User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Masters of American Psychiatry: Eric R. Kandel, MD
Pharmacologic and nonpharmacologic treatment options for panic disorder
Something smells different
CASE: Depressed and hopeless
Ms. D, age 69, has a 20-year history of bipolar II disorder, for which she is taking citalopram, 30 mg/d. She presents to her outpatient psychotherapist with a chief complaint of depressed mood. The therapist refers her for psychiatric hospitalization and electroconvulsive therapy consultation. Upon admission, Ms. D reports that her depressed mood has worsened over the past 5 weeks after a trip to the Dominican Republic. Ms. D had a negative encounter with airport security that she attributed to her 2 artificial knees and caused her to miss her flight. She endorses poor appetite, loss of energy, anhedonia, difficulty concentrating, poor memory, and feelings of hopelessness.
Ms. D reports increasingly frequent panic attacks as well as intermittent right-sided discomfort, unusual noxious smells, and increased falls. She says the falls likely are a result of new bilateral lower extremity weakness coupled with long-standing imbalance. Ms. D says she has experienced brief occasions of foul-smelling odors while showering without evidence of an offending substance. She also reports a mild, occipitally located headache.
Four years ago, Ms. D was hospitalized for a depressive episode without psychotic features and diagnosed with generalized anxiety disorder, for which she is taking clonazepam, 1.5 mg/d. Her last hypomanic episode was several years ago, and was characterized by increased energy with decreased need for sleep, flight of ideas, increased productivity, and impulsivity. Her medical history includes non-insulin dependent diabetes mellitus, chronic low back pain, hyperlipidemia, arthritis, and gastroesophageal reflux disease; her medications include pioglitazone, 30 mg/d, oxybutynin, 15 mg/d, rosuvastatin, 20 mg/d, losartan, 50 mg/d, and omeprazole, 20 mg/d. She also had bilateral knee replacements 9 years ago and an L4-S1 spinal fusion 11 years ago. She has no history of head injuries or seizures. Ms. D’s father had major depressive disorder, her mother died of a cerebrovascular accident at an unknown age, and her brother died of a myocardial infarction at age 52.
The authors’ observations
A striking aspect of Ms. D’s presenting complaints was her intermittent experience of foul smells. Although olfactory hallucinations can occur with psychotic and affective states, they also may be harbingers of an organic etiology involving the temporal lobe.1 Olfactory hallucinations associated with a psychiatric disorder often have an accompanying delusional belief regarding the cause of the smell.2
Olfactory hallucinations have been associated with migraines, epilepsy, and Parkinson’s disease.1-3 Neoplasms, cerebrovascular events, or traumatic brain injuries that result in focal mesial temporal lobe lesions can present as a partial complex seizure with olfactory or gustatory hallucinations and progress to automatisms.4 Characteristic odors in these hallucinations are unpleasant; patients with temporal lobe epilepsy describe the smells as “bad,” “rotten,” “sickening,” and “like burning food.”2 Ms. D’s report of unusual smells warranted consideration of an organic etiology for her mood change and a thorough neurologic examination.
EVALUATION: Neurologic signs
At the time of admission, Ms. D has a blood pressure of 127/68 mm Hg, heart rate of 74 beats per minute, respiratory rate of 16 breaths per minute, and temperature of 36.5°C. Neurologic examination reveals a left facial droop of unknown duration. Motor strength is weak throughout with left-sided focal weakness. Ms. D’s daughter notes that her mother’s smile appears “funny” in her admission photograph but is unsure when the asymmetry in her facial appearance began. Ms. D had been ambulatory before admission. Nursing staff observes Ms. D leans toward her left side and exhibits possible left-sided neglect during the first 12 hours of hospitalization.
When asked about her facial droop, Ms. D replies that she had not noticed any change in her appearance lately. She does not appear to be concerned about her worsening ambulation. On hospital day 2, Ms. D seems to have difficulty using utensils to eat breakfast. Ms. D is dismissive of her worsening motor function and asks to be left alone to finish her meal.
The authors’ observations
Ms. D’s focal neurologic deficits and complaint of a headache on admission were concerning because they could be caused by a cerebrovascular event or space-occupying brain lesion with potential for increased intracranial pressure. Neurologic examination with evaluation for papilledema is indicated, followed by medical transport to the closest medical center for emergent brain imaging. Neither Ms. D nor her daughter could pinpoint the onset of Ms. D’s left-sided facial droop, which precluded administering tissue plasminogen activator for a potential acute ischemic stroke.5
Ms. D’s case prompted us to consider what constitutes timely brain imaging in a patient who presents with psychiatric symptoms. Several neurologic conditions may present first with neurobehavioral symptoms before findings on physical exam. Two series of autopsies conducted >70 years ago at psychiatric hospitals found incidences of brain tumors of 3.45%6 and 13.5%.7 In a 5-year retrospective study, 21% of meningioma cases presented with psychiatric symptoms alone.8 These historical cases suggest that affective, behavioral, and psychotic symptoms may be the only clinical indicators of brain lesions that merit surgery.9-11
Imaging and radiation exposure
With the advent of CT scans in the 1970s, psychiatrists gained a new method of investigating potential structural CNS pathology in patients presenting with psychiatric symptoms. The dramatic increase in CT scan use in recent years and resulting radiation exposure is responsible for 1.5% to 2% of all cancers in the United States.12,13 Certainly, physicians must balance the advantage of early detection of brain lesions with cost-effectiveness and exposure to radiation.14
There is no consensus regarding use of brain imaging in a patient who presents with new-onset psychiatric symptoms. Certainly, patients with localizing neurologic deficits or symptoms of increased intracranial pressure should undergo brain imaging. As for psychiatric patients without neurologic findings, Filley and Kleinschmidt-DeMasters15 provide recommendations based on their 1995 case series, and other authors have recommended imaging for patients age ≥4016 vs ≥5017,18 who present with atypical mental status changes.
OUTCOME: Scan, then surgery
Ms. D’s head CT reveals a large right-sided temporoparietal low-density lesion with 8-mm left lower midline shift (Figure). She undergoes a right temporal craniotomy with resection of the mass, which is confirmed by surgical pathology to be a glioblastoma multiforme World Health Organization grade 4 tumor. Postoperative MRI shows evidence of infarction in the right posterior cerebral artery distribution and residual tumor is identified on follow-up imaging. Ms. D is referred to radiation oncology, where she receives a prognostic median life expectancy of 14 months with radiation and temozolomide treatment.19

Figure: Ms. D’s MRI results
MRI with contrast shows a large right temporal heterogeneous mass consistent with glioblastoma multiforme
The authors’ observations
Glioblastoma is a rare cancer that comprises 25% of all malignant nervous system tumors.20 It is associated with a poor prognosis, with a <30% relative survival rate for adults at 1 year and 3% at 5 years.20 Headaches, seizures, motor weakness, and progressive neurologic deficits are common symptoms of glioblastoma at diagnosis.20 Ms. D was offered the standard of care treatment for a high-grade glioma, including surgical resection followed by concomitant external-beam radiotherapy and chemotherapy.21
Consider structural brain lesions in patients who present with neurobehavioral symptoms, although most of these patients will be diagnosed with a primary psychiatric disorder. Ms. D had a known psychiatric disorder that predated the onset of neurologic symptoms and diagnosis of a rare brain cancer. Before she developed neurologic signs, Ms. D experienced symptoms uncharacteristic of her previous depressive episodes, including olfactory hallucinations, that provided an early indicator of a CNS lesion. Consider brain imaging in patients of any age who do not respond to medications targeting the presumed psychiatric diagnosis to ensure that insidious brain tumors are not missed (Table 1).15
Table 1
When to order neuroimaging for psychiatric patients
| Patient’s age | Most common types of brain tumor | MRI vs CT | Indications to image |
|---|---|---|---|
| ≥40 years | Metastases High-grade gliomas Meningiomas | Roughly equivalent for imaging common tumor types. Base on cost, availability, and relative patient contraindications | New-onset cognitive or emotional dysfunction. Patient is not responding to appropriate pharmacotherapy for psychiatric diagnosis |
| <40 years | Low-grade astrocytomas Oligodendrogliomas | MRI preferred | New-onset cognitive or emotional dysfunction with associated somatic symptoms (headache, nausea, vomiting, papilledema, seizures, or focal deficits). Patient is not responding to appropriate pharmacotherapy for the psychiatric diagnosis |
| Source: Reference 15 | |||
Compared with cerebrovascular lesions, neoplasms are more difficult to clinically correlate with their anatomic location. Neurobehavioral symptoms are more frequently associated with tumors originating in the frontal lobe or temporolimbic regions of the brain. The 3 types of frontal lobe syndromes are dorsolateral, orbitofrontal, and medial-frontal (Table 2).15 Temporolimbic tumors may present with hallucinations, mania, panic attacks, or amnesia. A meta-analysis found a statistically significant association between anorexia and hypothalamic tumors.22 Reports of neuropsychiatric symptoms that respond to pharmacologic treatment further confound the clinical picture.16
Table 2
Frontal lobe syndromes
| Syndrome | Characteristics |
|---|---|
| Dorsolateral | Deficits in executive functioning, including organization and behavior planning |
| Orbitofrontal | Prominent disinhibition |
| Medial-frontal | Apathy, abulia |
| Source: Reference 15 | |
It is uncommon for a patient with a long-standing mood disorder to develop a primary brain cancer. However, Ms. D’s case serves as an important reminder to consider medical comorbidities in our aging psychiatric population. In particular, a patient who develops unusual symptoms or does not respond to previously effective treatments should be more closely examined and the differential diagnosis broadened.
Related Resources
- MD Anderson Cancer Center. Brain tumor videos and podcasts. www.mdanderson.org/patient-and-cancer-information/cancer-information/cancer-types/brain-tumor/videos-and-podcasts/index.html.
- Braun CM, Dumont M, Duval J, et al. Brain modules of hallucination: an analysis of multiple patients with brain lesions. J Psychiatry Neurosci. 2003;28(6):432-449.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
- Losartan • Cozaar
- Omeprazole • Prilosec
- Oxybutynin • Ditropan
- Pioglitazone • Actos
- Rosuvastatin • Crestor
- Temozolomide • Temodar
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Assad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1986;143(9):1088-1097.
2. Carter JL. Visual somatosensory, olfactory, and gustatory hallucinations. Psychiatr Clin North Am. 1992;15(2):347-358.
3. Fuller GN, Guiloff RJ. Migrainous olfactory hallucinations. J Neurol Neurosurg Psychiatry. 1987;50(12):1688-1690.
4. Chang BS, Lowenstein DH. Mechanisms of disease: epilepsy. N Engl J Med. 2003;349(13):1257-1266.
5. Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
6. Hoffman JL. Intracranial neoplasms: their incidence and mental manifestations. Psychiatr Q. 1937;11(4):561-575.
7. Larson CP. Intracranial tumors in mental hospital patients. Am J Psychiatry. 1940;97(1):49-58.
8. Gupta RK, Kumar R. Benign brain tumours and psychiatric morbidity: a 5-years retrospective data analysis. Aust N Z J Psychiatry. 2004;38(5):316-319.
9. Chambers WR. Neurosurgical conditions masquerading as psychiatric diseases. Am J Psychiatry. 1955;112(5):387-389.
10. Trimble MR, Mendez MF, Cummings JL. Neuropsychiatric symptoms from the temporolimbic lobes. J Neuropsychiatry Clin Neurosci. 1997;9(3):429-438.
11. Uribe VM. Psychiatric symptoms and brain tumor. Am Fam Physician. 1986;34(2):95-98.
12. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(2):2277-2284.
13. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
14. Weinberger DR. Brain disease and psychiatric illness: when should a psychiatrist order a CAT scan? Am J Psychiatry. 1984;141(12):1521-1526.
15. Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995;163(1):19-25.
16. Moise D, Madhusoodanan S. Psychiatric symptoms associated with brain tumors: a clinical engima. CNS Spectr. 2006;11(1):28-31.
17. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
18. Hollister LE, Boutros N. Clinical use of CT and MR scans in psychiatric patients. J Psychiatr Neurosci. 1991;16(4):194-198.
19. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996.
20. Brandes AA, Tosoni A, Franceschi E, et al. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67(2):139-152.
21. Chandana SR, Movva S, Arora M, et al. Primary brain tumors in adults. Am Fam Physician. 2008;77(10):1423-1430.
22. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
CASE: Depressed and hopeless
Ms. D, age 69, has a 20-year history of bipolar II disorder, for which she is taking citalopram, 30 mg/d. She presents to her outpatient psychotherapist with a chief complaint of depressed mood. The therapist refers her for psychiatric hospitalization and electroconvulsive therapy consultation. Upon admission, Ms. D reports that her depressed mood has worsened over the past 5 weeks after a trip to the Dominican Republic. Ms. D had a negative encounter with airport security that she attributed to her 2 artificial knees and caused her to miss her flight. She endorses poor appetite, loss of energy, anhedonia, difficulty concentrating, poor memory, and feelings of hopelessness.
Ms. D reports increasingly frequent panic attacks as well as intermittent right-sided discomfort, unusual noxious smells, and increased falls. She says the falls likely are a result of new bilateral lower extremity weakness coupled with long-standing imbalance. Ms. D says she has experienced brief occasions of foul-smelling odors while showering without evidence of an offending substance. She also reports a mild, occipitally located headache.
Four years ago, Ms. D was hospitalized for a depressive episode without psychotic features and diagnosed with generalized anxiety disorder, for which she is taking clonazepam, 1.5 mg/d. Her last hypomanic episode was several years ago, and was characterized by increased energy with decreased need for sleep, flight of ideas, increased productivity, and impulsivity. Her medical history includes non-insulin dependent diabetes mellitus, chronic low back pain, hyperlipidemia, arthritis, and gastroesophageal reflux disease; her medications include pioglitazone, 30 mg/d, oxybutynin, 15 mg/d, rosuvastatin, 20 mg/d, losartan, 50 mg/d, and omeprazole, 20 mg/d. She also had bilateral knee replacements 9 years ago and an L4-S1 spinal fusion 11 years ago. She has no history of head injuries or seizures. Ms. D’s father had major depressive disorder, her mother died of a cerebrovascular accident at an unknown age, and her brother died of a myocardial infarction at age 52.
The authors’ observations
A striking aspect of Ms. D’s presenting complaints was her intermittent experience of foul smells. Although olfactory hallucinations can occur with psychotic and affective states, they also may be harbingers of an organic etiology involving the temporal lobe.1 Olfactory hallucinations associated with a psychiatric disorder often have an accompanying delusional belief regarding the cause of the smell.2
Olfactory hallucinations have been associated with migraines, epilepsy, and Parkinson’s disease.1-3 Neoplasms, cerebrovascular events, or traumatic brain injuries that result in focal mesial temporal lobe lesions can present as a partial complex seizure with olfactory or gustatory hallucinations and progress to automatisms.4 Characteristic odors in these hallucinations are unpleasant; patients with temporal lobe epilepsy describe the smells as “bad,” “rotten,” “sickening,” and “like burning food.”2 Ms. D’s report of unusual smells warranted consideration of an organic etiology for her mood change and a thorough neurologic examination.
EVALUATION: Neurologic signs
At the time of admission, Ms. D has a blood pressure of 127/68 mm Hg, heart rate of 74 beats per minute, respiratory rate of 16 breaths per minute, and temperature of 36.5°C. Neurologic examination reveals a left facial droop of unknown duration. Motor strength is weak throughout with left-sided focal weakness. Ms. D’s daughter notes that her mother’s smile appears “funny” in her admission photograph but is unsure when the asymmetry in her facial appearance began. Ms. D had been ambulatory before admission. Nursing staff observes Ms. D leans toward her left side and exhibits possible left-sided neglect during the first 12 hours of hospitalization.
When asked about her facial droop, Ms. D replies that she had not noticed any change in her appearance lately. She does not appear to be concerned about her worsening ambulation. On hospital day 2, Ms. D seems to have difficulty using utensils to eat breakfast. Ms. D is dismissive of her worsening motor function and asks to be left alone to finish her meal.
The authors’ observations
Ms. D’s focal neurologic deficits and complaint of a headache on admission were concerning because they could be caused by a cerebrovascular event or space-occupying brain lesion with potential for increased intracranial pressure. Neurologic examination with evaluation for papilledema is indicated, followed by medical transport to the closest medical center for emergent brain imaging. Neither Ms. D nor her daughter could pinpoint the onset of Ms. D’s left-sided facial droop, which precluded administering tissue plasminogen activator for a potential acute ischemic stroke.5
Ms. D’s case prompted us to consider what constitutes timely brain imaging in a patient who presents with psychiatric symptoms. Several neurologic conditions may present first with neurobehavioral symptoms before findings on physical exam. Two series of autopsies conducted >70 years ago at psychiatric hospitals found incidences of brain tumors of 3.45%6 and 13.5%.7 In a 5-year retrospective study, 21% of meningioma cases presented with psychiatric symptoms alone.8 These historical cases suggest that affective, behavioral, and psychotic symptoms may be the only clinical indicators of brain lesions that merit surgery.9-11
Imaging and radiation exposure
With the advent of CT scans in the 1970s, psychiatrists gained a new method of investigating potential structural CNS pathology in patients presenting with psychiatric symptoms. The dramatic increase in CT scan use in recent years and resulting radiation exposure is responsible for 1.5% to 2% of all cancers in the United States.12,13 Certainly, physicians must balance the advantage of early detection of brain lesions with cost-effectiveness and exposure to radiation.14
There is no consensus regarding use of brain imaging in a patient who presents with new-onset psychiatric symptoms. Certainly, patients with localizing neurologic deficits or symptoms of increased intracranial pressure should undergo brain imaging. As for psychiatric patients without neurologic findings, Filley and Kleinschmidt-DeMasters15 provide recommendations based on their 1995 case series, and other authors have recommended imaging for patients age ≥4016 vs ≥5017,18 who present with atypical mental status changes.
OUTCOME: Scan, then surgery
Ms. D’s head CT reveals a large right-sided temporoparietal low-density lesion with 8-mm left lower midline shift (Figure). She undergoes a right temporal craniotomy with resection of the mass, which is confirmed by surgical pathology to be a glioblastoma multiforme World Health Organization grade 4 tumor. Postoperative MRI shows evidence of infarction in the right posterior cerebral artery distribution and residual tumor is identified on follow-up imaging. Ms. D is referred to radiation oncology, where she receives a prognostic median life expectancy of 14 months with radiation and temozolomide treatment.19

Figure: Ms. D’s MRI results
MRI with contrast shows a large right temporal heterogeneous mass consistent with glioblastoma multiforme
The authors’ observations
Glioblastoma is a rare cancer that comprises 25% of all malignant nervous system tumors.20 It is associated with a poor prognosis, with a <30% relative survival rate for adults at 1 year and 3% at 5 years.20 Headaches, seizures, motor weakness, and progressive neurologic deficits are common symptoms of glioblastoma at diagnosis.20 Ms. D was offered the standard of care treatment for a high-grade glioma, including surgical resection followed by concomitant external-beam radiotherapy and chemotherapy.21
Consider structural brain lesions in patients who present with neurobehavioral symptoms, although most of these patients will be diagnosed with a primary psychiatric disorder. Ms. D had a known psychiatric disorder that predated the onset of neurologic symptoms and diagnosis of a rare brain cancer. Before she developed neurologic signs, Ms. D experienced symptoms uncharacteristic of her previous depressive episodes, including olfactory hallucinations, that provided an early indicator of a CNS lesion. Consider brain imaging in patients of any age who do not respond to medications targeting the presumed psychiatric diagnosis to ensure that insidious brain tumors are not missed (Table 1).15
Table 1
When to order neuroimaging for psychiatric patients
| Patient’s age | Most common types of brain tumor | MRI vs CT | Indications to image |
|---|---|---|---|
| ≥40 years | Metastases High-grade gliomas Meningiomas | Roughly equivalent for imaging common tumor types. Base on cost, availability, and relative patient contraindications | New-onset cognitive or emotional dysfunction. Patient is not responding to appropriate pharmacotherapy for psychiatric diagnosis |
| <40 years | Low-grade astrocytomas Oligodendrogliomas | MRI preferred | New-onset cognitive or emotional dysfunction with associated somatic symptoms (headache, nausea, vomiting, papilledema, seizures, or focal deficits). Patient is not responding to appropriate pharmacotherapy for the psychiatric diagnosis |
| Source: Reference 15 | |||
Compared with cerebrovascular lesions, neoplasms are more difficult to clinically correlate with their anatomic location. Neurobehavioral symptoms are more frequently associated with tumors originating in the frontal lobe or temporolimbic regions of the brain. The 3 types of frontal lobe syndromes are dorsolateral, orbitofrontal, and medial-frontal (Table 2).15 Temporolimbic tumors may present with hallucinations, mania, panic attacks, or amnesia. A meta-analysis found a statistically significant association between anorexia and hypothalamic tumors.22 Reports of neuropsychiatric symptoms that respond to pharmacologic treatment further confound the clinical picture.16
Table 2
Frontal lobe syndromes
| Syndrome | Characteristics |
|---|---|
| Dorsolateral | Deficits in executive functioning, including organization and behavior planning |
| Orbitofrontal | Prominent disinhibition |
| Medial-frontal | Apathy, abulia |
| Source: Reference 15 | |
It is uncommon for a patient with a long-standing mood disorder to develop a primary brain cancer. However, Ms. D’s case serves as an important reminder to consider medical comorbidities in our aging psychiatric population. In particular, a patient who develops unusual symptoms or does not respond to previously effective treatments should be more closely examined and the differential diagnosis broadened.
Related Resources
- MD Anderson Cancer Center. Brain tumor videos and podcasts. www.mdanderson.org/patient-and-cancer-information/cancer-information/cancer-types/brain-tumor/videos-and-podcasts/index.html.
- Braun CM, Dumont M, Duval J, et al. Brain modules of hallucination: an analysis of multiple patients with brain lesions. J Psychiatry Neurosci. 2003;28(6):432-449.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
- Losartan • Cozaar
- Omeprazole • Prilosec
- Oxybutynin • Ditropan
- Pioglitazone • Actos
- Rosuvastatin • Crestor
- Temozolomide • Temodar
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Depressed and hopeless
Ms. D, age 69, has a 20-year history of bipolar II disorder, for which she is taking citalopram, 30 mg/d. She presents to her outpatient psychotherapist with a chief complaint of depressed mood. The therapist refers her for psychiatric hospitalization and electroconvulsive therapy consultation. Upon admission, Ms. D reports that her depressed mood has worsened over the past 5 weeks after a trip to the Dominican Republic. Ms. D had a negative encounter with airport security that she attributed to her 2 artificial knees and caused her to miss her flight. She endorses poor appetite, loss of energy, anhedonia, difficulty concentrating, poor memory, and feelings of hopelessness.
Ms. D reports increasingly frequent panic attacks as well as intermittent right-sided discomfort, unusual noxious smells, and increased falls. She says the falls likely are a result of new bilateral lower extremity weakness coupled with long-standing imbalance. Ms. D says she has experienced brief occasions of foul-smelling odors while showering without evidence of an offending substance. She also reports a mild, occipitally located headache.
Four years ago, Ms. D was hospitalized for a depressive episode without psychotic features and diagnosed with generalized anxiety disorder, for which she is taking clonazepam, 1.5 mg/d. Her last hypomanic episode was several years ago, and was characterized by increased energy with decreased need for sleep, flight of ideas, increased productivity, and impulsivity. Her medical history includes non-insulin dependent diabetes mellitus, chronic low back pain, hyperlipidemia, arthritis, and gastroesophageal reflux disease; her medications include pioglitazone, 30 mg/d, oxybutynin, 15 mg/d, rosuvastatin, 20 mg/d, losartan, 50 mg/d, and omeprazole, 20 mg/d. She also had bilateral knee replacements 9 years ago and an L4-S1 spinal fusion 11 years ago. She has no history of head injuries or seizures. Ms. D’s father had major depressive disorder, her mother died of a cerebrovascular accident at an unknown age, and her brother died of a myocardial infarction at age 52.
The authors’ observations
A striking aspect of Ms. D’s presenting complaints was her intermittent experience of foul smells. Although olfactory hallucinations can occur with psychotic and affective states, they also may be harbingers of an organic etiology involving the temporal lobe.1 Olfactory hallucinations associated with a psychiatric disorder often have an accompanying delusional belief regarding the cause of the smell.2
Olfactory hallucinations have been associated with migraines, epilepsy, and Parkinson’s disease.1-3 Neoplasms, cerebrovascular events, or traumatic brain injuries that result in focal mesial temporal lobe lesions can present as a partial complex seizure with olfactory or gustatory hallucinations and progress to automatisms.4 Characteristic odors in these hallucinations are unpleasant; patients with temporal lobe epilepsy describe the smells as “bad,” “rotten,” “sickening,” and “like burning food.”2 Ms. D’s report of unusual smells warranted consideration of an organic etiology for her mood change and a thorough neurologic examination.
EVALUATION: Neurologic signs
At the time of admission, Ms. D has a blood pressure of 127/68 mm Hg, heart rate of 74 beats per minute, respiratory rate of 16 breaths per minute, and temperature of 36.5°C. Neurologic examination reveals a left facial droop of unknown duration. Motor strength is weak throughout with left-sided focal weakness. Ms. D’s daughter notes that her mother’s smile appears “funny” in her admission photograph but is unsure when the asymmetry in her facial appearance began. Ms. D had been ambulatory before admission. Nursing staff observes Ms. D leans toward her left side and exhibits possible left-sided neglect during the first 12 hours of hospitalization.
When asked about her facial droop, Ms. D replies that she had not noticed any change in her appearance lately. She does not appear to be concerned about her worsening ambulation. On hospital day 2, Ms. D seems to have difficulty using utensils to eat breakfast. Ms. D is dismissive of her worsening motor function and asks to be left alone to finish her meal.
The authors’ observations
Ms. D’s focal neurologic deficits and complaint of a headache on admission were concerning because they could be caused by a cerebrovascular event or space-occupying brain lesion with potential for increased intracranial pressure. Neurologic examination with evaluation for papilledema is indicated, followed by medical transport to the closest medical center for emergent brain imaging. Neither Ms. D nor her daughter could pinpoint the onset of Ms. D’s left-sided facial droop, which precluded administering tissue plasminogen activator for a potential acute ischemic stroke.5
Ms. D’s case prompted us to consider what constitutes timely brain imaging in a patient who presents with psychiatric symptoms. Several neurologic conditions may present first with neurobehavioral symptoms before findings on physical exam. Two series of autopsies conducted >70 years ago at psychiatric hospitals found incidences of brain tumors of 3.45%6 and 13.5%.7 In a 5-year retrospective study, 21% of meningioma cases presented with psychiatric symptoms alone.8 These historical cases suggest that affective, behavioral, and psychotic symptoms may be the only clinical indicators of brain lesions that merit surgery.9-11
Imaging and radiation exposure
With the advent of CT scans in the 1970s, psychiatrists gained a new method of investigating potential structural CNS pathology in patients presenting with psychiatric symptoms. The dramatic increase in CT scan use in recent years and resulting radiation exposure is responsible for 1.5% to 2% of all cancers in the United States.12,13 Certainly, physicians must balance the advantage of early detection of brain lesions with cost-effectiveness and exposure to radiation.14
There is no consensus regarding use of brain imaging in a patient who presents with new-onset psychiatric symptoms. Certainly, patients with localizing neurologic deficits or symptoms of increased intracranial pressure should undergo brain imaging. As for psychiatric patients without neurologic findings, Filley and Kleinschmidt-DeMasters15 provide recommendations based on their 1995 case series, and other authors have recommended imaging for patients age ≥4016 vs ≥5017,18 who present with atypical mental status changes.
OUTCOME: Scan, then surgery
Ms. D’s head CT reveals a large right-sided temporoparietal low-density lesion with 8-mm left lower midline shift (Figure). She undergoes a right temporal craniotomy with resection of the mass, which is confirmed by surgical pathology to be a glioblastoma multiforme World Health Organization grade 4 tumor. Postoperative MRI shows evidence of infarction in the right posterior cerebral artery distribution and residual tumor is identified on follow-up imaging. Ms. D is referred to radiation oncology, where she receives a prognostic median life expectancy of 14 months with radiation and temozolomide treatment.19

Figure: Ms. D’s MRI results
MRI with contrast shows a large right temporal heterogeneous mass consistent with glioblastoma multiforme
The authors’ observations
Glioblastoma is a rare cancer that comprises 25% of all malignant nervous system tumors.20 It is associated with a poor prognosis, with a <30% relative survival rate for adults at 1 year and 3% at 5 years.20 Headaches, seizures, motor weakness, and progressive neurologic deficits are common symptoms of glioblastoma at diagnosis.20 Ms. D was offered the standard of care treatment for a high-grade glioma, including surgical resection followed by concomitant external-beam radiotherapy and chemotherapy.21
Consider structural brain lesions in patients who present with neurobehavioral symptoms, although most of these patients will be diagnosed with a primary psychiatric disorder. Ms. D had a known psychiatric disorder that predated the onset of neurologic symptoms and diagnosis of a rare brain cancer. Before she developed neurologic signs, Ms. D experienced symptoms uncharacteristic of her previous depressive episodes, including olfactory hallucinations, that provided an early indicator of a CNS lesion. Consider brain imaging in patients of any age who do not respond to medications targeting the presumed psychiatric diagnosis to ensure that insidious brain tumors are not missed (Table 1).15
Table 1
When to order neuroimaging for psychiatric patients
| Patient’s age | Most common types of brain tumor | MRI vs CT | Indications to image |
|---|---|---|---|
| ≥40 years | Metastases High-grade gliomas Meningiomas | Roughly equivalent for imaging common tumor types. Base on cost, availability, and relative patient contraindications | New-onset cognitive or emotional dysfunction. Patient is not responding to appropriate pharmacotherapy for psychiatric diagnosis |
| <40 years | Low-grade astrocytomas Oligodendrogliomas | MRI preferred | New-onset cognitive or emotional dysfunction with associated somatic symptoms (headache, nausea, vomiting, papilledema, seizures, or focal deficits). Patient is not responding to appropriate pharmacotherapy for the psychiatric diagnosis |
| Source: Reference 15 | |||
Compared with cerebrovascular lesions, neoplasms are more difficult to clinically correlate with their anatomic location. Neurobehavioral symptoms are more frequently associated with tumors originating in the frontal lobe or temporolimbic regions of the brain. The 3 types of frontal lobe syndromes are dorsolateral, orbitofrontal, and medial-frontal (Table 2).15 Temporolimbic tumors may present with hallucinations, mania, panic attacks, or amnesia. A meta-analysis found a statistically significant association between anorexia and hypothalamic tumors.22 Reports of neuropsychiatric symptoms that respond to pharmacologic treatment further confound the clinical picture.16
Table 2
Frontal lobe syndromes
| Syndrome | Characteristics |
|---|---|
| Dorsolateral | Deficits in executive functioning, including organization and behavior planning |
| Orbitofrontal | Prominent disinhibition |
| Medial-frontal | Apathy, abulia |
| Source: Reference 15 | |
It is uncommon for a patient with a long-standing mood disorder to develop a primary brain cancer. However, Ms. D’s case serves as an important reminder to consider medical comorbidities in our aging psychiatric population. In particular, a patient who develops unusual symptoms or does not respond to previously effective treatments should be more closely examined and the differential diagnosis broadened.
Related Resources
- MD Anderson Cancer Center. Brain tumor videos and podcasts. www.mdanderson.org/patient-and-cancer-information/cancer-information/cancer-types/brain-tumor/videos-and-podcasts/index.html.
- Braun CM, Dumont M, Duval J, et al. Brain modules of hallucination: an analysis of multiple patients with brain lesions. J Psychiatry Neurosci. 2003;28(6):432-449.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
- Losartan • Cozaar
- Omeprazole • Prilosec
- Oxybutynin • Ditropan
- Pioglitazone • Actos
- Rosuvastatin • Crestor
- Temozolomide • Temodar
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Assad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1986;143(9):1088-1097.
2. Carter JL. Visual somatosensory, olfactory, and gustatory hallucinations. Psychiatr Clin North Am. 1992;15(2):347-358.
3. Fuller GN, Guiloff RJ. Migrainous olfactory hallucinations. J Neurol Neurosurg Psychiatry. 1987;50(12):1688-1690.
4. Chang BS, Lowenstein DH. Mechanisms of disease: epilepsy. N Engl J Med. 2003;349(13):1257-1266.
5. Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
6. Hoffman JL. Intracranial neoplasms: their incidence and mental manifestations. Psychiatr Q. 1937;11(4):561-575.
7. Larson CP. Intracranial tumors in mental hospital patients. Am J Psychiatry. 1940;97(1):49-58.
8. Gupta RK, Kumar R. Benign brain tumours and psychiatric morbidity: a 5-years retrospective data analysis. Aust N Z J Psychiatry. 2004;38(5):316-319.
9. Chambers WR. Neurosurgical conditions masquerading as psychiatric diseases. Am J Psychiatry. 1955;112(5):387-389.
10. Trimble MR, Mendez MF, Cummings JL. Neuropsychiatric symptoms from the temporolimbic lobes. J Neuropsychiatry Clin Neurosci. 1997;9(3):429-438.
11. Uribe VM. Psychiatric symptoms and brain tumor. Am Fam Physician. 1986;34(2):95-98.
12. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(2):2277-2284.
13. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
14. Weinberger DR. Brain disease and psychiatric illness: when should a psychiatrist order a CAT scan? Am J Psychiatry. 1984;141(12):1521-1526.
15. Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995;163(1):19-25.
16. Moise D, Madhusoodanan S. Psychiatric symptoms associated with brain tumors: a clinical engima. CNS Spectr. 2006;11(1):28-31.
17. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
18. Hollister LE, Boutros N. Clinical use of CT and MR scans in psychiatric patients. J Psychiatr Neurosci. 1991;16(4):194-198.
19. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996.
20. Brandes AA, Tosoni A, Franceschi E, et al. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67(2):139-152.
21. Chandana SR, Movva S, Arora M, et al. Primary brain tumors in adults. Am Fam Physician. 2008;77(10):1423-1430.
22. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
1. Assad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1986;143(9):1088-1097.
2. Carter JL. Visual somatosensory, olfactory, and gustatory hallucinations. Psychiatr Clin North Am. 1992;15(2):347-358.
3. Fuller GN, Guiloff RJ. Migrainous olfactory hallucinations. J Neurol Neurosurg Psychiatry. 1987;50(12):1688-1690.
4. Chang BS, Lowenstein DH. Mechanisms of disease: epilepsy. N Engl J Med. 2003;349(13):1257-1266.
5. Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
6. Hoffman JL. Intracranial neoplasms: their incidence and mental manifestations. Psychiatr Q. 1937;11(4):561-575.
7. Larson CP. Intracranial tumors in mental hospital patients. Am J Psychiatry. 1940;97(1):49-58.
8. Gupta RK, Kumar R. Benign brain tumours and psychiatric morbidity: a 5-years retrospective data analysis. Aust N Z J Psychiatry. 2004;38(5):316-319.
9. Chambers WR. Neurosurgical conditions masquerading as psychiatric diseases. Am J Psychiatry. 1955;112(5):387-389.
10. Trimble MR, Mendez MF, Cummings JL. Neuropsychiatric symptoms from the temporolimbic lobes. J Neuropsychiatry Clin Neurosci. 1997;9(3):429-438.
11. Uribe VM. Psychiatric symptoms and brain tumor. Am Fam Physician. 1986;34(2):95-98.
12. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(2):2277-2284.
13. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
14. Weinberger DR. Brain disease and psychiatric illness: when should a psychiatrist order a CAT scan? Am J Psychiatry. 1984;141(12):1521-1526.
15. Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995;163(1):19-25.
16. Moise D, Madhusoodanan S. Psychiatric symptoms associated with brain tumors: a clinical engima. CNS Spectr. 2006;11(1):28-31.
17. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
18. Hollister LE, Boutros N. Clinical use of CT and MR scans in psychiatric patients. J Psychiatr Neurosci. 1991;16(4):194-198.
19. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996.
20. Brandes AA, Tosoni A, Franceschi E, et al. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67(2):139-152.
21. Chandana SR, Movva S, Arora M, et al. Primary brain tumors in adults. Am Fam Physician. 2008;77(10):1423-1430.
22. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
Taking an extended leave: What to do before you go
Discuss this article at www.facebook.com/CurrentPsychiatry
Arranging coverage and adjusting workload duties before taking an extended leave of absence from clinical practice—eg, for vacation, family leave, medical illness—can be challenging. During extended absences, clinicians depend on colleagues for assistance. In clinical settings such as residency training programs, arranging coverage for a maternity leave could be complicated by differences in attitudes toward pregnancy.1 However, an anticipated leave allows for advanced planning that can help ease transfer of care.
A smooth transition
Begin planning far in advance of your leave date because complications may necessitate a sudden, early departure. All clinical documentation, such as progress notes, should be completed so that a covering colleague can seamlessly assume patient care. It may be helpful to create a spreadsheet of all patients’ information, including name, contact number, diagnoses, medications, and a risk category (eg, low to high), along with notes—eg, lab results that need to be followed up on or labs to be ordered. This spreadsheet can be updated weekly and kept in a secure location so colleagues can access it in case your leave begins earlier than anticipated. To reduce workload burden on covering colleagues, it may be helpful to see as many stable, medication-only patients as possible before you leave to ensure that you have provided enough refills to cover the duration of your leave, assuming these patients typically are seen every other month or less.
It may be helpful to arrange for colleagues to take on a greater proportion of new consultations within the practice as the leave draws closer, because usually this is not a good time to begin treating new patients. However, it may be desirable for you to see a greater proportion of 1-time consultations, such as pre-surgical evaluations and second-opinion consultations. If time allows, arrange meetings among yourself, the colleague who will be covering for you, and high-risk patients before your leave. This can help promote familiarity and comfort between patients and the covering physician and increase the likelihood that patients in crisis will reach out to the covering physician. In some cases it may be advisable to consider a patient’s diagnosis, treatment history, and past experiences when selecting which colleague will provide care, assuming a choice is available—ie, female patients with a history of sexual trauma may feel more comfortable with a female physician.
Although taking an extended leave of absence from clinical practice can present many practical challenges, working with colleagues in advance can help promote a smoother transition of care and decrease workload burden.
Disclosure
Dr. Troy reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
Reference
1. Tamburrino MB, Evans CL, Campbell NB, et al. Physician pregnancy: male and female colleagues’ attitudes. J Am Med Womens Assoc. 1992;47(3):82-84.
Discuss this article at www.facebook.com/CurrentPsychiatry
Arranging coverage and adjusting workload duties before taking an extended leave of absence from clinical practice—eg, for vacation, family leave, medical illness—can be challenging. During extended absences, clinicians depend on colleagues for assistance. In clinical settings such as residency training programs, arranging coverage for a maternity leave could be complicated by differences in attitudes toward pregnancy.1 However, an anticipated leave allows for advanced planning that can help ease transfer of care.
A smooth transition
Begin planning far in advance of your leave date because complications may necessitate a sudden, early departure. All clinical documentation, such as progress notes, should be completed so that a covering colleague can seamlessly assume patient care. It may be helpful to create a spreadsheet of all patients’ information, including name, contact number, diagnoses, medications, and a risk category (eg, low to high), along with notes—eg, lab results that need to be followed up on or labs to be ordered. This spreadsheet can be updated weekly and kept in a secure location so colleagues can access it in case your leave begins earlier than anticipated. To reduce workload burden on covering colleagues, it may be helpful to see as many stable, medication-only patients as possible before you leave to ensure that you have provided enough refills to cover the duration of your leave, assuming these patients typically are seen every other month or less.
It may be helpful to arrange for colleagues to take on a greater proportion of new consultations within the practice as the leave draws closer, because usually this is not a good time to begin treating new patients. However, it may be desirable for you to see a greater proportion of 1-time consultations, such as pre-surgical evaluations and second-opinion consultations. If time allows, arrange meetings among yourself, the colleague who will be covering for you, and high-risk patients before your leave. This can help promote familiarity and comfort between patients and the covering physician and increase the likelihood that patients in crisis will reach out to the covering physician. In some cases it may be advisable to consider a patient’s diagnosis, treatment history, and past experiences when selecting which colleague will provide care, assuming a choice is available—ie, female patients with a history of sexual trauma may feel more comfortable with a female physician.
Although taking an extended leave of absence from clinical practice can present many practical challenges, working with colleagues in advance can help promote a smoother transition of care and decrease workload burden.
Disclosure
Dr. Troy reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Arranging coverage and adjusting workload duties before taking an extended leave of absence from clinical practice—eg, for vacation, family leave, medical illness—can be challenging. During extended absences, clinicians depend on colleagues for assistance. In clinical settings such as residency training programs, arranging coverage for a maternity leave could be complicated by differences in attitudes toward pregnancy.1 However, an anticipated leave allows for advanced planning that can help ease transfer of care.
A smooth transition
Begin planning far in advance of your leave date because complications may necessitate a sudden, early departure. All clinical documentation, such as progress notes, should be completed so that a covering colleague can seamlessly assume patient care. It may be helpful to create a spreadsheet of all patients’ information, including name, contact number, diagnoses, medications, and a risk category (eg, low to high), along with notes—eg, lab results that need to be followed up on or labs to be ordered. This spreadsheet can be updated weekly and kept in a secure location so colleagues can access it in case your leave begins earlier than anticipated. To reduce workload burden on covering colleagues, it may be helpful to see as many stable, medication-only patients as possible before you leave to ensure that you have provided enough refills to cover the duration of your leave, assuming these patients typically are seen every other month or less.
It may be helpful to arrange for colleagues to take on a greater proportion of new consultations within the practice as the leave draws closer, because usually this is not a good time to begin treating new patients. However, it may be desirable for you to see a greater proportion of 1-time consultations, such as pre-surgical evaluations and second-opinion consultations. If time allows, arrange meetings among yourself, the colleague who will be covering for you, and high-risk patients before your leave. This can help promote familiarity and comfort between patients and the covering physician and increase the likelihood that patients in crisis will reach out to the covering physician. In some cases it may be advisable to consider a patient’s diagnosis, treatment history, and past experiences when selecting which colleague will provide care, assuming a choice is available—ie, female patients with a history of sexual trauma may feel more comfortable with a female physician.
Although taking an extended leave of absence from clinical practice can present many practical challenges, working with colleagues in advance can help promote a smoother transition of care and decrease workload burden.
Disclosure
Dr. Troy reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
Reference
1. Tamburrino MB, Evans CL, Campbell NB, et al. Physician pregnancy: male and female colleagues’ attitudes. J Am Med Womens Assoc. 1992;47(3):82-84.
Reference
1. Tamburrino MB, Evans CL, Campbell NB, et al. Physician pregnancy: male and female colleagues’ attitudes. J Am Med Womens Assoc. 1992;47(3):82-84.
Vitamin D deficiency in older adults
Low vitamin D levels can impact cognitive functioning in older adults.1 As vitamin D levels decrease, cognitive impairment increases.
Vitamin D deficiency can occur because few foods contain this nutrient2 and patients have limited exposure to sunlight—vitamin D is produced when sunlight strikes the skin.2 In addition to rickets, low levels of vitamin D have been linked to slower information processing in middle age and older men, cognitive decline, mood disorders, and altered brain development and function resulting in neurodegenerative diseases and other medical disorders.3
One study suggested that one-half of adults age >60 do not get sufficient vitamin D, with an even higher rate among women with Alzheimer’s disease.4 Patients in dementia units typically are not tested for vitamin D levels. These patients rarely leave the unit, which leaves them deprived of the vitamin D provided by sunlight. Even patients exposed to sunlight may receive minimal vitamin D because they use sunscreen.
The following protocol can help patients who may benefit from vitamin D supplementation and increased sun exposure.
Obtain and assess vitamin D levels. Evaluate your patient’s level in the context of physical or cognitive symptoms and other lab values:
- deficient: <12 ng/mL
- inadequate: 12 to 20 ng/mL
- adequate: ≥20 ng/mL.2
Order dietary assessment to identify foods that may increase vitamin D levels. The best sources are fish—salmon, tuna, and mackerel—fish oils, beef, liver, cheese, and egg yolks.2 Several food products, including milk and orange juice, are fortified with vitamin D.
Suggest a daily vitamin D supplement ranging from 400 IU/d to 1,000 IU/d. The Institute of Medicine suggests 600 IU/d for patients age 60 to 70 and 800 IU/d for those age ≥71. For vitamin D deficient patients, recommend >1,000 IU/d.1
Recommend 15 minutes per day in the sun without sunscreen from spring to autumn; late summer to fall is ideal because vitamin D’s half-life is 30 days. Midday is the best time to produce vitamin D.5
Recheck the patient’s Mini-Mental State Examination score every 4 months. Vitamin D supplementation is correlated with cognitive functioning.6
Disclosure
Dr. LaFerney reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
1. Mayo Clinic. Vitamin D. http://www.mayoclinic.com/health/vitamin-d/NS_patient-vitamind/DSECTION=dosing. Updated October 1 2011. Accessed September 26, 2012.
2. National Institutes of Health. Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional. Accessed September 26, 2012.
3. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
4. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
5. Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006;82(6):1697-1703.
6. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
Low vitamin D levels can impact cognitive functioning in older adults.1 As vitamin D levels decrease, cognitive impairment increases.
Vitamin D deficiency can occur because few foods contain this nutrient2 and patients have limited exposure to sunlight—vitamin D is produced when sunlight strikes the skin.2 In addition to rickets, low levels of vitamin D have been linked to slower information processing in middle age and older men, cognitive decline, mood disorders, and altered brain development and function resulting in neurodegenerative diseases and other medical disorders.3
One study suggested that one-half of adults age >60 do not get sufficient vitamin D, with an even higher rate among women with Alzheimer’s disease.4 Patients in dementia units typically are not tested for vitamin D levels. These patients rarely leave the unit, which leaves them deprived of the vitamin D provided by sunlight. Even patients exposed to sunlight may receive minimal vitamin D because they use sunscreen.
The following protocol can help patients who may benefit from vitamin D supplementation and increased sun exposure.
Obtain and assess vitamin D levels. Evaluate your patient’s level in the context of physical or cognitive symptoms and other lab values:
- deficient: <12 ng/mL
- inadequate: 12 to 20 ng/mL
- adequate: ≥20 ng/mL.2
Order dietary assessment to identify foods that may increase vitamin D levels. The best sources are fish—salmon, tuna, and mackerel—fish oils, beef, liver, cheese, and egg yolks.2 Several food products, including milk and orange juice, are fortified with vitamin D.
Suggest a daily vitamin D supplement ranging from 400 IU/d to 1,000 IU/d. The Institute of Medicine suggests 600 IU/d for patients age 60 to 70 and 800 IU/d for those age ≥71. For vitamin D deficient patients, recommend >1,000 IU/d.1
Recommend 15 minutes per day in the sun without sunscreen from spring to autumn; late summer to fall is ideal because vitamin D’s half-life is 30 days. Midday is the best time to produce vitamin D.5
Recheck the patient’s Mini-Mental State Examination score every 4 months. Vitamin D supplementation is correlated with cognitive functioning.6
Disclosure
Dr. LaFerney reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
Low vitamin D levels can impact cognitive functioning in older adults.1 As vitamin D levels decrease, cognitive impairment increases.
Vitamin D deficiency can occur because few foods contain this nutrient2 and patients have limited exposure to sunlight—vitamin D is produced when sunlight strikes the skin.2 In addition to rickets, low levels of vitamin D have been linked to slower information processing in middle age and older men, cognitive decline, mood disorders, and altered brain development and function resulting in neurodegenerative diseases and other medical disorders.3
One study suggested that one-half of adults age >60 do not get sufficient vitamin D, with an even higher rate among women with Alzheimer’s disease.4 Patients in dementia units typically are not tested for vitamin D levels. These patients rarely leave the unit, which leaves them deprived of the vitamin D provided by sunlight. Even patients exposed to sunlight may receive minimal vitamin D because they use sunscreen.
The following protocol can help patients who may benefit from vitamin D supplementation and increased sun exposure.
Obtain and assess vitamin D levels. Evaluate your patient’s level in the context of physical or cognitive symptoms and other lab values:
- deficient: <12 ng/mL
- inadequate: 12 to 20 ng/mL
- adequate: ≥20 ng/mL.2
Order dietary assessment to identify foods that may increase vitamin D levels. The best sources are fish—salmon, tuna, and mackerel—fish oils, beef, liver, cheese, and egg yolks.2 Several food products, including milk and orange juice, are fortified with vitamin D.
Suggest a daily vitamin D supplement ranging from 400 IU/d to 1,000 IU/d. The Institute of Medicine suggests 600 IU/d for patients age 60 to 70 and 800 IU/d for those age ≥71. For vitamin D deficient patients, recommend >1,000 IU/d.1
Recommend 15 minutes per day in the sun without sunscreen from spring to autumn; late summer to fall is ideal because vitamin D’s half-life is 30 days. Midday is the best time to produce vitamin D.5
Recheck the patient’s Mini-Mental State Examination score every 4 months. Vitamin D supplementation is correlated with cognitive functioning.6
Disclosure
Dr. LaFerney reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
1. Mayo Clinic. Vitamin D. http://www.mayoclinic.com/health/vitamin-d/NS_patient-vitamind/DSECTION=dosing. Updated October 1 2011. Accessed September 26, 2012.
2. National Institutes of Health. Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional. Accessed September 26, 2012.
3. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
4. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
5. Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006;82(6):1697-1703.
6. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
1. Mayo Clinic. Vitamin D. http://www.mayoclinic.com/health/vitamin-d/NS_patient-vitamind/DSECTION=dosing. Updated October 1 2011. Accessed September 26, 2012.
2. National Institutes of Health. Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional. Accessed September 26, 2012.
3. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
4. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
5. Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006;82(6):1697-1703.
6. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
Omega-3s for BPD
As a psychiatrist who incorporates diet and dietary supplements in my practice, I appreciated the excellent review of omega-3 fatty acids for psychiatric illness (Current Psychiatry, September 2012, p. 40-45; http://bit.ly/1ApTrXC). It’s far better to support normal biochemistry and avoid side effects whenever possible.
However, regarding treatment of borderline personality disorder (BPD), the author stated that omega-3 fatty acids are ineffective. I have found them clinically useful for BPD. In the 2003 study the author cited, Zanarini et al1 concluded “E-EPA [ethyl-eicosapentaenoic acid] is a nutriceutical agent that is both well tolerated and may be efficacious for the treatment of moderately disturbed women with borderline personality disorder. Ninety percent of those taking this compound were able to complete the entire 8-week trial and reported no clinically relevant side effects. Those treated with this compound also experienced a significantly greater reduction in their overall aggression as well as their depressive symptoms than those treated with placebo.” These results suggest that omega-3 fatty acids may be an effective monotherapy for women with moderately severe BPD.
Hyla Cass, MD
Private Practice
Pacific Palisades, CA
The author responds
Dr. Cass is correct. Zanarini et al did suggest that omega-3 fatty acids are beneficial in reducing aggression and depressive symptoms in women with moderate borderline personality disorder who were not prescribed other psychotropics. However, the study was small (N = 30), and further research is needed to support these findings.
Mary Morreale, MD
Assistant Professor
Department of Psychiatry
Wayne State University
Detroit, MI
As a psychiatrist who incorporates diet and dietary supplements in my practice, I appreciated the excellent review of omega-3 fatty acids for psychiatric illness (Current Psychiatry, September 2012, p. 40-45; http://bit.ly/1ApTrXC). It’s far better to support normal biochemistry and avoid side effects whenever possible.
However, regarding treatment of borderline personality disorder (BPD), the author stated that omega-3 fatty acids are ineffective. I have found them clinically useful for BPD. In the 2003 study the author cited, Zanarini et al1 concluded “E-EPA [ethyl-eicosapentaenoic acid] is a nutriceutical agent that is both well tolerated and may be efficacious for the treatment of moderately disturbed women with borderline personality disorder. Ninety percent of those taking this compound were able to complete the entire 8-week trial and reported no clinically relevant side effects. Those treated with this compound also experienced a significantly greater reduction in their overall aggression as well as their depressive symptoms than those treated with placebo.” These results suggest that omega-3 fatty acids may be an effective monotherapy for women with moderately severe BPD.
Hyla Cass, MD
Private Practice
Pacific Palisades, CA
The author responds
Dr. Cass is correct. Zanarini et al did suggest that omega-3 fatty acids are beneficial in reducing aggression and depressive symptoms in women with moderate borderline personality disorder who were not prescribed other psychotropics. However, the study was small (N = 30), and further research is needed to support these findings.
Mary Morreale, MD
Assistant Professor
Department of Psychiatry
Wayne State University
Detroit, MI
As a psychiatrist who incorporates diet and dietary supplements in my practice, I appreciated the excellent review of omega-3 fatty acids for psychiatric illness (Current Psychiatry, September 2012, p. 40-45; http://bit.ly/1ApTrXC). It’s far better to support normal biochemistry and avoid side effects whenever possible.
However, regarding treatment of borderline personality disorder (BPD), the author stated that omega-3 fatty acids are ineffective. I have found them clinically useful for BPD. In the 2003 study the author cited, Zanarini et al1 concluded “E-EPA [ethyl-eicosapentaenoic acid] is a nutriceutical agent that is both well tolerated and may be efficacious for the treatment of moderately disturbed women with borderline personality disorder. Ninety percent of those taking this compound were able to complete the entire 8-week trial and reported no clinically relevant side effects. Those treated with this compound also experienced a significantly greater reduction in their overall aggression as well as their depressive symptoms than those treated with placebo.” These results suggest that omega-3 fatty acids may be an effective monotherapy for women with moderately severe BPD.
Hyla Cass, MD
Private Practice
Pacific Palisades, CA
The author responds
Dr. Cass is correct. Zanarini et al did suggest that omega-3 fatty acids are beneficial in reducing aggression and depressive symptoms in women with moderate borderline personality disorder who were not prescribed other psychotropics. However, the study was small (N = 30), and further research is needed to support these findings.
Mary Morreale, MD
Assistant Professor
Department of Psychiatry
Wayne State University
Detroit, MI
Psychiatry’s ‘swords of Damocles’
Beneath the current haze of the election buzz, a national anticipatory panic is building up because of the looming “fiscal cliff,” when massive government budget cuts are expected to have grave ramifications and bleak economic and existential repercussions.
Individuals from all political affiliations are frantically demanding that lawmakers do something to avoid a disastrous plunge into chaos for government institutions.
Paradoxically and inexplicably, nothing is being done so far to circumvent this impending doom scenario. A sword of Damocles is hanging over the nation, held, as the legend goes, at the pommel by a single hair of a horse’s tail!
I often feel that’s also what is happening in psychiatry. We are facing not 1, but multiple serious and disruptive challenges, crises, and threats to our profession and our patients. It evokes a grim image of multiple Damocles’ swords hanging over us. We do not seem to be doing anything tangible to avoid these dangerous and injurious swords. I quietly fear that living in imminent danger has become the “new normal” for psychiatry. That’s actually a euphemism for “massive denial.” We all seem to be going on with our lives as if we are not heading to our own version of a “fiscal cliff.” The apathy, inaction, and lack of a sense of urgency by “organized psychiatry” are astonishing, given the critical need for urgent action.
Consider the following swords of Damocles hanging over psychiatry:
- Down-to-the-bone budget cuts in public psychiatry with inadequate resources in community mental health and public hospitals.
- Severe bed shortages: psychiatry has dropped from the most overbedded medical specialty in the 1960s to the most underbedded one, with devastating adverse effects on acutely ill patients who need inpatient treatment.
- Unabating incarceration and criminalization of seriously mentally ill patients as a substitute for hospitalization or care at residential facilities.
- A chronic shortage of psychiatrists, which is fueling the argument by some mental health professions with no medical or advanced nursing background that they should be permitted to “acquire” prescription privileges, as if prescribing is a skill independent from the extensive training of 4 years of medical school plus 4 years of psychiatric training. The consequences for disabled patients will be low-quality, even dangerous care.
- The low rate of medical students choosing psychiatry as a career. The shortage of psychiatrists will worsen if attrition from retirement or mortality is not offset by substantial annual infusions of newly minted psychiatrists.
- The deplorably short life span of patients with severe mental illness—25 years less than non-mentally ill individuals—mainly attributable to excess cardiovascular risk and lack of access to adequate primary care as part of community mental health management.
- An anemic pipeline of psychiatric residents choosing careers as teachers or researchers. Huge student loans and lack of mentorship are only some of the reasons residents select clinical work over academic careers.
- Withdrawal of several major pharmaceutical companies from research and development to discover new psychiatric drugs. It would be more judicious to engage and partner with them instead of demonizing them simply because they are the only entities that design, develop, test, and produce psychiatric medications. The unmet needs in psychiatry are enormous, especially because 80% of DSM-IV-TR psychiatric disorders have no FDA-approved medication.
- A lack of coalescence and cohesiveness of psychiatrists in the United States. We have fragmented into small organizations and factions, speaking with several faint voices instead of one powerful voice. Other disciplines are far better organized and can lobby more effectively for their causes.
- A lack of participation by psychiatrists in their professional organizations and low support for political action committees to navigate the agenda of psychiatry at the local, state, and national levels. The image and influence of psychiatry can be bolstered only by ongoing member donations and the reverse will occur without consistent member support.
- The persistent stigma of mental illness that continues to haunt our patients, inhibits treatment-seeking, and undermines treatment adherence. Despite magnificent advances in the medical basis of mental disorders, psychiatric illnesses are not regarded with the same acceptance and understanding as other medical disorders.
- A frustrating lag in translating the veritable explosion in basic neuroscience discoveries into clinical applications for diagnosis or therapeutics. Psychiatry could benefit greatly if a sufficient number of well-funded translational researchers get involved. A partnership between the National Institute of Mental Health (NIMH)and the pharmaceutical industry can expedite this translation, but has yet to happen.
This list of “dangling swords” may appear daunting and overwhelming but if we shed our apathy and unite, we can parry their threats and emerge stronger. I do not claim to have the answers but I do have an abiding faith in the collective wisdom and abilities of my fellow psychiatrists, if they decide to mobilize. The root of our “fiscal cliff” is our chronic inaction, passivity, and lack of cohesiveness and a passionate pursuit of our shared goals. We better wake up and act soon before these Damocles’ swords start falling and inflicting their exquisite pain.
Beneath the current haze of the election buzz, a national anticipatory panic is building up because of the looming “fiscal cliff,” when massive government budget cuts are expected to have grave ramifications and bleak economic and existential repercussions.
Individuals from all political affiliations are frantically demanding that lawmakers do something to avoid a disastrous plunge into chaos for government institutions.
Paradoxically and inexplicably, nothing is being done so far to circumvent this impending doom scenario. A sword of Damocles is hanging over the nation, held, as the legend goes, at the pommel by a single hair of a horse’s tail!
I often feel that’s also what is happening in psychiatry. We are facing not 1, but multiple serious and disruptive challenges, crises, and threats to our profession and our patients. It evokes a grim image of multiple Damocles’ swords hanging over us. We do not seem to be doing anything tangible to avoid these dangerous and injurious swords. I quietly fear that living in imminent danger has become the “new normal” for psychiatry. That’s actually a euphemism for “massive denial.” We all seem to be going on with our lives as if we are not heading to our own version of a “fiscal cliff.” The apathy, inaction, and lack of a sense of urgency by “organized psychiatry” are astonishing, given the critical need for urgent action.
Consider the following swords of Damocles hanging over psychiatry:
- Down-to-the-bone budget cuts in public psychiatry with inadequate resources in community mental health and public hospitals.
- Severe bed shortages: psychiatry has dropped from the most overbedded medical specialty in the 1960s to the most underbedded one, with devastating adverse effects on acutely ill patients who need inpatient treatment.
- Unabating incarceration and criminalization of seriously mentally ill patients as a substitute for hospitalization or care at residential facilities.
- A chronic shortage of psychiatrists, which is fueling the argument by some mental health professions with no medical or advanced nursing background that they should be permitted to “acquire” prescription privileges, as if prescribing is a skill independent from the extensive training of 4 years of medical school plus 4 years of psychiatric training. The consequences for disabled patients will be low-quality, even dangerous care.
- The low rate of medical students choosing psychiatry as a career. The shortage of psychiatrists will worsen if attrition from retirement or mortality is not offset by substantial annual infusions of newly minted psychiatrists.
- The deplorably short life span of patients with severe mental illness—25 years less than non-mentally ill individuals—mainly attributable to excess cardiovascular risk and lack of access to adequate primary care as part of community mental health management.
- An anemic pipeline of psychiatric residents choosing careers as teachers or researchers. Huge student loans and lack of mentorship are only some of the reasons residents select clinical work over academic careers.
- Withdrawal of several major pharmaceutical companies from research and development to discover new psychiatric drugs. It would be more judicious to engage and partner with them instead of demonizing them simply because they are the only entities that design, develop, test, and produce psychiatric medications. The unmet needs in psychiatry are enormous, especially because 80% of DSM-IV-TR psychiatric disorders have no FDA-approved medication.
- A lack of coalescence and cohesiveness of psychiatrists in the United States. We have fragmented into small organizations and factions, speaking with several faint voices instead of one powerful voice. Other disciplines are far better organized and can lobby more effectively for their causes.
- A lack of participation by psychiatrists in their professional organizations and low support for political action committees to navigate the agenda of psychiatry at the local, state, and national levels. The image and influence of psychiatry can be bolstered only by ongoing member donations and the reverse will occur without consistent member support.
- The persistent stigma of mental illness that continues to haunt our patients, inhibits treatment-seeking, and undermines treatment adherence. Despite magnificent advances in the medical basis of mental disorders, psychiatric illnesses are not regarded with the same acceptance and understanding as other medical disorders.
- A frustrating lag in translating the veritable explosion in basic neuroscience discoveries into clinical applications for diagnosis or therapeutics. Psychiatry could benefit greatly if a sufficient number of well-funded translational researchers get involved. A partnership between the National Institute of Mental Health (NIMH)and the pharmaceutical industry can expedite this translation, but has yet to happen.
This list of “dangling swords” may appear daunting and overwhelming but if we shed our apathy and unite, we can parry their threats and emerge stronger. I do not claim to have the answers but I do have an abiding faith in the collective wisdom and abilities of my fellow psychiatrists, if they decide to mobilize. The root of our “fiscal cliff” is our chronic inaction, passivity, and lack of cohesiveness and a passionate pursuit of our shared goals. We better wake up and act soon before these Damocles’ swords start falling and inflicting their exquisite pain.
Beneath the current haze of the election buzz, a national anticipatory panic is building up because of the looming “fiscal cliff,” when massive government budget cuts are expected to have grave ramifications and bleak economic and existential repercussions.
Individuals from all political affiliations are frantically demanding that lawmakers do something to avoid a disastrous plunge into chaos for government institutions.
Paradoxically and inexplicably, nothing is being done so far to circumvent this impending doom scenario. A sword of Damocles is hanging over the nation, held, as the legend goes, at the pommel by a single hair of a horse’s tail!
I often feel that’s also what is happening in psychiatry. We are facing not 1, but multiple serious and disruptive challenges, crises, and threats to our profession and our patients. It evokes a grim image of multiple Damocles’ swords hanging over us. We do not seem to be doing anything tangible to avoid these dangerous and injurious swords. I quietly fear that living in imminent danger has become the “new normal” for psychiatry. That’s actually a euphemism for “massive denial.” We all seem to be going on with our lives as if we are not heading to our own version of a “fiscal cliff.” The apathy, inaction, and lack of a sense of urgency by “organized psychiatry” are astonishing, given the critical need for urgent action.
Consider the following swords of Damocles hanging over psychiatry:
- Down-to-the-bone budget cuts in public psychiatry with inadequate resources in community mental health and public hospitals.
- Severe bed shortages: psychiatry has dropped from the most overbedded medical specialty in the 1960s to the most underbedded one, with devastating adverse effects on acutely ill patients who need inpatient treatment.
- Unabating incarceration and criminalization of seriously mentally ill patients as a substitute for hospitalization or care at residential facilities.
- A chronic shortage of psychiatrists, which is fueling the argument by some mental health professions with no medical or advanced nursing background that they should be permitted to “acquire” prescription privileges, as if prescribing is a skill independent from the extensive training of 4 years of medical school plus 4 years of psychiatric training. The consequences for disabled patients will be low-quality, even dangerous care.
- The low rate of medical students choosing psychiatry as a career. The shortage of psychiatrists will worsen if attrition from retirement or mortality is not offset by substantial annual infusions of newly minted psychiatrists.
- The deplorably short life span of patients with severe mental illness—25 years less than non-mentally ill individuals—mainly attributable to excess cardiovascular risk and lack of access to adequate primary care as part of community mental health management.
- An anemic pipeline of psychiatric residents choosing careers as teachers or researchers. Huge student loans and lack of mentorship are only some of the reasons residents select clinical work over academic careers.
- Withdrawal of several major pharmaceutical companies from research and development to discover new psychiatric drugs. It would be more judicious to engage and partner with them instead of demonizing them simply because they are the only entities that design, develop, test, and produce psychiatric medications. The unmet needs in psychiatry are enormous, especially because 80% of DSM-IV-TR psychiatric disorders have no FDA-approved medication.
- A lack of coalescence and cohesiveness of psychiatrists in the United States. We have fragmented into small organizations and factions, speaking with several faint voices instead of one powerful voice. Other disciplines are far better organized and can lobby more effectively for their causes.
- A lack of participation by psychiatrists in their professional organizations and low support for political action committees to navigate the agenda of psychiatry at the local, state, and national levels. The image and influence of psychiatry can be bolstered only by ongoing member donations and the reverse will occur without consistent member support.
- The persistent stigma of mental illness that continues to haunt our patients, inhibits treatment-seeking, and undermines treatment adherence. Despite magnificent advances in the medical basis of mental disorders, psychiatric illnesses are not regarded with the same acceptance and understanding as other medical disorders.
- A frustrating lag in translating the veritable explosion in basic neuroscience discoveries into clinical applications for diagnosis or therapeutics. Psychiatry could benefit greatly if a sufficient number of well-funded translational researchers get involved. A partnership between the National Institute of Mental Health (NIMH)and the pharmaceutical industry can expedite this translation, but has yet to happen.
This list of “dangling swords” may appear daunting and overwhelming but if we shed our apathy and unite, we can parry their threats and emerge stronger. I do not claim to have the answers but I do have an abiding faith in the collective wisdom and abilities of my fellow psychiatrists, if they decide to mobilize. The root of our “fiscal cliff” is our chronic inaction, passivity, and lack of cohesiveness and a passionate pursuit of our shared goals. We better wake up and act soon before these Damocles’ swords start falling and inflicting their exquisite pain.
An open-label trial of escitalopram for PPD: Considerations for research
Challenges in recruiting women to postpartum depression (PPD) antidepressant treatment trials, which we encountered when conducting a trial of escitalopram, contribute to the limited body of knowledge about PPD treatment. Here we discuss results from a preliminary trial of escitalopram for PPD, and challenges of research in this area.
Escitalopram, the S-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with high selectivity and potency that is FDA-approved for treating major depressive disorder (MDD) and generalized anxiety disorder. An agent with antidepressant and anxiolytic effects is particularly desirable for PPD because anxiety is more common in postpartum major depressive episodes than non-postpartum MDD.1 Anxiety and depressive disorders commonly are comorbid in postpartum women.2
We conducted an open-label trial of escitalopram for women with PPD and anxiety. We initially attempted to recruit 20 women.
Methods
Patients received 8 weeks of treatment with escitalopram, 10 to 20 mg/d (flexible dose). After completing the initial phone screen, patients had 5 follow-up visits, once every 2 weeks for 8 weeks. The institutional review board at Massachusetts General Hospital approved this study and we obtained written informed consent from all patients at the first visit. Twelve patients completed the phone screen and 7 eligible patients were enrolled in the study over 32 months. Reasons for ineligibility included having a history of psychosis, onset of symptoms >3 months postpartum, or presenting >6 months after onset. Others declined to participate because of concern about the time commitment or because they pursued nonpharmacologic treatments after the evaluation visit. One patient was lost to follow-up. Three patients completed the study. The study was halted because of the slow pace of recruitment.
Patient selection. Patients were screened for a major depressive episode with postpartum onset within 3 months of childbirth; depressive symptoms may have developed during pregnancy and worsened postpartum to meet criteria for MDD. Women were eligible for the study if they:
- were age 18 to 45
- experienced a major depressive episode with symptoms developing within 3 months of childbirth
- presented within 6 months of childbirth
- had a Montgomery-Åsberg Depression Rating Scale (MADRS) score >15
- had a Beck Anxiety Inventory (BAI) score >10.
Patients who were pregnant or breast-feeding were excluded from the study per an agreement with the sponsor. In addition, women were excluded if they had taken any psychotropic medication within 2 weeks of enrollment; had active suicidal ideation, homicidal ideation, or presence of psychotic symptoms; had chronic depression or dysthymia; had chronic or treatment-resistant anxiety disorders; had a history of mania or hypomania; or had active alcohol or substance abuse within the past year.
Treatment. Patients received escitalopram, 10 mg/d, after the baseline visit. At the investigator’s discretion, the dose could be increased to 20 mg/d or lowered to 5 mg/d if side effects occurred.
Measures. At the first visit, patients were assessed with the Mini-International Neuropsychiatric Interview to verify MDD and exclude diagnoses that would determine ineligibility. MADRS and Edinburgh Postnatal Depression Scale (EPDS) were used at each visit to measure depressive symptoms.3,4 The BAI was completed at each visit to measure anxiety symptoms. Obsessions and compulsions were measured with the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)5 at baseline, and at all following visits if the patient scored >8 at baseline. The Clinical Global Impression Scales for severity and improvement were completed at each visit.6
Results
Of 7 patients enrolled, 3 completed the study, 2 were ineligible after the baseline visit, and 2 did not participate after the baseline visit (1 selected to pursue psychotherapy, and 1 was lost to follow-up).
Two of 3 patients responded to escitalopram (≥50% decrease on MADRS), and both were remitters (MADRS score <7). All 3 patients were responders on EPDS and BAI. One patient had Y-BOCS >8 at baseline (Total Y-BOCS score of 9, and final Y-BOCS score of 8) (Table).
Table
Symptom rating scale scores at baseline and study end
| Baseline (Visit 1) | Final (Visit 5) | |||||
|---|---|---|---|---|---|---|
| Patient | MADRS | BAI | EPDS | MADRS | BAI | EPDS |
| Ms. A | 21 | 18 | 22 | 12 | 0 | 0 |
| Ms. B | 28 | 28 | 19 | 4 | 5 | 2 |
| Ms. C | 37 | 6 | 19 | 6 | 2 | 0 |
| BAI: Beck Anxiety Inventory; EPDS: Edinburgh Postnatal Depression Scale; MADRS: Montgomery-Åsberg Depression Rating Scale | ||||||
Discussion
Patients who stayed in treatment improved during the course of this study. Recruitment was difficult; we were able to recruit only 7 patients out of a projected 20 for the screening visit. We solicited feedback from local obstetrics health care providers and social workers on recruitment and attractiveness of the study as part of our routine collaboration with obstetrical services that screen for PPD. Primary reasons patients were not referred were that they were breast-feeding or they stated they would prefer to receive treatment from their primary care doctor. Recruitment difficulty in this study was in stark contrast to other recent studies completed at our center. For example, we have successfully recruited for menopausal depression and premenstrual dysphoric disorder treatment studies, and have completed large naturalistic studies of women with unipolar depression and bipolar disorder across pregnancy and postpartum. We suspect that many patients who were eligible for the study preferred to seek care from an obstetrician or primary care doctor with whom they already had a therapeutic alliance, and we also suspect that many women with PPD do not seek treatment at all, which is consistent with findings from other research groups.
Lessons learned from PPD research include:
- Including women who are breast-feeding is important because many women choose to breast-feed and suffer from PPD. Because antidepressant use during breast-feeding has been closely studied, it is appropriate to include potential research participants who are breast-feeding as long as they receive adequate information and are able to provide informed consent.
- Participants in PPD studies may require accommodations that take into account their role as a new mother, such as on-site childcare, home visits, or other strategies.
- Because of recruitment challenges in postpartum patients, multisite trials may be required to include adequate numbers of participants.
Related Resource
- Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-21.
Drug Brand Names
- Citalopram • Celexa
- Escitalopram • Lexapro
Disclosures
Dr. Freeman has received grant or research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, is on the advisory boards of Otsuka and Takeda/Lundbeck, and is a consultant for PamLab LLC.
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
This study was funded as an investigator-initiated trial by Forest Pharmaceuticals.
1. Bernstein IH, Rush AJ, Yonkers K, et al. Symptom features of postpartum depression: are they distinct? Depress Anxiety. 2008;25(1):20-26.
2. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
3. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
4. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
5. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
6. Guy W. ECDEU assessment manual for psychopharmacology. Rockville MD: US Department of Health and Human Services; 1976. Department of Health, Education, and Welfare Publication (ADM) 76–338.
Challenges in recruiting women to postpartum depression (PPD) antidepressant treatment trials, which we encountered when conducting a trial of escitalopram, contribute to the limited body of knowledge about PPD treatment. Here we discuss results from a preliminary trial of escitalopram for PPD, and challenges of research in this area.
Escitalopram, the S-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with high selectivity and potency that is FDA-approved for treating major depressive disorder (MDD) and generalized anxiety disorder. An agent with antidepressant and anxiolytic effects is particularly desirable for PPD because anxiety is more common in postpartum major depressive episodes than non-postpartum MDD.1 Anxiety and depressive disorders commonly are comorbid in postpartum women.2
We conducted an open-label trial of escitalopram for women with PPD and anxiety. We initially attempted to recruit 20 women.
Methods
Patients received 8 weeks of treatment with escitalopram, 10 to 20 mg/d (flexible dose). After completing the initial phone screen, patients had 5 follow-up visits, once every 2 weeks for 8 weeks. The institutional review board at Massachusetts General Hospital approved this study and we obtained written informed consent from all patients at the first visit. Twelve patients completed the phone screen and 7 eligible patients were enrolled in the study over 32 months. Reasons for ineligibility included having a history of psychosis, onset of symptoms >3 months postpartum, or presenting >6 months after onset. Others declined to participate because of concern about the time commitment or because they pursued nonpharmacologic treatments after the evaluation visit. One patient was lost to follow-up. Three patients completed the study. The study was halted because of the slow pace of recruitment.
Patient selection. Patients were screened for a major depressive episode with postpartum onset within 3 months of childbirth; depressive symptoms may have developed during pregnancy and worsened postpartum to meet criteria for MDD. Women were eligible for the study if they:
- were age 18 to 45
- experienced a major depressive episode with symptoms developing within 3 months of childbirth
- presented within 6 months of childbirth
- had a Montgomery-Åsberg Depression Rating Scale (MADRS) score >15
- had a Beck Anxiety Inventory (BAI) score >10.
Patients who were pregnant or breast-feeding were excluded from the study per an agreement with the sponsor. In addition, women were excluded if they had taken any psychotropic medication within 2 weeks of enrollment; had active suicidal ideation, homicidal ideation, or presence of psychotic symptoms; had chronic depression or dysthymia; had chronic or treatment-resistant anxiety disorders; had a history of mania or hypomania; or had active alcohol or substance abuse within the past year.
Treatment. Patients received escitalopram, 10 mg/d, after the baseline visit. At the investigator’s discretion, the dose could be increased to 20 mg/d or lowered to 5 mg/d if side effects occurred.
Measures. At the first visit, patients were assessed with the Mini-International Neuropsychiatric Interview to verify MDD and exclude diagnoses that would determine ineligibility. MADRS and Edinburgh Postnatal Depression Scale (EPDS) were used at each visit to measure depressive symptoms.3,4 The BAI was completed at each visit to measure anxiety symptoms. Obsessions and compulsions were measured with the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)5 at baseline, and at all following visits if the patient scored >8 at baseline. The Clinical Global Impression Scales for severity and improvement were completed at each visit.6
Results
Of 7 patients enrolled, 3 completed the study, 2 were ineligible after the baseline visit, and 2 did not participate after the baseline visit (1 selected to pursue psychotherapy, and 1 was lost to follow-up).
Two of 3 patients responded to escitalopram (≥50% decrease on MADRS), and both were remitters (MADRS score <7). All 3 patients were responders on EPDS and BAI. One patient had Y-BOCS >8 at baseline (Total Y-BOCS score of 9, and final Y-BOCS score of 8) (Table).
Table
Symptom rating scale scores at baseline and study end
| Baseline (Visit 1) | Final (Visit 5) | |||||
|---|---|---|---|---|---|---|
| Patient | MADRS | BAI | EPDS | MADRS | BAI | EPDS |
| Ms. A | 21 | 18 | 22 | 12 | 0 | 0 |
| Ms. B | 28 | 28 | 19 | 4 | 5 | 2 |
| Ms. C | 37 | 6 | 19 | 6 | 2 | 0 |
| BAI: Beck Anxiety Inventory; EPDS: Edinburgh Postnatal Depression Scale; MADRS: Montgomery-Åsberg Depression Rating Scale | ||||||
Discussion
Patients who stayed in treatment improved during the course of this study. Recruitment was difficult; we were able to recruit only 7 patients out of a projected 20 for the screening visit. We solicited feedback from local obstetrics health care providers and social workers on recruitment and attractiveness of the study as part of our routine collaboration with obstetrical services that screen for PPD. Primary reasons patients were not referred were that they were breast-feeding or they stated they would prefer to receive treatment from their primary care doctor. Recruitment difficulty in this study was in stark contrast to other recent studies completed at our center. For example, we have successfully recruited for menopausal depression and premenstrual dysphoric disorder treatment studies, and have completed large naturalistic studies of women with unipolar depression and bipolar disorder across pregnancy and postpartum. We suspect that many patients who were eligible for the study preferred to seek care from an obstetrician or primary care doctor with whom they already had a therapeutic alliance, and we also suspect that many women with PPD do not seek treatment at all, which is consistent with findings from other research groups.
Lessons learned from PPD research include:
- Including women who are breast-feeding is important because many women choose to breast-feed and suffer from PPD. Because antidepressant use during breast-feeding has been closely studied, it is appropriate to include potential research participants who are breast-feeding as long as they receive adequate information and are able to provide informed consent.
- Participants in PPD studies may require accommodations that take into account their role as a new mother, such as on-site childcare, home visits, or other strategies.
- Because of recruitment challenges in postpartum patients, multisite trials may be required to include adequate numbers of participants.
Related Resource
- Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-21.
Drug Brand Names
- Citalopram • Celexa
- Escitalopram • Lexapro
Disclosures
Dr. Freeman has received grant or research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, is on the advisory boards of Otsuka and Takeda/Lundbeck, and is a consultant for PamLab LLC.
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
This study was funded as an investigator-initiated trial by Forest Pharmaceuticals.
Challenges in recruiting women to postpartum depression (PPD) antidepressant treatment trials, which we encountered when conducting a trial of escitalopram, contribute to the limited body of knowledge about PPD treatment. Here we discuss results from a preliminary trial of escitalopram for PPD, and challenges of research in this area.
Escitalopram, the S-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with high selectivity and potency that is FDA-approved for treating major depressive disorder (MDD) and generalized anxiety disorder. An agent with antidepressant and anxiolytic effects is particularly desirable for PPD because anxiety is more common in postpartum major depressive episodes than non-postpartum MDD.1 Anxiety and depressive disorders commonly are comorbid in postpartum women.2
We conducted an open-label trial of escitalopram for women with PPD and anxiety. We initially attempted to recruit 20 women.
Methods
Patients received 8 weeks of treatment with escitalopram, 10 to 20 mg/d (flexible dose). After completing the initial phone screen, patients had 5 follow-up visits, once every 2 weeks for 8 weeks. The institutional review board at Massachusetts General Hospital approved this study and we obtained written informed consent from all patients at the first visit. Twelve patients completed the phone screen and 7 eligible patients were enrolled in the study over 32 months. Reasons for ineligibility included having a history of psychosis, onset of symptoms >3 months postpartum, or presenting >6 months after onset. Others declined to participate because of concern about the time commitment or because they pursued nonpharmacologic treatments after the evaluation visit. One patient was lost to follow-up. Three patients completed the study. The study was halted because of the slow pace of recruitment.
Patient selection. Patients were screened for a major depressive episode with postpartum onset within 3 months of childbirth; depressive symptoms may have developed during pregnancy and worsened postpartum to meet criteria for MDD. Women were eligible for the study if they:
- were age 18 to 45
- experienced a major depressive episode with symptoms developing within 3 months of childbirth
- presented within 6 months of childbirth
- had a Montgomery-Åsberg Depression Rating Scale (MADRS) score >15
- had a Beck Anxiety Inventory (BAI) score >10.
Patients who were pregnant or breast-feeding were excluded from the study per an agreement with the sponsor. In addition, women were excluded if they had taken any psychotropic medication within 2 weeks of enrollment; had active suicidal ideation, homicidal ideation, or presence of psychotic symptoms; had chronic depression or dysthymia; had chronic or treatment-resistant anxiety disorders; had a history of mania or hypomania; or had active alcohol or substance abuse within the past year.
Treatment. Patients received escitalopram, 10 mg/d, after the baseline visit. At the investigator’s discretion, the dose could be increased to 20 mg/d or lowered to 5 mg/d if side effects occurred.
Measures. At the first visit, patients were assessed with the Mini-International Neuropsychiatric Interview to verify MDD and exclude diagnoses that would determine ineligibility. MADRS and Edinburgh Postnatal Depression Scale (EPDS) were used at each visit to measure depressive symptoms.3,4 The BAI was completed at each visit to measure anxiety symptoms. Obsessions and compulsions were measured with the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)5 at baseline, and at all following visits if the patient scored >8 at baseline. The Clinical Global Impression Scales for severity and improvement were completed at each visit.6
Results
Of 7 patients enrolled, 3 completed the study, 2 were ineligible after the baseline visit, and 2 did not participate after the baseline visit (1 selected to pursue psychotherapy, and 1 was lost to follow-up).
Two of 3 patients responded to escitalopram (≥50% decrease on MADRS), and both were remitters (MADRS score <7). All 3 patients were responders on EPDS and BAI. One patient had Y-BOCS >8 at baseline (Total Y-BOCS score of 9, and final Y-BOCS score of 8) (Table).
Table
Symptom rating scale scores at baseline and study end
| Baseline (Visit 1) | Final (Visit 5) | |||||
|---|---|---|---|---|---|---|
| Patient | MADRS | BAI | EPDS | MADRS | BAI | EPDS |
| Ms. A | 21 | 18 | 22 | 12 | 0 | 0 |
| Ms. B | 28 | 28 | 19 | 4 | 5 | 2 |
| Ms. C | 37 | 6 | 19 | 6 | 2 | 0 |
| BAI: Beck Anxiety Inventory; EPDS: Edinburgh Postnatal Depression Scale; MADRS: Montgomery-Åsberg Depression Rating Scale | ||||||
Discussion
Patients who stayed in treatment improved during the course of this study. Recruitment was difficult; we were able to recruit only 7 patients out of a projected 20 for the screening visit. We solicited feedback from local obstetrics health care providers and social workers on recruitment and attractiveness of the study as part of our routine collaboration with obstetrical services that screen for PPD. Primary reasons patients were not referred were that they were breast-feeding or they stated they would prefer to receive treatment from their primary care doctor. Recruitment difficulty in this study was in stark contrast to other recent studies completed at our center. For example, we have successfully recruited for menopausal depression and premenstrual dysphoric disorder treatment studies, and have completed large naturalistic studies of women with unipolar depression and bipolar disorder across pregnancy and postpartum. We suspect that many patients who were eligible for the study preferred to seek care from an obstetrician or primary care doctor with whom they already had a therapeutic alliance, and we also suspect that many women with PPD do not seek treatment at all, which is consistent with findings from other research groups.
Lessons learned from PPD research include:
- Including women who are breast-feeding is important because many women choose to breast-feed and suffer from PPD. Because antidepressant use during breast-feeding has been closely studied, it is appropriate to include potential research participants who are breast-feeding as long as they receive adequate information and are able to provide informed consent.
- Participants in PPD studies may require accommodations that take into account their role as a new mother, such as on-site childcare, home visits, or other strategies.
- Because of recruitment challenges in postpartum patients, multisite trials may be required to include adequate numbers of participants.
Related Resource
- Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-21.
Drug Brand Names
- Citalopram • Celexa
- Escitalopram • Lexapro
Disclosures
Dr. Freeman has received grant or research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, is on the advisory boards of Otsuka and Takeda/Lundbeck, and is a consultant for PamLab LLC.
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
This study was funded as an investigator-initiated trial by Forest Pharmaceuticals.
1. Bernstein IH, Rush AJ, Yonkers K, et al. Symptom features of postpartum depression: are they distinct? Depress Anxiety. 2008;25(1):20-26.
2. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
3. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
4. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
5. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
6. Guy W. ECDEU assessment manual for psychopharmacology. Rockville MD: US Department of Health and Human Services; 1976. Department of Health, Education, and Welfare Publication (ADM) 76–338.
1. Bernstein IH, Rush AJ, Yonkers K, et al. Symptom features of postpartum depression: are they distinct? Depress Anxiety. 2008;25(1):20-26.
2. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
3. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
4. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
5. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
6. Guy W. ECDEU assessment manual for psychopharmacology. Rockville MD: US Department of Health and Human Services; 1976. Department of Health, Education, and Welfare Publication (ADM) 76–338.
Postpartum depression: Help patients find the right treatment
Discuss this article at www.facebook.com/CurrentPsychiatry
Postpartum depression (PPD)—emergence of a major depressive episode after childbirth—has broad negative consequences for the mother, baby, and other family members. The time of onset after delivery for a depressive episode to be considered postpartum is debatable, but the DSM-IV-TR specifier states that onset within 4 weeks of childbirth is considered postpartum. PPD can impact many aspects of child development, including mother-infant attachment, cognitive development, and behavior.1-3
An estimated 10% of women who have given birth experience PPD.4,5 The risk of PPD is particularly high among women who have had previous episodes of PPD or major depressive disorder (MDD). Other risk factors include stressful life events, depression and/or anxiety during pregnancy, family history of PPD, and obstetrical complications.6-8 Anxiety disorders are common in postpartum women, and anxiety symptoms often are prominent in PPD.9
Despite the prevalence of PPD and its serious consequences, few studies have addressed antidepressant treatment. In this article we discuss screening and treating PPD and considerations for breast-feeding mothers. Click here for results of an open-label trial of escitalopram for PPD we conducted in which patient recruitment was challenging.
Screening for PPD: A good start
Initiatives by state governments and health care providers have led to programs in which universal screening for PPD has been implemented. Screening provides a mechanism for early detection and intervention. The Edinburgh Postnatal Depression Scale10 is a self-rated, 10-item scale developed for the postpartum setting, and its use increases identification of PPD at postpartum obstetrics visits.11 Other screening tools such as the Patient Health Questionnaire-9 also are commonly used. Despite the success of screening programs in attempting the feasibility of screening, it is unclear if the identification of women who may be experiencing PPD increases their engagement in treatment. Studies have demonstrated that even when depressive symptoms suggesting a PPD episode are identified in the postpartum period, many women still do not receive treatment.12,13 Studies of PPD screening programs have not demonstrated that screening itself improves treatment engagement or improves outcomes.12,13
Psychotherapy: An effective option
Psychotherapy is an important first-line option for PPD, particularly because of considerations of medication exposure during breast-feeding and many women are reluctant to take antidepressants while breast-feeding.16 Interpersonal psychotherapy and cognitive-behavioral therapy (CBT) have been most studied for PPD, and both appear effective for prevention and acute treatment of PPD.17-20 Although psychotherapy alone may be sufficient for some women, for others, medication may be an important first-line treatment, depending on symptom severity, access to psychotherapy, and personal preference.
Evidence for antidepressants
Randomization to placebo is rare in PPD trials. Most trials have used open-label designs because placebo arms pose ethical dilemmas considering the impact of PPD on a mother and her baby. In a randomized study of sertraline or nortriptyline for PPD, both drugs were similarly efficacious.22 In another study comparing paroxetine monotherapy and paroxetine plus CBT for PPD, both groups experienced significant improvement in depression and anxiety symptoms, with no difference between groups at endpoint.23 Open-label trials have suggested antidepressants’ efficacy, although some studies have included small sample sizes (Table 1).20-27
Table 1
Antidepressants for PPD: Summary of the evidence
| Study | Design and size | Medication | Results |
|---|---|---|---|
| Appleby et al, 199720 | 12-week, placebo-controlled, N = 87 | Fluoxetine | Patients taking fluoxetine showed greater improvement than those taking placebo |
| Yonkers et al, 200821 | 8-week, placebo-controlled, N = 70 | Paroxetine | Both groups improved over time, but patients taking paroxetine had greater improvement in overall clinical severity |
| Wisner et al, 200622 | 8-week, RCT, N = 109 | Sertraline vs nortriptyline | Proportion of women who responded or remitted did not differ between those taking sertraline or nortriptyline |
| Misri et al, 200423 | 12-week, RCT, N = 35 | Paroxetine monotherapy vs paroxetine + CBT | Both groups showed significant improvement in mood and anxiety symptoms |
| Stowe et al, 199524 | 8-week, open-label, N = 21 | Sertraline | 20 patients experienced >50% reduction in SIGH-D score |
| Cohen et al, 199725 | Open-label, N = 15 | Venlafaxine | 12 patients achieved remission |
| Suri et al, 200126 | 8-week, open-label, N = 6 | Fluvoxamine | 4 patients became euthymic, with HDRS scores ranging from 2 to 5 |
| Nonacs et al, 200527 | 8-week, open-label, N = 8 | Bupropion | 6 patients had ≥50% decrease in HDRS score from baseline; 3 achieved remission |
| CBT: cognitive-behavioral therapy; HDRS: Hamilton Depression Rating Scale; PPD: postpartum depression; RCT: randomized controlled trial; SIGH-D: Structured Interview Guide for the Hamilton Depression Rating Scale | |||
Breast-feeding considerations
From a nutritional standpoint, breast-feeding is optimal for a newborn. However, for some women, breast-feeding is difficult and stressful, and new mothers may experience this difficulty as failure. Some women prefer not to breast-feed, and others may prefer to formula feed if they require pharmacotherapy, particularly if the medication has not been well studied in breast-feeding patients. Some women may decline to take medications if they are breast-feeding out of concern for the baby’s exposure via breast milk and prefer to try nonpharmacologic approaches first. Many mothers with PPD need to be reassured that stopping breast-feeding may be exactly what is needed if the experience is contributing to their PPD or making them uncomfortable accepting pharmacotherapy when indicated. Maternal mental health is more important than breast-feeding to the health and wellness of the mother-baby dyad.
Table 2
Considerations for antidepressant use during breast-feeding
| Drug(s) | Comments |
|---|---|
| Fluoxetine | Because of long half-life, may be more likely to be detected in infant serum, especially at higher doses. Reasonable for use during breast-feeding if a woman has had a good previous response to the drug or used it during pregnancy |
| Sertraline | Reports of low levels of exposure. Relatively large amount of data available |
| Citalopram, escitalopram | Less systematic study of mother-infant pairs compared with sertraline and paroxetine. Low levels of exposure to infant via breast-feeding observed |
| Paroxetine | Consistent reports of low levels of exposure and has been relatively well studied without reported adverse events. Use limited by commonly experienced withdrawal symptoms; may be more sedating than other SSRIs |
| Bupropion | Paucity of systematic study in newborns of nursing mothers; a few case reports in older infants demonstrated low levels of exposure via breast-feeding. May help women who smoke to quit or to maintain abstinence from smoking. Reasonable to use if a woman had good previous response. One case report of possible infant seizure; no other reported adverse events |
| Venlafaxine, desvenlafaxine | Higher levels of desvenlafaxine than venlafaxine found in breast milk. No adverse events reported. Patients may experience withdrawal with discontinuation or missed doses |
| Tricyclic antidepressants | Considered reasonable for breast-feeding mothers if use is clinically warranted; few adverse effects in babies and generally low levels of exposure reported |
| Mirtazapine, nefazodone, MAOIs, duloxetine | Systematic human data not available for breast-feeding patients. May be reasonable if a woman previously has responded best to 1 of these; advise patients that data are not available to guide decisions |
| MAOIs: monoamine oxidase inhibitors; SSRIs: selective serotonin reuptake inhibitors Source: References 29-31 | |
28,29
The psychiatrist’s role
PPD has great public health significance because it affects a large number of women and their families. Screening during obstetrical visits or in other settings may increase identification of women who are suffering from PPD. In order for this screening to lead to meaningful changes, women must receive timely and expert evaluations for PPD and treatment that is efficacious and accessible.
Diagnosis and treatment: 4 pearls
Verify the diagnosis. Many women who present with postpartum depressive symptoms may have previously unrecognized bipolar disorder, and many women presenting with a primary complaint of anxiety have PPD.33,34
Discuss breast-feeding. This topic is important in assessing the risks and benefits of antidepressants in postpartum women, but many women also experience breast-feeding as a topic with emotional valence of its own and may need support with infant feeding.
Meet the patient where she is. Patient preferences strongly influence PPD treatment decisions. Women with similar clinical presentations may have strong preferences for different treatments.
Make treatment accessible. Postpartum women may find it challenging to engage in treatment. Treatment plans need to be feasible for women who are depressed while caring for a newborn. On-site childcare, home visits, Internet communication, and other accommodations that may facilitate treatment should be considered at a systems level.
Related Resources
- American College of Obstetricians and Gynecologists. Screening for depression during and after pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Screening_for_Depression_During_and_After_Pregnancy.
- Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci. 2011;13(1):89-100.
- Dennis CL, Stewart DE. Treatment of postpartum depression, part 1: a critical review of biological interventions. J Clin Psychiatry. 2004;65(9):1242-1251.
- Dennis CL. Treatment of postpartum depression, part 2: a critical review of nonbiological interventions. J Clin Psychiatry. 2004;65(9):1252-1265.
- Cohen LS, Wang B, Nonacs R, et al. Treatment of mood disorders during pregnancy and postpartum. Psychiatr Clin North Am. 2010;33(2):273-293.
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Desvenlafaxine • Pristiq
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
1. Cicchetti D, Rogosch FA, Toth SL. Maternal depressive disorder and contextual risk: contributions to the development of attachment insecurity and behavior problems in toddlerhood. Dev Psychopathol. 1998;10(2):283-300.
2. Murray L, Fiori-Cowley A, Hooper R, et al. The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev. 1996;67(5):2512-2526.
3. Sharp D, Hay DF, Pawlby S, et al. The impact of postnatal depression on boys’ intellectual development. J Child Psychol Psychiatry. 1995;36(8):1315-1336.
4. Altshuler LL, Hendrick V, Cohen LS. Course of mood and anxiety disorders during pregnancy and the postpartum period. J Clin Psychiatry. 1998;59(suppl 2):29-33.
5. Pariser SF. Women and mood disorders. Menarche to menopause. Ann Clin Psychiatry. 1993;5(4):249-254.
6. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
7. Chaudron LH, Klein MH, Remington P, et al. Predictors, prodromes and incidence of postpartum depression. J Psychosom Obstet Gynaecol. 2001;22(2):103-112.
8. Heron J, O’Connor TG, Evans J, et al. ALSPAC Study Team. The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Affect Disord. 2004;80(1):65-73.
9. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
10. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
11. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
12. Flynn HA, O’Mahen HA, Massey L, et al. The impact of a brief obstetrics clinic-based intervention on treatment use for perinatal depression. J Womens Health (Larchmt). 2006;15(10):1195-1204.
13. Yonkers KA, Smith MV, Lin H, et al. Depression screening of perinatal women: an evaluation of the healthy start depression initiative. Psychiatr Serv. 2009;60(3):322-328.
14. van Schaik DJ, Klijn AF, van Hout HP, et al. Patients’ p in the treatment of depressive disorder in primary care. Gen Hosp Psychiatry. 2004;26(3):184-189.
15. Boath E, Bradley E, Henshaw C. Women’s views of antidepressants in the treatment of postnatal depression. J Psychosom Obstet Gynaecol. 2004;25(3-4):221-233.
16. Pearlstein TB, Zlotnick C, Battle CL, et al. Patient choice of treatment for postpartum depression: a pilot study. Arch Womens Ment Health. 2006;9(6):303-308.
17. Zlotnick C, Johnson SL, Miller IW, et al. Postpartum depression in women receiving public assistance: pilot study of an interpersonal-therapy-oriented group intervention. Am J Psychiatry. 2001;158(4):638-640.
18. Klier CM, Muzik M, Rosenblum KL, et al. Interpersonal psychotherapy adapted for the group setting in the treatment of postpartum depression. J Psychother Pract Res. 2001;10(2):124-131.
19. Stuart S, O’Hara MW, Gorman LL. The prevention and psychotherapeutic treatment of postpartum depression. Arch Womens Ment Health. 2003;6(suppl 2):S57-S69.
20. Appleby L, Warner R, Whitton A, et al. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085):932-936.
21. Yonkers KA, Lin H, Howell HB, et al. Pharmacologic treatment of postpartum women with new-onset major depressive disorder: a randomized controlled trial with paroxetine. J Clin Psychiatry. 2008;69(4):659-665.
22. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;(4)26:353-360.
23. Misri S, Reebye P, Corral M, et al. The use of paroxetine and cognitive-behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. J Clin Psychiatry. 2004;65(9):1236-1241.
24. Stowe ZN, Casarella J, Landry J, et al. Sertraline in the treatment of women with postpartum major depression. Depression. 1995;3(1-2):49-55.
25. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
26. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
27. Nonacs RM, Soares CN, Viguera AC, et al. Bupropion SR for the treatment of postpartum depression: a pilot study. Int J Neuropsychopharmacol. 2005;8(3):445-449.
28. Burt VK, Suri R, Altshuler L, et al. The use of psychotropic medications during breast-feeding. Am J Psychiatry. 2001;158(7):1001-1009.
29. Weissman AM, Levy BT, Hartz AJ, et al. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry. 2004;161(6):1066-1078.
30. Newport DJ, Ritchie JC, Knight BT, et al. Venlafaxine in human breast milk and nursing infant plasma: determination of exposure. J Clin Psychiatry. 2009;70(9):1304-1310.
31. Chaudron LH, Schoenecker CJ. Bupropion and breastfeeding: a case of a possible infant seizure. J Clin Psychiatry. 2004;65(6):881-882.
32. Hendrick V, Stowe ZN, Altshuler LL, et al. Fluoxetine and norfluoxetine concentrations in nursing infants and breast milk. Biol Psychiatry. 2001;50(10):775-782.
33. Sharma V, Khan M. Identification of bipolar disorder in women with postpartum depression. Bipolar Disord. 2010;12(3):335-340.
34. Austin MP, Hadzi-Pavlovic D, Priest SR, et al. Depressive and anxiety disorders in the postpartum period: how prevalent are they and can we improve their detection? Arch Womens Ment Health. 2010;13(5):395-401.
Discuss this article at www.facebook.com/CurrentPsychiatry
Postpartum depression (PPD)—emergence of a major depressive episode after childbirth—has broad negative consequences for the mother, baby, and other family members. The time of onset after delivery for a depressive episode to be considered postpartum is debatable, but the DSM-IV-TR specifier states that onset within 4 weeks of childbirth is considered postpartum. PPD can impact many aspects of child development, including mother-infant attachment, cognitive development, and behavior.1-3
An estimated 10% of women who have given birth experience PPD.4,5 The risk of PPD is particularly high among women who have had previous episodes of PPD or major depressive disorder (MDD). Other risk factors include stressful life events, depression and/or anxiety during pregnancy, family history of PPD, and obstetrical complications.6-8 Anxiety disorders are common in postpartum women, and anxiety symptoms often are prominent in PPD.9
Despite the prevalence of PPD and its serious consequences, few studies have addressed antidepressant treatment. In this article we discuss screening and treating PPD and considerations for breast-feeding mothers. Click here for results of an open-label trial of escitalopram for PPD we conducted in which patient recruitment was challenging.
Screening for PPD: A good start
Initiatives by state governments and health care providers have led to programs in which universal screening for PPD has been implemented. Screening provides a mechanism for early detection and intervention. The Edinburgh Postnatal Depression Scale10 is a self-rated, 10-item scale developed for the postpartum setting, and its use increases identification of PPD at postpartum obstetrics visits.11 Other screening tools such as the Patient Health Questionnaire-9 also are commonly used. Despite the success of screening programs in attempting the feasibility of screening, it is unclear if the identification of women who may be experiencing PPD increases their engagement in treatment. Studies have demonstrated that even when depressive symptoms suggesting a PPD episode are identified in the postpartum period, many women still do not receive treatment.12,13 Studies of PPD screening programs have not demonstrated that screening itself improves treatment engagement or improves outcomes.12,13
Psychotherapy: An effective option
Psychotherapy is an important first-line option for PPD, particularly because of considerations of medication exposure during breast-feeding and many women are reluctant to take antidepressants while breast-feeding.16 Interpersonal psychotherapy and cognitive-behavioral therapy (CBT) have been most studied for PPD, and both appear effective for prevention and acute treatment of PPD.17-20 Although psychotherapy alone may be sufficient for some women, for others, medication may be an important first-line treatment, depending on symptom severity, access to psychotherapy, and personal preference.
Evidence for antidepressants
Randomization to placebo is rare in PPD trials. Most trials have used open-label designs because placebo arms pose ethical dilemmas considering the impact of PPD on a mother and her baby. In a randomized study of sertraline or nortriptyline for PPD, both drugs were similarly efficacious.22 In another study comparing paroxetine monotherapy and paroxetine plus CBT for PPD, both groups experienced significant improvement in depression and anxiety symptoms, with no difference between groups at endpoint.23 Open-label trials have suggested antidepressants’ efficacy, although some studies have included small sample sizes (Table 1).20-27
Table 1
Antidepressants for PPD: Summary of the evidence
| Study | Design and size | Medication | Results |
|---|---|---|---|
| Appleby et al, 199720 | 12-week, placebo-controlled, N = 87 | Fluoxetine | Patients taking fluoxetine showed greater improvement than those taking placebo |
| Yonkers et al, 200821 | 8-week, placebo-controlled, N = 70 | Paroxetine | Both groups improved over time, but patients taking paroxetine had greater improvement in overall clinical severity |
| Wisner et al, 200622 | 8-week, RCT, N = 109 | Sertraline vs nortriptyline | Proportion of women who responded or remitted did not differ between those taking sertraline or nortriptyline |
| Misri et al, 200423 | 12-week, RCT, N = 35 | Paroxetine monotherapy vs paroxetine + CBT | Both groups showed significant improvement in mood and anxiety symptoms |
| Stowe et al, 199524 | 8-week, open-label, N = 21 | Sertraline | 20 patients experienced >50% reduction in SIGH-D score |
| Cohen et al, 199725 | Open-label, N = 15 | Venlafaxine | 12 patients achieved remission |
| Suri et al, 200126 | 8-week, open-label, N = 6 | Fluvoxamine | 4 patients became euthymic, with HDRS scores ranging from 2 to 5 |
| Nonacs et al, 200527 | 8-week, open-label, N = 8 | Bupropion | 6 patients had ≥50% decrease in HDRS score from baseline; 3 achieved remission |
| CBT: cognitive-behavioral therapy; HDRS: Hamilton Depression Rating Scale; PPD: postpartum depression; RCT: randomized controlled trial; SIGH-D: Structured Interview Guide for the Hamilton Depression Rating Scale | |||
Breast-feeding considerations
From a nutritional standpoint, breast-feeding is optimal for a newborn. However, for some women, breast-feeding is difficult and stressful, and new mothers may experience this difficulty as failure. Some women prefer not to breast-feed, and others may prefer to formula feed if they require pharmacotherapy, particularly if the medication has not been well studied in breast-feeding patients. Some women may decline to take medications if they are breast-feeding out of concern for the baby’s exposure via breast milk and prefer to try nonpharmacologic approaches first. Many mothers with PPD need to be reassured that stopping breast-feeding may be exactly what is needed if the experience is contributing to their PPD or making them uncomfortable accepting pharmacotherapy when indicated. Maternal mental health is more important than breast-feeding to the health and wellness of the mother-baby dyad.
Table 2
Considerations for antidepressant use during breast-feeding
| Drug(s) | Comments |
|---|---|
| Fluoxetine | Because of long half-life, may be more likely to be detected in infant serum, especially at higher doses. Reasonable for use during breast-feeding if a woman has had a good previous response to the drug or used it during pregnancy |
| Sertraline | Reports of low levels of exposure. Relatively large amount of data available |
| Citalopram, escitalopram | Less systematic study of mother-infant pairs compared with sertraline and paroxetine. Low levels of exposure to infant via breast-feeding observed |
| Paroxetine | Consistent reports of low levels of exposure and has been relatively well studied without reported adverse events. Use limited by commonly experienced withdrawal symptoms; may be more sedating than other SSRIs |
| Bupropion | Paucity of systematic study in newborns of nursing mothers; a few case reports in older infants demonstrated low levels of exposure via breast-feeding. May help women who smoke to quit or to maintain abstinence from smoking. Reasonable to use if a woman had good previous response. One case report of possible infant seizure; no other reported adverse events |
| Venlafaxine, desvenlafaxine | Higher levels of desvenlafaxine than venlafaxine found in breast milk. No adverse events reported. Patients may experience withdrawal with discontinuation or missed doses |
| Tricyclic antidepressants | Considered reasonable for breast-feeding mothers if use is clinically warranted; few adverse effects in babies and generally low levels of exposure reported |
| Mirtazapine, nefazodone, MAOIs, duloxetine | Systematic human data not available for breast-feeding patients. May be reasonable if a woman previously has responded best to 1 of these; advise patients that data are not available to guide decisions |
| MAOIs: monoamine oxidase inhibitors; SSRIs: selective serotonin reuptake inhibitors Source: References 29-31 | |
28,29
The psychiatrist’s role
PPD has great public health significance because it affects a large number of women and their families. Screening during obstetrical visits or in other settings may increase identification of women who are suffering from PPD. In order for this screening to lead to meaningful changes, women must receive timely and expert evaluations for PPD and treatment that is efficacious and accessible.
Diagnosis and treatment: 4 pearls
Verify the diagnosis. Many women who present with postpartum depressive symptoms may have previously unrecognized bipolar disorder, and many women presenting with a primary complaint of anxiety have PPD.33,34
Discuss breast-feeding. This topic is important in assessing the risks and benefits of antidepressants in postpartum women, but many women also experience breast-feeding as a topic with emotional valence of its own and may need support with infant feeding.
Meet the patient where she is. Patient preferences strongly influence PPD treatment decisions. Women with similar clinical presentations may have strong preferences for different treatments.
Make treatment accessible. Postpartum women may find it challenging to engage in treatment. Treatment plans need to be feasible for women who are depressed while caring for a newborn. On-site childcare, home visits, Internet communication, and other accommodations that may facilitate treatment should be considered at a systems level.
Related Resources
- American College of Obstetricians and Gynecologists. Screening for depression during and after pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Screening_for_Depression_During_and_After_Pregnancy.
- Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci. 2011;13(1):89-100.
- Dennis CL, Stewart DE. Treatment of postpartum depression, part 1: a critical review of biological interventions. J Clin Psychiatry. 2004;65(9):1242-1251.
- Dennis CL. Treatment of postpartum depression, part 2: a critical review of nonbiological interventions. J Clin Psychiatry. 2004;65(9):1252-1265.
- Cohen LS, Wang B, Nonacs R, et al. Treatment of mood disorders during pregnancy and postpartum. Psychiatr Clin North Am. 2010;33(2):273-293.
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Desvenlafaxine • Pristiq
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
Discuss this article at www.facebook.com/CurrentPsychiatry
Postpartum depression (PPD)—emergence of a major depressive episode after childbirth—has broad negative consequences for the mother, baby, and other family members. The time of onset after delivery for a depressive episode to be considered postpartum is debatable, but the DSM-IV-TR specifier states that onset within 4 weeks of childbirth is considered postpartum. PPD can impact many aspects of child development, including mother-infant attachment, cognitive development, and behavior.1-3
An estimated 10% of women who have given birth experience PPD.4,5 The risk of PPD is particularly high among women who have had previous episodes of PPD or major depressive disorder (MDD). Other risk factors include stressful life events, depression and/or anxiety during pregnancy, family history of PPD, and obstetrical complications.6-8 Anxiety disorders are common in postpartum women, and anxiety symptoms often are prominent in PPD.9
Despite the prevalence of PPD and its serious consequences, few studies have addressed antidepressant treatment. In this article we discuss screening and treating PPD and considerations for breast-feeding mothers. Click here for results of an open-label trial of escitalopram for PPD we conducted in which patient recruitment was challenging.
Screening for PPD: A good start
Initiatives by state governments and health care providers have led to programs in which universal screening for PPD has been implemented. Screening provides a mechanism for early detection and intervention. The Edinburgh Postnatal Depression Scale10 is a self-rated, 10-item scale developed for the postpartum setting, and its use increases identification of PPD at postpartum obstetrics visits.11 Other screening tools such as the Patient Health Questionnaire-9 also are commonly used. Despite the success of screening programs in attempting the feasibility of screening, it is unclear if the identification of women who may be experiencing PPD increases their engagement in treatment. Studies have demonstrated that even when depressive symptoms suggesting a PPD episode are identified in the postpartum period, many women still do not receive treatment.12,13 Studies of PPD screening programs have not demonstrated that screening itself improves treatment engagement or improves outcomes.12,13
Psychotherapy: An effective option
Psychotherapy is an important first-line option for PPD, particularly because of considerations of medication exposure during breast-feeding and many women are reluctant to take antidepressants while breast-feeding.16 Interpersonal psychotherapy and cognitive-behavioral therapy (CBT) have been most studied for PPD, and both appear effective for prevention and acute treatment of PPD.17-20 Although psychotherapy alone may be sufficient for some women, for others, medication may be an important first-line treatment, depending on symptom severity, access to psychotherapy, and personal preference.
Evidence for antidepressants
Randomization to placebo is rare in PPD trials. Most trials have used open-label designs because placebo arms pose ethical dilemmas considering the impact of PPD on a mother and her baby. In a randomized study of sertraline or nortriptyline for PPD, both drugs were similarly efficacious.22 In another study comparing paroxetine monotherapy and paroxetine plus CBT for PPD, both groups experienced significant improvement in depression and anxiety symptoms, with no difference between groups at endpoint.23 Open-label trials have suggested antidepressants’ efficacy, although some studies have included small sample sizes (Table 1).20-27
Table 1
Antidepressants for PPD: Summary of the evidence
| Study | Design and size | Medication | Results |
|---|---|---|---|
| Appleby et al, 199720 | 12-week, placebo-controlled, N = 87 | Fluoxetine | Patients taking fluoxetine showed greater improvement than those taking placebo |
| Yonkers et al, 200821 | 8-week, placebo-controlled, N = 70 | Paroxetine | Both groups improved over time, but patients taking paroxetine had greater improvement in overall clinical severity |
| Wisner et al, 200622 | 8-week, RCT, N = 109 | Sertraline vs nortriptyline | Proportion of women who responded or remitted did not differ between those taking sertraline or nortriptyline |
| Misri et al, 200423 | 12-week, RCT, N = 35 | Paroxetine monotherapy vs paroxetine + CBT | Both groups showed significant improvement in mood and anxiety symptoms |
| Stowe et al, 199524 | 8-week, open-label, N = 21 | Sertraline | 20 patients experienced >50% reduction in SIGH-D score |
| Cohen et al, 199725 | Open-label, N = 15 | Venlafaxine | 12 patients achieved remission |
| Suri et al, 200126 | 8-week, open-label, N = 6 | Fluvoxamine | 4 patients became euthymic, with HDRS scores ranging from 2 to 5 |
| Nonacs et al, 200527 | 8-week, open-label, N = 8 | Bupropion | 6 patients had ≥50% decrease in HDRS score from baseline; 3 achieved remission |
| CBT: cognitive-behavioral therapy; HDRS: Hamilton Depression Rating Scale; PPD: postpartum depression; RCT: randomized controlled trial; SIGH-D: Structured Interview Guide for the Hamilton Depression Rating Scale | |||
Breast-feeding considerations
From a nutritional standpoint, breast-feeding is optimal for a newborn. However, for some women, breast-feeding is difficult and stressful, and new mothers may experience this difficulty as failure. Some women prefer not to breast-feed, and others may prefer to formula feed if they require pharmacotherapy, particularly if the medication has not been well studied in breast-feeding patients. Some women may decline to take medications if they are breast-feeding out of concern for the baby’s exposure via breast milk and prefer to try nonpharmacologic approaches first. Many mothers with PPD need to be reassured that stopping breast-feeding may be exactly what is needed if the experience is contributing to their PPD or making them uncomfortable accepting pharmacotherapy when indicated. Maternal mental health is more important than breast-feeding to the health and wellness of the mother-baby dyad.
Table 2
Considerations for antidepressant use during breast-feeding
| Drug(s) | Comments |
|---|---|
| Fluoxetine | Because of long half-life, may be more likely to be detected in infant serum, especially at higher doses. Reasonable for use during breast-feeding if a woman has had a good previous response to the drug or used it during pregnancy |
| Sertraline | Reports of low levels of exposure. Relatively large amount of data available |
| Citalopram, escitalopram | Less systematic study of mother-infant pairs compared with sertraline and paroxetine. Low levels of exposure to infant via breast-feeding observed |
| Paroxetine | Consistent reports of low levels of exposure and has been relatively well studied without reported adverse events. Use limited by commonly experienced withdrawal symptoms; may be more sedating than other SSRIs |
| Bupropion | Paucity of systematic study in newborns of nursing mothers; a few case reports in older infants demonstrated low levels of exposure via breast-feeding. May help women who smoke to quit or to maintain abstinence from smoking. Reasonable to use if a woman had good previous response. One case report of possible infant seizure; no other reported adverse events |
| Venlafaxine, desvenlafaxine | Higher levels of desvenlafaxine than venlafaxine found in breast milk. No adverse events reported. Patients may experience withdrawal with discontinuation or missed doses |
| Tricyclic antidepressants | Considered reasonable for breast-feeding mothers if use is clinically warranted; few adverse effects in babies and generally low levels of exposure reported |
| Mirtazapine, nefazodone, MAOIs, duloxetine | Systematic human data not available for breast-feeding patients. May be reasonable if a woman previously has responded best to 1 of these; advise patients that data are not available to guide decisions |
| MAOIs: monoamine oxidase inhibitors; SSRIs: selective serotonin reuptake inhibitors Source: References 29-31 | |
28,29
The psychiatrist’s role
PPD has great public health significance because it affects a large number of women and their families. Screening during obstetrical visits or in other settings may increase identification of women who are suffering from PPD. In order for this screening to lead to meaningful changes, women must receive timely and expert evaluations for PPD and treatment that is efficacious and accessible.
Diagnosis and treatment: 4 pearls
Verify the diagnosis. Many women who present with postpartum depressive symptoms may have previously unrecognized bipolar disorder, and many women presenting with a primary complaint of anxiety have PPD.33,34
Discuss breast-feeding. This topic is important in assessing the risks and benefits of antidepressants in postpartum women, but many women also experience breast-feeding as a topic with emotional valence of its own and may need support with infant feeding.
Meet the patient where she is. Patient preferences strongly influence PPD treatment decisions. Women with similar clinical presentations may have strong preferences for different treatments.
Make treatment accessible. Postpartum women may find it challenging to engage in treatment. Treatment plans need to be feasible for women who are depressed while caring for a newborn. On-site childcare, home visits, Internet communication, and other accommodations that may facilitate treatment should be considered at a systems level.
Related Resources
- American College of Obstetricians and Gynecologists. Screening for depression during and after pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Screening_for_Depression_During_and_After_Pregnancy.
- Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci. 2011;13(1):89-100.
- Dennis CL, Stewart DE. Treatment of postpartum depression, part 1: a critical review of biological interventions. J Clin Psychiatry. 2004;65(9):1242-1251.
- Dennis CL. Treatment of postpartum depression, part 2: a critical review of nonbiological interventions. J Clin Psychiatry. 2004;65(9):1252-1265.
- Cohen LS, Wang B, Nonacs R, et al. Treatment of mood disorders during pregnancy and postpartum. Psychiatr Clin North Am. 2010;33(2):273-293.
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Desvenlafaxine • Pristiq
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
1. Cicchetti D, Rogosch FA, Toth SL. Maternal depressive disorder and contextual risk: contributions to the development of attachment insecurity and behavior problems in toddlerhood. Dev Psychopathol. 1998;10(2):283-300.
2. Murray L, Fiori-Cowley A, Hooper R, et al. The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev. 1996;67(5):2512-2526.
3. Sharp D, Hay DF, Pawlby S, et al. The impact of postnatal depression on boys’ intellectual development. J Child Psychol Psychiatry. 1995;36(8):1315-1336.
4. Altshuler LL, Hendrick V, Cohen LS. Course of mood and anxiety disorders during pregnancy and the postpartum period. J Clin Psychiatry. 1998;59(suppl 2):29-33.
5. Pariser SF. Women and mood disorders. Menarche to menopause. Ann Clin Psychiatry. 1993;5(4):249-254.
6. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
7. Chaudron LH, Klein MH, Remington P, et al. Predictors, prodromes and incidence of postpartum depression. J Psychosom Obstet Gynaecol. 2001;22(2):103-112.
8. Heron J, O’Connor TG, Evans J, et al. ALSPAC Study Team. The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Affect Disord. 2004;80(1):65-73.
9. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
10. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
11. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
12. Flynn HA, O’Mahen HA, Massey L, et al. The impact of a brief obstetrics clinic-based intervention on treatment use for perinatal depression. J Womens Health (Larchmt). 2006;15(10):1195-1204.
13. Yonkers KA, Smith MV, Lin H, et al. Depression screening of perinatal women: an evaluation of the healthy start depression initiative. Psychiatr Serv. 2009;60(3):322-328.
14. van Schaik DJ, Klijn AF, van Hout HP, et al. Patients’ p in the treatment of depressive disorder in primary care. Gen Hosp Psychiatry. 2004;26(3):184-189.
15. Boath E, Bradley E, Henshaw C. Women’s views of antidepressants in the treatment of postnatal depression. J Psychosom Obstet Gynaecol. 2004;25(3-4):221-233.
16. Pearlstein TB, Zlotnick C, Battle CL, et al. Patient choice of treatment for postpartum depression: a pilot study. Arch Womens Ment Health. 2006;9(6):303-308.
17. Zlotnick C, Johnson SL, Miller IW, et al. Postpartum depression in women receiving public assistance: pilot study of an interpersonal-therapy-oriented group intervention. Am J Psychiatry. 2001;158(4):638-640.
18. Klier CM, Muzik M, Rosenblum KL, et al. Interpersonal psychotherapy adapted for the group setting in the treatment of postpartum depression. J Psychother Pract Res. 2001;10(2):124-131.
19. Stuart S, O’Hara MW, Gorman LL. The prevention and psychotherapeutic treatment of postpartum depression. Arch Womens Ment Health. 2003;6(suppl 2):S57-S69.
20. Appleby L, Warner R, Whitton A, et al. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085):932-936.
21. Yonkers KA, Lin H, Howell HB, et al. Pharmacologic treatment of postpartum women with new-onset major depressive disorder: a randomized controlled trial with paroxetine. J Clin Psychiatry. 2008;69(4):659-665.
22. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;(4)26:353-360.
23. Misri S, Reebye P, Corral M, et al. The use of paroxetine and cognitive-behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. J Clin Psychiatry. 2004;65(9):1236-1241.
24. Stowe ZN, Casarella J, Landry J, et al. Sertraline in the treatment of women with postpartum major depression. Depression. 1995;3(1-2):49-55.
25. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
26. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
27. Nonacs RM, Soares CN, Viguera AC, et al. Bupropion SR for the treatment of postpartum depression: a pilot study. Int J Neuropsychopharmacol. 2005;8(3):445-449.
28. Burt VK, Suri R, Altshuler L, et al. The use of psychotropic medications during breast-feeding. Am J Psychiatry. 2001;158(7):1001-1009.
29. Weissman AM, Levy BT, Hartz AJ, et al. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry. 2004;161(6):1066-1078.
30. Newport DJ, Ritchie JC, Knight BT, et al. Venlafaxine in human breast milk and nursing infant plasma: determination of exposure. J Clin Psychiatry. 2009;70(9):1304-1310.
31. Chaudron LH, Schoenecker CJ. Bupropion and breastfeeding: a case of a possible infant seizure. J Clin Psychiatry. 2004;65(6):881-882.
32. Hendrick V, Stowe ZN, Altshuler LL, et al. Fluoxetine and norfluoxetine concentrations in nursing infants and breast milk. Biol Psychiatry. 2001;50(10):775-782.
33. Sharma V, Khan M. Identification of bipolar disorder in women with postpartum depression. Bipolar Disord. 2010;12(3):335-340.
34. Austin MP, Hadzi-Pavlovic D, Priest SR, et al. Depressive and anxiety disorders in the postpartum period: how prevalent are they and can we improve their detection? Arch Womens Ment Health. 2010;13(5):395-401.
1. Cicchetti D, Rogosch FA, Toth SL. Maternal depressive disorder and contextual risk: contributions to the development of attachment insecurity and behavior problems in toddlerhood. Dev Psychopathol. 1998;10(2):283-300.
2. Murray L, Fiori-Cowley A, Hooper R, et al. The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev. 1996;67(5):2512-2526.
3. Sharp D, Hay DF, Pawlby S, et al. The impact of postnatal depression on boys’ intellectual development. J Child Psychol Psychiatry. 1995;36(8):1315-1336.
4. Altshuler LL, Hendrick V, Cohen LS. Course of mood and anxiety disorders during pregnancy and the postpartum period. J Clin Psychiatry. 1998;59(suppl 2):29-33.
5. Pariser SF. Women and mood disorders. Menarche to menopause. Ann Clin Psychiatry. 1993;5(4):249-254.
6. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
7. Chaudron LH, Klein MH, Remington P, et al. Predictors, prodromes and incidence of postpartum depression. J Psychosom Obstet Gynaecol. 2001;22(2):103-112.
8. Heron J, O’Connor TG, Evans J, et al. ALSPAC Study Team. The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Affect Disord. 2004;80(1):65-73.
9. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
10. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
11. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
12. Flynn HA, O’Mahen HA, Massey L, et al. The impact of a brief obstetrics clinic-based intervention on treatment use for perinatal depression. J Womens Health (Larchmt). 2006;15(10):1195-1204.
13. Yonkers KA, Smith MV, Lin H, et al. Depression screening of perinatal women: an evaluation of the healthy start depression initiative. Psychiatr Serv. 2009;60(3):322-328.
14. van Schaik DJ, Klijn AF, van Hout HP, et al. Patients’ p in the treatment of depressive disorder in primary care. Gen Hosp Psychiatry. 2004;26(3):184-189.
15. Boath E, Bradley E, Henshaw C. Women’s views of antidepressants in the treatment of postnatal depression. J Psychosom Obstet Gynaecol. 2004;25(3-4):221-233.
16. Pearlstein TB, Zlotnick C, Battle CL, et al. Patient choice of treatment for postpartum depression: a pilot study. Arch Womens Ment Health. 2006;9(6):303-308.
17. Zlotnick C, Johnson SL, Miller IW, et al. Postpartum depression in women receiving public assistance: pilot study of an interpersonal-therapy-oriented group intervention. Am J Psychiatry. 2001;158(4):638-640.
18. Klier CM, Muzik M, Rosenblum KL, et al. Interpersonal psychotherapy adapted for the group setting in the treatment of postpartum depression. J Psychother Pract Res. 2001;10(2):124-131.
19. Stuart S, O’Hara MW, Gorman LL. The prevention and psychotherapeutic treatment of postpartum depression. Arch Womens Ment Health. 2003;6(suppl 2):S57-S69.
20. Appleby L, Warner R, Whitton A, et al. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085):932-936.
21. Yonkers KA, Lin H, Howell HB, et al. Pharmacologic treatment of postpartum women with new-onset major depressive disorder: a randomized controlled trial with paroxetine. J Clin Psychiatry. 2008;69(4):659-665.
22. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;(4)26:353-360.
23. Misri S, Reebye P, Corral M, et al. The use of paroxetine and cognitive-behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. J Clin Psychiatry. 2004;65(9):1236-1241.
24. Stowe ZN, Casarella J, Landry J, et al. Sertraline in the treatment of women with postpartum major depression. Depression. 1995;3(1-2):49-55.
25. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
26. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
27. Nonacs RM, Soares CN, Viguera AC, et al. Bupropion SR for the treatment of postpartum depression: a pilot study. Int J Neuropsychopharmacol. 2005;8(3):445-449.
28. Burt VK, Suri R, Altshuler L, et al. The use of psychotropic medications during breast-feeding. Am J Psychiatry. 2001;158(7):1001-1009.
29. Weissman AM, Levy BT, Hartz AJ, et al. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry. 2004;161(6):1066-1078.
30. Newport DJ, Ritchie JC, Knight BT, et al. Venlafaxine in human breast milk and nursing infant plasma: determination of exposure. J Clin Psychiatry. 2009;70(9):1304-1310.
31. Chaudron LH, Schoenecker CJ. Bupropion and breastfeeding: a case of a possible infant seizure. J Clin Psychiatry. 2004;65(6):881-882.
32. Hendrick V, Stowe ZN, Altshuler LL, et al. Fluoxetine and norfluoxetine concentrations in nursing infants and breast milk. Biol Psychiatry. 2001;50(10):775-782.
33. Sharma V, Khan M. Identification of bipolar disorder in women with postpartum depression. Bipolar Disord. 2010;12(3):335-340.
34. Austin MP, Hadzi-Pavlovic D, Priest SR, et al. Depressive and anxiety disorders in the postpartum period: how prevalent are they and can we improve their detection? Arch Womens Ment Health. 2010;13(5):395-401.
Panic disorder: Break the fear circuit
Ms. K, a 24-year-old waitress who lives with her boyfriend, was referred by her primary care physician for evaluation of panic attacks that began “out of nowhere” at work approximately 6 months ago. The unpredictable attacks occur multiple times per week, causing her to leave work and cancel shifts.
Ms. K reports that before the panic attacks began, she felt happy in her relationship, enjoyed hobbies, and was hopeful about the future. However, she has become concerned that a potentially catastrophic illness is causing her panic attacks. She researches her symptoms on the Internet, and is preoccupied with the possibility of sudden death due to an undiagnosed heart condition. Multiple visits to the emergency room have not identified any physical abnormalities. Her primary care doctor prescribed alprazolam, 0.5 mg as needed for panic attacks, which she reports is helpful, “but only in the moment of the attacks.” Ms. K avoids alcohol and illicit substances and limits her caffeine intake. She is not willing to accept that her life “feels so limited.” Her dream of earning a nursing degree and eventually starting a family now seems unattainable.
Panic disorder (PD) occurs in 3% to 5% of adults, with women affected at roughly twice the rate of men.1 Causing a broad range of distress and varying degrees of impairment, PD commonly occurs with other psychiatric disorders. For most patients, treatment is effective, but those who do not respond to initial approaches require a thoughtful, stepped approach to care. Key considerations include establishing an accurate diagnosis, clarifying comorbid illnesses, ascertaining patient beliefs and expectations, and providing appropriately dosed and maintained treatments.
Panic attacks vs PD
Panic attacks consist of rapid onset of intense anxiety, with prominent somatic symptoms, that peaks within 10 minutes (Figure).2 Attacks in which <4 of the listed symptoms occur are considered limited-symptom panic attacks.
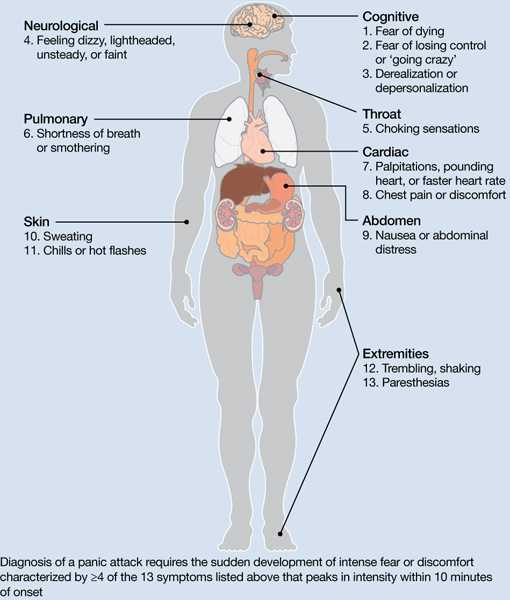
Figure: Body locations of panic attack symptoms
Diagnosis of a panic attack requires the sudden development of intense fear or discomfort characterized by ≥4 of the 13 symptoms listed above that peaks in intensity within 10 minutes of onset
Source: Reference 2
Panic attacks can occur with various disorders, including other anxiety disorders, mood disorders, and substance intoxication or withdrawal. Because serious medical conditions can present with panic-like symptoms, the initial occurrence of such symptoms warrants consideration of physiological causes. For a Box2 that describes the differential diagnosis of panic attacks, see this article at CurrentPsychiatry.com.
To meet diagnostic criteria for panic disorder, panic attacks must initially occur “out of the blue,” meaning no specific object or situation induced the attack. The differential diagnosis of panic attacks includes assessing for other psychiatric disorders that may involve panic attacks. Evaluation requires considering the context in which the panic attacks occur, including their start date, pattern of attacks, instigating situations, and associated thoughts.
Social phobia. Attacks occur only during or immediately before a social interaction in which the patient fears embarrassing himself or herself.
Obsessive-compulsive disorder (OCD). Attacks occur when the patient cannot avoid exposure to an obsessional fear or is prevented from performing a ritual that diffuses obsessional anxiety.
Posttraumatic stress disorder (PTSD). Attacks occur when confronted by a trauma-related memory or trigger.
Specific phobia. Attacks occur only when the patient encounters a specifically feared object, place, or situation, unrelated to social phobia, OCD, or PTSD.
Medical conditions. Conditions to consider include—but are not limited to—hyperthyroidism, pulmonary embolism, myocardial infarction, cardiac dysrhythmias, hypoglycemia, asthma, partial complex seizures, and pheochromocytoma.
Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000
A PD diagnosis requires that repeated panic attacks initially must occur from “out of the blue,” meaning no specific object or situation induced the attack. In addition, the diagnosis requires 1 of 3 types of psychological or behavioral changes as a result of the attacks (Table 1).2 Agoraphobia is diagnosed if 1 of the behavioral changes is avoidance of places or situations from which escape might be embarrassing or difficult should an attack occur. A patient can be diagnosed as having PD with agoraphobia, PD without agoraphobia, or agoraphobia without PD (ie, experiences only limited symptom panic attacks, but avoids situations or stimuli associated with them).
Table 1
Definitions of panic disorder and agoraphobia
| Panic disorder |
|---|
|
| Agoraphobia |
| Anxiety about, or avoidance of, being in places or situations from which escape might be difficult or embarrassing, or in which help may not be available in the event of having an unexpected or situationally predisposed panic attack or panic-like symptoms. Agoraphobic fears typically involve characteristic clusters of situations that include being outside the home alone, being in a crowd, standing in a line, being on a bridge, or traveling in a bus, train, or automobile |
| Source: Reference 2 |
Comorbidities are common in patients with PD and predict greater difficulty achieving remission (Box).1,3-6
The most common psychiatric conditions that co-occur with panic disorder (PD) are other anxiety disorders, mood disorders, personality disorders, and substance use disorders.1 Carefully assess the severity and degree of impairment or distress arising from each condition to prioritize treatment goals. For example, treating panic attacks would be a lower priority in a patient with untreated bipolar disorder.
Assessing comorbid substance abuse is important in selecting PD treatments. Benzodiazepines should almost always be avoided in patients with a history of drug abuse—illicit or prescribed. Although complete abstinence should not be a prerequisite for beginning PD treatment, detoxification and concomitant substance abuse treatment are essential.3
Comorbid mood disorders also affect the course of PD treatment. Antidepressants are effective for treating depression and PD, whereas benzodiazepines are not effective for depression.4 Antidepressants in patients with bipolar disorder are controversial because these medications might induce mixed or elevated mood states or rapid cycling. In these complicated patients, consider antidepressants lower in the treatment algorithm.5
Other conditions to consider before beginning treatment include pregnancy or the possibility of becoming pregnant in the near future and suicidal ideation. PD is associated with increased risk for suicidal ideation and progression to suicide attempts, particularly in patients with a comorbid mood or psychotic disorder.6 In addition, consider the potential impact of medications on comorbid medical conditions.
Treatment begins with education
The goal of treatment is remission of symptoms, ideally including an absence of panic attacks, agoraphobic avoidance, and anticipatory anxiety.1 The Panic Disorder Severity Scale self-report is a validated measure of panic symptoms that may be useful in clinical practice.7
The first step in treatment is educating patients about panic attacks, framing them as an overreactive fear circuit in the brain that produces physical symptoms that are not dangerous. Using a brain model that shows the location of the amygdala, hippocampus, and prefrontal cortex—which play crucial roles in generating and controlling anxiety and fear—can make this discussion more concrete.8 Although highly simplified, such models allow clinicians to demonstrate that excessive reactivity of limbic regions can be reduced by both top-down (cortico-limbic connections via cognitive-behavioral therapy [CBT]) and bottom-up (pharmacotherapy directly acting on limbic structures) approaches. Such discussions lead to treatment recommendations for CBT, pharmacotherapy, or their combination.
No single treatment has emerged as the definitive “best” for PD, and no reliable predictors can guide specific treatment for an individual.3 Combining CBT with pharmacotherapy produces higher short-term response rates than either treatment alone, but in the long term, combination treatment does not appear to be superior to CBT alone.9 Base the initial treatment selection for PD on patient preference, treatment availability and cost, and comorbid medical and psychiatric conditions. For an Algorithm to guide treatment decisions, see this article at CurrentPsychiatry.com.

Algorithm: Treatment for panic disorder: A suggested algorithm
aPoor response to an SSRI should lead to a switch to venlafaxine extended-release, and vice versa
bBenzodiazepines are relatively contraindicated in geriatric patients and patients with a history of substance abuse or dependence
CBT: cognitive-behavioral therapy; MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; Ven XR: venlafaxine extended-release
First-line treatments
Psychotherapy. CBT is the most efficacious psychotherapy for PD. Twelve to 15 sessions of CBT has demonstrated efficacy for PD, with additional effects on comorbid anxiety and depressive symptoms.10 No large clinical trials of CBT have used cognitive restructuring alone; all have included at least some component of exposure that requires the patient to confront feared physical sensations. Gains during treatment may be steady and gradual or sudden and uneven, with rapid improvement in some but not all symptoms. CBT and pharmacotherapy have demonstrated similar levels of benefit in short-term trials, but CBT has proven superior in most9 but not all11 trials evaluating long-term outcomes, particularly compared with pharmacotherapy that is discontinued during follow-up. Although less studied, group CBT also may be considered if a patient cannot afford individual CBT.
Pharmacotherapy. Evidence supports selective serotonin reuptake inhibitors (SSRIs), venlafaxine extended-release (XR), benzodiazepines, and tricyclic antidepressants (TCAs) as effective treatments for PD.3 No class of medication has demonstrated superiority over others in short-term treatment.3,12 Because of the medical risks associated with benzodiazepines and TCAs, an SSRI or venlafaxine XR should be the first medication option for most patients. Fluoxetine, paroxetine, sertraline, and venlafaxine XR are FDA-approved for PD. Paroxetine is associated with weight gain and may increase the risk for panic recurrence upon discontinuation more than sertraline, making it a less favorable option for many patients.13 Start doses at half the normal starting dose used for treating major depressive disorder and continue for 4 to 7 days, then increase to the minimal effective dose. For a Table3 that lists dosing recommendations for antidepressants to treat PD, see this article at CurrentPsychiatry.com. If there is no improvement by 4 weeks, increase the dose every 2 to 4 weeks until remission is achieved or side effects prevent further dose increases.
Table
Recommended doses for antidepressants used to treat panic disorder
| Medication | Starting dose (mg/d) | Therapeutic range (mg/d) |
|---|---|---|
| SSRIs | ||
| Citalopram | 10 | 20 to 40 |
| Escitalopram | 5 | 10 to 40 |
| Fluoxetine | 5 to 10 | 20 to 80 |
| Fluvoxamine | 25 | 100 to 300 |
| Paroxetine | 10 | 20 to 80 |
| Paroxetine CR | 12.5 | 25 to 50 |
| Sertraline | 25 | 100 to 200 |
| SNRIs | ||
| Duloxetine | 20 to 30 | 60 to 120 |
| Venlafaxine XR | 37.5 | 150 to 225 |
| TCAs | ||
| Clomipramine | 10 to 25 | 100 to 300 |
| Imipramine | 10 | 100 to 300 |
| MAOI | ||
| Phenelzine | 15 | 45 to 90 |
| CR: controlled release; MAOI: monoamine oxidase inhibitor; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants; XR: extended release Source: American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington, DC: American Psychiatric Association; 2009 | ||
Treatment nonresponse. True non-response needs to be distinguished from poor response caused by inadequate treatment delivery, eg, patients not completing homework assignments in CBT or not adhering to pharmacotherapy. Asking patients about adverse effects or personal and family beliefs about treatment may reveal reasons for nonadherence.
Second-line treatments
Little data are available to guide next-step treatment options in patients who don’t achieve remission from their initial treatment. Patients who benefit from an SSRI, venlafaxine XR, or CBT but still have symptoms should be started on combination treatment. For a patient who experiences complete non-response to the initial treatment, discontinue the first treatment and switch to the other modality. In general, completely ineffective treatments should be discontinued when another treatment is added, but when partial improvement (>30%) occurs, continue the original treatment and augment it with another approach.
For patients pursuing pharmacotherapy, poor response to an adequate SSRI trial usually should lead to a switch to venlafaxine XR, and vice versa. Failure to respond to both of these medication classes should prompt a switch to a benzodiazepine or TCA.
Benzodiazepines are a fast-acting, effective treatment for PD, with efficacy similar to SSRIs in acute and long-term treatment.14 Benzodiazepines may be prescribed with antidepressants at the beginning of treatment to improve response speed.15 Clonazepam and alprazolam are FDA-approved for treating PD. A high-potency, long-acting agent, clonazepam is the preferred initial benzodiazepine, dosed 0.5 to 4 mg/d on a fixed schedule. Although substantial data support using alprazolam for PD, it requires more frequent dosing and has a greater risk of rebound anxiety and abuse potential because of its more rapid onset of action. Compared with immediate-release alprazolam, alprazolam XR has a slower absorption rate and longer steady state in the blood, but this formulation does not have lower abuse potential or greater efficacy. Although not FDA-approved for PD, diazepam and lorazepam also have proven efficacy for PD.3
Benzodiazepines should be considered contraindicated in patients with a history of substance abuse, except in select cases.4 Benzodiazepines generally should be avoided in older patients because of increased risk for falls, cognitive impairment, and motor vehicle accidents. Table 2 lists situations in which benzodiazepines may be used to treat PD.
Table 2
Clinical scenarios in which to consider using benzodiazepines
| Coadministration for 2 to 4 weeks when initiating treatment with an SSRI or venlafaxine XR to achieve more rapid relief and mitigate potential antidepressant-induced anxiety |
| For patients who wish to avoid antidepressants because of concern about sexual dysfunction |
| For patients who need chronic aspirin or an NSAID, which may increase the risk for upper gastrointestinal bleeding when taken in combination with an SSRI |
| For patients with comorbid bipolar disorder or epilepsy |
| Next-step monotherapy or augmentation in patients who respond poorly to an SSRI, venlafaxine XR, TCA, or CBT |
| CBT: cognitive-behavioral therapy; NSAID: nonsteroidal anti-inflammatory drug; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; XR: extended release |
TCAs are effective as monotherapy for PD. Most support comes from studies of imipramine or clomipramine.12 Similar to SSRIs and venlafaxine XR, use a low initial dose and gradually increase until the patient remits or side effects prevent further increases. SSRI and TCA combinations rarely are used unless the TCA is a relatively specific norepinephrine reuptake inhibitor (eg, desipramine, nortriptyline). Because TCAs are metabolized via the cytochrome P450 2D6 system and some SSRIs—particularly fluoxetine and paroxetine—strongly inhibit 2D6, combinations of TCAs with these agents may lead to dangerously high plasma TCA levels, placing patients at risk for cardiac dysrhythmias and other side effects.16
Monoamine oxidase inhibitors (MAOIs)—particularly phenelzine—are underused for PD. They have the strongest efficacy data for any class of medications outside the first- and second-line agents and have a unique mechanism of action. In patients who can comply with the dietary and medication limitations, an MAOI generally should be the next step after nonresponse to other treatments.3
Alternative treatments
For patients who do not respond to any of the treatments described above, data from uncontrolled studies support mirtazapine, levetiracetam, and the serotonin-norepinephrine reuptake inhibitors duloxetine and milnacipran as monotherapy for PD.17 Pindolol—a beta blocker and 5-HT1A receptor antagonist—proved superior to placebo as an adjunctive agent to SSRIs in treatment-resistant PD in 1 of 2 trials.17 Minimal evidence supports the atypical antipsychotics risperidone and olanzapine in treatment-resistant PD, although a placebo-controlled trial of quetiapine SR coadministered with SSRIs recently was completed (NCT00619892; results pending). Atypical antipsychotics are best reserved for patients with a primary psychotic disorder or bipolar disorder who experience panic attacks.5
Panic-focused psychodynamic psychotherapy, a 12-week (approximately 24 sessions) form of psychotherapy, has demonstrated superiority vs applied relaxation therapy.18 This treatment could be considered for patients who do not respond to standard first-line treatments, but few community therapists are familiar with this method.
For many patients with PD, complementary and alternative medicine (CAM) approaches are appealing. See this article at CurrentPsychiatry.com for a Box that discusses CAM for PD.
Although no complementary and alternative medicine treatments have strong evidence of efficacy as monotherapy for panic disorder (PD), several have data that suggest benefit with little evidence of risk. These include bibliotherapy, yoga, aerobic exercise, and the dietary supplements kava and inositol.a Exercise as a treatment poses a challenge because it can induce symptoms that the patient fears, such as tachycardia and shortness of breath. In addition to any direct physiologic benefit from aerobic exercise, there is also an exposure component that can be harnessed by gradually increasing the exertion level.
Another approach undergoing extensive evaluation is Internet-provided cognitive-behavioral therapy (CBT). Using guided CBT modules with or without therapist support, Internet-provided CBT provides an option for motivated patients unable to complete in-person CBT because of logistical factors.b A helpful resource that reviews Internet self-help and psychotherapy guided programs for PD and other psychiatric conditions is http://beacon.anu.edu.au.
References
a. Antonacci DJ, Davis E, Bloch RM, et al. CAM for your anxious patient: what the evidence says. Current Psychiatry. 2010;9(10):42-52.
b. Johnston L, Titov N, Andrews G, et al. A RCT of a transdiagnostic internet-delivered treatment for three anxiety disorders: examination of support roles and disorder-specific outcomes. PLoS One. 2011;6(11):e28079.
Maintenance treatment
Patients who complete a course of CBT for PD often follow up with several “booster sessions” at monthly or longer intervals that focus on relapse prevention techniques. Few controlled trials have evaluated pharmacotherapy discontinuation in PD. Most guidelines recommend continuing treatment for ≥1 year after achieving remission to minimize the risk of relapse.3 Researchers are focusing on whether medication dosage can be reduced during maintenance without loss of efficacy.
Treatment discontinuation
In the absence of urgent medical need, taper medications for PD gradually over several months. PD patients are highly sensitive to unusual physical sensations, which can occur while discontinuing antidepressants or benzodiazepines. If a benzodiazepine is used in conjunction with an antidepressant, the benzodiazepine should be discontinued first, so that the antidepressant can help ease benzodiazepine-associated discontinuation symptoms. A brief course of CBT during pharmacotherapy discontinuation may increase the likelihood of successful tapering.19
CASE CONTINUED: A successful switch
Ms. K has to discontinue sequential trials of fluoxetine, 40 mg/d, and venlafaxine XR, 225 mg/d because of side effects, and she does not reduce the frequency of her alprazolam use. She agrees to switch from alprazolam to clonazepam, 0.5 mg every morning and 1 mg at bedtime, and to start CBT. Clonazepam reduces her anxiety sufficiently so she can address her symptoms in therapy. Through CBT she becomes motivated to monitor her thoughts and treat them as guesses rather than facts, reviewing the evidence for her thoughts and generating rational responses. She participates in exposure exercises, which she practices between sessions, and grows to tolerate uncomfortable sensations until they no longer signal danger. After 12 CBT sessions, she is panic-free. Despite some trepidation, she agrees to a slow taper off clonazepam, reducing the dose by 0.25 mg every 2 weeks. She continues booster sessions with her therapist to manage any re-emerging anxiety. After an additional 12 weeks, she successfully discontinues clonazepam and remains panic-free.
Related Resources
- American Psychiatric Association. Panic disorder. http://healthyminds.org/Main-Topic/Panic-Disorder.aspx.
- Anxiety and Depression Association of America. Panic disorder & agoraphobia. http://adaa.org/understanding-anxiety/panic-disorder-agoraphobia.
- Mayo Clinic. Panic attacks and panic disorder. www.mayoclinic.com/health/panic-attacks/DS00338.
- National Health Service Self-Help Guides. www.ntw.nhs.uk/pic/selfhelp.
- National Institute of Mental Health. Panic disorder. www.nimh.nih.gov/health/topics/panic-disorder/index.shtml.
Drug Brand Names
- Alprazolam • Xanax
- Alprazolam XR • Xanax XR
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Levetiracetam • Keppra
- Lorazepam • Ativan
- Milnacipran • Savella
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Paroxetine CR • Paxil CR
- Phenelzine • Nardil
- Pindolol • Visken
- Quetiapine SR • Seroquel SR
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine XR • Effexor XR
Disclosures
Dr. Dunlop receives research support from Bristol-Myers Squibb, GlaxoSmithKline, and the National Institute of Mental Health. He serves as a consultant to MedAvante and Roche.
Ms. Schneider and Dr. Gerardi report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Roy-Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet. 2006;368(9540):1023-1032.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
3. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington DC: American Psychiatric Association; 2009.
4. Dunlop BW, Davis PG. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: a review. Prim Care Companion J Clin Psychiatry. 2008;10(3):222-228.
5. Rakofsky JJ, Dunlop BW. Treating nonspecific anxiety and anxiety disorders in patients with bipolar disorder: a review. J Clin Psychiatry. 2011;72(1):81-90.
6. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
7. Houck PR, Spiegel DA, Shear MK, et al. Reliability of the self-report version of the panic disorder severity scale. Depress Anxiety. 2002;15(4):183-185.
8. Ninan PT, Dunlop BW. Neurobiology and etiology of panic disorder. J Clin Psychiatry. 2005;66(suppl 4):3-7.
9. Furukawa TA, Watanabe N, Churchill R. Psychotherapy plus antidepressant for panic disorder with or without agoraphobia: systematic review. Br J Psychiatry. 2006;188:305-312.
10. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
11. van Apeldoorn FJ, Timmerman ME, Mersch PP, et al. A randomized trial of cognitive-behavioral therapy or selective serotonin reuptake inhibitor or both combined for panic disorder with or without agoraphobia: treatment results through 1-year follow-up. J Clin Psychiatry. 2010;71(5):574-586.
12. Bakker A, van Balkom AJ, Spinhoven P. SSRIs vs. TCAs in the treatment of panic disorder: a meta-analysis. Acta Psychiatr Scand. 2002;106(3):163-167.
13. Bandelow B, Behnke K, Lenoir S, et al. Sertraline versus paroxetine in the treatment of panic disorder: an acute, double-blind noninferiority comparison. J Clin Psychiatry. 2004;65(3):405-413.
14. Nardi AE, Freire RC, Mochcovitch MD, et al. A randomized, naturalistic, parallel-group study for the long-term treatment of panic disorder with clonazepam or paroxetine. J Clin Psychopharmacol. 2012;32(1):120-126.
15. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
16. Preskorn SH, Shah R, Neff M, et al. The potential for clinically significant drug-drug interactions involving the CYP 2D6 system: effects with fluoxetine and paroxetine versus sertraline. J Psychiatr Pract. 2007;13(1):5-12.
17. Perna G, Guerriero G, Caldirola D. Emerging drugs for panic disorder. Expert Opin Emerg Drugs. 2011;16(4):631-645.
18. Milrod B, Leon AC, Busch F, et al. A randomized controlled clinical trial of psychoanalytic psychotherapy for panic disorder. Am J Psychiatry. 2007;164(2):265-272.
19. Otto MW, Pollack MH, Sachs GS, et al. Discontinuation of benzodiazepine treatment: efficacy of cognitive-behavioral therapy for patients with panic disorder. Am J Psychiatry. 1993;150(10):1485-1490.
Ms. K, a 24-year-old waitress who lives with her boyfriend, was referred by her primary care physician for evaluation of panic attacks that began “out of nowhere” at work approximately 6 months ago. The unpredictable attacks occur multiple times per week, causing her to leave work and cancel shifts.
Ms. K reports that before the panic attacks began, she felt happy in her relationship, enjoyed hobbies, and was hopeful about the future. However, she has become concerned that a potentially catastrophic illness is causing her panic attacks. She researches her symptoms on the Internet, and is preoccupied with the possibility of sudden death due to an undiagnosed heart condition. Multiple visits to the emergency room have not identified any physical abnormalities. Her primary care doctor prescribed alprazolam, 0.5 mg as needed for panic attacks, which she reports is helpful, “but only in the moment of the attacks.” Ms. K avoids alcohol and illicit substances and limits her caffeine intake. She is not willing to accept that her life “feels so limited.” Her dream of earning a nursing degree and eventually starting a family now seems unattainable.
Panic disorder (PD) occurs in 3% to 5% of adults, with women affected at roughly twice the rate of men.1 Causing a broad range of distress and varying degrees of impairment, PD commonly occurs with other psychiatric disorders. For most patients, treatment is effective, but those who do not respond to initial approaches require a thoughtful, stepped approach to care. Key considerations include establishing an accurate diagnosis, clarifying comorbid illnesses, ascertaining patient beliefs and expectations, and providing appropriately dosed and maintained treatments.
Panic attacks vs PD
Panic attacks consist of rapid onset of intense anxiety, with prominent somatic symptoms, that peaks within 10 minutes (Figure).2 Attacks in which <4 of the listed symptoms occur are considered limited-symptom panic attacks.

Figure: Body locations of panic attack symptoms
Diagnosis of a panic attack requires the sudden development of intense fear or discomfort characterized by ≥4 of the 13 symptoms listed above that peaks in intensity within 10 minutes of onset
Source: Reference 2
Panic attacks can occur with various disorders, including other anxiety disorders, mood disorders, and substance intoxication or withdrawal. Because serious medical conditions can present with panic-like symptoms, the initial occurrence of such symptoms warrants consideration of physiological causes. For a Box2 that describes the differential diagnosis of panic attacks, see this article at CurrentPsychiatry.com.
To meet diagnostic criteria for panic disorder, panic attacks must initially occur “out of the blue,” meaning no specific object or situation induced the attack. The differential diagnosis of panic attacks includes assessing for other psychiatric disorders that may involve panic attacks. Evaluation requires considering the context in which the panic attacks occur, including their start date, pattern of attacks, instigating situations, and associated thoughts.
Social phobia. Attacks occur only during or immediately before a social interaction in which the patient fears embarrassing himself or herself.
Obsessive-compulsive disorder (OCD). Attacks occur when the patient cannot avoid exposure to an obsessional fear or is prevented from performing a ritual that diffuses obsessional anxiety.
Posttraumatic stress disorder (PTSD). Attacks occur when confronted by a trauma-related memory or trigger.
Specific phobia. Attacks occur only when the patient encounters a specifically feared object, place, or situation, unrelated to social phobia, OCD, or PTSD.
Medical conditions. Conditions to consider include—but are not limited to—hyperthyroidism, pulmonary embolism, myocardial infarction, cardiac dysrhythmias, hypoglycemia, asthma, partial complex seizures, and pheochromocytoma.
Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000
A PD diagnosis requires that repeated panic attacks initially must occur from “out of the blue,” meaning no specific object or situation induced the attack. In addition, the diagnosis requires 1 of 3 types of psychological or behavioral changes as a result of the attacks (Table 1).2 Agoraphobia is diagnosed if 1 of the behavioral changes is avoidance of places or situations from which escape might be embarrassing or difficult should an attack occur. A patient can be diagnosed as having PD with agoraphobia, PD without agoraphobia, or agoraphobia without PD (ie, experiences only limited symptom panic attacks, but avoids situations or stimuli associated with them).
Table 1
Definitions of panic disorder and agoraphobia
| Panic disorder |
|---|
|
| Agoraphobia |
| Anxiety about, or avoidance of, being in places or situations from which escape might be difficult or embarrassing, or in which help may not be available in the event of having an unexpected or situationally predisposed panic attack or panic-like symptoms. Agoraphobic fears typically involve characteristic clusters of situations that include being outside the home alone, being in a crowd, standing in a line, being on a bridge, or traveling in a bus, train, or automobile |
| Source: Reference 2 |
Comorbidities are common in patients with PD and predict greater difficulty achieving remission (Box).1,3-6
The most common psychiatric conditions that co-occur with panic disorder (PD) are other anxiety disorders, mood disorders, personality disorders, and substance use disorders.1 Carefully assess the severity and degree of impairment or distress arising from each condition to prioritize treatment goals. For example, treating panic attacks would be a lower priority in a patient with untreated bipolar disorder.
Assessing comorbid substance abuse is important in selecting PD treatments. Benzodiazepines should almost always be avoided in patients with a history of drug abuse—illicit or prescribed. Although complete abstinence should not be a prerequisite for beginning PD treatment, detoxification and concomitant substance abuse treatment are essential.3
Comorbid mood disorders also affect the course of PD treatment. Antidepressants are effective for treating depression and PD, whereas benzodiazepines are not effective for depression.4 Antidepressants in patients with bipolar disorder are controversial because these medications might induce mixed or elevated mood states or rapid cycling. In these complicated patients, consider antidepressants lower in the treatment algorithm.5
Other conditions to consider before beginning treatment include pregnancy or the possibility of becoming pregnant in the near future and suicidal ideation. PD is associated with increased risk for suicidal ideation and progression to suicide attempts, particularly in patients with a comorbid mood or psychotic disorder.6 In addition, consider the potential impact of medications on comorbid medical conditions.
Treatment begins with education
The goal of treatment is remission of symptoms, ideally including an absence of panic attacks, agoraphobic avoidance, and anticipatory anxiety.1 The Panic Disorder Severity Scale self-report is a validated measure of panic symptoms that may be useful in clinical practice.7
The first step in treatment is educating patients about panic attacks, framing them as an overreactive fear circuit in the brain that produces physical symptoms that are not dangerous. Using a brain model that shows the location of the amygdala, hippocampus, and prefrontal cortex—which play crucial roles in generating and controlling anxiety and fear—can make this discussion more concrete.8 Although highly simplified, such models allow clinicians to demonstrate that excessive reactivity of limbic regions can be reduced by both top-down (cortico-limbic connections via cognitive-behavioral therapy [CBT]) and bottom-up (pharmacotherapy directly acting on limbic structures) approaches. Such discussions lead to treatment recommendations for CBT, pharmacotherapy, or their combination.
No single treatment has emerged as the definitive “best” for PD, and no reliable predictors can guide specific treatment for an individual.3 Combining CBT with pharmacotherapy produces higher short-term response rates than either treatment alone, but in the long term, combination treatment does not appear to be superior to CBT alone.9 Base the initial treatment selection for PD on patient preference, treatment availability and cost, and comorbid medical and psychiatric conditions. For an Algorithm to guide treatment decisions, see this article at CurrentPsychiatry.com.

Algorithm: Treatment for panic disorder: A suggested algorithm
aPoor response to an SSRI should lead to a switch to venlafaxine extended-release, and vice versa
bBenzodiazepines are relatively contraindicated in geriatric patients and patients with a history of substance abuse or dependence
CBT: cognitive-behavioral therapy; MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; Ven XR: venlafaxine extended-release
First-line treatments
Psychotherapy. CBT is the most efficacious psychotherapy for PD. Twelve to 15 sessions of CBT has demonstrated efficacy for PD, with additional effects on comorbid anxiety and depressive symptoms.10 No large clinical trials of CBT have used cognitive restructuring alone; all have included at least some component of exposure that requires the patient to confront feared physical sensations. Gains during treatment may be steady and gradual or sudden and uneven, with rapid improvement in some but not all symptoms. CBT and pharmacotherapy have demonstrated similar levels of benefit in short-term trials, but CBT has proven superior in most9 but not all11 trials evaluating long-term outcomes, particularly compared with pharmacotherapy that is discontinued during follow-up. Although less studied, group CBT also may be considered if a patient cannot afford individual CBT.
Pharmacotherapy. Evidence supports selective serotonin reuptake inhibitors (SSRIs), venlafaxine extended-release (XR), benzodiazepines, and tricyclic antidepressants (TCAs) as effective treatments for PD.3 No class of medication has demonstrated superiority over others in short-term treatment.3,12 Because of the medical risks associated with benzodiazepines and TCAs, an SSRI or venlafaxine XR should be the first medication option for most patients. Fluoxetine, paroxetine, sertraline, and venlafaxine XR are FDA-approved for PD. Paroxetine is associated with weight gain and may increase the risk for panic recurrence upon discontinuation more than sertraline, making it a less favorable option for many patients.13 Start doses at half the normal starting dose used for treating major depressive disorder and continue for 4 to 7 days, then increase to the minimal effective dose. For a Table3 that lists dosing recommendations for antidepressants to treat PD, see this article at CurrentPsychiatry.com. If there is no improvement by 4 weeks, increase the dose every 2 to 4 weeks until remission is achieved or side effects prevent further dose increases.
Table
Recommended doses for antidepressants used to treat panic disorder
| Medication | Starting dose (mg/d) | Therapeutic range (mg/d) |
|---|---|---|
| SSRIs | ||
| Citalopram | 10 | 20 to 40 |
| Escitalopram | 5 | 10 to 40 |
| Fluoxetine | 5 to 10 | 20 to 80 |
| Fluvoxamine | 25 | 100 to 300 |
| Paroxetine | 10 | 20 to 80 |
| Paroxetine CR | 12.5 | 25 to 50 |
| Sertraline | 25 | 100 to 200 |
| SNRIs | ||
| Duloxetine | 20 to 30 | 60 to 120 |
| Venlafaxine XR | 37.5 | 150 to 225 |
| TCAs | ||
| Clomipramine | 10 to 25 | 100 to 300 |
| Imipramine | 10 | 100 to 300 |
| MAOI | ||
| Phenelzine | 15 | 45 to 90 |
| CR: controlled release; MAOI: monoamine oxidase inhibitor; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants; XR: extended release Source: American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington, DC: American Psychiatric Association; 2009 | ||
Treatment nonresponse. True non-response needs to be distinguished from poor response caused by inadequate treatment delivery, eg, patients not completing homework assignments in CBT or not adhering to pharmacotherapy. Asking patients about adverse effects or personal and family beliefs about treatment may reveal reasons for nonadherence.
Second-line treatments
Little data are available to guide next-step treatment options in patients who don’t achieve remission from their initial treatment. Patients who benefit from an SSRI, venlafaxine XR, or CBT but still have symptoms should be started on combination treatment. For a patient who experiences complete non-response to the initial treatment, discontinue the first treatment and switch to the other modality. In general, completely ineffective treatments should be discontinued when another treatment is added, but when partial improvement (>30%) occurs, continue the original treatment and augment it with another approach.
For patients pursuing pharmacotherapy, poor response to an adequate SSRI trial usually should lead to a switch to venlafaxine XR, and vice versa. Failure to respond to both of these medication classes should prompt a switch to a benzodiazepine or TCA.
Benzodiazepines are a fast-acting, effective treatment for PD, with efficacy similar to SSRIs in acute and long-term treatment.14 Benzodiazepines may be prescribed with antidepressants at the beginning of treatment to improve response speed.15 Clonazepam and alprazolam are FDA-approved for treating PD. A high-potency, long-acting agent, clonazepam is the preferred initial benzodiazepine, dosed 0.5 to 4 mg/d on a fixed schedule. Although substantial data support using alprazolam for PD, it requires more frequent dosing and has a greater risk of rebound anxiety and abuse potential because of its more rapid onset of action. Compared with immediate-release alprazolam, alprazolam XR has a slower absorption rate and longer steady state in the blood, but this formulation does not have lower abuse potential or greater efficacy. Although not FDA-approved for PD, diazepam and lorazepam also have proven efficacy for PD.3
Benzodiazepines should be considered contraindicated in patients with a history of substance abuse, except in select cases.4 Benzodiazepines generally should be avoided in older patients because of increased risk for falls, cognitive impairment, and motor vehicle accidents. Table 2 lists situations in which benzodiazepines may be used to treat PD.
Table 2
Clinical scenarios in which to consider using benzodiazepines
| Coadministration for 2 to 4 weeks when initiating treatment with an SSRI or venlafaxine XR to achieve more rapid relief and mitigate potential antidepressant-induced anxiety |
| For patients who wish to avoid antidepressants because of concern about sexual dysfunction |
| For patients who need chronic aspirin or an NSAID, which may increase the risk for upper gastrointestinal bleeding when taken in combination with an SSRI |
| For patients with comorbid bipolar disorder or epilepsy |
| Next-step monotherapy or augmentation in patients who respond poorly to an SSRI, venlafaxine XR, TCA, or CBT |
| CBT: cognitive-behavioral therapy; NSAID: nonsteroidal anti-inflammatory drug; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; XR: extended release |
TCAs are effective as monotherapy for PD. Most support comes from studies of imipramine or clomipramine.12 Similar to SSRIs and venlafaxine XR, use a low initial dose and gradually increase until the patient remits or side effects prevent further increases. SSRI and TCA combinations rarely are used unless the TCA is a relatively specific norepinephrine reuptake inhibitor (eg, desipramine, nortriptyline). Because TCAs are metabolized via the cytochrome P450 2D6 system and some SSRIs—particularly fluoxetine and paroxetine—strongly inhibit 2D6, combinations of TCAs with these agents may lead to dangerously high plasma TCA levels, placing patients at risk for cardiac dysrhythmias and other side effects.16
Monoamine oxidase inhibitors (MAOIs)—particularly phenelzine—are underused for PD. They have the strongest efficacy data for any class of medications outside the first- and second-line agents and have a unique mechanism of action. In patients who can comply with the dietary and medication limitations, an MAOI generally should be the next step after nonresponse to other treatments.3
Alternative treatments
For patients who do not respond to any of the treatments described above, data from uncontrolled studies support mirtazapine, levetiracetam, and the serotonin-norepinephrine reuptake inhibitors duloxetine and milnacipran as monotherapy for PD.17 Pindolol—a beta blocker and 5-HT1A receptor antagonist—proved superior to placebo as an adjunctive agent to SSRIs in treatment-resistant PD in 1 of 2 trials.17 Minimal evidence supports the atypical antipsychotics risperidone and olanzapine in treatment-resistant PD, although a placebo-controlled trial of quetiapine SR coadministered with SSRIs recently was completed (NCT00619892; results pending). Atypical antipsychotics are best reserved for patients with a primary psychotic disorder or bipolar disorder who experience panic attacks.5
Panic-focused psychodynamic psychotherapy, a 12-week (approximately 24 sessions) form of psychotherapy, has demonstrated superiority vs applied relaxation therapy.18 This treatment could be considered for patients who do not respond to standard first-line treatments, but few community therapists are familiar with this method.
For many patients with PD, complementary and alternative medicine (CAM) approaches are appealing. See this article at CurrentPsychiatry.com for a Box that discusses CAM for PD.
Although no complementary and alternative medicine treatments have strong evidence of efficacy as monotherapy for panic disorder (PD), several have data that suggest benefit with little evidence of risk. These include bibliotherapy, yoga, aerobic exercise, and the dietary supplements kava and inositol.a Exercise as a treatment poses a challenge because it can induce symptoms that the patient fears, such as tachycardia and shortness of breath. In addition to any direct physiologic benefit from aerobic exercise, there is also an exposure component that can be harnessed by gradually increasing the exertion level.
Another approach undergoing extensive evaluation is Internet-provided cognitive-behavioral therapy (CBT). Using guided CBT modules with or without therapist support, Internet-provided CBT provides an option for motivated patients unable to complete in-person CBT because of logistical factors.b A helpful resource that reviews Internet self-help and psychotherapy guided programs for PD and other psychiatric conditions is http://beacon.anu.edu.au.
References
a. Antonacci DJ, Davis E, Bloch RM, et al. CAM for your anxious patient: what the evidence says. Current Psychiatry. 2010;9(10):42-52.
b. Johnston L, Titov N, Andrews G, et al. A RCT of a transdiagnostic internet-delivered treatment for three anxiety disorders: examination of support roles and disorder-specific outcomes. PLoS One. 2011;6(11):e28079.
Maintenance treatment
Patients who complete a course of CBT for PD often follow up with several “booster sessions” at monthly or longer intervals that focus on relapse prevention techniques. Few controlled trials have evaluated pharmacotherapy discontinuation in PD. Most guidelines recommend continuing treatment for ≥1 year after achieving remission to minimize the risk of relapse.3 Researchers are focusing on whether medication dosage can be reduced during maintenance without loss of efficacy.
Treatment discontinuation
In the absence of urgent medical need, taper medications for PD gradually over several months. PD patients are highly sensitive to unusual physical sensations, which can occur while discontinuing antidepressants or benzodiazepines. If a benzodiazepine is used in conjunction with an antidepressant, the benzodiazepine should be discontinued first, so that the antidepressant can help ease benzodiazepine-associated discontinuation symptoms. A brief course of CBT during pharmacotherapy discontinuation may increase the likelihood of successful tapering.19
CASE CONTINUED: A successful switch
Ms. K has to discontinue sequential trials of fluoxetine, 40 mg/d, and venlafaxine XR, 225 mg/d because of side effects, and she does not reduce the frequency of her alprazolam use. She agrees to switch from alprazolam to clonazepam, 0.5 mg every morning and 1 mg at bedtime, and to start CBT. Clonazepam reduces her anxiety sufficiently so she can address her symptoms in therapy. Through CBT she becomes motivated to monitor her thoughts and treat them as guesses rather than facts, reviewing the evidence for her thoughts and generating rational responses. She participates in exposure exercises, which she practices between sessions, and grows to tolerate uncomfortable sensations until they no longer signal danger. After 12 CBT sessions, she is panic-free. Despite some trepidation, she agrees to a slow taper off clonazepam, reducing the dose by 0.25 mg every 2 weeks. She continues booster sessions with her therapist to manage any re-emerging anxiety. After an additional 12 weeks, she successfully discontinues clonazepam and remains panic-free.
Related Resources
- American Psychiatric Association. Panic disorder. http://healthyminds.org/Main-Topic/Panic-Disorder.aspx.
- Anxiety and Depression Association of America. Panic disorder & agoraphobia. http://adaa.org/understanding-anxiety/panic-disorder-agoraphobia.
- Mayo Clinic. Panic attacks and panic disorder. www.mayoclinic.com/health/panic-attacks/DS00338.
- National Health Service Self-Help Guides. www.ntw.nhs.uk/pic/selfhelp.
- National Institute of Mental Health. Panic disorder. www.nimh.nih.gov/health/topics/panic-disorder/index.shtml.
Drug Brand Names
- Alprazolam • Xanax
- Alprazolam XR • Xanax XR
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Levetiracetam • Keppra
- Lorazepam • Ativan
- Milnacipran • Savella
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Paroxetine CR • Paxil CR
- Phenelzine • Nardil
- Pindolol • Visken
- Quetiapine SR • Seroquel SR
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine XR • Effexor XR
Disclosures
Dr. Dunlop receives research support from Bristol-Myers Squibb, GlaxoSmithKline, and the National Institute of Mental Health. He serves as a consultant to MedAvante and Roche.
Ms. Schneider and Dr. Gerardi report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. K, a 24-year-old waitress who lives with her boyfriend, was referred by her primary care physician for evaluation of panic attacks that began “out of nowhere” at work approximately 6 months ago. The unpredictable attacks occur multiple times per week, causing her to leave work and cancel shifts.
Ms. K reports that before the panic attacks began, she felt happy in her relationship, enjoyed hobbies, and was hopeful about the future. However, she has become concerned that a potentially catastrophic illness is causing her panic attacks. She researches her symptoms on the Internet, and is preoccupied with the possibility of sudden death due to an undiagnosed heart condition. Multiple visits to the emergency room have not identified any physical abnormalities. Her primary care doctor prescribed alprazolam, 0.5 mg as needed for panic attacks, which she reports is helpful, “but only in the moment of the attacks.” Ms. K avoids alcohol and illicit substances and limits her caffeine intake. She is not willing to accept that her life “feels so limited.” Her dream of earning a nursing degree and eventually starting a family now seems unattainable.
Panic disorder (PD) occurs in 3% to 5% of adults, with women affected at roughly twice the rate of men.1 Causing a broad range of distress and varying degrees of impairment, PD commonly occurs with other psychiatric disorders. For most patients, treatment is effective, but those who do not respond to initial approaches require a thoughtful, stepped approach to care. Key considerations include establishing an accurate diagnosis, clarifying comorbid illnesses, ascertaining patient beliefs and expectations, and providing appropriately dosed and maintained treatments.
Panic attacks vs PD
Panic attacks consist of rapid onset of intense anxiety, with prominent somatic symptoms, that peaks within 10 minutes (Figure).2 Attacks in which <4 of the listed symptoms occur are considered limited-symptom panic attacks.

Figure: Body locations of panic attack symptoms
Diagnosis of a panic attack requires the sudden development of intense fear or discomfort characterized by ≥4 of the 13 symptoms listed above that peaks in intensity within 10 minutes of onset
Source: Reference 2
Panic attacks can occur with various disorders, including other anxiety disorders, mood disorders, and substance intoxication or withdrawal. Because serious medical conditions can present with panic-like symptoms, the initial occurrence of such symptoms warrants consideration of physiological causes. For a Box2 that describes the differential diagnosis of panic attacks, see this article at CurrentPsychiatry.com.
To meet diagnostic criteria for panic disorder, panic attacks must initially occur “out of the blue,” meaning no specific object or situation induced the attack. The differential diagnosis of panic attacks includes assessing for other psychiatric disorders that may involve panic attacks. Evaluation requires considering the context in which the panic attacks occur, including their start date, pattern of attacks, instigating situations, and associated thoughts.
Social phobia. Attacks occur only during or immediately before a social interaction in which the patient fears embarrassing himself or herself.
Obsessive-compulsive disorder (OCD). Attacks occur when the patient cannot avoid exposure to an obsessional fear or is prevented from performing a ritual that diffuses obsessional anxiety.
Posttraumatic stress disorder (PTSD). Attacks occur when confronted by a trauma-related memory or trigger.
Specific phobia. Attacks occur only when the patient encounters a specifically feared object, place, or situation, unrelated to social phobia, OCD, or PTSD.
Medical conditions. Conditions to consider include—but are not limited to—hyperthyroidism, pulmonary embolism, myocardial infarction, cardiac dysrhythmias, hypoglycemia, asthma, partial complex seizures, and pheochromocytoma.
Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000
A PD diagnosis requires that repeated panic attacks initially must occur from “out of the blue,” meaning no specific object or situation induced the attack. In addition, the diagnosis requires 1 of 3 types of psychological or behavioral changes as a result of the attacks (Table 1).2 Agoraphobia is diagnosed if 1 of the behavioral changes is avoidance of places or situations from which escape might be embarrassing or difficult should an attack occur. A patient can be diagnosed as having PD with agoraphobia, PD without agoraphobia, or agoraphobia without PD (ie, experiences only limited symptom panic attacks, but avoids situations or stimuli associated with them).
Table 1
Definitions of panic disorder and agoraphobia
| Panic disorder |
|---|
|
| Agoraphobia |
| Anxiety about, or avoidance of, being in places or situations from which escape might be difficult or embarrassing, or in which help may not be available in the event of having an unexpected or situationally predisposed panic attack or panic-like symptoms. Agoraphobic fears typically involve characteristic clusters of situations that include being outside the home alone, being in a crowd, standing in a line, being on a bridge, or traveling in a bus, train, or automobile |
| Source: Reference 2 |
Comorbidities are common in patients with PD and predict greater difficulty achieving remission (Box).1,3-6
The most common psychiatric conditions that co-occur with panic disorder (PD) are other anxiety disorders, mood disorders, personality disorders, and substance use disorders.1 Carefully assess the severity and degree of impairment or distress arising from each condition to prioritize treatment goals. For example, treating panic attacks would be a lower priority in a patient with untreated bipolar disorder.
Assessing comorbid substance abuse is important in selecting PD treatments. Benzodiazepines should almost always be avoided in patients with a history of drug abuse—illicit or prescribed. Although complete abstinence should not be a prerequisite for beginning PD treatment, detoxification and concomitant substance abuse treatment are essential.3
Comorbid mood disorders also affect the course of PD treatment. Antidepressants are effective for treating depression and PD, whereas benzodiazepines are not effective for depression.4 Antidepressants in patients with bipolar disorder are controversial because these medications might induce mixed or elevated mood states or rapid cycling. In these complicated patients, consider antidepressants lower in the treatment algorithm.5
Other conditions to consider before beginning treatment include pregnancy or the possibility of becoming pregnant in the near future and suicidal ideation. PD is associated with increased risk for suicidal ideation and progression to suicide attempts, particularly in patients with a comorbid mood or psychotic disorder.6 In addition, consider the potential impact of medications on comorbid medical conditions.
Treatment begins with education
The goal of treatment is remission of symptoms, ideally including an absence of panic attacks, agoraphobic avoidance, and anticipatory anxiety.1 The Panic Disorder Severity Scale self-report is a validated measure of panic symptoms that may be useful in clinical practice.7
The first step in treatment is educating patients about panic attacks, framing them as an overreactive fear circuit in the brain that produces physical symptoms that are not dangerous. Using a brain model that shows the location of the amygdala, hippocampus, and prefrontal cortex—which play crucial roles in generating and controlling anxiety and fear—can make this discussion more concrete.8 Although highly simplified, such models allow clinicians to demonstrate that excessive reactivity of limbic regions can be reduced by both top-down (cortico-limbic connections via cognitive-behavioral therapy [CBT]) and bottom-up (pharmacotherapy directly acting on limbic structures) approaches. Such discussions lead to treatment recommendations for CBT, pharmacotherapy, or their combination.
No single treatment has emerged as the definitive “best” for PD, and no reliable predictors can guide specific treatment for an individual.3 Combining CBT with pharmacotherapy produces higher short-term response rates than either treatment alone, but in the long term, combination treatment does not appear to be superior to CBT alone.9 Base the initial treatment selection for PD on patient preference, treatment availability and cost, and comorbid medical and psychiatric conditions. For an Algorithm to guide treatment decisions, see this article at CurrentPsychiatry.com.

Algorithm: Treatment for panic disorder: A suggested algorithm
aPoor response to an SSRI should lead to a switch to venlafaxine extended-release, and vice versa
bBenzodiazepines are relatively contraindicated in geriatric patients and patients with a history of substance abuse or dependence
CBT: cognitive-behavioral therapy; MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; Ven XR: venlafaxine extended-release
First-line treatments
Psychotherapy. CBT is the most efficacious psychotherapy for PD. Twelve to 15 sessions of CBT has demonstrated efficacy for PD, with additional effects on comorbid anxiety and depressive symptoms.10 No large clinical trials of CBT have used cognitive restructuring alone; all have included at least some component of exposure that requires the patient to confront feared physical sensations. Gains during treatment may be steady and gradual or sudden and uneven, with rapid improvement in some but not all symptoms. CBT and pharmacotherapy have demonstrated similar levels of benefit in short-term trials, but CBT has proven superior in most9 but not all11 trials evaluating long-term outcomes, particularly compared with pharmacotherapy that is discontinued during follow-up. Although less studied, group CBT also may be considered if a patient cannot afford individual CBT.
Pharmacotherapy. Evidence supports selective serotonin reuptake inhibitors (SSRIs), venlafaxine extended-release (XR), benzodiazepines, and tricyclic antidepressants (TCAs) as effective treatments for PD.3 No class of medication has demonstrated superiority over others in short-term treatment.3,12 Because of the medical risks associated with benzodiazepines and TCAs, an SSRI or venlafaxine XR should be the first medication option for most patients. Fluoxetine, paroxetine, sertraline, and venlafaxine XR are FDA-approved for PD. Paroxetine is associated with weight gain and may increase the risk for panic recurrence upon discontinuation more than sertraline, making it a less favorable option for many patients.13 Start doses at half the normal starting dose used for treating major depressive disorder and continue for 4 to 7 days, then increase to the minimal effective dose. For a Table3 that lists dosing recommendations for antidepressants to treat PD, see this article at CurrentPsychiatry.com. If there is no improvement by 4 weeks, increase the dose every 2 to 4 weeks until remission is achieved or side effects prevent further dose increases.
Table
Recommended doses for antidepressants used to treat panic disorder
| Medication | Starting dose (mg/d) | Therapeutic range (mg/d) |
|---|---|---|
| SSRIs | ||
| Citalopram | 10 | 20 to 40 |
| Escitalopram | 5 | 10 to 40 |
| Fluoxetine | 5 to 10 | 20 to 80 |
| Fluvoxamine | 25 | 100 to 300 |
| Paroxetine | 10 | 20 to 80 |
| Paroxetine CR | 12.5 | 25 to 50 |
| Sertraline | 25 | 100 to 200 |
| SNRIs | ||
| Duloxetine | 20 to 30 | 60 to 120 |
| Venlafaxine XR | 37.5 | 150 to 225 |
| TCAs | ||
| Clomipramine | 10 to 25 | 100 to 300 |
| Imipramine | 10 | 100 to 300 |
| MAOI | ||
| Phenelzine | 15 | 45 to 90 |
| CR: controlled release; MAOI: monoamine oxidase inhibitor; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants; XR: extended release Source: American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington, DC: American Psychiatric Association; 2009 | ||
Treatment nonresponse. True non-response needs to be distinguished from poor response caused by inadequate treatment delivery, eg, patients not completing homework assignments in CBT or not adhering to pharmacotherapy. Asking patients about adverse effects or personal and family beliefs about treatment may reveal reasons for nonadherence.
Second-line treatments
Little data are available to guide next-step treatment options in patients who don’t achieve remission from their initial treatment. Patients who benefit from an SSRI, venlafaxine XR, or CBT but still have symptoms should be started on combination treatment. For a patient who experiences complete non-response to the initial treatment, discontinue the first treatment and switch to the other modality. In general, completely ineffective treatments should be discontinued when another treatment is added, but when partial improvement (>30%) occurs, continue the original treatment and augment it with another approach.
For patients pursuing pharmacotherapy, poor response to an adequate SSRI trial usually should lead to a switch to venlafaxine XR, and vice versa. Failure to respond to both of these medication classes should prompt a switch to a benzodiazepine or TCA.
Benzodiazepines are a fast-acting, effective treatment for PD, with efficacy similar to SSRIs in acute and long-term treatment.14 Benzodiazepines may be prescribed with antidepressants at the beginning of treatment to improve response speed.15 Clonazepam and alprazolam are FDA-approved for treating PD. A high-potency, long-acting agent, clonazepam is the preferred initial benzodiazepine, dosed 0.5 to 4 mg/d on a fixed schedule. Although substantial data support using alprazolam for PD, it requires more frequent dosing and has a greater risk of rebound anxiety and abuse potential because of its more rapid onset of action. Compared with immediate-release alprazolam, alprazolam XR has a slower absorption rate and longer steady state in the blood, but this formulation does not have lower abuse potential or greater efficacy. Although not FDA-approved for PD, diazepam and lorazepam also have proven efficacy for PD.3
Benzodiazepines should be considered contraindicated in patients with a history of substance abuse, except in select cases.4 Benzodiazepines generally should be avoided in older patients because of increased risk for falls, cognitive impairment, and motor vehicle accidents. Table 2 lists situations in which benzodiazepines may be used to treat PD.
Table 2
Clinical scenarios in which to consider using benzodiazepines
| Coadministration for 2 to 4 weeks when initiating treatment with an SSRI or venlafaxine XR to achieve more rapid relief and mitigate potential antidepressant-induced anxiety |
| For patients who wish to avoid antidepressants because of concern about sexual dysfunction |
| For patients who need chronic aspirin or an NSAID, which may increase the risk for upper gastrointestinal bleeding when taken in combination with an SSRI |
| For patients with comorbid bipolar disorder or epilepsy |
| Next-step monotherapy or augmentation in patients who respond poorly to an SSRI, venlafaxine XR, TCA, or CBT |
| CBT: cognitive-behavioral therapy; NSAID: nonsteroidal anti-inflammatory drug; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; XR: extended release |
TCAs are effective as monotherapy for PD. Most support comes from studies of imipramine or clomipramine.12 Similar to SSRIs and venlafaxine XR, use a low initial dose and gradually increase until the patient remits or side effects prevent further increases. SSRI and TCA combinations rarely are used unless the TCA is a relatively specific norepinephrine reuptake inhibitor (eg, desipramine, nortriptyline). Because TCAs are metabolized via the cytochrome P450 2D6 system and some SSRIs—particularly fluoxetine and paroxetine—strongly inhibit 2D6, combinations of TCAs with these agents may lead to dangerously high plasma TCA levels, placing patients at risk for cardiac dysrhythmias and other side effects.16
Monoamine oxidase inhibitors (MAOIs)—particularly phenelzine—are underused for PD. They have the strongest efficacy data for any class of medications outside the first- and second-line agents and have a unique mechanism of action. In patients who can comply with the dietary and medication limitations, an MAOI generally should be the next step after nonresponse to other treatments.3
Alternative treatments
For patients who do not respond to any of the treatments described above, data from uncontrolled studies support mirtazapine, levetiracetam, and the serotonin-norepinephrine reuptake inhibitors duloxetine and milnacipran as monotherapy for PD.17 Pindolol—a beta blocker and 5-HT1A receptor antagonist—proved superior to placebo as an adjunctive agent to SSRIs in treatment-resistant PD in 1 of 2 trials.17 Minimal evidence supports the atypical antipsychotics risperidone and olanzapine in treatment-resistant PD, although a placebo-controlled trial of quetiapine SR coadministered with SSRIs recently was completed (NCT00619892; results pending). Atypical antipsychotics are best reserved for patients with a primary psychotic disorder or bipolar disorder who experience panic attacks.5
Panic-focused psychodynamic psychotherapy, a 12-week (approximately 24 sessions) form of psychotherapy, has demonstrated superiority vs applied relaxation therapy.18 This treatment could be considered for patients who do not respond to standard first-line treatments, but few community therapists are familiar with this method.
For many patients with PD, complementary and alternative medicine (CAM) approaches are appealing. See this article at CurrentPsychiatry.com for a Box that discusses CAM for PD.
Although no complementary and alternative medicine treatments have strong evidence of efficacy as monotherapy for panic disorder (PD), several have data that suggest benefit with little evidence of risk. These include bibliotherapy, yoga, aerobic exercise, and the dietary supplements kava and inositol.a Exercise as a treatment poses a challenge because it can induce symptoms that the patient fears, such as tachycardia and shortness of breath. In addition to any direct physiologic benefit from aerobic exercise, there is also an exposure component that can be harnessed by gradually increasing the exertion level.
Another approach undergoing extensive evaluation is Internet-provided cognitive-behavioral therapy (CBT). Using guided CBT modules with or without therapist support, Internet-provided CBT provides an option for motivated patients unable to complete in-person CBT because of logistical factors.b A helpful resource that reviews Internet self-help and psychotherapy guided programs for PD and other psychiatric conditions is http://beacon.anu.edu.au.
References
a. Antonacci DJ, Davis E, Bloch RM, et al. CAM for your anxious patient: what the evidence says. Current Psychiatry. 2010;9(10):42-52.
b. Johnston L, Titov N, Andrews G, et al. A RCT of a transdiagnostic internet-delivered treatment for three anxiety disorders: examination of support roles and disorder-specific outcomes. PLoS One. 2011;6(11):e28079.
Maintenance treatment
Patients who complete a course of CBT for PD often follow up with several “booster sessions” at monthly or longer intervals that focus on relapse prevention techniques. Few controlled trials have evaluated pharmacotherapy discontinuation in PD. Most guidelines recommend continuing treatment for ≥1 year after achieving remission to minimize the risk of relapse.3 Researchers are focusing on whether medication dosage can be reduced during maintenance without loss of efficacy.
Treatment discontinuation
In the absence of urgent medical need, taper medications for PD gradually over several months. PD patients are highly sensitive to unusual physical sensations, which can occur while discontinuing antidepressants or benzodiazepines. If a benzodiazepine is used in conjunction with an antidepressant, the benzodiazepine should be discontinued first, so that the antidepressant can help ease benzodiazepine-associated discontinuation symptoms. A brief course of CBT during pharmacotherapy discontinuation may increase the likelihood of successful tapering.19
CASE CONTINUED: A successful switch
Ms. K has to discontinue sequential trials of fluoxetine, 40 mg/d, and venlafaxine XR, 225 mg/d because of side effects, and she does not reduce the frequency of her alprazolam use. She agrees to switch from alprazolam to clonazepam, 0.5 mg every morning and 1 mg at bedtime, and to start CBT. Clonazepam reduces her anxiety sufficiently so she can address her symptoms in therapy. Through CBT she becomes motivated to monitor her thoughts and treat them as guesses rather than facts, reviewing the evidence for her thoughts and generating rational responses. She participates in exposure exercises, which she practices between sessions, and grows to tolerate uncomfortable sensations until they no longer signal danger. After 12 CBT sessions, she is panic-free. Despite some trepidation, she agrees to a slow taper off clonazepam, reducing the dose by 0.25 mg every 2 weeks. She continues booster sessions with her therapist to manage any re-emerging anxiety. After an additional 12 weeks, she successfully discontinues clonazepam and remains panic-free.
Related Resources
- American Psychiatric Association. Panic disorder. http://healthyminds.org/Main-Topic/Panic-Disorder.aspx.
- Anxiety and Depression Association of America. Panic disorder & agoraphobia. http://adaa.org/understanding-anxiety/panic-disorder-agoraphobia.
- Mayo Clinic. Panic attacks and panic disorder. www.mayoclinic.com/health/panic-attacks/DS00338.
- National Health Service Self-Help Guides. www.ntw.nhs.uk/pic/selfhelp.
- National Institute of Mental Health. Panic disorder. www.nimh.nih.gov/health/topics/panic-disorder/index.shtml.
Drug Brand Names
- Alprazolam • Xanax
- Alprazolam XR • Xanax XR
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Levetiracetam • Keppra
- Lorazepam • Ativan
- Milnacipran • Savella
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Paroxetine CR • Paxil CR
- Phenelzine • Nardil
- Pindolol • Visken
- Quetiapine SR • Seroquel SR
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine XR • Effexor XR
Disclosures
Dr. Dunlop receives research support from Bristol-Myers Squibb, GlaxoSmithKline, and the National Institute of Mental Health. He serves as a consultant to MedAvante and Roche.
Ms. Schneider and Dr. Gerardi report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Roy-Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet. 2006;368(9540):1023-1032.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
3. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington DC: American Psychiatric Association; 2009.
4. Dunlop BW, Davis PG. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: a review. Prim Care Companion J Clin Psychiatry. 2008;10(3):222-228.
5. Rakofsky JJ, Dunlop BW. Treating nonspecific anxiety and anxiety disorders in patients with bipolar disorder: a review. J Clin Psychiatry. 2011;72(1):81-90.
6. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
7. Houck PR, Spiegel DA, Shear MK, et al. Reliability of the self-report version of the panic disorder severity scale. Depress Anxiety. 2002;15(4):183-185.
8. Ninan PT, Dunlop BW. Neurobiology and etiology of panic disorder. J Clin Psychiatry. 2005;66(suppl 4):3-7.
9. Furukawa TA, Watanabe N, Churchill R. Psychotherapy plus antidepressant for panic disorder with or without agoraphobia: systematic review. Br J Psychiatry. 2006;188:305-312.
10. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
11. van Apeldoorn FJ, Timmerman ME, Mersch PP, et al. A randomized trial of cognitive-behavioral therapy or selective serotonin reuptake inhibitor or both combined for panic disorder with or without agoraphobia: treatment results through 1-year follow-up. J Clin Psychiatry. 2010;71(5):574-586.
12. Bakker A, van Balkom AJ, Spinhoven P. SSRIs vs. TCAs in the treatment of panic disorder: a meta-analysis. Acta Psychiatr Scand. 2002;106(3):163-167.
13. Bandelow B, Behnke K, Lenoir S, et al. Sertraline versus paroxetine in the treatment of panic disorder: an acute, double-blind noninferiority comparison. J Clin Psychiatry. 2004;65(3):405-413.
14. Nardi AE, Freire RC, Mochcovitch MD, et al. A randomized, naturalistic, parallel-group study for the long-term treatment of panic disorder with clonazepam or paroxetine. J Clin Psychopharmacol. 2012;32(1):120-126.
15. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
16. Preskorn SH, Shah R, Neff M, et al. The potential for clinically significant drug-drug interactions involving the CYP 2D6 system: effects with fluoxetine and paroxetine versus sertraline. J Psychiatr Pract. 2007;13(1):5-12.
17. Perna G, Guerriero G, Caldirola D. Emerging drugs for panic disorder. Expert Opin Emerg Drugs. 2011;16(4):631-645.
18. Milrod B, Leon AC, Busch F, et al. A randomized controlled clinical trial of psychoanalytic psychotherapy for panic disorder. Am J Psychiatry. 2007;164(2):265-272.
19. Otto MW, Pollack MH, Sachs GS, et al. Discontinuation of benzodiazepine treatment: efficacy of cognitive-behavioral therapy for patients with panic disorder. Am J Psychiatry. 1993;150(10):1485-1490.
1. Roy-Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet. 2006;368(9540):1023-1032.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
3. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington DC: American Psychiatric Association; 2009.
4. Dunlop BW, Davis PG. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: a review. Prim Care Companion J Clin Psychiatry. 2008;10(3):222-228.
5. Rakofsky JJ, Dunlop BW. Treating nonspecific anxiety and anxiety disorders in patients with bipolar disorder: a review. J Clin Psychiatry. 2011;72(1):81-90.
6. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
7. Houck PR, Spiegel DA, Shear MK, et al. Reliability of the self-report version of the panic disorder severity scale. Depress Anxiety. 2002;15(4):183-185.
8. Ninan PT, Dunlop BW. Neurobiology and etiology of panic disorder. J Clin Psychiatry. 2005;66(suppl 4):3-7.
9. Furukawa TA, Watanabe N, Churchill R. Psychotherapy plus antidepressant for panic disorder with or without agoraphobia: systematic review. Br J Psychiatry. 2006;188:305-312.
10. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
11. van Apeldoorn FJ, Timmerman ME, Mersch PP, et al. A randomized trial of cognitive-behavioral therapy or selective serotonin reuptake inhibitor or both combined for panic disorder with or without agoraphobia: treatment results through 1-year follow-up. J Clin Psychiatry. 2010;71(5):574-586.
12. Bakker A, van Balkom AJ, Spinhoven P. SSRIs vs. TCAs in the treatment of panic disorder: a meta-analysis. Acta Psychiatr Scand. 2002;106(3):163-167.
13. Bandelow B, Behnke K, Lenoir S, et al. Sertraline versus paroxetine in the treatment of panic disorder: an acute, double-blind noninferiority comparison. J Clin Psychiatry. 2004;65(3):405-413.
14. Nardi AE, Freire RC, Mochcovitch MD, et al. A randomized, naturalistic, parallel-group study for the long-term treatment of panic disorder with clonazepam or paroxetine. J Clin Psychopharmacol. 2012;32(1):120-126.
15. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
16. Preskorn SH, Shah R, Neff M, et al. The potential for clinically significant drug-drug interactions involving the CYP 2D6 system: effects with fluoxetine and paroxetine versus sertraline. J Psychiatr Pract. 2007;13(1):5-12.
17. Perna G, Guerriero G, Caldirola D. Emerging drugs for panic disorder. Expert Opin Emerg Drugs. 2011;16(4):631-645.
18. Milrod B, Leon AC, Busch F, et al. A randomized controlled clinical trial of psychoanalytic psychotherapy for panic disorder. Am J Psychiatry. 2007;164(2):265-272.
19. Otto MW, Pollack MH, Sachs GS, et al. Discontinuation of benzodiazepine treatment: efficacy of cognitive-behavioral therapy for patients with panic disorder. Am J Psychiatry. 1993;150(10):1485-1490.