User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Emergency brain imaging: CT or MRI?
Discuss this article at www.facebook.com/CurrentPsychiatry
Together with a clinical assessment, neuroimaging increases diagnostic accuracy of detecting neuropathology. Direct patient benefit from scanning is best documented in those with overt, new clinical signs and symptoms of neurologic or psychiatric disease.1,2
Computerized tomography (CT) and magnetic resonance imaging (MRI) are the most common head scanning techniques used in emergency medicine.3 CT is quicker and cheaper, has less movement artifact, and is excellent at delineating acute hemorrhage, calcification, and bony anatomy.3,4 Unfortunately, CT exposes patients to radiation and poorly visualizes white matter or posterior fossa pathology.4
MRI is outstanding for well-defined tissue contrast in multiplanar views and excellent for identifying demyelination or metastatic processes,5 but may be contraindicated for patients with implanted metallic objects such as pacemakers, certain vascular clips or stents, and certain orthopedic devices.3-5 Some patients cannot tolerate the narrow space surrounding them during an MRI.4,5
Safety concerns with CT during pregnancy are well established, but are less clear with MRI. The opposite is true of contrast enhancement; gadolinium with MRI is better tolerated than CT procedures, for which contrast risks include allergy and renal dysfunction. When scanning for a hemorrhage, select a CT scan for patients in whom you suspect bleeding developed within the past 3 days; MRI may be better at screening for older bleeds.
For a list of indications for which a patient should undergo a CT or MRI, see the Table.1-5
Table
Indications for CT or MRI
| New or first-onset psychiatric illness |
| Recent head trauma |
| Recent or advancing cognitive dysfunction |
| New or worsening instances of syncope, vertigo, loss of consciousness, etc. |
| New, worsening, or altered pattern headaches |
| New signs of brain pathology, eg, seizure, paresis, or brain-related visual alteration |
| New neurologic examination abnormalities |
| Concerns about intracranial infection, inflammation, metastases, or increased pressure |
| Change in mental status in persons age >50 |
| Prescreening patients who are candidates for electroconvulsive therapy |
| Source: References 1-5 |
Disclosure
Dr. Lippmann reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pary R, Lippmann S. Clinical review of head CT scans in psychiatric patients. VA Practitioner. 1986;3:48-53.
2. Capote HA. Neuroimaging in psychiatry. Neurol Clin. 2009;27(1):237-249.
3. Malhi GS, Lagopoulos J. Making sense of neuroimaging in psychiatry. Acta Psychiatr Scand. 2008;117(2):100-117.
4. Small GW, Bookheimer SY, Thompson PM, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161-172.
5. Broderick DF. Neuroimaging in neuropsychiatry. Psychiatr Clin North Am. 2005;28(3):549-566,64.
Discuss this article at www.facebook.com/CurrentPsychiatry
Together with a clinical assessment, neuroimaging increases diagnostic accuracy of detecting neuropathology. Direct patient benefit from scanning is best documented in those with overt, new clinical signs and symptoms of neurologic or psychiatric disease.1,2
Computerized tomography (CT) and magnetic resonance imaging (MRI) are the most common head scanning techniques used in emergency medicine.3 CT is quicker and cheaper, has less movement artifact, and is excellent at delineating acute hemorrhage, calcification, and bony anatomy.3,4 Unfortunately, CT exposes patients to radiation and poorly visualizes white matter or posterior fossa pathology.4
MRI is outstanding for well-defined tissue contrast in multiplanar views and excellent for identifying demyelination or metastatic processes,5 but may be contraindicated for patients with implanted metallic objects such as pacemakers, certain vascular clips or stents, and certain orthopedic devices.3-5 Some patients cannot tolerate the narrow space surrounding them during an MRI.4,5
Safety concerns with CT during pregnancy are well established, but are less clear with MRI. The opposite is true of contrast enhancement; gadolinium with MRI is better tolerated than CT procedures, for which contrast risks include allergy and renal dysfunction. When scanning for a hemorrhage, select a CT scan for patients in whom you suspect bleeding developed within the past 3 days; MRI may be better at screening for older bleeds.
For a list of indications for which a patient should undergo a CT or MRI, see the Table.1-5
Table
Indications for CT or MRI
| New or first-onset psychiatric illness |
| Recent head trauma |
| Recent or advancing cognitive dysfunction |
| New or worsening instances of syncope, vertigo, loss of consciousness, etc. |
| New, worsening, or altered pattern headaches |
| New signs of brain pathology, eg, seizure, paresis, or brain-related visual alteration |
| New neurologic examination abnormalities |
| Concerns about intracranial infection, inflammation, metastases, or increased pressure |
| Change in mental status in persons age >50 |
| Prescreening patients who are candidates for electroconvulsive therapy |
| Source: References 1-5 |
Disclosure
Dr. Lippmann reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Together with a clinical assessment, neuroimaging increases diagnostic accuracy of detecting neuropathology. Direct patient benefit from scanning is best documented in those with overt, new clinical signs and symptoms of neurologic or psychiatric disease.1,2
Computerized tomography (CT) and magnetic resonance imaging (MRI) are the most common head scanning techniques used in emergency medicine.3 CT is quicker and cheaper, has less movement artifact, and is excellent at delineating acute hemorrhage, calcification, and bony anatomy.3,4 Unfortunately, CT exposes patients to radiation and poorly visualizes white matter or posterior fossa pathology.4
MRI is outstanding for well-defined tissue contrast in multiplanar views and excellent for identifying demyelination or metastatic processes,5 but may be contraindicated for patients with implanted metallic objects such as pacemakers, certain vascular clips or stents, and certain orthopedic devices.3-5 Some patients cannot tolerate the narrow space surrounding them during an MRI.4,5
Safety concerns with CT during pregnancy are well established, but are less clear with MRI. The opposite is true of contrast enhancement; gadolinium with MRI is better tolerated than CT procedures, for which contrast risks include allergy and renal dysfunction. When scanning for a hemorrhage, select a CT scan for patients in whom you suspect bleeding developed within the past 3 days; MRI may be better at screening for older bleeds.
For a list of indications for which a patient should undergo a CT or MRI, see the Table.1-5
Table
Indications for CT or MRI
| New or first-onset psychiatric illness |
| Recent head trauma |
| Recent or advancing cognitive dysfunction |
| New or worsening instances of syncope, vertigo, loss of consciousness, etc. |
| New, worsening, or altered pattern headaches |
| New signs of brain pathology, eg, seizure, paresis, or brain-related visual alteration |
| New neurologic examination abnormalities |
| Concerns about intracranial infection, inflammation, metastases, or increased pressure |
| Change in mental status in persons age >50 |
| Prescreening patients who are candidates for electroconvulsive therapy |
| Source: References 1-5 |
Disclosure
Dr. Lippmann reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pary R, Lippmann S. Clinical review of head CT scans in psychiatric patients. VA Practitioner. 1986;3:48-53.
2. Capote HA. Neuroimaging in psychiatry. Neurol Clin. 2009;27(1):237-249.
3. Malhi GS, Lagopoulos J. Making sense of neuroimaging in psychiatry. Acta Psychiatr Scand. 2008;117(2):100-117.
4. Small GW, Bookheimer SY, Thompson PM, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161-172.
5. Broderick DF. Neuroimaging in neuropsychiatry. Psychiatr Clin North Am. 2005;28(3):549-566,64.
1. Pary R, Lippmann S. Clinical review of head CT scans in psychiatric patients. VA Practitioner. 1986;3:48-53.
2. Capote HA. Neuroimaging in psychiatry. Neurol Clin. 2009;27(1):237-249.
3. Malhi GS, Lagopoulos J. Making sense of neuroimaging in psychiatry. Acta Psychiatr Scand. 2008;117(2):100-117.
4. Small GW, Bookheimer SY, Thompson PM, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161-172.
5. Broderick DF. Neuroimaging in neuropsychiatry. Psychiatr Clin North Am. 2005;28(3):549-566,64.
Better psychiatric documentation: From SOAP to PROMISE
Discuss this article at www.facebook.com/CurrentPsychiatry
Because documentation is an important part of medical practice,1 numerous tools have been developed to help physicians across all specialties, including the best-known acronym SOAP, which stands for Subjective, Objective, Assessment, and Plan. The SOAP note has been used in mental health settings,2 although this format may fall short for psychiatrists because objective tests are not diagnostic. Also, there’s no clear guidance to document specific information, such as behavioral risk assessment.
The acronym PROMISE—Problems, Resolved, Outcomes, Medications, Instructions, Safety, and Education—may be better suited for psychiatric documentation. The PROMISE note provides an easy-to-remember method to document specific information that might be overlooked in a less detailed format, such as normal findings, adherence and tolerability to medications, outcome ratings, and risk assessment.
Problems are described as ongoing symptoms, signs, and stressors. Resolved indicates improvement and normal findings. Outcome measures include patient or clinician rating scales. Medications documents the effectiveness and tolerability of current and past medications. Instructions are directives given; the rationale—cost-benefit analysis—can be documented in this section as well. Safety describes a behavioral risk assessment, including demographic, historical, clinical, and environmental risk and protective factors regarding suicidal or homicidal behavior. Education describes the verbal or written material shared with the patient.
Psychotherapists can use the same template. For them the M would stand for Methods of psychotherapy practiced in the session.
For an example of the PROMISE note used in practice, see the Table.
Table
Example of a patient’s PROMISE note
| Problems | Ongoing depressive symptoms: low mood, negative thinking, low interest level; patient has no insurance, pays out of pocket |
| Resolved | Mild improvement in motivation noted; sleeping and concentration both OK; continues to work full-time; spends time with parents |
| Outcomes | Clinical Global Impression-Severity Scale score: 4; PHQ-9 depression rating scale score: 12/27, indicating moderate depression (score 1 month ago was 15/27; 20% reduction) |
| Medications | Current treatment: citalopram, 20 mg/d, nortriptyline, 50 mg/d Prior medications: bupropion, citalopram, clomipramine, fluoxetine, MAOIs, sertraline, and venlafaxine. Patient’s adherence to medication is good Tolerability issues: sweating, constipation, dry mouth |
| Instructions | Increase both medications (20% improvement noted; recommend increase in nortriptyline; patient requests increase in citalopram). Ongoing moderate depression; initial side effects may subside |
| Safety | Identified risk or protective factors for suicidal, aggressive, or homicidal behavior: chronic depression without remission No current SI, HI, SIB, hopelessness, anxiety, agitation, insomnia, substance use, psychosis, or interpersonal aggression. No access to weapons. No history of suicide attempts. Good supports. Risk assessment: low |
| Education |
|
| HI: homicidal ideation; MAOIs: monoamine oxidase inhibitors; PHQ-9: 9-Question Patient Health Questionnaire; SI: suicidal ideation; SIB: self-injurious behavior | |
Disclosure
Dr. Bastiaens reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Because documentation is an important part of medical practice,1 numerous tools have been developed to help physicians across all specialties, including the best-known acronym SOAP, which stands for Subjective, Objective, Assessment, and Plan. The SOAP note has been used in mental health settings,2 although this format may fall short for psychiatrists because objective tests are not diagnostic. Also, there’s no clear guidance to document specific information, such as behavioral risk assessment.
The acronym PROMISE—Problems, Resolved, Outcomes, Medications, Instructions, Safety, and Education—may be better suited for psychiatric documentation. The PROMISE note provides an easy-to-remember method to document specific information that might be overlooked in a less detailed format, such as normal findings, adherence and tolerability to medications, outcome ratings, and risk assessment.
Problems are described as ongoing symptoms, signs, and stressors. Resolved indicates improvement and normal findings. Outcome measures include patient or clinician rating scales. Medications documents the effectiveness and tolerability of current and past medications. Instructions are directives given; the rationale—cost-benefit analysis—can be documented in this section as well. Safety describes a behavioral risk assessment, including demographic, historical, clinical, and environmental risk and protective factors regarding suicidal or homicidal behavior. Education describes the verbal or written material shared with the patient.
Psychotherapists can use the same template. For them the M would stand for Methods of psychotherapy practiced in the session.
For an example of the PROMISE note used in practice, see the Table.
Table
Example of a patient’s PROMISE note
| Problems | Ongoing depressive symptoms: low mood, negative thinking, low interest level; patient has no insurance, pays out of pocket |
| Resolved | Mild improvement in motivation noted; sleeping and concentration both OK; continues to work full-time; spends time with parents |
| Outcomes | Clinical Global Impression-Severity Scale score: 4; PHQ-9 depression rating scale score: 12/27, indicating moderate depression (score 1 month ago was 15/27; 20% reduction) |
| Medications | Current treatment: citalopram, 20 mg/d, nortriptyline, 50 mg/d Prior medications: bupropion, citalopram, clomipramine, fluoxetine, MAOIs, sertraline, and venlafaxine. Patient’s adherence to medication is good Tolerability issues: sweating, constipation, dry mouth |
| Instructions | Increase both medications (20% improvement noted; recommend increase in nortriptyline; patient requests increase in citalopram). Ongoing moderate depression; initial side effects may subside |
| Safety | Identified risk or protective factors for suicidal, aggressive, or homicidal behavior: chronic depression without remission No current SI, HI, SIB, hopelessness, anxiety, agitation, insomnia, substance use, psychosis, or interpersonal aggression. No access to weapons. No history of suicide attempts. Good supports. Risk assessment: low |
| Education |
|
| HI: homicidal ideation; MAOIs: monoamine oxidase inhibitors; PHQ-9: 9-Question Patient Health Questionnaire; SI: suicidal ideation; SIB: self-injurious behavior | |
Disclosure
Dr. Bastiaens reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Because documentation is an important part of medical practice,1 numerous tools have been developed to help physicians across all specialties, including the best-known acronym SOAP, which stands for Subjective, Objective, Assessment, and Plan. The SOAP note has been used in mental health settings,2 although this format may fall short for psychiatrists because objective tests are not diagnostic. Also, there’s no clear guidance to document specific information, such as behavioral risk assessment.
The acronym PROMISE—Problems, Resolved, Outcomes, Medications, Instructions, Safety, and Education—may be better suited for psychiatric documentation. The PROMISE note provides an easy-to-remember method to document specific information that might be overlooked in a less detailed format, such as normal findings, adherence and tolerability to medications, outcome ratings, and risk assessment.
Problems are described as ongoing symptoms, signs, and stressors. Resolved indicates improvement and normal findings. Outcome measures include patient or clinician rating scales. Medications documents the effectiveness and tolerability of current and past medications. Instructions are directives given; the rationale—cost-benefit analysis—can be documented in this section as well. Safety describes a behavioral risk assessment, including demographic, historical, clinical, and environmental risk and protective factors regarding suicidal or homicidal behavior. Education describes the verbal or written material shared with the patient.
Psychotherapists can use the same template. For them the M would stand for Methods of psychotherapy practiced in the session.
For an example of the PROMISE note used in practice, see the Table.
Table
Example of a patient’s PROMISE note
| Problems | Ongoing depressive symptoms: low mood, negative thinking, low interest level; patient has no insurance, pays out of pocket |
| Resolved | Mild improvement in motivation noted; sleeping and concentration both OK; continues to work full-time; spends time with parents |
| Outcomes | Clinical Global Impression-Severity Scale score: 4; PHQ-9 depression rating scale score: 12/27, indicating moderate depression (score 1 month ago was 15/27; 20% reduction) |
| Medications | Current treatment: citalopram, 20 mg/d, nortriptyline, 50 mg/d Prior medications: bupropion, citalopram, clomipramine, fluoxetine, MAOIs, sertraline, and venlafaxine. Patient’s adherence to medication is good Tolerability issues: sweating, constipation, dry mouth |
| Instructions | Increase both medications (20% improvement noted; recommend increase in nortriptyline; patient requests increase in citalopram). Ongoing moderate depression; initial side effects may subside |
| Safety | Identified risk or protective factors for suicidal, aggressive, or homicidal behavior: chronic depression without remission No current SI, HI, SIB, hopelessness, anxiety, agitation, insomnia, substance use, psychosis, or interpersonal aggression. No access to weapons. No history of suicide attempts. Good supports. Risk assessment: low |
| Education |
|
| HI: homicidal ideation; MAOIs: monoamine oxidase inhibitors; PHQ-9: 9-Question Patient Health Questionnaire; SI: suicidal ideation; SIB: self-injurious behavior | |
Disclosure
Dr. Bastiaens reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Psychiatric ‘holds’ for nonpsychiatric patients
Dear Dr. Mossman,
At the general hospital where I work, doctors and nurses sometimes ask me to fill out psychiatric “hold” documents to keep seriously ill medical or surgical patients from leaving the hospital. Last week, they asked me to stop Mr. J, a man with diabetes and a gangrenous lower leg, from leaving against medical advice (AMA). If he left, he would die. But if I filled out the psychiatric “hold,” I’d be saying the man needed civil commitment for a mental illness, which wasn’t true. If this happens again, what should I do?
Submitted by “Dr. Q”
“It is tempting, if the only tool you have is a hammer, to treat everything as if it were a nail,” wrote Abraham Maslow.1 The situation Dr. Q describes is one that psychiatrists frequently encounter because in some situations, a psychiatric “hold” can seem like the only way to stop a physically ill patient from leaving the hospital AMA. But pounding on this problem with a civil commitment hammer is the wrong response.
What’s wrong with using psychiatric holds in these situations? Do doctors have any other equipment in their medical toolbox for stopping an improvident AMA departure? To find out, we’ll look at:
- what a psychiatric hold does
- why holds don’t apply to medical-surgical treatment
- alternative responses to patients who lack capacity to refuse care.
Psychiatric holds
All states have laws that permit involuntary psychiatric hospitalization. The wording and procedural details in these laws vary across jurisdictions, but all states allow civil (ie, noncriminal) commitment of mentally ill persons who have gross impairments of judgment, behavior, reality-testing, or everyday functioning if their recent behavior show that they pose a danger because of their mental illness.2 Table 13 lists examples of the types of dangers that are potential reasons for civil commitment.
Table 1
Types of risks covered in civil commitment statutes
| All states |
|
| In some jurisdictions |
|
| Source: Adapted from reference 3 |
State laws also allow certain individuals (eg, police) to apprehend and transport mentally ill persons to facilities for psychiatric evaluation. Doctors may hold these persons temporarily until a court decides whether a longer involuntary hospitalization is justified. The documents used to initiate psychiatric holds have various informal names—”5150” (California), “pink slip” (Ohio), “pink paper” (Massachusetts), “Baker Act Form” (Florida)—but their function is the same: permitting lawful restraint of patients whose dangerousness results from their mental illness.
Urgent medical and surgical care
What about medical or surgical patients who refuse care despite being told they’ll die without it? Might involuntary psychiatric hospitalization procedures be a convenient way to keep them from coming to harm?
The answer: probably not, for 4 reasons:
- Once a psychiatric hold has been executed, the person who is subject to detention must be transferred to an appropriate facility within a specified period (usually 24 hours) for further evaluation and care.4,5 In this context, “appropriate facility” means a state-approved psychiatric treatment setting. A hospital’s medical or surgical unit usually would not qualify.
- The lawful use of a psychiatric hold is to declare that someone needs involuntary psychiatric examination for dangerousness arising “as a result of mental illness”—not for danger from a nonpsychiatric medical problem.6 Some civil commitment statutes specify that persons who have serious nonpsychiatric illness but no mental health problems that satisfy civil commitment criteria are to be offered voluntary treatment only.7
- A psychiatric hold only authorizes short-term detention. It does not allow forcing what patients such as Mr. J need: medical or surgical treatment. A psychiatric hold would not solve the problem that Mr. J’s doctors are facing.
- Doctors who execute psychiatric holds in good faith—sincerely believing a patient meets the legal criteria—enjoy statutory immunity from later accusations of malpractice or false imprisonment.8 Using civil commitment mechanisms when one does not actually believe those mechanisms apply might void this immunity.
Nonconsent: 2 varieties
For present purposes, let’s think of nonconsenting medical-surgical patients as coming in 2 varieties:
Variety 1: patients with compromised mental status. Often, medical-surgical patients cannot express objections to treatment because they are unconscious, delirious, or incoherent. Nurses and doctors assume such patients would want proper care and proceed with what they believe is in the patients’ best interest, often with input from family members.
Variety 2: lucid patients who refuse treatment. Patients who do not have obvious psychiatric problems may refuse necessary medical or surgical treatment for various reasons: obstinacy, distrust of doctors, fear, ignorance, incorrect but firmly held ideas about body functioning, cultural differences, or religious beliefs. None of these reasons is necessarily psychopathological, and none provides justification for a psychiatric hold.
Key determinant: Competence
Refusing treatment may be a bad choice and sometimes is evidence of a mental disorder, but it is not, by itself, a mental disorder. When a Variety 2 adult patient refuses care, the key question is, “Is this a competent refusal?” Assessment of a patient’s capacity to make medical decisions is not a skill unique to psychiatrists. Other specialists make judgments about capacity routinely—if only implicitly—when they elicit their patients’ informed consent for care. But when, as in Mr. J’s case, a seriously ill medical-surgical patient refuses lifesaving treatment, our medical colleagues often get psychiatrists involved. Consulting a psychiatrist in such circumstances makes sense, for at least 4 reasons:
- Although assessment of decision-making isn’t the special province of psychiatry, psychiatrists often have more experience assessing the capacity of persons whose thinking seems impaired.
- Psychiatrists also have more experience in detecting subtle indications of mental disorders (eg, mild dementia, depression, psychosis) that can compromise decision-making capacity.
- A nonpsychiatrist may believe that a patient is making a competent refusal but still wants a psychiatrist’s perspective to better understand the patient’s reasoning or to confirm the initial belief.
- Getting an independent opinion is a prudent way to make sure one’s emotions are not adversely influencing a critical judgment about a patient’s treatment.
Determining whether a patient has the requisite capacity to refuse care involves a situation-specific assessment of 4 aspects of mental functioning: expressing a choice coherently, understanding relevant information, appreciating this information, and using the information rationally. Table 29 describes these functional areas in more detail.
Table 2
Evaluating the quality of a patient’s decision: 4 dimensions
| 1. Can the patient communicate a choice and express a consistent preference? |
2. Can the patient grasp relevant information about:
|
| 3. Does the patient appreciate the illness and its consequences? Does he recognize he is ill and acknowledge how the information applies to his situation? |
| 4. Does the patient use the information rationally? Can he explain his decision-making and reasoning? Does he apply information to his situation in light of rational beliefs and desires? |
| Source: Adapted from reference 9 |
If capacity is lacking, what next?
As Judge Benjamin Cardozo ruled nearly a century ago, “Every human being of adult years and sound mind has a right to determine what shall be done with his own body.”10 In a case such as Mr. J’s, where a patient wants to leave the hospital or refuses medical treatment despite grave risk to himself, staff members should not let him leave until his treating doctors have tried to clarify his reasons for leaving and determined whether he has the capacity to give informed consent and refuse treatment. Psychiatrists may be consulted in this process, although the final judgment about capacity rests with the responsible physician. If an assessment shows that the patient has the capacity to make medical decisions, his treatment refusal is binding, even when it creates a clear risk of death.
What should happen if an assessment shows that a gravely ill patient lacks capacity to refuse treatment? Clinicians should consult with the hospital attorney about their facility’s policies and how to implement them properly.
Thinking about the possible legal implications of their actions, treating clinicians might worry that if they detain an unwilling patient without authorization from a court or guardian, they would risk being sued later for false imprisonment. But attorneys are likely to advise clinicians that they have more to fear liability-wise from letting incompetent patients leave the hospital than from detaining them for their own safety. As an Ohio court commented about a police officer who stopped a patient from leaving the hospital:
- What in the name of all that is reasonable should the officer have done? The court finds that the officer acted properly under the circumstances known to him at the time—and the reasonableness of an officer’s actions must be judged at the exigent split second on the street…11
Rather than allowing an incompetent patient to come to harm, attorneys may advise physicians to write an order to keep the patient in the hospital. Then, physicians can obtain consent for treatment from family members, making them aware of any physical or chemical restraint that might be needed to continue the patient’s treatment. Depending on the situation and the reasons for the lack of capacity, hospital staff members may later need to help a family member obtain a court’s authorization for emergency guardianship to allow non-urgent care to continue.
Treating physicians also should document the thinking and findings that support their actions. Table 3 provides an outline for this documentation.
Table 3
Detaining a patient for medical-surgical care: 7 components of documentation
| 1. Description of the patient’s refusal or efforts to leave the hospital |
| 2. Patient’s stated reasons for refusing or wanting to leave |
| 3. Reasonable alternatives to discharge that were offered |
| 4. Description of how refusing medical treatment would create a clear risk of physical harm or death |
| 5. Evidence that the patient lacks capacity to give informed consent or to refuse treatment |
| 6. Actions taken by the treating physician (eg, obtaining psychiatric consultation, enlisting other patient services, instituting physical restraint) |
| 7. Person who provided consent to continue treatment and that person’s relationship to patient |
Related Resources
- Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007; 357(18):1834-1840.
- Disability Rights California. Involuntary psychiatric treatment: California’s 72-hour hold and 14-day certification. www.disabilityrightsca.org/pubs/502401.pdf.
- Treatment Advocacy Center. Know the laws in your state. www.treatmentadvocacycenter.org/get-help/know-the-laws-in-your-state.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Dr. Mossman thanks David Schwallie, Esq, for his helpful insights about the topics discussed in this article.
1. Maslow AH. The psychology of science: a reconnaissance. New York NY: Harper & Row; 1966.
2. Mossman D, Schwartz AH, Elam ER. Risky business versus overt acts: what relevance do “actuarial” probabilistic risk assessments have for judicial decisions on involuntary psychiatric hospitalization? Houston Journal of Health Law & Policy. 2011;11:365-453.
3. Pinals DA, Mossman D. Evaluation for civil commitment. New York NY: Oxford University Press; 2012.
4. Ohio Revised Code § 5122.10.
5. Oregon Revised Statutes § 426.060.
6. California Welfare and Institutions Code § 5150.
7. Florida statutes § 394.463.
8. Cruze v National Psychiatric Services, Inc., 105 Cal. App. 4th 48 (2003).
9. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
10. Schloendorff v Society of New York Hospital, 211 N.Y. 125, 105 N.E. 92 (1914).
11. State v Clay, 43 Ohio Misc. 2d 5, 539 N.E.2d 1168 (1988).
Dear Dr. Mossman,
At the general hospital where I work, doctors and nurses sometimes ask me to fill out psychiatric “hold” documents to keep seriously ill medical or surgical patients from leaving the hospital. Last week, they asked me to stop Mr. J, a man with diabetes and a gangrenous lower leg, from leaving against medical advice (AMA). If he left, he would die. But if I filled out the psychiatric “hold,” I’d be saying the man needed civil commitment for a mental illness, which wasn’t true. If this happens again, what should I do?
Submitted by “Dr. Q”
“It is tempting, if the only tool you have is a hammer, to treat everything as if it were a nail,” wrote Abraham Maslow.1 The situation Dr. Q describes is one that psychiatrists frequently encounter because in some situations, a psychiatric “hold” can seem like the only way to stop a physically ill patient from leaving the hospital AMA. But pounding on this problem with a civil commitment hammer is the wrong response.
What’s wrong with using psychiatric holds in these situations? Do doctors have any other equipment in their medical toolbox for stopping an improvident AMA departure? To find out, we’ll look at:
- what a psychiatric hold does
- why holds don’t apply to medical-surgical treatment
- alternative responses to patients who lack capacity to refuse care.
Psychiatric holds
All states have laws that permit involuntary psychiatric hospitalization. The wording and procedural details in these laws vary across jurisdictions, but all states allow civil (ie, noncriminal) commitment of mentally ill persons who have gross impairments of judgment, behavior, reality-testing, or everyday functioning if their recent behavior show that they pose a danger because of their mental illness.2 Table 13 lists examples of the types of dangers that are potential reasons for civil commitment.
Table 1
Types of risks covered in civil commitment statutes
| All states |
|
| In some jurisdictions |
|
| Source: Adapted from reference 3 |
State laws also allow certain individuals (eg, police) to apprehend and transport mentally ill persons to facilities for psychiatric evaluation. Doctors may hold these persons temporarily until a court decides whether a longer involuntary hospitalization is justified. The documents used to initiate psychiatric holds have various informal names—”5150” (California), “pink slip” (Ohio), “pink paper” (Massachusetts), “Baker Act Form” (Florida)—but their function is the same: permitting lawful restraint of patients whose dangerousness results from their mental illness.
Urgent medical and surgical care
What about medical or surgical patients who refuse care despite being told they’ll die without it? Might involuntary psychiatric hospitalization procedures be a convenient way to keep them from coming to harm?
The answer: probably not, for 4 reasons:
- Once a psychiatric hold has been executed, the person who is subject to detention must be transferred to an appropriate facility within a specified period (usually 24 hours) for further evaluation and care.4,5 In this context, “appropriate facility” means a state-approved psychiatric treatment setting. A hospital’s medical or surgical unit usually would not qualify.
- The lawful use of a psychiatric hold is to declare that someone needs involuntary psychiatric examination for dangerousness arising “as a result of mental illness”—not for danger from a nonpsychiatric medical problem.6 Some civil commitment statutes specify that persons who have serious nonpsychiatric illness but no mental health problems that satisfy civil commitment criteria are to be offered voluntary treatment only.7
- A psychiatric hold only authorizes short-term detention. It does not allow forcing what patients such as Mr. J need: medical or surgical treatment. A psychiatric hold would not solve the problem that Mr. J’s doctors are facing.
- Doctors who execute psychiatric holds in good faith—sincerely believing a patient meets the legal criteria—enjoy statutory immunity from later accusations of malpractice or false imprisonment.8 Using civil commitment mechanisms when one does not actually believe those mechanisms apply might void this immunity.
Nonconsent: 2 varieties
For present purposes, let’s think of nonconsenting medical-surgical patients as coming in 2 varieties:
Variety 1: patients with compromised mental status. Often, medical-surgical patients cannot express objections to treatment because they are unconscious, delirious, or incoherent. Nurses and doctors assume such patients would want proper care and proceed with what they believe is in the patients’ best interest, often with input from family members.
Variety 2: lucid patients who refuse treatment. Patients who do not have obvious psychiatric problems may refuse necessary medical or surgical treatment for various reasons: obstinacy, distrust of doctors, fear, ignorance, incorrect but firmly held ideas about body functioning, cultural differences, or religious beliefs. None of these reasons is necessarily psychopathological, and none provides justification for a psychiatric hold.
Key determinant: Competence
Refusing treatment may be a bad choice and sometimes is evidence of a mental disorder, but it is not, by itself, a mental disorder. When a Variety 2 adult patient refuses care, the key question is, “Is this a competent refusal?” Assessment of a patient’s capacity to make medical decisions is not a skill unique to psychiatrists. Other specialists make judgments about capacity routinely—if only implicitly—when they elicit their patients’ informed consent for care. But when, as in Mr. J’s case, a seriously ill medical-surgical patient refuses lifesaving treatment, our medical colleagues often get psychiatrists involved. Consulting a psychiatrist in such circumstances makes sense, for at least 4 reasons:
- Although assessment of decision-making isn’t the special province of psychiatry, psychiatrists often have more experience assessing the capacity of persons whose thinking seems impaired.
- Psychiatrists also have more experience in detecting subtle indications of mental disorders (eg, mild dementia, depression, psychosis) that can compromise decision-making capacity.
- A nonpsychiatrist may believe that a patient is making a competent refusal but still wants a psychiatrist’s perspective to better understand the patient’s reasoning or to confirm the initial belief.
- Getting an independent opinion is a prudent way to make sure one’s emotions are not adversely influencing a critical judgment about a patient’s treatment.
Determining whether a patient has the requisite capacity to refuse care involves a situation-specific assessment of 4 aspects of mental functioning: expressing a choice coherently, understanding relevant information, appreciating this information, and using the information rationally. Table 29 describes these functional areas in more detail.
Table 2
Evaluating the quality of a patient’s decision: 4 dimensions
| 1. Can the patient communicate a choice and express a consistent preference? |
2. Can the patient grasp relevant information about:
|
| 3. Does the patient appreciate the illness and its consequences? Does he recognize he is ill and acknowledge how the information applies to his situation? |
| 4. Does the patient use the information rationally? Can he explain his decision-making and reasoning? Does he apply information to his situation in light of rational beliefs and desires? |
| Source: Adapted from reference 9 |
If capacity is lacking, what next?
As Judge Benjamin Cardozo ruled nearly a century ago, “Every human being of adult years and sound mind has a right to determine what shall be done with his own body.”10 In a case such as Mr. J’s, where a patient wants to leave the hospital or refuses medical treatment despite grave risk to himself, staff members should not let him leave until his treating doctors have tried to clarify his reasons for leaving and determined whether he has the capacity to give informed consent and refuse treatment. Psychiatrists may be consulted in this process, although the final judgment about capacity rests with the responsible physician. If an assessment shows that the patient has the capacity to make medical decisions, his treatment refusal is binding, even when it creates a clear risk of death.
What should happen if an assessment shows that a gravely ill patient lacks capacity to refuse treatment? Clinicians should consult with the hospital attorney about their facility’s policies and how to implement them properly.
Thinking about the possible legal implications of their actions, treating clinicians might worry that if they detain an unwilling patient without authorization from a court or guardian, they would risk being sued later for false imprisonment. But attorneys are likely to advise clinicians that they have more to fear liability-wise from letting incompetent patients leave the hospital than from detaining them for their own safety. As an Ohio court commented about a police officer who stopped a patient from leaving the hospital:
- What in the name of all that is reasonable should the officer have done? The court finds that the officer acted properly under the circumstances known to him at the time—and the reasonableness of an officer’s actions must be judged at the exigent split second on the street…11
Rather than allowing an incompetent patient to come to harm, attorneys may advise physicians to write an order to keep the patient in the hospital. Then, physicians can obtain consent for treatment from family members, making them aware of any physical or chemical restraint that might be needed to continue the patient’s treatment. Depending on the situation and the reasons for the lack of capacity, hospital staff members may later need to help a family member obtain a court’s authorization for emergency guardianship to allow non-urgent care to continue.
Treating physicians also should document the thinking and findings that support their actions. Table 3 provides an outline for this documentation.
Table 3
Detaining a patient for medical-surgical care: 7 components of documentation
| 1. Description of the patient’s refusal or efforts to leave the hospital |
| 2. Patient’s stated reasons for refusing or wanting to leave |
| 3. Reasonable alternatives to discharge that were offered |
| 4. Description of how refusing medical treatment would create a clear risk of physical harm or death |
| 5. Evidence that the patient lacks capacity to give informed consent or to refuse treatment |
| 6. Actions taken by the treating physician (eg, obtaining psychiatric consultation, enlisting other patient services, instituting physical restraint) |
| 7. Person who provided consent to continue treatment and that person’s relationship to patient |
Related Resources
- Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007; 357(18):1834-1840.
- Disability Rights California. Involuntary psychiatric treatment: California’s 72-hour hold and 14-day certification. www.disabilityrightsca.org/pubs/502401.pdf.
- Treatment Advocacy Center. Know the laws in your state. www.treatmentadvocacycenter.org/get-help/know-the-laws-in-your-state.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Dr. Mossman thanks David Schwallie, Esq, for his helpful insights about the topics discussed in this article.
Dear Dr. Mossman,
At the general hospital where I work, doctors and nurses sometimes ask me to fill out psychiatric “hold” documents to keep seriously ill medical or surgical patients from leaving the hospital. Last week, they asked me to stop Mr. J, a man with diabetes and a gangrenous lower leg, from leaving against medical advice (AMA). If he left, he would die. But if I filled out the psychiatric “hold,” I’d be saying the man needed civil commitment for a mental illness, which wasn’t true. If this happens again, what should I do?
Submitted by “Dr. Q”
“It is tempting, if the only tool you have is a hammer, to treat everything as if it were a nail,” wrote Abraham Maslow.1 The situation Dr. Q describes is one that psychiatrists frequently encounter because in some situations, a psychiatric “hold” can seem like the only way to stop a physically ill patient from leaving the hospital AMA. But pounding on this problem with a civil commitment hammer is the wrong response.
What’s wrong with using psychiatric holds in these situations? Do doctors have any other equipment in their medical toolbox for stopping an improvident AMA departure? To find out, we’ll look at:
- what a psychiatric hold does
- why holds don’t apply to medical-surgical treatment
- alternative responses to patients who lack capacity to refuse care.
Psychiatric holds
All states have laws that permit involuntary psychiatric hospitalization. The wording and procedural details in these laws vary across jurisdictions, but all states allow civil (ie, noncriminal) commitment of mentally ill persons who have gross impairments of judgment, behavior, reality-testing, or everyday functioning if their recent behavior show that they pose a danger because of their mental illness.2 Table 13 lists examples of the types of dangers that are potential reasons for civil commitment.
Table 1
Types of risks covered in civil commitment statutes
| All states |
|
| In some jurisdictions |
|
| Source: Adapted from reference 3 |
State laws also allow certain individuals (eg, police) to apprehend and transport mentally ill persons to facilities for psychiatric evaluation. Doctors may hold these persons temporarily until a court decides whether a longer involuntary hospitalization is justified. The documents used to initiate psychiatric holds have various informal names—”5150” (California), “pink slip” (Ohio), “pink paper” (Massachusetts), “Baker Act Form” (Florida)—but their function is the same: permitting lawful restraint of patients whose dangerousness results from their mental illness.
Urgent medical and surgical care
What about medical or surgical patients who refuse care despite being told they’ll die without it? Might involuntary psychiatric hospitalization procedures be a convenient way to keep them from coming to harm?
The answer: probably not, for 4 reasons:
- Once a psychiatric hold has been executed, the person who is subject to detention must be transferred to an appropriate facility within a specified period (usually 24 hours) for further evaluation and care.4,5 In this context, “appropriate facility” means a state-approved psychiatric treatment setting. A hospital’s medical or surgical unit usually would not qualify.
- The lawful use of a psychiatric hold is to declare that someone needs involuntary psychiatric examination for dangerousness arising “as a result of mental illness”—not for danger from a nonpsychiatric medical problem.6 Some civil commitment statutes specify that persons who have serious nonpsychiatric illness but no mental health problems that satisfy civil commitment criteria are to be offered voluntary treatment only.7
- A psychiatric hold only authorizes short-term detention. It does not allow forcing what patients such as Mr. J need: medical or surgical treatment. A psychiatric hold would not solve the problem that Mr. J’s doctors are facing.
- Doctors who execute psychiatric holds in good faith—sincerely believing a patient meets the legal criteria—enjoy statutory immunity from later accusations of malpractice or false imprisonment.8 Using civil commitment mechanisms when one does not actually believe those mechanisms apply might void this immunity.
Nonconsent: 2 varieties
For present purposes, let’s think of nonconsenting medical-surgical patients as coming in 2 varieties:
Variety 1: patients with compromised mental status. Often, medical-surgical patients cannot express objections to treatment because they are unconscious, delirious, or incoherent. Nurses and doctors assume such patients would want proper care and proceed with what they believe is in the patients’ best interest, often with input from family members.
Variety 2: lucid patients who refuse treatment. Patients who do not have obvious psychiatric problems may refuse necessary medical or surgical treatment for various reasons: obstinacy, distrust of doctors, fear, ignorance, incorrect but firmly held ideas about body functioning, cultural differences, or religious beliefs. None of these reasons is necessarily psychopathological, and none provides justification for a psychiatric hold.
Key determinant: Competence
Refusing treatment may be a bad choice and sometimes is evidence of a mental disorder, but it is not, by itself, a mental disorder. When a Variety 2 adult patient refuses care, the key question is, “Is this a competent refusal?” Assessment of a patient’s capacity to make medical decisions is not a skill unique to psychiatrists. Other specialists make judgments about capacity routinely—if only implicitly—when they elicit their patients’ informed consent for care. But when, as in Mr. J’s case, a seriously ill medical-surgical patient refuses lifesaving treatment, our medical colleagues often get psychiatrists involved. Consulting a psychiatrist in such circumstances makes sense, for at least 4 reasons:
- Although assessment of decision-making isn’t the special province of psychiatry, psychiatrists often have more experience assessing the capacity of persons whose thinking seems impaired.
- Psychiatrists also have more experience in detecting subtle indications of mental disorders (eg, mild dementia, depression, psychosis) that can compromise decision-making capacity.
- A nonpsychiatrist may believe that a patient is making a competent refusal but still wants a psychiatrist’s perspective to better understand the patient’s reasoning or to confirm the initial belief.
- Getting an independent opinion is a prudent way to make sure one’s emotions are not adversely influencing a critical judgment about a patient’s treatment.
Determining whether a patient has the requisite capacity to refuse care involves a situation-specific assessment of 4 aspects of mental functioning: expressing a choice coherently, understanding relevant information, appreciating this information, and using the information rationally. Table 29 describes these functional areas in more detail.
Table 2
Evaluating the quality of a patient’s decision: 4 dimensions
| 1. Can the patient communicate a choice and express a consistent preference? |
2. Can the patient grasp relevant information about:
|
| 3. Does the patient appreciate the illness and its consequences? Does he recognize he is ill and acknowledge how the information applies to his situation? |
| 4. Does the patient use the information rationally? Can he explain his decision-making and reasoning? Does he apply information to his situation in light of rational beliefs and desires? |
| Source: Adapted from reference 9 |
If capacity is lacking, what next?
As Judge Benjamin Cardozo ruled nearly a century ago, “Every human being of adult years and sound mind has a right to determine what shall be done with his own body.”10 In a case such as Mr. J’s, where a patient wants to leave the hospital or refuses medical treatment despite grave risk to himself, staff members should not let him leave until his treating doctors have tried to clarify his reasons for leaving and determined whether he has the capacity to give informed consent and refuse treatment. Psychiatrists may be consulted in this process, although the final judgment about capacity rests with the responsible physician. If an assessment shows that the patient has the capacity to make medical decisions, his treatment refusal is binding, even when it creates a clear risk of death.
What should happen if an assessment shows that a gravely ill patient lacks capacity to refuse treatment? Clinicians should consult with the hospital attorney about their facility’s policies and how to implement them properly.
Thinking about the possible legal implications of their actions, treating clinicians might worry that if they detain an unwilling patient without authorization from a court or guardian, they would risk being sued later for false imprisonment. But attorneys are likely to advise clinicians that they have more to fear liability-wise from letting incompetent patients leave the hospital than from detaining them for their own safety. As an Ohio court commented about a police officer who stopped a patient from leaving the hospital:
- What in the name of all that is reasonable should the officer have done? The court finds that the officer acted properly under the circumstances known to him at the time—and the reasonableness of an officer’s actions must be judged at the exigent split second on the street…11
Rather than allowing an incompetent patient to come to harm, attorneys may advise physicians to write an order to keep the patient in the hospital. Then, physicians can obtain consent for treatment from family members, making them aware of any physical or chemical restraint that might be needed to continue the patient’s treatment. Depending on the situation and the reasons for the lack of capacity, hospital staff members may later need to help a family member obtain a court’s authorization for emergency guardianship to allow non-urgent care to continue.
Treating physicians also should document the thinking and findings that support their actions. Table 3 provides an outline for this documentation.
Table 3
Detaining a patient for medical-surgical care: 7 components of documentation
| 1. Description of the patient’s refusal or efforts to leave the hospital |
| 2. Patient’s stated reasons for refusing or wanting to leave |
| 3. Reasonable alternatives to discharge that were offered |
| 4. Description of how refusing medical treatment would create a clear risk of physical harm or death |
| 5. Evidence that the patient lacks capacity to give informed consent or to refuse treatment |
| 6. Actions taken by the treating physician (eg, obtaining psychiatric consultation, enlisting other patient services, instituting physical restraint) |
| 7. Person who provided consent to continue treatment and that person’s relationship to patient |
Related Resources
- Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007; 357(18):1834-1840.
- Disability Rights California. Involuntary psychiatric treatment: California’s 72-hour hold and 14-day certification. www.disabilityrightsca.org/pubs/502401.pdf.
- Treatment Advocacy Center. Know the laws in your state. www.treatmentadvocacycenter.org/get-help/know-the-laws-in-your-state.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Dr. Mossman thanks David Schwallie, Esq, for his helpful insights about the topics discussed in this article.
1. Maslow AH. The psychology of science: a reconnaissance. New York NY: Harper & Row; 1966.
2. Mossman D, Schwartz AH, Elam ER. Risky business versus overt acts: what relevance do “actuarial” probabilistic risk assessments have for judicial decisions on involuntary psychiatric hospitalization? Houston Journal of Health Law & Policy. 2011;11:365-453.
3. Pinals DA, Mossman D. Evaluation for civil commitment. New York NY: Oxford University Press; 2012.
4. Ohio Revised Code § 5122.10.
5. Oregon Revised Statutes § 426.060.
6. California Welfare and Institutions Code § 5150.
7. Florida statutes § 394.463.
8. Cruze v National Psychiatric Services, Inc., 105 Cal. App. 4th 48 (2003).
9. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
10. Schloendorff v Society of New York Hospital, 211 N.Y. 125, 105 N.E. 92 (1914).
11. State v Clay, 43 Ohio Misc. 2d 5, 539 N.E.2d 1168 (1988).
1. Maslow AH. The psychology of science: a reconnaissance. New York NY: Harper & Row; 1966.
2. Mossman D, Schwartz AH, Elam ER. Risky business versus overt acts: what relevance do “actuarial” probabilistic risk assessments have for judicial decisions on involuntary psychiatric hospitalization? Houston Journal of Health Law & Policy. 2011;11:365-453.
3. Pinals DA, Mossman D. Evaluation for civil commitment. New York NY: Oxford University Press; 2012.
4. Ohio Revised Code § 5122.10.
5. Oregon Revised Statutes § 426.060.
6. California Welfare and Institutions Code § 5150.
7. Florida statutes § 394.463.
8. Cruze v National Psychiatric Services, Inc., 105 Cal. App. 4th 48 (2003).
9. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
10. Schloendorff v Society of New York Hospital, 211 N.Y. 125, 105 N.E. 92 (1914).
11. State v Clay, 43 Ohio Misc. 2d 5, 539 N.E.2d 1168 (1988).
Metabolic disturbance and dementia: A modifiable link
Discuss this article at www.facebook.com/CurrentPsychiatry
In addition to increasing patients’ risk for cardiovascular disease, stroke, and cancer, obesity and metabolic disturbance contribute to age-related cognitive decline and dementia. In particular, insulin resistance and hyperinsulinemia promote neurocognitive dysfunction and neurodegenerative changes during the extended, preclinical phase of Alzheimer’s disease (AD). However, with dietary modification it may be possible to resensitize insulin receptors, correct hyperinsulinemia, and improve memory function.
Metabolic disturbance and neurodegeneration
In the United States, 5.4 million people have AD, and there will be an estimated 16 million cases by 2050.1 Simultaneously we are experiencing an epidemic of metabolic disturbance and obesity. Approximately, 64% of adults in the United States are overweight (body mass index [BMI]: 25.0 to 29.9 kg/m2) and 34% are obese (BMI: ≥30 kg/m2).2 By 2030, 86% of adults will be overweight and 51% will be obese.3 This confluence of epidemics is not coincidental but instead reflects the fact that metabolic disturbance is a fundamental factor contributing to cognitive decline and neurodegeneration.4
Ninety-six percent of AD cases are classified as late onset, sporadic AD, occurring after age 64.1 Mild cognitive impairment (MCI) is a clinical construct that entails greater than expected memory impairment for the patient’s age and identifies older adults who are at increased risk for dementia. MCI represents the first clinical manifestation of neurodegeneration for a subset of patients who will progress to AD.5,6 MCI is distinguished from age-associated memory impairment (AAMI), which originally was conceptualized as normal or benign memory decline with aging.7,8 Recent data indicate that Alzheimer’s-type neuropathologic changes are the basis for subjective memory complaints and objectively assessed age-related cognitive decline,9 and early neurodegeneration is present in many patients with AAMI or MCI.10 This is consistent with the idea that an extended preclinical phase precedes AD onset. The preclinical phase can persist for a decade or more and precedes MCI and overt functional decline. However, neuropathologic changes accumulate during the preclinical phase of AD11 and during the preclinical phase of type 2 diabetes mellitus (T2DM).
Hyperinsulinemia and dementia
Insulin resistance and hyperinsulinemia occur in >40% of individuals age ≥60 and prevalence increases with age.4,12 Hyperinsulinemia develops to compensate for insulin resistance to overcome receptor insensitivity and maintain glucose homeostasis. Insulin receptors are densely expressed in brain regions vulnerable to neurodegeneration, including the medial temporal lobe and prefrontal cortex, which mediate long-term memory and working memory. However, insulin must be transported into the CNS from the periphery because little is synthesized in the brain. Paradoxically, peripheral compensatory hyperinsulinemia resulting from insulin resistance is associated with central (brain) hypoinsulinemia because of insensitivity and saturation of the receptor-mediated blood-brain barrier transport mechanism.13-15
Hyperinsulinemia is the precursor to T2DM. However, hyperinsulinemia is not well recognized in clinical contexts and generally is not a treatment target. Nonetheless, it contributes to several health problems, and insulin resistance in middle age is associated with age-related diseases such as hypertension, coronary artery disease, stroke, and cancer, while insulin sensitivity protects against such disorders.16
Chronic insulin resistance may contribute more to dementia development than T2DM because of the extended period of hyperinsulinemia that precedes T2DM onset. In population studies,17 insulin resistance syndrome increases risk for developing AD independent of apolipoprotein E (APOE e4) allele status, and in a longitudinal study,18 the risk for AD solely attributable to peripheral hyperinsulinemia was up to 39%. Being overweight in midlife increases risk for dementia in late life, and APOE e4 allele status does not contribute additional risk after accounting for BMI.19 Middle-aged individuals with hyperinsulinemia show memory decline, and obesity in middle age was associated with greater cognitive impairment after 6-year follow-up.20 Even in older adults who seem cognitively unimpaired, BMI and fasting insulin are positively correlated with atrophy in frontal, temporal, and subcortical brain regions, and obesity is an independent risk for atrophy in several brain regions, including the hippocampus.21
Compared with healthy older adults, individuals with AD have lower ratios of cerebrospinal fluid to plasma insulin.22 This lower ratio reflects the peripheral-to-central gradient of insulin levels in AD and suggests an etiological role for such metabolic disturbance. Insulin resistance has downstream effects that potentiate neurodegenerative factors, and central hypoinsulinemia can accelerate neurodegenerative processes and cognitive decline.4,23 Brain insulin plays a direct role in regulating proinflammatory cytokines and neurotrophic and neuroplastic factors essential for memory function. Insulin degrading enzyme, which varies with insulin levels,24 regulates the generation and clearance of amyloid β (Aβ) from the brain.25
Hyperinsulinemia typically is evident in increasing waist circumference and body weight.26 Waist circumference of ≥100 cm (39 inches) is a sensitive, specific, and independent predictor of hyperinsulinemia for men and women and a stronger predictor than BMI, waist-to-hip ratio, and other measures of body fat.27 Unpublished data derived from our clinical research with MCI subjects supports the association of metabolic disturbance with age-related cognitive decline. Our subjects are recruited from the community on the basis of mild memory decline and—other than excluding those with diabetes—weight and metabolic status are not considered in evaluating individuals for enrollment. The Table contains data on waist circumference and metabolic function in 122 older adults (age ≥68) with MCI. On average, these individuals exhibited fasting insulin values in the hyperinsulinemia range and elevated fasting glucose levels that indicated borderline diabetes. Waist circumference also was high, indicating excessive visceral fat deposition. We also observed a relationship between waist circumference and insulin, a consistent observation in older adults with memory decline. These data would not be surprising in any sample of older adults because of the population base rates for these conditions. However, we also found that waist circumference was a significant predictor of memory performance in patients with MCI. Abdominal adiposity is highly correlated with intrahepatic fat.28 Given this and recent indications that Alzheimer’s-type neuropathologic factors are generated in the liver,29,30 the predictive value of waist circumference to memory performance may reflect the fact that it is a proxy for downstream actions of liver fat.
Table
Waist circumference and metabolic factors in 122 older adults with MCIa
| Metabolic indicator | Value |
|---|---|
| Mean (SD) fasting glucose, mg/dL | 99.5 (11.2) |
| Mean (SD) fasting insulin, μIU/mL | 15.2 (8.1) |
| Mean (SD) waist, cm | 96.4 (13.3) |
| Waist-insulin correlation | r=0.51, P < .001 |
| aOlder adult patients (age ≥68) with subjective memory complaints were recruited from the community and screened with instruments assessing everyday functioning and objective memory performance to establish the presence of MCI MCI: mild cognitive impairment; SD: standard deviation | |
Dietary interventions
There is no cure for dementia, and it is not clear when effective therapy might be developed. Prevention and risk mitigation represent the best means of reducing the impact of this public health problem. Researchers have proposed that interventions initiated when individuals have predementia conditions such as AAMI and MCI might stall progression of cognitive decline, and MCI may be the last point when interventions might be effective because of the self-reinforcing neuropathologic cascades of AD.31 Because central hypoinsulinemia may promote central inflammation, Aβ generation, and reduced neuroplasticity, approaches aimed at improving metabolic function (and in particular correcting hyperinsulinemia) could influence fundamental neurodegenerative processes. Dietary approaches to preventing dementia are effective, low-risk, yet underutilized interventions. Reducing insulin by restricting calories32 or maintaining a ketogenic diet33 has been associated with improved memory function in middle-aged and older adults.
Carbohydrate consumption is the principal determinant of insulin secretion. Eliminating high-glycemic foods, including processed carbohydrates and sweets, would sensitize insulin receptors and correct hyperinsulinemia. In addition, replacing high glycemic foods with fruits and vegetables would increase polyphenol intake. Epidemiologic evidence supports the idea that greater consumption of polyphenol-containing vegetables and fruits mitigates risk for neurocognitive decline and dementia.34,35 Preclinical evidence suggests that such protection may be related to neuronal signaling effects and anti- inflammatory and antioxidant actions.36 In addition, certain polyphenol compounds, such as those found in berries, enhance metabolic function.37,38 In a 12-week pilot trial, older adults with early memory changes (N=9, mean age 76) who drank supplemental blueberry juice showed enhanced memory and improved metabolic parameters.39
Dietary changes that preserve insulin receptor sensitivity can help ensure general health with aging and substantially mitigate risk for neurodegeneration. The Western diet is particularly insulinogenic and dietary habits are difficult to change. However, the substantial benefits, absence of adverse effects, and low cost make dietary intervention the optimal means of protecting against neurodegeneration and other age-related diseases. Embarking on such a program early in life would be best, although late-life intervention can be effective.
Related Resources
- Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3(3):169-178.
- Luchsinger JA, Tang MX, Shea S, et al. Hyperinsulinemia and risk of Alzheimer’s disease. Neurology. 2004; 63(7):1187-1192.
- Krikorian R, Shidler MD, Dangelo K, et al. Dietary ketosis enhances memory in mild cognitive impairment. Neurbiol Aging. 2012;33(2):425.e19-e27.
Disclosure
Dr. Krikorian receives grant support from the National Institutes of Health, 1R01AG034617-01.
1. Alzheimer’s Association; Thies W, Bleiler L. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7(2):208-244.
2. Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235-241.
3. Wang Y, Beydoun MA, Liang L, et al. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring). 2008;16(10):2323-2330.
4. Craft S. Insulin resistance syndrome and Alzheimer’s disease: age- and obesity-related effect on memory amyloid, and inflammation. Neurobiol Aging. 2005;26(suppl 1):S65-S69.
5. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia – meta-analysis of 41 robust inception cohort studies. Acta Psychiat Scand. 2009;119(4):252-265.
6. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183-194.
7. Crook TH, Bartus RT, Ferris SH, et al. Age-associated memory impairment: proposed diagnostic criteria and measures of clinical change—report of a National Institute of Mental Health work group. Dev Neuropsychol. 1986;2(4):261-276.
8. Neilsen H, Lolk A, Kragh-Sørensen P. Age-associated memory impairment–pathological memory decline or normal aging? Scand J Psychol. 1998;39(1):33-37.
9. Wilson RS, Leurgans SE, Boyle PA, et al. Neurodegenerative basis of age related cognitive decline. Neurology. 2010;75(12):1070-1078.
10. Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834-842.
11. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
12. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356-359.
13. Baura GD, Foster DM, Kaiyala K, et al. Insulin transport from plasma into the central nervous system is inhibited by dexamethasone in dogs. Diabetes. 1996;45(1):86-90.
14. Wallum BJ, Taborsky GJ, Jr, Porte D Jr, et al. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocr Metab. 1987;64(1):190-194.
15. Woods SC, Seeley RJ, Baskin DG, et al. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9(10):795-800.
16. Facchini FS, Hua N, Abbasi F, et al. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86(8):3574-3578.
17. Kuusisto J, Koivisto K, Mykkänen L, et al. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype. BMJ. 1997;315(7115):1045-1049.
18. Luchsinger JA, Tang MX, Shea S, et al. Hyperinsulinemia and risk of Alzheimer’s disease. Neurology. 2004;63(7):1187-1192.
19. Hassing LB, Dahl AK, Thorvaldsson V, et al. Overweight in midlife and risk of dementia: a 40-year follow up study. Int J Obesity (Lond). 2009;33(8):893-898.
20. Young SE, Mainous AG 3rd, Carnemolla M. Hyperinsulinemia and cognitive decline in a middle-aged cohort. Diabetes Care. 2006;29(12):2688-2693.
21. Raji CA, Ho AJ, Parikshak NN, et al. Brain structure and obesity. Hum Brain Mapp. 2009;31(3):353-364.
22. Craft S, Peskind E, Schwartz MW, et al. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease. Neurology. 1998;50(1):164-168.
23. Craft S, Asthana S, Cook DG, et al. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28(6):809-822.
24. Zhao L, Teter B, Morihara T, et al. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer’s disease intervention. J Neurosci. 2004;24(49):11120-11126.
25. Farris W, Mansourian S, Chang Y, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100(7):4162-4167.
26. Tabata S, Yoshimitsu S, Hamachi T, et al. Waist circumference and insulin resistance: a cross-sectional study of Japanese men. BMC Endocr Disord. 2009;9:1.-doi: 10.1186/1472-6823-9-1.
27. Wahrenberg H, Hertel K, Leijonhufvud B, et al. Use of waist circumference to predict insulin resistance: retrospective study. BMJ. 2005;330(7504):1363-1364.
28. Jang S, Lee CH, Choi KM, et al. Correlation of fatty liver and abdominal fat distribution using a simple fat computed tomography protocol. World J Gastroenterol. 2011;17(28):3335-3341.
29. Sutcliffe JG, Hedlund PB, Thomas EA, et al. Peripheral reduction of ß-amyloid is sufficient to reduce brain ß-amyloid: implications for Alzheimer’s disease. J Neurosci Res. 2011;89(6):808-814.
30. Marques MA, Kulstad JJ, Savard CE, et al. Peripheral amyloid-β levels regulate amyloid-β clearance from the central nervous system. J Alzheimers Dis. 2009;16(2):325-329.
31. Cotman CW. Homeostatic processes in brain aging: the role of apoptosis inflammation, and oxidative stress in regulating healthy neural circuitry in the aging brain. In: Stern P, Carstensen L, eds. The aging mind: opportunities in cognitive research. Washington, DC: National Academy Press; 2000:114–143.
32. Witte AV, Fobker M, Gellner R, et al. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106(4):1255-1260.
33. Krikorian R, Shidler MD, Dangelo K, et al. Dietary ketosis enhances memory in mild cognitive impairment. Neurbiol Aging. 2012;33(2):425.e19-e27.
34. Letenneur L, Proust-Lima C, Le Gouge A, et al. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007;165(2):1364-1371.
35. Solfrizzi V, Panza F, Capurso A. The role of diet in cognitive decline. J Neural Transm. 2003;110(3):95-110.
36. Williams CM, El Mohsen MA, Vauzour D, et al. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radical Bio Med. 2008;45(3):295-305.
37. Martineau LC, Couture A, Spoor D, et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine. 2006;13(9-10):612-623.
38. Tsuda T. Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. J Agr Food Chem. 2008;56(3):642-646.
39. Krikorian R, Shidler MD, Nash TA, et al. Blueberry supplementation improves memory in older adults. J Agric Food Chem. 2010;58(7):3996-4000.
Discuss this article at www.facebook.com/CurrentPsychiatry
In addition to increasing patients’ risk for cardiovascular disease, stroke, and cancer, obesity and metabolic disturbance contribute to age-related cognitive decline and dementia. In particular, insulin resistance and hyperinsulinemia promote neurocognitive dysfunction and neurodegenerative changes during the extended, preclinical phase of Alzheimer’s disease (AD). However, with dietary modification it may be possible to resensitize insulin receptors, correct hyperinsulinemia, and improve memory function.
Metabolic disturbance and neurodegeneration
In the United States, 5.4 million people have AD, and there will be an estimated 16 million cases by 2050.1 Simultaneously we are experiencing an epidemic of metabolic disturbance and obesity. Approximately, 64% of adults in the United States are overweight (body mass index [BMI]: 25.0 to 29.9 kg/m2) and 34% are obese (BMI: ≥30 kg/m2).2 By 2030, 86% of adults will be overweight and 51% will be obese.3 This confluence of epidemics is not coincidental but instead reflects the fact that metabolic disturbance is a fundamental factor contributing to cognitive decline and neurodegeneration.4
Ninety-six percent of AD cases are classified as late onset, sporadic AD, occurring after age 64.1 Mild cognitive impairment (MCI) is a clinical construct that entails greater than expected memory impairment for the patient’s age and identifies older adults who are at increased risk for dementia. MCI represents the first clinical manifestation of neurodegeneration for a subset of patients who will progress to AD.5,6 MCI is distinguished from age-associated memory impairment (AAMI), which originally was conceptualized as normal or benign memory decline with aging.7,8 Recent data indicate that Alzheimer’s-type neuropathologic changes are the basis for subjective memory complaints and objectively assessed age-related cognitive decline,9 and early neurodegeneration is present in many patients with AAMI or MCI.10 This is consistent with the idea that an extended preclinical phase precedes AD onset. The preclinical phase can persist for a decade or more and precedes MCI and overt functional decline. However, neuropathologic changes accumulate during the preclinical phase of AD11 and during the preclinical phase of type 2 diabetes mellitus (T2DM).
Hyperinsulinemia and dementia
Insulin resistance and hyperinsulinemia occur in >40% of individuals age ≥60 and prevalence increases with age.4,12 Hyperinsulinemia develops to compensate for insulin resistance to overcome receptor insensitivity and maintain glucose homeostasis. Insulin receptors are densely expressed in brain regions vulnerable to neurodegeneration, including the medial temporal lobe and prefrontal cortex, which mediate long-term memory and working memory. However, insulin must be transported into the CNS from the periphery because little is synthesized in the brain. Paradoxically, peripheral compensatory hyperinsulinemia resulting from insulin resistance is associated with central (brain) hypoinsulinemia because of insensitivity and saturation of the receptor-mediated blood-brain barrier transport mechanism.13-15
Hyperinsulinemia is the precursor to T2DM. However, hyperinsulinemia is not well recognized in clinical contexts and generally is not a treatment target. Nonetheless, it contributes to several health problems, and insulin resistance in middle age is associated with age-related diseases such as hypertension, coronary artery disease, stroke, and cancer, while insulin sensitivity protects against such disorders.16
Chronic insulin resistance may contribute more to dementia development than T2DM because of the extended period of hyperinsulinemia that precedes T2DM onset. In population studies,17 insulin resistance syndrome increases risk for developing AD independent of apolipoprotein E (APOE e4) allele status, and in a longitudinal study,18 the risk for AD solely attributable to peripheral hyperinsulinemia was up to 39%. Being overweight in midlife increases risk for dementia in late life, and APOE e4 allele status does not contribute additional risk after accounting for BMI.19 Middle-aged individuals with hyperinsulinemia show memory decline, and obesity in middle age was associated with greater cognitive impairment after 6-year follow-up.20 Even in older adults who seem cognitively unimpaired, BMI and fasting insulin are positively correlated with atrophy in frontal, temporal, and subcortical brain regions, and obesity is an independent risk for atrophy in several brain regions, including the hippocampus.21
Compared with healthy older adults, individuals with AD have lower ratios of cerebrospinal fluid to plasma insulin.22 This lower ratio reflects the peripheral-to-central gradient of insulin levels in AD and suggests an etiological role for such metabolic disturbance. Insulin resistance has downstream effects that potentiate neurodegenerative factors, and central hypoinsulinemia can accelerate neurodegenerative processes and cognitive decline.4,23 Brain insulin plays a direct role in regulating proinflammatory cytokines and neurotrophic and neuroplastic factors essential for memory function. Insulin degrading enzyme, which varies with insulin levels,24 regulates the generation and clearance of amyloid β (Aβ) from the brain.25
Hyperinsulinemia typically is evident in increasing waist circumference and body weight.26 Waist circumference of ≥100 cm (39 inches) is a sensitive, specific, and independent predictor of hyperinsulinemia for men and women and a stronger predictor than BMI, waist-to-hip ratio, and other measures of body fat.27 Unpublished data derived from our clinical research with MCI subjects supports the association of metabolic disturbance with age-related cognitive decline. Our subjects are recruited from the community on the basis of mild memory decline and—other than excluding those with diabetes—weight and metabolic status are not considered in evaluating individuals for enrollment. The Table contains data on waist circumference and metabolic function in 122 older adults (age ≥68) with MCI. On average, these individuals exhibited fasting insulin values in the hyperinsulinemia range and elevated fasting glucose levels that indicated borderline diabetes. Waist circumference also was high, indicating excessive visceral fat deposition. We also observed a relationship between waist circumference and insulin, a consistent observation in older adults with memory decline. These data would not be surprising in any sample of older adults because of the population base rates for these conditions. However, we also found that waist circumference was a significant predictor of memory performance in patients with MCI. Abdominal adiposity is highly correlated with intrahepatic fat.28 Given this and recent indications that Alzheimer’s-type neuropathologic factors are generated in the liver,29,30 the predictive value of waist circumference to memory performance may reflect the fact that it is a proxy for downstream actions of liver fat.
Table
Waist circumference and metabolic factors in 122 older adults with MCIa
| Metabolic indicator | Value |
|---|---|
| Mean (SD) fasting glucose, mg/dL | 99.5 (11.2) |
| Mean (SD) fasting insulin, μIU/mL | 15.2 (8.1) |
| Mean (SD) waist, cm | 96.4 (13.3) |
| Waist-insulin correlation | r=0.51, P < .001 |
| aOlder adult patients (age ≥68) with subjective memory complaints were recruited from the community and screened with instruments assessing everyday functioning and objective memory performance to establish the presence of MCI MCI: mild cognitive impairment; SD: standard deviation | |
Dietary interventions
There is no cure for dementia, and it is not clear when effective therapy might be developed. Prevention and risk mitigation represent the best means of reducing the impact of this public health problem. Researchers have proposed that interventions initiated when individuals have predementia conditions such as AAMI and MCI might stall progression of cognitive decline, and MCI may be the last point when interventions might be effective because of the self-reinforcing neuropathologic cascades of AD.31 Because central hypoinsulinemia may promote central inflammation, Aβ generation, and reduced neuroplasticity, approaches aimed at improving metabolic function (and in particular correcting hyperinsulinemia) could influence fundamental neurodegenerative processes. Dietary approaches to preventing dementia are effective, low-risk, yet underutilized interventions. Reducing insulin by restricting calories32 or maintaining a ketogenic diet33 has been associated with improved memory function in middle-aged and older adults.
Carbohydrate consumption is the principal determinant of insulin secretion. Eliminating high-glycemic foods, including processed carbohydrates and sweets, would sensitize insulin receptors and correct hyperinsulinemia. In addition, replacing high glycemic foods with fruits and vegetables would increase polyphenol intake. Epidemiologic evidence supports the idea that greater consumption of polyphenol-containing vegetables and fruits mitigates risk for neurocognitive decline and dementia.34,35 Preclinical evidence suggests that such protection may be related to neuronal signaling effects and anti- inflammatory and antioxidant actions.36 In addition, certain polyphenol compounds, such as those found in berries, enhance metabolic function.37,38 In a 12-week pilot trial, older adults with early memory changes (N=9, mean age 76) who drank supplemental blueberry juice showed enhanced memory and improved metabolic parameters.39
Dietary changes that preserve insulin receptor sensitivity can help ensure general health with aging and substantially mitigate risk for neurodegeneration. The Western diet is particularly insulinogenic and dietary habits are difficult to change. However, the substantial benefits, absence of adverse effects, and low cost make dietary intervention the optimal means of protecting against neurodegeneration and other age-related diseases. Embarking on such a program early in life would be best, although late-life intervention can be effective.
Related Resources
- Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3(3):169-178.
- Luchsinger JA, Tang MX, Shea S, et al. Hyperinsulinemia and risk of Alzheimer’s disease. Neurology. 2004; 63(7):1187-1192.
- Krikorian R, Shidler MD, Dangelo K, et al. Dietary ketosis enhances memory in mild cognitive impairment. Neurbiol Aging. 2012;33(2):425.e19-e27.
Disclosure
Dr. Krikorian receives grant support from the National Institutes of Health, 1R01AG034617-01.
Discuss this article at www.facebook.com/CurrentPsychiatry
In addition to increasing patients’ risk for cardiovascular disease, stroke, and cancer, obesity and metabolic disturbance contribute to age-related cognitive decline and dementia. In particular, insulin resistance and hyperinsulinemia promote neurocognitive dysfunction and neurodegenerative changes during the extended, preclinical phase of Alzheimer’s disease (AD). However, with dietary modification it may be possible to resensitize insulin receptors, correct hyperinsulinemia, and improve memory function.
Metabolic disturbance and neurodegeneration
In the United States, 5.4 million people have AD, and there will be an estimated 16 million cases by 2050.1 Simultaneously we are experiencing an epidemic of metabolic disturbance and obesity. Approximately, 64% of adults in the United States are overweight (body mass index [BMI]: 25.0 to 29.9 kg/m2) and 34% are obese (BMI: ≥30 kg/m2).2 By 2030, 86% of adults will be overweight and 51% will be obese.3 This confluence of epidemics is not coincidental but instead reflects the fact that metabolic disturbance is a fundamental factor contributing to cognitive decline and neurodegeneration.4
Ninety-six percent of AD cases are classified as late onset, sporadic AD, occurring after age 64.1 Mild cognitive impairment (MCI) is a clinical construct that entails greater than expected memory impairment for the patient’s age and identifies older adults who are at increased risk for dementia. MCI represents the first clinical manifestation of neurodegeneration for a subset of patients who will progress to AD.5,6 MCI is distinguished from age-associated memory impairment (AAMI), which originally was conceptualized as normal or benign memory decline with aging.7,8 Recent data indicate that Alzheimer’s-type neuropathologic changes are the basis for subjective memory complaints and objectively assessed age-related cognitive decline,9 and early neurodegeneration is present in many patients with AAMI or MCI.10 This is consistent with the idea that an extended preclinical phase precedes AD onset. The preclinical phase can persist for a decade or more and precedes MCI and overt functional decline. However, neuropathologic changes accumulate during the preclinical phase of AD11 and during the preclinical phase of type 2 diabetes mellitus (T2DM).
Hyperinsulinemia and dementia
Insulin resistance and hyperinsulinemia occur in >40% of individuals age ≥60 and prevalence increases with age.4,12 Hyperinsulinemia develops to compensate for insulin resistance to overcome receptor insensitivity and maintain glucose homeostasis. Insulin receptors are densely expressed in brain regions vulnerable to neurodegeneration, including the medial temporal lobe and prefrontal cortex, which mediate long-term memory and working memory. However, insulin must be transported into the CNS from the periphery because little is synthesized in the brain. Paradoxically, peripheral compensatory hyperinsulinemia resulting from insulin resistance is associated with central (brain) hypoinsulinemia because of insensitivity and saturation of the receptor-mediated blood-brain barrier transport mechanism.13-15
Hyperinsulinemia is the precursor to T2DM. However, hyperinsulinemia is not well recognized in clinical contexts and generally is not a treatment target. Nonetheless, it contributes to several health problems, and insulin resistance in middle age is associated with age-related diseases such as hypertension, coronary artery disease, stroke, and cancer, while insulin sensitivity protects against such disorders.16
Chronic insulin resistance may contribute more to dementia development than T2DM because of the extended period of hyperinsulinemia that precedes T2DM onset. In population studies,17 insulin resistance syndrome increases risk for developing AD independent of apolipoprotein E (APOE e4) allele status, and in a longitudinal study,18 the risk for AD solely attributable to peripheral hyperinsulinemia was up to 39%. Being overweight in midlife increases risk for dementia in late life, and APOE e4 allele status does not contribute additional risk after accounting for BMI.19 Middle-aged individuals with hyperinsulinemia show memory decline, and obesity in middle age was associated with greater cognitive impairment after 6-year follow-up.20 Even in older adults who seem cognitively unimpaired, BMI and fasting insulin are positively correlated with atrophy in frontal, temporal, and subcortical brain regions, and obesity is an independent risk for atrophy in several brain regions, including the hippocampus.21
Compared with healthy older adults, individuals with AD have lower ratios of cerebrospinal fluid to plasma insulin.22 This lower ratio reflects the peripheral-to-central gradient of insulin levels in AD and suggests an etiological role for such metabolic disturbance. Insulin resistance has downstream effects that potentiate neurodegenerative factors, and central hypoinsulinemia can accelerate neurodegenerative processes and cognitive decline.4,23 Brain insulin plays a direct role in regulating proinflammatory cytokines and neurotrophic and neuroplastic factors essential for memory function. Insulin degrading enzyme, which varies with insulin levels,24 regulates the generation and clearance of amyloid β (Aβ) from the brain.25
Hyperinsulinemia typically is evident in increasing waist circumference and body weight.26 Waist circumference of ≥100 cm (39 inches) is a sensitive, specific, and independent predictor of hyperinsulinemia for men and women and a stronger predictor than BMI, waist-to-hip ratio, and other measures of body fat.27 Unpublished data derived from our clinical research with MCI subjects supports the association of metabolic disturbance with age-related cognitive decline. Our subjects are recruited from the community on the basis of mild memory decline and—other than excluding those with diabetes—weight and metabolic status are not considered in evaluating individuals for enrollment. The Table contains data on waist circumference and metabolic function in 122 older adults (age ≥68) with MCI. On average, these individuals exhibited fasting insulin values in the hyperinsulinemia range and elevated fasting glucose levels that indicated borderline diabetes. Waist circumference also was high, indicating excessive visceral fat deposition. We also observed a relationship between waist circumference and insulin, a consistent observation in older adults with memory decline. These data would not be surprising in any sample of older adults because of the population base rates for these conditions. However, we also found that waist circumference was a significant predictor of memory performance in patients with MCI. Abdominal adiposity is highly correlated with intrahepatic fat.28 Given this and recent indications that Alzheimer’s-type neuropathologic factors are generated in the liver,29,30 the predictive value of waist circumference to memory performance may reflect the fact that it is a proxy for downstream actions of liver fat.
Table
Waist circumference and metabolic factors in 122 older adults with MCIa
| Metabolic indicator | Value |
|---|---|
| Mean (SD) fasting glucose, mg/dL | 99.5 (11.2) |
| Mean (SD) fasting insulin, μIU/mL | 15.2 (8.1) |
| Mean (SD) waist, cm | 96.4 (13.3) |
| Waist-insulin correlation | r=0.51, P < .001 |
| aOlder adult patients (age ≥68) with subjective memory complaints were recruited from the community and screened with instruments assessing everyday functioning and objective memory performance to establish the presence of MCI MCI: mild cognitive impairment; SD: standard deviation | |
Dietary interventions
There is no cure for dementia, and it is not clear when effective therapy might be developed. Prevention and risk mitigation represent the best means of reducing the impact of this public health problem. Researchers have proposed that interventions initiated when individuals have predementia conditions such as AAMI and MCI might stall progression of cognitive decline, and MCI may be the last point when interventions might be effective because of the self-reinforcing neuropathologic cascades of AD.31 Because central hypoinsulinemia may promote central inflammation, Aβ generation, and reduced neuroplasticity, approaches aimed at improving metabolic function (and in particular correcting hyperinsulinemia) could influence fundamental neurodegenerative processes. Dietary approaches to preventing dementia are effective, low-risk, yet underutilized interventions. Reducing insulin by restricting calories32 or maintaining a ketogenic diet33 has been associated with improved memory function in middle-aged and older adults.
Carbohydrate consumption is the principal determinant of insulin secretion. Eliminating high-glycemic foods, including processed carbohydrates and sweets, would sensitize insulin receptors and correct hyperinsulinemia. In addition, replacing high glycemic foods with fruits and vegetables would increase polyphenol intake. Epidemiologic evidence supports the idea that greater consumption of polyphenol-containing vegetables and fruits mitigates risk for neurocognitive decline and dementia.34,35 Preclinical evidence suggests that such protection may be related to neuronal signaling effects and anti- inflammatory and antioxidant actions.36 In addition, certain polyphenol compounds, such as those found in berries, enhance metabolic function.37,38 In a 12-week pilot trial, older adults with early memory changes (N=9, mean age 76) who drank supplemental blueberry juice showed enhanced memory and improved metabolic parameters.39
Dietary changes that preserve insulin receptor sensitivity can help ensure general health with aging and substantially mitigate risk for neurodegeneration. The Western diet is particularly insulinogenic and dietary habits are difficult to change. However, the substantial benefits, absence of adverse effects, and low cost make dietary intervention the optimal means of protecting against neurodegeneration and other age-related diseases. Embarking on such a program early in life would be best, although late-life intervention can be effective.
Related Resources
- Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3(3):169-178.
- Luchsinger JA, Tang MX, Shea S, et al. Hyperinsulinemia and risk of Alzheimer’s disease. Neurology. 2004; 63(7):1187-1192.
- Krikorian R, Shidler MD, Dangelo K, et al. Dietary ketosis enhances memory in mild cognitive impairment. Neurbiol Aging. 2012;33(2):425.e19-e27.
Disclosure
Dr. Krikorian receives grant support from the National Institutes of Health, 1R01AG034617-01.
1. Alzheimer’s Association; Thies W, Bleiler L. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7(2):208-244.
2. Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235-241.
3. Wang Y, Beydoun MA, Liang L, et al. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring). 2008;16(10):2323-2330.
4. Craft S. Insulin resistance syndrome and Alzheimer’s disease: age- and obesity-related effect on memory amyloid, and inflammation. Neurobiol Aging. 2005;26(suppl 1):S65-S69.
5. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia – meta-analysis of 41 robust inception cohort studies. Acta Psychiat Scand. 2009;119(4):252-265.
6. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183-194.
7. Crook TH, Bartus RT, Ferris SH, et al. Age-associated memory impairment: proposed diagnostic criteria and measures of clinical change—report of a National Institute of Mental Health work group. Dev Neuropsychol. 1986;2(4):261-276.
8. Neilsen H, Lolk A, Kragh-Sørensen P. Age-associated memory impairment–pathological memory decline or normal aging? Scand J Psychol. 1998;39(1):33-37.
9. Wilson RS, Leurgans SE, Boyle PA, et al. Neurodegenerative basis of age related cognitive decline. Neurology. 2010;75(12):1070-1078.
10. Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834-842.
11. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
12. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356-359.
13. Baura GD, Foster DM, Kaiyala K, et al. Insulin transport from plasma into the central nervous system is inhibited by dexamethasone in dogs. Diabetes. 1996;45(1):86-90.
14. Wallum BJ, Taborsky GJ, Jr, Porte D Jr, et al. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocr Metab. 1987;64(1):190-194.
15. Woods SC, Seeley RJ, Baskin DG, et al. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9(10):795-800.
16. Facchini FS, Hua N, Abbasi F, et al. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86(8):3574-3578.
17. Kuusisto J, Koivisto K, Mykkänen L, et al. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype. BMJ. 1997;315(7115):1045-1049.
18. Luchsinger JA, Tang MX, Shea S, et al. Hyperinsulinemia and risk of Alzheimer’s disease. Neurology. 2004;63(7):1187-1192.
19. Hassing LB, Dahl AK, Thorvaldsson V, et al. Overweight in midlife and risk of dementia: a 40-year follow up study. Int J Obesity (Lond). 2009;33(8):893-898.
20. Young SE, Mainous AG 3rd, Carnemolla M. Hyperinsulinemia and cognitive decline in a middle-aged cohort. Diabetes Care. 2006;29(12):2688-2693.
21. Raji CA, Ho AJ, Parikshak NN, et al. Brain structure and obesity. Hum Brain Mapp. 2009;31(3):353-364.
22. Craft S, Peskind E, Schwartz MW, et al. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease. Neurology. 1998;50(1):164-168.
23. Craft S, Asthana S, Cook DG, et al. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28(6):809-822.
24. Zhao L, Teter B, Morihara T, et al. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer’s disease intervention. J Neurosci. 2004;24(49):11120-11126.
25. Farris W, Mansourian S, Chang Y, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100(7):4162-4167.
26. Tabata S, Yoshimitsu S, Hamachi T, et al. Waist circumference and insulin resistance: a cross-sectional study of Japanese men. BMC Endocr Disord. 2009;9:1.-doi: 10.1186/1472-6823-9-1.
27. Wahrenberg H, Hertel K, Leijonhufvud B, et al. Use of waist circumference to predict insulin resistance: retrospective study. BMJ. 2005;330(7504):1363-1364.
28. Jang S, Lee CH, Choi KM, et al. Correlation of fatty liver and abdominal fat distribution using a simple fat computed tomography protocol. World J Gastroenterol. 2011;17(28):3335-3341.
29. Sutcliffe JG, Hedlund PB, Thomas EA, et al. Peripheral reduction of ß-amyloid is sufficient to reduce brain ß-amyloid: implications for Alzheimer’s disease. J Neurosci Res. 2011;89(6):808-814.
30. Marques MA, Kulstad JJ, Savard CE, et al. Peripheral amyloid-β levels regulate amyloid-β clearance from the central nervous system. J Alzheimers Dis. 2009;16(2):325-329.
31. Cotman CW. Homeostatic processes in brain aging: the role of apoptosis inflammation, and oxidative stress in regulating healthy neural circuitry in the aging brain. In: Stern P, Carstensen L, eds. The aging mind: opportunities in cognitive research. Washington, DC: National Academy Press; 2000:114–143.
32. Witte AV, Fobker M, Gellner R, et al. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106(4):1255-1260.
33. Krikorian R, Shidler MD, Dangelo K, et al. Dietary ketosis enhances memory in mild cognitive impairment. Neurbiol Aging. 2012;33(2):425.e19-e27.
34. Letenneur L, Proust-Lima C, Le Gouge A, et al. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007;165(2):1364-1371.
35. Solfrizzi V, Panza F, Capurso A. The role of diet in cognitive decline. J Neural Transm. 2003;110(3):95-110.
36. Williams CM, El Mohsen MA, Vauzour D, et al. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radical Bio Med. 2008;45(3):295-305.
37. Martineau LC, Couture A, Spoor D, et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine. 2006;13(9-10):612-623.
38. Tsuda T. Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. J Agr Food Chem. 2008;56(3):642-646.
39. Krikorian R, Shidler MD, Nash TA, et al. Blueberry supplementation improves memory in older adults. J Agric Food Chem. 2010;58(7):3996-4000.
1. Alzheimer’s Association; Thies W, Bleiler L. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7(2):208-244.
2. Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235-241.
3. Wang Y, Beydoun MA, Liang L, et al. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring). 2008;16(10):2323-2330.
4. Craft S. Insulin resistance syndrome and Alzheimer’s disease: age- and obesity-related effect on memory amyloid, and inflammation. Neurobiol Aging. 2005;26(suppl 1):S65-S69.
5. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia – meta-analysis of 41 robust inception cohort studies. Acta Psychiat Scand. 2009;119(4):252-265.
6. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183-194.
7. Crook TH, Bartus RT, Ferris SH, et al. Age-associated memory impairment: proposed diagnostic criteria and measures of clinical change—report of a National Institute of Mental Health work group. Dev Neuropsychol. 1986;2(4):261-276.
8. Neilsen H, Lolk A, Kragh-Sørensen P. Age-associated memory impairment–pathological memory decline or normal aging? Scand J Psychol. 1998;39(1):33-37.
9. Wilson RS, Leurgans SE, Boyle PA, et al. Neurodegenerative basis of age related cognitive decline. Neurology. 2010;75(12):1070-1078.
10. Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834-842.
11. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
12. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356-359.
13. Baura GD, Foster DM, Kaiyala K, et al. Insulin transport from plasma into the central nervous system is inhibited by dexamethasone in dogs. Diabetes. 1996;45(1):86-90.
14. Wallum BJ, Taborsky GJ, Jr, Porte D Jr, et al. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocr Metab. 1987;64(1):190-194.
15. Woods SC, Seeley RJ, Baskin DG, et al. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9(10):795-800.
16. Facchini FS, Hua N, Abbasi F, et al. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86(8):3574-3578.
17. Kuusisto J, Koivisto K, Mykkänen L, et al. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype. BMJ. 1997;315(7115):1045-1049.
18. Luchsinger JA, Tang MX, Shea S, et al. Hyperinsulinemia and risk of Alzheimer’s disease. Neurology. 2004;63(7):1187-1192.
19. Hassing LB, Dahl AK, Thorvaldsson V, et al. Overweight in midlife and risk of dementia: a 40-year follow up study. Int J Obesity (Lond). 2009;33(8):893-898.
20. Young SE, Mainous AG 3rd, Carnemolla M. Hyperinsulinemia and cognitive decline in a middle-aged cohort. Diabetes Care. 2006;29(12):2688-2693.
21. Raji CA, Ho AJ, Parikshak NN, et al. Brain structure and obesity. Hum Brain Mapp. 2009;31(3):353-364.
22. Craft S, Peskind E, Schwartz MW, et al. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease. Neurology. 1998;50(1):164-168.
23. Craft S, Asthana S, Cook DG, et al. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28(6):809-822.
24. Zhao L, Teter B, Morihara T, et al. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer’s disease intervention. J Neurosci. 2004;24(49):11120-11126.
25. Farris W, Mansourian S, Chang Y, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100(7):4162-4167.
26. Tabata S, Yoshimitsu S, Hamachi T, et al. Waist circumference and insulin resistance: a cross-sectional study of Japanese men. BMC Endocr Disord. 2009;9:1.-doi: 10.1186/1472-6823-9-1.
27. Wahrenberg H, Hertel K, Leijonhufvud B, et al. Use of waist circumference to predict insulin resistance: retrospective study. BMJ. 2005;330(7504):1363-1364.
28. Jang S, Lee CH, Choi KM, et al. Correlation of fatty liver and abdominal fat distribution using a simple fat computed tomography protocol. World J Gastroenterol. 2011;17(28):3335-3341.
29. Sutcliffe JG, Hedlund PB, Thomas EA, et al. Peripheral reduction of ß-amyloid is sufficient to reduce brain ß-amyloid: implications for Alzheimer’s disease. J Neurosci Res. 2011;89(6):808-814.
30. Marques MA, Kulstad JJ, Savard CE, et al. Peripheral amyloid-β levels regulate amyloid-β clearance from the central nervous system. J Alzheimers Dis. 2009;16(2):325-329.
31. Cotman CW. Homeostatic processes in brain aging: the role of apoptosis inflammation, and oxidative stress in regulating healthy neural circuitry in the aging brain. In: Stern P, Carstensen L, eds. The aging mind: opportunities in cognitive research. Washington, DC: National Academy Press; 2000:114–143.
32. Witte AV, Fobker M, Gellner R, et al. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106(4):1255-1260.
33. Krikorian R, Shidler MD, Dangelo K, et al. Dietary ketosis enhances memory in mild cognitive impairment. Neurbiol Aging. 2012;33(2):425.e19-e27.
34. Letenneur L, Proust-Lima C, Le Gouge A, et al. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007;165(2):1364-1371.
35. Solfrizzi V, Panza F, Capurso A. The role of diet in cognitive decline. J Neural Transm. 2003;110(3):95-110.
36. Williams CM, El Mohsen MA, Vauzour D, et al. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radical Bio Med. 2008;45(3):295-305.
37. Martineau LC, Couture A, Spoor D, et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine. 2006;13(9-10):612-623.
38. Tsuda T. Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. J Agr Food Chem. 2008;56(3):642-646.
39. Krikorian R, Shidler MD, Nash TA, et al. Blueberry supplementation improves memory in older adults. J Agric Food Chem. 2010;58(7):3996-4000.
Brain and mind assessment in psychiatry
A mountain of evidence indicates that psychosis and bipolar disorder (BD) are brain disorders with an array of thought, mood, cognition, and behavioral aberrations.
Yet the clinical assessment of those neuropsychiatric disorders predominantly is restricted to evaluating mental and behavioral signs and symptoms. It’s time we comprehensively assess our psychiatric patients’ brains, not just describe their minds. This is the only way we can eventually identify the roots of serious mental illness and develop accurate and effective therapeutic interventions and preventions.
Consider the following brain probes, measures, and assessments that are rarely done in patients with first-episode schizophrenia, BD, or major depression. These clinical and technological cerebral evaluation methods all are available and feasible and are being routinely exploited in neurology and other medical specialties. Not using them represents missed opportunities to advance the scientific underpinnings of psychiatric diagnosis and treatment.
Complete neurologic examination, including cranial nerves, motor functions, sensory status, reflexes (including primitive reflexes), and soft neurologic signs. Psychiatrists rarely perform such examinations, although they can easily relearn and incorporate them in their critical initial assessment of severe psychiatric episodes. Researchers have identified many neurologic findings in drug-naïve psychotic patients before they receive medications in whom adverse effects may mask or add to motor or sensory abnormalities.
Neurocognitive testing. An extensive body of literature has definitively demonstrated severe cognitive deficits across multiple domains in schizophrenia, BD, and major depression. Yet, inexplicably, few first-episode patients are assessed with a standard battery of tests for memory, attention, visuospatial skills, or executive functions in clinical practice. Cognitive deficits are a product of abnormal neural pathways and neurocognitive tests can provide tremendous insight into regional and overall brain functions and provide clues for etiopathology and a road map for rehabilitation.
Neuroimaging. Multiple sophisticated techniques to assess brain structure and function are used in research but rarely in clinical practice. These include:
Morphological MRI,, which can provide exquisitely detailed anatomical information about cortical and subcortical structures. This can help identify lesions that cause mania, schizophrenia-like disorders, or depression secondary to a brain pathology. Even if no lesion is found, the pattern of atrophy, hypertrophy, ectopic gray matter, or hyperplasia can help identify subtypes of heterogeneous psychotic and mood disorders, and may lead to a specific diagnosis and treatment.
Magnetic resonance spectroscopy (MRS) is essentially a living biopsy of the brain in any region, detailing the spectrum and amount of various neurochemical substances (such as glutamine, γ-aminobutyric acid, creatine, N-acetylaspartic acid, or lactate) using proton spectroscopy, high energy phosphates such as adenosine diphosphate (ADP) or adenosine triphosphate (ATP) or membrane breakdown products (such as phosphomonoester and phosphodiester) using phosphorous MRS. Researchers are gradually “mapping” the regional chemistry of the brain in health and disease, which may provide profound insights for understanding the neurobiology of serious mental disorders.
Functional MRI, which can display the underactivation or overactivation of various brain regions at rest or while experiencing severe symptoms such as hallucinations or melancholia or while performing a cognitive task. Significant insights about brain pathways can be gleaned from this test.
Diffusion tensor imaging (DTI), which can assess myelin integrity and provide critical data about white matter tract pathology and intra- and inter-hemispheric disconnectivity. Pathological myelin findings in psychotic and mood disorders already are prompting novel treatments for these disabling brain illnesses.
Cerebrospinal fluid (CSF) examination. Psychiatrists rarely perform lumbar punctures (LP) in first-episode patients, although psychotic or bipolar disorders are as severe and disabling as multiple sclerosis or meningitis, where an LP is routine. This longstanding omission is the result of the antiquated notion that CSF in psychiatric patients is not abnormal and uninformative. But the fact is that CSF in patients with psychotic or mood disorders may contain many recently discovered biomarkers that shed light on the tremendous neurochemical changes during an acute psychotic, manic, or depressive episode. So the focus in psychiatry is not simply on red blood cells, white blood cells, glucose levels, or proteins, as in a routine LP, but on the emerging biomarkers of brain pathologies that have been implicated in the psychotic and mood disorders, including:
- inflammatory signaling and biomarkers (such as cytokines, interleukins, TNF-α)
- apoptotic (such as caspase-3, Fas, ARTS) and anti-apoptotic proteins (Bcl-2)
- neurotropic (growth) factor (such as BDNF, NGF, VEGF)
- oxidative stress biomarkers (such as TBARS, TRAP, PCC, SOD, and TAOP)
- myelin byproducts (such as S100B, oligodendrocytic proteins)
- glutamate/glutamine abnormalities
- lipodomic aberrations
- metabolomic profiles
- mitochondrial deficits (such as low glutathione and GPX)
- immunoglobulins (such as IgG, IgM).
If CSF analysis is done routinely, unprecedented discoveries can be made about the nature of brain pathologies and potential diagnostic biomarkers in various subtypes of serious psychiatric disorders, leading to specific and personalized treatments.
It’s time that we go beyond the current descriptive approach that includes a brief mental status exam. We must conduct a comprehensive investigation of our patients’ abnormal brains, which are responsible for their anomalous minds and impaired functioning. It’s time to capitalize on the amazing neuroscience advances to understand our patients’ brains. It’s time that we employ translational psychiatry to guide our diagnosis and treatment of severe mental disorders.
A mountain of evidence indicates that psychosis and bipolar disorder (BD) are brain disorders with an array of thought, mood, cognition, and behavioral aberrations.
Yet the clinical assessment of those neuropsychiatric disorders predominantly is restricted to evaluating mental and behavioral signs and symptoms. It’s time we comprehensively assess our psychiatric patients’ brains, not just describe their minds. This is the only way we can eventually identify the roots of serious mental illness and develop accurate and effective therapeutic interventions and preventions.
Consider the following brain probes, measures, and assessments that are rarely done in patients with first-episode schizophrenia, BD, or major depression. These clinical and technological cerebral evaluation methods all are available and feasible and are being routinely exploited in neurology and other medical specialties. Not using them represents missed opportunities to advance the scientific underpinnings of psychiatric diagnosis and treatment.
Complete neurologic examination, including cranial nerves, motor functions, sensory status, reflexes (including primitive reflexes), and soft neurologic signs. Psychiatrists rarely perform such examinations, although they can easily relearn and incorporate them in their critical initial assessment of severe psychiatric episodes. Researchers have identified many neurologic findings in drug-naïve psychotic patients before they receive medications in whom adverse effects may mask or add to motor or sensory abnormalities.
Neurocognitive testing. An extensive body of literature has definitively demonstrated severe cognitive deficits across multiple domains in schizophrenia, BD, and major depression. Yet, inexplicably, few first-episode patients are assessed with a standard battery of tests for memory, attention, visuospatial skills, or executive functions in clinical practice. Cognitive deficits are a product of abnormal neural pathways and neurocognitive tests can provide tremendous insight into regional and overall brain functions and provide clues for etiopathology and a road map for rehabilitation.
Neuroimaging. Multiple sophisticated techniques to assess brain structure and function are used in research but rarely in clinical practice. These include:
Morphological MRI,, which can provide exquisitely detailed anatomical information about cortical and subcortical structures. This can help identify lesions that cause mania, schizophrenia-like disorders, or depression secondary to a brain pathology. Even if no lesion is found, the pattern of atrophy, hypertrophy, ectopic gray matter, or hyperplasia can help identify subtypes of heterogeneous psychotic and mood disorders, and may lead to a specific diagnosis and treatment.
Magnetic resonance spectroscopy (MRS) is essentially a living biopsy of the brain in any region, detailing the spectrum and amount of various neurochemical substances (such as glutamine, γ-aminobutyric acid, creatine, N-acetylaspartic acid, or lactate) using proton spectroscopy, high energy phosphates such as adenosine diphosphate (ADP) or adenosine triphosphate (ATP) or membrane breakdown products (such as phosphomonoester and phosphodiester) using phosphorous MRS. Researchers are gradually “mapping” the regional chemistry of the brain in health and disease, which may provide profound insights for understanding the neurobiology of serious mental disorders.
Functional MRI, which can display the underactivation or overactivation of various brain regions at rest or while experiencing severe symptoms such as hallucinations or melancholia or while performing a cognitive task. Significant insights about brain pathways can be gleaned from this test.
Diffusion tensor imaging (DTI), which can assess myelin integrity and provide critical data about white matter tract pathology and intra- and inter-hemispheric disconnectivity. Pathological myelin findings in psychotic and mood disorders already are prompting novel treatments for these disabling brain illnesses.
Cerebrospinal fluid (CSF) examination. Psychiatrists rarely perform lumbar punctures (LP) in first-episode patients, although psychotic or bipolar disorders are as severe and disabling as multiple sclerosis or meningitis, where an LP is routine. This longstanding omission is the result of the antiquated notion that CSF in psychiatric patients is not abnormal and uninformative. But the fact is that CSF in patients with psychotic or mood disorders may contain many recently discovered biomarkers that shed light on the tremendous neurochemical changes during an acute psychotic, manic, or depressive episode. So the focus in psychiatry is not simply on red blood cells, white blood cells, glucose levels, or proteins, as in a routine LP, but on the emerging biomarkers of brain pathologies that have been implicated in the psychotic and mood disorders, including:
- inflammatory signaling and biomarkers (such as cytokines, interleukins, TNF-α)
- apoptotic (such as caspase-3, Fas, ARTS) and anti-apoptotic proteins (Bcl-2)
- neurotropic (growth) factor (such as BDNF, NGF, VEGF)
- oxidative stress biomarkers (such as TBARS, TRAP, PCC, SOD, and TAOP)
- myelin byproducts (such as S100B, oligodendrocytic proteins)
- glutamate/glutamine abnormalities
- lipodomic aberrations
- metabolomic profiles
- mitochondrial deficits (such as low glutathione and GPX)
- immunoglobulins (such as IgG, IgM).
If CSF analysis is done routinely, unprecedented discoveries can be made about the nature of brain pathologies and potential diagnostic biomarkers in various subtypes of serious psychiatric disorders, leading to specific and personalized treatments.
It’s time that we go beyond the current descriptive approach that includes a brief mental status exam. We must conduct a comprehensive investigation of our patients’ abnormal brains, which are responsible for their anomalous minds and impaired functioning. It’s time to capitalize on the amazing neuroscience advances to understand our patients’ brains. It’s time that we employ translational psychiatry to guide our diagnosis and treatment of severe mental disorders.
A mountain of evidence indicates that psychosis and bipolar disorder (BD) are brain disorders with an array of thought, mood, cognition, and behavioral aberrations.
Yet the clinical assessment of those neuropsychiatric disorders predominantly is restricted to evaluating mental and behavioral signs and symptoms. It’s time we comprehensively assess our psychiatric patients’ brains, not just describe their minds. This is the only way we can eventually identify the roots of serious mental illness and develop accurate and effective therapeutic interventions and preventions.
Consider the following brain probes, measures, and assessments that are rarely done in patients with first-episode schizophrenia, BD, or major depression. These clinical and technological cerebral evaluation methods all are available and feasible and are being routinely exploited in neurology and other medical specialties. Not using them represents missed opportunities to advance the scientific underpinnings of psychiatric diagnosis and treatment.
Complete neurologic examination, including cranial nerves, motor functions, sensory status, reflexes (including primitive reflexes), and soft neurologic signs. Psychiatrists rarely perform such examinations, although they can easily relearn and incorporate them in their critical initial assessment of severe psychiatric episodes. Researchers have identified many neurologic findings in drug-naïve psychotic patients before they receive medications in whom adverse effects may mask or add to motor or sensory abnormalities.
Neurocognitive testing. An extensive body of literature has definitively demonstrated severe cognitive deficits across multiple domains in schizophrenia, BD, and major depression. Yet, inexplicably, few first-episode patients are assessed with a standard battery of tests for memory, attention, visuospatial skills, or executive functions in clinical practice. Cognitive deficits are a product of abnormal neural pathways and neurocognitive tests can provide tremendous insight into regional and overall brain functions and provide clues for etiopathology and a road map for rehabilitation.
Neuroimaging. Multiple sophisticated techniques to assess brain structure and function are used in research but rarely in clinical practice. These include:
Morphological MRI,, which can provide exquisitely detailed anatomical information about cortical and subcortical structures. This can help identify lesions that cause mania, schizophrenia-like disorders, or depression secondary to a brain pathology. Even if no lesion is found, the pattern of atrophy, hypertrophy, ectopic gray matter, or hyperplasia can help identify subtypes of heterogeneous psychotic and mood disorders, and may lead to a specific diagnosis and treatment.
Magnetic resonance spectroscopy (MRS) is essentially a living biopsy of the brain in any region, detailing the spectrum and amount of various neurochemical substances (such as glutamine, γ-aminobutyric acid, creatine, N-acetylaspartic acid, or lactate) using proton spectroscopy, high energy phosphates such as adenosine diphosphate (ADP) or adenosine triphosphate (ATP) or membrane breakdown products (such as phosphomonoester and phosphodiester) using phosphorous MRS. Researchers are gradually “mapping” the regional chemistry of the brain in health and disease, which may provide profound insights for understanding the neurobiology of serious mental disorders.
Functional MRI, which can display the underactivation or overactivation of various brain regions at rest or while experiencing severe symptoms such as hallucinations or melancholia or while performing a cognitive task. Significant insights about brain pathways can be gleaned from this test.
Diffusion tensor imaging (DTI), which can assess myelin integrity and provide critical data about white matter tract pathology and intra- and inter-hemispheric disconnectivity. Pathological myelin findings in psychotic and mood disorders already are prompting novel treatments for these disabling brain illnesses.
Cerebrospinal fluid (CSF) examination. Psychiatrists rarely perform lumbar punctures (LP) in first-episode patients, although psychotic or bipolar disorders are as severe and disabling as multiple sclerosis or meningitis, where an LP is routine. This longstanding omission is the result of the antiquated notion that CSF in psychiatric patients is not abnormal and uninformative. But the fact is that CSF in patients with psychotic or mood disorders may contain many recently discovered biomarkers that shed light on the tremendous neurochemical changes during an acute psychotic, manic, or depressive episode. So the focus in psychiatry is not simply on red blood cells, white blood cells, glucose levels, or proteins, as in a routine LP, but on the emerging biomarkers of brain pathologies that have been implicated in the psychotic and mood disorders, including:
- inflammatory signaling and biomarkers (such as cytokines, interleukins, TNF-α)
- apoptotic (such as caspase-3, Fas, ARTS) and anti-apoptotic proteins (Bcl-2)
- neurotropic (growth) factor (such as BDNF, NGF, VEGF)
- oxidative stress biomarkers (such as TBARS, TRAP, PCC, SOD, and TAOP)
- myelin byproducts (such as S100B, oligodendrocytic proteins)
- glutamate/glutamine abnormalities
- lipodomic aberrations
- metabolomic profiles
- mitochondrial deficits (such as low glutathione and GPX)
- immunoglobulins (such as IgG, IgM).
If CSF analysis is done routinely, unprecedented discoveries can be made about the nature of brain pathologies and potential diagnostic biomarkers in various subtypes of serious psychiatric disorders, leading to specific and personalized treatments.
It’s time that we go beyond the current descriptive approach that includes a brief mental status exam. We must conduct a comprehensive investigation of our patients’ abnormal brains, which are responsible for their anomalous minds and impaired functioning. It’s time to capitalize on the amazing neuroscience advances to understand our patients’ brains. It’s time that we employ translational psychiatry to guide our diagnosis and treatment of severe mental disorders.
Can topiramate reduce nightmares in posttraumatic stress disorder?
Re-experiencing a previous life-threatening stress through nightmares or recurrent memories is a hallmark of posttraumatic stress disorder (PTSD). In the United States, the lifetime risk of PTSD is 10.1% and the 12-month prevalence is 3.7%.1 The selective serotonin reuptake inhibitors (SSRIs) sertraline and paroxetine are FDA-approved for treating PTSD; clinicians commonly use any SSRI for this disorder. Although SSRIs can alleviate many PTSD symptoms, at times patients experience only a partial response, which necessitates other interventions.
Rationale for using topiramate
The anticonvulsant topiramate blocks voltage-sensitive sodium channels, augments γ-aminobutyric acid type A, antagonizes the glutamate receptor, and inhibits carbonic anhydrase. Researchers have hypothesized that limbic nuclei become sensitized and “kindled” after exposure to a traumatic event. Anticonvulsants such as topiramate may help mitigate stress-activated kindling in PTSD.2,3
What does the evidence say?
Although less compelling than double-blind, placebo-controlled trials, small open-label studies and some case reports indicate a potential role for topiramate in PTSD for specific populations.4,5 In an 8-week open- label study, Alderman et al6 found adjunctive topiramate led to a statistically significant reduction in Clinician-Administered PTSD Scale (CAPS) scores and nightmares in 43 male veterans with combat-related PTSD. There was a nonsignificant decrease in high-risk alcohol use.
In a 2002 retrospective case series, Berlant et al7 found topiramate as monotherapy or adjunctive therapy reduced nightmares in 35 patients with chronic, non-combat PTSD. Nightmares decreased in 79% of patients and flashbacks decreased in 86%, with symptom improvement in a median of 4 days. Limitations of this study included lack of placebo control, a low number of participants, and a high dropout rate (9/35).
Two years later, Berlant8 used the PTSD Checklist-Civilian version (PCL-C) to assess response to topiramate in an open-label study of 33 patients with chronic, non-hallucinatory PTSD. Twenty-eight patients used topiramate as add-on therapy. PCL-C scores decreased by ≥30% in 77% of patients in 4 weeks, with a median dose of 50 mg/d and a median response time of 9 days.
In a double-blind, placebo-controlled trial, Tucker et al9 assessed 38 civilian patients who took topiramate monotherapy for PTSD. Using the CAPS, researchers concluded that topiramate reduced re-experiencing symptoms, but the effect was not statistically significant.9
Lindley et al10 conducted a randomized, double-blind, placebo-controlled trial to study the effect of add-on topiramate in 40 patients with chronic, combat-related PTSD. Because many patients in this study had a history of depression and substance use disorders, topiramate was added to antidepressants; no anticonvulsants, antipsychotics, or benzodiazepines were used. Similar to previous studies, researchers found no statistically significant effect on PTSD symptom severity or global symptom improvement. However, the small number of participants and a high dropout rate limited this study.10
In a 12-week, double-blind, placebo-controlled study of 35 men and women age 18 to 62 with PTSD, Yeh et al11 found that topiramate (mean dose: 102.94 mg/d) lead to a statistically significant overall CAPS score reduction, with significant improvements in re-experiencing symptoms, such as nightmares.
Our opinion
FDA-approved treatments such as SSRIs should be the first pharmacologic intervention for PTSD. If a patient’s response is partial or inadequate, consider additional treatment options. For patients with persistent re-experiencing symptoms, evidence and experience with prazosin and trazodone are more robust than that for topiramate.12
Using topiramate to reduce re-experiencing symptoms such as nightmares in PTSD is not supported by statistically significant evidence from double-blind, placebo- controlled trials. However, numerous open-label studies and case reports suggest that there may be a role for topiramate in PTSD patients who do not respond to other treatments. Data indicate that topiramate may be helpful for PTSD patients who have high-risk alcohol use6 or migraine headaches.13 Because some patients who take topiramate lose weight, the medication may be useful for PTSD patients who are overweight.13
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Related Resource
- U.S. Department of Veterans Affairs. Nightmares and PTSD. www.ptsd.va.gov/public/pages/nightmares.asp.
Drug Brand Names
- Paroxetine • Paxil
- Sertraline • Zoloft
- Prazosin • Minipress
- Topiramate • Topamax
- Trazodone • Desyrel, Oleptro
1. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169-184.
2. Berlin HA. Antiepileptic drugs for the treatment of post-traumatic stress disorder. Curr Psychiatry Rep. 2007;9(4):291-300.
3. Khan S, Liberzon I. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology (Berl). 2004;172(2):225-229.
4. Berlant JL. Topiramate in posttraumatic stress disorder: preliminary clinical observations. J Clin Psychiatry. 2001;62(suppl 17):60-63.
5. Tucker P, Masters B, Nawar O. Topiramate in the treatment of comorbid night eating syndrome and PTSD: a case study. Eat Disord. 2004;12(1):75-78.
6. Alderman CP, McCarthy LC, Condon JT, et al. Topiramate in combat-related posttraumatic stress disorder. Ann Pharmacother. 2009;43(4):635-641.
7. Berlant J, van Kammen DP. Open-label topiramate as primary or adjunctive therapy in chronic civilian posttraumatic stress disorder: a preliminary report. J Clin Psychiatry. 2002;63(1):15-20.
8. Berlant JL. Prospective open-label study of add-on and monotherapy topiramate in civilians with chronic nonhallucinatory posttraumatic stress disorder. BMC Psychiatry. 2004;4:24.-
9. Tucker P, Trautman RP, Wyatt DB, et al. Efficacy and safety of topiramate monotherapy in civilian posttraumatic stress disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(2):201-206.
10. Lindley SE, Carlson EB, Hill K. A randomized double-blind, placebo-controlled trial of augmentation topiramate for chronic combat-related posttraumatic stress disorder. J Clin Psychopharmacol. 2007;27(6):677-681.
11. Yeh MS, Mari JJ, Costa MC, et al. A double-blind randomized controlled trial to study the efficacy of topiramate in a civilian sample of PTSD. CNW Neurosci Ther. 2011;17(5):305-310.
12. Bajor LA, Ticlea AN, Osser DN. The Psychopharmacology Algorithm Project at the Harvard South Shore Program: an update on posttraumatic stress disorder. Harv Rev Psychiatry. 2011;19(5):240-258.
13. Topax [package insert]. Titusville NJ: Janssen Pharmaceuticals; 2009.
Re-experiencing a previous life-threatening stress through nightmares or recurrent memories is a hallmark of posttraumatic stress disorder (PTSD). In the United States, the lifetime risk of PTSD is 10.1% and the 12-month prevalence is 3.7%.1 The selective serotonin reuptake inhibitors (SSRIs) sertraline and paroxetine are FDA-approved for treating PTSD; clinicians commonly use any SSRI for this disorder. Although SSRIs can alleviate many PTSD symptoms, at times patients experience only a partial response, which necessitates other interventions.
Rationale for using topiramate
The anticonvulsant topiramate blocks voltage-sensitive sodium channels, augments γ-aminobutyric acid type A, antagonizes the glutamate receptor, and inhibits carbonic anhydrase. Researchers have hypothesized that limbic nuclei become sensitized and “kindled” after exposure to a traumatic event. Anticonvulsants such as topiramate may help mitigate stress-activated kindling in PTSD.2,3
What does the evidence say?
Although less compelling than double-blind, placebo-controlled trials, small open-label studies and some case reports indicate a potential role for topiramate in PTSD for specific populations.4,5 In an 8-week open- label study, Alderman et al6 found adjunctive topiramate led to a statistically significant reduction in Clinician-Administered PTSD Scale (CAPS) scores and nightmares in 43 male veterans with combat-related PTSD. There was a nonsignificant decrease in high-risk alcohol use.
In a 2002 retrospective case series, Berlant et al7 found topiramate as monotherapy or adjunctive therapy reduced nightmares in 35 patients with chronic, non-combat PTSD. Nightmares decreased in 79% of patients and flashbacks decreased in 86%, with symptom improvement in a median of 4 days. Limitations of this study included lack of placebo control, a low number of participants, and a high dropout rate (9/35).
Two years later, Berlant8 used the PTSD Checklist-Civilian version (PCL-C) to assess response to topiramate in an open-label study of 33 patients with chronic, non-hallucinatory PTSD. Twenty-eight patients used topiramate as add-on therapy. PCL-C scores decreased by ≥30% in 77% of patients in 4 weeks, with a median dose of 50 mg/d and a median response time of 9 days.
In a double-blind, placebo-controlled trial, Tucker et al9 assessed 38 civilian patients who took topiramate monotherapy for PTSD. Using the CAPS, researchers concluded that topiramate reduced re-experiencing symptoms, but the effect was not statistically significant.9
Lindley et al10 conducted a randomized, double-blind, placebo-controlled trial to study the effect of add-on topiramate in 40 patients with chronic, combat-related PTSD. Because many patients in this study had a history of depression and substance use disorders, topiramate was added to antidepressants; no anticonvulsants, antipsychotics, or benzodiazepines were used. Similar to previous studies, researchers found no statistically significant effect on PTSD symptom severity or global symptom improvement. However, the small number of participants and a high dropout rate limited this study.10
In a 12-week, double-blind, placebo-controlled study of 35 men and women age 18 to 62 with PTSD, Yeh et al11 found that topiramate (mean dose: 102.94 mg/d) lead to a statistically significant overall CAPS score reduction, with significant improvements in re-experiencing symptoms, such as nightmares.
Our opinion
FDA-approved treatments such as SSRIs should be the first pharmacologic intervention for PTSD. If a patient’s response is partial or inadequate, consider additional treatment options. For patients with persistent re-experiencing symptoms, evidence and experience with prazosin and trazodone are more robust than that for topiramate.12
Using topiramate to reduce re-experiencing symptoms such as nightmares in PTSD is not supported by statistically significant evidence from double-blind, placebo- controlled trials. However, numerous open-label studies and case reports suggest that there may be a role for topiramate in PTSD patients who do not respond to other treatments. Data indicate that topiramate may be helpful for PTSD patients who have high-risk alcohol use6 or migraine headaches.13 Because some patients who take topiramate lose weight, the medication may be useful for PTSD patients who are overweight.13
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Related Resource
- U.S. Department of Veterans Affairs. Nightmares and PTSD. www.ptsd.va.gov/public/pages/nightmares.asp.
Drug Brand Names
- Paroxetine • Paxil
- Sertraline • Zoloft
- Prazosin • Minipress
- Topiramate • Topamax
- Trazodone • Desyrel, Oleptro
Re-experiencing a previous life-threatening stress through nightmares or recurrent memories is a hallmark of posttraumatic stress disorder (PTSD). In the United States, the lifetime risk of PTSD is 10.1% and the 12-month prevalence is 3.7%.1 The selective serotonin reuptake inhibitors (SSRIs) sertraline and paroxetine are FDA-approved for treating PTSD; clinicians commonly use any SSRI for this disorder. Although SSRIs can alleviate many PTSD symptoms, at times patients experience only a partial response, which necessitates other interventions.
Rationale for using topiramate
The anticonvulsant topiramate blocks voltage-sensitive sodium channels, augments γ-aminobutyric acid type A, antagonizes the glutamate receptor, and inhibits carbonic anhydrase. Researchers have hypothesized that limbic nuclei become sensitized and “kindled” after exposure to a traumatic event. Anticonvulsants such as topiramate may help mitigate stress-activated kindling in PTSD.2,3
What does the evidence say?
Although less compelling than double-blind, placebo-controlled trials, small open-label studies and some case reports indicate a potential role for topiramate in PTSD for specific populations.4,5 In an 8-week open- label study, Alderman et al6 found adjunctive topiramate led to a statistically significant reduction in Clinician-Administered PTSD Scale (CAPS) scores and nightmares in 43 male veterans with combat-related PTSD. There was a nonsignificant decrease in high-risk alcohol use.
In a 2002 retrospective case series, Berlant et al7 found topiramate as monotherapy or adjunctive therapy reduced nightmares in 35 patients with chronic, non-combat PTSD. Nightmares decreased in 79% of patients and flashbacks decreased in 86%, with symptom improvement in a median of 4 days. Limitations of this study included lack of placebo control, a low number of participants, and a high dropout rate (9/35).
Two years later, Berlant8 used the PTSD Checklist-Civilian version (PCL-C) to assess response to topiramate in an open-label study of 33 patients with chronic, non-hallucinatory PTSD. Twenty-eight patients used topiramate as add-on therapy. PCL-C scores decreased by ≥30% in 77% of patients in 4 weeks, with a median dose of 50 mg/d and a median response time of 9 days.
In a double-blind, placebo-controlled trial, Tucker et al9 assessed 38 civilian patients who took topiramate monotherapy for PTSD. Using the CAPS, researchers concluded that topiramate reduced re-experiencing symptoms, but the effect was not statistically significant.9
Lindley et al10 conducted a randomized, double-blind, placebo-controlled trial to study the effect of add-on topiramate in 40 patients with chronic, combat-related PTSD. Because many patients in this study had a history of depression and substance use disorders, topiramate was added to antidepressants; no anticonvulsants, antipsychotics, or benzodiazepines were used. Similar to previous studies, researchers found no statistically significant effect on PTSD symptom severity or global symptom improvement. However, the small number of participants and a high dropout rate limited this study.10
In a 12-week, double-blind, placebo-controlled study of 35 men and women age 18 to 62 with PTSD, Yeh et al11 found that topiramate (mean dose: 102.94 mg/d) lead to a statistically significant overall CAPS score reduction, with significant improvements in re-experiencing symptoms, such as nightmares.
Our opinion
FDA-approved treatments such as SSRIs should be the first pharmacologic intervention for PTSD. If a patient’s response is partial or inadequate, consider additional treatment options. For patients with persistent re-experiencing symptoms, evidence and experience with prazosin and trazodone are more robust than that for topiramate.12
Using topiramate to reduce re-experiencing symptoms such as nightmares in PTSD is not supported by statistically significant evidence from double-blind, placebo- controlled trials. However, numerous open-label studies and case reports suggest that there may be a role for topiramate in PTSD patients who do not respond to other treatments. Data indicate that topiramate may be helpful for PTSD patients who have high-risk alcohol use6 or migraine headaches.13 Because some patients who take topiramate lose weight, the medication may be useful for PTSD patients who are overweight.13
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Related Resource
- U.S. Department of Veterans Affairs. Nightmares and PTSD. www.ptsd.va.gov/public/pages/nightmares.asp.
Drug Brand Names
- Paroxetine • Paxil
- Sertraline • Zoloft
- Prazosin • Minipress
- Topiramate • Topamax
- Trazodone • Desyrel, Oleptro
1. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169-184.
2. Berlin HA. Antiepileptic drugs for the treatment of post-traumatic stress disorder. Curr Psychiatry Rep. 2007;9(4):291-300.
3. Khan S, Liberzon I. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology (Berl). 2004;172(2):225-229.
4. Berlant JL. Topiramate in posttraumatic stress disorder: preliminary clinical observations. J Clin Psychiatry. 2001;62(suppl 17):60-63.
5. Tucker P, Masters B, Nawar O. Topiramate in the treatment of comorbid night eating syndrome and PTSD: a case study. Eat Disord. 2004;12(1):75-78.
6. Alderman CP, McCarthy LC, Condon JT, et al. Topiramate in combat-related posttraumatic stress disorder. Ann Pharmacother. 2009;43(4):635-641.
7. Berlant J, van Kammen DP. Open-label topiramate as primary or adjunctive therapy in chronic civilian posttraumatic stress disorder: a preliminary report. J Clin Psychiatry. 2002;63(1):15-20.
8. Berlant JL. Prospective open-label study of add-on and monotherapy topiramate in civilians with chronic nonhallucinatory posttraumatic stress disorder. BMC Psychiatry. 2004;4:24.-
9. Tucker P, Trautman RP, Wyatt DB, et al. Efficacy and safety of topiramate monotherapy in civilian posttraumatic stress disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(2):201-206.
10. Lindley SE, Carlson EB, Hill K. A randomized double-blind, placebo-controlled trial of augmentation topiramate for chronic combat-related posttraumatic stress disorder. J Clin Psychopharmacol. 2007;27(6):677-681.
11. Yeh MS, Mari JJ, Costa MC, et al. A double-blind randomized controlled trial to study the efficacy of topiramate in a civilian sample of PTSD. CNW Neurosci Ther. 2011;17(5):305-310.
12. Bajor LA, Ticlea AN, Osser DN. The Psychopharmacology Algorithm Project at the Harvard South Shore Program: an update on posttraumatic stress disorder. Harv Rev Psychiatry. 2011;19(5):240-258.
13. Topax [package insert]. Titusville NJ: Janssen Pharmaceuticals; 2009.
1. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169-184.
2. Berlin HA. Antiepileptic drugs for the treatment of post-traumatic stress disorder. Curr Psychiatry Rep. 2007;9(4):291-300.
3. Khan S, Liberzon I. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology (Berl). 2004;172(2):225-229.
4. Berlant JL. Topiramate in posttraumatic stress disorder: preliminary clinical observations. J Clin Psychiatry. 2001;62(suppl 17):60-63.
5. Tucker P, Masters B, Nawar O. Topiramate in the treatment of comorbid night eating syndrome and PTSD: a case study. Eat Disord. 2004;12(1):75-78.
6. Alderman CP, McCarthy LC, Condon JT, et al. Topiramate in combat-related posttraumatic stress disorder. Ann Pharmacother. 2009;43(4):635-641.
7. Berlant J, van Kammen DP. Open-label topiramate as primary or adjunctive therapy in chronic civilian posttraumatic stress disorder: a preliminary report. J Clin Psychiatry. 2002;63(1):15-20.
8. Berlant JL. Prospective open-label study of add-on and monotherapy topiramate in civilians with chronic nonhallucinatory posttraumatic stress disorder. BMC Psychiatry. 2004;4:24.-
9. Tucker P, Trautman RP, Wyatt DB, et al. Efficacy and safety of topiramate monotherapy in civilian posttraumatic stress disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(2):201-206.
10. Lindley SE, Carlson EB, Hill K. A randomized double-blind, placebo-controlled trial of augmentation topiramate for chronic combat-related posttraumatic stress disorder. J Clin Psychopharmacol. 2007;27(6):677-681.
11. Yeh MS, Mari JJ, Costa MC, et al. A double-blind randomized controlled trial to study the efficacy of topiramate in a civilian sample of PTSD. CNW Neurosci Ther. 2011;17(5):305-310.
12. Bajor LA, Ticlea AN, Osser DN. The Psychopharmacology Algorithm Project at the Harvard South Shore Program: an update on posttraumatic stress disorder. Harv Rev Psychiatry. 2011;19(5):240-258.
13. Topax [package insert]. Titusville NJ: Janssen Pharmaceuticals; 2009.
How to adapt cognitive-behavioral therapy for older adults
Some older patients with depression, anxiety, or insomnia may be reluctant to turn to pharmacotherapy and may prefer psychotherapeutic treatments.1 Evidence has established cognitive-behavioral therapy (CBT) as an effective intervention for several psychiatric disorders and CBT should be considered when treating geriatric patients (Table 1).2
Table 1
Indications for CBT
| Mild to moderate depression. In the case of severe depression, CBT can be combined with pharmacotherapy |
| Anxiety disorders, mixed anxiety states |
| Insomnia—both primary and comorbid with other medical and/or psychiatric conditions |
| CBT: cognitive-behavioral therapy |
Research evaluating the efficacy of CBT for depression in older adults was first published in the early 1980s. Since then, research and application of CBT with older adults has expanded to include other psychiatric disorders and researchers have suggested changes to increase the efficacy of CBT for these patients. This article provides:
- an overview of CBT’s efficacy for older adults with depression, anxiety, and insomnia
- modifications to employ when providing CBT to older patients.
The cognitive model of CBT
In the 1970s, Aaron T. Beck, MD, developed CBT while working with depressed patients. Beck’s patients reported thoughts characterized by inaccuracies and distortions in association with their depressed mood. He found these thoughts could be brought to the patient’s conscious attention and modified to improve the patient’s depression. This finding led to the development of CBT.
CBT is based on a cognitive model of the relationship among cognition, emotion, and behavior. Mood and behavior are viewed as determined by a person’s perception and interpretation of events, which manifest as a stream of automatically generated thoughts (Figure).3 These automatic thoughts have their origins in an underlying network of beliefs or schema. Patients with psychiatric disorders such as anxiety and depression typically have frequent automatic thoughts that characteristically lack validity because they arise from dysfunctional beliefs. The therapeutic process consists of helping the patient become aware of his or her internal stream of thoughts when distressed, and to identify and modify the dysfunctional thoughts. Behavioral techniques are used to bring about functional changes in behavior, regulate emotion, and help the cognitive restructuring process. Modifying the patient’s underlying dysfunctional beliefs leads to lasting improvements. In this structured therapy, the therapist and patient work collaboratively to use an approach that features reality testing and experimentation.4
Figure
The cognitive model of CBT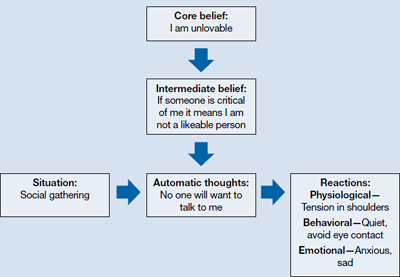
CBT: cognitive-behavioral therapy
Source: Adapted from reference 3
Indications for CBT in older adults
Depression. Among psychotherapies used in older adults, CBT has received the most research for late-life depression.5 Randomized controlled trials (RCTs) have found CBT is superior to treatment as usual in depressed adults age ≥60.6 It also has been found to be superior to wait-list control7 and talking as control.6,8 Meta-analyses have shown above-average effect sizes for CBT in treating late-life depression.9,10 A follow-up study found improvement was maintained up to 2 years after CBT, which suggests CBT’s impact is likely to be long lasting.11
Thompson et al12 compared 102 depressed patients age >60 who were treated with CBT alone, desipramine alone, or a combination of the 2. A combination of medication and CBT worked best for severely depressed patients; CBT alone or a combination of CBT and medication worked best for moderately depressed patients.
CBT is an option when treating depressed medically ill older adults. Research indicates that CBT could reduce depression in older patients with Parkinson’s disease13 and chronic obstructive pulmonary disease.14
As patients get older, cognitive impairment with comorbid depression can make treatment challenging. Limited research suggests CBT applied in a modified format that involves caregivers and uses problem solving and behavioral strategies can significantly reduce depression in patients with dementia.15
Anxiety. Researchers have examined the efficacy of variants of CBT in treating older adults with anxiety disorders—commonly, generalized anxiety disorder (GAD), panic disorder, agoraphobia, subjective anxiety, or a combination of these illnesses.16,17 Randomized trials have supported CBT’s efficacy for older patients with GAD and mixed anxiety states; gains made in CBT were maintained over a 1-year follow-up.18,19 In a meta-analysis of 15 studies using cognitive and behavioral methods of treating anxiety in older patients, Nordhus and Pallesen16 reported a significant effect size of 0.55. In a 2008 meta-analysis that included only RCTs, CBT was superior to wait-list conditions as well as active control conditions in treating anxious older patients.20
However, some research suggests that CBT for GAD may not be as effective for older adults as it is for younger adults. In a study of CBT for GAD in older adults, Stanley et al19 reported smaller effect sizes compared with CBT for younger adults. Researchers have found relatively few differences between CBT and comparison conditions—supportive psychotherapy or active control conditions—in treating GAD in older adults.21 Modified, more effective formats of CBT for GAD in older adults need to be established.22 Mohlman et al23 supplemented standard CBT for late-life GAD with memory and learning aids—weekly reading assignments, graphing exercises to chart mood ratings, reminder phone calls from therapists, and homework compliance requirement. This approach improved the response rate from 40% to 75%.23
Insomnia. Studies have found CBT to be an effective means of treating insomnia in geriatric patients. Although sleep problems occur more frequently among older patients, only 15% of chronic insomnia patients receive treatment; psychotherapy rarely is used.24 CBT for insomnia (CBT-I) should be considered for older adults because managing insomnia with medications may be problematic and these patients may prefer nonpharmacologic treatment.2 CBT-I typically incorporates cognitive strategies with established behavioral techniques, including sleep hygiene education, cognitive restructuring, relaxation training, stimulus control, and/or sleep restriction. The CBT-I multicomponent treatment package meets all criteria to be considered an evidence-based treatment for late-life insomnia.25
RCTs have reported significant improvements in late-life insomnia with CBT-I.26,27 Reviews and meta-analyses have also concluded that cognitive-behavioral treatments are effective for treating insomnia in older adults.25,28 Most insomnia cases in geriatric patients are reported to occur secondary to other medical or psychiatric conditions that are judged as causing the insomnia.25 In these cases, direct treatment of the insomnia usually is delayed or omitted.28 Studies evaluating the efficacy of CBT packages for treating insomnia occurring in conjunction with other medical or psychiatric illnesses have reported significant improvement of insomnia.28,29 Because insomnia frequently occurs in older patients with medical illnesses and psychiatric disorders, CBT-I could be beneficial for such patients.
Good candidates for CBT
Clinical experience indicates that older adults in relatively good health with no significant cognitive decline are good candidates for CBT. These patients tend to comply with their assignments, are interested in applying the learned strategies, and are motivated to read self-help books. CBT’s structured, goal-oriented approach makes it a short-term treatment, which makes it cost effective. Insomnia patients may improve after 6 to 8 CBT-I sessions and patients with anxiety or depression may need to undergo 15 to 20 CBT sessions. Patients age ≥65 have basic Medicare coverage that includes mental health care and psychotherapy.
There are no absolute contraindications for CBT, but the greater the cognitive impairment, the less the patient will benefit from CBT (Table 2). Similarly, severe depression and anxiety might make it difficult for patients to participate meaningfully, although CBT may be incorporated gradually as patients improve with medication. Severe medical illnesses and sensory losses such as visual and hearing loss would make it difficult to carry out CBT effectively.
Table 2
Contraindications for CBT
| High levels of cognitive impairment |
| Severe depression with psychotic features |
| Severe anxiety with high levels of agitation |
| Severe medical illness |
| Sensory losses |
| CBT: cognitive-behavioral therapy |
Adapting CBT for older patients
When using CBT with older patients, it is important to keep in mind characteristics that define the geriatric population. Laidlaw et al30 developed a model to help clinicians develop a more appropriate conceptualization of older patients that focuses on significant events and related cognitions associated with physical health, changes in role investments, and interactions with younger generations. It emphasizes the need to explore beliefs about aging viewed through each patient’s socio-cultural lens and examine cognitions in the context of the time period in which the individual has lived.
Losses and transitions. For many older patients, the latter years of life are characterized by losses and transitions.31 According to Thompson,31 these losses and transitions can trigger thoughts of missed opportunities or unresolved relationships and reflection on unachieved goals.31 CBT for older adults should focus on the meaning the patient gives to these losses and transitions. For example, depressed patients could view their retirement as a loss of self worth as they become less productive. CBT can help patients identify ways of thinking about the situation that will enable them to adapt to these losses and transitions.
Changes in cognition. Changes in cognitive functioning with aging are not universal and there’s considerable variability, but it’s important to make appropriate adaptations when needed. Patients may experience a decline in cognitive speed, working memory, selective attention, and fluid intelligence. This would require that information be presented slowly, with frequent repetitions and summaries. Also, it might be helpful to present information in alternate ways and to encourage patients to take notes during sessions. To accommodate for a decline in fluid intelligence, presenting new information in the context of previous experiences will help promote learning. Recordings of important information and conclusions from cognitive restructuring that patients can listen to between sessions could serve as helpful reminders that will help patients progress. Phone prompts or alarms can remind patients to carry out certain therapeutic measures, such as breathing exercises. Caretakers can attend sessions to become familiar with strategies performed during CBT and act as a co-therapist at home; however, their inclusion must be done with the consent of both parties and only if it’s viewed as necessary for the patient’s progress.
Additional strategies. For patients with substantial cognitive decline, cognitive restructuring might not be as effective as behavioral strategies—activity scheduling, graded task assignment, graded exposure, and rehearsals. Because older adults often have strengthened dysfunctional beliefs over a long time, modifying them takes longer, which is why the tapering process usually takes longer for older patients than for younger patients. The lengthier tapering ensures learning is well established and the process of modifying dysfunctional beliefs to functional beliefs continues. Collaborating with other professionals—physicians, social workers, and case managers—will help ensure a shared care process in which common goals are met.
The websites of the Academy of Cognitive Therapy, American Psychological Association, and Association for Behavioral and Cognitive Therapies can help clinicians who do not offer CBT to locate a qualified therapist for their patients (Related Resources).
- Academy of Cognitive Therapy. www.academyofct.org.
- American Psychological Association. www.apa.org.
- Association for Behavioral and Cognitive Therapies. www.abct.org.
- Laidlaw K, Thompson LW, Dick-Siskin L, et al. Cognitive behaviour therapy with older people. West Sussex, England: John Wiley & Sons, Ltd; 2003.
Drug Brand Name
- Desipramine • Norpramin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Landreville P, Landry J, Baillargeon L, et al. Older adults’ acceptance of psychological and pharmacological treatments for depression. J Gerontol B Psychol Sci Soc Sci. 2001;56(5):P285-P291.
2. Chambless DL, Ollendick TH. Empirically supported psychological interventions: controversies and evidence. Annu Rev Psychol. 2001;52:685-716.
3. Beck JS. Cognitive conceptualization. In: Cognitive therapy: basics and beyond. 2nd ed. New York NY: The Guilford Press; 2011:29–45.
4. Beck AT, Rush AJ, Shaw BF, et al. Cognitive therapy of depression. New York, NY: The Guilford Press; 1979.
5. Areán PA, Cook BL. Psychotherapy and combined psychotherapy/pharmacotherapy for late-life depression. Biol Psychiatry. 2002;52(3):293-303.
6. Laidlaw K, Davidson K, Toner H, et al. A randomised controlled trial of cognitive behaviour therapy vs treatment as usual in the treatment of mild to moderate late-life depression. Int J Geriatr Psychiatry. 2008;23(8):843-850.
7. Floyd M, Scogin F, McKendree-Smith NL, et al. Cognitive therapy for depression: a comparison of individual psychotherapy and bibliotherapy for depressed older adults. Behavior Modification. 2004;28(2):297-318.
8. Serfaty MA, Haworth D, Blanchard M, et al. Clinical effectiveness of individual cognitive behavioral therapy for depressed older people in primary care: a randomized controlled trial. Arch Gen Psychiatry. 2009;66(12):1332-1340.
9. Pinquart M, Sörensen S. How effective are psychotherapeutic and other psychosocial interventions with older adults? A meta-analysis. J Ment Health Aging. 2001;7(2):207-243.
10. Pinquart M, Duberstein PR, Lyness JM. Effects of psychotherapy and other behavioral interventions on clinically depressed older adults: a meta-analysis. Aging Ment Health. 2007;11(6):645-657.
11. Gallagher-Thompson D, Hanley-Peterson P, Thompson LW. Maintenance of gains versus relapse following brief psychotherapy for depression. J Consult Clin Psychol. 1990;58(3):371-374.
12. Thompson LW, Coon DW, Gallagher-Thompson D, et al. Comparison of desipramine and cognitive/behavioral therapy in the treatment of elderly outpatients with mild-to-moderate depression. Am J Geriatr Psychiatry. 2001;9(3):225-240.
13. Dobkin RD, Menza M, Allen LA, et al. Cognitive-behavioral therapy for depression in Parkinson’s disease: a randomized, controlled trial. Am J Psychiatry. 2011;168(10):1066-1074.
14. Kunik ME, Braun U, Stanley MA, et al. One session cognitive behavioural therapy for elderly patients with chronic obstructive pulmonary disease. Psychol Med. 2001;31(4):717-723.
15. Teri L, Logsdon RG, Uomoto J, et al. Behavioral treatment of depression in dementia patients: a controlled clinical trial. J Gerontol B Psychol Sci Soc Sci. 1997;52(4):P159-P166.
16. Nordhus IH, Pallesen S. Psychological treatment of late-life anxiety: an empirical review. J Consult Clin Psychol. 2003;71(4):643-651.
17. Gorenstein EE, Papp LA. Cognitive-behavioral therapy for anxiety in the elderly. Curr Psychiatry Rep. 2007;9(1):20-25.
18. Barrowclough C, King P, Colville J, et al. A randomized trial of the effectiveness of cognitive-behavioral therapy and supportive counseling for anxiety symptoms in older adults. J Consult Clin Psychol. 2001;69(5):756-762.
19. Stanley MA, Beck JG, Novy DM, et al. Cognitive-behavioral treatment of late-life generalized anxiety disorder. J Consult Clin Psychol. 2003;71(2):309-319.
20. Hendriks GJ, Oude Voshaar RC, Keijsers GP, et al. Cognitive-behavioural therapy for late-life anxiety disorders: a systematic review and meta-analysis. Acta Psychiatr Scand. 2008;117(6):403-411.
21. Wetherell JL, Gatz M, Craske MG. Treatment of generalized anxiety disorder in older adults. J Consult Clin Psychol. 2003;71(1):31-40.
22. Dugas MJ, Brillon P, Savard P, et al. A randomized clinical trial of cognitive-behavioral therapy and applied relaxation for adults with generalized anxiety disorder. Behav Ther. 2010;41(1):46-58.
23. Mohlman J, Gorenstein EE, Kleber M, et al. Standard and enhanced cognitive-behavior therapy for late-life generalized anxiety disorder: two pilot investigations. Am J Geriatr Psychiatry. 2003;11(1):24-32.
24. Flint AJ. Epidemiology and comorbidity of anxiety disorders in the elderly. Am J Psychiatry. 1994;151(5):640-649.
25. McCurry SM, Logsdon RG, Teri L, et al. Evidence-based psychological treatments for insomnia in older adults. Psychol Aging. 2007;22(1):18-27.
26. Sivertsen B, Omvik S, Pallesen S, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295(24):2851-2858.
27. Morgan K, Dixon S, Mathers N, et al. Psychological treatment for insomnia in the regulation of long-term hypnotic drug use. Health Technol Assess. 2004;8(8):iii iv, 1-68.
28. Nau SD, McCrae CS, Cook KG, et al. Treatment of insomnia in older adults. Clin Psychol Rev. 2005;25(5):645-672.
29. Rybarczyk B, Stepanski E, Fogg L, et al. A placebo-controlled test of cognitive-behavioral therapy for comorbid insomnia in older adults. J Consult Clin Psychol. 2005;73(6):1164-1174.
30. Laidlaw K, Thompson LW, Gallagher-Thompson D. Comprehensive conceptualization of cognitive behaviour therapy for late life depression. Behav Cogn Psychother. 2004;32(4):389-399.
31. Thompson LW. Cognitive-behavioral therapy and treatment for late-life depression. J Clin Psychiatry. 1996;57(suppl 5):29-37.
Some older patients with depression, anxiety, or insomnia may be reluctant to turn to pharmacotherapy and may prefer psychotherapeutic treatments.1 Evidence has established cognitive-behavioral therapy (CBT) as an effective intervention for several psychiatric disorders and CBT should be considered when treating geriatric patients (Table 1).2
Table 1
Indications for CBT
| Mild to moderate depression. In the case of severe depression, CBT can be combined with pharmacotherapy |
| Anxiety disorders, mixed anxiety states |
| Insomnia—both primary and comorbid with other medical and/or psychiatric conditions |
| CBT: cognitive-behavioral therapy |
Research evaluating the efficacy of CBT for depression in older adults was first published in the early 1980s. Since then, research and application of CBT with older adults has expanded to include other psychiatric disorders and researchers have suggested changes to increase the efficacy of CBT for these patients. This article provides:
- an overview of CBT’s efficacy for older adults with depression, anxiety, and insomnia
- modifications to employ when providing CBT to older patients.
The cognitive model of CBT
In the 1970s, Aaron T. Beck, MD, developed CBT while working with depressed patients. Beck’s patients reported thoughts characterized by inaccuracies and distortions in association with their depressed mood. He found these thoughts could be brought to the patient’s conscious attention and modified to improve the patient’s depression. This finding led to the development of CBT.
CBT is based on a cognitive model of the relationship among cognition, emotion, and behavior. Mood and behavior are viewed as determined by a person’s perception and interpretation of events, which manifest as a stream of automatically generated thoughts (Figure).3 These automatic thoughts have their origins in an underlying network of beliefs or schema. Patients with psychiatric disorders such as anxiety and depression typically have frequent automatic thoughts that characteristically lack validity because they arise from dysfunctional beliefs. The therapeutic process consists of helping the patient become aware of his or her internal stream of thoughts when distressed, and to identify and modify the dysfunctional thoughts. Behavioral techniques are used to bring about functional changes in behavior, regulate emotion, and help the cognitive restructuring process. Modifying the patient’s underlying dysfunctional beliefs leads to lasting improvements. In this structured therapy, the therapist and patient work collaboratively to use an approach that features reality testing and experimentation.4
Figure
The cognitive model of CBT
CBT: cognitive-behavioral therapy
Source: Adapted from reference 3
Indications for CBT in older adults
Depression. Among psychotherapies used in older adults, CBT has received the most research for late-life depression.5 Randomized controlled trials (RCTs) have found CBT is superior to treatment as usual in depressed adults age ≥60.6 It also has been found to be superior to wait-list control7 and talking as control.6,8 Meta-analyses have shown above-average effect sizes for CBT in treating late-life depression.9,10 A follow-up study found improvement was maintained up to 2 years after CBT, which suggests CBT’s impact is likely to be long lasting.11
Thompson et al12 compared 102 depressed patients age >60 who were treated with CBT alone, desipramine alone, or a combination of the 2. A combination of medication and CBT worked best for severely depressed patients; CBT alone or a combination of CBT and medication worked best for moderately depressed patients.
CBT is an option when treating depressed medically ill older adults. Research indicates that CBT could reduce depression in older patients with Parkinson’s disease13 and chronic obstructive pulmonary disease.14
As patients get older, cognitive impairment with comorbid depression can make treatment challenging. Limited research suggests CBT applied in a modified format that involves caregivers and uses problem solving and behavioral strategies can significantly reduce depression in patients with dementia.15
Anxiety. Researchers have examined the efficacy of variants of CBT in treating older adults with anxiety disorders—commonly, generalized anxiety disorder (GAD), panic disorder, agoraphobia, subjective anxiety, or a combination of these illnesses.16,17 Randomized trials have supported CBT’s efficacy for older patients with GAD and mixed anxiety states; gains made in CBT were maintained over a 1-year follow-up.18,19 In a meta-analysis of 15 studies using cognitive and behavioral methods of treating anxiety in older patients, Nordhus and Pallesen16 reported a significant effect size of 0.55. In a 2008 meta-analysis that included only RCTs, CBT was superior to wait-list conditions as well as active control conditions in treating anxious older patients.20
However, some research suggests that CBT for GAD may not be as effective for older adults as it is for younger adults. In a study of CBT for GAD in older adults, Stanley et al19 reported smaller effect sizes compared with CBT for younger adults. Researchers have found relatively few differences between CBT and comparison conditions—supportive psychotherapy or active control conditions—in treating GAD in older adults.21 Modified, more effective formats of CBT for GAD in older adults need to be established.22 Mohlman et al23 supplemented standard CBT for late-life GAD with memory and learning aids—weekly reading assignments, graphing exercises to chart mood ratings, reminder phone calls from therapists, and homework compliance requirement. This approach improved the response rate from 40% to 75%.23
Insomnia. Studies have found CBT to be an effective means of treating insomnia in geriatric patients. Although sleep problems occur more frequently among older patients, only 15% of chronic insomnia patients receive treatment; psychotherapy rarely is used.24 CBT for insomnia (CBT-I) should be considered for older adults because managing insomnia with medications may be problematic and these patients may prefer nonpharmacologic treatment.2 CBT-I typically incorporates cognitive strategies with established behavioral techniques, including sleep hygiene education, cognitive restructuring, relaxation training, stimulus control, and/or sleep restriction. The CBT-I multicomponent treatment package meets all criteria to be considered an evidence-based treatment for late-life insomnia.25
RCTs have reported significant improvements in late-life insomnia with CBT-I.26,27 Reviews and meta-analyses have also concluded that cognitive-behavioral treatments are effective for treating insomnia in older adults.25,28 Most insomnia cases in geriatric patients are reported to occur secondary to other medical or psychiatric conditions that are judged as causing the insomnia.25 In these cases, direct treatment of the insomnia usually is delayed or omitted.28 Studies evaluating the efficacy of CBT packages for treating insomnia occurring in conjunction with other medical or psychiatric illnesses have reported significant improvement of insomnia.28,29 Because insomnia frequently occurs in older patients with medical illnesses and psychiatric disorders, CBT-I could be beneficial for such patients.
Good candidates for CBT
Clinical experience indicates that older adults in relatively good health with no significant cognitive decline are good candidates for CBT. These patients tend to comply with their assignments, are interested in applying the learned strategies, and are motivated to read self-help books. CBT’s structured, goal-oriented approach makes it a short-term treatment, which makes it cost effective. Insomnia patients may improve after 6 to 8 CBT-I sessions and patients with anxiety or depression may need to undergo 15 to 20 CBT sessions. Patients age ≥65 have basic Medicare coverage that includes mental health care and psychotherapy.
There are no absolute contraindications for CBT, but the greater the cognitive impairment, the less the patient will benefit from CBT (Table 2). Similarly, severe depression and anxiety might make it difficult for patients to participate meaningfully, although CBT may be incorporated gradually as patients improve with medication. Severe medical illnesses and sensory losses such as visual and hearing loss would make it difficult to carry out CBT effectively.
Table 2
Contraindications for CBT
| High levels of cognitive impairment |
| Severe depression with psychotic features |
| Severe anxiety with high levels of agitation |
| Severe medical illness |
| Sensory losses |
| CBT: cognitive-behavioral therapy |
Adapting CBT for older patients
When using CBT with older patients, it is important to keep in mind characteristics that define the geriatric population. Laidlaw et al30 developed a model to help clinicians develop a more appropriate conceptualization of older patients that focuses on significant events and related cognitions associated with physical health, changes in role investments, and interactions with younger generations. It emphasizes the need to explore beliefs about aging viewed through each patient’s socio-cultural lens and examine cognitions in the context of the time period in which the individual has lived.
Losses and transitions. For many older patients, the latter years of life are characterized by losses and transitions.31 According to Thompson,31 these losses and transitions can trigger thoughts of missed opportunities or unresolved relationships and reflection on unachieved goals.31 CBT for older adults should focus on the meaning the patient gives to these losses and transitions. For example, depressed patients could view their retirement as a loss of self worth as they become less productive. CBT can help patients identify ways of thinking about the situation that will enable them to adapt to these losses and transitions.
Changes in cognition. Changes in cognitive functioning with aging are not universal and there’s considerable variability, but it’s important to make appropriate adaptations when needed. Patients may experience a decline in cognitive speed, working memory, selective attention, and fluid intelligence. This would require that information be presented slowly, with frequent repetitions and summaries. Also, it might be helpful to present information in alternate ways and to encourage patients to take notes during sessions. To accommodate for a decline in fluid intelligence, presenting new information in the context of previous experiences will help promote learning. Recordings of important information and conclusions from cognitive restructuring that patients can listen to between sessions could serve as helpful reminders that will help patients progress. Phone prompts or alarms can remind patients to carry out certain therapeutic measures, such as breathing exercises. Caretakers can attend sessions to become familiar with strategies performed during CBT and act as a co-therapist at home; however, their inclusion must be done with the consent of both parties and only if it’s viewed as necessary for the patient’s progress.
Additional strategies. For patients with substantial cognitive decline, cognitive restructuring might not be as effective as behavioral strategies—activity scheduling, graded task assignment, graded exposure, and rehearsals. Because older adults often have strengthened dysfunctional beliefs over a long time, modifying them takes longer, which is why the tapering process usually takes longer for older patients than for younger patients. The lengthier tapering ensures learning is well established and the process of modifying dysfunctional beliefs to functional beliefs continues. Collaborating with other professionals—physicians, social workers, and case managers—will help ensure a shared care process in which common goals are met.
The websites of the Academy of Cognitive Therapy, American Psychological Association, and Association for Behavioral and Cognitive Therapies can help clinicians who do not offer CBT to locate a qualified therapist for their patients (Related Resources).
- Academy of Cognitive Therapy. www.academyofct.org.
- American Psychological Association. www.apa.org.
- Association for Behavioral and Cognitive Therapies. www.abct.org.
- Laidlaw K, Thompson LW, Dick-Siskin L, et al. Cognitive behaviour therapy with older people. West Sussex, England: John Wiley & Sons, Ltd; 2003.
Drug Brand Name
- Desipramine • Norpramin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Some older patients with depression, anxiety, or insomnia may be reluctant to turn to pharmacotherapy and may prefer psychotherapeutic treatments.1 Evidence has established cognitive-behavioral therapy (CBT) as an effective intervention for several psychiatric disorders and CBT should be considered when treating geriatric patients (Table 1).2
Table 1
Indications for CBT
| Mild to moderate depression. In the case of severe depression, CBT can be combined with pharmacotherapy |
| Anxiety disorders, mixed anxiety states |
| Insomnia—both primary and comorbid with other medical and/or psychiatric conditions |
| CBT: cognitive-behavioral therapy |
Research evaluating the efficacy of CBT for depression in older adults was first published in the early 1980s. Since then, research and application of CBT with older adults has expanded to include other psychiatric disorders and researchers have suggested changes to increase the efficacy of CBT for these patients. This article provides:
- an overview of CBT’s efficacy for older adults with depression, anxiety, and insomnia
- modifications to employ when providing CBT to older patients.
The cognitive model of CBT
In the 1970s, Aaron T. Beck, MD, developed CBT while working with depressed patients. Beck’s patients reported thoughts characterized by inaccuracies and distortions in association with their depressed mood. He found these thoughts could be brought to the patient’s conscious attention and modified to improve the patient’s depression. This finding led to the development of CBT.
CBT is based on a cognitive model of the relationship among cognition, emotion, and behavior. Mood and behavior are viewed as determined by a person’s perception and interpretation of events, which manifest as a stream of automatically generated thoughts (Figure).3 These automatic thoughts have their origins in an underlying network of beliefs or schema. Patients with psychiatric disorders such as anxiety and depression typically have frequent automatic thoughts that characteristically lack validity because they arise from dysfunctional beliefs. The therapeutic process consists of helping the patient become aware of his or her internal stream of thoughts when distressed, and to identify and modify the dysfunctional thoughts. Behavioral techniques are used to bring about functional changes in behavior, regulate emotion, and help the cognitive restructuring process. Modifying the patient’s underlying dysfunctional beliefs leads to lasting improvements. In this structured therapy, the therapist and patient work collaboratively to use an approach that features reality testing and experimentation.4
Figure
The cognitive model of CBT
CBT: cognitive-behavioral therapy
Source: Adapted from reference 3
Indications for CBT in older adults
Depression. Among psychotherapies used in older adults, CBT has received the most research for late-life depression.5 Randomized controlled trials (RCTs) have found CBT is superior to treatment as usual in depressed adults age ≥60.6 It also has been found to be superior to wait-list control7 and talking as control.6,8 Meta-analyses have shown above-average effect sizes for CBT in treating late-life depression.9,10 A follow-up study found improvement was maintained up to 2 years after CBT, which suggests CBT’s impact is likely to be long lasting.11
Thompson et al12 compared 102 depressed patients age >60 who were treated with CBT alone, desipramine alone, or a combination of the 2. A combination of medication and CBT worked best for severely depressed patients; CBT alone or a combination of CBT and medication worked best for moderately depressed patients.
CBT is an option when treating depressed medically ill older adults. Research indicates that CBT could reduce depression in older patients with Parkinson’s disease13 and chronic obstructive pulmonary disease.14
As patients get older, cognitive impairment with comorbid depression can make treatment challenging. Limited research suggests CBT applied in a modified format that involves caregivers and uses problem solving and behavioral strategies can significantly reduce depression in patients with dementia.15
Anxiety. Researchers have examined the efficacy of variants of CBT in treating older adults with anxiety disorders—commonly, generalized anxiety disorder (GAD), panic disorder, agoraphobia, subjective anxiety, or a combination of these illnesses.16,17 Randomized trials have supported CBT’s efficacy for older patients with GAD and mixed anxiety states; gains made in CBT were maintained over a 1-year follow-up.18,19 In a meta-analysis of 15 studies using cognitive and behavioral methods of treating anxiety in older patients, Nordhus and Pallesen16 reported a significant effect size of 0.55. In a 2008 meta-analysis that included only RCTs, CBT was superior to wait-list conditions as well as active control conditions in treating anxious older patients.20
However, some research suggests that CBT for GAD may not be as effective for older adults as it is for younger adults. In a study of CBT for GAD in older adults, Stanley et al19 reported smaller effect sizes compared with CBT for younger adults. Researchers have found relatively few differences between CBT and comparison conditions—supportive psychotherapy or active control conditions—in treating GAD in older adults.21 Modified, more effective formats of CBT for GAD in older adults need to be established.22 Mohlman et al23 supplemented standard CBT for late-life GAD with memory and learning aids—weekly reading assignments, graphing exercises to chart mood ratings, reminder phone calls from therapists, and homework compliance requirement. This approach improved the response rate from 40% to 75%.23
Insomnia. Studies have found CBT to be an effective means of treating insomnia in geriatric patients. Although sleep problems occur more frequently among older patients, only 15% of chronic insomnia patients receive treatment; psychotherapy rarely is used.24 CBT for insomnia (CBT-I) should be considered for older adults because managing insomnia with medications may be problematic and these patients may prefer nonpharmacologic treatment.2 CBT-I typically incorporates cognitive strategies with established behavioral techniques, including sleep hygiene education, cognitive restructuring, relaxation training, stimulus control, and/or sleep restriction. The CBT-I multicomponent treatment package meets all criteria to be considered an evidence-based treatment for late-life insomnia.25
RCTs have reported significant improvements in late-life insomnia with CBT-I.26,27 Reviews and meta-analyses have also concluded that cognitive-behavioral treatments are effective for treating insomnia in older adults.25,28 Most insomnia cases in geriatric patients are reported to occur secondary to other medical or psychiatric conditions that are judged as causing the insomnia.25 In these cases, direct treatment of the insomnia usually is delayed or omitted.28 Studies evaluating the efficacy of CBT packages for treating insomnia occurring in conjunction with other medical or psychiatric illnesses have reported significant improvement of insomnia.28,29 Because insomnia frequently occurs in older patients with medical illnesses and psychiatric disorders, CBT-I could be beneficial for such patients.
Good candidates for CBT
Clinical experience indicates that older adults in relatively good health with no significant cognitive decline are good candidates for CBT. These patients tend to comply with their assignments, are interested in applying the learned strategies, and are motivated to read self-help books. CBT’s structured, goal-oriented approach makes it a short-term treatment, which makes it cost effective. Insomnia patients may improve after 6 to 8 CBT-I sessions and patients with anxiety or depression may need to undergo 15 to 20 CBT sessions. Patients age ≥65 have basic Medicare coverage that includes mental health care and psychotherapy.
There are no absolute contraindications for CBT, but the greater the cognitive impairment, the less the patient will benefit from CBT (Table 2). Similarly, severe depression and anxiety might make it difficult for patients to participate meaningfully, although CBT may be incorporated gradually as patients improve with medication. Severe medical illnesses and sensory losses such as visual and hearing loss would make it difficult to carry out CBT effectively.
Table 2
Contraindications for CBT
| High levels of cognitive impairment |
| Severe depression with psychotic features |
| Severe anxiety with high levels of agitation |
| Severe medical illness |
| Sensory losses |
| CBT: cognitive-behavioral therapy |
Adapting CBT for older patients
When using CBT with older patients, it is important to keep in mind characteristics that define the geriatric population. Laidlaw et al30 developed a model to help clinicians develop a more appropriate conceptualization of older patients that focuses on significant events and related cognitions associated with physical health, changes in role investments, and interactions with younger generations. It emphasizes the need to explore beliefs about aging viewed through each patient’s socio-cultural lens and examine cognitions in the context of the time period in which the individual has lived.
Losses and transitions. For many older patients, the latter years of life are characterized by losses and transitions.31 According to Thompson,31 these losses and transitions can trigger thoughts of missed opportunities or unresolved relationships and reflection on unachieved goals.31 CBT for older adults should focus on the meaning the patient gives to these losses and transitions. For example, depressed patients could view their retirement as a loss of self worth as they become less productive. CBT can help patients identify ways of thinking about the situation that will enable them to adapt to these losses and transitions.
Changes in cognition. Changes in cognitive functioning with aging are not universal and there’s considerable variability, but it’s important to make appropriate adaptations when needed. Patients may experience a decline in cognitive speed, working memory, selective attention, and fluid intelligence. This would require that information be presented slowly, with frequent repetitions and summaries. Also, it might be helpful to present information in alternate ways and to encourage patients to take notes during sessions. To accommodate for a decline in fluid intelligence, presenting new information in the context of previous experiences will help promote learning. Recordings of important information and conclusions from cognitive restructuring that patients can listen to between sessions could serve as helpful reminders that will help patients progress. Phone prompts or alarms can remind patients to carry out certain therapeutic measures, such as breathing exercises. Caretakers can attend sessions to become familiar with strategies performed during CBT and act as a co-therapist at home; however, their inclusion must be done with the consent of both parties and only if it’s viewed as necessary for the patient’s progress.
Additional strategies. For patients with substantial cognitive decline, cognitive restructuring might not be as effective as behavioral strategies—activity scheduling, graded task assignment, graded exposure, and rehearsals. Because older adults often have strengthened dysfunctional beliefs over a long time, modifying them takes longer, which is why the tapering process usually takes longer for older patients than for younger patients. The lengthier tapering ensures learning is well established and the process of modifying dysfunctional beliefs to functional beliefs continues. Collaborating with other professionals—physicians, social workers, and case managers—will help ensure a shared care process in which common goals are met.
The websites of the Academy of Cognitive Therapy, American Psychological Association, and Association for Behavioral and Cognitive Therapies can help clinicians who do not offer CBT to locate a qualified therapist for their patients (Related Resources).
- Academy of Cognitive Therapy. www.academyofct.org.
- American Psychological Association. www.apa.org.
- Association for Behavioral and Cognitive Therapies. www.abct.org.
- Laidlaw K, Thompson LW, Dick-Siskin L, et al. Cognitive behaviour therapy with older people. West Sussex, England: John Wiley & Sons, Ltd; 2003.
Drug Brand Name
- Desipramine • Norpramin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Landreville P, Landry J, Baillargeon L, et al. Older adults’ acceptance of psychological and pharmacological treatments for depression. J Gerontol B Psychol Sci Soc Sci. 2001;56(5):P285-P291.
2. Chambless DL, Ollendick TH. Empirically supported psychological interventions: controversies and evidence. Annu Rev Psychol. 2001;52:685-716.
3. Beck JS. Cognitive conceptualization. In: Cognitive therapy: basics and beyond. 2nd ed. New York NY: The Guilford Press; 2011:29–45.
4. Beck AT, Rush AJ, Shaw BF, et al. Cognitive therapy of depression. New York, NY: The Guilford Press; 1979.
5. Areán PA, Cook BL. Psychotherapy and combined psychotherapy/pharmacotherapy for late-life depression. Biol Psychiatry. 2002;52(3):293-303.
6. Laidlaw K, Davidson K, Toner H, et al. A randomised controlled trial of cognitive behaviour therapy vs treatment as usual in the treatment of mild to moderate late-life depression. Int J Geriatr Psychiatry. 2008;23(8):843-850.
7. Floyd M, Scogin F, McKendree-Smith NL, et al. Cognitive therapy for depression: a comparison of individual psychotherapy and bibliotherapy for depressed older adults. Behavior Modification. 2004;28(2):297-318.
8. Serfaty MA, Haworth D, Blanchard M, et al. Clinical effectiveness of individual cognitive behavioral therapy for depressed older people in primary care: a randomized controlled trial. Arch Gen Psychiatry. 2009;66(12):1332-1340.
9. Pinquart M, Sörensen S. How effective are psychotherapeutic and other psychosocial interventions with older adults? A meta-analysis. J Ment Health Aging. 2001;7(2):207-243.
10. Pinquart M, Duberstein PR, Lyness JM. Effects of psychotherapy and other behavioral interventions on clinically depressed older adults: a meta-analysis. Aging Ment Health. 2007;11(6):645-657.
11. Gallagher-Thompson D, Hanley-Peterson P, Thompson LW. Maintenance of gains versus relapse following brief psychotherapy for depression. J Consult Clin Psychol. 1990;58(3):371-374.
12. Thompson LW, Coon DW, Gallagher-Thompson D, et al. Comparison of desipramine and cognitive/behavioral therapy in the treatment of elderly outpatients with mild-to-moderate depression. Am J Geriatr Psychiatry. 2001;9(3):225-240.
13. Dobkin RD, Menza M, Allen LA, et al. Cognitive-behavioral therapy for depression in Parkinson’s disease: a randomized, controlled trial. Am J Psychiatry. 2011;168(10):1066-1074.
14. Kunik ME, Braun U, Stanley MA, et al. One session cognitive behavioural therapy for elderly patients with chronic obstructive pulmonary disease. Psychol Med. 2001;31(4):717-723.
15. Teri L, Logsdon RG, Uomoto J, et al. Behavioral treatment of depression in dementia patients: a controlled clinical trial. J Gerontol B Psychol Sci Soc Sci. 1997;52(4):P159-P166.
16. Nordhus IH, Pallesen S. Psychological treatment of late-life anxiety: an empirical review. J Consult Clin Psychol. 2003;71(4):643-651.
17. Gorenstein EE, Papp LA. Cognitive-behavioral therapy for anxiety in the elderly. Curr Psychiatry Rep. 2007;9(1):20-25.
18. Barrowclough C, King P, Colville J, et al. A randomized trial of the effectiveness of cognitive-behavioral therapy and supportive counseling for anxiety symptoms in older adults. J Consult Clin Psychol. 2001;69(5):756-762.
19. Stanley MA, Beck JG, Novy DM, et al. Cognitive-behavioral treatment of late-life generalized anxiety disorder. J Consult Clin Psychol. 2003;71(2):309-319.
20. Hendriks GJ, Oude Voshaar RC, Keijsers GP, et al. Cognitive-behavioural therapy for late-life anxiety disorders: a systematic review and meta-analysis. Acta Psychiatr Scand. 2008;117(6):403-411.
21. Wetherell JL, Gatz M, Craske MG. Treatment of generalized anxiety disorder in older adults. J Consult Clin Psychol. 2003;71(1):31-40.
22. Dugas MJ, Brillon P, Savard P, et al. A randomized clinical trial of cognitive-behavioral therapy and applied relaxation for adults with generalized anxiety disorder. Behav Ther. 2010;41(1):46-58.
23. Mohlman J, Gorenstein EE, Kleber M, et al. Standard and enhanced cognitive-behavior therapy for late-life generalized anxiety disorder: two pilot investigations. Am J Geriatr Psychiatry. 2003;11(1):24-32.
24. Flint AJ. Epidemiology and comorbidity of anxiety disorders in the elderly. Am J Psychiatry. 1994;151(5):640-649.
25. McCurry SM, Logsdon RG, Teri L, et al. Evidence-based psychological treatments for insomnia in older adults. Psychol Aging. 2007;22(1):18-27.
26. Sivertsen B, Omvik S, Pallesen S, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295(24):2851-2858.
27. Morgan K, Dixon S, Mathers N, et al. Psychological treatment for insomnia in the regulation of long-term hypnotic drug use. Health Technol Assess. 2004;8(8):iii iv, 1-68.
28. Nau SD, McCrae CS, Cook KG, et al. Treatment of insomnia in older adults. Clin Psychol Rev. 2005;25(5):645-672.
29. Rybarczyk B, Stepanski E, Fogg L, et al. A placebo-controlled test of cognitive-behavioral therapy for comorbid insomnia in older adults. J Consult Clin Psychol. 2005;73(6):1164-1174.
30. Laidlaw K, Thompson LW, Gallagher-Thompson D. Comprehensive conceptualization of cognitive behaviour therapy for late life depression. Behav Cogn Psychother. 2004;32(4):389-399.
31. Thompson LW. Cognitive-behavioral therapy and treatment for late-life depression. J Clin Psychiatry. 1996;57(suppl 5):29-37.
1. Landreville P, Landry J, Baillargeon L, et al. Older adults’ acceptance of psychological and pharmacological treatments for depression. J Gerontol B Psychol Sci Soc Sci. 2001;56(5):P285-P291.
2. Chambless DL, Ollendick TH. Empirically supported psychological interventions: controversies and evidence. Annu Rev Psychol. 2001;52:685-716.
3. Beck JS. Cognitive conceptualization. In: Cognitive therapy: basics and beyond. 2nd ed. New York NY: The Guilford Press; 2011:29–45.
4. Beck AT, Rush AJ, Shaw BF, et al. Cognitive therapy of depression. New York, NY: The Guilford Press; 1979.
5. Areán PA, Cook BL. Psychotherapy and combined psychotherapy/pharmacotherapy for late-life depression. Biol Psychiatry. 2002;52(3):293-303.
6. Laidlaw K, Davidson K, Toner H, et al. A randomised controlled trial of cognitive behaviour therapy vs treatment as usual in the treatment of mild to moderate late-life depression. Int J Geriatr Psychiatry. 2008;23(8):843-850.
7. Floyd M, Scogin F, McKendree-Smith NL, et al. Cognitive therapy for depression: a comparison of individual psychotherapy and bibliotherapy for depressed older adults. Behavior Modification. 2004;28(2):297-318.
8. Serfaty MA, Haworth D, Blanchard M, et al. Clinical effectiveness of individual cognitive behavioral therapy for depressed older people in primary care: a randomized controlled trial. Arch Gen Psychiatry. 2009;66(12):1332-1340.
9. Pinquart M, Sörensen S. How effective are psychotherapeutic and other psychosocial interventions with older adults? A meta-analysis. J Ment Health Aging. 2001;7(2):207-243.
10. Pinquart M, Duberstein PR, Lyness JM. Effects of psychotherapy and other behavioral interventions on clinically depressed older adults: a meta-analysis. Aging Ment Health. 2007;11(6):645-657.
11. Gallagher-Thompson D, Hanley-Peterson P, Thompson LW. Maintenance of gains versus relapse following brief psychotherapy for depression. J Consult Clin Psychol. 1990;58(3):371-374.
12. Thompson LW, Coon DW, Gallagher-Thompson D, et al. Comparison of desipramine and cognitive/behavioral therapy in the treatment of elderly outpatients with mild-to-moderate depression. Am J Geriatr Psychiatry. 2001;9(3):225-240.
13. Dobkin RD, Menza M, Allen LA, et al. Cognitive-behavioral therapy for depression in Parkinson’s disease: a randomized, controlled trial. Am J Psychiatry. 2011;168(10):1066-1074.
14. Kunik ME, Braun U, Stanley MA, et al. One session cognitive behavioural therapy for elderly patients with chronic obstructive pulmonary disease. Psychol Med. 2001;31(4):717-723.
15. Teri L, Logsdon RG, Uomoto J, et al. Behavioral treatment of depression in dementia patients: a controlled clinical trial. J Gerontol B Psychol Sci Soc Sci. 1997;52(4):P159-P166.
16. Nordhus IH, Pallesen S. Psychological treatment of late-life anxiety: an empirical review. J Consult Clin Psychol. 2003;71(4):643-651.
17. Gorenstein EE, Papp LA. Cognitive-behavioral therapy for anxiety in the elderly. Curr Psychiatry Rep. 2007;9(1):20-25.
18. Barrowclough C, King P, Colville J, et al. A randomized trial of the effectiveness of cognitive-behavioral therapy and supportive counseling for anxiety symptoms in older adults. J Consult Clin Psychol. 2001;69(5):756-762.
19. Stanley MA, Beck JG, Novy DM, et al. Cognitive-behavioral treatment of late-life generalized anxiety disorder. J Consult Clin Psychol. 2003;71(2):309-319.
20. Hendriks GJ, Oude Voshaar RC, Keijsers GP, et al. Cognitive-behavioural therapy for late-life anxiety disorders: a systematic review and meta-analysis. Acta Psychiatr Scand. 2008;117(6):403-411.
21. Wetherell JL, Gatz M, Craske MG. Treatment of generalized anxiety disorder in older adults. J Consult Clin Psychol. 2003;71(1):31-40.
22. Dugas MJ, Brillon P, Savard P, et al. A randomized clinical trial of cognitive-behavioral therapy and applied relaxation for adults with generalized anxiety disorder. Behav Ther. 2010;41(1):46-58.
23. Mohlman J, Gorenstein EE, Kleber M, et al. Standard and enhanced cognitive-behavior therapy for late-life generalized anxiety disorder: two pilot investigations. Am J Geriatr Psychiatry. 2003;11(1):24-32.
24. Flint AJ. Epidemiology and comorbidity of anxiety disorders in the elderly. Am J Psychiatry. 1994;151(5):640-649.
25. McCurry SM, Logsdon RG, Teri L, et al. Evidence-based psychological treatments for insomnia in older adults. Psychol Aging. 2007;22(1):18-27.
26. Sivertsen B, Omvik S, Pallesen S, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295(24):2851-2858.
27. Morgan K, Dixon S, Mathers N, et al. Psychological treatment for insomnia in the regulation of long-term hypnotic drug use. Health Technol Assess. 2004;8(8):iii iv, 1-68.
28. Nau SD, McCrae CS, Cook KG, et al. Treatment of insomnia in older adults. Clin Psychol Rev. 2005;25(5):645-672.
29. Rybarczyk B, Stepanski E, Fogg L, et al. A placebo-controlled test of cognitive-behavioral therapy for comorbid insomnia in older adults. J Consult Clin Psychol. 2005;73(6):1164-1174.
30. Laidlaw K, Thompson LW, Gallagher-Thompson D. Comprehensive conceptualization of cognitive behaviour therapy for late life depression. Behav Cogn Psychother. 2004;32(4):389-399.
31. Thompson LW. Cognitive-behavioral therapy and treatment for late-life depression. J Clin Psychiatry. 1996;57(suppl 5):29-37.
A taste for the unusual
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Nauseous and full
Ms. O, age 48, presents to the emergency department reporting a 3-day history of vomiting approximately 5 minutes after consuming solids or liquids. She’s had 10 vomiting episodes, which were associated with “fullness” and an “aching” sensation she rates as 6 on a 10-point scale pain scale that is diffuse over the upper epigastric area, with no palliative factors. Ms. O has not had a bowel movement for 3 days and her last menstrual period was 8 days ago. She is taking lorazepam, 1 mg/d. Her medical and psychiatric history includes anxiety, depression, personality disorder symptoms of affective dysregulation, obesity (270 lbs; medium height), and pica. She was 352 lbs when she underwent a Roux-en-Y gastric bypass 2 years ago. One year earlier, she had a laparoscopic gastric bezoar removal and an incisional hernia repair. Ms. O had no pica-related surgeries before undergoing gastric bypass surgery.
Ms. O denies shortness of breath, chest pain, allergies, smoking, or alcohol abuse, but reports uncontrollable cravings for paper products, specifically cardboard, which she describes as “just so delicious.” This craving led her to consume large amounts of cardboard and newspaper in the days before she began vomiting.
What may be causing Ms. O’s pica symptoms?
- iron deficiency anemia
- complications from gastric bypass surgery
- personality disorder
- generalized anxiety disorder (GAD)
The authors’ observations
DSM-IV-TR diagnostic criteria for pica include the persistent eating of non-nutritive substances for ≥1 month that is inappropriate for the level of a person’s development and not an acceptable part of one’s culture.1 If pica occurs with other mental disorders, it must be severe enough to indicate further clinical assessment to receive a separate diagnosis. Often associated with pregnancy, iron deficiency anemia, early development, and mental retardation, pica has been observed in post-gastric bypass surgery patients, all of whom presented with pagophagia (compulsive ice eating), and in one case was associated with a bezoar causing obstruction of the GI tract.1,2 With the dramatic increase in gastric bypass surgery and the required presurgical mental health evaluation, the consequences of failing to screen patients for pica behaviors can be devastating.
EVALUATION: Low iron
Ms. O’s vital signs on admission are stable, and physical exam is notable for mild abdominal distention with no guarding, tenderness, rigidity, or masses. No rebound tenderness is elicited. CT scan shows evidence of post-surgical changes involving the small bowel consistent with gastric bypass surgery and a hiatal hernia, but no obstruction, focal inflammation, free fluids, or gas. Lab values for amylase, lipase, urinalysis, coagulation studies, cardiac enzymes, and complete metabolic profile are within normal limits. Although not anemic, Ms. O is iron deficient, with ferritin, 10 ng/mL (normal 10 to 120 ng/mL); B12, 299 pg/mL (normal 100 to 700 pg/mL); and iron, 25 μg/dL (normal 50 to 170 μg/dL).
A foreign body is removed endoscopically and the specimen is sent to pathology. It is determined to be a gastric bezoar, yellowish-green in color, measuring 2.5 cm × 1 cm × 0.8 cm. After bezoar removal, Ms. O tolerates food and is discharged home on vitamin B12, 1,000 mcg/d for 2 weeks; folate, 1 mg/d for 1 month; calcium with vitamin D, 1 g/d; and esomeprazole, 40 mg/d for frequent heartburn. She is referred to psychiatry for behavioral modification therapy and medication management.
How would you treat Ms. O?
- start a selective serotonin reuptake inhibitor (SSRI)
- prescribe an atypical antipsychotic
- continue lorazepam
- begin behavioral therapy
HISTORY: Pica during pregnancy
During psychiatric workup, Ms. O admits to having pica urges most of her life, but experienced an uncontrollable exacerbation after gastric bypass surgery. This led to intense, chaotic periods of pica, resulting in a previous bezoar removal. She is particularly attracted to cardboard and newspaper cartoons, but notes she also has felt the urge to eat charcoal, moist soil, clay, chalk, pencils, and new shoes, which she chews on. In the past, her extreme anxiety and preoccupation with these urges had lead to diagnoses of personality disorder not otherwise specified, GAD, and obsessive-compulsive disorder.
Her first experience with pica was during her first pregnancy at age 15, when she had an impulse to eat soil. The urges briefly stopped until she became pregnant again. During each of her 5 pregnancies her pica symptoms returned. At one point during her last pregnancy she reports having felt out of control, eating 2 to 3 pencils with the eraser per day, after which she would feel intense relaxation. Her mother also exhibited symptoms of pica toward charcoal and soil. Ms. O had been taking unknown dosages of lorazepam for anxiety and fluoxetine for depression, both of which she stopped because she feared side effects during her last pregnancy. However, she never experienced any side effects.
The authors’ observations
Although pica is most commonly observed in young children, it sometimes is seen in pregnant women.1 Pica frequently is associated with other mental disorders, such as pervasive developmental disorder and mental retardation,1 and can be associated with premorbid psychosis and anxiety disorders. Occasional vitamin and mineral deficiencies, such as iron or zinc, have been reported, but usually patients’ lab values are normal. Treatment usually is initiated in the context of medical complications, such as iron deficiency anemia. In Ms. O’s case, the precipitating event was mechanical bowel obstruction due to a bezoar.
Several theories about the origins of pica have been proposed, but none truly are explanatory or satisfactory. The nutritional theory—that patients eat non-nutritive substances to compensate for mineral deficiencies—is popular because of pica’s frequent association with mineral deficiencies, but it is unknown whether pica is the cause or the result of the deficiency. An example of this is anemia due to eating clay instead of foods that contain iron. Another theory is that because pica is normal in early childhood development, it may be a manifestation of delayed development or mental retardation. The cultural theory is attractive because pregnant women in several cultures eat starch or clay as a part of their native rituals, and the incidence of pica is relatively high among pregnant African American women who live in rural areas.3 In the Roux-en-Y procedure, bypass of the duodenum and proximal jejunum can significantly decrease a patient’s iron uptake, leading to iron deficiency anemia, and could trigger pica in a susceptible patient.4
Exacerbation after gastric bypass
Kushner et al4 describes re-emergent pica after bariatric surgery in 2 patients with pagophagia associated with concomitant iron deficiency anemia. A 41-year-old white woman presented with pagophagia and a history of childhood consumption of dirt, chalk, and clay. Another patient, a 34-year-old African American woman, suffered from a lifelong desire to eat dirt, which she was able to resist, but experienced pagophagia during pregnancy and later when she developed iron deficiency anemia.4 In another case series, Kushner et al5 describes a 35-year-old woman with iron deficiency anemia with pagophagia presenting 2 years after Roux-en-Y. Her history was significant for eating clay as a child, but this new-onset pagophagia was so intense she purchased 2 snow cone machines, one for home and one for work, to feed her urges. Another patient, a 45-year-old African American woman, had an irresistible craving for calcium carbonate antacids, eating 40 to 50 a day, as well as several 30-ounce cups of ice.5 A third case report details a 33-year-old woman with iron deficiency anemia who presented with nocturnal pagophagia after Roux-en-Y anastomosis. She repeatedly rose during the night to eat the frost off the ice maker in her refrigerator.6 Another case described a female patient who ate cardboard after having a Roux-en-Y.2
Common themes in these case reports are female sex, Roux-en-Y, and dramatic resurgence of previously noted pica behaviors after gastric bypass surgery. Several studies have shown that pagophagia and pica in patients who are iron deficient or have iron deficiency anemia can be rapidly curbed with iron supplements.5 Ms. O, who has low iron, is taking iron supplementation, yet continues to experience pica cravings, albeit less severely. Her pica could be psychiatric in origin, perhaps related to her history of anxiety.
OUTCOME: Combination therapy
We start Ms. O on ziprasidone, 80 mg twice a day, restart lorazepam, 1 mg/d, and schedule monthly follow-up appointments to monitor her pica symptoms. We prescribe ziprasidone because it could treat paranoia and preoccupations and is considered to be weight-neutral. She continues her supplements, including ferrous sulfate, 325 mg 3 times daily. Ms. O attends weekly behavioral therapy sessions, during which the therapist monitors her mood and cravings with response prevention, which entails purposely avoiding behaviors after initiating a distressing stimulus. Ms. O responds well to medication and psychotherapy 1 month after the gastric bezoar removal, and she reports a decreased urge to eat cardboard. She is able to increase the amount of time she can go without eating non-nutritive substances—once daily, rather than repeatedly throughout the day.
The authors’ observations
Each patient with pica likely needs customized care. Children need to be supervised to prevent ingestion of lead-containing substances such as paint chips. Iron supplements are recommended for iron deficiency anemia and prophylaxis for iron deficiency anemia in Roux-en-Y patients.3,4 Pica in pregnant patients should be addressed to maintain adequate nutrition and prevent accidental poisonings.7 Behavioral intervention strategies are based on positive reinforcement and punishment (Table).8 A report of 3 young children with pica noted successful treatment of one with automatic reinforcement, and the other 2 with a combination of social and automatic reinforcement.9 There are no FDA-approved medications for pica. Positive effects have been seen with SSRIs, bupropion, atypical antipsychotics, buprenorphine, and chlorimipramine.10 Olanzapine has shown positive results as a treatment for pica.11 Most pica patients need concurrent psychotherapy.10
Table
Behavioral interventions for pica
| Intervention | Comments |
|---|---|
| Environmental enrichment | Providing additional stimulus to increase neuronal activity and focus behaviors |
| Noncontingent reinforcement | Presenting reinforcers according to a fixed schedule |
| Differential reinforcement | Desired behaviors are reinforced and inappropriate behaviors are ignored |
| Response blocking | Physically block a patient’s attempts to eat nonedible items |
| Source: Reference 8 | |
Related Resources
- Blinder BJ, Salama C. An update on pica: prevalence, contributing causes, and treatment. Psychiatric Times. www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008.
- Nurcombe B. Developmental disorders of attachment, feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
Drug Brand Names
- Buprenorphine • Subutex
- Bupropion • Wellbutrin, Zyban
- Chlorimipramine • Anafranil
- Esomeprazole • Nexium
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Patton W, Gibbs K. Cardboard bezoar complicating laparoscopic gastric bypass. Surg Obes Relat Dis. 2010;6(3):313-315.
3. Nurcombe B. Developmental disorders of attachment feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
4. Kushner F, Gleason B, Shanta-Retelny V. Reemergence of pica following gastric bypass surgery for obesity: a new presentation of an old problem. J Am Diet Assoc. 2004;104(9):1393-1397.
5. Kushner F, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15(10):1491-1495.
6. Marinella MA. Nocturnal pagophagia complicating gastric bypass. Mayo Clin Proc. 2008;83(8):961.-
7. Bernstein B, Weinstein M. Normal pregnancy & prenatal care. In: DeCherney AH Nathan L, Goodwin TM, et al, eds. CURRENT diagnosis & treatment obstetrics & gynecology. 10th ed. New York, NY: McGraw Hill; 2007.
8. Piazza C, Fisher W, Hanley P, et al. Treatment of pica through multiple analyses of its reinforcing functions. J Appl Behav Anal. 1998;31(2):165-189.
9. Williams DE, McAdam D. Assessment behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33(6):2050-2057.
10. Blinder BJ, Salama C. An update on pica: prevalence contributing causes, and treatment. Psychiatric Times. http://www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008. Accessed January 23, 2013.
11. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13(1):19.-
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Nauseous and full
Ms. O, age 48, presents to the emergency department reporting a 3-day history of vomiting approximately 5 minutes after consuming solids or liquids. She’s had 10 vomiting episodes, which were associated with “fullness” and an “aching” sensation she rates as 6 on a 10-point scale pain scale that is diffuse over the upper epigastric area, with no palliative factors. Ms. O has not had a bowel movement for 3 days and her last menstrual period was 8 days ago. She is taking lorazepam, 1 mg/d. Her medical and psychiatric history includes anxiety, depression, personality disorder symptoms of affective dysregulation, obesity (270 lbs; medium height), and pica. She was 352 lbs when she underwent a Roux-en-Y gastric bypass 2 years ago. One year earlier, she had a laparoscopic gastric bezoar removal and an incisional hernia repair. Ms. O had no pica-related surgeries before undergoing gastric bypass surgery.
Ms. O denies shortness of breath, chest pain, allergies, smoking, or alcohol abuse, but reports uncontrollable cravings for paper products, specifically cardboard, which she describes as “just so delicious.” This craving led her to consume large amounts of cardboard and newspaper in the days before she began vomiting.
What may be causing Ms. O’s pica symptoms?
- iron deficiency anemia
- complications from gastric bypass surgery
- personality disorder
- generalized anxiety disorder (GAD)
The authors’ observations
DSM-IV-TR diagnostic criteria for pica include the persistent eating of non-nutritive substances for ≥1 month that is inappropriate for the level of a person’s development and not an acceptable part of one’s culture.1 If pica occurs with other mental disorders, it must be severe enough to indicate further clinical assessment to receive a separate diagnosis. Often associated with pregnancy, iron deficiency anemia, early development, and mental retardation, pica has been observed in post-gastric bypass surgery patients, all of whom presented with pagophagia (compulsive ice eating), and in one case was associated with a bezoar causing obstruction of the GI tract.1,2 With the dramatic increase in gastric bypass surgery and the required presurgical mental health evaluation, the consequences of failing to screen patients for pica behaviors can be devastating.
EVALUATION: Low iron
Ms. O’s vital signs on admission are stable, and physical exam is notable for mild abdominal distention with no guarding, tenderness, rigidity, or masses. No rebound tenderness is elicited. CT scan shows evidence of post-surgical changes involving the small bowel consistent with gastric bypass surgery and a hiatal hernia, but no obstruction, focal inflammation, free fluids, or gas. Lab values for amylase, lipase, urinalysis, coagulation studies, cardiac enzymes, and complete metabolic profile are within normal limits. Although not anemic, Ms. O is iron deficient, with ferritin, 10 ng/mL (normal 10 to 120 ng/mL); B12, 299 pg/mL (normal 100 to 700 pg/mL); and iron, 25 μg/dL (normal 50 to 170 μg/dL).
A foreign body is removed endoscopically and the specimen is sent to pathology. It is determined to be a gastric bezoar, yellowish-green in color, measuring 2.5 cm × 1 cm × 0.8 cm. After bezoar removal, Ms. O tolerates food and is discharged home on vitamin B12, 1,000 mcg/d for 2 weeks; folate, 1 mg/d for 1 month; calcium with vitamin D, 1 g/d; and esomeprazole, 40 mg/d for frequent heartburn. She is referred to psychiatry for behavioral modification therapy and medication management.
How would you treat Ms. O?
- start a selective serotonin reuptake inhibitor (SSRI)
- prescribe an atypical antipsychotic
- continue lorazepam
- begin behavioral therapy
HISTORY: Pica during pregnancy
During psychiatric workup, Ms. O admits to having pica urges most of her life, but experienced an uncontrollable exacerbation after gastric bypass surgery. This led to intense, chaotic periods of pica, resulting in a previous bezoar removal. She is particularly attracted to cardboard and newspaper cartoons, but notes she also has felt the urge to eat charcoal, moist soil, clay, chalk, pencils, and new shoes, which she chews on. In the past, her extreme anxiety and preoccupation with these urges had lead to diagnoses of personality disorder not otherwise specified, GAD, and obsessive-compulsive disorder.
Her first experience with pica was during her first pregnancy at age 15, when she had an impulse to eat soil. The urges briefly stopped until she became pregnant again. During each of her 5 pregnancies her pica symptoms returned. At one point during her last pregnancy she reports having felt out of control, eating 2 to 3 pencils with the eraser per day, after which she would feel intense relaxation. Her mother also exhibited symptoms of pica toward charcoal and soil. Ms. O had been taking unknown dosages of lorazepam for anxiety and fluoxetine for depression, both of which she stopped because she feared side effects during her last pregnancy. However, she never experienced any side effects.
The authors’ observations
Although pica is most commonly observed in young children, it sometimes is seen in pregnant women.1 Pica frequently is associated with other mental disorders, such as pervasive developmental disorder and mental retardation,1 and can be associated with premorbid psychosis and anxiety disorders. Occasional vitamin and mineral deficiencies, such as iron or zinc, have been reported, but usually patients’ lab values are normal. Treatment usually is initiated in the context of medical complications, such as iron deficiency anemia. In Ms. O’s case, the precipitating event was mechanical bowel obstruction due to a bezoar.
Several theories about the origins of pica have been proposed, but none truly are explanatory or satisfactory. The nutritional theory—that patients eat non-nutritive substances to compensate for mineral deficiencies—is popular because of pica’s frequent association with mineral deficiencies, but it is unknown whether pica is the cause or the result of the deficiency. An example of this is anemia due to eating clay instead of foods that contain iron. Another theory is that because pica is normal in early childhood development, it may be a manifestation of delayed development or mental retardation. The cultural theory is attractive because pregnant women in several cultures eat starch or clay as a part of their native rituals, and the incidence of pica is relatively high among pregnant African American women who live in rural areas.3 In the Roux-en-Y procedure, bypass of the duodenum and proximal jejunum can significantly decrease a patient’s iron uptake, leading to iron deficiency anemia, and could trigger pica in a susceptible patient.4
Exacerbation after gastric bypass
Kushner et al4 describes re-emergent pica after bariatric surgery in 2 patients with pagophagia associated with concomitant iron deficiency anemia. A 41-year-old white woman presented with pagophagia and a history of childhood consumption of dirt, chalk, and clay. Another patient, a 34-year-old African American woman, suffered from a lifelong desire to eat dirt, which she was able to resist, but experienced pagophagia during pregnancy and later when she developed iron deficiency anemia.4 In another case series, Kushner et al5 describes a 35-year-old woman with iron deficiency anemia with pagophagia presenting 2 years after Roux-en-Y. Her history was significant for eating clay as a child, but this new-onset pagophagia was so intense she purchased 2 snow cone machines, one for home and one for work, to feed her urges. Another patient, a 45-year-old African American woman, had an irresistible craving for calcium carbonate antacids, eating 40 to 50 a day, as well as several 30-ounce cups of ice.5 A third case report details a 33-year-old woman with iron deficiency anemia who presented with nocturnal pagophagia after Roux-en-Y anastomosis. She repeatedly rose during the night to eat the frost off the ice maker in her refrigerator.6 Another case described a female patient who ate cardboard after having a Roux-en-Y.2
Common themes in these case reports are female sex, Roux-en-Y, and dramatic resurgence of previously noted pica behaviors after gastric bypass surgery. Several studies have shown that pagophagia and pica in patients who are iron deficient or have iron deficiency anemia can be rapidly curbed with iron supplements.5 Ms. O, who has low iron, is taking iron supplementation, yet continues to experience pica cravings, albeit less severely. Her pica could be psychiatric in origin, perhaps related to her history of anxiety.
OUTCOME: Combination therapy
We start Ms. O on ziprasidone, 80 mg twice a day, restart lorazepam, 1 mg/d, and schedule monthly follow-up appointments to monitor her pica symptoms. We prescribe ziprasidone because it could treat paranoia and preoccupations and is considered to be weight-neutral. She continues her supplements, including ferrous sulfate, 325 mg 3 times daily. Ms. O attends weekly behavioral therapy sessions, during which the therapist monitors her mood and cravings with response prevention, which entails purposely avoiding behaviors after initiating a distressing stimulus. Ms. O responds well to medication and psychotherapy 1 month after the gastric bezoar removal, and she reports a decreased urge to eat cardboard. She is able to increase the amount of time she can go without eating non-nutritive substances—once daily, rather than repeatedly throughout the day.
The authors’ observations
Each patient with pica likely needs customized care. Children need to be supervised to prevent ingestion of lead-containing substances such as paint chips. Iron supplements are recommended for iron deficiency anemia and prophylaxis for iron deficiency anemia in Roux-en-Y patients.3,4 Pica in pregnant patients should be addressed to maintain adequate nutrition and prevent accidental poisonings.7 Behavioral intervention strategies are based on positive reinforcement and punishment (Table).8 A report of 3 young children with pica noted successful treatment of one with automatic reinforcement, and the other 2 with a combination of social and automatic reinforcement.9 There are no FDA-approved medications for pica. Positive effects have been seen with SSRIs, bupropion, atypical antipsychotics, buprenorphine, and chlorimipramine.10 Olanzapine has shown positive results as a treatment for pica.11 Most pica patients need concurrent psychotherapy.10
Table
Behavioral interventions for pica
| Intervention | Comments |
|---|---|
| Environmental enrichment | Providing additional stimulus to increase neuronal activity and focus behaviors |
| Noncontingent reinforcement | Presenting reinforcers according to a fixed schedule |
| Differential reinforcement | Desired behaviors are reinforced and inappropriate behaviors are ignored |
| Response blocking | Physically block a patient’s attempts to eat nonedible items |
| Source: Reference 8 | |
Related Resources
- Blinder BJ, Salama C. An update on pica: prevalence, contributing causes, and treatment. Psychiatric Times. www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008.
- Nurcombe B. Developmental disorders of attachment, feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
Drug Brand Names
- Buprenorphine • Subutex
- Bupropion • Wellbutrin, Zyban
- Chlorimipramine • Anafranil
- Esomeprazole • Nexium
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Nauseous and full
Ms. O, age 48, presents to the emergency department reporting a 3-day history of vomiting approximately 5 minutes after consuming solids or liquids. She’s had 10 vomiting episodes, which were associated with “fullness” and an “aching” sensation she rates as 6 on a 10-point scale pain scale that is diffuse over the upper epigastric area, with no palliative factors. Ms. O has not had a bowel movement for 3 days and her last menstrual period was 8 days ago. She is taking lorazepam, 1 mg/d. Her medical and psychiatric history includes anxiety, depression, personality disorder symptoms of affective dysregulation, obesity (270 lbs; medium height), and pica. She was 352 lbs when she underwent a Roux-en-Y gastric bypass 2 years ago. One year earlier, she had a laparoscopic gastric bezoar removal and an incisional hernia repair. Ms. O had no pica-related surgeries before undergoing gastric bypass surgery.
Ms. O denies shortness of breath, chest pain, allergies, smoking, or alcohol abuse, but reports uncontrollable cravings for paper products, specifically cardboard, which she describes as “just so delicious.” This craving led her to consume large amounts of cardboard and newspaper in the days before she began vomiting.
What may be causing Ms. O’s pica symptoms?
- iron deficiency anemia
- complications from gastric bypass surgery
- personality disorder
- generalized anxiety disorder (GAD)
The authors’ observations
DSM-IV-TR diagnostic criteria for pica include the persistent eating of non-nutritive substances for ≥1 month that is inappropriate for the level of a person’s development and not an acceptable part of one’s culture.1 If pica occurs with other mental disorders, it must be severe enough to indicate further clinical assessment to receive a separate diagnosis. Often associated with pregnancy, iron deficiency anemia, early development, and mental retardation, pica has been observed in post-gastric bypass surgery patients, all of whom presented with pagophagia (compulsive ice eating), and in one case was associated with a bezoar causing obstruction of the GI tract.1,2 With the dramatic increase in gastric bypass surgery and the required presurgical mental health evaluation, the consequences of failing to screen patients for pica behaviors can be devastating.
EVALUATION: Low iron
Ms. O’s vital signs on admission are stable, and physical exam is notable for mild abdominal distention with no guarding, tenderness, rigidity, or masses. No rebound tenderness is elicited. CT scan shows evidence of post-surgical changes involving the small bowel consistent with gastric bypass surgery and a hiatal hernia, but no obstruction, focal inflammation, free fluids, or gas. Lab values for amylase, lipase, urinalysis, coagulation studies, cardiac enzymes, and complete metabolic profile are within normal limits. Although not anemic, Ms. O is iron deficient, with ferritin, 10 ng/mL (normal 10 to 120 ng/mL); B12, 299 pg/mL (normal 100 to 700 pg/mL); and iron, 25 μg/dL (normal 50 to 170 μg/dL).
A foreign body is removed endoscopically and the specimen is sent to pathology. It is determined to be a gastric bezoar, yellowish-green in color, measuring 2.5 cm × 1 cm × 0.8 cm. After bezoar removal, Ms. O tolerates food and is discharged home on vitamin B12, 1,000 mcg/d for 2 weeks; folate, 1 mg/d for 1 month; calcium with vitamin D, 1 g/d; and esomeprazole, 40 mg/d for frequent heartburn. She is referred to psychiatry for behavioral modification therapy and medication management.
How would you treat Ms. O?
- start a selective serotonin reuptake inhibitor (SSRI)
- prescribe an atypical antipsychotic
- continue lorazepam
- begin behavioral therapy
HISTORY: Pica during pregnancy
During psychiatric workup, Ms. O admits to having pica urges most of her life, but experienced an uncontrollable exacerbation after gastric bypass surgery. This led to intense, chaotic periods of pica, resulting in a previous bezoar removal. She is particularly attracted to cardboard and newspaper cartoons, but notes she also has felt the urge to eat charcoal, moist soil, clay, chalk, pencils, and new shoes, which she chews on. In the past, her extreme anxiety and preoccupation with these urges had lead to diagnoses of personality disorder not otherwise specified, GAD, and obsessive-compulsive disorder.
Her first experience with pica was during her first pregnancy at age 15, when she had an impulse to eat soil. The urges briefly stopped until she became pregnant again. During each of her 5 pregnancies her pica symptoms returned. At one point during her last pregnancy she reports having felt out of control, eating 2 to 3 pencils with the eraser per day, after which she would feel intense relaxation. Her mother also exhibited symptoms of pica toward charcoal and soil. Ms. O had been taking unknown dosages of lorazepam for anxiety and fluoxetine for depression, both of which she stopped because she feared side effects during her last pregnancy. However, she never experienced any side effects.
The authors’ observations
Although pica is most commonly observed in young children, it sometimes is seen in pregnant women.1 Pica frequently is associated with other mental disorders, such as pervasive developmental disorder and mental retardation,1 and can be associated with premorbid psychosis and anxiety disorders. Occasional vitamin and mineral deficiencies, such as iron or zinc, have been reported, but usually patients’ lab values are normal. Treatment usually is initiated in the context of medical complications, such as iron deficiency anemia. In Ms. O’s case, the precipitating event was mechanical bowel obstruction due to a bezoar.
Several theories about the origins of pica have been proposed, but none truly are explanatory or satisfactory. The nutritional theory—that patients eat non-nutritive substances to compensate for mineral deficiencies—is popular because of pica’s frequent association with mineral deficiencies, but it is unknown whether pica is the cause or the result of the deficiency. An example of this is anemia due to eating clay instead of foods that contain iron. Another theory is that because pica is normal in early childhood development, it may be a manifestation of delayed development or mental retardation. The cultural theory is attractive because pregnant women in several cultures eat starch or clay as a part of their native rituals, and the incidence of pica is relatively high among pregnant African American women who live in rural areas.3 In the Roux-en-Y procedure, bypass of the duodenum and proximal jejunum can significantly decrease a patient’s iron uptake, leading to iron deficiency anemia, and could trigger pica in a susceptible patient.4
Exacerbation after gastric bypass
Kushner et al4 describes re-emergent pica after bariatric surgery in 2 patients with pagophagia associated with concomitant iron deficiency anemia. A 41-year-old white woman presented with pagophagia and a history of childhood consumption of dirt, chalk, and clay. Another patient, a 34-year-old African American woman, suffered from a lifelong desire to eat dirt, which she was able to resist, but experienced pagophagia during pregnancy and later when she developed iron deficiency anemia.4 In another case series, Kushner et al5 describes a 35-year-old woman with iron deficiency anemia with pagophagia presenting 2 years after Roux-en-Y. Her history was significant for eating clay as a child, but this new-onset pagophagia was so intense she purchased 2 snow cone machines, one for home and one for work, to feed her urges. Another patient, a 45-year-old African American woman, had an irresistible craving for calcium carbonate antacids, eating 40 to 50 a day, as well as several 30-ounce cups of ice.5 A third case report details a 33-year-old woman with iron deficiency anemia who presented with nocturnal pagophagia after Roux-en-Y anastomosis. She repeatedly rose during the night to eat the frost off the ice maker in her refrigerator.6 Another case described a female patient who ate cardboard after having a Roux-en-Y.2
Common themes in these case reports are female sex, Roux-en-Y, and dramatic resurgence of previously noted pica behaviors after gastric bypass surgery. Several studies have shown that pagophagia and pica in patients who are iron deficient or have iron deficiency anemia can be rapidly curbed with iron supplements.5 Ms. O, who has low iron, is taking iron supplementation, yet continues to experience pica cravings, albeit less severely. Her pica could be psychiatric in origin, perhaps related to her history of anxiety.
OUTCOME: Combination therapy
We start Ms. O on ziprasidone, 80 mg twice a day, restart lorazepam, 1 mg/d, and schedule monthly follow-up appointments to monitor her pica symptoms. We prescribe ziprasidone because it could treat paranoia and preoccupations and is considered to be weight-neutral. She continues her supplements, including ferrous sulfate, 325 mg 3 times daily. Ms. O attends weekly behavioral therapy sessions, during which the therapist monitors her mood and cravings with response prevention, which entails purposely avoiding behaviors after initiating a distressing stimulus. Ms. O responds well to medication and psychotherapy 1 month after the gastric bezoar removal, and she reports a decreased urge to eat cardboard. She is able to increase the amount of time she can go without eating non-nutritive substances—once daily, rather than repeatedly throughout the day.
The authors’ observations
Each patient with pica likely needs customized care. Children need to be supervised to prevent ingestion of lead-containing substances such as paint chips. Iron supplements are recommended for iron deficiency anemia and prophylaxis for iron deficiency anemia in Roux-en-Y patients.3,4 Pica in pregnant patients should be addressed to maintain adequate nutrition and prevent accidental poisonings.7 Behavioral intervention strategies are based on positive reinforcement and punishment (Table).8 A report of 3 young children with pica noted successful treatment of one with automatic reinforcement, and the other 2 with a combination of social and automatic reinforcement.9 There are no FDA-approved medications for pica. Positive effects have been seen with SSRIs, bupropion, atypical antipsychotics, buprenorphine, and chlorimipramine.10 Olanzapine has shown positive results as a treatment for pica.11 Most pica patients need concurrent psychotherapy.10
Table
Behavioral interventions for pica
| Intervention | Comments |
|---|---|
| Environmental enrichment | Providing additional stimulus to increase neuronal activity and focus behaviors |
| Noncontingent reinforcement | Presenting reinforcers according to a fixed schedule |
| Differential reinforcement | Desired behaviors are reinforced and inappropriate behaviors are ignored |
| Response blocking | Physically block a patient’s attempts to eat nonedible items |
| Source: Reference 8 | |
Related Resources
- Blinder BJ, Salama C. An update on pica: prevalence, contributing causes, and treatment. Psychiatric Times. www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008.
- Nurcombe B. Developmental disorders of attachment, feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
Drug Brand Names
- Buprenorphine • Subutex
- Bupropion • Wellbutrin, Zyban
- Chlorimipramine • Anafranil
- Esomeprazole • Nexium
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Patton W, Gibbs K. Cardboard bezoar complicating laparoscopic gastric bypass. Surg Obes Relat Dis. 2010;6(3):313-315.
3. Nurcombe B. Developmental disorders of attachment feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
4. Kushner F, Gleason B, Shanta-Retelny V. Reemergence of pica following gastric bypass surgery for obesity: a new presentation of an old problem. J Am Diet Assoc. 2004;104(9):1393-1397.
5. Kushner F, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15(10):1491-1495.
6. Marinella MA. Nocturnal pagophagia complicating gastric bypass. Mayo Clin Proc. 2008;83(8):961.-
7. Bernstein B, Weinstein M. Normal pregnancy & prenatal care. In: DeCherney AH Nathan L, Goodwin TM, et al, eds. CURRENT diagnosis & treatment obstetrics & gynecology. 10th ed. New York, NY: McGraw Hill; 2007.
8. Piazza C, Fisher W, Hanley P, et al. Treatment of pica through multiple analyses of its reinforcing functions. J Appl Behav Anal. 1998;31(2):165-189.
9. Williams DE, McAdam D. Assessment behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33(6):2050-2057.
10. Blinder BJ, Salama C. An update on pica: prevalence contributing causes, and treatment. Psychiatric Times. http://www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008. Accessed January 23, 2013.
11. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13(1):19.-
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Patton W, Gibbs K. Cardboard bezoar complicating laparoscopic gastric bypass. Surg Obes Relat Dis. 2010;6(3):313-315.
3. Nurcombe B. Developmental disorders of attachment feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
4. Kushner F, Gleason B, Shanta-Retelny V. Reemergence of pica following gastric bypass surgery for obesity: a new presentation of an old problem. J Am Diet Assoc. 2004;104(9):1393-1397.
5. Kushner F, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15(10):1491-1495.
6. Marinella MA. Nocturnal pagophagia complicating gastric bypass. Mayo Clin Proc. 2008;83(8):961.-
7. Bernstein B, Weinstein M. Normal pregnancy & prenatal care. In: DeCherney AH Nathan L, Goodwin TM, et al, eds. CURRENT diagnosis & treatment obstetrics & gynecology. 10th ed. New York, NY: McGraw Hill; 2007.
8. Piazza C, Fisher W, Hanley P, et al. Treatment of pica through multiple analyses of its reinforcing functions. J Appl Behav Anal. 1998;31(2):165-189.
9. Williams DE, McAdam D. Assessment behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33(6):2050-2057.
10. Blinder BJ, Salama C. An update on pica: prevalence contributing causes, and treatment. Psychiatric Times. http://www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008. Accessed January 23, 2013.
11. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13(1):19.-
Working with NPs
I want to acknowledge the foresight of Current Psychiatry’s editorial staff in publishing "How to collaborate effectively with psychiatric nurse practitioners" (Current Psychiatry, November 2012, p. 49-53; http://bit.ly/1QTTtkK). Your article was the first of its kind that I have read published in a national psychiatry journal.
Having trained at Children’s Psychiatric Hospital at the University of Michigan, I also am proud of the article’s authors, who are associated with my alma mater’s psychiatry department. Nursing schools should increase their efforts to bring affordable, quality mental health services to all via the innovative role of collaborator.
Marianne Cannon, CNS, APRN
Private Practice
Beverly, MA
I want to acknowledge the foresight of Current Psychiatry’s editorial staff in publishing "How to collaborate effectively with psychiatric nurse practitioners" (Current Psychiatry, November 2012, p. 49-53; http://bit.ly/1QTTtkK). Your article was the first of its kind that I have read published in a national psychiatry journal.
Having trained at Children’s Psychiatric Hospital at the University of Michigan, I also am proud of the article’s authors, who are associated with my alma mater’s psychiatry department. Nursing schools should increase their efforts to bring affordable, quality mental health services to all via the innovative role of collaborator.
Marianne Cannon, CNS, APRN
Private Practice
Beverly, MA
I want to acknowledge the foresight of Current Psychiatry’s editorial staff in publishing "How to collaborate effectively with psychiatric nurse practitioners" (Current Psychiatry, November 2012, p. 49-53; http://bit.ly/1QTTtkK). Your article was the first of its kind that I have read published in a national psychiatry journal.
Having trained at Children’s Psychiatric Hospital at the University of Michigan, I also am proud of the article’s authors, who are associated with my alma mater’s psychiatry department. Nursing schools should increase their efforts to bring affordable, quality mental health services to all via the innovative role of collaborator.
Marianne Cannon, CNS, APRN
Private Practice
Beverly, MA
Paying for metabolic tests
Regarding Dr. Nasrallah’s excellent editorial ("Why are metabolic guidelines being ignored?"; Current Psychiatry, From the Editor, December 2012, p. 4-5; http://bit.ly/1GI2FFY): The next step is to get insurance companies, the Veterans Administration (VA), and the government to recognize that persons with “behavioral” illnesses may need a psychiatrist to identify and treat physical illnesses to comprehensively address—and in some cases, cure—the patient’s behavioral problem.
It is maddening that some insurance companies categorize mental illnesses apart from physical illnesses and will not process nonmental health service codes submitted by psychiatrists. Psychiatrists get chastised by insurance companies, the VA, and the government for ordering too many laboratory tests, as if there were no need for patients with mental illnesses to undergo metabolic monitoring. If the test is not reimbursed, then the test is not ordered. If psychiatrists are demeaned by mainstream medicine for holistically caring for their patients, then it’s no wonder psychiatry is a specialty on its way out.
Charles J. Mertz
Business Manager
Private Practice
Springfield, IL
Dr. Nasrallah responds
I thank Drs. Goldsmith and Vahabzadeh and Mr. Mertz for their letters.
Psychiatrists and nurse practitioners routinely order lab tests for patients as part of a physical assessment before starting any medication, whether at baseline or follow-up. Third-party payers cover complete blood counts, liver function tests, kidney function tests, and other tests. Thus, metabolic monitoring tests—including fasting glucose, fasting triglycerides, and fasting high-density lipoprotein—are no exception.
Any insurer who refuses to reimburse those tests for a patient receiving atypical antipsychotics can be liable in a court of law, especially in light of FDA recommendations.
Henry A. Nasrallah, MDEditor-in-Chief
Regarding Dr. Nasrallah’s excellent editorial ("Why are metabolic guidelines being ignored?"; Current Psychiatry, From the Editor, December 2012, p. 4-5; http://bit.ly/1GI2FFY): The next step is to get insurance companies, the Veterans Administration (VA), and the government to recognize that persons with “behavioral” illnesses may need a psychiatrist to identify and treat physical illnesses to comprehensively address—and in some cases, cure—the patient’s behavioral problem.
It is maddening that some insurance companies categorize mental illnesses apart from physical illnesses and will not process nonmental health service codes submitted by psychiatrists. Psychiatrists get chastised by insurance companies, the VA, and the government for ordering too many laboratory tests, as if there were no need for patients with mental illnesses to undergo metabolic monitoring. If the test is not reimbursed, then the test is not ordered. If psychiatrists are demeaned by mainstream medicine for holistically caring for their patients, then it’s no wonder psychiatry is a specialty on its way out.
Charles J. Mertz
Business Manager
Private Practice
Springfield, IL
Dr. Nasrallah responds
I thank Drs. Goldsmith and Vahabzadeh and Mr. Mertz for their letters.
Psychiatrists and nurse practitioners routinely order lab tests for patients as part of a physical assessment before starting any medication, whether at baseline or follow-up. Third-party payers cover complete blood counts, liver function tests, kidney function tests, and other tests. Thus, metabolic monitoring tests—including fasting glucose, fasting triglycerides, and fasting high-density lipoprotein—are no exception.
Any insurer who refuses to reimburse those tests for a patient receiving atypical antipsychotics can be liable in a court of law, especially in light of FDA recommendations.
Henry A. Nasrallah, MDEditor-in-Chief
Regarding Dr. Nasrallah’s excellent editorial ("Why are metabolic guidelines being ignored?"; Current Psychiatry, From the Editor, December 2012, p. 4-5; http://bit.ly/1GI2FFY): The next step is to get insurance companies, the Veterans Administration (VA), and the government to recognize that persons with “behavioral” illnesses may need a psychiatrist to identify and treat physical illnesses to comprehensively address—and in some cases, cure—the patient’s behavioral problem.
It is maddening that some insurance companies categorize mental illnesses apart from physical illnesses and will not process nonmental health service codes submitted by psychiatrists. Psychiatrists get chastised by insurance companies, the VA, and the government for ordering too many laboratory tests, as if there were no need for patients with mental illnesses to undergo metabolic monitoring. If the test is not reimbursed, then the test is not ordered. If psychiatrists are demeaned by mainstream medicine for holistically caring for their patients, then it’s no wonder psychiatry is a specialty on its way out.
Charles J. Mertz
Business Manager
Private Practice
Springfield, IL
Dr. Nasrallah responds
I thank Drs. Goldsmith and Vahabzadeh and Mr. Mertz for their letters.
Psychiatrists and nurse practitioners routinely order lab tests for patients as part of a physical assessment before starting any medication, whether at baseline or follow-up. Third-party payers cover complete blood counts, liver function tests, kidney function tests, and other tests. Thus, metabolic monitoring tests—including fasting glucose, fasting triglycerides, and fasting high-density lipoprotein—are no exception.
Any insurer who refuses to reimburse those tests for a patient receiving atypical antipsychotics can be liable in a court of law, especially in light of FDA recommendations.
Henry A. Nasrallah, MDEditor-in-Chief


