(1): 25-29.
16. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12): 941-948.
17. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
18. Dobmejer K. Delirium in elderly medical patients. Clinical Geriatrics. 1996;4:43-68.
19. Leentjens AF, Maclullich AM, Meagher DJ. Delirium, Cinderella no more...? J Psychosom Res. 2008;65(3):205.
20. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
21. Streubel PN, Ricci WM, Gardner MJ. Fragility fractures: preoperative, perioperative, and postoperative management. Current Orthopaedic Practice. 2009;20(5):482-489.
22. Spronk PE, Riekerk B, Hofhuis J, et al. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276-1280.
23. Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000;160(6):786-794.
24. Ganzini L. Care of patients with delirium at the end of life. Annals of Long-Term Care. 2007;15(3):35-40.
25. Bourne RS, Tahir TA, Borthwick M, et al. Drug treatment of delirium: past, present and future. J Psychosom Res. 2008;65(3):273-282.
26. Yokota H, Ogawa S, Kurokawa A, et al. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci. 2003;57(3):337-339.
27. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
28. Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5(2):132-148.
29. Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. 1994;97(3):278-288.
30. Laurila JV, Laakkonen ML, Tilvis RS, et al. Predisposing and precipitating factors for delirium in a frail geriatric population. J Psychosom Res. 2008;65(3):249-254.
31. Morandi A, McCurley J, Vasilevskis EE, et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005-2013.
32. Kazmierski J, Kowman M, Banach M, et al. The use of DSM-IV and ICD-10 criteria and diagnostic scales for delirium among cardiac surgery patients: results from the IPDACS study. J Neuropsychiatry Clin Neurosci. 2010; 22(4):426-432.
33. Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Rating Scale. J Pain Symptom Manage. 1997;13(3):128-137.
34. Wong CL, Holroyd-Leduc J, Simel DL, et al. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304(7):779-786.
35. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2011;49(5):516-522.
36. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628.
37. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007; 19(3):178-186.
38. Kratz A. Use of the acute confusion protocol: a research utilization project. J Nurs Care Qual. 2008;23(4):331-337.
39. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35(5):714-719.
40. Kaneko T, Cai J, Ishikura T, et al. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta Medica. 1999;42:179-184.
41. Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010;51(5):409-418.
42. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666.
43. Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116(5):987-997.
44. Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50(3): 206-217.
45. Short J. Use of dexmedetomidine for primary sedation in a general intensive care unit. Crit Care Nurse. 2010;30(1): 29-38; quiz 39.
46. Practice guideline for the treatment of patients with delirium. American Psychiatric Association [Comment in: Treatment of patients with delirium. Am J Psychiatry. 2000.]. Am J Psychiatry. 1999;156(suppl 5):1-20.
47. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis, and treatment. Crit Care Clin. 2008;24(4):657-722, vii.
48. Platt MM, Breitbart W, Smith M, et al. Efficacy of neuroleptics for hypoactive delirium. J Neuropsychiatry Clin Neurosci. 1994;6(1):66-67.
49. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594.
50. Seitz DP, Gill SS, van Zyl LT. Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68(1):11-21.
51. Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153(2):231-237.
52. Hu H, Deng W, Yang H, et al. Olanzapine and haloperidol for senile delirium: a randomized controlled observation. Chinese Journal of Clinical Rehabilitation. 2006;10(42): 188-190.
53. Friedman JH, Fernandez HH. Atypical antipsychotics in Parkinson-sensitive populations. J Geriatr Psychiatry Neurol. 2002;15(3):156-170.
54. Seitz DP, Gill SS. Neuroleptic malignant syndrome complicating antipsychotic treatment of delirium or agitation in medical and surgical patients: case reports and a review of the literature. Psychosomatics. 2009; 50(1):8-15.
55. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301.
56. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
57. Hermann N, Lanctôt KL. Atypical antipsychotics for neuropsychiatric symptoms of dementia: malignant or maligned? Drug Saf. 2006;29(10):833-843.
58. Noyan MA, Elbi H, Aksu H. Donepezil for anticholinergic drug intoxication: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(5):885-887.
59. Gleason OC. Donepezil for postoperative delirium. Psychosomatics. 2003;44(5):437-438.
60. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1): CD005317.
61. Davis MP. Does trazodone have a role in palliating symptoms? Support Care Cancer. 2007;15(2):221-224.
62. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002; 50(10):1723-1732.
63. Brajtman S, Wright D, Hogan D, et al. Developing guidelines for the assessment and treatment of delirium in older adults at the end of life. Can Geriatr J. 2011;14(2):40-50.
64. Caraceni A, Simonetti F. Palliating delirium in patients with cancer. Lancet Oncol. 2009;10(2):164-172.
65. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
66. Granja C, Gomes E, Amaro A, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med. 2008;36(10):2801-2809.
67. Ringdal GI, Ringdal K, Juliebø V, et al. Using the Mini- Mental State Examination to screen for delirium in elderly patients with hip fracture. Dement Geriatr Cogn Disord. 2011;32(6):394-400.
68. Olson RA, Chhanabhai T, McKenzie M. Feasibility study of the Montreal Cognitive Assessment (MoCA) in patients with brain metastases. Support Care Cancer. 2008;16(11):1273-1278.
A cognitive-behavioral strategy for preventing suicide
Many mental health practitioners have had training in cognitive-behavioral therapy (CBT)—short-term, evidence-based psychotherapy for treating a variety of psychiatric conditions (eg, posttraumatic stress disorder) and medical comorbidities (eg, insomnia)—but only some are knowledgeable about how to best use CBT with a suicidal patient. This article provides a clinician-friendly summary of a 10-session evidence-based outpatient1-3 and an adapted 6 to 8 session inpatient4,5 cognitive-behavioral protocol (known as Post-Admission Cognitive Therapy [PACT]) that is designed to help patients who have suicide-related thoughts and/or behaviors.
3 phases of CBT for suicide prevention
An average of 9 hours of individual CBT for the prevention of suicide has been reported to reduce the likelihood of repeat suicide attempts in approximately 50% of patients.1 Here, we introduce you to 3 phases of CBT for preventing suicide—phases that are the same for outpatients or inpatients. Our aim is to help you become familiar with CBT strategies that can be adapted for your treatment setting and used to intervene with vulnerable patients who are at risk for suicidal self-directed violence. A thorough assessment of the patient’s psychiatric diagnosis and history, presenting problems, and risk and protective factors for suicide must be completed before treatment begins.
Phase I. The patient is asked to tell a story associated with his (her) most recent episode of suicidal thoughts or behavior, or both. This narrative serves as 1) a foundation for planning treatment and 2) a model for understanding how best to deactivate the wish to die through the process of psychotherapy.
Phase II. The patient is assisted with modifying underdeveloped or overdeveloped skills that are most closely associated with the risk of triggering a suicidal crisis. For example, a patient with underdeveloped skills in regulating anger and hatred toward himself is taught to modulate these problematic emotions more effectively. In addition, effective problem-solving strategies are reviewed and practiced.
Phase III. The patient is guided through a relapse prevention task. The purpose of this exercise is to 1) highlight skills learned during therapy and 2) allow the patient to practice effective problem-solving strategies that are aimed at minimizing the recurrence of suicidal self-directed violence.
Theorectical basis for preventing suicide with CBT
Aaron Beck, in 1979,6 proposed that a person’s biopsychosocial vulnerabilities can interact with suicidal thoughts and behaviors to produce a state that Beck labeled the “suicide mode.” Once produced, a suicide mode can become activated by cognitive, affective, motivational, and behavioral systems.
The frequency and severity of suicide mode activation can increase over time, especially for persons who do not have protective factors and those who have a history of self-directed violence—in particular, attempted suicide. Moreover, some persons might experience a chronic state of suicide mode activation and, therefore, remain at elevated risk of suicide. Once a suicide-specific mode is activated, the person considers suicide the only option for solving his life problems. Suicide might be considered a rational decision at this point.6
The hypothesized mechanism of action associated with CBT for preventing suicide can be described as:
• deactivation of the suicide mode
• modification of the structure and content of the suicide mode
• construction and practice of more adaptive structural modes to promote a desire to live.
The underlying philosophy of this intervention is that the suicide mode occurs independently of psychiatric diagnoses and must be targeted directly; treatment therefore is transdiagnostic.7 In other words, instead of addressing a symptom of a psychiatric disorder, treatment directly targets suicide-related ideation and behaviors (Table 1).
Using that framework, psychiatric diagnoses are conceptualized in terms of how the associated symptoms contribute to the activation, maintenance, and exacerbation of the suicide mode.
Protocol for preventing suicide
The outpatient protocol1-3 comprises 10, 45- to 50-minute weekly individual psychotherapy sessions, with an allowance for booster sessions (as needed), until the patient is able to complete the relapse prevention task in Phase III. The inpatient protocol4,5 comprises 6, 90-minute individual psychotherapy sessions, with an allowance for 2 booster sessions (as needed) during the inpatient stay and as many as 4 telephone booster sessions after discharge.
Phase I: Tell the suicide story
Engage the patient in treatment. To increase adherence to treatment and minimize the risk of drop-out, practitioners are encouraged to establish a strong, early therapeutic alliance with the patient. Showing genuine empathy and providing a safe, supportive, and nonjudgmental environment are instrumental for engaging patients in treatment. The practitioner listens carefully to the patient’s narrative, provides periodic summaries to check on accurate understanding, and keeps interruptions to a minimum.
Collaboratively generate a safety plan. A crisis response plan or safety plan—an individualized, hierarchically arranged, written list of coping strategies to be implemented during a suicide crisis—is developed as soon as possible. Guidance on how to develop a structured safety plan has been provided by Stanley and Brown.8,9
Practitioners must ensure that the safety plan contains contact information that the patient can use to reach the practitioner, the clinic, the on-call provider (if available), the local 24-hour emergency department, and the 24/7 National Suicide Prevention Lifeline (800-273-TALK [8255]). Discussion of how to limit access to lethal means also is important.
Because safety planning is a collaborative process, it is imperative that practitioners check on the patient’s willingness to follow the safety plan and help him overcome perceived obstacles in implementation. Copies of the plan can be kept at different locations and shared with family members, friends, or both with the patient’s permission.
Develop a cognitive-behavioral conceptualization. The cognitive-behavioral conceptualization is an individualized map of a patient’s automatic thoughts (eg, “I am going to get fired today”), conditional assumptions (“If I get fired, then my life is over”), and core beliefs (“I am an utter failure”) that are activated before, during, and after suicidal self-directed violence. To develop that conceptualization, the patient is asked to tell a story about his (her) most recent suicidal crisis (the Box, offers a sample script) and to describe reactions to having survived a suicide attempt. (Note: Patients who report regret after an attempt are at greatest risk for dying by suicide.10)
This activity gives the patient an opportunity to disclose details surrounding his suicidal thoughts and actions, and might allow for a cathartic experience through storytelling. As practitioners listen to the suicide narrative, they collect data on the patient’s early childhood experiences (typically, suicide-activating events), associated automatic thoughts and images, emotional responses, and subsequent behaviors.
Based on this information, a cognitive-behavioral case conceptualization diagram (Figure 1, and Table 2) is generated collaboratively with the patienta and used to personalize treatment planning.
a Judith Beck offers sample case conceptualization diagrams in Cognitive behavior therapy: Basics and beyond, 2nd ed. New York, New York: Guilford Press; 2011.
Phase II: Build skills
Build skills to prevent episodes of suicidal self-directed violence. Information obtained from the conceptualization is used to generate an individualized cognitive-behavioral plan of intervention. The overall goal is to determine skill-based problem areas that are associated with the most recent episode of suicidal self-directed violence.
Practitioner and patient collaboratively identify skills that are underdeveloped and ones that are overdeveloped so that they can be addressed systematically. In general, based on our clinical experience with suicidal patients, we recommend focusing on skills captured within ≥1 of the deficit domains in Table 3. Explanation of the various cognitive-behavioral strategies used in this phase of treatment is beyond the scope of this article, but 2 activities that highlight the clinical work conducted in this phase are described in the following sections. We selected those activities because they are easy to implement and, we have found, receive overall patient acceptability.
For a detailed understanding of strategies used in Phase II of CBT for preventing suicide, see Related Resources. In addition, the book Choosing to live: How to defeat suicide through cognitive therapy11 can serve as a self-help guide for patients to follow through with CBT skill-building strategies.
Sample activity #1: Construct a ‘hope box.’ One activity that you can use to help a patient cope with suicide-activating core beliefs (eg, “My life is worthless”) involves construction of a so-called hope box. The box helps the patient directly challenge his extreme distress, by being reminded of previous successes, positive experiences, and reasons for living. The process of constructing a hope box allows the patient to work on modifying his problematic core beliefs (eg, worthlessness, helplessness, incapable of being loved).
It can be helpful to have the patient construct his hope box during a session, to ensure that everything that is put in the box is truly helpful and personalized. Items included vary from patient to patient, and might consist of pictures of loved ones, a favorite poem, a prayer, coping cards (see next section), or all of these. For example, one of our patients chose to include a picture of herself in her early 20s as a reminder of a positive, fulfilling time in her life; this gave her hope that it is possible to experience those feelings again.
Bush et alb at the National Center for Telehealth and Technology have developed a Virtual Hope Box, a free mobile application for tablets and smartphones (compatible with Android and iOS operating systems) that patients can use under the guidance of their practitioner.
bwww.t2.health.mil/apps/virtual-hope-box
Sample activity #2: Generate coping cards. Effective problem-solving skills can be promoted by having the patient construct coping cards —wallet-size cards generated collaboratively in session. Coping cards are note cards that a patient keeps nearby to cope better during a difficult situation. They provide an easily accessible way to jump-start adaptive thinking during a suicidal crisis. The patient is encouraged to use coping cards to practice adaptive thinking even when not in a crisis.
There are 3 kinds of coping cards:
• place a suicide-relevant automatic thought or core belief on one side of the card; on the other side, place an alternative, more adaptive response
• write a list of coping strategies
• write instructions to motivate or “activate” the patient toward completing a specific goal (Figure 2).
Phase III: Prevent relapse
Complete relapse prevention task. Relapse prevention is a common CBT strategy that aims to strengthen self-management to minimize likelihood of returning to a previously stopped behavior. For patients who present only with suicidal thoughts, relapse prevention is directed at identifying triggers and minimizing the occurrence and/ or intensity of such thoughts in the future. For patients who present with suicidal self-directed violence, relapse prevention is directed at identifying triggers for suicidal actions and reducing the likelihood of acting on suicidal urges. The brief guidance provided below will familiarize you with each of the relapse prevention steps, which may be completed in multiple sessions.
Step 1: Provide psychoeducation
Explain the difference between a lapse and a relapse. In general, you want the patient to understand that, although suicidal thoughts might persist and recur over time, suicidal self-directed violence must be prevented. Describe the purpose of the relapse prevention task (ie, to minimize the chance that suicidal thinking and actions will recur); address questions and concerns; and obtain permission to begin the procedure. Assure the patient that this is a collaborative activity and you will be in the room to ensure comfort and safety.
STEP 2: Retell the suicide story
Ask the patient to imagine the chain of events, thoughts, and feelings that led to the most recent episode of suicide ideation or suicidal self-directed violence. Tell the patient that you want him to construct a movie script to describe the chain of events that resulted in the suicide crisis —but to do so slowly, taking enough time to describe the details of each scene of the movie.
Step 3: Apply CBT skills
Ask the patient again to take you through the sequence of events leading to the most recent episode of suicide ideation or suicidal self-directed violence. This time, however, direct him to use the skills learned in therapy to appropriately respond cognitively, affectively, and behaviorally to move further away from the suicide outcome.
If the patient is moving too fast or neglecting important points, stop and ask about alternative ways of thinking, feeling, and behaving. Use as much time as needed until the patient is able to demonstrate solid learning of at least several learned CBT strategies to prevent suicidal self-directed violence.
Step 4: Generalize learning to prepare for future suicidal crises
In this stage —given your knowledge of the patient’s psychosocial history, cognitive- behavioral conceptualization, and suicide mode triggers —you collaboratively create a future scenario that is likely to activate suicidal self-directed violence. Question the patient about possible coping strategies, provide helpful feedback, guide him through each link in the chain of events, and propose additional alternative strategies if he is clearly neglecting important points of the intervention.
Step 5: Debrief and summarize lessons learned
Debrief the patient by providing a summary of the skills he has learned in therapy, congratulate him for completing this final therapeutic task, and assess overall emotional reaction to this activity. Remind him that mood fluctuations and future setbacks, in the form of lapses, are expected. Give him the option to request booster sessions and make plans for next steps in accomplishing general goals of therapy.
Treatment can be terminated when the patient is able to complete the relapse prevention task. If he is not ready or able to complete this exercise successfully, you can extend treatment. The duration of the extension is left to the practitioner’s judgment, based on the overall treatment plan. Brown and colleagues2 have reported a maximum number of 24 outpatient sessions (for patients who need additional booster sessions); based on clinical experience, it is reasonable to assume that it would be highly unlikely for a patient not to meet treatment objectives after a methodical course of outpatient CBT.
In cases in which goals of treatment have not been met, consultation with colleagues, review of adherence problems, and consideration of obstacles for treatment efficacy would be recommended.
A checklist can be used to determine whether a patient is ready to end treatment. Variables that can be considered in assessing readiness for termination include:
• reduced scores on self-report measures for a number of weeks
• evidence of enhanced problem-solving
• engagement in adjunctive health care services
• development of a social support system.
Post-Admission Cognitive Therapy (PACT)
An inpatient cognitive-behavioral protocol for the prevention of suicide, adapted from the efficacious outpatient model, is being evaluated at the Walter Reed National Military Medical Center, Bethesda, Maryland, and Fort Belvoir Community Hospital, Fort Belvoir, Virginia. The inpatient intervention is called PACT; components are summarized in Table 4.
Bottom Line
Cognitive-behavioral therapy for preventing suicide is an efficacious protocol for reducing the recurrence of suicidal self-directed violence. Post-Admission Cognitive Therapy is the adapted inpatient treatment package. You are encouraged to gain additional training and supervision on the delivery of these interventions to your high-risk suicidal patients.
Related Resources
• Academy of Cognitive Therapy. www.academyofct.org.
• National Suicide Prevention Lifeline. www.suicide preventionlifeline.org.
• Wenzel A, Brown GK, Beck AT. Cognitive therapy for suicidal patients: scientific and clinical applications. Washington, DC: American Psychological Association; 2009.
Disclosures
Support for research on inpatient cognitive-behavioral therapy for the prevention of suicide provided to Principal Investigator, Dr. Ghahramanlou-Holloway by the Department of Defense, Congressionally Directed Medical Research Program (W81XWH-08-2-0172), Military Operational Medicine Research Program (W81XWH-11-2-0106), and the National Alliance for Research on Schizophrenia and Depression (15219).
1. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005;294(5):563-570.
2. Brown GK, Henriques GR, Ratto C, et al. Cognitive therapy treatment manual for suicide attempters. Philadelphia, PA: University of Pennsylvania; 2002 (unpublished).
3. Berk MS, Henriques GR, Warman DM, et al. A cognitive therapy intervention for suicide attempters: an overview of the treatment and case examples. Cogn Behav Pract. 2004;11(3):265-277.
4. Ghahramanlou-Holloway M, Cox D, Greene F. Post-admission cognitive therapy: a brief intervention for psychiatric inpatients admitted after a suicide attempt. Cogn Behav Pract. 2012;19(2):233-244.
5. Neely L, Irwin K, Carreno Ponce JT, et al. Post Admission Cognitive Therapy (PACT) for the prevention of suicide in military personnel with histories of trauma: treatment development and case example. Clinical Case Studies. 2013;12(6):457-473.
6. Beck AT. Cognitive therapy and the emotional disorders. New York, NY: Penguin Group; 1979.
7. Ghahramanlou-Holloway M, Brown GK, Beck AT. Suicide. In: Whisman M, ed. Adapting cognitive therapy for depression: managing complexity and comorbidity. New York, NY: Guilford Press; 2008:159-184.
8. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012;19(2):256-264.
9. Stanley B, Brown GK. Safety plan treatment manual to reduce suicide risk: veteran version. http://www. mentalhealth.va.gov/docs/va_safety_planning_manual. pdf. Published August 20, 2008. Accessed July 2, 2014.
10. Henriques G, Wenzel A, Brown GK, et al. Suicide attempters’ reaction to survival as a risk factor for eventual suicide. Am J Psychiatry. 2005;162(11):2180-2182.
11. Ellis TE, Newman CF. Choosing to live: How to defeat suicide through cognitive therapy. Oakland, CA: New Harbinger Publications, Inc; 1996.
Many mental health practitioners have had training in cognitive-behavioral therapy (CBT)—short-term, evidence-based psychotherapy for treating a variety of psychiatric conditions (eg, posttraumatic stress disorder) and medical comorbidities (eg, insomnia)—but only some are knowledgeable about how to best use CBT with a suicidal patient. This article provides a clinician-friendly summary of a 10-session evidence-based outpatient1-3 and an adapted 6 to 8 session inpatient4,5 cognitive-behavioral protocol (known as Post-Admission Cognitive Therapy [PACT]) that is designed to help patients who have suicide-related thoughts and/or behaviors.
3 phases of CBT for suicide prevention
An average of 9 hours of individual CBT for the prevention of suicide has been reported to reduce the likelihood of repeat suicide attempts in approximately 50% of patients.1 Here, we introduce you to 3 phases of CBT for preventing suicide—phases that are the same for outpatients or inpatients. Our aim is to help you become familiar with CBT strategies that can be adapted for your treatment setting and used to intervene with vulnerable patients who are at risk for suicidal self-directed violence. A thorough assessment of the patient’s psychiatric diagnosis and history, presenting problems, and risk and protective factors for suicide must be completed before treatment begins.
Phase I. The patient is asked to tell a story associated with his (her) most recent episode of suicidal thoughts or behavior, or both. This narrative serves as 1) a foundation for planning treatment and 2) a model for understanding how best to deactivate the wish to die through the process of psychotherapy.
Phase II. The patient is assisted with modifying underdeveloped or overdeveloped skills that are most closely associated with the risk of triggering a suicidal crisis. For example, a patient with underdeveloped skills in regulating anger and hatred toward himself is taught to modulate these problematic emotions more effectively. In addition, effective problem-solving strategies are reviewed and practiced.
Phase III. The patient is guided through a relapse prevention task. The purpose of this exercise is to 1) highlight skills learned during therapy and 2) allow the patient to practice effective problem-solving strategies that are aimed at minimizing the recurrence of suicidal self-directed violence.
Theorectical basis for preventing suicide with CBT
Aaron Beck, in 1979,6 proposed that a person’s biopsychosocial vulnerabilities can interact with suicidal thoughts and behaviors to produce a state that Beck labeled the “suicide mode.” Once produced, a suicide mode can become activated by cognitive, affective, motivational, and behavioral systems.
The frequency and severity of suicide mode activation can increase over time, especially for persons who do not have protective factors and those who have a history of self-directed violence—in particular, attempted suicide. Moreover, some persons might experience a chronic state of suicide mode activation and, therefore, remain at elevated risk of suicide. Once a suicide-specific mode is activated, the person considers suicide the only option for solving his life problems. Suicide might be considered a rational decision at this point.6
The hypothesized mechanism of action associated with CBT for preventing suicide can be described as:
• deactivation of the suicide mode
• modification of the structure and content of the suicide mode
• construction and practice of more adaptive structural modes to promote a desire to live.
The underlying philosophy of this intervention is that the suicide mode occurs independently of psychiatric diagnoses and must be targeted directly; treatment therefore is transdiagnostic.7 In other words, instead of addressing a symptom of a psychiatric disorder, treatment directly targets suicide-related ideation and behaviors (Table 1).
Using that framework, psychiatric diagnoses are conceptualized in terms of how the associated symptoms contribute to the activation, maintenance, and exacerbation of the suicide mode.
Protocol for preventing suicide
The outpatient protocol1-3 comprises 10, 45- to 50-minute weekly individual psychotherapy sessions, with an allowance for booster sessions (as needed), until the patient is able to complete the relapse prevention task in Phase III. The inpatient protocol4,5 comprises 6, 90-minute individual psychotherapy sessions, with an allowance for 2 booster sessions (as needed) during the inpatient stay and as many as 4 telephone booster sessions after discharge.
Phase I: Tell the suicide story
Engage the patient in treatment. To increase adherence to treatment and minimize the risk of drop-out, practitioners are encouraged to establish a strong, early therapeutic alliance with the patient. Showing genuine empathy and providing a safe, supportive, and nonjudgmental environment are instrumental for engaging patients in treatment. The practitioner listens carefully to the patient’s narrative, provides periodic summaries to check on accurate understanding, and keeps interruptions to a minimum.
Collaboratively generate a safety plan. A crisis response plan or safety plan—an individualized, hierarchically arranged, written list of coping strategies to be implemented during a suicide crisis—is developed as soon as possible. Guidance on how to develop a structured safety plan has been provided by Stanley and Brown.8,9
Practitioners must ensure that the safety plan contains contact information that the patient can use to reach the practitioner, the clinic, the on-call provider (if available), the local 24-hour emergency department, and the 24/7 National Suicide Prevention Lifeline (800-273-TALK [8255]). Discussion of how to limit access to lethal means also is important.
Because safety planning is a collaborative process, it is imperative that practitioners check on the patient’s willingness to follow the safety plan and help him overcome perceived obstacles in implementation. Copies of the plan can be kept at different locations and shared with family members, friends, or both with the patient’s permission.
Develop a cognitive-behavioral conceptualization. The cognitive-behavioral conceptualization is an individualized map of a patient’s automatic thoughts (eg, “I am going to get fired today”), conditional assumptions (“If I get fired, then my life is over”), and core beliefs (“I am an utter failure”) that are activated before, during, and after suicidal self-directed violence. To develop that conceptualization, the patient is asked to tell a story about his (her) most recent suicidal crisis (the Box, offers a sample script) and to describe reactions to having survived a suicide attempt. (Note: Patients who report regret after an attempt are at greatest risk for dying by suicide.10)
This activity gives the patient an opportunity to disclose details surrounding his suicidal thoughts and actions, and might allow for a cathartic experience through storytelling. As practitioners listen to the suicide narrative, they collect data on the patient’s early childhood experiences (typically, suicide-activating events), associated automatic thoughts and images, emotional responses, and subsequent behaviors.
Based on this information, a cognitive-behavioral case conceptualization diagram (Figure 1, and Table 2) is generated collaboratively with the patienta and used to personalize treatment planning.
a Judith Beck offers sample case conceptualization diagrams in Cognitive behavior therapy: Basics and beyond, 2nd ed. New York, New York: Guilford Press; 2011.
Phase II: Build skills
Build skills to prevent episodes of suicidal self-directed violence. Information obtained from the conceptualization is used to generate an individualized cognitive-behavioral plan of intervention. The overall goal is to determine skill-based problem areas that are associated with the most recent episode of suicidal self-directed violence.
Practitioner and patient collaboratively identify skills that are underdeveloped and ones that are overdeveloped so that they can be addressed systematically. In general, based on our clinical experience with suicidal patients, we recommend focusing on skills captured within ≥1 of the deficit domains in Table 3. Explanation of the various cognitive-behavioral strategies used in this phase of treatment is beyond the scope of this article, but 2 activities that highlight the clinical work conducted in this phase are described in the following sections. We selected those activities because they are easy to implement and, we have found, receive overall patient acceptability.
For a detailed understanding of strategies used in Phase II of CBT for preventing suicide, see Related Resources. In addition, the book Choosing to live: How to defeat suicide through cognitive therapy11 can serve as a self-help guide for patients to follow through with CBT skill-building strategies.
Sample activity #1: Construct a ‘hope box.’ One activity that you can use to help a patient cope with suicide-activating core beliefs (eg, “My life is worthless”) involves construction of a so-called hope box. The box helps the patient directly challenge his extreme distress, by being reminded of previous successes, positive experiences, and reasons for living. The process of constructing a hope box allows the patient to work on modifying his problematic core beliefs (eg, worthlessness, helplessness, incapable of being loved).
It can be helpful to have the patient construct his hope box during a session, to ensure that everything that is put in the box is truly helpful and personalized. Items included vary from patient to patient, and might consist of pictures of loved ones, a favorite poem, a prayer, coping cards (see next section), or all of these. For example, one of our patients chose to include a picture of herself in her early 20s as a reminder of a positive, fulfilling time in her life; this gave her hope that it is possible to experience those feelings again.
Bush et alb at the National Center for Telehealth and Technology have developed a Virtual Hope Box, a free mobile application for tablets and smartphones (compatible with Android and iOS operating systems) that patients can use under the guidance of their practitioner.
bwww.t2.health.mil/apps/virtual-hope-box
Sample activity #2: Generate coping cards. Effective problem-solving skills can be promoted by having the patient construct coping cards —wallet-size cards generated collaboratively in session. Coping cards are note cards that a patient keeps nearby to cope better during a difficult situation. They provide an easily accessible way to jump-start adaptive thinking during a suicidal crisis. The patient is encouraged to use coping cards to practice adaptive thinking even when not in a crisis.
There are 3 kinds of coping cards:
• place a suicide-relevant automatic thought or core belief on one side of the card; on the other side, place an alternative, more adaptive response
• write a list of coping strategies
• write instructions to motivate or “activate” the patient toward completing a specific goal (Figure 2).
Phase III: Prevent relapse
Complete relapse prevention task. Relapse prevention is a common CBT strategy that aims to strengthen self-management to minimize likelihood of returning to a previously stopped behavior. For patients who present only with suicidal thoughts, relapse prevention is directed at identifying triggers and minimizing the occurrence and/ or intensity of such thoughts in the future. For patients who present with suicidal self-directed violence, relapse prevention is directed at identifying triggers for suicidal actions and reducing the likelihood of acting on suicidal urges. The brief guidance provided below will familiarize you with each of the relapse prevention steps, which may be completed in multiple sessions.
Step 1: Provide psychoeducation
Explain the difference between a lapse and a relapse. In general, you want the patient to understand that, although suicidal thoughts might persist and recur over time, suicidal self-directed violence must be prevented. Describe the purpose of the relapse prevention task (ie, to minimize the chance that suicidal thinking and actions will recur); address questions and concerns; and obtain permission to begin the procedure. Assure the patient that this is a collaborative activity and you will be in the room to ensure comfort and safety.
STEP 2: Retell the suicide story
Ask the patient to imagine the chain of events, thoughts, and feelings that led to the most recent episode of suicide ideation or suicidal self-directed violence. Tell the patient that you want him to construct a movie script to describe the chain of events that resulted in the suicide crisis —but to do so slowly, taking enough time to describe the details of each scene of the movie.
Step 3: Apply CBT skills
Ask the patient again to take you through the sequence of events leading to the most recent episode of suicide ideation or suicidal self-directed violence. This time, however, direct him to use the skills learned in therapy to appropriately respond cognitively, affectively, and behaviorally to move further away from the suicide outcome.
If the patient is moving too fast or neglecting important points, stop and ask about alternative ways of thinking, feeling, and behaving. Use as much time as needed until the patient is able to demonstrate solid learning of at least several learned CBT strategies to prevent suicidal self-directed violence.
Step 4: Generalize learning to prepare for future suicidal crises
In this stage —given your knowledge of the patient’s psychosocial history, cognitive- behavioral conceptualization, and suicide mode triggers —you collaboratively create a future scenario that is likely to activate suicidal self-directed violence. Question the patient about possible coping strategies, provide helpful feedback, guide him through each link in the chain of events, and propose additional alternative strategies if he is clearly neglecting important points of the intervention.
Step 5: Debrief and summarize lessons learned
Debrief the patient by providing a summary of the skills he has learned in therapy, congratulate him for completing this final therapeutic task, and assess overall emotional reaction to this activity. Remind him that mood fluctuations and future setbacks, in the form of lapses, are expected. Give him the option to request booster sessions and make plans for next steps in accomplishing general goals of therapy.
Treatment can be terminated when the patient is able to complete the relapse prevention task. If he is not ready or able to complete this exercise successfully, you can extend treatment. The duration of the extension is left to the practitioner’s judgment, based on the overall treatment plan. Brown and colleagues2 have reported a maximum number of 24 outpatient sessions (for patients who need additional booster sessions); based on clinical experience, it is reasonable to assume that it would be highly unlikely for a patient not to meet treatment objectives after a methodical course of outpatient CBT.
In cases in which goals of treatment have not been met, consultation with colleagues, review of adherence problems, and consideration of obstacles for treatment efficacy would be recommended.
A checklist can be used to determine whether a patient is ready to end treatment. Variables that can be considered in assessing readiness for termination include:
• reduced scores on self-report measures for a number of weeks
• evidence of enhanced problem-solving
• engagement in adjunctive health care services
• development of a social support system.
Post-Admission Cognitive Therapy (PACT)
An inpatient cognitive-behavioral protocol for the prevention of suicide, adapted from the efficacious outpatient model, is being evaluated at the Walter Reed National Military Medical Center, Bethesda, Maryland, and Fort Belvoir Community Hospital, Fort Belvoir, Virginia. The inpatient intervention is called PACT; components are summarized in Table 4.
Bottom Line
Cognitive-behavioral therapy for preventing suicide is an efficacious protocol for reducing the recurrence of suicidal self-directed violence. Post-Admission Cognitive Therapy is the adapted inpatient treatment package. You are encouraged to gain additional training and supervision on the delivery of these interventions to your high-risk suicidal patients.
Related Resources
• Academy of Cognitive Therapy. www.academyofct.org.
• National Suicide Prevention Lifeline. www.suicide preventionlifeline.org.
• Wenzel A, Brown GK, Beck AT. Cognitive therapy for suicidal patients: scientific and clinical applications. Washington, DC: American Psychological Association; 2009.
Disclosures
Support for research on inpatient cognitive-behavioral therapy for the prevention of suicide provided to Principal Investigator, Dr. Ghahramanlou-Holloway by the Department of Defense, Congressionally Directed Medical Research Program (W81XWH-08-2-0172), Military Operational Medicine Research Program (W81XWH-11-2-0106), and the National Alliance for Research on Schizophrenia and Depression (15219).
Many mental health practitioners have had training in cognitive-behavioral therapy (CBT)—short-term, evidence-based psychotherapy for treating a variety of psychiatric conditions (eg, posttraumatic stress disorder) and medical comorbidities (eg, insomnia)—but only some are knowledgeable about how to best use CBT with a suicidal patient. This article provides a clinician-friendly summary of a 10-session evidence-based outpatient1-3 and an adapted 6 to 8 session inpatient4,5 cognitive-behavioral protocol (known as Post-Admission Cognitive Therapy [PACT]) that is designed to help patients who have suicide-related thoughts and/or behaviors.
3 phases of CBT for suicide prevention
An average of 9 hours of individual CBT for the prevention of suicide has been reported to reduce the likelihood of repeat suicide attempts in approximately 50% of patients.1 Here, we introduce you to 3 phases of CBT for preventing suicide—phases that are the same for outpatients or inpatients. Our aim is to help you become familiar with CBT strategies that can be adapted for your treatment setting and used to intervene with vulnerable patients who are at risk for suicidal self-directed violence. A thorough assessment of the patient’s psychiatric diagnosis and history, presenting problems, and risk and protective factors for suicide must be completed before treatment begins.
Phase I. The patient is asked to tell a story associated with his (her) most recent episode of suicidal thoughts or behavior, or both. This narrative serves as 1) a foundation for planning treatment and 2) a model for understanding how best to deactivate the wish to die through the process of psychotherapy.
Phase II. The patient is assisted with modifying underdeveloped or overdeveloped skills that are most closely associated with the risk of triggering a suicidal crisis. For example, a patient with underdeveloped skills in regulating anger and hatred toward himself is taught to modulate these problematic emotions more effectively. In addition, effective problem-solving strategies are reviewed and practiced.
Phase III. The patient is guided through a relapse prevention task. The purpose of this exercise is to 1) highlight skills learned during therapy and 2) allow the patient to practice effective problem-solving strategies that are aimed at minimizing the recurrence of suicidal self-directed violence.
Theorectical basis for preventing suicide with CBT
Aaron Beck, in 1979,6 proposed that a person’s biopsychosocial vulnerabilities can interact with suicidal thoughts and behaviors to produce a state that Beck labeled the “suicide mode.” Once produced, a suicide mode can become activated by cognitive, affective, motivational, and behavioral systems.
The frequency and severity of suicide mode activation can increase over time, especially for persons who do not have protective factors and those who have a history of self-directed violence—in particular, attempted suicide. Moreover, some persons might experience a chronic state of suicide mode activation and, therefore, remain at elevated risk of suicide. Once a suicide-specific mode is activated, the person considers suicide the only option for solving his life problems. Suicide might be considered a rational decision at this point.6
The hypothesized mechanism of action associated with CBT for preventing suicide can be described as:
• deactivation of the suicide mode
• modification of the structure and content of the suicide mode
• construction and practice of more adaptive structural modes to promote a desire to live.
The underlying philosophy of this intervention is that the suicide mode occurs independently of psychiatric diagnoses and must be targeted directly; treatment therefore is transdiagnostic.7 In other words, instead of addressing a symptom of a psychiatric disorder, treatment directly targets suicide-related ideation and behaviors (Table 1).
Using that framework, psychiatric diagnoses are conceptualized in terms of how the associated symptoms contribute to the activation, maintenance, and exacerbation of the suicide mode.
Protocol for preventing suicide
The outpatient protocol1-3 comprises 10, 45- to 50-minute weekly individual psychotherapy sessions, with an allowance for booster sessions (as needed), until the patient is able to complete the relapse prevention task in Phase III. The inpatient protocol4,5 comprises 6, 90-minute individual psychotherapy sessions, with an allowance for 2 booster sessions (as needed) during the inpatient stay and as many as 4 telephone booster sessions after discharge.
Phase I: Tell the suicide story
Engage the patient in treatment. To increase adherence to treatment and minimize the risk of drop-out, practitioners are encouraged to establish a strong, early therapeutic alliance with the patient. Showing genuine empathy and providing a safe, supportive, and nonjudgmental environment are instrumental for engaging patients in treatment. The practitioner listens carefully to the patient’s narrative, provides periodic summaries to check on accurate understanding, and keeps interruptions to a minimum.
Collaboratively generate a safety plan. A crisis response plan or safety plan—an individualized, hierarchically arranged, written list of coping strategies to be implemented during a suicide crisis—is developed as soon as possible. Guidance on how to develop a structured safety plan has been provided by Stanley and Brown.8,9
Practitioners must ensure that the safety plan contains contact information that the patient can use to reach the practitioner, the clinic, the on-call provider (if available), the local 24-hour emergency department, and the 24/7 National Suicide Prevention Lifeline (800-273-TALK [8255]). Discussion of how to limit access to lethal means also is important.
Because safety planning is a collaborative process, it is imperative that practitioners check on the patient’s willingness to follow the safety plan and help him overcome perceived obstacles in implementation. Copies of the plan can be kept at different locations and shared with family members, friends, or both with the patient’s permission.
Develop a cognitive-behavioral conceptualization. The cognitive-behavioral conceptualization is an individualized map of a patient’s automatic thoughts (eg, “I am going to get fired today”), conditional assumptions (“If I get fired, then my life is over”), and core beliefs (“I am an utter failure”) that are activated before, during, and after suicidal self-directed violence. To develop that conceptualization, the patient is asked to tell a story about his (her) most recent suicidal crisis (the Box, offers a sample script) and to describe reactions to having survived a suicide attempt. (Note: Patients who report regret after an attempt are at greatest risk for dying by suicide.10)
This activity gives the patient an opportunity to disclose details surrounding his suicidal thoughts and actions, and might allow for a cathartic experience through storytelling. As practitioners listen to the suicide narrative, they collect data on the patient’s early childhood experiences (typically, suicide-activating events), associated automatic thoughts and images, emotional responses, and subsequent behaviors.
Based on this information, a cognitive-behavioral case conceptualization diagram (Figure 1, and Table 2) is generated collaboratively with the patienta and used to personalize treatment planning.
a Judith Beck offers sample case conceptualization diagrams in Cognitive behavior therapy: Basics and beyond, 2nd ed. New York, New York: Guilford Press; 2011.
Phase II: Build skills
Build skills to prevent episodes of suicidal self-directed violence. Information obtained from the conceptualization is used to generate an individualized cognitive-behavioral plan of intervention. The overall goal is to determine skill-based problem areas that are associated with the most recent episode of suicidal self-directed violence.
Practitioner and patient collaboratively identify skills that are underdeveloped and ones that are overdeveloped so that they can be addressed systematically. In general, based on our clinical experience with suicidal patients, we recommend focusing on skills captured within ≥1 of the deficit domains in Table 3. Explanation of the various cognitive-behavioral strategies used in this phase of treatment is beyond the scope of this article, but 2 activities that highlight the clinical work conducted in this phase are described in the following sections. We selected those activities because they are easy to implement and, we have found, receive overall patient acceptability.
For a detailed understanding of strategies used in Phase II of CBT for preventing suicide, see Related Resources. In addition, the book Choosing to live: How to defeat suicide through cognitive therapy11 can serve as a self-help guide for patients to follow through with CBT skill-building strategies.
Sample activity #1: Construct a ‘hope box.’ One activity that you can use to help a patient cope with suicide-activating core beliefs (eg, “My life is worthless”) involves construction of a so-called hope box. The box helps the patient directly challenge his extreme distress, by being reminded of previous successes, positive experiences, and reasons for living. The process of constructing a hope box allows the patient to work on modifying his problematic core beliefs (eg, worthlessness, helplessness, incapable of being loved).
It can be helpful to have the patient construct his hope box during a session, to ensure that everything that is put in the box is truly helpful and personalized. Items included vary from patient to patient, and might consist of pictures of loved ones, a favorite poem, a prayer, coping cards (see next section), or all of these. For example, one of our patients chose to include a picture of herself in her early 20s as a reminder of a positive, fulfilling time in her life; this gave her hope that it is possible to experience those feelings again.
Bush et alb at the National Center for Telehealth and Technology have developed a Virtual Hope Box, a free mobile application for tablets and smartphones (compatible with Android and iOS operating systems) that patients can use under the guidance of their practitioner.
bwww.t2.health.mil/apps/virtual-hope-box
Sample activity #2: Generate coping cards. Effective problem-solving skills can be promoted by having the patient construct coping cards —wallet-size cards generated collaboratively in session. Coping cards are note cards that a patient keeps nearby to cope better during a difficult situation. They provide an easily accessible way to jump-start adaptive thinking during a suicidal crisis. The patient is encouraged to use coping cards to practice adaptive thinking even when not in a crisis.
There are 3 kinds of coping cards:
• place a suicide-relevant automatic thought or core belief on one side of the card; on the other side, place an alternative, more adaptive response
• write a list of coping strategies
• write instructions to motivate or “activate” the patient toward completing a specific goal (Figure 2).
Phase III: Prevent relapse
Complete relapse prevention task. Relapse prevention is a common CBT strategy that aims to strengthen self-management to minimize likelihood of returning to a previously stopped behavior. For patients who present only with suicidal thoughts, relapse prevention is directed at identifying triggers and minimizing the occurrence and/ or intensity of such thoughts in the future. For patients who present with suicidal self-directed violence, relapse prevention is directed at identifying triggers for suicidal actions and reducing the likelihood of acting on suicidal urges. The brief guidance provided below will familiarize you with each of the relapse prevention steps, which may be completed in multiple sessions.
Step 1: Provide psychoeducation
Explain the difference between a lapse and a relapse. In general, you want the patient to understand that, although suicidal thoughts might persist and recur over time, suicidal self-directed violence must be prevented. Describe the purpose of the relapse prevention task (ie, to minimize the chance that suicidal thinking and actions will recur); address questions and concerns; and obtain permission to begin the procedure. Assure the patient that this is a collaborative activity and you will be in the room to ensure comfort and safety.
STEP 2: Retell the suicide story
Ask the patient to imagine the chain of events, thoughts, and feelings that led to the most recent episode of suicide ideation or suicidal self-directed violence. Tell the patient that you want him to construct a movie script to describe the chain of events that resulted in the suicide crisis —but to do so slowly, taking enough time to describe the details of each scene of the movie.
Step 3: Apply CBT skills
Ask the patient again to take you through the sequence of events leading to the most recent episode of suicide ideation or suicidal self-directed violence. This time, however, direct him to use the skills learned in therapy to appropriately respond cognitively, affectively, and behaviorally to move further away from the suicide outcome.
If the patient is moving too fast or neglecting important points, stop and ask about alternative ways of thinking, feeling, and behaving. Use as much time as needed until the patient is able to demonstrate solid learning of at least several learned CBT strategies to prevent suicidal self-directed violence.
Step 4: Generalize learning to prepare for future suicidal crises
In this stage —given your knowledge of the patient’s psychosocial history, cognitive- behavioral conceptualization, and suicide mode triggers —you collaboratively create a future scenario that is likely to activate suicidal self-directed violence. Question the patient about possible coping strategies, provide helpful feedback, guide him through each link in the chain of events, and propose additional alternative strategies if he is clearly neglecting important points of the intervention.
Step 5: Debrief and summarize lessons learned
Debrief the patient by providing a summary of the skills he has learned in therapy, congratulate him for completing this final therapeutic task, and assess overall emotional reaction to this activity. Remind him that mood fluctuations and future setbacks, in the form of lapses, are expected. Give him the option to request booster sessions and make plans for next steps in accomplishing general goals of therapy.
Treatment can be terminated when the patient is able to complete the relapse prevention task. If he is not ready or able to complete this exercise successfully, you can extend treatment. The duration of the extension is left to the practitioner’s judgment, based on the overall treatment plan. Brown and colleagues2 have reported a maximum number of 24 outpatient sessions (for patients who need additional booster sessions); based on clinical experience, it is reasonable to assume that it would be highly unlikely for a patient not to meet treatment objectives after a methodical course of outpatient CBT.
In cases in which goals of treatment have not been met, consultation with colleagues, review of adherence problems, and consideration of obstacles for treatment efficacy would be recommended.
A checklist can be used to determine whether a patient is ready to end treatment. Variables that can be considered in assessing readiness for termination include:
• reduced scores on self-report measures for a number of weeks
• evidence of enhanced problem-solving
• engagement in adjunctive health care services
• development of a social support system.
Post-Admission Cognitive Therapy (PACT)
An inpatient cognitive-behavioral protocol for the prevention of suicide, adapted from the efficacious outpatient model, is being evaluated at the Walter Reed National Military Medical Center, Bethesda, Maryland, and Fort Belvoir Community Hospital, Fort Belvoir, Virginia. The inpatient intervention is called PACT; components are summarized in Table 4.
Bottom Line
Cognitive-behavioral therapy for preventing suicide is an efficacious protocol for reducing the recurrence of suicidal self-directed violence. Post-Admission Cognitive Therapy is the adapted inpatient treatment package. You are encouraged to gain additional training and supervision on the delivery of these interventions to your high-risk suicidal patients.
Related Resources
• Academy of Cognitive Therapy. www.academyofct.org.
• National Suicide Prevention Lifeline. www.suicide preventionlifeline.org.
• Wenzel A, Brown GK, Beck AT. Cognitive therapy for suicidal patients: scientific and clinical applications. Washington, DC: American Psychological Association; 2009.
Disclosures
Support for research on inpatient cognitive-behavioral therapy for the prevention of suicide provided to Principal Investigator, Dr. Ghahramanlou-Holloway by the Department of Defense, Congressionally Directed Medical Research Program (W81XWH-08-2-0172), Military Operational Medicine Research Program (W81XWH-11-2-0106), and the National Alliance for Research on Schizophrenia and Depression (15219).
1. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005;294(5):563-570.
2. Brown GK, Henriques GR, Ratto C, et al. Cognitive therapy treatment manual for suicide attempters. Philadelphia, PA: University of Pennsylvania; 2002 (unpublished).
3. Berk MS, Henriques GR, Warman DM, et al. A cognitive therapy intervention for suicide attempters: an overview of the treatment and case examples. Cogn Behav Pract. 2004;11(3):265-277.
4. Ghahramanlou-Holloway M, Cox D, Greene F. Post-admission cognitive therapy: a brief intervention for psychiatric inpatients admitted after a suicide attempt. Cogn Behav Pract. 2012;19(2):233-244.
5. Neely L, Irwin K, Carreno Ponce JT, et al. Post Admission Cognitive Therapy (PACT) for the prevention of suicide in military personnel with histories of trauma: treatment development and case example. Clinical Case Studies. 2013;12(6):457-473.
6. Beck AT. Cognitive therapy and the emotional disorders. New York, NY: Penguin Group; 1979.
7. Ghahramanlou-Holloway M, Brown GK, Beck AT. Suicide. In: Whisman M, ed. Adapting cognitive therapy for depression: managing complexity and comorbidity. New York, NY: Guilford Press; 2008:159-184.
8. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012;19(2):256-264.
9. Stanley B, Brown GK. Safety plan treatment manual to reduce suicide risk: veteran version. http://www. mentalhealth.va.gov/docs/va_safety_planning_manual. pdf. Published August 20, 2008. Accessed July 2, 2014.
10. Henriques G, Wenzel A, Brown GK, et al. Suicide attempters’ reaction to survival as a risk factor for eventual suicide. Am J Psychiatry. 2005;162(11):2180-2182.
11. Ellis TE, Newman CF. Choosing to live: How to defeat suicide through cognitive therapy. Oakland, CA: New Harbinger Publications, Inc; 1996.
1. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005;294(5):563-570.
2. Brown GK, Henriques GR, Ratto C, et al. Cognitive therapy treatment manual for suicide attempters. Philadelphia, PA: University of Pennsylvania; 2002 (unpublished).
3. Berk MS, Henriques GR, Warman DM, et al. A cognitive therapy intervention for suicide attempters: an overview of the treatment and case examples. Cogn Behav Pract. 2004;11(3):265-277.
4. Ghahramanlou-Holloway M, Cox D, Greene F. Post-admission cognitive therapy: a brief intervention for psychiatric inpatients admitted after a suicide attempt. Cogn Behav Pract. 2012;19(2):233-244.
5. Neely L, Irwin K, Carreno Ponce JT, et al. Post Admission Cognitive Therapy (PACT) for the prevention of suicide in military personnel with histories of trauma: treatment development and case example. Clinical Case Studies. 2013;12(6):457-473.
6. Beck AT. Cognitive therapy and the emotional disorders. New York, NY: Penguin Group; 1979.
7. Ghahramanlou-Holloway M, Brown GK, Beck AT. Suicide. In: Whisman M, ed. Adapting cognitive therapy for depression: managing complexity and comorbidity. New York, NY: Guilford Press; 2008:159-184.
8. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012;19(2):256-264.
9. Stanley B, Brown GK. Safety plan treatment manual to reduce suicide risk: veteran version. http://www. mentalhealth.va.gov/docs/va_safety_planning_manual. pdf. Published August 20, 2008. Accessed July 2, 2014.
10. Henriques G, Wenzel A, Brown GK, et al. Suicide attempters’ reaction to survival as a risk factor for eventual suicide. Am J Psychiatry. 2005;162(11):2180-2182.
11. Ellis TE, Newman CF. Choosing to live: How to defeat suicide through cognitive therapy. Oakland, CA: New Harbinger Publications, Inc; 1996.
Opioid use remits, depression remains
Case Forgetful and depressed
Mr. B, age 55, has been a patient at our clinic for 8 years, where he has been under our care for treatment-resistant depression and opioid addiction [read about earlier events in his case in “A life of drugs and ‘downtime’” Current Psychiatry, August 2007, p. 98-103].1 He reports feeling intermittently depressed since his teens and has had 3 near-fatal suicide attempts.
Three years ago, Mr. B reported severe depressive symptoms and short-term memory loss, which undermined his job performance and contributed to interpersonal conflict with his wife. The episode has been continuously severe for 10 months. He was taking sertraline, 150 mg/d, and duloxetine, 60 mg/d, for major depressive disorder (MDD) and sublingual buprenorphine/naloxone, 20 mg/d, for opioid dependence, which was in sustained full remission.2 Mr. B scored 24/30 in the Mini- Mental State Examination, indicating mild cognitive deficit. Negative results of a complete routine laboratory workup rule out an organic cause for his deteriorating cognition.
How would you diagnose Mr. B’s condition at this point?
a) treatment-resistant MDD
b) cognitive disorder not otherwise specified
c) opioid use disorder
d) a and c
The authors' observations
Relapse is a core feature of substance use disorders (SUDs) that contributes significantly to the longstanding functional impairment in patients with a mood disorder. With the relapse rate following substance use treatment estimated at more than 60%,3 SUDs often are described as chronic relapsing conditions. In chronic stress, corticotropin-releasing factor (CRF) is over-sensitized; we believe that acute stress can cause an unhealthy response to an over-expressed CRF system.
To prevent relapse in patients with an over-expressed CRF system, it is crucial to manage stress. One treatment option to consider in preventing relapse is mindfulness-based interventions (MBI). Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and non-judgmentally.” In the event of a relapse, awareness and acceptance fostered by mindfulness may aid in recognizing and minimizing unhealthy responses, such as negative thinking that can increase the risk of relapse.
History Remission, then relapse
Mr. B was admitted to inpatient psychiatric unit after a near-fatal suicide attempt 8 years ago and given a diagnosis of MDD recurrent, severe without psychotic features. Trials of sertraline, bupropion, trazodone, quetiapine, and aripiprazole were ineffective.
Before he presented to our clinic 8 years ago, Mr. B had been taking venlafaxine, 75 mg/d, and mirtazapine, 30 mg at bedtime. His previous outpatient psychiatrist added methylphenidate, 40 mg/d, to augment the antidepressants, but this did not alleviate Mr. B’s depression.
At age 40, he entered a methadone program, began working steadily, and got married. Five years later, he stopped methadone (it is unclear from the chart if his psychiatrist initiated this change). Mr. B’s depression persisted while using opioids and became worse after stopping methadone.
We considered electroconvulsive therapy (ECT) at the time, but switching the antidepressant or starting ECT would address only the persistent depression; buprenorphine/naloxone would target opioid craving. We started a trial of buprenorphine/ naloxone, a partial μ opioid agonist and ĸ opioid antagonist; ĸ receptor antagonism serves as an antidepressant. He responded well to augmentation of his current regimen (mirtazapine, 30 mg at bedtime, and venlafaxine, 225 mg/d) with buprenorphine/naloxone, 16 mg/d.4,5 he reported no anergia and said he felt more motivated and productive.
Mr. B took buprenorphine/naloxone, 32 mg/d, for 4 years until, because of concern for daytime sedation, his outpatient psychiatrist reduced the dose to 20 mg/d. With the lower dosage of buprenorphine/naloxone initiated 4 years ago, Mr. B reported irritability, anhedonia, insomnia, increased self-criticism, and decreased self-care.
How would you treat Mr. B’s depression at this point?
a) switch to a daytime antidepressant
b) adjust the dosage of buprenorphine/ naloxone
c) try ECT
d) try mindfulness-based cognitive therapy
The authors’ observations
Mindfulness meditation (MM) is a meditation practice that cultivates awareness. While learning MM, the practitioner intentionally focuses on awareness—a way of purposely paying attention to the present moment, non-judgmentally, to nurture calmness and self-acceptance. Being conscious of what the practitioner is doing while he is doing it is the core of mindfulness practice.6
Mindfulness-based interventions. We recommended the following forms of MBI to treat Mr. B:
• Mindfulness-based cognitive therapy (MBCT). MBCT is designed to help people who suffer repeated bouts of depression and chronic unhappiness. It combines the ideas of cognitive-behavioral therapy (CBT) with MM practices and attitudes based on cultivating mindfulness.7
• Mindfulness-based stress reduction (MBSR). MBSR brings together MM and physical/breathing exercises to relax body and mind.6
Chronic stress and drug addiction
The literature demonstrates a significant association between acute and chronic stress and motivation to abuse substances. Stress mobilizes the CRF system to stimulate the hypothalamic-pituitary-adrenal (HPA) axis, and extra-hypothalamic actions of CRF can kindle the neuronal circuits responsible for stress-induced anxiety, dysphoria, and drug abuse behaviors.8
A study to evaluate effects of mindfulness on young adult romantic partners’ HPA responses to conflict stress showed that MM has sex-specific effects on neuroendocrine response to interpersonal stress.9 Research has shown that MM practice can decrease stress, increase well-being, and affect brain structure and function.10 Meta-analysis of studies of animal models and humans described how specific interventions intended to encourage pro-social behavior and well-being might produce plasticity-related changes in the brain.11 This work concluded that, by taking responsibility for the mind and the brain by participating in regular mental exercise, plastic changes in the brain promoted could produce lasting beneficial consequences for social and emotional behavior.11
What could be perpetuating Mr. B’s depression?
a) psychosocial stressors
b) over-expression of CRF gene due to psychosocial stressors
c) a and b
Treatment Mindfulness practice
Mr. B was started on CBT to manage anxiety symptoms and cognitive distortions. After 2 months, he reports no improvements in anxiety, depression, or cognitive distortions.
We consider MBI for Mr. B, which was developed by Segal et al7 to help prevent relapse of depression and gain the benefits of MM. There is evidence that MBI can prevent relapse of SUDs.12 Mr. B’s MBI practice is based on MBCT, as outlined by Segal et al.7 He attends biweekly, 45-minute therapy sessions at our outpatient clinic. During these sessions, MM is practiced for 10 minutes under a psychiatrist’s supervision. The MBCT manual calls for 45 minutes of MM practice but, during the 10-minute session, we instruct Mr. B to independently practice MM at home. Mr. B is assessed for relapses, and drug cravings; a urine toxicology screen is performed every 6 months.
We score Mr. B’s day-to-day level of mindfulness experience, depression, and anxiety symptoms before starting MBI and after 8 weeks of practicing MBI (Figure 1). Mindfulness is scored with the Mindful Attention Awareness Scale (MAAS), a valid, reliable scale.13 The MAAS comprises 15 items designed to reflect mindfulness in everyday experiences, including awareness and attention to thoughts, emotions, actions, and physical states. Items are rated on a 6-point Likert-type scale of 1 (“almost never”) to 6 (“almost always”). A typical item on MAAS is “I find myself doing things without paying attention.”
Depression and anxiety symptoms are measured using the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder Scale-7 (GAD-7) Item Scale. Mr. B scores a 23 on PHQ-9, indicating severe depression (he reports that he finds it ‘‘extremely difficult” to function) (Figure 2).
There is evidence to support the use of PHQ-9 for measurement-based care in the psychiatric population.14 PHQ-9 does not capture anxiety, which is a strong predicator of suicidal behavior; therefore, we use GAD-7 to measure the severity of Mr. B’s subjective anxiety.15 He scores a 14 on GAD-7 and reports that it is “very difficult” for him to function.
Mr. B is retested after 8 weeks. During those 8 weeks, he was instructed by audio guidance in body scan technique. He practices MBI techniques for 45 minutes every morning between 5 AM and 6 AM.6
After 3 months of MBI, Mr. B is promoted at work and reports that he is handling more responsibilities. He is stressed at his new job and, subsequently, experiences a relapse of anxiety symptoms and insomnia. Partly, this is because Mr. B is not able to consistently practice MBI and misses a few outpatient appointments. In the meantime, he has difficulties with sleep and concentration and anxiety symptoms.
The treating psychiatrist reassures Mr. B and provides support to restart MBI. He manages to attend outpatient clinic appointments consistently and shows interest in practicing MBI daily. Later, he reports practicing MBI consistently along with his routine treatment at our clinic. The timeline of Mr. B’s history and treatment are summarized in Figure 3.
The authors’ observations
Mr. B’s CRF may have been down-regulated by MBI. This, in turn, decreased his depressive and anxiety symptoms, thereby helping to prevent relapse of depression and substance abuse. He benefited from MBI practices in several areas of his life, which can be described with the acronym FACES.10
Flexible. Mr. B became more cognitively flexible. He started to realize that “thoughts are not facts.”7 This change was reflected in his relationship with his wife. His wife came to one of our sessions because she noticed significant change in his attitude toward her. Their marriage of 15 years was riddled with conflict and his wife was excited to see the improvement he achieved within the short time of practicing MBI.
Adaptive. He became more adaptive to changes at the work place and reported that he is enjoying his work. This is a change from his feeling that his job was a burden, as he observed in our earlier sessions.
Coherent. He became more cognitively rational. He reported improvement in his memory and concentration. Five months after initiation of MBI and MM training, he was promoted and could cope with the stress at work.
Energized. Initially, he had said that he never wanted to be part of his extended family. During a session toward the end of the treatment, he mentioned that he made an effort to contact his extended family and reported that he found it more meaningful now to be reconnected with them.
Stable. He became more emotionally stable. He did not have the urge to use drugs and he did not relapse.
As we hypothesized, for Mr. B, practicing MBI was associated with abstinence from substance use, increased mindfulness, acceptance of mental health problems, and remission of psychiatric symptoms.
Bottom Line
Mindfulness-based interventions provide patients with tools to target symptoms such as poor affect regulation, poor impulse control, and rumination. Evidence supports that using MBI in addition to the usual treatment can prevent relapse of a substance use disorder.
Related Resources
• Sipe WE, Eisendrath SJ. Mindfulness-based cognitive therapy: theory and practice. Can J Psychiatry. 2012;57(2):63-69.• Lau MA, Grabovac AD. Mindfulness-based interventions: Effective for depression and anxiety. Current Psychiatry. 2009;8(12):39-55.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Buprenorphine/naloxone • Quetiapine • Seroquel
Suboxone
Bupropion • Wellbutrin Sertraline • Zoloft
Duloxetine • Cymbalta Trazodone • Desyrel
Methadone • Dolophine Venlafaxine • Effexor
Methylphenidate • Ritalin, Concerta
Acknowledgement
The manuscript preparation of Maju Mathew Koola, MD, DPM was supported by the NIMH T32 grant MH067533-07 (PI: William T. Carpenter, MD) and the American Psychiatric Association/Kempf Fund Award for Research Development in Psychobiological Psychiatry (PI: Koola). The treating Psychiatrist PGY-5 (2011-2012) Addiction Psychiatry fellow (Dr. Varghese) was supervised by Dr. Eiger. Drs. Koola and Varghese contributed equally with the manuscript preparation and are joint first authors. Dr. Varghese received a second prize for a poster presentation of this case report at the 34th Indo American Psychiatric Association meeting in San Francisco, CA, May 19, 2013. Christina Mathew, MD, also contributed with manuscript preparation.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufactures of competing products.
1. Tan EM, Eiger RI, Roth JD. A life of drugs and ‘downtime.’ Current Psychiatry. 2007;6(8):98-103.
2. Diagnostic and statistical manual of mental disorders, 4th edition, text revision. Washington, DC, American Psychiatric Association; 2000.
3. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
4. Schreiber S, Bleich A, Pick CG. Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects—a possible opioid involvement in severe depression? J Mol Neurosci. 2002; 18(1-2):143-149.
5. Sikka P, Kaushik S, Kumar G, et al. Study of antinociceptive activity of SSRI (fluoxetine and escitalopram) and atypical antidepressants (venlafaxine and mirtazepine) and their interaction with morphine and naloxone in mice. J Pharm Bioallied Sci. 2011;3(3):412-416.
6. Kabat-Zinn J. Full catastrophe living. 15th ed. New York, NY: Bantam Books; 1990.
7. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
8. Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3-14.
9. Laurent H, Laurent S, Hertz R, et al. Sex-specific effects of mindfulness on romantic partners’ cortisol responses to conflict and relations with psychological adjustment. Psychoneuroendocrinology. 2013;38(12):2905-2913.
10. Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: W.W. Norton & Company; 2007.
11. Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15(5):689-695.
12. Bowen S, Chawla N, Collins SE, et al. Mindfulness-based prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 2009;30(4):295-305.
13. Grossman P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology’s (re)invention of mindfulness: comment on Brown et al. (2001). Psychol Assess. 2011;23(4):1034-1040; discussion 1041-1046.
14. Koola MM, Fawcett JA, Kelly DL. Case report on the management of depression in schizoaffective disorder, bipolar type focusing on lithium levels and measurement-based care. J Nerv Ment Dis. 2011;199(12):989-990.
15. Nock MK, Hwang I, Sampson N, et al. Cross-national analysis of the associations among mental disorders and suicidal behavior: findings from the WHO World Mental Health Surveys. PLoS Med. 2009;6(8):e1000123. doi: 10.1371/journal.pmed.1000123.
Case Forgetful and depressed
Mr. B, age 55, has been a patient at our clinic for 8 years, where he has been under our care for treatment-resistant depression and opioid addiction [read about earlier events in his case in “A life of drugs and ‘downtime’” Current Psychiatry, August 2007, p. 98-103].1 He reports feeling intermittently depressed since his teens and has had 3 near-fatal suicide attempts.
Three years ago, Mr. B reported severe depressive symptoms and short-term memory loss, which undermined his job performance and contributed to interpersonal conflict with his wife. The episode has been continuously severe for 10 months. He was taking sertraline, 150 mg/d, and duloxetine, 60 mg/d, for major depressive disorder (MDD) and sublingual buprenorphine/naloxone, 20 mg/d, for opioid dependence, which was in sustained full remission.2 Mr. B scored 24/30 in the Mini- Mental State Examination, indicating mild cognitive deficit. Negative results of a complete routine laboratory workup rule out an organic cause for his deteriorating cognition.
How would you diagnose Mr. B’s condition at this point?
a) treatment-resistant MDD
b) cognitive disorder not otherwise specified
c) opioid use disorder
d) a and c
The authors' observations
Relapse is a core feature of substance use disorders (SUDs) that contributes significantly to the longstanding functional impairment in patients with a mood disorder. With the relapse rate following substance use treatment estimated at more than 60%,3 SUDs often are described as chronic relapsing conditions. In chronic stress, corticotropin-releasing factor (CRF) is over-sensitized; we believe that acute stress can cause an unhealthy response to an over-expressed CRF system.
To prevent relapse in patients with an over-expressed CRF system, it is crucial to manage stress. One treatment option to consider in preventing relapse is mindfulness-based interventions (MBI). Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and non-judgmentally.” In the event of a relapse, awareness and acceptance fostered by mindfulness may aid in recognizing and minimizing unhealthy responses, such as negative thinking that can increase the risk of relapse.
History Remission, then relapse
Mr. B was admitted to inpatient psychiatric unit after a near-fatal suicide attempt 8 years ago and given a diagnosis of MDD recurrent, severe without psychotic features. Trials of sertraline, bupropion, trazodone, quetiapine, and aripiprazole were ineffective.
Before he presented to our clinic 8 years ago, Mr. B had been taking venlafaxine, 75 mg/d, and mirtazapine, 30 mg at bedtime. His previous outpatient psychiatrist added methylphenidate, 40 mg/d, to augment the antidepressants, but this did not alleviate Mr. B’s depression.
At age 40, he entered a methadone program, began working steadily, and got married. Five years later, he stopped methadone (it is unclear from the chart if his psychiatrist initiated this change). Mr. B’s depression persisted while using opioids and became worse after stopping methadone.
We considered electroconvulsive therapy (ECT) at the time, but switching the antidepressant or starting ECT would address only the persistent depression; buprenorphine/naloxone would target opioid craving. We started a trial of buprenorphine/ naloxone, a partial μ opioid agonist and ĸ opioid antagonist; ĸ receptor antagonism serves as an antidepressant. He responded well to augmentation of his current regimen (mirtazapine, 30 mg at bedtime, and venlafaxine, 225 mg/d) with buprenorphine/naloxone, 16 mg/d.4,5 he reported no anergia and said he felt more motivated and productive.
Mr. B took buprenorphine/naloxone, 32 mg/d, for 4 years until, because of concern for daytime sedation, his outpatient psychiatrist reduced the dose to 20 mg/d. With the lower dosage of buprenorphine/naloxone initiated 4 years ago, Mr. B reported irritability, anhedonia, insomnia, increased self-criticism, and decreased self-care.
How would you treat Mr. B’s depression at this point?
a) switch to a daytime antidepressant
b) adjust the dosage of buprenorphine/ naloxone
c) try ECT
d) try mindfulness-based cognitive therapy
The authors’ observations
Mindfulness meditation (MM) is a meditation practice that cultivates awareness. While learning MM, the practitioner intentionally focuses on awareness—a way of purposely paying attention to the present moment, non-judgmentally, to nurture calmness and self-acceptance. Being conscious of what the practitioner is doing while he is doing it is the core of mindfulness practice.6
Mindfulness-based interventions. We recommended the following forms of MBI to treat Mr. B:
• Mindfulness-based cognitive therapy (MBCT). MBCT is designed to help people who suffer repeated bouts of depression and chronic unhappiness. It combines the ideas of cognitive-behavioral therapy (CBT) with MM practices and attitudes based on cultivating mindfulness.7
• Mindfulness-based stress reduction (MBSR). MBSR brings together MM and physical/breathing exercises to relax body and mind.6
Chronic stress and drug addiction
The literature demonstrates a significant association between acute and chronic stress and motivation to abuse substances. Stress mobilizes the CRF system to stimulate the hypothalamic-pituitary-adrenal (HPA) axis, and extra-hypothalamic actions of CRF can kindle the neuronal circuits responsible for stress-induced anxiety, dysphoria, and drug abuse behaviors.8
A study to evaluate effects of mindfulness on young adult romantic partners’ HPA responses to conflict stress showed that MM has sex-specific effects on neuroendocrine response to interpersonal stress.9 Research has shown that MM practice can decrease stress, increase well-being, and affect brain structure and function.10 Meta-analysis of studies of animal models and humans described how specific interventions intended to encourage pro-social behavior and well-being might produce plasticity-related changes in the brain.11 This work concluded that, by taking responsibility for the mind and the brain by participating in regular mental exercise, plastic changes in the brain promoted could produce lasting beneficial consequences for social and emotional behavior.11
What could be perpetuating Mr. B’s depression?
a) psychosocial stressors
b) over-expression of CRF gene due to psychosocial stressors
c) a and b
Treatment Mindfulness practice
Mr. B was started on CBT to manage anxiety symptoms and cognitive distortions. After 2 months, he reports no improvements in anxiety, depression, or cognitive distortions.
We consider MBI for Mr. B, which was developed by Segal et al7 to help prevent relapse of depression and gain the benefits of MM. There is evidence that MBI can prevent relapse of SUDs.12 Mr. B’s MBI practice is based on MBCT, as outlined by Segal et al.7 He attends biweekly, 45-minute therapy sessions at our outpatient clinic. During these sessions, MM is practiced for 10 minutes under a psychiatrist’s supervision. The MBCT manual calls for 45 minutes of MM practice but, during the 10-minute session, we instruct Mr. B to independently practice MM at home. Mr. B is assessed for relapses, and drug cravings; a urine toxicology screen is performed every 6 months.
We score Mr. B’s day-to-day level of mindfulness experience, depression, and anxiety symptoms before starting MBI and after 8 weeks of practicing MBI (Figure 1). Mindfulness is scored with the Mindful Attention Awareness Scale (MAAS), a valid, reliable scale.13 The MAAS comprises 15 items designed to reflect mindfulness in everyday experiences, including awareness and attention to thoughts, emotions, actions, and physical states. Items are rated on a 6-point Likert-type scale of 1 (“almost never”) to 6 (“almost always”). A typical item on MAAS is “I find myself doing things without paying attention.”
Depression and anxiety symptoms are measured using the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder Scale-7 (GAD-7) Item Scale. Mr. B scores a 23 on PHQ-9, indicating severe depression (he reports that he finds it ‘‘extremely difficult” to function) (Figure 2).
There is evidence to support the use of PHQ-9 for measurement-based care in the psychiatric population.14 PHQ-9 does not capture anxiety, which is a strong predicator of suicidal behavior; therefore, we use GAD-7 to measure the severity of Mr. B’s subjective anxiety.15 He scores a 14 on GAD-7 and reports that it is “very difficult” for him to function.
Mr. B is retested after 8 weeks. During those 8 weeks, he was instructed by audio guidance in body scan technique. He practices MBI techniques for 45 minutes every morning between 5 AM and 6 AM.6
After 3 months of MBI, Mr. B is promoted at work and reports that he is handling more responsibilities. He is stressed at his new job and, subsequently, experiences a relapse of anxiety symptoms and insomnia. Partly, this is because Mr. B is not able to consistently practice MBI and misses a few outpatient appointments. In the meantime, he has difficulties with sleep and concentration and anxiety symptoms.
The treating psychiatrist reassures Mr. B and provides support to restart MBI. He manages to attend outpatient clinic appointments consistently and shows interest in practicing MBI daily. Later, he reports practicing MBI consistently along with his routine treatment at our clinic. The timeline of Mr. B’s history and treatment are summarized in Figure 3.
The authors’ observations
Mr. B’s CRF may have been down-regulated by MBI. This, in turn, decreased his depressive and anxiety symptoms, thereby helping to prevent relapse of depression and substance abuse. He benefited from MBI practices in several areas of his life, which can be described with the acronym FACES.10
Flexible. Mr. B became more cognitively flexible. He started to realize that “thoughts are not facts.”7 This change was reflected in his relationship with his wife. His wife came to one of our sessions because she noticed significant change in his attitude toward her. Their marriage of 15 years was riddled with conflict and his wife was excited to see the improvement he achieved within the short time of practicing MBI.
Adaptive. He became more adaptive to changes at the work place and reported that he is enjoying his work. This is a change from his feeling that his job was a burden, as he observed in our earlier sessions.
Coherent. He became more cognitively rational. He reported improvement in his memory and concentration. Five months after initiation of MBI and MM training, he was promoted and could cope with the stress at work.
Energized. Initially, he had said that he never wanted to be part of his extended family. During a session toward the end of the treatment, he mentioned that he made an effort to contact his extended family and reported that he found it more meaningful now to be reconnected with them.
Stable. He became more emotionally stable. He did not have the urge to use drugs and he did not relapse.
As we hypothesized, for Mr. B, practicing MBI was associated with abstinence from substance use, increased mindfulness, acceptance of mental health problems, and remission of psychiatric symptoms.
Bottom Line
Mindfulness-based interventions provide patients with tools to target symptoms such as poor affect regulation, poor impulse control, and rumination. Evidence supports that using MBI in addition to the usual treatment can prevent relapse of a substance use disorder.
Related Resources
• Sipe WE, Eisendrath SJ. Mindfulness-based cognitive therapy: theory and practice. Can J Psychiatry. 2012;57(2):63-69.• Lau MA, Grabovac AD. Mindfulness-based interventions: Effective for depression and anxiety. Current Psychiatry. 2009;8(12):39-55.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Buprenorphine/naloxone • Quetiapine • Seroquel
Suboxone
Bupropion • Wellbutrin Sertraline • Zoloft
Duloxetine • Cymbalta Trazodone • Desyrel
Methadone • Dolophine Venlafaxine • Effexor
Methylphenidate • Ritalin, Concerta
Acknowledgement
The manuscript preparation of Maju Mathew Koola, MD, DPM was supported by the NIMH T32 grant MH067533-07 (PI: William T. Carpenter, MD) and the American Psychiatric Association/Kempf Fund Award for Research Development in Psychobiological Psychiatry (PI: Koola). The treating Psychiatrist PGY-5 (2011-2012) Addiction Psychiatry fellow (Dr. Varghese) was supervised by Dr. Eiger. Drs. Koola and Varghese contributed equally with the manuscript preparation and are joint first authors. Dr. Varghese received a second prize for a poster presentation of this case report at the 34th Indo American Psychiatric Association meeting in San Francisco, CA, May 19, 2013. Christina Mathew, MD, also contributed with manuscript preparation.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufactures of competing products.
Case Forgetful and depressed
Mr. B, age 55, has been a patient at our clinic for 8 years, where he has been under our care for treatment-resistant depression and opioid addiction [read about earlier events in his case in “A life of drugs and ‘downtime’” Current Psychiatry, August 2007, p. 98-103].1 He reports feeling intermittently depressed since his teens and has had 3 near-fatal suicide attempts.
Three years ago, Mr. B reported severe depressive symptoms and short-term memory loss, which undermined his job performance and contributed to interpersonal conflict with his wife. The episode has been continuously severe for 10 months. He was taking sertraline, 150 mg/d, and duloxetine, 60 mg/d, for major depressive disorder (MDD) and sublingual buprenorphine/naloxone, 20 mg/d, for opioid dependence, which was in sustained full remission.2 Mr. B scored 24/30 in the Mini- Mental State Examination, indicating mild cognitive deficit. Negative results of a complete routine laboratory workup rule out an organic cause for his deteriorating cognition.
How would you diagnose Mr. B’s condition at this point?
a) treatment-resistant MDD
b) cognitive disorder not otherwise specified
c) opioid use disorder
d) a and c
The authors' observations
Relapse is a core feature of substance use disorders (SUDs) that contributes significantly to the longstanding functional impairment in patients with a mood disorder. With the relapse rate following substance use treatment estimated at more than 60%,3 SUDs often are described as chronic relapsing conditions. In chronic stress, corticotropin-releasing factor (CRF) is over-sensitized; we believe that acute stress can cause an unhealthy response to an over-expressed CRF system.
To prevent relapse in patients with an over-expressed CRF system, it is crucial to manage stress. One treatment option to consider in preventing relapse is mindfulness-based interventions (MBI). Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and non-judgmentally.” In the event of a relapse, awareness and acceptance fostered by mindfulness may aid in recognizing and minimizing unhealthy responses, such as negative thinking that can increase the risk of relapse.
History Remission, then relapse
Mr. B was admitted to inpatient psychiatric unit after a near-fatal suicide attempt 8 years ago and given a diagnosis of MDD recurrent, severe without psychotic features. Trials of sertraline, bupropion, trazodone, quetiapine, and aripiprazole were ineffective.
Before he presented to our clinic 8 years ago, Mr. B had been taking venlafaxine, 75 mg/d, and mirtazapine, 30 mg at bedtime. His previous outpatient psychiatrist added methylphenidate, 40 mg/d, to augment the antidepressants, but this did not alleviate Mr. B’s depression.
At age 40, he entered a methadone program, began working steadily, and got married. Five years later, he stopped methadone (it is unclear from the chart if his psychiatrist initiated this change). Mr. B’s depression persisted while using opioids and became worse after stopping methadone.
We considered electroconvulsive therapy (ECT) at the time, but switching the antidepressant or starting ECT would address only the persistent depression; buprenorphine/naloxone would target opioid craving. We started a trial of buprenorphine/ naloxone, a partial μ opioid agonist and ĸ opioid antagonist; ĸ receptor antagonism serves as an antidepressant. He responded well to augmentation of his current regimen (mirtazapine, 30 mg at bedtime, and venlafaxine, 225 mg/d) with buprenorphine/naloxone, 16 mg/d.4,5 he reported no anergia and said he felt more motivated and productive.
Mr. B took buprenorphine/naloxone, 32 mg/d, for 4 years until, because of concern for daytime sedation, his outpatient psychiatrist reduced the dose to 20 mg/d. With the lower dosage of buprenorphine/naloxone initiated 4 years ago, Mr. B reported irritability, anhedonia, insomnia, increased self-criticism, and decreased self-care.
How would you treat Mr. B’s depression at this point?
a) switch to a daytime antidepressant
b) adjust the dosage of buprenorphine/ naloxone
c) try ECT
d) try mindfulness-based cognitive therapy
The authors’ observations
Mindfulness meditation (MM) is a meditation practice that cultivates awareness. While learning MM, the practitioner intentionally focuses on awareness—a way of purposely paying attention to the present moment, non-judgmentally, to nurture calmness and self-acceptance. Being conscious of what the practitioner is doing while he is doing it is the core of mindfulness practice.6
Mindfulness-based interventions. We recommended the following forms of MBI to treat Mr. B:
• Mindfulness-based cognitive therapy (MBCT). MBCT is designed to help people who suffer repeated bouts of depression and chronic unhappiness. It combines the ideas of cognitive-behavioral therapy (CBT) with MM practices and attitudes based on cultivating mindfulness.7
• Mindfulness-based stress reduction (MBSR). MBSR brings together MM and physical/breathing exercises to relax body and mind.6
Chronic stress and drug addiction
The literature demonstrates a significant association between acute and chronic stress and motivation to abuse substances. Stress mobilizes the CRF system to stimulate the hypothalamic-pituitary-adrenal (HPA) axis, and extra-hypothalamic actions of CRF can kindle the neuronal circuits responsible for stress-induced anxiety, dysphoria, and drug abuse behaviors.8
A study to evaluate effects of mindfulness on young adult romantic partners’ HPA responses to conflict stress showed that MM has sex-specific effects on neuroendocrine response to interpersonal stress.9 Research has shown that MM practice can decrease stress, increase well-being, and affect brain structure and function.10 Meta-analysis of studies of animal models and humans described how specific interventions intended to encourage pro-social behavior and well-being might produce plasticity-related changes in the brain.11 This work concluded that, by taking responsibility for the mind and the brain by participating in regular mental exercise, plastic changes in the brain promoted could produce lasting beneficial consequences for social and emotional behavior.11
What could be perpetuating Mr. B’s depression?
a) psychosocial stressors
b) over-expression of CRF gene due to psychosocial stressors
c) a and b
Treatment Mindfulness practice
Mr. B was started on CBT to manage anxiety symptoms and cognitive distortions. After 2 months, he reports no improvements in anxiety, depression, or cognitive distortions.
We consider MBI for Mr. B, which was developed by Segal et al7 to help prevent relapse of depression and gain the benefits of MM. There is evidence that MBI can prevent relapse of SUDs.12 Mr. B’s MBI practice is based on MBCT, as outlined by Segal et al.7 He attends biweekly, 45-minute therapy sessions at our outpatient clinic. During these sessions, MM is practiced for 10 minutes under a psychiatrist’s supervision. The MBCT manual calls for 45 minutes of MM practice but, during the 10-minute session, we instruct Mr. B to independently practice MM at home. Mr. B is assessed for relapses, and drug cravings; a urine toxicology screen is performed every 6 months.
We score Mr. B’s day-to-day level of mindfulness experience, depression, and anxiety symptoms before starting MBI and after 8 weeks of practicing MBI (Figure 1). Mindfulness is scored with the Mindful Attention Awareness Scale (MAAS), a valid, reliable scale.13 The MAAS comprises 15 items designed to reflect mindfulness in everyday experiences, including awareness and attention to thoughts, emotions, actions, and physical states. Items are rated on a 6-point Likert-type scale of 1 (“almost never”) to 6 (“almost always”). A typical item on MAAS is “I find myself doing things without paying attention.”
Depression and anxiety symptoms are measured using the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder Scale-7 (GAD-7) Item Scale. Mr. B scores a 23 on PHQ-9, indicating severe depression (he reports that he finds it ‘‘extremely difficult” to function) (Figure 2).
There is evidence to support the use of PHQ-9 for measurement-based care in the psychiatric population.14 PHQ-9 does not capture anxiety, which is a strong predicator of suicidal behavior; therefore, we use GAD-7 to measure the severity of Mr. B’s subjective anxiety.15 He scores a 14 on GAD-7 and reports that it is “very difficult” for him to function.
Mr. B is retested after 8 weeks. During those 8 weeks, he was instructed by audio guidance in body scan technique. He practices MBI techniques for 45 minutes every morning between 5 AM and 6 AM.6
After 3 months of MBI, Mr. B is promoted at work and reports that he is handling more responsibilities. He is stressed at his new job and, subsequently, experiences a relapse of anxiety symptoms and insomnia. Partly, this is because Mr. B is not able to consistently practice MBI and misses a few outpatient appointments. In the meantime, he has difficulties with sleep and concentration and anxiety symptoms.
The treating psychiatrist reassures Mr. B and provides support to restart MBI. He manages to attend outpatient clinic appointments consistently and shows interest in practicing MBI daily. Later, he reports practicing MBI consistently along with his routine treatment at our clinic. The timeline of Mr. B’s history and treatment are summarized in Figure 3.
The authors’ observations
Mr. B’s CRF may have been down-regulated by MBI. This, in turn, decreased his depressive and anxiety symptoms, thereby helping to prevent relapse of depression and substance abuse. He benefited from MBI practices in several areas of his life, which can be described with the acronym FACES.10
Flexible. Mr. B became more cognitively flexible. He started to realize that “thoughts are not facts.”7 This change was reflected in his relationship with his wife. His wife came to one of our sessions because she noticed significant change in his attitude toward her. Their marriage of 15 years was riddled with conflict and his wife was excited to see the improvement he achieved within the short time of practicing MBI.
Adaptive. He became more adaptive to changes at the work place and reported that he is enjoying his work. This is a change from his feeling that his job was a burden, as he observed in our earlier sessions.
Coherent. He became more cognitively rational. He reported improvement in his memory and concentration. Five months after initiation of MBI and MM training, he was promoted and could cope with the stress at work.
Energized. Initially, he had said that he never wanted to be part of his extended family. During a session toward the end of the treatment, he mentioned that he made an effort to contact his extended family and reported that he found it more meaningful now to be reconnected with them.
Stable. He became more emotionally stable. He did not have the urge to use drugs and he did not relapse.
As we hypothesized, for Mr. B, practicing MBI was associated with abstinence from substance use, increased mindfulness, acceptance of mental health problems, and remission of psychiatric symptoms.
Bottom Line
Mindfulness-based interventions provide patients with tools to target symptoms such as poor affect regulation, poor impulse control, and rumination. Evidence supports that using MBI in addition to the usual treatment can prevent relapse of a substance use disorder.
Related Resources
• Sipe WE, Eisendrath SJ. Mindfulness-based cognitive therapy: theory and practice. Can J Psychiatry. 2012;57(2):63-69.• Lau MA, Grabovac AD. Mindfulness-based interventions: Effective for depression and anxiety. Current Psychiatry. 2009;8(12):39-55.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Buprenorphine/naloxone • Quetiapine • Seroquel
Suboxone
Bupropion • Wellbutrin Sertraline • Zoloft
Duloxetine • Cymbalta Trazodone • Desyrel
Methadone • Dolophine Venlafaxine • Effexor
Methylphenidate • Ritalin, Concerta
Acknowledgement
The manuscript preparation of Maju Mathew Koola, MD, DPM was supported by the NIMH T32 grant MH067533-07 (PI: William T. Carpenter, MD) and the American Psychiatric Association/Kempf Fund Award for Research Development in Psychobiological Psychiatry (PI: Koola). The treating Psychiatrist PGY-5 (2011-2012) Addiction Psychiatry fellow (Dr. Varghese) was supervised by Dr. Eiger. Drs. Koola and Varghese contributed equally with the manuscript preparation and are joint first authors. Dr. Varghese received a second prize for a poster presentation of this case report at the 34th Indo American Psychiatric Association meeting in San Francisco, CA, May 19, 2013. Christina Mathew, MD, also contributed with manuscript preparation.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufactures of competing products.
1. Tan EM, Eiger RI, Roth JD. A life of drugs and ‘downtime.’ Current Psychiatry. 2007;6(8):98-103.
2. Diagnostic and statistical manual of mental disorders, 4th edition, text revision. Washington, DC, American Psychiatric Association; 2000.
3. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
4. Schreiber S, Bleich A, Pick CG. Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects—a possible opioid involvement in severe depression? J Mol Neurosci. 2002; 18(1-2):143-149.
5. Sikka P, Kaushik S, Kumar G, et al. Study of antinociceptive activity of SSRI (fluoxetine and escitalopram) and atypical antidepressants (venlafaxine and mirtazepine) and their interaction with morphine and naloxone in mice. J Pharm Bioallied Sci. 2011;3(3):412-416.
6. Kabat-Zinn J. Full catastrophe living. 15th ed. New York, NY: Bantam Books; 1990.
7. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
8. Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3-14.
9. Laurent H, Laurent S, Hertz R, et al. Sex-specific effects of mindfulness on romantic partners’ cortisol responses to conflict and relations with psychological adjustment. Psychoneuroendocrinology. 2013;38(12):2905-2913.
10. Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: W.W. Norton & Company; 2007.
11. Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15(5):689-695.
12. Bowen S, Chawla N, Collins SE, et al. Mindfulness-based prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 2009;30(4):295-305.
13. Grossman P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology’s (re)invention of mindfulness: comment on Brown et al. (2001). Psychol Assess. 2011;23(4):1034-1040; discussion 1041-1046.
14. Koola MM, Fawcett JA, Kelly DL. Case report on the management of depression in schizoaffective disorder, bipolar type focusing on lithium levels and measurement-based care. J Nerv Ment Dis. 2011;199(12):989-990.
15. Nock MK, Hwang I, Sampson N, et al. Cross-national analysis of the associations among mental disorders and suicidal behavior: findings from the WHO World Mental Health Surveys. PLoS Med. 2009;6(8):e1000123. doi: 10.1371/journal.pmed.1000123.
1. Tan EM, Eiger RI, Roth JD. A life of drugs and ‘downtime.’ Current Psychiatry. 2007;6(8):98-103.
2. Diagnostic and statistical manual of mental disorders, 4th edition, text revision. Washington, DC, American Psychiatric Association; 2000.
3. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
4. Schreiber S, Bleich A, Pick CG. Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects—a possible opioid involvement in severe depression? J Mol Neurosci. 2002; 18(1-2):143-149.
5. Sikka P, Kaushik S, Kumar G, et al. Study of antinociceptive activity of SSRI (fluoxetine and escitalopram) and atypical antidepressants (venlafaxine and mirtazepine) and their interaction with morphine and naloxone in mice. J Pharm Bioallied Sci. 2011;3(3):412-416.
6. Kabat-Zinn J. Full catastrophe living. 15th ed. New York, NY: Bantam Books; 1990.
7. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
8. Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3-14.
9. Laurent H, Laurent S, Hertz R, et al. Sex-specific effects of mindfulness on romantic partners’ cortisol responses to conflict and relations with psychological adjustment. Psychoneuroendocrinology. 2013;38(12):2905-2913.
10. Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: W.W. Norton & Company; 2007.
11. Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15(5):689-695.
12. Bowen S, Chawla N, Collins SE, et al. Mindfulness-based prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 2009;30(4):295-305.
13. Grossman P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology’s (re)invention of mindfulness: comment on Brown et al. (2001). Psychol Assess. 2011;23(4):1034-1040; discussion 1041-1046.
14. Koola MM, Fawcett JA, Kelly DL. Case report on the management of depression in schizoaffective disorder, bipolar type focusing on lithium levels and measurement-based care. J Nerv Ment Dis. 2011;199(12):989-990.
15. Nock MK, Hwang I, Sampson N, et al. Cross-national analysis of the associations among mental disorders and suicidal behavior: findings from the WHO World Mental Health Surveys. PLoS Med. 2009;6(8):e1000123. doi: 10.1371/journal.pmed.1000123.
How to talk to patients and their family after a diagnosis of mild cognitive impairment
Mild cognitive impairment (MCI) is a transitional clinical stage between normal aging and dementia. Together with aging, it is considered the most significant risk factor for developing dementia, often the Alzheimer’s type.1
MCI is a challenging neuropsychiatric diagnosis to discuss with patients and their family because it is characterized by overlapping features of normal aging and because of its heterogeneity of etiology, clinical presentation, and outcome.2,3 The evolution to dementia and the lack of effective treatments for preventing or forestalling this outcome can be difficult to address—particularly when the patient is in good health and has been leading a productive life.
Successful communication is key
You can take steps to communicate in a helpful way, build a strong treatment alliance, and reduce the potential for the iatrogenic effects of disclosing this diagnosis and its prognostic implications.
Clarify that your findings are consistent with the patient’s or family’s report of sustained and concerning change in cognition and, depending on the patient, concurrent alterations in affect, behavior, or both. Emphasize that these changes are disproportionately severe relative to expectations for the patient’s age and are not caused by psychiatric or clear-cut medical factors.
Highlight contexts in which the patient’s symptoms are likely to become more disruptive and impaired, and situations in which the patient can be expected to function more effectively.
Provide evidence-based support for the rate of progression of symptoms and functional impairment.3
Emphasize that major lifestyle adjustments usually are unnecessary in the absence of progression, especially for patients who are retired or not involved in endeavors that involve significant cognitive and executive functioning demands.
Discuss the role that cognition-enhancing medications might play in managing symptoms.4
Address indications for additional services, including formal psychiatric care for patients who have concomitant affective or behavioral symptoms and who are highly distressed by the diagnosis. Pair these services with longitudinal monitoring for possible exacerbation of symptoms.
Identify psychiatric, medical, and lifestyle factors that can increase the risk of dementia. Depending on the patient’s history, this might include diabetes, hypertension, elevated lipid levels, obesity, smoking, head trauma, depression, physical inactivity, and lack of intellectual stimulation.
Review compensatory strategies. In MCI predominantly amnestic type, for example, having the patient make systematic lists for shopping and other activities of daily living, as well as establishing routines for organizaton, can bolster successful coping.
If psychometric testing was not utilized to establish the diagnosis, discussion can include the value of performing such an assessment for a more finely tuned profile of preserved and impaired neurobehavioral functions. Such a profile can include test patterns that 1) have prognostic value with regard to the likelihood of progression to dementia and 2) establish a baseline against which you can assess stability or progression over time.5
Disclosure
Dr. Pollak reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
2. Ellison JM, Harper DG, Berlow Y, et al. Beyond the “C” in MCI: noncognitive symptoms in amnestic and non-amnestic mild cognitive impairment. CNS Spectr. 2008;13(1):66-72.
3. Goveas JS, Dixon-Holbrook M, Kerwin D, et al. Mild cognitive impairment: how can you be sure? Current Psychiatry. 2008;7(4):36-40, 46-50.
4. Doody RS, Ferris SH, Salloway S, et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009;72(18):1555-1561.
5. Summers MJ, Saunders NL. Neuropsychological measures predict decline to Alzheimer’s dementia from mild cognitive impairment. Neuropsychology. 2012;26(4):498-508.
Mild cognitive impairment (MCI) is a transitional clinical stage between normal aging and dementia. Together with aging, it is considered the most significant risk factor for developing dementia, often the Alzheimer’s type.1
MCI is a challenging neuropsychiatric diagnosis to discuss with patients and their family because it is characterized by overlapping features of normal aging and because of its heterogeneity of etiology, clinical presentation, and outcome.2,3 The evolution to dementia and the lack of effective treatments for preventing or forestalling this outcome can be difficult to address—particularly when the patient is in good health and has been leading a productive life.
Successful communication is key
You can take steps to communicate in a helpful way, build a strong treatment alliance, and reduce the potential for the iatrogenic effects of disclosing this diagnosis and its prognostic implications.
Clarify that your findings are consistent with the patient’s or family’s report of sustained and concerning change in cognition and, depending on the patient, concurrent alterations in affect, behavior, or both. Emphasize that these changes are disproportionately severe relative to expectations for the patient’s age and are not caused by psychiatric or clear-cut medical factors.
Highlight contexts in which the patient’s symptoms are likely to become more disruptive and impaired, and situations in which the patient can be expected to function more effectively.
Provide evidence-based support for the rate of progression of symptoms and functional impairment.3
Emphasize that major lifestyle adjustments usually are unnecessary in the absence of progression, especially for patients who are retired or not involved in endeavors that involve significant cognitive and executive functioning demands.
Discuss the role that cognition-enhancing medications might play in managing symptoms.4
Address indications for additional services, including formal psychiatric care for patients who have concomitant affective or behavioral symptoms and who are highly distressed by the diagnosis. Pair these services with longitudinal monitoring for possible exacerbation of symptoms.
Identify psychiatric, medical, and lifestyle factors that can increase the risk of dementia. Depending on the patient’s history, this might include diabetes, hypertension, elevated lipid levels, obesity, smoking, head trauma, depression, physical inactivity, and lack of intellectual stimulation.
Review compensatory strategies. In MCI predominantly amnestic type, for example, having the patient make systematic lists for shopping and other activities of daily living, as well as establishing routines for organizaton, can bolster successful coping.
If psychometric testing was not utilized to establish the diagnosis, discussion can include the value of performing such an assessment for a more finely tuned profile of preserved and impaired neurobehavioral functions. Such a profile can include test patterns that 1) have prognostic value with regard to the likelihood of progression to dementia and 2) establish a baseline against which you can assess stability or progression over time.5
Disclosure
Dr. Pollak reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Mild cognitive impairment (MCI) is a transitional clinical stage between normal aging and dementia. Together with aging, it is considered the most significant risk factor for developing dementia, often the Alzheimer’s type.1
MCI is a challenging neuropsychiatric diagnosis to discuss with patients and their family because it is characterized by overlapping features of normal aging and because of its heterogeneity of etiology, clinical presentation, and outcome.2,3 The evolution to dementia and the lack of effective treatments for preventing or forestalling this outcome can be difficult to address—particularly when the patient is in good health and has been leading a productive life.
Successful communication is key
You can take steps to communicate in a helpful way, build a strong treatment alliance, and reduce the potential for the iatrogenic effects of disclosing this diagnosis and its prognostic implications.
Clarify that your findings are consistent with the patient’s or family’s report of sustained and concerning change in cognition and, depending on the patient, concurrent alterations in affect, behavior, or both. Emphasize that these changes are disproportionately severe relative to expectations for the patient’s age and are not caused by psychiatric or clear-cut medical factors.
Highlight contexts in which the patient’s symptoms are likely to become more disruptive and impaired, and situations in which the patient can be expected to function more effectively.
Provide evidence-based support for the rate of progression of symptoms and functional impairment.3
Emphasize that major lifestyle adjustments usually are unnecessary in the absence of progression, especially for patients who are retired or not involved in endeavors that involve significant cognitive and executive functioning demands.
Discuss the role that cognition-enhancing medications might play in managing symptoms.4
Address indications for additional services, including formal psychiatric care for patients who have concomitant affective or behavioral symptoms and who are highly distressed by the diagnosis. Pair these services with longitudinal monitoring for possible exacerbation of symptoms.
Identify psychiatric, medical, and lifestyle factors that can increase the risk of dementia. Depending on the patient’s history, this might include diabetes, hypertension, elevated lipid levels, obesity, smoking, head trauma, depression, physical inactivity, and lack of intellectual stimulation.
Review compensatory strategies. In MCI predominantly amnestic type, for example, having the patient make systematic lists for shopping and other activities of daily living, as well as establishing routines for organizaton, can bolster successful coping.
If psychometric testing was not utilized to establish the diagnosis, discussion can include the value of performing such an assessment for a more finely tuned profile of preserved and impaired neurobehavioral functions. Such a profile can include test patterns that 1) have prognostic value with regard to the likelihood of progression to dementia and 2) establish a baseline against which you can assess stability or progression over time.5
Disclosure
Dr. Pollak reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
2. Ellison JM, Harper DG, Berlow Y, et al. Beyond the “C” in MCI: noncognitive symptoms in amnestic and non-amnestic mild cognitive impairment. CNS Spectr. 2008;13(1):66-72.
3. Goveas JS, Dixon-Holbrook M, Kerwin D, et al. Mild cognitive impairment: how can you be sure? Current Psychiatry. 2008;7(4):36-40, 46-50.
4. Doody RS, Ferris SH, Salloway S, et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009;72(18):1555-1561.
5. Summers MJ, Saunders NL. Neuropsychological measures predict decline to Alzheimer’s dementia from mild cognitive impairment. Neuropsychology. 2012;26(4):498-508.
1. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
2. Ellison JM, Harper DG, Berlow Y, et al. Beyond the “C” in MCI: noncognitive symptoms in amnestic and non-amnestic mild cognitive impairment. CNS Spectr. 2008;13(1):66-72.
3. Goveas JS, Dixon-Holbrook M, Kerwin D, et al. Mild cognitive impairment: how can you be sure? Current Psychiatry. 2008;7(4):36-40, 46-50.
4. Doody RS, Ferris SH, Salloway S, et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009;72(18):1555-1561.
5. Summers MJ, Saunders NL. Neuropsychological measures predict decline to Alzheimer’s dementia from mild cognitive impairment. Neuropsychology. 2012;26(4):498-508.
Avoid hospitalization for severe and enduring anorexia nervosa by personalizing your care
Severe and enduring anorexia nervosa (SE-AN) is persistent anorexia nervosa (AN) lasting for ≥7 years with or without a history of treatment. Evidence points to the effectiveness of a patient-tailored plan for treating SE-AN over any universal fix. Proper medication, therapeutic alliance, and strategic discharge planning are the ingredients for treating SE-AN that avoids re-hospitalization (Table).
Nutritional support and pharmacotherapy required
Comprehensive metabolic analysis and initiating nutrition should be the first priority for the medical team. Starved-state patients can have electrolyte and metabolic derangements that place them at risk of fatal arrhythmias or multi-system organ failure. Do not hesitate to initiate nasogastric tube feeding under the observation of a certified nutritionist when necessary for survival. A double-blind, randomized controlled trial demonstrated the benefit of olanzapine compared with placebo to increase body mass index (BMI) of hospitalized AN patients. Olanzapine was titrated from 2.5 to 10 mg/d over a 13-week period, and was associated with higher patient achievement of a BMI > 18.5 kg/m2.1
Although the patient is receiving nutritional support in conjunction with psychotropic medication, the road to BMI recovery can be long. Don’t forget that SE-AN can be incapacitating. In SE-AN, the fear of gaining weight is so severe that the idea of starvation-induced death initially might seem more palatable. Although counterintuitive, as the patient recovers metabolically, self-image deteriorates. Statements praising any new weight gain can derail any therapeutic relationship.
Therapeutic alliance is key
Establishing high-quality therapeutic alliance, as measured by the Helping Relationships Questionnaire, has been shown to have a positive outcome on eating disorder symptoms and comorbid depressed mood in later phases of SE-AN treatment.2,3 Although therapeutic alliance is individualized, maintaining open communication and reiterating how it is the patient’s decision to consume whole food at a level at which the feeding tube can be discontinued are good places to start treatment.
Proper discharge timing and transition to outpatient care for SE-AN patients is paramount. In multicenter studies, treatment ends too early in 57.8% of patients; discharge at sub-ideal BMI is linked to rehospitalization.3 Slower weight gain and delayed establishment of therapeutic alliance are predictors of patients who exit treatment programs too early.3 Clinicians who remain vigilant for the above metrics are less likely to feed into the unacceptably high rate of treatment failure for SE-AN.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bissada H, Tasca GA, Barber AM, et al. Olanzapine in the treatment of low body weight and obsessive thinking in women with anorexia nervosa: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2008;165(10):1281-1288.
2. Stiles-Shields C, Touyz S, Hay P, et al. Therapeutic alliance in two treatments for adults with severe and enduring anorexia nervosa. Int J Eat Disord. 2013;46(8):783-789.
3. Sly R, Morgan JF, Mountford VA, et al. Predicting premature termination of hospitalised treatment for anorexia nervosa: the roles of therapeutic alliance, motivation, and behaviour change. Eat Behav. 2013;14(2):119-123.
Severe and enduring anorexia nervosa (SE-AN) is persistent anorexia nervosa (AN) lasting for ≥7 years with or without a history of treatment. Evidence points to the effectiveness of a patient-tailored plan for treating SE-AN over any universal fix. Proper medication, therapeutic alliance, and strategic discharge planning are the ingredients for treating SE-AN that avoids re-hospitalization (Table).
Nutritional support and pharmacotherapy required
Comprehensive metabolic analysis and initiating nutrition should be the first priority for the medical team. Starved-state patients can have electrolyte and metabolic derangements that place them at risk of fatal arrhythmias or multi-system organ failure. Do not hesitate to initiate nasogastric tube feeding under the observation of a certified nutritionist when necessary for survival. A double-blind, randomized controlled trial demonstrated the benefit of olanzapine compared with placebo to increase body mass index (BMI) of hospitalized AN patients. Olanzapine was titrated from 2.5 to 10 mg/d over a 13-week period, and was associated with higher patient achievement of a BMI > 18.5 kg/m2.1
Although the patient is receiving nutritional support in conjunction with psychotropic medication, the road to BMI recovery can be long. Don’t forget that SE-AN can be incapacitating. In SE-AN, the fear of gaining weight is so severe that the idea of starvation-induced death initially might seem more palatable. Although counterintuitive, as the patient recovers metabolically, self-image deteriorates. Statements praising any new weight gain can derail any therapeutic relationship.
Therapeutic alliance is key
Establishing high-quality therapeutic alliance, as measured by the Helping Relationships Questionnaire, has been shown to have a positive outcome on eating disorder symptoms and comorbid depressed mood in later phases of SE-AN treatment.2,3 Although therapeutic alliance is individualized, maintaining open communication and reiterating how it is the patient’s decision to consume whole food at a level at which the feeding tube can be discontinued are good places to start treatment.
Proper discharge timing and transition to outpatient care for SE-AN patients is paramount. In multicenter studies, treatment ends too early in 57.8% of patients; discharge at sub-ideal BMI is linked to rehospitalization.3 Slower weight gain and delayed establishment of therapeutic alliance are predictors of patients who exit treatment programs too early.3 Clinicians who remain vigilant for the above metrics are less likely to feed into the unacceptably high rate of treatment failure for SE-AN.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Severe and enduring anorexia nervosa (SE-AN) is persistent anorexia nervosa (AN) lasting for ≥7 years with or without a history of treatment. Evidence points to the effectiveness of a patient-tailored plan for treating SE-AN over any universal fix. Proper medication, therapeutic alliance, and strategic discharge planning are the ingredients for treating SE-AN that avoids re-hospitalization (Table).
Nutritional support and pharmacotherapy required
Comprehensive metabolic analysis and initiating nutrition should be the first priority for the medical team. Starved-state patients can have electrolyte and metabolic derangements that place them at risk of fatal arrhythmias or multi-system organ failure. Do not hesitate to initiate nasogastric tube feeding under the observation of a certified nutritionist when necessary for survival. A double-blind, randomized controlled trial demonstrated the benefit of olanzapine compared with placebo to increase body mass index (BMI) of hospitalized AN patients. Olanzapine was titrated from 2.5 to 10 mg/d over a 13-week period, and was associated with higher patient achievement of a BMI > 18.5 kg/m2.1
Although the patient is receiving nutritional support in conjunction with psychotropic medication, the road to BMI recovery can be long. Don’t forget that SE-AN can be incapacitating. In SE-AN, the fear of gaining weight is so severe that the idea of starvation-induced death initially might seem more palatable. Although counterintuitive, as the patient recovers metabolically, self-image deteriorates. Statements praising any new weight gain can derail any therapeutic relationship.
Therapeutic alliance is key
Establishing high-quality therapeutic alliance, as measured by the Helping Relationships Questionnaire, has been shown to have a positive outcome on eating disorder symptoms and comorbid depressed mood in later phases of SE-AN treatment.2,3 Although therapeutic alliance is individualized, maintaining open communication and reiterating how it is the patient’s decision to consume whole food at a level at which the feeding tube can be discontinued are good places to start treatment.
Proper discharge timing and transition to outpatient care for SE-AN patients is paramount. In multicenter studies, treatment ends too early in 57.8% of patients; discharge at sub-ideal BMI is linked to rehospitalization.3 Slower weight gain and delayed establishment of therapeutic alliance are predictors of patients who exit treatment programs too early.3 Clinicians who remain vigilant for the above metrics are less likely to feed into the unacceptably high rate of treatment failure for SE-AN.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bissada H, Tasca GA, Barber AM, et al. Olanzapine in the treatment of low body weight and obsessive thinking in women with anorexia nervosa: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2008;165(10):1281-1288.
2. Stiles-Shields C, Touyz S, Hay P, et al. Therapeutic alliance in two treatments for adults with severe and enduring anorexia nervosa. Int J Eat Disord. 2013;46(8):783-789.
3. Sly R, Morgan JF, Mountford VA, et al. Predicting premature termination of hospitalised treatment for anorexia nervosa: the roles of therapeutic alliance, motivation, and behaviour change. Eat Behav. 2013;14(2):119-123.
1. Bissada H, Tasca GA, Barber AM, et al. Olanzapine in the treatment of low body weight and obsessive thinking in women with anorexia nervosa: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2008;165(10):1281-1288.
2. Stiles-Shields C, Touyz S, Hay P, et al. Therapeutic alliance in two treatments for adults with severe and enduring anorexia nervosa. Int J Eat Disord. 2013;46(8):783-789.
3. Sly R, Morgan JF, Mountford VA, et al. Predicting premature termination of hospitalised treatment for anorexia nervosa: the roles of therapeutic alliance, motivation, and behaviour change. Eat Behav. 2013;14(2):119-123.
Take caution: Look for DISTURBED behaviors when you assess violence risk
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.