User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
FRISBEE: Does the study fly in the face of evidence-based medicine?
With the rapid emergence of novel therapies, psychiatrists face the challenge of deciphering the clinical application of published clinical trials. Although double-blind, randomized, placebo-controlled trials are the gold standard, their validity should be carefully examined.1 The FRISBEE mnemonic from Duke University’s psychiatry residency program can help you incorporate evidence-based medicine into your patient care.
Follow-up. Carefully interpret studies with inadequate follow-up or high drop-out rates. The reason for patient discontinuation might not be related to the studied intervention.
Randomization. To control for unknown confounding variables, patient assignment must be randomized.
Intent-to-treat analysis. ITT assumes that complete data are available during final analysis on every subject, but subjects often drop out. To compensate for drop-outs, researchers could:
- carry forward the last available measurement as the final result, known as last observation carried forward (LOCF).
- use data only from patients who complete entire study protocol (completer analysis method).
Both methods have statistical limitations, but LOCF generally is preferred because it accounts for every subject who enrolled in the study.2
Similar baseline. Compare known characteristics of the treatment and placebo groups at baseline. Confounding variables, such as illness severity or medical or psychiatric comorbidities, should appear equally among randomized patient groups. Not all variables will be similar because of random effects, however.
Blinding. With ineffective blinding, patients or researchers can tell which treatment was administered. If this occurs, the study’s outcome likely is biased by treatment expectations. To detect faulty blinding, some studies ask patients and/or providers if they can guess the intervention that was delivered.
Equal treatment. Even with proper randomization and blinding, other intervention-related treatments—such as blood work to monitor side effects or the duration or frequency of provider contact—might not be administered equally among patient groups. This can clue patients and researchers into which intervention was administered and create bias.
Equivalence to your patient. A typical study patient often has few medical and psychiatric comorbidities or psychosocial risk factors. Your patient might be substantially different. Carefully compare the patients in the study with the patient in your office before choosing a treatment.
1. Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA 2000;284(3):357-62.
2. Lachin JM. Statistical considerations in the intent to treat principle. Control Clin Trials 2000;21:167-89.
Dr. Xiong is assistant clinical professor at the University of California, Davis. Dr. Adams is clinical associate at Duke University Medical Center, Durham, NC.
With the rapid emergence of novel therapies, psychiatrists face the challenge of deciphering the clinical application of published clinical trials. Although double-blind, randomized, placebo-controlled trials are the gold standard, their validity should be carefully examined.1 The FRISBEE mnemonic from Duke University’s psychiatry residency program can help you incorporate evidence-based medicine into your patient care.
Follow-up. Carefully interpret studies with inadequate follow-up or high drop-out rates. The reason for patient discontinuation might not be related to the studied intervention.
Randomization. To control for unknown confounding variables, patient assignment must be randomized.
Intent-to-treat analysis. ITT assumes that complete data are available during final analysis on every subject, but subjects often drop out. To compensate for drop-outs, researchers could:
- carry forward the last available measurement as the final result, known as last observation carried forward (LOCF).
- use data only from patients who complete entire study protocol (completer analysis method).
Both methods have statistical limitations, but LOCF generally is preferred because it accounts for every subject who enrolled in the study.2
Similar baseline. Compare known characteristics of the treatment and placebo groups at baseline. Confounding variables, such as illness severity or medical or psychiatric comorbidities, should appear equally among randomized patient groups. Not all variables will be similar because of random effects, however.
Blinding. With ineffective blinding, patients or researchers can tell which treatment was administered. If this occurs, the study’s outcome likely is biased by treatment expectations. To detect faulty blinding, some studies ask patients and/or providers if they can guess the intervention that was delivered.
Equal treatment. Even with proper randomization and blinding, other intervention-related treatments—such as blood work to monitor side effects or the duration or frequency of provider contact—might not be administered equally among patient groups. This can clue patients and researchers into which intervention was administered and create bias.
Equivalence to your patient. A typical study patient often has few medical and psychiatric comorbidities or psychosocial risk factors. Your patient might be substantially different. Carefully compare the patients in the study with the patient in your office before choosing a treatment.
With the rapid emergence of novel therapies, psychiatrists face the challenge of deciphering the clinical application of published clinical trials. Although double-blind, randomized, placebo-controlled trials are the gold standard, their validity should be carefully examined.1 The FRISBEE mnemonic from Duke University’s psychiatry residency program can help you incorporate evidence-based medicine into your patient care.
Follow-up. Carefully interpret studies with inadequate follow-up or high drop-out rates. The reason for patient discontinuation might not be related to the studied intervention.
Randomization. To control for unknown confounding variables, patient assignment must be randomized.
Intent-to-treat analysis. ITT assumes that complete data are available during final analysis on every subject, but subjects often drop out. To compensate for drop-outs, researchers could:
- carry forward the last available measurement as the final result, known as last observation carried forward (LOCF).
- use data only from patients who complete entire study protocol (completer analysis method).
Both methods have statistical limitations, but LOCF generally is preferred because it accounts for every subject who enrolled in the study.2
Similar baseline. Compare known characteristics of the treatment and placebo groups at baseline. Confounding variables, such as illness severity or medical or psychiatric comorbidities, should appear equally among randomized patient groups. Not all variables will be similar because of random effects, however.
Blinding. With ineffective blinding, patients or researchers can tell which treatment was administered. If this occurs, the study’s outcome likely is biased by treatment expectations. To detect faulty blinding, some studies ask patients and/or providers if they can guess the intervention that was delivered.
Equal treatment. Even with proper randomization and blinding, other intervention-related treatments—such as blood work to monitor side effects or the duration or frequency of provider contact—might not be administered equally among patient groups. This can clue patients and researchers into which intervention was administered and create bias.
Equivalence to your patient. A typical study patient often has few medical and psychiatric comorbidities or psychosocial risk factors. Your patient might be substantially different. Carefully compare the patients in the study with the patient in your office before choosing a treatment.
1. Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA 2000;284(3):357-62.
2. Lachin JM. Statistical considerations in the intent to treat principle. Control Clin Trials 2000;21:167-89.
Dr. Xiong is assistant clinical professor at the University of California, Davis. Dr. Adams is clinical associate at Duke University Medical Center, Durham, NC.
1. Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA 2000;284(3):357-62.
2. Lachin JM. Statistical considerations in the intent to treat principle. Control Clin Trials 2000;21:167-89.
Dr. Xiong is assistant clinical professor at the University of California, Davis. Dr. Adams is clinical associate at Duke University Medical Center, Durham, NC.
Transdermal rivastigmine for dementia
The rivastigmine patch is the first transdermal treatment for symptoms of mild to moderate Alzheimer’s disease (AD) and mild to moderate Parkinson’s disease dementia (Table). Rivastigmine, a cholinesterase inhibitor, is the only therapy approved for both indications.
Table
Rivastigmine transdermal patch: Fast facts
| Brand name: Exelon Patch |
| Class: Cholinesterase inhibitor |
| Indication: Symptomatic treatment of mild to moderate Alzheimer’s-type dementia and mild to moderate dementia associated with Parkinson’s disease |
| Manufacturer: Novartis Pharmaceuticals, Inc. |
| Dosing forms: 4.6 and 9.5 mg/24 hours transdermal patches (5 cm2 and 10 cm2, respectively) |
| Recommended dosage: Start with 4.6 mg/24 hours patch for ≥4 weeks, followed by a one-step increase to the target dose 9.5 mg/24 hours patch* |
| *Unless the patient is taking oral rivastigmine (see ‘Transitioning to rivastigmine patch,’) |
Clinical implications
The rivastigmine patch offers continuous drug delivery through the skin into the bloodstream over 24 hours.1 This may reduce the incidence of side effects compared with oral rivastigmine,2 making optimal therapeutic doses easier to attain.3 The target dose 9.5 mg/24 hours patch provides efficacy similar to the highest recommended rivastigmine capsule dose (6 mg bid for a total of 12 mg/d).2
How it works
The rivastigmine patch uses matrix technology, which enables delivery of a large amount of drug from a small surface area.4 The patch is available in 2 dosage forms:
- a 5-cm2 size containing 9 mg of rivastigmine that delivers 4.6 mg/24 hours
- a 10-cm2 size containing 18 mg of rivastigmine that delivers 9.5 mg/24 hours.
Each patch consists of 4 layers: the backing layer, an acrylic drug matrix, a silicone adhesive matrix, and an overlapping release liner that is removed and discarded before the patch is applied.1
Cholinesterase inhibitors are believed to exert their effects by increasing available levels of the neurotransmitter acetylcholine in the brain. Two studies have demonstrated that cognitive improvements associated with rivastigmine treatment correlate significantly with cholinesterase inhibition.5,6 In 1 study, rivastigmine’s inhibitory effects on cholinesterase were sustained for 12 months.6
Pharmacokinetics
Rivastigmine is metabolized by its target cholinesterase enzymes to the decarbamylated metabolite NAP 226-90, which has minimal acetylcholinesterase inhibition and is excreted through the urine.1 As a result of its low accumulation potential and cytochrome P 450-independent metabolism, rivastigmine has low potential for pharmacokinetic drug–drug interactions. This lack of interaction has been confirmed for many drugs commonly taken by elderly patients, such as digoxin, nonsteroidal anti-inflammatory drugs, and estrogens.7
Rivastigmine has a half-life of 1 to 2 hours, so it is rapidly cleared.8 In the event of a serious reaction, significant clearance of rivastigmine from the body would occur within 3 hours of patch removal.
Centrally mediated cholinergic gastrointestinal (GI) side effects associated with oral rivastigmine are related to high maximum plasma concentrations (Cmax) and short time interval to Cmax (Tmax).9 In an open-label, parallel-group study of 51 AD patients that compared rivastigmine patches with rivastigmine capsules, transdermal administration was associated with slower increases to lower peak plasma concentrations (prolonged Tmax and reduced Cmax), and less fluctuation in plasma concentration.1 Despite these effects, the rivastigmine 9.5 mg/24 hours patch provided drug exposure comparable to the highest dose of capsules (6 mg bid for a total of 12 mg/d), with improved GI tolerability.3
Efficacy
Rivastigmine patch efficacy was evaluated in a single, 24-week, international, randomized, double-blind trial of 1,195 patients with AD.2 The study group represented typical patients with mild to moderate AD—age 50 to 85 years with Mini-Mental State Examination scores of 10 to 20 at baseline. Patients were randomly assigned to receive:
- 17.4 mg/24 hours rivastigmine patch (20-cm2 patch; n=303)
- 9.5 mg/24 hours rivastigmine patch (10-cm2 patch; n=293)
- 6 mg bid rivastigmine capsules (n=297)
- or placebo (n=302).
Data for the 17.4 mg/24 hours patch are not discussed here because this dose exceeds the FDA-approved maximum dosage (9.5 mg/24 hours) and is not available.
Patients in the 9.5 mg/24 hours patch group received a 4.6 mg/24 hours patch (5 cm2) for weeks 1 through 4, and then the 9.5 mg/24 hours patch for the remainder of the study. Patients in the capsule group started on 3 mg/d (1.5 mg bid) and were titrated every 4 weeks in steps of 3 mg/d to a maximum of 12 mg/d administered as 6 mg bid.
Primary outcomes were measured as mean change in score from baseline to endpoint on the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) and Alzheimer’s Disease Co-operative Study–Clinical Global Impression of Change (ADCS-CGIC). By study endpoint, the 9.5 mg/24 hours patch and capsules, 12 mg/d, showed comparable efficacy (Figure).2 Compared with those receiving placebo, patients in the 9.5 mg/24 hours patch and capsule groups showed significant improvements in dementia symptoms, including:
- cognition
- global performance
- attention
- activities of daily living.2
Based on my clinical experience, these improvements reflect small but clinically meaningful changes that are noted by patients and caregivers.
Figure
Efficacy of transdermal rivastigmine for Alzheimer’s symptoms
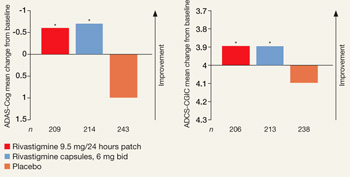
*P<0.05 vs placebo
ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale; ADCS-CGIC: Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change
Source: Adapted from reference 2
In a 24-week study, transdermal rivastigmine, 9.5 mg/24 hours, and the highest recommended dose of oral rivastigmine (6 mg bid) showed comparable efficacy as measured by mean change in score on scales commonly used in Alzheimer’s disease clinical trials. ADAS-Cog assesses orientation, memory, language, praxis, and visuospatial functions. ADCS-CGIC provides a single global rating of change from baseline based on interviews with the patient and caregiver.
Safety and tolerability
Adverse events associated with rivastigmine are predominantly cholinergic; GI side effects—nausea, vomiting, and diarrhea—are observed most frequently.2 These events occur less frequently with the patch than with capsules. In the efficacy trial, patients in the 9.5 mg/24 hours rivastigmine patch group had one-third as many reports of nausea (7.2% vs 23.1%) and vomiting (6.2% vs 17.0%) compared with the 6 mg bid capsule group.2
Diarrhea was reported by 6% of subjects receiving the 9.5 mg/24 hours patch, 5% of those taking 6-mg capsule bid, and 3% receiving placebo. Fewer subjects in the 9.5 mg/24 hours patch group (3%) experienced decreased weight compared with those in the capsule group (5%). The rate of decreased weight with placebo was 1%.
Dizziness affected 2% of those in the 9.5 mg/24 hours patch and placebo groups; incidence in the capsule group was significantly higher at 8%. Headache was similar with the 9.5 mg/24 hours patch (3%) and placebo (2%), with the capsule significantly higher at 6%.2
The proportion of patients who experienced no, slight, or mild skin irritation ranged from 90% to 98%.2 The most commonly reported moderate or severe skin irritations were erythema (8% rivastigmine patch vs 4% placebo) and pruritus (7% rivastigmine patch vs 3% placebo). Two percent of patients using active patch discontinued the trial because of skin irritation.
Rivastigmine appears not to produce adverse effects on cardiac function as assessed by ECG. In clinical trials of 2,791 patients, pooled 12-lead ECG data comparing oral rivastigmine and placebo groups did not differ significantly in heart rate or PR, QRS, and QTc intervals.10
Dosing
The rivastigmine patch is administered once daily, and the recommended maintenance dose is the 9.5 mg/24 hours patch. Start patients on a 4.6 mg/24 hours patch for at least 4 weeks and then increase to the 9.5 mg/24 hours target dose if the lower dose is well tolerated.
Dosage adjustment of rivastigmine is not necessary in patients with hepatic or renal disease because of minimal liver metabolism and the acetylcholinesterase-mediated hydrolysis of rivastigmine to the inactive decarbamylated metabolite NAP 226-90, which is excreted in the urine.11
Instruct patients or caregivers to apply the patch to clean, dry, hairless skin that is free of cuts, rashes, or irritation on the upper or lower back or upper arm or chest.1 The patch has shown good adhesive properties over 24 hours, remaining attached in a range of situations, including bathing and hot weather.2 In the 9.5 mg/24 hours group of the efficacy study, 96% of patches remained attached or had slight lifting of the edges (1,336 total patch evaluations).
Transitioning to rivastigmine patch
The efficacy study included an open-label extension, during which blinding was maintained. This provided information on patients beginning rivastigmine patch therapy directly from placebo2 or transitioning from rivastigmine capsules to the target dose 9.5 mg/24 hours patch.12 Based on these results, transition patients as follows:
- Patients taking oral rivastigmine, <6 mg/d: Switch to a 4.6 mg/24 hours patch for ≥4 weeks before increasing to a 9.5 mg/24 hours patch.
- Patients taking oral rivastigmine, 6 to 12 mg/d: Switch directly to a 9.5 mg/24 hours patch.
Apply the first patch the day after the last oral dose.
Related resource
- Rivastigmine transdermal system prescribing information. www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf.
Drug brand names
- Digoxin • Lanoxin
- Rivastigmine • Exelon
- Rivastigmine transdermal
- system • Exelon Patch
Disclosure
Dr. Sadowsky is a consultant to and speaker for Forest Pharmaceuticals and Novartis Pharmaceuticals.
Acknowledgment
The author thanks Christina Mackins, PhD, a medical writer for Alpha-Plus Medical Communications Ltd, for her editorial assistance with this article. Funding for her work was provided by Novartis Pharmaceuticals.
1. Lefèvre G, Sedek G, Jhee S, et al. Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice-daily capsules in Alzheimer’s disease patients. J Clin Pharmacol 2007;47:471-8.
2. Winblad B, Cummings J, Andreasen N, et al. A six-month, double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease—rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007;22:456-67.
3. Oertel W, Ross JS, Eggert K, Adler G. Rationale for transdermal drug administration in Alzheimer disease. Neurology 2007;69(suppl 1):S4-S9.
4. Petersen TA. Transdermal drug formulations and process development. Pharmaceut Technol 2003;(suppl):18-21.
5. Giacobini E, Spiegel R, Enz A, et al. Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: correlation with cognitive benefit. J Neural Transm 2002;109:1053-65.
6. Darreh-Shori T, Almkvist O, Guan ZZ, et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology 2002;59:563-72.
7. Grossberg GT, Stahelin HB, Messina JC, et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int J Geriatr Psychiatry 2000;15(3):242-7.
8. Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther 1998;20:634-47.
9. Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet 2002;41:719-39.
10. Morganroth J, Graham S, Hartman R, et al. Electrocardiographic effects of rivastigmine. J Clin Pharmacol 2002;42:558-68.
11. Exelon patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2007.
12. Frölich L, Barone P, Förstl H, et al. IDEAL: A 28-week open-label extension of a 24-week double-blind study of the first transdermal patch in Alzheimer’s disease. Poster presented at: 11th Congress of the European Federation of Neurological Societies; August 25-28, 2007; Brussels, Belgium.
Dr. Sadowsky is associate clinical professor of neurology, Nova Southeastern University, Fort Lauderdale, FL, and director, Premier Research Institute, Palm Beach Neurology, West Palm Beach, FL.
The rivastigmine patch is the first transdermal treatment for symptoms of mild to moderate Alzheimer’s disease (AD) and mild to moderate Parkinson’s disease dementia (Table). Rivastigmine, a cholinesterase inhibitor, is the only therapy approved for both indications.
Table
Rivastigmine transdermal patch: Fast facts
| Brand name: Exelon Patch |
| Class: Cholinesterase inhibitor |
| Indication: Symptomatic treatment of mild to moderate Alzheimer’s-type dementia and mild to moderate dementia associated with Parkinson’s disease |
| Manufacturer: Novartis Pharmaceuticals, Inc. |
| Dosing forms: 4.6 and 9.5 mg/24 hours transdermal patches (5 cm2 and 10 cm2, respectively) |
| Recommended dosage: Start with 4.6 mg/24 hours patch for ≥4 weeks, followed by a one-step increase to the target dose 9.5 mg/24 hours patch* |
| *Unless the patient is taking oral rivastigmine (see ‘Transitioning to rivastigmine patch,’) |
Clinical implications
The rivastigmine patch offers continuous drug delivery through the skin into the bloodstream over 24 hours.1 This may reduce the incidence of side effects compared with oral rivastigmine,2 making optimal therapeutic doses easier to attain.3 The target dose 9.5 mg/24 hours patch provides efficacy similar to the highest recommended rivastigmine capsule dose (6 mg bid for a total of 12 mg/d).2
How it works
The rivastigmine patch uses matrix technology, which enables delivery of a large amount of drug from a small surface area.4 The patch is available in 2 dosage forms:
- a 5-cm2 size containing 9 mg of rivastigmine that delivers 4.6 mg/24 hours
- a 10-cm2 size containing 18 mg of rivastigmine that delivers 9.5 mg/24 hours.
Each patch consists of 4 layers: the backing layer, an acrylic drug matrix, a silicone adhesive matrix, and an overlapping release liner that is removed and discarded before the patch is applied.1
Cholinesterase inhibitors are believed to exert their effects by increasing available levels of the neurotransmitter acetylcholine in the brain. Two studies have demonstrated that cognitive improvements associated with rivastigmine treatment correlate significantly with cholinesterase inhibition.5,6 In 1 study, rivastigmine’s inhibitory effects on cholinesterase were sustained for 12 months.6
Pharmacokinetics
Rivastigmine is metabolized by its target cholinesterase enzymes to the decarbamylated metabolite NAP 226-90, which has minimal acetylcholinesterase inhibition and is excreted through the urine.1 As a result of its low accumulation potential and cytochrome P 450-independent metabolism, rivastigmine has low potential for pharmacokinetic drug–drug interactions. This lack of interaction has been confirmed for many drugs commonly taken by elderly patients, such as digoxin, nonsteroidal anti-inflammatory drugs, and estrogens.7
Rivastigmine has a half-life of 1 to 2 hours, so it is rapidly cleared.8 In the event of a serious reaction, significant clearance of rivastigmine from the body would occur within 3 hours of patch removal.
Centrally mediated cholinergic gastrointestinal (GI) side effects associated with oral rivastigmine are related to high maximum plasma concentrations (Cmax) and short time interval to Cmax (Tmax).9 In an open-label, parallel-group study of 51 AD patients that compared rivastigmine patches with rivastigmine capsules, transdermal administration was associated with slower increases to lower peak plasma concentrations (prolonged Tmax and reduced Cmax), and less fluctuation in plasma concentration.1 Despite these effects, the rivastigmine 9.5 mg/24 hours patch provided drug exposure comparable to the highest dose of capsules (6 mg bid for a total of 12 mg/d), with improved GI tolerability.3
Efficacy
Rivastigmine patch efficacy was evaluated in a single, 24-week, international, randomized, double-blind trial of 1,195 patients with AD.2 The study group represented typical patients with mild to moderate AD—age 50 to 85 years with Mini-Mental State Examination scores of 10 to 20 at baseline. Patients were randomly assigned to receive:
- 17.4 mg/24 hours rivastigmine patch (20-cm2 patch; n=303)
- 9.5 mg/24 hours rivastigmine patch (10-cm2 patch; n=293)
- 6 mg bid rivastigmine capsules (n=297)
- or placebo (n=302).
Data for the 17.4 mg/24 hours patch are not discussed here because this dose exceeds the FDA-approved maximum dosage (9.5 mg/24 hours) and is not available.
Patients in the 9.5 mg/24 hours patch group received a 4.6 mg/24 hours patch (5 cm2) for weeks 1 through 4, and then the 9.5 mg/24 hours patch for the remainder of the study. Patients in the capsule group started on 3 mg/d (1.5 mg bid) and were titrated every 4 weeks in steps of 3 mg/d to a maximum of 12 mg/d administered as 6 mg bid.
Primary outcomes were measured as mean change in score from baseline to endpoint on the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) and Alzheimer’s Disease Co-operative Study–Clinical Global Impression of Change (ADCS-CGIC). By study endpoint, the 9.5 mg/24 hours patch and capsules, 12 mg/d, showed comparable efficacy (Figure).2 Compared with those receiving placebo, patients in the 9.5 mg/24 hours patch and capsule groups showed significant improvements in dementia symptoms, including:
- cognition
- global performance
- attention
- activities of daily living.2
Based on my clinical experience, these improvements reflect small but clinically meaningful changes that are noted by patients and caregivers.
Figure
Efficacy of transdermal rivastigmine for Alzheimer’s symptoms

*P<0.05 vs placebo
ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale; ADCS-CGIC: Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change
Source: Adapted from reference 2
In a 24-week study, transdermal rivastigmine, 9.5 mg/24 hours, and the highest recommended dose of oral rivastigmine (6 mg bid) showed comparable efficacy as measured by mean change in score on scales commonly used in Alzheimer’s disease clinical trials. ADAS-Cog assesses orientation, memory, language, praxis, and visuospatial functions. ADCS-CGIC provides a single global rating of change from baseline based on interviews with the patient and caregiver.
Safety and tolerability
Adverse events associated with rivastigmine are predominantly cholinergic; GI side effects—nausea, vomiting, and diarrhea—are observed most frequently.2 These events occur less frequently with the patch than with capsules. In the efficacy trial, patients in the 9.5 mg/24 hours rivastigmine patch group had one-third as many reports of nausea (7.2% vs 23.1%) and vomiting (6.2% vs 17.0%) compared with the 6 mg bid capsule group.2
Diarrhea was reported by 6% of subjects receiving the 9.5 mg/24 hours patch, 5% of those taking 6-mg capsule bid, and 3% receiving placebo. Fewer subjects in the 9.5 mg/24 hours patch group (3%) experienced decreased weight compared with those in the capsule group (5%). The rate of decreased weight with placebo was 1%.
Dizziness affected 2% of those in the 9.5 mg/24 hours patch and placebo groups; incidence in the capsule group was significantly higher at 8%. Headache was similar with the 9.5 mg/24 hours patch (3%) and placebo (2%), with the capsule significantly higher at 6%.2
The proportion of patients who experienced no, slight, or mild skin irritation ranged from 90% to 98%.2 The most commonly reported moderate or severe skin irritations were erythema (8% rivastigmine patch vs 4% placebo) and pruritus (7% rivastigmine patch vs 3% placebo). Two percent of patients using active patch discontinued the trial because of skin irritation.
Rivastigmine appears not to produce adverse effects on cardiac function as assessed by ECG. In clinical trials of 2,791 patients, pooled 12-lead ECG data comparing oral rivastigmine and placebo groups did not differ significantly in heart rate or PR, QRS, and QTc intervals.10
Dosing
The rivastigmine patch is administered once daily, and the recommended maintenance dose is the 9.5 mg/24 hours patch. Start patients on a 4.6 mg/24 hours patch for at least 4 weeks and then increase to the 9.5 mg/24 hours target dose if the lower dose is well tolerated.
Dosage adjustment of rivastigmine is not necessary in patients with hepatic or renal disease because of minimal liver metabolism and the acetylcholinesterase-mediated hydrolysis of rivastigmine to the inactive decarbamylated metabolite NAP 226-90, which is excreted in the urine.11
Instruct patients or caregivers to apply the patch to clean, dry, hairless skin that is free of cuts, rashes, or irritation on the upper or lower back or upper arm or chest.1 The patch has shown good adhesive properties over 24 hours, remaining attached in a range of situations, including bathing and hot weather.2 In the 9.5 mg/24 hours group of the efficacy study, 96% of patches remained attached or had slight lifting of the edges (1,336 total patch evaluations).
Transitioning to rivastigmine patch
The efficacy study included an open-label extension, during which blinding was maintained. This provided information on patients beginning rivastigmine patch therapy directly from placebo2 or transitioning from rivastigmine capsules to the target dose 9.5 mg/24 hours patch.12 Based on these results, transition patients as follows:
- Patients taking oral rivastigmine, <6 mg/d: Switch to a 4.6 mg/24 hours patch for ≥4 weeks before increasing to a 9.5 mg/24 hours patch.
- Patients taking oral rivastigmine, 6 to 12 mg/d: Switch directly to a 9.5 mg/24 hours patch.
Apply the first patch the day after the last oral dose.
Related resource
- Rivastigmine transdermal system prescribing information. www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf.
Drug brand names
- Digoxin • Lanoxin
- Rivastigmine • Exelon
- Rivastigmine transdermal
- system • Exelon Patch
Disclosure
Dr. Sadowsky is a consultant to and speaker for Forest Pharmaceuticals and Novartis Pharmaceuticals.
Acknowledgment
The author thanks Christina Mackins, PhD, a medical writer for Alpha-Plus Medical Communications Ltd, for her editorial assistance with this article. Funding for her work was provided by Novartis Pharmaceuticals.
The rivastigmine patch is the first transdermal treatment for symptoms of mild to moderate Alzheimer’s disease (AD) and mild to moderate Parkinson’s disease dementia (Table). Rivastigmine, a cholinesterase inhibitor, is the only therapy approved for both indications.
Table
Rivastigmine transdermal patch: Fast facts
| Brand name: Exelon Patch |
| Class: Cholinesterase inhibitor |
| Indication: Symptomatic treatment of mild to moderate Alzheimer’s-type dementia and mild to moderate dementia associated with Parkinson’s disease |
| Manufacturer: Novartis Pharmaceuticals, Inc. |
| Dosing forms: 4.6 and 9.5 mg/24 hours transdermal patches (5 cm2 and 10 cm2, respectively) |
| Recommended dosage: Start with 4.6 mg/24 hours patch for ≥4 weeks, followed by a one-step increase to the target dose 9.5 mg/24 hours patch* |
| *Unless the patient is taking oral rivastigmine (see ‘Transitioning to rivastigmine patch,’) |
Clinical implications
The rivastigmine patch offers continuous drug delivery through the skin into the bloodstream over 24 hours.1 This may reduce the incidence of side effects compared with oral rivastigmine,2 making optimal therapeutic doses easier to attain.3 The target dose 9.5 mg/24 hours patch provides efficacy similar to the highest recommended rivastigmine capsule dose (6 mg bid for a total of 12 mg/d).2
How it works
The rivastigmine patch uses matrix technology, which enables delivery of a large amount of drug from a small surface area.4 The patch is available in 2 dosage forms:
- a 5-cm2 size containing 9 mg of rivastigmine that delivers 4.6 mg/24 hours
- a 10-cm2 size containing 18 mg of rivastigmine that delivers 9.5 mg/24 hours.
Each patch consists of 4 layers: the backing layer, an acrylic drug matrix, a silicone adhesive matrix, and an overlapping release liner that is removed and discarded before the patch is applied.1
Cholinesterase inhibitors are believed to exert their effects by increasing available levels of the neurotransmitter acetylcholine in the brain. Two studies have demonstrated that cognitive improvements associated with rivastigmine treatment correlate significantly with cholinesterase inhibition.5,6 In 1 study, rivastigmine’s inhibitory effects on cholinesterase were sustained for 12 months.6
Pharmacokinetics
Rivastigmine is metabolized by its target cholinesterase enzymes to the decarbamylated metabolite NAP 226-90, which has minimal acetylcholinesterase inhibition and is excreted through the urine.1 As a result of its low accumulation potential and cytochrome P 450-independent metabolism, rivastigmine has low potential for pharmacokinetic drug–drug interactions. This lack of interaction has been confirmed for many drugs commonly taken by elderly patients, such as digoxin, nonsteroidal anti-inflammatory drugs, and estrogens.7
Rivastigmine has a half-life of 1 to 2 hours, so it is rapidly cleared.8 In the event of a serious reaction, significant clearance of rivastigmine from the body would occur within 3 hours of patch removal.
Centrally mediated cholinergic gastrointestinal (GI) side effects associated with oral rivastigmine are related to high maximum plasma concentrations (Cmax) and short time interval to Cmax (Tmax).9 In an open-label, parallel-group study of 51 AD patients that compared rivastigmine patches with rivastigmine capsules, transdermal administration was associated with slower increases to lower peak plasma concentrations (prolonged Tmax and reduced Cmax), and less fluctuation in plasma concentration.1 Despite these effects, the rivastigmine 9.5 mg/24 hours patch provided drug exposure comparable to the highest dose of capsules (6 mg bid for a total of 12 mg/d), with improved GI tolerability.3
Efficacy
Rivastigmine patch efficacy was evaluated in a single, 24-week, international, randomized, double-blind trial of 1,195 patients with AD.2 The study group represented typical patients with mild to moderate AD—age 50 to 85 years with Mini-Mental State Examination scores of 10 to 20 at baseline. Patients were randomly assigned to receive:
- 17.4 mg/24 hours rivastigmine patch (20-cm2 patch; n=303)
- 9.5 mg/24 hours rivastigmine patch (10-cm2 patch; n=293)
- 6 mg bid rivastigmine capsules (n=297)
- or placebo (n=302).
Data for the 17.4 mg/24 hours patch are not discussed here because this dose exceeds the FDA-approved maximum dosage (9.5 mg/24 hours) and is not available.
Patients in the 9.5 mg/24 hours patch group received a 4.6 mg/24 hours patch (5 cm2) for weeks 1 through 4, and then the 9.5 mg/24 hours patch for the remainder of the study. Patients in the capsule group started on 3 mg/d (1.5 mg bid) and were titrated every 4 weeks in steps of 3 mg/d to a maximum of 12 mg/d administered as 6 mg bid.
Primary outcomes were measured as mean change in score from baseline to endpoint on the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) and Alzheimer’s Disease Co-operative Study–Clinical Global Impression of Change (ADCS-CGIC). By study endpoint, the 9.5 mg/24 hours patch and capsules, 12 mg/d, showed comparable efficacy (Figure).2 Compared with those receiving placebo, patients in the 9.5 mg/24 hours patch and capsule groups showed significant improvements in dementia symptoms, including:
- cognition
- global performance
- attention
- activities of daily living.2
Based on my clinical experience, these improvements reflect small but clinically meaningful changes that are noted by patients and caregivers.
Figure
Efficacy of transdermal rivastigmine for Alzheimer’s symptoms

*P<0.05 vs placebo
ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale; ADCS-CGIC: Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change
Source: Adapted from reference 2
In a 24-week study, transdermal rivastigmine, 9.5 mg/24 hours, and the highest recommended dose of oral rivastigmine (6 mg bid) showed comparable efficacy as measured by mean change in score on scales commonly used in Alzheimer’s disease clinical trials. ADAS-Cog assesses orientation, memory, language, praxis, and visuospatial functions. ADCS-CGIC provides a single global rating of change from baseline based on interviews with the patient and caregiver.
Safety and tolerability
Adverse events associated with rivastigmine are predominantly cholinergic; GI side effects—nausea, vomiting, and diarrhea—are observed most frequently.2 These events occur less frequently with the patch than with capsules. In the efficacy trial, patients in the 9.5 mg/24 hours rivastigmine patch group had one-third as many reports of nausea (7.2% vs 23.1%) and vomiting (6.2% vs 17.0%) compared with the 6 mg bid capsule group.2
Diarrhea was reported by 6% of subjects receiving the 9.5 mg/24 hours patch, 5% of those taking 6-mg capsule bid, and 3% receiving placebo. Fewer subjects in the 9.5 mg/24 hours patch group (3%) experienced decreased weight compared with those in the capsule group (5%). The rate of decreased weight with placebo was 1%.
Dizziness affected 2% of those in the 9.5 mg/24 hours patch and placebo groups; incidence in the capsule group was significantly higher at 8%. Headache was similar with the 9.5 mg/24 hours patch (3%) and placebo (2%), with the capsule significantly higher at 6%.2
The proportion of patients who experienced no, slight, or mild skin irritation ranged from 90% to 98%.2 The most commonly reported moderate or severe skin irritations were erythema (8% rivastigmine patch vs 4% placebo) and pruritus (7% rivastigmine patch vs 3% placebo). Two percent of patients using active patch discontinued the trial because of skin irritation.
Rivastigmine appears not to produce adverse effects on cardiac function as assessed by ECG. In clinical trials of 2,791 patients, pooled 12-lead ECG data comparing oral rivastigmine and placebo groups did not differ significantly in heart rate or PR, QRS, and QTc intervals.10
Dosing
The rivastigmine patch is administered once daily, and the recommended maintenance dose is the 9.5 mg/24 hours patch. Start patients on a 4.6 mg/24 hours patch for at least 4 weeks and then increase to the 9.5 mg/24 hours target dose if the lower dose is well tolerated.
Dosage adjustment of rivastigmine is not necessary in patients with hepatic or renal disease because of minimal liver metabolism and the acetylcholinesterase-mediated hydrolysis of rivastigmine to the inactive decarbamylated metabolite NAP 226-90, which is excreted in the urine.11
Instruct patients or caregivers to apply the patch to clean, dry, hairless skin that is free of cuts, rashes, or irritation on the upper or lower back or upper arm or chest.1 The patch has shown good adhesive properties over 24 hours, remaining attached in a range of situations, including bathing and hot weather.2 In the 9.5 mg/24 hours group of the efficacy study, 96% of patches remained attached or had slight lifting of the edges (1,336 total patch evaluations).
Transitioning to rivastigmine patch
The efficacy study included an open-label extension, during which blinding was maintained. This provided information on patients beginning rivastigmine patch therapy directly from placebo2 or transitioning from rivastigmine capsules to the target dose 9.5 mg/24 hours patch.12 Based on these results, transition patients as follows:
- Patients taking oral rivastigmine, <6 mg/d: Switch to a 4.6 mg/24 hours patch for ≥4 weeks before increasing to a 9.5 mg/24 hours patch.
- Patients taking oral rivastigmine, 6 to 12 mg/d: Switch directly to a 9.5 mg/24 hours patch.
Apply the first patch the day after the last oral dose.
Related resource
- Rivastigmine transdermal system prescribing information. www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf.
Drug brand names
- Digoxin • Lanoxin
- Rivastigmine • Exelon
- Rivastigmine transdermal
- system • Exelon Patch
Disclosure
Dr. Sadowsky is a consultant to and speaker for Forest Pharmaceuticals and Novartis Pharmaceuticals.
Acknowledgment
The author thanks Christina Mackins, PhD, a medical writer for Alpha-Plus Medical Communications Ltd, for her editorial assistance with this article. Funding for her work was provided by Novartis Pharmaceuticals.
1. Lefèvre G, Sedek G, Jhee S, et al. Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice-daily capsules in Alzheimer’s disease patients. J Clin Pharmacol 2007;47:471-8.
2. Winblad B, Cummings J, Andreasen N, et al. A six-month, double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease—rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007;22:456-67.
3. Oertel W, Ross JS, Eggert K, Adler G. Rationale for transdermal drug administration in Alzheimer disease. Neurology 2007;69(suppl 1):S4-S9.
4. Petersen TA. Transdermal drug formulations and process development. Pharmaceut Technol 2003;(suppl):18-21.
5. Giacobini E, Spiegel R, Enz A, et al. Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: correlation with cognitive benefit. J Neural Transm 2002;109:1053-65.
6. Darreh-Shori T, Almkvist O, Guan ZZ, et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology 2002;59:563-72.
7. Grossberg GT, Stahelin HB, Messina JC, et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int J Geriatr Psychiatry 2000;15(3):242-7.
8. Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther 1998;20:634-47.
9. Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet 2002;41:719-39.
10. Morganroth J, Graham S, Hartman R, et al. Electrocardiographic effects of rivastigmine. J Clin Pharmacol 2002;42:558-68.
11. Exelon patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2007.
12. Frölich L, Barone P, Förstl H, et al. IDEAL: A 28-week open-label extension of a 24-week double-blind study of the first transdermal patch in Alzheimer’s disease. Poster presented at: 11th Congress of the European Federation of Neurological Societies; August 25-28, 2007; Brussels, Belgium.
Dr. Sadowsky is associate clinical professor of neurology, Nova Southeastern University, Fort Lauderdale, FL, and director, Premier Research Institute, Palm Beach Neurology, West Palm Beach, FL.
1. Lefèvre G, Sedek G, Jhee S, et al. Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice-daily capsules in Alzheimer’s disease patients. J Clin Pharmacol 2007;47:471-8.
2. Winblad B, Cummings J, Andreasen N, et al. A six-month, double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease—rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007;22:456-67.
3. Oertel W, Ross JS, Eggert K, Adler G. Rationale for transdermal drug administration in Alzheimer disease. Neurology 2007;69(suppl 1):S4-S9.
4. Petersen TA. Transdermal drug formulations and process development. Pharmaceut Technol 2003;(suppl):18-21.
5. Giacobini E, Spiegel R, Enz A, et al. Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: correlation with cognitive benefit. J Neural Transm 2002;109:1053-65.
6. Darreh-Shori T, Almkvist O, Guan ZZ, et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology 2002;59:563-72.
7. Grossberg GT, Stahelin HB, Messina JC, et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int J Geriatr Psychiatry 2000;15(3):242-7.
8. Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther 1998;20:634-47.
9. Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet 2002;41:719-39.
10. Morganroth J, Graham S, Hartman R, et al. Electrocardiographic effects of rivastigmine. J Clin Pharmacol 2002;42:558-68.
11. Exelon patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2007.
12. Frölich L, Barone P, Förstl H, et al. IDEAL: A 28-week open-label extension of a 24-week double-blind study of the first transdermal patch in Alzheimer’s disease. Poster presented at: 11th Congress of the European Federation of Neurological Societies; August 25-28, 2007; Brussels, Belgium.
Dr. Sadowsky is associate clinical professor of neurology, Nova Southeastern University, Fort Lauderdale, FL, and director, Premier Research Institute, Palm Beach Neurology, West Palm Beach, FL.
Help your patients keep appointments
Patients’ failure to keep appointments is a common problem. On average, patients miss approximately 15% of follow-up psychiatric appointments,1 but the percentage is much higher in some patient populations, such as patients with significant socioeconomic difficulties. Those who miss appointments often have worse outcomes and even a higher likelihood of psychiatric readmission.2
We present strategies to help patients keep appointments and to handle occasional and repeated absences. Although the problem of missed appointments will never go away, following these suggestions could help minimize it.
Prevent the problem
Explain to the patient why regular appointments are important. The most important point is that clinician and patient must agree that—to best help the patient—treatment requires that all appointments be kept, barring emergencies.
Communicate clearly. Avoid emphasizing rules, such as that patients must keep 80% of appointments, give 48-hours’ notice for cancellations, or pay a no-show fee. These suggest that patients may miss appointments as long as they follow the rules.
Fix structural problems in your practice that may be barriers to making, rescheduling, or cancelling appointments. Be clear with patients about:
- the phone number they should call for appointments
- if they or you must cancel, that person is to reschedule at the earliest opportunity.
If the patient is missing appointments because the frequency is too burdensome, in many cases less frequent but more regular visits may be better.
During your early sessions with patients, be sure they understand that you reserve specific times for them. Make sure, however, that patients don’t interpret this to mean that attending every appointment is for your benefit, rather than important for their treatment.
Emphasize responsibility. At the end of each session, set a goal with patients for the next appointment. With a patient who has missed appointments, ask for a commitment that he or she will come to the next session. We have found that stating that you are concerned the patient might not come to the next session can paradoxically be helpful.
Having your receptionist call and remind patients the day before their visits might not be a good idea in many cases. Patients might think these calls relieve them of the responsibility for remembering to keep appointments.
With patients you think might miss appointments—especially those on a medication that requires careful monitoring—consider writing prescriptions to last no longer than the next appointment.
Occasional missed appointments
Don’t just let it go when patients occasionally miss appointments without adequate reason. Ignoring the problem lets it progress.
Resist the temptation to be courteous and say, “That’s all right” when patients apologize or give a reason for missing the session. Doing so gives a subtle message that missing appointments is acceptable.
Discussing the patients’ reasons for missing appointments might solve the problem at times. For example, patients might not mention transportation or child care problems.
Note in the chart when a patient does not come to an appointment so you can calculate how many have been missed. This notation also will remind you to address these missed appointments during the next visit. Because discussing missed appointments at the start of the session might seem confrontational or punitive, inquire about the reasons for missing the previous appointment in a gentle manner and later in the session.
Remind patients that therapy is the tool to solve their emotional problems and thus has a special place in their lives. If patients want to solve other problems, they must start by regularly attending therapy.
Repeatedly missed appointments
When a patient misses appointments repeatedly, take 1 or more sessions to discuss it. This has to be done before therapy can proceed effectively (of course you might need to postpone this discussion if the patient has experienced major stressful events or has other pressing clinical issues).
When doing this, resist the temptation to become sidetracked by other issues the patient brings up. You can let the patient vent for a few minutes, but don’t let most of the session go by before addressing the missed appointments.
1. Mitchell AJ, Selmes T. A comparative survey of missed initial and follow-up appointments to psychiatric specialties in the United Kingdom. Psychiatr Serv 2007;58(6):868-71.
2. Killaspy H, Banerjee S, King M, Lloyd M. Prospective controlled study of psychiatric out-patient non-attendance. Characteristics and outcome. Br J Psychiatry 2000;176:160-5.
Dr. Mago is assistant professor of psychiatry and director of the mood disorders program; Dr. Mahajan is a research volunteer; and Dr. McFadden is an instructor and associate director of adult outpatient services, Thomas Jefferson University, Philadelphia.
Patients’ failure to keep appointments is a common problem. On average, patients miss approximately 15% of follow-up psychiatric appointments,1 but the percentage is much higher in some patient populations, such as patients with significant socioeconomic difficulties. Those who miss appointments often have worse outcomes and even a higher likelihood of psychiatric readmission.2
We present strategies to help patients keep appointments and to handle occasional and repeated absences. Although the problem of missed appointments will never go away, following these suggestions could help minimize it.
Prevent the problem
Explain to the patient why regular appointments are important. The most important point is that clinician and patient must agree that—to best help the patient—treatment requires that all appointments be kept, barring emergencies.
Communicate clearly. Avoid emphasizing rules, such as that patients must keep 80% of appointments, give 48-hours’ notice for cancellations, or pay a no-show fee. These suggest that patients may miss appointments as long as they follow the rules.
Fix structural problems in your practice that may be barriers to making, rescheduling, or cancelling appointments. Be clear with patients about:
- the phone number they should call for appointments
- if they or you must cancel, that person is to reschedule at the earliest opportunity.
If the patient is missing appointments because the frequency is too burdensome, in many cases less frequent but more regular visits may be better.
During your early sessions with patients, be sure they understand that you reserve specific times for them. Make sure, however, that patients don’t interpret this to mean that attending every appointment is for your benefit, rather than important for their treatment.
Emphasize responsibility. At the end of each session, set a goal with patients for the next appointment. With a patient who has missed appointments, ask for a commitment that he or she will come to the next session. We have found that stating that you are concerned the patient might not come to the next session can paradoxically be helpful.
Having your receptionist call and remind patients the day before their visits might not be a good idea in many cases. Patients might think these calls relieve them of the responsibility for remembering to keep appointments.
With patients you think might miss appointments—especially those on a medication that requires careful monitoring—consider writing prescriptions to last no longer than the next appointment.
Occasional missed appointments
Don’t just let it go when patients occasionally miss appointments without adequate reason. Ignoring the problem lets it progress.
Resist the temptation to be courteous and say, “That’s all right” when patients apologize or give a reason for missing the session. Doing so gives a subtle message that missing appointments is acceptable.
Discussing the patients’ reasons for missing appointments might solve the problem at times. For example, patients might not mention transportation or child care problems.
Note in the chart when a patient does not come to an appointment so you can calculate how many have been missed. This notation also will remind you to address these missed appointments during the next visit. Because discussing missed appointments at the start of the session might seem confrontational or punitive, inquire about the reasons for missing the previous appointment in a gentle manner and later in the session.
Remind patients that therapy is the tool to solve their emotional problems and thus has a special place in their lives. If patients want to solve other problems, they must start by regularly attending therapy.
Repeatedly missed appointments
When a patient misses appointments repeatedly, take 1 or more sessions to discuss it. This has to be done before therapy can proceed effectively (of course you might need to postpone this discussion if the patient has experienced major stressful events or has other pressing clinical issues).
When doing this, resist the temptation to become sidetracked by other issues the patient brings up. You can let the patient vent for a few minutes, but don’t let most of the session go by before addressing the missed appointments.
Patients’ failure to keep appointments is a common problem. On average, patients miss approximately 15% of follow-up psychiatric appointments,1 but the percentage is much higher in some patient populations, such as patients with significant socioeconomic difficulties. Those who miss appointments often have worse outcomes and even a higher likelihood of psychiatric readmission.2
We present strategies to help patients keep appointments and to handle occasional and repeated absences. Although the problem of missed appointments will never go away, following these suggestions could help minimize it.
Prevent the problem
Explain to the patient why regular appointments are important. The most important point is that clinician and patient must agree that—to best help the patient—treatment requires that all appointments be kept, barring emergencies.
Communicate clearly. Avoid emphasizing rules, such as that patients must keep 80% of appointments, give 48-hours’ notice for cancellations, or pay a no-show fee. These suggest that patients may miss appointments as long as they follow the rules.
Fix structural problems in your practice that may be barriers to making, rescheduling, or cancelling appointments. Be clear with patients about:
- the phone number they should call for appointments
- if they or you must cancel, that person is to reschedule at the earliest opportunity.
If the patient is missing appointments because the frequency is too burdensome, in many cases less frequent but more regular visits may be better.
During your early sessions with patients, be sure they understand that you reserve specific times for them. Make sure, however, that patients don’t interpret this to mean that attending every appointment is for your benefit, rather than important for their treatment.
Emphasize responsibility. At the end of each session, set a goal with patients for the next appointment. With a patient who has missed appointments, ask for a commitment that he or she will come to the next session. We have found that stating that you are concerned the patient might not come to the next session can paradoxically be helpful.
Having your receptionist call and remind patients the day before their visits might not be a good idea in many cases. Patients might think these calls relieve them of the responsibility for remembering to keep appointments.
With patients you think might miss appointments—especially those on a medication that requires careful monitoring—consider writing prescriptions to last no longer than the next appointment.
Occasional missed appointments
Don’t just let it go when patients occasionally miss appointments without adequate reason. Ignoring the problem lets it progress.
Resist the temptation to be courteous and say, “That’s all right” when patients apologize or give a reason for missing the session. Doing so gives a subtle message that missing appointments is acceptable.
Discussing the patients’ reasons for missing appointments might solve the problem at times. For example, patients might not mention transportation or child care problems.
Note in the chart when a patient does not come to an appointment so you can calculate how many have been missed. This notation also will remind you to address these missed appointments during the next visit. Because discussing missed appointments at the start of the session might seem confrontational or punitive, inquire about the reasons for missing the previous appointment in a gentle manner and later in the session.
Remind patients that therapy is the tool to solve their emotional problems and thus has a special place in their lives. If patients want to solve other problems, they must start by regularly attending therapy.
Repeatedly missed appointments
When a patient misses appointments repeatedly, take 1 or more sessions to discuss it. This has to be done before therapy can proceed effectively (of course you might need to postpone this discussion if the patient has experienced major stressful events or has other pressing clinical issues).
When doing this, resist the temptation to become sidetracked by other issues the patient brings up. You can let the patient vent for a few minutes, but don’t let most of the session go by before addressing the missed appointments.
1. Mitchell AJ, Selmes T. A comparative survey of missed initial and follow-up appointments to psychiatric specialties in the United Kingdom. Psychiatr Serv 2007;58(6):868-71.
2. Killaspy H, Banerjee S, King M, Lloyd M. Prospective controlled study of psychiatric out-patient non-attendance. Characteristics and outcome. Br J Psychiatry 2000;176:160-5.
Dr. Mago is assistant professor of psychiatry and director of the mood disorders program; Dr. Mahajan is a research volunteer; and Dr. McFadden is an instructor and associate director of adult outpatient services, Thomas Jefferson University, Philadelphia.
1. Mitchell AJ, Selmes T. A comparative survey of missed initial and follow-up appointments to psychiatric specialties in the United Kingdom. Psychiatr Serv 2007;58(6):868-71.
2. Killaspy H, Banerjee S, King M, Lloyd M. Prospective controlled study of psychiatric out-patient non-attendance. Characteristics and outcome. Br J Psychiatry 2000;176:160-5.
Dr. Mago is assistant professor of psychiatry and director of the mood disorders program; Dr. Mahajan is a research volunteer; and Dr. McFadden is an instructor and associate director of adult outpatient services, Thomas Jefferson University, Philadelphia.
How to protect patients’ confidentiality
Psychiatrist reveals patients’ information to another patient
Alameda County (CA) Superior Court
For several years 2 female patients were treated by the same psychiatrist. Jane Doe, age 56, read a breach of confidentiality report alleging sexual abuse filed by another patient of the psychiatrist. Jane Doe contacted the alleged victim, who informed her that the psychiatrist had disclosed information to her (the victim) regarding Jane Doe’s treatment, emotional problems, sexual preferences, and medication regimen.
Susan Doe, age 64, learned of the sexual abuse accusations against the psychiatrist in the same way and also contacted the alleged victim. She told Susan Doe that the psychiatrist had disclosed to her Susan Doe’s personal information regarding her dificult relationship with her daughter, depression, and instances when she stormed out of counseling sessions.
The patients brought separate claims, and their cases were later consolidated. The psychiatrist denied that he told the alleged sexual abuse victim details of the 2 patients’ treatments. The patients claimed that the victim could not have known their personal details unless the psychiatrist had told her.
- A jury returned a verdict in favor of the 2 patients. Jane Doe was awarded $225,000, and Susan Doe was awarded $47,000.
Dr. Grant’s observations
In the case of Jane Doe and Susan Doe, disclosing a patient’s personal information to another patient violates confidentiality. Patients must consent to the disclosure of information to third parties, and in this case these 2 patients apparently did not provide consent.
Medical practice—and particularly psychiatric practice—is based on the principle that communications between clinicians and patients are private. The Hippocratic oath states, “Whatever I see or hear in the lives of my patients, whether in connection with my professional practice or not, which ought not to be spoken of outside, I will keep secret, as considering all such things to be private.”1
According to the American Psychiatric Association’s (APA) code of ethics, “Psychiatric records, including even the identification of a person as a patient, must be protected with extreme care. Confidentiality is essential to psychiatric treatment, in part because of the special nature of psychiatric therapy. A psychiatrist may release confidential information only with the patient’s authorization or under proper legal compulsion.”2
Doctor-patient confidentiality is rooted in the belief that potential disclosure of information communicated during psychiatric diagnosis and treatment would discourage patients from seeking medical and mental health care (Table)
Table
Underlying values of confidentiality
| Proper doctor-patient confidentiality aims to: |
|
| Source: U.S. Department of Health and Human Services. Mental Health: A Report of the Surgeon General—Executive Summary. Rockville, MD: U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, National Institutes of Health, National Institute of Mental Health, 1999. |
When to disclose
There are circumstances, however, that override the requirement to maintain confidentiality and do not need a patient’s consent. Examples include:3
Duty to protect third parties. In 1976 the California Supreme Court ruled in the landmark Tarasoff case4 that a psychiatrist has a duty to do what is reasonably necessary to protect third parties if a patient presents a serious risk of violence to another person. The specific applications of this principle are governed by other states’ laws, which have extended or limited this duty.5 Be familiar with the law in your jurisdiction before disclosing confidential information to third parties who may be at risk of violence.
The APA’s position on this exception is consistent with legal standards. Its code of ethics states, “When, in the clinical judgment of the treating psychiatrist, the risk of danger is deemed to be significant, the psychiatrist may reveal confidential information disclosed by the patient.”6
Emergency release of information. Psychiatrists can release confidential information during a medical emergency. Releasing the information must be in the patient’s best interests, and the patient’s inability to consent to the release should be the result of a potentially reversible condition that leads the clinician to question the patient’s capacity to consent.3
For example, if a patient in an emergency room is delirious because of ingesting an unknown substance and is unable to consent, a physician can call family members to ask about the patient’s medical problems. Notifying family that the patient is in the hospital could violate confidentiality, however.
Reporting abuse. All clinicians are obligated to report suspected child abuse or neglect. Some state laws also may require physicians to disclose abuse of vulnerable groups such as the elderly or the disabled and report to the local department of health diagnosis of communicable diseases such as HIV.3
Circle of confidentiality. Certain parties— including clinical staff on an inpatient unit or a psychiatrist supervising a resident— are considered to be within a circle of confidentiality.3 You do not need a patient’s consent to share clinical information with those within the circle of confidentiality. Do not release a patient’s information to parties who are not in the circle of confidentiality—such as family members, attorneys representing the patient, and law enforcement personnel—unless you’ve first obtained the patient’s consent.
Document the reasoning behind your decision to disclose your patient’s personal information without the patient’s consent. Show that you engaged in a reasonable clinical decision-making process.3 For example, record the risks and benefits of your decision and how you arrived at your conclusion.3
Other scenarios
Multidisciplinary teams. Members of a multidisciplinary treatment team—such as physicians, nurses, or social workers—should only receive confidential information that is relevant to the patient’s care. Other clinicians who are not involved in the case—although they may be seeing other patients on the same unit—should not have access to the patient’s confidential information. Discussions with these team members must be private so that others do not overhear confidential information.
Insurance companies generally are not party to the patient’s records unless the patient agrees to allow access by signing a release. If the patient’s refusal to allow disclosure results in the insurance company’s refusal to pay, then the patient is responsible for resolving the issue.7
Scientific publications and presentations. When you present a case report for a scientific publication or at a meeting, alter the patient’s biographical data so that someone who knows the patient would be unable to identify him or her based on the information in the case report. If the information is so specific that you cannot prevent patient identification, either do not publish the case or offer the patient the right to veto the manuscript’s distribution. If necessary, have the patient sign a consent form to allow publication or presentation of the case report.
Confidentiality violations
Breach of confidentiality may be intentional, such as disclosing a patient’s personal information to a third party as in this case, or unintentional, such as talking about a patient to a colleague and having someone overhear your discussion.8 Violating confidentiality may result in litigation for malpractice (negligence), invasion of privacy, or breach of contract, and ethical sanctions.8
No aspect of psychiatric practice seems to generate stronger emotions than the potential legal repercussions of our work. Keeping up with patients’ needs, billing issues, and advancements in medicine leaves little time for tracking changing state and federal laws or case precedents. For the past 4 years it has been my pleasure to provide information on the legal issues psychiatrists face and provide possible means of avoiding legal pitfalls.
Although I have decided to pursue other projects, I wish to give readers my thanks and to suggest resources—only a few among many great ones—that may be useful guides for a variety of legal issues.
Jon E. Grant, JD, MD, MPH
- Journal of the American Academy of Psychiatry and the Law.
- Appelbaum PS, Gutheil TG. Clinical handbook of psychiatry and the law. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA:Harvard University Press; 1998.
- Simon RI, Shuman DW.Clinical manual of psychiatry and the law. Washington, DC: American Psychiatric Publishing, Inc. ; 2007.
Editor’s note
Current Psychiatry thanks Dr. Grant for writing the Malpractice Verdicts column since 2004. The column will continue in a new format in the February 2008 issue.
1. National Institutes of Health. The Hippocratic oath. Available at: http://www.nlm.nih.gov/hmd/greek/greek_oath.html. Accessed October 30, 2007.
2. Principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC: American Psychiatric Association; 2006: 6. Availableat: http://www.psych.org/psych_pract/ethics/ppaethics.pdf. Accessed October 30, 2007.
3. Lowenthal D. Case studies in confidentiality. J Psychiatr Prac 2002;8:151-9.
4. Tarasoff vs Regents of the University of California 551P 2d 334 (Cal 1976).
5. Appelbaum PS Taras off and the clinician: problems in fulfilling the duty to protect. Am J Psychiatry 1985;142:425-9.
6. Principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC: American Psychiatric Association; 2006:7. Availableat: http://www.psych.org/psych_pract/ethics/ppaethics.pdf. Accessed October 30, 2007.
7. Hilliard J. Liability issues with managed care. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA: Harvard University Press; 1998:44-51.
8. Berner M. Write smarter, not longer. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA: Harvard University Press; 1998:54-71.
Cases are selected by Current Psychiatry fromMedical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Psychiatrist reveals patients’ information to another patient
Alameda County (CA) Superior Court
For several years 2 female patients were treated by the same psychiatrist. Jane Doe, age 56, read a breach of confidentiality report alleging sexual abuse filed by another patient of the psychiatrist. Jane Doe contacted the alleged victim, who informed her that the psychiatrist had disclosed information to her (the victim) regarding Jane Doe’s treatment, emotional problems, sexual preferences, and medication regimen.
Susan Doe, age 64, learned of the sexual abuse accusations against the psychiatrist in the same way and also contacted the alleged victim. She told Susan Doe that the psychiatrist had disclosed to her Susan Doe’s personal information regarding her dificult relationship with her daughter, depression, and instances when she stormed out of counseling sessions.
The patients brought separate claims, and their cases were later consolidated. The psychiatrist denied that he told the alleged sexual abuse victim details of the 2 patients’ treatments. The patients claimed that the victim could not have known their personal details unless the psychiatrist had told her.
- A jury returned a verdict in favor of the 2 patients. Jane Doe was awarded $225,000, and Susan Doe was awarded $47,000.
Dr. Grant’s observations
In the case of Jane Doe and Susan Doe, disclosing a patient’s personal information to another patient violates confidentiality. Patients must consent to the disclosure of information to third parties, and in this case these 2 patients apparently did not provide consent.
Medical practice—and particularly psychiatric practice—is based on the principle that communications between clinicians and patients are private. The Hippocratic oath states, “Whatever I see or hear in the lives of my patients, whether in connection with my professional practice or not, which ought not to be spoken of outside, I will keep secret, as considering all such things to be private.”1
According to the American Psychiatric Association’s (APA) code of ethics, “Psychiatric records, including even the identification of a person as a patient, must be protected with extreme care. Confidentiality is essential to psychiatric treatment, in part because of the special nature of psychiatric therapy. A psychiatrist may release confidential information only with the patient’s authorization or under proper legal compulsion.”2
Doctor-patient confidentiality is rooted in the belief that potential disclosure of information communicated during psychiatric diagnosis and treatment would discourage patients from seeking medical and mental health care (Table)
Table
Underlying values of confidentiality
| Proper doctor-patient confidentiality aims to: |
|
| Source: U.S. Department of Health and Human Services. Mental Health: A Report of the Surgeon General—Executive Summary. Rockville, MD: U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, National Institutes of Health, National Institute of Mental Health, 1999. |
When to disclose
There are circumstances, however, that override the requirement to maintain confidentiality and do not need a patient’s consent. Examples include:3
Duty to protect third parties. In 1976 the California Supreme Court ruled in the landmark Tarasoff case4 that a psychiatrist has a duty to do what is reasonably necessary to protect third parties if a patient presents a serious risk of violence to another person. The specific applications of this principle are governed by other states’ laws, which have extended or limited this duty.5 Be familiar with the law in your jurisdiction before disclosing confidential information to third parties who may be at risk of violence.
The APA’s position on this exception is consistent with legal standards. Its code of ethics states, “When, in the clinical judgment of the treating psychiatrist, the risk of danger is deemed to be significant, the psychiatrist may reveal confidential information disclosed by the patient.”6
Emergency release of information. Psychiatrists can release confidential information during a medical emergency. Releasing the information must be in the patient’s best interests, and the patient’s inability to consent to the release should be the result of a potentially reversible condition that leads the clinician to question the patient’s capacity to consent.3
For example, if a patient in an emergency room is delirious because of ingesting an unknown substance and is unable to consent, a physician can call family members to ask about the patient’s medical problems. Notifying family that the patient is in the hospital could violate confidentiality, however.
Reporting abuse. All clinicians are obligated to report suspected child abuse or neglect. Some state laws also may require physicians to disclose abuse of vulnerable groups such as the elderly or the disabled and report to the local department of health diagnosis of communicable diseases such as HIV.3
Circle of confidentiality. Certain parties— including clinical staff on an inpatient unit or a psychiatrist supervising a resident— are considered to be within a circle of confidentiality.3 You do not need a patient’s consent to share clinical information with those within the circle of confidentiality. Do not release a patient’s information to parties who are not in the circle of confidentiality—such as family members, attorneys representing the patient, and law enforcement personnel—unless you’ve first obtained the patient’s consent.
Document the reasoning behind your decision to disclose your patient’s personal information without the patient’s consent. Show that you engaged in a reasonable clinical decision-making process.3 For example, record the risks and benefits of your decision and how you arrived at your conclusion.3
Other scenarios
Multidisciplinary teams. Members of a multidisciplinary treatment team—such as physicians, nurses, or social workers—should only receive confidential information that is relevant to the patient’s care. Other clinicians who are not involved in the case—although they may be seeing other patients on the same unit—should not have access to the patient’s confidential information. Discussions with these team members must be private so that others do not overhear confidential information.
Insurance companies generally are not party to the patient’s records unless the patient agrees to allow access by signing a release. If the patient’s refusal to allow disclosure results in the insurance company’s refusal to pay, then the patient is responsible for resolving the issue.7
Scientific publications and presentations. When you present a case report for a scientific publication or at a meeting, alter the patient’s biographical data so that someone who knows the patient would be unable to identify him or her based on the information in the case report. If the information is so specific that you cannot prevent patient identification, either do not publish the case or offer the patient the right to veto the manuscript’s distribution. If necessary, have the patient sign a consent form to allow publication or presentation of the case report.
Confidentiality violations
Breach of confidentiality may be intentional, such as disclosing a patient’s personal information to a third party as in this case, or unintentional, such as talking about a patient to a colleague and having someone overhear your discussion.8 Violating confidentiality may result in litigation for malpractice (negligence), invasion of privacy, or breach of contract, and ethical sanctions.8
No aspect of psychiatric practice seems to generate stronger emotions than the potential legal repercussions of our work. Keeping up with patients’ needs, billing issues, and advancements in medicine leaves little time for tracking changing state and federal laws or case precedents. For the past 4 years it has been my pleasure to provide information on the legal issues psychiatrists face and provide possible means of avoiding legal pitfalls.
Although I have decided to pursue other projects, I wish to give readers my thanks and to suggest resources—only a few among many great ones—that may be useful guides for a variety of legal issues.
Jon E. Grant, JD, MD, MPH
- Journal of the American Academy of Psychiatry and the Law.
- Appelbaum PS, Gutheil TG. Clinical handbook of psychiatry and the law. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA:Harvard University Press; 1998.
- Simon RI, Shuman DW.Clinical manual of psychiatry and the law. Washington, DC: American Psychiatric Publishing, Inc. ; 2007.
Editor’s note
Current Psychiatry thanks Dr. Grant for writing the Malpractice Verdicts column since 2004. The column will continue in a new format in the February 2008 issue.
Psychiatrist reveals patients’ information to another patient
Alameda County (CA) Superior Court
For several years 2 female patients were treated by the same psychiatrist. Jane Doe, age 56, read a breach of confidentiality report alleging sexual abuse filed by another patient of the psychiatrist. Jane Doe contacted the alleged victim, who informed her that the psychiatrist had disclosed information to her (the victim) regarding Jane Doe’s treatment, emotional problems, sexual preferences, and medication regimen.
Susan Doe, age 64, learned of the sexual abuse accusations against the psychiatrist in the same way and also contacted the alleged victim. She told Susan Doe that the psychiatrist had disclosed to her Susan Doe’s personal information regarding her dificult relationship with her daughter, depression, and instances when she stormed out of counseling sessions.
The patients brought separate claims, and their cases were later consolidated. The psychiatrist denied that he told the alleged sexual abuse victim details of the 2 patients’ treatments. The patients claimed that the victim could not have known their personal details unless the psychiatrist had told her.
- A jury returned a verdict in favor of the 2 patients. Jane Doe was awarded $225,000, and Susan Doe was awarded $47,000.
Dr. Grant’s observations
In the case of Jane Doe and Susan Doe, disclosing a patient’s personal information to another patient violates confidentiality. Patients must consent to the disclosure of information to third parties, and in this case these 2 patients apparently did not provide consent.
Medical practice—and particularly psychiatric practice—is based on the principle that communications between clinicians and patients are private. The Hippocratic oath states, “Whatever I see or hear in the lives of my patients, whether in connection with my professional practice or not, which ought not to be spoken of outside, I will keep secret, as considering all such things to be private.”1
According to the American Psychiatric Association’s (APA) code of ethics, “Psychiatric records, including even the identification of a person as a patient, must be protected with extreme care. Confidentiality is essential to psychiatric treatment, in part because of the special nature of psychiatric therapy. A psychiatrist may release confidential information only with the patient’s authorization or under proper legal compulsion.”2
Doctor-patient confidentiality is rooted in the belief that potential disclosure of information communicated during psychiatric diagnosis and treatment would discourage patients from seeking medical and mental health care (Table)
Table
Underlying values of confidentiality
| Proper doctor-patient confidentiality aims to: |
|
| Source: U.S. Department of Health and Human Services. Mental Health: A Report of the Surgeon General—Executive Summary. Rockville, MD: U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, National Institutes of Health, National Institute of Mental Health, 1999. |
When to disclose
There are circumstances, however, that override the requirement to maintain confidentiality and do not need a patient’s consent. Examples include:3
Duty to protect third parties. In 1976 the California Supreme Court ruled in the landmark Tarasoff case4 that a psychiatrist has a duty to do what is reasonably necessary to protect third parties if a patient presents a serious risk of violence to another person. The specific applications of this principle are governed by other states’ laws, which have extended or limited this duty.5 Be familiar with the law in your jurisdiction before disclosing confidential information to third parties who may be at risk of violence.
The APA’s position on this exception is consistent with legal standards. Its code of ethics states, “When, in the clinical judgment of the treating psychiatrist, the risk of danger is deemed to be significant, the psychiatrist may reveal confidential information disclosed by the patient.”6
Emergency release of information. Psychiatrists can release confidential information during a medical emergency. Releasing the information must be in the patient’s best interests, and the patient’s inability to consent to the release should be the result of a potentially reversible condition that leads the clinician to question the patient’s capacity to consent.3
For example, if a patient in an emergency room is delirious because of ingesting an unknown substance and is unable to consent, a physician can call family members to ask about the patient’s medical problems. Notifying family that the patient is in the hospital could violate confidentiality, however.
Reporting abuse. All clinicians are obligated to report suspected child abuse or neglect. Some state laws also may require physicians to disclose abuse of vulnerable groups such as the elderly or the disabled and report to the local department of health diagnosis of communicable diseases such as HIV.3
Circle of confidentiality. Certain parties— including clinical staff on an inpatient unit or a psychiatrist supervising a resident— are considered to be within a circle of confidentiality.3 You do not need a patient’s consent to share clinical information with those within the circle of confidentiality. Do not release a patient’s information to parties who are not in the circle of confidentiality—such as family members, attorneys representing the patient, and law enforcement personnel—unless you’ve first obtained the patient’s consent.
Document the reasoning behind your decision to disclose your patient’s personal information without the patient’s consent. Show that you engaged in a reasonable clinical decision-making process.3 For example, record the risks and benefits of your decision and how you arrived at your conclusion.3
Other scenarios
Multidisciplinary teams. Members of a multidisciplinary treatment team—such as physicians, nurses, or social workers—should only receive confidential information that is relevant to the patient’s care. Other clinicians who are not involved in the case—although they may be seeing other patients on the same unit—should not have access to the patient’s confidential information. Discussions with these team members must be private so that others do not overhear confidential information.
Insurance companies generally are not party to the patient’s records unless the patient agrees to allow access by signing a release. If the patient’s refusal to allow disclosure results in the insurance company’s refusal to pay, then the patient is responsible for resolving the issue.7
Scientific publications and presentations. When you present a case report for a scientific publication or at a meeting, alter the patient’s biographical data so that someone who knows the patient would be unable to identify him or her based on the information in the case report. If the information is so specific that you cannot prevent patient identification, either do not publish the case or offer the patient the right to veto the manuscript’s distribution. If necessary, have the patient sign a consent form to allow publication or presentation of the case report.
Confidentiality violations
Breach of confidentiality may be intentional, such as disclosing a patient’s personal information to a third party as in this case, or unintentional, such as talking about a patient to a colleague and having someone overhear your discussion.8 Violating confidentiality may result in litigation for malpractice (negligence), invasion of privacy, or breach of contract, and ethical sanctions.8
No aspect of psychiatric practice seems to generate stronger emotions than the potential legal repercussions of our work. Keeping up with patients’ needs, billing issues, and advancements in medicine leaves little time for tracking changing state and federal laws or case precedents. For the past 4 years it has been my pleasure to provide information on the legal issues psychiatrists face and provide possible means of avoiding legal pitfalls.
Although I have decided to pursue other projects, I wish to give readers my thanks and to suggest resources—only a few among many great ones—that may be useful guides for a variety of legal issues.
Jon E. Grant, JD, MD, MPH
- Journal of the American Academy of Psychiatry and the Law.
- Appelbaum PS, Gutheil TG. Clinical handbook of psychiatry and the law. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA:Harvard University Press; 1998.
- Simon RI, Shuman DW.Clinical manual of psychiatry and the law. Washington, DC: American Psychiatric Publishing, Inc. ; 2007.
Editor’s note
Current Psychiatry thanks Dr. Grant for writing the Malpractice Verdicts column since 2004. The column will continue in a new format in the February 2008 issue.
1. National Institutes of Health. The Hippocratic oath. Available at: http://www.nlm.nih.gov/hmd/greek/greek_oath.html. Accessed October 30, 2007.
2. Principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC: American Psychiatric Association; 2006: 6. Availableat: http://www.psych.org/psych_pract/ethics/ppaethics.pdf. Accessed October 30, 2007.
3. Lowenthal D. Case studies in confidentiality. J Psychiatr Prac 2002;8:151-9.
4. Tarasoff vs Regents of the University of California 551P 2d 334 (Cal 1976).
5. Appelbaum PS Taras off and the clinician: problems in fulfilling the duty to protect. Am J Psychiatry 1985;142:425-9.
6. Principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC: American Psychiatric Association; 2006:7. Availableat: http://www.psych.org/psych_pract/ethics/ppaethics.pdf. Accessed October 30, 2007.
7. Hilliard J. Liability issues with managed care. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA: Harvard University Press; 1998:44-51.
8. Berner M. Write smarter, not longer. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA: Harvard University Press; 1998:54-71.
Cases are selected by Current Psychiatry fromMedical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
1. National Institutes of Health. The Hippocratic oath. Available at: http://www.nlm.nih.gov/hmd/greek/greek_oath.html. Accessed October 30, 2007.
2. Principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC: American Psychiatric Association; 2006: 6. Availableat: http://www.psych.org/psych_pract/ethics/ppaethics.pdf. Accessed October 30, 2007.
3. Lowenthal D. Case studies in confidentiality. J Psychiatr Prac 2002;8:151-9.
4. Tarasoff vs Regents of the University of California 551P 2d 334 (Cal 1976).
5. Appelbaum PS Taras off and the clinician: problems in fulfilling the duty to protect. Am J Psychiatry 1985;142:425-9.
6. Principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC: American Psychiatric Association; 2006:7. Availableat: http://www.psych.org/psych_pract/ethics/ppaethics.pdf. Accessed October 30, 2007.
7. Hilliard J. Liability issues with managed care. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA: Harvard University Press; 1998:44-51.
8. Berner M. Write smarter, not longer. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA: Harvard University Press; 1998:54-71.
Cases are selected by Current Psychiatry fromMedical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Is schizophrenia a psychotic disorder?
Ask any mental health professional to give an example of a major psychotic disorder, and the most likely answer would be schizophrenia. But is schizophrenia really a psychotic disorder? And if not, then what is it, and how do you explain the psychotic symptoms associated with the disorder?
Research and clinical observation tell us that psychosis is a secondary feature of schizophrenia. This brain disease’s enduring and most disabling components are cognitive deficits and negative symptoms, both of which have been shown to precede the onset of psychotic symptoms. Because the core deficit is cognition—especially short-term memory and executive functions—individuals with schizophrenia are unable to return to the classroom or hold a job even when medications have suppressed their psychotic symptoms. Impaired social cognition can:
- masquerade as negative symptoms, such as poor social skills
- result in positive symptoms, such as ideas of reference or paranoid delusions, when the individual with schizophrenia misperceives ordinary social cues as “threats.”
Cognitive aberrations, including perceptual distortion, also contribute to delusions and hallucinations. Persons with schizophrenia are rarely identified as “ill” or hospitalized for acute psychiatric care until their behavior becomes bizarre with the appearance of psychotic symptoms. In fact, most practitioners do not apply the diagnostic label of schizophrenia until the individual manifests delusions, hallucinations, and bizarre behavior, and rarely—if ever—are cognitive functions assessed during initial evaluations (except in research settings). It may be that psychosis emerges as a consequence of cognitive deficits caused by adverse neurodevelopment and neurodegenerative and neurochemical changes.
Psychosis in medical disorders. Many genetically transmitted medical disorders can manifest with psychotic symptoms but are never labeled “psychotic disorders.” Examples include albinism, congenital adrenal hyperplasia, erythropoietic protoporphyria, Fabry’s disease, familial basal ganglia calcification, G6PD deficiency, Gaucher’s disease, hemochromatosis, Huntington’s chorea, hyperasparaginism, ichthyosis vulgaris, Kartagener’s syndrome, Klinefelter’s syndrome (karyotype 47,XXY), metachromatic leukodystrophy, Niemann-Pick disease, phenylketonuria, acute intermittent porphyria, Turner’s syndrome, and Wilson’s disease.
Medical disorders that include occasional psychotic symptoms may share neurobiologic features with schizophrenia and could provide clues about the neural pathways that generate delusions or hallucinations. But few of these disorders share schizophrenia’s core features of adverse neuroplastic changes and clusters of cognitive dysfunction and negative symptoms.
Targeting cognitive dysfunction in schizophrenia. Researchers are seeking ways to improve short-term memory and executive function in persons with schizophrenia, whose scores on these cognitive measures fall 1 to 3 standard deviations below the average of the general population. The National Institute of Mental Health, for example, has funded the MATRICS initiative (Measurement and Treatment Research to prove Cognition in Schizophrenia). Several candidate drugs that may serve as possible “cognitive enhancers” are being tested—for use in combination with antipsychotics—to help individuals with schizophrenia function better in social and employment settings.
Will negative symptoms—and even some positive symptoms—be ameliorated when cognition is improved? We’ll have to wait and see.
Perhaps DSM-V—planned to appear around 2012— should reconceptualize schizophrenia as a neurodevelopmental/neurodegenerative disorder characterized by a deficit syndrome and cognitive dysfunction, with intermittent secondary psychotic episodes. Or maybe we can go back to Kraepelin’s prescient nomenclature: dementia praecox!
Ask any mental health professional to give an example of a major psychotic disorder, and the most likely answer would be schizophrenia. But is schizophrenia really a psychotic disorder? And if not, then what is it, and how do you explain the psychotic symptoms associated with the disorder?
Research and clinical observation tell us that psychosis is a secondary feature of schizophrenia. This brain disease’s enduring and most disabling components are cognitive deficits and negative symptoms, both of which have been shown to precede the onset of psychotic symptoms. Because the core deficit is cognition—especially short-term memory and executive functions—individuals with schizophrenia are unable to return to the classroom or hold a job even when medications have suppressed their psychotic symptoms. Impaired social cognition can:
- masquerade as negative symptoms, such as poor social skills
- result in positive symptoms, such as ideas of reference or paranoid delusions, when the individual with schizophrenia misperceives ordinary social cues as “threats.”
Cognitive aberrations, including perceptual distortion, also contribute to delusions and hallucinations. Persons with schizophrenia are rarely identified as “ill” or hospitalized for acute psychiatric care until their behavior becomes bizarre with the appearance of psychotic symptoms. In fact, most practitioners do not apply the diagnostic label of schizophrenia until the individual manifests delusions, hallucinations, and bizarre behavior, and rarely—if ever—are cognitive functions assessed during initial evaluations (except in research settings). It may be that psychosis emerges as a consequence of cognitive deficits caused by adverse neurodevelopment and neurodegenerative and neurochemical changes.
Psychosis in medical disorders. Many genetically transmitted medical disorders can manifest with psychotic symptoms but are never labeled “psychotic disorders.” Examples include albinism, congenital adrenal hyperplasia, erythropoietic protoporphyria, Fabry’s disease, familial basal ganglia calcification, G6PD deficiency, Gaucher’s disease, hemochromatosis, Huntington’s chorea, hyperasparaginism, ichthyosis vulgaris, Kartagener’s syndrome, Klinefelter’s syndrome (karyotype 47,XXY), metachromatic leukodystrophy, Niemann-Pick disease, phenylketonuria, acute intermittent porphyria, Turner’s syndrome, and Wilson’s disease.
Medical disorders that include occasional psychotic symptoms may share neurobiologic features with schizophrenia and could provide clues about the neural pathways that generate delusions or hallucinations. But few of these disorders share schizophrenia’s core features of adverse neuroplastic changes and clusters of cognitive dysfunction and negative symptoms.
Targeting cognitive dysfunction in schizophrenia. Researchers are seeking ways to improve short-term memory and executive function in persons with schizophrenia, whose scores on these cognitive measures fall 1 to 3 standard deviations below the average of the general population. The National Institute of Mental Health, for example, has funded the MATRICS initiative (Measurement and Treatment Research to prove Cognition in Schizophrenia). Several candidate drugs that may serve as possible “cognitive enhancers” are being tested—for use in combination with antipsychotics—to help individuals with schizophrenia function better in social and employment settings.
Will negative symptoms—and even some positive symptoms—be ameliorated when cognition is improved? We’ll have to wait and see.
Perhaps DSM-V—planned to appear around 2012— should reconceptualize schizophrenia as a neurodevelopmental/neurodegenerative disorder characterized by a deficit syndrome and cognitive dysfunction, with intermittent secondary psychotic episodes. Or maybe we can go back to Kraepelin’s prescient nomenclature: dementia praecox!
Ask any mental health professional to give an example of a major psychotic disorder, and the most likely answer would be schizophrenia. But is schizophrenia really a psychotic disorder? And if not, then what is it, and how do you explain the psychotic symptoms associated with the disorder?
Research and clinical observation tell us that psychosis is a secondary feature of schizophrenia. This brain disease’s enduring and most disabling components are cognitive deficits and negative symptoms, both of which have been shown to precede the onset of psychotic symptoms. Because the core deficit is cognition—especially short-term memory and executive functions—individuals with schizophrenia are unable to return to the classroom or hold a job even when medications have suppressed their psychotic symptoms. Impaired social cognition can:
- masquerade as negative symptoms, such as poor social skills
- result in positive symptoms, such as ideas of reference or paranoid delusions, when the individual with schizophrenia misperceives ordinary social cues as “threats.”
Cognitive aberrations, including perceptual distortion, also contribute to delusions and hallucinations. Persons with schizophrenia are rarely identified as “ill” or hospitalized for acute psychiatric care until their behavior becomes bizarre with the appearance of psychotic symptoms. In fact, most practitioners do not apply the diagnostic label of schizophrenia until the individual manifests delusions, hallucinations, and bizarre behavior, and rarely—if ever—are cognitive functions assessed during initial evaluations (except in research settings). It may be that psychosis emerges as a consequence of cognitive deficits caused by adverse neurodevelopment and neurodegenerative and neurochemical changes.
Psychosis in medical disorders. Many genetically transmitted medical disorders can manifest with psychotic symptoms but are never labeled “psychotic disorders.” Examples include albinism, congenital adrenal hyperplasia, erythropoietic protoporphyria, Fabry’s disease, familial basal ganglia calcification, G6PD deficiency, Gaucher’s disease, hemochromatosis, Huntington’s chorea, hyperasparaginism, ichthyosis vulgaris, Kartagener’s syndrome, Klinefelter’s syndrome (karyotype 47,XXY), metachromatic leukodystrophy, Niemann-Pick disease, phenylketonuria, acute intermittent porphyria, Turner’s syndrome, and Wilson’s disease.
Medical disorders that include occasional psychotic symptoms may share neurobiologic features with schizophrenia and could provide clues about the neural pathways that generate delusions or hallucinations. But few of these disorders share schizophrenia’s core features of adverse neuroplastic changes and clusters of cognitive dysfunction and negative symptoms.
Targeting cognitive dysfunction in schizophrenia. Researchers are seeking ways to improve short-term memory and executive function in persons with schizophrenia, whose scores on these cognitive measures fall 1 to 3 standard deviations below the average of the general population. The National Institute of Mental Health, for example, has funded the MATRICS initiative (Measurement and Treatment Research to prove Cognition in Schizophrenia). Several candidate drugs that may serve as possible “cognitive enhancers” are being tested—for use in combination with antipsychotics—to help individuals with schizophrenia function better in social and employment settings.
Will negative symptoms—and even some positive symptoms—be ameliorated when cognition is improved? We’ll have to wait and see.
Perhaps DSM-V—planned to appear around 2012— should reconceptualize schizophrenia as a neurodevelopmental/neurodegenerative disorder characterized by a deficit syndrome and cognitive dysfunction, with intermittent secondary psychotic episodes. Or maybe we can go back to Kraepelin’s prescient nomenclature: dementia praecox!
Is she being abused or ‘acting out’?
HISTORY: ‘Unusual behavior’
Ms. L, age 44, has severe cerebral palsy and has used a wheelchair since childhood. Her mother, who had been her primary caretaker, died 12 years ago, and her stepsister has been caring for her since.
Ms. L’s primary care physician reports that the patient has been “acting out” lately and asks us to evaluate her “unusual behavior.” Six months ago, the physician prescribed escitalopram, 30 mg/d, to treat depressive symptoms stemming from her chronic neurologic disorder.
We interview Ms. L and her stepsister together. The patient says she has been depressed, irritable, and moody, and her stepsister confirms this. The patient shows no signs of distress during the interview, and her answers appear short and guarded.
The stepsister says she typically spends her day turning Ms. L to prevent bedsores, feeding and bathing her, replacing her urinary catheter and emptying her urinary bag, and helping her to the bathroom. At day’s end, the stepsister has little time to spend with her husband or for other activities. She says at times she resents tending to Ms. L’s constant needs and feels “stressed out.”
We diagnose Ms. L with a mood disorder caused by a general medical condition. We continue escitalopram, 30 mg/d, and add oxcarbazepine, 150 mg bid, to treat her irritability and lability.
FOLLOW-UP: ‘She’s abusing me’
At Ms. L’s follow-up visit 2 weeks later, we ask her stepsister to leave the examination room and interview the patient alone to gauge her emotional condition and insight.
Seconds later, Ms. L starts crying hysterically, then reports that for 12 years her stepsister has been beating her, usually after she requests something. Yesterday, she says, her stepsister started punching her after she asked to be taken to the park.
Ms. L says the abuse is escalating and now occurs daily. She says she is covered with bruises from the last beating, although no bruises are visible at first glance. Afraid to go home with her stepsister, she pleads for help.
- call the primary care physician for collateral information
- examine Ms. L for bruises
- get the stepsister’s side of the story
- contact state protective services
- all of the above
The authors’ observations
Is Ms. L being physically abused, or is a psychiatric condition driving her to fabricate these allegations?
We saw nothing suspicious during the first interview with the stepsister, although she acknowledged difficulty coping with Ms. L’s constant requests (Box 1).1 Caring for a severely disabled person day in and day out can be trying for both the caregiver and her family, and the stepsister could be taking her frustrations out on Ms. L.
Until proven otherwise, we must assume Ms. L is being harmed and seek more information. We also must watch for signs of a delusional or factitious disorder or malingering—any of which would suggest the allegations are false.
Is often a family member
Experiences stress brought on by the strain of caregiving coupled with marital problems, lack of money, overcrowded living conditions, or lack of needed health or social services
Often abuses alcohol and/or drugs
Might have emotional problems:
- Caregiver often resents patient’s dependency
- If patient is caregiver’s parent, caregiver might be retaliating for past mistreatment
Depends on vulnerable adult for basic needs such as money or housing
Might come from a family where abusive behavior is normal
Source: Reference 1
HISTORY: A second opinion
We ask Ms. L if we can discuss the allegations with her and her stepsister, but she fears retaliation and insists that we not speak to the caretaker.
We then call Ms. L’s primary care physician, who has been managing her care for several years. He says the patient has begged him numerous times for protection from her stepsister, but adds he has found no evidence of abuse. He notes that he has witnessed tension between the 2 women during office visits and cannot dismiss the possibility of abuse.
The attending psychiatrist performs a brief physical exam with the resident looking on but finds no bruises, excoriations, or unusual scarring on her arms and legs. Because our outpatient clinic lacks an examination room, we do not perform a whole-body exam.
We then notify state protective services. There, an agent tells us that in the past year, Ms. L has made 4 allegations of caretaker abuse, none of which were substantiated after extensive investigation. The agent says her office will assign a case worker but considers the case a low priority.
When we inform Ms. L of our findings, she frantically insists that her caretaker is beating her once a week and that the abuse has gone undetected. We become skeptical, recalling that Ms. L earlier said the beatings were daily.
Ms. L says she is afraid to go home and wonders where she can stay. Having no friends or other family members nearby, she requests hospitalization.
At this point, I would:
- discharge Ms. L to a safe house with close follow-up
- hospitalize her for safety and diagnostic clarification
- discharge her to her stepsister with close follow-up
The authors’ observations
Ms. L’s allegations pose a medical, ethical, and legal challenge. Physical examination and input from a protective services officer suggest Ms. L is fabricating the allegations. On the other hand, if the accusations are true, sending Ms. L home with her stepsister would endanger her.
We could hospitalize the patient and substantiate the allegations later, but we cannot justify taxing limited hospital resources when the need is questionable. We cannot send her to a safe house because of her severe physical disability, nor can we discuss the allegations with her stepsister because Ms. L instructed us not to.
- Caregiver.com (online magazine)
www.caregiver.com - Caring Today
www.caringtoday.com - National Alliance for Caregiving
www.caregiving.org - National Alliance on Mental Illness
www.nami.org (Click on “Find Support,” then “Education, Training, and Peer Support Programs”)
DISPOSITION: Going home
After meeting with hospital officials and clinic staff, we decide that Ms. L does not meet admission criteria. We discharge her to her stepsister and see the patient again the next day.
The authors’ observations
Legal duty. Our legal duty to protect a suspected abuse victim depends on the jurisdiction in which treatment is delivered.
Many states do not require physicians to report suspected abuse, but this complicates the decision process. If the suspicion is correct, not reporting it might constitute malpractice or negligence and could provoke future lawsuits or complaints to the state medical board. Worse, the abuse may escalate and cause irreparable harm to the patient. Conversely, reporting unfounded suspicions of abuse can destroy the doctor-patient relationship, prompt the caregiver to retaliate against the patient, or inspire patients or caregivers to sue the physician.
If you suspect patient abuse and your state mandates reporting, contact the state protective services agency at once (seeRelated Resources,). Base your report on a thorough history and physical, psychiatric evaluation, and—when available—collateral information.
If your state does not mandate reporting, obtain the patient’s consent to file a complaint with state protective services. By providing informed consent, the patient gives permission to disclose protected health information, and confidentiality is not breached.
Be careful when obtaining informed consent, especially when the patient is ambivalent about reporting because of:
- fear of retaliation from the abuser
- fear of the social stigma associated with abuse
- or the patient’s false belief that she deserves the abuse.
Ethical responsibility. Even if our legal responsibility is minimal, we should go further to do what is best for the patient.
Texas, for example, does not require physicians to hospitalize or find a safe environment for a suspected abuse victim.2 But if you see evidence of abuse, notify authorities and offer the patient information about local safe houses, support groups, and social services—even if not mandated by law. If resources are available, consider hospitalizing the patient and work with his or her social worker, therapist, or clergy to orchestrate outpatient services.
Whether or not abuse has occurred, empathizing with the caretaker about the difficulty of caring for the patient could diminish the caretaker’s stress and reduce the risk of abuse.
FOLLOW-UP: Truth or delusion?
At her appointment the next day, Ms. L says things are fine at home and does not bring up the abuse allegations. We then see her every 3 days for 2 weeks, weekly for 4 weeks, and every 3 weeks thereafter as the apparent risk of abuse diminishes. At each visit, she says her caretaker is not beating her but occasionally complains that she is verbally abusive.
Three weeks after her first follow-up, Ms. L enters the examination room agitated and frightened; she says another patient in the waiting room has just tried to strangle her for no apparent reason. Upon questioning, office staff say they saw no attack and note that the accused patient is a feeble woman with no history of violence; we doubt she assaulted Ms. L.
Ms. L suffers from:
- repeated physical abuse
- delusional disorder
- factitious disorder
- malingering
The authors’ observations
Although Ms. L clearly was not assaulted in the waiting room, this complaint is key to understanding her case. Although whether she is being abused at home remains unclear, evidence increasingly suggests that she suffers from delusions.
Delusions are beliefs that are fixed, false, and not ordinarily accepted by others in a patient’s culture or subculture.3 Delusional disorder is characterized by nonbizarre delusions lasting >1 month (>3 months according to ICD-10 criteria)4 with relatively preserved functioning and without prominent hallucinations. DSM-IV-TR defines bizarre delusions as “clearly implausible, not understandable, and not derived from ordinary life experience.”4,5
Ms. L most likely has a paranoid or persecutory type delusional disorder in which she is convinced she is being harmed. Her delusional thoughts might yield mood symptoms such as anger and irritability, and she might become assaultive. Often, such patients are extraordinarily determined to succeed against “the conspirators” and frequently appeal to the legal system or law enforcement.3
Differentiating between a patient’s delusions and reality can be difficult, leading clinicians to seek collateral information from family, past medical records, or providers to establish a diagnosis. The delusions might become less circumscribed over time, or additional information might clear the clinical picture.
Ms. L’s psychological makeup might help us rule out other diagnoses. Her request for hospitalization, for example, could suggest factitious illness, but she is disabled enough to play the sick role without manufacturing symptoms. Also, she seeks hospitalization because she has no family or friends to turn to. We rule out malingering because Ms. L has nothing to gain by accusing a stranger of choking her in the waiting room.
Treating delusional disorder
Pharmacotherapy and psychotherapy typically are used together to treat delusional disorder.
Pharmacotherapy. Antipsychotics such as olanzapine, 5 to 10 mg nightly, or risperidone, 1 to 2 mg nightly, can decrease the delusional thoughts’ intensity and frequency, allowing patients to function more appropriately.3 If 2 or more antipsychotic trials do not control delusional thoughts, consider starting clozapine at 300 mg/d and titrating to 900 mg/d.
Add an antidepressant if delusional thinking causes depression or anxiety. Selective serotonin reuptake inhibitors (SSRIs) such as paroxetine, 10 to 20 mg/d, or fluoxetine, 20 to 40 mg/d, are a good starting point. Consider other antidepressant types if SSRIs do not work.
Adjunctive benzodiazepines such as clonazepam, 1 to 2 mg/d, or lorazepam, 1 to 2 mg bid as needed, can help manage acute anxiety or agitation stemming from delusions.
Once rapport is established, consider challenging delusional beliefs by having the patient list evidence supporting or refuting the delusions. Be careful not to confront delusional thinking too quickly or aggressively, as this approach often does not change the patient’s beliefs and weakens the therapeutic alliance.3
TREATMENT: Fewer complaints
We still see Ms. L every 3 weeks for supportive psychotherapy and medication management. We continue oxcarbazepine, 150 mg bid, and escitalopram, 30 mg/d, and add risperidone, 1 mg at bedtime, to target her delusional thinking, lability, and irritability.
Over 6 months, Ms. L’s complaints of abuse become less emphatic. She endorses the abuse less frequently—every 3 to 4 visits—and only if the clinician specifically asks about it. Most often, she denies abuse is occurring but says it happened previously. At each visit, we document her statements and explain in her chart why we have not notified adult protective services or police.
- National Adult Protective Services Association. Links to adult protection agencies nationwide. www.apsnetwork.org.
- National Center on Elder Abuse. www.elderabusecenter.org.
Drug brand names
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Fairfax County, VA (June 15, 2006). Adult Protection Services. Available at: http://www.fairfaxcounty.gov/aaa/ombud/abuse.htm. Accessed October 26, 2007.
2. Texas medical jurisprudence manual, 15th ed. Austin, TX: Texas Medical Association; 2004;454–6.
3. Fennig S, Fochtman L, Bromet E. Chapter 12.16c Delusional disorder and shared psychotic disorder. In: Sadock B, Sadock V, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry, 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2005;1525–32.
4. International Classification of Diseases, 10th rev. Geneva, Switzerland: World Health Organization; 1992.
5. Diagnostic and statistical of mental disorders, 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
HISTORY: ‘Unusual behavior’
Ms. L, age 44, has severe cerebral palsy and has used a wheelchair since childhood. Her mother, who had been her primary caretaker, died 12 years ago, and her stepsister has been caring for her since.
Ms. L’s primary care physician reports that the patient has been “acting out” lately and asks us to evaluate her “unusual behavior.” Six months ago, the physician prescribed escitalopram, 30 mg/d, to treat depressive symptoms stemming from her chronic neurologic disorder.
We interview Ms. L and her stepsister together. The patient says she has been depressed, irritable, and moody, and her stepsister confirms this. The patient shows no signs of distress during the interview, and her answers appear short and guarded.
The stepsister says she typically spends her day turning Ms. L to prevent bedsores, feeding and bathing her, replacing her urinary catheter and emptying her urinary bag, and helping her to the bathroom. At day’s end, the stepsister has little time to spend with her husband or for other activities. She says at times she resents tending to Ms. L’s constant needs and feels “stressed out.”
We diagnose Ms. L with a mood disorder caused by a general medical condition. We continue escitalopram, 30 mg/d, and add oxcarbazepine, 150 mg bid, to treat her irritability and lability.
FOLLOW-UP: ‘She’s abusing me’
At Ms. L’s follow-up visit 2 weeks later, we ask her stepsister to leave the examination room and interview the patient alone to gauge her emotional condition and insight.
Seconds later, Ms. L starts crying hysterically, then reports that for 12 years her stepsister has been beating her, usually after she requests something. Yesterday, she says, her stepsister started punching her after she asked to be taken to the park.
Ms. L says the abuse is escalating and now occurs daily. She says she is covered with bruises from the last beating, although no bruises are visible at first glance. Afraid to go home with her stepsister, she pleads for help.
- call the primary care physician for collateral information
- examine Ms. L for bruises
- get the stepsister’s side of the story
- contact state protective services
- all of the above
The authors’ observations
Is Ms. L being physically abused, or is a psychiatric condition driving her to fabricate these allegations?
We saw nothing suspicious during the first interview with the stepsister, although she acknowledged difficulty coping with Ms. L’s constant requests (Box 1).1 Caring for a severely disabled person day in and day out can be trying for both the caregiver and her family, and the stepsister could be taking her frustrations out on Ms. L.
Until proven otherwise, we must assume Ms. L is being harmed and seek more information. We also must watch for signs of a delusional or factitious disorder or malingering—any of which would suggest the allegations are false.
Is often a family member
Experiences stress brought on by the strain of caregiving coupled with marital problems, lack of money, overcrowded living conditions, or lack of needed health or social services
Often abuses alcohol and/or drugs
Might have emotional problems:
- Caregiver often resents patient’s dependency
- If patient is caregiver’s parent, caregiver might be retaliating for past mistreatment
Depends on vulnerable adult for basic needs such as money or housing
Might come from a family where abusive behavior is normal
Source: Reference 1
HISTORY: A second opinion
We ask Ms. L if we can discuss the allegations with her and her stepsister, but she fears retaliation and insists that we not speak to the caretaker.
We then call Ms. L’s primary care physician, who has been managing her care for several years. He says the patient has begged him numerous times for protection from her stepsister, but adds he has found no evidence of abuse. He notes that he has witnessed tension between the 2 women during office visits and cannot dismiss the possibility of abuse.
The attending psychiatrist performs a brief physical exam with the resident looking on but finds no bruises, excoriations, or unusual scarring on her arms and legs. Because our outpatient clinic lacks an examination room, we do not perform a whole-body exam.
We then notify state protective services. There, an agent tells us that in the past year, Ms. L has made 4 allegations of caretaker abuse, none of which were substantiated after extensive investigation. The agent says her office will assign a case worker but considers the case a low priority.
When we inform Ms. L of our findings, she frantically insists that her caretaker is beating her once a week and that the abuse has gone undetected. We become skeptical, recalling that Ms. L earlier said the beatings were daily.
Ms. L says she is afraid to go home and wonders where she can stay. Having no friends or other family members nearby, she requests hospitalization.
At this point, I would:
- discharge Ms. L to a safe house with close follow-up
- hospitalize her for safety and diagnostic clarification
- discharge her to her stepsister with close follow-up
The authors’ observations
Ms. L’s allegations pose a medical, ethical, and legal challenge. Physical examination and input from a protective services officer suggest Ms. L is fabricating the allegations. On the other hand, if the accusations are true, sending Ms. L home with her stepsister would endanger her.
We could hospitalize the patient and substantiate the allegations later, but we cannot justify taxing limited hospital resources when the need is questionable. We cannot send her to a safe house because of her severe physical disability, nor can we discuss the allegations with her stepsister because Ms. L instructed us not to.
- Caregiver.com (online magazine)
www.caregiver.com - Caring Today
www.caringtoday.com - National Alliance for Caregiving
www.caregiving.org - National Alliance on Mental Illness
www.nami.org (Click on “Find Support,” then “Education, Training, and Peer Support Programs”)
DISPOSITION: Going home
After meeting with hospital officials and clinic staff, we decide that Ms. L does not meet admission criteria. We discharge her to her stepsister and see the patient again the next day.
The authors’ observations
Legal duty. Our legal duty to protect a suspected abuse victim depends on the jurisdiction in which treatment is delivered.
Many states do not require physicians to report suspected abuse, but this complicates the decision process. If the suspicion is correct, not reporting it might constitute malpractice or negligence and could provoke future lawsuits or complaints to the state medical board. Worse, the abuse may escalate and cause irreparable harm to the patient. Conversely, reporting unfounded suspicions of abuse can destroy the doctor-patient relationship, prompt the caregiver to retaliate against the patient, or inspire patients or caregivers to sue the physician.
If you suspect patient abuse and your state mandates reporting, contact the state protective services agency at once (seeRelated Resources,). Base your report on a thorough history and physical, psychiatric evaluation, and—when available—collateral information.
If your state does not mandate reporting, obtain the patient’s consent to file a complaint with state protective services. By providing informed consent, the patient gives permission to disclose protected health information, and confidentiality is not breached.
Be careful when obtaining informed consent, especially when the patient is ambivalent about reporting because of:
- fear of retaliation from the abuser
- fear of the social stigma associated with abuse
- or the patient’s false belief that she deserves the abuse.
Ethical responsibility. Even if our legal responsibility is minimal, we should go further to do what is best for the patient.
Texas, for example, does not require physicians to hospitalize or find a safe environment for a suspected abuse victim.2 But if you see evidence of abuse, notify authorities and offer the patient information about local safe houses, support groups, and social services—even if not mandated by law. If resources are available, consider hospitalizing the patient and work with his or her social worker, therapist, or clergy to orchestrate outpatient services.
Whether or not abuse has occurred, empathizing with the caretaker about the difficulty of caring for the patient could diminish the caretaker’s stress and reduce the risk of abuse.
FOLLOW-UP: Truth or delusion?
At her appointment the next day, Ms. L says things are fine at home and does not bring up the abuse allegations. We then see her every 3 days for 2 weeks, weekly for 4 weeks, and every 3 weeks thereafter as the apparent risk of abuse diminishes. At each visit, she says her caretaker is not beating her but occasionally complains that she is verbally abusive.
Three weeks after her first follow-up, Ms. L enters the examination room agitated and frightened; she says another patient in the waiting room has just tried to strangle her for no apparent reason. Upon questioning, office staff say they saw no attack and note that the accused patient is a feeble woman with no history of violence; we doubt she assaulted Ms. L.
Ms. L suffers from:
- repeated physical abuse
- delusional disorder
- factitious disorder
- malingering
The authors’ observations
Although Ms. L clearly was not assaulted in the waiting room, this complaint is key to understanding her case. Although whether she is being abused at home remains unclear, evidence increasingly suggests that she suffers from delusions.
Delusions are beliefs that are fixed, false, and not ordinarily accepted by others in a patient’s culture or subculture.3 Delusional disorder is characterized by nonbizarre delusions lasting >1 month (>3 months according to ICD-10 criteria)4 with relatively preserved functioning and without prominent hallucinations. DSM-IV-TR defines bizarre delusions as “clearly implausible, not understandable, and not derived from ordinary life experience.”4,5
Ms. L most likely has a paranoid or persecutory type delusional disorder in which she is convinced she is being harmed. Her delusional thoughts might yield mood symptoms such as anger and irritability, and she might become assaultive. Often, such patients are extraordinarily determined to succeed against “the conspirators” and frequently appeal to the legal system or law enforcement.3
Differentiating between a patient’s delusions and reality can be difficult, leading clinicians to seek collateral information from family, past medical records, or providers to establish a diagnosis. The delusions might become less circumscribed over time, or additional information might clear the clinical picture.
Ms. L’s psychological makeup might help us rule out other diagnoses. Her request for hospitalization, for example, could suggest factitious illness, but she is disabled enough to play the sick role without manufacturing symptoms. Also, she seeks hospitalization because she has no family or friends to turn to. We rule out malingering because Ms. L has nothing to gain by accusing a stranger of choking her in the waiting room.
Treating delusional disorder
Pharmacotherapy and psychotherapy typically are used together to treat delusional disorder.
Pharmacotherapy. Antipsychotics such as olanzapine, 5 to 10 mg nightly, or risperidone, 1 to 2 mg nightly, can decrease the delusional thoughts’ intensity and frequency, allowing patients to function more appropriately.3 If 2 or more antipsychotic trials do not control delusional thoughts, consider starting clozapine at 300 mg/d and titrating to 900 mg/d.
Add an antidepressant if delusional thinking causes depression or anxiety. Selective serotonin reuptake inhibitors (SSRIs) such as paroxetine, 10 to 20 mg/d, or fluoxetine, 20 to 40 mg/d, are a good starting point. Consider other antidepressant types if SSRIs do not work.
Adjunctive benzodiazepines such as clonazepam, 1 to 2 mg/d, or lorazepam, 1 to 2 mg bid as needed, can help manage acute anxiety or agitation stemming from delusions.
Once rapport is established, consider challenging delusional beliefs by having the patient list evidence supporting or refuting the delusions. Be careful not to confront delusional thinking too quickly or aggressively, as this approach often does not change the patient’s beliefs and weakens the therapeutic alliance.3
TREATMENT: Fewer complaints
We still see Ms. L every 3 weeks for supportive psychotherapy and medication management. We continue oxcarbazepine, 150 mg bid, and escitalopram, 30 mg/d, and add risperidone, 1 mg at bedtime, to target her delusional thinking, lability, and irritability.
Over 6 months, Ms. L’s complaints of abuse become less emphatic. She endorses the abuse less frequently—every 3 to 4 visits—and only if the clinician specifically asks about it. Most often, she denies abuse is occurring but says it happened previously. At each visit, we document her statements and explain in her chart why we have not notified adult protective services or police.
- National Adult Protective Services Association. Links to adult protection agencies nationwide. www.apsnetwork.org.
- National Center on Elder Abuse. www.elderabusecenter.org.
Drug brand names
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
HISTORY: ‘Unusual behavior’
Ms. L, age 44, has severe cerebral palsy and has used a wheelchair since childhood. Her mother, who had been her primary caretaker, died 12 years ago, and her stepsister has been caring for her since.
Ms. L’s primary care physician reports that the patient has been “acting out” lately and asks us to evaluate her “unusual behavior.” Six months ago, the physician prescribed escitalopram, 30 mg/d, to treat depressive symptoms stemming from her chronic neurologic disorder.
We interview Ms. L and her stepsister together. The patient says she has been depressed, irritable, and moody, and her stepsister confirms this. The patient shows no signs of distress during the interview, and her answers appear short and guarded.
The stepsister says she typically spends her day turning Ms. L to prevent bedsores, feeding and bathing her, replacing her urinary catheter and emptying her urinary bag, and helping her to the bathroom. At day’s end, the stepsister has little time to spend with her husband or for other activities. She says at times she resents tending to Ms. L’s constant needs and feels “stressed out.”
We diagnose Ms. L with a mood disorder caused by a general medical condition. We continue escitalopram, 30 mg/d, and add oxcarbazepine, 150 mg bid, to treat her irritability and lability.
FOLLOW-UP: ‘She’s abusing me’
At Ms. L’s follow-up visit 2 weeks later, we ask her stepsister to leave the examination room and interview the patient alone to gauge her emotional condition and insight.
Seconds later, Ms. L starts crying hysterically, then reports that for 12 years her stepsister has been beating her, usually after she requests something. Yesterday, she says, her stepsister started punching her after she asked to be taken to the park.
Ms. L says the abuse is escalating and now occurs daily. She says she is covered with bruises from the last beating, although no bruises are visible at first glance. Afraid to go home with her stepsister, she pleads for help.
- call the primary care physician for collateral information
- examine Ms. L for bruises
- get the stepsister’s side of the story
- contact state protective services
- all of the above
The authors’ observations
Is Ms. L being physically abused, or is a psychiatric condition driving her to fabricate these allegations?
We saw nothing suspicious during the first interview with the stepsister, although she acknowledged difficulty coping with Ms. L’s constant requests (Box 1).1 Caring for a severely disabled person day in and day out can be trying for both the caregiver and her family, and the stepsister could be taking her frustrations out on Ms. L.
Until proven otherwise, we must assume Ms. L is being harmed and seek more information. We also must watch for signs of a delusional or factitious disorder or malingering—any of which would suggest the allegations are false.
Is often a family member
Experiences stress brought on by the strain of caregiving coupled with marital problems, lack of money, overcrowded living conditions, or lack of needed health or social services
Often abuses alcohol and/or drugs
Might have emotional problems:
- Caregiver often resents patient’s dependency
- If patient is caregiver’s parent, caregiver might be retaliating for past mistreatment
Depends on vulnerable adult for basic needs such as money or housing
Might come from a family where abusive behavior is normal
Source: Reference 1
HISTORY: A second opinion
We ask Ms. L if we can discuss the allegations with her and her stepsister, but she fears retaliation and insists that we not speak to the caretaker.
We then call Ms. L’s primary care physician, who has been managing her care for several years. He says the patient has begged him numerous times for protection from her stepsister, but adds he has found no evidence of abuse. He notes that he has witnessed tension between the 2 women during office visits and cannot dismiss the possibility of abuse.
The attending psychiatrist performs a brief physical exam with the resident looking on but finds no bruises, excoriations, or unusual scarring on her arms and legs. Because our outpatient clinic lacks an examination room, we do not perform a whole-body exam.
We then notify state protective services. There, an agent tells us that in the past year, Ms. L has made 4 allegations of caretaker abuse, none of which were substantiated after extensive investigation. The agent says her office will assign a case worker but considers the case a low priority.
When we inform Ms. L of our findings, she frantically insists that her caretaker is beating her once a week and that the abuse has gone undetected. We become skeptical, recalling that Ms. L earlier said the beatings were daily.
Ms. L says she is afraid to go home and wonders where she can stay. Having no friends or other family members nearby, she requests hospitalization.
At this point, I would:
- discharge Ms. L to a safe house with close follow-up
- hospitalize her for safety and diagnostic clarification
- discharge her to her stepsister with close follow-up
The authors’ observations
Ms. L’s allegations pose a medical, ethical, and legal challenge. Physical examination and input from a protective services officer suggest Ms. L is fabricating the allegations. On the other hand, if the accusations are true, sending Ms. L home with her stepsister would endanger her.
We could hospitalize the patient and substantiate the allegations later, but we cannot justify taxing limited hospital resources when the need is questionable. We cannot send her to a safe house because of her severe physical disability, nor can we discuss the allegations with her stepsister because Ms. L instructed us not to.
- Caregiver.com (online magazine)
www.caregiver.com - Caring Today
www.caringtoday.com - National Alliance for Caregiving
www.caregiving.org - National Alliance on Mental Illness
www.nami.org (Click on “Find Support,” then “Education, Training, and Peer Support Programs”)
DISPOSITION: Going home
After meeting with hospital officials and clinic staff, we decide that Ms. L does not meet admission criteria. We discharge her to her stepsister and see the patient again the next day.
The authors’ observations
Legal duty. Our legal duty to protect a suspected abuse victim depends on the jurisdiction in which treatment is delivered.
Many states do not require physicians to report suspected abuse, but this complicates the decision process. If the suspicion is correct, not reporting it might constitute malpractice or negligence and could provoke future lawsuits or complaints to the state medical board. Worse, the abuse may escalate and cause irreparable harm to the patient. Conversely, reporting unfounded suspicions of abuse can destroy the doctor-patient relationship, prompt the caregiver to retaliate against the patient, or inspire patients or caregivers to sue the physician.
If you suspect patient abuse and your state mandates reporting, contact the state protective services agency at once (seeRelated Resources,). Base your report on a thorough history and physical, psychiatric evaluation, and—when available—collateral information.
If your state does not mandate reporting, obtain the patient’s consent to file a complaint with state protective services. By providing informed consent, the patient gives permission to disclose protected health information, and confidentiality is not breached.
Be careful when obtaining informed consent, especially when the patient is ambivalent about reporting because of:
- fear of retaliation from the abuser
- fear of the social stigma associated with abuse
- or the patient’s false belief that she deserves the abuse.
Ethical responsibility. Even if our legal responsibility is minimal, we should go further to do what is best for the patient.
Texas, for example, does not require physicians to hospitalize or find a safe environment for a suspected abuse victim.2 But if you see evidence of abuse, notify authorities and offer the patient information about local safe houses, support groups, and social services—even if not mandated by law. If resources are available, consider hospitalizing the patient and work with his or her social worker, therapist, or clergy to orchestrate outpatient services.
Whether or not abuse has occurred, empathizing with the caretaker about the difficulty of caring for the patient could diminish the caretaker’s stress and reduce the risk of abuse.
FOLLOW-UP: Truth or delusion?
At her appointment the next day, Ms. L says things are fine at home and does not bring up the abuse allegations. We then see her every 3 days for 2 weeks, weekly for 4 weeks, and every 3 weeks thereafter as the apparent risk of abuse diminishes. At each visit, she says her caretaker is not beating her but occasionally complains that she is verbally abusive.
Three weeks after her first follow-up, Ms. L enters the examination room agitated and frightened; she says another patient in the waiting room has just tried to strangle her for no apparent reason. Upon questioning, office staff say they saw no attack and note that the accused patient is a feeble woman with no history of violence; we doubt she assaulted Ms. L.
Ms. L suffers from:
- repeated physical abuse
- delusional disorder
- factitious disorder
- malingering
The authors’ observations
Although Ms. L clearly was not assaulted in the waiting room, this complaint is key to understanding her case. Although whether she is being abused at home remains unclear, evidence increasingly suggests that she suffers from delusions.
Delusions are beliefs that are fixed, false, and not ordinarily accepted by others in a patient’s culture or subculture.3 Delusional disorder is characterized by nonbizarre delusions lasting >1 month (>3 months according to ICD-10 criteria)4 with relatively preserved functioning and without prominent hallucinations. DSM-IV-TR defines bizarre delusions as “clearly implausible, not understandable, and not derived from ordinary life experience.”4,5
Ms. L most likely has a paranoid or persecutory type delusional disorder in which she is convinced she is being harmed. Her delusional thoughts might yield mood symptoms such as anger and irritability, and she might become assaultive. Often, such patients are extraordinarily determined to succeed against “the conspirators” and frequently appeal to the legal system or law enforcement.3
Differentiating between a patient’s delusions and reality can be difficult, leading clinicians to seek collateral information from family, past medical records, or providers to establish a diagnosis. The delusions might become less circumscribed over time, or additional information might clear the clinical picture.
Ms. L’s psychological makeup might help us rule out other diagnoses. Her request for hospitalization, for example, could suggest factitious illness, but she is disabled enough to play the sick role without manufacturing symptoms. Also, she seeks hospitalization because she has no family or friends to turn to. We rule out malingering because Ms. L has nothing to gain by accusing a stranger of choking her in the waiting room.
Treating delusional disorder
Pharmacotherapy and psychotherapy typically are used together to treat delusional disorder.
Pharmacotherapy. Antipsychotics such as olanzapine, 5 to 10 mg nightly, or risperidone, 1 to 2 mg nightly, can decrease the delusional thoughts’ intensity and frequency, allowing patients to function more appropriately.3 If 2 or more antipsychotic trials do not control delusional thoughts, consider starting clozapine at 300 mg/d and titrating to 900 mg/d.
Add an antidepressant if delusional thinking causes depression or anxiety. Selective serotonin reuptake inhibitors (SSRIs) such as paroxetine, 10 to 20 mg/d, or fluoxetine, 20 to 40 mg/d, are a good starting point. Consider other antidepressant types if SSRIs do not work.
Adjunctive benzodiazepines such as clonazepam, 1 to 2 mg/d, or lorazepam, 1 to 2 mg bid as needed, can help manage acute anxiety or agitation stemming from delusions.
Once rapport is established, consider challenging delusional beliefs by having the patient list evidence supporting or refuting the delusions. Be careful not to confront delusional thinking too quickly or aggressively, as this approach often does not change the patient’s beliefs and weakens the therapeutic alliance.3
TREATMENT: Fewer complaints
We still see Ms. L every 3 weeks for supportive psychotherapy and medication management. We continue oxcarbazepine, 150 mg bid, and escitalopram, 30 mg/d, and add risperidone, 1 mg at bedtime, to target her delusional thinking, lability, and irritability.
Over 6 months, Ms. L’s complaints of abuse become less emphatic. She endorses the abuse less frequently—every 3 to 4 visits—and only if the clinician specifically asks about it. Most often, she denies abuse is occurring but says it happened previously. At each visit, we document her statements and explain in her chart why we have not notified adult protective services or police.
- National Adult Protective Services Association. Links to adult protection agencies nationwide. www.apsnetwork.org.
- National Center on Elder Abuse. www.elderabusecenter.org.
Drug brand names
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Fairfax County, VA (June 15, 2006). Adult Protection Services. Available at: http://www.fairfaxcounty.gov/aaa/ombud/abuse.htm. Accessed October 26, 2007.
2. Texas medical jurisprudence manual, 15th ed. Austin, TX: Texas Medical Association; 2004;454–6.
3. Fennig S, Fochtman L, Bromet E. Chapter 12.16c Delusional disorder and shared psychotic disorder. In: Sadock B, Sadock V, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry, 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2005;1525–32.
4. International Classification of Diseases, 10th rev. Geneva, Switzerland: World Health Organization; 1992.
5. Diagnostic and statistical of mental disorders, 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
1. Fairfax County, VA (June 15, 2006). Adult Protection Services. Available at: http://www.fairfaxcounty.gov/aaa/ombud/abuse.htm. Accessed October 26, 2007.
2. Texas medical jurisprudence manual, 15th ed. Austin, TX: Texas Medical Association; 2004;454–6.
3. Fennig S, Fochtman L, Bromet E. Chapter 12.16c Delusional disorder and shared psychotic disorder. In: Sadock B, Sadock V, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry, 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2005;1525–32.
4. International Classification of Diseases, 10th rev. Geneva, Switzerland: World Health Organization; 1992.
5. Diagnostic and statistical of mental disorders, 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
5-step psychiatric workup of HIV patients
Mr. G, a 28-year-old heterosexual Puerto Rican man, is admitted to the hospital’s infectious diseases (ID) unit after 3 weeks of worsening bifrontal headaches. He has been treated as an outpatient for several years since becoming HIV-positive and was diagnosed with AIDS after an intracranial toxoplasmosis infection. Although he has not taken antiretrovirals for several months, Mr. G has adhered intermittently to his antiretroviral regimen and previously developed other opportunistic infections, including thrush and bacterial pneumonia.
Three days after Mr. G is admitted, ID clinicians become concerned that he appears severely depressed and request a psychiatric evaluation.
Psychiatric evaluation and diagnosis in patients with HIV can be a challenge because of:
- the myriad ways HIV can impact the CNS
- the proliferation of antiretroviral medications
- patients’ increasing lifespan as a result of highly active antiretroviral therapy (HAART)1
- the psychological repercussions of living with HIV infection.
In this case-based review, we outline a rational, 5-step approach to evaluating and diagnosing psychiatric symptoms in patients with HIV.
A wide differential diagnosis
Patients who are HIV-positive have disproportionately high rates of psychiatric disorders. One study of approximately 2,800 adults receiving care for HIV found that nearly one-half screened positive for major depression, dysthymia, generalized anxiety disorders, or panic attacks.2 Some psychiatric morbidity may be related to:
- the stress of having HIV
- stressors related to risk factors for acquiring HIV, including low socioeconomic status, homelessness, and discrimination and social stigma based on race and sexual orientation
- substance abuse, which is common among patients with HIV.2
Because of the range and variety of psychopathology encountered in HIV disease, keep a wide differential diagnosis in mind when evaluating patients with HIV.
A 5-step process can help you determine if symptoms in any patient—regardless of HIV status—are caused by a primary psychiatric disorder or CNS impairment (Box).
Table 1
HIV-associated CNS infections
| More common |
| Cryptococcus neoformans meningitis |
| Progressive multifocal leukoencephalopathy (polyomavirus JC) |
| Toxoplasma gondii |
| Less common |
| Aspergillosis |
| Coccidioidomycosis |
| Cytomegalovirus |
| Herpes simplex or varicella-zoster encephalitis |
| Histoplasmosis |
| Leptomeningeal tuberculosis |
| Source: References 5-8 |
Neuropsychiatric side effects of antiretroviral medications
| Medication | Potential side effect(s) |
|---|---|
| Abacavir | Depression, anxiety, psychosis |
| Amprenavir | Mood changes |
| Didanosine | Lethargy, nervousness, anxiety, confusion, sleep disturbances, mood disorders, psychosis |
| Efavirenz | Agitation, depersonalization, hallucinations, disturbed dreams, mood disorders, depression, suicidality, antisocial behavior, psychosis, catatonia, delirium |
| Enfuvirtide | Anxiety, depression |
| Indinavir | Mood changes |
| Lamivudine | Insomnia, mood disorders |
| Lopinavir+Ritonavir | Mood changes, agitation, anxiety |
| Nevirapine | Depression, cognitive impairment, psychosis |
| Ritonavir | Anxiety |
| Saquinavir | Depression, anxiety, sleep disturbances |
| Stavudine | Sleep disorders, mood disorders, delirium |
| Zalcitabine | Somnolence, impaired concentration, mood disorders, delirium |
| Zidovudine | Sleep disturbance, vivid dreams, agitation, mania, depression, psychotic symptoms, delirium |
| Source: References 9,10 | |
STEP 1 Perform initial exams
A careful diagnostic exam that includes a mental status examination with gross cognitive functioning testing is necessary to differentiate primary psychiatric disorders from HIV-related CNS pathology, including:
- HIV-associated dementia
- HIV-associated minor cognitive motor disorder (a less severe form of HIV-related cognitive and psychomotor impairment)
- opportunistic infections.
CASE CONTINUED
Mr. G sits in a chair alone in his room, looking out the window. He responds minimally to your initial greetings and has a staring expression and flat affect. Mr. G is calm and cooperative with the exam but has almost no spontaneous speech, answering questions with slow, imprecise 3- or 4-word responses. He is relaxed and does not seem guarded or paranoid.
Mr. G denies depressed mood or suicidal thinking and appears surprised to be asked about these symptoms. He also denies a history of manic or psychotic symptoms or problems with sleep, appetite, or energy. Bedside cognitive exam—focusing on alertness, orientation, attention, and memory—does not demonstrate any gross deficits.
Cognitive workup. Be vigilant for deficits in attention and orientation that might indicate an acute brain syndrome. In addition, look for discrepant patterns of symptoms or other features that may suggest CNS pathology. For example, Mr. G’s impoverished speech and lack of motivation—combined with a clear sensorium and lack of obvious patterns of mood, anxiety, or psychotic symptoms—suggest that a primary psychiatric disorder might not explain his presentation.
Although commonly used, the bedside Mini-Mental State Examination may be insensitive to cognitive deficits in HIV-associated dementia. The HIV-Dementia Scale is more sensitive to HIV’s typical subcortical features.
Physical workup. When evaluating symptoms in an immunocompromised patient at risk for opportunistic infections, it is important to conduct a comprehensive physical exam. Pay attention to evidence of secondary infection and to neurologic signs. Fever may suggest an opportunistic infection that could contribute to psychiatric symptoms. Immunocompromise in HIV may be associated with a variety of infectious meningitis forms, such as:
- cryptococcus
- aseptic meningitis (which may be caused by HIV)
- histoplasmosis
- coccidioidomycosis.
CASE CONTINUED
Physical exam reveals that Mr. G has a low-grade fever (100.2° F) and penile erosion consistent with herpes simplex infection. He has no meningeal signs and an otherwise normal neurologic examination.
STEP 2 Evaluate lab results
Use laboratory testing to search for potential medical causes of the patient’s presentation. Include a complete blood cell count, electrolytes, blood urea nitrogen and creatinine, and liver function tests to look for underlying metabolic problems.
CASE CONTINUED
Complete blood count, electrolytes, kidney function, and liver function tests are all within normal limits, and rapid plasma reagin (RPR) for syphilis is negative. Cerebrospinal fluid (CSF) analysis demonstrates normal opening pressures, protein, and glucose. India ink stain is negative for Cryptococcus neoformans, but 1 week later CSF cultures are positive for Cryptococcus. The patient has a CD4 count of 15 and a viral load of approximately 44,000.
The stepwise approach this article describes to evaluate and diagnose psychiatric symptoms in HIV-positive patients can be used in any patient to determine if psychiatric symptoms are the result of a primary psychiatric disorder or CNS impairment. This approach may be particularly helpful when evaluating patients with new-onset or unusual symptoms, as described in the following case.
Ms. K, 34, has a diagnosis of ophthalmic herpes and is hospitalized to control severe pain in her left eye. On the second day, she appears moderately anxious and somewhat restless. Although it is possible to recognize some words and connections between a few ideas, her speech is otherwise incomprehensible. The ophthalmologist requests a psychiatric consultation, concerned that the change in mental status represents emerging psychosis.
Because Ms. K is unable to provide information coherently, the psychiatrist carefully reviews her medical, social, and psychiatric histories and medications. Ms. K’s history includes tonsillectomy at age 2, arthroscopic knee surgery after a skiing accident in college, and the use of oral contraception.
STEP 1 During Ms. K’s mental status exam, she appears alert, attentive, and cooperative, although moderately anxious. Rather than tangentiality or loosening of associations, her speech is notable for pervasive word substitutions and paraphasic errors, such as saying “chair” when asked to identify the nightstand in her room.
Aside from her ocular lesion, Ms. K’s physical exam is normal.
STEP 2 Laboratory testing reveals normal electrolytes, renal functioning, liver function tests, thyroid functioning, and B12 and folate levels. Rapid plasma reagin for syphilis is negative.
STEP 3 The psychiatrist feels that her exam demonstrates aphasic features rather than psychotic thought process abnormalities and orders neuroimaging. Brain CT with contrast reveals that Ms. K has a ring-enhancing lesion in the left temporal-parietal area, consistent with toxoplasmosis or a glioblastoma. Biopsy confirms toxoplasmosis.
STEPS 4/5 Neuropsychological testing was not performed in this case. It would have revealed the aphasia. Putting all of the data together resulted in clarifying that the patient was not psychotic.
Because toxoplasmosis often develops in patients with severely compromised immune systems, the healthcare team advises Ms. K to undergo HIV testing. Her enzyme-linked immunoadsorbent assay is positive for HIV antibodies, and her HIV infection is confirmed with a Western blot test.
Treatment with pyrimethamine and sulfadiazine rapidly resolves her neurologic symptoms. When she is no longer aphasic, Ms. K gives a history of several sexual relationships in the last 4 years. She typically used condoms during sexual activity but recalled instances when the condom had ruptured during intercourse. She denies any other risk factors for contracting HIV. Ms. K fully recovers from toxoplasmosis with no signs of cognitive impairment. She is started on antiretroviral therapy and followed as an outpatient.
- HIV and syphilis share sexual risk factors
- having syphilis increases the likelihood of comorbid HIV infection 7- to 9-fold11
- syphilis may worsen the course of HIV infection12
- syphilis can mimic psychiatric symptoms.13,14
STEP 3 Order neuroimaging
Neuroimaging is an essential part of the workup of a patient for whom your clinical examination raises suspicion for CNS impairment. In patients with longstanding HIV infection, brain imaging may reveal cerebral atrophy, which may accompany the cognitive changes found in HIV-associated dementia. In addition, immunocompromised patients, particularly those with a CD4 count 15
CASE CONTINUED
Brain MRI shows moderate cerebral and cerebellar atrophy, which ID clinicians attribute to the long-term effects of HIV infection. No evidence of focal or mass lesions is seen.
By further investigating Mr. G’s medical records, you find a brain MRI performed when Mr. G initially presented with toxoplasmosis in 2001. This scan reveals a large ring-enhancing mass in the right frontal lobe. Although the patient had refused a brain biopsy, the radiologist determined the lesion was most consistent in appearance with intracranial toxoplasmosis.
STEP 4 Perform neuropsychological testing
When physical exam, mental status exam, or neuroimaging suggests a possible CNS cause for a patient’s psychiatric presentation, neuropsychological testing can help characterize which of the patient’s brain functions are compromised and determine their anatomic source. This testing allows for a more complete and precise assessment of brain function than can be achieved by a bedside cognitive exam. It typically includes the Trail Making Test Parts A and B and the Grooved Pegboard Test to evaluate executive and psychomotor functioning, as well as the Controlled Oral Word Association Test to evaluate cognitive speed.
CASE CONTINUED
A search of medical records reveals that Mr. G had recently undergone a brief neuropsychological assessment at the hospital’s outpatient HIV mental health clinic. The psychologist found evidence of frontal lobe dysfunction, including problems with shifting sets, verbal fluency, and naming the months of the year backwards. Mr. G’s performance demonstrated a subcortical dementia pattern that included prominent fine motor impairment.
STEP 5 Synthesize all data to make a diagnosis
Psychiatric illness in HIV-positive patients may involve factors at multiple biopsychosocial levels, including problems with social support, psychological stress, primary psychiatric illness, immunocompromise, and CNS disease. Consider data from all of these levels to arrive at a diagnosis.
CASE CONTINUED
After carefully considering Mr. G’s history, physical and mental status examinations, laboratory data, current and past neuroimaging, and neuropsychological testing, you and ID clinicians conclude that Mr. G’s neuropsychiatric presentation primarily represents the residual deficits from his large frontal lobe toxoplasmosis lesion diagnosed in 2001, with possible contribution from an underlying HIV-associated dementia. You feel that a depressive disorder can be ruled out with a high degree of certainty because the patient denied abnormalities of mood or hedonic tone, did not demonstrate deficits in neurovegetative functioning such as appetite, energy, and sleep, and did not show evidence of suicidality. You attribute the flat affect and amotivation that had prompted the psychiatric consult to his secondary neuropsychiatric deficits.
Table 3
Staging system for HIV-associated dementia
| Stage | Degree of severity | Clinical characteristics |
|---|---|---|
| 0 | Normal | Normal mental and motor function |
| 0.5 | Equivocal | Minimal or equivocal symptoms characteristic of cognitive or motor dysfunction, or mild signs (snout response or slowed extremity movements); no impairment of work or ADLs; gait and strength normal |
| 1 | Mild | Unequivocal evidence of functional, intellectual, or motor impairment (including symptoms, signs, or neuropsychological testing); can walk without assistance and perform all except more demanding aspects of work or ADLs |
| 2 | Moderate | Able to perform basic activities of self care but unable to work or maintain the more demanding ADLs; ambulatory but may require a single prop |
| 3 | Severe | Major intellectual incapacity (cannot follow news or personal events, cannot sustain complex conversation, considerable slowing of all outputs) or motor disability (unable to walk unassisted, requires walker or personal support, usually slowed and accompanied by clumsiness of arms) |
| 4 | End stage | A nearly vegetative state; intellectual and social comprehension and output are rudimentary; patient is nearly or absolutely mute and paraparetic or paraplegic, with urinary and fecal incontinence |
| ADLs: activities of daily living | ||
| Source: References 9,16 | ||
CASE CONTINUED
Because Mr. G had no evidence of a mood syndrome, you do not recommend antidepressants. You note that although a stimulant might improve the patient’s cognitive function and apathy, Mr. G’s history of heavy cocaine use is considered a contraindication.
Related Resources
- Aidsmap information on AIDS and HIV. www.aidsmap.com.
- America Psychiatric Association AIDS Resource Center. www.psych.org/AIDS.
- Abacavir • Ziagen
- Amprenavir • Agenerase
- Didanosine • Videx
- Efavirenz • Sustiva
- Enfuvirtide • Fuzeon
- Indinavir • Crixivan
- Lamivudine • Epivir
- Lopinavir/Ritonavir • Kaletra
- Nevirapine • Viramune
- Pyrimethamine • Daraprim
- Ritonavir • Norvir
- Saquinavir • Invirase
- Stavudine • Zerit
- Sulfadiazine • Microsulfon
- Zalcitabine • Hivid
- Zidovudine • Retrovir
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338(13):853-60.
2. Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry 2001;58(8):721-8.
3. Krikorian R, Wrobel AJ, Meinecke C, et al. Cognitive deficits associated with human immunodeficiency virus encephalopathy. J Neuropsychiatry Clin Neurosci 1990;2(3):256-60.
4. Clifford DB. Human immunodeficiency virus-associated dementia. Arch Neurol 2000;57(3):321-4.
5. Collazos J. Opportunistic infections of the CNS in patients with AIDS: diagnosis and management. CNS Drugs 2003;17(12):869-87.
6. Mischel PS, Vinters HV. Coccidioidomycosis of the CNS: neuropathological and vasculopathic manifestations and clinical correlates. Clin Infect Dis 1995;20(2):400-5.
7. Offiah CE, Turnbull IW. The imaging appearances of intracranial CNS infections in adult HIV and AIDS patients. Clin Radiol 2006;61(5):393-401.
8. Black KE, Baden LR. Fungal infections of the CNS: treatment strategies for the immunocompromised patient. CNS Drugs 2007;21(4):293-318.
9. Cespedes MS, Aberg JA. Neuropsychiatric complications of antiretroviral therapy. Drug Saf 2006;29(10):865-74.
10. Turjanski N, Lloyd GG. Psychiatric side-effects of medications: recent developments. Adv Psychiatr Treat 2005;11(1):58-70.
11. Quinn TC, Cannon RO, Glasser D, et al. The association of syphilis with risk of human immunodeficiency virus infection in patients attending sexually transmitted disease clinics. Arch Intern Med 1990;150(6):1297-1302.
12. Zetola NM, Klausner JD. Syphilis and HIV infection: an update. Clin Infect Dis 2007;44(9):1222-8.
13. Sobhan T, Rowe HM, Ryan WG, Munoz C. Unusual case report: three cases of psychiatric manifestations of neurosyphilis. Psychiatr Serv 2004;55(7):830-2.
14. Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry 2004;75(12):1727-30.
15. Camacho DLA, Smith JK, Castillo M. Differentiation of toxoplasmosis and lymphoma in AIDS patients by using apparent diffusion coefficients. AJNR Am J Neuroradiol 2003;24(4):633-7.
16. Price RW, Brew BJ. The AIDS dementia complex. J Infect Dis 1988;158:1079-83.
Mr. G, a 28-year-old heterosexual Puerto Rican man, is admitted to the hospital’s infectious diseases (ID) unit after 3 weeks of worsening bifrontal headaches. He has been treated as an outpatient for several years since becoming HIV-positive and was diagnosed with AIDS after an intracranial toxoplasmosis infection. Although he has not taken antiretrovirals for several months, Mr. G has adhered intermittently to his antiretroviral regimen and previously developed other opportunistic infections, including thrush and bacterial pneumonia.
Three days after Mr. G is admitted, ID clinicians become concerned that he appears severely depressed and request a psychiatric evaluation.
Psychiatric evaluation and diagnosis in patients with HIV can be a challenge because of:
- the myriad ways HIV can impact the CNS
- the proliferation of antiretroviral medications
- patients’ increasing lifespan as a result of highly active antiretroviral therapy (HAART)1
- the psychological repercussions of living with HIV infection.
In this case-based review, we outline a rational, 5-step approach to evaluating and diagnosing psychiatric symptoms in patients with HIV.
A wide differential diagnosis
Patients who are HIV-positive have disproportionately high rates of psychiatric disorders. One study of approximately 2,800 adults receiving care for HIV found that nearly one-half screened positive for major depression, dysthymia, generalized anxiety disorders, or panic attacks.2 Some psychiatric morbidity may be related to:
- the stress of having HIV
- stressors related to risk factors for acquiring HIV, including low socioeconomic status, homelessness, and discrimination and social stigma based on race and sexual orientation
- substance abuse, which is common among patients with HIV.2
Because of the range and variety of psychopathology encountered in HIV disease, keep a wide differential diagnosis in mind when evaluating patients with HIV.
A 5-step process can help you determine if symptoms in any patient—regardless of HIV status—are caused by a primary psychiatric disorder or CNS impairment (Box).
Table 1
HIV-associated CNS infections
| More common |
| Cryptococcus neoformans meningitis |
| Progressive multifocal leukoencephalopathy (polyomavirus JC) |
| Toxoplasma gondii |
| Less common |
| Aspergillosis |
| Coccidioidomycosis |
| Cytomegalovirus |
| Herpes simplex or varicella-zoster encephalitis |
| Histoplasmosis |
| Leptomeningeal tuberculosis |
| Source: References 5-8 |
Neuropsychiatric side effects of antiretroviral medications
| Medication | Potential side effect(s) |
|---|---|
| Abacavir | Depression, anxiety, psychosis |
| Amprenavir | Mood changes |
| Didanosine | Lethargy, nervousness, anxiety, confusion, sleep disturbances, mood disorders, psychosis |
| Efavirenz | Agitation, depersonalization, hallucinations, disturbed dreams, mood disorders, depression, suicidality, antisocial behavior, psychosis, catatonia, delirium |
| Enfuvirtide | Anxiety, depression |
| Indinavir | Mood changes |
| Lamivudine | Insomnia, mood disorders |
| Lopinavir+Ritonavir | Mood changes, agitation, anxiety |
| Nevirapine | Depression, cognitive impairment, psychosis |
| Ritonavir | Anxiety |
| Saquinavir | Depression, anxiety, sleep disturbances |
| Stavudine | Sleep disorders, mood disorders, delirium |
| Zalcitabine | Somnolence, impaired concentration, mood disorders, delirium |
| Zidovudine | Sleep disturbance, vivid dreams, agitation, mania, depression, psychotic symptoms, delirium |
| Source: References 9,10 | |
STEP 1 Perform initial exams
A careful diagnostic exam that includes a mental status examination with gross cognitive functioning testing is necessary to differentiate primary psychiatric disorders from HIV-related CNS pathology, including:
- HIV-associated dementia
- HIV-associated minor cognitive motor disorder (a less severe form of HIV-related cognitive and psychomotor impairment)
- opportunistic infections.
CASE CONTINUED
Mr. G sits in a chair alone in his room, looking out the window. He responds minimally to your initial greetings and has a staring expression and flat affect. Mr. G is calm and cooperative with the exam but has almost no spontaneous speech, answering questions with slow, imprecise 3- or 4-word responses. He is relaxed and does not seem guarded or paranoid.
Mr. G denies depressed mood or suicidal thinking and appears surprised to be asked about these symptoms. He also denies a history of manic or psychotic symptoms or problems with sleep, appetite, or energy. Bedside cognitive exam—focusing on alertness, orientation, attention, and memory—does not demonstrate any gross deficits.
Cognitive workup. Be vigilant for deficits in attention and orientation that might indicate an acute brain syndrome. In addition, look for discrepant patterns of symptoms or other features that may suggest CNS pathology. For example, Mr. G’s impoverished speech and lack of motivation—combined with a clear sensorium and lack of obvious patterns of mood, anxiety, or psychotic symptoms—suggest that a primary psychiatric disorder might not explain his presentation.
Although commonly used, the bedside Mini-Mental State Examination may be insensitive to cognitive deficits in HIV-associated dementia. The HIV-Dementia Scale is more sensitive to HIV’s typical subcortical features.
Physical workup. When evaluating symptoms in an immunocompromised patient at risk for opportunistic infections, it is important to conduct a comprehensive physical exam. Pay attention to evidence of secondary infection and to neurologic signs. Fever may suggest an opportunistic infection that could contribute to psychiatric symptoms. Immunocompromise in HIV may be associated with a variety of infectious meningitis forms, such as:
- cryptococcus
- aseptic meningitis (which may be caused by HIV)
- histoplasmosis
- coccidioidomycosis.
CASE CONTINUED
Physical exam reveals that Mr. G has a low-grade fever (100.2° F) and penile erosion consistent with herpes simplex infection. He has no meningeal signs and an otherwise normal neurologic examination.
STEP 2 Evaluate lab results
Use laboratory testing to search for potential medical causes of the patient’s presentation. Include a complete blood cell count, electrolytes, blood urea nitrogen and creatinine, and liver function tests to look for underlying metabolic problems.
CASE CONTINUED
Complete blood count, electrolytes, kidney function, and liver function tests are all within normal limits, and rapid plasma reagin (RPR) for syphilis is negative. Cerebrospinal fluid (CSF) analysis demonstrates normal opening pressures, protein, and glucose. India ink stain is negative for Cryptococcus neoformans, but 1 week later CSF cultures are positive for Cryptococcus. The patient has a CD4 count of 15 and a viral load of approximately 44,000.
The stepwise approach this article describes to evaluate and diagnose psychiatric symptoms in HIV-positive patients can be used in any patient to determine if psychiatric symptoms are the result of a primary psychiatric disorder or CNS impairment. This approach may be particularly helpful when evaluating patients with new-onset or unusual symptoms, as described in the following case.
Ms. K, 34, has a diagnosis of ophthalmic herpes and is hospitalized to control severe pain in her left eye. On the second day, she appears moderately anxious and somewhat restless. Although it is possible to recognize some words and connections between a few ideas, her speech is otherwise incomprehensible. The ophthalmologist requests a psychiatric consultation, concerned that the change in mental status represents emerging psychosis.
Because Ms. K is unable to provide information coherently, the psychiatrist carefully reviews her medical, social, and psychiatric histories and medications. Ms. K’s history includes tonsillectomy at age 2, arthroscopic knee surgery after a skiing accident in college, and the use of oral contraception.
STEP 1 During Ms. K’s mental status exam, she appears alert, attentive, and cooperative, although moderately anxious. Rather than tangentiality or loosening of associations, her speech is notable for pervasive word substitutions and paraphasic errors, such as saying “chair” when asked to identify the nightstand in her room.
Aside from her ocular lesion, Ms. K’s physical exam is normal.
STEP 2 Laboratory testing reveals normal electrolytes, renal functioning, liver function tests, thyroid functioning, and B12 and folate levels. Rapid plasma reagin for syphilis is negative.
STEP 3 The psychiatrist feels that her exam demonstrates aphasic features rather than psychotic thought process abnormalities and orders neuroimaging. Brain CT with contrast reveals that Ms. K has a ring-enhancing lesion in the left temporal-parietal area, consistent with toxoplasmosis or a glioblastoma. Biopsy confirms toxoplasmosis.
STEPS 4/5 Neuropsychological testing was not performed in this case. It would have revealed the aphasia. Putting all of the data together resulted in clarifying that the patient was not psychotic.
Because toxoplasmosis often develops in patients with severely compromised immune systems, the healthcare team advises Ms. K to undergo HIV testing. Her enzyme-linked immunoadsorbent assay is positive for HIV antibodies, and her HIV infection is confirmed with a Western blot test.
Treatment with pyrimethamine and sulfadiazine rapidly resolves her neurologic symptoms. When she is no longer aphasic, Ms. K gives a history of several sexual relationships in the last 4 years. She typically used condoms during sexual activity but recalled instances when the condom had ruptured during intercourse. She denies any other risk factors for contracting HIV. Ms. K fully recovers from toxoplasmosis with no signs of cognitive impairment. She is started on antiretroviral therapy and followed as an outpatient.
- HIV and syphilis share sexual risk factors
- having syphilis increases the likelihood of comorbid HIV infection 7- to 9-fold11
- syphilis may worsen the course of HIV infection12
- syphilis can mimic psychiatric symptoms.13,14
STEP 3 Order neuroimaging
Neuroimaging is an essential part of the workup of a patient for whom your clinical examination raises suspicion for CNS impairment. In patients with longstanding HIV infection, brain imaging may reveal cerebral atrophy, which may accompany the cognitive changes found in HIV-associated dementia. In addition, immunocompromised patients, particularly those with a CD4 count 15
CASE CONTINUED
Brain MRI shows moderate cerebral and cerebellar atrophy, which ID clinicians attribute to the long-term effects of HIV infection. No evidence of focal or mass lesions is seen.
By further investigating Mr. G’s medical records, you find a brain MRI performed when Mr. G initially presented with toxoplasmosis in 2001. This scan reveals a large ring-enhancing mass in the right frontal lobe. Although the patient had refused a brain biopsy, the radiologist determined the lesion was most consistent in appearance with intracranial toxoplasmosis.
STEP 4 Perform neuropsychological testing
When physical exam, mental status exam, or neuroimaging suggests a possible CNS cause for a patient’s psychiatric presentation, neuropsychological testing can help characterize which of the patient’s brain functions are compromised and determine their anatomic source. This testing allows for a more complete and precise assessment of brain function than can be achieved by a bedside cognitive exam. It typically includes the Trail Making Test Parts A and B and the Grooved Pegboard Test to evaluate executive and psychomotor functioning, as well as the Controlled Oral Word Association Test to evaluate cognitive speed.
CASE CONTINUED
A search of medical records reveals that Mr. G had recently undergone a brief neuropsychological assessment at the hospital’s outpatient HIV mental health clinic. The psychologist found evidence of frontal lobe dysfunction, including problems with shifting sets, verbal fluency, and naming the months of the year backwards. Mr. G’s performance demonstrated a subcortical dementia pattern that included prominent fine motor impairment.
STEP 5 Synthesize all data to make a diagnosis
Psychiatric illness in HIV-positive patients may involve factors at multiple biopsychosocial levels, including problems with social support, psychological stress, primary psychiatric illness, immunocompromise, and CNS disease. Consider data from all of these levels to arrive at a diagnosis.
CASE CONTINUED
After carefully considering Mr. G’s history, physical and mental status examinations, laboratory data, current and past neuroimaging, and neuropsychological testing, you and ID clinicians conclude that Mr. G’s neuropsychiatric presentation primarily represents the residual deficits from his large frontal lobe toxoplasmosis lesion diagnosed in 2001, with possible contribution from an underlying HIV-associated dementia. You feel that a depressive disorder can be ruled out with a high degree of certainty because the patient denied abnormalities of mood or hedonic tone, did not demonstrate deficits in neurovegetative functioning such as appetite, energy, and sleep, and did not show evidence of suicidality. You attribute the flat affect and amotivation that had prompted the psychiatric consult to his secondary neuropsychiatric deficits.
Table 3
Staging system for HIV-associated dementia
| Stage | Degree of severity | Clinical characteristics |
|---|---|---|
| 0 | Normal | Normal mental and motor function |
| 0.5 | Equivocal | Minimal or equivocal symptoms characteristic of cognitive or motor dysfunction, or mild signs (snout response or slowed extremity movements); no impairment of work or ADLs; gait and strength normal |
| 1 | Mild | Unequivocal evidence of functional, intellectual, or motor impairment (including symptoms, signs, or neuropsychological testing); can walk without assistance and perform all except more demanding aspects of work or ADLs |
| 2 | Moderate | Able to perform basic activities of self care but unable to work or maintain the more demanding ADLs; ambulatory but may require a single prop |
| 3 | Severe | Major intellectual incapacity (cannot follow news or personal events, cannot sustain complex conversation, considerable slowing of all outputs) or motor disability (unable to walk unassisted, requires walker or personal support, usually slowed and accompanied by clumsiness of arms) |
| 4 | End stage | A nearly vegetative state; intellectual and social comprehension and output are rudimentary; patient is nearly or absolutely mute and paraparetic or paraplegic, with urinary and fecal incontinence |
| ADLs: activities of daily living | ||
| Source: References 9,16 | ||
CASE CONTINUED
Because Mr. G had no evidence of a mood syndrome, you do not recommend antidepressants. You note that although a stimulant might improve the patient’s cognitive function and apathy, Mr. G’s history of heavy cocaine use is considered a contraindication.
Related Resources
- Aidsmap information on AIDS and HIV. www.aidsmap.com.
- America Psychiatric Association AIDS Resource Center. www.psych.org/AIDS.
- Abacavir • Ziagen
- Amprenavir • Agenerase
- Didanosine • Videx
- Efavirenz • Sustiva
- Enfuvirtide • Fuzeon
- Indinavir • Crixivan
- Lamivudine • Epivir
- Lopinavir/Ritonavir • Kaletra
- Nevirapine • Viramune
- Pyrimethamine • Daraprim
- Ritonavir • Norvir
- Saquinavir • Invirase
- Stavudine • Zerit
- Sulfadiazine • Microsulfon
- Zalcitabine • Hivid
- Zidovudine • Retrovir
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. G, a 28-year-old heterosexual Puerto Rican man, is admitted to the hospital’s infectious diseases (ID) unit after 3 weeks of worsening bifrontal headaches. He has been treated as an outpatient for several years since becoming HIV-positive and was diagnosed with AIDS after an intracranial toxoplasmosis infection. Although he has not taken antiretrovirals for several months, Mr. G has adhered intermittently to his antiretroviral regimen and previously developed other opportunistic infections, including thrush and bacterial pneumonia.
Three days after Mr. G is admitted, ID clinicians become concerned that he appears severely depressed and request a psychiatric evaluation.
Psychiatric evaluation and diagnosis in patients with HIV can be a challenge because of:
- the myriad ways HIV can impact the CNS
- the proliferation of antiretroviral medications
- patients’ increasing lifespan as a result of highly active antiretroviral therapy (HAART)1
- the psychological repercussions of living with HIV infection.
In this case-based review, we outline a rational, 5-step approach to evaluating and diagnosing psychiatric symptoms in patients with HIV.
A wide differential diagnosis
Patients who are HIV-positive have disproportionately high rates of psychiatric disorders. One study of approximately 2,800 adults receiving care for HIV found that nearly one-half screened positive for major depression, dysthymia, generalized anxiety disorders, or panic attacks.2 Some psychiatric morbidity may be related to:
- the stress of having HIV
- stressors related to risk factors for acquiring HIV, including low socioeconomic status, homelessness, and discrimination and social stigma based on race and sexual orientation
- substance abuse, which is common among patients with HIV.2
Because of the range and variety of psychopathology encountered in HIV disease, keep a wide differential diagnosis in mind when evaluating patients with HIV.
A 5-step process can help you determine if symptoms in any patient—regardless of HIV status—are caused by a primary psychiatric disorder or CNS impairment (Box).
Table 1
HIV-associated CNS infections
| More common |
| Cryptococcus neoformans meningitis |
| Progressive multifocal leukoencephalopathy (polyomavirus JC) |
| Toxoplasma gondii |
| Less common |
| Aspergillosis |
| Coccidioidomycosis |
| Cytomegalovirus |
| Herpes simplex or varicella-zoster encephalitis |
| Histoplasmosis |
| Leptomeningeal tuberculosis |
| Source: References 5-8 |
Neuropsychiatric side effects of antiretroviral medications
| Medication | Potential side effect(s) |
|---|---|
| Abacavir | Depression, anxiety, psychosis |
| Amprenavir | Mood changes |
| Didanosine | Lethargy, nervousness, anxiety, confusion, sleep disturbances, mood disorders, psychosis |
| Efavirenz | Agitation, depersonalization, hallucinations, disturbed dreams, mood disorders, depression, suicidality, antisocial behavior, psychosis, catatonia, delirium |
| Enfuvirtide | Anxiety, depression |
| Indinavir | Mood changes |
| Lamivudine | Insomnia, mood disorders |
| Lopinavir+Ritonavir | Mood changes, agitation, anxiety |
| Nevirapine | Depression, cognitive impairment, psychosis |
| Ritonavir | Anxiety |
| Saquinavir | Depression, anxiety, sleep disturbances |
| Stavudine | Sleep disorders, mood disorders, delirium |
| Zalcitabine | Somnolence, impaired concentration, mood disorders, delirium |
| Zidovudine | Sleep disturbance, vivid dreams, agitation, mania, depression, psychotic symptoms, delirium |
| Source: References 9,10 | |
STEP 1 Perform initial exams
A careful diagnostic exam that includes a mental status examination with gross cognitive functioning testing is necessary to differentiate primary psychiatric disorders from HIV-related CNS pathology, including:
- HIV-associated dementia
- HIV-associated minor cognitive motor disorder (a less severe form of HIV-related cognitive and psychomotor impairment)
- opportunistic infections.
CASE CONTINUED
Mr. G sits in a chair alone in his room, looking out the window. He responds minimally to your initial greetings and has a staring expression and flat affect. Mr. G is calm and cooperative with the exam but has almost no spontaneous speech, answering questions with slow, imprecise 3- or 4-word responses. He is relaxed and does not seem guarded or paranoid.
Mr. G denies depressed mood or suicidal thinking and appears surprised to be asked about these symptoms. He also denies a history of manic or psychotic symptoms or problems with sleep, appetite, or energy. Bedside cognitive exam—focusing on alertness, orientation, attention, and memory—does not demonstrate any gross deficits.
Cognitive workup. Be vigilant for deficits in attention and orientation that might indicate an acute brain syndrome. In addition, look for discrepant patterns of symptoms or other features that may suggest CNS pathology. For example, Mr. G’s impoverished speech and lack of motivation—combined with a clear sensorium and lack of obvious patterns of mood, anxiety, or psychotic symptoms—suggest that a primary psychiatric disorder might not explain his presentation.
Although commonly used, the bedside Mini-Mental State Examination may be insensitive to cognitive deficits in HIV-associated dementia. The HIV-Dementia Scale is more sensitive to HIV’s typical subcortical features.
Physical workup. When evaluating symptoms in an immunocompromised patient at risk for opportunistic infections, it is important to conduct a comprehensive physical exam. Pay attention to evidence of secondary infection and to neurologic signs. Fever may suggest an opportunistic infection that could contribute to psychiatric symptoms. Immunocompromise in HIV may be associated with a variety of infectious meningitis forms, such as:
- cryptococcus
- aseptic meningitis (which may be caused by HIV)
- histoplasmosis
- coccidioidomycosis.
CASE CONTINUED
Physical exam reveals that Mr. G has a low-grade fever (100.2° F) and penile erosion consistent with herpes simplex infection. He has no meningeal signs and an otherwise normal neurologic examination.
STEP 2 Evaluate lab results
Use laboratory testing to search for potential medical causes of the patient’s presentation. Include a complete blood cell count, electrolytes, blood urea nitrogen and creatinine, and liver function tests to look for underlying metabolic problems.
CASE CONTINUED
Complete blood count, electrolytes, kidney function, and liver function tests are all within normal limits, and rapid plasma reagin (RPR) for syphilis is negative. Cerebrospinal fluid (CSF) analysis demonstrates normal opening pressures, protein, and glucose. India ink stain is negative for Cryptococcus neoformans, but 1 week later CSF cultures are positive for Cryptococcus. The patient has a CD4 count of 15 and a viral load of approximately 44,000.
The stepwise approach this article describes to evaluate and diagnose psychiatric symptoms in HIV-positive patients can be used in any patient to determine if psychiatric symptoms are the result of a primary psychiatric disorder or CNS impairment. This approach may be particularly helpful when evaluating patients with new-onset or unusual symptoms, as described in the following case.
Ms. K, 34, has a diagnosis of ophthalmic herpes and is hospitalized to control severe pain in her left eye. On the second day, she appears moderately anxious and somewhat restless. Although it is possible to recognize some words and connections between a few ideas, her speech is otherwise incomprehensible. The ophthalmologist requests a psychiatric consultation, concerned that the change in mental status represents emerging psychosis.
Because Ms. K is unable to provide information coherently, the psychiatrist carefully reviews her medical, social, and psychiatric histories and medications. Ms. K’s history includes tonsillectomy at age 2, arthroscopic knee surgery after a skiing accident in college, and the use of oral contraception.
STEP 1 During Ms. K’s mental status exam, she appears alert, attentive, and cooperative, although moderately anxious. Rather than tangentiality or loosening of associations, her speech is notable for pervasive word substitutions and paraphasic errors, such as saying “chair” when asked to identify the nightstand in her room.
Aside from her ocular lesion, Ms. K’s physical exam is normal.
STEP 2 Laboratory testing reveals normal electrolytes, renal functioning, liver function tests, thyroid functioning, and B12 and folate levels. Rapid plasma reagin for syphilis is negative.
STEP 3 The psychiatrist feels that her exam demonstrates aphasic features rather than psychotic thought process abnormalities and orders neuroimaging. Brain CT with contrast reveals that Ms. K has a ring-enhancing lesion in the left temporal-parietal area, consistent with toxoplasmosis or a glioblastoma. Biopsy confirms toxoplasmosis.
STEPS 4/5 Neuropsychological testing was not performed in this case. It would have revealed the aphasia. Putting all of the data together resulted in clarifying that the patient was not psychotic.
Because toxoplasmosis often develops in patients with severely compromised immune systems, the healthcare team advises Ms. K to undergo HIV testing. Her enzyme-linked immunoadsorbent assay is positive for HIV antibodies, and her HIV infection is confirmed with a Western blot test.
Treatment with pyrimethamine and sulfadiazine rapidly resolves her neurologic symptoms. When she is no longer aphasic, Ms. K gives a history of several sexual relationships in the last 4 years. She typically used condoms during sexual activity but recalled instances when the condom had ruptured during intercourse. She denies any other risk factors for contracting HIV. Ms. K fully recovers from toxoplasmosis with no signs of cognitive impairment. She is started on antiretroviral therapy and followed as an outpatient.
- HIV and syphilis share sexual risk factors
- having syphilis increases the likelihood of comorbid HIV infection 7- to 9-fold11
- syphilis may worsen the course of HIV infection12
- syphilis can mimic psychiatric symptoms.13,14
STEP 3 Order neuroimaging
Neuroimaging is an essential part of the workup of a patient for whom your clinical examination raises suspicion for CNS impairment. In patients with longstanding HIV infection, brain imaging may reveal cerebral atrophy, which may accompany the cognitive changes found in HIV-associated dementia. In addition, immunocompromised patients, particularly those with a CD4 count 15
CASE CONTINUED
Brain MRI shows moderate cerebral and cerebellar atrophy, which ID clinicians attribute to the long-term effects of HIV infection. No evidence of focal or mass lesions is seen.
By further investigating Mr. G’s medical records, you find a brain MRI performed when Mr. G initially presented with toxoplasmosis in 2001. This scan reveals a large ring-enhancing mass in the right frontal lobe. Although the patient had refused a brain biopsy, the radiologist determined the lesion was most consistent in appearance with intracranial toxoplasmosis.
STEP 4 Perform neuropsychological testing
When physical exam, mental status exam, or neuroimaging suggests a possible CNS cause for a patient’s psychiatric presentation, neuropsychological testing can help characterize which of the patient’s brain functions are compromised and determine their anatomic source. This testing allows for a more complete and precise assessment of brain function than can be achieved by a bedside cognitive exam. It typically includes the Trail Making Test Parts A and B and the Grooved Pegboard Test to evaluate executive and psychomotor functioning, as well as the Controlled Oral Word Association Test to evaluate cognitive speed.
CASE CONTINUED
A search of medical records reveals that Mr. G had recently undergone a brief neuropsychological assessment at the hospital’s outpatient HIV mental health clinic. The psychologist found evidence of frontal lobe dysfunction, including problems with shifting sets, verbal fluency, and naming the months of the year backwards. Mr. G’s performance demonstrated a subcortical dementia pattern that included prominent fine motor impairment.
STEP 5 Synthesize all data to make a diagnosis
Psychiatric illness in HIV-positive patients may involve factors at multiple biopsychosocial levels, including problems with social support, psychological stress, primary psychiatric illness, immunocompromise, and CNS disease. Consider data from all of these levels to arrive at a diagnosis.
CASE CONTINUED
After carefully considering Mr. G’s history, physical and mental status examinations, laboratory data, current and past neuroimaging, and neuropsychological testing, you and ID clinicians conclude that Mr. G’s neuropsychiatric presentation primarily represents the residual deficits from his large frontal lobe toxoplasmosis lesion diagnosed in 2001, with possible contribution from an underlying HIV-associated dementia. You feel that a depressive disorder can be ruled out with a high degree of certainty because the patient denied abnormalities of mood or hedonic tone, did not demonstrate deficits in neurovegetative functioning such as appetite, energy, and sleep, and did not show evidence of suicidality. You attribute the flat affect and amotivation that had prompted the psychiatric consult to his secondary neuropsychiatric deficits.
Table 3
Staging system for HIV-associated dementia
| Stage | Degree of severity | Clinical characteristics |
|---|---|---|
| 0 | Normal | Normal mental and motor function |
| 0.5 | Equivocal | Minimal or equivocal symptoms characteristic of cognitive or motor dysfunction, or mild signs (snout response or slowed extremity movements); no impairment of work or ADLs; gait and strength normal |
| 1 | Mild | Unequivocal evidence of functional, intellectual, or motor impairment (including symptoms, signs, or neuropsychological testing); can walk without assistance and perform all except more demanding aspects of work or ADLs |
| 2 | Moderate | Able to perform basic activities of self care but unable to work or maintain the more demanding ADLs; ambulatory but may require a single prop |
| 3 | Severe | Major intellectual incapacity (cannot follow news or personal events, cannot sustain complex conversation, considerable slowing of all outputs) or motor disability (unable to walk unassisted, requires walker or personal support, usually slowed and accompanied by clumsiness of arms) |
| 4 | End stage | A nearly vegetative state; intellectual and social comprehension and output are rudimentary; patient is nearly or absolutely mute and paraparetic or paraplegic, with urinary and fecal incontinence |
| ADLs: activities of daily living | ||
| Source: References 9,16 | ||
CASE CONTINUED
Because Mr. G had no evidence of a mood syndrome, you do not recommend antidepressants. You note that although a stimulant might improve the patient’s cognitive function and apathy, Mr. G’s history of heavy cocaine use is considered a contraindication.
Related Resources
- Aidsmap information on AIDS and HIV. www.aidsmap.com.
- America Psychiatric Association AIDS Resource Center. www.psych.org/AIDS.
- Abacavir • Ziagen
- Amprenavir • Agenerase
- Didanosine • Videx
- Efavirenz • Sustiva
- Enfuvirtide • Fuzeon
- Indinavir • Crixivan
- Lamivudine • Epivir
- Lopinavir/Ritonavir • Kaletra
- Nevirapine • Viramune
- Pyrimethamine • Daraprim
- Ritonavir • Norvir
- Saquinavir • Invirase
- Stavudine • Zerit
- Sulfadiazine • Microsulfon
- Zalcitabine • Hivid
- Zidovudine • Retrovir
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338(13):853-60.
2. Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry 2001;58(8):721-8.
3. Krikorian R, Wrobel AJ, Meinecke C, et al. Cognitive deficits associated with human immunodeficiency virus encephalopathy. J Neuropsychiatry Clin Neurosci 1990;2(3):256-60.
4. Clifford DB. Human immunodeficiency virus-associated dementia. Arch Neurol 2000;57(3):321-4.
5. Collazos J. Opportunistic infections of the CNS in patients with AIDS: diagnosis and management. CNS Drugs 2003;17(12):869-87.
6. Mischel PS, Vinters HV. Coccidioidomycosis of the CNS: neuropathological and vasculopathic manifestations and clinical correlates. Clin Infect Dis 1995;20(2):400-5.
7. Offiah CE, Turnbull IW. The imaging appearances of intracranial CNS infections in adult HIV and AIDS patients. Clin Radiol 2006;61(5):393-401.
8. Black KE, Baden LR. Fungal infections of the CNS: treatment strategies for the immunocompromised patient. CNS Drugs 2007;21(4):293-318.
9. Cespedes MS, Aberg JA. Neuropsychiatric complications of antiretroviral therapy. Drug Saf 2006;29(10):865-74.
10. Turjanski N, Lloyd GG. Psychiatric side-effects of medications: recent developments. Adv Psychiatr Treat 2005;11(1):58-70.
11. Quinn TC, Cannon RO, Glasser D, et al. The association of syphilis with risk of human immunodeficiency virus infection in patients attending sexually transmitted disease clinics. Arch Intern Med 1990;150(6):1297-1302.
12. Zetola NM, Klausner JD. Syphilis and HIV infection: an update. Clin Infect Dis 2007;44(9):1222-8.
13. Sobhan T, Rowe HM, Ryan WG, Munoz C. Unusual case report: three cases of psychiatric manifestations of neurosyphilis. Psychiatr Serv 2004;55(7):830-2.
14. Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry 2004;75(12):1727-30.
15. Camacho DLA, Smith JK, Castillo M. Differentiation of toxoplasmosis and lymphoma in AIDS patients by using apparent diffusion coefficients. AJNR Am J Neuroradiol 2003;24(4):633-7.
16. Price RW, Brew BJ. The AIDS dementia complex. J Infect Dis 1988;158:1079-83.
1. Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338(13):853-60.
2. Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry 2001;58(8):721-8.
3. Krikorian R, Wrobel AJ, Meinecke C, et al. Cognitive deficits associated with human immunodeficiency virus encephalopathy. J Neuropsychiatry Clin Neurosci 1990;2(3):256-60.
4. Clifford DB. Human immunodeficiency virus-associated dementia. Arch Neurol 2000;57(3):321-4.
5. Collazos J. Opportunistic infections of the CNS in patients with AIDS: diagnosis and management. CNS Drugs 2003;17(12):869-87.
6. Mischel PS, Vinters HV. Coccidioidomycosis of the CNS: neuropathological and vasculopathic manifestations and clinical correlates. Clin Infect Dis 1995;20(2):400-5.
7. Offiah CE, Turnbull IW. The imaging appearances of intracranial CNS infections in adult HIV and AIDS patients. Clin Radiol 2006;61(5):393-401.
8. Black KE, Baden LR. Fungal infections of the CNS: treatment strategies for the immunocompromised patient. CNS Drugs 2007;21(4):293-318.
9. Cespedes MS, Aberg JA. Neuropsychiatric complications of antiretroviral therapy. Drug Saf 2006;29(10):865-74.
10. Turjanski N, Lloyd GG. Psychiatric side-effects of medications: recent developments. Adv Psychiatr Treat 2005;11(1):58-70.
11. Quinn TC, Cannon RO, Glasser D, et al. The association of syphilis with risk of human immunodeficiency virus infection in patients attending sexually transmitted disease clinics. Arch Intern Med 1990;150(6):1297-1302.
12. Zetola NM, Klausner JD. Syphilis and HIV infection: an update. Clin Infect Dis 2007;44(9):1222-8.
13. Sobhan T, Rowe HM, Ryan WG, Munoz C. Unusual case report: three cases of psychiatric manifestations of neurosyphilis. Psychiatr Serv 2004;55(7):830-2.
14. Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry 2004;75(12):1727-30.
15. Camacho DLA, Smith JK, Castillo M. Differentiation of toxoplasmosis and lymphoma in AIDS patients by using apparent diffusion coefficients. AJNR Am J Neuroradiol 2003;24(4):633-7.
16. Price RW, Brew BJ. The AIDS dementia complex. J Infect Dis 1988;158:1079-83.
Treatment-resistant insomnia? Ask yourself 8 questions
Although many patients with insomnia respond to standard treatments, some continue to experience insufficient sleep. When your patient appears “treatment-resistant,” you may be tempted to add another therapy or try an unorthodox medication. But choosing an appropriate next treatment is impossible without first looking back for a rationale:
- Have you overlooked one of insomnia’s many causes?
- Have you customized treatment for this patient?
- Is he or she unaware of behaviors that may be undermining attempts to sleep?
Refreshing sleep may elude some thoroughly evaluated and optimally treated patients, but they comprise a small minority. You can help most chronic insomnia sufferers by re-evaluating their behaviors, comorbidities, sleep-wake cycles, and medications (Table 1).
Table 1
Recommended approach to treatment-resistant insomnia
| Evaluation |
| Review your patient’s 24-hour sleep cycle, sleepiness, and sleeplessness, and note persistent patterns (a sleep log or diary may help) |
| Re-evaluate stimulating or sedating effects of prescribed and over-the-counter medications, caffeine, and alcohol |
Consider:
|
| Monitor insomnia-related daytime symptoms as key outcome measure |
| Treatment |
| Re-address sleep hygiene (Table 2) |
| Consider cognitive behavioral therapy for insomnia |
| Consider an FDA-approved medication for insomnia (Table 3), customized to your patient’s symptoms |
‘3 Ps’ and 8 questions
Thirty percent of adults experience insomnia at least occasionally, and 10% have persistent insomnia. Women, older persons, and patients with chronic medical conditions such as diabetes mellitus and lung disease have higher insomnia rates than the general population.1
An enormous variety of psychological and physiologic processes may influence sleep (Box 1). Multiple factors may contribute to an individual’s inability to achieve sufficient sleep, and the relative significance of these influences can shift over time. Factors that might trigger an insomnia episode are not necessarily those that maintain sleeplessness.
1 Does the patient have realistic goals for falling asleep and remaining asleep?
Patients view insomnia as being unable to sleep when they believe they should be sleeping. To be diagnosed as a disorder, insomnia must have daytime consequences associated with:
- difficulty falling asleep
- difficulty maintaining sleep
- awakening excessively early
- or experiencing nonrestorative sleep.
Recommendation. Determine how the patient defines “having insomnia” (there are no absolute thresholds). Ask how he or she is functioning during the day. Those who complain of imperfect nighttime sleep may admit that treatment has helped with the daytime symptoms that prompted them to seek treatment.
If daytime symptoms have diminished, reassure the patient that treatment apparently is helping. Patients are less likely to focus on perceived nighttime impairment when their distress about daytime functioning has eased.
Also determine if the patient has followed recommended treatment. Cognitive-behavioral therapy (CBT) may increase adherence to behavioral changes, sleep hygiene, and medication schedules.
2 Have I identified and optimally managed comorbidities?
Identifying comorbidities that may contribute to chronic insomnia is particularly important because managing these conditions may alleviate the sleep disturbance. Pain or discomfort caused by a medical condition may undermine sleep quality. Certain cardiovascular, pulmonary, endocrine, neurologic, rheumatologic, and orthopedic disorders are associated with insomnia.
Most patients experiencing exacerbations of mood and anxiety disorders suffer insomnia, and many other psychiatric disorders are associated with sleep disruption.
Diagnostic subtypes recognized by the American Academy of Sleep Medicine may suggest why recommended treatments have not relieved a patient’s symptoms. Insomnia may be:
- due to a mental disorder, medical condition, drug or substance
- adjustment-related (acute insomnia), psychophysiologic, paradoxical, or idiopathic
- related to inadequate sleep hygiene
- a behavioral characteristic of childhood
- organic (due to an unspecified physiologic condition)
- nonorganic, NOS (not due to a substance or known physiologic condition).
NOS: not otherwise specified
Insomnia often accompanies substance abuse and may continue after the patient stops abusing drugs or alcohol. Abused stimulants and sedatives can worsen sleep quality, and discontinuation can cause acute and chronic sleep disruption.
Recommendation. Treat mood and anxiety disorders independently of insomnia. Minimize pain and discomfort from medical conditions. Address substance abuse, and dispel patients’ notion that alcohol is a sleep aid.
Order sleep laboratory testing for patients at risk for sleep apnea, based on their history, physical exam—including obesity, upper airway anatomy, and neck circumference (collar size ≥17 inches)—and informant reports of snoring and breathing patterns.
3 Is the patient taking medications with stimulating effects?
Because insomnia is highly comorbid with mood and anxiety disorders, patients with insomnia often are prescribed antidepressants. Although some are sedating, antidepressants such as selective serotonin reuptake inhibitors are likely to be stimulating.
Recommendation. When insomnia persists, assess the potential effects of prescribed and over-the-counter (OTC) medications. Consider possible pharmacologic effects of aging that can make patients more sensitive to medications.
Also educate patients about the long-acting effects of caffeine and its varied sources, such as energy drinks and OTC products. Some patients will benefit from completely avoiding caffeine, whereas others may do fine restricting coffee to 1 or 2 cups in the morning. A good general practice is to avoid all caffeine after lunchtime.
Predisposing factors. Some personalities may be predisposed to insomnia. Persons who tend to be anxious, depressive, or emotionally reactive may be at increased risk for developing insomnia.
Precipitating factors may include situational crises, schedule changes, substance or medication use, and psychiatric, medical, and sleep disorders. A careful history allows you to consider precipitating events.
Perpetuating factors that may reinforce and maintain chronic insomnia include:
- maladaptive behaviors, such as napping or using alcohol as a sleep aid
- conditioned hyperarousal, whereby insomnia sufferers experience anxiety and tension associated with preparing for and getting into bed. Sleepless time in bed may reinforce the conditioning, contribute to anxiety and tension, and undermine sleep on future nights.
Source: Reference 2
4 Does the patient’s insomnia have a homeostatic component?
Circadian rhythms and a homeostatic sleep drive are temporally linked in regulating the normal routine of nighttime sleep alternating with day and evening wakefulness.5,6 The sleep drive promotes a sleep-to-waking ratio of approximately 1:2 (an average of 8 hours sleep per 24 hours). Adequate sleep, from the homeostatic perspective, could be achieved during any hours of the day or night.
Acute sleep deprivation may result from extended wakefulness—such as staying up all night to study for an exam. Chronic sleep deprivation may occur during successive 24-hour periods with insufficient sleep. Both patterns are associated with increasing subjective sleepiness and ultimately with cognitive impairment.
- is low throughout the daytime
- rises during the evening as bedtime approaches
- plateaus during nighttime sleep hours
- decreases as the normal morning wake time approaches.
Typically, people are more alert in the evening than at any other time in the 24-hour cycle. As bedtime approaches, rising melatonin interacts with SCN melatonin receptors and decreases circadian arousal. Normal sleep onset then can occur rapidly at bedtime, when the homeostatic sleep drive is unopposed.
Nighttime sleep initially is promoted by the homeostatic sleep drive. However, the homeostatic sleep pressure is reversed by sleep and thus decreases as sleep continues during the night. The circadian system promotes minimum stimulation during the latter sleep hours, sustaining total sleep for approximately 8 hours.
Consequences. Individual circadian timing tendencies may affect when people experience alertness and sleepiness and may be associated with persistent complaints of sleep onset difficulty or early morning awakening.7 Napping may reduce the homeostatic sleepiness available to aid bedtime sleep onset. Mismatched homeostatic and circadian processes often prevent shift workers from achieving satisfactory sleep.
Recommendation. Have the patient keep a sleep log to identify the time and duration of sleep episodes throughout the 24-hour cycle. Actigraphy may provide useful information about sleep-wake patterns.
5 Are circadian rhythm patterns contributing to insomnia?
Overlooking circadian rhythms’ effects on insomnia can lead to apparent treatment failure.8 Although the circadian system typically promotes sleep from about 10 pm to midnight until about 6 to 8 am, some individuals have long-standing predispositions for earlier or later sleep episodes.
An advanced circadian phase leads to sleepiness and the ability to fall asleep early in the evening, followed by a tendency to awaken spontaneously relatively early in the morning. In extreme cases, patients with these “lark” tendencies may be diagnosed with advanced sleep phase disorder. Persistent early morning awakening insomnia and sleep maintenance complaints are common.
A delayed circadian phase is associated with inability to fall asleep at a typical late evening bedtime and difficulty awakening at a desired time the following morning. In extreme cases, individuals may sleep from very late at night until the following afternoon. These markedly delayed schedules may be obvious, but the circadian contribution may not be recognized in less severe cases.
People with this predisposition may achieve optimum sleep by following their delayed circadian tendency, but school and work demands often conflict with this approach. They may develop chronic sleep deprivation from late sleep onset coupled with forced morning awakenings. Complaints of chronic difficulty with sleep onset are common.
Recommendation. Have the patient keep a sleep log to demonstrate advanced or delayed circadian phase tendencies. Determine if the patient is a shift worker who is attempting to sleep in the daytime. Consider prescribing ramelteon—a melatonin agonist—and providing strategic bright light exposure:
- in the evening for advanced circadian phase patients
- in the morning for delayed circadian phase patients.8
6 Is the patient following appropriate sleep hygiene?
Sleep hygiene will not necessarily cure chronic insomnia, but inattention to basic guidelines (Table 2) can undermine other treatments. When re-evaluating patients with chronic insomnia, give special attention to their alcohol and caffeine intake, regularity of bedtime and wake-up times, meal times, and the bedroom environment. Advise patients to remove televisions from the bedroom, for example.
CBT that is effective for chronic insomnia typically blends sleep hygiene with education, cognitive psychotherapy, and specific instructions regarding bedtime schedules.9,10 Relaxation techniques also may be beneficial.
Consider consulting with a sleep specialist if the patient has not been evaluated at a sleep center. Some sleep centers offer CBT.
Table 2
Patient education: Sleep hygiene guidelines
| Try to maintain a regular sleep–wake schedule |
| Avoid afternoon or evening napping |
| Allow yourself enough time in bed for adequate sleep duration (such as 11 PM to 7 AM) |
| Develop a relaxing evening routine for the hours before bedtime |
| Spend some idle time reflecting on the day’s events before going to bed; make a list of concerns and how some might be resolved |
| Reserve the bed for sleep and sex; do not do homework, pay bills, watch TV, or engage in serious domestic discussions in bed |
| Avoid alcohol in the evening |
| Avoid caffeine in the afternoon and evening |
| Minimize annoying noise, light, or temperature extremes |
| Consider a light snack before bedtime |
| Exercise regularly, but not late in the evening |
| Do not try harder and harder to fall asleep; if you can’t sleep, get out of bed and do something else, in another room if possible |
| Avoid smoking |
7 Does the patient regularly experience anxiety and tension as bedtime approaches or spend excessive wakeful time in bed?
Patients who tend to be anxious, depressive, or emotionally reactive are at increased risk for developing an insomnia episode. They then may develop conditioned hyperarousal associated with preparing for and getting into bed, which perpetuates insomnia.
Some patients spend long periods in bed, hoping to achieve any possible sleep that night. Extended time in bed can perpetuate insomnia by increasing frustrating time awake, thereby reinforcing the association between the bed and wakefulness.
Recommendation. CBT often helps ease these conditioned responses.
Stimulus control can help anxious individuals reassociate the bed, bedroom, and bedtime routines with sleep onset, rather than sleep-destructive tension. Advise patients to go to bed in the evening when they feel they can fall asleep. If they do not fall asleep within 10 to 15 minutes or experience their usual worry and frustration about not sleeping, instruct them to leave the bed and try again later. Also tell them to avoid daytime napping.
Sleep restriction therapy may help patients with excessive wakefulness in bed by limiting sleep opportunity to defined hours of the night. For example, a patient who reports getting 5 hours of sleep would be scheduled for 5 hours in bed. If his typical arising time is 7 am, he would not go to bed until 2 am. When his sleep log shows he has slept 90% of the time in bed for 5 consecutive nights, he can go to bed 15 to 30 minutes earlier. Over time, as this process is repeated, patients spend greater amounts of time sleeping while in bed.
Sleep restriction creates a degree of sleep deprivation that may enhance sleep onset and maintenance. Caution patients not to drive or perform hazardous activities while sleep-deprived.
8 Has the patient been prescribed appropriate doses of medications with appropriate indications?
Chronic insomnia sufferers often try to get more sleep by using alcohol, food supplement remedies, and OTC antihistamine sleep aids—none of which has demonstrated efficacy for treating insomnia. Although sedating prescription medications may be recommended for comorbid conditions, many also are prescribed off-label to promote sleep.
Examples include sedating antidepressants, antipsychotics, antihistamines, anticonvulsants, and benzodiazepines that are not indicated for insomnia. Little or no evidence supports these medications as safe and efficacious for treating insomnia, and important safety concerns are associated with their use.
The BZRA category includes 5 benzodiazepines and 4 nonbenzodiazepine formulations. Half-lives vary from approximately 1 hour to several days. Compared with benzodiazepines, nonbenzodiazepines have greater selectivity for GABAA receptor complexes incorporating the alpha-1 sub-unit subtype, which may confer some safety and tolerability advantages. One extended-release formulation is available. All may be beneficial for sleep onset, and some have indications for sleep maintenance difficulty.
Ramelteon is a nonsedating selective melatonin receptor agonist approved for treating insomnia characterized by sleep onset difficulty. This agent—which attenuates evening circadian arousal—may help promote sleep onset and enhance sleep during the early part of the night.
Administration. Inadequate dosing of insomnia medications may cause treatment to fail, but prescribing beyond approved ranges is rarely necessary. High sedative doses increase the risk of adverse effects, and patients may sleep no better. Adverse effects may include somnolence, headache, dizziness, nausea, diarrhea, and anterograde amnesia. Rarely patients may exhibit sleep walking or confused behaviors within a few hours after taking a hypnotic dose.
Recommendation. Customize your selection of FDA-approved insomnia medications. Consider whether your patient needs medication for sleep onset or sleep maintenance. In most cases, prescribe within dosing ranges listed in Table 3
Table 3
Insomnia treatment: FDA-approved medications
| Medication | Recommended dosage (mg) | Elimination half-life (hr) |
|---|---|---|
| Benzodiazepine receptor agonists | ||
| Immediate-release benzodiazepines | ||
| Estazolam | 1 to 2 | 8 to 24 |
| Flurazepam | 15 to 30 | 48 to 120 |
| Quazepam | 7.5 to 15 | 48 to 120 |
| Temazepam | 7.5 to 30 | 8 to 20 |
| Triazolam | 0.125 to 0.25 | 2 to 4 |
| Immediate-release nonbenzodiazepines | ||
| Eszopiclone | 1 to 3 | 5 to 7 |
| Zaleplon | 5 to 20 | 1 |
| Zolpidem | 5 to 10 | 1.5 to 2.4 |
| Extended-release nonbenzodiazepine | ||
| Zolpidem ER | 6.25 to 12.5 | 2.8 to 2.9 |
| Selective melatonin receptor agonist | ||
| Ramelteon | 8 | 1 to 2.6 |
Related Resources
- American Academy of Sleep Medicine. www.aasmnet.org.
- National Sleep Foundation. www.sleepfoundation.org.
- NIH National Center for Sleep Disorders Research. www.nhlbi.nih.gov/about/ncsdr.
Drug brand names
- Estazolam • ProSom
- Eszopiclone • Lunesta
- Flurazepam • Dalmane
- Quazepam • Doral
- Ramelteon • Rozerem
- Temazepam • Restoril
- Triazolam • Halcion
- Zaleplon • Sonata
- Zolpidem • Ambien
- Zolpidem ER • Ambien CR
Disclosure
Dr. Neubauer is a consultant to Neurocrine Biosciences, sanofi-aventis, and Takeda Pharmaceuticals North America and a speaker for sanofi-aventis and Takeda Pharmaceuticals North America.
1. National Institutes of Health. State of the Science Conference statement on manifestations and management of chronic insomnia in adults, June 13-15, 2005. Sleep 2005;28:1049-57.
2. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am 1987;10:541-53.
3. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000.
4. International Classification of Sleep Disorders: Diagnostic & Coding Manual, ICSD-2, 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005.
5. Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms 1999;14:557-68.
6. Richardson GS. The human circadian system in normal and disordered sleep. J Clin Psychiatry 2005;66:3-9.
7. Manthena P, Zee PC. Neurobiology of circadian rhythm sleep disorders. Curr Neurol Neurosci Rep 2006;6:163-8.
8. Zee PC, Manthena P. The brain’s master circadian clock: implications and opportunities for therapy of sleep disorders. Sleep Med Rev 2007;11(1):59-70.
9. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry 1994;151:1172-80.
10. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep 2006;29:1398-414.
Although many patients with insomnia respond to standard treatments, some continue to experience insufficient sleep. When your patient appears “treatment-resistant,” you may be tempted to add another therapy or try an unorthodox medication. But choosing an appropriate next treatment is impossible without first looking back for a rationale:
- Have you overlooked one of insomnia’s many causes?
- Have you customized treatment for this patient?
- Is he or she unaware of behaviors that may be undermining attempts to sleep?
Refreshing sleep may elude some thoroughly evaluated and optimally treated patients, but they comprise a small minority. You can help most chronic insomnia sufferers by re-evaluating their behaviors, comorbidities, sleep-wake cycles, and medications (Table 1).
Table 1
Recommended approach to treatment-resistant insomnia
| Evaluation |
| Review your patient’s 24-hour sleep cycle, sleepiness, and sleeplessness, and note persistent patterns (a sleep log or diary may help) |
| Re-evaluate stimulating or sedating effects of prescribed and over-the-counter medications, caffeine, and alcohol |
Consider:
|
| Monitor insomnia-related daytime symptoms as key outcome measure |
| Treatment |
| Re-address sleep hygiene (Table 2) |
| Consider cognitive behavioral therapy for insomnia |
| Consider an FDA-approved medication for insomnia (Table 3), customized to your patient’s symptoms |
‘3 Ps’ and 8 questions
Thirty percent of adults experience insomnia at least occasionally, and 10% have persistent insomnia. Women, older persons, and patients with chronic medical conditions such as diabetes mellitus and lung disease have higher insomnia rates than the general population.1
An enormous variety of psychological and physiologic processes may influence sleep (Box 1). Multiple factors may contribute to an individual’s inability to achieve sufficient sleep, and the relative significance of these influences can shift over time. Factors that might trigger an insomnia episode are not necessarily those that maintain sleeplessness.
1 Does the patient have realistic goals for falling asleep and remaining asleep?
Patients view insomnia as being unable to sleep when they believe they should be sleeping. To be diagnosed as a disorder, insomnia must have daytime consequences associated with:
- difficulty falling asleep
- difficulty maintaining sleep
- awakening excessively early
- or experiencing nonrestorative sleep.
Recommendation. Determine how the patient defines “having insomnia” (there are no absolute thresholds). Ask how he or she is functioning during the day. Those who complain of imperfect nighttime sleep may admit that treatment has helped with the daytime symptoms that prompted them to seek treatment.
If daytime symptoms have diminished, reassure the patient that treatment apparently is helping. Patients are less likely to focus on perceived nighttime impairment when their distress about daytime functioning has eased.
Also determine if the patient has followed recommended treatment. Cognitive-behavioral therapy (CBT) may increase adherence to behavioral changes, sleep hygiene, and medication schedules.
2 Have I identified and optimally managed comorbidities?
Identifying comorbidities that may contribute to chronic insomnia is particularly important because managing these conditions may alleviate the sleep disturbance. Pain or discomfort caused by a medical condition may undermine sleep quality. Certain cardiovascular, pulmonary, endocrine, neurologic, rheumatologic, and orthopedic disorders are associated with insomnia.
Most patients experiencing exacerbations of mood and anxiety disorders suffer insomnia, and many other psychiatric disorders are associated with sleep disruption.
Diagnostic subtypes recognized by the American Academy of Sleep Medicine may suggest why recommended treatments have not relieved a patient’s symptoms. Insomnia may be:
- due to a mental disorder, medical condition, drug or substance
- adjustment-related (acute insomnia), psychophysiologic, paradoxical, or idiopathic
- related to inadequate sleep hygiene
- a behavioral characteristic of childhood
- organic (due to an unspecified physiologic condition)
- nonorganic, NOS (not due to a substance or known physiologic condition).
NOS: not otherwise specified
Insomnia often accompanies substance abuse and may continue after the patient stops abusing drugs or alcohol. Abused stimulants and sedatives can worsen sleep quality, and discontinuation can cause acute and chronic sleep disruption.
Recommendation. Treat mood and anxiety disorders independently of insomnia. Minimize pain and discomfort from medical conditions. Address substance abuse, and dispel patients’ notion that alcohol is a sleep aid.
Order sleep laboratory testing for patients at risk for sleep apnea, based on their history, physical exam—including obesity, upper airway anatomy, and neck circumference (collar size ≥17 inches)—and informant reports of snoring and breathing patterns.
3 Is the patient taking medications with stimulating effects?
Because insomnia is highly comorbid with mood and anxiety disorders, patients with insomnia often are prescribed antidepressants. Although some are sedating, antidepressants such as selective serotonin reuptake inhibitors are likely to be stimulating.
Recommendation. When insomnia persists, assess the potential effects of prescribed and over-the-counter (OTC) medications. Consider possible pharmacologic effects of aging that can make patients more sensitive to medications.
Also educate patients about the long-acting effects of caffeine and its varied sources, such as energy drinks and OTC products. Some patients will benefit from completely avoiding caffeine, whereas others may do fine restricting coffee to 1 or 2 cups in the morning. A good general practice is to avoid all caffeine after lunchtime.
Predisposing factors. Some personalities may be predisposed to insomnia. Persons who tend to be anxious, depressive, or emotionally reactive may be at increased risk for developing insomnia.
Precipitating factors may include situational crises, schedule changes, substance or medication use, and psychiatric, medical, and sleep disorders. A careful history allows you to consider precipitating events.
Perpetuating factors that may reinforce and maintain chronic insomnia include:
- maladaptive behaviors, such as napping or using alcohol as a sleep aid
- conditioned hyperarousal, whereby insomnia sufferers experience anxiety and tension associated with preparing for and getting into bed. Sleepless time in bed may reinforce the conditioning, contribute to anxiety and tension, and undermine sleep on future nights.
Source: Reference 2
4 Does the patient’s insomnia have a homeostatic component?
Circadian rhythms and a homeostatic sleep drive are temporally linked in regulating the normal routine of nighttime sleep alternating with day and evening wakefulness.5,6 The sleep drive promotes a sleep-to-waking ratio of approximately 1:2 (an average of 8 hours sleep per 24 hours). Adequate sleep, from the homeostatic perspective, could be achieved during any hours of the day or night.
Acute sleep deprivation may result from extended wakefulness—such as staying up all night to study for an exam. Chronic sleep deprivation may occur during successive 24-hour periods with insufficient sleep. Both patterns are associated with increasing subjective sleepiness and ultimately with cognitive impairment.
- is low throughout the daytime
- rises during the evening as bedtime approaches
- plateaus during nighttime sleep hours
- decreases as the normal morning wake time approaches.
Typically, people are more alert in the evening than at any other time in the 24-hour cycle. As bedtime approaches, rising melatonin interacts with SCN melatonin receptors and decreases circadian arousal. Normal sleep onset then can occur rapidly at bedtime, when the homeostatic sleep drive is unopposed.
Nighttime sleep initially is promoted by the homeostatic sleep drive. However, the homeostatic sleep pressure is reversed by sleep and thus decreases as sleep continues during the night. The circadian system promotes minimum stimulation during the latter sleep hours, sustaining total sleep for approximately 8 hours.
Consequences. Individual circadian timing tendencies may affect when people experience alertness and sleepiness and may be associated with persistent complaints of sleep onset difficulty or early morning awakening.7 Napping may reduce the homeostatic sleepiness available to aid bedtime sleep onset. Mismatched homeostatic and circadian processes often prevent shift workers from achieving satisfactory sleep.
Recommendation. Have the patient keep a sleep log to identify the time and duration of sleep episodes throughout the 24-hour cycle. Actigraphy may provide useful information about sleep-wake patterns.
5 Are circadian rhythm patterns contributing to insomnia?
Overlooking circadian rhythms’ effects on insomnia can lead to apparent treatment failure.8 Although the circadian system typically promotes sleep from about 10 pm to midnight until about 6 to 8 am, some individuals have long-standing predispositions for earlier or later sleep episodes.
An advanced circadian phase leads to sleepiness and the ability to fall asleep early in the evening, followed by a tendency to awaken spontaneously relatively early in the morning. In extreme cases, patients with these “lark” tendencies may be diagnosed with advanced sleep phase disorder. Persistent early morning awakening insomnia and sleep maintenance complaints are common.
A delayed circadian phase is associated with inability to fall asleep at a typical late evening bedtime and difficulty awakening at a desired time the following morning. In extreme cases, individuals may sleep from very late at night until the following afternoon. These markedly delayed schedules may be obvious, but the circadian contribution may not be recognized in less severe cases.
People with this predisposition may achieve optimum sleep by following their delayed circadian tendency, but school and work demands often conflict with this approach. They may develop chronic sleep deprivation from late sleep onset coupled with forced morning awakenings. Complaints of chronic difficulty with sleep onset are common.
Recommendation. Have the patient keep a sleep log to demonstrate advanced or delayed circadian phase tendencies. Determine if the patient is a shift worker who is attempting to sleep in the daytime. Consider prescribing ramelteon—a melatonin agonist—and providing strategic bright light exposure:
- in the evening for advanced circadian phase patients
- in the morning for delayed circadian phase patients.8
6 Is the patient following appropriate sleep hygiene?
Sleep hygiene will not necessarily cure chronic insomnia, but inattention to basic guidelines (Table 2) can undermine other treatments. When re-evaluating patients with chronic insomnia, give special attention to their alcohol and caffeine intake, regularity of bedtime and wake-up times, meal times, and the bedroom environment. Advise patients to remove televisions from the bedroom, for example.
CBT that is effective for chronic insomnia typically blends sleep hygiene with education, cognitive psychotherapy, and specific instructions regarding bedtime schedules.9,10 Relaxation techniques also may be beneficial.
Consider consulting with a sleep specialist if the patient has not been evaluated at a sleep center. Some sleep centers offer CBT.
Table 2
Patient education: Sleep hygiene guidelines
| Try to maintain a regular sleep–wake schedule |
| Avoid afternoon or evening napping |
| Allow yourself enough time in bed for adequate sleep duration (such as 11 PM to 7 AM) |
| Develop a relaxing evening routine for the hours before bedtime |
| Spend some idle time reflecting on the day’s events before going to bed; make a list of concerns and how some might be resolved |
| Reserve the bed for sleep and sex; do not do homework, pay bills, watch TV, or engage in serious domestic discussions in bed |
| Avoid alcohol in the evening |
| Avoid caffeine in the afternoon and evening |
| Minimize annoying noise, light, or temperature extremes |
| Consider a light snack before bedtime |
| Exercise regularly, but not late in the evening |
| Do not try harder and harder to fall asleep; if you can’t sleep, get out of bed and do something else, in another room if possible |
| Avoid smoking |
7 Does the patient regularly experience anxiety and tension as bedtime approaches or spend excessive wakeful time in bed?
Patients who tend to be anxious, depressive, or emotionally reactive are at increased risk for developing an insomnia episode. They then may develop conditioned hyperarousal associated with preparing for and getting into bed, which perpetuates insomnia.
Some patients spend long periods in bed, hoping to achieve any possible sleep that night. Extended time in bed can perpetuate insomnia by increasing frustrating time awake, thereby reinforcing the association between the bed and wakefulness.
Recommendation. CBT often helps ease these conditioned responses.
Stimulus control can help anxious individuals reassociate the bed, bedroom, and bedtime routines with sleep onset, rather than sleep-destructive tension. Advise patients to go to bed in the evening when they feel they can fall asleep. If they do not fall asleep within 10 to 15 minutes or experience their usual worry and frustration about not sleeping, instruct them to leave the bed and try again later. Also tell them to avoid daytime napping.
Sleep restriction therapy may help patients with excessive wakefulness in bed by limiting sleep opportunity to defined hours of the night. For example, a patient who reports getting 5 hours of sleep would be scheduled for 5 hours in bed. If his typical arising time is 7 am, he would not go to bed until 2 am. When his sleep log shows he has slept 90% of the time in bed for 5 consecutive nights, he can go to bed 15 to 30 minutes earlier. Over time, as this process is repeated, patients spend greater amounts of time sleeping while in bed.
Sleep restriction creates a degree of sleep deprivation that may enhance sleep onset and maintenance. Caution patients not to drive or perform hazardous activities while sleep-deprived.
8 Has the patient been prescribed appropriate doses of medications with appropriate indications?
Chronic insomnia sufferers often try to get more sleep by using alcohol, food supplement remedies, and OTC antihistamine sleep aids—none of which has demonstrated efficacy for treating insomnia. Although sedating prescription medications may be recommended for comorbid conditions, many also are prescribed off-label to promote sleep.
Examples include sedating antidepressants, antipsychotics, antihistamines, anticonvulsants, and benzodiazepines that are not indicated for insomnia. Little or no evidence supports these medications as safe and efficacious for treating insomnia, and important safety concerns are associated with their use.
The BZRA category includes 5 benzodiazepines and 4 nonbenzodiazepine formulations. Half-lives vary from approximately 1 hour to several days. Compared with benzodiazepines, nonbenzodiazepines have greater selectivity for GABAA receptor complexes incorporating the alpha-1 sub-unit subtype, which may confer some safety and tolerability advantages. One extended-release formulation is available. All may be beneficial for sleep onset, and some have indications for sleep maintenance difficulty.
Ramelteon is a nonsedating selective melatonin receptor agonist approved for treating insomnia characterized by sleep onset difficulty. This agent—which attenuates evening circadian arousal—may help promote sleep onset and enhance sleep during the early part of the night.
Administration. Inadequate dosing of insomnia medications may cause treatment to fail, but prescribing beyond approved ranges is rarely necessary. High sedative doses increase the risk of adverse effects, and patients may sleep no better. Adverse effects may include somnolence, headache, dizziness, nausea, diarrhea, and anterograde amnesia. Rarely patients may exhibit sleep walking or confused behaviors within a few hours after taking a hypnotic dose.
Recommendation. Customize your selection of FDA-approved insomnia medications. Consider whether your patient needs medication for sleep onset or sleep maintenance. In most cases, prescribe within dosing ranges listed in Table 3
Table 3
Insomnia treatment: FDA-approved medications
| Medication | Recommended dosage (mg) | Elimination half-life (hr) |
|---|---|---|
| Benzodiazepine receptor agonists | ||
| Immediate-release benzodiazepines | ||
| Estazolam | 1 to 2 | 8 to 24 |
| Flurazepam | 15 to 30 | 48 to 120 |
| Quazepam | 7.5 to 15 | 48 to 120 |
| Temazepam | 7.5 to 30 | 8 to 20 |
| Triazolam | 0.125 to 0.25 | 2 to 4 |
| Immediate-release nonbenzodiazepines | ||
| Eszopiclone | 1 to 3 | 5 to 7 |
| Zaleplon | 5 to 20 | 1 |
| Zolpidem | 5 to 10 | 1.5 to 2.4 |
| Extended-release nonbenzodiazepine | ||
| Zolpidem ER | 6.25 to 12.5 | 2.8 to 2.9 |
| Selective melatonin receptor agonist | ||
| Ramelteon | 8 | 1 to 2.6 |
Related Resources
- American Academy of Sleep Medicine. www.aasmnet.org.
- National Sleep Foundation. www.sleepfoundation.org.
- NIH National Center for Sleep Disorders Research. www.nhlbi.nih.gov/about/ncsdr.
Drug brand names
- Estazolam • ProSom
- Eszopiclone • Lunesta
- Flurazepam • Dalmane
- Quazepam • Doral
- Ramelteon • Rozerem
- Temazepam • Restoril
- Triazolam • Halcion
- Zaleplon • Sonata
- Zolpidem • Ambien
- Zolpidem ER • Ambien CR
Disclosure
Dr. Neubauer is a consultant to Neurocrine Biosciences, sanofi-aventis, and Takeda Pharmaceuticals North America and a speaker for sanofi-aventis and Takeda Pharmaceuticals North America.
Although many patients with insomnia respond to standard treatments, some continue to experience insufficient sleep. When your patient appears “treatment-resistant,” you may be tempted to add another therapy or try an unorthodox medication. But choosing an appropriate next treatment is impossible without first looking back for a rationale:
- Have you overlooked one of insomnia’s many causes?
- Have you customized treatment for this patient?
- Is he or she unaware of behaviors that may be undermining attempts to sleep?
Refreshing sleep may elude some thoroughly evaluated and optimally treated patients, but they comprise a small minority. You can help most chronic insomnia sufferers by re-evaluating their behaviors, comorbidities, sleep-wake cycles, and medications (Table 1).
Table 1
Recommended approach to treatment-resistant insomnia
| Evaluation |
| Review your patient’s 24-hour sleep cycle, sleepiness, and sleeplessness, and note persistent patterns (a sleep log or diary may help) |
| Re-evaluate stimulating or sedating effects of prescribed and over-the-counter medications, caffeine, and alcohol |
Consider:
|
| Monitor insomnia-related daytime symptoms as key outcome measure |
| Treatment |
| Re-address sleep hygiene (Table 2) |
| Consider cognitive behavioral therapy for insomnia |
| Consider an FDA-approved medication for insomnia (Table 3), customized to your patient’s symptoms |
‘3 Ps’ and 8 questions
Thirty percent of adults experience insomnia at least occasionally, and 10% have persistent insomnia. Women, older persons, and patients with chronic medical conditions such as diabetes mellitus and lung disease have higher insomnia rates than the general population.1
An enormous variety of psychological and physiologic processes may influence sleep (Box 1). Multiple factors may contribute to an individual’s inability to achieve sufficient sleep, and the relative significance of these influences can shift over time. Factors that might trigger an insomnia episode are not necessarily those that maintain sleeplessness.
1 Does the patient have realistic goals for falling asleep and remaining asleep?
Patients view insomnia as being unable to sleep when they believe they should be sleeping. To be diagnosed as a disorder, insomnia must have daytime consequences associated with:
- difficulty falling asleep
- difficulty maintaining sleep
- awakening excessively early
- or experiencing nonrestorative sleep.
Recommendation. Determine how the patient defines “having insomnia” (there are no absolute thresholds). Ask how he or she is functioning during the day. Those who complain of imperfect nighttime sleep may admit that treatment has helped with the daytime symptoms that prompted them to seek treatment.
If daytime symptoms have diminished, reassure the patient that treatment apparently is helping. Patients are less likely to focus on perceived nighttime impairment when their distress about daytime functioning has eased.
Also determine if the patient has followed recommended treatment. Cognitive-behavioral therapy (CBT) may increase adherence to behavioral changes, sleep hygiene, and medication schedules.
2 Have I identified and optimally managed comorbidities?
Identifying comorbidities that may contribute to chronic insomnia is particularly important because managing these conditions may alleviate the sleep disturbance. Pain or discomfort caused by a medical condition may undermine sleep quality. Certain cardiovascular, pulmonary, endocrine, neurologic, rheumatologic, and orthopedic disorders are associated with insomnia.
Most patients experiencing exacerbations of mood and anxiety disorders suffer insomnia, and many other psychiatric disorders are associated with sleep disruption.
Diagnostic subtypes recognized by the American Academy of Sleep Medicine may suggest why recommended treatments have not relieved a patient’s symptoms. Insomnia may be:
- due to a mental disorder, medical condition, drug or substance
- adjustment-related (acute insomnia), psychophysiologic, paradoxical, or idiopathic
- related to inadequate sleep hygiene
- a behavioral characteristic of childhood
- organic (due to an unspecified physiologic condition)
- nonorganic, NOS (not due to a substance or known physiologic condition).
NOS: not otherwise specified
Insomnia often accompanies substance abuse and may continue after the patient stops abusing drugs or alcohol. Abused stimulants and sedatives can worsen sleep quality, and discontinuation can cause acute and chronic sleep disruption.
Recommendation. Treat mood and anxiety disorders independently of insomnia. Minimize pain and discomfort from medical conditions. Address substance abuse, and dispel patients’ notion that alcohol is a sleep aid.
Order sleep laboratory testing for patients at risk for sleep apnea, based on their history, physical exam—including obesity, upper airway anatomy, and neck circumference (collar size ≥17 inches)—and informant reports of snoring and breathing patterns.
3 Is the patient taking medications with stimulating effects?
Because insomnia is highly comorbid with mood and anxiety disorders, patients with insomnia often are prescribed antidepressants. Although some are sedating, antidepressants such as selective serotonin reuptake inhibitors are likely to be stimulating.
Recommendation. When insomnia persists, assess the potential effects of prescribed and over-the-counter (OTC) medications. Consider possible pharmacologic effects of aging that can make patients more sensitive to medications.
Also educate patients about the long-acting effects of caffeine and its varied sources, such as energy drinks and OTC products. Some patients will benefit from completely avoiding caffeine, whereas others may do fine restricting coffee to 1 or 2 cups in the morning. A good general practice is to avoid all caffeine after lunchtime.
Predisposing factors. Some personalities may be predisposed to insomnia. Persons who tend to be anxious, depressive, or emotionally reactive may be at increased risk for developing insomnia.
Precipitating factors may include situational crises, schedule changes, substance or medication use, and psychiatric, medical, and sleep disorders. A careful history allows you to consider precipitating events.
Perpetuating factors that may reinforce and maintain chronic insomnia include:
- maladaptive behaviors, such as napping or using alcohol as a sleep aid
- conditioned hyperarousal, whereby insomnia sufferers experience anxiety and tension associated with preparing for and getting into bed. Sleepless time in bed may reinforce the conditioning, contribute to anxiety and tension, and undermine sleep on future nights.
Source: Reference 2
4 Does the patient’s insomnia have a homeostatic component?
Circadian rhythms and a homeostatic sleep drive are temporally linked in regulating the normal routine of nighttime sleep alternating with day and evening wakefulness.5,6 The sleep drive promotes a sleep-to-waking ratio of approximately 1:2 (an average of 8 hours sleep per 24 hours). Adequate sleep, from the homeostatic perspective, could be achieved during any hours of the day or night.
Acute sleep deprivation may result from extended wakefulness—such as staying up all night to study for an exam. Chronic sleep deprivation may occur during successive 24-hour periods with insufficient sleep. Both patterns are associated with increasing subjective sleepiness and ultimately with cognitive impairment.
- is low throughout the daytime
- rises during the evening as bedtime approaches
- plateaus during nighttime sleep hours
- decreases as the normal morning wake time approaches.
Typically, people are more alert in the evening than at any other time in the 24-hour cycle. As bedtime approaches, rising melatonin interacts with SCN melatonin receptors and decreases circadian arousal. Normal sleep onset then can occur rapidly at bedtime, when the homeostatic sleep drive is unopposed.
Nighttime sleep initially is promoted by the homeostatic sleep drive. However, the homeostatic sleep pressure is reversed by sleep and thus decreases as sleep continues during the night. The circadian system promotes minimum stimulation during the latter sleep hours, sustaining total sleep for approximately 8 hours.
Consequences. Individual circadian timing tendencies may affect when people experience alertness and sleepiness and may be associated with persistent complaints of sleep onset difficulty or early morning awakening.7 Napping may reduce the homeostatic sleepiness available to aid bedtime sleep onset. Mismatched homeostatic and circadian processes often prevent shift workers from achieving satisfactory sleep.
Recommendation. Have the patient keep a sleep log to identify the time and duration of sleep episodes throughout the 24-hour cycle. Actigraphy may provide useful information about sleep-wake patterns.
5 Are circadian rhythm patterns contributing to insomnia?
Overlooking circadian rhythms’ effects on insomnia can lead to apparent treatment failure.8 Although the circadian system typically promotes sleep from about 10 pm to midnight until about 6 to 8 am, some individuals have long-standing predispositions for earlier or later sleep episodes.
An advanced circadian phase leads to sleepiness and the ability to fall asleep early in the evening, followed by a tendency to awaken spontaneously relatively early in the morning. In extreme cases, patients with these “lark” tendencies may be diagnosed with advanced sleep phase disorder. Persistent early morning awakening insomnia and sleep maintenance complaints are common.
A delayed circadian phase is associated with inability to fall asleep at a typical late evening bedtime and difficulty awakening at a desired time the following morning. In extreme cases, individuals may sleep from very late at night until the following afternoon. These markedly delayed schedules may be obvious, but the circadian contribution may not be recognized in less severe cases.
People with this predisposition may achieve optimum sleep by following their delayed circadian tendency, but school and work demands often conflict with this approach. They may develop chronic sleep deprivation from late sleep onset coupled with forced morning awakenings. Complaints of chronic difficulty with sleep onset are common.
Recommendation. Have the patient keep a sleep log to demonstrate advanced or delayed circadian phase tendencies. Determine if the patient is a shift worker who is attempting to sleep in the daytime. Consider prescribing ramelteon—a melatonin agonist—and providing strategic bright light exposure:
- in the evening for advanced circadian phase patients
- in the morning for delayed circadian phase patients.8
6 Is the patient following appropriate sleep hygiene?
Sleep hygiene will not necessarily cure chronic insomnia, but inattention to basic guidelines (Table 2) can undermine other treatments. When re-evaluating patients with chronic insomnia, give special attention to their alcohol and caffeine intake, regularity of bedtime and wake-up times, meal times, and the bedroom environment. Advise patients to remove televisions from the bedroom, for example.
CBT that is effective for chronic insomnia typically blends sleep hygiene with education, cognitive psychotherapy, and specific instructions regarding bedtime schedules.9,10 Relaxation techniques also may be beneficial.
Consider consulting with a sleep specialist if the patient has not been evaluated at a sleep center. Some sleep centers offer CBT.
Table 2
Patient education: Sleep hygiene guidelines
| Try to maintain a regular sleep–wake schedule |
| Avoid afternoon or evening napping |
| Allow yourself enough time in bed for adequate sleep duration (such as 11 PM to 7 AM) |
| Develop a relaxing evening routine for the hours before bedtime |
| Spend some idle time reflecting on the day’s events before going to bed; make a list of concerns and how some might be resolved |
| Reserve the bed for sleep and sex; do not do homework, pay bills, watch TV, or engage in serious domestic discussions in bed |
| Avoid alcohol in the evening |
| Avoid caffeine in the afternoon and evening |
| Minimize annoying noise, light, or temperature extremes |
| Consider a light snack before bedtime |
| Exercise regularly, but not late in the evening |
| Do not try harder and harder to fall asleep; if you can’t sleep, get out of bed and do something else, in another room if possible |
| Avoid smoking |
7 Does the patient regularly experience anxiety and tension as bedtime approaches or spend excessive wakeful time in bed?
Patients who tend to be anxious, depressive, or emotionally reactive are at increased risk for developing an insomnia episode. They then may develop conditioned hyperarousal associated with preparing for and getting into bed, which perpetuates insomnia.
Some patients spend long periods in bed, hoping to achieve any possible sleep that night. Extended time in bed can perpetuate insomnia by increasing frustrating time awake, thereby reinforcing the association between the bed and wakefulness.
Recommendation. CBT often helps ease these conditioned responses.
Stimulus control can help anxious individuals reassociate the bed, bedroom, and bedtime routines with sleep onset, rather than sleep-destructive tension. Advise patients to go to bed in the evening when they feel they can fall asleep. If they do not fall asleep within 10 to 15 minutes or experience their usual worry and frustration about not sleeping, instruct them to leave the bed and try again later. Also tell them to avoid daytime napping.
Sleep restriction therapy may help patients with excessive wakefulness in bed by limiting sleep opportunity to defined hours of the night. For example, a patient who reports getting 5 hours of sleep would be scheduled for 5 hours in bed. If his typical arising time is 7 am, he would not go to bed until 2 am. When his sleep log shows he has slept 90% of the time in bed for 5 consecutive nights, he can go to bed 15 to 30 minutes earlier. Over time, as this process is repeated, patients spend greater amounts of time sleeping while in bed.
Sleep restriction creates a degree of sleep deprivation that may enhance sleep onset and maintenance. Caution patients not to drive or perform hazardous activities while sleep-deprived.
8 Has the patient been prescribed appropriate doses of medications with appropriate indications?
Chronic insomnia sufferers often try to get more sleep by using alcohol, food supplement remedies, and OTC antihistamine sleep aids—none of which has demonstrated efficacy for treating insomnia. Although sedating prescription medications may be recommended for comorbid conditions, many also are prescribed off-label to promote sleep.
Examples include sedating antidepressants, antipsychotics, antihistamines, anticonvulsants, and benzodiazepines that are not indicated for insomnia. Little or no evidence supports these medications as safe and efficacious for treating insomnia, and important safety concerns are associated with their use.
The BZRA category includes 5 benzodiazepines and 4 nonbenzodiazepine formulations. Half-lives vary from approximately 1 hour to several days. Compared with benzodiazepines, nonbenzodiazepines have greater selectivity for GABAA receptor complexes incorporating the alpha-1 sub-unit subtype, which may confer some safety and tolerability advantages. One extended-release formulation is available. All may be beneficial for sleep onset, and some have indications for sleep maintenance difficulty.
Ramelteon is a nonsedating selective melatonin receptor agonist approved for treating insomnia characterized by sleep onset difficulty. This agent—which attenuates evening circadian arousal—may help promote sleep onset and enhance sleep during the early part of the night.
Administration. Inadequate dosing of insomnia medications may cause treatment to fail, but prescribing beyond approved ranges is rarely necessary. High sedative doses increase the risk of adverse effects, and patients may sleep no better. Adverse effects may include somnolence, headache, dizziness, nausea, diarrhea, and anterograde amnesia. Rarely patients may exhibit sleep walking or confused behaviors within a few hours after taking a hypnotic dose.
Recommendation. Customize your selection of FDA-approved insomnia medications. Consider whether your patient needs medication for sleep onset or sleep maintenance. In most cases, prescribe within dosing ranges listed in Table 3
Table 3
Insomnia treatment: FDA-approved medications
| Medication | Recommended dosage (mg) | Elimination half-life (hr) |
|---|---|---|
| Benzodiazepine receptor agonists | ||
| Immediate-release benzodiazepines | ||
| Estazolam | 1 to 2 | 8 to 24 |
| Flurazepam | 15 to 30 | 48 to 120 |
| Quazepam | 7.5 to 15 | 48 to 120 |
| Temazepam | 7.5 to 30 | 8 to 20 |
| Triazolam | 0.125 to 0.25 | 2 to 4 |
| Immediate-release nonbenzodiazepines | ||
| Eszopiclone | 1 to 3 | 5 to 7 |
| Zaleplon | 5 to 20 | 1 |
| Zolpidem | 5 to 10 | 1.5 to 2.4 |
| Extended-release nonbenzodiazepine | ||
| Zolpidem ER | 6.25 to 12.5 | 2.8 to 2.9 |
| Selective melatonin receptor agonist | ||
| Ramelteon | 8 | 1 to 2.6 |
Related Resources
- American Academy of Sleep Medicine. www.aasmnet.org.
- National Sleep Foundation. www.sleepfoundation.org.
- NIH National Center for Sleep Disorders Research. www.nhlbi.nih.gov/about/ncsdr.
Drug brand names
- Estazolam • ProSom
- Eszopiclone • Lunesta
- Flurazepam • Dalmane
- Quazepam • Doral
- Ramelteon • Rozerem
- Temazepam • Restoril
- Triazolam • Halcion
- Zaleplon • Sonata
- Zolpidem • Ambien
- Zolpidem ER • Ambien CR
Disclosure
Dr. Neubauer is a consultant to Neurocrine Biosciences, sanofi-aventis, and Takeda Pharmaceuticals North America and a speaker for sanofi-aventis and Takeda Pharmaceuticals North America.
1. National Institutes of Health. State of the Science Conference statement on manifestations and management of chronic insomnia in adults, June 13-15, 2005. Sleep 2005;28:1049-57.
2. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am 1987;10:541-53.
3. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000.
4. International Classification of Sleep Disorders: Diagnostic & Coding Manual, ICSD-2, 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005.
5. Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms 1999;14:557-68.
6. Richardson GS. The human circadian system in normal and disordered sleep. J Clin Psychiatry 2005;66:3-9.
7. Manthena P, Zee PC. Neurobiology of circadian rhythm sleep disorders. Curr Neurol Neurosci Rep 2006;6:163-8.
8. Zee PC, Manthena P. The brain’s master circadian clock: implications and opportunities for therapy of sleep disorders. Sleep Med Rev 2007;11(1):59-70.
9. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry 1994;151:1172-80.
10. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep 2006;29:1398-414.
1. National Institutes of Health. State of the Science Conference statement on manifestations and management of chronic insomnia in adults, June 13-15, 2005. Sleep 2005;28:1049-57.
2. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am 1987;10:541-53.
3. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000.
4. International Classification of Sleep Disorders: Diagnostic & Coding Manual, ICSD-2, 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005.
5. Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms 1999;14:557-68.
6. Richardson GS. The human circadian system in normal and disordered sleep. J Clin Psychiatry 2005;66:3-9.
7. Manthena P, Zee PC. Neurobiology of circadian rhythm sleep disorders. Curr Neurol Neurosci Rep 2006;6:163-8.
8. Zee PC, Manthena P. The brain’s master circadian clock: implications and opportunities for therapy of sleep disorders. Sleep Med Rev 2007;11(1):59-70.
9. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry 1994;151:1172-80.
10. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep 2006;29:1398-414.
Video games: When does play become pathology?
Nick, age 13, enjoys playing video games, but his parents think he may be “addicted.” His primary care doctor has referred Nick to you for evaluation.
Nick has played video games since age 7 and likes to share ideas with friends about to “beat” difficult games. Lately, though, he plays an online role-playing game, mostly alone, on the computer in his bedroom. Nick hasn’t seen his friends outside of school for 6 weeks.
Nick’s parents say he is growing short-tempered, and his grades have fallen for several months. He seems to worry a lot but becomes angry and storms out of the room when they try to talk with him about it.
Like Nick, 70% to 90% of American youths play video games, according to the American Medical Association (AMA).1 Most boys and girls find the games fun, entertaining, or relaxing (Table 1) and do not encounter difficulties as a result of their play.2 In some cases, however, they may:
- spend excessive time playing video games
- model inappropriate behavior from games
- over-invest in online relationships.
This article describes developmentally appropriate characteristics of play in general—and aspects of video game play in particular—to help you educate families about normative and excessive video game play.
Table 1
Top 10 reasons why children say they play video games
| Boys |
|
| Girls |
|
| * Response likely reflects the number of survey respondents living in a suburban/rural environment in which hunting is a popular leisure activity. |
| Source: Reference 1 |
An addiction?
Originally researchers believed video game play was not addictive and viewed excessive play as high engagement. More recently, efforts are being made to understand:
- how to classify excessive video game play that impairs psychosocial adjustment
- whether substance abuse models are appropriate for describing and treating pathologic video game play.
What is normative play?
Play is a motivating way for children to make sense of the world. By re-creating themes, relationships, places, or events in play children can control things that outside of play might be intimidating or overwhelming. Through play, children can explore situations in a setting that feels safe.4,5 Video games offer children play opportunities to explore roles and worlds that otherwise are unavailable to them.6
Video game play is one of the most popular leisure-time activities for middle-school students. Our group7 recently surveyed >1,200 students age 12 to 15 about their video game play and found:
- One-third of boys and two-thirds of girls played video games for ≤2 hours/week.
- One-third of boys and 11% of girls played video games 6 or 7 days each week.
- Boys played more than girls, with 45% of boys playing for ≥6 hours/week.
- 12.6% of boys played ≥15 hours/week.
- One-half listed ≥1 games rated M for mature (Table 2)7 among 5 games they played most frequently in the preceding 6 months.2
Pathologic behavior. Excessive video game playing can be viewed as pathologic if it involves an overwhelming need to play video games, with negative feelings and behaviors related to this need that lead to distress or functional impairment.9,10 Charlton et al
11 define pathologic video game play as incorporating high engagement plus core addiction characteristics such as interference with work or social life, failure to sleep, etc. In video game play, peripheral DSM addiction characteristics—such as high cognitive salience—may indicate high engagement. Characteristics of pathologic video game play, as identified by this group, are listed in Table 3.11
Table 2
ESRB video game ratings system and content descriptions*
| Rating | Content may be suitable for: | Examples |
|---|---|---|
| Early childhood | Age 3 and older; no material that parents would find inappropriate | Atari/others’ Dora the Explorer (series), Knowledge Adventure/Vivendi Universal’s Jump start (series) |
| Everyone | Age 6 and older; minimal cartoon, fantasy, or mild violence and/or infrequent use of mild language | Disney Interactive Studios/Buena Vista Games’ Hannah Montana (series), Taito Corporation’s Bubble Bobble |
| Everyone 10+ | Age 10 and older; more cartoon, fantasy, or mild violence, mild language and/or minimal suggestive themes | Electronic Arts’ Need for Speed: ProStreet, Ubisoft’s Rayman Raving Rabbids 2 |
| Teen | Age 13 and older; may contain violence, suggestive themes, crude humor, minimal blood, simulated gambling, and/or infrequent use of strong language | Midway Amusement Games’ Lord of the Rings Online: Shadows of Angmar (MMO), Sony Online Entertainment’s EverQuest (series; MMO) |
| Mature | Age 17 and older; may contain intense violence, blood and gore, sexual content, and/or strong language | Microsoft Corporation’s Halo (series), Rockstar Games’ Grand Theft Auto (most games in the series) |
| Adults only | Age 18 and older; may include prolonged scenes of intense violence and/or graphic sexual content and nudity | Vivendi Universal’s Leisure Suit Larry: Magna Cum Laude Uncut and Uncensored, Rockstar Games’ Grand Theft Auto: San Andreas |
| * On video game boxes, look for rating symbols on the front and content descriptions on the back. | ||
| ESRB: Entertainment Software Rating Board | ||
| MMO: massively-multiplayer online role-playing game | ||
| Source: Reference 7 | ||
Characteristics of ‘pathologic’ video game play
| Feeling agitated when not playing |
| Feeling “addicted” to play |
| Not being able to decrease time spent playing |
| Not sleeping because of video game play |
| Missing meals because of video game play |
| Being late because of video game play |
| Having arguments at home because of video game play |
| Letting video game play interfere with social relationships |
| Letting video game play interfere with schoolwork |
| Spending excessive amounts of money on video game play |
| Source: Reference 11 |
CASE CONTINUED: Going with the ‘flow’
Nick says he enjoys playing with people he’s met through a massively-multiplayer online role-playing game (MMORG, or also called MMO or MMP). The “guild” he has joined is a small community that collaborates to complete quests in the game. Nick describes his character—a healer—as a key figure who supports fellow players by replenishing their in-game health. Everyone in the guild thinks he’s important, and he likes to feel respected. Nick says this is quite different from how people treat him in “real” life. He says he often feels worthless and scared that his friends and family don’t think he’s good enough.
Video game play facilitates the experience of “flow”—a mental state of positive energy and effortless focus experienced while immersed in an activity over which one feels a sense of control. Video game play incorporates components of a flow experience (Table 4), including clear, focused goals that are attainable yet challenging and require a high level of concentration. Individuals who engage in artistic, athletic, or meditative activities often report experiencing flow.12
Flow can distort one’s sense of time, setting the stage for frustration on both sides when parents want their video game-playing child to engage in other activities. Their efforts to redirect their child’s attention—whether effective or not—disrupt the pleasurable feeling of flow.
Table 4
Characteristics of flow experiences related to video games
| Characteristic | Effect associated with video game play |
|---|---|
| Clear goals | Discernible objectives are appropriate to player’s abilities |
| Highly focused concentration | Allows player to become absorbed within a limited field of attention |
| Lack of self-consciousness | Player’s actions seem effortless |
| Distorted sense of time | Player lacks accurate sense of how long he/she has been playing |
| Direct and immediate feedback | Success and failure are quickly evident, allowing player to change strategies |
| Appropriate level of challenge | Difficulty is balanced with player’s ability |
| Control | Player has sense of control and self-efficacy |
| Source: Reference 7 | |
Types of games and devices
Role-playing games (such as Square Enix’s Final Fantasy series) involve players’ assuming identities and managing role-specific tasks and resources to progress through the game (for instance, a ranger befriending animals and tracking enemies in the wilderness).
Turn-based and real-time strategy games (such as Take 2’s Civilization series) and some simulation games (such as Atari’s Roller-Coaster Tycoon series) require players to manage resources to achieve larger goals—such as building an empire and negotiating with world leaders or constructing and maintaining a successful amusement park.
Video game play can be a social experience, involving friends or family in the same room or long-distance players online. Game consoles—such as Xbox 360, Play-Station 3, or Nintendo Wii—facilitate playing together in the same room, although they also support online play.
Games played on computers tend to be more solitary, although some games—particularly MMORPGs—also support online play. MMORPGs can connect hundreds or thousands of individuals around the world playing online. Examples include Blizzard Entertainment’s World of Warcraft or Midway Amusement Games’ Lord of the Rings Online: Shadows of Angmar. Most MMOs are intended for older audiences, but some (such as Walt Disney Internet Group’s Toontown) are designed for children.13
Maladaptive play
Children’s video game play becomes maladaptive or dysfunctional if it prevents them from engaging in developmentally appropriate activities and relationships—either because of excessive time spent playing or the possible influences of developmentally inappropriate content.14
Associated factors. Boys may be at particular risk of video game overuse. Compared with girls, boys spend more time playing—even normatively—and are more likely to play M-rated games.2 Sensation-seeking, boredom, animosity, poor academic achievement, and high family conflict also have been linked to excessive video game play.15,16 The 20% of middle school students who have a computer, game console, or television in their bedrooms are twice as likely as others to play video games ≥15 hours/week and to play M-rated games.2
Children who have experienced negative life events—trauma, family conflict, or social rejection by peers—also may spend excessive time playing video games. Gaming can interfere with more adaptive ways of coping with adversity, such as seeking support from friends and family.17,18 The draw of online relationships can be strong, especially for children who have grown up with video games and the Internet. Girls may be at particular risk for maladaptive online relationship patterns.19
Research has yet to show whether excessive video game play causes or results from these associated phenomena. Because any relationship that exists is probably transactional, pay attention to ways in which video game play may cause or result from distress or functional impairment when evaluating a patient for excessive video game play.
Violence and sexual content
Evidence is inconclusive but suggests that video games with violent content may influence children’s perceptions of aggression and violence, which may increase their likelihood of behaving aggressively or violently.20-22 Middle-school students who frequently play ≥1 M-rated games are somewhat more likely to:
- engage in physical fights
- beat someone up
- vandalize property for fun
- receive poor grades
- be threatened or injured with a weapon.23
- Does playing video games with violent content cause aggressive and violent behavior?
- Or does a tendency toward aggressive or violent behavior lead to the playing of video games with violent content?
Video game play with violent content may be analogous to rough-and-tumble play in early adolescence. In this way, it may serve boys’ developmentally appropriate needs for establishing social hierarchy—especially because video games with violent content often involve competition.13 Predispositions toward aggressive or violent behavior—such as neurologic impairments that result in poor impulse control or conduct disorders—may be exacerbated by playing violent video games.24
- talk with children to learn how these stereotypes may be influencing concerns about body image
- compare the positive and negative aspects of how men and women are portrayed in video games with adults the children know who model desired attitudes and behavior
- encourage children to internalize healthy perceptions of their physical appearance through healthy eating and physical activity.
Recommended approach
Explore whether a child’s behavior could be characterized as normative or excessive, in terms of how much time he or she spends playing video games. This can help put parents’ concerns in context. Regardless of how much time the patient spends playing video games, pay attention to whether his or her thoughts, emotions, and behaviors seem pathologic.
Try to determine if the child is experiencing distress or functional impairment because of video game play or if excessive time spent playing video games is exacerbating symptoms of a comorbid mood, anxiety, or disruptive behavior disorder. Assess overall functioning, participation in activities, engagement in relationships, and how the child perceives his or her play. Investigate the family environment, peer relationships, and history of trauma.
If these interventions fail to address excessive or pathologic video game play, or if comorbid disorders and functional impairment are severe, medication or residential treatment may be needed to effectively control video game exposure.
Table 5
Advice to parents for monitoring children’s video game use
| Keep computer and game consoles in a community area in the home |
| Check age-based ratings and content descriptors of games before renting or buying |
| Talk to your kids’ friends’ parents about the video games they play in their households |
| Talk with your kids about Internet safety, particularly if they play MMOs |
| Play games with your kids—have them teach you how to play and show you what they like about particular games |
| Engage in frequent casual conversations with your kids about the games they play and what the experience is like for them |
| Consult a mental health professional if you’re concerned about changes in your child’s mood, school performance, social relationships, or eating or sleeping habits |
| MMOs: massively-multiplayer online games |
- Entertainment Software Rating Board. Search for video game titles or publishers by rating, platform, and content descriptor. www.esrb.org.
- Jones G. Killing monsters: why children need fantasy, super heroes, and make-believe violence. New York: Basic Books; 2003.
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. AMA takes action on video games (news release). Chicago, IL: American Medical Association; June 27, 2007. Available at: http://www.ama-assn.org/ama/pub/category/17770.html. Accessed October 30, 2007.
2. Olson CK, Kutner LA, Warner DE, et al. Factors correlated with violent video game use by adolescent boys and girls. J Adolesc Health 2007;41(1):77-83.
3. Statement of the American Psychiatric Association on “video game addiction” (news release). Arlington, VA: American Psychiatric Association; June 21, 2007. Available at: http://psych.org/news_room/press_releases/07-47videogameaddiction_2_.pdf. Accessed October 30, 2007.
4. Ritvo S. Play and illusion. In: Solnit A, Cohen D, Neubauer P, eds. Many meanings of play: a psychoanalytic perspective. New Haven, CT: Yale University Press; 1993:234-51.
5. Scarlett WG, Naudeau S, Salonius-Pasternak DE, Ponte I. Children’s play. Thousand Oaks, CA: Sage Publications; 2004.
6. Gelfond HS, Salonius-Pasternak DE. The play’s the thing: a clinical-developmental perspective on video games. Child Adolesc Psychiatr Clin N Am 2005;14:491-508.
7. Entertainment Software Rating Board. About the ESRB. Available at: http://www.esrb.org/ratings/faq.jsp. Accessed October 30, 2007.
8. Roberts DF, Foehr UG, Rideout V. Generation M: Media in the lives of 8-18 year-olds. Menlo Park, CA: Kaiser Family Foundation; 2005.
9. Fisher S. Identifying video game addiction in children and adolescents. Addictive Behaviors 1994;19(5):545-53.
10. Salguero RA, Moran RM. Measuring problem video game playing in adolescents. Addiction 2002;97(12):1601-6.
11. Charlton JP, Danforth IDW. Distinguishing addiction and high engagement in the context of online game playing. Comput Human Behav 2007;23:1531-48.
12. Csikszentmihalyi M. Beyond boredom and anxiety. San Francisco: Jossey-Bass; 1975.
13. Warner DE, Raiter M. Social context in massively-multiplayer online games (MMOGs): ethical questions in shared space. International Review of Information Ethics 2006;4:46-51.
14. Stern SE. Addiction to technologies: a social psychological perspective of Internet addiction. Cyberpsychol Behav 1999;2(5):419-24.
15. Chui S, Lee J, Huang D. Video game addiction in children and teenagers in Taiwan. Cyberpsychol Behav 2004;7(5):571-81.
16. Feng Y, Yan X, Guo X, et al. Behavior problem and family environment of children with video game dependence. Chinese Mental Health Journal 2003;17(6):367-8.
17. Yang Z. Research on the correlation between life events and video game addiction in junior middle school students. Chinese Journal of Clinical Psychology 2005;13(2):192-3.
18. Keepers GA. Pathological preoccupation with video games. J Am Acad Child Adolesc Psychiatry 1990;29(1):49-50.
19. Leung L. Net-generation attributes and seductive properties of the Internet as predictors of online activities and Internet addiction. Cyberpsychol Behav 2004;7(3):343-8.
20. Funk JB, Baldacci HB, Pasold T, Baumgardner J. Violence exposure in real-life, video games, television, movies, and the Internet: is there desensitization? J Adolesc 2004;27:23-39.
21. Anderson CA, Bushman BJ. Effects of violent video games on aggressive behavior, aggressive cognition, aggressive affect, physiological arousal, and prosocial behavior: a meta-analytic review of the scientific literature. Psychol Sci 2001;12(5):353-9.
22. Gentile DA, Lynch PJ, Linder JR, et al. The effects of violent video game habits on adolescent hostility, aggressive behaviors, and school performance. J Adolesc 2004;27:5-22.
23. Olson CK, Kutner LA, Baer L, et al. M-rated video games and aggression. J Am Acad Child Adolesc Psychiatry. In press.
24. Salonius-Pasternak DE, Gelfond HS. The next level of research on electronic play: potential benefits and contextual influences for children and adolescents. Human Technology: An Interdisciplinary Journal on Humans in ICT Environments 2005;1(1):5-22.
25. Cassell J, Jenkins H. From Barbie to Mortal Kombat: gender and computer games. Cambridge, MA: MIT Press; 1998.
Nick, age 13, enjoys playing video games, but his parents think he may be “addicted.” His primary care doctor has referred Nick to you for evaluation.
Nick has played video games since age 7 and likes to share ideas with friends about to “beat” difficult games. Lately, though, he plays an online role-playing game, mostly alone, on the computer in his bedroom. Nick hasn’t seen his friends outside of school for 6 weeks.
Nick’s parents say he is growing short-tempered, and his grades have fallen for several months. He seems to worry a lot but becomes angry and storms out of the room when they try to talk with him about it.
Like Nick, 70% to 90% of American youths play video games, according to the American Medical Association (AMA).1 Most boys and girls find the games fun, entertaining, or relaxing (Table 1) and do not encounter difficulties as a result of their play.2 In some cases, however, they may:
- spend excessive time playing video games
- model inappropriate behavior from games
- over-invest in online relationships.
This article describes developmentally appropriate characteristics of play in general—and aspects of video game play in particular—to help you educate families about normative and excessive video game play.
Table 1
Top 10 reasons why children say they play video games
| Boys |
|
| Girls |
|
| * Response likely reflects the number of survey respondents living in a suburban/rural environment in which hunting is a popular leisure activity. |
| Source: Reference 1 |
An addiction?
Originally researchers believed video game play was not addictive and viewed excessive play as high engagement. More recently, efforts are being made to understand:
- how to classify excessive video game play that impairs psychosocial adjustment
- whether substance abuse models are appropriate for describing and treating pathologic video game play.
What is normative play?
Play is a motivating way for children to make sense of the world. By re-creating themes, relationships, places, or events in play children can control things that outside of play might be intimidating or overwhelming. Through play, children can explore situations in a setting that feels safe.4,5 Video games offer children play opportunities to explore roles and worlds that otherwise are unavailable to them.6
Video game play is one of the most popular leisure-time activities for middle-school students. Our group7 recently surveyed >1,200 students age 12 to 15 about their video game play and found:
- One-third of boys and two-thirds of girls played video games for ≤2 hours/week.
- One-third of boys and 11% of girls played video games 6 or 7 days each week.
- Boys played more than girls, with 45% of boys playing for ≥6 hours/week.
- 12.6% of boys played ≥15 hours/week.
- One-half listed ≥1 games rated M for mature (Table 2)7 among 5 games they played most frequently in the preceding 6 months.2
Pathologic behavior. Excessive video game playing can be viewed as pathologic if it involves an overwhelming need to play video games, with negative feelings and behaviors related to this need that lead to distress or functional impairment.9,10 Charlton et al
11 define pathologic video game play as incorporating high engagement plus core addiction characteristics such as interference with work or social life, failure to sleep, etc. In video game play, peripheral DSM addiction characteristics—such as high cognitive salience—may indicate high engagement. Characteristics of pathologic video game play, as identified by this group, are listed in Table 3.11
Table 2
ESRB video game ratings system and content descriptions*
| Rating | Content may be suitable for: | Examples |
|---|---|---|
| Early childhood | Age 3 and older; no material that parents would find inappropriate | Atari/others’ Dora the Explorer (series), Knowledge Adventure/Vivendi Universal’s Jump start (series) |
| Everyone | Age 6 and older; minimal cartoon, fantasy, or mild violence and/or infrequent use of mild language | Disney Interactive Studios/Buena Vista Games’ Hannah Montana (series), Taito Corporation’s Bubble Bobble |
| Everyone 10+ | Age 10 and older; more cartoon, fantasy, or mild violence, mild language and/or minimal suggestive themes | Electronic Arts’ Need for Speed: ProStreet, Ubisoft’s Rayman Raving Rabbids 2 |
| Teen | Age 13 and older; may contain violence, suggestive themes, crude humor, minimal blood, simulated gambling, and/or infrequent use of strong language | Midway Amusement Games’ Lord of the Rings Online: Shadows of Angmar (MMO), Sony Online Entertainment’s EverQuest (series; MMO) |
| Mature | Age 17 and older; may contain intense violence, blood and gore, sexual content, and/or strong language | Microsoft Corporation’s Halo (series), Rockstar Games’ Grand Theft Auto (most games in the series) |
| Adults only | Age 18 and older; may include prolonged scenes of intense violence and/or graphic sexual content and nudity | Vivendi Universal’s Leisure Suit Larry: Magna Cum Laude Uncut and Uncensored, Rockstar Games’ Grand Theft Auto: San Andreas |
| * On video game boxes, look for rating symbols on the front and content descriptions on the back. | ||
| ESRB: Entertainment Software Rating Board | ||
| MMO: massively-multiplayer online role-playing game | ||
| Source: Reference 7 | ||
Characteristics of ‘pathologic’ video game play
| Feeling agitated when not playing |
| Feeling “addicted” to play |
| Not being able to decrease time spent playing |
| Not sleeping because of video game play |
| Missing meals because of video game play |
| Being late because of video game play |
| Having arguments at home because of video game play |
| Letting video game play interfere with social relationships |
| Letting video game play interfere with schoolwork |
| Spending excessive amounts of money on video game play |
| Source: Reference 11 |
CASE CONTINUED: Going with the ‘flow’
Nick says he enjoys playing with people he’s met through a massively-multiplayer online role-playing game (MMORG, or also called MMO or MMP). The “guild” he has joined is a small community that collaborates to complete quests in the game. Nick describes his character—a healer—as a key figure who supports fellow players by replenishing their in-game health. Everyone in the guild thinks he’s important, and he likes to feel respected. Nick says this is quite different from how people treat him in “real” life. He says he often feels worthless and scared that his friends and family don’t think he’s good enough.
Video game play facilitates the experience of “flow”—a mental state of positive energy and effortless focus experienced while immersed in an activity over which one feels a sense of control. Video game play incorporates components of a flow experience (Table 4), including clear, focused goals that are attainable yet challenging and require a high level of concentration. Individuals who engage in artistic, athletic, or meditative activities often report experiencing flow.12
Flow can distort one’s sense of time, setting the stage for frustration on both sides when parents want their video game-playing child to engage in other activities. Their efforts to redirect their child’s attention—whether effective or not—disrupt the pleasurable feeling of flow.
Table 4
Characteristics of flow experiences related to video games
| Characteristic | Effect associated with video game play |
|---|---|
| Clear goals | Discernible objectives are appropriate to player’s abilities |
| Highly focused concentration | Allows player to become absorbed within a limited field of attention |
| Lack of self-consciousness | Player’s actions seem effortless |
| Distorted sense of time | Player lacks accurate sense of how long he/she has been playing |
| Direct and immediate feedback | Success and failure are quickly evident, allowing player to change strategies |
| Appropriate level of challenge | Difficulty is balanced with player’s ability |
| Control | Player has sense of control and self-efficacy |
| Source: Reference 7 | |
Types of games and devices
Role-playing games (such as Square Enix’s Final Fantasy series) involve players’ assuming identities and managing role-specific tasks and resources to progress through the game (for instance, a ranger befriending animals and tracking enemies in the wilderness).
Turn-based and real-time strategy games (such as Take 2’s Civilization series) and some simulation games (such as Atari’s Roller-Coaster Tycoon series) require players to manage resources to achieve larger goals—such as building an empire and negotiating with world leaders or constructing and maintaining a successful amusement park.
Video game play can be a social experience, involving friends or family in the same room or long-distance players online. Game consoles—such as Xbox 360, Play-Station 3, or Nintendo Wii—facilitate playing together in the same room, although they also support online play.
Games played on computers tend to be more solitary, although some games—particularly MMORPGs—also support online play. MMORPGs can connect hundreds or thousands of individuals around the world playing online. Examples include Blizzard Entertainment’s World of Warcraft or Midway Amusement Games’ Lord of the Rings Online: Shadows of Angmar. Most MMOs are intended for older audiences, but some (such as Walt Disney Internet Group’s Toontown) are designed for children.13
Maladaptive play
Children’s video game play becomes maladaptive or dysfunctional if it prevents them from engaging in developmentally appropriate activities and relationships—either because of excessive time spent playing or the possible influences of developmentally inappropriate content.14
Associated factors. Boys may be at particular risk of video game overuse. Compared with girls, boys spend more time playing—even normatively—and are more likely to play M-rated games.2 Sensation-seeking, boredom, animosity, poor academic achievement, and high family conflict also have been linked to excessive video game play.15,16 The 20% of middle school students who have a computer, game console, or television in their bedrooms are twice as likely as others to play video games ≥15 hours/week and to play M-rated games.2
Children who have experienced negative life events—trauma, family conflict, or social rejection by peers—also may spend excessive time playing video games. Gaming can interfere with more adaptive ways of coping with adversity, such as seeking support from friends and family.17,18 The draw of online relationships can be strong, especially for children who have grown up with video games and the Internet. Girls may be at particular risk for maladaptive online relationship patterns.19
Research has yet to show whether excessive video game play causes or results from these associated phenomena. Because any relationship that exists is probably transactional, pay attention to ways in which video game play may cause or result from distress or functional impairment when evaluating a patient for excessive video game play.
Violence and sexual content
Evidence is inconclusive but suggests that video games with violent content may influence children’s perceptions of aggression and violence, which may increase their likelihood of behaving aggressively or violently.20-22 Middle-school students who frequently play ≥1 M-rated games are somewhat more likely to:
- engage in physical fights
- beat someone up
- vandalize property for fun
- receive poor grades
- be threatened or injured with a weapon.23
- Does playing video games with violent content cause aggressive and violent behavior?
- Or does a tendency toward aggressive or violent behavior lead to the playing of video games with violent content?
Video game play with violent content may be analogous to rough-and-tumble play in early adolescence. In this way, it may serve boys’ developmentally appropriate needs for establishing social hierarchy—especially because video games with violent content often involve competition.13 Predispositions toward aggressive or violent behavior—such as neurologic impairments that result in poor impulse control or conduct disorders—may be exacerbated by playing violent video games.24
- talk with children to learn how these stereotypes may be influencing concerns about body image
- compare the positive and negative aspects of how men and women are portrayed in video games with adults the children know who model desired attitudes and behavior
- encourage children to internalize healthy perceptions of their physical appearance through healthy eating and physical activity.
Recommended approach
Explore whether a child’s behavior could be characterized as normative or excessive, in terms of how much time he or she spends playing video games. This can help put parents’ concerns in context. Regardless of how much time the patient spends playing video games, pay attention to whether his or her thoughts, emotions, and behaviors seem pathologic.
Try to determine if the child is experiencing distress or functional impairment because of video game play or if excessive time spent playing video games is exacerbating symptoms of a comorbid mood, anxiety, or disruptive behavior disorder. Assess overall functioning, participation in activities, engagement in relationships, and how the child perceives his or her play. Investigate the family environment, peer relationships, and history of trauma.
If these interventions fail to address excessive or pathologic video game play, or if comorbid disorders and functional impairment are severe, medication or residential treatment may be needed to effectively control video game exposure.
Table 5
Advice to parents for monitoring children’s video game use
| Keep computer and game consoles in a community area in the home |
| Check age-based ratings and content descriptors of games before renting or buying |
| Talk to your kids’ friends’ parents about the video games they play in their households |
| Talk with your kids about Internet safety, particularly if they play MMOs |
| Play games with your kids—have them teach you how to play and show you what they like about particular games |
| Engage in frequent casual conversations with your kids about the games they play and what the experience is like for them |
| Consult a mental health professional if you’re concerned about changes in your child’s mood, school performance, social relationships, or eating or sleeping habits |
| MMOs: massively-multiplayer online games |
- Entertainment Software Rating Board. Search for video game titles or publishers by rating, platform, and content descriptor. www.esrb.org.
- Jones G. Killing monsters: why children need fantasy, super heroes, and make-believe violence. New York: Basic Books; 2003.
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Nick, age 13, enjoys playing video games, but his parents think he may be “addicted.” His primary care doctor has referred Nick to you for evaluation.
Nick has played video games since age 7 and likes to share ideas with friends about to “beat” difficult games. Lately, though, he plays an online role-playing game, mostly alone, on the computer in his bedroom. Nick hasn’t seen his friends outside of school for 6 weeks.
Nick’s parents say he is growing short-tempered, and his grades have fallen for several months. He seems to worry a lot but becomes angry and storms out of the room when they try to talk with him about it.
Like Nick, 70% to 90% of American youths play video games, according to the American Medical Association (AMA).1 Most boys and girls find the games fun, entertaining, or relaxing (Table 1) and do not encounter difficulties as a result of their play.2 In some cases, however, they may:
- spend excessive time playing video games
- model inappropriate behavior from games
- over-invest in online relationships.
This article describes developmentally appropriate characteristics of play in general—and aspects of video game play in particular—to help you educate families about normative and excessive video game play.
Table 1
Top 10 reasons why children say they play video games
| Boys |
|
| Girls |
|
| * Response likely reflects the number of survey respondents living in a suburban/rural environment in which hunting is a popular leisure activity. |
| Source: Reference 1 |
An addiction?
Originally researchers believed video game play was not addictive and viewed excessive play as high engagement. More recently, efforts are being made to understand:
- how to classify excessive video game play that impairs psychosocial adjustment
- whether substance abuse models are appropriate for describing and treating pathologic video game play.
What is normative play?
Play is a motivating way for children to make sense of the world. By re-creating themes, relationships, places, or events in play children can control things that outside of play might be intimidating or overwhelming. Through play, children can explore situations in a setting that feels safe.4,5 Video games offer children play opportunities to explore roles and worlds that otherwise are unavailable to them.6
Video game play is one of the most popular leisure-time activities for middle-school students. Our group7 recently surveyed >1,200 students age 12 to 15 about their video game play and found:
- One-third of boys and two-thirds of girls played video games for ≤2 hours/week.
- One-third of boys and 11% of girls played video games 6 or 7 days each week.
- Boys played more than girls, with 45% of boys playing for ≥6 hours/week.
- 12.6% of boys played ≥15 hours/week.
- One-half listed ≥1 games rated M for mature (Table 2)7 among 5 games they played most frequently in the preceding 6 months.2
Pathologic behavior. Excessive video game playing can be viewed as pathologic if it involves an overwhelming need to play video games, with negative feelings and behaviors related to this need that lead to distress or functional impairment.9,10 Charlton et al
11 define pathologic video game play as incorporating high engagement plus core addiction characteristics such as interference with work or social life, failure to sleep, etc. In video game play, peripheral DSM addiction characteristics—such as high cognitive salience—may indicate high engagement. Characteristics of pathologic video game play, as identified by this group, are listed in Table 3.11
Table 2
ESRB video game ratings system and content descriptions*
| Rating | Content may be suitable for: | Examples |
|---|---|---|
| Early childhood | Age 3 and older; no material that parents would find inappropriate | Atari/others’ Dora the Explorer (series), Knowledge Adventure/Vivendi Universal’s Jump start (series) |
| Everyone | Age 6 and older; minimal cartoon, fantasy, or mild violence and/or infrequent use of mild language | Disney Interactive Studios/Buena Vista Games’ Hannah Montana (series), Taito Corporation’s Bubble Bobble |
| Everyone 10+ | Age 10 and older; more cartoon, fantasy, or mild violence, mild language and/or minimal suggestive themes | Electronic Arts’ Need for Speed: ProStreet, Ubisoft’s Rayman Raving Rabbids 2 |
| Teen | Age 13 and older; may contain violence, suggestive themes, crude humor, minimal blood, simulated gambling, and/or infrequent use of strong language | Midway Amusement Games’ Lord of the Rings Online: Shadows of Angmar (MMO), Sony Online Entertainment’s EverQuest (series; MMO) |
| Mature | Age 17 and older; may contain intense violence, blood and gore, sexual content, and/or strong language | Microsoft Corporation’s Halo (series), Rockstar Games’ Grand Theft Auto (most games in the series) |
| Adults only | Age 18 and older; may include prolonged scenes of intense violence and/or graphic sexual content and nudity | Vivendi Universal’s Leisure Suit Larry: Magna Cum Laude Uncut and Uncensored, Rockstar Games’ Grand Theft Auto: San Andreas |
| * On video game boxes, look for rating symbols on the front and content descriptions on the back. | ||
| ESRB: Entertainment Software Rating Board | ||
| MMO: massively-multiplayer online role-playing game | ||
| Source: Reference 7 | ||
Characteristics of ‘pathologic’ video game play
| Feeling agitated when not playing |
| Feeling “addicted” to play |
| Not being able to decrease time spent playing |
| Not sleeping because of video game play |
| Missing meals because of video game play |
| Being late because of video game play |
| Having arguments at home because of video game play |
| Letting video game play interfere with social relationships |
| Letting video game play interfere with schoolwork |
| Spending excessive amounts of money on video game play |
| Source: Reference 11 |
CASE CONTINUED: Going with the ‘flow’
Nick says he enjoys playing with people he’s met through a massively-multiplayer online role-playing game (MMORG, or also called MMO or MMP). The “guild” he has joined is a small community that collaborates to complete quests in the game. Nick describes his character—a healer—as a key figure who supports fellow players by replenishing their in-game health. Everyone in the guild thinks he’s important, and he likes to feel respected. Nick says this is quite different from how people treat him in “real” life. He says he often feels worthless and scared that his friends and family don’t think he’s good enough.
Video game play facilitates the experience of “flow”—a mental state of positive energy and effortless focus experienced while immersed in an activity over which one feels a sense of control. Video game play incorporates components of a flow experience (Table 4), including clear, focused goals that are attainable yet challenging and require a high level of concentration. Individuals who engage in artistic, athletic, or meditative activities often report experiencing flow.12
Flow can distort one’s sense of time, setting the stage for frustration on both sides when parents want their video game-playing child to engage in other activities. Their efforts to redirect their child’s attention—whether effective or not—disrupt the pleasurable feeling of flow.
Table 4
Characteristics of flow experiences related to video games
| Characteristic | Effect associated with video game play |
|---|---|
| Clear goals | Discernible objectives are appropriate to player’s abilities |
| Highly focused concentration | Allows player to become absorbed within a limited field of attention |
| Lack of self-consciousness | Player’s actions seem effortless |
| Distorted sense of time | Player lacks accurate sense of how long he/she has been playing |
| Direct and immediate feedback | Success and failure are quickly evident, allowing player to change strategies |
| Appropriate level of challenge | Difficulty is balanced with player’s ability |
| Control | Player has sense of control and self-efficacy |
| Source: Reference 7 | |
Types of games and devices
Role-playing games (such as Square Enix’s Final Fantasy series) involve players’ assuming identities and managing role-specific tasks and resources to progress through the game (for instance, a ranger befriending animals and tracking enemies in the wilderness).
Turn-based and real-time strategy games (such as Take 2’s Civilization series) and some simulation games (such as Atari’s Roller-Coaster Tycoon series) require players to manage resources to achieve larger goals—such as building an empire and negotiating with world leaders or constructing and maintaining a successful amusement park.
Video game play can be a social experience, involving friends or family in the same room or long-distance players online. Game consoles—such as Xbox 360, Play-Station 3, or Nintendo Wii—facilitate playing together in the same room, although they also support online play.
Games played on computers tend to be more solitary, although some games—particularly MMORPGs—also support online play. MMORPGs can connect hundreds or thousands of individuals around the world playing online. Examples include Blizzard Entertainment’s World of Warcraft or Midway Amusement Games’ Lord of the Rings Online: Shadows of Angmar. Most MMOs are intended for older audiences, but some (such as Walt Disney Internet Group’s Toontown) are designed for children.13
Maladaptive play
Children’s video game play becomes maladaptive or dysfunctional if it prevents them from engaging in developmentally appropriate activities and relationships—either because of excessive time spent playing or the possible influences of developmentally inappropriate content.14
Associated factors. Boys may be at particular risk of video game overuse. Compared with girls, boys spend more time playing—even normatively—and are more likely to play M-rated games.2 Sensation-seeking, boredom, animosity, poor academic achievement, and high family conflict also have been linked to excessive video game play.15,16 The 20% of middle school students who have a computer, game console, or television in their bedrooms are twice as likely as others to play video games ≥15 hours/week and to play M-rated games.2
Children who have experienced negative life events—trauma, family conflict, or social rejection by peers—also may spend excessive time playing video games. Gaming can interfere with more adaptive ways of coping with adversity, such as seeking support from friends and family.17,18 The draw of online relationships can be strong, especially for children who have grown up with video games and the Internet. Girls may be at particular risk for maladaptive online relationship patterns.19
Research has yet to show whether excessive video game play causes or results from these associated phenomena. Because any relationship that exists is probably transactional, pay attention to ways in which video game play may cause or result from distress or functional impairment when evaluating a patient for excessive video game play.
Violence and sexual content
Evidence is inconclusive but suggests that video games with violent content may influence children’s perceptions of aggression and violence, which may increase their likelihood of behaving aggressively or violently.20-22 Middle-school students who frequently play ≥1 M-rated games are somewhat more likely to:
- engage in physical fights
- beat someone up
- vandalize property for fun
- receive poor grades
- be threatened or injured with a weapon.23
- Does playing video games with violent content cause aggressive and violent behavior?
- Or does a tendency toward aggressive or violent behavior lead to the playing of video games with violent content?
Video game play with violent content may be analogous to rough-and-tumble play in early adolescence. In this way, it may serve boys’ developmentally appropriate needs for establishing social hierarchy—especially because video games with violent content often involve competition.13 Predispositions toward aggressive or violent behavior—such as neurologic impairments that result in poor impulse control or conduct disorders—may be exacerbated by playing violent video games.24
- talk with children to learn how these stereotypes may be influencing concerns about body image
- compare the positive and negative aspects of how men and women are portrayed in video games with adults the children know who model desired attitudes and behavior
- encourage children to internalize healthy perceptions of their physical appearance through healthy eating and physical activity.
Recommended approach
Explore whether a child’s behavior could be characterized as normative or excessive, in terms of how much time he or she spends playing video games. This can help put parents’ concerns in context. Regardless of how much time the patient spends playing video games, pay attention to whether his or her thoughts, emotions, and behaviors seem pathologic.
Try to determine if the child is experiencing distress or functional impairment because of video game play or if excessive time spent playing video games is exacerbating symptoms of a comorbid mood, anxiety, or disruptive behavior disorder. Assess overall functioning, participation in activities, engagement in relationships, and how the child perceives his or her play. Investigate the family environment, peer relationships, and history of trauma.
If these interventions fail to address excessive or pathologic video game play, or if comorbid disorders and functional impairment are severe, medication or residential treatment may be needed to effectively control video game exposure.
Table 5
Advice to parents for monitoring children’s video game use
| Keep computer and game consoles in a community area in the home |
| Check age-based ratings and content descriptors of games before renting or buying |
| Talk to your kids’ friends’ parents about the video games they play in their households |
| Talk with your kids about Internet safety, particularly if they play MMOs |
| Play games with your kids—have them teach you how to play and show you what they like about particular games |
| Engage in frequent casual conversations with your kids about the games they play and what the experience is like for them |
| Consult a mental health professional if you’re concerned about changes in your child’s mood, school performance, social relationships, or eating or sleeping habits |
| MMOs: massively-multiplayer online games |
- Entertainment Software Rating Board. Search for video game titles or publishers by rating, platform, and content descriptor. www.esrb.org.
- Jones G. Killing monsters: why children need fantasy, super heroes, and make-believe violence. New York: Basic Books; 2003.
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. AMA takes action on video games (news release). Chicago, IL: American Medical Association; June 27, 2007. Available at: http://www.ama-assn.org/ama/pub/category/17770.html. Accessed October 30, 2007.
2. Olson CK, Kutner LA, Warner DE, et al. Factors correlated with violent video game use by adolescent boys and girls. J Adolesc Health 2007;41(1):77-83.
3. Statement of the American Psychiatric Association on “video game addiction” (news release). Arlington, VA: American Psychiatric Association; June 21, 2007. Available at: http://psych.org/news_room/press_releases/07-47videogameaddiction_2_.pdf. Accessed October 30, 2007.
4. Ritvo S. Play and illusion. In: Solnit A, Cohen D, Neubauer P, eds. Many meanings of play: a psychoanalytic perspective. New Haven, CT: Yale University Press; 1993:234-51.
5. Scarlett WG, Naudeau S, Salonius-Pasternak DE, Ponte I. Children’s play. Thousand Oaks, CA: Sage Publications; 2004.
6. Gelfond HS, Salonius-Pasternak DE. The play’s the thing: a clinical-developmental perspective on video games. Child Adolesc Psychiatr Clin N Am 2005;14:491-508.
7. Entertainment Software Rating Board. About the ESRB. Available at: http://www.esrb.org/ratings/faq.jsp. Accessed October 30, 2007.
8. Roberts DF, Foehr UG, Rideout V. Generation M: Media in the lives of 8-18 year-olds. Menlo Park, CA: Kaiser Family Foundation; 2005.
9. Fisher S. Identifying video game addiction in children and adolescents. Addictive Behaviors 1994;19(5):545-53.
10. Salguero RA, Moran RM. Measuring problem video game playing in adolescents. Addiction 2002;97(12):1601-6.
11. Charlton JP, Danforth IDW. Distinguishing addiction and high engagement in the context of online game playing. Comput Human Behav 2007;23:1531-48.
12. Csikszentmihalyi M. Beyond boredom and anxiety. San Francisco: Jossey-Bass; 1975.
13. Warner DE, Raiter M. Social context in massively-multiplayer online games (MMOGs): ethical questions in shared space. International Review of Information Ethics 2006;4:46-51.
14. Stern SE. Addiction to technologies: a social psychological perspective of Internet addiction. Cyberpsychol Behav 1999;2(5):419-24.
15. Chui S, Lee J, Huang D. Video game addiction in children and teenagers in Taiwan. Cyberpsychol Behav 2004;7(5):571-81.
16. Feng Y, Yan X, Guo X, et al. Behavior problem and family environment of children with video game dependence. Chinese Mental Health Journal 2003;17(6):367-8.
17. Yang Z. Research on the correlation between life events and video game addiction in junior middle school students. Chinese Journal of Clinical Psychology 2005;13(2):192-3.
18. Keepers GA. Pathological preoccupation with video games. J Am Acad Child Adolesc Psychiatry 1990;29(1):49-50.
19. Leung L. Net-generation attributes and seductive properties of the Internet as predictors of online activities and Internet addiction. Cyberpsychol Behav 2004;7(3):343-8.
20. Funk JB, Baldacci HB, Pasold T, Baumgardner J. Violence exposure in real-life, video games, television, movies, and the Internet: is there desensitization? J Adolesc 2004;27:23-39.
21. Anderson CA, Bushman BJ. Effects of violent video games on aggressive behavior, aggressive cognition, aggressive affect, physiological arousal, and prosocial behavior: a meta-analytic review of the scientific literature. Psychol Sci 2001;12(5):353-9.
22. Gentile DA, Lynch PJ, Linder JR, et al. The effects of violent video game habits on adolescent hostility, aggressive behaviors, and school performance. J Adolesc 2004;27:5-22.
23. Olson CK, Kutner LA, Baer L, et al. M-rated video games and aggression. J Am Acad Child Adolesc Psychiatry. In press.
24. Salonius-Pasternak DE, Gelfond HS. The next level of research on electronic play: potential benefits and contextual influences for children and adolescents. Human Technology: An Interdisciplinary Journal on Humans in ICT Environments 2005;1(1):5-22.
25. Cassell J, Jenkins H. From Barbie to Mortal Kombat: gender and computer games. Cambridge, MA: MIT Press; 1998.
1. AMA takes action on video games (news release). Chicago, IL: American Medical Association; June 27, 2007. Available at: http://www.ama-assn.org/ama/pub/category/17770.html. Accessed October 30, 2007.
2. Olson CK, Kutner LA, Warner DE, et al. Factors correlated with violent video game use by adolescent boys and girls. J Adolesc Health 2007;41(1):77-83.
3. Statement of the American Psychiatric Association on “video game addiction” (news release). Arlington, VA: American Psychiatric Association; June 21, 2007. Available at: http://psych.org/news_room/press_releases/07-47videogameaddiction_2_.pdf. Accessed October 30, 2007.
4. Ritvo S. Play and illusion. In: Solnit A, Cohen D, Neubauer P, eds. Many meanings of play: a psychoanalytic perspective. New Haven, CT: Yale University Press; 1993:234-51.
5. Scarlett WG, Naudeau S, Salonius-Pasternak DE, Ponte I. Children’s play. Thousand Oaks, CA: Sage Publications; 2004.
6. Gelfond HS, Salonius-Pasternak DE. The play’s the thing: a clinical-developmental perspective on video games. Child Adolesc Psychiatr Clin N Am 2005;14:491-508.
7. Entertainment Software Rating Board. About the ESRB. Available at: http://www.esrb.org/ratings/faq.jsp. Accessed October 30, 2007.
8. Roberts DF, Foehr UG, Rideout V. Generation M: Media in the lives of 8-18 year-olds. Menlo Park, CA: Kaiser Family Foundation; 2005.
9. Fisher S. Identifying video game addiction in children and adolescents. Addictive Behaviors 1994;19(5):545-53.
10. Salguero RA, Moran RM. Measuring problem video game playing in adolescents. Addiction 2002;97(12):1601-6.
11. Charlton JP, Danforth IDW. Distinguishing addiction and high engagement in the context of online game playing. Comput Human Behav 2007;23:1531-48.
12. Csikszentmihalyi M. Beyond boredom and anxiety. San Francisco: Jossey-Bass; 1975.
13. Warner DE, Raiter M. Social context in massively-multiplayer online games (MMOGs): ethical questions in shared space. International Review of Information Ethics 2006;4:46-51.
14. Stern SE. Addiction to technologies: a social psychological perspective of Internet addiction. Cyberpsychol Behav 1999;2(5):419-24.
15. Chui S, Lee J, Huang D. Video game addiction in children and teenagers in Taiwan. Cyberpsychol Behav 2004;7(5):571-81.
16. Feng Y, Yan X, Guo X, et al. Behavior problem and family environment of children with video game dependence. Chinese Mental Health Journal 2003;17(6):367-8.
17. Yang Z. Research on the correlation between life events and video game addiction in junior middle school students. Chinese Journal of Clinical Psychology 2005;13(2):192-3.
18. Keepers GA. Pathological preoccupation with video games. J Am Acad Child Adolesc Psychiatry 1990;29(1):49-50.
19. Leung L. Net-generation attributes and seductive properties of the Internet as predictors of online activities and Internet addiction. Cyberpsychol Behav 2004;7(3):343-8.
20. Funk JB, Baldacci HB, Pasold T, Baumgardner J. Violence exposure in real-life, video games, television, movies, and the Internet: is there desensitization? J Adolesc 2004;27:23-39.
21. Anderson CA, Bushman BJ. Effects of violent video games on aggressive behavior, aggressive cognition, aggressive affect, physiological arousal, and prosocial behavior: a meta-analytic review of the scientific literature. Psychol Sci 2001;12(5):353-9.
22. Gentile DA, Lynch PJ, Linder JR, et al. The effects of violent video game habits on adolescent hostility, aggressive behaviors, and school performance. J Adolesc 2004;27:5-22.
23. Olson CK, Kutner LA, Baer L, et al. M-rated video games and aggression. J Am Acad Child Adolesc Psychiatry. In press.
24. Salonius-Pasternak DE, Gelfond HS. The next level of research on electronic play: potential benefits and contextual influences for children and adolescents. Human Technology: An Interdisciplinary Journal on Humans in ICT Environments 2005;1(1):5-22.
25. Cassell J, Jenkins H. From Barbie to Mortal Kombat: gender and computer games. Cambridge, MA: MIT Press; 1998.
Safe use of SSRIs in young adults: How strong is evidence for new suicide warning?
CASE: Life is 'not worth it'
Mr. B, age 20, has taken a semester leave from college because of gradually worsening depressed mood. Over the past 2 months he has lost interest in jogging and playing piano—which he usually enjoys. He reports reduced libido, middle insomnia, loss of appetite, feeling as if his head is “full of cotton,” trouble concentrating, and waking in the morning with a sense of dread. His anxiety dissipates during the day, but he continues to feel sad and sometimes weepy, which is unusual for him.
Mr. B reports feeling hopeless at times and has had vague thoughts about life being “not worth it if I continue to feel like this” but denies specific suicide plans. Your initial impression is that Mr. B is in the midst of a major depressive episode and that a selective serotonin reuptake inhibitor (SSRI) is indicated. As you finish taking his history, you run through your mind the pros and cons of the recommendation you will make to him.
Do SSRIs raise or lower the risk for suicidal behavior in young adults such as Mr. B? The answer is complicated and goes beyond an “either/or” question, as the FDA acknowledged in May 2007 when it:
- extended the black-box warning of increased suicidality risk with antidepressants to cover adults age 18 to 24 as well as children and adolescents
- included language in the warning about the benefits of treating depression and the suicide risk associated with untreated depression, given concerns about declining antidepressant prescriptions and rising suicides among youth.1
- To help you make informed decisions when treating depression in adults, this article reviews the studies leading up to and following the FDA’s meta-analysis of antidepressant trial data in patients age 18 and older. Our goal is to provide a framework for clinical treatment of adults age 18 to 24 and those age ≥25.
The FDA meta-analysis designed to investigate a reported association between antidepressants and suicidality in children and adolescents found contradictory results:
- Pooled adverse event data from 24 pediatric antidepressant trials totaling >4,400 patients showed a higher risk of suicidal ideation or behavior (no suicides occurred) with antidepressants (4%) vs placebo (2%).
- Systematically collected suicide-related item scores from 17 of the trials showed no evidence that antidepressants worsen suicidality or cause it to emerge.
One interpretation of these findings is that antidepressants’ effect on suicidality is small and therefore subject to measurement error.
Another is ascertainment bias; any side effect associated with active medication encourages discussion with the clinician and may distort the frequency of reported adverse events.
The FDA meta-analysis also found:
- Relative risk for suicidality ranged 10-fold among agents, from 0.9 with fluoxetine to 8.8 with venlafaxine.
- Most suicide-related events occurred in subjects having the highest baseline levels of suicidality.
- Hostility and agitation emerged with SSRI use, particularly during the first month of treatment.
- Patient age, sex, or history of suicide attempt/ideation did not affect the results.
Source: Reference 7
First hints of suicidality
SSRIs revolutionized depression treatment. From 1985 to 1999, annual U.S. antidepressant prescriptions quadrupled, with SSRIs accounting for 70% of the increase (see “Antidepressants and suicide risk, 1985 to 2007”). At the same time, the age-adjusted suicide rate:
- dropped 22.5% for women (who account for twice as many antidepressant prescriptions as men)
- dropped 12.8% for men (without change in the rank order of suicide methods).2
The debate rekindled in June 2003 when the British Committee on Safety of Medicines warned against using paroxetine or venlafaxine in children. After conducting its own meta-analysis, the FDA in 2004 ordered a black-box warning about suicidality and the use of antidepressants in children and adolescents ( Box ).7
After the pediatric ‘black box.’ Antidepressant prescriptions for children and adolescents declined in the years 2003 to 2004, as did diagnosis of pediatric depression.8-10 Antidepressant prescribing also showed signs of shifting from general practitioners to psychiatrists.8 At the same time, the suicide rate among youth age 11 In patients age >60, SSRI prescriptions continued to rise and suicide rates fell,9 a pattern of change consistent with antidepressants protecting against suicide.
An independent meta-analysis by Bridge et al12 examined the pediatric trial data used in the FDA meta-analysis plus 7 additional studies. Its findings differ in 2 important ways from those of the FDA review:
- Antidepressants—including others besides fluoxetine—showed efficacy in treating anxiety disorders and depression in children and adolescents.
- The frequency of suicide-related adverse events (no trial patients committed suicide) was approximately 3% on active medication—25% lower than the FDA estimated rate—and 2% on placebo, similar to the FDA estimate.
Antidepressants and suicide risk, 1985 to 2007
| 1985 | 1990 | 1991 | 1999 | 2003 | 2004 | 2006 | 2007 |
|---|---|---|---|---|---|---|---|
| Case reports suggest link between suicide and SSRI use | FDA analysis finds no association between SSRIs and increased suicide risk | UK agency warns of suicide-related events in children treated with paroxetine and venlafaxine | FDA conducts meta-analysis, requires black-box warnings of risk of suicidality in youth taking antidepressants | FDA meta-analysis finds age-dependent effect of antidepressants on suicidality risk in adults | FDA expands warning of increased suicidality risk with antidepressants to adults age | ||
| Antidepressant prescriptions quadruple; age-adjusted suicide rate drops 22.5% for women and 12.8% for men | Pediatric depression diagnoses and antidepressant prescriptions decline; suicides increase 11% | Bridge et al meta-analysis finds 25% lower rate of suicide-related events in youth than the FDA found | |||||
What about adults?
Overall effect. A subsequent FDA meta-analysis of antidepressant clinical trial data in adults13 found 8 suicides in 372 trials totaling nearly 100,000 persons. All occurred in the 295 trials with psychiatric indications. Among these psychiatric trials, 59% had a suicidal behavior/ideation event in either the test-drug or placebo arm, and 41% had none. Eleven antidepressants were included in the meta-analysis:
- 6 SSRIs (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline)
- 2 SNRIs (duloxetine and venlafaxine)
- 3 others (bupropion, mirtazapine, and nefazodone).
Age-specific findings. When the FDA analysis was stratified by age, however, antidepressants’ benefit appeared greater for patients age ≥25 than for those age 18 to 24. The data suggested:
- elevated suicidality risk among adults age
- neutral or possibly protective effect for adults age 25 to 64
- protective effect in adults age ≥65 ( Table 1 ).13
FDA meta-analysis: Suicide rates by age in antidepressant trials
| Age group (yr) | Suicide rate (%)(test drug/placebo) | Suicide attempt rate (%)(test drug/placebo) |
|---|---|---|
| 18 to 24 | 0.03/0.00 | 0.55/0.27 |
| 25 to 30 | 0.00/0.03 | 0.23/0.11 |
| 31 to 64 | 0.01/0.00 | 0.13/0.15 |
| ≥65 | 0.00/0.04 | 0.03/0.25 |
| Source: Reference 13 | ||
The odds ratio for suicidal behavior (preparatory acts, attempt, or suicide) for subacts, attempt, or suicide) for subjects age 18 to 24 on test drug vs placebo was 2.31 (95% CI: 1.02, 5.64) [event rate/sample: 23/3810 vs 8/2604]. NNH was 333, which means 333 adults in this age group would need to be treated with an antidepressant for 1 to experience a suicidal behavior event that would not have happened with placebo.
1. Kuehn BM. FDA panel seeks to balance risks in warnings for antidepressants. J Am Med Assoc 2007;297:573-4.
2. Grunebaum MF, Ellis SP, Li S, et al. Antidepressants and suicide risk in the United States, 1985-1999. J Clin Psychiatry 2004;65(11):1456-62.
3. Teicher MH, Glod C, Cole JO. Emergence of intense suicidal preoccupation during fluoxetine treatment. Am J Psychiatry 1990;147:207-10.
4. King RA, Riddle MA, Chappell PB, et al. Emergence of self-destructive phenomena in children and adolescents during fluoxetine treatment. J Am Acad Child Adolesc Psychiatry 1991;30(2):179-86.
5. Rothschild AJ, Locke CA. Reexposure to fluoxetine after serious suicide attempts by three patients: the role of akathisia. J Clin Psychiatry 1991;52:491-3.
6. Beasley CM, Dornseif BE, Bosomworth JC, et al. Fluoxetine and suicide: a meta-analysis of controlled trials of treatment for depression. BMJ 1991;303:685-92.
7. Hammad TA. Review and evaluation of clinical data. Food and Drug Administration. August 16, 2004. Available at: http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-10-TAB08-Hammads-Review.pdf. Accessed September 19, 2007.
8. Nemeroff CB, Kalali A, Keller MB, et al. Impact of publicity concerning pediatric suicidality data on physician practice patterns in the United States. Arch Gen Psychiatry 2007;64(4):466-72.
9. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry 2007;164:1356-63.
10. Libby AM, Brent DA, Morrato EH, et al. Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. Am J Psychiatry 2007;164(6):884-91.
11. US. Department of Health and Human Services. Centers for Disease Control and Prevention. Fatal injury reports. Web-based injury statistics query and reporting system. Available at: http://www.cdc.gov/NCIPC/wisqars. Accessed July 16, 2007.
12. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA 2007;297:1683-96.
13. Levenson M, Holland C. Statistical evaluation of suicidality in adults treated with antidepressants. In: Laughren TP. Memorandum: overview for December 13 meeting of PsychopharmacologicDrugs Advisory Committee (PDAC). Center for Drug Evaluation and Research, US Food and Drug Administration. November 16, 2006. Available at: http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdf. Accessed October 11, 2007.
14. Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am J Psychiatry 2000;157(12):1925-32.
15. Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, et al. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Arch Intern Med 2006;166(7):719-28.
16. Khan A, Khan S, Kolts R, Brown WA. Suicide rates in clinical trials of SSRIs, other antidepressants, and placebo: analysis of FDA reports. Am J Psychiatry 2003;160:790-2.
17. Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. J Am Med Assoc 2004;292(3):338-43.
18. Martinez C, Rietbrock S, Wise L, et al. Antidepressant treatment and the risk of fatal and non-fatal self harm in first episode depression: nested case-control study. BMJ 2005;330(7488):389.-
19. Gunnell D, Saperia J, Ashby D. Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA’s safety review. BMJ 2005;330(7488):385-9.
20. Fergusson D, Doucette S, Glass KC, et al. Association between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trials. BMJ 2005;330(7488):396.-
21. Juurlink DN, Mamdani MM, Kopp A, Redelmeier DA. The risk of suicide with selective serotonin reuptake inhibitors in the elderly. Am J Psychiatry 2006;163(5):813-21.
22. Isacsson G, Holmgren P, Ahlner J. Selective serotonin reuptake inhibitor antidepressants and the risk of suicide: a controlled forensic database study of 14,857 suicides. Acta Psychiatr Scand. 2005;111(4):286-90.
23. Simon GE, Savarino J, Operskalski B, Wang PS. Suicide risk during antidepressant treatment. Am J Psychiatry 2006;163(1):41-7.
24. Gibbons RD, Brown CH, Hur K, et al. Relationship between antidepressants and suicide attempts: an analysis of the Veterans Health Administration data sets. Am J Psychiatry 2007;164(7):1044-9.
25. Simon GE, Savarino J. Suicide attempts among patients starting depression treatment with medications or psychotherapy. Am J Psychiatry 2007;164(7):1029-34.
26. Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA 2005;294(16):2064-74.
27. Rich CL, Isacsson G. Suicide and antidepressants in South Alabama: evidence for improved treatment of depression. J Affect Disord 1997;45:135-42.
28. Isacsson G, Bergman U, Rich CL. Antidepressants, depression and suicide: an analysis of the San Diego study. J Affect Disord 1994;32:277-86.
29. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 2003;361(9358):653-61.
30. Gibbons RD, Hur K, Bhaumik DK, Mann JJ. The relationship between antidepressant medication use and rate of suicide. Arch Gen Psychiatry 2005;62(2):165-72.
31. Hall WD, Mant A, Mitchell PB, et al. Association between antidepressant prescribing and suicide in Australia, 1991-2000: trend analysis. BMJ 2003;326(7397):1008-11.
32. Nakagawa A, Grunebaum MF, Ellis SP, et al. Association of suicide and antidepressant prescription rates in Japan, 1999-2003. J Clin Psychiatry 2007;68(6):908-16.
33. Helgason T, Tomasson H, Zoega T. Antidepressants and public health in Iceland. Time series analysis of national data. Br J Psychiatry 2004;184:157-62.
34. March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA 2004;292(7):807-20.
CASE: Life is 'not worth it'
Mr. B, age 20, has taken a semester leave from college because of gradually worsening depressed mood. Over the past 2 months he has lost interest in jogging and playing piano—which he usually enjoys. He reports reduced libido, middle insomnia, loss of appetite, feeling as if his head is “full of cotton,” trouble concentrating, and waking in the morning with a sense of dread. His anxiety dissipates during the day, but he continues to feel sad and sometimes weepy, which is unusual for him.
Mr. B reports feeling hopeless at times and has had vague thoughts about life being “not worth it if I continue to feel like this” but denies specific suicide plans. Your initial impression is that Mr. B is in the midst of a major depressive episode and that a selective serotonin reuptake inhibitor (SSRI) is indicated. As you finish taking his history, you run through your mind the pros and cons of the recommendation you will make to him.
Do SSRIs raise or lower the risk for suicidal behavior in young adults such as Mr. B? The answer is complicated and goes beyond an “either/or” question, as the FDA acknowledged in May 2007 when it:
- extended the black-box warning of increased suicidality risk with antidepressants to cover adults age 18 to 24 as well as children and adolescents
- included language in the warning about the benefits of treating depression and the suicide risk associated with untreated depression, given concerns about declining antidepressant prescriptions and rising suicides among youth.1
- To help you make informed decisions when treating depression in adults, this article reviews the studies leading up to and following the FDA’s meta-analysis of antidepressant trial data in patients age 18 and older. Our goal is to provide a framework for clinical treatment of adults age 18 to 24 and those age ≥25.
The FDA meta-analysis designed to investigate a reported association between antidepressants and suicidality in children and adolescents found contradictory results:
- Pooled adverse event data from 24 pediatric antidepressant trials totaling >4,400 patients showed a higher risk of suicidal ideation or behavior (no suicides occurred) with antidepressants (4%) vs placebo (2%).
- Systematically collected suicide-related item scores from 17 of the trials showed no evidence that antidepressants worsen suicidality or cause it to emerge.
One interpretation of these findings is that antidepressants’ effect on suicidality is small and therefore subject to measurement error.
Another is ascertainment bias; any side effect associated with active medication encourages discussion with the clinician and may distort the frequency of reported adverse events.
The FDA meta-analysis also found:
- Relative risk for suicidality ranged 10-fold among agents, from 0.9 with fluoxetine to 8.8 with venlafaxine.
- Most suicide-related events occurred in subjects having the highest baseline levels of suicidality.
- Hostility and agitation emerged with SSRI use, particularly during the first month of treatment.
- Patient age, sex, or history of suicide attempt/ideation did not affect the results.
Source: Reference 7
First hints of suicidality
SSRIs revolutionized depression treatment. From 1985 to 1999, annual U.S. antidepressant prescriptions quadrupled, with SSRIs accounting for 70% of the increase (see “Antidepressants and suicide risk, 1985 to 2007”). At the same time, the age-adjusted suicide rate:
- dropped 22.5% for women (who account for twice as many antidepressant prescriptions as men)
- dropped 12.8% for men (without change in the rank order of suicide methods).2
The debate rekindled in June 2003 when the British Committee on Safety of Medicines warned against using paroxetine or venlafaxine in children. After conducting its own meta-analysis, the FDA in 2004 ordered a black-box warning about suicidality and the use of antidepressants in children and adolescents ( Box ).7
After the pediatric ‘black box.’ Antidepressant prescriptions for children and adolescents declined in the years 2003 to 2004, as did diagnosis of pediatric depression.8-10 Antidepressant prescribing also showed signs of shifting from general practitioners to psychiatrists.8 At the same time, the suicide rate among youth age 11 In patients age >60, SSRI prescriptions continued to rise and suicide rates fell,9 a pattern of change consistent with antidepressants protecting against suicide.
An independent meta-analysis by Bridge et al12 examined the pediatric trial data used in the FDA meta-analysis plus 7 additional studies. Its findings differ in 2 important ways from those of the FDA review:
- Antidepressants—including others besides fluoxetine—showed efficacy in treating anxiety disorders and depression in children and adolescents.
- The frequency of suicide-related adverse events (no trial patients committed suicide) was approximately 3% on active medication—25% lower than the FDA estimated rate—and 2% on placebo, similar to the FDA estimate.
Antidepressants and suicide risk, 1985 to 2007
| 1985 | 1990 | 1991 | 1999 | 2003 | 2004 | 2006 | 2007 |
|---|---|---|---|---|---|---|---|
| Case reports suggest link between suicide and SSRI use | FDA analysis finds no association between SSRIs and increased suicide risk | UK agency warns of suicide-related events in children treated with paroxetine and venlafaxine | FDA conducts meta-analysis, requires black-box warnings of risk of suicidality in youth taking antidepressants | FDA meta-analysis finds age-dependent effect of antidepressants on suicidality risk in adults | FDA expands warning of increased suicidality risk with antidepressants to adults age | ||
| Antidepressant prescriptions quadruple; age-adjusted suicide rate drops 22.5% for women and 12.8% for men | Pediatric depression diagnoses and antidepressant prescriptions decline; suicides increase 11% | Bridge et al meta-analysis finds 25% lower rate of suicide-related events in youth than the FDA found | |||||
What about adults?
Overall effect. A subsequent FDA meta-analysis of antidepressant clinical trial data in adults13 found 8 suicides in 372 trials totaling nearly 100,000 persons. All occurred in the 295 trials with psychiatric indications. Among these psychiatric trials, 59% had a suicidal behavior/ideation event in either the test-drug or placebo arm, and 41% had none. Eleven antidepressants were included in the meta-analysis:
- 6 SSRIs (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline)
- 2 SNRIs (duloxetine and venlafaxine)
- 3 others (bupropion, mirtazapine, and nefazodone).
Age-specific findings. When the FDA analysis was stratified by age, however, antidepressants’ benefit appeared greater for patients age ≥25 than for those age 18 to 24. The data suggested:
- elevated suicidality risk among adults age
- neutral or possibly protective effect for adults age 25 to 64
- protective effect in adults age ≥65 ( Table 1 ).13
FDA meta-analysis: Suicide rates by age in antidepressant trials
| Age group (yr) | Suicide rate (%)(test drug/placebo) | Suicide attempt rate (%)(test drug/placebo) |
|---|---|---|
| 18 to 24 | 0.03/0.00 | 0.55/0.27 |
| 25 to 30 | 0.00/0.03 | 0.23/0.11 |
| 31 to 64 | 0.01/0.00 | 0.13/0.15 |
| ≥65 | 0.00/0.04 | 0.03/0.25 |
| Source: Reference 13 | ||
The odds ratio for suicidal behavior (preparatory acts, attempt, or suicide) for subacts, attempt, or suicide) for subjects age 18 to 24 on test drug vs placebo was 2.31 (95% CI: 1.02, 5.64) [event rate/sample: 23/3810 vs 8/2604]. NNH was 333, which means 333 adults in this age group would need to be treated with an antidepressant for 1 to experience a suicidal behavior event that would not have happened with placebo.
CASE: Life is 'not worth it'
Mr. B, age 20, has taken a semester leave from college because of gradually worsening depressed mood. Over the past 2 months he has lost interest in jogging and playing piano—which he usually enjoys. He reports reduced libido, middle insomnia, loss of appetite, feeling as if his head is “full of cotton,” trouble concentrating, and waking in the morning with a sense of dread. His anxiety dissipates during the day, but he continues to feel sad and sometimes weepy, which is unusual for him.
Mr. B reports feeling hopeless at times and has had vague thoughts about life being “not worth it if I continue to feel like this” but denies specific suicide plans. Your initial impression is that Mr. B is in the midst of a major depressive episode and that a selective serotonin reuptake inhibitor (SSRI) is indicated. As you finish taking his history, you run through your mind the pros and cons of the recommendation you will make to him.
Do SSRIs raise or lower the risk for suicidal behavior in young adults such as Mr. B? The answer is complicated and goes beyond an “either/or” question, as the FDA acknowledged in May 2007 when it:
- extended the black-box warning of increased suicidality risk with antidepressants to cover adults age 18 to 24 as well as children and adolescents
- included language in the warning about the benefits of treating depression and the suicide risk associated with untreated depression, given concerns about declining antidepressant prescriptions and rising suicides among youth.1
- To help you make informed decisions when treating depression in adults, this article reviews the studies leading up to and following the FDA’s meta-analysis of antidepressant trial data in patients age 18 and older. Our goal is to provide a framework for clinical treatment of adults age 18 to 24 and those age ≥25.
The FDA meta-analysis designed to investigate a reported association between antidepressants and suicidality in children and adolescents found contradictory results:
- Pooled adverse event data from 24 pediatric antidepressant trials totaling >4,400 patients showed a higher risk of suicidal ideation or behavior (no suicides occurred) with antidepressants (4%) vs placebo (2%).
- Systematically collected suicide-related item scores from 17 of the trials showed no evidence that antidepressants worsen suicidality or cause it to emerge.
One interpretation of these findings is that antidepressants’ effect on suicidality is small and therefore subject to measurement error.
Another is ascertainment bias; any side effect associated with active medication encourages discussion with the clinician and may distort the frequency of reported adverse events.
The FDA meta-analysis also found:
- Relative risk for suicidality ranged 10-fold among agents, from 0.9 with fluoxetine to 8.8 with venlafaxine.
- Most suicide-related events occurred in subjects having the highest baseline levels of suicidality.
- Hostility and agitation emerged with SSRI use, particularly during the first month of treatment.
- Patient age, sex, or history of suicide attempt/ideation did not affect the results.
Source: Reference 7
First hints of suicidality
SSRIs revolutionized depression treatment. From 1985 to 1999, annual U.S. antidepressant prescriptions quadrupled, with SSRIs accounting for 70% of the increase (see “Antidepressants and suicide risk, 1985 to 2007”). At the same time, the age-adjusted suicide rate:
- dropped 22.5% for women (who account for twice as many antidepressant prescriptions as men)
- dropped 12.8% for men (without change in the rank order of suicide methods).2
The debate rekindled in June 2003 when the British Committee on Safety of Medicines warned against using paroxetine or venlafaxine in children. After conducting its own meta-analysis, the FDA in 2004 ordered a black-box warning about suicidality and the use of antidepressants in children and adolescents ( Box ).7
After the pediatric ‘black box.’ Antidepressant prescriptions for children and adolescents declined in the years 2003 to 2004, as did diagnosis of pediatric depression.8-10 Antidepressant prescribing also showed signs of shifting from general practitioners to psychiatrists.8 At the same time, the suicide rate among youth age 11 In patients age >60, SSRI prescriptions continued to rise and suicide rates fell,9 a pattern of change consistent with antidepressants protecting against suicide.
An independent meta-analysis by Bridge et al12 examined the pediatric trial data used in the FDA meta-analysis plus 7 additional studies. Its findings differ in 2 important ways from those of the FDA review:
- Antidepressants—including others besides fluoxetine—showed efficacy in treating anxiety disorders and depression in children and adolescents.
- The frequency of suicide-related adverse events (no trial patients committed suicide) was approximately 3% on active medication—25% lower than the FDA estimated rate—and 2% on placebo, similar to the FDA estimate.
Antidepressants and suicide risk, 1985 to 2007
| 1985 | 1990 | 1991 | 1999 | 2003 | 2004 | 2006 | 2007 |
|---|---|---|---|---|---|---|---|
| Case reports suggest link between suicide and SSRI use | FDA analysis finds no association between SSRIs and increased suicide risk | UK agency warns of suicide-related events in children treated with paroxetine and venlafaxine | FDA conducts meta-analysis, requires black-box warnings of risk of suicidality in youth taking antidepressants | FDA meta-analysis finds age-dependent effect of antidepressants on suicidality risk in adults | FDA expands warning of increased suicidality risk with antidepressants to adults age | ||
| Antidepressant prescriptions quadruple; age-adjusted suicide rate drops 22.5% for women and 12.8% for men | Pediatric depression diagnoses and antidepressant prescriptions decline; suicides increase 11% | Bridge et al meta-analysis finds 25% lower rate of suicide-related events in youth than the FDA found | |||||
What about adults?
Overall effect. A subsequent FDA meta-analysis of antidepressant clinical trial data in adults13 found 8 suicides in 372 trials totaling nearly 100,000 persons. All occurred in the 295 trials with psychiatric indications. Among these psychiatric trials, 59% had a suicidal behavior/ideation event in either the test-drug or placebo arm, and 41% had none. Eleven antidepressants were included in the meta-analysis:
- 6 SSRIs (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline)
- 2 SNRIs (duloxetine and venlafaxine)
- 3 others (bupropion, mirtazapine, and nefazodone).
Age-specific findings. When the FDA analysis was stratified by age, however, antidepressants’ benefit appeared greater for patients age ≥25 than for those age 18 to 24. The data suggested:
- elevated suicidality risk among adults age
- neutral or possibly protective effect for adults age 25 to 64
- protective effect in adults age ≥65 ( Table 1 ).13
FDA meta-analysis: Suicide rates by age in antidepressant trials
| Age group (yr) | Suicide rate (%)(test drug/placebo) | Suicide attempt rate (%)(test drug/placebo) |
|---|---|---|
| 18 to 24 | 0.03/0.00 | 0.55/0.27 |
| 25 to 30 | 0.00/0.03 | 0.23/0.11 |
| 31 to 64 | 0.01/0.00 | 0.13/0.15 |
| ≥65 | 0.00/0.04 | 0.03/0.25 |
| Source: Reference 13 | ||
The odds ratio for suicidal behavior (preparatory acts, attempt, or suicide) for subacts, attempt, or suicide) for subjects age 18 to 24 on test drug vs placebo was 2.31 (95% CI: 1.02, 5.64) [event rate/sample: 23/3810 vs 8/2604]. NNH was 333, which means 333 adults in this age group would need to be treated with an antidepressant for 1 to experience a suicidal behavior event that would not have happened with placebo.
1. Kuehn BM. FDA panel seeks to balance risks in warnings for antidepressants. J Am Med Assoc 2007;297:573-4.
2. Grunebaum MF, Ellis SP, Li S, et al. Antidepressants and suicide risk in the United States, 1985-1999. J Clin Psychiatry 2004;65(11):1456-62.
3. Teicher MH, Glod C, Cole JO. Emergence of intense suicidal preoccupation during fluoxetine treatment. Am J Psychiatry 1990;147:207-10.
4. King RA, Riddle MA, Chappell PB, et al. Emergence of self-destructive phenomena in children and adolescents during fluoxetine treatment. J Am Acad Child Adolesc Psychiatry 1991;30(2):179-86.
5. Rothschild AJ, Locke CA. Reexposure to fluoxetine after serious suicide attempts by three patients: the role of akathisia. J Clin Psychiatry 1991;52:491-3.
6. Beasley CM, Dornseif BE, Bosomworth JC, et al. Fluoxetine and suicide: a meta-analysis of controlled trials of treatment for depression. BMJ 1991;303:685-92.
7. Hammad TA. Review and evaluation of clinical data. Food and Drug Administration. August 16, 2004. Available at: http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-10-TAB08-Hammads-Review.pdf. Accessed September 19, 2007.
8. Nemeroff CB, Kalali A, Keller MB, et al. Impact of publicity concerning pediatric suicidality data on physician practice patterns in the United States. Arch Gen Psychiatry 2007;64(4):466-72.
9. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry 2007;164:1356-63.
10. Libby AM, Brent DA, Morrato EH, et al. Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. Am J Psychiatry 2007;164(6):884-91.
11. US. Department of Health and Human Services. Centers for Disease Control and Prevention. Fatal injury reports. Web-based injury statistics query and reporting system. Available at: http://www.cdc.gov/NCIPC/wisqars. Accessed July 16, 2007.
12. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA 2007;297:1683-96.
13. Levenson M, Holland C. Statistical evaluation of suicidality in adults treated with antidepressants. In: Laughren TP. Memorandum: overview for December 13 meeting of PsychopharmacologicDrugs Advisory Committee (PDAC). Center for Drug Evaluation and Research, US Food and Drug Administration. November 16, 2006. Available at: http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdf. Accessed October 11, 2007.
14. Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am J Psychiatry 2000;157(12):1925-32.
15. Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, et al. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Arch Intern Med 2006;166(7):719-28.
16. Khan A, Khan S, Kolts R, Brown WA. Suicide rates in clinical trials of SSRIs, other antidepressants, and placebo: analysis of FDA reports. Am J Psychiatry 2003;160:790-2.
17. Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. J Am Med Assoc 2004;292(3):338-43.
18. Martinez C, Rietbrock S, Wise L, et al. Antidepressant treatment and the risk of fatal and non-fatal self harm in first episode depression: nested case-control study. BMJ 2005;330(7488):389.-
19. Gunnell D, Saperia J, Ashby D. Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA’s safety review. BMJ 2005;330(7488):385-9.
20. Fergusson D, Doucette S, Glass KC, et al. Association between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trials. BMJ 2005;330(7488):396.-
21. Juurlink DN, Mamdani MM, Kopp A, Redelmeier DA. The risk of suicide with selective serotonin reuptake inhibitors in the elderly. Am J Psychiatry 2006;163(5):813-21.
22. Isacsson G, Holmgren P, Ahlner J. Selective serotonin reuptake inhibitor antidepressants and the risk of suicide: a controlled forensic database study of 14,857 suicides. Acta Psychiatr Scand. 2005;111(4):286-90.
23. Simon GE, Savarino J, Operskalski B, Wang PS. Suicide risk during antidepressant treatment. Am J Psychiatry 2006;163(1):41-7.
24. Gibbons RD, Brown CH, Hur K, et al. Relationship between antidepressants and suicide attempts: an analysis of the Veterans Health Administration data sets. Am J Psychiatry 2007;164(7):1044-9.
25. Simon GE, Savarino J. Suicide attempts among patients starting depression treatment with medications or psychotherapy. Am J Psychiatry 2007;164(7):1029-34.
26. Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA 2005;294(16):2064-74.
27. Rich CL, Isacsson G. Suicide and antidepressants in South Alabama: evidence for improved treatment of depression. J Affect Disord 1997;45:135-42.
28. Isacsson G, Bergman U, Rich CL. Antidepressants, depression and suicide: an analysis of the San Diego study. J Affect Disord 1994;32:277-86.
29. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 2003;361(9358):653-61.
30. Gibbons RD, Hur K, Bhaumik DK, Mann JJ. The relationship between antidepressant medication use and rate of suicide. Arch Gen Psychiatry 2005;62(2):165-72.
31. Hall WD, Mant A, Mitchell PB, et al. Association between antidepressant prescribing and suicide in Australia, 1991-2000: trend analysis. BMJ 2003;326(7397):1008-11.
32. Nakagawa A, Grunebaum MF, Ellis SP, et al. Association of suicide and antidepressant prescription rates in Japan, 1999-2003. J Clin Psychiatry 2007;68(6):908-16.
33. Helgason T, Tomasson H, Zoega T. Antidepressants and public health in Iceland. Time series analysis of national data. Br J Psychiatry 2004;184:157-62.
34. March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA 2004;292(7):807-20.
1. Kuehn BM. FDA panel seeks to balance risks in warnings for antidepressants. J Am Med Assoc 2007;297:573-4.
2. Grunebaum MF, Ellis SP, Li S, et al. Antidepressants and suicide risk in the United States, 1985-1999. J Clin Psychiatry 2004;65(11):1456-62.
3. Teicher MH, Glod C, Cole JO. Emergence of intense suicidal preoccupation during fluoxetine treatment. Am J Psychiatry 1990;147:207-10.
4. King RA, Riddle MA, Chappell PB, et al. Emergence of self-destructive phenomena in children and adolescents during fluoxetine treatment. J Am Acad Child Adolesc Psychiatry 1991;30(2):179-86.
5. Rothschild AJ, Locke CA. Reexposure to fluoxetine after serious suicide attempts by three patients: the role of akathisia. J Clin Psychiatry 1991;52:491-3.
6. Beasley CM, Dornseif BE, Bosomworth JC, et al. Fluoxetine and suicide: a meta-analysis of controlled trials of treatment for depression. BMJ 1991;303:685-92.
7. Hammad TA. Review and evaluation of clinical data. Food and Drug Administration. August 16, 2004. Available at: http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-10-TAB08-Hammads-Review.pdf. Accessed September 19, 2007.
8. Nemeroff CB, Kalali A, Keller MB, et al. Impact of publicity concerning pediatric suicidality data on physician practice patterns in the United States. Arch Gen Psychiatry 2007;64(4):466-72.
9. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry 2007;164:1356-63.
10. Libby AM, Brent DA, Morrato EH, et al. Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. Am J Psychiatry 2007;164(6):884-91.
11. US. Department of Health and Human Services. Centers for Disease Control and Prevention. Fatal injury reports. Web-based injury statistics query and reporting system. Available at: http://www.cdc.gov/NCIPC/wisqars. Accessed July 16, 2007.
12. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA 2007;297:1683-96.
13. Levenson M, Holland C. Statistical evaluation of suicidality in adults treated with antidepressants. In: Laughren TP. Memorandum: overview for December 13 meeting of PsychopharmacologicDrugs Advisory Committee (PDAC). Center for Drug Evaluation and Research, US Food and Drug Administration. November 16, 2006. Available at: http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdf. Accessed October 11, 2007.
14. Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am J Psychiatry 2000;157(12):1925-32.
15. Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, et al. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Arch Intern Med 2006;166(7):719-28.
16. Khan A, Khan S, Kolts R, Brown WA. Suicide rates in clinical trials of SSRIs, other antidepressants, and placebo: analysis of FDA reports. Am J Psychiatry 2003;160:790-2.
17. Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. J Am Med Assoc 2004;292(3):338-43.
18. Martinez C, Rietbrock S, Wise L, et al. Antidepressant treatment and the risk of fatal and non-fatal self harm in first episode depression: nested case-control study. BMJ 2005;330(7488):389.-
19. Gunnell D, Saperia J, Ashby D. Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA’s safety review. BMJ 2005;330(7488):385-9.
20. Fergusson D, Doucette S, Glass KC, et al. Association between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trials. BMJ 2005;330(7488):396.-
21. Juurlink DN, Mamdani MM, Kopp A, Redelmeier DA. The risk of suicide with selective serotonin reuptake inhibitors in the elderly. Am J Psychiatry 2006;163(5):813-21.
22. Isacsson G, Holmgren P, Ahlner J. Selective serotonin reuptake inhibitor antidepressants and the risk of suicide: a controlled forensic database study of 14,857 suicides. Acta Psychiatr Scand. 2005;111(4):286-90.
23. Simon GE, Savarino J, Operskalski B, Wang PS. Suicide risk during antidepressant treatment. Am J Psychiatry 2006;163(1):41-7.
24. Gibbons RD, Brown CH, Hur K, et al. Relationship between antidepressants and suicide attempts: an analysis of the Veterans Health Administration data sets. Am J Psychiatry 2007;164(7):1044-9.
25. Simon GE, Savarino J. Suicide attempts among patients starting depression treatment with medications or psychotherapy. Am J Psychiatry 2007;164(7):1029-34.
26. Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA 2005;294(16):2064-74.
27. Rich CL, Isacsson G. Suicide and antidepressants in South Alabama: evidence for improved treatment of depression. J Affect Disord 1997;45:135-42.
28. Isacsson G, Bergman U, Rich CL. Antidepressants, depression and suicide: an analysis of the San Diego study. J Affect Disord 1994;32:277-86.
29. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 2003;361(9358):653-61.
30. Gibbons RD, Hur K, Bhaumik DK, Mann JJ. The relationship between antidepressant medication use and rate of suicide. Arch Gen Psychiatry 2005;62(2):165-72.
31. Hall WD, Mant A, Mitchell PB, et al. Association between antidepressant prescribing and suicide in Australia, 1991-2000: trend analysis. BMJ 2003;326(7397):1008-11.
32. Nakagawa A, Grunebaum MF, Ellis SP, et al. Association of suicide and antidepressant prescription rates in Japan, 1999-2003. J Clin Psychiatry 2007;68(6):908-16.
33. Helgason T, Tomasson H, Zoega T. Antidepressants and public health in Iceland. Time series analysis of national data. Br J Psychiatry 2004;184:157-62.
34. March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA 2004;292(7):807-20.

