User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Telepsychiatry: Overcoming barriers to implementation
Discuss this article at www.facebook.com/CurrentPsychiatry
Although many states have substantial health services in urban areas, these services—particularly mental health care—are relatively scarce in rural areas.1 Telepsychiatry, in which clinicians provide mental health care from a distance in real time by using interactive, 2-way, audio-video communication (videoconferencing), could mitigate workforce shortages that affect remote and underserved areas.2 Psychiatry is one of the biggest users of telemedicine, which refers to any combination of communication technology and medicine.3-5 This article discusses telepsychiatry’s effectiveness in providing psychiatric diagnosis and treatment, and the clinical implications of this technology, including improving access, cost, and quality of mental health services.
Outcomes comparable to face-to-face care
Telepsychiatry is used primarily in rural areas or correctional institutions or with underserved populations such as veterans with posttraumatic stress disorder or children. Although the literature generally is weak, there has been more research on psychiatry than other medical specialties because psychiatric clinicians rely on mental status examinations and verbal communications, not physical exams. Telepsychiatry can be considered a part of an evolving “connected health” system that offers many benefits to patients and clinicians (Table).
Table
Benefits of telepsychiatry as part of a ‘connected health’ system
| Available to everyone |
| Health care is provided at the point of convenience |
| Patients are informed and empowered |
| Facilitates patient compliance, continuing education, ease of access into the health care system, and healthy behaviors |
| Clinical data are integrated with longitudinal electronic health records |
| Data are available to patients via his or her personal electronic medical record and authorized clinical providers |
| Data and transactions are secure to greatest practical extent |
| Other telehealth applications with demonstrated efficacy—eg, telephone, internet, e-mail, and text messaging interventions—can be used as well |
Barriers to implementation
Although telepsychiatry offers tremendous promise, implementation has not been widespread or easy. Potential barriers to implementation, such as cost and resistance to change, are associated with acceptance of new technology or practice in health care. In addition, there are several legal, regulatory, and technical barriers.
Institutional barriers. Physicians and other providers may not have access to timely, evidence-based information and may face challenges, such as time constraints, access to technical support, and complexity of large health care institutions, when integrating this information into clinical practice.16 Two studies17 found that after controlling for other barriers—eg, reimbursement and regulatory issues—providers are the most significant initial gatekeepers that affect telemedicine use. When designing a telemedicine system, project managers should prioritize providers’ needs, such as ease of use and incentives.18
States do not cover services provided by other mental health providers, except for Utah’s coverage for social workers. The American Psychiatric Association has 2 suggestions regarding this issue3:
- reimbursement for telepsychiatry services should follow customary charges for delivering the appropriate current procedural terminology code(s)
- a structure for reimbursement of collateral charges, such as technician and line time, should be identified.
Licensure. A physician conducting a telemedicine session with a patient in another state must be licensed in both his or her state and the patient’s state. Nurses and other allied health professionals have similar state licensing constraints. Sanders22 suggests 3 potential solutions:
- establishing a national licensing system
- assigning the responsibility of care to the referring physician, with the consulting physician’s opinion serving as “recommendation only”
- determining that the patient is being “electronically transmitted” to the consultant’s state.
Although telemedicine has embraced many communication technologies, live, interactive, 2-way, audio-video communication—called videoconferencing—is broadly synonymous with telemedicine and, more specifically, telepsychiatry.
Telepsychiatry primarily uses interactive audiovisual conferencing systems over high-bandwidth networks. The central component of interactive telepsychiatry is the codec (coder/decoder), which provides compression, decompression, and synchronization of audio and video signals; both patients and clinicians need a codec. A codec can be a separate device, but personal computer-based codecs are being used more frequently. A typical setup also includes a video camera, microphone, speakers or headset, and 1 or 2 display monitors at both the clinician’s and patient’s end of the system. Often, separate displays or a picture-in-picture display are used to see both outgoing and incoming video. Another consideration is pan-zoom-tilt control of video cameras. This allows clinicians to remotely control his or her view of the patient’s site or control the view being transmitted to the patient.
Historically, interactive telepsychiatry applications have used point-to-point network connections, usually as full or fractional T-1 or integrated services digital network circuits. However, the rapid diffusion of internet and ethernet networks has led to the development of videoconferencing systems that can work over internet protocol (IP) networks. If using an IP network, ensure security by using encrypted codecs or by setting up a virtual private network and/or a virtual local area network (LAN). The principal advantage of IP networks is that by implementing proper security solutions, they can be shared by several applications—eg, internet, e-mail, LAN, etc. This means that the telecommunications network costs can be shared or considered a sunk cost (ie, not an additional cost of the telepsychiatry application).
The U.S. Federal Communications Commission’s (FCC) Universal Service Fund (USF) subsidies can reduce the cost of telepsychiatry network connections. The FCC implemented the USF to bring high bandwidth telecommunications to rural schools, libraries, and health care providers. Funding for the USF is generated from fees paid by telecommunications providers. However, the USF subsidies are not being widely used for several reasons, including a cumbersome application process, limitations on eligible facilities and locations, and questions regarding costs to the health care provider.19
Individual states also have developed funding streams to support telemedicine. The Centers for Medicare and Medicaid Services will pay a facility site fee to the host site (where the patient is located), but only if the site is in a rural area. Providers can charge patients a fee to support telepsychiatry infrastructure and maintenance, but typically this arrangement is not affordable and is not standard practice.
The future
Telepsychiatry’s ability to improve access to mental health care to underserved populations is becoming more evident. Technology is adequate for most uses and is constantly advancing. Numerous applications already have been defined, and more are ripe for exploration. Barriers to implementation are primarily of the human variety and will require a combination of consumer, provider, and governmental advocacy to overcome.
- American Telemedicine Association. www.americantelemed.org.
- International Society for Telemedicine & eHealth. www.isfteh.org.
- American Psychiatric Association. Telepsychiatry internet resources. www.psychiatry.org/practice/professional-interests/underserved-communities/telepsychiatry-internet-resources.
- Mossman D. Practicing psychiatry via Skype: Medicolegal considerations. Current Psychiatry. 2011;10(12):30-32,39.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. President’s New Freedom Commission on Mental Health. Subcommittee on rural issues: background paper. Rockville MD: Substance Abuse and Mental Health Administration; 2004.
2. Antonacci DJ, Bloch RM, Saeed SA, et al. Empirical evidence on the use and effectiveness of telepsychiatry via videoconferencing: implications for forensic and correctional psychiatry. Behav Sci Law. 2008;26(3):253-269.
3. American Psychiatric Association. Resource document on telepsychiatry via videoconferencing. http://www.psychiatry.med.uwo.ca/ecp/info/toronto/telepsych/Appendix%20II.htm. Accessed November 5 2012.
4. Grigsby J, Rigby M, Hiemstra A, et al. Telemedicine/telehealth: an international perspective. The diffusion of telemedicine. Telemed J E Health. 2002;8(1):79-94.
5. Krupinski E, Nypaver M, Poropatich R, et al. Telemedicine/telehealth: an international perspective. Clinical applications in telemedicine/telehealth. Telemed J E Health. 2002;8(1):13-34.
6. Diamond JM, Bloch RM. Telepsychiatry assessments of child or adolescent behavior disorders: a review of evidence and issues. Telemed J E Health. 2010;16(6):712-716.
7. Buono S, Città S. Tele-assistance in intellectual disability. J Telemed Telecare. 2007;13(5):241-245.
8. Manguno-Mire GM, Thompson JW, Jr, Shore JH, et al. The use of telemedicine to evaluate competency to stand trial: a preliminary randomized controlled study. J Am Acad Psychiatry Law. 2007;35(4):481-489.
9. Fortney JC, Maciejewski ML, Tripathi SP, et al. A budget impact analysis of telemedicine-based collaborative care for depression. Med Care. 2011;49(9):872-880.
10. Pyne JM, Fortney JC, Tripathi SP, et al. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Arch Gen Psychiatry. 2010;67(8):812-821.
11. Morland LA, Greene CJ, Rosen CS, et al. Telemedicine for anger management therapy in a rural population of combat veterans with posttraumatic stress disorder: a randomized noninferiority trial. J Clin Psychiatry. 2010;71(7):855-863.
12. Mitchell JE, Crosby RD, Wonderlich SA, et al. A randomized trial comparing the efficacy of cognitive-behavioral therapy for bulimia nervosa delivered via telemedicine versus face-to-face. Behav Res Ther. 2008;46(5):581-592.
13. Steel K, Cox D, Garry H. Therapeutic videoconferencing interventions for the treatment of long-term conditions. J Telemed Telecare. 2011;17(3):109-117.
14. Greene CJ, Morland LA, Macdonald A, et al. How does tele-mental health affect group therapy process? Secondary analysis of a noninferiority trial. J Consult Clin Psychol. 2010;78(5):746-750.
15. Morland LA, Greene CJ, Grubbs K, et al. Therapist adherence to manualized cognitive-behavioral therapy for anger management delivered to veterans with PTSD via videoconferencing. J Clin Psychol. 2011;67(6):629-638.
16. Saeed SA, Diamond J, Bloch RM. Use of telepsychiatry to improve care for people with mental illness in rural North Carolina. N C Med J. 2011;72(3):219-222.
17. Whitten PS, Mackert MS. Addressing telehealth’s foremost barrier: provider as initial gatekeeper. Int J Technol Assess Health Care. 2005;21(4):517-521.
18. Coleman JR. HMOs and the future of telemedicine and telehealth: part 2. Case Manager. 2002;13(4):38-43.
19. Puskin DS. Telemedicine: follow the money modalities. Online J Issues Nurs. 2001;6(3):2.-
20. American Telemedicine Association. Medicare payment of telemedicine and telehealth services. http://www.americantelemed.org/files/public/membergroups/businessfinance/reimbursement/
BF_MedicarePaymentofTelemedicine.pdf. Published May 15 2006. Accessed November 5, 2012.
21. Fishbein M. Developing effective behavior change interventions: some lessons learned from behavioral research. NIDA Res Monogr. 1995;155:246-261.
22. Sanders JH. Telemedicine: challenges to implementation. Paper presented at: Rural Telemedicine Workshop; November 4 1993; Washington, DC.
23. Kumekawa JK. Health information privacy protection: crisis or common sense? Online J Issues Nurs. 2001;6(3):3.-
Discuss this article at www.facebook.com/CurrentPsychiatry
Although many states have substantial health services in urban areas, these services—particularly mental health care—are relatively scarce in rural areas.1 Telepsychiatry, in which clinicians provide mental health care from a distance in real time by using interactive, 2-way, audio-video communication (videoconferencing), could mitigate workforce shortages that affect remote and underserved areas.2 Psychiatry is one of the biggest users of telemedicine, which refers to any combination of communication technology and medicine.3-5 This article discusses telepsychiatry’s effectiveness in providing psychiatric diagnosis and treatment, and the clinical implications of this technology, including improving access, cost, and quality of mental health services.
Outcomes comparable to face-to-face care
Telepsychiatry is used primarily in rural areas or correctional institutions or with underserved populations such as veterans with posttraumatic stress disorder or children. Although the literature generally is weak, there has been more research on psychiatry than other medical specialties because psychiatric clinicians rely on mental status examinations and verbal communications, not physical exams. Telepsychiatry can be considered a part of an evolving “connected health” system that offers many benefits to patients and clinicians (Table).
Table
Benefits of telepsychiatry as part of a ‘connected health’ system
| Available to everyone |
| Health care is provided at the point of convenience |
| Patients are informed and empowered |
| Facilitates patient compliance, continuing education, ease of access into the health care system, and healthy behaviors |
| Clinical data are integrated with longitudinal electronic health records |
| Data are available to patients via his or her personal electronic medical record and authorized clinical providers |
| Data and transactions are secure to greatest practical extent |
| Other telehealth applications with demonstrated efficacy—eg, telephone, internet, e-mail, and text messaging interventions—can be used as well |
Barriers to implementation
Although telepsychiatry offers tremendous promise, implementation has not been widespread or easy. Potential barriers to implementation, such as cost and resistance to change, are associated with acceptance of new technology or practice in health care. In addition, there are several legal, regulatory, and technical barriers.
Institutional barriers. Physicians and other providers may not have access to timely, evidence-based information and may face challenges, such as time constraints, access to technical support, and complexity of large health care institutions, when integrating this information into clinical practice.16 Two studies17 found that after controlling for other barriers—eg, reimbursement and regulatory issues—providers are the most significant initial gatekeepers that affect telemedicine use. When designing a telemedicine system, project managers should prioritize providers’ needs, such as ease of use and incentives.18
States do not cover services provided by other mental health providers, except for Utah’s coverage for social workers. The American Psychiatric Association has 2 suggestions regarding this issue3:
- reimbursement for telepsychiatry services should follow customary charges for delivering the appropriate current procedural terminology code(s)
- a structure for reimbursement of collateral charges, such as technician and line time, should be identified.
Licensure. A physician conducting a telemedicine session with a patient in another state must be licensed in both his or her state and the patient’s state. Nurses and other allied health professionals have similar state licensing constraints. Sanders22 suggests 3 potential solutions:
- establishing a national licensing system
- assigning the responsibility of care to the referring physician, with the consulting physician’s opinion serving as “recommendation only”
- determining that the patient is being “electronically transmitted” to the consultant’s state.
Although telemedicine has embraced many communication technologies, live, interactive, 2-way, audio-video communication—called videoconferencing—is broadly synonymous with telemedicine and, more specifically, telepsychiatry.
Telepsychiatry primarily uses interactive audiovisual conferencing systems over high-bandwidth networks. The central component of interactive telepsychiatry is the codec (coder/decoder), which provides compression, decompression, and synchronization of audio and video signals; both patients and clinicians need a codec. A codec can be a separate device, but personal computer-based codecs are being used more frequently. A typical setup also includes a video camera, microphone, speakers or headset, and 1 or 2 display monitors at both the clinician’s and patient’s end of the system. Often, separate displays or a picture-in-picture display are used to see both outgoing and incoming video. Another consideration is pan-zoom-tilt control of video cameras. This allows clinicians to remotely control his or her view of the patient’s site or control the view being transmitted to the patient.
Historically, interactive telepsychiatry applications have used point-to-point network connections, usually as full or fractional T-1 or integrated services digital network circuits. However, the rapid diffusion of internet and ethernet networks has led to the development of videoconferencing systems that can work over internet protocol (IP) networks. If using an IP network, ensure security by using encrypted codecs or by setting up a virtual private network and/or a virtual local area network (LAN). The principal advantage of IP networks is that by implementing proper security solutions, they can be shared by several applications—eg, internet, e-mail, LAN, etc. This means that the telecommunications network costs can be shared or considered a sunk cost (ie, not an additional cost of the telepsychiatry application).
The U.S. Federal Communications Commission’s (FCC) Universal Service Fund (USF) subsidies can reduce the cost of telepsychiatry network connections. The FCC implemented the USF to bring high bandwidth telecommunications to rural schools, libraries, and health care providers. Funding for the USF is generated from fees paid by telecommunications providers. However, the USF subsidies are not being widely used for several reasons, including a cumbersome application process, limitations on eligible facilities and locations, and questions regarding costs to the health care provider.19
Individual states also have developed funding streams to support telemedicine. The Centers for Medicare and Medicaid Services will pay a facility site fee to the host site (where the patient is located), but only if the site is in a rural area. Providers can charge patients a fee to support telepsychiatry infrastructure and maintenance, but typically this arrangement is not affordable and is not standard practice.
The future
Telepsychiatry’s ability to improve access to mental health care to underserved populations is becoming more evident. Technology is adequate for most uses and is constantly advancing. Numerous applications already have been defined, and more are ripe for exploration. Barriers to implementation are primarily of the human variety and will require a combination of consumer, provider, and governmental advocacy to overcome.
- American Telemedicine Association. www.americantelemed.org.
- International Society for Telemedicine & eHealth. www.isfteh.org.
- American Psychiatric Association. Telepsychiatry internet resources. www.psychiatry.org/practice/professional-interests/underserved-communities/telepsychiatry-internet-resources.
- Mossman D. Practicing psychiatry via Skype: Medicolegal considerations. Current Psychiatry. 2011;10(12):30-32,39.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Although many states have substantial health services in urban areas, these services—particularly mental health care—are relatively scarce in rural areas.1 Telepsychiatry, in which clinicians provide mental health care from a distance in real time by using interactive, 2-way, audio-video communication (videoconferencing), could mitigate workforce shortages that affect remote and underserved areas.2 Psychiatry is one of the biggest users of telemedicine, which refers to any combination of communication technology and medicine.3-5 This article discusses telepsychiatry’s effectiveness in providing psychiatric diagnosis and treatment, and the clinical implications of this technology, including improving access, cost, and quality of mental health services.
Outcomes comparable to face-to-face care
Telepsychiatry is used primarily in rural areas or correctional institutions or with underserved populations such as veterans with posttraumatic stress disorder or children. Although the literature generally is weak, there has been more research on psychiatry than other medical specialties because psychiatric clinicians rely on mental status examinations and verbal communications, not physical exams. Telepsychiatry can be considered a part of an evolving “connected health” system that offers many benefits to patients and clinicians (Table).
Table
Benefits of telepsychiatry as part of a ‘connected health’ system
| Available to everyone |
| Health care is provided at the point of convenience |
| Patients are informed and empowered |
| Facilitates patient compliance, continuing education, ease of access into the health care system, and healthy behaviors |
| Clinical data are integrated with longitudinal electronic health records |
| Data are available to patients via his or her personal electronic medical record and authorized clinical providers |
| Data and transactions are secure to greatest practical extent |
| Other telehealth applications with demonstrated efficacy—eg, telephone, internet, e-mail, and text messaging interventions—can be used as well |
Barriers to implementation
Although telepsychiatry offers tremendous promise, implementation has not been widespread or easy. Potential barriers to implementation, such as cost and resistance to change, are associated with acceptance of new technology or practice in health care. In addition, there are several legal, regulatory, and technical barriers.
Institutional barriers. Physicians and other providers may not have access to timely, evidence-based information and may face challenges, such as time constraints, access to technical support, and complexity of large health care institutions, when integrating this information into clinical practice.16 Two studies17 found that after controlling for other barriers—eg, reimbursement and regulatory issues—providers are the most significant initial gatekeepers that affect telemedicine use. When designing a telemedicine system, project managers should prioritize providers’ needs, such as ease of use and incentives.18
States do not cover services provided by other mental health providers, except for Utah’s coverage for social workers. The American Psychiatric Association has 2 suggestions regarding this issue3:
- reimbursement for telepsychiatry services should follow customary charges for delivering the appropriate current procedural terminology code(s)
- a structure for reimbursement of collateral charges, such as technician and line time, should be identified.
Licensure. A physician conducting a telemedicine session with a patient in another state must be licensed in both his or her state and the patient’s state. Nurses and other allied health professionals have similar state licensing constraints. Sanders22 suggests 3 potential solutions:
- establishing a national licensing system
- assigning the responsibility of care to the referring physician, with the consulting physician’s opinion serving as “recommendation only”
- determining that the patient is being “electronically transmitted” to the consultant’s state.
Although telemedicine has embraced many communication technologies, live, interactive, 2-way, audio-video communication—called videoconferencing—is broadly synonymous with telemedicine and, more specifically, telepsychiatry.
Telepsychiatry primarily uses interactive audiovisual conferencing systems over high-bandwidth networks. The central component of interactive telepsychiatry is the codec (coder/decoder), which provides compression, decompression, and synchronization of audio and video signals; both patients and clinicians need a codec. A codec can be a separate device, but personal computer-based codecs are being used more frequently. A typical setup also includes a video camera, microphone, speakers or headset, and 1 or 2 display monitors at both the clinician’s and patient’s end of the system. Often, separate displays or a picture-in-picture display are used to see both outgoing and incoming video. Another consideration is pan-zoom-tilt control of video cameras. This allows clinicians to remotely control his or her view of the patient’s site or control the view being transmitted to the patient.
Historically, interactive telepsychiatry applications have used point-to-point network connections, usually as full or fractional T-1 or integrated services digital network circuits. However, the rapid diffusion of internet and ethernet networks has led to the development of videoconferencing systems that can work over internet protocol (IP) networks. If using an IP network, ensure security by using encrypted codecs or by setting up a virtual private network and/or a virtual local area network (LAN). The principal advantage of IP networks is that by implementing proper security solutions, they can be shared by several applications—eg, internet, e-mail, LAN, etc. This means that the telecommunications network costs can be shared or considered a sunk cost (ie, not an additional cost of the telepsychiatry application).
The U.S. Federal Communications Commission’s (FCC) Universal Service Fund (USF) subsidies can reduce the cost of telepsychiatry network connections. The FCC implemented the USF to bring high bandwidth telecommunications to rural schools, libraries, and health care providers. Funding for the USF is generated from fees paid by telecommunications providers. However, the USF subsidies are not being widely used for several reasons, including a cumbersome application process, limitations on eligible facilities and locations, and questions regarding costs to the health care provider.19
Individual states also have developed funding streams to support telemedicine. The Centers for Medicare and Medicaid Services will pay a facility site fee to the host site (where the patient is located), but only if the site is in a rural area. Providers can charge patients a fee to support telepsychiatry infrastructure and maintenance, but typically this arrangement is not affordable and is not standard practice.
The future
Telepsychiatry’s ability to improve access to mental health care to underserved populations is becoming more evident. Technology is adequate for most uses and is constantly advancing. Numerous applications already have been defined, and more are ripe for exploration. Barriers to implementation are primarily of the human variety and will require a combination of consumer, provider, and governmental advocacy to overcome.
- American Telemedicine Association. www.americantelemed.org.
- International Society for Telemedicine & eHealth. www.isfteh.org.
- American Psychiatric Association. Telepsychiatry internet resources. www.psychiatry.org/practice/professional-interests/underserved-communities/telepsychiatry-internet-resources.
- Mossman D. Practicing psychiatry via Skype: Medicolegal considerations. Current Psychiatry. 2011;10(12):30-32,39.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. President’s New Freedom Commission on Mental Health. Subcommittee on rural issues: background paper. Rockville MD: Substance Abuse and Mental Health Administration; 2004.
2. Antonacci DJ, Bloch RM, Saeed SA, et al. Empirical evidence on the use and effectiveness of telepsychiatry via videoconferencing: implications for forensic and correctional psychiatry. Behav Sci Law. 2008;26(3):253-269.
3. American Psychiatric Association. Resource document on telepsychiatry via videoconferencing. http://www.psychiatry.med.uwo.ca/ecp/info/toronto/telepsych/Appendix%20II.htm. Accessed November 5 2012.
4. Grigsby J, Rigby M, Hiemstra A, et al. Telemedicine/telehealth: an international perspective. The diffusion of telemedicine. Telemed J E Health. 2002;8(1):79-94.
5. Krupinski E, Nypaver M, Poropatich R, et al. Telemedicine/telehealth: an international perspective. Clinical applications in telemedicine/telehealth. Telemed J E Health. 2002;8(1):13-34.
6. Diamond JM, Bloch RM. Telepsychiatry assessments of child or adolescent behavior disorders: a review of evidence and issues. Telemed J E Health. 2010;16(6):712-716.
7. Buono S, Città S. Tele-assistance in intellectual disability. J Telemed Telecare. 2007;13(5):241-245.
8. Manguno-Mire GM, Thompson JW, Jr, Shore JH, et al. The use of telemedicine to evaluate competency to stand trial: a preliminary randomized controlled study. J Am Acad Psychiatry Law. 2007;35(4):481-489.
9. Fortney JC, Maciejewski ML, Tripathi SP, et al. A budget impact analysis of telemedicine-based collaborative care for depression. Med Care. 2011;49(9):872-880.
10. Pyne JM, Fortney JC, Tripathi SP, et al. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Arch Gen Psychiatry. 2010;67(8):812-821.
11. Morland LA, Greene CJ, Rosen CS, et al. Telemedicine for anger management therapy in a rural population of combat veterans with posttraumatic stress disorder: a randomized noninferiority trial. J Clin Psychiatry. 2010;71(7):855-863.
12. Mitchell JE, Crosby RD, Wonderlich SA, et al. A randomized trial comparing the efficacy of cognitive-behavioral therapy for bulimia nervosa delivered via telemedicine versus face-to-face. Behav Res Ther. 2008;46(5):581-592.
13. Steel K, Cox D, Garry H. Therapeutic videoconferencing interventions for the treatment of long-term conditions. J Telemed Telecare. 2011;17(3):109-117.
14. Greene CJ, Morland LA, Macdonald A, et al. How does tele-mental health affect group therapy process? Secondary analysis of a noninferiority trial. J Consult Clin Psychol. 2010;78(5):746-750.
15. Morland LA, Greene CJ, Grubbs K, et al. Therapist adherence to manualized cognitive-behavioral therapy for anger management delivered to veterans with PTSD via videoconferencing. J Clin Psychol. 2011;67(6):629-638.
16. Saeed SA, Diamond J, Bloch RM. Use of telepsychiatry to improve care for people with mental illness in rural North Carolina. N C Med J. 2011;72(3):219-222.
17. Whitten PS, Mackert MS. Addressing telehealth’s foremost barrier: provider as initial gatekeeper. Int J Technol Assess Health Care. 2005;21(4):517-521.
18. Coleman JR. HMOs and the future of telemedicine and telehealth: part 2. Case Manager. 2002;13(4):38-43.
19. Puskin DS. Telemedicine: follow the money modalities. Online J Issues Nurs. 2001;6(3):2.-
20. American Telemedicine Association. Medicare payment of telemedicine and telehealth services. http://www.americantelemed.org/files/public/membergroups/businessfinance/reimbursement/
BF_MedicarePaymentofTelemedicine.pdf. Published May 15 2006. Accessed November 5, 2012.
21. Fishbein M. Developing effective behavior change interventions: some lessons learned from behavioral research. NIDA Res Monogr. 1995;155:246-261.
22. Sanders JH. Telemedicine: challenges to implementation. Paper presented at: Rural Telemedicine Workshop; November 4 1993; Washington, DC.
23. Kumekawa JK. Health information privacy protection: crisis or common sense? Online J Issues Nurs. 2001;6(3):3.-
1. President’s New Freedom Commission on Mental Health. Subcommittee on rural issues: background paper. Rockville MD: Substance Abuse and Mental Health Administration; 2004.
2. Antonacci DJ, Bloch RM, Saeed SA, et al. Empirical evidence on the use and effectiveness of telepsychiatry via videoconferencing: implications for forensic and correctional psychiatry. Behav Sci Law. 2008;26(3):253-269.
3. American Psychiatric Association. Resource document on telepsychiatry via videoconferencing. http://www.psychiatry.med.uwo.ca/ecp/info/toronto/telepsych/Appendix%20II.htm. Accessed November 5 2012.
4. Grigsby J, Rigby M, Hiemstra A, et al. Telemedicine/telehealth: an international perspective. The diffusion of telemedicine. Telemed J E Health. 2002;8(1):79-94.
5. Krupinski E, Nypaver M, Poropatich R, et al. Telemedicine/telehealth: an international perspective. Clinical applications in telemedicine/telehealth. Telemed J E Health. 2002;8(1):13-34.
6. Diamond JM, Bloch RM. Telepsychiatry assessments of child or adolescent behavior disorders: a review of evidence and issues. Telemed J E Health. 2010;16(6):712-716.
7. Buono S, Città S. Tele-assistance in intellectual disability. J Telemed Telecare. 2007;13(5):241-245.
8. Manguno-Mire GM, Thompson JW, Jr, Shore JH, et al. The use of telemedicine to evaluate competency to stand trial: a preliminary randomized controlled study. J Am Acad Psychiatry Law. 2007;35(4):481-489.
9. Fortney JC, Maciejewski ML, Tripathi SP, et al. A budget impact analysis of telemedicine-based collaborative care for depression. Med Care. 2011;49(9):872-880.
10. Pyne JM, Fortney JC, Tripathi SP, et al. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Arch Gen Psychiatry. 2010;67(8):812-821.
11. Morland LA, Greene CJ, Rosen CS, et al. Telemedicine for anger management therapy in a rural population of combat veterans with posttraumatic stress disorder: a randomized noninferiority trial. J Clin Psychiatry. 2010;71(7):855-863.
12. Mitchell JE, Crosby RD, Wonderlich SA, et al. A randomized trial comparing the efficacy of cognitive-behavioral therapy for bulimia nervosa delivered via telemedicine versus face-to-face. Behav Res Ther. 2008;46(5):581-592.
13. Steel K, Cox D, Garry H. Therapeutic videoconferencing interventions for the treatment of long-term conditions. J Telemed Telecare. 2011;17(3):109-117.
14. Greene CJ, Morland LA, Macdonald A, et al. How does tele-mental health affect group therapy process? Secondary analysis of a noninferiority trial. J Consult Clin Psychol. 2010;78(5):746-750.
15. Morland LA, Greene CJ, Grubbs K, et al. Therapist adherence to manualized cognitive-behavioral therapy for anger management delivered to veterans with PTSD via videoconferencing. J Clin Psychol. 2011;67(6):629-638.
16. Saeed SA, Diamond J, Bloch RM. Use of telepsychiatry to improve care for people with mental illness in rural North Carolina. N C Med J. 2011;72(3):219-222.
17. Whitten PS, Mackert MS. Addressing telehealth’s foremost barrier: provider as initial gatekeeper. Int J Technol Assess Health Care. 2005;21(4):517-521.
18. Coleman JR. HMOs and the future of telemedicine and telehealth: part 2. Case Manager. 2002;13(4):38-43.
19. Puskin DS. Telemedicine: follow the money modalities. Online J Issues Nurs. 2001;6(3):2.-
20. American Telemedicine Association. Medicare payment of telemedicine and telehealth services. http://www.americantelemed.org/files/public/membergroups/businessfinance/reimbursement/
BF_MedicarePaymentofTelemedicine.pdf. Published May 15 2006. Accessed November 5, 2012.
21. Fishbein M. Developing effective behavior change interventions: some lessons learned from behavioral research. NIDA Res Monogr. 1995;155:246-261.
22. Sanders JH. Telemedicine: challenges to implementation. Paper presented at: Rural Telemedicine Workshop; November 4 1993; Washington, DC.
23. Kumekawa JK. Health information privacy protection: crisis or common sense? Online J Issues Nurs. 2001;6(3):3.-
Monoamine oxidase inhibitors: Forgotten treatment for depression
Discuss this article at www.facebook.com/CurrentPsychiatry
For patients with major depressive disorder (MDD), monoamine oxidase inhibitors (MAOIs) have efficacy comparable to that of other antidepressants. However, concerns about side effects—particularly hypertensive crisis—and drug-drug interactions have led clinicians to prescribe MAOIs less often than newer antidepressants. A 1999 survey of 573 Michigan psychiatrists found that 30% had not prescribed an MAOI within the past 3 years, and 12% had never prescribed an MAOI.1 Although there are challenges to using these agents, we prefer prescribing MAOIs to depressed patients who have not responded to previous antidepressant trials over trying untested antidepressant combinations.
Currently, MAOIs are used primarily for patients who have not responded to other antidepressant trials and are considered treatment resistant. Treatment-resistant depression (TRD) typically is defined as nonresponse to ≥3 adequate antidepressant trials. TRD is a major cause of disability and loss of productivity. These patients tend to do poorly over the long term, with high rates of hospitalization and suicide attempts. Several controlled trials have shown that patients who fail other antidepressants may respond to MAOIs.2-4
Our knowledge regarding MAOIs has grown considerably. We have learned more about depression subtypes that MAOIs may help. As we learned more about dietary restrictions for patients taking MAOIs, the list of “forbidden foods” has decreased. Advances in treating a hypertensive crisis have decreased the need for hospitalization. By educating ourselves and our patients about MAOIs, we can provide another option for treating MDD.
An older antidepressant class
MAOIs were introduced approximately 60 years ago. Their potential for treating depression was discovered when a tuberculosis treatment—iproniazid—was found to reduce depressive symptoms. Researchers determined iproniazid’s antidepressant effects were the result of blocking removal of the amine group by monoamine oxidase (MAO) from dopamine, norepinephrine, and serotonin.5 A second MAOI, tranylcypromine, was discovered when it was found to be ineffective for treating decongestion.6
MAOI use in psychiatric practice has undergone significant changes since these medications were introduced. The discovery of hypertensive crises related to tyramine consumption led to decreased MAOI use, as did the rise of tricyclic antidepressants (TCAs) shortly thereafter. In the 1960s, research compared the relative efficacy of MAOIs to TCAs, and they became second-line antidepressants after the TCAs. In the late 1980s, the introduction of fluoxetine and other selective serotonin reuptake inhibitors (SSRIs) resulted in a significant drop-off in MAOI use.
Pharmacologic effects
MAO is a class of enzymes that initiate oxidation of extracellular neurotransmitters such as serotonin, norepinephrine, and dopamine. MAOIs can be classified based on their relative affinity to MAO as well as their enzyme selectivity. The first distinguishing characteristic is whether the drug binds to MAO in a reversible or irreversible manner. Currently, all MAOIs that are FDA-approved for treating depression bind irreversibly to MAO. As a result, the body must renew its MAO levels before a patient is no longer at risk for a hypertensive crisis, a process that may take up to 2 weeks. Clinicians must take care to ensure their patients avoid foods that contain tyramine and medications contraindicated with MAOIs during this period.
MAOIs also differ from each other in enzyme selectivity. There are 2 subtypes of MAO enzymes—MAOA and MAOB. Generally, the antidepressant activity of MAOIs appears to be directed toward MAOA inhibition. MAOA has been found to be more specific for binding to serotonin and norepinephrine and MAOB to be more specific for phenylethylamine. Dopamine is equally deaminated by both MAOA and MAOB.
Reversible MAOA inhibitors require fewer restrictions on diet or concurrent medications, but efficacy data of reversible MAOA inhibitors is mixed.
Clinical use of MAOIs
Four MAOIs are available in the United States: tranylcypromine, phenelzine, isocarboxazid, and selegiline. Selegiline is the only MAOI available as a transdermal patch. Transdermal administration results in fewer effects on MAO in the gastrointestinal tract, which means no dietary restrictions at the 6 mg/d starting dose, although the manufacturer recommends patients follow the MAO diet at 9 mg/d and 12 mg/d doses.7 Although selegiline is selective for MAOB at low doses, it becomes nonselective at therapeutic doses for depression. Recommended dosages for MAOIs can be found in Table 1.8
Table 1
Recommended dosages of monoamine oxidase inhibitors
| Medication | Starting dosages | Usual therapeutic dosage |
|---|---|---|
| Isocarboxazid | 10 mg twice a day | 30 to 60 mg/d |
| Phenelzine | 15 mg twice a day | 45 to 90 mg/d |
| Selegiline transdermal | 6 mg patch/d | 6 to 12 mg patch/d |
| Tranylcypromine | 10 mg, 2 or 3 times a day | 30 to 60 mg/d |
| Source: Adapted from reference 8 | ||
10,11
Several controlled trials have found a better response rate to MAOI therapy in outpatients with MDD who have not responded to other antidepressants.2,12 In a 6-week, double-blind trial, Vallejo et al10 reported that the TCA imipramine and high-dose phenelzine were equally efficacious in 32 patients with major depression with melancholia. In 32 dysthymic patients, high-dose phenelzine was superior to imipramine. Himmelhoch et al13 compared efficacy of tranylcypromine with that of imipramine in treating anergic bipolar depressive illness. Patients receiving tranylcypromine experienced significantly greater symptomatic improvement and higher global response without increased risk of treatment-emergent hypomania or mania.
Serum monitoring of MAOIs is not clinically indicated and there are no correlations between drug levels and effectiveness.14 In a study that examined the correlation of inhibiting platelet MAO and MAOIs’ antidepressant effects, researchers found that a higher dose of phenelzine (60 mg/d) was significantly better in treating depression and anxiety than a lower dose (30 mg/d), and only the higher dose achieved 80% of platelet MAO inhibition.15 Further studies with other MAOIs did not reproduce this effect and platelet MAO inhibition is not regularly used to assess adequate dosing.
A refined view of side effects
In a hypertensive crisis, patients experience significant hypertension, headaches, tachycardia, diaphoresis, and vomiting. Intravenous phentolamine—an α-adrenergic receptor blocker—can be used as an antidote; often a single dose is effective.16 Alternatively, calcium channel blockers such as nifedipine can be prescribed. A patient can take 10 mg/hour and be observed in the emergency room until symptoms are relieved (usually only 1 or 2 doses are needed) without being admitted to the hospital.
Table 2
Food restrictions with MAOIs
| Severe |
| Aged cheeses Aged meats (pepperoni, sausage, salami) Sauerkraut Soy sauce Fava or broad bean pods Banana peels All beers on tap |
| Use in moderation (≤2 servings/d) |
| Red wine (4 oz) White wine (4 oz) Bottled or canned beers (12 oz) |
| Mild to none |
| Avocados Banana pulp Bouillon Chocolate Fresh cheeses (cottage cheese, cream cheese, processed cheese slices) Fresh or processed meat |
| MAOIs: monoamine oxidase inhibitors Source: Adapted from references 4,17,18 |
Orthostatic hypotension is a common cardiovascular side effect of MAOIs that may lead to dizziness or syncope. Typically this is seen 2 to 3 weeks after initiating MAOI treatment. If hypotension remains a problem, mineralocorticoids can be prescribed with monitoring of serum potassium for hypokalemia. Increasing doses of tranylcypromine can increase supine—but not standing—diastolic blood pressure.19 Distinguish this type of blood pressure elevation from a hypertensive crisis by monitoring blood pressure with the patient sitting and standing and before and after he or she walks for 60 seconds.
Insomnia and day-night shifting—sleeping during the day and staying awake at night—are common and patients often cite these as reasons for discontinuing MAOIs. Many patients who respond to MAOIs report periods of substantial sleepiness in the mid to late afternoon. Table 320 provides a more complete list of reported side effects and their frequencies.
Table 3
MAOIs: Stay vigilant for side effects
| Medication | Common side effects |
|---|---|
| Isocarboxazid | Anxiety, blurred vision, constipation, dizziness, headache, insomnia, mania, somnolence, weight gain, xerostomia |
| Phenelzine | Constipation, disorder of ejaculation and/or orgasm, dizziness, edema, fatigue, headache, hyperreflexia, impotence, elevated values on liver function tests, orthostatic hypotension, sleep disorders, somnolence, tremor, weight gain, xerostomia |
| Selegiline transdermal | Application site reaction, decreased systolic blood pressure, diarrhea, headache, indigestion, insomnia, orthostatic hypotension, weight loss, xerostomia |
| Tranylcypromine | Agitation, anxiety, constipation, diarrhea, dizziness, headache, impotence, insomnia, loss of appetite, mania, nausea, orthostatic hypotension, somnolence, weight gain, xerostomia |
| MAOIs: monoamine oxidase inhibitors Source: Adapted from reference 20 | |
Practice guidelines
The American Psychiatric Association’s practice guidelines for treating major depression state that MAOIs are effective in treating subgroups of patients with MDD with atypical features who have failed TCA trials.21 These guidelines also state that MAOIs have been shown to be effective treatment for some patients who have failed other antidepressants. However, for TRD patients who have not responded to SSRIs or SNRIs, the effectiveness of MAOIs compared with other strategies is unclear.22
MAOIs have been used for >6 decades, and published studies continue to document their efficacy and safety when patients are monitored appropriately.11,12,14,15,25 However, based on our observations we suspect MAOIs are underutilized in clinical practice today. We are concerned that such practices can trickle down into residency training programs. Psychiatric residents typically do not receive adequate training in prescribing MAOIs, largely because many training faculty are not prescribing MAOIs themselves. Despite MAOIs’ limitations, concerns about an increased risk of suicide in patients with TRD26 and the high likelihood of a poor outcome associated with persistent nonresponse to prior treatments must be weighed against the relatively low risk of a hypertensive event with MAOIs.6
- McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. Journal of Food Composition and Analysis. 2006;19(suppl):S58-S65.
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
- Clomipramine • Anafranil
- Epinephrine • Adrenalin, EpiPen
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Isocarboxazid • Marplan
- Lithium • Eskalith, Lithobid
- Nifedipine • Adalat, Afeditab
- Phenelzine • Nardil
- Phentolamine • OraVerse, Regitine
- Selegiline • EMSAM
- Tranylcypromine • Parnate
Dr. Kosinski reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rothschild receives grant or research support from Cyberonics, the National Institute of Mental Health, St. Jude Medical, and Takeda, and is a consultant to Allergan, Eli Lilly and Company, GlaxoSmithKline, Noven Pharmaceuticals, Pfizer Inc., Shire Pharmaceuticals, and Sunovion.
1. Balon R, Mufti R, Arfken CL. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatr Serv. 1999;50(7):945-947.
2. Nolen WA, van de Putte JJ, Dijken WA, et al. Treatment strategy in depression. II. MAO inhibitors in depression resistant to cyclic antidepressants: two controlled crossover studies with tranylcypromine versus L-5-hydroxytryptophan and nomifensine. Acta Psychiatr Scand. 1988;78(6):676-683.
3. McGrath PJ, Stewart JW, Harrison W, et al. Treatment of tricyclic refractory depression with a monoamine oxidase inhibitor antidepressant. Psychopharmacol Bull. 1987;23(1):169-172.
4. Amsterdam JD. Monoamine oxidase inhibitor therapy in severe and resistant depression. Psychiatr Ann. 2006;36(9):607-613.
5. Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122(5):509-522.
6. Kennedy SH, Holt A, Baker GB. Monoamine oxidase inhibitors. In: Sadock BJ Sadock VA, eds. Kaplan and Sadock’s comprehensive textbook of psychiatry. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005: 1076–1080.
7. EMSAM [package insert]. Napa CA: Dey Pharm LP; 2011.
8. Amsterdam JD, Chopra M. Monoamine oxidase inhibitors revisited. Psychiatric Ann. 2001;31(6):361-370.
9. Quitkin FM, Stewart JW, McGrath PJ, et al. Phenelzine versus imipramine in the treatment of probable atypical depression: defining syndrome boundaries of selective MAOI responders. Am J Psychiatry. 1988;145(3):306-311.
10. Vallejo J, Gasto C, Catalan R, et al. Double-blind study of imipramine versus phenelzine in melancholias and dysthymic disorders. Br J Psychiatry. 1987;151:639-642.
11. White K, Razani J, Cadow B, et al. Trancylpromine vs. nortriptyline vs. placebo in depressed outpatients: a controlled trial. Psychopharmacology (Berl). 1984;82(3):258-262.
12. Thase ME, Frank E, Mallinger AG, et al. Treatment of imipramine-resistant recurrent depression, III: efficacy of monoamine oxidase inhibitors. J Clin Psychiatry. 1992;53(1):5-11.
13. Himmelhoch JM, Thase ME, Mallinger AG, et al. Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry. 1991;148(7):910-916.
14. Rothschild AJ. ed. The evidence-based guide to antidepressant medications. Arlington, VA: American Psychiatric Publishing, Inc.; 2012:15–20.
15. Ravaris CL, Nies A, Robinson DS, et al. A multiple-dose, controlled study of phenelzine in depression-anxiety states. Arch Gen Psychiatry. 1976;33(3):347-350.
16. Cockhill LA, Remick RA. Blood pressure effects of monoamine oxidase inhibitors—the highs and lows. Can J Psychiatry. 1987;32(9):803-808.
17. Shulman KI, Walker SE. A reevaluation of dietary restrictions for irreversible monoamine oxidase inhibitors. Psychiatr Ann. 2001;31(6):378-384.
18. Gardner DM, Shulman KI, Walker SE, et al. The making of a user friendly MAOI diet. J Clin Psychiatry. 1996;57(3):99-104.
19. Keck PE, Jr, Carter WP, Nierenberg AA, et al. Acute cardiovascular effects of tranylcypromine: correlation with plasma drug, metabolite, norepinephrine, and MHPG levels. J Clin Psychiatry. 1991;52(6):250-254.
20. Micromedex Healthcare Series [UMass Memorial Healthcare Intranet System]. Version 5.1. Greenwood Village CO: Thomson Reuters (Healthcare) Inc.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder third edition. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1667485. Published October 2010. Accessed October 26, 2012.
22. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1541; quiz 1666.
23. Nelson JC, Byck R. Rapid response to lithium in phenelzine non-responders. Br J Psychiatry. 1982;141:85-86.
24. Guze BH, Baxter LR, Jr, Rego J. Refractory depression treated with high doses of monoamine oxidase inhibitor. J Clin Psychiatry. 1987;48(1):31-32.
25. Robinson DS, Gilmor ML, Yang Y, et al. Treatment effects of selegiline transdermal system on symptoms of major depressive disorder: a meta analysis of short term, placebo controlled, efficacy trials. Psychopharmacol Bull. 2007;40(3):15-28.
26. Keller MB, Lavori PW, Rice J, et al. The persistent risk of chronicity in recurrent episodes of nonbipolar major depressive disorder: a prospective follow-up. Am J Psychiatry. 1986;143(1):24-28.
Discuss this article at www.facebook.com/CurrentPsychiatry
For patients with major depressive disorder (MDD), monoamine oxidase inhibitors (MAOIs) have efficacy comparable to that of other antidepressants. However, concerns about side effects—particularly hypertensive crisis—and drug-drug interactions have led clinicians to prescribe MAOIs less often than newer antidepressants. A 1999 survey of 573 Michigan psychiatrists found that 30% had not prescribed an MAOI within the past 3 years, and 12% had never prescribed an MAOI.1 Although there are challenges to using these agents, we prefer prescribing MAOIs to depressed patients who have not responded to previous antidepressant trials over trying untested antidepressant combinations.
Currently, MAOIs are used primarily for patients who have not responded to other antidepressant trials and are considered treatment resistant. Treatment-resistant depression (TRD) typically is defined as nonresponse to ≥3 adequate antidepressant trials. TRD is a major cause of disability and loss of productivity. These patients tend to do poorly over the long term, with high rates of hospitalization and suicide attempts. Several controlled trials have shown that patients who fail other antidepressants may respond to MAOIs.2-4
Our knowledge regarding MAOIs has grown considerably. We have learned more about depression subtypes that MAOIs may help. As we learned more about dietary restrictions for patients taking MAOIs, the list of “forbidden foods” has decreased. Advances in treating a hypertensive crisis have decreased the need for hospitalization. By educating ourselves and our patients about MAOIs, we can provide another option for treating MDD.
An older antidepressant class
MAOIs were introduced approximately 60 years ago. Their potential for treating depression was discovered when a tuberculosis treatment—iproniazid—was found to reduce depressive symptoms. Researchers determined iproniazid’s antidepressant effects were the result of blocking removal of the amine group by monoamine oxidase (MAO) from dopamine, norepinephrine, and serotonin.5 A second MAOI, tranylcypromine, was discovered when it was found to be ineffective for treating decongestion.6
MAOI use in psychiatric practice has undergone significant changes since these medications were introduced. The discovery of hypertensive crises related to tyramine consumption led to decreased MAOI use, as did the rise of tricyclic antidepressants (TCAs) shortly thereafter. In the 1960s, research compared the relative efficacy of MAOIs to TCAs, and they became second-line antidepressants after the TCAs. In the late 1980s, the introduction of fluoxetine and other selective serotonin reuptake inhibitors (SSRIs) resulted in a significant drop-off in MAOI use.
Pharmacologic effects
MAO is a class of enzymes that initiate oxidation of extracellular neurotransmitters such as serotonin, norepinephrine, and dopamine. MAOIs can be classified based on their relative affinity to MAO as well as their enzyme selectivity. The first distinguishing characteristic is whether the drug binds to MAO in a reversible or irreversible manner. Currently, all MAOIs that are FDA-approved for treating depression bind irreversibly to MAO. As a result, the body must renew its MAO levels before a patient is no longer at risk for a hypertensive crisis, a process that may take up to 2 weeks. Clinicians must take care to ensure their patients avoid foods that contain tyramine and medications contraindicated with MAOIs during this period.
MAOIs also differ from each other in enzyme selectivity. There are 2 subtypes of MAO enzymes—MAOA and MAOB. Generally, the antidepressant activity of MAOIs appears to be directed toward MAOA inhibition. MAOA has been found to be more specific for binding to serotonin and norepinephrine and MAOB to be more specific for phenylethylamine. Dopamine is equally deaminated by both MAOA and MAOB.
Reversible MAOA inhibitors require fewer restrictions on diet or concurrent medications, but efficacy data of reversible MAOA inhibitors is mixed.
Clinical use of MAOIs
Four MAOIs are available in the United States: tranylcypromine, phenelzine, isocarboxazid, and selegiline. Selegiline is the only MAOI available as a transdermal patch. Transdermal administration results in fewer effects on MAO in the gastrointestinal tract, which means no dietary restrictions at the 6 mg/d starting dose, although the manufacturer recommends patients follow the MAO diet at 9 mg/d and 12 mg/d doses.7 Although selegiline is selective for MAOB at low doses, it becomes nonselective at therapeutic doses for depression. Recommended dosages for MAOIs can be found in Table 1.8
Table 1
Recommended dosages of monoamine oxidase inhibitors
| Medication | Starting dosages | Usual therapeutic dosage |
|---|---|---|
| Isocarboxazid | 10 mg twice a day | 30 to 60 mg/d |
| Phenelzine | 15 mg twice a day | 45 to 90 mg/d |
| Selegiline transdermal | 6 mg patch/d | 6 to 12 mg patch/d |
| Tranylcypromine | 10 mg, 2 or 3 times a day | 30 to 60 mg/d |
| Source: Adapted from reference 8 | ||
10,11
Several controlled trials have found a better response rate to MAOI therapy in outpatients with MDD who have not responded to other antidepressants.2,12 In a 6-week, double-blind trial, Vallejo et al10 reported that the TCA imipramine and high-dose phenelzine were equally efficacious in 32 patients with major depression with melancholia. In 32 dysthymic patients, high-dose phenelzine was superior to imipramine. Himmelhoch et al13 compared efficacy of tranylcypromine with that of imipramine in treating anergic bipolar depressive illness. Patients receiving tranylcypromine experienced significantly greater symptomatic improvement and higher global response without increased risk of treatment-emergent hypomania or mania.
Serum monitoring of MAOIs is not clinically indicated and there are no correlations between drug levels and effectiveness.14 In a study that examined the correlation of inhibiting platelet MAO and MAOIs’ antidepressant effects, researchers found that a higher dose of phenelzine (60 mg/d) was significantly better in treating depression and anxiety than a lower dose (30 mg/d), and only the higher dose achieved 80% of platelet MAO inhibition.15 Further studies with other MAOIs did not reproduce this effect and platelet MAO inhibition is not regularly used to assess adequate dosing.
A refined view of side effects
In a hypertensive crisis, patients experience significant hypertension, headaches, tachycardia, diaphoresis, and vomiting. Intravenous phentolamine—an α-adrenergic receptor blocker—can be used as an antidote; often a single dose is effective.16 Alternatively, calcium channel blockers such as nifedipine can be prescribed. A patient can take 10 mg/hour and be observed in the emergency room until symptoms are relieved (usually only 1 or 2 doses are needed) without being admitted to the hospital.
Table 2
Food restrictions with MAOIs
| Severe |
| Aged cheeses Aged meats (pepperoni, sausage, salami) Sauerkraut Soy sauce Fava or broad bean pods Banana peels All beers on tap |
| Use in moderation (≤2 servings/d) |
| Red wine (4 oz) White wine (4 oz) Bottled or canned beers (12 oz) |
| Mild to none |
| Avocados Banana pulp Bouillon Chocolate Fresh cheeses (cottage cheese, cream cheese, processed cheese slices) Fresh or processed meat |
| MAOIs: monoamine oxidase inhibitors Source: Adapted from references 4,17,18 |
Orthostatic hypotension is a common cardiovascular side effect of MAOIs that may lead to dizziness or syncope. Typically this is seen 2 to 3 weeks after initiating MAOI treatment. If hypotension remains a problem, mineralocorticoids can be prescribed with monitoring of serum potassium for hypokalemia. Increasing doses of tranylcypromine can increase supine—but not standing—diastolic blood pressure.19 Distinguish this type of blood pressure elevation from a hypertensive crisis by monitoring blood pressure with the patient sitting and standing and before and after he or she walks for 60 seconds.
Insomnia and day-night shifting—sleeping during the day and staying awake at night—are common and patients often cite these as reasons for discontinuing MAOIs. Many patients who respond to MAOIs report periods of substantial sleepiness in the mid to late afternoon. Table 320 provides a more complete list of reported side effects and their frequencies.
Table 3
MAOIs: Stay vigilant for side effects
| Medication | Common side effects |
|---|---|
| Isocarboxazid | Anxiety, blurred vision, constipation, dizziness, headache, insomnia, mania, somnolence, weight gain, xerostomia |
| Phenelzine | Constipation, disorder of ejaculation and/or orgasm, dizziness, edema, fatigue, headache, hyperreflexia, impotence, elevated values on liver function tests, orthostatic hypotension, sleep disorders, somnolence, tremor, weight gain, xerostomia |
| Selegiline transdermal | Application site reaction, decreased systolic blood pressure, diarrhea, headache, indigestion, insomnia, orthostatic hypotension, weight loss, xerostomia |
| Tranylcypromine | Agitation, anxiety, constipation, diarrhea, dizziness, headache, impotence, insomnia, loss of appetite, mania, nausea, orthostatic hypotension, somnolence, weight gain, xerostomia |
| MAOIs: monoamine oxidase inhibitors Source: Adapted from reference 20 | |
Practice guidelines
The American Psychiatric Association’s practice guidelines for treating major depression state that MAOIs are effective in treating subgroups of patients with MDD with atypical features who have failed TCA trials.21 These guidelines also state that MAOIs have been shown to be effective treatment for some patients who have failed other antidepressants. However, for TRD patients who have not responded to SSRIs or SNRIs, the effectiveness of MAOIs compared with other strategies is unclear.22
MAOIs have been used for >6 decades, and published studies continue to document their efficacy and safety when patients are monitored appropriately.11,12,14,15,25 However, based on our observations we suspect MAOIs are underutilized in clinical practice today. We are concerned that such practices can trickle down into residency training programs. Psychiatric residents typically do not receive adequate training in prescribing MAOIs, largely because many training faculty are not prescribing MAOIs themselves. Despite MAOIs’ limitations, concerns about an increased risk of suicide in patients with TRD26 and the high likelihood of a poor outcome associated with persistent nonresponse to prior treatments must be weighed against the relatively low risk of a hypertensive event with MAOIs.6
- McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. Journal of Food Composition and Analysis. 2006;19(suppl):S58-S65.
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
- Clomipramine • Anafranil
- Epinephrine • Adrenalin, EpiPen
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Isocarboxazid • Marplan
- Lithium • Eskalith, Lithobid
- Nifedipine • Adalat, Afeditab
- Phenelzine • Nardil
- Phentolamine • OraVerse, Regitine
- Selegiline • EMSAM
- Tranylcypromine • Parnate
Dr. Kosinski reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rothschild receives grant or research support from Cyberonics, the National Institute of Mental Health, St. Jude Medical, and Takeda, and is a consultant to Allergan, Eli Lilly and Company, GlaxoSmithKline, Noven Pharmaceuticals, Pfizer Inc., Shire Pharmaceuticals, and Sunovion.
Discuss this article at www.facebook.com/CurrentPsychiatry
For patients with major depressive disorder (MDD), monoamine oxidase inhibitors (MAOIs) have efficacy comparable to that of other antidepressants. However, concerns about side effects—particularly hypertensive crisis—and drug-drug interactions have led clinicians to prescribe MAOIs less often than newer antidepressants. A 1999 survey of 573 Michigan psychiatrists found that 30% had not prescribed an MAOI within the past 3 years, and 12% had never prescribed an MAOI.1 Although there are challenges to using these agents, we prefer prescribing MAOIs to depressed patients who have not responded to previous antidepressant trials over trying untested antidepressant combinations.
Currently, MAOIs are used primarily for patients who have not responded to other antidepressant trials and are considered treatment resistant. Treatment-resistant depression (TRD) typically is defined as nonresponse to ≥3 adequate antidepressant trials. TRD is a major cause of disability and loss of productivity. These patients tend to do poorly over the long term, with high rates of hospitalization and suicide attempts. Several controlled trials have shown that patients who fail other antidepressants may respond to MAOIs.2-4
Our knowledge regarding MAOIs has grown considerably. We have learned more about depression subtypes that MAOIs may help. As we learned more about dietary restrictions for patients taking MAOIs, the list of “forbidden foods” has decreased. Advances in treating a hypertensive crisis have decreased the need for hospitalization. By educating ourselves and our patients about MAOIs, we can provide another option for treating MDD.
An older antidepressant class
MAOIs were introduced approximately 60 years ago. Their potential for treating depression was discovered when a tuberculosis treatment—iproniazid—was found to reduce depressive symptoms. Researchers determined iproniazid’s antidepressant effects were the result of blocking removal of the amine group by monoamine oxidase (MAO) from dopamine, norepinephrine, and serotonin.5 A second MAOI, tranylcypromine, was discovered when it was found to be ineffective for treating decongestion.6
MAOI use in psychiatric practice has undergone significant changes since these medications were introduced. The discovery of hypertensive crises related to tyramine consumption led to decreased MAOI use, as did the rise of tricyclic antidepressants (TCAs) shortly thereafter. In the 1960s, research compared the relative efficacy of MAOIs to TCAs, and they became second-line antidepressants after the TCAs. In the late 1980s, the introduction of fluoxetine and other selective serotonin reuptake inhibitors (SSRIs) resulted in a significant drop-off in MAOI use.
Pharmacologic effects
MAO is a class of enzymes that initiate oxidation of extracellular neurotransmitters such as serotonin, norepinephrine, and dopamine. MAOIs can be classified based on their relative affinity to MAO as well as their enzyme selectivity. The first distinguishing characteristic is whether the drug binds to MAO in a reversible or irreversible manner. Currently, all MAOIs that are FDA-approved for treating depression bind irreversibly to MAO. As a result, the body must renew its MAO levels before a patient is no longer at risk for a hypertensive crisis, a process that may take up to 2 weeks. Clinicians must take care to ensure their patients avoid foods that contain tyramine and medications contraindicated with MAOIs during this period.
MAOIs also differ from each other in enzyme selectivity. There are 2 subtypes of MAO enzymes—MAOA and MAOB. Generally, the antidepressant activity of MAOIs appears to be directed toward MAOA inhibition. MAOA has been found to be more specific for binding to serotonin and norepinephrine and MAOB to be more specific for phenylethylamine. Dopamine is equally deaminated by both MAOA and MAOB.
Reversible MAOA inhibitors require fewer restrictions on diet or concurrent medications, but efficacy data of reversible MAOA inhibitors is mixed.
Clinical use of MAOIs
Four MAOIs are available in the United States: tranylcypromine, phenelzine, isocarboxazid, and selegiline. Selegiline is the only MAOI available as a transdermal patch. Transdermal administration results in fewer effects on MAO in the gastrointestinal tract, which means no dietary restrictions at the 6 mg/d starting dose, although the manufacturer recommends patients follow the MAO diet at 9 mg/d and 12 mg/d doses.7 Although selegiline is selective for MAOB at low doses, it becomes nonselective at therapeutic doses for depression. Recommended dosages for MAOIs can be found in Table 1.8
Table 1
Recommended dosages of monoamine oxidase inhibitors
| Medication | Starting dosages | Usual therapeutic dosage |
|---|---|---|
| Isocarboxazid | 10 mg twice a day | 30 to 60 mg/d |
| Phenelzine | 15 mg twice a day | 45 to 90 mg/d |
| Selegiline transdermal | 6 mg patch/d | 6 to 12 mg patch/d |
| Tranylcypromine | 10 mg, 2 or 3 times a day | 30 to 60 mg/d |
| Source: Adapted from reference 8 | ||
10,11
Several controlled trials have found a better response rate to MAOI therapy in outpatients with MDD who have not responded to other antidepressants.2,12 In a 6-week, double-blind trial, Vallejo et al10 reported that the TCA imipramine and high-dose phenelzine were equally efficacious in 32 patients with major depression with melancholia. In 32 dysthymic patients, high-dose phenelzine was superior to imipramine. Himmelhoch et al13 compared efficacy of tranylcypromine with that of imipramine in treating anergic bipolar depressive illness. Patients receiving tranylcypromine experienced significantly greater symptomatic improvement and higher global response without increased risk of treatment-emergent hypomania or mania.
Serum monitoring of MAOIs is not clinically indicated and there are no correlations between drug levels and effectiveness.14 In a study that examined the correlation of inhibiting platelet MAO and MAOIs’ antidepressant effects, researchers found that a higher dose of phenelzine (60 mg/d) was significantly better in treating depression and anxiety than a lower dose (30 mg/d), and only the higher dose achieved 80% of platelet MAO inhibition.15 Further studies with other MAOIs did not reproduce this effect and platelet MAO inhibition is not regularly used to assess adequate dosing.
A refined view of side effects
In a hypertensive crisis, patients experience significant hypertension, headaches, tachycardia, diaphoresis, and vomiting. Intravenous phentolamine—an α-adrenergic receptor blocker—can be used as an antidote; often a single dose is effective.16 Alternatively, calcium channel blockers such as nifedipine can be prescribed. A patient can take 10 mg/hour and be observed in the emergency room until symptoms are relieved (usually only 1 or 2 doses are needed) without being admitted to the hospital.
Table 2
Food restrictions with MAOIs
| Severe |
| Aged cheeses Aged meats (pepperoni, sausage, salami) Sauerkraut Soy sauce Fava or broad bean pods Banana peels All beers on tap |
| Use in moderation (≤2 servings/d) |
| Red wine (4 oz) White wine (4 oz) Bottled or canned beers (12 oz) |
| Mild to none |
| Avocados Banana pulp Bouillon Chocolate Fresh cheeses (cottage cheese, cream cheese, processed cheese slices) Fresh or processed meat |
| MAOIs: monoamine oxidase inhibitors Source: Adapted from references 4,17,18 |
Orthostatic hypotension is a common cardiovascular side effect of MAOIs that may lead to dizziness or syncope. Typically this is seen 2 to 3 weeks after initiating MAOI treatment. If hypotension remains a problem, mineralocorticoids can be prescribed with monitoring of serum potassium for hypokalemia. Increasing doses of tranylcypromine can increase supine—but not standing—diastolic blood pressure.19 Distinguish this type of blood pressure elevation from a hypertensive crisis by monitoring blood pressure with the patient sitting and standing and before and after he or she walks for 60 seconds.
Insomnia and day-night shifting—sleeping during the day and staying awake at night—are common and patients often cite these as reasons for discontinuing MAOIs. Many patients who respond to MAOIs report periods of substantial sleepiness in the mid to late afternoon. Table 320 provides a more complete list of reported side effects and their frequencies.
Table 3
MAOIs: Stay vigilant for side effects
| Medication | Common side effects |
|---|---|
| Isocarboxazid | Anxiety, blurred vision, constipation, dizziness, headache, insomnia, mania, somnolence, weight gain, xerostomia |
| Phenelzine | Constipation, disorder of ejaculation and/or orgasm, dizziness, edema, fatigue, headache, hyperreflexia, impotence, elevated values on liver function tests, orthostatic hypotension, sleep disorders, somnolence, tremor, weight gain, xerostomia |
| Selegiline transdermal | Application site reaction, decreased systolic blood pressure, diarrhea, headache, indigestion, insomnia, orthostatic hypotension, weight loss, xerostomia |
| Tranylcypromine | Agitation, anxiety, constipation, diarrhea, dizziness, headache, impotence, insomnia, loss of appetite, mania, nausea, orthostatic hypotension, somnolence, weight gain, xerostomia |
| MAOIs: monoamine oxidase inhibitors Source: Adapted from reference 20 | |
Practice guidelines
The American Psychiatric Association’s practice guidelines for treating major depression state that MAOIs are effective in treating subgroups of patients with MDD with atypical features who have failed TCA trials.21 These guidelines also state that MAOIs have been shown to be effective treatment for some patients who have failed other antidepressants. However, for TRD patients who have not responded to SSRIs or SNRIs, the effectiveness of MAOIs compared with other strategies is unclear.22
MAOIs have been used for >6 decades, and published studies continue to document their efficacy and safety when patients are monitored appropriately.11,12,14,15,25 However, based on our observations we suspect MAOIs are underutilized in clinical practice today. We are concerned that such practices can trickle down into residency training programs. Psychiatric residents typically do not receive adequate training in prescribing MAOIs, largely because many training faculty are not prescribing MAOIs themselves. Despite MAOIs’ limitations, concerns about an increased risk of suicide in patients with TRD26 and the high likelihood of a poor outcome associated with persistent nonresponse to prior treatments must be weighed against the relatively low risk of a hypertensive event with MAOIs.6
- McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. Journal of Food Composition and Analysis. 2006;19(suppl):S58-S65.
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
- Clomipramine • Anafranil
- Epinephrine • Adrenalin, EpiPen
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Isocarboxazid • Marplan
- Lithium • Eskalith, Lithobid
- Nifedipine • Adalat, Afeditab
- Phenelzine • Nardil
- Phentolamine • OraVerse, Regitine
- Selegiline • EMSAM
- Tranylcypromine • Parnate
Dr. Kosinski reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rothschild receives grant or research support from Cyberonics, the National Institute of Mental Health, St. Jude Medical, and Takeda, and is a consultant to Allergan, Eli Lilly and Company, GlaxoSmithKline, Noven Pharmaceuticals, Pfizer Inc., Shire Pharmaceuticals, and Sunovion.
1. Balon R, Mufti R, Arfken CL. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatr Serv. 1999;50(7):945-947.
2. Nolen WA, van de Putte JJ, Dijken WA, et al. Treatment strategy in depression. II. MAO inhibitors in depression resistant to cyclic antidepressants: two controlled crossover studies with tranylcypromine versus L-5-hydroxytryptophan and nomifensine. Acta Psychiatr Scand. 1988;78(6):676-683.
3. McGrath PJ, Stewart JW, Harrison W, et al. Treatment of tricyclic refractory depression with a monoamine oxidase inhibitor antidepressant. Psychopharmacol Bull. 1987;23(1):169-172.
4. Amsterdam JD. Monoamine oxidase inhibitor therapy in severe and resistant depression. Psychiatr Ann. 2006;36(9):607-613.
5. Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122(5):509-522.
6. Kennedy SH, Holt A, Baker GB. Monoamine oxidase inhibitors. In: Sadock BJ Sadock VA, eds. Kaplan and Sadock’s comprehensive textbook of psychiatry. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005: 1076–1080.
7. EMSAM [package insert]. Napa CA: Dey Pharm LP; 2011.
8. Amsterdam JD, Chopra M. Monoamine oxidase inhibitors revisited. Psychiatric Ann. 2001;31(6):361-370.
9. Quitkin FM, Stewart JW, McGrath PJ, et al. Phenelzine versus imipramine in the treatment of probable atypical depression: defining syndrome boundaries of selective MAOI responders. Am J Psychiatry. 1988;145(3):306-311.
10. Vallejo J, Gasto C, Catalan R, et al. Double-blind study of imipramine versus phenelzine in melancholias and dysthymic disorders. Br J Psychiatry. 1987;151:639-642.
11. White K, Razani J, Cadow B, et al. Trancylpromine vs. nortriptyline vs. placebo in depressed outpatients: a controlled trial. Psychopharmacology (Berl). 1984;82(3):258-262.
12. Thase ME, Frank E, Mallinger AG, et al. Treatment of imipramine-resistant recurrent depression, III: efficacy of monoamine oxidase inhibitors. J Clin Psychiatry. 1992;53(1):5-11.
13. Himmelhoch JM, Thase ME, Mallinger AG, et al. Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry. 1991;148(7):910-916.
14. Rothschild AJ. ed. The evidence-based guide to antidepressant medications. Arlington, VA: American Psychiatric Publishing, Inc.; 2012:15–20.
15. Ravaris CL, Nies A, Robinson DS, et al. A multiple-dose, controlled study of phenelzine in depression-anxiety states. Arch Gen Psychiatry. 1976;33(3):347-350.
16. Cockhill LA, Remick RA. Blood pressure effects of monoamine oxidase inhibitors—the highs and lows. Can J Psychiatry. 1987;32(9):803-808.
17. Shulman KI, Walker SE. A reevaluation of dietary restrictions for irreversible monoamine oxidase inhibitors. Psychiatr Ann. 2001;31(6):378-384.
18. Gardner DM, Shulman KI, Walker SE, et al. The making of a user friendly MAOI diet. J Clin Psychiatry. 1996;57(3):99-104.
19. Keck PE, Jr, Carter WP, Nierenberg AA, et al. Acute cardiovascular effects of tranylcypromine: correlation with plasma drug, metabolite, norepinephrine, and MHPG levels. J Clin Psychiatry. 1991;52(6):250-254.
20. Micromedex Healthcare Series [UMass Memorial Healthcare Intranet System]. Version 5.1. Greenwood Village CO: Thomson Reuters (Healthcare) Inc.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder third edition. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1667485. Published October 2010. Accessed October 26, 2012.
22. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1541; quiz 1666.
23. Nelson JC, Byck R. Rapid response to lithium in phenelzine non-responders. Br J Psychiatry. 1982;141:85-86.
24. Guze BH, Baxter LR, Jr, Rego J. Refractory depression treated with high doses of monoamine oxidase inhibitor. J Clin Psychiatry. 1987;48(1):31-32.
25. Robinson DS, Gilmor ML, Yang Y, et al. Treatment effects of selegiline transdermal system on symptoms of major depressive disorder: a meta analysis of short term, placebo controlled, efficacy trials. Psychopharmacol Bull. 2007;40(3):15-28.
26. Keller MB, Lavori PW, Rice J, et al. The persistent risk of chronicity in recurrent episodes of nonbipolar major depressive disorder: a prospective follow-up. Am J Psychiatry. 1986;143(1):24-28.
1. Balon R, Mufti R, Arfken CL. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatr Serv. 1999;50(7):945-947.
2. Nolen WA, van de Putte JJ, Dijken WA, et al. Treatment strategy in depression. II. MAO inhibitors in depression resistant to cyclic antidepressants: two controlled crossover studies with tranylcypromine versus L-5-hydroxytryptophan and nomifensine. Acta Psychiatr Scand. 1988;78(6):676-683.
3. McGrath PJ, Stewart JW, Harrison W, et al. Treatment of tricyclic refractory depression with a monoamine oxidase inhibitor antidepressant. Psychopharmacol Bull. 1987;23(1):169-172.
4. Amsterdam JD. Monoamine oxidase inhibitor therapy in severe and resistant depression. Psychiatr Ann. 2006;36(9):607-613.
5. Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122(5):509-522.
6. Kennedy SH, Holt A, Baker GB. Monoamine oxidase inhibitors. In: Sadock BJ Sadock VA, eds. Kaplan and Sadock’s comprehensive textbook of psychiatry. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005: 1076–1080.
7. EMSAM [package insert]. Napa CA: Dey Pharm LP; 2011.
8. Amsterdam JD, Chopra M. Monoamine oxidase inhibitors revisited. Psychiatric Ann. 2001;31(6):361-370.
9. Quitkin FM, Stewart JW, McGrath PJ, et al. Phenelzine versus imipramine in the treatment of probable atypical depression: defining syndrome boundaries of selective MAOI responders. Am J Psychiatry. 1988;145(3):306-311.
10. Vallejo J, Gasto C, Catalan R, et al. Double-blind study of imipramine versus phenelzine in melancholias and dysthymic disorders. Br J Psychiatry. 1987;151:639-642.
11. White K, Razani J, Cadow B, et al. Trancylpromine vs. nortriptyline vs. placebo in depressed outpatients: a controlled trial. Psychopharmacology (Berl). 1984;82(3):258-262.
12. Thase ME, Frank E, Mallinger AG, et al. Treatment of imipramine-resistant recurrent depression, III: efficacy of monoamine oxidase inhibitors. J Clin Psychiatry. 1992;53(1):5-11.
13. Himmelhoch JM, Thase ME, Mallinger AG, et al. Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry. 1991;148(7):910-916.
14. Rothschild AJ. ed. The evidence-based guide to antidepressant medications. Arlington, VA: American Psychiatric Publishing, Inc.; 2012:15–20.
15. Ravaris CL, Nies A, Robinson DS, et al. A multiple-dose, controlled study of phenelzine in depression-anxiety states. Arch Gen Psychiatry. 1976;33(3):347-350.
16. Cockhill LA, Remick RA. Blood pressure effects of monoamine oxidase inhibitors—the highs and lows. Can J Psychiatry. 1987;32(9):803-808.
17. Shulman KI, Walker SE. A reevaluation of dietary restrictions for irreversible monoamine oxidase inhibitors. Psychiatr Ann. 2001;31(6):378-384.
18. Gardner DM, Shulman KI, Walker SE, et al. The making of a user friendly MAOI diet. J Clin Psychiatry. 1996;57(3):99-104.
19. Keck PE, Jr, Carter WP, Nierenberg AA, et al. Acute cardiovascular effects of tranylcypromine: correlation with plasma drug, metabolite, norepinephrine, and MHPG levels. J Clin Psychiatry. 1991;52(6):250-254.
20. Micromedex Healthcare Series [UMass Memorial Healthcare Intranet System]. Version 5.1. Greenwood Village CO: Thomson Reuters (Healthcare) Inc.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder third edition. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1667485. Published October 2010. Accessed October 26, 2012.
22. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1541; quiz 1666.
23. Nelson JC, Byck R. Rapid response to lithium in phenelzine non-responders. Br J Psychiatry. 1982;141:85-86.
24. Guze BH, Baxter LR, Jr, Rego J. Refractory depression treated with high doses of monoamine oxidase inhibitor. J Clin Psychiatry. 1987;48(1):31-32.
25. Robinson DS, Gilmor ML, Yang Y, et al. Treatment effects of selegiline transdermal system on symptoms of major depressive disorder: a meta analysis of short term, placebo controlled, efficacy trials. Psychopharmacol Bull. 2007;40(3):15-28.
26. Keller MB, Lavori PW, Rice J, et al. The persistent risk of chronicity in recurrent episodes of nonbipolar major depressive disorder: a prospective follow-up. Am J Psychiatry. 1986;143(1):24-28.
QUIT: A mnemonic to help patients stop smoking
Discuss this article at www.facebook.com/CurrentPsychiatry
Research indicates that even brief physician advice on a regular basis can increase quit rates for patients who smoke.1 This is particularly important in mental health settings, where there are more smokers than in the general population (50% to 90% vs 25% to 27%, respectively) but quit rates are lower.2
There is no “one size fits all” solution to quitting smoking; there are many individual factors to take into account for each patient. In addition to environmental factors that can make quitting smoking more challenging—eg, the patient’s partner also smokes—a patient’s genetic makeup can make it easier or harder to become addicted or to quit smoking, and can make pharmacologic approaches to cessation more or less successful.3,4 A patient’s failed attempt to quit in the past does not indicate that quitting is impossible.
Although we encourage the use of traditional mnemonics such as the “5 A’s”5 and the “5 R’s,”5 we introduce QUIT as an easy-to-remember, compassionate, realistic way of discussing smoking cessation with patients.
Question each patient to understand the pros and cons of quitting. Ask your patients about the “benefits” of smoking and understand what role cigarettes serve in their lives. Remind patients of immediate benefits that would make quitting smoking a “trade” rather than a loss—eg, how would they use the extra $200 a month they would save by giving up cigarettes?
If patients say they are not interested in quitting, find out why they are not motivated to quit and collaborate with them to try to address their concerns. Additionally, ask if they would be comfortable discussing smoking cessation at each visit, even if they are not expressing interest.
Understand the nature of addiction. The trajectory of tobacco dependence—similar to other addictions—involves a chronic and relapsing course. Most patients require multiple quit attempts using several strategies before they succeed. Find out what they have tried in the past and build on previous successes. Be persistent in offering evidence-based treatments to help patients quit, even when motivation is low and patients have multiple failed attempts.
Keep in mind that only 4% to 7% of unaided quit attempts are successful.6 Most patients require counseling and/or medication, as well as help from a caring physician. By understanding the nature of addiction, you can be optimistic and supportive of your patients as they face the often disheartening process of quitting.
Identify risk factors and triggers. Studies have demonstrated that stimuli related to smoking increase a patient’s craving to smoke; this response is stronger than triggers encountered by patients addicted to alcohol or opiates.7 A plan for handling cravings and avoiding triggers can empower your patients and help them stay on track.
Talk with—not to—your patient. Discussing smoking can help clarify your patient’s feelings rather than avoiding them. Although patients may aspire to eventually quit smoking, the unspoken concerns they harbor combined with the “benefits” of smoking may lead to a failure to act.
Talk is powerful and with training, physicians can move patients toward change. Motivational interviewing is evidence-based and offers techniques that enable physicians to use conversation with their patients as a way of overcoming ambivalence about unhealthy behaviors and eliciting talk about changing these behaviors, and eventually help them to change.
You can make an impact
Physicians need to recognize their potential impact on this life-threatening behavior. Through an active, conversational style, develop a big-picture understanding of your patient’s pros and cons of quitting smoking; strengths and weaknesses; past failures and successes; barriers to success; available supports; etc. This information, combined with encouragement, support, and knowledge of evidence-based practices, can yield a thorough plan for quitting.
Although quitting smoking can be extremely challenging for clinicians and patients, expanding your knowledge in this area will allow you to help your patients make life-saving changes. The best care comes from direct communication and unconditional support.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lancaster T, Stead L, Silagy C, et al. Effectiveness of interventions to help people stop smoking: findings from the Cochrane Library. BMJ. 2000;321(7257):355-358.
2. Siru R, Hulse GK, Tait RJ. Assessing motivation to quit smoking in people with mental illness: a review. Addiction. 2009;104(5):719-733.
3. Amos CI, Spitz MR, Cinciripini P. Chipping away at the genetics of smoking behavior. Nat Genet. 2010;42(5):366-368.
4. Tillie-Louise H. Genetic determinants of smoking cessation. European Respiratory Disease. 2009;5(1):37-40.
5. U.S. Department of Health and Human Services. Treating tobacco use and dependence. Quick reference guide for clinicians. 2008 update. http://www.ahrq.gov/clinic/tobacco/tobaqrg.pdf. Accessed November 15, 2012.
6. Schroeder SA, Morris CD. Confronting a neglected epidemic: tobacco cessation for persons with mental illnesses and substance abuse problems. Annu Rev Public Health. 2010;31:297-314.
7. Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36(3):235-243.
Discuss this article at www.facebook.com/CurrentPsychiatry
Research indicates that even brief physician advice on a regular basis can increase quit rates for patients who smoke.1 This is particularly important in mental health settings, where there are more smokers than in the general population (50% to 90% vs 25% to 27%, respectively) but quit rates are lower.2
There is no “one size fits all” solution to quitting smoking; there are many individual factors to take into account for each patient. In addition to environmental factors that can make quitting smoking more challenging—eg, the patient’s partner also smokes—a patient’s genetic makeup can make it easier or harder to become addicted or to quit smoking, and can make pharmacologic approaches to cessation more or less successful.3,4 A patient’s failed attempt to quit in the past does not indicate that quitting is impossible.
Although we encourage the use of traditional mnemonics such as the “5 A’s”5 and the “5 R’s,”5 we introduce QUIT as an easy-to-remember, compassionate, realistic way of discussing smoking cessation with patients.
Question each patient to understand the pros and cons of quitting. Ask your patients about the “benefits” of smoking and understand what role cigarettes serve in their lives. Remind patients of immediate benefits that would make quitting smoking a “trade” rather than a loss—eg, how would they use the extra $200 a month they would save by giving up cigarettes?
If patients say they are not interested in quitting, find out why they are not motivated to quit and collaborate with them to try to address their concerns. Additionally, ask if they would be comfortable discussing smoking cessation at each visit, even if they are not expressing interest.
Understand the nature of addiction. The trajectory of tobacco dependence—similar to other addictions—involves a chronic and relapsing course. Most patients require multiple quit attempts using several strategies before they succeed. Find out what they have tried in the past and build on previous successes. Be persistent in offering evidence-based treatments to help patients quit, even when motivation is low and patients have multiple failed attempts.
Keep in mind that only 4% to 7% of unaided quit attempts are successful.6 Most patients require counseling and/or medication, as well as help from a caring physician. By understanding the nature of addiction, you can be optimistic and supportive of your patients as they face the often disheartening process of quitting.
Identify risk factors and triggers. Studies have demonstrated that stimuli related to smoking increase a patient’s craving to smoke; this response is stronger than triggers encountered by patients addicted to alcohol or opiates.7 A plan for handling cravings and avoiding triggers can empower your patients and help them stay on track.
Talk with—not to—your patient. Discussing smoking can help clarify your patient’s feelings rather than avoiding them. Although patients may aspire to eventually quit smoking, the unspoken concerns they harbor combined with the “benefits” of smoking may lead to a failure to act.
Talk is powerful and with training, physicians can move patients toward change. Motivational interviewing is evidence-based and offers techniques that enable physicians to use conversation with their patients as a way of overcoming ambivalence about unhealthy behaviors and eliciting talk about changing these behaviors, and eventually help them to change.
You can make an impact
Physicians need to recognize their potential impact on this life-threatening behavior. Through an active, conversational style, develop a big-picture understanding of your patient’s pros and cons of quitting smoking; strengths and weaknesses; past failures and successes; barriers to success; available supports; etc. This information, combined with encouragement, support, and knowledge of evidence-based practices, can yield a thorough plan for quitting.
Although quitting smoking can be extremely challenging for clinicians and patients, expanding your knowledge in this area will allow you to help your patients make life-saving changes. The best care comes from direct communication and unconditional support.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Research indicates that even brief physician advice on a regular basis can increase quit rates for patients who smoke.1 This is particularly important in mental health settings, where there are more smokers than in the general population (50% to 90% vs 25% to 27%, respectively) but quit rates are lower.2
There is no “one size fits all” solution to quitting smoking; there are many individual factors to take into account for each patient. In addition to environmental factors that can make quitting smoking more challenging—eg, the patient’s partner also smokes—a patient’s genetic makeup can make it easier or harder to become addicted or to quit smoking, and can make pharmacologic approaches to cessation more or less successful.3,4 A patient’s failed attempt to quit in the past does not indicate that quitting is impossible.
Although we encourage the use of traditional mnemonics such as the “5 A’s”5 and the “5 R’s,”5 we introduce QUIT as an easy-to-remember, compassionate, realistic way of discussing smoking cessation with patients.
Question each patient to understand the pros and cons of quitting. Ask your patients about the “benefits” of smoking and understand what role cigarettes serve in their lives. Remind patients of immediate benefits that would make quitting smoking a “trade” rather than a loss—eg, how would they use the extra $200 a month they would save by giving up cigarettes?
If patients say they are not interested in quitting, find out why they are not motivated to quit and collaborate with them to try to address their concerns. Additionally, ask if they would be comfortable discussing smoking cessation at each visit, even if they are not expressing interest.
Understand the nature of addiction. The trajectory of tobacco dependence—similar to other addictions—involves a chronic and relapsing course. Most patients require multiple quit attempts using several strategies before they succeed. Find out what they have tried in the past and build on previous successes. Be persistent in offering evidence-based treatments to help patients quit, even when motivation is low and patients have multiple failed attempts.
Keep in mind that only 4% to 7% of unaided quit attempts are successful.6 Most patients require counseling and/or medication, as well as help from a caring physician. By understanding the nature of addiction, you can be optimistic and supportive of your patients as they face the often disheartening process of quitting.
Identify risk factors and triggers. Studies have demonstrated that stimuli related to smoking increase a patient’s craving to smoke; this response is stronger than triggers encountered by patients addicted to alcohol or opiates.7 A plan for handling cravings and avoiding triggers can empower your patients and help them stay on track.
Talk with—not to—your patient. Discussing smoking can help clarify your patient’s feelings rather than avoiding them. Although patients may aspire to eventually quit smoking, the unspoken concerns they harbor combined with the “benefits” of smoking may lead to a failure to act.
Talk is powerful and with training, physicians can move patients toward change. Motivational interviewing is evidence-based and offers techniques that enable physicians to use conversation with their patients as a way of overcoming ambivalence about unhealthy behaviors and eliciting talk about changing these behaviors, and eventually help them to change.
You can make an impact
Physicians need to recognize their potential impact on this life-threatening behavior. Through an active, conversational style, develop a big-picture understanding of your patient’s pros and cons of quitting smoking; strengths and weaknesses; past failures and successes; barriers to success; available supports; etc. This information, combined with encouragement, support, and knowledge of evidence-based practices, can yield a thorough plan for quitting.
Although quitting smoking can be extremely challenging for clinicians and patients, expanding your knowledge in this area will allow you to help your patients make life-saving changes. The best care comes from direct communication and unconditional support.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lancaster T, Stead L, Silagy C, et al. Effectiveness of interventions to help people stop smoking: findings from the Cochrane Library. BMJ. 2000;321(7257):355-358.
2. Siru R, Hulse GK, Tait RJ. Assessing motivation to quit smoking in people with mental illness: a review. Addiction. 2009;104(5):719-733.
3. Amos CI, Spitz MR, Cinciripini P. Chipping away at the genetics of smoking behavior. Nat Genet. 2010;42(5):366-368.
4. Tillie-Louise H. Genetic determinants of smoking cessation. European Respiratory Disease. 2009;5(1):37-40.
5. U.S. Department of Health and Human Services. Treating tobacco use and dependence. Quick reference guide for clinicians. 2008 update. http://www.ahrq.gov/clinic/tobacco/tobaqrg.pdf. Accessed November 15, 2012.
6. Schroeder SA, Morris CD. Confronting a neglected epidemic: tobacco cessation for persons with mental illnesses and substance abuse problems. Annu Rev Public Health. 2010;31:297-314.
7. Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36(3):235-243.
1. Lancaster T, Stead L, Silagy C, et al. Effectiveness of interventions to help people stop smoking: findings from the Cochrane Library. BMJ. 2000;321(7257):355-358.
2. Siru R, Hulse GK, Tait RJ. Assessing motivation to quit smoking in people with mental illness: a review. Addiction. 2009;104(5):719-733.
3. Amos CI, Spitz MR, Cinciripini P. Chipping away at the genetics of smoking behavior. Nat Genet. 2010;42(5):366-368.
4. Tillie-Louise H. Genetic determinants of smoking cessation. European Respiratory Disease. 2009;5(1):37-40.
5. U.S. Department of Health and Human Services. Treating tobacco use and dependence. Quick reference guide for clinicians. 2008 update. http://www.ahrq.gov/clinic/tobacco/tobaqrg.pdf. Accessed November 15, 2012.
6. Schroeder SA, Morris CD. Confronting a neglected epidemic: tobacco cessation for persons with mental illnesses and substance abuse problems. Annu Rev Public Health. 2010;31:297-314.
7. Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36(3):235-243.
Teens, social media, and ‘sexting’: What to tell parents
Discuss this article at www.facebook.com/CurrentPsychiatry
Children and adolescents who have unrestricted use of the internet and cell phones are at increased risk for being exposed to sexually explicit material. One study found almost 1 in 5 high school students have “sexted”—sending a text message with sexually explicit pictures—and almost twice as many reported that they had received a sexually explicit picture via cell phone.1 More than 25% of students acknowledged forwarding a sexually explicit picture to others; >33% did so despite knowing the legal consequences, including being arrested and facing pornography charges.1
Concerned parents may seek advice on how to prevent their child from receiving or sending sexually inappropriate material on the internet or on their cell phones. You can help parents keep their children safe by sharing the following tips from The American Academy of Pediatrics (AAP)2:
Keep up with technology. Advise parents to become familiar with popular social networking websites such as Facebook. Creating their own Facebook page and “friending” their child may help them facilitate a conversation about their individual online experiences.
Enable privacy features. Instruct parents to install parental controls on their child’s computer. Explain to parents that these monitoring systems can help them check their child’s e-mail, chat records, and instant messages. Many social networking sites have privacy features that can help block unwanted users from contacting a child.
Check up on your children. Parents should let children know they are aware of their online presence and will be keeping an eye on them. They should periodically check a child’s chat logs, messages, e-mails, and social networking profiles for inappropriate content, friends, messages, and images. Instruct parents to teach their children that nothing is private once it’s posted on the internet. Suggest keeping the child’s computer in a public location such as the family room or kitchen.
Limit time spent online. Explain to parents that they should limit their child’s internet and cell phone access.
Combating ‘sexting’
Suggest to parents that they explain to their child in an age-appropriate manner what sexting is before giving their child a cell phone. The AAP2 recommends that parents make sure their children understand the legal ramifications of sexting. A child who is caught sexting could be arrested, which may hurt his or her chances of being accepted into college or getting a job. A simple way to reduce a child’s opportunities for sexting is to restrict his or her access to a cell phone during social situations where peer pressure could influence behavior.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Strassberg DS, McKinnon RK, Sustaíta MA. Sexting by high school students: an exploratory and descriptive study [published online June 7, 2012]. Arch Sex Behav. doi: 10.1007/s10508-012-9969-8.
2. American Academy of Pediatrics. Talking to kids and teens about social media and sexting. http://www.aap.org/en-us/about-the-aap/aap-press-room/news-features-and-safety-tips/pages/Talking-to-Kids-and-Teens-About-Social-Media-and-Sexting.aspx?. Published June 2009. Updated March 2, 2011. Accessed August 14, 2012.
Discuss this article at www.facebook.com/CurrentPsychiatry
Children and adolescents who have unrestricted use of the internet and cell phones are at increased risk for being exposed to sexually explicit material. One study found almost 1 in 5 high school students have “sexted”—sending a text message with sexually explicit pictures—and almost twice as many reported that they had received a sexually explicit picture via cell phone.1 More than 25% of students acknowledged forwarding a sexually explicit picture to others; >33% did so despite knowing the legal consequences, including being arrested and facing pornography charges.1
Concerned parents may seek advice on how to prevent their child from receiving or sending sexually inappropriate material on the internet or on their cell phones. You can help parents keep their children safe by sharing the following tips from The American Academy of Pediatrics (AAP)2:
Keep up with technology. Advise parents to become familiar with popular social networking websites such as Facebook. Creating their own Facebook page and “friending” their child may help them facilitate a conversation about their individual online experiences.
Enable privacy features. Instruct parents to install parental controls on their child’s computer. Explain to parents that these monitoring systems can help them check their child’s e-mail, chat records, and instant messages. Many social networking sites have privacy features that can help block unwanted users from contacting a child.
Check up on your children. Parents should let children know they are aware of their online presence and will be keeping an eye on them. They should periodically check a child’s chat logs, messages, e-mails, and social networking profiles for inappropriate content, friends, messages, and images. Instruct parents to teach their children that nothing is private once it’s posted on the internet. Suggest keeping the child’s computer in a public location such as the family room or kitchen.
Limit time spent online. Explain to parents that they should limit their child’s internet and cell phone access.
Combating ‘sexting’
Suggest to parents that they explain to their child in an age-appropriate manner what sexting is before giving their child a cell phone. The AAP2 recommends that parents make sure their children understand the legal ramifications of sexting. A child who is caught sexting could be arrested, which may hurt his or her chances of being accepted into college or getting a job. A simple way to reduce a child’s opportunities for sexting is to restrict his or her access to a cell phone during social situations where peer pressure could influence behavior.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Children and adolescents who have unrestricted use of the internet and cell phones are at increased risk for being exposed to sexually explicit material. One study found almost 1 in 5 high school students have “sexted”—sending a text message with sexually explicit pictures—and almost twice as many reported that they had received a sexually explicit picture via cell phone.1 More than 25% of students acknowledged forwarding a sexually explicit picture to others; >33% did so despite knowing the legal consequences, including being arrested and facing pornography charges.1
Concerned parents may seek advice on how to prevent their child from receiving or sending sexually inappropriate material on the internet or on their cell phones. You can help parents keep their children safe by sharing the following tips from The American Academy of Pediatrics (AAP)2:
Keep up with technology. Advise parents to become familiar with popular social networking websites such as Facebook. Creating their own Facebook page and “friending” their child may help them facilitate a conversation about their individual online experiences.
Enable privacy features. Instruct parents to install parental controls on their child’s computer. Explain to parents that these monitoring systems can help them check their child’s e-mail, chat records, and instant messages. Many social networking sites have privacy features that can help block unwanted users from contacting a child.
Check up on your children. Parents should let children know they are aware of their online presence and will be keeping an eye on them. They should periodically check a child’s chat logs, messages, e-mails, and social networking profiles for inappropriate content, friends, messages, and images. Instruct parents to teach their children that nothing is private once it’s posted on the internet. Suggest keeping the child’s computer in a public location such as the family room or kitchen.
Limit time spent online. Explain to parents that they should limit their child’s internet and cell phone access.
Combating ‘sexting’
Suggest to parents that they explain to their child in an age-appropriate manner what sexting is before giving their child a cell phone. The AAP2 recommends that parents make sure their children understand the legal ramifications of sexting. A child who is caught sexting could be arrested, which may hurt his or her chances of being accepted into college or getting a job. A simple way to reduce a child’s opportunities for sexting is to restrict his or her access to a cell phone during social situations where peer pressure could influence behavior.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Strassberg DS, McKinnon RK, Sustaíta MA. Sexting by high school students: an exploratory and descriptive study [published online June 7, 2012]. Arch Sex Behav. doi: 10.1007/s10508-012-9969-8.
2. American Academy of Pediatrics. Talking to kids and teens about social media and sexting. http://www.aap.org/en-us/about-the-aap/aap-press-room/news-features-and-safety-tips/pages/Talking-to-Kids-and-Teens-About-Social-Media-and-Sexting.aspx?. Published June 2009. Updated March 2, 2011. Accessed August 14, 2012.
1. Strassberg DS, McKinnon RK, Sustaíta MA. Sexting by high school students: an exploratory and descriptive study [published online June 7, 2012]. Arch Sex Behav. doi: 10.1007/s10508-012-9969-8.
2. American Academy of Pediatrics. Talking to kids and teens about social media and sexting. http://www.aap.org/en-us/about-the-aap/aap-press-room/news-features-and-safety-tips/pages/Talking-to-Kids-and-Teens-About-Social-Media-and-Sexting.aspx?. Published June 2009. Updated March 2, 2011. Accessed August 14, 2012.
How to provide culturally sensitive care to Arab American patients
Since September 11, 2001, many Arab Americans have faced increased discrimination, which puts them at greater risk for depression and low self-esteem.1 Children and adolescents in particular have been the victims of teasing and taunts. Many Muslim Arab Americans turned to their imams—a mosque’s spiritual leader—rather than a mental health clinician to help them deal with the national tragedy and the fallout that followed.2
Arab Americans may struggle to bridge their personal identity with their cultural one. Traditional Arab values stress the importance of family—both immediate and extended—loyalty to parents, religious adherence, and respect for elders and authority. Adapting those values to typical American values can cause dissonance as Arab Americans grapple to find a balance between renouncing their Arab culture in hopes of fitting in and feeling like outcasts in the country they call home.
Understanding cultural nuances
Be aware of the stigma of mental illness within Arab American communities. Unlike diabetes or heart disease, psychiatric disorders can carry a negative connotation for many Arab Americans.3 They may view mental illness as a personal shortcoming or ascribe their symptoms to supernatural spirits. The fear of being discriminated against for being culturally different and mentally ill may delay or prevent individuals from seeking care.
Understanding these dynamics, as well as Arab American culture, is the first step to evaluating these patients. Being aware of cultural nuances also is important. Patients may say they don’t smoke, but some prodding may reveal that they use a tobacco water pipe, or hookah.
Be cognizant of any preconceived notions that can seep into an assessment. It’s easy to assume that Arab American patients fall into stereotypical gender roles or are unhappy with what may be perceived as inadequate assimilation. Conversely, a patient’s appearance, devotion to cultural and religious values, and family support may lead to an assumption that the patient does not abuse substances or engage in high-risk behavior.
In addition, note that Arab Americans tend to present their mental illness as somatic complaints, which may make them more comfortable seeing a primary care physician than a psychiatrist.
Adjusting treatment
Many Arab Americans’ first choice is to seek support from family, friends, and religious leaders.4 A patient may need to be convinced to take psychotropics the same as they would other medications. Therefore, it may be necessary to involve family members to ensure treatment compliance. Clinicians may need to spend more time with Arab American patients, which can help the clinician grasp the complexity of their issues and allow patients to feel that they’re being cared for by a clinician who respects their cultural and religious beliefs. In conjunction, these steps will help you provide culturally sensitive care that best addresses Arab Americans’ mental health needs.
1. Amer MM, Hovey JD. Socio-demographic differences in acculturation and mental health for a sample of 2nd generation/early immigrant Arab Americans. J Immigr Minor Health. 2007;9(4):335-347.
2. Abu-Ras W, Gheith A, Cournos F. The imam’s role in mental health promotion: a study at 22 mosques in New York City’s Muslim community. J Muslim Ment Health. 2008;3(2):155-176.
3. Carolan MT, Bagherinia G, Juhari R, et al. Contemporary Muslim families: research and practice. Contemp Fam Ther. 2000;22(1):67-79.
4. Moradi B, Hasan NT. Arab American persons’ reported experiences of discrimination and mental health: the mediating role of personal control. J Couns Psychol. 2004;51(4):418-428.
Since September 11, 2001, many Arab Americans have faced increased discrimination, which puts them at greater risk for depression and low self-esteem.1 Children and adolescents in particular have been the victims of teasing and taunts. Many Muslim Arab Americans turned to their imams—a mosque’s spiritual leader—rather than a mental health clinician to help them deal with the national tragedy and the fallout that followed.2
Arab Americans may struggle to bridge their personal identity with their cultural one. Traditional Arab values stress the importance of family—both immediate and extended—loyalty to parents, religious adherence, and respect for elders and authority. Adapting those values to typical American values can cause dissonance as Arab Americans grapple to find a balance between renouncing their Arab culture in hopes of fitting in and feeling like outcasts in the country they call home.
Understanding cultural nuances
Be aware of the stigma of mental illness within Arab American communities. Unlike diabetes or heart disease, psychiatric disorders can carry a negative connotation for many Arab Americans.3 They may view mental illness as a personal shortcoming or ascribe their symptoms to supernatural spirits. The fear of being discriminated against for being culturally different and mentally ill may delay or prevent individuals from seeking care.
Understanding these dynamics, as well as Arab American culture, is the first step to evaluating these patients. Being aware of cultural nuances also is important. Patients may say they don’t smoke, but some prodding may reveal that they use a tobacco water pipe, or hookah.
Be cognizant of any preconceived notions that can seep into an assessment. It’s easy to assume that Arab American patients fall into stereotypical gender roles or are unhappy with what may be perceived as inadequate assimilation. Conversely, a patient’s appearance, devotion to cultural and religious values, and family support may lead to an assumption that the patient does not abuse substances or engage in high-risk behavior.
In addition, note that Arab Americans tend to present their mental illness as somatic complaints, which may make them more comfortable seeing a primary care physician than a psychiatrist.
Adjusting treatment
Many Arab Americans’ first choice is to seek support from family, friends, and religious leaders.4 A patient may need to be convinced to take psychotropics the same as they would other medications. Therefore, it may be necessary to involve family members to ensure treatment compliance. Clinicians may need to spend more time with Arab American patients, which can help the clinician grasp the complexity of their issues and allow patients to feel that they’re being cared for by a clinician who respects their cultural and religious beliefs. In conjunction, these steps will help you provide culturally sensitive care that best addresses Arab Americans’ mental health needs.
Since September 11, 2001, many Arab Americans have faced increased discrimination, which puts them at greater risk for depression and low self-esteem.1 Children and adolescents in particular have been the victims of teasing and taunts. Many Muslim Arab Americans turned to their imams—a mosque’s spiritual leader—rather than a mental health clinician to help them deal with the national tragedy and the fallout that followed.2
Arab Americans may struggle to bridge their personal identity with their cultural one. Traditional Arab values stress the importance of family—both immediate and extended—loyalty to parents, religious adherence, and respect for elders and authority. Adapting those values to typical American values can cause dissonance as Arab Americans grapple to find a balance between renouncing their Arab culture in hopes of fitting in and feeling like outcasts in the country they call home.
Understanding cultural nuances
Be aware of the stigma of mental illness within Arab American communities. Unlike diabetes or heart disease, psychiatric disorders can carry a negative connotation for many Arab Americans.3 They may view mental illness as a personal shortcoming or ascribe their symptoms to supernatural spirits. The fear of being discriminated against for being culturally different and mentally ill may delay or prevent individuals from seeking care.
Understanding these dynamics, as well as Arab American culture, is the first step to evaluating these patients. Being aware of cultural nuances also is important. Patients may say they don’t smoke, but some prodding may reveal that they use a tobacco water pipe, or hookah.
Be cognizant of any preconceived notions that can seep into an assessment. It’s easy to assume that Arab American patients fall into stereotypical gender roles or are unhappy with what may be perceived as inadequate assimilation. Conversely, a patient’s appearance, devotion to cultural and religious values, and family support may lead to an assumption that the patient does not abuse substances or engage in high-risk behavior.
In addition, note that Arab Americans tend to present their mental illness as somatic complaints, which may make them more comfortable seeing a primary care physician than a psychiatrist.
Adjusting treatment
Many Arab Americans’ first choice is to seek support from family, friends, and religious leaders.4 A patient may need to be convinced to take psychotropics the same as they would other medications. Therefore, it may be necessary to involve family members to ensure treatment compliance. Clinicians may need to spend more time with Arab American patients, which can help the clinician grasp the complexity of their issues and allow patients to feel that they’re being cared for by a clinician who respects their cultural and religious beliefs. In conjunction, these steps will help you provide culturally sensitive care that best addresses Arab Americans’ mental health needs.
1. Amer MM, Hovey JD. Socio-demographic differences in acculturation and mental health for a sample of 2nd generation/early immigrant Arab Americans. J Immigr Minor Health. 2007;9(4):335-347.
2. Abu-Ras W, Gheith A, Cournos F. The imam’s role in mental health promotion: a study at 22 mosques in New York City’s Muslim community. J Muslim Ment Health. 2008;3(2):155-176.
3. Carolan MT, Bagherinia G, Juhari R, et al. Contemporary Muslim families: research and practice. Contemp Fam Ther. 2000;22(1):67-79.
4. Moradi B, Hasan NT. Arab American persons’ reported experiences of discrimination and mental health: the mediating role of personal control. J Couns Psychol. 2004;51(4):418-428.
1. Amer MM, Hovey JD. Socio-demographic differences in acculturation and mental health for a sample of 2nd generation/early immigrant Arab Americans. J Immigr Minor Health. 2007;9(4):335-347.
2. Abu-Ras W, Gheith A, Cournos F. The imam’s role in mental health promotion: a study at 22 mosques in New York City’s Muslim community. J Muslim Ment Health. 2008;3(2):155-176.
3. Carolan MT, Bagherinia G, Juhari R, et al. Contemporary Muslim families: research and practice. Contemp Fam Ther. 2000;22(1):67-79.
4. Moradi B, Hasan NT. Arab American persons’ reported experiences of discrimination and mental health: the mediating role of personal control. J Couns Psychol. 2004;51(4):418-428.
When your patients disclose ‘insider information’
Discuss this article at www.facebook.com/CurrentPsychiatry
Dear Dr. Mossman:
My patient is an officer in a large corporation. During therapy, he sometimes talks about how the company is doing. Would I risk malpractice liability if I used this information in managing my retirement investments?
Submitted by “Dr. B”
As most physicians find out within a short time of finishing medical school, doctors learn all kinds of useful things from their patients, including information that can help them manage personal matters outside their practices. But are you allowed to use nonpublic business information to make investment decisions?
As this article explains, legal rules and case law suggest that if psychiatrists or therapists act on potentially profitable business information incidentally mentioned by a patient during treatment, they may be subject to serious legal problems. To explain why, we’ll begin with a brief overview of business terms, including “securities” and “insider trading.” Then, to answer Dr. B’s question, we’ll look at what kind of legal consequences may result if mental health professionals are found guilty of “misappropriating” confidential business information.
Securities and security rules
Approximately one-half to two-thirds of Americans have money invested in the stock market—either through their retirement plans, by owning mutual funds, or by holding stocks of individual companies.1 Stocks are a type of financial instrument, or security, that companies issue to raise capital. Companies also raise money by issuing debt, typically in the form of bonds that pay interest to the holder, who in buying the bond has in effect loaned money to the company. Derivatives refer to securities that have prices that move up or down depending on the value of some underlying asset, such as stock prices.2
Stock prices fluctuate in reaction to general economic developments—changes in the unemployment rate, in the cost of basic materials (eg, oil or metals used in manufacturing), or in government policies that influence consumers’ purchasing decisions. But the key factor in determining the price of a company’s stock is investors’ beliefs about the company’s future earnings.3 Because investors usually have to make educated guesses about a company’s future, actually knowing something about a company before the general public finds out would give an investor a huge—but possibly unfair—advantage over other investors.
Making markets fair for all investors is the key purpose of U.S. laws on trading securities. In the 1930s, Congress created the Securities and Exchange Commission (SEC), a federal agency charged with ensuring that companies report the truth about their financial situation and that potential investors receive full, fair disclosure of available public information.4 Among the many ways that the SEC does this is by enforcing regulations concerning “insider trading.”
‘Insider trading’
Corporate “insiders” (eg, directors or employees) often know a lot about how their businesses are doing, and they buy or sell stock in their own companies. Such trading is legal if the insiders follow federal regulations about the timing of their investments and report them publicly.
Insider trading is illegal, however, if an individual acquires material, nonpublic information about a corporation through a relationship that involves trust and confidence and then uses that information when buying or selling a security. The SEC has prosecuted corporate employees who traded securities after learning of confidential developments in their companies, friends and family members of corporate officers who bought or sold securities after getting such information, and employees of law firms who misused information they received while providing services to corporations whose securities they traded.5
To be guilty of insider trading, a person must:
- buy or sell a security based on information that the person realizes is material and nonpublic,6 and
- have received the confidential information under circumstances that create a duty of trust or confidence.7
If both of these conditions are met, the person has wrongfully used confidential information with which he was entrusted, or “misappropriated” that information for personal gain.8
Physicians sometimes gain information that, if used for investment decisions, might lead to accusations of insider trading. Stock prices of pharmaceutical companies rise before public announcements of clinical drug trials, which suggests that information about those results leaks out in advance.9 Recently, physicians have gotten into well-publicized legal trouble by making investment decisions based on information they obtained while participating on an institution’s board10 and from learning early results of clinical drug trials.11
But would it be wrong for a psychiatrist to make a potentially profitable investment based on information obtained incidentally during a treatment encounter? After all, it’s not as though the psychiatrist would be a corporate insider or would have acquired the information improperly. Yet courts have ruled that a psychiatrist’s trading on such information might constitute malpractice and could be grounds for even more serious legal consequences.
Potential malpractice issues
The federal court ruling in United States v Willis12 describes how a psychiatrist learned during treatment that a patient’s husband was seeking to become CEO of a large bank. Realizing that this development might make the bank more valuable, the psychiatrist told his broker what he had learned and purchased 13,000 shares of the bank’s stock for himself and his children. When the husband’s efforts were announced publicly a few weeks later, the psychiatrist sold the shares at a big profit.
Quoting the vow of confidentiality contained in the Hippocratic Oath (Box),13 the court held that the psychiatrist had an obligation to the patient not to disclose information learned during her treatment without her permission. The court said the patient “had an economic interest in preserving the confidentiality of the information disclosed,” and the psychiatrist’s actions “might have jeopardized her husband’s advancement” and financial benefits the wife would have gained. Also, the psychiatrist’s “disclosures jeopardized the psychiatrist-patient relationship,” which might negate the wife’s financial investment in her care, require her to find a new psychiatrist, or require additional treatment to deal with how the psychiatrist’s behavior had affected her.12
And about whatever I may see or hear in treatment, or even without treatment, in the life of human beings—things that should not ever be blurted out outside—I will remain silent, holding such things to be unutterable.
Source: Reference 13
More legal consequences
Dr. Willis had legal problems more serious than just a malpractice lawsuit. He faced criminal prosecution for insider trading and mail fraud, and the court refused to dismiss these charges. The court reasoned that the psychiatrist received the information while in a position of trust and confidence, and breached that trust when he used that confidential information for his personal benefit—behavior that meets the legal definition of “misappropriation.” Because the psychiatrist received stock trade confirmations through the U.S. mail, he also could face federal charges of mail fraud. Ultimately, Dr. Willis pled guilty and paid $137,000 in fines and penalties. Although Dr. Willis retained his New Jersey medical license and avoided a prison sentence, the district court sentenced him to 5 years of probation and required that he perform 3,000 hours of community service.14,15
In a second case,16 a licensed clinical social worker made investments through a broker based on information learned during a therapy session about upcoming business developments (the 1994 Lockheed-Martin Marietta merger). The social worker pled guilty to insider trading, forfeited the illegal gains, and paid a large fine.
Related Resources
- Insider trading versus medical professionalism. Lancet. 2005;366(9488):781.
- Nijm LM. The online message board controversy. Physicians hit with claims of libel and insider trading by their employers. J Leg Med. 2000;21(2):223-239.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jacobe D. In U.S., 54% have stock market investments, lowest since 1999. Gallup Economy. http://www.gallup.com/poll/147206/stock-market-investments-lowest-1999.aspx. Published April 20, 2011. Accessed October 9, 2012.
2. Roman S. Introduction to the mathematics of finance: from risk management to options pricing. New York NY: Springer-Verlag; 2004.
3. Elton EJ, Gruber MJ, Brown SJ, et al. Modern portfolio theory and investment analysis. Hoboken, NJ: John Wiley & Sons; 2010.
4. Keller E, Gehlmann GA. Introductory comment: a historical introduction to the Securities Act of 1933 and the Securities Exchange Act of 1934. Ohio State Law Journal. 1988;49:329-352.
5. U.S. Securities and Exchange Commission. Insider trading. http://www.sec.gov/answers/insider.htm. Published April 19, 2001. Accessed October 9, 2012.
6. 17 CFR 240. 10b5-1.
7. 17 CFR 240. 10b5-2.
8. United States v O’Hagan, 521 U.S. 642 (1997).
9. Rothenstein JM, Tomlinson G, Tannock IF, et al. Company stock prices before and after public announcements related to oncology drugs. J Natl Cancer Inst. 2011;103(20):1507-1512.
10. U.S. Securities and Exchange Commission. SEC charges five physicians with insider trading in stock of medical professional liability insurer. http://www.sec.gov/news/press/2012/2012-132.htm. Published July 10, 2012. Accessed October 9, 2012.
11. Two more are sentenced in insider trading cases. New York Times. December 21 2011:B9. http://www.nytimes.com/2011/12/22/business/in-crackdown-on-insider-trading-two-more-are-sentenced.html?_r=0. Accessed October 9, 2012.
12. United States v Willis, 737 F Supp 269 (SD NY 1990).
13. von Staden H. “In a pure and holy way”: personal and professional conduct in the Hippocratic Oath? J Hist Med Allied Sci. 1996;51(4):404-437.
14. 24 Sec Reg & L Rep (BNA) 7 (1992).
15. Psychiatrist is sentenced. New York Times. January 8 1992. http://www.nytimes.com/1992/01/08/business/credit-markets-psychiatrist-is-sentenced.html. Accessed November 5, 2012.
16. SEC v Cooper, Litigation Rel. No. 14754, 60 S.E.C. Docket 2430 (1995).
Discuss this article at www.facebook.com/CurrentPsychiatry
Dear Dr. Mossman:
My patient is an officer in a large corporation. During therapy, he sometimes talks about how the company is doing. Would I risk malpractice liability if I used this information in managing my retirement investments?
Submitted by “Dr. B”
As most physicians find out within a short time of finishing medical school, doctors learn all kinds of useful things from their patients, including information that can help them manage personal matters outside their practices. But are you allowed to use nonpublic business information to make investment decisions?
As this article explains, legal rules and case law suggest that if psychiatrists or therapists act on potentially profitable business information incidentally mentioned by a patient during treatment, they may be subject to serious legal problems. To explain why, we’ll begin with a brief overview of business terms, including “securities” and “insider trading.” Then, to answer Dr. B’s question, we’ll look at what kind of legal consequences may result if mental health professionals are found guilty of “misappropriating” confidential business information.
Securities and security rules
Approximately one-half to two-thirds of Americans have money invested in the stock market—either through their retirement plans, by owning mutual funds, or by holding stocks of individual companies.1 Stocks are a type of financial instrument, or security, that companies issue to raise capital. Companies also raise money by issuing debt, typically in the form of bonds that pay interest to the holder, who in buying the bond has in effect loaned money to the company. Derivatives refer to securities that have prices that move up or down depending on the value of some underlying asset, such as stock prices.2
Stock prices fluctuate in reaction to general economic developments—changes in the unemployment rate, in the cost of basic materials (eg, oil or metals used in manufacturing), or in government policies that influence consumers’ purchasing decisions. But the key factor in determining the price of a company’s stock is investors’ beliefs about the company’s future earnings.3 Because investors usually have to make educated guesses about a company’s future, actually knowing something about a company before the general public finds out would give an investor a huge—but possibly unfair—advantage over other investors.
Making markets fair for all investors is the key purpose of U.S. laws on trading securities. In the 1930s, Congress created the Securities and Exchange Commission (SEC), a federal agency charged with ensuring that companies report the truth about their financial situation and that potential investors receive full, fair disclosure of available public information.4 Among the many ways that the SEC does this is by enforcing regulations concerning “insider trading.”
‘Insider trading’
Corporate “insiders” (eg, directors or employees) often know a lot about how their businesses are doing, and they buy or sell stock in their own companies. Such trading is legal if the insiders follow federal regulations about the timing of their investments and report them publicly.
Insider trading is illegal, however, if an individual acquires material, nonpublic information about a corporation through a relationship that involves trust and confidence and then uses that information when buying or selling a security. The SEC has prosecuted corporate employees who traded securities after learning of confidential developments in their companies, friends and family members of corporate officers who bought or sold securities after getting such information, and employees of law firms who misused information they received while providing services to corporations whose securities they traded.5
To be guilty of insider trading, a person must:
- buy or sell a security based on information that the person realizes is material and nonpublic,6 and
- have received the confidential information under circumstances that create a duty of trust or confidence.7
If both of these conditions are met, the person has wrongfully used confidential information with which he was entrusted, or “misappropriated” that information for personal gain.8
Physicians sometimes gain information that, if used for investment decisions, might lead to accusations of insider trading. Stock prices of pharmaceutical companies rise before public announcements of clinical drug trials, which suggests that information about those results leaks out in advance.9 Recently, physicians have gotten into well-publicized legal trouble by making investment decisions based on information they obtained while participating on an institution’s board10 and from learning early results of clinical drug trials.11
But would it be wrong for a psychiatrist to make a potentially profitable investment based on information obtained incidentally during a treatment encounter? After all, it’s not as though the psychiatrist would be a corporate insider or would have acquired the information improperly. Yet courts have ruled that a psychiatrist’s trading on such information might constitute malpractice and could be grounds for even more serious legal consequences.
Potential malpractice issues
The federal court ruling in United States v Willis12 describes how a psychiatrist learned during treatment that a patient’s husband was seeking to become CEO of a large bank. Realizing that this development might make the bank more valuable, the psychiatrist told his broker what he had learned and purchased 13,000 shares of the bank’s stock for himself and his children. When the husband’s efforts were announced publicly a few weeks later, the psychiatrist sold the shares at a big profit.
Quoting the vow of confidentiality contained in the Hippocratic Oath (Box),13 the court held that the psychiatrist had an obligation to the patient not to disclose information learned during her treatment without her permission. The court said the patient “had an economic interest in preserving the confidentiality of the information disclosed,” and the psychiatrist’s actions “might have jeopardized her husband’s advancement” and financial benefits the wife would have gained. Also, the psychiatrist’s “disclosures jeopardized the psychiatrist-patient relationship,” which might negate the wife’s financial investment in her care, require her to find a new psychiatrist, or require additional treatment to deal with how the psychiatrist’s behavior had affected her.12
And about whatever I may see or hear in treatment, or even without treatment, in the life of human beings—things that should not ever be blurted out outside—I will remain silent, holding such things to be unutterable.
Source: Reference 13
More legal consequences
Dr. Willis had legal problems more serious than just a malpractice lawsuit. He faced criminal prosecution for insider trading and mail fraud, and the court refused to dismiss these charges. The court reasoned that the psychiatrist received the information while in a position of trust and confidence, and breached that trust when he used that confidential information for his personal benefit—behavior that meets the legal definition of “misappropriation.” Because the psychiatrist received stock trade confirmations through the U.S. mail, he also could face federal charges of mail fraud. Ultimately, Dr. Willis pled guilty and paid $137,000 in fines and penalties. Although Dr. Willis retained his New Jersey medical license and avoided a prison sentence, the district court sentenced him to 5 years of probation and required that he perform 3,000 hours of community service.14,15
In a second case,16 a licensed clinical social worker made investments through a broker based on information learned during a therapy session about upcoming business developments (the 1994 Lockheed-Martin Marietta merger). The social worker pled guilty to insider trading, forfeited the illegal gains, and paid a large fine.
Related Resources
- Insider trading versus medical professionalism. Lancet. 2005;366(9488):781.
- Nijm LM. The online message board controversy. Physicians hit with claims of libel and insider trading by their employers. J Leg Med. 2000;21(2):223-239.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Dear Dr. Mossman:
My patient is an officer in a large corporation. During therapy, he sometimes talks about how the company is doing. Would I risk malpractice liability if I used this information in managing my retirement investments?
Submitted by “Dr. B”
As most physicians find out within a short time of finishing medical school, doctors learn all kinds of useful things from their patients, including information that can help them manage personal matters outside their practices. But are you allowed to use nonpublic business information to make investment decisions?
As this article explains, legal rules and case law suggest that if psychiatrists or therapists act on potentially profitable business information incidentally mentioned by a patient during treatment, they may be subject to serious legal problems. To explain why, we’ll begin with a brief overview of business terms, including “securities” and “insider trading.” Then, to answer Dr. B’s question, we’ll look at what kind of legal consequences may result if mental health professionals are found guilty of “misappropriating” confidential business information.
Securities and security rules
Approximately one-half to two-thirds of Americans have money invested in the stock market—either through their retirement plans, by owning mutual funds, or by holding stocks of individual companies.1 Stocks are a type of financial instrument, or security, that companies issue to raise capital. Companies also raise money by issuing debt, typically in the form of bonds that pay interest to the holder, who in buying the bond has in effect loaned money to the company. Derivatives refer to securities that have prices that move up or down depending on the value of some underlying asset, such as stock prices.2
Stock prices fluctuate in reaction to general economic developments—changes in the unemployment rate, in the cost of basic materials (eg, oil or metals used in manufacturing), or in government policies that influence consumers’ purchasing decisions. But the key factor in determining the price of a company’s stock is investors’ beliefs about the company’s future earnings.3 Because investors usually have to make educated guesses about a company’s future, actually knowing something about a company before the general public finds out would give an investor a huge—but possibly unfair—advantage over other investors.
Making markets fair for all investors is the key purpose of U.S. laws on trading securities. In the 1930s, Congress created the Securities and Exchange Commission (SEC), a federal agency charged with ensuring that companies report the truth about their financial situation and that potential investors receive full, fair disclosure of available public information.4 Among the many ways that the SEC does this is by enforcing regulations concerning “insider trading.”
‘Insider trading’
Corporate “insiders” (eg, directors or employees) often know a lot about how their businesses are doing, and they buy or sell stock in their own companies. Such trading is legal if the insiders follow federal regulations about the timing of their investments and report them publicly.
Insider trading is illegal, however, if an individual acquires material, nonpublic information about a corporation through a relationship that involves trust and confidence and then uses that information when buying or selling a security. The SEC has prosecuted corporate employees who traded securities after learning of confidential developments in their companies, friends and family members of corporate officers who bought or sold securities after getting such information, and employees of law firms who misused information they received while providing services to corporations whose securities they traded.5
To be guilty of insider trading, a person must:
- buy or sell a security based on information that the person realizes is material and nonpublic,6 and
- have received the confidential information under circumstances that create a duty of trust or confidence.7
If both of these conditions are met, the person has wrongfully used confidential information with which he was entrusted, or “misappropriated” that information for personal gain.8
Physicians sometimes gain information that, if used for investment decisions, might lead to accusations of insider trading. Stock prices of pharmaceutical companies rise before public announcements of clinical drug trials, which suggests that information about those results leaks out in advance.9 Recently, physicians have gotten into well-publicized legal trouble by making investment decisions based on information they obtained while participating on an institution’s board10 and from learning early results of clinical drug trials.11
But would it be wrong for a psychiatrist to make a potentially profitable investment based on information obtained incidentally during a treatment encounter? After all, it’s not as though the psychiatrist would be a corporate insider or would have acquired the information improperly. Yet courts have ruled that a psychiatrist’s trading on such information might constitute malpractice and could be grounds for even more serious legal consequences.
Potential malpractice issues
The federal court ruling in United States v Willis12 describes how a psychiatrist learned during treatment that a patient’s husband was seeking to become CEO of a large bank. Realizing that this development might make the bank more valuable, the psychiatrist told his broker what he had learned and purchased 13,000 shares of the bank’s stock for himself and his children. When the husband’s efforts were announced publicly a few weeks later, the psychiatrist sold the shares at a big profit.
Quoting the vow of confidentiality contained in the Hippocratic Oath (Box),13 the court held that the psychiatrist had an obligation to the patient not to disclose information learned during her treatment without her permission. The court said the patient “had an economic interest in preserving the confidentiality of the information disclosed,” and the psychiatrist’s actions “might have jeopardized her husband’s advancement” and financial benefits the wife would have gained. Also, the psychiatrist’s “disclosures jeopardized the psychiatrist-patient relationship,” which might negate the wife’s financial investment in her care, require her to find a new psychiatrist, or require additional treatment to deal with how the psychiatrist’s behavior had affected her.12
And about whatever I may see or hear in treatment, or even without treatment, in the life of human beings—things that should not ever be blurted out outside—I will remain silent, holding such things to be unutterable.
Source: Reference 13
More legal consequences
Dr. Willis had legal problems more serious than just a malpractice lawsuit. He faced criminal prosecution for insider trading and mail fraud, and the court refused to dismiss these charges. The court reasoned that the psychiatrist received the information while in a position of trust and confidence, and breached that trust when he used that confidential information for his personal benefit—behavior that meets the legal definition of “misappropriation.” Because the psychiatrist received stock trade confirmations through the U.S. mail, he also could face federal charges of mail fraud. Ultimately, Dr. Willis pled guilty and paid $137,000 in fines and penalties. Although Dr. Willis retained his New Jersey medical license and avoided a prison sentence, the district court sentenced him to 5 years of probation and required that he perform 3,000 hours of community service.14,15
In a second case,16 a licensed clinical social worker made investments through a broker based on information learned during a therapy session about upcoming business developments (the 1994 Lockheed-Martin Marietta merger). The social worker pled guilty to insider trading, forfeited the illegal gains, and paid a large fine.
Related Resources
- Insider trading versus medical professionalism. Lancet. 2005;366(9488):781.
- Nijm LM. The online message board controversy. Physicians hit with claims of libel and insider trading by their employers. J Leg Med. 2000;21(2):223-239.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jacobe D. In U.S., 54% have stock market investments, lowest since 1999. Gallup Economy. http://www.gallup.com/poll/147206/stock-market-investments-lowest-1999.aspx. Published April 20, 2011. Accessed October 9, 2012.
2. Roman S. Introduction to the mathematics of finance: from risk management to options pricing. New York NY: Springer-Verlag; 2004.
3. Elton EJ, Gruber MJ, Brown SJ, et al. Modern portfolio theory and investment analysis. Hoboken, NJ: John Wiley & Sons; 2010.
4. Keller E, Gehlmann GA. Introductory comment: a historical introduction to the Securities Act of 1933 and the Securities Exchange Act of 1934. Ohio State Law Journal. 1988;49:329-352.
5. U.S. Securities and Exchange Commission. Insider trading. http://www.sec.gov/answers/insider.htm. Published April 19, 2001. Accessed October 9, 2012.
6. 17 CFR 240. 10b5-1.
7. 17 CFR 240. 10b5-2.
8. United States v O’Hagan, 521 U.S. 642 (1997).
9. Rothenstein JM, Tomlinson G, Tannock IF, et al. Company stock prices before and after public announcements related to oncology drugs. J Natl Cancer Inst. 2011;103(20):1507-1512.
10. U.S. Securities and Exchange Commission. SEC charges five physicians with insider trading in stock of medical professional liability insurer. http://www.sec.gov/news/press/2012/2012-132.htm. Published July 10, 2012. Accessed October 9, 2012.
11. Two more are sentenced in insider trading cases. New York Times. December 21 2011:B9. http://www.nytimes.com/2011/12/22/business/in-crackdown-on-insider-trading-two-more-are-sentenced.html?_r=0. Accessed October 9, 2012.
12. United States v Willis, 737 F Supp 269 (SD NY 1990).
13. von Staden H. “In a pure and holy way”: personal and professional conduct in the Hippocratic Oath? J Hist Med Allied Sci. 1996;51(4):404-437.
14. 24 Sec Reg & L Rep (BNA) 7 (1992).
15. Psychiatrist is sentenced. New York Times. January 8 1992. http://www.nytimes.com/1992/01/08/business/credit-markets-psychiatrist-is-sentenced.html. Accessed November 5, 2012.
16. SEC v Cooper, Litigation Rel. No. 14754, 60 S.E.C. Docket 2430 (1995).
1. Jacobe D. In U.S., 54% have stock market investments, lowest since 1999. Gallup Economy. http://www.gallup.com/poll/147206/stock-market-investments-lowest-1999.aspx. Published April 20, 2011. Accessed October 9, 2012.
2. Roman S. Introduction to the mathematics of finance: from risk management to options pricing. New York NY: Springer-Verlag; 2004.
3. Elton EJ, Gruber MJ, Brown SJ, et al. Modern portfolio theory and investment analysis. Hoboken, NJ: John Wiley & Sons; 2010.
4. Keller E, Gehlmann GA. Introductory comment: a historical introduction to the Securities Act of 1933 and the Securities Exchange Act of 1934. Ohio State Law Journal. 1988;49:329-352.
5. U.S. Securities and Exchange Commission. Insider trading. http://www.sec.gov/answers/insider.htm. Published April 19, 2001. Accessed October 9, 2012.
6. 17 CFR 240. 10b5-1.
7. 17 CFR 240. 10b5-2.
8. United States v O’Hagan, 521 U.S. 642 (1997).
9. Rothenstein JM, Tomlinson G, Tannock IF, et al. Company stock prices before and after public announcements related to oncology drugs. J Natl Cancer Inst. 2011;103(20):1507-1512.
10. U.S. Securities and Exchange Commission. SEC charges five physicians with insider trading in stock of medical professional liability insurer. http://www.sec.gov/news/press/2012/2012-132.htm. Published July 10, 2012. Accessed October 9, 2012.
11. Two more are sentenced in insider trading cases. New York Times. December 21 2011:B9. http://www.nytimes.com/2011/12/22/business/in-crackdown-on-insider-trading-two-more-are-sentenced.html?_r=0. Accessed October 9, 2012.
12. United States v Willis, 737 F Supp 269 (SD NY 1990).
13. von Staden H. “In a pure and holy way”: personal and professional conduct in the Hippocratic Oath? J Hist Med Allied Sci. 1996;51(4):404-437.
14. 24 Sec Reg & L Rep (BNA) 7 (1992).
15. Psychiatrist is sentenced. New York Times. January 8 1992. http://www.nytimes.com/1992/01/08/business/credit-markets-psychiatrist-is-sentenced.html. Accessed November 5, 2012.
16. SEC v Cooper, Litigation Rel. No. 14754, 60 S.E.C. Docket 2430 (1995).
Why are metabolic monitoring guidelines being ignored?
The vast majority of psychiatrists and psychiatric nurse practitioners in the United States are aware of the following facts:
- The unhealthy lifestyle of persons with chronic psychotic disorders (sedentary living, smoking, poor diet) is conducive to weight gain, metabolic disorders, high cardiovascular risk, and premature mortality. 1
- Second-generation antipsychotics (SGAs) are associated with metabolic dysregulation, including obesity, diabetes, dyslipidemia, and hypertension.2
- The dramatic increase in diabetes, diabetic ketoacidosis, and death in the late 1990s and early 2000s prompted the FDA in August 2003 to apply a class warning to the labels of all SGAs and require that practitioners monitor metabolic parameters in patients receiving SGAs, before and after initiating therapy.3
- The consensus statement published by the American Psychiatric Association (APA) and American Diabetes Association (ADA) in February 2004 reviewed the metabolic side-effects literature of SGAs and included guidelines for monitoring the metabolic status of patients at baseline and after initiating treatment, including family history of metabolic disorders, body mass index, waist circumference, blood pressure, fasting glucose, and fasting lipids.4,5
- The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study6 confirmed the APA/ADA findings and indicated that 43% of 1,460 outpatients with schizophrenia met criteria for metabolic syndrome7 when they enrolled in the study and the prevalence of metabolic syndrome increased over the duration of the study.8
- The CATIE study also reported that a substantial proportion of patients with schizophrenia had no access to primary care and never received standard treatment for hyperlipidemia, hypertension, and diabetes,9 prompting an angry Current Psychiatry editorial.10
And now for the grim facts. It is simply shocking that many practitioners are not monitoring the metabolic status of their patients consistently or at all. A recent systematic review and meta-analysis of 48 published studies showed that clinicians who prescribed antipsychotics are not abiding by guidelines to monitor metabolic risk in their seriously ill psychotic patients.11 The inconsistent monitoring was evident not only in the United States but also in Canada, the United Kingdom, Australia, and Spain. Thirty-nine of those studies were conducted on the pattern of monitoring before any guidelines were published and 9 were conducted after monitoring guidelines were published. All of them were equally dismal in their findings.
The most urgent question is why aren’t practitioners monitoring vulnerable, seriously mentally ill patients to protect them from potential iatrogenic harm? If we don’t do it, who will? Wouldn’t we be outraged if a family member was not receiving standard clinical monitoring for a serious medical condition? The language that the FDA inserted in SGA labels specifically states that intervention is required if hyperglycemia emerges during monitoring, including treatment or switch to a metabolically more benign antipsychotic agent. How can an intervention be implemented if the monitoring is not done in a timely manner?
Persons suffering from psychotic disorders such as schizophrenia are compromised mentally and physically. They rely on us to give them proper treatment and follow-up, driven by evidence as well as compassion and commitment. Our guiding principle is to treat while doing no harm. The potential benefit of antipsychotic pharmacotherapy is widely accepted and the potential harm also is well recognized; published guidelines help protect patients via early detection of metabolic dysregulation. There is absolutely no excuse for failing to provide patients who need antipsychotics with consistent, guideline-based monitoring. No excuses. None.
1. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334-1349.
2. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects. A comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
3. Food and Drug Administration. Warning about hyperglycemia and atypical antipsychotic drugs. http://www.accessdata.fda.gov/psn/printer.cfm?id=229. Accessed November 14 2012.
4. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267-272.
5. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
6. Lieberman JA, Stroup TS, McEvoy JP, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223.
7. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
8. Meyer JM, Davis VG, Goff DC, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res. 2008;101(1-3):273-286.
9. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
10. Nasrallah HA. Dying too young. Current Psychiatry. 2007;6(1):15-16.
11. Mitchell AJ, Delaffon V, Vancampfort D, et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med. 2012;42(1):125-147.
The vast majority of psychiatrists and psychiatric nurse practitioners in the United States are aware of the following facts:
- The unhealthy lifestyle of persons with chronic psychotic disorders (sedentary living, smoking, poor diet) is conducive to weight gain, metabolic disorders, high cardiovascular risk, and premature mortality. 1
- Second-generation antipsychotics (SGAs) are associated with metabolic dysregulation, including obesity, diabetes, dyslipidemia, and hypertension.2
- The dramatic increase in diabetes, diabetic ketoacidosis, and death in the late 1990s and early 2000s prompted the FDA in August 2003 to apply a class warning to the labels of all SGAs and require that practitioners monitor metabolic parameters in patients receiving SGAs, before and after initiating therapy.3
- The consensus statement published by the American Psychiatric Association (APA) and American Diabetes Association (ADA) in February 2004 reviewed the metabolic side-effects literature of SGAs and included guidelines for monitoring the metabolic status of patients at baseline and after initiating treatment, including family history of metabolic disorders, body mass index, waist circumference, blood pressure, fasting glucose, and fasting lipids.4,5
- The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study6 confirmed the APA/ADA findings and indicated that 43% of 1,460 outpatients with schizophrenia met criteria for metabolic syndrome7 when they enrolled in the study and the prevalence of metabolic syndrome increased over the duration of the study.8
- The CATIE study also reported that a substantial proportion of patients with schizophrenia had no access to primary care and never received standard treatment for hyperlipidemia, hypertension, and diabetes,9 prompting an angry Current Psychiatry editorial.10
And now for the grim facts. It is simply shocking that many practitioners are not monitoring the metabolic status of their patients consistently or at all. A recent systematic review and meta-analysis of 48 published studies showed that clinicians who prescribed antipsychotics are not abiding by guidelines to monitor metabolic risk in their seriously ill psychotic patients.11 The inconsistent monitoring was evident not only in the United States but also in Canada, the United Kingdom, Australia, and Spain. Thirty-nine of those studies were conducted on the pattern of monitoring before any guidelines were published and 9 were conducted after monitoring guidelines were published. All of them were equally dismal in their findings.
The most urgent question is why aren’t practitioners monitoring vulnerable, seriously mentally ill patients to protect them from potential iatrogenic harm? If we don’t do it, who will? Wouldn’t we be outraged if a family member was not receiving standard clinical monitoring for a serious medical condition? The language that the FDA inserted in SGA labels specifically states that intervention is required if hyperglycemia emerges during monitoring, including treatment or switch to a metabolically more benign antipsychotic agent. How can an intervention be implemented if the monitoring is not done in a timely manner?
Persons suffering from psychotic disorders such as schizophrenia are compromised mentally and physically. They rely on us to give them proper treatment and follow-up, driven by evidence as well as compassion and commitment. Our guiding principle is to treat while doing no harm. The potential benefit of antipsychotic pharmacotherapy is widely accepted and the potential harm also is well recognized; published guidelines help protect patients via early detection of metabolic dysregulation. There is absolutely no excuse for failing to provide patients who need antipsychotics with consistent, guideline-based monitoring. No excuses. None.
The vast majority of psychiatrists and psychiatric nurse practitioners in the United States are aware of the following facts:
- The unhealthy lifestyle of persons with chronic psychotic disorders (sedentary living, smoking, poor diet) is conducive to weight gain, metabolic disorders, high cardiovascular risk, and premature mortality. 1
- Second-generation antipsychotics (SGAs) are associated with metabolic dysregulation, including obesity, diabetes, dyslipidemia, and hypertension.2
- The dramatic increase in diabetes, diabetic ketoacidosis, and death in the late 1990s and early 2000s prompted the FDA in August 2003 to apply a class warning to the labels of all SGAs and require that practitioners monitor metabolic parameters in patients receiving SGAs, before and after initiating therapy.3
- The consensus statement published by the American Psychiatric Association (APA) and American Diabetes Association (ADA) in February 2004 reviewed the metabolic side-effects literature of SGAs and included guidelines for monitoring the metabolic status of patients at baseline and after initiating treatment, including family history of metabolic disorders, body mass index, waist circumference, blood pressure, fasting glucose, and fasting lipids.4,5
- The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study6 confirmed the APA/ADA findings and indicated that 43% of 1,460 outpatients with schizophrenia met criteria for metabolic syndrome7 when they enrolled in the study and the prevalence of metabolic syndrome increased over the duration of the study.8
- The CATIE study also reported that a substantial proportion of patients with schizophrenia had no access to primary care and never received standard treatment for hyperlipidemia, hypertension, and diabetes,9 prompting an angry Current Psychiatry editorial.10
And now for the grim facts. It is simply shocking that many practitioners are not monitoring the metabolic status of their patients consistently or at all. A recent systematic review and meta-analysis of 48 published studies showed that clinicians who prescribed antipsychotics are not abiding by guidelines to monitor metabolic risk in their seriously ill psychotic patients.11 The inconsistent monitoring was evident not only in the United States but also in Canada, the United Kingdom, Australia, and Spain. Thirty-nine of those studies were conducted on the pattern of monitoring before any guidelines were published and 9 were conducted after monitoring guidelines were published. All of them were equally dismal in their findings.
The most urgent question is why aren’t practitioners monitoring vulnerable, seriously mentally ill patients to protect them from potential iatrogenic harm? If we don’t do it, who will? Wouldn’t we be outraged if a family member was not receiving standard clinical monitoring for a serious medical condition? The language that the FDA inserted in SGA labels specifically states that intervention is required if hyperglycemia emerges during monitoring, including treatment or switch to a metabolically more benign antipsychotic agent. How can an intervention be implemented if the monitoring is not done in a timely manner?
Persons suffering from psychotic disorders such as schizophrenia are compromised mentally and physically. They rely on us to give them proper treatment and follow-up, driven by evidence as well as compassion and commitment. Our guiding principle is to treat while doing no harm. The potential benefit of antipsychotic pharmacotherapy is widely accepted and the potential harm also is well recognized; published guidelines help protect patients via early detection of metabolic dysregulation. There is absolutely no excuse for failing to provide patients who need antipsychotics with consistent, guideline-based monitoring. No excuses. None.
1. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334-1349.
2. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects. A comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
3. Food and Drug Administration. Warning about hyperglycemia and atypical antipsychotic drugs. http://www.accessdata.fda.gov/psn/printer.cfm?id=229. Accessed November 14 2012.
4. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267-272.
5. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
6. Lieberman JA, Stroup TS, McEvoy JP, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223.
7. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
8. Meyer JM, Davis VG, Goff DC, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res. 2008;101(1-3):273-286.
9. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
10. Nasrallah HA. Dying too young. Current Psychiatry. 2007;6(1):15-16.
11. Mitchell AJ, Delaffon V, Vancampfort D, et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med. 2012;42(1):125-147.
1. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334-1349.
2. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects. A comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
3. Food and Drug Administration. Warning about hyperglycemia and atypical antipsychotic drugs. http://www.accessdata.fda.gov/psn/printer.cfm?id=229. Accessed November 14 2012.
4. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267-272.
5. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
6. Lieberman JA, Stroup TS, McEvoy JP, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223.
7. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
8. Meyer JM, Davis VG, Goff DC, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res. 2008;101(1-3):273-286.
9. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
10. Nasrallah HA. Dying too young. Current Psychiatry. 2007;6(1):15-16.
11. Mitchell AJ, Delaffon V, Vancampfort D, et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med. 2012;42(1):125-147.
How to stabilize an acutely psychotic patient
Acute psychosis is a symptom that can be caused by many psychiatric and medical conditions. Psychotic patients might be unable to provide a history or participate in treatment if they are agitated, hostile, or violent. An appropriate workup may reveal the etiology of the psychosis; secondary causes, such as medical illness and substance use, are prevalent in the emergency room (ER) setting. If the patient has an underlying primary psychotic disorder, such as schizophrenia or mania, illness-specific intervention will help acutely and long-term. With agitated and uncooperative psychotic patients, clinicians often have to intervene quickly to ensure the safety of the patient and those nearby.
This article focuses on the initial evaluation and treatment of psychotic patients in the ER, either by a psychiatric emergency service or a psychiatric consultant. This process can be broken down into:
- triage or initial clinical assessment
- initial psychiatric stabilization, including pharmacologic interventions and agitation management
- diagnostic workup to evaluate medical and psychiatric conditions
- further psychiatric evaluation
- determining safe disposition.1
Triage determines the next step
Initial clinical assessment and triage are necessary to select the appropriate immediate intervention. When a patient arrives in the ER, determine if he or she requires urgent medical attention. Basic initial screening should include:
- vital signs
- finger stick blood glucose
- medical history
- signs or symptoms of intoxication or withdrawal
- signs of trauma (eg, neck ligature marks, gunshot wounds, lacerations)
- asking the patient to give a brief history leading up to the current presentation.
A review of medical records may reveal patients’ medical and psychiatric history and allergies. Collateral documentation—such as ambulance run sheets or police reports—may provide additional information. If no immediate medical intervention is warranted, determine if the patient can wait in an open, unlocked waiting area or if he or she needs to be in an unlocked area with a sitter, a locked open area, or a secluded room with access to restraints. In general, psychotic patients who pose a threat of harm to themselves or others or cannot care for themselves because of their psychosis need locked areas or observation.
Initial psychiatric stabilization
Agitation is diagnostically unspecific but can occur in patients with psychosis. Psychotic patients can become unpredictably and impulsively aggressive and assaultive. Rapid intervention is necessary to minimize risk of bodily harm to the patient and those around the patient. Physicians often must make quick interventions based on limited clinical information. It is important to recognize early signs and symptoms of agitation, including:
- restlessness (pacing, fidgeting, hand wringing, fist clenching, posturing)
- irritability
- decreased attention
- inappropriate or hostile behaviors.2
Pharmacologic interventions. The initial goals of pharmacologic treatment are to calm the patient without oversedation, thereby allowing the patient to take part in his or her care and begin treatment for the primary psychotic illness.3,4 Offering oral medications first and a choice of medications may help a patient feel more in control of the situation. If a patient has to be physically restrained, pharmacotherapy may limit the amount of time spent in restraints.
Medication choice depends on several factors, including onset of action, available formulation (eg, IM, liquid, rapidly dissolving), the patient’s previous medication response, side effect profile, allergies or adverse reactions to medications, and medical comorbidities.3 If a patient has a known psychotic illness, it may be helpful to administer the patient’s regular antipsychotic or anxiolytic medication. Some medications, such as lithium, are not effective in the acute setting and should be avoided. Additionally, benzodiazepines other than lorazepam or midazolam should not be administered IM because of erratic absorption.
Antipsychotics can be used for psychotic patients with or without agitation. Benzodiazepines may treat agitation, but are not specific for psychosis. Haloperidol can be used to treat acute psychosis and has proven efficacy for agitation. Benzodiazepines can decrease acute agitation and have efficacy similar to haloperidol, but with more sedation.5 A combination of lorazepam and haloperidol is thought to be superior to either medication alone.6 Lorazepam helps maintain sedation and decreases potential side effects caused by haloperidol. Consensus guidelines from 2001 and 2005 recommend combined haloperidol and lorazepam for first-line treatment of acute agitation.3,7 High-potency antipsychotics such as haloperidol have an increased risk for extrapyramidal symptoms (EPS), particularly acute dystonic reactions—involuntary, sustained muscle contractions—in susceptible patients (eg, antipsychotic-naïve patients); consider starting diphenhydramine, 25 to 50 mg, or benztropine, 0.5 to 2 mg, to prevent EPS from high-potency antipsychotics (Algorithm 1).
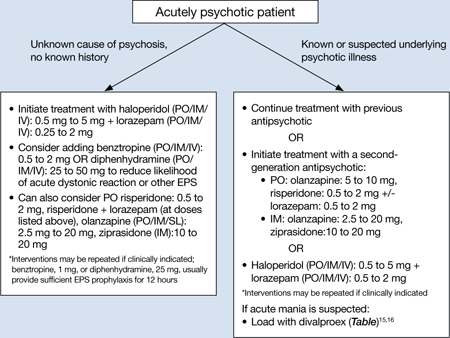
Algorithm 1: Treating acute psychosis: Choosing pharmacologic agents
EPS: extrapyramidal symptoms; PO: by mouth; SL: sublingual
Second-generation antipsychotics (SGAs) increasingly have been used for managing acute agitation in patients with an underlying psychotic disorder. Guidelines from a 2012 American Association for Emergency Psychiatry workgroup recommend using an SGA as monotherapy or in combination with another medication instead of haloperidol to treat agitated patients with a known psychotic disorder.8 Clinical policy guidelines from the American College of Emergency Physicians recommend antipsychotic monotherapy for agitation and initial treatment in patients with a known psychiatric illness for which antipsychotic treatment is indicated (eg, schizophrenia).9 For patients with known psychotic illness, expert opinion recommends oral risperidone or olanzapine.3,8 The combination of oral risperidone plus lorazepam may be as effective as the IM haloperidol and IM lorazepam combination.10 Patients who are too agitated to take oral doses may require parenteral medications. Ziprasidone, olanzapine, and aripiprazole are available in IM formulations. Ziprasidone, 20 mg IM, is well tolerated and has been shown to be effective in decreasing acute agitation symptoms in patients with psychotic disorders.11 Olanzapine is as effective as haloperidol in decreasing agitation in patients with schizophrenia, with lower rates of EPS.12 In a double-blind, placebo-controlled trial, psychotic symptoms in patients with schizophrenia or schizoaffective disorder decreased within 2 hours of IM olanzapine administration.13 Both IM ziprasidone and olanzapine have a relatively rapid onset of action (within 30 minutes), which makes them reasonable choices in the acute setting. Olanzapine has a long half-life (21 to 50 hours); therefore, patients’ comorbid medical conditions, such as cardiac abnormalities or hypotension, must be considered. If parenteral medication is required, IM olanzapine or IM ziprasidone is recommended.8 IM haloperidol with a benzodiazepine also can be considered.3
Coadministration of parenteral olanzapine and a benzodiazepine can lead to severe orthostatic hypotension and cardiac or respiratory depression and should be avoided in geriatric patients.14 Finally, it is important to rule out presentations that may worsen with antipsychotic treatment, including phencyclidine (PCP) toxicity (could worsen dystonic reactions), anticholinergic delirium, neuroleptic malignant syndrome (NMS), or catatonia.
If a patient does not respond to the initial dose of a medication, the dose may be repeated. However, doses should not be repeated until a patient is so sedated that he or she cannot take part in his or her care, or until he or she has developed significant EPS.
In addition to antipsychotics, consider loading with oral divalproex for patients who are acutely psychotic in the context of a manic episode (Table).15,16 Higher serum divalproex levels—target serum levels >94 μg/mL—are associated with greater efficacy as measured by change from baseline in Mania Rating Scale or Young Mania Rating Scale scores compared with placebo.15 For acutely psychotic schizophrenia patients, there is evidence of benefit with initial treatment with divalproex combined with an SGA. In a randomized, double-blind study, patients treated with divalproex plus olanzapine or risperidone showed quicker initial resolution of psychotic symptoms compared with olanzapine or risperidone monotherapy, but no better long-term benefit.16 Clinicians may consider this well-tolerated combination after an appropriate medical workup. This finding of early benefit was not replicated with divalproex extended-release.17
Table
Divalproex dosing for patients with acute psychosis and mania
| Initial dose | Titration | |
|---|---|---|
| Acute mania15 | Divalproex delayed-release: 750 mg/d Divalproex extended-release: 20 mg/kg/d | Increase to clinical effectiveness or maximum serum level of 125 μg/mL |
| Exacerbation of psychosis16 | Divalproex: 15 mg/kg/d (in 2 doses) | Increase to clinical effectiveness over 12 days or maximum dosage of 30 mg/kg/d |
Side effects and adverse reactions. Treatment with antipsychotics may cause QTc interval prolongation, which can lead to increased risk for torsades de pointes and sudden death due to ventricular fibrillation. However, there have been few cases of torsades de pointes after oral haloperidol and none with IM haloperidol compared with at least 30 cases of torsades de pointes after IV haloperidol treatment. Torsades de pointes after risperidone, olanzapine, or ziprasidone treatment has not been reported.18
Hypotension and bradycardia may occur in patients treated with olanzapine; however, these signs occur less frequently in agitated patients.18 Antipsychotic treatment increases risk for EPS, including acute dystonia, akathisia (subjective restlessness with desire to move), and parkinsonism (shuffling gait, resting tremor, rigidity and bradykinesia), as well as NMS.
Nonpharmacologic interventions. Verbal intervention to try to de-escalate an agitated, psychotic patient should be attempted first; however, this is not always possible. Other behavioral interventions include offering a meal, blanket, or pillow, or other comforting options to decrease the patient’s anxiety associated with psychosis.2 However, if agitated psychotic patients continue to display aggressive behaviors and pose a risk of harm to themselves or those around them, physical restraints should be considered because the clinician must balance protecting the patient’s rights with others’ safety. If physical restraints are used, medication also should be administered. Remove physical restraints as soon as safely possible; the Joint Commission has established standards for minimizing harm when using physical restraints.19
Diagnostic workup
Once a patient is medically stable in the ER, begin further workup of the etiology of the psychosis (Algorithm 2). All patients should have a physical exam, provided they are calm and in behavioral control. Monitor vital signs; patients at risk of withdrawal from substances should be monitored more frequently. Although there is no established standard for “medical clearance” of a psychiatric patient,20 all patients should undergo basic laboratory tests, including basic metabolic panel, complete blood count, and urine toxicology. The extent of the workup is determined by the clinical situation and suspected cause of psychosis.21
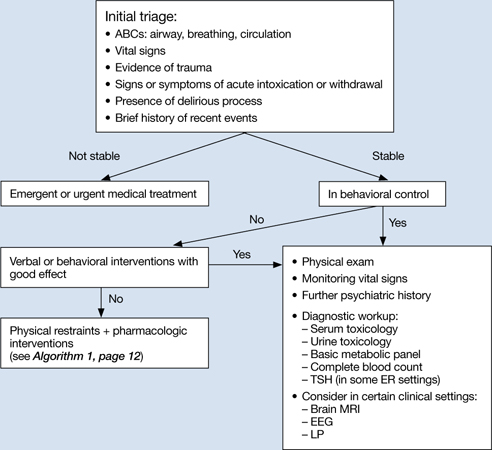
Algorithm 2: Diagnostic workup of an acutely psychotic patient
ER: emergency room; EEG: electroencephalography; LP: lumbar puncture; TSH: thyroid-stimulating hormone
If you suspect delirium, the underlying medical etiology must be identified and treated. Up to 40% of hospitalized patients with delirium may have psychosis.22 Psychosis in a delirious patient may be characterized by poorly formed delusions and visual hallucinations. Delirious patients often are inattentive, easily distracted, and disoriented, with a fluctuating clinical course. Patients with psychosis generally do not have impaired attention and are alert with intact memory. However, acutely psychotic patients may be quite disorganized and uncooperative, which makes it difficult to distinguish between these 2 diagnoses. Serial exams may help clarify the clinical picture. It is important to remember that patients with a history of a psychotic disorder may have a superimposed delirium.
In young patients (age 18 to 30) with new-onset psychosis, consider drug-induced psychosis; PCP, lysergic acid diethylamide, and methamphetamine intoxication and withdrawal can lead to psychotic presentations. Additionally, comorbid substance use is common among patients with primary psychotic disorders. One study found 37% of first-episode psychotic patients misused drugs or alcohol, similar to the lifetime rate of patients with chronic psychotic disorders.23,24 Check urine and serum toxicology screens and obtain relevant substance use history. Brain MRI may be considered for patients with first presentation of psychosis; however, there is little evidence to support head CT imaging unless there is known head trauma.25 Electroencephalography and lumbar puncture can be considered if clinically indicated.
Further psychiatric evaluation
Obtaining a psychiatric history is necessary to determine the etiology of the acute psychotic presentation. The timing and duration of psychotic symptoms are key. Acute symptom onset with fluctuating course and impaired attention suggests a delirious process. A gradual decline in functioning over several months to years in a young person suggests a first episode of a psychotic disorder (eg, schizophrenia). Drug abuse is common among young persons with a psychotic disorder and a positive drug screen for a psychogenic substance does not exclude a primary psychotic disorder.
If a patient has a history of schizophrenia, bipolar disorder, or psychotic depression, acutely worsening psychosis may be considered an acute or chronic presentation. Even in patients diagnosed with a psychotic illness, it is necessary to determine the cause of symptom exacerbation. Medication nonadherence (which can be partial), substance use, psychosocial stressors, or underlying medical illness should be considered. Collateral information from family or friends may be crucial to understanding a patient’s presentation.
Safe disposition
Patients who pose a risk of harm to themselves or others or who are so impaired by their psychosis that they cannot care for themselves generally should be admitted to an inpatient psychiatric facility. For some psychotic patients who are agreeable to treatment and not prone to violence, less restrictive settings—such as a crisis intervention unit or respite facility—may be appropriate. A patient with first-episode psychosis could be admitted for further diagnostic clarification and treatment initiation. Manic patients often have no insight into their illness and may need hospitalization for containment and assurance of medication adherence. Goals of inpatient care include initiating or resuming pharmacologic treatment to reduce psychotic symptoms and beginning the recovery process. Response rates—defined as ≥20% improvement in total score on a psychopathology scale such as the Positive and Negative Syndrome Scale—will vary, but can take ≥4 weeks in some patients with first-episode schizophrenia.26 However, most patients will be stabilized and ready for discharge before 4 weeks. Family education and alliance building with the patient and family are important during hospitalization.
Related Resources
- Schwartz S, Weathers, M. The psychotic patient. In: Riba MB, Ravindranath D, eds. Clinical manual of emergency psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2010:115-140.
- American Association for Emergency Psychiatry. http://emergencypsychiatry.org.
Drug Brand Names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Haloperidol • Haldol
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Midazolam • Versed
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Freudenreich receives grant or research support from Beacon Health Strategies, Global Medical Education, MGH Psychiatry Academy, Optimal Medicine, Pfizer Inc., and PsychoGenics.
Drs. Brown and Stoklosa report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Marco CA, Vaughan J. Emergency management of agitation in schizophrenia. Am J Emerg Med. 2005;23(6):767-776.
2. Freudenreich O. Emergency management of acute psychosis. In: Freudenreich O ed. Psychotic disorders: a practical guide. New York, NY: Wolter Kluwer/Lippincott Williams & Wilkins; 2008:72–78.
3. Allen MH, Currier GW, Carpenter D, et al. Expert Consensus Panel for Behavioral Emergencies 2005. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(1 suppl):S5-S108.
4. National Institute for Health and Clinical Excellence. Schizophrenia: core interventions in the treatment and management of schizophrenia in primary and secondary care. London United Kingdom: National Institute for Clinical Excellence; 2002.
5. Allen MH. Managing the agitated psychotic patient: a reappraisal of the evidence. J Clin Psychiatry. 2000;61 (14 suppl):S11-S20.
6. Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective, double-blind, emergency department study. Am J Emerg Med. 1997;15(4):335-340.
7. Allen MH, Currier GW, Hughes DH, et al. Expert Consensus Panel for Behavioral Emergencies. The expert consensus guideline series. Treatment of behavioral emergencies. Postgrad Med. 2001;(Spec no):1-88.
8. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of American Association for Emergency Psychiatry project BETA psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34.
9. Lukens TW, Wolf SJ, Edlow JA, et al. American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department. Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med. 2006;47(1):79-99.
10. Currier GW, Chou JC, Feifel D, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65(3):386-394.
11. Daniel DG, Potkin SG, Reeves KR, et al. Intramuscular (IM) ziprasidone 20 mg is effective in reducing agitation associated with psychosis: a double-blind, randomized trial. Psychopharmacology (Berl). 2001;155(2):128-134.
12. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatry. 2001;158(7):1149-1151.
13. Kapur S, Arenovic T, Agid O, et al. Evidence for onset of antipsychotic effects within the first 24 hours of treatment. Am J Psychiatry. 2005;162(5):939-946.
14. Marder SR, Sorasburu S, Dunayevic E, et al. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatry. 2010;71(4):433-441.
15. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
16. Casey DE, Daniel DG, Wassef AA, et al. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacology. 2003;28(1):182-192.
17. Casey DE, Daniel DG, Tamminga C, et al. Divalproex ER combined with olanzapine or risperidone for treatment of acute exacerbations of schizophrenia. Neuropsychopharmacology. 2009;34(5):1330-1338.
18. Currier GW, Allen MH, Bunney EB, et al. Safety of medications used to treat acute agitation. J Emerg Med. 2004;27(4 suppl):S19-S24.
19. The Joint Commission. Sentinel event alert. Preventing restraint deaths. Published November 18 1998. http://www.jointcommission.org/assets/1/18/SEA_8.pdf. Accessed October 26, 2012
20. Janiak BD, Atteberry S. Medical clearance of the psychiatric patient in the emergency department. J Emerg Med. 2012;43(5):866-870.
21. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
22. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
23. Cantwell R, Brewin J, Glazebrook C, et al. Prevalence of substance misuse in first-episode psychosis. Br J Psychiatry. 1999;174:150-153.
24. Green AI, Tohen MF, Hamer RM, et al. First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr Res. 2004;66(2-3):125-135.
25. Goulet K, Deschamps B, Evoy F, et al. Use of brain imaging (computed tomography and magnetic resonance imaging) in first-episode psychosis: review and retrospective study. Can J Psychiatry. 2009;54(7):493-501.
26. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
Acute psychosis is a symptom that can be caused by many psychiatric and medical conditions. Psychotic patients might be unable to provide a history or participate in treatment if they are agitated, hostile, or violent. An appropriate workup may reveal the etiology of the psychosis; secondary causes, such as medical illness and substance use, are prevalent in the emergency room (ER) setting. If the patient has an underlying primary psychotic disorder, such as schizophrenia or mania, illness-specific intervention will help acutely and long-term. With agitated and uncooperative psychotic patients, clinicians often have to intervene quickly to ensure the safety of the patient and those nearby.
This article focuses on the initial evaluation and treatment of psychotic patients in the ER, either by a psychiatric emergency service or a psychiatric consultant. This process can be broken down into:
- triage or initial clinical assessment
- initial psychiatric stabilization, including pharmacologic interventions and agitation management
- diagnostic workup to evaluate medical and psychiatric conditions
- further psychiatric evaluation
- determining safe disposition.1
Triage determines the next step
Initial clinical assessment and triage are necessary to select the appropriate immediate intervention. When a patient arrives in the ER, determine if he or she requires urgent medical attention. Basic initial screening should include:
- vital signs
- finger stick blood glucose
- medical history
- signs or symptoms of intoxication or withdrawal
- signs of trauma (eg, neck ligature marks, gunshot wounds, lacerations)
- asking the patient to give a brief history leading up to the current presentation.
A review of medical records may reveal patients’ medical and psychiatric history and allergies. Collateral documentation—such as ambulance run sheets or police reports—may provide additional information. If no immediate medical intervention is warranted, determine if the patient can wait in an open, unlocked waiting area or if he or she needs to be in an unlocked area with a sitter, a locked open area, or a secluded room with access to restraints. In general, psychotic patients who pose a threat of harm to themselves or others or cannot care for themselves because of their psychosis need locked areas or observation.
Initial psychiatric stabilization
Agitation is diagnostically unspecific but can occur in patients with psychosis. Psychotic patients can become unpredictably and impulsively aggressive and assaultive. Rapid intervention is necessary to minimize risk of bodily harm to the patient and those around the patient. Physicians often must make quick interventions based on limited clinical information. It is important to recognize early signs and symptoms of agitation, including:
- restlessness (pacing, fidgeting, hand wringing, fist clenching, posturing)
- irritability
- decreased attention
- inappropriate or hostile behaviors.2
Pharmacologic interventions. The initial goals of pharmacologic treatment are to calm the patient without oversedation, thereby allowing the patient to take part in his or her care and begin treatment for the primary psychotic illness.3,4 Offering oral medications first and a choice of medications may help a patient feel more in control of the situation. If a patient has to be physically restrained, pharmacotherapy may limit the amount of time spent in restraints.
Medication choice depends on several factors, including onset of action, available formulation (eg, IM, liquid, rapidly dissolving), the patient’s previous medication response, side effect profile, allergies or adverse reactions to medications, and medical comorbidities.3 If a patient has a known psychotic illness, it may be helpful to administer the patient’s regular antipsychotic or anxiolytic medication. Some medications, such as lithium, are not effective in the acute setting and should be avoided. Additionally, benzodiazepines other than lorazepam or midazolam should not be administered IM because of erratic absorption.
Antipsychotics can be used for psychotic patients with or without agitation. Benzodiazepines may treat agitation, but are not specific for psychosis. Haloperidol can be used to treat acute psychosis and has proven efficacy for agitation. Benzodiazepines can decrease acute agitation and have efficacy similar to haloperidol, but with more sedation.5 A combination of lorazepam and haloperidol is thought to be superior to either medication alone.6 Lorazepam helps maintain sedation and decreases potential side effects caused by haloperidol. Consensus guidelines from 2001 and 2005 recommend combined haloperidol and lorazepam for first-line treatment of acute agitation.3,7 High-potency antipsychotics such as haloperidol have an increased risk for extrapyramidal symptoms (EPS), particularly acute dystonic reactions—involuntary, sustained muscle contractions—in susceptible patients (eg, antipsychotic-naïve patients); consider starting diphenhydramine, 25 to 50 mg, or benztropine, 0.5 to 2 mg, to prevent EPS from high-potency antipsychotics (Algorithm 1).

Algorithm 1: Treating acute psychosis: Choosing pharmacologic agents
EPS: extrapyramidal symptoms; PO: by mouth; SL: sublingual
Second-generation antipsychotics (SGAs) increasingly have been used for managing acute agitation in patients with an underlying psychotic disorder. Guidelines from a 2012 American Association for Emergency Psychiatry workgroup recommend using an SGA as monotherapy or in combination with another medication instead of haloperidol to treat agitated patients with a known psychotic disorder.8 Clinical policy guidelines from the American College of Emergency Physicians recommend antipsychotic monotherapy for agitation and initial treatment in patients with a known psychiatric illness for which antipsychotic treatment is indicated (eg, schizophrenia).9 For patients with known psychotic illness, expert opinion recommends oral risperidone or olanzapine.3,8 The combination of oral risperidone plus lorazepam may be as effective as the IM haloperidol and IM lorazepam combination.10 Patients who are too agitated to take oral doses may require parenteral medications. Ziprasidone, olanzapine, and aripiprazole are available in IM formulations. Ziprasidone, 20 mg IM, is well tolerated and has been shown to be effective in decreasing acute agitation symptoms in patients with psychotic disorders.11 Olanzapine is as effective as haloperidol in decreasing agitation in patients with schizophrenia, with lower rates of EPS.12 In a double-blind, placebo-controlled trial, psychotic symptoms in patients with schizophrenia or schizoaffective disorder decreased within 2 hours of IM olanzapine administration.13 Both IM ziprasidone and olanzapine have a relatively rapid onset of action (within 30 minutes), which makes them reasonable choices in the acute setting. Olanzapine has a long half-life (21 to 50 hours); therefore, patients’ comorbid medical conditions, such as cardiac abnormalities or hypotension, must be considered. If parenteral medication is required, IM olanzapine or IM ziprasidone is recommended.8 IM haloperidol with a benzodiazepine also can be considered.3
Coadministration of parenteral olanzapine and a benzodiazepine can lead to severe orthostatic hypotension and cardiac or respiratory depression and should be avoided in geriatric patients.14 Finally, it is important to rule out presentations that may worsen with antipsychotic treatment, including phencyclidine (PCP) toxicity (could worsen dystonic reactions), anticholinergic delirium, neuroleptic malignant syndrome (NMS), or catatonia.
If a patient does not respond to the initial dose of a medication, the dose may be repeated. However, doses should not be repeated until a patient is so sedated that he or she cannot take part in his or her care, or until he or she has developed significant EPS.
In addition to antipsychotics, consider loading with oral divalproex for patients who are acutely psychotic in the context of a manic episode (Table).15,16 Higher serum divalproex levels—target serum levels >94 μg/mL—are associated with greater efficacy as measured by change from baseline in Mania Rating Scale or Young Mania Rating Scale scores compared with placebo.15 For acutely psychotic schizophrenia patients, there is evidence of benefit with initial treatment with divalproex combined with an SGA. In a randomized, double-blind study, patients treated with divalproex plus olanzapine or risperidone showed quicker initial resolution of psychotic symptoms compared with olanzapine or risperidone monotherapy, but no better long-term benefit.16 Clinicians may consider this well-tolerated combination after an appropriate medical workup. This finding of early benefit was not replicated with divalproex extended-release.17
Table
Divalproex dosing for patients with acute psychosis and mania
| Initial dose | Titration | |
|---|---|---|
| Acute mania15 | Divalproex delayed-release: 750 mg/d Divalproex extended-release: 20 mg/kg/d | Increase to clinical effectiveness or maximum serum level of 125 μg/mL |
| Exacerbation of psychosis16 | Divalproex: 15 mg/kg/d (in 2 doses) | Increase to clinical effectiveness over 12 days or maximum dosage of 30 mg/kg/d |
Side effects and adverse reactions. Treatment with antipsychotics may cause QTc interval prolongation, which can lead to increased risk for torsades de pointes and sudden death due to ventricular fibrillation. However, there have been few cases of torsades de pointes after oral haloperidol and none with IM haloperidol compared with at least 30 cases of torsades de pointes after IV haloperidol treatment. Torsades de pointes after risperidone, olanzapine, or ziprasidone treatment has not been reported.18
Hypotension and bradycardia may occur in patients treated with olanzapine; however, these signs occur less frequently in agitated patients.18 Antipsychotic treatment increases risk for EPS, including acute dystonia, akathisia (subjective restlessness with desire to move), and parkinsonism (shuffling gait, resting tremor, rigidity and bradykinesia), as well as NMS.
Nonpharmacologic interventions. Verbal intervention to try to de-escalate an agitated, psychotic patient should be attempted first; however, this is not always possible. Other behavioral interventions include offering a meal, blanket, or pillow, or other comforting options to decrease the patient’s anxiety associated with psychosis.2 However, if agitated psychotic patients continue to display aggressive behaviors and pose a risk of harm to themselves or those around them, physical restraints should be considered because the clinician must balance protecting the patient’s rights with others’ safety. If physical restraints are used, medication also should be administered. Remove physical restraints as soon as safely possible; the Joint Commission has established standards for minimizing harm when using physical restraints.19
Diagnostic workup
Once a patient is medically stable in the ER, begin further workup of the etiology of the psychosis (Algorithm 2). All patients should have a physical exam, provided they are calm and in behavioral control. Monitor vital signs; patients at risk of withdrawal from substances should be monitored more frequently. Although there is no established standard for “medical clearance” of a psychiatric patient,20 all patients should undergo basic laboratory tests, including basic metabolic panel, complete blood count, and urine toxicology. The extent of the workup is determined by the clinical situation and suspected cause of psychosis.21

Algorithm 2: Diagnostic workup of an acutely psychotic patient
ER: emergency room; EEG: electroencephalography; LP: lumbar puncture; TSH: thyroid-stimulating hormone
If you suspect delirium, the underlying medical etiology must be identified and treated. Up to 40% of hospitalized patients with delirium may have psychosis.22 Psychosis in a delirious patient may be characterized by poorly formed delusions and visual hallucinations. Delirious patients often are inattentive, easily distracted, and disoriented, with a fluctuating clinical course. Patients with psychosis generally do not have impaired attention and are alert with intact memory. However, acutely psychotic patients may be quite disorganized and uncooperative, which makes it difficult to distinguish between these 2 diagnoses. Serial exams may help clarify the clinical picture. It is important to remember that patients with a history of a psychotic disorder may have a superimposed delirium.
In young patients (age 18 to 30) with new-onset psychosis, consider drug-induced psychosis; PCP, lysergic acid diethylamide, and methamphetamine intoxication and withdrawal can lead to psychotic presentations. Additionally, comorbid substance use is common among patients with primary psychotic disorders. One study found 37% of first-episode psychotic patients misused drugs or alcohol, similar to the lifetime rate of patients with chronic psychotic disorders.23,24 Check urine and serum toxicology screens and obtain relevant substance use history. Brain MRI may be considered for patients with first presentation of psychosis; however, there is little evidence to support head CT imaging unless there is known head trauma.25 Electroencephalography and lumbar puncture can be considered if clinically indicated.
Further psychiatric evaluation
Obtaining a psychiatric history is necessary to determine the etiology of the acute psychotic presentation. The timing and duration of psychotic symptoms are key. Acute symptom onset with fluctuating course and impaired attention suggests a delirious process. A gradual decline in functioning over several months to years in a young person suggests a first episode of a psychotic disorder (eg, schizophrenia). Drug abuse is common among young persons with a psychotic disorder and a positive drug screen for a psychogenic substance does not exclude a primary psychotic disorder.
If a patient has a history of schizophrenia, bipolar disorder, or psychotic depression, acutely worsening psychosis may be considered an acute or chronic presentation. Even in patients diagnosed with a psychotic illness, it is necessary to determine the cause of symptom exacerbation. Medication nonadherence (which can be partial), substance use, psychosocial stressors, or underlying medical illness should be considered. Collateral information from family or friends may be crucial to understanding a patient’s presentation.
Safe disposition
Patients who pose a risk of harm to themselves or others or who are so impaired by their psychosis that they cannot care for themselves generally should be admitted to an inpatient psychiatric facility. For some psychotic patients who are agreeable to treatment and not prone to violence, less restrictive settings—such as a crisis intervention unit or respite facility—may be appropriate. A patient with first-episode psychosis could be admitted for further diagnostic clarification and treatment initiation. Manic patients often have no insight into their illness and may need hospitalization for containment and assurance of medication adherence. Goals of inpatient care include initiating or resuming pharmacologic treatment to reduce psychotic symptoms and beginning the recovery process. Response rates—defined as ≥20% improvement in total score on a psychopathology scale such as the Positive and Negative Syndrome Scale—will vary, but can take ≥4 weeks in some patients with first-episode schizophrenia.26 However, most patients will be stabilized and ready for discharge before 4 weeks. Family education and alliance building with the patient and family are important during hospitalization.
Related Resources
- Schwartz S, Weathers, M. The psychotic patient. In: Riba MB, Ravindranath D, eds. Clinical manual of emergency psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2010:115-140.
- American Association for Emergency Psychiatry. http://emergencypsychiatry.org.
Drug Brand Names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Haloperidol • Haldol
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Midazolam • Versed
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Freudenreich receives grant or research support from Beacon Health Strategies, Global Medical Education, MGH Psychiatry Academy, Optimal Medicine, Pfizer Inc., and PsychoGenics.
Drs. Brown and Stoklosa report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acute psychosis is a symptom that can be caused by many psychiatric and medical conditions. Psychotic patients might be unable to provide a history or participate in treatment if they are agitated, hostile, or violent. An appropriate workup may reveal the etiology of the psychosis; secondary causes, such as medical illness and substance use, are prevalent in the emergency room (ER) setting. If the patient has an underlying primary psychotic disorder, such as schizophrenia or mania, illness-specific intervention will help acutely and long-term. With agitated and uncooperative psychotic patients, clinicians often have to intervene quickly to ensure the safety of the patient and those nearby.
This article focuses on the initial evaluation and treatment of psychotic patients in the ER, either by a psychiatric emergency service or a psychiatric consultant. This process can be broken down into:
- triage or initial clinical assessment
- initial psychiatric stabilization, including pharmacologic interventions and agitation management
- diagnostic workup to evaluate medical and psychiatric conditions
- further psychiatric evaluation
- determining safe disposition.1
Triage determines the next step
Initial clinical assessment and triage are necessary to select the appropriate immediate intervention. When a patient arrives in the ER, determine if he or she requires urgent medical attention. Basic initial screening should include:
- vital signs
- finger stick blood glucose
- medical history
- signs or symptoms of intoxication or withdrawal
- signs of trauma (eg, neck ligature marks, gunshot wounds, lacerations)
- asking the patient to give a brief history leading up to the current presentation.
A review of medical records may reveal patients’ medical and psychiatric history and allergies. Collateral documentation—such as ambulance run sheets or police reports—may provide additional information. If no immediate medical intervention is warranted, determine if the patient can wait in an open, unlocked waiting area or if he or she needs to be in an unlocked area with a sitter, a locked open area, or a secluded room with access to restraints. In general, psychotic patients who pose a threat of harm to themselves or others or cannot care for themselves because of their psychosis need locked areas or observation.
Initial psychiatric stabilization
Agitation is diagnostically unspecific but can occur in patients with psychosis. Psychotic patients can become unpredictably and impulsively aggressive and assaultive. Rapid intervention is necessary to minimize risk of bodily harm to the patient and those around the patient. Physicians often must make quick interventions based on limited clinical information. It is important to recognize early signs and symptoms of agitation, including:
- restlessness (pacing, fidgeting, hand wringing, fist clenching, posturing)
- irritability
- decreased attention
- inappropriate or hostile behaviors.2
Pharmacologic interventions. The initial goals of pharmacologic treatment are to calm the patient without oversedation, thereby allowing the patient to take part in his or her care and begin treatment for the primary psychotic illness.3,4 Offering oral medications first and a choice of medications may help a patient feel more in control of the situation. If a patient has to be physically restrained, pharmacotherapy may limit the amount of time spent in restraints.
Medication choice depends on several factors, including onset of action, available formulation (eg, IM, liquid, rapidly dissolving), the patient’s previous medication response, side effect profile, allergies or adverse reactions to medications, and medical comorbidities.3 If a patient has a known psychotic illness, it may be helpful to administer the patient’s regular antipsychotic or anxiolytic medication. Some medications, such as lithium, are not effective in the acute setting and should be avoided. Additionally, benzodiazepines other than lorazepam or midazolam should not be administered IM because of erratic absorption.
Antipsychotics can be used for psychotic patients with or without agitation. Benzodiazepines may treat agitation, but are not specific for psychosis. Haloperidol can be used to treat acute psychosis and has proven efficacy for agitation. Benzodiazepines can decrease acute agitation and have efficacy similar to haloperidol, but with more sedation.5 A combination of lorazepam and haloperidol is thought to be superior to either medication alone.6 Lorazepam helps maintain sedation and decreases potential side effects caused by haloperidol. Consensus guidelines from 2001 and 2005 recommend combined haloperidol and lorazepam for first-line treatment of acute agitation.3,7 High-potency antipsychotics such as haloperidol have an increased risk for extrapyramidal symptoms (EPS), particularly acute dystonic reactions—involuntary, sustained muscle contractions—in susceptible patients (eg, antipsychotic-naïve patients); consider starting diphenhydramine, 25 to 50 mg, or benztropine, 0.5 to 2 mg, to prevent EPS from high-potency antipsychotics (Algorithm 1).

Algorithm 1: Treating acute psychosis: Choosing pharmacologic agents
EPS: extrapyramidal symptoms; PO: by mouth; SL: sublingual
Second-generation antipsychotics (SGAs) increasingly have been used for managing acute agitation in patients with an underlying psychotic disorder. Guidelines from a 2012 American Association for Emergency Psychiatry workgroup recommend using an SGA as monotherapy or in combination with another medication instead of haloperidol to treat agitated patients with a known psychotic disorder.8 Clinical policy guidelines from the American College of Emergency Physicians recommend antipsychotic monotherapy for agitation and initial treatment in patients with a known psychiatric illness for which antipsychotic treatment is indicated (eg, schizophrenia).9 For patients with known psychotic illness, expert opinion recommends oral risperidone or olanzapine.3,8 The combination of oral risperidone plus lorazepam may be as effective as the IM haloperidol and IM lorazepam combination.10 Patients who are too agitated to take oral doses may require parenteral medications. Ziprasidone, olanzapine, and aripiprazole are available in IM formulations. Ziprasidone, 20 mg IM, is well tolerated and has been shown to be effective in decreasing acute agitation symptoms in patients with psychotic disorders.11 Olanzapine is as effective as haloperidol in decreasing agitation in patients with schizophrenia, with lower rates of EPS.12 In a double-blind, placebo-controlled trial, psychotic symptoms in patients with schizophrenia or schizoaffective disorder decreased within 2 hours of IM olanzapine administration.13 Both IM ziprasidone and olanzapine have a relatively rapid onset of action (within 30 minutes), which makes them reasonable choices in the acute setting. Olanzapine has a long half-life (21 to 50 hours); therefore, patients’ comorbid medical conditions, such as cardiac abnormalities or hypotension, must be considered. If parenteral medication is required, IM olanzapine or IM ziprasidone is recommended.8 IM haloperidol with a benzodiazepine also can be considered.3
Coadministration of parenteral olanzapine and a benzodiazepine can lead to severe orthostatic hypotension and cardiac or respiratory depression and should be avoided in geriatric patients.14 Finally, it is important to rule out presentations that may worsen with antipsychotic treatment, including phencyclidine (PCP) toxicity (could worsen dystonic reactions), anticholinergic delirium, neuroleptic malignant syndrome (NMS), or catatonia.
If a patient does not respond to the initial dose of a medication, the dose may be repeated. However, doses should not be repeated until a patient is so sedated that he or she cannot take part in his or her care, or until he or she has developed significant EPS.
In addition to antipsychotics, consider loading with oral divalproex for patients who are acutely psychotic in the context of a manic episode (Table).15,16 Higher serum divalproex levels—target serum levels >94 μg/mL—are associated with greater efficacy as measured by change from baseline in Mania Rating Scale or Young Mania Rating Scale scores compared with placebo.15 For acutely psychotic schizophrenia patients, there is evidence of benefit with initial treatment with divalproex combined with an SGA. In a randomized, double-blind study, patients treated with divalproex plus olanzapine or risperidone showed quicker initial resolution of psychotic symptoms compared with olanzapine or risperidone monotherapy, but no better long-term benefit.16 Clinicians may consider this well-tolerated combination after an appropriate medical workup. This finding of early benefit was not replicated with divalproex extended-release.17
Table
Divalproex dosing for patients with acute psychosis and mania
| Initial dose | Titration | |
|---|---|---|
| Acute mania15 | Divalproex delayed-release: 750 mg/d Divalproex extended-release: 20 mg/kg/d | Increase to clinical effectiveness or maximum serum level of 125 μg/mL |
| Exacerbation of psychosis16 | Divalproex: 15 mg/kg/d (in 2 doses) | Increase to clinical effectiveness over 12 days or maximum dosage of 30 mg/kg/d |
Side effects and adverse reactions. Treatment with antipsychotics may cause QTc interval prolongation, which can lead to increased risk for torsades de pointes and sudden death due to ventricular fibrillation. However, there have been few cases of torsades de pointes after oral haloperidol and none with IM haloperidol compared with at least 30 cases of torsades de pointes after IV haloperidol treatment. Torsades de pointes after risperidone, olanzapine, or ziprasidone treatment has not been reported.18
Hypotension and bradycardia may occur in patients treated with olanzapine; however, these signs occur less frequently in agitated patients.18 Antipsychotic treatment increases risk for EPS, including acute dystonia, akathisia (subjective restlessness with desire to move), and parkinsonism (shuffling gait, resting tremor, rigidity and bradykinesia), as well as NMS.
Nonpharmacologic interventions. Verbal intervention to try to de-escalate an agitated, psychotic patient should be attempted first; however, this is not always possible. Other behavioral interventions include offering a meal, blanket, or pillow, or other comforting options to decrease the patient’s anxiety associated with psychosis.2 However, if agitated psychotic patients continue to display aggressive behaviors and pose a risk of harm to themselves or those around them, physical restraints should be considered because the clinician must balance protecting the patient’s rights with others’ safety. If physical restraints are used, medication also should be administered. Remove physical restraints as soon as safely possible; the Joint Commission has established standards for minimizing harm when using physical restraints.19
Diagnostic workup
Once a patient is medically stable in the ER, begin further workup of the etiology of the psychosis (Algorithm 2). All patients should have a physical exam, provided they are calm and in behavioral control. Monitor vital signs; patients at risk of withdrawal from substances should be monitored more frequently. Although there is no established standard for “medical clearance” of a psychiatric patient,20 all patients should undergo basic laboratory tests, including basic metabolic panel, complete blood count, and urine toxicology. The extent of the workup is determined by the clinical situation and suspected cause of psychosis.21

Algorithm 2: Diagnostic workup of an acutely psychotic patient
ER: emergency room; EEG: electroencephalography; LP: lumbar puncture; TSH: thyroid-stimulating hormone
If you suspect delirium, the underlying medical etiology must be identified and treated. Up to 40% of hospitalized patients with delirium may have psychosis.22 Psychosis in a delirious patient may be characterized by poorly formed delusions and visual hallucinations. Delirious patients often are inattentive, easily distracted, and disoriented, with a fluctuating clinical course. Patients with psychosis generally do not have impaired attention and are alert with intact memory. However, acutely psychotic patients may be quite disorganized and uncooperative, which makes it difficult to distinguish between these 2 diagnoses. Serial exams may help clarify the clinical picture. It is important to remember that patients with a history of a psychotic disorder may have a superimposed delirium.
In young patients (age 18 to 30) with new-onset psychosis, consider drug-induced psychosis; PCP, lysergic acid diethylamide, and methamphetamine intoxication and withdrawal can lead to psychotic presentations. Additionally, comorbid substance use is common among patients with primary psychotic disorders. One study found 37% of first-episode psychotic patients misused drugs or alcohol, similar to the lifetime rate of patients with chronic psychotic disorders.23,24 Check urine and serum toxicology screens and obtain relevant substance use history. Brain MRI may be considered for patients with first presentation of psychosis; however, there is little evidence to support head CT imaging unless there is known head trauma.25 Electroencephalography and lumbar puncture can be considered if clinically indicated.
Further psychiatric evaluation
Obtaining a psychiatric history is necessary to determine the etiology of the acute psychotic presentation. The timing and duration of psychotic symptoms are key. Acute symptom onset with fluctuating course and impaired attention suggests a delirious process. A gradual decline in functioning over several months to years in a young person suggests a first episode of a psychotic disorder (eg, schizophrenia). Drug abuse is common among young persons with a psychotic disorder and a positive drug screen for a psychogenic substance does not exclude a primary psychotic disorder.
If a patient has a history of schizophrenia, bipolar disorder, or psychotic depression, acutely worsening psychosis may be considered an acute or chronic presentation. Even in patients diagnosed with a psychotic illness, it is necessary to determine the cause of symptom exacerbation. Medication nonadherence (which can be partial), substance use, psychosocial stressors, or underlying medical illness should be considered. Collateral information from family or friends may be crucial to understanding a patient’s presentation.
Safe disposition
Patients who pose a risk of harm to themselves or others or who are so impaired by their psychosis that they cannot care for themselves generally should be admitted to an inpatient psychiatric facility. For some psychotic patients who are agreeable to treatment and not prone to violence, less restrictive settings—such as a crisis intervention unit or respite facility—may be appropriate. A patient with first-episode psychosis could be admitted for further diagnostic clarification and treatment initiation. Manic patients often have no insight into their illness and may need hospitalization for containment and assurance of medication adherence. Goals of inpatient care include initiating or resuming pharmacologic treatment to reduce psychotic symptoms and beginning the recovery process. Response rates—defined as ≥20% improvement in total score on a psychopathology scale such as the Positive and Negative Syndrome Scale—will vary, but can take ≥4 weeks in some patients with first-episode schizophrenia.26 However, most patients will be stabilized and ready for discharge before 4 weeks. Family education and alliance building with the patient and family are important during hospitalization.
Related Resources
- Schwartz S, Weathers, M. The psychotic patient. In: Riba MB, Ravindranath D, eds. Clinical manual of emergency psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2010:115-140.
- American Association for Emergency Psychiatry. http://emergencypsychiatry.org.
Drug Brand Names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Haloperidol • Haldol
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Midazolam • Versed
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Freudenreich receives grant or research support from Beacon Health Strategies, Global Medical Education, MGH Psychiatry Academy, Optimal Medicine, Pfizer Inc., and PsychoGenics.
Drs. Brown and Stoklosa report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Marco CA, Vaughan J. Emergency management of agitation in schizophrenia. Am J Emerg Med. 2005;23(6):767-776.
2. Freudenreich O. Emergency management of acute psychosis. In: Freudenreich O ed. Psychotic disorders: a practical guide. New York, NY: Wolter Kluwer/Lippincott Williams & Wilkins; 2008:72–78.
3. Allen MH, Currier GW, Carpenter D, et al. Expert Consensus Panel for Behavioral Emergencies 2005. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(1 suppl):S5-S108.
4. National Institute for Health and Clinical Excellence. Schizophrenia: core interventions in the treatment and management of schizophrenia in primary and secondary care. London United Kingdom: National Institute for Clinical Excellence; 2002.
5. Allen MH. Managing the agitated psychotic patient: a reappraisal of the evidence. J Clin Psychiatry. 2000;61 (14 suppl):S11-S20.
6. Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective, double-blind, emergency department study. Am J Emerg Med. 1997;15(4):335-340.
7. Allen MH, Currier GW, Hughes DH, et al. Expert Consensus Panel for Behavioral Emergencies. The expert consensus guideline series. Treatment of behavioral emergencies. Postgrad Med. 2001;(Spec no):1-88.
8. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of American Association for Emergency Psychiatry project BETA psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34.
9. Lukens TW, Wolf SJ, Edlow JA, et al. American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department. Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med. 2006;47(1):79-99.
10. Currier GW, Chou JC, Feifel D, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65(3):386-394.
11. Daniel DG, Potkin SG, Reeves KR, et al. Intramuscular (IM) ziprasidone 20 mg is effective in reducing agitation associated with psychosis: a double-blind, randomized trial. Psychopharmacology (Berl). 2001;155(2):128-134.
12. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatry. 2001;158(7):1149-1151.
13. Kapur S, Arenovic T, Agid O, et al. Evidence for onset of antipsychotic effects within the first 24 hours of treatment. Am J Psychiatry. 2005;162(5):939-946.
14. Marder SR, Sorasburu S, Dunayevic E, et al. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatry. 2010;71(4):433-441.
15. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
16. Casey DE, Daniel DG, Wassef AA, et al. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacology. 2003;28(1):182-192.
17. Casey DE, Daniel DG, Tamminga C, et al. Divalproex ER combined with olanzapine or risperidone for treatment of acute exacerbations of schizophrenia. Neuropsychopharmacology. 2009;34(5):1330-1338.
18. Currier GW, Allen MH, Bunney EB, et al. Safety of medications used to treat acute agitation. J Emerg Med. 2004;27(4 suppl):S19-S24.
19. The Joint Commission. Sentinel event alert. Preventing restraint deaths. Published November 18 1998. http://www.jointcommission.org/assets/1/18/SEA_8.pdf. Accessed October 26, 2012
20. Janiak BD, Atteberry S. Medical clearance of the psychiatric patient in the emergency department. J Emerg Med. 2012;43(5):866-870.
21. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
22. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
23. Cantwell R, Brewin J, Glazebrook C, et al. Prevalence of substance misuse in first-episode psychosis. Br J Psychiatry. 1999;174:150-153.
24. Green AI, Tohen MF, Hamer RM, et al. First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr Res. 2004;66(2-3):125-135.
25. Goulet K, Deschamps B, Evoy F, et al. Use of brain imaging (computed tomography and magnetic resonance imaging) in first-episode psychosis: review and retrospective study. Can J Psychiatry. 2009;54(7):493-501.
26. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
1. Marco CA, Vaughan J. Emergency management of agitation in schizophrenia. Am J Emerg Med. 2005;23(6):767-776.
2. Freudenreich O. Emergency management of acute psychosis. In: Freudenreich O ed. Psychotic disorders: a practical guide. New York, NY: Wolter Kluwer/Lippincott Williams & Wilkins; 2008:72–78.
3. Allen MH, Currier GW, Carpenter D, et al. Expert Consensus Panel for Behavioral Emergencies 2005. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(1 suppl):S5-S108.
4. National Institute for Health and Clinical Excellence. Schizophrenia: core interventions in the treatment and management of schizophrenia in primary and secondary care. London United Kingdom: National Institute for Clinical Excellence; 2002.
5. Allen MH. Managing the agitated psychotic patient: a reappraisal of the evidence. J Clin Psychiatry. 2000;61 (14 suppl):S11-S20.
6. Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective, double-blind, emergency department study. Am J Emerg Med. 1997;15(4):335-340.
7. Allen MH, Currier GW, Hughes DH, et al. Expert Consensus Panel for Behavioral Emergencies. The expert consensus guideline series. Treatment of behavioral emergencies. Postgrad Med. 2001;(Spec no):1-88.
8. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of American Association for Emergency Psychiatry project BETA psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34.
9. Lukens TW, Wolf SJ, Edlow JA, et al. American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department. Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med. 2006;47(1):79-99.
10. Currier GW, Chou JC, Feifel D, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65(3):386-394.
11. Daniel DG, Potkin SG, Reeves KR, et al. Intramuscular (IM) ziprasidone 20 mg is effective in reducing agitation associated with psychosis: a double-blind, randomized trial. Psychopharmacology (Berl). 2001;155(2):128-134.
12. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatry. 2001;158(7):1149-1151.
13. Kapur S, Arenovic T, Agid O, et al. Evidence for onset of antipsychotic effects within the first 24 hours of treatment. Am J Psychiatry. 2005;162(5):939-946.
14. Marder SR, Sorasburu S, Dunayevic E, et al. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatry. 2010;71(4):433-441.
15. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
16. Casey DE, Daniel DG, Wassef AA, et al. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacology. 2003;28(1):182-192.
17. Casey DE, Daniel DG, Tamminga C, et al. Divalproex ER combined with olanzapine or risperidone for treatment of acute exacerbations of schizophrenia. Neuropsychopharmacology. 2009;34(5):1330-1338.
18. Currier GW, Allen MH, Bunney EB, et al. Safety of medications used to treat acute agitation. J Emerg Med. 2004;27(4 suppl):S19-S24.
19. The Joint Commission. Sentinel event alert. Preventing restraint deaths. Published November 18 1998. http://www.jointcommission.org/assets/1/18/SEA_8.pdf. Accessed October 26, 2012
20. Janiak BD, Atteberry S. Medical clearance of the psychiatric patient in the emergency department. J Emerg Med. 2012;43(5):866-870.
21. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
22. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
23. Cantwell R, Brewin J, Glazebrook C, et al. Prevalence of substance misuse in first-episode psychosis. Br J Psychiatry. 1999;174:150-153.
24. Green AI, Tohen MF, Hamer RM, et al. First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr Res. 2004;66(2-3):125-135.
25. Goulet K, Deschamps B, Evoy F, et al. Use of brain imaging (computed tomography and magnetic resonance imaging) in first-episode psychosis: review and retrospective study. Can J Psychiatry. 2009;54(7):493-501.
26. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
Words of thanks
Thank you for making your wonderful journal available to all psychiatrists. I look forward to receiving each issue of Current Psychiatry. Please keep this educational, balanced journal coming.
A. Guillermo Pezzarossi, MDPrivate PracticeAlbuquerque, NM
Thank you for making your wonderful journal available to all psychiatrists. I look forward to receiving each issue of Current Psychiatry. Please keep this educational, balanced journal coming.
A. Guillermo Pezzarossi, MDPrivate PracticeAlbuquerque, NM
Thank you for making your wonderful journal available to all psychiatrists. I look forward to receiving each issue of Current Psychiatry. Please keep this educational, balanced journal coming.
A. Guillermo Pezzarossi, MDPrivate PracticeAlbuquerque, NM
Prison and the mentally ill
For years, I have been telling people what E. Fuller Torrey revealed in his 2010 report and what Dr. Nasrallah wrote about in his October 2012 editorial (“Psychiatry and the politics of incarceration,” From the Editor, Current Psychiatry, October 2012, p. 4-5; http://bit.ly/1JWYa87). It has been my experience that people falsely believe the mentally ill are dangerous and unpredictable or lazy and uncooperative and therefore are properly housed in prisons. Such a false idea seems to have pervaded Americans’ thoughts about the mentally ill to such a degree that a vast educational program would be needed to change this idea.
How sad is it that a nation founded on freedom has come to this.
Roxanne Lewis, PhDAssistant Professor of Behavioral SciencesDestiny University School of Medicine and Health SciencesRodney Bay, St. Lucia
For years, I have been telling people what E. Fuller Torrey revealed in his 2010 report and what Dr. Nasrallah wrote about in his October 2012 editorial (“Psychiatry and the politics of incarceration,” From the Editor, Current Psychiatry, October 2012, p. 4-5; http://bit.ly/1JWYa87). It has been my experience that people falsely believe the mentally ill are dangerous and unpredictable or lazy and uncooperative and therefore are properly housed in prisons. Such a false idea seems to have pervaded Americans’ thoughts about the mentally ill to such a degree that a vast educational program would be needed to change this idea.
How sad is it that a nation founded on freedom has come to this.
Roxanne Lewis, PhDAssistant Professor of Behavioral SciencesDestiny University School of Medicine and Health SciencesRodney Bay, St. Lucia
For years, I have been telling people what E. Fuller Torrey revealed in his 2010 report and what Dr. Nasrallah wrote about in his October 2012 editorial (“Psychiatry and the politics of incarceration,” From the Editor, Current Psychiatry, October 2012, p. 4-5; http://bit.ly/1JWYa87). It has been my experience that people falsely believe the mentally ill are dangerous and unpredictable or lazy and uncooperative and therefore are properly housed in prisons. Such a false idea seems to have pervaded Americans’ thoughts about the mentally ill to such a degree that a vast educational program would be needed to change this idea.
How sad is it that a nation founded on freedom has come to this.
Roxanne Lewis, PhDAssistant Professor of Behavioral SciencesDestiny University School of Medicine and Health SciencesRodney Bay, St. Lucia

