User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Identifying hyperthyroidism’s psychiatric presentations
Ms. A experienced an anxiety attack while driving home from work, with cardiac palpitations, tingling of the face, and fear of impending doom. Over the following 3 months she endured a “living hell,” consisting of basal anxiety, intermittent panic attacks, and agoraphobia, with exceptional difficulty even going to the grocery store.
A high-functioning career woman in her 30s, Ms. A also developed insomnia, depressed mood, and intrusive ego-dystonic thoughts. These symptoms emerged 10 years after a subtotal thyroidectomy for hyperthyroidism (Graves’ disease).
Hyperthyroidism’s association with psychiatric-spectrum symptoms is well-recognized (Box 1).1-4 Hyperthyroid patients are significantly more likely than controls to report feelings of isolation, impaired social functioning, anxiety, and mood disturbances5 and are more likely to be hospitalized with an affective disorder.6
Other individuals with subclinical or overt biochemical hyperthyroidism self-report above-average mood and lower-than-average anxiety.7
Ms. A’s is the first of three cases presented here to help you screen for and identify thyrotoxicosis (thyroid and nonthyroid causes of excessive thyroid hormone). Cases include:
- recurrent Graves’ disease with panic disorder and residual obsessive-compulsive disorder (Ms. A)
- undetected Graves’ hyperthyroidism in a bipolar-like mood syndrome with severe anxiety and cognitive decline (Ms. B)
- occult hyperthyroidism with occult anxiety (Mr. C).
These cases show that even when biochemical euthyroidism is restored, many formerly hyperthyroid patients with severe mood, anxiety, and/or cognitive symptoms continue to have significant residual symptoms that require ongoing psychiatric attention.6
Ms. A: Anxiety and thyrotoxicosis
Ms. A was greatly troubled by her intrusive ego-dystonic thoughts, which involved:
- violence to her beloved young children (for example, what would happen if someone started shooting her children with a gun)
- bizarre sexual ideations (for example, during dinner with an elderly woman she could not stop imagining her naked)
- paranoid ideations (for example, “Is my husband poisoning me?”).
She consulted a psychologist who told her that she suffered from an anxiety disorder and recommended psychotherapy, which was not helpful. She then sought endocrine consultation, and tests showed low-grade overt hyperthyroidism, with unmeasurably low thyroid stimulating hormone (TSH) concentrations and marginally elevated total and free levothyroxine (T4). Her levothyroxine replacement dosage was reduced from 100 to 50 mcg/d, then discontinued.
Without thyroid supplementation or replacement, she became biochemically euthyroid, with TSH 1.47 mIU/L and triiodothyronine (T3) and T4 in mid-normal range. Her panic anxiety resolved and her mood and sleep normalized, but the bizarre thoughts remained. The endocrinologist referred her to a psychiatrist, who diagnosed obsessive-compulsive disorder. Ms. A was effectively treated with fluvoxamine, 125 mg/d.
Discussion. Many patients with hyperthyroidism suffer from anxiety syndromes,8-10 including generalized anxiety disorder and social phobia (Table 1). “Nervousness” (including “feelings of apprehension and inability to concentrate”) is almost invariably present in the thyrotoxicosis of Graves’ disease.11
Hyperthyroidism-related anxiety syndromes are typically complicated by major depression and cognitive decline, such as in memory and attention.9 Thus, a pituitary-thyroid workup is an important step in the psychiatric evaluation of any patient with clinically significant anxiety (Box 2).3
The brain has among the highest expression of thyroid hormone receptors of any organ,1,2 and neurons are often more sensitive to thyroid abnormalities—including overt or subclinical hyperthyroidism and thyrotoxicosis, thyroiditis, and hypothyroidism3—than are other tissues.
Hyperthyroidism is often associated with anxiety, depression, mixed mood disorders, a hypomanic-like picture, emotional lability, mood swings, irritability/edginess, or cognitive deterioration with concentration problems. It also can manifest as psychosis or delirium.
Hyperthyroidism affects approximately 2.5% of the U.S. population (~7.5 million persons), according to the National Health and Nutrition Examination Survey (NHANES III). One-half of those afflicted (1.3%) do not know they are hyperthyroid, including 0.5% with overt symptoms and 0.8% with subclinical disease.
NHANES III defined hyperthyroidism as thyroid-stimulating hormone (TSH) <0.1 mIU/L with total thyroxine (T4) levels either elevated (overt hyperthyroidism) or normal (subclinical hyperthyroidism). Women are at least 5 times more likely than men to be hyperthyroid.4
CNS hypersensitivity to low-grade hyperthyroidism can manifest as an anxiety disorder before other Graves’ disease stigmata emerge. Panic disorder, for example, has been reported to precede Graves’ hyperthyroidism by 4 to 5 years in some cases,12 although how frequently this occurs is not known. Therefore, re-evaluate the thyroid status of any patient with severe anxiety who is biochemically euthyroid. Check yearly, for example, if anxiety is incompletely resolved.
Table 1
Psychiatric symptoms seen with hyperthyroidism
| Anxiety |
| Apathy (more often seen in older patients) |
| Cognitive impairment |
| Delirium |
| Depression |
| Emotional lability |
| Fatigue |
| Hypomania or mania |
| Impaired concentration |
| Insomnia |
| Irritability |
| Mood swings |
| Psychomotor agitation |
| Psychosis |
Causes of hyperthyroidism
Approximately 20 causes of thyrotoxicosis and hyperthyroxinemia have been characterized (see Related resources).11,13-15 The most common causes of hyperthyroidism are Graves’ disease, toxic multinodular goiter, and toxic thyroid adenoma. Another is thyroiditis, such as from lithium or iodine excess (such as from the cardiac drug amiodarone). A TSH-secreting pituitary adenoma is a rare cause of hyperthyroidism.16
A drug-induced thyrotoxic state can be seen with excess administration of exogenous thyroid hormone. This condition usually occurs inadvertently but is sometimes intentional, as in factitious disorder or malingering.
Graves’ disease is an autoimmune disorder that occurs when antibodies (thyroid-stimulating hormone immunoglobulins) stimulate thyroid TSH receptors, increasing thyroid hormone synthesis and secretion. Graves’ disease—seen in 60% to 85% of patients with thyrotoxicosis—is the most common cause of hyperthyroidism.15
Patients most often are women of childbearing years to middle age. Exophthalmos and other eye changes are common, along with diffuse goiter. Encephalopathy can be seen in Graves’ disease and Hashimoto’s thyroiditis because the brain can become an antibody target in autoimmune disorders.
Toxic multinodular goiter consists of autonomously functioning, circumscribed thyroid nodules with an enlarged (goitrous) thyroid, that typically emerge at length from simple (nontoxic) goiter—characterized by enlarged thyroid but normal thyroid-related biochemistry. Onset is typically later in life than Graves’ disease.11,17
Thyrotoxicosis is often relatively mild in toxic multinodular goiter, with marginal elevations in T4 and/or T3. Unlike in Graves’ disease, ophthalmologic changes are unusual. Tachycardia and weakness are common (Table 2).
Table 2
Nonpsychiatric symptoms seen with hyperthyroidism
| Metabolic |
| Heat intolerance (cold tolerance) |
| Increased perspiration |
| Weight loss (despite good appetite) |
| Endocrinologic |
| Goiter (enlarged thyroid gland) |
| Ophthalmologic |
| Exophthalmos |
| Lid lag |
| Stare/infrequent blinking |
| Ophthalmoplegia |
| Neurologic |
| Tremor |
| Hyperreflexia |
| Motor restlessness |
| Proximal muscle weakness/myopathy |
| Cardiologic |
| Tachycardia |
| Palpitations |
| Arrhythmia |
| Worsening or precipitation of angina, heart failure |
| Sexual |
| Oligomenorrhea/amenorrhea |
| Rapid ejaculation |
| Dermatologic |
| Warm, moist skin |
| Fine hair |
| Velvety skin texture |
| Onycholysis |
| Myxedema/leg swelling |
| Ruddy or erythemic skin/facial flushing |
| Eyelash loss |
| Hair loss |
| Premature graying (Graves’ disease) |
| Pruritus |
| Gastrointestinal |
| Frequent bowel movements |
| Diarrhea |
| Nausea |
| Orthopedic |
| Osteopenia or osteoporosis |
Adenomas. Toxic thyroid adenoma is a hyperfunctioning (“toxic”) benign tumor of the thyroid follicular cell. A TSH-secreting pituitary adenoma is a rare cause of hyperthyroidism.16
Thyroid storm is a rare, life-threatening thyrotoxicosis, usually seen in medical or surgical patients. Symptoms include fever, tachycardia, hypotension, irritability and restlessness, nausea and vomiting, delirium, and possibly coma.
Psychiatrists rarely see these cases, but propranolol (40 mg initial dose), fluids, and swift transport to an emergency room or critical care unit are indicated. Antithyroid agents and glucocorticoids are the usual treatment.
Thyrotoxic symptoms from thyroid hormone therapy. Thyroid hormone has been used in psychiatric patients as an antidepressant supplement,18 with therapeutic benefit reported to range from highly valuable19 to modestly helpful or no effect.20 In some patients thyroid hormone causes thyrotoxic symptoms such as tachycardia, gross tremulousness, restlessness, anxiety, inability to sleep, and impaired concentration.
Patients newly diagnosed with hypothyroidism can be exquisitely sensitive to exogenous thyroid hormone and develop acute thyrotoxic symptoms. When this occurs, a more measured titration of thyroid dose is indicated, rather than discontinuing hormone therapy. For example, patients whose optimal maintenance levothyroxine dosage proves to be >100 mcg/d might do better by first adapting to 75 mcg/d.
Thyroid hormone replacement can increase demand on the adrenal glands of chronically hypothyroid patients. For those who develop thyrotoxic-like symptoms, a pulse of glucocorticoids—such as a single 20-mg dose of prednisone (2 to 3 times the typical daily glucocorticoid maintenance requirement)—is sometimes very helpful. Severe eye pain and periorbital edema has been reported to respond to prednisone doses of 120 mg/d.13
Serum TSH is a sensitive screen. Low (<0.1 mIU/mL) or immeasurably low (<0.05 mIU/mL) circulating TSH usually means hyperthyroidism. A TSH screen is not foolproof, however; very low TSH can be seen with low circulating thyroid hormones in central hypothyroidism or in cases of laboratory error.
The recommended routine initial screen of the pituitary-thyroid axis in psychiatric patients includes TSH, free T4, and possibly free T3.3 Suppressed TSH with high serum free T3 and/or free T4 (accompanied by high total T4 and/or T3) is diagnostic of frank biochemical hyperthyroidism. If circulating thyroid hormone concentrations are normal, hyperthyroidism is considered compensated or subclinical. Although only free thyroid hormones are active, total T4 and total T3 are of interest to grossly estimate thyroid hormone output.
When you identify a thyrotoxic state, refer the patient for an endocrinologic evaluation. Antithyroid antibodies are often positive in Graves’ disease, but anti-TSH antibodies (which can be routinely ordered) are particularly diagnostic. If thyroid dysfunction is present—especially if autoimmune-based—screening tests are indicated to rule out adrenal, gonadal, and pancreatic (glucose regulation) dysfunction.
Ms. B: Hyperthyroidism and mood
Ms. B, age 35, an energetic clerical worker and fitness devotee, developed severe insomnia. She slept no more than 1 hour per night, with irritability, verbal explosiveness, “hot flashes,” and depressed mood. “Everything pisses me off violently,” she said.
She consulted a psychiatrist and was diagnosed with major depression. Over a period of years, she was serially prescribed selective serotonin reuptake inhibitors, serotonin/norepinephrine reuptake inhibitors, and older-generation sedating agents including trazodone and amitriptyline. She tolerated none of these because of side effects, including dysphoric hyperarousal and cognitive disruption.
“They all made me stupid,” she complained.
Zolpidem, 20 mg at night, helped temporarily as a hypnotic, but insomnia recurred within weeks. Diazepam was effective at high dosages but also dulled her cognition. The psychiatrist did not suspect a thyroid abnormality and did not perform a pituitary-thyroid laboratory evaluation.
Ms. B consulted a gynecologist, who prescribed estrogen for borderline low estradiol levels and with the hope that Ms. B’s symptoms represented early menopause. This partially ameliorated her irritability, possibly because estradiol binding of circulating T4 reduced free thyroid hormone levels.
Ms. B tried to continue working and exercising, but within 4 years her symptoms progressed to severe depression with frequent crying spells, feelings of general malaise, excessive sweating, occasional panic attacks, fatigue, sleepiness, deteriorating vision, and cognitive impairment. She struggled to read printed words and eventually took sick leave while consulting with physicians.
Finally, a routine thyroid screen before minor surgery revealed an undetectable TSH concentration. Further testing showed elevated thyroxine consistent with thyrotoxicosis. Graves’ disease was diagnosed, and euthyroidism was established with antithyroid medication.
Residual mood and anxiety symptoms persisted 1 year after euthyroidism was restored, and Ms. B sought psychiatric consultation.
Discussion. Hyperthyroidism can trigger or present as a hypomania or manic-like state, characterized by increased energy, hyperactivity, racing thoughts, hair-trigger verbal explosiveness, and decreased need for sleep.
Hypertalkativeness is common, even without pressured speech, as is irritability. Mood may be elevated, depressed, mixed, or cycling. A hyperthyroidism-related mixed syndrome of depression and hypomania can be confounding.
Mr. C: Occult hyperthyroidism
Mr. C, age 26, was apparently healthy when he was admitted into a neuroendocrine research protocol as a volunteer. His job performance was excellent, and his interactions with others were good; he was in good general health and taking no medication.
Formal psychiatric screening found no history of psychiatric disorders in Mr. C nor his family. His mental status was within normal limits. Physical exam revealed no significant abnormality. He was afebrile, normotensive, and had a resting pulse of 81 bpm.
His neurologic status was unremarkable, and laboratory screening tests showed normal CBC, liver and renal profiles, glucose, platelets and clotting times. Tests during the study, however, showed frankly elevated T4, free thyroxine (FT4), and T3 concentrations, along with undetectable TSH. Mr. C was informed of these results and referred to an endocrinologist.
Graves’ disease was diagnosed, and Mr. C received partial thyroid ablation therapy. He later reported that he had never felt better. In retrospect, he realized he had been anxious before he was treated for hyperthyroidism because he felt much more relaxed and able to concentrate after treatment.
Discussion. Subjective well-being in a patient with occult biochemical thyrotoxicosis can be misleading. Mr. C was much less anxious and able to concentrate after his return to euthyroidism.
Treatment
Refer your hyperthyroid patients to an endocrinologist for further workup and, in most cases, management. Hyperthyroidism is usually easy to treat using a form of ablation (antithyroid drugs, radioactive iodine, or partial thyroidectomy).
Remain involved in the patient’s care when psychiatric symptoms are prominent, however, as they are likely to persist even after thyrotoxicosis is corrected.6 Reasonable interventions include:
- control of acute thyrotoxic symptoms such as palpitations and tremulousness with propranolol, 20 to 40 mg as needed, or a 20-mg bolus of prednisone (especially if thyroiditis is present)
- address mood cycling, depression, edginess, anxiety, lability, insomnia, and/or irritability with lithium3
- oversee smoking cessation in patients with Graves’ disease (smoking exacerbates the autoimmune pathology).
Address and correct hyperthyroidism that is artifactual (caused by overuse or secret use by a patient) or iatrogenic (related to excessive prescribed hormone dosages).
Subclinical hyperthyroidism can be transient and resolve without treatment. Lithium can be helpful when a mood disorder coexists with sub clinical hyperthyroidism. Start with 300 to 600 mg every evening with dinner. If the mood disorder is mild, even as little as 300 to 450 mg of lithium may elevate a depressed mood and remove edginess and irritability.
Lithium is antithyroid, decreases thyroid hormone output, and increases serum TSH within 24 hours of initiation, but it can provoke autoimmune hyperthyroidism in some individuals.21
- For comprehensive tables of hyperthyroidism’s causes, refer to Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ 2006;332:1369-73, or Lazarus JH. Hyperthyroidism. Lancet 1997;349:339-43.
- Geracioti TD Jr. Identifying hypothyroidism’s psychiatric presentations. Current Psychiatry 2006;5(11):98-117.
- Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Molecular Psychiatry 2002;7:140-56.
Drug brand names
- Fluvoxamine • Luvox
- Lithium • Lithobid, others
- Levothyroxine • Synthroid, others
- Prednisone • Various brands
- Propranolol • Inderal
- Zolpidem • Ambien
Disclosures
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sakurai A, Nakai A, DeGroot LJ. Expression of three forms of thyroid hormone receptor in human tissues. Mol Endocrinol 1989;3:392-9.
2. Shahrara S, Drvota V, Sylven C. Organ specific expression of thyroid hormone receptor mRNA and protein in different human tissues. Biol Pharm Bull 1999;22:1027-33.
3. Geracioti TD, Jr. How to identify hypothyroidism’s psychiatric presentations. Current Psychiatry 2006;5(11):98-117.
4. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99.
5. Bianchi GP, Zaccheroni V, Vescini F, et al. Health-related quality of life in patients with thyroid disorders. Qual Life Res 2004;13:45-54.
6. Thomsen AF, Kvist TK, Andersen OK, Kessing LV. Increased risk of affective disorder following hospitalization with hyperthyroidism—a register-based study. Eur J Endocrinol 2005;152:535-43.
7. Grabe HJ, Volzke H, Ludermann J, et al. Mental and physical complaints in thyroid disorders in the general population. Acta Psychiatr Scand 2005;112:286-93.
8. Kathol RG, Delahunt JW. The relationship of anxiety and depression to symptoms of hyperthyroidism using operational criteria. Gen Hosp Psychiatry 1986;8:23-8.
9. Trzepacz PT, McCue M, Klein I, et al. A psychiatric and neuropsychological study of patients with untreated Graves’ disease. Gen Hosp Psychiatry 1988;10:49-55.
10. Bunevicius R, Velickiene D, Prange AJ, Jr. Mood and anxiety disorders in women with treated hyperthyroidism and ophthalmopathy caused by Graves’ disease. Gen Hosp Psychiatry 2005;27:133-9.
11. Larson PR, Davies TF, Hay ID. The thyroid gland. In: Wilson JD, Forster DW, Kronenberg HM, Larsen PR eds. Williams textbook of endocrinology. 9th ed. Philadelphia, PA: WB Saunders;1998:389-515.
12. Matsubayashi S, Tamai H, Matsumoto Y, et al. Graves’ disease after the onset of panic disorder. Psychother Psychosom 1996;65(5):277-80.
13. Lazarus JH. Hyperthyroidism. Lancet 1997;349:339-43.
14. Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ 2006;332:1369-73.
15. Utiger RD. The thyroid: physiology, thyrotoxicosis, hypothyroidism, and the painful thyroid. In: Felig P, Frohman LA, eds. Endocrinology and metabolism, 4th ed. New York, NY: McGraw-Hill; 2001:261-347.
16. Beckers A, Abs R, Mahler C, et al. Thyrotropin-secreting pituitary adenomas: report of seven cases. J Clin Endocrinol Metab 1991;72:477-83.
17. Kinder BK, Burrow GN. The thyroid: nodules and neoplasiaIn: Felig P, Frohman LA eds. Endocrinology and metabolism, 4th ed New York, NY: McGraw-Hill; 2001:349-383.
18. Prange AJ, Jr, Wilson IC, Rabon AM, Lipton MA. Enhancement of imipramine antidepressant activity by thyroid hormone. Am J Psychiatry 1969;126:457-69.
19. Geracioti TD, Jr, Loosen PT, Gold PW, Kling MA. Cortisol, thyroid hormone, and mood in atypical depression: a longitudinal case study. Biol Psychiatry 1992;31:515-9.
20. Geracioti TD, Kling MA, Post R, Gold PW. Antithyroid antibody-linked symptoms in borderline personality disorder. Endocrine 2003;21:153-8.
21. Bocchetta A, Mossa P, Velluzzi F, et al. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol 2001;21:594-8.
Ms. A experienced an anxiety attack while driving home from work, with cardiac palpitations, tingling of the face, and fear of impending doom. Over the following 3 months she endured a “living hell,” consisting of basal anxiety, intermittent panic attacks, and agoraphobia, with exceptional difficulty even going to the grocery store.
A high-functioning career woman in her 30s, Ms. A also developed insomnia, depressed mood, and intrusive ego-dystonic thoughts. These symptoms emerged 10 years after a subtotal thyroidectomy for hyperthyroidism (Graves’ disease).
Hyperthyroidism’s association with psychiatric-spectrum symptoms is well-recognized (Box 1).1-4 Hyperthyroid patients are significantly more likely than controls to report feelings of isolation, impaired social functioning, anxiety, and mood disturbances5 and are more likely to be hospitalized with an affective disorder.6
Other individuals with subclinical or overt biochemical hyperthyroidism self-report above-average mood and lower-than-average anxiety.7
Ms. A’s is the first of three cases presented here to help you screen for and identify thyrotoxicosis (thyroid and nonthyroid causes of excessive thyroid hormone). Cases include:
- recurrent Graves’ disease with panic disorder and residual obsessive-compulsive disorder (Ms. A)
- undetected Graves’ hyperthyroidism in a bipolar-like mood syndrome with severe anxiety and cognitive decline (Ms. B)
- occult hyperthyroidism with occult anxiety (Mr. C).
These cases show that even when biochemical euthyroidism is restored, many formerly hyperthyroid patients with severe mood, anxiety, and/or cognitive symptoms continue to have significant residual symptoms that require ongoing psychiatric attention.6
Ms. A: Anxiety and thyrotoxicosis
Ms. A was greatly troubled by her intrusive ego-dystonic thoughts, which involved:
- violence to her beloved young children (for example, what would happen if someone started shooting her children with a gun)
- bizarre sexual ideations (for example, during dinner with an elderly woman she could not stop imagining her naked)
- paranoid ideations (for example, “Is my husband poisoning me?”).
She consulted a psychologist who told her that she suffered from an anxiety disorder and recommended psychotherapy, which was not helpful. She then sought endocrine consultation, and tests showed low-grade overt hyperthyroidism, with unmeasurably low thyroid stimulating hormone (TSH) concentrations and marginally elevated total and free levothyroxine (T4). Her levothyroxine replacement dosage was reduced from 100 to 50 mcg/d, then discontinued.
Without thyroid supplementation or replacement, she became biochemically euthyroid, with TSH 1.47 mIU/L and triiodothyronine (T3) and T4 in mid-normal range. Her panic anxiety resolved and her mood and sleep normalized, but the bizarre thoughts remained. The endocrinologist referred her to a psychiatrist, who diagnosed obsessive-compulsive disorder. Ms. A was effectively treated with fluvoxamine, 125 mg/d.
Discussion. Many patients with hyperthyroidism suffer from anxiety syndromes,8-10 including generalized anxiety disorder and social phobia (Table 1). “Nervousness” (including “feelings of apprehension and inability to concentrate”) is almost invariably present in the thyrotoxicosis of Graves’ disease.11
Hyperthyroidism-related anxiety syndromes are typically complicated by major depression and cognitive decline, such as in memory and attention.9 Thus, a pituitary-thyroid workup is an important step in the psychiatric evaluation of any patient with clinically significant anxiety (Box 2).3
The brain has among the highest expression of thyroid hormone receptors of any organ,1,2 and neurons are often more sensitive to thyroid abnormalities—including overt or subclinical hyperthyroidism and thyrotoxicosis, thyroiditis, and hypothyroidism3—than are other tissues.
Hyperthyroidism is often associated with anxiety, depression, mixed mood disorders, a hypomanic-like picture, emotional lability, mood swings, irritability/edginess, or cognitive deterioration with concentration problems. It also can manifest as psychosis or delirium.
Hyperthyroidism affects approximately 2.5% of the U.S. population (~7.5 million persons), according to the National Health and Nutrition Examination Survey (NHANES III). One-half of those afflicted (1.3%) do not know they are hyperthyroid, including 0.5% with overt symptoms and 0.8% with subclinical disease.
NHANES III defined hyperthyroidism as thyroid-stimulating hormone (TSH) <0.1 mIU/L with total thyroxine (T4) levels either elevated (overt hyperthyroidism) or normal (subclinical hyperthyroidism). Women are at least 5 times more likely than men to be hyperthyroid.4
CNS hypersensitivity to low-grade hyperthyroidism can manifest as an anxiety disorder before other Graves’ disease stigmata emerge. Panic disorder, for example, has been reported to precede Graves’ hyperthyroidism by 4 to 5 years in some cases,12 although how frequently this occurs is not known. Therefore, re-evaluate the thyroid status of any patient with severe anxiety who is biochemically euthyroid. Check yearly, for example, if anxiety is incompletely resolved.
Table 1
Psychiatric symptoms seen with hyperthyroidism
| Anxiety |
| Apathy (more often seen in older patients) |
| Cognitive impairment |
| Delirium |
| Depression |
| Emotional lability |
| Fatigue |
| Hypomania or mania |
| Impaired concentration |
| Insomnia |
| Irritability |
| Mood swings |
| Psychomotor agitation |
| Psychosis |
Causes of hyperthyroidism
Approximately 20 causes of thyrotoxicosis and hyperthyroxinemia have been characterized (see Related resources).11,13-15 The most common causes of hyperthyroidism are Graves’ disease, toxic multinodular goiter, and toxic thyroid adenoma. Another is thyroiditis, such as from lithium or iodine excess (such as from the cardiac drug amiodarone). A TSH-secreting pituitary adenoma is a rare cause of hyperthyroidism.16
A drug-induced thyrotoxic state can be seen with excess administration of exogenous thyroid hormone. This condition usually occurs inadvertently but is sometimes intentional, as in factitious disorder or malingering.
Graves’ disease is an autoimmune disorder that occurs when antibodies (thyroid-stimulating hormone immunoglobulins) stimulate thyroid TSH receptors, increasing thyroid hormone synthesis and secretion. Graves’ disease—seen in 60% to 85% of patients with thyrotoxicosis—is the most common cause of hyperthyroidism.15
Patients most often are women of childbearing years to middle age. Exophthalmos and other eye changes are common, along with diffuse goiter. Encephalopathy can be seen in Graves’ disease and Hashimoto’s thyroiditis because the brain can become an antibody target in autoimmune disorders.
Toxic multinodular goiter consists of autonomously functioning, circumscribed thyroid nodules with an enlarged (goitrous) thyroid, that typically emerge at length from simple (nontoxic) goiter—characterized by enlarged thyroid but normal thyroid-related biochemistry. Onset is typically later in life than Graves’ disease.11,17
Thyrotoxicosis is often relatively mild in toxic multinodular goiter, with marginal elevations in T4 and/or T3. Unlike in Graves’ disease, ophthalmologic changes are unusual. Tachycardia and weakness are common (Table 2).
Table 2
Nonpsychiatric symptoms seen with hyperthyroidism
| Metabolic |
| Heat intolerance (cold tolerance) |
| Increased perspiration |
| Weight loss (despite good appetite) |
| Endocrinologic |
| Goiter (enlarged thyroid gland) |
| Ophthalmologic |
| Exophthalmos |
| Lid lag |
| Stare/infrequent blinking |
| Ophthalmoplegia |
| Neurologic |
| Tremor |
| Hyperreflexia |
| Motor restlessness |
| Proximal muscle weakness/myopathy |
| Cardiologic |
| Tachycardia |
| Palpitations |
| Arrhythmia |
| Worsening or precipitation of angina, heart failure |
| Sexual |
| Oligomenorrhea/amenorrhea |
| Rapid ejaculation |
| Dermatologic |
| Warm, moist skin |
| Fine hair |
| Velvety skin texture |
| Onycholysis |
| Myxedema/leg swelling |
| Ruddy or erythemic skin/facial flushing |
| Eyelash loss |
| Hair loss |
| Premature graying (Graves’ disease) |
| Pruritus |
| Gastrointestinal |
| Frequent bowel movements |
| Diarrhea |
| Nausea |
| Orthopedic |
| Osteopenia or osteoporosis |
Adenomas. Toxic thyroid adenoma is a hyperfunctioning (“toxic”) benign tumor of the thyroid follicular cell. A TSH-secreting pituitary adenoma is a rare cause of hyperthyroidism.16
Thyroid storm is a rare, life-threatening thyrotoxicosis, usually seen in medical or surgical patients. Symptoms include fever, tachycardia, hypotension, irritability and restlessness, nausea and vomiting, delirium, and possibly coma.
Psychiatrists rarely see these cases, but propranolol (40 mg initial dose), fluids, and swift transport to an emergency room or critical care unit are indicated. Antithyroid agents and glucocorticoids are the usual treatment.
Thyrotoxic symptoms from thyroid hormone therapy. Thyroid hormone has been used in psychiatric patients as an antidepressant supplement,18 with therapeutic benefit reported to range from highly valuable19 to modestly helpful or no effect.20 In some patients thyroid hormone causes thyrotoxic symptoms such as tachycardia, gross tremulousness, restlessness, anxiety, inability to sleep, and impaired concentration.
Patients newly diagnosed with hypothyroidism can be exquisitely sensitive to exogenous thyroid hormone and develop acute thyrotoxic symptoms. When this occurs, a more measured titration of thyroid dose is indicated, rather than discontinuing hormone therapy. For example, patients whose optimal maintenance levothyroxine dosage proves to be >100 mcg/d might do better by first adapting to 75 mcg/d.
Thyroid hormone replacement can increase demand on the adrenal glands of chronically hypothyroid patients. For those who develop thyrotoxic-like symptoms, a pulse of glucocorticoids—such as a single 20-mg dose of prednisone (2 to 3 times the typical daily glucocorticoid maintenance requirement)—is sometimes very helpful. Severe eye pain and periorbital edema has been reported to respond to prednisone doses of 120 mg/d.13
Serum TSH is a sensitive screen. Low (<0.1 mIU/mL) or immeasurably low (<0.05 mIU/mL) circulating TSH usually means hyperthyroidism. A TSH screen is not foolproof, however; very low TSH can be seen with low circulating thyroid hormones in central hypothyroidism or in cases of laboratory error.
The recommended routine initial screen of the pituitary-thyroid axis in psychiatric patients includes TSH, free T4, and possibly free T3.3 Suppressed TSH with high serum free T3 and/or free T4 (accompanied by high total T4 and/or T3) is diagnostic of frank biochemical hyperthyroidism. If circulating thyroid hormone concentrations are normal, hyperthyroidism is considered compensated or subclinical. Although only free thyroid hormones are active, total T4 and total T3 are of interest to grossly estimate thyroid hormone output.
When you identify a thyrotoxic state, refer the patient for an endocrinologic evaluation. Antithyroid antibodies are often positive in Graves’ disease, but anti-TSH antibodies (which can be routinely ordered) are particularly diagnostic. If thyroid dysfunction is present—especially if autoimmune-based—screening tests are indicated to rule out adrenal, gonadal, and pancreatic (glucose regulation) dysfunction.
Ms. B: Hyperthyroidism and mood
Ms. B, age 35, an energetic clerical worker and fitness devotee, developed severe insomnia. She slept no more than 1 hour per night, with irritability, verbal explosiveness, “hot flashes,” and depressed mood. “Everything pisses me off violently,” she said.
She consulted a psychiatrist and was diagnosed with major depression. Over a period of years, she was serially prescribed selective serotonin reuptake inhibitors, serotonin/norepinephrine reuptake inhibitors, and older-generation sedating agents including trazodone and amitriptyline. She tolerated none of these because of side effects, including dysphoric hyperarousal and cognitive disruption.
“They all made me stupid,” she complained.
Zolpidem, 20 mg at night, helped temporarily as a hypnotic, but insomnia recurred within weeks. Diazepam was effective at high dosages but also dulled her cognition. The psychiatrist did not suspect a thyroid abnormality and did not perform a pituitary-thyroid laboratory evaluation.
Ms. B consulted a gynecologist, who prescribed estrogen for borderline low estradiol levels and with the hope that Ms. B’s symptoms represented early menopause. This partially ameliorated her irritability, possibly because estradiol binding of circulating T4 reduced free thyroid hormone levels.
Ms. B tried to continue working and exercising, but within 4 years her symptoms progressed to severe depression with frequent crying spells, feelings of general malaise, excessive sweating, occasional panic attacks, fatigue, sleepiness, deteriorating vision, and cognitive impairment. She struggled to read printed words and eventually took sick leave while consulting with physicians.
Finally, a routine thyroid screen before minor surgery revealed an undetectable TSH concentration. Further testing showed elevated thyroxine consistent with thyrotoxicosis. Graves’ disease was diagnosed, and euthyroidism was established with antithyroid medication.
Residual mood and anxiety symptoms persisted 1 year after euthyroidism was restored, and Ms. B sought psychiatric consultation.
Discussion. Hyperthyroidism can trigger or present as a hypomania or manic-like state, characterized by increased energy, hyperactivity, racing thoughts, hair-trigger verbal explosiveness, and decreased need for sleep.
Hypertalkativeness is common, even without pressured speech, as is irritability. Mood may be elevated, depressed, mixed, or cycling. A hyperthyroidism-related mixed syndrome of depression and hypomania can be confounding.
Mr. C: Occult hyperthyroidism
Mr. C, age 26, was apparently healthy when he was admitted into a neuroendocrine research protocol as a volunteer. His job performance was excellent, and his interactions with others were good; he was in good general health and taking no medication.
Formal psychiatric screening found no history of psychiatric disorders in Mr. C nor his family. His mental status was within normal limits. Physical exam revealed no significant abnormality. He was afebrile, normotensive, and had a resting pulse of 81 bpm.
His neurologic status was unremarkable, and laboratory screening tests showed normal CBC, liver and renal profiles, glucose, platelets and clotting times. Tests during the study, however, showed frankly elevated T4, free thyroxine (FT4), and T3 concentrations, along with undetectable TSH. Mr. C was informed of these results and referred to an endocrinologist.
Graves’ disease was diagnosed, and Mr. C received partial thyroid ablation therapy. He later reported that he had never felt better. In retrospect, he realized he had been anxious before he was treated for hyperthyroidism because he felt much more relaxed and able to concentrate after treatment.
Discussion. Subjective well-being in a patient with occult biochemical thyrotoxicosis can be misleading. Mr. C was much less anxious and able to concentrate after his return to euthyroidism.
Treatment
Refer your hyperthyroid patients to an endocrinologist for further workup and, in most cases, management. Hyperthyroidism is usually easy to treat using a form of ablation (antithyroid drugs, radioactive iodine, or partial thyroidectomy).
Remain involved in the patient’s care when psychiatric symptoms are prominent, however, as they are likely to persist even after thyrotoxicosis is corrected.6 Reasonable interventions include:
- control of acute thyrotoxic symptoms such as palpitations and tremulousness with propranolol, 20 to 40 mg as needed, or a 20-mg bolus of prednisone (especially if thyroiditis is present)
- address mood cycling, depression, edginess, anxiety, lability, insomnia, and/or irritability with lithium3
- oversee smoking cessation in patients with Graves’ disease (smoking exacerbates the autoimmune pathology).
Address and correct hyperthyroidism that is artifactual (caused by overuse or secret use by a patient) or iatrogenic (related to excessive prescribed hormone dosages).
Subclinical hyperthyroidism can be transient and resolve without treatment. Lithium can be helpful when a mood disorder coexists with sub clinical hyperthyroidism. Start with 300 to 600 mg every evening with dinner. If the mood disorder is mild, even as little as 300 to 450 mg of lithium may elevate a depressed mood and remove edginess and irritability.
Lithium is antithyroid, decreases thyroid hormone output, and increases serum TSH within 24 hours of initiation, but it can provoke autoimmune hyperthyroidism in some individuals.21
- For comprehensive tables of hyperthyroidism’s causes, refer to Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ 2006;332:1369-73, or Lazarus JH. Hyperthyroidism. Lancet 1997;349:339-43.
- Geracioti TD Jr. Identifying hypothyroidism’s psychiatric presentations. Current Psychiatry 2006;5(11):98-117.
- Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Molecular Psychiatry 2002;7:140-56.
Drug brand names
- Fluvoxamine • Luvox
- Lithium • Lithobid, others
- Levothyroxine • Synthroid, others
- Prednisone • Various brands
- Propranolol • Inderal
- Zolpidem • Ambien
Disclosures
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. A experienced an anxiety attack while driving home from work, with cardiac palpitations, tingling of the face, and fear of impending doom. Over the following 3 months she endured a “living hell,” consisting of basal anxiety, intermittent panic attacks, and agoraphobia, with exceptional difficulty even going to the grocery store.
A high-functioning career woman in her 30s, Ms. A also developed insomnia, depressed mood, and intrusive ego-dystonic thoughts. These symptoms emerged 10 years after a subtotal thyroidectomy for hyperthyroidism (Graves’ disease).
Hyperthyroidism’s association with psychiatric-spectrum symptoms is well-recognized (Box 1).1-4 Hyperthyroid patients are significantly more likely than controls to report feelings of isolation, impaired social functioning, anxiety, and mood disturbances5 and are more likely to be hospitalized with an affective disorder.6
Other individuals with subclinical or overt biochemical hyperthyroidism self-report above-average mood and lower-than-average anxiety.7
Ms. A’s is the first of three cases presented here to help you screen for and identify thyrotoxicosis (thyroid and nonthyroid causes of excessive thyroid hormone). Cases include:
- recurrent Graves’ disease with panic disorder and residual obsessive-compulsive disorder (Ms. A)
- undetected Graves’ hyperthyroidism in a bipolar-like mood syndrome with severe anxiety and cognitive decline (Ms. B)
- occult hyperthyroidism with occult anxiety (Mr. C).
These cases show that even when biochemical euthyroidism is restored, many formerly hyperthyroid patients with severe mood, anxiety, and/or cognitive symptoms continue to have significant residual symptoms that require ongoing psychiatric attention.6
Ms. A: Anxiety and thyrotoxicosis
Ms. A was greatly troubled by her intrusive ego-dystonic thoughts, which involved:
- violence to her beloved young children (for example, what would happen if someone started shooting her children with a gun)
- bizarre sexual ideations (for example, during dinner with an elderly woman she could not stop imagining her naked)
- paranoid ideations (for example, “Is my husband poisoning me?”).
She consulted a psychologist who told her that she suffered from an anxiety disorder and recommended psychotherapy, which was not helpful. She then sought endocrine consultation, and tests showed low-grade overt hyperthyroidism, with unmeasurably low thyroid stimulating hormone (TSH) concentrations and marginally elevated total and free levothyroxine (T4). Her levothyroxine replacement dosage was reduced from 100 to 50 mcg/d, then discontinued.
Without thyroid supplementation or replacement, she became biochemically euthyroid, with TSH 1.47 mIU/L and triiodothyronine (T3) and T4 in mid-normal range. Her panic anxiety resolved and her mood and sleep normalized, but the bizarre thoughts remained. The endocrinologist referred her to a psychiatrist, who diagnosed obsessive-compulsive disorder. Ms. A was effectively treated with fluvoxamine, 125 mg/d.
Discussion. Many patients with hyperthyroidism suffer from anxiety syndromes,8-10 including generalized anxiety disorder and social phobia (Table 1). “Nervousness” (including “feelings of apprehension and inability to concentrate”) is almost invariably present in the thyrotoxicosis of Graves’ disease.11
Hyperthyroidism-related anxiety syndromes are typically complicated by major depression and cognitive decline, such as in memory and attention.9 Thus, a pituitary-thyroid workup is an important step in the psychiatric evaluation of any patient with clinically significant anxiety (Box 2).3
The brain has among the highest expression of thyroid hormone receptors of any organ,1,2 and neurons are often more sensitive to thyroid abnormalities—including overt or subclinical hyperthyroidism and thyrotoxicosis, thyroiditis, and hypothyroidism3—than are other tissues.
Hyperthyroidism is often associated with anxiety, depression, mixed mood disorders, a hypomanic-like picture, emotional lability, mood swings, irritability/edginess, or cognitive deterioration with concentration problems. It also can manifest as psychosis or delirium.
Hyperthyroidism affects approximately 2.5% of the U.S. population (~7.5 million persons), according to the National Health and Nutrition Examination Survey (NHANES III). One-half of those afflicted (1.3%) do not know they are hyperthyroid, including 0.5% with overt symptoms and 0.8% with subclinical disease.
NHANES III defined hyperthyroidism as thyroid-stimulating hormone (TSH) <0.1 mIU/L with total thyroxine (T4) levels either elevated (overt hyperthyroidism) or normal (subclinical hyperthyroidism). Women are at least 5 times more likely than men to be hyperthyroid.4
CNS hypersensitivity to low-grade hyperthyroidism can manifest as an anxiety disorder before other Graves’ disease stigmata emerge. Panic disorder, for example, has been reported to precede Graves’ hyperthyroidism by 4 to 5 years in some cases,12 although how frequently this occurs is not known. Therefore, re-evaluate the thyroid status of any patient with severe anxiety who is biochemically euthyroid. Check yearly, for example, if anxiety is incompletely resolved.
Table 1
Psychiatric symptoms seen with hyperthyroidism
| Anxiety |
| Apathy (more often seen in older patients) |
| Cognitive impairment |
| Delirium |
| Depression |
| Emotional lability |
| Fatigue |
| Hypomania or mania |
| Impaired concentration |
| Insomnia |
| Irritability |
| Mood swings |
| Psychomotor agitation |
| Psychosis |
Causes of hyperthyroidism
Approximately 20 causes of thyrotoxicosis and hyperthyroxinemia have been characterized (see Related resources).11,13-15 The most common causes of hyperthyroidism are Graves’ disease, toxic multinodular goiter, and toxic thyroid adenoma. Another is thyroiditis, such as from lithium or iodine excess (such as from the cardiac drug amiodarone). A TSH-secreting pituitary adenoma is a rare cause of hyperthyroidism.16
A drug-induced thyrotoxic state can be seen with excess administration of exogenous thyroid hormone. This condition usually occurs inadvertently but is sometimes intentional, as in factitious disorder or malingering.
Graves’ disease is an autoimmune disorder that occurs when antibodies (thyroid-stimulating hormone immunoglobulins) stimulate thyroid TSH receptors, increasing thyroid hormone synthesis and secretion. Graves’ disease—seen in 60% to 85% of patients with thyrotoxicosis—is the most common cause of hyperthyroidism.15
Patients most often are women of childbearing years to middle age. Exophthalmos and other eye changes are common, along with diffuse goiter. Encephalopathy can be seen in Graves’ disease and Hashimoto’s thyroiditis because the brain can become an antibody target in autoimmune disorders.
Toxic multinodular goiter consists of autonomously functioning, circumscribed thyroid nodules with an enlarged (goitrous) thyroid, that typically emerge at length from simple (nontoxic) goiter—characterized by enlarged thyroid but normal thyroid-related biochemistry. Onset is typically later in life than Graves’ disease.11,17
Thyrotoxicosis is often relatively mild in toxic multinodular goiter, with marginal elevations in T4 and/or T3. Unlike in Graves’ disease, ophthalmologic changes are unusual. Tachycardia and weakness are common (Table 2).
Table 2
Nonpsychiatric symptoms seen with hyperthyroidism
| Metabolic |
| Heat intolerance (cold tolerance) |
| Increased perspiration |
| Weight loss (despite good appetite) |
| Endocrinologic |
| Goiter (enlarged thyroid gland) |
| Ophthalmologic |
| Exophthalmos |
| Lid lag |
| Stare/infrequent blinking |
| Ophthalmoplegia |
| Neurologic |
| Tremor |
| Hyperreflexia |
| Motor restlessness |
| Proximal muscle weakness/myopathy |
| Cardiologic |
| Tachycardia |
| Palpitations |
| Arrhythmia |
| Worsening or precipitation of angina, heart failure |
| Sexual |
| Oligomenorrhea/amenorrhea |
| Rapid ejaculation |
| Dermatologic |
| Warm, moist skin |
| Fine hair |
| Velvety skin texture |
| Onycholysis |
| Myxedema/leg swelling |
| Ruddy or erythemic skin/facial flushing |
| Eyelash loss |
| Hair loss |
| Premature graying (Graves’ disease) |
| Pruritus |
| Gastrointestinal |
| Frequent bowel movements |
| Diarrhea |
| Nausea |
| Orthopedic |
| Osteopenia or osteoporosis |
Adenomas. Toxic thyroid adenoma is a hyperfunctioning (“toxic”) benign tumor of the thyroid follicular cell. A TSH-secreting pituitary adenoma is a rare cause of hyperthyroidism.16
Thyroid storm is a rare, life-threatening thyrotoxicosis, usually seen in medical or surgical patients. Symptoms include fever, tachycardia, hypotension, irritability and restlessness, nausea and vomiting, delirium, and possibly coma.
Psychiatrists rarely see these cases, but propranolol (40 mg initial dose), fluids, and swift transport to an emergency room or critical care unit are indicated. Antithyroid agents and glucocorticoids are the usual treatment.
Thyrotoxic symptoms from thyroid hormone therapy. Thyroid hormone has been used in psychiatric patients as an antidepressant supplement,18 with therapeutic benefit reported to range from highly valuable19 to modestly helpful or no effect.20 In some patients thyroid hormone causes thyrotoxic symptoms such as tachycardia, gross tremulousness, restlessness, anxiety, inability to sleep, and impaired concentration.
Patients newly diagnosed with hypothyroidism can be exquisitely sensitive to exogenous thyroid hormone and develop acute thyrotoxic symptoms. When this occurs, a more measured titration of thyroid dose is indicated, rather than discontinuing hormone therapy. For example, patients whose optimal maintenance levothyroxine dosage proves to be >100 mcg/d might do better by first adapting to 75 mcg/d.
Thyroid hormone replacement can increase demand on the adrenal glands of chronically hypothyroid patients. For those who develop thyrotoxic-like symptoms, a pulse of glucocorticoids—such as a single 20-mg dose of prednisone (2 to 3 times the typical daily glucocorticoid maintenance requirement)—is sometimes very helpful. Severe eye pain and periorbital edema has been reported to respond to prednisone doses of 120 mg/d.13
Serum TSH is a sensitive screen. Low (<0.1 mIU/mL) or immeasurably low (<0.05 mIU/mL) circulating TSH usually means hyperthyroidism. A TSH screen is not foolproof, however; very low TSH can be seen with low circulating thyroid hormones in central hypothyroidism or in cases of laboratory error.
The recommended routine initial screen of the pituitary-thyroid axis in psychiatric patients includes TSH, free T4, and possibly free T3.3 Suppressed TSH with high serum free T3 and/or free T4 (accompanied by high total T4 and/or T3) is diagnostic of frank biochemical hyperthyroidism. If circulating thyroid hormone concentrations are normal, hyperthyroidism is considered compensated or subclinical. Although only free thyroid hormones are active, total T4 and total T3 are of interest to grossly estimate thyroid hormone output.
When you identify a thyrotoxic state, refer the patient for an endocrinologic evaluation. Antithyroid antibodies are often positive in Graves’ disease, but anti-TSH antibodies (which can be routinely ordered) are particularly diagnostic. If thyroid dysfunction is present—especially if autoimmune-based—screening tests are indicated to rule out adrenal, gonadal, and pancreatic (glucose regulation) dysfunction.
Ms. B: Hyperthyroidism and mood
Ms. B, age 35, an energetic clerical worker and fitness devotee, developed severe insomnia. She slept no more than 1 hour per night, with irritability, verbal explosiveness, “hot flashes,” and depressed mood. “Everything pisses me off violently,” she said.
She consulted a psychiatrist and was diagnosed with major depression. Over a period of years, she was serially prescribed selective serotonin reuptake inhibitors, serotonin/norepinephrine reuptake inhibitors, and older-generation sedating agents including trazodone and amitriptyline. She tolerated none of these because of side effects, including dysphoric hyperarousal and cognitive disruption.
“They all made me stupid,” she complained.
Zolpidem, 20 mg at night, helped temporarily as a hypnotic, but insomnia recurred within weeks. Diazepam was effective at high dosages but also dulled her cognition. The psychiatrist did not suspect a thyroid abnormality and did not perform a pituitary-thyroid laboratory evaluation.
Ms. B consulted a gynecologist, who prescribed estrogen for borderline low estradiol levels and with the hope that Ms. B’s symptoms represented early menopause. This partially ameliorated her irritability, possibly because estradiol binding of circulating T4 reduced free thyroid hormone levels.
Ms. B tried to continue working and exercising, but within 4 years her symptoms progressed to severe depression with frequent crying spells, feelings of general malaise, excessive sweating, occasional panic attacks, fatigue, sleepiness, deteriorating vision, and cognitive impairment. She struggled to read printed words and eventually took sick leave while consulting with physicians.
Finally, a routine thyroid screen before minor surgery revealed an undetectable TSH concentration. Further testing showed elevated thyroxine consistent with thyrotoxicosis. Graves’ disease was diagnosed, and euthyroidism was established with antithyroid medication.
Residual mood and anxiety symptoms persisted 1 year after euthyroidism was restored, and Ms. B sought psychiatric consultation.
Discussion. Hyperthyroidism can trigger or present as a hypomania or manic-like state, characterized by increased energy, hyperactivity, racing thoughts, hair-trigger verbal explosiveness, and decreased need for sleep.
Hypertalkativeness is common, even without pressured speech, as is irritability. Mood may be elevated, depressed, mixed, or cycling. A hyperthyroidism-related mixed syndrome of depression and hypomania can be confounding.
Mr. C: Occult hyperthyroidism
Mr. C, age 26, was apparently healthy when he was admitted into a neuroendocrine research protocol as a volunteer. His job performance was excellent, and his interactions with others were good; he was in good general health and taking no medication.
Formal psychiatric screening found no history of psychiatric disorders in Mr. C nor his family. His mental status was within normal limits. Physical exam revealed no significant abnormality. He was afebrile, normotensive, and had a resting pulse of 81 bpm.
His neurologic status was unremarkable, and laboratory screening tests showed normal CBC, liver and renal profiles, glucose, platelets and clotting times. Tests during the study, however, showed frankly elevated T4, free thyroxine (FT4), and T3 concentrations, along with undetectable TSH. Mr. C was informed of these results and referred to an endocrinologist.
Graves’ disease was diagnosed, and Mr. C received partial thyroid ablation therapy. He later reported that he had never felt better. In retrospect, he realized he had been anxious before he was treated for hyperthyroidism because he felt much more relaxed and able to concentrate after treatment.
Discussion. Subjective well-being in a patient with occult biochemical thyrotoxicosis can be misleading. Mr. C was much less anxious and able to concentrate after his return to euthyroidism.
Treatment
Refer your hyperthyroid patients to an endocrinologist for further workup and, in most cases, management. Hyperthyroidism is usually easy to treat using a form of ablation (antithyroid drugs, radioactive iodine, or partial thyroidectomy).
Remain involved in the patient’s care when psychiatric symptoms are prominent, however, as they are likely to persist even after thyrotoxicosis is corrected.6 Reasonable interventions include:
- control of acute thyrotoxic symptoms such as palpitations and tremulousness with propranolol, 20 to 40 mg as needed, or a 20-mg bolus of prednisone (especially if thyroiditis is present)
- address mood cycling, depression, edginess, anxiety, lability, insomnia, and/or irritability with lithium3
- oversee smoking cessation in patients with Graves’ disease (smoking exacerbates the autoimmune pathology).
Address and correct hyperthyroidism that is artifactual (caused by overuse or secret use by a patient) or iatrogenic (related to excessive prescribed hormone dosages).
Subclinical hyperthyroidism can be transient and resolve without treatment. Lithium can be helpful when a mood disorder coexists with sub clinical hyperthyroidism. Start with 300 to 600 mg every evening with dinner. If the mood disorder is mild, even as little as 300 to 450 mg of lithium may elevate a depressed mood and remove edginess and irritability.
Lithium is antithyroid, decreases thyroid hormone output, and increases serum TSH within 24 hours of initiation, but it can provoke autoimmune hyperthyroidism in some individuals.21
- For comprehensive tables of hyperthyroidism’s causes, refer to Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ 2006;332:1369-73, or Lazarus JH. Hyperthyroidism. Lancet 1997;349:339-43.
- Geracioti TD Jr. Identifying hypothyroidism’s psychiatric presentations. Current Psychiatry 2006;5(11):98-117.
- Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Molecular Psychiatry 2002;7:140-56.
Drug brand names
- Fluvoxamine • Luvox
- Lithium • Lithobid, others
- Levothyroxine • Synthroid, others
- Prednisone • Various brands
- Propranolol • Inderal
- Zolpidem • Ambien
Disclosures
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sakurai A, Nakai A, DeGroot LJ. Expression of three forms of thyroid hormone receptor in human tissues. Mol Endocrinol 1989;3:392-9.
2. Shahrara S, Drvota V, Sylven C. Organ specific expression of thyroid hormone receptor mRNA and protein in different human tissues. Biol Pharm Bull 1999;22:1027-33.
3. Geracioti TD, Jr. How to identify hypothyroidism’s psychiatric presentations. Current Psychiatry 2006;5(11):98-117.
4. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99.
5. Bianchi GP, Zaccheroni V, Vescini F, et al. Health-related quality of life in patients with thyroid disorders. Qual Life Res 2004;13:45-54.
6. Thomsen AF, Kvist TK, Andersen OK, Kessing LV. Increased risk of affective disorder following hospitalization with hyperthyroidism—a register-based study. Eur J Endocrinol 2005;152:535-43.
7. Grabe HJ, Volzke H, Ludermann J, et al. Mental and physical complaints in thyroid disorders in the general population. Acta Psychiatr Scand 2005;112:286-93.
8. Kathol RG, Delahunt JW. The relationship of anxiety and depression to symptoms of hyperthyroidism using operational criteria. Gen Hosp Psychiatry 1986;8:23-8.
9. Trzepacz PT, McCue M, Klein I, et al. A psychiatric and neuropsychological study of patients with untreated Graves’ disease. Gen Hosp Psychiatry 1988;10:49-55.
10. Bunevicius R, Velickiene D, Prange AJ, Jr. Mood and anxiety disorders in women with treated hyperthyroidism and ophthalmopathy caused by Graves’ disease. Gen Hosp Psychiatry 2005;27:133-9.
11. Larson PR, Davies TF, Hay ID. The thyroid gland. In: Wilson JD, Forster DW, Kronenberg HM, Larsen PR eds. Williams textbook of endocrinology. 9th ed. Philadelphia, PA: WB Saunders;1998:389-515.
12. Matsubayashi S, Tamai H, Matsumoto Y, et al. Graves’ disease after the onset of panic disorder. Psychother Psychosom 1996;65(5):277-80.
13. Lazarus JH. Hyperthyroidism. Lancet 1997;349:339-43.
14. Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ 2006;332:1369-73.
15. Utiger RD. The thyroid: physiology, thyrotoxicosis, hypothyroidism, and the painful thyroid. In: Felig P, Frohman LA, eds. Endocrinology and metabolism, 4th ed. New York, NY: McGraw-Hill; 2001:261-347.
16. Beckers A, Abs R, Mahler C, et al. Thyrotropin-secreting pituitary adenomas: report of seven cases. J Clin Endocrinol Metab 1991;72:477-83.
17. Kinder BK, Burrow GN. The thyroid: nodules and neoplasiaIn: Felig P, Frohman LA eds. Endocrinology and metabolism, 4th ed New York, NY: McGraw-Hill; 2001:349-383.
18. Prange AJ, Jr, Wilson IC, Rabon AM, Lipton MA. Enhancement of imipramine antidepressant activity by thyroid hormone. Am J Psychiatry 1969;126:457-69.
19. Geracioti TD, Jr, Loosen PT, Gold PW, Kling MA. Cortisol, thyroid hormone, and mood in atypical depression: a longitudinal case study. Biol Psychiatry 1992;31:515-9.
20. Geracioti TD, Kling MA, Post R, Gold PW. Antithyroid antibody-linked symptoms in borderline personality disorder. Endocrine 2003;21:153-8.
21. Bocchetta A, Mossa P, Velluzzi F, et al. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol 2001;21:594-8.
1. Sakurai A, Nakai A, DeGroot LJ. Expression of three forms of thyroid hormone receptor in human tissues. Mol Endocrinol 1989;3:392-9.
2. Shahrara S, Drvota V, Sylven C. Organ specific expression of thyroid hormone receptor mRNA and protein in different human tissues. Biol Pharm Bull 1999;22:1027-33.
3. Geracioti TD, Jr. How to identify hypothyroidism’s psychiatric presentations. Current Psychiatry 2006;5(11):98-117.
4. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99.
5. Bianchi GP, Zaccheroni V, Vescini F, et al. Health-related quality of life in patients with thyroid disorders. Qual Life Res 2004;13:45-54.
6. Thomsen AF, Kvist TK, Andersen OK, Kessing LV. Increased risk of affective disorder following hospitalization with hyperthyroidism—a register-based study. Eur J Endocrinol 2005;152:535-43.
7. Grabe HJ, Volzke H, Ludermann J, et al. Mental and physical complaints in thyroid disorders in the general population. Acta Psychiatr Scand 2005;112:286-93.
8. Kathol RG, Delahunt JW. The relationship of anxiety and depression to symptoms of hyperthyroidism using operational criteria. Gen Hosp Psychiatry 1986;8:23-8.
9. Trzepacz PT, McCue M, Klein I, et al. A psychiatric and neuropsychological study of patients with untreated Graves’ disease. Gen Hosp Psychiatry 1988;10:49-55.
10. Bunevicius R, Velickiene D, Prange AJ, Jr. Mood and anxiety disorders in women with treated hyperthyroidism and ophthalmopathy caused by Graves’ disease. Gen Hosp Psychiatry 2005;27:133-9.
11. Larson PR, Davies TF, Hay ID. The thyroid gland. In: Wilson JD, Forster DW, Kronenberg HM, Larsen PR eds. Williams textbook of endocrinology. 9th ed. Philadelphia, PA: WB Saunders;1998:389-515.
12. Matsubayashi S, Tamai H, Matsumoto Y, et al. Graves’ disease after the onset of panic disorder. Psychother Psychosom 1996;65(5):277-80.
13. Lazarus JH. Hyperthyroidism. Lancet 1997;349:339-43.
14. Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ 2006;332:1369-73.
15. Utiger RD. The thyroid: physiology, thyrotoxicosis, hypothyroidism, and the painful thyroid. In: Felig P, Frohman LA, eds. Endocrinology and metabolism, 4th ed. New York, NY: McGraw-Hill; 2001:261-347.
16. Beckers A, Abs R, Mahler C, et al. Thyrotropin-secreting pituitary adenomas: report of seven cases. J Clin Endocrinol Metab 1991;72:477-83.
17. Kinder BK, Burrow GN. The thyroid: nodules and neoplasiaIn: Felig P, Frohman LA eds. Endocrinology and metabolism, 4th ed New York, NY: McGraw-Hill; 2001:349-383.
18. Prange AJ, Jr, Wilson IC, Rabon AM, Lipton MA. Enhancement of imipramine antidepressant activity by thyroid hormone. Am J Psychiatry 1969;126:457-69.
19. Geracioti TD, Jr, Loosen PT, Gold PW, Kling MA. Cortisol, thyroid hormone, and mood in atypical depression: a longitudinal case study. Biol Psychiatry 1992;31:515-9.
20. Geracioti TD, Kling MA, Post R, Gold PW. Antithyroid antibody-linked symptoms in borderline personality disorder. Endocrine 2003;21:153-8.
21. Bocchetta A, Mossa P, Velluzzi F, et al. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol 2001;21:594-8.
Solutions to school refusal for parents and kids
Case: 'He's okay on weekends'
Nathan, age 13, is referred by his parents for recent school refusal behavior. He has had difficulty adjusting to middle school and has been marked absent one-third of school days this academic year. These absences come in the form of tardiness, skipped classes, and full-day absences.
Nathan complains of headaches and stomachaches and says he feels upset and nervous while in school. His parents, however, complain that Nathan seems fine on weekends and holidays and seems to be embellishing symptoms to miss school. Nathan’s parents are concerned that their son may have some physical or mental condition that is preventing his school attendance and that might be remediated with medication.
Child-motivated refusal to attend school or remain in class an entire day is not uncommon, affecting 5% to 28% of youths at some time in their lives.1,2
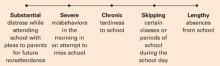
The behavior may be viewed along a spectrum of absenteeism (Figure), and a child may exhibit all forms of absenteeism at one time or another. In Nathan’s case, for example, he could be anxious during school on Monday, arrive late to school on Tuesday, skip afternoon classes on Wednesday, and fail to attend school completely on Thursday and Friday.
In this article you will learn characteristics of school refusal behavior to watch for and assess, and treatment strategies for youths ages 5 to 17. You will also find advice and techniques to offer parents.
Figure A child might exhibit each behavior on this spectrum at different times
Refusal behavior characteristics
School refusal behavior encompasses all subsets of problematic absenteeism, such as truancy, school phobia, and separation anxiety.3 Children and adolescents of all ages, boys and girls alike, can exhibit school refusal behavior. The most common age of onset is 10 to 13 years. Youths such as Nathan who are entering a school building for the first time—especially elementary and middle school—are at particular risk for school refusal behavior. Little information is available regarding ethnic differences, although school dropout rates for Hispanics are often considerably elevated compared with other ethnic groups.4,5
School refusal behavior covers a range of symptoms, diagnoses, somatic complaints, and medical conditions (Tables 1-3).6-12 Longitudinal studies indicate that school refusal behavior, if left unaddressed, can lead to serious short-term problems, such as distress, academic decline, alienation from peers, family conflict, and financial and legal consequences. Common long-term problems include school dropout, delinquent behaviors, economic deprivation, social isolation, marital problems, and difficulty maintaining employment. Approximately 52% of adolescents with school refusal behavior meet criteria for an anxiety, depressive, conduct-personality, or other psychiatric disorder later in life.13-16
Table 1
Common symptoms that could signal school refusal behavior
| Internalizing/covert symptoms | Externalizing/overt symptoms |
|---|---|
| Depression | Aggression |
| Fatigue/tiredness | Clinging to an adult |
| Fear and panic | Excessive reassurance-seeking behavior |
| General and social anxiety | Noncompliance and defiance |
| Self-consciousness | Refusal to move in the morning |
| Somatization | Running away from school or home |
| Worry | Temper tantrums and crying |
Table 2
Primary psychiatric disorders among youths with school refusal behavior
| Diagnosis | Percentage |
|---|---|
| None | 32.9% |
| Separation anxiety disorder | 22.4% |
| Generalized anxiety disorder | 10.5% |
| Oppositional defiant disorder | 8.4% |
| Major depression | 4.9% |
| Specific phobia | 4.2% |
| Social anxiety disorder | 3.5% |
| Conduct disorder | 2.8% |
| Attention deficit/hyperactivity disorder | 1.4% |
| Panic disorder | 1.4% |
| Enuresis | 0.7% |
| Posttraumatic stress disorder | 0.7% |
| Source: Reference 7 | |
Table 3
Somatic complaints and medical conditions
commonly associated with school refusal behavior
| Somatic complaints | Medical conditions |
|---|---|
| Diarrhea/irritable bowel | Allergic rhinitis |
| Fatigue | Asthma and respiratory illness |
| Headache and stomachache | Chronic pain and illness (notably cancer, Crohn’s disease, dyspepsia, hemophilia, chronic fatigue syndrome) |
| Nausea and vomiting | Diabetes |
| Palpitations and perspiration | Dysmenorrhea |
| Recurrent abdominal pain or other pain | Head louse infestation |
| Shaking or trembling | Influenza |
| Sleep problems | Orodental disease |
Finding a reason for school refusal
If a child has somatic complaints, you can expect to find that the child is:
- suffering from a true physical malady
- embellishing low-grade physical symptoms from stress or attention-seeking behavior
- reporting physical problems that have no medical basis.
A full medical examination is always recommended to rule out organic problems or to properly treat true medical conditions.
Four functions. If no medical condition is found, explore the reasons a particular child refuses school. A common model of conceptualizing school refusal behavior involves reinforcers:1,2
- to avoid school-based stimuli that provoke a sense of negative affectivity, or combined anxiety and depression; examples of key stimuli include teachers, peers, bus, cafeteria, classroom, and transitions between classes
- to escape aversive social or evaluative situations such as conversing or otherwise interacting with others or performing before others as in class presentations
- to pursue attention from significant others, such as wanting to stay home or go to work with parents
- to pursue tangible reinforcers outside of school, such as sleeping late, watching television, playing with friends, or engaging in delinquent behavior or substance use.
The first 2 functions are maintained by negative reinforcement or a desire to leave anxiety-provoking stimuli. The latter 2 functions are maintained by positive reinforcement, or a desire to pursue rewards outside of school. Youths may also refuse school for a combination of these reasons.17 In Nathan’s case, he was initially anxious about school in general (the first function). After his parents allowed him to stay home for a few days, however, he was refusing school to enjoy fun activities such as video games at home (the last function).
Violence on school campuses across the country naturally makes many parents skittish about possible copycat incidents. In fact, some parents acquiesce to their children’s pleas to remain home on school shooting anniversaries—particularly the Columbine tragedy of April 20, 1999.
Student and parental fears likely are exacerbated by new episodes of violence, such as three school shootings in 2006:
- On September 27, a 53-year-old man entered a high school in Bailey, Colorado, and shot one girl before killing himself.
- On September 29, a high school student near Madison, Wisconsin, killed his principal after being disciplined for carrying tobacco.
- On October 2, a heavily armed man barricaded himself in a one-room Amish schoolhouse in Paradise, Pennsylvania. He bound and shot 11 girls before killing himself, and five of the girls died.
Compared with highly publicized school violence, however, personal victimization is a much stronger factor in absenteeism.32 Specifically, school violence is related to school absenteeism especially for youths who have been previously victimized. The literature shows:
- Students who have been bullied are 2.1 times more likely than other students to feel unsafe at school.
- 20% of elementary school children report they would skip school to avoid being bullied.33
- High school students’ fear of attending classes because of violence is directly associated with victimization by teachers or other students.
- Missing school because of feeling unsafe is a strong risk factor for asthma and, potentially, being sent home early from school.34
Assessment scale. One method for quickly assessing the role of these functions is the School Refusal Assessment Scale-Revised.18,19 This scale poses 24 questions, the answers to which measure the relative strength of each of the 4 functions. Versions are available for children and parents, who complete their respective scales separately (see Related resources). Item means are calculated across the measures to help determine the primary reason for a child’s school refusal.
In addition to using the assessment scale, you may ask interview questions regarding the form and function of school refusal behavior (Tables 4,5). Take care to assess attendance history and patterns, comorbid conditions, instances of legitimate absenteeism, family disruption, and a child’s social and academic status. Specific questions about function can help narrow the reason for school refusal.
Assess specific school-related stimuli that provoke absenteeism such as social and evaluative situations, whether a child could attend school with a parent (evidence of attention-seeking), and what tangible rewards a child receives for absenteeism throughout the school day. Information about the form and function of school refusal behavior may also be evident during in-office observations of the family. Data from the School Refusal Assessment Scale-Revised, interviews, and observations can then be used to recommend particular treatment options.
Table 4
Questions related to forms of school refusal behavior
| What are the child’s specific forms of absenteeism, and how do these forms change daily? | What specific school-related stimuli are provoking the child’s concern about going to school? |
| Is a child’s school refusal behavior relatively acute or chronic in nature (in related fashion, how did the child’s school refusal behavior develop over time)? | Is the child’s refusal to attend school legitimate or understandable in some way (eg, school-based threat, bullying, inadequate school climate)? |
| What comorbid conditions occur with a child’s school refusal behavior (Table 3), including substance abuse? | What family disruption or conflict has occurred as a result of a child’s school refusal behavior? |
| What is the child’s degree of anxiety or misbehavior upon entering school, and what specific misbehaviors are present in the morning before school (Table 2)? | What is the child’s academic and social status? (This should include a review of academic records, formal evaluation reports, attendance records, and individualized education plans or 504 plans as applicable.) |
Table 5
Questions related to functions of school refusal behavior
| Have recent or traumatic home or school events influenced a child’s school refusal behavior? | Is the child willing to attend school if a parent accompanies him or her? |
| Are symptoms of school refusal behavior evident on weekends and holidays? | What specific tangible rewards does the child pursue outside of school that cause him or her to miss school? |
| Are there any nonschool situations where anxiety or attention-seeking behavior occurs? | Is the child willing to attend school if incentives are provided for attendance? |
| What specific social and/or evaluative situations at school are avoided? |
Treating youths who refuse school
Treatment success will be better assured if you work closely with school personnel and parents to gather and share information, coordinate a plan for returning a child to school, and address familial issues and the child’s comorbid medical problems that impact attendance.
Medications have proven useful in alleviating severe cases of anxiety and depression, and cognitive management techniques can be applied to the child, the parents, and the family together.
Anxiolytics or antidepressants. Pharmacotherapy research for school refusal behavior is in its infancy. Some investigators have found, however, that a tricyclic antidepressant (TCA) such as imipramine, 3 mg/kg/d, may be useful in some cases20,21—generally for youths ages 10 to 17 years with better attendance records and fewer symptoms of social avoidance and separation anxiety.22 Researchers speculate that TCAs, which are not always effective in children, may influence symptoms such as anhedonia or sleep problems that contribute to school refusal behavior.
With respect to substantial child anxiety and depression without school refusal behavior, researchers have focused on selective serotonin reuptake inhibitors (SSRIs). In particular, fluoxetine, 10 to 20 mg/d, fluvoxamine, 50 to 250 mg/d, sertraline, 85 to 160 mg/d, and paroxetine, 10 to 50 mg/d, have been useful for youths with symptoms of general and social anxiety and depression.23,24
Youths often do not respond to these medications as well as adults do, however, because of the fluid and amorphous nature of anxious and depressive symptomatology in children and adolescents. Careful monitoring is required when treating youth with SSRIs, which have been associated with an increased risk of suicidal behavior.
Psychological techniques. Sophisticated clinical controlled studies have addressed the treatment of diverse youths with school refusal behavior.25-28 Options for this population may be arranged according to function or the primary reinforcers maintaining absenteeism:
- child-based techniques to manage anxiety in a school setting
- parent-based techniques to manage contingencies for school attendance and nonattendance
- family-based techniques to manage incentives and disincentives for school attendance and nonattendance.
Child-based anxiety management techniques include relaxation training, breathing retraining, cognitive therapy (generally for youths ages 9 to 17), and exposure-based practices to gradually reintroduce a child to school. These techniques have been strongly supported by randomized controlled trials specific to school refusal behavior2 and are useful for treating general anxiety and depression as well.
Parent-based contingency management techniques include establishing morning and evening routines, modifying parental commands toward brevity and clarity, providing attention-based consequences for school nonattendance (such as early bedtime, limited time with a parent at night), reducing excessive child questioning or reassurance-seeking behavior, and engaging in forced school attendance under strict conditions. Parent-based techniques have received strong support in the literature in general29 but have been applied less frequently than child-based techniques to youths with school refusal behavior.
Family-based techniques include developing written contracts to increase incentives for school attendance and decrease incentives for nonattendance, escorting a child to school and classes, and teaching youths to refuse offers from peers to miss school.30 As with parent-based techniques, family-based techniques have received strong support in the literature in general, but have been applied less frequently than child-based techniques to youths with school refusal behavior.
Gradual reintroduction to school
A preferred approach to resolve school refusal behavior usually involves gradual reintegration to school and classes. This may include initial attendance at lunchtime, 1 or 2 favorite classes, or in an alternative classroom setting such as a guidance counselor’s office or school library. Gradual reintegration into regular classrooms may then proceed.
If possible, a child should remain in school during the day and not be sent home unless intense medical symptoms are present.30 A recommended list of intense symptoms includes:
- frequent vomiting
- bleeding
- temperature >100° F
- severe diarrhea
- lice
- acute flu-like symptoms
- extreme medical conditions such as intense pain.
Case continued: a full-time student.
A structured diagnostic interview and other behavioral assessment measures show that Nathan meets criteria for generalized anxiety disorder. He worries excessively about his social and academic performance at school and displays several somatic complaints related to anxiety. His treatment thus involves a two-pronged approach:
- sertraline, 50 mg/d, which has been found to significantly reduce symptoms of generalized anxiety disorder in youths ages 5 to 17.
- child-based anxiety management techniques and family therapy to increase incentives for school attendance and limit fun activities during a school day spent at home.
His therapist and family physician collaborate with school personnel to gradually reintroduce Nathan to a full-time academic schedule.
- Copies of the child and parent versions of the School Refusal Assessment Scale-Revised are available at www.jfponline.com/Pages.asp?AID=4322&UID=.
- King NJ, Bernstein GA. School refusal in children and adolescents: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 2001;40:197-205.
- Kearney CA. School refusal behavior in youth: a functional approach to assessment and treatment. Washington, DC: American Psychological Association; 2001.
- Kearney CA, Albano AM. When children refuse school: a cognitive-behavioral therapy approach. Parent workbook/therapist’s guide. New York: Oxford University Press; 2000.
Drug brand names
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Paroxetine • Paxil
- Sertraline • Zoloft
Acknowledgment
Adapted and reprinted with permission from The Journal of Family Practice, August 2006, p 685-92.
1. Kearney CA, Silverman WK. The evolution and reconciliation of taxonomic strategies for school refusal behavior. Clin Psychol: Sci Prac 1996;3:339-54.
2. Kearney CA. School refusal behavior in youth: a functional approach to assessment and treatment. Washington, DC: American Psychological Association; 2001.
3. Hansen C, Sanders SL, Massaro S, Last CG. Predictors of severity of absenteeism in children with anxiety-based school refusal. J Clin Child Psychol 1998;27:246-54.
4. Franklin CG, Soto I. Keeping Hispanic youths in school. Children & Schools 2002;24:139-43.
5. Egger HL, Costello EJ, Angold A. School refusal and psychiatric disorders: a community study. J Am Acad Child Adolesc Psychiatry 2003;42:797-807.
6. McShane G, Walter G, Rey JM. Characteristics of adolescents with school refusal. Aust New Zeal J Psychiatry 2001;35:822-6.
7. Kearney CA, Albano AM. The functional profiles of school refusal behavior: diagnostic aspects. Behav Modif 2004;28:147-61.
8. Bernstein GA, Massie ED, Thuras PD, Perwien AR, Borchardt CM, Crosby RD. Somatic symptoms in anxious-depressed school refusers. J Am Acad Child Adolesc Psychiatry 1997;36:661-8.
9. Gilliland FD, Berhane K, Islam T, et al. Environmental tobacco smoke and absenteeism related to respiratory illness in school children. Am J Epidemiology 2003;157:861-9.
10. Glaab LA, Brown R, Daneman D. School attendance in children with type I diabetes. Diabetic Med 2005;22:421-6.
11. Levy RL, Whitehead WE, Walker LS, et al. Increased somatic complaints and health-care utilization in children: effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterology 2004;99:2442-51.
12. Buitelaar JK, van Andel H, Duyx JHM, van Strien DC. Depressive and anxiety disorders in adolescence: a follow-up study of adolescents with school refusal. Acta Paedopsychiatrica 1994;56:249-53.
13. Flakierska-Praquin N, Lindstrom M, Gillberg C. School phobia with separation anxiety disorder: a comparative 20- to 29-year follow-up study of 35 school refusers. Comp Psychiatry 1997;38:17-22.
14. Hibbett A, Fogelman K. Future lives of truants: family formation and health-related behaviour. Brit J Educ Psychology 1990;60:171-9.
15. Hibbett A, Fogelman K, Manor O. Occupational outcomes of truancy. Brit J Educ Psychology 1990;60:23-36.
16. Kearney CA. Bridging the gap among professionals who address youth with school absenteeism: overview and suggestions for consensus. Prof Psychol Res Prac 2003;34:57-65.
17. King NJ, Heyne D, Tonge B, Gullone E, Ollendick TH. School refusal: categorical diagnoses, functional analysis and treatment planning. Clin Psychol Psychother 2001;8:352-60.
18. Kearney CA. Identifying the function of school refusal behavior: a revision of the School Refusal Assessment Scale. J Psychopathol Behav Assess 2002;24:235-45.
19. Kearney CA. Confirmatory factor analysis of the School Refusal Assessment Scale-Revised: child and parent versions. J Psychopathol Behav Assess 2006; in press.
20. Bernstein GA, Borchardt CM, Perwein AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Chil Adol Psychiatry 2000;39:276-83.
21. Kearney CA, Silverman WK. A critical review of pharmacotherapy for youth with anxiety disorders: things are not as they seem. J Anxiety Disord 1998;12:83-102.
22. Layne AE, Bernstein GA, Egan EA, Kushner MG. Predictors of treatment response in anxious-depressed adolescents with school refusal. J Am Acad Chil Adol Psychiatry 2003;42:319-26.
23. Compton SN, Grant PJ, Chrisman AK, et al. Sertraline in children and adolescents with social anxiety disorder: an open trial. J Am Acad Chil Adol Psychiatry 2001;40:564-71.
24. Whittington CJ, Kendall T, Fonagy T, et al. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004;363:1341-5.
25. Kearney CA, Silverman WK. Functionally-based prescriptive and nonprescriptive treatment for children and adolescents with school refusal behavior. Behav Ther 1999;30:673-95.
26. King NJ, Tonge BJ, Heyne D, et al. Cognitive-behavioral treatment of school-refusing children: A controlled evaluation. J Am Acad Chil Adol Psychiatry 1998;37:395-403.
27. Last CG, Hansen C, Franco N. Cognitive-behavioral treatment of school phobia. J Am Acad Chil Adol Psychiatry 1998;37:404-11.
28. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-20.
29. Kearney CA, Roblek TL. Parent training in the treatment of school refusal behavior. In: Briesmeister JM, Schaefer CE, eds. Handbook of parent training: parents as co-therapists for children’s behavior problems, 2nd ed. New York: Wiley; 1998:225-56.
30. Kearney CA, Albano AM. When children refuse school: A cognitive-behavioral therapy approach/Therapist’s guide. San Antonio: Psychological Corporation, 2000.
31. Kearney CA, Bates M. Addressing school refusal behavior: Suggestions for frontline professionals. Children & Schools 2005;27:207-16.
32. Astor RA, Benbenishty R, Zeira A, Vinokur A. School climate, observed risky behaviors, and victimization as predictors of high school students’ fear and judgments of school violence as a problem. Health Educ Behav 2002;29:716-36.
33. Glew GM, Fan M-Y, Katon W, Rivara FP, Kernic MA. Bullying, psychosocial adjustment, and academic performance in elementary school. Arch Pediatr Adolesc Med 2005;159:1026-31.
34. Swahn MH, Bossarte RM. The associations between victimization, feeling unsafe, and asthma episodes among US high-school students. Am J Public Health 2006;96:802-4.
Case: 'He's okay on weekends'
Nathan, age 13, is referred by his parents for recent school refusal behavior. He has had difficulty adjusting to middle school and has been marked absent one-third of school days this academic year. These absences come in the form of tardiness, skipped classes, and full-day absences.
Nathan complains of headaches and stomachaches and says he feels upset and nervous while in school. His parents, however, complain that Nathan seems fine on weekends and holidays and seems to be embellishing symptoms to miss school. Nathan’s parents are concerned that their son may have some physical or mental condition that is preventing his school attendance and that might be remediated with medication.
Child-motivated refusal to attend school or remain in class an entire day is not uncommon, affecting 5% to 28% of youths at some time in their lives.1,2
The behavior may be viewed along a spectrum of absenteeism (Figure), and a child may exhibit all forms of absenteeism at one time or another. In Nathan’s case, for example, he could be anxious during school on Monday, arrive late to school on Tuesday, skip afternoon classes on Wednesday, and fail to attend school completely on Thursday and Friday.
In this article you will learn characteristics of school refusal behavior to watch for and assess, and treatment strategies for youths ages 5 to 17. You will also find advice and techniques to offer parents.
Figure A child might exhibit each behavior on this spectrum at different times
Refusal behavior characteristics
School refusal behavior encompasses all subsets of problematic absenteeism, such as truancy, school phobia, and separation anxiety.3 Children and adolescents of all ages, boys and girls alike, can exhibit school refusal behavior. The most common age of onset is 10 to 13 years. Youths such as Nathan who are entering a school building for the first time—especially elementary and middle school—are at particular risk for school refusal behavior. Little information is available regarding ethnic differences, although school dropout rates for Hispanics are often considerably elevated compared with other ethnic groups.4,5
School refusal behavior covers a range of symptoms, diagnoses, somatic complaints, and medical conditions (Tables 1-3).6-12 Longitudinal studies indicate that school refusal behavior, if left unaddressed, can lead to serious short-term problems, such as distress, academic decline, alienation from peers, family conflict, and financial and legal consequences. Common long-term problems include school dropout, delinquent behaviors, economic deprivation, social isolation, marital problems, and difficulty maintaining employment. Approximately 52% of adolescents with school refusal behavior meet criteria for an anxiety, depressive, conduct-personality, or other psychiatric disorder later in life.13-16
Table 1
Common symptoms that could signal school refusal behavior
| Internalizing/covert symptoms | Externalizing/overt symptoms |
|---|---|
| Depression | Aggression |
| Fatigue/tiredness | Clinging to an adult |
| Fear and panic | Excessive reassurance-seeking behavior |
| General and social anxiety | Noncompliance and defiance |
| Self-consciousness | Refusal to move in the morning |
| Somatization | Running away from school or home |
| Worry | Temper tantrums and crying |
Table 2
Primary psychiatric disorders among youths with school refusal behavior
| Diagnosis | Percentage |
|---|---|
| None | 32.9% |
| Separation anxiety disorder | 22.4% |
| Generalized anxiety disorder | 10.5% |
| Oppositional defiant disorder | 8.4% |
| Major depression | 4.9% |
| Specific phobia | 4.2% |
| Social anxiety disorder | 3.5% |
| Conduct disorder | 2.8% |
| Attention deficit/hyperactivity disorder | 1.4% |
| Panic disorder | 1.4% |
| Enuresis | 0.7% |
| Posttraumatic stress disorder | 0.7% |
| Source: Reference 7 | |
Table 3
Somatic complaints and medical conditions
commonly associated with school refusal behavior
| Somatic complaints | Medical conditions |
|---|---|
| Diarrhea/irritable bowel | Allergic rhinitis |
| Fatigue | Asthma and respiratory illness |
| Headache and stomachache | Chronic pain and illness (notably cancer, Crohn’s disease, dyspepsia, hemophilia, chronic fatigue syndrome) |
| Nausea and vomiting | Diabetes |
| Palpitations and perspiration | Dysmenorrhea |
| Recurrent abdominal pain or other pain | Head louse infestation |
| Shaking or trembling | Influenza |
| Sleep problems | Orodental disease |
Finding a reason for school refusal
If a child has somatic complaints, you can expect to find that the child is:
- suffering from a true physical malady
- embellishing low-grade physical symptoms from stress or attention-seeking behavior
- reporting physical problems that have no medical basis.
A full medical examination is always recommended to rule out organic problems or to properly treat true medical conditions.
Four functions. If no medical condition is found, explore the reasons a particular child refuses school. A common model of conceptualizing school refusal behavior involves reinforcers:1,2
- to avoid school-based stimuli that provoke a sense of negative affectivity, or combined anxiety and depression; examples of key stimuli include teachers, peers, bus, cafeteria, classroom, and transitions between classes
- to escape aversive social or evaluative situations such as conversing or otherwise interacting with others or performing before others as in class presentations
- to pursue attention from significant others, such as wanting to stay home or go to work with parents
- to pursue tangible reinforcers outside of school, such as sleeping late, watching television, playing with friends, or engaging in delinquent behavior or substance use.
The first 2 functions are maintained by negative reinforcement or a desire to leave anxiety-provoking stimuli. The latter 2 functions are maintained by positive reinforcement, or a desire to pursue rewards outside of school. Youths may also refuse school for a combination of these reasons.17 In Nathan’s case, he was initially anxious about school in general (the first function). After his parents allowed him to stay home for a few days, however, he was refusing school to enjoy fun activities such as video games at home (the last function).
Violence on school campuses across the country naturally makes many parents skittish about possible copycat incidents. In fact, some parents acquiesce to their children’s pleas to remain home on school shooting anniversaries—particularly the Columbine tragedy of April 20, 1999.
Student and parental fears likely are exacerbated by new episodes of violence, such as three school shootings in 2006:
- On September 27, a 53-year-old man entered a high school in Bailey, Colorado, and shot one girl before killing himself.
- On September 29, a high school student near Madison, Wisconsin, killed his principal after being disciplined for carrying tobacco.
- On October 2, a heavily armed man barricaded himself in a one-room Amish schoolhouse in Paradise, Pennsylvania. He bound and shot 11 girls before killing himself, and five of the girls died.
Compared with highly publicized school violence, however, personal victimization is a much stronger factor in absenteeism.32 Specifically, school violence is related to school absenteeism especially for youths who have been previously victimized. The literature shows:
- Students who have been bullied are 2.1 times more likely than other students to feel unsafe at school.
- 20% of elementary school children report they would skip school to avoid being bullied.33
- High school students’ fear of attending classes because of violence is directly associated with victimization by teachers or other students.
- Missing school because of feeling unsafe is a strong risk factor for asthma and, potentially, being sent home early from school.34
Assessment scale. One method for quickly assessing the role of these functions is the School Refusal Assessment Scale-Revised.18,19 This scale poses 24 questions, the answers to which measure the relative strength of each of the 4 functions. Versions are available for children and parents, who complete their respective scales separately (see Related resources). Item means are calculated across the measures to help determine the primary reason for a child’s school refusal.
In addition to using the assessment scale, you may ask interview questions regarding the form and function of school refusal behavior (Tables 4,5). Take care to assess attendance history and patterns, comorbid conditions, instances of legitimate absenteeism, family disruption, and a child’s social and academic status. Specific questions about function can help narrow the reason for school refusal.
Assess specific school-related stimuli that provoke absenteeism such as social and evaluative situations, whether a child could attend school with a parent (evidence of attention-seeking), and what tangible rewards a child receives for absenteeism throughout the school day. Information about the form and function of school refusal behavior may also be evident during in-office observations of the family. Data from the School Refusal Assessment Scale-Revised, interviews, and observations can then be used to recommend particular treatment options.
Table 4
Questions related to forms of school refusal behavior
| What are the child’s specific forms of absenteeism, and how do these forms change daily? | What specific school-related stimuli are provoking the child’s concern about going to school? |
| Is a child’s school refusal behavior relatively acute or chronic in nature (in related fashion, how did the child’s school refusal behavior develop over time)? | Is the child’s refusal to attend school legitimate or understandable in some way (eg, school-based threat, bullying, inadequate school climate)? |
| What comorbid conditions occur with a child’s school refusal behavior (Table 3), including substance abuse? | What family disruption or conflict has occurred as a result of a child’s school refusal behavior? |
| What is the child’s degree of anxiety or misbehavior upon entering school, and what specific misbehaviors are present in the morning before school (Table 2)? | What is the child’s academic and social status? (This should include a review of academic records, formal evaluation reports, attendance records, and individualized education plans or 504 plans as applicable.) |
Table 5
Questions related to functions of school refusal behavior
| Have recent or traumatic home or school events influenced a child’s school refusal behavior? | Is the child willing to attend school if a parent accompanies him or her? |
| Are symptoms of school refusal behavior evident on weekends and holidays? | What specific tangible rewards does the child pursue outside of school that cause him or her to miss school? |
| Are there any nonschool situations where anxiety or attention-seeking behavior occurs? | Is the child willing to attend school if incentives are provided for attendance? |
| What specific social and/or evaluative situations at school are avoided? |
Treating youths who refuse school
Treatment success will be better assured if you work closely with school personnel and parents to gather and share information, coordinate a plan for returning a child to school, and address familial issues and the child’s comorbid medical problems that impact attendance.
Medications have proven useful in alleviating severe cases of anxiety and depression, and cognitive management techniques can be applied to the child, the parents, and the family together.
Anxiolytics or antidepressants. Pharmacotherapy research for school refusal behavior is in its infancy. Some investigators have found, however, that a tricyclic antidepressant (TCA) such as imipramine, 3 mg/kg/d, may be useful in some cases20,21—generally for youths ages 10 to 17 years with better attendance records and fewer symptoms of social avoidance and separation anxiety.22 Researchers speculate that TCAs, which are not always effective in children, may influence symptoms such as anhedonia or sleep problems that contribute to school refusal behavior.
With respect to substantial child anxiety and depression without school refusal behavior, researchers have focused on selective serotonin reuptake inhibitors (SSRIs). In particular, fluoxetine, 10 to 20 mg/d, fluvoxamine, 50 to 250 mg/d, sertraline, 85 to 160 mg/d, and paroxetine, 10 to 50 mg/d, have been useful for youths with symptoms of general and social anxiety and depression.23,24
Youths often do not respond to these medications as well as adults do, however, because of the fluid and amorphous nature of anxious and depressive symptomatology in children and adolescents. Careful monitoring is required when treating youth with SSRIs, which have been associated with an increased risk of suicidal behavior.
Psychological techniques. Sophisticated clinical controlled studies have addressed the treatment of diverse youths with school refusal behavior.25-28 Options for this population may be arranged according to function or the primary reinforcers maintaining absenteeism:
- child-based techniques to manage anxiety in a school setting
- parent-based techniques to manage contingencies for school attendance and nonattendance
- family-based techniques to manage incentives and disincentives for school attendance and nonattendance.
Child-based anxiety management techniques include relaxation training, breathing retraining, cognitive therapy (generally for youths ages 9 to 17), and exposure-based practices to gradually reintroduce a child to school. These techniques have been strongly supported by randomized controlled trials specific to school refusal behavior2 and are useful for treating general anxiety and depression as well.
Parent-based contingency management techniques include establishing morning and evening routines, modifying parental commands toward brevity and clarity, providing attention-based consequences for school nonattendance (such as early bedtime, limited time with a parent at night), reducing excessive child questioning or reassurance-seeking behavior, and engaging in forced school attendance under strict conditions. Parent-based techniques have received strong support in the literature in general29 but have been applied less frequently than child-based techniques to youths with school refusal behavior.
Family-based techniques include developing written contracts to increase incentives for school attendance and decrease incentives for nonattendance, escorting a child to school and classes, and teaching youths to refuse offers from peers to miss school.30 As with parent-based techniques, family-based techniques have received strong support in the literature in general, but have been applied less frequently than child-based techniques to youths with school refusal behavior.
Gradual reintroduction to school
A preferred approach to resolve school refusal behavior usually involves gradual reintegration to school and classes. This may include initial attendance at lunchtime, 1 or 2 favorite classes, or in an alternative classroom setting such as a guidance counselor’s office or school library. Gradual reintegration into regular classrooms may then proceed.
If possible, a child should remain in school during the day and not be sent home unless intense medical symptoms are present.30 A recommended list of intense symptoms includes:
- frequent vomiting
- bleeding
- temperature >100° F
- severe diarrhea
- lice
- acute flu-like symptoms
- extreme medical conditions such as intense pain.
Case continued: a full-time student.
A structured diagnostic interview and other behavioral assessment measures show that Nathan meets criteria for generalized anxiety disorder. He worries excessively about his social and academic performance at school and displays several somatic complaints related to anxiety. His treatment thus involves a two-pronged approach:
- sertraline, 50 mg/d, which has been found to significantly reduce symptoms of generalized anxiety disorder in youths ages 5 to 17.
- child-based anxiety management techniques and family therapy to increase incentives for school attendance and limit fun activities during a school day spent at home.
His therapist and family physician collaborate with school personnel to gradually reintroduce Nathan to a full-time academic schedule.
- Copies of the child and parent versions of the School Refusal Assessment Scale-Revised are available at www.jfponline.com/Pages.asp?AID=4322&UID=.
- King NJ, Bernstein GA. School refusal in children and adolescents: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 2001;40:197-205.
- Kearney CA. School refusal behavior in youth: a functional approach to assessment and treatment. Washington, DC: American Psychological Association; 2001.
- Kearney CA, Albano AM. When children refuse school: a cognitive-behavioral therapy approach. Parent workbook/therapist’s guide. New York: Oxford University Press; 2000.
Drug brand names
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Paroxetine • Paxil
- Sertraline • Zoloft
Acknowledgment
Adapted and reprinted with permission from The Journal of Family Practice, August 2006, p 685-92.
Case: 'He's okay on weekends'
Nathan, age 13, is referred by his parents for recent school refusal behavior. He has had difficulty adjusting to middle school and has been marked absent one-third of school days this academic year. These absences come in the form of tardiness, skipped classes, and full-day absences.
Nathan complains of headaches and stomachaches and says he feels upset and nervous while in school. His parents, however, complain that Nathan seems fine on weekends and holidays and seems to be embellishing symptoms to miss school. Nathan’s parents are concerned that their son may have some physical or mental condition that is preventing his school attendance and that might be remediated with medication.
Child-motivated refusal to attend school or remain in class an entire day is not uncommon, affecting 5% to 28% of youths at some time in their lives.1,2
The behavior may be viewed along a spectrum of absenteeism (Figure), and a child may exhibit all forms of absenteeism at one time or another. In Nathan’s case, for example, he could be anxious during school on Monday, arrive late to school on Tuesday, skip afternoon classes on Wednesday, and fail to attend school completely on Thursday and Friday.
In this article you will learn characteristics of school refusal behavior to watch for and assess, and treatment strategies for youths ages 5 to 17. You will also find advice and techniques to offer parents.
Figure A child might exhibit each behavior on this spectrum at different times
Refusal behavior characteristics
School refusal behavior encompasses all subsets of problematic absenteeism, such as truancy, school phobia, and separation anxiety.3 Children and adolescents of all ages, boys and girls alike, can exhibit school refusal behavior. The most common age of onset is 10 to 13 years. Youths such as Nathan who are entering a school building for the first time—especially elementary and middle school—are at particular risk for school refusal behavior. Little information is available regarding ethnic differences, although school dropout rates for Hispanics are often considerably elevated compared with other ethnic groups.4,5
School refusal behavior covers a range of symptoms, diagnoses, somatic complaints, and medical conditions (Tables 1-3).6-12 Longitudinal studies indicate that school refusal behavior, if left unaddressed, can lead to serious short-term problems, such as distress, academic decline, alienation from peers, family conflict, and financial and legal consequences. Common long-term problems include school dropout, delinquent behaviors, economic deprivation, social isolation, marital problems, and difficulty maintaining employment. Approximately 52% of adolescents with school refusal behavior meet criteria for an anxiety, depressive, conduct-personality, or other psychiatric disorder later in life.13-16
Table 1
Common symptoms that could signal school refusal behavior
| Internalizing/covert symptoms | Externalizing/overt symptoms |
|---|---|
| Depression | Aggression |
| Fatigue/tiredness | Clinging to an adult |
| Fear and panic | Excessive reassurance-seeking behavior |
| General and social anxiety | Noncompliance and defiance |
| Self-consciousness | Refusal to move in the morning |
| Somatization | Running away from school or home |
| Worry | Temper tantrums and crying |
Table 2
Primary psychiatric disorders among youths with school refusal behavior
| Diagnosis | Percentage |
|---|---|
| None | 32.9% |
| Separation anxiety disorder | 22.4% |
| Generalized anxiety disorder | 10.5% |
| Oppositional defiant disorder | 8.4% |
| Major depression | 4.9% |
| Specific phobia | 4.2% |
| Social anxiety disorder | 3.5% |
| Conduct disorder | 2.8% |
| Attention deficit/hyperactivity disorder | 1.4% |
| Panic disorder | 1.4% |
| Enuresis | 0.7% |
| Posttraumatic stress disorder | 0.7% |
| Source: Reference 7 | |
Table 3
Somatic complaints and medical conditions
commonly associated with school refusal behavior
| Somatic complaints | Medical conditions |
|---|---|
| Diarrhea/irritable bowel | Allergic rhinitis |
| Fatigue | Asthma and respiratory illness |
| Headache and stomachache | Chronic pain and illness (notably cancer, Crohn’s disease, dyspepsia, hemophilia, chronic fatigue syndrome) |
| Nausea and vomiting | Diabetes |
| Palpitations and perspiration | Dysmenorrhea |
| Recurrent abdominal pain or other pain | Head louse infestation |
| Shaking or trembling | Influenza |
| Sleep problems | Orodental disease |
Finding a reason for school refusal
If a child has somatic complaints, you can expect to find that the child is:
- suffering from a true physical malady
- embellishing low-grade physical symptoms from stress or attention-seeking behavior
- reporting physical problems that have no medical basis.
A full medical examination is always recommended to rule out organic problems or to properly treat true medical conditions.
Four functions. If no medical condition is found, explore the reasons a particular child refuses school. A common model of conceptualizing school refusal behavior involves reinforcers:1,2
- to avoid school-based stimuli that provoke a sense of negative affectivity, or combined anxiety and depression; examples of key stimuli include teachers, peers, bus, cafeteria, classroom, and transitions between classes
- to escape aversive social or evaluative situations such as conversing or otherwise interacting with others or performing before others as in class presentations
- to pursue attention from significant others, such as wanting to stay home or go to work with parents
- to pursue tangible reinforcers outside of school, such as sleeping late, watching television, playing with friends, or engaging in delinquent behavior or substance use.
The first 2 functions are maintained by negative reinforcement or a desire to leave anxiety-provoking stimuli. The latter 2 functions are maintained by positive reinforcement, or a desire to pursue rewards outside of school. Youths may also refuse school for a combination of these reasons.17 In Nathan’s case, he was initially anxious about school in general (the first function). After his parents allowed him to stay home for a few days, however, he was refusing school to enjoy fun activities such as video games at home (the last function).
Violence on school campuses across the country naturally makes many parents skittish about possible copycat incidents. In fact, some parents acquiesce to their children’s pleas to remain home on school shooting anniversaries—particularly the Columbine tragedy of April 20, 1999.
Student and parental fears likely are exacerbated by new episodes of violence, such as three school shootings in 2006:
- On September 27, a 53-year-old man entered a high school in Bailey, Colorado, and shot one girl before killing himself.
- On September 29, a high school student near Madison, Wisconsin, killed his principal after being disciplined for carrying tobacco.
- On October 2, a heavily armed man barricaded himself in a one-room Amish schoolhouse in Paradise, Pennsylvania. He bound and shot 11 girls before killing himself, and five of the girls died.
Compared with highly publicized school violence, however, personal victimization is a much stronger factor in absenteeism.32 Specifically, school violence is related to school absenteeism especially for youths who have been previously victimized. The literature shows:
- Students who have been bullied are 2.1 times more likely than other students to feel unsafe at school.
- 20% of elementary school children report they would skip school to avoid being bullied.33
- High school students’ fear of attending classes because of violence is directly associated with victimization by teachers or other students.
- Missing school because of feeling unsafe is a strong risk factor for asthma and, potentially, being sent home early from school.34
Assessment scale. One method for quickly assessing the role of these functions is the School Refusal Assessment Scale-Revised.18,19 This scale poses 24 questions, the answers to which measure the relative strength of each of the 4 functions. Versions are available for children and parents, who complete their respective scales separately (see Related resources). Item means are calculated across the measures to help determine the primary reason for a child’s school refusal.
In addition to using the assessment scale, you may ask interview questions regarding the form and function of school refusal behavior (Tables 4,5). Take care to assess attendance history and patterns, comorbid conditions, instances of legitimate absenteeism, family disruption, and a child’s social and academic status. Specific questions about function can help narrow the reason for school refusal.
Assess specific school-related stimuli that provoke absenteeism such as social and evaluative situations, whether a child could attend school with a parent (evidence of attention-seeking), and what tangible rewards a child receives for absenteeism throughout the school day. Information about the form and function of school refusal behavior may also be evident during in-office observations of the family. Data from the School Refusal Assessment Scale-Revised, interviews, and observations can then be used to recommend particular treatment options.
Table 4
Questions related to forms of school refusal behavior
| What are the child’s specific forms of absenteeism, and how do these forms change daily? | What specific school-related stimuli are provoking the child’s concern about going to school? |
| Is a child’s school refusal behavior relatively acute or chronic in nature (in related fashion, how did the child’s school refusal behavior develop over time)? | Is the child’s refusal to attend school legitimate or understandable in some way (eg, school-based threat, bullying, inadequate school climate)? |
| What comorbid conditions occur with a child’s school refusal behavior (Table 3), including substance abuse? | What family disruption or conflict has occurred as a result of a child’s school refusal behavior? |
| What is the child’s degree of anxiety or misbehavior upon entering school, and what specific misbehaviors are present in the morning before school (Table 2)? | What is the child’s academic and social status? (This should include a review of academic records, formal evaluation reports, attendance records, and individualized education plans or 504 plans as applicable.) |
Table 5
Questions related to functions of school refusal behavior
| Have recent or traumatic home or school events influenced a child’s school refusal behavior? | Is the child willing to attend school if a parent accompanies him or her? |
| Are symptoms of school refusal behavior evident on weekends and holidays? | What specific tangible rewards does the child pursue outside of school that cause him or her to miss school? |
| Are there any nonschool situations where anxiety or attention-seeking behavior occurs? | Is the child willing to attend school if incentives are provided for attendance? |
| What specific social and/or evaluative situations at school are avoided? |
Treating youths who refuse school
Treatment success will be better assured if you work closely with school personnel and parents to gather and share information, coordinate a plan for returning a child to school, and address familial issues and the child’s comorbid medical problems that impact attendance.
Medications have proven useful in alleviating severe cases of anxiety and depression, and cognitive management techniques can be applied to the child, the parents, and the family together.
Anxiolytics or antidepressants. Pharmacotherapy research for school refusal behavior is in its infancy. Some investigators have found, however, that a tricyclic antidepressant (TCA) such as imipramine, 3 mg/kg/d, may be useful in some cases20,21—generally for youths ages 10 to 17 years with better attendance records and fewer symptoms of social avoidance and separation anxiety.22 Researchers speculate that TCAs, which are not always effective in children, may influence symptoms such as anhedonia or sleep problems that contribute to school refusal behavior.
With respect to substantial child anxiety and depression without school refusal behavior, researchers have focused on selective serotonin reuptake inhibitors (SSRIs). In particular, fluoxetine, 10 to 20 mg/d, fluvoxamine, 50 to 250 mg/d, sertraline, 85 to 160 mg/d, and paroxetine, 10 to 50 mg/d, have been useful for youths with symptoms of general and social anxiety and depression.23,24
Youths often do not respond to these medications as well as adults do, however, because of the fluid and amorphous nature of anxious and depressive symptomatology in children and adolescents. Careful monitoring is required when treating youth with SSRIs, which have been associated with an increased risk of suicidal behavior.
Psychological techniques. Sophisticated clinical controlled studies have addressed the treatment of diverse youths with school refusal behavior.25-28 Options for this population may be arranged according to function or the primary reinforcers maintaining absenteeism:
- child-based techniques to manage anxiety in a school setting
- parent-based techniques to manage contingencies for school attendance and nonattendance
- family-based techniques to manage incentives and disincentives for school attendance and nonattendance.
Child-based anxiety management techniques include relaxation training, breathing retraining, cognitive therapy (generally for youths ages 9 to 17), and exposure-based practices to gradually reintroduce a child to school. These techniques have been strongly supported by randomized controlled trials specific to school refusal behavior2 and are useful for treating general anxiety and depression as well.
Parent-based contingency management techniques include establishing morning and evening routines, modifying parental commands toward brevity and clarity, providing attention-based consequences for school nonattendance (such as early bedtime, limited time with a parent at night), reducing excessive child questioning or reassurance-seeking behavior, and engaging in forced school attendance under strict conditions. Parent-based techniques have received strong support in the literature in general29 but have been applied less frequently than child-based techniques to youths with school refusal behavior.
Family-based techniques include developing written contracts to increase incentives for school attendance and decrease incentives for nonattendance, escorting a child to school and classes, and teaching youths to refuse offers from peers to miss school.30 As with parent-based techniques, family-based techniques have received strong support in the literature in general, but have been applied less frequently than child-based techniques to youths with school refusal behavior.
Gradual reintroduction to school
A preferred approach to resolve school refusal behavior usually involves gradual reintegration to school and classes. This may include initial attendance at lunchtime, 1 or 2 favorite classes, or in an alternative classroom setting such as a guidance counselor’s office or school library. Gradual reintegration into regular classrooms may then proceed.
If possible, a child should remain in school during the day and not be sent home unless intense medical symptoms are present.30 A recommended list of intense symptoms includes:
- frequent vomiting
- bleeding
- temperature >100° F
- severe diarrhea
- lice
- acute flu-like symptoms
- extreme medical conditions such as intense pain.
Case continued: a full-time student.
A structured diagnostic interview and other behavioral assessment measures show that Nathan meets criteria for generalized anxiety disorder. He worries excessively about his social and academic performance at school and displays several somatic complaints related to anxiety. His treatment thus involves a two-pronged approach:
- sertraline, 50 mg/d, which has been found to significantly reduce symptoms of generalized anxiety disorder in youths ages 5 to 17.
- child-based anxiety management techniques and family therapy to increase incentives for school attendance and limit fun activities during a school day spent at home.
His therapist and family physician collaborate with school personnel to gradually reintroduce Nathan to a full-time academic schedule.
- Copies of the child and parent versions of the School Refusal Assessment Scale-Revised are available at www.jfponline.com/Pages.asp?AID=4322&UID=.
- King NJ, Bernstein GA. School refusal in children and adolescents: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 2001;40:197-205.
- Kearney CA. School refusal behavior in youth: a functional approach to assessment and treatment. Washington, DC: American Psychological Association; 2001.
- Kearney CA, Albano AM. When children refuse school: a cognitive-behavioral therapy approach. Parent workbook/therapist’s guide. New York: Oxford University Press; 2000.
Drug brand names
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Paroxetine • Paxil
- Sertraline • Zoloft
Acknowledgment
Adapted and reprinted with permission from The Journal of Family Practice, August 2006, p 685-92.
1. Kearney CA, Silverman WK. The evolution and reconciliation of taxonomic strategies for school refusal behavior. Clin Psychol: Sci Prac 1996;3:339-54.
2. Kearney CA. School refusal behavior in youth: a functional approach to assessment and treatment. Washington, DC: American Psychological Association; 2001.
3. Hansen C, Sanders SL, Massaro S, Last CG. Predictors of severity of absenteeism in children with anxiety-based school refusal. J Clin Child Psychol 1998;27:246-54.
4. Franklin CG, Soto I. Keeping Hispanic youths in school. Children & Schools 2002;24:139-43.
5. Egger HL, Costello EJ, Angold A. School refusal and psychiatric disorders: a community study. J Am Acad Child Adolesc Psychiatry 2003;42:797-807.
6. McShane G, Walter G, Rey JM. Characteristics of adolescents with school refusal. Aust New Zeal J Psychiatry 2001;35:822-6.
7. Kearney CA, Albano AM. The functional profiles of school refusal behavior: diagnostic aspects. Behav Modif 2004;28:147-61.
8. Bernstein GA, Massie ED, Thuras PD, Perwien AR, Borchardt CM, Crosby RD. Somatic symptoms in anxious-depressed school refusers. J Am Acad Child Adolesc Psychiatry 1997;36:661-8.
9. Gilliland FD, Berhane K, Islam T, et al. Environmental tobacco smoke and absenteeism related to respiratory illness in school children. Am J Epidemiology 2003;157:861-9.
10. Glaab LA, Brown R, Daneman D. School attendance in children with type I diabetes. Diabetic Med 2005;22:421-6.
11. Levy RL, Whitehead WE, Walker LS, et al. Increased somatic complaints and health-care utilization in children: effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterology 2004;99:2442-51.
12. Buitelaar JK, van Andel H, Duyx JHM, van Strien DC. Depressive and anxiety disorders in adolescence: a follow-up study of adolescents with school refusal. Acta Paedopsychiatrica 1994;56:249-53.
13. Flakierska-Praquin N, Lindstrom M, Gillberg C. School phobia with separation anxiety disorder: a comparative 20- to 29-year follow-up study of 35 school refusers. Comp Psychiatry 1997;38:17-22.
14. Hibbett A, Fogelman K. Future lives of truants: family formation and health-related behaviour. Brit J Educ Psychology 1990;60:171-9.
15. Hibbett A, Fogelman K, Manor O. Occupational outcomes of truancy. Brit J Educ Psychology 1990;60:23-36.
16. Kearney CA. Bridging the gap among professionals who address youth with school absenteeism: overview and suggestions for consensus. Prof Psychol Res Prac 2003;34:57-65.
17. King NJ, Heyne D, Tonge B, Gullone E, Ollendick TH. School refusal: categorical diagnoses, functional analysis and treatment planning. Clin Psychol Psychother 2001;8:352-60.
18. Kearney CA. Identifying the function of school refusal behavior: a revision of the School Refusal Assessment Scale. J Psychopathol Behav Assess 2002;24:235-45.
19. Kearney CA. Confirmatory factor analysis of the School Refusal Assessment Scale-Revised: child and parent versions. J Psychopathol Behav Assess 2006; in press.
20. Bernstein GA, Borchardt CM, Perwein AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Chil Adol Psychiatry 2000;39:276-83.
21. Kearney CA, Silverman WK. A critical review of pharmacotherapy for youth with anxiety disorders: things are not as they seem. J Anxiety Disord 1998;12:83-102.
22. Layne AE, Bernstein GA, Egan EA, Kushner MG. Predictors of treatment response in anxious-depressed adolescents with school refusal. J Am Acad Chil Adol Psychiatry 2003;42:319-26.
23. Compton SN, Grant PJ, Chrisman AK, et al. Sertraline in children and adolescents with social anxiety disorder: an open trial. J Am Acad Chil Adol Psychiatry 2001;40:564-71.
24. Whittington CJ, Kendall T, Fonagy T, et al. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004;363:1341-5.
25. Kearney CA, Silverman WK. Functionally-based prescriptive and nonprescriptive treatment for children and adolescents with school refusal behavior. Behav Ther 1999;30:673-95.
26. King NJ, Tonge BJ, Heyne D, et al. Cognitive-behavioral treatment of school-refusing children: A controlled evaluation. J Am Acad Chil Adol Psychiatry 1998;37:395-403.
27. Last CG, Hansen C, Franco N. Cognitive-behavioral treatment of school phobia. J Am Acad Chil Adol Psychiatry 1998;37:404-11.
28. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-20.
29. Kearney CA, Roblek TL. Parent training in the treatment of school refusal behavior. In: Briesmeister JM, Schaefer CE, eds. Handbook of parent training: parents as co-therapists for children’s behavior problems, 2nd ed. New York: Wiley; 1998:225-56.
30. Kearney CA, Albano AM. When children refuse school: A cognitive-behavioral therapy approach/Therapist’s guide. San Antonio: Psychological Corporation, 2000.
31. Kearney CA, Bates M. Addressing school refusal behavior: Suggestions for frontline professionals. Children & Schools 2005;27:207-16.
32. Astor RA, Benbenishty R, Zeira A, Vinokur A. School climate, observed risky behaviors, and victimization as predictors of high school students’ fear and judgments of school violence as a problem. Health Educ Behav 2002;29:716-36.
33. Glew GM, Fan M-Y, Katon W, Rivara FP, Kernic MA. Bullying, psychosocial adjustment, and academic performance in elementary school. Arch Pediatr Adolesc Med 2005;159:1026-31.
34. Swahn MH, Bossarte RM. The associations between victimization, feeling unsafe, and asthma episodes among US high-school students. Am J Public Health 2006;96:802-4.
1. Kearney CA, Silverman WK. The evolution and reconciliation of taxonomic strategies for school refusal behavior. Clin Psychol: Sci Prac 1996;3:339-54.
2. Kearney CA. School refusal behavior in youth: a functional approach to assessment and treatment. Washington, DC: American Psychological Association; 2001.
3. Hansen C, Sanders SL, Massaro S, Last CG. Predictors of severity of absenteeism in children with anxiety-based school refusal. J Clin Child Psychol 1998;27:246-54.
4. Franklin CG, Soto I. Keeping Hispanic youths in school. Children & Schools 2002;24:139-43.
5. Egger HL, Costello EJ, Angold A. School refusal and psychiatric disorders: a community study. J Am Acad Child Adolesc Psychiatry 2003;42:797-807.
6. McShane G, Walter G, Rey JM. Characteristics of adolescents with school refusal. Aust New Zeal J Psychiatry 2001;35:822-6.
7. Kearney CA, Albano AM. The functional profiles of school refusal behavior: diagnostic aspects. Behav Modif 2004;28:147-61.
8. Bernstein GA, Massie ED, Thuras PD, Perwien AR, Borchardt CM, Crosby RD. Somatic symptoms in anxious-depressed school refusers. J Am Acad Child Adolesc Psychiatry 1997;36:661-8.
9. Gilliland FD, Berhane K, Islam T, et al. Environmental tobacco smoke and absenteeism related to respiratory illness in school children. Am J Epidemiology 2003;157:861-9.
10. Glaab LA, Brown R, Daneman D. School attendance in children with type I diabetes. Diabetic Med 2005;22:421-6.
11. Levy RL, Whitehead WE, Walker LS, et al. Increased somatic complaints and health-care utilization in children: effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterology 2004;99:2442-51.
12. Buitelaar JK, van Andel H, Duyx JHM, van Strien DC. Depressive and anxiety disorders in adolescence: a follow-up study of adolescents with school refusal. Acta Paedopsychiatrica 1994;56:249-53.
13. Flakierska-Praquin N, Lindstrom M, Gillberg C. School phobia with separation anxiety disorder: a comparative 20- to 29-year follow-up study of 35 school refusers. Comp Psychiatry 1997;38:17-22.
14. Hibbett A, Fogelman K. Future lives of truants: family formation and health-related behaviour. Brit J Educ Psychology 1990;60:171-9.
15. Hibbett A, Fogelman K, Manor O. Occupational outcomes of truancy. Brit J Educ Psychology 1990;60:23-36.
16. Kearney CA. Bridging the gap among professionals who address youth with school absenteeism: overview and suggestions for consensus. Prof Psychol Res Prac 2003;34:57-65.
17. King NJ, Heyne D, Tonge B, Gullone E, Ollendick TH. School refusal: categorical diagnoses, functional analysis and treatment planning. Clin Psychol Psychother 2001;8:352-60.
18. Kearney CA. Identifying the function of school refusal behavior: a revision of the School Refusal Assessment Scale. J Psychopathol Behav Assess 2002;24:235-45.
19. Kearney CA. Confirmatory factor analysis of the School Refusal Assessment Scale-Revised: child and parent versions. J Psychopathol Behav Assess 2006; in press.
20. Bernstein GA, Borchardt CM, Perwein AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Chil Adol Psychiatry 2000;39:276-83.
21. Kearney CA, Silverman WK. A critical review of pharmacotherapy for youth with anxiety disorders: things are not as they seem. J Anxiety Disord 1998;12:83-102.
22. Layne AE, Bernstein GA, Egan EA, Kushner MG. Predictors of treatment response in anxious-depressed adolescents with school refusal. J Am Acad Chil Adol Psychiatry 2003;42:319-26.
23. Compton SN, Grant PJ, Chrisman AK, et al. Sertraline in children and adolescents with social anxiety disorder: an open trial. J Am Acad Chil Adol Psychiatry 2001;40:564-71.
24. Whittington CJ, Kendall T, Fonagy T, et al. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004;363:1341-5.
25. Kearney CA, Silverman WK. Functionally-based prescriptive and nonprescriptive treatment for children and adolescents with school refusal behavior. Behav Ther 1999;30:673-95.
26. King NJ, Tonge BJ, Heyne D, et al. Cognitive-behavioral treatment of school-refusing children: A controlled evaluation. J Am Acad Chil Adol Psychiatry 1998;37:395-403.
27. Last CG, Hansen C, Franco N. Cognitive-behavioral treatment of school phobia. J Am Acad Chil Adol Psychiatry 1998;37:404-11.
28. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-20.
29. Kearney CA, Roblek TL. Parent training in the treatment of school refusal behavior. In: Briesmeister JM, Schaefer CE, eds. Handbook of parent training: parents as co-therapists for children’s behavior problems, 2nd ed. New York: Wiley; 1998:225-56.
30. Kearney CA, Albano AM. When children refuse school: A cognitive-behavioral therapy approach/Therapist’s guide. San Antonio: Psychological Corporation, 2000.
31. Kearney CA, Bates M. Addressing school refusal behavior: Suggestions for frontline professionals. Children & Schools 2005;27:207-16.
32. Astor RA, Benbenishty R, Zeira A, Vinokur A. School climate, observed risky behaviors, and victimization as predictors of high school students’ fear and judgments of school violence as a problem. Health Educ Behav 2002;29:716-36.
33. Glew GM, Fan M-Y, Katon W, Rivara FP, Kernic MA. Bullying, psychosocial adjustment, and academic performance in elementary school. Arch Pediatr Adolesc Med 2005;159:1026-31.
34. Swahn MH, Bossarte RM. The associations between victimization, feeling unsafe, and asthma episodes among US high-school students. Am J Public Health 2006;96:802-4.
Antipsychotics for patients without psychosis?
Controlled clinical trial results can help you make two prescribing decisions:
- Is an antipsychotic the right choice for this patient?
- If yes, which agent?
Prescribing antipsychotics off-label can be worthwhile when a patient gets better, but even then two worries remain:
- Most uses of antipsychotics for nonpsychotic illness are not evidence-based.
- This practice may expose clinicians to liability if the patient gets worse.
Consider the use of second-generation antipsychotics (SGAs) to manage acute behaviors in patients with dementia. The FDA ordered a black box warning in 2005 that SGAs may increase mortality risk in older patients. In October, the Clinical Antipsychotic Trials of Intervention Effectiveness-Alzheimer’s Disease (CATIE-AD) reported that SGAs’ side effects offset their benefits when compared with placebo (see Will CATIE-AD change dementia treatment?).1
What do you do when FDA-approved drugs fail to help your patient with dementia, unipolar depression, anxiety disorders, or other nonpsychotic symptoms, and SGAs may be the next consideration? The answers lie in managing side effects and knowing which antipsychotic uses are supported by data from controlled clinical trials, which we review here.
- FGAs showed efficacy for nonpsychotic disorders
- SGAs are associated with a lower risk of EPS and tardive dyskinesia at therapeutic dosages, compared with FGAs
- Many patients fail to respond adequately to medications approved for their illnesses
- Evidence on SGAs’ efficacy in nonpsychotic disorders has grown substantially in the past 10 years.
EPS: extrapyramidal symptoms
FGA: first-generation antipsychotic
SGA: second-generation antipsychotic
Prescribing considerations
For a variety of reasons (Box 1), SGAs have rapidly assumed a major role in treating nonpsychotic disorders. Thirty-one percent of psychotropics are dispensed off-label,2 and Buckley3 reported in a 3-state survey that 70% of SGA prescriptions were written for indications other than schizophrenia.
Using antipsychotics for nonpsychotic symptoms is a longstanding clinical practice. In schizophrenia patients, antipsychotics have been shown to improve psychotic and nonpsychotic symptoms: agitation, violence, negative symptoms, social isolation, depression, suicidality, anxiety, insomnia, poor appetite, compulsions, cognition, smoking, alcohol and drug use, polydipsia, tardive dyskinesia, and tardive dystonia. Some clinicians may view these reports as evidence that antipsychotics might relieve these symptoms in patients with nonpsychotic disorders as well, but the issue is more complicated than that (Box 2).4
Caveats. SGAs do offer clinicians unique tools; no other class of psychotropics can claim efficacy in psychotic disorders, bipolar disorder, depression, and other disorders we describe in this review. On the other hand:
- Although some SGAs are approved for bipolar disorder and one was recently approved to treat irritability in autism (Table 1), most SGA uses in nonpsychotic disorders are off-label and supported by few—if any—large, randomized, controlled trials.
- Antipsychotics can cause the very symptoms they relieve, including depression, obsessive-compulsive disorder (OCD), anxiety, poorer cognition, agitation, mania, insomnia, and abnormal movements.
- Few controlled studies have compared SGAs to usual first-line treatments; most have evaluated SGAs as adjuncts to other psychotropics—such as serotonin reuptake inhibitors (SRIs)—for patients with treatment-resistant disorders.
- Published head-to-head studies have rarely compared the efficacy of various SGAs in treating nonpsychotic disorders.
- Long-term safety studies of SGAs for nonpsychotic indications have not been done.
Table 1
Bipolar and other nonpsychotic indications FDA-approved for SGAs
| SGA | Bipolar mania | Bipolar depression | Bipolar maintenance | Other |
|---|---|---|---|---|
| Aripiprazole | Acute mania or mixed episodes | Bipolar I disorder, most recent episode manic or mixed | ||
| Clozapine | Risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorders | |||
| Olanzapine | Acute mania or mixed episodes; monotherapy or with lithium or valproate for manic episodes | Bipolar disorder maintenance monotherapy | ||
| Olanzapine/fluoxetine combination | Bipolar depressive episodes | |||
| Quetiapine | Acute manic episodes; monotherapy or with lithium or valproate | Bipolar depressive episodes | ||
| Risperidone | Acute mania or mixed episodes; monotherapy or with lithium or valproate | Irritability in autism | ||
| Ziprasidone | Acute manic or mixed episodes | |||
| SGA: second-generation antipsychotic (oral forms) | ||||
Prescribing decisions. SGA’s potential adverse effects complicate clinical decision-making. First you must decide whether to use an SGA for your patient with a nonpsychotic disorder.
Second-generation antipsychotics (SGAs) show efficacy in so many psychotic and nonpsychotic disorders that a specific therapeutic action for each disorder is highly doubtful. One might ask, then: What do they improve, and how do they do it?
The complete answer is beyond current understanding, unfortunately. We do know, however, that SGAs have not shown efficacy for treating nonpsychotic disorders that first-generation antipsychotics (FGAs) did not show—except perhaps for maintenance treatment in bipolar disorder.
Calming action. The major clinical action of SGAs appears to be in calming patients, which also was the first therapeutic effect attributed to the FGA chlorpromazine. This calming effect would explain SGAs’ efficacy in treating agitation, aggressiveness, anxiety, and possibly mania. Other clinical effects specific to psychosis and possibly to depression are possible.
Receptor-blocking action. SGAs’ D2 and 5-HT2A receptor-blocking activity may explain much of the drugs’ therapeutic effect. However, if SGAs’ effect on nonpsychotic symptoms derives from their action on nondopaminergic receptors, then individual SGAs would vary widely in efficacy and pure dopaminergic agents such as amisulpride would be ineffective.
SGAs also bind at other receptor sites, and the clinical importance of this may vary from patient to patient, drug to drug, and dose to dose.4
Knowing, for example, that antipsychotics have been shown to increase mortality and cerebrovascular events in older patients might make you less likely to prescribe an SGA for a patient with dementia-related agitation. No other pharmacologic treatment has shown clear efficacy for these patients, however, so other factors are important to consider, including:
- patient history and clinical characteristics
- potential side effects
- individual therapeutic response to previous medications.
Table 2
SGA uses in nonpsychotic disorders supported by evidence
from published double-blind clinical trials*
| SGA | Unipolar depression | OCD | Anxiety disorders | Dementia | Developmental disorders | Borderline personality disorder |
|---|---|---|---|---|---|---|
| Aripiprazole | Yes | |||||
| Clozapine | ||||||
| Olanzapine | Yes | Yes | Yes | Yes | Yes | |
| Quetiapine | Yes | |||||
| Risperidone | Yes | Yes | Yes | Yes | ||
| Ziprasidone | ||||||
| * Not including studies of bipolar disorder | ||||||
| OCD: obsessive-compulsive disorder | ||||||
| SGA: second-generation antipsychotic | ||||||
Dementia
Most Alzheimer’s patients—75% to 90%—experience behavioral problems during this progressive dementia. Double-blind studies have found risperidone (mean dosage ~1 mg/d) and olanzapine (mean dosage 5 to 10 mg/d) effective in reducing agitation and aggression, even in nonpsychotic patients with Alzheimer’s disease or vascular dementia.5,6 Quetiapine, ≤100 mg/d, was not more effective than placebo in reducing agitation.7 One study comparing IM olanzapine with IM lorazepam and placebo in acute agitation found both active treatments more effective than placebo.8
CATIE-AD—sponsored by the National Institute of Mental Health—compared olanzapine, risperidone, and quetiapine with placebo in 421 outpatients with behavioral symptoms such as psychosis, agitation, or aggressiveness.1 No significant differences were seen in overall effectiveness (measured as discontinuation for any cause9), although patients receiving olanzapine (mean dosage 5.5 mg/d) or risperidone (mean dosage 1 mg/d) had lower discontinuation rates for lack of efficacy than those receiving placebo.
Unfortunately, the results of the first phase of CATIE-AD provide no clear guidance on the therapeutic strategy to use in dementia. Its findings do suggest two secondary conclusions, however, about using SGAs for patients with dementia:
- Because quetiapine, mean dosage 56.5 mg/d, was not more effective than placebo on any measures, consider higher dosages when using this agent.
- Close attention to preventing and treating SGAs’ side effects is the key to effectively treating agitation and psychosis in dementia.
Recommendation. Nonpharmacologic interventions are an important part of treating behavioral problems in patients with dementia.13,14 Antipsychotics—particularly SGAs—have shown efficacy for psychosis and agitation in these patients and remain the first therapeutic option. The CATIE-AD investigators recommend that clinicians evaluate potential risks and benefits of pharmacotherapy and discuss these with patients and caregivers.1 Also:
- Consider which SGAs have the lowest risk of causing side effects for an individual patient.
- Start with low dosages and increase as needed, based on efficacy and tolerability.
Bipolar disorder
Acute mania. Five SGAs—aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone—are FDA-approved for acute mania (Table 1). Large double-blind studies supporting this indication show that SGAs have efficacy in treating mania as monotherapy and in combination with lithium or divalproex.15 These clinical trials included patients who were not psychotic at baseline.
Antipsychotic dosages in these studies were within the ranges used in schizophrenia treatment studies. Combining an SGA with lithium or divalproex generally yields greater reductions in mania rating scale scores, higher response rates, and higher remission rates than using lithium or divalproex alone. No published study has compared SGAs with each other in mania, but differences in efficacy among these compounds are likely to be small.16
Bipolar depression. SGAs’ efficacy in bipolar depression has been evaluated in double-blind studies, and quetiapine and the olanzapine/fluoxetine combination are FDA-approved for this indication.
Olanzapine plus fluoxetine was more effective in improving depressive symptoms than olanzapine alone in a double-blind study of 833 adults with depressive symptoms of bipolar I disorder, as measured by Montgomery-Åsburg Depression Rating Scale (MADRS) scores. Olanzapine alone was more effective than placebo. Mean dosages were olanzapine, 7.4 mg/d, and fluoxetine, 39.3 mg/d, in combination therapy and olanzapine, 9.7 mg/d, as monotherapy.
MADRS scores indicated that combination therapy—but not olanzapine alone—improved core depressive symptoms such as sadness, lassitude, inability to feel, and pessimistic thoughts.17
Quetiapine. A double-blind, placebo-controlled trial (BOLDER I) evaluated quetiapine in 542 outpatients experiencing a major depressive episode associated with bipolar I or II disorder. After 8 weeks, quetiapine at 300 or 600 mg/d was more effective than placebo in reducing depressive symptoms, as measured by MADRS score changes.
Response rates were 58% with quetiapine and 36% with placebo; remission rates were 53% with quetiapine and 28% with placebo. Most symptoms, including core depression items, improved significantly with quetiapine, compared with placebo.18 Results of a second double-blind study (BOLDER II) have been presented at conferences but have not been fully published.
Risperidone. A smaller double-blind study compared risperidone plus placebo, paroxetine plus placebo, and risperidone plus paroxetine in 30 patients in the depressed phase of bipolar I or II disorder. Patients continued taking mood stabilizers during the study. After 12 weeks, depressive symptoms improved significantly in all three groups, with no significant differences.19
Maintenance therapy. Olanzapine and aripiprazole are FDA-approved for maintenance therapy in bipolar disorder (Table 1).
Unipolar depression
FGAs have shown efficacy in depression in multiple controlled studies.20 SGAs have been evaluated mostly as add-on therapies in antidepressant-resistant depression.
Olanzapine. Shelton et al21 compared olanzapine monotherapy, fluoxetine monotherapy, and combined treatment in 34 nonpsychotic, treatment-resistant depressed subjects. Olanzapine plus fluoxetine was more effective than either agent alone. A subsequent double-blind study, however, showed similar efficacy after 8 weeks among the three treatments and nortriptyline monotherapy. Patients in the double-blind trial appeared to respond more rapidly to combined treatment than to the monotherapies.22
Risperidone. A multiphase study of the efficacy of risperidone augmentation in treatment-resistant major depression began when 489 outpatients (2% with psychotic symptoms) received open-label citalopram, 20 to 60 mg/d. After 4 to 6 weeks, 386 nonresponders entered the augmentation phase with open-label risperidone, 0.25 to 2 mg/d. After 4 to 6 weeks of combination therapy, 241 (63%) patients whose symptoms resolved entered a double-blind discontinuation phase, in which they were randomly assigned to augmentation with risperidone or placebo, while on citalopram.
Median time to relapse during the double-blind phase was 102 days with risperidone augmentation and 85 days with placebo—not a statistically significant difference. Relapse rates after 24 weeks were 53.3% and 54.6%, respectively.23 This study showed that the improvement observed after adding risperidone was not sustained over time.
Quetiapine. In a prospective single-blind study, paroxetine augmented with quetiapine, 200 mg/d, was compared to paroxetine alone in major depression with anxiety.24 Combination therapy was more effective in improving anxiety and depression symptoms.
Others. Open-label, add-on studies indicate that aripiprazole and ziprasidone can improve treatment-resistant depression.25-27
Anxiety disorders
OCD. SGAs also have been investigated as augmentation therapy for patients with OCD resistant to SRIs. A single-blind study of 27 patients found adjunctive quetiapine more effective than placebo in improving OCD symptoms.28 SGAs were more effective than placebo as augmentation therapy to SRIs for treatment-refractory OCD in double-blind, placebo-controlled studies using mean dosages of:
In other trials:
- A small double-blind study of patients with social anxiety disorder found olanzapine monotherapy more effective than placebo.34
- Low-dose risperidone (mean dosage 1.1 mg/d) improved core symptoms of generalized anxiety disorder in a 5-week, double-blind, placebo-controlled trial.35
- Some authors have reported clinical improvement of panic disorder with olanzapine augmentation.36
Developmental disorders
Antipsychotics represent one-third of all filled psychotropic prescriptions for individuals with pervasive developmental disorders (PDD).37 Haloperidol and thioridazine are the only two FDA-approved FGAs for severe behavioral problems in PDD (and for hyperactivity with conduct disorders). Recently, risperidone received FDA approval for the treatment of irritability associated with autistic disorder in children.
Risperidone—the most-studied SGA in the PDD population—has shown efficacy in autism and in PDD not otherwise specified. Risperidone at dosages >3 mg/d improved repetitive behavior and aggression in adult patients.38
In children with autism, risperidone can improve tantrums, aggression, and self-injury. In a study of risperidone’s effect on autism’s core symptoms, the authors reported improvements in repetitive and stereotyped behavior but not in social relatedness or verbal communication.39
Double-blind studies have shown positive effects on aggression and behavioral disturbances in children with conduct disorder, oppositional defiant disorder, and other disruptive disorders, developmentally delayed adolescents, and mentally retarded subjects of various ages.40-42 Children and adolescents appear to be more sensitive than adults to risperidone’s side effects such as weight gain, EPS, and pancreatitis.
Personality disorders
Antipsychotics have been recommended for paranoid ideas and psychotic-like symptoms in borderline personality disorder and in paranoid personality disorder.43
Olanzapine. A 24-week, double-blind study found low-dose olanzapine (mean dosage 5.3 mg/d) more effective than placebo for anxiety, interpersonal sensitivity, paranoia, and anger/hostility in women with borderline personality disorder.44
In another double-blind study, 12 weeks of olanzapine therapy (mean 6.9 mg/d) was more effective than placebo for inappropriate anger in borderline personality disorder, as measured by a modified Clinical Global Impression scale.45
Others. Anger and hostility improved more with aripiprazole, 15 mg/d, than with placebo in an 8-week double-blind study of patients with borderline personality disorder.46 Quetiapine, risperidone, ziprasidone, and clozapine have shown efficacy in open-label studies and case reports.
Related resources
- Boos J. Off label use–label off use? Ann Oncol 2003;14(1):1-5.
- Blum RS. Legal considerations in off-label medication prescribing. Arch Intern Med 2002;162(15):1777-9.
- Jeste DV, Dolder CR. Treatment of non-schizophrenic disorders: focus on atypical antipsychotics. J Psychiatr Res 2004;38(1):73-103.
- Food and Drug Administration. Searchable catalog of FDA-approved drug products. www.accessdata.fda.gov/scripts/cder/drugsatfda.
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Nortriptyline • Pamelor, Aventyl
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Trémeau receives grant/research support from Eli Lilly and Company.
Dr. Citrome receives grant/research support from AstraZeneca, Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Janssen Research Foundation, and Pfizer; is a consultant to Bristol-Myers Squibb, Eli Lilly and Company, Pfizer, Jazz Pharmaceuticals, and GlaxoSmithKline; and is a speaker for Abbott Laboratories, AstraZeneca, Eli Lilly and Company, and Pfizer.
1. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525-38.
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med 2006;166:1021-6.
3. Buckley PF. New antipsychotic agents: emerging clinical profiles. J Clin Psychiatry 1999;60(suppl 1):12-7.
4. Shayegan DK, Stahl SM. Atypical antipsychotics: matching receptor profile to individual patient’s clinical profile. CNS Spectr 2004;9(10 suppl 11):6-14.
5. Jeste DV, Dolder CR, Nayak GV, Salzman C. Atypical antipsychotics in elderly patients with dementia or schizophrenia: review of recent literature. Harv Rev Psychiatry 2005;13(6):340-51.
6. Carson S, McDonagh MS, Peterson K. A systematic review of the efficacy and safety of atypical antipsychotics in patients with psychological and behavioral symptoms of dementia. J Am Geriatr Soc 2006;54(2):354-61.
7. Ballard C, Margallo-Lana M, Juszczak E, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. BMJ 2005;330(7496):874.-
8. Meehan KM, Wang H, David SR, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: a double-blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology 2002;26(4):494-504.
9. Schneider LS, Tariot PN, Lyketsos CG, et al. National Institute of Mental Health Antipsychotic Trials of Intervention Effectiveness (CATIE). Alzheimer disease trial methodology. Am J Geriatr Psychiatry 2001;9:346-60.
10. Kennedy J, Deberdt W, Siegal A, et al. Olanzapine does not enhance cognition in non-agitated and non-psychotic patients with mild to moderate Alzheimer’s dementia. Int J Geriatr Psychiatry 2005;20(11):1020-7.
11. U.S. Food and Drug Administration. Center for Drug Evaluation and Research. FDA public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. April 11, 2005. Available at: http://www.fda.gov/Cder/drug/advisory/antipsychotics.htm. Accessed October 17, 2006.
12. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353(22):2335-41.
13. Rabins P, Bland W, Bright-Long L, et al. from the Work Group on Alzheimer’s disease and related dementias. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias of late life. American Psychiatric Association Practice Guideline 1997. Available at http://www.psych.org/psych_pract/ treatg/pg/prac_guide.cfm. Accessed November 15, 2006.
14. Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer’s disease: a random control trial. JAMA 1996;276:1725-31.
15. Citrome L, Goldberg JF, Stahl SM. Toward convergence in the medication treatment of bipolar disorder and schizophrenia. Harv Rev Psychiatry 2005;13(1):28-42.
16. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry 2006;67(4):509-16.
17. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60(11):1079-88.
18. Calabrese JR, Keck PE, Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 2005;162(7):1351-60.
19. Shelton RC, Stahl SM. Risperidone and paroxetine given singly and in combination for bipolar depression. J Clin Psychiatry 2004;65(12):1715-9.
20. Robertson MM, Trimble MR. Major tranquillisers used as antidepressants. A review. J Affect Disord 1982;4(3):173-93.
21. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158(1):131-4.
22. Shelton RC, Williamson DJ, Corya SA, et al. Olanzapine/fluoxetine combination for treatment-resistant depression: a controlled study of SSRI and nortriptyline resistance. J Clin Psychiatry 2005;66(10):1289-97.
23. Rapaport MH, Gharabawi GM, Canuso CM, et al. Effects of risperidone augmentation in patients with treatment-resistant depression: results of open-label treatment followed by double-blind continuation. Neuropsychopharmacology 2006;31(11):2505-13.
24. Yargic LI, Corapcioglu A, Kocabasoglu N, et al. A prospective randomized single-blind, multicenter trial comparing the efficacy and safety of paroxetine with and without quetiapine therapy in depression associated with anxiety. Int J Psychiatry Clin Pract 2004;8:205-11.
25. Papakostas GI, Petersen TJ, Kinrys G, et al. Aripiprazole augmentation of selective serotonin reuptake inhibitors for treatment-resistant major depressive disorder. J Clin Psychiatry 2005;66(10):1326-30.
26. Papakostas GI, Petersen TJ, Nierenberg AA, et al. Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J Clin Psychiatry 2004;65(2):217-21.
27. Simon JS, Nemeroff CB. Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder. J Clin Psychiatry 2005;66(10):1216-20.
28. Atmaca M, Kuloglu M, Tezcan E, Gecici O. Quetiapine augmentation in patients with treatment resistant obsessive-compulsive disorder: a single-blind, placebo-controlled study. Int Clin Psychopharmacol 2002;17(3):115-9.
29. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 2000;57(8):794-801.
30. Bystritsky A, Ackerman DL, Rosen RM, et al. Augmentation of serotonin reuptake inhibitors in refractory obsessive-compulsive disorder using adjunctive olanzapine: a placebo-controlled trial. J Clin Psychiatry 2004;65(4):565-8.
31. Denys D, de Geus F, van Megen HJ, Westenberg HG. A double-blind, randomized, placebo-controlled trial of quetiapine addition in patients with obsessive-compulsive disorder refractory to serotonin reuptake inhibitors. J Clin Psychiatry 2004;65(8):1040-8.
32. Bartzokis G, Lu PH, Turner J, et al. Adjunctive risperidone in the treatment of chronic combat-related posttraumatic stress disorder. Biol Psychiatry 2005;57(5):474-9.
33. Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry 2002;159(10):1777-9.
34. Barnett SD, Kramer ML, Casat CD, et al. Efficacy of olanzapine in social anxiety disorder: a pilot study. J Psychopharmacol 2002;16(4):365-8.
35. Brawman-Mintzer O, Knapp RG, Nietert PJ. Adjunctive risperidone in generalized anxiety disorder: a double-blind, placebo-controlled study. J Clin Psychiatry 2005;66(10):1321-5.
36. Khaldi S, Kornreich C, Dan B, Pelc I. Usefulness of olanzapine in refractory panic attacks. J Clin Psychopharmacol 2003;23(1):100-1.
37. Lott IT, McGregor M, Engelman L, et al. Longitudinal prescribing patterns for psychoactive medications in community-based individuals with developmental disabilities: utilization of pharmacy records. J Intellect Disabil Res 2004;48(Pt 6):563-71.
38. McDougle CJ, Holmes JP, Carlson DC, et al. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry 1998;55(7):633-41.
39. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005;162(6):1142-8.
40. Vanden Borre R, Vermote R, Buttiens M, et al. Risperidone as addon therapy in behavioural disturbances in mental retardation: a double-blind placebo-controlled cross-over study. Acta Psychiatr Scand 1993;87(3):167-71.
41. Buitelaar JK, van der Gaag RJ, Cohen-Kettenis P, Melman CT. A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. J Clin Psychiatry 2001;62(4):239-48.
42. Snyder R, Turgay A, Aman M, et al; Risperidone Conduct Study Group. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. J Am Acad Child Adolesc Psychiatry 2002;41(9):1026-36.
43. Oldham JM, Gabbard GO, Goin MK, et al, from the workgroup on borderline personality disorder. Practice guideline for the treatment of patients with borderline personality disorder. American Psychiatric Association Practice Guideline 2001. Available at http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm. Accessed November 15, 2006.
44. Zanarini MC, Frankenburg FR. Olanzapine treatment of female borderline personality disorder patients: a double-blind, placebo-controlled pilot study. J Clin Psychiatry 2001;62(11):849-54.
45. Bogenschutz MP, George Nurnberg H. Olanzapine versus placebo in the treatment of borderline personality disorder. J Clin Psychiatry 2004;65(1):104-9.
46. Nickel MK, Muehlbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry 2006;163(5):833-8.
Controlled clinical trial results can help you make two prescribing decisions:
- Is an antipsychotic the right choice for this patient?
- If yes, which agent?
Prescribing antipsychotics off-label can be worthwhile when a patient gets better, but even then two worries remain:
- Most uses of antipsychotics for nonpsychotic illness are not evidence-based.
- This practice may expose clinicians to liability if the patient gets worse.
Consider the use of second-generation antipsychotics (SGAs) to manage acute behaviors in patients with dementia. The FDA ordered a black box warning in 2005 that SGAs may increase mortality risk in older patients. In October, the Clinical Antipsychotic Trials of Intervention Effectiveness-Alzheimer’s Disease (CATIE-AD) reported that SGAs’ side effects offset their benefits when compared with placebo (see Will CATIE-AD change dementia treatment?).1
What do you do when FDA-approved drugs fail to help your patient with dementia, unipolar depression, anxiety disorders, or other nonpsychotic symptoms, and SGAs may be the next consideration? The answers lie in managing side effects and knowing which antipsychotic uses are supported by data from controlled clinical trials, which we review here.
- FGAs showed efficacy for nonpsychotic disorders
- SGAs are associated with a lower risk of EPS and tardive dyskinesia at therapeutic dosages, compared with FGAs
- Many patients fail to respond adequately to medications approved for their illnesses
- Evidence on SGAs’ efficacy in nonpsychotic disorders has grown substantially in the past 10 years.
EPS: extrapyramidal symptoms
FGA: first-generation antipsychotic
SGA: second-generation antipsychotic
Prescribing considerations
For a variety of reasons (Box 1), SGAs have rapidly assumed a major role in treating nonpsychotic disorders. Thirty-one percent of psychotropics are dispensed off-label,2 and Buckley3 reported in a 3-state survey that 70% of SGA prescriptions were written for indications other than schizophrenia.
Using antipsychotics for nonpsychotic symptoms is a longstanding clinical practice. In schizophrenia patients, antipsychotics have been shown to improve psychotic and nonpsychotic symptoms: agitation, violence, negative symptoms, social isolation, depression, suicidality, anxiety, insomnia, poor appetite, compulsions, cognition, smoking, alcohol and drug use, polydipsia, tardive dyskinesia, and tardive dystonia. Some clinicians may view these reports as evidence that antipsychotics might relieve these symptoms in patients with nonpsychotic disorders as well, but the issue is more complicated than that (Box 2).4
Caveats. SGAs do offer clinicians unique tools; no other class of psychotropics can claim efficacy in psychotic disorders, bipolar disorder, depression, and other disorders we describe in this review. On the other hand:
- Although some SGAs are approved for bipolar disorder and one was recently approved to treat irritability in autism (Table 1), most SGA uses in nonpsychotic disorders are off-label and supported by few—if any—large, randomized, controlled trials.
- Antipsychotics can cause the very symptoms they relieve, including depression, obsessive-compulsive disorder (OCD), anxiety, poorer cognition, agitation, mania, insomnia, and abnormal movements.
- Few controlled studies have compared SGAs to usual first-line treatments; most have evaluated SGAs as adjuncts to other psychotropics—such as serotonin reuptake inhibitors (SRIs)—for patients with treatment-resistant disorders.
- Published head-to-head studies have rarely compared the efficacy of various SGAs in treating nonpsychotic disorders.
- Long-term safety studies of SGAs for nonpsychotic indications have not been done.
Table 1
Bipolar and other nonpsychotic indications FDA-approved for SGAs
| SGA | Bipolar mania | Bipolar depression | Bipolar maintenance | Other |
|---|---|---|---|---|
| Aripiprazole | Acute mania or mixed episodes | Bipolar I disorder, most recent episode manic or mixed | ||
| Clozapine | Risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorders | |||
| Olanzapine | Acute mania or mixed episodes; monotherapy or with lithium or valproate for manic episodes | Bipolar disorder maintenance monotherapy | ||
| Olanzapine/fluoxetine combination | Bipolar depressive episodes | |||
| Quetiapine | Acute manic episodes; monotherapy or with lithium or valproate | Bipolar depressive episodes | ||
| Risperidone | Acute mania or mixed episodes; monotherapy or with lithium or valproate | Irritability in autism | ||
| Ziprasidone | Acute manic or mixed episodes | |||
| SGA: second-generation antipsychotic (oral forms) | ||||
Prescribing decisions. SGA’s potential adverse effects complicate clinical decision-making. First you must decide whether to use an SGA for your patient with a nonpsychotic disorder.
Second-generation antipsychotics (SGAs) show efficacy in so many psychotic and nonpsychotic disorders that a specific therapeutic action for each disorder is highly doubtful. One might ask, then: What do they improve, and how do they do it?
The complete answer is beyond current understanding, unfortunately. We do know, however, that SGAs have not shown efficacy for treating nonpsychotic disorders that first-generation antipsychotics (FGAs) did not show—except perhaps for maintenance treatment in bipolar disorder.
Calming action. The major clinical action of SGAs appears to be in calming patients, which also was the first therapeutic effect attributed to the FGA chlorpromazine. This calming effect would explain SGAs’ efficacy in treating agitation, aggressiveness, anxiety, and possibly mania. Other clinical effects specific to psychosis and possibly to depression are possible.
Receptor-blocking action. SGAs’ D2 and 5-HT2A receptor-blocking activity may explain much of the drugs’ therapeutic effect. However, if SGAs’ effect on nonpsychotic symptoms derives from their action on nondopaminergic receptors, then individual SGAs would vary widely in efficacy and pure dopaminergic agents such as amisulpride would be ineffective.
SGAs also bind at other receptor sites, and the clinical importance of this may vary from patient to patient, drug to drug, and dose to dose.4
Knowing, for example, that antipsychotics have been shown to increase mortality and cerebrovascular events in older patients might make you less likely to prescribe an SGA for a patient with dementia-related agitation. No other pharmacologic treatment has shown clear efficacy for these patients, however, so other factors are important to consider, including:
- patient history and clinical characteristics
- potential side effects
- individual therapeutic response to previous medications.
Table 2
SGA uses in nonpsychotic disorders supported by evidence
from published double-blind clinical trials*
| SGA | Unipolar depression | OCD | Anxiety disorders | Dementia | Developmental disorders | Borderline personality disorder |
|---|---|---|---|---|---|---|
| Aripiprazole | Yes | |||||
| Clozapine | ||||||
| Olanzapine | Yes | Yes | Yes | Yes | Yes | |
| Quetiapine | Yes | |||||
| Risperidone | Yes | Yes | Yes | Yes | ||
| Ziprasidone | ||||||
| * Not including studies of bipolar disorder | ||||||
| OCD: obsessive-compulsive disorder | ||||||
| SGA: second-generation antipsychotic | ||||||
Dementia
Most Alzheimer’s patients—75% to 90%—experience behavioral problems during this progressive dementia. Double-blind studies have found risperidone (mean dosage ~1 mg/d) and olanzapine (mean dosage 5 to 10 mg/d) effective in reducing agitation and aggression, even in nonpsychotic patients with Alzheimer’s disease or vascular dementia.5,6 Quetiapine, ≤100 mg/d, was not more effective than placebo in reducing agitation.7 One study comparing IM olanzapine with IM lorazepam and placebo in acute agitation found both active treatments more effective than placebo.8
CATIE-AD—sponsored by the National Institute of Mental Health—compared olanzapine, risperidone, and quetiapine with placebo in 421 outpatients with behavioral symptoms such as psychosis, agitation, or aggressiveness.1 No significant differences were seen in overall effectiveness (measured as discontinuation for any cause9), although patients receiving olanzapine (mean dosage 5.5 mg/d) or risperidone (mean dosage 1 mg/d) had lower discontinuation rates for lack of efficacy than those receiving placebo.
Unfortunately, the results of the first phase of CATIE-AD provide no clear guidance on the therapeutic strategy to use in dementia. Its findings do suggest two secondary conclusions, however, about using SGAs for patients with dementia:
- Because quetiapine, mean dosage 56.5 mg/d, was not more effective than placebo on any measures, consider higher dosages when using this agent.
- Close attention to preventing and treating SGAs’ side effects is the key to effectively treating agitation and psychosis in dementia.
Recommendation. Nonpharmacologic interventions are an important part of treating behavioral problems in patients with dementia.13,14 Antipsychotics—particularly SGAs—have shown efficacy for psychosis and agitation in these patients and remain the first therapeutic option. The CATIE-AD investigators recommend that clinicians evaluate potential risks and benefits of pharmacotherapy and discuss these with patients and caregivers.1 Also:
- Consider which SGAs have the lowest risk of causing side effects for an individual patient.
- Start with low dosages and increase as needed, based on efficacy and tolerability.
Bipolar disorder
Acute mania. Five SGAs—aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone—are FDA-approved for acute mania (Table 1). Large double-blind studies supporting this indication show that SGAs have efficacy in treating mania as monotherapy and in combination with lithium or divalproex.15 These clinical trials included patients who were not psychotic at baseline.
Antipsychotic dosages in these studies were within the ranges used in schizophrenia treatment studies. Combining an SGA with lithium or divalproex generally yields greater reductions in mania rating scale scores, higher response rates, and higher remission rates than using lithium or divalproex alone. No published study has compared SGAs with each other in mania, but differences in efficacy among these compounds are likely to be small.16
Bipolar depression. SGAs’ efficacy in bipolar depression has been evaluated in double-blind studies, and quetiapine and the olanzapine/fluoxetine combination are FDA-approved for this indication.
Olanzapine plus fluoxetine was more effective in improving depressive symptoms than olanzapine alone in a double-blind study of 833 adults with depressive symptoms of bipolar I disorder, as measured by Montgomery-Åsburg Depression Rating Scale (MADRS) scores. Olanzapine alone was more effective than placebo. Mean dosages were olanzapine, 7.4 mg/d, and fluoxetine, 39.3 mg/d, in combination therapy and olanzapine, 9.7 mg/d, as monotherapy.
MADRS scores indicated that combination therapy—but not olanzapine alone—improved core depressive symptoms such as sadness, lassitude, inability to feel, and pessimistic thoughts.17
Quetiapine. A double-blind, placebo-controlled trial (BOLDER I) evaluated quetiapine in 542 outpatients experiencing a major depressive episode associated with bipolar I or II disorder. After 8 weeks, quetiapine at 300 or 600 mg/d was more effective than placebo in reducing depressive symptoms, as measured by MADRS score changes.
Response rates were 58% with quetiapine and 36% with placebo; remission rates were 53% with quetiapine and 28% with placebo. Most symptoms, including core depression items, improved significantly with quetiapine, compared with placebo.18 Results of a second double-blind study (BOLDER II) have been presented at conferences but have not been fully published.
Risperidone. A smaller double-blind study compared risperidone plus placebo, paroxetine plus placebo, and risperidone plus paroxetine in 30 patients in the depressed phase of bipolar I or II disorder. Patients continued taking mood stabilizers during the study. After 12 weeks, depressive symptoms improved significantly in all three groups, with no significant differences.19
Maintenance therapy. Olanzapine and aripiprazole are FDA-approved for maintenance therapy in bipolar disorder (Table 1).
Unipolar depression
FGAs have shown efficacy in depression in multiple controlled studies.20 SGAs have been evaluated mostly as add-on therapies in antidepressant-resistant depression.
Olanzapine. Shelton et al21 compared olanzapine monotherapy, fluoxetine monotherapy, and combined treatment in 34 nonpsychotic, treatment-resistant depressed subjects. Olanzapine plus fluoxetine was more effective than either agent alone. A subsequent double-blind study, however, showed similar efficacy after 8 weeks among the three treatments and nortriptyline monotherapy. Patients in the double-blind trial appeared to respond more rapidly to combined treatment than to the monotherapies.22
Risperidone. A multiphase study of the efficacy of risperidone augmentation in treatment-resistant major depression began when 489 outpatients (2% with psychotic symptoms) received open-label citalopram, 20 to 60 mg/d. After 4 to 6 weeks, 386 nonresponders entered the augmentation phase with open-label risperidone, 0.25 to 2 mg/d. After 4 to 6 weeks of combination therapy, 241 (63%) patients whose symptoms resolved entered a double-blind discontinuation phase, in which they were randomly assigned to augmentation with risperidone or placebo, while on citalopram.
Median time to relapse during the double-blind phase was 102 days with risperidone augmentation and 85 days with placebo—not a statistically significant difference. Relapse rates after 24 weeks were 53.3% and 54.6%, respectively.23 This study showed that the improvement observed after adding risperidone was not sustained over time.
Quetiapine. In a prospective single-blind study, paroxetine augmented with quetiapine, 200 mg/d, was compared to paroxetine alone in major depression with anxiety.24 Combination therapy was more effective in improving anxiety and depression symptoms.
Others. Open-label, add-on studies indicate that aripiprazole and ziprasidone can improve treatment-resistant depression.25-27
Anxiety disorders
OCD. SGAs also have been investigated as augmentation therapy for patients with OCD resistant to SRIs. A single-blind study of 27 patients found adjunctive quetiapine more effective than placebo in improving OCD symptoms.28 SGAs were more effective than placebo as augmentation therapy to SRIs for treatment-refractory OCD in double-blind, placebo-controlled studies using mean dosages of:
In other trials:
- A small double-blind study of patients with social anxiety disorder found olanzapine monotherapy more effective than placebo.34
- Low-dose risperidone (mean dosage 1.1 mg/d) improved core symptoms of generalized anxiety disorder in a 5-week, double-blind, placebo-controlled trial.35
- Some authors have reported clinical improvement of panic disorder with olanzapine augmentation.36
Developmental disorders
Antipsychotics represent one-third of all filled psychotropic prescriptions for individuals with pervasive developmental disorders (PDD).37 Haloperidol and thioridazine are the only two FDA-approved FGAs for severe behavioral problems in PDD (and for hyperactivity with conduct disorders). Recently, risperidone received FDA approval for the treatment of irritability associated with autistic disorder in children.
Risperidone—the most-studied SGA in the PDD population—has shown efficacy in autism and in PDD not otherwise specified. Risperidone at dosages >3 mg/d improved repetitive behavior and aggression in adult patients.38
In children with autism, risperidone can improve tantrums, aggression, and self-injury. In a study of risperidone’s effect on autism’s core symptoms, the authors reported improvements in repetitive and stereotyped behavior but not in social relatedness or verbal communication.39
Double-blind studies have shown positive effects on aggression and behavioral disturbances in children with conduct disorder, oppositional defiant disorder, and other disruptive disorders, developmentally delayed adolescents, and mentally retarded subjects of various ages.40-42 Children and adolescents appear to be more sensitive than adults to risperidone’s side effects such as weight gain, EPS, and pancreatitis.
Personality disorders
Antipsychotics have been recommended for paranoid ideas and psychotic-like symptoms in borderline personality disorder and in paranoid personality disorder.43
Olanzapine. A 24-week, double-blind study found low-dose olanzapine (mean dosage 5.3 mg/d) more effective than placebo for anxiety, interpersonal sensitivity, paranoia, and anger/hostility in women with borderline personality disorder.44
In another double-blind study, 12 weeks of olanzapine therapy (mean 6.9 mg/d) was more effective than placebo for inappropriate anger in borderline personality disorder, as measured by a modified Clinical Global Impression scale.45
Others. Anger and hostility improved more with aripiprazole, 15 mg/d, than with placebo in an 8-week double-blind study of patients with borderline personality disorder.46 Quetiapine, risperidone, ziprasidone, and clozapine have shown efficacy in open-label studies and case reports.
Related resources
- Boos J. Off label use–label off use? Ann Oncol 2003;14(1):1-5.
- Blum RS. Legal considerations in off-label medication prescribing. Arch Intern Med 2002;162(15):1777-9.
- Jeste DV, Dolder CR. Treatment of non-schizophrenic disorders: focus on atypical antipsychotics. J Psychiatr Res 2004;38(1):73-103.
- Food and Drug Administration. Searchable catalog of FDA-approved drug products. www.accessdata.fda.gov/scripts/cder/drugsatfda.
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Nortriptyline • Pamelor, Aventyl
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Trémeau receives grant/research support from Eli Lilly and Company.
Dr. Citrome receives grant/research support from AstraZeneca, Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Janssen Research Foundation, and Pfizer; is a consultant to Bristol-Myers Squibb, Eli Lilly and Company, Pfizer, Jazz Pharmaceuticals, and GlaxoSmithKline; and is a speaker for Abbott Laboratories, AstraZeneca, Eli Lilly and Company, and Pfizer.
Controlled clinical trial results can help you make two prescribing decisions:
- Is an antipsychotic the right choice for this patient?
- If yes, which agent?
Prescribing antipsychotics off-label can be worthwhile when a patient gets better, but even then two worries remain:
- Most uses of antipsychotics for nonpsychotic illness are not evidence-based.
- This practice may expose clinicians to liability if the patient gets worse.
Consider the use of second-generation antipsychotics (SGAs) to manage acute behaviors in patients with dementia. The FDA ordered a black box warning in 2005 that SGAs may increase mortality risk in older patients. In October, the Clinical Antipsychotic Trials of Intervention Effectiveness-Alzheimer’s Disease (CATIE-AD) reported that SGAs’ side effects offset their benefits when compared with placebo (see Will CATIE-AD change dementia treatment?).1
What do you do when FDA-approved drugs fail to help your patient with dementia, unipolar depression, anxiety disorders, or other nonpsychotic symptoms, and SGAs may be the next consideration? The answers lie in managing side effects and knowing which antipsychotic uses are supported by data from controlled clinical trials, which we review here.
- FGAs showed efficacy for nonpsychotic disorders
- SGAs are associated with a lower risk of EPS and tardive dyskinesia at therapeutic dosages, compared with FGAs
- Many patients fail to respond adequately to medications approved for their illnesses
- Evidence on SGAs’ efficacy in nonpsychotic disorders has grown substantially in the past 10 years.
EPS: extrapyramidal symptoms
FGA: first-generation antipsychotic
SGA: second-generation antipsychotic
Prescribing considerations
For a variety of reasons (Box 1), SGAs have rapidly assumed a major role in treating nonpsychotic disorders. Thirty-one percent of psychotropics are dispensed off-label,2 and Buckley3 reported in a 3-state survey that 70% of SGA prescriptions were written for indications other than schizophrenia.
Using antipsychotics for nonpsychotic symptoms is a longstanding clinical practice. In schizophrenia patients, antipsychotics have been shown to improve psychotic and nonpsychotic symptoms: agitation, violence, negative symptoms, social isolation, depression, suicidality, anxiety, insomnia, poor appetite, compulsions, cognition, smoking, alcohol and drug use, polydipsia, tardive dyskinesia, and tardive dystonia. Some clinicians may view these reports as evidence that antipsychotics might relieve these symptoms in patients with nonpsychotic disorders as well, but the issue is more complicated than that (Box 2).4
Caveats. SGAs do offer clinicians unique tools; no other class of psychotropics can claim efficacy in psychotic disorders, bipolar disorder, depression, and other disorders we describe in this review. On the other hand:
- Although some SGAs are approved for bipolar disorder and one was recently approved to treat irritability in autism (Table 1), most SGA uses in nonpsychotic disorders are off-label and supported by few—if any—large, randomized, controlled trials.
- Antipsychotics can cause the very symptoms they relieve, including depression, obsessive-compulsive disorder (OCD), anxiety, poorer cognition, agitation, mania, insomnia, and abnormal movements.
- Few controlled studies have compared SGAs to usual first-line treatments; most have evaluated SGAs as adjuncts to other psychotropics—such as serotonin reuptake inhibitors (SRIs)—for patients with treatment-resistant disorders.
- Published head-to-head studies have rarely compared the efficacy of various SGAs in treating nonpsychotic disorders.
- Long-term safety studies of SGAs for nonpsychotic indications have not been done.
Table 1
Bipolar and other nonpsychotic indications FDA-approved for SGAs
| SGA | Bipolar mania | Bipolar depression | Bipolar maintenance | Other |
|---|---|---|---|---|
| Aripiprazole | Acute mania or mixed episodes | Bipolar I disorder, most recent episode manic or mixed | ||
| Clozapine | Risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorders | |||
| Olanzapine | Acute mania or mixed episodes; monotherapy or with lithium or valproate for manic episodes | Bipolar disorder maintenance monotherapy | ||
| Olanzapine/fluoxetine combination | Bipolar depressive episodes | |||
| Quetiapine | Acute manic episodes; monotherapy or with lithium or valproate | Bipolar depressive episodes | ||
| Risperidone | Acute mania or mixed episodes; monotherapy or with lithium or valproate | Irritability in autism | ||
| Ziprasidone | Acute manic or mixed episodes | |||
| SGA: second-generation antipsychotic (oral forms) | ||||
Prescribing decisions. SGA’s potential adverse effects complicate clinical decision-making. First you must decide whether to use an SGA for your patient with a nonpsychotic disorder.
Second-generation antipsychotics (SGAs) show efficacy in so many psychotic and nonpsychotic disorders that a specific therapeutic action for each disorder is highly doubtful. One might ask, then: What do they improve, and how do they do it?
The complete answer is beyond current understanding, unfortunately. We do know, however, that SGAs have not shown efficacy for treating nonpsychotic disorders that first-generation antipsychotics (FGAs) did not show—except perhaps for maintenance treatment in bipolar disorder.
Calming action. The major clinical action of SGAs appears to be in calming patients, which also was the first therapeutic effect attributed to the FGA chlorpromazine. This calming effect would explain SGAs’ efficacy in treating agitation, aggressiveness, anxiety, and possibly mania. Other clinical effects specific to psychosis and possibly to depression are possible.
Receptor-blocking action. SGAs’ D2 and 5-HT2A receptor-blocking activity may explain much of the drugs’ therapeutic effect. However, if SGAs’ effect on nonpsychotic symptoms derives from their action on nondopaminergic receptors, then individual SGAs would vary widely in efficacy and pure dopaminergic agents such as amisulpride would be ineffective.
SGAs also bind at other receptor sites, and the clinical importance of this may vary from patient to patient, drug to drug, and dose to dose.4
Knowing, for example, that antipsychotics have been shown to increase mortality and cerebrovascular events in older patients might make you less likely to prescribe an SGA for a patient with dementia-related agitation. No other pharmacologic treatment has shown clear efficacy for these patients, however, so other factors are important to consider, including:
- patient history and clinical characteristics
- potential side effects
- individual therapeutic response to previous medications.
Table 2
SGA uses in nonpsychotic disorders supported by evidence
from published double-blind clinical trials*
| SGA | Unipolar depression | OCD | Anxiety disorders | Dementia | Developmental disorders | Borderline personality disorder |
|---|---|---|---|---|---|---|
| Aripiprazole | Yes | |||||
| Clozapine | ||||||
| Olanzapine | Yes | Yes | Yes | Yes | Yes | |
| Quetiapine | Yes | |||||
| Risperidone | Yes | Yes | Yes | Yes | ||
| Ziprasidone | ||||||
| * Not including studies of bipolar disorder | ||||||
| OCD: obsessive-compulsive disorder | ||||||
| SGA: second-generation antipsychotic | ||||||
Dementia
Most Alzheimer’s patients—75% to 90%—experience behavioral problems during this progressive dementia. Double-blind studies have found risperidone (mean dosage ~1 mg/d) and olanzapine (mean dosage 5 to 10 mg/d) effective in reducing agitation and aggression, even in nonpsychotic patients with Alzheimer’s disease or vascular dementia.5,6 Quetiapine, ≤100 mg/d, was not more effective than placebo in reducing agitation.7 One study comparing IM olanzapine with IM lorazepam and placebo in acute agitation found both active treatments more effective than placebo.8
CATIE-AD—sponsored by the National Institute of Mental Health—compared olanzapine, risperidone, and quetiapine with placebo in 421 outpatients with behavioral symptoms such as psychosis, agitation, or aggressiveness.1 No significant differences were seen in overall effectiveness (measured as discontinuation for any cause9), although patients receiving olanzapine (mean dosage 5.5 mg/d) or risperidone (mean dosage 1 mg/d) had lower discontinuation rates for lack of efficacy than those receiving placebo.
Unfortunately, the results of the first phase of CATIE-AD provide no clear guidance on the therapeutic strategy to use in dementia. Its findings do suggest two secondary conclusions, however, about using SGAs for patients with dementia:
- Because quetiapine, mean dosage 56.5 mg/d, was not more effective than placebo on any measures, consider higher dosages when using this agent.
- Close attention to preventing and treating SGAs’ side effects is the key to effectively treating agitation and psychosis in dementia.
Recommendation. Nonpharmacologic interventions are an important part of treating behavioral problems in patients with dementia.13,14 Antipsychotics—particularly SGAs—have shown efficacy for psychosis and agitation in these patients and remain the first therapeutic option. The CATIE-AD investigators recommend that clinicians evaluate potential risks and benefits of pharmacotherapy and discuss these with patients and caregivers.1 Also:
- Consider which SGAs have the lowest risk of causing side effects for an individual patient.
- Start with low dosages and increase as needed, based on efficacy and tolerability.
Bipolar disorder
Acute mania. Five SGAs—aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone—are FDA-approved for acute mania (Table 1). Large double-blind studies supporting this indication show that SGAs have efficacy in treating mania as monotherapy and in combination with lithium or divalproex.15 These clinical trials included patients who were not psychotic at baseline.
Antipsychotic dosages in these studies were within the ranges used in schizophrenia treatment studies. Combining an SGA with lithium or divalproex generally yields greater reductions in mania rating scale scores, higher response rates, and higher remission rates than using lithium or divalproex alone. No published study has compared SGAs with each other in mania, but differences in efficacy among these compounds are likely to be small.16
Bipolar depression. SGAs’ efficacy in bipolar depression has been evaluated in double-blind studies, and quetiapine and the olanzapine/fluoxetine combination are FDA-approved for this indication.
Olanzapine plus fluoxetine was more effective in improving depressive symptoms than olanzapine alone in a double-blind study of 833 adults with depressive symptoms of bipolar I disorder, as measured by Montgomery-Åsburg Depression Rating Scale (MADRS) scores. Olanzapine alone was more effective than placebo. Mean dosages were olanzapine, 7.4 mg/d, and fluoxetine, 39.3 mg/d, in combination therapy and olanzapine, 9.7 mg/d, as monotherapy.
MADRS scores indicated that combination therapy—but not olanzapine alone—improved core depressive symptoms such as sadness, lassitude, inability to feel, and pessimistic thoughts.17
Quetiapine. A double-blind, placebo-controlled trial (BOLDER I) evaluated quetiapine in 542 outpatients experiencing a major depressive episode associated with bipolar I or II disorder. After 8 weeks, quetiapine at 300 or 600 mg/d was more effective than placebo in reducing depressive symptoms, as measured by MADRS score changes.
Response rates were 58% with quetiapine and 36% with placebo; remission rates were 53% with quetiapine and 28% with placebo. Most symptoms, including core depression items, improved significantly with quetiapine, compared with placebo.18 Results of a second double-blind study (BOLDER II) have been presented at conferences but have not been fully published.
Risperidone. A smaller double-blind study compared risperidone plus placebo, paroxetine plus placebo, and risperidone plus paroxetine in 30 patients in the depressed phase of bipolar I or II disorder. Patients continued taking mood stabilizers during the study. After 12 weeks, depressive symptoms improved significantly in all three groups, with no significant differences.19
Maintenance therapy. Olanzapine and aripiprazole are FDA-approved for maintenance therapy in bipolar disorder (Table 1).
Unipolar depression
FGAs have shown efficacy in depression in multiple controlled studies.20 SGAs have been evaluated mostly as add-on therapies in antidepressant-resistant depression.
Olanzapine. Shelton et al21 compared olanzapine monotherapy, fluoxetine monotherapy, and combined treatment in 34 nonpsychotic, treatment-resistant depressed subjects. Olanzapine plus fluoxetine was more effective than either agent alone. A subsequent double-blind study, however, showed similar efficacy after 8 weeks among the three treatments and nortriptyline monotherapy. Patients in the double-blind trial appeared to respond more rapidly to combined treatment than to the monotherapies.22
Risperidone. A multiphase study of the efficacy of risperidone augmentation in treatment-resistant major depression began when 489 outpatients (2% with psychotic symptoms) received open-label citalopram, 20 to 60 mg/d. After 4 to 6 weeks, 386 nonresponders entered the augmentation phase with open-label risperidone, 0.25 to 2 mg/d. After 4 to 6 weeks of combination therapy, 241 (63%) patients whose symptoms resolved entered a double-blind discontinuation phase, in which they were randomly assigned to augmentation with risperidone or placebo, while on citalopram.
Median time to relapse during the double-blind phase was 102 days with risperidone augmentation and 85 days with placebo—not a statistically significant difference. Relapse rates after 24 weeks were 53.3% and 54.6%, respectively.23 This study showed that the improvement observed after adding risperidone was not sustained over time.
Quetiapine. In a prospective single-blind study, paroxetine augmented with quetiapine, 200 mg/d, was compared to paroxetine alone in major depression with anxiety.24 Combination therapy was more effective in improving anxiety and depression symptoms.
Others. Open-label, add-on studies indicate that aripiprazole and ziprasidone can improve treatment-resistant depression.25-27
Anxiety disorders
OCD. SGAs also have been investigated as augmentation therapy for patients with OCD resistant to SRIs. A single-blind study of 27 patients found adjunctive quetiapine more effective than placebo in improving OCD symptoms.28 SGAs were more effective than placebo as augmentation therapy to SRIs for treatment-refractory OCD in double-blind, placebo-controlled studies using mean dosages of:
In other trials:
- A small double-blind study of patients with social anxiety disorder found olanzapine monotherapy more effective than placebo.34
- Low-dose risperidone (mean dosage 1.1 mg/d) improved core symptoms of generalized anxiety disorder in a 5-week, double-blind, placebo-controlled trial.35
- Some authors have reported clinical improvement of panic disorder with olanzapine augmentation.36
Developmental disorders
Antipsychotics represent one-third of all filled psychotropic prescriptions for individuals with pervasive developmental disorders (PDD).37 Haloperidol and thioridazine are the only two FDA-approved FGAs for severe behavioral problems in PDD (and for hyperactivity with conduct disorders). Recently, risperidone received FDA approval for the treatment of irritability associated with autistic disorder in children.
Risperidone—the most-studied SGA in the PDD population—has shown efficacy in autism and in PDD not otherwise specified. Risperidone at dosages >3 mg/d improved repetitive behavior and aggression in adult patients.38
In children with autism, risperidone can improve tantrums, aggression, and self-injury. In a study of risperidone’s effect on autism’s core symptoms, the authors reported improvements in repetitive and stereotyped behavior but not in social relatedness or verbal communication.39
Double-blind studies have shown positive effects on aggression and behavioral disturbances in children with conduct disorder, oppositional defiant disorder, and other disruptive disorders, developmentally delayed adolescents, and mentally retarded subjects of various ages.40-42 Children and adolescents appear to be more sensitive than adults to risperidone’s side effects such as weight gain, EPS, and pancreatitis.
Personality disorders
Antipsychotics have been recommended for paranoid ideas and psychotic-like symptoms in borderline personality disorder and in paranoid personality disorder.43
Olanzapine. A 24-week, double-blind study found low-dose olanzapine (mean dosage 5.3 mg/d) more effective than placebo for anxiety, interpersonal sensitivity, paranoia, and anger/hostility in women with borderline personality disorder.44
In another double-blind study, 12 weeks of olanzapine therapy (mean 6.9 mg/d) was more effective than placebo for inappropriate anger in borderline personality disorder, as measured by a modified Clinical Global Impression scale.45
Others. Anger and hostility improved more with aripiprazole, 15 mg/d, than with placebo in an 8-week double-blind study of patients with borderline personality disorder.46 Quetiapine, risperidone, ziprasidone, and clozapine have shown efficacy in open-label studies and case reports.
Related resources
- Boos J. Off label use–label off use? Ann Oncol 2003;14(1):1-5.
- Blum RS. Legal considerations in off-label medication prescribing. Arch Intern Med 2002;162(15):1777-9.
- Jeste DV, Dolder CR. Treatment of non-schizophrenic disorders: focus on atypical antipsychotics. J Psychiatr Res 2004;38(1):73-103.
- Food and Drug Administration. Searchable catalog of FDA-approved drug products. www.accessdata.fda.gov/scripts/cder/drugsatfda.
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Nortriptyline • Pamelor, Aventyl
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Trémeau receives grant/research support from Eli Lilly and Company.
Dr. Citrome receives grant/research support from AstraZeneca, Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Janssen Research Foundation, and Pfizer; is a consultant to Bristol-Myers Squibb, Eli Lilly and Company, Pfizer, Jazz Pharmaceuticals, and GlaxoSmithKline; and is a speaker for Abbott Laboratories, AstraZeneca, Eli Lilly and Company, and Pfizer.
1. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525-38.
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med 2006;166:1021-6.
3. Buckley PF. New antipsychotic agents: emerging clinical profiles. J Clin Psychiatry 1999;60(suppl 1):12-7.
4. Shayegan DK, Stahl SM. Atypical antipsychotics: matching receptor profile to individual patient’s clinical profile. CNS Spectr 2004;9(10 suppl 11):6-14.
5. Jeste DV, Dolder CR, Nayak GV, Salzman C. Atypical antipsychotics in elderly patients with dementia or schizophrenia: review of recent literature. Harv Rev Psychiatry 2005;13(6):340-51.
6. Carson S, McDonagh MS, Peterson K. A systematic review of the efficacy and safety of atypical antipsychotics in patients with psychological and behavioral symptoms of dementia. J Am Geriatr Soc 2006;54(2):354-61.
7. Ballard C, Margallo-Lana M, Juszczak E, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. BMJ 2005;330(7496):874.-
8. Meehan KM, Wang H, David SR, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: a double-blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology 2002;26(4):494-504.
9. Schneider LS, Tariot PN, Lyketsos CG, et al. National Institute of Mental Health Antipsychotic Trials of Intervention Effectiveness (CATIE). Alzheimer disease trial methodology. Am J Geriatr Psychiatry 2001;9:346-60.
10. Kennedy J, Deberdt W, Siegal A, et al. Olanzapine does not enhance cognition in non-agitated and non-psychotic patients with mild to moderate Alzheimer’s dementia. Int J Geriatr Psychiatry 2005;20(11):1020-7.
11. U.S. Food and Drug Administration. Center for Drug Evaluation and Research. FDA public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. April 11, 2005. Available at: http://www.fda.gov/Cder/drug/advisory/antipsychotics.htm. Accessed October 17, 2006.
12. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353(22):2335-41.
13. Rabins P, Bland W, Bright-Long L, et al. from the Work Group on Alzheimer’s disease and related dementias. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias of late life. American Psychiatric Association Practice Guideline 1997. Available at http://www.psych.org/psych_pract/ treatg/pg/prac_guide.cfm. Accessed November 15, 2006.
14. Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer’s disease: a random control trial. JAMA 1996;276:1725-31.
15. Citrome L, Goldberg JF, Stahl SM. Toward convergence in the medication treatment of bipolar disorder and schizophrenia. Harv Rev Psychiatry 2005;13(1):28-42.
16. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry 2006;67(4):509-16.
17. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60(11):1079-88.
18. Calabrese JR, Keck PE, Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 2005;162(7):1351-60.
19. Shelton RC, Stahl SM. Risperidone and paroxetine given singly and in combination for bipolar depression. J Clin Psychiatry 2004;65(12):1715-9.
20. Robertson MM, Trimble MR. Major tranquillisers used as antidepressants. A review. J Affect Disord 1982;4(3):173-93.
21. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158(1):131-4.
22. Shelton RC, Williamson DJ, Corya SA, et al. Olanzapine/fluoxetine combination for treatment-resistant depression: a controlled study of SSRI and nortriptyline resistance. J Clin Psychiatry 2005;66(10):1289-97.
23. Rapaport MH, Gharabawi GM, Canuso CM, et al. Effects of risperidone augmentation in patients with treatment-resistant depression: results of open-label treatment followed by double-blind continuation. Neuropsychopharmacology 2006;31(11):2505-13.
24. Yargic LI, Corapcioglu A, Kocabasoglu N, et al. A prospective randomized single-blind, multicenter trial comparing the efficacy and safety of paroxetine with and without quetiapine therapy in depression associated with anxiety. Int J Psychiatry Clin Pract 2004;8:205-11.
25. Papakostas GI, Petersen TJ, Kinrys G, et al. Aripiprazole augmentation of selective serotonin reuptake inhibitors for treatment-resistant major depressive disorder. J Clin Psychiatry 2005;66(10):1326-30.
26. Papakostas GI, Petersen TJ, Nierenberg AA, et al. Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J Clin Psychiatry 2004;65(2):217-21.
27. Simon JS, Nemeroff CB. Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder. J Clin Psychiatry 2005;66(10):1216-20.
28. Atmaca M, Kuloglu M, Tezcan E, Gecici O. Quetiapine augmentation in patients with treatment resistant obsessive-compulsive disorder: a single-blind, placebo-controlled study. Int Clin Psychopharmacol 2002;17(3):115-9.
29. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 2000;57(8):794-801.
30. Bystritsky A, Ackerman DL, Rosen RM, et al. Augmentation of serotonin reuptake inhibitors in refractory obsessive-compulsive disorder using adjunctive olanzapine: a placebo-controlled trial. J Clin Psychiatry 2004;65(4):565-8.
31. Denys D, de Geus F, van Megen HJ, Westenberg HG. A double-blind, randomized, placebo-controlled trial of quetiapine addition in patients with obsessive-compulsive disorder refractory to serotonin reuptake inhibitors. J Clin Psychiatry 2004;65(8):1040-8.
32. Bartzokis G, Lu PH, Turner J, et al. Adjunctive risperidone in the treatment of chronic combat-related posttraumatic stress disorder. Biol Psychiatry 2005;57(5):474-9.
33. Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry 2002;159(10):1777-9.
34. Barnett SD, Kramer ML, Casat CD, et al. Efficacy of olanzapine in social anxiety disorder: a pilot study. J Psychopharmacol 2002;16(4):365-8.
35. Brawman-Mintzer O, Knapp RG, Nietert PJ. Adjunctive risperidone in generalized anxiety disorder: a double-blind, placebo-controlled study. J Clin Psychiatry 2005;66(10):1321-5.
36. Khaldi S, Kornreich C, Dan B, Pelc I. Usefulness of olanzapine in refractory panic attacks. J Clin Psychopharmacol 2003;23(1):100-1.
37. Lott IT, McGregor M, Engelman L, et al. Longitudinal prescribing patterns for psychoactive medications in community-based individuals with developmental disabilities: utilization of pharmacy records. J Intellect Disabil Res 2004;48(Pt 6):563-71.
38. McDougle CJ, Holmes JP, Carlson DC, et al. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry 1998;55(7):633-41.
39. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005;162(6):1142-8.
40. Vanden Borre R, Vermote R, Buttiens M, et al. Risperidone as addon therapy in behavioural disturbances in mental retardation: a double-blind placebo-controlled cross-over study. Acta Psychiatr Scand 1993;87(3):167-71.
41. Buitelaar JK, van der Gaag RJ, Cohen-Kettenis P, Melman CT. A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. J Clin Psychiatry 2001;62(4):239-48.
42. Snyder R, Turgay A, Aman M, et al; Risperidone Conduct Study Group. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. J Am Acad Child Adolesc Psychiatry 2002;41(9):1026-36.
43. Oldham JM, Gabbard GO, Goin MK, et al, from the workgroup on borderline personality disorder. Practice guideline for the treatment of patients with borderline personality disorder. American Psychiatric Association Practice Guideline 2001. Available at http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm. Accessed November 15, 2006.
44. Zanarini MC, Frankenburg FR. Olanzapine treatment of female borderline personality disorder patients: a double-blind, placebo-controlled pilot study. J Clin Psychiatry 2001;62(11):849-54.
45. Bogenschutz MP, George Nurnberg H. Olanzapine versus placebo in the treatment of borderline personality disorder. J Clin Psychiatry 2004;65(1):104-9.
46. Nickel MK, Muehlbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry 2006;163(5):833-8.
1. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525-38.
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med 2006;166:1021-6.
3. Buckley PF. New antipsychotic agents: emerging clinical profiles. J Clin Psychiatry 1999;60(suppl 1):12-7.
4. Shayegan DK, Stahl SM. Atypical antipsychotics: matching receptor profile to individual patient’s clinical profile. CNS Spectr 2004;9(10 suppl 11):6-14.
5. Jeste DV, Dolder CR, Nayak GV, Salzman C. Atypical antipsychotics in elderly patients with dementia or schizophrenia: review of recent literature. Harv Rev Psychiatry 2005;13(6):340-51.
6. Carson S, McDonagh MS, Peterson K. A systematic review of the efficacy and safety of atypical antipsychotics in patients with psychological and behavioral symptoms of dementia. J Am Geriatr Soc 2006;54(2):354-61.
7. Ballard C, Margallo-Lana M, Juszczak E, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. BMJ 2005;330(7496):874.-
8. Meehan KM, Wang H, David SR, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: a double-blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology 2002;26(4):494-504.
9. Schneider LS, Tariot PN, Lyketsos CG, et al. National Institute of Mental Health Antipsychotic Trials of Intervention Effectiveness (CATIE). Alzheimer disease trial methodology. Am J Geriatr Psychiatry 2001;9:346-60.
10. Kennedy J, Deberdt W, Siegal A, et al. Olanzapine does not enhance cognition in non-agitated and non-psychotic patients with mild to moderate Alzheimer’s dementia. Int J Geriatr Psychiatry 2005;20(11):1020-7.
11. U.S. Food and Drug Administration. Center for Drug Evaluation and Research. FDA public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. April 11, 2005. Available at: http://www.fda.gov/Cder/drug/advisory/antipsychotics.htm. Accessed October 17, 2006.
12. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353(22):2335-41.
13. Rabins P, Bland W, Bright-Long L, et al. from the Work Group on Alzheimer’s disease and related dementias. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias of late life. American Psychiatric Association Practice Guideline 1997. Available at http://www.psych.org/psych_pract/ treatg/pg/prac_guide.cfm. Accessed November 15, 2006.
14. Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer’s disease: a random control trial. JAMA 1996;276:1725-31.
15. Citrome L, Goldberg JF, Stahl SM. Toward convergence in the medication treatment of bipolar disorder and schizophrenia. Harv Rev Psychiatry 2005;13(1):28-42.
16. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry 2006;67(4):509-16.
17. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60(11):1079-88.
18. Calabrese JR, Keck PE, Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 2005;162(7):1351-60.
19. Shelton RC, Stahl SM. Risperidone and paroxetine given singly and in combination for bipolar depression. J Clin Psychiatry 2004;65(12):1715-9.
20. Robertson MM, Trimble MR. Major tranquillisers used as antidepressants. A review. J Affect Disord 1982;4(3):173-93.
21. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158(1):131-4.
22. Shelton RC, Williamson DJ, Corya SA, et al. Olanzapine/fluoxetine combination for treatment-resistant depression: a controlled study of SSRI and nortriptyline resistance. J Clin Psychiatry 2005;66(10):1289-97.
23. Rapaport MH, Gharabawi GM, Canuso CM, et al. Effects of risperidone augmentation in patients with treatment-resistant depression: results of open-label treatment followed by double-blind continuation. Neuropsychopharmacology 2006;31(11):2505-13.
24. Yargic LI, Corapcioglu A, Kocabasoglu N, et al. A prospective randomized single-blind, multicenter trial comparing the efficacy and safety of paroxetine with and without quetiapine therapy in depression associated with anxiety. Int J Psychiatry Clin Pract 2004;8:205-11.
25. Papakostas GI, Petersen TJ, Kinrys G, et al. Aripiprazole augmentation of selective serotonin reuptake inhibitors for treatment-resistant major depressive disorder. J Clin Psychiatry 2005;66(10):1326-30.
26. Papakostas GI, Petersen TJ, Nierenberg AA, et al. Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J Clin Psychiatry 2004;65(2):217-21.
27. Simon JS, Nemeroff CB. Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder. J Clin Psychiatry 2005;66(10):1216-20.
28. Atmaca M, Kuloglu M, Tezcan E, Gecici O. Quetiapine augmentation in patients with treatment resistant obsessive-compulsive disorder: a single-blind, placebo-controlled study. Int Clin Psychopharmacol 2002;17(3):115-9.
29. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 2000;57(8):794-801.
30. Bystritsky A, Ackerman DL, Rosen RM, et al. Augmentation of serotonin reuptake inhibitors in refractory obsessive-compulsive disorder using adjunctive olanzapine: a placebo-controlled trial. J Clin Psychiatry 2004;65(4):565-8.
31. Denys D, de Geus F, van Megen HJ, Westenberg HG. A double-blind, randomized, placebo-controlled trial of quetiapine addition in patients with obsessive-compulsive disorder refractory to serotonin reuptake inhibitors. J Clin Psychiatry 2004;65(8):1040-8.
32. Bartzokis G, Lu PH, Turner J, et al. Adjunctive risperidone in the treatment of chronic combat-related posttraumatic stress disorder. Biol Psychiatry 2005;57(5):474-9.
33. Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry 2002;159(10):1777-9.
34. Barnett SD, Kramer ML, Casat CD, et al. Efficacy of olanzapine in social anxiety disorder: a pilot study. J Psychopharmacol 2002;16(4):365-8.
35. Brawman-Mintzer O, Knapp RG, Nietert PJ. Adjunctive risperidone in generalized anxiety disorder: a double-blind, placebo-controlled study. J Clin Psychiatry 2005;66(10):1321-5.
36. Khaldi S, Kornreich C, Dan B, Pelc I. Usefulness of olanzapine in refractory panic attacks. J Clin Psychopharmacol 2003;23(1):100-1.
37. Lott IT, McGregor M, Engelman L, et al. Longitudinal prescribing patterns for psychoactive medications in community-based individuals with developmental disabilities: utilization of pharmacy records. J Intellect Disabil Res 2004;48(Pt 6):563-71.
38. McDougle CJ, Holmes JP, Carlson DC, et al. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry 1998;55(7):633-41.
39. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005;162(6):1142-8.
40. Vanden Borre R, Vermote R, Buttiens M, et al. Risperidone as addon therapy in behavioural disturbances in mental retardation: a double-blind placebo-controlled cross-over study. Acta Psychiatr Scand 1993;87(3):167-71.
41. Buitelaar JK, van der Gaag RJ, Cohen-Kettenis P, Melman CT. A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. J Clin Psychiatry 2001;62(4):239-48.
42. Snyder R, Turgay A, Aman M, et al; Risperidone Conduct Study Group. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. J Am Acad Child Adolesc Psychiatry 2002;41(9):1026-36.
43. Oldham JM, Gabbard GO, Goin MK, et al, from the workgroup on borderline personality disorder. Practice guideline for the treatment of patients with borderline personality disorder. American Psychiatric Association Practice Guideline 2001. Available at http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm. Accessed November 15, 2006.
44. Zanarini MC, Frankenburg FR. Olanzapine treatment of female borderline personality disorder patients: a double-blind, placebo-controlled pilot study. J Clin Psychiatry 2001;62(11):849-54.
45. Bogenschutz MP, George Nurnberg H. Olanzapine versus placebo in the treatment of borderline personality disorder. J Clin Psychiatry 2004;65(1):104-9.
46. Nickel MK, Muehlbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry 2006;163(5):833-8.
Inpatient treatment planning: Consider 6 preadmission patterns
Emergency admissions to psychiatric units usually are hectic, with ample opportunities to miss valuable clinical information. In these circumstances, failing to identify key features of the patient’s preadmission history can waste time and misinform treatment.
In our experience, psychiatric inpatients show characteristic trajectories and can be grouped into 6 categories based on preadmission course. Identifying which category best describes a particular patient can help direct your assessment and predict appropriate treatment (Table 1).
Table 1
Treatment planning at psychiatric admission: 6 patient categories*
| Patient category | Prior admission? | Outpatient treatment adherence? | Stressful life event? | Progressive deterioration? | Comprehensive workup indicated? |
|---|---|---|---|---|---|
| 1 | No; first episode | Yes | |||
| 2 | Yes | No care | No; re-establish previously successful treatment, and promote adherence | ||
| 3 | Yes | Incomplete adherence | |||
| 4 | Yes | Yes | Yes | No; provide psychosocial support to address stressful event | |
| 5 | Yes | Yes | No | Yes | Yes |
| 6 | Malingering patients either have no psychiatric illness or have psychiatric illness and exaggerate symptoms for secondary gain | No; discharge (or do not admit) those without mental illness; provide psychosocial support for others | |||
| * These general categories are not intended to apply to all patients and do not consider predictors of length of stay such as diagnoses, presence of suicidal thoughts/plans, or behavioral disturbances. | |||||
Why this system?
In busy academic psychiatry departments—with rotating inpatient attending physicians and multiple outpatient providers—inpatient teams need a treatment framework that will decrease redundant assessments, unnecessary laboratory exams, and diagnostic confusion. We developed the following guide for residents, medical students, and attending physicians who practice in our 20-bed inpatient unit in an urban public hospital, where beds are always at a premium.
No controlled data support this approach. It does not consider predictors of length of stay—such as diagnoses or if patients have suicidal thoughts or behavioral disturbances. This model can be used across a broad range of diagnostic, symptomatic, and behavioral presentations, however, and our residents have found it helps them organize inpatient care.
Category 1: first-time admissions
Patients who never have had psychiatric treatment and are admitted to an inpatient unit usually are experiencing an index psychotic or mood episode. They represent a small fraction of all admitted patients but deserve the most comprehensive medical and psychiatric evaluation. The goal is to establish an accurate diagnosis, which will determine the treatment course.
Evaluation. Obtain a clinical history from the patient and from individuals with corroborating information. Do a comprehensive physical examination, and order a baseline laboratory evaluation, including a complete blood count with differential, comprehensive metabolic panel (CMP), thyroid-stimulating hormone (TSH), and toxicology screen. Add other laboratory tests as indicated by the history and physical exam.
Always obtain brain CT or MRI for an atypical psychosis and probably even for a typical presentation of an index psychotic episode. CT and/or MRI are indicated if mental status, physical, or neurologic examinations reveal focal neurologic deficits.
Order an EEG if you suspect seizures—especially complex partial seizures—or metabolic encephalopathy. Order a lumbar puncture with cerebrospinal fluid analysis and culture if CNS infection is a possibility.
Neuropsychological tests. Psychological testing may include personality tests such as the Minnesota Multiphasic Personality Inventory (MMPI-2) or Millon Clinical Multiaxial Inventory-III and rating scales and instruments to quantify symptom severity (Table 2).
Always administer a Mini-Mental State Examination (MMSE) to assess for cognitive impairment, and use the Blessed Dementia Scale (BDS) if needed.
For suspected delirium, consider the Delirium Rating Scale (DRS) or the updated version, DRS-98. Further neuropsychological testing may be indicated if the patient shows cognitive dysfunction, as may be seen in delirium or dementia (Table 3).
Table 2
How severe are psychiatric symptoms? Consider these rating scales
| Symptom | Clinically useful instruments |
|---|---|
| Anxiety | Hamilton Anxiety Rating Scale |
| Bipolar mania | Young-Mania Rating Scale (Y-MRS) |
| Depression | Hamilton Rating Scale for Depression (HRSD) |
| Beck Depression Inventory (BDI) | |
| Montgomery-Åsburg Depression Rating Scale (MADRS) | |
| OCD | Yale-Brown Obsessive Compulsive Scale (Y-BOCS) |
| Psychosis | Brief Psychiatric Rating Scale (BPRS) |
| Positive and Negative Syndrome Scale (PANSS) | |
| Scale for the Assessment of Positive Symptoms (SAPS) | |
| Scale for the Assessment of Negative Symptoms (SANS) | |
| Overall symptomatology | Clinical Global Impressions (CGI) Scale |
Tests for patients with cognitive dysfunction, as in delirium or dementia
| Symptom domain | Neuropsychological tests |
|---|---|
| Attention and concentration | Trail Making Test, parts A and B |
| Wechsler Adult Intelligence Scale (WAIS-III): verbal IQ subtests | |
| Boston Naming Test | |
| Memory | Wechsler Memory Scale-III |
| Three Words-Three Shapes memory test | |
| Visual-spatial constructional ability | Rey-Osterrieth Complex Figure Test |
| Benton Visual Form Discrimination Test | |
| WAIS-III performance IQ subtests | |
| Executive function and abstract thinking | Wisconsin Card Sorting Test |
| Stroop Color-Word Test | |
| WAIS-III similarities and comprehension subtests |
Categories 2 & 3: readmission for nonadherence
Without outpatient follow-up. Although inpatients are almost always referred for outpatient care after discharge, many do not keep even one outpatient appointment. Patients who have no outpatient follow-up after discharge are twice as likely to be rehospitalized the same year, compared with patients who kept at least one outpatient appointment.1
With outpatient follow-up. Patients who relapse and are readmitted after a period of outpatient treatment probably account for the largest group of psychiatric inpatients. Reasons why outpatients become nonadherent2 and relapse after a period of stable remission are legion (Table 4).3-10
Table 4
Factors that interfere with patient adherence to psychiatric treatment
|
| Source: References 3-10 |
Patients being readmitted often are less-than-honest about treatment adherence, so seek corroboration from family, case managers, other caregivers, or outpatient clinicians. Measuring blood levels of medications or hormones (such as prolactin) that are influenced by medications may help you gauge adherence.
Promote treatment adherence. After you re-establish treatment, the second goal is to promote future adherence. Contact the outpatient psychiatrist to explore the patient’s involvement with outpatient care.
If the patient was nonadherent because of poor insight or misconceptions about the medication—such as fear of dependence, stigma of mental illness, or denial—educate the patient and family/caregivers. If patient and family education prove ineffective or are not possible, consider depot psychotropics.
For nonadherence caused by side effects, consider changing the dosage or rhythm of administration. Simplified dosing schedules might help. Pay close attention to drug-drug interactions with nonpsychotropic medications.
Because drug or alcohol abuse is a common reason for outpatient nonadherence,7,8 consider chemical-dependency treatment programs for patients with addictions or those whose nondependent substance use causes psychiatric relapse.
Links to outpatient care. In an ideal system, the links between inpatient and outpatient treatment would be seamless. Many real world systems do not work as well, however. Too often patients are admitted and discharged before the outpatient doctor has heard of the admission.
Three inpatient interventions have been shown to more than triple the likelihood for successful linkage to outpatient care:
- communication between inpatient and outpatient clinicians about discharge plans
- patients starting outpatient programs before discharge
- involving family during the hospital stay.6
Category 4: readmission after stressful event
Even in patients who have been completely adherent with outpatient treatment, a sudden stressful life event can exacerbate psychiatric symptoms and require inpatient care. Examples include:
- death of a family member
- departure of a trusted caregiver
- onset of an intercurrent medical illness
- loss of a job or other financial hardship
- loss of housing
- anniversary of a traumatic life event.
Although we are trained to look for a “precipitating event” in formulating psychiatric illness, patients might be unable to talk about such preadmission changes. If this information is not readily available, symptomatic deterioration may be misinterpreted as treatment nonadherence, incorrect diagnosis, or other etiology.
Treatment goals. For a patient who has deteriorated because of a psychosocial change, the goal of admission is to address this stressor and return the patient to function. These patients do not require extensive medical or psychiatric assessment, and their previously successful psychotropics probably do not need to be changed.
Patients in this category typically do not need or benefit from diagnostic reassessment or comprehensive laboratory, radiologic, or psychological investigations. Even so, consider the possibility that the patient might be experiencing a comorbid medical illness, drug side effect, or other biomedical change, even in cases of an apparent intercurrent stressful event.
Inpatient treatment is supportive, encompassing grief work and individual and group therapy. Involve the social worker immediately to address adverse changes in the patient’s income, financial status, and residential circumstances.
Continue previous outpatient medications, and modify dosages if indicated by symptom severity. Patients in this group usually require only short hospital stays until the acute symptoms recede and they rebuild sufficient coping skills to address the new stressors.
Category 5: progressive deterioration
Patients who worsen despite adherence with out-patient treatment and have not experienced a new psychosocial stressor are difficult to treat. Similar to patients with an index psychotic episode, deteriorating patients require extensive re-evaluation of diagnosis and treatment trajectory going back several years.
As described for category 1 patients, perform an extensive physical and laboratory examination, psychological testing, and additional or specialized radiologic testing such as MRI, fMRI, SPECT, or (if possible) PET, as needed. Focus on the possibility of diagnostic reassignment and/or the presence of comorbidities. Seek clinical consultation, especially if academic specialty programs are available in the vicinity. Consultation from medical, neurology, and neuropsychology colleagues can help clarify diagnostic possibilities.
Unlike patients in categories 2, 3, and 4, deteriorating patients often require wholesale changes in medication management because:
- adherence with previous regimens has not produced ongoing remission
- illness is worse or progressive and requires a new or more intensive approach.
Category 6: malingering
Resnick11 characterized 5 motivations for malingering psychosis and probably mental illness in general (Table 5). Psychiatric malingerers fall into two categories:
- those who have no illness but fake one
- those who have mental illness but grossly exaggerate the intensity and gravity of symptoms for secondary gain.
In general, malingering patients should not be hospitalized. If malingering is discovered after admission, discharge those without illness. In those with psychiatric illness, exaggerating symptoms may represent comorbid illness (especially Axis II disorders) or increased dependency because of a psychosocial change, such as loss of housing.
Address the latter with psychosocial support and social work/case management services, as with patients in category 4.
Table 5
5 motivations for psychiatric malingering
| To avoid punishment for criminal behavior |
| To avoid military conscription or combat |
| To obtain financial gain for disability or lawsuits |
| To obtain drugs or “do easier time” while incarcerated |
| To gain hospital admission to avoid arrest or obtain free room and board |
| Source: Reference 11 |
5 clinical factors that suggest malingering
| Absence of active or subtle signs of psychosis |
| Marked inconsistencies, contradictions |
| Patient endorses improbable psychiatric symptoms |
|
| Patient is evasive or uncooperative |
|
| Psychological testing (SIRS, M-FAST, MMPI-2) indicates malingering SIRS: Structured Interview of Reported Symptoms M-FAST: Miller Forensic Assessment of Symptoms Test MMPI-2: Minnesota Multiphasic Personality Inventory, Revised |
| Source: Reference 12 |
- Cuffel BJ, Held M, Goldman W. Predictive models and the effectiveness of strategies for improving outpatient follow-up under managed care. Psychiatr Serv 2002;53(11):1438-43.
- Lien L. Are readmission rates influenced by how psychiatric services are organized? Nord J Psychiatry 2002;56(1):23-8.
- Maruish ME, ed. The use of psychological testing for treatment planning and outcomes assessment, 3rd ed. Mahwah, NJ: Lawrence Erlbaum Assoc; 2004.
1. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv 2000;51(7):885-9.
2. Cohen LJ. The importance of staying on therapy. Psychiatric Times 2006;23(4):15-22.
3. Fleck DE, Keck PE Jr, Corey KB, Strakowski SM. Factors associated with medication adherence in African American and white patients with bipolar disorder. J Clin Psychiatry 2005;66(5):646-52.
4. Byrne N, Regan C, Livingston G. Adherence to treatment in mood disorders. Curr Opin Psychiatry 2006;19(1):44-9.
5. Sher I, McGinn L, Sirey JA, Meyers B. Effects of caregivers’ perceived stigma and causal beliefs on patients’ adherence to antidepressant treatment. Psychiatr Serv 2005;56(5):564-9.
6. Boyer CA, McAlpine DD, Pottick KJ, Olfson M. Identifying risk factors and key strategies in linkage to outpatient psychiatric care. Am J Psychiatry 2000;157(10):1592-8.
7. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv 2000;51(2):216-22.
8. Cooper C, Carpenter I, Katona C, et al. The AdHOC Study of older adults’ adherence to medication in 11 countries. Am J Geriatr Psychiatry 2005;13(12):1067-76.
9. Melartin TK, Rytsala HJ, Leskela US, et al. Continuity is the main challenge in treating major depressive disorder in psychiatric care. J Clin Psychiatry 2005;66(2):220-7.
10. Misdrahi D, Llorca PM, Lancon C, Bayle FJ. Compliance in schizophrenia: predictive factors, therapeutic considerations and research implications. Encephale 2002;28(3 Pt 1):266-72.
11. Resnick PJ. The detection of malingered psychosis. Psychiatr Clin North Am 1999;22(1):159-72.
12. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry 2005;4(11):12-25.
Emergency admissions to psychiatric units usually are hectic, with ample opportunities to miss valuable clinical information. In these circumstances, failing to identify key features of the patient’s preadmission history can waste time and misinform treatment.
In our experience, psychiatric inpatients show characteristic trajectories and can be grouped into 6 categories based on preadmission course. Identifying which category best describes a particular patient can help direct your assessment and predict appropriate treatment (Table 1).
Table 1
Treatment planning at psychiatric admission: 6 patient categories*
| Patient category | Prior admission? | Outpatient treatment adherence? | Stressful life event? | Progressive deterioration? | Comprehensive workup indicated? |
|---|---|---|---|---|---|
| 1 | No; first episode | Yes | |||
| 2 | Yes | No care | No; re-establish previously successful treatment, and promote adherence | ||
| 3 | Yes | Incomplete adherence | |||
| 4 | Yes | Yes | Yes | No; provide psychosocial support to address stressful event | |
| 5 | Yes | Yes | No | Yes | Yes |
| 6 | Malingering patients either have no psychiatric illness or have psychiatric illness and exaggerate symptoms for secondary gain | No; discharge (or do not admit) those without mental illness; provide psychosocial support for others | |||
| * These general categories are not intended to apply to all patients and do not consider predictors of length of stay such as diagnoses, presence of suicidal thoughts/plans, or behavioral disturbances. | |||||
Why this system?
In busy academic psychiatry departments—with rotating inpatient attending physicians and multiple outpatient providers—inpatient teams need a treatment framework that will decrease redundant assessments, unnecessary laboratory exams, and diagnostic confusion. We developed the following guide for residents, medical students, and attending physicians who practice in our 20-bed inpatient unit in an urban public hospital, where beds are always at a premium.
No controlled data support this approach. It does not consider predictors of length of stay—such as diagnoses or if patients have suicidal thoughts or behavioral disturbances. This model can be used across a broad range of diagnostic, symptomatic, and behavioral presentations, however, and our residents have found it helps them organize inpatient care.
Category 1: first-time admissions
Patients who never have had psychiatric treatment and are admitted to an inpatient unit usually are experiencing an index psychotic or mood episode. They represent a small fraction of all admitted patients but deserve the most comprehensive medical and psychiatric evaluation. The goal is to establish an accurate diagnosis, which will determine the treatment course.
Evaluation. Obtain a clinical history from the patient and from individuals with corroborating information. Do a comprehensive physical examination, and order a baseline laboratory evaluation, including a complete blood count with differential, comprehensive metabolic panel (CMP), thyroid-stimulating hormone (TSH), and toxicology screen. Add other laboratory tests as indicated by the history and physical exam.
Always obtain brain CT or MRI for an atypical psychosis and probably even for a typical presentation of an index psychotic episode. CT and/or MRI are indicated if mental status, physical, or neurologic examinations reveal focal neurologic deficits.
Order an EEG if you suspect seizures—especially complex partial seizures—or metabolic encephalopathy. Order a lumbar puncture with cerebrospinal fluid analysis and culture if CNS infection is a possibility.
Neuropsychological tests. Psychological testing may include personality tests such as the Minnesota Multiphasic Personality Inventory (MMPI-2) or Millon Clinical Multiaxial Inventory-III and rating scales and instruments to quantify symptom severity (Table 2).
Always administer a Mini-Mental State Examination (MMSE) to assess for cognitive impairment, and use the Blessed Dementia Scale (BDS) if needed.
For suspected delirium, consider the Delirium Rating Scale (DRS) or the updated version, DRS-98. Further neuropsychological testing may be indicated if the patient shows cognitive dysfunction, as may be seen in delirium or dementia (Table 3).
Table 2
How severe are psychiatric symptoms? Consider these rating scales
| Symptom | Clinically useful instruments |
|---|---|
| Anxiety | Hamilton Anxiety Rating Scale |
| Bipolar mania | Young-Mania Rating Scale (Y-MRS) |
| Depression | Hamilton Rating Scale for Depression (HRSD) |
| Beck Depression Inventory (BDI) | |
| Montgomery-Åsburg Depression Rating Scale (MADRS) | |
| OCD | Yale-Brown Obsessive Compulsive Scale (Y-BOCS) |
| Psychosis | Brief Psychiatric Rating Scale (BPRS) |
| Positive and Negative Syndrome Scale (PANSS) | |
| Scale for the Assessment of Positive Symptoms (SAPS) | |
| Scale for the Assessment of Negative Symptoms (SANS) | |
| Overall symptomatology | Clinical Global Impressions (CGI) Scale |
Tests for patients with cognitive dysfunction, as in delirium or dementia
| Symptom domain | Neuropsychological tests |
|---|---|
| Attention and concentration | Trail Making Test, parts A and B |
| Wechsler Adult Intelligence Scale (WAIS-III): verbal IQ subtests | |
| Boston Naming Test | |
| Memory | Wechsler Memory Scale-III |
| Three Words-Three Shapes memory test | |
| Visual-spatial constructional ability | Rey-Osterrieth Complex Figure Test |
| Benton Visual Form Discrimination Test | |
| WAIS-III performance IQ subtests | |
| Executive function and abstract thinking | Wisconsin Card Sorting Test |
| Stroop Color-Word Test | |
| WAIS-III similarities and comprehension subtests |
Categories 2 & 3: readmission for nonadherence
Without outpatient follow-up. Although inpatients are almost always referred for outpatient care after discharge, many do not keep even one outpatient appointment. Patients who have no outpatient follow-up after discharge are twice as likely to be rehospitalized the same year, compared with patients who kept at least one outpatient appointment.1
With outpatient follow-up. Patients who relapse and are readmitted after a period of outpatient treatment probably account for the largest group of psychiatric inpatients. Reasons why outpatients become nonadherent2 and relapse after a period of stable remission are legion (Table 4).3-10
Table 4
Factors that interfere with patient adherence to psychiatric treatment
|
| Source: References 3-10 |
Patients being readmitted often are less-than-honest about treatment adherence, so seek corroboration from family, case managers, other caregivers, or outpatient clinicians. Measuring blood levels of medications or hormones (such as prolactin) that are influenced by medications may help you gauge adherence.
Promote treatment adherence. After you re-establish treatment, the second goal is to promote future adherence. Contact the outpatient psychiatrist to explore the patient’s involvement with outpatient care.
If the patient was nonadherent because of poor insight or misconceptions about the medication—such as fear of dependence, stigma of mental illness, or denial—educate the patient and family/caregivers. If patient and family education prove ineffective or are not possible, consider depot psychotropics.
For nonadherence caused by side effects, consider changing the dosage or rhythm of administration. Simplified dosing schedules might help. Pay close attention to drug-drug interactions with nonpsychotropic medications.
Because drug or alcohol abuse is a common reason for outpatient nonadherence,7,8 consider chemical-dependency treatment programs for patients with addictions or those whose nondependent substance use causes psychiatric relapse.
Links to outpatient care. In an ideal system, the links between inpatient and outpatient treatment would be seamless. Many real world systems do not work as well, however. Too often patients are admitted and discharged before the outpatient doctor has heard of the admission.
Three inpatient interventions have been shown to more than triple the likelihood for successful linkage to outpatient care:
- communication between inpatient and outpatient clinicians about discharge plans
- patients starting outpatient programs before discharge
- involving family during the hospital stay.6
Category 4: readmission after stressful event
Even in patients who have been completely adherent with outpatient treatment, a sudden stressful life event can exacerbate psychiatric symptoms and require inpatient care. Examples include:
- death of a family member
- departure of a trusted caregiver
- onset of an intercurrent medical illness
- loss of a job or other financial hardship
- loss of housing
- anniversary of a traumatic life event.
Although we are trained to look for a “precipitating event” in formulating psychiatric illness, patients might be unable to talk about such preadmission changes. If this information is not readily available, symptomatic deterioration may be misinterpreted as treatment nonadherence, incorrect diagnosis, or other etiology.
Treatment goals. For a patient who has deteriorated because of a psychosocial change, the goal of admission is to address this stressor and return the patient to function. These patients do not require extensive medical or psychiatric assessment, and their previously successful psychotropics probably do not need to be changed.
Patients in this category typically do not need or benefit from diagnostic reassessment or comprehensive laboratory, radiologic, or psychological investigations. Even so, consider the possibility that the patient might be experiencing a comorbid medical illness, drug side effect, or other biomedical change, even in cases of an apparent intercurrent stressful event.
Inpatient treatment is supportive, encompassing grief work and individual and group therapy. Involve the social worker immediately to address adverse changes in the patient’s income, financial status, and residential circumstances.
Continue previous outpatient medications, and modify dosages if indicated by symptom severity. Patients in this group usually require only short hospital stays until the acute symptoms recede and they rebuild sufficient coping skills to address the new stressors.
Category 5: progressive deterioration
Patients who worsen despite adherence with out-patient treatment and have not experienced a new psychosocial stressor are difficult to treat. Similar to patients with an index psychotic episode, deteriorating patients require extensive re-evaluation of diagnosis and treatment trajectory going back several years.
As described for category 1 patients, perform an extensive physical and laboratory examination, psychological testing, and additional or specialized radiologic testing such as MRI, fMRI, SPECT, or (if possible) PET, as needed. Focus on the possibility of diagnostic reassignment and/or the presence of comorbidities. Seek clinical consultation, especially if academic specialty programs are available in the vicinity. Consultation from medical, neurology, and neuropsychology colleagues can help clarify diagnostic possibilities.
Unlike patients in categories 2, 3, and 4, deteriorating patients often require wholesale changes in medication management because:
- adherence with previous regimens has not produced ongoing remission
- illness is worse or progressive and requires a new or more intensive approach.
Category 6: malingering
Resnick11 characterized 5 motivations for malingering psychosis and probably mental illness in general (Table 5). Psychiatric malingerers fall into two categories:
- those who have no illness but fake one
- those who have mental illness but grossly exaggerate the intensity and gravity of symptoms for secondary gain.
In general, malingering patients should not be hospitalized. If malingering is discovered after admission, discharge those without illness. In those with psychiatric illness, exaggerating symptoms may represent comorbid illness (especially Axis II disorders) or increased dependency because of a psychosocial change, such as loss of housing.
Address the latter with psychosocial support and social work/case management services, as with patients in category 4.
Table 5
5 motivations for psychiatric malingering
| To avoid punishment for criminal behavior |
| To avoid military conscription or combat |
| To obtain financial gain for disability or lawsuits |
| To obtain drugs or “do easier time” while incarcerated |
| To gain hospital admission to avoid arrest or obtain free room and board |
| Source: Reference 11 |
5 clinical factors that suggest malingering
| Absence of active or subtle signs of psychosis |
| Marked inconsistencies, contradictions |
| Patient endorses improbable psychiatric symptoms |
|
| Patient is evasive or uncooperative |
|
| Psychological testing (SIRS, M-FAST, MMPI-2) indicates malingering SIRS: Structured Interview of Reported Symptoms M-FAST: Miller Forensic Assessment of Symptoms Test MMPI-2: Minnesota Multiphasic Personality Inventory, Revised |
| Source: Reference 12 |
- Cuffel BJ, Held M, Goldman W. Predictive models and the effectiveness of strategies for improving outpatient follow-up under managed care. Psychiatr Serv 2002;53(11):1438-43.
- Lien L. Are readmission rates influenced by how psychiatric services are organized? Nord J Psychiatry 2002;56(1):23-8.
- Maruish ME, ed. The use of psychological testing for treatment planning and outcomes assessment, 3rd ed. Mahwah, NJ: Lawrence Erlbaum Assoc; 2004.
Emergency admissions to psychiatric units usually are hectic, with ample opportunities to miss valuable clinical information. In these circumstances, failing to identify key features of the patient’s preadmission history can waste time and misinform treatment.
In our experience, psychiatric inpatients show characteristic trajectories and can be grouped into 6 categories based on preadmission course. Identifying which category best describes a particular patient can help direct your assessment and predict appropriate treatment (Table 1).
Table 1
Treatment planning at psychiatric admission: 6 patient categories*
| Patient category | Prior admission? | Outpatient treatment adherence? | Stressful life event? | Progressive deterioration? | Comprehensive workup indicated? |
|---|---|---|---|---|---|
| 1 | No; first episode | Yes | |||
| 2 | Yes | No care | No; re-establish previously successful treatment, and promote adherence | ||
| 3 | Yes | Incomplete adherence | |||
| 4 | Yes | Yes | Yes | No; provide psychosocial support to address stressful event | |
| 5 | Yes | Yes | No | Yes | Yes |
| 6 | Malingering patients either have no psychiatric illness or have psychiatric illness and exaggerate symptoms for secondary gain | No; discharge (or do not admit) those without mental illness; provide psychosocial support for others | |||
| * These general categories are not intended to apply to all patients and do not consider predictors of length of stay such as diagnoses, presence of suicidal thoughts/plans, or behavioral disturbances. | |||||
Why this system?
In busy academic psychiatry departments—with rotating inpatient attending physicians and multiple outpatient providers—inpatient teams need a treatment framework that will decrease redundant assessments, unnecessary laboratory exams, and diagnostic confusion. We developed the following guide for residents, medical students, and attending physicians who practice in our 20-bed inpatient unit in an urban public hospital, where beds are always at a premium.
No controlled data support this approach. It does not consider predictors of length of stay—such as diagnoses or if patients have suicidal thoughts or behavioral disturbances. This model can be used across a broad range of diagnostic, symptomatic, and behavioral presentations, however, and our residents have found it helps them organize inpatient care.
Category 1: first-time admissions
Patients who never have had psychiatric treatment and are admitted to an inpatient unit usually are experiencing an index psychotic or mood episode. They represent a small fraction of all admitted patients but deserve the most comprehensive medical and psychiatric evaluation. The goal is to establish an accurate diagnosis, which will determine the treatment course.
Evaluation. Obtain a clinical history from the patient and from individuals with corroborating information. Do a comprehensive physical examination, and order a baseline laboratory evaluation, including a complete blood count with differential, comprehensive metabolic panel (CMP), thyroid-stimulating hormone (TSH), and toxicology screen. Add other laboratory tests as indicated by the history and physical exam.
Always obtain brain CT or MRI for an atypical psychosis and probably even for a typical presentation of an index psychotic episode. CT and/or MRI are indicated if mental status, physical, or neurologic examinations reveal focal neurologic deficits.
Order an EEG if you suspect seizures—especially complex partial seizures—or metabolic encephalopathy. Order a lumbar puncture with cerebrospinal fluid analysis and culture if CNS infection is a possibility.
Neuropsychological tests. Psychological testing may include personality tests such as the Minnesota Multiphasic Personality Inventory (MMPI-2) or Millon Clinical Multiaxial Inventory-III and rating scales and instruments to quantify symptom severity (Table 2).
Always administer a Mini-Mental State Examination (MMSE) to assess for cognitive impairment, and use the Blessed Dementia Scale (BDS) if needed.
For suspected delirium, consider the Delirium Rating Scale (DRS) or the updated version, DRS-98. Further neuropsychological testing may be indicated if the patient shows cognitive dysfunction, as may be seen in delirium or dementia (Table 3).
Table 2
How severe are psychiatric symptoms? Consider these rating scales
| Symptom | Clinically useful instruments |
|---|---|
| Anxiety | Hamilton Anxiety Rating Scale |
| Bipolar mania | Young-Mania Rating Scale (Y-MRS) |
| Depression | Hamilton Rating Scale for Depression (HRSD) |
| Beck Depression Inventory (BDI) | |
| Montgomery-Åsburg Depression Rating Scale (MADRS) | |
| OCD | Yale-Brown Obsessive Compulsive Scale (Y-BOCS) |
| Psychosis | Brief Psychiatric Rating Scale (BPRS) |
| Positive and Negative Syndrome Scale (PANSS) | |
| Scale for the Assessment of Positive Symptoms (SAPS) | |
| Scale for the Assessment of Negative Symptoms (SANS) | |
| Overall symptomatology | Clinical Global Impressions (CGI) Scale |
Tests for patients with cognitive dysfunction, as in delirium or dementia
| Symptom domain | Neuropsychological tests |
|---|---|
| Attention and concentration | Trail Making Test, parts A and B |
| Wechsler Adult Intelligence Scale (WAIS-III): verbal IQ subtests | |
| Boston Naming Test | |
| Memory | Wechsler Memory Scale-III |
| Three Words-Three Shapes memory test | |
| Visual-spatial constructional ability | Rey-Osterrieth Complex Figure Test |
| Benton Visual Form Discrimination Test | |
| WAIS-III performance IQ subtests | |
| Executive function and abstract thinking | Wisconsin Card Sorting Test |
| Stroop Color-Word Test | |
| WAIS-III similarities and comprehension subtests |
Categories 2 & 3: readmission for nonadherence
Without outpatient follow-up. Although inpatients are almost always referred for outpatient care after discharge, many do not keep even one outpatient appointment. Patients who have no outpatient follow-up after discharge are twice as likely to be rehospitalized the same year, compared with patients who kept at least one outpatient appointment.1
With outpatient follow-up. Patients who relapse and are readmitted after a period of outpatient treatment probably account for the largest group of psychiatric inpatients. Reasons why outpatients become nonadherent2 and relapse after a period of stable remission are legion (Table 4).3-10
Table 4
Factors that interfere with patient adherence to psychiatric treatment
|
| Source: References 3-10 |
Patients being readmitted often are less-than-honest about treatment adherence, so seek corroboration from family, case managers, other caregivers, or outpatient clinicians. Measuring blood levels of medications or hormones (such as prolactin) that are influenced by medications may help you gauge adherence.
Promote treatment adherence. After you re-establish treatment, the second goal is to promote future adherence. Contact the outpatient psychiatrist to explore the patient’s involvement with outpatient care.
If the patient was nonadherent because of poor insight or misconceptions about the medication—such as fear of dependence, stigma of mental illness, or denial—educate the patient and family/caregivers. If patient and family education prove ineffective or are not possible, consider depot psychotropics.
For nonadherence caused by side effects, consider changing the dosage or rhythm of administration. Simplified dosing schedules might help. Pay close attention to drug-drug interactions with nonpsychotropic medications.
Because drug or alcohol abuse is a common reason for outpatient nonadherence,7,8 consider chemical-dependency treatment programs for patients with addictions or those whose nondependent substance use causes psychiatric relapse.
Links to outpatient care. In an ideal system, the links between inpatient and outpatient treatment would be seamless. Many real world systems do not work as well, however. Too often patients are admitted and discharged before the outpatient doctor has heard of the admission.
Three inpatient interventions have been shown to more than triple the likelihood for successful linkage to outpatient care:
- communication between inpatient and outpatient clinicians about discharge plans
- patients starting outpatient programs before discharge
- involving family during the hospital stay.6
Category 4: readmission after stressful event
Even in patients who have been completely adherent with outpatient treatment, a sudden stressful life event can exacerbate psychiatric symptoms and require inpatient care. Examples include:
- death of a family member
- departure of a trusted caregiver
- onset of an intercurrent medical illness
- loss of a job or other financial hardship
- loss of housing
- anniversary of a traumatic life event.
Although we are trained to look for a “precipitating event” in formulating psychiatric illness, patients might be unable to talk about such preadmission changes. If this information is not readily available, symptomatic deterioration may be misinterpreted as treatment nonadherence, incorrect diagnosis, or other etiology.
Treatment goals. For a patient who has deteriorated because of a psychosocial change, the goal of admission is to address this stressor and return the patient to function. These patients do not require extensive medical or psychiatric assessment, and their previously successful psychotropics probably do not need to be changed.
Patients in this category typically do not need or benefit from diagnostic reassessment or comprehensive laboratory, radiologic, or psychological investigations. Even so, consider the possibility that the patient might be experiencing a comorbid medical illness, drug side effect, or other biomedical change, even in cases of an apparent intercurrent stressful event.
Inpatient treatment is supportive, encompassing grief work and individual and group therapy. Involve the social worker immediately to address adverse changes in the patient’s income, financial status, and residential circumstances.
Continue previous outpatient medications, and modify dosages if indicated by symptom severity. Patients in this group usually require only short hospital stays until the acute symptoms recede and they rebuild sufficient coping skills to address the new stressors.
Category 5: progressive deterioration
Patients who worsen despite adherence with out-patient treatment and have not experienced a new psychosocial stressor are difficult to treat. Similar to patients with an index psychotic episode, deteriorating patients require extensive re-evaluation of diagnosis and treatment trajectory going back several years.
As described for category 1 patients, perform an extensive physical and laboratory examination, psychological testing, and additional or specialized radiologic testing such as MRI, fMRI, SPECT, or (if possible) PET, as needed. Focus on the possibility of diagnostic reassignment and/or the presence of comorbidities. Seek clinical consultation, especially if academic specialty programs are available in the vicinity. Consultation from medical, neurology, and neuropsychology colleagues can help clarify diagnostic possibilities.
Unlike patients in categories 2, 3, and 4, deteriorating patients often require wholesale changes in medication management because:
- adherence with previous regimens has not produced ongoing remission
- illness is worse or progressive and requires a new or more intensive approach.
Category 6: malingering
Resnick11 characterized 5 motivations for malingering psychosis and probably mental illness in general (Table 5). Psychiatric malingerers fall into two categories:
- those who have no illness but fake one
- those who have mental illness but grossly exaggerate the intensity and gravity of symptoms for secondary gain.
In general, malingering patients should not be hospitalized. If malingering is discovered after admission, discharge those without illness. In those with psychiatric illness, exaggerating symptoms may represent comorbid illness (especially Axis II disorders) or increased dependency because of a psychosocial change, such as loss of housing.
Address the latter with psychosocial support and social work/case management services, as with patients in category 4.
Table 5
5 motivations for psychiatric malingering
| To avoid punishment for criminal behavior |
| To avoid military conscription or combat |
| To obtain financial gain for disability or lawsuits |
| To obtain drugs or “do easier time” while incarcerated |
| To gain hospital admission to avoid arrest or obtain free room and board |
| Source: Reference 11 |
5 clinical factors that suggest malingering
| Absence of active or subtle signs of psychosis |
| Marked inconsistencies, contradictions |
| Patient endorses improbable psychiatric symptoms |
|
| Patient is evasive or uncooperative |
|
| Psychological testing (SIRS, M-FAST, MMPI-2) indicates malingering SIRS: Structured Interview of Reported Symptoms M-FAST: Miller Forensic Assessment of Symptoms Test MMPI-2: Minnesota Multiphasic Personality Inventory, Revised |
| Source: Reference 12 |
- Cuffel BJ, Held M, Goldman W. Predictive models and the effectiveness of strategies for improving outpatient follow-up under managed care. Psychiatr Serv 2002;53(11):1438-43.
- Lien L. Are readmission rates influenced by how psychiatric services are organized? Nord J Psychiatry 2002;56(1):23-8.
- Maruish ME, ed. The use of psychological testing for treatment planning and outcomes assessment, 3rd ed. Mahwah, NJ: Lawrence Erlbaum Assoc; 2004.
1. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv 2000;51(7):885-9.
2. Cohen LJ. The importance of staying on therapy. Psychiatric Times 2006;23(4):15-22.
3. Fleck DE, Keck PE Jr, Corey KB, Strakowski SM. Factors associated with medication adherence in African American and white patients with bipolar disorder. J Clin Psychiatry 2005;66(5):646-52.
4. Byrne N, Regan C, Livingston G. Adherence to treatment in mood disorders. Curr Opin Psychiatry 2006;19(1):44-9.
5. Sher I, McGinn L, Sirey JA, Meyers B. Effects of caregivers’ perceived stigma and causal beliefs on patients’ adherence to antidepressant treatment. Psychiatr Serv 2005;56(5):564-9.
6. Boyer CA, McAlpine DD, Pottick KJ, Olfson M. Identifying risk factors and key strategies in linkage to outpatient psychiatric care. Am J Psychiatry 2000;157(10):1592-8.
7. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv 2000;51(2):216-22.
8. Cooper C, Carpenter I, Katona C, et al. The AdHOC Study of older adults’ adherence to medication in 11 countries. Am J Geriatr Psychiatry 2005;13(12):1067-76.
9. Melartin TK, Rytsala HJ, Leskela US, et al. Continuity is the main challenge in treating major depressive disorder in psychiatric care. J Clin Psychiatry 2005;66(2):220-7.
10. Misdrahi D, Llorca PM, Lancon C, Bayle FJ. Compliance in schizophrenia: predictive factors, therapeutic considerations and research implications. Encephale 2002;28(3 Pt 1):266-72.
11. Resnick PJ. The detection of malingered psychosis. Psychiatr Clin North Am 1999;22(1):159-72.
12. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry 2005;4(11):12-25.
1. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv 2000;51(7):885-9.
2. Cohen LJ. The importance of staying on therapy. Psychiatric Times 2006;23(4):15-22.
3. Fleck DE, Keck PE Jr, Corey KB, Strakowski SM. Factors associated with medication adherence in African American and white patients with bipolar disorder. J Clin Psychiatry 2005;66(5):646-52.
4. Byrne N, Regan C, Livingston G. Adherence to treatment in mood disorders. Curr Opin Psychiatry 2006;19(1):44-9.
5. Sher I, McGinn L, Sirey JA, Meyers B. Effects of caregivers’ perceived stigma and causal beliefs on patients’ adherence to antidepressant treatment. Psychiatr Serv 2005;56(5):564-9.
6. Boyer CA, McAlpine DD, Pottick KJ, Olfson M. Identifying risk factors and key strategies in linkage to outpatient psychiatric care. Am J Psychiatry 2000;157(10):1592-8.
7. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv 2000;51(2):216-22.
8. Cooper C, Carpenter I, Katona C, et al. The AdHOC Study of older adults’ adherence to medication in 11 countries. Am J Geriatr Psychiatry 2005;13(12):1067-76.
9. Melartin TK, Rytsala HJ, Leskela US, et al. Continuity is the main challenge in treating major depressive disorder in psychiatric care. J Clin Psychiatry 2005;66(2):220-7.
10. Misdrahi D, Llorca PM, Lancon C, Bayle FJ. Compliance in schizophrenia: predictive factors, therapeutic considerations and research implications. Encephale 2002;28(3 Pt 1):266-72.
11. Resnick PJ. The detection of malingered psychosis. Psychiatr Clin North Am 1999;22(1):159-72.
12. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry 2005;4(11):12-25.
PDA prescription program pros and cons
Personal digital assistant (PDA)-based drug reference software can help you make informed point-of-care prescription decisions, but accuracy, usability, and comprehensiveness vary greatly among programs.
This article looks at the benefits and drawbacks of popular PDA drug reference programs and offers advice on choosing the right one for your practice.
PDA Program benefits
Equipping your PDA with drug reference software makes prescription information portable and accessible. PDA-based drug guides also:
- are easy to update. Most PDA-based systems automatically update as part of routine synchronization procedures between the device and its host computer, keeping the information up-to-date. This is an important feature as some drug databases are updated daily.
- list potential side effects. Most programs list all potential medication side effects and distinguish between common and uncommon adverse events.
- list possible drug-drug interactions. This feature gives PDA-based drug guides a clear advantage over print textbooks. Users enter two or more medications, and the program searches for potential interactions between them.
- list interactions with alternative medications. Many databases include herbal supplements and other nontraditional pharmaceuticals.
- offer additional tools, such as medical calculators, treatment algorithms, and other handy features.
Drawbacks
Because PDA screens are so small, PDA-based drug guides must compromise between level of detail and ease of use. Unlike standard drug reference books, for example, PDA programs rarely list rates of side effects or comparisons with placebo.
PDA-based drug guides usually do not display references or analyses of the data behind the lists. For example, many programs provide dosing suggestions for special populations—such as the elderly, medically ill, and children—but the basis for these dose adjustments often is unclear or does not jibe with other programs. Most PDA drug software excludes other specific information, such as the evidence behind drug indications.
Researchers’ ratings
Some PDA-based drug databases, such as mobilePDR, evolved from written pharmacy databases. Others, such as ePharmacopoeia or Epocrates, were developed from more-general reference tools aimed at students and physicians. Overall, the former type of drug guide is more comprehensive and the latter is easier to use, although all PDA drug software offers some degree of comprehensiveness and usability.
PDA-based programs differ greatly, however, and the following researchers have explored the differences.
- Enders et al1 in 2001 rated Lexi-Drugs Platinum the most comprehensive and accurate among nine programs.
- Galt and colleagues,2 placing more weight on safety concerns than ease of use, in 2005 also rated Lexi-Drugs Platinum number one among 11 programs.
- Clauson et al3 in 2004 rated Lexi-Drugs number one among 10 programs, but noted that other products were catching up.
- Knollmann et al4 in 2004 rated 11 PDA-based drug databases on ease of use, comprehensiveness, and accuracy. They entered three pairs of drug names into each program; one pair included an herbal supplement.
- Perkins et al5 in 2004 entered 37 pairs of drug names into eight programs to test their ability to detect drug-drug interactions. They found that Epocrates offered the best combination of sensitivity (identifying all potential interactions) and specificity (reporting only important interactions). Lexi-Drugs had perfect sensitivity but comparatively poor specificity.
Which program should you choose?
No PDA-based drug reference will provide everything you need, so be clear on what you desire most when choosing a program:
- If drug safety is your primary concern, Lexi-Drugs might be best, although it tends to report clinically insignificant interactions.
- If you want only clinically significant interactions, consider Epocrates or Epocrates RX Pro.
- If a comprehensive database is critical, Clinical Pharmacology OnHand has the most extensive drug database.
- If you’re looking for the best combination of accuracy, comprehensiveness, and physician-friendly features, Lexi-Drugs and PEPID PDC might be most effective, though Epocrates is also a reasonable performer.
- If you wish to understand the evidence behind each recommendation, books and online pharmaceutical references remain better options.
Cost is another factor. This might explain why Epocrates—which offers a free version for physicians and medical students—is more popular than Lexi-Drugs, which is one of the most expensive programs at $70 for the basic version and $285 for the comprehensive suite. Most programs have two options: a less expensive—or even free—drug database that costs up to $75 and a suite of features such as treatment algorithms or diagnosis databases that range from $60 to almost $300.
Personal experience is the best way to determine which product is best. Most manufacturers let you try their programs before buying.
Related resources
Chan CH, Luo JS, Kennedy RS. Concise Guide to Computers in Clinical Psychiatry. Washington DC: American Psychiatric Press; 2002
American Association for Technology in Psychiatry. www.techpsych.org
1. Enders SJ, Enders JM, Holstad SG. Drug-information software for Palm operating system personal digital assistants: breadth, clinical dependability, and ease of use. Pharmacotherapy 2002;22:1036-40.
2. Galt KA, Rule AM, Houghton B, et al. Personal digital assistant-based drug information sources: potential to improve medication safety. J Med Librar Assoc. 2005;93:229-36.
3. Clauson KA, Seamon MJ, Clauson AS, et al. Evaluation of drug information databases for personal digital assistants. Am J Health Syst Pharm 2004;61:1015-24.
4. Knollmann BC, Smyth BJ, Garnett CE, et al. Personal digital assistant-based drug reference software as tools to improve rational prescribing: benchmark criteria and performance. Clin Pharmacol Ther 2005;78:7-18.
5. Perkins NA, Murphy JE, Malone DC, Armstrong EP. Performance of drug-drug interaction software for personal digital assistants. Ann Pharmacother 2006;40:850-5.
Personal digital assistant (PDA)-based drug reference software can help you make informed point-of-care prescription decisions, but accuracy, usability, and comprehensiveness vary greatly among programs.
This article looks at the benefits and drawbacks of popular PDA drug reference programs and offers advice on choosing the right one for your practice.
PDA Program benefits
Equipping your PDA with drug reference software makes prescription information portable and accessible. PDA-based drug guides also:
- are easy to update. Most PDA-based systems automatically update as part of routine synchronization procedures between the device and its host computer, keeping the information up-to-date. This is an important feature as some drug databases are updated daily.
- list potential side effects. Most programs list all potential medication side effects and distinguish between common and uncommon adverse events.
- list possible drug-drug interactions. This feature gives PDA-based drug guides a clear advantage over print textbooks. Users enter two or more medications, and the program searches for potential interactions between them.
- list interactions with alternative medications. Many databases include herbal supplements and other nontraditional pharmaceuticals.
- offer additional tools, such as medical calculators, treatment algorithms, and other handy features.
Drawbacks
Because PDA screens are so small, PDA-based drug guides must compromise between level of detail and ease of use. Unlike standard drug reference books, for example, PDA programs rarely list rates of side effects or comparisons with placebo.
PDA-based drug guides usually do not display references or analyses of the data behind the lists. For example, many programs provide dosing suggestions for special populations—such as the elderly, medically ill, and children—but the basis for these dose adjustments often is unclear or does not jibe with other programs. Most PDA drug software excludes other specific information, such as the evidence behind drug indications.
Researchers’ ratings
Some PDA-based drug databases, such as mobilePDR, evolved from written pharmacy databases. Others, such as ePharmacopoeia or Epocrates, were developed from more-general reference tools aimed at students and physicians. Overall, the former type of drug guide is more comprehensive and the latter is easier to use, although all PDA drug software offers some degree of comprehensiveness and usability.
PDA-based programs differ greatly, however, and the following researchers have explored the differences.
- Enders et al1 in 2001 rated Lexi-Drugs Platinum the most comprehensive and accurate among nine programs.
- Galt and colleagues,2 placing more weight on safety concerns than ease of use, in 2005 also rated Lexi-Drugs Platinum number one among 11 programs.
- Clauson et al3 in 2004 rated Lexi-Drugs number one among 10 programs, but noted that other products were catching up.
- Knollmann et al4 in 2004 rated 11 PDA-based drug databases on ease of use, comprehensiveness, and accuracy. They entered three pairs of drug names into each program; one pair included an herbal supplement.
- Perkins et al5 in 2004 entered 37 pairs of drug names into eight programs to test their ability to detect drug-drug interactions. They found that Epocrates offered the best combination of sensitivity (identifying all potential interactions) and specificity (reporting only important interactions). Lexi-Drugs had perfect sensitivity but comparatively poor specificity.
Which program should you choose?
No PDA-based drug reference will provide everything you need, so be clear on what you desire most when choosing a program:
- If drug safety is your primary concern, Lexi-Drugs might be best, although it tends to report clinically insignificant interactions.
- If you want only clinically significant interactions, consider Epocrates or Epocrates RX Pro.
- If a comprehensive database is critical, Clinical Pharmacology OnHand has the most extensive drug database.
- If you’re looking for the best combination of accuracy, comprehensiveness, and physician-friendly features, Lexi-Drugs and PEPID PDC might be most effective, though Epocrates is also a reasonable performer.
- If you wish to understand the evidence behind each recommendation, books and online pharmaceutical references remain better options.
Cost is another factor. This might explain why Epocrates—which offers a free version for physicians and medical students—is more popular than Lexi-Drugs, which is one of the most expensive programs at $70 for the basic version and $285 for the comprehensive suite. Most programs have two options: a less expensive—or even free—drug database that costs up to $75 and a suite of features such as treatment algorithms or diagnosis databases that range from $60 to almost $300.
Personal experience is the best way to determine which product is best. Most manufacturers let you try their programs before buying.
Related resources
Chan CH, Luo JS, Kennedy RS. Concise Guide to Computers in Clinical Psychiatry. Washington DC: American Psychiatric Press; 2002
American Association for Technology in Psychiatry. www.techpsych.org
Personal digital assistant (PDA)-based drug reference software can help you make informed point-of-care prescription decisions, but accuracy, usability, and comprehensiveness vary greatly among programs.
This article looks at the benefits and drawbacks of popular PDA drug reference programs and offers advice on choosing the right one for your practice.
PDA Program benefits
Equipping your PDA with drug reference software makes prescription information portable and accessible. PDA-based drug guides also:
- are easy to update. Most PDA-based systems automatically update as part of routine synchronization procedures between the device and its host computer, keeping the information up-to-date. This is an important feature as some drug databases are updated daily.
- list potential side effects. Most programs list all potential medication side effects and distinguish between common and uncommon adverse events.
- list possible drug-drug interactions. This feature gives PDA-based drug guides a clear advantage over print textbooks. Users enter two or more medications, and the program searches for potential interactions between them.
- list interactions with alternative medications. Many databases include herbal supplements and other nontraditional pharmaceuticals.
- offer additional tools, such as medical calculators, treatment algorithms, and other handy features.
Drawbacks
Because PDA screens are so small, PDA-based drug guides must compromise between level of detail and ease of use. Unlike standard drug reference books, for example, PDA programs rarely list rates of side effects or comparisons with placebo.
PDA-based drug guides usually do not display references or analyses of the data behind the lists. For example, many programs provide dosing suggestions for special populations—such as the elderly, medically ill, and children—but the basis for these dose adjustments often is unclear or does not jibe with other programs. Most PDA drug software excludes other specific information, such as the evidence behind drug indications.
Researchers’ ratings
Some PDA-based drug databases, such as mobilePDR, evolved from written pharmacy databases. Others, such as ePharmacopoeia or Epocrates, were developed from more-general reference tools aimed at students and physicians. Overall, the former type of drug guide is more comprehensive and the latter is easier to use, although all PDA drug software offers some degree of comprehensiveness and usability.
PDA-based programs differ greatly, however, and the following researchers have explored the differences.
- Enders et al1 in 2001 rated Lexi-Drugs Platinum the most comprehensive and accurate among nine programs.
- Galt and colleagues,2 placing more weight on safety concerns than ease of use, in 2005 also rated Lexi-Drugs Platinum number one among 11 programs.
- Clauson et al3 in 2004 rated Lexi-Drugs number one among 10 programs, but noted that other products were catching up.
- Knollmann et al4 in 2004 rated 11 PDA-based drug databases on ease of use, comprehensiveness, and accuracy. They entered three pairs of drug names into each program; one pair included an herbal supplement.
- Perkins et al5 in 2004 entered 37 pairs of drug names into eight programs to test their ability to detect drug-drug interactions. They found that Epocrates offered the best combination of sensitivity (identifying all potential interactions) and specificity (reporting only important interactions). Lexi-Drugs had perfect sensitivity but comparatively poor specificity.
Which program should you choose?
No PDA-based drug reference will provide everything you need, so be clear on what you desire most when choosing a program:
- If drug safety is your primary concern, Lexi-Drugs might be best, although it tends to report clinically insignificant interactions.
- If you want only clinically significant interactions, consider Epocrates or Epocrates RX Pro.
- If a comprehensive database is critical, Clinical Pharmacology OnHand has the most extensive drug database.
- If you’re looking for the best combination of accuracy, comprehensiveness, and physician-friendly features, Lexi-Drugs and PEPID PDC might be most effective, though Epocrates is also a reasonable performer.
- If you wish to understand the evidence behind each recommendation, books and online pharmaceutical references remain better options.
Cost is another factor. This might explain why Epocrates—which offers a free version for physicians and medical students—is more popular than Lexi-Drugs, which is one of the most expensive programs at $70 for the basic version and $285 for the comprehensive suite. Most programs have two options: a less expensive—or even free—drug database that costs up to $75 and a suite of features such as treatment algorithms or diagnosis databases that range from $60 to almost $300.
Personal experience is the best way to determine which product is best. Most manufacturers let you try their programs before buying.
Related resources
Chan CH, Luo JS, Kennedy RS. Concise Guide to Computers in Clinical Psychiatry. Washington DC: American Psychiatric Press; 2002
American Association for Technology in Psychiatry. www.techpsych.org
1. Enders SJ, Enders JM, Holstad SG. Drug-information software for Palm operating system personal digital assistants: breadth, clinical dependability, and ease of use. Pharmacotherapy 2002;22:1036-40.
2. Galt KA, Rule AM, Houghton B, et al. Personal digital assistant-based drug information sources: potential to improve medication safety. J Med Librar Assoc. 2005;93:229-36.
3. Clauson KA, Seamon MJ, Clauson AS, et al. Evaluation of drug information databases for personal digital assistants. Am J Health Syst Pharm 2004;61:1015-24.
4. Knollmann BC, Smyth BJ, Garnett CE, et al. Personal digital assistant-based drug reference software as tools to improve rational prescribing: benchmark criteria and performance. Clin Pharmacol Ther 2005;78:7-18.
5. Perkins NA, Murphy JE, Malone DC, Armstrong EP. Performance of drug-drug interaction software for personal digital assistants. Ann Pharmacother 2006;40:850-5.
1. Enders SJ, Enders JM, Holstad SG. Drug-information software for Palm operating system personal digital assistants: breadth, clinical dependability, and ease of use. Pharmacotherapy 2002;22:1036-40.
2. Galt KA, Rule AM, Houghton B, et al. Personal digital assistant-based drug information sources: potential to improve medication safety. J Med Librar Assoc. 2005;93:229-36.
3. Clauson KA, Seamon MJ, Clauson AS, et al. Evaluation of drug information databases for personal digital assistants. Am J Health Syst Pharm 2004;61:1015-24.
4. Knollmann BC, Smyth BJ, Garnett CE, et al. Personal digital assistant-based drug reference software as tools to improve rational prescribing: benchmark criteria and performance. Clin Pharmacol Ther 2005;78:7-18.
5. Perkins NA, Murphy JE, Malone DC, Armstrong EP. Performance of drug-drug interaction software for personal digital assistants. Ann Pharmacother 2006;40:850-5.
Paliperidone
Paliperidone, a second-generation antipsychotic (SGA), was awaiting FDA approval for treating schizophrenia (Table 1) at press time. FDA issued an approvable letter September 29.
The long-acting oral medication can be given once daily, without the plasma level peaks and troughs associated with other SGAs.
Table 1
Paliperidone: Fast facts
| Class: Second-generation antipsychotic Prospective indication: Schizophrenia FDA action: Issued approvable letter September 29, 2006 Manufacturer: Janssen Pharmaceutica Dosing forms: Not determined Recommended dosage: Data suggest that 6 mg/d is a suitable starting dosage for most patients.1-3 No formal recommendation issued |
Clinical implications
Paliperidone—the active metabolite of risperidone (9-hydroxy risperidone)—produces the same effects as its parent compound. Because it is metabolized less by the liver, however, paliperidone will likely have a lower risk of drug-drug interactions than risperidone.
Paliperidone can help patients with hallucinations, delusions, and other florid psychotic symptoms. Once-daily dosing could also make it easier for patients with schizophrenia to adhere to treatment.
Paliperidone caused relatively few side effects in clinical trials, indicating that the drug could be started at therapeutic dosages. A 6-mg dose reaches clinically effective plasma levels in approximately 22 hours; a higher dosage might take less time.
How it works
As with all antipsychotics, paliperidone blocks dopamine uptake (D2 receptors) and—as with other newer SGAs—it has a high affinity for 5-HT (serotonin) receptors. This serotonergic action may help modulate side effects.
Paliperidone has shown antipsychotic effectiveness at 3 to 15 mg/d, and a 6-mg dosage is no more likely to cause extrapyramidal symptoms (EPS) or other adverse events than olanzapine, 10 mg/d, or placebo.1-3 This finding suggests that 6 mg/d might be a suitable starting dosage for most patients.
Clinical findings
Efficacy. The drug showed efficacy for treating acute schizophrenia in three randomized, double-blind, controlled trials.
In a 6-week study,1 444 patients who were experiencing acute schizophrenia episodes and had Positive and Negative Syndrome Scale (PANSS) scores between 70 and 120 received paliperidone, 6 or 12 mg/d, olanzapine, 10 mg/d, or placebo. The study was powered to compare paliperidone and placebo; the olanzapine group was included to confirm study sensitivity.
Mean baseline total and negative symptom PANSS scores improved twice as much among the paliperidone and olanzapine groups compared with placebo (Table 2). Personal and Social Performance (PSP) scale scores also improved significantly among patients receiving paliperidone, 6 mg/d. The PSP scale gauges function, ability to perform socially useful activities such as self-care and work, and disturbing and aggressive behavior.
In two similarly designed, 6-week studies (N=1,248),2,3 paliperidone at 3, 6, 9, or 12 mg/d produced statistically significant improvement in total and negative symptom PANSS scores and PSP scale scores compared with placebo.
Table 2
Mean PANSS score reductions among patients taking paliperidone, olanzapine, or placebo
| Paliperidone, 6 mg/d | Paliperidone, 12 mg/d | Olanzapine, 10 mg/d | Placebo | |
|---|---|---|---|---|
| Total PANSS score | 17.5 | 17.5 | 18.4 | 8.0 |
| Negative symptom PANSS score | 4.4 | 3.9 | 4.4 | 2.2 |
| Source: Reference 1 | ||||
Relapse prevention. Kramer et al4 measured paliperidone’s ability to prevent or delay schizophrenia recurrence among 205 patients experiencing an episode. Participants had been diagnosed with schizophrenia at least 1 year earlier and had PANSS total scores between 70 and 120. Patients with substance dependence or other axis I disorders were excluded.
Over an 8-week run-in period, patients were hospitalized for 2 weeks and started on open-label paliperidone, 9 mg/d; dosages then were titrated to 3 to 15 mg/d depending on efficacy and tolerability. Once stable for 2 weeks, subjects were discharged and maintained on paliperidone for 6 weeks, then were randomized to a double-blind phase during which they received a similar dosage of paliperidone or placebo. Regimen duration varied during the double-blind phase.
The study was stopped after 2 months when an interim analysis showed significant efficacy for paliperidone. Among patients who relapsed, mean time to relapse was 68 days among patients taking paliperidone (n=14, 25%), compared with 25 days in the placebo group (n=29, 53%).
Recurrence was defined as:
- psychiatric hospitalization
- total PANSS score ≥40 at randomization decreased by 25% for 2 days, or total PANSS score
- Clinical Global Impression of Severity (CGI-S) score ≥3 at randomization increased to ≥4 for 2 days, or CGI-S score ≥4 at randomization rose to ≥5
- individual-item PANSS baseline score ≥3 increased to ≥5 for 2 days, or baseline score ≥4 rose to ≥6
- self injury, suicidal or homicidal thoughts, or clinically significant aggressive behavior.
A final analysis showed a 22% relapse rate among patients taking paliperidone vs. 52% of the placebo group. Paliperidone was associated with improvements in PANSS, CGI-S, PSP, and quality of life measures at all dosages.
Safety
Compared with placebo, mean prolactin levels in the long-term study were four times as high among men (40 vs. 10 ng/mL) and five times as high among women (100 vs. 20 ng/mL) who received paliperidone at any dosage.4 Also, EPS such as dystonia and hyperkinesis were more prevalent at 12 mg/d (10%) than at 6 mg/d (5%).1,2
During paliperidone therapy, patients should be asked if they are experiencing abnormal movements, sexual dysfunction, breast enlargement, or irregular menstruation (women). If so, decrease the dosage by 3 mg and monitor for side effects and clinical efficacy.
Among other reported adverse effects in the efficacy studies:
- somnolence was less prevalent among patients receiving either paliperidone, 3 to 12 mg/d, or placebo (13%) than among those receiving olanzapine, 10 mg/d (25%)3
- tachycardia was more prevalent among the paliperidone and olanzapine groups (14% to 20%) compared with placebo (0%)1-3
- headache prevalence (10% to 20%) was similar in all groups1-3
- mean body weight changes after 6 weeks were more pronounced in the olanzapine group (1.3±2.8 kg) than among patients receiving placebo (–0.7±2.4 kg) or paliperidone at 6 mg/d (0.2±2.4 kg), 9 mg/d (0.6±2.7 kg), or 12 mg/d (0.6±2.6 kg).2
In the relapse prevention study,4 potential neuroleptic malignant syndrome was reported in 1 patient taking paliperidone 3 days after the patient withdrew from the study. The patient had received paliperidone for 19 days and was treated for EPS with an unknown medication during the run-in period. Medical status returned to normal at endpoint except for elevated creatine kinase.
Discontinuation rates in the relapse prevention study’s double-blind phase were greater among the paliperidone group (n=20, 19%) compared with placebo (n=8, 8%).4 The reasons paliperidone group patients withdrew consent were not available.
Related resources
- Karlsson P, Dencker E, Nyberg S, et al. Pharmacokinetics, dopamine D2 and serotonin 5-HT2A receptor occupancy and safety profile of paliperidone extended-release in healthy subjects. Poster presented at: Winter Workshop on Schizophrenia (WWS); February 5-11, 2006; Davos, Switzerland.
Drug brand names
- Olanzapine • Zyprexa
- Risperidone • Risperdal
Disclosures
Dr. Simpson receives grant support from AstraZeneca Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, and Johnson & Johnson. He is a consultant to Janssen Pharmaceutica, Johnson & Johnson, Merck and Co., and Pfizer, and is a speaker for Janssen Pharmaceutica and Pfizer.
1. Marder S, Kramer M, Ford L, et al. Efficacy and safety of oral paliperidone extended-release tablets in treatment of schizophrenia: a 6-week placebo control study. Poster presented at: Winter Workshop on Schizophrenia (WWS); February 5-11, 2006; Davos, Switzerland.
2. Kane J, Kramer J, Ford L, et al. Treatment of schizophrenia using oral paliperidone extended-release tablets: a 6-week placebo-controlled study. Poster presented at: WWS; February 5-11, 2006; Davos, Switzerland.
3. Davidson M, Emsley R, Kramer M, et al. Efficacy, safety and effect on functioning of paliperidone extended-release in healthy subjects. Poster presented at: WWS; February 5-11, 2006; Davos, Switzerland.
4. Kramer M, Simpson G, Maciulis V, et al. Paliperidone extended release tablets for prevention of symptom recurrence in patients with schizophrenia: A randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol (in press).
Paliperidone, a second-generation antipsychotic (SGA), was awaiting FDA approval for treating schizophrenia (Table 1) at press time. FDA issued an approvable letter September 29.
The long-acting oral medication can be given once daily, without the plasma level peaks and troughs associated with other SGAs.
Table 1
Paliperidone: Fast facts
| Class: Second-generation antipsychotic Prospective indication: Schizophrenia FDA action: Issued approvable letter September 29, 2006 Manufacturer: Janssen Pharmaceutica Dosing forms: Not determined Recommended dosage: Data suggest that 6 mg/d is a suitable starting dosage for most patients.1-3 No formal recommendation issued |
Clinical implications
Paliperidone—the active metabolite of risperidone (9-hydroxy risperidone)—produces the same effects as its parent compound. Because it is metabolized less by the liver, however, paliperidone will likely have a lower risk of drug-drug interactions than risperidone.
Paliperidone can help patients with hallucinations, delusions, and other florid psychotic symptoms. Once-daily dosing could also make it easier for patients with schizophrenia to adhere to treatment.
Paliperidone caused relatively few side effects in clinical trials, indicating that the drug could be started at therapeutic dosages. A 6-mg dose reaches clinically effective plasma levels in approximately 22 hours; a higher dosage might take less time.
How it works
As with all antipsychotics, paliperidone blocks dopamine uptake (D2 receptors) and—as with other newer SGAs—it has a high affinity for 5-HT (serotonin) receptors. This serotonergic action may help modulate side effects.
Paliperidone has shown antipsychotic effectiveness at 3 to 15 mg/d, and a 6-mg dosage is no more likely to cause extrapyramidal symptoms (EPS) or other adverse events than olanzapine, 10 mg/d, or placebo.1-3 This finding suggests that 6 mg/d might be a suitable starting dosage for most patients.
Clinical findings
Efficacy. The drug showed efficacy for treating acute schizophrenia in three randomized, double-blind, controlled trials.
In a 6-week study,1 444 patients who were experiencing acute schizophrenia episodes and had Positive and Negative Syndrome Scale (PANSS) scores between 70 and 120 received paliperidone, 6 or 12 mg/d, olanzapine, 10 mg/d, or placebo. The study was powered to compare paliperidone and placebo; the olanzapine group was included to confirm study sensitivity.
Mean baseline total and negative symptom PANSS scores improved twice as much among the paliperidone and olanzapine groups compared with placebo (Table 2). Personal and Social Performance (PSP) scale scores also improved significantly among patients receiving paliperidone, 6 mg/d. The PSP scale gauges function, ability to perform socially useful activities such as self-care and work, and disturbing and aggressive behavior.
In two similarly designed, 6-week studies (N=1,248),2,3 paliperidone at 3, 6, 9, or 12 mg/d produced statistically significant improvement in total and negative symptom PANSS scores and PSP scale scores compared with placebo.
Table 2
Mean PANSS score reductions among patients taking paliperidone, olanzapine, or placebo
| Paliperidone, 6 mg/d | Paliperidone, 12 mg/d | Olanzapine, 10 mg/d | Placebo | |
|---|---|---|---|---|
| Total PANSS score | 17.5 | 17.5 | 18.4 | 8.0 |
| Negative symptom PANSS score | 4.4 | 3.9 | 4.4 | 2.2 |
| Source: Reference 1 | ||||
Relapse prevention. Kramer et al4 measured paliperidone’s ability to prevent or delay schizophrenia recurrence among 205 patients experiencing an episode. Participants had been diagnosed with schizophrenia at least 1 year earlier and had PANSS total scores between 70 and 120. Patients with substance dependence or other axis I disorders were excluded.
Over an 8-week run-in period, patients were hospitalized for 2 weeks and started on open-label paliperidone, 9 mg/d; dosages then were titrated to 3 to 15 mg/d depending on efficacy and tolerability. Once stable for 2 weeks, subjects were discharged and maintained on paliperidone for 6 weeks, then were randomized to a double-blind phase during which they received a similar dosage of paliperidone or placebo. Regimen duration varied during the double-blind phase.
The study was stopped after 2 months when an interim analysis showed significant efficacy for paliperidone. Among patients who relapsed, mean time to relapse was 68 days among patients taking paliperidone (n=14, 25%), compared with 25 days in the placebo group (n=29, 53%).
Recurrence was defined as:
- psychiatric hospitalization
- total PANSS score ≥40 at randomization decreased by 25% for 2 days, or total PANSS score
- Clinical Global Impression of Severity (CGI-S) score ≥3 at randomization increased to ≥4 for 2 days, or CGI-S score ≥4 at randomization rose to ≥5
- individual-item PANSS baseline score ≥3 increased to ≥5 for 2 days, or baseline score ≥4 rose to ≥6
- self injury, suicidal or homicidal thoughts, or clinically significant aggressive behavior.
A final analysis showed a 22% relapse rate among patients taking paliperidone vs. 52% of the placebo group. Paliperidone was associated with improvements in PANSS, CGI-S, PSP, and quality of life measures at all dosages.
Safety
Compared with placebo, mean prolactin levels in the long-term study were four times as high among men (40 vs. 10 ng/mL) and five times as high among women (100 vs. 20 ng/mL) who received paliperidone at any dosage.4 Also, EPS such as dystonia and hyperkinesis were more prevalent at 12 mg/d (10%) than at 6 mg/d (5%).1,2
During paliperidone therapy, patients should be asked if they are experiencing abnormal movements, sexual dysfunction, breast enlargement, or irregular menstruation (women). If so, decrease the dosage by 3 mg and monitor for side effects and clinical efficacy.
Among other reported adverse effects in the efficacy studies:
- somnolence was less prevalent among patients receiving either paliperidone, 3 to 12 mg/d, or placebo (13%) than among those receiving olanzapine, 10 mg/d (25%)3
- tachycardia was more prevalent among the paliperidone and olanzapine groups (14% to 20%) compared with placebo (0%)1-3
- headache prevalence (10% to 20%) was similar in all groups1-3
- mean body weight changes after 6 weeks were more pronounced in the olanzapine group (1.3±2.8 kg) than among patients receiving placebo (–0.7±2.4 kg) or paliperidone at 6 mg/d (0.2±2.4 kg), 9 mg/d (0.6±2.7 kg), or 12 mg/d (0.6±2.6 kg).2
In the relapse prevention study,4 potential neuroleptic malignant syndrome was reported in 1 patient taking paliperidone 3 days after the patient withdrew from the study. The patient had received paliperidone for 19 days and was treated for EPS with an unknown medication during the run-in period. Medical status returned to normal at endpoint except for elevated creatine kinase.
Discontinuation rates in the relapse prevention study’s double-blind phase were greater among the paliperidone group (n=20, 19%) compared with placebo (n=8, 8%).4 The reasons paliperidone group patients withdrew consent were not available.
Related resources
- Karlsson P, Dencker E, Nyberg S, et al. Pharmacokinetics, dopamine D2 and serotonin 5-HT2A receptor occupancy and safety profile of paliperidone extended-release in healthy subjects. Poster presented at: Winter Workshop on Schizophrenia (WWS); February 5-11, 2006; Davos, Switzerland.
Drug brand names
- Olanzapine • Zyprexa
- Risperidone • Risperdal
Disclosures
Dr. Simpson receives grant support from AstraZeneca Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, and Johnson & Johnson. He is a consultant to Janssen Pharmaceutica, Johnson & Johnson, Merck and Co., and Pfizer, and is a speaker for Janssen Pharmaceutica and Pfizer.
Paliperidone, a second-generation antipsychotic (SGA), was awaiting FDA approval for treating schizophrenia (Table 1) at press time. FDA issued an approvable letter September 29.
The long-acting oral medication can be given once daily, without the plasma level peaks and troughs associated with other SGAs.
Table 1
Paliperidone: Fast facts
| Class: Second-generation antipsychotic Prospective indication: Schizophrenia FDA action: Issued approvable letter September 29, 2006 Manufacturer: Janssen Pharmaceutica Dosing forms: Not determined Recommended dosage: Data suggest that 6 mg/d is a suitable starting dosage for most patients.1-3 No formal recommendation issued |
Clinical implications
Paliperidone—the active metabolite of risperidone (9-hydroxy risperidone)—produces the same effects as its parent compound. Because it is metabolized less by the liver, however, paliperidone will likely have a lower risk of drug-drug interactions than risperidone.
Paliperidone can help patients with hallucinations, delusions, and other florid psychotic symptoms. Once-daily dosing could also make it easier for patients with schizophrenia to adhere to treatment.
Paliperidone caused relatively few side effects in clinical trials, indicating that the drug could be started at therapeutic dosages. A 6-mg dose reaches clinically effective plasma levels in approximately 22 hours; a higher dosage might take less time.
How it works
As with all antipsychotics, paliperidone blocks dopamine uptake (D2 receptors) and—as with other newer SGAs—it has a high affinity for 5-HT (serotonin) receptors. This serotonergic action may help modulate side effects.
Paliperidone has shown antipsychotic effectiveness at 3 to 15 mg/d, and a 6-mg dosage is no more likely to cause extrapyramidal symptoms (EPS) or other adverse events than olanzapine, 10 mg/d, or placebo.1-3 This finding suggests that 6 mg/d might be a suitable starting dosage for most patients.
Clinical findings
Efficacy. The drug showed efficacy for treating acute schizophrenia in three randomized, double-blind, controlled trials.
In a 6-week study,1 444 patients who were experiencing acute schizophrenia episodes and had Positive and Negative Syndrome Scale (PANSS) scores between 70 and 120 received paliperidone, 6 or 12 mg/d, olanzapine, 10 mg/d, or placebo. The study was powered to compare paliperidone and placebo; the olanzapine group was included to confirm study sensitivity.
Mean baseline total and negative symptom PANSS scores improved twice as much among the paliperidone and olanzapine groups compared with placebo (Table 2). Personal and Social Performance (PSP) scale scores also improved significantly among patients receiving paliperidone, 6 mg/d. The PSP scale gauges function, ability to perform socially useful activities such as self-care and work, and disturbing and aggressive behavior.
In two similarly designed, 6-week studies (N=1,248),2,3 paliperidone at 3, 6, 9, or 12 mg/d produced statistically significant improvement in total and negative symptom PANSS scores and PSP scale scores compared with placebo.
Table 2
Mean PANSS score reductions among patients taking paliperidone, olanzapine, or placebo
| Paliperidone, 6 mg/d | Paliperidone, 12 mg/d | Olanzapine, 10 mg/d | Placebo | |
|---|---|---|---|---|
| Total PANSS score | 17.5 | 17.5 | 18.4 | 8.0 |
| Negative symptom PANSS score | 4.4 | 3.9 | 4.4 | 2.2 |
| Source: Reference 1 | ||||
Relapse prevention. Kramer et al4 measured paliperidone’s ability to prevent or delay schizophrenia recurrence among 205 patients experiencing an episode. Participants had been diagnosed with schizophrenia at least 1 year earlier and had PANSS total scores between 70 and 120. Patients with substance dependence or other axis I disorders were excluded.
Over an 8-week run-in period, patients were hospitalized for 2 weeks and started on open-label paliperidone, 9 mg/d; dosages then were titrated to 3 to 15 mg/d depending on efficacy and tolerability. Once stable for 2 weeks, subjects were discharged and maintained on paliperidone for 6 weeks, then were randomized to a double-blind phase during which they received a similar dosage of paliperidone or placebo. Regimen duration varied during the double-blind phase.
The study was stopped after 2 months when an interim analysis showed significant efficacy for paliperidone. Among patients who relapsed, mean time to relapse was 68 days among patients taking paliperidone (n=14, 25%), compared with 25 days in the placebo group (n=29, 53%).
Recurrence was defined as:
- psychiatric hospitalization
- total PANSS score ≥40 at randomization decreased by 25% for 2 days, or total PANSS score
- Clinical Global Impression of Severity (CGI-S) score ≥3 at randomization increased to ≥4 for 2 days, or CGI-S score ≥4 at randomization rose to ≥5
- individual-item PANSS baseline score ≥3 increased to ≥5 for 2 days, or baseline score ≥4 rose to ≥6
- self injury, suicidal or homicidal thoughts, or clinically significant aggressive behavior.
A final analysis showed a 22% relapse rate among patients taking paliperidone vs. 52% of the placebo group. Paliperidone was associated with improvements in PANSS, CGI-S, PSP, and quality of life measures at all dosages.
Safety
Compared with placebo, mean prolactin levels in the long-term study were four times as high among men (40 vs. 10 ng/mL) and five times as high among women (100 vs. 20 ng/mL) who received paliperidone at any dosage.4 Also, EPS such as dystonia and hyperkinesis were more prevalent at 12 mg/d (10%) than at 6 mg/d (5%).1,2
During paliperidone therapy, patients should be asked if they are experiencing abnormal movements, sexual dysfunction, breast enlargement, or irregular menstruation (women). If so, decrease the dosage by 3 mg and monitor for side effects and clinical efficacy.
Among other reported adverse effects in the efficacy studies:
- somnolence was less prevalent among patients receiving either paliperidone, 3 to 12 mg/d, or placebo (13%) than among those receiving olanzapine, 10 mg/d (25%)3
- tachycardia was more prevalent among the paliperidone and olanzapine groups (14% to 20%) compared with placebo (0%)1-3
- headache prevalence (10% to 20%) was similar in all groups1-3
- mean body weight changes after 6 weeks were more pronounced in the olanzapine group (1.3±2.8 kg) than among patients receiving placebo (–0.7±2.4 kg) or paliperidone at 6 mg/d (0.2±2.4 kg), 9 mg/d (0.6±2.7 kg), or 12 mg/d (0.6±2.6 kg).2
In the relapse prevention study,4 potential neuroleptic malignant syndrome was reported in 1 patient taking paliperidone 3 days after the patient withdrew from the study. The patient had received paliperidone for 19 days and was treated for EPS with an unknown medication during the run-in period. Medical status returned to normal at endpoint except for elevated creatine kinase.
Discontinuation rates in the relapse prevention study’s double-blind phase were greater among the paliperidone group (n=20, 19%) compared with placebo (n=8, 8%).4 The reasons paliperidone group patients withdrew consent were not available.
Related resources
- Karlsson P, Dencker E, Nyberg S, et al. Pharmacokinetics, dopamine D2 and serotonin 5-HT2A receptor occupancy and safety profile of paliperidone extended-release in healthy subjects. Poster presented at: Winter Workshop on Schizophrenia (WWS); February 5-11, 2006; Davos, Switzerland.
Drug brand names
- Olanzapine • Zyprexa
- Risperidone • Risperdal
Disclosures
Dr. Simpson receives grant support from AstraZeneca Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, and Johnson & Johnson. He is a consultant to Janssen Pharmaceutica, Johnson & Johnson, Merck and Co., and Pfizer, and is a speaker for Janssen Pharmaceutica and Pfizer.
1. Marder S, Kramer M, Ford L, et al. Efficacy and safety of oral paliperidone extended-release tablets in treatment of schizophrenia: a 6-week placebo control study. Poster presented at: Winter Workshop on Schizophrenia (WWS); February 5-11, 2006; Davos, Switzerland.
2. Kane J, Kramer J, Ford L, et al. Treatment of schizophrenia using oral paliperidone extended-release tablets: a 6-week placebo-controlled study. Poster presented at: WWS; February 5-11, 2006; Davos, Switzerland.
3. Davidson M, Emsley R, Kramer M, et al. Efficacy, safety and effect on functioning of paliperidone extended-release in healthy subjects. Poster presented at: WWS; February 5-11, 2006; Davos, Switzerland.
4. Kramer M, Simpson G, Maciulis V, et al. Paliperidone extended release tablets for prevention of symptom recurrence in patients with schizophrenia: A randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol (in press).
1. Marder S, Kramer M, Ford L, et al. Efficacy and safety of oral paliperidone extended-release tablets in treatment of schizophrenia: a 6-week placebo control study. Poster presented at: Winter Workshop on Schizophrenia (WWS); February 5-11, 2006; Davos, Switzerland.
2. Kane J, Kramer J, Ford L, et al. Treatment of schizophrenia using oral paliperidone extended-release tablets: a 6-week placebo-controlled study. Poster presented at: WWS; February 5-11, 2006; Davos, Switzerland.
3. Davidson M, Emsley R, Kramer M, et al. Efficacy, safety and effect on functioning of paliperidone extended-release in healthy subjects. Poster presented at: WWS; February 5-11, 2006; Davos, Switzerland.
4. Kramer M, Simpson G, Maciulis V, et al. Paliperidone extended release tablets for prevention of symptom recurrence in patients with schizophrenia: A randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol (in press).
10 tips for working with a drug formulary
Medicare, private HMOs, and Veterans Affairs medical centers justify drug formularies by claiming lower co-payments and insurance premiums and cost-effective health care without diminishing quality. But some physicians believe having to prescribe from a list interferes in medical practice and reduces clinical autonomy.
You may view having to request a consult as a hassle when you wish to prescribe a nonformulary drug. A thoroughly researched nonformulary request can expedite the process, however.
5 strategies for nonformulary requests
- Reference evidence-based information to support your request.
- Document your patient’s drug trials, dosages, clinical response failure, and adverse effects to expedite review of nonformulary requests.
- Ask the patient about family history of response to a medication. Such pharmacogenetic data may predict response in first-degree relatives and support a nonformulary request.
- Document pharmacokinetic or pharmacodynamic interactions between the formulary drug and other medications the patient is taking.
- List known interactions with foods and diseases.
5 tips for practicing within a formulary
Physicians are not alone in being leery of drug formularies. Patients encouraged by direct-to-consumer advertising ask for the newest—and often most expensive medications—may share that wariness.1 To help us work within the restrictions of a drug formulary system and provide appropriate patient care, we suggest the following helpful strategies:
- Understand that formularies are well intentioned and highly calculated. Lists are updated frequently to represent physicians’ and other experts’ clinical judgment on the use of safe, appropriate, and cost-effective medications, therapies, and health products that best serve patients.2
- Address patient concerns to help them accept and adhere to prescribed formulary drugs. Emphasize that a medication’s cost does not determine its efficacy.
- Remember that your duty to provide proper treatment supersedes cost considerations.
- Ask for a physician review when your request for a nonformulary drug is denied.
- Use nonpharmacologic treatments such as sleep hygiene, cognitive-behavioral therapy, or relaxation techniques when possible. This approach reduces polypharmacy and keeps costs low.
Medicare, private HMOs, and Veterans Affairs medical centers justify drug formularies by claiming lower co-payments and insurance premiums and cost-effective health care without diminishing quality. But some physicians believe having to prescribe from a list interferes in medical practice and reduces clinical autonomy.
You may view having to request a consult as a hassle when you wish to prescribe a nonformulary drug. A thoroughly researched nonformulary request can expedite the process, however.
5 strategies for nonformulary requests
- Reference evidence-based information to support your request.
- Document your patient’s drug trials, dosages, clinical response failure, and adverse effects to expedite review of nonformulary requests.
- Ask the patient about family history of response to a medication. Such pharmacogenetic data may predict response in first-degree relatives and support a nonformulary request.
- Document pharmacokinetic or pharmacodynamic interactions between the formulary drug and other medications the patient is taking.
- List known interactions with foods and diseases.
5 tips for practicing within a formulary
Physicians are not alone in being leery of drug formularies. Patients encouraged by direct-to-consumer advertising ask for the newest—and often most expensive medications—may share that wariness.1 To help us work within the restrictions of a drug formulary system and provide appropriate patient care, we suggest the following helpful strategies:
- Understand that formularies are well intentioned and highly calculated. Lists are updated frequently to represent physicians’ and other experts’ clinical judgment on the use of safe, appropriate, and cost-effective medications, therapies, and health products that best serve patients.2
- Address patient concerns to help them accept and adhere to prescribed formulary drugs. Emphasize that a medication’s cost does not determine its efficacy.
- Remember that your duty to provide proper treatment supersedes cost considerations.
- Ask for a physician review when your request for a nonformulary drug is denied.
- Use nonpharmacologic treatments such as sleep hygiene, cognitive-behavioral therapy, or relaxation techniques when possible. This approach reduces polypharmacy and keeps costs low.
Medicare, private HMOs, and Veterans Affairs medical centers justify drug formularies by claiming lower co-payments and insurance premiums and cost-effective health care without diminishing quality. But some physicians believe having to prescribe from a list interferes in medical practice and reduces clinical autonomy.
You may view having to request a consult as a hassle when you wish to prescribe a nonformulary drug. A thoroughly researched nonformulary request can expedite the process, however.
5 strategies for nonformulary requests
- Reference evidence-based information to support your request.
- Document your patient’s drug trials, dosages, clinical response failure, and adverse effects to expedite review of nonformulary requests.
- Ask the patient about family history of response to a medication. Such pharmacogenetic data may predict response in first-degree relatives and support a nonformulary request.
- Document pharmacokinetic or pharmacodynamic interactions between the formulary drug and other medications the patient is taking.
- List known interactions with foods and diseases.
5 tips for practicing within a formulary
Physicians are not alone in being leery of drug formularies. Patients encouraged by direct-to-consumer advertising ask for the newest—and often most expensive medications—may share that wariness.1 To help us work within the restrictions of a drug formulary system and provide appropriate patient care, we suggest the following helpful strategies:
- Understand that formularies are well intentioned and highly calculated. Lists are updated frequently to represent physicians’ and other experts’ clinical judgment on the use of safe, appropriate, and cost-effective medications, therapies, and health products that best serve patients.2
- Address patient concerns to help them accept and adhere to prescribed formulary drugs. Emphasize that a medication’s cost does not determine its efficacy.
- Remember that your duty to provide proper treatment supersedes cost considerations.
- Ask for a physician review when your request for a nonformulary drug is denied.
- Use nonpharmacologic treatments such as sleep hygiene, cognitive-behavioral therapy, or relaxation techniques when possible. This approach reduces polypharmacy and keeps costs low.
6 safety rules for tapering antidepressants
Side effects to discontinuing serotonin reuptake inhibitor (SRI) treatment are common and may be severe.1 Patients who are not prepared for these reactions may attribute symptoms to other causes such as a medical illness. Educate your patient about potential side effects to mitigate problems such as relapse or patient distress.
1 Warn your patient
Alert patients to potential discontinuation reactions when prescribing any antidepressant, particularly SRIs because they seem to cause more discontinuation problems than other classes of drugs. Although reactions appear to be more common and severe with short-acting drugs such as venlafaxine and paroxetine, they can occur with longer half-life agents such as fluoxetine and sertraline.
Warn patients about discontinuation effects when starting treatment and before the planned drug taper. In particular, caution them against missing doses or stopping a drug without informing you.
2 Know the symptoms
Common discontinuation symptoms can be grouped into six areas:
- Neurosensory—vertigo, paresthesias, shock-like reactions, myalgia
- Neuromotor—tremor, myoclonus, ataxia, visual changes, piloerection
- Gastrointestinal—nausea, vomiting, diarrhea
- Psychiatric—anxiety, depressed mood, suicidal ideation, irritability
- Vasomotor—flushing, diaphoresis
- Other neuropsychiatric—anorexia, insomnia, vivid dreams, asthenia, chills.2
3 Distinguish discontinuation reactions from relapse
Although depressed mood and anxiety may occur during taper, these symptoms tend to be transient in most patients. Severe or persistent symptoms—including emerging suicidal ideation—may indicate a relapse.
4 Reduce medication slowly
Tapering is recommended for all antidepressants but should be particularly slow for certain drugs—including venlafaxine, paroxetine, and clomipramine—which can cause significant discontinuation effects. For example, venlafaxine at 225 mg/d could be reduced by 75 mg/d every 1 to 2 weeks, with a final step at 37.5 mg/d for at least 1 week.
If discontinuation reactions are a problem, ultimate discontinuation may require substituting a longer-acting medication such as fluoxetine during the tapering period. For example, add fluoxetine, 10 to 20 mg/d, for 1 week, then stop both antidepressants or discontinue the first medication and continue fluoxetine for 2 to 3 weeks until the risk of reactions passes.
Clinicians often are concerned about serotonin syndrome caused by combining SRIs. Isolated cases have been reported, but the small, finite chance of serotonin syndrome is much lower than the risk of severe discontinuation reactions.
5 Titrate up and taper down
When switching to another SRI, titrate the second drug upward while tapering off the first. Remember that changing to a drug that does not act on serotonin, such as bupropion, can protect against discontinuation effects.
6 Allow for pregnancy
Infants born to mothers taking antidepressants can exhibit discontinuation symptoms,2 particularly with shorter-acting drugs such as paroxetine or venlafaxine. Consider tapering the antidepressant early in the patient’s third trimester, then re-institute treatment after delivery. If an antidepressant is required during pregnancy, try using one with a longer half-life such as fluoxetine.
Drug brand names
- Bupropion • Wellbutrin
- Clomipramine • Anafranil
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
1. Schatzberg AF, Blier P, Delgado PL, et al. Antidepressant discontinuation syndrome: consensus panel recommendations for clinical management and additional research. J Clin Psychiatry 2006;67 (suppl 4):27-30.
2. Shelton RC. The nature of the discontinuation syndrome associated with antidepressant drugs. J Clin Psychiatry 2006;67(suppl 4):3-7.
Dr. Shelton is the James G. Blakemore Research Professor and vice-chair for research, department of psychiatry, and professor of pharmacology, Vanderbilt University, Nashville, TN.
Side effects to discontinuing serotonin reuptake inhibitor (SRI) treatment are common and may be severe.1 Patients who are not prepared for these reactions may attribute symptoms to other causes such as a medical illness. Educate your patient about potential side effects to mitigate problems such as relapse or patient distress.
1 Warn your patient
Alert patients to potential discontinuation reactions when prescribing any antidepressant, particularly SRIs because they seem to cause more discontinuation problems than other classes of drugs. Although reactions appear to be more common and severe with short-acting drugs such as venlafaxine and paroxetine, they can occur with longer half-life agents such as fluoxetine and sertraline.
Warn patients about discontinuation effects when starting treatment and before the planned drug taper. In particular, caution them against missing doses or stopping a drug without informing you.
2 Know the symptoms
Common discontinuation symptoms can be grouped into six areas:
- Neurosensory—vertigo, paresthesias, shock-like reactions, myalgia
- Neuromotor—tremor, myoclonus, ataxia, visual changes, piloerection
- Gastrointestinal—nausea, vomiting, diarrhea
- Psychiatric—anxiety, depressed mood, suicidal ideation, irritability
- Vasomotor—flushing, diaphoresis
- Other neuropsychiatric—anorexia, insomnia, vivid dreams, asthenia, chills.2
3 Distinguish discontinuation reactions from relapse
Although depressed mood and anxiety may occur during taper, these symptoms tend to be transient in most patients. Severe or persistent symptoms—including emerging suicidal ideation—may indicate a relapse.
4 Reduce medication slowly
Tapering is recommended for all antidepressants but should be particularly slow for certain drugs—including venlafaxine, paroxetine, and clomipramine—which can cause significant discontinuation effects. For example, venlafaxine at 225 mg/d could be reduced by 75 mg/d every 1 to 2 weeks, with a final step at 37.5 mg/d for at least 1 week.
If discontinuation reactions are a problem, ultimate discontinuation may require substituting a longer-acting medication such as fluoxetine during the tapering period. For example, add fluoxetine, 10 to 20 mg/d, for 1 week, then stop both antidepressants or discontinue the first medication and continue fluoxetine for 2 to 3 weeks until the risk of reactions passes.
Clinicians often are concerned about serotonin syndrome caused by combining SRIs. Isolated cases have been reported, but the small, finite chance of serotonin syndrome is much lower than the risk of severe discontinuation reactions.
5 Titrate up and taper down
When switching to another SRI, titrate the second drug upward while tapering off the first. Remember that changing to a drug that does not act on serotonin, such as bupropion, can protect against discontinuation effects.
6 Allow for pregnancy
Infants born to mothers taking antidepressants can exhibit discontinuation symptoms,2 particularly with shorter-acting drugs such as paroxetine or venlafaxine. Consider tapering the antidepressant early in the patient’s third trimester, then re-institute treatment after delivery. If an antidepressant is required during pregnancy, try using one with a longer half-life such as fluoxetine.
Drug brand names
- Bupropion • Wellbutrin
- Clomipramine • Anafranil
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Side effects to discontinuing serotonin reuptake inhibitor (SRI) treatment are common and may be severe.1 Patients who are not prepared for these reactions may attribute symptoms to other causes such as a medical illness. Educate your patient about potential side effects to mitigate problems such as relapse or patient distress.
1 Warn your patient
Alert patients to potential discontinuation reactions when prescribing any antidepressant, particularly SRIs because they seem to cause more discontinuation problems than other classes of drugs. Although reactions appear to be more common and severe with short-acting drugs such as venlafaxine and paroxetine, they can occur with longer half-life agents such as fluoxetine and sertraline.
Warn patients about discontinuation effects when starting treatment and before the planned drug taper. In particular, caution them against missing doses or stopping a drug without informing you.
2 Know the symptoms
Common discontinuation symptoms can be grouped into six areas:
- Neurosensory—vertigo, paresthesias, shock-like reactions, myalgia
- Neuromotor—tremor, myoclonus, ataxia, visual changes, piloerection
- Gastrointestinal—nausea, vomiting, diarrhea
- Psychiatric—anxiety, depressed mood, suicidal ideation, irritability
- Vasomotor—flushing, diaphoresis
- Other neuropsychiatric—anorexia, insomnia, vivid dreams, asthenia, chills.2
3 Distinguish discontinuation reactions from relapse
Although depressed mood and anxiety may occur during taper, these symptoms tend to be transient in most patients. Severe or persistent symptoms—including emerging suicidal ideation—may indicate a relapse.
4 Reduce medication slowly
Tapering is recommended for all antidepressants but should be particularly slow for certain drugs—including venlafaxine, paroxetine, and clomipramine—which can cause significant discontinuation effects. For example, venlafaxine at 225 mg/d could be reduced by 75 mg/d every 1 to 2 weeks, with a final step at 37.5 mg/d for at least 1 week.
If discontinuation reactions are a problem, ultimate discontinuation may require substituting a longer-acting medication such as fluoxetine during the tapering period. For example, add fluoxetine, 10 to 20 mg/d, for 1 week, then stop both antidepressants or discontinue the first medication and continue fluoxetine for 2 to 3 weeks until the risk of reactions passes.
Clinicians often are concerned about serotonin syndrome caused by combining SRIs. Isolated cases have been reported, but the small, finite chance of serotonin syndrome is much lower than the risk of severe discontinuation reactions.
5 Titrate up and taper down
When switching to another SRI, titrate the second drug upward while tapering off the first. Remember that changing to a drug that does not act on serotonin, such as bupropion, can protect against discontinuation effects.
6 Allow for pregnancy
Infants born to mothers taking antidepressants can exhibit discontinuation symptoms,2 particularly with shorter-acting drugs such as paroxetine or venlafaxine. Consider tapering the antidepressant early in the patient’s third trimester, then re-institute treatment after delivery. If an antidepressant is required during pregnancy, try using one with a longer half-life such as fluoxetine.
Drug brand names
- Bupropion • Wellbutrin
- Clomipramine • Anafranil
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
1. Schatzberg AF, Blier P, Delgado PL, et al. Antidepressant discontinuation syndrome: consensus panel recommendations for clinical management and additional research. J Clin Psychiatry 2006;67 (suppl 4):27-30.
2. Shelton RC. The nature of the discontinuation syndrome associated with antidepressant drugs. J Clin Psychiatry 2006;67(suppl 4):3-7.
Dr. Shelton is the James G. Blakemore Research Professor and vice-chair for research, department of psychiatry, and professor of pharmacology, Vanderbilt University, Nashville, TN.
1. Schatzberg AF, Blier P, Delgado PL, et al. Antidepressant discontinuation syndrome: consensus panel recommendations for clinical management and additional research. J Clin Psychiatry 2006;67 (suppl 4):27-30.
2. Shelton RC. The nature of the discontinuation syndrome associated with antidepressant drugs. J Clin Psychiatry 2006;67(suppl 4):3-7.
Dr. Shelton is the James G. Blakemore Research Professor and vice-chair for research, department of psychiatry, and professor of pharmacology, Vanderbilt University, Nashville, TN.
Eye-opening behaviors help diagnose nonepileptic seizures
On average, 7 years elapse between a patient’s first psychological nonepileptic seizure (PNES) and the correct diagnosis.1
PNES can be difficult to distinguish from epileptic seizures (ES), with both showing alterations in behavior, consciousness, sensation, and perception.2 Delayed diagnosis could lead to:
- adverse effects from unneeded antiepileptic drugs
- iatrogenic complications from invasive procedures in continuous PNES
- medical costs due to unnecessary hospitalization treatment and workup
- delayed referral to appropriate psychiatric treatment
- employment difficulties and disability. Fortunately, researchers are discovering some clinically useful differentiating features to use as adjuncts to video EEG, the diagnostic gold standard.3,4
Behavioral differences
Differentiating PNES from ES (Table 1) is the first step toward appropriate treatment,5 and observing seizure characteristics can be helpful.
Table 1
Behaviors to distinguish psychological nonepileptic and epileptic seizures
| Behavior | Psychological nonepileptic seizure | Epileptic seizure |
|---|---|---|
| Eye movement | Eyes closed at onset and during seizure; geotropic eye movement may be observed | Eyes open during seizure onset; may close briefly |
| Post-ictal nose rubbing and cough | Not present | May be present |
| Weeping | May be present | Not present |
| Body movements | Pelvic thrusting; out-of-phase or side-to-side oscillatory movements; chaotic and disorganized thrashing; ictal stuttering; post-ictal whispering | Pelvic thrusting; quick, tonic posturing; vocalization |
| Self-injury | May be present | May be present |
| Tongue laceration | May be present | May be present |
| Incontinence | May be present | May be present |
| Source: References 6-12,16,17 | ||
Eyes open or closed? Using data from video-EEG monitoring, researchers found that:
- 50 of 52 PNES patients (96%) closed their eyes during the seizure
- 152 of 156 of ES patients (97%) had their eyes open at the beginning of their seizures.6
Observing a patient’s eyes during a violent seizure could be difficult, but this information might help clinicians differentiate between PNES and ES, particularly when the two types of seizures occur in the same patient. Also, other observers, such as family members, could report to physicians if the patient’s eyes were open or closed during the ictal event.
Patients with PNES may also exhibit geotropic eye movements, in which the eyes deviate downward to the side that the head is turned.7 Eyelids are typically closed for a longer duration (20 seconds) compared with temporal lobe epilepsy (TLE) or frontal lobe seizures (FLS) (~2 seconds).8 Weeping also is a characteristic with PNES.9,10 Ictal stuttering and post-ictal whispering are seen in PNES.11,12 Post-ictal nose rubbing and cough have been observed in TLE but not in PNES.13
Pelvic thrusting reportedly is as common in FLS as in PNES. Other ictal features associated with PNES are out-of-phase or side-to-side oscillatory movements or chaotic and disorganized thrashing.2 In contrast, FLS typically arise from sleep, are brief, and often involve vocalization and quick, tonic posturing.14,15 Occasionally, whole body trembling may be observed with PNES. These behaviors may wax, wane, and change over many minutes, which is atypical for ES.
Injury. Physical injury during an ictus was once thought to occur only in patients with epilepsy, but research shows more than one-half of patients with PNES are injured during seizures.16 Tongue biting, self-injury, and incontinence are commonly associated with ES but are also reported by two-thirds of PNES patients, rendering these signs less specific than once thought.17
Diagnostic measures
EEG. PNES diagnosis is most accurately established by registering EEG neurophysiologic testing with video. Video-EEG—where the patient’s seizure is observed visually with simultaneous EEG—allows data about neurobehavior to be coupled with EEG rhythms. The absence of expected ictal patterns during the behavioral event points to a PNES diagnosis. Rarely, EEG-negative epilepsy occurs, where a partial simple seizure, a FLS, or a TLS does not generate an ictal epileptic pattern. Without video-EEG, neurologists’ ability to differentiate ES from PNES by history alone has a specificity of 50%.18
Neuroimaging. Structural neuroimaging abnormalities neither confirm nor exclude ES or PNES. PNES may occur in the presence of focal lesions, as confirmed by:
- case reports of PNES patients who have CNS lesions19
- a study showing that 10% of patients with PNES alone have structural abnormalities on MRI.20
A negative ictal single-photon emission computed tomography (SPECT) scan does not imply a diagnosis of PNES, nor does an abnormal scan mean that epilepsy is present. A small series of ictal and interictal SPECT scans of patients with PNES revealed a few scans with lateralized perfusion abnormalities, but the findings did not change when the ictal and interictal images were compared.21 Patients with epilepsy, in contrast, have dynamic changes when ictal and interictal changes on functional neuroimaging are compared.
Neurohumoral testing. Serum prolactin drawn within 30 minutes of ictus onset is helpful for differentiating generalized tonic clonic seizures and partial complex seizures from PNES, as summarized in a recent report from the American Academy of Neurology.22
Pnes characteristics
Patient characteristics and neuropsychological testing are helpful adjuncts to video EEG to diagnose PNES.
Family and patient traits. Studies comparing family functioning in patients with ES and PNES reveal:
- individuals with PNES view their families as more dysfunctional, particularly in regard to communication23
- family members of patients with PNES reported difficulties defining roles23
- patients with PNES score higher on measures of somatic complaints when compared with other seizure patients.24
Pain disorders are also common in patients with PNES. Among epilepsy clinic patients, a diagnosis of fibromyalgia or chronic pain has an 85% positive predictive value for PNES.25
Neuropsychological measures. A number of studies describe the cognitive, emotional, personality, and psychomotor differences between ES and PNES cohorts (Table 2).26-29 Patients with ES and PNES perform about the same on neuropsychological measures but worse than healthy controls. Patients with PNES appear to suffer from cognitive and somatic distress and anxiety. Studies reveal they also have difficulties expressing this distress to family members and others.
Table 2
Neuropsychological (NP) differences between PNES and ES
| Feature | Differences |
|---|---|
| Cognitive ability | Patients with ES and PNES show no significant differences on tests of intelligence, learning, and memory but score lower than healthy control subjects26 |
| Psychomotor skills | Patients with PNES show reduced motor speed and grip strength, compared with healthy controls27 |
| Motivation | Patients with PNES score lower on motivational measures than ES patients, perhaps reflecting a lack of psychological resources necessary to persist with a challenging NP battery; frank malingering is thought to occur rarely in PNES28 |
| Personality | Minnesota Multiphasic Personality Inventory (MMPI-2) studies show elevations in hypochondria, hysteria, and depression scores in patients with PNES29 |
1. Reuber M, Fernandez G, Bauer J, et al. Diagnostic delay in psychogenic nonepileptic seizures. Neurology 2002;58(3):493-5.
2. Gates JR, Ramani V, Whalen S, Loewenson R. Ictal characteristics of pseudoseizures. Arch Neurol 1985;42(12):1183-7.
3. LaFrance WC, Jr, Benbadis SR. Avoiding the costs of unrecognized psychological nonepileptic seizures. Neurology 2006;66(11):1730-1.
4. Cragar DE, Berry DT, Fakhory TA, et al. A review of diagnostic techniques in the differential diagnosis of epileptic and nonepileptic seizures. Neuropsychol Rev 2002;(1):31-64.
5. LaFrance WC, Jr, Devinsky O. Treatment of nonepileptic seizures. Epilepsy Behav 2002;3(suppl):S19-S23.
6. Chung SS, Gerber P, Kirlin KA. Ictal eye closure is a reliable indicator for psychogenic nonepileptic seizures. Neurology 2006;66(11):1730-1.
7. Henry JA, Woodruff GHA. A diagnostic sign in states of apparent unconsciousness. Lancet 1978;2(8096):920-1.
8. Donati F, Kollar M, Pihan H, Mathis J. Eyelids position during epileptic versus psychogenic seizures. J Neurol Sciences 2005;238(suppl 1):S82-S83.
9. Flügel D, Bauer J, Kaseborn U, et al. Closed eyes during a seizure indicate psychogenic etiology: A study with suggestive seizure provocation. J Epilepsy 1996;9(3):165-9.
10. Bergen D, Ristanovic R. Weeping as a common element of pseudoseizures. Arch Neurol 1993;50(10):1059-60.
11. Vossler DG, Haltiner AM, Schepp SK, et al. Ictal stuttering: a sign suggestive of psychogenic nonepileptic seizures. Neurology 2004;63(3):516-9.
12. Chabola DR, Shih JJ. Postictal behaviors associated with psychogenic nonepileptic seizures. Epilepsy Behav 2006;9(2):307-11.
13. Wennberg R. Postictal coughing and nose rubbing coexist in temporal lobe epilepsy. Neurology 2001;56(1):133-4.
14. Kanner AM, Morris HH, Luders H, et al. Supplementary motor seizures mimicking pseudoseizures: some clinical differences. Neurology 1990;40(9):1404-7.
15. Jobst BC, Williamson PD. Frontal lobe seizures. Psychiatr Clin North Am 2005;28(3):635-51.
16. Kanner AM. Psychogenic nonepileptic seizures are bad for your health. Epilepsy Curr 2003;3(5):181-2.
17. de Timary P, Fouchet P, Sylin M, et al. Nonepileptic seizures: delayed diagnosis in patients presenting with electroencephalo-graphic (EEG) or clinical signs of epileptic seizures. Seizure 2002;11:193-7.
18. Deacon C, Wiebe S, Blume WT, et al. Seizure identification by clinical description in temporal lobe epilepsy: how accurate are we? Neurology 2003;61(12):1686-9.
19. Lowe MR, De Toledo JC, Rabinstein AA, Giulla MF. Correspondence: MRI evidence of mesial temporal sclerosis in patients with psychogenic nonepileptic seizures. Neurology 2001;56(6):821-3.
20. Reuber M, Fernandez G, Helmstaedter C, et al. Evidence of brain abnormality in patients with psychogenic nonepileptic seizures. Epilepsy Behav 2002;3(3):249-54.
21. Ettinger AB, Coyle PK, Jandorf L, et al. Postictal SPECT in epileptic versus nonepileptic seizures. J Epilepsy 1998;11:67-73.
22. Chen DK, So YT, Fisher RS. Use of serum prolactin in diagnosing epileptic seizures: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005;65(5):668-75.
23. Krawetz P, Fleisher W, Pillay N, et al. Family functioning in subjects with pseudoseizures and epilepsy. J Nerv Ment Dis 2001;189(1):38-43.
24. van Merode T, Twellaar M, Kotsopoulos IA, et al. Psychological characteristics of patients with newly developed psychogenic seizures. J Neurol Neurosurg Psychiatry 2004;75(8):1175-7.
25. Benbadis SR. A spell in the epilepsy clinic and a history of “chronic pain” or “fibromyalgia” independently predict a diagnosis of psychogenic seizures. Epilepsy Behav 2005;6(2):264-5.
26. Binder LM, Kindermann SS, Heaton RK, Salinsky MC. Neuropsychologic impairment in patients with nonepileptic seizures. Arch Clin Neuropsychol 1998;13(6):513-22.
27. Kalogjera-Sackellares D, Sackellares JC. Impaired motor function in patients with psychogenic pseudoseizures. Epilepsia 2001;42(12):1600-6.
28. Binder LM, Salinsky MC, Smith SP. Psychological correlates of psychogenic seizures. J Clin Exp Neuropsychol 1994;16(4):524-30.
29. Cragar DE, Berry DT, Fakhoury TA, et al. A review of diagnostic techniques in the differential diagnosis of epileptic and nonepileptic seizures. Neuropsychol Rev 2002;12(1):31-64.
On average, 7 years elapse between a patient’s first psychological nonepileptic seizure (PNES) and the correct diagnosis.1
PNES can be difficult to distinguish from epileptic seizures (ES), with both showing alterations in behavior, consciousness, sensation, and perception.2 Delayed diagnosis could lead to:
- adverse effects from unneeded antiepileptic drugs
- iatrogenic complications from invasive procedures in continuous PNES
- medical costs due to unnecessary hospitalization treatment and workup
- delayed referral to appropriate psychiatric treatment
- employment difficulties and disability. Fortunately, researchers are discovering some clinically useful differentiating features to use as adjuncts to video EEG, the diagnostic gold standard.3,4
Behavioral differences
Differentiating PNES from ES (Table 1) is the first step toward appropriate treatment,5 and observing seizure characteristics can be helpful.
Table 1
Behaviors to distinguish psychological nonepileptic and epileptic seizures
| Behavior | Psychological nonepileptic seizure | Epileptic seizure |
|---|---|---|
| Eye movement | Eyes closed at onset and during seizure; geotropic eye movement may be observed | Eyes open during seizure onset; may close briefly |
| Post-ictal nose rubbing and cough | Not present | May be present |
| Weeping | May be present | Not present |
| Body movements | Pelvic thrusting; out-of-phase or side-to-side oscillatory movements; chaotic and disorganized thrashing; ictal stuttering; post-ictal whispering | Pelvic thrusting; quick, tonic posturing; vocalization |
| Self-injury | May be present | May be present |
| Tongue laceration | May be present | May be present |
| Incontinence | May be present | May be present |
| Source: References 6-12,16,17 | ||
Eyes open or closed? Using data from video-EEG monitoring, researchers found that:
- 50 of 52 PNES patients (96%) closed their eyes during the seizure
- 152 of 156 of ES patients (97%) had their eyes open at the beginning of their seizures.6
Observing a patient’s eyes during a violent seizure could be difficult, but this information might help clinicians differentiate between PNES and ES, particularly when the two types of seizures occur in the same patient. Also, other observers, such as family members, could report to physicians if the patient’s eyes were open or closed during the ictal event.
Patients with PNES may also exhibit geotropic eye movements, in which the eyes deviate downward to the side that the head is turned.7 Eyelids are typically closed for a longer duration (20 seconds) compared with temporal lobe epilepsy (TLE) or frontal lobe seizures (FLS) (~2 seconds).8 Weeping also is a characteristic with PNES.9,10 Ictal stuttering and post-ictal whispering are seen in PNES.11,12 Post-ictal nose rubbing and cough have been observed in TLE but not in PNES.13
Pelvic thrusting reportedly is as common in FLS as in PNES. Other ictal features associated with PNES are out-of-phase or side-to-side oscillatory movements or chaotic and disorganized thrashing.2 In contrast, FLS typically arise from sleep, are brief, and often involve vocalization and quick, tonic posturing.14,15 Occasionally, whole body trembling may be observed with PNES. These behaviors may wax, wane, and change over many minutes, which is atypical for ES.
Injury. Physical injury during an ictus was once thought to occur only in patients with epilepsy, but research shows more than one-half of patients with PNES are injured during seizures.16 Tongue biting, self-injury, and incontinence are commonly associated with ES but are also reported by two-thirds of PNES patients, rendering these signs less specific than once thought.17
Diagnostic measures
EEG. PNES diagnosis is most accurately established by registering EEG neurophysiologic testing with video. Video-EEG—where the patient’s seizure is observed visually with simultaneous EEG—allows data about neurobehavior to be coupled with EEG rhythms. The absence of expected ictal patterns during the behavioral event points to a PNES diagnosis. Rarely, EEG-negative epilepsy occurs, where a partial simple seizure, a FLS, or a TLS does not generate an ictal epileptic pattern. Without video-EEG, neurologists’ ability to differentiate ES from PNES by history alone has a specificity of 50%.18
Neuroimaging. Structural neuroimaging abnormalities neither confirm nor exclude ES or PNES. PNES may occur in the presence of focal lesions, as confirmed by:
- case reports of PNES patients who have CNS lesions19
- a study showing that 10% of patients with PNES alone have structural abnormalities on MRI.20
A negative ictal single-photon emission computed tomography (SPECT) scan does not imply a diagnosis of PNES, nor does an abnormal scan mean that epilepsy is present. A small series of ictal and interictal SPECT scans of patients with PNES revealed a few scans with lateralized perfusion abnormalities, but the findings did not change when the ictal and interictal images were compared.21 Patients with epilepsy, in contrast, have dynamic changes when ictal and interictal changes on functional neuroimaging are compared.
Neurohumoral testing. Serum prolactin drawn within 30 minutes of ictus onset is helpful for differentiating generalized tonic clonic seizures and partial complex seizures from PNES, as summarized in a recent report from the American Academy of Neurology.22
Pnes characteristics
Patient characteristics and neuropsychological testing are helpful adjuncts to video EEG to diagnose PNES.
Family and patient traits. Studies comparing family functioning in patients with ES and PNES reveal:
- individuals with PNES view their families as more dysfunctional, particularly in regard to communication23
- family members of patients with PNES reported difficulties defining roles23
- patients with PNES score higher on measures of somatic complaints when compared with other seizure patients.24
Pain disorders are also common in patients with PNES. Among epilepsy clinic patients, a diagnosis of fibromyalgia or chronic pain has an 85% positive predictive value for PNES.25
Neuropsychological measures. A number of studies describe the cognitive, emotional, personality, and psychomotor differences between ES and PNES cohorts (Table 2).26-29 Patients with ES and PNES perform about the same on neuropsychological measures but worse than healthy controls. Patients with PNES appear to suffer from cognitive and somatic distress and anxiety. Studies reveal they also have difficulties expressing this distress to family members and others.
Table 2
Neuropsychological (NP) differences between PNES and ES
| Feature | Differences |
|---|---|
| Cognitive ability | Patients with ES and PNES show no significant differences on tests of intelligence, learning, and memory but score lower than healthy control subjects26 |
| Psychomotor skills | Patients with PNES show reduced motor speed and grip strength, compared with healthy controls27 |
| Motivation | Patients with PNES score lower on motivational measures than ES patients, perhaps reflecting a lack of psychological resources necessary to persist with a challenging NP battery; frank malingering is thought to occur rarely in PNES28 |
| Personality | Minnesota Multiphasic Personality Inventory (MMPI-2) studies show elevations in hypochondria, hysteria, and depression scores in patients with PNES29 |
On average, 7 years elapse between a patient’s first psychological nonepileptic seizure (PNES) and the correct diagnosis.1
PNES can be difficult to distinguish from epileptic seizures (ES), with both showing alterations in behavior, consciousness, sensation, and perception.2 Delayed diagnosis could lead to:
- adverse effects from unneeded antiepileptic drugs
- iatrogenic complications from invasive procedures in continuous PNES
- medical costs due to unnecessary hospitalization treatment and workup
- delayed referral to appropriate psychiatric treatment
- employment difficulties and disability. Fortunately, researchers are discovering some clinically useful differentiating features to use as adjuncts to video EEG, the diagnostic gold standard.3,4
Behavioral differences
Differentiating PNES from ES (Table 1) is the first step toward appropriate treatment,5 and observing seizure characteristics can be helpful.
Table 1
Behaviors to distinguish psychological nonepileptic and epileptic seizures
| Behavior | Psychological nonepileptic seizure | Epileptic seizure |
|---|---|---|
| Eye movement | Eyes closed at onset and during seizure; geotropic eye movement may be observed | Eyes open during seizure onset; may close briefly |
| Post-ictal nose rubbing and cough | Not present | May be present |
| Weeping | May be present | Not present |
| Body movements | Pelvic thrusting; out-of-phase or side-to-side oscillatory movements; chaotic and disorganized thrashing; ictal stuttering; post-ictal whispering | Pelvic thrusting; quick, tonic posturing; vocalization |
| Self-injury | May be present | May be present |
| Tongue laceration | May be present | May be present |
| Incontinence | May be present | May be present |
| Source: References 6-12,16,17 | ||
Eyes open or closed? Using data from video-EEG monitoring, researchers found that:
- 50 of 52 PNES patients (96%) closed their eyes during the seizure
- 152 of 156 of ES patients (97%) had their eyes open at the beginning of their seizures.6
Observing a patient’s eyes during a violent seizure could be difficult, but this information might help clinicians differentiate between PNES and ES, particularly when the two types of seizures occur in the same patient. Also, other observers, such as family members, could report to physicians if the patient’s eyes were open or closed during the ictal event.
Patients with PNES may also exhibit geotropic eye movements, in which the eyes deviate downward to the side that the head is turned.7 Eyelids are typically closed for a longer duration (20 seconds) compared with temporal lobe epilepsy (TLE) or frontal lobe seizures (FLS) (~2 seconds).8 Weeping also is a characteristic with PNES.9,10 Ictal stuttering and post-ictal whispering are seen in PNES.11,12 Post-ictal nose rubbing and cough have been observed in TLE but not in PNES.13
Pelvic thrusting reportedly is as common in FLS as in PNES. Other ictal features associated with PNES are out-of-phase or side-to-side oscillatory movements or chaotic and disorganized thrashing.2 In contrast, FLS typically arise from sleep, are brief, and often involve vocalization and quick, tonic posturing.14,15 Occasionally, whole body trembling may be observed with PNES. These behaviors may wax, wane, and change over many minutes, which is atypical for ES.
Injury. Physical injury during an ictus was once thought to occur only in patients with epilepsy, but research shows more than one-half of patients with PNES are injured during seizures.16 Tongue biting, self-injury, and incontinence are commonly associated with ES but are also reported by two-thirds of PNES patients, rendering these signs less specific than once thought.17
Diagnostic measures
EEG. PNES diagnosis is most accurately established by registering EEG neurophysiologic testing with video. Video-EEG—where the patient’s seizure is observed visually with simultaneous EEG—allows data about neurobehavior to be coupled with EEG rhythms. The absence of expected ictal patterns during the behavioral event points to a PNES diagnosis. Rarely, EEG-negative epilepsy occurs, where a partial simple seizure, a FLS, or a TLS does not generate an ictal epileptic pattern. Without video-EEG, neurologists’ ability to differentiate ES from PNES by history alone has a specificity of 50%.18
Neuroimaging. Structural neuroimaging abnormalities neither confirm nor exclude ES or PNES. PNES may occur in the presence of focal lesions, as confirmed by:
- case reports of PNES patients who have CNS lesions19
- a study showing that 10% of patients with PNES alone have structural abnormalities on MRI.20
A negative ictal single-photon emission computed tomography (SPECT) scan does not imply a diagnosis of PNES, nor does an abnormal scan mean that epilepsy is present. A small series of ictal and interictal SPECT scans of patients with PNES revealed a few scans with lateralized perfusion abnormalities, but the findings did not change when the ictal and interictal images were compared.21 Patients with epilepsy, in contrast, have dynamic changes when ictal and interictal changes on functional neuroimaging are compared.
Neurohumoral testing. Serum prolactin drawn within 30 minutes of ictus onset is helpful for differentiating generalized tonic clonic seizures and partial complex seizures from PNES, as summarized in a recent report from the American Academy of Neurology.22
Pnes characteristics
Patient characteristics and neuropsychological testing are helpful adjuncts to video EEG to diagnose PNES.
Family and patient traits. Studies comparing family functioning in patients with ES and PNES reveal:
- individuals with PNES view their families as more dysfunctional, particularly in regard to communication23
- family members of patients with PNES reported difficulties defining roles23
- patients with PNES score higher on measures of somatic complaints when compared with other seizure patients.24
Pain disorders are also common in patients with PNES. Among epilepsy clinic patients, a diagnosis of fibromyalgia or chronic pain has an 85% positive predictive value for PNES.25
Neuropsychological measures. A number of studies describe the cognitive, emotional, personality, and psychomotor differences between ES and PNES cohorts (Table 2).26-29 Patients with ES and PNES perform about the same on neuropsychological measures but worse than healthy controls. Patients with PNES appear to suffer from cognitive and somatic distress and anxiety. Studies reveal they also have difficulties expressing this distress to family members and others.
Table 2
Neuropsychological (NP) differences between PNES and ES
| Feature | Differences |
|---|---|
| Cognitive ability | Patients with ES and PNES show no significant differences on tests of intelligence, learning, and memory but score lower than healthy control subjects26 |
| Psychomotor skills | Patients with PNES show reduced motor speed and grip strength, compared with healthy controls27 |
| Motivation | Patients with PNES score lower on motivational measures than ES patients, perhaps reflecting a lack of psychological resources necessary to persist with a challenging NP battery; frank malingering is thought to occur rarely in PNES28 |
| Personality | Minnesota Multiphasic Personality Inventory (MMPI-2) studies show elevations in hypochondria, hysteria, and depression scores in patients with PNES29 |
1. Reuber M, Fernandez G, Bauer J, et al. Diagnostic delay in psychogenic nonepileptic seizures. Neurology 2002;58(3):493-5.
2. Gates JR, Ramani V, Whalen S, Loewenson R. Ictal characteristics of pseudoseizures. Arch Neurol 1985;42(12):1183-7.
3. LaFrance WC, Jr, Benbadis SR. Avoiding the costs of unrecognized psychological nonepileptic seizures. Neurology 2006;66(11):1730-1.
4. Cragar DE, Berry DT, Fakhory TA, et al. A review of diagnostic techniques in the differential diagnosis of epileptic and nonepileptic seizures. Neuropsychol Rev 2002;(1):31-64.
5. LaFrance WC, Jr, Devinsky O. Treatment of nonepileptic seizures. Epilepsy Behav 2002;3(suppl):S19-S23.
6. Chung SS, Gerber P, Kirlin KA. Ictal eye closure is a reliable indicator for psychogenic nonepileptic seizures. Neurology 2006;66(11):1730-1.
7. Henry JA, Woodruff GHA. A diagnostic sign in states of apparent unconsciousness. Lancet 1978;2(8096):920-1.
8. Donati F, Kollar M, Pihan H, Mathis J. Eyelids position during epileptic versus psychogenic seizures. J Neurol Sciences 2005;238(suppl 1):S82-S83.
9. Flügel D, Bauer J, Kaseborn U, et al. Closed eyes during a seizure indicate psychogenic etiology: A study with suggestive seizure provocation. J Epilepsy 1996;9(3):165-9.
10. Bergen D, Ristanovic R. Weeping as a common element of pseudoseizures. Arch Neurol 1993;50(10):1059-60.
11. Vossler DG, Haltiner AM, Schepp SK, et al. Ictal stuttering: a sign suggestive of psychogenic nonepileptic seizures. Neurology 2004;63(3):516-9.
12. Chabola DR, Shih JJ. Postictal behaviors associated with psychogenic nonepileptic seizures. Epilepsy Behav 2006;9(2):307-11.
13. Wennberg R. Postictal coughing and nose rubbing coexist in temporal lobe epilepsy. Neurology 2001;56(1):133-4.
14. Kanner AM, Morris HH, Luders H, et al. Supplementary motor seizures mimicking pseudoseizures: some clinical differences. Neurology 1990;40(9):1404-7.
15. Jobst BC, Williamson PD. Frontal lobe seizures. Psychiatr Clin North Am 2005;28(3):635-51.
16. Kanner AM. Psychogenic nonepileptic seizures are bad for your health. Epilepsy Curr 2003;3(5):181-2.
17. de Timary P, Fouchet P, Sylin M, et al. Nonepileptic seizures: delayed diagnosis in patients presenting with electroencephalo-graphic (EEG) or clinical signs of epileptic seizures. Seizure 2002;11:193-7.
18. Deacon C, Wiebe S, Blume WT, et al. Seizure identification by clinical description in temporal lobe epilepsy: how accurate are we? Neurology 2003;61(12):1686-9.
19. Lowe MR, De Toledo JC, Rabinstein AA, Giulla MF. Correspondence: MRI evidence of mesial temporal sclerosis in patients with psychogenic nonepileptic seizures. Neurology 2001;56(6):821-3.
20. Reuber M, Fernandez G, Helmstaedter C, et al. Evidence of brain abnormality in patients with psychogenic nonepileptic seizures. Epilepsy Behav 2002;3(3):249-54.
21. Ettinger AB, Coyle PK, Jandorf L, et al. Postictal SPECT in epileptic versus nonepileptic seizures. J Epilepsy 1998;11:67-73.
22. Chen DK, So YT, Fisher RS. Use of serum prolactin in diagnosing epileptic seizures: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005;65(5):668-75.
23. Krawetz P, Fleisher W, Pillay N, et al. Family functioning in subjects with pseudoseizures and epilepsy. J Nerv Ment Dis 2001;189(1):38-43.
24. van Merode T, Twellaar M, Kotsopoulos IA, et al. Psychological characteristics of patients with newly developed psychogenic seizures. J Neurol Neurosurg Psychiatry 2004;75(8):1175-7.
25. Benbadis SR. A spell in the epilepsy clinic and a history of “chronic pain” or “fibromyalgia” independently predict a diagnosis of psychogenic seizures. Epilepsy Behav 2005;6(2):264-5.
26. Binder LM, Kindermann SS, Heaton RK, Salinsky MC. Neuropsychologic impairment in patients with nonepileptic seizures. Arch Clin Neuropsychol 1998;13(6):513-22.
27. Kalogjera-Sackellares D, Sackellares JC. Impaired motor function in patients with psychogenic pseudoseizures. Epilepsia 2001;42(12):1600-6.
28. Binder LM, Salinsky MC, Smith SP. Psychological correlates of psychogenic seizures. J Clin Exp Neuropsychol 1994;16(4):524-30.
29. Cragar DE, Berry DT, Fakhoury TA, et al. A review of diagnostic techniques in the differential diagnosis of epileptic and nonepileptic seizures. Neuropsychol Rev 2002;12(1):31-64.
1. Reuber M, Fernandez G, Bauer J, et al. Diagnostic delay in psychogenic nonepileptic seizures. Neurology 2002;58(3):493-5.
2. Gates JR, Ramani V, Whalen S, Loewenson R. Ictal characteristics of pseudoseizures. Arch Neurol 1985;42(12):1183-7.
3. LaFrance WC, Jr, Benbadis SR. Avoiding the costs of unrecognized psychological nonepileptic seizures. Neurology 2006;66(11):1730-1.
4. Cragar DE, Berry DT, Fakhory TA, et al. A review of diagnostic techniques in the differential diagnosis of epileptic and nonepileptic seizures. Neuropsychol Rev 2002;(1):31-64.
5. LaFrance WC, Jr, Devinsky O. Treatment of nonepileptic seizures. Epilepsy Behav 2002;3(suppl):S19-S23.
6. Chung SS, Gerber P, Kirlin KA. Ictal eye closure is a reliable indicator for psychogenic nonepileptic seizures. Neurology 2006;66(11):1730-1.
7. Henry JA, Woodruff GHA. A diagnostic sign in states of apparent unconsciousness. Lancet 1978;2(8096):920-1.
8. Donati F, Kollar M, Pihan H, Mathis J. Eyelids position during epileptic versus psychogenic seizures. J Neurol Sciences 2005;238(suppl 1):S82-S83.
9. Flügel D, Bauer J, Kaseborn U, et al. Closed eyes during a seizure indicate psychogenic etiology: A study with suggestive seizure provocation. J Epilepsy 1996;9(3):165-9.
10. Bergen D, Ristanovic R. Weeping as a common element of pseudoseizures. Arch Neurol 1993;50(10):1059-60.
11. Vossler DG, Haltiner AM, Schepp SK, et al. Ictal stuttering: a sign suggestive of psychogenic nonepileptic seizures. Neurology 2004;63(3):516-9.
12. Chabola DR, Shih JJ. Postictal behaviors associated with psychogenic nonepileptic seizures. Epilepsy Behav 2006;9(2):307-11.
13. Wennberg R. Postictal coughing and nose rubbing coexist in temporal lobe epilepsy. Neurology 2001;56(1):133-4.
14. Kanner AM, Morris HH, Luders H, et al. Supplementary motor seizures mimicking pseudoseizures: some clinical differences. Neurology 1990;40(9):1404-7.
15. Jobst BC, Williamson PD. Frontal lobe seizures. Psychiatr Clin North Am 2005;28(3):635-51.
16. Kanner AM. Psychogenic nonepileptic seizures are bad for your health. Epilepsy Curr 2003;3(5):181-2.
17. de Timary P, Fouchet P, Sylin M, et al. Nonepileptic seizures: delayed diagnosis in patients presenting with electroencephalo-graphic (EEG) or clinical signs of epileptic seizures. Seizure 2002;11:193-7.
18. Deacon C, Wiebe S, Blume WT, et al. Seizure identification by clinical description in temporal lobe epilepsy: how accurate are we? Neurology 2003;61(12):1686-9.
19. Lowe MR, De Toledo JC, Rabinstein AA, Giulla MF. Correspondence: MRI evidence of mesial temporal sclerosis in patients with psychogenic nonepileptic seizures. Neurology 2001;56(6):821-3.
20. Reuber M, Fernandez G, Helmstaedter C, et al. Evidence of brain abnormality in patients with psychogenic nonepileptic seizures. Epilepsy Behav 2002;3(3):249-54.
21. Ettinger AB, Coyle PK, Jandorf L, et al. Postictal SPECT in epileptic versus nonepileptic seizures. J Epilepsy 1998;11:67-73.
22. Chen DK, So YT, Fisher RS. Use of serum prolactin in diagnosing epileptic seizures: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005;65(5):668-75.
23. Krawetz P, Fleisher W, Pillay N, et al. Family functioning in subjects with pseudoseizures and epilepsy. J Nerv Ment Dis 2001;189(1):38-43.
24. van Merode T, Twellaar M, Kotsopoulos IA, et al. Psychological characteristics of patients with newly developed psychogenic seizures. J Neurol Neurosurg Psychiatry 2004;75(8):1175-7.
25. Benbadis SR. A spell in the epilepsy clinic and a history of “chronic pain” or “fibromyalgia” independently predict a diagnosis of psychogenic seizures. Epilepsy Behav 2005;6(2):264-5.
26. Binder LM, Kindermann SS, Heaton RK, Salinsky MC. Neuropsychologic impairment in patients with nonepileptic seizures. Arch Clin Neuropsychol 1998;13(6):513-22.
27. Kalogjera-Sackellares D, Sackellares JC. Impaired motor function in patients with psychogenic pseudoseizures. Epilepsia 2001;42(12):1600-6.
28. Binder LM, Salinsky MC, Smith SP. Psychological correlates of psychogenic seizures. J Clin Exp Neuropsychol 1994;16(4):524-30.
29. Cragar DE, Berry DT, Fakhoury TA, et al. A review of diagnostic techniques in the differential diagnosis of epileptic and nonepileptic seizures. Neuropsychol Rev 2002;12(1):31-64.
Hepatitis C: How to manage mood during interferon treatment
Mr. R, age 39, is found to have elevated liver function during a routine physical exam by his primary care physician. Subsequent testing reveals chronic hepatitis C viral (HCV) infection.
Starting at age 17, Mr. R abused alcohol and drugs and occasionally shared IV needles. He stopped using street drugs at age 28 when he lost contact with his drug abusing friends and is now married and has two children. In the past 10 years he has had two episodes of major depression, successfully treated with fluoxetine, 40 mg/d. He has no physical or psychiatric symptoms of HCV infection.
IV drug use causes >40% of HCV infections in the United States,1 and substance abusers have increased rates of psychiatric illness, particularly major depression. But substance use does not account fully for the link between HCV infection and depression. A depressive syndrome may explain why depression’s mood and somatic symptoms are seen in significantly more HCV-infected drug users than in noninfected drug users.2
Psychiatrists are often called on to treat HCV-associated depression and other psychiatric symptoms—irritability, insomnia, and impaired concentration—and to support patients who pursue a cure through lengthy interferon treatment. To help you collaborate in the medical/psychiatric care of these patients, this article discusses:
- hepatitis C’s natural history
- diagnostic evaluation
- treatment options
- how to manage treatment’s psychiatric side effects.
Table 1
How Americans contract hepatitis C viral infection
| Risk factor | Percentage of U.S. cases* |
|---|---|
| IV drug use | 42% |
| Having >1 sexual partner | 27% |
| Surgery | 19% |
| Sexual contact with a hepatitis C patient | 14% |
| Household contact with a hepatitis C patient | 6% |
| Percutaneous injury (needlestick) | 5% |
| Employment in medical/dental field | 4% |
| Hemodialysis | |
| Blood transfusion | |
| * Patients could have more than one risk factor for hepatitis C transmission | |
| Source: Centers for Disease Control and Prevention. Hepatitis surveillance report. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. no. 61. | |
Course of Chronic HCV
Mr. R’s primary care physician refers him to a gastroenterologist for liver function evaluation and treatment. Polymerase chain reaction testing reveals a detectable viral level, genotyping indicates that he has HCV type 1a, and liver biopsy shows moderate fibrosis.
As part of the clinic’s treatment protocol, Mr. R is referred to a psychiatrist for evaluation.
The typical interval from HCV infection to diagnosis is 10 to 30 years. Patients with unrecognized HCV infection usually are first treated by primary care physicians, who notice elevated liver function and refer them to a hepatologist or gastroenterologist.
In the United States, HCV is transmitted most frequently through IV drug use, sexual activity, and surgery (Table 1). Nearly all IV drug abusers (65% to 90%) have been exposed to HCV.1 After exposure, 70% of patients develop chronic HCV infection. The disease often is asymptomatic for many years, and some patients never show symptoms. If symptoms develop, they are usually nonspecific, such as fatigue, abdominal discomfort, and nausea, and rarely jaundice and dark urine (Box).
Over time, the disease can progress to cirrhosis and hepatocellular carcinoma. Ten percent to 20% of HCV patients develop cirrhosis a mean 20 years after infection. Serious complications develop more rapidly in patients who:
- are age >40 when infected
- abuse alcohol
- have HIV or coexistent liver disease.
Mood Symptoms with IFN
Significant depressive symptoms occur in 21% to 58% of patients receiving interferon, with major depressive disorder developing at a mean 12 weeks (range 1 to 32 weeks) after therapy begins.3 Other patients develop depressive symptoms that do not meet DSM-IV-TR criteria for major depression.
Manic and hypomanic symptoms also may emerge, such as elevated mood, irritability, inflated self-esteem, insomnia, talkativeness, racing thoughts, distractibility, agitation, and excessive pursuit of pleasurable activities.
The mechanism for psychiatric side effects with IFN is unknown, but nutritional and metabolic alterations are thought to be responsible. One theory holds that IFN decreases CNS tryptophan levels by disrupting the transporter that ferries this essential amino acid across the blood-brain barrier. Deficient tryptophan—the rate-limiting step in serotonin synthesis—results in decreased serotonin levels.4 Another possible explanation is that interferon disrupts the hypothalamic-pituitary axis or more directly alters neural functioning.
Patient history of depression. One study asserted that patients with a history of depression or increased depressive symptoms at baseline are more susceptible to IFN-related psychiatric side effects such as irritability, insomnia, depression, and impaired concentration.5 Other studies, however, show no statistically significant difference in neuropsychiatric symptoms during IFN therapy in patients with preexisting psychiatric disorders and those without such a history.6,7
- HCV affects 2% of the U.S. population but 20% of persons with severe mental illness
- Average annual new infections declined to 36,000 in 1996 from a high of 230,000 in the 1980s, which for reasons that are unclear correlates with a decrease in cases among IV drug users
- Progression of HCV infection is the leading cause of liver transplants in the United States
- Persons infected with HCV are at an increased risk for disease progression if they drink alcohol (>2 drinks/day for men under age 65, >1 drink/day for nonpregnant women and all persons over age 66), are age >40 years at time of infection, or are HIV-positive
- Deaths from acute liver failure are rare
- Chronic HCV infection causes 8,000 to 10,000 deaths per year
Psychiatric assessment. Assess all IFN candidates for present or past psychiatric disorders, including:
- depression
- suicidal thoughts (in one study, 43% of patients on IFN therapy reported suicidal ideation)12
- bipolar disorder (selective serotonin reuptake inhibitors [SSRIs] could induce mania or aggravate cycling)
- chemical dependency (substance abuse may represent the patient’s attempt to self-medicate underlying mood and anxiety symptoms).
Case Continued: Getting Ready
Although Mr. R no longer uses street drugs, he tells the psychiatrist he drinks 2 to 3 beers nightly. Because alcohol use stresses a compromised liver and could undermine IFN therapy’s effectiveness, he agrees to complete a chemical dependency program, demonstrate 6 months of sobriety before starting HCV treatment, and enroll in a chemical dependency relapse prevention program where unannounced drug and alcohol screenings are conducted.
As his IFN treatment approaches, Mr. R agrees to begin prophylactic citalopram, 20 mg/d, because he may be at increased risk for IFN-induced depression. Although Mr. R’s past depressive episodes responded well to fluoxetine, the psychiatrist chooses citalopram during IFN treatment because of its lower risk of drug-drug interactions.
Alcohol and IFN. Continued alcohol use can accelerate HCV-induced liver disease and reduce the likelihood of viral clearance with IFN treatment. One study showed that individuals who enrolled in a substance abuse treatment program were more likely to complete HCV treatment.13
This study also reported that HCV-seropositive patients were more likely to complete a 28-day chemical dependency treatment and remain abstinent 6 months after program discharge, compared with HCV-seronegative patients.13 This suggests that a chronic hepatitis C diagnosis motivates patients to address chemical dependency as a pre-requisite for hepatitis C treatment.
Table 2
Psychiatric side effects with interferon/ribavirin treatment*
| Side effect | Prevalence |
|---|---|
| Irritability, anxiety | 33% to 45% |
| Insomnia | 30% to 40% |
| Depression | 20% to 31% |
| Impaired concentration | 10% to 17% |
| Aggressive behavior | |
| Psychotic disorder | |
| Suicide | |
| * In patients without a history of psychiatric disorders | |
| Source: References 19 and 20 | |
Start antidepressants 2 to 4 weeks before antiviral therapy begins to allow the medication to reach therapeutic efficacy. SSRIs such as paroxetine, 10 to 50 mg/d, and citalopram, 20 to 40 mg/d, have been reported to be effective and do not interact with HCV therapies.5,14 In our experience, dual-action antidepressants such as duloxetine, venlafaxine, or bupropion also can be beneficial.
IFN Treatment Protocol
Mr. R begins a 48-week IFN protocol. To maximize the treatment’s effectiveness, he is given long-acting pegylated interferon, 180 mcg injected weekly, and takes oral ribavirin, 600 mg twice daily.
IFN plus ribavirin. The mainstay of HCV therapy is IFN, a cytokine immunotherapeutic agent. A long-acting IFN administered weekly—called pegylated because the compound is bound to polyethylene glycol—doubles the sustained viral response rate and is now widely used.
Pegylated interferon is often combined with ribavirin—an oral nucleoside analog that has been shown to improve outcomes. Ribavirin increases the risk of hemolysis, however, which mandates frequent blood count monitoring. The NIH recommends pegylated interferon and ribavirin for patients with:
- detectable HCV RNA viral loads >50 copies per ml of blood
- liver biopsy with portal or bridging fibrosis
- and at least moderate inflammation and necrosis.
Forty-eight weeks of treatment are recommended for patients with HCV genotype 1 (70% of patients) and 24 weeks for those with HCV genotypes 2 and 3 (15% to 25% patients).
Some patients—particularly those with genotype 1b—do not respond to IFN therapy. For nonresponders, repeated trials of longer duration or different types of IFN may be tried. Higher IFN dosages or more frequent administration are not viewed as beneficial.
Side effects. Sustained response rates 6 months after patients complete interferon treatment are:
Many of IFN’s early side effects are neurovegetative and overlap with psychiatric symptoms. The more specific psychiatric side effects of irritability, anxiety, insomnia, depression, and impaired concentration develop in 1 to 32 weeks of treatment (mean 12.1 weeks).19,20 Fatigue and depression are the main reasons 10% to 14% of outpatients in large randomized trials discontinue HCV treatment.21
Table 3
HCV testing protocols
| HCVab | If positive then do a HCV Riba |
| HCV Riba | If positive 2 bands or more, then do a HCV Genotype and HCV PCR |
| HCV Genotype | Genotype determines duration of treatment |
| HCV PCR Qualitative and/or Quantitative | Confirms presence of the virus |
| Liver biopsy | Determines the extent of liver damage from fibrosis or cirrhosis |
Case Continued: Preventing Relapse
During therapy, Mr. R completes the BDI and Fatigue Severity Scale (FSS) weekly. His pretreatment BDI score of 9 (normal) increases over time to 22 (mild to moderate depression), and his FSS scores range from 4 to 6, indicating fatigue sufficient to impair daily functioning. Medical and psychiatric staff address his symptoms during weekly treatment assessments.
After 12 weeks of treatment his viral levels are undetectable, but he develops severe fatigue and mild irritability that contribute to arguments with his wife. He is referred to supportive counseling, and citalopram is increased to 40 mg/d. His wife tests negative for HCV.
Monitor patients closely during IFN treatment, regardless of whether an antidepressant is prescribed. If depression abruptly worsens or mania emerges, IFN might need to be discontinued until the patient’s psychiatric disorder is stabilized. Adding an atypical antipsychotic—such as olanzapine, 10 to 20 mg/d—can help patients with psychosis, mania, mood lability, impulsivity, or irritability.22
For patients with substantial fatigue, we may supplement antidepressants with modafinil, 100 to 200 mg/d, which caused some improvement in an open trial as measured by the FSS.23 Support groups and cognitive-behavioral therapy have shown modest benefit.
Case Continued: Staying Healthy
Mr. R completes treatment, and his prognosis for remaining virus-free remains good. His fatigue and irritability resolve, and his BDI score returns to 9. One year later, he maintains a negative viral load. He periodically returns to his gastroenterologist for monitoring and continues in a chemical dependency relapse prevention program.
Mr. R acknowledges that his HCV-seronegative status motivates him to stay sober. Citalopram was withdrawn 1 month after he completed antiviral treatment, and his wife has not noted resurgent irritability. He has returned to work, and his supervisors report satisfactory task completion.
Related resources
- American Liver Foundation. www.liverfoundation.org.
- U.S. Centers for Disease Control and Prevention. Viral Hepatitis. www.cdc.gov/hepatitis.
- Hep C Connection. www.hepc-connection.org.
- Hepatitis C Support Project. www.hcvadvocate.org.
- National Institute of Mental Health. Depression. www.nimh.nih.gov/healthinformation/depressionmenu.cfm.
- U.S. Department of Veterans Affairs National Hepatitis C Program. www.hepatitis.va.gov.
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Modafinil • Provigil
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Venlafaxine • Effexor
Dr. Martin, Dr. Krahn, and Ms. Rosati report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Balan receives research grants from Novartis, Roche Pharmaceuticals, Schering-Plough, InterMune, SciClone Pharmaceuticals, and Human Genome Sciences.
1. McCarthy JJ, Flynn N. Hepatitis C in methadone maintenance patients: prevalence and public policy implications. J Addict Dis. 2001;20(1):19-31.
2. Johnson ME, Fisher DG, Fenaughty A, Theno SA. Hepatitis C virus and depression in drug users. Am J Gastroenterol. 1998;93:85-9.
3. Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7(9):942-7.
4. Capuron L, Ravaud A, Neveu PJ, et al. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7(5):468-73.
5. Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon alpha. N Engl J Med. 2001;29;344(13):961-6.
6. Ho SB, Nguyen H, Tetrick LL, et al. Influence of psychiatric diagnoses on interferon-alpha treatment for chronic hepatitis C in a veteran population. Am J Gastroenterol. 2001;96(1):157-64.
7. Pariante CM, Landau S, Carpiniello B. Cagliari Group. Interferon alfa-induced adverse effects in patients with a psychiatric diagnosis (letter). N Engl J Med. 2002;11;347(2):148-9.
8. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa 2b plus ribavirin for initial treatment of chronic hepatitis C: A randomized trial. Lancet. 2001;22;358(9286):958-65.
9. Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-alfa 2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44(2):104-12.
10. Horikawa N, Yamazaki T, Izumi N, Uchihara M. Incidence and clinical course of major depression in patients with chronic hepatitis type C undergoing interferon-alpha therapy: A prospective study. Gen Hosp Psychiatry. 2003;24:34-8.
11. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;26;347(13):975-82.
12. Dieperink E, Ho SB, Tetrick L, et al. Suicidal ideation during interferon-alpha2b and ribavirin treatment of patients with chronic hepatitis C. Gen Hosp Psychiatry. 2004;26(3):237-40.
13. Rifai MA, Moles JK, Lehman LP, Van der Linden BJ. Hepatitis C screening and treatment outcomes in patients with substance use/dependence disorders. Psychosomatics. 2006;(2):112-21.
14. Gleason OC, Yates WR, Isbell MD, Philipsen MA. An open-label trial of citalopram for major depression in patients with hepatitis C. J Clin Psychiatry. 2002;63(3):194-8.
15. Rosenberg SD, Swanson JW, Wolford GL, et al. Blood borne infections and persons with mental illness: the five-site health and risk study of blood-borne infections among persons with severe mental illness. Psychiatr Serv. 2003;54:827-35.
16. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41:88-96.
17. Bini EJ, Brau N, Currie S, et al. Prospective multicenter study of eligibility for antiviral therapy among 4,084 U.S. veterans with chronic hepatitis C infection. Am J Gastroenterol. 2005;100:1772-9.
18. Rifai MA, Moles JK, Short DD. Hepatitis C treatment eligibility and outcomes in patients with psychiatric illness. Psychiatr Serv. 2006;57:(4):570-2.
19. Pegasys [package insert] Roche Pharmaceuticals, Nutley, NJ, 2002.
20. PEG-Intron [package insert] Schering Corp, Kenilworth, NJ, 2001.
21. Geppert CM, Dettmer E, Jakiche A. Ethical challenges in the care of persons with hepatitis C infection: a pilot study to enhance informed consent with veterans. Psychosomatics. 2005;46(5):392-401.
22. D’Innella P, Zaccala G, Terazzi M, Olgiati P, Torre E. Protective effect of olanzapine in psychotic disorder induced by interferon-alpha. Recenti Prog Med. 2003;4(7-8):343-4.
23. Martin KA, Krahn LE, Rosati MJ, Balan V. Modafinil’s use in combating interferon induced fatigue. Dig Dis Sci. In press.
24. Centers for Disease Control and Prevention Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR. 1998;47(RR-19):1-54.
25. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-7.
Mr. R, age 39, is found to have elevated liver function during a routine physical exam by his primary care physician. Subsequent testing reveals chronic hepatitis C viral (HCV) infection.
Starting at age 17, Mr. R abused alcohol and drugs and occasionally shared IV needles. He stopped using street drugs at age 28 when he lost contact with his drug abusing friends and is now married and has two children. In the past 10 years he has had two episodes of major depression, successfully treated with fluoxetine, 40 mg/d. He has no physical or psychiatric symptoms of HCV infection.
IV drug use causes >40% of HCV infections in the United States,1 and substance abusers have increased rates of psychiatric illness, particularly major depression. But substance use does not account fully for the link between HCV infection and depression. A depressive syndrome may explain why depression’s mood and somatic symptoms are seen in significantly more HCV-infected drug users than in noninfected drug users.2
Psychiatrists are often called on to treat HCV-associated depression and other psychiatric symptoms—irritability, insomnia, and impaired concentration—and to support patients who pursue a cure through lengthy interferon treatment. To help you collaborate in the medical/psychiatric care of these patients, this article discusses:
- hepatitis C’s natural history
- diagnostic evaluation
- treatment options
- how to manage treatment’s psychiatric side effects.
Table 1
How Americans contract hepatitis C viral infection
| Risk factor | Percentage of U.S. cases* |
|---|---|
| IV drug use | 42% |
| Having >1 sexual partner | 27% |
| Surgery | 19% |
| Sexual contact with a hepatitis C patient | 14% |
| Household contact with a hepatitis C patient | 6% |
| Percutaneous injury (needlestick) | 5% |
| Employment in medical/dental field | 4% |
| Hemodialysis | |
| Blood transfusion | |
| * Patients could have more than one risk factor for hepatitis C transmission | |
| Source: Centers for Disease Control and Prevention. Hepatitis surveillance report. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. no. 61. | |
Course of Chronic HCV
Mr. R’s primary care physician refers him to a gastroenterologist for liver function evaluation and treatment. Polymerase chain reaction testing reveals a detectable viral level, genotyping indicates that he has HCV type 1a, and liver biopsy shows moderate fibrosis.
As part of the clinic’s treatment protocol, Mr. R is referred to a psychiatrist for evaluation.
The typical interval from HCV infection to diagnosis is 10 to 30 years. Patients with unrecognized HCV infection usually are first treated by primary care physicians, who notice elevated liver function and refer them to a hepatologist or gastroenterologist.
In the United States, HCV is transmitted most frequently through IV drug use, sexual activity, and surgery (Table 1). Nearly all IV drug abusers (65% to 90%) have been exposed to HCV.1 After exposure, 70% of patients develop chronic HCV infection. The disease often is asymptomatic for many years, and some patients never show symptoms. If symptoms develop, they are usually nonspecific, such as fatigue, abdominal discomfort, and nausea, and rarely jaundice and dark urine (Box).
Over time, the disease can progress to cirrhosis and hepatocellular carcinoma. Ten percent to 20% of HCV patients develop cirrhosis a mean 20 years after infection. Serious complications develop more rapidly in patients who:
- are age >40 when infected
- abuse alcohol
- have HIV or coexistent liver disease.
Mood Symptoms with IFN
Significant depressive symptoms occur in 21% to 58% of patients receiving interferon, with major depressive disorder developing at a mean 12 weeks (range 1 to 32 weeks) after therapy begins.3 Other patients develop depressive symptoms that do not meet DSM-IV-TR criteria for major depression.
Manic and hypomanic symptoms also may emerge, such as elevated mood, irritability, inflated self-esteem, insomnia, talkativeness, racing thoughts, distractibility, agitation, and excessive pursuit of pleasurable activities.
The mechanism for psychiatric side effects with IFN is unknown, but nutritional and metabolic alterations are thought to be responsible. One theory holds that IFN decreases CNS tryptophan levels by disrupting the transporter that ferries this essential amino acid across the blood-brain barrier. Deficient tryptophan—the rate-limiting step in serotonin synthesis—results in decreased serotonin levels.4 Another possible explanation is that interferon disrupts the hypothalamic-pituitary axis or more directly alters neural functioning.
Patient history of depression. One study asserted that patients with a history of depression or increased depressive symptoms at baseline are more susceptible to IFN-related psychiatric side effects such as irritability, insomnia, depression, and impaired concentration.5 Other studies, however, show no statistically significant difference in neuropsychiatric symptoms during IFN therapy in patients with preexisting psychiatric disorders and those without such a history.6,7
- HCV affects 2% of the U.S. population but 20% of persons with severe mental illness
- Average annual new infections declined to 36,000 in 1996 from a high of 230,000 in the 1980s, which for reasons that are unclear correlates with a decrease in cases among IV drug users
- Progression of HCV infection is the leading cause of liver transplants in the United States
- Persons infected with HCV are at an increased risk for disease progression if they drink alcohol (>2 drinks/day for men under age 65, >1 drink/day for nonpregnant women and all persons over age 66), are age >40 years at time of infection, or are HIV-positive
- Deaths from acute liver failure are rare
- Chronic HCV infection causes 8,000 to 10,000 deaths per year
Psychiatric assessment. Assess all IFN candidates for present or past psychiatric disorders, including:
- depression
- suicidal thoughts (in one study, 43% of patients on IFN therapy reported suicidal ideation)12
- bipolar disorder (selective serotonin reuptake inhibitors [SSRIs] could induce mania or aggravate cycling)
- chemical dependency (substance abuse may represent the patient’s attempt to self-medicate underlying mood and anxiety symptoms).
Case Continued: Getting Ready
Although Mr. R no longer uses street drugs, he tells the psychiatrist he drinks 2 to 3 beers nightly. Because alcohol use stresses a compromised liver and could undermine IFN therapy’s effectiveness, he agrees to complete a chemical dependency program, demonstrate 6 months of sobriety before starting HCV treatment, and enroll in a chemical dependency relapse prevention program where unannounced drug and alcohol screenings are conducted.
As his IFN treatment approaches, Mr. R agrees to begin prophylactic citalopram, 20 mg/d, because he may be at increased risk for IFN-induced depression. Although Mr. R’s past depressive episodes responded well to fluoxetine, the psychiatrist chooses citalopram during IFN treatment because of its lower risk of drug-drug interactions.
Alcohol and IFN. Continued alcohol use can accelerate HCV-induced liver disease and reduce the likelihood of viral clearance with IFN treatment. One study showed that individuals who enrolled in a substance abuse treatment program were more likely to complete HCV treatment.13
This study also reported that HCV-seropositive patients were more likely to complete a 28-day chemical dependency treatment and remain abstinent 6 months after program discharge, compared with HCV-seronegative patients.13 This suggests that a chronic hepatitis C diagnosis motivates patients to address chemical dependency as a pre-requisite for hepatitis C treatment.
Table 2
Psychiatric side effects with interferon/ribavirin treatment*
| Side effect | Prevalence |
|---|---|
| Irritability, anxiety | 33% to 45% |
| Insomnia | 30% to 40% |
| Depression | 20% to 31% |
| Impaired concentration | 10% to 17% |
| Aggressive behavior | |
| Psychotic disorder | |
| Suicide | |
| * In patients without a history of psychiatric disorders | |
| Source: References 19 and 20 | |
Start antidepressants 2 to 4 weeks before antiviral therapy begins to allow the medication to reach therapeutic efficacy. SSRIs such as paroxetine, 10 to 50 mg/d, and citalopram, 20 to 40 mg/d, have been reported to be effective and do not interact with HCV therapies.5,14 In our experience, dual-action antidepressants such as duloxetine, venlafaxine, or bupropion also can be beneficial.
IFN Treatment Protocol
Mr. R begins a 48-week IFN protocol. To maximize the treatment’s effectiveness, he is given long-acting pegylated interferon, 180 mcg injected weekly, and takes oral ribavirin, 600 mg twice daily.
IFN plus ribavirin. The mainstay of HCV therapy is IFN, a cytokine immunotherapeutic agent. A long-acting IFN administered weekly—called pegylated because the compound is bound to polyethylene glycol—doubles the sustained viral response rate and is now widely used.
Pegylated interferon is often combined with ribavirin—an oral nucleoside analog that has been shown to improve outcomes. Ribavirin increases the risk of hemolysis, however, which mandates frequent blood count monitoring. The NIH recommends pegylated interferon and ribavirin for patients with:
- detectable HCV RNA viral loads >50 copies per ml of blood
- liver biopsy with portal or bridging fibrosis
- and at least moderate inflammation and necrosis.
Forty-eight weeks of treatment are recommended for patients with HCV genotype 1 (70% of patients) and 24 weeks for those with HCV genotypes 2 and 3 (15% to 25% patients).
Some patients—particularly those with genotype 1b—do not respond to IFN therapy. For nonresponders, repeated trials of longer duration or different types of IFN may be tried. Higher IFN dosages or more frequent administration are not viewed as beneficial.
Side effects. Sustained response rates 6 months after patients complete interferon treatment are:
Many of IFN’s early side effects are neurovegetative and overlap with psychiatric symptoms. The more specific psychiatric side effects of irritability, anxiety, insomnia, depression, and impaired concentration develop in 1 to 32 weeks of treatment (mean 12.1 weeks).19,20 Fatigue and depression are the main reasons 10% to 14% of outpatients in large randomized trials discontinue HCV treatment.21
Table 3
HCV testing protocols
| HCVab | If positive then do a HCV Riba |
| HCV Riba | If positive 2 bands or more, then do a HCV Genotype and HCV PCR |
| HCV Genotype | Genotype determines duration of treatment |
| HCV PCR Qualitative and/or Quantitative | Confirms presence of the virus |
| Liver biopsy | Determines the extent of liver damage from fibrosis or cirrhosis |
Case Continued: Preventing Relapse
During therapy, Mr. R completes the BDI and Fatigue Severity Scale (FSS) weekly. His pretreatment BDI score of 9 (normal) increases over time to 22 (mild to moderate depression), and his FSS scores range from 4 to 6, indicating fatigue sufficient to impair daily functioning. Medical and psychiatric staff address his symptoms during weekly treatment assessments.
After 12 weeks of treatment his viral levels are undetectable, but he develops severe fatigue and mild irritability that contribute to arguments with his wife. He is referred to supportive counseling, and citalopram is increased to 40 mg/d. His wife tests negative for HCV.
Monitor patients closely during IFN treatment, regardless of whether an antidepressant is prescribed. If depression abruptly worsens or mania emerges, IFN might need to be discontinued until the patient’s psychiatric disorder is stabilized. Adding an atypical antipsychotic—such as olanzapine, 10 to 20 mg/d—can help patients with psychosis, mania, mood lability, impulsivity, or irritability.22
For patients with substantial fatigue, we may supplement antidepressants with modafinil, 100 to 200 mg/d, which caused some improvement in an open trial as measured by the FSS.23 Support groups and cognitive-behavioral therapy have shown modest benefit.
Case Continued: Staying Healthy
Mr. R completes treatment, and his prognosis for remaining virus-free remains good. His fatigue and irritability resolve, and his BDI score returns to 9. One year later, he maintains a negative viral load. He periodically returns to his gastroenterologist for monitoring and continues in a chemical dependency relapse prevention program.
Mr. R acknowledges that his HCV-seronegative status motivates him to stay sober. Citalopram was withdrawn 1 month after he completed antiviral treatment, and his wife has not noted resurgent irritability. He has returned to work, and his supervisors report satisfactory task completion.
Related resources
- American Liver Foundation. www.liverfoundation.org.
- U.S. Centers for Disease Control and Prevention. Viral Hepatitis. www.cdc.gov/hepatitis.
- Hep C Connection. www.hepc-connection.org.
- Hepatitis C Support Project. www.hcvadvocate.org.
- National Institute of Mental Health. Depression. www.nimh.nih.gov/healthinformation/depressionmenu.cfm.
- U.S. Department of Veterans Affairs National Hepatitis C Program. www.hepatitis.va.gov.
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Modafinil • Provigil
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Venlafaxine • Effexor
Dr. Martin, Dr. Krahn, and Ms. Rosati report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Balan receives research grants from Novartis, Roche Pharmaceuticals, Schering-Plough, InterMune, SciClone Pharmaceuticals, and Human Genome Sciences.
Mr. R, age 39, is found to have elevated liver function during a routine physical exam by his primary care physician. Subsequent testing reveals chronic hepatitis C viral (HCV) infection.
Starting at age 17, Mr. R abused alcohol and drugs and occasionally shared IV needles. He stopped using street drugs at age 28 when he lost contact with his drug abusing friends and is now married and has two children. In the past 10 years he has had two episodes of major depression, successfully treated with fluoxetine, 40 mg/d. He has no physical or psychiatric symptoms of HCV infection.
IV drug use causes >40% of HCV infections in the United States,1 and substance abusers have increased rates of psychiatric illness, particularly major depression. But substance use does not account fully for the link between HCV infection and depression. A depressive syndrome may explain why depression’s mood and somatic symptoms are seen in significantly more HCV-infected drug users than in noninfected drug users.2
Psychiatrists are often called on to treat HCV-associated depression and other psychiatric symptoms—irritability, insomnia, and impaired concentration—and to support patients who pursue a cure through lengthy interferon treatment. To help you collaborate in the medical/psychiatric care of these patients, this article discusses:
- hepatitis C’s natural history
- diagnostic evaluation
- treatment options
- how to manage treatment’s psychiatric side effects.
Table 1
How Americans contract hepatitis C viral infection
| Risk factor | Percentage of U.S. cases* |
|---|---|
| IV drug use | 42% |
| Having >1 sexual partner | 27% |
| Surgery | 19% |
| Sexual contact with a hepatitis C patient | 14% |
| Household contact with a hepatitis C patient | 6% |
| Percutaneous injury (needlestick) | 5% |
| Employment in medical/dental field | 4% |
| Hemodialysis | |
| Blood transfusion | |
| * Patients could have more than one risk factor for hepatitis C transmission | |
| Source: Centers for Disease Control and Prevention. Hepatitis surveillance report. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. no. 61. | |
Course of Chronic HCV
Mr. R’s primary care physician refers him to a gastroenterologist for liver function evaluation and treatment. Polymerase chain reaction testing reveals a detectable viral level, genotyping indicates that he has HCV type 1a, and liver biopsy shows moderate fibrosis.
As part of the clinic’s treatment protocol, Mr. R is referred to a psychiatrist for evaluation.
The typical interval from HCV infection to diagnosis is 10 to 30 years. Patients with unrecognized HCV infection usually are first treated by primary care physicians, who notice elevated liver function and refer them to a hepatologist or gastroenterologist.
In the United States, HCV is transmitted most frequently through IV drug use, sexual activity, and surgery (Table 1). Nearly all IV drug abusers (65% to 90%) have been exposed to HCV.1 After exposure, 70% of patients develop chronic HCV infection. The disease often is asymptomatic for many years, and some patients never show symptoms. If symptoms develop, they are usually nonspecific, such as fatigue, abdominal discomfort, and nausea, and rarely jaundice and dark urine (Box).
Over time, the disease can progress to cirrhosis and hepatocellular carcinoma. Ten percent to 20% of HCV patients develop cirrhosis a mean 20 years after infection. Serious complications develop more rapidly in patients who:
- are age >40 when infected
- abuse alcohol
- have HIV or coexistent liver disease.
Mood Symptoms with IFN
Significant depressive symptoms occur in 21% to 58% of patients receiving interferon, with major depressive disorder developing at a mean 12 weeks (range 1 to 32 weeks) after therapy begins.3 Other patients develop depressive symptoms that do not meet DSM-IV-TR criteria for major depression.
Manic and hypomanic symptoms also may emerge, such as elevated mood, irritability, inflated self-esteem, insomnia, talkativeness, racing thoughts, distractibility, agitation, and excessive pursuit of pleasurable activities.
The mechanism for psychiatric side effects with IFN is unknown, but nutritional and metabolic alterations are thought to be responsible. One theory holds that IFN decreases CNS tryptophan levels by disrupting the transporter that ferries this essential amino acid across the blood-brain barrier. Deficient tryptophan—the rate-limiting step in serotonin synthesis—results in decreased serotonin levels.4 Another possible explanation is that interferon disrupts the hypothalamic-pituitary axis or more directly alters neural functioning.
Patient history of depression. One study asserted that patients with a history of depression or increased depressive symptoms at baseline are more susceptible to IFN-related psychiatric side effects such as irritability, insomnia, depression, and impaired concentration.5 Other studies, however, show no statistically significant difference in neuropsychiatric symptoms during IFN therapy in patients with preexisting psychiatric disorders and those without such a history.6,7
- HCV affects 2% of the U.S. population but 20% of persons with severe mental illness
- Average annual new infections declined to 36,000 in 1996 from a high of 230,000 in the 1980s, which for reasons that are unclear correlates with a decrease in cases among IV drug users
- Progression of HCV infection is the leading cause of liver transplants in the United States
- Persons infected with HCV are at an increased risk for disease progression if they drink alcohol (>2 drinks/day for men under age 65, >1 drink/day for nonpregnant women and all persons over age 66), are age >40 years at time of infection, or are HIV-positive
- Deaths from acute liver failure are rare
- Chronic HCV infection causes 8,000 to 10,000 deaths per year
Psychiatric assessment. Assess all IFN candidates for present or past psychiatric disorders, including:
- depression
- suicidal thoughts (in one study, 43% of patients on IFN therapy reported suicidal ideation)12
- bipolar disorder (selective serotonin reuptake inhibitors [SSRIs] could induce mania or aggravate cycling)
- chemical dependency (substance abuse may represent the patient’s attempt to self-medicate underlying mood and anxiety symptoms).
Case Continued: Getting Ready
Although Mr. R no longer uses street drugs, he tells the psychiatrist he drinks 2 to 3 beers nightly. Because alcohol use stresses a compromised liver and could undermine IFN therapy’s effectiveness, he agrees to complete a chemical dependency program, demonstrate 6 months of sobriety before starting HCV treatment, and enroll in a chemical dependency relapse prevention program where unannounced drug and alcohol screenings are conducted.
As his IFN treatment approaches, Mr. R agrees to begin prophylactic citalopram, 20 mg/d, because he may be at increased risk for IFN-induced depression. Although Mr. R’s past depressive episodes responded well to fluoxetine, the psychiatrist chooses citalopram during IFN treatment because of its lower risk of drug-drug interactions.
Alcohol and IFN. Continued alcohol use can accelerate HCV-induced liver disease and reduce the likelihood of viral clearance with IFN treatment. One study showed that individuals who enrolled in a substance abuse treatment program were more likely to complete HCV treatment.13
This study also reported that HCV-seropositive patients were more likely to complete a 28-day chemical dependency treatment and remain abstinent 6 months after program discharge, compared with HCV-seronegative patients.13 This suggests that a chronic hepatitis C diagnosis motivates patients to address chemical dependency as a pre-requisite for hepatitis C treatment.
Table 2
Psychiatric side effects with interferon/ribavirin treatment*
| Side effect | Prevalence |
|---|---|
| Irritability, anxiety | 33% to 45% |
| Insomnia | 30% to 40% |
| Depression | 20% to 31% |
| Impaired concentration | 10% to 17% |
| Aggressive behavior | |
| Psychotic disorder | |
| Suicide | |
| * In patients without a history of psychiatric disorders | |
| Source: References 19 and 20 | |
Start antidepressants 2 to 4 weeks before antiviral therapy begins to allow the medication to reach therapeutic efficacy. SSRIs such as paroxetine, 10 to 50 mg/d, and citalopram, 20 to 40 mg/d, have been reported to be effective and do not interact with HCV therapies.5,14 In our experience, dual-action antidepressants such as duloxetine, venlafaxine, or bupropion also can be beneficial.
IFN Treatment Protocol
Mr. R begins a 48-week IFN protocol. To maximize the treatment’s effectiveness, he is given long-acting pegylated interferon, 180 mcg injected weekly, and takes oral ribavirin, 600 mg twice daily.
IFN plus ribavirin. The mainstay of HCV therapy is IFN, a cytokine immunotherapeutic agent. A long-acting IFN administered weekly—called pegylated because the compound is bound to polyethylene glycol—doubles the sustained viral response rate and is now widely used.
Pegylated interferon is often combined with ribavirin—an oral nucleoside analog that has been shown to improve outcomes. Ribavirin increases the risk of hemolysis, however, which mandates frequent blood count monitoring. The NIH recommends pegylated interferon and ribavirin for patients with:
- detectable HCV RNA viral loads >50 copies per ml of blood
- liver biopsy with portal or bridging fibrosis
- and at least moderate inflammation and necrosis.
Forty-eight weeks of treatment are recommended for patients with HCV genotype 1 (70% of patients) and 24 weeks for those with HCV genotypes 2 and 3 (15% to 25% patients).
Some patients—particularly those with genotype 1b—do not respond to IFN therapy. For nonresponders, repeated trials of longer duration or different types of IFN may be tried. Higher IFN dosages or more frequent administration are not viewed as beneficial.
Side effects. Sustained response rates 6 months after patients complete interferon treatment are:
Many of IFN’s early side effects are neurovegetative and overlap with psychiatric symptoms. The more specific psychiatric side effects of irritability, anxiety, insomnia, depression, and impaired concentration develop in 1 to 32 weeks of treatment (mean 12.1 weeks).19,20 Fatigue and depression are the main reasons 10% to 14% of outpatients in large randomized trials discontinue HCV treatment.21
Table 3
HCV testing protocols
| HCVab | If positive then do a HCV Riba |
| HCV Riba | If positive 2 bands or more, then do a HCV Genotype and HCV PCR |
| HCV Genotype | Genotype determines duration of treatment |
| HCV PCR Qualitative and/or Quantitative | Confirms presence of the virus |
| Liver biopsy | Determines the extent of liver damage from fibrosis or cirrhosis |
Case Continued: Preventing Relapse
During therapy, Mr. R completes the BDI and Fatigue Severity Scale (FSS) weekly. His pretreatment BDI score of 9 (normal) increases over time to 22 (mild to moderate depression), and his FSS scores range from 4 to 6, indicating fatigue sufficient to impair daily functioning. Medical and psychiatric staff address his symptoms during weekly treatment assessments.
After 12 weeks of treatment his viral levels are undetectable, but he develops severe fatigue and mild irritability that contribute to arguments with his wife. He is referred to supportive counseling, and citalopram is increased to 40 mg/d. His wife tests negative for HCV.
Monitor patients closely during IFN treatment, regardless of whether an antidepressant is prescribed. If depression abruptly worsens or mania emerges, IFN might need to be discontinued until the patient’s psychiatric disorder is stabilized. Adding an atypical antipsychotic—such as olanzapine, 10 to 20 mg/d—can help patients with psychosis, mania, mood lability, impulsivity, or irritability.22
For patients with substantial fatigue, we may supplement antidepressants with modafinil, 100 to 200 mg/d, which caused some improvement in an open trial as measured by the FSS.23 Support groups and cognitive-behavioral therapy have shown modest benefit.
Case Continued: Staying Healthy
Mr. R completes treatment, and his prognosis for remaining virus-free remains good. His fatigue and irritability resolve, and his BDI score returns to 9. One year later, he maintains a negative viral load. He periodically returns to his gastroenterologist for monitoring and continues in a chemical dependency relapse prevention program.
Mr. R acknowledges that his HCV-seronegative status motivates him to stay sober. Citalopram was withdrawn 1 month after he completed antiviral treatment, and his wife has not noted resurgent irritability. He has returned to work, and his supervisors report satisfactory task completion.
Related resources
- American Liver Foundation. www.liverfoundation.org.
- U.S. Centers for Disease Control and Prevention. Viral Hepatitis. www.cdc.gov/hepatitis.
- Hep C Connection. www.hepc-connection.org.
- Hepatitis C Support Project. www.hcvadvocate.org.
- National Institute of Mental Health. Depression. www.nimh.nih.gov/healthinformation/depressionmenu.cfm.
- U.S. Department of Veterans Affairs National Hepatitis C Program. www.hepatitis.va.gov.
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Modafinil • Provigil
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Venlafaxine • Effexor
Dr. Martin, Dr. Krahn, and Ms. Rosati report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Balan receives research grants from Novartis, Roche Pharmaceuticals, Schering-Plough, InterMune, SciClone Pharmaceuticals, and Human Genome Sciences.
1. McCarthy JJ, Flynn N. Hepatitis C in methadone maintenance patients: prevalence and public policy implications. J Addict Dis. 2001;20(1):19-31.
2. Johnson ME, Fisher DG, Fenaughty A, Theno SA. Hepatitis C virus and depression in drug users. Am J Gastroenterol. 1998;93:85-9.
3. Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7(9):942-7.
4. Capuron L, Ravaud A, Neveu PJ, et al. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7(5):468-73.
5. Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon alpha. N Engl J Med. 2001;29;344(13):961-6.
6. Ho SB, Nguyen H, Tetrick LL, et al. Influence of psychiatric diagnoses on interferon-alpha treatment for chronic hepatitis C in a veteran population. Am J Gastroenterol. 2001;96(1):157-64.
7. Pariante CM, Landau S, Carpiniello B. Cagliari Group. Interferon alfa-induced adverse effects in patients with a psychiatric diagnosis (letter). N Engl J Med. 2002;11;347(2):148-9.
8. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa 2b plus ribavirin for initial treatment of chronic hepatitis C: A randomized trial. Lancet. 2001;22;358(9286):958-65.
9. Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-alfa 2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44(2):104-12.
10. Horikawa N, Yamazaki T, Izumi N, Uchihara M. Incidence and clinical course of major depression in patients with chronic hepatitis type C undergoing interferon-alpha therapy: A prospective study. Gen Hosp Psychiatry. 2003;24:34-8.
11. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;26;347(13):975-82.
12. Dieperink E, Ho SB, Tetrick L, et al. Suicidal ideation during interferon-alpha2b and ribavirin treatment of patients with chronic hepatitis C. Gen Hosp Psychiatry. 2004;26(3):237-40.
13. Rifai MA, Moles JK, Lehman LP, Van der Linden BJ. Hepatitis C screening and treatment outcomes in patients with substance use/dependence disorders. Psychosomatics. 2006;(2):112-21.
14. Gleason OC, Yates WR, Isbell MD, Philipsen MA. An open-label trial of citalopram for major depression in patients with hepatitis C. J Clin Psychiatry. 2002;63(3):194-8.
15. Rosenberg SD, Swanson JW, Wolford GL, et al. Blood borne infections and persons with mental illness: the five-site health and risk study of blood-borne infections among persons with severe mental illness. Psychiatr Serv. 2003;54:827-35.
16. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41:88-96.
17. Bini EJ, Brau N, Currie S, et al. Prospective multicenter study of eligibility for antiviral therapy among 4,084 U.S. veterans with chronic hepatitis C infection. Am J Gastroenterol. 2005;100:1772-9.
18. Rifai MA, Moles JK, Short DD. Hepatitis C treatment eligibility and outcomes in patients with psychiatric illness. Psychiatr Serv. 2006;57:(4):570-2.
19. Pegasys [package insert] Roche Pharmaceuticals, Nutley, NJ, 2002.
20. PEG-Intron [package insert] Schering Corp, Kenilworth, NJ, 2001.
21. Geppert CM, Dettmer E, Jakiche A. Ethical challenges in the care of persons with hepatitis C infection: a pilot study to enhance informed consent with veterans. Psychosomatics. 2005;46(5):392-401.
22. D’Innella P, Zaccala G, Terazzi M, Olgiati P, Torre E. Protective effect of olanzapine in psychotic disorder induced by interferon-alpha. Recenti Prog Med. 2003;4(7-8):343-4.
23. Martin KA, Krahn LE, Rosati MJ, Balan V. Modafinil’s use in combating interferon induced fatigue. Dig Dis Sci. In press.
24. Centers for Disease Control and Prevention Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR. 1998;47(RR-19):1-54.
25. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-7.
1. McCarthy JJ, Flynn N. Hepatitis C in methadone maintenance patients: prevalence and public policy implications. J Addict Dis. 2001;20(1):19-31.
2. Johnson ME, Fisher DG, Fenaughty A, Theno SA. Hepatitis C virus and depression in drug users. Am J Gastroenterol. 1998;93:85-9.
3. Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7(9):942-7.
4. Capuron L, Ravaud A, Neveu PJ, et al. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7(5):468-73.
5. Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon alpha. N Engl J Med. 2001;29;344(13):961-6.
6. Ho SB, Nguyen H, Tetrick LL, et al. Influence of psychiatric diagnoses on interferon-alpha treatment for chronic hepatitis C in a veteran population. Am J Gastroenterol. 2001;96(1):157-64.
7. Pariante CM, Landau S, Carpiniello B. Cagliari Group. Interferon alfa-induced adverse effects in patients with a psychiatric diagnosis (letter). N Engl J Med. 2002;11;347(2):148-9.
8. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa 2b plus ribavirin for initial treatment of chronic hepatitis C: A randomized trial. Lancet. 2001;22;358(9286):958-65.
9. Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-alfa 2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44(2):104-12.
10. Horikawa N, Yamazaki T, Izumi N, Uchihara M. Incidence and clinical course of major depression in patients with chronic hepatitis type C undergoing interferon-alpha therapy: A prospective study. Gen Hosp Psychiatry. 2003;24:34-8.
11. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;26;347(13):975-82.
12. Dieperink E, Ho SB, Tetrick L, et al. Suicidal ideation during interferon-alpha2b and ribavirin treatment of patients with chronic hepatitis C. Gen Hosp Psychiatry. 2004;26(3):237-40.
13. Rifai MA, Moles JK, Lehman LP, Van der Linden BJ. Hepatitis C screening and treatment outcomes in patients with substance use/dependence disorders. Psychosomatics. 2006;(2):112-21.
14. Gleason OC, Yates WR, Isbell MD, Philipsen MA. An open-label trial of citalopram for major depression in patients with hepatitis C. J Clin Psychiatry. 2002;63(3):194-8.
15. Rosenberg SD, Swanson JW, Wolford GL, et al. Blood borne infections and persons with mental illness: the five-site health and risk study of blood-borne infections among persons with severe mental illness. Psychiatr Serv. 2003;54:827-35.
16. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41:88-96.
17. Bini EJ, Brau N, Currie S, et al. Prospective multicenter study of eligibility for antiviral therapy among 4,084 U.S. veterans with chronic hepatitis C infection. Am J Gastroenterol. 2005;100:1772-9.
18. Rifai MA, Moles JK, Short DD. Hepatitis C treatment eligibility and outcomes in patients with psychiatric illness. Psychiatr Serv. 2006;57:(4):570-2.
19. Pegasys [package insert] Roche Pharmaceuticals, Nutley, NJ, 2002.
20. PEG-Intron [package insert] Schering Corp, Kenilworth, NJ, 2001.
21. Geppert CM, Dettmer E, Jakiche A. Ethical challenges in the care of persons with hepatitis C infection: a pilot study to enhance informed consent with veterans. Psychosomatics. 2005;46(5):392-401.
22. D’Innella P, Zaccala G, Terazzi M, Olgiati P, Torre E. Protective effect of olanzapine in psychotic disorder induced by interferon-alpha. Recenti Prog Med. 2003;4(7-8):343-4.
23. Martin KA, Krahn LE, Rosati MJ, Balan V. Modafinil’s use in combating interferon induced fatigue. Dig Dis Sci. In press.
24. Centers for Disease Control and Prevention Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR. 1998;47(RR-19):1-54.
25. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-7.