User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Cautions when prescribing lithium
I was astonished that Dr. Melvin G. McInnis’ article on using lithium to treat bipolar disorder (BD) (Current Psychiatry, June 2014, p. 38-44; [http://bit.ly/1sszAUr]) did not address all the potential hazards of the medication. He discussed side effects, but only how to manage them so that patients will adhere to treatment.
I have used lithium for patients with BD, and often it is efficacious, although hazardous in overdose. Lithium toxicity can cause cardiac arrhythmias, and must be monitored closely. In addition, the effects of hydration and exercise on the lithium level, especially during summer, often are ignored.
Two of my patients, an adolescent and an adult, were well-maintained on lithium, adhered to treatment, and had no concurrent medical problems, but developed significant toxicity for no reason that I could determine. The adult had a lithium level of 2.0 mEq/L in the emergency room; the adolescent had a lithium level of 1.8 mEq/L. Levels this high are considered potentially lethal, and because it happened without warning and without a cause that I could determine, I consider lithium to be one of the riskier mood stabilizers. I still prescribe it, but with great caution.
Dr. McInnis also did not mention the possibility of lithium-induced diabetes insipidus, a condition in which the kidneys are no longer able to concentrate urine and that is marked by excessive urination, concomitant water intake, and low urine specific gravity. It is uncommon, but I have seen it 3 times in 30 years, in a practice that specializes in psychotherapy and does not see a high percentage of patients with BD. I consider it a condition that must be kept in mind as we follow our patients in long-term treatment.
Mary Davis, MD
Lancaster, Pennsylvania
Dr. McInnis responds
Dr. Davis raises the issue of lithium toxicity and provides examples of 2 patients who developed levels of 2.0 mEq/L and 1.8 mEq/L. These levels clearly are well beyond the toxicity threshold of 1.3 mEq/L, and the patients wisely sought urgent care. These scenarios exemplify the need for regular monitoring of the lithium level—in particular, when there is any change in physical or mental health status. Development of significant toxicity generally has some lead-time with emerging short-term side effects (outlined in Table 2 of my article), which underscores the importance of discussing the nature of emerging side effects with your patient.
Dr. Davis is correct in noting that the practitioner must be aware of long-term side effects of lithium. I find it helpful to discuss these effects with the patient in the context of short-term (days or weeks), intermediate (weeks or months), and long-term (months or years) time frames (Table 2). Diabetes insipidus is listed as an intermediate side effect.
I am grateful to Dr. Davis for raising the issue of hydration and summer heat, a concern among parents and coaches when student athletes practice strenuously for extended hours.1 Miller et al2 found that the concentration of lithium was between 1.2- and 4.6-fold in forearm sweat compared with serum levels, with the implication that heat-induced sweating may lower lithium levels. Jefferson et al3 studied 4 athletes after a 20-km race and found that all had become dehydrated but had a decrease in the serum lithium level. This is contrary to the widely held belief that excessive sweating predisposes to lithium toxicity.
BD is among the more lethal psychiatric disorders, and lithium is among the few medications shown to mitigate suicidal behavior.4 As with any medication, lithium is not without risk, and there is a clear need for informed medical management. Any notable change in health status or physical activity in a patient taking lithium is worthy of review, with recommendations based on knowledge of the patient and medical science.
1. Reardon CL, Factor RM. Sport psychiatry: a systematic review of diagnosis and medical treatment of mental illness in athletes. Sports Med. 2010;40:961-980.
2. Miller EB, Pain RW, Skripal PJ. Sweat lithium in manic-depression. Br J Psychiatry. 1978;133:477-478.
3. Jefferson JW, Greist JH, Clagnaz PJ, et al. Effect of strenuous exercise on serum lithium level in man. Am J Psychiatry. 1982;139(12):1593-1595.
4. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473.
I was astonished that Dr. Melvin G. McInnis’ article on using lithium to treat bipolar disorder (BD) (Current Psychiatry, June 2014, p. 38-44; [http://bit.ly/1sszAUr]) did not address all the potential hazards of the medication. He discussed side effects, but only how to manage them so that patients will adhere to treatment.
I have used lithium for patients with BD, and often it is efficacious, although hazardous in overdose. Lithium toxicity can cause cardiac arrhythmias, and must be monitored closely. In addition, the effects of hydration and exercise on the lithium level, especially during summer, often are ignored.
Two of my patients, an adolescent and an adult, were well-maintained on lithium, adhered to treatment, and had no concurrent medical problems, but developed significant toxicity for no reason that I could determine. The adult had a lithium level of 2.0 mEq/L in the emergency room; the adolescent had a lithium level of 1.8 mEq/L. Levels this high are considered potentially lethal, and because it happened without warning and without a cause that I could determine, I consider lithium to be one of the riskier mood stabilizers. I still prescribe it, but with great caution.
Dr. McInnis also did not mention the possibility of lithium-induced diabetes insipidus, a condition in which the kidneys are no longer able to concentrate urine and that is marked by excessive urination, concomitant water intake, and low urine specific gravity. It is uncommon, but I have seen it 3 times in 30 years, in a practice that specializes in psychotherapy and does not see a high percentage of patients with BD. I consider it a condition that must be kept in mind as we follow our patients in long-term treatment.
Mary Davis, MD
Lancaster, Pennsylvania
Dr. McInnis responds
Dr. Davis raises the issue of lithium toxicity and provides examples of 2 patients who developed levels of 2.0 mEq/L and 1.8 mEq/L. These levels clearly are well beyond the toxicity threshold of 1.3 mEq/L, and the patients wisely sought urgent care. These scenarios exemplify the need for regular monitoring of the lithium level—in particular, when there is any change in physical or mental health status. Development of significant toxicity generally has some lead-time with emerging short-term side effects (outlined in Table 2 of my article), which underscores the importance of discussing the nature of emerging side effects with your patient.
Dr. Davis is correct in noting that the practitioner must be aware of long-term side effects of lithium. I find it helpful to discuss these effects with the patient in the context of short-term (days or weeks), intermediate (weeks or months), and long-term (months or years) time frames (Table 2). Diabetes insipidus is listed as an intermediate side effect.
I am grateful to Dr. Davis for raising the issue of hydration and summer heat, a concern among parents and coaches when student athletes practice strenuously for extended hours.1 Miller et al2 found that the concentration of lithium was between 1.2- and 4.6-fold in forearm sweat compared with serum levels, with the implication that heat-induced sweating may lower lithium levels. Jefferson et al3 studied 4 athletes after a 20-km race and found that all had become dehydrated but had a decrease in the serum lithium level. This is contrary to the widely held belief that excessive sweating predisposes to lithium toxicity.
BD is among the more lethal psychiatric disorders, and lithium is among the few medications shown to mitigate suicidal behavior.4 As with any medication, lithium is not without risk, and there is a clear need for informed medical management. Any notable change in health status or physical activity in a patient taking lithium is worthy of review, with recommendations based on knowledge of the patient and medical science.
I was astonished that Dr. Melvin G. McInnis’ article on using lithium to treat bipolar disorder (BD) (Current Psychiatry, June 2014, p. 38-44; [http://bit.ly/1sszAUr]) did not address all the potential hazards of the medication. He discussed side effects, but only how to manage them so that patients will adhere to treatment.
I have used lithium for patients with BD, and often it is efficacious, although hazardous in overdose. Lithium toxicity can cause cardiac arrhythmias, and must be monitored closely. In addition, the effects of hydration and exercise on the lithium level, especially during summer, often are ignored.
Two of my patients, an adolescent and an adult, were well-maintained on lithium, adhered to treatment, and had no concurrent medical problems, but developed significant toxicity for no reason that I could determine. The adult had a lithium level of 2.0 mEq/L in the emergency room; the adolescent had a lithium level of 1.8 mEq/L. Levels this high are considered potentially lethal, and because it happened without warning and without a cause that I could determine, I consider lithium to be one of the riskier mood stabilizers. I still prescribe it, but with great caution.
Dr. McInnis also did not mention the possibility of lithium-induced diabetes insipidus, a condition in which the kidneys are no longer able to concentrate urine and that is marked by excessive urination, concomitant water intake, and low urine specific gravity. It is uncommon, but I have seen it 3 times in 30 years, in a practice that specializes in psychotherapy and does not see a high percentage of patients with BD. I consider it a condition that must be kept in mind as we follow our patients in long-term treatment.
Mary Davis, MD
Lancaster, Pennsylvania
Dr. McInnis responds
Dr. Davis raises the issue of lithium toxicity and provides examples of 2 patients who developed levels of 2.0 mEq/L and 1.8 mEq/L. These levels clearly are well beyond the toxicity threshold of 1.3 mEq/L, and the patients wisely sought urgent care. These scenarios exemplify the need for regular monitoring of the lithium level—in particular, when there is any change in physical or mental health status. Development of significant toxicity generally has some lead-time with emerging short-term side effects (outlined in Table 2 of my article), which underscores the importance of discussing the nature of emerging side effects with your patient.
Dr. Davis is correct in noting that the practitioner must be aware of long-term side effects of lithium. I find it helpful to discuss these effects with the patient in the context of short-term (days or weeks), intermediate (weeks or months), and long-term (months or years) time frames (Table 2). Diabetes insipidus is listed as an intermediate side effect.
I am grateful to Dr. Davis for raising the issue of hydration and summer heat, a concern among parents and coaches when student athletes practice strenuously for extended hours.1 Miller et al2 found that the concentration of lithium was between 1.2- and 4.6-fold in forearm sweat compared with serum levels, with the implication that heat-induced sweating may lower lithium levels. Jefferson et al3 studied 4 athletes after a 20-km race and found that all had become dehydrated but had a decrease in the serum lithium level. This is contrary to the widely held belief that excessive sweating predisposes to lithium toxicity.
BD is among the more lethal psychiatric disorders, and lithium is among the few medications shown to mitigate suicidal behavior.4 As with any medication, lithium is not without risk, and there is a clear need for informed medical management. Any notable change in health status or physical activity in a patient taking lithium is worthy of review, with recommendations based on knowledge of the patient and medical science.
1. Reardon CL, Factor RM. Sport psychiatry: a systematic review of diagnosis and medical treatment of mental illness in athletes. Sports Med. 2010;40:961-980.
2. Miller EB, Pain RW, Skripal PJ. Sweat lithium in manic-depression. Br J Psychiatry. 1978;133:477-478.
3. Jefferson JW, Greist JH, Clagnaz PJ, et al. Effect of strenuous exercise on serum lithium level in man. Am J Psychiatry. 1982;139(12):1593-1595.
4. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473.
1. Reardon CL, Factor RM. Sport psychiatry: a systematic review of diagnosis and medical treatment of mental illness in athletes. Sports Med. 2010;40:961-980.
2. Miller EB, Pain RW, Skripal PJ. Sweat lithium in manic-depression. Br J Psychiatry. 1978;133:477-478.
3. Jefferson JW, Greist JH, Clagnaz PJ, et al. Effect of strenuous exercise on serum lithium level in man. Am J Psychiatry. 1982;139(12):1593-1595.
4. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473.
What makes you responsible during a screening call?
Help your patient with hoarding disorder move the clutter to the curb
Hoarding disorder (HD), categorized in DSM-5 under obsessive-compulsive and related disorders, is defined as the “persistent difficulty discarding or parting with possessions, regardless of their actual value.”1 Hoarders feel that they need to save items, and experience distress when discarding them. Prevalence of HD among the general population is 2% to 5%.
Compulsive hoarders usually keep old items in their home that they do not intend to use. In severe cases, the clutter is so great that areas of the home cannot be used or entered. Hoarders tend to isolate themselves and usually do not invite people home, perhaps because they are embarrassed about the clutter or anxious that someone might try to clean the house. Hoarders may travel long distances to collect items others have discarded.
Hoarding can lead to psychiatric disorders and social problems. Hoarders tend to not develop attachment with people because they are more attached to their possessions. They may avoid social interactions; in turn, others avoid them. This isolation can lead to depression, anxiety, and substance abuse. Hoarders may be evicted from their home if the clutter makes the house dangerous or unfit to live in it. Compulsive hoarding is detrimental to the hoarder and the health and well-being of family members. Hoarding can coexist or can be result of other psychiatric disorders (Table).
Neural mechanism in hoarding
Hoarders may start to accumulate and store large quantities of items because of a cognitive deficit, such as trouble making decisions or poor recognition or acknowledgement of the situation, or maladaptive thoughts. Tolin et al1 found the anterior cingulate cortex and insula was stimulus-dependent in patients with HD. Functional MRI showed when patients with HD were shown an item that was their possession, they exhibited an abnormal brain activity, compared with low activity when the items shown were not theirs.
Interventions
Choice of treatment depends on the age of the patient and severity of illness: behavioral, medical, or a combination of both. For an uncomplicated case, management can begin with behavioral modification.
Behavioral modifications. HD can stem from any of several variables, including greater response latency for decision-making about possessions and maladaptive beliefs about, and emotional attachment to, possessions, which can lead to intense emotional experiences about the prospect of losing those possessions.2 Cognitive-behavioral therapy has shown promising results for treating HD by addressing the aforementioned factors. A step-by-step approach usually is feasible and convenient for the therapist and patient. It involves gradual mental detachment from items to accommodate the patient’s pace.2
Pharmacotherapy. There is no clear evidence for treating HD with any particular drug. Hoarders are less likely to use psychotropics, possibly because of poor insight (eg, they do not realize the potentially dangerous living conditions hoarding creates).3 Because HD is related to obsessive-compulsive disorder, it is intuitive to consider a selective serotonin reuptake inhibitor.
There is still a need for more research on management of HD.
Disclosure
Dr. Silman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tolin DF, Stevens MC, Villavicencio AL, et al. Neural mechanism of decision making in hoarding disorder. Arch Gen Psychiatry. 2012;69(8):832-841.
2. Tolin DF, Frost RO, Steketee G. An open trial of cognitivebehavioral therapy for compulsive hoarding. Behav Res Ther. 2007;45(7):1461-1470.
3. Brakoulias V, Starcevic V, Berle D, et al. The use of psychotropic agents for the symptoms of obsessivecompulsive disorder. Australas Psychiatry. 2013;21(2): 117-121.
Hoarding disorder (HD), categorized in DSM-5 under obsessive-compulsive and related disorders, is defined as the “persistent difficulty discarding or parting with possessions, regardless of their actual value.”1 Hoarders feel that they need to save items, and experience distress when discarding them. Prevalence of HD among the general population is 2% to 5%.
Compulsive hoarders usually keep old items in their home that they do not intend to use. In severe cases, the clutter is so great that areas of the home cannot be used or entered. Hoarders tend to isolate themselves and usually do not invite people home, perhaps because they are embarrassed about the clutter or anxious that someone might try to clean the house. Hoarders may travel long distances to collect items others have discarded.
Hoarding can lead to psychiatric disorders and social problems. Hoarders tend to not develop attachment with people because they are more attached to their possessions. They may avoid social interactions; in turn, others avoid them. This isolation can lead to depression, anxiety, and substance abuse. Hoarders may be evicted from their home if the clutter makes the house dangerous or unfit to live in it. Compulsive hoarding is detrimental to the hoarder and the health and well-being of family members. Hoarding can coexist or can be result of other psychiatric disorders (Table).
Neural mechanism in hoarding
Hoarders may start to accumulate and store large quantities of items because of a cognitive deficit, such as trouble making decisions or poor recognition or acknowledgement of the situation, or maladaptive thoughts. Tolin et al1 found the anterior cingulate cortex and insula was stimulus-dependent in patients with HD. Functional MRI showed when patients with HD were shown an item that was their possession, they exhibited an abnormal brain activity, compared with low activity when the items shown were not theirs.
Interventions
Choice of treatment depends on the age of the patient and severity of illness: behavioral, medical, or a combination of both. For an uncomplicated case, management can begin with behavioral modification.
Behavioral modifications. HD can stem from any of several variables, including greater response latency for decision-making about possessions and maladaptive beliefs about, and emotional attachment to, possessions, which can lead to intense emotional experiences about the prospect of losing those possessions.2 Cognitive-behavioral therapy has shown promising results for treating HD by addressing the aforementioned factors. A step-by-step approach usually is feasible and convenient for the therapist and patient. It involves gradual mental detachment from items to accommodate the patient’s pace.2
Pharmacotherapy. There is no clear evidence for treating HD with any particular drug. Hoarders are less likely to use psychotropics, possibly because of poor insight (eg, they do not realize the potentially dangerous living conditions hoarding creates).3 Because HD is related to obsessive-compulsive disorder, it is intuitive to consider a selective serotonin reuptake inhibitor.
There is still a need for more research on management of HD.
Disclosure
Dr. Silman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Hoarding disorder (HD), categorized in DSM-5 under obsessive-compulsive and related disorders, is defined as the “persistent difficulty discarding or parting with possessions, regardless of their actual value.”1 Hoarders feel that they need to save items, and experience distress when discarding them. Prevalence of HD among the general population is 2% to 5%.
Compulsive hoarders usually keep old items in their home that they do not intend to use. In severe cases, the clutter is so great that areas of the home cannot be used or entered. Hoarders tend to isolate themselves and usually do not invite people home, perhaps because they are embarrassed about the clutter or anxious that someone might try to clean the house. Hoarders may travel long distances to collect items others have discarded.
Hoarding can lead to psychiatric disorders and social problems. Hoarders tend to not develop attachment with people because they are more attached to their possessions. They may avoid social interactions; in turn, others avoid them. This isolation can lead to depression, anxiety, and substance abuse. Hoarders may be evicted from their home if the clutter makes the house dangerous or unfit to live in it. Compulsive hoarding is detrimental to the hoarder and the health and well-being of family members. Hoarding can coexist or can be result of other psychiatric disorders (Table).
Neural mechanism in hoarding
Hoarders may start to accumulate and store large quantities of items because of a cognitive deficit, such as trouble making decisions or poor recognition or acknowledgement of the situation, or maladaptive thoughts. Tolin et al1 found the anterior cingulate cortex and insula was stimulus-dependent in patients with HD. Functional MRI showed when patients with HD were shown an item that was their possession, they exhibited an abnormal brain activity, compared with low activity when the items shown were not theirs.
Interventions
Choice of treatment depends on the age of the patient and severity of illness: behavioral, medical, or a combination of both. For an uncomplicated case, management can begin with behavioral modification.
Behavioral modifications. HD can stem from any of several variables, including greater response latency for decision-making about possessions and maladaptive beliefs about, and emotional attachment to, possessions, which can lead to intense emotional experiences about the prospect of losing those possessions.2 Cognitive-behavioral therapy has shown promising results for treating HD by addressing the aforementioned factors. A step-by-step approach usually is feasible and convenient for the therapist and patient. It involves gradual mental detachment from items to accommodate the patient’s pace.2
Pharmacotherapy. There is no clear evidence for treating HD with any particular drug. Hoarders are less likely to use psychotropics, possibly because of poor insight (eg, they do not realize the potentially dangerous living conditions hoarding creates).3 Because HD is related to obsessive-compulsive disorder, it is intuitive to consider a selective serotonin reuptake inhibitor.
There is still a need for more research on management of HD.
Disclosure
Dr. Silman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tolin DF, Stevens MC, Villavicencio AL, et al. Neural mechanism of decision making in hoarding disorder. Arch Gen Psychiatry. 2012;69(8):832-841.
2. Tolin DF, Frost RO, Steketee G. An open trial of cognitivebehavioral therapy for compulsive hoarding. Behav Res Ther. 2007;45(7):1461-1470.
3. Brakoulias V, Starcevic V, Berle D, et al. The use of psychotropic agents for the symptoms of obsessivecompulsive disorder. Australas Psychiatry. 2013;21(2): 117-121.
1. Tolin DF, Stevens MC, Villavicencio AL, et al. Neural mechanism of decision making in hoarding disorder. Arch Gen Psychiatry. 2012;69(8):832-841.
2. Tolin DF, Frost RO, Steketee G. An open trial of cognitivebehavioral therapy for compulsive hoarding. Behav Res Ther. 2007;45(7):1461-1470.
3. Brakoulias V, Starcevic V, Berle D, et al. The use of psychotropic agents for the symptoms of obsessivecompulsive disorder. Australas Psychiatry. 2013;21(2): 117-121.
8 tests rolled into a mnemonic to detect weakness in suspected conversion disorder
DSM-5 criteria for conversion disorder (or functional neurological symptom disorder) requires findings that are incompatible with recognized neurologic or medical conditions.1 Knowledge of signs specific to conversion disorder may help you diagnose the illness with confidence.
We review signs suggestive of conversion disorder. These can be remembered using the mnemonic How About Finding Some Conversion Weakness [in an otherwise] Strong Guy/Gal? (Table2).
Inconsistencies in motor function can be observed on examination. Signs may be consciously or unconsciously produced. Although most of the tests mentioned have high positive and negative predictive values (noted in the Table2) they have limited sensitivity and specificity,3 and the presence of a positive sign does not exclude the possibility of comorbid disease.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM- 5: conversion disorder. Am J Psychiatry. 2010;167(6):626-627.
2. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85(2):180-190.
3. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
DSM-5 criteria for conversion disorder (or functional neurological symptom disorder) requires findings that are incompatible with recognized neurologic or medical conditions.1 Knowledge of signs specific to conversion disorder may help you diagnose the illness with confidence.
We review signs suggestive of conversion disorder. These can be remembered using the mnemonic How About Finding Some Conversion Weakness [in an otherwise] Strong Guy/Gal? (Table2).
Inconsistencies in motor function can be observed on examination. Signs may be consciously or unconsciously produced. Although most of the tests mentioned have high positive and negative predictive values (noted in the Table2) they have limited sensitivity and specificity,3 and the presence of a positive sign does not exclude the possibility of comorbid disease.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
DSM-5 criteria for conversion disorder (or functional neurological symptom disorder) requires findings that are incompatible with recognized neurologic or medical conditions.1 Knowledge of signs specific to conversion disorder may help you diagnose the illness with confidence.
We review signs suggestive of conversion disorder. These can be remembered using the mnemonic How About Finding Some Conversion Weakness [in an otherwise] Strong Guy/Gal? (Table2).
Inconsistencies in motor function can be observed on examination. Signs may be consciously or unconsciously produced. Although most of the tests mentioned have high positive and negative predictive values (noted in the Table2) they have limited sensitivity and specificity,3 and the presence of a positive sign does not exclude the possibility of comorbid disease.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM- 5: conversion disorder. Am J Psychiatry. 2010;167(6):626-627.
2. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85(2):180-190.
3. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
1. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM- 5: conversion disorder. Am J Psychiatry. 2010;167(6):626-627.
2. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85(2):180-190.
3. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
What are your responsibilities after a screening call?
Dear Dr. Mossman,
When I take a call from a treatment-seeker at our outpatient clinic, I ask brief screening questions to determine whether our services would be appropriate. Shortly after I screened one caller, Ms. C, she called back requesting a medication refill and asking about her diagnosis.
What obligation do I have to Ms. C? Is she my patient? Would I be liable if I didn’t help her out and something bad happened to her?
Submitted by “Dr. S”
Office and hospital Web sites, LinkedIn profiles, and Facebook pages are just a few of the ways that people find physicians and learn about their services. But most 21st century doctor-patient relationships still start with 19th century technology: a telephone call.
Talking with prospective patients before setting up an appointment makes sense. A short conversation can clarify whether you offer the services that a caller needs and increases the show-up rate for initial appointments.1
But if you ask for some personal history and information about symptoms in a screening interview, does that make the caller your patient? Ms. C seemed to have thought so. To find out whether Ms. C was right and to learn how Dr. S should handle initial telephone calls, we’ll look at:
• the rationale for screening callers before initiating treatment
• features of screening that can create a doctor-patient relationship
• how to fulfill duties that result from screening.
Why screen prospective patients?
Mental health treatment has become more diversified and specialized over the past 30 years. No psychiatrist nowadays has all the therapeutic skills that all potential patients might need.
Before speaking to you, a treatment-seeker often won’t know whether your practice style will fit his (her) needs. You might prefer not to provide medication management for another clinician’s psychotherapy patient or, if you’re like most psychiatrists, you might not offer psychotherapy.
In the absence of prior obligation (eg, agreeing to provide coverage for an emergency room), physicians may structure their practices and contract for their services as they see fit2—but this leaves you with some obligation to screen potential patients for appropriate mutual fit. In years past, some psychiatrists saw potential patients for an in-office evaluation to decide whether to provide treatment—a practicethat remains acceptable if the person is told, when the appointment is made, that the first meeting is “to meet each other and see if you want to establish a treatment relationship.”3
Good treatment plans take into account patients’ temperament, emotional state, cognitive capacity, culture, family circumstances, substance use, and medical history.4 Common mental conditions often can be identified in a telephone call.5,6 Although the diagnostic accuracy of such efforts is uncertain,7 such calls can help practitioners determine whether they offer the right services for callers. Good decisions about initiating care always take financial pressures and constraints into account,8 and a pre-appointment telephone call can address those issues, too.
For all these reasons, talking to a prospective patient before he comes to see you makes sense. Screening lets you decide:
• whether you’re the right clinician for his needs
• who the right clinician is if you are not
• whether he should seek emergency evaluation when the situation sounds urgent.
Do phone calls start treatment?
As Dr. S’s questions show, telephone screenings might leave some callers thinking that treatment has started, even before their first office appointment. Having a treatment relationship is a prerequisite to malpractice liability,9 and courts have concluded that, under the right circumstances, telephone assessments do create physician-patient relationships.
Creating a physician-patient relationship
How or when might telephone screening make someone your patient? This question doesn’t have a precise answer, but how courts decided similar questions has depended on the questions the physician asked and whether the physician offered what sounded like medical advice.10,11 A physician-patient relationship forms when the physician takes some implied or affirmative action to treat, see, examine, care for, or offer a diagnosis to the patient,9,12,13 such as:
• knowingly accepting someone as a patient14
• explicitly agreeing to treat a person
• “acting in some other way such that the patient might reasonably be led to assume a doctor-patient relationship has been established.”15
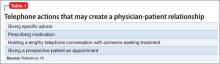
Also, the “fact that a physician does not deal directly with a patient does not necessarily preclude the existence of a physician-patient relationship,”12 so a telephone conversation can create such a relationship if it contains the right elements. Table 116 highlights actions that, during the course of screening, might constitute initiation of a physician-patient relationship. Table 2 offers suggestions for managing initial telephone contacts to reduce the chance of inadvertently creating a physician-patient relationship.
In the eyes of the law, whether a physician-patient relationship was formed depends on specific facts of the situation and may be decided by a jury.13,14 In the case of Ms. C, Dr. S might avoid premature creation of a physician-patient relationship by refraining from offering a diagnosis at the conclusion of the screening call.17
Prescribing
Although features of the original screening interview indicated that Ms. C was not yet Dr. S’s patient, prescribing certainly would commence a physician-patient relationship.18 But even if the screening had made Ms. C a patient, refilling her prescription now probably is a bad idea.
Assuming that a physician-patient relationship exists, it is unlikely that a short telephone interview gave Dr. S enough information about Ms. C’s medical history and present mental status to ensure that his diagnostic reasoning would not be faulty. It also is unlikely that telephone screening allowed Dr. S to meet the standard of care for prescribing—a process that involves choosing medications suitable to the patient’s clinical needs, checking the results of any necessary lab tests, and obtaining appropriate informed consent.19
Satisfying duties
Outpatient facilities can instruct telephone screeners to conduct interviews in ways that reduce inadvertent establishment of a treatment relationship, but establishing such a relationship cannot be avoided in all cases. If a caller is distraught or in crisis, for example, compassion dictates helping him, and some callers (eg, Ms. C) may feel they have a firmer treatment relationship than actually exists.
Once you have created a physician-patient relationship, you must continue that relationship until you end it appropriately.3 That does not mean you have to provide definitive treatment; you simply need to exercise “reasonable care according to the standards of the profession.”16,20 If a caller telephones in an emergency situation, for example, the screening clinician should take appropriate steps to ensure safety, which might include calling law enforcement or facilitating hospitalization.3
One way to fulfill the duties of a physician-patient relationship inadvertently established during initial screening is through explicit discharge (if medically appropriate) or transfer of care to another physician.15 A prudent clinic or practitioner will describe other mental health resources in the community and sometimes assist with referral if the inquiring potential patient needs services that the provider does not offer.
In many communities, finding appropriate mental health resources is difficult. Creative approaches to this problem include transitional psychiatry or crisis support clinics that serve as a “bridge” to longer-term services,21,22 preliminary process groups,23 and telepsychiatry transitional clinics.24 When a clinic does not accept a person as a patient, the clinic should clearly document 1) key features of the contact and 2) the rationale for that decision
Bottom Line
You have a right and a responsibility to screen prospective patients for good fit to your treatment services. In doing so, however, you might inadvertently create a physician-patient relationship. If this happens, you should fulfill your clinical responsibilities, as you would for any patient, by helping the patient get appropriate care from you or another provider.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Shoffner J, Staudt M, Marcus S, et al. Using telephone reminders to increase attendance at psychiatric appointments: findings of a pilot study in rural Appalachia. Psychiatr Serv. 2007;58(6):872-875.
2. Hiser v Randolph, 1980 617 P2d 774 (Ariz App).
3. American Psychiatric Association. Practice management for early career psychiatrists: a reference guide, 6th edition. http://www.psych.org/practice/managing-a-practice/ starting-a-practice. Published October 16, 2006. Accessed July 8, 2014.
4. Delgado SV, Strawn JR. Difficult psychiatric consultations: an integrated approach. New York, NY: Springer; 2014.
5. Aziz MA, Kenford S. Comparability of telephone and face-to-face interviews in assessing patients with posttraumatic stress disorder. J Psychiatric Pract. 2004;10(5): 307-313.
6. Michel C, Schimmelmann BG, Kupferschmid S, et al. Reliability of telephone assessments of at-risk criteria of psychosis: a comparison to face-to-face interviews. Schizophr Res. 2014;153(1-3):251-253.
7. Muskens EM, Lucassen P, Groenleer W, et al. Psychiatric diagnosis by telephone: is it an opportunity [published online March 15, 2014]? Soc Psychiatry Psychiatr Epidemiol. doi: 10.1007/s00127-014-0861-9.
8. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802.
9. Roberts v Sankey, 2004 813 NE2d 1195 (Ind App).
10. O’Neill v Montefiore Hospital, 1960 202 NYS 2d 436 (NY App).
11. McKinney v Schlatter, 1997 692 NE2d 1045 (Ohio App).
12. Dehn v Edgecombe, 865 A2d 603 (Md 2005).
13. Kelley v Middle Tennessee Emergency Physicians, 133 SW3d 587 (Tenn 2004).
14. Oliver v Brock, 342 So2d 1 (Ala 1976).
15. Appelbaum PS, Gutheil TG. Malpractice and other forms of liability. In: Appelbaum PS, Gutheil TG, eds. Clinical Handbook of Psychiatry and the Law, 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2007:115-116.
16. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
17. Torres A, Wagner R. Establishing the physician-patient relationship. J Dermatol Surg Oncol. 1993;19(2):147-149.
18. Aboff BM, Collier VU, Farber NJ, et al. Residents’ prescription writing for nonpatients. JAMA. 2002;288(3):381-385.
19. Edersheim JG, Stern TA. Liability associated with prescribing medications. Prim Care Companion J Clin Psychiatry. 2009;11(3):115-119.
20. Brown v Koulizakis, 331 SE2d 440 (Va 1985).
21. University of Michigan Department of Psychiatry. Crisis support clinic. http://www.psych.med.umich.edu/patient-care/crisis-support-clinic. Accessed July 9, 2014.
22. UAB Department of Psychiatry. http://www.uab.edu/ medicine/psychiatry. Accessed July 9, 2014.
23. Stone WN, Klein EB. The waiting-list group. Int J Group Psychother. 1999;49(4):417-428.
24. Detweiler MB, Arif S, Candelario J, et al. A telepsychiatry transition clinic: the first 12 months experience. J Telemed Telecare. 2011;17(6):293-297.
Dear Dr. Mossman,
When I take a call from a treatment-seeker at our outpatient clinic, I ask brief screening questions to determine whether our services would be appropriate. Shortly after I screened one caller, Ms. C, she called back requesting a medication refill and asking about her diagnosis.
What obligation do I have to Ms. C? Is she my patient? Would I be liable if I didn’t help her out and something bad happened to her?
Submitted by “Dr. S”
Office and hospital Web sites, LinkedIn profiles, and Facebook pages are just a few of the ways that people find physicians and learn about their services. But most 21st century doctor-patient relationships still start with 19th century technology: a telephone call.
Talking with prospective patients before setting up an appointment makes sense. A short conversation can clarify whether you offer the services that a caller needs and increases the show-up rate for initial appointments.1
But if you ask for some personal history and information about symptoms in a screening interview, does that make the caller your patient? Ms. C seemed to have thought so. To find out whether Ms. C was right and to learn how Dr. S should handle initial telephone calls, we’ll look at:
• the rationale for screening callers before initiating treatment
• features of screening that can create a doctor-patient relationship
• how to fulfill duties that result from screening.
Why screen prospective patients?
Mental health treatment has become more diversified and specialized over the past 30 years. No psychiatrist nowadays has all the therapeutic skills that all potential patients might need.
Before speaking to you, a treatment-seeker often won’t know whether your practice style will fit his (her) needs. You might prefer not to provide medication management for another clinician’s psychotherapy patient or, if you’re like most psychiatrists, you might not offer psychotherapy.
In the absence of prior obligation (eg, agreeing to provide coverage for an emergency room), physicians may structure their practices and contract for their services as they see fit2—but this leaves you with some obligation to screen potential patients for appropriate mutual fit. In years past, some psychiatrists saw potential patients for an in-office evaluation to decide whether to provide treatment—a practicethat remains acceptable if the person is told, when the appointment is made, that the first meeting is “to meet each other and see if you want to establish a treatment relationship.”3
Good treatment plans take into account patients’ temperament, emotional state, cognitive capacity, culture, family circumstances, substance use, and medical history.4 Common mental conditions often can be identified in a telephone call.5,6 Although the diagnostic accuracy of such efforts is uncertain,7 such calls can help practitioners determine whether they offer the right services for callers. Good decisions about initiating care always take financial pressures and constraints into account,8 and a pre-appointment telephone call can address those issues, too.
For all these reasons, talking to a prospective patient before he comes to see you makes sense. Screening lets you decide:
• whether you’re the right clinician for his needs
• who the right clinician is if you are not
• whether he should seek emergency evaluation when the situation sounds urgent.
Do phone calls start treatment?
As Dr. S’s questions show, telephone screenings might leave some callers thinking that treatment has started, even before their first office appointment. Having a treatment relationship is a prerequisite to malpractice liability,9 and courts have concluded that, under the right circumstances, telephone assessments do create physician-patient relationships.
Creating a physician-patient relationship
How or when might telephone screening make someone your patient? This question doesn’t have a precise answer, but how courts decided similar questions has depended on the questions the physician asked and whether the physician offered what sounded like medical advice.10,11 A physician-patient relationship forms when the physician takes some implied or affirmative action to treat, see, examine, care for, or offer a diagnosis to the patient,9,12,13 such as:
• knowingly accepting someone as a patient14
• explicitly agreeing to treat a person
• “acting in some other way such that the patient might reasonably be led to assume a doctor-patient relationship has been established.”15
Also, the “fact that a physician does not deal directly with a patient does not necessarily preclude the existence of a physician-patient relationship,”12 so a telephone conversation can create such a relationship if it contains the right elements. Table 116 highlights actions that, during the course of screening, might constitute initiation of a physician-patient relationship. Table 2 offers suggestions for managing initial telephone contacts to reduce the chance of inadvertently creating a physician-patient relationship.
In the eyes of the law, whether a physician-patient relationship was formed depends on specific facts of the situation and may be decided by a jury.13,14 In the case of Ms. C, Dr. S might avoid premature creation of a physician-patient relationship by refraining from offering a diagnosis at the conclusion of the screening call.17
Prescribing
Although features of the original screening interview indicated that Ms. C was not yet Dr. S’s patient, prescribing certainly would commence a physician-patient relationship.18 But even if the screening had made Ms. C a patient, refilling her prescription now probably is a bad idea.
Assuming that a physician-patient relationship exists, it is unlikely that a short telephone interview gave Dr. S enough information about Ms. C’s medical history and present mental status to ensure that his diagnostic reasoning would not be faulty. It also is unlikely that telephone screening allowed Dr. S to meet the standard of care for prescribing—a process that involves choosing medications suitable to the patient’s clinical needs, checking the results of any necessary lab tests, and obtaining appropriate informed consent.19
Satisfying duties
Outpatient facilities can instruct telephone screeners to conduct interviews in ways that reduce inadvertent establishment of a treatment relationship, but establishing such a relationship cannot be avoided in all cases. If a caller is distraught or in crisis, for example, compassion dictates helping him, and some callers (eg, Ms. C) may feel they have a firmer treatment relationship than actually exists.
Once you have created a physician-patient relationship, you must continue that relationship until you end it appropriately.3 That does not mean you have to provide definitive treatment; you simply need to exercise “reasonable care according to the standards of the profession.”16,20 If a caller telephones in an emergency situation, for example, the screening clinician should take appropriate steps to ensure safety, which might include calling law enforcement or facilitating hospitalization.3
One way to fulfill the duties of a physician-patient relationship inadvertently established during initial screening is through explicit discharge (if medically appropriate) or transfer of care to another physician.15 A prudent clinic or practitioner will describe other mental health resources in the community and sometimes assist with referral if the inquiring potential patient needs services that the provider does not offer.
In many communities, finding appropriate mental health resources is difficult. Creative approaches to this problem include transitional psychiatry or crisis support clinics that serve as a “bridge” to longer-term services,21,22 preliminary process groups,23 and telepsychiatry transitional clinics.24 When a clinic does not accept a person as a patient, the clinic should clearly document 1) key features of the contact and 2) the rationale for that decision
Bottom Line
You have a right and a responsibility to screen prospective patients for good fit to your treatment services. In doing so, however, you might inadvertently create a physician-patient relationship. If this happens, you should fulfill your clinical responsibilities, as you would for any patient, by helping the patient get appropriate care from you or another provider.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dear Dr. Mossman,
When I take a call from a treatment-seeker at our outpatient clinic, I ask brief screening questions to determine whether our services would be appropriate. Shortly after I screened one caller, Ms. C, she called back requesting a medication refill and asking about her diagnosis.
What obligation do I have to Ms. C? Is she my patient? Would I be liable if I didn’t help her out and something bad happened to her?
Submitted by “Dr. S”
Office and hospital Web sites, LinkedIn profiles, and Facebook pages are just a few of the ways that people find physicians and learn about their services. But most 21st century doctor-patient relationships still start with 19th century technology: a telephone call.
Talking with prospective patients before setting up an appointment makes sense. A short conversation can clarify whether you offer the services that a caller needs and increases the show-up rate for initial appointments.1
But if you ask for some personal history and information about symptoms in a screening interview, does that make the caller your patient? Ms. C seemed to have thought so. To find out whether Ms. C was right and to learn how Dr. S should handle initial telephone calls, we’ll look at:
• the rationale for screening callers before initiating treatment
• features of screening that can create a doctor-patient relationship
• how to fulfill duties that result from screening.
Why screen prospective patients?
Mental health treatment has become more diversified and specialized over the past 30 years. No psychiatrist nowadays has all the therapeutic skills that all potential patients might need.
Before speaking to you, a treatment-seeker often won’t know whether your practice style will fit his (her) needs. You might prefer not to provide medication management for another clinician’s psychotherapy patient or, if you’re like most psychiatrists, you might not offer psychotherapy.
In the absence of prior obligation (eg, agreeing to provide coverage for an emergency room), physicians may structure their practices and contract for their services as they see fit2—but this leaves you with some obligation to screen potential patients for appropriate mutual fit. In years past, some psychiatrists saw potential patients for an in-office evaluation to decide whether to provide treatment—a practicethat remains acceptable if the person is told, when the appointment is made, that the first meeting is “to meet each other and see if you want to establish a treatment relationship.”3
Good treatment plans take into account patients’ temperament, emotional state, cognitive capacity, culture, family circumstances, substance use, and medical history.4 Common mental conditions often can be identified in a telephone call.5,6 Although the diagnostic accuracy of such efforts is uncertain,7 such calls can help practitioners determine whether they offer the right services for callers. Good decisions about initiating care always take financial pressures and constraints into account,8 and a pre-appointment telephone call can address those issues, too.
For all these reasons, talking to a prospective patient before he comes to see you makes sense. Screening lets you decide:
• whether you’re the right clinician for his needs
• who the right clinician is if you are not
• whether he should seek emergency evaluation when the situation sounds urgent.
Do phone calls start treatment?
As Dr. S’s questions show, telephone screenings might leave some callers thinking that treatment has started, even before their first office appointment. Having a treatment relationship is a prerequisite to malpractice liability,9 and courts have concluded that, under the right circumstances, telephone assessments do create physician-patient relationships.
Creating a physician-patient relationship
How or when might telephone screening make someone your patient? This question doesn’t have a precise answer, but how courts decided similar questions has depended on the questions the physician asked and whether the physician offered what sounded like medical advice.10,11 A physician-patient relationship forms when the physician takes some implied or affirmative action to treat, see, examine, care for, or offer a diagnosis to the patient,9,12,13 such as:
• knowingly accepting someone as a patient14
• explicitly agreeing to treat a person
• “acting in some other way such that the patient might reasonably be led to assume a doctor-patient relationship has been established.”15
Also, the “fact that a physician does not deal directly with a patient does not necessarily preclude the existence of a physician-patient relationship,”12 so a telephone conversation can create such a relationship if it contains the right elements. Table 116 highlights actions that, during the course of screening, might constitute initiation of a physician-patient relationship. Table 2 offers suggestions for managing initial telephone contacts to reduce the chance of inadvertently creating a physician-patient relationship.
In the eyes of the law, whether a physician-patient relationship was formed depends on specific facts of the situation and may be decided by a jury.13,14 In the case of Ms. C, Dr. S might avoid premature creation of a physician-patient relationship by refraining from offering a diagnosis at the conclusion of the screening call.17
Prescribing
Although features of the original screening interview indicated that Ms. C was not yet Dr. S’s patient, prescribing certainly would commence a physician-patient relationship.18 But even if the screening had made Ms. C a patient, refilling her prescription now probably is a bad idea.
Assuming that a physician-patient relationship exists, it is unlikely that a short telephone interview gave Dr. S enough information about Ms. C’s medical history and present mental status to ensure that his diagnostic reasoning would not be faulty. It also is unlikely that telephone screening allowed Dr. S to meet the standard of care for prescribing—a process that involves choosing medications suitable to the patient’s clinical needs, checking the results of any necessary lab tests, and obtaining appropriate informed consent.19
Satisfying duties
Outpatient facilities can instruct telephone screeners to conduct interviews in ways that reduce inadvertent establishment of a treatment relationship, but establishing such a relationship cannot be avoided in all cases. If a caller is distraught or in crisis, for example, compassion dictates helping him, and some callers (eg, Ms. C) may feel they have a firmer treatment relationship than actually exists.
Once you have created a physician-patient relationship, you must continue that relationship until you end it appropriately.3 That does not mean you have to provide definitive treatment; you simply need to exercise “reasonable care according to the standards of the profession.”16,20 If a caller telephones in an emergency situation, for example, the screening clinician should take appropriate steps to ensure safety, which might include calling law enforcement or facilitating hospitalization.3
One way to fulfill the duties of a physician-patient relationship inadvertently established during initial screening is through explicit discharge (if medically appropriate) or transfer of care to another physician.15 A prudent clinic or practitioner will describe other mental health resources in the community and sometimes assist with referral if the inquiring potential patient needs services that the provider does not offer.
In many communities, finding appropriate mental health resources is difficult. Creative approaches to this problem include transitional psychiatry or crisis support clinics that serve as a “bridge” to longer-term services,21,22 preliminary process groups,23 and telepsychiatry transitional clinics.24 When a clinic does not accept a person as a patient, the clinic should clearly document 1) key features of the contact and 2) the rationale for that decision
Bottom Line
You have a right and a responsibility to screen prospective patients for good fit to your treatment services. In doing so, however, you might inadvertently create a physician-patient relationship. If this happens, you should fulfill your clinical responsibilities, as you would for any patient, by helping the patient get appropriate care from you or another provider.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Shoffner J, Staudt M, Marcus S, et al. Using telephone reminders to increase attendance at psychiatric appointments: findings of a pilot study in rural Appalachia. Psychiatr Serv. 2007;58(6):872-875.
2. Hiser v Randolph, 1980 617 P2d 774 (Ariz App).
3. American Psychiatric Association. Practice management for early career psychiatrists: a reference guide, 6th edition. http://www.psych.org/practice/managing-a-practice/ starting-a-practice. Published October 16, 2006. Accessed July 8, 2014.
4. Delgado SV, Strawn JR. Difficult psychiatric consultations: an integrated approach. New York, NY: Springer; 2014.
5. Aziz MA, Kenford S. Comparability of telephone and face-to-face interviews in assessing patients with posttraumatic stress disorder. J Psychiatric Pract. 2004;10(5): 307-313.
6. Michel C, Schimmelmann BG, Kupferschmid S, et al. Reliability of telephone assessments of at-risk criteria of psychosis: a comparison to face-to-face interviews. Schizophr Res. 2014;153(1-3):251-253.
7. Muskens EM, Lucassen P, Groenleer W, et al. Psychiatric diagnosis by telephone: is it an opportunity [published online March 15, 2014]? Soc Psychiatry Psychiatr Epidemiol. doi: 10.1007/s00127-014-0861-9.
8. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802.
9. Roberts v Sankey, 2004 813 NE2d 1195 (Ind App).
10. O’Neill v Montefiore Hospital, 1960 202 NYS 2d 436 (NY App).
11. McKinney v Schlatter, 1997 692 NE2d 1045 (Ohio App).
12. Dehn v Edgecombe, 865 A2d 603 (Md 2005).
13. Kelley v Middle Tennessee Emergency Physicians, 133 SW3d 587 (Tenn 2004).
14. Oliver v Brock, 342 So2d 1 (Ala 1976).
15. Appelbaum PS, Gutheil TG. Malpractice and other forms of liability. In: Appelbaum PS, Gutheil TG, eds. Clinical Handbook of Psychiatry and the Law, 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2007:115-116.
16. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
17. Torres A, Wagner R. Establishing the physician-patient relationship. J Dermatol Surg Oncol. 1993;19(2):147-149.
18. Aboff BM, Collier VU, Farber NJ, et al. Residents’ prescription writing for nonpatients. JAMA. 2002;288(3):381-385.
19. Edersheim JG, Stern TA. Liability associated with prescribing medications. Prim Care Companion J Clin Psychiatry. 2009;11(3):115-119.
20. Brown v Koulizakis, 331 SE2d 440 (Va 1985).
21. University of Michigan Department of Psychiatry. Crisis support clinic. http://www.psych.med.umich.edu/patient-care/crisis-support-clinic. Accessed July 9, 2014.
22. UAB Department of Psychiatry. http://www.uab.edu/ medicine/psychiatry. Accessed July 9, 2014.
23. Stone WN, Klein EB. The waiting-list group. Int J Group Psychother. 1999;49(4):417-428.
24. Detweiler MB, Arif S, Candelario J, et al. A telepsychiatry transition clinic: the first 12 months experience. J Telemed Telecare. 2011;17(6):293-297.
1. Shoffner J, Staudt M, Marcus S, et al. Using telephone reminders to increase attendance at psychiatric appointments: findings of a pilot study in rural Appalachia. Psychiatr Serv. 2007;58(6):872-875.
2. Hiser v Randolph, 1980 617 P2d 774 (Ariz App).
3. American Psychiatric Association. Practice management for early career psychiatrists: a reference guide, 6th edition. http://www.psych.org/practice/managing-a-practice/ starting-a-practice. Published October 16, 2006. Accessed July 8, 2014.
4. Delgado SV, Strawn JR. Difficult psychiatric consultations: an integrated approach. New York, NY: Springer; 2014.
5. Aziz MA, Kenford S. Comparability of telephone and face-to-face interviews in assessing patients with posttraumatic stress disorder. J Psychiatric Pract. 2004;10(5): 307-313.
6. Michel C, Schimmelmann BG, Kupferschmid S, et al. Reliability of telephone assessments of at-risk criteria of psychosis: a comparison to face-to-face interviews. Schizophr Res. 2014;153(1-3):251-253.
7. Muskens EM, Lucassen P, Groenleer W, et al. Psychiatric diagnosis by telephone: is it an opportunity [published online March 15, 2014]? Soc Psychiatry Psychiatr Epidemiol. doi: 10.1007/s00127-014-0861-9.
8. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802.
9. Roberts v Sankey, 2004 813 NE2d 1195 (Ind App).
10. O’Neill v Montefiore Hospital, 1960 202 NYS 2d 436 (NY App).
11. McKinney v Schlatter, 1997 692 NE2d 1045 (Ohio App).
12. Dehn v Edgecombe, 865 A2d 603 (Md 2005).
13. Kelley v Middle Tennessee Emergency Physicians, 133 SW3d 587 (Tenn 2004).
14. Oliver v Brock, 342 So2d 1 (Ala 1976).
15. Appelbaum PS, Gutheil TG. Malpractice and other forms of liability. In: Appelbaum PS, Gutheil TG, eds. Clinical Handbook of Psychiatry and the Law, 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2007:115-116.
16. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
17. Torres A, Wagner R. Establishing the physician-patient relationship. J Dermatol Surg Oncol. 1993;19(2):147-149.
18. Aboff BM, Collier VU, Farber NJ, et al. Residents’ prescription writing for nonpatients. JAMA. 2002;288(3):381-385.
19. Edersheim JG, Stern TA. Liability associated with prescribing medications. Prim Care Companion J Clin Psychiatry. 2009;11(3):115-119.
20. Brown v Koulizakis, 331 SE2d 440 (Va 1985).
21. University of Michigan Department of Psychiatry. Crisis support clinic. http://www.psych.med.umich.edu/patient-care/crisis-support-clinic. Accessed July 9, 2014.
22. UAB Department of Psychiatry. http://www.uab.edu/ medicine/psychiatry. Accessed July 9, 2014.
23. Stone WN, Klein EB. The waiting-list group. Int J Group Psychother. 1999;49(4):417-428.
24. Detweiler MB, Arif S, Candelario J, et al. A telepsychiatry transition clinic: the first 12 months experience. J Telemed Telecare. 2011;17(6):293-297.
A young man with psychosis whose heart is racing
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
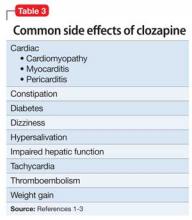
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
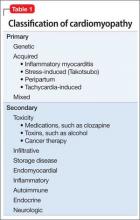
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.
Blueprint for building a psychiatrist: How residency has prepared us
Becoming a psychiatrist entails a shift in how we see ourselves and those around us. We learn—sometimes the hard way—about cultural self-assessment and the relative nature of our perspective on social, cultural, and clinical matters. What do I mean?
As psychiatrists in the making, we are unaware that we’ve been given this persona-suit, so to speak, with its social expectations and misperceptions. We start noticing how telling people what we do shapes our interactions at cocktail parties, informal gatherings, and in day-to-day life. A new acquaintance might disclose more about herself than she otherwise would or, on the contrary, might become reserved, even guarded. Awkward jokes sometimes are thrown into the mix to lighten the mood. All this is part of the package we’ve been handed, because we chose to specialize in the diagnosis and treatment of mental illness and brain disorders.
So, as I enter my final year of training, I find myself reflecting on just how intense a journey residency has been.
We were physicians first…
We’re psychiatrists now, but first we learned the germ theory of disease, the pathophysiology of every well-known illness, and the scientific basis of the practice of medicine. Many of us weren’t fully aware of the challenges that come with psychiatric training when we signed up. But we powered through— trading set measures and laboratory values for subjective experiences and nonverbal cues. Along the way, we realized that we had to master not only an array of neuropsychiatric facts but other implicit skills: “active listening,” the capacity to make on-the-go complex ethical decisions, and the difficult task of being empathetically detached.
It might be only in retrospect that we can appreciate how residency has shaped us in a personal way—almost as much as it has professionally.
We think of physicians broadly as healers who save lives. Psychiatrists are no different; preventing the most hopeless from dying is something that we do the same way a cardiologist prevents a patient from dying of a massive heart attack. Winning the battle over mortality, by whatever imprecise measures of risk we use, ranks at the top of our therapeutic priorities. We find ourselves scrambling so that catastrophe never happens on our watch. Sometimes, we don’t stop to realize how much of a lifesaver we are— especially because, as junior residents, we’re too pressed for time to reflect and are focused on mastering clinical skills.
New tool to measure success in residency
The Accreditation Council for Graduate Medical Education (ACGME) recently released the “Milestones Project,”1 a thorough evaluation system for residency programs to apply to their trainees. This is a great effort to push for more field-specific evaluation measures among the specialties.
In psychiatry, subjects now considered when evaluating a resident’s progress and preparedness for promotion include competence in applied neuroscience; the practitioner’s emotional response to patients’ problems; and regulatory compliance. Ways doctors learn are changing: Emphasis is now on problem-based learning.2 Patient safety is a priority; to respect that, we are betting strongly on the physician’s aptitude to provide good care by decreasing burnout.3 I am pleased to learn that there are ongoing efforts to improve the way we prepare psychiatrists.
In line with ACGME practices, residency programs also need to continue revisiting their didactic curricula to include innovative, emerging topics. Social media, the antipsychiatry movement, Internet forums, opinionated bloggers, and public figures gone viral—these are some current issues that shouldn’t be ignored during training just because they aren’t discussed in texts or academic journals. Programs that teach and stimulate the inclusion of social sciences and critical thinking should yield better, more holistic psychiatrists.4
For me, these avenues of study have made a huge difference. I feel incredibly grateful for the opportunities that my residency program has provided to me as a psychiatrist-in-training, including a year-long course that touches on novel topics, a weekly process group for all residents, and a broad support network to depend on when personal matters arise.
Mentoring: Invaluable part of the process
As part of the journey through residency, we have the opportunity to work alongside renowned academic psychiatrists, most of who also happen to be amazing people. Mentoring has incredible value at this stage of professional development; don’t shy from taking advantage of that opportunity!
Mentors help us make more informed decisions about our career path. I love hearing the personal stories that my attending physicians tell. On hectic days, when we are beleaguered by managed care and electronic health records, those stories touch us in ways that abstract learning cannot. Internalizing our role models is a conscious and an unconscious element of the process of becoming a psychiatrist.
About that process: It’s far from perfect, always changing, and only the start of our mastery over the tough but rewarding daily tasks of listening… reflecting… prescribing, and, well, saving lives.
Disclosure
Dr. Jovel reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Accreditation Council for Graduate Medical Education and American Board of Psychiatry and Neurology. The Psychiatry Milestone Project. http://acgme.org/acgmeweb/ Portals/0/PDFs/Milestones/PsychiatryMilestones.pdf. Published November 2013. Accessed June 23, 2014.
2. Koh GC, Khoo HE, Wong ML, et al. The effects of problem-based learning during medical school on physician competency: a systematic review. CMAJ. 2008;178(1):34-41.
3. Block L, Wu AW, Feldman L, et al. Residency schedule, burnout and patient care among first-year residents. Postgrad Med J. 2013;89(1055):495-500.
4. Bromley E, Braslow J. Teaching critical thinking in psychiatric training: a role for the social sciences. Am J Psychiatry. 2008; 165(11):1396-1401.
Becoming a psychiatrist entails a shift in how we see ourselves and those around us. We learn—sometimes the hard way—about cultural self-assessment and the relative nature of our perspective on social, cultural, and clinical matters. What do I mean?
As psychiatrists in the making, we are unaware that we’ve been given this persona-suit, so to speak, with its social expectations and misperceptions. We start noticing how telling people what we do shapes our interactions at cocktail parties, informal gatherings, and in day-to-day life. A new acquaintance might disclose more about herself than she otherwise would or, on the contrary, might become reserved, even guarded. Awkward jokes sometimes are thrown into the mix to lighten the mood. All this is part of the package we’ve been handed, because we chose to specialize in the diagnosis and treatment of mental illness and brain disorders.
So, as I enter my final year of training, I find myself reflecting on just how intense a journey residency has been.
We were physicians first…
We’re psychiatrists now, but first we learned the germ theory of disease, the pathophysiology of every well-known illness, and the scientific basis of the practice of medicine. Many of us weren’t fully aware of the challenges that come with psychiatric training when we signed up. But we powered through— trading set measures and laboratory values for subjective experiences and nonverbal cues. Along the way, we realized that we had to master not only an array of neuropsychiatric facts but other implicit skills: “active listening,” the capacity to make on-the-go complex ethical decisions, and the difficult task of being empathetically detached.
It might be only in retrospect that we can appreciate how residency has shaped us in a personal way—almost as much as it has professionally.
We think of physicians broadly as healers who save lives. Psychiatrists are no different; preventing the most hopeless from dying is something that we do the same way a cardiologist prevents a patient from dying of a massive heart attack. Winning the battle over mortality, by whatever imprecise measures of risk we use, ranks at the top of our therapeutic priorities. We find ourselves scrambling so that catastrophe never happens on our watch. Sometimes, we don’t stop to realize how much of a lifesaver we are— especially because, as junior residents, we’re too pressed for time to reflect and are focused on mastering clinical skills.
New tool to measure success in residency
The Accreditation Council for Graduate Medical Education (ACGME) recently released the “Milestones Project,”1 a thorough evaluation system for residency programs to apply to their trainees. This is a great effort to push for more field-specific evaluation measures among the specialties.
In psychiatry, subjects now considered when evaluating a resident’s progress and preparedness for promotion include competence in applied neuroscience; the practitioner’s emotional response to patients’ problems; and regulatory compliance. Ways doctors learn are changing: Emphasis is now on problem-based learning.2 Patient safety is a priority; to respect that, we are betting strongly on the physician’s aptitude to provide good care by decreasing burnout.3 I am pleased to learn that there are ongoing efforts to improve the way we prepare psychiatrists.
In line with ACGME practices, residency programs also need to continue revisiting their didactic curricula to include innovative, emerging topics. Social media, the antipsychiatry movement, Internet forums, opinionated bloggers, and public figures gone viral—these are some current issues that shouldn’t be ignored during training just because they aren’t discussed in texts or academic journals. Programs that teach and stimulate the inclusion of social sciences and critical thinking should yield better, more holistic psychiatrists.4
For me, these avenues of study have made a huge difference. I feel incredibly grateful for the opportunities that my residency program has provided to me as a psychiatrist-in-training, including a year-long course that touches on novel topics, a weekly process group for all residents, and a broad support network to depend on when personal matters arise.
Mentoring: Invaluable part of the process
As part of the journey through residency, we have the opportunity to work alongside renowned academic psychiatrists, most of who also happen to be amazing people. Mentoring has incredible value at this stage of professional development; don’t shy from taking advantage of that opportunity!
Mentors help us make more informed decisions about our career path. I love hearing the personal stories that my attending physicians tell. On hectic days, when we are beleaguered by managed care and electronic health records, those stories touch us in ways that abstract learning cannot. Internalizing our role models is a conscious and an unconscious element of the process of becoming a psychiatrist.
About that process: It’s far from perfect, always changing, and only the start of our mastery over the tough but rewarding daily tasks of listening… reflecting… prescribing, and, well, saving lives.
Disclosure
Dr. Jovel reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Becoming a psychiatrist entails a shift in how we see ourselves and those around us. We learn—sometimes the hard way—about cultural self-assessment and the relative nature of our perspective on social, cultural, and clinical matters. What do I mean?
As psychiatrists in the making, we are unaware that we’ve been given this persona-suit, so to speak, with its social expectations and misperceptions. We start noticing how telling people what we do shapes our interactions at cocktail parties, informal gatherings, and in day-to-day life. A new acquaintance might disclose more about herself than she otherwise would or, on the contrary, might become reserved, even guarded. Awkward jokes sometimes are thrown into the mix to lighten the mood. All this is part of the package we’ve been handed, because we chose to specialize in the diagnosis and treatment of mental illness and brain disorders.
So, as I enter my final year of training, I find myself reflecting on just how intense a journey residency has been.
We were physicians first…
We’re psychiatrists now, but first we learned the germ theory of disease, the pathophysiology of every well-known illness, and the scientific basis of the practice of medicine. Many of us weren’t fully aware of the challenges that come with psychiatric training when we signed up. But we powered through— trading set measures and laboratory values for subjective experiences and nonverbal cues. Along the way, we realized that we had to master not only an array of neuropsychiatric facts but other implicit skills: “active listening,” the capacity to make on-the-go complex ethical decisions, and the difficult task of being empathetically detached.
It might be only in retrospect that we can appreciate how residency has shaped us in a personal way—almost as much as it has professionally.
We think of physicians broadly as healers who save lives. Psychiatrists are no different; preventing the most hopeless from dying is something that we do the same way a cardiologist prevents a patient from dying of a massive heart attack. Winning the battle over mortality, by whatever imprecise measures of risk we use, ranks at the top of our therapeutic priorities. We find ourselves scrambling so that catastrophe never happens on our watch. Sometimes, we don’t stop to realize how much of a lifesaver we are— especially because, as junior residents, we’re too pressed for time to reflect and are focused on mastering clinical skills.
New tool to measure success in residency
The Accreditation Council for Graduate Medical Education (ACGME) recently released the “Milestones Project,”1 a thorough evaluation system for residency programs to apply to their trainees. This is a great effort to push for more field-specific evaluation measures among the specialties.
In psychiatry, subjects now considered when evaluating a resident’s progress and preparedness for promotion include competence in applied neuroscience; the practitioner’s emotional response to patients’ problems; and regulatory compliance. Ways doctors learn are changing: Emphasis is now on problem-based learning.2 Patient safety is a priority; to respect that, we are betting strongly on the physician’s aptitude to provide good care by decreasing burnout.3 I am pleased to learn that there are ongoing efforts to improve the way we prepare psychiatrists.
In line with ACGME practices, residency programs also need to continue revisiting their didactic curricula to include innovative, emerging topics. Social media, the antipsychiatry movement, Internet forums, opinionated bloggers, and public figures gone viral—these are some current issues that shouldn’t be ignored during training just because they aren’t discussed in texts or academic journals. Programs that teach and stimulate the inclusion of social sciences and critical thinking should yield better, more holistic psychiatrists.4
For me, these avenues of study have made a huge difference. I feel incredibly grateful for the opportunities that my residency program has provided to me as a psychiatrist-in-training, including a year-long course that touches on novel topics, a weekly process group for all residents, and a broad support network to depend on when personal matters arise.
Mentoring: Invaluable part of the process
As part of the journey through residency, we have the opportunity to work alongside renowned academic psychiatrists, most of who also happen to be amazing people. Mentoring has incredible value at this stage of professional development; don’t shy from taking advantage of that opportunity!
Mentors help us make more informed decisions about our career path. I love hearing the personal stories that my attending physicians tell. On hectic days, when we are beleaguered by managed care and electronic health records, those stories touch us in ways that abstract learning cannot. Internalizing our role models is a conscious and an unconscious element of the process of becoming a psychiatrist.
About that process: It’s far from perfect, always changing, and only the start of our mastery over the tough but rewarding daily tasks of listening… reflecting… prescribing, and, well, saving lives.
Disclosure
Dr. Jovel reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Accreditation Council for Graduate Medical Education and American Board of Psychiatry and Neurology. The Psychiatry Milestone Project. http://acgme.org/acgmeweb/ Portals/0/PDFs/Milestones/PsychiatryMilestones.pdf. Published November 2013. Accessed June 23, 2014.
2. Koh GC, Khoo HE, Wong ML, et al. The effects of problem-based learning during medical school on physician competency: a systematic review. CMAJ. 2008;178(1):34-41.
3. Block L, Wu AW, Feldman L, et al. Residency schedule, burnout and patient care among first-year residents. Postgrad Med J. 2013;89(1055):495-500.
4. Bromley E, Braslow J. Teaching critical thinking in psychiatric training: a role for the social sciences. Am J Psychiatry. 2008; 165(11):1396-1401.
1. Accreditation Council for Graduate Medical Education and American Board of Psychiatry and Neurology. The Psychiatry Milestone Project. http://acgme.org/acgmeweb/ Portals/0/PDFs/Milestones/PsychiatryMilestones.pdf. Published November 2013. Accessed June 23, 2014.
2. Koh GC, Khoo HE, Wong ML, et al. The effects of problem-based learning during medical school on physician competency: a systematic review. CMAJ. 2008;178(1):34-41.
3. Block L, Wu AW, Feldman L, et al. Residency schedule, burnout and patient care among first-year residents. Postgrad Med J. 2013;89(1055):495-500.
4. Bromley E, Braslow J. Teaching critical thinking in psychiatric training: a role for the social sciences. Am J Psychiatry. 2008; 165(11):1396-1401.
Treating methamphetamine abuse disorder: Experience from research and practice
Methamphetamine and other amphetamine-type stimulants are the world’s second most widely used group of illicit substances (after Cannabis), with prevalence of abuse varying by region and by locales within nations. As prescription use of stimulants has grown dramatically in recent years, so has abuse of these substances.
Given the widespread and growing misuse of amphetamine-type stimulants (Box,1-3), clinicians are faced with the need to learn how to recognize and manage methamphetamine abuse. Both prescribed and non-prescribed uses of stimulants present complex challenges; in this article, we examine effects, manifestations, and current evidence-based behavioral and medical treatments of methamphetamine misuse and abuse, and look ahead to investigational therapies that hold promise for improving the limited existing approaches to management.
Effects and manifestations of methamphetamine use
Different routes of administration produce different consequences, in terms of medical comorbidity and propensity to induce addiction. Smoked or injected, methamphetamine enters the brain in seconds; snorted or taken by mouth, the drug produces its effects in several minutes and a half hour, respectively.
Rapid uptake and effects of methamphetamine result from its ability to cross the blood−brain barrier. Its primary effects are caused by inhibition of dopamine storage and release of intracellular dopamine.
Methamphetamine stimulates the CNS and the cardiovascular system through release of dopamine and norepinephrine, which increases blood pressure, body temperature, and heart rate, and, occasionally, induces arrhythmia that can contribute to heart attack and stroke. Users experience euphoria, hypervigilance, suppressed appetite, and increased libido.
Binge use is common to sustain euphoria and other reinforcing effects, which subside with rapidly developing tolerance. After days of repeated dosing, elevated methamphetamine blood levels can lead to mood disturbances, repetitive motor activities, and psychotic symptoms such as hallucinations, delusions, and paranoia. Acute psychosis can bring on violence and other injurious behaviors that involve law enforcement and emergency medical services.
When methamphetamine is used over months or years, health consequences include anorexia, tremor, so-called meth mouth (broken teeth, infections, cavities, burns), insomnia, panic attacks, confusion, depression, irritability, and impaired memory and other cognitive processes.
Treating methamphetamine intoxication and withdrawal
At initial clinical contact with a person who abuses methamphetamine, practitioners may face several acute consequences requiring attention. Prominent among presenting conditions, especially during acute intoxication, are agitation, anxiety, and psychotic symptoms, which may improve by providing the patient with calming reassurance in a quiet space. In more severe cases, a benzodiazepine, antipsychotic, or both might be indicated4,5 (Table 1).
Methamphetamine withdrawal is characterized by anxiety, depression, and insomnia. These symptoms generally resolve in a matter of days after the start of withdrawal without pharmacotherapy. In some cases, depression or psychosis becomes chronic, as a result of methamphetamine use itself6 or as an emergent concomitant psychiatric condition.
A sedative-hypnotic medication or an anxiolytic can be used as necessary to ameliorate insomnia or anxiety, respectively. Prolonged depression can be treated with an antidepressant. An antipsychotic might be indicated for long-term management of patients who have persistent psychosis.
Therapy for methamphetamine abuse
Treatment of methamphetamine abuse— with the goal of stopping drug use—is a complicated matter on 2 counts:
• No medications are FDA-approved for treating methamphetamine addiction.
• There are no accepted substitution medications (ie, stimulants that can be used in place of methamphetamine, as is available for opioid addiction).
Pharmacotherapeutic possibilities. The rationale for considering replacement pharmacotherapy is that psychostimulants can counter the cravings, dysphoria, and fatigue produced by methamphetamine withdrawal and can alleviate methamphetamine-related cognitive impairment. Although dextroamphetamine and other psychostimulants have been evaluated in small trials as replacement medication, most countries are reluctant to consider their use, because of the potential for abuse and accompanying liability.
After decades of medication research, several drugs have shown promise for reducing methamphetamine abuse, although results have not been robust (Table 2):
• Bupropion has shown benefit in reducing methamphetamine use among users with less severe addiction.7,8
• Methylphenidate, a psychostimulant FDA-approved for attention-deficit/hyperactivity disorder, was found to reduce methamphetamine use compared with placebo in a European sample of amphetamine injectors who had attained abstinence in a residential program.9 Those results were not replicated in a recent study by Miles et al, however.10 A study with a more clinically realistic approach (ie, not requiring daily clinic attendance, as in the Miles trial) vs placebo for methamphetamine abuse was recently published, with promising results that require confirmation in further study.11
• Mirtazapine, an antidepressant, has demonstrated efficacy in reducing methamphetamine use compared with placebo.12
• Modafinil, another medication with stimulant properties, reduced methamphetamine use in a subgroup analysis of heavy users, compared with placebo.13
• Dextroamphetamine, 60 mg/d, showed no difference in reducing methamphetamine compared with placebo, but did diminish cravings and withdrawal symptoms.14
A trial of the phosphodiesterase inhibitor ibudilast (not available in the United States) for methamphetamine abuse is underway. Ibudilast has anti-inflammatory activity in the peripheral immune system and the central nervous system, including modulating the activity of glial cells.15
Many medications have yielded negligible results in studies: selegiline, baclofen, sertraline, topiramate, gabapentin, rivastigmine, risperidone, and ondansetron.16 Recent evaluation of disulfiram, vigabatrin, and lobeline also has yielded inconsistent findings.17
No drug has proved effective for preventing relapse; research continues, focusing on several types of compounds that target various mechanisms: the dopamine system, the opioid system (by way of the γ-aminobutyric acid inhibitory system), and cortico-limbic reward circuitry.
Once-monthly injectable naltrexone has potential for ameliorating craving and relapse by modulating the opioid receptor system. However, the drug has not been adequately explored in generalizable settings of methamphetamine users.
Trials of oral naltrexone in Sweden have shown encouraging results, including reduced subjective effects and amphetamine use in open-label trials18,19; results were replicated in a subsequent placebo-controlled trial.20 In an unpublished study, however, no differences in amphetamine use were found among users randomized to depot naltrexone or placebo.21
Depot naltrexone with assured dosing might have a role in treating methamphetamine abuse, however; a combination of depot naltrexone and oral bupropion is being examined in a National Institute on Drug Abuse Clinical Trials Networks study that commenced in 2013. Pairing medications that have different mechanistic targets might work toward promoting cessation of methamphetamine abuse and reducing relapse once patients are abstinent.
In an early phase of research, but showing promise based on their ability to target different systems, are:
• N-acetylcysteine, modulator of the glutamate system
• D3 antagonists and partial agonists22
• varenicline.23
Potential “vaccines” against methamphetamine are in preclinical development, including use of a protein carrier or other immune-stimulating molecule to create antibodies that bind methamphetamine in the bloodstream and block its psychoactive effects.24,25
Sigma receptor effects are being studied in rodents as potential targets to mitigate effects of methamphetamine. The ligand AZ66, a sigma receptor antagonist, has demonstrated efficacy in reducing methamphetamine-induced cognitive impairment—suggesting that the sigma receptor has a potential role in ameliorating methamphetamine-related neurotoxicity.26
Psychosocial and behavioral interventions. Among the non-drug treatments that have demonstrated efficacy for treating methamphetamine abuse, cognitive-behavioral therapy (CBT) and contingency management (CM) have been most widely studied and applied in treatment settings.
CBT involves individual or group counseling that focuses on relapse prevention skills, including identification of relapse triggers, strategies to diminish cravings, and engagement in alternative non-drug activities27,28 (Table 3).
CM, which is based on positive reinforcement, offers tangible reinforcers, or rewards, for behaviors (eg, clinic attendance, providing a drug-free urine sample) according to guidelines set by the practitioner. CM-based interventions are the most reliably documented approaches for treating methamphetamine abuse,29,30 but their utility might prove to be most efficient in combination with medication— once suitable pharmacotherapeutic options emerge.
Although CBT and CM remain accepted standard treatments for methamphetamine abuse, outcomes are suboptimal.27 Both interventions have a high rate of dropout during the first month of treatment and a >50% relapse rate 6 to 19 months after treatment ends.31-33
As with treatment of other substance use disorders, patients who abuse methamphetamine can benefit from residential treatment in a drug-free setting for ≥30 days.34 In the residential approach, removing access to drugs, drug cues, and drug-using acquaintances combined with group and individual counseling reaches an inevitable end: discharge into the community. Then the patient’s battle to avoid relapse begins.
Because cognitive impairment is common among patients who abuse methamphetamine, even after they stop using,35 researchers have examined the potential for increasing participation in psychosocial interventions such as CBT by using medications that might have potential to increase cognitive function, such as modafinil.36 Increased attention and concentration afforded by medication could enhance efficacy of CBT. Results of trials and new drug development have been mixed37; no clear candidate for preventing relapse through any of the putative mechanisms of action has emerged.
Relapse is a problematic target for treatment
Ending methamphetamine abuse and sustaining abstinence from stimulants require a change in the cognitive associations that have been laid down in a drug user’s memory. Relapse occurs because of recalled memories that can be cued, or triggered, by internal or external stimuli. Eliminating drug memories, perhaps assisted by medications such as d-cycloserine (an antagonist of the N-methyl-d-aspartate receptor), could be useful for suppressing the inclination to relapse.
Last, alternative, non-drug forms of cognitive amendment have shown efficacy in preventing relapse: for example, incorporating mindfulness meditation, which has shown promise in managing craving for methamphetamine and decreasing reactivity to environmental cues for drug use.38
Bottom Line
Practitioners who work in emergency, inpatient, and outpatient settings will be called on more and more to treat acute stimulant intoxication and withdrawal, stimulant-induced psychosis, and methamphetamine abuse. Few evidence-based treatments and no FDA-approved medications are available to treat this addiction; many drugs and a few psychotherapeutic techniques have shown promise. Ongoing research promises to deliver medical and behavioral interventions to help patients quit using methamphetamine.
Related Resources
• Karch SB, Drummer O. Karch’s pathology of drug abuse, fifth ed. Boca Raton, FL: CRC Press/Taylor & David; 2013.
• Roll J, Rawson RA, Ling W, eds. Methamphetamine addiction: from basic science to treatment. New York, NY: Guilford Press; 2009.
• Sheff D. Beautiful boy: a father’s journey through his son’s addiction. New York, NY: Houghton Mifflin Harcourt Publishing Company; 2008.
• Sheff N. Tweak: growing up on methamphetamines. New York, NY: Antheneum Books for Young Readers; 2007.
• National Institute on Drug Abuse. Drugs of abuse. www. drugabuse.gov/drugs-abuse/methamphetamine.
Drug Brand Names
Baclofen • Lioresal Naltrexone (depot) • Vivitrol
Bupropion • Wellbutrin Naltrexone (oral) • ReVia
D-cycloserine • Seromycin Ondansetron • Zofran
Dexreoamphetaime • Adderall Risperidone • Risperadal
Disulfiram • Antabuse Rivastigimine • Exelon
Gabapentin • Neurontin Selegiline• EMSAM
Methylphenidate • Ritalin Sertraline • Zoloft
Mirtazapine • Remeron Topiramate • Topamax
Modafinil • Provigil Varenicline • Chantix
N-acetylcysteine • Mucomyst Vigabatrin • Sabril
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. UNODC. World Drug Report 2012 (United Nations publication, Sales No. E.12.XI.1). http://www.unodc. org/documents/data-and-analysis/WDR2012/ WDR_2012_web_small.pdf. Published 2012. Accessed August 4, 2014.
2. UNODC. World Drug Report 2010 (United Nations publication, Sales No. E.10.XI.13). http://www.unodc. org/documents/wdr/WDR_2010/World_Drug_ Report_2010_lo-res.pdf. Published 2010. Accessed August 4, 2014.
3. Rawson RA, Gonzales R, Brecht M, et al. Evaluation of the California Outcomes Measurement System (CalOMS): Final Report 2008. http://www.uclaisap.org/assets/documents/ California-ADP-DHCS-Evals/2007-2008_CalOMS%20 Report.pdf. Published 2008. Accessed August 4, 2014.
4. Shoptaw SJ, Kao U, Ling W. Treatment for amphetamine psychosis. Cochrane Database Syst Rev. 2009;(1):CD003026.
5. Leelahanaj T, Kongsakon R, Netrakom P. A 4-week, double-blind comparison of olanzapine with haloperidol in the treatment of amphetamine psychosis. J Med Assoc Thai. 2005;88(suppl 3):S43-S52.
6. McKetin R, McLaren J, Lubman D, et al. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101(10):1473-1478.
7. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
8. McCann DJ, Li SH. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS Neurosci Ther. 2012;18(5):414-418.
9. Tiihonen J, Kuoppasalmi K, Föhr J, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164(1): 160-162.
10. Miles SW, Sheridan J, Russell B, et al. Extended-release methylphenidate for treatment of amphetamine/ methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108(7): 1279-1286.
11. Ling W, Chang L, Hillhouse M, et al. Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder. Addiction. 2014;109(9): 1489-1500.
12. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175.
13. Heinzerling KG, Swanson AN, Kim S, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109(1-3):20-29.
14. Galloway GP, Buscemi R, Coyle JR, et al. A randomized, placebo-controlled trial of sustained-release dextro-amphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther. 2011;89(2):276-282.
15. Snider SE, Hendrick ES, Beardsley PM. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur J Pharmacol. 2013;701(1-3):124-130.
16. Ling W, Rawson R, Shoptaw S. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep. 2006;8(5):345-354.
17. Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24(6):541-550.
18. Jayaram-Lindström N, Wennberg P, Beck O, et al. An open clinical trial of naltrexone for amphetamine dependence: compliance and tolerability. Nord J Psychiatry. 2005;59(3):167-171.
19. Jayaram-Lindström N, Konstenius M, Eksborg S, et al. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2007;33(8):1856-1863.
20. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448.
21. Woody GE, Tyrfingsoon P. Symposium XI: Emerging data on efficacy and clinical applications of extended-release naltrexone formulations. 75th Annual Meeting, College on Problems of Drug Dependence. June 19, 2013; San Diego, CA.
22. Newman AH, Blaylock BL, Nader MA, et al. Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem Pharmacol. 2012;84(7):882-890.
23. Verrico CD, Mahoney JJ 3rd, Thompson-Lake DG, et al. Safety and efficacy of varenicline to reduce positive subjective effects produced by methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol. 2014;17(2):223-233.
24. Miller ML, Moreno AY, Aarde S, et al. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatry. 2013;73(8):721-728.
25. Shen XY, Kosten TA, Lopez AY, et al. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013;129(1-2):41-48.
26. Seminerio MJ, Robson MJ, Abdelazeem AH, et al. Synthesis and pharmacological characterization of a novel sigma receptor ligand with improved metabolic stability and antagonistic effects against methamphetamine. AAPS J. 2012;14(1):43-51.
27. Rawson RA, Marinelli-Casey P, Anglin M, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99(6):708-717.
28. Vocci FJ, Montoya ID. Psychological treatments for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr Opin Psychiatry. 2009;22(3):263-268.
29. Rawson RA, McCann MJ, Flammino F, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267-274.
30. Roll JM, Petry NM, Stitzer ML, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry. 2006;163(11):1993-1999.
31. Brecht ML, von Mayrhauser C, Anglin MD. Predictors of relapse after treatment for methamphetamine use. J Psychoactive Drugs. 2000;32(2):211-220.
32. Smout MF, Longo M, Harrison S, et al. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and Acceptance and Commitment Therapy. Subst Abus. 2010;31(2):98-107.
33. Wang G, Shi J, Chen N, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;24;8(7):e68791. doi: 10.1371/ journal.pone.0068791.
34. McKetin R, Lubman DI, Baker AL, et al. Dose-related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry. 2013;70(3):319-324.
35. Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35(6):593-598.
36. Dean AC, Sevak RJ, Monterosso JR, et al. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. J Stud Alcohol Drugs. 2011;72(6):943-953.
37. Zullino DF, Benguettat D, Khazaal Y. Improvement of cognitive performance by topiramate: blockage of automatic processes may be the underlying mechanism [Comment on: Effects of topiramate on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition in recently abstinent methamphetamine-dependent individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2007.] Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):787.
38. Witkiewitz K, Lustyk M, Bowen S. Retraining the addicted brain: a review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol Addict Behav. 2013;27(2):351-365.
Methamphetamine and other amphetamine-type stimulants are the world’s second most widely used group of illicit substances (after Cannabis), with prevalence of abuse varying by region and by locales within nations. As prescription use of stimulants has grown dramatically in recent years, so has abuse of these substances.
Given the widespread and growing misuse of amphetamine-type stimulants (Box,1-3), clinicians are faced with the need to learn how to recognize and manage methamphetamine abuse. Both prescribed and non-prescribed uses of stimulants present complex challenges; in this article, we examine effects, manifestations, and current evidence-based behavioral and medical treatments of methamphetamine misuse and abuse, and look ahead to investigational therapies that hold promise for improving the limited existing approaches to management.
Effects and manifestations of methamphetamine use
Different routes of administration produce different consequences, in terms of medical comorbidity and propensity to induce addiction. Smoked or injected, methamphetamine enters the brain in seconds; snorted or taken by mouth, the drug produces its effects in several minutes and a half hour, respectively.
Rapid uptake and effects of methamphetamine result from its ability to cross the blood−brain barrier. Its primary effects are caused by inhibition of dopamine storage and release of intracellular dopamine.
Methamphetamine stimulates the CNS and the cardiovascular system through release of dopamine and norepinephrine, which increases blood pressure, body temperature, and heart rate, and, occasionally, induces arrhythmia that can contribute to heart attack and stroke. Users experience euphoria, hypervigilance, suppressed appetite, and increased libido.
Binge use is common to sustain euphoria and other reinforcing effects, which subside with rapidly developing tolerance. After days of repeated dosing, elevated methamphetamine blood levels can lead to mood disturbances, repetitive motor activities, and psychotic symptoms such as hallucinations, delusions, and paranoia. Acute psychosis can bring on violence and other injurious behaviors that involve law enforcement and emergency medical services.
When methamphetamine is used over months or years, health consequences include anorexia, tremor, so-called meth mouth (broken teeth, infections, cavities, burns), insomnia, panic attacks, confusion, depression, irritability, and impaired memory and other cognitive processes.
Treating methamphetamine intoxication and withdrawal
At initial clinical contact with a person who abuses methamphetamine, practitioners may face several acute consequences requiring attention. Prominent among presenting conditions, especially during acute intoxication, are agitation, anxiety, and psychotic symptoms, which may improve by providing the patient with calming reassurance in a quiet space. In more severe cases, a benzodiazepine, antipsychotic, or both might be indicated4,5 (Table 1).
Methamphetamine withdrawal is characterized by anxiety, depression, and insomnia. These symptoms generally resolve in a matter of days after the start of withdrawal without pharmacotherapy. In some cases, depression or psychosis becomes chronic, as a result of methamphetamine use itself6 or as an emergent concomitant psychiatric condition.
A sedative-hypnotic medication or an anxiolytic can be used as necessary to ameliorate insomnia or anxiety, respectively. Prolonged depression can be treated with an antidepressant. An antipsychotic might be indicated for long-term management of patients who have persistent psychosis.
Therapy for methamphetamine abuse
Treatment of methamphetamine abuse— with the goal of stopping drug use—is a complicated matter on 2 counts:
• No medications are FDA-approved for treating methamphetamine addiction.
• There are no accepted substitution medications (ie, stimulants that can be used in place of methamphetamine, as is available for opioid addiction).
Pharmacotherapeutic possibilities. The rationale for considering replacement pharmacotherapy is that psychostimulants can counter the cravings, dysphoria, and fatigue produced by methamphetamine withdrawal and can alleviate methamphetamine-related cognitive impairment. Although dextroamphetamine and other psychostimulants have been evaluated in small trials as replacement medication, most countries are reluctant to consider their use, because of the potential for abuse and accompanying liability.
After decades of medication research, several drugs have shown promise for reducing methamphetamine abuse, although results have not been robust (Table 2):
• Bupropion has shown benefit in reducing methamphetamine use among users with less severe addiction.7,8
• Methylphenidate, a psychostimulant FDA-approved for attention-deficit/hyperactivity disorder, was found to reduce methamphetamine use compared with placebo in a European sample of amphetamine injectors who had attained abstinence in a residential program.9 Those results were not replicated in a recent study by Miles et al, however.10 A study with a more clinically realistic approach (ie, not requiring daily clinic attendance, as in the Miles trial) vs placebo for methamphetamine abuse was recently published, with promising results that require confirmation in further study.11
• Mirtazapine, an antidepressant, has demonstrated efficacy in reducing methamphetamine use compared with placebo.12
• Modafinil, another medication with stimulant properties, reduced methamphetamine use in a subgroup analysis of heavy users, compared with placebo.13
• Dextroamphetamine, 60 mg/d, showed no difference in reducing methamphetamine compared with placebo, but did diminish cravings and withdrawal symptoms.14
A trial of the phosphodiesterase inhibitor ibudilast (not available in the United States) for methamphetamine abuse is underway. Ibudilast has anti-inflammatory activity in the peripheral immune system and the central nervous system, including modulating the activity of glial cells.15
Many medications have yielded negligible results in studies: selegiline, baclofen, sertraline, topiramate, gabapentin, rivastigmine, risperidone, and ondansetron.16 Recent evaluation of disulfiram, vigabatrin, and lobeline also has yielded inconsistent findings.17
No drug has proved effective for preventing relapse; research continues, focusing on several types of compounds that target various mechanisms: the dopamine system, the opioid system (by way of the γ-aminobutyric acid inhibitory system), and cortico-limbic reward circuitry.
Once-monthly injectable naltrexone has potential for ameliorating craving and relapse by modulating the opioid receptor system. However, the drug has not been adequately explored in generalizable settings of methamphetamine users.
Trials of oral naltrexone in Sweden have shown encouraging results, including reduced subjective effects and amphetamine use in open-label trials18,19; results were replicated in a subsequent placebo-controlled trial.20 In an unpublished study, however, no differences in amphetamine use were found among users randomized to depot naltrexone or placebo.21
Depot naltrexone with assured dosing might have a role in treating methamphetamine abuse, however; a combination of depot naltrexone and oral bupropion is being examined in a National Institute on Drug Abuse Clinical Trials Networks study that commenced in 2013. Pairing medications that have different mechanistic targets might work toward promoting cessation of methamphetamine abuse and reducing relapse once patients are abstinent.
In an early phase of research, but showing promise based on their ability to target different systems, are:
• N-acetylcysteine, modulator of the glutamate system
• D3 antagonists and partial agonists22
• varenicline.23
Potential “vaccines” against methamphetamine are in preclinical development, including use of a protein carrier or other immune-stimulating molecule to create antibodies that bind methamphetamine in the bloodstream and block its psychoactive effects.24,25
Sigma receptor effects are being studied in rodents as potential targets to mitigate effects of methamphetamine. The ligand AZ66, a sigma receptor antagonist, has demonstrated efficacy in reducing methamphetamine-induced cognitive impairment—suggesting that the sigma receptor has a potential role in ameliorating methamphetamine-related neurotoxicity.26
Psychosocial and behavioral interventions. Among the non-drug treatments that have demonstrated efficacy for treating methamphetamine abuse, cognitive-behavioral therapy (CBT) and contingency management (CM) have been most widely studied and applied in treatment settings.
CBT involves individual or group counseling that focuses on relapse prevention skills, including identification of relapse triggers, strategies to diminish cravings, and engagement in alternative non-drug activities27,28 (Table 3).
CM, which is based on positive reinforcement, offers tangible reinforcers, or rewards, for behaviors (eg, clinic attendance, providing a drug-free urine sample) according to guidelines set by the practitioner. CM-based interventions are the most reliably documented approaches for treating methamphetamine abuse,29,30 but their utility might prove to be most efficient in combination with medication— once suitable pharmacotherapeutic options emerge.
Although CBT and CM remain accepted standard treatments for methamphetamine abuse, outcomes are suboptimal.27 Both interventions have a high rate of dropout during the first month of treatment and a >50% relapse rate 6 to 19 months after treatment ends.31-33
As with treatment of other substance use disorders, patients who abuse methamphetamine can benefit from residential treatment in a drug-free setting for ≥30 days.34 In the residential approach, removing access to drugs, drug cues, and drug-using acquaintances combined with group and individual counseling reaches an inevitable end: discharge into the community. Then the patient’s battle to avoid relapse begins.
Because cognitive impairment is common among patients who abuse methamphetamine, even after they stop using,35 researchers have examined the potential for increasing participation in psychosocial interventions such as CBT by using medications that might have potential to increase cognitive function, such as modafinil.36 Increased attention and concentration afforded by medication could enhance efficacy of CBT. Results of trials and new drug development have been mixed37; no clear candidate for preventing relapse through any of the putative mechanisms of action has emerged.
Relapse is a problematic target for treatment
Ending methamphetamine abuse and sustaining abstinence from stimulants require a change in the cognitive associations that have been laid down in a drug user’s memory. Relapse occurs because of recalled memories that can be cued, or triggered, by internal or external stimuli. Eliminating drug memories, perhaps assisted by medications such as d-cycloserine (an antagonist of the N-methyl-d-aspartate receptor), could be useful for suppressing the inclination to relapse.
Last, alternative, non-drug forms of cognitive amendment have shown efficacy in preventing relapse: for example, incorporating mindfulness meditation, which has shown promise in managing craving for methamphetamine and decreasing reactivity to environmental cues for drug use.38
Bottom Line
Practitioners who work in emergency, inpatient, and outpatient settings will be called on more and more to treat acute stimulant intoxication and withdrawal, stimulant-induced psychosis, and methamphetamine abuse. Few evidence-based treatments and no FDA-approved medications are available to treat this addiction; many drugs and a few psychotherapeutic techniques have shown promise. Ongoing research promises to deliver medical and behavioral interventions to help patients quit using methamphetamine.
Related Resources
• Karch SB, Drummer O. Karch’s pathology of drug abuse, fifth ed. Boca Raton, FL: CRC Press/Taylor & David; 2013.
• Roll J, Rawson RA, Ling W, eds. Methamphetamine addiction: from basic science to treatment. New York, NY: Guilford Press; 2009.
• Sheff D. Beautiful boy: a father’s journey through his son’s addiction. New York, NY: Houghton Mifflin Harcourt Publishing Company; 2008.
• Sheff N. Tweak: growing up on methamphetamines. New York, NY: Antheneum Books for Young Readers; 2007.
• National Institute on Drug Abuse. Drugs of abuse. www. drugabuse.gov/drugs-abuse/methamphetamine.
Drug Brand Names
Baclofen • Lioresal Naltrexone (depot) • Vivitrol
Bupropion • Wellbutrin Naltrexone (oral) • ReVia
D-cycloserine • Seromycin Ondansetron • Zofran
Dexreoamphetaime • Adderall Risperidone • Risperadal
Disulfiram • Antabuse Rivastigimine • Exelon
Gabapentin • Neurontin Selegiline• EMSAM
Methylphenidate • Ritalin Sertraline • Zoloft
Mirtazapine • Remeron Topiramate • Topamax
Modafinil • Provigil Varenicline • Chantix
N-acetylcysteine • Mucomyst Vigabatrin • Sabril
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Methamphetamine and other amphetamine-type stimulants are the world’s second most widely used group of illicit substances (after Cannabis), with prevalence of abuse varying by region and by locales within nations. As prescription use of stimulants has grown dramatically in recent years, so has abuse of these substances.
Given the widespread and growing misuse of amphetamine-type stimulants (Box,1-3), clinicians are faced with the need to learn how to recognize and manage methamphetamine abuse. Both prescribed and non-prescribed uses of stimulants present complex challenges; in this article, we examine effects, manifestations, and current evidence-based behavioral and medical treatments of methamphetamine misuse and abuse, and look ahead to investigational therapies that hold promise for improving the limited existing approaches to management.
Effects and manifestations of methamphetamine use
Different routes of administration produce different consequences, in terms of medical comorbidity and propensity to induce addiction. Smoked or injected, methamphetamine enters the brain in seconds; snorted or taken by mouth, the drug produces its effects in several minutes and a half hour, respectively.
Rapid uptake and effects of methamphetamine result from its ability to cross the blood−brain barrier. Its primary effects are caused by inhibition of dopamine storage and release of intracellular dopamine.
Methamphetamine stimulates the CNS and the cardiovascular system through release of dopamine and norepinephrine, which increases blood pressure, body temperature, and heart rate, and, occasionally, induces arrhythmia that can contribute to heart attack and stroke. Users experience euphoria, hypervigilance, suppressed appetite, and increased libido.
Binge use is common to sustain euphoria and other reinforcing effects, which subside with rapidly developing tolerance. After days of repeated dosing, elevated methamphetamine blood levels can lead to mood disturbances, repetitive motor activities, and psychotic symptoms such as hallucinations, delusions, and paranoia. Acute psychosis can bring on violence and other injurious behaviors that involve law enforcement and emergency medical services.
When methamphetamine is used over months or years, health consequences include anorexia, tremor, so-called meth mouth (broken teeth, infections, cavities, burns), insomnia, panic attacks, confusion, depression, irritability, and impaired memory and other cognitive processes.
Treating methamphetamine intoxication and withdrawal
At initial clinical contact with a person who abuses methamphetamine, practitioners may face several acute consequences requiring attention. Prominent among presenting conditions, especially during acute intoxication, are agitation, anxiety, and psychotic symptoms, which may improve by providing the patient with calming reassurance in a quiet space. In more severe cases, a benzodiazepine, antipsychotic, or both might be indicated4,5 (Table 1).
Methamphetamine withdrawal is characterized by anxiety, depression, and insomnia. These symptoms generally resolve in a matter of days after the start of withdrawal without pharmacotherapy. In some cases, depression or psychosis becomes chronic, as a result of methamphetamine use itself6 or as an emergent concomitant psychiatric condition.
A sedative-hypnotic medication or an anxiolytic can be used as necessary to ameliorate insomnia or anxiety, respectively. Prolonged depression can be treated with an antidepressant. An antipsychotic might be indicated for long-term management of patients who have persistent psychosis.
Therapy for methamphetamine abuse
Treatment of methamphetamine abuse— with the goal of stopping drug use—is a complicated matter on 2 counts:
• No medications are FDA-approved for treating methamphetamine addiction.
• There are no accepted substitution medications (ie, stimulants that can be used in place of methamphetamine, as is available for opioid addiction).
Pharmacotherapeutic possibilities. The rationale for considering replacement pharmacotherapy is that psychostimulants can counter the cravings, dysphoria, and fatigue produced by methamphetamine withdrawal and can alleviate methamphetamine-related cognitive impairment. Although dextroamphetamine and other psychostimulants have been evaluated in small trials as replacement medication, most countries are reluctant to consider their use, because of the potential for abuse and accompanying liability.
After decades of medication research, several drugs have shown promise for reducing methamphetamine abuse, although results have not been robust (Table 2):
• Bupropion has shown benefit in reducing methamphetamine use among users with less severe addiction.7,8
• Methylphenidate, a psychostimulant FDA-approved for attention-deficit/hyperactivity disorder, was found to reduce methamphetamine use compared with placebo in a European sample of amphetamine injectors who had attained abstinence in a residential program.9 Those results were not replicated in a recent study by Miles et al, however.10 A study with a more clinically realistic approach (ie, not requiring daily clinic attendance, as in the Miles trial) vs placebo for methamphetamine abuse was recently published, with promising results that require confirmation in further study.11
• Mirtazapine, an antidepressant, has demonstrated efficacy in reducing methamphetamine use compared with placebo.12
• Modafinil, another medication with stimulant properties, reduced methamphetamine use in a subgroup analysis of heavy users, compared with placebo.13
• Dextroamphetamine, 60 mg/d, showed no difference in reducing methamphetamine compared with placebo, but did diminish cravings and withdrawal symptoms.14
A trial of the phosphodiesterase inhibitor ibudilast (not available in the United States) for methamphetamine abuse is underway. Ibudilast has anti-inflammatory activity in the peripheral immune system and the central nervous system, including modulating the activity of glial cells.15
Many medications have yielded negligible results in studies: selegiline, baclofen, sertraline, topiramate, gabapentin, rivastigmine, risperidone, and ondansetron.16 Recent evaluation of disulfiram, vigabatrin, and lobeline also has yielded inconsistent findings.17
No drug has proved effective for preventing relapse; research continues, focusing on several types of compounds that target various mechanisms: the dopamine system, the opioid system (by way of the γ-aminobutyric acid inhibitory system), and cortico-limbic reward circuitry.
Once-monthly injectable naltrexone has potential for ameliorating craving and relapse by modulating the opioid receptor system. However, the drug has not been adequately explored in generalizable settings of methamphetamine users.
Trials of oral naltrexone in Sweden have shown encouraging results, including reduced subjective effects and amphetamine use in open-label trials18,19; results were replicated in a subsequent placebo-controlled trial.20 In an unpublished study, however, no differences in amphetamine use were found among users randomized to depot naltrexone or placebo.21
Depot naltrexone with assured dosing might have a role in treating methamphetamine abuse, however; a combination of depot naltrexone and oral bupropion is being examined in a National Institute on Drug Abuse Clinical Trials Networks study that commenced in 2013. Pairing medications that have different mechanistic targets might work toward promoting cessation of methamphetamine abuse and reducing relapse once patients are abstinent.
In an early phase of research, but showing promise based on their ability to target different systems, are:
• N-acetylcysteine, modulator of the glutamate system
• D3 antagonists and partial agonists22
• varenicline.23
Potential “vaccines” against methamphetamine are in preclinical development, including use of a protein carrier or other immune-stimulating molecule to create antibodies that bind methamphetamine in the bloodstream and block its psychoactive effects.24,25
Sigma receptor effects are being studied in rodents as potential targets to mitigate effects of methamphetamine. The ligand AZ66, a sigma receptor antagonist, has demonstrated efficacy in reducing methamphetamine-induced cognitive impairment—suggesting that the sigma receptor has a potential role in ameliorating methamphetamine-related neurotoxicity.26
Psychosocial and behavioral interventions. Among the non-drug treatments that have demonstrated efficacy for treating methamphetamine abuse, cognitive-behavioral therapy (CBT) and contingency management (CM) have been most widely studied and applied in treatment settings.
CBT involves individual or group counseling that focuses on relapse prevention skills, including identification of relapse triggers, strategies to diminish cravings, and engagement in alternative non-drug activities27,28 (Table 3).
CM, which is based on positive reinforcement, offers tangible reinforcers, or rewards, for behaviors (eg, clinic attendance, providing a drug-free urine sample) according to guidelines set by the practitioner. CM-based interventions are the most reliably documented approaches for treating methamphetamine abuse,29,30 but their utility might prove to be most efficient in combination with medication— once suitable pharmacotherapeutic options emerge.
Although CBT and CM remain accepted standard treatments for methamphetamine abuse, outcomes are suboptimal.27 Both interventions have a high rate of dropout during the first month of treatment and a >50% relapse rate 6 to 19 months after treatment ends.31-33
As with treatment of other substance use disorders, patients who abuse methamphetamine can benefit from residential treatment in a drug-free setting for ≥30 days.34 In the residential approach, removing access to drugs, drug cues, and drug-using acquaintances combined with group and individual counseling reaches an inevitable end: discharge into the community. Then the patient’s battle to avoid relapse begins.
Because cognitive impairment is common among patients who abuse methamphetamine, even after they stop using,35 researchers have examined the potential for increasing participation in psychosocial interventions such as CBT by using medications that might have potential to increase cognitive function, such as modafinil.36 Increased attention and concentration afforded by medication could enhance efficacy of CBT. Results of trials and new drug development have been mixed37; no clear candidate for preventing relapse through any of the putative mechanisms of action has emerged.
Relapse is a problematic target for treatment
Ending methamphetamine abuse and sustaining abstinence from stimulants require a change in the cognitive associations that have been laid down in a drug user’s memory. Relapse occurs because of recalled memories that can be cued, or triggered, by internal or external stimuli. Eliminating drug memories, perhaps assisted by medications such as d-cycloserine (an antagonist of the N-methyl-d-aspartate receptor), could be useful for suppressing the inclination to relapse.
Last, alternative, non-drug forms of cognitive amendment have shown efficacy in preventing relapse: for example, incorporating mindfulness meditation, which has shown promise in managing craving for methamphetamine and decreasing reactivity to environmental cues for drug use.38
Bottom Line
Practitioners who work in emergency, inpatient, and outpatient settings will be called on more and more to treat acute stimulant intoxication and withdrawal, stimulant-induced psychosis, and methamphetamine abuse. Few evidence-based treatments and no FDA-approved medications are available to treat this addiction; many drugs and a few psychotherapeutic techniques have shown promise. Ongoing research promises to deliver medical and behavioral interventions to help patients quit using methamphetamine.
Related Resources
• Karch SB, Drummer O. Karch’s pathology of drug abuse, fifth ed. Boca Raton, FL: CRC Press/Taylor & David; 2013.
• Roll J, Rawson RA, Ling W, eds. Methamphetamine addiction: from basic science to treatment. New York, NY: Guilford Press; 2009.
• Sheff D. Beautiful boy: a father’s journey through his son’s addiction. New York, NY: Houghton Mifflin Harcourt Publishing Company; 2008.
• Sheff N. Tweak: growing up on methamphetamines. New York, NY: Antheneum Books for Young Readers; 2007.
• National Institute on Drug Abuse. Drugs of abuse. www. drugabuse.gov/drugs-abuse/methamphetamine.
Drug Brand Names
Baclofen • Lioresal Naltrexone (depot) • Vivitrol
Bupropion • Wellbutrin Naltrexone (oral) • ReVia
D-cycloserine • Seromycin Ondansetron • Zofran
Dexreoamphetaime • Adderall Risperidone • Risperadal
Disulfiram • Antabuse Rivastigimine • Exelon
Gabapentin • Neurontin Selegiline• EMSAM
Methylphenidate • Ritalin Sertraline • Zoloft
Mirtazapine • Remeron Topiramate • Topamax
Modafinil • Provigil Varenicline • Chantix
N-acetylcysteine • Mucomyst Vigabatrin • Sabril
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. UNODC. World Drug Report 2012 (United Nations publication, Sales No. E.12.XI.1). http://www.unodc. org/documents/data-and-analysis/WDR2012/ WDR_2012_web_small.pdf. Published 2012. Accessed August 4, 2014.
2. UNODC. World Drug Report 2010 (United Nations publication, Sales No. E.10.XI.13). http://www.unodc. org/documents/wdr/WDR_2010/World_Drug_ Report_2010_lo-res.pdf. Published 2010. Accessed August 4, 2014.
3. Rawson RA, Gonzales R, Brecht M, et al. Evaluation of the California Outcomes Measurement System (CalOMS): Final Report 2008. http://www.uclaisap.org/assets/documents/ California-ADP-DHCS-Evals/2007-2008_CalOMS%20 Report.pdf. Published 2008. Accessed August 4, 2014.
4. Shoptaw SJ, Kao U, Ling W. Treatment for amphetamine psychosis. Cochrane Database Syst Rev. 2009;(1):CD003026.
5. Leelahanaj T, Kongsakon R, Netrakom P. A 4-week, double-blind comparison of olanzapine with haloperidol in the treatment of amphetamine psychosis. J Med Assoc Thai. 2005;88(suppl 3):S43-S52.
6. McKetin R, McLaren J, Lubman D, et al. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101(10):1473-1478.
7. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
8. McCann DJ, Li SH. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS Neurosci Ther. 2012;18(5):414-418.
9. Tiihonen J, Kuoppasalmi K, Föhr J, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164(1): 160-162.
10. Miles SW, Sheridan J, Russell B, et al. Extended-release methylphenidate for treatment of amphetamine/ methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108(7): 1279-1286.
11. Ling W, Chang L, Hillhouse M, et al. Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder. Addiction. 2014;109(9): 1489-1500.
12. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175.
13. Heinzerling KG, Swanson AN, Kim S, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109(1-3):20-29.
14. Galloway GP, Buscemi R, Coyle JR, et al. A randomized, placebo-controlled trial of sustained-release dextro-amphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther. 2011;89(2):276-282.
15. Snider SE, Hendrick ES, Beardsley PM. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur J Pharmacol. 2013;701(1-3):124-130.
16. Ling W, Rawson R, Shoptaw S. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep. 2006;8(5):345-354.
17. Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24(6):541-550.
18. Jayaram-Lindström N, Wennberg P, Beck O, et al. An open clinical trial of naltrexone for amphetamine dependence: compliance and tolerability. Nord J Psychiatry. 2005;59(3):167-171.
19. Jayaram-Lindström N, Konstenius M, Eksborg S, et al. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2007;33(8):1856-1863.
20. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448.
21. Woody GE, Tyrfingsoon P. Symposium XI: Emerging data on efficacy and clinical applications of extended-release naltrexone formulations. 75th Annual Meeting, College on Problems of Drug Dependence. June 19, 2013; San Diego, CA.
22. Newman AH, Blaylock BL, Nader MA, et al. Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem Pharmacol. 2012;84(7):882-890.
23. Verrico CD, Mahoney JJ 3rd, Thompson-Lake DG, et al. Safety and efficacy of varenicline to reduce positive subjective effects produced by methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol. 2014;17(2):223-233.
24. Miller ML, Moreno AY, Aarde S, et al. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatry. 2013;73(8):721-728.
25. Shen XY, Kosten TA, Lopez AY, et al. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013;129(1-2):41-48.
26. Seminerio MJ, Robson MJ, Abdelazeem AH, et al. Synthesis and pharmacological characterization of a novel sigma receptor ligand with improved metabolic stability and antagonistic effects against methamphetamine. AAPS J. 2012;14(1):43-51.
27. Rawson RA, Marinelli-Casey P, Anglin M, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99(6):708-717.
28. Vocci FJ, Montoya ID. Psychological treatments for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr Opin Psychiatry. 2009;22(3):263-268.
29. Rawson RA, McCann MJ, Flammino F, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267-274.
30. Roll JM, Petry NM, Stitzer ML, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry. 2006;163(11):1993-1999.
31. Brecht ML, von Mayrhauser C, Anglin MD. Predictors of relapse after treatment for methamphetamine use. J Psychoactive Drugs. 2000;32(2):211-220.
32. Smout MF, Longo M, Harrison S, et al. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and Acceptance and Commitment Therapy. Subst Abus. 2010;31(2):98-107.
33. Wang G, Shi J, Chen N, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;24;8(7):e68791. doi: 10.1371/ journal.pone.0068791.
34. McKetin R, Lubman DI, Baker AL, et al. Dose-related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry. 2013;70(3):319-324.
35. Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35(6):593-598.
36. Dean AC, Sevak RJ, Monterosso JR, et al. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. J Stud Alcohol Drugs. 2011;72(6):943-953.
37. Zullino DF, Benguettat D, Khazaal Y. Improvement of cognitive performance by topiramate: blockage of automatic processes may be the underlying mechanism [Comment on: Effects of topiramate on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition in recently abstinent methamphetamine-dependent individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2007.] Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):787.
38. Witkiewitz K, Lustyk M, Bowen S. Retraining the addicted brain: a review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol Addict Behav. 2013;27(2):351-365.
1. UNODC. World Drug Report 2012 (United Nations publication, Sales No. E.12.XI.1). http://www.unodc. org/documents/data-and-analysis/WDR2012/ WDR_2012_web_small.pdf. Published 2012. Accessed August 4, 2014.
2. UNODC. World Drug Report 2010 (United Nations publication, Sales No. E.10.XI.13). http://www.unodc. org/documents/wdr/WDR_2010/World_Drug_ Report_2010_lo-res.pdf. Published 2010. Accessed August 4, 2014.
3. Rawson RA, Gonzales R, Brecht M, et al. Evaluation of the California Outcomes Measurement System (CalOMS): Final Report 2008. http://www.uclaisap.org/assets/documents/ California-ADP-DHCS-Evals/2007-2008_CalOMS%20 Report.pdf. Published 2008. Accessed August 4, 2014.
4. Shoptaw SJ, Kao U, Ling W. Treatment for amphetamine psychosis. Cochrane Database Syst Rev. 2009;(1):CD003026.
5. Leelahanaj T, Kongsakon R, Netrakom P. A 4-week, double-blind comparison of olanzapine with haloperidol in the treatment of amphetamine psychosis. J Med Assoc Thai. 2005;88(suppl 3):S43-S52.
6. McKetin R, McLaren J, Lubman D, et al. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101(10):1473-1478.
7. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
8. McCann DJ, Li SH. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS Neurosci Ther. 2012;18(5):414-418.
9. Tiihonen J, Kuoppasalmi K, Föhr J, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164(1): 160-162.
10. Miles SW, Sheridan J, Russell B, et al. Extended-release methylphenidate for treatment of amphetamine/ methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108(7): 1279-1286.
11. Ling W, Chang L, Hillhouse M, et al. Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder. Addiction. 2014;109(9): 1489-1500.
12. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175.
13. Heinzerling KG, Swanson AN, Kim S, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109(1-3):20-29.
14. Galloway GP, Buscemi R, Coyle JR, et al. A randomized, placebo-controlled trial of sustained-release dextro-amphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther. 2011;89(2):276-282.
15. Snider SE, Hendrick ES, Beardsley PM. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur J Pharmacol. 2013;701(1-3):124-130.
16. Ling W, Rawson R, Shoptaw S. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep. 2006;8(5):345-354.
17. Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24(6):541-550.
18. Jayaram-Lindström N, Wennberg P, Beck O, et al. An open clinical trial of naltrexone for amphetamine dependence: compliance and tolerability. Nord J Psychiatry. 2005;59(3):167-171.
19. Jayaram-Lindström N, Konstenius M, Eksborg S, et al. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2007;33(8):1856-1863.
20. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448.
21. Woody GE, Tyrfingsoon P. Symposium XI: Emerging data on efficacy and clinical applications of extended-release naltrexone formulations. 75th Annual Meeting, College on Problems of Drug Dependence. June 19, 2013; San Diego, CA.
22. Newman AH, Blaylock BL, Nader MA, et al. Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem Pharmacol. 2012;84(7):882-890.
23. Verrico CD, Mahoney JJ 3rd, Thompson-Lake DG, et al. Safety and efficacy of varenicline to reduce positive subjective effects produced by methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol. 2014;17(2):223-233.
24. Miller ML, Moreno AY, Aarde S, et al. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatry. 2013;73(8):721-728.
25. Shen XY, Kosten TA, Lopez AY, et al. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013;129(1-2):41-48.
26. Seminerio MJ, Robson MJ, Abdelazeem AH, et al. Synthesis and pharmacological characterization of a novel sigma receptor ligand with improved metabolic stability and antagonistic effects against methamphetamine. AAPS J. 2012;14(1):43-51.
27. Rawson RA, Marinelli-Casey P, Anglin M, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99(6):708-717.
28. Vocci FJ, Montoya ID. Psychological treatments for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr Opin Psychiatry. 2009;22(3):263-268.
29. Rawson RA, McCann MJ, Flammino F, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267-274.
30. Roll JM, Petry NM, Stitzer ML, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry. 2006;163(11):1993-1999.
31. Brecht ML, von Mayrhauser C, Anglin MD. Predictors of relapse after treatment for methamphetamine use. J Psychoactive Drugs. 2000;32(2):211-220.
32. Smout MF, Longo M, Harrison S, et al. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and Acceptance and Commitment Therapy. Subst Abus. 2010;31(2):98-107.
33. Wang G, Shi J, Chen N, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;24;8(7):e68791. doi: 10.1371/ journal.pone.0068791.
34. McKetin R, Lubman DI, Baker AL, et al. Dose-related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry. 2013;70(3):319-324.
35. Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35(6):593-598.
36. Dean AC, Sevak RJ, Monterosso JR, et al. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. J Stud Alcohol Drugs. 2011;72(6):943-953.
37. Zullino DF, Benguettat D, Khazaal Y. Improvement of cognitive performance by topiramate: blockage of automatic processes may be the underlying mechanism [Comment on: Effects of topiramate on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition in recently abstinent methamphetamine-dependent individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2007.] Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):787.
38. Witkiewitz K, Lustyk M, Bowen S. Retraining the addicted brain: a review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol Addict Behav. 2013;27(2):351-365.
Can what we learned about reducing no-shows in our clinic work for you?
The no-show rate is high in ambulatory psychiatric clinics, especially those associated with academic medical institutions, which usually accept all public insurance providers and do not maintain a strict rule by which patients are charged a penalty when they fail to keep a scheduled appointment—a policy that, to the contrary, is customary in private practice. The University of Texas (UT) Health Sciences Center at Houston is primarily an academic medical center with resident-managed, faculty-supervised clinics that provide care to a large volume of patients.
At the UT clinics, we have struggled with a high no-show rate, and were challenged to reduce that rate. Our study of the problem, formulation and application of strategies to reduce that rate, and a discussion of our results are provided here for the benefit of psychiatric clinicians who struggle with this problem, to the detriment of their patients’ health and the financial well-being of the practice.
For patients who have a severe psychiatric illness, such as schizophrenia or bipolar disorder, 60% to 70% of the direct cost of their care is attributable to inpatient services.1,2 Poor medication adherence is a critical factor: It results in exacerbation of symptoms, relapse, and hospitalization. The matter is compounded by patients’ failure to show up for scheduled follow-up appointments.
Studies show that failure to attend routinely scheduled outpatient appointments increases the risk of hospitalization. Recent research has shown that, among all causes of hospitalization, length of stay and relapse hospitalization are increased in patients with low adherence to their treatment regimen.3 Patients who miss an appointment also are more unwell and more functionally impaired—also contributing to a higher risk and rate of rehospitalization.4,5
To begin to address the problem at UT, we acknowledged that an elevated no-show rate is linked to medication nonadherence, increased risk of re-hospitalization, and increased costs associated with poor care.
Impact of nonadherence
Significant evidence supports the efficacy of antipsychotic medications for treating schizophrenia, of course,6 but that success story is undermined by the mean rate of medication nonadherence among schizophrenia patients, which can be as high as 49% in studies.7 (The actual rate might be higher because those studies do not account for persons who refuse treatment or drop out.)
Nonadherence increases the risk of relapse 3.7-fold, compared with what is seen in patients who adhere to treatment.8 Nonadherence to a medication regimen also can increase patients’ risk of engaging in assault and other dangerous behaviors, especially during periods of psychosis.8 Variables consistently associated with nonadherence include poor insight, negative attitude or subjective response toward medication, previous nonadherence, substance abuse, shorter duration of illness, inadequate discharge planning or after-care environment, and poorer therapeutic alliance.7,8
Investigation of medication adherence in bipolar disorder suggests that 1 in 3 patients fail to take at least 30% of their medication.9 In such patients, medication nonadherence can lead to mania, depression, hospital readmission, suicide, increased substance abuse, and nonresponse to treatment.10,11
Depression also is associated with an increased rate of health care utilization and severe limitation in daily functioning.12 Compared with non-depressed patients, depressed patients are 3 times more likely to be nonadherent with medical treatment recommendations.13 Estimates of medication nonadherence for unipolar and bipolar disorders range from 10% to 60% (median, 40%). This prevalence has not changed significantly with the introduction of new medications.14
Our literature review of research devoted to reducing no-shows found that few studies have explored this critical treatment concern. The no-show rate was higher among younger patients and slightly higher among women, but varied by diagnosis.15 The most common reason psychiatric patients gave for missing an appointment was “forgetting”—a response heard twice as often among no-show patients in psychiatry than in other specialties.4
Little has been tried to solve the problem. Often, community mental health centers and private practices double-book appointments. Double-booking is intended to reduce the financial burden on the practice when a patient misses an appointment. This approach fails to address nonadherence or the poor care that usually results when a patient misses regular outpatient appointments.
Several methods have been employed to improve adherence, such as electronic pill dispensing.16 Increasing medication adherence appears to be a key factor in improving quality-of-life measures in patients with schizophrenia.6
The UT project
Methods. This project was completed at the ambulatory psychiatry clinic at the UT Medical School at Houston. The clinic staff comprises residents and faculty members who provide outpatient care. During the study period, the clinic was scheduling as many as 800 office visits a month, including a mix of new and follow-up appointments. Two weeks’ retrospective data revealed a no-show rate of 31%.
For the project, we defined no-show rate as the total number of patients who missed an appointment or canceled fewer than 24 hours before the scheduled time, divided by the total number of patients scheduled that day.
Table 1 demonstrates the no-show rate calculations for 1 of the weeks preceding the start of the project. Given approximately 800 patient appointments a month, a 31% no-show rate meant that, first, 248 patients failed to receive recommended care and, second, 248 appointment slots were wasted.
Besides undermining such components of quality care as patient safety and medication compliance, the high no-show rate also harms employee morale and productivity; impairs medical education; and, possibly, increases the use of emergency and after-hour services.
We agreed that our current no-show rate of 31% was too high.
We then formed a team of residents, faculty members, therapists, front office staff, an office manager, and an office nurse. We explored and hypothesized what could be contributing to the high no-show rate (Table 2).
Several interventions were then devised and implemented:
• Patients. We increased patient education about 1) the need for regular follow-up and 2) risks associated with medication nonadherence.
• Environment. We explored environmental limitations to access and agreed that certain static factors could not be modified—eg, location of the clinic and lack of access to public transportation. We were able to make some changes to the environment (explained later) to reduce wait time.
• Staff. Some patients had complained of long wait times, which could hinder active participation in treatment. We agreed that the clinic nurse would make rounds through the waiting room every hour and talk to patients. The nurse would identify patients who had been waiting for longer than 30 minutes after their scheduled appointment time and notify the doctor accordingly. We also agreed to revise patient appointment reminder practices: instead of using an automated answering service, one of the staff members called patients personally to remind them about their appointments. (This also allowed us to update telephone numbers for many patients; numbers on record often were outdated.) We initially recruited summer interns and provided a written script to follow during calls to patients, which allowed patients to confirm, cancel, or reschedule their appointment. Once we demonstrated positive results from the change to personal calls, the department agreed to absorb the cost, and front desk personnel began making reminder calls.
• Policies and procedures. Although some practices are able to charge a small fine for missed appointments, this was not allowed at our institution. Instead, we had several departmental policies on the books, such as discharging patients from our clinics if they missed 3 consecutive appointments and limiting prescription refills to a maximum of 6 months. These policies were neither communicated to patients and staff, nor were they implemented. We decided to educate patients and staff and implement the policies.
• Transparency. We posted the no-show rate in common areas so that the team could review and follow the progression of that rate as we implemented the changes. This allowed team members to take ownership of the project and facilitated active participation.
By implementing these changes, we aimed to reduce the no-show rate to 20%.
Results. We were able to reduce the no-show rate from a documented average of 31% to an average of 12% during the study period after implementing all the proposed changes in the outpatient clinics.
We calculated the no-show rate (as shown in Table 1 for May 2013), then collected the daily no-show rate from June to September 2013 (Figure). With these calculations, we demonstrated a reduction in the no-show rate to 12%. Because of the time and effort required, we reduced data collection from daily to weekly, beginning in September.
Applying the changes required consistent effort and substantial input from various stakeholders—front desk staff, residents, the nurse, therapists, and faculty. Gradually, we were able to implement all the changes.
Keeping the no-show rate low required consistent effort and monitoring of the newly implemented procedures because even a slight change, such as failure to make reminder calls, resulted in a sudden increase in the no-show rate (that was the case in October of the study period, when we were short-staffed and could not call every patient). Patients told us that it was difficult to ignore a personal call; if they were not planning to keep the appointment, the call allowed them to reschedule on the spot.
We also made sure that current no-show rates were posted in common areas, visible to team members every day.
Discussion
We attempted a literature review of research exploring approaches to reducing the no-show rate but found few studies that explored this critical concern in patient treatment.15 Some data suggested that, in the setting studied, the no-show rate:
• was higher among younger patients (age 20 to 39) than older ones (age 60 to 79)
• was slightly higher in women than in men
• varied by diagnosis.
We found a paucity of data regarding interventions that can reduce the no-show rate.
Among the changes we made, the one that had the greatest impact was personalized appointment reminder calls, as evidenced by our patients’ reports and the increase in the no-show rate when personal calls were not made.
We also realized that, although we had several departmental policies in place regarding appointments, they were not being followed. Raising awareness among team members and their patients also was an effective deterrent to a no-show for an appointment. For example, patients were informed that 3 consecutive no-shows could lead to termination of care. Often, they reacted with surprise to this caution but also voiced a desire to improve their attendance to avoid such an outcome.
We found that establishing common operational definitions is important. It also was important to have a cohesive team, with every member agreeing on goals and changes to operational policies that needed to be implemented. Support from the department chair and the administration, we learned, is vital to the success of such an intervention.
A note about limitations. The goal of the project was limited to reducing the no-show rate. We demonstrated that this is possible among patients who have a severe mental illness, and that reducing the associated waste of time and resources can improve finances in an academic department of psychiatry. We would need additional measures, however, to quantify medication adherence and hospitalization; a larger, more inclusive project is needed to demonstrate that reducing the no-show rate reduces the symptomatic burden of psychiatric illness.
Comments in conclusion
This project was designed and conducted as a required part of a Clinical Safety and Effectiveness Program at Memorial Hermann Texas Medical Center and the UT Medical School at Houston.17 Although there was initial hesitancy about attempting to reduce the no-show rate in a chronically mentally ill population, the success of this project—indeed, it surpassed its proposed goals—demonstrates that operational changes in any clinic can reduce the no-show rate. It also is important to maintain operational changes, however; without consistent effort, desired results cannot be sustained.
Last, it is possible to replicate the methodology of this project and thereby attempt to reduce the no-show rate in other divisions of medicine that offer care to chronically ill patients, such as pediatrics and family medicine.
Bottom Line
Failure to attend routinely scheduled outpatient appointments increases a patient’s functional impairment and risk of hospitalization. Patient education, appointment reminder phone calls, revised policies and procedures, and transparency regarding the no-show rate can reduce the number of missed appointments and improve patient outcomes.
Related Resources
• Mitchell AJ, Selmes T. Why don’t patients attend their appointments? Maintaining engagement with psychiatric services. Advances in Psychiatric Treatment. 2007;13:423-434.
• Molfenter T. Reducing appointment no-shows: going from theory to practice. Subst Use Misuse. 2013;48(9):743-749.
• Williston MA, Block-Lerner J, Wolanin A, et al. Brief acceptance-based intervention for increasing intake attendance at a community mental health center. Psychol Serv. 2014;11(3):324-332.
Disclosure
Dr. Gajwani receives grant or research support from the National Institute on Mental Health, the National Institute of Drug Abuse, The Stanley Foundation, and Forest Laboratories, Inc. He is a member of the speakers’ bureau of AstraZeneca, Merck, Otsuka America Pharmaceutical, and Sunovion Pharmaceuticals.
1. Wyatt RJ, Henter I. An economic evaluation of manic-depressive illness—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5):213-219.
2. Wyatt RJ, Henter I, Leary MC, et al. An economic evaluation of schizophrenia—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5);196-205.
3. Offord S, Lin J, Wong B, et al. Impact of oral antipsychotic medication adherence on healthcare resource utilization among schizophrenic patients with medicare coverage. Community Ment Health J. 2013;49(6):625-629.
4. Killaspy H, Banerjee S, King M, et al. Prospective controlled study of psychiatric out-patient non-attendance: characteristics and outcome. Br J Psychiatry. 2000;176:160- 165.
5. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv. 2000;51(7):885-889.
6. Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ. 1998;317(7167):1181-1184.
7. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892-909.
8. Fenton WS, Blyler C, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
9. Scott J, Pope M. Self-reported adherence to treatment with mood stabilizers, plasma levels, and psychiatric hospitalization. Am J Psychiatry. 2002;159(11):1927-1929.
10. Adams J, Scott J. Predicting medication adherence in severe mental disorders. Acta Psychiatr Scand. 2000;101(2):119-124.
11. Müller-Oerlinghausen B, Müser-Causemann B, Volk J. Suicides and parasuicides in a high-risk patient group on and off lithium long-term treatment. J Affect Disord. 1992;25(4):261-269.
12. Manning WG Jr, Wells KB. The effects of psychological distress and psychological well-being on use of medical services. Med Care. 1992;30(6):541-553.
13. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
14. Lingam R, Scott J. Treatment non‐adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164-172.
15. Allan AT. No-shows at a community mental health clinic: a pilot study. Int J Soc Psychiatry. 1988;34(1):40-46.
16. Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv. 1998;49(2):196-201.
17. Gajwani P. Improving quality of care: reducing no-show rate in ambulatory psychiatry clinic. Poster presented at: American Psychiatric Association 166th Annual Meeting; May 18-22, 2013; San Francisco, CA.
The no-show rate is high in ambulatory psychiatric clinics, especially those associated with academic medical institutions, which usually accept all public insurance providers and do not maintain a strict rule by which patients are charged a penalty when they fail to keep a scheduled appointment—a policy that, to the contrary, is customary in private practice. The University of Texas (UT) Health Sciences Center at Houston is primarily an academic medical center with resident-managed, faculty-supervised clinics that provide care to a large volume of patients.
At the UT clinics, we have struggled with a high no-show rate, and were challenged to reduce that rate. Our study of the problem, formulation and application of strategies to reduce that rate, and a discussion of our results are provided here for the benefit of psychiatric clinicians who struggle with this problem, to the detriment of their patients’ health and the financial well-being of the practice.
For patients who have a severe psychiatric illness, such as schizophrenia or bipolar disorder, 60% to 70% of the direct cost of their care is attributable to inpatient services.1,2 Poor medication adherence is a critical factor: It results in exacerbation of symptoms, relapse, and hospitalization. The matter is compounded by patients’ failure to show up for scheduled follow-up appointments.
Studies show that failure to attend routinely scheduled outpatient appointments increases the risk of hospitalization. Recent research has shown that, among all causes of hospitalization, length of stay and relapse hospitalization are increased in patients with low adherence to their treatment regimen.3 Patients who miss an appointment also are more unwell and more functionally impaired—also contributing to a higher risk and rate of rehospitalization.4,5
To begin to address the problem at UT, we acknowledged that an elevated no-show rate is linked to medication nonadherence, increased risk of re-hospitalization, and increased costs associated with poor care.
Impact of nonadherence
Significant evidence supports the efficacy of antipsychotic medications for treating schizophrenia, of course,6 but that success story is undermined by the mean rate of medication nonadherence among schizophrenia patients, which can be as high as 49% in studies.7 (The actual rate might be higher because those studies do not account for persons who refuse treatment or drop out.)
Nonadherence increases the risk of relapse 3.7-fold, compared with what is seen in patients who adhere to treatment.8 Nonadherence to a medication regimen also can increase patients’ risk of engaging in assault and other dangerous behaviors, especially during periods of psychosis.8 Variables consistently associated with nonadherence include poor insight, negative attitude or subjective response toward medication, previous nonadherence, substance abuse, shorter duration of illness, inadequate discharge planning or after-care environment, and poorer therapeutic alliance.7,8
Investigation of medication adherence in bipolar disorder suggests that 1 in 3 patients fail to take at least 30% of their medication.9 In such patients, medication nonadherence can lead to mania, depression, hospital readmission, suicide, increased substance abuse, and nonresponse to treatment.10,11
Depression also is associated with an increased rate of health care utilization and severe limitation in daily functioning.12 Compared with non-depressed patients, depressed patients are 3 times more likely to be nonadherent with medical treatment recommendations.13 Estimates of medication nonadherence for unipolar and bipolar disorders range from 10% to 60% (median, 40%). This prevalence has not changed significantly with the introduction of new medications.14
Our literature review of research devoted to reducing no-shows found that few studies have explored this critical treatment concern. The no-show rate was higher among younger patients and slightly higher among women, but varied by diagnosis.15 The most common reason psychiatric patients gave for missing an appointment was “forgetting”—a response heard twice as often among no-show patients in psychiatry than in other specialties.4
Little has been tried to solve the problem. Often, community mental health centers and private practices double-book appointments. Double-booking is intended to reduce the financial burden on the practice when a patient misses an appointment. This approach fails to address nonadherence or the poor care that usually results when a patient misses regular outpatient appointments.
Several methods have been employed to improve adherence, such as electronic pill dispensing.16 Increasing medication adherence appears to be a key factor in improving quality-of-life measures in patients with schizophrenia.6
The UT project
Methods. This project was completed at the ambulatory psychiatry clinic at the UT Medical School at Houston. The clinic staff comprises residents and faculty members who provide outpatient care. During the study period, the clinic was scheduling as many as 800 office visits a month, including a mix of new and follow-up appointments. Two weeks’ retrospective data revealed a no-show rate of 31%.
For the project, we defined no-show rate as the total number of patients who missed an appointment or canceled fewer than 24 hours before the scheduled time, divided by the total number of patients scheduled that day.
Table 1 demonstrates the no-show rate calculations for 1 of the weeks preceding the start of the project. Given approximately 800 patient appointments a month, a 31% no-show rate meant that, first, 248 patients failed to receive recommended care and, second, 248 appointment slots were wasted.
Besides undermining such components of quality care as patient safety and medication compliance, the high no-show rate also harms employee morale and productivity; impairs medical education; and, possibly, increases the use of emergency and after-hour services.
We agreed that our current no-show rate of 31% was too high.
We then formed a team of residents, faculty members, therapists, front office staff, an office manager, and an office nurse. We explored and hypothesized what could be contributing to the high no-show rate (Table 2).
Several interventions were then devised and implemented:
• Patients. We increased patient education about 1) the need for regular follow-up and 2) risks associated with medication nonadherence.
• Environment. We explored environmental limitations to access and agreed that certain static factors could not be modified—eg, location of the clinic and lack of access to public transportation. We were able to make some changes to the environment (explained later) to reduce wait time.
• Staff. Some patients had complained of long wait times, which could hinder active participation in treatment. We agreed that the clinic nurse would make rounds through the waiting room every hour and talk to patients. The nurse would identify patients who had been waiting for longer than 30 minutes after their scheduled appointment time and notify the doctor accordingly. We also agreed to revise patient appointment reminder practices: instead of using an automated answering service, one of the staff members called patients personally to remind them about their appointments. (This also allowed us to update telephone numbers for many patients; numbers on record often were outdated.) We initially recruited summer interns and provided a written script to follow during calls to patients, which allowed patients to confirm, cancel, or reschedule their appointment. Once we demonstrated positive results from the change to personal calls, the department agreed to absorb the cost, and front desk personnel began making reminder calls.
• Policies and procedures. Although some practices are able to charge a small fine for missed appointments, this was not allowed at our institution. Instead, we had several departmental policies on the books, such as discharging patients from our clinics if they missed 3 consecutive appointments and limiting prescription refills to a maximum of 6 months. These policies were neither communicated to patients and staff, nor were they implemented. We decided to educate patients and staff and implement the policies.
• Transparency. We posted the no-show rate in common areas so that the team could review and follow the progression of that rate as we implemented the changes. This allowed team members to take ownership of the project and facilitated active participation.
By implementing these changes, we aimed to reduce the no-show rate to 20%.
Results. We were able to reduce the no-show rate from a documented average of 31% to an average of 12% during the study period after implementing all the proposed changes in the outpatient clinics.
We calculated the no-show rate (as shown in Table 1 for May 2013), then collected the daily no-show rate from June to September 2013 (Figure). With these calculations, we demonstrated a reduction in the no-show rate to 12%. Because of the time and effort required, we reduced data collection from daily to weekly, beginning in September.
Applying the changes required consistent effort and substantial input from various stakeholders—front desk staff, residents, the nurse, therapists, and faculty. Gradually, we were able to implement all the changes.
Keeping the no-show rate low required consistent effort and monitoring of the newly implemented procedures because even a slight change, such as failure to make reminder calls, resulted in a sudden increase in the no-show rate (that was the case in October of the study period, when we were short-staffed and could not call every patient). Patients told us that it was difficult to ignore a personal call; if they were not planning to keep the appointment, the call allowed them to reschedule on the spot.
We also made sure that current no-show rates were posted in common areas, visible to team members every day.
Discussion
We attempted a literature review of research exploring approaches to reducing the no-show rate but found few studies that explored this critical concern in patient treatment.15 Some data suggested that, in the setting studied, the no-show rate:
• was higher among younger patients (age 20 to 39) than older ones (age 60 to 79)
• was slightly higher in women than in men
• varied by diagnosis.
We found a paucity of data regarding interventions that can reduce the no-show rate.
Among the changes we made, the one that had the greatest impact was personalized appointment reminder calls, as evidenced by our patients’ reports and the increase in the no-show rate when personal calls were not made.
We also realized that, although we had several departmental policies in place regarding appointments, they were not being followed. Raising awareness among team members and their patients also was an effective deterrent to a no-show for an appointment. For example, patients were informed that 3 consecutive no-shows could lead to termination of care. Often, they reacted with surprise to this caution but also voiced a desire to improve their attendance to avoid such an outcome.
We found that establishing common operational definitions is important. It also was important to have a cohesive team, with every member agreeing on goals and changes to operational policies that needed to be implemented. Support from the department chair and the administration, we learned, is vital to the success of such an intervention.
A note about limitations. The goal of the project was limited to reducing the no-show rate. We demonstrated that this is possible among patients who have a severe mental illness, and that reducing the associated waste of time and resources can improve finances in an academic department of psychiatry. We would need additional measures, however, to quantify medication adherence and hospitalization; a larger, more inclusive project is needed to demonstrate that reducing the no-show rate reduces the symptomatic burden of psychiatric illness.
Comments in conclusion
This project was designed and conducted as a required part of a Clinical Safety and Effectiveness Program at Memorial Hermann Texas Medical Center and the UT Medical School at Houston.17 Although there was initial hesitancy about attempting to reduce the no-show rate in a chronically mentally ill population, the success of this project—indeed, it surpassed its proposed goals—demonstrates that operational changes in any clinic can reduce the no-show rate. It also is important to maintain operational changes, however; without consistent effort, desired results cannot be sustained.
Last, it is possible to replicate the methodology of this project and thereby attempt to reduce the no-show rate in other divisions of medicine that offer care to chronically ill patients, such as pediatrics and family medicine.
Bottom Line
Failure to attend routinely scheduled outpatient appointments increases a patient’s functional impairment and risk of hospitalization. Patient education, appointment reminder phone calls, revised policies and procedures, and transparency regarding the no-show rate can reduce the number of missed appointments and improve patient outcomes.
Related Resources
• Mitchell AJ, Selmes T. Why don’t patients attend their appointments? Maintaining engagement with psychiatric services. Advances in Psychiatric Treatment. 2007;13:423-434.
• Molfenter T. Reducing appointment no-shows: going from theory to practice. Subst Use Misuse. 2013;48(9):743-749.
• Williston MA, Block-Lerner J, Wolanin A, et al. Brief acceptance-based intervention for increasing intake attendance at a community mental health center. Psychol Serv. 2014;11(3):324-332.
Disclosure
Dr. Gajwani receives grant or research support from the National Institute on Mental Health, the National Institute of Drug Abuse, The Stanley Foundation, and Forest Laboratories, Inc. He is a member of the speakers’ bureau of AstraZeneca, Merck, Otsuka America Pharmaceutical, and Sunovion Pharmaceuticals.
The no-show rate is high in ambulatory psychiatric clinics, especially those associated with academic medical institutions, which usually accept all public insurance providers and do not maintain a strict rule by which patients are charged a penalty when they fail to keep a scheduled appointment—a policy that, to the contrary, is customary in private practice. The University of Texas (UT) Health Sciences Center at Houston is primarily an academic medical center with resident-managed, faculty-supervised clinics that provide care to a large volume of patients.
At the UT clinics, we have struggled with a high no-show rate, and were challenged to reduce that rate. Our study of the problem, formulation and application of strategies to reduce that rate, and a discussion of our results are provided here for the benefit of psychiatric clinicians who struggle with this problem, to the detriment of their patients’ health and the financial well-being of the practice.
For patients who have a severe psychiatric illness, such as schizophrenia or bipolar disorder, 60% to 70% of the direct cost of their care is attributable to inpatient services.1,2 Poor medication adherence is a critical factor: It results in exacerbation of symptoms, relapse, and hospitalization. The matter is compounded by patients’ failure to show up for scheduled follow-up appointments.
Studies show that failure to attend routinely scheduled outpatient appointments increases the risk of hospitalization. Recent research has shown that, among all causes of hospitalization, length of stay and relapse hospitalization are increased in patients with low adherence to their treatment regimen.3 Patients who miss an appointment also are more unwell and more functionally impaired—also contributing to a higher risk and rate of rehospitalization.4,5
To begin to address the problem at UT, we acknowledged that an elevated no-show rate is linked to medication nonadherence, increased risk of re-hospitalization, and increased costs associated with poor care.
Impact of nonadherence
Significant evidence supports the efficacy of antipsychotic medications for treating schizophrenia, of course,6 but that success story is undermined by the mean rate of medication nonadherence among schizophrenia patients, which can be as high as 49% in studies.7 (The actual rate might be higher because those studies do not account for persons who refuse treatment or drop out.)
Nonadherence increases the risk of relapse 3.7-fold, compared with what is seen in patients who adhere to treatment.8 Nonadherence to a medication regimen also can increase patients’ risk of engaging in assault and other dangerous behaviors, especially during periods of psychosis.8 Variables consistently associated with nonadherence include poor insight, negative attitude or subjective response toward medication, previous nonadherence, substance abuse, shorter duration of illness, inadequate discharge planning or after-care environment, and poorer therapeutic alliance.7,8
Investigation of medication adherence in bipolar disorder suggests that 1 in 3 patients fail to take at least 30% of their medication.9 In such patients, medication nonadherence can lead to mania, depression, hospital readmission, suicide, increased substance abuse, and nonresponse to treatment.10,11
Depression also is associated with an increased rate of health care utilization and severe limitation in daily functioning.12 Compared with non-depressed patients, depressed patients are 3 times more likely to be nonadherent with medical treatment recommendations.13 Estimates of medication nonadherence for unipolar and bipolar disorders range from 10% to 60% (median, 40%). This prevalence has not changed significantly with the introduction of new medications.14
Our literature review of research devoted to reducing no-shows found that few studies have explored this critical treatment concern. The no-show rate was higher among younger patients and slightly higher among women, but varied by diagnosis.15 The most common reason psychiatric patients gave for missing an appointment was “forgetting”—a response heard twice as often among no-show patients in psychiatry than in other specialties.4
Little has been tried to solve the problem. Often, community mental health centers and private practices double-book appointments. Double-booking is intended to reduce the financial burden on the practice when a patient misses an appointment. This approach fails to address nonadherence or the poor care that usually results when a patient misses regular outpatient appointments.
Several methods have been employed to improve adherence, such as electronic pill dispensing.16 Increasing medication adherence appears to be a key factor in improving quality-of-life measures in patients with schizophrenia.6
The UT project
Methods. This project was completed at the ambulatory psychiatry clinic at the UT Medical School at Houston. The clinic staff comprises residents and faculty members who provide outpatient care. During the study period, the clinic was scheduling as many as 800 office visits a month, including a mix of new and follow-up appointments. Two weeks’ retrospective data revealed a no-show rate of 31%.
For the project, we defined no-show rate as the total number of patients who missed an appointment or canceled fewer than 24 hours before the scheduled time, divided by the total number of patients scheduled that day.
Table 1 demonstrates the no-show rate calculations for 1 of the weeks preceding the start of the project. Given approximately 800 patient appointments a month, a 31% no-show rate meant that, first, 248 patients failed to receive recommended care and, second, 248 appointment slots were wasted.
Besides undermining such components of quality care as patient safety and medication compliance, the high no-show rate also harms employee morale and productivity; impairs medical education; and, possibly, increases the use of emergency and after-hour services.
We agreed that our current no-show rate of 31% was too high.
We then formed a team of residents, faculty members, therapists, front office staff, an office manager, and an office nurse. We explored and hypothesized what could be contributing to the high no-show rate (Table 2).
Several interventions were then devised and implemented:
• Patients. We increased patient education about 1) the need for regular follow-up and 2) risks associated with medication nonadherence.
• Environment. We explored environmental limitations to access and agreed that certain static factors could not be modified—eg, location of the clinic and lack of access to public transportation. We were able to make some changes to the environment (explained later) to reduce wait time.
• Staff. Some patients had complained of long wait times, which could hinder active participation in treatment. We agreed that the clinic nurse would make rounds through the waiting room every hour and talk to patients. The nurse would identify patients who had been waiting for longer than 30 minutes after their scheduled appointment time and notify the doctor accordingly. We also agreed to revise patient appointment reminder practices: instead of using an automated answering service, one of the staff members called patients personally to remind them about their appointments. (This also allowed us to update telephone numbers for many patients; numbers on record often were outdated.) We initially recruited summer interns and provided a written script to follow during calls to patients, which allowed patients to confirm, cancel, or reschedule their appointment. Once we demonstrated positive results from the change to personal calls, the department agreed to absorb the cost, and front desk personnel began making reminder calls.
• Policies and procedures. Although some practices are able to charge a small fine for missed appointments, this was not allowed at our institution. Instead, we had several departmental policies on the books, such as discharging patients from our clinics if they missed 3 consecutive appointments and limiting prescription refills to a maximum of 6 months. These policies were neither communicated to patients and staff, nor were they implemented. We decided to educate patients and staff and implement the policies.
• Transparency. We posted the no-show rate in common areas so that the team could review and follow the progression of that rate as we implemented the changes. This allowed team members to take ownership of the project and facilitated active participation.
By implementing these changes, we aimed to reduce the no-show rate to 20%.
Results. We were able to reduce the no-show rate from a documented average of 31% to an average of 12% during the study period after implementing all the proposed changes in the outpatient clinics.
We calculated the no-show rate (as shown in Table 1 for May 2013), then collected the daily no-show rate from June to September 2013 (Figure). With these calculations, we demonstrated a reduction in the no-show rate to 12%. Because of the time and effort required, we reduced data collection from daily to weekly, beginning in September.
Applying the changes required consistent effort and substantial input from various stakeholders—front desk staff, residents, the nurse, therapists, and faculty. Gradually, we were able to implement all the changes.
Keeping the no-show rate low required consistent effort and monitoring of the newly implemented procedures because even a slight change, such as failure to make reminder calls, resulted in a sudden increase in the no-show rate (that was the case in October of the study period, when we were short-staffed and could not call every patient). Patients told us that it was difficult to ignore a personal call; if they were not planning to keep the appointment, the call allowed them to reschedule on the spot.
We also made sure that current no-show rates were posted in common areas, visible to team members every day.
Discussion
We attempted a literature review of research exploring approaches to reducing the no-show rate but found few studies that explored this critical concern in patient treatment.15 Some data suggested that, in the setting studied, the no-show rate:
• was higher among younger patients (age 20 to 39) than older ones (age 60 to 79)
• was slightly higher in women than in men
• varied by diagnosis.
We found a paucity of data regarding interventions that can reduce the no-show rate.
Among the changes we made, the one that had the greatest impact was personalized appointment reminder calls, as evidenced by our patients’ reports and the increase in the no-show rate when personal calls were not made.
We also realized that, although we had several departmental policies in place regarding appointments, they were not being followed. Raising awareness among team members and their patients also was an effective deterrent to a no-show for an appointment. For example, patients were informed that 3 consecutive no-shows could lead to termination of care. Often, they reacted with surprise to this caution but also voiced a desire to improve their attendance to avoid such an outcome.
We found that establishing common operational definitions is important. It also was important to have a cohesive team, with every member agreeing on goals and changes to operational policies that needed to be implemented. Support from the department chair and the administration, we learned, is vital to the success of such an intervention.
A note about limitations. The goal of the project was limited to reducing the no-show rate. We demonstrated that this is possible among patients who have a severe mental illness, and that reducing the associated waste of time and resources can improve finances in an academic department of psychiatry. We would need additional measures, however, to quantify medication adherence and hospitalization; a larger, more inclusive project is needed to demonstrate that reducing the no-show rate reduces the symptomatic burden of psychiatric illness.
Comments in conclusion
This project was designed and conducted as a required part of a Clinical Safety and Effectiveness Program at Memorial Hermann Texas Medical Center and the UT Medical School at Houston.17 Although there was initial hesitancy about attempting to reduce the no-show rate in a chronically mentally ill population, the success of this project—indeed, it surpassed its proposed goals—demonstrates that operational changes in any clinic can reduce the no-show rate. It also is important to maintain operational changes, however; without consistent effort, desired results cannot be sustained.
Last, it is possible to replicate the methodology of this project and thereby attempt to reduce the no-show rate in other divisions of medicine that offer care to chronically ill patients, such as pediatrics and family medicine.
Bottom Line
Failure to attend routinely scheduled outpatient appointments increases a patient’s functional impairment and risk of hospitalization. Patient education, appointment reminder phone calls, revised policies and procedures, and transparency regarding the no-show rate can reduce the number of missed appointments and improve patient outcomes.
Related Resources
• Mitchell AJ, Selmes T. Why don’t patients attend their appointments? Maintaining engagement with psychiatric services. Advances in Psychiatric Treatment. 2007;13:423-434.
• Molfenter T. Reducing appointment no-shows: going from theory to practice. Subst Use Misuse. 2013;48(9):743-749.
• Williston MA, Block-Lerner J, Wolanin A, et al. Brief acceptance-based intervention for increasing intake attendance at a community mental health center. Psychol Serv. 2014;11(3):324-332.
Disclosure
Dr. Gajwani receives grant or research support from the National Institute on Mental Health, the National Institute of Drug Abuse, The Stanley Foundation, and Forest Laboratories, Inc. He is a member of the speakers’ bureau of AstraZeneca, Merck, Otsuka America Pharmaceutical, and Sunovion Pharmaceuticals.
1. Wyatt RJ, Henter I. An economic evaluation of manic-depressive illness—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5):213-219.
2. Wyatt RJ, Henter I, Leary MC, et al. An economic evaluation of schizophrenia—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5);196-205.
3. Offord S, Lin J, Wong B, et al. Impact of oral antipsychotic medication adherence on healthcare resource utilization among schizophrenic patients with medicare coverage. Community Ment Health J. 2013;49(6):625-629.
4. Killaspy H, Banerjee S, King M, et al. Prospective controlled study of psychiatric out-patient non-attendance: characteristics and outcome. Br J Psychiatry. 2000;176:160- 165.
5. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv. 2000;51(7):885-889.
6. Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ. 1998;317(7167):1181-1184.
7. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892-909.
8. Fenton WS, Blyler C, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
9. Scott J, Pope M. Self-reported adherence to treatment with mood stabilizers, plasma levels, and psychiatric hospitalization. Am J Psychiatry. 2002;159(11):1927-1929.
10. Adams J, Scott J. Predicting medication adherence in severe mental disorders. Acta Psychiatr Scand. 2000;101(2):119-124.
11. Müller-Oerlinghausen B, Müser-Causemann B, Volk J. Suicides and parasuicides in a high-risk patient group on and off lithium long-term treatment. J Affect Disord. 1992;25(4):261-269.
12. Manning WG Jr, Wells KB. The effects of psychological distress and psychological well-being on use of medical services. Med Care. 1992;30(6):541-553.
13. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
14. Lingam R, Scott J. Treatment non‐adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164-172.
15. Allan AT. No-shows at a community mental health clinic: a pilot study. Int J Soc Psychiatry. 1988;34(1):40-46.
16. Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv. 1998;49(2):196-201.
17. Gajwani P. Improving quality of care: reducing no-show rate in ambulatory psychiatry clinic. Poster presented at: American Psychiatric Association 166th Annual Meeting; May 18-22, 2013; San Francisco, CA.
1. Wyatt RJ, Henter I. An economic evaluation of manic-depressive illness—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5):213-219.
2. Wyatt RJ, Henter I, Leary MC, et al. An economic evaluation of schizophrenia—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5);196-205.
3. Offord S, Lin J, Wong B, et al. Impact of oral antipsychotic medication adherence on healthcare resource utilization among schizophrenic patients with medicare coverage. Community Ment Health J. 2013;49(6):625-629.
4. Killaspy H, Banerjee S, King M, et al. Prospective controlled study of psychiatric out-patient non-attendance: characteristics and outcome. Br J Psychiatry. 2000;176:160- 165.
5. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv. 2000;51(7):885-889.
6. Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ. 1998;317(7167):1181-1184.
7. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892-909.
8. Fenton WS, Blyler C, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
9. Scott J, Pope M. Self-reported adherence to treatment with mood stabilizers, plasma levels, and psychiatric hospitalization. Am J Psychiatry. 2002;159(11):1927-1929.
10. Adams J, Scott J. Predicting medication adherence in severe mental disorders. Acta Psychiatr Scand. 2000;101(2):119-124.
11. Müller-Oerlinghausen B, Müser-Causemann B, Volk J. Suicides and parasuicides in a high-risk patient group on and off lithium long-term treatment. J Affect Disord. 1992;25(4):261-269.
12. Manning WG Jr, Wells KB. The effects of psychological distress and psychological well-being on use of medical services. Med Care. 1992;30(6):541-553.
13. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
14. Lingam R, Scott J. Treatment non‐adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164-172.
15. Allan AT. No-shows at a community mental health clinic: a pilot study. Int J Soc Psychiatry. 1988;34(1):40-46.
16. Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv. 1998;49(2):196-201.
17. Gajwani P. Improving quality of care: reducing no-show rate in ambulatory psychiatry clinic. Poster presented at: American Psychiatric Association 166th Annual Meeting; May 18-22, 2013; San Francisco, CA.
Can philanthropy fill the unmet needs of psychiatry?
Recent examples come quickly to mind:
• $100 million from the Lieber family to fund the Lieber Institute for Brain Development at Johns Hopkins University
• $300 million from Microsoft co-founder Paul G. Allen to create the Allen Institute for Brain Science
• $650 million from Ted Stanley for the Stanley Center at the Broad Institute.
Such generosity is cause for celebration by psychiatrists and their long-suffering patients who are disabled by a brain disorder. Private money supplements research funding by the National Institutes of Health and will bolster the war against mental illness,1 which costs >$300 billion annually (Box,2page 12).
Although philanthropy will help, many needs in psychiatry are unmet, and not all can be addressed with money. Consider a number of areas of need.
Unmet clinical needs
Models of disease. Psychiatry is in desperate need of an objective, valid diagnostic system that transcends the DSM model of symptom clusters. The Research Domain Criteria3 represents the effort to find an alternative. To achieve that goal, it’s necessary to identify biomarkers and establish their utility—a task that requires a huge amount of funding.
Therapeutics. Clinicians are hungry for innovative, safe pharmaceuticals and non-drug treatments that modify disease, not just alleviate symptoms. Obsessive-compulsive disorder always has lacked such therapies; so have dementia, schizophrenia, personality disorders, dissociative disorders, and sexual pathologies. In fact, >80% of DSM disorders do not have an FDA-approved, evidence-based treatment,4 and many available medications are only partially efficacious, poorly tolerated, or unsafe.
There are more unmet needs in therapeutics:
• Development of promising non-drug therapies, such as neuromodulation, proceeds slowly.
• Research into neurobiological mechanisms of psychotherapy is in its infancy.
• A foolproof method to monitor adherence does not exist, and countless patients relapse needlessly and deteriorate functionally.
• Effective, evidence-based rehabilitation for serious psychiatric disorders is used narrowly and vastly underfunded.
• Insurers continue to thumb their nose at laws that require parity for treating mental illness—thus impeding access to, delaying, or truncating psychiatric care.
Unmet scientific needs
Translational investigators. Despite increased funding for basic neuroscientific study and breathtaking discoveries in animal molecular neurobiology, a trickle of findings has been applied to clinical medicine. This translational gap has many causes, including a shortage of translational neuroscientists (MD-PhD psychiatrists and neurologists), insufficient long-term funding to develop such clinician-researchers, and complex regulatory oversight of human research.
Stalled progress in drug development. Development of novel-mechanism therapeutics for brain disorders is languishing. Some pharmaceutical manufacturers have abandoned the development of drugs that act on the CNS in favor of less complex, more lucrative areas such as oncology and cardiology; others have reduced their investment in CNS products. Developing treatments for knotty disorders of the most complex structure in the known universe requires mammoth investment. Why are stakeholders bailing out on the greatest challenge for science and medicine for easier endeavors?
Discovering new genes for every devastating neuropsychiatric syndrome, such as schizophrenia, is cause for celebration, but the champagne won’t flow until the coding of every gene is unscrambled so that specific biological interventions can be developed. The cost of the chase might be orders of magnitude greater than what is invested in research today. Conceptualizing new models of brain disorders is a critical part of scientific progress and an antidote to the inertia of perpetual group-think. Depression, for example, is being reconceptualized as a disorder of impaired neuroplasticity and neurotropic deficiency, rather than a shortage of serotonin and norepinephrine. Rapid reversal of severe depression to euthymia—in 1 or 2 hours—with IV ketamine shattered the dogma that depression takes weeks to lift, and is ushering in unprecedented new thinking and models likely to revolutionize treatment of severe depression. We need such breakthroughs for other psychiatric brain disorders.
Unmet professional and sociopolitical needs
Broadening of training. Psychiatrists have focused on the mind but insufficiently attended to the biology of the brain. For psychiatry to rise to the next level as a medical specialty and brain discipline, training must incorporate more neurology than it does now. The converse is true in neurology.
Hospitalization not incarceration. It is unconscionable that people suffering from a medical illness that impairs their judgment and behavior are locked up as criminals. Psychiatry must forcefully lobby so that the seriously mentally ill are treated in secure hospitals staffed by physicians, nurses, and mental health professionals.
That’s right: Bring back the asylum to address this unmet medical, political, and ethical need for psychiatric patients.a A serious mental disorder must be accepted as a fault-free illness.
aTo read more about this, I recommend my March 2008 editorial, “Bring back the asylums?,” at CurrentPsychiatry.com, and Dr. George Paulson’s excellent book, Closing the asylums: Causes and consequences of the deinstitutionalization movement (Jefferson, NC: McFarland & Co. Inc; 2012).
Full integration of psychiatry into the rest of medicine remains an unmet need, despite good progress. Because almost every medical illness can cause psychiatric symptoms, DSM-5 mandates that general medical conditions be ruled out before a primary psychiatric diagnosis is made.
Along the same lines, most severely mentally ill persons suffer from medical and neurologic ailments before their first episode,5 and many die prematurely from cardiovascular causes that often are the result of unhealthy lifestyle; iatrogenic complications; and lack of primary care interventions.6 Psychiatric patients must always receive standard general medical evaluation and management, side by side with their psychiatric care.
Philanthropy for psychiatry
Philanthropic support of psychiatry is a salutary trend. Some unmet needs in psychiatry, however, require not only money but a change in attitude (such as eliminating the absurd and discriminatory stigma of mental illness), better training, and forceful political activism by all of us.
1. Licinio J, Wong ML. Launching the ‘war on mental illness’. Mol Psychiatry. 2014;19(1):1-5.
2. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427.
3. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders. The majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
4. Cuthbert BN. The RDoC framework: facilitating transition from ICD/OSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28-35.
5. Sørensen HJ, Nielsen PR, Benros ME, et al. Somatic diseases and conditions before the first diagnosis of schizophrenia: a nationwide population-based cohort study in more than 900000 individuals [published online July 25, 2014]. Schizophr Bull. doi: 10.1093/schbul/sbu110.
6. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
Recent examples come quickly to mind:
• $100 million from the Lieber family to fund the Lieber Institute for Brain Development at Johns Hopkins University
• $300 million from Microsoft co-founder Paul G. Allen to create the Allen Institute for Brain Science
• $650 million from Ted Stanley for the Stanley Center at the Broad Institute.
Such generosity is cause for celebration by psychiatrists and their long-suffering patients who are disabled by a brain disorder. Private money supplements research funding by the National Institutes of Health and will bolster the war against mental illness,1 which costs >$300 billion annually (Box,2page 12).
Although philanthropy will help, many needs in psychiatry are unmet, and not all can be addressed with money. Consider a number of areas of need.
Unmet clinical needs
Models of disease. Psychiatry is in desperate need of an objective, valid diagnostic system that transcends the DSM model of symptom clusters. The Research Domain Criteria3 represents the effort to find an alternative. To achieve that goal, it’s necessary to identify biomarkers and establish their utility—a task that requires a huge amount of funding.
Therapeutics. Clinicians are hungry for innovative, safe pharmaceuticals and non-drug treatments that modify disease, not just alleviate symptoms. Obsessive-compulsive disorder always has lacked such therapies; so have dementia, schizophrenia, personality disorders, dissociative disorders, and sexual pathologies. In fact, >80% of DSM disorders do not have an FDA-approved, evidence-based treatment,4 and many available medications are only partially efficacious, poorly tolerated, or unsafe.
There are more unmet needs in therapeutics:
• Development of promising non-drug therapies, such as neuromodulation, proceeds slowly.
• Research into neurobiological mechanisms of psychotherapy is in its infancy.
• A foolproof method to monitor adherence does not exist, and countless patients relapse needlessly and deteriorate functionally.
• Effective, evidence-based rehabilitation for serious psychiatric disorders is used narrowly and vastly underfunded.
• Insurers continue to thumb their nose at laws that require parity for treating mental illness—thus impeding access to, delaying, or truncating psychiatric care.
Unmet scientific needs
Translational investigators. Despite increased funding for basic neuroscientific study and breathtaking discoveries in animal molecular neurobiology, a trickle of findings has been applied to clinical medicine. This translational gap has many causes, including a shortage of translational neuroscientists (MD-PhD psychiatrists and neurologists), insufficient long-term funding to develop such clinician-researchers, and complex regulatory oversight of human research.
Stalled progress in drug development. Development of novel-mechanism therapeutics for brain disorders is languishing. Some pharmaceutical manufacturers have abandoned the development of drugs that act on the CNS in favor of less complex, more lucrative areas such as oncology and cardiology; others have reduced their investment in CNS products. Developing treatments for knotty disorders of the most complex structure in the known universe requires mammoth investment. Why are stakeholders bailing out on the greatest challenge for science and medicine for easier endeavors?
Discovering new genes for every devastating neuropsychiatric syndrome, such as schizophrenia, is cause for celebration, but the champagne won’t flow until the coding of every gene is unscrambled so that specific biological interventions can be developed. The cost of the chase might be orders of magnitude greater than what is invested in research today. Conceptualizing new models of brain disorders is a critical part of scientific progress and an antidote to the inertia of perpetual group-think. Depression, for example, is being reconceptualized as a disorder of impaired neuroplasticity and neurotropic deficiency, rather than a shortage of serotonin and norepinephrine. Rapid reversal of severe depression to euthymia—in 1 or 2 hours—with IV ketamine shattered the dogma that depression takes weeks to lift, and is ushering in unprecedented new thinking and models likely to revolutionize treatment of severe depression. We need such breakthroughs for other psychiatric brain disorders.
Unmet professional and sociopolitical needs
Broadening of training. Psychiatrists have focused on the mind but insufficiently attended to the biology of the brain. For psychiatry to rise to the next level as a medical specialty and brain discipline, training must incorporate more neurology than it does now. The converse is true in neurology.
Hospitalization not incarceration. It is unconscionable that people suffering from a medical illness that impairs their judgment and behavior are locked up as criminals. Psychiatry must forcefully lobby so that the seriously mentally ill are treated in secure hospitals staffed by physicians, nurses, and mental health professionals.
That’s right: Bring back the asylum to address this unmet medical, political, and ethical need for psychiatric patients.a A serious mental disorder must be accepted as a fault-free illness.
aTo read more about this, I recommend my March 2008 editorial, “Bring back the asylums?,” at CurrentPsychiatry.com, and Dr. George Paulson’s excellent book, Closing the asylums: Causes and consequences of the deinstitutionalization movement (Jefferson, NC: McFarland & Co. Inc; 2012).
Full integration of psychiatry into the rest of medicine remains an unmet need, despite good progress. Because almost every medical illness can cause psychiatric symptoms, DSM-5 mandates that general medical conditions be ruled out before a primary psychiatric diagnosis is made.
Along the same lines, most severely mentally ill persons suffer from medical and neurologic ailments before their first episode,5 and many die prematurely from cardiovascular causes that often are the result of unhealthy lifestyle; iatrogenic complications; and lack of primary care interventions.6 Psychiatric patients must always receive standard general medical evaluation and management, side by side with their psychiatric care.
Philanthropy for psychiatry
Philanthropic support of psychiatry is a salutary trend. Some unmet needs in psychiatry, however, require not only money but a change in attitude (such as eliminating the absurd and discriminatory stigma of mental illness), better training, and forceful political activism by all of us.
Recent examples come quickly to mind:
• $100 million from the Lieber family to fund the Lieber Institute for Brain Development at Johns Hopkins University
• $300 million from Microsoft co-founder Paul G. Allen to create the Allen Institute for Brain Science
• $650 million from Ted Stanley for the Stanley Center at the Broad Institute.
Such generosity is cause for celebration by psychiatrists and their long-suffering patients who are disabled by a brain disorder. Private money supplements research funding by the National Institutes of Health and will bolster the war against mental illness,1 which costs >$300 billion annually (Box,2page 12).
Although philanthropy will help, many needs in psychiatry are unmet, and not all can be addressed with money. Consider a number of areas of need.
Unmet clinical needs
Models of disease. Psychiatry is in desperate need of an objective, valid diagnostic system that transcends the DSM model of symptom clusters. The Research Domain Criteria3 represents the effort to find an alternative. To achieve that goal, it’s necessary to identify biomarkers and establish their utility—a task that requires a huge amount of funding.
Therapeutics. Clinicians are hungry for innovative, safe pharmaceuticals and non-drug treatments that modify disease, not just alleviate symptoms. Obsessive-compulsive disorder always has lacked such therapies; so have dementia, schizophrenia, personality disorders, dissociative disorders, and sexual pathologies. In fact, >80% of DSM disorders do not have an FDA-approved, evidence-based treatment,4 and many available medications are only partially efficacious, poorly tolerated, or unsafe.
There are more unmet needs in therapeutics:
• Development of promising non-drug therapies, such as neuromodulation, proceeds slowly.
• Research into neurobiological mechanisms of psychotherapy is in its infancy.
• A foolproof method to monitor adherence does not exist, and countless patients relapse needlessly and deteriorate functionally.
• Effective, evidence-based rehabilitation for serious psychiatric disorders is used narrowly and vastly underfunded.
• Insurers continue to thumb their nose at laws that require parity for treating mental illness—thus impeding access to, delaying, or truncating psychiatric care.
Unmet scientific needs
Translational investigators. Despite increased funding for basic neuroscientific study and breathtaking discoveries in animal molecular neurobiology, a trickle of findings has been applied to clinical medicine. This translational gap has many causes, including a shortage of translational neuroscientists (MD-PhD psychiatrists and neurologists), insufficient long-term funding to develop such clinician-researchers, and complex regulatory oversight of human research.
Stalled progress in drug development. Development of novel-mechanism therapeutics for brain disorders is languishing. Some pharmaceutical manufacturers have abandoned the development of drugs that act on the CNS in favor of less complex, more lucrative areas such as oncology and cardiology; others have reduced their investment in CNS products. Developing treatments for knotty disorders of the most complex structure in the known universe requires mammoth investment. Why are stakeholders bailing out on the greatest challenge for science and medicine for easier endeavors?
Discovering new genes for every devastating neuropsychiatric syndrome, such as schizophrenia, is cause for celebration, but the champagne won’t flow until the coding of every gene is unscrambled so that specific biological interventions can be developed. The cost of the chase might be orders of magnitude greater than what is invested in research today. Conceptualizing new models of brain disorders is a critical part of scientific progress and an antidote to the inertia of perpetual group-think. Depression, for example, is being reconceptualized as a disorder of impaired neuroplasticity and neurotropic deficiency, rather than a shortage of serotonin and norepinephrine. Rapid reversal of severe depression to euthymia—in 1 or 2 hours—with IV ketamine shattered the dogma that depression takes weeks to lift, and is ushering in unprecedented new thinking and models likely to revolutionize treatment of severe depression. We need such breakthroughs for other psychiatric brain disorders.
Unmet professional and sociopolitical needs
Broadening of training. Psychiatrists have focused on the mind but insufficiently attended to the biology of the brain. For psychiatry to rise to the next level as a medical specialty and brain discipline, training must incorporate more neurology than it does now. The converse is true in neurology.
Hospitalization not incarceration. It is unconscionable that people suffering from a medical illness that impairs their judgment and behavior are locked up as criminals. Psychiatry must forcefully lobby so that the seriously mentally ill are treated in secure hospitals staffed by physicians, nurses, and mental health professionals.
That’s right: Bring back the asylum to address this unmet medical, political, and ethical need for psychiatric patients.a A serious mental disorder must be accepted as a fault-free illness.
aTo read more about this, I recommend my March 2008 editorial, “Bring back the asylums?,” at CurrentPsychiatry.com, and Dr. George Paulson’s excellent book, Closing the asylums: Causes and consequences of the deinstitutionalization movement (Jefferson, NC: McFarland & Co. Inc; 2012).
Full integration of psychiatry into the rest of medicine remains an unmet need, despite good progress. Because almost every medical illness can cause psychiatric symptoms, DSM-5 mandates that general medical conditions be ruled out before a primary psychiatric diagnosis is made.
Along the same lines, most severely mentally ill persons suffer from medical and neurologic ailments before their first episode,5 and many die prematurely from cardiovascular causes that often are the result of unhealthy lifestyle; iatrogenic complications; and lack of primary care interventions.6 Psychiatric patients must always receive standard general medical evaluation and management, side by side with their psychiatric care.
Philanthropy for psychiatry
Philanthropic support of psychiatry is a salutary trend. Some unmet needs in psychiatry, however, require not only money but a change in attitude (such as eliminating the absurd and discriminatory stigma of mental illness), better training, and forceful political activism by all of us.
1. Licinio J, Wong ML. Launching the ‘war on mental illness’. Mol Psychiatry. 2014;19(1):1-5.
2. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427.
3. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders. The majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
4. Cuthbert BN. The RDoC framework: facilitating transition from ICD/OSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28-35.
5. Sørensen HJ, Nielsen PR, Benros ME, et al. Somatic diseases and conditions before the first diagnosis of schizophrenia: a nationwide population-based cohort study in more than 900000 individuals [published online July 25, 2014]. Schizophr Bull. doi: 10.1093/schbul/sbu110.
6. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
1. Licinio J, Wong ML. Launching the ‘war on mental illness’. Mol Psychiatry. 2014;19(1):1-5.
2. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427.
3. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders. The majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
4. Cuthbert BN. The RDoC framework: facilitating transition from ICD/OSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28-35.
5. Sørensen HJ, Nielsen PR, Benros ME, et al. Somatic diseases and conditions before the first diagnosis of schizophrenia: a nationwide population-based cohort study in more than 900000 individuals [published online July 25, 2014]. Schizophr Bull. doi: 10.1093/schbul/sbu110.
6. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.