User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Correction
The article, “For women only: Hormones may prevent addiction relapse” (Current Psychiatry, August 2006) contained an error on page 42.
Reference 7 supports the statement that “higher stress responsiveness is associated with increased cocaine craving,” but reference 8 shows that—unlike in cocaine addiction—lower HPA axis responsiveness in alcoholics is associated with an increased risk of relapse.
The article, “For women only: Hormones may prevent addiction relapse” (Current Psychiatry, August 2006) contained an error on page 42.
Reference 7 supports the statement that “higher stress responsiveness is associated with increased cocaine craving,” but reference 8 shows that—unlike in cocaine addiction—lower HPA axis responsiveness in alcoholics is associated with an increased risk of relapse.
The article, “For women only: Hormones may prevent addiction relapse” (Current Psychiatry, August 2006) contained an error on page 42.
Reference 7 supports the statement that “higher stress responsiveness is associated with increased cocaine craving,” but reference 8 shows that—unlike in cocaine addiction—lower HPA axis responsiveness in alcoholics is associated with an increased risk of relapse.
Prescription pad packs a punch
I found Dr. Richard C. Christensen’s article (“‘Prescribing’ behavioral and lifestyle changes,” Current Psychiatry, July 2006) very useful. I am a therapist, but I think it is important for physicians to remember that the power of the prescription pad can go beyond prescribing medications. Thank you for this insightful article.
Richard Cloyd
Dandridge, TN
I found Dr. Richard C. Christensen’s article (“‘Prescribing’ behavioral and lifestyle changes,” Current Psychiatry, July 2006) very useful. I am a therapist, but I think it is important for physicians to remember that the power of the prescription pad can go beyond prescribing medications. Thank you for this insightful article.
Richard Cloyd
Dandridge, TN
I found Dr. Richard C. Christensen’s article (“‘Prescribing’ behavioral and lifestyle changes,” Current Psychiatry, July 2006) very useful. I am a therapist, but I think it is important for physicians to remember that the power of the prescription pad can go beyond prescribing medications. Thank you for this insightful article.
Richard Cloyd
Dandridge, TN
Hail to the chief
I’m glad to see Dr. Henry Nasrallah at the helm of Current Psychiatry. It is one of the few journals I read cover to cover. Although it does not publish original research, the journal interprets important studies like CATIE in a way that is interesting and useable.
Robert Karp, MD
Medical director, vice president
Maumee Valley Guidance Center
Defiance, OH
I’m glad to see Dr. Henry Nasrallah at the helm of Current Psychiatry. It is one of the few journals I read cover to cover. Although it does not publish original research, the journal interprets important studies like CATIE in a way that is interesting and useable.
Robert Karp, MD
Medical director, vice president
Maumee Valley Guidance Center
Defiance, OH
I’m glad to see Dr. Henry Nasrallah at the helm of Current Psychiatry. It is one of the few journals I read cover to cover. Although it does not publish original research, the journal interprets important studies like CATIE in a way that is interesting and useable.
Robert Karp, MD
Medical director, vice president
Maumee Valley Guidance Center
Defiance, OH
Clonazepam versus buspirone
“Cases That Test Your Skills: Is it anxiety, depression, or bipolar disorder?” (Current Psychiatry, August 2006) succinctly captures a common problem found in clinical practice. The authors’ methodical and meticulous teasing out of the differential diagnosis was based on clinical findings rather than a hunch. Various tables and the authors’ method to clarify the diagnosis and treat the patient effectively were helpful.
I am curious why the authors did not consider whether clonazepam or other benzodiazepines—with or without buspirone—would have resolved the patient’s anxiety faster than buspirone alone. According to the article, clonazepam had not been tried during the patient’s previous treatments and she did not have a history of substance abuse.
Vasudev N. Makhija, MD
Linden, NJ
Dr. Singh responds
Besides my preference to use nonbenzodiazepine drugs as a first-line treatment for anxiety disorders, medications like clonazepam1,2 act as antianxiety and mood stabilizing agents. If the patient had responded to clonazepam, it would not have been clear whether bipolar disorder or anxiety disorder was the correct diagnosis. We decided to use buspirone because it is an antianxiety agent that does not affect mood.
Tanvir Singh, MD
Assistant professor of psychiatry
University of Toledo, Health Science Campus
Toledo, OH
“Cases That Test Your Skills: Is it anxiety, depression, or bipolar disorder?” (Current Psychiatry, August 2006) succinctly captures a common problem found in clinical practice. The authors’ methodical and meticulous teasing out of the differential diagnosis was based on clinical findings rather than a hunch. Various tables and the authors’ method to clarify the diagnosis and treat the patient effectively were helpful.
I am curious why the authors did not consider whether clonazepam or other benzodiazepines—with or without buspirone—would have resolved the patient’s anxiety faster than buspirone alone. According to the article, clonazepam had not been tried during the patient’s previous treatments and she did not have a history of substance abuse.
Vasudev N. Makhija, MD
Linden, NJ
Dr. Singh responds
Besides my preference to use nonbenzodiazepine drugs as a first-line treatment for anxiety disorders, medications like clonazepam1,2 act as antianxiety and mood stabilizing agents. If the patient had responded to clonazepam, it would not have been clear whether bipolar disorder or anxiety disorder was the correct diagnosis. We decided to use buspirone because it is an antianxiety agent that does not affect mood.
Tanvir Singh, MD
Assistant professor of psychiatry
University of Toledo, Health Science Campus
Toledo, OH
“Cases That Test Your Skills: Is it anxiety, depression, or bipolar disorder?” (Current Psychiatry, August 2006) succinctly captures a common problem found in clinical practice. The authors’ methodical and meticulous teasing out of the differential diagnosis was based on clinical findings rather than a hunch. Various tables and the authors’ method to clarify the diagnosis and treat the patient effectively were helpful.
I am curious why the authors did not consider whether clonazepam or other benzodiazepines—with or without buspirone—would have resolved the patient’s anxiety faster than buspirone alone. According to the article, clonazepam had not been tried during the patient’s previous treatments and she did not have a history of substance abuse.
Vasudev N. Makhija, MD
Linden, NJ
Dr. Singh responds
Besides my preference to use nonbenzodiazepine drugs as a first-line treatment for anxiety disorders, medications like clonazepam1,2 act as antianxiety and mood stabilizing agents. If the patient had responded to clonazepam, it would not have been clear whether bipolar disorder or anxiety disorder was the correct diagnosis. We decided to use buspirone because it is an antianxiety agent that does not affect mood.
Tanvir Singh, MD
Assistant professor of psychiatry
University of Toledo, Health Science Campus
Toledo, OH
Protect yourself against patient assault
Wayne Fenton, MD, an associate director of the National Institute of Mental Health (NIMH), was murdered September 3—allegedly by a patient—in his Bethesda, MD, office. The case has led other mental health professionals to wonder how susceptible they are to assault and whether they are doing all they can to protect themselves.
To explore these safety issues, Current Psychiatry Deputy Editor Lois E. Krahn, MD, talked with John Battaglia, MD, medical director of the Program of Assertive Community Treatment (PACT) in Madison, WI.
Dr. Battaglia’s work takes him into the community to treat patients with severe chronic mental illnesses. The Madison PACT program uses an intensive, team-based approach for patients who have been inadequately treated in usual mental health services. Patients with complicated psychiatric, social, and legal problems are seen in their homes, at work, or on the streets in an assertive and comprehensive style of case management.
Dr. Krahn: Dr. Fenton’s death was a tremendous loss to the psychiatric community.
Dr. Battaglia: We were all shaken; my first reaction was horror and sadness.
Dr. Krahn: Dr. Fenton was a very experienced psychiatrist (Box 1). His murder makes us think about our own vulnerability and wonder if such an assault could happen to us.
Dr. Battaglia: Yes, it’s very common for psychiatrists or mental health providers to be assaulted (Box 2).
Dr. Fenton devoted his life to schizophrenia, through his compassion for those afflicted and his research that aided untold numbers of the mentally ill and their caregivers.
So it was especially sad that Dr. Fenton died while reaching out to a patient in need. On September 3, the NIMH associate director answered an urgent call to help a distressed, psychotic young man. A short time later, Dr. Fenton was found beaten to death at his Bethesda, MD, office.
Dr. Fenton was just 53 when he died, but his accomplishments were great. He joined NIMH in 1999, helping the organization find new treatments to enable schizophrenia patients to function in society. In this role, he galvanized colleagues nationwide to tackle the complex issue of difficult-to-treat schizophrenia. Before joining NIMH, Dr. Fenton was director and CEO of the Chestnut Lodge Hospital in Rockville, MD, where he did pivotal long-term studies of therapies for schizophrenia. From 2000 to 2005, he was deputy editor-in-chief of the journal Schizophrenia Bulletin. He served on numerous boards and in advocacy roles and won numerous awards.
In addition to these responsibilities, Dr. Fenton made time for his patients. And he gave his life, as he had lived it, trying to help. His obituary in the Washington Post included this quotation from Dr. Fenton, whom the newspaper interviewed in 2002:
All one has to do is walk through a downtown area to appreciate that the availability of adequate treatment for patients with schizophrenia and other mental illnesses is a serious problem for the country. We wouldn’t let our 80-year-old mother with Alzheimer’s live on a grate. Why is it all right for a 30-year-old daughter with schizophrenia?
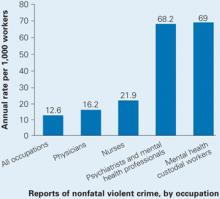
In one study, more than 50% of psychiatrists and 75% of mental health nurses reported experiencing an act or threat of violence within the past year.1
Dr. Krahn: Have you been assaulted by a patient?
Dr. Battaglia: Yes I have, and I think we need to define assault. A 15-year analysis of assaults on staff in a Massachusetts mental health system divided the acts into four types: physical, sexual, nonverbal threats/intimidation, and verbal assault.2 And you might think physical assault would be worse than verbal assaults. But a threat from a patient—especially one aimed toward your family—can leave you feeling vulnerable, stressed, and hypervigilant. Every sound at night makes you wonder if that person is coming after your family.
Dr. Krahn: What kinds of patients are associated with violence and assault?
Dr. Battaglia: The DSM-IV-TR diagnosis that comes up most often is schizophrenia, but it’s debatable whether diagnosis alone increases the risk of violence.
A study in Sweden published this year found a definite correlation between severe mental illness and violent crime. The authors concluded that about 5% of violent crimes in that country were committed by persons with severe mental illness.3
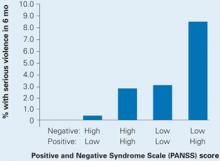
Also this year, a study of data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) found an increased risk of violence in schizophrenia patients with positive psychotic symptoms but a decreased risk in those with predominantly negative symptoms such as social withdrawal. Those with a combination of above-median positive and below-median negative symptoms were at highest risk for serious violence (Box 3).
Among a sample of 1,410 chronic schizophrenia patients enrolled in the NIMH-sponsored CATIE, 19% were involved in either minor or serious violent behavior in the past 6 months and 3.6% in serious violent behavior.4
Nobody argues that someone with schizophrenia is clearly at higher risk of becoming violent when in a high arousal state with positive symptoms or unpleasant delusions or hallucinations. A person with schizophrenia who is in an agitated, aroused psychotic state with active paranoid delusions and hallucinations is clearly at higher risk for committing violence.5,6 The patient who has been charged in the beating death of Dr. Fenton was a 19-year-old man with severe psychosis.
Dr. Krahn: Are there other disorders, such as bipolar mania, that are high risk for patient violence?
Dr. Battaglia: Acute manic states are higher risk.7 But, again, the diagnosis of bipolar disorder in and of itself does not show an increased incidence of violence. Personality disorders can be higher risk, as can nonspecific neurologic abnormalities, such as abnormal EEGs or neurologic “soft signs” by exam or testing.
Dr. Krahn: What about substance abuse?
Dr. Battaglia: The risk of violence is higher in patients who are under the influence of certain stimulants such as cocaine and methamphetamines, as opposed to marijuana or sedatives.8
Dr. Krahn: How can we predict whether a patient is at high risk for assault?
Dr. Battaglia: The best predictor is a history of violence, especially when the act was unprovoked or resulted in injury.9 A small number of patients is responsible for the majority of aggression. One study showed that recidivists committed 53% of all violent acts in a health care setting.10
Dr. Krahn: What if the patient’s history is unknown?
Dr. Battaglia: Most assaults in health care occur in high arousal states. Planned, methodical assaults are significantly less frequent. So, in the case of patients making threats against staff—let’s say you terminated your relationship with a patient and obtained a restraining order—very commonly that patient’s passion toward the clinic will wane over time.
Dr. Krahn: But not every arousal state results in assault.
Dr. Battaglia: Right. I have a colleague who says, “Risk factors make you worry more, and nothing makes you worry less.” That’s the attitude to have. Nothing should make you lower your antenna.
Source: U.S. Department of Justice, National Crime Victimization Survey, 1993 to 1999
Dr. Krahn: Is the risk higher with a new patient, or does it go down as you establish a relationship?
Dr. Battaglia: Clearly, untreated patients in high arousal states are a much greater risk. Does risk go down with somebody you’ve known for a while? I don’t know. My own experiences with assault have sometimes occurred with people I’ve grown to trust and when I let my guard down.
Dr. Krahn: So we might relax once we know the patient, but then we might be more vulnerable. Any clues that should put us on high alert?
Dr. Battaglia: The first clue—and this is going to sound obvious—is our internal, visceral, emotional sense of impending danger. In my experience, psychiatrists have a very good sense of that, but we override or don’t pay attention to it. Part of that inattention is an occupational hazard; we have to turn off our sense of danger again and again so that we can stay in situations that would repulse most people.
For instance, medical students with no psychiatric experience might sit in an interview with an agitated patient and feel an intense need to flee. Their antennae are telling them the situation looks dangerous. Seasoned psychiatrists, however, will calm themselves and stay through the interview. We are so used to being healers and helpers that we often turn off or dampen our sense of danger.
Dr. Krahn: Can you elaborate?
Dr. Battaglia: A nurse and I were with a patient who was highly agitated. He was labile; he was angry; he was spitting as he was speaking. In any other context, people would be keeping their distance because the signals were so powerful. Instead, the nurse leaned in, held his hand, and started telling him, “Come on now (Bob), you need to settle down. This is scaring us.”
That’s what I call the “leaning-in response.” We do that day in and day out. We turn off our danger signals in order to be therapeutic, and that makes us vulnerable.
Dr. Krahn: So, how do we keep our signals tuned?
Dr. Battaglia: When our senses are telling us we’re scared or we’re noticing a feeling of wanting to flee, we have to shift away from the goal of being therapeutic and focus on the goal of harm reduction. In assault cases, two clinician errors I see are:
- people had a sense that something was dangerous, but they ignored or dampened it
- people were passive when tension was mounting and didn’t abort an assault situation.
Anger is easy to recognize. Raised voice, inappropriate staring, clenched fists, agitation, and verbal threats are common before a violent episode. This seems self-evident, yet it’s surprising—even when these signs are obvious—that clinicians often took no de-escalation measures to ward off violence. A verbal threat is a red flag to prepare for violence.
Dr. Krahn: So, your senses are tingling. What do you do?
Dr. Battaglia: If the patient is threatening you and is in a negative affective arousal state that does not allow verbal redirection, you need to get away. Before you make your move, however, announce your behavior so that the patient will not interpret it as an attack (“Bob, I am standing up now because I need to leave the room”).
Schizophrenia symptoms associated with violent behavior
Schizophrenia patients with combined low negative and high positive PANSS scores were at highest risk to cause bodily injury or harm someone with a weapon in the past 6 months.
Dr. Krahn: Can that be a difficult call?
Dr. Battaglia: I think you learn when to shift gears. You undergo a number of incidents where you question yourself, and you go to an experienced colleague and say, “I was in a session with this patient. Here’s what I did. Do you think I was exposing myself unnecessarily?” Go over the incident in detail with someone who is supportive and understanding but also has a critical eye.
Dr. Krahn: Any suggestions as to how the room or other staff can be positioned to keep the risk as low as possible? Do you recommend alarms inside offices?
Dr. Battaglia: I think it’s smart to have an alarm system. And you need to think about the physical layout of the room ahead of time. You and the patient may need to have equal access to the door. If the patient is high-risk, you might want to arrange seating at a 90-degree angle rather than face-to-face to limit sustained confrontational eye contact. You might want to place your chair greater than an arm swing or leg kick away. You need to decide whether it’s safe to be alone, and whether to have the door open or to have security posted.
Dr. Krahn: What kind of training should staff be given?
Dr. Battaglia: Every office should have policies and protocols for handling behavioral emergencies. Who calls 911? What are each person’s responsibilities? Also, staff should be confident but not confrontational. That, in itself, may dissuade a patient from acting out.
Everyone should be taught de-escalation techniques. Body language can send threatening signals or they can signal a person that you’re not a threat and you’re going to work with them.
Dr. Krahn: Can you give an example where training might have helped?
Dr. Battaglia: I recently reviewed an incident where a nurse and a psychologist had a delusional, paranoid patient in their office and he wanted to leave. He was relapsed and clearly agitated; he was psychotic; he needed to be hospitalized. He wanted to escape, and they barred the door because they wanted to get him in the hospital.
The patient punched the nurse. If you bar someone’s escape, you’re very likely to get hurt. Let the patient go and call the police, who are trained to bring people in.
Dr. Krahn: What about building security? I know of a situation where a patient was found waiting for a psychiatrist in the parking garage. If there are threats, should an escort system be in place?
Dr. Battaglia: Security needs to work with the staff to come up with a plan.
Dr. Krahn: If someone in your office is assaulted, how do you handle the aftermath?
Dr. Battaglia: The person who is assaulted needs to get help. Crisis debriefing has been debated in trauma treatment, but there’s no debate about the benefit of “psychological first aid.” It provides an opportunity for the person to talk in confidence with another professional about what’s happened and how it may be affecting him or her.
Dr. Krahn: Can you continue to treat someone who has assaulted you?
Dr. Battaglia: That decision has to be made on a case-by-case basis. The main question is whether you feel safe enough to be therapeutic with the person in the future. Outside of a controlled setting, I don’t think you can effectively treat a patient you fear.
Dr. Krahn: Dr. Fenton’s death brings home that we need to be vigilant each day. We meet new patients every week, and any of them may have the disorders and risk factors that can lead to violence.
Dr. Battaglia: That’s true, yet being in a constant state of fear can impair mental health professionals’ ability to do our work. It’s a dynamic balance—we attempt a measured calmness in our work yet pay attention to external and visceral cues of impending danger.
Dr. Krahn: I think some psychiatrists feel patient violence occurs only in correctional settings or emergency rooms—not in their world. But Dr. Fenton’s death shows that it can happen anywhere. You just don’t know.
Related resources
- Joint Commission on Accreditation of HealthCare Organizations (JCAHO). Rules on application of seclusion and restraint. www.jointcommission.org.
Acknowledgment
This article was edited by Lynn Waltz, a medical writer and editor in Norfolk, VA, from the transcript of the September 29, 2006 interview of Dr. Battaglia by Dr. Krahn.
1. Nolan P, Dallender J, Soares J, et al. Violence in mental health care: the experiences of mental health nurses and psychiatrists. J Adv Nurs 1999;30:934-41.
2. Flannery RB, Jr, Juliano J, Cronin S, Walker AP. Characteristics of assaultive psychiatric patients: fifteen-year analysis of the Assaulted Staff Action Program (ASAP). Psychiatr Q 2006;77(3):239-49.
3. Fazel S, Grann M. The population impact of severe mental illness on violent crime. Am J Psychiatry 2006;163(8):1397-403.
4. Swanson JW, Swartz MS, Van Dorn RA, et al. A national study of violent behavior in persons with schizophrenia. Arch Gen Psychiatry 2006;63(5):490-9.
5. Cheung P, Schweitzer I, Crowley K, et al. Violence in schizophrenia: role of hallucinations and delusions. Schizophr Res 1997;26:181-90.
6. Binder R, McNiel D. Effects of diagnosis and context on dangerousness. Am J Psychiatry 1988;145:728-32.
7. Hyman S. The violent patient. In: Hyman S (ed). Manual of psychiatric emergencies. Boston: Little, Brown and Co, 1988;23-31.
8. Swartz M, Swanson J, Hiday V, et al. Violence and severe mental illness: the effects of substance abuse and nonadherence to medication. Am J Psychiatry 1998;155:226-31.
9. Convit A, Isay D, Otis D, et al. Characteristics of repeatedly assaultive psychiatric inpatients. Hosp Community Psychiatry 1990;41:1112-5.
10. Taylor P. Motives for offending among violent and psychotic men. Br J Psychiatry 1985;147:491-8.
Wayne Fenton, MD, an associate director of the National Institute of Mental Health (NIMH), was murdered September 3—allegedly by a patient—in his Bethesda, MD, office. The case has led other mental health professionals to wonder how susceptible they are to assault and whether they are doing all they can to protect themselves.
To explore these safety issues, Current Psychiatry Deputy Editor Lois E. Krahn, MD, talked with John Battaglia, MD, medical director of the Program of Assertive Community Treatment (PACT) in Madison, WI.
Dr. Battaglia’s work takes him into the community to treat patients with severe chronic mental illnesses. The Madison PACT program uses an intensive, team-based approach for patients who have been inadequately treated in usual mental health services. Patients with complicated psychiatric, social, and legal problems are seen in their homes, at work, or on the streets in an assertive and comprehensive style of case management.
Dr. Krahn: Dr. Fenton’s death was a tremendous loss to the psychiatric community.
Dr. Battaglia: We were all shaken; my first reaction was horror and sadness.
Dr. Krahn: Dr. Fenton was a very experienced psychiatrist (Box 1). His murder makes us think about our own vulnerability and wonder if such an assault could happen to us.
Dr. Battaglia: Yes, it’s very common for psychiatrists or mental health providers to be assaulted (Box 2).
Dr. Fenton devoted his life to schizophrenia, through his compassion for those afflicted and his research that aided untold numbers of the mentally ill and their caregivers.
So it was especially sad that Dr. Fenton died while reaching out to a patient in need. On September 3, the NIMH associate director answered an urgent call to help a distressed, psychotic young man. A short time later, Dr. Fenton was found beaten to death at his Bethesda, MD, office.
Dr. Fenton was just 53 when he died, but his accomplishments were great. He joined NIMH in 1999, helping the organization find new treatments to enable schizophrenia patients to function in society. In this role, he galvanized colleagues nationwide to tackle the complex issue of difficult-to-treat schizophrenia. Before joining NIMH, Dr. Fenton was director and CEO of the Chestnut Lodge Hospital in Rockville, MD, where he did pivotal long-term studies of therapies for schizophrenia. From 2000 to 2005, he was deputy editor-in-chief of the journal Schizophrenia Bulletin. He served on numerous boards and in advocacy roles and won numerous awards.
In addition to these responsibilities, Dr. Fenton made time for his patients. And he gave his life, as he had lived it, trying to help. His obituary in the Washington Post included this quotation from Dr. Fenton, whom the newspaper interviewed in 2002:
All one has to do is walk through a downtown area to appreciate that the availability of adequate treatment for patients with schizophrenia and other mental illnesses is a serious problem for the country. We wouldn’t let our 80-year-old mother with Alzheimer’s live on a grate. Why is it all right for a 30-year-old daughter with schizophrenia?
In one study, more than 50% of psychiatrists and 75% of mental health nurses reported experiencing an act or threat of violence within the past year.1
Dr. Krahn: Have you been assaulted by a patient?
Dr. Battaglia: Yes I have, and I think we need to define assault. A 15-year analysis of assaults on staff in a Massachusetts mental health system divided the acts into four types: physical, sexual, nonverbal threats/intimidation, and verbal assault.2 And you might think physical assault would be worse than verbal assaults. But a threat from a patient—especially one aimed toward your family—can leave you feeling vulnerable, stressed, and hypervigilant. Every sound at night makes you wonder if that person is coming after your family.
Dr. Krahn: What kinds of patients are associated with violence and assault?
Dr. Battaglia: The DSM-IV-TR diagnosis that comes up most often is schizophrenia, but it’s debatable whether diagnosis alone increases the risk of violence.
A study in Sweden published this year found a definite correlation between severe mental illness and violent crime. The authors concluded that about 5% of violent crimes in that country were committed by persons with severe mental illness.3
Also this year, a study of data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) found an increased risk of violence in schizophrenia patients with positive psychotic symptoms but a decreased risk in those with predominantly negative symptoms such as social withdrawal. Those with a combination of above-median positive and below-median negative symptoms were at highest risk for serious violence (Box 3).
Among a sample of 1,410 chronic schizophrenia patients enrolled in the NIMH-sponsored CATIE, 19% were involved in either minor or serious violent behavior in the past 6 months and 3.6% in serious violent behavior.4
Nobody argues that someone with schizophrenia is clearly at higher risk of becoming violent when in a high arousal state with positive symptoms or unpleasant delusions or hallucinations. A person with schizophrenia who is in an agitated, aroused psychotic state with active paranoid delusions and hallucinations is clearly at higher risk for committing violence.5,6 The patient who has been charged in the beating death of Dr. Fenton was a 19-year-old man with severe psychosis.
Dr. Krahn: Are there other disorders, such as bipolar mania, that are high risk for patient violence?
Dr. Battaglia: Acute manic states are higher risk.7 But, again, the diagnosis of bipolar disorder in and of itself does not show an increased incidence of violence. Personality disorders can be higher risk, as can nonspecific neurologic abnormalities, such as abnormal EEGs or neurologic “soft signs” by exam or testing.
Dr. Krahn: What about substance abuse?
Dr. Battaglia: The risk of violence is higher in patients who are under the influence of certain stimulants such as cocaine and methamphetamines, as opposed to marijuana or sedatives.8
Dr. Krahn: How can we predict whether a patient is at high risk for assault?
Dr. Battaglia: The best predictor is a history of violence, especially when the act was unprovoked or resulted in injury.9 A small number of patients is responsible for the majority of aggression. One study showed that recidivists committed 53% of all violent acts in a health care setting.10
Dr. Krahn: What if the patient’s history is unknown?
Dr. Battaglia: Most assaults in health care occur in high arousal states. Planned, methodical assaults are significantly less frequent. So, in the case of patients making threats against staff—let’s say you terminated your relationship with a patient and obtained a restraining order—very commonly that patient’s passion toward the clinic will wane over time.
Dr. Krahn: But not every arousal state results in assault.
Dr. Battaglia: Right. I have a colleague who says, “Risk factors make you worry more, and nothing makes you worry less.” That’s the attitude to have. Nothing should make you lower your antenna.
Source: U.S. Department of Justice, National Crime Victimization Survey, 1993 to 1999
Dr. Krahn: Is the risk higher with a new patient, or does it go down as you establish a relationship?
Dr. Battaglia: Clearly, untreated patients in high arousal states are a much greater risk. Does risk go down with somebody you’ve known for a while? I don’t know. My own experiences with assault have sometimes occurred with people I’ve grown to trust and when I let my guard down.
Dr. Krahn: So we might relax once we know the patient, but then we might be more vulnerable. Any clues that should put us on high alert?
Dr. Battaglia: The first clue—and this is going to sound obvious—is our internal, visceral, emotional sense of impending danger. In my experience, psychiatrists have a very good sense of that, but we override or don’t pay attention to it. Part of that inattention is an occupational hazard; we have to turn off our sense of danger again and again so that we can stay in situations that would repulse most people.
For instance, medical students with no psychiatric experience might sit in an interview with an agitated patient and feel an intense need to flee. Their antennae are telling them the situation looks dangerous. Seasoned psychiatrists, however, will calm themselves and stay through the interview. We are so used to being healers and helpers that we often turn off or dampen our sense of danger.
Dr. Krahn: Can you elaborate?
Dr. Battaglia: A nurse and I were with a patient who was highly agitated. He was labile; he was angry; he was spitting as he was speaking. In any other context, people would be keeping their distance because the signals were so powerful. Instead, the nurse leaned in, held his hand, and started telling him, “Come on now (Bob), you need to settle down. This is scaring us.”
That’s what I call the “leaning-in response.” We do that day in and day out. We turn off our danger signals in order to be therapeutic, and that makes us vulnerable.
Dr. Krahn: So, how do we keep our signals tuned?
Dr. Battaglia: When our senses are telling us we’re scared or we’re noticing a feeling of wanting to flee, we have to shift away from the goal of being therapeutic and focus on the goal of harm reduction. In assault cases, two clinician errors I see are:
- people had a sense that something was dangerous, but they ignored or dampened it
- people were passive when tension was mounting and didn’t abort an assault situation.
Anger is easy to recognize. Raised voice, inappropriate staring, clenched fists, agitation, and verbal threats are common before a violent episode. This seems self-evident, yet it’s surprising—even when these signs are obvious—that clinicians often took no de-escalation measures to ward off violence. A verbal threat is a red flag to prepare for violence.
Dr. Krahn: So, your senses are tingling. What do you do?
Dr. Battaglia: If the patient is threatening you and is in a negative affective arousal state that does not allow verbal redirection, you need to get away. Before you make your move, however, announce your behavior so that the patient will not interpret it as an attack (“Bob, I am standing up now because I need to leave the room”).
Schizophrenia symptoms associated with violent behavior
Schizophrenia patients with combined low negative and high positive PANSS scores were at highest risk to cause bodily injury or harm someone with a weapon in the past 6 months.
Dr. Krahn: Can that be a difficult call?
Dr. Battaglia: I think you learn when to shift gears. You undergo a number of incidents where you question yourself, and you go to an experienced colleague and say, “I was in a session with this patient. Here’s what I did. Do you think I was exposing myself unnecessarily?” Go over the incident in detail with someone who is supportive and understanding but also has a critical eye.
Dr. Krahn: Any suggestions as to how the room or other staff can be positioned to keep the risk as low as possible? Do you recommend alarms inside offices?
Dr. Battaglia: I think it’s smart to have an alarm system. And you need to think about the physical layout of the room ahead of time. You and the patient may need to have equal access to the door. If the patient is high-risk, you might want to arrange seating at a 90-degree angle rather than face-to-face to limit sustained confrontational eye contact. You might want to place your chair greater than an arm swing or leg kick away. You need to decide whether it’s safe to be alone, and whether to have the door open or to have security posted.
Dr. Krahn: What kind of training should staff be given?
Dr. Battaglia: Every office should have policies and protocols for handling behavioral emergencies. Who calls 911? What are each person’s responsibilities? Also, staff should be confident but not confrontational. That, in itself, may dissuade a patient from acting out.
Everyone should be taught de-escalation techniques. Body language can send threatening signals or they can signal a person that you’re not a threat and you’re going to work with them.
Dr. Krahn: Can you give an example where training might have helped?
Dr. Battaglia: I recently reviewed an incident where a nurse and a psychologist had a delusional, paranoid patient in their office and he wanted to leave. He was relapsed and clearly agitated; he was psychotic; he needed to be hospitalized. He wanted to escape, and they barred the door because they wanted to get him in the hospital.
The patient punched the nurse. If you bar someone’s escape, you’re very likely to get hurt. Let the patient go and call the police, who are trained to bring people in.
Dr. Krahn: What about building security? I know of a situation where a patient was found waiting for a psychiatrist in the parking garage. If there are threats, should an escort system be in place?
Dr. Battaglia: Security needs to work with the staff to come up with a plan.
Dr. Krahn: If someone in your office is assaulted, how do you handle the aftermath?
Dr. Battaglia: The person who is assaulted needs to get help. Crisis debriefing has been debated in trauma treatment, but there’s no debate about the benefit of “psychological first aid.” It provides an opportunity for the person to talk in confidence with another professional about what’s happened and how it may be affecting him or her.
Dr. Krahn: Can you continue to treat someone who has assaulted you?
Dr. Battaglia: That decision has to be made on a case-by-case basis. The main question is whether you feel safe enough to be therapeutic with the person in the future. Outside of a controlled setting, I don’t think you can effectively treat a patient you fear.
Dr. Krahn: Dr. Fenton’s death brings home that we need to be vigilant each day. We meet new patients every week, and any of them may have the disorders and risk factors that can lead to violence.
Dr. Battaglia: That’s true, yet being in a constant state of fear can impair mental health professionals’ ability to do our work. It’s a dynamic balance—we attempt a measured calmness in our work yet pay attention to external and visceral cues of impending danger.
Dr. Krahn: I think some psychiatrists feel patient violence occurs only in correctional settings or emergency rooms—not in their world. But Dr. Fenton’s death shows that it can happen anywhere. You just don’t know.
Related resources
- Joint Commission on Accreditation of HealthCare Organizations (JCAHO). Rules on application of seclusion and restraint. www.jointcommission.org.
Acknowledgment
This article was edited by Lynn Waltz, a medical writer and editor in Norfolk, VA, from the transcript of the September 29, 2006 interview of Dr. Battaglia by Dr. Krahn.
Wayne Fenton, MD, an associate director of the National Institute of Mental Health (NIMH), was murdered September 3—allegedly by a patient—in his Bethesda, MD, office. The case has led other mental health professionals to wonder how susceptible they are to assault and whether they are doing all they can to protect themselves.
To explore these safety issues, Current Psychiatry Deputy Editor Lois E. Krahn, MD, talked with John Battaglia, MD, medical director of the Program of Assertive Community Treatment (PACT) in Madison, WI.
Dr. Battaglia’s work takes him into the community to treat patients with severe chronic mental illnesses. The Madison PACT program uses an intensive, team-based approach for patients who have been inadequately treated in usual mental health services. Patients with complicated psychiatric, social, and legal problems are seen in their homes, at work, or on the streets in an assertive and comprehensive style of case management.
Dr. Krahn: Dr. Fenton’s death was a tremendous loss to the psychiatric community.
Dr. Battaglia: We were all shaken; my first reaction was horror and sadness.
Dr. Krahn: Dr. Fenton was a very experienced psychiatrist (Box 1). His murder makes us think about our own vulnerability and wonder if such an assault could happen to us.
Dr. Battaglia: Yes, it’s very common for psychiatrists or mental health providers to be assaulted (Box 2).
Dr. Fenton devoted his life to schizophrenia, through his compassion for those afflicted and his research that aided untold numbers of the mentally ill and their caregivers.
So it was especially sad that Dr. Fenton died while reaching out to a patient in need. On September 3, the NIMH associate director answered an urgent call to help a distressed, psychotic young man. A short time later, Dr. Fenton was found beaten to death at his Bethesda, MD, office.
Dr. Fenton was just 53 when he died, but his accomplishments were great. He joined NIMH in 1999, helping the organization find new treatments to enable schizophrenia patients to function in society. In this role, he galvanized colleagues nationwide to tackle the complex issue of difficult-to-treat schizophrenia. Before joining NIMH, Dr. Fenton was director and CEO of the Chestnut Lodge Hospital in Rockville, MD, where he did pivotal long-term studies of therapies for schizophrenia. From 2000 to 2005, he was deputy editor-in-chief of the journal Schizophrenia Bulletin. He served on numerous boards and in advocacy roles and won numerous awards.
In addition to these responsibilities, Dr. Fenton made time for his patients. And he gave his life, as he had lived it, trying to help. His obituary in the Washington Post included this quotation from Dr. Fenton, whom the newspaper interviewed in 2002:
All one has to do is walk through a downtown area to appreciate that the availability of adequate treatment for patients with schizophrenia and other mental illnesses is a serious problem for the country. We wouldn’t let our 80-year-old mother with Alzheimer’s live on a grate. Why is it all right for a 30-year-old daughter with schizophrenia?
In one study, more than 50% of psychiatrists and 75% of mental health nurses reported experiencing an act or threat of violence within the past year.1
Dr. Krahn: Have you been assaulted by a patient?
Dr. Battaglia: Yes I have, and I think we need to define assault. A 15-year analysis of assaults on staff in a Massachusetts mental health system divided the acts into four types: physical, sexual, nonverbal threats/intimidation, and verbal assault.2 And you might think physical assault would be worse than verbal assaults. But a threat from a patient—especially one aimed toward your family—can leave you feeling vulnerable, stressed, and hypervigilant. Every sound at night makes you wonder if that person is coming after your family.
Dr. Krahn: What kinds of patients are associated with violence and assault?
Dr. Battaglia: The DSM-IV-TR diagnosis that comes up most often is schizophrenia, but it’s debatable whether diagnosis alone increases the risk of violence.
A study in Sweden published this year found a definite correlation between severe mental illness and violent crime. The authors concluded that about 5% of violent crimes in that country were committed by persons with severe mental illness.3
Also this year, a study of data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) found an increased risk of violence in schizophrenia patients with positive psychotic symptoms but a decreased risk in those with predominantly negative symptoms such as social withdrawal. Those with a combination of above-median positive and below-median negative symptoms were at highest risk for serious violence (Box 3).
Among a sample of 1,410 chronic schizophrenia patients enrolled in the NIMH-sponsored CATIE, 19% were involved in either minor or serious violent behavior in the past 6 months and 3.6% in serious violent behavior.4
Nobody argues that someone with schizophrenia is clearly at higher risk of becoming violent when in a high arousal state with positive symptoms or unpleasant delusions or hallucinations. A person with schizophrenia who is in an agitated, aroused psychotic state with active paranoid delusions and hallucinations is clearly at higher risk for committing violence.5,6 The patient who has been charged in the beating death of Dr. Fenton was a 19-year-old man with severe psychosis.
Dr. Krahn: Are there other disorders, such as bipolar mania, that are high risk for patient violence?
Dr. Battaglia: Acute manic states are higher risk.7 But, again, the diagnosis of bipolar disorder in and of itself does not show an increased incidence of violence. Personality disorders can be higher risk, as can nonspecific neurologic abnormalities, such as abnormal EEGs or neurologic “soft signs” by exam or testing.
Dr. Krahn: What about substance abuse?
Dr. Battaglia: The risk of violence is higher in patients who are under the influence of certain stimulants such as cocaine and methamphetamines, as opposed to marijuana or sedatives.8
Dr. Krahn: How can we predict whether a patient is at high risk for assault?
Dr. Battaglia: The best predictor is a history of violence, especially when the act was unprovoked or resulted in injury.9 A small number of patients is responsible for the majority of aggression. One study showed that recidivists committed 53% of all violent acts in a health care setting.10
Dr. Krahn: What if the patient’s history is unknown?
Dr. Battaglia: Most assaults in health care occur in high arousal states. Planned, methodical assaults are significantly less frequent. So, in the case of patients making threats against staff—let’s say you terminated your relationship with a patient and obtained a restraining order—very commonly that patient’s passion toward the clinic will wane over time.
Dr. Krahn: But not every arousal state results in assault.
Dr. Battaglia: Right. I have a colleague who says, “Risk factors make you worry more, and nothing makes you worry less.” That’s the attitude to have. Nothing should make you lower your antenna.
Source: U.S. Department of Justice, National Crime Victimization Survey, 1993 to 1999
Dr. Krahn: Is the risk higher with a new patient, or does it go down as you establish a relationship?
Dr. Battaglia: Clearly, untreated patients in high arousal states are a much greater risk. Does risk go down with somebody you’ve known for a while? I don’t know. My own experiences with assault have sometimes occurred with people I’ve grown to trust and when I let my guard down.
Dr. Krahn: So we might relax once we know the patient, but then we might be more vulnerable. Any clues that should put us on high alert?
Dr. Battaglia: The first clue—and this is going to sound obvious—is our internal, visceral, emotional sense of impending danger. In my experience, psychiatrists have a very good sense of that, but we override or don’t pay attention to it. Part of that inattention is an occupational hazard; we have to turn off our sense of danger again and again so that we can stay in situations that would repulse most people.
For instance, medical students with no psychiatric experience might sit in an interview with an agitated patient and feel an intense need to flee. Their antennae are telling them the situation looks dangerous. Seasoned psychiatrists, however, will calm themselves and stay through the interview. We are so used to being healers and helpers that we often turn off or dampen our sense of danger.
Dr. Krahn: Can you elaborate?
Dr. Battaglia: A nurse and I were with a patient who was highly agitated. He was labile; he was angry; he was spitting as he was speaking. In any other context, people would be keeping their distance because the signals were so powerful. Instead, the nurse leaned in, held his hand, and started telling him, “Come on now (Bob), you need to settle down. This is scaring us.”
That’s what I call the “leaning-in response.” We do that day in and day out. We turn off our danger signals in order to be therapeutic, and that makes us vulnerable.
Dr. Krahn: So, how do we keep our signals tuned?
Dr. Battaglia: When our senses are telling us we’re scared or we’re noticing a feeling of wanting to flee, we have to shift away from the goal of being therapeutic and focus on the goal of harm reduction. In assault cases, two clinician errors I see are:
- people had a sense that something was dangerous, but they ignored or dampened it
- people were passive when tension was mounting and didn’t abort an assault situation.
Anger is easy to recognize. Raised voice, inappropriate staring, clenched fists, agitation, and verbal threats are common before a violent episode. This seems self-evident, yet it’s surprising—even when these signs are obvious—that clinicians often took no de-escalation measures to ward off violence. A verbal threat is a red flag to prepare for violence.
Dr. Krahn: So, your senses are tingling. What do you do?
Dr. Battaglia: If the patient is threatening you and is in a negative affective arousal state that does not allow verbal redirection, you need to get away. Before you make your move, however, announce your behavior so that the patient will not interpret it as an attack (“Bob, I am standing up now because I need to leave the room”).
Schizophrenia symptoms associated with violent behavior
Schizophrenia patients with combined low negative and high positive PANSS scores were at highest risk to cause bodily injury or harm someone with a weapon in the past 6 months.
Dr. Krahn: Can that be a difficult call?
Dr. Battaglia: I think you learn when to shift gears. You undergo a number of incidents where you question yourself, and you go to an experienced colleague and say, “I was in a session with this patient. Here’s what I did. Do you think I was exposing myself unnecessarily?” Go over the incident in detail with someone who is supportive and understanding but also has a critical eye.
Dr. Krahn: Any suggestions as to how the room or other staff can be positioned to keep the risk as low as possible? Do you recommend alarms inside offices?
Dr. Battaglia: I think it’s smart to have an alarm system. And you need to think about the physical layout of the room ahead of time. You and the patient may need to have equal access to the door. If the patient is high-risk, you might want to arrange seating at a 90-degree angle rather than face-to-face to limit sustained confrontational eye contact. You might want to place your chair greater than an arm swing or leg kick away. You need to decide whether it’s safe to be alone, and whether to have the door open or to have security posted.
Dr. Krahn: What kind of training should staff be given?
Dr. Battaglia: Every office should have policies and protocols for handling behavioral emergencies. Who calls 911? What are each person’s responsibilities? Also, staff should be confident but not confrontational. That, in itself, may dissuade a patient from acting out.
Everyone should be taught de-escalation techniques. Body language can send threatening signals or they can signal a person that you’re not a threat and you’re going to work with them.
Dr. Krahn: Can you give an example where training might have helped?
Dr. Battaglia: I recently reviewed an incident where a nurse and a psychologist had a delusional, paranoid patient in their office and he wanted to leave. He was relapsed and clearly agitated; he was psychotic; he needed to be hospitalized. He wanted to escape, and they barred the door because they wanted to get him in the hospital.
The patient punched the nurse. If you bar someone’s escape, you’re very likely to get hurt. Let the patient go and call the police, who are trained to bring people in.
Dr. Krahn: What about building security? I know of a situation where a patient was found waiting for a psychiatrist in the parking garage. If there are threats, should an escort system be in place?
Dr. Battaglia: Security needs to work with the staff to come up with a plan.
Dr. Krahn: If someone in your office is assaulted, how do you handle the aftermath?
Dr. Battaglia: The person who is assaulted needs to get help. Crisis debriefing has been debated in trauma treatment, but there’s no debate about the benefit of “psychological first aid.” It provides an opportunity for the person to talk in confidence with another professional about what’s happened and how it may be affecting him or her.
Dr. Krahn: Can you continue to treat someone who has assaulted you?
Dr. Battaglia: That decision has to be made on a case-by-case basis. The main question is whether you feel safe enough to be therapeutic with the person in the future. Outside of a controlled setting, I don’t think you can effectively treat a patient you fear.
Dr. Krahn: Dr. Fenton’s death brings home that we need to be vigilant each day. We meet new patients every week, and any of them may have the disorders and risk factors that can lead to violence.
Dr. Battaglia: That’s true, yet being in a constant state of fear can impair mental health professionals’ ability to do our work. It’s a dynamic balance—we attempt a measured calmness in our work yet pay attention to external and visceral cues of impending danger.
Dr. Krahn: I think some psychiatrists feel patient violence occurs only in correctional settings or emergency rooms—not in their world. But Dr. Fenton’s death shows that it can happen anywhere. You just don’t know.
Related resources
- Joint Commission on Accreditation of HealthCare Organizations (JCAHO). Rules on application of seclusion and restraint. www.jointcommission.org.
Acknowledgment
This article was edited by Lynn Waltz, a medical writer and editor in Norfolk, VA, from the transcript of the September 29, 2006 interview of Dr. Battaglia by Dr. Krahn.
1. Nolan P, Dallender J, Soares J, et al. Violence in mental health care: the experiences of mental health nurses and psychiatrists. J Adv Nurs 1999;30:934-41.
2. Flannery RB, Jr, Juliano J, Cronin S, Walker AP. Characteristics of assaultive psychiatric patients: fifteen-year analysis of the Assaulted Staff Action Program (ASAP). Psychiatr Q 2006;77(3):239-49.
3. Fazel S, Grann M. The population impact of severe mental illness on violent crime. Am J Psychiatry 2006;163(8):1397-403.
4. Swanson JW, Swartz MS, Van Dorn RA, et al. A national study of violent behavior in persons with schizophrenia. Arch Gen Psychiatry 2006;63(5):490-9.
5. Cheung P, Schweitzer I, Crowley K, et al. Violence in schizophrenia: role of hallucinations and delusions. Schizophr Res 1997;26:181-90.
6. Binder R, McNiel D. Effects of diagnosis and context on dangerousness. Am J Psychiatry 1988;145:728-32.
7. Hyman S. The violent patient. In: Hyman S (ed). Manual of psychiatric emergencies. Boston: Little, Brown and Co, 1988;23-31.
8. Swartz M, Swanson J, Hiday V, et al. Violence and severe mental illness: the effects of substance abuse and nonadherence to medication. Am J Psychiatry 1998;155:226-31.
9. Convit A, Isay D, Otis D, et al. Characteristics of repeatedly assaultive psychiatric inpatients. Hosp Community Psychiatry 1990;41:1112-5.
10. Taylor P. Motives for offending among violent and psychotic men. Br J Psychiatry 1985;147:491-8.
1. Nolan P, Dallender J, Soares J, et al. Violence in mental health care: the experiences of mental health nurses and psychiatrists. J Adv Nurs 1999;30:934-41.
2. Flannery RB, Jr, Juliano J, Cronin S, Walker AP. Characteristics of assaultive psychiatric patients: fifteen-year analysis of the Assaulted Staff Action Program (ASAP). Psychiatr Q 2006;77(3):239-49.
3. Fazel S, Grann M. The population impact of severe mental illness on violent crime. Am J Psychiatry 2006;163(8):1397-403.
4. Swanson JW, Swartz MS, Van Dorn RA, et al. A national study of violent behavior in persons with schizophrenia. Arch Gen Psychiatry 2006;63(5):490-9.
5. Cheung P, Schweitzer I, Crowley K, et al. Violence in schizophrenia: role of hallucinations and delusions. Schizophr Res 1997;26:181-90.
6. Binder R, McNiel D. Effects of diagnosis and context on dangerousness. Am J Psychiatry 1988;145:728-32.
7. Hyman S. The violent patient. In: Hyman S (ed). Manual of psychiatric emergencies. Boston: Little, Brown and Co, 1988;23-31.
8. Swartz M, Swanson J, Hiday V, et al. Violence and severe mental illness: the effects of substance abuse and nonadherence to medication. Am J Psychiatry 1998;155:226-31.
9. Convit A, Isay D, Otis D, et al. Characteristics of repeatedly assaultive psychiatric inpatients. Hosp Community Psychiatry 1990;41:1112-5.
10. Taylor P. Motives for offending among violent and psychotic men. Br J Psychiatry 1985;147:491-8.
Pathways of pleasure and pain: How well do you know dopamine?
How many dopamine functions do you know?
1 to 3: Fair
4 to 6: Good
7 to 9: Very Good
10 to 13: Outstanding
There is much more to dopamine than schizophrenia and Parkinson’s disease. Research has discovered a fascinating array of dopamine functions, some as triggers for neuropsychiatric disorders and others critical for healthy living.
Here’s a quiz; how many of the following functions of dopamine’s six receptors and four pathways do you know?
Motor coordination. Most of us take for granted moving or handling objects, thanks to normal dopamine levels in our nigrostriatal tracts. However, one look at a person afflicted with Parkinson’s disease trying to walk or hold a cup of coffee should make us grateful to dopamine for freedom of movement.
Abnormal vs. reality testing. Normal dopamine activity in the mesolimbic tract is vital to maintaining a healthy, adaptive awareness of our surroundings. Persons with abnormally heightened dopamine activity in the medial temporal region—for example, abusers of dopamine agonists such as methamphetamine (see "The 'meth' epidemic")—can be tormented by ideas of reference and delusions about neutral environmental stimuli.
Reward and pleasure. It is hard to imagine life without experiencing fun doing pleasurable activities. So give credit to dopamine in the ventral striatum for all the activities you enjoy, such as playing golf, watching an opera, or socializing with good friends. The dopamine receptor gene has been referred to as “the reward gene.”
Cognition and decision-making. Critical cognitive tasks such as memory, attention, and executive functions depend on an intact mesocortical dopamine pathway. Going to school or holding a job is impossible if mesocortical dopamine is deficient, which is why persons with schizophrenia become disabled.
Placebo effect. Psychiatrists appreciate the high placebo response rates associated with treating depression, anxiety, insomnia, or pain. Research implicates the release of dopamine in the reward pathways that probably are involved in alleviating psychiatric and physical symptoms. Even patients with Parkinson’s disease receiving sham deep brain stimulation or striatal tissue transplants in controlled clinical trials may improve initially because of dopamine release.
Depression. Dopamine is a monoamine that modulates mood, and a decrement in dopamine leads to dysphoria or major depression. Dopamine deficiency states such as Parkinson’s disease are frequently associated with depression and can be treated with dopaminergic antidepressants.
Social defeat. People—and animals as well—can develop permanent aversion to social contact if exposed to unrelenting abuse and violence. Mesolimbic dopamine pathways are implicated in this syndrome of “social defeat,” which can be reversed by chronic administration of antidepressants.
Alcohol dependence. Dopamine receptor dysfunction has been linked with a propensity to abuse alcohol and to reduced sensitivity to reward. The mesolimbic dopamine pathway appears to be critical for experiencing pleasure from abused drugs and is thought to be a common denominator for addictions and drug-seeking behavior.
Hypersexuality. When levodopa [L-dopa] was first used to treat Parkinson’s disease, one of its earliest side effects was increased sexual drive. This effect has been observed with other dopamine agonists and is attributed to potentiation of reward pathways that reinforce pleasurable activities. Other factors also may be involved, as in impulse control disorders.
Pathologic gambling. Treating Parkinson’s disease with dopamine agonists has been associated with an increase in pathologic gambling, an impulse control disorder. The incidence approaches 10%.
Compulsive shopping. Excessive, inappropriate buying is another impulse control disorder that emerges during dopamine agonist therapy.
ADHD. A cortical/frontal dopamine deficit is believed important in the pathophysiology of attention-deficit/hyperactivity disorder. ADHD symptoms can be alleviated with dopamine agonists (stimulants).
Dopamine can enhance health but also disrupt movement, mood, and behavior and lead to neuropsychiatric disorders. This neurotransmitter can reward or torment us. I invite you to send me e-mail ([email protected]) to comment on the 12 functions I’ve listed above or on other disorders you have observed that result from excessive or deficient dopamine activity.
P.S. Three days after I wrote this editorial, researchers announced another potential role for dopamine: regulating the sleep-wake cycle.1 Excess dopamine in mice allowed rapid-eye movement (REM) sleep (i.e., dreaming) to intrude on wakefulness! This may explain the dreamlike hallucinations in schizophrenia or L-dopa psychosis. Mice with depleted dopamine stopped having REM, even during sleep, but injecting them with D2 receptor agonists restored REM.
1. Dzirasa K, Ribeiro S, Costa R, et al. Dopaminergic control of sleep–wake states. J Neurosci 2006 Oct. 11;26:10577-89.
How many dopamine functions do you know?
1 to 3: Fair
4 to 6: Good
7 to 9: Very Good
10 to 13: Outstanding
There is much more to dopamine than schizophrenia and Parkinson’s disease. Research has discovered a fascinating array of dopamine functions, some as triggers for neuropsychiatric disorders and others critical for healthy living.
Here’s a quiz; how many of the following functions of dopamine’s six receptors and four pathways do you know?
Motor coordination. Most of us take for granted moving or handling objects, thanks to normal dopamine levels in our nigrostriatal tracts. However, one look at a person afflicted with Parkinson’s disease trying to walk or hold a cup of coffee should make us grateful to dopamine for freedom of movement.
Abnormal vs. reality testing. Normal dopamine activity in the mesolimbic tract is vital to maintaining a healthy, adaptive awareness of our surroundings. Persons with abnormally heightened dopamine activity in the medial temporal region—for example, abusers of dopamine agonists such as methamphetamine (see "The 'meth' epidemic")—can be tormented by ideas of reference and delusions about neutral environmental stimuli.
Reward and pleasure. It is hard to imagine life without experiencing fun doing pleasurable activities. So give credit to dopamine in the ventral striatum for all the activities you enjoy, such as playing golf, watching an opera, or socializing with good friends. The dopamine receptor gene has been referred to as “the reward gene.”
Cognition and decision-making. Critical cognitive tasks such as memory, attention, and executive functions depend on an intact mesocortical dopamine pathway. Going to school or holding a job is impossible if mesocortical dopamine is deficient, which is why persons with schizophrenia become disabled.
Placebo effect. Psychiatrists appreciate the high placebo response rates associated with treating depression, anxiety, insomnia, or pain. Research implicates the release of dopamine in the reward pathways that probably are involved in alleviating psychiatric and physical symptoms. Even patients with Parkinson’s disease receiving sham deep brain stimulation or striatal tissue transplants in controlled clinical trials may improve initially because of dopamine release.
Depression. Dopamine is a monoamine that modulates mood, and a decrement in dopamine leads to dysphoria or major depression. Dopamine deficiency states such as Parkinson’s disease are frequently associated with depression and can be treated with dopaminergic antidepressants.
Social defeat. People—and animals as well—can develop permanent aversion to social contact if exposed to unrelenting abuse and violence. Mesolimbic dopamine pathways are implicated in this syndrome of “social defeat,” which can be reversed by chronic administration of antidepressants.
Alcohol dependence. Dopamine receptor dysfunction has been linked with a propensity to abuse alcohol and to reduced sensitivity to reward. The mesolimbic dopamine pathway appears to be critical for experiencing pleasure from abused drugs and is thought to be a common denominator for addictions and drug-seeking behavior.
Hypersexuality. When levodopa [L-dopa] was first used to treat Parkinson’s disease, one of its earliest side effects was increased sexual drive. This effect has been observed with other dopamine agonists and is attributed to potentiation of reward pathways that reinforce pleasurable activities. Other factors also may be involved, as in impulse control disorders.
Pathologic gambling. Treating Parkinson’s disease with dopamine agonists has been associated with an increase in pathologic gambling, an impulse control disorder. The incidence approaches 10%.
Compulsive shopping. Excessive, inappropriate buying is another impulse control disorder that emerges during dopamine agonist therapy.
ADHD. A cortical/frontal dopamine deficit is believed important in the pathophysiology of attention-deficit/hyperactivity disorder. ADHD symptoms can be alleviated with dopamine agonists (stimulants).
Dopamine can enhance health but also disrupt movement, mood, and behavior and lead to neuropsychiatric disorders. This neurotransmitter can reward or torment us. I invite you to send me e-mail ([email protected]) to comment on the 12 functions I’ve listed above or on other disorders you have observed that result from excessive or deficient dopamine activity.
P.S. Three days after I wrote this editorial, researchers announced another potential role for dopamine: regulating the sleep-wake cycle.1 Excess dopamine in mice allowed rapid-eye movement (REM) sleep (i.e., dreaming) to intrude on wakefulness! This may explain the dreamlike hallucinations in schizophrenia or L-dopa psychosis. Mice with depleted dopamine stopped having REM, even during sleep, but injecting them with D2 receptor agonists restored REM.
How many dopamine functions do you know?
1 to 3: Fair
4 to 6: Good
7 to 9: Very Good
10 to 13: Outstanding
There is much more to dopamine than schizophrenia and Parkinson’s disease. Research has discovered a fascinating array of dopamine functions, some as triggers for neuropsychiatric disorders and others critical for healthy living.
Here’s a quiz; how many of the following functions of dopamine’s six receptors and four pathways do you know?
Motor coordination. Most of us take for granted moving or handling objects, thanks to normal dopamine levels in our nigrostriatal tracts. However, one look at a person afflicted with Parkinson’s disease trying to walk or hold a cup of coffee should make us grateful to dopamine for freedom of movement.
Abnormal vs. reality testing. Normal dopamine activity in the mesolimbic tract is vital to maintaining a healthy, adaptive awareness of our surroundings. Persons with abnormally heightened dopamine activity in the medial temporal region—for example, abusers of dopamine agonists such as methamphetamine (see "The 'meth' epidemic")—can be tormented by ideas of reference and delusions about neutral environmental stimuli.
Reward and pleasure. It is hard to imagine life without experiencing fun doing pleasurable activities. So give credit to dopamine in the ventral striatum for all the activities you enjoy, such as playing golf, watching an opera, or socializing with good friends. The dopamine receptor gene has been referred to as “the reward gene.”
Cognition and decision-making. Critical cognitive tasks such as memory, attention, and executive functions depend on an intact mesocortical dopamine pathway. Going to school or holding a job is impossible if mesocortical dopamine is deficient, which is why persons with schizophrenia become disabled.
Placebo effect. Psychiatrists appreciate the high placebo response rates associated with treating depression, anxiety, insomnia, or pain. Research implicates the release of dopamine in the reward pathways that probably are involved in alleviating psychiatric and physical symptoms. Even patients with Parkinson’s disease receiving sham deep brain stimulation or striatal tissue transplants in controlled clinical trials may improve initially because of dopamine release.
Depression. Dopamine is a monoamine that modulates mood, and a decrement in dopamine leads to dysphoria or major depression. Dopamine deficiency states such as Parkinson’s disease are frequently associated with depression and can be treated with dopaminergic antidepressants.
Social defeat. People—and animals as well—can develop permanent aversion to social contact if exposed to unrelenting abuse and violence. Mesolimbic dopamine pathways are implicated in this syndrome of “social defeat,” which can be reversed by chronic administration of antidepressants.
Alcohol dependence. Dopamine receptor dysfunction has been linked with a propensity to abuse alcohol and to reduced sensitivity to reward. The mesolimbic dopamine pathway appears to be critical for experiencing pleasure from abused drugs and is thought to be a common denominator for addictions and drug-seeking behavior.
Hypersexuality. When levodopa [L-dopa] was first used to treat Parkinson’s disease, one of its earliest side effects was increased sexual drive. This effect has been observed with other dopamine agonists and is attributed to potentiation of reward pathways that reinforce pleasurable activities. Other factors also may be involved, as in impulse control disorders.
Pathologic gambling. Treating Parkinson’s disease with dopamine agonists has been associated with an increase in pathologic gambling, an impulse control disorder. The incidence approaches 10%.
Compulsive shopping. Excessive, inappropriate buying is another impulse control disorder that emerges during dopamine agonist therapy.
ADHD. A cortical/frontal dopamine deficit is believed important in the pathophysiology of attention-deficit/hyperactivity disorder. ADHD symptoms can be alleviated with dopamine agonists (stimulants).
Dopamine can enhance health but also disrupt movement, mood, and behavior and lead to neuropsychiatric disorders. This neurotransmitter can reward or torment us. I invite you to send me e-mail ([email protected]) to comment on the 12 functions I’ve listed above or on other disorders you have observed that result from excessive or deficient dopamine activity.
P.S. Three days after I wrote this editorial, researchers announced another potential role for dopamine: regulating the sleep-wake cycle.1 Excess dopamine in mice allowed rapid-eye movement (REM) sleep (i.e., dreaming) to intrude on wakefulness! This may explain the dreamlike hallucinations in schizophrenia or L-dopa psychosis. Mice with depleted dopamine stopped having REM, even during sleep, but injecting them with D2 receptor agonists restored REM.
1. Dzirasa K, Ribeiro S, Costa R, et al. Dopaminergic control of sleep–wake states. J Neurosci 2006 Oct. 11;26:10577-89.
1. Dzirasa K, Ribeiro S, Costa R, et al. Dopaminergic control of sleep–wake states. J Neurosci 2006 Oct. 11;26:10577-89.
The consequences of sipping ‘tea’
History: a ‘negative’ view
Mr. J, a 50-year-old native of Fiji, has had depression and substance abuse disorder for more than 10 years, marked by irritability, poor sleep, hopelessness, and suicidality. He also suffered a traumatic brain injury in the military 25 years ago.
Police bring Mr. J to the ER after they find him wandering near traffic and speaking incoherently. His feet and hands jerk on the way to the hospital, leading police to suspect that Mr. J has suffered a grand mal seizure.
In the ER, Mr. J appears confused, has visual hallucinations, and moves his hands and feet involuntarily. His head and arms move erratically during the ER psychiatrist’s interview, and he says that his pelvis is arching forward and preventing him from walking steadily. The day before, he says, he saw frightening “visions” of a being who looked “like a photo negative.”
Mr. J has been seeing an outpatient psychiatrist, who has prescribed citalopram, 40 mg/d, for depression and clonazepam, 1 mg three times daily, for related anxiety symptoms.
The patient is disoriented and inattentive during the mental status examination. His cognitive deficits fluctuate in severity; at times he is aware of his surroundings, then suddenly loses this awareness.
Vital signs are stable. Physical exam shows Mr. J is approximately 30 lb underweight (97 lb) with a body mass index of 16.9 kg/m2—nearly 2 kg/m2 below normal. He says he has been skipping meals because of poor appetite. He also has strikingly lizard-like, scaly skin.
Urine drug screen shows no signs of recent alcohol or substance abuse. Complete metabolic profile shows elevated liver enzymes, suggesting alcohol or illicit substance toxicity, medication toxicity, hepatitis, thyroid disorder, muscle disease, or a rare liver condition. EEG shows mild encephalopathy but no ictal activity.
poll here
The authors’ observations
Our psychiatric differential diagnosis is broad:
- visual and auditory hallucinations are concurrent in numerous disorders, including schizophrenia and depression
- visual hallucinations alone suggest dementia, delirium, or psychosis resulting from a medical condition, medication, or substance(s) of abuse1
- Mr. J’s past head injury increases his risk of dementia and delirium
- his abrupt symptom onset and inattention suggest delirium.
Choreoathetosis can result from:
- medications such as stimulants and levodopa
- toxins
- systemic diseases such as systemic lupus erythematosus, thyrotoxicosis, or stroke
- degenerative brain diseases such as Huntington’s disease
- or focal brain diseases such as tumors.2
Although the test results narrow the differential diagnosis, we still have to consider numerous medical conditions that can cause delirium, such as trauma, cerebral vascular accident, intracerebral masses, CNS infection, and inflammatory disease.
poll here
History: collateral contributions
We refer Mr. J for lumbar puncture to rule out CNS infection and MRI to rule out tumor, abscess, or other structural brain abnormalities that could cause seizure. Results are unremarkable.
We then speak with Mr. J’s outpatient psychiatrist, who reports that Mr. J has had no residual cognitive impairment from his head injury. She adds, though, that he often develops cognitive problems after consuming large amounts of a traditional South Pacific beverage containing kava (Piper methysticum). She explains that Mr. J socializes with fellow Fijians who drink kava at gatherings, and that he often drinks kava to excess. She attributes his dry, scaly skin to excessive kava use.
Upon questioning, Mr. J says he consumes about a half-pound of kava root per day. He says he uses the root to make a tea-like beverage that, like alcohol, induces euphoria and relaxation. He says he began doing this in his youth back in Fiji, and now drinks “many cups” of kava per day.
Mr. J states that his current episode of strange movements and visual hallucinations began hours after he drank several cups of kava the day before police brought him to the ER. He considers his new psychiatric symptoms Jesus’ punishment for drinking kava.
The authors’ observations
Mr. J’s persecutory delusions suggest that he does not fully associate his symptoms with excessive kava use, but his abnormal movements, weight loss, skin changes, liver function abnormalities, and mental status changes are known adverse effects of kava.3 We diagnose substance-induced delirium rather than substance intoxication or substance-induced psychosis because:
- Mr. J’s cognitive symptoms are more severe than those caused by kava intoxication
- his psychotic symptoms occur only when he is delirious
- his disturbed consciousness, cognitive, and perceptual disturbances and the temporal relationship between symptom onset and massive kava use match DSM-IV-TR criteria for substance-induced delirium.4
Being aware of cultural customs and beliefs in your practice area can alert you to herbal substance use in various populations, such as kava by patients from the South Pacific or echinacea, goldenseal, and burdock by some Native Americans (see Related resources).
Medicinal use. Patients often use kava and other herbal supplements—including fatty acids, ginkgo biloba, ginseng, St. John’s wort, valerian, and others—with or instead of prescription drugs to alleviate psychiatric symptoms. Complementary and alternative medicine practitioners use kava to treat anxiety, for example (Box).
Anxiety and depression are among the most common reasons persons seek complementary or alternative treatment. In a national survey, 57% of respondents who suffered “anxiety attacks” and 54% of those with “severe depression” reported using such therapies.8 Nearly 1 in 5 persons who take prescription drugs also take herbs and/or high-dose vitamin supplements.9
Herbal products have been shown to cause adverse effects (Table 1).10 Kava, for example, has been associated with hepatotoxicity, dermopathy, movement disorders, GI disturbance, and weight loss. Standardized extracts such as capsules and tinctures appear more likely to cause adverse effects than traditional extractions, such as a beverage made by infusing kava root.5
Kava toxicity has been reported among heavy users. Although the dosage at which kava becomes dangerous is unknown, the FDA recommends that users not exceed typical dosages (50 to 280 mg/d) and use kava only under a physician’s supervision.11
Also, interactions between herbal products and allopathic medications can cause substantial morbidity (Table 2). St. John’s wort, for example, can lead to serotonin syndrome when combined with selective serotonin reuptake inhibitors (SSRIs)12 and can reduce blood levels of psychotropics metabolized by the cytochrome P-450 3A4 isoenzyme, such as alprazolam and carbamazepine.
Mr. J combined kava with clonazepam. Both substances affect gamma-aminobutyric (GABA) receptors, increasing the risk of sedation by depressing the CNS.
Table 1
Possible adverse effects of herbal supplements
used for psychiatric symptoms
| Medication | Psychiatric uses | Adverse effects |
|---|---|---|
| Fatty acids | Depression, mania | GI upset |
| 5-HTP (5-hydroxytryptophan) | Depression, anxiety | Agitation, ataxia, blurred vision, bradycardia, dyspnea, eosinophilia, headache, hypotension, insomnia, mania, psychosis, tremulousness |
| Ginkgo (Ginkgo biloba) | Cognitive enhancement | Bleeding, dizziness, GI upset, headache, palpitations, Stevens-Johnson syndrome |
| Ginseng (Panax ginseng) | Cognitive enhancement | Estrogenic effects, insomnia, mania |
| Kava (Piper methysticum) | Anxiety | Dermopathy, drowsiness, dry mouth, GI disturbance, hepatotoxicity, weight loss, movement disorders |
| SAM-e (S-adenosyl-L-methionine) | Depression, fibromyalgia | Constipation, diarrhea, increased salivation, headache, nausea, urinary frequency, mania in patients with bipolar disorder |
| St. John’s wort (Hypericum perforatum) | Depression | Anorexia, anorgasmia, anxiety, constipation, dizziness, dry mouth, fatigue, GI upset, mania, photosensitivity, pruritis, restlessness, urinary frequency |
| Valerian (Valeriana officinalis) | Insomnia | Drowsiness, GI upset, headache, hepatotoxicity |
| Source: Reference 10 | ||
Potential adverse interactions between psychotropics and complementary/alternative medications
| Herb | Interacts with… | Interaction can cause… |
|---|---|---|
| 5-HTP | carbidopa, MAOIs, SSRIs | delirium, serotonin syndrome |
| Ginseng | MAOIs | mania |
| Kava | first- and second-generation antipsychotics, benzodiazepines, MAOIs | sedation |
| SAM-E | TCAs | serotonin syndrome |
| St. John’s wort | benzodiazepines, beta blockers, buspirone, carbamazepine, clozapine, MAOIs, SSRIs, TCAs, trazodone | serotonin syndrome (w/SSRIs) |
| reduced plasma levels of cytochrome P-450 3A4 substrates, diminishing their effectiveness | ||
| Valerian | benzodiazepines | sedation |
| MAOIs: Monoamine oxidase inhibitors | ||
| SSRIs: Selective serotonin reuptake inhibitors | ||
| TCAs: Tricyclic antidepressants | ||
Kava, extracted from the roots of Piper methysticum, acts as a muscle relaxant, anesthetic, and anxiolytic.5 It is among the most commonly used alternative treatments for psychiatric symptoms, with sales estimated at $17 million in the United States in 2004.6
Kava lactones, the pharmacologically active components of kava, might act via several pathways, including GABA-A receptor binding and dopaminergic antagonism.7 This GABAergic CNS activity affects similar receptors as do benzodiazepines and produces kava’s anxiolytic effects.
Kava is available in health food stores as capsules, tinctures, and fluid extracts and can be obtained without a prescription. The amount of active ingredient varies greatly from preparation to preparation.
Ask about alternative medicine use
According to a national survey,13 many patients do not tell allopathic physicians they are using complementary or alternative medications because:
- “It wasn’t important for the doctor to know.”
- “The doctor never asked.”
- “It was none of the doctor’s business.”
- “The doctor would not understand.”
- or “The doctor would disapprove or discourage CAM use.”
- routinely question patients about use of alternative therapies
- discuss safety and efficacy of commonly used alternative treatments
- discuss merits of alternative treatments
- provide information on the effectiveness and risks of various treatments
- learn about alternative therapies by consulting the Physicians’ Desk Reference (PDR) for Herbal Medicines or similar references
- help patients make decisions about alternative treatments, such as finding a qualified, licensed alternative provider.
Treatment: quick resolution
We admit Mr. J to the inpatient psychiatry unit. There, we continue his outpatient prescription medications at the same dosages and block access to nonprescription substances. His symptoms begin to improve during the first day of hospitalization. His choreoathetosis, hallucinations, and confusion resolve within 48 hours, and he is medically stable.
We discharge Mr. J after 2 days and continue citalopram and clonazepam at the same dosages.
The authors’ observations
Kava reaches peak plasma levels 1.8 hours after oral dosing and has a short (9-hour) elimination half-life. As a result, kava intoxication symptoms tend to resolve rapidly, as in Mr. J’s case.
Although Mr. J is medically stable, liver damage associated with kava use can be irreversible, prompting some European countries to ban its sale. Make sure patients who report kava use are aware of its hepatotoxicity risk.
Follow-up: kicking the kava habit
Two weeks after Mr. J’s discharge, his psychiatrist notes that his mental status has returned to baseline and that his skin has improved dramatically. The patient is following his citalopram and clonazepam regimen, and he seems more aware of kava’s potential adverse effects.
Mr. J reports that he has not consumed kava since his hospitalization. He has been eating four meals per day and has gained 9 lb. He is pleased with his improved appetite and is motivated to continue abstaining from kava.
- National Center for Complementary and Alternative Medicine. http://nccam.nih.gov.
- Physicians Desk Reference (PDR) for herbal medicines, 3rd ed. Montvale, NJ: Thomson PDR; 2004.
- Ernst E, Pittler MH, Stevinson C, et al. The desktop guide to complementary and alternative medicine. Edinburgh, UK: Mosby; 2001.
- Alprazolam • Xanax
- Buspirone • BuSpar
- Carbamazepine • Equetro, others
- Carbidopa • Lodosyn
- Citalopram • Celexa
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Trazodone • Desyrel
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Moore DA, Jefferson J. Handbook of medical psychiatry, 2nd ed. New York: Elsevier/Mosby; 2004:8.
2. Nagagopal V. Movement disorders. In: Noble J, Greene HL, Levinson W, eds. Textbook of primary care medicine, 3rd ed. New York: Elsevier/Mosby; 2001:1530-1.
3. De Smet PA. Health risks of herbal remedies: an update. Clin Pharmacol Ther 2004;76:1-17.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000;143-5.
5. Whitton PA, Lau A, Salisbury J, et al. Kava lactones and the kavakava controversy. Phytochemistry 2003;64:673-9.
6. Nutrition Business Journal: Top 100 selling U.S. supplements sales & growth, 1999-2004. Available at: http://tdaf.nutrition4texas.org/meetings/files/2006TDA_Paul_Thomas_Top100.pdf. Accessed October 13, 2006.
7. Pittler MH, Ernst E. Efficacy of kava extract for treating anxiety: systematic review and meta-analysis. J Clin Psychopharmacol 2000;20:84-9.
8. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry 2001;158:289-94.
9. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA 1998;280:1569-75.
10. Werneke U, Turner T, Priebe S. Complementary medicines in psychiatry: review of effectiveness and safety. Br J Psychiatry 2006;188:109-21.
11. Medline Plus. Kava (Piper methysticum G. Forst). Available at: http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient-kava.html. Accessed October 13, 2006.
12. Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs 2001;61:2163-75.
13. Eisenberg DM, Kessler RC, Van Rompany MI, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med 2001;135:344-51.
14. Yager J, Siegfreid SL, DiMatteo TL. Use of alternative remedies by psychiatric patients: illustrative vignettes and a discussion of the issues. Am J Psychiatry 1999;156:1432-8.
History: a ‘negative’ view
Mr. J, a 50-year-old native of Fiji, has had depression and substance abuse disorder for more than 10 years, marked by irritability, poor sleep, hopelessness, and suicidality. He also suffered a traumatic brain injury in the military 25 years ago.
Police bring Mr. J to the ER after they find him wandering near traffic and speaking incoherently. His feet and hands jerk on the way to the hospital, leading police to suspect that Mr. J has suffered a grand mal seizure.
In the ER, Mr. J appears confused, has visual hallucinations, and moves his hands and feet involuntarily. His head and arms move erratically during the ER psychiatrist’s interview, and he says that his pelvis is arching forward and preventing him from walking steadily. The day before, he says, he saw frightening “visions” of a being who looked “like a photo negative.”
Mr. J has been seeing an outpatient psychiatrist, who has prescribed citalopram, 40 mg/d, for depression and clonazepam, 1 mg three times daily, for related anxiety symptoms.
The patient is disoriented and inattentive during the mental status examination. His cognitive deficits fluctuate in severity; at times he is aware of his surroundings, then suddenly loses this awareness.
Vital signs are stable. Physical exam shows Mr. J is approximately 30 lb underweight (97 lb) with a body mass index of 16.9 kg/m2—nearly 2 kg/m2 below normal. He says he has been skipping meals because of poor appetite. He also has strikingly lizard-like, scaly skin.
Urine drug screen shows no signs of recent alcohol or substance abuse. Complete metabolic profile shows elevated liver enzymes, suggesting alcohol or illicit substance toxicity, medication toxicity, hepatitis, thyroid disorder, muscle disease, or a rare liver condition. EEG shows mild encephalopathy but no ictal activity.
poll here
The authors’ observations
Our psychiatric differential diagnosis is broad:
- visual and auditory hallucinations are concurrent in numerous disorders, including schizophrenia and depression
- visual hallucinations alone suggest dementia, delirium, or psychosis resulting from a medical condition, medication, or substance(s) of abuse1
- Mr. J’s past head injury increases his risk of dementia and delirium
- his abrupt symptom onset and inattention suggest delirium.
Choreoathetosis can result from:
- medications such as stimulants and levodopa
- toxins
- systemic diseases such as systemic lupus erythematosus, thyrotoxicosis, or stroke
- degenerative brain diseases such as Huntington’s disease
- or focal brain diseases such as tumors.2
Although the test results narrow the differential diagnosis, we still have to consider numerous medical conditions that can cause delirium, such as trauma, cerebral vascular accident, intracerebral masses, CNS infection, and inflammatory disease.
poll here
History: collateral contributions
We refer Mr. J for lumbar puncture to rule out CNS infection and MRI to rule out tumor, abscess, or other structural brain abnormalities that could cause seizure. Results are unremarkable.
We then speak with Mr. J’s outpatient psychiatrist, who reports that Mr. J has had no residual cognitive impairment from his head injury. She adds, though, that he often develops cognitive problems after consuming large amounts of a traditional South Pacific beverage containing kava (Piper methysticum). She explains that Mr. J socializes with fellow Fijians who drink kava at gatherings, and that he often drinks kava to excess. She attributes his dry, scaly skin to excessive kava use.
Upon questioning, Mr. J says he consumes about a half-pound of kava root per day. He says he uses the root to make a tea-like beverage that, like alcohol, induces euphoria and relaxation. He says he began doing this in his youth back in Fiji, and now drinks “many cups” of kava per day.
Mr. J states that his current episode of strange movements and visual hallucinations began hours after he drank several cups of kava the day before police brought him to the ER. He considers his new psychiatric symptoms Jesus’ punishment for drinking kava.
The authors’ observations
Mr. J’s persecutory delusions suggest that he does not fully associate his symptoms with excessive kava use, but his abnormal movements, weight loss, skin changes, liver function abnormalities, and mental status changes are known adverse effects of kava.3 We diagnose substance-induced delirium rather than substance intoxication or substance-induced psychosis because:
- Mr. J’s cognitive symptoms are more severe than those caused by kava intoxication
- his psychotic symptoms occur only when he is delirious
- his disturbed consciousness, cognitive, and perceptual disturbances and the temporal relationship between symptom onset and massive kava use match DSM-IV-TR criteria for substance-induced delirium.4
Being aware of cultural customs and beliefs in your practice area can alert you to herbal substance use in various populations, such as kava by patients from the South Pacific or echinacea, goldenseal, and burdock by some Native Americans (see Related resources).
Medicinal use. Patients often use kava and other herbal supplements—including fatty acids, ginkgo biloba, ginseng, St. John’s wort, valerian, and others—with or instead of prescription drugs to alleviate psychiatric symptoms. Complementary and alternative medicine practitioners use kava to treat anxiety, for example (Box).
Anxiety and depression are among the most common reasons persons seek complementary or alternative treatment. In a national survey, 57% of respondents who suffered “anxiety attacks” and 54% of those with “severe depression” reported using such therapies.8 Nearly 1 in 5 persons who take prescription drugs also take herbs and/or high-dose vitamin supplements.9
Herbal products have been shown to cause adverse effects (Table 1).10 Kava, for example, has been associated with hepatotoxicity, dermopathy, movement disorders, GI disturbance, and weight loss. Standardized extracts such as capsules and tinctures appear more likely to cause adverse effects than traditional extractions, such as a beverage made by infusing kava root.5
Kava toxicity has been reported among heavy users. Although the dosage at which kava becomes dangerous is unknown, the FDA recommends that users not exceed typical dosages (50 to 280 mg/d) and use kava only under a physician’s supervision.11
Also, interactions between herbal products and allopathic medications can cause substantial morbidity (Table 2). St. John’s wort, for example, can lead to serotonin syndrome when combined with selective serotonin reuptake inhibitors (SSRIs)12 and can reduce blood levels of psychotropics metabolized by the cytochrome P-450 3A4 isoenzyme, such as alprazolam and carbamazepine.
Mr. J combined kava with clonazepam. Both substances affect gamma-aminobutyric (GABA) receptors, increasing the risk of sedation by depressing the CNS.
Table 1
Possible adverse effects of herbal supplements
used for psychiatric symptoms
| Medication | Psychiatric uses | Adverse effects |
|---|---|---|
| Fatty acids | Depression, mania | GI upset |
| 5-HTP (5-hydroxytryptophan) | Depression, anxiety | Agitation, ataxia, blurred vision, bradycardia, dyspnea, eosinophilia, headache, hypotension, insomnia, mania, psychosis, tremulousness |
| Ginkgo (Ginkgo biloba) | Cognitive enhancement | Bleeding, dizziness, GI upset, headache, palpitations, Stevens-Johnson syndrome |
| Ginseng (Panax ginseng) | Cognitive enhancement | Estrogenic effects, insomnia, mania |
| Kava (Piper methysticum) | Anxiety | Dermopathy, drowsiness, dry mouth, GI disturbance, hepatotoxicity, weight loss, movement disorders |
| SAM-e (S-adenosyl-L-methionine) | Depression, fibromyalgia | Constipation, diarrhea, increased salivation, headache, nausea, urinary frequency, mania in patients with bipolar disorder |
| St. John’s wort (Hypericum perforatum) | Depression | Anorexia, anorgasmia, anxiety, constipation, dizziness, dry mouth, fatigue, GI upset, mania, photosensitivity, pruritis, restlessness, urinary frequency |
| Valerian (Valeriana officinalis) | Insomnia | Drowsiness, GI upset, headache, hepatotoxicity |
| Source: Reference 10 | ||
Potential adverse interactions between psychotropics and complementary/alternative medications
| Herb | Interacts with… | Interaction can cause… |
|---|---|---|
| 5-HTP | carbidopa, MAOIs, SSRIs | delirium, serotonin syndrome |
| Ginseng | MAOIs | mania |
| Kava | first- and second-generation antipsychotics, benzodiazepines, MAOIs | sedation |
| SAM-E | TCAs | serotonin syndrome |
| St. John’s wort | benzodiazepines, beta blockers, buspirone, carbamazepine, clozapine, MAOIs, SSRIs, TCAs, trazodone | serotonin syndrome (w/SSRIs) |
| reduced plasma levels of cytochrome P-450 3A4 substrates, diminishing their effectiveness | ||
| Valerian | benzodiazepines | sedation |
| MAOIs: Monoamine oxidase inhibitors | ||
| SSRIs: Selective serotonin reuptake inhibitors | ||
| TCAs: Tricyclic antidepressants | ||
Kava, extracted from the roots of Piper methysticum, acts as a muscle relaxant, anesthetic, and anxiolytic.5 It is among the most commonly used alternative treatments for psychiatric symptoms, with sales estimated at $17 million in the United States in 2004.6
Kava lactones, the pharmacologically active components of kava, might act via several pathways, including GABA-A receptor binding and dopaminergic antagonism.7 This GABAergic CNS activity affects similar receptors as do benzodiazepines and produces kava’s anxiolytic effects.
Kava is available in health food stores as capsules, tinctures, and fluid extracts and can be obtained without a prescription. The amount of active ingredient varies greatly from preparation to preparation.
Ask about alternative medicine use
According to a national survey,13 many patients do not tell allopathic physicians they are using complementary or alternative medications because:
- “It wasn’t important for the doctor to know.”
- “The doctor never asked.”
- “It was none of the doctor’s business.”
- “The doctor would not understand.”
- or “The doctor would disapprove or discourage CAM use.”
- routinely question patients about use of alternative therapies
- discuss safety and efficacy of commonly used alternative treatments
- discuss merits of alternative treatments
- provide information on the effectiveness and risks of various treatments
- learn about alternative therapies by consulting the Physicians’ Desk Reference (PDR) for Herbal Medicines or similar references
- help patients make decisions about alternative treatments, such as finding a qualified, licensed alternative provider.
Treatment: quick resolution
We admit Mr. J to the inpatient psychiatry unit. There, we continue his outpatient prescription medications at the same dosages and block access to nonprescription substances. His symptoms begin to improve during the first day of hospitalization. His choreoathetosis, hallucinations, and confusion resolve within 48 hours, and he is medically stable.
We discharge Mr. J after 2 days and continue citalopram and clonazepam at the same dosages.
The authors’ observations
Kava reaches peak plasma levels 1.8 hours after oral dosing and has a short (9-hour) elimination half-life. As a result, kava intoxication symptoms tend to resolve rapidly, as in Mr. J’s case.
Although Mr. J is medically stable, liver damage associated with kava use can be irreversible, prompting some European countries to ban its sale. Make sure patients who report kava use are aware of its hepatotoxicity risk.
Follow-up: kicking the kava habit
Two weeks after Mr. J’s discharge, his psychiatrist notes that his mental status has returned to baseline and that his skin has improved dramatically. The patient is following his citalopram and clonazepam regimen, and he seems more aware of kava’s potential adverse effects.
Mr. J reports that he has not consumed kava since his hospitalization. He has been eating four meals per day and has gained 9 lb. He is pleased with his improved appetite and is motivated to continue abstaining from kava.
- National Center for Complementary and Alternative Medicine. http://nccam.nih.gov.
- Physicians Desk Reference (PDR) for herbal medicines, 3rd ed. Montvale, NJ: Thomson PDR; 2004.
- Ernst E, Pittler MH, Stevinson C, et al. The desktop guide to complementary and alternative medicine. Edinburgh, UK: Mosby; 2001.
- Alprazolam • Xanax
- Buspirone • BuSpar
- Carbamazepine • Equetro, others
- Carbidopa • Lodosyn
- Citalopram • Celexa
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Trazodone • Desyrel
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
History: a ‘negative’ view
Mr. J, a 50-year-old native of Fiji, has had depression and substance abuse disorder for more than 10 years, marked by irritability, poor sleep, hopelessness, and suicidality. He also suffered a traumatic brain injury in the military 25 years ago.
Police bring Mr. J to the ER after they find him wandering near traffic and speaking incoherently. His feet and hands jerk on the way to the hospital, leading police to suspect that Mr. J has suffered a grand mal seizure.
In the ER, Mr. J appears confused, has visual hallucinations, and moves his hands and feet involuntarily. His head and arms move erratically during the ER psychiatrist’s interview, and he says that his pelvis is arching forward and preventing him from walking steadily. The day before, he says, he saw frightening “visions” of a being who looked “like a photo negative.”
Mr. J has been seeing an outpatient psychiatrist, who has prescribed citalopram, 40 mg/d, for depression and clonazepam, 1 mg three times daily, for related anxiety symptoms.
The patient is disoriented and inattentive during the mental status examination. His cognitive deficits fluctuate in severity; at times he is aware of his surroundings, then suddenly loses this awareness.
Vital signs are stable. Physical exam shows Mr. J is approximately 30 lb underweight (97 lb) with a body mass index of 16.9 kg/m2—nearly 2 kg/m2 below normal. He says he has been skipping meals because of poor appetite. He also has strikingly lizard-like, scaly skin.
Urine drug screen shows no signs of recent alcohol or substance abuse. Complete metabolic profile shows elevated liver enzymes, suggesting alcohol or illicit substance toxicity, medication toxicity, hepatitis, thyroid disorder, muscle disease, or a rare liver condition. EEG shows mild encephalopathy but no ictal activity.
poll here
The authors’ observations
Our psychiatric differential diagnosis is broad:
- visual and auditory hallucinations are concurrent in numerous disorders, including schizophrenia and depression
- visual hallucinations alone suggest dementia, delirium, or psychosis resulting from a medical condition, medication, or substance(s) of abuse1
- Mr. J’s past head injury increases his risk of dementia and delirium
- his abrupt symptom onset and inattention suggest delirium.
Choreoathetosis can result from:
- medications such as stimulants and levodopa
- toxins
- systemic diseases such as systemic lupus erythematosus, thyrotoxicosis, or stroke
- degenerative brain diseases such as Huntington’s disease
- or focal brain diseases such as tumors.2
Although the test results narrow the differential diagnosis, we still have to consider numerous medical conditions that can cause delirium, such as trauma, cerebral vascular accident, intracerebral masses, CNS infection, and inflammatory disease.
poll here
History: collateral contributions
We refer Mr. J for lumbar puncture to rule out CNS infection and MRI to rule out tumor, abscess, or other structural brain abnormalities that could cause seizure. Results are unremarkable.
We then speak with Mr. J’s outpatient psychiatrist, who reports that Mr. J has had no residual cognitive impairment from his head injury. She adds, though, that he often develops cognitive problems after consuming large amounts of a traditional South Pacific beverage containing kava (Piper methysticum). She explains that Mr. J socializes with fellow Fijians who drink kava at gatherings, and that he often drinks kava to excess. She attributes his dry, scaly skin to excessive kava use.
Upon questioning, Mr. J says he consumes about a half-pound of kava root per day. He says he uses the root to make a tea-like beverage that, like alcohol, induces euphoria and relaxation. He says he began doing this in his youth back in Fiji, and now drinks “many cups” of kava per day.
Mr. J states that his current episode of strange movements and visual hallucinations began hours after he drank several cups of kava the day before police brought him to the ER. He considers his new psychiatric symptoms Jesus’ punishment for drinking kava.
The authors’ observations
Mr. J’s persecutory delusions suggest that he does not fully associate his symptoms with excessive kava use, but his abnormal movements, weight loss, skin changes, liver function abnormalities, and mental status changes are known adverse effects of kava.3 We diagnose substance-induced delirium rather than substance intoxication or substance-induced psychosis because:
- Mr. J’s cognitive symptoms are more severe than those caused by kava intoxication
- his psychotic symptoms occur only when he is delirious
- his disturbed consciousness, cognitive, and perceptual disturbances and the temporal relationship between symptom onset and massive kava use match DSM-IV-TR criteria for substance-induced delirium.4
Being aware of cultural customs and beliefs in your practice area can alert you to herbal substance use in various populations, such as kava by patients from the South Pacific or echinacea, goldenseal, and burdock by some Native Americans (see Related resources).
Medicinal use. Patients often use kava and other herbal supplements—including fatty acids, ginkgo biloba, ginseng, St. John’s wort, valerian, and others—with or instead of prescription drugs to alleviate psychiatric symptoms. Complementary and alternative medicine practitioners use kava to treat anxiety, for example (Box).
Anxiety and depression are among the most common reasons persons seek complementary or alternative treatment. In a national survey, 57% of respondents who suffered “anxiety attacks” and 54% of those with “severe depression” reported using such therapies.8 Nearly 1 in 5 persons who take prescription drugs also take herbs and/or high-dose vitamin supplements.9
Herbal products have been shown to cause adverse effects (Table 1).10 Kava, for example, has been associated with hepatotoxicity, dermopathy, movement disorders, GI disturbance, and weight loss. Standardized extracts such as capsules and tinctures appear more likely to cause adverse effects than traditional extractions, such as a beverage made by infusing kava root.5
Kava toxicity has been reported among heavy users. Although the dosage at which kava becomes dangerous is unknown, the FDA recommends that users not exceed typical dosages (50 to 280 mg/d) and use kava only under a physician’s supervision.11
Also, interactions between herbal products and allopathic medications can cause substantial morbidity (Table 2). St. John’s wort, for example, can lead to serotonin syndrome when combined with selective serotonin reuptake inhibitors (SSRIs)12 and can reduce blood levels of psychotropics metabolized by the cytochrome P-450 3A4 isoenzyme, such as alprazolam and carbamazepine.
Mr. J combined kava with clonazepam. Both substances affect gamma-aminobutyric (GABA) receptors, increasing the risk of sedation by depressing the CNS.
Table 1
Possible adverse effects of herbal supplements
used for psychiatric symptoms
| Medication | Psychiatric uses | Adverse effects |
|---|---|---|
| Fatty acids | Depression, mania | GI upset |
| 5-HTP (5-hydroxytryptophan) | Depression, anxiety | Agitation, ataxia, blurred vision, bradycardia, dyspnea, eosinophilia, headache, hypotension, insomnia, mania, psychosis, tremulousness |
| Ginkgo (Ginkgo biloba) | Cognitive enhancement | Bleeding, dizziness, GI upset, headache, palpitations, Stevens-Johnson syndrome |
| Ginseng (Panax ginseng) | Cognitive enhancement | Estrogenic effects, insomnia, mania |
| Kava (Piper methysticum) | Anxiety | Dermopathy, drowsiness, dry mouth, GI disturbance, hepatotoxicity, weight loss, movement disorders |
| SAM-e (S-adenosyl-L-methionine) | Depression, fibromyalgia | Constipation, diarrhea, increased salivation, headache, nausea, urinary frequency, mania in patients with bipolar disorder |
| St. John’s wort (Hypericum perforatum) | Depression | Anorexia, anorgasmia, anxiety, constipation, dizziness, dry mouth, fatigue, GI upset, mania, photosensitivity, pruritis, restlessness, urinary frequency |
| Valerian (Valeriana officinalis) | Insomnia | Drowsiness, GI upset, headache, hepatotoxicity |
| Source: Reference 10 | ||
Potential adverse interactions between psychotropics and complementary/alternative medications
| Herb | Interacts with… | Interaction can cause… |
|---|---|---|
| 5-HTP | carbidopa, MAOIs, SSRIs | delirium, serotonin syndrome |
| Ginseng | MAOIs | mania |
| Kava | first- and second-generation antipsychotics, benzodiazepines, MAOIs | sedation |
| SAM-E | TCAs | serotonin syndrome |
| St. John’s wort | benzodiazepines, beta blockers, buspirone, carbamazepine, clozapine, MAOIs, SSRIs, TCAs, trazodone | serotonin syndrome (w/SSRIs) |
| reduced plasma levels of cytochrome P-450 3A4 substrates, diminishing their effectiveness | ||
| Valerian | benzodiazepines | sedation |
| MAOIs: Monoamine oxidase inhibitors | ||
| SSRIs: Selective serotonin reuptake inhibitors | ||
| TCAs: Tricyclic antidepressants | ||
Kava, extracted from the roots of Piper methysticum, acts as a muscle relaxant, anesthetic, and anxiolytic.5 It is among the most commonly used alternative treatments for psychiatric symptoms, with sales estimated at $17 million in the United States in 2004.6
Kava lactones, the pharmacologically active components of kava, might act via several pathways, including GABA-A receptor binding and dopaminergic antagonism.7 This GABAergic CNS activity affects similar receptors as do benzodiazepines and produces kava’s anxiolytic effects.
Kava is available in health food stores as capsules, tinctures, and fluid extracts and can be obtained without a prescription. The amount of active ingredient varies greatly from preparation to preparation.
Ask about alternative medicine use
According to a national survey,13 many patients do not tell allopathic physicians they are using complementary or alternative medications because:
- “It wasn’t important for the doctor to know.”
- “The doctor never asked.”
- “It was none of the doctor’s business.”
- “The doctor would not understand.”
- or “The doctor would disapprove or discourage CAM use.”
- routinely question patients about use of alternative therapies
- discuss safety and efficacy of commonly used alternative treatments
- discuss merits of alternative treatments
- provide information on the effectiveness and risks of various treatments
- learn about alternative therapies by consulting the Physicians’ Desk Reference (PDR) for Herbal Medicines or similar references
- help patients make decisions about alternative treatments, such as finding a qualified, licensed alternative provider.
Treatment: quick resolution
We admit Mr. J to the inpatient psychiatry unit. There, we continue his outpatient prescription medications at the same dosages and block access to nonprescription substances. His symptoms begin to improve during the first day of hospitalization. His choreoathetosis, hallucinations, and confusion resolve within 48 hours, and he is medically stable.
We discharge Mr. J after 2 days and continue citalopram and clonazepam at the same dosages.
The authors’ observations
Kava reaches peak plasma levels 1.8 hours after oral dosing and has a short (9-hour) elimination half-life. As a result, kava intoxication symptoms tend to resolve rapidly, as in Mr. J’s case.
Although Mr. J is medically stable, liver damage associated with kava use can be irreversible, prompting some European countries to ban its sale. Make sure patients who report kava use are aware of its hepatotoxicity risk.
Follow-up: kicking the kava habit
Two weeks after Mr. J’s discharge, his psychiatrist notes that his mental status has returned to baseline and that his skin has improved dramatically. The patient is following his citalopram and clonazepam regimen, and he seems more aware of kava’s potential adverse effects.
Mr. J reports that he has not consumed kava since his hospitalization. He has been eating four meals per day and has gained 9 lb. He is pleased with his improved appetite and is motivated to continue abstaining from kava.
- National Center for Complementary and Alternative Medicine. http://nccam.nih.gov.
- Physicians Desk Reference (PDR) for herbal medicines, 3rd ed. Montvale, NJ: Thomson PDR; 2004.
- Ernst E, Pittler MH, Stevinson C, et al. The desktop guide to complementary and alternative medicine. Edinburgh, UK: Mosby; 2001.
- Alprazolam • Xanax
- Buspirone • BuSpar
- Carbamazepine • Equetro, others
- Carbidopa • Lodosyn
- Citalopram • Celexa
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Trazodone • Desyrel
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Moore DA, Jefferson J. Handbook of medical psychiatry, 2nd ed. New York: Elsevier/Mosby; 2004:8.
2. Nagagopal V. Movement disorders. In: Noble J, Greene HL, Levinson W, eds. Textbook of primary care medicine, 3rd ed. New York: Elsevier/Mosby; 2001:1530-1.
3. De Smet PA. Health risks of herbal remedies: an update. Clin Pharmacol Ther 2004;76:1-17.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000;143-5.
5. Whitton PA, Lau A, Salisbury J, et al. Kava lactones and the kavakava controversy. Phytochemistry 2003;64:673-9.
6. Nutrition Business Journal: Top 100 selling U.S. supplements sales & growth, 1999-2004. Available at: http://tdaf.nutrition4texas.org/meetings/files/2006TDA_Paul_Thomas_Top100.pdf. Accessed October 13, 2006.
7. Pittler MH, Ernst E. Efficacy of kava extract for treating anxiety: systematic review and meta-analysis. J Clin Psychopharmacol 2000;20:84-9.
8. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry 2001;158:289-94.
9. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA 1998;280:1569-75.
10. Werneke U, Turner T, Priebe S. Complementary medicines in psychiatry: review of effectiveness and safety. Br J Psychiatry 2006;188:109-21.
11. Medline Plus. Kava (Piper methysticum G. Forst). Available at: http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient-kava.html. Accessed October 13, 2006.
12. Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs 2001;61:2163-75.
13. Eisenberg DM, Kessler RC, Van Rompany MI, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med 2001;135:344-51.
14. Yager J, Siegfreid SL, DiMatteo TL. Use of alternative remedies by psychiatric patients: illustrative vignettes and a discussion of the issues. Am J Psychiatry 1999;156:1432-8.
1. Moore DA, Jefferson J. Handbook of medical psychiatry, 2nd ed. New York: Elsevier/Mosby; 2004:8.
2. Nagagopal V. Movement disorders. In: Noble J, Greene HL, Levinson W, eds. Textbook of primary care medicine, 3rd ed. New York: Elsevier/Mosby; 2001:1530-1.
3. De Smet PA. Health risks of herbal remedies: an update. Clin Pharmacol Ther 2004;76:1-17.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000;143-5.
5. Whitton PA, Lau A, Salisbury J, et al. Kava lactones and the kavakava controversy. Phytochemistry 2003;64:673-9.
6. Nutrition Business Journal: Top 100 selling U.S. supplements sales & growth, 1999-2004. Available at: http://tdaf.nutrition4texas.org/meetings/files/2006TDA_Paul_Thomas_Top100.pdf. Accessed October 13, 2006.
7. Pittler MH, Ernst E. Efficacy of kava extract for treating anxiety: systematic review and meta-analysis. J Clin Psychopharmacol 2000;20:84-9.
8. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry 2001;158:289-94.
9. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA 1998;280:1569-75.
10. Werneke U, Turner T, Priebe S. Complementary medicines in psychiatry: review of effectiveness and safety. Br J Psychiatry 2006;188:109-21.
11. Medline Plus. Kava (Piper methysticum G. Forst). Available at: http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient-kava.html. Accessed October 13, 2006.
12. Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs 2001;61:2163-75.
13. Eisenberg DM, Kessler RC, Van Rompany MI, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med 2001;135:344-51.
14. Yager J, Siegfreid SL, DiMatteo TL. Use of alternative remedies by psychiatric patients: illustrative vignettes and a discussion of the issues. Am J Psychiatry 1999;156:1432-8.
Identifying hypothyroidism’s psychiatric presentations
Hypothyroidism—even when occult or subclinical—can cause subtle or frank changes in energy, mood, anxiety level, or cognition. Some patients’ affective symptoms remit with thyroid hormone replacement or with antidepressants only after a euthyroid state is established.
To help you recognize hypothyroidism in patients presenting with psychiatric illnesses and provide effective treatment, this article describes:
- hypothyroidism’s signs and symptoms
- primary and subclinical hypothyroidism, thyroiditis, central hypothyroidism, and thyroid hormone resistance
- laboratory screening for thyroid dysfunction in patients with psychiatric symptoms.
Overlapping clinical signs
Thyroid hormone is required for the metabolic activity of every cell in the body. When patients experience symptoms related to abnormal functioning of the hypothalamic-pituitary-thyroid axis (Figure), psychiatrists often are the first professionals they consult.
Diagnosis of thyroid disorders is based on biochemical and clinical data (Box 1),1 which might not be congruent. Clinical symptoms of hypothyroidism, for example, are notoriously variable. Severe biochemical hypothyroidism may be associated with mild clinical symptoms, whereas mild biochemical hypothyroidism may be associated with severe symptoms.2
Patients with thyroid disturbance and psychiatric symptoms most often are diagnosed with a depressive-spectrum syndrome. Most common are:
- atypical depression (which may present as dysthymia)
- bipolar spectrum syndrome (including manic-depression, mixed mania, bipolar depression, rapid-cycling bipolar disorder, cyclothymia, and premenstrual syndromes)
- borderline personality disorder
- or psychotic disorder (typically paranoid psychosis).
Psychiatric symptoms of hypothyroidism (Table 1) are often prominent or even primary. Patients commonly show:
- impaired attention, concentration, learning, and memory
- psychomotor slowing
- and/or mental dullness.
Table 1
Hypothyroidism’s psychiatric signs and symptoms
| Cognitive changes | Impaired memory, psychomotor slowing, reduced attention span |
| Vegetative symptoms | Hypersomnia, sleep apnea, fatigue, lethargy, apathy, anergia, low libido |
| Mood changes | Depression, mood instability, mania, anxiety |
| Other | Psychosis |
Psychiatric patients—particularly those with mood disorders—are more likely to exhibit biochemical evidence of frank or subclinical hypothyroidism, hyperthyroidism, and autoimmune thyroiditis than the general population. In a study of 17,533 Americans,1 approximately 12% had thyroid abnormalities and 80% of these were hypothyroid. An additional 17% of women and 9% of men tested positive for antithyroid antibodies.
Biochemical hypothyroidism was defined as TSH >4.5 mIU/L, with low total T4, and subclinical hypothyroidism as TSH >4.5 mIU/L with normal T4 levels. Population studies and other data have led some endocrinologists to regard serum TSH levels >2.5 mIU/L as abnormally high.
Figure Thyroid hormone regulation: The hypothalamic-pituitary-thyroid axis
Thyroid-stimulating hormone (TSH, thyrotropin) released into circulation by the pituitary stimulates the thyroid to synthesize and release thyroid hormone, including triiodothyronine (T3) and thyroxine (T4). TSH is regulated by hypothalamic elaboration of thyrotropin-releasing hormone (TRH), which stimulates TSH release in a dose-dependent manner. Negative feedback from T3 and T4 downregulates TRH gene expression and restrains the pituitary from responding to TSH. Most T3—the active hormone—is formed in circulation or in target tissues by deiodination of T4 by selenium-containing enzymes.
Illustration for Current Psychiatry by Rich LaroccoThe degree of impairment may depend on the patient’s normal functional level. For example, a well-educated patient of mine with thyroiditis-related hypothyroidism reported word-finding difficulties. Instead of asking her husband to take a bottle of wine from the rack, she asked him to take a bottle of wine from the “thing.”
Loss of vitality, fatigue, lethargy, hypersomnia (especially if sleep apnea is present), and depressed mood also are commonly seen.
Depressive symptoms. Hypothyroid patients usually meet several criteria for a major depressive episode—such as concentration difficulties, lassitude, low libido, and sometimes pessimism or sadness—and symptoms improve after sustained thyroid hormone replacement therapy.3 Women with mild hypothyroidism who screen negative for a psychiatric syndrome show statistically significant mood improvement and improved verbal fluency after 6 months of levothyroxine replacement therapy.4
In some patients with no clear evidence of a biochemical or clinical thyroid disorder, mood symptoms nevertheless respond to thyroid hormone augmentation of antidepressants.5
Anxiety symptoms. Occasionally thyroid dysfunction is seen in patients with anxiety disorders, including panic disorder, agoraphobia, social phobia, performance anxiety, post-traumatic stress disorder, and generalized anxiety disorder.6
It may seem counterintuitive, but hypothyroidism is probably as common as hyperthyroidism in extremely anxious patients. Both hypoand hyperthyroidism are seen much more often in patients with panic-level anxiety than in the general population.
In a sample of 144 consecutive female psychiatric patients with a lifetime history of panic disorder and/or agoraphobia:
- 27% had a history of thyroid disorder
- 17% had hypothyroidism
- 8% had hyperthyroidism.7
Hypothyroid symptoms. Associated symptoms of hypothyroidism (Table 2) may include cold intolerance, lack of or reduced perspiration, dry skin, constipation, lethargy, psychomotor slowness, and subjective paresthesias and muscle pains. Edema is often present. The face typically is swollen or “puffy” in the morning, but by evening the lower legs (and not the face) are edematous.
Deep tendon reflexes usually relax slowly after initial stimulation. Vascular resistance is increased, but hypertension is not usual. Noradrenergic systems become more active in a compensatory, counter-regulatory manner; however, bradycardia—when present—is sometimes profound. Weight gain can occur but often is conspicuously absent.
Severe hypothyroidism presents with paradoxical tremendous agitation, paranoia, and aggressiveness. The skin is leathery, and facies are characteristically rough. Myxedema is fairly common, even in high-functioning patients. I have seen only one case of so-called “myxedema madness;” the female patient’s hyperarousal, yelling, cursing, grossly poor cognitive ability, and loosely conceived paranoid delusions are unforgettable.
Galactorrhea (related to hyperprolactinemia) can be a symptom of severe hypothyroidism, presumably from increased hypothalamic thyrotropin-releasing hormone (TRH) drive. TRH is the main known secretagogue for pituitary prolactin secretion. Infertility, oligomenorrhea, or amenorrhea could be part of the hypothyroid clinical picture.
Other symptoms. Macroglossia and hypertrophy of the uvula are possible; in a recent report, dysarthria resulting from these oral changes was the only presenting symptom of a hypothyroid man.8 Dysarthria promptly corrected after levothyroxine replacement.
Hypothyroidism is a primary cause of central sleep apnea caused by dysfunction of ventilatory control and/or reduction in airway aperture.
Table 2
Hypothyroidism’s other signs and symptoms
| Metabolic | Low basal body temperature/cool skin, diminished perspiration, weight gain or difficulty losing weight |
| Cardiovascular | Bradycardia, dizziness |
| Dermatologic | Dry, rough, or scaly skin; brittle nails; coarse or thinning hair (especially in women); pallor; dependent edema; myxedema/skin mucinosis (classically pretibial) |
| Digestive | Nausea, constipation, enlarged tongue |
| Reproductive | Oligomenorrhea, amenorrhea, infertility, miscarriage, delayed ejaculation |
| Musculoskeletal and peripheral nervous system | Muscle cramps, joint pain, paresthesias, numbness, weakness, reduced exercise tolerance, delayed ankle reflex |
| Sensory | Upper eyelid drooping, dysarthria, hoarseness, diminished hearing, diminished taste (hypogustia) |
Causes of thyroid disorders
Primary hypothyroidism results from thyroid gland failure. The many causes include iodine deficiency, but most cases of adult-onset or acquired hypothyroidism are attributed to autoimmune thyroiditis. In primary hypothyroidism, low serum T4 and/or T3 are seen in combination with elevated serum TSH. Circulating free T4 diminishes sooner than free T3 does as the body attempts to preserve active hormone levels. In turn, total T4 and T3 diminish sooner than free hormone concentrations. A compensatory increase in pituitary-elaborated TSH is seen as the brain and pituitary attempt to stimulate the thyroid to produce adequate thyroid hormone.
In subclinical hypothyroidism, circulating free thyroid hormone concentrations are within the normal laboratory range (typically in the lower range), but TSH is elevated. TSH concentrations >3.0 mIU/mL call for repeat or follow-up biochemistry and clinical correlation.
The decision to treat subclinical hypothyroidism with thyroid hormone replacement is less controversial in psychiatry than in endocrinology. Psychiatric patients with subclinical hypothyroidism—especially those with incomplete responses to psychotropic therapy—should usually be treated with thyroid hormone (Box 2).1
Free T3 levels in the lower 20% of the laboratory’s normal range are cause for pause in a patient with a mood or psychotic disorder and any of hypothyroidism’s clinical stigmata, even if thyroxine and TSH concentrations are normal.
Thyroiditis is characterized by thyroid gland inflammation. The thyroid may be painful or nonpainful, enlarged, fleshy, goitrous, normal in size, or atrophic and fibrotic (especially late in the course).
Postulated precipitants include viruses and other infectious agents, vaccines, iodine excess, lithium therapy (Box 3),9 tobacco smoke, environmental chemicals or toxins, irradiation, and—arguably—cortically-mediated (psychological) stress in vulnerable individuals.
Clinically, thyroiditis syndromes often have a long prodromal phase, wax and wane in severity, have an insidious and sometimes silent course, and can be serially associated with hyperthyroidism (especially early in the course), euthyroidism, or hypothyroidism (especially late in the course). Most thyroiditis syndromes appear to resolve spontaneously, but many become chronic or show evidence of subtle thyroid dysfunction years after the first occurrence or diagnosis.
Most presentations are nonspecific; symptoms may be limited to lethargy, fatigue, and depression. Increased antithyroid antibody titers have been linked with psychotic and depressive syndromes in borderline personality disorder.10
In early thyroiditis, thyroxine and triiodothyronine secretion is often elevated, with low or suppressed TSH. However, antithyroid antibody production is associated with a significantly increased risk of eventual subclinical or frank hypothyroidism. Permanent hypothyroidism develops eventually in at least one-half of women with histories of postpartum thyroiditis.11
Central hypothyroidism stems from TSH deficiency. Both pituitary thyrotrophic failure and hypothalamic failure—secondary and tertiary hypothyroidism, respectively—are considered central (or “secondary”). Hypothyroidism of pituitary and hypothalamic origins are lumped together as “central” because it is often very difficult to differentiate these pathologies.
Thyroid hormone resistance, in which end-organ or cellular resistance to thyroid hormone signals is seen, is an increasingly recognized syndrome in clinical medicine. The typical case is an euthyroid or hypothyroid individual with elevated T4 and T3 and nonsuppressed or even frankly elevated TSH. Inappropriately elevated TSH combined with high thyroid hormone levels also can be seen in TSH-secreting pituitary tumors, although the clinical picture in this case is one of hyperthyroidism.
Thyroid hormone resistance ranges from euthyroid and clinically transparent to profoundly hypothyroid, and different organs in the same patient may show different sensitivities to thyroid hormone. Clinical features vary, depending on the strength of thyroid hormone resistance.
Early emergence of resistance (as would be expected in someone with an inherited thyroid hormone receptor abnormality) leads to developmental problems, including:
- short stature
- mental or learning disabilities (including attention deficits).
In a study of 18 families with strong history of generalized resistance to thyroid hormone, 70% of affected children met diagnostic criteria for attention-deficit/hyperactivity disorder.12
Target psychiatric symptoms for prescribing replacement thyroid hormone are depression; mood cycling or instability; low energy, fatigue, or lethargy; cognitive impairment (Table 1); and psychosis, if present. Because these symptoms are not specific to thyroid dysfunction, institute thyroid hormone only when biochemical evidence of compromised or suboptimal thyroid function is also present.
Exceptions to this rule may include patients with target psychiatric symptoms and:
Patients taking lithium for mood stabilization will likely need supplemental thyroid hormone eventually because lithium is thyrotoxic. Also, patients with bipolar mood symptoms often have coexisting thyroid abnormalities, and giving supraphysiologic thyroid hormone dosages sometimes converts those who do not respond to mood stabilizers into responders.
Thyrotropin concentrations increase within 1 day after patients start taking lithium carbonate, but without commensurate increases in T3 or T4. More often, T3 and T4 concentrations decrease in the presence of lithium. Among 150 patients maintained on lithium for 10 years, hypothyroidism, autoimmune thyroiditis, or goiter developed at rates of 1.7%, 1.4%, and 2.1% per year, respectively. The study authors suggested that long-term lithium may increase the risk for hypothyroidism in women and in patients with thyroid autoimmunity.9
Laboratory screening
TSH and thyroid hormones. The basic thyroid screen is a combination of serum levels of TSH (“sensitive TSH”) and free thyroid hormones.
TSH has a circadian rhythm, with a nocturnal surge amounting to a 50% to 200% increase over daytime levels, beginning at around 6 to 8 PM. Peak TSH pulsatile activity and levels are seen after sleep begins—usually after midnight—with trough levels and fewest TSH pulses in late morning and early afternoon.13,14 This rhythm implies that the most accurate TSH measurement may be obtained by drawing blood in the morning before 9 AM. I have seen subclinical hypothyroidism missed when clinicians relied on afternoon TSH levels.
In general medicine, TSH alone frequently is used as a routine screening tool. In psychiatric practice, however, I recommend supplementing TSH with free T3 and free T4 because thyroid system dysfunction is frequent in psychiatric syndromes. If laboratory costs are a concern, free T4 with TSH usually suffices for initial screening.
Although the unbound, free fractions of T3 and T4 are of primary interest, total T4 and total T3 are necessary to assess the thyroid gland’s synthetic capacity. When clinical evidence suggests abnormal thyroid hormone function, order repeated or serial biochemical testing. Marginal biochemical results also mandate repeat thyroid function studies, expanded to include:
- total thyroid hormone concentrations (ideally T4 and T3)
- antithyroid antibodies
- serum cholesterol
- prolactin.
Antithyroid antibodies. Obtain antithyroglobulin and antithyroid microsomal antibody titers if:
- thyroid hormone indices are abnormal or marginal—in either direction
- or the patient now has, has had, or has a family history of autoimmune-mediated symptoms, such as lupus erythematosus or rheumatoid arthritis.
Negative or low antithyroid autoantibody titers do not rule out thyroiditis as a cause of hypothyroidism, as these titers are most likely to be generated during periods of active inflammation.
Many autoantibodies react with thyroid-related elements. Most clinical laboratories can quantify antibodies directed against thyroid peroxidase—also called antimicrosomal antibodies—and thyroglobulin. The presence of these antibodies is associated with thyroid inflammation and a risk of progression to thyroid failure and hypothyroidism.
Other laboratory findings of hypothyroidism include hypercholesterolemia, mild hyperprolactinemia, and types of anemia (including iron-deficiency anemia with low ferritin levels).
Related resources
- American Thyroid Association. www.thyroid.org.
- Dunn JT. Guarding our nation’s thyroid health. J Clin Endocrinol Metab 2001;872:486-8.
- Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med 2003;348:2646-55.
- Sullivan GM, Mann JJ, Oquendo MA, et al. Low cerebrospinal fluid transthyretin levels in depression: correlations with suicidal ideation and low serotonin function. Biol Psychiatry 2006;60(5):500-6.
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99.
2. Zulewski H, Muller B, Exer P, et al. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab 1997;82:771-6.
3. Gunnarsson T, Sjoberg S, Eriksson M, Nordin C. Depressive symptoms in hyothyroid disorder with some observations on biochemical correlates. Neuropsychobiology 2001;43:70-4.
4. Bono G, Fancellu R, Blandini F, Santor G, Mauri M. Cognitive and affective status in mild hypothyroidism and interactions with L-thyroxine treatment. Acta Neurol Scand 2004;110:59-66
5. Iosifescu DV. ‘Supercharge’ antidepressants by adding thyroid hormones: Why hormones help, and new data on SSRI augmentation. Current Psychiatry 2006;5(7):15-25.
6. Tancer ME, Stein MB, Gelernter CS, Uhde TW. The hypothalamic-pituitary-thyroid axis in social phobia. Am J Psychiatry 1990;147(7):929-33
7. Orenstein H, Peskind A, Raskind MA. Thyroid disorders in female psychiatric patients with panic disorder or agoraphobia. Am J Psychiatry 1988;145:1428-30.
8. Stollberger C, Finsterer J, Brand E, Tschabitscher D. Dysarthria as the leading symptom of hypothyroidism. Am J Otolaryngol 2001;22:70-2.
9. Bocchetta A, Mossa P, Velluzzi F, et al. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol 2001;21(6):594-8.
10. Geracioti TD, Kling MA, Post R, Gold PW. Antithyroid antibody-linked symptoms in borderline personality disorder. Endocrine 2003;21:153-8.
11. Premawardhana LD, Parkes AB, Ammari F, et al. Postpartum thyroiditis and long-term thyroid status: prognostic influence of thyroid peroxidase antibodies and ultrasound echogenicity. J Clin Endocrinol Metab 2000;85:71-5.
12. Hauser P, Zametkin AJ, Martinez P, et al. Attention deficithyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med 1993;328:997-1001.
13. Ghizzoni L, Mastorakos G, Ziveri M, et al. Interactions of leptin and thyrotropin 24-hour secretory profiles in short normal children. J Clin Endocrinol Metab 2001;86(5):2065-72.
14. Mantzoros CS, Ozata M, Negrao AB, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab 2001;86(7):3284-91.
15. Chopra IJ, Sabatino L. Nature and sources of circulating thyroid hormones. In: Braverman LE, Utiger RD, eds. The thyroid. A fundamental and clinical text. Philadelphia: Lippincott; 2000:120-35.
Hypothyroidism—even when occult or subclinical—can cause subtle or frank changes in energy, mood, anxiety level, or cognition. Some patients’ affective symptoms remit with thyroid hormone replacement or with antidepressants only after a euthyroid state is established.
To help you recognize hypothyroidism in patients presenting with psychiatric illnesses and provide effective treatment, this article describes:
- hypothyroidism’s signs and symptoms
- primary and subclinical hypothyroidism, thyroiditis, central hypothyroidism, and thyroid hormone resistance
- laboratory screening for thyroid dysfunction in patients with psychiatric symptoms.
Overlapping clinical signs
Thyroid hormone is required for the metabolic activity of every cell in the body. When patients experience symptoms related to abnormal functioning of the hypothalamic-pituitary-thyroid axis (Figure), psychiatrists often are the first professionals they consult.
Diagnosis of thyroid disorders is based on biochemical and clinical data (Box 1),1 which might not be congruent. Clinical symptoms of hypothyroidism, for example, are notoriously variable. Severe biochemical hypothyroidism may be associated with mild clinical symptoms, whereas mild biochemical hypothyroidism may be associated with severe symptoms.2
Patients with thyroid disturbance and psychiatric symptoms most often are diagnosed with a depressive-spectrum syndrome. Most common are:
- atypical depression (which may present as dysthymia)
- bipolar spectrum syndrome (including manic-depression, mixed mania, bipolar depression, rapid-cycling bipolar disorder, cyclothymia, and premenstrual syndromes)
- borderline personality disorder
- or psychotic disorder (typically paranoid psychosis).
Psychiatric symptoms of hypothyroidism (Table 1) are often prominent or even primary. Patients commonly show:
- impaired attention, concentration, learning, and memory
- psychomotor slowing
- and/or mental dullness.
Table 1
Hypothyroidism’s psychiatric signs and symptoms
| Cognitive changes | Impaired memory, psychomotor slowing, reduced attention span |
| Vegetative symptoms | Hypersomnia, sleep apnea, fatigue, lethargy, apathy, anergia, low libido |
| Mood changes | Depression, mood instability, mania, anxiety |
| Other | Psychosis |
Psychiatric patients—particularly those with mood disorders—are more likely to exhibit biochemical evidence of frank or subclinical hypothyroidism, hyperthyroidism, and autoimmune thyroiditis than the general population. In a study of 17,533 Americans,1 approximately 12% had thyroid abnormalities and 80% of these were hypothyroid. An additional 17% of women and 9% of men tested positive for antithyroid antibodies.
Biochemical hypothyroidism was defined as TSH >4.5 mIU/L, with low total T4, and subclinical hypothyroidism as TSH >4.5 mIU/L with normal T4 levels. Population studies and other data have led some endocrinologists to regard serum TSH levels >2.5 mIU/L as abnormally high.
Figure Thyroid hormone regulation: The hypothalamic-pituitary-thyroid axis
Thyroid-stimulating hormone (TSH, thyrotropin) released into circulation by the pituitary stimulates the thyroid to synthesize and release thyroid hormone, including triiodothyronine (T3) and thyroxine (T4). TSH is regulated by hypothalamic elaboration of thyrotropin-releasing hormone (TRH), which stimulates TSH release in a dose-dependent manner. Negative feedback from T3 and T4 downregulates TRH gene expression and restrains the pituitary from responding to TSH. Most T3—the active hormone—is formed in circulation or in target tissues by deiodination of T4 by selenium-containing enzymes.
Illustration for Current Psychiatry by Rich LaroccoThe degree of impairment may depend on the patient’s normal functional level. For example, a well-educated patient of mine with thyroiditis-related hypothyroidism reported word-finding difficulties. Instead of asking her husband to take a bottle of wine from the rack, she asked him to take a bottle of wine from the “thing.”
Loss of vitality, fatigue, lethargy, hypersomnia (especially if sleep apnea is present), and depressed mood also are commonly seen.
Depressive symptoms. Hypothyroid patients usually meet several criteria for a major depressive episode—such as concentration difficulties, lassitude, low libido, and sometimes pessimism or sadness—and symptoms improve after sustained thyroid hormone replacement therapy.3 Women with mild hypothyroidism who screen negative for a psychiatric syndrome show statistically significant mood improvement and improved verbal fluency after 6 months of levothyroxine replacement therapy.4
In some patients with no clear evidence of a biochemical or clinical thyroid disorder, mood symptoms nevertheless respond to thyroid hormone augmentation of antidepressants.5
Anxiety symptoms. Occasionally thyroid dysfunction is seen in patients with anxiety disorders, including panic disorder, agoraphobia, social phobia, performance anxiety, post-traumatic stress disorder, and generalized anxiety disorder.6
It may seem counterintuitive, but hypothyroidism is probably as common as hyperthyroidism in extremely anxious patients. Both hypoand hyperthyroidism are seen much more often in patients with panic-level anxiety than in the general population.
In a sample of 144 consecutive female psychiatric patients with a lifetime history of panic disorder and/or agoraphobia:
- 27% had a history of thyroid disorder
- 17% had hypothyroidism
- 8% had hyperthyroidism.7
Hypothyroid symptoms. Associated symptoms of hypothyroidism (Table 2) may include cold intolerance, lack of or reduced perspiration, dry skin, constipation, lethargy, psychomotor slowness, and subjective paresthesias and muscle pains. Edema is often present. The face typically is swollen or “puffy” in the morning, but by evening the lower legs (and not the face) are edematous.
Deep tendon reflexes usually relax slowly after initial stimulation. Vascular resistance is increased, but hypertension is not usual. Noradrenergic systems become more active in a compensatory, counter-regulatory manner; however, bradycardia—when present—is sometimes profound. Weight gain can occur but often is conspicuously absent.
Severe hypothyroidism presents with paradoxical tremendous agitation, paranoia, and aggressiveness. The skin is leathery, and facies are characteristically rough. Myxedema is fairly common, even in high-functioning patients. I have seen only one case of so-called “myxedema madness;” the female patient’s hyperarousal, yelling, cursing, grossly poor cognitive ability, and loosely conceived paranoid delusions are unforgettable.
Galactorrhea (related to hyperprolactinemia) can be a symptom of severe hypothyroidism, presumably from increased hypothalamic thyrotropin-releasing hormone (TRH) drive. TRH is the main known secretagogue for pituitary prolactin secretion. Infertility, oligomenorrhea, or amenorrhea could be part of the hypothyroid clinical picture.
Other symptoms. Macroglossia and hypertrophy of the uvula are possible; in a recent report, dysarthria resulting from these oral changes was the only presenting symptom of a hypothyroid man.8 Dysarthria promptly corrected after levothyroxine replacement.
Hypothyroidism is a primary cause of central sleep apnea caused by dysfunction of ventilatory control and/or reduction in airway aperture.
Table 2
Hypothyroidism’s other signs and symptoms
| Metabolic | Low basal body temperature/cool skin, diminished perspiration, weight gain or difficulty losing weight |
| Cardiovascular | Bradycardia, dizziness |
| Dermatologic | Dry, rough, or scaly skin; brittle nails; coarse or thinning hair (especially in women); pallor; dependent edema; myxedema/skin mucinosis (classically pretibial) |
| Digestive | Nausea, constipation, enlarged tongue |
| Reproductive | Oligomenorrhea, amenorrhea, infertility, miscarriage, delayed ejaculation |
| Musculoskeletal and peripheral nervous system | Muscle cramps, joint pain, paresthesias, numbness, weakness, reduced exercise tolerance, delayed ankle reflex |
| Sensory | Upper eyelid drooping, dysarthria, hoarseness, diminished hearing, diminished taste (hypogustia) |
Causes of thyroid disorders
Primary hypothyroidism results from thyroid gland failure. The many causes include iodine deficiency, but most cases of adult-onset or acquired hypothyroidism are attributed to autoimmune thyroiditis. In primary hypothyroidism, low serum T4 and/or T3 are seen in combination with elevated serum TSH. Circulating free T4 diminishes sooner than free T3 does as the body attempts to preserve active hormone levels. In turn, total T4 and T3 diminish sooner than free hormone concentrations. A compensatory increase in pituitary-elaborated TSH is seen as the brain and pituitary attempt to stimulate the thyroid to produce adequate thyroid hormone.
In subclinical hypothyroidism, circulating free thyroid hormone concentrations are within the normal laboratory range (typically in the lower range), but TSH is elevated. TSH concentrations >3.0 mIU/mL call for repeat or follow-up biochemistry and clinical correlation.
The decision to treat subclinical hypothyroidism with thyroid hormone replacement is less controversial in psychiatry than in endocrinology. Psychiatric patients with subclinical hypothyroidism—especially those with incomplete responses to psychotropic therapy—should usually be treated with thyroid hormone (Box 2).1
Free T3 levels in the lower 20% of the laboratory’s normal range are cause for pause in a patient with a mood or psychotic disorder and any of hypothyroidism’s clinical stigmata, even if thyroxine and TSH concentrations are normal.
Thyroiditis is characterized by thyroid gland inflammation. The thyroid may be painful or nonpainful, enlarged, fleshy, goitrous, normal in size, or atrophic and fibrotic (especially late in the course).
Postulated precipitants include viruses and other infectious agents, vaccines, iodine excess, lithium therapy (Box 3),9 tobacco smoke, environmental chemicals or toxins, irradiation, and—arguably—cortically-mediated (psychological) stress in vulnerable individuals.
Clinically, thyroiditis syndromes often have a long prodromal phase, wax and wane in severity, have an insidious and sometimes silent course, and can be serially associated with hyperthyroidism (especially early in the course), euthyroidism, or hypothyroidism (especially late in the course). Most thyroiditis syndromes appear to resolve spontaneously, but many become chronic or show evidence of subtle thyroid dysfunction years after the first occurrence or diagnosis.
Most presentations are nonspecific; symptoms may be limited to lethargy, fatigue, and depression. Increased antithyroid antibody titers have been linked with psychotic and depressive syndromes in borderline personality disorder.10
In early thyroiditis, thyroxine and triiodothyronine secretion is often elevated, with low or suppressed TSH. However, antithyroid antibody production is associated with a significantly increased risk of eventual subclinical or frank hypothyroidism. Permanent hypothyroidism develops eventually in at least one-half of women with histories of postpartum thyroiditis.11
Central hypothyroidism stems from TSH deficiency. Both pituitary thyrotrophic failure and hypothalamic failure—secondary and tertiary hypothyroidism, respectively—are considered central (or “secondary”). Hypothyroidism of pituitary and hypothalamic origins are lumped together as “central” because it is often very difficult to differentiate these pathologies.
Thyroid hormone resistance, in which end-organ or cellular resistance to thyroid hormone signals is seen, is an increasingly recognized syndrome in clinical medicine. The typical case is an euthyroid or hypothyroid individual with elevated T4 and T3 and nonsuppressed or even frankly elevated TSH. Inappropriately elevated TSH combined with high thyroid hormone levels also can be seen in TSH-secreting pituitary tumors, although the clinical picture in this case is one of hyperthyroidism.
Thyroid hormone resistance ranges from euthyroid and clinically transparent to profoundly hypothyroid, and different organs in the same patient may show different sensitivities to thyroid hormone. Clinical features vary, depending on the strength of thyroid hormone resistance.
Early emergence of resistance (as would be expected in someone with an inherited thyroid hormone receptor abnormality) leads to developmental problems, including:
- short stature
- mental or learning disabilities (including attention deficits).
In a study of 18 families with strong history of generalized resistance to thyroid hormone, 70% of affected children met diagnostic criteria for attention-deficit/hyperactivity disorder.12
Target psychiatric symptoms for prescribing replacement thyroid hormone are depression; mood cycling or instability; low energy, fatigue, or lethargy; cognitive impairment (Table 1); and psychosis, if present. Because these symptoms are not specific to thyroid dysfunction, institute thyroid hormone only when biochemical evidence of compromised or suboptimal thyroid function is also present.
Exceptions to this rule may include patients with target psychiatric symptoms and:
Patients taking lithium for mood stabilization will likely need supplemental thyroid hormone eventually because lithium is thyrotoxic. Also, patients with bipolar mood symptoms often have coexisting thyroid abnormalities, and giving supraphysiologic thyroid hormone dosages sometimes converts those who do not respond to mood stabilizers into responders.
Thyrotropin concentrations increase within 1 day after patients start taking lithium carbonate, but without commensurate increases in T3 or T4. More often, T3 and T4 concentrations decrease in the presence of lithium. Among 150 patients maintained on lithium for 10 years, hypothyroidism, autoimmune thyroiditis, or goiter developed at rates of 1.7%, 1.4%, and 2.1% per year, respectively. The study authors suggested that long-term lithium may increase the risk for hypothyroidism in women and in patients with thyroid autoimmunity.9
Laboratory screening
TSH and thyroid hormones. The basic thyroid screen is a combination of serum levels of TSH (“sensitive TSH”) and free thyroid hormones.
TSH has a circadian rhythm, with a nocturnal surge amounting to a 50% to 200% increase over daytime levels, beginning at around 6 to 8 PM. Peak TSH pulsatile activity and levels are seen after sleep begins—usually after midnight—with trough levels and fewest TSH pulses in late morning and early afternoon.13,14 This rhythm implies that the most accurate TSH measurement may be obtained by drawing blood in the morning before 9 AM. I have seen subclinical hypothyroidism missed when clinicians relied on afternoon TSH levels.
In general medicine, TSH alone frequently is used as a routine screening tool. In psychiatric practice, however, I recommend supplementing TSH with free T3 and free T4 because thyroid system dysfunction is frequent in psychiatric syndromes. If laboratory costs are a concern, free T4 with TSH usually suffices for initial screening.
Although the unbound, free fractions of T3 and T4 are of primary interest, total T4 and total T3 are necessary to assess the thyroid gland’s synthetic capacity. When clinical evidence suggests abnormal thyroid hormone function, order repeated or serial biochemical testing. Marginal biochemical results also mandate repeat thyroid function studies, expanded to include:
- total thyroid hormone concentrations (ideally T4 and T3)
- antithyroid antibodies
- serum cholesterol
- prolactin.
Antithyroid antibodies. Obtain antithyroglobulin and antithyroid microsomal antibody titers if:
- thyroid hormone indices are abnormal or marginal—in either direction
- or the patient now has, has had, or has a family history of autoimmune-mediated symptoms, such as lupus erythematosus or rheumatoid arthritis.
Negative or low antithyroid autoantibody titers do not rule out thyroiditis as a cause of hypothyroidism, as these titers are most likely to be generated during periods of active inflammation.
Many autoantibodies react with thyroid-related elements. Most clinical laboratories can quantify antibodies directed against thyroid peroxidase—also called antimicrosomal antibodies—and thyroglobulin. The presence of these antibodies is associated with thyroid inflammation and a risk of progression to thyroid failure and hypothyroidism.
Other laboratory findings of hypothyroidism include hypercholesterolemia, mild hyperprolactinemia, and types of anemia (including iron-deficiency anemia with low ferritin levels).
Related resources
- American Thyroid Association. www.thyroid.org.
- Dunn JT. Guarding our nation’s thyroid health. J Clin Endocrinol Metab 2001;872:486-8.
- Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med 2003;348:2646-55.
- Sullivan GM, Mann JJ, Oquendo MA, et al. Low cerebrospinal fluid transthyretin levels in depression: correlations with suicidal ideation and low serotonin function. Biol Psychiatry 2006;60(5):500-6.
Hypothyroidism—even when occult or subclinical—can cause subtle or frank changes in energy, mood, anxiety level, or cognition. Some patients’ affective symptoms remit with thyroid hormone replacement or with antidepressants only after a euthyroid state is established.
To help you recognize hypothyroidism in patients presenting with psychiatric illnesses and provide effective treatment, this article describes:
- hypothyroidism’s signs and symptoms
- primary and subclinical hypothyroidism, thyroiditis, central hypothyroidism, and thyroid hormone resistance
- laboratory screening for thyroid dysfunction in patients with psychiatric symptoms.
Overlapping clinical signs
Thyroid hormone is required for the metabolic activity of every cell in the body. When patients experience symptoms related to abnormal functioning of the hypothalamic-pituitary-thyroid axis (Figure), psychiatrists often are the first professionals they consult.
Diagnosis of thyroid disorders is based on biochemical and clinical data (Box 1),1 which might not be congruent. Clinical symptoms of hypothyroidism, for example, are notoriously variable. Severe biochemical hypothyroidism may be associated with mild clinical symptoms, whereas mild biochemical hypothyroidism may be associated with severe symptoms.2
Patients with thyroid disturbance and psychiatric symptoms most often are diagnosed with a depressive-spectrum syndrome. Most common are:
- atypical depression (which may present as dysthymia)
- bipolar spectrum syndrome (including manic-depression, mixed mania, bipolar depression, rapid-cycling bipolar disorder, cyclothymia, and premenstrual syndromes)
- borderline personality disorder
- or psychotic disorder (typically paranoid psychosis).
Psychiatric symptoms of hypothyroidism (Table 1) are often prominent or even primary. Patients commonly show:
- impaired attention, concentration, learning, and memory
- psychomotor slowing
- and/or mental dullness.
Table 1
Hypothyroidism’s psychiatric signs and symptoms
| Cognitive changes | Impaired memory, psychomotor slowing, reduced attention span |
| Vegetative symptoms | Hypersomnia, sleep apnea, fatigue, lethargy, apathy, anergia, low libido |
| Mood changes | Depression, mood instability, mania, anxiety |
| Other | Psychosis |
Psychiatric patients—particularly those with mood disorders—are more likely to exhibit biochemical evidence of frank or subclinical hypothyroidism, hyperthyroidism, and autoimmune thyroiditis than the general population. In a study of 17,533 Americans,1 approximately 12% had thyroid abnormalities and 80% of these were hypothyroid. An additional 17% of women and 9% of men tested positive for antithyroid antibodies.
Biochemical hypothyroidism was defined as TSH >4.5 mIU/L, with low total T4, and subclinical hypothyroidism as TSH >4.5 mIU/L with normal T4 levels. Population studies and other data have led some endocrinologists to regard serum TSH levels >2.5 mIU/L as abnormally high.
Figure Thyroid hormone regulation: The hypothalamic-pituitary-thyroid axis
Thyroid-stimulating hormone (TSH, thyrotropin) released into circulation by the pituitary stimulates the thyroid to synthesize and release thyroid hormone, including triiodothyronine (T3) and thyroxine (T4). TSH is regulated by hypothalamic elaboration of thyrotropin-releasing hormone (TRH), which stimulates TSH release in a dose-dependent manner. Negative feedback from T3 and T4 downregulates TRH gene expression and restrains the pituitary from responding to TSH. Most T3—the active hormone—is formed in circulation or in target tissues by deiodination of T4 by selenium-containing enzymes.
Illustration for Current Psychiatry by Rich LaroccoThe degree of impairment may depend on the patient’s normal functional level. For example, a well-educated patient of mine with thyroiditis-related hypothyroidism reported word-finding difficulties. Instead of asking her husband to take a bottle of wine from the rack, she asked him to take a bottle of wine from the “thing.”
Loss of vitality, fatigue, lethargy, hypersomnia (especially if sleep apnea is present), and depressed mood also are commonly seen.
Depressive symptoms. Hypothyroid patients usually meet several criteria for a major depressive episode—such as concentration difficulties, lassitude, low libido, and sometimes pessimism or sadness—and symptoms improve after sustained thyroid hormone replacement therapy.3 Women with mild hypothyroidism who screen negative for a psychiatric syndrome show statistically significant mood improvement and improved verbal fluency after 6 months of levothyroxine replacement therapy.4
In some patients with no clear evidence of a biochemical or clinical thyroid disorder, mood symptoms nevertheless respond to thyroid hormone augmentation of antidepressants.5
Anxiety symptoms. Occasionally thyroid dysfunction is seen in patients with anxiety disorders, including panic disorder, agoraphobia, social phobia, performance anxiety, post-traumatic stress disorder, and generalized anxiety disorder.6
It may seem counterintuitive, but hypothyroidism is probably as common as hyperthyroidism in extremely anxious patients. Both hypoand hyperthyroidism are seen much more often in patients with panic-level anxiety than in the general population.
In a sample of 144 consecutive female psychiatric patients with a lifetime history of panic disorder and/or agoraphobia:
- 27% had a history of thyroid disorder
- 17% had hypothyroidism
- 8% had hyperthyroidism.7
Hypothyroid symptoms. Associated symptoms of hypothyroidism (Table 2) may include cold intolerance, lack of or reduced perspiration, dry skin, constipation, lethargy, psychomotor slowness, and subjective paresthesias and muscle pains. Edema is often present. The face typically is swollen or “puffy” in the morning, but by evening the lower legs (and not the face) are edematous.
Deep tendon reflexes usually relax slowly after initial stimulation. Vascular resistance is increased, but hypertension is not usual. Noradrenergic systems become more active in a compensatory, counter-regulatory manner; however, bradycardia—when present—is sometimes profound. Weight gain can occur but often is conspicuously absent.
Severe hypothyroidism presents with paradoxical tremendous agitation, paranoia, and aggressiveness. The skin is leathery, and facies are characteristically rough. Myxedema is fairly common, even in high-functioning patients. I have seen only one case of so-called “myxedema madness;” the female patient’s hyperarousal, yelling, cursing, grossly poor cognitive ability, and loosely conceived paranoid delusions are unforgettable.
Galactorrhea (related to hyperprolactinemia) can be a symptom of severe hypothyroidism, presumably from increased hypothalamic thyrotropin-releasing hormone (TRH) drive. TRH is the main known secretagogue for pituitary prolactin secretion. Infertility, oligomenorrhea, or amenorrhea could be part of the hypothyroid clinical picture.
Other symptoms. Macroglossia and hypertrophy of the uvula are possible; in a recent report, dysarthria resulting from these oral changes was the only presenting symptom of a hypothyroid man.8 Dysarthria promptly corrected after levothyroxine replacement.
Hypothyroidism is a primary cause of central sleep apnea caused by dysfunction of ventilatory control and/or reduction in airway aperture.
Table 2
Hypothyroidism’s other signs and symptoms
| Metabolic | Low basal body temperature/cool skin, diminished perspiration, weight gain or difficulty losing weight |
| Cardiovascular | Bradycardia, dizziness |
| Dermatologic | Dry, rough, or scaly skin; brittle nails; coarse or thinning hair (especially in women); pallor; dependent edema; myxedema/skin mucinosis (classically pretibial) |
| Digestive | Nausea, constipation, enlarged tongue |
| Reproductive | Oligomenorrhea, amenorrhea, infertility, miscarriage, delayed ejaculation |
| Musculoskeletal and peripheral nervous system | Muscle cramps, joint pain, paresthesias, numbness, weakness, reduced exercise tolerance, delayed ankle reflex |
| Sensory | Upper eyelid drooping, dysarthria, hoarseness, diminished hearing, diminished taste (hypogustia) |
Causes of thyroid disorders
Primary hypothyroidism results from thyroid gland failure. The many causes include iodine deficiency, but most cases of adult-onset or acquired hypothyroidism are attributed to autoimmune thyroiditis. In primary hypothyroidism, low serum T4 and/or T3 are seen in combination with elevated serum TSH. Circulating free T4 diminishes sooner than free T3 does as the body attempts to preserve active hormone levels. In turn, total T4 and T3 diminish sooner than free hormone concentrations. A compensatory increase in pituitary-elaborated TSH is seen as the brain and pituitary attempt to stimulate the thyroid to produce adequate thyroid hormone.
In subclinical hypothyroidism, circulating free thyroid hormone concentrations are within the normal laboratory range (typically in the lower range), but TSH is elevated. TSH concentrations >3.0 mIU/mL call for repeat or follow-up biochemistry and clinical correlation.
The decision to treat subclinical hypothyroidism with thyroid hormone replacement is less controversial in psychiatry than in endocrinology. Psychiatric patients with subclinical hypothyroidism—especially those with incomplete responses to psychotropic therapy—should usually be treated with thyroid hormone (Box 2).1
Free T3 levels in the lower 20% of the laboratory’s normal range are cause for pause in a patient with a mood or psychotic disorder and any of hypothyroidism’s clinical stigmata, even if thyroxine and TSH concentrations are normal.
Thyroiditis is characterized by thyroid gland inflammation. The thyroid may be painful or nonpainful, enlarged, fleshy, goitrous, normal in size, or atrophic and fibrotic (especially late in the course).
Postulated precipitants include viruses and other infectious agents, vaccines, iodine excess, lithium therapy (Box 3),9 tobacco smoke, environmental chemicals or toxins, irradiation, and—arguably—cortically-mediated (psychological) stress in vulnerable individuals.
Clinically, thyroiditis syndromes often have a long prodromal phase, wax and wane in severity, have an insidious and sometimes silent course, and can be serially associated with hyperthyroidism (especially early in the course), euthyroidism, or hypothyroidism (especially late in the course). Most thyroiditis syndromes appear to resolve spontaneously, but many become chronic or show evidence of subtle thyroid dysfunction years after the first occurrence or diagnosis.
Most presentations are nonspecific; symptoms may be limited to lethargy, fatigue, and depression. Increased antithyroid antibody titers have been linked with psychotic and depressive syndromes in borderline personality disorder.10
In early thyroiditis, thyroxine and triiodothyronine secretion is often elevated, with low or suppressed TSH. However, antithyroid antibody production is associated with a significantly increased risk of eventual subclinical or frank hypothyroidism. Permanent hypothyroidism develops eventually in at least one-half of women with histories of postpartum thyroiditis.11
Central hypothyroidism stems from TSH deficiency. Both pituitary thyrotrophic failure and hypothalamic failure—secondary and tertiary hypothyroidism, respectively—are considered central (or “secondary”). Hypothyroidism of pituitary and hypothalamic origins are lumped together as “central” because it is often very difficult to differentiate these pathologies.
Thyroid hormone resistance, in which end-organ or cellular resistance to thyroid hormone signals is seen, is an increasingly recognized syndrome in clinical medicine. The typical case is an euthyroid or hypothyroid individual with elevated T4 and T3 and nonsuppressed or even frankly elevated TSH. Inappropriately elevated TSH combined with high thyroid hormone levels also can be seen in TSH-secreting pituitary tumors, although the clinical picture in this case is one of hyperthyroidism.
Thyroid hormone resistance ranges from euthyroid and clinically transparent to profoundly hypothyroid, and different organs in the same patient may show different sensitivities to thyroid hormone. Clinical features vary, depending on the strength of thyroid hormone resistance.
Early emergence of resistance (as would be expected in someone with an inherited thyroid hormone receptor abnormality) leads to developmental problems, including:
- short stature
- mental or learning disabilities (including attention deficits).
In a study of 18 families with strong history of generalized resistance to thyroid hormone, 70% of affected children met diagnostic criteria for attention-deficit/hyperactivity disorder.12
Target psychiatric symptoms for prescribing replacement thyroid hormone are depression; mood cycling or instability; low energy, fatigue, or lethargy; cognitive impairment (Table 1); and psychosis, if present. Because these symptoms are not specific to thyroid dysfunction, institute thyroid hormone only when biochemical evidence of compromised or suboptimal thyroid function is also present.
Exceptions to this rule may include patients with target psychiatric symptoms and:
Patients taking lithium for mood stabilization will likely need supplemental thyroid hormone eventually because lithium is thyrotoxic. Also, patients with bipolar mood symptoms often have coexisting thyroid abnormalities, and giving supraphysiologic thyroid hormone dosages sometimes converts those who do not respond to mood stabilizers into responders.
Thyrotropin concentrations increase within 1 day after patients start taking lithium carbonate, but without commensurate increases in T3 or T4. More often, T3 and T4 concentrations decrease in the presence of lithium. Among 150 patients maintained on lithium for 10 years, hypothyroidism, autoimmune thyroiditis, or goiter developed at rates of 1.7%, 1.4%, and 2.1% per year, respectively. The study authors suggested that long-term lithium may increase the risk for hypothyroidism in women and in patients with thyroid autoimmunity.9
Laboratory screening
TSH and thyroid hormones. The basic thyroid screen is a combination of serum levels of TSH (“sensitive TSH”) and free thyroid hormones.
TSH has a circadian rhythm, with a nocturnal surge amounting to a 50% to 200% increase over daytime levels, beginning at around 6 to 8 PM. Peak TSH pulsatile activity and levels are seen after sleep begins—usually after midnight—with trough levels and fewest TSH pulses in late morning and early afternoon.13,14 This rhythm implies that the most accurate TSH measurement may be obtained by drawing blood in the morning before 9 AM. I have seen subclinical hypothyroidism missed when clinicians relied on afternoon TSH levels.
In general medicine, TSH alone frequently is used as a routine screening tool. In psychiatric practice, however, I recommend supplementing TSH with free T3 and free T4 because thyroid system dysfunction is frequent in psychiatric syndromes. If laboratory costs are a concern, free T4 with TSH usually suffices for initial screening.
Although the unbound, free fractions of T3 and T4 are of primary interest, total T4 and total T3 are necessary to assess the thyroid gland’s synthetic capacity. When clinical evidence suggests abnormal thyroid hormone function, order repeated or serial biochemical testing. Marginal biochemical results also mandate repeat thyroid function studies, expanded to include:
- total thyroid hormone concentrations (ideally T4 and T3)
- antithyroid antibodies
- serum cholesterol
- prolactin.
Antithyroid antibodies. Obtain antithyroglobulin and antithyroid microsomal antibody titers if:
- thyroid hormone indices are abnormal or marginal—in either direction
- or the patient now has, has had, or has a family history of autoimmune-mediated symptoms, such as lupus erythematosus or rheumatoid arthritis.
Negative or low antithyroid autoantibody titers do not rule out thyroiditis as a cause of hypothyroidism, as these titers are most likely to be generated during periods of active inflammation.
Many autoantibodies react with thyroid-related elements. Most clinical laboratories can quantify antibodies directed against thyroid peroxidase—also called antimicrosomal antibodies—and thyroglobulin. The presence of these antibodies is associated with thyroid inflammation and a risk of progression to thyroid failure and hypothyroidism.
Other laboratory findings of hypothyroidism include hypercholesterolemia, mild hyperprolactinemia, and types of anemia (including iron-deficiency anemia with low ferritin levels).
Related resources
- American Thyroid Association. www.thyroid.org.
- Dunn JT. Guarding our nation’s thyroid health. J Clin Endocrinol Metab 2001;872:486-8.
- Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med 2003;348:2646-55.
- Sullivan GM, Mann JJ, Oquendo MA, et al. Low cerebrospinal fluid transthyretin levels in depression: correlations with suicidal ideation and low serotonin function. Biol Psychiatry 2006;60(5):500-6.
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99.
2. Zulewski H, Muller B, Exer P, et al. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab 1997;82:771-6.
3. Gunnarsson T, Sjoberg S, Eriksson M, Nordin C. Depressive symptoms in hyothyroid disorder with some observations on biochemical correlates. Neuropsychobiology 2001;43:70-4.
4. Bono G, Fancellu R, Blandini F, Santor G, Mauri M. Cognitive and affective status in mild hypothyroidism and interactions with L-thyroxine treatment. Acta Neurol Scand 2004;110:59-66
5. Iosifescu DV. ‘Supercharge’ antidepressants by adding thyroid hormones: Why hormones help, and new data on SSRI augmentation. Current Psychiatry 2006;5(7):15-25.
6. Tancer ME, Stein MB, Gelernter CS, Uhde TW. The hypothalamic-pituitary-thyroid axis in social phobia. Am J Psychiatry 1990;147(7):929-33
7. Orenstein H, Peskind A, Raskind MA. Thyroid disorders in female psychiatric patients with panic disorder or agoraphobia. Am J Psychiatry 1988;145:1428-30.
8. Stollberger C, Finsterer J, Brand E, Tschabitscher D. Dysarthria as the leading symptom of hypothyroidism. Am J Otolaryngol 2001;22:70-2.
9. Bocchetta A, Mossa P, Velluzzi F, et al. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol 2001;21(6):594-8.
10. Geracioti TD, Kling MA, Post R, Gold PW. Antithyroid antibody-linked symptoms in borderline personality disorder. Endocrine 2003;21:153-8.
11. Premawardhana LD, Parkes AB, Ammari F, et al. Postpartum thyroiditis and long-term thyroid status: prognostic influence of thyroid peroxidase antibodies and ultrasound echogenicity. J Clin Endocrinol Metab 2000;85:71-5.
12. Hauser P, Zametkin AJ, Martinez P, et al. Attention deficithyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med 1993;328:997-1001.
13. Ghizzoni L, Mastorakos G, Ziveri M, et al. Interactions of leptin and thyrotropin 24-hour secretory profiles in short normal children. J Clin Endocrinol Metab 2001;86(5):2065-72.
14. Mantzoros CS, Ozata M, Negrao AB, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab 2001;86(7):3284-91.
15. Chopra IJ, Sabatino L. Nature and sources of circulating thyroid hormones. In: Braverman LE, Utiger RD, eds. The thyroid. A fundamental and clinical text. Philadelphia: Lippincott; 2000:120-35.
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99.
2. Zulewski H, Muller B, Exer P, et al. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab 1997;82:771-6.
3. Gunnarsson T, Sjoberg S, Eriksson M, Nordin C. Depressive symptoms in hyothyroid disorder with some observations on biochemical correlates. Neuropsychobiology 2001;43:70-4.
4. Bono G, Fancellu R, Blandini F, Santor G, Mauri M. Cognitive and affective status in mild hypothyroidism and interactions with L-thyroxine treatment. Acta Neurol Scand 2004;110:59-66
5. Iosifescu DV. ‘Supercharge’ antidepressants by adding thyroid hormones: Why hormones help, and new data on SSRI augmentation. Current Psychiatry 2006;5(7):15-25.
6. Tancer ME, Stein MB, Gelernter CS, Uhde TW. The hypothalamic-pituitary-thyroid axis in social phobia. Am J Psychiatry 1990;147(7):929-33
7. Orenstein H, Peskind A, Raskind MA. Thyroid disorders in female psychiatric patients with panic disorder or agoraphobia. Am J Psychiatry 1988;145:1428-30.
8. Stollberger C, Finsterer J, Brand E, Tschabitscher D. Dysarthria as the leading symptom of hypothyroidism. Am J Otolaryngol 2001;22:70-2.
9. Bocchetta A, Mossa P, Velluzzi F, et al. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol 2001;21(6):594-8.
10. Geracioti TD, Kling MA, Post R, Gold PW. Antithyroid antibody-linked symptoms in borderline personality disorder. Endocrine 2003;21:153-8.
11. Premawardhana LD, Parkes AB, Ammari F, et al. Postpartum thyroiditis and long-term thyroid status: prognostic influence of thyroid peroxidase antibodies and ultrasound echogenicity. J Clin Endocrinol Metab 2000;85:71-5.
12. Hauser P, Zametkin AJ, Martinez P, et al. Attention deficithyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med 1993;328:997-1001.
13. Ghizzoni L, Mastorakos G, Ziveri M, et al. Interactions of leptin and thyrotropin 24-hour secretory profiles in short normal children. J Clin Endocrinol Metab 2001;86(5):2065-72.
14. Mantzoros CS, Ozata M, Negrao AB, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab 2001;86(7):3284-91.
15. Chopra IJ, Sabatino L. Nature and sources of circulating thyroid hormones. In: Braverman LE, Utiger RD, eds. The thyroid. A fundamental and clinical text. Philadelphia: Lippincott; 2000:120-35.
The ‘meth’ epidemic: Managing acute psychosis, agitation, and suicide risk
Methamphetamine abuse has spread to every region of the United States in the past 10 years (Table 1).1 Its long-lasting, difficult-to-treat medical effects destroy lives and create psychiatric and physical comorbidities that confound clinicians in emergency rooms and community practice settings.
This first in a series of two articles describes methamphetamine’s growing use and offers guidance to identify abusers and manage acute “meth” intoxication. Methamphetamine-abusing patients can appear in any area of acute psychiatric practice—during emergency department (ED) evaluations, medical-surgical consultations, and inpatient psychiatric admissions. Using case examples, we describe key clinical principles to help you assess patients in each of these settings.
Table 1
10-year growth in hospitalization rates
for methamphetamine/amphetamine use*
| Year | ||
|---|---|---|
| State | 1993 | 2003 |
| U.S. national rate | 13 | 56 |
| Northeast | ||
| Connecticut | 1 | 4 |
| Maine | 2 | 5 |
| Massachusetts | <1 | 2 |
| New Hampshire | <1 | 2 |
| New Jersey | 3 | 2 |
| New York | 2 | 4 |
| Pennsylvania | 3 | 2 |
| Rhode Island | 2 | 2 |
| Vermont | 5 | 4 |
| South | ||
| Alabama | 1 | 45 |
| Arkansas | 13 | 130 |
| Delaware | 2 | 2 |
| District of Columbia | — | 2 |
| Florida | 2 | 7 |
| Georgia | 3 | 39 |
| Kentucky | — | 20 |
| Louisiana | 4 | 21 |
| Maryland | 1 | 3 |
| Mississippi | — | 23 |
| North Carolina | <1 | 4 |
| Oklahoma | 19 | 117 |
| South Carolina | 1 | 9 |
| Tennessee | † | 6 |
| Texas | 7 | 17 |
| Virginia | 1 | 4 |
| West Virginia | <1 | — |
| Midwest | ||
| Illinois | 1 | 19 |
| Indiana | 3 | 28 |
| Iowa | 13 | 213 |
| Kansas | 15 | 65 |
| Michigan | 2 | 7 |
| Minnesota | 8 | 100 |
| Missouri | 7 | 84 |
| Nebraska | 8 | 117 |
| North Dakota | 3 | 44 |
| Ohio | 3 | 3 |
| South Dakota | 5 | 90 |
| Wisconsin | <1 | 5 |
| West | ||
| Alaska | 4 | 13 |
| Arizona | — | 36 |
| California | 66 | 212 |
| Colorado | 18 | 86 |
| Hawaii | 52 | 241 |
| Idaho | 20 | 72 |
| Montana | 30 | 133 |
| Nevada | 59 | 176 |
| New Mexico | 7 | 10 |
| Oregon | 98 | 251 |
| Utah | 16 | 186 |
| Washington | 18 | 143 |
| Wyoming | 15 | 209 |
| * Per 100,000 population aged 12 or older, with methamphetamine/amphetamine use as the primary diagnosis. Percentages in boldface exceed the national rate for that year. | ||
| † <0.05% | ||
| –No data available | ||
| Source: Reference 1 | ||
Scourge of the Heartland
A stimulant first synthesized in Japan,2 methamphetamine is the primary drug of abuse in Asia3 and the leading drug threat in the United States, according to U.S. law enforcement officials.4 Although most methamphetamine used in the United States is manufactured in “super-labs” along the U.S.-Mexican border,4 the drug is also easily made from common ingredients in small-scale home laboratories.
These smaller domestic “meth labs” have devastated rural communities and altered demographic patterns of methamphetamine abuse (Figure 1).5 Two aspects of rural life—relative isolation and availability of ingredients for production—proved critical in the initial spread of methamphetamine production and use in the United States. As a result, production by smaller labs is being targeted by state and federal law enforcement officers, who have had some success in eradicating this scourge (Box 1, Figure 2).6-9
Figure 1 Substance abuse treatment admission rates
for methamphetamine-related diagnoses
Substance abuse treatment centers in rural areas had the highest admission rates for methamphetamine/amphetamine-related diagnoses in 2004. Admission rates in nonmetropolitan regions containing cities with populations >10,000 were triple those of suburbs, nearly twice those of big cities, and twice the U.S. average.
Figure 2 Methamphetamine clandestine laboratory incidents,* 2005
* Incidents include chemicals, contaminated glass, and equipment used in methamphetamine production found by law enforcement agencies at laboratories or dump sites.
Source: Drug Enforcement Administration database, reference 9Box 1
Dangerous recipes. The ease with which methamphetamine can be “cooked” in a home kitchen from ingredients available in pharmacies and hardware stores has contributed to the drug’s rapid spread. Meth users produce their own “fixes” using recipes readily available on the Internet and passed on by other “cooks.”6 The combination of inexperienced or intoxicated cooks, homemade equipment, and highly flammable ingredients results in frequent fires and explosions, often with injuries to home occupants and emergency responders.7
Meth labs have been estimated to produce 6 pounds of toxic waste for each 1 pound of methamphetamine produced. Composed of acid, lye, and phosphorus, this waste typically is dumped into ditches, rivers, yards, and drains. The fine-particulate methamphetamine residue generated during home production settles on exposed household surfaces, leading to absorption by children and others who come into contact with it.6,8
Disastrous results. Methamphetamine cooking has caused a social, environmental, and medical disaster—particularly in the Midwest (Figure 2), although the situation has improved in the past 2 years. Many states have passed laws restricting and monitoring sales of the methamphetamine ingredients ephedrine and pseudoephedrine. A change in U.S. law prohibiting pseudoephedrine imports in bulk from Canada has decreased domestic “superlab” production.9 Although these laws appear to have slowed U.S. manufacturing, the drug is still readily available, predominantly smuggled in from large-scale producers in Mexico.
Symptoms of ‘Meth’ Use
Physiologic effects. Methamphetamine is taken because it induces euphoria, anorexia, and increased energy, sexual stimulation, and alertness. Initial use evolves into abuse because of the drug’s highly addictive properties. Available in multiple forms and carrying a variety of labels (see Related resources), methamphetamine causes CNS release of monoamines—particularly dopamine—and damages dopaminergic neurons in the striatum and serotonergic neurons in the frontal lobes, striatum, and hippocampus.10,11
Through sympathetic nervous system activation, methamphetamine can cause reversible or irreversible damage to organ systems (Table 2).12-17
Table 2
Physiologic signs of methamphetamine abuse
| Vital signs | Tachycardia |
| Hypertension | |
| Pyrexia | |
| Laboratory abnormalities | Metabolic acidosis |
| Evidence of rhabdomyolysis | |
| Organ damage | Cardiomyopathy |
| Acute coronary syndrome | |
| Pulmonary edema | |
| Stigmata of chronic use | Premature aging |
| Cachexia | |
| Discolored and fractured teeth | |
| Skin lesions from stereotypical scratching related to formication (“meth bugs”) and/or compulsive picking | |
| Source: References 12-17 | |
Psychiatric effects. Methamphetamine abusers frequently report depressive symptoms, including irritability, anxiety, social isolation, and suicidal ideation.10,18 These patients may show:
- signs of psychosis, including paranoia, hallucinations, and homicidal thoughts
- neurocognitive changes, including poor attention, impaired verbal memory, and decreased executive functioning.19
Agitation is frequent, and its severity appears to correlate directly with methamphetamine blood levels.20 Violent behavior is common. In 1,016 previous users, 40% of men and 46% of women described difficulty controlling their behavior when under methamphetamine’s influence.18
In acute clinical practice, differentiating a primary thought disorder from methamphetamine-induced psychosis is challenging—especially when a patient shows signs of both.21 Methamphetamine also can contribute profoundly to depressive and anxiety disorders. Users may experience residual psychotic symptoms years after the original abuse ends, particularly when stressed. Their positive and negative symptoms are strikingly similar to those seen in schizophrenia.21
Longitudinal illness course, recent history, collateral information, and laboratory and physical data may all inform clinical presentations and comorbidity.
Emergent Evaluation
Gathering data. Police bring Mr. J, age 22, to the ED after his parents said he talked about killing himself and the mother of his 4-year-old child. Police report that Mr. J’s parents said he and his friends abuse methamphetamine, but no first-hand information is available.
Disheveled and uncooperative, Mr. J threatens to harm ED staff. His speech is pressured, and he appears to be responding to internal stimuli. Vital signs include temperature 37.8° C, pulse rate 105 bpm, blood pressure 140/85 mm Hg, and respiration rate 18 breaths per minute.
Mr. J refuses to provide blood or urine for drug screening or to provide a history to the ED physician. He attempts to walk out and is placed in restraints after he tries to punch the ED security officer.
Options for containing uncooperative and agitated patients such as Mr. J are extremely limited, and the overriding concern with violently intoxicated patients is to minimize damage to self, others, and property. Methamphetamine abusers have a propensity for impulsivity and violence;18 many are brought to the hospital by police and have criminal histories.1 In emergent evaluation, begin by searching patients and their belongings for weapons.
Because laboratory results and patient history are not immediately available, methamphetamine abuse often is not included in the initial differential diagnosis—particularly for patients with pre-existing primary affective or psychotic disorders. It is critical to remember that methamphetamine abuse might be complicating a patient’s psychiatric presentation.
Managing agitation. When agitation is prominent, secure the patient in a quiet room to reduce stimulation. Have on hand adequate staffing and benzodiazepines, antipsychotics, or both.
In theory, using an antipsychotic to control methamphetamine-induced agitation is problematic because synergy between the two agents might adversely affect cardiac function.15 On the other hand, acute treatment of agitation often leads to salutary declines in pulse rate, blood pressure, respiration rate, and body temperature.
Benzodiazepines vs neuroleptics. Evidence for acute treatment of agitation is limited,22 especially when agitation was induced by methamphetamine. A randomized, controlled comparison of lorazepam and droperidol (a neuroleptic not routinely prescribed for psychosis) suggested that droperidol could be used safely to control agitation in ED patients, with methamphetamine toxicity.23 Droperidol provided more rapid and effective sedation than lorazepam.
Droperidol use has decreased dramatically since 2001, however, when the FDA ordered a black-box warning about potential for cardiac dysrhythmias.24-25 After that warning, the American College of Emergency Physicians26 examined the evidence to identify the most effective pharmacologic treatment for agitation of unknown etiology. Its recommendation—felt to represent “moderate” clinical certainty—was monotherapy with either:
- a benzodiazepine (lorazepam or midazolam)
- or a conventional antipsychotic (droperidol or haloperidol).
The level of certainty for combining a benzodiazepine with an antipsychotic was lower. In our experience, psychiatrists tend to favor haloperidol and lorazepam over droperidol and midazolam.
Evidence on treating methamphetamine-induced agitation is limited (Box 2).22-26 Before you prescribe any medication, keep in mind its side effect profile, the patient’s age and physical condition, and the possibility that other substances might be contributing to emergent presentations.
We have repeatedly and effectively treated acutely agitated patients in the ED with haloperidol and lorazepam without observing adverse effects. Psychiatrists generally favor haloperidol and lorazepam over droperidol and midazolam. When a patient can cooperate with treatment, we recommend an ECG to rule out prolonged QTc interval, an uncommon complication. Telemetry and a cardiology consultation are indicated with a QTc interval >450 msec or >25% over previous ECGs, particularly if you plan to continue haloperidol treatment.27
Physical examination. Because methamphetamine use can cause substantial physical morbidity, we recommend a thorough physical exam aimed at identifying its stigmata (Table 2). Look especially for injuries resulting from violence, and test for sexually-transmitted diseases. Drug testing in the ED is essential to diagnosis and for planning treatment (Box 3).28,29
Medical-Surgical Consultation
Ensuring safety. Ms. A, age 41, is admitted to the trauma surgery service after a motor vehicle accident in which she was the driver. She has long-standing methamphetamine dependence and is severely agitated. Urine drug testing is positive for methamphetamine, marijuana, and alcohol. Her alcohol serum level of 165 mg/dL exceeds the legal threshold for intoxication.
Tibial and fibular fractures sustained in the car accident require open reduction and internal fixation. On the postsurgical floor 2 days later, Ms. A remains “extremely irritable, dysphoric, and suicidal,” according to the trauma surgery consultation. Staff is concerned about her boyfriend’s behavior: “We think he’s using drugs and might be bringing her drugs.”
Understanding Ms. A’s behavior requires us to consider a broad range of diagnostic contributors, including:
- untreated withdrawal from alcohol or other drugs
- delirium from ongoing effects of the trauma or corrective operation
- inadequate pain control, particularly given her history of substance dependence
- psychiatric comorbidity.
Management includes:
- monitoring for withdrawal and treating it if symptoms emerge
- identifying and minimizing medical factors contributing to confusion, and medicating agitation with psychotropics
- providing adequate analgesia, mindful that dosing may need to be aggressive—particularly if the abused substances include narcotics
- assessing for pre-existing and methamphetamine-induced psychiatric disorders.
If the patient is cognitively able to cooperate, perform a thorough suicide assessment and provide initial supportive and cognitive-behavioral therapy to target suicidal behavior. Consider one-to-one monitoring, depending on the potential for deliberate self-injury, and guard against impulsive actions occurring in a drug- or treatment-induced delirium that could endanger the patient or staff.
A one-to-one monitor also can watch for smuggled contraband. When hospitalized, patients who are chronic substance abusers are prone to continue using illicit substances smuggled in by associates, such as the boyfriend in this case. Consider further testing for illicit drugs if you suspect smuggling.
Acute Psychiatric Inpatient
Initial diagnosis and treatment planning. Miss G, age 23 and homeless, is admitted directly to the inpatient psychiatric unit from an urgent care clinic. She reports being “depressed and suicidal.” An intermittent methamphetamine abuser, she says she last used the drug the previous day.
Miss G reveals that she is on probation for forged checks and drug use. She believes she failed a random urinalysis given earlier in the day as a condition of her probation, and she fears being sent back to jail. Her history includes childhood sexual abuse and emotional abuse in a relationship that ended the previous year.
Take the long-term view. Emergency room physicians and psychiatrists often disagree about drug testing in the ED. Emergency medicine physicians argue that the yield is low and results do not affect short-term ED management. However, we believe that drug testing is essential during the initial evaluation and that, at a minimum, urine toxicology screening must be performed to aid diagnosis and subsequent treatment planning.
A positive toxicology screen provides nearly irrefutable evidence with which to confront a resistant patient who is likely to be involved with the criminal justice system. In a study by Perrone et al,28 the patient history combined with drug testing was most likely to identify substance abuse. Overreliance on either the history or testing alone was flawed.
Objective data. In our experience, patients with legal problems often deny drug abuse. A toxicology screen provides objective data on concomitant use of other substances abused by many methamphetamine users to temper methamphetamine-related insomnia, anxiety, and overstimulation. Hair testing, a promising tool being investigated, may allow more substance abuse to be detected and possibly determine the level of use.29
Physical examination shows multiple erythematous excoriations on her arms from repetitive picking at her skin, poor dentition, and cachexia. She reports multiple recent sexual partners without using condoms. She cannot remember when she last menstruated, and she doesn’t recall ever being tested for sexually transmitted disease.
As in any medical setting involving methamphetamine abusers, acute management of psychiatric inpatients includes careful attention to methamphetamine-related physical conditions—in Miss G’s case possible sexually transmitted diseases, pregnancy, cellulitis, and dental disease.
Mood and anxiety disorders. Methamphetamine users may present with depressive symptoms and suicidality.18,30 In a study of Taiwanese methamphetamine abusers who had recently quit the drug, depressive symptoms were common on cessation but often resolved without antidepressants within 2 to 3 weeks.30 Evidence on antidepressant use in the methamphetamine-dependent patient is limited, and the existing studies have yielded conflicting results (as we will detail in part 2 of this article).
For patients previously diagnosed with mood or anxiety disorders, do not restart psychotropics until you have considered how methamphetamine use is contributing to the immediate presentation. We recommend initial observation for several weeks before starting an antidepressant if there is no pre-methamphetamine history of mood or anxiety symptoms.
Psychosocial treatments. Involve social services in assessing the patient’s need for community resources. Miss G’s ability to benefit from these programs will depend on her cognitive capacity, education level, trauma history, and comorbid psychiatric illness.
For patients who relapse to methamphetamine use, previous successful treatment and abstinence may be a hopeful prognostic sign and warrant referral to a program for recidivists. The patient’s legal status may limit some options in the community but open others in the criminal justice system.
Methamphetamine users often have multiple problems that require attention. For example, compared with other mothers under investigation by child welfare services in California, methamphetamine-abusing mothers were younger and less educated on average, less likely to have had substance-abuse treatment, and more likely to have criminal records.31 These findings underscore the challenge of coordinating a response that integrates separate and complex systems—psychiatric/substance abuse treatment, child welfare, and criminal justice.
- Methresources. Web site pooling information from multiple agencies for communities, law enforcement, and policy makers. www.methresources.gov.
- Methamphetamine. National drug threat assessment, with information on methamphetamine production, trafficking, and patterns of use. U.S. Department of Justice. Drug Enforcement Administration. www.dea.gov/concern/18862/meth.htm.
- Substance Abuse and Mental Health Services Administration. Drug and Alcohol Services Information System. Trends in methamphetamine/amphetamine admissions to treatment: 1993-2003. The DASIS Report 2006; Issue 9. www.oas.samhsa.gov/2k6/methTX/methTX.pdf.
Drug brand names
- Droperidol • Inapsine
- Haloperidol • Haldol
- Lorazepam • Ativan
- Midazolam • Versed
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Substance Abuse and Mental Health Services Administration Drug and Alcohol Services Information System. Trends in methamphetamine/amphetamine admissions to treatment: 1993-2003. The DASIS Report 2006; Issue 9. Available at: http://www.oas.samhsa.gov/2k6/methTX/methTX.htm. Accessed September 4, 2006.
2. Suwaki H, Fukui S, Konuma K. Methamphetamine abuse in Japan: its 45 year history and the current situation. In: Klee H, ed. Amphetamine misuse: international perspectives on current trends Amsterdam: Harwood Academic Publishers; 1997:199-214.
3. Greenfeld KT. The need for speed. Time (Asia ed) February 26, 2001. Available at: http://www.time.com/time/asia/news/magazine/0,9754,100581,00.html. Accessed September 4, 2006.
4. Williams P. Meth trade moves south of the border. Tighter restrictions reduce U.S. meth labs, so Mexican drug lords fill gap. NBC News August 25, 2006. Available at: http://www.msnbc.msn.com/id/14500890/. Accessed September 4, 2006.
5. Substance Abuse and Mental Health Services Administration Drug and Alcohol Services Information System. Methamphetamine/amphetamine treatment admissions in urban and rural areas: 2004. The DASIS Report 2006; Issue 27. Available at: http://www.oas.samhsa.gov/2k6/methRuralTX/methRuralTX.htm. Accessed September 4, 2006.
6. Research Overview: Methamphetamine Production Precursor Chemicals and Child Endangerment Albuquerque, NM: New Mexico Sentencing Commission; January 2004. Available at: http://www.nmsc.state.nm.us/DWIDrugReports.htm. Accessed October 1, 2006.
7. Santos AP, Wilson AK, Hornung CA, et al. Methamphetamine laboratory explosions: a new and emerging burn injury. J Burn Care Rehabil 2005;26(3):228-32.
8. Martyny JW, Arbuckle SL, McCammon CS, et al. Chemical exposures associated with clandestine methamphetamine laboratories Denver, CO: The National Jewish Medical and Research Center. Available at: http://www.njc.org/pdf/chemical_exposures.pdf. Accessed September 4, 2006.
9. U.S. Department of Justice. Drug Enforcement Administration. Maps of methamphetamine lab incidents. Available at: http://www.usdoj.gov/dea/concern/map_lab_seizures.html. Accessed September 4, 2006.
10. Anglin MD, Burke C, Perrochet B, et al. History of the methamphetamine problem. J Psychoactive Drugs 2000;32(2):137-41.
11. National Institutes of Health National Institute on Drug Abuse. Methamphetamine abuse and addiction. NIH Publication No 02-4210. Research Report Series January 2002. Available at: http://www.nida.nih.gov/ResearchReports/Methamph/Methamph.html. Accessed October 1, 2006.
12. Burchell SA, Ho HC, Yu M, Margulies DR. Effects of methamphetamine on trauma patients: a cause of severe metabolic acidosis? Crit Care Med 2000;28(6):2112-5.
13. Richards JR, Johnson EB, Stark RW, Derlet RW. Methamphetamine abuse and rhabdomyolysis in the ED: a 5-year study. Am J Emerg Med 1999;17(7):681-5.
14. Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine-associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol 2003;41(7):981-6.
15. Turnipseed SD, Richards JR, Kirk JD, et al. Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. J Emerg Med 2003;24(4):369-73.
16. Nestor TA, Tamamoto WI, Kam TH, Schultz T. Acute pulmonary oedema caused by crystalline methamphetamine. Lancet 1989;2(8674):1277-8.
17. Venker D. Crystal methamphetamine and the dental patient. Iowa Dent J 1999;85(4):34.-
18. Zweben JE, Cohen JB, Christian D, et al. Psychiatric symptoms in methamphetamine users. Am J Addict 2004;13(2):181-90.
19. Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci 2003;15(3):317-25.
20. Batki SL, Harris DS. Quantitative drug levels in stimulant psychosis: relationship to symptom severity, catecholamines and hyperkinesia. Am J Addict 2004;13(5):461-70.
21. Yui K, Ikemoto S, Ishiguro T, Goto K. Studies of amphetamine or methamphetamine psychosis in Japan: relation of methamphetamine psychosis to schizophrenia. Ann N Y Acad Sci 2000;914:1-12.
22. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry 2006;67(suppl10):13-21.
23. Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med 1998;16(4):567-73.
24. Shale JH, Shale CM, Mastin WD. Safety of droperidol in behavioural emergencies. Expert Opin Drug Saf 2004;3(4):369-78.
25. Jacoby JL, Fulton J, Cesta M, Heller M. After the black box warning: dramatic changes in ED use of droperidol. Am J Emerg Med 2005;23(2):196.-Letter.
26. Lukens TW, Wolf SJ, Edlow JA, et al. American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med 2006;47(1):79-99.
27. American Psychiatric Association Practice guideline for the treatment of patients with delirium. Practice guidelines for the treatment of psychiatric disorders, compendium 2004 Washington, DC: American Psychiatric Publishing; 29-66.
28. Perrone J, De Roos F, Jayaraman S, Hollander JE. Drug screening versus history in detection of substance use in ED psychiatric patients. Am J Emerg Med 2001;19(1):49-51.
29. Mieczkowski T. Hair analysis for detection of psychotropic drug use [letter]. Mayo Clin Proc 2006;81(4):568-9.
30. McGregor C, Srisurapanont M, Jittiwutikarn J, et al. The nature, time course and severity of methamphetamine withdrawal. Addiction 2005;100(9):1320-9.
31. Grella CE, Hser YI, Huang YC. Mothers in substance abuse treatment: differences in characteristics based on involvement with child welfare services. Child Abuse Negl 2006;30(1):55-73.
Methamphetamine abuse has spread to every region of the United States in the past 10 years (Table 1).1 Its long-lasting, difficult-to-treat medical effects destroy lives and create psychiatric and physical comorbidities that confound clinicians in emergency rooms and community practice settings.
This first in a series of two articles describes methamphetamine’s growing use and offers guidance to identify abusers and manage acute “meth” intoxication. Methamphetamine-abusing patients can appear in any area of acute psychiatric practice—during emergency department (ED) evaluations, medical-surgical consultations, and inpatient psychiatric admissions. Using case examples, we describe key clinical principles to help you assess patients in each of these settings.
Table 1
10-year growth in hospitalization rates
for methamphetamine/amphetamine use*
| Year | ||
|---|---|---|
| State | 1993 | 2003 |
| U.S. national rate | 13 | 56 |
| Northeast | ||
| Connecticut | 1 | 4 |
| Maine | 2 | 5 |
| Massachusetts | <1 | 2 |
| New Hampshire | <1 | 2 |
| New Jersey | 3 | 2 |
| New York | 2 | 4 |
| Pennsylvania | 3 | 2 |
| Rhode Island | 2 | 2 |
| Vermont | 5 | 4 |
| South | ||
| Alabama | 1 | 45 |
| Arkansas | 13 | 130 |
| Delaware | 2 | 2 |
| District of Columbia | — | 2 |
| Florida | 2 | 7 |
| Georgia | 3 | 39 |
| Kentucky | — | 20 |
| Louisiana | 4 | 21 |
| Maryland | 1 | 3 |
| Mississippi | — | 23 |
| North Carolina | <1 | 4 |
| Oklahoma | 19 | 117 |
| South Carolina | 1 | 9 |
| Tennessee | † | 6 |
| Texas | 7 | 17 |
| Virginia | 1 | 4 |
| West Virginia | <1 | — |
| Midwest | ||
| Illinois | 1 | 19 |
| Indiana | 3 | 28 |
| Iowa | 13 | 213 |
| Kansas | 15 | 65 |
| Michigan | 2 | 7 |
| Minnesota | 8 | 100 |
| Missouri | 7 | 84 |
| Nebraska | 8 | 117 |
| North Dakota | 3 | 44 |
| Ohio | 3 | 3 |
| South Dakota | 5 | 90 |
| Wisconsin | <1 | 5 |
| West | ||
| Alaska | 4 | 13 |
| Arizona | — | 36 |
| California | 66 | 212 |
| Colorado | 18 | 86 |
| Hawaii | 52 | 241 |
| Idaho | 20 | 72 |
| Montana | 30 | 133 |
| Nevada | 59 | 176 |
| New Mexico | 7 | 10 |
| Oregon | 98 | 251 |
| Utah | 16 | 186 |
| Washington | 18 | 143 |
| Wyoming | 15 | 209 |
| * Per 100,000 population aged 12 or older, with methamphetamine/amphetamine use as the primary diagnosis. Percentages in boldface exceed the national rate for that year. | ||
| † <0.05% | ||
| –No data available | ||
| Source: Reference 1 | ||
Scourge of the Heartland
A stimulant first synthesized in Japan,2 methamphetamine is the primary drug of abuse in Asia3 and the leading drug threat in the United States, according to U.S. law enforcement officials.4 Although most methamphetamine used in the United States is manufactured in “super-labs” along the U.S.-Mexican border,4 the drug is also easily made from common ingredients in small-scale home laboratories.
These smaller domestic “meth labs” have devastated rural communities and altered demographic patterns of methamphetamine abuse (Figure 1).5 Two aspects of rural life—relative isolation and availability of ingredients for production—proved critical in the initial spread of methamphetamine production and use in the United States. As a result, production by smaller labs is being targeted by state and federal law enforcement officers, who have had some success in eradicating this scourge (Box 1, Figure 2).6-9
Figure 1 Substance abuse treatment admission rates
for methamphetamine-related diagnoses
Substance abuse treatment centers in rural areas had the highest admission rates for methamphetamine/amphetamine-related diagnoses in 2004. Admission rates in nonmetropolitan regions containing cities with populations >10,000 were triple those of suburbs, nearly twice those of big cities, and twice the U.S. average.
Figure 2 Methamphetamine clandestine laboratory incidents,* 2005
* Incidents include chemicals, contaminated glass, and equipment used in methamphetamine production found by law enforcement agencies at laboratories or dump sites.
Source: Drug Enforcement Administration database, reference 9Box 1
Dangerous recipes. The ease with which methamphetamine can be “cooked” in a home kitchen from ingredients available in pharmacies and hardware stores has contributed to the drug’s rapid spread. Meth users produce their own “fixes” using recipes readily available on the Internet and passed on by other “cooks.”6 The combination of inexperienced or intoxicated cooks, homemade equipment, and highly flammable ingredients results in frequent fires and explosions, often with injuries to home occupants and emergency responders.7
Meth labs have been estimated to produce 6 pounds of toxic waste for each 1 pound of methamphetamine produced. Composed of acid, lye, and phosphorus, this waste typically is dumped into ditches, rivers, yards, and drains. The fine-particulate methamphetamine residue generated during home production settles on exposed household surfaces, leading to absorption by children and others who come into contact with it.6,8
Disastrous results. Methamphetamine cooking has caused a social, environmental, and medical disaster—particularly in the Midwest (Figure 2), although the situation has improved in the past 2 years. Many states have passed laws restricting and monitoring sales of the methamphetamine ingredients ephedrine and pseudoephedrine. A change in U.S. law prohibiting pseudoephedrine imports in bulk from Canada has decreased domestic “superlab” production.9 Although these laws appear to have slowed U.S. manufacturing, the drug is still readily available, predominantly smuggled in from large-scale producers in Mexico.
Symptoms of ‘Meth’ Use
Physiologic effects. Methamphetamine is taken because it induces euphoria, anorexia, and increased energy, sexual stimulation, and alertness. Initial use evolves into abuse because of the drug’s highly addictive properties. Available in multiple forms and carrying a variety of labels (see Related resources), methamphetamine causes CNS release of monoamines—particularly dopamine—and damages dopaminergic neurons in the striatum and serotonergic neurons in the frontal lobes, striatum, and hippocampus.10,11
Through sympathetic nervous system activation, methamphetamine can cause reversible or irreversible damage to organ systems (Table 2).12-17
Table 2
Physiologic signs of methamphetamine abuse
| Vital signs | Tachycardia |
| Hypertension | |
| Pyrexia | |
| Laboratory abnormalities | Metabolic acidosis |
| Evidence of rhabdomyolysis | |
| Organ damage | Cardiomyopathy |
| Acute coronary syndrome | |
| Pulmonary edema | |
| Stigmata of chronic use | Premature aging |
| Cachexia | |
| Discolored and fractured teeth | |
| Skin lesions from stereotypical scratching related to formication (“meth bugs”) and/or compulsive picking | |
| Source: References 12-17 | |
Psychiatric effects. Methamphetamine abusers frequently report depressive symptoms, including irritability, anxiety, social isolation, and suicidal ideation.10,18 These patients may show:
- signs of psychosis, including paranoia, hallucinations, and homicidal thoughts
- neurocognitive changes, including poor attention, impaired verbal memory, and decreased executive functioning.19
Agitation is frequent, and its severity appears to correlate directly with methamphetamine blood levels.20 Violent behavior is common. In 1,016 previous users, 40% of men and 46% of women described difficulty controlling their behavior when under methamphetamine’s influence.18
In acute clinical practice, differentiating a primary thought disorder from methamphetamine-induced psychosis is challenging—especially when a patient shows signs of both.21 Methamphetamine also can contribute profoundly to depressive and anxiety disorders. Users may experience residual psychotic symptoms years after the original abuse ends, particularly when stressed. Their positive and negative symptoms are strikingly similar to those seen in schizophrenia.21
Longitudinal illness course, recent history, collateral information, and laboratory and physical data may all inform clinical presentations and comorbidity.
Emergent Evaluation
Gathering data. Police bring Mr. J, age 22, to the ED after his parents said he talked about killing himself and the mother of his 4-year-old child. Police report that Mr. J’s parents said he and his friends abuse methamphetamine, but no first-hand information is available.
Disheveled and uncooperative, Mr. J threatens to harm ED staff. His speech is pressured, and he appears to be responding to internal stimuli. Vital signs include temperature 37.8° C, pulse rate 105 bpm, blood pressure 140/85 mm Hg, and respiration rate 18 breaths per minute.
Mr. J refuses to provide blood or urine for drug screening or to provide a history to the ED physician. He attempts to walk out and is placed in restraints after he tries to punch the ED security officer.
Options for containing uncooperative and agitated patients such as Mr. J are extremely limited, and the overriding concern with violently intoxicated patients is to minimize damage to self, others, and property. Methamphetamine abusers have a propensity for impulsivity and violence;18 many are brought to the hospital by police and have criminal histories.1 In emergent evaluation, begin by searching patients and their belongings for weapons.
Because laboratory results and patient history are not immediately available, methamphetamine abuse often is not included in the initial differential diagnosis—particularly for patients with pre-existing primary affective or psychotic disorders. It is critical to remember that methamphetamine abuse might be complicating a patient’s psychiatric presentation.
Managing agitation. When agitation is prominent, secure the patient in a quiet room to reduce stimulation. Have on hand adequate staffing and benzodiazepines, antipsychotics, or both.
In theory, using an antipsychotic to control methamphetamine-induced agitation is problematic because synergy between the two agents might adversely affect cardiac function.15 On the other hand, acute treatment of agitation often leads to salutary declines in pulse rate, blood pressure, respiration rate, and body temperature.
Benzodiazepines vs neuroleptics. Evidence for acute treatment of agitation is limited,22 especially when agitation was induced by methamphetamine. A randomized, controlled comparison of lorazepam and droperidol (a neuroleptic not routinely prescribed for psychosis) suggested that droperidol could be used safely to control agitation in ED patients, with methamphetamine toxicity.23 Droperidol provided more rapid and effective sedation than lorazepam.
Droperidol use has decreased dramatically since 2001, however, when the FDA ordered a black-box warning about potential for cardiac dysrhythmias.24-25 After that warning, the American College of Emergency Physicians26 examined the evidence to identify the most effective pharmacologic treatment for agitation of unknown etiology. Its recommendation—felt to represent “moderate” clinical certainty—was monotherapy with either:
- a benzodiazepine (lorazepam or midazolam)
- or a conventional antipsychotic (droperidol or haloperidol).
The level of certainty for combining a benzodiazepine with an antipsychotic was lower. In our experience, psychiatrists tend to favor haloperidol and lorazepam over droperidol and midazolam.
Evidence on treating methamphetamine-induced agitation is limited (Box 2).22-26 Before you prescribe any medication, keep in mind its side effect profile, the patient’s age and physical condition, and the possibility that other substances might be contributing to emergent presentations.
We have repeatedly and effectively treated acutely agitated patients in the ED with haloperidol and lorazepam without observing adverse effects. Psychiatrists generally favor haloperidol and lorazepam over droperidol and midazolam. When a patient can cooperate with treatment, we recommend an ECG to rule out prolonged QTc interval, an uncommon complication. Telemetry and a cardiology consultation are indicated with a QTc interval >450 msec or >25% over previous ECGs, particularly if you plan to continue haloperidol treatment.27
Physical examination. Because methamphetamine use can cause substantial physical morbidity, we recommend a thorough physical exam aimed at identifying its stigmata (Table 2). Look especially for injuries resulting from violence, and test for sexually-transmitted diseases. Drug testing in the ED is essential to diagnosis and for planning treatment (Box 3).28,29
Medical-Surgical Consultation
Ensuring safety. Ms. A, age 41, is admitted to the trauma surgery service after a motor vehicle accident in which she was the driver. She has long-standing methamphetamine dependence and is severely agitated. Urine drug testing is positive for methamphetamine, marijuana, and alcohol. Her alcohol serum level of 165 mg/dL exceeds the legal threshold for intoxication.
Tibial and fibular fractures sustained in the car accident require open reduction and internal fixation. On the postsurgical floor 2 days later, Ms. A remains “extremely irritable, dysphoric, and suicidal,” according to the trauma surgery consultation. Staff is concerned about her boyfriend’s behavior: “We think he’s using drugs and might be bringing her drugs.”
Understanding Ms. A’s behavior requires us to consider a broad range of diagnostic contributors, including:
- untreated withdrawal from alcohol or other drugs
- delirium from ongoing effects of the trauma or corrective operation
- inadequate pain control, particularly given her history of substance dependence
- psychiatric comorbidity.
Management includes:
- monitoring for withdrawal and treating it if symptoms emerge
- identifying and minimizing medical factors contributing to confusion, and medicating agitation with psychotropics
- providing adequate analgesia, mindful that dosing may need to be aggressive—particularly if the abused substances include narcotics
- assessing for pre-existing and methamphetamine-induced psychiatric disorders.
If the patient is cognitively able to cooperate, perform a thorough suicide assessment and provide initial supportive and cognitive-behavioral therapy to target suicidal behavior. Consider one-to-one monitoring, depending on the potential for deliberate self-injury, and guard against impulsive actions occurring in a drug- or treatment-induced delirium that could endanger the patient or staff.
A one-to-one monitor also can watch for smuggled contraband. When hospitalized, patients who are chronic substance abusers are prone to continue using illicit substances smuggled in by associates, such as the boyfriend in this case. Consider further testing for illicit drugs if you suspect smuggling.
Acute Psychiatric Inpatient
Initial diagnosis and treatment planning. Miss G, age 23 and homeless, is admitted directly to the inpatient psychiatric unit from an urgent care clinic. She reports being “depressed and suicidal.” An intermittent methamphetamine abuser, she says she last used the drug the previous day.
Miss G reveals that she is on probation for forged checks and drug use. She believes she failed a random urinalysis given earlier in the day as a condition of her probation, and she fears being sent back to jail. Her history includes childhood sexual abuse and emotional abuse in a relationship that ended the previous year.
Take the long-term view. Emergency room physicians and psychiatrists often disagree about drug testing in the ED. Emergency medicine physicians argue that the yield is low and results do not affect short-term ED management. However, we believe that drug testing is essential during the initial evaluation and that, at a minimum, urine toxicology screening must be performed to aid diagnosis and subsequent treatment planning.
A positive toxicology screen provides nearly irrefutable evidence with which to confront a resistant patient who is likely to be involved with the criminal justice system. In a study by Perrone et al,28 the patient history combined with drug testing was most likely to identify substance abuse. Overreliance on either the history or testing alone was flawed.
Objective data. In our experience, patients with legal problems often deny drug abuse. A toxicology screen provides objective data on concomitant use of other substances abused by many methamphetamine users to temper methamphetamine-related insomnia, anxiety, and overstimulation. Hair testing, a promising tool being investigated, may allow more substance abuse to be detected and possibly determine the level of use.29
Physical examination shows multiple erythematous excoriations on her arms from repetitive picking at her skin, poor dentition, and cachexia. She reports multiple recent sexual partners without using condoms. She cannot remember when she last menstruated, and she doesn’t recall ever being tested for sexually transmitted disease.
As in any medical setting involving methamphetamine abusers, acute management of psychiatric inpatients includes careful attention to methamphetamine-related physical conditions—in Miss G’s case possible sexually transmitted diseases, pregnancy, cellulitis, and dental disease.
Mood and anxiety disorders. Methamphetamine users may present with depressive symptoms and suicidality.18,30 In a study of Taiwanese methamphetamine abusers who had recently quit the drug, depressive symptoms were common on cessation but often resolved without antidepressants within 2 to 3 weeks.30 Evidence on antidepressant use in the methamphetamine-dependent patient is limited, and the existing studies have yielded conflicting results (as we will detail in part 2 of this article).
For patients previously diagnosed with mood or anxiety disorders, do not restart psychotropics until you have considered how methamphetamine use is contributing to the immediate presentation. We recommend initial observation for several weeks before starting an antidepressant if there is no pre-methamphetamine history of mood or anxiety symptoms.
Psychosocial treatments. Involve social services in assessing the patient’s need for community resources. Miss G’s ability to benefit from these programs will depend on her cognitive capacity, education level, trauma history, and comorbid psychiatric illness.
For patients who relapse to methamphetamine use, previous successful treatment and abstinence may be a hopeful prognostic sign and warrant referral to a program for recidivists. The patient’s legal status may limit some options in the community but open others in the criminal justice system.
Methamphetamine users often have multiple problems that require attention. For example, compared with other mothers under investigation by child welfare services in California, methamphetamine-abusing mothers were younger and less educated on average, less likely to have had substance-abuse treatment, and more likely to have criminal records.31 These findings underscore the challenge of coordinating a response that integrates separate and complex systems—psychiatric/substance abuse treatment, child welfare, and criminal justice.
- Methresources. Web site pooling information from multiple agencies for communities, law enforcement, and policy makers. www.methresources.gov.
- Methamphetamine. National drug threat assessment, with information on methamphetamine production, trafficking, and patterns of use. U.S. Department of Justice. Drug Enforcement Administration. www.dea.gov/concern/18862/meth.htm.
- Substance Abuse and Mental Health Services Administration. Drug and Alcohol Services Information System. Trends in methamphetamine/amphetamine admissions to treatment: 1993-2003. The DASIS Report 2006; Issue 9. www.oas.samhsa.gov/2k6/methTX/methTX.pdf.
Drug brand names
- Droperidol • Inapsine
- Haloperidol • Haldol
- Lorazepam • Ativan
- Midazolam • Versed
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Methamphetamine abuse has spread to every region of the United States in the past 10 years (Table 1).1 Its long-lasting, difficult-to-treat medical effects destroy lives and create psychiatric and physical comorbidities that confound clinicians in emergency rooms and community practice settings.
This first in a series of two articles describes methamphetamine’s growing use and offers guidance to identify abusers and manage acute “meth” intoxication. Methamphetamine-abusing patients can appear in any area of acute psychiatric practice—during emergency department (ED) evaluations, medical-surgical consultations, and inpatient psychiatric admissions. Using case examples, we describe key clinical principles to help you assess patients in each of these settings.
Table 1
10-year growth in hospitalization rates
for methamphetamine/amphetamine use*
| Year | ||
|---|---|---|
| State | 1993 | 2003 |
| U.S. national rate | 13 | 56 |
| Northeast | ||
| Connecticut | 1 | 4 |
| Maine | 2 | 5 |
| Massachusetts | <1 | 2 |
| New Hampshire | <1 | 2 |
| New Jersey | 3 | 2 |
| New York | 2 | 4 |
| Pennsylvania | 3 | 2 |
| Rhode Island | 2 | 2 |
| Vermont | 5 | 4 |
| South | ||
| Alabama | 1 | 45 |
| Arkansas | 13 | 130 |
| Delaware | 2 | 2 |
| District of Columbia | — | 2 |
| Florida | 2 | 7 |
| Georgia | 3 | 39 |
| Kentucky | — | 20 |
| Louisiana | 4 | 21 |
| Maryland | 1 | 3 |
| Mississippi | — | 23 |
| North Carolina | <1 | 4 |
| Oklahoma | 19 | 117 |
| South Carolina | 1 | 9 |
| Tennessee | † | 6 |
| Texas | 7 | 17 |
| Virginia | 1 | 4 |
| West Virginia | <1 | — |
| Midwest | ||
| Illinois | 1 | 19 |
| Indiana | 3 | 28 |
| Iowa | 13 | 213 |
| Kansas | 15 | 65 |
| Michigan | 2 | 7 |
| Minnesota | 8 | 100 |
| Missouri | 7 | 84 |
| Nebraska | 8 | 117 |
| North Dakota | 3 | 44 |
| Ohio | 3 | 3 |
| South Dakota | 5 | 90 |
| Wisconsin | <1 | 5 |
| West | ||
| Alaska | 4 | 13 |
| Arizona | — | 36 |
| California | 66 | 212 |
| Colorado | 18 | 86 |
| Hawaii | 52 | 241 |
| Idaho | 20 | 72 |
| Montana | 30 | 133 |
| Nevada | 59 | 176 |
| New Mexico | 7 | 10 |
| Oregon | 98 | 251 |
| Utah | 16 | 186 |
| Washington | 18 | 143 |
| Wyoming | 15 | 209 |
| * Per 100,000 population aged 12 or older, with methamphetamine/amphetamine use as the primary diagnosis. Percentages in boldface exceed the national rate for that year. | ||
| † <0.05% | ||
| –No data available | ||
| Source: Reference 1 | ||
Scourge of the Heartland
A stimulant first synthesized in Japan,2 methamphetamine is the primary drug of abuse in Asia3 and the leading drug threat in the United States, according to U.S. law enforcement officials.4 Although most methamphetamine used in the United States is manufactured in “super-labs” along the U.S.-Mexican border,4 the drug is also easily made from common ingredients in small-scale home laboratories.
These smaller domestic “meth labs” have devastated rural communities and altered demographic patterns of methamphetamine abuse (Figure 1).5 Two aspects of rural life—relative isolation and availability of ingredients for production—proved critical in the initial spread of methamphetamine production and use in the United States. As a result, production by smaller labs is being targeted by state and federal law enforcement officers, who have had some success in eradicating this scourge (Box 1, Figure 2).6-9
Figure 1 Substance abuse treatment admission rates
for methamphetamine-related diagnoses
Substance abuse treatment centers in rural areas had the highest admission rates for methamphetamine/amphetamine-related diagnoses in 2004. Admission rates in nonmetropolitan regions containing cities with populations >10,000 were triple those of suburbs, nearly twice those of big cities, and twice the U.S. average.
Figure 2 Methamphetamine clandestine laboratory incidents,* 2005
* Incidents include chemicals, contaminated glass, and equipment used in methamphetamine production found by law enforcement agencies at laboratories or dump sites.
Source: Drug Enforcement Administration database, reference 9Box 1
Dangerous recipes. The ease with which methamphetamine can be “cooked” in a home kitchen from ingredients available in pharmacies and hardware stores has contributed to the drug’s rapid spread. Meth users produce their own “fixes” using recipes readily available on the Internet and passed on by other “cooks.”6 The combination of inexperienced or intoxicated cooks, homemade equipment, and highly flammable ingredients results in frequent fires and explosions, often with injuries to home occupants and emergency responders.7
Meth labs have been estimated to produce 6 pounds of toxic waste for each 1 pound of methamphetamine produced. Composed of acid, lye, and phosphorus, this waste typically is dumped into ditches, rivers, yards, and drains. The fine-particulate methamphetamine residue generated during home production settles on exposed household surfaces, leading to absorption by children and others who come into contact with it.6,8
Disastrous results. Methamphetamine cooking has caused a social, environmental, and medical disaster—particularly in the Midwest (Figure 2), although the situation has improved in the past 2 years. Many states have passed laws restricting and monitoring sales of the methamphetamine ingredients ephedrine and pseudoephedrine. A change in U.S. law prohibiting pseudoephedrine imports in bulk from Canada has decreased domestic “superlab” production.9 Although these laws appear to have slowed U.S. manufacturing, the drug is still readily available, predominantly smuggled in from large-scale producers in Mexico.
Symptoms of ‘Meth’ Use
Physiologic effects. Methamphetamine is taken because it induces euphoria, anorexia, and increased energy, sexual stimulation, and alertness. Initial use evolves into abuse because of the drug’s highly addictive properties. Available in multiple forms and carrying a variety of labels (see Related resources), methamphetamine causes CNS release of monoamines—particularly dopamine—and damages dopaminergic neurons in the striatum and serotonergic neurons in the frontal lobes, striatum, and hippocampus.10,11
Through sympathetic nervous system activation, methamphetamine can cause reversible or irreversible damage to organ systems (Table 2).12-17
Table 2
Physiologic signs of methamphetamine abuse
| Vital signs | Tachycardia |
| Hypertension | |
| Pyrexia | |
| Laboratory abnormalities | Metabolic acidosis |
| Evidence of rhabdomyolysis | |
| Organ damage | Cardiomyopathy |
| Acute coronary syndrome | |
| Pulmonary edema | |
| Stigmata of chronic use | Premature aging |
| Cachexia | |
| Discolored and fractured teeth | |
| Skin lesions from stereotypical scratching related to formication (“meth bugs”) and/or compulsive picking | |
| Source: References 12-17 | |
Psychiatric effects. Methamphetamine abusers frequently report depressive symptoms, including irritability, anxiety, social isolation, and suicidal ideation.10,18 These patients may show:
- signs of psychosis, including paranoia, hallucinations, and homicidal thoughts
- neurocognitive changes, including poor attention, impaired verbal memory, and decreased executive functioning.19
Agitation is frequent, and its severity appears to correlate directly with methamphetamine blood levels.20 Violent behavior is common. In 1,016 previous users, 40% of men and 46% of women described difficulty controlling their behavior when under methamphetamine’s influence.18
In acute clinical practice, differentiating a primary thought disorder from methamphetamine-induced psychosis is challenging—especially when a patient shows signs of both.21 Methamphetamine also can contribute profoundly to depressive and anxiety disorders. Users may experience residual psychotic symptoms years after the original abuse ends, particularly when stressed. Their positive and negative symptoms are strikingly similar to those seen in schizophrenia.21
Longitudinal illness course, recent history, collateral information, and laboratory and physical data may all inform clinical presentations and comorbidity.
Emergent Evaluation
Gathering data. Police bring Mr. J, age 22, to the ED after his parents said he talked about killing himself and the mother of his 4-year-old child. Police report that Mr. J’s parents said he and his friends abuse methamphetamine, but no first-hand information is available.
Disheveled and uncooperative, Mr. J threatens to harm ED staff. His speech is pressured, and he appears to be responding to internal stimuli. Vital signs include temperature 37.8° C, pulse rate 105 bpm, blood pressure 140/85 mm Hg, and respiration rate 18 breaths per minute.
Mr. J refuses to provide blood or urine for drug screening or to provide a history to the ED physician. He attempts to walk out and is placed in restraints after he tries to punch the ED security officer.
Options for containing uncooperative and agitated patients such as Mr. J are extremely limited, and the overriding concern with violently intoxicated patients is to minimize damage to self, others, and property. Methamphetamine abusers have a propensity for impulsivity and violence;18 many are brought to the hospital by police and have criminal histories.1 In emergent evaluation, begin by searching patients and their belongings for weapons.
Because laboratory results and patient history are not immediately available, methamphetamine abuse often is not included in the initial differential diagnosis—particularly for patients with pre-existing primary affective or psychotic disorders. It is critical to remember that methamphetamine abuse might be complicating a patient’s psychiatric presentation.
Managing agitation. When agitation is prominent, secure the patient in a quiet room to reduce stimulation. Have on hand adequate staffing and benzodiazepines, antipsychotics, or both.
In theory, using an antipsychotic to control methamphetamine-induced agitation is problematic because synergy between the two agents might adversely affect cardiac function.15 On the other hand, acute treatment of agitation often leads to salutary declines in pulse rate, blood pressure, respiration rate, and body temperature.
Benzodiazepines vs neuroleptics. Evidence for acute treatment of agitation is limited,22 especially when agitation was induced by methamphetamine. A randomized, controlled comparison of lorazepam and droperidol (a neuroleptic not routinely prescribed for psychosis) suggested that droperidol could be used safely to control agitation in ED patients, with methamphetamine toxicity.23 Droperidol provided more rapid and effective sedation than lorazepam.
Droperidol use has decreased dramatically since 2001, however, when the FDA ordered a black-box warning about potential for cardiac dysrhythmias.24-25 After that warning, the American College of Emergency Physicians26 examined the evidence to identify the most effective pharmacologic treatment for agitation of unknown etiology. Its recommendation—felt to represent “moderate” clinical certainty—was monotherapy with either:
- a benzodiazepine (lorazepam or midazolam)
- or a conventional antipsychotic (droperidol or haloperidol).
The level of certainty for combining a benzodiazepine with an antipsychotic was lower. In our experience, psychiatrists tend to favor haloperidol and lorazepam over droperidol and midazolam.
Evidence on treating methamphetamine-induced agitation is limited (Box 2).22-26 Before you prescribe any medication, keep in mind its side effect profile, the patient’s age and physical condition, and the possibility that other substances might be contributing to emergent presentations.
We have repeatedly and effectively treated acutely agitated patients in the ED with haloperidol and lorazepam without observing adverse effects. Psychiatrists generally favor haloperidol and lorazepam over droperidol and midazolam. When a patient can cooperate with treatment, we recommend an ECG to rule out prolonged QTc interval, an uncommon complication. Telemetry and a cardiology consultation are indicated with a QTc interval >450 msec or >25% over previous ECGs, particularly if you plan to continue haloperidol treatment.27
Physical examination. Because methamphetamine use can cause substantial physical morbidity, we recommend a thorough physical exam aimed at identifying its stigmata (Table 2). Look especially for injuries resulting from violence, and test for sexually-transmitted diseases. Drug testing in the ED is essential to diagnosis and for planning treatment (Box 3).28,29
Medical-Surgical Consultation
Ensuring safety. Ms. A, age 41, is admitted to the trauma surgery service after a motor vehicle accident in which she was the driver. She has long-standing methamphetamine dependence and is severely agitated. Urine drug testing is positive for methamphetamine, marijuana, and alcohol. Her alcohol serum level of 165 mg/dL exceeds the legal threshold for intoxication.
Tibial and fibular fractures sustained in the car accident require open reduction and internal fixation. On the postsurgical floor 2 days later, Ms. A remains “extremely irritable, dysphoric, and suicidal,” according to the trauma surgery consultation. Staff is concerned about her boyfriend’s behavior: “We think he’s using drugs and might be bringing her drugs.”
Understanding Ms. A’s behavior requires us to consider a broad range of diagnostic contributors, including:
- untreated withdrawal from alcohol or other drugs
- delirium from ongoing effects of the trauma or corrective operation
- inadequate pain control, particularly given her history of substance dependence
- psychiatric comorbidity.
Management includes:
- monitoring for withdrawal and treating it if symptoms emerge
- identifying and minimizing medical factors contributing to confusion, and medicating agitation with psychotropics
- providing adequate analgesia, mindful that dosing may need to be aggressive—particularly if the abused substances include narcotics
- assessing for pre-existing and methamphetamine-induced psychiatric disorders.
If the patient is cognitively able to cooperate, perform a thorough suicide assessment and provide initial supportive and cognitive-behavioral therapy to target suicidal behavior. Consider one-to-one monitoring, depending on the potential for deliberate self-injury, and guard against impulsive actions occurring in a drug- or treatment-induced delirium that could endanger the patient or staff.
A one-to-one monitor also can watch for smuggled contraband. When hospitalized, patients who are chronic substance abusers are prone to continue using illicit substances smuggled in by associates, such as the boyfriend in this case. Consider further testing for illicit drugs if you suspect smuggling.
Acute Psychiatric Inpatient
Initial diagnosis and treatment planning. Miss G, age 23 and homeless, is admitted directly to the inpatient psychiatric unit from an urgent care clinic. She reports being “depressed and suicidal.” An intermittent methamphetamine abuser, she says she last used the drug the previous day.
Miss G reveals that she is on probation for forged checks and drug use. She believes she failed a random urinalysis given earlier in the day as a condition of her probation, and she fears being sent back to jail. Her history includes childhood sexual abuse and emotional abuse in a relationship that ended the previous year.
Take the long-term view. Emergency room physicians and psychiatrists often disagree about drug testing in the ED. Emergency medicine physicians argue that the yield is low and results do not affect short-term ED management. However, we believe that drug testing is essential during the initial evaluation and that, at a minimum, urine toxicology screening must be performed to aid diagnosis and subsequent treatment planning.
A positive toxicology screen provides nearly irrefutable evidence with which to confront a resistant patient who is likely to be involved with the criminal justice system. In a study by Perrone et al,28 the patient history combined with drug testing was most likely to identify substance abuse. Overreliance on either the history or testing alone was flawed.
Objective data. In our experience, patients with legal problems often deny drug abuse. A toxicology screen provides objective data on concomitant use of other substances abused by many methamphetamine users to temper methamphetamine-related insomnia, anxiety, and overstimulation. Hair testing, a promising tool being investigated, may allow more substance abuse to be detected and possibly determine the level of use.29
Physical examination shows multiple erythematous excoriations on her arms from repetitive picking at her skin, poor dentition, and cachexia. She reports multiple recent sexual partners without using condoms. She cannot remember when she last menstruated, and she doesn’t recall ever being tested for sexually transmitted disease.
As in any medical setting involving methamphetamine abusers, acute management of psychiatric inpatients includes careful attention to methamphetamine-related physical conditions—in Miss G’s case possible sexually transmitted diseases, pregnancy, cellulitis, and dental disease.
Mood and anxiety disorders. Methamphetamine users may present with depressive symptoms and suicidality.18,30 In a study of Taiwanese methamphetamine abusers who had recently quit the drug, depressive symptoms were common on cessation but often resolved without antidepressants within 2 to 3 weeks.30 Evidence on antidepressant use in the methamphetamine-dependent patient is limited, and the existing studies have yielded conflicting results (as we will detail in part 2 of this article).
For patients previously diagnosed with mood or anxiety disorders, do not restart psychotropics until you have considered how methamphetamine use is contributing to the immediate presentation. We recommend initial observation for several weeks before starting an antidepressant if there is no pre-methamphetamine history of mood or anxiety symptoms.
Psychosocial treatments. Involve social services in assessing the patient’s need for community resources. Miss G’s ability to benefit from these programs will depend on her cognitive capacity, education level, trauma history, and comorbid psychiatric illness.
For patients who relapse to methamphetamine use, previous successful treatment and abstinence may be a hopeful prognostic sign and warrant referral to a program for recidivists. The patient’s legal status may limit some options in the community but open others in the criminal justice system.
Methamphetamine users often have multiple problems that require attention. For example, compared with other mothers under investigation by child welfare services in California, methamphetamine-abusing mothers were younger and less educated on average, less likely to have had substance-abuse treatment, and more likely to have criminal records.31 These findings underscore the challenge of coordinating a response that integrates separate and complex systems—psychiatric/substance abuse treatment, child welfare, and criminal justice.
- Methresources. Web site pooling information from multiple agencies for communities, law enforcement, and policy makers. www.methresources.gov.
- Methamphetamine. National drug threat assessment, with information on methamphetamine production, trafficking, and patterns of use. U.S. Department of Justice. Drug Enforcement Administration. www.dea.gov/concern/18862/meth.htm.
- Substance Abuse and Mental Health Services Administration. Drug and Alcohol Services Information System. Trends in methamphetamine/amphetamine admissions to treatment: 1993-2003. The DASIS Report 2006; Issue 9. www.oas.samhsa.gov/2k6/methTX/methTX.pdf.
Drug brand names
- Droperidol • Inapsine
- Haloperidol • Haldol
- Lorazepam • Ativan
- Midazolam • Versed
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Substance Abuse and Mental Health Services Administration Drug and Alcohol Services Information System. Trends in methamphetamine/amphetamine admissions to treatment: 1993-2003. The DASIS Report 2006; Issue 9. Available at: http://www.oas.samhsa.gov/2k6/methTX/methTX.htm. Accessed September 4, 2006.
2. Suwaki H, Fukui S, Konuma K. Methamphetamine abuse in Japan: its 45 year history and the current situation. In: Klee H, ed. Amphetamine misuse: international perspectives on current trends Amsterdam: Harwood Academic Publishers; 1997:199-214.
3. Greenfeld KT. The need for speed. Time (Asia ed) February 26, 2001. Available at: http://www.time.com/time/asia/news/magazine/0,9754,100581,00.html. Accessed September 4, 2006.
4. Williams P. Meth trade moves south of the border. Tighter restrictions reduce U.S. meth labs, so Mexican drug lords fill gap. NBC News August 25, 2006. Available at: http://www.msnbc.msn.com/id/14500890/. Accessed September 4, 2006.
5. Substance Abuse and Mental Health Services Administration Drug and Alcohol Services Information System. Methamphetamine/amphetamine treatment admissions in urban and rural areas: 2004. The DASIS Report 2006; Issue 27. Available at: http://www.oas.samhsa.gov/2k6/methRuralTX/methRuralTX.htm. Accessed September 4, 2006.
6. Research Overview: Methamphetamine Production Precursor Chemicals and Child Endangerment Albuquerque, NM: New Mexico Sentencing Commission; January 2004. Available at: http://www.nmsc.state.nm.us/DWIDrugReports.htm. Accessed October 1, 2006.
7. Santos AP, Wilson AK, Hornung CA, et al. Methamphetamine laboratory explosions: a new and emerging burn injury. J Burn Care Rehabil 2005;26(3):228-32.
8. Martyny JW, Arbuckle SL, McCammon CS, et al. Chemical exposures associated with clandestine methamphetamine laboratories Denver, CO: The National Jewish Medical and Research Center. Available at: http://www.njc.org/pdf/chemical_exposures.pdf. Accessed September 4, 2006.
9. U.S. Department of Justice. Drug Enforcement Administration. Maps of methamphetamine lab incidents. Available at: http://www.usdoj.gov/dea/concern/map_lab_seizures.html. Accessed September 4, 2006.
10. Anglin MD, Burke C, Perrochet B, et al. History of the methamphetamine problem. J Psychoactive Drugs 2000;32(2):137-41.
11. National Institutes of Health National Institute on Drug Abuse. Methamphetamine abuse and addiction. NIH Publication No 02-4210. Research Report Series January 2002. Available at: http://www.nida.nih.gov/ResearchReports/Methamph/Methamph.html. Accessed October 1, 2006.
12. Burchell SA, Ho HC, Yu M, Margulies DR. Effects of methamphetamine on trauma patients: a cause of severe metabolic acidosis? Crit Care Med 2000;28(6):2112-5.
13. Richards JR, Johnson EB, Stark RW, Derlet RW. Methamphetamine abuse and rhabdomyolysis in the ED: a 5-year study. Am J Emerg Med 1999;17(7):681-5.
14. Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine-associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol 2003;41(7):981-6.
15. Turnipseed SD, Richards JR, Kirk JD, et al. Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. J Emerg Med 2003;24(4):369-73.
16. Nestor TA, Tamamoto WI, Kam TH, Schultz T. Acute pulmonary oedema caused by crystalline methamphetamine. Lancet 1989;2(8674):1277-8.
17. Venker D. Crystal methamphetamine and the dental patient. Iowa Dent J 1999;85(4):34.-
18. Zweben JE, Cohen JB, Christian D, et al. Psychiatric symptoms in methamphetamine users. Am J Addict 2004;13(2):181-90.
19. Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci 2003;15(3):317-25.
20. Batki SL, Harris DS. Quantitative drug levels in stimulant psychosis: relationship to symptom severity, catecholamines and hyperkinesia. Am J Addict 2004;13(5):461-70.
21. Yui K, Ikemoto S, Ishiguro T, Goto K. Studies of amphetamine or methamphetamine psychosis in Japan: relation of methamphetamine psychosis to schizophrenia. Ann N Y Acad Sci 2000;914:1-12.
22. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry 2006;67(suppl10):13-21.
23. Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med 1998;16(4):567-73.
24. Shale JH, Shale CM, Mastin WD. Safety of droperidol in behavioural emergencies. Expert Opin Drug Saf 2004;3(4):369-78.
25. Jacoby JL, Fulton J, Cesta M, Heller M. After the black box warning: dramatic changes in ED use of droperidol. Am J Emerg Med 2005;23(2):196.-Letter.
26. Lukens TW, Wolf SJ, Edlow JA, et al. American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med 2006;47(1):79-99.
27. American Psychiatric Association Practice guideline for the treatment of patients with delirium. Practice guidelines for the treatment of psychiatric disorders, compendium 2004 Washington, DC: American Psychiatric Publishing; 29-66.
28. Perrone J, De Roos F, Jayaraman S, Hollander JE. Drug screening versus history in detection of substance use in ED psychiatric patients. Am J Emerg Med 2001;19(1):49-51.
29. Mieczkowski T. Hair analysis for detection of psychotropic drug use [letter]. Mayo Clin Proc 2006;81(4):568-9.
30. McGregor C, Srisurapanont M, Jittiwutikarn J, et al. The nature, time course and severity of methamphetamine withdrawal. Addiction 2005;100(9):1320-9.
31. Grella CE, Hser YI, Huang YC. Mothers in substance abuse treatment: differences in characteristics based on involvement with child welfare services. Child Abuse Negl 2006;30(1):55-73.
1. Substance Abuse and Mental Health Services Administration Drug and Alcohol Services Information System. Trends in methamphetamine/amphetamine admissions to treatment: 1993-2003. The DASIS Report 2006; Issue 9. Available at: http://www.oas.samhsa.gov/2k6/methTX/methTX.htm. Accessed September 4, 2006.
2. Suwaki H, Fukui S, Konuma K. Methamphetamine abuse in Japan: its 45 year history and the current situation. In: Klee H, ed. Amphetamine misuse: international perspectives on current trends Amsterdam: Harwood Academic Publishers; 1997:199-214.
3. Greenfeld KT. The need for speed. Time (Asia ed) February 26, 2001. Available at: http://www.time.com/time/asia/news/magazine/0,9754,100581,00.html. Accessed September 4, 2006.
4. Williams P. Meth trade moves south of the border. Tighter restrictions reduce U.S. meth labs, so Mexican drug lords fill gap. NBC News August 25, 2006. Available at: http://www.msnbc.msn.com/id/14500890/. Accessed September 4, 2006.
5. Substance Abuse and Mental Health Services Administration Drug and Alcohol Services Information System. Methamphetamine/amphetamine treatment admissions in urban and rural areas: 2004. The DASIS Report 2006; Issue 27. Available at: http://www.oas.samhsa.gov/2k6/methRuralTX/methRuralTX.htm. Accessed September 4, 2006.
6. Research Overview: Methamphetamine Production Precursor Chemicals and Child Endangerment Albuquerque, NM: New Mexico Sentencing Commission; January 2004. Available at: http://www.nmsc.state.nm.us/DWIDrugReports.htm. Accessed October 1, 2006.
7. Santos AP, Wilson AK, Hornung CA, et al. Methamphetamine laboratory explosions: a new and emerging burn injury. J Burn Care Rehabil 2005;26(3):228-32.
8. Martyny JW, Arbuckle SL, McCammon CS, et al. Chemical exposures associated with clandestine methamphetamine laboratories Denver, CO: The National Jewish Medical and Research Center. Available at: http://www.njc.org/pdf/chemical_exposures.pdf. Accessed September 4, 2006.
9. U.S. Department of Justice. Drug Enforcement Administration. Maps of methamphetamine lab incidents. Available at: http://www.usdoj.gov/dea/concern/map_lab_seizures.html. Accessed September 4, 2006.
10. Anglin MD, Burke C, Perrochet B, et al. History of the methamphetamine problem. J Psychoactive Drugs 2000;32(2):137-41.
11. National Institutes of Health National Institute on Drug Abuse. Methamphetamine abuse and addiction. NIH Publication No 02-4210. Research Report Series January 2002. Available at: http://www.nida.nih.gov/ResearchReports/Methamph/Methamph.html. Accessed October 1, 2006.
12. Burchell SA, Ho HC, Yu M, Margulies DR. Effects of methamphetamine on trauma patients: a cause of severe metabolic acidosis? Crit Care Med 2000;28(6):2112-5.
13. Richards JR, Johnson EB, Stark RW, Derlet RW. Methamphetamine abuse and rhabdomyolysis in the ED: a 5-year study. Am J Emerg Med 1999;17(7):681-5.
14. Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine-associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol 2003;41(7):981-6.
15. Turnipseed SD, Richards JR, Kirk JD, et al. Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. J Emerg Med 2003;24(4):369-73.
16. Nestor TA, Tamamoto WI, Kam TH, Schultz T. Acute pulmonary oedema caused by crystalline methamphetamine. Lancet 1989;2(8674):1277-8.
17. Venker D. Crystal methamphetamine and the dental patient. Iowa Dent J 1999;85(4):34.-
18. Zweben JE, Cohen JB, Christian D, et al. Psychiatric symptoms in methamphetamine users. Am J Addict 2004;13(2):181-90.
19. Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci 2003;15(3):317-25.
20. Batki SL, Harris DS. Quantitative drug levels in stimulant psychosis: relationship to symptom severity, catecholamines and hyperkinesia. Am J Addict 2004;13(5):461-70.
21. Yui K, Ikemoto S, Ishiguro T, Goto K. Studies of amphetamine or methamphetamine psychosis in Japan: relation of methamphetamine psychosis to schizophrenia. Ann N Y Acad Sci 2000;914:1-12.
22. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry 2006;67(suppl10):13-21.
23. Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med 1998;16(4):567-73.
24. Shale JH, Shale CM, Mastin WD. Safety of droperidol in behavioural emergencies. Expert Opin Drug Saf 2004;3(4):369-78.
25. Jacoby JL, Fulton J, Cesta M, Heller M. After the black box warning: dramatic changes in ED use of droperidol. Am J Emerg Med 2005;23(2):196.-Letter.
26. Lukens TW, Wolf SJ, Edlow JA, et al. American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med 2006;47(1):79-99.
27. American Psychiatric Association Practice guideline for the treatment of patients with delirium. Practice guidelines for the treatment of psychiatric disorders, compendium 2004 Washington, DC: American Psychiatric Publishing; 29-66.
28. Perrone J, De Roos F, Jayaraman S, Hollander JE. Drug screening versus history in detection of substance use in ED psychiatric patients. Am J Emerg Med 2001;19(1):49-51.
29. Mieczkowski T. Hair analysis for detection of psychotropic drug use [letter]. Mayo Clin Proc 2006;81(4):568-9.
30. McGregor C, Srisurapanont M, Jittiwutikarn J, et al. The nature, time course and severity of methamphetamine withdrawal. Addiction 2005;100(9):1320-9.
31. Grella CE, Hser YI, Huang YC. Mothers in substance abuse treatment: differences in characteristics based on involvement with child welfare services. Child Abuse Negl 2006;30(1):55-73.
Avoiding EPS is key to realizing ‘atypical’ benefits
Many findings of the Clinical Antipsychotic Trials of Intervention Effectiveness in schizophrenia (CATIE) were unexpected,1,2 but one was arguably the most surprising. It was that schizophrenia patients showed similar rates of extrapyramidal symptoms (EPS), whether treated with a first-generation antipsychotic (FGA) or any of four second-generation antipsychotics (SGAs).
This finding in CATIE phase 1 runs contrary to the understanding that SGAs, compared with FGAs, provide a broader spectrum of efficacy with significantly fewer motor side effects. A substantial body of evidence and virtually all schizophrenia treatment guidelines3-5 support this prevailing view.
Did earlier schizophrenia treatment studies misinform us, or was CATIE’s comparison of FGAs and SGAs “flawed”?6,7 This article attempts to reconcile the divergent findings about antipsychotics and EPS and reveals a clinical pearl that suggests how to provide optimum antipsychotic therapy to schizophrenia patients.
What did catie find?
CATIE was a three-phase, 18-month, randomized controlled clinical trial designed to evaluate the effectiveness of five SGAs (risperidone, olanzapine, quetiapine, ziprasidone, and clozapine) and two FGAs (perphenazine and fluphenazine) in treating schizophrenia. Findings from phases 1 and 2 have been published or presented (Table 1),2,8-9 and results from phase 3 are awaited.
Table 1
5 key findings from CATIE phases 1 and 2
|
CATIE phase 1 found no difference in efficacy, safety/tolerability, or effectiveness among perphenazine, risperidone, ziprasidone, and quetiapine. Soon-to-be-published data also will show no significant difference in cognitive effects among patients receiving perphenazine or any of four SGAs (risperidone, olanzapine, quetiapine, or ziprasidone).8 Because no FGA was used in CATIE phase 2,9-11 its results added little to phase 1 observations about how “typical” and “atypical” antipsychotics compare.
‘Atypicals’ and EPS. By definition, a reduced tendency to cause EPS (such as parkinsonism, dystonia, akathisia, and akinesia) distinguishes SGAs from FGAs. In fact, SGAs were called “atypical” because they disproved the belief that EPS are an unavoidable consequence of drugs that produce an antipsychotic effect.12,13 The CATIE trial’s inability to detect a difference in EPS rates between typical and atypical antipsychotics (Table 2)2 is therefore the study’s most surprising finding.
Table 2
CATIE: Similar EPS rates with perphenazine and SGAs*
| EPS measurement | Perphenazine-treated patients | SGA-treated patients |
|---|---|---|
| Increased mean Simpson-Angus Scale score | 6% | 4% to 8% |
| Increased AIMS global severity score | 17% | 13% to 16% |
| Increased Barnes Akathisia Rating Scale score | 7% | 5% to 9% |
| Anticholinergic added | 10% | 3% to 9% |
| * Differences were not statistically significant | ||
| EPS: extrapyramidal side effects | ||
| SGA: second-generation antipsychotic | ||
| AIMS: Abnormal Involuntary Movement Scale | ||
| Source: Reference 2 | ||
Making sense of catie
Most studies suggest consistent differences between FGAs and SGAs in risk of EPS and tardive dyskinesia.14-16 One explanation for CATIE’s discrepant findings may be that the use of high-dose, high-potency haloperidol as the typical comparator in pre-CATIE studies magnified differences between FGAs and SGAs.17,18
Conversely, CATIE researchers minimized this difference by studying a population of schizophrenia patients at an unusually low risk for EPS. The study design:
- assigned 231 patients with a history of tardive dyskinesia to an SGA, without the opportunity to be randomly assigned to an FGA
- excluded patients with first-episode schizophrenia
- enrolled patients who had been treated with antipsychotics for an average of 14 years without a history of significant adverse effects from study treatments.19
Just as prior studies might have exaggerated the EPS advantage for SGAs, CATIE might have minimized the FGA-SGA difference by studying a low-risk cohort in a way that reduced the trial’s ability to detect such differences.
Interpretation. How can we reconcile the absence of a difference between FGAs and SGAs in EPS liability in CATIE with the preponderance of data suggesting otherwise? It appears that SGAs may be less likely to cause EPS than FGAs, but this difference is not evident in all populations. Furthermore, SGAs and FGAs differ in their ability to provide an adequate antipsychotic effect without EPS.
Among FGAs, low-potency agents are less likely to cause EPS or require concomitant anticholinergics than high-potency agents. Among SGAs, the gradient of EPS liability appears to be risperidone > olanzapine, aripiprazole, ziprasidone > quetiapine > clozapine (Figure). Clinically, these pharmacologic differences interact with physiologic differences in EPS vulnerability—some patients are more liable to develop EPS than others. Individuals who are more susceptible to developing EPS are more likely to benefit from antipsychotics with lower EPS liability.
CATIE found no difference among the various FGAs and SGAs with regard to overall efficacy, effects on cognition, and occurrence of tardive dyskinesia in treating chronic schizophrenia. Perhaps it was CATIE’s failure to find a difference in EPS that explains its inability to demonstrate FGA-SGA differences in cognition and other effectiveness domains.
Figure Dose-response curves: Antipsychotic vs extrapyramidal effects
All FGAs and SGAs produce an equivalent antipsychotic effect (red), but they vary in the degree of separation between dosages at which their antipsychotic and extrapyramidal effects occur.
Source: Adapted from reference 13
What catie tells us
The exaggerated view of SGAs as uniformly more efficacious, safer, and better tolerated than FGAs needs to be revised. At the same time, however, the results of CATIE should not be over-interpreted. They tell us that if the four phase 1 SGAs and the FGA perphenazine are used at certain dosages in a particular manner in a specific schizophrenia population—chronic, moderately ill, without tardive dyskinesia—then no differences might be expected among these antipsychotics. But CATIE’s findings might not generalize beyond individuals with schizophrenia at low risk for EPS.
CATIE underlines the importance of achieving an adequate antipsychotic effect without EPS and without using anticholinergics. Clinical consequences of EPS extend beyond motor manifestations and include:
- worse cognition (bradyphrenia)
- worse negative symptoms (neuroleptic-induced deficit syndrome)
- worse depression and suicidality (neuroleptic dysphoria)
- higher risk of tardive dyskinesia.20
SGAs’ presumed ability to provide broader efficacy—cognition, negative symptoms, dysphoria—and lower risk of tardive dyskinesia appears to be driven by their lower EPS liability in association with an equivalent antipsychotic effect. Evidence for an SGA advantage independent of this effect is weak.21,22
Thus, CATIE’s inability to find an FGA-SGA difference in EPS might explain its failure to observe an FGA-SGA difference in cognition and other effectiveness domains.
The clinical pearl
Avoiding EPS and anticholinergics appears to be the key to improving cognition, dysphoria, and negative symptoms with FGAs and SGAs. SGAs’ ability to achieve an equivalent antipsychotic effect without EPS also seems related to their lower risk of tardive dyskinesia.
SGAs’ main advantage may be their greater ease of achieving an adequate antipsychotic effect without EPS or the need to add an anticholinergic to treat or prevent EPS. This comes from the broader separation between dosages at which SGAs produce their antipsychotic versus EPS effects, compared with FGAs (Figure).13
In clinical practice, then, we must achieve an adequate antipsychotic effect for our patients without EPS—whether we are using FGAs or SGAs—to obtain “atypical” benefits. The purported benefits of an “atypical” antipsychotic are not unique to a particular class of agents but relate to achieving a good antipsychotic effect without EPS—and the SGAs are better able to accomplish this than the FGAs.
Careful EPS monitoring is crucial to achieving optimal antipsychotic therapy. Reduced emphasis on EPS in the past decade (in awareness of EPS and training to detect symptoms) and overlap between behavioral aspects of EPS and psychopathology need to be addressed.
CATIE confirms clinical observations that:
Different agents are associated with different adverse effects, which can make achieving maximum efficacy and safety/tolerability challenging.
But differences among antipsychotics and heterogeneity in individual response and vulnerabilities may allow us to optimize treatment.
Different agents at different dosages may provide the best outcomes for individual patients, and the optimal agent and/or dosage can vary in the same patient at different stages of the illness. The CATIE trial contributes to evidence that guides our efforts to provide optimal antipsychotic treatment of schizophrenia (Table 3). Its “surprising” findings are most useful when considered in the context of the database to which it adds.25
Table 3
Treating chronic schizophrenia: 4 clinical tips from CATIE
| Minimizing extrapyramidal symptoms (EPS) is essential, whether using FGAs or SGAs |
| Avoiding EPS and not using adjunctive anticholinergics is the key to SGAs’ purported benefits, such better cognition, less dysphoria, lower negative symptom burden, and lower risk of tardive dyskinesia |
| Antipsychotic dosing is key to accomplishing an adequate antipsychotic effect without EPS |
| Match the antipsychotic choice and dosage to the individual patient’s vulnerability, then make adjustments based on response |
Related resources
- Tandon R. Comparative effectiveness of antipsychotics in the treatment of schizophrenia: What does CATIE tell us? Parts 1 and 2. Int Drug Ther Newsl 2006;41:51-8;67-74.
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia study. www.catie.unc.edu/schizophrenia.
Drug brand names
- Clozapine • Clozaril
- Fluphenazine • Permitil
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Risperidone • Risperdal
- Quetiapine • Seroquel
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Nasrallah HA. CATIE’s surprises. Current Psychiatry 2006;5(2):48-65.
2. Lieberman JA, Stroup ST, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
3. Kane JM, Leucht S, Carpenter D, et al. The expert consensus guideline series: optimizing pharmacologic treatment of psychotic disorders. J Clin Psychiatry 2003;64(suppl 12):1-100.
4. Miller AL, Hall CS, Buchanan RW, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2003 update. J Clin Psychiatry 2004;65:500-8.
5. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, 2nd ed. Am J Psychiatry 2004;161(suppl 2):1-56.
6. Kane JM. Commentary on the CATIE trial. J Clin Psychiatry 2006;67:831-2.
7. Glick ID. Understanding the results of CATIE in the context of the field. CNS Spectrums 2006;1(suppl 7):40-7.
8. Keefe RSE. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Paper presented at: 61st Society of Biological Psychiatry annual meeting; May 18-20, 2006; Toronto, Canada.
9. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
10. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
11. Buckley PF. Which antipsychotic do I choose next? CATIE phase 2 offers insights on efficacy and tolerability. Current Psychiatry 2006;5(9):27-43.
12. Casey DE. Motor and mental aspects of EPS. Int Clin Psychopharmacol 1995;10:105-14.
13. Jibson MD, Tandon R. New atypical antipsychotic medications. J Psychiatr Res 1998;32:215-28.
14. Pierre JM. Extrapyramidal symptoms with atypical antipsychotics. Drug Safety 2005;28:191-208.
15. Kelly DL, Conley RR, Carpenter WT. First-episode schizophrenia: A focus on pharmacological treatment and safety considerations. Drugs 2005;65:1113-38.
16. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 2004;161:414-25.
17. Geddes J, Freemantle N, Harrison P, et al. Atypical antipsychotics in the treatment of schizophrenia: a systematic overview and meta-regression analysis. BMJ 2000;231:1371-6.
18. Hugenholtz GW, Heerdink ER, Stolker JJ, et al. Haloperidol dose when used as active comparator in randomized controlled trials with atypical antipsychotics in schizophrenia: comparison with officially recommended doses. J Clin Psychiatry 2006;67:897-903.
19. Casey D. Implications of the CATIE trial on treatment: extrapyramidal symptoms. CNS Spectrums 2006;11(suppl 7):25-31.
20. Tandon R. Jibson MD: Extrapyramidal side effects of antipsychotic treatment: Scope of problem and impact on outcome. Ann Clin Psychiatry 2002;14:123-9.
21. Thornton AE, Snellenberg JXV, Sepehry AA, Honer WG. The impact of atypical antipsychotic medications on long-term memory dysfunction in schizophrenia spectrum disorder: a quantitative review. J Psychopharmacol 2006;20:335-46.
22. Carpenter WT, Gold JM. Another view of therapy for cognition in schizophrenia. Biol Psychiatry 2002;51:972-8.
23. Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol 2004;24:192-208.
24. Tandon R, Nasrallah HA. Subjecting meta-analyses to closer scrutiny: Little support for differential efficacy among second-generation antipsychotics at equivalent doses. Arch Gen Psychiatry 2006;62:935-7.
25. Tandon R. Comparing antipsychotic efficacy. Am J Psychiatry 2006;163:1645.-
Many findings of the Clinical Antipsychotic Trials of Intervention Effectiveness in schizophrenia (CATIE) were unexpected,1,2 but one was arguably the most surprising. It was that schizophrenia patients showed similar rates of extrapyramidal symptoms (EPS), whether treated with a first-generation antipsychotic (FGA) or any of four second-generation antipsychotics (SGAs).
This finding in CATIE phase 1 runs contrary to the understanding that SGAs, compared with FGAs, provide a broader spectrum of efficacy with significantly fewer motor side effects. A substantial body of evidence and virtually all schizophrenia treatment guidelines3-5 support this prevailing view.
Did earlier schizophrenia treatment studies misinform us, or was CATIE’s comparison of FGAs and SGAs “flawed”?6,7 This article attempts to reconcile the divergent findings about antipsychotics and EPS and reveals a clinical pearl that suggests how to provide optimum antipsychotic therapy to schizophrenia patients.
What did catie find?
CATIE was a three-phase, 18-month, randomized controlled clinical trial designed to evaluate the effectiveness of five SGAs (risperidone, olanzapine, quetiapine, ziprasidone, and clozapine) and two FGAs (perphenazine and fluphenazine) in treating schizophrenia. Findings from phases 1 and 2 have been published or presented (Table 1),2,8-9 and results from phase 3 are awaited.
Table 1
5 key findings from CATIE phases 1 and 2
|
CATIE phase 1 found no difference in efficacy, safety/tolerability, or effectiveness among perphenazine, risperidone, ziprasidone, and quetiapine. Soon-to-be-published data also will show no significant difference in cognitive effects among patients receiving perphenazine or any of four SGAs (risperidone, olanzapine, quetiapine, or ziprasidone).8 Because no FGA was used in CATIE phase 2,9-11 its results added little to phase 1 observations about how “typical” and “atypical” antipsychotics compare.
‘Atypicals’ and EPS. By definition, a reduced tendency to cause EPS (such as parkinsonism, dystonia, akathisia, and akinesia) distinguishes SGAs from FGAs. In fact, SGAs were called “atypical” because they disproved the belief that EPS are an unavoidable consequence of drugs that produce an antipsychotic effect.12,13 The CATIE trial’s inability to detect a difference in EPS rates between typical and atypical antipsychotics (Table 2)2 is therefore the study’s most surprising finding.
Table 2
CATIE: Similar EPS rates with perphenazine and SGAs*
| EPS measurement | Perphenazine-treated patients | SGA-treated patients |
|---|---|---|
| Increased mean Simpson-Angus Scale score | 6% | 4% to 8% |
| Increased AIMS global severity score | 17% | 13% to 16% |
| Increased Barnes Akathisia Rating Scale score | 7% | 5% to 9% |
| Anticholinergic added | 10% | 3% to 9% |
| * Differences were not statistically significant | ||
| EPS: extrapyramidal side effects | ||
| SGA: second-generation antipsychotic | ||
| AIMS: Abnormal Involuntary Movement Scale | ||
| Source: Reference 2 | ||
Making sense of catie
Most studies suggest consistent differences between FGAs and SGAs in risk of EPS and tardive dyskinesia.14-16 One explanation for CATIE’s discrepant findings may be that the use of high-dose, high-potency haloperidol as the typical comparator in pre-CATIE studies magnified differences between FGAs and SGAs.17,18
Conversely, CATIE researchers minimized this difference by studying a population of schizophrenia patients at an unusually low risk for EPS. The study design:
- assigned 231 patients with a history of tardive dyskinesia to an SGA, without the opportunity to be randomly assigned to an FGA
- excluded patients with first-episode schizophrenia
- enrolled patients who had been treated with antipsychotics for an average of 14 years without a history of significant adverse effects from study treatments.19
Just as prior studies might have exaggerated the EPS advantage for SGAs, CATIE might have minimized the FGA-SGA difference by studying a low-risk cohort in a way that reduced the trial’s ability to detect such differences.
Interpretation. How can we reconcile the absence of a difference between FGAs and SGAs in EPS liability in CATIE with the preponderance of data suggesting otherwise? It appears that SGAs may be less likely to cause EPS than FGAs, but this difference is not evident in all populations. Furthermore, SGAs and FGAs differ in their ability to provide an adequate antipsychotic effect without EPS.
Among FGAs, low-potency agents are less likely to cause EPS or require concomitant anticholinergics than high-potency agents. Among SGAs, the gradient of EPS liability appears to be risperidone > olanzapine, aripiprazole, ziprasidone > quetiapine > clozapine (Figure). Clinically, these pharmacologic differences interact with physiologic differences in EPS vulnerability—some patients are more liable to develop EPS than others. Individuals who are more susceptible to developing EPS are more likely to benefit from antipsychotics with lower EPS liability.
CATIE found no difference among the various FGAs and SGAs with regard to overall efficacy, effects on cognition, and occurrence of tardive dyskinesia in treating chronic schizophrenia. Perhaps it was CATIE’s failure to find a difference in EPS that explains its inability to demonstrate FGA-SGA differences in cognition and other effectiveness domains.
Figure Dose-response curves: Antipsychotic vs extrapyramidal effects
All FGAs and SGAs produce an equivalent antipsychotic effect (red), but they vary in the degree of separation between dosages at which their antipsychotic and extrapyramidal effects occur.
Source: Adapted from reference 13
What catie tells us
The exaggerated view of SGAs as uniformly more efficacious, safer, and better tolerated than FGAs needs to be revised. At the same time, however, the results of CATIE should not be over-interpreted. They tell us that if the four phase 1 SGAs and the FGA perphenazine are used at certain dosages in a particular manner in a specific schizophrenia population—chronic, moderately ill, without tardive dyskinesia—then no differences might be expected among these antipsychotics. But CATIE’s findings might not generalize beyond individuals with schizophrenia at low risk for EPS.
CATIE underlines the importance of achieving an adequate antipsychotic effect without EPS and without using anticholinergics. Clinical consequences of EPS extend beyond motor manifestations and include:
- worse cognition (bradyphrenia)
- worse negative symptoms (neuroleptic-induced deficit syndrome)
- worse depression and suicidality (neuroleptic dysphoria)
- higher risk of tardive dyskinesia.20
SGAs’ presumed ability to provide broader efficacy—cognition, negative symptoms, dysphoria—and lower risk of tardive dyskinesia appears to be driven by their lower EPS liability in association with an equivalent antipsychotic effect. Evidence for an SGA advantage independent of this effect is weak.21,22
Thus, CATIE’s inability to find an FGA-SGA difference in EPS might explain its failure to observe an FGA-SGA difference in cognition and other effectiveness domains.
The clinical pearl
Avoiding EPS and anticholinergics appears to be the key to improving cognition, dysphoria, and negative symptoms with FGAs and SGAs. SGAs’ ability to achieve an equivalent antipsychotic effect without EPS also seems related to their lower risk of tardive dyskinesia.
SGAs’ main advantage may be their greater ease of achieving an adequate antipsychotic effect without EPS or the need to add an anticholinergic to treat or prevent EPS. This comes from the broader separation between dosages at which SGAs produce their antipsychotic versus EPS effects, compared with FGAs (Figure).13
In clinical practice, then, we must achieve an adequate antipsychotic effect for our patients without EPS—whether we are using FGAs or SGAs—to obtain “atypical” benefits. The purported benefits of an “atypical” antipsychotic are not unique to a particular class of agents but relate to achieving a good antipsychotic effect without EPS—and the SGAs are better able to accomplish this than the FGAs.
Careful EPS monitoring is crucial to achieving optimal antipsychotic therapy. Reduced emphasis on EPS in the past decade (in awareness of EPS and training to detect symptoms) and overlap between behavioral aspects of EPS and psychopathology need to be addressed.
CATIE confirms clinical observations that:
Different agents are associated with different adverse effects, which can make achieving maximum efficacy and safety/tolerability challenging.
But differences among antipsychotics and heterogeneity in individual response and vulnerabilities may allow us to optimize treatment.
Different agents at different dosages may provide the best outcomes for individual patients, and the optimal agent and/or dosage can vary in the same patient at different stages of the illness. The CATIE trial contributes to evidence that guides our efforts to provide optimal antipsychotic treatment of schizophrenia (Table 3). Its “surprising” findings are most useful when considered in the context of the database to which it adds.25
Table 3
Treating chronic schizophrenia: 4 clinical tips from CATIE
| Minimizing extrapyramidal symptoms (EPS) is essential, whether using FGAs or SGAs |
| Avoiding EPS and not using adjunctive anticholinergics is the key to SGAs’ purported benefits, such better cognition, less dysphoria, lower negative symptom burden, and lower risk of tardive dyskinesia |
| Antipsychotic dosing is key to accomplishing an adequate antipsychotic effect without EPS |
| Match the antipsychotic choice and dosage to the individual patient’s vulnerability, then make adjustments based on response |
Related resources
- Tandon R. Comparative effectiveness of antipsychotics in the treatment of schizophrenia: What does CATIE tell us? Parts 1 and 2. Int Drug Ther Newsl 2006;41:51-8;67-74.
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia study. www.catie.unc.edu/schizophrenia.
Drug brand names
- Clozapine • Clozaril
- Fluphenazine • Permitil
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Risperidone • Risperdal
- Quetiapine • Seroquel
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Many findings of the Clinical Antipsychotic Trials of Intervention Effectiveness in schizophrenia (CATIE) were unexpected,1,2 but one was arguably the most surprising. It was that schizophrenia patients showed similar rates of extrapyramidal symptoms (EPS), whether treated with a first-generation antipsychotic (FGA) or any of four second-generation antipsychotics (SGAs).
This finding in CATIE phase 1 runs contrary to the understanding that SGAs, compared with FGAs, provide a broader spectrum of efficacy with significantly fewer motor side effects. A substantial body of evidence and virtually all schizophrenia treatment guidelines3-5 support this prevailing view.
Did earlier schizophrenia treatment studies misinform us, or was CATIE’s comparison of FGAs and SGAs “flawed”?6,7 This article attempts to reconcile the divergent findings about antipsychotics and EPS and reveals a clinical pearl that suggests how to provide optimum antipsychotic therapy to schizophrenia patients.
What did catie find?
CATIE was a three-phase, 18-month, randomized controlled clinical trial designed to evaluate the effectiveness of five SGAs (risperidone, olanzapine, quetiapine, ziprasidone, and clozapine) and two FGAs (perphenazine and fluphenazine) in treating schizophrenia. Findings from phases 1 and 2 have been published or presented (Table 1),2,8-9 and results from phase 3 are awaited.
Table 1
5 key findings from CATIE phases 1 and 2
|
CATIE phase 1 found no difference in efficacy, safety/tolerability, or effectiveness among perphenazine, risperidone, ziprasidone, and quetiapine. Soon-to-be-published data also will show no significant difference in cognitive effects among patients receiving perphenazine or any of four SGAs (risperidone, olanzapine, quetiapine, or ziprasidone).8 Because no FGA was used in CATIE phase 2,9-11 its results added little to phase 1 observations about how “typical” and “atypical” antipsychotics compare.
‘Atypicals’ and EPS. By definition, a reduced tendency to cause EPS (such as parkinsonism, dystonia, akathisia, and akinesia) distinguishes SGAs from FGAs. In fact, SGAs were called “atypical” because they disproved the belief that EPS are an unavoidable consequence of drugs that produce an antipsychotic effect.12,13 The CATIE trial’s inability to detect a difference in EPS rates between typical and atypical antipsychotics (Table 2)2 is therefore the study’s most surprising finding.
Table 2
CATIE: Similar EPS rates with perphenazine and SGAs*
| EPS measurement | Perphenazine-treated patients | SGA-treated patients |
|---|---|---|
| Increased mean Simpson-Angus Scale score | 6% | 4% to 8% |
| Increased AIMS global severity score | 17% | 13% to 16% |
| Increased Barnes Akathisia Rating Scale score | 7% | 5% to 9% |
| Anticholinergic added | 10% | 3% to 9% |
| * Differences were not statistically significant | ||
| EPS: extrapyramidal side effects | ||
| SGA: second-generation antipsychotic | ||
| AIMS: Abnormal Involuntary Movement Scale | ||
| Source: Reference 2 | ||
Making sense of catie
Most studies suggest consistent differences between FGAs and SGAs in risk of EPS and tardive dyskinesia.14-16 One explanation for CATIE’s discrepant findings may be that the use of high-dose, high-potency haloperidol as the typical comparator in pre-CATIE studies magnified differences between FGAs and SGAs.17,18
Conversely, CATIE researchers minimized this difference by studying a population of schizophrenia patients at an unusually low risk for EPS. The study design:
- assigned 231 patients with a history of tardive dyskinesia to an SGA, without the opportunity to be randomly assigned to an FGA
- excluded patients with first-episode schizophrenia
- enrolled patients who had been treated with antipsychotics for an average of 14 years without a history of significant adverse effects from study treatments.19
Just as prior studies might have exaggerated the EPS advantage for SGAs, CATIE might have minimized the FGA-SGA difference by studying a low-risk cohort in a way that reduced the trial’s ability to detect such differences.
Interpretation. How can we reconcile the absence of a difference between FGAs and SGAs in EPS liability in CATIE with the preponderance of data suggesting otherwise? It appears that SGAs may be less likely to cause EPS than FGAs, but this difference is not evident in all populations. Furthermore, SGAs and FGAs differ in their ability to provide an adequate antipsychotic effect without EPS.
Among FGAs, low-potency agents are less likely to cause EPS or require concomitant anticholinergics than high-potency agents. Among SGAs, the gradient of EPS liability appears to be risperidone > olanzapine, aripiprazole, ziprasidone > quetiapine > clozapine (Figure). Clinically, these pharmacologic differences interact with physiologic differences in EPS vulnerability—some patients are more liable to develop EPS than others. Individuals who are more susceptible to developing EPS are more likely to benefit from antipsychotics with lower EPS liability.
CATIE found no difference among the various FGAs and SGAs with regard to overall efficacy, effects on cognition, and occurrence of tardive dyskinesia in treating chronic schizophrenia. Perhaps it was CATIE’s failure to find a difference in EPS that explains its inability to demonstrate FGA-SGA differences in cognition and other effectiveness domains.
Figure Dose-response curves: Antipsychotic vs extrapyramidal effects
All FGAs and SGAs produce an equivalent antipsychotic effect (red), but they vary in the degree of separation between dosages at which their antipsychotic and extrapyramidal effects occur.
Source: Adapted from reference 13
What catie tells us
The exaggerated view of SGAs as uniformly more efficacious, safer, and better tolerated than FGAs needs to be revised. At the same time, however, the results of CATIE should not be over-interpreted. They tell us that if the four phase 1 SGAs and the FGA perphenazine are used at certain dosages in a particular manner in a specific schizophrenia population—chronic, moderately ill, without tardive dyskinesia—then no differences might be expected among these antipsychotics. But CATIE’s findings might not generalize beyond individuals with schizophrenia at low risk for EPS.
CATIE underlines the importance of achieving an adequate antipsychotic effect without EPS and without using anticholinergics. Clinical consequences of EPS extend beyond motor manifestations and include:
- worse cognition (bradyphrenia)
- worse negative symptoms (neuroleptic-induced deficit syndrome)
- worse depression and suicidality (neuroleptic dysphoria)
- higher risk of tardive dyskinesia.20
SGAs’ presumed ability to provide broader efficacy—cognition, negative symptoms, dysphoria—and lower risk of tardive dyskinesia appears to be driven by their lower EPS liability in association with an equivalent antipsychotic effect. Evidence for an SGA advantage independent of this effect is weak.21,22
Thus, CATIE’s inability to find an FGA-SGA difference in EPS might explain its failure to observe an FGA-SGA difference in cognition and other effectiveness domains.
The clinical pearl
Avoiding EPS and anticholinergics appears to be the key to improving cognition, dysphoria, and negative symptoms with FGAs and SGAs. SGAs’ ability to achieve an equivalent antipsychotic effect without EPS also seems related to their lower risk of tardive dyskinesia.
SGAs’ main advantage may be their greater ease of achieving an adequate antipsychotic effect without EPS or the need to add an anticholinergic to treat or prevent EPS. This comes from the broader separation between dosages at which SGAs produce their antipsychotic versus EPS effects, compared with FGAs (Figure).13
In clinical practice, then, we must achieve an adequate antipsychotic effect for our patients without EPS—whether we are using FGAs or SGAs—to obtain “atypical” benefits. The purported benefits of an “atypical” antipsychotic are not unique to a particular class of agents but relate to achieving a good antipsychotic effect without EPS—and the SGAs are better able to accomplish this than the FGAs.
Careful EPS monitoring is crucial to achieving optimal antipsychotic therapy. Reduced emphasis on EPS in the past decade (in awareness of EPS and training to detect symptoms) and overlap between behavioral aspects of EPS and psychopathology need to be addressed.
CATIE confirms clinical observations that:
Different agents are associated with different adverse effects, which can make achieving maximum efficacy and safety/tolerability challenging.
But differences among antipsychotics and heterogeneity in individual response and vulnerabilities may allow us to optimize treatment.
Different agents at different dosages may provide the best outcomes for individual patients, and the optimal agent and/or dosage can vary in the same patient at different stages of the illness. The CATIE trial contributes to evidence that guides our efforts to provide optimal antipsychotic treatment of schizophrenia (Table 3). Its “surprising” findings are most useful when considered in the context of the database to which it adds.25
Table 3
Treating chronic schizophrenia: 4 clinical tips from CATIE
| Minimizing extrapyramidal symptoms (EPS) is essential, whether using FGAs or SGAs |
| Avoiding EPS and not using adjunctive anticholinergics is the key to SGAs’ purported benefits, such better cognition, less dysphoria, lower negative symptom burden, and lower risk of tardive dyskinesia |
| Antipsychotic dosing is key to accomplishing an adequate antipsychotic effect without EPS |
| Match the antipsychotic choice and dosage to the individual patient’s vulnerability, then make adjustments based on response |
Related resources
- Tandon R. Comparative effectiveness of antipsychotics in the treatment of schizophrenia: What does CATIE tell us? Parts 1 and 2. Int Drug Ther Newsl 2006;41:51-8;67-74.
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia study. www.catie.unc.edu/schizophrenia.
Drug brand names
- Clozapine • Clozaril
- Fluphenazine • Permitil
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Risperidone • Risperdal
- Quetiapine • Seroquel
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Nasrallah HA. CATIE’s surprises. Current Psychiatry 2006;5(2):48-65.
2. Lieberman JA, Stroup ST, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
3. Kane JM, Leucht S, Carpenter D, et al. The expert consensus guideline series: optimizing pharmacologic treatment of psychotic disorders. J Clin Psychiatry 2003;64(suppl 12):1-100.
4. Miller AL, Hall CS, Buchanan RW, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2003 update. J Clin Psychiatry 2004;65:500-8.
5. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, 2nd ed. Am J Psychiatry 2004;161(suppl 2):1-56.
6. Kane JM. Commentary on the CATIE trial. J Clin Psychiatry 2006;67:831-2.
7. Glick ID. Understanding the results of CATIE in the context of the field. CNS Spectrums 2006;1(suppl 7):40-7.
8. Keefe RSE. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Paper presented at: 61st Society of Biological Psychiatry annual meeting; May 18-20, 2006; Toronto, Canada.
9. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
10. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
11. Buckley PF. Which antipsychotic do I choose next? CATIE phase 2 offers insights on efficacy and tolerability. Current Psychiatry 2006;5(9):27-43.
12. Casey DE. Motor and mental aspects of EPS. Int Clin Psychopharmacol 1995;10:105-14.
13. Jibson MD, Tandon R. New atypical antipsychotic medications. J Psychiatr Res 1998;32:215-28.
14. Pierre JM. Extrapyramidal symptoms with atypical antipsychotics. Drug Safety 2005;28:191-208.
15. Kelly DL, Conley RR, Carpenter WT. First-episode schizophrenia: A focus on pharmacological treatment and safety considerations. Drugs 2005;65:1113-38.
16. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 2004;161:414-25.
17. Geddes J, Freemantle N, Harrison P, et al. Atypical antipsychotics in the treatment of schizophrenia: a systematic overview and meta-regression analysis. BMJ 2000;231:1371-6.
18. Hugenholtz GW, Heerdink ER, Stolker JJ, et al. Haloperidol dose when used as active comparator in randomized controlled trials with atypical antipsychotics in schizophrenia: comparison with officially recommended doses. J Clin Psychiatry 2006;67:897-903.
19. Casey D. Implications of the CATIE trial on treatment: extrapyramidal symptoms. CNS Spectrums 2006;11(suppl 7):25-31.
20. Tandon R. Jibson MD: Extrapyramidal side effects of antipsychotic treatment: Scope of problem and impact on outcome. Ann Clin Psychiatry 2002;14:123-9.
21. Thornton AE, Snellenberg JXV, Sepehry AA, Honer WG. The impact of atypical antipsychotic medications on long-term memory dysfunction in schizophrenia spectrum disorder: a quantitative review. J Psychopharmacol 2006;20:335-46.
22. Carpenter WT, Gold JM. Another view of therapy for cognition in schizophrenia. Biol Psychiatry 2002;51:972-8.
23. Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol 2004;24:192-208.
24. Tandon R, Nasrallah HA. Subjecting meta-analyses to closer scrutiny: Little support for differential efficacy among second-generation antipsychotics at equivalent doses. Arch Gen Psychiatry 2006;62:935-7.
25. Tandon R. Comparing antipsychotic efficacy. Am J Psychiatry 2006;163:1645.-
1. Nasrallah HA. CATIE’s surprises. Current Psychiatry 2006;5(2):48-65.
2. Lieberman JA, Stroup ST, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
3. Kane JM, Leucht S, Carpenter D, et al. The expert consensus guideline series: optimizing pharmacologic treatment of psychotic disorders. J Clin Psychiatry 2003;64(suppl 12):1-100.
4. Miller AL, Hall CS, Buchanan RW, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2003 update. J Clin Psychiatry 2004;65:500-8.
5. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, 2nd ed. Am J Psychiatry 2004;161(suppl 2):1-56.
6. Kane JM. Commentary on the CATIE trial. J Clin Psychiatry 2006;67:831-2.
7. Glick ID. Understanding the results of CATIE in the context of the field. CNS Spectrums 2006;1(suppl 7):40-7.
8. Keefe RSE. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Paper presented at: 61st Society of Biological Psychiatry annual meeting; May 18-20, 2006; Toronto, Canada.
9. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
10. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
11. Buckley PF. Which antipsychotic do I choose next? CATIE phase 2 offers insights on efficacy and tolerability. Current Psychiatry 2006;5(9):27-43.
12. Casey DE. Motor and mental aspects of EPS. Int Clin Psychopharmacol 1995;10:105-14.
13. Jibson MD, Tandon R. New atypical antipsychotic medications. J Psychiatr Res 1998;32:215-28.
14. Pierre JM. Extrapyramidal symptoms with atypical antipsychotics. Drug Safety 2005;28:191-208.
15. Kelly DL, Conley RR, Carpenter WT. First-episode schizophrenia: A focus on pharmacological treatment and safety considerations. Drugs 2005;65:1113-38.
16. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 2004;161:414-25.
17. Geddes J, Freemantle N, Harrison P, et al. Atypical antipsychotics in the treatment of schizophrenia: a systematic overview and meta-regression analysis. BMJ 2000;231:1371-6.
18. Hugenholtz GW, Heerdink ER, Stolker JJ, et al. Haloperidol dose when used as active comparator in randomized controlled trials with atypical antipsychotics in schizophrenia: comparison with officially recommended doses. J Clin Psychiatry 2006;67:897-903.
19. Casey D. Implications of the CATIE trial on treatment: extrapyramidal symptoms. CNS Spectrums 2006;11(suppl 7):25-31.
20. Tandon R. Jibson MD: Extrapyramidal side effects of antipsychotic treatment: Scope of problem and impact on outcome. Ann Clin Psychiatry 2002;14:123-9.
21. Thornton AE, Snellenberg JXV, Sepehry AA, Honer WG. The impact of atypical antipsychotic medications on long-term memory dysfunction in schizophrenia spectrum disorder: a quantitative review. J Psychopharmacol 2006;20:335-46.
22. Carpenter WT, Gold JM. Another view of therapy for cognition in schizophrenia. Biol Psychiatry 2002;51:972-8.
23. Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol 2004;24:192-208.
24. Tandon R, Nasrallah HA. Subjecting meta-analyses to closer scrutiny: Little support for differential efficacy among second-generation antipsychotics at equivalent doses. Arch Gen Psychiatry 2006;62:935-7.
25. Tandon R. Comparing antipsychotic efficacy. Am J Psychiatry 2006;163:1645.-