User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
When clozapine is not an option
History: ‘leaving town’
Mr. S, age 58, escaped repeatedly from his group home over 4 weeks. During one episode, he removed mail from neighbors’ mailboxes and tried to direct midday traffic. He would disappear for a few hours, sometimes overnight, before returning or being brought back by police.
The patient—who has had schizophrenia with catatonic features for 30 years—offered assorted explanations for escaping, most of them based on delusional beliefs, such as “I’m leaving town to get married” or “I’m late for engineering class.”
Since his last escape 3 weeks ago, Mr. S has remained in the group home without incident but has not been reporting for his usual outpatient psychiatric care. One day, he finally presents to us at the group home sponsor’s urging.
On evaluation, Mr. S shows stereotyped speech, staring, posturing, speech-prompt mutism, and odd mannerisms such as saluting. He has not been bathing or sleeping and smiles inappropriately. He speaks only when spoken to and answers with short phrases punctuated with ”By the grace of the good Lord.”
The authors’ observations
DSM-IV-TR requires at least two features to diagnose catatonic schizophrenia:
- peculiar voluntary movements
- extreme negativism
- excessive motor activity
- echolalia or echopraxia
- motoric immobility.1
Catatonia is common among the chronic mentally ill,2 yet it often goes undiagnosed.3 As a form of psychosis, catatonia might lead to greater functional impairment if not treated.
Treatment: time to try clozapine?
Over 10 years, numerous antipsychotic regimens plus adjunctive valproic acid, 500 mg tid, or lorazepam, up to 2 mg tid, have not lessened Mr. S’ psychosis and impulsivity. We start clozapine, 400 mg/d, and order twice-monthly blood tests to check for clozapine-induced agranulocytosis.
After nearly 6 months, some catatonic features improve gradually based on clinical interview. Serum clozapine is 363 ng/mL.
poll here
The authors’ observations
Second-generation antipsychotics (SGAs) are favored over first-generation antipsychotics to treat schizophrenia with catatonic features (Table),4,5 but no drug in either class has worked for Mr. S.
ECT can alleviate catatonic schizophrenia,4,6 but this option often is not available because the clinician fears a negative outcome would prompt legal action, or the guardian or next of kin do not consent to the procedure.3 We considered referring Mr. S to an ECT provider, but he has no legal guardian to provide consent. The group home sponsor also objected to ECT because Mr. S would have been sent out of town for treatment.
Catatonia patients who are immobile, physically compromised, and refuse food and drink typically are considered ECT candidates. Mr. S eats and drinks regularly and is physically able.
Lorazepam can produce rapid response, but it can be addictive.2 Also, an adjunctive 2 mg/d dosage showed no effect.
Clozapine monotherapy has shown effectiveness in catatonic schizophrenia7 and might be an option after other antipsychotics have failed.
Table 1
Treatments for catatonia: risks and benefits
| Medication | Use | Rationale | Benefits | Risks |
|---|---|---|---|---|
| First-generation antipsychotics (FGAs) | Often used for schizophrenia | Control positive symptoms | Well-established | Catatonia might be difficult to distinguish from NMS |
| Less expensive than other medications | ||||
| Second-generation antipsychotics (SGAs) | Beneficial in catatonia | Less likely than FGAs to worsen catatonia because of low D2 blockade | Some studies suggest greater efficacy than with FGAs | Metabolic syndrome, agranulocytosis with clozapine |
| Benzodiazepines | Lorazepam helpful in acute catatonia | Can be added to any antipsychotic | Safe, first-line treatment for catatonia | Respiratory compromise, incoordination, sedation, potential for abuse |
| Electroconvulsive therapy | ||||
| Electroconvulsive therapy | Beneficial in malignant catatonia | Effective in catatonia, NMS | Useful for treatment-refractory catatonia | Concerns with anesthesia, informed consent, availability |
| Rapid onset of action | ||||
| NMS: Neuroleptic malignant syndrome | ||||
Complication: agranulocytosis, then nms
Six months after starting clozapine, Mr. S starts having diaphoresis and night sweats, suggesting neutropenia. Blood testing shows a white blood cell count (WBC) of 3.6/μL, down from 4.6/μL 2 weeks before (normal range, 4.6 to 11/μL).
One week later, Mr. S’ WBC is 1.6/μL with a 46% relative neutrophil value (normal range, 50% to 70%) and an absolute neutrophil count of 736 (normal range, 2,500 to 7,000).
We diagnose agranulocytosis and stop clozapine, but Mr. S’ WBC continues to fall over 2 weeks to 0.8/μL with a 16% relative and 128 absolute neutrophil count. After 1 more week, his WBC increases to 2.6/μL and returns to normal 1 week later—4 weeks after stopping clozapine
We then target Mr. S’ catatonia with intramuscular haloperidol, 100 mg/d for 4 weeks, and ziprasidone, 80 mg bid with food. He tolerates this combination but gradually develops tremor and rigidity. Six weeks later, we add levodopa/carbidopa, 25/250 mg bid for his movement problems.
Two weeks later, Mr. S is sweating profusely, disoriented, rigid, and febrile (104.6°F). We diagnose neuroleptic malignant syndrome (NMS), stop both antipsychotics, and admit him for treatment. We start lorazepam, 1 mg tid for catatonia; bromocriptine, 250 mg bid for rigidity; and continue levodopa/carbidopa at the same dosage. We also add dantrolene, 25 mg tid for 5 days for fever and rigidity, and provide a cooling blanket for hyperthermia.
Mr. S’ fever, autonomic changes, and diaphoresis diminish within 3 days. Rigidity and mental status improve gradually over 2 weeks. We discharge him after 10 days.
poll here
The author’s observations
Catatonia is a recognized risk factor for NMS. White and Robins8 described 17 patients with a catatonic syndrome that developed into NMS within 5 to 96 hours of starting a neuroleptic. Sachdev developed an NMS rating scale that includes catatonic symptoms.9
Northoff,10 however, associates NMS with D2 receptor blockage in the basal ganglia and relates catatonia to a frontocortical gamma-aminobutyric acid (GABA) dysfunction. Based on this theory, haloperidol—which offers a higher D2 blockade than do SGAs such as ziprasidone—might have contributed to Mr. S’ NMS.
Some evidence suggests that lorazepam—which works on gamma-aminobutyric acid ionotropic type A (GABAA) receptors—helps treat catatonia in NMS and improves rigidity, hyperthermia, and autonomic signs.11
Treatment: which agents will work?
Three weeks after his discharge, we restart ziprasidone, 40 mg bid for Mr. S’ catatonic schizophrenia. He remains free of NMS symptoms but still has mannerisms (posturing, staring, immobility, stereotypic scratching on his face).
Over 1 year, Mr. S is hospitalized repeatedly because of persistent impulsivity and delusions. He has failed numerous antipsychotic regimens lasting 1 month or longer, including olanzapine, up to 30 mg/d; quetiapine, 300 mg tid; and risperidone, 2 mg tid. Adding a first-generation antipsychotic either does not help (as with perphenazine, 12 mg/d) or diminishes his memory (as with chlorpromazine, 250 mg/d). The anticholinergic benztropine, 2 mg bid, also is ineffective.
Combination quetiapine, 300 mg/d, and the antiviral amantadine, 100 mg tid, improve Mr. S’ stereotypy at first, but his delusions intensify within 1 week. His Bush-Francis Catatonia Rating Scale scores range from 9 (indicating moderate catatonia) to 16 (persistent catatonic features).12
poll here
The authors’ observations
Catatonic schizophrenia’s pathophysiology and response to medication might differ compared with other schizophrenia forms.13 Dopamine D2 hypoactivity, glutamate N-methyl-D-aspartate (NMDA) hyperactivity, or GABAA hypoactivity are believed to cause catatonia.3,6,7 GABA agonists, anticonvulsants, dopamine agonists, SGAs, and NMDA antagonists target these pathophysiologies, but patients with a catatonia subtype often respond to only one type of medication.
Lorazepam exerts an anticatatonic effect by binding to GABAA receptors and increasing GABA activity. Lorazepam can help some patients with schizophrenia but has not shown benefit when added to an antipsychotic for chronic catatonia.6,14
SGAs can provide marked improvement in patients with catatonic schizophrenia.5
Salokangas et al15 note that “atypicals” pass more dopamine to the D2 receptor when dopamine is low in the basal ganglia. This suggests that SGAs with low D2 binding—such as clozapine, olanzapine, and quetiapine—are more beneficial than other SGAs for catatonia. Serotonin binding or other mechanisms might add to these drugs’ anticatatonic effect.7
Anticonvulsants. Adjunctive anticonvulsant therapy might alleviate catatonia by increasing GABA activity or by causing a modest antiglutaminergic effect, as reported with carbamazepine or valproic acid.16 Anticholinergics also might help treat neuroleptic-induced catatonia.17
Amantadine—FDA-approved to treat Parkinson’s disease and extrapyramidal disease—can alleviate catatonia by blocking hyperglutamatergic excitotoxicity in neurons, thus blocking NMDA receptors.18 As with Mr. S, however, amantadine can worsen psychosis by increasing dopamine release.
Memantine—an NMDA receptor antagonist indicated for moderate to severe Alzheimer’s disease—also blocks hyperglutamatergic excitotoxicity in neurons. The medication has shown effectiveness for treating catatonic schizophrenia in case reports,19-21 but 3 patients have reported memantine-induced psychosis and seizures.21
Some might argue that Mr. S’ delusions are predominant and more compelling than his catatonia, but these did not hamper his ability to live in a group home. His catatonia-related negativism, impulsivity, and inability to cooperate are what led to frequent hospitalization.
Follow-up: treatment change
We stop amantadine, add memantine, 10 mg bid, and titrate quetiapine over 2 weeks to 900 mg/d. Mr. S’ catatonia improves but some delusions persist. We add olanzapine, 7.5 mg bid, and within 2 weeks Mr. S is less delusional and more cooperative.
We discharge Mr. S on the above medications, plus:
- lorazepam, 1 mg each morning and 2 mg nightly, which he has been taking for catatonia for about 1 year
- trazodone, 150 mg bid, which we added 6 months ago to help him sleep and reduce psychomotor excitement
- ranitidine, 150 mg bid, for gastroesophageal reflux disorder
- and levothyroxine, 0.5 mg/d, for comobrid hypothyroidism. His thyroid-stimulating hormone level is normal.
We see Mr. S monthly. He is still impulsive at times, occasionally collecting his neighbors’ newspapers and mail despite instructions from group home staff not to do so. Yet his sponsors say Mr. S is “like a new person.” He talks spontaneously, interacts, and is cooperative. He has not been hospitalized for more than 1 year.
The authors’ observations
Mr. S responded favorably to clozapine but cannot tolerate it. With a combination of two other SGAs, a patient might gain the benefits of clozapine without the need for frequent blood draws or the risk of agranulocytosis, other side effects, or interactions between clozapine and other drugs. Adding memantine was necessary to improve the catatonic features that prevented his return to the group home.
Related resources
- World Federation of Societies of Biological Psychiatry. www.wfsbp.com.
- Neuroleptic Malignant Syndrome Information Service. www.nmsis.org.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions, 2nd ed. Arlington, VA: American Psychiatric Press; 2003:1-44.
- Ungvari GS (ed). Catatonia-an anthology of classical contributions. Hong Kong: Scientific Communications International; 2006.
- Amantadine • Symmetrel
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Carbamazepine • Equetro, others
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Haloperidol • Haldol
- Levodopa/carbidopa • Sinemet
- Levothyroxine • Synthroid
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Risperidone • Risperdal
- Trazodone • Desyrel
- Valproic acid • Depakene
- Ziprasidone • Geodon
Dr. Carroll is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers-Squibb Co., Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer.
Dr. Thomas receives grant support from Pfizer and is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, and Pfizer.
Dr. Tugrul is a consultant to and speaker for Bristol Myers-Squibb Co. and Eli Lilly and Co.
Dr. Jayanti reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Acknowledgment
The authors thank Francisco José Appiani, MD, chairman, psychiatry department, Military Hospital of Campo de Mayo, Buenos Aires, Argentina, and Vijay Jayanti, BS, medical student, The Ohio State University, Columbus, for their help with this article.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:204.
2. Ungvari GS, Leung SK, Ng FS, et al. Schizophrenia with prominent catatonic features (“catatonic schizophrenia”) I. Demographic and clinical correlates in the chronic phase. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:27-38.
3. Dhossche D, Wing L, Ohta M, Neumarker K (eds). Catatonia in autism spectrum disorders. International review of neurobiology, vol. 72. San Diego: Elsevier/Academic Press; 2006.
4. Falkai P, Wobrock T, Lieberman J. WFSBP guidelines for biological treatment of schizophrenia, part 1. Acute treatment of schizophrenia. World J Biol Psychiatry 2005;6:32-91.
5. Van Dalfsen F, Van Hecke J, Van Dalfsen A, et al. The use of atypical antipsychotics in the treatment of catatonia. Eur Psychiatry 2005;20:422-9.
6. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
7. Dursun SM, Hallak JE, Haddad P, et al. Clozapine monotherapy for catatonic schizophrenia: should clozapine be the treatment of choice, with catatonia rather than psychosis as the main therapeutic index? J Psychopharmacol 2005;19:432-3.
8. White DAC, Robbins AH. An analysis of 17 catatonic patients diagnosed with neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
9. Sachdev PS. A rating scale for neuroleptic malignant syndrome. Psychiatry Res 2005;135:249-56.
10. Northoff G. Catatonia and neuroleptic malignant syndrome: psychopathology and pathophysiology. J Neural Transm 2002;109:1453-67.
11. Francis A, Chandragiri S, Rizvi S, et al. Is lorazepam a treatment for neuroleptic malignant syndrome? CNS Spectrums 2000;5:54-7.
12. Bush G, Fink M, Petrides, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
13. Carroll BT, Thomas C, Jayanti K, et al. Schizophrenia with catatonic features deserves further study. World J Biol Psychiatry 2005;6(4):267-8.
14. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: A random, double blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999;142:393-8.
15. Salokangas R, Honkonen T, Stengard E, et al. Negative symptoms and neuroleptics in catatonic schizophrenia. Schizophr Res 2003;59:73-6.
16. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press; 2003.
17. Franz M, Gallhofer B, Kanzow WT. Treatment of catatonia with intravenous biperidine. Br J Psychiatry 1994;164:847-8.
18. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
19. Thomas C, Carroll BT, Maley JT, et al. Memantine in catatonic schizophrenia. Am J Psychiatry 2005;162:656.
20. Carroll BT, Thomas C, Jayanti K. Amantadine and memantine in catatonic schizophrenia. Ann Clin Psychiatry 2006;18:133-4.
21. Carpenter SS, Hatchett AD, Fuller MA. Catatonic schizophrenia and the use of memantine. Ann Pharmacother 2006;40:344-6.
History: ‘leaving town’
Mr. S, age 58, escaped repeatedly from his group home over 4 weeks. During one episode, he removed mail from neighbors’ mailboxes and tried to direct midday traffic. He would disappear for a few hours, sometimes overnight, before returning or being brought back by police.
The patient—who has had schizophrenia with catatonic features for 30 years—offered assorted explanations for escaping, most of them based on delusional beliefs, such as “I’m leaving town to get married” or “I’m late for engineering class.”
Since his last escape 3 weeks ago, Mr. S has remained in the group home without incident but has not been reporting for his usual outpatient psychiatric care. One day, he finally presents to us at the group home sponsor’s urging.
On evaluation, Mr. S shows stereotyped speech, staring, posturing, speech-prompt mutism, and odd mannerisms such as saluting. He has not been bathing or sleeping and smiles inappropriately. He speaks only when spoken to and answers with short phrases punctuated with ”By the grace of the good Lord.”
The authors’ observations
DSM-IV-TR requires at least two features to diagnose catatonic schizophrenia:
- peculiar voluntary movements
- extreme negativism
- excessive motor activity
- echolalia or echopraxia
- motoric immobility.1
Catatonia is common among the chronic mentally ill,2 yet it often goes undiagnosed.3 As a form of psychosis, catatonia might lead to greater functional impairment if not treated.
Treatment: time to try clozapine?
Over 10 years, numerous antipsychotic regimens plus adjunctive valproic acid, 500 mg tid, or lorazepam, up to 2 mg tid, have not lessened Mr. S’ psychosis and impulsivity. We start clozapine, 400 mg/d, and order twice-monthly blood tests to check for clozapine-induced agranulocytosis.
After nearly 6 months, some catatonic features improve gradually based on clinical interview. Serum clozapine is 363 ng/mL.
poll here
The authors’ observations
Second-generation antipsychotics (SGAs) are favored over first-generation antipsychotics to treat schizophrenia with catatonic features (Table),4,5 but no drug in either class has worked for Mr. S.
ECT can alleviate catatonic schizophrenia,4,6 but this option often is not available because the clinician fears a negative outcome would prompt legal action, or the guardian or next of kin do not consent to the procedure.3 We considered referring Mr. S to an ECT provider, but he has no legal guardian to provide consent. The group home sponsor also objected to ECT because Mr. S would have been sent out of town for treatment.
Catatonia patients who are immobile, physically compromised, and refuse food and drink typically are considered ECT candidates. Mr. S eats and drinks regularly and is physically able.
Lorazepam can produce rapid response, but it can be addictive.2 Also, an adjunctive 2 mg/d dosage showed no effect.
Clozapine monotherapy has shown effectiveness in catatonic schizophrenia7 and might be an option after other antipsychotics have failed.
Table 1
Treatments for catatonia: risks and benefits
| Medication | Use | Rationale | Benefits | Risks |
|---|---|---|---|---|
| First-generation antipsychotics (FGAs) | Often used for schizophrenia | Control positive symptoms | Well-established | Catatonia might be difficult to distinguish from NMS |
| Less expensive than other medications | ||||
| Second-generation antipsychotics (SGAs) | Beneficial in catatonia | Less likely than FGAs to worsen catatonia because of low D2 blockade | Some studies suggest greater efficacy than with FGAs | Metabolic syndrome, agranulocytosis with clozapine |
| Benzodiazepines | Lorazepam helpful in acute catatonia | Can be added to any antipsychotic | Safe, first-line treatment for catatonia | Respiratory compromise, incoordination, sedation, potential for abuse |
| Electroconvulsive therapy | ||||
| Electroconvulsive therapy | Beneficial in malignant catatonia | Effective in catatonia, NMS | Useful for treatment-refractory catatonia | Concerns with anesthesia, informed consent, availability |
| Rapid onset of action | ||||
| NMS: Neuroleptic malignant syndrome | ||||
Complication: agranulocytosis, then nms
Six months after starting clozapine, Mr. S starts having diaphoresis and night sweats, suggesting neutropenia. Blood testing shows a white blood cell count (WBC) of 3.6/μL, down from 4.6/μL 2 weeks before (normal range, 4.6 to 11/μL).
One week later, Mr. S’ WBC is 1.6/μL with a 46% relative neutrophil value (normal range, 50% to 70%) and an absolute neutrophil count of 736 (normal range, 2,500 to 7,000).
We diagnose agranulocytosis and stop clozapine, but Mr. S’ WBC continues to fall over 2 weeks to 0.8/μL with a 16% relative and 128 absolute neutrophil count. After 1 more week, his WBC increases to 2.6/μL and returns to normal 1 week later—4 weeks after stopping clozapine
We then target Mr. S’ catatonia with intramuscular haloperidol, 100 mg/d for 4 weeks, and ziprasidone, 80 mg bid with food. He tolerates this combination but gradually develops tremor and rigidity. Six weeks later, we add levodopa/carbidopa, 25/250 mg bid for his movement problems.
Two weeks later, Mr. S is sweating profusely, disoriented, rigid, and febrile (104.6°F). We diagnose neuroleptic malignant syndrome (NMS), stop both antipsychotics, and admit him for treatment. We start lorazepam, 1 mg tid for catatonia; bromocriptine, 250 mg bid for rigidity; and continue levodopa/carbidopa at the same dosage. We also add dantrolene, 25 mg tid for 5 days for fever and rigidity, and provide a cooling blanket for hyperthermia.
Mr. S’ fever, autonomic changes, and diaphoresis diminish within 3 days. Rigidity and mental status improve gradually over 2 weeks. We discharge him after 10 days.
poll here
The author’s observations
Catatonia is a recognized risk factor for NMS. White and Robins8 described 17 patients with a catatonic syndrome that developed into NMS within 5 to 96 hours of starting a neuroleptic. Sachdev developed an NMS rating scale that includes catatonic symptoms.9
Northoff,10 however, associates NMS with D2 receptor blockage in the basal ganglia and relates catatonia to a frontocortical gamma-aminobutyric acid (GABA) dysfunction. Based on this theory, haloperidol—which offers a higher D2 blockade than do SGAs such as ziprasidone—might have contributed to Mr. S’ NMS.
Some evidence suggests that lorazepam—which works on gamma-aminobutyric acid ionotropic type A (GABAA) receptors—helps treat catatonia in NMS and improves rigidity, hyperthermia, and autonomic signs.11
Treatment: which agents will work?
Three weeks after his discharge, we restart ziprasidone, 40 mg bid for Mr. S’ catatonic schizophrenia. He remains free of NMS symptoms but still has mannerisms (posturing, staring, immobility, stereotypic scratching on his face).
Over 1 year, Mr. S is hospitalized repeatedly because of persistent impulsivity and delusions. He has failed numerous antipsychotic regimens lasting 1 month or longer, including olanzapine, up to 30 mg/d; quetiapine, 300 mg tid; and risperidone, 2 mg tid. Adding a first-generation antipsychotic either does not help (as with perphenazine, 12 mg/d) or diminishes his memory (as with chlorpromazine, 250 mg/d). The anticholinergic benztropine, 2 mg bid, also is ineffective.
Combination quetiapine, 300 mg/d, and the antiviral amantadine, 100 mg tid, improve Mr. S’ stereotypy at first, but his delusions intensify within 1 week. His Bush-Francis Catatonia Rating Scale scores range from 9 (indicating moderate catatonia) to 16 (persistent catatonic features).12
poll here
The authors’ observations
Catatonic schizophrenia’s pathophysiology and response to medication might differ compared with other schizophrenia forms.13 Dopamine D2 hypoactivity, glutamate N-methyl-D-aspartate (NMDA) hyperactivity, or GABAA hypoactivity are believed to cause catatonia.3,6,7 GABA agonists, anticonvulsants, dopamine agonists, SGAs, and NMDA antagonists target these pathophysiologies, but patients with a catatonia subtype often respond to only one type of medication.
Lorazepam exerts an anticatatonic effect by binding to GABAA receptors and increasing GABA activity. Lorazepam can help some patients with schizophrenia but has not shown benefit when added to an antipsychotic for chronic catatonia.6,14
SGAs can provide marked improvement in patients with catatonic schizophrenia.5
Salokangas et al15 note that “atypicals” pass more dopamine to the D2 receptor when dopamine is low in the basal ganglia. This suggests that SGAs with low D2 binding—such as clozapine, olanzapine, and quetiapine—are more beneficial than other SGAs for catatonia. Serotonin binding or other mechanisms might add to these drugs’ anticatatonic effect.7
Anticonvulsants. Adjunctive anticonvulsant therapy might alleviate catatonia by increasing GABA activity or by causing a modest antiglutaminergic effect, as reported with carbamazepine or valproic acid.16 Anticholinergics also might help treat neuroleptic-induced catatonia.17
Amantadine—FDA-approved to treat Parkinson’s disease and extrapyramidal disease—can alleviate catatonia by blocking hyperglutamatergic excitotoxicity in neurons, thus blocking NMDA receptors.18 As with Mr. S, however, amantadine can worsen psychosis by increasing dopamine release.
Memantine—an NMDA receptor antagonist indicated for moderate to severe Alzheimer’s disease—also blocks hyperglutamatergic excitotoxicity in neurons. The medication has shown effectiveness for treating catatonic schizophrenia in case reports,19-21 but 3 patients have reported memantine-induced psychosis and seizures.21
Some might argue that Mr. S’ delusions are predominant and more compelling than his catatonia, but these did not hamper his ability to live in a group home. His catatonia-related negativism, impulsivity, and inability to cooperate are what led to frequent hospitalization.
Follow-up: treatment change
We stop amantadine, add memantine, 10 mg bid, and titrate quetiapine over 2 weeks to 900 mg/d. Mr. S’ catatonia improves but some delusions persist. We add olanzapine, 7.5 mg bid, and within 2 weeks Mr. S is less delusional and more cooperative.
We discharge Mr. S on the above medications, plus:
- lorazepam, 1 mg each morning and 2 mg nightly, which he has been taking for catatonia for about 1 year
- trazodone, 150 mg bid, which we added 6 months ago to help him sleep and reduce psychomotor excitement
- ranitidine, 150 mg bid, for gastroesophageal reflux disorder
- and levothyroxine, 0.5 mg/d, for comobrid hypothyroidism. His thyroid-stimulating hormone level is normal.
We see Mr. S monthly. He is still impulsive at times, occasionally collecting his neighbors’ newspapers and mail despite instructions from group home staff not to do so. Yet his sponsors say Mr. S is “like a new person.” He talks spontaneously, interacts, and is cooperative. He has not been hospitalized for more than 1 year.
The authors’ observations
Mr. S responded favorably to clozapine but cannot tolerate it. With a combination of two other SGAs, a patient might gain the benefits of clozapine without the need for frequent blood draws or the risk of agranulocytosis, other side effects, or interactions between clozapine and other drugs. Adding memantine was necessary to improve the catatonic features that prevented his return to the group home.
Related resources
- World Federation of Societies of Biological Psychiatry. www.wfsbp.com.
- Neuroleptic Malignant Syndrome Information Service. www.nmsis.org.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions, 2nd ed. Arlington, VA: American Psychiatric Press; 2003:1-44.
- Ungvari GS (ed). Catatonia-an anthology of classical contributions. Hong Kong: Scientific Communications International; 2006.
- Amantadine • Symmetrel
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Carbamazepine • Equetro, others
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Haloperidol • Haldol
- Levodopa/carbidopa • Sinemet
- Levothyroxine • Synthroid
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Risperidone • Risperdal
- Trazodone • Desyrel
- Valproic acid • Depakene
- Ziprasidone • Geodon
Dr. Carroll is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers-Squibb Co., Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer.
Dr. Thomas receives grant support from Pfizer and is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, and Pfizer.
Dr. Tugrul is a consultant to and speaker for Bristol Myers-Squibb Co. and Eli Lilly and Co.
Dr. Jayanti reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Acknowledgment
The authors thank Francisco José Appiani, MD, chairman, psychiatry department, Military Hospital of Campo de Mayo, Buenos Aires, Argentina, and Vijay Jayanti, BS, medical student, The Ohio State University, Columbus, for their help with this article.
History: ‘leaving town’
Mr. S, age 58, escaped repeatedly from his group home over 4 weeks. During one episode, he removed mail from neighbors’ mailboxes and tried to direct midday traffic. He would disappear for a few hours, sometimes overnight, before returning or being brought back by police.
The patient—who has had schizophrenia with catatonic features for 30 years—offered assorted explanations for escaping, most of them based on delusional beliefs, such as “I’m leaving town to get married” or “I’m late for engineering class.”
Since his last escape 3 weeks ago, Mr. S has remained in the group home without incident but has not been reporting for his usual outpatient psychiatric care. One day, he finally presents to us at the group home sponsor’s urging.
On evaluation, Mr. S shows stereotyped speech, staring, posturing, speech-prompt mutism, and odd mannerisms such as saluting. He has not been bathing or sleeping and smiles inappropriately. He speaks only when spoken to and answers with short phrases punctuated with ”By the grace of the good Lord.”
The authors’ observations
DSM-IV-TR requires at least two features to diagnose catatonic schizophrenia:
- peculiar voluntary movements
- extreme negativism
- excessive motor activity
- echolalia or echopraxia
- motoric immobility.1
Catatonia is common among the chronic mentally ill,2 yet it often goes undiagnosed.3 As a form of psychosis, catatonia might lead to greater functional impairment if not treated.
Treatment: time to try clozapine?
Over 10 years, numerous antipsychotic regimens plus adjunctive valproic acid, 500 mg tid, or lorazepam, up to 2 mg tid, have not lessened Mr. S’ psychosis and impulsivity. We start clozapine, 400 mg/d, and order twice-monthly blood tests to check for clozapine-induced agranulocytosis.
After nearly 6 months, some catatonic features improve gradually based on clinical interview. Serum clozapine is 363 ng/mL.
poll here
The authors’ observations
Second-generation antipsychotics (SGAs) are favored over first-generation antipsychotics to treat schizophrenia with catatonic features (Table),4,5 but no drug in either class has worked for Mr. S.
ECT can alleviate catatonic schizophrenia,4,6 but this option often is not available because the clinician fears a negative outcome would prompt legal action, or the guardian or next of kin do not consent to the procedure.3 We considered referring Mr. S to an ECT provider, but he has no legal guardian to provide consent. The group home sponsor also objected to ECT because Mr. S would have been sent out of town for treatment.
Catatonia patients who are immobile, physically compromised, and refuse food and drink typically are considered ECT candidates. Mr. S eats and drinks regularly and is physically able.
Lorazepam can produce rapid response, but it can be addictive.2 Also, an adjunctive 2 mg/d dosage showed no effect.
Clozapine monotherapy has shown effectiveness in catatonic schizophrenia7 and might be an option after other antipsychotics have failed.
Table 1
Treatments for catatonia: risks and benefits
| Medication | Use | Rationale | Benefits | Risks |
|---|---|---|---|---|
| First-generation antipsychotics (FGAs) | Often used for schizophrenia | Control positive symptoms | Well-established | Catatonia might be difficult to distinguish from NMS |
| Less expensive than other medications | ||||
| Second-generation antipsychotics (SGAs) | Beneficial in catatonia | Less likely than FGAs to worsen catatonia because of low D2 blockade | Some studies suggest greater efficacy than with FGAs | Metabolic syndrome, agranulocytosis with clozapine |
| Benzodiazepines | Lorazepam helpful in acute catatonia | Can be added to any antipsychotic | Safe, first-line treatment for catatonia | Respiratory compromise, incoordination, sedation, potential for abuse |
| Electroconvulsive therapy | ||||
| Electroconvulsive therapy | Beneficial in malignant catatonia | Effective in catatonia, NMS | Useful for treatment-refractory catatonia | Concerns with anesthesia, informed consent, availability |
| Rapid onset of action | ||||
| NMS: Neuroleptic malignant syndrome | ||||
Complication: agranulocytosis, then nms
Six months after starting clozapine, Mr. S starts having diaphoresis and night sweats, suggesting neutropenia. Blood testing shows a white blood cell count (WBC) of 3.6/μL, down from 4.6/μL 2 weeks before (normal range, 4.6 to 11/μL).
One week later, Mr. S’ WBC is 1.6/μL with a 46% relative neutrophil value (normal range, 50% to 70%) and an absolute neutrophil count of 736 (normal range, 2,500 to 7,000).
We diagnose agranulocytosis and stop clozapine, but Mr. S’ WBC continues to fall over 2 weeks to 0.8/μL with a 16% relative and 128 absolute neutrophil count. After 1 more week, his WBC increases to 2.6/μL and returns to normal 1 week later—4 weeks after stopping clozapine
We then target Mr. S’ catatonia with intramuscular haloperidol, 100 mg/d for 4 weeks, and ziprasidone, 80 mg bid with food. He tolerates this combination but gradually develops tremor and rigidity. Six weeks later, we add levodopa/carbidopa, 25/250 mg bid for his movement problems.
Two weeks later, Mr. S is sweating profusely, disoriented, rigid, and febrile (104.6°F). We diagnose neuroleptic malignant syndrome (NMS), stop both antipsychotics, and admit him for treatment. We start lorazepam, 1 mg tid for catatonia; bromocriptine, 250 mg bid for rigidity; and continue levodopa/carbidopa at the same dosage. We also add dantrolene, 25 mg tid for 5 days for fever and rigidity, and provide a cooling blanket for hyperthermia.
Mr. S’ fever, autonomic changes, and diaphoresis diminish within 3 days. Rigidity and mental status improve gradually over 2 weeks. We discharge him after 10 days.
poll here
The author’s observations
Catatonia is a recognized risk factor for NMS. White and Robins8 described 17 patients with a catatonic syndrome that developed into NMS within 5 to 96 hours of starting a neuroleptic. Sachdev developed an NMS rating scale that includes catatonic symptoms.9
Northoff,10 however, associates NMS with D2 receptor blockage in the basal ganglia and relates catatonia to a frontocortical gamma-aminobutyric acid (GABA) dysfunction. Based on this theory, haloperidol—which offers a higher D2 blockade than do SGAs such as ziprasidone—might have contributed to Mr. S’ NMS.
Some evidence suggests that lorazepam—which works on gamma-aminobutyric acid ionotropic type A (GABAA) receptors—helps treat catatonia in NMS and improves rigidity, hyperthermia, and autonomic signs.11
Treatment: which agents will work?
Three weeks after his discharge, we restart ziprasidone, 40 mg bid for Mr. S’ catatonic schizophrenia. He remains free of NMS symptoms but still has mannerisms (posturing, staring, immobility, stereotypic scratching on his face).
Over 1 year, Mr. S is hospitalized repeatedly because of persistent impulsivity and delusions. He has failed numerous antipsychotic regimens lasting 1 month or longer, including olanzapine, up to 30 mg/d; quetiapine, 300 mg tid; and risperidone, 2 mg tid. Adding a first-generation antipsychotic either does not help (as with perphenazine, 12 mg/d) or diminishes his memory (as with chlorpromazine, 250 mg/d). The anticholinergic benztropine, 2 mg bid, also is ineffective.
Combination quetiapine, 300 mg/d, and the antiviral amantadine, 100 mg tid, improve Mr. S’ stereotypy at first, but his delusions intensify within 1 week. His Bush-Francis Catatonia Rating Scale scores range from 9 (indicating moderate catatonia) to 16 (persistent catatonic features).12
poll here
The authors’ observations
Catatonic schizophrenia’s pathophysiology and response to medication might differ compared with other schizophrenia forms.13 Dopamine D2 hypoactivity, glutamate N-methyl-D-aspartate (NMDA) hyperactivity, or GABAA hypoactivity are believed to cause catatonia.3,6,7 GABA agonists, anticonvulsants, dopamine agonists, SGAs, and NMDA antagonists target these pathophysiologies, but patients with a catatonia subtype often respond to only one type of medication.
Lorazepam exerts an anticatatonic effect by binding to GABAA receptors and increasing GABA activity. Lorazepam can help some patients with schizophrenia but has not shown benefit when added to an antipsychotic for chronic catatonia.6,14
SGAs can provide marked improvement in patients with catatonic schizophrenia.5
Salokangas et al15 note that “atypicals” pass more dopamine to the D2 receptor when dopamine is low in the basal ganglia. This suggests that SGAs with low D2 binding—such as clozapine, olanzapine, and quetiapine—are more beneficial than other SGAs for catatonia. Serotonin binding or other mechanisms might add to these drugs’ anticatatonic effect.7
Anticonvulsants. Adjunctive anticonvulsant therapy might alleviate catatonia by increasing GABA activity or by causing a modest antiglutaminergic effect, as reported with carbamazepine or valproic acid.16 Anticholinergics also might help treat neuroleptic-induced catatonia.17
Amantadine—FDA-approved to treat Parkinson’s disease and extrapyramidal disease—can alleviate catatonia by blocking hyperglutamatergic excitotoxicity in neurons, thus blocking NMDA receptors.18 As with Mr. S, however, amantadine can worsen psychosis by increasing dopamine release.
Memantine—an NMDA receptor antagonist indicated for moderate to severe Alzheimer’s disease—also blocks hyperglutamatergic excitotoxicity in neurons. The medication has shown effectiveness for treating catatonic schizophrenia in case reports,19-21 but 3 patients have reported memantine-induced psychosis and seizures.21
Some might argue that Mr. S’ delusions are predominant and more compelling than his catatonia, but these did not hamper his ability to live in a group home. His catatonia-related negativism, impulsivity, and inability to cooperate are what led to frequent hospitalization.
Follow-up: treatment change
We stop amantadine, add memantine, 10 mg bid, and titrate quetiapine over 2 weeks to 900 mg/d. Mr. S’ catatonia improves but some delusions persist. We add olanzapine, 7.5 mg bid, and within 2 weeks Mr. S is less delusional and more cooperative.
We discharge Mr. S on the above medications, plus:
- lorazepam, 1 mg each morning and 2 mg nightly, which he has been taking for catatonia for about 1 year
- trazodone, 150 mg bid, which we added 6 months ago to help him sleep and reduce psychomotor excitement
- ranitidine, 150 mg bid, for gastroesophageal reflux disorder
- and levothyroxine, 0.5 mg/d, for comobrid hypothyroidism. His thyroid-stimulating hormone level is normal.
We see Mr. S monthly. He is still impulsive at times, occasionally collecting his neighbors’ newspapers and mail despite instructions from group home staff not to do so. Yet his sponsors say Mr. S is “like a new person.” He talks spontaneously, interacts, and is cooperative. He has not been hospitalized for more than 1 year.
The authors’ observations
Mr. S responded favorably to clozapine but cannot tolerate it. With a combination of two other SGAs, a patient might gain the benefits of clozapine without the need for frequent blood draws or the risk of agranulocytosis, other side effects, or interactions between clozapine and other drugs. Adding memantine was necessary to improve the catatonic features that prevented his return to the group home.
Related resources
- World Federation of Societies of Biological Psychiatry. www.wfsbp.com.
- Neuroleptic Malignant Syndrome Information Service. www.nmsis.org.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions, 2nd ed. Arlington, VA: American Psychiatric Press; 2003:1-44.
- Ungvari GS (ed). Catatonia-an anthology of classical contributions. Hong Kong: Scientific Communications International; 2006.
- Amantadine • Symmetrel
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Carbamazepine • Equetro, others
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Haloperidol • Haldol
- Levodopa/carbidopa • Sinemet
- Levothyroxine • Synthroid
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Risperidone • Risperdal
- Trazodone • Desyrel
- Valproic acid • Depakene
- Ziprasidone • Geodon
Dr. Carroll is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers-Squibb Co., Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer.
Dr. Thomas receives grant support from Pfizer and is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, and Pfizer.
Dr. Tugrul is a consultant to and speaker for Bristol Myers-Squibb Co. and Eli Lilly and Co.
Dr. Jayanti reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Acknowledgment
The authors thank Francisco José Appiani, MD, chairman, psychiatry department, Military Hospital of Campo de Mayo, Buenos Aires, Argentina, and Vijay Jayanti, BS, medical student, The Ohio State University, Columbus, for their help with this article.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:204.
2. Ungvari GS, Leung SK, Ng FS, et al. Schizophrenia with prominent catatonic features (“catatonic schizophrenia”) I. Demographic and clinical correlates in the chronic phase. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:27-38.
3. Dhossche D, Wing L, Ohta M, Neumarker K (eds). Catatonia in autism spectrum disorders. International review of neurobiology, vol. 72. San Diego: Elsevier/Academic Press; 2006.
4. Falkai P, Wobrock T, Lieberman J. WFSBP guidelines for biological treatment of schizophrenia, part 1. Acute treatment of schizophrenia. World J Biol Psychiatry 2005;6:32-91.
5. Van Dalfsen F, Van Hecke J, Van Dalfsen A, et al. The use of atypical antipsychotics in the treatment of catatonia. Eur Psychiatry 2005;20:422-9.
6. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
7. Dursun SM, Hallak JE, Haddad P, et al. Clozapine monotherapy for catatonic schizophrenia: should clozapine be the treatment of choice, with catatonia rather than psychosis as the main therapeutic index? J Psychopharmacol 2005;19:432-3.
8. White DAC, Robbins AH. An analysis of 17 catatonic patients diagnosed with neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
9. Sachdev PS. A rating scale for neuroleptic malignant syndrome. Psychiatry Res 2005;135:249-56.
10. Northoff G. Catatonia and neuroleptic malignant syndrome: psychopathology and pathophysiology. J Neural Transm 2002;109:1453-67.
11. Francis A, Chandragiri S, Rizvi S, et al. Is lorazepam a treatment for neuroleptic malignant syndrome? CNS Spectrums 2000;5:54-7.
12. Bush G, Fink M, Petrides, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
13. Carroll BT, Thomas C, Jayanti K, et al. Schizophrenia with catatonic features deserves further study. World J Biol Psychiatry 2005;6(4):267-8.
14. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: A random, double blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999;142:393-8.
15. Salokangas R, Honkonen T, Stengard E, et al. Negative symptoms and neuroleptics in catatonic schizophrenia. Schizophr Res 2003;59:73-6.
16. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press; 2003.
17. Franz M, Gallhofer B, Kanzow WT. Treatment of catatonia with intravenous biperidine. Br J Psychiatry 1994;164:847-8.
18. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
19. Thomas C, Carroll BT, Maley JT, et al. Memantine in catatonic schizophrenia. Am J Psychiatry 2005;162:656.
20. Carroll BT, Thomas C, Jayanti K. Amantadine and memantine in catatonic schizophrenia. Ann Clin Psychiatry 2006;18:133-4.
21. Carpenter SS, Hatchett AD, Fuller MA. Catatonic schizophrenia and the use of memantine. Ann Pharmacother 2006;40:344-6.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:204.
2. Ungvari GS, Leung SK, Ng FS, et al. Schizophrenia with prominent catatonic features (“catatonic schizophrenia”) I. Demographic and clinical correlates in the chronic phase. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:27-38.
3. Dhossche D, Wing L, Ohta M, Neumarker K (eds). Catatonia in autism spectrum disorders. International review of neurobiology, vol. 72. San Diego: Elsevier/Academic Press; 2006.
4. Falkai P, Wobrock T, Lieberman J. WFSBP guidelines for biological treatment of schizophrenia, part 1. Acute treatment of schizophrenia. World J Biol Psychiatry 2005;6:32-91.
5. Van Dalfsen F, Van Hecke J, Van Dalfsen A, et al. The use of atypical antipsychotics in the treatment of catatonia. Eur Psychiatry 2005;20:422-9.
6. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
7. Dursun SM, Hallak JE, Haddad P, et al. Clozapine monotherapy for catatonic schizophrenia: should clozapine be the treatment of choice, with catatonia rather than psychosis as the main therapeutic index? J Psychopharmacol 2005;19:432-3.
8. White DAC, Robbins AH. An analysis of 17 catatonic patients diagnosed with neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
9. Sachdev PS. A rating scale for neuroleptic malignant syndrome. Psychiatry Res 2005;135:249-56.
10. Northoff G. Catatonia and neuroleptic malignant syndrome: psychopathology and pathophysiology. J Neural Transm 2002;109:1453-67.
11. Francis A, Chandragiri S, Rizvi S, et al. Is lorazepam a treatment for neuroleptic malignant syndrome? CNS Spectrums 2000;5:54-7.
12. Bush G, Fink M, Petrides, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
13. Carroll BT, Thomas C, Jayanti K, et al. Schizophrenia with catatonic features deserves further study. World J Biol Psychiatry 2005;6(4):267-8.
14. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: A random, double blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999;142:393-8.
15. Salokangas R, Honkonen T, Stengard E, et al. Negative symptoms and neuroleptics in catatonic schizophrenia. Schizophr Res 2003;59:73-6.
16. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press; 2003.
17. Franz M, Gallhofer B, Kanzow WT. Treatment of catatonia with intravenous biperidine. Br J Psychiatry 1994;164:847-8.
18. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
19. Thomas C, Carroll BT, Maley JT, et al. Memantine in catatonic schizophrenia. Am J Psychiatry 2005;162:656.
20. Carroll BT, Thomas C, Jayanti K. Amantadine and memantine in catatonic schizophrenia. Ann Clin Psychiatry 2006;18:133-4.
21. Carpenter SS, Hatchett AD, Fuller MA. Catatonic schizophrenia and the use of memantine. Ann Pharmacother 2006;40:344-6.
Making an IMPACT on late-life depression
Few depressed older adults seek help from psychiatrists. Those who receive mental health treatment most likely do so in primary care settings. Yet primary care physicians (PCPs) often are ill-equipped to effectively treat depression while managing older patients’ numerous acute and chronic medical conditions.
If depressed older patients won’t go to a psychiatrist, why not bring the psychiatrist to the patients? This article describes a clinically tested approach called project IMPACT that links psychiatrists to primary care teams and dramatically improves depression treatment in older adults.
Untreated geriatric depression
Preference for primary care. Major depression is rare in community-living older adults (1% to 3% prevalence), but:
- 5% to 10% of older primary care patients meet DSM-IV-TR criteria for major depression1
- approximately one-half of depressed older adults report a primary care visit when a mental health problem was addressed during the past year.2
- Prefer treatment in primary care instead of mental health settings
- Only 50% follow through on mental health referrals
- Only 20% improve when treated in primary care without a team approach
- Chronic medical illnesses often complicate diagnosis and treatment
- Often complain of somatic symptoms of depression
- May believe depression is a ‘normal’ part of aging
- May receive subtherapeutic antidepressant dosages because of physician concerns about side effects
Source: References 2-4
Depression is rarely the only illness an older adult is experiencing:
- 10% to 25% of adults with chronic medical illnesses such as diabetes or heart disease have major depression.
- Medical illness is associated with increased rates of depression, and depression is associated with poorer physical health.6
Depression diminishes self-care, which is key to managing chronic medical illnesses in late life. Depressed patients have higher rates of obesity and smoking. They are less likely to exercise, eat well, or adhere to complex treatment regimens with oral hypoglycemics, antihypertensives, and lipid-lowering drugs.7
Depression also substantially increases total health care costs among older adults.8
Only 8% of depressed older adults visit a mental health specialist in a given year, compared with 25% of depressed younger adults.2 Even when PCPs refer older patients to a mental health specialist, only 50% follow through (Box 1).3,4
Barriers to effective care. Depression diagnosis and treatment by PCPs has improved, but a recent survey suggests that with usual treatment:
- only 1 in 5 depressed older adults treated in a primary care practice experiences substantial improvement over 12 months
- only 1 in 10 becomes symptom-free.5
Many depressed older adults do not realize they have depression and visit their PCPs complaining of physical symptoms (Box 2).6-8 Their limited knowledge about depression or fear of being labeled “mentally ill” deters them from disclosing a depressed mood. They and their PCPs may think depression is inevitable with aging.
PCPs also may lack training to differentiate mood disorders, transitory reactions to life-events, or depression caused by medical illness. Their busy schedules limit time to address and prioritize patient concerns about acute and chronic medical problems (Box 3). Thus depression “falls through the cracks.”
Prescribing concerns. PCPs who feel uncomfortable prescribing antidepressants to older patients may be concerned about side effects and maintain dosages at low starting levels instead of titrating up to a therapeutic range.
Collaborative care
One way to overcome these barriers is to integrate mental health providers into primary care to support and augment PCP-prescribed depression treatment. Collaborative care can become an effective, efficient way to provide high-quality depression care to older patients who might otherwise go untreated.9
- Concerns about discussing a socially stigmatized condition with older adults
- Buying into the fallacy that depression is ‘normal’ in late life
- Missing the diagnosis because of medical comorbidity and older adults’ focus on physical versus emotional symptoms
- Unfamiliarity with how to prescribe antidepressants, particularly for patients with complex medical comorbidity
- Time constraints may discourage opening ‘Pandora’s box’ of depression
Project IMPACT. One such model—project IMPACT (Improving Mood: Promoting Access to Collaborative Treatment for Late-life Depression)—was developed with support from the John A. Hartford Foundation and California Healthcare Foundation. At its heart is a depression care manager or depression clinical specialist—typically a nurse, social worker, or psychologist—who works in a primary care practice. Other team members include the patient’s PCP, the patient, and a consulting psychiatrist.
The care manager works closely with the PCP by:
- educating patients about depression
- coaching patients in pleasant events scheduling/behavioral activation
- supporting the PCP’s antidepressant management
- offering patients a brief course of evidence-based counseling, such as Problem Solving Treatment in Primary Care10
- measuring patients’ depressive symptoms at treatment onset and regularly thereafter with a tool such as the 9-item depression scale of the Patient Health Questionnaire (PHQ-9).11
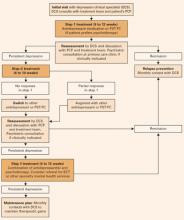
Consulting psychiatrist’s role. If a patient is not sufficiently improved after 8 to 10 weeks, the care manager works with the PCP and psychiatrist to change treatment according to an evidence-based algorithm (Figure).12 In large health care systems, the psychiatrist meets weekly with the care manager to review treatment plans for approximately 100 depressed older adults, with particular attention to those who are not improving. Psychiatrists may see patients in person or facilitate other specialty mental health treatment, as indicated.
Patients are encouraged to choose an antidepressant prescribed by their PCP, psychotherapy provided by the care manager in the primary care setting, or both. The patient and PCP make treatment decisions with support from the care manager and psychiatrist.
After depressive symptoms remit, the care manager completes a relapse prevention plan with the patient. This includes:
- steps the patient can take to prevent a relapse
- identifying relapse warning signs
- an action plan if depressive symptoms recur.
Training for clinicians in the IMPACT model is available at workshops and on the Internet (see Related resources). Successful implementation requires addressing operational and financing issues, and potential funding sources for primary care-based management have been described.13
Figure 3-step IMPACT intervention for a typical older patient with depression
PST-PC: Problem Solving Treatment in Primary Care
ECT: Electroconvulsive therapy
Source: Adapted and reprinted with permission from Unützer J, Katon WJ, Williams JW, et al. Improving primary care for depression in late life: the design of a multi-center randomized trial. Medical Care 2001;39:785.
How impact was tested
The IMPACT team care model has been tested in a randomized trial with 1,801 depressed older adults from 18 primary care clinics in 8 U.S. health care organizations. These included feefor-service plans, health maintenance organizations, and Veterans Affairs clinics with more than 450 PCPs in rural and urban settings.
Patients were randomly assigned to receive IMPACT care for 12 months or care as usual (in usual care, the patient and provider were informed that the patient met diagnostic criteria for major depression or dysthymia). Patients assigned to usual care could engage in any depression treatment the provider normally used, including referral to specialty mental health services.14
Result: Better outcomes. IMPACT was more effective than usual care for late-life depression in all 8 organizations over 2 years.15,16 Overall, IMPACT doubled the effect of usual care. IMPACT patients:
- experienced >100 additional depression-free days17
- showed substantial improvements in physical and social functioning15,17 and quality of life,15 even 12 months after IMPACT resources were withdrawn18
- experienced less pain and pain-related functional impairment19
- had significantly less suicidal ideation.20
IMPACT care also was more cost-effective than usual care for depression in older adults with and without comorbid medical illnesses.17
From research to practice
Based on the robust study outcomes, researchers received a grant from the John A. Hartford Foundation to provide materials, training, and technical assistance to organizations interested in adopting IMPACT. More than 20 health care organizations have used IMPACT, and several have completed program evaluations showing outcomes matching the original IMPACT trial’s.
Kaiser Permanente of Southern California serves 3 million members. Before adopting collaborative care for depression, Kaiser conducted a pilot study of the project IMPACT model modified to fit its health care system. Adaptations included:
- expanding the program to serve depressed adults of all ages
- adding medical assistants to the care team to help with patient follow-up
- adding a “depression class” to offer group-based patient education
- providing psychiatric consultation to the care manager and primary care providers by telephone.
Kaiser investigators compared the outcomes of 300 patients who experienced the adapted program with outcomes in 140 usual-care patients and 140 intervention patients in the original IMPACT study. The effects on depression symptoms were equal to those achieved in the original IMPACT study, with 68% of depressed older adults showing substantial improvement (at least a 50% reduction in depression symptoms) at 6 months.21
- Project IMPACT. www.impact-uw.org.
- American Association of Geriatric Psychiatry. www.aagpgpa.org.
- Positive Aging Resource Center. http://positiveaging.org.
Acknowledgment
This paper is based on a presentation by Dr. Unützer for the Distinguished Scientist Award at the American Association of Geriatric Psychiatry annual meeting in Puerto Rico, March 10, 2006.
1. Lyness JM, Caine ED, King DA, et al. Psychiatric disorders in older primary care patients. J Gen Intern Med 1999;14(4):249-54.
2. Klap R, Unroe KT, Unützer J. Caring for mental illness in the United States: a focus on older adults. Am J Geriatr Psychiatry 2003;11(5):517-24.
3. Callahan CM, Hendrie HC, Dittus RS, et al. Improving treatment of late life depression in primary care: a randomized clinical trial. J Am Geriatr Soc 1994;42(8):839-46.
4. Bartels SJ, Coakley EH, Zubritsky C, et al. Improving access to geriatric mental health services: a randomized trial comparing treatment engagement with integrated versus enhanced referral care for depression, anxiety, and at-risk alcohol use. Am J Psychiatry 2004;161(8):1455-62.
5. Crystal S, Sambamoorthi U, Walkup JT, et al. Diagnosis and treatment of depression in the elderly medicare population: predictors, disparities, and trends. J Am Geriatr Soc 2003;51(12):1718-28.
6. Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry 2003;54(3):216-26.
7. Lin EH, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care 2004;27(9):2154-60.
8. Unützer J, Patrick DL, Simon G, et al. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older. A 4-year prospective study. JAMA 1997;277(20):1618-23.
9. Oxman TE, Dietrich AJ, Schulberg HC. Evidence-based models of integrated management of depression in primary care. Psychiatr Clin North Am 2005;28(4):1061-77.
10. Hegel MT, Dietrich AJ, Seville JL, et al. Training residents in problem-solving treatment of depression: a pilot feasibility and impact study. Fam Med 2004;36(3):204-8.
11. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606-13.
12. Unützer J, Katon WJ, Williams JW, et al. Improving primary care for depression in late life: the design of a multi-center randomized trial. Medical Care 2001;39:785.-
13. Bachman J, Pincus HA, Houtsinger JK, Unützer J. Funding mechanisms for depression care management: opportunities and challenges. Gen Hosp Psychiatry 2006;28(4):278-88.
14. Unützer J, Katon W, Williams JW, Jr, et al. Improving primary care for depression in late life: the design of a multicenter randomized trial. Med Care 2001;39(8):785-99.
15. Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 2002;288(22):2836-45.
16. Unützer J, Powers D, Katon W, et al. From establishing an evidence-based practice to implementation in real-world settings: IMPACT as a case study. Psychiatr Clin North Am 2005;28(4):1079-92.
17. Katon WJ, Schoenbaum M, Fan MY, et al. Cost-effectiveness of improving primary care treatment of late-life depression. Arch Gen Psychiatry 2005;62(12):1313-20.
18. Hunkeler EM, Katon W, Tang L, et al. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ 2006;332(7536):259-63.
19. Lin EH, Katon W, Von Korff M, et al. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. JAMA 2003;290(18):2428-9.
20. Bruce ML, Ten Have TR, Reynolds CF, 3rd, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA 2004;291(9):1081-91.
21. Grypma L, Haverkamp R, Little S, et al. Taking an evidence-based model of depression care from research to practice: making lemonade out of depression. Gen Hosp Psychiatry 2006;28(2):101-7.
Few depressed older adults seek help from psychiatrists. Those who receive mental health treatment most likely do so in primary care settings. Yet primary care physicians (PCPs) often are ill-equipped to effectively treat depression while managing older patients’ numerous acute and chronic medical conditions.
If depressed older patients won’t go to a psychiatrist, why not bring the psychiatrist to the patients? This article describes a clinically tested approach called project IMPACT that links psychiatrists to primary care teams and dramatically improves depression treatment in older adults.
Untreated geriatric depression
Preference for primary care. Major depression is rare in community-living older adults (1% to 3% prevalence), but:
- 5% to 10% of older primary care patients meet DSM-IV-TR criteria for major depression1
- approximately one-half of depressed older adults report a primary care visit when a mental health problem was addressed during the past year.2
- Prefer treatment in primary care instead of mental health settings
- Only 50% follow through on mental health referrals
- Only 20% improve when treated in primary care without a team approach
- Chronic medical illnesses often complicate diagnosis and treatment
- Often complain of somatic symptoms of depression
- May believe depression is a ‘normal’ part of aging
- May receive subtherapeutic antidepressant dosages because of physician concerns about side effects
Source: References 2-4
Depression is rarely the only illness an older adult is experiencing:
- 10% to 25% of adults with chronic medical illnesses such as diabetes or heart disease have major depression.
- Medical illness is associated with increased rates of depression, and depression is associated with poorer physical health.6
Depression diminishes self-care, which is key to managing chronic medical illnesses in late life. Depressed patients have higher rates of obesity and smoking. They are less likely to exercise, eat well, or adhere to complex treatment regimens with oral hypoglycemics, antihypertensives, and lipid-lowering drugs.7
Depression also substantially increases total health care costs among older adults.8
Only 8% of depressed older adults visit a mental health specialist in a given year, compared with 25% of depressed younger adults.2 Even when PCPs refer older patients to a mental health specialist, only 50% follow through (Box 1).3,4
Barriers to effective care. Depression diagnosis and treatment by PCPs has improved, but a recent survey suggests that with usual treatment:
- only 1 in 5 depressed older adults treated in a primary care practice experiences substantial improvement over 12 months
- only 1 in 10 becomes symptom-free.5
Many depressed older adults do not realize they have depression and visit their PCPs complaining of physical symptoms (Box 2).6-8 Their limited knowledge about depression or fear of being labeled “mentally ill” deters them from disclosing a depressed mood. They and their PCPs may think depression is inevitable with aging.
PCPs also may lack training to differentiate mood disorders, transitory reactions to life-events, or depression caused by medical illness. Their busy schedules limit time to address and prioritize patient concerns about acute and chronic medical problems (Box 3). Thus depression “falls through the cracks.”
Prescribing concerns. PCPs who feel uncomfortable prescribing antidepressants to older patients may be concerned about side effects and maintain dosages at low starting levels instead of titrating up to a therapeutic range.
Collaborative care
One way to overcome these barriers is to integrate mental health providers into primary care to support and augment PCP-prescribed depression treatment. Collaborative care can become an effective, efficient way to provide high-quality depression care to older patients who might otherwise go untreated.9
- Concerns about discussing a socially stigmatized condition with older adults
- Buying into the fallacy that depression is ‘normal’ in late life
- Missing the diagnosis because of medical comorbidity and older adults’ focus on physical versus emotional symptoms
- Unfamiliarity with how to prescribe antidepressants, particularly for patients with complex medical comorbidity
- Time constraints may discourage opening ‘Pandora’s box’ of depression
Project IMPACT. One such model—project IMPACT (Improving Mood: Promoting Access to Collaborative Treatment for Late-life Depression)—was developed with support from the John A. Hartford Foundation and California Healthcare Foundation. At its heart is a depression care manager or depression clinical specialist—typically a nurse, social worker, or psychologist—who works in a primary care practice. Other team members include the patient’s PCP, the patient, and a consulting psychiatrist.
The care manager works closely with the PCP by:
- educating patients about depression
- coaching patients in pleasant events scheduling/behavioral activation
- supporting the PCP’s antidepressant management
- offering patients a brief course of evidence-based counseling, such as Problem Solving Treatment in Primary Care10
- measuring patients’ depressive symptoms at treatment onset and regularly thereafter with a tool such as the 9-item depression scale of the Patient Health Questionnaire (PHQ-9).11
Consulting psychiatrist’s role. If a patient is not sufficiently improved after 8 to 10 weeks, the care manager works with the PCP and psychiatrist to change treatment according to an evidence-based algorithm (Figure).12 In large health care systems, the psychiatrist meets weekly with the care manager to review treatment plans for approximately 100 depressed older adults, with particular attention to those who are not improving. Psychiatrists may see patients in person or facilitate other specialty mental health treatment, as indicated.
Patients are encouraged to choose an antidepressant prescribed by their PCP, psychotherapy provided by the care manager in the primary care setting, or both. The patient and PCP make treatment decisions with support from the care manager and psychiatrist.
After depressive symptoms remit, the care manager completes a relapse prevention plan with the patient. This includes:
- steps the patient can take to prevent a relapse
- identifying relapse warning signs
- an action plan if depressive symptoms recur.
Training for clinicians in the IMPACT model is available at workshops and on the Internet (see Related resources). Successful implementation requires addressing operational and financing issues, and potential funding sources for primary care-based management have been described.13
Figure 3-step IMPACT intervention for a typical older patient with depression
PST-PC: Problem Solving Treatment in Primary Care
ECT: Electroconvulsive therapy
Source: Adapted and reprinted with permission from Unützer J, Katon WJ, Williams JW, et al. Improving primary care for depression in late life: the design of a multi-center randomized trial. Medical Care 2001;39:785.
How impact was tested
The IMPACT team care model has been tested in a randomized trial with 1,801 depressed older adults from 18 primary care clinics in 8 U.S. health care organizations. These included feefor-service plans, health maintenance organizations, and Veterans Affairs clinics with more than 450 PCPs in rural and urban settings.
Patients were randomly assigned to receive IMPACT care for 12 months or care as usual (in usual care, the patient and provider were informed that the patient met diagnostic criteria for major depression or dysthymia). Patients assigned to usual care could engage in any depression treatment the provider normally used, including referral to specialty mental health services.14
Result: Better outcomes. IMPACT was more effective than usual care for late-life depression in all 8 organizations over 2 years.15,16 Overall, IMPACT doubled the effect of usual care. IMPACT patients:
- experienced >100 additional depression-free days17
- showed substantial improvements in physical and social functioning15,17 and quality of life,15 even 12 months after IMPACT resources were withdrawn18
- experienced less pain and pain-related functional impairment19
- had significantly less suicidal ideation.20
IMPACT care also was more cost-effective than usual care for depression in older adults with and without comorbid medical illnesses.17
From research to practice
Based on the robust study outcomes, researchers received a grant from the John A. Hartford Foundation to provide materials, training, and technical assistance to organizations interested in adopting IMPACT. More than 20 health care organizations have used IMPACT, and several have completed program evaluations showing outcomes matching the original IMPACT trial’s.
Kaiser Permanente of Southern California serves 3 million members. Before adopting collaborative care for depression, Kaiser conducted a pilot study of the project IMPACT model modified to fit its health care system. Adaptations included:
- expanding the program to serve depressed adults of all ages
- adding medical assistants to the care team to help with patient follow-up
- adding a “depression class” to offer group-based patient education
- providing psychiatric consultation to the care manager and primary care providers by telephone.
Kaiser investigators compared the outcomes of 300 patients who experienced the adapted program with outcomes in 140 usual-care patients and 140 intervention patients in the original IMPACT study. The effects on depression symptoms were equal to those achieved in the original IMPACT study, with 68% of depressed older adults showing substantial improvement (at least a 50% reduction in depression symptoms) at 6 months.21
- Project IMPACT. www.impact-uw.org.
- American Association of Geriatric Psychiatry. www.aagpgpa.org.
- Positive Aging Resource Center. http://positiveaging.org.
Acknowledgment
This paper is based on a presentation by Dr. Unützer for the Distinguished Scientist Award at the American Association of Geriatric Psychiatry annual meeting in Puerto Rico, March 10, 2006.
Few depressed older adults seek help from psychiatrists. Those who receive mental health treatment most likely do so in primary care settings. Yet primary care physicians (PCPs) often are ill-equipped to effectively treat depression while managing older patients’ numerous acute and chronic medical conditions.
If depressed older patients won’t go to a psychiatrist, why not bring the psychiatrist to the patients? This article describes a clinically tested approach called project IMPACT that links psychiatrists to primary care teams and dramatically improves depression treatment in older adults.
Untreated geriatric depression
Preference for primary care. Major depression is rare in community-living older adults (1% to 3% prevalence), but:
- 5% to 10% of older primary care patients meet DSM-IV-TR criteria for major depression1
- approximately one-half of depressed older adults report a primary care visit when a mental health problem was addressed during the past year.2
- Prefer treatment in primary care instead of mental health settings
- Only 50% follow through on mental health referrals
- Only 20% improve when treated in primary care without a team approach
- Chronic medical illnesses often complicate diagnosis and treatment
- Often complain of somatic symptoms of depression
- May believe depression is a ‘normal’ part of aging
- May receive subtherapeutic antidepressant dosages because of physician concerns about side effects
Source: References 2-4
Depression is rarely the only illness an older adult is experiencing:
- 10% to 25% of adults with chronic medical illnesses such as diabetes or heart disease have major depression.
- Medical illness is associated with increased rates of depression, and depression is associated with poorer physical health.6
Depression diminishes self-care, which is key to managing chronic medical illnesses in late life. Depressed patients have higher rates of obesity and smoking. They are less likely to exercise, eat well, or adhere to complex treatment regimens with oral hypoglycemics, antihypertensives, and lipid-lowering drugs.7
Depression also substantially increases total health care costs among older adults.8
Only 8% of depressed older adults visit a mental health specialist in a given year, compared with 25% of depressed younger adults.2 Even when PCPs refer older patients to a mental health specialist, only 50% follow through (Box 1).3,4
Barriers to effective care. Depression diagnosis and treatment by PCPs has improved, but a recent survey suggests that with usual treatment:
- only 1 in 5 depressed older adults treated in a primary care practice experiences substantial improvement over 12 months
- only 1 in 10 becomes symptom-free.5
Many depressed older adults do not realize they have depression and visit their PCPs complaining of physical symptoms (Box 2).6-8 Their limited knowledge about depression or fear of being labeled “mentally ill” deters them from disclosing a depressed mood. They and their PCPs may think depression is inevitable with aging.
PCPs also may lack training to differentiate mood disorders, transitory reactions to life-events, or depression caused by medical illness. Their busy schedules limit time to address and prioritize patient concerns about acute and chronic medical problems (Box 3). Thus depression “falls through the cracks.”
Prescribing concerns. PCPs who feel uncomfortable prescribing antidepressants to older patients may be concerned about side effects and maintain dosages at low starting levels instead of titrating up to a therapeutic range.
Collaborative care
One way to overcome these barriers is to integrate mental health providers into primary care to support and augment PCP-prescribed depression treatment. Collaborative care can become an effective, efficient way to provide high-quality depression care to older patients who might otherwise go untreated.9
- Concerns about discussing a socially stigmatized condition with older adults
- Buying into the fallacy that depression is ‘normal’ in late life
- Missing the diagnosis because of medical comorbidity and older adults’ focus on physical versus emotional symptoms
- Unfamiliarity with how to prescribe antidepressants, particularly for patients with complex medical comorbidity
- Time constraints may discourage opening ‘Pandora’s box’ of depression
Project IMPACT. One such model—project IMPACT (Improving Mood: Promoting Access to Collaborative Treatment for Late-life Depression)—was developed with support from the John A. Hartford Foundation and California Healthcare Foundation. At its heart is a depression care manager or depression clinical specialist—typically a nurse, social worker, or psychologist—who works in a primary care practice. Other team members include the patient’s PCP, the patient, and a consulting psychiatrist.
The care manager works closely with the PCP by:
- educating patients about depression
- coaching patients in pleasant events scheduling/behavioral activation
- supporting the PCP’s antidepressant management
- offering patients a brief course of evidence-based counseling, such as Problem Solving Treatment in Primary Care10
- measuring patients’ depressive symptoms at treatment onset and regularly thereafter with a tool such as the 9-item depression scale of the Patient Health Questionnaire (PHQ-9).11
Consulting psychiatrist’s role. If a patient is not sufficiently improved after 8 to 10 weeks, the care manager works with the PCP and psychiatrist to change treatment according to an evidence-based algorithm (Figure).12 In large health care systems, the psychiatrist meets weekly with the care manager to review treatment plans for approximately 100 depressed older adults, with particular attention to those who are not improving. Psychiatrists may see patients in person or facilitate other specialty mental health treatment, as indicated.
Patients are encouraged to choose an antidepressant prescribed by their PCP, psychotherapy provided by the care manager in the primary care setting, or both. The patient and PCP make treatment decisions with support from the care manager and psychiatrist.
After depressive symptoms remit, the care manager completes a relapse prevention plan with the patient. This includes:
- steps the patient can take to prevent a relapse
- identifying relapse warning signs
- an action plan if depressive symptoms recur.
Training for clinicians in the IMPACT model is available at workshops and on the Internet (see Related resources). Successful implementation requires addressing operational and financing issues, and potential funding sources for primary care-based management have been described.13
Figure 3-step IMPACT intervention for a typical older patient with depression
PST-PC: Problem Solving Treatment in Primary Care
ECT: Electroconvulsive therapy
Source: Adapted and reprinted with permission from Unützer J, Katon WJ, Williams JW, et al. Improving primary care for depression in late life: the design of a multi-center randomized trial. Medical Care 2001;39:785.
How impact was tested
The IMPACT team care model has been tested in a randomized trial with 1,801 depressed older adults from 18 primary care clinics in 8 U.S. health care organizations. These included feefor-service plans, health maintenance organizations, and Veterans Affairs clinics with more than 450 PCPs in rural and urban settings.
Patients were randomly assigned to receive IMPACT care for 12 months or care as usual (in usual care, the patient and provider were informed that the patient met diagnostic criteria for major depression or dysthymia). Patients assigned to usual care could engage in any depression treatment the provider normally used, including referral to specialty mental health services.14
Result: Better outcomes. IMPACT was more effective than usual care for late-life depression in all 8 organizations over 2 years.15,16 Overall, IMPACT doubled the effect of usual care. IMPACT patients:
- experienced >100 additional depression-free days17
- showed substantial improvements in physical and social functioning15,17 and quality of life,15 even 12 months after IMPACT resources were withdrawn18
- experienced less pain and pain-related functional impairment19
- had significantly less suicidal ideation.20
IMPACT care also was more cost-effective than usual care for depression in older adults with and without comorbid medical illnesses.17
From research to practice
Based on the robust study outcomes, researchers received a grant from the John A. Hartford Foundation to provide materials, training, and technical assistance to organizations interested in adopting IMPACT. More than 20 health care organizations have used IMPACT, and several have completed program evaluations showing outcomes matching the original IMPACT trial’s.
Kaiser Permanente of Southern California serves 3 million members. Before adopting collaborative care for depression, Kaiser conducted a pilot study of the project IMPACT model modified to fit its health care system. Adaptations included:
- expanding the program to serve depressed adults of all ages
- adding medical assistants to the care team to help with patient follow-up
- adding a “depression class” to offer group-based patient education
- providing psychiatric consultation to the care manager and primary care providers by telephone.
Kaiser investigators compared the outcomes of 300 patients who experienced the adapted program with outcomes in 140 usual-care patients and 140 intervention patients in the original IMPACT study. The effects on depression symptoms were equal to those achieved in the original IMPACT study, with 68% of depressed older adults showing substantial improvement (at least a 50% reduction in depression symptoms) at 6 months.21
- Project IMPACT. www.impact-uw.org.
- American Association of Geriatric Psychiatry. www.aagpgpa.org.
- Positive Aging Resource Center. http://positiveaging.org.
Acknowledgment
This paper is based on a presentation by Dr. Unützer for the Distinguished Scientist Award at the American Association of Geriatric Psychiatry annual meeting in Puerto Rico, March 10, 2006.
1. Lyness JM, Caine ED, King DA, et al. Psychiatric disorders in older primary care patients. J Gen Intern Med 1999;14(4):249-54.
2. Klap R, Unroe KT, Unützer J. Caring for mental illness in the United States: a focus on older adults. Am J Geriatr Psychiatry 2003;11(5):517-24.
3. Callahan CM, Hendrie HC, Dittus RS, et al. Improving treatment of late life depression in primary care: a randomized clinical trial. J Am Geriatr Soc 1994;42(8):839-46.
4. Bartels SJ, Coakley EH, Zubritsky C, et al. Improving access to geriatric mental health services: a randomized trial comparing treatment engagement with integrated versus enhanced referral care for depression, anxiety, and at-risk alcohol use. Am J Psychiatry 2004;161(8):1455-62.
5. Crystal S, Sambamoorthi U, Walkup JT, et al. Diagnosis and treatment of depression in the elderly medicare population: predictors, disparities, and trends. J Am Geriatr Soc 2003;51(12):1718-28.
6. Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry 2003;54(3):216-26.
7. Lin EH, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care 2004;27(9):2154-60.
8. Unützer J, Patrick DL, Simon G, et al. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older. A 4-year prospective study. JAMA 1997;277(20):1618-23.
9. Oxman TE, Dietrich AJ, Schulberg HC. Evidence-based models of integrated management of depression in primary care. Psychiatr Clin North Am 2005;28(4):1061-77.
10. Hegel MT, Dietrich AJ, Seville JL, et al. Training residents in problem-solving treatment of depression: a pilot feasibility and impact study. Fam Med 2004;36(3):204-8.
11. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606-13.
12. Unützer J, Katon WJ, Williams JW, et al. Improving primary care for depression in late life: the design of a multi-center randomized trial. Medical Care 2001;39:785.-
13. Bachman J, Pincus HA, Houtsinger JK, Unützer J. Funding mechanisms for depression care management: opportunities and challenges. Gen Hosp Psychiatry 2006;28(4):278-88.
14. Unützer J, Katon W, Williams JW, Jr, et al. Improving primary care for depression in late life: the design of a multicenter randomized trial. Med Care 2001;39(8):785-99.
15. Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 2002;288(22):2836-45.
16. Unützer J, Powers D, Katon W, et al. From establishing an evidence-based practice to implementation in real-world settings: IMPACT as a case study. Psychiatr Clin North Am 2005;28(4):1079-92.
17. Katon WJ, Schoenbaum M, Fan MY, et al. Cost-effectiveness of improving primary care treatment of late-life depression. Arch Gen Psychiatry 2005;62(12):1313-20.
18. Hunkeler EM, Katon W, Tang L, et al. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ 2006;332(7536):259-63.
19. Lin EH, Katon W, Von Korff M, et al. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. JAMA 2003;290(18):2428-9.
20. Bruce ML, Ten Have TR, Reynolds CF, 3rd, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA 2004;291(9):1081-91.
21. Grypma L, Haverkamp R, Little S, et al. Taking an evidence-based model of depression care from research to practice: making lemonade out of depression. Gen Hosp Psychiatry 2006;28(2):101-7.
1. Lyness JM, Caine ED, King DA, et al. Psychiatric disorders in older primary care patients. J Gen Intern Med 1999;14(4):249-54.
2. Klap R, Unroe KT, Unützer J. Caring for mental illness in the United States: a focus on older adults. Am J Geriatr Psychiatry 2003;11(5):517-24.
3. Callahan CM, Hendrie HC, Dittus RS, et al. Improving treatment of late life depression in primary care: a randomized clinical trial. J Am Geriatr Soc 1994;42(8):839-46.
4. Bartels SJ, Coakley EH, Zubritsky C, et al. Improving access to geriatric mental health services: a randomized trial comparing treatment engagement with integrated versus enhanced referral care for depression, anxiety, and at-risk alcohol use. Am J Psychiatry 2004;161(8):1455-62.
5. Crystal S, Sambamoorthi U, Walkup JT, et al. Diagnosis and treatment of depression in the elderly medicare population: predictors, disparities, and trends. J Am Geriatr Soc 2003;51(12):1718-28.
6. Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry 2003;54(3):216-26.
7. Lin EH, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care 2004;27(9):2154-60.
8. Unützer J, Patrick DL, Simon G, et al. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older. A 4-year prospective study. JAMA 1997;277(20):1618-23.
9. Oxman TE, Dietrich AJ, Schulberg HC. Evidence-based models of integrated management of depression in primary care. Psychiatr Clin North Am 2005;28(4):1061-77.
10. Hegel MT, Dietrich AJ, Seville JL, et al. Training residents in problem-solving treatment of depression: a pilot feasibility and impact study. Fam Med 2004;36(3):204-8.
11. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606-13.
12. Unützer J, Katon WJ, Williams JW, et al. Improving primary care for depression in late life: the design of a multi-center randomized trial. Medical Care 2001;39:785.-
13. Bachman J, Pincus HA, Houtsinger JK, Unützer J. Funding mechanisms for depression care management: opportunities and challenges. Gen Hosp Psychiatry 2006;28(4):278-88.
14. Unützer J, Katon W, Williams JW, Jr, et al. Improving primary care for depression in late life: the design of a multicenter randomized trial. Med Care 2001;39(8):785-99.
15. Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 2002;288(22):2836-45.
16. Unützer J, Powers D, Katon W, et al. From establishing an evidence-based practice to implementation in real-world settings: IMPACT as a case study. Psychiatr Clin North Am 2005;28(4):1079-92.
17. Katon WJ, Schoenbaum M, Fan MY, et al. Cost-effectiveness of improving primary care treatment of late-life depression. Arch Gen Psychiatry 2005;62(12):1313-20.
18. Hunkeler EM, Katon W, Tang L, et al. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ 2006;332(7536):259-63.
19. Lin EH, Katon W, Von Korff M, et al. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. JAMA 2003;290(18):2428-9.
20. Bruce ML, Ten Have TR, Reynolds CF, 3rd, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA 2004;291(9):1081-91.
21. Grypma L, Haverkamp R, Little S, et al. Taking an evidence-based model of depression care from research to practice: making lemonade out of depression. Gen Hosp Psychiatry 2006;28(2):101-7.
Reducing guesswork in schizophrenia treatment
The Positive and Negative Syndrome Scale (PANSS) is moving from research into clinical practice as demand grows for objective rating scales. We see the PANSS becoming a treatment and planning tool for psychiatry, just as the electrocardiogram evolved into a measure of cardiac status in medical practice.
Based on our experience in co-authoring (LA Opler) and using the PANSS, we describe how you can use it to:
- identify psychotic symptoms for targeted treatment
- predict with greater accuracy how patients will respond to the treatment you provide.
Standardized Assessments
The PANSS first gained stature in studies that established the efficacy of second-generation antipsychotics (SGAs).1-6 But its authors7 also envisioned the scale as a useful tool to help practicing clinicians treat patients with schizophrenia and other psychotic disorders.
Twenty years of experience has shown the PANSS to be a reliable and valid severity symptom scale for schizophrenia, bipolar disorder, and other serious mental illnesses. It is particularly useful to track changes in positive and negative symptoms.8
Traditionally, psychiatric evaluation has been impressionistic and subjective, but standardized tools provide a common language while introducing objective, empiric measures of clinical status. Because patients with mental disorders are treated by providers from psychiatry, psychology, social work, nursing, and other mental health disciplines, having standardized benchmarks to assess symptom severity can facilitate an integrated approach. And because the PANSS has been translated into some 40 languages and is being adopted in clinical settings worldwide, it provides a universal means of communicating information about a patient’s clinical status.
Panss Scoring System
The PANSS includes 30 items, each rated from 1 (absent) to 7 (extreme). In theory, a patient rated “absent” (or 1) on all items would receive a total score of 30, and a patient rated “extreme” (or 7) on all items would receive a total score of 210. In the real world, though, no one sees these extremes. Stable outpatients usually score 60 to 80. Inpatients’ scores rarely exceed 80 to 150, even in “treatment refractory” cases.
The 30 items are arranged as 7 positive symptom subscale items (P1-P7), 7 negative symptom subscale items (N1-N7), and 16 general psychopathology symptom items (G1-G16) (Table 1). Each item has a definition and a basis for rating. The first question you need to answer when rating a patient is whether the item is absent or present.
How it works. For example, the PANSS defines delusions as “beliefs that are unfounded, unrealistic, and idiosyncratic,” and the basis for rating is “thought content expressed during the interview and its influence on the patient’s social relations and behavior as reported from primary care workers or family.” If the definition does not apply to your patient, you rate this item 1 or absent. If the definition does apply, “anchoring points” for each level of severity are provided (Table 2), and you decide which anchoring point best describes the patient’s functioning during the interview and the preceding week.
Time required. In research, gathering informant information, conducting the interview, and generating reliable ratings takes 45 to 60 minutes. In clinical settings, if you know your patient and can function as informant and interviewer, you probably can obtain accurate ratings in 30 to 45 minutes.
Ideally, you would use the Structured Clinical Interview for the PANSS (SCI-PANSS), though clinicians who know this instrument well may prefer a less structured interview that covers all areas of inquiry. Accurate PANSS scores are easy to generate on all 30 items by combining information from the interview with information about how the patient has functioned in the past week.
PANSS ratings are not meant to be obtained after every patient contact but rather as often as needed to guide clinical treatment. For example, you might obtain a PANSS rating:
- when an inpatient is first admitted
- before starting a new medication
- weeks or months later to gauge the new treatment’s effect.
The PANSS manual—a complete individual kit costs approximately $200—or licenses to use multiple copies are available from the copyright holder, MultiHealth Systems, Inc. (see Related resources).
Table 1
Subscales of the 30-item Positive and Negative Syndrome Scale (PANSS)
| 7 Positive symptom subscale items | 7 Negative symptom subscale items |
| P1. Delusions | N1. Blunted affect |
| P2. Conceptual disorganization | N2. Emotional withdrawal |
| P3. Hallucinatory behavior | N3. Poor rapport |
| P4. Excitement | N4. Passive/apathetic social withdrawal |
| P5. Grandiosity | N5. Difficulty in abstract thinking |
| P6. Suspiciousness/persecution | N6. Lack of spontaneity and flow of conversation |
| P7. Hostility | N7. Stereotyped thinking |
| 16 General psychopathology symptoms | |
| G1. Somatic concern | G9. Unusual thought content |
| G2. Anxiety | G10. Disorientation |
| G3. Guilt feelings | G11. Poor attention |
| G4. Tension | G12. Lack of judgment and insight |
| G5. Mannerisms and posturing | G13. Disturbance of volition |
| G6. Depression | G14. Poor impulse control |
| G7. Motor retardation | G15. Preoccupation |
| G8. Uncooperativeness | G16. Active social avoidance |
Table 2
7 levels of severity on the PANSS for characterizing delusions
| Severity level (“anchoring point”) | Description of patient function |
|---|---|
| 1 - Absent | The definition does not apply |
| 2 - Minimal | Questionable pathology; the patient may be at the upper extreme of normal limits |
| 3 - Mild | Presence of one or two delusions that are vague, uncrystallized, and not tenaciously held. The delusions do not interfere with the patient’s thinking, social relations, or behavior |
| 4 - Moderate | Presence of either a kaleidoscopic array of poorly formed, unstable delusions, or a few well-formed delusions that occasionally interfere with the patient’s thinking, social relations, or behavior |
| 5 - Moderate severe | Presence of numerous well-formed delusions that are tenaciously held and occasionally interfere with the patient’s thinking, social relations, or behavior |
| 6 - Severe | Presence of a stable set of delusions that are crystallized, possibly systematized, tenaciously held, and clearly interfere with the patient’s thinking, social relations, or behavior |
| 7 - Extreme | Presence of a stable set of delusions that are either highly systematized or very numerous, and that dominate major facets of the patient’s life. This behavior frequently results in inappropriate and irresponsible action that may jeopardize the safety of the patient or others |
Gauging Symptom Severity
Treatment planning. Clinicians at the Rochester (New York) Psychiatric Center use the PANSS to assess symptom severity in inpatients with schizophrenia and other psychotic disorders.
Within 1 week of admission, patients are evaluated on the 30 items by a team of experienced PANSS raters. Symptoms identified by the PANSS become targets in individualized treatment plans. Follow-up PANSS assessments help determine if treatment has improved the selected symptoms.
Tracking patient progress. Florida State Hospital uses the PANSS to track progress of patients with serious mental illnesses. Data collected over 8 years from >19,000 PANSS assessments in a multilingual, multicultural population suggests that the PANSS:
- aids in decision making for medical and nonmedical aspects of care for individual patients
- can help determine if changes in agency prescribing practices affect patient symptom profiles and severity, one indicator of how policy and guidelines translate into patient care.9
Predicting Outcomes
The PANSS has been shown to predict course of illness and treatment response, functional outcomes (including aggression), and long-term outcomes (including deterioration). Adjusting treatments to achieve optimal PANSS scores also can help clinicians achieve remission of their patients’ psychotic symptoms (Box).11,12
Remission. Achieving and maintaining remission of schizophrenia has been hampered by a lack of specificity in existing scales. Andreasen et al11 recommend using selected items from the PANSS and other rating scales, including the Brief Psychiatric Rating Scale (BPRS), Scale for Assessment of Negative Symptoms (SANS), and Scale for Assessment of Positive Symptoms (SAPS).
Creating agreed-upon criteria will mean that clinicians will know what is meant by symptom remission, allowing for better communication and a standard to achieve.
Costs. Eventually, rating scales such as PANSS may provide “financial prognoses” to predict treatment costs over time. Mohr et al12 used PANSS scores to group 663 patients from public and private psychiatric hospitals into eight categories based on symptom severity. When each disease state was correlated with annual treatment costs, baseline assessment was a significant predictor of annualized cost as well as clinical outcome.
Functional outcomes. Steinert et al14 used the PANSS to rate 199 inpatients within 24 hours of admission into an acute psychiatric ward. After discharge, each patient was assessed retrospectively for aggressive behavior. The conceptual disorganization and hostility items from the positive sub-scale could predict violent behaviors during inpatient treatment with statistical significance.
Long-term outcomes. White et al15 assessed older schizophrenia inpatients, using the PANSS at baseline and after 1 year. The researchers looked specifically at the “activation factor”—six PANSS items including hostility, poor impulse control, excitement, uncooperativeness, poor rapport, and tension. Poor outcome and low discharge rates were directly correlated with high baseline scores on the PANSS activation factor (PANSS-AF).
Deterioration. Goetz et al16 showed that residual positive symptoms were significantly related to deteriorating course of illness, even when patients adhered to their medications. These results suggest that even subtle symptom elevations as measured by the PANSS can predict deterioration.
- The PANSS Institute. Information on how to attain, maintain, and retain high reliability as a PANSS rater. www.panss.org.
- MultiHealth Systems, Inc. (publisher and copyright holder) to purchase the Positive and Negative Syndrome Scale (PANSS). www.mhs.com.
- Opler LA, Ramirez PM, Mougios VA. Measuring outcome in serious mental illness. In: IsHak WW, Burt T, Sederer L (eds). Outcome measurement in psychiatry: a critical review. Washington, DC: American Psychiatric Press; 2002.
Dr. Lewis A. Opler receives royalties from MultiHealth Systems, Inc. on sales of the Positive and Negative Syndrome Scale (PANSS) Manual, the Structured Clinical Interview for the PANSS (SCI-PANSS), and the Informant Questionnaire for the PANSS (IQ-PANSS).
Dr. Mark G. Opler is Executive Director of The PANSS Institute.
Acknowledgement
This work was supported in part by NIMH grant K24 MH01699 (DM).
1. van Kammen DP, McEvoy JP, Targum SD, et al. A randomized, controlled, dose-ranging trial of sertindole in patients with schizophrenia. Psychopharmacology (Berl) 1996;124(1-2):168-75.
2. Weiden PJ, Simpson GM, Potkin SG, et al. Effectiveness of switching to ziprasidone for stable but symptomatic outpatients with schizophrenia. J Clin Psychiatry 2003;64(5):580-8.
3. Duggan L, Fenton M, Rathbone J, et al. Olanzapine for schizophrenia (Cochrane Review). Cochrane Database of Systematic Reviews 2005, Issue 2. Article No. CD001359.
4. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 2002;63(9):763-71.
5. Lasser R, Bossie CA, Gharabawi G, et al. Efficacy and safety of long-acting risperidone in stable patients with schizoaffective disorder. J Affect Disord 2004;83(2-3):263-75.
6. Zalsman G, Carmon E, Martin A, et al. Effectiveness, safety, and tolerability of risperidone in adolescents with schizophrenia: an open-label study. J Child Adol Psychopharmacol 2003;13(3):319-27.
7. Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) manual. Toronto, Ontario: MultiHealth Systems, Inc.; 2006.
8. Kay SR. Positive-negative symptom assessment in schizophrenia: psychometric issues and scale comparison. Psychiatr Q 1990;61(3):163-78.
9. Annis LV. Implementation of the Positive and Negative Syndrome Scale in a state psychiatric hospital: eight years of data and experience. Paper presented at: 16th Annual Conference on State Mental Health Agency Services Research, Program Evaluation and Policy, 2006.
10. Zalsman G, Posmanik S, Fisch T, et al. Psychosocial situations, quality of depression and schizophrenia in adolescents. Psychiatry Res 2003;129:149-157.
11. Andreason N, Carpenter W, Kane J, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 2005;162:441-9.
12. Mohr PE, Cheng CM, Claxton K, et al. The heterogeneity of schizophrenia in disease states. Schizophr Res 2004;71:83-95.
13. Hatta K, Nakamura H, Matsuzaki I. Acute-phase treatment in general hospitals: clinical psychopharmacologic evaluation in first-episode schizophrenia patients. Gen Hosp Psychiatry 2003;25:39-45.
14. Steinert T, Wolfle M, Gebhardt R-P. Measurement of violence during inpatient treatment and association with psychopathology. Acta Psychiatr Scand 2000;102:107-12.
15. White L, Opler L, Harvey P, et al. Activation symptoms and discharge in early chronic schizophrenia inpatients. J Nerv Ment Dis 2004;192(12):880-3.
16. Goetz D, Goetz R, Yale S, et al. Comparing early and chronic psychosis clinical characteristics. Schizophr Res 2004;70:120.-
The Positive and Negative Syndrome Scale (PANSS) is moving from research into clinical practice as demand grows for objective rating scales. We see the PANSS becoming a treatment and planning tool for psychiatry, just as the electrocardiogram evolved into a measure of cardiac status in medical practice.
Based on our experience in co-authoring (LA Opler) and using the PANSS, we describe how you can use it to:
- identify psychotic symptoms for targeted treatment
- predict with greater accuracy how patients will respond to the treatment you provide.
Standardized Assessments
The PANSS first gained stature in studies that established the efficacy of second-generation antipsychotics (SGAs).1-6 But its authors7 also envisioned the scale as a useful tool to help practicing clinicians treat patients with schizophrenia and other psychotic disorders.
Twenty years of experience has shown the PANSS to be a reliable and valid severity symptom scale for schizophrenia, bipolar disorder, and other serious mental illnesses. It is particularly useful to track changes in positive and negative symptoms.8
Traditionally, psychiatric evaluation has been impressionistic and subjective, but standardized tools provide a common language while introducing objective, empiric measures of clinical status. Because patients with mental disorders are treated by providers from psychiatry, psychology, social work, nursing, and other mental health disciplines, having standardized benchmarks to assess symptom severity can facilitate an integrated approach. And because the PANSS has been translated into some 40 languages and is being adopted in clinical settings worldwide, it provides a universal means of communicating information about a patient’s clinical status.
Panss Scoring System
The PANSS includes 30 items, each rated from 1 (absent) to 7 (extreme). In theory, a patient rated “absent” (or 1) on all items would receive a total score of 30, and a patient rated “extreme” (or 7) on all items would receive a total score of 210. In the real world, though, no one sees these extremes. Stable outpatients usually score 60 to 80. Inpatients’ scores rarely exceed 80 to 150, even in “treatment refractory” cases.
The 30 items are arranged as 7 positive symptom subscale items (P1-P7), 7 negative symptom subscale items (N1-N7), and 16 general psychopathology symptom items (G1-G16) (Table 1). Each item has a definition and a basis for rating. The first question you need to answer when rating a patient is whether the item is absent or present.
How it works. For example, the PANSS defines delusions as “beliefs that are unfounded, unrealistic, and idiosyncratic,” and the basis for rating is “thought content expressed during the interview and its influence on the patient’s social relations and behavior as reported from primary care workers or family.” If the definition does not apply to your patient, you rate this item 1 or absent. If the definition does apply, “anchoring points” for each level of severity are provided (Table 2), and you decide which anchoring point best describes the patient’s functioning during the interview and the preceding week.
Time required. In research, gathering informant information, conducting the interview, and generating reliable ratings takes 45 to 60 minutes. In clinical settings, if you know your patient and can function as informant and interviewer, you probably can obtain accurate ratings in 30 to 45 minutes.
Ideally, you would use the Structured Clinical Interview for the PANSS (SCI-PANSS), though clinicians who know this instrument well may prefer a less structured interview that covers all areas of inquiry. Accurate PANSS scores are easy to generate on all 30 items by combining information from the interview with information about how the patient has functioned in the past week.
PANSS ratings are not meant to be obtained after every patient contact but rather as often as needed to guide clinical treatment. For example, you might obtain a PANSS rating:
- when an inpatient is first admitted
- before starting a new medication
- weeks or months later to gauge the new treatment’s effect.
The PANSS manual—a complete individual kit costs approximately $200—or licenses to use multiple copies are available from the copyright holder, MultiHealth Systems, Inc. (see Related resources).
Table 1
Subscales of the 30-item Positive and Negative Syndrome Scale (PANSS)
| 7 Positive symptom subscale items | 7 Negative symptom subscale items |
| P1. Delusions | N1. Blunted affect |
| P2. Conceptual disorganization | N2. Emotional withdrawal |
| P3. Hallucinatory behavior | N3. Poor rapport |
| P4. Excitement | N4. Passive/apathetic social withdrawal |
| P5. Grandiosity | N5. Difficulty in abstract thinking |
| P6. Suspiciousness/persecution | N6. Lack of spontaneity and flow of conversation |
| P7. Hostility | N7. Stereotyped thinking |
| 16 General psychopathology symptoms | |
| G1. Somatic concern | G9. Unusual thought content |
| G2. Anxiety | G10. Disorientation |
| G3. Guilt feelings | G11. Poor attention |
| G4. Tension | G12. Lack of judgment and insight |
| G5. Mannerisms and posturing | G13. Disturbance of volition |
| G6. Depression | G14. Poor impulse control |
| G7. Motor retardation | G15. Preoccupation |
| G8. Uncooperativeness | G16. Active social avoidance |
Table 2
7 levels of severity on the PANSS for characterizing delusions
| Severity level (“anchoring point”) | Description of patient function |
|---|---|
| 1 - Absent | The definition does not apply |
| 2 - Minimal | Questionable pathology; the patient may be at the upper extreme of normal limits |
| 3 - Mild | Presence of one or two delusions that are vague, uncrystallized, and not tenaciously held. The delusions do not interfere with the patient’s thinking, social relations, or behavior |
| 4 - Moderate | Presence of either a kaleidoscopic array of poorly formed, unstable delusions, or a few well-formed delusions that occasionally interfere with the patient’s thinking, social relations, or behavior |
| 5 - Moderate severe | Presence of numerous well-formed delusions that are tenaciously held and occasionally interfere with the patient’s thinking, social relations, or behavior |
| 6 - Severe | Presence of a stable set of delusions that are crystallized, possibly systematized, tenaciously held, and clearly interfere with the patient’s thinking, social relations, or behavior |
| 7 - Extreme | Presence of a stable set of delusions that are either highly systematized or very numerous, and that dominate major facets of the patient’s life. This behavior frequently results in inappropriate and irresponsible action that may jeopardize the safety of the patient or others |
Gauging Symptom Severity
Treatment planning. Clinicians at the Rochester (New York) Psychiatric Center use the PANSS to assess symptom severity in inpatients with schizophrenia and other psychotic disorders.
Within 1 week of admission, patients are evaluated on the 30 items by a team of experienced PANSS raters. Symptoms identified by the PANSS become targets in individualized treatment plans. Follow-up PANSS assessments help determine if treatment has improved the selected symptoms.
Tracking patient progress. Florida State Hospital uses the PANSS to track progress of patients with serious mental illnesses. Data collected over 8 years from >19,000 PANSS assessments in a multilingual, multicultural population suggests that the PANSS:
- aids in decision making for medical and nonmedical aspects of care for individual patients
- can help determine if changes in agency prescribing practices affect patient symptom profiles and severity, one indicator of how policy and guidelines translate into patient care.9
Predicting Outcomes
The PANSS has been shown to predict course of illness and treatment response, functional outcomes (including aggression), and long-term outcomes (including deterioration). Adjusting treatments to achieve optimal PANSS scores also can help clinicians achieve remission of their patients’ psychotic symptoms (Box).11,12
Remission. Achieving and maintaining remission of schizophrenia has been hampered by a lack of specificity in existing scales. Andreasen et al11 recommend using selected items from the PANSS and other rating scales, including the Brief Psychiatric Rating Scale (BPRS), Scale for Assessment of Negative Symptoms (SANS), and Scale for Assessment of Positive Symptoms (SAPS).
Creating agreed-upon criteria will mean that clinicians will know what is meant by symptom remission, allowing for better communication and a standard to achieve.
Costs. Eventually, rating scales such as PANSS may provide “financial prognoses” to predict treatment costs over time. Mohr et al12 used PANSS scores to group 663 patients from public and private psychiatric hospitals into eight categories based on symptom severity. When each disease state was correlated with annual treatment costs, baseline assessment was a significant predictor of annualized cost as well as clinical outcome.
Functional outcomes. Steinert et al14 used the PANSS to rate 199 inpatients within 24 hours of admission into an acute psychiatric ward. After discharge, each patient was assessed retrospectively for aggressive behavior. The conceptual disorganization and hostility items from the positive sub-scale could predict violent behaviors during inpatient treatment with statistical significance.
Long-term outcomes. White et al15 assessed older schizophrenia inpatients, using the PANSS at baseline and after 1 year. The researchers looked specifically at the “activation factor”—six PANSS items including hostility, poor impulse control, excitement, uncooperativeness, poor rapport, and tension. Poor outcome and low discharge rates were directly correlated with high baseline scores on the PANSS activation factor (PANSS-AF).
Deterioration. Goetz et al16 showed that residual positive symptoms were significantly related to deteriorating course of illness, even when patients adhered to their medications. These results suggest that even subtle symptom elevations as measured by the PANSS can predict deterioration.
- The PANSS Institute. Information on how to attain, maintain, and retain high reliability as a PANSS rater. www.panss.org.
- MultiHealth Systems, Inc. (publisher and copyright holder) to purchase the Positive and Negative Syndrome Scale (PANSS). www.mhs.com.
- Opler LA, Ramirez PM, Mougios VA. Measuring outcome in serious mental illness. In: IsHak WW, Burt T, Sederer L (eds). Outcome measurement in psychiatry: a critical review. Washington, DC: American Psychiatric Press; 2002.
Dr. Lewis A. Opler receives royalties from MultiHealth Systems, Inc. on sales of the Positive and Negative Syndrome Scale (PANSS) Manual, the Structured Clinical Interview for the PANSS (SCI-PANSS), and the Informant Questionnaire for the PANSS (IQ-PANSS).
Dr. Mark G. Opler is Executive Director of The PANSS Institute.
Acknowledgement
This work was supported in part by NIMH grant K24 MH01699 (DM).
The Positive and Negative Syndrome Scale (PANSS) is moving from research into clinical practice as demand grows for objective rating scales. We see the PANSS becoming a treatment and planning tool for psychiatry, just as the electrocardiogram evolved into a measure of cardiac status in medical practice.
Based on our experience in co-authoring (LA Opler) and using the PANSS, we describe how you can use it to:
- identify psychotic symptoms for targeted treatment
- predict with greater accuracy how patients will respond to the treatment you provide.
Standardized Assessments
The PANSS first gained stature in studies that established the efficacy of second-generation antipsychotics (SGAs).1-6 But its authors7 also envisioned the scale as a useful tool to help practicing clinicians treat patients with schizophrenia and other psychotic disorders.
Twenty years of experience has shown the PANSS to be a reliable and valid severity symptom scale for schizophrenia, bipolar disorder, and other serious mental illnesses. It is particularly useful to track changes in positive and negative symptoms.8
Traditionally, psychiatric evaluation has been impressionistic and subjective, but standardized tools provide a common language while introducing objective, empiric measures of clinical status. Because patients with mental disorders are treated by providers from psychiatry, psychology, social work, nursing, and other mental health disciplines, having standardized benchmarks to assess symptom severity can facilitate an integrated approach. And because the PANSS has been translated into some 40 languages and is being adopted in clinical settings worldwide, it provides a universal means of communicating information about a patient’s clinical status.
Panss Scoring System
The PANSS includes 30 items, each rated from 1 (absent) to 7 (extreme). In theory, a patient rated “absent” (or 1) on all items would receive a total score of 30, and a patient rated “extreme” (or 7) on all items would receive a total score of 210. In the real world, though, no one sees these extremes. Stable outpatients usually score 60 to 80. Inpatients’ scores rarely exceed 80 to 150, even in “treatment refractory” cases.
The 30 items are arranged as 7 positive symptom subscale items (P1-P7), 7 negative symptom subscale items (N1-N7), and 16 general psychopathology symptom items (G1-G16) (Table 1). Each item has a definition and a basis for rating. The first question you need to answer when rating a patient is whether the item is absent or present.
How it works. For example, the PANSS defines delusions as “beliefs that are unfounded, unrealistic, and idiosyncratic,” and the basis for rating is “thought content expressed during the interview and its influence on the patient’s social relations and behavior as reported from primary care workers or family.” If the definition does not apply to your patient, you rate this item 1 or absent. If the definition does apply, “anchoring points” for each level of severity are provided (Table 2), and you decide which anchoring point best describes the patient’s functioning during the interview and the preceding week.
Time required. In research, gathering informant information, conducting the interview, and generating reliable ratings takes 45 to 60 minutes. In clinical settings, if you know your patient and can function as informant and interviewer, you probably can obtain accurate ratings in 30 to 45 minutes.
Ideally, you would use the Structured Clinical Interview for the PANSS (SCI-PANSS), though clinicians who know this instrument well may prefer a less structured interview that covers all areas of inquiry. Accurate PANSS scores are easy to generate on all 30 items by combining information from the interview with information about how the patient has functioned in the past week.
PANSS ratings are not meant to be obtained after every patient contact but rather as often as needed to guide clinical treatment. For example, you might obtain a PANSS rating:
- when an inpatient is first admitted
- before starting a new medication
- weeks or months later to gauge the new treatment’s effect.
The PANSS manual—a complete individual kit costs approximately $200—or licenses to use multiple copies are available from the copyright holder, MultiHealth Systems, Inc. (see Related resources).
Table 1
Subscales of the 30-item Positive and Negative Syndrome Scale (PANSS)
| 7 Positive symptom subscale items | 7 Negative symptom subscale items |
| P1. Delusions | N1. Blunted affect |
| P2. Conceptual disorganization | N2. Emotional withdrawal |
| P3. Hallucinatory behavior | N3. Poor rapport |
| P4. Excitement | N4. Passive/apathetic social withdrawal |
| P5. Grandiosity | N5. Difficulty in abstract thinking |
| P6. Suspiciousness/persecution | N6. Lack of spontaneity and flow of conversation |
| P7. Hostility | N7. Stereotyped thinking |
| 16 General psychopathology symptoms | |
| G1. Somatic concern | G9. Unusual thought content |
| G2. Anxiety | G10. Disorientation |
| G3. Guilt feelings | G11. Poor attention |
| G4. Tension | G12. Lack of judgment and insight |
| G5. Mannerisms and posturing | G13. Disturbance of volition |
| G6. Depression | G14. Poor impulse control |
| G7. Motor retardation | G15. Preoccupation |
| G8. Uncooperativeness | G16. Active social avoidance |
Table 2
7 levels of severity on the PANSS for characterizing delusions
| Severity level (“anchoring point”) | Description of patient function |
|---|---|
| 1 - Absent | The definition does not apply |
| 2 - Minimal | Questionable pathology; the patient may be at the upper extreme of normal limits |
| 3 - Mild | Presence of one or two delusions that are vague, uncrystallized, and not tenaciously held. The delusions do not interfere with the patient’s thinking, social relations, or behavior |
| 4 - Moderate | Presence of either a kaleidoscopic array of poorly formed, unstable delusions, or a few well-formed delusions that occasionally interfere with the patient’s thinking, social relations, or behavior |
| 5 - Moderate severe | Presence of numerous well-formed delusions that are tenaciously held and occasionally interfere with the patient’s thinking, social relations, or behavior |
| 6 - Severe | Presence of a stable set of delusions that are crystallized, possibly systematized, tenaciously held, and clearly interfere with the patient’s thinking, social relations, or behavior |
| 7 - Extreme | Presence of a stable set of delusions that are either highly systematized or very numerous, and that dominate major facets of the patient’s life. This behavior frequently results in inappropriate and irresponsible action that may jeopardize the safety of the patient or others |
Gauging Symptom Severity
Treatment planning. Clinicians at the Rochester (New York) Psychiatric Center use the PANSS to assess symptom severity in inpatients with schizophrenia and other psychotic disorders.
Within 1 week of admission, patients are evaluated on the 30 items by a team of experienced PANSS raters. Symptoms identified by the PANSS become targets in individualized treatment plans. Follow-up PANSS assessments help determine if treatment has improved the selected symptoms.
Tracking patient progress. Florida State Hospital uses the PANSS to track progress of patients with serious mental illnesses. Data collected over 8 years from >19,000 PANSS assessments in a multilingual, multicultural population suggests that the PANSS:
- aids in decision making for medical and nonmedical aspects of care for individual patients
- can help determine if changes in agency prescribing practices affect patient symptom profiles and severity, one indicator of how policy and guidelines translate into patient care.9
Predicting Outcomes
The PANSS has been shown to predict course of illness and treatment response, functional outcomes (including aggression), and long-term outcomes (including deterioration). Adjusting treatments to achieve optimal PANSS scores also can help clinicians achieve remission of their patients’ psychotic symptoms (Box).11,12
Remission. Achieving and maintaining remission of schizophrenia has been hampered by a lack of specificity in existing scales. Andreasen et al11 recommend using selected items from the PANSS and other rating scales, including the Brief Psychiatric Rating Scale (BPRS), Scale for Assessment of Negative Symptoms (SANS), and Scale for Assessment of Positive Symptoms (SAPS).
Creating agreed-upon criteria will mean that clinicians will know what is meant by symptom remission, allowing for better communication and a standard to achieve.
Costs. Eventually, rating scales such as PANSS may provide “financial prognoses” to predict treatment costs over time. Mohr et al12 used PANSS scores to group 663 patients from public and private psychiatric hospitals into eight categories based on symptom severity. When each disease state was correlated with annual treatment costs, baseline assessment was a significant predictor of annualized cost as well as clinical outcome.
Functional outcomes. Steinert et al14 used the PANSS to rate 199 inpatients within 24 hours of admission into an acute psychiatric ward. After discharge, each patient was assessed retrospectively for aggressive behavior. The conceptual disorganization and hostility items from the positive sub-scale could predict violent behaviors during inpatient treatment with statistical significance.
Long-term outcomes. White et al15 assessed older schizophrenia inpatients, using the PANSS at baseline and after 1 year. The researchers looked specifically at the “activation factor”—six PANSS items including hostility, poor impulse control, excitement, uncooperativeness, poor rapport, and tension. Poor outcome and low discharge rates were directly correlated with high baseline scores on the PANSS activation factor (PANSS-AF).
Deterioration. Goetz et al16 showed that residual positive symptoms were significantly related to deteriorating course of illness, even when patients adhered to their medications. These results suggest that even subtle symptom elevations as measured by the PANSS can predict deterioration.
- The PANSS Institute. Information on how to attain, maintain, and retain high reliability as a PANSS rater. www.panss.org.
- MultiHealth Systems, Inc. (publisher and copyright holder) to purchase the Positive and Negative Syndrome Scale (PANSS). www.mhs.com.
- Opler LA, Ramirez PM, Mougios VA. Measuring outcome in serious mental illness. In: IsHak WW, Burt T, Sederer L (eds). Outcome measurement in psychiatry: a critical review. Washington, DC: American Psychiatric Press; 2002.
Dr. Lewis A. Opler receives royalties from MultiHealth Systems, Inc. on sales of the Positive and Negative Syndrome Scale (PANSS) Manual, the Structured Clinical Interview for the PANSS (SCI-PANSS), and the Informant Questionnaire for the PANSS (IQ-PANSS).
Dr. Mark G. Opler is Executive Director of The PANSS Institute.
Acknowledgement
This work was supported in part by NIMH grant K24 MH01699 (DM).
1. van Kammen DP, McEvoy JP, Targum SD, et al. A randomized, controlled, dose-ranging trial of sertindole in patients with schizophrenia. Psychopharmacology (Berl) 1996;124(1-2):168-75.
2. Weiden PJ, Simpson GM, Potkin SG, et al. Effectiveness of switching to ziprasidone for stable but symptomatic outpatients with schizophrenia. J Clin Psychiatry 2003;64(5):580-8.
3. Duggan L, Fenton M, Rathbone J, et al. Olanzapine for schizophrenia (Cochrane Review). Cochrane Database of Systematic Reviews 2005, Issue 2. Article No. CD001359.
4. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 2002;63(9):763-71.
5. Lasser R, Bossie CA, Gharabawi G, et al. Efficacy and safety of long-acting risperidone in stable patients with schizoaffective disorder. J Affect Disord 2004;83(2-3):263-75.
6. Zalsman G, Carmon E, Martin A, et al. Effectiveness, safety, and tolerability of risperidone in adolescents with schizophrenia: an open-label study. J Child Adol Psychopharmacol 2003;13(3):319-27.
7. Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) manual. Toronto, Ontario: MultiHealth Systems, Inc.; 2006.
8. Kay SR. Positive-negative symptom assessment in schizophrenia: psychometric issues and scale comparison. Psychiatr Q 1990;61(3):163-78.
9. Annis LV. Implementation of the Positive and Negative Syndrome Scale in a state psychiatric hospital: eight years of data and experience. Paper presented at: 16th Annual Conference on State Mental Health Agency Services Research, Program Evaluation and Policy, 2006.
10. Zalsman G, Posmanik S, Fisch T, et al. Psychosocial situations, quality of depression and schizophrenia in adolescents. Psychiatry Res 2003;129:149-157.
11. Andreason N, Carpenter W, Kane J, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 2005;162:441-9.
12. Mohr PE, Cheng CM, Claxton K, et al. The heterogeneity of schizophrenia in disease states. Schizophr Res 2004;71:83-95.
13. Hatta K, Nakamura H, Matsuzaki I. Acute-phase treatment in general hospitals: clinical psychopharmacologic evaluation in first-episode schizophrenia patients. Gen Hosp Psychiatry 2003;25:39-45.
14. Steinert T, Wolfle M, Gebhardt R-P. Measurement of violence during inpatient treatment and association with psychopathology. Acta Psychiatr Scand 2000;102:107-12.
15. White L, Opler L, Harvey P, et al. Activation symptoms and discharge in early chronic schizophrenia inpatients. J Nerv Ment Dis 2004;192(12):880-3.
16. Goetz D, Goetz R, Yale S, et al. Comparing early and chronic psychosis clinical characteristics. Schizophr Res 2004;70:120.-
1. van Kammen DP, McEvoy JP, Targum SD, et al. A randomized, controlled, dose-ranging trial of sertindole in patients with schizophrenia. Psychopharmacology (Berl) 1996;124(1-2):168-75.
2. Weiden PJ, Simpson GM, Potkin SG, et al. Effectiveness of switching to ziprasidone for stable but symptomatic outpatients with schizophrenia. J Clin Psychiatry 2003;64(5):580-8.
3. Duggan L, Fenton M, Rathbone J, et al. Olanzapine for schizophrenia (Cochrane Review). Cochrane Database of Systematic Reviews 2005, Issue 2. Article No. CD001359.
4. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 2002;63(9):763-71.
5. Lasser R, Bossie CA, Gharabawi G, et al. Efficacy and safety of long-acting risperidone in stable patients with schizoaffective disorder. J Affect Disord 2004;83(2-3):263-75.
6. Zalsman G, Carmon E, Martin A, et al. Effectiveness, safety, and tolerability of risperidone in adolescents with schizophrenia: an open-label study. J Child Adol Psychopharmacol 2003;13(3):319-27.
7. Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) manual. Toronto, Ontario: MultiHealth Systems, Inc.; 2006.
8. Kay SR. Positive-negative symptom assessment in schizophrenia: psychometric issues and scale comparison. Psychiatr Q 1990;61(3):163-78.
9. Annis LV. Implementation of the Positive and Negative Syndrome Scale in a state psychiatric hospital: eight years of data and experience. Paper presented at: 16th Annual Conference on State Mental Health Agency Services Research, Program Evaluation and Policy, 2006.
10. Zalsman G, Posmanik S, Fisch T, et al. Psychosocial situations, quality of depression and schizophrenia in adolescents. Psychiatry Res 2003;129:149-157.
11. Andreason N, Carpenter W, Kane J, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 2005;162:441-9.
12. Mohr PE, Cheng CM, Claxton K, et al. The heterogeneity of schizophrenia in disease states. Schizophr Res 2004;71:83-95.
13. Hatta K, Nakamura H, Matsuzaki I. Acute-phase treatment in general hospitals: clinical psychopharmacologic evaluation in first-episode schizophrenia patients. Gen Hosp Psychiatry 2003;25:39-45.
14. Steinert T, Wolfle M, Gebhardt R-P. Measurement of violence during inpatient treatment and association with psychopathology. Acta Psychiatr Scand 2000;102:107-12.
15. White L, Opler L, Harvey P, et al. Activation symptoms and discharge in early chronic schizophrenia inpatients. J Nerv Ment Dis 2004;192(12):880-3.
16. Goetz D, Goetz R, Yale S, et al. Comparing early and chronic psychosis clinical characteristics. Schizophr Res 2004;70:120.-
5 questions guide care of patients with psychosis
Keeping track of outpatients with psychosis can be challenging when multiple clinicians provide care and social services are disjointed. To ensure continuity of care and achieve optimal functional outcome, answer these five questions periodically during long-term treatment.
1. Are you acquainted with the patient’s primary care physician? If not, introduce yourself by e-mail, letter, or telephone.
For some patients, you are the primary physician by default, so know some medical monitoring guidelines such as the Mount Sinai Consensus Conference Recommendations for Physical Health Monitoring in Schizophrenia.1 Also encourage the patient to exercise, stop smoking, and lose weight, if necessary.2
2. When did you last request a written consultation from a specialist? Documenting consultations helps other clinicians follow the steps you took to care for the patient. Requesting and preparing the written consultation also forces you and the consultant to think about the exact nature of the patient’s problem.
3. How strong is the patient’s social network? To identify who can help with the patient’s care, draw a schematic of his or her family tree. Determine who lives with the patient or lives nearby and regularly sees him or her. Repeat this exercise every year because family networks change.
4. Do you have data regarding the patient’s complaints? Get as much information as possible to determine a cause. For example, if the patient has:
- Insomnia—Have the patient or caregiver complete a sleep log and count the number of caffeinated drinks and cigarettes the patient consumes daily.
- Overweight/obesity—Weigh the patient at each visit and suggest that the patient or caregiver keep a food diary.
- Worsening psychosis—Use a psychopathology rating scale such as the Brief Psychiatric Rating Scale. Count pills, and order antipsychotic blood levels and urine drug screens if necessary to test for medication non-adherence or unrecognized drug use.
- Possible cognitive problems—Order neuropsychological tests, particularly to assess memory, attention, and executive function.
5. How much progress can you expect in 1 year? To help you set feasible rehabilitation goals, make a schedule of a typical week in the patient’s life. This schedule will suggest how to engage the patient in work, family, and the community. Have the patient bring in a resume or work history as a starting point for discussion. Repeat this exercise annually.
Reference
1. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004;16:1334-49.
2. Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia; guidelines for psychiatrists. J Clin Psychiatry 2005;66:183-94.
Dr. Freudenreich is director, first episode and early psychosis program, Massachusetts General Hospital, Boston, MA.
Dr. Querques is an assistant in psychiatry, Massachusetts General Hospital.
Dr. Kontos is associate director consultationliaison psychiatry, Cambridge Health Alliance, Cambridge, MA.
Keeping track of outpatients with psychosis can be challenging when multiple clinicians provide care and social services are disjointed. To ensure continuity of care and achieve optimal functional outcome, answer these five questions periodically during long-term treatment.
1. Are you acquainted with the patient’s primary care physician? If not, introduce yourself by e-mail, letter, or telephone.
For some patients, you are the primary physician by default, so know some medical monitoring guidelines such as the Mount Sinai Consensus Conference Recommendations for Physical Health Monitoring in Schizophrenia.1 Also encourage the patient to exercise, stop smoking, and lose weight, if necessary.2
2. When did you last request a written consultation from a specialist? Documenting consultations helps other clinicians follow the steps you took to care for the patient. Requesting and preparing the written consultation also forces you and the consultant to think about the exact nature of the patient’s problem.
3. How strong is the patient’s social network? To identify who can help with the patient’s care, draw a schematic of his or her family tree. Determine who lives with the patient or lives nearby and regularly sees him or her. Repeat this exercise every year because family networks change.
4. Do you have data regarding the patient’s complaints? Get as much information as possible to determine a cause. For example, if the patient has:
- Insomnia—Have the patient or caregiver complete a sleep log and count the number of caffeinated drinks and cigarettes the patient consumes daily.
- Overweight/obesity—Weigh the patient at each visit and suggest that the patient or caregiver keep a food diary.
- Worsening psychosis—Use a psychopathology rating scale such as the Brief Psychiatric Rating Scale. Count pills, and order antipsychotic blood levels and urine drug screens if necessary to test for medication non-adherence or unrecognized drug use.
- Possible cognitive problems—Order neuropsychological tests, particularly to assess memory, attention, and executive function.
5. How much progress can you expect in 1 year? To help you set feasible rehabilitation goals, make a schedule of a typical week in the patient’s life. This schedule will suggest how to engage the patient in work, family, and the community. Have the patient bring in a resume or work history as a starting point for discussion. Repeat this exercise annually.
Keeping track of outpatients with psychosis can be challenging when multiple clinicians provide care and social services are disjointed. To ensure continuity of care and achieve optimal functional outcome, answer these five questions periodically during long-term treatment.
1. Are you acquainted with the patient’s primary care physician? If not, introduce yourself by e-mail, letter, or telephone.
For some patients, you are the primary physician by default, so know some medical monitoring guidelines such as the Mount Sinai Consensus Conference Recommendations for Physical Health Monitoring in Schizophrenia.1 Also encourage the patient to exercise, stop smoking, and lose weight, if necessary.2
2. When did you last request a written consultation from a specialist? Documenting consultations helps other clinicians follow the steps you took to care for the patient. Requesting and preparing the written consultation also forces you and the consultant to think about the exact nature of the patient’s problem.
3. How strong is the patient’s social network? To identify who can help with the patient’s care, draw a schematic of his or her family tree. Determine who lives with the patient or lives nearby and regularly sees him or her. Repeat this exercise every year because family networks change.
4. Do you have data regarding the patient’s complaints? Get as much information as possible to determine a cause. For example, if the patient has:
- Insomnia—Have the patient or caregiver complete a sleep log and count the number of caffeinated drinks and cigarettes the patient consumes daily.
- Overweight/obesity—Weigh the patient at each visit and suggest that the patient or caregiver keep a food diary.
- Worsening psychosis—Use a psychopathology rating scale such as the Brief Psychiatric Rating Scale. Count pills, and order antipsychotic blood levels and urine drug screens if necessary to test for medication non-adherence or unrecognized drug use.
- Possible cognitive problems—Order neuropsychological tests, particularly to assess memory, attention, and executive function.
5. How much progress can you expect in 1 year? To help you set feasible rehabilitation goals, make a schedule of a typical week in the patient’s life. This schedule will suggest how to engage the patient in work, family, and the community. Have the patient bring in a resume or work history as a starting point for discussion. Repeat this exercise annually.
Reference
1. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004;16:1334-49.
2. Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia; guidelines for psychiatrists. J Clin Psychiatry 2005;66:183-94.
Dr. Freudenreich is director, first episode and early psychosis program, Massachusetts General Hospital, Boston, MA.
Dr. Querques is an assistant in psychiatry, Massachusetts General Hospital.
Dr. Kontos is associate director consultationliaison psychiatry, Cambridge Health Alliance, Cambridge, MA.
Reference
1. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004;16:1334-49.
2. Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia; guidelines for psychiatrists. J Clin Psychiatry 2005;66:183-94.
Dr. Freudenreich is director, first episode and early psychosis program, Massachusetts General Hospital, Boston, MA.
Dr. Querques is an assistant in psychiatry, Massachusetts General Hospital.
Dr. Kontos is associate director consultationliaison psychiatry, Cambridge Health Alliance, Cambridge, MA.
‘WEED’ out false-positive urine drug screens
Numerous medications and other substances can appear in a urine drug screen (UDS) as an illicit narcotic (Table). These false positives can:
- lead to incorrect diagnosis and inappropriate intervention, particularly if the result determines treatment
- endanger the therapeutic alliance by making the patient uncomfortable and defensive.
Table
Substances that may trigger a false urinary drug screen result
| Prescription drugs | Nonprescription drugs | Could appear in urinary drug screen as |
|---|---|---|
| Amphetamines | Nasal decongestants | Amphetamines |
| Methamphetamines | MDMA | |
| Bupropion | Pseudoephedrine | |
| Fluoxetine | ||
| Ranitidine | ||
| Trazodone | ||
| Nefazodone | ||
| Diazepam | None | Alcohol |
| Sertraline | None | Benzodiazepines |
| Oxaprozin | ||
| Amoxicillin | NSAIDs | Cocaine |
| Most antibiotics | ||
| MS Contin (false negative) | Poppy seeds | Heroin (morphine) |
| Quinolones | ||
| Rifampin | (6-Acetylmorphine) | |
| Codeine | ||
| Oxycodone (false negative) | ||
| Dronabinol | Visine eye drops (false negative) | Marijuana |
| Pantoprazole | Hemp seeds (false negative) | |
| Diazepam (false negative) | Nyquil | Methadone |
| Dextromethorphan | PCP | |
| Source: References 1,4-6 | ||
Why Drug Screens Are Sometimes Wrong
A UDS for recreational drug use is commonly performed when the patient presents to the ER with acute changes in mental or behavioral status.
Ms. A, age 57, presents to the ER with fluctuating consciousness. The cause is unknown.
Surgical removal of a pituitary tumor 39 years earlier caused hormone deficiencies, seizures, and excessive sleepiness. Symptoms of panhypopituitarism have been managed with medication, and her current regimen includes thyroxine, phenytoin, the proton pump inhibitor pantoprazole, and prednisone. Recently, comorbid depression caused her to skip doses.
ER physicians order a UDS because of Ms. A’s mental status changes. The enzyme-linked immunosorbent (ELISA) toxicology test for alcohol, amphetamines, barbiturates, benzodiazepines, cocaine, opiates, marijuana, and phencyclidine (PCP) is positive for marijuana. When the attending psychiatrist informs Ms. A of the result, she is shocked. She tells the psychiatrist she is active in church and opposes recreational use of narcotics. She adamantly denies using marijuana or other street drugs, alcohol, nicotine, or caffeine.
Eventually, physicians attributed Ms. A’s mental status changes to several underlying medical issues, including Addison’s disease. A thorough review of the case revealed that the proton pump inhibitor pantoprazole caused the false-positive UDS.
UDS tests are sensitive but not highly specific. A medication or other substance with a chemical structure similar to that of the suspected drug can cause a false positive.1-3
The “WEED” mnemonic spells out steps for critically evaluating UDS test results to ensure appropriate care:
- Write out a list of the patient’s medications. This list may explain the symptoms or help interpret UDS results. If a narcotic dose was recently increased, for example, a UDS might not be needed to confirm what caused the change in mental status.
- Examine the patient carefully. Evaluate physical signs, take a thorough medical history, and consider the potential for drug use. Although not impossible, for example, PCP intoxication is not a likely cause of psychosis in nursing home patients.
- Equate UDS results with presenting complaints and symptoms. For example, if a patient with sudden syncope tests positive for marijuana, the syncopal symptoms demand further investigation because marijuana is not the likely cause.
- Duplicate the UDS screen with confirmatory tests if the result will determine treatment. When UDS results are ambiguous, use highly specific tests such as gas chromatography with mass spectrometry and high-performance liquid chromatography. Although expensive and time consuming, these tests confirm the presence or absence of substances with few false results.
1. Casavant MJ. Urine drug screening in adolescents. Pediatr Clin North Am 2002;49(2):317-27.
2. Baden LR, Horowitz G, Jacoby H, Eliopoulos GM. Quinolones and false-positive urine screening for opiates by immunoassay technology. JAMA 2001;286:3115-9.
3. Fraser AD, Howell P. Oxaprozin cross-reactivity in three commercial immunoassays for benzodiazepines in urine. J Anal Toxicol 1998;22:50-4.
4. Pearson SD, Ash KO, Urry FM. Mechanism of false-negative urine cannabinoid immunoassay screens by Visine eyedrops. Clin Chem 1989;35:636-8.
5. The Merck Index: An encyclopedia of chemicals, drugs, and biologicals. Whitehouse Station, NJ: Merck Research Laboratories; 2001.
6. Baselt RC. Disposition of toxic drugs and chemicals in man. Foster City, CA: Chemical Toxicology Institute; 2000.
Dr. Rapuri is a family practice resident, Memorial Medical Center, Johnstown, PA, and clinical attache, VA Medical Center, Omaha, NE.
Dr. Ramaswamy is an instructor, Creighton University, and staff psychiatrist, VA Medical Center, Omaha, NE.
Dr. Madaan is a fellow in child and adolescent psychiatry, Creighton University, Omaha, NE.
Dr. Rasimas is chief resident, department of psychiatry and psychology, Mayo Clinic, Rochester, MN.
Dr. Krahn is deputy editor, Current Psychiatry, and chair, psychiatry department, Mayo Clinic, Scottsdale, AZ
Numerous medications and other substances can appear in a urine drug screen (UDS) as an illicit narcotic (Table). These false positives can:
- lead to incorrect diagnosis and inappropriate intervention, particularly if the result determines treatment
- endanger the therapeutic alliance by making the patient uncomfortable and defensive.
Table
Substances that may trigger a false urinary drug screen result
| Prescription drugs | Nonprescription drugs | Could appear in urinary drug screen as |
|---|---|---|
| Amphetamines | Nasal decongestants | Amphetamines |
| Methamphetamines | MDMA | |
| Bupropion | Pseudoephedrine | |
| Fluoxetine | ||
| Ranitidine | ||
| Trazodone | ||
| Nefazodone | ||
| Diazepam | None | Alcohol |
| Sertraline | None | Benzodiazepines |
| Oxaprozin | ||
| Amoxicillin | NSAIDs | Cocaine |
| Most antibiotics | ||
| MS Contin (false negative) | Poppy seeds | Heroin (morphine) |
| Quinolones | ||
| Rifampin | (6-Acetylmorphine) | |
| Codeine | ||
| Oxycodone (false negative) | ||
| Dronabinol | Visine eye drops (false negative) | Marijuana |
| Pantoprazole | Hemp seeds (false negative) | |
| Diazepam (false negative) | Nyquil | Methadone |
| Dextromethorphan | PCP | |
| Source: References 1,4-6 | ||
Why Drug Screens Are Sometimes Wrong
A UDS for recreational drug use is commonly performed when the patient presents to the ER with acute changes in mental or behavioral status.
Ms. A, age 57, presents to the ER with fluctuating consciousness. The cause is unknown.
Surgical removal of a pituitary tumor 39 years earlier caused hormone deficiencies, seizures, and excessive sleepiness. Symptoms of panhypopituitarism have been managed with medication, and her current regimen includes thyroxine, phenytoin, the proton pump inhibitor pantoprazole, and prednisone. Recently, comorbid depression caused her to skip doses.
ER physicians order a UDS because of Ms. A’s mental status changes. The enzyme-linked immunosorbent (ELISA) toxicology test for alcohol, amphetamines, barbiturates, benzodiazepines, cocaine, opiates, marijuana, and phencyclidine (PCP) is positive for marijuana. When the attending psychiatrist informs Ms. A of the result, she is shocked. She tells the psychiatrist she is active in church and opposes recreational use of narcotics. She adamantly denies using marijuana or other street drugs, alcohol, nicotine, or caffeine.
Eventually, physicians attributed Ms. A’s mental status changes to several underlying medical issues, including Addison’s disease. A thorough review of the case revealed that the proton pump inhibitor pantoprazole caused the false-positive UDS.
UDS tests are sensitive but not highly specific. A medication or other substance with a chemical structure similar to that of the suspected drug can cause a false positive.1-3
The “WEED” mnemonic spells out steps for critically evaluating UDS test results to ensure appropriate care:
- Write out a list of the patient’s medications. This list may explain the symptoms or help interpret UDS results. If a narcotic dose was recently increased, for example, a UDS might not be needed to confirm what caused the change in mental status.
- Examine the patient carefully. Evaluate physical signs, take a thorough medical history, and consider the potential for drug use. Although not impossible, for example, PCP intoxication is not a likely cause of psychosis in nursing home patients.
- Equate UDS results with presenting complaints and symptoms. For example, if a patient with sudden syncope tests positive for marijuana, the syncopal symptoms demand further investigation because marijuana is not the likely cause.
- Duplicate the UDS screen with confirmatory tests if the result will determine treatment. When UDS results are ambiguous, use highly specific tests such as gas chromatography with mass spectrometry and high-performance liquid chromatography. Although expensive and time consuming, these tests confirm the presence or absence of substances with few false results.
Numerous medications and other substances can appear in a urine drug screen (UDS) as an illicit narcotic (Table). These false positives can:
- lead to incorrect diagnosis and inappropriate intervention, particularly if the result determines treatment
- endanger the therapeutic alliance by making the patient uncomfortable and defensive.
Table
Substances that may trigger a false urinary drug screen result
| Prescription drugs | Nonprescription drugs | Could appear in urinary drug screen as |
|---|---|---|
| Amphetamines | Nasal decongestants | Amphetamines |
| Methamphetamines | MDMA | |
| Bupropion | Pseudoephedrine | |
| Fluoxetine | ||
| Ranitidine | ||
| Trazodone | ||
| Nefazodone | ||
| Diazepam | None | Alcohol |
| Sertraline | None | Benzodiazepines |
| Oxaprozin | ||
| Amoxicillin | NSAIDs | Cocaine |
| Most antibiotics | ||
| MS Contin (false negative) | Poppy seeds | Heroin (morphine) |
| Quinolones | ||
| Rifampin | (6-Acetylmorphine) | |
| Codeine | ||
| Oxycodone (false negative) | ||
| Dronabinol | Visine eye drops (false negative) | Marijuana |
| Pantoprazole | Hemp seeds (false negative) | |
| Diazepam (false negative) | Nyquil | Methadone |
| Dextromethorphan | PCP | |
| Source: References 1,4-6 | ||
Why Drug Screens Are Sometimes Wrong
A UDS for recreational drug use is commonly performed when the patient presents to the ER with acute changes in mental or behavioral status.
Ms. A, age 57, presents to the ER with fluctuating consciousness. The cause is unknown.
Surgical removal of a pituitary tumor 39 years earlier caused hormone deficiencies, seizures, and excessive sleepiness. Symptoms of panhypopituitarism have been managed with medication, and her current regimen includes thyroxine, phenytoin, the proton pump inhibitor pantoprazole, and prednisone. Recently, comorbid depression caused her to skip doses.
ER physicians order a UDS because of Ms. A’s mental status changes. The enzyme-linked immunosorbent (ELISA) toxicology test for alcohol, amphetamines, barbiturates, benzodiazepines, cocaine, opiates, marijuana, and phencyclidine (PCP) is positive for marijuana. When the attending psychiatrist informs Ms. A of the result, she is shocked. She tells the psychiatrist she is active in church and opposes recreational use of narcotics. She adamantly denies using marijuana or other street drugs, alcohol, nicotine, or caffeine.
Eventually, physicians attributed Ms. A’s mental status changes to several underlying medical issues, including Addison’s disease. A thorough review of the case revealed that the proton pump inhibitor pantoprazole caused the false-positive UDS.
UDS tests are sensitive but not highly specific. A medication or other substance with a chemical structure similar to that of the suspected drug can cause a false positive.1-3
The “WEED” mnemonic spells out steps for critically evaluating UDS test results to ensure appropriate care:
- Write out a list of the patient’s medications. This list may explain the symptoms or help interpret UDS results. If a narcotic dose was recently increased, for example, a UDS might not be needed to confirm what caused the change in mental status.
- Examine the patient carefully. Evaluate physical signs, take a thorough medical history, and consider the potential for drug use. Although not impossible, for example, PCP intoxication is not a likely cause of psychosis in nursing home patients.
- Equate UDS results with presenting complaints and symptoms. For example, if a patient with sudden syncope tests positive for marijuana, the syncopal symptoms demand further investigation because marijuana is not the likely cause.
- Duplicate the UDS screen with confirmatory tests if the result will determine treatment. When UDS results are ambiguous, use highly specific tests such as gas chromatography with mass spectrometry and high-performance liquid chromatography. Although expensive and time consuming, these tests confirm the presence or absence of substances with few false results.
1. Casavant MJ. Urine drug screening in adolescents. Pediatr Clin North Am 2002;49(2):317-27.
2. Baden LR, Horowitz G, Jacoby H, Eliopoulos GM. Quinolones and false-positive urine screening for opiates by immunoassay technology. JAMA 2001;286:3115-9.
3. Fraser AD, Howell P. Oxaprozin cross-reactivity in three commercial immunoassays for benzodiazepines in urine. J Anal Toxicol 1998;22:50-4.
4. Pearson SD, Ash KO, Urry FM. Mechanism of false-negative urine cannabinoid immunoassay screens by Visine eyedrops. Clin Chem 1989;35:636-8.
5. The Merck Index: An encyclopedia of chemicals, drugs, and biologicals. Whitehouse Station, NJ: Merck Research Laboratories; 2001.
6. Baselt RC. Disposition of toxic drugs and chemicals in man. Foster City, CA: Chemical Toxicology Institute; 2000.
Dr. Rapuri is a family practice resident, Memorial Medical Center, Johnstown, PA, and clinical attache, VA Medical Center, Omaha, NE.
Dr. Ramaswamy is an instructor, Creighton University, and staff psychiatrist, VA Medical Center, Omaha, NE.
Dr. Madaan is a fellow in child and adolescent psychiatry, Creighton University, Omaha, NE.
Dr. Rasimas is chief resident, department of psychiatry and psychology, Mayo Clinic, Rochester, MN.
Dr. Krahn is deputy editor, Current Psychiatry, and chair, psychiatry department, Mayo Clinic, Scottsdale, AZ
1. Casavant MJ. Urine drug screening in adolescents. Pediatr Clin North Am 2002;49(2):317-27.
2. Baden LR, Horowitz G, Jacoby H, Eliopoulos GM. Quinolones and false-positive urine screening for opiates by immunoassay technology. JAMA 2001;286:3115-9.
3. Fraser AD, Howell P. Oxaprozin cross-reactivity in three commercial immunoassays for benzodiazepines in urine. J Anal Toxicol 1998;22:50-4.
4. Pearson SD, Ash KO, Urry FM. Mechanism of false-negative urine cannabinoid immunoassay screens by Visine eyedrops. Clin Chem 1989;35:636-8.
5. The Merck Index: An encyclopedia of chemicals, drugs, and biologicals. Whitehouse Station, NJ: Merck Research Laboratories; 2001.
6. Baselt RC. Disposition of toxic drugs and chemicals in man. Foster City, CA: Chemical Toxicology Institute; 2000.
Dr. Rapuri is a family practice resident, Memorial Medical Center, Johnstown, PA, and clinical attache, VA Medical Center, Omaha, NE.
Dr. Ramaswamy is an instructor, Creighton University, and staff psychiatrist, VA Medical Center, Omaha, NE.
Dr. Madaan is a fellow in child and adolescent psychiatry, Creighton University, Omaha, NE.
Dr. Rasimas is chief resident, department of psychiatry and psychology, Mayo Clinic, Rochester, MN.
Dr. Krahn is deputy editor, Current Psychiatry, and chair, psychiatry department, Mayo Clinic, Scottsdale, AZ
Hepatitis C and interferon: Watch for hostility, impulsivity
Pegylated interferon-alpha with ribavirin is the most effective therapy for chronic hepatitis C infection,1,2 but psychiatric patients often discontinue IFN-alpha because of its mood side effects.3 Most studies describe depressive states, although manic symptoms—irritability, aggression, anger, hostility, emotional lability, anxiety, panic attacks, and insomnia—also have been reported.4-6
To help you manage adverse mood changes and prevent IFN-alpha treatment discontinuation in patients with hepatitis C, this article:
- reviews studies of patients with a history mood disorders who were treated with IFN-alpha
- explains how to recognize and treat IFN-alpha-induced mood disturbances when antidepressants are contraindicated.
Psychiatric Patients and IFN Therapy
Chronic hepatitis C infection is common among psychiatric patients. Routine screening among 1,556 patients admitted to a U.S. public psychiatric hospital across 3 years identified 133 patients (8.5%) who were positive for hepatitis C virus.7
Patients with psychopathologic symptoms before starting IFN therapy may suffer more-severe adverse psychiatric effects during treatment than those without psychopathology.8 In fact, mood disorders were considered an absolute contraindication to IFN therapy until recently.
Now that the National Institutes of Health (NIH) has recommended extending hepatitis C research and treatment to psychiatric patients, IFN-alpha-induced mental illness could become more common in clinical practice. Before the 2002 NIH consensus statement, patients with mental illness and substance use disorders—who represent >50% of candidates who need IFN therapy—were excluded from research protocols.
Safely using IFN. Some reports and studies suggest that patients with past or existing psychiatric disorders can be treated safely and effectively with IFN-alpha.
In a prospective open-label study, 29 of 31 patients with co-existing chronic hepatitis C and psychiatric illness completed 6 months of IFN therapy, 5 million units (MU) three times/week or 5 MU daily. Patients continued maintenance psychotropics during IFN treatment, and a psychiatrist monitored psychiatric symptoms.
Psychiatric illness worsened in four patients, and two discontinued IFN treatment. Serum alanine aminotransferase returned to normal in 22 patients (71%), and hepatitis C virus RNA cleared from the sera of 15 (48%).9
Another prospective study of 50 patients treated with IFN for chronic hepatitis found that those with a pre-existing mood or anxiety disorder were not more likely than others to discontinue IFN therapy.10
Unique to hepatitis therapy? IFN-induced mood disturbances are probably different in patients with chronic hepatitis C than in those receiving IFN for other diseases because of differences in regimens and effects of the underlying pathologies. For example, prescribing a preventative antidepressant before starting IFN treatment might help cancer patients but not patients with hepatitis C.11
This distinction could be particularly important when giving IFN-alpha to patients who are vulnerable to psychiatric illness with impulsive features. To emphasize this point, we describe clinical features and treatment response in patients with hepatitis C who were treated in our department.
IFN-Alpha-Induced Moods
In our prospective study of 93 patients, 30 (32%) developed IFN-alpha-induced mood disorders during the first 12 weeks of treatment.12 Contrary to previous studies focusing on depression, most of our patients had a mix of manic/hypomanic and depressive symptoms. Twenty-four (13 women and 11 men, mean age 43) accepted referral to a psychiatrist specializing in mood disorders to characterize their symptoms.
Mood characteristics. Using DSM-IV criteria, the psychiatrist determined that IFN-alpha induced both manic/hypomanic and depressive symptoms in many patients, and the manic/hypomanic features predominated. Five manic symptoms and three depressive symptoms were present in >50% of patients (Figure 1, Table 1).
Three patients presented with a manic episode,15 with hypomania with prevalent irritability and dysphoric features, and 6 with a mixed depressive state (major depressive episode with at least 3 hypomania symptoms).13 Four patients (17%) suffered a relapse of alcohol or cannabis abuse.
Nearly all (84%) reported paroxysmal anxiety, and all had emotional hyperreactivity that the psychiatrist described as a main symptom of a manic or mixed state.14 Most felt extremely impulsive and feared losing control. Two faced legal difficulties, and one was in prison.
The patients’ mean Montgomery-Åsburg Depression Rating Scale (MADRS) score was 16.12 (±1.6), indicating a mild to moderate depressive state. The highest-scoring items were inner tension, reduced sleep, reduced appetite, and lassitude (Figure 2).
Their mean Bech-Rafaelsen Mania Scale15 score was 14.33 (±1.3), indicating hypomanic or moderate manic symptoms. Hostility was by far the predominant manic symptom (mean score 3.2/4). Other manic symptom scores ranged from 0.2/4 to 2.1/4 (Figure 3).
Figure 1 Mood symptoms identified in 24 patients after 12 weeks of IFN-alpha treatment
IFN: Interferon
Source: Reference 12
Figure 2 Depressive symptoms in 24 patients with IFN-induced mood disorder
IFN: Interferon
Source: Reference 12
Figure 3 Manic symptoms in 24 patients with IFN-induced mood disorder
IFN: Interferon
Source: Reference 12Table 1
Most-prevalent IFN-induced mood symptoms in 24 patients treated for hepatitis C
| 5 manic symptoms (% of patients) | 3 depressive symptoms (% of patients) |
| Irritability (100%) | Insomnia or hypersomnia (100%) |
| Racing thoughts (87%) | Poor appetite or weight loss (92%) |
| Distractibility (87%) | Psychomotor agitation or retardation (54%) |
| Insomnia (58%) | |
| Agitation (70%) | |
| IFN: Interferon | |
| Source: Reference 12 | |
Treatment. Given the predominance of manic/hypomanic symptoms, we treated the 24 patients with low-to-moderate dosages of an atypical antipsychotic (amisulpride, 100 to 600 mg/d). This medication—not available in the United States—would be similar to using risperidone, 1 to 6 mg/d. Low-dose benzodiazepines (clonazepam or alprazolam) were added as needed to manage insomnia. Antipsychotic treatment enabled 23 of 24 patients (96%) to continue antiviral therapy.
Five patients had received selective serotonin reuptake inhibitors (SSRIs) for 1 to 4 weeks before referral to the psychiatrist. Antidepressants worsened their mood symptoms, which included impulsivity, agitation, and insomnia. Emotional hyper-reactivity, irritability, hostility, and impulsiveness improved in all 5 patients within 1 to 2 weeks of stopping SSRIs and starting the atypical antipsychotic.
Discussion
Accurately characterizing IFN-induced mood states confirmed our published data showing a mix of manic/hypomanic and depressive symptoms in psychiatric patients treated for chronic hepatitis C. Irritability and hostility were the two most prominent symptoms.
These findings differ from those of investigations that identified depressive symptoms as the hallmark of IFN’s psychiatric side effects.6 Our data were thoroughly characterized according to DSM-IV-TR criteria, two mood-rating scales, and a psychiatrist experienced in mood disorders.
Why is mania missed? One possible explanation for these different findings is that IFN-alpha-induced fatigue and flu-like symptoms could be misinterpreted as depressive symptoms. Also, most researchers have used depression rating scales—but not mania scales—and have not considered other psychiatric diagnostics.16
Self-report questionnaires for evaluating depression also take into account somatic side effects, resulting in higher rating scores. Moreover, few studies have included clinical psychiatric interviews and even fewer diagnostic confirmation by a psychiatrist.
Finally, clinical experience indicates that patients who present with both manic/hypomanic and depressive symptoms are more likely to complain about depression than about manic symptoms. Therefore, clinicians may miss manic symptoms if they don’t actively seek them.
Irritability and hostility. Previous studies have described irritability, mood lability, and anger/hostility as frequent symptoms4,5 but failed to consider them as possible manifestations of mania or hypomania. Many of our patients reported irritability severe enough to interfere with their work, social, and family relationships.
Hostility was the symptom with the highest score on the Bech-Rafaelsen Mania Scale. This objective evidence suggests that investigating the consequences to patients of increased irritability, impulsivity, or hostility might reveal some frightening behavior
How to treat these patients. Considerable evidence from characterizing IFN-induced side effects in patients with hepatitis C points to a syndrome of depressive and manic/hypomanic symptoms13,17-19 that does not fulfill criteria for a mixed state. This disorder is not described in DSM-IV-TR and, unfortunately, usually is misdiagnosed as a depressive state.
Antidepressants can worsen depression by causing agitation and impulsivity and can increase risk of suicide.17,18,20 By contrast, atypical antipsychotics have been shown to improve bipolar depression.21,22
We used an atypical antipsychotic to treat patients with prevalent manic or hypomanic symptoms and those who did not respond to SSRIs. Their IFN-induced mood disorders improved rapidly on low dosages of amisulpride, and antiviral therapy discontinuation rates were low (1/24; 4%). By comparison, other studies have reported antiviral treatment discontinuation rates of 30% to 40% in patients taking antidepressants.23,24
The atypical antipsychotic did not improve our patients’ fatigue and other neurovegetative symptoms, but antidepressants likewise do not improve these symptoms.8
Recommendations
This study leads us to warn clinicians that antidepressants can worsen IFN-alpha-induced mood disorders and to recommend that antipsychotics be considered:
- at least before you decide to discontinue a hepatitis C patient’s IFN-alpha therapy
- or in patients with high irritability and hostility.
To determine whether to use an antidepressant or antimanic agent as first-line treatment, carefully diagnose the patient’s symptoms as a manic/hypomanic state, depressive mixed state, or depression. Successful IFN therapy requires:
- psychological support
- medication for psychiatric adverse effects
- collaboration between the psychiatrist and hepatologist.
IFN-alpha-induced mood disorders are triggered exogenously, do not correspond to typical features of any classic psychiatric disorder, and require further study. IFN-induced impulsivity and hostility also need to be better characterized, particularly as more patients with pre-existing psychiatric disorders are treated for hepatitis C.
Related resources
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345(1):41-52.
- Onyike CU, Bonner JO, Lyketsos CG, Treisman GJ. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry 2004;161:3.
Drug brand names
- Alprazolam • Xanax
- Amisulpride • (not available in the United States)
- Clonazepam • Klonopin
- Risperidone • Risperdal
Acknowledgment
This study was conducted with support from the Centre d’Investigation Clinique INSERM/CHU de Bordeaux.
1. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-65.
2. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-82.
3. Zdilar D, Franco-Bronson K, Buchler N, et al. Hepatitis C, interferon alfa, and depression. Hepatology. 2000;31:1207-11.
4. Schaefer M, Engelbrecht MA, Gut O, et al. Interferon alpha (INF-alpha) and psychiatric syndromes: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:731-46.
5. Trask PC, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000;18:2316-26.
6. Kraus MR, Schäfer A, Faler H, et al. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2B therapy. J Clin Psychiatry. 2003;64:6.-
7. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-4.
8. Capuron L, Gummick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643-52.
9. Van Thiel DH, Friedlander L, Molloy PJ, et al. Interferon-alpha can be used successfully in patients with hepatitis C virus-positive chronic hepatitis who have a psychiatric illness. Eur J Gastroenterol Hepatol. 1995;7(2):165-8.
10. Pariante CM, Landau S, Carpiniello B. Interferon alfa-induced adverse effects in patients with a psychiatric diagnosis. N Engl J Med. 2002;347(2):148-9.
11. Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon-alfa. N Engl J Med. 2001;344(13):961-6.
12. Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms J Clin Psychiatry. 2005;66;8:1050-7.
13. Benazzi F. Major depressive episodes with hypomanic symptoms are common among depressed outpatients. Comprehensive Psychiatry. 2001;42(2):139-43.
14. Henry C, Swendsen J, Van Den Bulke D, et al. Emotional hyperreactivity as the fundamental mood characteristic of manic and mixed states. Eur Psychiat. 2003;18:124-8.
15. Bech P, Rafaelsen OJ, Kramp P, Bolwig TG. The mania rating scale: scale construction and inter-observer agreement. Neuropharmacology. 1978;17:430-1.
16. Castera L, Constant A, Henry C, et al. Incidence, risk factors and treatment of mood disorders associated with peginterferon and ribavirin therapy in patients with chronic hepatitis C: results of a prospective study (abstract). Hepatology. 2003;38 (suppl.1):735A.-
17. Koukopoulos A, Koukopoulos A. Agitated depression as a mixed state and the problem of melancholia. Bipolarity: beyond classic mania. Psychiatr Clin North Am. 1999;22(3):564-74.
18. Benazzi F, Koukopoulos A, Akiskal HS. Toward a validation of a new definition of agitated depression as a bipolar mixed state (mixed depression). European Psychiatry. 2004;19:85-90.
19. Suppes T, Mintz J, McElroy SL, et al. Mixed hypomania in 908 patients with bipolar disorder evaluated prospectively in the Stanley Foundation Bipolar Treatment Network. A sex-specific phenomenon. Arch Gen Psychiatry. 2005;62:1089-96.
20. Henry C, Demotes-Mainard J. Avoiding drug-induced switching in patients with bipolar depression. Drug Safety. 2003;26:337-51.
21. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry. 2003;60(11):1079-88.
22. Calabrese JR, Keck PE, Jr, MacFadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression, Am J Psychiatry. 2005;162(7):1351-60.
23. Kraus MR, Schafer A, Faller H, et al. Paroxetine for the treatment of interferon-alpha-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002;16:1091-9.
24. Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942-7.
Pegylated interferon-alpha with ribavirin is the most effective therapy for chronic hepatitis C infection,1,2 but psychiatric patients often discontinue IFN-alpha because of its mood side effects.3 Most studies describe depressive states, although manic symptoms—irritability, aggression, anger, hostility, emotional lability, anxiety, panic attacks, and insomnia—also have been reported.4-6
To help you manage adverse mood changes and prevent IFN-alpha treatment discontinuation in patients with hepatitis C, this article:
- reviews studies of patients with a history mood disorders who were treated with IFN-alpha
- explains how to recognize and treat IFN-alpha-induced mood disturbances when antidepressants are contraindicated.
Psychiatric Patients and IFN Therapy
Chronic hepatitis C infection is common among psychiatric patients. Routine screening among 1,556 patients admitted to a U.S. public psychiatric hospital across 3 years identified 133 patients (8.5%) who were positive for hepatitis C virus.7
Patients with psychopathologic symptoms before starting IFN therapy may suffer more-severe adverse psychiatric effects during treatment than those without psychopathology.8 In fact, mood disorders were considered an absolute contraindication to IFN therapy until recently.
Now that the National Institutes of Health (NIH) has recommended extending hepatitis C research and treatment to psychiatric patients, IFN-alpha-induced mental illness could become more common in clinical practice. Before the 2002 NIH consensus statement, patients with mental illness and substance use disorders—who represent >50% of candidates who need IFN therapy—were excluded from research protocols.
Safely using IFN. Some reports and studies suggest that patients with past or existing psychiatric disorders can be treated safely and effectively with IFN-alpha.
In a prospective open-label study, 29 of 31 patients with co-existing chronic hepatitis C and psychiatric illness completed 6 months of IFN therapy, 5 million units (MU) three times/week or 5 MU daily. Patients continued maintenance psychotropics during IFN treatment, and a psychiatrist monitored psychiatric symptoms.
Psychiatric illness worsened in four patients, and two discontinued IFN treatment. Serum alanine aminotransferase returned to normal in 22 patients (71%), and hepatitis C virus RNA cleared from the sera of 15 (48%).9
Another prospective study of 50 patients treated with IFN for chronic hepatitis found that those with a pre-existing mood or anxiety disorder were not more likely than others to discontinue IFN therapy.10
Unique to hepatitis therapy? IFN-induced mood disturbances are probably different in patients with chronic hepatitis C than in those receiving IFN for other diseases because of differences in regimens and effects of the underlying pathologies. For example, prescribing a preventative antidepressant before starting IFN treatment might help cancer patients but not patients with hepatitis C.11
This distinction could be particularly important when giving IFN-alpha to patients who are vulnerable to psychiatric illness with impulsive features. To emphasize this point, we describe clinical features and treatment response in patients with hepatitis C who were treated in our department.
IFN-Alpha-Induced Moods
In our prospective study of 93 patients, 30 (32%) developed IFN-alpha-induced mood disorders during the first 12 weeks of treatment.12 Contrary to previous studies focusing on depression, most of our patients had a mix of manic/hypomanic and depressive symptoms. Twenty-four (13 women and 11 men, mean age 43) accepted referral to a psychiatrist specializing in mood disorders to characterize their symptoms.
Mood characteristics. Using DSM-IV criteria, the psychiatrist determined that IFN-alpha induced both manic/hypomanic and depressive symptoms in many patients, and the manic/hypomanic features predominated. Five manic symptoms and three depressive symptoms were present in >50% of patients (Figure 1, Table 1).
Three patients presented with a manic episode,15 with hypomania with prevalent irritability and dysphoric features, and 6 with a mixed depressive state (major depressive episode with at least 3 hypomania symptoms).13 Four patients (17%) suffered a relapse of alcohol or cannabis abuse.
Nearly all (84%) reported paroxysmal anxiety, and all had emotional hyperreactivity that the psychiatrist described as a main symptom of a manic or mixed state.14 Most felt extremely impulsive and feared losing control. Two faced legal difficulties, and one was in prison.
The patients’ mean Montgomery-Åsburg Depression Rating Scale (MADRS) score was 16.12 (±1.6), indicating a mild to moderate depressive state. The highest-scoring items were inner tension, reduced sleep, reduced appetite, and lassitude (Figure 2).
Their mean Bech-Rafaelsen Mania Scale15 score was 14.33 (±1.3), indicating hypomanic or moderate manic symptoms. Hostility was by far the predominant manic symptom (mean score 3.2/4). Other manic symptom scores ranged from 0.2/4 to 2.1/4 (Figure 3).
Figure 1 Mood symptoms identified in 24 patients after 12 weeks of IFN-alpha treatment
IFN: Interferon
Source: Reference 12
Figure 2 Depressive symptoms in 24 patients with IFN-induced mood disorder
IFN: Interferon
Source: Reference 12
Figure 3 Manic symptoms in 24 patients with IFN-induced mood disorder
IFN: Interferon
Source: Reference 12Table 1
Most-prevalent IFN-induced mood symptoms in 24 patients treated for hepatitis C
| 5 manic symptoms (% of patients) | 3 depressive symptoms (% of patients) |
| Irritability (100%) | Insomnia or hypersomnia (100%) |
| Racing thoughts (87%) | Poor appetite or weight loss (92%) |
| Distractibility (87%) | Psychomotor agitation or retardation (54%) |
| Insomnia (58%) | |
| Agitation (70%) | |
| IFN: Interferon | |
| Source: Reference 12 | |
Treatment. Given the predominance of manic/hypomanic symptoms, we treated the 24 patients with low-to-moderate dosages of an atypical antipsychotic (amisulpride, 100 to 600 mg/d). This medication—not available in the United States—would be similar to using risperidone, 1 to 6 mg/d. Low-dose benzodiazepines (clonazepam or alprazolam) were added as needed to manage insomnia. Antipsychotic treatment enabled 23 of 24 patients (96%) to continue antiviral therapy.
Five patients had received selective serotonin reuptake inhibitors (SSRIs) for 1 to 4 weeks before referral to the psychiatrist. Antidepressants worsened their mood symptoms, which included impulsivity, agitation, and insomnia. Emotional hyper-reactivity, irritability, hostility, and impulsiveness improved in all 5 patients within 1 to 2 weeks of stopping SSRIs and starting the atypical antipsychotic.
Discussion
Accurately characterizing IFN-induced mood states confirmed our published data showing a mix of manic/hypomanic and depressive symptoms in psychiatric patients treated for chronic hepatitis C. Irritability and hostility were the two most prominent symptoms.
These findings differ from those of investigations that identified depressive symptoms as the hallmark of IFN’s psychiatric side effects.6 Our data were thoroughly characterized according to DSM-IV-TR criteria, two mood-rating scales, and a psychiatrist experienced in mood disorders.
Why is mania missed? One possible explanation for these different findings is that IFN-alpha-induced fatigue and flu-like symptoms could be misinterpreted as depressive symptoms. Also, most researchers have used depression rating scales—but not mania scales—and have not considered other psychiatric diagnostics.16
Self-report questionnaires for evaluating depression also take into account somatic side effects, resulting in higher rating scores. Moreover, few studies have included clinical psychiatric interviews and even fewer diagnostic confirmation by a psychiatrist.
Finally, clinical experience indicates that patients who present with both manic/hypomanic and depressive symptoms are more likely to complain about depression than about manic symptoms. Therefore, clinicians may miss manic symptoms if they don’t actively seek them.
Irritability and hostility. Previous studies have described irritability, mood lability, and anger/hostility as frequent symptoms4,5 but failed to consider them as possible manifestations of mania or hypomania. Many of our patients reported irritability severe enough to interfere with their work, social, and family relationships.
Hostility was the symptom with the highest score on the Bech-Rafaelsen Mania Scale. This objective evidence suggests that investigating the consequences to patients of increased irritability, impulsivity, or hostility might reveal some frightening behavior
How to treat these patients. Considerable evidence from characterizing IFN-induced side effects in patients with hepatitis C points to a syndrome of depressive and manic/hypomanic symptoms13,17-19 that does not fulfill criteria for a mixed state. This disorder is not described in DSM-IV-TR and, unfortunately, usually is misdiagnosed as a depressive state.
Antidepressants can worsen depression by causing agitation and impulsivity and can increase risk of suicide.17,18,20 By contrast, atypical antipsychotics have been shown to improve bipolar depression.21,22
We used an atypical antipsychotic to treat patients with prevalent manic or hypomanic symptoms and those who did not respond to SSRIs. Their IFN-induced mood disorders improved rapidly on low dosages of amisulpride, and antiviral therapy discontinuation rates were low (1/24; 4%). By comparison, other studies have reported antiviral treatment discontinuation rates of 30% to 40% in patients taking antidepressants.23,24
The atypical antipsychotic did not improve our patients’ fatigue and other neurovegetative symptoms, but antidepressants likewise do not improve these symptoms.8
Recommendations
This study leads us to warn clinicians that antidepressants can worsen IFN-alpha-induced mood disorders and to recommend that antipsychotics be considered:
- at least before you decide to discontinue a hepatitis C patient’s IFN-alpha therapy
- or in patients with high irritability and hostility.
To determine whether to use an antidepressant or antimanic agent as first-line treatment, carefully diagnose the patient’s symptoms as a manic/hypomanic state, depressive mixed state, or depression. Successful IFN therapy requires:
- psychological support
- medication for psychiatric adverse effects
- collaboration between the psychiatrist and hepatologist.
IFN-alpha-induced mood disorders are triggered exogenously, do not correspond to typical features of any classic psychiatric disorder, and require further study. IFN-induced impulsivity and hostility also need to be better characterized, particularly as more patients with pre-existing psychiatric disorders are treated for hepatitis C.
Related resources
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345(1):41-52.
- Onyike CU, Bonner JO, Lyketsos CG, Treisman GJ. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry 2004;161:3.
Drug brand names
- Alprazolam • Xanax
- Amisulpride • (not available in the United States)
- Clonazepam • Klonopin
- Risperidone • Risperdal
Acknowledgment
This study was conducted with support from the Centre d’Investigation Clinique INSERM/CHU de Bordeaux.
Pegylated interferon-alpha with ribavirin is the most effective therapy for chronic hepatitis C infection,1,2 but psychiatric patients often discontinue IFN-alpha because of its mood side effects.3 Most studies describe depressive states, although manic symptoms—irritability, aggression, anger, hostility, emotional lability, anxiety, panic attacks, and insomnia—also have been reported.4-6
To help you manage adverse mood changes and prevent IFN-alpha treatment discontinuation in patients with hepatitis C, this article:
- reviews studies of patients with a history mood disorders who were treated with IFN-alpha
- explains how to recognize and treat IFN-alpha-induced mood disturbances when antidepressants are contraindicated.
Psychiatric Patients and IFN Therapy
Chronic hepatitis C infection is common among psychiatric patients. Routine screening among 1,556 patients admitted to a U.S. public psychiatric hospital across 3 years identified 133 patients (8.5%) who were positive for hepatitis C virus.7
Patients with psychopathologic symptoms before starting IFN therapy may suffer more-severe adverse psychiatric effects during treatment than those without psychopathology.8 In fact, mood disorders were considered an absolute contraindication to IFN therapy until recently.
Now that the National Institutes of Health (NIH) has recommended extending hepatitis C research and treatment to psychiatric patients, IFN-alpha-induced mental illness could become more common in clinical practice. Before the 2002 NIH consensus statement, patients with mental illness and substance use disorders—who represent >50% of candidates who need IFN therapy—were excluded from research protocols.
Safely using IFN. Some reports and studies suggest that patients with past or existing psychiatric disorders can be treated safely and effectively with IFN-alpha.
In a prospective open-label study, 29 of 31 patients with co-existing chronic hepatitis C and psychiatric illness completed 6 months of IFN therapy, 5 million units (MU) three times/week or 5 MU daily. Patients continued maintenance psychotropics during IFN treatment, and a psychiatrist monitored psychiatric symptoms.
Psychiatric illness worsened in four patients, and two discontinued IFN treatment. Serum alanine aminotransferase returned to normal in 22 patients (71%), and hepatitis C virus RNA cleared from the sera of 15 (48%).9
Another prospective study of 50 patients treated with IFN for chronic hepatitis found that those with a pre-existing mood or anxiety disorder were not more likely than others to discontinue IFN therapy.10
Unique to hepatitis therapy? IFN-induced mood disturbances are probably different in patients with chronic hepatitis C than in those receiving IFN for other diseases because of differences in regimens and effects of the underlying pathologies. For example, prescribing a preventative antidepressant before starting IFN treatment might help cancer patients but not patients with hepatitis C.11
This distinction could be particularly important when giving IFN-alpha to patients who are vulnerable to psychiatric illness with impulsive features. To emphasize this point, we describe clinical features and treatment response in patients with hepatitis C who were treated in our department.
IFN-Alpha-Induced Moods
In our prospective study of 93 patients, 30 (32%) developed IFN-alpha-induced mood disorders during the first 12 weeks of treatment.12 Contrary to previous studies focusing on depression, most of our patients had a mix of manic/hypomanic and depressive symptoms. Twenty-four (13 women and 11 men, mean age 43) accepted referral to a psychiatrist specializing in mood disorders to characterize their symptoms.
Mood characteristics. Using DSM-IV criteria, the psychiatrist determined that IFN-alpha induced both manic/hypomanic and depressive symptoms in many patients, and the manic/hypomanic features predominated. Five manic symptoms and three depressive symptoms were present in >50% of patients (Figure 1, Table 1).
Three patients presented with a manic episode,15 with hypomania with prevalent irritability and dysphoric features, and 6 with a mixed depressive state (major depressive episode with at least 3 hypomania symptoms).13 Four patients (17%) suffered a relapse of alcohol or cannabis abuse.
Nearly all (84%) reported paroxysmal anxiety, and all had emotional hyperreactivity that the psychiatrist described as a main symptom of a manic or mixed state.14 Most felt extremely impulsive and feared losing control. Two faced legal difficulties, and one was in prison.
The patients’ mean Montgomery-Åsburg Depression Rating Scale (MADRS) score was 16.12 (±1.6), indicating a mild to moderate depressive state. The highest-scoring items were inner tension, reduced sleep, reduced appetite, and lassitude (Figure 2).
Their mean Bech-Rafaelsen Mania Scale15 score was 14.33 (±1.3), indicating hypomanic or moderate manic symptoms. Hostility was by far the predominant manic symptom (mean score 3.2/4). Other manic symptom scores ranged from 0.2/4 to 2.1/4 (Figure 3).
Figure 1 Mood symptoms identified in 24 patients after 12 weeks of IFN-alpha treatment
IFN: Interferon
Source: Reference 12
Figure 2 Depressive symptoms in 24 patients with IFN-induced mood disorder
IFN: Interferon
Source: Reference 12
Figure 3 Manic symptoms in 24 patients with IFN-induced mood disorder
IFN: Interferon
Source: Reference 12Table 1
Most-prevalent IFN-induced mood symptoms in 24 patients treated for hepatitis C
| 5 manic symptoms (% of patients) | 3 depressive symptoms (% of patients) |
| Irritability (100%) | Insomnia or hypersomnia (100%) |
| Racing thoughts (87%) | Poor appetite or weight loss (92%) |
| Distractibility (87%) | Psychomotor agitation or retardation (54%) |
| Insomnia (58%) | |
| Agitation (70%) | |
| IFN: Interferon | |
| Source: Reference 12 | |
Treatment. Given the predominance of manic/hypomanic symptoms, we treated the 24 patients with low-to-moderate dosages of an atypical antipsychotic (amisulpride, 100 to 600 mg/d). This medication—not available in the United States—would be similar to using risperidone, 1 to 6 mg/d. Low-dose benzodiazepines (clonazepam or alprazolam) were added as needed to manage insomnia. Antipsychotic treatment enabled 23 of 24 patients (96%) to continue antiviral therapy.
Five patients had received selective serotonin reuptake inhibitors (SSRIs) for 1 to 4 weeks before referral to the psychiatrist. Antidepressants worsened their mood symptoms, which included impulsivity, agitation, and insomnia. Emotional hyper-reactivity, irritability, hostility, and impulsiveness improved in all 5 patients within 1 to 2 weeks of stopping SSRIs and starting the atypical antipsychotic.
Discussion
Accurately characterizing IFN-induced mood states confirmed our published data showing a mix of manic/hypomanic and depressive symptoms in psychiatric patients treated for chronic hepatitis C. Irritability and hostility were the two most prominent symptoms.
These findings differ from those of investigations that identified depressive symptoms as the hallmark of IFN’s psychiatric side effects.6 Our data were thoroughly characterized according to DSM-IV-TR criteria, two mood-rating scales, and a psychiatrist experienced in mood disorders.
Why is mania missed? One possible explanation for these different findings is that IFN-alpha-induced fatigue and flu-like symptoms could be misinterpreted as depressive symptoms. Also, most researchers have used depression rating scales—but not mania scales—and have not considered other psychiatric diagnostics.16
Self-report questionnaires for evaluating depression also take into account somatic side effects, resulting in higher rating scores. Moreover, few studies have included clinical psychiatric interviews and even fewer diagnostic confirmation by a psychiatrist.
Finally, clinical experience indicates that patients who present with both manic/hypomanic and depressive symptoms are more likely to complain about depression than about manic symptoms. Therefore, clinicians may miss manic symptoms if they don’t actively seek them.
Irritability and hostility. Previous studies have described irritability, mood lability, and anger/hostility as frequent symptoms4,5 but failed to consider them as possible manifestations of mania or hypomania. Many of our patients reported irritability severe enough to interfere with their work, social, and family relationships.
Hostility was the symptom with the highest score on the Bech-Rafaelsen Mania Scale. This objective evidence suggests that investigating the consequences to patients of increased irritability, impulsivity, or hostility might reveal some frightening behavior
How to treat these patients. Considerable evidence from characterizing IFN-induced side effects in patients with hepatitis C points to a syndrome of depressive and manic/hypomanic symptoms13,17-19 that does not fulfill criteria for a mixed state. This disorder is not described in DSM-IV-TR and, unfortunately, usually is misdiagnosed as a depressive state.
Antidepressants can worsen depression by causing agitation and impulsivity and can increase risk of suicide.17,18,20 By contrast, atypical antipsychotics have been shown to improve bipolar depression.21,22
We used an atypical antipsychotic to treat patients with prevalent manic or hypomanic symptoms and those who did not respond to SSRIs. Their IFN-induced mood disorders improved rapidly on low dosages of amisulpride, and antiviral therapy discontinuation rates were low (1/24; 4%). By comparison, other studies have reported antiviral treatment discontinuation rates of 30% to 40% in patients taking antidepressants.23,24
The atypical antipsychotic did not improve our patients’ fatigue and other neurovegetative symptoms, but antidepressants likewise do not improve these symptoms.8
Recommendations
This study leads us to warn clinicians that antidepressants can worsen IFN-alpha-induced mood disorders and to recommend that antipsychotics be considered:
- at least before you decide to discontinue a hepatitis C patient’s IFN-alpha therapy
- or in patients with high irritability and hostility.
To determine whether to use an antidepressant or antimanic agent as first-line treatment, carefully diagnose the patient’s symptoms as a manic/hypomanic state, depressive mixed state, or depression. Successful IFN therapy requires:
- psychological support
- medication for psychiatric adverse effects
- collaboration between the psychiatrist and hepatologist.
IFN-alpha-induced mood disorders are triggered exogenously, do not correspond to typical features of any classic psychiatric disorder, and require further study. IFN-induced impulsivity and hostility also need to be better characterized, particularly as more patients with pre-existing psychiatric disorders are treated for hepatitis C.
Related resources
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345(1):41-52.
- Onyike CU, Bonner JO, Lyketsos CG, Treisman GJ. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry 2004;161:3.
Drug brand names
- Alprazolam • Xanax
- Amisulpride • (not available in the United States)
- Clonazepam • Klonopin
- Risperidone • Risperdal
Acknowledgment
This study was conducted with support from the Centre d’Investigation Clinique INSERM/CHU de Bordeaux.
1. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-65.
2. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-82.
3. Zdilar D, Franco-Bronson K, Buchler N, et al. Hepatitis C, interferon alfa, and depression. Hepatology. 2000;31:1207-11.
4. Schaefer M, Engelbrecht MA, Gut O, et al. Interferon alpha (INF-alpha) and psychiatric syndromes: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:731-46.
5. Trask PC, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000;18:2316-26.
6. Kraus MR, Schäfer A, Faler H, et al. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2B therapy. J Clin Psychiatry. 2003;64:6.-
7. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-4.
8. Capuron L, Gummick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643-52.
9. Van Thiel DH, Friedlander L, Molloy PJ, et al. Interferon-alpha can be used successfully in patients with hepatitis C virus-positive chronic hepatitis who have a psychiatric illness. Eur J Gastroenterol Hepatol. 1995;7(2):165-8.
10. Pariante CM, Landau S, Carpiniello B. Interferon alfa-induced adverse effects in patients with a psychiatric diagnosis. N Engl J Med. 2002;347(2):148-9.
11. Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon-alfa. N Engl J Med. 2001;344(13):961-6.
12. Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms J Clin Psychiatry. 2005;66;8:1050-7.
13. Benazzi F. Major depressive episodes with hypomanic symptoms are common among depressed outpatients. Comprehensive Psychiatry. 2001;42(2):139-43.
14. Henry C, Swendsen J, Van Den Bulke D, et al. Emotional hyperreactivity as the fundamental mood characteristic of manic and mixed states. Eur Psychiat. 2003;18:124-8.
15. Bech P, Rafaelsen OJ, Kramp P, Bolwig TG. The mania rating scale: scale construction and inter-observer agreement. Neuropharmacology. 1978;17:430-1.
16. Castera L, Constant A, Henry C, et al. Incidence, risk factors and treatment of mood disorders associated with peginterferon and ribavirin therapy in patients with chronic hepatitis C: results of a prospective study (abstract). Hepatology. 2003;38 (suppl.1):735A.-
17. Koukopoulos A, Koukopoulos A. Agitated depression as a mixed state and the problem of melancholia. Bipolarity: beyond classic mania. Psychiatr Clin North Am. 1999;22(3):564-74.
18. Benazzi F, Koukopoulos A, Akiskal HS. Toward a validation of a new definition of agitated depression as a bipolar mixed state (mixed depression). European Psychiatry. 2004;19:85-90.
19. Suppes T, Mintz J, McElroy SL, et al. Mixed hypomania in 908 patients with bipolar disorder evaluated prospectively in the Stanley Foundation Bipolar Treatment Network. A sex-specific phenomenon. Arch Gen Psychiatry. 2005;62:1089-96.
20. Henry C, Demotes-Mainard J. Avoiding drug-induced switching in patients with bipolar depression. Drug Safety. 2003;26:337-51.
21. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry. 2003;60(11):1079-88.
22. Calabrese JR, Keck PE, Jr, MacFadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression, Am J Psychiatry. 2005;162(7):1351-60.
23. Kraus MR, Schafer A, Faller H, et al. Paroxetine for the treatment of interferon-alpha-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002;16:1091-9.
24. Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942-7.
1. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-65.
2. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-82.
3. Zdilar D, Franco-Bronson K, Buchler N, et al. Hepatitis C, interferon alfa, and depression. Hepatology. 2000;31:1207-11.
4. Schaefer M, Engelbrecht MA, Gut O, et al. Interferon alpha (INF-alpha) and psychiatric syndromes: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:731-46.
5. Trask PC, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000;18:2316-26.
6. Kraus MR, Schäfer A, Faler H, et al. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2B therapy. J Clin Psychiatry. 2003;64:6.-
7. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-4.
8. Capuron L, Gummick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643-52.
9. Van Thiel DH, Friedlander L, Molloy PJ, et al. Interferon-alpha can be used successfully in patients with hepatitis C virus-positive chronic hepatitis who have a psychiatric illness. Eur J Gastroenterol Hepatol. 1995;7(2):165-8.
10. Pariante CM, Landau S, Carpiniello B. Interferon alfa-induced adverse effects in patients with a psychiatric diagnosis. N Engl J Med. 2002;347(2):148-9.
11. Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon-alfa. N Engl J Med. 2001;344(13):961-6.
12. Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms J Clin Psychiatry. 2005;66;8:1050-7.
13. Benazzi F. Major depressive episodes with hypomanic symptoms are common among depressed outpatients. Comprehensive Psychiatry. 2001;42(2):139-43.
14. Henry C, Swendsen J, Van Den Bulke D, et al. Emotional hyperreactivity as the fundamental mood characteristic of manic and mixed states. Eur Psychiat. 2003;18:124-8.
15. Bech P, Rafaelsen OJ, Kramp P, Bolwig TG. The mania rating scale: scale construction and inter-observer agreement. Neuropharmacology. 1978;17:430-1.
16. Castera L, Constant A, Henry C, et al. Incidence, risk factors and treatment of mood disorders associated with peginterferon and ribavirin therapy in patients with chronic hepatitis C: results of a prospective study (abstract). Hepatology. 2003;38 (suppl.1):735A.-
17. Koukopoulos A, Koukopoulos A. Agitated depression as a mixed state and the problem of melancholia. Bipolarity: beyond classic mania. Psychiatr Clin North Am. 1999;22(3):564-74.
18. Benazzi F, Koukopoulos A, Akiskal HS. Toward a validation of a new definition of agitated depression as a bipolar mixed state (mixed depression). European Psychiatry. 2004;19:85-90.
19. Suppes T, Mintz J, McElroy SL, et al. Mixed hypomania in 908 patients with bipolar disorder evaluated prospectively in the Stanley Foundation Bipolar Treatment Network. A sex-specific phenomenon. Arch Gen Psychiatry. 2005;62:1089-96.
20. Henry C, Demotes-Mainard J. Avoiding drug-induced switching in patients with bipolar depression. Drug Safety. 2003;26:337-51.
21. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry. 2003;60(11):1079-88.
22. Calabrese JR, Keck PE, Jr, MacFadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression, Am J Psychiatry. 2005;162(7):1351-60.
23. Kraus MR, Schafer A, Faller H, et al. Paroxetine for the treatment of interferon-alpha-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002;16:1091-9.
24. Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942-7.
ECT wipes out 30 years of memories
Woman loses 30 years of memories after electroconvulsive therapy
Richland County (SC) Circuit Court
A 55-year old woman with a history of depression underwent successful electroconvulsive therapy (ECT) after her husband and father died. Six months later she became depressed, and a new psychiatrist referred her to his partner for additional ECT treatments.
The partner administered outpatient ECT at a hospital daily for 10 days. The referring psychiatrist wrote in the patient’s chart that the patient experienced memory loss and severe cognitive problems during the initial ECT regimen but did not report this development to his partner and allegedly encouraged the patient to continue ECT.
After the second round of ECT treatments, the patient suffered brain damage and lost all her memories from the past 30 years—including the births of her children and her job skills—leaving her unable to work.
In court, the patient claimed ECT should be administered no more than three times a week, and the referring psychiatrist should have told his partner about the patient’s memory problems.
- The case was settled for $18,000
Dr. Grant’s observations
Although this case concerns ECT, the claim is based on negligence—that is, the psychiatrist did not fulfill his duty to care for the patient. The negligence claim focused on how the treatment was implemented, not whether ECT was appropriate for this woman’s depression.
ECT’s response rate ranges from 50% to 60%1 among patients who did not respond to one or more antidepressant trials. Symptomatic improvement usually is faster with ECT than with pharmacotherapy2 when ECT is administered three times per week. Mortality rates with ECT are similar to those associated with minor surgery.1
In addition to being an effective and safe treatment for depression, ECT rarely is a basis for malpractice. One study found that only 4 (0.2%) of 1,700 psychiatric malpractice claims filed between 1984 and 1990 concerned ECT’s side effects, complications, or appropriateness.3 Few patients who receive ECT file a malpractice claim because most are satisfied with the treatment; approximately 80% of ECT patients say they would consent to ECT again.4,5 In fact, one might consider withholding ECT from severely depressed patients grounds for malpractice.
Although safe and effective, ECT could present health risks that you need to discuss with patients. In particular, cognitive problems such as delirium and impaired attention and memory may result.1
Cognitive impairment risk in ect
ECT’s more severe cognitive side effects stem from:
- bilateral electrode placement
- sine wave stimulation
- suprathreshold stimulus intensity
- administration >3 times per week
- large numbers of treatments, usually >20 in an acute treatment course
- some medications, such as lithium carbonate and anticholinergics6
- pre-existing neurologic diseases such as Alzheimer’s or Parkinson’s disease.1
The magnitude of retrograde amnesia often is greatest immediately after treatment. Patients are more likely to forget public information such as current events than personal information.10 The effects usually subside over time, and older memories are more likely to be recovered than more recent ones. ECT can cause permanent memory loss, particularly after bilateral electrode placement, suprathreshold stimulus intensity, sine wave stimulation, or large numbers of treatments—usually more than 20.
Ensuring adequate informed consent when delivering ECT or before referring a patient for treatment can help prevent a malpractice claim. Although specific requirements for ECT consent vary by jurisdiction, follow these general principles:1
- Provide the patient adequate information. Explain the reasons for ECT, describe the procedure including choice of stimulus electrode placement, offer alternative treatments, and explain the risks, benefits, anticipated number of treatments, relapse risk, and need for continuing treatment.
- Make sure the patient is capable of understanding and acting reasonably on this information and knows he or she can refuse treatment at any time.
- Tell the patient that a successful outcome is not guaranteed.
- Describe the likelihood and potential severity of major risks associated with ECT, including mortality, cardiovascular and CNS problems, and minor side effects such as headache, muscle aches, or nausea.
- Be sure the patient understands that consent is voluntary and can be withdrawn. The patient should know that he or she is also consenting to emergency treatment.
- Tell patients about possible behavioral restrictions—such as needing a friend or family member to monitor the patient or not being able to drive a car—that may be necessary during evaluation, treatment, and recuperation.
Although ECT might impair memory, it can improve neuropsychological domains such as global cognitive status and measures of general intelligence.11 Also, there is no evidence that ECT causes lasting problems in executive functioning, abstract reasoning, creativity, semantic memory, implicit memory, or skill acquisition or retention. Long-term negative effects on ability to learn and retain new information are unlikely.1
Avoiding an ect related malpractice claim
To reduce the possibility of a malpractice claim after ECT:
- Inform the patient about the risk of cognitive side effects as part of the informed consent process (Box).
- Assess the patient’s orientation and memory functions before and throughout ECT. In the above case, the referring psychiatrist had a duty to inform the psychiatrist administering ECT about the patient’s memory problems and recommend decreasing or discontinuing ECT.
- Consider a patient’s mood state, which may influence how ECT patients rate their memory.12 Ask about symptoms of depression. Patients with cognitive complaints such as subjective memory loss are more likely than those without such problems to have depression symptoms.1
- Do not administer ECT more than 3 times per week. No evidence supports more frequent use, and daily ECT may increase cognitive problems.1 The psychiatrist in the above case was negligent in providing a treatment frequency with no scientific support or medical rationale.
- Verify that the physician is qualified to perform ECT. Hospitals must ensure ECT quality and safety and should have a written plan for providing and maintaining ECT privileges.
- Involve the family when appropriate. Family members often care for patients during outpatient ECT. Give patients and family members literature describing ECT. Allow them time to consider the procedure, then schedule an appointment to answer questions.
1. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging, 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
2. Nobler MS, Sackeim HA, Moeller JR, et al. Quantifying the speed of symptomatic improvement with electroconvulsive therapy: comparison of alternative statistical methods. Convuls Ther 1997;13:208-21.
3. Slawson P. Psychiatric malpractice and ECT: a review of 1,700 claims. Convuls Ther 1991;7:255-61.
4. Freeman CP, Cheshire KE. Attitude studies on electroconvulsive therapy. Convuls Ther. 1986;2:31-42.
5. Pettinati HM, Tanburello TA, Ruetsch CR, et al. Patient attitudes toward electroconvulsive therapy. Psychopharmacol Bull. 1994;30:471-5.
6. Small JG, Kellams JJ, Milstein V, et al. Complications with electroconvulsive treatment combined with lithium. Biol Psychiatry 1980;15:103-12.
7. Sobin C, Sackeim HA, Prudic J, et al. Predictors of retrograde amnesia following ECT. Am J Psychiatry 1995;152:995-1001.
8. Donahue JC. Electroconvulsive therapy and memory loss: anatomy of a debate. J ECT 2000;16:133-43.
9. Sackeim HA. Memory and ECT: from polarization to reconciliation. J ECT 2000;16:87-96.
10. Lisanby SH, Maddox JH, Prudic J, et al. The effects of electroconvulsive therapy on memory of autobiographical and public events. Arch Gen Psychiatry 2000;57:581-90.
11. Sackeim HA, Prudic J, Devanand DP, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med 1993;328:839-46.
12. Coleman EA, Sackeim HA, Prudic J, et al. Subjective memory complaints before and after electroconvulsive therapy. Biol Psychiatry 1996;39:346-56.
Woman loses 30 years of memories after electroconvulsive therapy
Richland County (SC) Circuit Court
A 55-year old woman with a history of depression underwent successful electroconvulsive therapy (ECT) after her husband and father died. Six months later she became depressed, and a new psychiatrist referred her to his partner for additional ECT treatments.
The partner administered outpatient ECT at a hospital daily for 10 days. The referring psychiatrist wrote in the patient’s chart that the patient experienced memory loss and severe cognitive problems during the initial ECT regimen but did not report this development to his partner and allegedly encouraged the patient to continue ECT.
After the second round of ECT treatments, the patient suffered brain damage and lost all her memories from the past 30 years—including the births of her children and her job skills—leaving her unable to work.
In court, the patient claimed ECT should be administered no more than three times a week, and the referring psychiatrist should have told his partner about the patient’s memory problems.
- The case was settled for $18,000
Dr. Grant’s observations
Although this case concerns ECT, the claim is based on negligence—that is, the psychiatrist did not fulfill his duty to care for the patient. The negligence claim focused on how the treatment was implemented, not whether ECT was appropriate for this woman’s depression.
ECT’s response rate ranges from 50% to 60%1 among patients who did not respond to one or more antidepressant trials. Symptomatic improvement usually is faster with ECT than with pharmacotherapy2 when ECT is administered three times per week. Mortality rates with ECT are similar to those associated with minor surgery.1
In addition to being an effective and safe treatment for depression, ECT rarely is a basis for malpractice. One study found that only 4 (0.2%) of 1,700 psychiatric malpractice claims filed between 1984 and 1990 concerned ECT’s side effects, complications, or appropriateness.3 Few patients who receive ECT file a malpractice claim because most are satisfied with the treatment; approximately 80% of ECT patients say they would consent to ECT again.4,5 In fact, one might consider withholding ECT from severely depressed patients grounds for malpractice.
Although safe and effective, ECT could present health risks that you need to discuss with patients. In particular, cognitive problems such as delirium and impaired attention and memory may result.1
Cognitive impairment risk in ect
ECT’s more severe cognitive side effects stem from:
- bilateral electrode placement
- sine wave stimulation
- suprathreshold stimulus intensity
- administration >3 times per week
- large numbers of treatments, usually >20 in an acute treatment course
- some medications, such as lithium carbonate and anticholinergics6
- pre-existing neurologic diseases such as Alzheimer’s or Parkinson’s disease.1
The magnitude of retrograde amnesia often is greatest immediately after treatment. Patients are more likely to forget public information such as current events than personal information.10 The effects usually subside over time, and older memories are more likely to be recovered than more recent ones. ECT can cause permanent memory loss, particularly after bilateral electrode placement, suprathreshold stimulus intensity, sine wave stimulation, or large numbers of treatments—usually more than 20.
Ensuring adequate informed consent when delivering ECT or before referring a patient for treatment can help prevent a malpractice claim. Although specific requirements for ECT consent vary by jurisdiction, follow these general principles:1
- Provide the patient adequate information. Explain the reasons for ECT, describe the procedure including choice of stimulus electrode placement, offer alternative treatments, and explain the risks, benefits, anticipated number of treatments, relapse risk, and need for continuing treatment.
- Make sure the patient is capable of understanding and acting reasonably on this information and knows he or she can refuse treatment at any time.
- Tell the patient that a successful outcome is not guaranteed.
- Describe the likelihood and potential severity of major risks associated with ECT, including mortality, cardiovascular and CNS problems, and minor side effects such as headache, muscle aches, or nausea.
- Be sure the patient understands that consent is voluntary and can be withdrawn. The patient should know that he or she is also consenting to emergency treatment.
- Tell patients about possible behavioral restrictions—such as needing a friend or family member to monitor the patient or not being able to drive a car—that may be necessary during evaluation, treatment, and recuperation.
Although ECT might impair memory, it can improve neuropsychological domains such as global cognitive status and measures of general intelligence.11 Also, there is no evidence that ECT causes lasting problems in executive functioning, abstract reasoning, creativity, semantic memory, implicit memory, or skill acquisition or retention. Long-term negative effects on ability to learn and retain new information are unlikely.1
Avoiding an ect related malpractice claim
To reduce the possibility of a malpractice claim after ECT:
- Inform the patient about the risk of cognitive side effects as part of the informed consent process (Box).
- Assess the patient’s orientation and memory functions before and throughout ECT. In the above case, the referring psychiatrist had a duty to inform the psychiatrist administering ECT about the patient’s memory problems and recommend decreasing or discontinuing ECT.
- Consider a patient’s mood state, which may influence how ECT patients rate their memory.12 Ask about symptoms of depression. Patients with cognitive complaints such as subjective memory loss are more likely than those without such problems to have depression symptoms.1
- Do not administer ECT more than 3 times per week. No evidence supports more frequent use, and daily ECT may increase cognitive problems.1 The psychiatrist in the above case was negligent in providing a treatment frequency with no scientific support or medical rationale.
- Verify that the physician is qualified to perform ECT. Hospitals must ensure ECT quality and safety and should have a written plan for providing and maintaining ECT privileges.
- Involve the family when appropriate. Family members often care for patients during outpatient ECT. Give patients and family members literature describing ECT. Allow them time to consider the procedure, then schedule an appointment to answer questions.
Woman loses 30 years of memories after electroconvulsive therapy
Richland County (SC) Circuit Court
A 55-year old woman with a history of depression underwent successful electroconvulsive therapy (ECT) after her husband and father died. Six months later she became depressed, and a new psychiatrist referred her to his partner for additional ECT treatments.
The partner administered outpatient ECT at a hospital daily for 10 days. The referring psychiatrist wrote in the patient’s chart that the patient experienced memory loss and severe cognitive problems during the initial ECT regimen but did not report this development to his partner and allegedly encouraged the patient to continue ECT.
After the second round of ECT treatments, the patient suffered brain damage and lost all her memories from the past 30 years—including the births of her children and her job skills—leaving her unable to work.
In court, the patient claimed ECT should be administered no more than three times a week, and the referring psychiatrist should have told his partner about the patient’s memory problems.
- The case was settled for $18,000
Dr. Grant’s observations
Although this case concerns ECT, the claim is based on negligence—that is, the psychiatrist did not fulfill his duty to care for the patient. The negligence claim focused on how the treatment was implemented, not whether ECT was appropriate for this woman’s depression.
ECT’s response rate ranges from 50% to 60%1 among patients who did not respond to one or more antidepressant trials. Symptomatic improvement usually is faster with ECT than with pharmacotherapy2 when ECT is administered three times per week. Mortality rates with ECT are similar to those associated with minor surgery.1
In addition to being an effective and safe treatment for depression, ECT rarely is a basis for malpractice. One study found that only 4 (0.2%) of 1,700 psychiatric malpractice claims filed between 1984 and 1990 concerned ECT’s side effects, complications, or appropriateness.3 Few patients who receive ECT file a malpractice claim because most are satisfied with the treatment; approximately 80% of ECT patients say they would consent to ECT again.4,5 In fact, one might consider withholding ECT from severely depressed patients grounds for malpractice.
Although safe and effective, ECT could present health risks that you need to discuss with patients. In particular, cognitive problems such as delirium and impaired attention and memory may result.1
Cognitive impairment risk in ect
ECT’s more severe cognitive side effects stem from:
- bilateral electrode placement
- sine wave stimulation
- suprathreshold stimulus intensity
- administration >3 times per week
- large numbers of treatments, usually >20 in an acute treatment course
- some medications, such as lithium carbonate and anticholinergics6
- pre-existing neurologic diseases such as Alzheimer’s or Parkinson’s disease.1
The magnitude of retrograde amnesia often is greatest immediately after treatment. Patients are more likely to forget public information such as current events than personal information.10 The effects usually subside over time, and older memories are more likely to be recovered than more recent ones. ECT can cause permanent memory loss, particularly after bilateral electrode placement, suprathreshold stimulus intensity, sine wave stimulation, or large numbers of treatments—usually more than 20.
Ensuring adequate informed consent when delivering ECT or before referring a patient for treatment can help prevent a malpractice claim. Although specific requirements for ECT consent vary by jurisdiction, follow these general principles:1
- Provide the patient adequate information. Explain the reasons for ECT, describe the procedure including choice of stimulus electrode placement, offer alternative treatments, and explain the risks, benefits, anticipated number of treatments, relapse risk, and need for continuing treatment.
- Make sure the patient is capable of understanding and acting reasonably on this information and knows he or she can refuse treatment at any time.
- Tell the patient that a successful outcome is not guaranteed.
- Describe the likelihood and potential severity of major risks associated with ECT, including mortality, cardiovascular and CNS problems, and minor side effects such as headache, muscle aches, or nausea.
- Be sure the patient understands that consent is voluntary and can be withdrawn. The patient should know that he or she is also consenting to emergency treatment.
- Tell patients about possible behavioral restrictions—such as needing a friend or family member to monitor the patient or not being able to drive a car—that may be necessary during evaluation, treatment, and recuperation.
Although ECT might impair memory, it can improve neuropsychological domains such as global cognitive status and measures of general intelligence.11 Also, there is no evidence that ECT causes lasting problems in executive functioning, abstract reasoning, creativity, semantic memory, implicit memory, or skill acquisition or retention. Long-term negative effects on ability to learn and retain new information are unlikely.1
Avoiding an ect related malpractice claim
To reduce the possibility of a malpractice claim after ECT:
- Inform the patient about the risk of cognitive side effects as part of the informed consent process (Box).
- Assess the patient’s orientation and memory functions before and throughout ECT. In the above case, the referring psychiatrist had a duty to inform the psychiatrist administering ECT about the patient’s memory problems and recommend decreasing or discontinuing ECT.
- Consider a patient’s mood state, which may influence how ECT patients rate their memory.12 Ask about symptoms of depression. Patients with cognitive complaints such as subjective memory loss are more likely than those without such problems to have depression symptoms.1
- Do not administer ECT more than 3 times per week. No evidence supports more frequent use, and daily ECT may increase cognitive problems.1 The psychiatrist in the above case was negligent in providing a treatment frequency with no scientific support or medical rationale.
- Verify that the physician is qualified to perform ECT. Hospitals must ensure ECT quality and safety and should have a written plan for providing and maintaining ECT privileges.
- Involve the family when appropriate. Family members often care for patients during outpatient ECT. Give patients and family members literature describing ECT. Allow them time to consider the procedure, then schedule an appointment to answer questions.
1. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging, 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
2. Nobler MS, Sackeim HA, Moeller JR, et al. Quantifying the speed of symptomatic improvement with electroconvulsive therapy: comparison of alternative statistical methods. Convuls Ther 1997;13:208-21.
3. Slawson P. Psychiatric malpractice and ECT: a review of 1,700 claims. Convuls Ther 1991;7:255-61.
4. Freeman CP, Cheshire KE. Attitude studies on electroconvulsive therapy. Convuls Ther. 1986;2:31-42.
5. Pettinati HM, Tanburello TA, Ruetsch CR, et al. Patient attitudes toward electroconvulsive therapy. Psychopharmacol Bull. 1994;30:471-5.
6. Small JG, Kellams JJ, Milstein V, et al. Complications with electroconvulsive treatment combined with lithium. Biol Psychiatry 1980;15:103-12.
7. Sobin C, Sackeim HA, Prudic J, et al. Predictors of retrograde amnesia following ECT. Am J Psychiatry 1995;152:995-1001.
8. Donahue JC. Electroconvulsive therapy and memory loss: anatomy of a debate. J ECT 2000;16:133-43.
9. Sackeim HA. Memory and ECT: from polarization to reconciliation. J ECT 2000;16:87-96.
10. Lisanby SH, Maddox JH, Prudic J, et al. The effects of electroconvulsive therapy on memory of autobiographical and public events. Arch Gen Psychiatry 2000;57:581-90.
11. Sackeim HA, Prudic J, Devanand DP, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med 1993;328:839-46.
12. Coleman EA, Sackeim HA, Prudic J, et al. Subjective memory complaints before and after electroconvulsive therapy. Biol Psychiatry 1996;39:346-56.
1. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging, 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
2. Nobler MS, Sackeim HA, Moeller JR, et al. Quantifying the speed of symptomatic improvement with electroconvulsive therapy: comparison of alternative statistical methods. Convuls Ther 1997;13:208-21.
3. Slawson P. Psychiatric malpractice and ECT: a review of 1,700 claims. Convuls Ther 1991;7:255-61.
4. Freeman CP, Cheshire KE. Attitude studies on electroconvulsive therapy. Convuls Ther. 1986;2:31-42.
5. Pettinati HM, Tanburello TA, Ruetsch CR, et al. Patient attitudes toward electroconvulsive therapy. Psychopharmacol Bull. 1994;30:471-5.
6. Small JG, Kellams JJ, Milstein V, et al. Complications with electroconvulsive treatment combined with lithium. Biol Psychiatry 1980;15:103-12.
7. Sobin C, Sackeim HA, Prudic J, et al. Predictors of retrograde amnesia following ECT. Am J Psychiatry 1995;152:995-1001.
8. Donahue JC. Electroconvulsive therapy and memory loss: anatomy of a debate. J ECT 2000;16:133-43.
9. Sackeim HA. Memory and ECT: from polarization to reconciliation. J ECT 2000;16:87-96.
10. Lisanby SH, Maddox JH, Prudic J, et al. The effects of electroconvulsive therapy on memory of autobiographical and public events. Arch Gen Psychiatry 2000;57:581-90.
11. Sackeim HA, Prudic J, Devanand DP, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med 1993;328:839-46.
12. Coleman EA, Sackeim HA, Prudic J, et al. Subjective memory complaints before and after electroconvulsive therapy. Biol Psychiatry 1996;39:346-56.
Correction
The article “Female sexual dysfunction: don’t assume it’s a side effect” (Med/Psych Update, July 2006,) contained an error on pages 56-7. It should have stated that elevated thyroid-stimulating hormone (TSH) could point to hypothyroidism and low TSH could signal hyperthyroidism.
The article “Female sexual dysfunction: don’t assume it’s a side effect” (Med/Psych Update, July 2006,) contained an error on pages 56-7. It should have stated that elevated thyroid-stimulating hormone (TSH) could point to hypothyroidism and low TSH could signal hyperthyroidism.
The article “Female sexual dysfunction: don’t assume it’s a side effect” (Med/Psych Update, July 2006,) contained an error on pages 56-7. It should have stated that elevated thyroid-stimulating hormone (TSH) could point to hypothyroidism and low TSH could signal hyperthyroidism.
Opioids: Pain-management option for some older patients
In their article on managing dementia-related behaviors (Current Psychiatry, May 2006), Dr. Bruce Sutor and colleagues advise against using opioids to manage pain in older patients (p. 88). I was disheartened by their comments.
American Geriatrics Society guidelines clearly state that opiate medications are an appropriate choice for treating moderate to severe pain in some elderly persons, including those with dementia.1
Refusing to consider opiates for pain management can lead to severe suffering, especially for a patient who is incapable of communication. If an elder is at risk for falls, perhaps a full pain assessment is warranted and other CNS suppressants such as benzodiazepines can be eliminated. That way, we can treat pain and reduce the risk of falls.
Unfortunately, the myth that certain groups are not appropriate candidates for opiates lives on. We need to assess and treat all elders for pain, whether they are cognitively intact or impaired. As health care professionals, we are obligated to reduce pain and suffering in all patients, especially those who have trouble communicating.
Teresa Keane, RN, MSN, PMHNP
Psychiatric nurse practitioner, Kaiser Permanente Northwest
Adjunct faculty member, Oregon Health & Science University
Portland, OR
Dr. Sutor responds
Ms. Keane’s letter raises important points regarding managing pain in dementia patients.
We agree wholeheartedly that a full pain assessment is warranted when pain is suspected. All patients need and deserve to have their pain managed. There is, however, a danger that a dementia patient with behavior problems may be presumed to have pain when he or she does not and may be given narcotics reflexively.
Ms. Keane’s contention that CNS suppressants (such as benzodiazepines) can be eliminated is germane to our point about opiate analgesics and supports our argument that opiates should be used warily, if at all. Eliminating and avoiding opiates, when possible, reduces the risks of falls, fractures, agitation, delirium, and diminished cognitive function.
Our article should have stated clearly that opiates should be avoided if the cause of pain can be ameliorated or eliminated, or if other “rungs” on the pain ladder effectively manage pain.
It is no myth that some dementia patients are not appropriate candidates for opiates. Those who have suffered falls, fractures, delirium, confusion, and CNS obtundation support the contention that opiates can do more harm than good for some dementia patients.
Bruce Sutor, MD
Assistant professor of psychiatry
Department of psychiatry and psychology
Mayo Clinic College of Medicine
Rochester, MN
Reference
1. American Geriatrics Society Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002;50:1-20.
In their article on managing dementia-related behaviors (Current Psychiatry, May 2006), Dr. Bruce Sutor and colleagues advise against using opioids to manage pain in older patients (p. 88). I was disheartened by their comments.
American Geriatrics Society guidelines clearly state that opiate medications are an appropriate choice for treating moderate to severe pain in some elderly persons, including those with dementia.1
Refusing to consider opiates for pain management can lead to severe suffering, especially for a patient who is incapable of communication. If an elder is at risk for falls, perhaps a full pain assessment is warranted and other CNS suppressants such as benzodiazepines can be eliminated. That way, we can treat pain and reduce the risk of falls.
Unfortunately, the myth that certain groups are not appropriate candidates for opiates lives on. We need to assess and treat all elders for pain, whether they are cognitively intact or impaired. As health care professionals, we are obligated to reduce pain and suffering in all patients, especially those who have trouble communicating.
Teresa Keane, RN, MSN, PMHNP
Psychiatric nurse practitioner, Kaiser Permanente Northwest
Adjunct faculty member, Oregon Health & Science University
Portland, OR
Dr. Sutor responds
Ms. Keane’s letter raises important points regarding managing pain in dementia patients.
We agree wholeheartedly that a full pain assessment is warranted when pain is suspected. All patients need and deserve to have their pain managed. There is, however, a danger that a dementia patient with behavior problems may be presumed to have pain when he or she does not and may be given narcotics reflexively.
Ms. Keane’s contention that CNS suppressants (such as benzodiazepines) can be eliminated is germane to our point about opiate analgesics and supports our argument that opiates should be used warily, if at all. Eliminating and avoiding opiates, when possible, reduces the risks of falls, fractures, agitation, delirium, and diminished cognitive function.
Our article should have stated clearly that opiates should be avoided if the cause of pain can be ameliorated or eliminated, or if other “rungs” on the pain ladder effectively manage pain.
It is no myth that some dementia patients are not appropriate candidates for opiates. Those who have suffered falls, fractures, delirium, confusion, and CNS obtundation support the contention that opiates can do more harm than good for some dementia patients.
Bruce Sutor, MD
Assistant professor of psychiatry
Department of psychiatry and psychology
Mayo Clinic College of Medicine
Rochester, MN
In their article on managing dementia-related behaviors (Current Psychiatry, May 2006), Dr. Bruce Sutor and colleagues advise against using opioids to manage pain in older patients (p. 88). I was disheartened by their comments.
American Geriatrics Society guidelines clearly state that opiate medications are an appropriate choice for treating moderate to severe pain in some elderly persons, including those with dementia.1
Refusing to consider opiates for pain management can lead to severe suffering, especially for a patient who is incapable of communication. If an elder is at risk for falls, perhaps a full pain assessment is warranted and other CNS suppressants such as benzodiazepines can be eliminated. That way, we can treat pain and reduce the risk of falls.
Unfortunately, the myth that certain groups are not appropriate candidates for opiates lives on. We need to assess and treat all elders for pain, whether they are cognitively intact or impaired. As health care professionals, we are obligated to reduce pain and suffering in all patients, especially those who have trouble communicating.
Teresa Keane, RN, MSN, PMHNP
Psychiatric nurse practitioner, Kaiser Permanente Northwest
Adjunct faculty member, Oregon Health & Science University
Portland, OR
Dr. Sutor responds
Ms. Keane’s letter raises important points regarding managing pain in dementia patients.
We agree wholeheartedly that a full pain assessment is warranted when pain is suspected. All patients need and deserve to have their pain managed. There is, however, a danger that a dementia patient with behavior problems may be presumed to have pain when he or she does not and may be given narcotics reflexively.
Ms. Keane’s contention that CNS suppressants (such as benzodiazepines) can be eliminated is germane to our point about opiate analgesics and supports our argument that opiates should be used warily, if at all. Eliminating and avoiding opiates, when possible, reduces the risks of falls, fractures, agitation, delirium, and diminished cognitive function.
Our article should have stated clearly that opiates should be avoided if the cause of pain can be ameliorated or eliminated, or if other “rungs” on the pain ladder effectively manage pain.
It is no myth that some dementia patients are not appropriate candidates for opiates. Those who have suffered falls, fractures, delirium, confusion, and CNS obtundation support the contention that opiates can do more harm than good for some dementia patients.
Bruce Sutor, MD
Assistant professor of psychiatry
Department of psychiatry and psychology
Mayo Clinic College of Medicine
Rochester, MN
Reference
1. American Geriatrics Society Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002;50:1-20.
Reference
1. American Geriatrics Society Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002;50:1-20.
My valedictory address
Valedictory: a statement of farewell or leave taking. From pp. stem of L. valedicere “bid farewell,” from vale, imperative of valere “be well” + dicere “to say”
Dear Readers,
I am graduating from my position as editor-in-chief of Current Psychiatry. Four years is enough for high school, college, medical school, or residency training (at least in psychiatry). It is also the right amount of time to have been editor of this journal (although it has taken me closer to 5 years to finish).
Since January 2002, Current Psychiatry has become one of the most widely read journals in its field. It is the only journal I read cover to cover, and I know that is the case for many of you as well. Wherever I go, I hear extremely positive comments from psychiatrists and psychiatric nurse practitioners. I like to think that Current Psychiatry—more than other journals—has put our readers at the center and has always tried to give you “news you can use this week” in your clinical practices.
I am very happy that our editor, Alice Luddington, and the rest of the excellent Quadrant HealthCom Inc. staff will continue to work with you on this journal. I am also happy that my good friend, Henry A. Nasrallah, MD, is assuming the role of editor-in-chief as of next month’s issue.
I plan to work on several exciting new projects this summer. I also plan to read Current Psychiatry for a long time to come. Be well!
Editor’s note: To recognize Dr. Hillard’s many contributions as Current Psychiatry’s founding editor-in-chief, we will list him on our masthead as Editor-in-Chief Emeritus beginning in September.
Valedictory: a statement of farewell or leave taking. From pp. stem of L. valedicere “bid farewell,” from vale, imperative of valere “be well” + dicere “to say”
Dear Readers,
I am graduating from my position as editor-in-chief of Current Psychiatry. Four years is enough for high school, college, medical school, or residency training (at least in psychiatry). It is also the right amount of time to have been editor of this journal (although it has taken me closer to 5 years to finish).
Since January 2002, Current Psychiatry has become one of the most widely read journals in its field. It is the only journal I read cover to cover, and I know that is the case for many of you as well. Wherever I go, I hear extremely positive comments from psychiatrists and psychiatric nurse practitioners. I like to think that Current Psychiatry—more than other journals—has put our readers at the center and has always tried to give you “news you can use this week” in your clinical practices.
I am very happy that our editor, Alice Luddington, and the rest of the excellent Quadrant HealthCom Inc. staff will continue to work with you on this journal. I am also happy that my good friend, Henry A. Nasrallah, MD, is assuming the role of editor-in-chief as of next month’s issue.
I plan to work on several exciting new projects this summer. I also plan to read Current Psychiatry for a long time to come. Be well!
Valedictory: a statement of farewell or leave taking. From pp. stem of L. valedicere “bid farewell,” from vale, imperative of valere “be well” + dicere “to say”
Dear Readers,
I am graduating from my position as editor-in-chief of Current Psychiatry. Four years is enough for high school, college, medical school, or residency training (at least in psychiatry). It is also the right amount of time to have been editor of this journal (although it has taken me closer to 5 years to finish).
Since January 2002, Current Psychiatry has become one of the most widely read journals in its field. It is the only journal I read cover to cover, and I know that is the case for many of you as well. Wherever I go, I hear extremely positive comments from psychiatrists and psychiatric nurse practitioners. I like to think that Current Psychiatry—more than other journals—has put our readers at the center and has always tried to give you “news you can use this week” in your clinical practices.
I am very happy that our editor, Alice Luddington, and the rest of the excellent Quadrant HealthCom Inc. staff will continue to work with you on this journal. I am also happy that my good friend, Henry A. Nasrallah, MD, is assuming the role of editor-in-chief as of next month’s issue.
I plan to work on several exciting new projects this summer. I also plan to read Current Psychiatry for a long time to come. Be well!
Editor’s note: To recognize Dr. Hillard’s many contributions as Current Psychiatry’s founding editor-in-chief, we will list him on our masthead as Editor-in-Chief Emeritus beginning in September.
Editor’s note: To recognize Dr. Hillard’s many contributions as Current Psychiatry’s founding editor-in-chief, we will list him on our masthead as Editor-in-Chief Emeritus beginning in September.