User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Facebook and boundaries
Drs. Douglas Mossman and Helen M. Farrell’s article on the social networking Web site Facebook (“Facebook: Social networking meets professional duty,” Malpractice Rx, Current Psychiatry , March 2012, p. 34-37; http://bit.ly/1JFSxP3) deserves some expansion.
We shouldn’t give Facebook more credit than it deserves, nor our patients less just because they are mentally ill. Even severely mentally disturbed patients often possess a fair degree of knowledge when it comes to social media. Most patients can and often do obtain information about us from Internet searches without ever having to “friend” us on Facebook. What psychiatrist hasn’t seen patients who say they “found us on the Internet”?
Drs. Mossman and Farrell are correct that there are Web sites that provide our academic, personal, family, legal, and military information with the click of a mouse; patients don’t have to go to Facebook. Because Facebook has a number of security and privacy settings, anyone who does not take the time to learn about these settings shouldn’t be on Facebook.
Handling a Facebook friend request can be a tool to educate or exploit. A psychiatrist might have a Facebook presence that has nothing to do with mental health, but devoted to his or her hobby. A patient may have the same hobby; however, a friend request such as this cannot be honored because it is a personal/boundary issue.
We’ve all seen patients at gas stations, supermarkets, post offices, banks, movie theaters, and libraries. We don’t change banks, gas stations, or supermarkets just because a patient patronizes the same business we do. We don’t refuse to be interviewed for newspaper or magazine articles, radio programs, or television shows just because a patient might read, listen, or watch. We treat these unintended encounters as a natural by-product of our chosen discipline with respect and integrity that maintains the therapeutic relationship without crossing professional boundaries, or having to completely alter one’s lifestyle. Should we react differently if a patient is on Facebook or Twitter? Until the American Psychiatric Association makes a definitive ruling on this issue, it is an individual matter of cautious judgment.
Roland S. Jefferson, MD
Private Practice
Los Angeles, CA
Drs. Douglas Mossman and Helen M. Farrell’s article on the social networking Web site Facebook (“Facebook: Social networking meets professional duty,” Malpractice Rx, Current Psychiatry , March 2012, p. 34-37; http://bit.ly/1JFSxP3) deserves some expansion.
We shouldn’t give Facebook more credit than it deserves, nor our patients less just because they are mentally ill. Even severely mentally disturbed patients often possess a fair degree of knowledge when it comes to social media. Most patients can and often do obtain information about us from Internet searches without ever having to “friend” us on Facebook. What psychiatrist hasn’t seen patients who say they “found us on the Internet”?
Drs. Mossman and Farrell are correct that there are Web sites that provide our academic, personal, family, legal, and military information with the click of a mouse; patients don’t have to go to Facebook. Because Facebook has a number of security and privacy settings, anyone who does not take the time to learn about these settings shouldn’t be on Facebook.
Handling a Facebook friend request can be a tool to educate or exploit. A psychiatrist might have a Facebook presence that has nothing to do with mental health, but devoted to his or her hobby. A patient may have the same hobby; however, a friend request such as this cannot be honored because it is a personal/boundary issue.
We’ve all seen patients at gas stations, supermarkets, post offices, banks, movie theaters, and libraries. We don’t change banks, gas stations, or supermarkets just because a patient patronizes the same business we do. We don’t refuse to be interviewed for newspaper or magazine articles, radio programs, or television shows just because a patient might read, listen, or watch. We treat these unintended encounters as a natural by-product of our chosen discipline with respect and integrity that maintains the therapeutic relationship without crossing professional boundaries, or having to completely alter one’s lifestyle. Should we react differently if a patient is on Facebook or Twitter? Until the American Psychiatric Association makes a definitive ruling on this issue, it is an individual matter of cautious judgment.
Roland S. Jefferson, MD
Private Practice
Los Angeles, CA
Drs. Douglas Mossman and Helen M. Farrell’s article on the social networking Web site Facebook (“Facebook: Social networking meets professional duty,” Malpractice Rx, Current Psychiatry , March 2012, p. 34-37; http://bit.ly/1JFSxP3) deserves some expansion.
We shouldn’t give Facebook more credit than it deserves, nor our patients less just because they are mentally ill. Even severely mentally disturbed patients often possess a fair degree of knowledge when it comes to social media. Most patients can and often do obtain information about us from Internet searches without ever having to “friend” us on Facebook. What psychiatrist hasn’t seen patients who say they “found us on the Internet”?
Drs. Mossman and Farrell are correct that there are Web sites that provide our academic, personal, family, legal, and military information with the click of a mouse; patients don’t have to go to Facebook. Because Facebook has a number of security and privacy settings, anyone who does not take the time to learn about these settings shouldn’t be on Facebook.
Handling a Facebook friend request can be a tool to educate or exploit. A psychiatrist might have a Facebook presence that has nothing to do with mental health, but devoted to his or her hobby. A patient may have the same hobby; however, a friend request such as this cannot be honored because it is a personal/boundary issue.
We’ve all seen patients at gas stations, supermarkets, post offices, banks, movie theaters, and libraries. We don’t change banks, gas stations, or supermarkets just because a patient patronizes the same business we do. We don’t refuse to be interviewed for newspaper or magazine articles, radio programs, or television shows just because a patient might read, listen, or watch. We treat these unintended encounters as a natural by-product of our chosen discipline with respect and integrity that maintains the therapeutic relationship without crossing professional boundaries, or having to completely alter one’s lifestyle. Should we react differently if a patient is on Facebook or Twitter? Until the American Psychiatric Association makes a definitive ruling on this issue, it is an individual matter of cautious judgment.
Roland S. Jefferson, MD
Private Practice
Los Angeles, CA
Changing terminology
I applaud Dr. Nasrallah’s visionary April editorial. One of the biggest obstacles to lifting stigma is the mental-physical categorization. “Mental” is the last slur still commonly used, instantly bringing to mind its synonyms: “crazy,” “looney,” and “nuts.” This term should be extinguished from the psychiatric nomenclature. I suggest the label “neuriatry” in place of psychiatry because “neuro” gets us away from the stigmatizing term “psyche” while creating a linkage with our sister specialty, neurology.
Stefan Lerner, MDPrivate PracticePrinceton, NJ
I applaud Dr. Nasrallah’s visionary April editorial. One of the biggest obstacles to lifting stigma is the mental-physical categorization. “Mental” is the last slur still commonly used, instantly bringing to mind its synonyms: “crazy,” “looney,” and “nuts.” This term should be extinguished from the psychiatric nomenclature. I suggest the label “neuriatry” in place of psychiatry because “neuro” gets us away from the stigmatizing term “psyche” while creating a linkage with our sister specialty, neurology.
Stefan Lerner, MDPrivate PracticePrinceton, NJ
I applaud Dr. Nasrallah’s visionary April editorial. One of the biggest obstacles to lifting stigma is the mental-physical categorization. “Mental” is the last slur still commonly used, instantly bringing to mind its synonyms: “crazy,” “looney,” and “nuts.” This term should be extinguished from the psychiatric nomenclature. I suggest the label “neuriatry” in place of psychiatry because “neuro” gets us away from the stigmatizing term “psyche” while creating a linkage with our sister specialty, neurology.
Stefan Lerner, MDPrivate PracticePrinceton, NJ
Consider market forces
I found Dr. Nasrallah’s April editorial interesting because I think morphing our profession is exciting. However, the reasons for the need for transformation were not laid out concretely.
We are always interested in the development of new diagnostic models and novel treatments; however, how can the mental health delivery system be changed? It is huge, poorly funded, and generally not run on the medical model. Community mental health programs tend to be lead by nonpsychiatrists at non-university hospitals. Insurance companies and private payees are reluctant to pay psychiatrists and reimbursements are comparatively lower than other medical specialties, so why would they pay for double-boarded psychiatrists? It doesn’t appear that the market would support further psychiatric education when more lucrative medical professions exist.
Finally, our government and patients want more access to medical care and lower costs. Would our patients and government support and reimburse us for more specialization?
Alexander Fariborzian, MD
Private Practice
Gainesville, FL
I found Dr. Nasrallah’s April editorial interesting because I think morphing our profession is exciting. However, the reasons for the need for transformation were not laid out concretely.
We are always interested in the development of new diagnostic models and novel treatments; however, how can the mental health delivery system be changed? It is huge, poorly funded, and generally not run on the medical model. Community mental health programs tend to be lead by nonpsychiatrists at non-university hospitals. Insurance companies and private payees are reluctant to pay psychiatrists and reimbursements are comparatively lower than other medical specialties, so why would they pay for double-boarded psychiatrists? It doesn’t appear that the market would support further psychiatric education when more lucrative medical professions exist.
Finally, our government and patients want more access to medical care and lower costs. Would our patients and government support and reimburse us for more specialization?
Alexander Fariborzian, MD
Private Practice
Gainesville, FL
I found Dr. Nasrallah’s April editorial interesting because I think morphing our profession is exciting. However, the reasons for the need for transformation were not laid out concretely.
We are always interested in the development of new diagnostic models and novel treatments; however, how can the mental health delivery system be changed? It is huge, poorly funded, and generally not run on the medical model. Community mental health programs tend to be lead by nonpsychiatrists at non-university hospitals. Insurance companies and private payees are reluctant to pay psychiatrists and reimbursements are comparatively lower than other medical specialties, so why would they pay for double-boarded psychiatrists? It doesn’t appear that the market would support further psychiatric education when more lucrative medical professions exist.
Finally, our government and patients want more access to medical care and lower costs. Would our patients and government support and reimburse us for more specialization?
Alexander Fariborzian, MD
Private Practice
Gainesville, FL
Masters of American Psychiatry: Glen O. Gabbard, MD
Mimics of depression
Overwhelmed by side effects
CASE: Medication sensitivity
Mrs. C, age 48, is admitted to a tertiary care inpatient mood disorder unit for evaluation of severe depression characterized by depressed mood, anhedonia, and insomnia. Her initial Hamilton Rating Scale for Depression 17-Item (HRSD-17) score is 30, indicating severe depression. Her medications are fluoxetine, 10 mg/d, and diazepam, 0.5 mg/d.
Mrs. C describes a 10-month history of depression and extreme anxiety in the context of several psychosocial stressors. Her father recently died and she is having difficulty with the demands of administering her father’s estate. She is intensely obsessive and focused on nihilistic themes, her diagnosis, somatic themes, and medications side effects. Her husband confirms our observations. No history or current symptoms of typical compulsions (eg, washing hands or checking doors) are elicited. She has limited insight into her obsessive tendencies.
Mrs. C had no psychiatric history before her depressive and obsessive symptoms developed 10 months ago. However, in the past 10 months, she has been hospitalized in a psychiatric facility twice. She also received a series of 8 electroconvulsive therapy treatments, but reported minimal improvement of her depressive symptoms. Mrs. C had a few cognitive-behavioral therapy (CBT) sessions with a psychotherapist, but she said they didn’t help much.
Mrs. C has substantial difficulty adhering to medications, even at subtherapeutic doses. She states she is “extremely sensitive” to all medications. Mrs. C says she develops dizziness, increased anxiety, insomnia, nausea, and other vague reactions whenever she attempts to increase her psychotropics to therapeutic doses. She took sertraline, 10 mg/d, for 4 days, but discontinued it because of unspecified side effects. She then received escitalopram, 2.5 mg/d, for 10 days, but again stopped it because of vague side effects. She was taking paroxetine, 10 mg/d, for 2 days, but experienced vomiting and discontinued the drug. She tried venlafaxine at a low dose and also discontinued it because of vomiting. Mrs. C stayed on mirtazapine, 22.5 mg/d, for 3 months, but stopped it because of lack of efficacy and she was unwilling to increase the dose. Other unsuccessful trials include citalopram and doxepin. Mrs. C is hesitant to try another medication or increase to therapeutic doses any of the previous medications.
The authors’ observations
Before initiating another treatment, the treatment team considered Mrs. C’s pervasive medication intolerance. Her enzymatic activity may be genetically compromised, which could lead to high blood levels of medications and significant side effects when she takes very low doses. Individual variations in response to psychotropics are influenced by genetic factors.1 Variants in the cytochrome P450 (CYP450) genes produce enzymes with increased activity, normal activity, reduced activity, or no activity, creating phenotypes of ultrarapid metabolizers, extensive metabolizers, intermediate metabolizers, and poor metabolizers, respectively. These genetic variations can affect blood levels of medications that employ these enzymes in their metabolic pathways.2 Mrs. C could be a poor metabolizer of common CYP450 variant enzymes, which led to her exquisite sensitivity to psychotropics. We felt this was a reasonable hypothesis given her tumultuous 10-month course of psychiatric treatment and multiple failed medication trials.
An alternative hypothesis is that Mrs. C’s somatic obsessions about drug side effects were the primary clinical issue that led to her severe medication intolerance. Mrs. C spends hours questioning the inpatient staff about her diagnosis (eg, “Are you sure I don’t have bipolar disorder?”), medications (eg, “Are you sure this medication won’t make me sick?”), somatic themes (eg, “Are you sure I don’t have Meniere’s disease with all my dizziness?”), and nihilistic themes (eg, “What if I never get better?”). Mrs. C’s husband attested that she has spent hours researching her new medications on the Internet and reading the medication handouts from the pharmacy. She admits to mentally cycling through the DSM-IV-TR criteria for hours at a time to “figure out” if she has bipolar disorder (BD).
We initiated pharmacogenomic testing to help distinguish between these hypotheses. Mrs. C’s results are presented in Table 1. Genotype results were applied using an interpretive algorithm (Figure) in which 26 psychiatric medications were placed in categories of “use as directed” (green column), “use with caution” (yellow column), and “use with caution or more frequent monitoring” (red column). The algorithm incorporates the genetic information with the known pharmacologic profile for each of the medications in the panel. Highlights of Mrs. C’s interpretive report are shown in Table 2.
Table 1
Mrs. C’s genotype results
| Gene | Allele | Predicted phenotype |
|---|---|---|
| CYP2D6 | *1/*4 | Intermediate metabolizer |
| CYP2C19 | *1/*1 | Extensive metabolizer |
| SLC6A4 | S/S | Low activity |
| 5HTR2A | G/G | Reduced activity |
Figure
Genotype-phenotype integration into Mrs. C’s interpretive report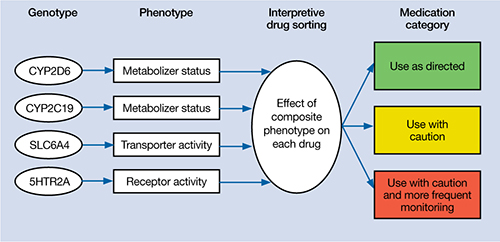
Table 2
Mrs. C’s pharmacogenomic-based interpretive report
| Use as directed | Use with caution | Use with caution and more frequent monitoring |
|---|---|---|
| Antidepressants: Duloxetine, mirtazapine Antipsychotics: Clozapine, olanzapine, quetiapine, ziprasidone | Antidepressants: Amitriptyline,a,b bupropion,a citalopram,c clomipramine,a,b desipramine,a,b escitalopram,c fluoxetine,a fluvoxamine,c imipramine,a,b nortriptyline,a,b sertraline,c paroxetine,c trazodone,a venlafaxinea Antipsychotics: Aripiprazole,a haloperidol,a perphenazine,a risperidonea | None |
| aSerum level may be too high, lower doses may be required bSerum levels may be outside of optimal range cGenotype suggests less than optimal response | ||
The authors’ observations
Mrs. C’s genotype might explain some sensitivity to medications metabolized by CYP2D6 (eg, venlafaxine, paroxetine, fluoxetine), but does not explain her acute sensitivity to all of the medications she has taken. For example, she is an extensive metabolizer for CYP2C19, which metabolizes escitalopram; therefore, it is unlikely escitalopram, 2.5 mg/d, would result in high blood levels and side effects.3 Regardless of the next step in treatment, we deemed her somatic obsessions to be the most important clinical issue. It seems unlikely that Mrs. C would adhere to any medication regimen until this underlying problem was addressed.
The focus of treatment shifted to Mrs. C’s obsessions about her medications and their side effects. Mrs. C was fixated on the content of her obsessions (eg, medications, side effects) rather than the process of her obsessional thinking. The goal was to help Mrs. C identify, label, and ultimately create distance from her obsessive thoughts associated with side effects. The treatment team employed an acceptance and commitment therapy (ACT) model of observing and defusing thoughts in the inpatient setting (Table 3).4 ACT is based on mindfulness and committed, values-based action.5 When patients are “fused” with their thoughts, they believe these thoughts are important and representative of reality. In Mrs. C’s case, she fused with the concept that her medications were making her sick and the idea that she may have BD. The treatment team thought these fused thoughts were the major problem that resulted in 10 months of protracted illness.
Conversely, in a “defused” state, patients can separate from their thoughts and observe them as disparate sounds, words, stories, or bits of language. The goal is to observe and allow the patient’s thoughts to simply be thoughts rather than trying to determine if they are “true.” Mrs. C was fused with the idea that her medications were making her ill, so this belief became the story underlying her obsessional thinking. Helping her disengage from this story became the focus of her treatment.
Table 3
6 core principles of acceptance and commitment therapy
| Defusion | Learning to step back and observe thoughts as separate from the self |
| Acceptance | Allowing unpleasant thoughts to come and go without trying to control them |
| Contact with the present moment | Full awareness and engagement with present experiences |
| Observing the present self | Accessing a transcendent sense of self |
| Values | Clarifying what is most important to the patient |
| Committed action | Setting goals and taking action to achieve them |
| Source: Reference 4 | |
Results guide pharmacotherapy
In addition to helping change the focus of Mrs. C’s psychotherapy, we used the pharmacogenomic results to guide medication treatment. We initially prescribed fluvoxamine, 50 mg/d, because her partially compromised CYP2D6 pathway probably would play only a minor role in metabolizing the drug.1 Smoking induces CYP1A2, which is fluvoxamine’s primary metabolic pathway; however, Mrs. C does not smoke.6 When we saw Mrs. C in January 2009, the author (JGW) was unaware of any available genetic testing for CYP1A2, although now such testing is clinically available.
Mirtazapine is in the “use as directed” category for Mrs. C’s genotype (Table 2) and was the only medication she had adhered to at a therapeutic dose for more than a few days. However, she indicated that she would not adhere to this medication if we prescribed it again. Duloxetine also is in the “use as directed” category; however, given the entire clinical picture, we chose fluvoxamine because of Mrs. C’s obsessive symptomatology and because she had never reached a therapeutic dose of a selective serotonin reuptake inhibitor.
OUTCOME: Obsessions abate
Given Mrs. C’s lack of insight, we initiate a family approach to help broach the topic of obsessions as the focus of treatment. With her husband’s help, she participates in defusion techniques as an inpatient and follows up with an acceptance-based psychotherapist after discharge. After we share the pharmacogenomic information with Mrs. C, she agrees to try fluvoxamine, which is titrated to 100 mg/d. She maintains this dose at her 4-week follow-up visit. Notably, this was only the second time Mrs. C adhered to a medication trial since illness onset. Upon admission, Mrs. C had an HRSD-17 score of 30, indicating severe depression; at 4 weeks, her HRSD-17 score is 8, indicating mild depression.
The authors’ observations
In a complementary case, the author (JGW) consulted on a patient who was taking paroxetine and experiencing anorgasmia, weight gain, and loss of libido. Pharmacogenomic testing revealed that the patient was a poor metabolizer of CYP2D6. Paroxetine is substantially metabolized by CYP2D6; therefore, it was likely that high blood levels were contributing to the side effects.3,7 The key clinical distinction is that although this patient was bothered by intrusive side effects, he was not fixated on them like Mrs. C. His pharmacogenomic test results were used to identify a metabolic issue that was causing the side effects. This is in contrast with Mrs. C, for whom the pharmacogenomic information ruled out a metabolic issue as the primary problem and helped guide the next step in treatment.
Mrs. C’s case illustrates how pharmacogenomics and ACT complemented each other to create a desirable outcome. Pharmacogenomic testing originally was developed as a safety mechanism for medication choice and dosing, but clinical applications have grown as astute clinicians utilize it to help care for their patients.8 ACT can be a powerful tool for patients who have difficulties creating distance from their thoughts. Both pharmacogenomic testing and ACT are noninvasive interventions that can be implemented as part of a multi-faceted treatment approach.
Related Resources
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2011.
- Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
Drug Brand Names
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Desipramine • Norpramin
- Diazepam • Valium
- Doxepin • Adapin, Silenor
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Nortriptyline • Pamelor
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel, Oleptro
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors are employed by AssureRx Health, Inc., the provider of the pharmacogenomic testing used in this article.
1. Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
2. Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9(5):442-473.
3. Kircheiner J, Brøsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand. 2001;104(3):173-192.
4. Harris R. Embracing your demons: an overview of acceptance and commitment therapy. Psychotherapy in Australia. 2006;12(4):2-8.
5. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: an experiential approach to behavior change. New York, NY: Guilford Press; 2003.
6. Luvox CR [package insert] Palo Alto CA: Jazz Pharmaceuticals, Inc.; 2011.
7. Kaneda Y, Kawamura I, Fujii A, et al. Serotonin syndrome– ‘potential’ role of the CYP2D6 genetic polymorphism in Asians. Int J Neuropsychopharmacol. 2002;5(1):105-106.
8. Kung S, Li X. The clinical use of pharmacogenomic testing in treatment-resistant depression. Primary Psychiatry. 2010;17(5):46-51.
CASE: Medication sensitivity
Mrs. C, age 48, is admitted to a tertiary care inpatient mood disorder unit for evaluation of severe depression characterized by depressed mood, anhedonia, and insomnia. Her initial Hamilton Rating Scale for Depression 17-Item (HRSD-17) score is 30, indicating severe depression. Her medications are fluoxetine, 10 mg/d, and diazepam, 0.5 mg/d.
Mrs. C describes a 10-month history of depression and extreme anxiety in the context of several psychosocial stressors. Her father recently died and she is having difficulty with the demands of administering her father’s estate. She is intensely obsessive and focused on nihilistic themes, her diagnosis, somatic themes, and medications side effects. Her husband confirms our observations. No history or current symptoms of typical compulsions (eg, washing hands or checking doors) are elicited. She has limited insight into her obsessive tendencies.
Mrs. C had no psychiatric history before her depressive and obsessive symptoms developed 10 months ago. However, in the past 10 months, she has been hospitalized in a psychiatric facility twice. She also received a series of 8 electroconvulsive therapy treatments, but reported minimal improvement of her depressive symptoms. Mrs. C had a few cognitive-behavioral therapy (CBT) sessions with a psychotherapist, but she said they didn’t help much.
Mrs. C has substantial difficulty adhering to medications, even at subtherapeutic doses. She states she is “extremely sensitive” to all medications. Mrs. C says she develops dizziness, increased anxiety, insomnia, nausea, and other vague reactions whenever she attempts to increase her psychotropics to therapeutic doses. She took sertraline, 10 mg/d, for 4 days, but discontinued it because of unspecified side effects. She then received escitalopram, 2.5 mg/d, for 10 days, but again stopped it because of vague side effects. She was taking paroxetine, 10 mg/d, for 2 days, but experienced vomiting and discontinued the drug. She tried venlafaxine at a low dose and also discontinued it because of vomiting. Mrs. C stayed on mirtazapine, 22.5 mg/d, for 3 months, but stopped it because of lack of efficacy and she was unwilling to increase the dose. Other unsuccessful trials include citalopram and doxepin. Mrs. C is hesitant to try another medication or increase to therapeutic doses any of the previous medications.
The authors’ observations
Before initiating another treatment, the treatment team considered Mrs. C’s pervasive medication intolerance. Her enzymatic activity may be genetically compromised, which could lead to high blood levels of medications and significant side effects when she takes very low doses. Individual variations in response to psychotropics are influenced by genetic factors.1 Variants in the cytochrome P450 (CYP450) genes produce enzymes with increased activity, normal activity, reduced activity, or no activity, creating phenotypes of ultrarapid metabolizers, extensive metabolizers, intermediate metabolizers, and poor metabolizers, respectively. These genetic variations can affect blood levels of medications that employ these enzymes in their metabolic pathways.2 Mrs. C could be a poor metabolizer of common CYP450 variant enzymes, which led to her exquisite sensitivity to psychotropics. We felt this was a reasonable hypothesis given her tumultuous 10-month course of psychiatric treatment and multiple failed medication trials.
An alternative hypothesis is that Mrs. C’s somatic obsessions about drug side effects were the primary clinical issue that led to her severe medication intolerance. Mrs. C spends hours questioning the inpatient staff about her diagnosis (eg, “Are you sure I don’t have bipolar disorder?”), medications (eg, “Are you sure this medication won’t make me sick?”), somatic themes (eg, “Are you sure I don’t have Meniere’s disease with all my dizziness?”), and nihilistic themes (eg, “What if I never get better?”). Mrs. C’s husband attested that she has spent hours researching her new medications on the Internet and reading the medication handouts from the pharmacy. She admits to mentally cycling through the DSM-IV-TR criteria for hours at a time to “figure out” if she has bipolar disorder (BD).
We initiated pharmacogenomic testing to help distinguish between these hypotheses. Mrs. C’s results are presented in Table 1. Genotype results were applied using an interpretive algorithm (Figure) in which 26 psychiatric medications were placed in categories of “use as directed” (green column), “use with caution” (yellow column), and “use with caution or more frequent monitoring” (red column). The algorithm incorporates the genetic information with the known pharmacologic profile for each of the medications in the panel. Highlights of Mrs. C’s interpretive report are shown in Table 2.
Table 1
Mrs. C’s genotype results
| Gene | Allele | Predicted phenotype |
|---|---|---|
| CYP2D6 | *1/*4 | Intermediate metabolizer |
| CYP2C19 | *1/*1 | Extensive metabolizer |
| SLC6A4 | S/S | Low activity |
| 5HTR2A | G/G | Reduced activity |
Figure
Genotype-phenotype integration into Mrs. C’s interpretive report
Table 2
Mrs. C’s pharmacogenomic-based interpretive report
| Use as directed | Use with caution | Use with caution and more frequent monitoring |
|---|---|---|
| Antidepressants: Duloxetine, mirtazapine Antipsychotics: Clozapine, olanzapine, quetiapine, ziprasidone | Antidepressants: Amitriptyline,a,b bupropion,a citalopram,c clomipramine,a,b desipramine,a,b escitalopram,c fluoxetine,a fluvoxamine,c imipramine,a,b nortriptyline,a,b sertraline,c paroxetine,c trazodone,a venlafaxinea Antipsychotics: Aripiprazole,a haloperidol,a perphenazine,a risperidonea | None |
| aSerum level may be too high, lower doses may be required bSerum levels may be outside of optimal range cGenotype suggests less than optimal response | ||
The authors’ observations
Mrs. C’s genotype might explain some sensitivity to medications metabolized by CYP2D6 (eg, venlafaxine, paroxetine, fluoxetine), but does not explain her acute sensitivity to all of the medications she has taken. For example, she is an extensive metabolizer for CYP2C19, which metabolizes escitalopram; therefore, it is unlikely escitalopram, 2.5 mg/d, would result in high blood levels and side effects.3 Regardless of the next step in treatment, we deemed her somatic obsessions to be the most important clinical issue. It seems unlikely that Mrs. C would adhere to any medication regimen until this underlying problem was addressed.
The focus of treatment shifted to Mrs. C’s obsessions about her medications and their side effects. Mrs. C was fixated on the content of her obsessions (eg, medications, side effects) rather than the process of her obsessional thinking. The goal was to help Mrs. C identify, label, and ultimately create distance from her obsessive thoughts associated with side effects. The treatment team employed an acceptance and commitment therapy (ACT) model of observing and defusing thoughts in the inpatient setting (Table 3).4 ACT is based on mindfulness and committed, values-based action.5 When patients are “fused” with their thoughts, they believe these thoughts are important and representative of reality. In Mrs. C’s case, she fused with the concept that her medications were making her sick and the idea that she may have BD. The treatment team thought these fused thoughts were the major problem that resulted in 10 months of protracted illness.
Conversely, in a “defused” state, patients can separate from their thoughts and observe them as disparate sounds, words, stories, or bits of language. The goal is to observe and allow the patient’s thoughts to simply be thoughts rather than trying to determine if they are “true.” Mrs. C was fused with the idea that her medications were making her ill, so this belief became the story underlying her obsessional thinking. Helping her disengage from this story became the focus of her treatment.
Table 3
6 core principles of acceptance and commitment therapy
| Defusion | Learning to step back and observe thoughts as separate from the self |
| Acceptance | Allowing unpleasant thoughts to come and go without trying to control them |
| Contact with the present moment | Full awareness and engagement with present experiences |
| Observing the present self | Accessing a transcendent sense of self |
| Values | Clarifying what is most important to the patient |
| Committed action | Setting goals and taking action to achieve them |
| Source: Reference 4 | |
Results guide pharmacotherapy
In addition to helping change the focus of Mrs. C’s psychotherapy, we used the pharmacogenomic results to guide medication treatment. We initially prescribed fluvoxamine, 50 mg/d, because her partially compromised CYP2D6 pathway probably would play only a minor role in metabolizing the drug.1 Smoking induces CYP1A2, which is fluvoxamine’s primary metabolic pathway; however, Mrs. C does not smoke.6 When we saw Mrs. C in January 2009, the author (JGW) was unaware of any available genetic testing for CYP1A2, although now such testing is clinically available.
Mirtazapine is in the “use as directed” category for Mrs. C’s genotype (Table 2) and was the only medication she had adhered to at a therapeutic dose for more than a few days. However, she indicated that she would not adhere to this medication if we prescribed it again. Duloxetine also is in the “use as directed” category; however, given the entire clinical picture, we chose fluvoxamine because of Mrs. C’s obsessive symptomatology and because she had never reached a therapeutic dose of a selective serotonin reuptake inhibitor.
OUTCOME: Obsessions abate
Given Mrs. C’s lack of insight, we initiate a family approach to help broach the topic of obsessions as the focus of treatment. With her husband’s help, she participates in defusion techniques as an inpatient and follows up with an acceptance-based psychotherapist after discharge. After we share the pharmacogenomic information with Mrs. C, she agrees to try fluvoxamine, which is titrated to 100 mg/d. She maintains this dose at her 4-week follow-up visit. Notably, this was only the second time Mrs. C adhered to a medication trial since illness onset. Upon admission, Mrs. C had an HRSD-17 score of 30, indicating severe depression; at 4 weeks, her HRSD-17 score is 8, indicating mild depression.
The authors’ observations
In a complementary case, the author (JGW) consulted on a patient who was taking paroxetine and experiencing anorgasmia, weight gain, and loss of libido. Pharmacogenomic testing revealed that the patient was a poor metabolizer of CYP2D6. Paroxetine is substantially metabolized by CYP2D6; therefore, it was likely that high blood levels were contributing to the side effects.3,7 The key clinical distinction is that although this patient was bothered by intrusive side effects, he was not fixated on them like Mrs. C. His pharmacogenomic test results were used to identify a metabolic issue that was causing the side effects. This is in contrast with Mrs. C, for whom the pharmacogenomic information ruled out a metabolic issue as the primary problem and helped guide the next step in treatment.
Mrs. C’s case illustrates how pharmacogenomics and ACT complemented each other to create a desirable outcome. Pharmacogenomic testing originally was developed as a safety mechanism for medication choice and dosing, but clinical applications have grown as astute clinicians utilize it to help care for their patients.8 ACT can be a powerful tool for patients who have difficulties creating distance from their thoughts. Both pharmacogenomic testing and ACT are noninvasive interventions that can be implemented as part of a multi-faceted treatment approach.
Related Resources
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2011.
- Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
Drug Brand Names
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Desipramine • Norpramin
- Diazepam • Valium
- Doxepin • Adapin, Silenor
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Nortriptyline • Pamelor
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel, Oleptro
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors are employed by AssureRx Health, Inc., the provider of the pharmacogenomic testing used in this article.
CASE: Medication sensitivity
Mrs. C, age 48, is admitted to a tertiary care inpatient mood disorder unit for evaluation of severe depression characterized by depressed mood, anhedonia, and insomnia. Her initial Hamilton Rating Scale for Depression 17-Item (HRSD-17) score is 30, indicating severe depression. Her medications are fluoxetine, 10 mg/d, and diazepam, 0.5 mg/d.
Mrs. C describes a 10-month history of depression and extreme anxiety in the context of several psychosocial stressors. Her father recently died and she is having difficulty with the demands of administering her father’s estate. She is intensely obsessive and focused on nihilistic themes, her diagnosis, somatic themes, and medications side effects. Her husband confirms our observations. No history or current symptoms of typical compulsions (eg, washing hands or checking doors) are elicited. She has limited insight into her obsessive tendencies.
Mrs. C had no psychiatric history before her depressive and obsessive symptoms developed 10 months ago. However, in the past 10 months, she has been hospitalized in a psychiatric facility twice. She also received a series of 8 electroconvulsive therapy treatments, but reported minimal improvement of her depressive symptoms. Mrs. C had a few cognitive-behavioral therapy (CBT) sessions with a psychotherapist, but she said they didn’t help much.
Mrs. C has substantial difficulty adhering to medications, even at subtherapeutic doses. She states she is “extremely sensitive” to all medications. Mrs. C says she develops dizziness, increased anxiety, insomnia, nausea, and other vague reactions whenever she attempts to increase her psychotropics to therapeutic doses. She took sertraline, 10 mg/d, for 4 days, but discontinued it because of unspecified side effects. She then received escitalopram, 2.5 mg/d, for 10 days, but again stopped it because of vague side effects. She was taking paroxetine, 10 mg/d, for 2 days, but experienced vomiting and discontinued the drug. She tried venlafaxine at a low dose and also discontinued it because of vomiting. Mrs. C stayed on mirtazapine, 22.5 mg/d, for 3 months, but stopped it because of lack of efficacy and she was unwilling to increase the dose. Other unsuccessful trials include citalopram and doxepin. Mrs. C is hesitant to try another medication or increase to therapeutic doses any of the previous medications.
The authors’ observations
Before initiating another treatment, the treatment team considered Mrs. C’s pervasive medication intolerance. Her enzymatic activity may be genetically compromised, which could lead to high blood levels of medications and significant side effects when she takes very low doses. Individual variations in response to psychotropics are influenced by genetic factors.1 Variants in the cytochrome P450 (CYP450) genes produce enzymes with increased activity, normal activity, reduced activity, or no activity, creating phenotypes of ultrarapid metabolizers, extensive metabolizers, intermediate metabolizers, and poor metabolizers, respectively. These genetic variations can affect blood levels of medications that employ these enzymes in their metabolic pathways.2 Mrs. C could be a poor metabolizer of common CYP450 variant enzymes, which led to her exquisite sensitivity to psychotropics. We felt this was a reasonable hypothesis given her tumultuous 10-month course of psychiatric treatment and multiple failed medication trials.
An alternative hypothesis is that Mrs. C’s somatic obsessions about drug side effects were the primary clinical issue that led to her severe medication intolerance. Mrs. C spends hours questioning the inpatient staff about her diagnosis (eg, “Are you sure I don’t have bipolar disorder?”), medications (eg, “Are you sure this medication won’t make me sick?”), somatic themes (eg, “Are you sure I don’t have Meniere’s disease with all my dizziness?”), and nihilistic themes (eg, “What if I never get better?”). Mrs. C’s husband attested that she has spent hours researching her new medications on the Internet and reading the medication handouts from the pharmacy. She admits to mentally cycling through the DSM-IV-TR criteria for hours at a time to “figure out” if she has bipolar disorder (BD).
We initiated pharmacogenomic testing to help distinguish between these hypotheses. Mrs. C’s results are presented in Table 1. Genotype results were applied using an interpretive algorithm (Figure) in which 26 psychiatric medications were placed in categories of “use as directed” (green column), “use with caution” (yellow column), and “use with caution or more frequent monitoring” (red column). The algorithm incorporates the genetic information with the known pharmacologic profile for each of the medications in the panel. Highlights of Mrs. C’s interpretive report are shown in Table 2.
Table 1
Mrs. C’s genotype results
| Gene | Allele | Predicted phenotype |
|---|---|---|
| CYP2D6 | *1/*4 | Intermediate metabolizer |
| CYP2C19 | *1/*1 | Extensive metabolizer |
| SLC6A4 | S/S | Low activity |
| 5HTR2A | G/G | Reduced activity |
Figure
Genotype-phenotype integration into Mrs. C’s interpretive report
Table 2
Mrs. C’s pharmacogenomic-based interpretive report
| Use as directed | Use with caution | Use with caution and more frequent monitoring |
|---|---|---|
| Antidepressants: Duloxetine, mirtazapine Antipsychotics: Clozapine, olanzapine, quetiapine, ziprasidone | Antidepressants: Amitriptyline,a,b bupropion,a citalopram,c clomipramine,a,b desipramine,a,b escitalopram,c fluoxetine,a fluvoxamine,c imipramine,a,b nortriptyline,a,b sertraline,c paroxetine,c trazodone,a venlafaxinea Antipsychotics: Aripiprazole,a haloperidol,a perphenazine,a risperidonea | None |
| aSerum level may be too high, lower doses may be required bSerum levels may be outside of optimal range cGenotype suggests less than optimal response | ||
The authors’ observations
Mrs. C’s genotype might explain some sensitivity to medications metabolized by CYP2D6 (eg, venlafaxine, paroxetine, fluoxetine), but does not explain her acute sensitivity to all of the medications she has taken. For example, she is an extensive metabolizer for CYP2C19, which metabolizes escitalopram; therefore, it is unlikely escitalopram, 2.5 mg/d, would result in high blood levels and side effects.3 Regardless of the next step in treatment, we deemed her somatic obsessions to be the most important clinical issue. It seems unlikely that Mrs. C would adhere to any medication regimen until this underlying problem was addressed.
The focus of treatment shifted to Mrs. C’s obsessions about her medications and their side effects. Mrs. C was fixated on the content of her obsessions (eg, medications, side effects) rather than the process of her obsessional thinking. The goal was to help Mrs. C identify, label, and ultimately create distance from her obsessive thoughts associated with side effects. The treatment team employed an acceptance and commitment therapy (ACT) model of observing and defusing thoughts in the inpatient setting (Table 3).4 ACT is based on mindfulness and committed, values-based action.5 When patients are “fused” with their thoughts, they believe these thoughts are important and representative of reality. In Mrs. C’s case, she fused with the concept that her medications were making her sick and the idea that she may have BD. The treatment team thought these fused thoughts were the major problem that resulted in 10 months of protracted illness.
Conversely, in a “defused” state, patients can separate from their thoughts and observe them as disparate sounds, words, stories, or bits of language. The goal is to observe and allow the patient’s thoughts to simply be thoughts rather than trying to determine if they are “true.” Mrs. C was fused with the idea that her medications were making her ill, so this belief became the story underlying her obsessional thinking. Helping her disengage from this story became the focus of her treatment.
Table 3
6 core principles of acceptance and commitment therapy
| Defusion | Learning to step back and observe thoughts as separate from the self |
| Acceptance | Allowing unpleasant thoughts to come and go without trying to control them |
| Contact with the present moment | Full awareness and engagement with present experiences |
| Observing the present self | Accessing a transcendent sense of self |
| Values | Clarifying what is most important to the patient |
| Committed action | Setting goals and taking action to achieve them |
| Source: Reference 4 | |
Results guide pharmacotherapy
In addition to helping change the focus of Mrs. C’s psychotherapy, we used the pharmacogenomic results to guide medication treatment. We initially prescribed fluvoxamine, 50 mg/d, because her partially compromised CYP2D6 pathway probably would play only a minor role in metabolizing the drug.1 Smoking induces CYP1A2, which is fluvoxamine’s primary metabolic pathway; however, Mrs. C does not smoke.6 When we saw Mrs. C in January 2009, the author (JGW) was unaware of any available genetic testing for CYP1A2, although now such testing is clinically available.
Mirtazapine is in the “use as directed” category for Mrs. C’s genotype (Table 2) and was the only medication she had adhered to at a therapeutic dose for more than a few days. However, she indicated that she would not adhere to this medication if we prescribed it again. Duloxetine also is in the “use as directed” category; however, given the entire clinical picture, we chose fluvoxamine because of Mrs. C’s obsessive symptomatology and because she had never reached a therapeutic dose of a selective serotonin reuptake inhibitor.
OUTCOME: Obsessions abate
Given Mrs. C’s lack of insight, we initiate a family approach to help broach the topic of obsessions as the focus of treatment. With her husband’s help, she participates in defusion techniques as an inpatient and follows up with an acceptance-based psychotherapist after discharge. After we share the pharmacogenomic information with Mrs. C, she agrees to try fluvoxamine, which is titrated to 100 mg/d. She maintains this dose at her 4-week follow-up visit. Notably, this was only the second time Mrs. C adhered to a medication trial since illness onset. Upon admission, Mrs. C had an HRSD-17 score of 30, indicating severe depression; at 4 weeks, her HRSD-17 score is 8, indicating mild depression.
The authors’ observations
In a complementary case, the author (JGW) consulted on a patient who was taking paroxetine and experiencing anorgasmia, weight gain, and loss of libido. Pharmacogenomic testing revealed that the patient was a poor metabolizer of CYP2D6. Paroxetine is substantially metabolized by CYP2D6; therefore, it was likely that high blood levels were contributing to the side effects.3,7 The key clinical distinction is that although this patient was bothered by intrusive side effects, he was not fixated on them like Mrs. C. His pharmacogenomic test results were used to identify a metabolic issue that was causing the side effects. This is in contrast with Mrs. C, for whom the pharmacogenomic information ruled out a metabolic issue as the primary problem and helped guide the next step in treatment.
Mrs. C’s case illustrates how pharmacogenomics and ACT complemented each other to create a desirable outcome. Pharmacogenomic testing originally was developed as a safety mechanism for medication choice and dosing, but clinical applications have grown as astute clinicians utilize it to help care for their patients.8 ACT can be a powerful tool for patients who have difficulties creating distance from their thoughts. Both pharmacogenomic testing and ACT are noninvasive interventions that can be implemented as part of a multi-faceted treatment approach.
Related Resources
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2011.
- Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
Drug Brand Names
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Desipramine • Norpramin
- Diazepam • Valium
- Doxepin • Adapin, Silenor
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Nortriptyline • Pamelor
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel, Oleptro
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors are employed by AssureRx Health, Inc., the provider of the pharmacogenomic testing used in this article.
1. Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
2. Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9(5):442-473.
3. Kircheiner J, Brøsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand. 2001;104(3):173-192.
4. Harris R. Embracing your demons: an overview of acceptance and commitment therapy. Psychotherapy in Australia. 2006;12(4):2-8.
5. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: an experiential approach to behavior change. New York, NY: Guilford Press; 2003.
6. Luvox CR [package insert] Palo Alto CA: Jazz Pharmaceuticals, Inc.; 2011.
7. Kaneda Y, Kawamura I, Fujii A, et al. Serotonin syndrome– ‘potential’ role of the CYP2D6 genetic polymorphism in Asians. Int J Neuropsychopharmacol. 2002;5(1):105-106.
8. Kung S, Li X. The clinical use of pharmacogenomic testing in treatment-resistant depression. Primary Psychiatry. 2010;17(5):46-51.
1. Mrazek DA. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010.
2. Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9(5):442-473.
3. Kircheiner J, Brøsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand. 2001;104(3):173-192.
4. Harris R. Embracing your demons: an overview of acceptance and commitment therapy. Psychotherapy in Australia. 2006;12(4):2-8.
5. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: an experiential approach to behavior change. New York, NY: Guilford Press; 2003.
6. Luvox CR [package insert] Palo Alto CA: Jazz Pharmaceuticals, Inc.; 2011.
7. Kaneda Y, Kawamura I, Fujii A, et al. Serotonin syndrome– ‘potential’ role of the CYP2D6 genetic polymorphism in Asians. Int J Neuropsychopharmacol. 2002;5(1):105-106.
8. Kung S, Li X. The clinical use of pharmacogenomic testing in treatment-resistant depression. Primary Psychiatry. 2010;17(5):46-51.
PTSD nightmares: Prazosin and atypical antipsychotics
• Prazosin is recommended as a first-line therapy for nighttime PTSD symptoms, such as nightmares or sleep disturbances—especially among veterans—because of superior long-term effectiveness.
• Risk of metabolic syndrome, which has been reported with low-dose atypical antipsychotics used for treating insomnia, limits their use for PTSD-related nightmares.
Mr. S, a 45-year-old veteran, was diagnosed with posttraumatic stress disorder (PTSD) 18 years ago after a tour of duty in the Persian Gulf. He had combat-related flashbacks triggered by the smell of gasoline or smoke from a fire, was easily startled, and began to isolate himself socially. However, his symptoms improved when he started volunteering at his local Veterans Affairs Medical Center. After he lost his job 3 years ago, Mr. S started experiencing flashbacks. He was irritable, easily startled, and avoided things that reminded him of his time in the Persian Gulf. His psychiatrist prescribed sertraline, titrated to 200 mg/d. The drug reduced the severity of his avoidance and hyperarousal symptoms and improved his mood.
During a clinic visit, Mr. S says he is doing well and can fall asleep at night but is having recurring nightmares about traumatic events that occurred during combat. These nightmares wake him up and have become more frequent, occurring once per night for the past month. Mr. S says he has been watching more news programs about conflicts in Afghanistan and Iraq since the nightmares began. His psychiatrist starts quetiapine, 50 mg at bedtime for 7 nights then 100 mg at bedtime, but after 6 weeks Mr. S says his nightmares continue.
PTSD occurs in approximately 19% of Vietnam war combat veterans1 and 14% of service members returning from Iraq and Afghanistan.2 PTSD symptoms are classified into clusters: intrusive/re-experiencing; avoidant/numbing; and hyperarousal.3 Nightmares are part of the intrusive/re-experiencing cluster, which is Criterion B in DSM-IV-TR. See Table 1 for a description of DSM-IV-TR PTSD criteria. Among PTSD patients, 50% to 70% report PTSD-associated nightmares.4 Despite adequate treatment targeted to improve PTSD’s core symptoms, symptoms such as sleep disturbances or nightmares often persist.
Table 1
DSM-IV-TR diagnostic criteria for posttraumatic stress disorder
|
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
Nightmares and other sleep disturbances are associated with significant distress and daytime impairment and can interfere with PTSD recovery4-8 by disrupting sleep-dependent processing of emotional experiences and causing repeated resensitization to trauma cues (Table 2).8
Table 2
Psychosocial consequences of sleep disruption in PTSD
| Increased reactivity to emotional cues |
| Compromised ability to function in social and occupational roles |
| Negative psychiatric outcomes, including suicidal ideation or worsening of depression or psychosis |
| Interference of natural recovery from trauma exposure |
| Repeated resensitization to trauma cues |
| Neurocognitive deficits |
| Neuroendocrine abnormalities |
| PTSD: posttraumatic stress disorder Source: Adapted from reference 8 |
Few randomized controlled medication trials specifically address PTSD-related nightmares. Most PTSD studies do not examine sleep outcomes as a primary measure, and comprehensive literature reviews could not offer evidence-based recommendations.9,10 The American Academy of Sleep Medicine (AASM) also noted a paucity of PTSD studies that identified nightmares as a primary outcome measure.11 See Table 3 for a list of recommended medication options for PTSD-associated nightmares.
Table 3
Recommended medication treatments for PTSD-associated nightmares
| Evidence level | Medication | Evidence |
|---|---|---|
| Recommended for treating PTSD-associated nightmares | ||
| 1, 4 | Prazosin | In 3 level 1 studies, adding prazosin (mean dose 3 mg/d) significantly decreased trauma-related nightmares according to the CAPS “recurrent distressing dreams” item after 3 to 9 weeks of treatment vs placebo in veteran and civilian patients (N = 57) |
| Not suggested for treating PTSD-associated nightmares | ||
| 1 | Venlafaxine | No difference between extended-release venlafaxine (37.5 to 300 mg/d) and placebo in the CAPS-SX17 “distressing dreams” item at 12 weeks in 340 PTSD patients |
| May be considered for treating PTSD-associated nightmares | ||
| 4 | Clonidine | Reduced the number of nightmares in 11 of 13 refugees for 2 weeks to 3 months (dose: 0.2 to 0.6 mg/d) |
| May be considered for treating PTSD-associated nightmares, but data are low grade and sparse | ||
| 4 | Trazodone | Although trazodone (25 to 600 mg) significantly decreased nightmare frequency in veteran patients during an 8-week hospital stay (N = 60), 19% discontinued therapy because of side effects |
| 4 | Olanzapine | Adjunctive olanzapine (10 to 20 mg) rapidly improved sleep in a case series of combat-related PTSD patients resistant to SSRIs and benzodiazepines (N = 5) |
| 4 | Risperidone | In case series, risperidone (0.5 to 3 mg) significantly decreased CAPS scores for recurrent distressing dreams and proportion of traumatic dreams documented in diaries of combat veterans over 6 weeks (N = 17), and improved nightmares in adult burn patients taking pain medications after 1 to 2 days (N = 10) |
| 4 | Aripiprazole | In a case series, aripiprazole (15 to 30 mg at bedtime) with CBT or sertraline significantly improved nightmares in 4 of 5 combat-related PTSD patients |
| 4 | Topiramate | Topiramate reduced nightmares in 79% of civilians with PTSD and fully suppressed nightmares in 50% of patients in a case series (N = 35) |
| 4 | Low-dose cortisol | Significant decrease in frequency but not intensity of nightmares with low-dose cortisol (10 mg/d) in civilians with PTSD (N = 3) |
| 4 | Fluvoxamine | In 2 case series, fluvoxamine (up to 300 mg/d) significantly decreased the IES-R level of “dreams about combat trauma” but not the SRRS “bad dreams” rating at 10 weeks (N = 21). During 4 to 12 weeks of follow-up there was a qualitative decrease in reported nightmares in veteran patients (n = 12) |
| 2 | Triazolam/nitrazepam | Limited data showed triazolam (0.5 mg) and nitrazepam (5 mg) provide equal efficacy in decreasing the number of patients who experience unpleasant dreams over 1 night |
| 4 | Phenelzine | One study showed phenelzine monotherapy (30 to 90 mg) resulted in elimination of nightmares within 1 month (N = 5); another reported “moderately reduced traumatic dreams” (N = 21) in veterans. Therapy was discontinued because of short-lived efficacy or plateau effect |
| 4 | Gabapentin | Adjunctive gabapentin (300 to 3,600 mg/d) improved insomnia and decreased nightmare frequency and/or intensity over 1 to 36 months in 30 veterans with PTSD |
| 4 | Cyproheptadine | Conflicting data ranges from eliminating nightmares to no changes in the presence or intensity of nightmares |
| 4 | TCAs | Among 10 Cambodian concentration camp survivors treated with TCAs, 4 reported their nightmares ceased and 4 reported improvement after 1-year follow-up |
| 4 | Nefazodone | Reduced nightmare occurrence in 3 open-label studies as monotherapy (386 to 600 mg/d). Not recommended first line because of hepatotoxicity risk |
| No recommendation because of sparse data | ||
| 2 | Clonazepam | Clonazepam (1 to 2 mg/d) was ineffective in decreasing frequency or intensity of combat-related PTSD nightmares in veterans (N = 6) |
Evidence levels:
| ||
| CAPS: Clinician-Administered PTSD Scale; CAPS-SX17: 17-item Clinician-Administered PTSD Scale; CBT: cognitive-behavioral therapy; IES-R: Impact of Event Scale-Revised; PTSD: posttraumatic stress disorder; SRRS: Stress Response Rating Scale; SSRI: selective serotonin reuptake inhibitor; TCAs: tricyclic antidepressants Source: Adapted from Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401 | ||
CASE CONTINUED: Medication change, improvement
After reviewing AASM’s treatment recommendations, we prescribe prazosin, 1 mg at bedtime for 7 nights, then increase by 1 mg at bedtime each week until Mr. S’s nightmares improve. He reports a substantial improvement in nightmare severity and frequency after a few weeks of treatment with prazosin, 5 mg at bedtime.
Prazosin
Prazosin is an α1-adrenergic receptor antagonist with good CNS penetrability. The rationale for reducing adrenergic activity to address intrusive PTSD symptoms has been well documented.12,13 In open-label trials,14-18 a chart review,19 and placebo-controlled trials,20-22prazosin reduced trauma nightmares and improved sleep quality and global clinical status more than placebo (Table 4). In these studies, prazosin doses ranged from 1 to 20 mg/d, with an average of 3 mg at bedtime and a starting dose of 1 mg. Prazosin is the only agent recommended in the AASM’s Best Practice Guide for treating PTSD-related nightmares.11
Table 4
RCTs of prazosin for trauma-related nightmares
| Study | Design | Patients | Results |
|---|---|---|---|
| Raskind et al, 200320 | 20-week, double-blind, placebo-controlled, crossover study (mean dose 9.5 mg/d at bedtime) | 10 Vietnam veterans with chronic PTSD and severe trauma-related nightmares | Prazosin was superior to placebo on scores on the recurrent distressing dreams item and difficulty falling/staying asleep item of the CAPS and change in PTSD severity and functional status on the CGI-C |
| Raskind et al, 200721 | 8-week, placebo-controlled, parallel study (mean dose 13.3 ± 3 mg/d in the evening) | 40 veterans with chronic PTSD, distressing trauma nightmares, and sleep disturbance | Prazosin was superior to placebo in reducing trauma nightmares and improving sleep quality and global clinical status; prazosin also shifted dream characteristics of trauma-related nightmares to those typical of normal dreams |
| Taylor et al, 200822 | 7-week, randomized, placebo-controlled, crossover trial (mean dose 3.1 ± 1.3 mg) | 13 outpatients with chronic civilian trauma PTSD, frequent nightmares, and sleep disturbance | Prazosin significantly increased total sleep time and REM sleep time; reduced trauma-related nightmares, distressed awakenings, and total PCL-C scores; improved CGI-I scores; and changed PDRS scores toward normal dreaming |
| CAPS: Clinician-Administered PTSD Scale; CGI-C: Clinical Global Impression of Change; CGI-I: Clinical Global Impression of Improvement; PCL-C: PTSD Checklist-Civilian; PDRS: PTSD Dream Rating Scale; PTSD: posttraumatic stress disorder; RCTs: randomized controlled trials; REM: rapid eye movement | |||
Atypical antipsychotics
Atypical antipsychotics have been used to reduce nightmares in PTSD; however, most of the evidence from studies evaluated in the AASM’s Best Practice Guide were considered to be low quality.11 Quetiapine and ziprasidone were not included in the AASM review. See (Table 5) for a review of the evidence for atypical antipsychotics for treating PTSD nightmares.
Table 5
Combat-related nightmares: Evidence for atypical antipsychotics
| Study | Design | Patients/dosage | Results |
|---|---|---|---|
| Aripiprazole | |||
| Lambert, 2006 a | Case report | 4 veterans with combat-related PTSD (3 male, 1 female; age 22 to 24); dose: 15 to 30 mg; concurrent treatment sertraline or CBT | Decreased frequency of weekly nightmares and agitated sleep by at least 50% |
| Olanzapine | |||
| Stein et al, 2002 b | 8-week, double-blind, placebo-controlled study | 19 male veterans with combat-related PTSD (olanzapine group mean age: 55.2 ± 6.6; placebo group 51.1 ± 8.1); mean dose: 15 mg/d | Significantly greater reduction in sleep disturbances (PSQI: -3.29 vs 1.57; P = .01); significantly higher weight gain (13.2 lbs vs -3 lbs; P = .001) |
| Jakovljevic et al, 2003 c | Case reports | 5 veterans with combat-related PTSD for 6 to 7 years (age: 28 to 50); dose: 10 to 20 mg; adjunct treatment | Decreased frequency of nightmares within 3 days |
| Labbate et al, 2000 d | Case report | 1 male veteran (age: 58) with a 20-year history of combat-related PTSD; dose: 5 mg at bedtime; concurrent treatment with sertraline (200 mg/d), bupropion (150 mg/d), and diazepam (15 mg/d) | Eliminated nightmares after 1 week and improved sleep quality |
| Quetiapine | |||
| Ahearn et al, 2006 e | 8-week, open-label trial | 15 PTSD patients (8 male; 7 female; 5 with combat-related PTSD; mean age: 49); mean dose: 216 mg/d (100 to 400 mg/d) | Significantly improved re-experiencing (CAPS: 10 vs 23; P = .0012) and sleep (PSQI: 17.5 vs 30; P = .0044) at 8 weeks compared with baseline |
| Robert et al, 2005 f | 6-week, open-label trial | 19 combat veterans; mean dose: 100 ± 70 mg/d (25 to 300 mg/d); adjunct treatment | Significantly improved sleep quality (PSQI: 1.67 vs 2.41; P = .006), latency (PSQI: 1.5 vs 2.65; P = .002), duration (PSQI: 1.31 vs 2.71; P < .001), and sleep disturbances (PSQI: 1.22 vs 1.71; P = .034) and decreased terror episodes (PSQI-A: 0.73 vs 0.91; P = .040) and acting out dreams (PSQI-A: 1.07 vs 1.35; P = .013); however, no difference in nightmares caused by trauma (PSQI-A: 1.53 vs 2.06) |
| Sokolski et al, 2003 g | Retrospective chart review | 68 male Vietnam War combat veterans (mean age: 55 ± 3.5); mean dose: 155 ± 130 mg (25 to 700 mg); adjunct treatment | Improved sleep disturbances in 62% and nightmares in 25% of patients |
| Ahearn et al, 2003 h | Case report | 2 male patients with combat-related PTSD (age 53, 72); dose: 25 to 50 mg; adjunct to SSRI therapy | Decreased frequency of nightmares with increased sleep duration |
| Risperidone | |||
| David et al, 2006 i | 6-week, open-label trial | 17 male veterans with combat-related PTSD (mean age: 53.7 ± 3.8); mean maximum dose: 2.3 ± 0.6 mg (range: 1 to 3 mg) | Improved recurrent distressing dreams (CAPS B-2: 3.8 vs 5.4; P = .04), but not with the PSQI subscale (PSQI bad dreams: 2.5 vs 2.7; NS). Decreased nighttime awakenings (1.9 vs 2.8; P = .003) and trauma dreams (19% vs 38%; P = .04) |
| Leyba et al, 1998 j | Case reports | 3 male patients (age 43 to 46); dose: 1 to 3 mg; adjunct therapy | Decreased occurrence of nightmares |
| Ziprasidone | |||
| Siddiqui et al, 2005 k | Case report | 1 male veteran with chronic combat-related PTSD (age 55); dose: 80 to 120 mg/d; adjunct with trazodone (100 mg) and topiramate | Improved occurrence of nightmares up to 4 months |
CAPS: Clinician-Administered PTSD Scale; CAPS B-2: Clinician-Administered PTSD Scale B-2 (recurrent distressing dreams of the event); CBT: cognitive-behavioral therapy; PSQI: Pittsburgh Sleep Quality Index; PSQI-A: Pittsburgh Sleep Quality Index Addendum for PTSD; NS: not significant; PTSD: posttraumatic stress disorder; SSRI: selective serotonin reuptake inhibitor References
| |||
Comparing prazosin and quetiapine. A historical prospective cohort study of 237 veterans with PTSD receiving prazosin or quetiapine for nighttime PTSD symptoms demonstrated that although the 2 drugs have similar efficacy (defined as symptomatic improvement) for short-term, 6-month treatment (61% vs 62%; P=.54), a higher percentage of patients continued prazosin long-term (3 to 6 years) than those taking quetiapine (48% vs 24%; P < .001).23 Twenty-five percent of patients taking quetiapine switched to prazosin during the study, and approximately one-half of these patients remained on prazosin until the study’s end. Only 8% of prazosin patients switched to quetiapine, and none continued this therapy until study end.23 Patients in the quetiapine group were more likely to discontinue the drug because of lack of efficacy (13% vs 3%; P=.03) and adverse effects (35% vs 18%; P=.008), specifically sedation (21% vs 2%; P < .001) and metabolic effects (9% vs 0%; P=.014), compared with prazosin. Although this trial may be the only published comparison study of prazosin and quetiapine, its methodological quality has been questioned, which makes it difficult to draw definitive conclusions.
Metabolic syndrome—elevated diastolic blood pressure, increased waist circumference, and low high-density lipoprotein cholesterol—is common among PTSD patients treated with antipsychotics.24 This syndrome may be caused by medications, lifestyle factors, or long-term overactivation of stress-response pathways. A retrospective chart review at a community mental health center revealed that patients taking even low doses of quetiapine for insomnia gained an average of 5 lbs (P=.037).25 Another retrospective chart review at 2 military hospitals reported that patients receiving low-dose quetiapine (≤100 mg/d) gained an average of slightly less than 1 lb per month, which adds up to approximately 10 lbs per year (P < .001).26 The benefit of using atypical antipsychotics may be outweighed by metabolic risks such as obesity, new-onset diabetes, and dyslipidemia.27
Prazosin is considered a first-line treatment for sleep disturbances and nightmares in PTSD because of its superior long-term efficacy and decreased adverse effects compared with quetiapine.
Related Resources
- American Psychiatric Association. Practice guidelines for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Arlington, VA: American Psychiatric Publishing, Inc.; 2004.
- Veterans Affairs/Department of Defense clinical practice guidelines. Management of traumatic stress disorder and acute stress reaction. www.healthquality.va.gov/Post_Traumatic_Stress_Disorder_PTSD.asp.
Drug Brand Names
- Prazosin • Minipress
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dohrenwend BP, Turner JB, Turse NA, et al. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
2. Tanielian T, Jaycox L. eds. Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. Santa Monica, CA: RAND Corporation; 2008.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. Wittmann L, Schredl M, Kramer M. Dreaming in posttraumatic stress disorder: a critical review of phenomenology psychophysiology and treatment. Psychother Psychosom. 2007;76(1):25-39.
5. Clum GA, Nishith P, Resick PA. Trauma-related sleep disturbance and self-reported physical health symptoms in treatment-seeking female rape victims. J Nerv Ment Dis. 2001;189(9):618-622.
6. Kramer TL, Booth BM, Han X, et al. Service utilization and outcomes in medically ill veterans with posttraumatic stress and depressive disorders. J Trauma Stress. 2003;16(3):211-219.
7. Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929-933.
8. Nappi CM, Drummond SP, Hall JM. Treating nightmares and insomnia in posttraumatic stress disorder: a review of current evidence. Neuropharmacology. 2012;62(2):576-585.
9. Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
10. van Liempt S, Vermetten E, Geuze E, et al. Pharmacotherapy for disordered sleep in post-traumatic stress disorder: a systematic review. Int Clin Psychopharmacol. 2006;21(4):193-202.
11. Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
12. Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract. 2007;13(2):72-78.
13. Strawn JR, Geracioti TD, Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260-271.
14. Calohan J, Peterson K, Peskind ER, et al. Prazosin treatment of trauma nightmares and sleep disturbance in soldiers deployed in Iraq. J Trauma Stress. 2010;23(5):645-648.
15. Daly CM, Doyle ME, Radkind M, et al. Clinical case series: the use of Prazosin for combat-related recurrent nightmares among Operation Iraqi Freedom combat veterans. Mil Med. 2005;170(6):513-515.
16. Peskind ER, Bonner LT, Hoff DJ, et al. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16(3):165-171.
17. Raskind MA, Dobie DJ, Kanter ED, et al. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
18. Taylor F, Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol. 2002;22(1):82-85.
19. Raskind MA, Thompson C, Petrie EC, et al. Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder. J Clin Psychiatry. 2002;63(7):565-568.
20. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
21. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
22. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
23. Byers MG, Allison KM, Wendel CS, et al. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J Clin Psychopharmacol. 2010;30(3):225-229.
24. Jin H, Lanouette NM, Mudaliar S, et al. Association of posttraumatic stress disorder with increased prevalence of metabolic syndrome. J Clin Psychopharmacol. 2009;29(3):210-215.
25. Cates ME, Jackson CW, Feldman JM, et al. Metabolic consequences of using low-dose quetiapine for insomnia in psychiatric patients. Community Ment Health J. 2009;45(4):251-254.
26. Williams SG, Alinejad NA, Williams JA, et al. Statistically significant increase in weight caused by low-dose quetiapine. Pharmacotherapy. 2010;30(10):1011-1015.
27. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267-272.
• Prazosin is recommended as a first-line therapy for nighttime PTSD symptoms, such as nightmares or sleep disturbances—especially among veterans—because of superior long-term effectiveness.
• Risk of metabolic syndrome, which has been reported with low-dose atypical antipsychotics used for treating insomnia, limits their use for PTSD-related nightmares.
Mr. S, a 45-year-old veteran, was diagnosed with posttraumatic stress disorder (PTSD) 18 years ago after a tour of duty in the Persian Gulf. He had combat-related flashbacks triggered by the smell of gasoline or smoke from a fire, was easily startled, and began to isolate himself socially. However, his symptoms improved when he started volunteering at his local Veterans Affairs Medical Center. After he lost his job 3 years ago, Mr. S started experiencing flashbacks. He was irritable, easily startled, and avoided things that reminded him of his time in the Persian Gulf. His psychiatrist prescribed sertraline, titrated to 200 mg/d. The drug reduced the severity of his avoidance and hyperarousal symptoms and improved his mood.
During a clinic visit, Mr. S says he is doing well and can fall asleep at night but is having recurring nightmares about traumatic events that occurred during combat. These nightmares wake him up and have become more frequent, occurring once per night for the past month. Mr. S says he has been watching more news programs about conflicts in Afghanistan and Iraq since the nightmares began. His psychiatrist starts quetiapine, 50 mg at bedtime for 7 nights then 100 mg at bedtime, but after 6 weeks Mr. S says his nightmares continue.
PTSD occurs in approximately 19% of Vietnam war combat veterans1 and 14% of service members returning from Iraq and Afghanistan.2 PTSD symptoms are classified into clusters: intrusive/re-experiencing; avoidant/numbing; and hyperarousal.3 Nightmares are part of the intrusive/re-experiencing cluster, which is Criterion B in DSM-IV-TR. See Table 1 for a description of DSM-IV-TR PTSD criteria. Among PTSD patients, 50% to 70% report PTSD-associated nightmares.4 Despite adequate treatment targeted to improve PTSD’s core symptoms, symptoms such as sleep disturbances or nightmares often persist.
Table 1
DSM-IV-TR diagnostic criteria for posttraumatic stress disorder
|
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
Nightmares and other sleep disturbances are associated with significant distress and daytime impairment and can interfere with PTSD recovery4-8 by disrupting sleep-dependent processing of emotional experiences and causing repeated resensitization to trauma cues (Table 2).8
Table 2
Psychosocial consequences of sleep disruption in PTSD
| Increased reactivity to emotional cues |
| Compromised ability to function in social and occupational roles |
| Negative psychiatric outcomes, including suicidal ideation or worsening of depression or psychosis |
| Interference of natural recovery from trauma exposure |
| Repeated resensitization to trauma cues |
| Neurocognitive deficits |
| Neuroendocrine abnormalities |
| PTSD: posttraumatic stress disorder Source: Adapted from reference 8 |
Few randomized controlled medication trials specifically address PTSD-related nightmares. Most PTSD studies do not examine sleep outcomes as a primary measure, and comprehensive literature reviews could not offer evidence-based recommendations.9,10 The American Academy of Sleep Medicine (AASM) also noted a paucity of PTSD studies that identified nightmares as a primary outcome measure.11 See Table 3 for a list of recommended medication options for PTSD-associated nightmares.
Table 3
Recommended medication treatments for PTSD-associated nightmares
| Evidence level | Medication | Evidence |
|---|---|---|
| Recommended for treating PTSD-associated nightmares | ||
| 1, 4 | Prazosin | In 3 level 1 studies, adding prazosin (mean dose 3 mg/d) significantly decreased trauma-related nightmares according to the CAPS “recurrent distressing dreams” item after 3 to 9 weeks of treatment vs placebo in veteran and civilian patients (N = 57) |
| Not suggested for treating PTSD-associated nightmares | ||
| 1 | Venlafaxine | No difference between extended-release venlafaxine (37.5 to 300 mg/d) and placebo in the CAPS-SX17 “distressing dreams” item at 12 weeks in 340 PTSD patients |
| May be considered for treating PTSD-associated nightmares | ||
| 4 | Clonidine | Reduced the number of nightmares in 11 of 13 refugees for 2 weeks to 3 months (dose: 0.2 to 0.6 mg/d) |
| May be considered for treating PTSD-associated nightmares, but data are low grade and sparse | ||
| 4 | Trazodone | Although trazodone (25 to 600 mg) significantly decreased nightmare frequency in veteran patients during an 8-week hospital stay (N = 60), 19% discontinued therapy because of side effects |
| 4 | Olanzapine | Adjunctive olanzapine (10 to 20 mg) rapidly improved sleep in a case series of combat-related PTSD patients resistant to SSRIs and benzodiazepines (N = 5) |
| 4 | Risperidone | In case series, risperidone (0.5 to 3 mg) significantly decreased CAPS scores for recurrent distressing dreams and proportion of traumatic dreams documented in diaries of combat veterans over 6 weeks (N = 17), and improved nightmares in adult burn patients taking pain medications after 1 to 2 days (N = 10) |
| 4 | Aripiprazole | In a case series, aripiprazole (15 to 30 mg at bedtime) with CBT or sertraline significantly improved nightmares in 4 of 5 combat-related PTSD patients |
| 4 | Topiramate | Topiramate reduced nightmares in 79% of civilians with PTSD and fully suppressed nightmares in 50% of patients in a case series (N = 35) |
| 4 | Low-dose cortisol | Significant decrease in frequency but not intensity of nightmares with low-dose cortisol (10 mg/d) in civilians with PTSD (N = 3) |
| 4 | Fluvoxamine | In 2 case series, fluvoxamine (up to 300 mg/d) significantly decreased the IES-R level of “dreams about combat trauma” but not the SRRS “bad dreams” rating at 10 weeks (N = 21). During 4 to 12 weeks of follow-up there was a qualitative decrease in reported nightmares in veteran patients (n = 12) |
| 2 | Triazolam/nitrazepam | Limited data showed triazolam (0.5 mg) and nitrazepam (5 mg) provide equal efficacy in decreasing the number of patients who experience unpleasant dreams over 1 night |
| 4 | Phenelzine | One study showed phenelzine monotherapy (30 to 90 mg) resulted in elimination of nightmares within 1 month (N = 5); another reported “moderately reduced traumatic dreams” (N = 21) in veterans. Therapy was discontinued because of short-lived efficacy or plateau effect |
| 4 | Gabapentin | Adjunctive gabapentin (300 to 3,600 mg/d) improved insomnia and decreased nightmare frequency and/or intensity over 1 to 36 months in 30 veterans with PTSD |
| 4 | Cyproheptadine | Conflicting data ranges from eliminating nightmares to no changes in the presence or intensity of nightmares |
| 4 | TCAs | Among 10 Cambodian concentration camp survivors treated with TCAs, 4 reported their nightmares ceased and 4 reported improvement after 1-year follow-up |
| 4 | Nefazodone | Reduced nightmare occurrence in 3 open-label studies as monotherapy (386 to 600 mg/d). Not recommended first line because of hepatotoxicity risk |
| No recommendation because of sparse data | ||
| 2 | Clonazepam | Clonazepam (1 to 2 mg/d) was ineffective in decreasing frequency or intensity of combat-related PTSD nightmares in veterans (N = 6) |
Evidence levels:
| ||
| CAPS: Clinician-Administered PTSD Scale; CAPS-SX17: 17-item Clinician-Administered PTSD Scale; CBT: cognitive-behavioral therapy; IES-R: Impact of Event Scale-Revised; PTSD: posttraumatic stress disorder; SRRS: Stress Response Rating Scale; SSRI: selective serotonin reuptake inhibitor; TCAs: tricyclic antidepressants Source: Adapted from Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401 | ||
CASE CONTINUED: Medication change, improvement
After reviewing AASM’s treatment recommendations, we prescribe prazosin, 1 mg at bedtime for 7 nights, then increase by 1 mg at bedtime each week until Mr. S’s nightmares improve. He reports a substantial improvement in nightmare severity and frequency after a few weeks of treatment with prazosin, 5 mg at bedtime.
Prazosin
Prazosin is an α1-adrenergic receptor antagonist with good CNS penetrability. The rationale for reducing adrenergic activity to address intrusive PTSD symptoms has been well documented.12,13 In open-label trials,14-18 a chart review,19 and placebo-controlled trials,20-22prazosin reduced trauma nightmares and improved sleep quality and global clinical status more than placebo (Table 4). In these studies, prazosin doses ranged from 1 to 20 mg/d, with an average of 3 mg at bedtime and a starting dose of 1 mg. Prazosin is the only agent recommended in the AASM’s Best Practice Guide for treating PTSD-related nightmares.11
Table 4
RCTs of prazosin for trauma-related nightmares
| Study | Design | Patients | Results |
|---|---|---|---|
| Raskind et al, 200320 | 20-week, double-blind, placebo-controlled, crossover study (mean dose 9.5 mg/d at bedtime) | 10 Vietnam veterans with chronic PTSD and severe trauma-related nightmares | Prazosin was superior to placebo on scores on the recurrent distressing dreams item and difficulty falling/staying asleep item of the CAPS and change in PTSD severity and functional status on the CGI-C |
| Raskind et al, 200721 | 8-week, placebo-controlled, parallel study (mean dose 13.3 ± 3 mg/d in the evening) | 40 veterans with chronic PTSD, distressing trauma nightmares, and sleep disturbance | Prazosin was superior to placebo in reducing trauma nightmares and improving sleep quality and global clinical status; prazosin also shifted dream characteristics of trauma-related nightmares to those typical of normal dreams |
| Taylor et al, 200822 | 7-week, randomized, placebo-controlled, crossover trial (mean dose 3.1 ± 1.3 mg) | 13 outpatients with chronic civilian trauma PTSD, frequent nightmares, and sleep disturbance | Prazosin significantly increased total sleep time and REM sleep time; reduced trauma-related nightmares, distressed awakenings, and total PCL-C scores; improved CGI-I scores; and changed PDRS scores toward normal dreaming |
| CAPS: Clinician-Administered PTSD Scale; CGI-C: Clinical Global Impression of Change; CGI-I: Clinical Global Impression of Improvement; PCL-C: PTSD Checklist-Civilian; PDRS: PTSD Dream Rating Scale; PTSD: posttraumatic stress disorder; RCTs: randomized controlled trials; REM: rapid eye movement | |||
Atypical antipsychotics
Atypical antipsychotics have been used to reduce nightmares in PTSD; however, most of the evidence from studies evaluated in the AASM’s Best Practice Guide were considered to be low quality.11 Quetiapine and ziprasidone were not included in the AASM review. See (Table 5) for a review of the evidence for atypical antipsychotics for treating PTSD nightmares.
Table 5
Combat-related nightmares: Evidence for atypical antipsychotics
| Study | Design | Patients/dosage | Results |
|---|---|---|---|
| Aripiprazole | |||
| Lambert, 2006 a | Case report | 4 veterans with combat-related PTSD (3 male, 1 female; age 22 to 24); dose: 15 to 30 mg; concurrent treatment sertraline or CBT | Decreased frequency of weekly nightmares and agitated sleep by at least 50% |
| Olanzapine | |||
| Stein et al, 2002 b | 8-week, double-blind, placebo-controlled study | 19 male veterans with combat-related PTSD (olanzapine group mean age: 55.2 ± 6.6; placebo group 51.1 ± 8.1); mean dose: 15 mg/d | Significantly greater reduction in sleep disturbances (PSQI: -3.29 vs 1.57; P = .01); significantly higher weight gain (13.2 lbs vs -3 lbs; P = .001) |
| Jakovljevic et al, 2003 c | Case reports | 5 veterans with combat-related PTSD for 6 to 7 years (age: 28 to 50); dose: 10 to 20 mg; adjunct treatment | Decreased frequency of nightmares within 3 days |
| Labbate et al, 2000 d | Case report | 1 male veteran (age: 58) with a 20-year history of combat-related PTSD; dose: 5 mg at bedtime; concurrent treatment with sertraline (200 mg/d), bupropion (150 mg/d), and diazepam (15 mg/d) | Eliminated nightmares after 1 week and improved sleep quality |
| Quetiapine | |||
| Ahearn et al, 2006 e | 8-week, open-label trial | 15 PTSD patients (8 male; 7 female; 5 with combat-related PTSD; mean age: 49); mean dose: 216 mg/d (100 to 400 mg/d) | Significantly improved re-experiencing (CAPS: 10 vs 23; P = .0012) and sleep (PSQI: 17.5 vs 30; P = .0044) at 8 weeks compared with baseline |
| Robert et al, 2005 f | 6-week, open-label trial | 19 combat veterans; mean dose: 100 ± 70 mg/d (25 to 300 mg/d); adjunct treatment | Significantly improved sleep quality (PSQI: 1.67 vs 2.41; P = .006), latency (PSQI: 1.5 vs 2.65; P = .002), duration (PSQI: 1.31 vs 2.71; P < .001), and sleep disturbances (PSQI: 1.22 vs 1.71; P = .034) and decreased terror episodes (PSQI-A: 0.73 vs 0.91; P = .040) and acting out dreams (PSQI-A: 1.07 vs 1.35; P = .013); however, no difference in nightmares caused by trauma (PSQI-A: 1.53 vs 2.06) |
| Sokolski et al, 2003 g | Retrospective chart review | 68 male Vietnam War combat veterans (mean age: 55 ± 3.5); mean dose: 155 ± 130 mg (25 to 700 mg); adjunct treatment | Improved sleep disturbances in 62% and nightmares in 25% of patients |
| Ahearn et al, 2003 h | Case report | 2 male patients with combat-related PTSD (age 53, 72); dose: 25 to 50 mg; adjunct to SSRI therapy | Decreased frequency of nightmares with increased sleep duration |
| Risperidone | |||
| David et al, 2006 i | 6-week, open-label trial | 17 male veterans with combat-related PTSD (mean age: 53.7 ± 3.8); mean maximum dose: 2.3 ± 0.6 mg (range: 1 to 3 mg) | Improved recurrent distressing dreams (CAPS B-2: 3.8 vs 5.4; P = .04), but not with the PSQI subscale (PSQI bad dreams: 2.5 vs 2.7; NS). Decreased nighttime awakenings (1.9 vs 2.8; P = .003) and trauma dreams (19% vs 38%; P = .04) |
| Leyba et al, 1998 j | Case reports | 3 male patients (age 43 to 46); dose: 1 to 3 mg; adjunct therapy | Decreased occurrence of nightmares |
| Ziprasidone | |||
| Siddiqui et al, 2005 k | Case report | 1 male veteran with chronic combat-related PTSD (age 55); dose: 80 to 120 mg/d; adjunct with trazodone (100 mg) and topiramate | Improved occurrence of nightmares up to 4 months |
CAPS: Clinician-Administered PTSD Scale; CAPS B-2: Clinician-Administered PTSD Scale B-2 (recurrent distressing dreams of the event); CBT: cognitive-behavioral therapy; PSQI: Pittsburgh Sleep Quality Index; PSQI-A: Pittsburgh Sleep Quality Index Addendum for PTSD; NS: not significant; PTSD: posttraumatic stress disorder; SSRI: selective serotonin reuptake inhibitor References
| |||
Comparing prazosin and quetiapine. A historical prospective cohort study of 237 veterans with PTSD receiving prazosin or quetiapine for nighttime PTSD symptoms demonstrated that although the 2 drugs have similar efficacy (defined as symptomatic improvement) for short-term, 6-month treatment (61% vs 62%; P=.54), a higher percentage of patients continued prazosin long-term (3 to 6 years) than those taking quetiapine (48% vs 24%; P < .001).23 Twenty-five percent of patients taking quetiapine switched to prazosin during the study, and approximately one-half of these patients remained on prazosin until the study’s end. Only 8% of prazosin patients switched to quetiapine, and none continued this therapy until study end.23 Patients in the quetiapine group were more likely to discontinue the drug because of lack of efficacy (13% vs 3%; P=.03) and adverse effects (35% vs 18%; P=.008), specifically sedation (21% vs 2%; P < .001) and metabolic effects (9% vs 0%; P=.014), compared with prazosin. Although this trial may be the only published comparison study of prazosin and quetiapine, its methodological quality has been questioned, which makes it difficult to draw definitive conclusions.
Metabolic syndrome—elevated diastolic blood pressure, increased waist circumference, and low high-density lipoprotein cholesterol—is common among PTSD patients treated with antipsychotics.24 This syndrome may be caused by medications, lifestyle factors, or long-term overactivation of stress-response pathways. A retrospective chart review at a community mental health center revealed that patients taking even low doses of quetiapine for insomnia gained an average of 5 lbs (P=.037).25 Another retrospective chart review at 2 military hospitals reported that patients receiving low-dose quetiapine (≤100 mg/d) gained an average of slightly less than 1 lb per month, which adds up to approximately 10 lbs per year (P < .001).26 The benefit of using atypical antipsychotics may be outweighed by metabolic risks such as obesity, new-onset diabetes, and dyslipidemia.27
Prazosin is considered a first-line treatment for sleep disturbances and nightmares in PTSD because of its superior long-term efficacy and decreased adverse effects compared with quetiapine.
Related Resources
- American Psychiatric Association. Practice guidelines for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Arlington, VA: American Psychiatric Publishing, Inc.; 2004.
- Veterans Affairs/Department of Defense clinical practice guidelines. Management of traumatic stress disorder and acute stress reaction. www.healthquality.va.gov/Post_Traumatic_Stress_Disorder_PTSD.asp.
Drug Brand Names
- Prazosin • Minipress
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
• Prazosin is recommended as a first-line therapy for nighttime PTSD symptoms, such as nightmares or sleep disturbances—especially among veterans—because of superior long-term effectiveness.
• Risk of metabolic syndrome, which has been reported with low-dose atypical antipsychotics used for treating insomnia, limits their use for PTSD-related nightmares.
Mr. S, a 45-year-old veteran, was diagnosed with posttraumatic stress disorder (PTSD) 18 years ago after a tour of duty in the Persian Gulf. He had combat-related flashbacks triggered by the smell of gasoline or smoke from a fire, was easily startled, and began to isolate himself socially. However, his symptoms improved when he started volunteering at his local Veterans Affairs Medical Center. After he lost his job 3 years ago, Mr. S started experiencing flashbacks. He was irritable, easily startled, and avoided things that reminded him of his time in the Persian Gulf. His psychiatrist prescribed sertraline, titrated to 200 mg/d. The drug reduced the severity of his avoidance and hyperarousal symptoms and improved his mood.
During a clinic visit, Mr. S says he is doing well and can fall asleep at night but is having recurring nightmares about traumatic events that occurred during combat. These nightmares wake him up and have become more frequent, occurring once per night for the past month. Mr. S says he has been watching more news programs about conflicts in Afghanistan and Iraq since the nightmares began. His psychiatrist starts quetiapine, 50 mg at bedtime for 7 nights then 100 mg at bedtime, but after 6 weeks Mr. S says his nightmares continue.
PTSD occurs in approximately 19% of Vietnam war combat veterans1 and 14% of service members returning from Iraq and Afghanistan.2 PTSD symptoms are classified into clusters: intrusive/re-experiencing; avoidant/numbing; and hyperarousal.3 Nightmares are part of the intrusive/re-experiencing cluster, which is Criterion B in DSM-IV-TR. See Table 1 for a description of DSM-IV-TR PTSD criteria. Among PTSD patients, 50% to 70% report PTSD-associated nightmares.4 Despite adequate treatment targeted to improve PTSD’s core symptoms, symptoms such as sleep disturbances or nightmares often persist.
Table 1
DSM-IV-TR diagnostic criteria for posttraumatic stress disorder
|
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
Nightmares and other sleep disturbances are associated with significant distress and daytime impairment and can interfere with PTSD recovery4-8 by disrupting sleep-dependent processing of emotional experiences and causing repeated resensitization to trauma cues (Table 2).8
Table 2
Psychosocial consequences of sleep disruption in PTSD
| Increased reactivity to emotional cues |
| Compromised ability to function in social and occupational roles |
| Negative psychiatric outcomes, including suicidal ideation or worsening of depression or psychosis |
| Interference of natural recovery from trauma exposure |
| Repeated resensitization to trauma cues |
| Neurocognitive deficits |
| Neuroendocrine abnormalities |
| PTSD: posttraumatic stress disorder Source: Adapted from reference 8 |
Few randomized controlled medication trials specifically address PTSD-related nightmares. Most PTSD studies do not examine sleep outcomes as a primary measure, and comprehensive literature reviews could not offer evidence-based recommendations.9,10 The American Academy of Sleep Medicine (AASM) also noted a paucity of PTSD studies that identified nightmares as a primary outcome measure.11 See Table 3 for a list of recommended medication options for PTSD-associated nightmares.
Table 3
Recommended medication treatments for PTSD-associated nightmares
| Evidence level | Medication | Evidence |
|---|---|---|
| Recommended for treating PTSD-associated nightmares | ||
| 1, 4 | Prazosin | In 3 level 1 studies, adding prazosin (mean dose 3 mg/d) significantly decreased trauma-related nightmares according to the CAPS “recurrent distressing dreams” item after 3 to 9 weeks of treatment vs placebo in veteran and civilian patients (N = 57) |
| Not suggested for treating PTSD-associated nightmares | ||
| 1 | Venlafaxine | No difference between extended-release venlafaxine (37.5 to 300 mg/d) and placebo in the CAPS-SX17 “distressing dreams” item at 12 weeks in 340 PTSD patients |
| May be considered for treating PTSD-associated nightmares | ||
| 4 | Clonidine | Reduced the number of nightmares in 11 of 13 refugees for 2 weeks to 3 months (dose: 0.2 to 0.6 mg/d) |
| May be considered for treating PTSD-associated nightmares, but data are low grade and sparse | ||
| 4 | Trazodone | Although trazodone (25 to 600 mg) significantly decreased nightmare frequency in veteran patients during an 8-week hospital stay (N = 60), 19% discontinued therapy because of side effects |
| 4 | Olanzapine | Adjunctive olanzapine (10 to 20 mg) rapidly improved sleep in a case series of combat-related PTSD patients resistant to SSRIs and benzodiazepines (N = 5) |
| 4 | Risperidone | In case series, risperidone (0.5 to 3 mg) significantly decreased CAPS scores for recurrent distressing dreams and proportion of traumatic dreams documented in diaries of combat veterans over 6 weeks (N = 17), and improved nightmares in adult burn patients taking pain medications after 1 to 2 days (N = 10) |
| 4 | Aripiprazole | In a case series, aripiprazole (15 to 30 mg at bedtime) with CBT or sertraline significantly improved nightmares in 4 of 5 combat-related PTSD patients |
| 4 | Topiramate | Topiramate reduced nightmares in 79% of civilians with PTSD and fully suppressed nightmares in 50% of patients in a case series (N = 35) |
| 4 | Low-dose cortisol | Significant decrease in frequency but not intensity of nightmares with low-dose cortisol (10 mg/d) in civilians with PTSD (N = 3) |
| 4 | Fluvoxamine | In 2 case series, fluvoxamine (up to 300 mg/d) significantly decreased the IES-R level of “dreams about combat trauma” but not the SRRS “bad dreams” rating at 10 weeks (N = 21). During 4 to 12 weeks of follow-up there was a qualitative decrease in reported nightmares in veteran patients (n = 12) |
| 2 | Triazolam/nitrazepam | Limited data showed triazolam (0.5 mg) and nitrazepam (5 mg) provide equal efficacy in decreasing the number of patients who experience unpleasant dreams over 1 night |
| 4 | Phenelzine | One study showed phenelzine monotherapy (30 to 90 mg) resulted in elimination of nightmares within 1 month (N = 5); another reported “moderately reduced traumatic dreams” (N = 21) in veterans. Therapy was discontinued because of short-lived efficacy or plateau effect |
| 4 | Gabapentin | Adjunctive gabapentin (300 to 3,600 mg/d) improved insomnia and decreased nightmare frequency and/or intensity over 1 to 36 months in 30 veterans with PTSD |
| 4 | Cyproheptadine | Conflicting data ranges from eliminating nightmares to no changes in the presence or intensity of nightmares |
| 4 | TCAs | Among 10 Cambodian concentration camp survivors treated with TCAs, 4 reported their nightmares ceased and 4 reported improvement after 1-year follow-up |
| 4 | Nefazodone | Reduced nightmare occurrence in 3 open-label studies as monotherapy (386 to 600 mg/d). Not recommended first line because of hepatotoxicity risk |
| No recommendation because of sparse data | ||
| 2 | Clonazepam | Clonazepam (1 to 2 mg/d) was ineffective in decreasing frequency or intensity of combat-related PTSD nightmares in veterans (N = 6) |
Evidence levels:
| ||
| CAPS: Clinician-Administered PTSD Scale; CAPS-SX17: 17-item Clinician-Administered PTSD Scale; CBT: cognitive-behavioral therapy; IES-R: Impact of Event Scale-Revised; PTSD: posttraumatic stress disorder; SRRS: Stress Response Rating Scale; SSRI: selective serotonin reuptake inhibitor; TCAs: tricyclic antidepressants Source: Adapted from Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401 | ||
CASE CONTINUED: Medication change, improvement
After reviewing AASM’s treatment recommendations, we prescribe prazosin, 1 mg at bedtime for 7 nights, then increase by 1 mg at bedtime each week until Mr. S’s nightmares improve. He reports a substantial improvement in nightmare severity and frequency after a few weeks of treatment with prazosin, 5 mg at bedtime.
Prazosin
Prazosin is an α1-adrenergic receptor antagonist with good CNS penetrability. The rationale for reducing adrenergic activity to address intrusive PTSD symptoms has been well documented.12,13 In open-label trials,14-18 a chart review,19 and placebo-controlled trials,20-22prazosin reduced trauma nightmares and improved sleep quality and global clinical status more than placebo (Table 4). In these studies, prazosin doses ranged from 1 to 20 mg/d, with an average of 3 mg at bedtime and a starting dose of 1 mg. Prazosin is the only agent recommended in the AASM’s Best Practice Guide for treating PTSD-related nightmares.11
Table 4
RCTs of prazosin for trauma-related nightmares
| Study | Design | Patients | Results |
|---|---|---|---|
| Raskind et al, 200320 | 20-week, double-blind, placebo-controlled, crossover study (mean dose 9.5 mg/d at bedtime) | 10 Vietnam veterans with chronic PTSD and severe trauma-related nightmares | Prazosin was superior to placebo on scores on the recurrent distressing dreams item and difficulty falling/staying asleep item of the CAPS and change in PTSD severity and functional status on the CGI-C |
| Raskind et al, 200721 | 8-week, placebo-controlled, parallel study (mean dose 13.3 ± 3 mg/d in the evening) | 40 veterans with chronic PTSD, distressing trauma nightmares, and sleep disturbance | Prazosin was superior to placebo in reducing trauma nightmares and improving sleep quality and global clinical status; prazosin also shifted dream characteristics of trauma-related nightmares to those typical of normal dreams |
| Taylor et al, 200822 | 7-week, randomized, placebo-controlled, crossover trial (mean dose 3.1 ± 1.3 mg) | 13 outpatients with chronic civilian trauma PTSD, frequent nightmares, and sleep disturbance | Prazosin significantly increased total sleep time and REM sleep time; reduced trauma-related nightmares, distressed awakenings, and total PCL-C scores; improved CGI-I scores; and changed PDRS scores toward normal dreaming |
| CAPS: Clinician-Administered PTSD Scale; CGI-C: Clinical Global Impression of Change; CGI-I: Clinical Global Impression of Improvement; PCL-C: PTSD Checklist-Civilian; PDRS: PTSD Dream Rating Scale; PTSD: posttraumatic stress disorder; RCTs: randomized controlled trials; REM: rapid eye movement | |||
Atypical antipsychotics
Atypical antipsychotics have been used to reduce nightmares in PTSD; however, most of the evidence from studies evaluated in the AASM’s Best Practice Guide were considered to be low quality.11 Quetiapine and ziprasidone were not included in the AASM review. See (Table 5) for a review of the evidence for atypical antipsychotics for treating PTSD nightmares.
Table 5
Combat-related nightmares: Evidence for atypical antipsychotics
| Study | Design | Patients/dosage | Results |
|---|---|---|---|
| Aripiprazole | |||
| Lambert, 2006 a | Case report | 4 veterans with combat-related PTSD (3 male, 1 female; age 22 to 24); dose: 15 to 30 mg; concurrent treatment sertraline or CBT | Decreased frequency of weekly nightmares and agitated sleep by at least 50% |
| Olanzapine | |||
| Stein et al, 2002 b | 8-week, double-blind, placebo-controlled study | 19 male veterans with combat-related PTSD (olanzapine group mean age: 55.2 ± 6.6; placebo group 51.1 ± 8.1); mean dose: 15 mg/d | Significantly greater reduction in sleep disturbances (PSQI: -3.29 vs 1.57; P = .01); significantly higher weight gain (13.2 lbs vs -3 lbs; P = .001) |
| Jakovljevic et al, 2003 c | Case reports | 5 veterans with combat-related PTSD for 6 to 7 years (age: 28 to 50); dose: 10 to 20 mg; adjunct treatment | Decreased frequency of nightmares within 3 days |
| Labbate et al, 2000 d | Case report | 1 male veteran (age: 58) with a 20-year history of combat-related PTSD; dose: 5 mg at bedtime; concurrent treatment with sertraline (200 mg/d), bupropion (150 mg/d), and diazepam (15 mg/d) | Eliminated nightmares after 1 week and improved sleep quality |
| Quetiapine | |||
| Ahearn et al, 2006 e | 8-week, open-label trial | 15 PTSD patients (8 male; 7 female; 5 with combat-related PTSD; mean age: 49); mean dose: 216 mg/d (100 to 400 mg/d) | Significantly improved re-experiencing (CAPS: 10 vs 23; P = .0012) and sleep (PSQI: 17.5 vs 30; P = .0044) at 8 weeks compared with baseline |
| Robert et al, 2005 f | 6-week, open-label trial | 19 combat veterans; mean dose: 100 ± 70 mg/d (25 to 300 mg/d); adjunct treatment | Significantly improved sleep quality (PSQI: 1.67 vs 2.41; P = .006), latency (PSQI: 1.5 vs 2.65; P = .002), duration (PSQI: 1.31 vs 2.71; P < .001), and sleep disturbances (PSQI: 1.22 vs 1.71; P = .034) and decreased terror episodes (PSQI-A: 0.73 vs 0.91; P = .040) and acting out dreams (PSQI-A: 1.07 vs 1.35; P = .013); however, no difference in nightmares caused by trauma (PSQI-A: 1.53 vs 2.06) |
| Sokolski et al, 2003 g | Retrospective chart review | 68 male Vietnam War combat veterans (mean age: 55 ± 3.5); mean dose: 155 ± 130 mg (25 to 700 mg); adjunct treatment | Improved sleep disturbances in 62% and nightmares in 25% of patients |
| Ahearn et al, 2003 h | Case report | 2 male patients with combat-related PTSD (age 53, 72); dose: 25 to 50 mg; adjunct to SSRI therapy | Decreased frequency of nightmares with increased sleep duration |
| Risperidone | |||
| David et al, 2006 i | 6-week, open-label trial | 17 male veterans with combat-related PTSD (mean age: 53.7 ± 3.8); mean maximum dose: 2.3 ± 0.6 mg (range: 1 to 3 mg) | Improved recurrent distressing dreams (CAPS B-2: 3.8 vs 5.4; P = .04), but not with the PSQI subscale (PSQI bad dreams: 2.5 vs 2.7; NS). Decreased nighttime awakenings (1.9 vs 2.8; P = .003) and trauma dreams (19% vs 38%; P = .04) |
| Leyba et al, 1998 j | Case reports | 3 male patients (age 43 to 46); dose: 1 to 3 mg; adjunct therapy | Decreased occurrence of nightmares |
| Ziprasidone | |||
| Siddiqui et al, 2005 k | Case report | 1 male veteran with chronic combat-related PTSD (age 55); dose: 80 to 120 mg/d; adjunct with trazodone (100 mg) and topiramate | Improved occurrence of nightmares up to 4 months |
CAPS: Clinician-Administered PTSD Scale; CAPS B-2: Clinician-Administered PTSD Scale B-2 (recurrent distressing dreams of the event); CBT: cognitive-behavioral therapy; PSQI: Pittsburgh Sleep Quality Index; PSQI-A: Pittsburgh Sleep Quality Index Addendum for PTSD; NS: not significant; PTSD: posttraumatic stress disorder; SSRI: selective serotonin reuptake inhibitor References
| |||
Comparing prazosin and quetiapine. A historical prospective cohort study of 237 veterans with PTSD receiving prazosin or quetiapine for nighttime PTSD symptoms demonstrated that although the 2 drugs have similar efficacy (defined as symptomatic improvement) for short-term, 6-month treatment (61% vs 62%; P=.54), a higher percentage of patients continued prazosin long-term (3 to 6 years) than those taking quetiapine (48% vs 24%; P < .001).23 Twenty-five percent of patients taking quetiapine switched to prazosin during the study, and approximately one-half of these patients remained on prazosin until the study’s end. Only 8% of prazosin patients switched to quetiapine, and none continued this therapy until study end.23 Patients in the quetiapine group were more likely to discontinue the drug because of lack of efficacy (13% vs 3%; P=.03) and adverse effects (35% vs 18%; P=.008), specifically sedation (21% vs 2%; P < .001) and metabolic effects (9% vs 0%; P=.014), compared with prazosin. Although this trial may be the only published comparison study of prazosin and quetiapine, its methodological quality has been questioned, which makes it difficult to draw definitive conclusions.
Metabolic syndrome—elevated diastolic blood pressure, increased waist circumference, and low high-density lipoprotein cholesterol—is common among PTSD patients treated with antipsychotics.24 This syndrome may be caused by medications, lifestyle factors, or long-term overactivation of stress-response pathways. A retrospective chart review at a community mental health center revealed that patients taking even low doses of quetiapine for insomnia gained an average of 5 lbs (P=.037).25 Another retrospective chart review at 2 military hospitals reported that patients receiving low-dose quetiapine (≤100 mg/d) gained an average of slightly less than 1 lb per month, which adds up to approximately 10 lbs per year (P < .001).26 The benefit of using atypical antipsychotics may be outweighed by metabolic risks such as obesity, new-onset diabetes, and dyslipidemia.27
Prazosin is considered a first-line treatment for sleep disturbances and nightmares in PTSD because of its superior long-term efficacy and decreased adverse effects compared with quetiapine.
Related Resources
- American Psychiatric Association. Practice guidelines for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Arlington, VA: American Psychiatric Publishing, Inc.; 2004.
- Veterans Affairs/Department of Defense clinical practice guidelines. Management of traumatic stress disorder and acute stress reaction. www.healthquality.va.gov/Post_Traumatic_Stress_Disorder_PTSD.asp.
Drug Brand Names
- Prazosin • Minipress
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dohrenwend BP, Turner JB, Turse NA, et al. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
2. Tanielian T, Jaycox L. eds. Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. Santa Monica, CA: RAND Corporation; 2008.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. Wittmann L, Schredl M, Kramer M. Dreaming in posttraumatic stress disorder: a critical review of phenomenology psychophysiology and treatment. Psychother Psychosom. 2007;76(1):25-39.
5. Clum GA, Nishith P, Resick PA. Trauma-related sleep disturbance and self-reported physical health symptoms in treatment-seeking female rape victims. J Nerv Ment Dis. 2001;189(9):618-622.
6. Kramer TL, Booth BM, Han X, et al. Service utilization and outcomes in medically ill veterans with posttraumatic stress and depressive disorders. J Trauma Stress. 2003;16(3):211-219.
7. Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929-933.
8. Nappi CM, Drummond SP, Hall JM. Treating nightmares and insomnia in posttraumatic stress disorder: a review of current evidence. Neuropharmacology. 2012;62(2):576-585.
9. Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
10. van Liempt S, Vermetten E, Geuze E, et al. Pharmacotherapy for disordered sleep in post-traumatic stress disorder: a systematic review. Int Clin Psychopharmacol. 2006;21(4):193-202.
11. Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
12. Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract. 2007;13(2):72-78.
13. Strawn JR, Geracioti TD, Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260-271.
14. Calohan J, Peterson K, Peskind ER, et al. Prazosin treatment of trauma nightmares and sleep disturbance in soldiers deployed in Iraq. J Trauma Stress. 2010;23(5):645-648.
15. Daly CM, Doyle ME, Radkind M, et al. Clinical case series: the use of Prazosin for combat-related recurrent nightmares among Operation Iraqi Freedom combat veterans. Mil Med. 2005;170(6):513-515.
16. Peskind ER, Bonner LT, Hoff DJ, et al. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16(3):165-171.
17. Raskind MA, Dobie DJ, Kanter ED, et al. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
18. Taylor F, Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol. 2002;22(1):82-85.
19. Raskind MA, Thompson C, Petrie EC, et al. Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder. J Clin Psychiatry. 2002;63(7):565-568.
20. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
21. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
22. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
23. Byers MG, Allison KM, Wendel CS, et al. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J Clin Psychopharmacol. 2010;30(3):225-229.
24. Jin H, Lanouette NM, Mudaliar S, et al. Association of posttraumatic stress disorder with increased prevalence of metabolic syndrome. J Clin Psychopharmacol. 2009;29(3):210-215.
25. Cates ME, Jackson CW, Feldman JM, et al. Metabolic consequences of using low-dose quetiapine for insomnia in psychiatric patients. Community Ment Health J. 2009;45(4):251-254.
26. Williams SG, Alinejad NA, Williams JA, et al. Statistically significant increase in weight caused by low-dose quetiapine. Pharmacotherapy. 2010;30(10):1011-1015.
27. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267-272.
1. Dohrenwend BP, Turner JB, Turse NA, et al. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
2. Tanielian T, Jaycox L. eds. Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. Santa Monica, CA: RAND Corporation; 2008.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. Wittmann L, Schredl M, Kramer M. Dreaming in posttraumatic stress disorder: a critical review of phenomenology psychophysiology and treatment. Psychother Psychosom. 2007;76(1):25-39.
5. Clum GA, Nishith P, Resick PA. Trauma-related sleep disturbance and self-reported physical health symptoms in treatment-seeking female rape victims. J Nerv Ment Dis. 2001;189(9):618-622.
6. Kramer TL, Booth BM, Han X, et al. Service utilization and outcomes in medically ill veterans with posttraumatic stress and depressive disorders. J Trauma Stress. 2003;16(3):211-219.
7. Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929-933.
8. Nappi CM, Drummond SP, Hall JM. Treating nightmares and insomnia in posttraumatic stress disorder: a review of current evidence. Neuropharmacology. 2012;62(2):576-585.
9. Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
10. van Liempt S, Vermetten E, Geuze E, et al. Pharmacotherapy for disordered sleep in post-traumatic stress disorder: a systematic review. Int Clin Psychopharmacol. 2006;21(4):193-202.
11. Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
12. Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract. 2007;13(2):72-78.
13. Strawn JR, Geracioti TD, Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260-271.
14. Calohan J, Peterson K, Peskind ER, et al. Prazosin treatment of trauma nightmares and sleep disturbance in soldiers deployed in Iraq. J Trauma Stress. 2010;23(5):645-648.
15. Daly CM, Doyle ME, Radkind M, et al. Clinical case series: the use of Prazosin for combat-related recurrent nightmares among Operation Iraqi Freedom combat veterans. Mil Med. 2005;170(6):513-515.
16. Peskind ER, Bonner LT, Hoff DJ, et al. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16(3):165-171.
17. Raskind MA, Dobie DJ, Kanter ED, et al. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
18. Taylor F, Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol. 2002;22(1):82-85.
19. Raskind MA, Thompson C, Petrie EC, et al. Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder. J Clin Psychiatry. 2002;63(7):565-568.
20. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
21. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
22. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
23. Byers MG, Allison KM, Wendel CS, et al. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J Clin Psychopharmacol. 2010;30(3):225-229.
24. Jin H, Lanouette NM, Mudaliar S, et al. Association of posttraumatic stress disorder with increased prevalence of metabolic syndrome. J Clin Psychopharmacol. 2009;29(3):210-215.
25. Cates ME, Jackson CW, Feldman JM, et al. Metabolic consequences of using low-dose quetiapine for insomnia in psychiatric patients. Community Ment Health J. 2009;45(4):251-254.
26. Williams SG, Alinejad NA, Williams JA, et al. Statistically significant increase in weight caused by low-dose quetiapine. Pharmacotherapy. 2010;30(10):1011-1015.
27. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267-272.
How to respond to an in-flight emergency
Discuss this article at www.facebook.com/CurrentPsychiatry
Requests for a physician to assist during in-flight medical emergencies are becoming more common as travelers age and more people have access to air travel.1 Yet in this age of medical specialization, all physicians are not created equal, particularly in providing acute care. Responding to in-flight medical emergencies can be stressful, particularly for specialists who may doubt their skill base in a medical crisis.
The in-flight medical consulting service Medlink reported that only 3.5% of calls it receives are related to psychiatric emergencies.2 This means that psychiatrists who answer an in-flight distress call will almost invariably find themselves confronting a medical issue.
Discomfort with possibly having to deliver acute or invasive medical care may deter psychiatrists from responding. However, psychiatrists should be familiar with many medical problems and their basic management, particularly if their institution requires up-to-date advanced cardiac life support certification. Understanding your role and conceptualizing a general approach before you find yourself in the midst of an in-flight medical emergency can be helpful. We suggest the following principles for responding to in-flight emergencies:
- Respond to a call only if you are a licensed, currently practicing physician.
- Defer to other physicians present who may have more experience delivering acute medical care.3
- If you have been drinking alcohol, do not respond unless there are no other health care providers on board. If you do respond, know your limitations and make them known to the air crew and patient.
- Identify yourself to the patient. Give him or her your name and tell the patient that you are a psychiatrist. If the chief complaint is not something you regularly deal with, tell the patient and crew.
- Perform the best history and physical exam you can, given the setting. Obtain vital signs. Document your findings for your records and for medical personnel who may later assume patient care.
- Do not attempt procedures you are unfamiliar with or not qualified to perform (eg, starting an IV, intubations). Administer only treatments you are comfortable with.
- If you are concerned the patient may face significant morbidity or death, advise the crew to divert the flight to the closest hospital.
- Realize that it is not your role to take leadership of the situation, unless you are the only physician present. Do not be afraid to ask for help from other physicians or health care providers—including nurses or emergency medical technicians—who may not have heard or acknowledged the call or a ground-based medical consulting service. Also, once another physician has taken over, you still can contribute by stabilizing the ill patient’s emotions and behavior.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article of with manufacturers of competing products.
1. Gendreau MA, DeJohn C. Responding to medical events during commercial airline flights. N Engl J Med. 2002;346(14):1067-1073.
2. Matsumoto K, Goebert D. In-flight psychiatric emergencies. Aviat Space Environ Med. 2001;72(10):919-923.
3. Macleod S. “If there is a doctor aboard this flight…”: issues and advice for the passenger-psychiatrist. Australas Psychiatry. 2008;16(4):233-237.
Discuss this article at www.facebook.com/CurrentPsychiatry
Requests for a physician to assist during in-flight medical emergencies are becoming more common as travelers age and more people have access to air travel.1 Yet in this age of medical specialization, all physicians are not created equal, particularly in providing acute care. Responding to in-flight medical emergencies can be stressful, particularly for specialists who may doubt their skill base in a medical crisis.
The in-flight medical consulting service Medlink reported that only 3.5% of calls it receives are related to psychiatric emergencies.2 This means that psychiatrists who answer an in-flight distress call will almost invariably find themselves confronting a medical issue.
Discomfort with possibly having to deliver acute or invasive medical care may deter psychiatrists from responding. However, psychiatrists should be familiar with many medical problems and their basic management, particularly if their institution requires up-to-date advanced cardiac life support certification. Understanding your role and conceptualizing a general approach before you find yourself in the midst of an in-flight medical emergency can be helpful. We suggest the following principles for responding to in-flight emergencies:
- Respond to a call only if you are a licensed, currently practicing physician.
- Defer to other physicians present who may have more experience delivering acute medical care.3
- If you have been drinking alcohol, do not respond unless there are no other health care providers on board. If you do respond, know your limitations and make them known to the air crew and patient.
- Identify yourself to the patient. Give him or her your name and tell the patient that you are a psychiatrist. If the chief complaint is not something you regularly deal with, tell the patient and crew.
- Perform the best history and physical exam you can, given the setting. Obtain vital signs. Document your findings for your records and for medical personnel who may later assume patient care.
- Do not attempt procedures you are unfamiliar with or not qualified to perform (eg, starting an IV, intubations). Administer only treatments you are comfortable with.
- If you are concerned the patient may face significant morbidity or death, advise the crew to divert the flight to the closest hospital.
- Realize that it is not your role to take leadership of the situation, unless you are the only physician present. Do not be afraid to ask for help from other physicians or health care providers—including nurses or emergency medical technicians—who may not have heard or acknowledged the call or a ground-based medical consulting service. Also, once another physician has taken over, you still can contribute by stabilizing the ill patient’s emotions and behavior.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article of with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Requests for a physician to assist during in-flight medical emergencies are becoming more common as travelers age and more people have access to air travel.1 Yet in this age of medical specialization, all physicians are not created equal, particularly in providing acute care. Responding to in-flight medical emergencies can be stressful, particularly for specialists who may doubt their skill base in a medical crisis.
The in-flight medical consulting service Medlink reported that only 3.5% of calls it receives are related to psychiatric emergencies.2 This means that psychiatrists who answer an in-flight distress call will almost invariably find themselves confronting a medical issue.
Discomfort with possibly having to deliver acute or invasive medical care may deter psychiatrists from responding. However, psychiatrists should be familiar with many medical problems and their basic management, particularly if their institution requires up-to-date advanced cardiac life support certification. Understanding your role and conceptualizing a general approach before you find yourself in the midst of an in-flight medical emergency can be helpful. We suggest the following principles for responding to in-flight emergencies:
- Respond to a call only if you are a licensed, currently practicing physician.
- Defer to other physicians present who may have more experience delivering acute medical care.3
- If you have been drinking alcohol, do not respond unless there are no other health care providers on board. If you do respond, know your limitations and make them known to the air crew and patient.
- Identify yourself to the patient. Give him or her your name and tell the patient that you are a psychiatrist. If the chief complaint is not something you regularly deal with, tell the patient and crew.
- Perform the best history and physical exam you can, given the setting. Obtain vital signs. Document your findings for your records and for medical personnel who may later assume patient care.
- Do not attempt procedures you are unfamiliar with or not qualified to perform (eg, starting an IV, intubations). Administer only treatments you are comfortable with.
- If you are concerned the patient may face significant morbidity or death, advise the crew to divert the flight to the closest hospital.
- Realize that it is not your role to take leadership of the situation, unless you are the only physician present. Do not be afraid to ask for help from other physicians or health care providers—including nurses or emergency medical technicians—who may not have heard or acknowledged the call or a ground-based medical consulting service. Also, once another physician has taken over, you still can contribute by stabilizing the ill patient’s emotions and behavior.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article of with manufacturers of competing products.
1. Gendreau MA, DeJohn C. Responding to medical events during commercial airline flights. N Engl J Med. 2002;346(14):1067-1073.
2. Matsumoto K, Goebert D. In-flight psychiatric emergencies. Aviat Space Environ Med. 2001;72(10):919-923.
3. Macleod S. “If there is a doctor aboard this flight…”: issues and advice for the passenger-psychiatrist. Australas Psychiatry. 2008;16(4):233-237.
1. Gendreau MA, DeJohn C. Responding to medical events during commercial airline flights. N Engl J Med. 2002;346(14):1067-1073.
2. Matsumoto K, Goebert D. In-flight psychiatric emergencies. Aviat Space Environ Med. 2001;72(10):919-923.
3. Macleod S. “If there is a doctor aboard this flight…”: issues and advice for the passenger-psychiatrist. Australas Psychiatry. 2008;16(4):233-237.
Implementing a smoking ban: Tips for success
Discuss this article at www.facebook.com/CurrentPsychiatry
The prevalence of tobacco use among psychiatric patients is up to 4 times greater than that of the general population.1 Increasing numbers of psychiatric facilities have implemented policies that ban smoking to eliminate secondhand smoke, achieve a cleaner environment, encourage healthier lifestyles for patients and staff, and reduce patient smoke breaks, which allows more time for treatment.2 The potential benefits of tobacco-free psychiatric institutions has led some clinicians to call for the total exclusion of tobacco from psychiatric and addiction settings.3
New Hampshire Hospital is a 152-bed acute inpatient psychiatric facility that has approximately 2,400 patient admissions per year. Most patients have psychotic or mood disorders, often with a co-occurring substance use or personality disorder. We report our experience in planning and implementing a campus-wide “total” smoking ban—a ban on all tobacco products in the hospital building and on hospital grounds.
Implementation and results
Our hospital’s interdisciplinary Tobacco-Free Campus Task Force developed specific recommendations and a timeline for implementing the total smoking ban. Hospital staff voiced concerns that banning smoking would lead to increased episodes of aggressive behavior. We reviewed data on the use of seclusion and restraints, patient assaults, and smoking contraband before and after initiating the total smoking ban. We found no evidence of an increase in the use of seclusion or restraints or in patient assaults with staff injury after implementing the smoking ban. However, we did see an initial increase in smoking contraband. These rates peaked and then tapered to pre-smoking ban rates within 2 years.
Why we succeeded
Several factors contributed to the successful implementation of our total smoking ban:
- Hospital administration supported having a smoke-free campus, and executive leadership allowed staff to develop strategies, programs, treatment options, and groups to maximize the possibility of success.
- Extensive communication with outside agencies, advocacy groups, and care providers allowed for discussion of potential difficulties, such as concerns regarding individuals not having access to tobacco during their hospital stay and how this could affect their treatment.
- The hospital’s Tobacco-Free Campus Task Force helped develop strategies that allowed for an effective transition to a smoke-free campus, such as increasing the number of smoking cessation groups for patients and staff and eliminating the sale of tobacco products at the hospital’s visitor shop.
- Extensive preparation, clear timelines, and achievable goals created a positive climate for a “culture of change.”
Recommendations
If you are considering a total smoking ban at your facility, we recommend the following steps:
- set a clear target date
- allow adequate time for hospital staff and administration to develop strategies for implementation
- make sure hospital administration is supportive
- involve all hospital disciplines—psychiatry, nursing, rehabilitation, psychology, social work, etc.
- address staff concerns regarding patient and staff safety
- ensure adequate nicotine replacement therapy options for patients and staff
- anticipate an initial increase in smoking-related contraband
- understand there may be differing opinions regarding the smoking ban, but remain committed to the change.
Dr. Folks is a consultant to Independent Medical Experts Consulting Services and Medical Care Management Corporation.
Drs. de Nesnera and Rauter report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Morisano D, Bacher I, Audrain-McGovern J, et al. Mechanisms underlying the comorbidity of tobacco use in mental health and addictive disorders. Can J Psychiatry. 2009;54(6):356-367.
2. Etter M, Khan AN, Etter JF. Acceptability and impact of a partial smoking ban followed by a total smoking ban in a psychiatric hospital. Prev Med. 2008;46(6):572-578.
3. Moss TG, Weinberger AH, Vessicchio JC, et al. A tobacco reconceptualization in psychiatry: toward the development of tobacco-free psychiatric facilities. Am J Addict. 2010;19(4):293-311.
Discuss this article at www.facebook.com/CurrentPsychiatry
The prevalence of tobacco use among psychiatric patients is up to 4 times greater than that of the general population.1 Increasing numbers of psychiatric facilities have implemented policies that ban smoking to eliminate secondhand smoke, achieve a cleaner environment, encourage healthier lifestyles for patients and staff, and reduce patient smoke breaks, which allows more time for treatment.2 The potential benefits of tobacco-free psychiatric institutions has led some clinicians to call for the total exclusion of tobacco from psychiatric and addiction settings.3
New Hampshire Hospital is a 152-bed acute inpatient psychiatric facility that has approximately 2,400 patient admissions per year. Most patients have psychotic or mood disorders, often with a co-occurring substance use or personality disorder. We report our experience in planning and implementing a campus-wide “total” smoking ban—a ban on all tobacco products in the hospital building and on hospital grounds.
Implementation and results
Our hospital’s interdisciplinary Tobacco-Free Campus Task Force developed specific recommendations and a timeline for implementing the total smoking ban. Hospital staff voiced concerns that banning smoking would lead to increased episodes of aggressive behavior. We reviewed data on the use of seclusion and restraints, patient assaults, and smoking contraband before and after initiating the total smoking ban. We found no evidence of an increase in the use of seclusion or restraints or in patient assaults with staff injury after implementing the smoking ban. However, we did see an initial increase in smoking contraband. These rates peaked and then tapered to pre-smoking ban rates within 2 years.
Why we succeeded
Several factors contributed to the successful implementation of our total smoking ban:
- Hospital administration supported having a smoke-free campus, and executive leadership allowed staff to develop strategies, programs, treatment options, and groups to maximize the possibility of success.
- Extensive communication with outside agencies, advocacy groups, and care providers allowed for discussion of potential difficulties, such as concerns regarding individuals not having access to tobacco during their hospital stay and how this could affect their treatment.
- The hospital’s Tobacco-Free Campus Task Force helped develop strategies that allowed for an effective transition to a smoke-free campus, such as increasing the number of smoking cessation groups for patients and staff and eliminating the sale of tobacco products at the hospital’s visitor shop.
- Extensive preparation, clear timelines, and achievable goals created a positive climate for a “culture of change.”
Recommendations
If you are considering a total smoking ban at your facility, we recommend the following steps:
- set a clear target date
- allow adequate time for hospital staff and administration to develop strategies for implementation
- make sure hospital administration is supportive
- involve all hospital disciplines—psychiatry, nursing, rehabilitation, psychology, social work, etc.
- address staff concerns regarding patient and staff safety
- ensure adequate nicotine replacement therapy options for patients and staff
- anticipate an initial increase in smoking-related contraband
- understand there may be differing opinions regarding the smoking ban, but remain committed to the change.
Dr. Folks is a consultant to Independent Medical Experts Consulting Services and Medical Care Management Corporation.
Drs. de Nesnera and Rauter report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
The prevalence of tobacco use among psychiatric patients is up to 4 times greater than that of the general population.1 Increasing numbers of psychiatric facilities have implemented policies that ban smoking to eliminate secondhand smoke, achieve a cleaner environment, encourage healthier lifestyles for patients and staff, and reduce patient smoke breaks, which allows more time for treatment.2 The potential benefits of tobacco-free psychiatric institutions has led some clinicians to call for the total exclusion of tobacco from psychiatric and addiction settings.3
New Hampshire Hospital is a 152-bed acute inpatient psychiatric facility that has approximately 2,400 patient admissions per year. Most patients have psychotic or mood disorders, often with a co-occurring substance use or personality disorder. We report our experience in planning and implementing a campus-wide “total” smoking ban—a ban on all tobacco products in the hospital building and on hospital grounds.
Implementation and results
Our hospital’s interdisciplinary Tobacco-Free Campus Task Force developed specific recommendations and a timeline for implementing the total smoking ban. Hospital staff voiced concerns that banning smoking would lead to increased episodes of aggressive behavior. We reviewed data on the use of seclusion and restraints, patient assaults, and smoking contraband before and after initiating the total smoking ban. We found no evidence of an increase in the use of seclusion or restraints or in patient assaults with staff injury after implementing the smoking ban. However, we did see an initial increase in smoking contraband. These rates peaked and then tapered to pre-smoking ban rates within 2 years.
Why we succeeded
Several factors contributed to the successful implementation of our total smoking ban:
- Hospital administration supported having a smoke-free campus, and executive leadership allowed staff to develop strategies, programs, treatment options, and groups to maximize the possibility of success.
- Extensive communication with outside agencies, advocacy groups, and care providers allowed for discussion of potential difficulties, such as concerns regarding individuals not having access to tobacco during their hospital stay and how this could affect their treatment.
- The hospital’s Tobacco-Free Campus Task Force helped develop strategies that allowed for an effective transition to a smoke-free campus, such as increasing the number of smoking cessation groups for patients and staff and eliminating the sale of tobacco products at the hospital’s visitor shop.
- Extensive preparation, clear timelines, and achievable goals created a positive climate for a “culture of change.”
Recommendations
If you are considering a total smoking ban at your facility, we recommend the following steps:
- set a clear target date
- allow adequate time for hospital staff and administration to develop strategies for implementation
- make sure hospital administration is supportive
- involve all hospital disciplines—psychiatry, nursing, rehabilitation, psychology, social work, etc.
- address staff concerns regarding patient and staff safety
- ensure adequate nicotine replacement therapy options for patients and staff
- anticipate an initial increase in smoking-related contraband
- understand there may be differing opinions regarding the smoking ban, but remain committed to the change.
Dr. Folks is a consultant to Independent Medical Experts Consulting Services and Medical Care Management Corporation.
Drs. de Nesnera and Rauter report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Morisano D, Bacher I, Audrain-McGovern J, et al. Mechanisms underlying the comorbidity of tobacco use in mental health and addictive disorders. Can J Psychiatry. 2009;54(6):356-367.
2. Etter M, Khan AN, Etter JF. Acceptability and impact of a partial smoking ban followed by a total smoking ban in a psychiatric hospital. Prev Med. 2008;46(6):572-578.
3. Moss TG, Weinberger AH, Vessicchio JC, et al. A tobacco reconceptualization in psychiatry: toward the development of tobacco-free psychiatric facilities. Am J Addict. 2010;19(4):293-311.
1. Morisano D, Bacher I, Audrain-McGovern J, et al. Mechanisms underlying the comorbidity of tobacco use in mental health and addictive disorders. Can J Psychiatry. 2009;54(6):356-367.
2. Etter M, Khan AN, Etter JF. Acceptability and impact of a partial smoking ban followed by a total smoking ban in a psychiatric hospital. Prev Med. 2008;46(6):572-578.
3. Moss TG, Weinberger AH, Vessicchio JC, et al. A tobacco reconceptualization in psychiatry: toward the development of tobacco-free psychiatric facilities. Am J Addict. 2010;19(4):293-311.
‘Curbside’ consults: Know your liability
Discuss this article at www.facebook.com/CurrentPsychiatry
Dear Dr. Mossman:
Could providing a “curbside” consultation to a colleague leave me medico legally vulnerable if an adverse event leads to a malpractice lawsuit? If so, what can I do to address this risk?—Submitted by “Dr. W”
Medicine is a collaborative profession. Surgeons often combine skills to perform complex operations together, and specialists pool their expertise when they collectively manage patients with several medical problems. Doctors share their knowledge when they give lectures to medical audiences, write reports to referring physicians, or respond verbally to colleagues’ requests for information or advice.1
Doctors use the phrase “curbside consult” to refer (with humor and self-deprecation) to informal conversations with colleagues about patients’ medical management—advice-seeking that falls short of asking a colleague to make recommendations based on a formal, personal examination. Many physicians seek or provide curbside advice several times a month.2 Curbside consults transmit knowledge and cement professional bonds among physicians, making them “an integral part of our medical culture.”3
More than a dozen legal decisions mention curbside consultations. Judges think informal information-sharing improves medical practice and don’t want doctors to stop soliciting ideas or offering suggestions because they fear lawsuits.4,5 However, courts have found that, under certain conditions, giving advice can create liability for a bad outcome, even though the doctor never met the patient who was harmed.
In this article, we’ll look at:
- when such liability might occur, and
- what you can do to minimize it.
A doctor-patient relationship?
Legally, doctors are obligated to provide competent care for just 1 group of people: their patients. Therefore, to decide if plaintiffs could pursue malpractice claims in cases where doctors offered comments about patients they did not personally examine, courts have asked whether the circumstances, actions undertaken, or nature of information that was exchanged created a professional relationship.
Reynolds v Decatur Memorial Hospital4 describes an informal consultation that did not create a physician-patient relationship. In this case, a boy was admitted to a hospital after he had fallen. The treating pediatrician telephoned a neurosurgeon, who asked whether the boy’s neck was stiff, discussed diagnostic possibilities with the pediatrician, and suggested doing a lumbar puncture. The neurosurgeon offered to see the boy if requested, but he never did, and he did not bill for the telephone consultation. Guillain-Barré syndrome was first suspected, but a spinal cord injury was discovered after the boy—who developed quadriplegia—was transferred to another hospital.
In a subsequent lawsuit, the boy’s mother claimed her son’s paralysis resulted from negligence by the first hospital and its doctors, but the trial court dismissed the case against the neurosurgeon. Affirming the trial court’s ruling, an Illinois appeals court explained that the neurosurgeon had not been asked to provide medical services, conduct tests, or interpret test results. “A doctor who gives an informal opinion at the request of a treating physician does not owe a duty of care to the patient whose case was discussed,” the Reynolds court said.
Campbell v Haber6 describes circumstances that differed slightly from those described in the Reynolds decision but appeared to create a doctor-patient relationship. Campbell concerned a patient who came to an emergency room (ER) complaining of chest pain. The ER physician’s findings indicated possible heart muscle damage, so he telephoned a cardiologist (whom the ER doctor believed was “on call”) and described the patient’s symptoms and test results. The cardiologist thought the test results were not consistent with a cardiac event. The ER physician told the patient and his wife about the cardiologist’s opinion and, relying on what the cardiologist said, discharged the patient. Shortly after, the patient had a heart attack.
The patient sued not just the ER physician, but the cardiologist, who sought dismissal from the suit because he never saw the patient, had no treatment relationship with him, and never billed for services. However, the trial judge ruled that the patient could sue the cardiologist and the appellate court agreed, saying that a jury had to decide whether the cardiologist had incurred a doctor-patient relationship and might be liable. “An implied physician-patient relationship may arise when a physician gives advice to a patient,” the appeals court said, “even if that advice is communicated through another health care professional.”
Telling the difference
So what differentiates a no-liability curbside consult from a medical discussion that creates a doctor-patient duty and potential for liability for adverse results?
You create a physician-patient relationship when you assume responsibility to diagnose or treat someone.7 Although typically this requires an in-person encounter with a patient, it can happen indirectly—electronically (through e-mail), by telephone, or through a family member or another professional. But if you do nothing that implies consent to act for the patient’s benefit, you should have no actual malpractice liability if something goes wrong.3,8 As a Kansas Supreme Court decision explains, you “cannot be liable for medical malpractice” if you “merely consult with a treating physician and [do] nothing more.”5
Several legal cases discuss doctors’ efforts to extricate themselves from lawsuits arising from clinical encounters that the doctors mistakenly thought were just curbside consults. Table 18-12 lists situations in which talking about patients goes beyond just being “curbsided.”
Table 1
When it’s not a ‘curbside consultation’
| Situation | Why it’s not a curbside consultation |
|---|---|
| On call | If you are “on call” for an emergency room, get called about a patient with an emergency condition, and discuss the patient’s symptoms, possible diagnosis, or treatment, you have a relationship with the patient that entails a duty of care8,9 |
| Covering | If you have agreed to “cover” patients for a colleague, you have assumed a duty to properly care for the colleague’s patients: they’re your patients during the colleague’s absence. Getting asked questions about managing those patients is not a curbside consultation, even if you’ve never met or spoken to the patient10,11 |
| Supervising | Physician assistants, residents in training, and nurse practitioners do not practice independently of their supervising physicians. If you’re a supervisor and get a call about managing a patient, you may bear vicarious liability for adverse results12 |
| Specifics and reliance | If responding to the informal consult requires you to give specific advice that the consulting colleague will rely on to make a diagnosis or select treatment, you are participating in the patient’s care11 |
How to respond
Should you decline to provide curbside consultations to keep yourself out of lawsuits? Some authors think so, pointing out that informally transmitted clinical data may be faulty, which means you may give bad advice based on incomplete information or a verbal misunderstanding.13-16 These authors suggest that if you’re curbsided you should ask to see the patient for a formal consultation, decline to give informal advice, or provide a response that lacks specifics.
Other authors feel that these approaches are needlessly cautious and would harm patients by impeding doctors’ ability to help and learn from each other.3,17 These authors think the risk of incurring liability from a curbside consult is low. Also, getting advice from a colleague is a valuable risk management strategy; it helps you make sure you’re on the right track, and it shows you are a thoughtful clinician whose patients benefit from your own and your colleagues’ medical expertise.
Even if you’re comfortable soliciting and providing curbside advice, sometimes circumstances make it wise to follow-up an informal initial inquiry with a formal consultation. Table 23,17 lists examples of when you should follow-up with a formal consultation.
Table 2
Considerations that favor formal consultation
| Complicated diagnostic situations |
| The consulted or requesting physician feels that giving good advice requires a personal examination |
| Advice is based on a detailed discussion and is specific to a patient’s situation |
| The patient requested the consultation |
| The consultant will make a report for the patient’s record |
| The consult bills for the consultation |
Documentation
Experts disagree about whether the requesting or receiving physician should document a curbside consultation, and if so, how. On one hand, making a notation in a patient’s record documents the treating doctor’s diligence and may provide a measure of liability protection in a malpractice action. Doing this, however, exposes the identity of the consultant, who might be named among the defendants in a lawsuit.
One commonly recommended strategy is to request the consultant’s permission before identifying him or her in the record,13,16,17 a position that is defensible on grounds of courtesy alone. But omitting a consultant’s name from record does not guarantee that the consultant’s involvement won’t be discovered in the course of litigation.3 For example, treating doctors who get sued often are asked during their depositions about whether they talked with anyone about the case, and they have to answer honestly.
If a consulted doctor makes written notes, it might suggest that the consultation was more than the sort of informal information-sharing implied by the term “curbside.” However, in the unlikely event that a lawsuit arose and included the consultant as a defendant, documentation of advice given would help the consultant recall and defend what was said.
Related Resources
- Grant-Kels JM, Kels BD. The curbside consultation: legal, moral, and ethical considerations. J Am Acad Dermatol. 2012;66(5):827-829.
- Kreichelt R, Hilbert ML, Shinn D. Minimizing the legal risk with ‘curbside’ consultation. J Healthc Risk Manag. 2008;28(1):27-29.
- Atkinson L. Curbside consults: what is your liability risk? Iowa Med. 2003;93(4):15.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Perley CM. Physician use of the curbside consultation to address information needs: report on a collective case study. J Med Libr Assoc. 2006;94(2):137-144.
2. Kuo D, Gifford DR, Stein MD. Curbside consultation practices and attitudes among primary care physicians and medical subspecialists. JAMA. 1998;280(10):905-909.
3. Cotton VR. Legal risks of “curbside” consults. Am J Cardiol. 2010;106(1):135-138.
4. Reynolds v Decatur Memorial Hospital, 277 Ill App 3d 80 (Ill App Ct 4th Dist 1996).
5. Irvin v Smith, 272 Kan 112 (Kan 2001).
6. Campbell v Haber, 274 A.D.2d 946 (NY App Div 4th Dep’t 2000).
7. Sterling v Johns Hopkins Hospital, 802 A.2d 440 (Md Ct Spec App 2002), cert den, 808 A.2d 808 (Md 2002).
8. Emergency Medical Treatment and Active Labor Act, 42 USC § 1395DD.
9. Lownsbury v VanBuren, 94 Ohio St 3d 231, 762 NE 2d 354 (2002).
10. Blazo v McLaren Regional Medical Center, 2002 Mich App LEXIS 752 (Mich Ct App 2002).
11. Kelley v Middle Tennessee Emergency Physicians, PC, 133 SW3d 587 (Tenn 2004).
12. Hammonds v Jewish Hospital, 899 SW2d 527 (Mo Ct App 1995).
13. MAG Mutual Insurance Company, Inc. Curbing the curbside consult—a risk management perspective. J Med Assoc Ga. 2008;97(1):50.-
14. Burns CD. Death of the curbside consult? J Ky Med Assoc. 2006;104(1):27.-
15. Hendel T. Informal consultations: do new risks exist with this age-old tradition? J Med Pract Manage. 2002;17(6):308-311.
16. Manian FA, Janssen DA. Curbside consultations. A closer look at a common practice. JAMA. 1996;275(2):145-147.
17. Curbside consultations. Psychiatry (Edgmont). 2010;7(5):51-53.
Discuss this article at www.facebook.com/CurrentPsychiatry
Dear Dr. Mossman:
Could providing a “curbside” consultation to a colleague leave me medico legally vulnerable if an adverse event leads to a malpractice lawsuit? If so, what can I do to address this risk?—Submitted by “Dr. W”
Medicine is a collaborative profession. Surgeons often combine skills to perform complex operations together, and specialists pool their expertise when they collectively manage patients with several medical problems. Doctors share their knowledge when they give lectures to medical audiences, write reports to referring physicians, or respond verbally to colleagues’ requests for information or advice.1
Doctors use the phrase “curbside consult” to refer (with humor and self-deprecation) to informal conversations with colleagues about patients’ medical management—advice-seeking that falls short of asking a colleague to make recommendations based on a formal, personal examination. Many physicians seek or provide curbside advice several times a month.2 Curbside consults transmit knowledge and cement professional bonds among physicians, making them “an integral part of our medical culture.”3
More than a dozen legal decisions mention curbside consultations. Judges think informal information-sharing improves medical practice and don’t want doctors to stop soliciting ideas or offering suggestions because they fear lawsuits.4,5 However, courts have found that, under certain conditions, giving advice can create liability for a bad outcome, even though the doctor never met the patient who was harmed.
In this article, we’ll look at:
- when such liability might occur, and
- what you can do to minimize it.
A doctor-patient relationship?
Legally, doctors are obligated to provide competent care for just 1 group of people: their patients. Therefore, to decide if plaintiffs could pursue malpractice claims in cases where doctors offered comments about patients they did not personally examine, courts have asked whether the circumstances, actions undertaken, or nature of information that was exchanged created a professional relationship.
Reynolds v Decatur Memorial Hospital4 describes an informal consultation that did not create a physician-patient relationship. In this case, a boy was admitted to a hospital after he had fallen. The treating pediatrician telephoned a neurosurgeon, who asked whether the boy’s neck was stiff, discussed diagnostic possibilities with the pediatrician, and suggested doing a lumbar puncture. The neurosurgeon offered to see the boy if requested, but he never did, and he did not bill for the telephone consultation. Guillain-Barré syndrome was first suspected, but a spinal cord injury was discovered after the boy—who developed quadriplegia—was transferred to another hospital.
In a subsequent lawsuit, the boy’s mother claimed her son’s paralysis resulted from negligence by the first hospital and its doctors, but the trial court dismissed the case against the neurosurgeon. Affirming the trial court’s ruling, an Illinois appeals court explained that the neurosurgeon had not been asked to provide medical services, conduct tests, or interpret test results. “A doctor who gives an informal opinion at the request of a treating physician does not owe a duty of care to the patient whose case was discussed,” the Reynolds court said.
Campbell v Haber6 describes circumstances that differed slightly from those described in the Reynolds decision but appeared to create a doctor-patient relationship. Campbell concerned a patient who came to an emergency room (ER) complaining of chest pain. The ER physician’s findings indicated possible heart muscle damage, so he telephoned a cardiologist (whom the ER doctor believed was “on call”) and described the patient’s symptoms and test results. The cardiologist thought the test results were not consistent with a cardiac event. The ER physician told the patient and his wife about the cardiologist’s opinion and, relying on what the cardiologist said, discharged the patient. Shortly after, the patient had a heart attack.
The patient sued not just the ER physician, but the cardiologist, who sought dismissal from the suit because he never saw the patient, had no treatment relationship with him, and never billed for services. However, the trial judge ruled that the patient could sue the cardiologist and the appellate court agreed, saying that a jury had to decide whether the cardiologist had incurred a doctor-patient relationship and might be liable. “An implied physician-patient relationship may arise when a physician gives advice to a patient,” the appeals court said, “even if that advice is communicated through another health care professional.”
Telling the difference
So what differentiates a no-liability curbside consult from a medical discussion that creates a doctor-patient duty and potential for liability for adverse results?
You create a physician-patient relationship when you assume responsibility to diagnose or treat someone.7 Although typically this requires an in-person encounter with a patient, it can happen indirectly—electronically (through e-mail), by telephone, or through a family member or another professional. But if you do nothing that implies consent to act for the patient’s benefit, you should have no actual malpractice liability if something goes wrong.3,8 As a Kansas Supreme Court decision explains, you “cannot be liable for medical malpractice” if you “merely consult with a treating physician and [do] nothing more.”5
Several legal cases discuss doctors’ efforts to extricate themselves from lawsuits arising from clinical encounters that the doctors mistakenly thought were just curbside consults. Table 18-12 lists situations in which talking about patients goes beyond just being “curbsided.”
Table 1
When it’s not a ‘curbside consultation’
| Situation | Why it’s not a curbside consultation |
|---|---|
| On call | If you are “on call” for an emergency room, get called about a patient with an emergency condition, and discuss the patient’s symptoms, possible diagnosis, or treatment, you have a relationship with the patient that entails a duty of care8,9 |
| Covering | If you have agreed to “cover” patients for a colleague, you have assumed a duty to properly care for the colleague’s patients: they’re your patients during the colleague’s absence. Getting asked questions about managing those patients is not a curbside consultation, even if you’ve never met or spoken to the patient10,11 |
| Supervising | Physician assistants, residents in training, and nurse practitioners do not practice independently of their supervising physicians. If you’re a supervisor and get a call about managing a patient, you may bear vicarious liability for adverse results12 |
| Specifics and reliance | If responding to the informal consult requires you to give specific advice that the consulting colleague will rely on to make a diagnosis or select treatment, you are participating in the patient’s care11 |
How to respond
Should you decline to provide curbside consultations to keep yourself out of lawsuits? Some authors think so, pointing out that informally transmitted clinical data may be faulty, which means you may give bad advice based on incomplete information or a verbal misunderstanding.13-16 These authors suggest that if you’re curbsided you should ask to see the patient for a formal consultation, decline to give informal advice, or provide a response that lacks specifics.
Other authors feel that these approaches are needlessly cautious and would harm patients by impeding doctors’ ability to help and learn from each other.3,17 These authors think the risk of incurring liability from a curbside consult is low. Also, getting advice from a colleague is a valuable risk management strategy; it helps you make sure you’re on the right track, and it shows you are a thoughtful clinician whose patients benefit from your own and your colleagues’ medical expertise.
Even if you’re comfortable soliciting and providing curbside advice, sometimes circumstances make it wise to follow-up an informal initial inquiry with a formal consultation. Table 23,17 lists examples of when you should follow-up with a formal consultation.
Table 2
Considerations that favor formal consultation
| Complicated diagnostic situations |
| The consulted or requesting physician feels that giving good advice requires a personal examination |
| Advice is based on a detailed discussion and is specific to a patient’s situation |
| The patient requested the consultation |
| The consultant will make a report for the patient’s record |
| The consult bills for the consultation |
Documentation
Experts disagree about whether the requesting or receiving physician should document a curbside consultation, and if so, how. On one hand, making a notation in a patient’s record documents the treating doctor’s diligence and may provide a measure of liability protection in a malpractice action. Doing this, however, exposes the identity of the consultant, who might be named among the defendants in a lawsuit.
One commonly recommended strategy is to request the consultant’s permission before identifying him or her in the record,13,16,17 a position that is defensible on grounds of courtesy alone. But omitting a consultant’s name from record does not guarantee that the consultant’s involvement won’t be discovered in the course of litigation.3 For example, treating doctors who get sued often are asked during their depositions about whether they talked with anyone about the case, and they have to answer honestly.
If a consulted doctor makes written notes, it might suggest that the consultation was more than the sort of informal information-sharing implied by the term “curbside.” However, in the unlikely event that a lawsuit arose and included the consultant as a defendant, documentation of advice given would help the consultant recall and defend what was said.
Related Resources
- Grant-Kels JM, Kels BD. The curbside consultation: legal, moral, and ethical considerations. J Am Acad Dermatol. 2012;66(5):827-829.
- Kreichelt R, Hilbert ML, Shinn D. Minimizing the legal risk with ‘curbside’ consultation. J Healthc Risk Manag. 2008;28(1):27-29.
- Atkinson L. Curbside consults: what is your liability risk? Iowa Med. 2003;93(4):15.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Dear Dr. Mossman:
Could providing a “curbside” consultation to a colleague leave me medico legally vulnerable if an adverse event leads to a malpractice lawsuit? If so, what can I do to address this risk?—Submitted by “Dr. W”
Medicine is a collaborative profession. Surgeons often combine skills to perform complex operations together, and specialists pool their expertise when they collectively manage patients with several medical problems. Doctors share their knowledge when they give lectures to medical audiences, write reports to referring physicians, or respond verbally to colleagues’ requests for information or advice.1
Doctors use the phrase “curbside consult” to refer (with humor and self-deprecation) to informal conversations with colleagues about patients’ medical management—advice-seeking that falls short of asking a colleague to make recommendations based on a formal, personal examination. Many physicians seek or provide curbside advice several times a month.2 Curbside consults transmit knowledge and cement professional bonds among physicians, making them “an integral part of our medical culture.”3
More than a dozen legal decisions mention curbside consultations. Judges think informal information-sharing improves medical practice and don’t want doctors to stop soliciting ideas or offering suggestions because they fear lawsuits.4,5 However, courts have found that, under certain conditions, giving advice can create liability for a bad outcome, even though the doctor never met the patient who was harmed.
In this article, we’ll look at:
- when such liability might occur, and
- what you can do to minimize it.
A doctor-patient relationship?
Legally, doctors are obligated to provide competent care for just 1 group of people: their patients. Therefore, to decide if plaintiffs could pursue malpractice claims in cases where doctors offered comments about patients they did not personally examine, courts have asked whether the circumstances, actions undertaken, or nature of information that was exchanged created a professional relationship.
Reynolds v Decatur Memorial Hospital4 describes an informal consultation that did not create a physician-patient relationship. In this case, a boy was admitted to a hospital after he had fallen. The treating pediatrician telephoned a neurosurgeon, who asked whether the boy’s neck was stiff, discussed diagnostic possibilities with the pediatrician, and suggested doing a lumbar puncture. The neurosurgeon offered to see the boy if requested, but he never did, and he did not bill for the telephone consultation. Guillain-Barré syndrome was first suspected, but a spinal cord injury was discovered after the boy—who developed quadriplegia—was transferred to another hospital.
In a subsequent lawsuit, the boy’s mother claimed her son’s paralysis resulted from negligence by the first hospital and its doctors, but the trial court dismissed the case against the neurosurgeon. Affirming the trial court’s ruling, an Illinois appeals court explained that the neurosurgeon had not been asked to provide medical services, conduct tests, or interpret test results. “A doctor who gives an informal opinion at the request of a treating physician does not owe a duty of care to the patient whose case was discussed,” the Reynolds court said.
Campbell v Haber6 describes circumstances that differed slightly from those described in the Reynolds decision but appeared to create a doctor-patient relationship. Campbell concerned a patient who came to an emergency room (ER) complaining of chest pain. The ER physician’s findings indicated possible heart muscle damage, so he telephoned a cardiologist (whom the ER doctor believed was “on call”) and described the patient’s symptoms and test results. The cardiologist thought the test results were not consistent with a cardiac event. The ER physician told the patient and his wife about the cardiologist’s opinion and, relying on what the cardiologist said, discharged the patient. Shortly after, the patient had a heart attack.
The patient sued not just the ER physician, but the cardiologist, who sought dismissal from the suit because he never saw the patient, had no treatment relationship with him, and never billed for services. However, the trial judge ruled that the patient could sue the cardiologist and the appellate court agreed, saying that a jury had to decide whether the cardiologist had incurred a doctor-patient relationship and might be liable. “An implied physician-patient relationship may arise when a physician gives advice to a patient,” the appeals court said, “even if that advice is communicated through another health care professional.”
Telling the difference
So what differentiates a no-liability curbside consult from a medical discussion that creates a doctor-patient duty and potential for liability for adverse results?
You create a physician-patient relationship when you assume responsibility to diagnose or treat someone.7 Although typically this requires an in-person encounter with a patient, it can happen indirectly—electronically (through e-mail), by telephone, or through a family member or another professional. But if you do nothing that implies consent to act for the patient’s benefit, you should have no actual malpractice liability if something goes wrong.3,8 As a Kansas Supreme Court decision explains, you “cannot be liable for medical malpractice” if you “merely consult with a treating physician and [do] nothing more.”5
Several legal cases discuss doctors’ efforts to extricate themselves from lawsuits arising from clinical encounters that the doctors mistakenly thought were just curbside consults. Table 18-12 lists situations in which talking about patients goes beyond just being “curbsided.”
Table 1
When it’s not a ‘curbside consultation’
| Situation | Why it’s not a curbside consultation |
|---|---|
| On call | If you are “on call” for an emergency room, get called about a patient with an emergency condition, and discuss the patient’s symptoms, possible diagnosis, or treatment, you have a relationship with the patient that entails a duty of care8,9 |
| Covering | If you have agreed to “cover” patients for a colleague, you have assumed a duty to properly care for the colleague’s patients: they’re your patients during the colleague’s absence. Getting asked questions about managing those patients is not a curbside consultation, even if you’ve never met or spoken to the patient10,11 |
| Supervising | Physician assistants, residents in training, and nurse practitioners do not practice independently of their supervising physicians. If you’re a supervisor and get a call about managing a patient, you may bear vicarious liability for adverse results12 |
| Specifics and reliance | If responding to the informal consult requires you to give specific advice that the consulting colleague will rely on to make a diagnosis or select treatment, you are participating in the patient’s care11 |
How to respond
Should you decline to provide curbside consultations to keep yourself out of lawsuits? Some authors think so, pointing out that informally transmitted clinical data may be faulty, which means you may give bad advice based on incomplete information or a verbal misunderstanding.13-16 These authors suggest that if you’re curbsided you should ask to see the patient for a formal consultation, decline to give informal advice, or provide a response that lacks specifics.
Other authors feel that these approaches are needlessly cautious and would harm patients by impeding doctors’ ability to help and learn from each other.3,17 These authors think the risk of incurring liability from a curbside consult is low. Also, getting advice from a colleague is a valuable risk management strategy; it helps you make sure you’re on the right track, and it shows you are a thoughtful clinician whose patients benefit from your own and your colleagues’ medical expertise.
Even if you’re comfortable soliciting and providing curbside advice, sometimes circumstances make it wise to follow-up an informal initial inquiry with a formal consultation. Table 23,17 lists examples of when you should follow-up with a formal consultation.
Table 2
Considerations that favor formal consultation
| Complicated diagnostic situations |
| The consulted or requesting physician feels that giving good advice requires a personal examination |
| Advice is based on a detailed discussion and is specific to a patient’s situation |
| The patient requested the consultation |
| The consultant will make a report for the patient’s record |
| The consult bills for the consultation |
Documentation
Experts disagree about whether the requesting or receiving physician should document a curbside consultation, and if so, how. On one hand, making a notation in a patient’s record documents the treating doctor’s diligence and may provide a measure of liability protection in a malpractice action. Doing this, however, exposes the identity of the consultant, who might be named among the defendants in a lawsuit.
One commonly recommended strategy is to request the consultant’s permission before identifying him or her in the record,13,16,17 a position that is defensible on grounds of courtesy alone. But omitting a consultant’s name from record does not guarantee that the consultant’s involvement won’t be discovered in the course of litigation.3 For example, treating doctors who get sued often are asked during their depositions about whether they talked with anyone about the case, and they have to answer honestly.
If a consulted doctor makes written notes, it might suggest that the consultation was more than the sort of informal information-sharing implied by the term “curbside.” However, in the unlikely event that a lawsuit arose and included the consultant as a defendant, documentation of advice given would help the consultant recall and defend what was said.
Related Resources
- Grant-Kels JM, Kels BD. The curbside consultation: legal, moral, and ethical considerations. J Am Acad Dermatol. 2012;66(5):827-829.
- Kreichelt R, Hilbert ML, Shinn D. Minimizing the legal risk with ‘curbside’ consultation. J Healthc Risk Manag. 2008;28(1):27-29.
- Atkinson L. Curbside consults: what is your liability risk? Iowa Med. 2003;93(4):15.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Perley CM. Physician use of the curbside consultation to address information needs: report on a collective case study. J Med Libr Assoc. 2006;94(2):137-144.
2. Kuo D, Gifford DR, Stein MD. Curbside consultation practices and attitudes among primary care physicians and medical subspecialists. JAMA. 1998;280(10):905-909.
3. Cotton VR. Legal risks of “curbside” consults. Am J Cardiol. 2010;106(1):135-138.
4. Reynolds v Decatur Memorial Hospital, 277 Ill App 3d 80 (Ill App Ct 4th Dist 1996).
5. Irvin v Smith, 272 Kan 112 (Kan 2001).
6. Campbell v Haber, 274 A.D.2d 946 (NY App Div 4th Dep’t 2000).
7. Sterling v Johns Hopkins Hospital, 802 A.2d 440 (Md Ct Spec App 2002), cert den, 808 A.2d 808 (Md 2002).
8. Emergency Medical Treatment and Active Labor Act, 42 USC § 1395DD.
9. Lownsbury v VanBuren, 94 Ohio St 3d 231, 762 NE 2d 354 (2002).
10. Blazo v McLaren Regional Medical Center, 2002 Mich App LEXIS 752 (Mich Ct App 2002).
11. Kelley v Middle Tennessee Emergency Physicians, PC, 133 SW3d 587 (Tenn 2004).
12. Hammonds v Jewish Hospital, 899 SW2d 527 (Mo Ct App 1995).
13. MAG Mutual Insurance Company, Inc. Curbing the curbside consult—a risk management perspective. J Med Assoc Ga. 2008;97(1):50.-
14. Burns CD. Death of the curbside consult? J Ky Med Assoc. 2006;104(1):27.-
15. Hendel T. Informal consultations: do new risks exist with this age-old tradition? J Med Pract Manage. 2002;17(6):308-311.
16. Manian FA, Janssen DA. Curbside consultations. A closer look at a common practice. JAMA. 1996;275(2):145-147.
17. Curbside consultations. Psychiatry (Edgmont). 2010;7(5):51-53.
1. Perley CM. Physician use of the curbside consultation to address information needs: report on a collective case study. J Med Libr Assoc. 2006;94(2):137-144.
2. Kuo D, Gifford DR, Stein MD. Curbside consultation practices and attitudes among primary care physicians and medical subspecialists. JAMA. 1998;280(10):905-909.
3. Cotton VR. Legal risks of “curbside” consults. Am J Cardiol. 2010;106(1):135-138.
4. Reynolds v Decatur Memorial Hospital, 277 Ill App 3d 80 (Ill App Ct 4th Dist 1996).
5. Irvin v Smith, 272 Kan 112 (Kan 2001).
6. Campbell v Haber, 274 A.D.2d 946 (NY App Div 4th Dep’t 2000).
7. Sterling v Johns Hopkins Hospital, 802 A.2d 440 (Md Ct Spec App 2002), cert den, 808 A.2d 808 (Md 2002).
8. Emergency Medical Treatment and Active Labor Act, 42 USC § 1395DD.
9. Lownsbury v VanBuren, 94 Ohio St 3d 231, 762 NE 2d 354 (2002).
10. Blazo v McLaren Regional Medical Center, 2002 Mich App LEXIS 752 (Mich Ct App 2002).
11. Kelley v Middle Tennessee Emergency Physicians, PC, 133 SW3d 587 (Tenn 2004).
12. Hammonds v Jewish Hospital, 899 SW2d 527 (Mo Ct App 1995).
13. MAG Mutual Insurance Company, Inc. Curbing the curbside consult—a risk management perspective. J Med Assoc Ga. 2008;97(1):50.-
14. Burns CD. Death of the curbside consult? J Ky Med Assoc. 2006;104(1):27.-
15. Hendel T. Informal consultations: do new risks exist with this age-old tradition? J Med Pract Manage. 2002;17(6):308-311.
16. Manian FA, Janssen DA. Curbside consultations. A closer look at a common practice. JAMA. 1996;275(2):145-147.
17. Curbside consultations. Psychiatry (Edgmont). 2010;7(5):51-53.


