User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Beyond psychiatry’s reach: Fringe behaviors that defy treatment
Human nature is amazingly diverse. It is replete with behavioral quirks, oddities, eccentricities, and foibles. Most of these are idiosyncrasies that are outside the realm of psychopathology.
Frankly, the world would be a rather boring place without them. Contrary to the allegations that psychiatry medicalizes too many human behaviors that are in the “normal” range, only a small fraction of behavioral deviations are included in axis II of DSM-IV-TR. Unlike common peculiarities, the enduring personality disorders that psychiatrists diagnose and valiantly attempt to treat (no effective pharmacologic treatment has been approved for any of them) usually are significantly maladaptive and could lead to social or vocational dysfunction.
Many individuals exhibit “deplorable” behaviors that generally are frowned upon as a source of concern or are unlawful. Consider greed, bullying, chauvinism, fanaticism, extortion, demagoguery, or corruption. Very few, if any, of those who exhibit such fringe behaviors would consider seeking psychiatric help, although persons irked or injured by them wish they would. And what about bribery, power-seeking, malice, infidelity, laziness, mendacity, cheating, cruelty, narrow-mindedness, ruthlessness, and deceptiveness? Everyone has friends or relatives who exhibit such traits and flaunt them openly as a personal “brand.” Few such individuals would ever regard themselves as truly “flawed” or in need of change or professional help. In fact, those afflicted with such behavioral aberrations that are well outside the socially acceptable norms often are “successful” individuals who occupy prominent business or community roles despite their unendearing, even loathsome conduct. It sometimes appears that they have thrived despite their glaring lack of desirable social traits such as empathy, honesty, generosity, selflessness, and kindness.
Sometimes, it is not the oddities of behavior but extremes of political beliefs that can be most intriguing. They are reminiscent of delusions although they do not meet the DSM definition of delusional thinking (a fixed false belief inconsistent with one’s ethnic, cultural, educational, or religious background). Although real delusions are treatable with medications, para-delusional beliefs are not! A good example is the escalating prevalence of political fanaticism that has permeated society and dominated much of what used to be civilized discourse, even though most of the population continues to uphold middle-of-the-road political beliefs. The ideological polarization has even “infected” some media outlets, especially the free-wheeling blogosphere, where potentially venomous ideas are continuously spawned and instantly disseminated. Those who take diametrically opposite views often extend their conflicts over beliefs and ideas to personal hostilities and ad hominem attacks on “the other side.” This is an excellent example of how the entrenchment of beliefs and attitudes can directly shape behavior and transform an opinion into an “article of faith.”
Fanaticism is a long-standing human trait that has sparked wars and perpetuated prejudice and discrimination. Europe was ravaged by century-long wars instigated and fueled by religious fanaticism, proving that extreme beliefs can not only disrupt individual behavior but can impact cities, countries, and even the world, as depicted by World War II. Interestingly, when bizarre and fringe beliefs and behaviors are manifested on a large scale, it is regarded as political rather than psychopathological. Similarly, when religious beliefs become extreme, as sometimes happens in persons with acute bipolar mania, the unusual verbal output is regarded as a variant of religious exuberance rather than a psychiatric condition, which may delay medical treatment. It seems that in an age of intensifying secularism in developed western countries, political zealotry is the new religion!
Homo sapiens (wise man) is the only species that develops and harbors beliefs and whose behavior is inevitably guided, shaped, and determined by those beliefs. Obviously, some of our species’ beliefs are not consistently “wise,” but tenets can be vital to the health and survival of human individuals and communities. Émile Durkheim, the famous French sociologist, proposed in 1897 that “anomie,” or the lack of norms, values, and beliefs, is the main underpinning of suicide.1 On the other hand, psychiatrists generally attribute suicide to multiple other factors and anomie rarely is mentioned. However, Durkheim, like all humans, is entitled to his idiosyncratic belief!
Reference
1. Durkheim É. Le suicide; étude de sociologie. Paris, France: F. Alcan; 1897.
Human nature is amazingly diverse. It is replete with behavioral quirks, oddities, eccentricities, and foibles. Most of these are idiosyncrasies that are outside the realm of psychopathology.
Frankly, the world would be a rather boring place without them. Contrary to the allegations that psychiatry medicalizes too many human behaviors that are in the “normal” range, only a small fraction of behavioral deviations are included in axis II of DSM-IV-TR. Unlike common peculiarities, the enduring personality disorders that psychiatrists diagnose and valiantly attempt to treat (no effective pharmacologic treatment has been approved for any of them) usually are significantly maladaptive and could lead to social or vocational dysfunction.
Many individuals exhibit “deplorable” behaviors that generally are frowned upon as a source of concern or are unlawful. Consider greed, bullying, chauvinism, fanaticism, extortion, demagoguery, or corruption. Very few, if any, of those who exhibit such fringe behaviors would consider seeking psychiatric help, although persons irked or injured by them wish they would. And what about bribery, power-seeking, malice, infidelity, laziness, mendacity, cheating, cruelty, narrow-mindedness, ruthlessness, and deceptiveness? Everyone has friends or relatives who exhibit such traits and flaunt them openly as a personal “brand.” Few such individuals would ever regard themselves as truly “flawed” or in need of change or professional help. In fact, those afflicted with such behavioral aberrations that are well outside the socially acceptable norms often are “successful” individuals who occupy prominent business or community roles despite their unendearing, even loathsome conduct. It sometimes appears that they have thrived despite their glaring lack of desirable social traits such as empathy, honesty, generosity, selflessness, and kindness.
Sometimes, it is not the oddities of behavior but extremes of political beliefs that can be most intriguing. They are reminiscent of delusions although they do not meet the DSM definition of delusional thinking (a fixed false belief inconsistent with one’s ethnic, cultural, educational, or religious background). Although real delusions are treatable with medications, para-delusional beliefs are not! A good example is the escalating prevalence of political fanaticism that has permeated society and dominated much of what used to be civilized discourse, even though most of the population continues to uphold middle-of-the-road political beliefs. The ideological polarization has even “infected” some media outlets, especially the free-wheeling blogosphere, where potentially venomous ideas are continuously spawned and instantly disseminated. Those who take diametrically opposite views often extend their conflicts over beliefs and ideas to personal hostilities and ad hominem attacks on “the other side.” This is an excellent example of how the entrenchment of beliefs and attitudes can directly shape behavior and transform an opinion into an “article of faith.”
Fanaticism is a long-standing human trait that has sparked wars and perpetuated prejudice and discrimination. Europe was ravaged by century-long wars instigated and fueled by religious fanaticism, proving that extreme beliefs can not only disrupt individual behavior but can impact cities, countries, and even the world, as depicted by World War II. Interestingly, when bizarre and fringe beliefs and behaviors are manifested on a large scale, it is regarded as political rather than psychopathological. Similarly, when religious beliefs become extreme, as sometimes happens in persons with acute bipolar mania, the unusual verbal output is regarded as a variant of religious exuberance rather than a psychiatric condition, which may delay medical treatment. It seems that in an age of intensifying secularism in developed western countries, political zealotry is the new religion!
Homo sapiens (wise man) is the only species that develops and harbors beliefs and whose behavior is inevitably guided, shaped, and determined by those beliefs. Obviously, some of our species’ beliefs are not consistently “wise,” but tenets can be vital to the health and survival of human individuals and communities. Émile Durkheim, the famous French sociologist, proposed in 1897 that “anomie,” or the lack of norms, values, and beliefs, is the main underpinning of suicide.1 On the other hand, psychiatrists generally attribute suicide to multiple other factors and anomie rarely is mentioned. However, Durkheim, like all humans, is entitled to his idiosyncratic belief!
Human nature is amazingly diverse. It is replete with behavioral quirks, oddities, eccentricities, and foibles. Most of these are idiosyncrasies that are outside the realm of psychopathology.
Frankly, the world would be a rather boring place without them. Contrary to the allegations that psychiatry medicalizes too many human behaviors that are in the “normal” range, only a small fraction of behavioral deviations are included in axis II of DSM-IV-TR. Unlike common peculiarities, the enduring personality disorders that psychiatrists diagnose and valiantly attempt to treat (no effective pharmacologic treatment has been approved for any of them) usually are significantly maladaptive and could lead to social or vocational dysfunction.
Many individuals exhibit “deplorable” behaviors that generally are frowned upon as a source of concern or are unlawful. Consider greed, bullying, chauvinism, fanaticism, extortion, demagoguery, or corruption. Very few, if any, of those who exhibit such fringe behaviors would consider seeking psychiatric help, although persons irked or injured by them wish they would. And what about bribery, power-seeking, malice, infidelity, laziness, mendacity, cheating, cruelty, narrow-mindedness, ruthlessness, and deceptiveness? Everyone has friends or relatives who exhibit such traits and flaunt them openly as a personal “brand.” Few such individuals would ever regard themselves as truly “flawed” or in need of change or professional help. In fact, those afflicted with such behavioral aberrations that are well outside the socially acceptable norms often are “successful” individuals who occupy prominent business or community roles despite their unendearing, even loathsome conduct. It sometimes appears that they have thrived despite their glaring lack of desirable social traits such as empathy, honesty, generosity, selflessness, and kindness.
Sometimes, it is not the oddities of behavior but extremes of political beliefs that can be most intriguing. They are reminiscent of delusions although they do not meet the DSM definition of delusional thinking (a fixed false belief inconsistent with one’s ethnic, cultural, educational, or religious background). Although real delusions are treatable with medications, para-delusional beliefs are not! A good example is the escalating prevalence of political fanaticism that has permeated society and dominated much of what used to be civilized discourse, even though most of the population continues to uphold middle-of-the-road political beliefs. The ideological polarization has even “infected” some media outlets, especially the free-wheeling blogosphere, where potentially venomous ideas are continuously spawned and instantly disseminated. Those who take diametrically opposite views often extend their conflicts over beliefs and ideas to personal hostilities and ad hominem attacks on “the other side.” This is an excellent example of how the entrenchment of beliefs and attitudes can directly shape behavior and transform an opinion into an “article of faith.”
Fanaticism is a long-standing human trait that has sparked wars and perpetuated prejudice and discrimination. Europe was ravaged by century-long wars instigated and fueled by religious fanaticism, proving that extreme beliefs can not only disrupt individual behavior but can impact cities, countries, and even the world, as depicted by World War II. Interestingly, when bizarre and fringe beliefs and behaviors are manifested on a large scale, it is regarded as political rather than psychopathological. Similarly, when religious beliefs become extreme, as sometimes happens in persons with acute bipolar mania, the unusual verbal output is regarded as a variant of religious exuberance rather than a psychiatric condition, which may delay medical treatment. It seems that in an age of intensifying secularism in developed western countries, political zealotry is the new religion!
Homo sapiens (wise man) is the only species that develops and harbors beliefs and whose behavior is inevitably guided, shaped, and determined by those beliefs. Obviously, some of our species’ beliefs are not consistently “wise,” but tenets can be vital to the health and survival of human individuals and communities. Émile Durkheim, the famous French sociologist, proposed in 1897 that “anomie,” or the lack of norms, values, and beliefs, is the main underpinning of suicide.1 On the other hand, psychiatrists generally attribute suicide to multiple other factors and anomie rarely is mentioned. However, Durkheim, like all humans, is entitled to his idiosyncratic belief!
Reference
1. Durkheim É. Le suicide; étude de sociologie. Paris, France: F. Alcan; 1897.
Reference
1. Durkheim É. Le suicide; étude de sociologie. Paris, France: F. Alcan; 1897.
A paranoid, violent teenager
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Sleepless and paranoid
Ms. V, age 16, is referred to our psychiatric hospital from a juvenile detention center after she is charged with killing her sister with a hammer. She reports paranoid delusions, including believing that her sister was poisoning her food. Ms. V’s troubling behavior increased in the 6 months before the murder. She began to ask her mother to smell her food for possible poison. Her school grades dropped and she experienced decreased sleep and appetite. According to her mother, Ms. V’s insomnia worsened recently because of her paranoid thinking, which was evident when she noticed that her daughter slept with a hammer. Ms. V stopped socializing with her peers and no longer went to the gym.
Ms. V’s mother describes her daughter’s negative symptoms as consisting of social isolation and a flat affect. There was no evidence of auditory or visual hallucinations. After noticing the change in her daughter’s behavior, Ms. V’s mother attempted to schedule an appointment with a mental health professional, but there was a 2-month waiting list.
Ms. V cleaned her room before the murder, which was uncharacteristic of her routine behavior. On the day of the murder, Ms. V approached her sister while she was sleeping on the sofa and struck her on the head several times with a hammer. After the sister died, neighbors spotted Ms. V washing blood off her hands in their backyard with a sprinkler. Soaked in blood, she approached one of the neighbors and said that someone had been killed in the house. The neighbors called the police and Ms. V was arrested. She did not express remorse. She did not exhibit physical aggression toward others before the murder. Ms. V’s sense of entitlement and grandiosity persisted after the murder.
The authors’ observations
Paranoid delusions are fixed false beliefs with severe fears of others that may impair functioning at school or work, in personal relationships, and in other social dimensions. Paranoid thinking can have diverse presentations, ranging from social concerns such as fear of rejection to severe threat perceptions of people trying to cause substantial physical harm.1 Paranoid thoughts can be a result of misinterpretation of language, a personality disorder, anxiety, or psychosis.
Feelings of low self-esteem2 and anger1 may develop in a patient experiencing paranoid ideations. When anger begins to escalate, it may erupt into violent behavior. In Ms. V’s case, her paranoid ideations increased until she killed her younger sister. Ms. V’s case is similar to a mass shooting near Tucson, AZ on January 8, 2011 in that it possibly could have been prevented with earlier psychiatric intervention (Box).3-6
On January 8, 2011, a mass shooting occurred near Tucson, AZ that killed 6 and wounded 13. The suspect, 22-year-old Jared Lee Loughner, refused to cooperate with authorities by invoking his right to remain silent.3 Although the motives behind this crime remain undisclosed, mental illness appears to be a contributing factor.
Reports indicate that Mr. Loughner was abusing drugs and those close to him had noticed personality changes.4,5 The college he was attending advised Mr. Loughner to undergo a mental health evaluation, but he refused and dropped out of school.4,5 While in custody after the shooting, Mr. Loughner was diagnosed with paranoid schizophrenia, deemed incompetent to stand trial, and ordered to receive psychiatric treatment.6
This tragic mass shooting and similar incidents have led to questions regarding the adequacy of the mental health care infrastructure in United States. Experts suggest that this tragedy could have been prevented with more aggressive psychiatric prevention and intervention. Critical analysis of similar recent cases and expert opinions are needed to address this problem effectively.
EVALUATION: Remorseless
At admission, Ms. V’s affect is restricted and, at times, inappropriate. She is guarded about discussing the homicide but describes paranoid thoughts about her sister poisoning her. She is eager to learn if the police had found poison in her food. Her speech is soft with good articulation. Based on her presentation, her intelligence is average. She shows no evidence of remorse and is preoccupied with her sister poisoning her.
The Rorschach Inkblot Technique reveals positive evidence for a severe thought disorder. Ms. V’s thinking seems regressed. Ms. V’s medical workup, including MRI, electroencephalogram, and laboratory tests, are all within normal limits.
In the 5th grade, Ms. V’s primary care provider prescribed amphetamine and dextroamphetamine for attention-deficit/hyperactivity disorder, but she discontinued the drug after 1 year. Ms. V has never been hospitalized for psychiatric illness. She had no chronic medical conditions and no developmental delays.
Ms. V also has a history of periodic temper problems characterized by verbal aggression such as threatening the assistant principal at her school, and throwing her cellphone at her mother a few weeks before the murder, but no other aggressive episodes. Ms. V’s history does not suggest conduct disorder. She has no history of suicidal ideation or suicide attempts. Ms. V has used alcohol since age 15, but her mother reports that she was not a heavy or frequent user. Her last reported alcohol use was 10 days before the murder. A maternal uncle had been diagnosed with schizophrenia.
Before the murder, Ms. V lived with her sister and mother. Her parents were divorced. At age 9, Ms. V was sexually abused by a soccer coach; however, she denied symptoms of posttraumatic stress disorder related to the sexual abuse. She had no criminal history before the murder.
The authors’ observations
Based on Ms. V’s presentation and history, schizophrenia, paranoid type seems to be the most likely diagnosis because of her negative symptoms, including affective flattening, positive family history for schizophrenia, and paranoid delusions leading to dysfunction (Table).7 Delusional disorder seems less likely because Ms. V is young and has negative symptoms. Because she is generally healthy and her medical workup is negative, psychotic disorder due to a general medical condition is ruled out. She does not appear to be over-reporting, malingering, or exaggerating symptoms. In the context of psychosis, adolescent psychopathy does not seem likely even though there is evidence of grandiosity and a lack of remorse.
Table
DSM-IV-TR criteria for schizophrenia
A. Characteristic symptoms: ≥2 of the following, each present for a significant portion of time during a 1-month period:
|
| B. Social/occupational dysfunction: For a significant portion of the time since the onset of the disturbance, ≥1 major areas of functioning such as work, interpersonal relations, or self-care are markedly below the level achieved prior to the onset |
| C. Duration: Continuous signs of the disturbance persist for at least 6 months. This 6-month period must include at least 1 month of symptoms that meet Criterion A and may include periods of prodromal or residual symptoms |
| D. Schizoaffective and mood disorder exclusion: Schizoaffective disorder and mood disorder with psychotic features have been ruled out because either (1) no major depressive, manic, or mixed episodes have occurred concurrently with the active-phase symptoms; or (2) if mood episodes have occurred during active-phase symptoms, their total duration has been brief relative to the duration of the active and residual periods |
| E. Substance/general medical condition exclusion: The disturbance is not due to the direct physiological effects of a substance or a general medical condition |
| F. Relationship to a pervasive developmental disorder: If there is a history of autistic disorder or another pervasive developmental disorder, the additional diagnosis of schizophrenia is made only if prominent delusions or hallucinations are also present for at least 1 month |
| Diagnostic criteria for paranoid type: A type of schizophrenia in which the following criteria are met: A. Preoccupation with ≥1 delusions or frequent auditory hallucinations B. None of the following are prominent: disorganized speech, disorganized or catatonic behavior, or flat or inappropriate affect |
| Source: Reference 7 |
The authors’ observations
Various treatments can be used for paranoia with aggression, but the severity of the paranoia should be assessed before initiating treatment. Although categorizing paranoid ideations as mild, moderate, and severe is mainly a clinical judgment, Freeman et al1 have attempted to design a paranoia hierarchy from social concerns to severe threats. CBT8 and antipsychotic medication may help reduce mild to moderate paranoid delusions, particularly those associated with schizophrenia or mood disorders. For severe paranoia, hospitalization should carefully be considered.
When a patient exhibits moderate paranoia, the probability of progressing to severe symptoms or improving to mild symptoms depends on several variables. Pharmacologic treatment, family insight, and social support may be important variables in such circumstances. Psychoeducation for the family is vital.
In patients experiencing paranoia, violence may be prevented by proper assessment and treatment. The patient’s family should be educated about paranoid ideation and the need for treatment to improve symptoms and ensure safety. The long-term effects of untreated paranoia and types of treatment modalities available should be discussed with the family and the patient. During these teaching sessions, focus on improving the overall insight of the family and the patient about the psychotic illness to improve treatment adherence.9 This step may be challenging if the family is resistant to the patient receiving mental health treatment.
Gaining a detailed clinical history of a patient’s paranoia is important. A clinician should look for changes in behavior, such as the patient becoming quieter or more hostile, and impaired academic or social functioning. After gathering sufficient evidence contrary to the delusion, clinicians can help patients improve their reality testing.
Rule out medical and neurologic conditions that may be contributing to paranoia and aggression.
TREATMENT: Some improvement
Ms. V is started on risperidone, 1 mg/d, which leads to a partial response. She starts interacting more with staff and her peers on the unit, but her delusions of her sister poisioning her persist. Given the severity of the crime, Ms. V is sent to adult court, where she is found not guilty by reason of insanity and committed to a state hospital.
The authors’ observations
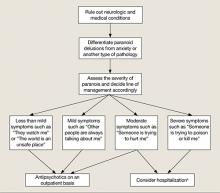
New-onset paranoia is a serious symptom that requires immediate evaluation and treatment. We recommend an approach presened in a flowchart (Figure) that highlights the importance of early intervention and aggressive treatment.
The MacArthur Violence Risk Assessment Study10 indicated that a “suspicious” attitude toward others can be a precipitating cause for increased violence in some cases. In light of ongoing controversy regarding the link between violence and mental illnesses such as schizophrenia,10-12 addressing an individual’s psychiatric illness early is preferable to prevent possible complications such as violent crimes. Because patients with paranoid ideations may have severely impaired ego control, they may be at risk for acting out aggressive and/or destructive urges. Therefore, new-onset paranoia should be thought of as a medical emergency similar to chest pain. Although accurately predicting and preventing violence may be impossible, in Ms. V’s case, earlier mental health treatment and intervention may have been able to prevent a murder.
Figure: Paranoia: A suggested approach to treatment
aBased on clinical judgment and extent of social support
Symptoms may become less severe or more severe (bidirectional). Strong social support has a positive effect on all levels and complements therapy. Regular counseling sessions and enhanced family insight about the patient’s paranoia helps strengthen social support
- Marneros A, Pillmann F, Wustmann T. Delusional disorders—are they simply paranoid schizophrenia? [published online ahead of print November 15, 2010]. Schizophr Bull. doi: 10.1093/schbul/sbq125.
Drug Brand Names
Amphetamine and dextroamphetamine • Adderall
Risperidone • Risperdal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Personal and clinical details of this case were altered to maintain patient confidentiality.
1. Freeman D, Garety PA, Bebbington PE, et al. Psychological investigation of the structure of paranoia in a non-clinical population. Br J Psychiatry. 2005;186:427-435.
2. Kendler KS, Hays P. Paranoid psychosis (delusional disorder) and schizophrenia. A family history study. Arch Gen Psychiatry. 1981;38(5):547-551.
3. CNN Wire Staff. Police “actively pursuing” second person in Tucson shooting. CNN. http://us.cnn.com/2011/CRIME/01/08/arizona.shooting. Published January 9 2011. Accessed January 9, 2012.
4. Lipton E, Savage C, Shane S. Arizona suspect’s recent acts offer hints of alienation. The New York Times. January 8 2011:A8. http://www.nytimes.com/2011/01/09/us/politics/09shooter.html. Accessed January 10, 2012.
5. Berger J. Mental health warnings preceded rampage as Arizona gunman likely went untreated. http://www.foxnews.com/us/2011/01/10/mental-health-warnings-preceded-arizona-rampage-evidence-gunman-sought. Published January 10, 2011. Accessed January 11, 2012.
6. Lacey M. Suspect in shooting of Giffords ruled unfit for trial. The New York Times. May 25 2011:A1. http://www.nytimes.com/2011/05/26/us/26loughner.html. Accessed January 5, 2012.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
8. Turkington D, Kingdon D, Weiden PJ. Cognitive behavior therapy for schizophrenia. Am J Psychiatry. 2006;163(3):365-373.
9. Smith CM, Barzman DH, Pristach CA. Effect of patient and family insight on compliance of schizophrenic patients. J Clin Pharmacol. 1997;37(2):147-154.
10. Appelbaum PS, Robbins PC, Monahan J. Violence and delusions: data from the MacArthur Violence Risk Assessment Study. Am J Psychiatry. 2000;157(4):566-572.
11. Mullen PE. A reassessment of the link between mental disorder and violent behaviour and its implications for clinical practice. Aust N Z J Psychiatry. 1997;31(1):3-11.
12. Fazel S, Gulati G, Linsell L, et al. Schizophrenia and violence: systematic review and meta-analysis. PLoS Med. 2009;6(8):e1000120.-
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Sleepless and paranoid
Ms. V, age 16, is referred to our psychiatric hospital from a juvenile detention center after she is charged with killing her sister with a hammer. She reports paranoid delusions, including believing that her sister was poisoning her food. Ms. V’s troubling behavior increased in the 6 months before the murder. She began to ask her mother to smell her food for possible poison. Her school grades dropped and she experienced decreased sleep and appetite. According to her mother, Ms. V’s insomnia worsened recently because of her paranoid thinking, which was evident when she noticed that her daughter slept with a hammer. Ms. V stopped socializing with her peers and no longer went to the gym.
Ms. V’s mother describes her daughter’s negative symptoms as consisting of social isolation and a flat affect. There was no evidence of auditory or visual hallucinations. After noticing the change in her daughter’s behavior, Ms. V’s mother attempted to schedule an appointment with a mental health professional, but there was a 2-month waiting list.
Ms. V cleaned her room before the murder, which was uncharacteristic of her routine behavior. On the day of the murder, Ms. V approached her sister while she was sleeping on the sofa and struck her on the head several times with a hammer. After the sister died, neighbors spotted Ms. V washing blood off her hands in their backyard with a sprinkler. Soaked in blood, she approached one of the neighbors and said that someone had been killed in the house. The neighbors called the police and Ms. V was arrested. She did not express remorse. She did not exhibit physical aggression toward others before the murder. Ms. V’s sense of entitlement and grandiosity persisted after the murder.
The authors’ observations
Paranoid delusions are fixed false beliefs with severe fears of others that may impair functioning at school or work, in personal relationships, and in other social dimensions. Paranoid thinking can have diverse presentations, ranging from social concerns such as fear of rejection to severe threat perceptions of people trying to cause substantial physical harm.1 Paranoid thoughts can be a result of misinterpretation of language, a personality disorder, anxiety, or psychosis.
Feelings of low self-esteem2 and anger1 may develop in a patient experiencing paranoid ideations. When anger begins to escalate, it may erupt into violent behavior. In Ms. V’s case, her paranoid ideations increased until she killed her younger sister. Ms. V’s case is similar to a mass shooting near Tucson, AZ on January 8, 2011 in that it possibly could have been prevented with earlier psychiatric intervention (Box).3-6
On January 8, 2011, a mass shooting occurred near Tucson, AZ that killed 6 and wounded 13. The suspect, 22-year-old Jared Lee Loughner, refused to cooperate with authorities by invoking his right to remain silent.3 Although the motives behind this crime remain undisclosed, mental illness appears to be a contributing factor.
Reports indicate that Mr. Loughner was abusing drugs and those close to him had noticed personality changes.4,5 The college he was attending advised Mr. Loughner to undergo a mental health evaluation, but he refused and dropped out of school.4,5 While in custody after the shooting, Mr. Loughner was diagnosed with paranoid schizophrenia, deemed incompetent to stand trial, and ordered to receive psychiatric treatment.6
This tragic mass shooting and similar incidents have led to questions regarding the adequacy of the mental health care infrastructure in United States. Experts suggest that this tragedy could have been prevented with more aggressive psychiatric prevention and intervention. Critical analysis of similar recent cases and expert opinions are needed to address this problem effectively.
EVALUATION: Remorseless
At admission, Ms. V’s affect is restricted and, at times, inappropriate. She is guarded about discussing the homicide but describes paranoid thoughts about her sister poisoning her. She is eager to learn if the police had found poison in her food. Her speech is soft with good articulation. Based on her presentation, her intelligence is average. She shows no evidence of remorse and is preoccupied with her sister poisoning her.
The Rorschach Inkblot Technique reveals positive evidence for a severe thought disorder. Ms. V’s thinking seems regressed. Ms. V’s medical workup, including MRI, electroencephalogram, and laboratory tests, are all within normal limits.
In the 5th grade, Ms. V’s primary care provider prescribed amphetamine and dextroamphetamine for attention-deficit/hyperactivity disorder, but she discontinued the drug after 1 year. Ms. V has never been hospitalized for psychiatric illness. She had no chronic medical conditions and no developmental delays.
Ms. V also has a history of periodic temper problems characterized by verbal aggression such as threatening the assistant principal at her school, and throwing her cellphone at her mother a few weeks before the murder, but no other aggressive episodes. Ms. V’s history does not suggest conduct disorder. She has no history of suicidal ideation or suicide attempts. Ms. V has used alcohol since age 15, but her mother reports that she was not a heavy or frequent user. Her last reported alcohol use was 10 days before the murder. A maternal uncle had been diagnosed with schizophrenia.
Before the murder, Ms. V lived with her sister and mother. Her parents were divorced. At age 9, Ms. V was sexually abused by a soccer coach; however, she denied symptoms of posttraumatic stress disorder related to the sexual abuse. She had no criminal history before the murder.
The authors’ observations
Based on Ms. V’s presentation and history, schizophrenia, paranoid type seems to be the most likely diagnosis because of her negative symptoms, including affective flattening, positive family history for schizophrenia, and paranoid delusions leading to dysfunction (Table).7 Delusional disorder seems less likely because Ms. V is young and has negative symptoms. Because she is generally healthy and her medical workup is negative, psychotic disorder due to a general medical condition is ruled out. She does not appear to be over-reporting, malingering, or exaggerating symptoms. In the context of psychosis, adolescent psychopathy does not seem likely even though there is evidence of grandiosity and a lack of remorse.
Table
DSM-IV-TR criteria for schizophrenia
A. Characteristic symptoms: ≥2 of the following, each present for a significant portion of time during a 1-month period:
|
| B. Social/occupational dysfunction: For a significant portion of the time since the onset of the disturbance, ≥1 major areas of functioning such as work, interpersonal relations, or self-care are markedly below the level achieved prior to the onset |
| C. Duration: Continuous signs of the disturbance persist for at least 6 months. This 6-month period must include at least 1 month of symptoms that meet Criterion A and may include periods of prodromal or residual symptoms |
| D. Schizoaffective and mood disorder exclusion: Schizoaffective disorder and mood disorder with psychotic features have been ruled out because either (1) no major depressive, manic, or mixed episodes have occurred concurrently with the active-phase symptoms; or (2) if mood episodes have occurred during active-phase symptoms, their total duration has been brief relative to the duration of the active and residual periods |
| E. Substance/general medical condition exclusion: The disturbance is not due to the direct physiological effects of a substance or a general medical condition |
| F. Relationship to a pervasive developmental disorder: If there is a history of autistic disorder or another pervasive developmental disorder, the additional diagnosis of schizophrenia is made only if prominent delusions or hallucinations are also present for at least 1 month |
| Diagnostic criteria for paranoid type: A type of schizophrenia in which the following criteria are met: A. Preoccupation with ≥1 delusions or frequent auditory hallucinations B. None of the following are prominent: disorganized speech, disorganized or catatonic behavior, or flat or inappropriate affect |
| Source: Reference 7 |
The authors’ observations
Various treatments can be used for paranoia with aggression, but the severity of the paranoia should be assessed before initiating treatment. Although categorizing paranoid ideations as mild, moderate, and severe is mainly a clinical judgment, Freeman et al1 have attempted to design a paranoia hierarchy from social concerns to severe threats. CBT8 and antipsychotic medication may help reduce mild to moderate paranoid delusions, particularly those associated with schizophrenia or mood disorders. For severe paranoia, hospitalization should carefully be considered.
When a patient exhibits moderate paranoia, the probability of progressing to severe symptoms or improving to mild symptoms depends on several variables. Pharmacologic treatment, family insight, and social support may be important variables in such circumstances. Psychoeducation for the family is vital.
In patients experiencing paranoia, violence may be prevented by proper assessment and treatment. The patient’s family should be educated about paranoid ideation and the need for treatment to improve symptoms and ensure safety. The long-term effects of untreated paranoia and types of treatment modalities available should be discussed with the family and the patient. During these teaching sessions, focus on improving the overall insight of the family and the patient about the psychotic illness to improve treatment adherence.9 This step may be challenging if the family is resistant to the patient receiving mental health treatment.
Gaining a detailed clinical history of a patient’s paranoia is important. A clinician should look for changes in behavior, such as the patient becoming quieter or more hostile, and impaired academic or social functioning. After gathering sufficient evidence contrary to the delusion, clinicians can help patients improve their reality testing.
Rule out medical and neurologic conditions that may be contributing to paranoia and aggression.
TREATMENT: Some improvement
Ms. V is started on risperidone, 1 mg/d, which leads to a partial response. She starts interacting more with staff and her peers on the unit, but her delusions of her sister poisioning her persist. Given the severity of the crime, Ms. V is sent to adult court, where she is found not guilty by reason of insanity and committed to a state hospital.
The authors’ observations
New-onset paranoia is a serious symptom that requires immediate evaluation and treatment. We recommend an approach presened in a flowchart (Figure) that highlights the importance of early intervention and aggressive treatment.
The MacArthur Violence Risk Assessment Study10 indicated that a “suspicious” attitude toward others can be a precipitating cause for increased violence in some cases. In light of ongoing controversy regarding the link between violence and mental illnesses such as schizophrenia,10-12 addressing an individual’s psychiatric illness early is preferable to prevent possible complications such as violent crimes. Because patients with paranoid ideations may have severely impaired ego control, they may be at risk for acting out aggressive and/or destructive urges. Therefore, new-onset paranoia should be thought of as a medical emergency similar to chest pain. Although accurately predicting and preventing violence may be impossible, in Ms. V’s case, earlier mental health treatment and intervention may have been able to prevent a murder.
Figure: Paranoia: A suggested approach to treatment
aBased on clinical judgment and extent of social support
Symptoms may become less severe or more severe (bidirectional). Strong social support has a positive effect on all levels and complements therapy. Regular counseling sessions and enhanced family insight about the patient’s paranoia helps strengthen social support
- Marneros A, Pillmann F, Wustmann T. Delusional disorders—are they simply paranoid schizophrenia? [published online ahead of print November 15, 2010]. Schizophr Bull. doi: 10.1093/schbul/sbq125.
Drug Brand Names
Amphetamine and dextroamphetamine • Adderall
Risperidone • Risperdal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Personal and clinical details of this case were altered to maintain patient confidentiality.
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Sleepless and paranoid
Ms. V, age 16, is referred to our psychiatric hospital from a juvenile detention center after she is charged with killing her sister with a hammer. She reports paranoid delusions, including believing that her sister was poisoning her food. Ms. V’s troubling behavior increased in the 6 months before the murder. She began to ask her mother to smell her food for possible poison. Her school grades dropped and she experienced decreased sleep and appetite. According to her mother, Ms. V’s insomnia worsened recently because of her paranoid thinking, which was evident when she noticed that her daughter slept with a hammer. Ms. V stopped socializing with her peers and no longer went to the gym.
Ms. V’s mother describes her daughter’s negative symptoms as consisting of social isolation and a flat affect. There was no evidence of auditory or visual hallucinations. After noticing the change in her daughter’s behavior, Ms. V’s mother attempted to schedule an appointment with a mental health professional, but there was a 2-month waiting list.
Ms. V cleaned her room before the murder, which was uncharacteristic of her routine behavior. On the day of the murder, Ms. V approached her sister while she was sleeping on the sofa and struck her on the head several times with a hammer. After the sister died, neighbors spotted Ms. V washing blood off her hands in their backyard with a sprinkler. Soaked in blood, she approached one of the neighbors and said that someone had been killed in the house. The neighbors called the police and Ms. V was arrested. She did not express remorse. She did not exhibit physical aggression toward others before the murder. Ms. V’s sense of entitlement and grandiosity persisted after the murder.
The authors’ observations
Paranoid delusions are fixed false beliefs with severe fears of others that may impair functioning at school or work, in personal relationships, and in other social dimensions. Paranoid thinking can have diverse presentations, ranging from social concerns such as fear of rejection to severe threat perceptions of people trying to cause substantial physical harm.1 Paranoid thoughts can be a result of misinterpretation of language, a personality disorder, anxiety, or psychosis.
Feelings of low self-esteem2 and anger1 may develop in a patient experiencing paranoid ideations. When anger begins to escalate, it may erupt into violent behavior. In Ms. V’s case, her paranoid ideations increased until she killed her younger sister. Ms. V’s case is similar to a mass shooting near Tucson, AZ on January 8, 2011 in that it possibly could have been prevented with earlier psychiatric intervention (Box).3-6
On January 8, 2011, a mass shooting occurred near Tucson, AZ that killed 6 and wounded 13. The suspect, 22-year-old Jared Lee Loughner, refused to cooperate with authorities by invoking his right to remain silent.3 Although the motives behind this crime remain undisclosed, mental illness appears to be a contributing factor.
Reports indicate that Mr. Loughner was abusing drugs and those close to him had noticed personality changes.4,5 The college he was attending advised Mr. Loughner to undergo a mental health evaluation, but he refused and dropped out of school.4,5 While in custody after the shooting, Mr. Loughner was diagnosed with paranoid schizophrenia, deemed incompetent to stand trial, and ordered to receive psychiatric treatment.6
This tragic mass shooting and similar incidents have led to questions regarding the adequacy of the mental health care infrastructure in United States. Experts suggest that this tragedy could have been prevented with more aggressive psychiatric prevention and intervention. Critical analysis of similar recent cases and expert opinions are needed to address this problem effectively.
EVALUATION: Remorseless
At admission, Ms. V’s affect is restricted and, at times, inappropriate. She is guarded about discussing the homicide but describes paranoid thoughts about her sister poisoning her. She is eager to learn if the police had found poison in her food. Her speech is soft with good articulation. Based on her presentation, her intelligence is average. She shows no evidence of remorse and is preoccupied with her sister poisoning her.
The Rorschach Inkblot Technique reveals positive evidence for a severe thought disorder. Ms. V’s thinking seems regressed. Ms. V’s medical workup, including MRI, electroencephalogram, and laboratory tests, are all within normal limits.
In the 5th grade, Ms. V’s primary care provider prescribed amphetamine and dextroamphetamine for attention-deficit/hyperactivity disorder, but she discontinued the drug after 1 year. Ms. V has never been hospitalized for psychiatric illness. She had no chronic medical conditions and no developmental delays.
Ms. V also has a history of periodic temper problems characterized by verbal aggression such as threatening the assistant principal at her school, and throwing her cellphone at her mother a few weeks before the murder, but no other aggressive episodes. Ms. V’s history does not suggest conduct disorder. She has no history of suicidal ideation or suicide attempts. Ms. V has used alcohol since age 15, but her mother reports that she was not a heavy or frequent user. Her last reported alcohol use was 10 days before the murder. A maternal uncle had been diagnosed with schizophrenia.
Before the murder, Ms. V lived with her sister and mother. Her parents were divorced. At age 9, Ms. V was sexually abused by a soccer coach; however, she denied symptoms of posttraumatic stress disorder related to the sexual abuse. She had no criminal history before the murder.
The authors’ observations
Based on Ms. V’s presentation and history, schizophrenia, paranoid type seems to be the most likely diagnosis because of her negative symptoms, including affective flattening, positive family history for schizophrenia, and paranoid delusions leading to dysfunction (Table).7 Delusional disorder seems less likely because Ms. V is young and has negative symptoms. Because she is generally healthy and her medical workup is negative, psychotic disorder due to a general medical condition is ruled out. She does not appear to be over-reporting, malingering, or exaggerating symptoms. In the context of psychosis, adolescent psychopathy does not seem likely even though there is evidence of grandiosity and a lack of remorse.
Table
DSM-IV-TR criteria for schizophrenia
A. Characteristic symptoms: ≥2 of the following, each present for a significant portion of time during a 1-month period:
|
| B. Social/occupational dysfunction: For a significant portion of the time since the onset of the disturbance, ≥1 major areas of functioning such as work, interpersonal relations, or self-care are markedly below the level achieved prior to the onset |
| C. Duration: Continuous signs of the disturbance persist for at least 6 months. This 6-month period must include at least 1 month of symptoms that meet Criterion A and may include periods of prodromal or residual symptoms |
| D. Schizoaffective and mood disorder exclusion: Schizoaffective disorder and mood disorder with psychotic features have been ruled out because either (1) no major depressive, manic, or mixed episodes have occurred concurrently with the active-phase symptoms; or (2) if mood episodes have occurred during active-phase symptoms, their total duration has been brief relative to the duration of the active and residual periods |
| E. Substance/general medical condition exclusion: The disturbance is not due to the direct physiological effects of a substance or a general medical condition |
| F. Relationship to a pervasive developmental disorder: If there is a history of autistic disorder or another pervasive developmental disorder, the additional diagnosis of schizophrenia is made only if prominent delusions or hallucinations are also present for at least 1 month |
| Diagnostic criteria for paranoid type: A type of schizophrenia in which the following criteria are met: A. Preoccupation with ≥1 delusions or frequent auditory hallucinations B. None of the following are prominent: disorganized speech, disorganized or catatonic behavior, or flat or inappropriate affect |
| Source: Reference 7 |
The authors’ observations
Various treatments can be used for paranoia with aggression, but the severity of the paranoia should be assessed before initiating treatment. Although categorizing paranoid ideations as mild, moderate, and severe is mainly a clinical judgment, Freeman et al1 have attempted to design a paranoia hierarchy from social concerns to severe threats. CBT8 and antipsychotic medication may help reduce mild to moderate paranoid delusions, particularly those associated with schizophrenia or mood disorders. For severe paranoia, hospitalization should carefully be considered.
When a patient exhibits moderate paranoia, the probability of progressing to severe symptoms or improving to mild symptoms depends on several variables. Pharmacologic treatment, family insight, and social support may be important variables in such circumstances. Psychoeducation for the family is vital.
In patients experiencing paranoia, violence may be prevented by proper assessment and treatment. The patient’s family should be educated about paranoid ideation and the need for treatment to improve symptoms and ensure safety. The long-term effects of untreated paranoia and types of treatment modalities available should be discussed with the family and the patient. During these teaching sessions, focus on improving the overall insight of the family and the patient about the psychotic illness to improve treatment adherence.9 This step may be challenging if the family is resistant to the patient receiving mental health treatment.
Gaining a detailed clinical history of a patient’s paranoia is important. A clinician should look for changes in behavior, such as the patient becoming quieter or more hostile, and impaired academic or social functioning. After gathering sufficient evidence contrary to the delusion, clinicians can help patients improve their reality testing.
Rule out medical and neurologic conditions that may be contributing to paranoia and aggression.
TREATMENT: Some improvement
Ms. V is started on risperidone, 1 mg/d, which leads to a partial response. She starts interacting more with staff and her peers on the unit, but her delusions of her sister poisioning her persist. Given the severity of the crime, Ms. V is sent to adult court, where she is found not guilty by reason of insanity and committed to a state hospital.
The authors’ observations
New-onset paranoia is a serious symptom that requires immediate evaluation and treatment. We recommend an approach presened in a flowchart (Figure) that highlights the importance of early intervention and aggressive treatment.
The MacArthur Violence Risk Assessment Study10 indicated that a “suspicious” attitude toward others can be a precipitating cause for increased violence in some cases. In light of ongoing controversy regarding the link between violence and mental illnesses such as schizophrenia,10-12 addressing an individual’s psychiatric illness early is preferable to prevent possible complications such as violent crimes. Because patients with paranoid ideations may have severely impaired ego control, they may be at risk for acting out aggressive and/or destructive urges. Therefore, new-onset paranoia should be thought of as a medical emergency similar to chest pain. Although accurately predicting and preventing violence may be impossible, in Ms. V’s case, earlier mental health treatment and intervention may have been able to prevent a murder.
Figure: Paranoia: A suggested approach to treatment
aBased on clinical judgment and extent of social support
Symptoms may become less severe or more severe (bidirectional). Strong social support has a positive effect on all levels and complements therapy. Regular counseling sessions and enhanced family insight about the patient’s paranoia helps strengthen social support
- Marneros A, Pillmann F, Wustmann T. Delusional disorders—are they simply paranoid schizophrenia? [published online ahead of print November 15, 2010]. Schizophr Bull. doi: 10.1093/schbul/sbq125.
Drug Brand Names
Amphetamine and dextroamphetamine • Adderall
Risperidone • Risperdal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Personal and clinical details of this case were altered to maintain patient confidentiality.
1. Freeman D, Garety PA, Bebbington PE, et al. Psychological investigation of the structure of paranoia in a non-clinical population. Br J Psychiatry. 2005;186:427-435.
2. Kendler KS, Hays P. Paranoid psychosis (delusional disorder) and schizophrenia. A family history study. Arch Gen Psychiatry. 1981;38(5):547-551.
3. CNN Wire Staff. Police “actively pursuing” second person in Tucson shooting. CNN. http://us.cnn.com/2011/CRIME/01/08/arizona.shooting. Published January 9 2011. Accessed January 9, 2012.
4. Lipton E, Savage C, Shane S. Arizona suspect’s recent acts offer hints of alienation. The New York Times. January 8 2011:A8. http://www.nytimes.com/2011/01/09/us/politics/09shooter.html. Accessed January 10, 2012.
5. Berger J. Mental health warnings preceded rampage as Arizona gunman likely went untreated. http://www.foxnews.com/us/2011/01/10/mental-health-warnings-preceded-arizona-rampage-evidence-gunman-sought. Published January 10, 2011. Accessed January 11, 2012.
6. Lacey M. Suspect in shooting of Giffords ruled unfit for trial. The New York Times. May 25 2011:A1. http://www.nytimes.com/2011/05/26/us/26loughner.html. Accessed January 5, 2012.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
8. Turkington D, Kingdon D, Weiden PJ. Cognitive behavior therapy for schizophrenia. Am J Psychiatry. 2006;163(3):365-373.
9. Smith CM, Barzman DH, Pristach CA. Effect of patient and family insight on compliance of schizophrenic patients. J Clin Pharmacol. 1997;37(2):147-154.
10. Appelbaum PS, Robbins PC, Monahan J. Violence and delusions: data from the MacArthur Violence Risk Assessment Study. Am J Psychiatry. 2000;157(4):566-572.
11. Mullen PE. A reassessment of the link between mental disorder and violent behaviour and its implications for clinical practice. Aust N Z J Psychiatry. 1997;31(1):3-11.
12. Fazel S, Gulati G, Linsell L, et al. Schizophrenia and violence: systematic review and meta-analysis. PLoS Med. 2009;6(8):e1000120.-
1. Freeman D, Garety PA, Bebbington PE, et al. Psychological investigation of the structure of paranoia in a non-clinical population. Br J Psychiatry. 2005;186:427-435.
2. Kendler KS, Hays P. Paranoid psychosis (delusional disorder) and schizophrenia. A family history study. Arch Gen Psychiatry. 1981;38(5):547-551.
3. CNN Wire Staff. Police “actively pursuing” second person in Tucson shooting. CNN. http://us.cnn.com/2011/CRIME/01/08/arizona.shooting. Published January 9 2011. Accessed January 9, 2012.
4. Lipton E, Savage C, Shane S. Arizona suspect’s recent acts offer hints of alienation. The New York Times. January 8 2011:A8. http://www.nytimes.com/2011/01/09/us/politics/09shooter.html. Accessed January 10, 2012.
5. Berger J. Mental health warnings preceded rampage as Arizona gunman likely went untreated. http://www.foxnews.com/us/2011/01/10/mental-health-warnings-preceded-arizona-rampage-evidence-gunman-sought. Published January 10, 2011. Accessed January 11, 2012.
6. Lacey M. Suspect in shooting of Giffords ruled unfit for trial. The New York Times. May 25 2011:A1. http://www.nytimes.com/2011/05/26/us/26loughner.html. Accessed January 5, 2012.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
8. Turkington D, Kingdon D, Weiden PJ. Cognitive behavior therapy for schizophrenia. Am J Psychiatry. 2006;163(3):365-373.
9. Smith CM, Barzman DH, Pristach CA. Effect of patient and family insight on compliance of schizophrenic patients. J Clin Pharmacol. 1997;37(2):147-154.
10. Appelbaum PS, Robbins PC, Monahan J. Violence and delusions: data from the MacArthur Violence Risk Assessment Study. Am J Psychiatry. 2000;157(4):566-572.
11. Mullen PE. A reassessment of the link between mental disorder and violent behaviour and its implications for clinical practice. Aust N Z J Psychiatry. 1997;31(1):3-11.
12. Fazel S, Gulati G, Linsell L, et al. Schizophrenia and violence: systematic review and meta-analysis. PLoS Med. 2009;6(8):e1000120.-
Identifying and treating factors that put patients at risk for suicide
Enhancing psychiatric care: A decade of progress
One of the greatest challenges for busy clinicians, such as Current Psychiatry readers, is keeping up with advances in the field of psychiatry in our limited available time. Because psychiatry is one of the most rapidly expanding medical specialties, psychiatrists, psychiatric nurse practitioners, and other mental health clinicians recognize the importance of ongoing self-driven learning to make sure they are practicing the latest standards of care in diagnosis and treatment.
Although continuously acquiring new knowledge that can improve patient care is stimulating and necessary, it also may be intimidating because most medical journals are packed with studies with arcane methodology, complicated designs, complex statistics, dense tables, and busy figures. Wading through the literature can be time-consuming—and even exhausting—for a busy clinician with limited time to learn the latest research findings, and this approach is not always guaranteed to provide the relevant “take-home nuggets” that can enhance clinical practice.
Enter Current Psychiatry. Established in 2002 as the brainchild of former University of Cincinnati Department of Psychiatry Chair Randy Hillard, MD (now Editor-in-Chief Emeritus) and publisher Thomas Pizor, Current Psychiatry was designed precisely to fill this vital unmet need for busy clinical psychiatrists.1 Current Psychiatry provides practical, peer-reviewed, evidence-based reviews that are highly relevant to the realities of clinical psychiatric practice. Nationally recognized experts write articles about topics identified as “valued” by practitioners based on systematic surveys of clinicians in various community settings. A diverse editorial board of prominent academic teachers and researchers provides an ongoing stream of diverse articles and perspectives that distill the emerging science and practice of psychiatry into immediately useful applications.
From our first issue, Current Psychiatry has presented information that readers could use to care for their patients, in articles such as:
- Using antipsychotics in patients with dementia (Kasckow JW, et al; February 2004)
- How to reduce mania risk when prescribing stimulants (Dubovsky SL, et al; October 2005)
- Hypnotics and driving: FDA action, clinical trials show need for precautions (Freeman B, et al; April 2007)
- Fibromyalgia: Psychiatric drugs target CNS-linked symptoms (Stanford SB; March 2009)
- The re-emerging role of therapeutic neuromodulation (Janicak PG, et al; November 2010)
The impact has been spectacular. As Current Psychiatry celebrates its 10th anniversary this month, we can relish its remarkable growth and success. In independent readership surveys, Current Psychiatry has grown to become the most widely read non-tabloid psychiatry journal.2 As Editor-in-Chief, I derive great satisfaction from the rave reviews I receive from my colleagues around the country about how they regard Current Psychiatry as their number 1 resource for clinical updates. Stellar feedback such as this indicates that Current Psychiatry clearly meets clinicians’ educational needs.
However, we are not resting on our laurels. Current Psychiatry has continued to innovate and develop new approaches to ongoing self-education by growing its online presence. The offerings at CurrentPsychiatry.com have steadily increased to include the following features:
Online-exclusive content. CurrentPsychiatry.com provides additional resources such as tables, boxes, algorithms, and/or figures related to articles from the printed edition. Later this year, as our 10th anniversary initiative for you, our readers, Current Psychiatry will offer complete online-exclusive articles that will be listed on the table of contents but published only on CurrentPsychiatry.com.
Multimedia library. Every month, the author of 1 article is invited to participate in a brief (5- to 10-minute) audiocast in which he or she provides additional commentary on clinical topics related to the article. This library currently houses nearly 50 audiocasts. Available at CurrentPsychiatry.com/pages.asp?id=6412.
Continuing Medical Education (CME). This section of our Web site provides peer-reviewed education programs that offer visitors the opportunity to earn free CME credits on a range of clinical topics, including schizophrenia, bipolar disorder, depression, and more. Available at CurrentPsychiatry.com/pages_cme.asp.
Supplements. Recent topics covered in these peer-reviewed, non-CME programs include managing schizophrenia, transcranial magnetic stimulation for depression, and more. Available at CurrentPsychiatry.com/pages_supplement.asp.
Going beyond our printed and online content, Current Psychiatry serves our readers’ educational needs through CME meetings such as the annual Psychiatry Update, which is hosted in conjunction with the American Academy of Clinical Psychiatrists. The next meeting will take place March 29 to 31, 2012 in Chicago, IL and will offer a maximum of 18 AMA PRA Category 1 CreditsTM (For more information, click here.). In addition, Current Psychiatry co-sponsors the University of Cincinnati’s Annual Psychopharmacology Update, which is scheduled for October 20, 2012 in Cincinnati, OH, offering AMA PRA Category 1 CreditsTM.
One of the gratifying aspects of producing a highly relevant educational vehicle such as Current Psychiatry is that my trainees at the University of Cincinnati tell me how useful they find it for their clinical practice. I am glad that they already have developed the good habit of reading Current Psychiatry from cover to cover during their training!
On behalf of Current Psychiatry’s Deputy Editor, Joseph F. Goldberg, MD, our Editorial Consultants, Section Editors, Associate Editors, editorial staff, and publishing staff, we thank you, our loyal readers, for valuing what we do and using the knowledge provided by Current Psychiatry to manage various psychiatric populations with the latest nosological and therapeutic advances. We invite you to continue interacting with us in person, by e-mail, or via CurrentPsychiatry.com and tell us how we can continue to meet your educational needs. We find it very rewarding to hear from you.
1. Hillard JR. Here is why we do need a new psychiatry journal. Current Psychiatry. 2002;1(1):7. -http://www.currentpsychiatry.com/article_pages.asp?AID=465&UID=44140
2. Kantar Media. June 2011 Medical/Surgical Readership Study. Psychiatry. New York, NY: Kantar Media; 2011.
One of the greatest challenges for busy clinicians, such as Current Psychiatry readers, is keeping up with advances in the field of psychiatry in our limited available time. Because psychiatry is one of the most rapidly expanding medical specialties, psychiatrists, psychiatric nurse practitioners, and other mental health clinicians recognize the importance of ongoing self-driven learning to make sure they are practicing the latest standards of care in diagnosis and treatment.
Although continuously acquiring new knowledge that can improve patient care is stimulating and necessary, it also may be intimidating because most medical journals are packed with studies with arcane methodology, complicated designs, complex statistics, dense tables, and busy figures. Wading through the literature can be time-consuming—and even exhausting—for a busy clinician with limited time to learn the latest research findings, and this approach is not always guaranteed to provide the relevant “take-home nuggets” that can enhance clinical practice.
Enter Current Psychiatry. Established in 2002 as the brainchild of former University of Cincinnati Department of Psychiatry Chair Randy Hillard, MD (now Editor-in-Chief Emeritus) and publisher Thomas Pizor, Current Psychiatry was designed precisely to fill this vital unmet need for busy clinical psychiatrists.1 Current Psychiatry provides practical, peer-reviewed, evidence-based reviews that are highly relevant to the realities of clinical psychiatric practice. Nationally recognized experts write articles about topics identified as “valued” by practitioners based on systematic surveys of clinicians in various community settings. A diverse editorial board of prominent academic teachers and researchers provides an ongoing stream of diverse articles and perspectives that distill the emerging science and practice of psychiatry into immediately useful applications.
From our first issue, Current Psychiatry has presented information that readers could use to care for their patients, in articles such as:
- Using antipsychotics in patients with dementia (Kasckow JW, et al; February 2004)
- How to reduce mania risk when prescribing stimulants (Dubovsky SL, et al; October 2005)
- Hypnotics and driving: FDA action, clinical trials show need for precautions (Freeman B, et al; April 2007)
- Fibromyalgia: Psychiatric drugs target CNS-linked symptoms (Stanford SB; March 2009)
- The re-emerging role of therapeutic neuromodulation (Janicak PG, et al; November 2010)
The impact has been spectacular. As Current Psychiatry celebrates its 10th anniversary this month, we can relish its remarkable growth and success. In independent readership surveys, Current Psychiatry has grown to become the most widely read non-tabloid psychiatry journal.2 As Editor-in-Chief, I derive great satisfaction from the rave reviews I receive from my colleagues around the country about how they regard Current Psychiatry as their number 1 resource for clinical updates. Stellar feedback such as this indicates that Current Psychiatry clearly meets clinicians’ educational needs.
However, we are not resting on our laurels. Current Psychiatry has continued to innovate and develop new approaches to ongoing self-education by growing its online presence. The offerings at CurrentPsychiatry.com have steadily increased to include the following features:
Online-exclusive content. CurrentPsychiatry.com provides additional resources such as tables, boxes, algorithms, and/or figures related to articles from the printed edition. Later this year, as our 10th anniversary initiative for you, our readers, Current Psychiatry will offer complete online-exclusive articles that will be listed on the table of contents but published only on CurrentPsychiatry.com.
Multimedia library. Every month, the author of 1 article is invited to participate in a brief (5- to 10-minute) audiocast in which he or she provides additional commentary on clinical topics related to the article. This library currently houses nearly 50 audiocasts. Available at CurrentPsychiatry.com/pages.asp?id=6412.
Continuing Medical Education (CME). This section of our Web site provides peer-reviewed education programs that offer visitors the opportunity to earn free CME credits on a range of clinical topics, including schizophrenia, bipolar disorder, depression, and more. Available at CurrentPsychiatry.com/pages_cme.asp.
Supplements. Recent topics covered in these peer-reviewed, non-CME programs include managing schizophrenia, transcranial magnetic stimulation for depression, and more. Available at CurrentPsychiatry.com/pages_supplement.asp.
Going beyond our printed and online content, Current Psychiatry serves our readers’ educational needs through CME meetings such as the annual Psychiatry Update, which is hosted in conjunction with the American Academy of Clinical Psychiatrists. The next meeting will take place March 29 to 31, 2012 in Chicago, IL and will offer a maximum of 18 AMA PRA Category 1 CreditsTM (For more information, click here.). In addition, Current Psychiatry co-sponsors the University of Cincinnati’s Annual Psychopharmacology Update, which is scheduled for October 20, 2012 in Cincinnati, OH, offering AMA PRA Category 1 CreditsTM.
One of the gratifying aspects of producing a highly relevant educational vehicle such as Current Psychiatry is that my trainees at the University of Cincinnati tell me how useful they find it for their clinical practice. I am glad that they already have developed the good habit of reading Current Psychiatry from cover to cover during their training!
On behalf of Current Psychiatry’s Deputy Editor, Joseph F. Goldberg, MD, our Editorial Consultants, Section Editors, Associate Editors, editorial staff, and publishing staff, we thank you, our loyal readers, for valuing what we do and using the knowledge provided by Current Psychiatry to manage various psychiatric populations with the latest nosological and therapeutic advances. We invite you to continue interacting with us in person, by e-mail, or via CurrentPsychiatry.com and tell us how we can continue to meet your educational needs. We find it very rewarding to hear from you.
One of the greatest challenges for busy clinicians, such as Current Psychiatry readers, is keeping up with advances in the field of psychiatry in our limited available time. Because psychiatry is one of the most rapidly expanding medical specialties, psychiatrists, psychiatric nurse practitioners, and other mental health clinicians recognize the importance of ongoing self-driven learning to make sure they are practicing the latest standards of care in diagnosis and treatment.
Although continuously acquiring new knowledge that can improve patient care is stimulating and necessary, it also may be intimidating because most medical journals are packed with studies with arcane methodology, complicated designs, complex statistics, dense tables, and busy figures. Wading through the literature can be time-consuming—and even exhausting—for a busy clinician with limited time to learn the latest research findings, and this approach is not always guaranteed to provide the relevant “take-home nuggets” that can enhance clinical practice.
Enter Current Psychiatry. Established in 2002 as the brainchild of former University of Cincinnati Department of Psychiatry Chair Randy Hillard, MD (now Editor-in-Chief Emeritus) and publisher Thomas Pizor, Current Psychiatry was designed precisely to fill this vital unmet need for busy clinical psychiatrists.1 Current Psychiatry provides practical, peer-reviewed, evidence-based reviews that are highly relevant to the realities of clinical psychiatric practice. Nationally recognized experts write articles about topics identified as “valued” by practitioners based on systematic surveys of clinicians in various community settings. A diverse editorial board of prominent academic teachers and researchers provides an ongoing stream of diverse articles and perspectives that distill the emerging science and practice of psychiatry into immediately useful applications.
From our first issue, Current Psychiatry has presented information that readers could use to care for their patients, in articles such as:
- Using antipsychotics in patients with dementia (Kasckow JW, et al; February 2004)
- How to reduce mania risk when prescribing stimulants (Dubovsky SL, et al; October 2005)
- Hypnotics and driving: FDA action, clinical trials show need for precautions (Freeman B, et al; April 2007)
- Fibromyalgia: Psychiatric drugs target CNS-linked symptoms (Stanford SB; March 2009)
- The re-emerging role of therapeutic neuromodulation (Janicak PG, et al; November 2010)
The impact has been spectacular. As Current Psychiatry celebrates its 10th anniversary this month, we can relish its remarkable growth and success. In independent readership surveys, Current Psychiatry has grown to become the most widely read non-tabloid psychiatry journal.2 As Editor-in-Chief, I derive great satisfaction from the rave reviews I receive from my colleagues around the country about how they regard Current Psychiatry as their number 1 resource for clinical updates. Stellar feedback such as this indicates that Current Psychiatry clearly meets clinicians’ educational needs.
However, we are not resting on our laurels. Current Psychiatry has continued to innovate and develop new approaches to ongoing self-education by growing its online presence. The offerings at CurrentPsychiatry.com have steadily increased to include the following features:
Online-exclusive content. CurrentPsychiatry.com provides additional resources such as tables, boxes, algorithms, and/or figures related to articles from the printed edition. Later this year, as our 10th anniversary initiative for you, our readers, Current Psychiatry will offer complete online-exclusive articles that will be listed on the table of contents but published only on CurrentPsychiatry.com.
Multimedia library. Every month, the author of 1 article is invited to participate in a brief (5- to 10-minute) audiocast in which he or she provides additional commentary on clinical topics related to the article. This library currently houses nearly 50 audiocasts. Available at CurrentPsychiatry.com/pages.asp?id=6412.
Continuing Medical Education (CME). This section of our Web site provides peer-reviewed education programs that offer visitors the opportunity to earn free CME credits on a range of clinical topics, including schizophrenia, bipolar disorder, depression, and more. Available at CurrentPsychiatry.com/pages_cme.asp.
Supplements. Recent topics covered in these peer-reviewed, non-CME programs include managing schizophrenia, transcranial magnetic stimulation for depression, and more. Available at CurrentPsychiatry.com/pages_supplement.asp.
Going beyond our printed and online content, Current Psychiatry serves our readers’ educational needs through CME meetings such as the annual Psychiatry Update, which is hosted in conjunction with the American Academy of Clinical Psychiatrists. The next meeting will take place March 29 to 31, 2012 in Chicago, IL and will offer a maximum of 18 AMA PRA Category 1 CreditsTM (For more information, click here.). In addition, Current Psychiatry co-sponsors the University of Cincinnati’s Annual Psychopharmacology Update, which is scheduled for October 20, 2012 in Cincinnati, OH, offering AMA PRA Category 1 CreditsTM.
One of the gratifying aspects of producing a highly relevant educational vehicle such as Current Psychiatry is that my trainees at the University of Cincinnati tell me how useful they find it for their clinical practice. I am glad that they already have developed the good habit of reading Current Psychiatry from cover to cover during their training!
On behalf of Current Psychiatry’s Deputy Editor, Joseph F. Goldberg, MD, our Editorial Consultants, Section Editors, Associate Editors, editorial staff, and publishing staff, we thank you, our loyal readers, for valuing what we do and using the knowledge provided by Current Psychiatry to manage various psychiatric populations with the latest nosological and therapeutic advances. We invite you to continue interacting with us in person, by e-mail, or via CurrentPsychiatry.com and tell us how we can continue to meet your educational needs. We find it very rewarding to hear from you.
1. Hillard JR. Here is why we do need a new psychiatry journal. Current Psychiatry. 2002;1(1):7. -http://www.currentpsychiatry.com/article_pages.asp?AID=465&UID=44140
2. Kantar Media. June 2011 Medical/Surgical Readership Study. Psychiatry. New York, NY: Kantar Media; 2011.
1. Hillard JR. Here is why we do need a new psychiatry journal. Current Psychiatry. 2002;1(1):7. -http://www.currentpsychiatry.com/article_pages.asp?AID=465&UID=44140
2. Kantar Media. June 2011 Medical/Surgical Readership Study. Psychiatry. New York, NY: Kantar Media; 2011.
The delirious substance abuser
CASE: Hurt and confused
Emergency medical services (EMS) are called to Ms. K’s apartment after her roommate found her lying on the floor moaning. The roommate tells EMS that Ms. K, age 29, appeared confused and was slurring her words, and reports that this change in her awareness progressed rapidly over a few hours. EMS personnel find that Ms. K has multiple contusions on her arms and face, which they presume to be self-inflicted. A marijuana pipe is discovered at Ms. K’s apartment.
In the emergency room (ER), Ms. K is inattentive and has difficulty following simple commands. Her speech is mumbled and her thoughts are disorganized. She displays psychomotor restlessness in the form of combativeness. Ms. K cannot provide meaningful historical data and is disoriented to place and time. The ER staff requests a psychiatric consultation.
Family members reveal that Ms. K has no preexisting medical conditions, is not taking prescription medications, but has a history of substance abuse (sporadic cocaine and cannabis use). Her family is unaware of recent substance use.
Physical examination reveals tachycardia (heart rate 110 to 120 beats per minute), hypotension (blood pressure 78/49 mm Hg), hypothermia (temperature 88ºF), and peripheral pulse oximetry of 84%. Her pupils are dilated and reactive to light; no conjunctival injection is noted. Her lung fields are clear on auscultation, but she is noted to have a rapid, irregular heartbeat. The abdomen is positive for bowel sounds, soft on palpation, and without any repositioning or notable overt signs of tenderness. Ms. K’s toes show purple discoloration with poor capillary refill. The dorsalis pedis pulses are reported to be 1+ bilaterally; however, the remainder of the arterial pulse examination is normal.
Her sodium, potassium, and chloride values are normal, but she has an abnormal anion gap (28.1 mEq/L), blood urea nitrogen (53 mg/dL), creatinine (2.9 mg/dL), creatine kinase (10,857 U/L), creatine kinase MB (432.6 ng/mL), and hyperglycemia (glucose 425 mg/dL). Arterial blood gas reveals hypoxia (Po2 of 55 mm Hg), with metabolic acidosis (sodium bicarbonate 10 with compensatory Pco2 of 33 mm Hg). Her urine is cloudy, positive for protein, ketones, hemoglobin, and glucose. She is thought to have a high anion gap acidosis related to dehydration, lactic acidosis (lactic acid 20 mEq/L), and hyperglycemia. Urine toxicology is positive for cannabinoids; ethylene glycol and methanol screen negatively, which rules these out as potential contributors to her high anion gap acidosis.
Ms. K is intubated and IV fluids are initiated for rhabdomyolysis and acute renal failure. Dialysis is implemented on a short-term basis. Her mental state improves gradually over 3 days.
The authors’ observations
Based on the abrupt onset of inattention and confusion, disorganized speech, memory impairments, and psychomotor agitation, we made an initial diagnosis of delirium; however, the precise etiology remained unclear. DSM-IV-TR diagnostic criteria for delirium are described in Table 1. Although delirium due to multiple etiologies does not have a DSM-IV-TR coding designation, we speculated that multiple causes contributed to Ms. K’s presentation. Acute renal failure secondary to dehydration as well as rhabdomyolysis, hypoxia, and hyperglycemia were implicated as general medical conditions etiologically linked to delirium. Because Ms. K has no preexisting medical conditions and her roommate and family stated she had a history of substance abuse, we also considered a presumptive diagnosis of substance-induced delirium. The medical team speculated that, based on information provided by her family, Ms. K may have had a seizure or may have fallen, which would account for her multiple contusions, and could have led to muscle injury and breakdown and the resultant rhabdomyolysis.
The possibility of cannabinoid-induced delirium has been reported, albeit rarely.1-3 However, Ms. K’s presentation—hypothermia, variable heart rate, lack of dry mucous membranes—was not consistent with significant anticholinergic toxicity or cannabinoid intoxication (Table 2).
By contrast, cocaine-induced delirium has been reported and initially appeared to be a plausible cause of Ms. K’s symptoms (Table 2). Delirium related to excess ingestion of cocaine may be related to the drug’s secondary effects resulting in rhabdomyolysis and renal dysfunction.4-6 Although several mechanisms underlying this relationship have been proposed, no single specific mechanism has been identified. The basis for cocaine ingestion and the resultant metabolic and renal effects, as observed in Ms. K’s case, likely are multifactorial. Mechanisms of the rhabdomyolysis might include:
- blockade of synaptic catecholamine reuptake and induction of adrenergic agonism, resulting in vasoconstriction and ischemia and leading to muscle damage
- cocaine-induced seizures and/or prolonged unconsciousness, leading to muscle compression and breakdown of muscle tissue
- a period of exertion induced by cocaine, precipitating an excited delirium and associated rhabdomyolysis
- a surge in dopamine concentrations, similar to neuroleptic malignant syndrome, precipitates hyperthermia, muscle rigidity, and psychomotor agitation, disrupting neuromuscular homeostasis and leading to rhabdomyolysis.
We were uncertain about the plausibility that acute cocaine intoxication caused Ms. K’s medical sequelae, in light of her toxicology findings. If cocaine use was the inciting event, and because the delirium reportedly had developed over several hours, we would expect cocaine to be detected in the toxicology screen. However, it was not detected. Cocaine can remain detectable in urine for 2 to 4 days,7 which raised our speculation that remote cocaine abuse could account for Ms. K’s current presentation and the timeline the roommate initially relayed to EMS personnel was inaccurate. We needed to clarify the timeline and progression of Ms. K’s symptoms with the roommate. In addition, we suggested to the medical team that alternative substances of abuse could be causing Ms. K’s symptoms and the roommate might be the only person who could unveil this possibility.
Table 1
DSM-IV-TR criteria for delirium due to multiple etiologies
| A. Disturbance of consciousness (ie, reduced clarity of awareness of the environment) with reduced ability to focus, sustain, or shift attention |
| B. A change in cognition (such as memory deficit, disorientation, language disturbances) or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia |
| C. The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of the day |
| D. There is evidence from the history, physical examination, or laboratory findings that the delirium has >1 etiology (eg, >1 etiological general medical condition, a general medical condition plus substance intoxication or medication side effect) |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
Table 2
Diagnostic criteria for cannabis and cocaine intoxication
| Diagnostic criteria | Cannabis intoxication | Cocaine intoxication |
|---|---|---|
| Recurrent use | + | + |
| Symptom onset | During or shortly after use | During or shortly after use |
| Behavioral changes | Impaired motor coordination | Hypervigilance, stereotyped behaviors |
| Psychological changes | Euphoria, anxiety, sensation of slowed time, social withdrawal, impaired judgment | Euphoria, anxiety, tension, anger, changes in sociability, interpersonal sensitivity, impaired social or occupational functioning |
| Associated criteria (≥2) | Conjunctival injection, increased appetite, dry mouth, tachycardia | Tachycardia or bradycardia, papillary dilation, elevated or lowered blood pressure, chills/perspiration, nausea/vomiting, evidence of weight loss, psychomotor changes, muscular weakness, chest pain, cardiac arrhythmias, seizure, dyskinesia, dystonia, delirium, coma |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | ||
HISTORY: Unknown substance
Ms. K’s roommate is contacted for supplemental history. The roommate reports that recently he observed Ms. K “snorting” a brown/tan-colored substance. He had not seen her use this substance previously, and when he asked her what it was, she reportedly said that it was “PeeVee” (also called “bath salts”) purchased over the Internet.
The authors’ observations
MDPV is a novel chemical compound that is used as a recreational drug (Table 3).8 It commonly is acquired from Internet sources and sold as “bath salts.” Its use first emerged in approximately 2004, and its popularity has been increasing because of its easy availability and relatively low cost.9 The American Association of Poison Control Centers received 302 calls related to MDPV toxicity in 2010 and 5,625 calls related to MDPV use between January 1 and October 31, 2011.10,11
MDPV has psychoactive properties, with stimulant effects acting as a norepinephrine-dopamine reuptake inhibitor.8,9,12 When snorted, ingested orally, or inserted rectally, the agent produces effects comparable to cocaine or psychostimulants such as methylphenidate or dextroamphetamine.
Acute effects of MDPV include heightened alertness, diminished need for sleep, hyperarousal, and euphoria.8,9 These symptoms often are accompanied by increases in heart rate and blood pressure, sweating, and peripheral vasoconstriction. Individuals may abuse MDPV to acquire sustained attention, reduce their need for sleep, or for aphrodisiac effects. In many cases, anxiety and irritability can accompany the desired euphoric effects. For some, the euphoric effects can be superseded by anxiety or agitation. Mood and attention effects are estimated to last 3 to 4 hours; however, tachycardia and hypertension can persist for 6 to 8 hours.
MDPV use can trigger cravings and lead to binging. Euphoric stimulation with MDPV can become dysphoric as the dose and duration of use increase. Extended use has been associated with agitation, irritability, aggression, panic and marked anxiety, psychosis, and delirium.8,9 Anxiety can range from mild dysphoric stimulation to extreme panic-like states. In moderate forms, a state of sympathetic discharge can occur, producing physiologic effects resembling panic attacks, including hypertension, tachycardia, sweating, and peripheral vasoconstriction. In more severe cases, users may experience a feeling of impending doom, marked distress, and frank psychosis. Patients may experience disorientation and unsystematized paranoid delusions. Case reports of intoxication have described self-injurious behaviors, such as cutting, which may account for the contusions observed on Ms. K’s face and arms. Increasingly, MDPV use has resulted in ER presentations with patients manifesting abrupt onset confusion, anxiety, and self-injurious behaviors.
The mechanisms underlying MDPV-induced delirium have not been definitively identified. Given the similarities in mechanism of action between MDPV and cocaine, causes for delirium related to MDPV are similarly presumed to be multifactorial. The course of delirium associated with MDPV intoxication is self-limited and requires supportive measures.8,9
Suspect MDPV abuse in patients who present with signs or symptoms of stimulant intoxication but have a negative toxicology screen for cocaine and other psychostimulants. MDPV is not detected on routine toxicology assessments; however, it can be identified through laboratories with gas chromatography/mass spectroscopy capabilities. However, the time needed to obtain the results may exceed the clinical course of the patient’s delirium. One of the limitations in Ms. K’s case was the lack of gas chromatography/mass spectroscopy to confirm MDPV ingestion. Ms. K’s roommate could not locate any unused brown powder within their apartment to bring in for laboratory investigations. Recently, screening assessments for MDPV have become commercially available (see Related Resources).
Table 3
Overview of MDPV features
| Chemical name | 3,4-methylenedioxypyrovalerone |
| Popular names | MDPV, PV, PeeVee, Super coke, Magic |
| Sources | Sold as “bath salts” by Internet sources, “head shops,” and gas stations |
| Mode of use | Oral, snorting, smoking, rectal insertion, intravenous |
| Acute effects | Increased energy, perception of heightened alertness/attention, aphrodisiac properties, increased sociability |
| Adverse psychological effects | Anxiety (panic attacks), irritability, agitation, confusion, suicidal ideations, visual distortions |
| Adverse physical effects | Insomnia/overstimulation, bruxism, muscle twitching, pupil dilation/blurred vision, anorexia, headache, nausea/vomiting, hyperthermia, irregular heart beat, tachycardia, dyspnea, fatigue |
| Effects of protracted use | Dysphoria, depression, anhedonia |
| LD50 | Unknown |
| LD50: lethal dose; MDPV: methylenedioxypyrovalerone Source: Reference 8 | |
OUTCOME: Referral to treatment
Dialysis is discontinued within 1 day of hospitalization. Ms. K’s peripheral arterial perfusion improves, as does her thermoregulatory status. Her mental status improvements coincide with improvements in her physical and metabolic status.
Ms. K is able to sustain attention when speaking with interviewers. She is aware of her surroundings and is no longer distracted by extraneous stimuli. Her speech is articulate and her thoughts are linear. There is no evidence of any residual thought disorganization, delusions, or hallucinations.
Initially, Ms. K is reluctant to acknowledge her substance use, but eventually, she concedes to acquiring a stimulant from an Internet source and abusing it in undetermined amounts. She had no experience with using MDPV and did not know how to avoid ingesting dangerous amounts. We educate Ms. K about the dangers she faced during this hospitalization and the potential life-threatening outcomes. She is amenable to pursuing outpatient substance abuse treatment. Her roommate is enlisted to facilitate her follow-up with this treatment.
The authors’ observations
Managing MDPV toxicity presents a diagnostic dilemma for medical personnel and psychiatrists when evaluating and managing acute delirium. MDPV ingestion may go unrecognized in clinical settings because toxicology assessments for it are not readily available and patients’ historical information may be unreliable.
Because of the seriousness of sequelae associated with MDPV use, state and federal agencies have intervened. Until recently, bath salts did not have a controlled substance designation. In October 2011, the US Drug Enforcement Administration (DEA) ruled to make MDPV a controlled substance for 1 year, with the possibility of a 6-month extension.13 Although this ruling is temporary, it makes possession, sale, or distribution of these chemicals, or the products that contain them, illegal in the United States. In the interim, the DEA and the US Department of Health and Human Services will determine whether MDPV should remain a controlled substance.
- American Screening Corp. (MDPV screening). www.americanscreeningcorp.com.
- U.S. Drug Enforcement Administration. 3, 4-Methylenedioxypyrovalerone (MDPV). www.deadiversion.usdoj.gov/drugs_concern/mdpv.pdf.
- Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones [published online ahead of print November 23, 2011]. J Med Toxicol. doi: 10.1007/s13181-011-0193-z.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. André C, Jaber-Filho JA, Bento RM, et al. Delirium following ingestion of marijuana in chocolate cookies. CNS Spectr. 2006;11(4):262-264.
2. Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38(1):1-20.
3. Meyer ME. Psychiatric consequences of marijuana use: the state of the evidence. In: Tinklenberg JR ed. Marijuana and health hazards: methodologic issues in current research. New York, NY: Academic Press; 1975:33–152.
4. Ruttenber AJ, Lawler-Heavner J, Yin M, et al. Fatal excited delirium following cocaine use: epidemiologic findings provide new evidence for mechanisms of cocaine toxicity. J Forensic Sci. 1997;42(1):25-31.
5. Ruttenber AJ, McAnally HB, Wetli CV. Cocaine-associated rhabdomyolysis and excited delirium: different stages of the same syndrome. Am J Forensic Med Pathol. 1999;20(2):120-127.
6. Singhal PC, Rubin RB, Peters A, et al. Rhabdomyolysis and acute renal failure associated with cocaine abuse. J Toxicol Clin Toxicol. 1990;28(3):321-330.
7. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76.
8. Psychonaut WebMapping Research Group. MDPV report. London United Kingdom: Institute of Psychiatry, King’s College. http://www.psychonautproject.eu/documents/reports/MDPV.pdf. Accessed November 23, 2011.
9. Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365(10):967-968.
10. American Association of Poison Control Centers. Bath salts data. http://www.aapcc.org/dnn/Portals/0/Bath%20Salts%20Data%20for%20Website%2011.03.2011.pdf. Updated November 3 2011. Accessed November 23, 2011.
11. Centers for Disease Control and Prevention. Emergency department visits after use of a drug sold as “bath salts”—Michigan November 13, 2010-March 31, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(19):624-627.
12. Westphal F, Junge T, Rösner P, et al. Mass and NMR spectroscopic characterization of 3, 4-methylenedioxypyrovalerone: a designer drug with α-pyrrolidinophenone structure. Forensic Sci Int. 2009;190(1-3):1-8.
13. U.S. Drug Enforcement Administration. Chemicals used in “bath salts” now under federal control and regulation. http://www.justice.gov/dea/pubs/pressrel/pr102111.html. Accessed November 23, 2011.
CASE: Hurt and confused
Emergency medical services (EMS) are called to Ms. K’s apartment after her roommate found her lying on the floor moaning. The roommate tells EMS that Ms. K, age 29, appeared confused and was slurring her words, and reports that this change in her awareness progressed rapidly over a few hours. EMS personnel find that Ms. K has multiple contusions on her arms and face, which they presume to be self-inflicted. A marijuana pipe is discovered at Ms. K’s apartment.
In the emergency room (ER), Ms. K is inattentive and has difficulty following simple commands. Her speech is mumbled and her thoughts are disorganized. She displays psychomotor restlessness in the form of combativeness. Ms. K cannot provide meaningful historical data and is disoriented to place and time. The ER staff requests a psychiatric consultation.
Family members reveal that Ms. K has no preexisting medical conditions, is not taking prescription medications, but has a history of substance abuse (sporadic cocaine and cannabis use). Her family is unaware of recent substance use.
Physical examination reveals tachycardia (heart rate 110 to 120 beats per minute), hypotension (blood pressure 78/49 mm Hg), hypothermia (temperature 88ºF), and peripheral pulse oximetry of 84%. Her pupils are dilated and reactive to light; no conjunctival injection is noted. Her lung fields are clear on auscultation, but she is noted to have a rapid, irregular heartbeat. The abdomen is positive for bowel sounds, soft on palpation, and without any repositioning or notable overt signs of tenderness. Ms. K’s toes show purple discoloration with poor capillary refill. The dorsalis pedis pulses are reported to be 1+ bilaterally; however, the remainder of the arterial pulse examination is normal.
Her sodium, potassium, and chloride values are normal, but she has an abnormal anion gap (28.1 mEq/L), blood urea nitrogen (53 mg/dL), creatinine (2.9 mg/dL), creatine kinase (10,857 U/L), creatine kinase MB (432.6 ng/mL), and hyperglycemia (glucose 425 mg/dL). Arterial blood gas reveals hypoxia (Po2 of 55 mm Hg), with metabolic acidosis (sodium bicarbonate 10 with compensatory Pco2 of 33 mm Hg). Her urine is cloudy, positive for protein, ketones, hemoglobin, and glucose. She is thought to have a high anion gap acidosis related to dehydration, lactic acidosis (lactic acid 20 mEq/L), and hyperglycemia. Urine toxicology is positive for cannabinoids; ethylene glycol and methanol screen negatively, which rules these out as potential contributors to her high anion gap acidosis.
Ms. K is intubated and IV fluids are initiated for rhabdomyolysis and acute renal failure. Dialysis is implemented on a short-term basis. Her mental state improves gradually over 3 days.
The authors’ observations
Based on the abrupt onset of inattention and confusion, disorganized speech, memory impairments, and psychomotor agitation, we made an initial diagnosis of delirium; however, the precise etiology remained unclear. DSM-IV-TR diagnostic criteria for delirium are described in Table 1. Although delirium due to multiple etiologies does not have a DSM-IV-TR coding designation, we speculated that multiple causes contributed to Ms. K’s presentation. Acute renal failure secondary to dehydration as well as rhabdomyolysis, hypoxia, and hyperglycemia were implicated as general medical conditions etiologically linked to delirium. Because Ms. K has no preexisting medical conditions and her roommate and family stated she had a history of substance abuse, we also considered a presumptive diagnosis of substance-induced delirium. The medical team speculated that, based on information provided by her family, Ms. K may have had a seizure or may have fallen, which would account for her multiple contusions, and could have led to muscle injury and breakdown and the resultant rhabdomyolysis.
The possibility of cannabinoid-induced delirium has been reported, albeit rarely.1-3 However, Ms. K’s presentation—hypothermia, variable heart rate, lack of dry mucous membranes—was not consistent with significant anticholinergic toxicity or cannabinoid intoxication (Table 2).
By contrast, cocaine-induced delirium has been reported and initially appeared to be a plausible cause of Ms. K’s symptoms (Table 2). Delirium related to excess ingestion of cocaine may be related to the drug’s secondary effects resulting in rhabdomyolysis and renal dysfunction.4-6 Although several mechanisms underlying this relationship have been proposed, no single specific mechanism has been identified. The basis for cocaine ingestion and the resultant metabolic and renal effects, as observed in Ms. K’s case, likely are multifactorial. Mechanisms of the rhabdomyolysis might include:
- blockade of synaptic catecholamine reuptake and induction of adrenergic agonism, resulting in vasoconstriction and ischemia and leading to muscle damage
- cocaine-induced seizures and/or prolonged unconsciousness, leading to muscle compression and breakdown of muscle tissue
- a period of exertion induced by cocaine, precipitating an excited delirium and associated rhabdomyolysis
- a surge in dopamine concentrations, similar to neuroleptic malignant syndrome, precipitates hyperthermia, muscle rigidity, and psychomotor agitation, disrupting neuromuscular homeostasis and leading to rhabdomyolysis.
We were uncertain about the plausibility that acute cocaine intoxication caused Ms. K’s medical sequelae, in light of her toxicology findings. If cocaine use was the inciting event, and because the delirium reportedly had developed over several hours, we would expect cocaine to be detected in the toxicology screen. However, it was not detected. Cocaine can remain detectable in urine for 2 to 4 days,7 which raised our speculation that remote cocaine abuse could account for Ms. K’s current presentation and the timeline the roommate initially relayed to EMS personnel was inaccurate. We needed to clarify the timeline and progression of Ms. K’s symptoms with the roommate. In addition, we suggested to the medical team that alternative substances of abuse could be causing Ms. K’s symptoms and the roommate might be the only person who could unveil this possibility.
Table 1
DSM-IV-TR criteria for delirium due to multiple etiologies
| A. Disturbance of consciousness (ie, reduced clarity of awareness of the environment) with reduced ability to focus, sustain, or shift attention |
| B. A change in cognition (such as memory deficit, disorientation, language disturbances) or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia |
| C. The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of the day |
| D. There is evidence from the history, physical examination, or laboratory findings that the delirium has >1 etiology (eg, >1 etiological general medical condition, a general medical condition plus substance intoxication or medication side effect) |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
Table 2
Diagnostic criteria for cannabis and cocaine intoxication
| Diagnostic criteria | Cannabis intoxication | Cocaine intoxication |
|---|---|---|
| Recurrent use | + | + |
| Symptom onset | During or shortly after use | During or shortly after use |
| Behavioral changes | Impaired motor coordination | Hypervigilance, stereotyped behaviors |
| Psychological changes | Euphoria, anxiety, sensation of slowed time, social withdrawal, impaired judgment | Euphoria, anxiety, tension, anger, changes in sociability, interpersonal sensitivity, impaired social or occupational functioning |
| Associated criteria (≥2) | Conjunctival injection, increased appetite, dry mouth, tachycardia | Tachycardia or bradycardia, papillary dilation, elevated or lowered blood pressure, chills/perspiration, nausea/vomiting, evidence of weight loss, psychomotor changes, muscular weakness, chest pain, cardiac arrhythmias, seizure, dyskinesia, dystonia, delirium, coma |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | ||
HISTORY: Unknown substance
Ms. K’s roommate is contacted for supplemental history. The roommate reports that recently he observed Ms. K “snorting” a brown/tan-colored substance. He had not seen her use this substance previously, and when he asked her what it was, she reportedly said that it was “PeeVee” (also called “bath salts”) purchased over the Internet.
The authors’ observations
MDPV is a novel chemical compound that is used as a recreational drug (Table 3).8 It commonly is acquired from Internet sources and sold as “bath salts.” Its use first emerged in approximately 2004, and its popularity has been increasing because of its easy availability and relatively low cost.9 The American Association of Poison Control Centers received 302 calls related to MDPV toxicity in 2010 and 5,625 calls related to MDPV use between January 1 and October 31, 2011.10,11
MDPV has psychoactive properties, with stimulant effects acting as a norepinephrine-dopamine reuptake inhibitor.8,9,12 When snorted, ingested orally, or inserted rectally, the agent produces effects comparable to cocaine or psychostimulants such as methylphenidate or dextroamphetamine.
Acute effects of MDPV include heightened alertness, diminished need for sleep, hyperarousal, and euphoria.8,9 These symptoms often are accompanied by increases in heart rate and blood pressure, sweating, and peripheral vasoconstriction. Individuals may abuse MDPV to acquire sustained attention, reduce their need for sleep, or for aphrodisiac effects. In many cases, anxiety and irritability can accompany the desired euphoric effects. For some, the euphoric effects can be superseded by anxiety or agitation. Mood and attention effects are estimated to last 3 to 4 hours; however, tachycardia and hypertension can persist for 6 to 8 hours.
MDPV use can trigger cravings and lead to binging. Euphoric stimulation with MDPV can become dysphoric as the dose and duration of use increase. Extended use has been associated with agitation, irritability, aggression, panic and marked anxiety, psychosis, and delirium.8,9 Anxiety can range from mild dysphoric stimulation to extreme panic-like states. In moderate forms, a state of sympathetic discharge can occur, producing physiologic effects resembling panic attacks, including hypertension, tachycardia, sweating, and peripheral vasoconstriction. In more severe cases, users may experience a feeling of impending doom, marked distress, and frank psychosis. Patients may experience disorientation and unsystematized paranoid delusions. Case reports of intoxication have described self-injurious behaviors, such as cutting, which may account for the contusions observed on Ms. K’s face and arms. Increasingly, MDPV use has resulted in ER presentations with patients manifesting abrupt onset confusion, anxiety, and self-injurious behaviors.
The mechanisms underlying MDPV-induced delirium have not been definitively identified. Given the similarities in mechanism of action between MDPV and cocaine, causes for delirium related to MDPV are similarly presumed to be multifactorial. The course of delirium associated with MDPV intoxication is self-limited and requires supportive measures.8,9
Suspect MDPV abuse in patients who present with signs or symptoms of stimulant intoxication but have a negative toxicology screen for cocaine and other psychostimulants. MDPV is not detected on routine toxicology assessments; however, it can be identified through laboratories with gas chromatography/mass spectroscopy capabilities. However, the time needed to obtain the results may exceed the clinical course of the patient’s delirium. One of the limitations in Ms. K’s case was the lack of gas chromatography/mass spectroscopy to confirm MDPV ingestion. Ms. K’s roommate could not locate any unused brown powder within their apartment to bring in for laboratory investigations. Recently, screening assessments for MDPV have become commercially available (see Related Resources).
Table 3
Overview of MDPV features
| Chemical name | 3,4-methylenedioxypyrovalerone |
| Popular names | MDPV, PV, PeeVee, Super coke, Magic |
| Sources | Sold as “bath salts” by Internet sources, “head shops,” and gas stations |
| Mode of use | Oral, snorting, smoking, rectal insertion, intravenous |
| Acute effects | Increased energy, perception of heightened alertness/attention, aphrodisiac properties, increased sociability |
| Adverse psychological effects | Anxiety (panic attacks), irritability, agitation, confusion, suicidal ideations, visual distortions |
| Adverse physical effects | Insomnia/overstimulation, bruxism, muscle twitching, pupil dilation/blurred vision, anorexia, headache, nausea/vomiting, hyperthermia, irregular heart beat, tachycardia, dyspnea, fatigue |
| Effects of protracted use | Dysphoria, depression, anhedonia |
| LD50 | Unknown |
| LD50: lethal dose; MDPV: methylenedioxypyrovalerone Source: Reference 8 | |
OUTCOME: Referral to treatment
Dialysis is discontinued within 1 day of hospitalization. Ms. K’s peripheral arterial perfusion improves, as does her thermoregulatory status. Her mental status improvements coincide with improvements in her physical and metabolic status.
Ms. K is able to sustain attention when speaking with interviewers. She is aware of her surroundings and is no longer distracted by extraneous stimuli. Her speech is articulate and her thoughts are linear. There is no evidence of any residual thought disorganization, delusions, or hallucinations.
Initially, Ms. K is reluctant to acknowledge her substance use, but eventually, she concedes to acquiring a stimulant from an Internet source and abusing it in undetermined amounts. She had no experience with using MDPV and did not know how to avoid ingesting dangerous amounts. We educate Ms. K about the dangers she faced during this hospitalization and the potential life-threatening outcomes. She is amenable to pursuing outpatient substance abuse treatment. Her roommate is enlisted to facilitate her follow-up with this treatment.
The authors’ observations
Managing MDPV toxicity presents a diagnostic dilemma for medical personnel and psychiatrists when evaluating and managing acute delirium. MDPV ingestion may go unrecognized in clinical settings because toxicology assessments for it are not readily available and patients’ historical information may be unreliable.
Because of the seriousness of sequelae associated with MDPV use, state and federal agencies have intervened. Until recently, bath salts did not have a controlled substance designation. In October 2011, the US Drug Enforcement Administration (DEA) ruled to make MDPV a controlled substance for 1 year, with the possibility of a 6-month extension.13 Although this ruling is temporary, it makes possession, sale, or distribution of these chemicals, or the products that contain them, illegal in the United States. In the interim, the DEA and the US Department of Health and Human Services will determine whether MDPV should remain a controlled substance.
- American Screening Corp. (MDPV screening). www.americanscreeningcorp.com.
- U.S. Drug Enforcement Administration. 3, 4-Methylenedioxypyrovalerone (MDPV). www.deadiversion.usdoj.gov/drugs_concern/mdpv.pdf.
- Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones [published online ahead of print November 23, 2011]. J Med Toxicol. doi: 10.1007/s13181-011-0193-z.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Hurt and confused
Emergency medical services (EMS) are called to Ms. K’s apartment after her roommate found her lying on the floor moaning. The roommate tells EMS that Ms. K, age 29, appeared confused and was slurring her words, and reports that this change in her awareness progressed rapidly over a few hours. EMS personnel find that Ms. K has multiple contusions on her arms and face, which they presume to be self-inflicted. A marijuana pipe is discovered at Ms. K’s apartment.
In the emergency room (ER), Ms. K is inattentive and has difficulty following simple commands. Her speech is mumbled and her thoughts are disorganized. She displays psychomotor restlessness in the form of combativeness. Ms. K cannot provide meaningful historical data and is disoriented to place and time. The ER staff requests a psychiatric consultation.
Family members reveal that Ms. K has no preexisting medical conditions, is not taking prescription medications, but has a history of substance abuse (sporadic cocaine and cannabis use). Her family is unaware of recent substance use.
Physical examination reveals tachycardia (heart rate 110 to 120 beats per minute), hypotension (blood pressure 78/49 mm Hg), hypothermia (temperature 88ºF), and peripheral pulse oximetry of 84%. Her pupils are dilated and reactive to light; no conjunctival injection is noted. Her lung fields are clear on auscultation, but she is noted to have a rapid, irregular heartbeat. The abdomen is positive for bowel sounds, soft on palpation, and without any repositioning or notable overt signs of tenderness. Ms. K’s toes show purple discoloration with poor capillary refill. The dorsalis pedis pulses are reported to be 1+ bilaterally; however, the remainder of the arterial pulse examination is normal.
Her sodium, potassium, and chloride values are normal, but she has an abnormal anion gap (28.1 mEq/L), blood urea nitrogen (53 mg/dL), creatinine (2.9 mg/dL), creatine kinase (10,857 U/L), creatine kinase MB (432.6 ng/mL), and hyperglycemia (glucose 425 mg/dL). Arterial blood gas reveals hypoxia (Po2 of 55 mm Hg), with metabolic acidosis (sodium bicarbonate 10 with compensatory Pco2 of 33 mm Hg). Her urine is cloudy, positive for protein, ketones, hemoglobin, and glucose. She is thought to have a high anion gap acidosis related to dehydration, lactic acidosis (lactic acid 20 mEq/L), and hyperglycemia. Urine toxicology is positive for cannabinoids; ethylene glycol and methanol screen negatively, which rules these out as potential contributors to her high anion gap acidosis.
Ms. K is intubated and IV fluids are initiated for rhabdomyolysis and acute renal failure. Dialysis is implemented on a short-term basis. Her mental state improves gradually over 3 days.
The authors’ observations
Based on the abrupt onset of inattention and confusion, disorganized speech, memory impairments, and psychomotor agitation, we made an initial diagnosis of delirium; however, the precise etiology remained unclear. DSM-IV-TR diagnostic criteria for delirium are described in Table 1. Although delirium due to multiple etiologies does not have a DSM-IV-TR coding designation, we speculated that multiple causes contributed to Ms. K’s presentation. Acute renal failure secondary to dehydration as well as rhabdomyolysis, hypoxia, and hyperglycemia were implicated as general medical conditions etiologically linked to delirium. Because Ms. K has no preexisting medical conditions and her roommate and family stated she had a history of substance abuse, we also considered a presumptive diagnosis of substance-induced delirium. The medical team speculated that, based on information provided by her family, Ms. K may have had a seizure or may have fallen, which would account for her multiple contusions, and could have led to muscle injury and breakdown and the resultant rhabdomyolysis.
The possibility of cannabinoid-induced delirium has been reported, albeit rarely.1-3 However, Ms. K’s presentation—hypothermia, variable heart rate, lack of dry mucous membranes—was not consistent with significant anticholinergic toxicity or cannabinoid intoxication (Table 2).
By contrast, cocaine-induced delirium has been reported and initially appeared to be a plausible cause of Ms. K’s symptoms (Table 2). Delirium related to excess ingestion of cocaine may be related to the drug’s secondary effects resulting in rhabdomyolysis and renal dysfunction.4-6 Although several mechanisms underlying this relationship have been proposed, no single specific mechanism has been identified. The basis for cocaine ingestion and the resultant metabolic and renal effects, as observed in Ms. K’s case, likely are multifactorial. Mechanisms of the rhabdomyolysis might include:
- blockade of synaptic catecholamine reuptake and induction of adrenergic agonism, resulting in vasoconstriction and ischemia and leading to muscle damage
- cocaine-induced seizures and/or prolonged unconsciousness, leading to muscle compression and breakdown of muscle tissue
- a period of exertion induced by cocaine, precipitating an excited delirium and associated rhabdomyolysis
- a surge in dopamine concentrations, similar to neuroleptic malignant syndrome, precipitates hyperthermia, muscle rigidity, and psychomotor agitation, disrupting neuromuscular homeostasis and leading to rhabdomyolysis.
We were uncertain about the plausibility that acute cocaine intoxication caused Ms. K’s medical sequelae, in light of her toxicology findings. If cocaine use was the inciting event, and because the delirium reportedly had developed over several hours, we would expect cocaine to be detected in the toxicology screen. However, it was not detected. Cocaine can remain detectable in urine for 2 to 4 days,7 which raised our speculation that remote cocaine abuse could account for Ms. K’s current presentation and the timeline the roommate initially relayed to EMS personnel was inaccurate. We needed to clarify the timeline and progression of Ms. K’s symptoms with the roommate. In addition, we suggested to the medical team that alternative substances of abuse could be causing Ms. K’s symptoms and the roommate might be the only person who could unveil this possibility.
Table 1
DSM-IV-TR criteria for delirium due to multiple etiologies
| A. Disturbance of consciousness (ie, reduced clarity of awareness of the environment) with reduced ability to focus, sustain, or shift attention |
| B. A change in cognition (such as memory deficit, disorientation, language disturbances) or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia |
| C. The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of the day |
| D. There is evidence from the history, physical examination, or laboratory findings that the delirium has >1 etiology (eg, >1 etiological general medical condition, a general medical condition plus substance intoxication or medication side effect) |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
Table 2
Diagnostic criteria for cannabis and cocaine intoxication
| Diagnostic criteria | Cannabis intoxication | Cocaine intoxication |
|---|---|---|
| Recurrent use | + | + |
| Symptom onset | During or shortly after use | During or shortly after use |
| Behavioral changes | Impaired motor coordination | Hypervigilance, stereotyped behaviors |
| Psychological changes | Euphoria, anxiety, sensation of slowed time, social withdrawal, impaired judgment | Euphoria, anxiety, tension, anger, changes in sociability, interpersonal sensitivity, impaired social or occupational functioning |
| Associated criteria (≥2) | Conjunctival injection, increased appetite, dry mouth, tachycardia | Tachycardia or bradycardia, papillary dilation, elevated or lowered blood pressure, chills/perspiration, nausea/vomiting, evidence of weight loss, psychomotor changes, muscular weakness, chest pain, cardiac arrhythmias, seizure, dyskinesia, dystonia, delirium, coma |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | ||
HISTORY: Unknown substance
Ms. K’s roommate is contacted for supplemental history. The roommate reports that recently he observed Ms. K “snorting” a brown/tan-colored substance. He had not seen her use this substance previously, and when he asked her what it was, she reportedly said that it was “PeeVee” (also called “bath salts”) purchased over the Internet.
The authors’ observations
MDPV is a novel chemical compound that is used as a recreational drug (Table 3).8 It commonly is acquired from Internet sources and sold as “bath salts.” Its use first emerged in approximately 2004, and its popularity has been increasing because of its easy availability and relatively low cost.9 The American Association of Poison Control Centers received 302 calls related to MDPV toxicity in 2010 and 5,625 calls related to MDPV use between January 1 and October 31, 2011.10,11
MDPV has psychoactive properties, with stimulant effects acting as a norepinephrine-dopamine reuptake inhibitor.8,9,12 When snorted, ingested orally, or inserted rectally, the agent produces effects comparable to cocaine or psychostimulants such as methylphenidate or dextroamphetamine.
Acute effects of MDPV include heightened alertness, diminished need for sleep, hyperarousal, and euphoria.8,9 These symptoms often are accompanied by increases in heart rate and blood pressure, sweating, and peripheral vasoconstriction. Individuals may abuse MDPV to acquire sustained attention, reduce their need for sleep, or for aphrodisiac effects. In many cases, anxiety and irritability can accompany the desired euphoric effects. For some, the euphoric effects can be superseded by anxiety or agitation. Mood and attention effects are estimated to last 3 to 4 hours; however, tachycardia and hypertension can persist for 6 to 8 hours.
MDPV use can trigger cravings and lead to binging. Euphoric stimulation with MDPV can become dysphoric as the dose and duration of use increase. Extended use has been associated with agitation, irritability, aggression, panic and marked anxiety, psychosis, and delirium.8,9 Anxiety can range from mild dysphoric stimulation to extreme panic-like states. In moderate forms, a state of sympathetic discharge can occur, producing physiologic effects resembling panic attacks, including hypertension, tachycardia, sweating, and peripheral vasoconstriction. In more severe cases, users may experience a feeling of impending doom, marked distress, and frank psychosis. Patients may experience disorientation and unsystematized paranoid delusions. Case reports of intoxication have described self-injurious behaviors, such as cutting, which may account for the contusions observed on Ms. K’s face and arms. Increasingly, MDPV use has resulted in ER presentations with patients manifesting abrupt onset confusion, anxiety, and self-injurious behaviors.
The mechanisms underlying MDPV-induced delirium have not been definitively identified. Given the similarities in mechanism of action between MDPV and cocaine, causes for delirium related to MDPV are similarly presumed to be multifactorial. The course of delirium associated with MDPV intoxication is self-limited and requires supportive measures.8,9
Suspect MDPV abuse in patients who present with signs or symptoms of stimulant intoxication but have a negative toxicology screen for cocaine and other psychostimulants. MDPV is not detected on routine toxicology assessments; however, it can be identified through laboratories with gas chromatography/mass spectroscopy capabilities. However, the time needed to obtain the results may exceed the clinical course of the patient’s delirium. One of the limitations in Ms. K’s case was the lack of gas chromatography/mass spectroscopy to confirm MDPV ingestion. Ms. K’s roommate could not locate any unused brown powder within their apartment to bring in for laboratory investigations. Recently, screening assessments for MDPV have become commercially available (see Related Resources).
Table 3
Overview of MDPV features
| Chemical name | 3,4-methylenedioxypyrovalerone |
| Popular names | MDPV, PV, PeeVee, Super coke, Magic |
| Sources | Sold as “bath salts” by Internet sources, “head shops,” and gas stations |
| Mode of use | Oral, snorting, smoking, rectal insertion, intravenous |
| Acute effects | Increased energy, perception of heightened alertness/attention, aphrodisiac properties, increased sociability |
| Adverse psychological effects | Anxiety (panic attacks), irritability, agitation, confusion, suicidal ideations, visual distortions |
| Adverse physical effects | Insomnia/overstimulation, bruxism, muscle twitching, pupil dilation/blurred vision, anorexia, headache, nausea/vomiting, hyperthermia, irregular heart beat, tachycardia, dyspnea, fatigue |
| Effects of protracted use | Dysphoria, depression, anhedonia |
| LD50 | Unknown |
| LD50: lethal dose; MDPV: methylenedioxypyrovalerone Source: Reference 8 | |
OUTCOME: Referral to treatment
Dialysis is discontinued within 1 day of hospitalization. Ms. K’s peripheral arterial perfusion improves, as does her thermoregulatory status. Her mental status improvements coincide with improvements in her physical and metabolic status.
Ms. K is able to sustain attention when speaking with interviewers. She is aware of her surroundings and is no longer distracted by extraneous stimuli. Her speech is articulate and her thoughts are linear. There is no evidence of any residual thought disorganization, delusions, or hallucinations.
Initially, Ms. K is reluctant to acknowledge her substance use, but eventually, she concedes to acquiring a stimulant from an Internet source and abusing it in undetermined amounts. She had no experience with using MDPV and did not know how to avoid ingesting dangerous amounts. We educate Ms. K about the dangers she faced during this hospitalization and the potential life-threatening outcomes. She is amenable to pursuing outpatient substance abuse treatment. Her roommate is enlisted to facilitate her follow-up with this treatment.
The authors’ observations
Managing MDPV toxicity presents a diagnostic dilemma for medical personnel and psychiatrists when evaluating and managing acute delirium. MDPV ingestion may go unrecognized in clinical settings because toxicology assessments for it are not readily available and patients’ historical information may be unreliable.
Because of the seriousness of sequelae associated with MDPV use, state and federal agencies have intervened. Until recently, bath salts did not have a controlled substance designation. In October 2011, the US Drug Enforcement Administration (DEA) ruled to make MDPV a controlled substance for 1 year, with the possibility of a 6-month extension.13 Although this ruling is temporary, it makes possession, sale, or distribution of these chemicals, or the products that contain them, illegal in the United States. In the interim, the DEA and the US Department of Health and Human Services will determine whether MDPV should remain a controlled substance.
- American Screening Corp. (MDPV screening). www.americanscreeningcorp.com.
- U.S. Drug Enforcement Administration. 3, 4-Methylenedioxypyrovalerone (MDPV). www.deadiversion.usdoj.gov/drugs_concern/mdpv.pdf.
- Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones [published online ahead of print November 23, 2011]. J Med Toxicol. doi: 10.1007/s13181-011-0193-z.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. André C, Jaber-Filho JA, Bento RM, et al. Delirium following ingestion of marijuana in chocolate cookies. CNS Spectr. 2006;11(4):262-264.
2. Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38(1):1-20.
3. Meyer ME. Psychiatric consequences of marijuana use: the state of the evidence. In: Tinklenberg JR ed. Marijuana and health hazards: methodologic issues in current research. New York, NY: Academic Press; 1975:33–152.
4. Ruttenber AJ, Lawler-Heavner J, Yin M, et al. Fatal excited delirium following cocaine use: epidemiologic findings provide new evidence for mechanisms of cocaine toxicity. J Forensic Sci. 1997;42(1):25-31.
5. Ruttenber AJ, McAnally HB, Wetli CV. Cocaine-associated rhabdomyolysis and excited delirium: different stages of the same syndrome. Am J Forensic Med Pathol. 1999;20(2):120-127.
6. Singhal PC, Rubin RB, Peters A, et al. Rhabdomyolysis and acute renal failure associated with cocaine abuse. J Toxicol Clin Toxicol. 1990;28(3):321-330.
7. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76.
8. Psychonaut WebMapping Research Group. MDPV report. London United Kingdom: Institute of Psychiatry, King’s College. http://www.psychonautproject.eu/documents/reports/MDPV.pdf. Accessed November 23, 2011.
9. Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365(10):967-968.
10. American Association of Poison Control Centers. Bath salts data. http://www.aapcc.org/dnn/Portals/0/Bath%20Salts%20Data%20for%20Website%2011.03.2011.pdf. Updated November 3 2011. Accessed November 23, 2011.
11. Centers for Disease Control and Prevention. Emergency department visits after use of a drug sold as “bath salts”—Michigan November 13, 2010-March 31, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(19):624-627.
12. Westphal F, Junge T, Rösner P, et al. Mass and NMR spectroscopic characterization of 3, 4-methylenedioxypyrovalerone: a designer drug with α-pyrrolidinophenone structure. Forensic Sci Int. 2009;190(1-3):1-8.
13. U.S. Drug Enforcement Administration. Chemicals used in “bath salts” now under federal control and regulation. http://www.justice.gov/dea/pubs/pressrel/pr102111.html. Accessed November 23, 2011.
1. André C, Jaber-Filho JA, Bento RM, et al. Delirium following ingestion of marijuana in chocolate cookies. CNS Spectr. 2006;11(4):262-264.
2. Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38(1):1-20.
3. Meyer ME. Psychiatric consequences of marijuana use: the state of the evidence. In: Tinklenberg JR ed. Marijuana and health hazards: methodologic issues in current research. New York, NY: Academic Press; 1975:33–152.
4. Ruttenber AJ, Lawler-Heavner J, Yin M, et al. Fatal excited delirium following cocaine use: epidemiologic findings provide new evidence for mechanisms of cocaine toxicity. J Forensic Sci. 1997;42(1):25-31.
5. Ruttenber AJ, McAnally HB, Wetli CV. Cocaine-associated rhabdomyolysis and excited delirium: different stages of the same syndrome. Am J Forensic Med Pathol. 1999;20(2):120-127.
6. Singhal PC, Rubin RB, Peters A, et al. Rhabdomyolysis and acute renal failure associated with cocaine abuse. J Toxicol Clin Toxicol. 1990;28(3):321-330.
7. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76.
8. Psychonaut WebMapping Research Group. MDPV report. London United Kingdom: Institute of Psychiatry, King’s College. http://www.psychonautproject.eu/documents/reports/MDPV.pdf. Accessed November 23, 2011.
9. Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365(10):967-968.
10. American Association of Poison Control Centers. Bath salts data. http://www.aapcc.org/dnn/Portals/0/Bath%20Salts%20Data%20for%20Website%2011.03.2011.pdf. Updated November 3 2011. Accessed November 23, 2011.
11. Centers for Disease Control and Prevention. Emergency department visits after use of a drug sold as “bath salts”—Michigan November 13, 2010-March 31, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(19):624-627.
12. Westphal F, Junge T, Rösner P, et al. Mass and NMR spectroscopic characterization of 3, 4-methylenedioxypyrovalerone: a designer drug with α-pyrrolidinophenone structure. Forensic Sci Int. 2009;190(1-3):1-8.
13. U.S. Drug Enforcement Administration. Chemicals used in “bath salts” now under federal control and regulation. http://www.justice.gov/dea/pubs/pressrel/pr102111.html. Accessed November 23, 2011.
Additional traits
I find Current Psychiatry to be exceedingly useful for myself and the physician assistant students I teach. I agree with the 7 domains in Dr. Nasrallah’s editorial (“The model psychiatrist: 7 domains of excellence,” From the Editor, Current Psychiatry, November 2011, p. 5-6). However, I would like to add 2 more traits:
- The role that a psychiatrist plays in his or her family, especially with their children, because ignoring one’s family in the pursuit of clinical sainthood is not a mark of greatness
- The psychiatrist today is more of a team member than team leader. Failure to recognize this role creates intolerable stresses on the treatment environment in which the psychiatrist works. This does not minimize the need for personal excellence, but it certainly helps decrease destructive narcissism.
Kim J. Masters, MD
Medical Director
Three Rivers Midlands Campus Residential Treatment Center
West Columbia, SC
I find Current Psychiatry to be exceedingly useful for myself and the physician assistant students I teach. I agree with the 7 domains in Dr. Nasrallah’s editorial (“The model psychiatrist: 7 domains of excellence,” From the Editor, Current Psychiatry, November 2011, p. 5-6). However, I would like to add 2 more traits:
- The role that a psychiatrist plays in his or her family, especially with their children, because ignoring one’s family in the pursuit of clinical sainthood is not a mark of greatness
- The psychiatrist today is more of a team member than team leader. Failure to recognize this role creates intolerable stresses on the treatment environment in which the psychiatrist works. This does not minimize the need for personal excellence, but it certainly helps decrease destructive narcissism.
Kim J. Masters, MD
Medical Director
Three Rivers Midlands Campus Residential Treatment Center
West Columbia, SC
I find Current Psychiatry to be exceedingly useful for myself and the physician assistant students I teach. I agree with the 7 domains in Dr. Nasrallah’s editorial (“The model psychiatrist: 7 domains of excellence,” From the Editor, Current Psychiatry, November 2011, p. 5-6). However, I would like to add 2 more traits:
- The role that a psychiatrist plays in his or her family, especially with their children, because ignoring one’s family in the pursuit of clinical sainthood is not a mark of greatness
- The psychiatrist today is more of a team member than team leader. Failure to recognize this role creates intolerable stresses on the treatment environment in which the psychiatrist works. This does not minimize the need for personal excellence, but it certainly helps decrease destructive narcissism.
Kim J. Masters, MD
Medical Director
Three Rivers Midlands Campus Residential Treatment Center
West Columbia, SC
Domains of excellence
I want to thank Dr. Nasrallah, whose monthly comments I find interesting and provocative, for his first-of-its-kind description of the “ideal” psychiatrist’s role and identity (“The model psychiatrist: 7 domains of excellence,” From the Editor, Current Psychiatry, November 2011, p. 5-6). This article should be mandatory reading and discussion material for every psychiatry residency program. For this elder psychiatrist, it was a thoughtful review of where I have been and where I am going in my field.
Ronald Blank, MD
Private Practice
Easthampton, MA
I want to thank Dr. Nasrallah, whose monthly comments I find interesting and provocative, for his first-of-its-kind description of the “ideal” psychiatrist’s role and identity (“The model psychiatrist: 7 domains of excellence,” From the Editor, Current Psychiatry, November 2011, p. 5-6). This article should be mandatory reading and discussion material for every psychiatry residency program. For this elder psychiatrist, it was a thoughtful review of where I have been and where I am going in my field.
Ronald Blank, MD
Private Practice
Easthampton, MA
I want to thank Dr. Nasrallah, whose monthly comments I find interesting and provocative, for his first-of-its-kind description of the “ideal” psychiatrist’s role and identity (“The model psychiatrist: 7 domains of excellence,” From the Editor, Current Psychiatry, November 2011, p. 5-6). This article should be mandatory reading and discussion material for every psychiatry residency program. For this elder psychiatrist, it was a thoughtful review of where I have been and where I am going in my field.
Ronald Blank, MD
Private Practice
Easthampton, MA
Concerns about valproate
I read Dr. Jain and Ms. Beste’s Pearl on treating alopecia developing during valproate use ("Valproate-induced hair loss: What to tell patients," Current Psychiatry, November 2011, p. 74) with some dismay.
Valproate is a valuable drug that has demonstrated efficacy in treating bipolar disorder; however, valproate use is associated with substantial side effects for women and developing fetuses.
I take no issue with any of the points made in the article, but I am concerned about the failure to mention critical side effects associated with valproate, including:
- weight gain and metabolic side effects
- for women, polycystic ovary syndrome—a serious and difficult-to-treat complication
- danger to fetuses—recent research suggests marked reductions in intelligence quotient in babies exposed to valproate in utero.1
We would be wise to remind ourselves of these issues whenever considering initiating or continuing valproate therapy.
Edward Pontius, MD, DFAPA
Private Practice
Brunswick, ME
References
1. Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360(16):1597-1605.
The authors respond
The concerns expressed by Dr. Pontius regarding clinical use of valproate are genuine and worthy. The purpose of our article was to call attention to a lesser-known side effect of valproate and how to intervene. We assumed that clinicians would discuss with patients the teratogenicity of valproate, along with other common side effects—weight gain, pancreatitis, effect on liver function tests, thrombocytopenia, and polycystic ovary syndrome—before initiating the drug. Such discussion about valproate was beyond the scope of our article, but we thank Dr. Pontius for bringing these concerns to our attention.
Shailesh Jain, MD, MPH, ABDA
Regional Chair
Associate Professor
Department of Psychiatry
Beth Beste, MS
Fourth-Year Medical Student
Texas Tech University Health Sciences Center, Permian Basin
Odessa, TX
I read Dr. Jain and Ms. Beste’s Pearl on treating alopecia developing during valproate use ("Valproate-induced hair loss: What to tell patients," Current Psychiatry, November 2011, p. 74) with some dismay.
Valproate is a valuable drug that has demonstrated efficacy in treating bipolar disorder; however, valproate use is associated with substantial side effects for women and developing fetuses.
I take no issue with any of the points made in the article, but I am concerned about the failure to mention critical side effects associated with valproate, including:
- weight gain and metabolic side effects
- for women, polycystic ovary syndrome—a serious and difficult-to-treat complication
- danger to fetuses—recent research suggests marked reductions in intelligence quotient in babies exposed to valproate in utero.1
We would be wise to remind ourselves of these issues whenever considering initiating or continuing valproate therapy.
Edward Pontius, MD, DFAPA
Private Practice
Brunswick, ME
References
1. Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360(16):1597-1605.
The authors respond
The concerns expressed by Dr. Pontius regarding clinical use of valproate are genuine and worthy. The purpose of our article was to call attention to a lesser-known side effect of valproate and how to intervene. We assumed that clinicians would discuss with patients the teratogenicity of valproate, along with other common side effects—weight gain, pancreatitis, effect on liver function tests, thrombocytopenia, and polycystic ovary syndrome—before initiating the drug. Such discussion about valproate was beyond the scope of our article, but we thank Dr. Pontius for bringing these concerns to our attention.
Shailesh Jain, MD, MPH, ABDA
Regional Chair
Associate Professor
Department of Psychiatry
Beth Beste, MS
Fourth-Year Medical Student
Texas Tech University Health Sciences Center, Permian Basin
Odessa, TX
I read Dr. Jain and Ms. Beste’s Pearl on treating alopecia developing during valproate use ("Valproate-induced hair loss: What to tell patients," Current Psychiatry, November 2011, p. 74) with some dismay.
Valproate is a valuable drug that has demonstrated efficacy in treating bipolar disorder; however, valproate use is associated with substantial side effects for women and developing fetuses.
I take no issue with any of the points made in the article, but I am concerned about the failure to mention critical side effects associated with valproate, including:
- weight gain and metabolic side effects
- for women, polycystic ovary syndrome—a serious and difficult-to-treat complication
- danger to fetuses—recent research suggests marked reductions in intelligence quotient in babies exposed to valproate in utero.1
We would be wise to remind ourselves of these issues whenever considering initiating or continuing valproate therapy.
Edward Pontius, MD, DFAPA
Private Practice
Brunswick, ME
References
1. Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360(16):1597-1605.
The authors respond
The concerns expressed by Dr. Pontius regarding clinical use of valproate are genuine and worthy. The purpose of our article was to call attention to a lesser-known side effect of valproate and how to intervene. We assumed that clinicians would discuss with patients the teratogenicity of valproate, along with other common side effects—weight gain, pancreatitis, effect on liver function tests, thrombocytopenia, and polycystic ovary syndrome—before initiating the drug. Such discussion about valproate was beyond the scope of our article, but we thank Dr. Pontius for bringing these concerns to our attention.
Shailesh Jain, MD, MPH, ABDA
Regional Chair
Associate Professor
Department of Psychiatry
Beth Beste, MS
Fourth-Year Medical Student
Texas Tech University Health Sciences Center, Permian Basin
Odessa, TX
Comments & Controversies
Concerns about valproate
I read Dr. Jain and Ms. Beste’s Pearl on treating alopecia developing during valproate use ("Valproate-induced hair loss: What to tell patients," Current Psychiatry, November 2011, p. 74) with some dismay.
Valproate is a valuable drug that has demonstrated efficacy in treating bipolar disorder; however, valproate use is associated with substantial side effects for women and developing fetuses.
I take no issue with any of the points made in the article, but I am concerned about the failure to mention critical side effects associated with valproate, including:
- weight gain and metabolic side effects
- for women, polycystic ovary syndrome—a serious and difficult-to-treat complication
- danger to fetuses—recent research suggests marked reductions in intelligence quotient in babies exposed to valproate in utero.1
We would be wise to remind ourselves of these issues whenever considering initiating or continuing valproate therapy.
Edward Pontius, MD, DFAPA
Private Practice
Brunswick, ME
The authors respond
The concerns expressed by Dr. Pontius regarding clinical use of valproate are genuine and worthy. The purpose of our article was to call attention to a lesser-known side effect of valproate and how to intervene. We assumed that clinicians would discuss with patients the teratogenicity of valproate, along with other common side effects—weight gain, pancreatitis, effect on liver function tests, thrombocytopenia, and polycystic ovary syndrome—before initiating the drug. Such discussion about valproate was beyond the scope of our article, but we thank Dr. Pontius for bringing these concerns to our attention.
Shailesh Jain, MD, MPH, ABDA
Regional Chair
Associate Professor
Department of Psychiatry
Beth Beste, MS
Fourth-Year Medical Student
Texas Tech University Health Sciences Center, Permian Basin
Odessa, TX
Domains of excellence
I want to thank Dr. Nasrallah, whose monthly comments I find interesting and provocative, for his first-of-its-kind description of the “ideal” psychiatrist’s role and identity (“The model psychiatrist: 7 domains of excellence,” From the Editor, Current Psychiatry, November 2011, p. 5-6). This article should be mandatory reading and discussion material for every psychiatry residency program. For this elder psychiatrist, it was a thoughtful review of where I have been and where I am going in my field.
Ronald Blank, MD
Private Practice
Easthampton, MA
Additional traits
I find Current Psychiatry to be exceedingly useful for myself and the physician assistant students I teach. I agree with the 7 domains in Dr. Nasrallah’s editorial (“The model psychiatrist: 7 domains of excellence,” From the Editor, Current Psychiatry, November 2011, p. 5-6). However, I would like to add 2 more traits:
- The role that a psychiatrist plays in his or her family, especially with their children, because ignoring one’s family in the pursuit of clinical sainthood is not a mark of greatness
- The psychiatrist today is more of a team member than team leader. Failure to recognize this role creates intolerable stresses on the treatment environment in which the psychiatrist works. This does not minimize the need for personal excellence, but it certainly helps decrease destructive narcissism.
Kim J. Masters, MD
Medical Director
Three Rivers Midlands Campus Residential Treatment Center
West Columbia, SC
Concerns about valproate
I read Dr. Jain and Ms. Beste’s Pearl on treating alopecia developing during valproate use ("Valproate-induced hair loss: What to tell patients," Current Psychiatry, November 2011, p. 74) with some dismay.
Valproate is a valuable drug that has demonstrated efficacy in treating bipolar disorder; however, valproate use is associated with substantial side effects for women and developing fetuses.
I take no issue with any of the points made in the article, but I am concerned about the failure to mention critical side effects associated with valproate, including:
- weight gain and metabolic side effects
- for women, polycystic ovary syndrome—a serious and difficult-to-treat complication
- danger to fetuses—recent research suggests marked reductions in intelligence quotient in babies exposed to valproate in utero.1
We would be wise to remind ourselves of these issues whenever considering initiating or continuing valproate therapy.
Edward Pontius, MD, DFAPA
Private Practice
Brunswick, ME
The authors respond
The concerns expressed by Dr. Pontius regarding clinical use of valproate are genuine and worthy. The purpose of our article was to call attention to a lesser-known side effect of valproate and how to intervene. We assumed that clinicians would discuss with patients the teratogenicity of valproate, along with other common side effects—weight gain, pancreatitis, effect on liver function tests, thrombocytopenia, and polycystic ovary syndrome—before initiating the drug. Such discussion about valproate was beyond the scope of our article, but we thank Dr. Pontius for bringing these concerns to our attention.
Shailesh Jain, MD, MPH, ABDA
Regional Chair
Associate Professor
Department of Psychiatry
Beth Beste, MS
Fourth-Year Medical Student
Texas Tech University Health Sciences Center, Permian Basin
Odessa, TX
Domains of excellence
I want to thank Dr. Nasrallah, whose monthly comments I find interesting and provocative, for his first-of-its-kind description of the “ideal” psychiatrist’s role and identity (“The model psychiatrist: 7 domains of excellence,” From the Editor, Current Psychiatry, November 2011, p. 5-6). This article should be mandatory reading and discussion material for every psychiatry residency program. For this elder psychiatrist, it was a thoughtful review of where I have been and where I am going in my field.
Ronald Blank, MD
Private Practice
Easthampton, MA
Additional traits
I find Current Psychiatry to be exceedingly useful for myself and the physician assistant students I teach. I agree with the 7 domains in Dr. Nasrallah’s editorial (“The model psychiatrist: 7 domains of excellence,” From the Editor, Current Psychiatry, November 2011, p. 5-6). However, I would like to add 2 more traits:
- The role that a psychiatrist plays in his or her family, especially with their children, because ignoring one’s family in the pursuit of clinical sainthood is not a mark of greatness
- The psychiatrist today is more of a team member than team leader. Failure to recognize this role creates intolerable stresses on the treatment environment in which the psychiatrist works. This does not minimize the need for personal excellence, but it certainly helps decrease destructive narcissism.
Kim J. Masters, MD
Medical Director
Three Rivers Midlands Campus Residential Treatment Center
West Columbia, SC
Concerns about valproate
I read Dr. Jain and Ms. Beste’s Pearl on treating alopecia developing during valproate use ("Valproate-induced hair loss: What to tell patients," Current Psychiatry, November 2011, p. 74) with some dismay.
Valproate is a valuable drug that has demonstrated efficacy in treating bipolar disorder; however, valproate use is associated with substantial side effects for women and developing fetuses.
I take no issue with any of the points made in the article, but I am concerned about the failure to mention critical side effects associated with valproate, including:
- weight gain and metabolic side effects
- for women, polycystic ovary syndrome—a serious and difficult-to-treat complication
- danger to fetuses—recent research suggests marked reductions in intelligence quotient in babies exposed to valproate in utero.1
We would be wise to remind ourselves of these issues whenever considering initiating or continuing valproate therapy.
Edward Pontius, MD, DFAPA
Private Practice
Brunswick, ME
The authors respond
The concerns expressed by Dr. Pontius regarding clinical use of valproate are genuine and worthy. The purpose of our article was to call attention to a lesser-known side effect of valproate and how to intervene. We assumed that clinicians would discuss with patients the teratogenicity of valproate, along with other common side effects—weight gain, pancreatitis, effect on liver function tests, thrombocytopenia, and polycystic ovary syndrome—before initiating the drug. Such discussion about valproate was beyond the scope of our article, but we thank Dr. Pontius for bringing these concerns to our attention.
Shailesh Jain, MD, MPH, ABDA
Regional Chair
Associate Professor
Department of Psychiatry
Beth Beste, MS
Fourth-Year Medical Student
Texas Tech University Health Sciences Center, Permian Basin
Odessa, TX
Domains of excellence
I want to thank Dr. Nasrallah, whose monthly comments I find interesting and provocative, for his first-of-its-kind description of the “ideal” psychiatrist’s role and identity (“The model psychiatrist: 7 domains of excellence,” From the Editor, Current Psychiatry, November 2011, p. 5-6). This article should be mandatory reading and discussion material for every psychiatry residency program. For this elder psychiatrist, it was a thoughtful review of where I have been and where I am going in my field.
Ronald Blank, MD
Private Practice
Easthampton, MA
Additional traits
I find Current Psychiatry to be exceedingly useful for myself and the physician assistant students I teach. I agree with the 7 domains in Dr. Nasrallah’s editorial (“The model psychiatrist: 7 domains of excellence,” From the Editor, Current Psychiatry, November 2011, p. 5-6). However, I would like to add 2 more traits:
- The role that a psychiatrist plays in his or her family, especially with their children, because ignoring one’s family in the pursuit of clinical sainthood is not a mark of greatness
- The psychiatrist today is more of a team member than team leader. Failure to recognize this role creates intolerable stresses on the treatment environment in which the psychiatrist works. This does not minimize the need for personal excellence, but it certainly helps decrease destructive narcissism.
Kim J. Masters, MD
Medical Director
Three Rivers Midlands Campus Residential Treatment Center
West Columbia, SC
Smoking cessation: What to tell patients about over-the-counter treatments
Discuss this article at www.facebook.com/CurrentPsychiatry
• Over-the-counter smoking cessation products likely will be the most appropriate first-line choice for many individuals before trying prescription products.
• Instruct patients to avoid smoking while using nicotine replacement therapy and educate them about the immediate and long-term benefits of quitting.
• Encourage patients to seek psychosocial counseling along with pharmacotherapy.
• Urge patients to engage in other quitting strategies by referring them to online and telephone resources (Related Resources). Also, encourage them to attend follow-up appointments to assess cessation therapy.
Mr. T, age 56, has major depressive disorder that is well controlled with fluoxetine, 40 mg/d. He has smoked ≥1 packs of cigarettes per day for the last 25 years. On a recent visit, he indicates that he has begun using a 21-mg nicotine patch as advised by his pharmacist and that things are going OK, although he has had some “slip ups.” He is on week 7 of his quitting regimen and now is stepping down the patch dosage.
Upon further questioning he says that he has been cutting the 21-mg patches in half to save money. Mr. T also explains that occasionally he has given in to a strong urge to smoke because it was “too much to handle.” He states that he does not think this is a big deal because he uses electronic cigarettes and has heard that these products don’t contain “the bad cancer stuff.” At the end of Mr. T’s visit, he asks for something to help him sleep because has been unable to sleep consistently and has been having vivid dreams since starting the patch. He also wants to know how to reduce itching from the patch.
Approximately 46 million Americans smoke and cigarette smoking accounts for 1 of every 5 deaths in the United States each year.1 Since the advent of “Stop Smoking” campaigns, bans on smoking in public buildings, over-the-counter (OTC) nicotine replacement products, and Surgeon General recommendations, discussing smoking cessation with patients has become standard practice.
Research suggests that treatment to quit smoking should include a combination of pharmacotherapy and counseling, such as cognitive-behavioral strategies, support groups, and quitting hotlines.2 Pharmacotherapy consists of OTC nicotine replacement therapy (NRT) products and prescription medications. This article briefly highlights how to counsel patients about using OTC NRT products (Table 1).2-5 See Table 2 for a summary of prescription smoking cessation agents
Table 1
Over-the-counter nicotine replacement therapy products
| Product | Dosage | Side effects | Amount of nicotine | Costa | Comments |
|---|---|---|---|---|---|
| Nicotine transdermal patches | For patients who smoked >.5 PPD: 21 mg/d for 6 weeks; 14 mg/d for 2 weeks; 7 mg/d for 2 weeks | Local skin irritation, sleep disturbances, and vivid dreams | 7, 14, or 21 mg /d | 21 mg: $32 for 14 patches 14 mg: $32 for 14 patches 7 mg: $19 for 7 patches | Alternate sites. Do not cut. Do not leave on skin for longer than life of patch (24 hours). Washing, bathing, swimming are OK. Remove patch if undergoing MRI because of reports of burns |
| For patients who smoked <.5 PPD: 14 mg/d for 6 weeks; 7 mg/d for 2 weeks | |||||
| Nicotine polacrilex gum | For patients who smoked ≥1.25 PPD: 4 mg | Unpleasant taste, jaw soreness, hiccups, dyspepsia, hypersalivation, and nausea (from chewing gum too quickly) | 2 or 4 mg per piece | 4 mg: $50 for 170 pieces 2 mg: $50 for 170 pieces | Use “chew and park” method. As part of combination therapy, use only as needed. No more than 24 pieces per day; use caution with patients with jaw or mouth conditions |
| For patients who smoked <1.25 PPD: 2 mg Weeks 1 to 6: 1 piece every 1 to 2 hours Weeks 7 to 9: 1 piece every 2 to 4 hours Weeks 10 to 12: 1 piece every 4 to 8 hours | |||||
| Nicotine polacrilex lozenge | For patients who smoked 1st cigarette within 30 minutes of waking: 4 mg | Mouth irritation, hiccups, nausea, cough, and insomnia | 2 or 4 mg per lozenge | 4 mg: $43 for 72 lozenges 2 mg: $43 for 72 lozenges | Dissolve lozenge in mouth for 20 to 30 minutes. Rotate lozenge to different parts of mouth occasionally. Do not chew. No more than 5 lozenges in 6 hours or 20 per day. Same taper schedule as nicotine gum |
| For patients who smoked 1st cigarette >30 minutes after waking: 2 mg | |||||
| aAll prices taken from drugstore.com on September 26, 2011 PPD: packs per day Source: References 2-5 | |||||
Patches
Nicotine replacement patches are best used for maintenance treatment of nicotine cravings. They deliver a fixed amount of nicotine over 24 hours.3 Patches have a specially formulated transdermal matrix system and should not be cut. Doing so damages the drug delivery system and could lead to drug evaporation from the cut edges.4 Mr. T’s psychiatrist advises him not to cut patches but instead purchase the 14-mg patch because he is at this step of the smoking cessation regimen.
Skin irritation caused by adhesive is a common adverse event from nicotine patches. Rotating the location of each patch to a different hairless body area is the best way to prevent or combat skin irritation. If rotating the location of the patch does not relieve irritation, patients can apply a thin layer of an OTC hydrocortisone 1% cream to the affected site 2 to 4 times a day after gently washing the area.5 Instruct patients to avoid using occlusive dressings over the topical application.
Nicotine replacement patches also have been reported to cause vivid dreams and insomnia.3 These side effects may be caused by nighttime nicotine absorption, which might be avoided by switching to a different NRT product or removing the 24-hour patch when going to bed.4
Combining treatments
Many patients experience nicotine cravings while using the nicotine replacement patch. Stressful situations and events can trigger a patient’s desire for nicotine and withdrawal symptoms that a patch that delivers a continuous amount of nicotine over 24 hours cannot alleviate. Combining different forms of treatment could combat these symptoms.2,3,5
Combination therapy might consist of using sustained-release bupropion or a nicotine patch with rapid-acting NRT products such as a lozenge, gum, nasal spray, or inhaler. In Mr. T’s case, clinicians recommend that he use nicotine polacrilex gum in addition to the patch to quell his cravings. Also, he is instructed to stop using electronic cigarettes because they are considered tobacco products, are not regulated by the FDA, and may contain toxic substances.6
Instruct patients who use nicotine gum to employ the “chew and park” method.4 First, they should chew the gum very slowly until they notice a minty taste or tingling feeling, then “park” the gum between the cheek and gums for 1 to 2 minutes to allow nicotine to be absorbed across the gum lining. After 2 minutes or when tingling ceases, patients should slowly resume chewing until a tingling or minty taste returns and then “park” the gum again in a different area of the gums. Tell patients to repeat the “chew and park” method until there is no more taste or tingling (approximately 30 minutes). Explain that chewing the gum too fast may result in nausea or lightheadedness and patients should refrain from eating or drinking 15 minutes before or while using the gum. Mr. T is instructed to use the gum only when the urge to smoke is overbearing, and not regularly.
The nicotine polacrilex gum is more viscous than ordinary chewing gum and may stick to or possibly damage dental work such as fillings, dentures, crowns, and braces. An acceptable alternative is the nicotine polacrilex lozenge. Advise patients who want to try lozenges to:
- place the lozenge in the mouth and allow it to dissolve slowly over 20 to 30 minutes (during this time patients may experience a tingling sensation as nicotine is released)
- rotate the lozenge to different areas of the mouth every few minutes to lessen irritation
- avoid chewing or swallowing the lozenge because doing so will lead to improper release of nicotine and side effects, including nausea, hiccups, and heartburn
- refrain from eating or drinking 15 minutes before or while using the lozenge.
For many patients, the breadth of pharmacologic agents available for smoking cessation has made quitting a more attainable goal. OTC smoking cessation products are available in most drug stores, which gives smokers easy access to taking this important step. Counseling patients on the proper use of OTC products may help them successfully stop smoking.7
Although a patient’s medical history, including cardiac status, must be considered before starting specific agents, in many instances patient preference is the prevailing factor when choosing therapy. Often, the risks of continued smoking outweigh the risks of using smoking cessation products. OTC smoking cessation products may be an appropriate first-line treatment for many individuals before trying prescription medications, such as bupropion or varenicline.
For patients
- Treat Tobacco. www.treatobacco.net.
- Smokefree.gov mobile application. http://smokefree.gov/apps.
- Medline Plus. Quitting smoking. www.nlm.nih.gov/medlineplus/quittingsmoking.html.
- Quit for Life Program. www.quitnow.net.
- American Lung Association. Stop Smoking. www.lungusa.org/stop-smoking.
For clinicians
- Agency for Health Research and Quality. Treating tobacco use and dependence: 2008 update. www.ahrq.gov/path/tobacco.htm#clinicians.
Drug Brand Names
- Bupropion SR • Zyban, Wellbutrin SR
- Fluoxetine • Prozac
- Varenicline • Chantix
Disclosure
Dr. Ellingrod receives grant/research support from the National Institute of Mental Health.
Dr. Burghardt reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table 2
Prescription smoking cessation productsa
| Product | Dosage | Side effects | Amount of nicotine | Costb | Notes |
|---|---|---|---|---|---|
| Nicotine inhaler | 6 to 16 cartridges/d | Throat/mouth irritation and cough | 10 mg cartridges deliver 4 mg of nicotine | 10 mg inhaler with 168 cartridges: $213 | Vapor, not smoke, is released and deposited in mouth. Similar mechanism of action to nicotine gum. Continuously puff for ~20 minutes. Gradually reduce dosage over 12 weeks. Helps with patients who need the “action” of smoking. Caution in patients who have a history of bronchospastic disease because of potential airway irritation |
| Nicotine nasal spray | 10 mg/ml bottle 8 to 40 doses/d One dose is a spray to each nostril | Initial (~10 week) watery eyes, coughing, and nasal and throat irritation | 0.5 mg/spray | 10 ml bottle: $186 | Fastest delivery of nicotine vs other products. Tilt head back slightly when delivering spray. Do not sniff, swallow, or inhale through the nose. Continue treatment for 3 to 6 months with an individualized reduction in usage |
| Bupropion SR | 150 mg/d for 3 days, then 300 mg/d for 7 to 12 weeks or longer | Weight change, constipation, confusion, headache, and insomnia | N/A | 60 tablets: $106 | Patients should stop smoking during the second week of treatment. Combination treatment has achieved higher cessation rates. Avoid bedtime dosing to minimize insomnia (eg, 7 AM and 3 PM dosing strategy). Avoid in patients with seizure disorders |
| Varenicline | Days 1 to 3: 0.5 mg/d Days 4 to 7: 1 mg/d Day 8 to end of treatment: 2 mg/d Start treatment 1 week before quitting and continue for 3 to 6 months | Constipation, flatulence, nausea, vomiting, insomnia, and headache | N/A | Starting pack: $179 Continuing pack: $177 | Partial agonist of nicotinic acetylcholine receptor. Superiority to placebo has been shown but more studies are needed to show superiority to NRT. Safety and efficacy of combination therapy has not been established. Pack titrates dosage to 2 mg/d to decrease nausea. Take with water and food. Has a “black-box” warning for serious neuropsychiatric events, including suicidal ideations and behavior |
1. Centers for Disease Control and Prevention Smoking and Tobacco Use. Adult cigarette smoking in the United States: current estimate. http://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm. Accessed November 29 2011.
2. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med. 2008;35(2):158-176.
3. Physicians’ Desk Reference. 64th ed. Montvale, NJ: Thomson PDR; 2010.
4. Kroon LA, Hudmon KS, Corelli RL. Smoking cessation. In: Berardi RR Ferreri SP, Hume AL, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 16th ed. Washington, DC: American Pharmacists Association; 2009:883–916.
5. Doering PL, Kennedy WK, Boothby LA. Substance-related disorders: alcohol nicotine, and caffeine. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: a pathophysiologic approach. 7th ed. New York, NY: McGraw-Hill; 2008:1083–1098.
6. U.S. Food and Drug Administration. Electronic cigarettes. http://www.fda.gov/NewsEvents/PublicHealthFocus/ucm172906.htm. Accessed November 29, 2011.
7. Prokhorov AV, Hudmon KS, Marani S, et al. Engaging physicians and pharmacists in providing smoking cessation counseling. Arch Intern Med. 2010;170(18):1640-1646.
Discuss this article at www.facebook.com/CurrentPsychiatry
• Over-the-counter smoking cessation products likely will be the most appropriate first-line choice for many individuals before trying prescription products.
• Instruct patients to avoid smoking while using nicotine replacement therapy and educate them about the immediate and long-term benefits of quitting.
• Encourage patients to seek psychosocial counseling along with pharmacotherapy.
• Urge patients to engage in other quitting strategies by referring them to online and telephone resources (Related Resources). Also, encourage them to attend follow-up appointments to assess cessation therapy.
Mr. T, age 56, has major depressive disorder that is well controlled with fluoxetine, 40 mg/d. He has smoked ≥1 packs of cigarettes per day for the last 25 years. On a recent visit, he indicates that he has begun using a 21-mg nicotine patch as advised by his pharmacist and that things are going OK, although he has had some “slip ups.” He is on week 7 of his quitting regimen and now is stepping down the patch dosage.
Upon further questioning he says that he has been cutting the 21-mg patches in half to save money. Mr. T also explains that occasionally he has given in to a strong urge to smoke because it was “too much to handle.” He states that he does not think this is a big deal because he uses electronic cigarettes and has heard that these products don’t contain “the bad cancer stuff.” At the end of Mr. T’s visit, he asks for something to help him sleep because has been unable to sleep consistently and has been having vivid dreams since starting the patch. He also wants to know how to reduce itching from the patch.
Approximately 46 million Americans smoke and cigarette smoking accounts for 1 of every 5 deaths in the United States each year.1 Since the advent of “Stop Smoking” campaigns, bans on smoking in public buildings, over-the-counter (OTC) nicotine replacement products, and Surgeon General recommendations, discussing smoking cessation with patients has become standard practice.
Research suggests that treatment to quit smoking should include a combination of pharmacotherapy and counseling, such as cognitive-behavioral strategies, support groups, and quitting hotlines.2 Pharmacotherapy consists of OTC nicotine replacement therapy (NRT) products and prescription medications. This article briefly highlights how to counsel patients about using OTC NRT products (Table 1).2-5 See Table 2 for a summary of prescription smoking cessation agents
Table 1
Over-the-counter nicotine replacement therapy products
| Product | Dosage | Side effects | Amount of nicotine | Costa | Comments |
|---|---|---|---|---|---|
| Nicotine transdermal patches | For patients who smoked >.5 PPD: 21 mg/d for 6 weeks; 14 mg/d for 2 weeks; 7 mg/d for 2 weeks | Local skin irritation, sleep disturbances, and vivid dreams | 7, 14, or 21 mg /d | 21 mg: $32 for 14 patches 14 mg: $32 for 14 patches 7 mg: $19 for 7 patches | Alternate sites. Do not cut. Do not leave on skin for longer than life of patch (24 hours). Washing, bathing, swimming are OK. Remove patch if undergoing MRI because of reports of burns |
| For patients who smoked <.5 PPD: 14 mg/d for 6 weeks; 7 mg/d for 2 weeks | |||||
| Nicotine polacrilex gum | For patients who smoked ≥1.25 PPD: 4 mg | Unpleasant taste, jaw soreness, hiccups, dyspepsia, hypersalivation, and nausea (from chewing gum too quickly) | 2 or 4 mg per piece | 4 mg: $50 for 170 pieces 2 mg: $50 for 170 pieces | Use “chew and park” method. As part of combination therapy, use only as needed. No more than 24 pieces per day; use caution with patients with jaw or mouth conditions |
| For patients who smoked <1.25 PPD: 2 mg Weeks 1 to 6: 1 piece every 1 to 2 hours Weeks 7 to 9: 1 piece every 2 to 4 hours Weeks 10 to 12: 1 piece every 4 to 8 hours | |||||
| Nicotine polacrilex lozenge | For patients who smoked 1st cigarette within 30 minutes of waking: 4 mg | Mouth irritation, hiccups, nausea, cough, and insomnia | 2 or 4 mg per lozenge | 4 mg: $43 for 72 lozenges 2 mg: $43 for 72 lozenges | Dissolve lozenge in mouth for 20 to 30 minutes. Rotate lozenge to different parts of mouth occasionally. Do not chew. No more than 5 lozenges in 6 hours or 20 per day. Same taper schedule as nicotine gum |
| For patients who smoked 1st cigarette >30 minutes after waking: 2 mg | |||||
| aAll prices taken from drugstore.com on September 26, 2011 PPD: packs per day Source: References 2-5 | |||||
Patches
Nicotine replacement patches are best used for maintenance treatment of nicotine cravings. They deliver a fixed amount of nicotine over 24 hours.3 Patches have a specially formulated transdermal matrix system and should not be cut. Doing so damages the drug delivery system and could lead to drug evaporation from the cut edges.4 Mr. T’s psychiatrist advises him not to cut patches but instead purchase the 14-mg patch because he is at this step of the smoking cessation regimen.
Skin irritation caused by adhesive is a common adverse event from nicotine patches. Rotating the location of each patch to a different hairless body area is the best way to prevent or combat skin irritation. If rotating the location of the patch does not relieve irritation, patients can apply a thin layer of an OTC hydrocortisone 1% cream to the affected site 2 to 4 times a day after gently washing the area.5 Instruct patients to avoid using occlusive dressings over the topical application.
Nicotine replacement patches also have been reported to cause vivid dreams and insomnia.3 These side effects may be caused by nighttime nicotine absorption, which might be avoided by switching to a different NRT product or removing the 24-hour patch when going to bed.4
Combining treatments
Many patients experience nicotine cravings while using the nicotine replacement patch. Stressful situations and events can trigger a patient’s desire for nicotine and withdrawal symptoms that a patch that delivers a continuous amount of nicotine over 24 hours cannot alleviate. Combining different forms of treatment could combat these symptoms.2,3,5
Combination therapy might consist of using sustained-release bupropion or a nicotine patch with rapid-acting NRT products such as a lozenge, gum, nasal spray, or inhaler. In Mr. T’s case, clinicians recommend that he use nicotine polacrilex gum in addition to the patch to quell his cravings. Also, he is instructed to stop using electronic cigarettes because they are considered tobacco products, are not regulated by the FDA, and may contain toxic substances.6
Instruct patients who use nicotine gum to employ the “chew and park” method.4 First, they should chew the gum very slowly until they notice a minty taste or tingling feeling, then “park” the gum between the cheek and gums for 1 to 2 minutes to allow nicotine to be absorbed across the gum lining. After 2 minutes or when tingling ceases, patients should slowly resume chewing until a tingling or minty taste returns and then “park” the gum again in a different area of the gums. Tell patients to repeat the “chew and park” method until there is no more taste or tingling (approximately 30 minutes). Explain that chewing the gum too fast may result in nausea or lightheadedness and patients should refrain from eating or drinking 15 minutes before or while using the gum. Mr. T is instructed to use the gum only when the urge to smoke is overbearing, and not regularly.
The nicotine polacrilex gum is more viscous than ordinary chewing gum and may stick to or possibly damage dental work such as fillings, dentures, crowns, and braces. An acceptable alternative is the nicotine polacrilex lozenge. Advise patients who want to try lozenges to:
- place the lozenge in the mouth and allow it to dissolve slowly over 20 to 30 minutes (during this time patients may experience a tingling sensation as nicotine is released)
- rotate the lozenge to different areas of the mouth every few minutes to lessen irritation
- avoid chewing or swallowing the lozenge because doing so will lead to improper release of nicotine and side effects, including nausea, hiccups, and heartburn
- refrain from eating or drinking 15 minutes before or while using the lozenge.
For many patients, the breadth of pharmacologic agents available for smoking cessation has made quitting a more attainable goal. OTC smoking cessation products are available in most drug stores, which gives smokers easy access to taking this important step. Counseling patients on the proper use of OTC products may help them successfully stop smoking.7
Although a patient’s medical history, including cardiac status, must be considered before starting specific agents, in many instances patient preference is the prevailing factor when choosing therapy. Often, the risks of continued smoking outweigh the risks of using smoking cessation products. OTC smoking cessation products may be an appropriate first-line treatment for many individuals before trying prescription medications, such as bupropion or varenicline.
For patients
- Treat Tobacco. www.treatobacco.net.
- Smokefree.gov mobile application. http://smokefree.gov/apps.
- Medline Plus. Quitting smoking. www.nlm.nih.gov/medlineplus/quittingsmoking.html.
- Quit for Life Program. www.quitnow.net.
- American Lung Association. Stop Smoking. www.lungusa.org/stop-smoking.
For clinicians
- Agency for Health Research and Quality. Treating tobacco use and dependence: 2008 update. www.ahrq.gov/path/tobacco.htm#clinicians.
Drug Brand Names
- Bupropion SR • Zyban, Wellbutrin SR
- Fluoxetine • Prozac
- Varenicline • Chantix
Disclosure
Dr. Ellingrod receives grant/research support from the National Institute of Mental Health.
Dr. Burghardt reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table 2
Prescription smoking cessation productsa
| Product | Dosage | Side effects | Amount of nicotine | Costb | Notes |
|---|---|---|---|---|---|
| Nicotine inhaler | 6 to 16 cartridges/d | Throat/mouth irritation and cough | 10 mg cartridges deliver 4 mg of nicotine | 10 mg inhaler with 168 cartridges: $213 | Vapor, not smoke, is released and deposited in mouth. Similar mechanism of action to nicotine gum. Continuously puff for ~20 minutes. Gradually reduce dosage over 12 weeks. Helps with patients who need the “action” of smoking. Caution in patients who have a history of bronchospastic disease because of potential airway irritation |
| Nicotine nasal spray | 10 mg/ml bottle 8 to 40 doses/d One dose is a spray to each nostril | Initial (~10 week) watery eyes, coughing, and nasal and throat irritation | 0.5 mg/spray | 10 ml bottle: $186 | Fastest delivery of nicotine vs other products. Tilt head back slightly when delivering spray. Do not sniff, swallow, or inhale through the nose. Continue treatment for 3 to 6 months with an individualized reduction in usage |
| Bupropion SR | 150 mg/d for 3 days, then 300 mg/d for 7 to 12 weeks or longer | Weight change, constipation, confusion, headache, and insomnia | N/A | 60 tablets: $106 | Patients should stop smoking during the second week of treatment. Combination treatment has achieved higher cessation rates. Avoid bedtime dosing to minimize insomnia (eg, 7 AM and 3 PM dosing strategy). Avoid in patients with seizure disorders |
| Varenicline | Days 1 to 3: 0.5 mg/d Days 4 to 7: 1 mg/d Day 8 to end of treatment: 2 mg/d Start treatment 1 week before quitting and continue for 3 to 6 months | Constipation, flatulence, nausea, vomiting, insomnia, and headache | N/A | Starting pack: $179 Continuing pack: $177 | Partial agonist of nicotinic acetylcholine receptor. Superiority to placebo has been shown but more studies are needed to show superiority to NRT. Safety and efficacy of combination therapy has not been established. Pack titrates dosage to 2 mg/d to decrease nausea. Take with water and food. Has a “black-box” warning for serious neuropsychiatric events, including suicidal ideations and behavior |
Discuss this article at www.facebook.com/CurrentPsychiatry
• Over-the-counter smoking cessation products likely will be the most appropriate first-line choice for many individuals before trying prescription products.
• Instruct patients to avoid smoking while using nicotine replacement therapy and educate them about the immediate and long-term benefits of quitting.
• Encourage patients to seek psychosocial counseling along with pharmacotherapy.
• Urge patients to engage in other quitting strategies by referring them to online and telephone resources (Related Resources). Also, encourage them to attend follow-up appointments to assess cessation therapy.
Mr. T, age 56, has major depressive disorder that is well controlled with fluoxetine, 40 mg/d. He has smoked ≥1 packs of cigarettes per day for the last 25 years. On a recent visit, he indicates that he has begun using a 21-mg nicotine patch as advised by his pharmacist and that things are going OK, although he has had some “slip ups.” He is on week 7 of his quitting regimen and now is stepping down the patch dosage.
Upon further questioning he says that he has been cutting the 21-mg patches in half to save money. Mr. T also explains that occasionally he has given in to a strong urge to smoke because it was “too much to handle.” He states that he does not think this is a big deal because he uses electronic cigarettes and has heard that these products don’t contain “the bad cancer stuff.” At the end of Mr. T’s visit, he asks for something to help him sleep because has been unable to sleep consistently and has been having vivid dreams since starting the patch. He also wants to know how to reduce itching from the patch.
Approximately 46 million Americans smoke and cigarette smoking accounts for 1 of every 5 deaths in the United States each year.1 Since the advent of “Stop Smoking” campaigns, bans on smoking in public buildings, over-the-counter (OTC) nicotine replacement products, and Surgeon General recommendations, discussing smoking cessation with patients has become standard practice.
Research suggests that treatment to quit smoking should include a combination of pharmacotherapy and counseling, such as cognitive-behavioral strategies, support groups, and quitting hotlines.2 Pharmacotherapy consists of OTC nicotine replacement therapy (NRT) products and prescription medications. This article briefly highlights how to counsel patients about using OTC NRT products (Table 1).2-5 See Table 2 for a summary of prescription smoking cessation agents
Table 1
Over-the-counter nicotine replacement therapy products
| Product | Dosage | Side effects | Amount of nicotine | Costa | Comments |
|---|---|---|---|---|---|
| Nicotine transdermal patches | For patients who smoked >.5 PPD: 21 mg/d for 6 weeks; 14 mg/d for 2 weeks; 7 mg/d for 2 weeks | Local skin irritation, sleep disturbances, and vivid dreams | 7, 14, or 21 mg /d | 21 mg: $32 for 14 patches 14 mg: $32 for 14 patches 7 mg: $19 for 7 patches | Alternate sites. Do not cut. Do not leave on skin for longer than life of patch (24 hours). Washing, bathing, swimming are OK. Remove patch if undergoing MRI because of reports of burns |
| For patients who smoked <.5 PPD: 14 mg/d for 6 weeks; 7 mg/d for 2 weeks | |||||
| Nicotine polacrilex gum | For patients who smoked ≥1.25 PPD: 4 mg | Unpleasant taste, jaw soreness, hiccups, dyspepsia, hypersalivation, and nausea (from chewing gum too quickly) | 2 or 4 mg per piece | 4 mg: $50 for 170 pieces 2 mg: $50 for 170 pieces | Use “chew and park” method. As part of combination therapy, use only as needed. No more than 24 pieces per day; use caution with patients with jaw or mouth conditions |
| For patients who smoked <1.25 PPD: 2 mg Weeks 1 to 6: 1 piece every 1 to 2 hours Weeks 7 to 9: 1 piece every 2 to 4 hours Weeks 10 to 12: 1 piece every 4 to 8 hours | |||||
| Nicotine polacrilex lozenge | For patients who smoked 1st cigarette within 30 minutes of waking: 4 mg | Mouth irritation, hiccups, nausea, cough, and insomnia | 2 or 4 mg per lozenge | 4 mg: $43 for 72 lozenges 2 mg: $43 for 72 lozenges | Dissolve lozenge in mouth for 20 to 30 minutes. Rotate lozenge to different parts of mouth occasionally. Do not chew. No more than 5 lozenges in 6 hours or 20 per day. Same taper schedule as nicotine gum |
| For patients who smoked 1st cigarette >30 minutes after waking: 2 mg | |||||
| aAll prices taken from drugstore.com on September 26, 2011 PPD: packs per day Source: References 2-5 | |||||
Patches
Nicotine replacement patches are best used for maintenance treatment of nicotine cravings. They deliver a fixed amount of nicotine over 24 hours.3 Patches have a specially formulated transdermal matrix system and should not be cut. Doing so damages the drug delivery system and could lead to drug evaporation from the cut edges.4 Mr. T’s psychiatrist advises him not to cut patches but instead purchase the 14-mg patch because he is at this step of the smoking cessation regimen.
Skin irritation caused by adhesive is a common adverse event from nicotine patches. Rotating the location of each patch to a different hairless body area is the best way to prevent or combat skin irritation. If rotating the location of the patch does not relieve irritation, patients can apply a thin layer of an OTC hydrocortisone 1% cream to the affected site 2 to 4 times a day after gently washing the area.5 Instruct patients to avoid using occlusive dressings over the topical application.
Nicotine replacement patches also have been reported to cause vivid dreams and insomnia.3 These side effects may be caused by nighttime nicotine absorption, which might be avoided by switching to a different NRT product or removing the 24-hour patch when going to bed.4
Combining treatments
Many patients experience nicotine cravings while using the nicotine replacement patch. Stressful situations and events can trigger a patient’s desire for nicotine and withdrawal symptoms that a patch that delivers a continuous amount of nicotine over 24 hours cannot alleviate. Combining different forms of treatment could combat these symptoms.2,3,5
Combination therapy might consist of using sustained-release bupropion or a nicotine patch with rapid-acting NRT products such as a lozenge, gum, nasal spray, or inhaler. In Mr. T’s case, clinicians recommend that he use nicotine polacrilex gum in addition to the patch to quell his cravings. Also, he is instructed to stop using electronic cigarettes because they are considered tobacco products, are not regulated by the FDA, and may contain toxic substances.6
Instruct patients who use nicotine gum to employ the “chew and park” method.4 First, they should chew the gum very slowly until they notice a minty taste or tingling feeling, then “park” the gum between the cheek and gums for 1 to 2 minutes to allow nicotine to be absorbed across the gum lining. After 2 minutes or when tingling ceases, patients should slowly resume chewing until a tingling or minty taste returns and then “park” the gum again in a different area of the gums. Tell patients to repeat the “chew and park” method until there is no more taste or tingling (approximately 30 minutes). Explain that chewing the gum too fast may result in nausea or lightheadedness and patients should refrain from eating or drinking 15 minutes before or while using the gum. Mr. T is instructed to use the gum only when the urge to smoke is overbearing, and not regularly.
The nicotine polacrilex gum is more viscous than ordinary chewing gum and may stick to or possibly damage dental work such as fillings, dentures, crowns, and braces. An acceptable alternative is the nicotine polacrilex lozenge. Advise patients who want to try lozenges to:
- place the lozenge in the mouth and allow it to dissolve slowly over 20 to 30 minutes (during this time patients may experience a tingling sensation as nicotine is released)
- rotate the lozenge to different areas of the mouth every few minutes to lessen irritation
- avoid chewing or swallowing the lozenge because doing so will lead to improper release of nicotine and side effects, including nausea, hiccups, and heartburn
- refrain from eating or drinking 15 minutes before or while using the lozenge.
For many patients, the breadth of pharmacologic agents available for smoking cessation has made quitting a more attainable goal. OTC smoking cessation products are available in most drug stores, which gives smokers easy access to taking this important step. Counseling patients on the proper use of OTC products may help them successfully stop smoking.7
Although a patient’s medical history, including cardiac status, must be considered before starting specific agents, in many instances patient preference is the prevailing factor when choosing therapy. Often, the risks of continued smoking outweigh the risks of using smoking cessation products. OTC smoking cessation products may be an appropriate first-line treatment for many individuals before trying prescription medications, such as bupropion or varenicline.
For patients
- Treat Tobacco. www.treatobacco.net.
- Smokefree.gov mobile application. http://smokefree.gov/apps.
- Medline Plus. Quitting smoking. www.nlm.nih.gov/medlineplus/quittingsmoking.html.
- Quit for Life Program. www.quitnow.net.
- American Lung Association. Stop Smoking. www.lungusa.org/stop-smoking.
For clinicians
- Agency for Health Research and Quality. Treating tobacco use and dependence: 2008 update. www.ahrq.gov/path/tobacco.htm#clinicians.
Drug Brand Names
- Bupropion SR • Zyban, Wellbutrin SR
- Fluoxetine • Prozac
- Varenicline • Chantix
Disclosure
Dr. Ellingrod receives grant/research support from the National Institute of Mental Health.
Dr. Burghardt reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table 2
Prescription smoking cessation productsa
| Product | Dosage | Side effects | Amount of nicotine | Costb | Notes |
|---|---|---|---|---|---|
| Nicotine inhaler | 6 to 16 cartridges/d | Throat/mouth irritation and cough | 10 mg cartridges deliver 4 mg of nicotine | 10 mg inhaler with 168 cartridges: $213 | Vapor, not smoke, is released and deposited in mouth. Similar mechanism of action to nicotine gum. Continuously puff for ~20 minutes. Gradually reduce dosage over 12 weeks. Helps with patients who need the “action” of smoking. Caution in patients who have a history of bronchospastic disease because of potential airway irritation |
| Nicotine nasal spray | 10 mg/ml bottle 8 to 40 doses/d One dose is a spray to each nostril | Initial (~10 week) watery eyes, coughing, and nasal and throat irritation | 0.5 mg/spray | 10 ml bottle: $186 | Fastest delivery of nicotine vs other products. Tilt head back slightly when delivering spray. Do not sniff, swallow, or inhale through the nose. Continue treatment for 3 to 6 months with an individualized reduction in usage |
| Bupropion SR | 150 mg/d for 3 days, then 300 mg/d for 7 to 12 weeks or longer | Weight change, constipation, confusion, headache, and insomnia | N/A | 60 tablets: $106 | Patients should stop smoking during the second week of treatment. Combination treatment has achieved higher cessation rates. Avoid bedtime dosing to minimize insomnia (eg, 7 AM and 3 PM dosing strategy). Avoid in patients with seizure disorders |
| Varenicline | Days 1 to 3: 0.5 mg/d Days 4 to 7: 1 mg/d Day 8 to end of treatment: 2 mg/d Start treatment 1 week before quitting and continue for 3 to 6 months | Constipation, flatulence, nausea, vomiting, insomnia, and headache | N/A | Starting pack: $179 Continuing pack: $177 | Partial agonist of nicotinic acetylcholine receptor. Superiority to placebo has been shown but more studies are needed to show superiority to NRT. Safety and efficacy of combination therapy has not been established. Pack titrates dosage to 2 mg/d to decrease nausea. Take with water and food. Has a “black-box” warning for serious neuropsychiatric events, including suicidal ideations and behavior |
1. Centers for Disease Control and Prevention Smoking and Tobacco Use. Adult cigarette smoking in the United States: current estimate. http://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm. Accessed November 29 2011.
2. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med. 2008;35(2):158-176.
3. Physicians’ Desk Reference. 64th ed. Montvale, NJ: Thomson PDR; 2010.
4. Kroon LA, Hudmon KS, Corelli RL. Smoking cessation. In: Berardi RR Ferreri SP, Hume AL, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 16th ed. Washington, DC: American Pharmacists Association; 2009:883–916.
5. Doering PL, Kennedy WK, Boothby LA. Substance-related disorders: alcohol nicotine, and caffeine. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: a pathophysiologic approach. 7th ed. New York, NY: McGraw-Hill; 2008:1083–1098.
6. U.S. Food and Drug Administration. Electronic cigarettes. http://www.fda.gov/NewsEvents/PublicHealthFocus/ucm172906.htm. Accessed November 29, 2011.
7. Prokhorov AV, Hudmon KS, Marani S, et al. Engaging physicians and pharmacists in providing smoking cessation counseling. Arch Intern Med. 2010;170(18):1640-1646.
1. Centers for Disease Control and Prevention Smoking and Tobacco Use. Adult cigarette smoking in the United States: current estimate. http://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm. Accessed November 29 2011.
2. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med. 2008;35(2):158-176.
3. Physicians’ Desk Reference. 64th ed. Montvale, NJ: Thomson PDR; 2010.
4. Kroon LA, Hudmon KS, Corelli RL. Smoking cessation. In: Berardi RR Ferreri SP, Hume AL, et al, eds. Handbook of nonprescription drugs: an interactive approach to self-care. 16th ed. Washington, DC: American Pharmacists Association; 2009:883–916.
5. Doering PL, Kennedy WK, Boothby LA. Substance-related disorders: alcohol nicotine, and caffeine. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: a pathophysiologic approach. 7th ed. New York, NY: McGraw-Hill; 2008:1083–1098.
6. U.S. Food and Drug Administration. Electronic cigarettes. http://www.fda.gov/NewsEvents/PublicHealthFocus/ucm172906.htm. Accessed November 29, 2011.
7. Prokhorov AV, Hudmon KS, Marani S, et al. Engaging physicians and pharmacists in providing smoking cessation counseling. Arch Intern Med. 2010;170(18):1640-1646.