User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Can medications prevent PTSD in trauma victims?
Posttraumatic stress disorder (PTSD) is a preventable mental illness—without trauma, the illness does not occur. Primary prevention (such as eliminating war, rape, physical assaults, child abuse, or motor vehicle accidents) would be effective but is an unrealistic goal. Secondary prevention (such as preventing PTSD after individuals have been exposed to trauma) may be attainable.
No medication is FDA-approved to prevent PTSD, but patients recently exposed to trauma might benefit from drugs approved for other indications. Possibilities include noradrenergics such as propranolol, corticosteroids that affect the hypothalamic-pituitary-adrenal (HPA) axis, opioids, benzodiazepines, and antidepressants. Some investigational agents also might block the process that turns a traumatic experience into PTSD.
This article discusses these intriguing ideas and suggests which trauma victims might benefit now from acute pharmacologic PTSD prevention.
Who might be treated?
An estimated 8% to 10% of the U.S. population experiences PTSD at some point in life (Box 1).1,2 A person’s risk of developing PTSD after a traumatic event depends on the type of trauma. For example, 10% of motor vehicle accident survivors develop PTSD, compared with 60% of rape survivors.1
Targeting anyone who has experienced trauma for secondary PTSD prevention would expose large groups of people to medications they do not need. Targeting selected persons who are at the highest risk would be more efficient and cost-effective. In a group of acute trauma-exposed persons, 2 selection criteria could be considered simultaneously:
- Which patients may be most predisposed to PTSD?
- Which patients are showing early symptoms that may predict PTSD?
More than half of all American adults have been exposed to at least one traumatic event at some point in their lives.1 In most persons, the posttraumatic stress reaction causes short-term distress, with hyperarousal, agitation, intrusive memories, and exaggerated startle. Although these symptoms usually subside relatively quickly, they persist and evolve into posttraumatic stress disorder (PTSD) in a substantial number of trauma victims.
An estimated 8% to 10% of the U.S. population experiences PTSD at some point in life.2 Emotional distress, social and occupational disability, and persistent decrements in quality of life make PTSD a major public health problem.
Risk factors and resiliency. Certain factors have been shown to increase a person’s vulnerability for PTSD (Table 1).3 Other proposed risk factors include:
- personality types4
- psychophysiologic factors such as reactivity, conditionability, and resistance to extinction/habituation.5
Strong evidence also indicates that acute trauma-related symptoms—including excessive arousal and fear,6 peritraumatic dissociation, and depression—predict the later development of PTSD.
Once identified, individuals predisposed to developing PTSD could be given treatment to increase their resiliency after they have been exposed to trauma. Early evidence suggests that you also could consider giving these patients medications as secondary prevention (Table 2).
Table 1
Who develops PTSD? Risk and resiliency factors
Risk factors that may increase vulnerability for PTSD
|
Resiliency factors that may protect against PTSD
|
| Source: Reference 3 |
Table 2
Medications being studied for PTSD prevention
| Mechanism of action | Medication | FDA-approved indications | ||
|---|---|---|---|---|
| Psychiatric | Nonpsychiatric | |||
| Noradrenergic | Clonidine | No | Yes | |
| Guanfacine | No | Yes | ||
| Prazosin | No | Yes | ||
| Propranolol | No | Yes | ||
| Hypothalamic-pituitary-adrenal axis | Hydrocortisone | No | Yes | |
| Opioid | Morphine | No | Yes | |
| Antidepressant | Dual action | Duloxetine | Yes | Yes |
| Venlafaxine | Yes | No | ||
| SSRIs | Citalopram | Yes | No | |
| Fluoxetine | Yes | No | ||
| Paroxetine | Yes | No | ||
| Sertraline | Yes | No | ||
| TCAs | Amitriptyline | Yes | No | |
| Imipramine | Yes | No | ||
| GABA-benzodiazepine | Alprazolam | Yes | No | |
| Temazepam | Yes | No | ||
| Corticotropin-releasing hormone (CRH) | CRH antagonist | Investigational | ||
| Substance P | Substance P antagonist | Investigational | ||
| Neuropeptide Y | Neuropeptide Y agonist | Investigational | ||
| SSRIs: selective serotonin reuptake inhibitors | ||||
| TCAs: tricyclic antidepressants | ||||
Targeting noradrenergic activity
Increased noradrenergic activity has been associated with persistent memories and PTSD. Therefore, medications that reduce noradrenergic tone by blocking receptors or reduce norepinephrine release are being explored for PTSD prevention.
Propranolol. Three small studies have examined whether the beta-noradrenergic receptor blocker propranolol can prevent PTSD.
In a randomized, double-blind, placebo-controlled trial,7 41 emergency department patients who had a heart rate of ≥ 80 bpm within 6 hours of a traumatic accident received propranolol, 40 mg qid, or placebo for 10 days. After 1 month, the 11 patients who completed propranolol treatment showed a nonsignificant trend toward lower scores on the Clinician-Administered PTSD Scale (CAPS), compared with 20 patients taking placebo. At 3 months, the propranolol group had less physiologic reactivity (as measured by heart rate and skin conductance) to trauma-related cues than the placebo group.
In a nonrandomized study,8 PTSD developed within 2 months in 1 of 11 trauma victims who agreed to take propranolol, 40 mg tid, immediately after the trauma, compared with 3 of 8 victims who refused the medication.
In an unpublished randomized, double-blind trial,9 48 patients admitted to a level I trauma center received propranolol, 40 mg tid; gabapentin, 400 mg tid; or placebo for PTSD prevention. Gabapentin was chosen because it has few side effects or metabolic interactions and preliminary evidence of anxiolytic efficacy.
Neither propranolol nor gabapentin showed statistically significant benefit in preventing PTSD compared with placebo. Effect sizes with the 2 treatments were too small to suggest that larger samples would produce a statistically significant result.
Prazosin—an alpha-1 adrenergic receptor antagonist—has been evaluated in 3 controlled studies and found to reduce intrusive nightmares typical of chronic PTSD.
Ten combat veterans with chronic PTSD showed significantly improved sleep, fewer severe nightmares, and improved global clinical status after receiving prazosin (mean dose 9.5 mg at bedtime) in a 20-week, placebo-controlled, double-blind, crossover study.10
In a larger randomized, parallel group trial,11 the same authors compared prazosin with placebo in 40 combat veterans (mean age 56) with chronic PTSD. After 8 weeks, veterans taking prazosin (mean 13.3 ± 3 mg) had significantly fewer trauma nightmares, improved sleep (including return of normal dreams), and improved global clinical status vs placebo. Overall CAP scores did not decline significantly, however.
In a third placebo-controlled study,12 a midmorning dose of prazosin was added to the regimens of 11 civilian trauma patients already taking the drug at bedtime to suppress trauma-related nightmares. Their daytime PTSD symptoms improved, as shown by reduced psychological distress in response to verbal trauma cues.
Prazosin can reduce chronic PTSD manifestations of nightmares and disturbed sleep, but it has not been shown to ameliorate the full PTSD syndrome. Prazosin has not been studied as an early PTSD intervention.
Other antiadrenergics that reduce the release of norepinephrine—including clonidine and guanfacine—have been studied in open trials as treatment for PTSD. The only controlled study13 showed no benefit from guanfacine for PTSD prevention.
De-stressing the HPA axis
Hydrocortisone has been proposed to prevent PTSD by reducing HPA axis activation, acting as a countermeasure to elevated corticotropin-releasing factor found in patients with chronic PTSD.
IV hydrocortisone’s effect on the development of PTSD was compared with placebo in 20 septic shock survivors after discharge from intensive care.14 One of 9 patients (11%) in the hydrocortisone group was diagnosed with PTSD at follow-up (mean 31 months), compared with 7 of 11 (64%) in the placebo group.
In a similar study, the same researchers gave patients hydrocortisone before, during, and after cardiac surgery. Follow-up interviews revealed significantly lower PTSD and chronic stress symptom scores in the treatment group vs the placebo group.15
These studies—although provocative—are limited by the narrow range of trauma related to severe medical illness or extensive medical procedures.
Norepinephrine-blocking opioids
When the noradrenergic system is activated, one physiologic response is the activation of endogenous opioid systems, which may promote recovery by inhibiting the HPA axis. Opioid systems might be involved in PTSD, as suggested by:
- preclinical evidence that opioids modulate memory16
- studies showing low pain thresholds17 and abnormal beta-endorphin (an opioid peptide neurotransmitter)18 and methionine enkephalin (an opioid peptide)19 levels in PTSD patients.
In theory, opioid administration immediately after trauma may attenuate norepinephrine release, thus thwarting arousal-charged memory consolidation, hyperarousal, and re-experiencing.
One uncontrolled report of pediatric burn victims found a significant association between the morphine dose given for pain during hospitalization and reduced PTSD symptoms 6 months later.20 Decreased pain did not explain the reduction in PTSD, as no significant correlation was seen between pain symptoms and PTSD outcome measures. Similarly, a longitudinal study of substance use among Vietnam War veterans with PTSD found decreased hyperarousal symptoms in heroin users.21
Using opioids to prevent PTSD would be feasible and efficient in acute care settings because 80% to 90% of traumatically-injured patients are discharged on opioid analgesics (compared with <10% on beta blockers or corticosteroids).22 However, 20% to 40% of physically injured inpatients are diagnosed with a substance use disorder at some point in life, making the use of opioid analgesics a practical concern.23
GABA-benzodiazepine paradox
The GABA-benzodiazepine system plays an important role in mediating anxiety, which is consistent with the potent anxiolytic effects of benzodiazepines. Even so, trials of benzodiazepines have found these drugs surprisingly unhelpful—and perhaps harmful—in patients with acute trauma.
Alprazolam did not reduce PTSD symptoms in a small randomized, double-blind study.24 Another trial found that receiving benzodiazepines shortly after trauma exposure was associated with increased PTSD risk in trauma survivors. Nine of 13 patients (69%) who received alprazolam or clonazepam met PTSD diagnostic criteria 6 months after the trauma, compared with 3 of 13 controls (15%).25
Similarly, in a randomized controlled trial, 22 patients were given temazepam for 7 nights, starting approximately 14 days after exposure to a traumatic event. Six weeks later, 55% of those receiving temazepam and 27% of those receiving placebo met criteria for PTSD.26
In summary, benzodiazepines might be helpful when given for a few days after traumatization to control overwhelming anxiety but could be harmful over a longer term.
Other agents for PTSD
Antidepressants. Early trauma-related symptoms of depression predict later development of PTSD.27 Thus, antidepressants have been proposed for early intervention in addition to their well-established role as first-line treatment of PTSD.28
One study supports this idea: a 7-day randomized double-blind trial that compared the tricyclic antidepressant imipramine with chloral hydrate in pediatric burn patients with acute stress disorder (ASD). Imipramine was more effective (83% response) than chloral hydrate (38% response) in reducing ASD symptoms.29
Drugs in development. Three new medications being explored for treating anxiety and depression also might be useful for PTSD prevention. Neuropeptide Y (NPY) agonists,30 substance P antagonists,31 and CRH-antagonists32 are thought to hold promise because of their more proximate roles—compared with monoamine neurotransmitters such as dopamine, norepinephrine and serotonin—in mediating the stress response.
Manage the post-trauma environment:
- Move the victim to safety.
- Treat pain effectively.
- Avoid stress from interrogations, separation from loved ones, or unstable housing.
Avoid crisis incident stress debriefing (CISD), which could enhance physiologic hyperarousal and is not recommended as first-line treatment for most trauma victims. CISD was designed for and is best received by emergency personnel.
Consider prescribing antidepressants for patients thought to be particularly vulnerable to develop posttraumatic stress disorder (PTSD). Risk factors include:
- history of PTSD, depression, or anxiety disorder
- severe trauma (such as from sexual assault or torture)
- physical injury, when antidepressants with analgesic properties might be useful.
Analyzing the evidence
Insufficient evidence exists to determine which strategies might be most effective to prevent PTSD, what optimal dosing might be, and which traumatized individuals might be best targeted with these approaches.
- Beta-blockers and corticosteroids—the most theoretically compelling strategies—are the most difficult agents to use for PTSD prevention because they have the most medical contraindications. In addition, evidence supporting their ability to prevent PTSD is meager at best.
- Prazosin is intriguing but has contra-indications similar to those of beta blockers, no studies of secondary prevention, and no clear indication that it works for the overall PTSD syndrome.
- Opioids are restricted agents with substantial contraindications.
- Evidence is limited but points most strongly toward earlier use of antidepressants. Early trauma-related symptoms of depression predict later development of PTSD,27 and a number of selective serotonin reuptake inhibitors—such as citalopram, fluoxetine, paroxetine, and sertraline—are FDA-approved or used off-label for treating PTSD.33
Prescribing recommendations. Consider practicality, ease of use, and safety of the proposed medication when choosing a drug for PTSD prevention (Table 3).22 Based on the evidence, the most reasonable posttrauma approach (Box 2) might be to consider starting an approved antidepressant for individuals thought to be particularly vulnerable to PTSD because of:
- past history of PTSD, depression, or anxiety disorder
- severity of the trauma (such as in cases of sexual assault or torture)
- pain (antidepressants with analgesic properties—such as venlafaxine or duloxetine—might be useful in patients whose trauma is associated with physical injury, although neither is FDA-approved to treat PTSD).
Table 3
4 considerations when choosing a drug for PTSD prevention
| Potential benefits | Practicality, ease of use, and safety of the proposed medication |
| Potential drug-drug or drug-disease interactions | Asthma, diabetes, and trauma are relative contraindications to the use of antiadrenergics and corticosteroids |
| Psychiatric comorbidities | A patient’s history of substance use disorder makes opioid analgesics a concern |
| Clinical experience | Agents already prescribed safely and broadly in clinical practice are easiest to test and to use |
Related resources
- Mental health and mass violence: Evidence-based early psychological intervention for victims/survivors of mass violence. A workshop to reach consensus on best practices. Rockland, MD: National Institute of Mental Health; 2002. www.nimh.nih.gov.
- Post-traumatic stress disorder: the management of PTSD in adults and children in primary and secondary care (clinical guideline 26). London, UK: National Institute for Clinical Excellence; 2005. www.nice.org.uk.
- Ursano RJ, Bell C, Eth S, et al. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Am J Psychiatry 2004;161(suppl 11):3-31.
Drug brand names
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Citalopram • Celexa
- Clonazepam • Klonopin
- Clonidine • Catapres
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Guanfacine • Tenex
- Imipramine • Tofranil
- Lamotrigine • Lamictal
- Paroxetine • Paxil
- Prazosin • Minipress
- Propranolol • Inderal
- Sertraline • Zoloft
- Temazepam • Restoril
- Venlafaxine • Effexor
Disclosure
Dr. Bennett and Dr. Zatzick report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Roy-Byrne is a consultant to Jazz Pharmaceuticals and Solvay and has received speaker honoraria from Wyeth and Forrest Pharmaceuticals.
1. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995;52(12):1048-60.
2. Zatzick D. Posttraumatic stress, functional impairment, and service utilization after injury: a public health approach. Semin Clin Neuropsychiatry 2003;8(3):149-57.
3. Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann NY Acad Sci 2006;1071:379-96.
4. Schnurr P, Vielhauer M. Personality as a risk factor for PTSD. In: Yehuda R, ed. Risk factors for post-traumatic stress disorder. Washington, DC: American Psychiatric Publishing; 1999:191-222.
5. Shalev A. Psychophysiological expression of risk factors for PTSD. In: Yehuda R, ed. Risk factors for posttraumatic stress disorder. Washington, DC: American Psychiatric Publishing; 1995.
6. Bryant RA, Harvey AG, Guthrie RM, Moulds ML. A prospective study of psychophysiological arousal, acute stress disorder, and posttraumatic stress disorder. J Abnorm Psychol 2000;109(2):341-4.
7. Pitman RK, Sanders KM, Zusman RM, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry 2002;51(2):189-92.
8. Vaiva G, Ducrocq F, Jezequel K, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry 2003;54(9):947-9.
9. Stein M. Pharmacoprevention of adverse psychiatric sequelae of physical injury. Paper presented at: 21st Annual Meeting of the International Society for Traumatic Stress Studies; November 2-5, 2005; Toronto, Ontario, Canada.
10. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry 2003;160(2):371-3.
11. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry 2007;61(8):928-34.
12. Taylor FB, Lowe K, Thompson C, et al. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol Psychiatry 2006;59(7):577-81.
13. Neylan TC, Lenoci M, Samuelson KW, et al. No improvement of posttraumatic stress disorder symptoms with guanfacine treatment. Am J Psychiatry 2006;163(12):2186-8.
14. Schelling G, Briegel J, Roozendaal B, et al. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry 2001;50(12):978-85.
15. Weis F, Kilger E, Roozendaal B, et al. Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. J Thorac Cardiovasc Surg 2006;131(2):277-82.
16. McGaugh JL, Introini-Collison IB, Nagahara AH, et al. Involvement of the amygdaloid complex in neuromodulatory influences on memory storage. Neurosci Biobehav Rev 1990;14(4):425-31.
17. Shalev AY, Peri T, Canetti L, Schreiber S. Predictors of PTSD in injured trauma survivors: a prospective study. Am J Psychiatry 1996;153(2):219-25.
18. Baker DG, West SA, Orth DN, et al. Cerebrospinal fluid and plasma beta-endorphin in combat veterans with post-traumatic stress disorder. Psychoneuroendocrinol 1997;22(7):517-29.
19. Wolf ME, Mosnaim AD, Puente J, Ignacio R. Plasma methionine-enkephalin in PTSD. Biol Psychiatry 1991;29(3):305-7.
20. Saxe G, Stoddard F, Courtney D, et al. Relationship between acute morphine and the course of PTSD in children with burns. J Am Acad Child Adolesc Psychiatry 2001;40(8):915-21.
21. Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry 1996;153(3):369-75.
22. Zatzick D, Roy-Byrne PP. From bedside to bench: how the epidemiology of clinical practice can inform the secondary prevention of PTSD. Psychiatr Serv 2006;57(12):1726-30.
23. Zatzick D, Jurkovich G, Russo J, et al. Posttraumatic distress, alcohol disorders, and recurrent trauma across level 1 trauma centers. J Trauma 2004;57(2):360-6.
24. Braun P, Greenberg D, Dasberg H, Lerer B. Core symptoms of posttraumatic stress disorder unimproved by alprazolam treatment. J Clin Psychiatry 1990;51(6):236-8.
25. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry 1996;57(9):390-4.
26. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry 2002;63(12):1183-4.
27. Freedman SA, Brandes D, Peri T, Shalev A. Predictors of chronic post-traumatic stress disorder. A prospective study. Br J Psychiatry 1999;174:353-9.
28. Davidson JR. Pharmacologic treatment of acute and chronic stress following trauma. J Clin Psychiatry 2006;67(suppl 2):34-9.
29. Robert R, Blakeney PE, Villarreal C, et al. Imipramine treatment in pediatric burn patients with symptoms of acute stress disorder: a pilot study. J Am Acad Child Adolesc Psychiatry 1999;38(7):873-82.
30. Morgan CA, Wang S, Southwick SM, et al. Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol Psychiatry 2000;47(10):902-9.
31. Geracioti TD, Carpenter LL, Owens MJ, et al. Elevated cerebrospinal fluid substance P concentrations in posttraumatic stress disorder and major depression. Am J Psychiatry 2006;163(4):637-43.
32. Zobel AW, Nickel T, Künzel HE, et al. Effects of the highaffinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res 2000;34(3):171-81.
33. Davidson JR. Treatment of posttraumatic stress disorder: the impact of paroxetine. Psychopharmacol Bull 2003;37(suppl 1):76-88.
Posttraumatic stress disorder (PTSD) is a preventable mental illness—without trauma, the illness does not occur. Primary prevention (such as eliminating war, rape, physical assaults, child abuse, or motor vehicle accidents) would be effective but is an unrealistic goal. Secondary prevention (such as preventing PTSD after individuals have been exposed to trauma) may be attainable.
No medication is FDA-approved to prevent PTSD, but patients recently exposed to trauma might benefit from drugs approved for other indications. Possibilities include noradrenergics such as propranolol, corticosteroids that affect the hypothalamic-pituitary-adrenal (HPA) axis, opioids, benzodiazepines, and antidepressants. Some investigational agents also might block the process that turns a traumatic experience into PTSD.
This article discusses these intriguing ideas and suggests which trauma victims might benefit now from acute pharmacologic PTSD prevention.
Who might be treated?
An estimated 8% to 10% of the U.S. population experiences PTSD at some point in life (Box 1).1,2 A person’s risk of developing PTSD after a traumatic event depends on the type of trauma. For example, 10% of motor vehicle accident survivors develop PTSD, compared with 60% of rape survivors.1
Targeting anyone who has experienced trauma for secondary PTSD prevention would expose large groups of people to medications they do not need. Targeting selected persons who are at the highest risk would be more efficient and cost-effective. In a group of acute trauma-exposed persons, 2 selection criteria could be considered simultaneously:
- Which patients may be most predisposed to PTSD?
- Which patients are showing early symptoms that may predict PTSD?
More than half of all American adults have been exposed to at least one traumatic event at some point in their lives.1 In most persons, the posttraumatic stress reaction causes short-term distress, with hyperarousal, agitation, intrusive memories, and exaggerated startle. Although these symptoms usually subside relatively quickly, they persist and evolve into posttraumatic stress disorder (PTSD) in a substantial number of trauma victims.
An estimated 8% to 10% of the U.S. population experiences PTSD at some point in life.2 Emotional distress, social and occupational disability, and persistent decrements in quality of life make PTSD a major public health problem.
Risk factors and resiliency. Certain factors have been shown to increase a person’s vulnerability for PTSD (Table 1).3 Other proposed risk factors include:
- personality types4
- psychophysiologic factors such as reactivity, conditionability, and resistance to extinction/habituation.5
Strong evidence also indicates that acute trauma-related symptoms—including excessive arousal and fear,6 peritraumatic dissociation, and depression—predict the later development of PTSD.
Once identified, individuals predisposed to developing PTSD could be given treatment to increase their resiliency after they have been exposed to trauma. Early evidence suggests that you also could consider giving these patients medications as secondary prevention (Table 2).
Table 1
Who develops PTSD? Risk and resiliency factors
Risk factors that may increase vulnerability for PTSD
|
Resiliency factors that may protect against PTSD
|
| Source: Reference 3 |
Table 2
Medications being studied for PTSD prevention
| Mechanism of action | Medication | FDA-approved indications | ||
|---|---|---|---|---|
| Psychiatric | Nonpsychiatric | |||
| Noradrenergic | Clonidine | No | Yes | |
| Guanfacine | No | Yes | ||
| Prazosin | No | Yes | ||
| Propranolol | No | Yes | ||
| Hypothalamic-pituitary-adrenal axis | Hydrocortisone | No | Yes | |
| Opioid | Morphine | No | Yes | |
| Antidepressant | Dual action | Duloxetine | Yes | Yes |
| Venlafaxine | Yes | No | ||
| SSRIs | Citalopram | Yes | No | |
| Fluoxetine | Yes | No | ||
| Paroxetine | Yes | No | ||
| Sertraline | Yes | No | ||
| TCAs | Amitriptyline | Yes | No | |
| Imipramine | Yes | No | ||
| GABA-benzodiazepine | Alprazolam | Yes | No | |
| Temazepam | Yes | No | ||
| Corticotropin-releasing hormone (CRH) | CRH antagonist | Investigational | ||
| Substance P | Substance P antagonist | Investigational | ||
| Neuropeptide Y | Neuropeptide Y agonist | Investigational | ||
| SSRIs: selective serotonin reuptake inhibitors | ||||
| TCAs: tricyclic antidepressants | ||||
Targeting noradrenergic activity
Increased noradrenergic activity has been associated with persistent memories and PTSD. Therefore, medications that reduce noradrenergic tone by blocking receptors or reduce norepinephrine release are being explored for PTSD prevention.
Propranolol. Three small studies have examined whether the beta-noradrenergic receptor blocker propranolol can prevent PTSD.
In a randomized, double-blind, placebo-controlled trial,7 41 emergency department patients who had a heart rate of ≥ 80 bpm within 6 hours of a traumatic accident received propranolol, 40 mg qid, or placebo for 10 days. After 1 month, the 11 patients who completed propranolol treatment showed a nonsignificant trend toward lower scores on the Clinician-Administered PTSD Scale (CAPS), compared with 20 patients taking placebo. At 3 months, the propranolol group had less physiologic reactivity (as measured by heart rate and skin conductance) to trauma-related cues than the placebo group.
In a nonrandomized study,8 PTSD developed within 2 months in 1 of 11 trauma victims who agreed to take propranolol, 40 mg tid, immediately after the trauma, compared with 3 of 8 victims who refused the medication.
In an unpublished randomized, double-blind trial,9 48 patients admitted to a level I trauma center received propranolol, 40 mg tid; gabapentin, 400 mg tid; or placebo for PTSD prevention. Gabapentin was chosen because it has few side effects or metabolic interactions and preliminary evidence of anxiolytic efficacy.
Neither propranolol nor gabapentin showed statistically significant benefit in preventing PTSD compared with placebo. Effect sizes with the 2 treatments were too small to suggest that larger samples would produce a statistically significant result.
Prazosin—an alpha-1 adrenergic receptor antagonist—has been evaluated in 3 controlled studies and found to reduce intrusive nightmares typical of chronic PTSD.
Ten combat veterans with chronic PTSD showed significantly improved sleep, fewer severe nightmares, and improved global clinical status after receiving prazosin (mean dose 9.5 mg at bedtime) in a 20-week, placebo-controlled, double-blind, crossover study.10
In a larger randomized, parallel group trial,11 the same authors compared prazosin with placebo in 40 combat veterans (mean age 56) with chronic PTSD. After 8 weeks, veterans taking prazosin (mean 13.3 ± 3 mg) had significantly fewer trauma nightmares, improved sleep (including return of normal dreams), and improved global clinical status vs placebo. Overall CAP scores did not decline significantly, however.
In a third placebo-controlled study,12 a midmorning dose of prazosin was added to the regimens of 11 civilian trauma patients already taking the drug at bedtime to suppress trauma-related nightmares. Their daytime PTSD symptoms improved, as shown by reduced psychological distress in response to verbal trauma cues.
Prazosin can reduce chronic PTSD manifestations of nightmares and disturbed sleep, but it has not been shown to ameliorate the full PTSD syndrome. Prazosin has not been studied as an early PTSD intervention.
Other antiadrenergics that reduce the release of norepinephrine—including clonidine and guanfacine—have been studied in open trials as treatment for PTSD. The only controlled study13 showed no benefit from guanfacine for PTSD prevention.
De-stressing the HPA axis
Hydrocortisone has been proposed to prevent PTSD by reducing HPA axis activation, acting as a countermeasure to elevated corticotropin-releasing factor found in patients with chronic PTSD.
IV hydrocortisone’s effect on the development of PTSD was compared with placebo in 20 septic shock survivors after discharge from intensive care.14 One of 9 patients (11%) in the hydrocortisone group was diagnosed with PTSD at follow-up (mean 31 months), compared with 7 of 11 (64%) in the placebo group.
In a similar study, the same researchers gave patients hydrocortisone before, during, and after cardiac surgery. Follow-up interviews revealed significantly lower PTSD and chronic stress symptom scores in the treatment group vs the placebo group.15
These studies—although provocative—are limited by the narrow range of trauma related to severe medical illness or extensive medical procedures.
Norepinephrine-blocking opioids
When the noradrenergic system is activated, one physiologic response is the activation of endogenous opioid systems, which may promote recovery by inhibiting the HPA axis. Opioid systems might be involved in PTSD, as suggested by:
- preclinical evidence that opioids modulate memory16
- studies showing low pain thresholds17 and abnormal beta-endorphin (an opioid peptide neurotransmitter)18 and methionine enkephalin (an opioid peptide)19 levels in PTSD patients.
In theory, opioid administration immediately after trauma may attenuate norepinephrine release, thus thwarting arousal-charged memory consolidation, hyperarousal, and re-experiencing.
One uncontrolled report of pediatric burn victims found a significant association between the morphine dose given for pain during hospitalization and reduced PTSD symptoms 6 months later.20 Decreased pain did not explain the reduction in PTSD, as no significant correlation was seen between pain symptoms and PTSD outcome measures. Similarly, a longitudinal study of substance use among Vietnam War veterans with PTSD found decreased hyperarousal symptoms in heroin users.21
Using opioids to prevent PTSD would be feasible and efficient in acute care settings because 80% to 90% of traumatically-injured patients are discharged on opioid analgesics (compared with <10% on beta blockers or corticosteroids).22 However, 20% to 40% of physically injured inpatients are diagnosed with a substance use disorder at some point in life, making the use of opioid analgesics a practical concern.23
GABA-benzodiazepine paradox
The GABA-benzodiazepine system plays an important role in mediating anxiety, which is consistent with the potent anxiolytic effects of benzodiazepines. Even so, trials of benzodiazepines have found these drugs surprisingly unhelpful—and perhaps harmful—in patients with acute trauma.
Alprazolam did not reduce PTSD symptoms in a small randomized, double-blind study.24 Another trial found that receiving benzodiazepines shortly after trauma exposure was associated with increased PTSD risk in trauma survivors. Nine of 13 patients (69%) who received alprazolam or clonazepam met PTSD diagnostic criteria 6 months after the trauma, compared with 3 of 13 controls (15%).25
Similarly, in a randomized controlled trial, 22 patients were given temazepam for 7 nights, starting approximately 14 days after exposure to a traumatic event. Six weeks later, 55% of those receiving temazepam and 27% of those receiving placebo met criteria for PTSD.26
In summary, benzodiazepines might be helpful when given for a few days after traumatization to control overwhelming anxiety but could be harmful over a longer term.
Other agents for PTSD
Antidepressants. Early trauma-related symptoms of depression predict later development of PTSD.27 Thus, antidepressants have been proposed for early intervention in addition to their well-established role as first-line treatment of PTSD.28
One study supports this idea: a 7-day randomized double-blind trial that compared the tricyclic antidepressant imipramine with chloral hydrate in pediatric burn patients with acute stress disorder (ASD). Imipramine was more effective (83% response) than chloral hydrate (38% response) in reducing ASD symptoms.29
Drugs in development. Three new medications being explored for treating anxiety and depression also might be useful for PTSD prevention. Neuropeptide Y (NPY) agonists,30 substance P antagonists,31 and CRH-antagonists32 are thought to hold promise because of their more proximate roles—compared with monoamine neurotransmitters such as dopamine, norepinephrine and serotonin—in mediating the stress response.
Manage the post-trauma environment:
- Move the victim to safety.
- Treat pain effectively.
- Avoid stress from interrogations, separation from loved ones, or unstable housing.
Avoid crisis incident stress debriefing (CISD), which could enhance physiologic hyperarousal and is not recommended as first-line treatment for most trauma victims. CISD was designed for and is best received by emergency personnel.
Consider prescribing antidepressants for patients thought to be particularly vulnerable to develop posttraumatic stress disorder (PTSD). Risk factors include:
- history of PTSD, depression, or anxiety disorder
- severe trauma (such as from sexual assault or torture)
- physical injury, when antidepressants with analgesic properties might be useful.
Analyzing the evidence
Insufficient evidence exists to determine which strategies might be most effective to prevent PTSD, what optimal dosing might be, and which traumatized individuals might be best targeted with these approaches.
- Beta-blockers and corticosteroids—the most theoretically compelling strategies—are the most difficult agents to use for PTSD prevention because they have the most medical contraindications. In addition, evidence supporting their ability to prevent PTSD is meager at best.
- Prazosin is intriguing but has contra-indications similar to those of beta blockers, no studies of secondary prevention, and no clear indication that it works for the overall PTSD syndrome.
- Opioids are restricted agents with substantial contraindications.
- Evidence is limited but points most strongly toward earlier use of antidepressants. Early trauma-related symptoms of depression predict later development of PTSD,27 and a number of selective serotonin reuptake inhibitors—such as citalopram, fluoxetine, paroxetine, and sertraline—are FDA-approved or used off-label for treating PTSD.33
Prescribing recommendations. Consider practicality, ease of use, and safety of the proposed medication when choosing a drug for PTSD prevention (Table 3).22 Based on the evidence, the most reasonable posttrauma approach (Box 2) might be to consider starting an approved antidepressant for individuals thought to be particularly vulnerable to PTSD because of:
- past history of PTSD, depression, or anxiety disorder
- severity of the trauma (such as in cases of sexual assault or torture)
- pain (antidepressants with analgesic properties—such as venlafaxine or duloxetine—might be useful in patients whose trauma is associated with physical injury, although neither is FDA-approved to treat PTSD).
Table 3
4 considerations when choosing a drug for PTSD prevention
| Potential benefits | Practicality, ease of use, and safety of the proposed medication |
| Potential drug-drug or drug-disease interactions | Asthma, diabetes, and trauma are relative contraindications to the use of antiadrenergics and corticosteroids |
| Psychiatric comorbidities | A patient’s history of substance use disorder makes opioid analgesics a concern |
| Clinical experience | Agents already prescribed safely and broadly in clinical practice are easiest to test and to use |
Related resources
- Mental health and mass violence: Evidence-based early psychological intervention for victims/survivors of mass violence. A workshop to reach consensus on best practices. Rockland, MD: National Institute of Mental Health; 2002. www.nimh.nih.gov.
- Post-traumatic stress disorder: the management of PTSD in adults and children in primary and secondary care (clinical guideline 26). London, UK: National Institute for Clinical Excellence; 2005. www.nice.org.uk.
- Ursano RJ, Bell C, Eth S, et al. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Am J Psychiatry 2004;161(suppl 11):3-31.
Drug brand names
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Citalopram • Celexa
- Clonazepam • Klonopin
- Clonidine • Catapres
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Guanfacine • Tenex
- Imipramine • Tofranil
- Lamotrigine • Lamictal
- Paroxetine • Paxil
- Prazosin • Minipress
- Propranolol • Inderal
- Sertraline • Zoloft
- Temazepam • Restoril
- Venlafaxine • Effexor
Disclosure
Dr. Bennett and Dr. Zatzick report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Roy-Byrne is a consultant to Jazz Pharmaceuticals and Solvay and has received speaker honoraria from Wyeth and Forrest Pharmaceuticals.
Posttraumatic stress disorder (PTSD) is a preventable mental illness—without trauma, the illness does not occur. Primary prevention (such as eliminating war, rape, physical assaults, child abuse, or motor vehicle accidents) would be effective but is an unrealistic goal. Secondary prevention (such as preventing PTSD after individuals have been exposed to trauma) may be attainable.
No medication is FDA-approved to prevent PTSD, but patients recently exposed to trauma might benefit from drugs approved for other indications. Possibilities include noradrenergics such as propranolol, corticosteroids that affect the hypothalamic-pituitary-adrenal (HPA) axis, opioids, benzodiazepines, and antidepressants. Some investigational agents also might block the process that turns a traumatic experience into PTSD.
This article discusses these intriguing ideas and suggests which trauma victims might benefit now from acute pharmacologic PTSD prevention.
Who might be treated?
An estimated 8% to 10% of the U.S. population experiences PTSD at some point in life (Box 1).1,2 A person’s risk of developing PTSD after a traumatic event depends on the type of trauma. For example, 10% of motor vehicle accident survivors develop PTSD, compared with 60% of rape survivors.1
Targeting anyone who has experienced trauma for secondary PTSD prevention would expose large groups of people to medications they do not need. Targeting selected persons who are at the highest risk would be more efficient and cost-effective. In a group of acute trauma-exposed persons, 2 selection criteria could be considered simultaneously:
- Which patients may be most predisposed to PTSD?
- Which patients are showing early symptoms that may predict PTSD?
More than half of all American adults have been exposed to at least one traumatic event at some point in their lives.1 In most persons, the posttraumatic stress reaction causes short-term distress, with hyperarousal, agitation, intrusive memories, and exaggerated startle. Although these symptoms usually subside relatively quickly, they persist and evolve into posttraumatic stress disorder (PTSD) in a substantial number of trauma victims.
An estimated 8% to 10% of the U.S. population experiences PTSD at some point in life.2 Emotional distress, social and occupational disability, and persistent decrements in quality of life make PTSD a major public health problem.
Risk factors and resiliency. Certain factors have been shown to increase a person’s vulnerability for PTSD (Table 1).3 Other proposed risk factors include:
- personality types4
- psychophysiologic factors such as reactivity, conditionability, and resistance to extinction/habituation.5
Strong evidence also indicates that acute trauma-related symptoms—including excessive arousal and fear,6 peritraumatic dissociation, and depression—predict the later development of PTSD.
Once identified, individuals predisposed to developing PTSD could be given treatment to increase their resiliency after they have been exposed to trauma. Early evidence suggests that you also could consider giving these patients medications as secondary prevention (Table 2).
Table 1
Who develops PTSD? Risk and resiliency factors
Risk factors that may increase vulnerability for PTSD
|
Resiliency factors that may protect against PTSD
|
| Source: Reference 3 |
Table 2
Medications being studied for PTSD prevention
| Mechanism of action | Medication | FDA-approved indications | ||
|---|---|---|---|---|
| Psychiatric | Nonpsychiatric | |||
| Noradrenergic | Clonidine | No | Yes | |
| Guanfacine | No | Yes | ||
| Prazosin | No | Yes | ||
| Propranolol | No | Yes | ||
| Hypothalamic-pituitary-adrenal axis | Hydrocortisone | No | Yes | |
| Opioid | Morphine | No | Yes | |
| Antidepressant | Dual action | Duloxetine | Yes | Yes |
| Venlafaxine | Yes | No | ||
| SSRIs | Citalopram | Yes | No | |
| Fluoxetine | Yes | No | ||
| Paroxetine | Yes | No | ||
| Sertraline | Yes | No | ||
| TCAs | Amitriptyline | Yes | No | |
| Imipramine | Yes | No | ||
| GABA-benzodiazepine | Alprazolam | Yes | No | |
| Temazepam | Yes | No | ||
| Corticotropin-releasing hormone (CRH) | CRH antagonist | Investigational | ||
| Substance P | Substance P antagonist | Investigational | ||
| Neuropeptide Y | Neuropeptide Y agonist | Investigational | ||
| SSRIs: selective serotonin reuptake inhibitors | ||||
| TCAs: tricyclic antidepressants | ||||
Targeting noradrenergic activity
Increased noradrenergic activity has been associated with persistent memories and PTSD. Therefore, medications that reduce noradrenergic tone by blocking receptors or reduce norepinephrine release are being explored for PTSD prevention.
Propranolol. Three small studies have examined whether the beta-noradrenergic receptor blocker propranolol can prevent PTSD.
In a randomized, double-blind, placebo-controlled trial,7 41 emergency department patients who had a heart rate of ≥ 80 bpm within 6 hours of a traumatic accident received propranolol, 40 mg qid, or placebo for 10 days. After 1 month, the 11 patients who completed propranolol treatment showed a nonsignificant trend toward lower scores on the Clinician-Administered PTSD Scale (CAPS), compared with 20 patients taking placebo. At 3 months, the propranolol group had less physiologic reactivity (as measured by heart rate and skin conductance) to trauma-related cues than the placebo group.
In a nonrandomized study,8 PTSD developed within 2 months in 1 of 11 trauma victims who agreed to take propranolol, 40 mg tid, immediately after the trauma, compared with 3 of 8 victims who refused the medication.
In an unpublished randomized, double-blind trial,9 48 patients admitted to a level I trauma center received propranolol, 40 mg tid; gabapentin, 400 mg tid; or placebo for PTSD prevention. Gabapentin was chosen because it has few side effects or metabolic interactions and preliminary evidence of anxiolytic efficacy.
Neither propranolol nor gabapentin showed statistically significant benefit in preventing PTSD compared with placebo. Effect sizes with the 2 treatments were too small to suggest that larger samples would produce a statistically significant result.
Prazosin—an alpha-1 adrenergic receptor antagonist—has been evaluated in 3 controlled studies and found to reduce intrusive nightmares typical of chronic PTSD.
Ten combat veterans with chronic PTSD showed significantly improved sleep, fewer severe nightmares, and improved global clinical status after receiving prazosin (mean dose 9.5 mg at bedtime) in a 20-week, placebo-controlled, double-blind, crossover study.10
In a larger randomized, parallel group trial,11 the same authors compared prazosin with placebo in 40 combat veterans (mean age 56) with chronic PTSD. After 8 weeks, veterans taking prazosin (mean 13.3 ± 3 mg) had significantly fewer trauma nightmares, improved sleep (including return of normal dreams), and improved global clinical status vs placebo. Overall CAP scores did not decline significantly, however.
In a third placebo-controlled study,12 a midmorning dose of prazosin was added to the regimens of 11 civilian trauma patients already taking the drug at bedtime to suppress trauma-related nightmares. Their daytime PTSD symptoms improved, as shown by reduced psychological distress in response to verbal trauma cues.
Prazosin can reduce chronic PTSD manifestations of nightmares and disturbed sleep, but it has not been shown to ameliorate the full PTSD syndrome. Prazosin has not been studied as an early PTSD intervention.
Other antiadrenergics that reduce the release of norepinephrine—including clonidine and guanfacine—have been studied in open trials as treatment for PTSD. The only controlled study13 showed no benefit from guanfacine for PTSD prevention.
De-stressing the HPA axis
Hydrocortisone has been proposed to prevent PTSD by reducing HPA axis activation, acting as a countermeasure to elevated corticotropin-releasing factor found in patients with chronic PTSD.
IV hydrocortisone’s effect on the development of PTSD was compared with placebo in 20 septic shock survivors after discharge from intensive care.14 One of 9 patients (11%) in the hydrocortisone group was diagnosed with PTSD at follow-up (mean 31 months), compared with 7 of 11 (64%) in the placebo group.
In a similar study, the same researchers gave patients hydrocortisone before, during, and after cardiac surgery. Follow-up interviews revealed significantly lower PTSD and chronic stress symptom scores in the treatment group vs the placebo group.15
These studies—although provocative—are limited by the narrow range of trauma related to severe medical illness or extensive medical procedures.
Norepinephrine-blocking opioids
When the noradrenergic system is activated, one physiologic response is the activation of endogenous opioid systems, which may promote recovery by inhibiting the HPA axis. Opioid systems might be involved in PTSD, as suggested by:
- preclinical evidence that opioids modulate memory16
- studies showing low pain thresholds17 and abnormal beta-endorphin (an opioid peptide neurotransmitter)18 and methionine enkephalin (an opioid peptide)19 levels in PTSD patients.
In theory, opioid administration immediately after trauma may attenuate norepinephrine release, thus thwarting arousal-charged memory consolidation, hyperarousal, and re-experiencing.
One uncontrolled report of pediatric burn victims found a significant association between the morphine dose given for pain during hospitalization and reduced PTSD symptoms 6 months later.20 Decreased pain did not explain the reduction in PTSD, as no significant correlation was seen between pain symptoms and PTSD outcome measures. Similarly, a longitudinal study of substance use among Vietnam War veterans with PTSD found decreased hyperarousal symptoms in heroin users.21
Using opioids to prevent PTSD would be feasible and efficient in acute care settings because 80% to 90% of traumatically-injured patients are discharged on opioid analgesics (compared with <10% on beta blockers or corticosteroids).22 However, 20% to 40% of physically injured inpatients are diagnosed with a substance use disorder at some point in life, making the use of opioid analgesics a practical concern.23
GABA-benzodiazepine paradox
The GABA-benzodiazepine system plays an important role in mediating anxiety, which is consistent with the potent anxiolytic effects of benzodiazepines. Even so, trials of benzodiazepines have found these drugs surprisingly unhelpful—and perhaps harmful—in patients with acute trauma.
Alprazolam did not reduce PTSD symptoms in a small randomized, double-blind study.24 Another trial found that receiving benzodiazepines shortly after trauma exposure was associated with increased PTSD risk in trauma survivors. Nine of 13 patients (69%) who received alprazolam or clonazepam met PTSD diagnostic criteria 6 months after the trauma, compared with 3 of 13 controls (15%).25
Similarly, in a randomized controlled trial, 22 patients were given temazepam for 7 nights, starting approximately 14 days after exposure to a traumatic event. Six weeks later, 55% of those receiving temazepam and 27% of those receiving placebo met criteria for PTSD.26
In summary, benzodiazepines might be helpful when given for a few days after traumatization to control overwhelming anxiety but could be harmful over a longer term.
Other agents for PTSD
Antidepressants. Early trauma-related symptoms of depression predict later development of PTSD.27 Thus, antidepressants have been proposed for early intervention in addition to their well-established role as first-line treatment of PTSD.28
One study supports this idea: a 7-day randomized double-blind trial that compared the tricyclic antidepressant imipramine with chloral hydrate in pediatric burn patients with acute stress disorder (ASD). Imipramine was more effective (83% response) than chloral hydrate (38% response) in reducing ASD symptoms.29
Drugs in development. Three new medications being explored for treating anxiety and depression also might be useful for PTSD prevention. Neuropeptide Y (NPY) agonists,30 substance P antagonists,31 and CRH-antagonists32 are thought to hold promise because of their more proximate roles—compared with monoamine neurotransmitters such as dopamine, norepinephrine and serotonin—in mediating the stress response.
Manage the post-trauma environment:
- Move the victim to safety.
- Treat pain effectively.
- Avoid stress from interrogations, separation from loved ones, or unstable housing.
Avoid crisis incident stress debriefing (CISD), which could enhance physiologic hyperarousal and is not recommended as first-line treatment for most trauma victims. CISD was designed for and is best received by emergency personnel.
Consider prescribing antidepressants for patients thought to be particularly vulnerable to develop posttraumatic stress disorder (PTSD). Risk factors include:
- history of PTSD, depression, or anxiety disorder
- severe trauma (such as from sexual assault or torture)
- physical injury, when antidepressants with analgesic properties might be useful.
Analyzing the evidence
Insufficient evidence exists to determine which strategies might be most effective to prevent PTSD, what optimal dosing might be, and which traumatized individuals might be best targeted with these approaches.
- Beta-blockers and corticosteroids—the most theoretically compelling strategies—are the most difficult agents to use for PTSD prevention because they have the most medical contraindications. In addition, evidence supporting their ability to prevent PTSD is meager at best.
- Prazosin is intriguing but has contra-indications similar to those of beta blockers, no studies of secondary prevention, and no clear indication that it works for the overall PTSD syndrome.
- Opioids are restricted agents with substantial contraindications.
- Evidence is limited but points most strongly toward earlier use of antidepressants. Early trauma-related symptoms of depression predict later development of PTSD,27 and a number of selective serotonin reuptake inhibitors—such as citalopram, fluoxetine, paroxetine, and sertraline—are FDA-approved or used off-label for treating PTSD.33
Prescribing recommendations. Consider practicality, ease of use, and safety of the proposed medication when choosing a drug for PTSD prevention (Table 3).22 Based on the evidence, the most reasonable posttrauma approach (Box 2) might be to consider starting an approved antidepressant for individuals thought to be particularly vulnerable to PTSD because of:
- past history of PTSD, depression, or anxiety disorder
- severity of the trauma (such as in cases of sexual assault or torture)
- pain (antidepressants with analgesic properties—such as venlafaxine or duloxetine—might be useful in patients whose trauma is associated with physical injury, although neither is FDA-approved to treat PTSD).
Table 3
4 considerations when choosing a drug for PTSD prevention
| Potential benefits | Practicality, ease of use, and safety of the proposed medication |
| Potential drug-drug or drug-disease interactions | Asthma, diabetes, and trauma are relative contraindications to the use of antiadrenergics and corticosteroids |
| Psychiatric comorbidities | A patient’s history of substance use disorder makes opioid analgesics a concern |
| Clinical experience | Agents already prescribed safely and broadly in clinical practice are easiest to test and to use |
Related resources
- Mental health and mass violence: Evidence-based early psychological intervention for victims/survivors of mass violence. A workshop to reach consensus on best practices. Rockland, MD: National Institute of Mental Health; 2002. www.nimh.nih.gov.
- Post-traumatic stress disorder: the management of PTSD in adults and children in primary and secondary care (clinical guideline 26). London, UK: National Institute for Clinical Excellence; 2005. www.nice.org.uk.
- Ursano RJ, Bell C, Eth S, et al. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Am J Psychiatry 2004;161(suppl 11):3-31.
Drug brand names
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Citalopram • Celexa
- Clonazepam • Klonopin
- Clonidine • Catapres
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Guanfacine • Tenex
- Imipramine • Tofranil
- Lamotrigine • Lamictal
- Paroxetine • Paxil
- Prazosin • Minipress
- Propranolol • Inderal
- Sertraline • Zoloft
- Temazepam • Restoril
- Venlafaxine • Effexor
Disclosure
Dr. Bennett and Dr. Zatzick report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Roy-Byrne is a consultant to Jazz Pharmaceuticals and Solvay and has received speaker honoraria from Wyeth and Forrest Pharmaceuticals.
1. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995;52(12):1048-60.
2. Zatzick D. Posttraumatic stress, functional impairment, and service utilization after injury: a public health approach. Semin Clin Neuropsychiatry 2003;8(3):149-57.
3. Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann NY Acad Sci 2006;1071:379-96.
4. Schnurr P, Vielhauer M. Personality as a risk factor for PTSD. In: Yehuda R, ed. Risk factors for post-traumatic stress disorder. Washington, DC: American Psychiatric Publishing; 1999:191-222.
5. Shalev A. Psychophysiological expression of risk factors for PTSD. In: Yehuda R, ed. Risk factors for posttraumatic stress disorder. Washington, DC: American Psychiatric Publishing; 1995.
6. Bryant RA, Harvey AG, Guthrie RM, Moulds ML. A prospective study of psychophysiological arousal, acute stress disorder, and posttraumatic stress disorder. J Abnorm Psychol 2000;109(2):341-4.
7. Pitman RK, Sanders KM, Zusman RM, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry 2002;51(2):189-92.
8. Vaiva G, Ducrocq F, Jezequel K, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry 2003;54(9):947-9.
9. Stein M. Pharmacoprevention of adverse psychiatric sequelae of physical injury. Paper presented at: 21st Annual Meeting of the International Society for Traumatic Stress Studies; November 2-5, 2005; Toronto, Ontario, Canada.
10. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry 2003;160(2):371-3.
11. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry 2007;61(8):928-34.
12. Taylor FB, Lowe K, Thompson C, et al. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol Psychiatry 2006;59(7):577-81.
13. Neylan TC, Lenoci M, Samuelson KW, et al. No improvement of posttraumatic stress disorder symptoms with guanfacine treatment. Am J Psychiatry 2006;163(12):2186-8.
14. Schelling G, Briegel J, Roozendaal B, et al. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry 2001;50(12):978-85.
15. Weis F, Kilger E, Roozendaal B, et al. Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. J Thorac Cardiovasc Surg 2006;131(2):277-82.
16. McGaugh JL, Introini-Collison IB, Nagahara AH, et al. Involvement of the amygdaloid complex in neuromodulatory influences on memory storage. Neurosci Biobehav Rev 1990;14(4):425-31.
17. Shalev AY, Peri T, Canetti L, Schreiber S. Predictors of PTSD in injured trauma survivors: a prospective study. Am J Psychiatry 1996;153(2):219-25.
18. Baker DG, West SA, Orth DN, et al. Cerebrospinal fluid and plasma beta-endorphin in combat veterans with post-traumatic stress disorder. Psychoneuroendocrinol 1997;22(7):517-29.
19. Wolf ME, Mosnaim AD, Puente J, Ignacio R. Plasma methionine-enkephalin in PTSD. Biol Psychiatry 1991;29(3):305-7.
20. Saxe G, Stoddard F, Courtney D, et al. Relationship between acute morphine and the course of PTSD in children with burns. J Am Acad Child Adolesc Psychiatry 2001;40(8):915-21.
21. Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry 1996;153(3):369-75.
22. Zatzick D, Roy-Byrne PP. From bedside to bench: how the epidemiology of clinical practice can inform the secondary prevention of PTSD. Psychiatr Serv 2006;57(12):1726-30.
23. Zatzick D, Jurkovich G, Russo J, et al. Posttraumatic distress, alcohol disorders, and recurrent trauma across level 1 trauma centers. J Trauma 2004;57(2):360-6.
24. Braun P, Greenberg D, Dasberg H, Lerer B. Core symptoms of posttraumatic stress disorder unimproved by alprazolam treatment. J Clin Psychiatry 1990;51(6):236-8.
25. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry 1996;57(9):390-4.
26. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry 2002;63(12):1183-4.
27. Freedman SA, Brandes D, Peri T, Shalev A. Predictors of chronic post-traumatic stress disorder. A prospective study. Br J Psychiatry 1999;174:353-9.
28. Davidson JR. Pharmacologic treatment of acute and chronic stress following trauma. J Clin Psychiatry 2006;67(suppl 2):34-9.
29. Robert R, Blakeney PE, Villarreal C, et al. Imipramine treatment in pediatric burn patients with symptoms of acute stress disorder: a pilot study. J Am Acad Child Adolesc Psychiatry 1999;38(7):873-82.
30. Morgan CA, Wang S, Southwick SM, et al. Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol Psychiatry 2000;47(10):902-9.
31. Geracioti TD, Carpenter LL, Owens MJ, et al. Elevated cerebrospinal fluid substance P concentrations in posttraumatic stress disorder and major depression. Am J Psychiatry 2006;163(4):637-43.
32. Zobel AW, Nickel T, Künzel HE, et al. Effects of the highaffinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res 2000;34(3):171-81.
33. Davidson JR. Treatment of posttraumatic stress disorder: the impact of paroxetine. Psychopharmacol Bull 2003;37(suppl 1):76-88.
1. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995;52(12):1048-60.
2. Zatzick D. Posttraumatic stress, functional impairment, and service utilization after injury: a public health approach. Semin Clin Neuropsychiatry 2003;8(3):149-57.
3. Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann NY Acad Sci 2006;1071:379-96.
4. Schnurr P, Vielhauer M. Personality as a risk factor for PTSD. In: Yehuda R, ed. Risk factors for post-traumatic stress disorder. Washington, DC: American Psychiatric Publishing; 1999:191-222.
5. Shalev A. Psychophysiological expression of risk factors for PTSD. In: Yehuda R, ed. Risk factors for posttraumatic stress disorder. Washington, DC: American Psychiatric Publishing; 1995.
6. Bryant RA, Harvey AG, Guthrie RM, Moulds ML. A prospective study of psychophysiological arousal, acute stress disorder, and posttraumatic stress disorder. J Abnorm Psychol 2000;109(2):341-4.
7. Pitman RK, Sanders KM, Zusman RM, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry 2002;51(2):189-92.
8. Vaiva G, Ducrocq F, Jezequel K, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry 2003;54(9):947-9.
9. Stein M. Pharmacoprevention of adverse psychiatric sequelae of physical injury. Paper presented at: 21st Annual Meeting of the International Society for Traumatic Stress Studies; November 2-5, 2005; Toronto, Ontario, Canada.
10. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry 2003;160(2):371-3.
11. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry 2007;61(8):928-34.
12. Taylor FB, Lowe K, Thompson C, et al. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol Psychiatry 2006;59(7):577-81.
13. Neylan TC, Lenoci M, Samuelson KW, et al. No improvement of posttraumatic stress disorder symptoms with guanfacine treatment. Am J Psychiatry 2006;163(12):2186-8.
14. Schelling G, Briegel J, Roozendaal B, et al. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry 2001;50(12):978-85.
15. Weis F, Kilger E, Roozendaal B, et al. Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. J Thorac Cardiovasc Surg 2006;131(2):277-82.
16. McGaugh JL, Introini-Collison IB, Nagahara AH, et al. Involvement of the amygdaloid complex in neuromodulatory influences on memory storage. Neurosci Biobehav Rev 1990;14(4):425-31.
17. Shalev AY, Peri T, Canetti L, Schreiber S. Predictors of PTSD in injured trauma survivors: a prospective study. Am J Psychiatry 1996;153(2):219-25.
18. Baker DG, West SA, Orth DN, et al. Cerebrospinal fluid and plasma beta-endorphin in combat veterans with post-traumatic stress disorder. Psychoneuroendocrinol 1997;22(7):517-29.
19. Wolf ME, Mosnaim AD, Puente J, Ignacio R. Plasma methionine-enkephalin in PTSD. Biol Psychiatry 1991;29(3):305-7.
20. Saxe G, Stoddard F, Courtney D, et al. Relationship between acute morphine and the course of PTSD in children with burns. J Am Acad Child Adolesc Psychiatry 2001;40(8):915-21.
21. Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry 1996;153(3):369-75.
22. Zatzick D, Roy-Byrne PP. From bedside to bench: how the epidemiology of clinical practice can inform the secondary prevention of PTSD. Psychiatr Serv 2006;57(12):1726-30.
23. Zatzick D, Jurkovich G, Russo J, et al. Posttraumatic distress, alcohol disorders, and recurrent trauma across level 1 trauma centers. J Trauma 2004;57(2):360-6.
24. Braun P, Greenberg D, Dasberg H, Lerer B. Core symptoms of posttraumatic stress disorder unimproved by alprazolam treatment. J Clin Psychiatry 1990;51(6):236-8.
25. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry 1996;57(9):390-4.
26. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry 2002;63(12):1183-4.
27. Freedman SA, Brandes D, Peri T, Shalev A. Predictors of chronic post-traumatic stress disorder. A prospective study. Br J Psychiatry 1999;174:353-9.
28. Davidson JR. Pharmacologic treatment of acute and chronic stress following trauma. J Clin Psychiatry 2006;67(suppl 2):34-9.
29. Robert R, Blakeney PE, Villarreal C, et al. Imipramine treatment in pediatric burn patients with symptoms of acute stress disorder: a pilot study. J Am Acad Child Adolesc Psychiatry 1999;38(7):873-82.
30. Morgan CA, Wang S, Southwick SM, et al. Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol Psychiatry 2000;47(10):902-9.
31. Geracioti TD, Carpenter LL, Owens MJ, et al. Elevated cerebrospinal fluid substance P concentrations in posttraumatic stress disorder and major depression. Am J Psychiatry 2006;163(4):637-43.
32. Zobel AW, Nickel T, Künzel HE, et al. Effects of the highaffinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res 2000;34(3):171-81.
33. Davidson JR. Treatment of posttraumatic stress disorder: the impact of paroxetine. Psychopharmacol Bull 2003;37(suppl 1):76-88.
Suicide intervention: How to recognize risk, focus on patient safety
More than 50% of psychiatrists have experienced the death of a patient by suicide.1 For many of us, suicide represents the most feared outcome of a patient’s mental illness and makes managing suicide risk critical to everyday practice.
Unfortunately, we have little ability to predict suicide. Research into risk factors and the use of suicide rating scales have produced no consistently definitive methods to determine who will and who will not attempt or complete suicide.2 The purpose of suicide assessment, then, is not to predict suicide but to help us understand the sources of a patient’s suicidality and develop an informed intervention.
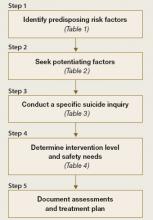
This article describes a practical, commonly accepted approach to suicide risk assessment and intervention, based on the B-SAFE model (Basic Suicide Assessment Five-step Evaluation) proposed by Jacobs et al (Figure).3 Using this method to assess suicide risk can help you answer questions such as:
- Which factors are most important to consider when evaluating suicide risk in my patient?
- What questions should I ask my patient to find out if he or she is suicidal?
- How do I know if a patient is at risk for suicide?
- What emergent interventions are called for when managing the acutely suicidal patient?
- How should I document a suicide risk assessment?
Figure Basic Suicide Assessment Five-step Evaluation (B-SAFE)
Source: Created from information in references 2,11
Why ask about suicide?
No single risk factor or combination of risk factors can predict or preclude suicide. Even so, attempting to evaluate an individual’s risk by asking about suicidal thinking, reviewing risk factors, or using clinical rating scales helps you determine the next appropriate action (discharge, medication, psychiatric referral, consultation, or hospitalization).
While talking to patients and evaluating their risk for suicide, you may begin to understand their suffering—described as the most common denominator in suicide2 and perhaps the most important clue to heightened suicide risk. Such an exploration allows you to identify potential:
- risk factors that can be modified
- preventative factors to promote.
Don’t be afraid to ask. Asking about suicidal thoughts is necessary—but not enough—to understand an individual’s potential for suicide. Never be afraid to ask patients about suicide, believing that doing so will “put ideas into their heads.” By the same token, a patient who denies thoughts or plans for suicide may still be at risk.
Identifying at-risk patients is much more difficult than just asking if they are considering suicide. Opening a concerned dialogue can provide a sense of relief to the patient while allowing you to explore:
- the extent and seriousness of the suicidal thoughts
- associated risk factors or conditions, such as depression.
Stepwise risk assessment
The first 3 steps of Jacobs’ B-SAFE model focus on identifying predisposing and protective factors for suicide.3 For an in-depth discussion, consult the American Psychiatric Association practice guideline for the assessment and treatment of patients with suicidal behaviors4 (available at http://www.psych.org/psych_pract/treatg/pg/suicidalbehavior_05-15-06.pdf).
STEP 1: Risk factors. Use the patient interview, medical records, and collateral information to uncover potential suicide risk factors (Table 1).2
Psychopathology. Focus on depression, bipolar disorder, schizophrenia, substance abuse, and personality disorders, which are strongly associated with suicide. These disorders are considered modifiable risk factors—diagnosis and appropriate treatment can diminish suicide risk.
Suicidality has been associated with early depression or bipolar disorder, often before patients receive a diagnosis or effective treatment. Recovery and immediate post-discharge periods also are thought to be times of heightened suicide risk.
Psychosocial variables. Demographic and psychosocial variables may influence suicide risk estimation. A retrospective study of 100 patients who attempted suicide suggests that the most predictive factors for suicide are:
- living alone
- being aged 17 to 35 (although in other studies, more advanced age also has been linked to increased suicide risk3)
- complaints of severe hopelessness, anhedonia, and insomnia.5
Physical illness may potentiate suicide risk. Medical illnesses that produce great pain, disfigurement, limited function, or fear of dependence may reduce a person’s will to live and increase suicide risk.6 Epilepsy has been associated with a 4- to 5-fold increase in suicide risk7 and is the only medical diagnosis to carry a documented increase in suicide among children and adolescents.8 Often these medical disorders coexist with psychiatric disorders, complicating the task of determining independent risk.
Severity of attempts or self-mutilation. When evaluating self-injurious or suicidal behavior in the emergency setting, consider the severity of the attempt as part of overall suicide assessment. Self-injurious behavior (cutting or burning) or impulsive suicide attempts (planned for <3 hours, committed in the presence others, or where discovery is very probable) appear to carry less severity or intent to die than do carefully planned and/or hidden suicide attempts.9 However, consider at high risk for suicide any patient with self-mutilating or suicidal behavior who expresses persistent intent to die; acute stabilization on an in-patient unit may be necessary.
STEP 2: ‘Protective’ factors. Discover and discuss internal and external factors that might help prevent the individual with suicidal thoughts from converting those thoughts into action (Table 2).2 When discussing these potentially protective effects, emphasize the patient’s:
- resilience during past personal crises
- family responsibilities
- religious or spiritual beliefs.
‘No-harm contracts.’ Suicide (or “no-harm”) contracts with patients might help open communication about factors that promote or mitigate suicide risk. Such contacts do not prevent suicide or lessen medicolegal risk in the event of a patient suicide, however.10
STEP 3: Suicide plans. Ask about suicide thoughts, plans, and behaviors (Table 3).11 Probe gently to allow the individual to discuss his or her feelings and to explore the next appropriate avenue of care.
In my experience, patients who reveal passive suicidal ideation (such as, “I sometimes wish I would just die in my sleep”) and strong deterrents to acting on thoughts of suicide (such as, “My children need me,” or “It’s against my religion”) should continue outpatient treatment. Those without deterrents or who discuss active and imminent thoughts and recent actions—writing suicide notes, buying a weapon, stockpiling pills—require emergent evaluation for psychiatric admission. Ask about thoughts of self-injury or mutilation (such as cutting or burning), as well as homicidal ideation.
Recognizing that patients with suicidal thoughts are almost always ambivalent about suicide to some extent—conflicted by simultaneous desires to live and to die—gives you the opportunity to intervene by allying with the part of the patient that wants to live. Creating a therapeutic connection also will help you determine the level of intervention required.
STEP 4: Intervention. Understanding why a patient feels suicidal—gathered in Steps 1 to 3—can help you choose the appropriate intervention. Among the 5 steps, Step 4 relies most heavily on clinical judgment:
- Is the suicidality acute or chronic?
- How great is the risk for suicide?
- To keep the patient safe, how urgent is the required intervention?
Acute risk. Suicidality related to Axis I psychiatric disorders tends to be acute, with prominent pain, anguish, and a desire to escape. Patients may describe a driven quality to the suicidality, which commands a treatment plan that maintains patient safety until suicidal feelings remit.
Hospitalization is often needed, plus focused treatments such as medication, psychotherapy, or electroconvulsive therapy. Intensive outpatient follow-up or partial hospitalization programs might be considered for patients:
- with whom you have a strong therapeutic alliance
- who have sturdy psychosocial support
- whose precipitating factors for suicidality have resolved.
Chronic risk. Suicide risk tends to be more chronic and has an impulsive quality for patients with suicidality related to personality disorders and environmental factors. Personality disordered patients may report feelings of anger, rage, or vengeance connected with their suicidal thoughts.
Hospitalization might become necessary, although multiple hospitalizations can be counter-therapeutic. Attempting in therapy to teach the patient to cope with suicidal thoughts and feelings might be a more effective intervention.
Malingering. Use your best judgment when patients make suicide threats that could represent malingering to achieve hospitalization.
Step 5: Documentation. Document your assessment of the suicidal patient and decision making to:
- clarify the treatment plan
- communicate to other caregivers
- manage medicolegal risk.
Include a brief summary (Box) that is timely, legible, and communicates the estimated degree of risk, known data, diagnosis, and planned interventions such as medications, tests, consultations, and follow-up reassessments.
This 46-year-old, recently divorced man is experiencing his second episode of major depression associated with clear-cut panic attacks and suspected psychotic features. Although he denies current suicidal ideation, the treatment team believes he is at moderate to high risk for suicide because of known past history of serious suicide attempt with first depression, the presence of panic/anxiety, and possibly psychotic features. Additional risk is posed by loss of marital support and his inability to verbalize meaningful protective factors.
The plan is to convert from observation status on the inpatient unit to full admission, as the suicide risk precludes discharge at present. Further medication management and consideration for electroconvulsive therapy will take place, with daily reassessments. Suicide precautions ordered.
Table 1
Factors associated with potential for increased suicide risk
| Variable | Risk Factors |
|---|---|
| Demographic | Male gender, Caucasian race, rural residence, possibly age (varies among studies) |
| Imprisoned; widowed, divorced, or separated; living alone; no children or no children living in the home | |
| Psychosocial | Lack or loss of social supports, recent loss of employment, decrease in socioeconomic status or poverty, hopelessness |
| History of victimization (physical or sexual abuse), psychological turmoil, severe relationship conflict, aggressive or impulsive traits | |
| Writing suicide notes; family history of suicide, previous attempts, ‘imitation’ suicide, gun ownership | |
| Occupational risk (physicians, dentists, nurses, pharmacists, veterinarians, farmers) | |
| Psychiatric | Psychiatric diagnosis of recent onset |
| Mood disorder, particularly major depression and bipolar disorder | |
| Schizophrenia; alcohol or other substance abuse or addiction; personality disorder; panic attacks or severe psychic anxiety | |
| Insomnia; poor concentration or confusion; anhedonia | |
| Medical | Huntington’s disease, stroke, multiple sclerosis, head injury, spinal cord injury, systemic lupus erythematosus, AIDS |
| Epilepsy, pain, malignant neoplasms, peptic ulcer disease, renal disease | |
| Source: Adapted with permission from reference 3 | |
Table 2
Potentially protective factors against suicide
| Internal |
| Successful past responses to stress |
| Positive coping skills |
| Spirituality |
| Capacity for reality testing |
| Frustration tolerance/optimism |
| Overall individual resiliency |
| External |
| Children or pets in the home |
| Religious prohibition or beliefs |
| Positive therapeutic relationships |
| Sense of responsibility to family |
| Social supports and connections |
| Financial incentives or deterrents |
| Source: Adapted from reference 11 |
Table 3
Evaluating suicide risk: Questions to ask patients
| Have you felt so sad or depressed that you thought life is not worth living? |
| Have you thought about hurting yourself or taking your own life? |
| Have you thought about a way or plan to kill yourself? |
| Do you have the means to complete the plan? (such as, do you have access to weapons or pills?) |
| Have you practiced or rehearsed this plan to end your own life? |
| Do you have a location picked out? |
| What has stopped you from following through with the plan? |
| Have you ever attempted suicide? |
| Has anyone in your family ever attempted or committed suicide? |
| Source: Adapted with permission from reference 3 |
Interventions for suicidal patients
Physical protection. Take decisive action when you determine that suicide risk is elevated and imminent. Pursue urgent psychiatric hospitalization, with or without patient consent, in accordance with local probate and involuntary commitment statutes.
The logistics of protective action can be challenging; transportation is often required, and the patient is not always cooperative with admission. Table 4 lists measures and precautions that can help keep the suicidal patient safe.
Disease-specific interventions. Because suicidal ideation is often symptomatic of a primary psychiatric disorder, rapidly identifying major depression, bipolar disorder, or a psychotic illness is crucial to reducing suicidal thoughts and behaviors. Prescribe appropriate antidepressants, mood stabilizers, and antipsychotics at adequate doses and for sufficient duration.
Be vigilant for distressing symptoms that may be elevating the patient’s suicide risk, such as anxiety, panic, agitation, insomnia, or pain. Pharmacotherapies—such as anxiolytics, sedative-hypnotics, antipsychotics, or analgesics—may rapidly reduce suffering.
Impulsivity associated with substance use disorders—particularly during intoxication and withdrawal syndromes—requires aggressive attempts by the treatment team to engage the patient in detoxification and rehabilitation.
Direct antisuicide therapy. Clozapine carries an FDA-approved indication for preventing suicide in patients with schizophrenia or schizoaffective disorder. The mechanism by which clozapine helps prevent suicide is not known, but its anti-suicidal effects appear to be independent of its antipsychotic effects.12
Lithium has been reported to reduce risk of suicide and suicide attempts in patients with bipolar disorder, perhaps by as much as 80%.13 Such benefit has not been observed with other mood stabilizers, suggesting that lithium confers protective effects against suicide beyond its mood-stabilizing effects. Suicide risk is known to increase after lithium is discontinued.14
Lithium’s antisuicidal effects may arise from its ability to enhance serotonin. This theory, although unproven, is consistent with observations associating central serotonergic deficiency with suicidal and aggressive behaviors.
Psychosocial measures. Address psychosocial variables that may increase suicide risk (Table 1). Recruit and involve the patient’s support system, augmented with a close follow-up plan. Case management to explore housing and job opportunities can help. Work with the patient’s family or others to remove guns from the patient’s access. Individual, marital, and family therapies can reduce conflicts and strengthen coping skills.
Table 4
Safety measures to protect the suicidal patient
| Hospitalize—voluntarily or involuntarily—on a locked psychiatric unit |
| Provide constant 1-to-1 observation by staff |
| Transport the patient, accompanied by adequate personnel |
| Use physical restraints or seclusion while maintaining continuous observation |
| Employ metal detector to remove dangerous, hidden objects |
| Remove and secure patient’s belongings (bags, coats, purses may contain pills or weapons) |
| Search visitors’ belongings before allowing access to unit |
| Ensure that inpatient unit meets all coded safety regulations |
Related resources
- National Suicide Prevention Lifeline, sponsored by the Substance Abuse & Mental Health Services Administration: 1-800-SUICIDE or 1-800-273-TALK (8255); www.suicidepreventionlifeline.org.
- American Foundation for Suicide Prevention (AFSP) 1-888-333-AFSP; www.afsp.org.
- Simon RI, Hales RE. Textbook of suicide assessment and management. Washington, DC: American Psychiatric Publishing; 2006.
Drug brand names
- Clozapine • Clozaril
- Lithium • Eskalith, Lithobid, others
Disclosure
Dr. Muzina has received grants from or served as a consultant to Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Novartis Pharmaceuticals Corp., Pfizer, and Repligen.
1. Chemtob CM, Hamada RS, Bauer G, et al. Patients’ suicides: frequency and impact on psychiatrists. Am J Psychiatry 1988;145(2):224-8.
2. Muzina DJ. What physicians can do to prevent suicide. Cleve Clin J Med 2004;71(3):242-50.
3. Jacobs DG, Brewer ML, Klein-Benheim M. Suicide assessment: an overview and recommended protocol. In: Jacobs DG, ed. The Harvard Medical School guide to suicide assessment and intervention.. San Francisco, CA: Jossey-Bass Publishers; 1999:3-39.
4. Jacobs DG, Baldessarini RJ, Conwell Y, et al. American Psychiatric Association practice guideline for the assessment and treatment of patients with suicidal behaviors. Available at: http://www.psych.org/psych_pract/treatg/pg/suicidalbehavior_05-15-06.pdf. Accessed May 14, 2007.
5. Hall RC, Platt DE, Hall RC. Suicide risk assessment: a review of risk factors for suicide in 100 patients who made severe suicide attempts. Evaluation of suicide risk in a time of managed care. Psychosomatics 1999;40(1):18-27.
6. Mackenzie TB, Popkin MK. Suicide in the medical patient. Int J Psychiatry Med 1987;17(1):3-22.
7. Barraclough BM. The suicide rate of epilepsy. Acta Psychiatr Scand 1987;76(4):339-45
8. Brent DA, Kolko DJ, Allan MJ, Brown RV. Suicidality in affectively disordered adolescent inpatients. J Am Acad Child Adolesc Psychiatry 1990;29(4):586-93.
9. Polewka A, Mikolaszek-Boba M, Chrostek Maj J, Groszek B. The characteristics of suicide attempts based on the suicidal intent scale scores. Przegl Lek 2005;62(6):415-8.
10. Lewis LM. No-harm contracts: a review of what we know. Suicide Life Threat Behav 2007;37(1):50-7.
11. Jacobs DG. A resource guide for implementing the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) 2007 patient safety goals on suicide. Available at: http://www.sprc.org/library/jcahosafetygoals.pdf. Accessed May 14, 2007.
12. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003;60(1):82-91.
13. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord 2006;8(5 Pt 2):625-39.
14. Baldessarini RJ, Tondo L, Viguera AC. Discontinuing lithium maintenance treatment in bipolar disorders: risks and implications. Bipolar Disord 1999;1(1):17-24.
More than 50% of psychiatrists have experienced the death of a patient by suicide.1 For many of us, suicide represents the most feared outcome of a patient’s mental illness and makes managing suicide risk critical to everyday practice.
Unfortunately, we have little ability to predict suicide. Research into risk factors and the use of suicide rating scales have produced no consistently definitive methods to determine who will and who will not attempt or complete suicide.2 The purpose of suicide assessment, then, is not to predict suicide but to help us understand the sources of a patient’s suicidality and develop an informed intervention.
This article describes a practical, commonly accepted approach to suicide risk assessment and intervention, based on the B-SAFE model (Basic Suicide Assessment Five-step Evaluation) proposed by Jacobs et al (Figure).3 Using this method to assess suicide risk can help you answer questions such as:
- Which factors are most important to consider when evaluating suicide risk in my patient?
- What questions should I ask my patient to find out if he or she is suicidal?
- How do I know if a patient is at risk for suicide?
- What emergent interventions are called for when managing the acutely suicidal patient?
- How should I document a suicide risk assessment?
Figure Basic Suicide Assessment Five-step Evaluation (B-SAFE)
Source: Created from information in references 2,11
Why ask about suicide?
No single risk factor or combination of risk factors can predict or preclude suicide. Even so, attempting to evaluate an individual’s risk by asking about suicidal thinking, reviewing risk factors, or using clinical rating scales helps you determine the next appropriate action (discharge, medication, psychiatric referral, consultation, or hospitalization).
While talking to patients and evaluating their risk for suicide, you may begin to understand their suffering—described as the most common denominator in suicide2 and perhaps the most important clue to heightened suicide risk. Such an exploration allows you to identify potential:
- risk factors that can be modified
- preventative factors to promote.
Don’t be afraid to ask. Asking about suicidal thoughts is necessary—but not enough—to understand an individual’s potential for suicide. Never be afraid to ask patients about suicide, believing that doing so will “put ideas into their heads.” By the same token, a patient who denies thoughts or plans for suicide may still be at risk.
Identifying at-risk patients is much more difficult than just asking if they are considering suicide. Opening a concerned dialogue can provide a sense of relief to the patient while allowing you to explore:
- the extent and seriousness of the suicidal thoughts
- associated risk factors or conditions, such as depression.
Stepwise risk assessment
The first 3 steps of Jacobs’ B-SAFE model focus on identifying predisposing and protective factors for suicide.3 For an in-depth discussion, consult the American Psychiatric Association practice guideline for the assessment and treatment of patients with suicidal behaviors4 (available at http://www.psych.org/psych_pract/treatg/pg/suicidalbehavior_05-15-06.pdf).
STEP 1: Risk factors. Use the patient interview, medical records, and collateral information to uncover potential suicide risk factors (Table 1).2
Psychopathology. Focus on depression, bipolar disorder, schizophrenia, substance abuse, and personality disorders, which are strongly associated with suicide. These disorders are considered modifiable risk factors—diagnosis and appropriate treatment can diminish suicide risk.
Suicidality has been associated with early depression or bipolar disorder, often before patients receive a diagnosis or effective treatment. Recovery and immediate post-discharge periods also are thought to be times of heightened suicide risk.
Psychosocial variables. Demographic and psychosocial variables may influence suicide risk estimation. A retrospective study of 100 patients who attempted suicide suggests that the most predictive factors for suicide are:
- living alone
- being aged 17 to 35 (although in other studies, more advanced age also has been linked to increased suicide risk3)
- complaints of severe hopelessness, anhedonia, and insomnia.5
Physical illness may potentiate suicide risk. Medical illnesses that produce great pain, disfigurement, limited function, or fear of dependence may reduce a person’s will to live and increase suicide risk.6 Epilepsy has been associated with a 4- to 5-fold increase in suicide risk7 and is the only medical diagnosis to carry a documented increase in suicide among children and adolescents.8 Often these medical disorders coexist with psychiatric disorders, complicating the task of determining independent risk.
Severity of attempts or self-mutilation. When evaluating self-injurious or suicidal behavior in the emergency setting, consider the severity of the attempt as part of overall suicide assessment. Self-injurious behavior (cutting or burning) or impulsive suicide attempts (planned for <3 hours, committed in the presence others, or where discovery is very probable) appear to carry less severity or intent to die than do carefully planned and/or hidden suicide attempts.9 However, consider at high risk for suicide any patient with self-mutilating or suicidal behavior who expresses persistent intent to die; acute stabilization on an in-patient unit may be necessary.
STEP 2: ‘Protective’ factors. Discover and discuss internal and external factors that might help prevent the individual with suicidal thoughts from converting those thoughts into action (Table 2).2 When discussing these potentially protective effects, emphasize the patient’s:
- resilience during past personal crises
- family responsibilities
- religious or spiritual beliefs.
‘No-harm contracts.’ Suicide (or “no-harm”) contracts with patients might help open communication about factors that promote or mitigate suicide risk. Such contacts do not prevent suicide or lessen medicolegal risk in the event of a patient suicide, however.10
STEP 3: Suicide plans. Ask about suicide thoughts, plans, and behaviors (Table 3).11 Probe gently to allow the individual to discuss his or her feelings and to explore the next appropriate avenue of care.
In my experience, patients who reveal passive suicidal ideation (such as, “I sometimes wish I would just die in my sleep”) and strong deterrents to acting on thoughts of suicide (such as, “My children need me,” or “It’s against my religion”) should continue outpatient treatment. Those without deterrents or who discuss active and imminent thoughts and recent actions—writing suicide notes, buying a weapon, stockpiling pills—require emergent evaluation for psychiatric admission. Ask about thoughts of self-injury or mutilation (such as cutting or burning), as well as homicidal ideation.
Recognizing that patients with suicidal thoughts are almost always ambivalent about suicide to some extent—conflicted by simultaneous desires to live and to die—gives you the opportunity to intervene by allying with the part of the patient that wants to live. Creating a therapeutic connection also will help you determine the level of intervention required.
STEP 4: Intervention. Understanding why a patient feels suicidal—gathered in Steps 1 to 3—can help you choose the appropriate intervention. Among the 5 steps, Step 4 relies most heavily on clinical judgment:
- Is the suicidality acute or chronic?
- How great is the risk for suicide?
- To keep the patient safe, how urgent is the required intervention?
Acute risk. Suicidality related to Axis I psychiatric disorders tends to be acute, with prominent pain, anguish, and a desire to escape. Patients may describe a driven quality to the suicidality, which commands a treatment plan that maintains patient safety until suicidal feelings remit.
Hospitalization is often needed, plus focused treatments such as medication, psychotherapy, or electroconvulsive therapy. Intensive outpatient follow-up or partial hospitalization programs might be considered for patients:
- with whom you have a strong therapeutic alliance
- who have sturdy psychosocial support
- whose precipitating factors for suicidality have resolved.
Chronic risk. Suicide risk tends to be more chronic and has an impulsive quality for patients with suicidality related to personality disorders and environmental factors. Personality disordered patients may report feelings of anger, rage, or vengeance connected with their suicidal thoughts.
Hospitalization might become necessary, although multiple hospitalizations can be counter-therapeutic. Attempting in therapy to teach the patient to cope with suicidal thoughts and feelings might be a more effective intervention.
Malingering. Use your best judgment when patients make suicide threats that could represent malingering to achieve hospitalization.
Step 5: Documentation. Document your assessment of the suicidal patient and decision making to:
- clarify the treatment plan
- communicate to other caregivers
- manage medicolegal risk.
Include a brief summary (Box) that is timely, legible, and communicates the estimated degree of risk, known data, diagnosis, and planned interventions such as medications, tests, consultations, and follow-up reassessments.
This 46-year-old, recently divorced man is experiencing his second episode of major depression associated with clear-cut panic attacks and suspected psychotic features. Although he denies current suicidal ideation, the treatment team believes he is at moderate to high risk for suicide because of known past history of serious suicide attempt with first depression, the presence of panic/anxiety, and possibly psychotic features. Additional risk is posed by loss of marital support and his inability to verbalize meaningful protective factors.
The plan is to convert from observation status on the inpatient unit to full admission, as the suicide risk precludes discharge at present. Further medication management and consideration for electroconvulsive therapy will take place, with daily reassessments. Suicide precautions ordered.
Table 1
Factors associated with potential for increased suicide risk
| Variable | Risk Factors |
|---|---|
| Demographic | Male gender, Caucasian race, rural residence, possibly age (varies among studies) |
| Imprisoned; widowed, divorced, or separated; living alone; no children or no children living in the home | |
| Psychosocial | Lack or loss of social supports, recent loss of employment, decrease in socioeconomic status or poverty, hopelessness |
| History of victimization (physical or sexual abuse), psychological turmoil, severe relationship conflict, aggressive or impulsive traits | |
| Writing suicide notes; family history of suicide, previous attempts, ‘imitation’ suicide, gun ownership | |
| Occupational risk (physicians, dentists, nurses, pharmacists, veterinarians, farmers) | |
| Psychiatric | Psychiatric diagnosis of recent onset |
| Mood disorder, particularly major depression and bipolar disorder | |
| Schizophrenia; alcohol or other substance abuse or addiction; personality disorder; panic attacks or severe psychic anxiety | |
| Insomnia; poor concentration or confusion; anhedonia | |
| Medical | Huntington’s disease, stroke, multiple sclerosis, head injury, spinal cord injury, systemic lupus erythematosus, AIDS |
| Epilepsy, pain, malignant neoplasms, peptic ulcer disease, renal disease | |
| Source: Adapted with permission from reference 3 | |
Table 2
Potentially protective factors against suicide
| Internal |
| Successful past responses to stress |
| Positive coping skills |
| Spirituality |
| Capacity for reality testing |
| Frustration tolerance/optimism |
| Overall individual resiliency |
| External |
| Children or pets in the home |
| Religious prohibition or beliefs |
| Positive therapeutic relationships |
| Sense of responsibility to family |
| Social supports and connections |
| Financial incentives or deterrents |
| Source: Adapted from reference 11 |
Table 3
Evaluating suicide risk: Questions to ask patients
| Have you felt so sad or depressed that you thought life is not worth living? |
| Have you thought about hurting yourself or taking your own life? |
| Have you thought about a way or plan to kill yourself? |
| Do you have the means to complete the plan? (such as, do you have access to weapons or pills?) |
| Have you practiced or rehearsed this plan to end your own life? |
| Do you have a location picked out? |
| What has stopped you from following through with the plan? |
| Have you ever attempted suicide? |
| Has anyone in your family ever attempted or committed suicide? |
| Source: Adapted with permission from reference 3 |
Interventions for suicidal patients
Physical protection. Take decisive action when you determine that suicide risk is elevated and imminent. Pursue urgent psychiatric hospitalization, with or without patient consent, in accordance with local probate and involuntary commitment statutes.
The logistics of protective action can be challenging; transportation is often required, and the patient is not always cooperative with admission. Table 4 lists measures and precautions that can help keep the suicidal patient safe.
Disease-specific interventions. Because suicidal ideation is often symptomatic of a primary psychiatric disorder, rapidly identifying major depression, bipolar disorder, or a psychotic illness is crucial to reducing suicidal thoughts and behaviors. Prescribe appropriate antidepressants, mood stabilizers, and antipsychotics at adequate doses and for sufficient duration.
Be vigilant for distressing symptoms that may be elevating the patient’s suicide risk, such as anxiety, panic, agitation, insomnia, or pain. Pharmacotherapies—such as anxiolytics, sedative-hypnotics, antipsychotics, or analgesics—may rapidly reduce suffering.
Impulsivity associated with substance use disorders—particularly during intoxication and withdrawal syndromes—requires aggressive attempts by the treatment team to engage the patient in detoxification and rehabilitation.
Direct antisuicide therapy. Clozapine carries an FDA-approved indication for preventing suicide in patients with schizophrenia or schizoaffective disorder. The mechanism by which clozapine helps prevent suicide is not known, but its anti-suicidal effects appear to be independent of its antipsychotic effects.12
Lithium has been reported to reduce risk of suicide and suicide attempts in patients with bipolar disorder, perhaps by as much as 80%.13 Such benefit has not been observed with other mood stabilizers, suggesting that lithium confers protective effects against suicide beyond its mood-stabilizing effects. Suicide risk is known to increase after lithium is discontinued.14
Lithium’s antisuicidal effects may arise from its ability to enhance serotonin. This theory, although unproven, is consistent with observations associating central serotonergic deficiency with suicidal and aggressive behaviors.
Psychosocial measures. Address psychosocial variables that may increase suicide risk (Table 1). Recruit and involve the patient’s support system, augmented with a close follow-up plan. Case management to explore housing and job opportunities can help. Work with the patient’s family or others to remove guns from the patient’s access. Individual, marital, and family therapies can reduce conflicts and strengthen coping skills.
Table 4
Safety measures to protect the suicidal patient
| Hospitalize—voluntarily or involuntarily—on a locked psychiatric unit |
| Provide constant 1-to-1 observation by staff |
| Transport the patient, accompanied by adequate personnel |
| Use physical restraints or seclusion while maintaining continuous observation |
| Employ metal detector to remove dangerous, hidden objects |
| Remove and secure patient’s belongings (bags, coats, purses may contain pills or weapons) |
| Search visitors’ belongings before allowing access to unit |
| Ensure that inpatient unit meets all coded safety regulations |
Related resources
- National Suicide Prevention Lifeline, sponsored by the Substance Abuse & Mental Health Services Administration: 1-800-SUICIDE or 1-800-273-TALK (8255); www.suicidepreventionlifeline.org.
- American Foundation for Suicide Prevention (AFSP) 1-888-333-AFSP; www.afsp.org.
- Simon RI, Hales RE. Textbook of suicide assessment and management. Washington, DC: American Psychiatric Publishing; 2006.
Drug brand names
- Clozapine • Clozaril
- Lithium • Eskalith, Lithobid, others
Disclosure
Dr. Muzina has received grants from or served as a consultant to Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Novartis Pharmaceuticals Corp., Pfizer, and Repligen.
More than 50% of psychiatrists have experienced the death of a patient by suicide.1 For many of us, suicide represents the most feared outcome of a patient’s mental illness and makes managing suicide risk critical to everyday practice.
Unfortunately, we have little ability to predict suicide. Research into risk factors and the use of suicide rating scales have produced no consistently definitive methods to determine who will and who will not attempt or complete suicide.2 The purpose of suicide assessment, then, is not to predict suicide but to help us understand the sources of a patient’s suicidality and develop an informed intervention.
This article describes a practical, commonly accepted approach to suicide risk assessment and intervention, based on the B-SAFE model (Basic Suicide Assessment Five-step Evaluation) proposed by Jacobs et al (Figure).3 Using this method to assess suicide risk can help you answer questions such as:
- Which factors are most important to consider when evaluating suicide risk in my patient?
- What questions should I ask my patient to find out if he or she is suicidal?
- How do I know if a patient is at risk for suicide?
- What emergent interventions are called for when managing the acutely suicidal patient?
- How should I document a suicide risk assessment?
Figure Basic Suicide Assessment Five-step Evaluation (B-SAFE)
Source: Created from information in references 2,11
Why ask about suicide?
No single risk factor or combination of risk factors can predict or preclude suicide. Even so, attempting to evaluate an individual’s risk by asking about suicidal thinking, reviewing risk factors, or using clinical rating scales helps you determine the next appropriate action (discharge, medication, psychiatric referral, consultation, or hospitalization).
While talking to patients and evaluating their risk for suicide, you may begin to understand their suffering—described as the most common denominator in suicide2 and perhaps the most important clue to heightened suicide risk. Such an exploration allows you to identify potential:
- risk factors that can be modified
- preventative factors to promote.
Don’t be afraid to ask. Asking about suicidal thoughts is necessary—but not enough—to understand an individual’s potential for suicide. Never be afraid to ask patients about suicide, believing that doing so will “put ideas into their heads.” By the same token, a patient who denies thoughts or plans for suicide may still be at risk.
Identifying at-risk patients is much more difficult than just asking if they are considering suicide. Opening a concerned dialogue can provide a sense of relief to the patient while allowing you to explore:
- the extent and seriousness of the suicidal thoughts
- associated risk factors or conditions, such as depression.
Stepwise risk assessment
The first 3 steps of Jacobs’ B-SAFE model focus on identifying predisposing and protective factors for suicide.3 For an in-depth discussion, consult the American Psychiatric Association practice guideline for the assessment and treatment of patients with suicidal behaviors4 (available at http://www.psych.org/psych_pract/treatg/pg/suicidalbehavior_05-15-06.pdf).
STEP 1: Risk factors. Use the patient interview, medical records, and collateral information to uncover potential suicide risk factors (Table 1).2
Psychopathology. Focus on depression, bipolar disorder, schizophrenia, substance abuse, and personality disorders, which are strongly associated with suicide. These disorders are considered modifiable risk factors—diagnosis and appropriate treatment can diminish suicide risk.
Suicidality has been associated with early depression or bipolar disorder, often before patients receive a diagnosis or effective treatment. Recovery and immediate post-discharge periods also are thought to be times of heightened suicide risk.
Psychosocial variables. Demographic and psychosocial variables may influence suicide risk estimation. A retrospective study of 100 patients who attempted suicide suggests that the most predictive factors for suicide are:
- living alone
- being aged 17 to 35 (although in other studies, more advanced age also has been linked to increased suicide risk3)
- complaints of severe hopelessness, anhedonia, and insomnia.5
Physical illness may potentiate suicide risk. Medical illnesses that produce great pain, disfigurement, limited function, or fear of dependence may reduce a person’s will to live and increase suicide risk.6 Epilepsy has been associated with a 4- to 5-fold increase in suicide risk7 and is the only medical diagnosis to carry a documented increase in suicide among children and adolescents.8 Often these medical disorders coexist with psychiatric disorders, complicating the task of determining independent risk.
Severity of attempts or self-mutilation. When evaluating self-injurious or suicidal behavior in the emergency setting, consider the severity of the attempt as part of overall suicide assessment. Self-injurious behavior (cutting or burning) or impulsive suicide attempts (planned for <3 hours, committed in the presence others, or where discovery is very probable) appear to carry less severity or intent to die than do carefully planned and/or hidden suicide attempts.9 However, consider at high risk for suicide any patient with self-mutilating or suicidal behavior who expresses persistent intent to die; acute stabilization on an in-patient unit may be necessary.
STEP 2: ‘Protective’ factors. Discover and discuss internal and external factors that might help prevent the individual with suicidal thoughts from converting those thoughts into action (Table 2).2 When discussing these potentially protective effects, emphasize the patient’s:
- resilience during past personal crises
- family responsibilities
- religious or spiritual beliefs.
‘No-harm contracts.’ Suicide (or “no-harm”) contracts with patients might help open communication about factors that promote or mitigate suicide risk. Such contacts do not prevent suicide or lessen medicolegal risk in the event of a patient suicide, however.10
STEP 3: Suicide plans. Ask about suicide thoughts, plans, and behaviors (Table 3).11 Probe gently to allow the individual to discuss his or her feelings and to explore the next appropriate avenue of care.
In my experience, patients who reveal passive suicidal ideation (such as, “I sometimes wish I would just die in my sleep”) and strong deterrents to acting on thoughts of suicide (such as, “My children need me,” or “It’s against my religion”) should continue outpatient treatment. Those without deterrents or who discuss active and imminent thoughts and recent actions—writing suicide notes, buying a weapon, stockpiling pills—require emergent evaluation for psychiatric admission. Ask about thoughts of self-injury or mutilation (such as cutting or burning), as well as homicidal ideation.
Recognizing that patients with suicidal thoughts are almost always ambivalent about suicide to some extent—conflicted by simultaneous desires to live and to die—gives you the opportunity to intervene by allying with the part of the patient that wants to live. Creating a therapeutic connection also will help you determine the level of intervention required.
STEP 4: Intervention. Understanding why a patient feels suicidal—gathered in Steps 1 to 3—can help you choose the appropriate intervention. Among the 5 steps, Step 4 relies most heavily on clinical judgment:
- Is the suicidality acute or chronic?
- How great is the risk for suicide?
- To keep the patient safe, how urgent is the required intervention?
Acute risk. Suicidality related to Axis I psychiatric disorders tends to be acute, with prominent pain, anguish, and a desire to escape. Patients may describe a driven quality to the suicidality, which commands a treatment plan that maintains patient safety until suicidal feelings remit.
Hospitalization is often needed, plus focused treatments such as medication, psychotherapy, or electroconvulsive therapy. Intensive outpatient follow-up or partial hospitalization programs might be considered for patients:
- with whom you have a strong therapeutic alliance
- who have sturdy psychosocial support
- whose precipitating factors for suicidality have resolved.
Chronic risk. Suicide risk tends to be more chronic and has an impulsive quality for patients with suicidality related to personality disorders and environmental factors. Personality disordered patients may report feelings of anger, rage, or vengeance connected with their suicidal thoughts.
Hospitalization might become necessary, although multiple hospitalizations can be counter-therapeutic. Attempting in therapy to teach the patient to cope with suicidal thoughts and feelings might be a more effective intervention.
Malingering. Use your best judgment when patients make suicide threats that could represent malingering to achieve hospitalization.
Step 5: Documentation. Document your assessment of the suicidal patient and decision making to:
- clarify the treatment plan
- communicate to other caregivers
- manage medicolegal risk.
Include a brief summary (Box) that is timely, legible, and communicates the estimated degree of risk, known data, diagnosis, and planned interventions such as medications, tests, consultations, and follow-up reassessments.
This 46-year-old, recently divorced man is experiencing his second episode of major depression associated with clear-cut panic attacks and suspected psychotic features. Although he denies current suicidal ideation, the treatment team believes he is at moderate to high risk for suicide because of known past history of serious suicide attempt with first depression, the presence of panic/anxiety, and possibly psychotic features. Additional risk is posed by loss of marital support and his inability to verbalize meaningful protective factors.
The plan is to convert from observation status on the inpatient unit to full admission, as the suicide risk precludes discharge at present. Further medication management and consideration for electroconvulsive therapy will take place, with daily reassessments. Suicide precautions ordered.
Table 1
Factors associated with potential for increased suicide risk
| Variable | Risk Factors |
|---|---|
| Demographic | Male gender, Caucasian race, rural residence, possibly age (varies among studies) |
| Imprisoned; widowed, divorced, or separated; living alone; no children or no children living in the home | |
| Psychosocial | Lack or loss of social supports, recent loss of employment, decrease in socioeconomic status or poverty, hopelessness |
| History of victimization (physical or sexual abuse), psychological turmoil, severe relationship conflict, aggressive or impulsive traits | |
| Writing suicide notes; family history of suicide, previous attempts, ‘imitation’ suicide, gun ownership | |
| Occupational risk (physicians, dentists, nurses, pharmacists, veterinarians, farmers) | |
| Psychiatric | Psychiatric diagnosis of recent onset |
| Mood disorder, particularly major depression and bipolar disorder | |
| Schizophrenia; alcohol or other substance abuse or addiction; personality disorder; panic attacks or severe psychic anxiety | |
| Insomnia; poor concentration or confusion; anhedonia | |
| Medical | Huntington’s disease, stroke, multiple sclerosis, head injury, spinal cord injury, systemic lupus erythematosus, AIDS |
| Epilepsy, pain, malignant neoplasms, peptic ulcer disease, renal disease | |
| Source: Adapted with permission from reference 3 | |
Table 2
Potentially protective factors against suicide
| Internal |
| Successful past responses to stress |
| Positive coping skills |
| Spirituality |
| Capacity for reality testing |
| Frustration tolerance/optimism |
| Overall individual resiliency |
| External |
| Children or pets in the home |
| Religious prohibition or beliefs |
| Positive therapeutic relationships |
| Sense of responsibility to family |
| Social supports and connections |
| Financial incentives or deterrents |
| Source: Adapted from reference 11 |
Table 3
Evaluating suicide risk: Questions to ask patients
| Have you felt so sad or depressed that you thought life is not worth living? |
| Have you thought about hurting yourself or taking your own life? |
| Have you thought about a way or plan to kill yourself? |
| Do you have the means to complete the plan? (such as, do you have access to weapons or pills?) |
| Have you practiced or rehearsed this plan to end your own life? |
| Do you have a location picked out? |
| What has stopped you from following through with the plan? |
| Have you ever attempted suicide? |
| Has anyone in your family ever attempted or committed suicide? |
| Source: Adapted with permission from reference 3 |
Interventions for suicidal patients
Physical protection. Take decisive action when you determine that suicide risk is elevated and imminent. Pursue urgent psychiatric hospitalization, with or without patient consent, in accordance with local probate and involuntary commitment statutes.
The logistics of protective action can be challenging; transportation is often required, and the patient is not always cooperative with admission. Table 4 lists measures and precautions that can help keep the suicidal patient safe.
Disease-specific interventions. Because suicidal ideation is often symptomatic of a primary psychiatric disorder, rapidly identifying major depression, bipolar disorder, or a psychotic illness is crucial to reducing suicidal thoughts and behaviors. Prescribe appropriate antidepressants, mood stabilizers, and antipsychotics at adequate doses and for sufficient duration.
Be vigilant for distressing symptoms that may be elevating the patient’s suicide risk, such as anxiety, panic, agitation, insomnia, or pain. Pharmacotherapies—such as anxiolytics, sedative-hypnotics, antipsychotics, or analgesics—may rapidly reduce suffering.
Impulsivity associated with substance use disorders—particularly during intoxication and withdrawal syndromes—requires aggressive attempts by the treatment team to engage the patient in detoxification and rehabilitation.
Direct antisuicide therapy. Clozapine carries an FDA-approved indication for preventing suicide in patients with schizophrenia or schizoaffective disorder. The mechanism by which clozapine helps prevent suicide is not known, but its anti-suicidal effects appear to be independent of its antipsychotic effects.12
Lithium has been reported to reduce risk of suicide and suicide attempts in patients with bipolar disorder, perhaps by as much as 80%.13 Such benefit has not been observed with other mood stabilizers, suggesting that lithium confers protective effects against suicide beyond its mood-stabilizing effects. Suicide risk is known to increase after lithium is discontinued.14
Lithium’s antisuicidal effects may arise from its ability to enhance serotonin. This theory, although unproven, is consistent with observations associating central serotonergic deficiency with suicidal and aggressive behaviors.
Psychosocial measures. Address psychosocial variables that may increase suicide risk (Table 1). Recruit and involve the patient’s support system, augmented with a close follow-up plan. Case management to explore housing and job opportunities can help. Work with the patient’s family or others to remove guns from the patient’s access. Individual, marital, and family therapies can reduce conflicts and strengthen coping skills.
Table 4
Safety measures to protect the suicidal patient
| Hospitalize—voluntarily or involuntarily—on a locked psychiatric unit |
| Provide constant 1-to-1 observation by staff |
| Transport the patient, accompanied by adequate personnel |
| Use physical restraints or seclusion while maintaining continuous observation |
| Employ metal detector to remove dangerous, hidden objects |
| Remove and secure patient’s belongings (bags, coats, purses may contain pills or weapons) |
| Search visitors’ belongings before allowing access to unit |
| Ensure that inpatient unit meets all coded safety regulations |
Related resources
- National Suicide Prevention Lifeline, sponsored by the Substance Abuse & Mental Health Services Administration: 1-800-SUICIDE or 1-800-273-TALK (8255); www.suicidepreventionlifeline.org.
- American Foundation for Suicide Prevention (AFSP) 1-888-333-AFSP; www.afsp.org.
- Simon RI, Hales RE. Textbook of suicide assessment and management. Washington, DC: American Psychiatric Publishing; 2006.
Drug brand names
- Clozapine • Clozaril
- Lithium • Eskalith, Lithobid, others
Disclosure
Dr. Muzina has received grants from or served as a consultant to Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Novartis Pharmaceuticals Corp., Pfizer, and Repligen.
1. Chemtob CM, Hamada RS, Bauer G, et al. Patients’ suicides: frequency and impact on psychiatrists. Am J Psychiatry 1988;145(2):224-8.
2. Muzina DJ. What physicians can do to prevent suicide. Cleve Clin J Med 2004;71(3):242-50.
3. Jacobs DG, Brewer ML, Klein-Benheim M. Suicide assessment: an overview and recommended protocol. In: Jacobs DG, ed. The Harvard Medical School guide to suicide assessment and intervention.. San Francisco, CA: Jossey-Bass Publishers; 1999:3-39.
4. Jacobs DG, Baldessarini RJ, Conwell Y, et al. American Psychiatric Association practice guideline for the assessment and treatment of patients with suicidal behaviors. Available at: http://www.psych.org/psych_pract/treatg/pg/suicidalbehavior_05-15-06.pdf. Accessed May 14, 2007.
5. Hall RC, Platt DE, Hall RC. Suicide risk assessment: a review of risk factors for suicide in 100 patients who made severe suicide attempts. Evaluation of suicide risk in a time of managed care. Psychosomatics 1999;40(1):18-27.
6. Mackenzie TB, Popkin MK. Suicide in the medical patient. Int J Psychiatry Med 1987;17(1):3-22.
7. Barraclough BM. The suicide rate of epilepsy. Acta Psychiatr Scand 1987;76(4):339-45
8. Brent DA, Kolko DJ, Allan MJ, Brown RV. Suicidality in affectively disordered adolescent inpatients. J Am Acad Child Adolesc Psychiatry 1990;29(4):586-93.
9. Polewka A, Mikolaszek-Boba M, Chrostek Maj J, Groszek B. The characteristics of suicide attempts based on the suicidal intent scale scores. Przegl Lek 2005;62(6):415-8.
10. Lewis LM. No-harm contracts: a review of what we know. Suicide Life Threat Behav 2007;37(1):50-7.
11. Jacobs DG. A resource guide for implementing the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) 2007 patient safety goals on suicide. Available at: http://www.sprc.org/library/jcahosafetygoals.pdf. Accessed May 14, 2007.
12. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003;60(1):82-91.
13. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord 2006;8(5 Pt 2):625-39.
14. Baldessarini RJ, Tondo L, Viguera AC. Discontinuing lithium maintenance treatment in bipolar disorders: risks and implications. Bipolar Disord 1999;1(1):17-24.
1. Chemtob CM, Hamada RS, Bauer G, et al. Patients’ suicides: frequency and impact on psychiatrists. Am J Psychiatry 1988;145(2):224-8.
2. Muzina DJ. What physicians can do to prevent suicide. Cleve Clin J Med 2004;71(3):242-50.
3. Jacobs DG, Brewer ML, Klein-Benheim M. Suicide assessment: an overview and recommended protocol. In: Jacobs DG, ed. The Harvard Medical School guide to suicide assessment and intervention.. San Francisco, CA: Jossey-Bass Publishers; 1999:3-39.
4. Jacobs DG, Baldessarini RJ, Conwell Y, et al. American Psychiatric Association practice guideline for the assessment and treatment of patients with suicidal behaviors. Available at: http://www.psych.org/psych_pract/treatg/pg/suicidalbehavior_05-15-06.pdf. Accessed May 14, 2007.
5. Hall RC, Platt DE, Hall RC. Suicide risk assessment: a review of risk factors for suicide in 100 patients who made severe suicide attempts. Evaluation of suicide risk in a time of managed care. Psychosomatics 1999;40(1):18-27.
6. Mackenzie TB, Popkin MK. Suicide in the medical patient. Int J Psychiatry Med 1987;17(1):3-22.
7. Barraclough BM. The suicide rate of epilepsy. Acta Psychiatr Scand 1987;76(4):339-45
8. Brent DA, Kolko DJ, Allan MJ, Brown RV. Suicidality in affectively disordered adolescent inpatients. J Am Acad Child Adolesc Psychiatry 1990;29(4):586-93.
9. Polewka A, Mikolaszek-Boba M, Chrostek Maj J, Groszek B. The characteristics of suicide attempts based on the suicidal intent scale scores. Przegl Lek 2005;62(6):415-8.
10. Lewis LM. No-harm contracts: a review of what we know. Suicide Life Threat Behav 2007;37(1):50-7.
11. Jacobs DG. A resource guide for implementing the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) 2007 patient safety goals on suicide. Available at: http://www.sprc.org/library/jcahosafetygoals.pdf. Accessed May 14, 2007.
12. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003;60(1):82-91.
13. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord 2006;8(5 Pt 2):625-39.
14. Baldessarini RJ, Tondo L, Viguera AC. Discontinuing lithium maintenance treatment in bipolar disorders: risks and implications. Bipolar Disord 1999;1(1):17-24.
Managing anxiety in patients with implanted cardiac defibrillators
Mr. W, age 51, received an implantable cardioverter defibrillator (ICD) after a diagnosis of ventricular tachycardia. He has lived more than 2 years without an ICD discharge and had been functioning well.
One evening, while watching his favorite football team lose a close, intense game on television, Mr. W receives his first ICD “shock.” He becomes extremely anxious and immediately associates the shock with the anger and frustration he was feeling while watching the game. He also suspects he might have caused the shock by using the remote control to change channels when he realized his team would lose.
Anxiety, depression, and feeling a “loss of control” are common among ICD patients. Although the devices provide cardiac patients with a better quality of life than the use of antiarrhythmia drugs,1 ICD recipients may live in fear of receiving a powerful shock at any moment.
This article explains how to break the “fear of fear” cycle that causes ICD patients to avoid activities they associate with ICD activation. To help you preserve ICD patients’ functioning, we describe:
- common psychiatric symptoms in ICD recipients
- when and how to assess for ICD-related anxiety
- cognitive-behavioral and pharmacotherapeutic options.
A psychological jolt
An ICD acts to prevent sudden cardiac death (Box 1)2-7 when a patient develops a sustained, potentially life-threatening arrhythmia. The device restores normal cardiac rhythm by delivering high-energy electrical pulses (shocks) to the appropriate chamber in the patient’s heart (Box 2).
Patients usually perceive ICD shocks as painful and unpleasant, which can cause fear, anger, anxiety, helplessness, and depression.8-11 The ICD recipient’s cardiac status, history of psychiatric illness, and other factors increase the risk of experiencing these symptoms (Table 1).8,9
Undergoing the implantation of an ICD may cause patients to feel a loss of personal, social, and material resources. The higher the ICD recipient’s sense of loss of financial or physical well-being, the higher the risk of depression and anxiety.9
Inadequate social support, poor physical functioning, a
history of depression, and greater length of time
since ICD implantation also may contribute
to emotional distress.9
Besides having undergone cardiac surgery, patients with newly implanted ICDs face other stressors, including:
- expensive medical bills
- possible disability
- driving restrictions.
Patients’ personal relationships may become strained because of changes in their ability to maintain previous physical, social, and sexual activity. Depression or anxiety can cause patients to be withdrawn or irritable.
Some ICD recipients become concerned with body image because the silhouette of the device may be visible under the skin. After ICD implantation, patients may become more aware that they may experience life-threatening arrhythmias.
Finally—and perhaps most important to the patient and psychiatrist—a recipient might be constantly afraid that the device is about to deliver a shock.8
Sudden cardiac death (SCD)—one of the leading causes of mortality in the United States2,3—is an unexpected death from cardiac causes that occurs in ≤1 hour. Causes include ventricular tachycardia, ventricular fibrillation, and bradycardia. Most SCD victims have coronary artery disease.2
Each year approximately 330,000 people die from SCD outside a hospital or emergency department.2 The incidence increases with age and is higher in men than women and in African-Americans than Caucasians.3
Implantable cardioverter defibrillators (ICDs) are a first-line prophylactic therapy for patients at risk for SCD.4,5 Annual ICD implants increased by 11-fold from 1990 to 2000, and this trend continues as the U.S. population ages.6 ICDs prevent sudden cardiac death in >90% of cardiac rhythm disturbances.7
Table 1
5 risk factors for ICD-related psychiatric symptoms
| Age <50 years |
| Limited social support |
| Poor cardiac status |
| History of psychiatric illness |
| Receiving frequent ICD discharges |
| Source: References 8,9 |
Fear impairs functioning
Experiencing an ICD discharge can affect patients’ appraisal of their situation and impair functioning.
CASE CONTINUED: Anxiety leads to avoidance
After receiving the ICD shock, Mr. W stops watching his favorite teams on television. He later ceases viewing sporting events, and over the next several months stops watching television altogether.
Mr. W continues to experience severe anxiety and ruminates about potential shocks. He believes that as long as he avoids becoming “excited” he can reduce his risk of shock. He comes to believe that his avoidance accounts for the absence of subsequent shocks and therefore continues to alter his lifestyle. Mr. W begins to eliminate other behaviors, including sex and exercise, that he fears might induce an ICD shock.
Avoiding activities. After receiving a shock, patients often develop fear that causes them to avoid the activity they were doing when the discharge occurred. This classical conditioning process often leads patients to continually avoid the activity, which may reduce anxiety but is negatively reinforcing (operant conditioning).
These fears and subsequent avoidance behaviors may increase with the number of shocks.8 Over time, fear and avoidance may adversely alter lifestyle and diminish quality of life.
Hypervigilance to physical sensations. Patients typically are troubled by ICDs’ uncontrollable, unpredictable nature and feel compelled to try to predict when the device will fire. Often patients become attuned to minor physical sensations and incorrectly interpret normal sympathetic sensations as precursors of an ICD discharge. Fearful assessment of these symptoms can activate anxiety-related sympathetic arousal, creating a “fear of fear” cycle that mimics the catastrophic interpretation of panic disorder patients.10
Cognitive distortions. After receiving an ICD discharge, patients commonly “catastrophize” about future events. They might overestimate the negative consequences of a discharge by, for example, thinking that if they are shocked during an important event, it will ruin the event for everyone.
“Overgeneralizing” occurs when patients believe a rare occurrence (an ICD discharge) will happen frequently, which may contribute to avoidance. For example, patients might think they should avoid the grocery store if they previously received a shock while shopping.
Phantom shocks. A patient with an ICD may insist he has experienced a shock even though the device provides no evidence of delivering one. Patients may report feeling kicked, shaken, or jolted out of sleep.
Because re-experiencing an event (in this case, through phantom shocks), avoidance, and hyperarousal are part of DSM-IV-TR criteria for posttraumatic stress disorder (PTSD), some clinicians equate patients’ post-shock experience to PTSD.11 The more often patients are shocked, the more likely they will experience these outcomes.
CASE CONTINUED: Delayed diagnosis
As a result of his drastic lifestyle changes, Mr. W experiences considerable relationship, vocational, and financial problems. Unfortunately, Mr. W’s primary care physician doesn’t recognize the impact of these symptoms until Mr. W becomes severely depressed. Then Mr. W is referred for psychiatric treatment.
During implantation, the surgeon places the ICD under the skin in the upper chest beneath the patient’s collarbone or abdominal muscles. Each lead is threaded through the subclavian or cephalic vein, passed into the appropriate cardiac chamber, and anchored with a soft prong or screw.
Table 2
Self-rated Florida Shock Anxiety Scale for ICD patients*
| 1. | I am scared to exercise because I am scared that it will increase my heart rate and cause my device to fire. |
| 2. | I am afraid of being alone when the ICD fires and I will need help. |
| 3. | I do not get angry or upset because it may cause the ICD to fire. |
| 4. | It bothers me that I do not know when the ICD will fire. |
| 5. | I worry about the ICD not firing sometime when it should. |
| 6. | I am afraid to touch others for fear that I will shock them if the ICD fires. |
| 7. | I worry about the ICD firing and causing a scene. |
| 8. | When I note my heart beating rapidly, I worry that the ICD will fire. |
| 9. | I have unwanted thoughts of my ICD firing. |
| 10. | I do not engage in sexual activity because it will cause my ICD to fire. |
| *Patients rate each item as 1=Not at all; 2=Rarely; 3=Some of the time; 4=Most of the time; or 5=All of the time. A patient who scores 3 or higher on any item should be referred for further discussion of his specific concerns. | |
| Source: Adapted with permission of Blackwell Publishing Ltd. from Kuhl E, Dixit N, Walker R, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-8. | |
Frequent assessment is key
ICD patients should be assessed for psychiatric symptoms soon after the device is implanted and consistently as a part of routine medical care. At all follow-up visits, primary care clinicians should screen patients for anxiety, depression, and difficulty adjusting to receiving an ICD, and refer those who exhibit signs and symptoms of emotional distress to a mental health professional for evaluation and treatment.
ICD patients might not readily report psychiatric symptoms because of embarrassment, lack of insight, or the circumscribed nature of their symptoms. Because patients may have symptoms that do not meet diagnostic criteria for a specific DSM-IV-TR disorder but impair quality of life and functioning, assess for subclinical distress. Assess patients regularly even if they have not experienced a shock because device-related distress can occur in the absence of shock.
Frequently used screening instruments for anxiety and depression—such as the Beck Depression Inventory, Beck Anxiety Inventory, Brief Symptom Inventory-18 Item, and the Patient Health Questionnaire—can be helpful. Some patients’ distress is limited to their ICDs, however, and cannot be identified by more general measures.
As with Mr. W, traditional inventories might not detect the severity of ICD patients’ psychiatric symptoms until symptoms are severe. Moreover, initial symptoms can be very specific to the ICD and, to some clinicians, may seem relatively benign. Using an instrument designed specifically for assessing ICD patients can lead to earlier diagnosis and treatment.
ICD-specific tools. The Florida Shock Anxiety Scale (FSAS) is a 10-item questionnaire measuring ICD-specific fears about shock (Table 2).12 The FSAS contains statements, such as “I have unwanted thoughts of my ICD firing” and “When I note my heart beating rapidly, I worry that the ICD will fire,” that patients rate on a scale of 1 (Not at all) to 5 (All of the time). A patient who scores 3 or higher on any item should receive counseling related to his specific concerns.
Another useful instrument is the ICD Patient Concerns Questionnaire,13 a 20-item questionnaire to assess the number and severity of ICD recipients’ concerns. Both instruments can help identify targets for intervention.
Targeting underlying beliefs
The initial treatment goal is to relieve anxiety and depressive symptoms. These are likely to persist, however, if the patient’s irrational beliefs, avoidance, and conditioning are not addressed.
Treatment often involves a combination of medication, psychotherapy, and support. Anxiolytics and antidepressants may prove helpful. Choose medications—in collaboration with the patient’s electrophysiologist— based on the patient’s medical history, psychiatric history, and other factors.
Cognitive-behavioral therapy (CBT) often is used to identify and correct maladaptive or irrational beliefs about ICDs and shocks and to eliminate avoidance behaviors that serve as negative reinforcement. CBT typically begins with psychoeducation about the ICD to help patients realize their thoughts about the device might be irrational. Strategies include keeping a daily log of ICD-related thoughts and cognitive re-structuring. Exposure therapy can help patients re-engage in activities they have been avoiding because of irrational fears.
One study found that ICD patients who received CBT that included psychoeducation, stress management, addressing distorted cognitions and avoidance behavior, and resuming work and social activities had less depression and anxiety and better overall adjustment than those who did not get CBT, whether or not their ICD had ever delivered a shock.14
Smith et al15 demonstrated the effectiveness of CBT in patients with ICDs who suffer from panic disorder with agoraphobia and depression. This treatment included:
- interoceptive exposure to target somatic hypervigilance
- relaxation techniques
- cognitive restructuring.
Other therapeutic interventions that can help patients cope and improve their quality of life include support groups, relaxation training, biofeedback, and couples’ counseling.16-19
CASE CONTINUED: CBT helps patient resume activities CBT helps patient resume activities
During 8 CBT sessions, Mr. W learns to identify and challenge the irrational and maladaptive beliefs that were leading him to avoid numerous activities. He eventually accepts that watching television is not likely by itself to trigger an ICD discharge. Through a combination of exposure therapy and relaxation training, Mr. W can resume most activities, which improves his personal relationships and quality of life.
Related resources
- Frizelle D, Lewin B, Kaye G, Moniz-Cook E. Development of a measure of the concerns held by people with implanted cardioverter defibrillators: the ICDC. Br J Health Psychol 2006;11:293-301.
- Hecksel KA, Bostwick JM. Getting to the heart of his 'shocking' trauma. Current Psychiatry 2007;6(6):84-95.
- Kuhl E, Dixit N, Walker R, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-8.
- Thomas S, Friedmann E, Kao C, et al. Quality of life and psychological status of patients with implantable cardioverter defibrillators. Am J Crit Care 2006;15(4):389-98.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. American Heart Association Sudden cardiac death 2007. Available at: http://www.americanheart.org/presenter.jhtml?identifier=14. Accessed April 16, 2007.
2. Zheng Z, Croft J, Giles W, Mehsah G. Sudden cardiac death in the United States, 1989 to 1998. Circulation 2001;104:2158-63.
3. The Antiarrhythmic versus Implantable Defibrillator Study (AVID) Investigators. A comparison of antiarrhythmicdrug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. Circulation 1997;337:1576-83.
4. Moss A, Zareba W, Hall W, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877-83.
5. Kadish A, Dyer A, Daubert J, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004;350:2151-8.
6. Maisel W, Sweeney M, Stevenson W, et al. Recalls and safety alerts involving pacemakers and implantable cardioverter defibrillator generators. JAMA 2001;286(7):793-9.
7. Groeneveld P, Matta M, Suh J, et al. Costs and quality-of-life effects of implantable cardioverter-defibrillators. Am J Cardiol 2006;98:1409-14.
8. Sears S, Kovacs A, Azzarello L, et al. Innovations in health psychology: the psychosocial care of adults with implantable cardioverter defibrillators. Prof Psychol Res Pr 2004;35:520-6.
9. Luyster F, Hughes J, Waechter D, Josephson R. Resource loss predicts depression and anxiety among patients treated with an implantable cardioverter defibrillator. Psychosom Med 2006;68:794-800.
10. Pauli P, Wiedemannn G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76.
11. Hamner M, Hunt N, Gee J, et al. PTSD and automatic implantable cardioverter defibrillators. Psychosomatics 1999;40:82-5.
12. Kuhl E, Dixit N, Walker R, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-8.
13. Frizelle D, Lewin B, Kaye G, Moniz-Cook E. Development of a measure of the concerns held by people with implanted cardioverter defibrillators: the ICDC. Br J Health Psychol 2006;11:293-301.
14. Kohn C, Petrucci R, Baessler C, et al. The effect of psychological intervention on patients’ long-term adjustment to the ICD: a prospective study. Pacing Clin Electrophysiol 2000;23(4):450-6.
15. Smith LC, Fogel D, Friedman S. Cognitive-behavioral treatment of panic disorder with agoraphobia triggered by AICD implant activity. Psychosomatics 1999;39(5):474-7.
16. Fitchet A, Doherty PJ, Bundy C, et al. Comprehensive cardiac rehabilitation program for implantable cardioverter-defibrillator patients: a randomized controlled trial. Heart 2003;89:155-60.
17. Sears SF, Conti JB. Quality of life and psychological functioning of ICD patients. Heart 2002;87:488-93.
18. Kranitz L, Lehrer P. Biofeedback applications in the treatment of cardiovascular diseases. Cardiol Rev 2004;12(3):177-81.
19. O’Brien MC, Langberg J, Valderrama AL, et al. Implantable cardioverter defibrillator storm nursing care issues for patients and families. Crit Care Nurs Clin North Am 2005;17:9-6.
Mr. W, age 51, received an implantable cardioverter defibrillator (ICD) after a diagnosis of ventricular tachycardia. He has lived more than 2 years without an ICD discharge and had been functioning well.
One evening, while watching his favorite football team lose a close, intense game on television, Mr. W receives his first ICD “shock.” He becomes extremely anxious and immediately associates the shock with the anger and frustration he was feeling while watching the game. He also suspects he might have caused the shock by using the remote control to change channels when he realized his team would lose.
Anxiety, depression, and feeling a “loss of control” are common among ICD patients. Although the devices provide cardiac patients with a better quality of life than the use of antiarrhythmia drugs,1 ICD recipients may live in fear of receiving a powerful shock at any moment.
This article explains how to break the “fear of fear” cycle that causes ICD patients to avoid activities they associate with ICD activation. To help you preserve ICD patients’ functioning, we describe:
- common psychiatric symptoms in ICD recipients
- when and how to assess for ICD-related anxiety
- cognitive-behavioral and pharmacotherapeutic options.
A psychological jolt
An ICD acts to prevent sudden cardiac death (Box 1)2-7 when a patient develops a sustained, potentially life-threatening arrhythmia. The device restores normal cardiac rhythm by delivering high-energy electrical pulses (shocks) to the appropriate chamber in the patient’s heart (Box 2).
Patients usually perceive ICD shocks as painful and unpleasant, which can cause fear, anger, anxiety, helplessness, and depression.8-11 The ICD recipient’s cardiac status, history of psychiatric illness, and other factors increase the risk of experiencing these symptoms (Table 1).8,9
Undergoing the implantation of an ICD may cause patients to feel a loss of personal, social, and material resources. The higher the ICD recipient’s sense of loss of financial or physical well-being, the higher the risk of depression and anxiety.9
Inadequate social support, poor physical functioning, a
history of depression, and greater length of time
since ICD implantation also may contribute
to emotional distress.9
Besides having undergone cardiac surgery, patients with newly implanted ICDs face other stressors, including:
- expensive medical bills
- possible disability
- driving restrictions.
Patients’ personal relationships may become strained because of changes in their ability to maintain previous physical, social, and sexual activity. Depression or anxiety can cause patients to be withdrawn or irritable.
Some ICD recipients become concerned with body image because the silhouette of the device may be visible under the skin. After ICD implantation, patients may become more aware that they may experience life-threatening arrhythmias.
Finally—and perhaps most important to the patient and psychiatrist—a recipient might be constantly afraid that the device is about to deliver a shock.8
Sudden cardiac death (SCD)—one of the leading causes of mortality in the United States2,3—is an unexpected death from cardiac causes that occurs in ≤1 hour. Causes include ventricular tachycardia, ventricular fibrillation, and bradycardia. Most SCD victims have coronary artery disease.2
Each year approximately 330,000 people die from SCD outside a hospital or emergency department.2 The incidence increases with age and is higher in men than women and in African-Americans than Caucasians.3
Implantable cardioverter defibrillators (ICDs) are a first-line prophylactic therapy for patients at risk for SCD.4,5 Annual ICD implants increased by 11-fold from 1990 to 2000, and this trend continues as the U.S. population ages.6 ICDs prevent sudden cardiac death in >90% of cardiac rhythm disturbances.7
Table 1
5 risk factors for ICD-related psychiatric symptoms
| Age <50 years |
| Limited social support |
| Poor cardiac status |
| History of psychiatric illness |
| Receiving frequent ICD discharges |
| Source: References 8,9 |
Fear impairs functioning
Experiencing an ICD discharge can affect patients’ appraisal of their situation and impair functioning.
CASE CONTINUED: Anxiety leads to avoidance
After receiving the ICD shock, Mr. W stops watching his favorite teams on television. He later ceases viewing sporting events, and over the next several months stops watching television altogether.
Mr. W continues to experience severe anxiety and ruminates about potential shocks. He believes that as long as he avoids becoming “excited” he can reduce his risk of shock. He comes to believe that his avoidance accounts for the absence of subsequent shocks and therefore continues to alter his lifestyle. Mr. W begins to eliminate other behaviors, including sex and exercise, that he fears might induce an ICD shock.
Avoiding activities. After receiving a shock, patients often develop fear that causes them to avoid the activity they were doing when the discharge occurred. This classical conditioning process often leads patients to continually avoid the activity, which may reduce anxiety but is negatively reinforcing (operant conditioning).
These fears and subsequent avoidance behaviors may increase with the number of shocks.8 Over time, fear and avoidance may adversely alter lifestyle and diminish quality of life.
Hypervigilance to physical sensations. Patients typically are troubled by ICDs’ uncontrollable, unpredictable nature and feel compelled to try to predict when the device will fire. Often patients become attuned to minor physical sensations and incorrectly interpret normal sympathetic sensations as precursors of an ICD discharge. Fearful assessment of these symptoms can activate anxiety-related sympathetic arousal, creating a “fear of fear” cycle that mimics the catastrophic interpretation of panic disorder patients.10
Cognitive distortions. After receiving an ICD discharge, patients commonly “catastrophize” about future events. They might overestimate the negative consequences of a discharge by, for example, thinking that if they are shocked during an important event, it will ruin the event for everyone.
“Overgeneralizing” occurs when patients believe a rare occurrence (an ICD discharge) will happen frequently, which may contribute to avoidance. For example, patients might think they should avoid the grocery store if they previously received a shock while shopping.
Phantom shocks. A patient with an ICD may insist he has experienced a shock even though the device provides no evidence of delivering one. Patients may report feeling kicked, shaken, or jolted out of sleep.
Because re-experiencing an event (in this case, through phantom shocks), avoidance, and hyperarousal are part of DSM-IV-TR criteria for posttraumatic stress disorder (PTSD), some clinicians equate patients’ post-shock experience to PTSD.11 The more often patients are shocked, the more likely they will experience these outcomes.
CASE CONTINUED: Delayed diagnosis
As a result of his drastic lifestyle changes, Mr. W experiences considerable relationship, vocational, and financial problems. Unfortunately, Mr. W’s primary care physician doesn’t recognize the impact of these symptoms until Mr. W becomes severely depressed. Then Mr. W is referred for psychiatric treatment.
During implantation, the surgeon places the ICD under the skin in the upper chest beneath the patient’s collarbone or abdominal muscles. Each lead is threaded through the subclavian or cephalic vein, passed into the appropriate cardiac chamber, and anchored with a soft prong or screw.
Table 2
Self-rated Florida Shock Anxiety Scale for ICD patients*
| 1. | I am scared to exercise because I am scared that it will increase my heart rate and cause my device to fire. |
| 2. | I am afraid of being alone when the ICD fires and I will need help. |
| 3. | I do not get angry or upset because it may cause the ICD to fire. |
| 4. | It bothers me that I do not know when the ICD will fire. |
| 5. | I worry about the ICD not firing sometime when it should. |
| 6. | I am afraid to touch others for fear that I will shock them if the ICD fires. |
| 7. | I worry about the ICD firing and causing a scene. |
| 8. | When I note my heart beating rapidly, I worry that the ICD will fire. |
| 9. | I have unwanted thoughts of my ICD firing. |
| 10. | I do not engage in sexual activity because it will cause my ICD to fire. |
| *Patients rate each item as 1=Not at all; 2=Rarely; 3=Some of the time; 4=Most of the time; or 5=All of the time. A patient who scores 3 or higher on any item should be referred for further discussion of his specific concerns. | |
| Source: Adapted with permission of Blackwell Publishing Ltd. from Kuhl E, Dixit N, Walker R, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-8. | |
Frequent assessment is key
ICD patients should be assessed for psychiatric symptoms soon after the device is implanted and consistently as a part of routine medical care. At all follow-up visits, primary care clinicians should screen patients for anxiety, depression, and difficulty adjusting to receiving an ICD, and refer those who exhibit signs and symptoms of emotional distress to a mental health professional for evaluation and treatment.
ICD patients might not readily report psychiatric symptoms because of embarrassment, lack of insight, or the circumscribed nature of their symptoms. Because patients may have symptoms that do not meet diagnostic criteria for a specific DSM-IV-TR disorder but impair quality of life and functioning, assess for subclinical distress. Assess patients regularly even if they have not experienced a shock because device-related distress can occur in the absence of shock.
Frequently used screening instruments for anxiety and depression—such as the Beck Depression Inventory, Beck Anxiety Inventory, Brief Symptom Inventory-18 Item, and the Patient Health Questionnaire—can be helpful. Some patients’ distress is limited to their ICDs, however, and cannot be identified by more general measures.
As with Mr. W, traditional inventories might not detect the severity of ICD patients’ psychiatric symptoms until symptoms are severe. Moreover, initial symptoms can be very specific to the ICD and, to some clinicians, may seem relatively benign. Using an instrument designed specifically for assessing ICD patients can lead to earlier diagnosis and treatment.
ICD-specific tools. The Florida Shock Anxiety Scale (FSAS) is a 10-item questionnaire measuring ICD-specific fears about shock (Table 2).12 The FSAS contains statements, such as “I have unwanted thoughts of my ICD firing” and “When I note my heart beating rapidly, I worry that the ICD will fire,” that patients rate on a scale of 1 (Not at all) to 5 (All of the time). A patient who scores 3 or higher on any item should receive counseling related to his specific concerns.
Another useful instrument is the ICD Patient Concerns Questionnaire,13 a 20-item questionnaire to assess the number and severity of ICD recipients’ concerns. Both instruments can help identify targets for intervention.
Targeting underlying beliefs
The initial treatment goal is to relieve anxiety and depressive symptoms. These are likely to persist, however, if the patient’s irrational beliefs, avoidance, and conditioning are not addressed.
Treatment often involves a combination of medication, psychotherapy, and support. Anxiolytics and antidepressants may prove helpful. Choose medications—in collaboration with the patient’s electrophysiologist— based on the patient’s medical history, psychiatric history, and other factors.
Cognitive-behavioral therapy (CBT) often is used to identify and correct maladaptive or irrational beliefs about ICDs and shocks and to eliminate avoidance behaviors that serve as negative reinforcement. CBT typically begins with psychoeducation about the ICD to help patients realize their thoughts about the device might be irrational. Strategies include keeping a daily log of ICD-related thoughts and cognitive re-structuring. Exposure therapy can help patients re-engage in activities they have been avoiding because of irrational fears.
One study found that ICD patients who received CBT that included psychoeducation, stress management, addressing distorted cognitions and avoidance behavior, and resuming work and social activities had less depression and anxiety and better overall adjustment than those who did not get CBT, whether or not their ICD had ever delivered a shock.14
Smith et al15 demonstrated the effectiveness of CBT in patients with ICDs who suffer from panic disorder with agoraphobia and depression. This treatment included:
- interoceptive exposure to target somatic hypervigilance
- relaxation techniques
- cognitive restructuring.
Other therapeutic interventions that can help patients cope and improve their quality of life include support groups, relaxation training, biofeedback, and couples’ counseling.16-19
CASE CONTINUED: CBT helps patient resume activities CBT helps patient resume activities
During 8 CBT sessions, Mr. W learns to identify and challenge the irrational and maladaptive beliefs that were leading him to avoid numerous activities. He eventually accepts that watching television is not likely by itself to trigger an ICD discharge. Through a combination of exposure therapy and relaxation training, Mr. W can resume most activities, which improves his personal relationships and quality of life.
Related resources
- Frizelle D, Lewin B, Kaye G, Moniz-Cook E. Development of a measure of the concerns held by people with implanted cardioverter defibrillators: the ICDC. Br J Health Psychol 2006;11:293-301.
- Hecksel KA, Bostwick JM. Getting to the heart of his 'shocking' trauma. Current Psychiatry 2007;6(6):84-95.
- Kuhl E, Dixit N, Walker R, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-8.
- Thomas S, Friedmann E, Kao C, et al. Quality of life and psychological status of patients with implantable cardioverter defibrillators. Am J Crit Care 2006;15(4):389-98.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Mr. W, age 51, received an implantable cardioverter defibrillator (ICD) after a diagnosis of ventricular tachycardia. He has lived more than 2 years without an ICD discharge and had been functioning well.
One evening, while watching his favorite football team lose a close, intense game on television, Mr. W receives his first ICD “shock.” He becomes extremely anxious and immediately associates the shock with the anger and frustration he was feeling while watching the game. He also suspects he might have caused the shock by using the remote control to change channels when he realized his team would lose.
Anxiety, depression, and feeling a “loss of control” are common among ICD patients. Although the devices provide cardiac patients with a better quality of life than the use of antiarrhythmia drugs,1 ICD recipients may live in fear of receiving a powerful shock at any moment.
This article explains how to break the “fear of fear” cycle that causes ICD patients to avoid activities they associate with ICD activation. To help you preserve ICD patients’ functioning, we describe:
- common psychiatric symptoms in ICD recipients
- when and how to assess for ICD-related anxiety
- cognitive-behavioral and pharmacotherapeutic options.
A psychological jolt
An ICD acts to prevent sudden cardiac death (Box 1)2-7 when a patient develops a sustained, potentially life-threatening arrhythmia. The device restores normal cardiac rhythm by delivering high-energy electrical pulses (shocks) to the appropriate chamber in the patient’s heart (Box 2).
Patients usually perceive ICD shocks as painful and unpleasant, which can cause fear, anger, anxiety, helplessness, and depression.8-11 The ICD recipient’s cardiac status, history of psychiatric illness, and other factors increase the risk of experiencing these symptoms (Table 1).8,9
Undergoing the implantation of an ICD may cause patients to feel a loss of personal, social, and material resources. The higher the ICD recipient’s sense of loss of financial or physical well-being, the higher the risk of depression and anxiety.9
Inadequate social support, poor physical functioning, a
history of depression, and greater length of time
since ICD implantation also may contribute
to emotional distress.9
Besides having undergone cardiac surgery, patients with newly implanted ICDs face other stressors, including:
- expensive medical bills
- possible disability
- driving restrictions.
Patients’ personal relationships may become strained because of changes in their ability to maintain previous physical, social, and sexual activity. Depression or anxiety can cause patients to be withdrawn or irritable.
Some ICD recipients become concerned with body image because the silhouette of the device may be visible under the skin. After ICD implantation, patients may become more aware that they may experience life-threatening arrhythmias.
Finally—and perhaps most important to the patient and psychiatrist—a recipient might be constantly afraid that the device is about to deliver a shock.8
Sudden cardiac death (SCD)—one of the leading causes of mortality in the United States2,3—is an unexpected death from cardiac causes that occurs in ≤1 hour. Causes include ventricular tachycardia, ventricular fibrillation, and bradycardia. Most SCD victims have coronary artery disease.2
Each year approximately 330,000 people die from SCD outside a hospital or emergency department.2 The incidence increases with age and is higher in men than women and in African-Americans than Caucasians.3
Implantable cardioverter defibrillators (ICDs) are a first-line prophylactic therapy for patients at risk for SCD.4,5 Annual ICD implants increased by 11-fold from 1990 to 2000, and this trend continues as the U.S. population ages.6 ICDs prevent sudden cardiac death in >90% of cardiac rhythm disturbances.7
Table 1
5 risk factors for ICD-related psychiatric symptoms
| Age <50 years |
| Limited social support |
| Poor cardiac status |
| History of psychiatric illness |
| Receiving frequent ICD discharges |
| Source: References 8,9 |
Fear impairs functioning
Experiencing an ICD discharge can affect patients’ appraisal of their situation and impair functioning.
CASE CONTINUED: Anxiety leads to avoidance
After receiving the ICD shock, Mr. W stops watching his favorite teams on television. He later ceases viewing sporting events, and over the next several months stops watching television altogether.
Mr. W continues to experience severe anxiety and ruminates about potential shocks. He believes that as long as he avoids becoming “excited” he can reduce his risk of shock. He comes to believe that his avoidance accounts for the absence of subsequent shocks and therefore continues to alter his lifestyle. Mr. W begins to eliminate other behaviors, including sex and exercise, that he fears might induce an ICD shock.
Avoiding activities. After receiving a shock, patients often develop fear that causes them to avoid the activity they were doing when the discharge occurred. This classical conditioning process often leads patients to continually avoid the activity, which may reduce anxiety but is negatively reinforcing (operant conditioning).
These fears and subsequent avoidance behaviors may increase with the number of shocks.8 Over time, fear and avoidance may adversely alter lifestyle and diminish quality of life.
Hypervigilance to physical sensations. Patients typically are troubled by ICDs’ uncontrollable, unpredictable nature and feel compelled to try to predict when the device will fire. Often patients become attuned to minor physical sensations and incorrectly interpret normal sympathetic sensations as precursors of an ICD discharge. Fearful assessment of these symptoms can activate anxiety-related sympathetic arousal, creating a “fear of fear” cycle that mimics the catastrophic interpretation of panic disorder patients.10
Cognitive distortions. After receiving an ICD discharge, patients commonly “catastrophize” about future events. They might overestimate the negative consequences of a discharge by, for example, thinking that if they are shocked during an important event, it will ruin the event for everyone.
“Overgeneralizing” occurs when patients believe a rare occurrence (an ICD discharge) will happen frequently, which may contribute to avoidance. For example, patients might think they should avoid the grocery store if they previously received a shock while shopping.
Phantom shocks. A patient with an ICD may insist he has experienced a shock even though the device provides no evidence of delivering one. Patients may report feeling kicked, shaken, or jolted out of sleep.
Because re-experiencing an event (in this case, through phantom shocks), avoidance, and hyperarousal are part of DSM-IV-TR criteria for posttraumatic stress disorder (PTSD), some clinicians equate patients’ post-shock experience to PTSD.11 The more often patients are shocked, the more likely they will experience these outcomes.
CASE CONTINUED: Delayed diagnosis
As a result of his drastic lifestyle changes, Mr. W experiences considerable relationship, vocational, and financial problems. Unfortunately, Mr. W’s primary care physician doesn’t recognize the impact of these symptoms until Mr. W becomes severely depressed. Then Mr. W is referred for psychiatric treatment.
During implantation, the surgeon places the ICD under the skin in the upper chest beneath the patient’s collarbone or abdominal muscles. Each lead is threaded through the subclavian or cephalic vein, passed into the appropriate cardiac chamber, and anchored with a soft prong or screw.
Table 2
Self-rated Florida Shock Anxiety Scale for ICD patients*
| 1. | I am scared to exercise because I am scared that it will increase my heart rate and cause my device to fire. |
| 2. | I am afraid of being alone when the ICD fires and I will need help. |
| 3. | I do not get angry or upset because it may cause the ICD to fire. |
| 4. | It bothers me that I do not know when the ICD will fire. |
| 5. | I worry about the ICD not firing sometime when it should. |
| 6. | I am afraid to touch others for fear that I will shock them if the ICD fires. |
| 7. | I worry about the ICD firing and causing a scene. |
| 8. | When I note my heart beating rapidly, I worry that the ICD will fire. |
| 9. | I have unwanted thoughts of my ICD firing. |
| 10. | I do not engage in sexual activity because it will cause my ICD to fire. |
| *Patients rate each item as 1=Not at all; 2=Rarely; 3=Some of the time; 4=Most of the time; or 5=All of the time. A patient who scores 3 or higher on any item should be referred for further discussion of his specific concerns. | |
| Source: Adapted with permission of Blackwell Publishing Ltd. from Kuhl E, Dixit N, Walker R, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-8. | |
Frequent assessment is key
ICD patients should be assessed for psychiatric symptoms soon after the device is implanted and consistently as a part of routine medical care. At all follow-up visits, primary care clinicians should screen patients for anxiety, depression, and difficulty adjusting to receiving an ICD, and refer those who exhibit signs and symptoms of emotional distress to a mental health professional for evaluation and treatment.
ICD patients might not readily report psychiatric symptoms because of embarrassment, lack of insight, or the circumscribed nature of their symptoms. Because patients may have symptoms that do not meet diagnostic criteria for a specific DSM-IV-TR disorder but impair quality of life and functioning, assess for subclinical distress. Assess patients regularly even if they have not experienced a shock because device-related distress can occur in the absence of shock.
Frequently used screening instruments for anxiety and depression—such as the Beck Depression Inventory, Beck Anxiety Inventory, Brief Symptom Inventory-18 Item, and the Patient Health Questionnaire—can be helpful. Some patients’ distress is limited to their ICDs, however, and cannot be identified by more general measures.
As with Mr. W, traditional inventories might not detect the severity of ICD patients’ psychiatric symptoms until symptoms are severe. Moreover, initial symptoms can be very specific to the ICD and, to some clinicians, may seem relatively benign. Using an instrument designed specifically for assessing ICD patients can lead to earlier diagnosis and treatment.
ICD-specific tools. The Florida Shock Anxiety Scale (FSAS) is a 10-item questionnaire measuring ICD-specific fears about shock (Table 2).12 The FSAS contains statements, such as “I have unwanted thoughts of my ICD firing” and “When I note my heart beating rapidly, I worry that the ICD will fire,” that patients rate on a scale of 1 (Not at all) to 5 (All of the time). A patient who scores 3 or higher on any item should receive counseling related to his specific concerns.
Another useful instrument is the ICD Patient Concerns Questionnaire,13 a 20-item questionnaire to assess the number and severity of ICD recipients’ concerns. Both instruments can help identify targets for intervention.
Targeting underlying beliefs
The initial treatment goal is to relieve anxiety and depressive symptoms. These are likely to persist, however, if the patient’s irrational beliefs, avoidance, and conditioning are not addressed.
Treatment often involves a combination of medication, psychotherapy, and support. Anxiolytics and antidepressants may prove helpful. Choose medications—in collaboration with the patient’s electrophysiologist— based on the patient’s medical history, psychiatric history, and other factors.
Cognitive-behavioral therapy (CBT) often is used to identify and correct maladaptive or irrational beliefs about ICDs and shocks and to eliminate avoidance behaviors that serve as negative reinforcement. CBT typically begins with psychoeducation about the ICD to help patients realize their thoughts about the device might be irrational. Strategies include keeping a daily log of ICD-related thoughts and cognitive re-structuring. Exposure therapy can help patients re-engage in activities they have been avoiding because of irrational fears.
One study found that ICD patients who received CBT that included psychoeducation, stress management, addressing distorted cognitions and avoidance behavior, and resuming work and social activities had less depression and anxiety and better overall adjustment than those who did not get CBT, whether or not their ICD had ever delivered a shock.14
Smith et al15 demonstrated the effectiveness of CBT in patients with ICDs who suffer from panic disorder with agoraphobia and depression. This treatment included:
- interoceptive exposure to target somatic hypervigilance
- relaxation techniques
- cognitive restructuring.
Other therapeutic interventions that can help patients cope and improve their quality of life include support groups, relaxation training, biofeedback, and couples’ counseling.16-19
CASE CONTINUED: CBT helps patient resume activities CBT helps patient resume activities
During 8 CBT sessions, Mr. W learns to identify and challenge the irrational and maladaptive beliefs that were leading him to avoid numerous activities. He eventually accepts that watching television is not likely by itself to trigger an ICD discharge. Through a combination of exposure therapy and relaxation training, Mr. W can resume most activities, which improves his personal relationships and quality of life.
Related resources
- Frizelle D, Lewin B, Kaye G, Moniz-Cook E. Development of a measure of the concerns held by people with implanted cardioverter defibrillators: the ICDC. Br J Health Psychol 2006;11:293-301.
- Hecksel KA, Bostwick JM. Getting to the heart of his 'shocking' trauma. Current Psychiatry 2007;6(6):84-95.
- Kuhl E, Dixit N, Walker R, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-8.
- Thomas S, Friedmann E, Kao C, et al. Quality of life and psychological status of patients with implantable cardioverter defibrillators. Am J Crit Care 2006;15(4):389-98.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. American Heart Association Sudden cardiac death 2007. Available at: http://www.americanheart.org/presenter.jhtml?identifier=14. Accessed April 16, 2007.
2. Zheng Z, Croft J, Giles W, Mehsah G. Sudden cardiac death in the United States, 1989 to 1998. Circulation 2001;104:2158-63.
3. The Antiarrhythmic versus Implantable Defibrillator Study (AVID) Investigators. A comparison of antiarrhythmicdrug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. Circulation 1997;337:1576-83.
4. Moss A, Zareba W, Hall W, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877-83.
5. Kadish A, Dyer A, Daubert J, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004;350:2151-8.
6. Maisel W, Sweeney M, Stevenson W, et al. Recalls and safety alerts involving pacemakers and implantable cardioverter defibrillator generators. JAMA 2001;286(7):793-9.
7. Groeneveld P, Matta M, Suh J, et al. Costs and quality-of-life effects of implantable cardioverter-defibrillators. Am J Cardiol 2006;98:1409-14.
8. Sears S, Kovacs A, Azzarello L, et al. Innovations in health psychology: the psychosocial care of adults with implantable cardioverter defibrillators. Prof Psychol Res Pr 2004;35:520-6.
9. Luyster F, Hughes J, Waechter D, Josephson R. Resource loss predicts depression and anxiety among patients treated with an implantable cardioverter defibrillator. Psychosom Med 2006;68:794-800.
10. Pauli P, Wiedemannn G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76.
11. Hamner M, Hunt N, Gee J, et al. PTSD and automatic implantable cardioverter defibrillators. Psychosomatics 1999;40:82-5.
12. Kuhl E, Dixit N, Walker R, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-8.
13. Frizelle D, Lewin B, Kaye G, Moniz-Cook E. Development of a measure of the concerns held by people with implanted cardioverter defibrillators: the ICDC. Br J Health Psychol 2006;11:293-301.
14. Kohn C, Petrucci R, Baessler C, et al. The effect of psychological intervention on patients’ long-term adjustment to the ICD: a prospective study. Pacing Clin Electrophysiol 2000;23(4):450-6.
15. Smith LC, Fogel D, Friedman S. Cognitive-behavioral treatment of panic disorder with agoraphobia triggered by AICD implant activity. Psychosomatics 1999;39(5):474-7.
16. Fitchet A, Doherty PJ, Bundy C, et al. Comprehensive cardiac rehabilitation program for implantable cardioverter-defibrillator patients: a randomized controlled trial. Heart 2003;89:155-60.
17. Sears SF, Conti JB. Quality of life and psychological functioning of ICD patients. Heart 2002;87:488-93.
18. Kranitz L, Lehrer P. Biofeedback applications in the treatment of cardiovascular diseases. Cardiol Rev 2004;12(3):177-81.
19. O’Brien MC, Langberg J, Valderrama AL, et al. Implantable cardioverter defibrillator storm nursing care issues for patients and families. Crit Care Nurs Clin North Am 2005;17:9-6.
1. American Heart Association Sudden cardiac death 2007. Available at: http://www.americanheart.org/presenter.jhtml?identifier=14. Accessed April 16, 2007.
2. Zheng Z, Croft J, Giles W, Mehsah G. Sudden cardiac death in the United States, 1989 to 1998. Circulation 2001;104:2158-63.
3. The Antiarrhythmic versus Implantable Defibrillator Study (AVID) Investigators. A comparison of antiarrhythmicdrug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. Circulation 1997;337:1576-83.
4. Moss A, Zareba W, Hall W, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877-83.
5. Kadish A, Dyer A, Daubert J, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004;350:2151-8.
6. Maisel W, Sweeney M, Stevenson W, et al. Recalls and safety alerts involving pacemakers and implantable cardioverter defibrillator generators. JAMA 2001;286(7):793-9.
7. Groeneveld P, Matta M, Suh J, et al. Costs and quality-of-life effects of implantable cardioverter-defibrillators. Am J Cardiol 2006;98:1409-14.
8. Sears S, Kovacs A, Azzarello L, et al. Innovations in health psychology: the psychosocial care of adults with implantable cardioverter defibrillators. Prof Psychol Res Pr 2004;35:520-6.
9. Luyster F, Hughes J, Waechter D, Josephson R. Resource loss predicts depression and anxiety among patients treated with an implantable cardioverter defibrillator. Psychosom Med 2006;68:794-800.
10. Pauli P, Wiedemannn G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76.
11. Hamner M, Hunt N, Gee J, et al. PTSD and automatic implantable cardioverter defibrillators. Psychosomatics 1999;40:82-5.
12. Kuhl E, Dixit N, Walker R, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-8.
13. Frizelle D, Lewin B, Kaye G, Moniz-Cook E. Development of a measure of the concerns held by people with implanted cardioverter defibrillators: the ICDC. Br J Health Psychol 2006;11:293-301.
14. Kohn C, Petrucci R, Baessler C, et al. The effect of psychological intervention on patients’ long-term adjustment to the ICD: a prospective study. Pacing Clin Electrophysiol 2000;23(4):450-6.
15. Smith LC, Fogel D, Friedman S. Cognitive-behavioral treatment of panic disorder with agoraphobia triggered by AICD implant activity. Psychosomatics 1999;39(5):474-7.
16. Fitchet A, Doherty PJ, Bundy C, et al. Comprehensive cardiac rehabilitation program for implantable cardioverter-defibrillator patients: a randomized controlled trial. Heart 2003;89:155-60.
17. Sears SF, Conti JB. Quality of life and psychological functioning of ICD patients. Heart 2002;87:488-93.
18. Kranitz L, Lehrer P. Biofeedback applications in the treatment of cardiovascular diseases. Cardiol Rev 2004;12(3):177-81.
19. O’Brien MC, Langberg J, Valderrama AL, et al. Implantable cardioverter defibrillator storm nursing care issues for patients and families. Crit Care Nurs Clin North Am 2005;17:9-6.
Neuroleptic malignant syndrome: Don’t let your guard down yet
When second-generation antipsychotics (SGAs) were introduced, clinicians hoped the drugs would not have the potential to cause neuroleptic malignant syndrome (NMS).1 Since then, however, case reports have made it clear that SGAs—like first-generation antipsychotics (FGAs)—can precipitate this life-threatening neurologic emergency.
To help you protect your patients receiving SGAs, this article explains how to:
- identify those at risk
- recognize the different NMS presentations associated with each SGA
- continue antipsychotic treatment for a patient with a history of NMS.
CASE STUDY: A drug-induced disorder
Mrs. Z, age 39, has a history of multiple hospitalizations for schizoaffective disorder complicated by poor compliance and a history of benzodiazepine abuse. This time she was admitted with increased auditory hallucinations and paranoid delusions of her family trying to poison her. Despite multiple haloperidol injections (5 mg IM q4h prn), Mrs. Z continued to have hallucinations and remained agitated.
Haloperidol was discontinued and ziprasidone (20 mg IM q4h prn) was started. After 3 days, Mrs. Z became less agitated and had fewer hallucinations. The IM route was discontinued and oral ziprasidone was started at 40 mg bid, then titrated to 80 mg bid after 2 days. On the third day after titration, Mrs. Z fell twice. She hit her head in one fall, but a brain CT to rule out bleeding was normal.
The next day, Mrs. Z became more confused and developed fever, tremor, urinary incontinence, and a severe headache. She became obtunded, was intubated, and was transferred to the intensive care unit of a tertiary care center.
On admission, her temperature was 103° F (39.4° C); she had severe muscle rigidity and blood pressure of 85/60 mm Hg. Creatine phosphokinase (CPK) was 2,559 U/L (normal 24 to 170 U/L). Liver enzymes were elevated: alanine transaminase was 202 U/L (normal 13 to 50 U/L), and aspartate transaminase (AST) was 190 U/L (normal 15 to 46 U/L). At 140 μg/dL, Mrs. Z’s serum iron was within normal limits (40 to 150 μg/dL).
Neuroleptic malignant syndrome
Clinical manifestations of NMS range from typical—as defined by the DSM-IV-TR (Table 1)2,3—to atypical, without:
Table 1
DSM-IV-TR definition of NMS*
| Hyperthermia (>38° C) and |
| Muscle rigidity and |
At least 2 of the following:
|
| * Symptoms must be associated with the use of neuroleptic medication, and other central and systemic causes of hyperthermia must be excluded. |
| CPK: creatine phosphokinase; NMS: neuroleptic malignant syndrome |
| Source: DSM-IV-TR |
Many conditions resemble NMS (Table 2). Because NMS can be fatal without emergent diagnosis and treatment, maintain a high index of suspicion for this condition whenever you prescribe antipsychotics.
Table 2
NMS differential diagnosis
| Primary CNS disorders |
| CNS vasculitis |
| Infarctions |
| Infections |
| Parkinson’s disease |
| Status epilepticus |
| Trauma |
| Tumors |
| Systemic disorders |
| Acute porphyria |
| Autoimmune disorders |
| Dehydration |
| Heat stroke |
| Hyperthyroidism |
| Infections |
| Pheochromocytoma |
| Tetanus |
| Psychiatric disorders |
| Idiopathic lethal catatonia |
| Medication-related disorders |
| Anticholinergic syndrome |
| Drug intoxication |
| Levodopa syndrome |
| Malignant hyperthermia |
| Serotonin syndrome |
NMS is believed to be caused by reduced dopamine activity in the brain associated with dopamine antagonists, interruptions in nigrostriatal dopamine pathways, or withdrawal of dopaminergic medications.3 However, dopamine D2 receptor blocking potential is not directly linked to the occurrence of NMS.6 Other mechanisms include genetic susceptibility and different CNS neurotransmitter disturbances.7
NMS develops in an estimated 0.02% to 2.5% of patients treated with antipsychotics.8-10 The syndrome appears to occur slightly less frequently with SGAs than with FGAs.6,10
Risk factors. NMS can develop at any age, in men and women, and in patients with psychiatric or medical illness.11,12 In addition to antipsychotics, other medications—including antiemetics and sedatives—can cause NMS. The syndrome has been triggered when Parkinson’s disease patients stop taking or reduce the dose of a dopamine agonist or switch from 1 dopamine agonist to another.13,14
Symptoms usually develop during the first 2 weeks of pharmacotherapy but may start after the initial dose or during long-term stable therapy.15 Although some studies found NMS development to be dose-independent, multiple cases have demonstrated an association with dose changes. Death occurs from dysautonomic manifestations and systemic complications.
An elevated risk for NMS may exist in patients with:
- mood disorders
- preexisting catatonia16
- complicated medical and neurologic disorders, such as encephalitis or mental retardation17
- poor functional and physiologic status3
- concurrent lithium treatment
- IM injection of an antipsychotic
- use of a high-potency antipsychotic, such as haloperidol
- psychomotor agitation.
Other potential risk factors include dehydration, adolescent age, male gender, low serum iron concentrations, relatively high antipsychotic dosages, and mental retardation or prior structural brain injury.18-20
NMS and SGAs
We reviewed 88 reports of NMS cases associated with 6 SGAs: olanzapine, clozapine, risperidone, ziprasidone, quetiapine, and aripiprazole. In this article, we cite representative cases only; readers interested in the full literature search can find this evidence and its references in the Case Reports Table.
NMS cases were fairly evenly distributed across all age groups (Figure 1). SGAs were implicated in NMS when used as monotherapy in 9 cases (10%) and in combination with other psychotropics in 41 cases (47%). We could not find medication regimen data for 38 cases (43%).
Figure 1
NMS incidence across age groups
Incidence is dispersed fairly evenly; elderly patients may be less likely to be prescribed an antipsychotic than other age groups.
Source: Reference 5Our review suggests that a history of NMS is a risk factor for developing another episode. Twenty cases showed a clear history of NMS, and 2 cases reported 3 different NMS episodes in each patient.19,21
In the cases we reviewed, NMS developed more often among men than women (Figure 2). The reason is not clear. One hypothesis suggests that men are more likely to present with severe agitation that requires aggressive antipsychotic treatment.14,22
Figure 2
NMS: More common in men
Men may be at higher risk because they are more likely to present with severe agitation and receive larger doses of potent antipsychotics.
Source: References 14,22Previous reports suggested that parenteral antipsychotic administration might increase NMS risk. Most NMS cases in our review involved oral administration, perhaps because parenteral SGAs have become available only recently. In the future, increased use of parenteral SGAs might increase the incidence of NMS.
The NMS mortality rate associated with SGAs was lower than that linked to FGAs.6 This finding, however, may be influenced by increasing awareness of NMS among physicians, resulting in earlier diagnosis and treatment.6
Findings for specific SGAs
Aripiprazole. Because aripiprazole is the newest SGA, data on its association with NMS are limited. Our review looked at 2 cases. Both patients had atypical NMS features, including absence of fever and mild CPK elevation. In 1 case, aripiprazole was used to treat agitation in a 13-year-old girl with history of NMS. This resulted in a mild increase in tachycardia and brief worsening of serum CPK but did not significantly affect temperature, respiratory rate, or blood pressure.
Clozapine. Several NMS cases have been connected to clozapine monotherapy (6 cases) or combination therapy (22 cases). Compared with NMS caused by other antipsychotics, clozapine-induced NMS occurred sooner after patients started the drug or restarted it after discontinuation. NMS has developed in patients receiving chronic steady doses of clozapine, after dosage increases, and after other medications have been added.
Clozapine-treated patients need to be closely monitored for agranulocytosis symptoms, so any other adverse effects—such as initial symptoms of NMS—likely will be detected early. Some reports suggested that clozapine-induced NMS may feature fewer extrapyramidal side effects and a lower-than-typical increase in CPK. In the cases we reviewed, however, NMS presentations ranged from typical—with a highly elevated CPK—to mild with no rigidity and mild or no CPK elevation. Two of 28 cases reported neurologic sequelae, including severe truncal ataxia and dysmetria.
Clozapine has been used to treat patients with a history of NMS who experience psychotic relapse. In several cases, however, NMS recurred after clozapine was started. In 1 case, a third rechallenge with slow titration of clozapine was successful.
Olanzapine. Some studies have found olanzapine-induced NMS to be rare (rate ≤0.01%), but our review found 36 such cases. Ten patients (30%) had a history of NMS. Olanzapine dosing did not correlate with NMS—in 11 cases NMS occurred with daily doses ≤10 mg.
As with clozapine, the presentation of olanzapine-induced NMS varies widely. Onset from within 8 hours of starting olanzapine to after 2½ years of stable olanzapine dosing has been reported. Some cases have featured a typical NMS presentation. Atypical presentations have included:
- extremely elevated serum sodium
- absence of rigidity
- normal CPK
- generalized tonic-clonic seizures preceding NMS onset
- anterograde amnesia
- deficits in learning verbal information.
Olanzapine challenge for patients with a history of NMS often has triggered recurring NMS.
Quetiapine. NMS has been reported in patients receiving quetiapine monotherapy and combination therapy. Patients who previously experienced NMS after taking an FGA have developed quetiapine-induced NMS, as have some with a history of Lewy body disease. Two patients treated with quetiapine developed CPK elevations to almost 9,000 U/L (normal <171 U/L)—without other NMS features—that improved after discontinuing the medication.
Risperidone. NMS among patients taking risperidone occurs more frequently in those with history of NMS or who restart risperidone after discontinuation. Time to NMS occurrence after starting risperidone varies from hours to months. Atypical presentations include delayed fever, delayed muscle rigidity, massive intestinal bleeding, massive CPK elevation (such as 46,420 U/L), and hyponatremia instead of hypernatremia.
Ziprasidone. Administering IM ziprasidone or combining any form of the drug with other psychotropics increases NMS risk. Although most cases featured typical presentations, 1 case reported absence of muscle rigidity, which is present in >90% of patients with NMS associated with FGAs.
NMS sequelae related to SGAs
Brain injury following NMS can cause truncal ataxia, limb ataxia, athetosis, hemiballismus, dysmetria, dysarthria, sensory function problems, balance problems, persistent amnesia, difficulties comprehending commands, attention problems, and electroencephalograph or MRI abnormalities.23,24 Postmortem studies of patients with NMS have revealed cerebellar degeneration, reduction of Purkinje and granule cells, and gliosis in the dentate nucleus.25,26
Why some patients develop sequelae after NMS while others recover is unknown. Sustained hyperpyrexia, preexisting medical or neurologic disorders, polypharmacy, prolonged courses, and delayed diagnosis may play a role.25-27
CASE CONTINUED: A complicated illness
Mrs. Z was diagnosed with NMS. Ziprasidone was discontinued, and supportive treatment, bromocriptine (2.5 mg po qid), and lorazepam (2 mg IV qid) were started. Temperatures of 101° to 103° F (38.3° to 39.4° C) persisted for the next 2 days. This hyperthermia was difficult to control because of suspected meningitis.
The team started ceftriaxone (2 gm IV q12h) while awaiting lumbar puncture results. CSF showed mild white blood cell elevation of 20/cu mm (normal 0 to 5/cu mm) with 62% neutrophils (normal 0 to 6%), normal protein, normal glucose, and negative cultures. After 2 days of antibiotic therapy, the patient developed diarrhea and was diagnosed with Clostridium difficile-associated colitis, a side effect of the antibiotic.
Treatment is mainly supportive
Recognizing NMS signs is the first and most important step to quick diagnosis and early medical intervention. Recommendations for medical treatment of NMS vary widely, but most stress stopping the triggering drug and initiating supportive care (Table 3).27-29
Several medications have been used off-label to treat NMS based on anecdotal clinical reports. Benzodiazepines such as parenteral lorazepam, 1 to 2 mg every 6 to 8 hours, have been used to treat catatonic symptoms.30 Dopamine agonists—including bromocriptine, 2.5 mg every 8 hours—have reduced the duration and mortality of NMS but have the potential to worsen psychotic symptoms and cause hypotension and emesis.30
Table 3
Treating NMS: Where to start
| Stop offending agent(s) |
Provide intensive hemodynamic and supportive care:
|
CASE CONTINUED: Resuming antipsychotic Tx
Five days after intubation, Mrs. Z started to improve and was extubated successfully. However, she developed severe truncal ataxia, upper extremity tremors (resting and intentional), athetosis, hemiballismus, dysmetria, and dystonia. She continued to experience hallucinations after transfer back to the psychiatric floor.
Oral olanzapine challenge was started at 2.5 mg/d and titrated up to 10 mg/d over the next 7 days. Her psychotic symptoms showed mild improvement but her ataxic movements worsened and she fell frequently. Benztropine, 1 mg po bid, was added to her regimen and helped with the tremor. She was transferred for rehabilitation and eventually discharged home.
If a patient needs antipsychotics
If a patient who has experienced NMS continues to need pharmacotherapy for psychosis, wait 1 or 2 weeks after NMS symptoms resolve before restarting any antipsychotic.31 Although most patients can be treated safely with an antipsychotic after having NMS, clearly document the indications and your discussions with the patients and their families.
No conclusive evidence indicates which antipsychotic might lower a patient’s risk of recurrent NMS. Using an FGA in patients who recover from NMS carries a 30% risk of recurrent episodes.3 Data on the recurrence of NMS with SGAs are inconclusive. No relationship was found between relapse rate and patients’ age or sex.32
Regardless of which drug you choose, start with a low dosage and titrate slowly. You also can protect patients by reducing risk factors for NMS, such as dehydration, and considering alternate therapies such as electroconvulsive therapy, when appropriate.
This paper was among those entered in the 2007 Promising New Investigators competition sponsored by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition was “New insights on psychotropic drug safety and side effects.”
Current Psychiatry is honored to publish this peer-reviewed, evidenced-based article on a clinically important topic for practicing psychiatrists.
NMSIS is dedicated to reducing morbidity and mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing support information for medical professionals, patients, and families; and improving scientific understanding of these conditions through research.
Related resources
- Neuroleptic Malignant Syndrome Information Service. http://nmsis.org.
- National Institute of Neurological Disorders and Stroke. Neuroleptic malignant syndrome information page www.ninds.nih.gov/disorders/neuroleptic_syndrome/neuroleptic_syndrome.htm.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Ceftriaxone • Rocephin
- Clozapine • Clozaril
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Delay J, Pichot P, Lemperiere T, et al. Un neuroleptique majeur non-phenothiazine et non reserpinique, l’haloperidol, dans le traitement des psychoses. Annales Medico-Psychologique 1960;118:145-52.
2. Thornberg SA, Ereshefsky L. Neuroleptic malignant syndrome associated with clozapine monotherapy. Pharmacotherapy 1993;13:510-4.
3. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
4. Rodriguez OP, Dowell MS. A case report of neuroleptic malignant syndrome without fever in a patient given aripiprazole. J Okla State Med Assoc 2006;9(7):435-8.
5. Kogoj A, Velikonja I. Olanzapine induced neuroleptic malignant syndrome—a case review. Hum Psychopharmacol 2003;18(4):301-9.
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65(4):464-70.
7. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatric Annals 2000;30:314-21.
8. Levenson JL. Neuroleptic malignant syndrome. Am J Psychiatry 1985;142:1137.-
9. Addonizio G, Susman VL, Roth SD. Symptoms of neuroleptic malignant syndrome in 82 consecutive inpatients. Am J Psychiatry 1986;143:1587-90.
10. Pope HG, Keck PE, McElroy SL. Frequency and presentation of neuroleptic malignant syndrome in a large psychiatric hospital. Am J Psychiatry 1986;143:1227-33.
11. Chungh DS, Kim BN, Cho SC. Neuroleptic malignant syndrome due to three atypical antipsychotics in a child. J Psychopharmacol 2005;19(4):422-5.
12. Suh H, Bronson B, Martin R. Neuroleptic malignant syndrome and low-dose olanzapine. Am J Psychiatry 2003;160(4):796.-
13. Shalev A, Hermesh H, Munitz H. Mortality from neuroleptic malignant syndrome. J Clin Psychiatry 1989;50:18.-
14. Velamoor VR. Neuroleptic malignant syndrome. Recognition, prevention and management. Drug Saf 1998;19:73.-
15. Pope HG, Jr, Aizley HG, Keck PE, Jr, McElroy SL. Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry 1991;52:208.-
16. White DAC, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry 1991;158:419-21.
17. Caroff SN, Mann SC, McCarthy M, et al. Acute infectious encephalitis complicated by neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18:349-51.
18. Apple JE, Van Hauer G. Neuroleptic malignant syndrome associated with olanzapine therapy. Psychosomatics 1999;40(3):267-8.
19. Margolese HC, Chouinard G. Olanzapine-induced neuroleptic malignant syndrome with mental retardation. Am J Psychiatry 1999;156(7):1115-6.
20. Boyd RD. Neuroleptic malignant syndrome and mental retardation: review and analysis of 29 cases. Am J Ment Retard 1993;98:143-55.
21. Malyuk R, Gibson B, Procyshyn RM, Kang N. Olanzapine associated weight gain, hyperglycemia and neuroleptic malignant syndrome: case report. Int J Geriatr Psychiatry 2002;17(4):326-8.
22. Zun LS. A prospective study of the complication rate of use of patient restraint in the emergency department. J Emerg Med 2003;24(2):119-24.
23. Labuda A, Cullen N. Brain injury following neuroleptic malignant syndrome: case report and review of the literature. Brain Inj 2006;20(7):775-8.
24. Manto M, Goldman S, Hildebrand J. Cerebellar gait ataxia following neuroleptic malignant syndrome. J Neurol 1996;243(1):101-2.
25. Lee S, Merriam A, Kim TS, et al. Cerebellar degeneration in neuroleptic malignant syndrome: neuropathologic findings and review of the literature concerning heat-related nervous system injury. J Neurol Neurosurg Psychiatry 1989;52(3):387-91.
26. Naramoto A, Koizumi N, Itoh N, Shigematsu H. An autopsy case of cerebellar degeneration following lithium intoxication with neuroleptic malignant syndrome. Acta Pathol Jpn 1993;43(1-2):55-8.
27. Gratz SS, Levinson DF, Simpson GM. The treatment and management of neuroleptic malignant syndrome. Prog Neuropsychopharmacol Biol Psychiatry 1992;16(4):425-43.
28. Scheftner WA, Shulman RB. Treatment choice in neuroleptic malignant syndrome. Convuls Ther 1992;8:267-79.
29. Harsch HH. Neuroleptic malignant syndrome: physiological and laboratory findings in a series of nine cases. J Clin Psychiatry 1987;48:328-33.
30. Caroff SN. Neuroleptic malignant syndrome: still a risk, but which patients may be in danger? Current Psychiatry 2003;2:36-42.
31. Wells AJ, Sommi RW, Crismon ML. Neuroleptic rechallenge after neuroleptic malignant syndrome: case report and literature review. Drug Intell Clin Pharm 1988;22:475-80.
32. Susman VL, Addonizio G. Recurrence of neuroleptic malignant syndrome. J Nerv Ment Dis 1988;176:234-41.
When second-generation antipsychotics (SGAs) were introduced, clinicians hoped the drugs would not have the potential to cause neuroleptic malignant syndrome (NMS).1 Since then, however, case reports have made it clear that SGAs—like first-generation antipsychotics (FGAs)—can precipitate this life-threatening neurologic emergency.
To help you protect your patients receiving SGAs, this article explains how to:
- identify those at risk
- recognize the different NMS presentations associated with each SGA
- continue antipsychotic treatment for a patient with a history of NMS.
CASE STUDY: A drug-induced disorder
Mrs. Z, age 39, has a history of multiple hospitalizations for schizoaffective disorder complicated by poor compliance and a history of benzodiazepine abuse. This time she was admitted with increased auditory hallucinations and paranoid delusions of her family trying to poison her. Despite multiple haloperidol injections (5 mg IM q4h prn), Mrs. Z continued to have hallucinations and remained agitated.
Haloperidol was discontinued and ziprasidone (20 mg IM q4h prn) was started. After 3 days, Mrs. Z became less agitated and had fewer hallucinations. The IM route was discontinued and oral ziprasidone was started at 40 mg bid, then titrated to 80 mg bid after 2 days. On the third day after titration, Mrs. Z fell twice. She hit her head in one fall, but a brain CT to rule out bleeding was normal.
The next day, Mrs. Z became more confused and developed fever, tremor, urinary incontinence, and a severe headache. She became obtunded, was intubated, and was transferred to the intensive care unit of a tertiary care center.
On admission, her temperature was 103° F (39.4° C); she had severe muscle rigidity and blood pressure of 85/60 mm Hg. Creatine phosphokinase (CPK) was 2,559 U/L (normal 24 to 170 U/L). Liver enzymes were elevated: alanine transaminase was 202 U/L (normal 13 to 50 U/L), and aspartate transaminase (AST) was 190 U/L (normal 15 to 46 U/L). At 140 μg/dL, Mrs. Z’s serum iron was within normal limits (40 to 150 μg/dL).
Neuroleptic malignant syndrome
Clinical manifestations of NMS range from typical—as defined by the DSM-IV-TR (Table 1)2,3—to atypical, without:
Table 1
DSM-IV-TR definition of NMS*
| Hyperthermia (>38° C) and |
| Muscle rigidity and |
At least 2 of the following:
|
| * Symptoms must be associated with the use of neuroleptic medication, and other central and systemic causes of hyperthermia must be excluded. |
| CPK: creatine phosphokinase; NMS: neuroleptic malignant syndrome |
| Source: DSM-IV-TR |
Many conditions resemble NMS (Table 2). Because NMS can be fatal without emergent diagnosis and treatment, maintain a high index of suspicion for this condition whenever you prescribe antipsychotics.
Table 2
NMS differential diagnosis
| Primary CNS disorders |
| CNS vasculitis |
| Infarctions |
| Infections |
| Parkinson’s disease |
| Status epilepticus |
| Trauma |
| Tumors |
| Systemic disorders |
| Acute porphyria |
| Autoimmune disorders |
| Dehydration |
| Heat stroke |
| Hyperthyroidism |
| Infections |
| Pheochromocytoma |
| Tetanus |
| Psychiatric disorders |
| Idiopathic lethal catatonia |
| Medication-related disorders |
| Anticholinergic syndrome |
| Drug intoxication |
| Levodopa syndrome |
| Malignant hyperthermia |
| Serotonin syndrome |
NMS is believed to be caused by reduced dopamine activity in the brain associated with dopamine antagonists, interruptions in nigrostriatal dopamine pathways, or withdrawal of dopaminergic medications.3 However, dopamine D2 receptor blocking potential is not directly linked to the occurrence of NMS.6 Other mechanisms include genetic susceptibility and different CNS neurotransmitter disturbances.7
NMS develops in an estimated 0.02% to 2.5% of patients treated with antipsychotics.8-10 The syndrome appears to occur slightly less frequently with SGAs than with FGAs.6,10
Risk factors. NMS can develop at any age, in men and women, and in patients with psychiatric or medical illness.11,12 In addition to antipsychotics, other medications—including antiemetics and sedatives—can cause NMS. The syndrome has been triggered when Parkinson’s disease patients stop taking or reduce the dose of a dopamine agonist or switch from 1 dopamine agonist to another.13,14
Symptoms usually develop during the first 2 weeks of pharmacotherapy but may start after the initial dose or during long-term stable therapy.15 Although some studies found NMS development to be dose-independent, multiple cases have demonstrated an association with dose changes. Death occurs from dysautonomic manifestations and systemic complications.
An elevated risk for NMS may exist in patients with:
- mood disorders
- preexisting catatonia16
- complicated medical and neurologic disorders, such as encephalitis or mental retardation17
- poor functional and physiologic status3
- concurrent lithium treatment
- IM injection of an antipsychotic
- use of a high-potency antipsychotic, such as haloperidol
- psychomotor agitation.
Other potential risk factors include dehydration, adolescent age, male gender, low serum iron concentrations, relatively high antipsychotic dosages, and mental retardation or prior structural brain injury.18-20
NMS and SGAs
We reviewed 88 reports of NMS cases associated with 6 SGAs: olanzapine, clozapine, risperidone, ziprasidone, quetiapine, and aripiprazole. In this article, we cite representative cases only; readers interested in the full literature search can find this evidence and its references in the Case Reports Table.
NMS cases were fairly evenly distributed across all age groups (Figure 1). SGAs were implicated in NMS when used as monotherapy in 9 cases (10%) and in combination with other psychotropics in 41 cases (47%). We could not find medication regimen data for 38 cases (43%).
Figure 1
NMS incidence across age groups
Incidence is dispersed fairly evenly; elderly patients may be less likely to be prescribed an antipsychotic than other age groups.
Source: Reference 5Our review suggests that a history of NMS is a risk factor for developing another episode. Twenty cases showed a clear history of NMS, and 2 cases reported 3 different NMS episodes in each patient.19,21
In the cases we reviewed, NMS developed more often among men than women (Figure 2). The reason is not clear. One hypothesis suggests that men are more likely to present with severe agitation that requires aggressive antipsychotic treatment.14,22
Figure 2
NMS: More common in men
Men may be at higher risk because they are more likely to present with severe agitation and receive larger doses of potent antipsychotics.
Source: References 14,22Previous reports suggested that parenteral antipsychotic administration might increase NMS risk. Most NMS cases in our review involved oral administration, perhaps because parenteral SGAs have become available only recently. In the future, increased use of parenteral SGAs might increase the incidence of NMS.
The NMS mortality rate associated with SGAs was lower than that linked to FGAs.6 This finding, however, may be influenced by increasing awareness of NMS among physicians, resulting in earlier diagnosis and treatment.6
Findings for specific SGAs
Aripiprazole. Because aripiprazole is the newest SGA, data on its association with NMS are limited. Our review looked at 2 cases. Both patients had atypical NMS features, including absence of fever and mild CPK elevation. In 1 case, aripiprazole was used to treat agitation in a 13-year-old girl with history of NMS. This resulted in a mild increase in tachycardia and brief worsening of serum CPK but did not significantly affect temperature, respiratory rate, or blood pressure.
Clozapine. Several NMS cases have been connected to clozapine monotherapy (6 cases) or combination therapy (22 cases). Compared with NMS caused by other antipsychotics, clozapine-induced NMS occurred sooner after patients started the drug or restarted it after discontinuation. NMS has developed in patients receiving chronic steady doses of clozapine, after dosage increases, and after other medications have been added.
Clozapine-treated patients need to be closely monitored for agranulocytosis symptoms, so any other adverse effects—such as initial symptoms of NMS—likely will be detected early. Some reports suggested that clozapine-induced NMS may feature fewer extrapyramidal side effects and a lower-than-typical increase in CPK. In the cases we reviewed, however, NMS presentations ranged from typical—with a highly elevated CPK—to mild with no rigidity and mild or no CPK elevation. Two of 28 cases reported neurologic sequelae, including severe truncal ataxia and dysmetria.
Clozapine has been used to treat patients with a history of NMS who experience psychotic relapse. In several cases, however, NMS recurred after clozapine was started. In 1 case, a third rechallenge with slow titration of clozapine was successful.
Olanzapine. Some studies have found olanzapine-induced NMS to be rare (rate ≤0.01%), but our review found 36 such cases. Ten patients (30%) had a history of NMS. Olanzapine dosing did not correlate with NMS—in 11 cases NMS occurred with daily doses ≤10 mg.
As with clozapine, the presentation of olanzapine-induced NMS varies widely. Onset from within 8 hours of starting olanzapine to after 2½ years of stable olanzapine dosing has been reported. Some cases have featured a typical NMS presentation. Atypical presentations have included:
- extremely elevated serum sodium
- absence of rigidity
- normal CPK
- generalized tonic-clonic seizures preceding NMS onset
- anterograde amnesia
- deficits in learning verbal information.
Olanzapine challenge for patients with a history of NMS often has triggered recurring NMS.
Quetiapine. NMS has been reported in patients receiving quetiapine monotherapy and combination therapy. Patients who previously experienced NMS after taking an FGA have developed quetiapine-induced NMS, as have some with a history of Lewy body disease. Two patients treated with quetiapine developed CPK elevations to almost 9,000 U/L (normal <171 U/L)—without other NMS features—that improved after discontinuing the medication.
Risperidone. NMS among patients taking risperidone occurs more frequently in those with history of NMS or who restart risperidone after discontinuation. Time to NMS occurrence after starting risperidone varies from hours to months. Atypical presentations include delayed fever, delayed muscle rigidity, massive intestinal bleeding, massive CPK elevation (such as 46,420 U/L), and hyponatremia instead of hypernatremia.
Ziprasidone. Administering IM ziprasidone or combining any form of the drug with other psychotropics increases NMS risk. Although most cases featured typical presentations, 1 case reported absence of muscle rigidity, which is present in >90% of patients with NMS associated with FGAs.
NMS sequelae related to SGAs
Brain injury following NMS can cause truncal ataxia, limb ataxia, athetosis, hemiballismus, dysmetria, dysarthria, sensory function problems, balance problems, persistent amnesia, difficulties comprehending commands, attention problems, and electroencephalograph or MRI abnormalities.23,24 Postmortem studies of patients with NMS have revealed cerebellar degeneration, reduction of Purkinje and granule cells, and gliosis in the dentate nucleus.25,26
Why some patients develop sequelae after NMS while others recover is unknown. Sustained hyperpyrexia, preexisting medical or neurologic disorders, polypharmacy, prolonged courses, and delayed diagnosis may play a role.25-27
CASE CONTINUED: A complicated illness
Mrs. Z was diagnosed with NMS. Ziprasidone was discontinued, and supportive treatment, bromocriptine (2.5 mg po qid), and lorazepam (2 mg IV qid) were started. Temperatures of 101° to 103° F (38.3° to 39.4° C) persisted for the next 2 days. This hyperthermia was difficult to control because of suspected meningitis.
The team started ceftriaxone (2 gm IV q12h) while awaiting lumbar puncture results. CSF showed mild white blood cell elevation of 20/cu mm (normal 0 to 5/cu mm) with 62% neutrophils (normal 0 to 6%), normal protein, normal glucose, and negative cultures. After 2 days of antibiotic therapy, the patient developed diarrhea and was diagnosed with Clostridium difficile-associated colitis, a side effect of the antibiotic.
Treatment is mainly supportive
Recognizing NMS signs is the first and most important step to quick diagnosis and early medical intervention. Recommendations for medical treatment of NMS vary widely, but most stress stopping the triggering drug and initiating supportive care (Table 3).27-29
Several medications have been used off-label to treat NMS based on anecdotal clinical reports. Benzodiazepines such as parenteral lorazepam, 1 to 2 mg every 6 to 8 hours, have been used to treat catatonic symptoms.30 Dopamine agonists—including bromocriptine, 2.5 mg every 8 hours—have reduced the duration and mortality of NMS but have the potential to worsen psychotic symptoms and cause hypotension and emesis.30
Table 3
Treating NMS: Where to start
| Stop offending agent(s) |
Provide intensive hemodynamic and supportive care:
|
CASE CONTINUED: Resuming antipsychotic Tx
Five days after intubation, Mrs. Z started to improve and was extubated successfully. However, she developed severe truncal ataxia, upper extremity tremors (resting and intentional), athetosis, hemiballismus, dysmetria, and dystonia. She continued to experience hallucinations after transfer back to the psychiatric floor.
Oral olanzapine challenge was started at 2.5 mg/d and titrated up to 10 mg/d over the next 7 days. Her psychotic symptoms showed mild improvement but her ataxic movements worsened and she fell frequently. Benztropine, 1 mg po bid, was added to her regimen and helped with the tremor. She was transferred for rehabilitation and eventually discharged home.
If a patient needs antipsychotics
If a patient who has experienced NMS continues to need pharmacotherapy for psychosis, wait 1 or 2 weeks after NMS symptoms resolve before restarting any antipsychotic.31 Although most patients can be treated safely with an antipsychotic after having NMS, clearly document the indications and your discussions with the patients and their families.
No conclusive evidence indicates which antipsychotic might lower a patient’s risk of recurrent NMS. Using an FGA in patients who recover from NMS carries a 30% risk of recurrent episodes.3 Data on the recurrence of NMS with SGAs are inconclusive. No relationship was found between relapse rate and patients’ age or sex.32
Regardless of which drug you choose, start with a low dosage and titrate slowly. You also can protect patients by reducing risk factors for NMS, such as dehydration, and considering alternate therapies such as electroconvulsive therapy, when appropriate.
This paper was among those entered in the 2007 Promising New Investigators competition sponsored by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition was “New insights on psychotropic drug safety and side effects.”
Current Psychiatry is honored to publish this peer-reviewed, evidenced-based article on a clinically important topic for practicing psychiatrists.
NMSIS is dedicated to reducing morbidity and mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing support information for medical professionals, patients, and families; and improving scientific understanding of these conditions through research.
Related resources
- Neuroleptic Malignant Syndrome Information Service. http://nmsis.org.
- National Institute of Neurological Disorders and Stroke. Neuroleptic malignant syndrome information page www.ninds.nih.gov/disorders/neuroleptic_syndrome/neuroleptic_syndrome.htm.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Ceftriaxone • Rocephin
- Clozapine • Clozaril
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
When second-generation antipsychotics (SGAs) were introduced, clinicians hoped the drugs would not have the potential to cause neuroleptic malignant syndrome (NMS).1 Since then, however, case reports have made it clear that SGAs—like first-generation antipsychotics (FGAs)—can precipitate this life-threatening neurologic emergency.
To help you protect your patients receiving SGAs, this article explains how to:
- identify those at risk
- recognize the different NMS presentations associated with each SGA
- continue antipsychotic treatment for a patient with a history of NMS.
CASE STUDY: A drug-induced disorder
Mrs. Z, age 39, has a history of multiple hospitalizations for schizoaffective disorder complicated by poor compliance and a history of benzodiazepine abuse. This time she was admitted with increased auditory hallucinations and paranoid delusions of her family trying to poison her. Despite multiple haloperidol injections (5 mg IM q4h prn), Mrs. Z continued to have hallucinations and remained agitated.
Haloperidol was discontinued and ziprasidone (20 mg IM q4h prn) was started. After 3 days, Mrs. Z became less agitated and had fewer hallucinations. The IM route was discontinued and oral ziprasidone was started at 40 mg bid, then titrated to 80 mg bid after 2 days. On the third day after titration, Mrs. Z fell twice. She hit her head in one fall, but a brain CT to rule out bleeding was normal.
The next day, Mrs. Z became more confused and developed fever, tremor, urinary incontinence, and a severe headache. She became obtunded, was intubated, and was transferred to the intensive care unit of a tertiary care center.
On admission, her temperature was 103° F (39.4° C); she had severe muscle rigidity and blood pressure of 85/60 mm Hg. Creatine phosphokinase (CPK) was 2,559 U/L (normal 24 to 170 U/L). Liver enzymes were elevated: alanine transaminase was 202 U/L (normal 13 to 50 U/L), and aspartate transaminase (AST) was 190 U/L (normal 15 to 46 U/L). At 140 μg/dL, Mrs. Z’s serum iron was within normal limits (40 to 150 μg/dL).
Neuroleptic malignant syndrome
Clinical manifestations of NMS range from typical—as defined by the DSM-IV-TR (Table 1)2,3—to atypical, without:
Table 1
DSM-IV-TR definition of NMS*
| Hyperthermia (>38° C) and |
| Muscle rigidity and |
At least 2 of the following:
|
| * Symptoms must be associated with the use of neuroleptic medication, and other central and systemic causes of hyperthermia must be excluded. |
| CPK: creatine phosphokinase; NMS: neuroleptic malignant syndrome |
| Source: DSM-IV-TR |
Many conditions resemble NMS (Table 2). Because NMS can be fatal without emergent diagnosis and treatment, maintain a high index of suspicion for this condition whenever you prescribe antipsychotics.
Table 2
NMS differential diagnosis
| Primary CNS disorders |
| CNS vasculitis |
| Infarctions |
| Infections |
| Parkinson’s disease |
| Status epilepticus |
| Trauma |
| Tumors |
| Systemic disorders |
| Acute porphyria |
| Autoimmune disorders |
| Dehydration |
| Heat stroke |
| Hyperthyroidism |
| Infections |
| Pheochromocytoma |
| Tetanus |
| Psychiatric disorders |
| Idiopathic lethal catatonia |
| Medication-related disorders |
| Anticholinergic syndrome |
| Drug intoxication |
| Levodopa syndrome |
| Malignant hyperthermia |
| Serotonin syndrome |
NMS is believed to be caused by reduced dopamine activity in the brain associated with dopamine antagonists, interruptions in nigrostriatal dopamine pathways, or withdrawal of dopaminergic medications.3 However, dopamine D2 receptor blocking potential is not directly linked to the occurrence of NMS.6 Other mechanisms include genetic susceptibility and different CNS neurotransmitter disturbances.7
NMS develops in an estimated 0.02% to 2.5% of patients treated with antipsychotics.8-10 The syndrome appears to occur slightly less frequently with SGAs than with FGAs.6,10
Risk factors. NMS can develop at any age, in men and women, and in patients with psychiatric or medical illness.11,12 In addition to antipsychotics, other medications—including antiemetics and sedatives—can cause NMS. The syndrome has been triggered when Parkinson’s disease patients stop taking or reduce the dose of a dopamine agonist or switch from 1 dopamine agonist to another.13,14
Symptoms usually develop during the first 2 weeks of pharmacotherapy but may start after the initial dose or during long-term stable therapy.15 Although some studies found NMS development to be dose-independent, multiple cases have demonstrated an association with dose changes. Death occurs from dysautonomic manifestations and systemic complications.
An elevated risk for NMS may exist in patients with:
- mood disorders
- preexisting catatonia16
- complicated medical and neurologic disorders, such as encephalitis or mental retardation17
- poor functional and physiologic status3
- concurrent lithium treatment
- IM injection of an antipsychotic
- use of a high-potency antipsychotic, such as haloperidol
- psychomotor agitation.
Other potential risk factors include dehydration, adolescent age, male gender, low serum iron concentrations, relatively high antipsychotic dosages, and mental retardation or prior structural brain injury.18-20
NMS and SGAs
We reviewed 88 reports of NMS cases associated with 6 SGAs: olanzapine, clozapine, risperidone, ziprasidone, quetiapine, and aripiprazole. In this article, we cite representative cases only; readers interested in the full literature search can find this evidence and its references in the Case Reports Table.
NMS cases were fairly evenly distributed across all age groups (Figure 1). SGAs were implicated in NMS when used as monotherapy in 9 cases (10%) and in combination with other psychotropics in 41 cases (47%). We could not find medication regimen data for 38 cases (43%).
Figure 1
NMS incidence across age groups
Incidence is dispersed fairly evenly; elderly patients may be less likely to be prescribed an antipsychotic than other age groups.
Source: Reference 5Our review suggests that a history of NMS is a risk factor for developing another episode. Twenty cases showed a clear history of NMS, and 2 cases reported 3 different NMS episodes in each patient.19,21
In the cases we reviewed, NMS developed more often among men than women (Figure 2). The reason is not clear. One hypothesis suggests that men are more likely to present with severe agitation that requires aggressive antipsychotic treatment.14,22
Figure 2
NMS: More common in men
Men may be at higher risk because they are more likely to present with severe agitation and receive larger doses of potent antipsychotics.
Source: References 14,22Previous reports suggested that parenteral antipsychotic administration might increase NMS risk. Most NMS cases in our review involved oral administration, perhaps because parenteral SGAs have become available only recently. In the future, increased use of parenteral SGAs might increase the incidence of NMS.
The NMS mortality rate associated with SGAs was lower than that linked to FGAs.6 This finding, however, may be influenced by increasing awareness of NMS among physicians, resulting in earlier diagnosis and treatment.6
Findings for specific SGAs
Aripiprazole. Because aripiprazole is the newest SGA, data on its association with NMS are limited. Our review looked at 2 cases. Both patients had atypical NMS features, including absence of fever and mild CPK elevation. In 1 case, aripiprazole was used to treat agitation in a 13-year-old girl with history of NMS. This resulted in a mild increase in tachycardia and brief worsening of serum CPK but did not significantly affect temperature, respiratory rate, or blood pressure.
Clozapine. Several NMS cases have been connected to clozapine monotherapy (6 cases) or combination therapy (22 cases). Compared with NMS caused by other antipsychotics, clozapine-induced NMS occurred sooner after patients started the drug or restarted it after discontinuation. NMS has developed in patients receiving chronic steady doses of clozapine, after dosage increases, and after other medications have been added.
Clozapine-treated patients need to be closely monitored for agranulocytosis symptoms, so any other adverse effects—such as initial symptoms of NMS—likely will be detected early. Some reports suggested that clozapine-induced NMS may feature fewer extrapyramidal side effects and a lower-than-typical increase in CPK. In the cases we reviewed, however, NMS presentations ranged from typical—with a highly elevated CPK—to mild with no rigidity and mild or no CPK elevation. Two of 28 cases reported neurologic sequelae, including severe truncal ataxia and dysmetria.
Clozapine has been used to treat patients with a history of NMS who experience psychotic relapse. In several cases, however, NMS recurred after clozapine was started. In 1 case, a third rechallenge with slow titration of clozapine was successful.
Olanzapine. Some studies have found olanzapine-induced NMS to be rare (rate ≤0.01%), but our review found 36 such cases. Ten patients (30%) had a history of NMS. Olanzapine dosing did not correlate with NMS—in 11 cases NMS occurred with daily doses ≤10 mg.
As with clozapine, the presentation of olanzapine-induced NMS varies widely. Onset from within 8 hours of starting olanzapine to after 2½ years of stable olanzapine dosing has been reported. Some cases have featured a typical NMS presentation. Atypical presentations have included:
- extremely elevated serum sodium
- absence of rigidity
- normal CPK
- generalized tonic-clonic seizures preceding NMS onset
- anterograde amnesia
- deficits in learning verbal information.
Olanzapine challenge for patients with a history of NMS often has triggered recurring NMS.
Quetiapine. NMS has been reported in patients receiving quetiapine monotherapy and combination therapy. Patients who previously experienced NMS after taking an FGA have developed quetiapine-induced NMS, as have some with a history of Lewy body disease. Two patients treated with quetiapine developed CPK elevations to almost 9,000 U/L (normal <171 U/L)—without other NMS features—that improved after discontinuing the medication.
Risperidone. NMS among patients taking risperidone occurs more frequently in those with history of NMS or who restart risperidone after discontinuation. Time to NMS occurrence after starting risperidone varies from hours to months. Atypical presentations include delayed fever, delayed muscle rigidity, massive intestinal bleeding, massive CPK elevation (such as 46,420 U/L), and hyponatremia instead of hypernatremia.
Ziprasidone. Administering IM ziprasidone or combining any form of the drug with other psychotropics increases NMS risk. Although most cases featured typical presentations, 1 case reported absence of muscle rigidity, which is present in >90% of patients with NMS associated with FGAs.
NMS sequelae related to SGAs
Brain injury following NMS can cause truncal ataxia, limb ataxia, athetosis, hemiballismus, dysmetria, dysarthria, sensory function problems, balance problems, persistent amnesia, difficulties comprehending commands, attention problems, and electroencephalograph or MRI abnormalities.23,24 Postmortem studies of patients with NMS have revealed cerebellar degeneration, reduction of Purkinje and granule cells, and gliosis in the dentate nucleus.25,26
Why some patients develop sequelae after NMS while others recover is unknown. Sustained hyperpyrexia, preexisting medical or neurologic disorders, polypharmacy, prolonged courses, and delayed diagnosis may play a role.25-27
CASE CONTINUED: A complicated illness
Mrs. Z was diagnosed with NMS. Ziprasidone was discontinued, and supportive treatment, bromocriptine (2.5 mg po qid), and lorazepam (2 mg IV qid) were started. Temperatures of 101° to 103° F (38.3° to 39.4° C) persisted for the next 2 days. This hyperthermia was difficult to control because of suspected meningitis.
The team started ceftriaxone (2 gm IV q12h) while awaiting lumbar puncture results. CSF showed mild white blood cell elevation of 20/cu mm (normal 0 to 5/cu mm) with 62% neutrophils (normal 0 to 6%), normal protein, normal glucose, and negative cultures. After 2 days of antibiotic therapy, the patient developed diarrhea and was diagnosed with Clostridium difficile-associated colitis, a side effect of the antibiotic.
Treatment is mainly supportive
Recognizing NMS signs is the first and most important step to quick diagnosis and early medical intervention. Recommendations for medical treatment of NMS vary widely, but most stress stopping the triggering drug and initiating supportive care (Table 3).27-29
Several medications have been used off-label to treat NMS based on anecdotal clinical reports. Benzodiazepines such as parenteral lorazepam, 1 to 2 mg every 6 to 8 hours, have been used to treat catatonic symptoms.30 Dopamine agonists—including bromocriptine, 2.5 mg every 8 hours—have reduced the duration and mortality of NMS but have the potential to worsen psychotic symptoms and cause hypotension and emesis.30
Table 3
Treating NMS: Where to start
| Stop offending agent(s) |
Provide intensive hemodynamic and supportive care:
|
CASE CONTINUED: Resuming antipsychotic Tx
Five days after intubation, Mrs. Z started to improve and was extubated successfully. However, she developed severe truncal ataxia, upper extremity tremors (resting and intentional), athetosis, hemiballismus, dysmetria, and dystonia. She continued to experience hallucinations after transfer back to the psychiatric floor.
Oral olanzapine challenge was started at 2.5 mg/d and titrated up to 10 mg/d over the next 7 days. Her psychotic symptoms showed mild improvement but her ataxic movements worsened and she fell frequently. Benztropine, 1 mg po bid, was added to her regimen and helped with the tremor. She was transferred for rehabilitation and eventually discharged home.
If a patient needs antipsychotics
If a patient who has experienced NMS continues to need pharmacotherapy for psychosis, wait 1 or 2 weeks after NMS symptoms resolve before restarting any antipsychotic.31 Although most patients can be treated safely with an antipsychotic after having NMS, clearly document the indications and your discussions with the patients and their families.
No conclusive evidence indicates which antipsychotic might lower a patient’s risk of recurrent NMS. Using an FGA in patients who recover from NMS carries a 30% risk of recurrent episodes.3 Data on the recurrence of NMS with SGAs are inconclusive. No relationship was found between relapse rate and patients’ age or sex.32
Regardless of which drug you choose, start with a low dosage and titrate slowly. You also can protect patients by reducing risk factors for NMS, such as dehydration, and considering alternate therapies such as electroconvulsive therapy, when appropriate.
This paper was among those entered in the 2007 Promising New Investigators competition sponsored by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition was “New insights on psychotropic drug safety and side effects.”
Current Psychiatry is honored to publish this peer-reviewed, evidenced-based article on a clinically important topic for practicing psychiatrists.
NMSIS is dedicated to reducing morbidity and mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing support information for medical professionals, patients, and families; and improving scientific understanding of these conditions through research.
Related resources
- Neuroleptic Malignant Syndrome Information Service. http://nmsis.org.
- National Institute of Neurological Disorders and Stroke. Neuroleptic malignant syndrome information page www.ninds.nih.gov/disorders/neuroleptic_syndrome/neuroleptic_syndrome.htm.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Ceftriaxone • Rocephin
- Clozapine • Clozaril
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Delay J, Pichot P, Lemperiere T, et al. Un neuroleptique majeur non-phenothiazine et non reserpinique, l’haloperidol, dans le traitement des psychoses. Annales Medico-Psychologique 1960;118:145-52.
2. Thornberg SA, Ereshefsky L. Neuroleptic malignant syndrome associated with clozapine monotherapy. Pharmacotherapy 1993;13:510-4.
3. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
4. Rodriguez OP, Dowell MS. A case report of neuroleptic malignant syndrome without fever in a patient given aripiprazole. J Okla State Med Assoc 2006;9(7):435-8.
5. Kogoj A, Velikonja I. Olanzapine induced neuroleptic malignant syndrome—a case review. Hum Psychopharmacol 2003;18(4):301-9.
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65(4):464-70.
7. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatric Annals 2000;30:314-21.
8. Levenson JL. Neuroleptic malignant syndrome. Am J Psychiatry 1985;142:1137.-
9. Addonizio G, Susman VL, Roth SD. Symptoms of neuroleptic malignant syndrome in 82 consecutive inpatients. Am J Psychiatry 1986;143:1587-90.
10. Pope HG, Keck PE, McElroy SL. Frequency and presentation of neuroleptic malignant syndrome in a large psychiatric hospital. Am J Psychiatry 1986;143:1227-33.
11. Chungh DS, Kim BN, Cho SC. Neuroleptic malignant syndrome due to three atypical antipsychotics in a child. J Psychopharmacol 2005;19(4):422-5.
12. Suh H, Bronson B, Martin R. Neuroleptic malignant syndrome and low-dose olanzapine. Am J Psychiatry 2003;160(4):796.-
13. Shalev A, Hermesh H, Munitz H. Mortality from neuroleptic malignant syndrome. J Clin Psychiatry 1989;50:18.-
14. Velamoor VR. Neuroleptic malignant syndrome. Recognition, prevention and management. Drug Saf 1998;19:73.-
15. Pope HG, Jr, Aizley HG, Keck PE, Jr, McElroy SL. Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry 1991;52:208.-
16. White DAC, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry 1991;158:419-21.
17. Caroff SN, Mann SC, McCarthy M, et al. Acute infectious encephalitis complicated by neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18:349-51.
18. Apple JE, Van Hauer G. Neuroleptic malignant syndrome associated with olanzapine therapy. Psychosomatics 1999;40(3):267-8.
19. Margolese HC, Chouinard G. Olanzapine-induced neuroleptic malignant syndrome with mental retardation. Am J Psychiatry 1999;156(7):1115-6.
20. Boyd RD. Neuroleptic malignant syndrome and mental retardation: review and analysis of 29 cases. Am J Ment Retard 1993;98:143-55.
21. Malyuk R, Gibson B, Procyshyn RM, Kang N. Olanzapine associated weight gain, hyperglycemia and neuroleptic malignant syndrome: case report. Int J Geriatr Psychiatry 2002;17(4):326-8.
22. Zun LS. A prospective study of the complication rate of use of patient restraint in the emergency department. J Emerg Med 2003;24(2):119-24.
23. Labuda A, Cullen N. Brain injury following neuroleptic malignant syndrome: case report and review of the literature. Brain Inj 2006;20(7):775-8.
24. Manto M, Goldman S, Hildebrand J. Cerebellar gait ataxia following neuroleptic malignant syndrome. J Neurol 1996;243(1):101-2.
25. Lee S, Merriam A, Kim TS, et al. Cerebellar degeneration in neuroleptic malignant syndrome: neuropathologic findings and review of the literature concerning heat-related nervous system injury. J Neurol Neurosurg Psychiatry 1989;52(3):387-91.
26. Naramoto A, Koizumi N, Itoh N, Shigematsu H. An autopsy case of cerebellar degeneration following lithium intoxication with neuroleptic malignant syndrome. Acta Pathol Jpn 1993;43(1-2):55-8.
27. Gratz SS, Levinson DF, Simpson GM. The treatment and management of neuroleptic malignant syndrome. Prog Neuropsychopharmacol Biol Psychiatry 1992;16(4):425-43.
28. Scheftner WA, Shulman RB. Treatment choice in neuroleptic malignant syndrome. Convuls Ther 1992;8:267-79.
29. Harsch HH. Neuroleptic malignant syndrome: physiological and laboratory findings in a series of nine cases. J Clin Psychiatry 1987;48:328-33.
30. Caroff SN. Neuroleptic malignant syndrome: still a risk, but which patients may be in danger? Current Psychiatry 2003;2:36-42.
31. Wells AJ, Sommi RW, Crismon ML. Neuroleptic rechallenge after neuroleptic malignant syndrome: case report and literature review. Drug Intell Clin Pharm 1988;22:475-80.
32. Susman VL, Addonizio G. Recurrence of neuroleptic malignant syndrome. J Nerv Ment Dis 1988;176:234-41.
1. Delay J, Pichot P, Lemperiere T, et al. Un neuroleptique majeur non-phenothiazine et non reserpinique, l’haloperidol, dans le traitement des psychoses. Annales Medico-Psychologique 1960;118:145-52.
2. Thornberg SA, Ereshefsky L. Neuroleptic malignant syndrome associated with clozapine monotherapy. Pharmacotherapy 1993;13:510-4.
3. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
4. Rodriguez OP, Dowell MS. A case report of neuroleptic malignant syndrome without fever in a patient given aripiprazole. J Okla State Med Assoc 2006;9(7):435-8.
5. Kogoj A, Velikonja I. Olanzapine induced neuroleptic malignant syndrome—a case review. Hum Psychopharmacol 2003;18(4):301-9.
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65(4):464-70.
7. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatric Annals 2000;30:314-21.
8. Levenson JL. Neuroleptic malignant syndrome. Am J Psychiatry 1985;142:1137.-
9. Addonizio G, Susman VL, Roth SD. Symptoms of neuroleptic malignant syndrome in 82 consecutive inpatients. Am J Psychiatry 1986;143:1587-90.
10. Pope HG, Keck PE, McElroy SL. Frequency and presentation of neuroleptic malignant syndrome in a large psychiatric hospital. Am J Psychiatry 1986;143:1227-33.
11. Chungh DS, Kim BN, Cho SC. Neuroleptic malignant syndrome due to three atypical antipsychotics in a child. J Psychopharmacol 2005;19(4):422-5.
12. Suh H, Bronson B, Martin R. Neuroleptic malignant syndrome and low-dose olanzapine. Am J Psychiatry 2003;160(4):796.-
13. Shalev A, Hermesh H, Munitz H. Mortality from neuroleptic malignant syndrome. J Clin Psychiatry 1989;50:18.-
14. Velamoor VR. Neuroleptic malignant syndrome. Recognition, prevention and management. Drug Saf 1998;19:73.-
15. Pope HG, Jr, Aizley HG, Keck PE, Jr, McElroy SL. Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry 1991;52:208.-
16. White DAC, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry 1991;158:419-21.
17. Caroff SN, Mann SC, McCarthy M, et al. Acute infectious encephalitis complicated by neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18:349-51.
18. Apple JE, Van Hauer G. Neuroleptic malignant syndrome associated with olanzapine therapy. Psychosomatics 1999;40(3):267-8.
19. Margolese HC, Chouinard G. Olanzapine-induced neuroleptic malignant syndrome with mental retardation. Am J Psychiatry 1999;156(7):1115-6.
20. Boyd RD. Neuroleptic malignant syndrome and mental retardation: review and analysis of 29 cases. Am J Ment Retard 1993;98:143-55.
21. Malyuk R, Gibson B, Procyshyn RM, Kang N. Olanzapine associated weight gain, hyperglycemia and neuroleptic malignant syndrome: case report. Int J Geriatr Psychiatry 2002;17(4):326-8.
22. Zun LS. A prospective study of the complication rate of use of patient restraint in the emergency department. J Emerg Med 2003;24(2):119-24.
23. Labuda A, Cullen N. Brain injury following neuroleptic malignant syndrome: case report and review of the literature. Brain Inj 2006;20(7):775-8.
24. Manto M, Goldman S, Hildebrand J. Cerebellar gait ataxia following neuroleptic malignant syndrome. J Neurol 1996;243(1):101-2.
25. Lee S, Merriam A, Kim TS, et al. Cerebellar degeneration in neuroleptic malignant syndrome: neuropathologic findings and review of the literature concerning heat-related nervous system injury. J Neurol Neurosurg Psychiatry 1989;52(3):387-91.
26. Naramoto A, Koizumi N, Itoh N, Shigematsu H. An autopsy case of cerebellar degeneration following lithium intoxication with neuroleptic malignant syndrome. Acta Pathol Jpn 1993;43(1-2):55-8.
27. Gratz SS, Levinson DF, Simpson GM. The treatment and management of neuroleptic malignant syndrome. Prog Neuropsychopharmacol Biol Psychiatry 1992;16(4):425-43.
28. Scheftner WA, Shulman RB. Treatment choice in neuroleptic malignant syndrome. Convuls Ther 1992;8:267-79.
29. Harsch HH. Neuroleptic malignant syndrome: physiological and laboratory findings in a series of nine cases. J Clin Psychiatry 1987;48:328-33.
30. Caroff SN. Neuroleptic malignant syndrome: still a risk, but which patients may be in danger? Current Psychiatry 2003;2:36-42.
31. Wells AJ, Sommi RW, Crismon ML. Neuroleptic rechallenge after neuroleptic malignant syndrome: case report and literature review. Drug Intell Clin Pharm 1988;22:475-80.
32. Susman VL, Addonizio G. Recurrence of neuroleptic malignant syndrome. J Nerv Ment Dis 1988;176:234-41.
‘DARE’ to spot borderline personality disorder
Patients with borderline personality disorder (BPD) exhibit a pattern of instability in interpersonal relation-ships, self-image, and affects, and marked impulsivity beginning in early adulthood.1 These patients may experience other symptoms, such as mood swings or transient psychotic episodes, that are exacerbated by stress.
A BPD patient likely has additional diagnoses from previous clinicians—such as bipolar disorder, dysthymic disorder, panic disorder, major recurrent depression, substance abuse, posttraumatic stress disorder, intermittent explosive disorder, or any variety of adjustment, anxiety, eating, impulse control, mood, somatoform, or personality disorders.2 However, a BPD diagnosis best describes many of these patients, and the mnemonic “DARE” enumerates the most commonly encountered clinical picture.
Depression, Destruction, Denial. Chronic low-grade depression is the baseline mood for BPD. The patients might not report suicidal ideation or might deny a desire to die. But the predilection and potential for risky behavior that could result in accidental injury or death tends to confirm the presence of an underlying self-destructive wish.
Anger, Abandonment, Abuse. Typically, BPD patients are angry at the world. Anger simmers just below the threshold of self-control. When it boils over, BPD patients are apt to take their anger out on themselves by committing suicide or a self-mutilative act, or on others with passive aggression or the kind of physical or emotional abuse they themselves suffered.
BPD patients’ histories often include physical or emotional abandonment or abuse.
Relationships, Regrets, Repetition. Repeated patterns of unstable relationships are characteristic. Often BPD patients have multiple romantic partners, frequent job turnover, interrupted education, and few long-term, mature friendships. These patients’ friends and partners frequently suffer from similar problematic personality characteristics. BPD patients seem unable to break free of their unsuccessful patterns and repeatedly fail to maintain healthy, productive relationships.
Extremes, Emergencies, Ennui. Overuse of prescription drugs, alcohol, or other substances result in frequent emergency room visits. Bulimia, sexual promiscuity, and multiple body piercings or tattoos are emblematic of BPD. Ennui—a feeling of weariness or discontent—is fought off by engaging in extreme behaviors, such as reckless driving.
Many BPD patients improve and show greater stability in jobs and relationships with therapeutic intervention, although they often have a lifelong tendency toward impulsivity and intense relationships and emotions.
Dr. Roth is attending psychiatrist, Department of Veterans Affairs Medical Center, North Chicago, IL.
Patients with borderline personality disorder (BPD) exhibit a pattern of instability in interpersonal relation-ships, self-image, and affects, and marked impulsivity beginning in early adulthood.1 These patients may experience other symptoms, such as mood swings or transient psychotic episodes, that are exacerbated by stress.
A BPD patient likely has additional diagnoses from previous clinicians—such as bipolar disorder, dysthymic disorder, panic disorder, major recurrent depression, substance abuse, posttraumatic stress disorder, intermittent explosive disorder, or any variety of adjustment, anxiety, eating, impulse control, mood, somatoform, or personality disorders.2 However, a BPD diagnosis best describes many of these patients, and the mnemonic “DARE” enumerates the most commonly encountered clinical picture.
Depression, Destruction, Denial. Chronic low-grade depression is the baseline mood for BPD. The patients might not report suicidal ideation or might deny a desire to die. But the predilection and potential for risky behavior that could result in accidental injury or death tends to confirm the presence of an underlying self-destructive wish.
Anger, Abandonment, Abuse. Typically, BPD patients are angry at the world. Anger simmers just below the threshold of self-control. When it boils over, BPD patients are apt to take their anger out on themselves by committing suicide or a self-mutilative act, or on others with passive aggression or the kind of physical or emotional abuse they themselves suffered.
BPD patients’ histories often include physical or emotional abandonment or abuse.
Relationships, Regrets, Repetition. Repeated patterns of unstable relationships are characteristic. Often BPD patients have multiple romantic partners, frequent job turnover, interrupted education, and few long-term, mature friendships. These patients’ friends and partners frequently suffer from similar problematic personality characteristics. BPD patients seem unable to break free of their unsuccessful patterns and repeatedly fail to maintain healthy, productive relationships.
Extremes, Emergencies, Ennui. Overuse of prescription drugs, alcohol, or other substances result in frequent emergency room visits. Bulimia, sexual promiscuity, and multiple body piercings or tattoos are emblematic of BPD. Ennui—a feeling of weariness or discontent—is fought off by engaging in extreme behaviors, such as reckless driving.
Many BPD patients improve and show greater stability in jobs and relationships with therapeutic intervention, although they often have a lifelong tendency toward impulsivity and intense relationships and emotions.
Dr. Roth is attending psychiatrist, Department of Veterans Affairs Medical Center, North Chicago, IL.
Patients with borderline personality disorder (BPD) exhibit a pattern of instability in interpersonal relation-ships, self-image, and affects, and marked impulsivity beginning in early adulthood.1 These patients may experience other symptoms, such as mood swings or transient psychotic episodes, that are exacerbated by stress.
A BPD patient likely has additional diagnoses from previous clinicians—such as bipolar disorder, dysthymic disorder, panic disorder, major recurrent depression, substance abuse, posttraumatic stress disorder, intermittent explosive disorder, or any variety of adjustment, anxiety, eating, impulse control, mood, somatoform, or personality disorders.2 However, a BPD diagnosis best describes many of these patients, and the mnemonic “DARE” enumerates the most commonly encountered clinical picture.
Depression, Destruction, Denial. Chronic low-grade depression is the baseline mood for BPD. The patients might not report suicidal ideation or might deny a desire to die. But the predilection and potential for risky behavior that could result in accidental injury or death tends to confirm the presence of an underlying self-destructive wish.
Anger, Abandonment, Abuse. Typically, BPD patients are angry at the world. Anger simmers just below the threshold of self-control. When it boils over, BPD patients are apt to take their anger out on themselves by committing suicide or a self-mutilative act, or on others with passive aggression or the kind of physical or emotional abuse they themselves suffered.
BPD patients’ histories often include physical or emotional abandonment or abuse.
Relationships, Regrets, Repetition. Repeated patterns of unstable relationships are characteristic. Often BPD patients have multiple romantic partners, frequent job turnover, interrupted education, and few long-term, mature friendships. These patients’ friends and partners frequently suffer from similar problematic personality characteristics. BPD patients seem unable to break free of their unsuccessful patterns and repeatedly fail to maintain healthy, productive relationships.
Extremes, Emergencies, Ennui. Overuse of prescription drugs, alcohol, or other substances result in frequent emergency room visits. Bulimia, sexual promiscuity, and multiple body piercings or tattoos are emblematic of BPD. Ennui—a feeling of weariness or discontent—is fought off by engaging in extreme behaviors, such as reckless driving.
Many BPD patients improve and show greater stability in jobs and relationships with therapeutic intervention, although they often have a lifelong tendency toward impulsivity and intense relationships and emotions.
Dr. Roth is attending psychiatrist, Department of Veterans Affairs Medical Center, North Chicago, IL.
How to monitor medication side effects
Woman prescribed a stimulant suffers stroke and disability
Harris County (TX) District Court
A 39-year-old patient was diagnosed with attention-deficit/hyperactivity disorder (ADHD) by a psychologist, who referred her to a psychiatrist. The psychiatrist prescribed amphetamine/dextroamphetamine, which the patient took for 9 months. During this time her blood pressure and other vital signs were not monitored. The patient then suffered a stroke, is now a paraplegic, and must use a wheelchair.
The patient claimed that negligent misdiagnosis and monitoring caused the stroke. The psychiatrist maintained that diagnosis and monitoring were appropriate, and the drug did not cause the stroke. The psychiatrist also claimed that the patient had a transient ischemic attack (TIA) before taking amphetamine/dextroamphetamine and another stroke after discontinuing the drug.
- A defense verdict was returned
Improper dose of lamotrigine blamed for liver failure
San Diego County (CA) Superior Court
The patient, age 35, was involuntarily admitted to an inpatient psychiatric facility after the police found her acting bizarrely and hallucinating. The admitting and treating psychiatrist learned that the patient had been admitted for psychiatric treatment 9 times in the previous 12 months, had a long history of polysubstance abuse, and had been largely nonadherent with medication. The psychiatrist diagnosed rapid-cycling bipolar disorder and started the patient on lamotrigine with an escalating dosage schedule. The patient was released from the psychiatric facility.
Later that month, the patient developed a urinary tract infection and was readmitted to the hospital. She agreed to lab testing and all results were within normal limits, but throughout a 2-month stay the patient intermittently complained of a sore throat, cough, and nausea. Two weeks later, the psychiatrist reviewed lab tests that showed a mild elevation of the patient’s liver enzymes.
The next day the patient reported a rash on her chest and a high fever. She was transferred to an acute care facility. The patient’s liver enzymes continued to rise, and the psychiatrist discontinued lamotrigine. The patient continued to deteriorate and was transferred to another hospital to consult with a liver specialist. About 3 weeks later the patient went into a coma and died.
Autopsy showed massive liver necrosis. The patient’s family claimed the psychiatrist was negligent in giving the patient lamotrigine, which caused the liver failure. They contended the dose prescribed was too high, the patient was not properly monitored, and other psychiatric drugs could have been used with more gradual increases.
The psychiatrist maintained that the lamotrigine dosage used was appropriate, lamotrigine was not known to cause liver problems, and it did not cause the patient’s liver failure.
- A defense verdict was returned
Dr. Grant’s observations
These cases reflect a clinician’s worst nightmare—using an appropriate medication, experiencing a disastrous outcome, and then being sued for malpractice. Clinicians need to remember:
- anyone can be sued
- a lawsuit does not mean that the clinician did anything inappropriate.
Meeting standards of care
Medical malpractice claims could be based on a physician diverging from 1 of 2 standards of care:
- The “average practitioner” or “customary practice” standard means the treatment practice is consistent with others in the field. Courts might allow the medical profession to define the standard of care according to medical custom.
- The “reasonably prudent physician” standard means what a reasonable physician would have done under the circumstances. The jury determines if the physician acted reasonably, not whether the physician conformed to existing standards.1
In these cases, using amphetamine/ dextroamphetamine for ADHD and lamotrigine for bipolar disorder appears to meet either standard. These 2 drugs are FDA-approved to treat the disorders for which they were prescribed. Although we do not know what doses the physicians prescribed in these 2 cases, in general if the dosing adheres to the FDA-approved range or can be based on credible research, the treatment will meet the 2 standards.
Choosing a treatment plan
The American Psychiatric Association’s practice guidelines (available at http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm) state “the ultimate judgment regarding a particular clinical procedure or treatment plan must be made by the psychiatrist in light of the clinical data presented by the patient and the diagnostic and treatment options available.”3
Regardless of the treatment used—even if the medication is “off-label” and not FDA-approved for a particular disorder or the dose is not within the FDA-approved dosing range—you should be able to document your rationale for using a medication and dosing by showing that it is part of good clinical practice.
A clinician’s scientific rationale for medication and dosing choice should be based on the psychiatric evaluation and known risks and benefits of the treatment. In addition, the patient should:
- understand pertinent information regarding the medication and its side effects
- and freely give consent to treatment.4
Monitoring for side effects
In these cases, the court also had to determine whether clinicians’ monitoring for side effects was appropriate. For several years, case reports have raised speculation about a link between strokes and amphetamine/ dextroamphetamine4,5 In 2005, Adderall XR was taken off the Canadian market because of reports of strokes and sudden deaths.7
The FDA’s Adverse Event Reporting System database identified 12 cases of sudden death in pediatric patents treated for ADHD with Adderall or Adderall XR.8 lthough the drug has returned to the Canadian market and a clear link between stroke or sudden death and Adderall has not been established, The Physicians’ Desk Reference (PDR)9 advises physicians to monitor blood pressure in individuals taking amphetamine/dextroamphetamine, particularly those with hypertension. The FDA has issued new labeling instructions for all stimulants advising prescribing clinicians to monitor blood pressure regularly.10
Even so, if you fail to monitor blood pressure and a patient has a stroke—such as in the first case—you are not necessarily negligent. Successful malpractice cases need to demonstrate causation. The plaintiff must prove:
- The physician’s act or omission was the cause-in-fact of the harm. Without the act, the harm would not have occurred.
- The act was the proximate cause of the harm. In a natural, unbroken sequence of events, the act produces a foreseeable result. A physician should not be liable for the far-reaching and improbable consequences of an act or omission.1
- lack of foreseeability—the consequences of the act were not reasonably foreseeable, or
- an intervening event that supersedes all others in causing the injury.1
Foreseeability
A defendant may be liable only if the consequences of the act or omission were reasonably foreseeable. Foreseeability is a vague legal concept and is not the same as predictability. Foreseeability should be understood in context of what information was available at the time. For example, the FDA black box warnings about the link between stimulants and stroke or sudden death did not appear until 2006.11 What light be considered foreseeable now might not have been before 2006 (it is unclear when the above case was litigated).
Intervening events
An intervening event is one that takes effect after the defendant’s negligence and breaks the chain of causation. In the first case, the patient had a history of TIAs before taking amphetamine/dextroamphetamine. The condition that caused the TIAs, such as atherosclerosis in an artery, may also have caused the stroke independent of the use of stimulants, and therefore could be considered an intervening event.
In the lamotrigine case, elevations of aspartate transaminase and alanine transaminase are infrequent or rare. Several case reports have discussed possible hepatotoxicity associated with the drug.13
A reasonably prudent physician should warn patients about and monitor for symptoms of Stevens-Johnson syndrome, a serious disorder of the skin and mucous membranes sometimes seen with lamotrigine that can begin with cough, fever, and sore throat. Although hepatitis is a possible complication of Stevens-Johnson, the first step of treatment is to hospitalize the patient in an intensive care unit, which the physician did. The PDR and FDA guidelines do not recommend monitoring liver function tests as a way to assess for Stevens-Johnson or for liver dysfunction as an independent problem with lamotrigine.9,12
Given the lack of guidelines and the scant literature on this topic, the psychiatrist in this case would not have been expected to monitor liver function, which would meet either the “average practitioner” or “reasonably prudent physician” standard. Although the literature suggests that liver toxicity might have been foreseeable, the patient had a history of polysubstance abuse, which may be determined to be an intervening event. Substance abuse could have caused liver toxicity, depending on the drugs the patient abused.
Drug brand names
- Amphetamine/Dextroamphetamine • Adderall
- Lamotrigine • Lamictal
1. Knoll J, Gerbasi J. Psychiatric malpractice case analysis: striving for objectivity. J Am Acad Psychiatry Law 2006;34:215-223.
2. Lewis MH, Gohagan JK, Merensteine DJ. The locality rule and the physician’s dilemma: local medical practices vs the national standard of care. JAMA 2007;297:2633-7.
3. American Psychiatric Association Practice guidelines. Available at: http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm. Accessed June 27, 2007.
4. Berner M. Informed consent. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA: Harvard University Press 1998:23-43.
5. Toffol GJ, Biller J, Adams HP. Nontraumtic intracerebral hemorrhage in young adults. Arch Neurol 1987;44:483-5.
6. Bakheit AM. Intracerebral haemorrhage in previously healthy young adults. Postgrad Med J 1999;75:499-500.
7. McMillen M. Adderall: a stroke of bad news. The Washington Post. February 15, 2005. Available at: http://www.washingtonpost.com/wp-dyn/articles/A24764-2005Feb14.html. Accessed June 27, 2007.
8. U.S. Food and Drug Administration. Alert for healthcare professionals Adderall and Adderall XT (amphetamines). September 23, 2005. Available at: http://www.fda.gov/cder/drug/infosheets/hcp/adderalhcp.htm. Accessed July 5, 2007.
9. Physicians’ desk reference Montvale, NJ: Thompson PDR; 2007.
10. U.S. Food and Drug Administration. Adderall and Adderall XR (amphetamines) information. February 22, 2007. Available at: http://www.fda.gov/cder/drug/infopage/adderall/default.htm. Accessed June 27, 2007.
11. Charatan F. FDA committee votes for warning labels on stimulant drugs. BMJ 2006;332:380-
12. Lamictal prescribing information. Food and Drug Administration Web site. Available at http://www.fda.gov/cder/foi/label/2006020241s10s21s25s26s27,020764s3s14s18s19s20lbl.pdf. Accessed July 9, 2007.
13. Overstreet K, Costanza C, Behling C, et al. Fatal progressive hepatic necrosis associated with lamotrigine treatment: a case report and literature review. Dig Dis Sci 2002;47:1921-5.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Woman prescribed a stimulant suffers stroke and disability
Harris County (TX) District Court
A 39-year-old patient was diagnosed with attention-deficit/hyperactivity disorder (ADHD) by a psychologist, who referred her to a psychiatrist. The psychiatrist prescribed amphetamine/dextroamphetamine, which the patient took for 9 months. During this time her blood pressure and other vital signs were not monitored. The patient then suffered a stroke, is now a paraplegic, and must use a wheelchair.
The patient claimed that negligent misdiagnosis and monitoring caused the stroke. The psychiatrist maintained that diagnosis and monitoring were appropriate, and the drug did not cause the stroke. The psychiatrist also claimed that the patient had a transient ischemic attack (TIA) before taking amphetamine/dextroamphetamine and another stroke after discontinuing the drug.
- A defense verdict was returned
Improper dose of lamotrigine blamed for liver failure
San Diego County (CA) Superior Court
The patient, age 35, was involuntarily admitted to an inpatient psychiatric facility after the police found her acting bizarrely and hallucinating. The admitting and treating psychiatrist learned that the patient had been admitted for psychiatric treatment 9 times in the previous 12 months, had a long history of polysubstance abuse, and had been largely nonadherent with medication. The psychiatrist diagnosed rapid-cycling bipolar disorder and started the patient on lamotrigine with an escalating dosage schedule. The patient was released from the psychiatric facility.
Later that month, the patient developed a urinary tract infection and was readmitted to the hospital. She agreed to lab testing and all results were within normal limits, but throughout a 2-month stay the patient intermittently complained of a sore throat, cough, and nausea. Two weeks later, the psychiatrist reviewed lab tests that showed a mild elevation of the patient’s liver enzymes.
The next day the patient reported a rash on her chest and a high fever. She was transferred to an acute care facility. The patient’s liver enzymes continued to rise, and the psychiatrist discontinued lamotrigine. The patient continued to deteriorate and was transferred to another hospital to consult with a liver specialist. About 3 weeks later the patient went into a coma and died.
Autopsy showed massive liver necrosis. The patient’s family claimed the psychiatrist was negligent in giving the patient lamotrigine, which caused the liver failure. They contended the dose prescribed was too high, the patient was not properly monitored, and other psychiatric drugs could have been used with more gradual increases.
The psychiatrist maintained that the lamotrigine dosage used was appropriate, lamotrigine was not known to cause liver problems, and it did not cause the patient’s liver failure.
- A defense verdict was returned
Dr. Grant’s observations
These cases reflect a clinician’s worst nightmare—using an appropriate medication, experiencing a disastrous outcome, and then being sued for malpractice. Clinicians need to remember:
- anyone can be sued
- a lawsuit does not mean that the clinician did anything inappropriate.
Meeting standards of care
Medical malpractice claims could be based on a physician diverging from 1 of 2 standards of care:
- The “average practitioner” or “customary practice” standard means the treatment practice is consistent with others in the field. Courts might allow the medical profession to define the standard of care according to medical custom.
- The “reasonably prudent physician” standard means what a reasonable physician would have done under the circumstances. The jury determines if the physician acted reasonably, not whether the physician conformed to existing standards.1
In these cases, using amphetamine/ dextroamphetamine for ADHD and lamotrigine for bipolar disorder appears to meet either standard. These 2 drugs are FDA-approved to treat the disorders for which they were prescribed. Although we do not know what doses the physicians prescribed in these 2 cases, in general if the dosing adheres to the FDA-approved range or can be based on credible research, the treatment will meet the 2 standards.
Choosing a treatment plan
The American Psychiatric Association’s practice guidelines (available at http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm) state “the ultimate judgment regarding a particular clinical procedure or treatment plan must be made by the psychiatrist in light of the clinical data presented by the patient and the diagnostic and treatment options available.”3
Regardless of the treatment used—even if the medication is “off-label” and not FDA-approved for a particular disorder or the dose is not within the FDA-approved dosing range—you should be able to document your rationale for using a medication and dosing by showing that it is part of good clinical practice.
A clinician’s scientific rationale for medication and dosing choice should be based on the psychiatric evaluation and known risks and benefits of the treatment. In addition, the patient should:
- understand pertinent information regarding the medication and its side effects
- and freely give consent to treatment.4
Monitoring for side effects
In these cases, the court also had to determine whether clinicians’ monitoring for side effects was appropriate. For several years, case reports have raised speculation about a link between strokes and amphetamine/ dextroamphetamine4,5 In 2005, Adderall XR was taken off the Canadian market because of reports of strokes and sudden deaths.7
The FDA’s Adverse Event Reporting System database identified 12 cases of sudden death in pediatric patents treated for ADHD with Adderall or Adderall XR.8 lthough the drug has returned to the Canadian market and a clear link between stroke or sudden death and Adderall has not been established, The Physicians’ Desk Reference (PDR)9 advises physicians to monitor blood pressure in individuals taking amphetamine/dextroamphetamine, particularly those with hypertension. The FDA has issued new labeling instructions for all stimulants advising prescribing clinicians to monitor blood pressure regularly.10
Even so, if you fail to monitor blood pressure and a patient has a stroke—such as in the first case—you are not necessarily negligent. Successful malpractice cases need to demonstrate causation. The plaintiff must prove:
- The physician’s act or omission was the cause-in-fact of the harm. Without the act, the harm would not have occurred.
- The act was the proximate cause of the harm. In a natural, unbroken sequence of events, the act produces a foreseeable result. A physician should not be liable for the far-reaching and improbable consequences of an act or omission.1
- lack of foreseeability—the consequences of the act were not reasonably foreseeable, or
- an intervening event that supersedes all others in causing the injury.1
Foreseeability
A defendant may be liable only if the consequences of the act or omission were reasonably foreseeable. Foreseeability is a vague legal concept and is not the same as predictability. Foreseeability should be understood in context of what information was available at the time. For example, the FDA black box warnings about the link between stimulants and stroke or sudden death did not appear until 2006.11 What light be considered foreseeable now might not have been before 2006 (it is unclear when the above case was litigated).
Intervening events
An intervening event is one that takes effect after the defendant’s negligence and breaks the chain of causation. In the first case, the patient had a history of TIAs before taking amphetamine/dextroamphetamine. The condition that caused the TIAs, such as atherosclerosis in an artery, may also have caused the stroke independent of the use of stimulants, and therefore could be considered an intervening event.
In the lamotrigine case, elevations of aspartate transaminase and alanine transaminase are infrequent or rare. Several case reports have discussed possible hepatotoxicity associated with the drug.13
A reasonably prudent physician should warn patients about and monitor for symptoms of Stevens-Johnson syndrome, a serious disorder of the skin and mucous membranes sometimes seen with lamotrigine that can begin with cough, fever, and sore throat. Although hepatitis is a possible complication of Stevens-Johnson, the first step of treatment is to hospitalize the patient in an intensive care unit, which the physician did. The PDR and FDA guidelines do not recommend monitoring liver function tests as a way to assess for Stevens-Johnson or for liver dysfunction as an independent problem with lamotrigine.9,12
Given the lack of guidelines and the scant literature on this topic, the psychiatrist in this case would not have been expected to monitor liver function, which would meet either the “average practitioner” or “reasonably prudent physician” standard. Although the literature suggests that liver toxicity might have been foreseeable, the patient had a history of polysubstance abuse, which may be determined to be an intervening event. Substance abuse could have caused liver toxicity, depending on the drugs the patient abused.
Drug brand names
- Amphetamine/Dextroamphetamine • Adderall
- Lamotrigine • Lamictal
Woman prescribed a stimulant suffers stroke and disability
Harris County (TX) District Court
A 39-year-old patient was diagnosed with attention-deficit/hyperactivity disorder (ADHD) by a psychologist, who referred her to a psychiatrist. The psychiatrist prescribed amphetamine/dextroamphetamine, which the patient took for 9 months. During this time her blood pressure and other vital signs were not monitored. The patient then suffered a stroke, is now a paraplegic, and must use a wheelchair.
The patient claimed that negligent misdiagnosis and monitoring caused the stroke. The psychiatrist maintained that diagnosis and monitoring were appropriate, and the drug did not cause the stroke. The psychiatrist also claimed that the patient had a transient ischemic attack (TIA) before taking amphetamine/dextroamphetamine and another stroke after discontinuing the drug.
- A defense verdict was returned
Improper dose of lamotrigine blamed for liver failure
San Diego County (CA) Superior Court
The patient, age 35, was involuntarily admitted to an inpatient psychiatric facility after the police found her acting bizarrely and hallucinating. The admitting and treating psychiatrist learned that the patient had been admitted for psychiatric treatment 9 times in the previous 12 months, had a long history of polysubstance abuse, and had been largely nonadherent with medication. The psychiatrist diagnosed rapid-cycling bipolar disorder and started the patient on lamotrigine with an escalating dosage schedule. The patient was released from the psychiatric facility.
Later that month, the patient developed a urinary tract infection and was readmitted to the hospital. She agreed to lab testing and all results were within normal limits, but throughout a 2-month stay the patient intermittently complained of a sore throat, cough, and nausea. Two weeks later, the psychiatrist reviewed lab tests that showed a mild elevation of the patient’s liver enzymes.
The next day the patient reported a rash on her chest and a high fever. She was transferred to an acute care facility. The patient’s liver enzymes continued to rise, and the psychiatrist discontinued lamotrigine. The patient continued to deteriorate and was transferred to another hospital to consult with a liver specialist. About 3 weeks later the patient went into a coma and died.
Autopsy showed massive liver necrosis. The patient’s family claimed the psychiatrist was negligent in giving the patient lamotrigine, which caused the liver failure. They contended the dose prescribed was too high, the patient was not properly monitored, and other psychiatric drugs could have been used with more gradual increases.
The psychiatrist maintained that the lamotrigine dosage used was appropriate, lamotrigine was not known to cause liver problems, and it did not cause the patient’s liver failure.
- A defense verdict was returned
Dr. Grant’s observations
These cases reflect a clinician’s worst nightmare—using an appropriate medication, experiencing a disastrous outcome, and then being sued for malpractice. Clinicians need to remember:
- anyone can be sued
- a lawsuit does not mean that the clinician did anything inappropriate.
Meeting standards of care
Medical malpractice claims could be based on a physician diverging from 1 of 2 standards of care:
- The “average practitioner” or “customary practice” standard means the treatment practice is consistent with others in the field. Courts might allow the medical profession to define the standard of care according to medical custom.
- The “reasonably prudent physician” standard means what a reasonable physician would have done under the circumstances. The jury determines if the physician acted reasonably, not whether the physician conformed to existing standards.1
In these cases, using amphetamine/ dextroamphetamine for ADHD and lamotrigine for bipolar disorder appears to meet either standard. These 2 drugs are FDA-approved to treat the disorders for which they were prescribed. Although we do not know what doses the physicians prescribed in these 2 cases, in general if the dosing adheres to the FDA-approved range or can be based on credible research, the treatment will meet the 2 standards.
Choosing a treatment plan
The American Psychiatric Association’s practice guidelines (available at http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm) state “the ultimate judgment regarding a particular clinical procedure or treatment plan must be made by the psychiatrist in light of the clinical data presented by the patient and the diagnostic and treatment options available.”3
Regardless of the treatment used—even if the medication is “off-label” and not FDA-approved for a particular disorder or the dose is not within the FDA-approved dosing range—you should be able to document your rationale for using a medication and dosing by showing that it is part of good clinical practice.
A clinician’s scientific rationale for medication and dosing choice should be based on the psychiatric evaluation and known risks and benefits of the treatment. In addition, the patient should:
- understand pertinent information regarding the medication and its side effects
- and freely give consent to treatment.4
Monitoring for side effects
In these cases, the court also had to determine whether clinicians’ monitoring for side effects was appropriate. For several years, case reports have raised speculation about a link between strokes and amphetamine/ dextroamphetamine4,5 In 2005, Adderall XR was taken off the Canadian market because of reports of strokes and sudden deaths.7
The FDA’s Adverse Event Reporting System database identified 12 cases of sudden death in pediatric patents treated for ADHD with Adderall or Adderall XR.8 lthough the drug has returned to the Canadian market and a clear link between stroke or sudden death and Adderall has not been established, The Physicians’ Desk Reference (PDR)9 advises physicians to monitor blood pressure in individuals taking amphetamine/dextroamphetamine, particularly those with hypertension. The FDA has issued new labeling instructions for all stimulants advising prescribing clinicians to monitor blood pressure regularly.10
Even so, if you fail to monitor blood pressure and a patient has a stroke—such as in the first case—you are not necessarily negligent. Successful malpractice cases need to demonstrate causation. The plaintiff must prove:
- The physician’s act or omission was the cause-in-fact of the harm. Without the act, the harm would not have occurred.
- The act was the proximate cause of the harm. In a natural, unbroken sequence of events, the act produces a foreseeable result. A physician should not be liable for the far-reaching and improbable consequences of an act or omission.1
- lack of foreseeability—the consequences of the act were not reasonably foreseeable, or
- an intervening event that supersedes all others in causing the injury.1
Foreseeability
A defendant may be liable only if the consequences of the act or omission were reasonably foreseeable. Foreseeability is a vague legal concept and is not the same as predictability. Foreseeability should be understood in context of what information was available at the time. For example, the FDA black box warnings about the link between stimulants and stroke or sudden death did not appear until 2006.11 What light be considered foreseeable now might not have been before 2006 (it is unclear when the above case was litigated).
Intervening events
An intervening event is one that takes effect after the defendant’s negligence and breaks the chain of causation. In the first case, the patient had a history of TIAs before taking amphetamine/dextroamphetamine. The condition that caused the TIAs, such as atherosclerosis in an artery, may also have caused the stroke independent of the use of stimulants, and therefore could be considered an intervening event.
In the lamotrigine case, elevations of aspartate transaminase and alanine transaminase are infrequent or rare. Several case reports have discussed possible hepatotoxicity associated with the drug.13
A reasonably prudent physician should warn patients about and monitor for symptoms of Stevens-Johnson syndrome, a serious disorder of the skin and mucous membranes sometimes seen with lamotrigine that can begin with cough, fever, and sore throat. Although hepatitis is a possible complication of Stevens-Johnson, the first step of treatment is to hospitalize the patient in an intensive care unit, which the physician did. The PDR and FDA guidelines do not recommend monitoring liver function tests as a way to assess for Stevens-Johnson or for liver dysfunction as an independent problem with lamotrigine.9,12
Given the lack of guidelines and the scant literature on this topic, the psychiatrist in this case would not have been expected to monitor liver function, which would meet either the “average practitioner” or “reasonably prudent physician” standard. Although the literature suggests that liver toxicity might have been foreseeable, the patient had a history of polysubstance abuse, which may be determined to be an intervening event. Substance abuse could have caused liver toxicity, depending on the drugs the patient abused.
Drug brand names
- Amphetamine/Dextroamphetamine • Adderall
- Lamotrigine • Lamictal
1. Knoll J, Gerbasi J. Psychiatric malpractice case analysis: striving for objectivity. J Am Acad Psychiatry Law 2006;34:215-223.
2. Lewis MH, Gohagan JK, Merensteine DJ. The locality rule and the physician’s dilemma: local medical practices vs the national standard of care. JAMA 2007;297:2633-7.
3. American Psychiatric Association Practice guidelines. Available at: http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm. Accessed June 27, 2007.
4. Berner M. Informed consent. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA: Harvard University Press 1998:23-43.
5. Toffol GJ, Biller J, Adams HP. Nontraumtic intracerebral hemorrhage in young adults. Arch Neurol 1987;44:483-5.
6. Bakheit AM. Intracerebral haemorrhage in previously healthy young adults. Postgrad Med J 1999;75:499-500.
7. McMillen M. Adderall: a stroke of bad news. The Washington Post. February 15, 2005. Available at: http://www.washingtonpost.com/wp-dyn/articles/A24764-2005Feb14.html. Accessed June 27, 2007.
8. U.S. Food and Drug Administration. Alert for healthcare professionals Adderall and Adderall XT (amphetamines). September 23, 2005. Available at: http://www.fda.gov/cder/drug/infosheets/hcp/adderalhcp.htm. Accessed July 5, 2007.
9. Physicians’ desk reference Montvale, NJ: Thompson PDR; 2007.
10. U.S. Food and Drug Administration. Adderall and Adderall XR (amphetamines) information. February 22, 2007. Available at: http://www.fda.gov/cder/drug/infopage/adderall/default.htm. Accessed June 27, 2007.
11. Charatan F. FDA committee votes for warning labels on stimulant drugs. BMJ 2006;332:380-
12. Lamictal prescribing information. Food and Drug Administration Web site. Available at http://www.fda.gov/cder/foi/label/2006020241s10s21s25s26s27,020764s3s14s18s19s20lbl.pdf. Accessed July 9, 2007.
13. Overstreet K, Costanza C, Behling C, et al. Fatal progressive hepatic necrosis associated with lamotrigine treatment: a case report and literature review. Dig Dis Sci 2002;47:1921-5.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
1. Knoll J, Gerbasi J. Psychiatric malpractice case analysis: striving for objectivity. J Am Acad Psychiatry Law 2006;34:215-223.
2. Lewis MH, Gohagan JK, Merensteine DJ. The locality rule and the physician’s dilemma: local medical practices vs the national standard of care. JAMA 2007;297:2633-7.
3. American Psychiatric Association Practice guidelines. Available at: http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm. Accessed June 27, 2007.
4. Berner M. Informed consent. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA: Harvard University Press 1998:23-43.
5. Toffol GJ, Biller J, Adams HP. Nontraumtic intracerebral hemorrhage in young adults. Arch Neurol 1987;44:483-5.
6. Bakheit AM. Intracerebral haemorrhage in previously healthy young adults. Postgrad Med J 1999;75:499-500.
7. McMillen M. Adderall: a stroke of bad news. The Washington Post. February 15, 2005. Available at: http://www.washingtonpost.com/wp-dyn/articles/A24764-2005Feb14.html. Accessed June 27, 2007.
8. U.S. Food and Drug Administration. Alert for healthcare professionals Adderall and Adderall XT (amphetamines). September 23, 2005. Available at: http://www.fda.gov/cder/drug/infosheets/hcp/adderalhcp.htm. Accessed July 5, 2007.
9. Physicians’ desk reference Montvale, NJ: Thompson PDR; 2007.
10. U.S. Food and Drug Administration. Adderall and Adderall XR (amphetamines) information. February 22, 2007. Available at: http://www.fda.gov/cder/drug/infopage/adderall/default.htm. Accessed June 27, 2007.
11. Charatan F. FDA committee votes for warning labels on stimulant drugs. BMJ 2006;332:380-
12. Lamictal prescribing information. Food and Drug Administration Web site. Available at http://www.fda.gov/cder/foi/label/2006020241s10s21s25s26s27,020764s3s14s18s19s20lbl.pdf. Accessed July 9, 2007.
13. Overstreet K, Costanza C, Behling C, et al. Fatal progressive hepatic necrosis associated with lamotrigine treatment: a case report and literature review. Dig Dis Sci 2002;47:1921-5.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
What causes PTSD?
The article “6 keys to resilience for PTSD and everyday stress,” (Current Psychiatry, April 2007) makes only a small reference to post-traumatic stress disorder’s (PTSD) biologic factors. My knowledge of PTSD comes from my experience as a psychiatrist for Special Forces soldiers from 1964 to 1966 and follow-up studies by Peter Bourne, MD, and Colonel Lewellyn Legters, MD.
During the Vietnam War, Dr. Bourne noted that there was no rise in cortisol levels in the blood of Special Forces soldiers in battle. The casualty rate at that time was 1 in 10 wounded in a year. Pentagon or Special Operations soldiers placed in Special Forces had a casualty rate of 1 in 2 killed in Vietnam in 7 months.
Only 3% of the population will come through stressful experiences without some emotional sequelae. So what were the resilience factors in Special Forces troops that prevented combat fatigue, shell shock, or PTSD? Special Forces soldiers often had a long history of accepting and accomplishing challenges. This is different from mastering childhood adversity such as deaths, relocations, or family job loss.
Leonard R. Friedman, MD
Revere, MA
The article “6 keys to resilience for PTSD and everyday stress,” (Current Psychiatry, April 2007) makes only a small reference to post-traumatic stress disorder’s (PTSD) biologic factors. My knowledge of PTSD comes from my experience as a psychiatrist for Special Forces soldiers from 1964 to 1966 and follow-up studies by Peter Bourne, MD, and Colonel Lewellyn Legters, MD.
During the Vietnam War, Dr. Bourne noted that there was no rise in cortisol levels in the blood of Special Forces soldiers in battle. The casualty rate at that time was 1 in 10 wounded in a year. Pentagon or Special Operations soldiers placed in Special Forces had a casualty rate of 1 in 2 killed in Vietnam in 7 months.
Only 3% of the population will come through stressful experiences without some emotional sequelae. So what were the resilience factors in Special Forces troops that prevented combat fatigue, shell shock, or PTSD? Special Forces soldiers often had a long history of accepting and accomplishing challenges. This is different from mastering childhood adversity such as deaths, relocations, or family job loss.
Leonard R. Friedman, MD
Revere, MA
The article “6 keys to resilience for PTSD and everyday stress,” (Current Psychiatry, April 2007) makes only a small reference to post-traumatic stress disorder’s (PTSD) biologic factors. My knowledge of PTSD comes from my experience as a psychiatrist for Special Forces soldiers from 1964 to 1966 and follow-up studies by Peter Bourne, MD, and Colonel Lewellyn Legters, MD.
During the Vietnam War, Dr. Bourne noted that there was no rise in cortisol levels in the blood of Special Forces soldiers in battle. The casualty rate at that time was 1 in 10 wounded in a year. Pentagon or Special Operations soldiers placed in Special Forces had a casualty rate of 1 in 2 killed in Vietnam in 7 months.
Only 3% of the population will come through stressful experiences without some emotional sequelae. So what were the resilience factors in Special Forces troops that prevented combat fatigue, shell shock, or PTSD? Special Forces soldiers often had a long history of accepting and accomplishing challenges. This is different from mastering childhood adversity such as deaths, relocations, or family job loss.
Leonard R. Friedman, MD
Revere, MA
Gender misconceptions
Sad to say, the source Dr. Thomas K. Nelson cites in his letter “The meaning of gender” (Letters, Current Psychiatry, May 2007) is a very prejudiced work in regard to transsexualism. I should know; I am a postoperative, male-to-female trans-sexual, although I now identify myself as a normal woman.
Gender identity disorder (GID) starts quite young, usually at age 3 or 4, and is a persistent knowledge that the body’s physical characteristics are wrong for the gender our brain determines. Please note that this occurs well before the onset of puberty.
It is sad that therapists often act as “gatekeepers” and can impose their standards of who is female or male as the case may be. There is, of course, no way a therapist can detect who is lying or mimicking having GID because these individuals might be guilty of self-deception. Usually the start of hormone replacement therapy and loss of libido discourages these individuals, but a few do slip through.
For me the greatest sense of relief and happiness occurred immediately after the operation, and I realized that for the first time in my life my body was congruent with my brain’s gender.
Pamela J.S. Dunn
Tampa, FL
Sad to say, the source Dr. Thomas K. Nelson cites in his letter “The meaning of gender” (Letters, Current Psychiatry, May 2007) is a very prejudiced work in regard to transsexualism. I should know; I am a postoperative, male-to-female trans-sexual, although I now identify myself as a normal woman.
Gender identity disorder (GID) starts quite young, usually at age 3 or 4, and is a persistent knowledge that the body’s physical characteristics are wrong for the gender our brain determines. Please note that this occurs well before the onset of puberty.
It is sad that therapists often act as “gatekeepers” and can impose their standards of who is female or male as the case may be. There is, of course, no way a therapist can detect who is lying or mimicking having GID because these individuals might be guilty of self-deception. Usually the start of hormone replacement therapy and loss of libido discourages these individuals, but a few do slip through.
For me the greatest sense of relief and happiness occurred immediately after the operation, and I realized that for the first time in my life my body was congruent with my brain’s gender.
Pamela J.S. Dunn
Tampa, FL
Sad to say, the source Dr. Thomas K. Nelson cites in his letter “The meaning of gender” (Letters, Current Psychiatry, May 2007) is a very prejudiced work in regard to transsexualism. I should know; I am a postoperative, male-to-female trans-sexual, although I now identify myself as a normal woman.
Gender identity disorder (GID) starts quite young, usually at age 3 or 4, and is a persistent knowledge that the body’s physical characteristics are wrong for the gender our brain determines. Please note that this occurs well before the onset of puberty.
It is sad that therapists often act as “gatekeepers” and can impose their standards of who is female or male as the case may be. There is, of course, no way a therapist can detect who is lying or mimicking having GID because these individuals might be guilty of self-deception. Usually the start of hormone replacement therapy and loss of libido discourages these individuals, but a few do slip through.
For me the greatest sense of relief and happiness occurred immediately after the operation, and I realized that for the first time in my life my body was congruent with my brain’s gender.
Pamela J.S. Dunn
Tampa, FL
Cards promote depression awareness on campuses
I enjoyed Dr. Henry Nasrallah’s editorial addressing mental illness on college campuses (“Mental illness on campus: What have we learned?” Current Psychiatry, June 2007). I am a senior psychology/premed student at Southern Methodist University in Dallas. As part of a psychology project, I created a card about the size of a credit card that lists the signs of depression. The cards are designed for students to carry in their wallets for convenient referral.
More than 300 college student health centers across the United States have requested more than 20,000 of my cards on depression. These cards can be customized with mental health resources available at each campus for no charge.
Jennifer Rosemore
Dallas, TX
I enjoyed Dr. Henry Nasrallah’s editorial addressing mental illness on college campuses (“Mental illness on campus: What have we learned?” Current Psychiatry, June 2007). I am a senior psychology/premed student at Southern Methodist University in Dallas. As part of a psychology project, I created a card about the size of a credit card that lists the signs of depression. The cards are designed for students to carry in their wallets for convenient referral.
More than 300 college student health centers across the United States have requested more than 20,000 of my cards on depression. These cards can be customized with mental health resources available at each campus for no charge.
Jennifer Rosemore
Dallas, TX
I enjoyed Dr. Henry Nasrallah’s editorial addressing mental illness on college campuses (“Mental illness on campus: What have we learned?” Current Psychiatry, June 2007). I am a senior psychology/premed student at Southern Methodist University in Dallas. As part of a psychology project, I created a card about the size of a credit card that lists the signs of depression. The cards are designed for students to carry in their wallets for convenient referral.
More than 300 college student health centers across the United States have requested more than 20,000 of my cards on depression. These cards can be customized with mental health resources available at each campus for no charge.
Jennifer Rosemore
Dallas, TX
Financial disclosures: Readers’ right to know
In “Stimulant danger diffused” (Letters, Current Psychiatry, June 2007), Drs. Lenard Adler and Anthony Rostain rebut a reader’s concern that Shire Pharmaceutical’s Daytrana transdermal methylphenidate patch could lead to toxic levels of methylphenidate in certain circumstances (“Dangers of stimulant patch misuse,” Letters, Current Psychiatry, March 2007). Although the doctor’s arguments have merit, many readers would have been interested to know that Drs. Adler and Rostain have financial interests that may have affected the tenor of their commentary.
In a recent Medscape interview supported by Shire, Dr. Adler disclosed financial relationships with Abbott Laboratories, Bristol-Myers Squibb, Cephalon, Cortex Pharmaceuticals, Eli Lilly and Company, Kyowa Pharmaceuticals, McNeil/Johnson & Johnson, Merck, Neurosearch, Novartis Pharmaceuticals, Pfizer Labs, and Shire. Similarly, Shire sponsored Dr. Rostain to speak at a professional development program produced by the American College Health Association, where he disclosed that he is a speaker for Eli Lilly and Company and Ortho-McNeil and a consultant for Shire.
I note that Current Psychiatry appears to have an inconsistent policy regarding disclosure requirements. For example, although most of the “evidence-based reviews” list disclosures, some do not. For example, in Adler and Rostain’s original article on stimulants (“New warnings on stimulants for ADHD: Cause for alarm?” Current Psychiatry, October 2006), no disclosures were listed.
Given increasing concerns regarding the effects of pharmaceutical industry funding on medical education, I urge Current Psychiatry to require financial disclosures for all letters and articles that mention commercial products, which is the standard for most psychiatric journals. Disclosing this information would allow readers to gauge the probability that an author’s conclusions might be affected by competing financial interests.
Daniel Carlat, MD
Newburyport, MA
Editor’s response
Drs. Adler and Rostain did, in fact, provide our required financial disclosure statements that listed the relationships described in Dr. Carlat’s letter. I regret that I forgot to include the disclosures with this particular article in our October 2006 issue.
Since its founding, Current Psychiatry has had a policy requiring authors of evidence-based reviews and those participating in interviews to complete disclosure forms reporting financial relationships “with any company whose products are mentioned” in their articles or “with manufacturers of competing products.” We believe our readers have a right to this information.
I appreciate Dr. Carlat’s vigilance in reminding us to make sure we take the information from these forms and include it with the articles. We have added the disclosures to the archived version of the Adler/Rostain interview on our Web site at www.currentpsychiatry.com.
Given this opportunity, I also wish to assure readers that our editorial board and professional staff choose topics and invite authors independently. No pharmaceutical company participated in developing the Adler/Rostain interview or any other article in Current Psychiatry.
Alice V. Luddington, ELS
Editor
In “Stimulant danger diffused” (Letters, Current Psychiatry, June 2007), Drs. Lenard Adler and Anthony Rostain rebut a reader’s concern that Shire Pharmaceutical’s Daytrana transdermal methylphenidate patch could lead to toxic levels of methylphenidate in certain circumstances (“Dangers of stimulant patch misuse,” Letters, Current Psychiatry, March 2007). Although the doctor’s arguments have merit, many readers would have been interested to know that Drs. Adler and Rostain have financial interests that may have affected the tenor of their commentary.
In a recent Medscape interview supported by Shire, Dr. Adler disclosed financial relationships with Abbott Laboratories, Bristol-Myers Squibb, Cephalon, Cortex Pharmaceuticals, Eli Lilly and Company, Kyowa Pharmaceuticals, McNeil/Johnson & Johnson, Merck, Neurosearch, Novartis Pharmaceuticals, Pfizer Labs, and Shire. Similarly, Shire sponsored Dr. Rostain to speak at a professional development program produced by the American College Health Association, where he disclosed that he is a speaker for Eli Lilly and Company and Ortho-McNeil and a consultant for Shire.
I note that Current Psychiatry appears to have an inconsistent policy regarding disclosure requirements. For example, although most of the “evidence-based reviews” list disclosures, some do not. For example, in Adler and Rostain’s original article on stimulants (“New warnings on stimulants for ADHD: Cause for alarm?” Current Psychiatry, October 2006), no disclosures were listed.
Given increasing concerns regarding the effects of pharmaceutical industry funding on medical education, I urge Current Psychiatry to require financial disclosures for all letters and articles that mention commercial products, which is the standard for most psychiatric journals. Disclosing this information would allow readers to gauge the probability that an author’s conclusions might be affected by competing financial interests.
Daniel Carlat, MD
Newburyport, MA
Editor’s response
Drs. Adler and Rostain did, in fact, provide our required financial disclosure statements that listed the relationships described in Dr. Carlat’s letter. I regret that I forgot to include the disclosures with this particular article in our October 2006 issue.
Since its founding, Current Psychiatry has had a policy requiring authors of evidence-based reviews and those participating in interviews to complete disclosure forms reporting financial relationships “with any company whose products are mentioned” in their articles or “with manufacturers of competing products.” We believe our readers have a right to this information.
I appreciate Dr. Carlat’s vigilance in reminding us to make sure we take the information from these forms and include it with the articles. We have added the disclosures to the archived version of the Adler/Rostain interview on our Web site at www.currentpsychiatry.com.
Given this opportunity, I also wish to assure readers that our editorial board and professional staff choose topics and invite authors independently. No pharmaceutical company participated in developing the Adler/Rostain interview or any other article in Current Psychiatry.
Alice V. Luddington, ELS
Editor
In “Stimulant danger diffused” (Letters, Current Psychiatry, June 2007), Drs. Lenard Adler and Anthony Rostain rebut a reader’s concern that Shire Pharmaceutical’s Daytrana transdermal methylphenidate patch could lead to toxic levels of methylphenidate in certain circumstances (“Dangers of stimulant patch misuse,” Letters, Current Psychiatry, March 2007). Although the doctor’s arguments have merit, many readers would have been interested to know that Drs. Adler and Rostain have financial interests that may have affected the tenor of their commentary.
In a recent Medscape interview supported by Shire, Dr. Adler disclosed financial relationships with Abbott Laboratories, Bristol-Myers Squibb, Cephalon, Cortex Pharmaceuticals, Eli Lilly and Company, Kyowa Pharmaceuticals, McNeil/Johnson & Johnson, Merck, Neurosearch, Novartis Pharmaceuticals, Pfizer Labs, and Shire. Similarly, Shire sponsored Dr. Rostain to speak at a professional development program produced by the American College Health Association, where he disclosed that he is a speaker for Eli Lilly and Company and Ortho-McNeil and a consultant for Shire.
I note that Current Psychiatry appears to have an inconsistent policy regarding disclosure requirements. For example, although most of the “evidence-based reviews” list disclosures, some do not. For example, in Adler and Rostain’s original article on stimulants (“New warnings on stimulants for ADHD: Cause for alarm?” Current Psychiatry, October 2006), no disclosures were listed.
Given increasing concerns regarding the effects of pharmaceutical industry funding on medical education, I urge Current Psychiatry to require financial disclosures for all letters and articles that mention commercial products, which is the standard for most psychiatric journals. Disclosing this information would allow readers to gauge the probability that an author’s conclusions might be affected by competing financial interests.
Daniel Carlat, MD
Newburyport, MA
Editor’s response
Drs. Adler and Rostain did, in fact, provide our required financial disclosure statements that listed the relationships described in Dr. Carlat’s letter. I regret that I forgot to include the disclosures with this particular article in our October 2006 issue.
Since its founding, Current Psychiatry has had a policy requiring authors of evidence-based reviews and those participating in interviews to complete disclosure forms reporting financial relationships “with any company whose products are mentioned” in their articles or “with manufacturers of competing products.” We believe our readers have a right to this information.
I appreciate Dr. Carlat’s vigilance in reminding us to make sure we take the information from these forms and include it with the articles. We have added the disclosures to the archived version of the Adler/Rostain interview on our Web site at www.currentpsychiatry.com.
Given this opportunity, I also wish to assure readers that our editorial board and professional staff choose topics and invite authors independently. No pharmaceutical company participated in developing the Adler/Rostain interview or any other article in Current Psychiatry.
Alice V. Luddington, ELS
Editor