User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
People who live in ethical ‘glass houses’
Psychiatrists often have to deal with “ethically challenged” entities or individuals. This can be of concern because, ultimately, unethical policies or behavior can harm our patients. However, psychiatrists recognize that what may appear to be unethical to them may not be considered as such by nonpsychiatrists.
Ethics (from Ethos, which means customs) is defined as the standards and rules of conduct that govern a set of human actions by a specific group, profession, or culture. Thus, there are medical ethics, corporate ethics, Christian ethics, student ethics, fishing ethics, etc. Ethics is, therefore, not an absolute standard and may exist only “in the eye of the beholder.” For example, psychiatry as a medical specialty has its own rigorous ethical code that other medical specialties do not uphold (such as prohibiting socializing with patients).
Who are the entities whose ethical transgressions may affect psychiatrists’ work? Consider the following examples:
Insurance companies. Some of their business practices outrage psychiatrists, including:
- vehemently opposing parity for psychiatric brain disorders with other medical disorders
- refusing to cover preexisting conditions
- the preauthorization farce, which costs psychiatrists a large amount of uncompensated time and effort (essentially an unfunded mandate)
- low reimbursement rate for psychiatric care and a bias against coverage for psychotherapy
- forcing stable patients to switch to a cheaper medication that may not work as well, thus potentially destabilizing the patient.
Pharmaceutical companies. Because of intense scrutiny by regulatory and compliance bodies, pharmaceutical companies have largely discontinued questionable practices such as:
- not publishing unfavorable drug data
- minimizing serious side effects such as obesity and diabetes until after their drug is widely used.
However, some companies continue to disconcert psychiatrists and trigger their umbrage by:
- abandoning psychiatric drug development despite the tremendous unmet need and shifting resources to more profitable therapeutic areas
- direct-to-consumer advertising that disrupts the doctor-patient relationship and undermines psychiatrists’ clinical judgment.
The FDA. This key government agency plays an important role in protecting the public, but its policies occasionally spawn ethical dilemmas.
For example, why does it insist that new psychiatric medications be indicated for a DSM “diagnosis” instead of common “symptoms” such as agitation, depression, delusions, hallucinations, anxiety, or impulsivity? DSM diagnoses are arbitrary, committee-created constructs that may change drastically from edition to edition. There is extensive evidence for overlapping symptoms of many psychiatric “diagnoses,” which implies that a drug approved and deemed safe and effective for 1 psychiatric syndrome (eg, psychosis, depression) can help other disorders that share symptoms.
Why doesn’t the FDA channel the billions of dollars in penalties they have imposed on pharmaceutical companies to the National Institutes of Health (NIH) instead of to the government’s general fund? These valuable funds are being siphoned from research; ethically, from a public health perspective, they should be kept in research. These billions can help one of the NIH institutes such the National Institute of Mental Health (NIMH) establish a psychiatric drug development section to translate biologic discoveries into novel treatments. No such capability exists at the NIMH due to lack of funds.
Legislators. There generally is considerable cynicism about the ethical conduct of politicians, but from psychiatry’s point of view, consider the following:
- Why not use the force of law to enforce parity in insurance coverage?
- Why are legislators willing to appropriate funds to build prisons but not long-term psychiatric hospitals? Is it ethical to criminalize mental illness and incarcerate persons with brain disorders side-by-side with hardened criminals instead of providing them with a dignified and safe medical facility?
- Why don’t legislators fix the broken public mental health system that is underfunded, ineffective, and too bureaucratic for patients and families to navigate?
The media. Although significant improvement has taken place in portraying mental illness compared with a few decades ago, the following unacceptable patterns continue:
- depicting the mentally ill as dangerous killers and “psychos”
- continuing to mock mental illness and addictions as character frailties rather than view them as legitimate illnesses
- failing to expose the injustices that afflict persons with psychiatric brain disorders, including stigma, neglect of physical health needs, inadequate treatment resources (such as availability of inpatient psychiatric beds), or imprisonment in lieu of hospitalization.
Communities. It is regrettable that the negative attitude toward mental illness still is intense enough to perpetuate the vociferous “not in my backyard” (NIMBY) opposition to mental health clinics, residential facilities, or halfway houses. Although the NIMBY syndrome is driven by lack of education and/or understanding of mental illness, ignorance is a poor excuse for ethical shortcomings.
Non-psychiatric physicians. It is quite disheartening to see how prejudiced some internists and surgeons can be toward mentally ill individuals. Most developed a distorted view of psychiatry from being trained decades ago, before the momentous neuroscience advances in psychiatry. But more worrisome are the barriers mentally ill persons face in health care1-4 that lead to underutilization of routine primary care5 and underdiagnosis of serious health conditions.2,6 Psychiatric patients are less likely to undergo coronary revascularization procedures after a myocardial infarction7 or to be properly treated for chronic conditions such as arthritis.8 Limited or inadequate medical care has led to early mortality.9,10 But there is good news from the U.S. Department of Veterans Affairs, where psychiatric patients with diabetes receive as good care as veterans without mental illness11 and have no barriers to nutrition and exercise counseling.12
What about our own ethical conduct?
Finally, perhaps psychiatrists should think twice before throwing stones because we, too, may live in ethical “glass houses.” Although we try to adhere to our ethical standards, some of us occasionally may commit ethical peccadilloes, such as:
- continuing to use haloperidol, a 45-year-old drug that has been shown to be neurotoxic in >20 studies over the past decade13
- ignoring tier I evidence-based treatments and using unproven modalities that may delay illness resolution
- not regularly monitoring patients for metabolic complications of antipsychotics14
- not using depot antipsychotics for patients who exhibit violent behavior each time they relapse due to nonadherence
- requiring a drug company representative to bring lunch to the entire clinic staff in return for access to the prescriber.
The quandary with ethics is that they can be too nuanced, enabling almost anyone who breaches an ethical boundary to find a justification. The most unambiguous ethical standards have long been moved from a moral philosophy to codified and legally enforced laws (robbery, assault, rape, homicide, etc.). Psychiatry deals with many groups that have their own version of an “ethics compass.” We psychiatrists have our own ethics standards, which we always aspire to uphold. However, are we so ethically infallible that we can smugly throw stones at people who live in ethical “glass houses?” Doesn’t our ethical “brick house” have glass windows?
Reference
1. Druss BG. The mental health/primary care interface in the United States: history structure, and context. Gen Hosp Psychiatry. 2002;24(4):197-202.
2. Druss BG, Rosenheck RA. Use of medical services by veterans with mental disorders. Psychosomatics. 1997;38(5):451-458.
3. Druss BG, Rosenheck RA. Mental disorders and access to medical care in the United States. Am J Psychiatry. 1998;155(12):1775-1777.
4. Levinson Miller C, Druss BG, Dombrowski EA, et al. Barriers to primary medical care among patients at a community mental health center. Psychiatr Serv. 2003;54(8):1158-1160.
5. Bosworth HB, Calhoun PS, Stechuchak KM, et al. Use of psychiatric and medical health care by veterans with severe mental illness. Psychiatr Serv. 2004;55(6):708-710.
6. Cradock-O’Leary J, Young AS, Yano EM, et al. Use of general medical services by VA patients with psychiatric disorders. Psychiatr Serv. 2002;53(7):874-878.
7. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
8. Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338(21):1516-1520.
9. Casey DE, Hansen TE. Excessive mortality and morbidity associated with schizophrenia. In: Meyer JM Nasrallah HA, eds. Medical illness and schizophrenia. Arlington, VA: American Psychiatric Publishing, Inc; 2008;17-35.
10. Enger C, Weatherby L, Reynolds RF, et al. Serious cardiovascular events and mortality among patients with schizophrenia. J Nerv Ment Dis. 2004;192(1):19-27.
11. Desai MM, Rosenheck RA, Druss BG, et al. Mental disorders and quality of diabetes care in the veterans health administration. Am J Psychiatry. 2002;159(9):1584-1590.
12. Desai MM, Rosenheck RA, Druss BG, et al. Receipt of nutrition and exercise counseling among medical outpatients with psychiatric and substance use disorders. J Gen Intern Med. 2002;17(7):556-560.
13. Nasrallah HA. Invisible tattoos: the stigmata of psychiatry. Current Psychiatry. 2011;10(9):18-19.
14. Buckley PF, Miller DD, Singer B, et al. Clinicians’ recognition of the metabolic adverse effects of antipsychotic medications. Schizophr Res. 2005;79(2-3):281-288.
Psychiatrists often have to deal with “ethically challenged” entities or individuals. This can be of concern because, ultimately, unethical policies or behavior can harm our patients. However, psychiatrists recognize that what may appear to be unethical to them may not be considered as such by nonpsychiatrists.
Ethics (from Ethos, which means customs) is defined as the standards and rules of conduct that govern a set of human actions by a specific group, profession, or culture. Thus, there are medical ethics, corporate ethics, Christian ethics, student ethics, fishing ethics, etc. Ethics is, therefore, not an absolute standard and may exist only “in the eye of the beholder.” For example, psychiatry as a medical specialty has its own rigorous ethical code that other medical specialties do not uphold (such as prohibiting socializing with patients).
Who are the entities whose ethical transgressions may affect psychiatrists’ work? Consider the following examples:
Insurance companies. Some of their business practices outrage psychiatrists, including:
- vehemently opposing parity for psychiatric brain disorders with other medical disorders
- refusing to cover preexisting conditions
- the preauthorization farce, which costs psychiatrists a large amount of uncompensated time and effort (essentially an unfunded mandate)
- low reimbursement rate for psychiatric care and a bias against coverage for psychotherapy
- forcing stable patients to switch to a cheaper medication that may not work as well, thus potentially destabilizing the patient.
Pharmaceutical companies. Because of intense scrutiny by regulatory and compliance bodies, pharmaceutical companies have largely discontinued questionable practices such as:
- not publishing unfavorable drug data
- minimizing serious side effects such as obesity and diabetes until after their drug is widely used.
However, some companies continue to disconcert psychiatrists and trigger their umbrage by:
- abandoning psychiatric drug development despite the tremendous unmet need and shifting resources to more profitable therapeutic areas
- direct-to-consumer advertising that disrupts the doctor-patient relationship and undermines psychiatrists’ clinical judgment.
The FDA. This key government agency plays an important role in protecting the public, but its policies occasionally spawn ethical dilemmas.
For example, why does it insist that new psychiatric medications be indicated for a DSM “diagnosis” instead of common “symptoms” such as agitation, depression, delusions, hallucinations, anxiety, or impulsivity? DSM diagnoses are arbitrary, committee-created constructs that may change drastically from edition to edition. There is extensive evidence for overlapping symptoms of many psychiatric “diagnoses,” which implies that a drug approved and deemed safe and effective for 1 psychiatric syndrome (eg, psychosis, depression) can help other disorders that share symptoms.
Why doesn’t the FDA channel the billions of dollars in penalties they have imposed on pharmaceutical companies to the National Institutes of Health (NIH) instead of to the government’s general fund? These valuable funds are being siphoned from research; ethically, from a public health perspective, they should be kept in research. These billions can help one of the NIH institutes such the National Institute of Mental Health (NIMH) establish a psychiatric drug development section to translate biologic discoveries into novel treatments. No such capability exists at the NIMH due to lack of funds.
Legislators. There generally is considerable cynicism about the ethical conduct of politicians, but from psychiatry’s point of view, consider the following:
- Why not use the force of law to enforce parity in insurance coverage?
- Why are legislators willing to appropriate funds to build prisons but not long-term psychiatric hospitals? Is it ethical to criminalize mental illness and incarcerate persons with brain disorders side-by-side with hardened criminals instead of providing them with a dignified and safe medical facility?
- Why don’t legislators fix the broken public mental health system that is underfunded, ineffective, and too bureaucratic for patients and families to navigate?
The media. Although significant improvement has taken place in portraying mental illness compared with a few decades ago, the following unacceptable patterns continue:
- depicting the mentally ill as dangerous killers and “psychos”
- continuing to mock mental illness and addictions as character frailties rather than view them as legitimate illnesses
- failing to expose the injustices that afflict persons with psychiatric brain disorders, including stigma, neglect of physical health needs, inadequate treatment resources (such as availability of inpatient psychiatric beds), or imprisonment in lieu of hospitalization.
Communities. It is regrettable that the negative attitude toward mental illness still is intense enough to perpetuate the vociferous “not in my backyard” (NIMBY) opposition to mental health clinics, residential facilities, or halfway houses. Although the NIMBY syndrome is driven by lack of education and/or understanding of mental illness, ignorance is a poor excuse for ethical shortcomings.
Non-psychiatric physicians. It is quite disheartening to see how prejudiced some internists and surgeons can be toward mentally ill individuals. Most developed a distorted view of psychiatry from being trained decades ago, before the momentous neuroscience advances in psychiatry. But more worrisome are the barriers mentally ill persons face in health care1-4 that lead to underutilization of routine primary care5 and underdiagnosis of serious health conditions.2,6 Psychiatric patients are less likely to undergo coronary revascularization procedures after a myocardial infarction7 or to be properly treated for chronic conditions such as arthritis.8 Limited or inadequate medical care has led to early mortality.9,10 But there is good news from the U.S. Department of Veterans Affairs, where psychiatric patients with diabetes receive as good care as veterans without mental illness11 and have no barriers to nutrition and exercise counseling.12
What about our own ethical conduct?
Finally, perhaps psychiatrists should think twice before throwing stones because we, too, may live in ethical “glass houses.” Although we try to adhere to our ethical standards, some of us occasionally may commit ethical peccadilloes, such as:
- continuing to use haloperidol, a 45-year-old drug that has been shown to be neurotoxic in >20 studies over the past decade13
- ignoring tier I evidence-based treatments and using unproven modalities that may delay illness resolution
- not regularly monitoring patients for metabolic complications of antipsychotics14
- not using depot antipsychotics for patients who exhibit violent behavior each time they relapse due to nonadherence
- requiring a drug company representative to bring lunch to the entire clinic staff in return for access to the prescriber.
The quandary with ethics is that they can be too nuanced, enabling almost anyone who breaches an ethical boundary to find a justification. The most unambiguous ethical standards have long been moved from a moral philosophy to codified and legally enforced laws (robbery, assault, rape, homicide, etc.). Psychiatry deals with many groups that have their own version of an “ethics compass.” We psychiatrists have our own ethics standards, which we always aspire to uphold. However, are we so ethically infallible that we can smugly throw stones at people who live in ethical “glass houses?” Doesn’t our ethical “brick house” have glass windows?
Psychiatrists often have to deal with “ethically challenged” entities or individuals. This can be of concern because, ultimately, unethical policies or behavior can harm our patients. However, psychiatrists recognize that what may appear to be unethical to them may not be considered as such by nonpsychiatrists.
Ethics (from Ethos, which means customs) is defined as the standards and rules of conduct that govern a set of human actions by a specific group, profession, or culture. Thus, there are medical ethics, corporate ethics, Christian ethics, student ethics, fishing ethics, etc. Ethics is, therefore, not an absolute standard and may exist only “in the eye of the beholder.” For example, psychiatry as a medical specialty has its own rigorous ethical code that other medical specialties do not uphold (such as prohibiting socializing with patients).
Who are the entities whose ethical transgressions may affect psychiatrists’ work? Consider the following examples:
Insurance companies. Some of their business practices outrage psychiatrists, including:
- vehemently opposing parity for psychiatric brain disorders with other medical disorders
- refusing to cover preexisting conditions
- the preauthorization farce, which costs psychiatrists a large amount of uncompensated time and effort (essentially an unfunded mandate)
- low reimbursement rate for psychiatric care and a bias against coverage for psychotherapy
- forcing stable patients to switch to a cheaper medication that may not work as well, thus potentially destabilizing the patient.
Pharmaceutical companies. Because of intense scrutiny by regulatory and compliance bodies, pharmaceutical companies have largely discontinued questionable practices such as:
- not publishing unfavorable drug data
- minimizing serious side effects such as obesity and diabetes until after their drug is widely used.
However, some companies continue to disconcert psychiatrists and trigger their umbrage by:
- abandoning psychiatric drug development despite the tremendous unmet need and shifting resources to more profitable therapeutic areas
- direct-to-consumer advertising that disrupts the doctor-patient relationship and undermines psychiatrists’ clinical judgment.
The FDA. This key government agency plays an important role in protecting the public, but its policies occasionally spawn ethical dilemmas.
For example, why does it insist that new psychiatric medications be indicated for a DSM “diagnosis” instead of common “symptoms” such as agitation, depression, delusions, hallucinations, anxiety, or impulsivity? DSM diagnoses are arbitrary, committee-created constructs that may change drastically from edition to edition. There is extensive evidence for overlapping symptoms of many psychiatric “diagnoses,” which implies that a drug approved and deemed safe and effective for 1 psychiatric syndrome (eg, psychosis, depression) can help other disorders that share symptoms.
Why doesn’t the FDA channel the billions of dollars in penalties they have imposed on pharmaceutical companies to the National Institutes of Health (NIH) instead of to the government’s general fund? These valuable funds are being siphoned from research; ethically, from a public health perspective, they should be kept in research. These billions can help one of the NIH institutes such the National Institute of Mental Health (NIMH) establish a psychiatric drug development section to translate biologic discoveries into novel treatments. No such capability exists at the NIMH due to lack of funds.
Legislators. There generally is considerable cynicism about the ethical conduct of politicians, but from psychiatry’s point of view, consider the following:
- Why not use the force of law to enforce parity in insurance coverage?
- Why are legislators willing to appropriate funds to build prisons but not long-term psychiatric hospitals? Is it ethical to criminalize mental illness and incarcerate persons with brain disorders side-by-side with hardened criminals instead of providing them with a dignified and safe medical facility?
- Why don’t legislators fix the broken public mental health system that is underfunded, ineffective, and too bureaucratic for patients and families to navigate?
The media. Although significant improvement has taken place in portraying mental illness compared with a few decades ago, the following unacceptable patterns continue:
- depicting the mentally ill as dangerous killers and “psychos”
- continuing to mock mental illness and addictions as character frailties rather than view them as legitimate illnesses
- failing to expose the injustices that afflict persons with psychiatric brain disorders, including stigma, neglect of physical health needs, inadequate treatment resources (such as availability of inpatient psychiatric beds), or imprisonment in lieu of hospitalization.
Communities. It is regrettable that the negative attitude toward mental illness still is intense enough to perpetuate the vociferous “not in my backyard” (NIMBY) opposition to mental health clinics, residential facilities, or halfway houses. Although the NIMBY syndrome is driven by lack of education and/or understanding of mental illness, ignorance is a poor excuse for ethical shortcomings.
Non-psychiatric physicians. It is quite disheartening to see how prejudiced some internists and surgeons can be toward mentally ill individuals. Most developed a distorted view of psychiatry from being trained decades ago, before the momentous neuroscience advances in psychiatry. But more worrisome are the barriers mentally ill persons face in health care1-4 that lead to underutilization of routine primary care5 and underdiagnosis of serious health conditions.2,6 Psychiatric patients are less likely to undergo coronary revascularization procedures after a myocardial infarction7 or to be properly treated for chronic conditions such as arthritis.8 Limited or inadequate medical care has led to early mortality.9,10 But there is good news from the U.S. Department of Veterans Affairs, where psychiatric patients with diabetes receive as good care as veterans without mental illness11 and have no barriers to nutrition and exercise counseling.12
What about our own ethical conduct?
Finally, perhaps psychiatrists should think twice before throwing stones because we, too, may live in ethical “glass houses.” Although we try to adhere to our ethical standards, some of us occasionally may commit ethical peccadilloes, such as:
- continuing to use haloperidol, a 45-year-old drug that has been shown to be neurotoxic in >20 studies over the past decade13
- ignoring tier I evidence-based treatments and using unproven modalities that may delay illness resolution
- not regularly monitoring patients for metabolic complications of antipsychotics14
- not using depot antipsychotics for patients who exhibit violent behavior each time they relapse due to nonadherence
- requiring a drug company representative to bring lunch to the entire clinic staff in return for access to the prescriber.
The quandary with ethics is that they can be too nuanced, enabling almost anyone who breaches an ethical boundary to find a justification. The most unambiguous ethical standards have long been moved from a moral philosophy to codified and legally enforced laws (robbery, assault, rape, homicide, etc.). Psychiatry deals with many groups that have their own version of an “ethics compass.” We psychiatrists have our own ethics standards, which we always aspire to uphold. However, are we so ethically infallible that we can smugly throw stones at people who live in ethical “glass houses?” Doesn’t our ethical “brick house” have glass windows?
Reference
1. Druss BG. The mental health/primary care interface in the United States: history structure, and context. Gen Hosp Psychiatry. 2002;24(4):197-202.
2. Druss BG, Rosenheck RA. Use of medical services by veterans with mental disorders. Psychosomatics. 1997;38(5):451-458.
3. Druss BG, Rosenheck RA. Mental disorders and access to medical care in the United States. Am J Psychiatry. 1998;155(12):1775-1777.
4. Levinson Miller C, Druss BG, Dombrowski EA, et al. Barriers to primary medical care among patients at a community mental health center. Psychiatr Serv. 2003;54(8):1158-1160.
5. Bosworth HB, Calhoun PS, Stechuchak KM, et al. Use of psychiatric and medical health care by veterans with severe mental illness. Psychiatr Serv. 2004;55(6):708-710.
6. Cradock-O’Leary J, Young AS, Yano EM, et al. Use of general medical services by VA patients with psychiatric disorders. Psychiatr Serv. 2002;53(7):874-878.
7. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
8. Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338(21):1516-1520.
9. Casey DE, Hansen TE. Excessive mortality and morbidity associated with schizophrenia. In: Meyer JM Nasrallah HA, eds. Medical illness and schizophrenia. Arlington, VA: American Psychiatric Publishing, Inc; 2008;17-35.
10. Enger C, Weatherby L, Reynolds RF, et al. Serious cardiovascular events and mortality among patients with schizophrenia. J Nerv Ment Dis. 2004;192(1):19-27.
11. Desai MM, Rosenheck RA, Druss BG, et al. Mental disorders and quality of diabetes care in the veterans health administration. Am J Psychiatry. 2002;159(9):1584-1590.
12. Desai MM, Rosenheck RA, Druss BG, et al. Receipt of nutrition and exercise counseling among medical outpatients with psychiatric and substance use disorders. J Gen Intern Med. 2002;17(7):556-560.
13. Nasrallah HA. Invisible tattoos: the stigmata of psychiatry. Current Psychiatry. 2011;10(9):18-19.
14. Buckley PF, Miller DD, Singer B, et al. Clinicians’ recognition of the metabolic adverse effects of antipsychotic medications. Schizophr Res. 2005;79(2-3):281-288.
Reference
1. Druss BG. The mental health/primary care interface in the United States: history structure, and context. Gen Hosp Psychiatry. 2002;24(4):197-202.
2. Druss BG, Rosenheck RA. Use of medical services by veterans with mental disorders. Psychosomatics. 1997;38(5):451-458.
3. Druss BG, Rosenheck RA. Mental disorders and access to medical care in the United States. Am J Psychiatry. 1998;155(12):1775-1777.
4. Levinson Miller C, Druss BG, Dombrowski EA, et al. Barriers to primary medical care among patients at a community mental health center. Psychiatr Serv. 2003;54(8):1158-1160.
5. Bosworth HB, Calhoun PS, Stechuchak KM, et al. Use of psychiatric and medical health care by veterans with severe mental illness. Psychiatr Serv. 2004;55(6):708-710.
6. Cradock-O’Leary J, Young AS, Yano EM, et al. Use of general medical services by VA patients with psychiatric disorders. Psychiatr Serv. 2002;53(7):874-878.
7. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
8. Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338(21):1516-1520.
9. Casey DE, Hansen TE. Excessive mortality and morbidity associated with schizophrenia. In: Meyer JM Nasrallah HA, eds. Medical illness and schizophrenia. Arlington, VA: American Psychiatric Publishing, Inc; 2008;17-35.
10. Enger C, Weatherby L, Reynolds RF, et al. Serious cardiovascular events and mortality among patients with schizophrenia. J Nerv Ment Dis. 2004;192(1):19-27.
11. Desai MM, Rosenheck RA, Druss BG, et al. Mental disorders and quality of diabetes care in the veterans health administration. Am J Psychiatry. 2002;159(9):1584-1590.
12. Desai MM, Rosenheck RA, Druss BG, et al. Receipt of nutrition and exercise counseling among medical outpatients with psychiatric and substance use disorders. J Gen Intern Med. 2002;17(7):556-560.
13. Nasrallah HA. Invisible tattoos: the stigmata of psychiatry. Current Psychiatry. 2011;10(9):18-19.
14. Buckley PF, Miller DD, Singer B, et al. Clinicians’ recognition of the metabolic adverse effects of antipsychotic medications. Schizophr Res. 2005;79(2-3):281-288.
Personalizing depression treatment: 2 clinical tools
Approximately one-half of patients with major depressive disorder (MDD) will have partial or nonresponse to first-line antidepressant monotherapy, despite receiving an adequate dosage and sufficient duration of treatment.1 This has led to the definition of treatment-resistant depression (TRD) as a depressive episode that has shown insufficient response to ≥1 trial of an antidepressant that has demonstrated efficacy in clinical trials.1 Depressed patients should be treated to full remission because absence of complete remission is associated with:
- a more recurrent and chronic illness course2,3
- increased medical and psychiatric comorbidities
- greater functional burden
- increased social and economic costs linked with impaired social functioning.4
Clinicians need to properly identify MDD and treatment resistance to guide optimal treatment choices. Additional tools are necessary to accurately identify, document, and communicate about symptoms commonly experienced by depressed patients but not fully characterized by DSM-IV-TR MDD criteria.5 Finally, in many cases, trait or situational factors might obfuscate accurate diagnosis and the natural course of illness, and tools that can be implemented practically will help identify patients with MDD.
Our group has created and implemented 2 clinician-administered tools—the SAFER Interview and the Antidepressant Treatment Response Questionnaire (ATRQ)—to enrich the qualitative assessment of MDD and treatment resistance.
SAFER: Assessing the diagnosis, symptom severity
The SAFER interview refines the diagnosis of depressed patients by assessing the state vs trait nature of the symptoms, their assessability, their face and ecological validity, and if they pass the rule of the 3 Ps: pervasiveness, persistence, and pathological nature of the current MDD episode (Table 1 and Table 2).6 This reliable assessment of the patient’s diagnosis and symptom severity is made in a way that reflects the illness in a real-world setting.
Clinical application of SAFER. Implementing SAFER in clinical settings promotes a personalized, dimensional approach by taking into account a varying degree of symptom severity in depressed patients, in contrast to relying on symptom lists as found in the DSM-IV-TR. Using the SAFER interview deepens the typical psychiatric diagnostic process, allows for a more precise understanding of the patient’s situation, and may help clinicians select effective treatments that target specific symptoms, thus resulting in more rapid alleviation of MDD.6
Table 1
The SAFER interview: Assessing depression in a real-world setting
State vs trait nature of the symptoms
|
Assessability
|
Face validity
|
Ecological validity
|
Rule of the 3 Ps
|
| © Massachusetts General Hospital Source: Reference 6 |
Table 2
The SAFER criteria: Rule of the 3 Ps
| Pervasive—Do the major symptoms affect the patient across multiple arenas of life (work, relationships, school, chores, etc.)? |
| Persistent—Do the main symptoms occur most days, most of the day during the current episode? |
| Pathological—Do the symptoms of the present episode interfere with functioning? |
| © Massachusetts General Hospital Source: Reference 6 |
CASE REPORT: Worsening symptoms
Ms. Y, age 53, has been depressed for 30 years. She hardly remembers a time in her life when she felt good for more than a few days. However, 2 months ago she noted her symptoms got worse. She presents with many MDD symptoms as assessed by the Hamilton Depression Rating Scale, eg, ongoing depressive mood, feelings of guilt, major sleep disturbances, and work impairments.
Using SAFER to evaluate Ms. Y, a clinician would ask: Does she have symptoms that are present primarily during an episode of acute illness? Does the episode constitute a measurable exacerbation of preexisting symptoms? This clinical vignette illustrates the importance of the first SAFER criterion, state vs trait nature of the symptoms. Ms. Y is a SAFER “pass”—meaning consistent with a major depressive episode—because exacerbation of preexisting symptoms is measurable. However, if her symptoms represented a chronic, long-standing trait, she would be a SAFER “fail” based on this criterion, and her symptoms likely would not improve during a brief pharmacologic intervention. For such patients, SAFER would have oriented the clinician toward alternative therapies such as psychotherapy or a combination of longer, more complex pharmacologic treatment and psychotherapy.
By helping clinicians refine MDD diagnoses, SAFER draws attention to specific depression entities (eg, MDD or dysthymia) and directs them toward appropriate guidelines, treatment algorithms, and precautions (eg, when treated with pharmacotherapy, dysthymic patients are particularly sensitive to unwanted effects and adherence is a serious issue).7 The SAFER interview also can help identify when what appears to be a depressive episode clearly is precipitated by situational factors, and is more likely to be influenced by changes in the patient’s life than by treatment. For such patients, first consider nonpharmacologic interventions to avoid unnecessary medication exposure.
ATRQ: Efficacy and adequacy
The ATRQ examines the efficacy (improvement from 0% [not improved at all] to 100% [completely improved]), and adequacy (adequate duration and dose) of any antidepressant treatment in a step-by-step procedure.1,8,9 For a copy of the ATRQ, click here.
While conducting the interview, clinicians ask about treatment adherence to each medication trial. A treatment-resistant patient may go through many types of trials, from monotherapy to combination to augmentation.10 For each trial, the ATRQ systematically reviews 4 strategies to enhance treatment response:
- increasing the initial antidepressant dosage11
- combining the initial antidepressant with another antidepressant, typically from another class12
- augmenting the initial antidepressant with a nonantidepressant12
- switching from the initial antidepressant to another antidepressant.13
These strategies also are applied in cases of lost sustained antidepressant efficacy or depressive relapse/recurrence, although empirical evidence supporting these strategies is lacking, with the possible exception of dose increase.14,15
In the convention our group adopted, an adequate antidepressant trial must be ≥6 weeks in total length, with a dose within an adequate range as specified in the medication’s package insert. In addition, for the purposes of conducting TRD trials, we have considered a patient treatment-resistant if response to adequate dose and duration is <50%. On the ATRQ, 50% improvement refers to 50% symptom reduction from baseline without achieving remission. In an initial clinical trial that lasts ≥6 weeks, any dose increase (for ≥4 weeks) represents optimization and is not considered a new or separate trial, whereas augmentation or combination therapy (for ≥3 weeks) or a switch to another antidepressant (for ≥6 weeks) are considered new trials/treatments.
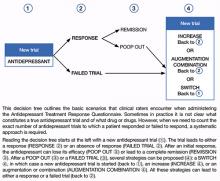
Decision tree for ATRQ. Because antidepressant treatment always is constrained by personal (eg, adherence, insurance coverage, etc.) and clinical (eg, contraindications due to comorbid conditions, side effects, etc.) considerations, we propose a decision tree to help clinicians determine the number of failed antidepressant trials their patient experienced (Figure). Although this tree does not represent all treatment scenarios, we hope it could help clinicians implement TRD treatment strategies because it highlights proper assessment of treatment duration, dosage, and changes before applying a TRD diagnosis.
The ATRQ meticulously examines patients’ antidepressant history to identify:
- pseudo-resistance, to guide adequate dosing and/or duration, and
- resistance, to propose next-step treatment.
Pseudo-resistance refers to treatment failures that can be attributed to factors such as inadequate treatment dosing or duration, atypical pharmacokinetics that reduce agents’ effectiveness, patient nonadherence (eg, due to adverse effects), or misdiagnosis of the primary disorder (ie, other mood disorders or depressive subsets such as dysthymia or minor depression mistreated as unipolar depression).16,17 Studies show that many patients with MDD referred to specialty settings are undertreated and receive inadequate antidepressant doses,18 which suggests that many referrals for TRD are in fact pseudo-resistance.19 Despite the lack of consensus on criteria for TRD,1 standardization of what constitutes treatment adequacy during antidepressant trials (eg, adherence, dose, duration) is indispensable.
Clinical application of the ATRQ. Because TRD may require specific interventions, we first need to properly identify treatment resistance. Also, systematic use of a classification system enhances the ability of clinicians and patients to provide meaningful descriptions of antidepressant resistance.
In clinical practice, choice of treatment strategy is based on factors that include partial or nonresponse, tolerability, avoiding withdrawal symptoms, the need to target side effects of a current antidepressant by administering another drug, cost, avoiding drug-drug interactions, and patient preference. Because a treatment-resistant patient may go through many types of trials, it is essential to obtain information about all treatments to determine the number of failed clinical trials a patient may have had for the current MDD episode and lifetime episodes. The importance of asking about adherence to each trial cannot be overemphasized.
Figure: Using the Massachusetts General Hospital Antidepressant Treatment History Questionnaire: A decision tree
CASE REPORT: Limited improvement
Mr. T, age 45, reported that his current depressive episode started several years ago. The first antidepressant trial he received, sertraline, 100 mg/d for 3 months, resulted in 0% improvement. Next he received citalopram, 20 mg/d, for 1 month, without any improvement. The next trial, venlafaxine, 75 mg/d, for 7 weeks, resulted in a 30% response. More recently Mr. B tried duloxetine, up to 90 mg/d, for 2 years with an 80% improvement during the first 3 months and then a decrease of response to <40%. He then received aripiprazole, 10 mg/d, in combination with duloxetine for approximately 4 weeks with no response.
Using the ATRQ to evaluate Mr. B, a clinician would consider the sertraline trial as a failed trial. The citalopram treatment would not be considered an adequate trial because it was too short. The venlafaxine course wouldn’t count as an adequate trial because the dosage was too low. The trial with duloxetine would count as a response (80% improvement) followed by a “poop out” or tachyphylaxis (40% improvement). The fifth trial with the combination of duloxetine and aripiprazole would count as a failed trial. The ATRQ highlights which drugs have been used for too short a duration or at too low a dosage. In Mr. B’s case, using the ATRQ revealed that of 5 trials, only 2 showed antidepressant resistance.
The ATRQ and decision tree are meant to provide clinicians with user-friendly tools to more precisely determine the number of failed antidepressant trials a patient experienced. By assessing if an antidepressant trial had an adequate dose and duration, the ATRQ can help suggest the next treatment options. For example, if a trial was inadequate in dose and/or duration but the patient tolerated the medication, then optimizing treatment with the current drug would be a logical next step. If a patient does not respond to an adequate trial, clinicians have several options, such as switching to another antidepressant, using a combination of medications or an augmentation strategy, or increasing the dose of the original antidepressant.
Limitations of the ATRQ. Historical rating of treatment is not as accurate as a prospective trial. Another limitation of the ATRQ is that the minimum effective dose is accepted as adequate; many clinicians would suggest that such a dose is inadequate. Also, the duration specified for augmentation, dose increase, and monotherapy are based on expert consensus1 rather than systematic research. Nonetheless, this method of documenting prior trials and treatment adequacy is an important advance.
The ATRQ lacks a place to indicate discontinuation due to intolerance. Knowing if adverse events caused treatment nonadherence or discontinuation is relevant to selecting treatment.
The ATRQ considers only pharmacotherapy and electroconvulsive therapy. Comprehensive assessment of treatment resistance requires asking about depression-specific, evidence-based psychotherapies, including cognitive-behavioral therapy and interpersonal therapy. Another precaution is that the ATRQ and SAFER should be used in conjunction with structured or semi-structured clinical interviews and other clinical tools to rule out other primary diagnoses (eg, bipolar disorder, posttraumatic stress disorder, obsessive-compulsive disorder, or surreptitious substance abuse).20
Using SAFER and ATRQ allows clinicians to make a proper MDD diagnosis with an accurate, reliable assessment of symptom severity and a precise count of antidepressant trials of adequate duration and dose. SAFER helps differentiate MDD from other depressive disorders and can be used to separate MDD from a similar presentation of a reaction to external circumstances that may remit if these circumstances change.
These 2 clinical tools focus on MDD and TRD. Compared with treatment resistance in MDD, treatment resistance in other forms of depression, such as minor depressive disorder or dysthymia, has been inadequately researched21,22 and should be addressed in large studies. However, to help patients achieve remission of depressive disorders—especially MDD—clinicians should use patient information gathered via measurement-based care in combination with algorithm recommendations.23 Persistence of clinical care of MDD is essential because several treatment steps might be necessary for some patients to achieve remission. Throughout treatment, patients’ symptoms, adverse events secondary to ongoing treatment, and medication adherence should be assessed with appropriate tools. ATRQ and SAFER offer a clinician support in assessing treatment resistance history and a patient’s likelihood to respond to pharmacologic treatment.
Related Resources
- Desseilles M, Witte J, Chang TE, et al. Assessing the adequacy of past antidepressant trials: a clinician’s guide to the Antidepressant Treatment Response Questionnaire (ATRQ). J Clin Psychiatry. 2011;72(8):1152-1154.
- Targum SD, Pollack MH, Fava M. Redefining affective disorders: relevance for drug development. CNS Neurosci Ther. 2008;14(1):2-9.
- Depression Clinical and Research Program. www.massgeneral.org/psychiatry/services/dcrp_home.aspx.
Drug Brand Names
- Aripiprazole • Abilify
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Desseilles was supported by the National Funds for Scientific Research of Belgium. Additional support came from the Belgian American Educational Foundation.
Dr. Fava receives grant/research support from, is a consultant to, and/or is a speaker for Abbott Laboratories, Adamed, Advanced Meeting Partners, Affectis Pharmaceuticals, Alkermes, Amarin Pharma, American Psychiatric Association, American Society of Clinical Psychopharmacology, Aspect Medical Systems, AstraZeneca, Auspex Pharmaceuticals, Bayer, Belvoir Media Group, Best Practice Project Management, BioMarin, BioResearch, Biovail, Boehringer Ingelheim, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Clinical Trials Solutions, Clintara, CME Institute/Physicians Postgraduate Press, CNS Response, Compellis Pharmaceuticals, Covance, Covidien, Cypress Pharmaceutical, DiagnoSearch Life Sciences, Dainippon Sumitomo Pharma, Dov Pharmaceuticals, Edgemont Pharmaceuticals, Eisai, Eli Lilly and Company, EnVivo Pharmaceuticals, ePharmaSolutions, EPIX Pharmaceuticals, Euthymics Bioscience, Fabre-Kramer Pharmaceuticals, Forest Pharmaceuticals, Ganeden Biotech, GenOmind, GlaxoSmithKline, Grünenthal GmbH, Icon Clinical Research, Imedex, i3 Innovus/Ingenix, Janssen Pharmaceutica, Johnson & Johnson Pharmaceutical Research & Development, Knoll Pharmaceuticals, Labopharm, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Lundbeck, MedAvante, Merck, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed Elsevier, MSI Methylation Sciences, National Alliance for Research on Schizophrenia & Depression, National Center for Complementary and Alternative Medicine, National Institute of Drug Abuse, National Institute of Mental Health, Naurex, Neuronetics, NextWave Pharmaceuticals, Novartis, Nutrition 21, Orexigen Therapeutics, Organon Pharmaceuticals, Otsuka Pharmaceuticals, PamLab, Pfizer, PharmaStar, Pharmavite®, PharmoRx Therapeutics, Photothera, Precision Human Biolaboratory, Prexa Pharmaceuticals, Puretech Ventures, PsychoGenics, Psylin Neurosciences, RCT Logic, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Pharmaceuticals, sanofi-aventis US, Schering-Plough, Sepracor, Servier Laboratories, Shire, Solvay Pharmaceuticals, Somaxon Pharmaceuticals, Somerset Pharmaceuticals, Sunovion Pharmaceuticals, Supernus Pharmaceuticals, Synthelabo, Takeda Pharmaceutical, Tal Medical, Tetragenex Pharmaceuticals, Transcept Pharmaceuticals, TransForm Pharmaceuticals, United BioSource, Vanda Pharmaceuticals, and Wyeth-Ayerst Laboratories.
Over the past year, Dr. Mischoulon has received research support from the Bowman Family Foundation, FisherWallace, Ganeden, and Nordic Naturals. He has received honoraria for speaking from Nordic Naturals. He has received royalties from Lippincott Williams & Wilkins for the book Natural medications for psychiatric disorders: Considering the alternatives. No payment has exceeded $10,000.
Dr. Freeman has received research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, and served on a one-time advisory board to Bristol-Myers Squibb.
Acknowledgment
The authors acknowledge Janet Witte, MD, MPH, Trina E. Chang, MD, MPH, Nadia Iovieno, MD, PhD, Christina Dording, MD, Heidi Ashih, MD, PhD, Maren Nyer, PhD, and Marasha-Fiona De Jong, MD from the Clinical Trials Network and Institute at Massachusetts General Hospital, Boston, MA.
1. Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649-659.
2. Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50(2-3):97-108.
3. Van Londen L, Molenaar RP, Goekoop JG, et al. Three- to 5-year prospective follow-up of outcome in major depression. Psychol Med. 1998;28(3):731-735.
4. Paykel ES. Achieving gains beyond response. Acta Psychiatr Scand Suppl. 2002;(415):12-17.
5. Cassano P, Fava M. Depression and public health: an overview. J Psychosom Res. 2002;53(4):849-857.
6. Targum SD, Pollack MH, Fava M. Redefining affective disorders: relevance for drug development. CNS Neurosci Ther. 2008;14(1):2-9.
7. Versiani M, Nardi AE, Figueira I. Pharmacotherapy of dysthymia: review and new findings. Eur Psychiatry. 1998;13(4):203-209.
8. Chandler GM, Iosifescu DV, Pollack MH, et al. RESEARCH: validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322-325.
9. Desseilles M, Witte J, Chang TE, et al. Assessing the adequacy of past antidepressant trials: a clinician’s guide to the antidepressant treatment response questionnaire. J Clin Psychiatry. 2011;72(8):1152-1154.
10. Fava M. Augmentation and combination strategies for complicated depression. J Clin Psychiatry. 2009;70(11):e40.-
11. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
12. Fava M, Rush AJ. Current status of augmentation and combination treatments for major depressive disorder: a literature review and a proposal for a novel approach to improve practice. Psychother Psychosom. 2006;75(3):139-153.
13. Wohlreich MM, Mallinckrodt CH, Watkin JG, et al. Immediate switching of antidepressant therapy: results from a clinical trial of duloxetine. Ann Clin Psychiatry. 2005;17(4):259-268.
14. Schmidt ME, Fava M, Zhang S, et al. Treatment approaches to major depressive disorder relapse. Part 1: dose increase. Psychother Psychosom. 2002;71(4):190-194.
15. Fava M, Detke MJ, Balestrieri M, et al. Management of depression relapse: re-initiation of duloxetine treatment or dose increase. J Psychiatr Res. 2006;40(4):328-336.
16. Kornstein SG, Schneider RK. Clinical features of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):18-25.
17. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
18. Hirschfeld RM, Keller MB, Panico S, et al. The National Depressive and Manic-Depressive Association consensus statement on the undertreatment of depression. JAMA. 1997;277(4):333-340.
19. Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10-17.
20. Parker G, Malhi GS, Crawford JG, et al. Identifying “paradigm failures” contributing to treatment-resistant depression. J Affect Disord. 2005;87(2-3):185-191.
21. Harris SJ, Parent M. Patient with chronic and apparently treatment-resistant dysthymia. Am J Psychiatry. 1986;143(2):260-261.
22. Amsterdam J, Hornig M, Nierenberg AA. Treatment-resistant mood disorders. Cambridge United Kingdom: Cambridge University Press; 2001.
23. Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry. 2009;70(suppl 6):26-31.
Approximately one-half of patients with major depressive disorder (MDD) will have partial or nonresponse to first-line antidepressant monotherapy, despite receiving an adequate dosage and sufficient duration of treatment.1 This has led to the definition of treatment-resistant depression (TRD) as a depressive episode that has shown insufficient response to ≥1 trial of an antidepressant that has demonstrated efficacy in clinical trials.1 Depressed patients should be treated to full remission because absence of complete remission is associated with:
- a more recurrent and chronic illness course2,3
- increased medical and psychiatric comorbidities
- greater functional burden
- increased social and economic costs linked with impaired social functioning.4
Clinicians need to properly identify MDD and treatment resistance to guide optimal treatment choices. Additional tools are necessary to accurately identify, document, and communicate about symptoms commonly experienced by depressed patients but not fully characterized by DSM-IV-TR MDD criteria.5 Finally, in many cases, trait or situational factors might obfuscate accurate diagnosis and the natural course of illness, and tools that can be implemented practically will help identify patients with MDD.
Our group has created and implemented 2 clinician-administered tools—the SAFER Interview and the Antidepressant Treatment Response Questionnaire (ATRQ)—to enrich the qualitative assessment of MDD and treatment resistance.
SAFER: Assessing the diagnosis, symptom severity
The SAFER interview refines the diagnosis of depressed patients by assessing the state vs trait nature of the symptoms, their assessability, their face and ecological validity, and if they pass the rule of the 3 Ps: pervasiveness, persistence, and pathological nature of the current MDD episode (Table 1 and Table 2).6 This reliable assessment of the patient’s diagnosis and symptom severity is made in a way that reflects the illness in a real-world setting.
Clinical application of SAFER. Implementing SAFER in clinical settings promotes a personalized, dimensional approach by taking into account a varying degree of symptom severity in depressed patients, in contrast to relying on symptom lists as found in the DSM-IV-TR. Using the SAFER interview deepens the typical psychiatric diagnostic process, allows for a more precise understanding of the patient’s situation, and may help clinicians select effective treatments that target specific symptoms, thus resulting in more rapid alleviation of MDD.6
Table 1
The SAFER interview: Assessing depression in a real-world setting
State vs trait nature of the symptoms
|
Assessability
|
Face validity
|
Ecological validity
|
Rule of the 3 Ps
|
| © Massachusetts General Hospital Source: Reference 6 |
Table 2
The SAFER criteria: Rule of the 3 Ps
| Pervasive—Do the major symptoms affect the patient across multiple arenas of life (work, relationships, school, chores, etc.)? |
| Persistent—Do the main symptoms occur most days, most of the day during the current episode? |
| Pathological—Do the symptoms of the present episode interfere with functioning? |
| © Massachusetts General Hospital Source: Reference 6 |
CASE REPORT: Worsening symptoms
Ms. Y, age 53, has been depressed for 30 years. She hardly remembers a time in her life when she felt good for more than a few days. However, 2 months ago she noted her symptoms got worse. She presents with many MDD symptoms as assessed by the Hamilton Depression Rating Scale, eg, ongoing depressive mood, feelings of guilt, major sleep disturbances, and work impairments.
Using SAFER to evaluate Ms. Y, a clinician would ask: Does she have symptoms that are present primarily during an episode of acute illness? Does the episode constitute a measurable exacerbation of preexisting symptoms? This clinical vignette illustrates the importance of the first SAFER criterion, state vs trait nature of the symptoms. Ms. Y is a SAFER “pass”—meaning consistent with a major depressive episode—because exacerbation of preexisting symptoms is measurable. However, if her symptoms represented a chronic, long-standing trait, she would be a SAFER “fail” based on this criterion, and her symptoms likely would not improve during a brief pharmacologic intervention. For such patients, SAFER would have oriented the clinician toward alternative therapies such as psychotherapy or a combination of longer, more complex pharmacologic treatment and psychotherapy.
By helping clinicians refine MDD diagnoses, SAFER draws attention to specific depression entities (eg, MDD or dysthymia) and directs them toward appropriate guidelines, treatment algorithms, and precautions (eg, when treated with pharmacotherapy, dysthymic patients are particularly sensitive to unwanted effects and adherence is a serious issue).7 The SAFER interview also can help identify when what appears to be a depressive episode clearly is precipitated by situational factors, and is more likely to be influenced by changes in the patient’s life than by treatment. For such patients, first consider nonpharmacologic interventions to avoid unnecessary medication exposure.
ATRQ: Efficacy and adequacy
The ATRQ examines the efficacy (improvement from 0% [not improved at all] to 100% [completely improved]), and adequacy (adequate duration and dose) of any antidepressant treatment in a step-by-step procedure.1,8,9 For a copy of the ATRQ, click here.
While conducting the interview, clinicians ask about treatment adherence to each medication trial. A treatment-resistant patient may go through many types of trials, from monotherapy to combination to augmentation.10 For each trial, the ATRQ systematically reviews 4 strategies to enhance treatment response:
- increasing the initial antidepressant dosage11
- combining the initial antidepressant with another antidepressant, typically from another class12
- augmenting the initial antidepressant with a nonantidepressant12
- switching from the initial antidepressant to another antidepressant.13
These strategies also are applied in cases of lost sustained antidepressant efficacy or depressive relapse/recurrence, although empirical evidence supporting these strategies is lacking, with the possible exception of dose increase.14,15
In the convention our group adopted, an adequate antidepressant trial must be ≥6 weeks in total length, with a dose within an adequate range as specified in the medication’s package insert. In addition, for the purposes of conducting TRD trials, we have considered a patient treatment-resistant if response to adequate dose and duration is <50%. On the ATRQ, 50% improvement refers to 50% symptom reduction from baseline without achieving remission. In an initial clinical trial that lasts ≥6 weeks, any dose increase (for ≥4 weeks) represents optimization and is not considered a new or separate trial, whereas augmentation or combination therapy (for ≥3 weeks) or a switch to another antidepressant (for ≥6 weeks) are considered new trials/treatments.
Decision tree for ATRQ. Because antidepressant treatment always is constrained by personal (eg, adherence, insurance coverage, etc.) and clinical (eg, contraindications due to comorbid conditions, side effects, etc.) considerations, we propose a decision tree to help clinicians determine the number of failed antidepressant trials their patient experienced (Figure). Although this tree does not represent all treatment scenarios, we hope it could help clinicians implement TRD treatment strategies because it highlights proper assessment of treatment duration, dosage, and changes before applying a TRD diagnosis.
The ATRQ meticulously examines patients’ antidepressant history to identify:
- pseudo-resistance, to guide adequate dosing and/or duration, and
- resistance, to propose next-step treatment.
Pseudo-resistance refers to treatment failures that can be attributed to factors such as inadequate treatment dosing or duration, atypical pharmacokinetics that reduce agents’ effectiveness, patient nonadherence (eg, due to adverse effects), or misdiagnosis of the primary disorder (ie, other mood disorders or depressive subsets such as dysthymia or minor depression mistreated as unipolar depression).16,17 Studies show that many patients with MDD referred to specialty settings are undertreated and receive inadequate antidepressant doses,18 which suggests that many referrals for TRD are in fact pseudo-resistance.19 Despite the lack of consensus on criteria for TRD,1 standardization of what constitutes treatment adequacy during antidepressant trials (eg, adherence, dose, duration) is indispensable.
Clinical application of the ATRQ. Because TRD may require specific interventions, we first need to properly identify treatment resistance. Also, systematic use of a classification system enhances the ability of clinicians and patients to provide meaningful descriptions of antidepressant resistance.
In clinical practice, choice of treatment strategy is based on factors that include partial or nonresponse, tolerability, avoiding withdrawal symptoms, the need to target side effects of a current antidepressant by administering another drug, cost, avoiding drug-drug interactions, and patient preference. Because a treatment-resistant patient may go through many types of trials, it is essential to obtain information about all treatments to determine the number of failed clinical trials a patient may have had for the current MDD episode and lifetime episodes. The importance of asking about adherence to each trial cannot be overemphasized.
Figure: Using the Massachusetts General Hospital Antidepressant Treatment History Questionnaire: A decision tree
CASE REPORT: Limited improvement
Mr. T, age 45, reported that his current depressive episode started several years ago. The first antidepressant trial he received, sertraline, 100 mg/d for 3 months, resulted in 0% improvement. Next he received citalopram, 20 mg/d, for 1 month, without any improvement. The next trial, venlafaxine, 75 mg/d, for 7 weeks, resulted in a 30% response. More recently Mr. B tried duloxetine, up to 90 mg/d, for 2 years with an 80% improvement during the first 3 months and then a decrease of response to <40%. He then received aripiprazole, 10 mg/d, in combination with duloxetine for approximately 4 weeks with no response.
Using the ATRQ to evaluate Mr. B, a clinician would consider the sertraline trial as a failed trial. The citalopram treatment would not be considered an adequate trial because it was too short. The venlafaxine course wouldn’t count as an adequate trial because the dosage was too low. The trial with duloxetine would count as a response (80% improvement) followed by a “poop out” or tachyphylaxis (40% improvement). The fifth trial with the combination of duloxetine and aripiprazole would count as a failed trial. The ATRQ highlights which drugs have been used for too short a duration or at too low a dosage. In Mr. B’s case, using the ATRQ revealed that of 5 trials, only 2 showed antidepressant resistance.
The ATRQ and decision tree are meant to provide clinicians with user-friendly tools to more precisely determine the number of failed antidepressant trials a patient experienced. By assessing if an antidepressant trial had an adequate dose and duration, the ATRQ can help suggest the next treatment options. For example, if a trial was inadequate in dose and/or duration but the patient tolerated the medication, then optimizing treatment with the current drug would be a logical next step. If a patient does not respond to an adequate trial, clinicians have several options, such as switching to another antidepressant, using a combination of medications or an augmentation strategy, or increasing the dose of the original antidepressant.
Limitations of the ATRQ. Historical rating of treatment is not as accurate as a prospective trial. Another limitation of the ATRQ is that the minimum effective dose is accepted as adequate; many clinicians would suggest that such a dose is inadequate. Also, the duration specified for augmentation, dose increase, and monotherapy are based on expert consensus1 rather than systematic research. Nonetheless, this method of documenting prior trials and treatment adequacy is an important advance.
The ATRQ lacks a place to indicate discontinuation due to intolerance. Knowing if adverse events caused treatment nonadherence or discontinuation is relevant to selecting treatment.
The ATRQ considers only pharmacotherapy and electroconvulsive therapy. Comprehensive assessment of treatment resistance requires asking about depression-specific, evidence-based psychotherapies, including cognitive-behavioral therapy and interpersonal therapy. Another precaution is that the ATRQ and SAFER should be used in conjunction with structured or semi-structured clinical interviews and other clinical tools to rule out other primary diagnoses (eg, bipolar disorder, posttraumatic stress disorder, obsessive-compulsive disorder, or surreptitious substance abuse).20
Using SAFER and ATRQ allows clinicians to make a proper MDD diagnosis with an accurate, reliable assessment of symptom severity and a precise count of antidepressant trials of adequate duration and dose. SAFER helps differentiate MDD from other depressive disorders and can be used to separate MDD from a similar presentation of a reaction to external circumstances that may remit if these circumstances change.
These 2 clinical tools focus on MDD and TRD. Compared with treatment resistance in MDD, treatment resistance in other forms of depression, such as minor depressive disorder or dysthymia, has been inadequately researched21,22 and should be addressed in large studies. However, to help patients achieve remission of depressive disorders—especially MDD—clinicians should use patient information gathered via measurement-based care in combination with algorithm recommendations.23 Persistence of clinical care of MDD is essential because several treatment steps might be necessary for some patients to achieve remission. Throughout treatment, patients’ symptoms, adverse events secondary to ongoing treatment, and medication adherence should be assessed with appropriate tools. ATRQ and SAFER offer a clinician support in assessing treatment resistance history and a patient’s likelihood to respond to pharmacologic treatment.
Related Resources
- Desseilles M, Witte J, Chang TE, et al. Assessing the adequacy of past antidepressant trials: a clinician’s guide to the Antidepressant Treatment Response Questionnaire (ATRQ). J Clin Psychiatry. 2011;72(8):1152-1154.
- Targum SD, Pollack MH, Fava M. Redefining affective disorders: relevance for drug development. CNS Neurosci Ther. 2008;14(1):2-9.
- Depression Clinical and Research Program. www.massgeneral.org/psychiatry/services/dcrp_home.aspx.
Drug Brand Names
- Aripiprazole • Abilify
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Desseilles was supported by the National Funds for Scientific Research of Belgium. Additional support came from the Belgian American Educational Foundation.
Dr. Fava receives grant/research support from, is a consultant to, and/or is a speaker for Abbott Laboratories, Adamed, Advanced Meeting Partners, Affectis Pharmaceuticals, Alkermes, Amarin Pharma, American Psychiatric Association, American Society of Clinical Psychopharmacology, Aspect Medical Systems, AstraZeneca, Auspex Pharmaceuticals, Bayer, Belvoir Media Group, Best Practice Project Management, BioMarin, BioResearch, Biovail, Boehringer Ingelheim, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Clinical Trials Solutions, Clintara, CME Institute/Physicians Postgraduate Press, CNS Response, Compellis Pharmaceuticals, Covance, Covidien, Cypress Pharmaceutical, DiagnoSearch Life Sciences, Dainippon Sumitomo Pharma, Dov Pharmaceuticals, Edgemont Pharmaceuticals, Eisai, Eli Lilly and Company, EnVivo Pharmaceuticals, ePharmaSolutions, EPIX Pharmaceuticals, Euthymics Bioscience, Fabre-Kramer Pharmaceuticals, Forest Pharmaceuticals, Ganeden Biotech, GenOmind, GlaxoSmithKline, Grünenthal GmbH, Icon Clinical Research, Imedex, i3 Innovus/Ingenix, Janssen Pharmaceutica, Johnson & Johnson Pharmaceutical Research & Development, Knoll Pharmaceuticals, Labopharm, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Lundbeck, MedAvante, Merck, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed Elsevier, MSI Methylation Sciences, National Alliance for Research on Schizophrenia & Depression, National Center for Complementary and Alternative Medicine, National Institute of Drug Abuse, National Institute of Mental Health, Naurex, Neuronetics, NextWave Pharmaceuticals, Novartis, Nutrition 21, Orexigen Therapeutics, Organon Pharmaceuticals, Otsuka Pharmaceuticals, PamLab, Pfizer, PharmaStar, Pharmavite®, PharmoRx Therapeutics, Photothera, Precision Human Biolaboratory, Prexa Pharmaceuticals, Puretech Ventures, PsychoGenics, Psylin Neurosciences, RCT Logic, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Pharmaceuticals, sanofi-aventis US, Schering-Plough, Sepracor, Servier Laboratories, Shire, Solvay Pharmaceuticals, Somaxon Pharmaceuticals, Somerset Pharmaceuticals, Sunovion Pharmaceuticals, Supernus Pharmaceuticals, Synthelabo, Takeda Pharmaceutical, Tal Medical, Tetragenex Pharmaceuticals, Transcept Pharmaceuticals, TransForm Pharmaceuticals, United BioSource, Vanda Pharmaceuticals, and Wyeth-Ayerst Laboratories.
Over the past year, Dr. Mischoulon has received research support from the Bowman Family Foundation, FisherWallace, Ganeden, and Nordic Naturals. He has received honoraria for speaking from Nordic Naturals. He has received royalties from Lippincott Williams & Wilkins for the book Natural medications for psychiatric disorders: Considering the alternatives. No payment has exceeded $10,000.
Dr. Freeman has received research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, and served on a one-time advisory board to Bristol-Myers Squibb.
Acknowledgment
The authors acknowledge Janet Witte, MD, MPH, Trina E. Chang, MD, MPH, Nadia Iovieno, MD, PhD, Christina Dording, MD, Heidi Ashih, MD, PhD, Maren Nyer, PhD, and Marasha-Fiona De Jong, MD from the Clinical Trials Network and Institute at Massachusetts General Hospital, Boston, MA.
Approximately one-half of patients with major depressive disorder (MDD) will have partial or nonresponse to first-line antidepressant monotherapy, despite receiving an adequate dosage and sufficient duration of treatment.1 This has led to the definition of treatment-resistant depression (TRD) as a depressive episode that has shown insufficient response to ≥1 trial of an antidepressant that has demonstrated efficacy in clinical trials.1 Depressed patients should be treated to full remission because absence of complete remission is associated with:
- a more recurrent and chronic illness course2,3
- increased medical and psychiatric comorbidities
- greater functional burden
- increased social and economic costs linked with impaired social functioning.4
Clinicians need to properly identify MDD and treatment resistance to guide optimal treatment choices. Additional tools are necessary to accurately identify, document, and communicate about symptoms commonly experienced by depressed patients but not fully characterized by DSM-IV-TR MDD criteria.5 Finally, in many cases, trait or situational factors might obfuscate accurate diagnosis and the natural course of illness, and tools that can be implemented practically will help identify patients with MDD.
Our group has created and implemented 2 clinician-administered tools—the SAFER Interview and the Antidepressant Treatment Response Questionnaire (ATRQ)—to enrich the qualitative assessment of MDD and treatment resistance.
SAFER: Assessing the diagnosis, symptom severity
The SAFER interview refines the diagnosis of depressed patients by assessing the state vs trait nature of the symptoms, their assessability, their face and ecological validity, and if they pass the rule of the 3 Ps: pervasiveness, persistence, and pathological nature of the current MDD episode (Table 1 and Table 2).6 This reliable assessment of the patient’s diagnosis and symptom severity is made in a way that reflects the illness in a real-world setting.
Clinical application of SAFER. Implementing SAFER in clinical settings promotes a personalized, dimensional approach by taking into account a varying degree of symptom severity in depressed patients, in contrast to relying on symptom lists as found in the DSM-IV-TR. Using the SAFER interview deepens the typical psychiatric diagnostic process, allows for a more precise understanding of the patient’s situation, and may help clinicians select effective treatments that target specific symptoms, thus resulting in more rapid alleviation of MDD.6
Table 1
The SAFER interview: Assessing depression in a real-world setting
State vs trait nature of the symptoms
|
Assessability
|
Face validity
|
Ecological validity
|
Rule of the 3 Ps
|
| © Massachusetts General Hospital Source: Reference 6 |
Table 2
The SAFER criteria: Rule of the 3 Ps
| Pervasive—Do the major symptoms affect the patient across multiple arenas of life (work, relationships, school, chores, etc.)? |
| Persistent—Do the main symptoms occur most days, most of the day during the current episode? |
| Pathological—Do the symptoms of the present episode interfere with functioning? |
| © Massachusetts General Hospital Source: Reference 6 |
CASE REPORT: Worsening symptoms
Ms. Y, age 53, has been depressed for 30 years. She hardly remembers a time in her life when she felt good for more than a few days. However, 2 months ago she noted her symptoms got worse. She presents with many MDD symptoms as assessed by the Hamilton Depression Rating Scale, eg, ongoing depressive mood, feelings of guilt, major sleep disturbances, and work impairments.
Using SAFER to evaluate Ms. Y, a clinician would ask: Does she have symptoms that are present primarily during an episode of acute illness? Does the episode constitute a measurable exacerbation of preexisting symptoms? This clinical vignette illustrates the importance of the first SAFER criterion, state vs trait nature of the symptoms. Ms. Y is a SAFER “pass”—meaning consistent with a major depressive episode—because exacerbation of preexisting symptoms is measurable. However, if her symptoms represented a chronic, long-standing trait, she would be a SAFER “fail” based on this criterion, and her symptoms likely would not improve during a brief pharmacologic intervention. For such patients, SAFER would have oriented the clinician toward alternative therapies such as psychotherapy or a combination of longer, more complex pharmacologic treatment and psychotherapy.
By helping clinicians refine MDD diagnoses, SAFER draws attention to specific depression entities (eg, MDD or dysthymia) and directs them toward appropriate guidelines, treatment algorithms, and precautions (eg, when treated with pharmacotherapy, dysthymic patients are particularly sensitive to unwanted effects and adherence is a serious issue).7 The SAFER interview also can help identify when what appears to be a depressive episode clearly is precipitated by situational factors, and is more likely to be influenced by changes in the patient’s life than by treatment. For such patients, first consider nonpharmacologic interventions to avoid unnecessary medication exposure.
ATRQ: Efficacy and adequacy
The ATRQ examines the efficacy (improvement from 0% [not improved at all] to 100% [completely improved]), and adequacy (adequate duration and dose) of any antidepressant treatment in a step-by-step procedure.1,8,9 For a copy of the ATRQ, click here.
While conducting the interview, clinicians ask about treatment adherence to each medication trial. A treatment-resistant patient may go through many types of trials, from monotherapy to combination to augmentation.10 For each trial, the ATRQ systematically reviews 4 strategies to enhance treatment response:
- increasing the initial antidepressant dosage11
- combining the initial antidepressant with another antidepressant, typically from another class12
- augmenting the initial antidepressant with a nonantidepressant12
- switching from the initial antidepressant to another antidepressant.13
These strategies also are applied in cases of lost sustained antidepressant efficacy or depressive relapse/recurrence, although empirical evidence supporting these strategies is lacking, with the possible exception of dose increase.14,15
In the convention our group adopted, an adequate antidepressant trial must be ≥6 weeks in total length, with a dose within an adequate range as specified in the medication’s package insert. In addition, for the purposes of conducting TRD trials, we have considered a patient treatment-resistant if response to adequate dose and duration is <50%. On the ATRQ, 50% improvement refers to 50% symptom reduction from baseline without achieving remission. In an initial clinical trial that lasts ≥6 weeks, any dose increase (for ≥4 weeks) represents optimization and is not considered a new or separate trial, whereas augmentation or combination therapy (for ≥3 weeks) or a switch to another antidepressant (for ≥6 weeks) are considered new trials/treatments.
Decision tree for ATRQ. Because antidepressant treatment always is constrained by personal (eg, adherence, insurance coverage, etc.) and clinical (eg, contraindications due to comorbid conditions, side effects, etc.) considerations, we propose a decision tree to help clinicians determine the number of failed antidepressant trials their patient experienced (Figure). Although this tree does not represent all treatment scenarios, we hope it could help clinicians implement TRD treatment strategies because it highlights proper assessment of treatment duration, dosage, and changes before applying a TRD diagnosis.
The ATRQ meticulously examines patients’ antidepressant history to identify:
- pseudo-resistance, to guide adequate dosing and/or duration, and
- resistance, to propose next-step treatment.
Pseudo-resistance refers to treatment failures that can be attributed to factors such as inadequate treatment dosing or duration, atypical pharmacokinetics that reduce agents’ effectiveness, patient nonadherence (eg, due to adverse effects), or misdiagnosis of the primary disorder (ie, other mood disorders or depressive subsets such as dysthymia or minor depression mistreated as unipolar depression).16,17 Studies show that many patients with MDD referred to specialty settings are undertreated and receive inadequate antidepressant doses,18 which suggests that many referrals for TRD are in fact pseudo-resistance.19 Despite the lack of consensus on criteria for TRD,1 standardization of what constitutes treatment adequacy during antidepressant trials (eg, adherence, dose, duration) is indispensable.
Clinical application of the ATRQ. Because TRD may require specific interventions, we first need to properly identify treatment resistance. Also, systematic use of a classification system enhances the ability of clinicians and patients to provide meaningful descriptions of antidepressant resistance.
In clinical practice, choice of treatment strategy is based on factors that include partial or nonresponse, tolerability, avoiding withdrawal symptoms, the need to target side effects of a current antidepressant by administering another drug, cost, avoiding drug-drug interactions, and patient preference. Because a treatment-resistant patient may go through many types of trials, it is essential to obtain information about all treatments to determine the number of failed clinical trials a patient may have had for the current MDD episode and lifetime episodes. The importance of asking about adherence to each trial cannot be overemphasized.
Figure: Using the Massachusetts General Hospital Antidepressant Treatment History Questionnaire: A decision tree
CASE REPORT: Limited improvement
Mr. T, age 45, reported that his current depressive episode started several years ago. The first antidepressant trial he received, sertraline, 100 mg/d for 3 months, resulted in 0% improvement. Next he received citalopram, 20 mg/d, for 1 month, without any improvement. The next trial, venlafaxine, 75 mg/d, for 7 weeks, resulted in a 30% response. More recently Mr. B tried duloxetine, up to 90 mg/d, for 2 years with an 80% improvement during the first 3 months and then a decrease of response to <40%. He then received aripiprazole, 10 mg/d, in combination with duloxetine for approximately 4 weeks with no response.
Using the ATRQ to evaluate Mr. B, a clinician would consider the sertraline trial as a failed trial. The citalopram treatment would not be considered an adequate trial because it was too short. The venlafaxine course wouldn’t count as an adequate trial because the dosage was too low. The trial with duloxetine would count as a response (80% improvement) followed by a “poop out” or tachyphylaxis (40% improvement). The fifth trial with the combination of duloxetine and aripiprazole would count as a failed trial. The ATRQ highlights which drugs have been used for too short a duration or at too low a dosage. In Mr. B’s case, using the ATRQ revealed that of 5 trials, only 2 showed antidepressant resistance.
The ATRQ and decision tree are meant to provide clinicians with user-friendly tools to more precisely determine the number of failed antidepressant trials a patient experienced. By assessing if an antidepressant trial had an adequate dose and duration, the ATRQ can help suggest the next treatment options. For example, if a trial was inadequate in dose and/or duration but the patient tolerated the medication, then optimizing treatment with the current drug would be a logical next step. If a patient does not respond to an adequate trial, clinicians have several options, such as switching to another antidepressant, using a combination of medications or an augmentation strategy, or increasing the dose of the original antidepressant.
Limitations of the ATRQ. Historical rating of treatment is not as accurate as a prospective trial. Another limitation of the ATRQ is that the minimum effective dose is accepted as adequate; many clinicians would suggest that such a dose is inadequate. Also, the duration specified for augmentation, dose increase, and monotherapy are based on expert consensus1 rather than systematic research. Nonetheless, this method of documenting prior trials and treatment adequacy is an important advance.
The ATRQ lacks a place to indicate discontinuation due to intolerance. Knowing if adverse events caused treatment nonadherence or discontinuation is relevant to selecting treatment.
The ATRQ considers only pharmacotherapy and electroconvulsive therapy. Comprehensive assessment of treatment resistance requires asking about depression-specific, evidence-based psychotherapies, including cognitive-behavioral therapy and interpersonal therapy. Another precaution is that the ATRQ and SAFER should be used in conjunction with structured or semi-structured clinical interviews and other clinical tools to rule out other primary diagnoses (eg, bipolar disorder, posttraumatic stress disorder, obsessive-compulsive disorder, or surreptitious substance abuse).20
Using SAFER and ATRQ allows clinicians to make a proper MDD diagnosis with an accurate, reliable assessment of symptom severity and a precise count of antidepressant trials of adequate duration and dose. SAFER helps differentiate MDD from other depressive disorders and can be used to separate MDD from a similar presentation of a reaction to external circumstances that may remit if these circumstances change.
These 2 clinical tools focus on MDD and TRD. Compared with treatment resistance in MDD, treatment resistance in other forms of depression, such as minor depressive disorder or dysthymia, has been inadequately researched21,22 and should be addressed in large studies. However, to help patients achieve remission of depressive disorders—especially MDD—clinicians should use patient information gathered via measurement-based care in combination with algorithm recommendations.23 Persistence of clinical care of MDD is essential because several treatment steps might be necessary for some patients to achieve remission. Throughout treatment, patients’ symptoms, adverse events secondary to ongoing treatment, and medication adherence should be assessed with appropriate tools. ATRQ and SAFER offer a clinician support in assessing treatment resistance history and a patient’s likelihood to respond to pharmacologic treatment.
Related Resources
- Desseilles M, Witte J, Chang TE, et al. Assessing the adequacy of past antidepressant trials: a clinician’s guide to the Antidepressant Treatment Response Questionnaire (ATRQ). J Clin Psychiatry. 2011;72(8):1152-1154.
- Targum SD, Pollack MH, Fava M. Redefining affective disorders: relevance for drug development. CNS Neurosci Ther. 2008;14(1):2-9.
- Depression Clinical and Research Program. www.massgeneral.org/psychiatry/services/dcrp_home.aspx.
Drug Brand Names
- Aripiprazole • Abilify
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Desseilles was supported by the National Funds for Scientific Research of Belgium. Additional support came from the Belgian American Educational Foundation.
Dr. Fava receives grant/research support from, is a consultant to, and/or is a speaker for Abbott Laboratories, Adamed, Advanced Meeting Partners, Affectis Pharmaceuticals, Alkermes, Amarin Pharma, American Psychiatric Association, American Society of Clinical Psychopharmacology, Aspect Medical Systems, AstraZeneca, Auspex Pharmaceuticals, Bayer, Belvoir Media Group, Best Practice Project Management, BioMarin, BioResearch, Biovail, Boehringer Ingelheim, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Clinical Trials Solutions, Clintara, CME Institute/Physicians Postgraduate Press, CNS Response, Compellis Pharmaceuticals, Covance, Covidien, Cypress Pharmaceutical, DiagnoSearch Life Sciences, Dainippon Sumitomo Pharma, Dov Pharmaceuticals, Edgemont Pharmaceuticals, Eisai, Eli Lilly and Company, EnVivo Pharmaceuticals, ePharmaSolutions, EPIX Pharmaceuticals, Euthymics Bioscience, Fabre-Kramer Pharmaceuticals, Forest Pharmaceuticals, Ganeden Biotech, GenOmind, GlaxoSmithKline, Grünenthal GmbH, Icon Clinical Research, Imedex, i3 Innovus/Ingenix, Janssen Pharmaceutica, Johnson & Johnson Pharmaceutical Research & Development, Knoll Pharmaceuticals, Labopharm, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Lundbeck, MedAvante, Merck, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed Elsevier, MSI Methylation Sciences, National Alliance for Research on Schizophrenia & Depression, National Center for Complementary and Alternative Medicine, National Institute of Drug Abuse, National Institute of Mental Health, Naurex, Neuronetics, NextWave Pharmaceuticals, Novartis, Nutrition 21, Orexigen Therapeutics, Organon Pharmaceuticals, Otsuka Pharmaceuticals, PamLab, Pfizer, PharmaStar, Pharmavite®, PharmoRx Therapeutics, Photothera, Precision Human Biolaboratory, Prexa Pharmaceuticals, Puretech Ventures, PsychoGenics, Psylin Neurosciences, RCT Logic, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Pharmaceuticals, sanofi-aventis US, Schering-Plough, Sepracor, Servier Laboratories, Shire, Solvay Pharmaceuticals, Somaxon Pharmaceuticals, Somerset Pharmaceuticals, Sunovion Pharmaceuticals, Supernus Pharmaceuticals, Synthelabo, Takeda Pharmaceutical, Tal Medical, Tetragenex Pharmaceuticals, Transcept Pharmaceuticals, TransForm Pharmaceuticals, United BioSource, Vanda Pharmaceuticals, and Wyeth-Ayerst Laboratories.
Over the past year, Dr. Mischoulon has received research support from the Bowman Family Foundation, FisherWallace, Ganeden, and Nordic Naturals. He has received honoraria for speaking from Nordic Naturals. He has received royalties from Lippincott Williams & Wilkins for the book Natural medications for psychiatric disorders: Considering the alternatives. No payment has exceeded $10,000.
Dr. Freeman has received research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, and served on a one-time advisory board to Bristol-Myers Squibb.
Acknowledgment
The authors acknowledge Janet Witte, MD, MPH, Trina E. Chang, MD, MPH, Nadia Iovieno, MD, PhD, Christina Dording, MD, Heidi Ashih, MD, PhD, Maren Nyer, PhD, and Marasha-Fiona De Jong, MD from the Clinical Trials Network and Institute at Massachusetts General Hospital, Boston, MA.
1. Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649-659.
2. Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50(2-3):97-108.
3. Van Londen L, Molenaar RP, Goekoop JG, et al. Three- to 5-year prospective follow-up of outcome in major depression. Psychol Med. 1998;28(3):731-735.
4. Paykel ES. Achieving gains beyond response. Acta Psychiatr Scand Suppl. 2002;(415):12-17.
5. Cassano P, Fava M. Depression and public health: an overview. J Psychosom Res. 2002;53(4):849-857.
6. Targum SD, Pollack MH, Fava M. Redefining affective disorders: relevance for drug development. CNS Neurosci Ther. 2008;14(1):2-9.
7. Versiani M, Nardi AE, Figueira I. Pharmacotherapy of dysthymia: review and new findings. Eur Psychiatry. 1998;13(4):203-209.
8. Chandler GM, Iosifescu DV, Pollack MH, et al. RESEARCH: validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322-325.
9. Desseilles M, Witte J, Chang TE, et al. Assessing the adequacy of past antidepressant trials: a clinician’s guide to the antidepressant treatment response questionnaire. J Clin Psychiatry. 2011;72(8):1152-1154.
10. Fava M. Augmentation and combination strategies for complicated depression. J Clin Psychiatry. 2009;70(11):e40.-
11. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
12. Fava M, Rush AJ. Current status of augmentation and combination treatments for major depressive disorder: a literature review and a proposal for a novel approach to improve practice. Psychother Psychosom. 2006;75(3):139-153.
13. Wohlreich MM, Mallinckrodt CH, Watkin JG, et al. Immediate switching of antidepressant therapy: results from a clinical trial of duloxetine. Ann Clin Psychiatry. 2005;17(4):259-268.
14. Schmidt ME, Fava M, Zhang S, et al. Treatment approaches to major depressive disorder relapse. Part 1: dose increase. Psychother Psychosom. 2002;71(4):190-194.
15. Fava M, Detke MJ, Balestrieri M, et al. Management of depression relapse: re-initiation of duloxetine treatment or dose increase. J Psychiatr Res. 2006;40(4):328-336.
16. Kornstein SG, Schneider RK. Clinical features of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):18-25.
17. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
18. Hirschfeld RM, Keller MB, Panico S, et al. The National Depressive and Manic-Depressive Association consensus statement on the undertreatment of depression. JAMA. 1997;277(4):333-340.
19. Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10-17.
20. Parker G, Malhi GS, Crawford JG, et al. Identifying “paradigm failures” contributing to treatment-resistant depression. J Affect Disord. 2005;87(2-3):185-191.
21. Harris SJ, Parent M. Patient with chronic and apparently treatment-resistant dysthymia. Am J Psychiatry. 1986;143(2):260-261.
22. Amsterdam J, Hornig M, Nierenberg AA. Treatment-resistant mood disorders. Cambridge United Kingdom: Cambridge University Press; 2001.
23. Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry. 2009;70(suppl 6):26-31.
1. Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649-659.
2. Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50(2-3):97-108.
3. Van Londen L, Molenaar RP, Goekoop JG, et al. Three- to 5-year prospective follow-up of outcome in major depression. Psychol Med. 1998;28(3):731-735.
4. Paykel ES. Achieving gains beyond response. Acta Psychiatr Scand Suppl. 2002;(415):12-17.
5. Cassano P, Fava M. Depression and public health: an overview. J Psychosom Res. 2002;53(4):849-857.
6. Targum SD, Pollack MH, Fava M. Redefining affective disorders: relevance for drug development. CNS Neurosci Ther. 2008;14(1):2-9.
7. Versiani M, Nardi AE, Figueira I. Pharmacotherapy of dysthymia: review and new findings. Eur Psychiatry. 1998;13(4):203-209.
8. Chandler GM, Iosifescu DV, Pollack MH, et al. RESEARCH: validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322-325.
9. Desseilles M, Witte J, Chang TE, et al. Assessing the adequacy of past antidepressant trials: a clinician’s guide to the antidepressant treatment response questionnaire. J Clin Psychiatry. 2011;72(8):1152-1154.
10. Fava M. Augmentation and combination strategies for complicated depression. J Clin Psychiatry. 2009;70(11):e40.-
11. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
12. Fava M, Rush AJ. Current status of augmentation and combination treatments for major depressive disorder: a literature review and a proposal for a novel approach to improve practice. Psychother Psychosom. 2006;75(3):139-153.
13. Wohlreich MM, Mallinckrodt CH, Watkin JG, et al. Immediate switching of antidepressant therapy: results from a clinical trial of duloxetine. Ann Clin Psychiatry. 2005;17(4):259-268.
14. Schmidt ME, Fava M, Zhang S, et al. Treatment approaches to major depressive disorder relapse. Part 1: dose increase. Psychother Psychosom. 2002;71(4):190-194.
15. Fava M, Detke MJ, Balestrieri M, et al. Management of depression relapse: re-initiation of duloxetine treatment or dose increase. J Psychiatr Res. 2006;40(4):328-336.
16. Kornstein SG, Schneider RK. Clinical features of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):18-25.
17. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
18. Hirschfeld RM, Keller MB, Panico S, et al. The National Depressive and Manic-Depressive Association consensus statement on the undertreatment of depression. JAMA. 1997;277(4):333-340.
19. Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10-17.
20. Parker G, Malhi GS, Crawford JG, et al. Identifying “paradigm failures” contributing to treatment-resistant depression. J Affect Disord. 2005;87(2-3):185-191.
21. Harris SJ, Parent M. Patient with chronic and apparently treatment-resistant dysthymia. Am J Psychiatry. 1986;143(2):260-261.
22. Amsterdam J, Hornig M, Nierenberg AA. Treatment-resistant mood disorders. Cambridge United Kingdom: Cambridge University Press; 2001.
23. Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry. 2009;70(suppl 6):26-31.
New Alzheimer’s disease guidelines: Implications for clinicians
Discuss this article at www.facebook.com/CurrentPsychiatry
In 2011, a workgroup of experts from the Alzheimer’s Association and the National Institute on Aging published new criteria and guidelines for diagnosing Alzheimer’s disease (AD), the first new AD guidelines since 1984.1-4 These criteria reflect data that suggest AD is not synonymous with dementia of the Alzheimer’s type (DAT) but is a disease that slowly develops over many years as a result of accumulated neuropathologic changes, with dementia representing only the final phase of the disease (Figure).1-4
Figure: Cognitive decline in AD over time
AD: Alzheimer’s disease; MCI: mild cognitive impairment
Source: Adapted from reference 2
This article highlights the similarities and differences of the 1984 and 2011 AD diagnosis guidelines. We also discuss the new guidelines’ limitations and clinical implications.
The 1984 AD criteria
Both the 1984 AD criteria5 and DSM-IV-TR criteria6 rely on the concept that AD is a clinical diagnosis made after a patient develops dementia. That is, diagnosis rests on the physician’s clinical judgment about the etiology of the patient’s symptoms, taking into account reports from the patient, family, and friends, as well as results of neurocognitive testing and mental status evaluation. The 1984 criteria were developed with the expectation that if a patient who met clinical criteria for AD were to undergo an autopsy, he or she likely would have evidence of AD pathology as the underlying etiology. These criteria were developed before researchers discovered that in AD, pathologic changes occur over many years and clinical dementia is the end product of accumulated pathology. The 1984 criteria did not address important phases that precede clinical dementia—such as mild cognitive impairment (MCI). See the Table for a summary of the 1984 AD criteria.
Table
The 1984 NINCDS-ADRDA criteria for clinical diagnosis of AD
|
| AD: Alzheimer’s disease; NINCDS-ADRDA: National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association Source: McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944 |
The 2011 AD criteria
The new AD criteria differ from the 1984 criteria in 2 major ways:
- expansion of AD into 3 phases, only 1 of which is characterized by dementia
- incorporation of biomarkers to provide information regarding pathophysiologic changes underlying the disease state (Table 1).1-5
The 3 phases. The 2011 criteria expand the definition of AD to include an asymptomatic, preclinical phase; a symptomatic, pre-dementia phase; and a dementia phase. In the initial phase, neuronal toxins such as beta-amyloid (Aβ) plaques and elevated tau first become detectable. Patients in this phase are asymptomatic or have subtle symptoms. This phase should be viewed as part of a continuum and includes patients who may, for instance, develop Aβ plaques but do not progress to further neurodegeneration.2 The diagnostic criteria of this phase are intended for research purposes only.1,2
Patients in the symptomatic, pre-dementia phase—also known as the MCI phase—exhibit mild decline in memory, attention, and thinking. Although this decline is more than what is expected for the patient’s age and education, it does not compromise everyday activity and functioning.
A patient who develops cognitive or behavioral problems that interfere with his or her ability to function at work or in everyday activities has entered the dementia phase. Similar to the 1984 guidelines, the 2011 criteria classify patients into probable and possible AD dementia. All patients who would have satisfied criteria for probable AD under the 1984 guidelines will satisfy criteria for probable AD dementia under the 2011 criteria.4 The same is not true for possible AD dementia. The 2011 criteria include 2 other major categories for patients with AD dementia: probable and possible AD dementia with evidence of the AD pathophysiological process. These categories are intended for research purposes only, whereas the criteria for the MCI and dementia phases are intended to guide diagnosis in the clinical setting.
By incorporating phases of AD that precede dementia into the disease spectrum, the new guidelines are designed to move clinicians toward earlier diagnosis and treatment.1-3 Similar to how early, pre-symptomatic detection and treatment of conditions such as diabetes and cancer can reduce mortality, improving diagnosis of AD in its early phases may allow clinicians to better test potential therapies and eventually use them to treat at-risk individuals.2,3 Most pharmacotherapies for AD are FDA-approved only for patients diagnosed with clinical dementia. Furthermore, current pharmacotherapies do not alter the course of AD; they have a modest effect in slowing cognitive and functional decline.7,8 If patients in the earlier phases of AD could be recruited for research studies, we may be able to develop new treatments to stop or reverse AD pathology and its clinical manifestations.
Biomarkers. The new criteria incorporate biomarkers to provide information about pathophysiologic changes underlying the disease process. These criteria define biomarkers as physiologic, biochemical, or anatomic parameters that can be measured in vivo and reflect specific features of disease-related pathophysiologic processes.1 Presently, there are no cutoff values to demarcate “normal” levels from “abnormal,” and biomarkers are proposed primarily as research tools because they have not been studied adequately in community settings and laboratory techniques to measure biomarkers have not been standardized.1-4,9
The 5 biomarkers incorporated into the new criteria are divided into 2 categories: biomarkers of Aβ accumulation and those of neuronal degeneration or injury (Table 2).1-4 In the initial, preclinical phase, biomarkers are used to detect changes in the brain—such as amyloid accumulation and nerve cell degeneration—that may already be in process in an individual whose clinical symptoms are subtle or not yet evident.1,2 In this phase, progressive evidence of biomarkers, such as both Aβ accumulation and neuronal injury rather than Aβ accumulation alone, may increase the probability that a patient will decline quickly into the MCI phase.2 Biomarkers of neuronal degeneration or injury especially correlate with the likelihood that the disease will progress to clinical dementia.1 Subtle cognitive symptoms in the preclinical phase also might predict rapid decline into MCI.2
In the MCI and dementia phases, biomarkers are used to determine the level of certainty that AD is responsible for the patient’s symptoms.1,3,4 For example, a patient could meet criteria for a non-AD dementia such as dementia with Lewy bodies, but also meet pathologic criteria for AD on autopsy.3 The diagnostic category of possible AD dementia with evidence of the AD pathophysiologic process is intended for this type of scenario.4 For the MCI phase, the criteria propose levels of certainty that a patient’s MCI syndrome is caused by AD, ranging from MCI due to AD-high likelihood to MCI-unlikely due to AD.3
Research has demonstrated that a patient’s clinical picture doesn’t necessarily reflect the extent of the underlying pathology. For example, a patient could have extensive AD pathology, such as diffuse amyloid plaques, without any obvious clinical symptoms.3 Conversely, although both Aβ deposition and elevated tau are hallmarks of AD, variations in these proteins can be seen in neuropsychiatric disorders other than AD.10 That said, it appears that worsening of clinical symptoms often parallels worsening of neurodegenerative biomarkers.1
Under the 2011 guidelines, biomarkers would not be used to diagnose or exclude AD or MCI, but instead would help improve diagnostic accuracy in individuals with cognitive decline.1,3,4 In other words, AD remains a clinical diagnosis, but these biomarkers could raise or lower the positive predictive value of a clinician’s judgment about the etiology of a patient’s symptoms.
See the Box for a description of the potential risks and benefits of using the new diagnostic criteria.
Table 1
Comparing the 1984 and 2011 AD criteria
| 1984 criteria | 2011 criteria |
|---|---|
| AD is a clinical diagnosis | AD remains a clinical diagnosis but biomarkers serve to improve the accuracy of diagnosis of the disease |
| There is only 1 phase of AD—dementia. | AD is expanded into 3 phases: an asymptomatic, preclinical phase; a symptomatic, pre-dementia phase; and a dementia phase |
| A patient who meets the clinical criteria for AD would be expected to have AD pathology as the underlying etiology were he/she to undergo a brain autopsy | Presently, biomarkers are proposed as research tools only and are not intended to be applied in the clinical setting. However, eventually clinicians will be able to diagnose AD in all 3 phases, as biomarker testing becomes standardized and reliable enough to be accurately applied in clinical settings |
| Little consideration is given to specific neuropathologic changes underlying the disease process | Biomarkers provide information regarding the pathophysiologic changes underlying the disease state |
| Little consideration is given to the idea that pathologic changes occur over many years | Inherent in dividing AD into 3 phases is the concept that AD develops slowly over many years and has a long prodromal phase that is clinically silent |
| AD: Alzheimer’s disease Source: References 1-5 | |
Table 2
5 biomarkers incorporated into the 2011 AD criteria
| Category | Biomarkers |
|---|---|
| Biomarkers of Aβ accumulation | Abnormal tracer retention on amyloid PET imaging |
| Low CSF Aβ42 | |
| Biomarkers of neuronal degeneration or injury | Elevated CSF tau (total and phosphorylated tau) |
| Decreased fluorodeoxyglucose uptake on PET | |
| Atrophy on structural magnetic resonance imaging | |
| Aβ: beta-amyloid; AD: Alzheimer’s disease; CSF: cerebrospinal fluid; PET: positron emission tomography Source: References 1-4 | |
The earlier an Alzheimer’s disease (AD) diagnosis is made, the less certain it is AD.a Biomarkers typically found in individuals with AD also can be found in patients with dementia not caused by AD, such as vascular dementia, as well as in individuals who may never develop dementia.b Additionally, there is no certainty that a patient in an early phase of AD will develop clinical dementia. Falsely diagnosing a patient with AD may lead the individual and their family to feel helpless, hopeless, depressed, anxious, or ashamed and to spend money and other resources preparing for a prognosis that may never come to fruition. Clinicians may feel compelled to assess for biomarkers using expensive, invasive tests that are not yet standardized in an attempt to support the AD diagnosis.
Early diagnosis of AD has many benefits that should not be overlooked, however. It provides patients and their families an opportunity to become familiar with the disease course, which may help some patients cope with the diagnosis. Patients diagnosed in the early stages would be able to make important decisions regarding health care, social, and financial planning before they develop pathology that limits their executive planning abilities or become functionally impaired.
Diagnosing an illness when there are no disease-modifying therapies available is not futile. Some patients with newly diagnosed AD in the pre-dementia phases may want to participate in clinical research trials to help develop therapies for AD. Some data suggest that AD treatment appears to provide the greatest benefit when initiated early in the disease course and maintained over a long duration.c Eventually, we may be able to tailor specific AD treatments in different phases of the disease. For instance, we may discover treatments for patients who show evidence of beta-amyloid plaques but not neuronal injury, or vice versa. Patients also may benefit from education on nonpharmacologic treatments, including reducing vascular risk factors to help improve brain aging,d reducing stress, and learning cognitive strategies such as using mnemonics to aid memory.
In many clinical settings, patients are being clinically diagnosed with mild cognitive impairment (MCI). Research indicates that patients with MCI are at near-term risk of developing dementia, particularly dementia of the Alzheimer’s type.d,e Presently, no definite transition points demarcate MCI from dementia; this progression is based upon clinical judgment.
In the last decade, researchers have begun to describe a syndrome of subjective cognitive impairment (SCI), which may be a phase that precedes the MCI phase of AD.f Patients with SCI report cognitive deficits (eg, forgetfulness and word-finding difficulties) but have no objective evidence of cognitive impairment on neuropsychological tests. Cognitive problems associated with SCI do not cause functional decline.g SCI may reflect the minimal cognitive complaints mentioned in the research criteria for the preclinical phase of AD. Eventually, biomarkers may be able to help clinicians more accurately predict which patients with SCI are most likely to progress to the MCI or dementia phase of AD.
References
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
- Galasko D. Biomarkers in non-Alzheimer dementias. Clinical Neuroscience Research. 2004;3(6):375-381.
- Geldmacher DS. Treatment guidelines for Alzheimer’s disease: redefining perceptions in primary care. Prim Care Companion J Clin Psychiatry. 2007;9(2):113-121.
- Chertkow H, Massoud F, Nasreddine Z, et al. Diagnosis and treatment of dementia: 3. Mild cognitive impairment and cognitive impairment without dementia. CMAJ. 2008;178(10):1273-1285.
- Rosenberg PB, Lyketsos C. Mild cognitive impairment: searching for the prodrome of Alzheimer’s disease. World Psychiatry. 2008;7(2):72-78.
- Reisberg B, Shulman MB, Torossian C, et al. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6(1):11-24.
- Desai AK, Schwarz L. Subjective cognitive impairment: when to be concerned about ‘senior moments.’ Current Psychiatry. 2011;10(4):31-44.
Clinical applications
Although pharmacologic therapies for the early phases of AD are not yet available, research supports implementing nonpharmacologic modalities in older adults with MCI as well as those without any cognitive impairment (Table 3).8,11 Growing evidence suggests physicians should encourage patients to lead an active and socially integrated lifestyle that includes leisure activities, cognitive stimulation, meditation, a balanced diet, and daily exercise.8 Practitioners should treat vascular risk factors in geriatric patients with and without cognitive impairment to optimize healthy brain aging and reduce the risk of cardiovascular disease and stroke.11 By raising awareness of available treatments for early phases of AD, we may be able to reduce the anxiety and sense of helplessness or hopelessness that may accompany an AD diagnosis.
Depression and AD. Having depression nearly doubles one’s risk of developing AD later in life, and depression may exacerbate AD.12 Although the precise mechanism linking depression to AD is unclear, depression seems to exert a toxic effect on the hippocampus.13 Treating depression may prevent or mitigate the rate of memory impairment and overall AD severity and improve a patient’s quality of life, overall health, and ability to function.
Almost one-third of family caregivers become depressed while helping a family member with DAT.14 Directing caregivers to peer support groups and providing them with tips on how to take care of themselves physically, emotionally, and psychologically can be extremely beneficial. Data suggest that improving the psychological and emotional well-being of caretakers may delay nursing home placement of patients with DAT.15 Delaying nursing home placement can substantially improve quality of life and reduce the financial strain on patients and caregivers.
Patients and families often turn to clinicians for advice on what problems they or their loved ones may encounter if they suffer from cognitive impairment. One benefit of the new guidelines is that they can help us become educated about the early phases of AD as well as the long and often difficult course of the disease. In turn, we can better educate our patients and their families about the disease.
As early screening of AD improves, patients in the early phases will have an opportunity to take part in clinical trials for potential pharmacologic treatments of the disease. Our role as clinicians will be to guide patients and their families to such trials and give them the opportunity to help change our understanding of and approach to treating AD. It is important to keep in mind that the new guidelines should not be considered final, but rather as a work in progress that periodically will be revised as AD research progresses.3
Table 3
Promoting healthy brain aging
| Healthy diet (eg, Mediterranean diet) |
| Adequate sleep |
| Daily exercise |
| Smoking cessation |
| Active, socially integrated lifestyle |
| Leisure activities |
| Cognitive stimulation |
| Optimize treatment of depression and other mental illnesses |
| Meditation and other mindfulness strategies (eg, yoga) |
| Spiritual activities |
| Controlling vascular risk factors (hypertension, diabetes, dyslipidemia, and obesity) |
| Source: References 8,11 |
Related Resources
- Alzheimer’s Association. www.alz.org.
- National Institute on Aging. Alzheimer’s disease education and referral center. www.nia.nih.gov/alzheimers.
Disclosures
Drs. Kimchi and Desai report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg is a consultant to Baxter, Forest Laboratories, Merck, Otsuka, and Novartis.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257-262.
2. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
3. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
4. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
5. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944.
6. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
7. Ihl R, Frölich L, Winblad B, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of Alzheimer’s disease and other dementias. World J Biol Psychiatry. 2011;12(1):2-32.
8. Chertkow H, Massoud F, Nasreddine Z, et al. Diagnosis and treatment of dementia: 3. Mild cognitive impairment and cognitive impairment without dementia. CMAJ. 2008;178(10):1273-1285.
9. McKhann GM. Changing concepts of Alzheimer disease. JAMA. 2011;305(23):2458-2459.
10. Galasko D. Biomarkers in non-Alzheimer’s dementias. Clinical Neuroscience Research. 2004;3(6):375-381.
11. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
12. Wilson RS, Hoganson GM, Rajan KB, et al. Temporal course of depressive symptoms during the development of Alzheimer disease. Neurology. 2010;75(1):21-26.
13. Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115-118.
14. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090-2097.
15. Mittelman MS, Haley WE, Clay OJ, et al. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592-1599.
Discuss this article at www.facebook.com/CurrentPsychiatry
In 2011, a workgroup of experts from the Alzheimer’s Association and the National Institute on Aging published new criteria and guidelines for diagnosing Alzheimer’s disease (AD), the first new AD guidelines since 1984.1-4 These criteria reflect data that suggest AD is not synonymous with dementia of the Alzheimer’s type (DAT) but is a disease that slowly develops over many years as a result of accumulated neuropathologic changes, with dementia representing only the final phase of the disease (Figure).1-4
Figure: Cognitive decline in AD over time
AD: Alzheimer’s disease; MCI: mild cognitive impairment
Source: Adapted from reference 2
This article highlights the similarities and differences of the 1984 and 2011 AD diagnosis guidelines. We also discuss the new guidelines’ limitations and clinical implications.
The 1984 AD criteria
Both the 1984 AD criteria5 and DSM-IV-TR criteria6 rely on the concept that AD is a clinical diagnosis made after a patient develops dementia. That is, diagnosis rests on the physician’s clinical judgment about the etiology of the patient’s symptoms, taking into account reports from the patient, family, and friends, as well as results of neurocognitive testing and mental status evaluation. The 1984 criteria were developed with the expectation that if a patient who met clinical criteria for AD were to undergo an autopsy, he or she likely would have evidence of AD pathology as the underlying etiology. These criteria were developed before researchers discovered that in AD, pathologic changes occur over many years and clinical dementia is the end product of accumulated pathology. The 1984 criteria did not address important phases that precede clinical dementia—such as mild cognitive impairment (MCI). See the Table for a summary of the 1984 AD criteria.
Table
The 1984 NINCDS-ADRDA criteria for clinical diagnosis of AD
|
| AD: Alzheimer’s disease; NINCDS-ADRDA: National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association Source: McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944 |
The 2011 AD criteria
The new AD criteria differ from the 1984 criteria in 2 major ways:
- expansion of AD into 3 phases, only 1 of which is characterized by dementia
- incorporation of biomarkers to provide information regarding pathophysiologic changes underlying the disease state (Table 1).1-5
The 3 phases. The 2011 criteria expand the definition of AD to include an asymptomatic, preclinical phase; a symptomatic, pre-dementia phase; and a dementia phase. In the initial phase, neuronal toxins such as beta-amyloid (Aβ) plaques and elevated tau first become detectable. Patients in this phase are asymptomatic or have subtle symptoms. This phase should be viewed as part of a continuum and includes patients who may, for instance, develop Aβ plaques but do not progress to further neurodegeneration.2 The diagnostic criteria of this phase are intended for research purposes only.1,2
Patients in the symptomatic, pre-dementia phase—also known as the MCI phase—exhibit mild decline in memory, attention, and thinking. Although this decline is more than what is expected for the patient’s age and education, it does not compromise everyday activity and functioning.
A patient who develops cognitive or behavioral problems that interfere with his or her ability to function at work or in everyday activities has entered the dementia phase. Similar to the 1984 guidelines, the 2011 criteria classify patients into probable and possible AD dementia. All patients who would have satisfied criteria for probable AD under the 1984 guidelines will satisfy criteria for probable AD dementia under the 2011 criteria.4 The same is not true for possible AD dementia. The 2011 criteria include 2 other major categories for patients with AD dementia: probable and possible AD dementia with evidence of the AD pathophysiological process. These categories are intended for research purposes only, whereas the criteria for the MCI and dementia phases are intended to guide diagnosis in the clinical setting.
By incorporating phases of AD that precede dementia into the disease spectrum, the new guidelines are designed to move clinicians toward earlier diagnosis and treatment.1-3 Similar to how early, pre-symptomatic detection and treatment of conditions such as diabetes and cancer can reduce mortality, improving diagnosis of AD in its early phases may allow clinicians to better test potential therapies and eventually use them to treat at-risk individuals.2,3 Most pharmacotherapies for AD are FDA-approved only for patients diagnosed with clinical dementia. Furthermore, current pharmacotherapies do not alter the course of AD; they have a modest effect in slowing cognitive and functional decline.7,8 If patients in the earlier phases of AD could be recruited for research studies, we may be able to develop new treatments to stop or reverse AD pathology and its clinical manifestations.
Biomarkers. The new criteria incorporate biomarkers to provide information about pathophysiologic changes underlying the disease process. These criteria define biomarkers as physiologic, biochemical, or anatomic parameters that can be measured in vivo and reflect specific features of disease-related pathophysiologic processes.1 Presently, there are no cutoff values to demarcate “normal” levels from “abnormal,” and biomarkers are proposed primarily as research tools because they have not been studied adequately in community settings and laboratory techniques to measure biomarkers have not been standardized.1-4,9
The 5 biomarkers incorporated into the new criteria are divided into 2 categories: biomarkers of Aβ accumulation and those of neuronal degeneration or injury (Table 2).1-4 In the initial, preclinical phase, biomarkers are used to detect changes in the brain—such as amyloid accumulation and nerve cell degeneration—that may already be in process in an individual whose clinical symptoms are subtle or not yet evident.1,2 In this phase, progressive evidence of biomarkers, such as both Aβ accumulation and neuronal injury rather than Aβ accumulation alone, may increase the probability that a patient will decline quickly into the MCI phase.2 Biomarkers of neuronal degeneration or injury especially correlate with the likelihood that the disease will progress to clinical dementia.1 Subtle cognitive symptoms in the preclinical phase also might predict rapid decline into MCI.2
In the MCI and dementia phases, biomarkers are used to determine the level of certainty that AD is responsible for the patient’s symptoms.1,3,4 For example, a patient could meet criteria for a non-AD dementia such as dementia with Lewy bodies, but also meet pathologic criteria for AD on autopsy.3 The diagnostic category of possible AD dementia with evidence of the AD pathophysiologic process is intended for this type of scenario.4 For the MCI phase, the criteria propose levels of certainty that a patient’s MCI syndrome is caused by AD, ranging from MCI due to AD-high likelihood to MCI-unlikely due to AD.3
Research has demonstrated that a patient’s clinical picture doesn’t necessarily reflect the extent of the underlying pathology. For example, a patient could have extensive AD pathology, such as diffuse amyloid plaques, without any obvious clinical symptoms.3 Conversely, although both Aβ deposition and elevated tau are hallmarks of AD, variations in these proteins can be seen in neuropsychiatric disorders other than AD.10 That said, it appears that worsening of clinical symptoms often parallels worsening of neurodegenerative biomarkers.1
Under the 2011 guidelines, biomarkers would not be used to diagnose or exclude AD or MCI, but instead would help improve diagnostic accuracy in individuals with cognitive decline.1,3,4 In other words, AD remains a clinical diagnosis, but these biomarkers could raise or lower the positive predictive value of a clinician’s judgment about the etiology of a patient’s symptoms.
See the Box for a description of the potential risks and benefits of using the new diagnostic criteria.
Table 1
Comparing the 1984 and 2011 AD criteria
| 1984 criteria | 2011 criteria |
|---|---|
| AD is a clinical diagnosis | AD remains a clinical diagnosis but biomarkers serve to improve the accuracy of diagnosis of the disease |
| There is only 1 phase of AD—dementia. | AD is expanded into 3 phases: an asymptomatic, preclinical phase; a symptomatic, pre-dementia phase; and a dementia phase |
| A patient who meets the clinical criteria for AD would be expected to have AD pathology as the underlying etiology were he/she to undergo a brain autopsy | Presently, biomarkers are proposed as research tools only and are not intended to be applied in the clinical setting. However, eventually clinicians will be able to diagnose AD in all 3 phases, as biomarker testing becomes standardized and reliable enough to be accurately applied in clinical settings |
| Little consideration is given to specific neuropathologic changes underlying the disease process | Biomarkers provide information regarding the pathophysiologic changes underlying the disease state |
| Little consideration is given to the idea that pathologic changes occur over many years | Inherent in dividing AD into 3 phases is the concept that AD develops slowly over many years and has a long prodromal phase that is clinically silent |
| AD: Alzheimer’s disease Source: References 1-5 | |
Table 2
5 biomarkers incorporated into the 2011 AD criteria
| Category | Biomarkers |
|---|---|
| Biomarkers of Aβ accumulation | Abnormal tracer retention on amyloid PET imaging |
| Low CSF Aβ42 | |
| Biomarkers of neuronal degeneration or injury | Elevated CSF tau (total and phosphorylated tau) |
| Decreased fluorodeoxyglucose uptake on PET | |
| Atrophy on structural magnetic resonance imaging | |
| Aβ: beta-amyloid; AD: Alzheimer’s disease; CSF: cerebrospinal fluid; PET: positron emission tomography Source: References 1-4 | |
The earlier an Alzheimer’s disease (AD) diagnosis is made, the less certain it is AD.a Biomarkers typically found in individuals with AD also can be found in patients with dementia not caused by AD, such as vascular dementia, as well as in individuals who may never develop dementia.b Additionally, there is no certainty that a patient in an early phase of AD will develop clinical dementia. Falsely diagnosing a patient with AD may lead the individual and their family to feel helpless, hopeless, depressed, anxious, or ashamed and to spend money and other resources preparing for a prognosis that may never come to fruition. Clinicians may feel compelled to assess for biomarkers using expensive, invasive tests that are not yet standardized in an attempt to support the AD diagnosis.
Early diagnosis of AD has many benefits that should not be overlooked, however. It provides patients and their families an opportunity to become familiar with the disease course, which may help some patients cope with the diagnosis. Patients diagnosed in the early stages would be able to make important decisions regarding health care, social, and financial planning before they develop pathology that limits their executive planning abilities or become functionally impaired.
Diagnosing an illness when there are no disease-modifying therapies available is not futile. Some patients with newly diagnosed AD in the pre-dementia phases may want to participate in clinical research trials to help develop therapies for AD. Some data suggest that AD treatment appears to provide the greatest benefit when initiated early in the disease course and maintained over a long duration.c Eventually, we may be able to tailor specific AD treatments in different phases of the disease. For instance, we may discover treatments for patients who show evidence of beta-amyloid plaques but not neuronal injury, or vice versa. Patients also may benefit from education on nonpharmacologic treatments, including reducing vascular risk factors to help improve brain aging,d reducing stress, and learning cognitive strategies such as using mnemonics to aid memory.
In many clinical settings, patients are being clinically diagnosed with mild cognitive impairment (MCI). Research indicates that patients with MCI are at near-term risk of developing dementia, particularly dementia of the Alzheimer’s type.d,e Presently, no definite transition points demarcate MCI from dementia; this progression is based upon clinical judgment.
In the last decade, researchers have begun to describe a syndrome of subjective cognitive impairment (SCI), which may be a phase that precedes the MCI phase of AD.f Patients with SCI report cognitive deficits (eg, forgetfulness and word-finding difficulties) but have no objective evidence of cognitive impairment on neuropsychological tests. Cognitive problems associated with SCI do not cause functional decline.g SCI may reflect the minimal cognitive complaints mentioned in the research criteria for the preclinical phase of AD. Eventually, biomarkers may be able to help clinicians more accurately predict which patients with SCI are most likely to progress to the MCI or dementia phase of AD.
References
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
- Galasko D. Biomarkers in non-Alzheimer dementias. Clinical Neuroscience Research. 2004;3(6):375-381.
- Geldmacher DS. Treatment guidelines for Alzheimer’s disease: redefining perceptions in primary care. Prim Care Companion J Clin Psychiatry. 2007;9(2):113-121.
- Chertkow H, Massoud F, Nasreddine Z, et al. Diagnosis and treatment of dementia: 3. Mild cognitive impairment and cognitive impairment without dementia. CMAJ. 2008;178(10):1273-1285.
- Rosenberg PB, Lyketsos C. Mild cognitive impairment: searching for the prodrome of Alzheimer’s disease. World Psychiatry. 2008;7(2):72-78.
- Reisberg B, Shulman MB, Torossian C, et al. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6(1):11-24.
- Desai AK, Schwarz L. Subjective cognitive impairment: when to be concerned about ‘senior moments.’ Current Psychiatry. 2011;10(4):31-44.
Clinical applications
Although pharmacologic therapies for the early phases of AD are not yet available, research supports implementing nonpharmacologic modalities in older adults with MCI as well as those without any cognitive impairment (Table 3).8,11 Growing evidence suggests physicians should encourage patients to lead an active and socially integrated lifestyle that includes leisure activities, cognitive stimulation, meditation, a balanced diet, and daily exercise.8 Practitioners should treat vascular risk factors in geriatric patients with and without cognitive impairment to optimize healthy brain aging and reduce the risk of cardiovascular disease and stroke.11 By raising awareness of available treatments for early phases of AD, we may be able to reduce the anxiety and sense of helplessness or hopelessness that may accompany an AD diagnosis.
Depression and AD. Having depression nearly doubles one’s risk of developing AD later in life, and depression may exacerbate AD.12 Although the precise mechanism linking depression to AD is unclear, depression seems to exert a toxic effect on the hippocampus.13 Treating depression may prevent or mitigate the rate of memory impairment and overall AD severity and improve a patient’s quality of life, overall health, and ability to function.
Almost one-third of family caregivers become depressed while helping a family member with DAT.14 Directing caregivers to peer support groups and providing them with tips on how to take care of themselves physically, emotionally, and psychologically can be extremely beneficial. Data suggest that improving the psychological and emotional well-being of caretakers may delay nursing home placement of patients with DAT.15 Delaying nursing home placement can substantially improve quality of life and reduce the financial strain on patients and caregivers.
Patients and families often turn to clinicians for advice on what problems they or their loved ones may encounter if they suffer from cognitive impairment. One benefit of the new guidelines is that they can help us become educated about the early phases of AD as well as the long and often difficult course of the disease. In turn, we can better educate our patients and their families about the disease.
As early screening of AD improves, patients in the early phases will have an opportunity to take part in clinical trials for potential pharmacologic treatments of the disease. Our role as clinicians will be to guide patients and their families to such trials and give them the opportunity to help change our understanding of and approach to treating AD. It is important to keep in mind that the new guidelines should not be considered final, but rather as a work in progress that periodically will be revised as AD research progresses.3
Table 3
Promoting healthy brain aging
| Healthy diet (eg, Mediterranean diet) |
| Adequate sleep |
| Daily exercise |
| Smoking cessation |
| Active, socially integrated lifestyle |
| Leisure activities |
| Cognitive stimulation |
| Optimize treatment of depression and other mental illnesses |
| Meditation and other mindfulness strategies (eg, yoga) |
| Spiritual activities |
| Controlling vascular risk factors (hypertension, diabetes, dyslipidemia, and obesity) |
| Source: References 8,11 |
Related Resources
- Alzheimer’s Association. www.alz.org.
- National Institute on Aging. Alzheimer’s disease education and referral center. www.nia.nih.gov/alzheimers.
Disclosures
Drs. Kimchi and Desai report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg is a consultant to Baxter, Forest Laboratories, Merck, Otsuka, and Novartis.
Discuss this article at www.facebook.com/CurrentPsychiatry
In 2011, a workgroup of experts from the Alzheimer’s Association and the National Institute on Aging published new criteria and guidelines for diagnosing Alzheimer’s disease (AD), the first new AD guidelines since 1984.1-4 These criteria reflect data that suggest AD is not synonymous with dementia of the Alzheimer’s type (DAT) but is a disease that slowly develops over many years as a result of accumulated neuropathologic changes, with dementia representing only the final phase of the disease (Figure).1-4
Figure: Cognitive decline in AD over time
AD: Alzheimer’s disease; MCI: mild cognitive impairment
Source: Adapted from reference 2
This article highlights the similarities and differences of the 1984 and 2011 AD diagnosis guidelines. We also discuss the new guidelines’ limitations and clinical implications.
The 1984 AD criteria
Both the 1984 AD criteria5 and DSM-IV-TR criteria6 rely on the concept that AD is a clinical diagnosis made after a patient develops dementia. That is, diagnosis rests on the physician’s clinical judgment about the etiology of the patient’s symptoms, taking into account reports from the patient, family, and friends, as well as results of neurocognitive testing and mental status evaluation. The 1984 criteria were developed with the expectation that if a patient who met clinical criteria for AD were to undergo an autopsy, he or she likely would have evidence of AD pathology as the underlying etiology. These criteria were developed before researchers discovered that in AD, pathologic changes occur over many years and clinical dementia is the end product of accumulated pathology. The 1984 criteria did not address important phases that precede clinical dementia—such as mild cognitive impairment (MCI). See the Table for a summary of the 1984 AD criteria.
Table
The 1984 NINCDS-ADRDA criteria for clinical diagnosis of AD
|
| AD: Alzheimer’s disease; NINCDS-ADRDA: National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association Source: McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944 |
The 2011 AD criteria
The new AD criteria differ from the 1984 criteria in 2 major ways:
- expansion of AD into 3 phases, only 1 of which is characterized by dementia
- incorporation of biomarkers to provide information regarding pathophysiologic changes underlying the disease state (Table 1).1-5
The 3 phases. The 2011 criteria expand the definition of AD to include an asymptomatic, preclinical phase; a symptomatic, pre-dementia phase; and a dementia phase. In the initial phase, neuronal toxins such as beta-amyloid (Aβ) plaques and elevated tau first become detectable. Patients in this phase are asymptomatic or have subtle symptoms. This phase should be viewed as part of a continuum and includes patients who may, for instance, develop Aβ plaques but do not progress to further neurodegeneration.2 The diagnostic criteria of this phase are intended for research purposes only.1,2
Patients in the symptomatic, pre-dementia phase—also known as the MCI phase—exhibit mild decline in memory, attention, and thinking. Although this decline is more than what is expected for the patient’s age and education, it does not compromise everyday activity and functioning.
A patient who develops cognitive or behavioral problems that interfere with his or her ability to function at work or in everyday activities has entered the dementia phase. Similar to the 1984 guidelines, the 2011 criteria classify patients into probable and possible AD dementia. All patients who would have satisfied criteria for probable AD under the 1984 guidelines will satisfy criteria for probable AD dementia under the 2011 criteria.4 The same is not true for possible AD dementia. The 2011 criteria include 2 other major categories for patients with AD dementia: probable and possible AD dementia with evidence of the AD pathophysiological process. These categories are intended for research purposes only, whereas the criteria for the MCI and dementia phases are intended to guide diagnosis in the clinical setting.
By incorporating phases of AD that precede dementia into the disease spectrum, the new guidelines are designed to move clinicians toward earlier diagnosis and treatment.1-3 Similar to how early, pre-symptomatic detection and treatment of conditions such as diabetes and cancer can reduce mortality, improving diagnosis of AD in its early phases may allow clinicians to better test potential therapies and eventually use them to treat at-risk individuals.2,3 Most pharmacotherapies for AD are FDA-approved only for patients diagnosed with clinical dementia. Furthermore, current pharmacotherapies do not alter the course of AD; they have a modest effect in slowing cognitive and functional decline.7,8 If patients in the earlier phases of AD could be recruited for research studies, we may be able to develop new treatments to stop or reverse AD pathology and its clinical manifestations.
Biomarkers. The new criteria incorporate biomarkers to provide information about pathophysiologic changes underlying the disease process. These criteria define biomarkers as physiologic, biochemical, or anatomic parameters that can be measured in vivo and reflect specific features of disease-related pathophysiologic processes.1 Presently, there are no cutoff values to demarcate “normal” levels from “abnormal,” and biomarkers are proposed primarily as research tools because they have not been studied adequately in community settings and laboratory techniques to measure biomarkers have not been standardized.1-4,9
The 5 biomarkers incorporated into the new criteria are divided into 2 categories: biomarkers of Aβ accumulation and those of neuronal degeneration or injury (Table 2).1-4 In the initial, preclinical phase, biomarkers are used to detect changes in the brain—such as amyloid accumulation and nerve cell degeneration—that may already be in process in an individual whose clinical symptoms are subtle or not yet evident.1,2 In this phase, progressive evidence of biomarkers, such as both Aβ accumulation and neuronal injury rather than Aβ accumulation alone, may increase the probability that a patient will decline quickly into the MCI phase.2 Biomarkers of neuronal degeneration or injury especially correlate with the likelihood that the disease will progress to clinical dementia.1 Subtle cognitive symptoms in the preclinical phase also might predict rapid decline into MCI.2
In the MCI and dementia phases, biomarkers are used to determine the level of certainty that AD is responsible for the patient’s symptoms.1,3,4 For example, a patient could meet criteria for a non-AD dementia such as dementia with Lewy bodies, but also meet pathologic criteria for AD on autopsy.3 The diagnostic category of possible AD dementia with evidence of the AD pathophysiologic process is intended for this type of scenario.4 For the MCI phase, the criteria propose levels of certainty that a patient’s MCI syndrome is caused by AD, ranging from MCI due to AD-high likelihood to MCI-unlikely due to AD.3
Research has demonstrated that a patient’s clinical picture doesn’t necessarily reflect the extent of the underlying pathology. For example, a patient could have extensive AD pathology, such as diffuse amyloid plaques, without any obvious clinical symptoms.3 Conversely, although both Aβ deposition and elevated tau are hallmarks of AD, variations in these proteins can be seen in neuropsychiatric disorders other than AD.10 That said, it appears that worsening of clinical symptoms often parallels worsening of neurodegenerative biomarkers.1
Under the 2011 guidelines, biomarkers would not be used to diagnose or exclude AD or MCI, but instead would help improve diagnostic accuracy in individuals with cognitive decline.1,3,4 In other words, AD remains a clinical diagnosis, but these biomarkers could raise or lower the positive predictive value of a clinician’s judgment about the etiology of a patient’s symptoms.
See the Box for a description of the potential risks and benefits of using the new diagnostic criteria.
Table 1
Comparing the 1984 and 2011 AD criteria
| 1984 criteria | 2011 criteria |
|---|---|
| AD is a clinical diagnosis | AD remains a clinical diagnosis but biomarkers serve to improve the accuracy of diagnosis of the disease |
| There is only 1 phase of AD—dementia. | AD is expanded into 3 phases: an asymptomatic, preclinical phase; a symptomatic, pre-dementia phase; and a dementia phase |
| A patient who meets the clinical criteria for AD would be expected to have AD pathology as the underlying etiology were he/she to undergo a brain autopsy | Presently, biomarkers are proposed as research tools only and are not intended to be applied in the clinical setting. However, eventually clinicians will be able to diagnose AD in all 3 phases, as biomarker testing becomes standardized and reliable enough to be accurately applied in clinical settings |
| Little consideration is given to specific neuropathologic changes underlying the disease process | Biomarkers provide information regarding the pathophysiologic changes underlying the disease state |
| Little consideration is given to the idea that pathologic changes occur over many years | Inherent in dividing AD into 3 phases is the concept that AD develops slowly over many years and has a long prodromal phase that is clinically silent |
| AD: Alzheimer’s disease Source: References 1-5 | |
Table 2
5 biomarkers incorporated into the 2011 AD criteria
| Category | Biomarkers |
|---|---|
| Biomarkers of Aβ accumulation | Abnormal tracer retention on amyloid PET imaging |
| Low CSF Aβ42 | |
| Biomarkers of neuronal degeneration or injury | Elevated CSF tau (total and phosphorylated tau) |
| Decreased fluorodeoxyglucose uptake on PET | |
| Atrophy on structural magnetic resonance imaging | |
| Aβ: beta-amyloid; AD: Alzheimer’s disease; CSF: cerebrospinal fluid; PET: positron emission tomography Source: References 1-4 | |
The earlier an Alzheimer’s disease (AD) diagnosis is made, the less certain it is AD.a Biomarkers typically found in individuals with AD also can be found in patients with dementia not caused by AD, such as vascular dementia, as well as in individuals who may never develop dementia.b Additionally, there is no certainty that a patient in an early phase of AD will develop clinical dementia. Falsely diagnosing a patient with AD may lead the individual and their family to feel helpless, hopeless, depressed, anxious, or ashamed and to spend money and other resources preparing for a prognosis that may never come to fruition. Clinicians may feel compelled to assess for biomarkers using expensive, invasive tests that are not yet standardized in an attempt to support the AD diagnosis.
Early diagnosis of AD has many benefits that should not be overlooked, however. It provides patients and their families an opportunity to become familiar with the disease course, which may help some patients cope with the diagnosis. Patients diagnosed in the early stages would be able to make important decisions regarding health care, social, and financial planning before they develop pathology that limits their executive planning abilities or become functionally impaired.
Diagnosing an illness when there are no disease-modifying therapies available is not futile. Some patients with newly diagnosed AD in the pre-dementia phases may want to participate in clinical research trials to help develop therapies for AD. Some data suggest that AD treatment appears to provide the greatest benefit when initiated early in the disease course and maintained over a long duration.c Eventually, we may be able to tailor specific AD treatments in different phases of the disease. For instance, we may discover treatments for patients who show evidence of beta-amyloid plaques but not neuronal injury, or vice versa. Patients also may benefit from education on nonpharmacologic treatments, including reducing vascular risk factors to help improve brain aging,d reducing stress, and learning cognitive strategies such as using mnemonics to aid memory.
In many clinical settings, patients are being clinically diagnosed with mild cognitive impairment (MCI). Research indicates that patients with MCI are at near-term risk of developing dementia, particularly dementia of the Alzheimer’s type.d,e Presently, no definite transition points demarcate MCI from dementia; this progression is based upon clinical judgment.
In the last decade, researchers have begun to describe a syndrome of subjective cognitive impairment (SCI), which may be a phase that precedes the MCI phase of AD.f Patients with SCI report cognitive deficits (eg, forgetfulness and word-finding difficulties) but have no objective evidence of cognitive impairment on neuropsychological tests. Cognitive problems associated with SCI do not cause functional decline.g SCI may reflect the minimal cognitive complaints mentioned in the research criteria for the preclinical phase of AD. Eventually, biomarkers may be able to help clinicians more accurately predict which patients with SCI are most likely to progress to the MCI or dementia phase of AD.
References
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
- Galasko D. Biomarkers in non-Alzheimer dementias. Clinical Neuroscience Research. 2004;3(6):375-381.
- Geldmacher DS. Treatment guidelines for Alzheimer’s disease: redefining perceptions in primary care. Prim Care Companion J Clin Psychiatry. 2007;9(2):113-121.
- Chertkow H, Massoud F, Nasreddine Z, et al. Diagnosis and treatment of dementia: 3. Mild cognitive impairment and cognitive impairment without dementia. CMAJ. 2008;178(10):1273-1285.
- Rosenberg PB, Lyketsos C. Mild cognitive impairment: searching for the prodrome of Alzheimer’s disease. World Psychiatry. 2008;7(2):72-78.
- Reisberg B, Shulman MB, Torossian C, et al. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6(1):11-24.
- Desai AK, Schwarz L. Subjective cognitive impairment: when to be concerned about ‘senior moments.’ Current Psychiatry. 2011;10(4):31-44.
Clinical applications
Although pharmacologic therapies for the early phases of AD are not yet available, research supports implementing nonpharmacologic modalities in older adults with MCI as well as those without any cognitive impairment (Table 3).8,11 Growing evidence suggests physicians should encourage patients to lead an active and socially integrated lifestyle that includes leisure activities, cognitive stimulation, meditation, a balanced diet, and daily exercise.8 Practitioners should treat vascular risk factors in geriatric patients with and without cognitive impairment to optimize healthy brain aging and reduce the risk of cardiovascular disease and stroke.11 By raising awareness of available treatments for early phases of AD, we may be able to reduce the anxiety and sense of helplessness or hopelessness that may accompany an AD diagnosis.
Depression and AD. Having depression nearly doubles one’s risk of developing AD later in life, and depression may exacerbate AD.12 Although the precise mechanism linking depression to AD is unclear, depression seems to exert a toxic effect on the hippocampus.13 Treating depression may prevent or mitigate the rate of memory impairment and overall AD severity and improve a patient’s quality of life, overall health, and ability to function.
Almost one-third of family caregivers become depressed while helping a family member with DAT.14 Directing caregivers to peer support groups and providing them with tips on how to take care of themselves physically, emotionally, and psychologically can be extremely beneficial. Data suggest that improving the psychological and emotional well-being of caretakers may delay nursing home placement of patients with DAT.15 Delaying nursing home placement can substantially improve quality of life and reduce the financial strain on patients and caregivers.
Patients and families often turn to clinicians for advice on what problems they or their loved ones may encounter if they suffer from cognitive impairment. One benefit of the new guidelines is that they can help us become educated about the early phases of AD as well as the long and often difficult course of the disease. In turn, we can better educate our patients and their families about the disease.
As early screening of AD improves, patients in the early phases will have an opportunity to take part in clinical trials for potential pharmacologic treatments of the disease. Our role as clinicians will be to guide patients and their families to such trials and give them the opportunity to help change our understanding of and approach to treating AD. It is important to keep in mind that the new guidelines should not be considered final, but rather as a work in progress that periodically will be revised as AD research progresses.3
Table 3
Promoting healthy brain aging
| Healthy diet (eg, Mediterranean diet) |
| Adequate sleep |
| Daily exercise |
| Smoking cessation |
| Active, socially integrated lifestyle |
| Leisure activities |
| Cognitive stimulation |
| Optimize treatment of depression and other mental illnesses |
| Meditation and other mindfulness strategies (eg, yoga) |
| Spiritual activities |
| Controlling vascular risk factors (hypertension, diabetes, dyslipidemia, and obesity) |
| Source: References 8,11 |
Related Resources
- Alzheimer’s Association. www.alz.org.
- National Institute on Aging. Alzheimer’s disease education and referral center. www.nia.nih.gov/alzheimers.
Disclosures
Drs. Kimchi and Desai report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg is a consultant to Baxter, Forest Laboratories, Merck, Otsuka, and Novartis.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257-262.
2. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
3. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
4. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
5. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944.
6. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
7. Ihl R, Frölich L, Winblad B, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of Alzheimer’s disease and other dementias. World J Biol Psychiatry. 2011;12(1):2-32.
8. Chertkow H, Massoud F, Nasreddine Z, et al. Diagnosis and treatment of dementia: 3. Mild cognitive impairment and cognitive impairment without dementia. CMAJ. 2008;178(10):1273-1285.
9. McKhann GM. Changing concepts of Alzheimer disease. JAMA. 2011;305(23):2458-2459.
10. Galasko D. Biomarkers in non-Alzheimer’s dementias. Clinical Neuroscience Research. 2004;3(6):375-381.
11. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
12. Wilson RS, Hoganson GM, Rajan KB, et al. Temporal course of depressive symptoms during the development of Alzheimer disease. Neurology. 2010;75(1):21-26.
13. Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115-118.
14. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090-2097.
15. Mittelman MS, Haley WE, Clay OJ, et al. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592-1599.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257-262.
2. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
3. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
4. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
5. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944.
6. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
7. Ihl R, Frölich L, Winblad B, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of Alzheimer’s disease and other dementias. World J Biol Psychiatry. 2011;12(1):2-32.
8. Chertkow H, Massoud F, Nasreddine Z, et al. Diagnosis and treatment of dementia: 3. Mild cognitive impairment and cognitive impairment without dementia. CMAJ. 2008;178(10):1273-1285.
9. McKhann GM. Changing concepts of Alzheimer disease. JAMA. 2011;305(23):2458-2459.
10. Galasko D. Biomarkers in non-Alzheimer’s dementias. Clinical Neuroscience Research. 2004;3(6):375-381.
11. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
12. Wilson RS, Hoganson GM, Rajan KB, et al. Temporal course of depressive symptoms during the development of Alzheimer disease. Neurology. 2010;75(1):21-26.
13. Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115-118.
14. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090-2097.
15. Mittelman MS, Haley WE, Clay OJ, et al. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592-1599.
The impact of perinatal illness on parenting and fetal/infant development
Getting ready for DSM-5: Part 1
Discuss this article at www.facebook.com/CurrentPsychiatry
Work on the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)—scheduled to be published in May 2013—has been ongoing for more than a decade. Momentous advances in genetics and brain imaging since publication of DSM-IV in 1994 have generated optimism that an improved understanding of the neurobiologic underpinnings of psychiatric disorders might lead to a paradigm shift from the current descriptive classification system to a more scientific etiopathophysiological system similar to that used by other medical specialities.1
Some fear that any changes to our current classification system may be premature and could make an already complex system even more unwieldy.2 Scores of articles about the content and process of DSM-5 and several critiques and commentaries on the topic have been published. The American Psychiatric Association (APA) has made the DSM-5 process transparent by posting frequent updates to the DSM-5 Development Web site (www.dsm5.org), seeking feedback from the psychiatric community and the public, and presenting progress reports by members of the DSM-5 Task Force at scientific meetings.
There have been few discussions on the implications of DSM-5 from the practicing clinician’s vantage point, which I seek to present in this series of articles, the remainder of which will be published here, at CurrentPsychiatry.com. In this article, I:
- provide a brief history of psychiatric classification, focusing on the origins and evolution of the DSM system
- summarize the limitations of DSM-IV
- note the challenges and tensions in the construction of DSM-5
- review the DSM-5 process
- outline its current status
- discuss the organization and content of future articles in this series.
Although I am a member of the DSM-5 Psychotic Disorders Work Group, I am solely responsible for the content and any opinions that I offer in this article and series. All details of DSM-5 that I discuss are publicly available at www.dsm5.org. I’ve been a clinician and clinical researcher for >25 years, and my opinions are colored by the need for clarity, rigor, clinical relevance, and a disdain for overly speculative thinking.
Evolution of DSM
A nosological system (system of classification of disease) enables clinicians to provide specific treatments for medical causes of human disease and/or disability with precise and predictable effects and guide patients and families about the likely course and outcome. Such classification systems also are used by:
- researchers, to learn more about the nature of the conditions being classified and develop better treatments for them
- health care systems, to provide optimal health care and track its appropriate provision
- insurance companies, to provide appropriate reimbursement for health care
- health product developers, including pharmaceutical companies, to develop health care products and promote their appropriate utilization
- government agencies, to determine health priorities and apportionment of health care resources
- public health agencies, to track the distribution of health and disease in communities around the world.
An ideal classification system would meet all constituents’ needs while perfectly mapping natural disease entities with distinct etiology and pathophysiology (validity), consistently allow all users to reach the same diagnosis (reliability), and provide clinicians with clear guidance about treatment and likely course for each of the entities (utility), with the list of entities being mutually exclusive and collectively exhaustive (coverage).
The current nosological system for psychiatric disorders originated in the late 19th and early 20th centuries and culminated in the first edition of the Diagnostic and Statistical Manual of Mental Disorders3 released in 1952 and a section related to mental disorders (section V) in the sixth revision of the International Classification of Disease (ICD).4 Whereas DSM focuses exclusively on mental disorders, the ICD is a general medical classification system that began covering mental disorders with its sixth revision in 1949. In subsequent revisions (ICD-7 through -10 and DSM-II through -IV), substantial changes in diagnostic criteria have been made, although the systems’ basic structure has been retained. Table 1 describes major changes from DSM-I through DSM-IV-TR.3,5-9 DSM and ICD both are being revised; DSM-5 is scheduled to be released in 2013 and ICD-11 is to be finalized by 2016.
Table 1
Conceptual development of DSM-I to DSM-IV-TR
| Version | Comments |
|---|---|
| DSM-I (1952)3 | Presumed etiology. 106 diagnoses |
| DSM-II (1968)5 | Glossary definitions. 185 diagnoses |
| DSM-III (1980)6 | Paradigm shift. Explicit criteria. Emphasis on reliability. 265 diagnoses |
| DSM-III-R (1987)7 | Modest changes. Blunted hierarchies. Clarifications. 292 diagnoses |
| DSM-IV (1994)8 | Modest changes. More blunted hierarchies. 361 diagnoses |
| DSM-IV-TR (2000)9 | Only text revision. 361 diagnostic conditions |
What do clinicians need?
Similar to ICD-10, DSM-IV is marked by considerable complexity, variable validity, limited clinical and research utility, and problems of burgeoning comorbidity.10 Efforts to revise DSM seek to address these limitations. From a clinician’s perspective, the most challenging aspects of DSM-IV derive from its complexity—which makes clinical application difficult—and its limited clinical utility, which is exemplified by artificial comorbidity,11 frequent use of “not otherwise specified” (NOS), and relative treatment nonspecificity with reference to diagnosis.
As clinicians, we want a nosological system that is easy to use, can guide treatment decisions, provides useful information about likely disease course and outcomes, and allows us to easily communicate about disease nature with patients, families, payers, and health care administrators. Additionally, although good validity and reliability are desirable for clinicians, adequate coverage of psychiatric disease—the listed conditions should be collectively exhaustive and mutually exclusive—is particularly valued. Finally, we want a diagnostic system that allows us to explain the reasoning behind psychiatric diagnoses and related treatment in lay terms to patients and their families.
DSM-5 development to date
DSM-5 development has been a collaborative effort led by the APA and involves the National Institute of Mental Health, the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and the World Health Organization (WHO). Between 1999 and 2002, 3 work conferences resulted in a series of white papers that identified gaps and research needs.1 Between 2003 and 2008, the American Psychiatric Institute for Research and Education, the National Institute of Health, and the WHO organized 13 international conferences to review a wide range of nosologic issues; the proceedings have been compiled into 13 monographs (11 published and 2 in press) and >125 scientific articles that serve as key reference sources for the DSM-5 process. For a continually updated list of these publications, see www.dsm5.org/Research.
In 2006, DSM-5 Task Force Chair David J. Kupfer, MD and Vice Chair Darrel A. Regier, MD, MPH were appointed and began selecting members of the DSM-5 Task Force, a process that was completed in 2007. Members of the 13 diagnostic area Work Groups (Table 2) were selected and the Work Groups were constituted in 2008. All 168 Task Force and Work Group members were vetted to ensure that they met standards of minimum conflict of interest and broad representation. Membership includes diverse professional representation from academia and mental health; 75% of members are from the United States. Six cross-cutting study groups have deliberated on a range of common issues, including:
- spectrum disorders
- lifespan and development
- gender and cross-cultural
- psychiatric/general medicine interface
- impairment and disability assessment
- diagnostic measurement and assessment.
Additionally, >300 external advisors with special expertise have participated in the process. Since 2008, each of the Work Groups has conducted extensive literature reviews of all assigned disorders, evaluated what works and what doesn’t work in DSM-IV-TR, assessed new research developments and clinical issues that have arisen since publication of DSM-IV-TR in 1994, and developed research plans to investigate critical issues utilizing systematic reviews and secondary data analyses. Based on these analyses, each Work Group proposed draft diagnostic criteria for its disorders, using a strict protocol for criteria revisions such as addition or deletion of disorders and changes to existing diagnostic criteria. These draft diagnostic criteria were first presented on www.dsm5.org in late 2009 through early 2010. Based on input from other Work Groups, the Task Force, several external groups, and the public, the Work Groups revised these criteria and prioritized necessary field trials to evaluate key recommendations. Phase I of the field trials began in 2010.12 Results of these field trials are being compiled and analyzed.
The DSM-5 Work Groups have met via teleconference 1 to 2 times a month and in-person twice a year, with significant communication between meetings. Work Group chairs are members of the Task Force, which has equally frequent meetings. Reports of DSM-5 deliberations have been presented at hundreds of professional meetings and described in >200 scientific publications. Comprehensive information and ongoing updates on DSM-5 and a list of publications and meetings are provided at www.dsm5.org.
Public input has been sought and the Work Groups have received and processed >10,000 comments. In 2010, the APA Board of Trustees appointed a Scientific Review Committee to evaluate the scientific merit and clinical impact of the Work Group recommendations and comment on the strength of the evidence advanced in support of each proposed revision. In 2011, the Board of Trustees appointed a Clinical and Public Health Committee to evaluate the clinical utility and public health significance of the proposed revisions. The APA and WHO have shared information and assessments in an effort to harmonize diagnostic criteria between DSM-5 and ICD-11.
Initial hopes that DSM-5 could represent a paradigm shift toward an etiopathophysiological classification of psychiatric disorders have been tempered by recognition of the limitations of our current neurobiologic understanding of psychiatric disorders. Therefore, the focus for DSM-5 has shifted from validity enhancements to improved clinical utility while building a framework that better lends itself to a future etiopathophysiological nosology.13-18 Whereas dimensional assessments are likely to be added across various diagnostic categories, a primarily categorical nosology will be retained and the proposed criterion changes are relatively modest. The results of our enhanced knowledge about the neurobiologic underpinnings of psychiatric disorders will not be reflected in diagnostic criteria, but in the significant revisions to the DSM text.
Our DSM-5 series
Subsequent articles in this series—which will be published here, at CurrentPsychiatry.com—will discuss specific proposed DSM-5 changes in 13 groups of disorders (Table 2) and their clinical implications. These articles also will address the relationship of DSM to ICD, issues with dimensional classification, and the importance of and challenges in precise diagnostic measurement.
Table 2
DSM-5 Work Groups
| Attention-deficit/hyperactivity disorder and disruptive behaviors |
| Anxiety, obsessive-compulsive, posttraumatic, and dissociative disorders |
| Disorders in childhood and adolescence |
| Eating disorders |
| Mood disorders |
| Neurocognitive disorders |
| Neurodevelopmental disorders |
| Personality and personality disorders |
| Psychotic disorders |
| Sexual and gender identity disorders |
| Sleep-wake disorders |
| Somatic distress disorders |
| Substance-related disorders |
Related Resources
- American Psychiatric Association. DSM-5 Development. www.dsm5.org.
- Black DW, Zimmerman M. Redefining personality disorders: Proposed revisions for DSM-5. Current Psychiatry. 2011;10(9):26-38.
Disclosure
Dr. Tandon is a member of the DSM-5 Psychotic Disorders Work Group. He reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kupfer DJ, First MB, Regier DA. eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
2. Frances A. Whither DSM-V? Br J Psychiatry. 2009;195(5):391-392.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington DC: American Psychiatric Association; 1952.
4. World Health Organization. Manual of the international statistical classification of diseases injuries and causes of death, 6th revision (ICD-6). Geneva, Switzerland: World Health Organization; 1949.
5. Diagnostic and statistical manual of mental disorders, 2nd ed. Washington DC: American Psychiatric Association; 1968.
6. Diagnostic and statistical manual of mental disorders, 3rd ed. Washington DC: American Psychiatric Association; 1980.
7. Diagnostic and statistical manual of mental disorders, 3rd ed, rev. Washington, DC: American Psychiatric Association; 1987.
8. Diagnostic and statistical manual of mental disorders, 4th ed. Washington DC: American Psychiatric Association; 1994.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
10. Kendell RE, Jablensky A. Distinguishing between the validity and utility of psychiatric diagnoses. Am J Psychiatry. 2003;160(1):4-12.
11. Maj M. “Psychiatric comorbidity”: an artifact of current diagnostic systems? Br J Psychiatry. 2005;186:182-184.
12. Kraemer HC, Kupfer DJ, Narrow WE, et al. Moving toward DSM-5: the field trials. Am J Psychiatry. 2010;167(10):1158-1160.
13. Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010;6:155-179.
14. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1-23.
15. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
16. Kendler KS, First MB. Alternative futures for the DSM revision process: iteration versus paradigm shift. Br J Psychiatry. 2010;197(4):263-265.
17. Kupfer DJ, Regier DA. Neuroscience clinical evidence, and the future of psychiatric classification in DSM-5. Am J Psychiatry. 2011;168(7):672-674.
18. Regier DA, Kuhl EA, Narrow WE, et al. Research planning for the future of psychiatric diagnosis [published online ahead of print June 13, 2011]. Eur Psychiatry. doi:10.1016/j.eurpsy.2009.11.013.
Discuss this article at www.facebook.com/CurrentPsychiatry
Work on the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)—scheduled to be published in May 2013—has been ongoing for more than a decade. Momentous advances in genetics and brain imaging since publication of DSM-IV in 1994 have generated optimism that an improved understanding of the neurobiologic underpinnings of psychiatric disorders might lead to a paradigm shift from the current descriptive classification system to a more scientific etiopathophysiological system similar to that used by other medical specialities.1
Some fear that any changes to our current classification system may be premature and could make an already complex system even more unwieldy.2 Scores of articles about the content and process of DSM-5 and several critiques and commentaries on the topic have been published. The American Psychiatric Association (APA) has made the DSM-5 process transparent by posting frequent updates to the DSM-5 Development Web site (www.dsm5.org), seeking feedback from the psychiatric community and the public, and presenting progress reports by members of the DSM-5 Task Force at scientific meetings.
There have been few discussions on the implications of DSM-5 from the practicing clinician’s vantage point, which I seek to present in this series of articles, the remainder of which will be published here, at CurrentPsychiatry.com. In this article, I:
- provide a brief history of psychiatric classification, focusing on the origins and evolution of the DSM system
- summarize the limitations of DSM-IV
- note the challenges and tensions in the construction of DSM-5
- review the DSM-5 process
- outline its current status
- discuss the organization and content of future articles in this series.
Although I am a member of the DSM-5 Psychotic Disorders Work Group, I am solely responsible for the content and any opinions that I offer in this article and series. All details of DSM-5 that I discuss are publicly available at www.dsm5.org. I’ve been a clinician and clinical researcher for >25 years, and my opinions are colored by the need for clarity, rigor, clinical relevance, and a disdain for overly speculative thinking.
Evolution of DSM
A nosological system (system of classification of disease) enables clinicians to provide specific treatments for medical causes of human disease and/or disability with precise and predictable effects and guide patients and families about the likely course and outcome. Such classification systems also are used by:
- researchers, to learn more about the nature of the conditions being classified and develop better treatments for them
- health care systems, to provide optimal health care and track its appropriate provision
- insurance companies, to provide appropriate reimbursement for health care
- health product developers, including pharmaceutical companies, to develop health care products and promote their appropriate utilization
- government agencies, to determine health priorities and apportionment of health care resources
- public health agencies, to track the distribution of health and disease in communities around the world.
An ideal classification system would meet all constituents’ needs while perfectly mapping natural disease entities with distinct etiology and pathophysiology (validity), consistently allow all users to reach the same diagnosis (reliability), and provide clinicians with clear guidance about treatment and likely course for each of the entities (utility), with the list of entities being mutually exclusive and collectively exhaustive (coverage).
The current nosological system for psychiatric disorders originated in the late 19th and early 20th centuries and culminated in the first edition of the Diagnostic and Statistical Manual of Mental Disorders3 released in 1952 and a section related to mental disorders (section V) in the sixth revision of the International Classification of Disease (ICD).4 Whereas DSM focuses exclusively on mental disorders, the ICD is a general medical classification system that began covering mental disorders with its sixth revision in 1949. In subsequent revisions (ICD-7 through -10 and DSM-II through -IV), substantial changes in diagnostic criteria have been made, although the systems’ basic structure has been retained. Table 1 describes major changes from DSM-I through DSM-IV-TR.3,5-9 DSM and ICD both are being revised; DSM-5 is scheduled to be released in 2013 and ICD-11 is to be finalized by 2016.
Table 1
Conceptual development of DSM-I to DSM-IV-TR
| Version | Comments |
|---|---|
| DSM-I (1952)3 | Presumed etiology. 106 diagnoses |
| DSM-II (1968)5 | Glossary definitions. 185 diagnoses |
| DSM-III (1980)6 | Paradigm shift. Explicit criteria. Emphasis on reliability. 265 diagnoses |
| DSM-III-R (1987)7 | Modest changes. Blunted hierarchies. Clarifications. 292 diagnoses |
| DSM-IV (1994)8 | Modest changes. More blunted hierarchies. 361 diagnoses |
| DSM-IV-TR (2000)9 | Only text revision. 361 diagnostic conditions |
What do clinicians need?
Similar to ICD-10, DSM-IV is marked by considerable complexity, variable validity, limited clinical and research utility, and problems of burgeoning comorbidity.10 Efforts to revise DSM seek to address these limitations. From a clinician’s perspective, the most challenging aspects of DSM-IV derive from its complexity—which makes clinical application difficult—and its limited clinical utility, which is exemplified by artificial comorbidity,11 frequent use of “not otherwise specified” (NOS), and relative treatment nonspecificity with reference to diagnosis.
As clinicians, we want a nosological system that is easy to use, can guide treatment decisions, provides useful information about likely disease course and outcomes, and allows us to easily communicate about disease nature with patients, families, payers, and health care administrators. Additionally, although good validity and reliability are desirable for clinicians, adequate coverage of psychiatric disease—the listed conditions should be collectively exhaustive and mutually exclusive—is particularly valued. Finally, we want a diagnostic system that allows us to explain the reasoning behind psychiatric diagnoses and related treatment in lay terms to patients and their families.
DSM-5 development to date
DSM-5 development has been a collaborative effort led by the APA and involves the National Institute of Mental Health, the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and the World Health Organization (WHO). Between 1999 and 2002, 3 work conferences resulted in a series of white papers that identified gaps and research needs.1 Between 2003 and 2008, the American Psychiatric Institute for Research and Education, the National Institute of Health, and the WHO organized 13 international conferences to review a wide range of nosologic issues; the proceedings have been compiled into 13 monographs (11 published and 2 in press) and >125 scientific articles that serve as key reference sources for the DSM-5 process. For a continually updated list of these publications, see www.dsm5.org/Research.
In 2006, DSM-5 Task Force Chair David J. Kupfer, MD and Vice Chair Darrel A. Regier, MD, MPH were appointed and began selecting members of the DSM-5 Task Force, a process that was completed in 2007. Members of the 13 diagnostic area Work Groups (Table 2) were selected and the Work Groups were constituted in 2008. All 168 Task Force and Work Group members were vetted to ensure that they met standards of minimum conflict of interest and broad representation. Membership includes diverse professional representation from academia and mental health; 75% of members are from the United States. Six cross-cutting study groups have deliberated on a range of common issues, including:
- spectrum disorders
- lifespan and development
- gender and cross-cultural
- psychiatric/general medicine interface
- impairment and disability assessment
- diagnostic measurement and assessment.
Additionally, >300 external advisors with special expertise have participated in the process. Since 2008, each of the Work Groups has conducted extensive literature reviews of all assigned disorders, evaluated what works and what doesn’t work in DSM-IV-TR, assessed new research developments and clinical issues that have arisen since publication of DSM-IV-TR in 1994, and developed research plans to investigate critical issues utilizing systematic reviews and secondary data analyses. Based on these analyses, each Work Group proposed draft diagnostic criteria for its disorders, using a strict protocol for criteria revisions such as addition or deletion of disorders and changes to existing diagnostic criteria. These draft diagnostic criteria were first presented on www.dsm5.org in late 2009 through early 2010. Based on input from other Work Groups, the Task Force, several external groups, and the public, the Work Groups revised these criteria and prioritized necessary field trials to evaluate key recommendations. Phase I of the field trials began in 2010.12 Results of these field trials are being compiled and analyzed.
The DSM-5 Work Groups have met via teleconference 1 to 2 times a month and in-person twice a year, with significant communication between meetings. Work Group chairs are members of the Task Force, which has equally frequent meetings. Reports of DSM-5 deliberations have been presented at hundreds of professional meetings and described in >200 scientific publications. Comprehensive information and ongoing updates on DSM-5 and a list of publications and meetings are provided at www.dsm5.org.
Public input has been sought and the Work Groups have received and processed >10,000 comments. In 2010, the APA Board of Trustees appointed a Scientific Review Committee to evaluate the scientific merit and clinical impact of the Work Group recommendations and comment on the strength of the evidence advanced in support of each proposed revision. In 2011, the Board of Trustees appointed a Clinical and Public Health Committee to evaluate the clinical utility and public health significance of the proposed revisions. The APA and WHO have shared information and assessments in an effort to harmonize diagnostic criteria between DSM-5 and ICD-11.
Initial hopes that DSM-5 could represent a paradigm shift toward an etiopathophysiological classification of psychiatric disorders have been tempered by recognition of the limitations of our current neurobiologic understanding of psychiatric disorders. Therefore, the focus for DSM-5 has shifted from validity enhancements to improved clinical utility while building a framework that better lends itself to a future etiopathophysiological nosology.13-18 Whereas dimensional assessments are likely to be added across various diagnostic categories, a primarily categorical nosology will be retained and the proposed criterion changes are relatively modest. The results of our enhanced knowledge about the neurobiologic underpinnings of psychiatric disorders will not be reflected in diagnostic criteria, but in the significant revisions to the DSM text.
Our DSM-5 series
Subsequent articles in this series—which will be published here, at CurrentPsychiatry.com—will discuss specific proposed DSM-5 changes in 13 groups of disorders (Table 2) and their clinical implications. These articles also will address the relationship of DSM to ICD, issues with dimensional classification, and the importance of and challenges in precise diagnostic measurement.
Table 2
DSM-5 Work Groups
| Attention-deficit/hyperactivity disorder and disruptive behaviors |
| Anxiety, obsessive-compulsive, posttraumatic, and dissociative disorders |
| Disorders in childhood and adolescence |
| Eating disorders |
| Mood disorders |
| Neurocognitive disorders |
| Neurodevelopmental disorders |
| Personality and personality disorders |
| Psychotic disorders |
| Sexual and gender identity disorders |
| Sleep-wake disorders |
| Somatic distress disorders |
| Substance-related disorders |
Related Resources
- American Psychiatric Association. DSM-5 Development. www.dsm5.org.
- Black DW, Zimmerman M. Redefining personality disorders: Proposed revisions for DSM-5. Current Psychiatry. 2011;10(9):26-38.
Disclosure
Dr. Tandon is a member of the DSM-5 Psychotic Disorders Work Group. He reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Work on the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)—scheduled to be published in May 2013—has been ongoing for more than a decade. Momentous advances in genetics and brain imaging since publication of DSM-IV in 1994 have generated optimism that an improved understanding of the neurobiologic underpinnings of psychiatric disorders might lead to a paradigm shift from the current descriptive classification system to a more scientific etiopathophysiological system similar to that used by other medical specialities.1
Some fear that any changes to our current classification system may be premature and could make an already complex system even more unwieldy.2 Scores of articles about the content and process of DSM-5 and several critiques and commentaries on the topic have been published. The American Psychiatric Association (APA) has made the DSM-5 process transparent by posting frequent updates to the DSM-5 Development Web site (www.dsm5.org), seeking feedback from the psychiatric community and the public, and presenting progress reports by members of the DSM-5 Task Force at scientific meetings.
There have been few discussions on the implications of DSM-5 from the practicing clinician’s vantage point, which I seek to present in this series of articles, the remainder of which will be published here, at CurrentPsychiatry.com. In this article, I:
- provide a brief history of psychiatric classification, focusing on the origins and evolution of the DSM system
- summarize the limitations of DSM-IV
- note the challenges and tensions in the construction of DSM-5
- review the DSM-5 process
- outline its current status
- discuss the organization and content of future articles in this series.
Although I am a member of the DSM-5 Psychotic Disorders Work Group, I am solely responsible for the content and any opinions that I offer in this article and series. All details of DSM-5 that I discuss are publicly available at www.dsm5.org. I’ve been a clinician and clinical researcher for >25 years, and my opinions are colored by the need for clarity, rigor, clinical relevance, and a disdain for overly speculative thinking.
Evolution of DSM
A nosological system (system of classification of disease) enables clinicians to provide specific treatments for medical causes of human disease and/or disability with precise and predictable effects and guide patients and families about the likely course and outcome. Such classification systems also are used by:
- researchers, to learn more about the nature of the conditions being classified and develop better treatments for them
- health care systems, to provide optimal health care and track its appropriate provision
- insurance companies, to provide appropriate reimbursement for health care
- health product developers, including pharmaceutical companies, to develop health care products and promote their appropriate utilization
- government agencies, to determine health priorities and apportionment of health care resources
- public health agencies, to track the distribution of health and disease in communities around the world.
An ideal classification system would meet all constituents’ needs while perfectly mapping natural disease entities with distinct etiology and pathophysiology (validity), consistently allow all users to reach the same diagnosis (reliability), and provide clinicians with clear guidance about treatment and likely course for each of the entities (utility), with the list of entities being mutually exclusive and collectively exhaustive (coverage).
The current nosological system for psychiatric disorders originated in the late 19th and early 20th centuries and culminated in the first edition of the Diagnostic and Statistical Manual of Mental Disorders3 released in 1952 and a section related to mental disorders (section V) in the sixth revision of the International Classification of Disease (ICD).4 Whereas DSM focuses exclusively on mental disorders, the ICD is a general medical classification system that began covering mental disorders with its sixth revision in 1949. In subsequent revisions (ICD-7 through -10 and DSM-II through -IV), substantial changes in diagnostic criteria have been made, although the systems’ basic structure has been retained. Table 1 describes major changes from DSM-I through DSM-IV-TR.3,5-9 DSM and ICD both are being revised; DSM-5 is scheduled to be released in 2013 and ICD-11 is to be finalized by 2016.
Table 1
Conceptual development of DSM-I to DSM-IV-TR
| Version | Comments |
|---|---|
| DSM-I (1952)3 | Presumed etiology. 106 diagnoses |
| DSM-II (1968)5 | Glossary definitions. 185 diagnoses |
| DSM-III (1980)6 | Paradigm shift. Explicit criteria. Emphasis on reliability. 265 diagnoses |
| DSM-III-R (1987)7 | Modest changes. Blunted hierarchies. Clarifications. 292 diagnoses |
| DSM-IV (1994)8 | Modest changes. More blunted hierarchies. 361 diagnoses |
| DSM-IV-TR (2000)9 | Only text revision. 361 diagnostic conditions |
What do clinicians need?
Similar to ICD-10, DSM-IV is marked by considerable complexity, variable validity, limited clinical and research utility, and problems of burgeoning comorbidity.10 Efforts to revise DSM seek to address these limitations. From a clinician’s perspective, the most challenging aspects of DSM-IV derive from its complexity—which makes clinical application difficult—and its limited clinical utility, which is exemplified by artificial comorbidity,11 frequent use of “not otherwise specified” (NOS), and relative treatment nonspecificity with reference to diagnosis.
As clinicians, we want a nosological system that is easy to use, can guide treatment decisions, provides useful information about likely disease course and outcomes, and allows us to easily communicate about disease nature with patients, families, payers, and health care administrators. Additionally, although good validity and reliability are desirable for clinicians, adequate coverage of psychiatric disease—the listed conditions should be collectively exhaustive and mutually exclusive—is particularly valued. Finally, we want a diagnostic system that allows us to explain the reasoning behind psychiatric diagnoses and related treatment in lay terms to patients and their families.
DSM-5 development to date
DSM-5 development has been a collaborative effort led by the APA and involves the National Institute of Mental Health, the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and the World Health Organization (WHO). Between 1999 and 2002, 3 work conferences resulted in a series of white papers that identified gaps and research needs.1 Between 2003 and 2008, the American Psychiatric Institute for Research and Education, the National Institute of Health, and the WHO organized 13 international conferences to review a wide range of nosologic issues; the proceedings have been compiled into 13 monographs (11 published and 2 in press) and >125 scientific articles that serve as key reference sources for the DSM-5 process. For a continually updated list of these publications, see www.dsm5.org/Research.
In 2006, DSM-5 Task Force Chair David J. Kupfer, MD and Vice Chair Darrel A. Regier, MD, MPH were appointed and began selecting members of the DSM-5 Task Force, a process that was completed in 2007. Members of the 13 diagnostic area Work Groups (Table 2) were selected and the Work Groups were constituted in 2008. All 168 Task Force and Work Group members were vetted to ensure that they met standards of minimum conflict of interest and broad representation. Membership includes diverse professional representation from academia and mental health; 75% of members are from the United States. Six cross-cutting study groups have deliberated on a range of common issues, including:
- spectrum disorders
- lifespan and development
- gender and cross-cultural
- psychiatric/general medicine interface
- impairment and disability assessment
- diagnostic measurement and assessment.
Additionally, >300 external advisors with special expertise have participated in the process. Since 2008, each of the Work Groups has conducted extensive literature reviews of all assigned disorders, evaluated what works and what doesn’t work in DSM-IV-TR, assessed new research developments and clinical issues that have arisen since publication of DSM-IV-TR in 1994, and developed research plans to investigate critical issues utilizing systematic reviews and secondary data analyses. Based on these analyses, each Work Group proposed draft diagnostic criteria for its disorders, using a strict protocol for criteria revisions such as addition or deletion of disorders and changes to existing diagnostic criteria. These draft diagnostic criteria were first presented on www.dsm5.org in late 2009 through early 2010. Based on input from other Work Groups, the Task Force, several external groups, and the public, the Work Groups revised these criteria and prioritized necessary field trials to evaluate key recommendations. Phase I of the field trials began in 2010.12 Results of these field trials are being compiled and analyzed.
The DSM-5 Work Groups have met via teleconference 1 to 2 times a month and in-person twice a year, with significant communication between meetings. Work Group chairs are members of the Task Force, which has equally frequent meetings. Reports of DSM-5 deliberations have been presented at hundreds of professional meetings and described in >200 scientific publications. Comprehensive information and ongoing updates on DSM-5 and a list of publications and meetings are provided at www.dsm5.org.
Public input has been sought and the Work Groups have received and processed >10,000 comments. In 2010, the APA Board of Trustees appointed a Scientific Review Committee to evaluate the scientific merit and clinical impact of the Work Group recommendations and comment on the strength of the evidence advanced in support of each proposed revision. In 2011, the Board of Trustees appointed a Clinical and Public Health Committee to evaluate the clinical utility and public health significance of the proposed revisions. The APA and WHO have shared information and assessments in an effort to harmonize diagnostic criteria between DSM-5 and ICD-11.
Initial hopes that DSM-5 could represent a paradigm shift toward an etiopathophysiological classification of psychiatric disorders have been tempered by recognition of the limitations of our current neurobiologic understanding of psychiatric disorders. Therefore, the focus for DSM-5 has shifted from validity enhancements to improved clinical utility while building a framework that better lends itself to a future etiopathophysiological nosology.13-18 Whereas dimensional assessments are likely to be added across various diagnostic categories, a primarily categorical nosology will be retained and the proposed criterion changes are relatively modest. The results of our enhanced knowledge about the neurobiologic underpinnings of psychiatric disorders will not be reflected in diagnostic criteria, but in the significant revisions to the DSM text.
Our DSM-5 series
Subsequent articles in this series—which will be published here, at CurrentPsychiatry.com—will discuss specific proposed DSM-5 changes in 13 groups of disorders (Table 2) and their clinical implications. These articles also will address the relationship of DSM to ICD, issues with dimensional classification, and the importance of and challenges in precise diagnostic measurement.
Table 2
DSM-5 Work Groups
| Attention-deficit/hyperactivity disorder and disruptive behaviors |
| Anxiety, obsessive-compulsive, posttraumatic, and dissociative disorders |
| Disorders in childhood and adolescence |
| Eating disorders |
| Mood disorders |
| Neurocognitive disorders |
| Neurodevelopmental disorders |
| Personality and personality disorders |
| Psychotic disorders |
| Sexual and gender identity disorders |
| Sleep-wake disorders |
| Somatic distress disorders |
| Substance-related disorders |
Related Resources
- American Psychiatric Association. DSM-5 Development. www.dsm5.org.
- Black DW, Zimmerman M. Redefining personality disorders: Proposed revisions for DSM-5. Current Psychiatry. 2011;10(9):26-38.
Disclosure
Dr. Tandon is a member of the DSM-5 Psychotic Disorders Work Group. He reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kupfer DJ, First MB, Regier DA. eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
2. Frances A. Whither DSM-V? Br J Psychiatry. 2009;195(5):391-392.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington DC: American Psychiatric Association; 1952.
4. World Health Organization. Manual of the international statistical classification of diseases injuries and causes of death, 6th revision (ICD-6). Geneva, Switzerland: World Health Organization; 1949.
5. Diagnostic and statistical manual of mental disorders, 2nd ed. Washington DC: American Psychiatric Association; 1968.
6. Diagnostic and statistical manual of mental disorders, 3rd ed. Washington DC: American Psychiatric Association; 1980.
7. Diagnostic and statistical manual of mental disorders, 3rd ed, rev. Washington, DC: American Psychiatric Association; 1987.
8. Diagnostic and statistical manual of mental disorders, 4th ed. Washington DC: American Psychiatric Association; 1994.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
10. Kendell RE, Jablensky A. Distinguishing between the validity and utility of psychiatric diagnoses. Am J Psychiatry. 2003;160(1):4-12.
11. Maj M. “Psychiatric comorbidity”: an artifact of current diagnostic systems? Br J Psychiatry. 2005;186:182-184.
12. Kraemer HC, Kupfer DJ, Narrow WE, et al. Moving toward DSM-5: the field trials. Am J Psychiatry. 2010;167(10):1158-1160.
13. Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010;6:155-179.
14. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1-23.
15. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
16. Kendler KS, First MB. Alternative futures for the DSM revision process: iteration versus paradigm shift. Br J Psychiatry. 2010;197(4):263-265.
17. Kupfer DJ, Regier DA. Neuroscience clinical evidence, and the future of psychiatric classification in DSM-5. Am J Psychiatry. 2011;168(7):672-674.
18. Regier DA, Kuhl EA, Narrow WE, et al. Research planning for the future of psychiatric diagnosis [published online ahead of print June 13, 2011]. Eur Psychiatry. doi:10.1016/j.eurpsy.2009.11.013.
1. Kupfer DJ, First MB, Regier DA. eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
2. Frances A. Whither DSM-V? Br J Psychiatry. 2009;195(5):391-392.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington DC: American Psychiatric Association; 1952.
4. World Health Organization. Manual of the international statistical classification of diseases injuries and causes of death, 6th revision (ICD-6). Geneva, Switzerland: World Health Organization; 1949.
5. Diagnostic and statistical manual of mental disorders, 2nd ed. Washington DC: American Psychiatric Association; 1968.
6. Diagnostic and statistical manual of mental disorders, 3rd ed. Washington DC: American Psychiatric Association; 1980.
7. Diagnostic and statistical manual of mental disorders, 3rd ed, rev. Washington, DC: American Psychiatric Association; 1987.
8. Diagnostic and statistical manual of mental disorders, 4th ed. Washington DC: American Psychiatric Association; 1994.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
10. Kendell RE, Jablensky A. Distinguishing between the validity and utility of psychiatric diagnoses. Am J Psychiatry. 2003;160(1):4-12.
11. Maj M. “Psychiatric comorbidity”: an artifact of current diagnostic systems? Br J Psychiatry. 2005;186:182-184.
12. Kraemer HC, Kupfer DJ, Narrow WE, et al. Moving toward DSM-5: the field trials. Am J Psychiatry. 2010;167(10):1158-1160.
13. Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010;6:155-179.
14. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1-23.
15. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
16. Kendler KS, First MB. Alternative futures for the DSM revision process: iteration versus paradigm shift. Br J Psychiatry. 2010;197(4):263-265.
17. Kupfer DJ, Regier DA. Neuroscience clinical evidence, and the future of psychiatric classification in DSM-5. Am J Psychiatry. 2011;168(7):672-674.
18. Regier DA, Kuhl EA, Narrow WE, et al. Research planning for the future of psychiatric diagnosis [published online ahead of print June 13, 2011]. Eur Psychiatry. doi:10.1016/j.eurpsy.2009.11.013.
Differentiating restless legs syndrome from psychotropic side effects
Discuss this article at www.facebook.com/CurrentPsychiatry
Patients who complain of unpleasant paresthesias present a challenge to psychiatrists who need to differentiate between what could be psychotropic side effects from restless legs syndrome (RLS). Reconizing the differences between the 2 conditions can guide intervention. A previously unknown RLS diagnosis may shed light on a patient’s comorbid sleep disturbances and insomnia.
Signs that suggest RLS
Symptoms consistent with akathisia temporally related to initiating an antipsychotic or antidepressant should not be considered RLS-induced. In less clear cases, subtleties of the symptoms can help you decide.
RLS often presents as discomfort in the legs that patients describe as creeping, crawling, pulling, or itching; movement typically relieves this discomfort. Feelings of akathisia also have been described as an inner restlessness and a need to get up and move to relieve the tension. However, RLS has the following defining characteristics:
- occurs specifically in the lower extremities
- has a circadian rhythm and is worse at night
- can be accompanied by paresthesias and myoclonic jerks while awake.1
Other factors that support an RLS diagnosis are a family history of RLS,1 positive response to dopaminergic drugs,1 and low ferritin levels. Also consider conditions that put patients at risk for RLS, including end stage renal disease, diabetes mellitus, multiple sclerosis, Parkinson’s disease, anemia, rheumatic disease, venous insufficiency, and pregnancy.
3 substances that can worsen RLS
Ask patients about their intake of caffeine, nicotine, and alcohol. Use of these substances is common among psychiatric patients and can worsen RLS symptoms. Making a connection between these substances and RLS symptoms can help motivate patients to temper their use.
In addition to mimicking the subjective experience of RLS, many psychotropics, including antidepressants, neuroleptics, and antihistamines,2-4 can worsen RLS symptoms in patients with a known RLS diagnosis.
The next step
RLS is a clinical diagnosis that’s usually made based on a patient’s medical history. Polysomnography is not necessary to make an RLS diagnosis but may be helpful if a patient is treatment-resistant or to monitor periodic leg movement disorders. Serum ferritin levels should be checked because normal hemoglobin levels do not rule out iron deficiency.
Disclosure
Dr. Baker reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101-119.
2. Hoque R, Chesson AL, Jr. Pharmacologically induced/exacerbated restless legs syndrome periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010;6(1):79-83.
3. O’Sullivan RL, Greenberg DB. H2 antagonists restless leg syndrome, and movement disorders. Psychosomatics. 1993;34(6):530-532.
4. Terao T, Terao M, Yoshimura R, et al. Restless legs syndrome induced by lithium. Biol Psychiatry. 1991;30(11):1167-1170.
Discuss this article at www.facebook.com/CurrentPsychiatry
Patients who complain of unpleasant paresthesias present a challenge to psychiatrists who need to differentiate between what could be psychotropic side effects from restless legs syndrome (RLS). Reconizing the differences between the 2 conditions can guide intervention. A previously unknown RLS diagnosis may shed light on a patient’s comorbid sleep disturbances and insomnia.
Signs that suggest RLS
Symptoms consistent with akathisia temporally related to initiating an antipsychotic or antidepressant should not be considered RLS-induced. In less clear cases, subtleties of the symptoms can help you decide.
RLS often presents as discomfort in the legs that patients describe as creeping, crawling, pulling, or itching; movement typically relieves this discomfort. Feelings of akathisia also have been described as an inner restlessness and a need to get up and move to relieve the tension. However, RLS has the following defining characteristics:
- occurs specifically in the lower extremities
- has a circadian rhythm and is worse at night
- can be accompanied by paresthesias and myoclonic jerks while awake.1
Other factors that support an RLS diagnosis are a family history of RLS,1 positive response to dopaminergic drugs,1 and low ferritin levels. Also consider conditions that put patients at risk for RLS, including end stage renal disease, diabetes mellitus, multiple sclerosis, Parkinson’s disease, anemia, rheumatic disease, venous insufficiency, and pregnancy.
3 substances that can worsen RLS
Ask patients about their intake of caffeine, nicotine, and alcohol. Use of these substances is common among psychiatric patients and can worsen RLS symptoms. Making a connection between these substances and RLS symptoms can help motivate patients to temper their use.
In addition to mimicking the subjective experience of RLS, many psychotropics, including antidepressants, neuroleptics, and antihistamines,2-4 can worsen RLS symptoms in patients with a known RLS diagnosis.
The next step
RLS is a clinical diagnosis that’s usually made based on a patient’s medical history. Polysomnography is not necessary to make an RLS diagnosis but may be helpful if a patient is treatment-resistant or to monitor periodic leg movement disorders. Serum ferritin levels should be checked because normal hemoglobin levels do not rule out iron deficiency.
Disclosure
Dr. Baker reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Patients who complain of unpleasant paresthesias present a challenge to psychiatrists who need to differentiate between what could be psychotropic side effects from restless legs syndrome (RLS). Reconizing the differences between the 2 conditions can guide intervention. A previously unknown RLS diagnosis may shed light on a patient’s comorbid sleep disturbances and insomnia.
Signs that suggest RLS
Symptoms consistent with akathisia temporally related to initiating an antipsychotic or antidepressant should not be considered RLS-induced. In less clear cases, subtleties of the symptoms can help you decide.
RLS often presents as discomfort in the legs that patients describe as creeping, crawling, pulling, or itching; movement typically relieves this discomfort. Feelings of akathisia also have been described as an inner restlessness and a need to get up and move to relieve the tension. However, RLS has the following defining characteristics:
- occurs specifically in the lower extremities
- has a circadian rhythm and is worse at night
- can be accompanied by paresthesias and myoclonic jerks while awake.1
Other factors that support an RLS diagnosis are a family history of RLS,1 positive response to dopaminergic drugs,1 and low ferritin levels. Also consider conditions that put patients at risk for RLS, including end stage renal disease, diabetes mellitus, multiple sclerosis, Parkinson’s disease, anemia, rheumatic disease, venous insufficiency, and pregnancy.
3 substances that can worsen RLS
Ask patients about their intake of caffeine, nicotine, and alcohol. Use of these substances is common among psychiatric patients and can worsen RLS symptoms. Making a connection between these substances and RLS symptoms can help motivate patients to temper their use.
In addition to mimicking the subjective experience of RLS, many psychotropics, including antidepressants, neuroleptics, and antihistamines,2-4 can worsen RLS symptoms in patients with a known RLS diagnosis.
The next step
RLS is a clinical diagnosis that’s usually made based on a patient’s medical history. Polysomnography is not necessary to make an RLS diagnosis but may be helpful if a patient is treatment-resistant or to monitor periodic leg movement disorders. Serum ferritin levels should be checked because normal hemoglobin levels do not rule out iron deficiency.
Disclosure
Dr. Baker reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101-119.
2. Hoque R, Chesson AL, Jr. Pharmacologically induced/exacerbated restless legs syndrome periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010;6(1):79-83.
3. O’Sullivan RL, Greenberg DB. H2 antagonists restless leg syndrome, and movement disorders. Psychosomatics. 1993;34(6):530-532.
4. Terao T, Terao M, Yoshimura R, et al. Restless legs syndrome induced by lithium. Biol Psychiatry. 1991;30(11):1167-1170.
1. Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101-119.
2. Hoque R, Chesson AL, Jr. Pharmacologically induced/exacerbated restless legs syndrome periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010;6(1):79-83.
3. O’Sullivan RL, Greenberg DB. H2 antagonists restless leg syndrome, and movement disorders. Psychosomatics. 1993;34(6):530-532.
4. Terao T, Terao M, Yoshimura R, et al. Restless legs syndrome induced by lithium. Biol Psychiatry. 1991;30(11):1167-1170.
Psychiatric illness during pregnancy
Perinatal psychopathology is a common and undertreated problem with wide-ranging consequences for both mother and child.1-4 Women at risk for psychopathology are more likely to engage in unhealthy behaviors such as smoking and substance abuse and have difficulty engaging in treatment and attending psychiatric and obstetrics appointments.5 In addition, many of these women have trouble attaching to and caring for their infants and struggle with everyday stressors during pregnancy and postpartum.6
Routine prenatal screening for mental illness coupled with non-judgmental, collaborative, and individualized care delivered by a multidisciplinary team is critical for treatment engagement and adherence. Providers should be aware of risk factors for perinatal psychiatric illness—including a history of mental illness, stressful life events, and interpersonal conflict—and should be versed in current treatment guidelines.
CASE REPORT: Difficulty coping
Ms. A, age 28, is referred to our High Risk Perinatal Team by her obstetrician when she is approximately 6 weeks pregnant. She is single, has 3 other children (age 10, 4, and 2), a history of depression, and chronic pain related to an auto accident 3 years ago. She reports that this pregnancy likely is the result of a sexual assault, but she has decided to keep the baby. Ms. A describes severe depressive symptoms, including insomnia, low appetite, feelings of worthlessness, and thoughts of harming herself. In addition, she has incapacitating panic attacks and constantly worries about her children’s safety when she is not with them. She schedules an appointment with the perinatal team, but does not show up twice.
When our team finally sees Ms. A, she is well into her second trimester and brings her 2 youngest children with her. She says she recently was fired from her job as a cashier because she missed too many days of work, and is applying for Medicaid. Recently, her back and shoulder pain have worsened, and she is running out of her prescription for acetaminophen/hydrocodone. Ms. A’s affect is flat, her mood depressed, and she has difficulty explaining her history because her 2-year-old son interrupts the interview. She has never been in psychotherapy, and is reluctant to take antidepressants. Despite a difficult first visit, she engages with the clinician and agrees to schedule a second appointment.
What complicates pregnancy?
Women are at higher risk for developing depression during puberty, the perinatal period (ie, pregnancy and first year postpartum), and perimenopause.7 These times often are fraught with unfamiliar hormonal fluctuations, role transitions, emotional upheaval, and physical changes. However, because these times are expected to be stressful, serious mood changes often go unnoticed by patients and untreated by clinicians.8 Women are expected to celebrate, thrive, and “glow” during pregnancy, and those who suffer from depression and anxiety frequently do so in silence. Social stigma surrounding perinatal depression or anxiety leads many women to believe they are alone in their struggle and hesitant to seek help.9
Most pregnant women who develop psychiatric illness do not present for treatment.10 One study found that 86% of pregnant women who screened positive for depression in an obstetrics (OB) setting did not receive treatment.11 Some women are reluctant to take antidepressants out of concern for their infant’s safety,8 and psychotherapy or alternative approaches are not available in all areas.12 Transportation, childcare issues, or ongoing life stressors may prevent women from seeking help (Box 1).9
Diagnostic uncertainty among professionals may aggravate undertreatment. Clinicians who are unfamiliar with the presentation of perinatal mental illness may mislabel depressive features—such as irritability, loss of interest in activities, low energy, increased anxiety, difficulty sleeping, or appetite dysregulation—as normative experiences during pregnancy or adjustment after childbirth. Concerned about fetal exposure to potentially teratogenic compounds, clinicians may under-dose otherwise effective medications, which can lead to treatment resistance. Even if treated aggressively, depression in pregnancy may persist because of other factors, such as comorbid anxiety, somatization, pain, substance use/dependence, undiagnosed bipolar illness, or the presence of severe psychosocial stress or trauma.
Maternal suicide and/or harm to the infant—the most severe result of untreated perinatal psychopathology—is rare.13 Common negative outcomes of untreated depression or anxiety in pregnant women include inadequate weight gain, preeclampsia, difficulty bonding with their unborn baby, premature labor, and lack of follow through with prenatal care.14,15 Symptoms become harder to treat when aggravated by psychosocial stressors such as poor social support, ambivalence about the pregnancy, and/or substance abuse.
The key to successful intervention is finding a balance between managing psychiatric concerns, facilitating adequate coping with psychosocial stressors, and, if necessary, aggressively treating pregnancy-related physical illnesses. Successful treatment response depends on early detection and initiating individualized care as soon as possible.
Lack of insurance, childcare, or transportation can make it difficult for a pregnant woman to receive psychiatric treatment. All pregnant women are eligible for Medicaid if private insurance is unavailable to them, and clinicians can help patients apply for assistance. Some programs—for example, Michigan’s state-funded Maternal Infant Health Programs—offer help with transportation to appointments, such as cabs and reimbursement for gas, in addition to nutrition guidance, counseling, home visits, and referrals to community resources such as childbirth classes, infant mental health specialists, and/or substance abuse treatment (see Related Resources ).
Offering childcare during psychotherapy sessions can be particularly helpful, and may provide valuable experience for a student or resident interested in working with at-risk children. Women may be more likely to engage in care if psychotherapy sessions are conducted by phone or in their homes. A positive experience with mental health care during pregnancy may increase the likelihood that women will remain engaged in treatment after childbirth, therefore lessening the negative effects of perinatal psychopathology on mother and child.
Early detection. Women’s health care providers play a fundamental role in guiding decision-making about mental health care, providing referrals, and most important, allowing women to talk about perinatal psychopathology without fear of stigma.
When a woman becomes pregnant, it is critical to determine if she is at risk for developing psychopathology or presents with active illness. Many OB clinics screen for depression several times during pregnancy and early postpartum. The most commonly used screening tool is the Edinburgh Postpartum Depression Scale (EPDS),16 a 10-item self-report measure that is sensitive to cognitive and affective symptoms of depression. If a woman scores >15 during pregnancy or >13 postpartum, further assessment is indicated.17 The anxiety subscale (items 5 and 6) of the EPDS has been validated for screening perinatal anxiety using a cut-off score >4.18 Depression can be quickly assessed using the 2-question Patient Health Questionnaire (PHQ-2) or the 9-question PHQ-9.19,20 All 3 scales are free and available on the Internet (Table 1).21
These screening tools offer clinicians an opportunity to assess for risk factors that may increase the likelihood of illness onset or worsened prognosis (Table 2).5,22 All women who present with pregnancy-related medical illness, such as preeclampsia or gestational diabetes, should be screened for co-occurring depression or anxiety because psychiatric comorbidity is common.
Individualized care. Have an open mind about the kind of care to offer and collaborate with the patient when discussing treatment options.5 Some pregnant women may reject traditional treatments, such as pharmacotherapy or psychotherapy, because of concern about harm to the unborn baby or reluctance to work through past or present conflicts in therapy during a vulnerable time.9 Women may assume that medication will be the only treatment offered, or even fear that they will be forced to take antidepressants. Women often do not follow through on mental health referrals, even when they are appropriately screened and identified to be at risk, and an OB nurse explains the risks of untreated psychopathology.11
A multidisciplinary, collaborative care model is vital for positive pregnancy outcomes. Connecting obstetricians and midwives with psychologists, psychiatrists, social workers, and infant mental health specialists to coordinate treatment ensures that at-risk pregnant and postpartum women get the care they need. A nonjudgmental approach is essential to engage pregnant women in care. Assure women that pharmacotherapy is not required when receiving mental health treatment, but is an option they can choose.
Table 1
Screening for psychiatric illness during pregnancy
| Screening tool | Sensitivity/specificity | Administration | Availability |
|---|---|---|---|
| Edinburgh Postpartum Depression Scale | Sensitivity = 0.86 Specificity = 0.78 Positive screen: >10 | Self-administered in 5 to 10 minutes. Could be self-scored | http://bit.ly/PPDscale |
| Patient Health Questionnaire-2 (PHQ-2) | Sensitivity = 0.83 Specificity = 0.92 Positive screen: >3 | Self- or clinician-administered in <1 minute | www.phqscreeners.com The 2 questions from the PHQ-9 for mood and anhedonia are used |
| Patient Health Questionnaire-9 (PHQ-9) | Sensitivity = 0.88 Specificity = 0.88 Positive screen: >10 | Self-administered and self-scored, 5 to 10 minutes | www.phqscreeners.com |
| Source: Reference 21 | |||
Treatment choices
Pharmacotherapy. If a woman has only mild symptoms or has been symptom-free for ≥6 months, it may be safe to decrease or discontinue antidepressants during pregnancy or while trying to conceive, but such patients should be monitored closely for signs of relapse.23 In a study of 201 depressed pregnant women, 68% of those who discontinued medication experienced symptom relapse compared with 26% of those who continued medication.24 If a depressed woman has a history of relapse or severe symptoms, including suicide attempts and inpatient psychiatric admissions, it is recommended that she remain on antidepressants or mood stabilizers, regardless of pregnancy status.25 If medications are necessary during pregnancy— ie, the benefits to the mother outweigh the risks to the unborn baby—the following precautions could help decrease fetal exposure:23
- keep the medication regimen simple and at the lowest effective dose
- use monotherapy when appropriate
- if possible, do not change medications during pregnancy.
When considering pharmacotherapy, evaluate each woman’s risk for disease exacerbation and consequences for pregnancy and neonatal outcomes, and ask the woman how she views reproductive risk vs disease benefit.
Developing fetuses are exposed to either the effects of the mother’s untreated mental illness or the medication.26 A recent study comparing birth and neonatal outcomes among women with untreated depression vs those taking selective serotonin reuptake inhibitors (SSRIs) found similar adverse outcomes.27 Babies continously exposed to either prenatal depression or SSRIs were more likely to be born prematurely, but partial exposure to either condition did not increase this risk.27 In addition, women who were not taking SSRIs had more depressive symptoms and more trouble functioning, which can interfere with bonding between mother and baby, both in-utero and postpartum.6,27 Neither SSRIs nor depression exposure increased risk for minor physical anomalies.27
A careful process of informed consent and documentation is essential when prescribing medications during pregnancy. Women should understand the risks of pharmacotherapy as well as the risks of undertreated illness.
Electroconvulsive therapy can safely help pregnant women with treatment-resistant, life-threatening, or psychotic depression.28,29
Table 2
Risk factors for perinatal psychopathology
| Pregnancy during adolescence |
| Previous diagnosis of depression, anxiety, psychosis, or bipolar disorder |
| Trauma history, including physical, emotional, or sexual abuse |
| Current or past substance abuse/dependence, including cigarette smoking |
| Lack of social support |
| Single parenthood |
| Low socioeconomic status |
| History of sexual assault or domestic violence |
| Unstable home environment |
| Stopping antidepressants during pregnancy |
| Financial problems |
| Ambivalence about pregnancy |
| Source: References 5,22 |
Psychotherapy. The American College of Obstetricians and Gynecologists treatment guidelines22 favor psychotherapy over medication for women with mild depressive symptoms and no loss of function, suicidality, or psychotic experiences; pharmacotherapy is suggested for women who have moderate to severely impaired functioning, recurrent depressive symptoms, or suicidal thinking (Table 3).22
Interpersonal psychotherapy or cognitive-behavioral therapy can be safe and effective during pregnancy.30,31 Other psychotherapeutic modalities and alternative/complementary treatments offer potential benefit without substantial risk, and could help prevent relapse when discontinuing mood stabilizers or antidepressants after conception (Box 2).32-35
Table 3
ACOG guidelines for treating depression during pregnancy
| Women who are thinking about getting pregnant |
| For women on medication with mild or no symptoms for ≥6 months, it may be appropriate to taper and discontinue medication before becoming pregnant |
| Medication discontinuation may not be appropriate in women with a history of severe, recurrent depression or who have psychosis, bipolar disorder, other psychiatric illness requiring medication, or a history of suicide attempts |
| Pregnant women currently taking medication for depression |
| Psychiatrically stable women who prefer to stay on medication may be able to do so after consultation between their psychiatrist and obstetrician to discuss risks and benefits |
| Women who want to discontinue medication may attempt to taper and discontinue if they are not experiencing symptoms, depending on their psychiatric history. Women with a history of recurrent depression are at a high risk of relapse if medication is discontinued |
| Women with recurrent depression or who have symptoms despite medication may benefit from psychotherapy to replace or augment medication |
| Women with severe depression (with suicide attempts, functional incapacitation, or weight loss) should remain on medication. If a patient refuses medication, alternative treatment and monitoring should be in place, preferably before discontinuation |
| Pregnant and not currently on medication for depression |
| Psychotherapy may be beneficial for women who prefer to avoid antidepressants |
| For women who want to take medication, risks and benefits of treatment choices should be evaluated and discussed, including factors such as stage of gestation, symptoms, history of depression, and other conditions and circumstances (eg, smoking, difficulty gaining weight) |
| All pregnant women |
| Regardless of circumstances, a woman with suicidal or psychotic symptoms should immediately see a psychiatrist |
| ACOG: American College of Obstetricians and Gynecologists Source: Reference 22 |
Mind-body approaches such as mindfulness-based stress reduction, yoga, and progressive relaxation and supplements such as fish oil may be good adjuncts to psychotherapy. Many pregnant women prefer mindfulness yoga to other mind-body techniques.32 A pilot study found that mindfulness yoga significantly decreased depressive symptoms and increased maternal-fetal attachment, particularly in mildly depressed women.33 For women who do not wish to engage in traditional treatments, alternative approaches such as progressive relaxation are easily taught and can help reduce depressive symptoms.34 Regular exercise may improve self-esteem and reduce symptoms of depression and anxiety in pregnant women.35
CASE CONTINUED: Healthy baby boy
Ms. A either doesn’t show up or cancels her weekly appointments about once a month, but seems to be making progress. Her therapist makes accommodations for Ms. A, such as offering childcare in an adjacent room during sessions, conducting brief sessions by phone when Ms. A is unable to come to the clinic, and helping her enroll in the state’s Maternal Infant Health Program. Ms. A’s therapist has referred her to a specialized OB clinic that can manage her pain medication and monitor for signs of abuse and keeps in regular contact with her obstetrician.
At 26 weeks gestation, Ms. A still is reluctant to try psychotropics, so her therapist works with her to integrate psychotherapy with alternative approaches such as mindfulness meditation and yoga. During therapy, Ms. A learns ways to manage her depressive symptoms, improve her social functioning, adjust to role transitions, and work through her traumatic experiences. Ms. A enrolls in a prenatal yoga class with a mindfulness focus, which allows her to interact with other pregnant women at risk for psychopathology and learn new ways to cope with her depressed mood and chronic pain.
Ms. A delivers a healthy boy at 38 weeks gestation. During labor, she uses many of the yoga poses she learned to manage pain, but elects to have an epidural after 30 hours of labor. Her baby tests positive for hydrocodone, which can cause ongoing mild irritability and occasional jitteriness. He is observed in the hospital for signs of withdrawal for 48 hours and then discharged home with his mother. Ms. A starts breast-feeding in the hospital and plans to continue at home.
Ms. A’s therapist continues to stay in touch with her by phone until she schedules another appointment and assists with referrals to other community resources.
- University of Michigan Department of Psychiatry Depression Center Women’s Mental Health and Infants Program. Women and depression. www.psych.med.umich.edu/wimhc.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
- Michigan Families Medicaid Project. Year 1 final report. Includes a list of maternal and child health services similar to Michigan’s Maternal Infant Health Program, for all 50 states. http://1.usa.gov/yCzdca.
Drug Brand Name
- Acetaminophen/hydrocodone • Vicodin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
2. Zuckerman B, Bauchner H, Parker S, et al. Maternal depressive symptoms during pregnancy, and newborn irritability. J Dev Behav Pediatr. 1990;11(4):190-194.
3. Field T, Healy B, Goldstein S, et al. Infants of depressed mothers show depressed behavior even with non-depressed adults. Child Dev. 1988;59(6):1569-1579.
4. Coghill SR, Caplan HL, Alexandra H, et al. Impact of maternal postnatal depression on cognitive development of young children. Br Med J (Clin Res Ed). 1986;292(6529):1165-1167.
5. Muzik M, Marcus S, Heringhausen J, et al. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788, ix–x.
6. Bifulco A, Figueiredo B, Guedeney N, et al. Maternal attachment style and depression associated with childbirth: preliminary results from a European and US cross-cultural study. Br J Psychiatry Suppl. 2004;46:s31-s37.
7. National Institute of Mental Health. Women and depression: discovering hope. http://www.nimh.nih.gov/health/publications/women-and-depression-discovering-hope/complete-index.shtml. Accessed December 27 2011.
8. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
9. O’Mahen HA, Flynn HA. P and perceived barriers to treatment for depression during the perinatal period. J Womens Health (Larchmt). 2008;17(8):1301-1309.
10. Flynn HA, O’Mahen HA, Massey L, et al. The impact of a brief obstetrics clinic based intervention on treatment use for perinatal depression. J Womens Health (Larchmt). 2006;15(10):1195-1204.
11. Marcus SM, Flynn HA, Blow FC, et al. Depressive symptoms among pregnant women screened in obstetrics settings. J Womens Health (Larchmt). 2003;12(4):373-380.
12. Olfson M, Marcus SC. National trends in outpatient psychotherapy. Am J Psychiatry. 2010;167(12):1456-1463.
13. Schiff MA, Grossman DC. Adverse perinatal outcomes and risk for postpartum suicide attempt in Washington state 1987-2001. Pediatrics. 2006;118(3):669-675.
14. Kurki T, Hiilesmaa V, Raitasalo R, et al. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95:(4)487-490.
15. McKee MD, Cunningham M, Jankowski KR, et al. Health-related functional status in pregnancy: relationship to depression and social support in a multiethnic population. Obstet Gynecol. 2001;97(6):988-993.
16. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
17. Matthey S, Henshaw C, Elliott S, et al. Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale: implications for clinical and research practice. Arch Womens Ment Health. 2006;9(6):309-315.
18. Chaudron LH, Szilagyi PG, Tang W, et al. Accuracy of depression screening tools for identifying postpartum depression among urban mothers. Pediatrics. 2010;125(3):609-617.
19. Ross AS, Hall RW, Frost K, et al. Antenatal and neonatal guidelines, education and learning system. J Ark Med Soc. 2006;102(12):328-330.
20. Muzik M, Klier CK, Rosenblum KL, et al. Are commonly used self-report inventories suitable for screening postpartum depression and anxiety disorders? Acta Psychiatr Scand. 2000;102(1):71-73.
21. Muzik M, Thelen K, Rosenblum KL. Perinatal depression: detection and treatment. Neuropsychiatry. 2011;1(2):179-195.
22. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
23. ACOG Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001-1020.
24. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
25. Gonsalves L, Schuermeyer I. Treating depression in pregnancy: practical suggestions. Cleve Clin J Med. 2006;73(12):1098-1104.
26. Misri S, Kendrick K. Treatment of perinatal mood and anxiety disorders: a review. Can J Psychiatry. 2007;52(8):489-498.
27. Wisner KL, Sit DY, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557-566.
28. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71(2):235-242.
29. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry. 1994;45(5):444-450.
30. O’Hara MW, Stuart S, Gorman LL, et al. Efficacy of interpersonal psychotherapy for postpartum depression. Arch Gen Psychiatry. 2000;57(11):1039-1045.
31. Bhatia SC, Bhatia SK. Depression in women: diagnostic and treatment considerations. Am Fam Physician. 1999;60(1):225-240.
32. Battle CL, Uebelacker LA, Howard M, et al. Prenatal yoga and depression during pregnancy. Birth. 2010;37(4):353-354.
33. Muzik M, Hamilton SE, Waxler EG, et al. Mindfulness yoga during pregnancy for women with depression and PTSD: preliminary results from a pilot feasibility study. Paper presented at: 36th Annual Meeting of the Association for Women in Psychology; March 5, 2011; Philadelphia, PA.
34. Deligiannidis KM, Freeman MP. Complementary and alternative medicine for the treatment of depressive disorders in women. Psychiatr Clin North Am. 2010;33(2):441-463.
35. Shivakumar G, Brandon AR, Snell PG, et al. Antenatal depression: a rationale for studying exercise. Depress Anxiety. 2011;28(3):234-242.
Perinatal psychopathology is a common and undertreated problem with wide-ranging consequences for both mother and child.1-4 Women at risk for psychopathology are more likely to engage in unhealthy behaviors such as smoking and substance abuse and have difficulty engaging in treatment and attending psychiatric and obstetrics appointments.5 In addition, many of these women have trouble attaching to and caring for their infants and struggle with everyday stressors during pregnancy and postpartum.6
Routine prenatal screening for mental illness coupled with non-judgmental, collaborative, and individualized care delivered by a multidisciplinary team is critical for treatment engagement and adherence. Providers should be aware of risk factors for perinatal psychiatric illness—including a history of mental illness, stressful life events, and interpersonal conflict—and should be versed in current treatment guidelines.
CASE REPORT: Difficulty coping
Ms. A, age 28, is referred to our High Risk Perinatal Team by her obstetrician when she is approximately 6 weeks pregnant. She is single, has 3 other children (age 10, 4, and 2), a history of depression, and chronic pain related to an auto accident 3 years ago. She reports that this pregnancy likely is the result of a sexual assault, but she has decided to keep the baby. Ms. A describes severe depressive symptoms, including insomnia, low appetite, feelings of worthlessness, and thoughts of harming herself. In addition, she has incapacitating panic attacks and constantly worries about her children’s safety when she is not with them. She schedules an appointment with the perinatal team, but does not show up twice.
When our team finally sees Ms. A, she is well into her second trimester and brings her 2 youngest children with her. She says she recently was fired from her job as a cashier because she missed too many days of work, and is applying for Medicaid. Recently, her back and shoulder pain have worsened, and she is running out of her prescription for acetaminophen/hydrocodone. Ms. A’s affect is flat, her mood depressed, and she has difficulty explaining her history because her 2-year-old son interrupts the interview. She has never been in psychotherapy, and is reluctant to take antidepressants. Despite a difficult first visit, she engages with the clinician and agrees to schedule a second appointment.
What complicates pregnancy?
Women are at higher risk for developing depression during puberty, the perinatal period (ie, pregnancy and first year postpartum), and perimenopause.7 These times often are fraught with unfamiliar hormonal fluctuations, role transitions, emotional upheaval, and physical changes. However, because these times are expected to be stressful, serious mood changes often go unnoticed by patients and untreated by clinicians.8 Women are expected to celebrate, thrive, and “glow” during pregnancy, and those who suffer from depression and anxiety frequently do so in silence. Social stigma surrounding perinatal depression or anxiety leads many women to believe they are alone in their struggle and hesitant to seek help.9
Most pregnant women who develop psychiatric illness do not present for treatment.10 One study found that 86% of pregnant women who screened positive for depression in an obstetrics (OB) setting did not receive treatment.11 Some women are reluctant to take antidepressants out of concern for their infant’s safety,8 and psychotherapy or alternative approaches are not available in all areas.12 Transportation, childcare issues, or ongoing life stressors may prevent women from seeking help (Box 1).9
Diagnostic uncertainty among professionals may aggravate undertreatment. Clinicians who are unfamiliar with the presentation of perinatal mental illness may mislabel depressive features—such as irritability, loss of interest in activities, low energy, increased anxiety, difficulty sleeping, or appetite dysregulation—as normative experiences during pregnancy or adjustment after childbirth. Concerned about fetal exposure to potentially teratogenic compounds, clinicians may under-dose otherwise effective medications, which can lead to treatment resistance. Even if treated aggressively, depression in pregnancy may persist because of other factors, such as comorbid anxiety, somatization, pain, substance use/dependence, undiagnosed bipolar illness, or the presence of severe psychosocial stress or trauma.
Maternal suicide and/or harm to the infant—the most severe result of untreated perinatal psychopathology—is rare.13 Common negative outcomes of untreated depression or anxiety in pregnant women include inadequate weight gain, preeclampsia, difficulty bonding with their unborn baby, premature labor, and lack of follow through with prenatal care.14,15 Symptoms become harder to treat when aggravated by psychosocial stressors such as poor social support, ambivalence about the pregnancy, and/or substance abuse.
The key to successful intervention is finding a balance between managing psychiatric concerns, facilitating adequate coping with psychosocial stressors, and, if necessary, aggressively treating pregnancy-related physical illnesses. Successful treatment response depends on early detection and initiating individualized care as soon as possible.
Lack of insurance, childcare, or transportation can make it difficult for a pregnant woman to receive psychiatric treatment. All pregnant women are eligible for Medicaid if private insurance is unavailable to them, and clinicians can help patients apply for assistance. Some programs—for example, Michigan’s state-funded Maternal Infant Health Programs—offer help with transportation to appointments, such as cabs and reimbursement for gas, in addition to nutrition guidance, counseling, home visits, and referrals to community resources such as childbirth classes, infant mental health specialists, and/or substance abuse treatment (see Related Resources ).
Offering childcare during psychotherapy sessions can be particularly helpful, and may provide valuable experience for a student or resident interested in working with at-risk children. Women may be more likely to engage in care if psychotherapy sessions are conducted by phone or in their homes. A positive experience with mental health care during pregnancy may increase the likelihood that women will remain engaged in treatment after childbirth, therefore lessening the negative effects of perinatal psychopathology on mother and child.
Early detection. Women’s health care providers play a fundamental role in guiding decision-making about mental health care, providing referrals, and most important, allowing women to talk about perinatal psychopathology without fear of stigma.
When a woman becomes pregnant, it is critical to determine if she is at risk for developing psychopathology or presents with active illness. Many OB clinics screen for depression several times during pregnancy and early postpartum. The most commonly used screening tool is the Edinburgh Postpartum Depression Scale (EPDS),16 a 10-item self-report measure that is sensitive to cognitive and affective symptoms of depression. If a woman scores >15 during pregnancy or >13 postpartum, further assessment is indicated.17 The anxiety subscale (items 5 and 6) of the EPDS has been validated for screening perinatal anxiety using a cut-off score >4.18 Depression can be quickly assessed using the 2-question Patient Health Questionnaire (PHQ-2) or the 9-question PHQ-9.19,20 All 3 scales are free and available on the Internet (Table 1).21
These screening tools offer clinicians an opportunity to assess for risk factors that may increase the likelihood of illness onset or worsened prognosis (Table 2).5,22 All women who present with pregnancy-related medical illness, such as preeclampsia or gestational diabetes, should be screened for co-occurring depression or anxiety because psychiatric comorbidity is common.
Individualized care. Have an open mind about the kind of care to offer and collaborate with the patient when discussing treatment options.5 Some pregnant women may reject traditional treatments, such as pharmacotherapy or psychotherapy, because of concern about harm to the unborn baby or reluctance to work through past or present conflicts in therapy during a vulnerable time.9 Women may assume that medication will be the only treatment offered, or even fear that they will be forced to take antidepressants. Women often do not follow through on mental health referrals, even when they are appropriately screened and identified to be at risk, and an OB nurse explains the risks of untreated psychopathology.11
A multidisciplinary, collaborative care model is vital for positive pregnancy outcomes. Connecting obstetricians and midwives with psychologists, psychiatrists, social workers, and infant mental health specialists to coordinate treatment ensures that at-risk pregnant and postpartum women get the care they need. A nonjudgmental approach is essential to engage pregnant women in care. Assure women that pharmacotherapy is not required when receiving mental health treatment, but is an option they can choose.
Table 1
Screening for psychiatric illness during pregnancy
| Screening tool | Sensitivity/specificity | Administration | Availability |
|---|---|---|---|
| Edinburgh Postpartum Depression Scale | Sensitivity = 0.86 Specificity = 0.78 Positive screen: >10 | Self-administered in 5 to 10 minutes. Could be self-scored | http://bit.ly/PPDscale |
| Patient Health Questionnaire-2 (PHQ-2) | Sensitivity = 0.83 Specificity = 0.92 Positive screen: >3 | Self- or clinician-administered in <1 minute | www.phqscreeners.com The 2 questions from the PHQ-9 for mood and anhedonia are used |
| Patient Health Questionnaire-9 (PHQ-9) | Sensitivity = 0.88 Specificity = 0.88 Positive screen: >10 | Self-administered and self-scored, 5 to 10 minutes | www.phqscreeners.com |
| Source: Reference 21 | |||
Treatment choices
Pharmacotherapy. If a woman has only mild symptoms or has been symptom-free for ≥6 months, it may be safe to decrease or discontinue antidepressants during pregnancy or while trying to conceive, but such patients should be monitored closely for signs of relapse.23 In a study of 201 depressed pregnant women, 68% of those who discontinued medication experienced symptom relapse compared with 26% of those who continued medication.24 If a depressed woman has a history of relapse or severe symptoms, including suicide attempts and inpatient psychiatric admissions, it is recommended that she remain on antidepressants or mood stabilizers, regardless of pregnancy status.25 If medications are necessary during pregnancy— ie, the benefits to the mother outweigh the risks to the unborn baby—the following precautions could help decrease fetal exposure:23
- keep the medication regimen simple and at the lowest effective dose
- use monotherapy when appropriate
- if possible, do not change medications during pregnancy.
When considering pharmacotherapy, evaluate each woman’s risk for disease exacerbation and consequences for pregnancy and neonatal outcomes, and ask the woman how she views reproductive risk vs disease benefit.
Developing fetuses are exposed to either the effects of the mother’s untreated mental illness or the medication.26 A recent study comparing birth and neonatal outcomes among women with untreated depression vs those taking selective serotonin reuptake inhibitors (SSRIs) found similar adverse outcomes.27 Babies continously exposed to either prenatal depression or SSRIs were more likely to be born prematurely, but partial exposure to either condition did not increase this risk.27 In addition, women who were not taking SSRIs had more depressive symptoms and more trouble functioning, which can interfere with bonding between mother and baby, both in-utero and postpartum.6,27 Neither SSRIs nor depression exposure increased risk for minor physical anomalies.27
A careful process of informed consent and documentation is essential when prescribing medications during pregnancy. Women should understand the risks of pharmacotherapy as well as the risks of undertreated illness.
Electroconvulsive therapy can safely help pregnant women with treatment-resistant, life-threatening, or psychotic depression.28,29
Table 2
Risk factors for perinatal psychopathology
| Pregnancy during adolescence |
| Previous diagnosis of depression, anxiety, psychosis, or bipolar disorder |
| Trauma history, including physical, emotional, or sexual abuse |
| Current or past substance abuse/dependence, including cigarette smoking |
| Lack of social support |
| Single parenthood |
| Low socioeconomic status |
| History of sexual assault or domestic violence |
| Unstable home environment |
| Stopping antidepressants during pregnancy |
| Financial problems |
| Ambivalence about pregnancy |
| Source: References 5,22 |
Psychotherapy. The American College of Obstetricians and Gynecologists treatment guidelines22 favor psychotherapy over medication for women with mild depressive symptoms and no loss of function, suicidality, or psychotic experiences; pharmacotherapy is suggested for women who have moderate to severely impaired functioning, recurrent depressive symptoms, or suicidal thinking (Table 3).22
Interpersonal psychotherapy or cognitive-behavioral therapy can be safe and effective during pregnancy.30,31 Other psychotherapeutic modalities and alternative/complementary treatments offer potential benefit without substantial risk, and could help prevent relapse when discontinuing mood stabilizers or antidepressants after conception (Box 2).32-35
Table 3
ACOG guidelines for treating depression during pregnancy
| Women who are thinking about getting pregnant |
| For women on medication with mild or no symptoms for ≥6 months, it may be appropriate to taper and discontinue medication before becoming pregnant |
| Medication discontinuation may not be appropriate in women with a history of severe, recurrent depression or who have psychosis, bipolar disorder, other psychiatric illness requiring medication, or a history of suicide attempts |
| Pregnant women currently taking medication for depression |
| Psychiatrically stable women who prefer to stay on medication may be able to do so after consultation between their psychiatrist and obstetrician to discuss risks and benefits |
| Women who want to discontinue medication may attempt to taper and discontinue if they are not experiencing symptoms, depending on their psychiatric history. Women with a history of recurrent depression are at a high risk of relapse if medication is discontinued |
| Women with recurrent depression or who have symptoms despite medication may benefit from psychotherapy to replace or augment medication |
| Women with severe depression (with suicide attempts, functional incapacitation, or weight loss) should remain on medication. If a patient refuses medication, alternative treatment and monitoring should be in place, preferably before discontinuation |
| Pregnant and not currently on medication for depression |
| Psychotherapy may be beneficial for women who prefer to avoid antidepressants |
| For women who want to take medication, risks and benefits of treatment choices should be evaluated and discussed, including factors such as stage of gestation, symptoms, history of depression, and other conditions and circumstances (eg, smoking, difficulty gaining weight) |
| All pregnant women |
| Regardless of circumstances, a woman with suicidal or psychotic symptoms should immediately see a psychiatrist |
| ACOG: American College of Obstetricians and Gynecologists Source: Reference 22 |
Mind-body approaches such as mindfulness-based stress reduction, yoga, and progressive relaxation and supplements such as fish oil may be good adjuncts to psychotherapy. Many pregnant women prefer mindfulness yoga to other mind-body techniques.32 A pilot study found that mindfulness yoga significantly decreased depressive symptoms and increased maternal-fetal attachment, particularly in mildly depressed women.33 For women who do not wish to engage in traditional treatments, alternative approaches such as progressive relaxation are easily taught and can help reduce depressive symptoms.34 Regular exercise may improve self-esteem and reduce symptoms of depression and anxiety in pregnant women.35
CASE CONTINUED: Healthy baby boy
Ms. A either doesn’t show up or cancels her weekly appointments about once a month, but seems to be making progress. Her therapist makes accommodations for Ms. A, such as offering childcare in an adjacent room during sessions, conducting brief sessions by phone when Ms. A is unable to come to the clinic, and helping her enroll in the state’s Maternal Infant Health Program. Ms. A’s therapist has referred her to a specialized OB clinic that can manage her pain medication and monitor for signs of abuse and keeps in regular contact with her obstetrician.
At 26 weeks gestation, Ms. A still is reluctant to try psychotropics, so her therapist works with her to integrate psychotherapy with alternative approaches such as mindfulness meditation and yoga. During therapy, Ms. A learns ways to manage her depressive symptoms, improve her social functioning, adjust to role transitions, and work through her traumatic experiences. Ms. A enrolls in a prenatal yoga class with a mindfulness focus, which allows her to interact with other pregnant women at risk for psychopathology and learn new ways to cope with her depressed mood and chronic pain.
Ms. A delivers a healthy boy at 38 weeks gestation. During labor, she uses many of the yoga poses she learned to manage pain, but elects to have an epidural after 30 hours of labor. Her baby tests positive for hydrocodone, which can cause ongoing mild irritability and occasional jitteriness. He is observed in the hospital for signs of withdrawal for 48 hours and then discharged home with his mother. Ms. A starts breast-feeding in the hospital and plans to continue at home.
Ms. A’s therapist continues to stay in touch with her by phone until she schedules another appointment and assists with referrals to other community resources.
- University of Michigan Department of Psychiatry Depression Center Women’s Mental Health and Infants Program. Women and depression. www.psych.med.umich.edu/wimhc.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
- Michigan Families Medicaid Project. Year 1 final report. Includes a list of maternal and child health services similar to Michigan’s Maternal Infant Health Program, for all 50 states. http://1.usa.gov/yCzdca.
Drug Brand Name
- Acetaminophen/hydrocodone • Vicodin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Perinatal psychopathology is a common and undertreated problem with wide-ranging consequences for both mother and child.1-4 Women at risk for psychopathology are more likely to engage in unhealthy behaviors such as smoking and substance abuse and have difficulty engaging in treatment and attending psychiatric and obstetrics appointments.5 In addition, many of these women have trouble attaching to and caring for their infants and struggle with everyday stressors during pregnancy and postpartum.6
Routine prenatal screening for mental illness coupled with non-judgmental, collaborative, and individualized care delivered by a multidisciplinary team is critical for treatment engagement and adherence. Providers should be aware of risk factors for perinatal psychiatric illness—including a history of mental illness, stressful life events, and interpersonal conflict—and should be versed in current treatment guidelines.
CASE REPORT: Difficulty coping
Ms. A, age 28, is referred to our High Risk Perinatal Team by her obstetrician when she is approximately 6 weeks pregnant. She is single, has 3 other children (age 10, 4, and 2), a history of depression, and chronic pain related to an auto accident 3 years ago. She reports that this pregnancy likely is the result of a sexual assault, but she has decided to keep the baby. Ms. A describes severe depressive symptoms, including insomnia, low appetite, feelings of worthlessness, and thoughts of harming herself. In addition, she has incapacitating panic attacks and constantly worries about her children’s safety when she is not with them. She schedules an appointment with the perinatal team, but does not show up twice.
When our team finally sees Ms. A, she is well into her second trimester and brings her 2 youngest children with her. She says she recently was fired from her job as a cashier because she missed too many days of work, and is applying for Medicaid. Recently, her back and shoulder pain have worsened, and she is running out of her prescription for acetaminophen/hydrocodone. Ms. A’s affect is flat, her mood depressed, and she has difficulty explaining her history because her 2-year-old son interrupts the interview. She has never been in psychotherapy, and is reluctant to take antidepressants. Despite a difficult first visit, she engages with the clinician and agrees to schedule a second appointment.
What complicates pregnancy?
Women are at higher risk for developing depression during puberty, the perinatal period (ie, pregnancy and first year postpartum), and perimenopause.7 These times often are fraught with unfamiliar hormonal fluctuations, role transitions, emotional upheaval, and physical changes. However, because these times are expected to be stressful, serious mood changes often go unnoticed by patients and untreated by clinicians.8 Women are expected to celebrate, thrive, and “glow” during pregnancy, and those who suffer from depression and anxiety frequently do so in silence. Social stigma surrounding perinatal depression or anxiety leads many women to believe they are alone in their struggle and hesitant to seek help.9
Most pregnant women who develop psychiatric illness do not present for treatment.10 One study found that 86% of pregnant women who screened positive for depression in an obstetrics (OB) setting did not receive treatment.11 Some women are reluctant to take antidepressants out of concern for their infant’s safety,8 and psychotherapy or alternative approaches are not available in all areas.12 Transportation, childcare issues, or ongoing life stressors may prevent women from seeking help (Box 1).9
Diagnostic uncertainty among professionals may aggravate undertreatment. Clinicians who are unfamiliar with the presentation of perinatal mental illness may mislabel depressive features—such as irritability, loss of interest in activities, low energy, increased anxiety, difficulty sleeping, or appetite dysregulation—as normative experiences during pregnancy or adjustment after childbirth. Concerned about fetal exposure to potentially teratogenic compounds, clinicians may under-dose otherwise effective medications, which can lead to treatment resistance. Even if treated aggressively, depression in pregnancy may persist because of other factors, such as comorbid anxiety, somatization, pain, substance use/dependence, undiagnosed bipolar illness, or the presence of severe psychosocial stress or trauma.
Maternal suicide and/or harm to the infant—the most severe result of untreated perinatal psychopathology—is rare.13 Common negative outcomes of untreated depression or anxiety in pregnant women include inadequate weight gain, preeclampsia, difficulty bonding with their unborn baby, premature labor, and lack of follow through with prenatal care.14,15 Symptoms become harder to treat when aggravated by psychosocial stressors such as poor social support, ambivalence about the pregnancy, and/or substance abuse.
The key to successful intervention is finding a balance between managing psychiatric concerns, facilitating adequate coping with psychosocial stressors, and, if necessary, aggressively treating pregnancy-related physical illnesses. Successful treatment response depends on early detection and initiating individualized care as soon as possible.
Lack of insurance, childcare, or transportation can make it difficult for a pregnant woman to receive psychiatric treatment. All pregnant women are eligible for Medicaid if private insurance is unavailable to them, and clinicians can help patients apply for assistance. Some programs—for example, Michigan’s state-funded Maternal Infant Health Programs—offer help with transportation to appointments, such as cabs and reimbursement for gas, in addition to nutrition guidance, counseling, home visits, and referrals to community resources such as childbirth classes, infant mental health specialists, and/or substance abuse treatment (see Related Resources ).
Offering childcare during psychotherapy sessions can be particularly helpful, and may provide valuable experience for a student or resident interested in working with at-risk children. Women may be more likely to engage in care if psychotherapy sessions are conducted by phone or in their homes. A positive experience with mental health care during pregnancy may increase the likelihood that women will remain engaged in treatment after childbirth, therefore lessening the negative effects of perinatal psychopathology on mother and child.
Early detection. Women’s health care providers play a fundamental role in guiding decision-making about mental health care, providing referrals, and most important, allowing women to talk about perinatal psychopathology without fear of stigma.
When a woman becomes pregnant, it is critical to determine if she is at risk for developing psychopathology or presents with active illness. Many OB clinics screen for depression several times during pregnancy and early postpartum. The most commonly used screening tool is the Edinburgh Postpartum Depression Scale (EPDS),16 a 10-item self-report measure that is sensitive to cognitive and affective symptoms of depression. If a woman scores >15 during pregnancy or >13 postpartum, further assessment is indicated.17 The anxiety subscale (items 5 and 6) of the EPDS has been validated for screening perinatal anxiety using a cut-off score >4.18 Depression can be quickly assessed using the 2-question Patient Health Questionnaire (PHQ-2) or the 9-question PHQ-9.19,20 All 3 scales are free and available on the Internet (Table 1).21
These screening tools offer clinicians an opportunity to assess for risk factors that may increase the likelihood of illness onset or worsened prognosis (Table 2).5,22 All women who present with pregnancy-related medical illness, such as preeclampsia or gestational diabetes, should be screened for co-occurring depression or anxiety because psychiatric comorbidity is common.
Individualized care. Have an open mind about the kind of care to offer and collaborate with the patient when discussing treatment options.5 Some pregnant women may reject traditional treatments, such as pharmacotherapy or psychotherapy, because of concern about harm to the unborn baby or reluctance to work through past or present conflicts in therapy during a vulnerable time.9 Women may assume that medication will be the only treatment offered, or even fear that they will be forced to take antidepressants. Women often do not follow through on mental health referrals, even when they are appropriately screened and identified to be at risk, and an OB nurse explains the risks of untreated psychopathology.11
A multidisciplinary, collaborative care model is vital for positive pregnancy outcomes. Connecting obstetricians and midwives with psychologists, psychiatrists, social workers, and infant mental health specialists to coordinate treatment ensures that at-risk pregnant and postpartum women get the care they need. A nonjudgmental approach is essential to engage pregnant women in care. Assure women that pharmacotherapy is not required when receiving mental health treatment, but is an option they can choose.
Table 1
Screening for psychiatric illness during pregnancy
| Screening tool | Sensitivity/specificity | Administration | Availability |
|---|---|---|---|
| Edinburgh Postpartum Depression Scale | Sensitivity = 0.86 Specificity = 0.78 Positive screen: >10 | Self-administered in 5 to 10 minutes. Could be self-scored | http://bit.ly/PPDscale |
| Patient Health Questionnaire-2 (PHQ-2) | Sensitivity = 0.83 Specificity = 0.92 Positive screen: >3 | Self- or clinician-administered in <1 minute | www.phqscreeners.com The 2 questions from the PHQ-9 for mood and anhedonia are used |
| Patient Health Questionnaire-9 (PHQ-9) | Sensitivity = 0.88 Specificity = 0.88 Positive screen: >10 | Self-administered and self-scored, 5 to 10 minutes | www.phqscreeners.com |
| Source: Reference 21 | |||
Treatment choices
Pharmacotherapy. If a woman has only mild symptoms or has been symptom-free for ≥6 months, it may be safe to decrease or discontinue antidepressants during pregnancy or while trying to conceive, but such patients should be monitored closely for signs of relapse.23 In a study of 201 depressed pregnant women, 68% of those who discontinued medication experienced symptom relapse compared with 26% of those who continued medication.24 If a depressed woman has a history of relapse or severe symptoms, including suicide attempts and inpatient psychiatric admissions, it is recommended that she remain on antidepressants or mood stabilizers, regardless of pregnancy status.25 If medications are necessary during pregnancy— ie, the benefits to the mother outweigh the risks to the unborn baby—the following precautions could help decrease fetal exposure:23
- keep the medication regimen simple and at the lowest effective dose
- use monotherapy when appropriate
- if possible, do not change medications during pregnancy.
When considering pharmacotherapy, evaluate each woman’s risk for disease exacerbation and consequences for pregnancy and neonatal outcomes, and ask the woman how she views reproductive risk vs disease benefit.
Developing fetuses are exposed to either the effects of the mother’s untreated mental illness or the medication.26 A recent study comparing birth and neonatal outcomes among women with untreated depression vs those taking selective serotonin reuptake inhibitors (SSRIs) found similar adverse outcomes.27 Babies continously exposed to either prenatal depression or SSRIs were more likely to be born prematurely, but partial exposure to either condition did not increase this risk.27 In addition, women who were not taking SSRIs had more depressive symptoms and more trouble functioning, which can interfere with bonding between mother and baby, both in-utero and postpartum.6,27 Neither SSRIs nor depression exposure increased risk for minor physical anomalies.27
A careful process of informed consent and documentation is essential when prescribing medications during pregnancy. Women should understand the risks of pharmacotherapy as well as the risks of undertreated illness.
Electroconvulsive therapy can safely help pregnant women with treatment-resistant, life-threatening, or psychotic depression.28,29
Table 2
Risk factors for perinatal psychopathology
| Pregnancy during adolescence |
| Previous diagnosis of depression, anxiety, psychosis, or bipolar disorder |
| Trauma history, including physical, emotional, or sexual abuse |
| Current or past substance abuse/dependence, including cigarette smoking |
| Lack of social support |
| Single parenthood |
| Low socioeconomic status |
| History of sexual assault or domestic violence |
| Unstable home environment |
| Stopping antidepressants during pregnancy |
| Financial problems |
| Ambivalence about pregnancy |
| Source: References 5,22 |
Psychotherapy. The American College of Obstetricians and Gynecologists treatment guidelines22 favor psychotherapy over medication for women with mild depressive symptoms and no loss of function, suicidality, or psychotic experiences; pharmacotherapy is suggested for women who have moderate to severely impaired functioning, recurrent depressive symptoms, or suicidal thinking (Table 3).22
Interpersonal psychotherapy or cognitive-behavioral therapy can be safe and effective during pregnancy.30,31 Other psychotherapeutic modalities and alternative/complementary treatments offer potential benefit without substantial risk, and could help prevent relapse when discontinuing mood stabilizers or antidepressants after conception (Box 2).32-35
Table 3
ACOG guidelines for treating depression during pregnancy
| Women who are thinking about getting pregnant |
| For women on medication with mild or no symptoms for ≥6 months, it may be appropriate to taper and discontinue medication before becoming pregnant |
| Medication discontinuation may not be appropriate in women with a history of severe, recurrent depression or who have psychosis, bipolar disorder, other psychiatric illness requiring medication, or a history of suicide attempts |
| Pregnant women currently taking medication for depression |
| Psychiatrically stable women who prefer to stay on medication may be able to do so after consultation between their psychiatrist and obstetrician to discuss risks and benefits |
| Women who want to discontinue medication may attempt to taper and discontinue if they are not experiencing symptoms, depending on their psychiatric history. Women with a history of recurrent depression are at a high risk of relapse if medication is discontinued |
| Women with recurrent depression or who have symptoms despite medication may benefit from psychotherapy to replace or augment medication |
| Women with severe depression (with suicide attempts, functional incapacitation, or weight loss) should remain on medication. If a patient refuses medication, alternative treatment and monitoring should be in place, preferably before discontinuation |
| Pregnant and not currently on medication for depression |
| Psychotherapy may be beneficial for women who prefer to avoid antidepressants |
| For women who want to take medication, risks and benefits of treatment choices should be evaluated and discussed, including factors such as stage of gestation, symptoms, history of depression, and other conditions and circumstances (eg, smoking, difficulty gaining weight) |
| All pregnant women |
| Regardless of circumstances, a woman with suicidal or psychotic symptoms should immediately see a psychiatrist |
| ACOG: American College of Obstetricians and Gynecologists Source: Reference 22 |
Mind-body approaches such as mindfulness-based stress reduction, yoga, and progressive relaxation and supplements such as fish oil may be good adjuncts to psychotherapy. Many pregnant women prefer mindfulness yoga to other mind-body techniques.32 A pilot study found that mindfulness yoga significantly decreased depressive symptoms and increased maternal-fetal attachment, particularly in mildly depressed women.33 For women who do not wish to engage in traditional treatments, alternative approaches such as progressive relaxation are easily taught and can help reduce depressive symptoms.34 Regular exercise may improve self-esteem and reduce symptoms of depression and anxiety in pregnant women.35
CASE CONTINUED: Healthy baby boy
Ms. A either doesn’t show up or cancels her weekly appointments about once a month, but seems to be making progress. Her therapist makes accommodations for Ms. A, such as offering childcare in an adjacent room during sessions, conducting brief sessions by phone when Ms. A is unable to come to the clinic, and helping her enroll in the state’s Maternal Infant Health Program. Ms. A’s therapist has referred her to a specialized OB clinic that can manage her pain medication and monitor for signs of abuse and keeps in regular contact with her obstetrician.
At 26 weeks gestation, Ms. A still is reluctant to try psychotropics, so her therapist works with her to integrate psychotherapy with alternative approaches such as mindfulness meditation and yoga. During therapy, Ms. A learns ways to manage her depressive symptoms, improve her social functioning, adjust to role transitions, and work through her traumatic experiences. Ms. A enrolls in a prenatal yoga class with a mindfulness focus, which allows her to interact with other pregnant women at risk for psychopathology and learn new ways to cope with her depressed mood and chronic pain.
Ms. A delivers a healthy boy at 38 weeks gestation. During labor, she uses many of the yoga poses she learned to manage pain, but elects to have an epidural after 30 hours of labor. Her baby tests positive for hydrocodone, which can cause ongoing mild irritability and occasional jitteriness. He is observed in the hospital for signs of withdrawal for 48 hours and then discharged home with his mother. Ms. A starts breast-feeding in the hospital and plans to continue at home.
Ms. A’s therapist continues to stay in touch with her by phone until she schedules another appointment and assists with referrals to other community resources.
- University of Michigan Department of Psychiatry Depression Center Women’s Mental Health and Infants Program. Women and depression. www.psych.med.umich.edu/wimhc.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
- Michigan Families Medicaid Project. Year 1 final report. Includes a list of maternal and child health services similar to Michigan’s Maternal Infant Health Program, for all 50 states. http://1.usa.gov/yCzdca.
Drug Brand Name
- Acetaminophen/hydrocodone • Vicodin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
2. Zuckerman B, Bauchner H, Parker S, et al. Maternal depressive symptoms during pregnancy, and newborn irritability. J Dev Behav Pediatr. 1990;11(4):190-194.
3. Field T, Healy B, Goldstein S, et al. Infants of depressed mothers show depressed behavior even with non-depressed adults. Child Dev. 1988;59(6):1569-1579.
4. Coghill SR, Caplan HL, Alexandra H, et al. Impact of maternal postnatal depression on cognitive development of young children. Br Med J (Clin Res Ed). 1986;292(6529):1165-1167.
5. Muzik M, Marcus S, Heringhausen J, et al. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788, ix–x.
6. Bifulco A, Figueiredo B, Guedeney N, et al. Maternal attachment style and depression associated with childbirth: preliminary results from a European and US cross-cultural study. Br J Psychiatry Suppl. 2004;46:s31-s37.
7. National Institute of Mental Health. Women and depression: discovering hope. http://www.nimh.nih.gov/health/publications/women-and-depression-discovering-hope/complete-index.shtml. Accessed December 27 2011.
8. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
9. O’Mahen HA, Flynn HA. P and perceived barriers to treatment for depression during the perinatal period. J Womens Health (Larchmt). 2008;17(8):1301-1309.
10. Flynn HA, O’Mahen HA, Massey L, et al. The impact of a brief obstetrics clinic based intervention on treatment use for perinatal depression. J Womens Health (Larchmt). 2006;15(10):1195-1204.
11. Marcus SM, Flynn HA, Blow FC, et al. Depressive symptoms among pregnant women screened in obstetrics settings. J Womens Health (Larchmt). 2003;12(4):373-380.
12. Olfson M, Marcus SC. National trends in outpatient psychotherapy. Am J Psychiatry. 2010;167(12):1456-1463.
13. Schiff MA, Grossman DC. Adverse perinatal outcomes and risk for postpartum suicide attempt in Washington state 1987-2001. Pediatrics. 2006;118(3):669-675.
14. Kurki T, Hiilesmaa V, Raitasalo R, et al. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95:(4)487-490.
15. McKee MD, Cunningham M, Jankowski KR, et al. Health-related functional status in pregnancy: relationship to depression and social support in a multiethnic population. Obstet Gynecol. 2001;97(6):988-993.
16. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
17. Matthey S, Henshaw C, Elliott S, et al. Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale: implications for clinical and research practice. Arch Womens Ment Health. 2006;9(6):309-315.
18. Chaudron LH, Szilagyi PG, Tang W, et al. Accuracy of depression screening tools for identifying postpartum depression among urban mothers. Pediatrics. 2010;125(3):609-617.
19. Ross AS, Hall RW, Frost K, et al. Antenatal and neonatal guidelines, education and learning system. J Ark Med Soc. 2006;102(12):328-330.
20. Muzik M, Klier CK, Rosenblum KL, et al. Are commonly used self-report inventories suitable for screening postpartum depression and anxiety disorders? Acta Psychiatr Scand. 2000;102(1):71-73.
21. Muzik M, Thelen K, Rosenblum KL. Perinatal depression: detection and treatment. Neuropsychiatry. 2011;1(2):179-195.
22. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
23. ACOG Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001-1020.
24. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
25. Gonsalves L, Schuermeyer I. Treating depression in pregnancy: practical suggestions. Cleve Clin J Med. 2006;73(12):1098-1104.
26. Misri S, Kendrick K. Treatment of perinatal mood and anxiety disorders: a review. Can J Psychiatry. 2007;52(8):489-498.
27. Wisner KL, Sit DY, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557-566.
28. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71(2):235-242.
29. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry. 1994;45(5):444-450.
30. O’Hara MW, Stuart S, Gorman LL, et al. Efficacy of interpersonal psychotherapy for postpartum depression. Arch Gen Psychiatry. 2000;57(11):1039-1045.
31. Bhatia SC, Bhatia SK. Depression in women: diagnostic and treatment considerations. Am Fam Physician. 1999;60(1):225-240.
32. Battle CL, Uebelacker LA, Howard M, et al. Prenatal yoga and depression during pregnancy. Birth. 2010;37(4):353-354.
33. Muzik M, Hamilton SE, Waxler EG, et al. Mindfulness yoga during pregnancy for women with depression and PTSD: preliminary results from a pilot feasibility study. Paper presented at: 36th Annual Meeting of the Association for Women in Psychology; March 5, 2011; Philadelphia, PA.
34. Deligiannidis KM, Freeman MP. Complementary and alternative medicine for the treatment of depressive disorders in women. Psychiatr Clin North Am. 2010;33(2):441-463.
35. Shivakumar G, Brandon AR, Snell PG, et al. Antenatal depression: a rationale for studying exercise. Depress Anxiety. 2011;28(3):234-242.
1. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
2. Zuckerman B, Bauchner H, Parker S, et al. Maternal depressive symptoms during pregnancy, and newborn irritability. J Dev Behav Pediatr. 1990;11(4):190-194.
3. Field T, Healy B, Goldstein S, et al. Infants of depressed mothers show depressed behavior even with non-depressed adults. Child Dev. 1988;59(6):1569-1579.
4. Coghill SR, Caplan HL, Alexandra H, et al. Impact of maternal postnatal depression on cognitive development of young children. Br Med J (Clin Res Ed). 1986;292(6529):1165-1167.
5. Muzik M, Marcus S, Heringhausen J, et al. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788, ix–x.
6. Bifulco A, Figueiredo B, Guedeney N, et al. Maternal attachment style and depression associated with childbirth: preliminary results from a European and US cross-cultural study. Br J Psychiatry Suppl. 2004;46:s31-s37.
7. National Institute of Mental Health. Women and depression: discovering hope. http://www.nimh.nih.gov/health/publications/women-and-depression-discovering-hope/complete-index.shtml. Accessed December 27 2011.
8. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
9. O’Mahen HA, Flynn HA. P and perceived barriers to treatment for depression during the perinatal period. J Womens Health (Larchmt). 2008;17(8):1301-1309.
10. Flynn HA, O’Mahen HA, Massey L, et al. The impact of a brief obstetrics clinic based intervention on treatment use for perinatal depression. J Womens Health (Larchmt). 2006;15(10):1195-1204.
11. Marcus SM, Flynn HA, Blow FC, et al. Depressive symptoms among pregnant women screened in obstetrics settings. J Womens Health (Larchmt). 2003;12(4):373-380.
12. Olfson M, Marcus SC. National trends in outpatient psychotherapy. Am J Psychiatry. 2010;167(12):1456-1463.
13. Schiff MA, Grossman DC. Adverse perinatal outcomes and risk for postpartum suicide attempt in Washington state 1987-2001. Pediatrics. 2006;118(3):669-675.
14. Kurki T, Hiilesmaa V, Raitasalo R, et al. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95:(4)487-490.
15. McKee MD, Cunningham M, Jankowski KR, et al. Health-related functional status in pregnancy: relationship to depression and social support in a multiethnic population. Obstet Gynecol. 2001;97(6):988-993.
16. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
17. Matthey S, Henshaw C, Elliott S, et al. Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale: implications for clinical and research practice. Arch Womens Ment Health. 2006;9(6):309-315.
18. Chaudron LH, Szilagyi PG, Tang W, et al. Accuracy of depression screening tools for identifying postpartum depression among urban mothers. Pediatrics. 2010;125(3):609-617.
19. Ross AS, Hall RW, Frost K, et al. Antenatal and neonatal guidelines, education and learning system. J Ark Med Soc. 2006;102(12):328-330.
20. Muzik M, Klier CK, Rosenblum KL, et al. Are commonly used self-report inventories suitable for screening postpartum depression and anxiety disorders? Acta Psychiatr Scand. 2000;102(1):71-73.
21. Muzik M, Thelen K, Rosenblum KL. Perinatal depression: detection and treatment. Neuropsychiatry. 2011;1(2):179-195.
22. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
23. ACOG Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001-1020.
24. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
25. Gonsalves L, Schuermeyer I. Treating depression in pregnancy: practical suggestions. Cleve Clin J Med. 2006;73(12):1098-1104.
26. Misri S, Kendrick K. Treatment of perinatal mood and anxiety disorders: a review. Can J Psychiatry. 2007;52(8):489-498.
27. Wisner KL, Sit DY, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557-566.
28. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71(2):235-242.
29. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry. 1994;45(5):444-450.
30. O’Hara MW, Stuart S, Gorman LL, et al. Efficacy of interpersonal psychotherapy for postpartum depression. Arch Gen Psychiatry. 2000;57(11):1039-1045.
31. Bhatia SC, Bhatia SK. Depression in women: diagnostic and treatment considerations. Am Fam Physician. 1999;60(1):225-240.
32. Battle CL, Uebelacker LA, Howard M, et al. Prenatal yoga and depression during pregnancy. Birth. 2010;37(4):353-354.
33. Muzik M, Hamilton SE, Waxler EG, et al. Mindfulness yoga during pregnancy for women with depression and PTSD: preliminary results from a pilot feasibility study. Paper presented at: 36th Annual Meeting of the Association for Women in Psychology; March 5, 2011; Philadelphia, PA.
34. Deligiannidis KM, Freeman MP. Complementary and alternative medicine for the treatment of depressive disorders in women. Psychiatr Clin North Am. 2010;33(2):441-463.
35. Shivakumar G, Brandon AR, Snell PG, et al. Antenatal depression: a rationale for studying exercise. Depress Anxiety. 2011;28(3):234-242.
Managing chronic pain: Consider psychotropics and other non-opioids
Discuss this article at www.facebook.com/CurrentPsychiatry
Of the 56 million American adults who report living with chronic pain almost 60% also exhibit psychiatric disorders such as depression or anxiety.1,2 Because patients with chronic pain suffer from a mixture of physical and psychological components, managing such conditions is complicated, and using opioids is tempting. However, treatment needs to address the underlying pathology along with social and psychological factors.
Because substance abuse treatment admissions increased by 400% from 1998 to 2008,3 many physicians look to non-opioids and other treatment modalities to control chronic non-cancer pain. Common pharmacologic therapies used to treat chronic pain include tricyclic antidepressants (TCAs), serotonin-norepinephrine reuptake inhibitors (SNRIs), antiepileptic drugs (AEDs), nonsteroidal anti-inflammatory drugs (NSAIDs), and, to a lesser extent, atypical antipsychotics. TCAs, SNRIs, AEDs, NSAIDs, and atypical antipsychotics influence a variety of presumed underlying pathophysiological processes, including inflammatory mediators, activity of N-methyl-d-aspartate (NMDA) receptors, and voltage-gated calcium channels. In addition, they increase activity of descending inhibitory pain pathways. Animal studies suggest dysfunction of these inhibitory mechanisms contributes to the central sensitization and hyperexcitability of pain transmitting pathways.4
In this article, we discuss psychotropics and other non-opioid agents for treating pain. However, no single solution is best for all patients with chronic pain and this article is not a “how to” guide to avoid administering opioid medication. Also incorporate a multimodal, non-pharmacologic approach whenever possible.
Tricyclic antidepressants
Although this class acts primarily by increasing serotonin levels, norepinephrine and dopamine also are affected depending on the particular medication. Studies have shown that amitriptyline, nortriptyline, and desipramine function well as analgesics independent of their antidepressant effects.5 TCAs may improve pain symptoms at lower therapeutic dosages than those used for treating depression.5
Although researchers have not elucidated TCAs’ mechanism of action with regards to analgesia, they are thought to act within the concept of the gating theory of pain control,6 which functions by activation and inhibition of pain signal transmission. It is believed TCAs act on nociceptive pathways by blocking serotonin and norepinephrine reuptake. Although researchers previously thought that TCAs’ analgesic mechanism was correlated to serotonin reuptake inhibition, this theory has changed. Selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine have not demonstrated substantial effectiveness in neuropathic pain when compared with TCAs and SNRIs. Recent studies have shown that TCAs may work by blocking sodium channels, similar to local anesthetics and antiarrhythmic agents.7
Psychiatrists prescribe TCAs infrequently because of these drugs’ unfavorable side effect profile compared with SSRIs and SNRIs. However, TCAs often are prescribed for pain management as an adjunct to other medications for neuropathic conditions and at lower dosages than those used for treating depression (Table 1).8
Table 1
Tricyclic antidepressants used to treat pain
| Drug | Dosage range for pain (off-label) | Comments |
|---|---|---|
| Amitriptyline | 10 to 100 mg/d | High sedation, high anticholinergic side effects |
| Amoxapine | 50 to 100 mg/d | Low sedation, moderate anticholinergic side effects |
| Clomipramine | 25 to 100 mg/d | Low sedation, low anticholinergic side effects |
| Desipramine | 25 to 100 mg/d | Low sedation, low anticholinergic side effects |
| Imipramine | 25 to 100 mg/d | Moderate sedation, moderate anticholinergic side effects |
| Nortriptyline | 10 to 75 mg/d | Moderate sedation, low anticholinergic side effects |
| Source: Reference 8 | ||
SNRIs
Evidence supports using duloxetine, a potent SNRI that mediates pain inhibition in the descending pathways, for 4 chronic pain conditions:
- diabetic peripheral neuropathic pain
- fibromyalgia
- mechanical low back pain
- pain associated with osteoarthritis.9
Titrate the dosage to 60 mg/d and maintain the patient at this dose for at least 4 weeks. Thereafter, according to patient response, the dosage may be titrated to 120 mg/d (off-label) with appropriate vital sign monitoring and routine lab analysis.
Venlafaxine also can mediate pain response in a similar manner to duloxetine, but is not FDA-approved for treating pain. Use caution when prescribing venlafaxine for patients with a history of hypertension. Milnacipran is a relatively new SNRI that has been shown to be effective in treating fibromyalgia in divided doses of 100 to 200 mg/d (Table 2).9-11
Table 2
Treating pain with serotonin-norepinephrine reuptake inhibitors
| Drug | Dosage range for pain | Comments |
|---|---|---|
| Duloxetine | 60 to 120 mg/d9 | FDA maximum recommended dose is 60 mg/d |
| Milnacipran | 25 to 200 mg/d10 | Approved for treating depression outside the United States |
| Venlafaxine | 75 to 225 mg/d11 | Monitor blood pressure, LFTs, and kidney function |
| LFTs: liver function tests | ||
Antiepileptic drugs
Several AEDs are used for pain management (Table 3).12-16 Gabapentin and pregabalin work by binding to voltage-gated calcium channels and decreasing excitatory neurotransmitter release. Along with TCAs, they are considered a first-line treatment for managing neuropathic pain.17 Gabapentin is FDA-approved for seizures and postherpetic neuralgia, but evidence supports its use in most types of neuropathic pain. Pregabalin is FDA-approved for treating seizures, diabetic peripheral neuropathy, central neuropathic pain, postherpetic neuralgia, and fibromyalgia.
Topiramate inhibits excitatory neurotransmission by enhancing the effects of gamma-aminobutyric acid, and also by blocking NMDA receptors. Topiramate is FDA-approved for seizures and migraine prophylaxis, and is used off-label for treating neuropathic pain. A 12-week trial of topiramate for diabetic neuropathy found significant analgesia in 50% of patients taking the drug, compared with 34% receiving placebo.18
Lamotrigine is approved for several types of seizures and maintenance of bipolar I disorder, and is used off-label for neuropathic pain. A recent Cochrane database review concluded that lamotrigine is ineffective for neuropathic pain14; however, some guidelines recommend using lamotrigine to treat neuropathies that do not respond to treatment with carbamazepine.19
Carbamazepine is a complex AED that is structurally similar to TCAs. It blocks sodium channels and has various pharmacologic properties, including anticholinergic, muscle relaxant, antidepressant, and sedative effects. Carbamazepine has analgesic effects through blockade of synaptic transmission in the trigeminal nucleus and is FDA-approved for seizures, bipolar disorder, neuropathic pain, and trigeminal neuralgia. In a systematic review of 12 trials of carbamazepine that included 4 placebo-controlled trials for trigeminal neuralgia, 2 studies showed a number needed to treat (NNT) of 1.8.20 For diabetic neuropathy, there was insufficient data to calculate NNT.
Oxcarbazepine, an analog of carbamazepine, also is FDA-approved for seizures and is used off-label for neuropathic pain. In the only double-blind trial with positive results, oxcarbazepine titrated to 1,800 mg/d reduced diabetic neuropathy pain scores on a visual analog scale by 24 points—roughly 25%.15
Table 3
Antiepileptic drugs for pain treatment
| Drug | Dosage range for pain | Comments |
|---|---|---|
| Carbamazepine | Starting dose: 100 mg twice a day, doses titrated to 400 to 800 mg/d usually are adequate. Maximum of 1,200 mg/d12 | Anticholinergic effects, blood dyscrasias, hyponatremia, increase in LFTs, ECG changes. CYP450 inducer, many DDIs |
| Gabapentin | Starting dose: 100 to 300 mg at bedtime or 100 to 300 mg 3 times a day, slow titration, maximum of 3,600 mg/d13 | Dizziness, sedation, weight gain, peripheral edema. Adjust dose in renal insufficiency |
| Lamotrigine | 200 to 400 mg/d14 | Sedation, headache, dizziness, ataxia, GI upset, blurred vision. Risk of life-threatening rash |
| Oxcarbazepine | Starting dose: 300 mg/d, then titrated as tolerated to a maximum of 1,800 mg/d15 | Adverse drug reactions similar to carbamazepine, less anticholinergic effects, more hyponatremia. Fewer DDIs than carbamazepine |
| Pregabalin | Starting dose: 50 mg 3 times a day or 75 mg twice a day, may increase every 3 to 7 days as tolerated, maximum of 600 mg/d13 | Same adverse drug reactions as gabapentin, less sedation. Adjust dose in renal insufficiency. More costly than gabapentin |
| Topiramate | Starting dose: 12.5 to 25 mg once or twice a day for 4 weeks; then double the dose every 4 weeks to reach a maximum dose of 100 to 200 mg/d in divided doses16 | Weight loss, anorexia, nephrolithiasis, cognitive impairment |
| CYP450: cytochrome P450; DDIs: drug-drug interactions; GI: gastrointestinal; LFTs: liver function tests | ||
Non-opioid analgesics
NSAIDs have antipyretic, analgesic, and anti-inflammatory effects and are used for fever, headache, mild-to-moderate pain, musculoskeletal pain, menstrual pain, and dental pain. They are particularly useful in treating acute pain, often in combination with opioid analgesics. NSAIDs exert their analgesic action through blockade of prostaglandin production via reversible inhibition of cyclooxygenase-1 and cyclooxygenase-2.
The most common side effects of NSAIDs are the result of gastrointestinal (GI) toxicity and include dyspepsia, heartburn, nausea, anorexia, and epigastric pain.21 GI ulceration and bleeding are rare but serious complications. To decrease these risks, tell patients to take NSAIDs with food. Add a GI protective agent, such as an H2 blocker or proton pump inhibitor, for patients at higher risk for GI complications.22
In addition, inhibition of renal prostaglandins by NSAIDs can cause renal toxicity, fluid retention, and edema, potentially exacerbating existing cardiovascular conditions such as hypertension and heart failure. NSAIDs may increase the risk of serious thrombotic events such as myocardial infarction and stroke. Use NSAIDs at the lowest effective dose for the shortest duration possible and generally avoid prescribing in patients at high risk for cardiovascular disease and pregnant women, especially those in their third trimester.23,24
NSAIDs may cause pharmacodynamic and pharmacokinetic drug-drug interactions. The risk of GI toxicity and bleeding increases when NSAIDs are administered with drugs that also irritate the gastric mucosa or have antiplatelet/anticoagulant effects.21 Plasma concentrations of drugs with a narrow therapeutic index that are renally eliminated, such as methotrexate and lithium, can increase to potentially toxic levels with concurrent NSAID use because NSAIDs decrease renal perfusion.21 Also, the therapeutic effects of antihypertensives may be attenuated because NSAIDs cause fluid retention.25
Acetaminophen (APAP) is available in several dosage forms as a single ingredient and in combination with opioids in prescription products. For more information about APAP, see the Box below.
Atypical antipsychotics
Although atypical antipsychotics are not often used to treat pain, studies indicate that fibromyalgia patients may benefit from ziprasidone26 and olanzapine,27 most often as an adjunctive treatment rather than monotherapy. Randomized controlled studies indicate poor tolerability with several atypical antipsychotics. Weight gain, akathisia, and somnolence are side effects of some atypical antipsychotics. Additionally, ziprasidone has been associated with QTc prolongation. For chronic pain patients, atypical antipsychotics are most useful for treating psychiatric comorbidities.
Although its mechanism of action is not well understood, acetaminophen (APAP) works by blocking prostaglandin syntheses via inhibition of cyclooxygenase-1 and cyclooxygenase-2 in the CNS.a Therefore, in contrast to NSAIDs, APAP does not possess peripheral anti-inflammatory effects or affect platelet function and is effective for treating fever, headache, and acute and chronic mild pain. The American Geriatrics Society recommends APAP for minor and persistent pain in older patientsb and the American College of Rheumatology recommends it as first-line therapy for osteoarthritis of the hip or knee.c
APAP has few clinically significant drug interactions, an excellent safety profile, and a long history of safe and effective use. When used within the recommended dosage range, APAP has few side effects. However, overuse of APAP is the leading cause of acute liver failure in the United States.d APAP hepatotoxicity can be accompanied by nephrotoxicity, is dose-dependent, and can be caused by acute overdose or chronic ingestion at doses over the recommended maximum of 4 g/d. Patients have experienced elevated liver transaminases with coadministration of APAP with phenytoin and phenobarbital.e,f Alcohol and other potentially hepatotoxic drugs also can increase the risk of liver toxicity when combined with APAP.d APAP is pregnancy category B and is considered the drug of choice for treating pain or fever during pregnancy and breast-feeding.g
References
- Amadio P Jr. Peripherally acting analgesics. Am J Med. 1984;77(3A):17-26.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43(9):1905-1915.
- Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364-1372.
- Pirotte JH. Apparent potentiation of hepatotoxicity from small doses of acetaminophen by phenobarbital. Ann Intern Med. 1984;101(3):403.
- Brackett CC, Bloch JD. Phenytoin as a possible cause of acetaminophen hepatotoxicity: case report and review of the literature. Pharmacotherapy. 2000;20(2):229-233.
- Hersh EV, Moore PA, Ross GL. Over-the-counter analgesics and antipyretics: a critical assessment. Clin Ther. 2000; 22(5):500-548.
Related Resources
- Leo RJ. Chronic nonmalignant pain: How to ‘turn down’ its physiologic triggers. Current Psychiatry. 2008;7(8):19-36.
- Nikolaus T, Zeyfang A. Pharmacological treatments for persistent non-malignant pain in older persons. Drugs Aging. 2004;21(1):19-41.
- World Health Organization. WHO’s pain ladder. www.who.int/cancer/palliative/painladder/en.
Drug Brand Names
- Acetaminophen • Tylenol
- Amitriptyline • Elavil, others
- Amoxapine • Asendin
- Carbamazepine • Tegretol, Carbatrol, others
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin, Gralise
- Imipramine • Tofranil
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Methotrexate • Rheumatrex, Trexall
- Milnacipran • Savella
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Pregabalin • Lyrica
- Topiramate • Topamax, Topiragen
- Venlafaxine • Effexor
- iprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg. 2007;105(1):205-221.
2. Thieme K, Turk DC, Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: relationship to somatic and psychosocial variables. Psychosom Med. 2004;66(6):837-844.
3. Substance Abuse and Mental Health Services Administration, Office of Applied Studies Treatment episode data set (TEDS). 1998-2008. National admissions to substance abuse treatment services. Rockville MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2010.
4. Iyengar S, Webster AA, Hemrick-Luecke SK, et al. Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther. 2004;311(2):576-584.
5. Guay DR. Adjunctive agents in the management of chronic pain. Pharmacotherapy. 2001;21(9):1070-1081.
6. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399-409.
7. Dick IE, Brochu RM, Purohit Y, et al. Sodium channel blockade may contribute to the analgesic efficacy of antidepressants. J Pain. 2007;8(4):315-324.
8. Stahl SM. Essential psychopharmacology: the prescriber’s guide. New York NY: Cambridge University Press; 2006.
9. Skljarevski V, Desaiah D, Liu-Seifert H, et al. Efficacy and safety of duloxetine in patients with chronic low back pain. Spine (Phila Pa 1976). 2010;35(13):E578-E585.
10. Hsu ES. Acute and chronic pain management in fibromyalgia: updates on pharmacotherapy. Am J Ther. 2011;18(6):487-509.
11. Bomholt SF, Mikkelsen JD, Blackburn-Munro G. Antinociceptive effects of the antidepressants amitriptyline duloxetine, mirtazapine and citalopram in animal models of acute, persistent and neuropathic pain. Neuropharmacology. 2005;48(2):252-263.
12. Campbell FG, Graham JG, Zilkha KJ. Clinical trial of carbazepine (tegretol) in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1966;29(3):265-267.
13. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(10 suppl):S22-S32.
14. Dogra S, Beydoun S, Mazzola J, et al. Oxcarbazepine in painful diabetic neuropathy: a randomized, placebo-controlled study. Eur J Pain. 2005;9(5):543-554.
15. Kline KM, Carroll DG, Malnar KF. Painful diabetic peripheral neuropathy relieved with use of oral topiramate. South Med J. 2003;96(6):602-605.
16. Wiffen PJ, Derry S, Moore RA. Lamotrigine for acute and chronic pain. Cochrane Database Syst Rev. 2011;(2):CD006044.-
17. Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 suppl):S3-S14.
18. Raskin P, Donofrio PD, Rosenthal NR, et al. Topiramate vs placebo in painful diabetic neuropathy: analgesic and metabolic effects. Neurology. 2004;63(5):865-873.
19. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13-21.
20. Wiffen PJ, Derry S, Moore RA, et al. Carbamazepine for acute and chronic pain in adults. Cochrane Database Syst Rev. 2011;(1):CD005451.-
21. Hersh EV, Moore PA, Ross GL. Over-the-counter analgesics and antipyretics: a critical assessment. Clin Ther. 2000;22(5):500-548.
22. Lanas AI. Current approaches to reducing gastrointestinal toxicity of low-dose aspirin. Am J Med. 2001;110(1A):70S-73S.
23. Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634-1642.
24. Briggs G, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation. 8th ed. Baltimore MD: Lippincott Williams and Wilkins; 2008.
25. Frishman WH. Effects of nonsteroidal anti-inflammatory drug therapy on blood pressure and peripheral edema. Am J Cardiol. 2002;89(6A):18D-25D.
26. Calandre EP, Hidalgo J, Rico-Villademoros F. Use of ziprasidone in patients with fibromyalgia: a case series. Rheumatol Int. 2007;27(5):473-476.
27. Rico-Villademoros F, Hidalgo J, Dominguez I, et al. Atypical antipsychotics in the treatment of fibromyalgia: a case series with olanzapine. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(1):161-164.
Discuss this article at www.facebook.com/CurrentPsychiatry
Of the 56 million American adults who report living with chronic pain almost 60% also exhibit psychiatric disorders such as depression or anxiety.1,2 Because patients with chronic pain suffer from a mixture of physical and psychological components, managing such conditions is complicated, and using opioids is tempting. However, treatment needs to address the underlying pathology along with social and psychological factors.
Because substance abuse treatment admissions increased by 400% from 1998 to 2008,3 many physicians look to non-opioids and other treatment modalities to control chronic non-cancer pain. Common pharmacologic therapies used to treat chronic pain include tricyclic antidepressants (TCAs), serotonin-norepinephrine reuptake inhibitors (SNRIs), antiepileptic drugs (AEDs), nonsteroidal anti-inflammatory drugs (NSAIDs), and, to a lesser extent, atypical antipsychotics. TCAs, SNRIs, AEDs, NSAIDs, and atypical antipsychotics influence a variety of presumed underlying pathophysiological processes, including inflammatory mediators, activity of N-methyl-d-aspartate (NMDA) receptors, and voltage-gated calcium channels. In addition, they increase activity of descending inhibitory pain pathways. Animal studies suggest dysfunction of these inhibitory mechanisms contributes to the central sensitization and hyperexcitability of pain transmitting pathways.4
In this article, we discuss psychotropics and other non-opioid agents for treating pain. However, no single solution is best for all patients with chronic pain and this article is not a “how to” guide to avoid administering opioid medication. Also incorporate a multimodal, non-pharmacologic approach whenever possible.
Tricyclic antidepressants
Although this class acts primarily by increasing serotonin levels, norepinephrine and dopamine also are affected depending on the particular medication. Studies have shown that amitriptyline, nortriptyline, and desipramine function well as analgesics independent of their antidepressant effects.5 TCAs may improve pain symptoms at lower therapeutic dosages than those used for treating depression.5
Although researchers have not elucidated TCAs’ mechanism of action with regards to analgesia, they are thought to act within the concept of the gating theory of pain control,6 which functions by activation and inhibition of pain signal transmission. It is believed TCAs act on nociceptive pathways by blocking serotonin and norepinephrine reuptake. Although researchers previously thought that TCAs’ analgesic mechanism was correlated to serotonin reuptake inhibition, this theory has changed. Selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine have not demonstrated substantial effectiveness in neuropathic pain when compared with TCAs and SNRIs. Recent studies have shown that TCAs may work by blocking sodium channels, similar to local anesthetics and antiarrhythmic agents.7
Psychiatrists prescribe TCAs infrequently because of these drugs’ unfavorable side effect profile compared with SSRIs and SNRIs. However, TCAs often are prescribed for pain management as an adjunct to other medications for neuropathic conditions and at lower dosages than those used for treating depression (Table 1).8
Table 1
Tricyclic antidepressants used to treat pain
| Drug | Dosage range for pain (off-label) | Comments |
|---|---|---|
| Amitriptyline | 10 to 100 mg/d | High sedation, high anticholinergic side effects |
| Amoxapine | 50 to 100 mg/d | Low sedation, moderate anticholinergic side effects |
| Clomipramine | 25 to 100 mg/d | Low sedation, low anticholinergic side effects |
| Desipramine | 25 to 100 mg/d | Low sedation, low anticholinergic side effects |
| Imipramine | 25 to 100 mg/d | Moderate sedation, moderate anticholinergic side effects |
| Nortriptyline | 10 to 75 mg/d | Moderate sedation, low anticholinergic side effects |
| Source: Reference 8 | ||
SNRIs
Evidence supports using duloxetine, a potent SNRI that mediates pain inhibition in the descending pathways, for 4 chronic pain conditions:
- diabetic peripheral neuropathic pain
- fibromyalgia
- mechanical low back pain
- pain associated with osteoarthritis.9
Titrate the dosage to 60 mg/d and maintain the patient at this dose for at least 4 weeks. Thereafter, according to patient response, the dosage may be titrated to 120 mg/d (off-label) with appropriate vital sign monitoring and routine lab analysis.
Venlafaxine also can mediate pain response in a similar manner to duloxetine, but is not FDA-approved for treating pain. Use caution when prescribing venlafaxine for patients with a history of hypertension. Milnacipran is a relatively new SNRI that has been shown to be effective in treating fibromyalgia in divided doses of 100 to 200 mg/d (Table 2).9-11
Table 2
Treating pain with serotonin-norepinephrine reuptake inhibitors
| Drug | Dosage range for pain | Comments |
|---|---|---|
| Duloxetine | 60 to 120 mg/d9 | FDA maximum recommended dose is 60 mg/d |
| Milnacipran | 25 to 200 mg/d10 | Approved for treating depression outside the United States |
| Venlafaxine | 75 to 225 mg/d11 | Monitor blood pressure, LFTs, and kidney function |
| LFTs: liver function tests | ||
Antiepileptic drugs
Several AEDs are used for pain management (Table 3).12-16 Gabapentin and pregabalin work by binding to voltage-gated calcium channels and decreasing excitatory neurotransmitter release. Along with TCAs, they are considered a first-line treatment for managing neuropathic pain.17 Gabapentin is FDA-approved for seizures and postherpetic neuralgia, but evidence supports its use in most types of neuropathic pain. Pregabalin is FDA-approved for treating seizures, diabetic peripheral neuropathy, central neuropathic pain, postherpetic neuralgia, and fibromyalgia.
Topiramate inhibits excitatory neurotransmission by enhancing the effects of gamma-aminobutyric acid, and also by blocking NMDA receptors. Topiramate is FDA-approved for seizures and migraine prophylaxis, and is used off-label for treating neuropathic pain. A 12-week trial of topiramate for diabetic neuropathy found significant analgesia in 50% of patients taking the drug, compared with 34% receiving placebo.18
Lamotrigine is approved for several types of seizures and maintenance of bipolar I disorder, and is used off-label for neuropathic pain. A recent Cochrane database review concluded that lamotrigine is ineffective for neuropathic pain14; however, some guidelines recommend using lamotrigine to treat neuropathies that do not respond to treatment with carbamazepine.19
Carbamazepine is a complex AED that is structurally similar to TCAs. It blocks sodium channels and has various pharmacologic properties, including anticholinergic, muscle relaxant, antidepressant, and sedative effects. Carbamazepine has analgesic effects through blockade of synaptic transmission in the trigeminal nucleus and is FDA-approved for seizures, bipolar disorder, neuropathic pain, and trigeminal neuralgia. In a systematic review of 12 trials of carbamazepine that included 4 placebo-controlled trials for trigeminal neuralgia, 2 studies showed a number needed to treat (NNT) of 1.8.20 For diabetic neuropathy, there was insufficient data to calculate NNT.
Oxcarbazepine, an analog of carbamazepine, also is FDA-approved for seizures and is used off-label for neuropathic pain. In the only double-blind trial with positive results, oxcarbazepine titrated to 1,800 mg/d reduced diabetic neuropathy pain scores on a visual analog scale by 24 points—roughly 25%.15
Table 3
Antiepileptic drugs for pain treatment
| Drug | Dosage range for pain | Comments |
|---|---|---|
| Carbamazepine | Starting dose: 100 mg twice a day, doses titrated to 400 to 800 mg/d usually are adequate. Maximum of 1,200 mg/d12 | Anticholinergic effects, blood dyscrasias, hyponatremia, increase in LFTs, ECG changes. CYP450 inducer, many DDIs |
| Gabapentin | Starting dose: 100 to 300 mg at bedtime or 100 to 300 mg 3 times a day, slow titration, maximum of 3,600 mg/d13 | Dizziness, sedation, weight gain, peripheral edema. Adjust dose in renal insufficiency |
| Lamotrigine | 200 to 400 mg/d14 | Sedation, headache, dizziness, ataxia, GI upset, blurred vision. Risk of life-threatening rash |
| Oxcarbazepine | Starting dose: 300 mg/d, then titrated as tolerated to a maximum of 1,800 mg/d15 | Adverse drug reactions similar to carbamazepine, less anticholinergic effects, more hyponatremia. Fewer DDIs than carbamazepine |
| Pregabalin | Starting dose: 50 mg 3 times a day or 75 mg twice a day, may increase every 3 to 7 days as tolerated, maximum of 600 mg/d13 | Same adverse drug reactions as gabapentin, less sedation. Adjust dose in renal insufficiency. More costly than gabapentin |
| Topiramate | Starting dose: 12.5 to 25 mg once or twice a day for 4 weeks; then double the dose every 4 weeks to reach a maximum dose of 100 to 200 mg/d in divided doses16 | Weight loss, anorexia, nephrolithiasis, cognitive impairment |
| CYP450: cytochrome P450; DDIs: drug-drug interactions; GI: gastrointestinal; LFTs: liver function tests | ||
Non-opioid analgesics
NSAIDs have antipyretic, analgesic, and anti-inflammatory effects and are used for fever, headache, mild-to-moderate pain, musculoskeletal pain, menstrual pain, and dental pain. They are particularly useful in treating acute pain, often in combination with opioid analgesics. NSAIDs exert their analgesic action through blockade of prostaglandin production via reversible inhibition of cyclooxygenase-1 and cyclooxygenase-2.
The most common side effects of NSAIDs are the result of gastrointestinal (GI) toxicity and include dyspepsia, heartburn, nausea, anorexia, and epigastric pain.21 GI ulceration and bleeding are rare but serious complications. To decrease these risks, tell patients to take NSAIDs with food. Add a GI protective agent, such as an H2 blocker or proton pump inhibitor, for patients at higher risk for GI complications.22
In addition, inhibition of renal prostaglandins by NSAIDs can cause renal toxicity, fluid retention, and edema, potentially exacerbating existing cardiovascular conditions such as hypertension and heart failure. NSAIDs may increase the risk of serious thrombotic events such as myocardial infarction and stroke. Use NSAIDs at the lowest effective dose for the shortest duration possible and generally avoid prescribing in patients at high risk for cardiovascular disease and pregnant women, especially those in their third trimester.23,24
NSAIDs may cause pharmacodynamic and pharmacokinetic drug-drug interactions. The risk of GI toxicity and bleeding increases when NSAIDs are administered with drugs that also irritate the gastric mucosa or have antiplatelet/anticoagulant effects.21 Plasma concentrations of drugs with a narrow therapeutic index that are renally eliminated, such as methotrexate and lithium, can increase to potentially toxic levels with concurrent NSAID use because NSAIDs decrease renal perfusion.21 Also, the therapeutic effects of antihypertensives may be attenuated because NSAIDs cause fluid retention.25
Acetaminophen (APAP) is available in several dosage forms as a single ingredient and in combination with opioids in prescription products. For more information about APAP, see the Box below.
Atypical antipsychotics
Although atypical antipsychotics are not often used to treat pain, studies indicate that fibromyalgia patients may benefit from ziprasidone26 and olanzapine,27 most often as an adjunctive treatment rather than monotherapy. Randomized controlled studies indicate poor tolerability with several atypical antipsychotics. Weight gain, akathisia, and somnolence are side effects of some atypical antipsychotics. Additionally, ziprasidone has been associated with QTc prolongation. For chronic pain patients, atypical antipsychotics are most useful for treating psychiatric comorbidities.
Although its mechanism of action is not well understood, acetaminophen (APAP) works by blocking prostaglandin syntheses via inhibition of cyclooxygenase-1 and cyclooxygenase-2 in the CNS.a Therefore, in contrast to NSAIDs, APAP does not possess peripheral anti-inflammatory effects or affect platelet function and is effective for treating fever, headache, and acute and chronic mild pain. The American Geriatrics Society recommends APAP for minor and persistent pain in older patientsb and the American College of Rheumatology recommends it as first-line therapy for osteoarthritis of the hip or knee.c
APAP has few clinically significant drug interactions, an excellent safety profile, and a long history of safe and effective use. When used within the recommended dosage range, APAP has few side effects. However, overuse of APAP is the leading cause of acute liver failure in the United States.d APAP hepatotoxicity can be accompanied by nephrotoxicity, is dose-dependent, and can be caused by acute overdose or chronic ingestion at doses over the recommended maximum of 4 g/d. Patients have experienced elevated liver transaminases with coadministration of APAP with phenytoin and phenobarbital.e,f Alcohol and other potentially hepatotoxic drugs also can increase the risk of liver toxicity when combined with APAP.d APAP is pregnancy category B and is considered the drug of choice for treating pain or fever during pregnancy and breast-feeding.g
References
- Amadio P Jr. Peripherally acting analgesics. Am J Med. 1984;77(3A):17-26.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43(9):1905-1915.
- Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364-1372.
- Pirotte JH. Apparent potentiation of hepatotoxicity from small doses of acetaminophen by phenobarbital. Ann Intern Med. 1984;101(3):403.
- Brackett CC, Bloch JD. Phenytoin as a possible cause of acetaminophen hepatotoxicity: case report and review of the literature. Pharmacotherapy. 2000;20(2):229-233.
- Hersh EV, Moore PA, Ross GL. Over-the-counter analgesics and antipyretics: a critical assessment. Clin Ther. 2000; 22(5):500-548.
Related Resources
- Leo RJ. Chronic nonmalignant pain: How to ‘turn down’ its physiologic triggers. Current Psychiatry. 2008;7(8):19-36.
- Nikolaus T, Zeyfang A. Pharmacological treatments for persistent non-malignant pain in older persons. Drugs Aging. 2004;21(1):19-41.
- World Health Organization. WHO’s pain ladder. www.who.int/cancer/palliative/painladder/en.
Drug Brand Names
- Acetaminophen • Tylenol
- Amitriptyline • Elavil, others
- Amoxapine • Asendin
- Carbamazepine • Tegretol, Carbatrol, others
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin, Gralise
- Imipramine • Tofranil
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Methotrexate • Rheumatrex, Trexall
- Milnacipran • Savella
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Pregabalin • Lyrica
- Topiramate • Topamax, Topiragen
- Venlafaxine • Effexor
- iprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Of the 56 million American adults who report living with chronic pain almost 60% also exhibit psychiatric disorders such as depression or anxiety.1,2 Because patients with chronic pain suffer from a mixture of physical and psychological components, managing such conditions is complicated, and using opioids is tempting. However, treatment needs to address the underlying pathology along with social and psychological factors.
Because substance abuse treatment admissions increased by 400% from 1998 to 2008,3 many physicians look to non-opioids and other treatment modalities to control chronic non-cancer pain. Common pharmacologic therapies used to treat chronic pain include tricyclic antidepressants (TCAs), serotonin-norepinephrine reuptake inhibitors (SNRIs), antiepileptic drugs (AEDs), nonsteroidal anti-inflammatory drugs (NSAIDs), and, to a lesser extent, atypical antipsychotics. TCAs, SNRIs, AEDs, NSAIDs, and atypical antipsychotics influence a variety of presumed underlying pathophysiological processes, including inflammatory mediators, activity of N-methyl-d-aspartate (NMDA) receptors, and voltage-gated calcium channels. In addition, they increase activity of descending inhibitory pain pathways. Animal studies suggest dysfunction of these inhibitory mechanisms contributes to the central sensitization and hyperexcitability of pain transmitting pathways.4
In this article, we discuss psychotropics and other non-opioid agents for treating pain. However, no single solution is best for all patients with chronic pain and this article is not a “how to” guide to avoid administering opioid medication. Also incorporate a multimodal, non-pharmacologic approach whenever possible.
Tricyclic antidepressants
Although this class acts primarily by increasing serotonin levels, norepinephrine and dopamine also are affected depending on the particular medication. Studies have shown that amitriptyline, nortriptyline, and desipramine function well as analgesics independent of their antidepressant effects.5 TCAs may improve pain symptoms at lower therapeutic dosages than those used for treating depression.5
Although researchers have not elucidated TCAs’ mechanism of action with regards to analgesia, they are thought to act within the concept of the gating theory of pain control,6 which functions by activation and inhibition of pain signal transmission. It is believed TCAs act on nociceptive pathways by blocking serotonin and norepinephrine reuptake. Although researchers previously thought that TCAs’ analgesic mechanism was correlated to serotonin reuptake inhibition, this theory has changed. Selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine have not demonstrated substantial effectiveness in neuropathic pain when compared with TCAs and SNRIs. Recent studies have shown that TCAs may work by blocking sodium channels, similar to local anesthetics and antiarrhythmic agents.7
Psychiatrists prescribe TCAs infrequently because of these drugs’ unfavorable side effect profile compared with SSRIs and SNRIs. However, TCAs often are prescribed for pain management as an adjunct to other medications for neuropathic conditions and at lower dosages than those used for treating depression (Table 1).8
Table 1
Tricyclic antidepressants used to treat pain
| Drug | Dosage range for pain (off-label) | Comments |
|---|---|---|
| Amitriptyline | 10 to 100 mg/d | High sedation, high anticholinergic side effects |
| Amoxapine | 50 to 100 mg/d | Low sedation, moderate anticholinergic side effects |
| Clomipramine | 25 to 100 mg/d | Low sedation, low anticholinergic side effects |
| Desipramine | 25 to 100 mg/d | Low sedation, low anticholinergic side effects |
| Imipramine | 25 to 100 mg/d | Moderate sedation, moderate anticholinergic side effects |
| Nortriptyline | 10 to 75 mg/d | Moderate sedation, low anticholinergic side effects |
| Source: Reference 8 | ||
SNRIs
Evidence supports using duloxetine, a potent SNRI that mediates pain inhibition in the descending pathways, for 4 chronic pain conditions:
- diabetic peripheral neuropathic pain
- fibromyalgia
- mechanical low back pain
- pain associated with osteoarthritis.9
Titrate the dosage to 60 mg/d and maintain the patient at this dose for at least 4 weeks. Thereafter, according to patient response, the dosage may be titrated to 120 mg/d (off-label) with appropriate vital sign monitoring and routine lab analysis.
Venlafaxine also can mediate pain response in a similar manner to duloxetine, but is not FDA-approved for treating pain. Use caution when prescribing venlafaxine for patients with a history of hypertension. Milnacipran is a relatively new SNRI that has been shown to be effective in treating fibromyalgia in divided doses of 100 to 200 mg/d (Table 2).9-11
Table 2
Treating pain with serotonin-norepinephrine reuptake inhibitors
| Drug | Dosage range for pain | Comments |
|---|---|---|
| Duloxetine | 60 to 120 mg/d9 | FDA maximum recommended dose is 60 mg/d |
| Milnacipran | 25 to 200 mg/d10 | Approved for treating depression outside the United States |
| Venlafaxine | 75 to 225 mg/d11 | Monitor blood pressure, LFTs, and kidney function |
| LFTs: liver function tests | ||
Antiepileptic drugs
Several AEDs are used for pain management (Table 3).12-16 Gabapentin and pregabalin work by binding to voltage-gated calcium channels and decreasing excitatory neurotransmitter release. Along with TCAs, they are considered a first-line treatment for managing neuropathic pain.17 Gabapentin is FDA-approved for seizures and postherpetic neuralgia, but evidence supports its use in most types of neuropathic pain. Pregabalin is FDA-approved for treating seizures, diabetic peripheral neuropathy, central neuropathic pain, postherpetic neuralgia, and fibromyalgia.
Topiramate inhibits excitatory neurotransmission by enhancing the effects of gamma-aminobutyric acid, and also by blocking NMDA receptors. Topiramate is FDA-approved for seizures and migraine prophylaxis, and is used off-label for treating neuropathic pain. A 12-week trial of topiramate for diabetic neuropathy found significant analgesia in 50% of patients taking the drug, compared with 34% receiving placebo.18
Lamotrigine is approved for several types of seizures and maintenance of bipolar I disorder, and is used off-label for neuropathic pain. A recent Cochrane database review concluded that lamotrigine is ineffective for neuropathic pain14; however, some guidelines recommend using lamotrigine to treat neuropathies that do not respond to treatment with carbamazepine.19
Carbamazepine is a complex AED that is structurally similar to TCAs. It blocks sodium channels and has various pharmacologic properties, including anticholinergic, muscle relaxant, antidepressant, and sedative effects. Carbamazepine has analgesic effects through blockade of synaptic transmission in the trigeminal nucleus and is FDA-approved for seizures, bipolar disorder, neuropathic pain, and trigeminal neuralgia. In a systematic review of 12 trials of carbamazepine that included 4 placebo-controlled trials for trigeminal neuralgia, 2 studies showed a number needed to treat (NNT) of 1.8.20 For diabetic neuropathy, there was insufficient data to calculate NNT.
Oxcarbazepine, an analog of carbamazepine, also is FDA-approved for seizures and is used off-label for neuropathic pain. In the only double-blind trial with positive results, oxcarbazepine titrated to 1,800 mg/d reduced diabetic neuropathy pain scores on a visual analog scale by 24 points—roughly 25%.15
Table 3
Antiepileptic drugs for pain treatment
| Drug | Dosage range for pain | Comments |
|---|---|---|
| Carbamazepine | Starting dose: 100 mg twice a day, doses titrated to 400 to 800 mg/d usually are adequate. Maximum of 1,200 mg/d12 | Anticholinergic effects, blood dyscrasias, hyponatremia, increase in LFTs, ECG changes. CYP450 inducer, many DDIs |
| Gabapentin | Starting dose: 100 to 300 mg at bedtime or 100 to 300 mg 3 times a day, slow titration, maximum of 3,600 mg/d13 | Dizziness, sedation, weight gain, peripheral edema. Adjust dose in renal insufficiency |
| Lamotrigine | 200 to 400 mg/d14 | Sedation, headache, dizziness, ataxia, GI upset, blurred vision. Risk of life-threatening rash |
| Oxcarbazepine | Starting dose: 300 mg/d, then titrated as tolerated to a maximum of 1,800 mg/d15 | Adverse drug reactions similar to carbamazepine, less anticholinergic effects, more hyponatremia. Fewer DDIs than carbamazepine |
| Pregabalin | Starting dose: 50 mg 3 times a day or 75 mg twice a day, may increase every 3 to 7 days as tolerated, maximum of 600 mg/d13 | Same adverse drug reactions as gabapentin, less sedation. Adjust dose in renal insufficiency. More costly than gabapentin |
| Topiramate | Starting dose: 12.5 to 25 mg once or twice a day for 4 weeks; then double the dose every 4 weeks to reach a maximum dose of 100 to 200 mg/d in divided doses16 | Weight loss, anorexia, nephrolithiasis, cognitive impairment |
| CYP450: cytochrome P450; DDIs: drug-drug interactions; GI: gastrointestinal; LFTs: liver function tests | ||
Non-opioid analgesics
NSAIDs have antipyretic, analgesic, and anti-inflammatory effects and are used for fever, headache, mild-to-moderate pain, musculoskeletal pain, menstrual pain, and dental pain. They are particularly useful in treating acute pain, often in combination with opioid analgesics. NSAIDs exert their analgesic action through blockade of prostaglandin production via reversible inhibition of cyclooxygenase-1 and cyclooxygenase-2.
The most common side effects of NSAIDs are the result of gastrointestinal (GI) toxicity and include dyspepsia, heartburn, nausea, anorexia, and epigastric pain.21 GI ulceration and bleeding are rare but serious complications. To decrease these risks, tell patients to take NSAIDs with food. Add a GI protective agent, such as an H2 blocker or proton pump inhibitor, for patients at higher risk for GI complications.22
In addition, inhibition of renal prostaglandins by NSAIDs can cause renal toxicity, fluid retention, and edema, potentially exacerbating existing cardiovascular conditions such as hypertension and heart failure. NSAIDs may increase the risk of serious thrombotic events such as myocardial infarction and stroke. Use NSAIDs at the lowest effective dose for the shortest duration possible and generally avoid prescribing in patients at high risk for cardiovascular disease and pregnant women, especially those in their third trimester.23,24
NSAIDs may cause pharmacodynamic and pharmacokinetic drug-drug interactions. The risk of GI toxicity and bleeding increases when NSAIDs are administered with drugs that also irritate the gastric mucosa or have antiplatelet/anticoagulant effects.21 Plasma concentrations of drugs with a narrow therapeutic index that are renally eliminated, such as methotrexate and lithium, can increase to potentially toxic levels with concurrent NSAID use because NSAIDs decrease renal perfusion.21 Also, the therapeutic effects of antihypertensives may be attenuated because NSAIDs cause fluid retention.25
Acetaminophen (APAP) is available in several dosage forms as a single ingredient and in combination with opioids in prescription products. For more information about APAP, see the Box below.
Atypical antipsychotics
Although atypical antipsychotics are not often used to treat pain, studies indicate that fibromyalgia patients may benefit from ziprasidone26 and olanzapine,27 most often as an adjunctive treatment rather than monotherapy. Randomized controlled studies indicate poor tolerability with several atypical antipsychotics. Weight gain, akathisia, and somnolence are side effects of some atypical antipsychotics. Additionally, ziprasidone has been associated with QTc prolongation. For chronic pain patients, atypical antipsychotics are most useful for treating psychiatric comorbidities.
Although its mechanism of action is not well understood, acetaminophen (APAP) works by blocking prostaglandin syntheses via inhibition of cyclooxygenase-1 and cyclooxygenase-2 in the CNS.a Therefore, in contrast to NSAIDs, APAP does not possess peripheral anti-inflammatory effects or affect platelet function and is effective for treating fever, headache, and acute and chronic mild pain. The American Geriatrics Society recommends APAP for minor and persistent pain in older patientsb and the American College of Rheumatology recommends it as first-line therapy for osteoarthritis of the hip or knee.c
APAP has few clinically significant drug interactions, an excellent safety profile, and a long history of safe and effective use. When used within the recommended dosage range, APAP has few side effects. However, overuse of APAP is the leading cause of acute liver failure in the United States.d APAP hepatotoxicity can be accompanied by nephrotoxicity, is dose-dependent, and can be caused by acute overdose or chronic ingestion at doses over the recommended maximum of 4 g/d. Patients have experienced elevated liver transaminases with coadministration of APAP with phenytoin and phenobarbital.e,f Alcohol and other potentially hepatotoxic drugs also can increase the risk of liver toxicity when combined with APAP.d APAP is pregnancy category B and is considered the drug of choice for treating pain or fever during pregnancy and breast-feeding.g
References
- Amadio P Jr. Peripherally acting analgesics. Am J Med. 1984;77(3A):17-26.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43(9):1905-1915.
- Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364-1372.
- Pirotte JH. Apparent potentiation of hepatotoxicity from small doses of acetaminophen by phenobarbital. Ann Intern Med. 1984;101(3):403.
- Brackett CC, Bloch JD. Phenytoin as a possible cause of acetaminophen hepatotoxicity: case report and review of the literature. Pharmacotherapy. 2000;20(2):229-233.
- Hersh EV, Moore PA, Ross GL. Over-the-counter analgesics and antipyretics: a critical assessment. Clin Ther. 2000; 22(5):500-548.
Related Resources
- Leo RJ. Chronic nonmalignant pain: How to ‘turn down’ its physiologic triggers. Current Psychiatry. 2008;7(8):19-36.
- Nikolaus T, Zeyfang A. Pharmacological treatments for persistent non-malignant pain in older persons. Drugs Aging. 2004;21(1):19-41.
- World Health Organization. WHO’s pain ladder. www.who.int/cancer/palliative/painladder/en.
Drug Brand Names
- Acetaminophen • Tylenol
- Amitriptyline • Elavil, others
- Amoxapine • Asendin
- Carbamazepine • Tegretol, Carbatrol, others
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin, Gralise
- Imipramine • Tofranil
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Methotrexate • Rheumatrex, Trexall
- Milnacipran • Savella
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Pregabalin • Lyrica
- Topiramate • Topamax, Topiragen
- Venlafaxine • Effexor
- iprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg. 2007;105(1):205-221.
2. Thieme K, Turk DC, Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: relationship to somatic and psychosocial variables. Psychosom Med. 2004;66(6):837-844.
3. Substance Abuse and Mental Health Services Administration, Office of Applied Studies Treatment episode data set (TEDS). 1998-2008. National admissions to substance abuse treatment services. Rockville MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2010.
4. Iyengar S, Webster AA, Hemrick-Luecke SK, et al. Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther. 2004;311(2):576-584.
5. Guay DR. Adjunctive agents in the management of chronic pain. Pharmacotherapy. 2001;21(9):1070-1081.
6. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399-409.
7. Dick IE, Brochu RM, Purohit Y, et al. Sodium channel blockade may contribute to the analgesic efficacy of antidepressants. J Pain. 2007;8(4):315-324.
8. Stahl SM. Essential psychopharmacology: the prescriber’s guide. New York NY: Cambridge University Press; 2006.
9. Skljarevski V, Desaiah D, Liu-Seifert H, et al. Efficacy and safety of duloxetine in patients with chronic low back pain. Spine (Phila Pa 1976). 2010;35(13):E578-E585.
10. Hsu ES. Acute and chronic pain management in fibromyalgia: updates on pharmacotherapy. Am J Ther. 2011;18(6):487-509.
11. Bomholt SF, Mikkelsen JD, Blackburn-Munro G. Antinociceptive effects of the antidepressants amitriptyline duloxetine, mirtazapine and citalopram in animal models of acute, persistent and neuropathic pain. Neuropharmacology. 2005;48(2):252-263.
12. Campbell FG, Graham JG, Zilkha KJ. Clinical trial of carbazepine (tegretol) in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1966;29(3):265-267.
13. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(10 suppl):S22-S32.
14. Dogra S, Beydoun S, Mazzola J, et al. Oxcarbazepine in painful diabetic neuropathy: a randomized, placebo-controlled study. Eur J Pain. 2005;9(5):543-554.
15. Kline KM, Carroll DG, Malnar KF. Painful diabetic peripheral neuropathy relieved with use of oral topiramate. South Med J. 2003;96(6):602-605.
16. Wiffen PJ, Derry S, Moore RA. Lamotrigine for acute and chronic pain. Cochrane Database Syst Rev. 2011;(2):CD006044.-
17. Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 suppl):S3-S14.
18. Raskin P, Donofrio PD, Rosenthal NR, et al. Topiramate vs placebo in painful diabetic neuropathy: analgesic and metabolic effects. Neurology. 2004;63(5):865-873.
19. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13-21.
20. Wiffen PJ, Derry S, Moore RA, et al. Carbamazepine for acute and chronic pain in adults. Cochrane Database Syst Rev. 2011;(1):CD005451.-
21. Hersh EV, Moore PA, Ross GL. Over-the-counter analgesics and antipyretics: a critical assessment. Clin Ther. 2000;22(5):500-548.
22. Lanas AI. Current approaches to reducing gastrointestinal toxicity of low-dose aspirin. Am J Med. 2001;110(1A):70S-73S.
23. Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634-1642.
24. Briggs G, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation. 8th ed. Baltimore MD: Lippincott Williams and Wilkins; 2008.
25. Frishman WH. Effects of nonsteroidal anti-inflammatory drug therapy on blood pressure and peripheral edema. Am J Cardiol. 2002;89(6A):18D-25D.
26. Calandre EP, Hidalgo J, Rico-Villademoros F. Use of ziprasidone in patients with fibromyalgia: a case series. Rheumatol Int. 2007;27(5):473-476.
27. Rico-Villademoros F, Hidalgo J, Dominguez I, et al. Atypical antipsychotics in the treatment of fibromyalgia: a case series with olanzapine. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(1):161-164.
1. Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg. 2007;105(1):205-221.
2. Thieme K, Turk DC, Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: relationship to somatic and psychosocial variables. Psychosom Med. 2004;66(6):837-844.
3. Substance Abuse and Mental Health Services Administration, Office of Applied Studies Treatment episode data set (TEDS). 1998-2008. National admissions to substance abuse treatment services. Rockville MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2010.
4. Iyengar S, Webster AA, Hemrick-Luecke SK, et al. Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther. 2004;311(2):576-584.
5. Guay DR. Adjunctive agents in the management of chronic pain. Pharmacotherapy. 2001;21(9):1070-1081.
6. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399-409.
7. Dick IE, Brochu RM, Purohit Y, et al. Sodium channel blockade may contribute to the analgesic efficacy of antidepressants. J Pain. 2007;8(4):315-324.
8. Stahl SM. Essential psychopharmacology: the prescriber’s guide. New York NY: Cambridge University Press; 2006.
9. Skljarevski V, Desaiah D, Liu-Seifert H, et al. Efficacy and safety of duloxetine in patients with chronic low back pain. Spine (Phila Pa 1976). 2010;35(13):E578-E585.
10. Hsu ES. Acute and chronic pain management in fibromyalgia: updates on pharmacotherapy. Am J Ther. 2011;18(6):487-509.
11. Bomholt SF, Mikkelsen JD, Blackburn-Munro G. Antinociceptive effects of the antidepressants amitriptyline duloxetine, mirtazapine and citalopram in animal models of acute, persistent and neuropathic pain. Neuropharmacology. 2005;48(2):252-263.
12. Campbell FG, Graham JG, Zilkha KJ. Clinical trial of carbazepine (tegretol) in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1966;29(3):265-267.
13. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(10 suppl):S22-S32.
14. Dogra S, Beydoun S, Mazzola J, et al. Oxcarbazepine in painful diabetic neuropathy: a randomized, placebo-controlled study. Eur J Pain. 2005;9(5):543-554.
15. Kline KM, Carroll DG, Malnar KF. Painful diabetic peripheral neuropathy relieved with use of oral topiramate. South Med J. 2003;96(6):602-605.
16. Wiffen PJ, Derry S, Moore RA. Lamotrigine for acute and chronic pain. Cochrane Database Syst Rev. 2011;(2):CD006044.-
17. Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 suppl):S3-S14.
18. Raskin P, Donofrio PD, Rosenthal NR, et al. Topiramate vs placebo in painful diabetic neuropathy: analgesic and metabolic effects. Neurology. 2004;63(5):865-873.
19. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13-21.
20. Wiffen PJ, Derry S, Moore RA, et al. Carbamazepine for acute and chronic pain in adults. Cochrane Database Syst Rev. 2011;(1):CD005451.-
21. Hersh EV, Moore PA, Ross GL. Over-the-counter analgesics and antipyretics: a critical assessment. Clin Ther. 2000;22(5):500-548.
22. Lanas AI. Current approaches to reducing gastrointestinal toxicity of low-dose aspirin. Am J Med. 2001;110(1A):70S-73S.
23. Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634-1642.
24. Briggs G, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation. 8th ed. Baltimore MD: Lippincott Williams and Wilkins; 2008.
25. Frishman WH. Effects of nonsteroidal anti-inflammatory drug therapy on blood pressure and peripheral edema. Am J Cardiol. 2002;89(6A):18D-25D.
26. Calandre EP, Hidalgo J, Rico-Villademoros F. Use of ziprasidone in patients with fibromyalgia: a case series. Rheumatol Int. 2007;27(5):473-476.
27. Rico-Villademoros F, Hidalgo J, Dominguez I, et al. Atypical antipsychotics in the treatment of fibromyalgia: a case series with olanzapine. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(1):161-164.
Mood instability in ADHD
Discuss this article at www.facebook.com/CurrentPsychiatry
Dr. Goldberg makes an important point that not all mood lability indicates bipolar disorder (BD) in “Ultra-rapid cycling bipolar disorder: A critical look” (Current Psychiatry, December 2011, p. 42-52).
However, there was 1 significant diagnostic omission. Patients with adult attention-deficit/hyperactivity disorder (ADHD) can present with an unremarkable mental status exam, yet can give a history of abrupt episodes of dyscontrol, often in interpersonal situations. As opposed to children manifesting ADHD, where comorbidity with BD is substantial, adults may primarily display impulsivity rather than hyperactivity or inattention. By ignoring this diagnostic consideration, important pharmacotherapeutic options have been discarded, although cognitive-behavioral therapy and dialectical behavior therapy for “borderline” patients are always relevant. Regardless of diagnostic terms and the fate of DSM-5, our treatment approach serves to strengthen prefrontal cortex inhibitory activity and block limbic system reactivity.
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
Dr. Goldberg responds
Drs. Bunt and Barris each raise the clinically and theoretically interesting observation that in patients whose childhood attention-deficit/hyperactivity disorder (ADHD) persists into adulthood, affective instability may be a prominent feature. Consequently, they advise that complaints of frequent mood swings within 1 day should alert clinicians to consider ADHD in their differential diagnosis.
Importantly, emotional dysregulation is not an established criterion for ADHD, although investigators have begun to study impaired emotional processing in adults with ADHD.1 Because observational research examining emotional dysregulation in adult ADHD is preliminary, I cannot concur with Dr. Bunt’s assertion that “an omission of this sort does a disservice to the field.”
To the contrary, it would seem premature to counsel practitioners to look for mood instability as a red flag for adult ADHD. In fact, given the nontrivial rates of comorbid mood disorders with ADHD as cited by Dr. Bunt, it’s plausible that mood instability co-occurring with ADHD simply may be the epiphenomenon of a psychiatric comorbidity such as borderline personality disorder,2 a disruptive behavior disorder,3 or substance abuse.3
Moreover, endophenotype studies suggest that emotional lability and ADHD do not cosegregate in families.3 Further research is needed to determine whether moment-to-moment mood fluctuations are an intrinsic feature of ADHD that is not better accounted for by another accompanying condition.
Dr. Bunt appears to have misconstrued my use of the term “validation” with respect to ultra-rapid cycling (URC) as if I had been referring to validation of URC as a diagnosis—which I never suggested—rather than as a putative course modifier or specifier in an otherwise-diagnosed bipolar disorder patient—as was the case when researchers empirically validated rapid cycling (RC) as a bipolar course specifier, leading to its inclusion in DSM-IV.4 To my knowledge there’s no movement to consider URC as a bipolar course specifier in DSM-5, which would be a difficult undertaking in the absence of field trials such as those conducted for bipolar RC.
Drs. Barris, Bunt, and I seem to agree that mood shifts occurring on a daily or more frequent basis constitute a non-pathognomonic phenomenon for which “careful evaluation” is necessary to discern the broader psychopathologic condition and context in which it arises.
Joseph F. Goldberg, MD
Associate Clinical Professor of Psychiatry
Mt. Sinai School of Medicine
New York, NY
References
1. Herrmann MJ, Biehl SC, Jacob C, et al. Neurobiological and psychophysiological correlates of emotional dysregulation in ADHD patients. Atten Defic Hyperact Disord. 2010;2(4):233-239.
2. Philipsen A, Feige B, Hesslinger B, et al. Borderline typical symptoms in adult patients with attention deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2009;1(1):11-18.
3. Sobanski E, Banaschewski T, Asherson P, et al. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry. 2010;51(8):915-923.
4. Bauer MS, Calabrese J, Dunner DL, et al. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151(4):506-515.
Discuss this article at www.facebook.com/CurrentPsychiatry
Dr. Goldberg makes an important point that not all mood lability indicates bipolar disorder (BD) in “Ultra-rapid cycling bipolar disorder: A critical look” (Current Psychiatry, December 2011, p. 42-52).
However, there was 1 significant diagnostic omission. Patients with adult attention-deficit/hyperactivity disorder (ADHD) can present with an unremarkable mental status exam, yet can give a history of abrupt episodes of dyscontrol, often in interpersonal situations. As opposed to children manifesting ADHD, where comorbidity with BD is substantial, adults may primarily display impulsivity rather than hyperactivity or inattention. By ignoring this diagnostic consideration, important pharmacotherapeutic options have been discarded, although cognitive-behavioral therapy and dialectical behavior therapy for “borderline” patients are always relevant. Regardless of diagnostic terms and the fate of DSM-5, our treatment approach serves to strengthen prefrontal cortex inhibitory activity and block limbic system reactivity.
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
Dr. Goldberg responds
Drs. Bunt and Barris each raise the clinically and theoretically interesting observation that in patients whose childhood attention-deficit/hyperactivity disorder (ADHD) persists into adulthood, affective instability may be a prominent feature. Consequently, they advise that complaints of frequent mood swings within 1 day should alert clinicians to consider ADHD in their differential diagnosis.
Importantly, emotional dysregulation is not an established criterion for ADHD, although investigators have begun to study impaired emotional processing in adults with ADHD.1 Because observational research examining emotional dysregulation in adult ADHD is preliminary, I cannot concur with Dr. Bunt’s assertion that “an omission of this sort does a disservice to the field.”
To the contrary, it would seem premature to counsel practitioners to look for mood instability as a red flag for adult ADHD. In fact, given the nontrivial rates of comorbid mood disorders with ADHD as cited by Dr. Bunt, it’s plausible that mood instability co-occurring with ADHD simply may be the epiphenomenon of a psychiatric comorbidity such as borderline personality disorder,2 a disruptive behavior disorder,3 or substance abuse.3
Moreover, endophenotype studies suggest that emotional lability and ADHD do not cosegregate in families.3 Further research is needed to determine whether moment-to-moment mood fluctuations are an intrinsic feature of ADHD that is not better accounted for by another accompanying condition.
Dr. Bunt appears to have misconstrued my use of the term “validation” with respect to ultra-rapid cycling (URC) as if I had been referring to validation of URC as a diagnosis—which I never suggested—rather than as a putative course modifier or specifier in an otherwise-diagnosed bipolar disorder patient—as was the case when researchers empirically validated rapid cycling (RC) as a bipolar course specifier, leading to its inclusion in DSM-IV.4 To my knowledge there’s no movement to consider URC as a bipolar course specifier in DSM-5, which would be a difficult undertaking in the absence of field trials such as those conducted for bipolar RC.
Drs. Barris, Bunt, and I seem to agree that mood shifts occurring on a daily or more frequent basis constitute a non-pathognomonic phenomenon for which “careful evaluation” is necessary to discern the broader psychopathologic condition and context in which it arises.
Joseph F. Goldberg, MD
Associate Clinical Professor of Psychiatry
Mt. Sinai School of Medicine
New York, NY
References
1. Herrmann MJ, Biehl SC, Jacob C, et al. Neurobiological and psychophysiological correlates of emotional dysregulation in ADHD patients. Atten Defic Hyperact Disord. 2010;2(4):233-239.
2. Philipsen A, Feige B, Hesslinger B, et al. Borderline typical symptoms in adult patients with attention deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2009;1(1):11-18.
3. Sobanski E, Banaschewski T, Asherson P, et al. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry. 2010;51(8):915-923.
4. Bauer MS, Calabrese J, Dunner DL, et al. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151(4):506-515.
Discuss this article at www.facebook.com/CurrentPsychiatry
Dr. Goldberg makes an important point that not all mood lability indicates bipolar disorder (BD) in “Ultra-rapid cycling bipolar disorder: A critical look” (Current Psychiatry, December 2011, p. 42-52).
However, there was 1 significant diagnostic omission. Patients with adult attention-deficit/hyperactivity disorder (ADHD) can present with an unremarkable mental status exam, yet can give a history of abrupt episodes of dyscontrol, often in interpersonal situations. As opposed to children manifesting ADHD, where comorbidity with BD is substantial, adults may primarily display impulsivity rather than hyperactivity or inattention. By ignoring this diagnostic consideration, important pharmacotherapeutic options have been discarded, although cognitive-behavioral therapy and dialectical behavior therapy for “borderline” patients are always relevant. Regardless of diagnostic terms and the fate of DSM-5, our treatment approach serves to strengthen prefrontal cortex inhibitory activity and block limbic system reactivity.
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
Dr. Goldberg responds
Drs. Bunt and Barris each raise the clinically and theoretically interesting observation that in patients whose childhood attention-deficit/hyperactivity disorder (ADHD) persists into adulthood, affective instability may be a prominent feature. Consequently, they advise that complaints of frequent mood swings within 1 day should alert clinicians to consider ADHD in their differential diagnosis.
Importantly, emotional dysregulation is not an established criterion for ADHD, although investigators have begun to study impaired emotional processing in adults with ADHD.1 Because observational research examining emotional dysregulation in adult ADHD is preliminary, I cannot concur with Dr. Bunt’s assertion that “an omission of this sort does a disservice to the field.”
To the contrary, it would seem premature to counsel practitioners to look for mood instability as a red flag for adult ADHD. In fact, given the nontrivial rates of comorbid mood disorders with ADHD as cited by Dr. Bunt, it’s plausible that mood instability co-occurring with ADHD simply may be the epiphenomenon of a psychiatric comorbidity such as borderline personality disorder,2 a disruptive behavior disorder,3 or substance abuse.3
Moreover, endophenotype studies suggest that emotional lability and ADHD do not cosegregate in families.3 Further research is needed to determine whether moment-to-moment mood fluctuations are an intrinsic feature of ADHD that is not better accounted for by another accompanying condition.
Dr. Bunt appears to have misconstrued my use of the term “validation” with respect to ultra-rapid cycling (URC) as if I had been referring to validation of URC as a diagnosis—which I never suggested—rather than as a putative course modifier or specifier in an otherwise-diagnosed bipolar disorder patient—as was the case when researchers empirically validated rapid cycling (RC) as a bipolar course specifier, leading to its inclusion in DSM-IV.4 To my knowledge there’s no movement to consider URC as a bipolar course specifier in DSM-5, which would be a difficult undertaking in the absence of field trials such as those conducted for bipolar RC.
Drs. Barris, Bunt, and I seem to agree that mood shifts occurring on a daily or more frequent basis constitute a non-pathognomonic phenomenon for which “careful evaluation” is necessary to discern the broader psychopathologic condition and context in which it arises.
Joseph F. Goldberg, MD
Associate Clinical Professor of Psychiatry
Mt. Sinai School of Medicine
New York, NY
References
1. Herrmann MJ, Biehl SC, Jacob C, et al. Neurobiological and psychophysiological correlates of emotional dysregulation in ADHD patients. Atten Defic Hyperact Disord. 2010;2(4):233-239.
2. Philipsen A, Feige B, Hesslinger B, et al. Borderline typical symptoms in adult patients with attention deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2009;1(1):11-18.
3. Sobanski E, Banaschewski T, Asherson P, et al. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry. 2010;51(8):915-923.
4. Bauer MS, Calabrese J, Dunner DL, et al. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151(4):506-515.
Ultra-rapid cycling in BD
I feel Dr. Goldberg’s article addressing ultra-rapid cycling (URC) bipolar disorder (BD) (“Ultra-rapid cycling bipolar disorder: A critical look,” Current Psychiatry, December 2011, p. 42-52), fell short in 2 critical regards. First, I believe evidence would have supported much stronger or less ambiguous conclusions. Although URC clearly is an observable symptomatic phenomenon, it’s not a valid construct within the BD spectrum, per se. To include it as such would only detract from the homogeneity that has been achieved with the resolution of that group to date, thereby dissipating the usefulness of the group from both a clinical and research standpoint. Even the Bottom Line stated that URC “has not been validated as a distinct clinical entity,” but “careful evaluation” is recommended “to differentiate URC from affective lability seen in other conditions,” thus implicitly validating using the term as a diagnostic entity.
Second, I am disturbed by the article’s absence of adult attention-deficit/hyperactivity disorder (ADHD), the secondary features of which easily rival BD in accounting for a significant proportion of symptoms commonly attributed to URC, if not the preponderance thereof. Notably, ADHD shares the “trait feature” status the article cites as unique to BD. A commonly cited figure places the prevalence of adult ADHD at 4.4% (using DSM-IV criteria) with 75% to 80% of those patients untreated and undiagnosed.Ultra-rapid cycling bipolar disorder: A critical look” (Current Psychiatry, December 2011, p. 42-52).
However, there was 1 significant diagnostic omission. Patients with adult attention-deficit/hyperactivity disorder (ADHD) can present with an unremarkable mental status exam, yet can give a history of abrupt episodes of dyscontrol, often in interpersonal situations. As opposed to children manifesting ADHD, where comorbidity with BD is substantial, adults may primarily display impulsivity rather than hyperactivity or inattention. By ignoring this diagnostic consideration, important pharmacotherapeutic options have been discarded, although cognitive-behavioral therapy and dialectical behavior therapy for “borderline” patients are always relevant. Regardless of diagnostic terms and the fate of DSM-5, our treatment approach serves to strengthen prefrontal cortex inhibitory activity and block limbic system reactivity.
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
Dr. Goldberg responds
Drs. Bunt and Barris each raise the clinically and theoretically interesting observation that in patients whose childhood attention-deficit/hyperactivity disorder (ADHD) persists into adulthood, affective instability may be a prominent feature. Consequently, they advise that complaints of frequent mood swings within 1 day should alert clinicians to consider ADHD in their differential diagnosis.
Importantly, emotional dysregulation is not an established criterion for ADHD, although investigators have begun to study impaired emotional processing in adults with ADHD.1 Because observational research examining emotional dysregulation in adult ADHD is preliminary, I cannot concur with Dr. Bunt’s assertion that “an omission of this sort does a disservice to the field.”
To the contrary, it would seem premature to counsel practitioners to look for mood instability as a red flag for adult ADHD. In fact, given the nontrivial rates of comorbid mood disorders with ADHD as cited by Dr. Bunt, it’s plausible that mood instability co-occurring with ADHD simply may be the epiphenomenon of a psychiatric comorbidity such as borderline personality disorder,2 a disruptive behavior disorder,3 or substance abuse.3
Moreover, endophenotype studies suggest that emotional lability and ADHD do not cosegregate in families.3 Further research is needed to determine whether moment-to-moment mood fluctuations are an intrinsic feature of ADHD that is not better accounted for by another accompanying condition.
Dr. Bunt appears to have misconstrued my use of the term “validation” with respect to ultra-rapid cycling (URC) as if I had been referring to validation of URC as a diagnosis—which I never suggested—rather than as a putative course modifier or specifier in an otherwise-diagnosed bipolar disorder patient—as was the case when researchers empirically validated rapid cycling (RC) as a bipolar course specifier, leading to its inclusion in DSM-IV.4 To my knowledge there’s no movement to consider URC as a bipolar course specifier in DSM-5, which would be a difficult undertaking in the absence of field trials such as those conducted for bipolar RC.
Drs. Barris, Bunt, and I seem to agree that mood shifts occurring on a daily or more frequent basis constitute a non-pathognomonic phenomenon for which “careful evaluation” is necessary to discern the broader psychopathologic condition and context in which it arises.
Joseph F. Goldberg, MD
Associate Clinical Professor of Psychiatry
Mt. Sinai School of Medicine
New York, NY
I feel Dr. Goldberg’s article addressing ultra-rapid cycling (URC) bipolar disorder (BD) (“Ultra-rapid cycling bipolar disorder: A critical look,” Current Psychiatry, December 2011, p. 42-52), fell short in 2 critical regards. First, I believe evidence would have supported much stronger or less ambiguous conclusions. Although URC clearly is an observable symptomatic phenomenon, it’s not a valid construct within the BD spectrum, per se. To include it as such would only detract from the homogeneity that has been achieved with the resolution of that group to date, thereby dissipating the usefulness of the group from both a clinical and research standpoint. Even the Bottom Line stated that URC “has not been validated as a distinct clinical entity,” but “careful evaluation” is recommended “to differentiate URC from affective lability seen in other conditions,” thus implicitly validating using the term as a diagnostic entity.
Second, I am disturbed by the article’s absence of adult attention-deficit/hyperactivity disorder (ADHD), the secondary features of which easily rival BD in accounting for a significant proportion of symptoms commonly attributed to URC, if not the preponderance thereof. Notably, ADHD shares the “trait feature” status the article cites as unique to BD. A commonly cited figure places the prevalence of adult ADHD at 4.4% (using DSM-IV criteria) with 75% to 80% of those patients untreated and undiagnosed.Ultra-rapid cycling bipolar disorder: A critical look” (Current Psychiatry, December 2011, p. 42-52).
However, there was 1 significant diagnostic omission. Patients with adult attention-deficit/hyperactivity disorder (ADHD) can present with an unremarkable mental status exam, yet can give a history of abrupt episodes of dyscontrol, often in interpersonal situations. As opposed to children manifesting ADHD, where comorbidity with BD is substantial, adults may primarily display impulsivity rather than hyperactivity or inattention. By ignoring this diagnostic consideration, important pharmacotherapeutic options have been discarded, although cognitive-behavioral therapy and dialectical behavior therapy for “borderline” patients are always relevant. Regardless of diagnostic terms and the fate of DSM-5, our treatment approach serves to strengthen prefrontal cortex inhibitory activity and block limbic system reactivity.
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
Dr. Goldberg responds
Drs. Bunt and Barris each raise the clinically and theoretically interesting observation that in patients whose childhood attention-deficit/hyperactivity disorder (ADHD) persists into adulthood, affective instability may be a prominent feature. Consequently, they advise that complaints of frequent mood swings within 1 day should alert clinicians to consider ADHD in their differential diagnosis.
Importantly, emotional dysregulation is not an established criterion for ADHD, although investigators have begun to study impaired emotional processing in adults with ADHD.1 Because observational research examining emotional dysregulation in adult ADHD is preliminary, I cannot concur with Dr. Bunt’s assertion that “an omission of this sort does a disservice to the field.”
To the contrary, it would seem premature to counsel practitioners to look for mood instability as a red flag for adult ADHD. In fact, given the nontrivial rates of comorbid mood disorders with ADHD as cited by Dr. Bunt, it’s plausible that mood instability co-occurring with ADHD simply may be the epiphenomenon of a psychiatric comorbidity such as borderline personality disorder,2 a disruptive behavior disorder,3 or substance abuse.3
Moreover, endophenotype studies suggest that emotional lability and ADHD do not cosegregate in families.3 Further research is needed to determine whether moment-to-moment mood fluctuations are an intrinsic feature of ADHD that is not better accounted for by another accompanying condition.
Dr. Bunt appears to have misconstrued my use of the term “validation” with respect to ultra-rapid cycling (URC) as if I had been referring to validation of URC as a diagnosis—which I never suggested—rather than as a putative course modifier or specifier in an otherwise-diagnosed bipolar disorder patient—as was the case when researchers empirically validated rapid cycling (RC) as a bipolar course specifier, leading to its inclusion in DSM-IV.4 To my knowledge there’s no movement to consider URC as a bipolar course specifier in DSM-5, which would be a difficult undertaking in the absence of field trials such as those conducted for bipolar RC.
Drs. Barris, Bunt, and I seem to agree that mood shifts occurring on a daily or more frequent basis constitute a non-pathognomonic phenomenon for which “careful evaluation” is necessary to discern the broader psychopathologic condition and context in which it arises.
Joseph F. Goldberg, MD
Associate Clinical Professor of Psychiatry
Mt. Sinai School of Medicine
New York, NY
I feel Dr. Goldberg’s article addressing ultra-rapid cycling (URC) bipolar disorder (BD) (“Ultra-rapid cycling bipolar disorder: A critical look,” Current Psychiatry, December 2011, p. 42-52), fell short in 2 critical regards. First, I believe evidence would have supported much stronger or less ambiguous conclusions. Although URC clearly is an observable symptomatic phenomenon, it’s not a valid construct within the BD spectrum, per se. To include it as such would only detract from the homogeneity that has been achieved with the resolution of that group to date, thereby dissipating the usefulness of the group from both a clinical and research standpoint. Even the Bottom Line stated that URC “has not been validated as a distinct clinical entity,” but “careful evaluation” is recommended “to differentiate URC from affective lability seen in other conditions,” thus implicitly validating using the term as a diagnostic entity.
Second, I am disturbed by the article’s absence of adult attention-deficit/hyperactivity disorder (ADHD), the secondary features of which easily rival BD in accounting for a significant proportion of symptoms commonly attributed to URC, if not the preponderance thereof. Notably, ADHD shares the “trait feature” status the article cites as unique to BD. A commonly cited figure places the prevalence of adult ADHD at 4.4% (using DSM-IV criteria) with 75% to 80% of those patients untreated and undiagnosed.Ultra-rapid cycling bipolar disorder: A critical look” (Current Psychiatry, December 2011, p. 42-52).
However, there was 1 significant diagnostic omission. Patients with adult attention-deficit/hyperactivity disorder (ADHD) can present with an unremarkable mental status exam, yet can give a history of abrupt episodes of dyscontrol, often in interpersonal situations. As opposed to children manifesting ADHD, where comorbidity with BD is substantial, adults may primarily display impulsivity rather than hyperactivity or inattention. By ignoring this diagnostic consideration, important pharmacotherapeutic options have been discarded, although cognitive-behavioral therapy and dialectical behavior therapy for “borderline” patients are always relevant. Regardless of diagnostic terms and the fate of DSM-5, our treatment approach serves to strengthen prefrontal cortex inhibitory activity and block limbic system reactivity.
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
Dr. Goldberg responds
Drs. Bunt and Barris each raise the clinically and theoretically interesting observation that in patients whose childhood attention-deficit/hyperactivity disorder (ADHD) persists into adulthood, affective instability may be a prominent feature. Consequently, they advise that complaints of frequent mood swings within 1 day should alert clinicians to consider ADHD in their differential diagnosis.
Importantly, emotional dysregulation is not an established criterion for ADHD, although investigators have begun to study impaired emotional processing in adults with ADHD.1 Because observational research examining emotional dysregulation in adult ADHD is preliminary, I cannot concur with Dr. Bunt’s assertion that “an omission of this sort does a disservice to the field.”
To the contrary, it would seem premature to counsel practitioners to look for mood instability as a red flag for adult ADHD. In fact, given the nontrivial rates of comorbid mood disorders with ADHD as cited by Dr. Bunt, it’s plausible that mood instability co-occurring with ADHD simply may be the epiphenomenon of a psychiatric comorbidity such as borderline personality disorder,2 a disruptive behavior disorder,3 or substance abuse.3
Moreover, endophenotype studies suggest that emotional lability and ADHD do not cosegregate in families.3 Further research is needed to determine whether moment-to-moment mood fluctuations are an intrinsic feature of ADHD that is not better accounted for by another accompanying condition.
Dr. Bunt appears to have misconstrued my use of the term “validation” with respect to ultra-rapid cycling (URC) as if I had been referring to validation of URC as a diagnosis—which I never suggested—rather than as a putative course modifier or specifier in an otherwise-diagnosed bipolar disorder patient—as was the case when researchers empirically validated rapid cycling (RC) as a bipolar course specifier, leading to its inclusion in DSM-IV.4 To my knowledge there’s no movement to consider URC as a bipolar course specifier in DSM-5, which would be a difficult undertaking in the absence of field trials such as those conducted for bipolar RC.
Drs. Barris, Bunt, and I seem to agree that mood shifts occurring on a daily or more frequent basis constitute a non-pathognomonic phenomenon for which “careful evaluation” is necessary to discern the broader psychopathologic condition and context in which it arises.
Joseph F. Goldberg, MD
Associate Clinical Professor of Psychiatry
Mt. Sinai School of Medicine
New York, NY