User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Is this patient dangerous?
Will this patient turn violent?” Psychiatrists face this tough question every day. Although predicting a complex behavior such as violence is nearly impossible, we can prepare for dangerous behavior and improve our safety by:
- knowing the risk factors for patient violence
- assessing individuals for violence potential before clinical encounters
- controlling situations to reduce injury risk.
In one study, more than 50% of psychiatrists and 75% of mental health nurses reported an act or threat of violence from patients within the past year.1 To help you avoid becoming a statistic, this article provides a 5-step procedure (Box) to quickly assess and respond to risk of violence in a psychiatric patient.
1 Seek patient history of violence; if positive, obtain details of violent behavior
2 Evaluate the context, including the patient’s psychosocial stressors, recent behavior
3 Identify arousal states such as fear, anger, confusion, or humiliation that are risk factors for violence
4 Structure the interview for safety, with attention to the treatment environment
5 Evaluate the patient in a structured clinical encounter
Step 1: Seek Patient History
A careful review of past events and those immediately preceding the clinical encounter is the best tool for assessing potential for violence. The more you can learn from the patient chart and other sources before you see the patient, the better (Table 1). Valuable clues can be obtained from interviews with family members, outpatient providers, police officers, and others who have had pertinent social contact with the patient.
Table 1
Will this patient become violent?
Questions to consider before a clinical encounter
| Long-term behavior What violent acts has this patient committed? Were conditions similar with each episode? Any unprovoked acts? Was violence associated with alcohol or drug use? How has the patient behaved with health care providers in the hospital? In outpatient settings? |
| Immediate situation What are the patient’s immediate stressors? Did he or she arrive with family or in police custody? How did the patient behave while en route? |
A minority of patients account for most aggressive acts in clinical encounters. One study showed that recidivists committed 53% of all violent behaviors in a health care setting.4 A patient’s history of violence should be flagged in the chart and verbally passed on to staff to alert providers of increased risk.
However, not having a violent history does not guarantee that a patient will not become dangerous during a clinical encounter. All patients with a violent past had an initial violent episode, and that first time can occur in a practice setting.
Psychotic states by themselves appear to increase the risk of violence, although the literature is mixed.5,6 Clearly, however, psychotic states associated with arousal or agitation do predispose patients to violence, especially if the psychosis involves active paranoid delusions or hallucinations associated with negative affect (anger, sadness, anxiety).7
Increased rates of violence have also been reported in psychiatric patients with:
- acute manic states associated with arousal or agitation8
- nonspecific neurologic abnormalities such as abnormal EEGs, localizing neurologic signs, or “soft signs” (impaired face-hand test, graphesthesia, stereognosis).9
Psychiatric diagnoses associated with increased risk of violence include schizophrenia, bipolar mania, alcohol and other substance abuse, and personality disorders.11-13 In clinical practice, however, I find psychiatric diagnoses less useful in predicting violence than the patient’s arousal state and the other risk factors discussed above.
Step 2: Evaluate the Context
In addition to evidence-supported risk factors (Table 2), context—or the broader situation in which a patient is embedded at the time of psychiatric evaluation—plays a prominent role in potentially violent situations. For example, if “divorce” is listed as a presenting factor:
- Is the patient recently divorced, or did it occur years ago?
- Does he hate all women or just his ex-wife?
- Was she having an affair, and did he just learn about this?
Table 2
Risk factors for violence among psychiatric patients*
|
| *As identified in the literature |
Step 3: Identify Arousal States
Patients rarely commit violent acts when their anxiety and moods are well controlled. They are more likely to become aggressive in high arousal states.
Fear is probably an element of most situations where patients act out violently. Because the fearful patient may not exhibit easily interpreted danger signals, however, you may unwittingly provoke an assault by violating his or her personal space. A fearful, paranoid patient requires a greater-than-usual “intimate zone,” although this need for increased space may not be obvious.
Minimize provocation by explaining your actions and behaviors in advance (such as, “I would like to enter the room, sit down, and talk with you for about 20 minutes”). Be businesslike with paranoid patients. Avoid exuding warmth, as they may view attempts at warmth as having sinister intent.
Clinicians are sometimes injured when trying to prevent a fearful, paranoid patient from fleeing. To avoid injury, don’t stand between the patient and the door. Let the patient escape from the immediate situation, and enlist security or police in further intervention attempts.
Anger is easy to recognize by signs of mounting tension. Loud voice, inappropriate staring, banging objects, clenched fists, agitated pacing, and verbal threats are common in the angry patient before a violent episode. Although this seems self-evident, it is surprising how many violent acts occur when these signs are obvious and noted by staff, yet no de-escalation measures are taken.
A patient’s verbal threats can actually help the clinician. This “red flag” alerts staff to focus on de-escalation techniques and prepare for a violent situation.
Confusion can be an underlying risk factor in patients with delirium or nonspecific organic brain syndrome. These patients may strike out unexpectedly when health care personnel are attempting to do routine procedures, and clinicians are sometimes caught off-guard when operating in a care-giving rather than defensive mode.
Clinicians can often avoid arousing confused patients by using orienting techniques and explaining their actions. For example, a nurse might say, “Hello Mr. X, I am a nurse and you are in this hospital for treatment of your illness. I will need to use this machine to check your blood pressure.”
Humiliation. Men in particular can react aggressively to loss of self-esteem and feelings of powerlessness. Take note if a man has been humiliated in front of family before being brought for evaluation; for example, was he removed by police in an emergency detention situation? This patient may need to act out violently to restore his sense of self.
Staff can lessen a patient’s potential to act on humiliation by using a therapeutic, esteem-building interview technique. For example, address the patient as “Mr.” instead of by first name, and highlight his strengths or accomplishments early in the interview.
Step 4: Structure the Interview for Safety
The time you take before an interview to learn about a patient’s violence history, context, and arousal state is time well-spent and more patient-specific than past diagnoses. This information allows you to prepare for a safe intervention.
Interview environment. The physical and social environment where you interview the patient may contribute to violence potential.
- Is the patient being interviewed in a cramped room or an open hallway?
- Is the evaluation unit overcrowded?
- Are security personnel visible?
- Is the examiner of the same race or ethnic background as the patient?
Take control of the interview and treatment situation. Use the physical space and personnel as you would any other intervention tool—to increase safety and decrease potential for violent behavior. For example, some patients do better when interviewed in a small, private setting. Other interviews must be conducted in a triage area while police escorts hold the patient and handcuffs remain on.
Ideally, you and the patient should have equal access to the door if you conduct the psychiatric interview in an enclosed room. With high-risk patients, arrange your seating at a 90-degree angle—rather than face-to-face-to limit sustained, confrontational eye contact. Sit at greater than an arm swing or leg kick away from the patient, and require him or her to remain seated during the interview (or you will promptly leave).
In the outpatient practice, terminate the interview or evaluation session if a patient in a negative affective arousal state does not allow verbal redirection. Before you make any movement to exit, however, announce, “I am leaving the room now.”
Trust your intuition. I do not enter a closed, private space with a patient unless I feel safe. If I feel afraid, I take that as a valuable warning that further safety measures are necessary.
Use restraints as needed. When patients with a history of violence are brought to the hospital in high arousal states, I let them remain in restraint with security present during the initial interview. If the patient cannot have a back-and-forth conversation with me, I keep the security force present until I believe my verbal interactions have a substantial effect.
Patients must be responsive to talking interventions before restraint, security, or other environmental safety measures are removed. Some patients do not reach this point until after tranquilizing medications are given.
Step 5: The Clinical Encounter
When discussing how to assess the likelihood of patient violence during a clinical encounter, a psychiatric colleague once commented, “Risk factors make you worry more; nothing makes you worry less.”
In other words, keep your guard up. Let clinical judgment take precedence over statistics when you are evaluating any patient. Statistics represent frequencies or averages; they may or may not apply to any one individual.
Techniques for assessing and treating violent patients are beyond the scope of this article, but at the very least:
- obtain training in safety/treatment protocols for violent patients
- ensure that your hospital/clinic has procedures in place to improve safety and to handle violent situations.
For every violent act that requires staff intervention, automatically schedule a debriefing session for those involved to assess the incident and allow them to express their feelings.
Related resources
- American Association for Emergency Psychiatry. www.emergencypsychiatry.org.
- Volavka J. The neurobiology of violence: an update. J Neuropsychiatry Clin Neurosci 1999;11:307-14.
- McNiel DE, Eisner JP, Binder RL. The relationship between command hallucinations and violence. Psychiatr Serv 2000;51: 1288-92.
1. Nolan P, Dallender J, Soares J, et al. Violence in mental health care: the experiences of mental health nurses and psychiatrists. J Adv Nurs 1999;30:934-41.
2. Blomhoff S, Seim S, Friis S. Can prediction of violence among psychiatric inpatients be improved? Hosp Community Psychiatry 1990;41:771-5.
3. Convit A, Isay D, Otis D, et al. Characteristics of repeatedly assaultive psychiatric inpatients. Hosp Community Psychiatry 1990;41:1112-5.
4. Taylor P. Motives for offending among violent and psychotic men. Br J Psychiatry 1985;147:491-8.
5. Junginger J, Parks-Levy J, McGuire L. Delusions and symptom-consistent violence. Psychiatr Serv 1998;49:218-20.
6. Cheung P, Schweitzer I, Crowley K, et al. Violence in schizophrenia: role of hallucinations and delusions. Schizophr Res 1997;26:181-90.
7. Binder R, McNiel D. Effects of diagnosis and context on dangerousness. Am J Psychiatry 1988;145:728-32.
8. Convit A, Jaeger J, Pin Lin S, et al. Predicting assaultiveness in psychiatric inpatients: A pilot study. Hosp Community Psychiatry 1988;39:429-34.
9. Hyman S. The violent patient. In: Hyman S, ed. Manual of psychiatric emergencies. Boston: Little, Brown and Co.; 1988:23-31.
10. Swartz M, Swanson J, Hiday V, et al. Violence and severe mental illness: the effects of substance abuse and nonadherence to medication. Am J Psychiatry 1998;155:226-31.
11. Owen C, Tarantello C, Jones M, et al. Repetitively violent patients in psychiatric units. Psychiatr Serv 1998;49:1458-61.
12. Citrome L, Volavka J. Clinical management of persistent aggressive behavior in schizophrenia, part I. Definitions, epidemiology, assessment and acute treatment. Essen Psychopharmacol 2002;5:1-16.
13. Abeyasinghe R, Jayasekera R. Violence in a general hospital psychiatry unit for men. Ceylon Med J 2003;48(2):45-7.
Will this patient turn violent?” Psychiatrists face this tough question every day. Although predicting a complex behavior such as violence is nearly impossible, we can prepare for dangerous behavior and improve our safety by:
- knowing the risk factors for patient violence
- assessing individuals for violence potential before clinical encounters
- controlling situations to reduce injury risk.
In one study, more than 50% of psychiatrists and 75% of mental health nurses reported an act or threat of violence from patients within the past year.1 To help you avoid becoming a statistic, this article provides a 5-step procedure (Box) to quickly assess and respond to risk of violence in a psychiatric patient.
1 Seek patient history of violence; if positive, obtain details of violent behavior
2 Evaluate the context, including the patient’s psychosocial stressors, recent behavior
3 Identify arousal states such as fear, anger, confusion, or humiliation that are risk factors for violence
4 Structure the interview for safety, with attention to the treatment environment
5 Evaluate the patient in a structured clinical encounter
Step 1: Seek Patient History
A careful review of past events and those immediately preceding the clinical encounter is the best tool for assessing potential for violence. The more you can learn from the patient chart and other sources before you see the patient, the better (Table 1). Valuable clues can be obtained from interviews with family members, outpatient providers, police officers, and others who have had pertinent social contact with the patient.
Table 1
Will this patient become violent?
Questions to consider before a clinical encounter
| Long-term behavior What violent acts has this patient committed? Were conditions similar with each episode? Any unprovoked acts? Was violence associated with alcohol or drug use? How has the patient behaved with health care providers in the hospital? In outpatient settings? |
| Immediate situation What are the patient’s immediate stressors? Did he or she arrive with family or in police custody? How did the patient behave while en route? |
A minority of patients account for most aggressive acts in clinical encounters. One study showed that recidivists committed 53% of all violent behaviors in a health care setting.4 A patient’s history of violence should be flagged in the chart and verbally passed on to staff to alert providers of increased risk.
However, not having a violent history does not guarantee that a patient will not become dangerous during a clinical encounter. All patients with a violent past had an initial violent episode, and that first time can occur in a practice setting.
Psychotic states by themselves appear to increase the risk of violence, although the literature is mixed.5,6 Clearly, however, psychotic states associated with arousal or agitation do predispose patients to violence, especially if the psychosis involves active paranoid delusions or hallucinations associated with negative affect (anger, sadness, anxiety).7
Increased rates of violence have also been reported in psychiatric patients with:
- acute manic states associated with arousal or agitation8
- nonspecific neurologic abnormalities such as abnormal EEGs, localizing neurologic signs, or “soft signs” (impaired face-hand test, graphesthesia, stereognosis).9
Psychiatric diagnoses associated with increased risk of violence include schizophrenia, bipolar mania, alcohol and other substance abuse, and personality disorders.11-13 In clinical practice, however, I find psychiatric diagnoses less useful in predicting violence than the patient’s arousal state and the other risk factors discussed above.
Step 2: Evaluate the Context
In addition to evidence-supported risk factors (Table 2), context—or the broader situation in which a patient is embedded at the time of psychiatric evaluation—plays a prominent role in potentially violent situations. For example, if “divorce” is listed as a presenting factor:
- Is the patient recently divorced, or did it occur years ago?
- Does he hate all women or just his ex-wife?
- Was she having an affair, and did he just learn about this?
Table 2
Risk factors for violence among psychiatric patients*
|
| *As identified in the literature |
Step 3: Identify Arousal States
Patients rarely commit violent acts when their anxiety and moods are well controlled. They are more likely to become aggressive in high arousal states.
Fear is probably an element of most situations where patients act out violently. Because the fearful patient may not exhibit easily interpreted danger signals, however, you may unwittingly provoke an assault by violating his or her personal space. A fearful, paranoid patient requires a greater-than-usual “intimate zone,” although this need for increased space may not be obvious.
Minimize provocation by explaining your actions and behaviors in advance (such as, “I would like to enter the room, sit down, and talk with you for about 20 minutes”). Be businesslike with paranoid patients. Avoid exuding warmth, as they may view attempts at warmth as having sinister intent.
Clinicians are sometimes injured when trying to prevent a fearful, paranoid patient from fleeing. To avoid injury, don’t stand between the patient and the door. Let the patient escape from the immediate situation, and enlist security or police in further intervention attempts.
Anger is easy to recognize by signs of mounting tension. Loud voice, inappropriate staring, banging objects, clenched fists, agitated pacing, and verbal threats are common in the angry patient before a violent episode. Although this seems self-evident, it is surprising how many violent acts occur when these signs are obvious and noted by staff, yet no de-escalation measures are taken.
A patient’s verbal threats can actually help the clinician. This “red flag” alerts staff to focus on de-escalation techniques and prepare for a violent situation.
Confusion can be an underlying risk factor in patients with delirium or nonspecific organic brain syndrome. These patients may strike out unexpectedly when health care personnel are attempting to do routine procedures, and clinicians are sometimes caught off-guard when operating in a care-giving rather than defensive mode.
Clinicians can often avoid arousing confused patients by using orienting techniques and explaining their actions. For example, a nurse might say, “Hello Mr. X, I am a nurse and you are in this hospital for treatment of your illness. I will need to use this machine to check your blood pressure.”
Humiliation. Men in particular can react aggressively to loss of self-esteem and feelings of powerlessness. Take note if a man has been humiliated in front of family before being brought for evaluation; for example, was he removed by police in an emergency detention situation? This patient may need to act out violently to restore his sense of self.
Staff can lessen a patient’s potential to act on humiliation by using a therapeutic, esteem-building interview technique. For example, address the patient as “Mr.” instead of by first name, and highlight his strengths or accomplishments early in the interview.
Step 4: Structure the Interview for Safety
The time you take before an interview to learn about a patient’s violence history, context, and arousal state is time well-spent and more patient-specific than past diagnoses. This information allows you to prepare for a safe intervention.
Interview environment. The physical and social environment where you interview the patient may contribute to violence potential.
- Is the patient being interviewed in a cramped room or an open hallway?
- Is the evaluation unit overcrowded?
- Are security personnel visible?
- Is the examiner of the same race or ethnic background as the patient?
Take control of the interview and treatment situation. Use the physical space and personnel as you would any other intervention tool—to increase safety and decrease potential for violent behavior. For example, some patients do better when interviewed in a small, private setting. Other interviews must be conducted in a triage area while police escorts hold the patient and handcuffs remain on.
Ideally, you and the patient should have equal access to the door if you conduct the psychiatric interview in an enclosed room. With high-risk patients, arrange your seating at a 90-degree angle—rather than face-to-face-to limit sustained, confrontational eye contact. Sit at greater than an arm swing or leg kick away from the patient, and require him or her to remain seated during the interview (or you will promptly leave).
In the outpatient practice, terminate the interview or evaluation session if a patient in a negative affective arousal state does not allow verbal redirection. Before you make any movement to exit, however, announce, “I am leaving the room now.”
Trust your intuition. I do not enter a closed, private space with a patient unless I feel safe. If I feel afraid, I take that as a valuable warning that further safety measures are necessary.
Use restraints as needed. When patients with a history of violence are brought to the hospital in high arousal states, I let them remain in restraint with security present during the initial interview. If the patient cannot have a back-and-forth conversation with me, I keep the security force present until I believe my verbal interactions have a substantial effect.
Patients must be responsive to talking interventions before restraint, security, or other environmental safety measures are removed. Some patients do not reach this point until after tranquilizing medications are given.
Step 5: The Clinical Encounter
When discussing how to assess the likelihood of patient violence during a clinical encounter, a psychiatric colleague once commented, “Risk factors make you worry more; nothing makes you worry less.”
In other words, keep your guard up. Let clinical judgment take precedence over statistics when you are evaluating any patient. Statistics represent frequencies or averages; they may or may not apply to any one individual.
Techniques for assessing and treating violent patients are beyond the scope of this article, but at the very least:
- obtain training in safety/treatment protocols for violent patients
- ensure that your hospital/clinic has procedures in place to improve safety and to handle violent situations.
For every violent act that requires staff intervention, automatically schedule a debriefing session for those involved to assess the incident and allow them to express their feelings.
Related resources
- American Association for Emergency Psychiatry. www.emergencypsychiatry.org.
- Volavka J. The neurobiology of violence: an update. J Neuropsychiatry Clin Neurosci 1999;11:307-14.
- McNiel DE, Eisner JP, Binder RL. The relationship between command hallucinations and violence. Psychiatr Serv 2000;51: 1288-92.
Will this patient turn violent?” Psychiatrists face this tough question every day. Although predicting a complex behavior such as violence is nearly impossible, we can prepare for dangerous behavior and improve our safety by:
- knowing the risk factors for patient violence
- assessing individuals for violence potential before clinical encounters
- controlling situations to reduce injury risk.
In one study, more than 50% of psychiatrists and 75% of mental health nurses reported an act or threat of violence from patients within the past year.1 To help you avoid becoming a statistic, this article provides a 5-step procedure (Box) to quickly assess and respond to risk of violence in a psychiatric patient.
1 Seek patient history of violence; if positive, obtain details of violent behavior
2 Evaluate the context, including the patient’s psychosocial stressors, recent behavior
3 Identify arousal states such as fear, anger, confusion, or humiliation that are risk factors for violence
4 Structure the interview for safety, with attention to the treatment environment
5 Evaluate the patient in a structured clinical encounter
Step 1: Seek Patient History
A careful review of past events and those immediately preceding the clinical encounter is the best tool for assessing potential for violence. The more you can learn from the patient chart and other sources before you see the patient, the better (Table 1). Valuable clues can be obtained from interviews with family members, outpatient providers, police officers, and others who have had pertinent social contact with the patient.
Table 1
Will this patient become violent?
Questions to consider before a clinical encounter
| Long-term behavior What violent acts has this patient committed? Were conditions similar with each episode? Any unprovoked acts? Was violence associated with alcohol or drug use? How has the patient behaved with health care providers in the hospital? In outpatient settings? |
| Immediate situation What are the patient’s immediate stressors? Did he or she arrive with family or in police custody? How did the patient behave while en route? |
A minority of patients account for most aggressive acts in clinical encounters. One study showed that recidivists committed 53% of all violent behaviors in a health care setting.4 A patient’s history of violence should be flagged in the chart and verbally passed on to staff to alert providers of increased risk.
However, not having a violent history does not guarantee that a patient will not become dangerous during a clinical encounter. All patients with a violent past had an initial violent episode, and that first time can occur in a practice setting.
Psychotic states by themselves appear to increase the risk of violence, although the literature is mixed.5,6 Clearly, however, psychotic states associated with arousal or agitation do predispose patients to violence, especially if the psychosis involves active paranoid delusions or hallucinations associated with negative affect (anger, sadness, anxiety).7
Increased rates of violence have also been reported in psychiatric patients with:
- acute manic states associated with arousal or agitation8
- nonspecific neurologic abnormalities such as abnormal EEGs, localizing neurologic signs, or “soft signs” (impaired face-hand test, graphesthesia, stereognosis).9
Psychiatric diagnoses associated with increased risk of violence include schizophrenia, bipolar mania, alcohol and other substance abuse, and personality disorders.11-13 In clinical practice, however, I find psychiatric diagnoses less useful in predicting violence than the patient’s arousal state and the other risk factors discussed above.
Step 2: Evaluate the Context
In addition to evidence-supported risk factors (Table 2), context—or the broader situation in which a patient is embedded at the time of psychiatric evaluation—plays a prominent role in potentially violent situations. For example, if “divorce” is listed as a presenting factor:
- Is the patient recently divorced, or did it occur years ago?
- Does he hate all women or just his ex-wife?
- Was she having an affair, and did he just learn about this?
Table 2
Risk factors for violence among psychiatric patients*
|
| *As identified in the literature |
Step 3: Identify Arousal States
Patients rarely commit violent acts when their anxiety and moods are well controlled. They are more likely to become aggressive in high arousal states.
Fear is probably an element of most situations where patients act out violently. Because the fearful patient may not exhibit easily interpreted danger signals, however, you may unwittingly provoke an assault by violating his or her personal space. A fearful, paranoid patient requires a greater-than-usual “intimate zone,” although this need for increased space may not be obvious.
Minimize provocation by explaining your actions and behaviors in advance (such as, “I would like to enter the room, sit down, and talk with you for about 20 minutes”). Be businesslike with paranoid patients. Avoid exuding warmth, as they may view attempts at warmth as having sinister intent.
Clinicians are sometimes injured when trying to prevent a fearful, paranoid patient from fleeing. To avoid injury, don’t stand between the patient and the door. Let the patient escape from the immediate situation, and enlist security or police in further intervention attempts.
Anger is easy to recognize by signs of mounting tension. Loud voice, inappropriate staring, banging objects, clenched fists, agitated pacing, and verbal threats are common in the angry patient before a violent episode. Although this seems self-evident, it is surprising how many violent acts occur when these signs are obvious and noted by staff, yet no de-escalation measures are taken.
A patient’s verbal threats can actually help the clinician. This “red flag” alerts staff to focus on de-escalation techniques and prepare for a violent situation.
Confusion can be an underlying risk factor in patients with delirium or nonspecific organic brain syndrome. These patients may strike out unexpectedly when health care personnel are attempting to do routine procedures, and clinicians are sometimes caught off-guard when operating in a care-giving rather than defensive mode.
Clinicians can often avoid arousing confused patients by using orienting techniques and explaining their actions. For example, a nurse might say, “Hello Mr. X, I am a nurse and you are in this hospital for treatment of your illness. I will need to use this machine to check your blood pressure.”
Humiliation. Men in particular can react aggressively to loss of self-esteem and feelings of powerlessness. Take note if a man has been humiliated in front of family before being brought for evaluation; for example, was he removed by police in an emergency detention situation? This patient may need to act out violently to restore his sense of self.
Staff can lessen a patient’s potential to act on humiliation by using a therapeutic, esteem-building interview technique. For example, address the patient as “Mr.” instead of by first name, and highlight his strengths or accomplishments early in the interview.
Step 4: Structure the Interview for Safety
The time you take before an interview to learn about a patient’s violence history, context, and arousal state is time well-spent and more patient-specific than past diagnoses. This information allows you to prepare for a safe intervention.
Interview environment. The physical and social environment where you interview the patient may contribute to violence potential.
- Is the patient being interviewed in a cramped room or an open hallway?
- Is the evaluation unit overcrowded?
- Are security personnel visible?
- Is the examiner of the same race or ethnic background as the patient?
Take control of the interview and treatment situation. Use the physical space and personnel as you would any other intervention tool—to increase safety and decrease potential for violent behavior. For example, some patients do better when interviewed in a small, private setting. Other interviews must be conducted in a triage area while police escorts hold the patient and handcuffs remain on.
Ideally, you and the patient should have equal access to the door if you conduct the psychiatric interview in an enclosed room. With high-risk patients, arrange your seating at a 90-degree angle—rather than face-to-face-to limit sustained, confrontational eye contact. Sit at greater than an arm swing or leg kick away from the patient, and require him or her to remain seated during the interview (or you will promptly leave).
In the outpatient practice, terminate the interview or evaluation session if a patient in a negative affective arousal state does not allow verbal redirection. Before you make any movement to exit, however, announce, “I am leaving the room now.”
Trust your intuition. I do not enter a closed, private space with a patient unless I feel safe. If I feel afraid, I take that as a valuable warning that further safety measures are necessary.
Use restraints as needed. When patients with a history of violence are brought to the hospital in high arousal states, I let them remain in restraint with security present during the initial interview. If the patient cannot have a back-and-forth conversation with me, I keep the security force present until I believe my verbal interactions have a substantial effect.
Patients must be responsive to talking interventions before restraint, security, or other environmental safety measures are removed. Some patients do not reach this point until after tranquilizing medications are given.
Step 5: The Clinical Encounter
When discussing how to assess the likelihood of patient violence during a clinical encounter, a psychiatric colleague once commented, “Risk factors make you worry more; nothing makes you worry less.”
In other words, keep your guard up. Let clinical judgment take precedence over statistics when you are evaluating any patient. Statistics represent frequencies or averages; they may or may not apply to any one individual.
Techniques for assessing and treating violent patients are beyond the scope of this article, but at the very least:
- obtain training in safety/treatment protocols for violent patients
- ensure that your hospital/clinic has procedures in place to improve safety and to handle violent situations.
For every violent act that requires staff intervention, automatically schedule a debriefing session for those involved to assess the incident and allow them to express their feelings.
Related resources
- American Association for Emergency Psychiatry. www.emergencypsychiatry.org.
- Volavka J. The neurobiology of violence: an update. J Neuropsychiatry Clin Neurosci 1999;11:307-14.
- McNiel DE, Eisner JP, Binder RL. The relationship between command hallucinations and violence. Psychiatr Serv 2000;51: 1288-92.
1. Nolan P, Dallender J, Soares J, et al. Violence in mental health care: the experiences of mental health nurses and psychiatrists. J Adv Nurs 1999;30:934-41.
2. Blomhoff S, Seim S, Friis S. Can prediction of violence among psychiatric inpatients be improved? Hosp Community Psychiatry 1990;41:771-5.
3. Convit A, Isay D, Otis D, et al. Characteristics of repeatedly assaultive psychiatric inpatients. Hosp Community Psychiatry 1990;41:1112-5.
4. Taylor P. Motives for offending among violent and psychotic men. Br J Psychiatry 1985;147:491-8.
5. Junginger J, Parks-Levy J, McGuire L. Delusions and symptom-consistent violence. Psychiatr Serv 1998;49:218-20.
6. Cheung P, Schweitzer I, Crowley K, et al. Violence in schizophrenia: role of hallucinations and delusions. Schizophr Res 1997;26:181-90.
7. Binder R, McNiel D. Effects of diagnosis and context on dangerousness. Am J Psychiatry 1988;145:728-32.
8. Convit A, Jaeger J, Pin Lin S, et al. Predicting assaultiveness in psychiatric inpatients: A pilot study. Hosp Community Psychiatry 1988;39:429-34.
9. Hyman S. The violent patient. In: Hyman S, ed. Manual of psychiatric emergencies. Boston: Little, Brown and Co.; 1988:23-31.
10. Swartz M, Swanson J, Hiday V, et al. Violence and severe mental illness: the effects of substance abuse and nonadherence to medication. Am J Psychiatry 1998;155:226-31.
11. Owen C, Tarantello C, Jones M, et al. Repetitively violent patients in psychiatric units. Psychiatr Serv 1998;49:1458-61.
12. Citrome L, Volavka J. Clinical management of persistent aggressive behavior in schizophrenia, part I. Definitions, epidemiology, assessment and acute treatment. Essen Psychopharmacol 2002;5:1-16.
13. Abeyasinghe R, Jayasekera R. Violence in a general hospital psychiatry unit for men. Ceylon Med J 2003;48(2):45-7.
1. Nolan P, Dallender J, Soares J, et al. Violence in mental health care: the experiences of mental health nurses and psychiatrists. J Adv Nurs 1999;30:934-41.
2. Blomhoff S, Seim S, Friis S. Can prediction of violence among psychiatric inpatients be improved? Hosp Community Psychiatry 1990;41:771-5.
3. Convit A, Isay D, Otis D, et al. Characteristics of repeatedly assaultive psychiatric inpatients. Hosp Community Psychiatry 1990;41:1112-5.
4. Taylor P. Motives for offending among violent and psychotic men. Br J Psychiatry 1985;147:491-8.
5. Junginger J, Parks-Levy J, McGuire L. Delusions and symptom-consistent violence. Psychiatr Serv 1998;49:218-20.
6. Cheung P, Schweitzer I, Crowley K, et al. Violence in schizophrenia: role of hallucinations and delusions. Schizophr Res 1997;26:181-90.
7. Binder R, McNiel D. Effects of diagnosis and context on dangerousness. Am J Psychiatry 1988;145:728-32.
8. Convit A, Jaeger J, Pin Lin S, et al. Predicting assaultiveness in psychiatric inpatients: A pilot study. Hosp Community Psychiatry 1988;39:429-34.
9. Hyman S. The violent patient. In: Hyman S, ed. Manual of psychiatric emergencies. Boston: Little, Brown and Co.; 1988:23-31.
10. Swartz M, Swanson J, Hiday V, et al. Violence and severe mental illness: the effects of substance abuse and nonadherence to medication. Am J Psychiatry 1998;155:226-31.
11. Owen C, Tarantello C, Jones M, et al. Repetitively violent patients in psychiatric units. Psychiatr Serv 1998;49:1458-61.
12. Citrome L, Volavka J. Clinical management of persistent aggressive behavior in schizophrenia, part I. Definitions, epidemiology, assessment and acute treatment. Essen Psychopharmacol 2002;5:1-16.
13. Abeyasinghe R, Jayasekera R. Violence in a general hospital psychiatry unit for men. Ceylon Med J 2003;48(2):45-7.
Drink to your health? Beware energy drinks’ risks
Energy drinks’ popularity has soared among consumers who crave an energy boost from the highly caffeinated herbal concoctions. Clinicians should ask patients about energy drink consumption because:
- caffeine abuse can exacerbate mood and anxiety disorders and disrupt sleep patterns
- herbal additives can cause physical and/or psychiatric side effects
- some ingredients interact with prescription or OTC medications
- effects of energy drinks may contribute to a patient’s presenting complaint.
Approximately 70% of patients1 do not report energy drink use because they believe these products are natural and safe. Ask patients if they consume energy drinks so you can alert them to the health risks.
A Stimulating recipe
Two main ingredients in most energy drinks are caffeine and carbohydrates in the form of glucose, sucrose, fructose, galactose, and maltodextrins (Table).
Table
Caffeine and carbohydrate content of popular energy drinks
| Drink name | Caffeine content | Carbohydrate content |
|---|---|---|
| Red Bull (8.5 oz) | 80 mg | 28 g |
| Full Throttle (16 oz) | 144 mg | 57 g |
| SoBe No Fear (16 oz) | 158 mg | 66 g |
| Source: American Beverage Association, SoBe Beverages, Red Bull | ||
Caffeine. Energy drinks contain 75 to 158 mg of caffeine per can—8 ounces of coffee has 150 mg of caffeine. Also, some products include the South American plant extracts guarana or yerba mate, which contain an unknown amount of caffeine.
High caffeine intake—≥300 mg/d—might exacerbate bipolar disorder’s manic symptoms. Also find out about additional caffeine intake from coffee, tea, soda, and some OTC medications, such as Excedrin.
Herbal ingredients. Ginseng or ginkgo biloba can cause patients to feel jittery or anxious. When taken in large amounts—the FDA has no guidelines on safe dosages—these ingredients can aggravate manic or psychotic symptoms.
Significant energy drink consumption—3 or more 8-oz drinks per day—can bring about physical side effects such as tachycardia and insomnia. Advise patients to limit their intake to 2 drinks per day, and suggest safer ways to increase energy levels such as moderate exercise and adequate sleep.
1. Ciocon JO, Ciocon DG, Galindo DJ. Dietary supplements in primary care: botanicals can affect surgical outcomes and follow-up. Geriatrics 2004;59(9):20-4.
Dr. Berigan is a contracting psychiatrist at Behavioral Health Services, Fort Huachuca, AZ.
Energy drinks’ popularity has soared among consumers who crave an energy boost from the highly caffeinated herbal concoctions. Clinicians should ask patients about energy drink consumption because:
- caffeine abuse can exacerbate mood and anxiety disorders and disrupt sleep patterns
- herbal additives can cause physical and/or psychiatric side effects
- some ingredients interact with prescription or OTC medications
- effects of energy drinks may contribute to a patient’s presenting complaint.
Approximately 70% of patients1 do not report energy drink use because they believe these products are natural and safe. Ask patients if they consume energy drinks so you can alert them to the health risks.
A Stimulating recipe
Two main ingredients in most energy drinks are caffeine and carbohydrates in the form of glucose, sucrose, fructose, galactose, and maltodextrins (Table).
Table
Caffeine and carbohydrate content of popular energy drinks
| Drink name | Caffeine content | Carbohydrate content |
|---|---|---|
| Red Bull (8.5 oz) | 80 mg | 28 g |
| Full Throttle (16 oz) | 144 mg | 57 g |
| SoBe No Fear (16 oz) | 158 mg | 66 g |
| Source: American Beverage Association, SoBe Beverages, Red Bull | ||
Caffeine. Energy drinks contain 75 to 158 mg of caffeine per can—8 ounces of coffee has 150 mg of caffeine. Also, some products include the South American plant extracts guarana or yerba mate, which contain an unknown amount of caffeine.
High caffeine intake—≥300 mg/d—might exacerbate bipolar disorder’s manic symptoms. Also find out about additional caffeine intake from coffee, tea, soda, and some OTC medications, such as Excedrin.
Herbal ingredients. Ginseng or ginkgo biloba can cause patients to feel jittery or anxious. When taken in large amounts—the FDA has no guidelines on safe dosages—these ingredients can aggravate manic or psychotic symptoms.
Significant energy drink consumption—3 or more 8-oz drinks per day—can bring about physical side effects such as tachycardia and insomnia. Advise patients to limit their intake to 2 drinks per day, and suggest safer ways to increase energy levels such as moderate exercise and adequate sleep.
Energy drinks’ popularity has soared among consumers who crave an energy boost from the highly caffeinated herbal concoctions. Clinicians should ask patients about energy drink consumption because:
- caffeine abuse can exacerbate mood and anxiety disorders and disrupt sleep patterns
- herbal additives can cause physical and/or psychiatric side effects
- some ingredients interact with prescription or OTC medications
- effects of energy drinks may contribute to a patient’s presenting complaint.
Approximately 70% of patients1 do not report energy drink use because they believe these products are natural and safe. Ask patients if they consume energy drinks so you can alert them to the health risks.
A Stimulating recipe
Two main ingredients in most energy drinks are caffeine and carbohydrates in the form of glucose, sucrose, fructose, galactose, and maltodextrins (Table).
Table
Caffeine and carbohydrate content of popular energy drinks
| Drink name | Caffeine content | Carbohydrate content |
|---|---|---|
| Red Bull (8.5 oz) | 80 mg | 28 g |
| Full Throttle (16 oz) | 144 mg | 57 g |
| SoBe No Fear (16 oz) | 158 mg | 66 g |
| Source: American Beverage Association, SoBe Beverages, Red Bull | ||
Caffeine. Energy drinks contain 75 to 158 mg of caffeine per can—8 ounces of coffee has 150 mg of caffeine. Also, some products include the South American plant extracts guarana or yerba mate, which contain an unknown amount of caffeine.
High caffeine intake—≥300 mg/d—might exacerbate bipolar disorder’s manic symptoms. Also find out about additional caffeine intake from coffee, tea, soda, and some OTC medications, such as Excedrin.
Herbal ingredients. Ginseng or ginkgo biloba can cause patients to feel jittery or anxious. When taken in large amounts—the FDA has no guidelines on safe dosages—these ingredients can aggravate manic or psychotic symptoms.
Significant energy drink consumption—3 or more 8-oz drinks per day—can bring about physical side effects such as tachycardia and insomnia. Advise patients to limit their intake to 2 drinks per day, and suggest safer ways to increase energy levels such as moderate exercise and adequate sleep.
1. Ciocon JO, Ciocon DG, Galindo DJ. Dietary supplements in primary care: botanicals can affect surgical outcomes and follow-up. Geriatrics 2004;59(9):20-4.
Dr. Berigan is a contracting psychiatrist at Behavioral Health Services, Fort Huachuca, AZ.
1. Ciocon JO, Ciocon DG, Galindo DJ. Dietary supplements in primary care: botanicals can affect surgical outcomes and follow-up. Geriatrics 2004;59(9):20-4.
Dr. Berigan is a contracting psychiatrist at Behavioral Health Services, Fort Huachuca, AZ.
10 delirium myths debunked
A consultation-liaison psychiatrist is called in to help manage “schizophrenia” in a middle-aged attorney who is recovering from a complicated cardiac bypass procedure. This patient is not mentally ill, the psychiatrist realizes, but has delirium with rapid-onset neuropsychiatric symptoms noted by fluctuations in arousal and associated changes in sleep, mood, personality, and cognition. This example illustrates 1 of 10 myths about delirium:
1. My patient is paranoid, therefore he or she must be schizophrenic
Patients with delirium may present with perceptual disturbances such as hallucinations, delusions, or paranoia. An otherwise highly functioning individual with symptom onset while in a medical setting likely has delirium, not a chronic mental disorder such as schizophrenia.
2. Delirium is rare
Delirium is found frequently in medically ill populations. In some groups, such as stem-cell transplant patients, rates may approach 50%. Risk factors include medical severity and advanced patient age.1
3. Delirium is not serious
Delirium is associated with increased morbidity and mortality. It is a marker for “cerebral insufficiency”—reversible impairment in brain function—and requires prompt treatment.2-4
4. Sleep deprivation causes delirium
Disrupted sleep in hospitalized patients—otherwise known as “ICU psychosis”—is more likely the result of delirium than the cause. Patients’ delirium and sleep both improve when they move from the ICU to a general medical floor, a reflection of their improved medical condition.5
5. Delirium goes away rapidly
Delirium usually lasts for days or weeks. Many patients—although superficially improved—still have subtle cognitive deficits and difficulty with daily activities.
6. The patient’s medical problem has been treated, so the delirium should resolve
A patient’s CNS often needs time to recover, and delirium may persist after the underlying medical cause has been treated. Delirium can be caused by factors other than medical illness, such as sedating, analgesic, or antiemetic medications.
7. My delirious patient cannot make medical decisions
Many patients with delirium can make decisions during more lucid periods. As their delirium improves, they should be able to participate in decision-making.
8. My patient cannot be delirious because he or she is oriented to time and place
Simple orientation questions can miss subtle signs of delirium. Watch for an inability to function cognitively at the individual’s baseline level. For example, a software engineer who is unable to draw a clock could be delirious.
9. My patient has depression, not delirium, because he or she is not getting out of bed
Delirium can present with changes of mood, energy, and personality that mimic depression. Even severely depressed individuals can function at a basic cognitive level and maintain daytime wakefulness, whereas patients with delirium may not.
10. Delirium cannot be treated
Manage delirium by evaluating and treating underlying medical precipitants such as metabolic derangement, infection, dehydration, hypoxia, pain, or medication effects. Also consider CNS injuries including stroke, head injury, or neoplasm. Research suggests antipsychotic medications at low dosages6,7 can safely treat delirium. Improving orientation and comfort by reassuring the patient, assessing for anxiety, and reducing excessive noise and stimulation also help.
1. Fann JR, Roth-Roemer S, Burington BE, et al. Delirium in patients undergoing hematopoietic stem cell transplantation. Cancer 2002;95(9):1971-81.
2. Engel GL, Romano J. Delirium, a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci 2004;16(4):526-38.
3. Thomason JW, Shintani A, Peterson JF, et al. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care. [serial online]. 2005;9(4):R375-81.
4. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291(14):1753-62.
5. Justic M. Does “ICU psychosis” really exist? Crit Care Nurse 2000;20(3):28-37.
6. Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics 2002;43(3):171-4.
7. Hassaballa HA, Balk RA. Torsade de pointes associated with the administration of intravenous haloperidol: a review of the literature and practical guidelines for use. Expert Opin Drug Saf 2003;2(6):543-7.
Dr. Levy is assistant professor in psychiatry, University of Washington, Seattle.
A consultation-liaison psychiatrist is called in to help manage “schizophrenia” in a middle-aged attorney who is recovering from a complicated cardiac bypass procedure. This patient is not mentally ill, the psychiatrist realizes, but has delirium with rapid-onset neuropsychiatric symptoms noted by fluctuations in arousal and associated changes in sleep, mood, personality, and cognition. This example illustrates 1 of 10 myths about delirium:
1. My patient is paranoid, therefore he or she must be schizophrenic
Patients with delirium may present with perceptual disturbances such as hallucinations, delusions, or paranoia. An otherwise highly functioning individual with symptom onset while in a medical setting likely has delirium, not a chronic mental disorder such as schizophrenia.
2. Delirium is rare
Delirium is found frequently in medically ill populations. In some groups, such as stem-cell transplant patients, rates may approach 50%. Risk factors include medical severity and advanced patient age.1
3. Delirium is not serious
Delirium is associated with increased morbidity and mortality. It is a marker for “cerebral insufficiency”—reversible impairment in brain function—and requires prompt treatment.2-4
4. Sleep deprivation causes delirium
Disrupted sleep in hospitalized patients—otherwise known as “ICU psychosis”—is more likely the result of delirium than the cause. Patients’ delirium and sleep both improve when they move from the ICU to a general medical floor, a reflection of their improved medical condition.5
5. Delirium goes away rapidly
Delirium usually lasts for days or weeks. Many patients—although superficially improved—still have subtle cognitive deficits and difficulty with daily activities.
6. The patient’s medical problem has been treated, so the delirium should resolve
A patient’s CNS often needs time to recover, and delirium may persist after the underlying medical cause has been treated. Delirium can be caused by factors other than medical illness, such as sedating, analgesic, or antiemetic medications.
7. My delirious patient cannot make medical decisions
Many patients with delirium can make decisions during more lucid periods. As their delirium improves, they should be able to participate in decision-making.
8. My patient cannot be delirious because he or she is oriented to time and place
Simple orientation questions can miss subtle signs of delirium. Watch for an inability to function cognitively at the individual’s baseline level. For example, a software engineer who is unable to draw a clock could be delirious.
9. My patient has depression, not delirium, because he or she is not getting out of bed
Delirium can present with changes of mood, energy, and personality that mimic depression. Even severely depressed individuals can function at a basic cognitive level and maintain daytime wakefulness, whereas patients with delirium may not.
10. Delirium cannot be treated
Manage delirium by evaluating and treating underlying medical precipitants such as metabolic derangement, infection, dehydration, hypoxia, pain, or medication effects. Also consider CNS injuries including stroke, head injury, or neoplasm. Research suggests antipsychotic medications at low dosages6,7 can safely treat delirium. Improving orientation and comfort by reassuring the patient, assessing for anxiety, and reducing excessive noise and stimulation also help.
A consultation-liaison psychiatrist is called in to help manage “schizophrenia” in a middle-aged attorney who is recovering from a complicated cardiac bypass procedure. This patient is not mentally ill, the psychiatrist realizes, but has delirium with rapid-onset neuropsychiatric symptoms noted by fluctuations in arousal and associated changes in sleep, mood, personality, and cognition. This example illustrates 1 of 10 myths about delirium:
1. My patient is paranoid, therefore he or she must be schizophrenic
Patients with delirium may present with perceptual disturbances such as hallucinations, delusions, or paranoia. An otherwise highly functioning individual with symptom onset while in a medical setting likely has delirium, not a chronic mental disorder such as schizophrenia.
2. Delirium is rare
Delirium is found frequently in medically ill populations. In some groups, such as stem-cell transplant patients, rates may approach 50%. Risk factors include medical severity and advanced patient age.1
3. Delirium is not serious
Delirium is associated with increased morbidity and mortality. It is a marker for “cerebral insufficiency”—reversible impairment in brain function—and requires prompt treatment.2-4
4. Sleep deprivation causes delirium
Disrupted sleep in hospitalized patients—otherwise known as “ICU psychosis”—is more likely the result of delirium than the cause. Patients’ delirium and sleep both improve when they move from the ICU to a general medical floor, a reflection of their improved medical condition.5
5. Delirium goes away rapidly
Delirium usually lasts for days or weeks. Many patients—although superficially improved—still have subtle cognitive deficits and difficulty with daily activities.
6. The patient’s medical problem has been treated, so the delirium should resolve
A patient’s CNS often needs time to recover, and delirium may persist after the underlying medical cause has been treated. Delirium can be caused by factors other than medical illness, such as sedating, analgesic, or antiemetic medications.
7. My delirious patient cannot make medical decisions
Many patients with delirium can make decisions during more lucid periods. As their delirium improves, they should be able to participate in decision-making.
8. My patient cannot be delirious because he or she is oriented to time and place
Simple orientation questions can miss subtle signs of delirium. Watch for an inability to function cognitively at the individual’s baseline level. For example, a software engineer who is unable to draw a clock could be delirious.
9. My patient has depression, not delirium, because he or she is not getting out of bed
Delirium can present with changes of mood, energy, and personality that mimic depression. Even severely depressed individuals can function at a basic cognitive level and maintain daytime wakefulness, whereas patients with delirium may not.
10. Delirium cannot be treated
Manage delirium by evaluating and treating underlying medical precipitants such as metabolic derangement, infection, dehydration, hypoxia, pain, or medication effects. Also consider CNS injuries including stroke, head injury, or neoplasm. Research suggests antipsychotic medications at low dosages6,7 can safely treat delirium. Improving orientation and comfort by reassuring the patient, assessing for anxiety, and reducing excessive noise and stimulation also help.
1. Fann JR, Roth-Roemer S, Burington BE, et al. Delirium in patients undergoing hematopoietic stem cell transplantation. Cancer 2002;95(9):1971-81.
2. Engel GL, Romano J. Delirium, a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci 2004;16(4):526-38.
3. Thomason JW, Shintani A, Peterson JF, et al. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care. [serial online]. 2005;9(4):R375-81.
4. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291(14):1753-62.
5. Justic M. Does “ICU psychosis” really exist? Crit Care Nurse 2000;20(3):28-37.
6. Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics 2002;43(3):171-4.
7. Hassaballa HA, Balk RA. Torsade de pointes associated with the administration of intravenous haloperidol: a review of the literature and practical guidelines for use. Expert Opin Drug Saf 2003;2(6):543-7.
Dr. Levy is assistant professor in psychiatry, University of Washington, Seattle.
1. Fann JR, Roth-Roemer S, Burington BE, et al. Delirium in patients undergoing hematopoietic stem cell transplantation. Cancer 2002;95(9):1971-81.
2. Engel GL, Romano J. Delirium, a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci 2004;16(4):526-38.
3. Thomason JW, Shintani A, Peterson JF, et al. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care. [serial online]. 2005;9(4):R375-81.
4. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291(14):1753-62.
5. Justic M. Does “ICU psychosis” really exist? Crit Care Nurse 2000;20(3):28-37.
6. Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics 2002;43(3):171-4.
7. Hassaballa HA, Balk RA. Torsade de pointes associated with the administration of intravenous haloperidol: a review of the literature and practical guidelines for use. Expert Opin Drug Saf 2003;2(6):543-7.
Dr. Levy is assistant professor in psychiatry, University of Washington, Seattle.
Treating psychiatric reactions to medical illness
Mrs. M, 35, is undergoing breast cancer treatment. She is referred to the consultation-liaison service by an emergency room physician for evaluation of suicidal thoughts.
Mrs. M has been crying for 2 hours. She awoke this morning feeling that she could no longer deal with “the unknown.” She has lost her hair and 30 pounds during chemotherapy and hardly recognized herself in the bathroom mirror. She thought about killing herself until one of her children walked by. After they went to school, she drove to the emergency room.
When you ask if she has had other thoughts of hurting herself, she says, “I would never do anything like that to my family, but the fact that I was thinking about it really scared me.”
Each patient responds uniquely to the emotional trauma of having a chronic or life-threatening medical illness. Coping styles depend on medical, psychological, and social factors as well as the person’s personality and experiences. Reactions range from mature to psychotic.
To help you guide patients such as Mrs. M through difficult medical treatments and decisions, we describe:
- a psychiatric workup to identify maladaptive response to illness
- typical emotional and behavioral responses to chronic illness
- how to provide psychotherapy tailored to the needs of 7 personality styles of medically ill patients.
Psychiatric Workup
Mrs. M is married with 3 sons. She found a lump on her breast 4 months ago but decided to wait “because, I thought, ‘it can’t be anything bad; it will go away.’” When the lump remained 3 months later, she consulted a specialist who diagnosed breast cancer.
Mrs. M underwent a lumpectomy and has been receiving weekly chemotherapy. Besides losing her hair, she says she has no appetite. Looking at food or driving close to the hospital makes her nauseous. She starts vomiting before she arrives for chemotherapy.
Though her family supports and encourages her, she can’t stop thinking about death. She feels isolated, lonely, and cries often. “No one understands what I am going through,” she says. “How can I share these feelings with them?”
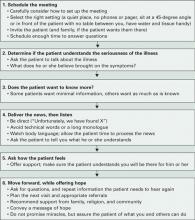
Physicians such as the specialist who diagnosed Mrs. M’s cancer often must communicate difficult information. Careful planning when delivering bad news (Box 1)1,2 can set the stage for a healthier emotional and behavioral response.
Chronic illness causes depression in up to 25% of patients.3 At particular risk of developing depressive symptoms are patients with:
- poor physical condition
- poorly controlled pain
- advanced illness
- history of major depressive disorder
- family history of depression and suicide
- certain cancers, such as of the pancreas, lung, head or neck.4
Mechanisms of depression in illness. Depression can be the first symptom of some medical illnesses, such as pancreatic or liver cancer. Diseases that directly affect the brain—such as Parkinson’s disease, multiple sclerosis, and systemic lupus erythematosus—can cause depression. Patients disabled by spinal cord injury, stroke, or cancer also are at risk for depression.
Treatment is the same, whether illness-related chemical changes or the patient’s emotional response to disability is causing the depression. When diagnosing depression in medically ill patients, DSM-IV-TR recommends using criteria for major depression and providing treatment, whatever the cause.
Mrs. M has trouble sharing feelings of hopelessness with her family. Her changes in demeanor and isolation from everyday activities are clues to clinical depression and anxiety.
Depression can be difficult to diagnose when medical illness causes depression-like symptoms.5 To make the diagnosis, ask patients with chronic illness about depression’s emotional symptoms (hopelessness, withdrawal from others, sadness, ruminating thoughts, frequent crying spells), rather than its physical symptoms of poor eating, disturbed sleep, or low energy that may stem from the medical illness. Patients might not be as hard on themselves if they feel the medical illness—not poor coping on their part—caused their depression.
Box 1 6 steps for delivering bad medical news
Source: Adapted from references 1, 2Workup. Our workup of Mrs. M includes thyroid function tests, CBC, comprehensive metabolic panel, and brain MRI to rule out metastasis. All are negative. In her history, Mrs. M reports a previous episode of major depression 5 years ago that was successfully treated with sertraline for 1 year.
Consistent with her workup and symptoms, we diagnose major depression without psychosis. Cancer patients who report hopelessness are at increased risk of suicide. Because of her family support and religious beliefs, however, we feel she can safely go home and return for follow-up the next week.
Emotional response to illness
Mrs. M starts weekly outpatient appointments at our clinic. Because sertraline has worked for her in the past, it is our first choice to increase her energy, improve her mood, and decrease her hopelessness. Initial dosage is 25 mg/d for the first 3 days, with an increase to 50 mg/d for 1 month, and 100 mg/d thereafter. She reports no side effects.
In some patients, sertraline can cause adverse gastrointestinal effects such as upset stomach or nausea. Other antidepressants such as mirtazapine could improve patients’ sleep and decrease nausea.
Mrs. M also begins cognitive-behavioral therapy (CBT) to help her deal with negative thoughts, and relaxation training to combat her anxiety before chemotherapy. We recommend a local breast cancer support group, and Mrs. M starts going twice a month. She feels relieved that other patients are experiencing feelings similar to hers.
Medication plus CBT has been shown to be the most effective treatment for patients who meet criteria for depression and anxiety disorders. CBT has been shown to help patients manage physical symptoms and reframe negative thoughts associated with many chronic illnesses, including breast cancer, Parkinson’s disease, epilepsy, rheumatoid arthritis, and multiple sclerosis.6-10
Core emotions. Medically ill patients experience a range of core emotions, which Lazarus11 identified as anger, anxiety, guilt, fright, shame, sadness, happiness, envy, relief, and hope. Identifying the source of these emotions is important to counseling patients effectively.
For example, a patient experiencing fright might fear death, pain, disability, stigma, disfigurement, or other eventualities. Mrs. M has said she can’t stop thinking about dying. By knowing what the patient fears (Table 1),12 we can more effectively reassure and offer support, even when little else can be done.
Table 1
Common fears of patients reacting to diagnosis of chronic illness
|
| Source: Reference 12 |
Defensive behaviors. Patient’s behavioral responses may be adaptive or maladaptive; treatment nonadherence is one maladaptive response (Box 2).13,14 The patient history can suggest how well a person has adapted to past losses and disappointments. Patients may try to protect themselves against emotional and physical pain with psychotic, immature, neurotic, or mature defense mechanisms.15
Psychotic defenses are characterized by regression until patients lose touch with reality. Delusions and fantasy isolate them from the harshness of a serious medical condition. Antipsychotic medication and patience are often indicated.16
Immature defenses—as seen in patients with borderline personality disorder—can irritate and alienate the medical team. Physicians may not understand why their best efforts are thwarted or negated. Well-intentioned, caring doctors often try harder when the verbal attacks begin, but soon even the hardiest can wither under the patient’s criticism and threats.
Depressed medically ill patients adhere poorly to treatment schedules and other recommendations, which may cut their chances of survival.13 Up to 2% of hospital discharges are initiated by patients against medical advice.
Causes of refusing or discontinuing treatment may include anger towards the medical team or caregiver,14 anxiety, or withdrawal from addictive substances. Some patients who sign themselves out may be psychotic or confused by delirium or dementia. Others may be in denial of their illness.
Untreated psychiatric disorders can cause illness-specific nonadherence. Patients with:
- depression might not have the energy or motivation to follow the treatment.
- bipolar disorder might feel they don’t need the treatment.
- psychotic disorders may feel threatened by the treatment or the doctor.
Psychiatric patients may avoid taking medications prescribed for medical illness if they fear side effects or interactions with their psychotropics. Some don’t tell their medical practitioners about their psychiatric diagnoses because of the stigma associated with mental illness.
Psychiatrists can counsel the medical team to:
- pull back and focus on setting treatment objectives
- encourage the patient to work as a team member to ensure the best possible care
- communicate with the patient and care team to prevent divisions among the staff.
Neurotic defenses are in play when patients blame themselves and suffer internally. Give them ample opportunities to explore their feelings.
Mature defenses are seen in those who show concern for others, may express humor, and can adaptively plan, thus gaining the respect of others. Spirituality and optimism allow them to feel more at peace and less controlled by the illness.
Support these patients by encouraging their coping skills. For example, if spirituality has helped before, it may again strengthen them and their families.
Improving coping skills. By recognizing which patients are struggling, you can provide support to strengthen their coping abilities. Initially, Mrs. M was using neurotic defense mechanisms and internalizing her emotions. With the help of CBT, she begins to rely on more mature defenses. Her improved coping skills allow her to share her feelings during group therapy and with her family.
Encouraging adaptive behavior
Mrs. M is in remission when chemotherapy ends, but she worries that the cancer will come back. “The fear is still there,” she says. “You can’t be called a survivor for 5 years.”
She continues biweekly psychotherapy for the next year, and sertraline, 100 mg/d. She is doing well and has volunteered to help other newly-diagnosed patients at the cancer center.
Personality styles. Kahana and Bibring17 described 7 personality types that affect how patients cope with illnesses. Based on our experience, we suggest how to observe these styles, identify the emotional pain behind them, and respond in ways that will help each type of patient (Table 2).17
These personality types are not necessarily personality disorders; rather, they describe pervasive characteristics of coping styles. Some individuals will not match the descriptions of any of these types, and others may fall into more than one. Mrs. M, for example, shows traits of more than one personality style, including avoidant and mild schizoid features.
We helped her by engaging her in psychiatric treatment, helping her better understand her medical situation, and restoring her sense of control in making medical decisions. We discussed psycho-therapy as a two-way street, outlining her responsibilities to practice new CBT skills to use during treatment and in remission.
Table 2
Recommended treatment approaches for 7 patient personalities
| Personality | Patients who… | Often feel… | Are helped by… |
|---|---|---|---|
| Dependent | ask many questions, making it hard for you to end conversations or leave the room | afraid you won’t find them worthy, won’t want to care for them | regular, brief sessions (set tactful limits that will reassure patient and not annoy staff) |
| Obsessive | are insistent, detail-oriented | angry when they can’t control their illness, the staff, and the schedule | detailed explanations (provide choices whenever possible, try to use patient input collaboratively) |
| Narcissistic | are self-centered, criticize others, believe no one is qualified to care for them | fearful, threatened, and vulnerable | avoiding confrontation but emphasizing that they deserve the best care staff can provide (keep patient informed; be sure all staff provides the same message) |
| Suffering victim | always have symptoms and request much attention; might not follow recommendations | suffering is their role; views illness and its treatments as punishment, but hopes doctor will keep trying | regular visits, no matter how variable the complaints |
| Paranoid | do not trust doctors, refuse to participate in treatment plans or sign out | taken advantage of by others or purposefully neglected or harmed | staying calm (don’t argue; offer understanding of patient’s position, provide clear recommendations |
| Histrionic | are flirtatious, want to call doctors by first names | need to be ‘special’ to the physician; fear illness will invalidate them or make them unattractive | encouraging patient to verbalize concerns (set boundaries for relationship; remain courteous and objective) |
| Schizoid | are very lonely, tend to avoid medical care | doctors are invading their privacy | engaging patient in making medical decisions |
| Source: Adapted from reference 17 | |||
Supportive therapy. Encourage patients to seek support from family and friends.18 Some benefit from meeting other patients with the same needs, fears, and questions.19 The Internet is a good resource to find local support groups.
Becoming sick or disabled and having to change one’s lifestyle can damage a person’s self esteem. Encourage patients to help others by volunteering, participating in research, or engaging in other activities that provide hope, gratification, and a sense of service.20
Some patients regain control and battle their fears by becoming experts on their diseases. This approach will not help those who become anxious learning about prognosis and side effects, however, and prefer to have limited information about their illnesses. Your knowledge of a patient’s personality type can help you determine whether added information might alleviate or worsen that patient’s stress.
Personal transformation can occur when patients face mortality. Their perceptions about what is important and how to achieve their goals can change dramatically.
Related resources
- National Cancer Institute. Coping with cancer. www.cancer.gov/cancertopics/coping.
- American Psychiatric Association. Patient education site with links to topics such as coping with AIDS/HIV, postpartum depression, alcohol abuse, mental health of the elderly, and common childhood disorders. www.healthyminds.org.
- Groves M, Muskin P. Psychological responses to illness. In: Bourgeois JA, Hales RE, Shahrokh N (eds). Textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005:68-88.
Drug brand names
- Mirtazapine • Remeron
- Sertraline • Zoloft
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bailey W, Buckman R, Lenzi R, et al. SPIKES - a six-step protocol for delivering bad news; application to the patient with cancer. Oncologist 2000;5:4,302-11.
2. Koenig HG, Larson DB, Larson SS. Religion and coping with serious medical illness. Ann Pharmacother 2001;35(3):352-9.
3. Faller H, Schmidt M. Prognostic value of depressive coping and depression in survival of lung cancer patients. Psychooncology 2004;13(5):359-63.
4. Holland JC. Psychological care of patients: psycho-oncology’s contribution. J Clin Oncol 2003;21(23 suppl):253s-265s.
5. Lovejoy NC, Tabor D, Matteis M, Lillis P. Cancer-related depression: Part I. Neurologic alterations and cognitive-behavioral therapy. Oncol Nurs Forum 2000;27(4):667-78.
6. Cole K, Vaughan FL. The feasibility of using cognitive behaviour therapy for depression associated with Parkinson’s disease: a literature review. Parkinsonism Relat Disord 2005;11(5):269-76
7. Goldstein LH, McAlpine M, Deale A, et al. Cognitive behaviour therapy with adults with intractable epilepsy and psychiatric co-morbidity: preliminary observations on changes in psychological state and seizure frequency. Behav Res Ther 2003;41(4):447-60.
8. Evers AW, Kraaimaat FW, van Riel PL, de Jong AJ. Tailored cognitive-behavioral therapy in early rheumatoid arthritis for patients at risk: a randomized controlled trial. Pain 2002;100(1-2):141-53.
9. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol 2000;68(2):356-61.
10. Tatrow K. Montgomery GH. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. J Behav Med 2006;29(1):17-27.
11. Lazarus RS, Folkman S. Stress, appraisal and coping. New York: Springer, 1984.
12. Gazzola L, Muskin PR. The impact of stress and the objective of psychosocial interventions. In: Schein LA, Bernard HS, Spitz HI, Muskin PR (eds). Psychosocial treatment for medical conditions: principles and techniques. New York: Brunner-Routledge; 2003: 373-406.
13. Uitterhoeve RJ, Vernooy M, Litjens M, et al. Psychosocial interventions for patients with advanced cancer - a systematic review of the literature. Br J Cancer 2004;91(6):1050-62.
14. Perry S, Viederman M. Management of emotional reactions to acute medical illness. Med Clin North Am 1981;65:3-14.
15. Gabbard G. Major modalities: psychoanalytic/psychodynamic. In: Gabbard G, Beck J, Holmes J, eds. Oxford textbook of psychotherapy. New York: Oxford University Press; 2005:3-13.
16. Groves M, Muskin P. Psychological responses to illness In: Bourgeois JA, Hales RE, Shahrokh N (eds). Textbook of psychosomatic medicine Washington, DC: American Psychiatric Publishing; 2005:68-88.
17. Kahana RJ, Bibring G. Personality types in medical management. In: Zinberg NE (ed). Psychiatry and medical practice in a general hospital. New York: International Universities Press, 1964:108-23.
18. Johnson KS, Elbert-Avila KI, Tulsky JA. The influence of spiritual beliefs and practices on the treatment p of African Americans: a review of the literature. J Am Geriatr Soc 2005;53(4):711-19.
19. Vos PJ, Visser AP, Garssen B, et al. Effects of delayed psychosocial interventions versus early psychosocial interventions for women with early stage breast cancer. Patient Educ Couns 2006;60(2):212-9
20. Breitbart W, Gibson C, Poppito SR, Berg A. Psychotherapeutic interventions at the end of life: a focus on meaning and spirituality. Can J Psychiatry 2004;49(6):366-72.
Mrs. M, 35, is undergoing breast cancer treatment. She is referred to the consultation-liaison service by an emergency room physician for evaluation of suicidal thoughts.
Mrs. M has been crying for 2 hours. She awoke this morning feeling that she could no longer deal with “the unknown.” She has lost her hair and 30 pounds during chemotherapy and hardly recognized herself in the bathroom mirror. She thought about killing herself until one of her children walked by. After they went to school, she drove to the emergency room.
When you ask if she has had other thoughts of hurting herself, she says, “I would never do anything like that to my family, but the fact that I was thinking about it really scared me.”
Each patient responds uniquely to the emotional trauma of having a chronic or life-threatening medical illness. Coping styles depend on medical, psychological, and social factors as well as the person’s personality and experiences. Reactions range from mature to psychotic.
To help you guide patients such as Mrs. M through difficult medical treatments and decisions, we describe:
- a psychiatric workup to identify maladaptive response to illness
- typical emotional and behavioral responses to chronic illness
- how to provide psychotherapy tailored to the needs of 7 personality styles of medically ill patients.
Psychiatric Workup
Mrs. M is married with 3 sons. She found a lump on her breast 4 months ago but decided to wait “because, I thought, ‘it can’t be anything bad; it will go away.’” When the lump remained 3 months later, she consulted a specialist who diagnosed breast cancer.
Mrs. M underwent a lumpectomy and has been receiving weekly chemotherapy. Besides losing her hair, she says she has no appetite. Looking at food or driving close to the hospital makes her nauseous. She starts vomiting before she arrives for chemotherapy.
Though her family supports and encourages her, she can’t stop thinking about death. She feels isolated, lonely, and cries often. “No one understands what I am going through,” she says. “How can I share these feelings with them?”
Physicians such as the specialist who diagnosed Mrs. M’s cancer often must communicate difficult information. Careful planning when delivering bad news (Box 1)1,2 can set the stage for a healthier emotional and behavioral response.
Chronic illness causes depression in up to 25% of patients.3 At particular risk of developing depressive symptoms are patients with:
- poor physical condition
- poorly controlled pain
- advanced illness
- history of major depressive disorder
- family history of depression and suicide
- certain cancers, such as of the pancreas, lung, head or neck.4
Mechanisms of depression in illness. Depression can be the first symptom of some medical illnesses, such as pancreatic or liver cancer. Diseases that directly affect the brain—such as Parkinson’s disease, multiple sclerosis, and systemic lupus erythematosus—can cause depression. Patients disabled by spinal cord injury, stroke, or cancer also are at risk for depression.
Treatment is the same, whether illness-related chemical changes or the patient’s emotional response to disability is causing the depression. When diagnosing depression in medically ill patients, DSM-IV-TR recommends using criteria for major depression and providing treatment, whatever the cause.
Mrs. M has trouble sharing feelings of hopelessness with her family. Her changes in demeanor and isolation from everyday activities are clues to clinical depression and anxiety.
Depression can be difficult to diagnose when medical illness causes depression-like symptoms.5 To make the diagnosis, ask patients with chronic illness about depression’s emotional symptoms (hopelessness, withdrawal from others, sadness, ruminating thoughts, frequent crying spells), rather than its physical symptoms of poor eating, disturbed sleep, or low energy that may stem from the medical illness. Patients might not be as hard on themselves if they feel the medical illness—not poor coping on their part—caused their depression.
Box 1 6 steps for delivering bad medical news
Source: Adapted from references 1, 2Workup. Our workup of Mrs. M includes thyroid function tests, CBC, comprehensive metabolic panel, and brain MRI to rule out metastasis. All are negative. In her history, Mrs. M reports a previous episode of major depression 5 years ago that was successfully treated with sertraline for 1 year.
Consistent with her workup and symptoms, we diagnose major depression without psychosis. Cancer patients who report hopelessness are at increased risk of suicide. Because of her family support and religious beliefs, however, we feel she can safely go home and return for follow-up the next week.
Emotional response to illness
Mrs. M starts weekly outpatient appointments at our clinic. Because sertraline has worked for her in the past, it is our first choice to increase her energy, improve her mood, and decrease her hopelessness. Initial dosage is 25 mg/d for the first 3 days, with an increase to 50 mg/d for 1 month, and 100 mg/d thereafter. She reports no side effects.
In some patients, sertraline can cause adverse gastrointestinal effects such as upset stomach or nausea. Other antidepressants such as mirtazapine could improve patients’ sleep and decrease nausea.
Mrs. M also begins cognitive-behavioral therapy (CBT) to help her deal with negative thoughts, and relaxation training to combat her anxiety before chemotherapy. We recommend a local breast cancer support group, and Mrs. M starts going twice a month. She feels relieved that other patients are experiencing feelings similar to hers.
Medication plus CBT has been shown to be the most effective treatment for patients who meet criteria for depression and anxiety disorders. CBT has been shown to help patients manage physical symptoms and reframe negative thoughts associated with many chronic illnesses, including breast cancer, Parkinson’s disease, epilepsy, rheumatoid arthritis, and multiple sclerosis.6-10
Core emotions. Medically ill patients experience a range of core emotions, which Lazarus11 identified as anger, anxiety, guilt, fright, shame, sadness, happiness, envy, relief, and hope. Identifying the source of these emotions is important to counseling patients effectively.
For example, a patient experiencing fright might fear death, pain, disability, stigma, disfigurement, or other eventualities. Mrs. M has said she can’t stop thinking about dying. By knowing what the patient fears (Table 1),12 we can more effectively reassure and offer support, even when little else can be done.
Table 1
Common fears of patients reacting to diagnosis of chronic illness
|
| Source: Reference 12 |
Defensive behaviors. Patient’s behavioral responses may be adaptive or maladaptive; treatment nonadherence is one maladaptive response (Box 2).13,14 The patient history can suggest how well a person has adapted to past losses and disappointments. Patients may try to protect themselves against emotional and physical pain with psychotic, immature, neurotic, or mature defense mechanisms.15
Psychotic defenses are characterized by regression until patients lose touch with reality. Delusions and fantasy isolate them from the harshness of a serious medical condition. Antipsychotic medication and patience are often indicated.16
Immature defenses—as seen in patients with borderline personality disorder—can irritate and alienate the medical team. Physicians may not understand why their best efforts are thwarted or negated. Well-intentioned, caring doctors often try harder when the verbal attacks begin, but soon even the hardiest can wither under the patient’s criticism and threats.
Depressed medically ill patients adhere poorly to treatment schedules and other recommendations, which may cut their chances of survival.13 Up to 2% of hospital discharges are initiated by patients against medical advice.
Causes of refusing or discontinuing treatment may include anger towards the medical team or caregiver,14 anxiety, or withdrawal from addictive substances. Some patients who sign themselves out may be psychotic or confused by delirium or dementia. Others may be in denial of their illness.
Untreated psychiatric disorders can cause illness-specific nonadherence. Patients with:
- depression might not have the energy or motivation to follow the treatment.
- bipolar disorder might feel they don’t need the treatment.
- psychotic disorders may feel threatened by the treatment or the doctor.
Psychiatric patients may avoid taking medications prescribed for medical illness if they fear side effects or interactions with their psychotropics. Some don’t tell their medical practitioners about their psychiatric diagnoses because of the stigma associated with mental illness.
Psychiatrists can counsel the medical team to:
- pull back and focus on setting treatment objectives
- encourage the patient to work as a team member to ensure the best possible care
- communicate with the patient and care team to prevent divisions among the staff.
Neurotic defenses are in play when patients blame themselves and suffer internally. Give them ample opportunities to explore their feelings.
Mature defenses are seen in those who show concern for others, may express humor, and can adaptively plan, thus gaining the respect of others. Spirituality and optimism allow them to feel more at peace and less controlled by the illness.
Support these patients by encouraging their coping skills. For example, if spirituality has helped before, it may again strengthen them and their families.
Improving coping skills. By recognizing which patients are struggling, you can provide support to strengthen their coping abilities. Initially, Mrs. M was using neurotic defense mechanisms and internalizing her emotions. With the help of CBT, she begins to rely on more mature defenses. Her improved coping skills allow her to share her feelings during group therapy and with her family.
Encouraging adaptive behavior
Mrs. M is in remission when chemotherapy ends, but she worries that the cancer will come back. “The fear is still there,” she says. “You can’t be called a survivor for 5 years.”
She continues biweekly psychotherapy for the next year, and sertraline, 100 mg/d. She is doing well and has volunteered to help other newly-diagnosed patients at the cancer center.
Personality styles. Kahana and Bibring17 described 7 personality types that affect how patients cope with illnesses. Based on our experience, we suggest how to observe these styles, identify the emotional pain behind them, and respond in ways that will help each type of patient (Table 2).17
These personality types are not necessarily personality disorders; rather, they describe pervasive characteristics of coping styles. Some individuals will not match the descriptions of any of these types, and others may fall into more than one. Mrs. M, for example, shows traits of more than one personality style, including avoidant and mild schizoid features.
We helped her by engaging her in psychiatric treatment, helping her better understand her medical situation, and restoring her sense of control in making medical decisions. We discussed psycho-therapy as a two-way street, outlining her responsibilities to practice new CBT skills to use during treatment and in remission.
Table 2
Recommended treatment approaches for 7 patient personalities
| Personality | Patients who… | Often feel… | Are helped by… |
|---|---|---|---|
| Dependent | ask many questions, making it hard for you to end conversations or leave the room | afraid you won’t find them worthy, won’t want to care for them | regular, brief sessions (set tactful limits that will reassure patient and not annoy staff) |
| Obsessive | are insistent, detail-oriented | angry when they can’t control their illness, the staff, and the schedule | detailed explanations (provide choices whenever possible, try to use patient input collaboratively) |
| Narcissistic | are self-centered, criticize others, believe no one is qualified to care for them | fearful, threatened, and vulnerable | avoiding confrontation but emphasizing that they deserve the best care staff can provide (keep patient informed; be sure all staff provides the same message) |
| Suffering victim | always have symptoms and request much attention; might not follow recommendations | suffering is their role; views illness and its treatments as punishment, but hopes doctor will keep trying | regular visits, no matter how variable the complaints |
| Paranoid | do not trust doctors, refuse to participate in treatment plans or sign out | taken advantage of by others or purposefully neglected or harmed | staying calm (don’t argue; offer understanding of patient’s position, provide clear recommendations |
| Histrionic | are flirtatious, want to call doctors by first names | need to be ‘special’ to the physician; fear illness will invalidate them or make them unattractive | encouraging patient to verbalize concerns (set boundaries for relationship; remain courteous and objective) |
| Schizoid | are very lonely, tend to avoid medical care | doctors are invading their privacy | engaging patient in making medical decisions |
| Source: Adapted from reference 17 | |||
Supportive therapy. Encourage patients to seek support from family and friends.18 Some benefit from meeting other patients with the same needs, fears, and questions.19 The Internet is a good resource to find local support groups.
Becoming sick or disabled and having to change one’s lifestyle can damage a person’s self esteem. Encourage patients to help others by volunteering, participating in research, or engaging in other activities that provide hope, gratification, and a sense of service.20
Some patients regain control and battle their fears by becoming experts on their diseases. This approach will not help those who become anxious learning about prognosis and side effects, however, and prefer to have limited information about their illnesses. Your knowledge of a patient’s personality type can help you determine whether added information might alleviate or worsen that patient’s stress.
Personal transformation can occur when patients face mortality. Their perceptions about what is important and how to achieve their goals can change dramatically.
Related resources
- National Cancer Institute. Coping with cancer. www.cancer.gov/cancertopics/coping.
- American Psychiatric Association. Patient education site with links to topics such as coping with AIDS/HIV, postpartum depression, alcohol abuse, mental health of the elderly, and common childhood disorders. www.healthyminds.org.
- Groves M, Muskin P. Psychological responses to illness. In: Bourgeois JA, Hales RE, Shahrokh N (eds). Textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005:68-88.
Drug brand names
- Mirtazapine • Remeron
- Sertraline • Zoloft
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Mrs. M, 35, is undergoing breast cancer treatment. She is referred to the consultation-liaison service by an emergency room physician for evaluation of suicidal thoughts.
Mrs. M has been crying for 2 hours. She awoke this morning feeling that she could no longer deal with “the unknown.” She has lost her hair and 30 pounds during chemotherapy and hardly recognized herself in the bathroom mirror. She thought about killing herself until one of her children walked by. After they went to school, she drove to the emergency room.
When you ask if she has had other thoughts of hurting herself, she says, “I would never do anything like that to my family, but the fact that I was thinking about it really scared me.”
Each patient responds uniquely to the emotional trauma of having a chronic or life-threatening medical illness. Coping styles depend on medical, psychological, and social factors as well as the person’s personality and experiences. Reactions range from mature to psychotic.
To help you guide patients such as Mrs. M through difficult medical treatments and decisions, we describe:
- a psychiatric workup to identify maladaptive response to illness
- typical emotional and behavioral responses to chronic illness
- how to provide psychotherapy tailored to the needs of 7 personality styles of medically ill patients.
Psychiatric Workup
Mrs. M is married with 3 sons. She found a lump on her breast 4 months ago but decided to wait “because, I thought, ‘it can’t be anything bad; it will go away.’” When the lump remained 3 months later, she consulted a specialist who diagnosed breast cancer.
Mrs. M underwent a lumpectomy and has been receiving weekly chemotherapy. Besides losing her hair, she says she has no appetite. Looking at food or driving close to the hospital makes her nauseous. She starts vomiting before she arrives for chemotherapy.
Though her family supports and encourages her, she can’t stop thinking about death. She feels isolated, lonely, and cries often. “No one understands what I am going through,” she says. “How can I share these feelings with them?”
Physicians such as the specialist who diagnosed Mrs. M’s cancer often must communicate difficult information. Careful planning when delivering bad news (Box 1)1,2 can set the stage for a healthier emotional and behavioral response.
Chronic illness causes depression in up to 25% of patients.3 At particular risk of developing depressive symptoms are patients with:
- poor physical condition
- poorly controlled pain
- advanced illness
- history of major depressive disorder
- family history of depression and suicide
- certain cancers, such as of the pancreas, lung, head or neck.4
Mechanisms of depression in illness. Depression can be the first symptom of some medical illnesses, such as pancreatic or liver cancer. Diseases that directly affect the brain—such as Parkinson’s disease, multiple sclerosis, and systemic lupus erythematosus—can cause depression. Patients disabled by spinal cord injury, stroke, or cancer also are at risk for depression.
Treatment is the same, whether illness-related chemical changes or the patient’s emotional response to disability is causing the depression. When diagnosing depression in medically ill patients, DSM-IV-TR recommends using criteria for major depression and providing treatment, whatever the cause.
Mrs. M has trouble sharing feelings of hopelessness with her family. Her changes in demeanor and isolation from everyday activities are clues to clinical depression and anxiety.
Depression can be difficult to diagnose when medical illness causes depression-like symptoms.5 To make the diagnosis, ask patients with chronic illness about depression’s emotional symptoms (hopelessness, withdrawal from others, sadness, ruminating thoughts, frequent crying spells), rather than its physical symptoms of poor eating, disturbed sleep, or low energy that may stem from the medical illness. Patients might not be as hard on themselves if they feel the medical illness—not poor coping on their part—caused their depression.
Box 1 6 steps for delivering bad medical news
Source: Adapted from references 1, 2Workup. Our workup of Mrs. M includes thyroid function tests, CBC, comprehensive metabolic panel, and brain MRI to rule out metastasis. All are negative. In her history, Mrs. M reports a previous episode of major depression 5 years ago that was successfully treated with sertraline for 1 year.
Consistent with her workup and symptoms, we diagnose major depression without psychosis. Cancer patients who report hopelessness are at increased risk of suicide. Because of her family support and religious beliefs, however, we feel she can safely go home and return for follow-up the next week.
Emotional response to illness
Mrs. M starts weekly outpatient appointments at our clinic. Because sertraline has worked for her in the past, it is our first choice to increase her energy, improve her mood, and decrease her hopelessness. Initial dosage is 25 mg/d for the first 3 days, with an increase to 50 mg/d for 1 month, and 100 mg/d thereafter. She reports no side effects.
In some patients, sertraline can cause adverse gastrointestinal effects such as upset stomach or nausea. Other antidepressants such as mirtazapine could improve patients’ sleep and decrease nausea.
Mrs. M also begins cognitive-behavioral therapy (CBT) to help her deal with negative thoughts, and relaxation training to combat her anxiety before chemotherapy. We recommend a local breast cancer support group, and Mrs. M starts going twice a month. She feels relieved that other patients are experiencing feelings similar to hers.
Medication plus CBT has been shown to be the most effective treatment for patients who meet criteria for depression and anxiety disorders. CBT has been shown to help patients manage physical symptoms and reframe negative thoughts associated with many chronic illnesses, including breast cancer, Parkinson’s disease, epilepsy, rheumatoid arthritis, and multiple sclerosis.6-10
Core emotions. Medically ill patients experience a range of core emotions, which Lazarus11 identified as anger, anxiety, guilt, fright, shame, sadness, happiness, envy, relief, and hope. Identifying the source of these emotions is important to counseling patients effectively.
For example, a patient experiencing fright might fear death, pain, disability, stigma, disfigurement, or other eventualities. Mrs. M has said she can’t stop thinking about dying. By knowing what the patient fears (Table 1),12 we can more effectively reassure and offer support, even when little else can be done.
Table 1
Common fears of patients reacting to diagnosis of chronic illness
|
| Source: Reference 12 |
Defensive behaviors. Patient’s behavioral responses may be adaptive or maladaptive; treatment nonadherence is one maladaptive response (Box 2).13,14 The patient history can suggest how well a person has adapted to past losses and disappointments. Patients may try to protect themselves against emotional and physical pain with psychotic, immature, neurotic, or mature defense mechanisms.15
Psychotic defenses are characterized by regression until patients lose touch with reality. Delusions and fantasy isolate them from the harshness of a serious medical condition. Antipsychotic medication and patience are often indicated.16
Immature defenses—as seen in patients with borderline personality disorder—can irritate and alienate the medical team. Physicians may not understand why their best efforts are thwarted or negated. Well-intentioned, caring doctors often try harder when the verbal attacks begin, but soon even the hardiest can wither under the patient’s criticism and threats.
Depressed medically ill patients adhere poorly to treatment schedules and other recommendations, which may cut their chances of survival.13 Up to 2% of hospital discharges are initiated by patients against medical advice.
Causes of refusing or discontinuing treatment may include anger towards the medical team or caregiver,14 anxiety, or withdrawal from addictive substances. Some patients who sign themselves out may be psychotic or confused by delirium or dementia. Others may be in denial of their illness.
Untreated psychiatric disorders can cause illness-specific nonadherence. Patients with:
- depression might not have the energy or motivation to follow the treatment.
- bipolar disorder might feel they don’t need the treatment.
- psychotic disorders may feel threatened by the treatment or the doctor.
Psychiatric patients may avoid taking medications prescribed for medical illness if they fear side effects or interactions with their psychotropics. Some don’t tell their medical practitioners about their psychiatric diagnoses because of the stigma associated with mental illness.
Psychiatrists can counsel the medical team to:
- pull back and focus on setting treatment objectives
- encourage the patient to work as a team member to ensure the best possible care
- communicate with the patient and care team to prevent divisions among the staff.
Neurotic defenses are in play when patients blame themselves and suffer internally. Give them ample opportunities to explore their feelings.
Mature defenses are seen in those who show concern for others, may express humor, and can adaptively plan, thus gaining the respect of others. Spirituality and optimism allow them to feel more at peace and less controlled by the illness.
Support these patients by encouraging their coping skills. For example, if spirituality has helped before, it may again strengthen them and their families.
Improving coping skills. By recognizing which patients are struggling, you can provide support to strengthen their coping abilities. Initially, Mrs. M was using neurotic defense mechanisms and internalizing her emotions. With the help of CBT, she begins to rely on more mature defenses. Her improved coping skills allow her to share her feelings during group therapy and with her family.
Encouraging adaptive behavior
Mrs. M is in remission when chemotherapy ends, but she worries that the cancer will come back. “The fear is still there,” she says. “You can’t be called a survivor for 5 years.”
She continues biweekly psychotherapy for the next year, and sertraline, 100 mg/d. She is doing well and has volunteered to help other newly-diagnosed patients at the cancer center.
Personality styles. Kahana and Bibring17 described 7 personality types that affect how patients cope with illnesses. Based on our experience, we suggest how to observe these styles, identify the emotional pain behind them, and respond in ways that will help each type of patient (Table 2).17
These personality types are not necessarily personality disorders; rather, they describe pervasive characteristics of coping styles. Some individuals will not match the descriptions of any of these types, and others may fall into more than one. Mrs. M, for example, shows traits of more than one personality style, including avoidant and mild schizoid features.
We helped her by engaging her in psychiatric treatment, helping her better understand her medical situation, and restoring her sense of control in making medical decisions. We discussed psycho-therapy as a two-way street, outlining her responsibilities to practice new CBT skills to use during treatment and in remission.
Table 2
Recommended treatment approaches for 7 patient personalities
| Personality | Patients who… | Often feel… | Are helped by… |
|---|---|---|---|
| Dependent | ask many questions, making it hard for you to end conversations or leave the room | afraid you won’t find them worthy, won’t want to care for them | regular, brief sessions (set tactful limits that will reassure patient and not annoy staff) |
| Obsessive | are insistent, detail-oriented | angry when they can’t control their illness, the staff, and the schedule | detailed explanations (provide choices whenever possible, try to use patient input collaboratively) |
| Narcissistic | are self-centered, criticize others, believe no one is qualified to care for them | fearful, threatened, and vulnerable | avoiding confrontation but emphasizing that they deserve the best care staff can provide (keep patient informed; be sure all staff provides the same message) |
| Suffering victim | always have symptoms and request much attention; might not follow recommendations | suffering is their role; views illness and its treatments as punishment, but hopes doctor will keep trying | regular visits, no matter how variable the complaints |
| Paranoid | do not trust doctors, refuse to participate in treatment plans or sign out | taken advantage of by others or purposefully neglected or harmed | staying calm (don’t argue; offer understanding of patient’s position, provide clear recommendations |
| Histrionic | are flirtatious, want to call doctors by first names | need to be ‘special’ to the physician; fear illness will invalidate them or make them unattractive | encouraging patient to verbalize concerns (set boundaries for relationship; remain courteous and objective) |
| Schizoid | are very lonely, tend to avoid medical care | doctors are invading their privacy | engaging patient in making medical decisions |
| Source: Adapted from reference 17 | |||
Supportive therapy. Encourage patients to seek support from family and friends.18 Some benefit from meeting other patients with the same needs, fears, and questions.19 The Internet is a good resource to find local support groups.
Becoming sick or disabled and having to change one’s lifestyle can damage a person’s self esteem. Encourage patients to help others by volunteering, participating in research, or engaging in other activities that provide hope, gratification, and a sense of service.20
Some patients regain control and battle their fears by becoming experts on their diseases. This approach will not help those who become anxious learning about prognosis and side effects, however, and prefer to have limited information about their illnesses. Your knowledge of a patient’s personality type can help you determine whether added information might alleviate or worsen that patient’s stress.
Personal transformation can occur when patients face mortality. Their perceptions about what is important and how to achieve their goals can change dramatically.
Related resources
- National Cancer Institute. Coping with cancer. www.cancer.gov/cancertopics/coping.
- American Psychiatric Association. Patient education site with links to topics such as coping with AIDS/HIV, postpartum depression, alcohol abuse, mental health of the elderly, and common childhood disorders. www.healthyminds.org.
- Groves M, Muskin P. Psychological responses to illness. In: Bourgeois JA, Hales RE, Shahrokh N (eds). Textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005:68-88.
Drug brand names
- Mirtazapine • Remeron
- Sertraline • Zoloft
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bailey W, Buckman R, Lenzi R, et al. SPIKES - a six-step protocol for delivering bad news; application to the patient with cancer. Oncologist 2000;5:4,302-11.
2. Koenig HG, Larson DB, Larson SS. Religion and coping with serious medical illness. Ann Pharmacother 2001;35(3):352-9.
3. Faller H, Schmidt M. Prognostic value of depressive coping and depression in survival of lung cancer patients. Psychooncology 2004;13(5):359-63.
4. Holland JC. Psychological care of patients: psycho-oncology’s contribution. J Clin Oncol 2003;21(23 suppl):253s-265s.
5. Lovejoy NC, Tabor D, Matteis M, Lillis P. Cancer-related depression: Part I. Neurologic alterations and cognitive-behavioral therapy. Oncol Nurs Forum 2000;27(4):667-78.
6. Cole K, Vaughan FL. The feasibility of using cognitive behaviour therapy for depression associated with Parkinson’s disease: a literature review. Parkinsonism Relat Disord 2005;11(5):269-76
7. Goldstein LH, McAlpine M, Deale A, et al. Cognitive behaviour therapy with adults with intractable epilepsy and psychiatric co-morbidity: preliminary observations on changes in psychological state and seizure frequency. Behav Res Ther 2003;41(4):447-60.
8. Evers AW, Kraaimaat FW, van Riel PL, de Jong AJ. Tailored cognitive-behavioral therapy in early rheumatoid arthritis for patients at risk: a randomized controlled trial. Pain 2002;100(1-2):141-53.
9. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol 2000;68(2):356-61.
10. Tatrow K. Montgomery GH. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. J Behav Med 2006;29(1):17-27.
11. Lazarus RS, Folkman S. Stress, appraisal and coping. New York: Springer, 1984.
12. Gazzola L, Muskin PR. The impact of stress and the objective of psychosocial interventions. In: Schein LA, Bernard HS, Spitz HI, Muskin PR (eds). Psychosocial treatment for medical conditions: principles and techniques. New York: Brunner-Routledge; 2003: 373-406.
13. Uitterhoeve RJ, Vernooy M, Litjens M, et al. Psychosocial interventions for patients with advanced cancer - a systematic review of the literature. Br J Cancer 2004;91(6):1050-62.
14. Perry S, Viederman M. Management of emotional reactions to acute medical illness. Med Clin North Am 1981;65:3-14.
15. Gabbard G. Major modalities: psychoanalytic/psychodynamic. In: Gabbard G, Beck J, Holmes J, eds. Oxford textbook of psychotherapy. New York: Oxford University Press; 2005:3-13.
16. Groves M, Muskin P. Psychological responses to illness In: Bourgeois JA, Hales RE, Shahrokh N (eds). Textbook of psychosomatic medicine Washington, DC: American Psychiatric Publishing; 2005:68-88.
17. Kahana RJ, Bibring G. Personality types in medical management. In: Zinberg NE (ed). Psychiatry and medical practice in a general hospital. New York: International Universities Press, 1964:108-23.
18. Johnson KS, Elbert-Avila KI, Tulsky JA. The influence of spiritual beliefs and practices on the treatment p of African Americans: a review of the literature. J Am Geriatr Soc 2005;53(4):711-19.
19. Vos PJ, Visser AP, Garssen B, et al. Effects of delayed psychosocial interventions versus early psychosocial interventions for women with early stage breast cancer. Patient Educ Couns 2006;60(2):212-9
20. Breitbart W, Gibson C, Poppito SR, Berg A. Psychotherapeutic interventions at the end of life: a focus on meaning and spirituality. Can J Psychiatry 2004;49(6):366-72.
1. Bailey W, Buckman R, Lenzi R, et al. SPIKES - a six-step protocol for delivering bad news; application to the patient with cancer. Oncologist 2000;5:4,302-11.
2. Koenig HG, Larson DB, Larson SS. Religion and coping with serious medical illness. Ann Pharmacother 2001;35(3):352-9.
3. Faller H, Schmidt M. Prognostic value of depressive coping and depression in survival of lung cancer patients. Psychooncology 2004;13(5):359-63.
4. Holland JC. Psychological care of patients: psycho-oncology’s contribution. J Clin Oncol 2003;21(23 suppl):253s-265s.
5. Lovejoy NC, Tabor D, Matteis M, Lillis P. Cancer-related depression: Part I. Neurologic alterations and cognitive-behavioral therapy. Oncol Nurs Forum 2000;27(4):667-78.
6. Cole K, Vaughan FL. The feasibility of using cognitive behaviour therapy for depression associated with Parkinson’s disease: a literature review. Parkinsonism Relat Disord 2005;11(5):269-76
7. Goldstein LH, McAlpine M, Deale A, et al. Cognitive behaviour therapy with adults with intractable epilepsy and psychiatric co-morbidity: preliminary observations on changes in psychological state and seizure frequency. Behav Res Ther 2003;41(4):447-60.
8. Evers AW, Kraaimaat FW, van Riel PL, de Jong AJ. Tailored cognitive-behavioral therapy in early rheumatoid arthritis for patients at risk: a randomized controlled trial. Pain 2002;100(1-2):141-53.
9. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol 2000;68(2):356-61.
10. Tatrow K. Montgomery GH. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. J Behav Med 2006;29(1):17-27.
11. Lazarus RS, Folkman S. Stress, appraisal and coping. New York: Springer, 1984.
12. Gazzola L, Muskin PR. The impact of stress and the objective of psychosocial interventions. In: Schein LA, Bernard HS, Spitz HI, Muskin PR (eds). Psychosocial treatment for medical conditions: principles and techniques. New York: Brunner-Routledge; 2003: 373-406.
13. Uitterhoeve RJ, Vernooy M, Litjens M, et al. Psychosocial interventions for patients with advanced cancer - a systematic review of the literature. Br J Cancer 2004;91(6):1050-62.
14. Perry S, Viederman M. Management of emotional reactions to acute medical illness. Med Clin North Am 1981;65:3-14.
15. Gabbard G. Major modalities: psychoanalytic/psychodynamic. In: Gabbard G, Beck J, Holmes J, eds. Oxford textbook of psychotherapy. New York: Oxford University Press; 2005:3-13.
16. Groves M, Muskin P. Psychological responses to illness In: Bourgeois JA, Hales RE, Shahrokh N (eds). Textbook of psychosomatic medicine Washington, DC: American Psychiatric Publishing; 2005:68-88.
17. Kahana RJ, Bibring G. Personality types in medical management. In: Zinberg NE (ed). Psychiatry and medical practice in a general hospital. New York: International Universities Press, 1964:108-23.
18. Johnson KS, Elbert-Avila KI, Tulsky JA. The influence of spiritual beliefs and practices on the treatment p of African Americans: a review of the literature. J Am Geriatr Soc 2005;53(4):711-19.
19. Vos PJ, Visser AP, Garssen B, et al. Effects of delayed psychosocial interventions versus early psychosocial interventions for women with early stage breast cancer. Patient Educ Couns 2006;60(2):212-9
20. Breitbart W, Gibson C, Poppito SR, Berg A. Psychotherapeutic interventions at the end of life: a focus on meaning and spirituality. Can J Psychiatry 2004;49(6):366-72.
Prudent prescribing for patients with addictions
Did benzodiazepines prescribed to patient
with addiction cause delirium?
Maricopa County (AZ) Superior Court
A 40-year-old woman addicted to diazepam sought treatment from a psychiatric nurse who performed a psychological evaluation. The patient claimed that the nurse negligently prescribed benzodiazepines and other medications for anxiety, panic attacks, and depression.
The patient claimed that the prescriptions caused a drug-induced delirium, during which she put a nonlethal amount of the medication on her two minor daughters’ ice cream, then attempted suicide by overdosing with her prescriptions. The patient and her daughters survived.
The patient was charged with two counts of attempted murder and was incarcerated for 18 months while awaiting trail. She was acquitted of the charges but lost custody of her daughters.
The psychiatric nurse argued that the medication prescribed was appropriate and the patient was not in a drug-induced delirium when she tried to kill herself and her daughters. The defense alleged that other factors caused the patient to attempt suicide/homicide, including a pending divorce and financial problems.
- A defense verdict was returned
Woman claims she was prescribed narcotics
despite alprazolam addiction
Multnomah County (OR) Superior Court
The patient, age 57, began seeing a psychiatrist in March 1993 for anxiety and panic attacks. She had kicked a 10-year alprazolam addiction and had been drug-free for more than 6 months when she first visited the psychiatrist.
The patient claimed that over the next 11 years she developed an intimate friendship with the psychiatrist. The patient visited the psychiatrist’s office almost weekly—sometimes twice a week—and incurred almost $100,000 in fees. The patient says that the psychiatrist prescribed her narcotics, then sought the drugs from her for his personal use, and was negligent in his treatment.
- A $593,000 verdict was returned, which included $200,000 in punitive damages
- Try prescribing nonaddictive alternate medication first.
- Prescribe a limited amount for a short time when an abusable substance is clinically warranted.
- Document in the patient’s chart specific treatment needs that will be addressed by the medication, potential benefits and risks, the dosage, and date of the prescription.
- Use medication in combination with an ongoing discussion of the patient’s anxiety, history of addiction, and the clinician’s attempts to prevent future addictions.
- If prescription drug abuse develops, identify the problem and help the patient find appropriate treatment, such as detoxification inpatient chemical dependency treatment, or intensive outpatient dependency treatment.
Dr. Grant’s observations
Should benzodiazepines or other addictive substances be prescribed to a patient with a history of substance abuse? Little evidence guides clinicians,1,2 and limited research has examined whether former substance abusers are more likely than other patients to abuse benzodiazepines or if these medications increase the risk of substance abuse relapse.2
A psychiatrist can prescribe medication whenever a medical basis exists. In the first case a patient with anxiety and panic attacks was given benzodiazepines, an appropriate treatment for anxiety disorder.3 But what if the patient has a history of substance abuse? When is prescribing these medications negligent?
The fiduciary relationship between psychiatrist and patient states that the therapist is the patient’s ally and should always act in the patient’s best interest. With limited data, clinicians have no clear rule for a standard of care.
On one hand, benzodiazepine misuse is a problem and these medications must be prescribed cautiously. In 2004 roughly 300,000 Americans reported using prescription sedatives for nonmedical purposes.4 Many addiction specialists believe benzodiazepines are contraindicated for patients with current alcohol or drug abuse problems and for those in recovery. In this scenario, the clinician could choose an appropriate alternative to a benzodiazepine such as an antidepressant, buspirone, beta blocker, or anticonvulsant. Explain to the patient that these medications’ clinical effect is slower than that of benzodiazepines. Also consider psychotherapy to address anxiety.
On the other hand, benzodiazepines might be underused because of fear of addiction.5 Clinicians must consider whether their prescribing practices are designed to protect themselves or are in the patients’ best interests (Box 1). Of course, when treating a patient with a benzodiazepine addiction, the risk-benefit analysis shifts and abuse concerns may be more appropriate.
In the first case, the patient attempted suicide by overdosing on the prescribed medication. This fact might support the patient’s argument that she was not an appropriate candidate for benzodiazepines and the psychiatric nurse could be held liable—even though in this case she was not. One court found that a psychiatrist writing prescriptions for large amounts of controlled substances to someone addicted to drugs could be held liable for the patient’s suicide.6
In the second case, a psychiatrist prescribed narcotics to a patient with a history of addiction. The code of medical ethics is clear: A psychiatrist who regularly practices outside his or her area of professional competence should be considered as having acted in an unethical manner.7 So if you wish to prescribe narcotics, you must follow internal medicine’s ethical standards (Box 2).
Responsibility of care
Although the nurse in the first case could be liable for her actions, the psychiatrist who supervised the nurse might also be partially responsible. The law assumes that those who work under a physician’s supervision act as his or her agents. Nurses working for a physician are the physician’s agents, and the physician is responsible for a nurse’s acts. This legal principle is respondeat superior, or “let the master reply.”
Generally, the physician’s lack of knowledge about what the nurse prescribes is not a defense for a malpractice claim. In fact, the law requires that the physician know whether his or her agents meet the profession’s standard of care. In cases where a nurse prescribes an inappropriate medication, the psychiatrist can be charged with negligent supervision—that is, failing to provide to the nurse proper guidance and instruction.
Ethical conduct
Relationships with patients. The second case raises several egregious issues in patient care. Although intimate relationships with patients are prohibited, the fact that these cases still come before licensing boards and courts suggests that physicians are not getting the message. Although the report of this case is vague about what “intimate” means, several points are raised:
- Sexual relationships with current or former patients are not allowed.7 A patient is vulnerable, and the power differential makes it difficult for the patient to resist the therapist’s requests.
- Nonsexual, intimate relationships likely would be seen as a boundary violation, akin to a sexual relationship. In the case presented, the boundary violation is obvious even though the relationship may not have been sexual.
- Establish a patient-physician relationship.
- Perform and document a medical history and physical exam to justify the medication prescribed.
- Medication must be warranted and consistent with the physician’s diagnosis.
- Dosages and prolonged prescriptions need to be within the usual course of medical practice.
- Maintain accurate and complete treatment records.
Source: Snyder L, Leffler C. American College of Physicians ethics manual, 5th ed. Available at: http://www.acponline.org/ethics/ethicman5th.htm. Accessed August 30, 2006.
Medical ethics prohibit this behavior and state that psychiatrists should not:7
- use the unique position afforded by the psychotherapeutic situation to influence the patient in any way that is not directly relevant to treatment goals
- exploit information furnished by patients.
State medical boards have varying procedures in place to handle a physician’s substance abuse.
These programs’ goal is to assist recovery, eliminate risk to the public, and allow the physician to return to work. Clinicians should be aware of such programs in their jurisdictions.
Drug brand names
- Alprazolam • Xanax
- Buspirone • BuSpar
- Diazepam • Valium
1. Brunette MF, Noordsy DL, Xie H, et al. Benzodiazepine use and abuse among patients with severe mental illness and co-occurring substance use disorders. Psychiatr Serv 2003;54:1395-401.
2. Posternak MA, Mueller TI. Assessing the risks and benefits of benzodiazepines for anxiety disorders in patients with a history of substance abuse or dependence. Am J Addict 2001;10:48-68.
3. Uhlenhuth EH, Balter MB, Ban TA, et al. International study of expert judgment on therapeutic use of benzodiazepines and other psychotherapeutic medications: IV. Therapeutic dose dependence and abuse liability of benzodiazepines in the long-term treatment of anxiety disorders. J Clin Psychopharmacol 1999;19(suppl 2):23S-29S.
4. U.S. Department of Health and Human Services. National Survey on Drug Use and Health. http://www.oas.samhsa.gov/nhsda.htm; accessed August 23, 2006.
5. American Psychiatric Association Practice guideline for the treatment of patients with panic disorder. Washington, DC: American Psychiatric Association; 1998.
6. Argus v Scheppegrell 472 So. 2d 573 (La. 1985).
7. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry, 2006 edition. Available at: http://www.psych.org/psych_pract/ethics/ppaethics.cfm. Accessed August 28, 2006.
8. Patten SB, Love EJ. Neuropsychiatric adverse drug reactions: passive reports to Health and Welfare Canada’s adverse reaction database (1965-present). Int J Psychiatry Med 1994;24:45-62.
9. Michel L, Lang JP. Benzodiazepines and forensic aspects. Encephale 2003;29:479-85.
10. Hughes PH, Brandenburg N, Baldwin DC, et al. Prevalence of substance abuse among US physicians. JAMA 1992;267:2333-9.
Did benzodiazepines prescribed to patient
with addiction cause delirium?
Maricopa County (AZ) Superior Court
A 40-year-old woman addicted to diazepam sought treatment from a psychiatric nurse who performed a psychological evaluation. The patient claimed that the nurse negligently prescribed benzodiazepines and other medications for anxiety, panic attacks, and depression.
The patient claimed that the prescriptions caused a drug-induced delirium, during which she put a nonlethal amount of the medication on her two minor daughters’ ice cream, then attempted suicide by overdosing with her prescriptions. The patient and her daughters survived.
The patient was charged with two counts of attempted murder and was incarcerated for 18 months while awaiting trail. She was acquitted of the charges but lost custody of her daughters.
The psychiatric nurse argued that the medication prescribed was appropriate and the patient was not in a drug-induced delirium when she tried to kill herself and her daughters. The defense alleged that other factors caused the patient to attempt suicide/homicide, including a pending divorce and financial problems.
- A defense verdict was returned
Woman claims she was prescribed narcotics
despite alprazolam addiction
Multnomah County (OR) Superior Court
The patient, age 57, began seeing a psychiatrist in March 1993 for anxiety and panic attacks. She had kicked a 10-year alprazolam addiction and had been drug-free for more than 6 months when she first visited the psychiatrist.
The patient claimed that over the next 11 years she developed an intimate friendship with the psychiatrist. The patient visited the psychiatrist’s office almost weekly—sometimes twice a week—and incurred almost $100,000 in fees. The patient says that the psychiatrist prescribed her narcotics, then sought the drugs from her for his personal use, and was negligent in his treatment.
- A $593,000 verdict was returned, which included $200,000 in punitive damages
- Try prescribing nonaddictive alternate medication first.
- Prescribe a limited amount for a short time when an abusable substance is clinically warranted.
- Document in the patient’s chart specific treatment needs that will be addressed by the medication, potential benefits and risks, the dosage, and date of the prescription.
- Use medication in combination with an ongoing discussion of the patient’s anxiety, history of addiction, and the clinician’s attempts to prevent future addictions.
- If prescription drug abuse develops, identify the problem and help the patient find appropriate treatment, such as detoxification inpatient chemical dependency treatment, or intensive outpatient dependency treatment.
Dr. Grant’s observations
Should benzodiazepines or other addictive substances be prescribed to a patient with a history of substance abuse? Little evidence guides clinicians,1,2 and limited research has examined whether former substance abusers are more likely than other patients to abuse benzodiazepines or if these medications increase the risk of substance abuse relapse.2
A psychiatrist can prescribe medication whenever a medical basis exists. In the first case a patient with anxiety and panic attacks was given benzodiazepines, an appropriate treatment for anxiety disorder.3 But what if the patient has a history of substance abuse? When is prescribing these medications negligent?
The fiduciary relationship between psychiatrist and patient states that the therapist is the patient’s ally and should always act in the patient’s best interest. With limited data, clinicians have no clear rule for a standard of care.
On one hand, benzodiazepine misuse is a problem and these medications must be prescribed cautiously. In 2004 roughly 300,000 Americans reported using prescription sedatives for nonmedical purposes.4 Many addiction specialists believe benzodiazepines are contraindicated for patients with current alcohol or drug abuse problems and for those in recovery. In this scenario, the clinician could choose an appropriate alternative to a benzodiazepine such as an antidepressant, buspirone, beta blocker, or anticonvulsant. Explain to the patient that these medications’ clinical effect is slower than that of benzodiazepines. Also consider psychotherapy to address anxiety.
On the other hand, benzodiazepines might be underused because of fear of addiction.5 Clinicians must consider whether their prescribing practices are designed to protect themselves or are in the patients’ best interests (Box 1). Of course, when treating a patient with a benzodiazepine addiction, the risk-benefit analysis shifts and abuse concerns may be more appropriate.
In the first case, the patient attempted suicide by overdosing on the prescribed medication. This fact might support the patient’s argument that she was not an appropriate candidate for benzodiazepines and the psychiatric nurse could be held liable—even though in this case she was not. One court found that a psychiatrist writing prescriptions for large amounts of controlled substances to someone addicted to drugs could be held liable for the patient’s suicide.6
In the second case, a psychiatrist prescribed narcotics to a patient with a history of addiction. The code of medical ethics is clear: A psychiatrist who regularly practices outside his or her area of professional competence should be considered as having acted in an unethical manner.7 So if you wish to prescribe narcotics, you must follow internal medicine’s ethical standards (Box 2).
Responsibility of care
Although the nurse in the first case could be liable for her actions, the psychiatrist who supervised the nurse might also be partially responsible. The law assumes that those who work under a physician’s supervision act as his or her agents. Nurses working for a physician are the physician’s agents, and the physician is responsible for a nurse’s acts. This legal principle is respondeat superior, or “let the master reply.”
Generally, the physician’s lack of knowledge about what the nurse prescribes is not a defense for a malpractice claim. In fact, the law requires that the physician know whether his or her agents meet the profession’s standard of care. In cases where a nurse prescribes an inappropriate medication, the psychiatrist can be charged with negligent supervision—that is, failing to provide to the nurse proper guidance and instruction.
Ethical conduct
Relationships with patients. The second case raises several egregious issues in patient care. Although intimate relationships with patients are prohibited, the fact that these cases still come before licensing boards and courts suggests that physicians are not getting the message. Although the report of this case is vague about what “intimate” means, several points are raised:
- Sexual relationships with current or former patients are not allowed.7 A patient is vulnerable, and the power differential makes it difficult for the patient to resist the therapist’s requests.
- Nonsexual, intimate relationships likely would be seen as a boundary violation, akin to a sexual relationship. In the case presented, the boundary violation is obvious even though the relationship may not have been sexual.
- Establish a patient-physician relationship.
- Perform and document a medical history and physical exam to justify the medication prescribed.
- Medication must be warranted and consistent with the physician’s diagnosis.
- Dosages and prolonged prescriptions need to be within the usual course of medical practice.
- Maintain accurate and complete treatment records.
Source: Snyder L, Leffler C. American College of Physicians ethics manual, 5th ed. Available at: http://www.acponline.org/ethics/ethicman5th.htm. Accessed August 30, 2006.
Medical ethics prohibit this behavior and state that psychiatrists should not:7
- use the unique position afforded by the psychotherapeutic situation to influence the patient in any way that is not directly relevant to treatment goals
- exploit information furnished by patients.
State medical boards have varying procedures in place to handle a physician’s substance abuse.
These programs’ goal is to assist recovery, eliminate risk to the public, and allow the physician to return to work. Clinicians should be aware of such programs in their jurisdictions.
Drug brand names
- Alprazolam • Xanax
- Buspirone • BuSpar
- Diazepam • Valium
Did benzodiazepines prescribed to patient
with addiction cause delirium?
Maricopa County (AZ) Superior Court
A 40-year-old woman addicted to diazepam sought treatment from a psychiatric nurse who performed a psychological evaluation. The patient claimed that the nurse negligently prescribed benzodiazepines and other medications for anxiety, panic attacks, and depression.
The patient claimed that the prescriptions caused a drug-induced delirium, during which she put a nonlethal amount of the medication on her two minor daughters’ ice cream, then attempted suicide by overdosing with her prescriptions. The patient and her daughters survived.
The patient was charged with two counts of attempted murder and was incarcerated for 18 months while awaiting trail. She was acquitted of the charges but lost custody of her daughters.
The psychiatric nurse argued that the medication prescribed was appropriate and the patient was not in a drug-induced delirium when she tried to kill herself and her daughters. The defense alleged that other factors caused the patient to attempt suicide/homicide, including a pending divorce and financial problems.
- A defense verdict was returned
Woman claims she was prescribed narcotics
despite alprazolam addiction
Multnomah County (OR) Superior Court
The patient, age 57, began seeing a psychiatrist in March 1993 for anxiety and panic attacks. She had kicked a 10-year alprazolam addiction and had been drug-free for more than 6 months when she first visited the psychiatrist.
The patient claimed that over the next 11 years she developed an intimate friendship with the psychiatrist. The patient visited the psychiatrist’s office almost weekly—sometimes twice a week—and incurred almost $100,000 in fees. The patient says that the psychiatrist prescribed her narcotics, then sought the drugs from her for his personal use, and was negligent in his treatment.
- A $593,000 verdict was returned, which included $200,000 in punitive damages
- Try prescribing nonaddictive alternate medication first.
- Prescribe a limited amount for a short time when an abusable substance is clinically warranted.
- Document in the patient’s chart specific treatment needs that will be addressed by the medication, potential benefits and risks, the dosage, and date of the prescription.
- Use medication in combination with an ongoing discussion of the patient’s anxiety, history of addiction, and the clinician’s attempts to prevent future addictions.
- If prescription drug abuse develops, identify the problem and help the patient find appropriate treatment, such as detoxification inpatient chemical dependency treatment, or intensive outpatient dependency treatment.
Dr. Grant’s observations
Should benzodiazepines or other addictive substances be prescribed to a patient with a history of substance abuse? Little evidence guides clinicians,1,2 and limited research has examined whether former substance abusers are more likely than other patients to abuse benzodiazepines or if these medications increase the risk of substance abuse relapse.2
A psychiatrist can prescribe medication whenever a medical basis exists. In the first case a patient with anxiety and panic attacks was given benzodiazepines, an appropriate treatment for anxiety disorder.3 But what if the patient has a history of substance abuse? When is prescribing these medications negligent?
The fiduciary relationship between psychiatrist and patient states that the therapist is the patient’s ally and should always act in the patient’s best interest. With limited data, clinicians have no clear rule for a standard of care.
On one hand, benzodiazepine misuse is a problem and these medications must be prescribed cautiously. In 2004 roughly 300,000 Americans reported using prescription sedatives for nonmedical purposes.4 Many addiction specialists believe benzodiazepines are contraindicated for patients with current alcohol or drug abuse problems and for those in recovery. In this scenario, the clinician could choose an appropriate alternative to a benzodiazepine such as an antidepressant, buspirone, beta blocker, or anticonvulsant. Explain to the patient that these medications’ clinical effect is slower than that of benzodiazepines. Also consider psychotherapy to address anxiety.
On the other hand, benzodiazepines might be underused because of fear of addiction.5 Clinicians must consider whether their prescribing practices are designed to protect themselves or are in the patients’ best interests (Box 1). Of course, when treating a patient with a benzodiazepine addiction, the risk-benefit analysis shifts and abuse concerns may be more appropriate.
In the first case, the patient attempted suicide by overdosing on the prescribed medication. This fact might support the patient’s argument that she was not an appropriate candidate for benzodiazepines and the psychiatric nurse could be held liable—even though in this case she was not. One court found that a psychiatrist writing prescriptions for large amounts of controlled substances to someone addicted to drugs could be held liable for the patient’s suicide.6
In the second case, a psychiatrist prescribed narcotics to a patient with a history of addiction. The code of medical ethics is clear: A psychiatrist who regularly practices outside his or her area of professional competence should be considered as having acted in an unethical manner.7 So if you wish to prescribe narcotics, you must follow internal medicine’s ethical standards (Box 2).
Responsibility of care
Although the nurse in the first case could be liable for her actions, the psychiatrist who supervised the nurse might also be partially responsible. The law assumes that those who work under a physician’s supervision act as his or her agents. Nurses working for a physician are the physician’s agents, and the physician is responsible for a nurse’s acts. This legal principle is respondeat superior, or “let the master reply.”
Generally, the physician’s lack of knowledge about what the nurse prescribes is not a defense for a malpractice claim. In fact, the law requires that the physician know whether his or her agents meet the profession’s standard of care. In cases where a nurse prescribes an inappropriate medication, the psychiatrist can be charged with negligent supervision—that is, failing to provide to the nurse proper guidance and instruction.
Ethical conduct
Relationships with patients. The second case raises several egregious issues in patient care. Although intimate relationships with patients are prohibited, the fact that these cases still come before licensing boards and courts suggests that physicians are not getting the message. Although the report of this case is vague about what “intimate” means, several points are raised:
- Sexual relationships with current or former patients are not allowed.7 A patient is vulnerable, and the power differential makes it difficult for the patient to resist the therapist’s requests.
- Nonsexual, intimate relationships likely would be seen as a boundary violation, akin to a sexual relationship. In the case presented, the boundary violation is obvious even though the relationship may not have been sexual.
- Establish a patient-physician relationship.
- Perform and document a medical history and physical exam to justify the medication prescribed.
- Medication must be warranted and consistent with the physician’s diagnosis.
- Dosages and prolonged prescriptions need to be within the usual course of medical practice.
- Maintain accurate and complete treatment records.
Source: Snyder L, Leffler C. American College of Physicians ethics manual, 5th ed. Available at: http://www.acponline.org/ethics/ethicman5th.htm. Accessed August 30, 2006.
Medical ethics prohibit this behavior and state that psychiatrists should not:7
- use the unique position afforded by the psychotherapeutic situation to influence the patient in any way that is not directly relevant to treatment goals
- exploit information furnished by patients.
State medical boards have varying procedures in place to handle a physician’s substance abuse.
These programs’ goal is to assist recovery, eliminate risk to the public, and allow the physician to return to work. Clinicians should be aware of such programs in their jurisdictions.
Drug brand names
- Alprazolam • Xanax
- Buspirone • BuSpar
- Diazepam • Valium
1. Brunette MF, Noordsy DL, Xie H, et al. Benzodiazepine use and abuse among patients with severe mental illness and co-occurring substance use disorders. Psychiatr Serv 2003;54:1395-401.
2. Posternak MA, Mueller TI. Assessing the risks and benefits of benzodiazepines for anxiety disorders in patients with a history of substance abuse or dependence. Am J Addict 2001;10:48-68.
3. Uhlenhuth EH, Balter MB, Ban TA, et al. International study of expert judgment on therapeutic use of benzodiazepines and other psychotherapeutic medications: IV. Therapeutic dose dependence and abuse liability of benzodiazepines in the long-term treatment of anxiety disorders. J Clin Psychopharmacol 1999;19(suppl 2):23S-29S.
4. U.S. Department of Health and Human Services. National Survey on Drug Use and Health. http://www.oas.samhsa.gov/nhsda.htm; accessed August 23, 2006.
5. American Psychiatric Association Practice guideline for the treatment of patients with panic disorder. Washington, DC: American Psychiatric Association; 1998.
6. Argus v Scheppegrell 472 So. 2d 573 (La. 1985).
7. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry, 2006 edition. Available at: http://www.psych.org/psych_pract/ethics/ppaethics.cfm. Accessed August 28, 2006.
8. Patten SB, Love EJ. Neuropsychiatric adverse drug reactions: passive reports to Health and Welfare Canada’s adverse reaction database (1965-present). Int J Psychiatry Med 1994;24:45-62.
9. Michel L, Lang JP. Benzodiazepines and forensic aspects. Encephale 2003;29:479-85.
10. Hughes PH, Brandenburg N, Baldwin DC, et al. Prevalence of substance abuse among US physicians. JAMA 1992;267:2333-9.
1. Brunette MF, Noordsy DL, Xie H, et al. Benzodiazepine use and abuse among patients with severe mental illness and co-occurring substance use disorders. Psychiatr Serv 2003;54:1395-401.
2. Posternak MA, Mueller TI. Assessing the risks and benefits of benzodiazepines for anxiety disorders in patients with a history of substance abuse or dependence. Am J Addict 2001;10:48-68.
3. Uhlenhuth EH, Balter MB, Ban TA, et al. International study of expert judgment on therapeutic use of benzodiazepines and other psychotherapeutic medications: IV. Therapeutic dose dependence and abuse liability of benzodiazepines in the long-term treatment of anxiety disorders. J Clin Psychopharmacol 1999;19(suppl 2):23S-29S.
4. U.S. Department of Health and Human Services. National Survey on Drug Use and Health. http://www.oas.samhsa.gov/nhsda.htm; accessed August 23, 2006.
5. American Psychiatric Association Practice guideline for the treatment of patients with panic disorder. Washington, DC: American Psychiatric Association; 1998.
6. Argus v Scheppegrell 472 So. 2d 573 (La. 1985).
7. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry, 2006 edition. Available at: http://www.psych.org/psych_pract/ethics/ppaethics.cfm. Accessed August 28, 2006.
8. Patten SB, Love EJ. Neuropsychiatric adverse drug reactions: passive reports to Health and Welfare Canada’s adverse reaction database (1965-present). Int J Psychiatry Med 1994;24:45-62.
9. Michel L, Lang JP. Benzodiazepines and forensic aspects. Encephale 2003;29:479-85.
10. Hughes PH, Brandenburg N, Baldwin DC, et al. Prevalence of substance abuse among US physicians. JAMA 1992;267:2333-9.
ECT and memory loss
The headline “ECT wipes out 30 years of memories” (Current Psychiatry, August 2006) is unsupported by data presented in the article. I assume the decision to sensationalize the subject was an editorial one, as Dr. Jon Grant’s comments otherwise are suitably cautious, measured, and correct.
The case presented did not state whether unilateral or bilateral electrode placement was used to administer daily electroconvulsive therapy (ECT) for 10 days, but daily bilateral placement is not recommended by any authority or textbook and is not considered standard. My research has shown that daily unilateral ECT administered 5 days per week for a total of 20 treatments does not affect memory adversely.1
The claim that “the patient suffered severe brain damage and lost all her memories for the past 30 years” also is unsupported. In fact, there is no published evidence that any form of ECT can cause brain damage or permanent memory loss, a subject I have reviewed in considerable detail.2
Richard Abrams, MD
Professor of psychiatry (retired)
Rosalind Franklin University of Medicine and Science
Director of Somatics LLC, manufacturer of ECT equipment
Chicago, IL
References
1. Abrams R. Daily administration of unilateral ECT. Amer J Psychiat 1967;124(3):384-6.
2. Abrams R. Electroconvulsive therapy. 4th ed. New York, NY: Oxford University Press; 2002.
Dr. Grant responds
Dr. Abrams’ letter reflects the ongoing controversy regarding ECT-associated memory loss.
Although Dr. Abrams states that there is no evidence of permanent memory loss associated with ECT, rare cases such as the one presented in the article—where a patient reports losing memories dating back 30 years—call into question ECT’s potential cognitive side effects. Sackeim points to evidence of severe, persistent retrograde amnesia in some ECT cases, which may extend several years prior to treatment.1
In a published, first-person account, an ECT patient describes memory loss of major life events dating back 8 to 9 years.2 Although the memory loss caused significant impairment, the author says she would undergo ECT again.
Further research ultimately may clarify this ongoing controversy. In fact, the first large prospective study of ECT’s cognitive effects has been published recently.3 In a study of 347 patients in 7 facilities, the researchers found that sine wave stimulation resulted in pronounced reaction time slowing, both immediately and 6 months following ECT. Bilateral ECT resulted in more-severe, persistent retrograde amnesia than did right unilateral ECT. Advanced age, lower premorbid intellectual function, and female gender were associated with greater cognitive deficits.
Given the ongoing controversy about ECT’s cognitive side effects, clinicians should make patients aware of this ongoing discussion and the evidence on both sides of the debate.
Jon E. Grant, JD, MD, MPH
Associate professor of psychiatry
University of Minnesota Medical Center, Minneapolis
1. Sackeim HA. Memory and ECT: from polarization to reconciliation. J ECT 2000;16:87-96.
2. Donahue AB. Electroconvulsive therapy and memory loss: a personal journey. J ECT 2000;16:133-43.
3. Sackeim HA, Prudic J, Fuller R, et al. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology [serial online] August 23, 2006. Available at: www.nature.com/npp/journal/vaop/ncurrent/full/1301180a.html. Accessed September 12, 2006.
The headline “ECT wipes out 30 years of memories” (Current Psychiatry, August 2006) is unsupported by data presented in the article. I assume the decision to sensationalize the subject was an editorial one, as Dr. Jon Grant’s comments otherwise are suitably cautious, measured, and correct.
The case presented did not state whether unilateral or bilateral electrode placement was used to administer daily electroconvulsive therapy (ECT) for 10 days, but daily bilateral placement is not recommended by any authority or textbook and is not considered standard. My research has shown that daily unilateral ECT administered 5 days per week for a total of 20 treatments does not affect memory adversely.1
The claim that “the patient suffered severe brain damage and lost all her memories for the past 30 years” also is unsupported. In fact, there is no published evidence that any form of ECT can cause brain damage or permanent memory loss, a subject I have reviewed in considerable detail.2
Richard Abrams, MD
Professor of psychiatry (retired)
Rosalind Franklin University of Medicine and Science
Director of Somatics LLC, manufacturer of ECT equipment
Chicago, IL
References
1. Abrams R. Daily administration of unilateral ECT. Amer J Psychiat 1967;124(3):384-6.
2. Abrams R. Electroconvulsive therapy. 4th ed. New York, NY: Oxford University Press; 2002.
Dr. Grant responds
Dr. Abrams’ letter reflects the ongoing controversy regarding ECT-associated memory loss.
Although Dr. Abrams states that there is no evidence of permanent memory loss associated with ECT, rare cases such as the one presented in the article—where a patient reports losing memories dating back 30 years—call into question ECT’s potential cognitive side effects. Sackeim points to evidence of severe, persistent retrograde amnesia in some ECT cases, which may extend several years prior to treatment.1
In a published, first-person account, an ECT patient describes memory loss of major life events dating back 8 to 9 years.2 Although the memory loss caused significant impairment, the author says she would undergo ECT again.
Further research ultimately may clarify this ongoing controversy. In fact, the first large prospective study of ECT’s cognitive effects has been published recently.3 In a study of 347 patients in 7 facilities, the researchers found that sine wave stimulation resulted in pronounced reaction time slowing, both immediately and 6 months following ECT. Bilateral ECT resulted in more-severe, persistent retrograde amnesia than did right unilateral ECT. Advanced age, lower premorbid intellectual function, and female gender were associated with greater cognitive deficits.
Given the ongoing controversy about ECT’s cognitive side effects, clinicians should make patients aware of this ongoing discussion and the evidence on both sides of the debate.
Jon E. Grant, JD, MD, MPH
Associate professor of psychiatry
University of Minnesota Medical Center, Minneapolis
The headline “ECT wipes out 30 years of memories” (Current Psychiatry, August 2006) is unsupported by data presented in the article. I assume the decision to sensationalize the subject was an editorial one, as Dr. Jon Grant’s comments otherwise are suitably cautious, measured, and correct.
The case presented did not state whether unilateral or bilateral electrode placement was used to administer daily electroconvulsive therapy (ECT) for 10 days, but daily bilateral placement is not recommended by any authority or textbook and is not considered standard. My research has shown that daily unilateral ECT administered 5 days per week for a total of 20 treatments does not affect memory adversely.1
The claim that “the patient suffered severe brain damage and lost all her memories for the past 30 years” also is unsupported. In fact, there is no published evidence that any form of ECT can cause brain damage or permanent memory loss, a subject I have reviewed in considerable detail.2
Richard Abrams, MD
Professor of psychiatry (retired)
Rosalind Franklin University of Medicine and Science
Director of Somatics LLC, manufacturer of ECT equipment
Chicago, IL
References
1. Abrams R. Daily administration of unilateral ECT. Amer J Psychiat 1967;124(3):384-6.
2. Abrams R. Electroconvulsive therapy. 4th ed. New York, NY: Oxford University Press; 2002.
Dr. Grant responds
Dr. Abrams’ letter reflects the ongoing controversy regarding ECT-associated memory loss.
Although Dr. Abrams states that there is no evidence of permanent memory loss associated with ECT, rare cases such as the one presented in the article—where a patient reports losing memories dating back 30 years—call into question ECT’s potential cognitive side effects. Sackeim points to evidence of severe, persistent retrograde amnesia in some ECT cases, which may extend several years prior to treatment.1
In a published, first-person account, an ECT patient describes memory loss of major life events dating back 8 to 9 years.2 Although the memory loss caused significant impairment, the author says she would undergo ECT again.
Further research ultimately may clarify this ongoing controversy. In fact, the first large prospective study of ECT’s cognitive effects has been published recently.3 In a study of 347 patients in 7 facilities, the researchers found that sine wave stimulation resulted in pronounced reaction time slowing, both immediately and 6 months following ECT. Bilateral ECT resulted in more-severe, persistent retrograde amnesia than did right unilateral ECT. Advanced age, lower premorbid intellectual function, and female gender were associated with greater cognitive deficits.
Given the ongoing controversy about ECT’s cognitive side effects, clinicians should make patients aware of this ongoing discussion and the evidence on both sides of the debate.
Jon E. Grant, JD, MD, MPH
Associate professor of psychiatry
University of Minnesota Medical Center, Minneapolis
1. Sackeim HA. Memory and ECT: from polarization to reconciliation. J ECT 2000;16:87-96.
2. Donahue AB. Electroconvulsive therapy and memory loss: a personal journey. J ECT 2000;16:133-43.
3. Sackeim HA, Prudic J, Fuller R, et al. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology [serial online] August 23, 2006. Available at: www.nature.com/npp/journal/vaop/ncurrent/full/1301180a.html. Accessed September 12, 2006.
1. Sackeim HA. Memory and ECT: from polarization to reconciliation. J ECT 2000;16:87-96.
2. Donahue AB. Electroconvulsive therapy and memory loss: a personal journey. J ECT 2000;16:133-43.
3. Sackeim HA, Prudic J, Fuller R, et al. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology [serial online] August 23, 2006. Available at: www.nature.com/npp/journal/vaop/ncurrent/full/1301180a.html. Accessed September 12, 2006.
Correction
Reference 20 in “Making an IMPACT on late-life depression” (September 2006) should have been Unützer J, Tang L, Oishi S, et al. Reducing suicidal ideation in depressed older primary care patients. J Am Geriatr Soc [serial online]. Sept. 8, 2006. Available at: www.blackwellsynergy.com/toc/jgs/0/0. The article “ECT wipes out 30 years of memories” (Malpractice Verdicts, August 2006) misstated that the case was settled for $18,000. The jury awarded $625,200 to the patient and found the referring psychiatrist solely liable.
Reference 20 in “Making an IMPACT on late-life depression” (September 2006) should have been Unützer J, Tang L, Oishi S, et al. Reducing suicidal ideation in depressed older primary care patients. J Am Geriatr Soc [serial online]. Sept. 8, 2006. Available at: www.blackwellsynergy.com/toc/jgs/0/0. The article “ECT wipes out 30 years of memories” (Malpractice Verdicts, August 2006) misstated that the case was settled for $18,000. The jury awarded $625,200 to the patient and found the referring psychiatrist solely liable.
Reference 20 in “Making an IMPACT on late-life depression” (September 2006) should have been Unützer J, Tang L, Oishi S, et al. Reducing suicidal ideation in depressed older primary care patients. J Am Geriatr Soc [serial online]. Sept. 8, 2006. Available at: www.blackwellsynergy.com/toc/jgs/0/0. The article “ECT wipes out 30 years of memories” (Malpractice Verdicts, August 2006) misstated that the case was settled for $18,000. The jury awarded $625,200 to the patient and found the referring psychiatrist solely liable.
Medications with psychotherapy: A synergy to heal the brain
Pharmacotherapy works for psychiatric disorders, and so does psychotherapy. That’s why I provide both to each of my patients and why Current Psychiatry articles address combination therapy (for example, see “Treating psychiatric reactions to medical illness,”). Many studies have concluded that combining pharmacotherapy and psychotherapy produces better outcomes than either treatment alone.
The apparent synergy between verbal therapy and medications is often observed but rarely investigated. Does it occur because two different mechanisms of action work better than one, such as when we combine a dopamine blocker (antipsychotic) with a GABA agonist (mood stabilizer) in a patient with bipolar mania? Or do drug therapy and psychotherapy share a common pathway that is enhanced when they are administered together, such as when we combine two antidepressants to manage a patient with treatment-resistant depression?
What is psychotherapy’s mechanism of action anyway, and do various modalities—cognitive-behavioral, interpersonal, or supportive—have the same psychobiological mechanism of action?
Psychotherapy’s mechanism
Given the limited evidence and lack of clear answers in this area, it is reasonable to use recent advances in neuroscience to speculate on how two ostensibly different treatment modalities improve clinical manifestations—the mood, thought, and behavior alterations—of psychiatric brain disorders.
Emerging discoveries over the past few years suggest the common pathway of psychotherapy and pharmacotherapy may be neuroplasticity—synaptogenesis, dendritic spines, and neurogenesis. In other words, talking to a person or giving that person a psychotropic are both likely to modify that person’s brain structure.
Unlike any other organ, the brain changes continuously in response to external and internal stimuli, such as verbal, visual, tactile, and olfactory perceptions as well as chemical stimuli. Neural tissue also changes in response to experiences—stressful or pleasurable, real or imagined, emotional or cognitive. Psychotherapy represents a targeted, strategic, and tactical approach to stimulate specific feelings, recollections, and insights. These are encoded into the recipient’s neurobiological pathways and ultimately translate into a change in behavior or symptoms.
Every therapeutic encounter produces neuroplasticity, and—as with drug therapy—the cumulative effect of repeated doses of psychotherapy consolidates the improvement. One way to conceptualize this mechanism is that psychotherapy refurbishes the patient’s brain structure at the molecular level, restoring resilience to a brain/mind system that was compromised by genetic factors or environmental stress.
‘Re-Wiring’ the brain
Where does pharmacotherapy fit into this model? Here, too, evidence is emerging that psychotropics may exert their therapeutic effect not only through neurochemical pathways but also by stimulating neurotropic factors and inducing beneficial neuroplastic changes.
Antidepressants, mood stabilizers, and atypical antipsychotics (but not conventional neuroleptics) have been shown to induce synaptogenesis, neurite extension, and neurogenesis. These actions result in “re-wiring” and “re-sculpting” brain regions such as the hippocampus and subventricular zone.
Psychotropics are known to trigger gene expression within hours for some genes and after days or weeks for others (which may explain short-term alleviation of some symptoms but delayed response of others). Psychotropics’ neuroprotective and neuroplastic effects also appear to help “replenish” brain tissue destroyed by the neurotoxic effects of repetitive psychotic, manic, or depressive relapses. Increases in nerve growth factor, brain-derived neurotropic factor, and fibroblast growth factor have been implicated in brain tissue regeneration in serious psychiatric disorders. Animal and human studies support this model, but more research is needed.
Accelerating Neuroplasticity
Using this neuroplasticity model, we can reasonably postulate that the convergence of psychotherapy with pharmacotherapy may accelerate and expedite the clinical improvement that hinges on “therapeutic restructuring” of certain neural pathways. I find it intriguing to think that by repairing brain tissue with two different stimuli—talking and medicating—we can mend the fractured mind of an ailing brain.
As neuroscientists explore more deeply this area of research, we clinicians will continue to use concomitant pharmacotherapy and psychotherapy to help our patients navigate the road to recovery.
Pharmacotherapy works for psychiatric disorders, and so does psychotherapy. That’s why I provide both to each of my patients and why Current Psychiatry articles address combination therapy (for example, see “Treating psychiatric reactions to medical illness,”). Many studies have concluded that combining pharmacotherapy and psychotherapy produces better outcomes than either treatment alone.
The apparent synergy between verbal therapy and medications is often observed but rarely investigated. Does it occur because two different mechanisms of action work better than one, such as when we combine a dopamine blocker (antipsychotic) with a GABA agonist (mood stabilizer) in a patient with bipolar mania? Or do drug therapy and psychotherapy share a common pathway that is enhanced when they are administered together, such as when we combine two antidepressants to manage a patient with treatment-resistant depression?
What is psychotherapy’s mechanism of action anyway, and do various modalities—cognitive-behavioral, interpersonal, or supportive—have the same psychobiological mechanism of action?
Psychotherapy’s mechanism
Given the limited evidence and lack of clear answers in this area, it is reasonable to use recent advances in neuroscience to speculate on how two ostensibly different treatment modalities improve clinical manifestations—the mood, thought, and behavior alterations—of psychiatric brain disorders.
Emerging discoveries over the past few years suggest the common pathway of psychotherapy and pharmacotherapy may be neuroplasticity—synaptogenesis, dendritic spines, and neurogenesis. In other words, talking to a person or giving that person a psychotropic are both likely to modify that person’s brain structure.
Unlike any other organ, the brain changes continuously in response to external and internal stimuli, such as verbal, visual, tactile, and olfactory perceptions as well as chemical stimuli. Neural tissue also changes in response to experiences—stressful or pleasurable, real or imagined, emotional or cognitive. Psychotherapy represents a targeted, strategic, and tactical approach to stimulate specific feelings, recollections, and insights. These are encoded into the recipient’s neurobiological pathways and ultimately translate into a change in behavior or symptoms.
Every therapeutic encounter produces neuroplasticity, and—as with drug therapy—the cumulative effect of repeated doses of psychotherapy consolidates the improvement. One way to conceptualize this mechanism is that psychotherapy refurbishes the patient’s brain structure at the molecular level, restoring resilience to a brain/mind system that was compromised by genetic factors or environmental stress.
‘Re-Wiring’ the brain
Where does pharmacotherapy fit into this model? Here, too, evidence is emerging that psychotropics may exert their therapeutic effect not only through neurochemical pathways but also by stimulating neurotropic factors and inducing beneficial neuroplastic changes.
Antidepressants, mood stabilizers, and atypical antipsychotics (but not conventional neuroleptics) have been shown to induce synaptogenesis, neurite extension, and neurogenesis. These actions result in “re-wiring” and “re-sculpting” brain regions such as the hippocampus and subventricular zone.
Psychotropics are known to trigger gene expression within hours for some genes and after days or weeks for others (which may explain short-term alleviation of some symptoms but delayed response of others). Psychotropics’ neuroprotective and neuroplastic effects also appear to help “replenish” brain tissue destroyed by the neurotoxic effects of repetitive psychotic, manic, or depressive relapses. Increases in nerve growth factor, brain-derived neurotropic factor, and fibroblast growth factor have been implicated in brain tissue regeneration in serious psychiatric disorders. Animal and human studies support this model, but more research is needed.
Accelerating Neuroplasticity
Using this neuroplasticity model, we can reasonably postulate that the convergence of psychotherapy with pharmacotherapy may accelerate and expedite the clinical improvement that hinges on “therapeutic restructuring” of certain neural pathways. I find it intriguing to think that by repairing brain tissue with two different stimuli—talking and medicating—we can mend the fractured mind of an ailing brain.
As neuroscientists explore more deeply this area of research, we clinicians will continue to use concomitant pharmacotherapy and psychotherapy to help our patients navigate the road to recovery.
Pharmacotherapy works for psychiatric disorders, and so does psychotherapy. That’s why I provide both to each of my patients and why Current Psychiatry articles address combination therapy (for example, see “Treating psychiatric reactions to medical illness,”). Many studies have concluded that combining pharmacotherapy and psychotherapy produces better outcomes than either treatment alone.
The apparent synergy between verbal therapy and medications is often observed but rarely investigated. Does it occur because two different mechanisms of action work better than one, such as when we combine a dopamine blocker (antipsychotic) with a GABA agonist (mood stabilizer) in a patient with bipolar mania? Or do drug therapy and psychotherapy share a common pathway that is enhanced when they are administered together, such as when we combine two antidepressants to manage a patient with treatment-resistant depression?
What is psychotherapy’s mechanism of action anyway, and do various modalities—cognitive-behavioral, interpersonal, or supportive—have the same psychobiological mechanism of action?
Psychotherapy’s mechanism
Given the limited evidence and lack of clear answers in this area, it is reasonable to use recent advances in neuroscience to speculate on how two ostensibly different treatment modalities improve clinical manifestations—the mood, thought, and behavior alterations—of psychiatric brain disorders.
Emerging discoveries over the past few years suggest the common pathway of psychotherapy and pharmacotherapy may be neuroplasticity—synaptogenesis, dendritic spines, and neurogenesis. In other words, talking to a person or giving that person a psychotropic are both likely to modify that person’s brain structure.
Unlike any other organ, the brain changes continuously in response to external and internal stimuli, such as verbal, visual, tactile, and olfactory perceptions as well as chemical stimuli. Neural tissue also changes in response to experiences—stressful or pleasurable, real or imagined, emotional or cognitive. Psychotherapy represents a targeted, strategic, and tactical approach to stimulate specific feelings, recollections, and insights. These are encoded into the recipient’s neurobiological pathways and ultimately translate into a change in behavior or symptoms.
Every therapeutic encounter produces neuroplasticity, and—as with drug therapy—the cumulative effect of repeated doses of psychotherapy consolidates the improvement. One way to conceptualize this mechanism is that psychotherapy refurbishes the patient’s brain structure at the molecular level, restoring resilience to a brain/mind system that was compromised by genetic factors or environmental stress.
‘Re-Wiring’ the brain
Where does pharmacotherapy fit into this model? Here, too, evidence is emerging that psychotropics may exert their therapeutic effect not only through neurochemical pathways but also by stimulating neurotropic factors and inducing beneficial neuroplastic changes.
Antidepressants, mood stabilizers, and atypical antipsychotics (but not conventional neuroleptics) have been shown to induce synaptogenesis, neurite extension, and neurogenesis. These actions result in “re-wiring” and “re-sculpting” brain regions such as the hippocampus and subventricular zone.
Psychotropics are known to trigger gene expression within hours for some genes and after days or weeks for others (which may explain short-term alleviation of some symptoms but delayed response of others). Psychotropics’ neuroprotective and neuroplastic effects also appear to help “replenish” brain tissue destroyed by the neurotoxic effects of repetitive psychotic, manic, or depressive relapses. Increases in nerve growth factor, brain-derived neurotropic factor, and fibroblast growth factor have been implicated in brain tissue regeneration in serious psychiatric disorders. Animal and human studies support this model, but more research is needed.
Accelerating Neuroplasticity
Using this neuroplasticity model, we can reasonably postulate that the convergence of psychotherapy with pharmacotherapy may accelerate and expedite the clinical improvement that hinges on “therapeutic restructuring” of certain neural pathways. I find it intriguing to think that by repairing brain tissue with two different stimuli—talking and medicating—we can mend the fractured mind of an ailing brain.
As neuroscientists explore more deeply this area of research, we clinicians will continue to use concomitant pharmacotherapy and psychotherapy to help our patients navigate the road to recovery.
Anxiously looking for love
History: a lovelorn life
Ms. F, age 33, presents with one complaint: “I want to know how to maintain a relationship.” Problem is, social situations have made her feel anxious since childhood. She has trouble keeping a boyfriend; she left two intimate, extended relationships at different times.
She says she is too ashamed to invite people over because she cannot keep her apartment neat. She is also sick of her job as a filing clerk and wants a new career.
Ms. F reports no other anxiety symptoms or mood changes but often cannot concentrate. She denies impulsivity or poor judgment but admits that she makes decisions without getting important facts. For example, she enrolled at a community college without knowing what skills her new career would require. About 6 months ago, she left her boyfriend after realizing—18 months into the relationship—that he does not share her interests.
poll here
The authors’ observations
Information on all the above factors is crucial to diagnosing a socialization problem. Outline your differential diagnosis as the interview progresses.
Ask the patient:
How did you fare in school? A childhood history of pervasive inattention or impulsivity in at least two settings (at home and in school, for example) can signal attention-deficit/hyperactivity disorder (ADHD).fragile X syndrome). Boys with the fragile X premutation have a higher rate of ADHD symptoms and autism spectrum disorders than do boys without this premutation.3 Ms. F’s test showed two normal alleles, thus ruling out fragile X premutation.
Table 1
Mental status examination signs that suggest a PDD
| Little direct or sustained eye contact Eyes flit around the room Patient talks without looking at anyone |
| Few facial expressions Flat affect |
| Impaired speech production Although prosody (intonation) is normal, rate is rapid, with cluttered bursts followed by long pauses and occasional unusual emphasis on certain words |
| Tangential thought process Patient changes topics quickly without transition Non-sequitur responses |
| Brief responses to questions, offering little spontaneous information |
| Very detailed answers that include irrelevant information |
| Pedantic phrasing |
| Repetitive use of language |
| Does not pick up on nonquestions |
| Concrete answers to questions about emotion Patient cannot describe how emotions “feel” |
| Appears uncomfortable during conversation with examiner Rapport strained; patient does not seem to enjoy interaction |
| PDD: pervasive developmental disorder |
Treatment: medication and exploration
Ms. F agrees to an ADOS test. Her total score of 9 (7 in social, 2 in communication, and 0 in stereotyped/repetitive behavior) suggest a moderate PDD. We rule out autism based on the test score and Asperger’s syndrome because of her early language development delays (Table 2).
We start escitalopram, 10 mg/d, to address Ms. F’s anxiety. We see her weekly for medication management and start weekly psychotherapy to explore her two previous relationships and her desire to find a partner.
Ms. F, however, reacts anxiously to the therapist’s exploratory techniques. She has difficulty taking the lead and becomes extremely uncomfortable with silences in the conversation. The therapist tries cognitive-behavioral tactics to engage her, but Ms. F does not respond.
The therapist then conceptualizes her role as “coach” and tries a more-direct, problem-solving approach. She addresses specific challenges, such as an overwhelming class assignment, but Ms. F does not discuss or follow through on the problem.
After 6 months, Ms. F asks to stop psychotherapy because she has made little progress. She also asks to reduce medication checks to monthly, saying that weekly sessions interfere with her schoolwork. She says she would consider resuming psychotherapy.
At this point, Ms. F’s anxiety is significantly improved based on clinical impression. She continues to do well 6 months after stopping psychotherapy, though she is still without a boyfriend.
poll hereTable 2
Autism or Asperger’s? Watch for these distinguishing features
| Clinical feature | Autism | Asperger’s syndrome |
|---|---|---|
| Impaired nonverbal behavior | + | + |
| Language delay | + | – |
| Stereotyped behavior (routines, mannerisms) | + | + |
| Impaired social relationships | + | + |
| Cognitive delay | ± | – |
| +: Present –: absent ±: Might be present | ||
The authors’ observations
The ability to possess a theory of mind—or “mentalize”—helps us understand others’ beliefs, desires, thoughts, intentions, and knowledge. Attributing mental states to self and others helps explain and predict behavior, which is critical to social interaction.
A therapeutic relationship can help teach patients to handle social situations.4 In autism or PDD,5,6 however, theory of mind deficits typically frustrate relationship building.4 Because ability to mentalize is critical to psychodynamic psychotherapy,7 exploration does not help patients with PDD. By contrast, therapists can be more successful by being active in sessions and giving directions, suggestions, and information.
Which psychotherapy models work? Limited data address psychotherapy for adults with PDD; most studies have followed children.
CBT for persons with autism or PDD is directive, problem-focused, and targets automatic reactions.8 Social skills groups and CBT focusing on day-to-day problem solving can help older children and adolescents.9 A 20-week social skills intervention employing a CBT approach, paired with psychoeducation for parents, has helped boys ages 8 to 12 with autism, PDD, or Asperger’s syndrome.10
Other interventions use pictures, cartoons, and other visuals to help patients identify and correct misperceptions and determine how different responses might affect people’s thoughts and feelings.9,11 Role play allows the patient to practice social interaction but requires make-believe,11 so getting a PDD patient to participate can be challenging.
Medication can help manage comorbid anxiety, obsessive-compulsive, and mood symptoms in PDD. Limited data support using selective serotonin reuptake inhibitors for this purpose.12
Related resources
- Ozonoff S, Dawson G, McPartland J. A parent’s guide to Asperger syndrome & high-functioning autism: how to meet the challenges and help your child thrive. New York: Guilford Press; 2002.
- MAAP Services. A global information and support network for more advanced persons with autism and Asperger syndrome. www.asperger.org.
- Escitalopram • Lexapro
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000;30:205-23.
3. Farzin F, Perry H, Hessl D, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr 2006;27(S2):S137-S144.
4. Ramsay JR, Brodkin ES, Cohen MR, et al. “Better strangers:” using the relationship in psychotherapy for adult patients with Asperger syndrome. Psychotherapy: Theory, Research, Practice, Training 2005;42:483-93.
5. Hill E, Frith U. Understanding autism: insights from mind and brain. Philos Trans R Soc Lond B Biol Sci 2003;358:281-9.
6. Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 2002;125:1839-49.
7. Gabbard GO. Psychodynamic psychiatry in clinical practice, 4th ed. Arlington, VA: American Psychiatric Publishing; 2005:60.
8. Beebe DW, Risi S. Treatment of adolescents and young adults with high-functioning autism or Asperger syndrome. In: Reinecke MA, Dattilio FM, Freeman A, eds. Cognitive therapy with children and adolescents. A casebook for clinical practice, 2nd ed. New York: Guilford Press; 2003.
9. Atwood T. Frameworks for behavioral interventions. Child Adolesc Psychiatr Clin N Am 2003;12:65-86.
10. Solomon M, Goodlin-Jones BL, Anders T. A social adjustment enhancement intervention for high functioning autism, Asperger’s syndrome, and pervasive developmental disorder NOS. J Autism Dev Disord 2004;34:649-68.
11. Rajendran G, Mitchell P, Rickards H. How do individuals with Asperger syndrome respond to nonliteral language and inappropriate requests in computer-mediated communication? J Autism Dev Disord 2005;35:429-43.
12. Namerow LB, Thomas P, Bostic JQ, et al. Use of citalopram in pervasive developmental disorders. J Dev Behav Pediatr 2003;24:104-8.
History: a lovelorn life
Ms. F, age 33, presents with one complaint: “I want to know how to maintain a relationship.” Problem is, social situations have made her feel anxious since childhood. She has trouble keeping a boyfriend; she left two intimate, extended relationships at different times.
She says she is too ashamed to invite people over because she cannot keep her apartment neat. She is also sick of her job as a filing clerk and wants a new career.
Ms. F reports no other anxiety symptoms or mood changes but often cannot concentrate. She denies impulsivity or poor judgment but admits that she makes decisions without getting important facts. For example, she enrolled at a community college without knowing what skills her new career would require. About 6 months ago, she left her boyfriend after realizing—18 months into the relationship—that he does not share her interests.
poll here
The authors’ observations
Information on all the above factors is crucial to diagnosing a socialization problem. Outline your differential diagnosis as the interview progresses.
Ask the patient:
How did you fare in school? A childhood history of pervasive inattention or impulsivity in at least two settings (at home and in school, for example) can signal attention-deficit/hyperactivity disorder (ADHD).fragile X syndrome). Boys with the fragile X premutation have a higher rate of ADHD symptoms and autism spectrum disorders than do boys without this premutation.3 Ms. F’s test showed two normal alleles, thus ruling out fragile X premutation.
Table 1
Mental status examination signs that suggest a PDD
| Little direct or sustained eye contact Eyes flit around the room Patient talks without looking at anyone |
| Few facial expressions Flat affect |
| Impaired speech production Although prosody (intonation) is normal, rate is rapid, with cluttered bursts followed by long pauses and occasional unusual emphasis on certain words |
| Tangential thought process Patient changes topics quickly without transition Non-sequitur responses |
| Brief responses to questions, offering little spontaneous information |
| Very detailed answers that include irrelevant information |
| Pedantic phrasing |
| Repetitive use of language |
| Does not pick up on nonquestions |
| Concrete answers to questions about emotion Patient cannot describe how emotions “feel” |
| Appears uncomfortable during conversation with examiner Rapport strained; patient does not seem to enjoy interaction |
| PDD: pervasive developmental disorder |
Treatment: medication and exploration
Ms. F agrees to an ADOS test. Her total score of 9 (7 in social, 2 in communication, and 0 in stereotyped/repetitive behavior) suggest a moderate PDD. We rule out autism based on the test score and Asperger’s syndrome because of her early language development delays (Table 2).
We start escitalopram, 10 mg/d, to address Ms. F’s anxiety. We see her weekly for medication management and start weekly psychotherapy to explore her two previous relationships and her desire to find a partner.
Ms. F, however, reacts anxiously to the therapist’s exploratory techniques. She has difficulty taking the lead and becomes extremely uncomfortable with silences in the conversation. The therapist tries cognitive-behavioral tactics to engage her, but Ms. F does not respond.
The therapist then conceptualizes her role as “coach” and tries a more-direct, problem-solving approach. She addresses specific challenges, such as an overwhelming class assignment, but Ms. F does not discuss or follow through on the problem.
After 6 months, Ms. F asks to stop psychotherapy because she has made little progress. She also asks to reduce medication checks to monthly, saying that weekly sessions interfere with her schoolwork. She says she would consider resuming psychotherapy.
At this point, Ms. F’s anxiety is significantly improved based on clinical impression. She continues to do well 6 months after stopping psychotherapy, though she is still without a boyfriend.
poll hereTable 2
Autism or Asperger’s? Watch for these distinguishing features
| Clinical feature | Autism | Asperger’s syndrome |
|---|---|---|
| Impaired nonverbal behavior | + | + |
| Language delay | + | – |
| Stereotyped behavior (routines, mannerisms) | + | + |
| Impaired social relationships | + | + |
| Cognitive delay | ± | – |
| +: Present –: absent ±: Might be present | ||
The authors’ observations
The ability to possess a theory of mind—or “mentalize”—helps us understand others’ beliefs, desires, thoughts, intentions, and knowledge. Attributing mental states to self and others helps explain and predict behavior, which is critical to social interaction.
A therapeutic relationship can help teach patients to handle social situations.4 In autism or PDD,5,6 however, theory of mind deficits typically frustrate relationship building.4 Because ability to mentalize is critical to psychodynamic psychotherapy,7 exploration does not help patients with PDD. By contrast, therapists can be more successful by being active in sessions and giving directions, suggestions, and information.
Which psychotherapy models work? Limited data address psychotherapy for adults with PDD; most studies have followed children.
CBT for persons with autism or PDD is directive, problem-focused, and targets automatic reactions.8 Social skills groups and CBT focusing on day-to-day problem solving can help older children and adolescents.9 A 20-week social skills intervention employing a CBT approach, paired with psychoeducation for parents, has helped boys ages 8 to 12 with autism, PDD, or Asperger’s syndrome.10
Other interventions use pictures, cartoons, and other visuals to help patients identify and correct misperceptions and determine how different responses might affect people’s thoughts and feelings.9,11 Role play allows the patient to practice social interaction but requires make-believe,11 so getting a PDD patient to participate can be challenging.
Medication can help manage comorbid anxiety, obsessive-compulsive, and mood symptoms in PDD. Limited data support using selective serotonin reuptake inhibitors for this purpose.12
Related resources
- Ozonoff S, Dawson G, McPartland J. A parent’s guide to Asperger syndrome & high-functioning autism: how to meet the challenges and help your child thrive. New York: Guilford Press; 2002.
- MAAP Services. A global information and support network for more advanced persons with autism and Asperger syndrome. www.asperger.org.
- Escitalopram • Lexapro
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
History: a lovelorn life
Ms. F, age 33, presents with one complaint: “I want to know how to maintain a relationship.” Problem is, social situations have made her feel anxious since childhood. She has trouble keeping a boyfriend; she left two intimate, extended relationships at different times.
She says she is too ashamed to invite people over because she cannot keep her apartment neat. She is also sick of her job as a filing clerk and wants a new career.
Ms. F reports no other anxiety symptoms or mood changes but often cannot concentrate. She denies impulsivity or poor judgment but admits that she makes decisions without getting important facts. For example, she enrolled at a community college without knowing what skills her new career would require. About 6 months ago, she left her boyfriend after realizing—18 months into the relationship—that he does not share her interests.
poll here
The authors’ observations
Information on all the above factors is crucial to diagnosing a socialization problem. Outline your differential diagnosis as the interview progresses.
Ask the patient:
How did you fare in school? A childhood history of pervasive inattention or impulsivity in at least two settings (at home and in school, for example) can signal attention-deficit/hyperactivity disorder (ADHD).fragile X syndrome). Boys with the fragile X premutation have a higher rate of ADHD symptoms and autism spectrum disorders than do boys without this premutation.3 Ms. F’s test showed two normal alleles, thus ruling out fragile X premutation.
Table 1
Mental status examination signs that suggest a PDD
| Little direct or sustained eye contact Eyes flit around the room Patient talks without looking at anyone |
| Few facial expressions Flat affect |
| Impaired speech production Although prosody (intonation) is normal, rate is rapid, with cluttered bursts followed by long pauses and occasional unusual emphasis on certain words |
| Tangential thought process Patient changes topics quickly without transition Non-sequitur responses |
| Brief responses to questions, offering little spontaneous information |
| Very detailed answers that include irrelevant information |
| Pedantic phrasing |
| Repetitive use of language |
| Does not pick up on nonquestions |
| Concrete answers to questions about emotion Patient cannot describe how emotions “feel” |
| Appears uncomfortable during conversation with examiner Rapport strained; patient does not seem to enjoy interaction |
| PDD: pervasive developmental disorder |
Treatment: medication and exploration
Ms. F agrees to an ADOS test. Her total score of 9 (7 in social, 2 in communication, and 0 in stereotyped/repetitive behavior) suggest a moderate PDD. We rule out autism based on the test score and Asperger’s syndrome because of her early language development delays (Table 2).
We start escitalopram, 10 mg/d, to address Ms. F’s anxiety. We see her weekly for medication management and start weekly psychotherapy to explore her two previous relationships and her desire to find a partner.
Ms. F, however, reacts anxiously to the therapist’s exploratory techniques. She has difficulty taking the lead and becomes extremely uncomfortable with silences in the conversation. The therapist tries cognitive-behavioral tactics to engage her, but Ms. F does not respond.
The therapist then conceptualizes her role as “coach” and tries a more-direct, problem-solving approach. She addresses specific challenges, such as an overwhelming class assignment, but Ms. F does not discuss or follow through on the problem.
After 6 months, Ms. F asks to stop psychotherapy because she has made little progress. She also asks to reduce medication checks to monthly, saying that weekly sessions interfere with her schoolwork. She says she would consider resuming psychotherapy.
At this point, Ms. F’s anxiety is significantly improved based on clinical impression. She continues to do well 6 months after stopping psychotherapy, though she is still without a boyfriend.
poll hereTable 2
Autism or Asperger’s? Watch for these distinguishing features
| Clinical feature | Autism | Asperger’s syndrome |
|---|---|---|
| Impaired nonverbal behavior | + | + |
| Language delay | + | – |
| Stereotyped behavior (routines, mannerisms) | + | + |
| Impaired social relationships | + | + |
| Cognitive delay | ± | – |
| +: Present –: absent ±: Might be present | ||
The authors’ observations
The ability to possess a theory of mind—or “mentalize”—helps us understand others’ beliefs, desires, thoughts, intentions, and knowledge. Attributing mental states to self and others helps explain and predict behavior, which is critical to social interaction.
A therapeutic relationship can help teach patients to handle social situations.4 In autism or PDD,5,6 however, theory of mind deficits typically frustrate relationship building.4 Because ability to mentalize is critical to psychodynamic psychotherapy,7 exploration does not help patients with PDD. By contrast, therapists can be more successful by being active in sessions and giving directions, suggestions, and information.
Which psychotherapy models work? Limited data address psychotherapy for adults with PDD; most studies have followed children.
CBT for persons with autism or PDD is directive, problem-focused, and targets automatic reactions.8 Social skills groups and CBT focusing on day-to-day problem solving can help older children and adolescents.9 A 20-week social skills intervention employing a CBT approach, paired with psychoeducation for parents, has helped boys ages 8 to 12 with autism, PDD, or Asperger’s syndrome.10
Other interventions use pictures, cartoons, and other visuals to help patients identify and correct misperceptions and determine how different responses might affect people’s thoughts and feelings.9,11 Role play allows the patient to practice social interaction but requires make-believe,11 so getting a PDD patient to participate can be challenging.
Medication can help manage comorbid anxiety, obsessive-compulsive, and mood symptoms in PDD. Limited data support using selective serotonin reuptake inhibitors for this purpose.12
Related resources
- Ozonoff S, Dawson G, McPartland J. A parent’s guide to Asperger syndrome & high-functioning autism: how to meet the challenges and help your child thrive. New York: Guilford Press; 2002.
- MAAP Services. A global information and support network for more advanced persons with autism and Asperger syndrome. www.asperger.org.
- Escitalopram • Lexapro
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000;30:205-23.
3. Farzin F, Perry H, Hessl D, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr 2006;27(S2):S137-S144.
4. Ramsay JR, Brodkin ES, Cohen MR, et al. “Better strangers:” using the relationship in psychotherapy for adult patients with Asperger syndrome. Psychotherapy: Theory, Research, Practice, Training 2005;42:483-93.
5. Hill E, Frith U. Understanding autism: insights from mind and brain. Philos Trans R Soc Lond B Biol Sci 2003;358:281-9.
6. Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 2002;125:1839-49.
7. Gabbard GO. Psychodynamic psychiatry in clinical practice, 4th ed. Arlington, VA: American Psychiatric Publishing; 2005:60.
8. Beebe DW, Risi S. Treatment of adolescents and young adults with high-functioning autism or Asperger syndrome. In: Reinecke MA, Dattilio FM, Freeman A, eds. Cognitive therapy with children and adolescents. A casebook for clinical practice, 2nd ed. New York: Guilford Press; 2003.
9. Atwood T. Frameworks for behavioral interventions. Child Adolesc Psychiatr Clin N Am 2003;12:65-86.
10. Solomon M, Goodlin-Jones BL, Anders T. A social adjustment enhancement intervention for high functioning autism, Asperger’s syndrome, and pervasive developmental disorder NOS. J Autism Dev Disord 2004;34:649-68.
11. Rajendran G, Mitchell P, Rickards H. How do individuals with Asperger syndrome respond to nonliteral language and inappropriate requests in computer-mediated communication? J Autism Dev Disord 2005;35:429-43.
12. Namerow LB, Thomas P, Bostic JQ, et al. Use of citalopram in pervasive developmental disorders. J Dev Behav Pediatr 2003;24:104-8.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000;30:205-23.
3. Farzin F, Perry H, Hessl D, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr 2006;27(S2):S137-S144.
4. Ramsay JR, Brodkin ES, Cohen MR, et al. “Better strangers:” using the relationship in psychotherapy for adult patients with Asperger syndrome. Psychotherapy: Theory, Research, Practice, Training 2005;42:483-93.
5. Hill E, Frith U. Understanding autism: insights from mind and brain. Philos Trans R Soc Lond B Biol Sci 2003;358:281-9.
6. Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 2002;125:1839-49.
7. Gabbard GO. Psychodynamic psychiatry in clinical practice, 4th ed. Arlington, VA: American Psychiatric Publishing; 2005:60.
8. Beebe DW, Risi S. Treatment of adolescents and young adults with high-functioning autism or Asperger syndrome. In: Reinecke MA, Dattilio FM, Freeman A, eds. Cognitive therapy with children and adolescents. A casebook for clinical practice, 2nd ed. New York: Guilford Press; 2003.
9. Atwood T. Frameworks for behavioral interventions. Child Adolesc Psychiatr Clin N Am 2003;12:65-86.
10. Solomon M, Goodlin-Jones BL, Anders T. A social adjustment enhancement intervention for high functioning autism, Asperger’s syndrome, and pervasive developmental disorder NOS. J Autism Dev Disord 2004;34:649-68.
11. Rajendran G, Mitchell P, Rickards H. How do individuals with Asperger syndrome respond to nonliteral language and inappropriate requests in computer-mediated communication? J Autism Dev Disord 2005;35:429-43.
12. Namerow LB, Thomas P, Bostic JQ, et al. Use of citalopram in pervasive developmental disorders. J Dev Behav Pediatr 2003;24:104-8.
Managing maladaptive behaviors in fragile X patients
Psychotropics1,2 are used to manage maladaptive and interfering behaviors in 70% of patients with fragile X syndrome (FXS), the leading cause of hereditary mental retardation. Treatment tends to follow a developmental course:
- In children, stimulants and alpha-2 agonists are used for attention-deficit/hyperactivity disorder (ADHD)-like symptoms.
- In adolescents and adults, selective serotonin reuptake inhibitors (SSRIs) are used for anxiety/repetitive phenomena and second-generation antipsychotics (SGAs) for irritability.
This course—which is often effective—is based primarily on anecdotal descriptions and on rationales borrowed from studies of ADHD, obsessive-compulsive disorder (OCD), and autistic disorder/related pervasive developmental disorders (PDDs).3 Disease-modifying agents to target the underlying brain dysregulation inherent in FXS (Box)1,4-10 are being investigated. For now, psychotropics can help you manage three common FXS symptom clusters: inattention and hyperactivity, anxiety, and aggression and self-injurious behavior (SIB).
The term “fragile X” describes how the X chromosome of affected individuals fractures in a folate-deprived medium. This most common form of inherited mental retardation affects 1 in 2,000 to 4,000 males and 1 in 4,000 to 8,000 females.4 One in four individuals with fragile X syndrome (FXS) also meets diagnostic criteria for autistic disorder (Table 1), with social skill and communication delays and interfering repetitive behaviors.5
Genetic profile. FXS results from a triplet repeat expansion in the fragile X mental retardation-1 gene.6 This mutation causes underproduction of fragile X mental retardation protein (FMRP), an inhibitor of the metabotropic glutamate receptor (mGluR). In theory, insufficient FMRP allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype: mental retardation, increased seizure risk, behavioral symptoms, and stereotypic movements.7,8
Behavioral difficulties cluster in three categories: attention-deficit/hyperactivity disorder-like symptoms, anxiety symptoms, and aggression and self-injurious behaviors.1,4,9 These are thought to be more prevalent in persons with FXS than would be expected from the degree of cognitive delay alone.1 Potential differences in the behavioral phenotypes of FXS patients with and without comorbid autism continue to be defined.10
Table 1
Clinical characteristics of patients with fragile X syndrome
| Physical features (seen in some males) | Long, narrow face |
| High, arched palate | |
| Narrow inter-eye distance | |
| Enlarged ears | |
| Macro-orchidism | |
| Behavioral symptoms | Inattention |
| Hyperactivity | |
| Anxiety | |
| Repetitive behaviors | |
| Aggression and self-injurious behaviors (increased in adolescence and adulthood) | |
| Comorbidities | Mental retardation (mean IQ for affected males in moderate range) |
| Comorbid autism (25% of affected individuals) | |
| Frequent seizures (10% to 20% of affected males) | |
| Hypersensitivity to sensory Stimuli |
Inattention and hyperactivity
Mike, age 6, has fragile X syndrome. He has been attending first grade for 4 months, and his teacher reports he does not sit still, runs throughout the classroom, and cannot focus on class work. Mike’s hyperactivity has been evident for 2 years but did not cause problems until first grade, his parents report.
Psychostimulants are the most frequently prescribed agents for inattention and hyperactivity in FXS, particularly in boys and male adolescents.1 Among FXS patients prescribed ≥ 1 psychotropic, approximately 70% are taking a stimulant.1,2
Efficacy. A clinical chart review found a 75% response rate in FXS children and adolescents who were given a stimulant for inattention and/or hyperactivity.1 This is higher than the 25% to 49% stimulant response rate reported in patients with PDDs.11,12
A 3-week, placebo-controlled, crossover trial of methylphenidate and dextroamphetamine noted a statistically significant response only to methylphenidate, with a positive response reported in 10 of 15 children (67%).13
Side effects. To date, limited information has described the rate of intolerable side effects associated with stimulant use in FXS,14 but in patients with PDD:
- 154 of 268 (57.5%) patient trials in a retrospective naturalistic study showed significant adverse effects with stimulant use.11
- 13 of 72 (18%) subjects in a controlled trial discontinued methylphenidate because of adverse events (most commonly irritability).12
Antiadrenergics. The alpha-2 agonists clonidine and guanfacine are the second most-used class of agents for inattention and hyperactivity in FXS. As with stimulants, boys and male adolescents are most likely to receive alpha-2 agonists, with administration rates of 10% to 20%.1,2
Efficacy. In one survey, nearly two-thirds (63%) of parents described clonidine as “very beneficial” to 35 children (mean age 6.6) with FXS.15 This is similar to a 70% response rate described for these alpha-2 agonists in a chart review.1 These rates are much higher than the 24% response rate reported with guanfacine in a retrospective chart review of 80 children and adolescents with a PDD.16 In that review, guanfacine use was associated with reduced hyperactivity, insomnia, and tics, and increased attention.15
Side effects associated with alpha-2 agonists include lowered blood pressure and sedation.
L-acetylcarnitine—a carnitine derivative required for neuronal use and transport of fatty acids—is being investigated to treat hyperactivity in FXS. Hyperactive symptoms improved significantly with L-acetylcarnitine, as measured by the Conners’ Abbreviated Parent-Teacher Questionnaire, in a 1-year, placebo-controlled trial of 20 boys (mean age 9.2) with FXS.17
Discussion. Supporting evidence is limited, but clinicians are treating ADHD-like symptoms with stimulants and alpha-2 agonists in many FXS patients. Preliminary data indicate that stimulants may be more effective and better tolerated in individuals with FXS than in those with PDD.
Trying a stimulant or alpha-2 agonist for inattention or hyperactivity symptoms in a child or adolescent with FXS appears clinically appropriate, given the available evidence. Additional data based on placebo-controlled and standardized measures of treatment response are needed to help guide treatment.
We start Mike on methylphenidate, 5 mg in the morning, for inattention and hyperactivity. He tolerates this well, and after 2 weeks we increase the dosage to 5 mg bid. Several weeks into treatment, his teacher comments that he is beginning to stay in his seat and attends to some assigned tasks in the classroom.
Mike continued to tolerate methylphenidate over the next 4 years. We gradually increased the dosage as he grew and when he periodically developed breakthrough interfering symptoms in the classroom.
Anxiety symptoms
In grade school, Mike became increasingly nervous around schoolmates, teachers, and friends. His teachers commented that he repeated phrases when he appeared anxious. Other children in his special education class began to shun him; they found his perseveration odd and sometimes threatening.
Now that Mike is age 10 and in fifth grade, his parents decide that his anxiety, particularly in social settings, is interfering with his life.
Anxiety symptoms—including generalized nervousness and OCD-like obsessions and perseverations—are common psychotropic targets in FXS. Boys may be the FXS patients most often prescribed drugs for inattention and hyperactivity, but they are the least likely to receive antidepressants for anxiety symptoms.1,2
Efficacy. More than 50% of female patients and men with FXS are prescribed SSRIs for anxiety (Table 2), and the reported response rate of 50% to 60%1 is similar to that seen with SSRIs in autism and related disorders.18 In autism, a developmental approach is warranted, as SSRIs tend to be less effective and cause more side effects in children and adolescents than in adults.18
Adverse effects reported with SSRIs in FXS include behavioral activation, appetite changes, insomnia, and nausea.1 In a study of fluoxetine for FXS symptoms, 10 of 35 patients (29%) had persistent side effects, most commonly weight loss and weight gain.19 One patient with pre-existing suicidal ideation worsened.
Watch for emergence or worsening of suicidal thoughts in all children and adolescents receiving antidepressants, whatever their target symptoms.
Mike is taking methylphenidate, 15 mg bid, for comorbid ADHD, and we add fluoxetine, 10 mg/d, for anxiety. This regimen is well-tolerated, so we increase fluoxetine to 20 mg/d at his 4-week follow-up appointment. After about 8 weeks, Mike’s parents report that his anxiety-associated symptoms are less severe.
Mike still appears nervous sometimes, but he uses markedly fewer perseverative phrases. This allows him to interact more meaningfully with peers and contributes to his social development.
Table 2
Target symptoms and treatment options for fragile X syndrome
| Medication class | Target symptom cluster | Evidence for use of drug class in FXS |
|---|---|---|
| Stimulants | Inattention, hyperactivity | One placebo-controlled trial, two large clinic surveys |
| Alpha-2 agonists | Inattention, hyperactivity | One parent-interview report, two large clinic surveys |
| SSRIs | Anxiety-related symptoms | One mailed survey, two large clinic surveys |
| Atypical antipsychotics | Aggression, self-injury | Two large clinic surveys, several controlled trials in PDDs |
| FXS: fragile X syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
| PDDs: pervasive developmental disorders. | ||
Aggression and self-injury
Mike, now age 20 and participating daily in a vocational workshop, begins yelling profanities at coworkers. At his group home, he has been hitting staff at least twice a week when redirected.
He is no longer taking stimulants, having been weaned from methylphenidate several years ago, but he continues to take fluoxetine, 40 mg/d.
Fluoxetine19 and clonidine15 can decrease irritability in FXS, but atypical antipsychotics are most commonly used for aggression and SIB.1,2 SGAs are prescribed to 10% to 20% of FXS patients who are taking medication1,2—particularly to men—and have produced response rates of 60% to 100% when used for aggression and SIB.1
Risperidone. No published reports have addressed using specific SGAs in FXS. In the PDD literature, most controlled data concerns risperidone.20
The largest randomized, placebo-controlled trial enrolled 101 children ages 5 to 17 with autistic disorder accompanied by severe tantrums, aggression, or self-injurious behavior. Among the 49 children taking risperidone, 0.5 to 3.5 mg/d for 8 weeks, 34 (69%) were judged as treatment responders with significantly reduced irritable behavior, compared with 6 of 52 (12%) taking placebo.21 Risperidone therapy was associated with average weight gain of 2.7±2.9 kg, compared with 0.8±2.2 kg with placebo.
Besides weight gain, other significant side effects associated with risperidone include sedation and elevated serum prolactin. These effects often are more pronounced in children and adolescents than in adults with PDDs.20
Other antipsychotics. Future use of SGAs in FXS will likely mirror the pattern seen in PDDs, where clinicians are moving towards weight-neutral antipsychotics such as ziprasidone and aripiprazole. In a preliminary report, aripiprazole reduced irritability in 5 youths with PDD.22 Our group is conducting a double-blind, placebo-controlled trial of aripiprazole in autism, targeting aggression, SIB, and irritability.
Discussion. SGAs are used most often in FXS to treat aggression and SIB, based on data from studies on treating similar symptoms in PDDs. Closely monitor patients for sedation, weight gain, and lipid, glucose, and prolactin elevations when using SGAs (Table 3). Be especially vigilant when children gain weight rapidly or show hyperprolactinemia signs while taking these drugs.
After being suspended from the vocational workshop, Mike is treated at a local mental health center for aggressive behaviors. He tolerates an initial dosage of aripiprazole,2.5 mg/d, which is titrated in 2.5-mg increments biweekly to 10 mg/d. At this dosage, he stops hitting staff members and his yelling of profanities is greatly reduced. Over several months, Mike returns to his vocational workshop and maintains residence at his group home.
Table 3
Medication side effects and recommended monitoring
| Medication class | Side effects | Medication monitoring |
|---|---|---|
| Stimulants | Anorexia, insomnia, agitation, exacerbation of tics | Observe closely when starting treatment and increasing dosage |
| Alpha-2 agonists | Lowered blood pressure, sedation, dizziness | Observe closely when starting treatment and increasing dosage |
| Check blood pressure with all dosage changes and at all clinic visits | ||
| SSRIs | Irritability, mood lability, nausea, sleep and appetite disturbances, suicidality | Observe closely when starting treatment and increasing dosage |
| Atypical antipsychotics | Sedation, weight gain, hyperglycemia, hyperlipidemia, hyperprolactinemia, EPS, NMS, tardive dyskinesia | Obtain metabolic profile, including fasting lipids, glucose, and prolactin levels |
| Monitor for weight gain and signs of EPS | ||
| EPS: extrapyramidal symptoms | ||
| NMS: neuroleptic malignant syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
Genetic-related treatments
Studies are needed to investigate the use of stimulants, SSRIs, and antipsychotics in patients with FXS unaccompanied by generalized anxiety disorder, OCD, ADHD, or PDDs. How FXS patients without those comorbidities will respond to drug treatment is unknown. Also, little also is known about possible side effects associated with combining drug treatments in individuals with FXS.
Future drug treatment in FXS will likely focus on agents that target the underlying neurochemical dysregulation associated with the FXS genotype. This approach might reduce interfering behaviors and alter the course of cognitive dysfunction—including mental retardation—associated with FXS.
Past attempts to correct FXS’ neurochemical abnormalities focused on using folic acid. The term “fragile X” describes how the X chromosome of individuals with FXS fractures in a folate-deprived medium. Many controlled trials of folic acid in FXS did not support earlier positive reports, however.4
Greater understanding of fragile X mental retardation protein (FMRP) function has led to the metabotropic glutamate receptor (mGluR) theory.7 It holds that FMRP underproduction allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype. Researchers now are attempting to reverse the neurochemical impact of insufficient FMRP with two medication classes:
- selective group 1 mGluR receptor antagonists (mGluR5 antagonists, in particular). The mGluR5 receptor antagonist MPEP has shown the ability to rescue normal behaviors in animal models of FXS. MPEP and lithium have reversed behaviors associated with FXS and—at the microscopic level—rescued synaptic plasticity.23,24 In the drosophila fly model of FXS, lithium reduced activity in the mGluR cascade, thus compensating for lack of FMRP.23
- positive AMPA receptor modulators (ampakines) that promote activity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.9 Excessive mGluR activity appears to impair AMPA receptors’ ability to promote cortical development, memory, and learning.7 Reduced AMPA receptors have been shown in the FXS mouse model,25 and an ampakine is being investigated in a study of men with FXS and autism.1
- FRAXA: The Fragile X Research Foundation. Founded by parents of children with fragile X syndrome to increase funding for research toward effective treatments. www.fraxa.org.
- The National Fragile X Foundation. Provides educational and emotional support for fragile X families and promotes public and professional awareness. www.fragilex.org.
- Hagerman RJ, Hagerman PJ, eds. Fragile X syndrome: diagnosis, treatment, and research, 3rd ed. Baltimore, MD: The Johns Hopkins University Press; 2002.
- Aripiprazole • Abilify
- Clonidine • Catapres
- Dextroamphetamine • Dexedrine
- Fluoxetine • Prozac
- Guanfacine • Tenex
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Erickson reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Stigler receives grant/research support from Bristol-Myers Squibb Co. and Janssen Pharmaceutica.
Dr. Posey receives grant/research support from Forest Pharmaceuticals and Janssen Pharmaceutica and is a consultant to Forest Pharmaceuticals.
Dr. McDougle receives grant/research support from Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., and Eli Lilly and Co., and is a consultant to or speaker for Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., Eli Lilly and Co., and Pfizer Inc.
1. Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome-present and future. Ment Retard Dev Disabil Res Rev 2004;10(1):42-8.
2. Amaria RN, Billeisen LL, Hagerman RJ. Medication use in fragile X syndrome. Ment Health Aspects Dev Disabil 2001;4(4):143-7.
3. McDougle CJ, Posey DJ, Stigler KA. Pharmacological treatments. In: Moldin SO, Rubenstein JLR, eds. Understanding autism: from basic neuroscience to treatment. Boca Raton, FL: CRC/Taylor & Frances; 2006:417-42.
4. Tsiouris JA, Brown WT. Neuropsychiatric symptoms of fragile X syndrome: pathophysiology and pharmacotherapy. CNS Drugs 2004;18(11):687-703.
5. Hatton DD, Sideris J, Skinner M, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A 2006;140A(17):1804-13.
6. Jin P, Warren ST. Understanding the molecular basis of fragile X syndrome. Hum Mol Genet 2000;9(6):901-8.
7. Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci 2004;27(7):370-7.
8. Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav 2005;4(6):393-8.
9. Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr 2006;27(1):63-74.
10. Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr 2001;22(6):409-17.
11. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
12. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry 2005;62(11):1266-74.
13. Hagerman RJ, Murphy MA, Wittenberger MD. A controlled trial of stimulant medication in children with the fragile X syndrome. Am J Med Genet 1988;30(12):377-92.
14. Berry-Kravis E, Potanos K. Stimulant therapy in fragile X syndrome. Ann Neurol 2003;54:S150.-
15. Hagerman RJ, Riddle JE, Roberts LS, et al. Survey of the efficacy of clonidine in fragile X syndrome. Dev Brain Dysfunct 1995;8(4-6):336-44.
16. Posey DJ, Puntney JI, Sasher TM, et al. Guanfacine treatment of hyperactivity and inattention in pervasive developmental disorders: a retrospective analysis of 80 cases. J Child Adolesc Psychopharmacol 2004;14(2):233-41.
17. Torrioli MG, Vernacotola S, Mariotti P, et al. Double-blind, placebo-controlled study of L-acetylcarnitine for the treatment of hyperactive behavior in fragile X syndrome. Am J Med Genet 1999;87(4):366-8.
18. Posey DJ, Erickson CA, Stigler KA, McDougle CJ. The use of selective serotonin reuptake inhibitors in autism and related disorders. J Child Adolesc Psychopharmacol 2006;16(1-2):181-6.
19. Hagerman RJ, Fulton MJ, Leaman A, et al. A survey of fluoxetine therapy in fragile X syndrome. Dev Brain Dysfunct 1994;7:155-64.
20. Erickson CA, Stigler KA, Posey DJ, McDougle CJ. Risperidone in pervasive developmental disorders. Expert Rev Neurother 2005;5(6):713-9.
21. McCracken JT, McGough J, Shah B, et al, and the Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
22. Stigler KA, Posey DJ, McDougle CJ. Aripiprazole for maladaptive behavior in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(3):455-63.
23. McBride SM, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 2005;45(5):753-64.
24. Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 2005;49(7):1053-66.
25. Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci 2002;19(2):138-51.
Psychotropics1,2 are used to manage maladaptive and interfering behaviors in 70% of patients with fragile X syndrome (FXS), the leading cause of hereditary mental retardation. Treatment tends to follow a developmental course:
- In children, stimulants and alpha-2 agonists are used for attention-deficit/hyperactivity disorder (ADHD)-like symptoms.
- In adolescents and adults, selective serotonin reuptake inhibitors (SSRIs) are used for anxiety/repetitive phenomena and second-generation antipsychotics (SGAs) for irritability.
This course—which is often effective—is based primarily on anecdotal descriptions and on rationales borrowed from studies of ADHD, obsessive-compulsive disorder (OCD), and autistic disorder/related pervasive developmental disorders (PDDs).3 Disease-modifying agents to target the underlying brain dysregulation inherent in FXS (Box)1,4-10 are being investigated. For now, psychotropics can help you manage three common FXS symptom clusters: inattention and hyperactivity, anxiety, and aggression and self-injurious behavior (SIB).
The term “fragile X” describes how the X chromosome of affected individuals fractures in a folate-deprived medium. This most common form of inherited mental retardation affects 1 in 2,000 to 4,000 males and 1 in 4,000 to 8,000 females.4 One in four individuals with fragile X syndrome (FXS) also meets diagnostic criteria for autistic disorder (Table 1), with social skill and communication delays and interfering repetitive behaviors.5
Genetic profile. FXS results from a triplet repeat expansion in the fragile X mental retardation-1 gene.6 This mutation causes underproduction of fragile X mental retardation protein (FMRP), an inhibitor of the metabotropic glutamate receptor (mGluR). In theory, insufficient FMRP allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype: mental retardation, increased seizure risk, behavioral symptoms, and stereotypic movements.7,8
Behavioral difficulties cluster in three categories: attention-deficit/hyperactivity disorder-like symptoms, anxiety symptoms, and aggression and self-injurious behaviors.1,4,9 These are thought to be more prevalent in persons with FXS than would be expected from the degree of cognitive delay alone.1 Potential differences in the behavioral phenotypes of FXS patients with and without comorbid autism continue to be defined.10
Table 1
Clinical characteristics of patients with fragile X syndrome
| Physical features (seen in some males) | Long, narrow face |
| High, arched palate | |
| Narrow inter-eye distance | |
| Enlarged ears | |
| Macro-orchidism | |
| Behavioral symptoms | Inattention |
| Hyperactivity | |
| Anxiety | |
| Repetitive behaviors | |
| Aggression and self-injurious behaviors (increased in adolescence and adulthood) | |
| Comorbidities | Mental retardation (mean IQ for affected males in moderate range) |
| Comorbid autism (25% of affected individuals) | |
| Frequent seizures (10% to 20% of affected males) | |
| Hypersensitivity to sensory Stimuli |
Inattention and hyperactivity
Mike, age 6, has fragile X syndrome. He has been attending first grade for 4 months, and his teacher reports he does not sit still, runs throughout the classroom, and cannot focus on class work. Mike’s hyperactivity has been evident for 2 years but did not cause problems until first grade, his parents report.
Psychostimulants are the most frequently prescribed agents for inattention and hyperactivity in FXS, particularly in boys and male adolescents.1 Among FXS patients prescribed ≥ 1 psychotropic, approximately 70% are taking a stimulant.1,2
Efficacy. A clinical chart review found a 75% response rate in FXS children and adolescents who were given a stimulant for inattention and/or hyperactivity.1 This is higher than the 25% to 49% stimulant response rate reported in patients with PDDs.11,12
A 3-week, placebo-controlled, crossover trial of methylphenidate and dextroamphetamine noted a statistically significant response only to methylphenidate, with a positive response reported in 10 of 15 children (67%).13
Side effects. To date, limited information has described the rate of intolerable side effects associated with stimulant use in FXS,14 but in patients with PDD:
- 154 of 268 (57.5%) patient trials in a retrospective naturalistic study showed significant adverse effects with stimulant use.11
- 13 of 72 (18%) subjects in a controlled trial discontinued methylphenidate because of adverse events (most commonly irritability).12
Antiadrenergics. The alpha-2 agonists clonidine and guanfacine are the second most-used class of agents for inattention and hyperactivity in FXS. As with stimulants, boys and male adolescents are most likely to receive alpha-2 agonists, with administration rates of 10% to 20%.1,2
Efficacy. In one survey, nearly two-thirds (63%) of parents described clonidine as “very beneficial” to 35 children (mean age 6.6) with FXS.15 This is similar to a 70% response rate described for these alpha-2 agonists in a chart review.1 These rates are much higher than the 24% response rate reported with guanfacine in a retrospective chart review of 80 children and adolescents with a PDD.16 In that review, guanfacine use was associated with reduced hyperactivity, insomnia, and tics, and increased attention.15
Side effects associated with alpha-2 agonists include lowered blood pressure and sedation.
L-acetylcarnitine—a carnitine derivative required for neuronal use and transport of fatty acids—is being investigated to treat hyperactivity in FXS. Hyperactive symptoms improved significantly with L-acetylcarnitine, as measured by the Conners’ Abbreviated Parent-Teacher Questionnaire, in a 1-year, placebo-controlled trial of 20 boys (mean age 9.2) with FXS.17
Discussion. Supporting evidence is limited, but clinicians are treating ADHD-like symptoms with stimulants and alpha-2 agonists in many FXS patients. Preliminary data indicate that stimulants may be more effective and better tolerated in individuals with FXS than in those with PDD.
Trying a stimulant or alpha-2 agonist for inattention or hyperactivity symptoms in a child or adolescent with FXS appears clinically appropriate, given the available evidence. Additional data based on placebo-controlled and standardized measures of treatment response are needed to help guide treatment.
We start Mike on methylphenidate, 5 mg in the morning, for inattention and hyperactivity. He tolerates this well, and after 2 weeks we increase the dosage to 5 mg bid. Several weeks into treatment, his teacher comments that he is beginning to stay in his seat and attends to some assigned tasks in the classroom.
Mike continued to tolerate methylphenidate over the next 4 years. We gradually increased the dosage as he grew and when he periodically developed breakthrough interfering symptoms in the classroom.
Anxiety symptoms
In grade school, Mike became increasingly nervous around schoolmates, teachers, and friends. His teachers commented that he repeated phrases when he appeared anxious. Other children in his special education class began to shun him; they found his perseveration odd and sometimes threatening.
Now that Mike is age 10 and in fifth grade, his parents decide that his anxiety, particularly in social settings, is interfering with his life.
Anxiety symptoms—including generalized nervousness and OCD-like obsessions and perseverations—are common psychotropic targets in FXS. Boys may be the FXS patients most often prescribed drugs for inattention and hyperactivity, but they are the least likely to receive antidepressants for anxiety symptoms.1,2
Efficacy. More than 50% of female patients and men with FXS are prescribed SSRIs for anxiety (Table 2), and the reported response rate of 50% to 60%1 is similar to that seen with SSRIs in autism and related disorders.18 In autism, a developmental approach is warranted, as SSRIs tend to be less effective and cause more side effects in children and adolescents than in adults.18
Adverse effects reported with SSRIs in FXS include behavioral activation, appetite changes, insomnia, and nausea.1 In a study of fluoxetine for FXS symptoms, 10 of 35 patients (29%) had persistent side effects, most commonly weight loss and weight gain.19 One patient with pre-existing suicidal ideation worsened.
Watch for emergence or worsening of suicidal thoughts in all children and adolescents receiving antidepressants, whatever their target symptoms.
Mike is taking methylphenidate, 15 mg bid, for comorbid ADHD, and we add fluoxetine, 10 mg/d, for anxiety. This regimen is well-tolerated, so we increase fluoxetine to 20 mg/d at his 4-week follow-up appointment. After about 8 weeks, Mike’s parents report that his anxiety-associated symptoms are less severe.
Mike still appears nervous sometimes, but he uses markedly fewer perseverative phrases. This allows him to interact more meaningfully with peers and contributes to his social development.
Table 2
Target symptoms and treatment options for fragile X syndrome
| Medication class | Target symptom cluster | Evidence for use of drug class in FXS |
|---|---|---|
| Stimulants | Inattention, hyperactivity | One placebo-controlled trial, two large clinic surveys |
| Alpha-2 agonists | Inattention, hyperactivity | One parent-interview report, two large clinic surveys |
| SSRIs | Anxiety-related symptoms | One mailed survey, two large clinic surveys |
| Atypical antipsychotics | Aggression, self-injury | Two large clinic surveys, several controlled trials in PDDs |
| FXS: fragile X syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
| PDDs: pervasive developmental disorders. | ||
Aggression and self-injury
Mike, now age 20 and participating daily in a vocational workshop, begins yelling profanities at coworkers. At his group home, he has been hitting staff at least twice a week when redirected.
He is no longer taking stimulants, having been weaned from methylphenidate several years ago, but he continues to take fluoxetine, 40 mg/d.
Fluoxetine19 and clonidine15 can decrease irritability in FXS, but atypical antipsychotics are most commonly used for aggression and SIB.1,2 SGAs are prescribed to 10% to 20% of FXS patients who are taking medication1,2—particularly to men—and have produced response rates of 60% to 100% when used for aggression and SIB.1
Risperidone. No published reports have addressed using specific SGAs in FXS. In the PDD literature, most controlled data concerns risperidone.20
The largest randomized, placebo-controlled trial enrolled 101 children ages 5 to 17 with autistic disorder accompanied by severe tantrums, aggression, or self-injurious behavior. Among the 49 children taking risperidone, 0.5 to 3.5 mg/d for 8 weeks, 34 (69%) were judged as treatment responders with significantly reduced irritable behavior, compared with 6 of 52 (12%) taking placebo.21 Risperidone therapy was associated with average weight gain of 2.7±2.9 kg, compared with 0.8±2.2 kg with placebo.
Besides weight gain, other significant side effects associated with risperidone include sedation and elevated serum prolactin. These effects often are more pronounced in children and adolescents than in adults with PDDs.20
Other antipsychotics. Future use of SGAs in FXS will likely mirror the pattern seen in PDDs, where clinicians are moving towards weight-neutral antipsychotics such as ziprasidone and aripiprazole. In a preliminary report, aripiprazole reduced irritability in 5 youths with PDD.22 Our group is conducting a double-blind, placebo-controlled trial of aripiprazole in autism, targeting aggression, SIB, and irritability.
Discussion. SGAs are used most often in FXS to treat aggression and SIB, based on data from studies on treating similar symptoms in PDDs. Closely monitor patients for sedation, weight gain, and lipid, glucose, and prolactin elevations when using SGAs (Table 3). Be especially vigilant when children gain weight rapidly or show hyperprolactinemia signs while taking these drugs.
After being suspended from the vocational workshop, Mike is treated at a local mental health center for aggressive behaviors. He tolerates an initial dosage of aripiprazole,2.5 mg/d, which is titrated in 2.5-mg increments biweekly to 10 mg/d. At this dosage, he stops hitting staff members and his yelling of profanities is greatly reduced. Over several months, Mike returns to his vocational workshop and maintains residence at his group home.
Table 3
Medication side effects and recommended monitoring
| Medication class | Side effects | Medication monitoring |
|---|---|---|
| Stimulants | Anorexia, insomnia, agitation, exacerbation of tics | Observe closely when starting treatment and increasing dosage |
| Alpha-2 agonists | Lowered blood pressure, sedation, dizziness | Observe closely when starting treatment and increasing dosage |
| Check blood pressure with all dosage changes and at all clinic visits | ||
| SSRIs | Irritability, mood lability, nausea, sleep and appetite disturbances, suicidality | Observe closely when starting treatment and increasing dosage |
| Atypical antipsychotics | Sedation, weight gain, hyperglycemia, hyperlipidemia, hyperprolactinemia, EPS, NMS, tardive dyskinesia | Obtain metabolic profile, including fasting lipids, glucose, and prolactin levels |
| Monitor for weight gain and signs of EPS | ||
| EPS: extrapyramidal symptoms | ||
| NMS: neuroleptic malignant syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
Genetic-related treatments
Studies are needed to investigate the use of stimulants, SSRIs, and antipsychotics in patients with FXS unaccompanied by generalized anxiety disorder, OCD, ADHD, or PDDs. How FXS patients without those comorbidities will respond to drug treatment is unknown. Also, little also is known about possible side effects associated with combining drug treatments in individuals with FXS.
Future drug treatment in FXS will likely focus on agents that target the underlying neurochemical dysregulation associated with the FXS genotype. This approach might reduce interfering behaviors and alter the course of cognitive dysfunction—including mental retardation—associated with FXS.
Past attempts to correct FXS’ neurochemical abnormalities focused on using folic acid. The term “fragile X” describes how the X chromosome of individuals with FXS fractures in a folate-deprived medium. Many controlled trials of folic acid in FXS did not support earlier positive reports, however.4
Greater understanding of fragile X mental retardation protein (FMRP) function has led to the metabotropic glutamate receptor (mGluR) theory.7 It holds that FMRP underproduction allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype. Researchers now are attempting to reverse the neurochemical impact of insufficient FMRP with two medication classes:
- selective group 1 mGluR receptor antagonists (mGluR5 antagonists, in particular). The mGluR5 receptor antagonist MPEP has shown the ability to rescue normal behaviors in animal models of FXS. MPEP and lithium have reversed behaviors associated with FXS and—at the microscopic level—rescued synaptic plasticity.23,24 In the drosophila fly model of FXS, lithium reduced activity in the mGluR cascade, thus compensating for lack of FMRP.23
- positive AMPA receptor modulators (ampakines) that promote activity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.9 Excessive mGluR activity appears to impair AMPA receptors’ ability to promote cortical development, memory, and learning.7 Reduced AMPA receptors have been shown in the FXS mouse model,25 and an ampakine is being investigated in a study of men with FXS and autism.1
- FRAXA: The Fragile X Research Foundation. Founded by parents of children with fragile X syndrome to increase funding for research toward effective treatments. www.fraxa.org.
- The National Fragile X Foundation. Provides educational and emotional support for fragile X families and promotes public and professional awareness. www.fragilex.org.
- Hagerman RJ, Hagerman PJ, eds. Fragile X syndrome: diagnosis, treatment, and research, 3rd ed. Baltimore, MD: The Johns Hopkins University Press; 2002.
- Aripiprazole • Abilify
- Clonidine • Catapres
- Dextroamphetamine • Dexedrine
- Fluoxetine • Prozac
- Guanfacine • Tenex
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Erickson reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Stigler receives grant/research support from Bristol-Myers Squibb Co. and Janssen Pharmaceutica.
Dr. Posey receives grant/research support from Forest Pharmaceuticals and Janssen Pharmaceutica and is a consultant to Forest Pharmaceuticals.
Dr. McDougle receives grant/research support from Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., and Eli Lilly and Co., and is a consultant to or speaker for Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., Eli Lilly and Co., and Pfizer Inc.
Psychotropics1,2 are used to manage maladaptive and interfering behaviors in 70% of patients with fragile X syndrome (FXS), the leading cause of hereditary mental retardation. Treatment tends to follow a developmental course:
- In children, stimulants and alpha-2 agonists are used for attention-deficit/hyperactivity disorder (ADHD)-like symptoms.
- In adolescents and adults, selective serotonin reuptake inhibitors (SSRIs) are used for anxiety/repetitive phenomena and second-generation antipsychotics (SGAs) for irritability.
This course—which is often effective—is based primarily on anecdotal descriptions and on rationales borrowed from studies of ADHD, obsessive-compulsive disorder (OCD), and autistic disorder/related pervasive developmental disorders (PDDs).3 Disease-modifying agents to target the underlying brain dysregulation inherent in FXS (Box)1,4-10 are being investigated. For now, psychotropics can help you manage three common FXS symptom clusters: inattention and hyperactivity, anxiety, and aggression and self-injurious behavior (SIB).
The term “fragile X” describes how the X chromosome of affected individuals fractures in a folate-deprived medium. This most common form of inherited mental retardation affects 1 in 2,000 to 4,000 males and 1 in 4,000 to 8,000 females.4 One in four individuals with fragile X syndrome (FXS) also meets diagnostic criteria for autistic disorder (Table 1), with social skill and communication delays and interfering repetitive behaviors.5
Genetic profile. FXS results from a triplet repeat expansion in the fragile X mental retardation-1 gene.6 This mutation causes underproduction of fragile X mental retardation protein (FMRP), an inhibitor of the metabotropic glutamate receptor (mGluR). In theory, insufficient FMRP allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype: mental retardation, increased seizure risk, behavioral symptoms, and stereotypic movements.7,8
Behavioral difficulties cluster in three categories: attention-deficit/hyperactivity disorder-like symptoms, anxiety symptoms, and aggression and self-injurious behaviors.1,4,9 These are thought to be more prevalent in persons with FXS than would be expected from the degree of cognitive delay alone.1 Potential differences in the behavioral phenotypes of FXS patients with and without comorbid autism continue to be defined.10
Table 1
Clinical characteristics of patients with fragile X syndrome
| Physical features (seen in some males) | Long, narrow face |
| High, arched palate | |
| Narrow inter-eye distance | |
| Enlarged ears | |
| Macro-orchidism | |
| Behavioral symptoms | Inattention |
| Hyperactivity | |
| Anxiety | |
| Repetitive behaviors | |
| Aggression and self-injurious behaviors (increased in adolescence and adulthood) | |
| Comorbidities | Mental retardation (mean IQ for affected males in moderate range) |
| Comorbid autism (25% of affected individuals) | |
| Frequent seizures (10% to 20% of affected males) | |
| Hypersensitivity to sensory Stimuli |
Inattention and hyperactivity
Mike, age 6, has fragile X syndrome. He has been attending first grade for 4 months, and his teacher reports he does not sit still, runs throughout the classroom, and cannot focus on class work. Mike’s hyperactivity has been evident for 2 years but did not cause problems until first grade, his parents report.
Psychostimulants are the most frequently prescribed agents for inattention and hyperactivity in FXS, particularly in boys and male adolescents.1 Among FXS patients prescribed ≥ 1 psychotropic, approximately 70% are taking a stimulant.1,2
Efficacy. A clinical chart review found a 75% response rate in FXS children and adolescents who were given a stimulant for inattention and/or hyperactivity.1 This is higher than the 25% to 49% stimulant response rate reported in patients with PDDs.11,12
A 3-week, placebo-controlled, crossover trial of methylphenidate and dextroamphetamine noted a statistically significant response only to methylphenidate, with a positive response reported in 10 of 15 children (67%).13
Side effects. To date, limited information has described the rate of intolerable side effects associated with stimulant use in FXS,14 but in patients with PDD:
- 154 of 268 (57.5%) patient trials in a retrospective naturalistic study showed significant adverse effects with stimulant use.11
- 13 of 72 (18%) subjects in a controlled trial discontinued methylphenidate because of adverse events (most commonly irritability).12
Antiadrenergics. The alpha-2 agonists clonidine and guanfacine are the second most-used class of agents for inattention and hyperactivity in FXS. As with stimulants, boys and male adolescents are most likely to receive alpha-2 agonists, with administration rates of 10% to 20%.1,2
Efficacy. In one survey, nearly two-thirds (63%) of parents described clonidine as “very beneficial” to 35 children (mean age 6.6) with FXS.15 This is similar to a 70% response rate described for these alpha-2 agonists in a chart review.1 These rates are much higher than the 24% response rate reported with guanfacine in a retrospective chart review of 80 children and adolescents with a PDD.16 In that review, guanfacine use was associated with reduced hyperactivity, insomnia, and tics, and increased attention.15
Side effects associated with alpha-2 agonists include lowered blood pressure and sedation.
L-acetylcarnitine—a carnitine derivative required for neuronal use and transport of fatty acids—is being investigated to treat hyperactivity in FXS. Hyperactive symptoms improved significantly with L-acetylcarnitine, as measured by the Conners’ Abbreviated Parent-Teacher Questionnaire, in a 1-year, placebo-controlled trial of 20 boys (mean age 9.2) with FXS.17
Discussion. Supporting evidence is limited, but clinicians are treating ADHD-like symptoms with stimulants and alpha-2 agonists in many FXS patients. Preliminary data indicate that stimulants may be more effective and better tolerated in individuals with FXS than in those with PDD.
Trying a stimulant or alpha-2 agonist for inattention or hyperactivity symptoms in a child or adolescent with FXS appears clinically appropriate, given the available evidence. Additional data based on placebo-controlled and standardized measures of treatment response are needed to help guide treatment.
We start Mike on methylphenidate, 5 mg in the morning, for inattention and hyperactivity. He tolerates this well, and after 2 weeks we increase the dosage to 5 mg bid. Several weeks into treatment, his teacher comments that he is beginning to stay in his seat and attends to some assigned tasks in the classroom.
Mike continued to tolerate methylphenidate over the next 4 years. We gradually increased the dosage as he grew and when he periodically developed breakthrough interfering symptoms in the classroom.
Anxiety symptoms
In grade school, Mike became increasingly nervous around schoolmates, teachers, and friends. His teachers commented that he repeated phrases when he appeared anxious. Other children in his special education class began to shun him; they found his perseveration odd and sometimes threatening.
Now that Mike is age 10 and in fifth grade, his parents decide that his anxiety, particularly in social settings, is interfering with his life.
Anxiety symptoms—including generalized nervousness and OCD-like obsessions and perseverations—are common psychotropic targets in FXS. Boys may be the FXS patients most often prescribed drugs for inattention and hyperactivity, but they are the least likely to receive antidepressants for anxiety symptoms.1,2
Efficacy. More than 50% of female patients and men with FXS are prescribed SSRIs for anxiety (Table 2), and the reported response rate of 50% to 60%1 is similar to that seen with SSRIs in autism and related disorders.18 In autism, a developmental approach is warranted, as SSRIs tend to be less effective and cause more side effects in children and adolescents than in adults.18
Adverse effects reported with SSRIs in FXS include behavioral activation, appetite changes, insomnia, and nausea.1 In a study of fluoxetine for FXS symptoms, 10 of 35 patients (29%) had persistent side effects, most commonly weight loss and weight gain.19 One patient with pre-existing suicidal ideation worsened.
Watch for emergence or worsening of suicidal thoughts in all children and adolescents receiving antidepressants, whatever their target symptoms.
Mike is taking methylphenidate, 15 mg bid, for comorbid ADHD, and we add fluoxetine, 10 mg/d, for anxiety. This regimen is well-tolerated, so we increase fluoxetine to 20 mg/d at his 4-week follow-up appointment. After about 8 weeks, Mike’s parents report that his anxiety-associated symptoms are less severe.
Mike still appears nervous sometimes, but he uses markedly fewer perseverative phrases. This allows him to interact more meaningfully with peers and contributes to his social development.
Table 2
Target symptoms and treatment options for fragile X syndrome
| Medication class | Target symptom cluster | Evidence for use of drug class in FXS |
|---|---|---|
| Stimulants | Inattention, hyperactivity | One placebo-controlled trial, two large clinic surveys |
| Alpha-2 agonists | Inattention, hyperactivity | One parent-interview report, two large clinic surveys |
| SSRIs | Anxiety-related symptoms | One mailed survey, two large clinic surveys |
| Atypical antipsychotics | Aggression, self-injury | Two large clinic surveys, several controlled trials in PDDs |
| FXS: fragile X syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
| PDDs: pervasive developmental disorders. | ||
Aggression and self-injury
Mike, now age 20 and participating daily in a vocational workshop, begins yelling profanities at coworkers. At his group home, he has been hitting staff at least twice a week when redirected.
He is no longer taking stimulants, having been weaned from methylphenidate several years ago, but he continues to take fluoxetine, 40 mg/d.
Fluoxetine19 and clonidine15 can decrease irritability in FXS, but atypical antipsychotics are most commonly used for aggression and SIB.1,2 SGAs are prescribed to 10% to 20% of FXS patients who are taking medication1,2—particularly to men—and have produced response rates of 60% to 100% when used for aggression and SIB.1
Risperidone. No published reports have addressed using specific SGAs in FXS. In the PDD literature, most controlled data concerns risperidone.20
The largest randomized, placebo-controlled trial enrolled 101 children ages 5 to 17 with autistic disorder accompanied by severe tantrums, aggression, or self-injurious behavior. Among the 49 children taking risperidone, 0.5 to 3.5 mg/d for 8 weeks, 34 (69%) were judged as treatment responders with significantly reduced irritable behavior, compared with 6 of 52 (12%) taking placebo.21 Risperidone therapy was associated with average weight gain of 2.7±2.9 kg, compared with 0.8±2.2 kg with placebo.
Besides weight gain, other significant side effects associated with risperidone include sedation and elevated serum prolactin. These effects often are more pronounced in children and adolescents than in adults with PDDs.20
Other antipsychotics. Future use of SGAs in FXS will likely mirror the pattern seen in PDDs, where clinicians are moving towards weight-neutral antipsychotics such as ziprasidone and aripiprazole. In a preliminary report, aripiprazole reduced irritability in 5 youths with PDD.22 Our group is conducting a double-blind, placebo-controlled trial of aripiprazole in autism, targeting aggression, SIB, and irritability.
Discussion. SGAs are used most often in FXS to treat aggression and SIB, based on data from studies on treating similar symptoms in PDDs. Closely monitor patients for sedation, weight gain, and lipid, glucose, and prolactin elevations when using SGAs (Table 3). Be especially vigilant when children gain weight rapidly or show hyperprolactinemia signs while taking these drugs.
After being suspended from the vocational workshop, Mike is treated at a local mental health center for aggressive behaviors. He tolerates an initial dosage of aripiprazole,2.5 mg/d, which is titrated in 2.5-mg increments biweekly to 10 mg/d. At this dosage, he stops hitting staff members and his yelling of profanities is greatly reduced. Over several months, Mike returns to his vocational workshop and maintains residence at his group home.
Table 3
Medication side effects and recommended monitoring
| Medication class | Side effects | Medication monitoring |
|---|---|---|
| Stimulants | Anorexia, insomnia, agitation, exacerbation of tics | Observe closely when starting treatment and increasing dosage |
| Alpha-2 agonists | Lowered blood pressure, sedation, dizziness | Observe closely when starting treatment and increasing dosage |
| Check blood pressure with all dosage changes and at all clinic visits | ||
| SSRIs | Irritability, mood lability, nausea, sleep and appetite disturbances, suicidality | Observe closely when starting treatment and increasing dosage |
| Atypical antipsychotics | Sedation, weight gain, hyperglycemia, hyperlipidemia, hyperprolactinemia, EPS, NMS, tardive dyskinesia | Obtain metabolic profile, including fasting lipids, glucose, and prolactin levels |
| Monitor for weight gain and signs of EPS | ||
| EPS: extrapyramidal symptoms | ||
| NMS: neuroleptic malignant syndrome | ||
| SSRIs: selective serotonin reuptake inhibitors | ||
Genetic-related treatments
Studies are needed to investigate the use of stimulants, SSRIs, and antipsychotics in patients with FXS unaccompanied by generalized anxiety disorder, OCD, ADHD, or PDDs. How FXS patients without those comorbidities will respond to drug treatment is unknown. Also, little also is known about possible side effects associated with combining drug treatments in individuals with FXS.
Future drug treatment in FXS will likely focus on agents that target the underlying neurochemical dysregulation associated with the FXS genotype. This approach might reduce interfering behaviors and alter the course of cognitive dysfunction—including mental retardation—associated with FXS.
Past attempts to correct FXS’ neurochemical abnormalities focused on using folic acid. The term “fragile X” describes how the X chromosome of individuals with FXS fractures in a folate-deprived medium. Many controlled trials of folic acid in FXS did not support earlier positive reports, however.4
Greater understanding of fragile X mental retardation protein (FMRP) function has led to the metabotropic glutamate receptor (mGluR) theory.7 It holds that FMRP underproduction allows exaggerated group 1 mGluR activity and leads to the FXS neurobehavioral phenotype. Researchers now are attempting to reverse the neurochemical impact of insufficient FMRP with two medication classes:
- selective group 1 mGluR receptor antagonists (mGluR5 antagonists, in particular). The mGluR5 receptor antagonist MPEP has shown the ability to rescue normal behaviors in animal models of FXS. MPEP and lithium have reversed behaviors associated with FXS and—at the microscopic level—rescued synaptic plasticity.23,24 In the drosophila fly model of FXS, lithium reduced activity in the mGluR cascade, thus compensating for lack of FMRP.23
- positive AMPA receptor modulators (ampakines) that promote activity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.9 Excessive mGluR activity appears to impair AMPA receptors’ ability to promote cortical development, memory, and learning.7 Reduced AMPA receptors have been shown in the FXS mouse model,25 and an ampakine is being investigated in a study of men with FXS and autism.1
- FRAXA: The Fragile X Research Foundation. Founded by parents of children with fragile X syndrome to increase funding for research toward effective treatments. www.fraxa.org.
- The National Fragile X Foundation. Provides educational and emotional support for fragile X families and promotes public and professional awareness. www.fragilex.org.
- Hagerman RJ, Hagerman PJ, eds. Fragile X syndrome: diagnosis, treatment, and research, 3rd ed. Baltimore, MD: The Johns Hopkins University Press; 2002.
- Aripiprazole • Abilify
- Clonidine • Catapres
- Dextroamphetamine • Dexedrine
- Fluoxetine • Prozac
- Guanfacine • Tenex
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Erickson reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Stigler receives grant/research support from Bristol-Myers Squibb Co. and Janssen Pharmaceutica.
Dr. Posey receives grant/research support from Forest Pharmaceuticals and Janssen Pharmaceutica and is a consultant to Forest Pharmaceuticals.
Dr. McDougle receives grant/research support from Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., and Eli Lilly and Co., and is a consultant to or speaker for Forest Pharmaceuticals, Janssen Pharmaceutica, Bristol-Myers Squibb Co., Eli Lilly and Co., and Pfizer Inc.
1. Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome-present and future. Ment Retard Dev Disabil Res Rev 2004;10(1):42-8.
2. Amaria RN, Billeisen LL, Hagerman RJ. Medication use in fragile X syndrome. Ment Health Aspects Dev Disabil 2001;4(4):143-7.
3. McDougle CJ, Posey DJ, Stigler KA. Pharmacological treatments. In: Moldin SO, Rubenstein JLR, eds. Understanding autism: from basic neuroscience to treatment. Boca Raton, FL: CRC/Taylor & Frances; 2006:417-42.
4. Tsiouris JA, Brown WT. Neuropsychiatric symptoms of fragile X syndrome: pathophysiology and pharmacotherapy. CNS Drugs 2004;18(11):687-703.
5. Hatton DD, Sideris J, Skinner M, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A 2006;140A(17):1804-13.
6. Jin P, Warren ST. Understanding the molecular basis of fragile X syndrome. Hum Mol Genet 2000;9(6):901-8.
7. Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci 2004;27(7):370-7.
8. Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav 2005;4(6):393-8.
9. Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr 2006;27(1):63-74.
10. Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr 2001;22(6):409-17.
11. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
12. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry 2005;62(11):1266-74.
13. Hagerman RJ, Murphy MA, Wittenberger MD. A controlled trial of stimulant medication in children with the fragile X syndrome. Am J Med Genet 1988;30(12):377-92.
14. Berry-Kravis E, Potanos K. Stimulant therapy in fragile X syndrome. Ann Neurol 2003;54:S150.-
15. Hagerman RJ, Riddle JE, Roberts LS, et al. Survey of the efficacy of clonidine in fragile X syndrome. Dev Brain Dysfunct 1995;8(4-6):336-44.
16. Posey DJ, Puntney JI, Sasher TM, et al. Guanfacine treatment of hyperactivity and inattention in pervasive developmental disorders: a retrospective analysis of 80 cases. J Child Adolesc Psychopharmacol 2004;14(2):233-41.
17. Torrioli MG, Vernacotola S, Mariotti P, et al. Double-blind, placebo-controlled study of L-acetylcarnitine for the treatment of hyperactive behavior in fragile X syndrome. Am J Med Genet 1999;87(4):366-8.
18. Posey DJ, Erickson CA, Stigler KA, McDougle CJ. The use of selective serotonin reuptake inhibitors in autism and related disorders. J Child Adolesc Psychopharmacol 2006;16(1-2):181-6.
19. Hagerman RJ, Fulton MJ, Leaman A, et al. A survey of fluoxetine therapy in fragile X syndrome. Dev Brain Dysfunct 1994;7:155-64.
20. Erickson CA, Stigler KA, Posey DJ, McDougle CJ. Risperidone in pervasive developmental disorders. Expert Rev Neurother 2005;5(6):713-9.
21. McCracken JT, McGough J, Shah B, et al, and the Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
22. Stigler KA, Posey DJ, McDougle CJ. Aripiprazole for maladaptive behavior in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(3):455-63.
23. McBride SM, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 2005;45(5):753-64.
24. Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 2005;49(7):1053-66.
25. Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci 2002;19(2):138-51.
1. Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome-present and future. Ment Retard Dev Disabil Res Rev 2004;10(1):42-8.
2. Amaria RN, Billeisen LL, Hagerman RJ. Medication use in fragile X syndrome. Ment Health Aspects Dev Disabil 2001;4(4):143-7.
3. McDougle CJ, Posey DJ, Stigler KA. Pharmacological treatments. In: Moldin SO, Rubenstein JLR, eds. Understanding autism: from basic neuroscience to treatment. Boca Raton, FL: CRC/Taylor & Frances; 2006:417-42.
4. Tsiouris JA, Brown WT. Neuropsychiatric symptoms of fragile X syndrome: pathophysiology and pharmacotherapy. CNS Drugs 2004;18(11):687-703.
5. Hatton DD, Sideris J, Skinner M, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A 2006;140A(17):1804-13.
6. Jin P, Warren ST. Understanding the molecular basis of fragile X syndrome. Hum Mol Genet 2000;9(6):901-8.
7. Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci 2004;27(7):370-7.
8. Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav 2005;4(6):393-8.
9. Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr 2006;27(1):63-74.
10. Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr 2001;22(6):409-17.
11. Stigler KA, Desmond LA, Posey DJ, et al. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(1):49-56.
12. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry 2005;62(11):1266-74.
13. Hagerman RJ, Murphy MA, Wittenberger MD. A controlled trial of stimulant medication in children with the fragile X syndrome. Am J Med Genet 1988;30(12):377-92.
14. Berry-Kravis E, Potanos K. Stimulant therapy in fragile X syndrome. Ann Neurol 2003;54:S150.-
15. Hagerman RJ, Riddle JE, Roberts LS, et al. Survey of the efficacy of clonidine in fragile X syndrome. Dev Brain Dysfunct 1995;8(4-6):336-44.
16. Posey DJ, Puntney JI, Sasher TM, et al. Guanfacine treatment of hyperactivity and inattention in pervasive developmental disorders: a retrospective analysis of 80 cases. J Child Adolesc Psychopharmacol 2004;14(2):233-41.
17. Torrioli MG, Vernacotola S, Mariotti P, et al. Double-blind, placebo-controlled study of L-acetylcarnitine for the treatment of hyperactive behavior in fragile X syndrome. Am J Med Genet 1999;87(4):366-8.
18. Posey DJ, Erickson CA, Stigler KA, McDougle CJ. The use of selective serotonin reuptake inhibitors in autism and related disorders. J Child Adolesc Psychopharmacol 2006;16(1-2):181-6.
19. Hagerman RJ, Fulton MJ, Leaman A, et al. A survey of fluoxetine therapy in fragile X syndrome. Dev Brain Dysfunct 1994;7:155-64.
20. Erickson CA, Stigler KA, Posey DJ, McDougle CJ. Risperidone in pervasive developmental disorders. Expert Rev Neurother 2005;5(6):713-9.
21. McCracken JT, McGough J, Shah B, et al, and the Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
22. Stigler KA, Posey DJ, McDougle CJ. Aripiprazole for maladaptive behavior in pervasive developmental disorders. J Child Adolesc Psychopharmacol 2004;14(3):455-63.
23. McBride SM, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 2005;45(5):753-64.
24. Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 2005;49(7):1053-66.
25. Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci 2002;19(2):138-51.