User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Understanding dreams: Tapping a rich resource
Dreams are a rich resource for understanding how the mind integrates waking experience into older memory networks. Any psychiatrist who doubts dreams’ therapeutic value has probably not attended closely to his or her own dreams or become aware of exciting new evidence.
Recent understandings of how memory is processed during sleep are bringing dreams back into clinical importance. Patients can gather clinically useful data while sleeping—not in laboratories but in their own beds. Detecting and interpreting patterns in that data can help you treat patients not responding adequately to other therapies.
CHARTING DREAM SEQUENCES
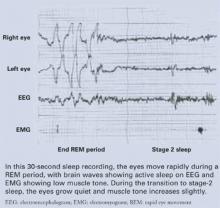
The rate at which the eyes move during rapid-eye movement (REM) sleep (Figure) has been associated with memory consolidation. Eye movements increase during REM sleep, and waking performance improves after intensive learning periods. When eye movements are sparse, patients report dreams with less visual imagery and blander emotional content.1
Though all sleep stages contribute to learning and memory, REM sleep appears to allow wider, easier access to memories2 than does slow-wave sleep or waking. In other words, dreams are far from meaningless. They constitute a continuing mental operation that allows us to modify memory networks of emotional importance to us.
Dreams’ emotional tone tends to shift from negative to positive as the night goes on:3
- Dream-to-dream down-regulation of negative feelings is seen when a person’s waking concerns are strong but not overwhelming.
- Conversely, a dream sequence may show no progress within the night4—and the last dream may be as negative as the first—if the person has reached a point of resignation while awake.
This “sequential hypothesis”5 holds that knowledge of dreams as they occur—one after the other within the night—is a valuable resource for observing how a person is relating waking experience to the past. Dreams thus can give the therapist a “heads up” about a patient’s progress in organizing troublesome feelings.
Figure REM sleep: Dreaming’s prime time
CASE EXAMPLE: NIGHTMARES FOR 13 YEARS
Ms. R, a newlywed at age 30, presents for help with repetitive nightmares that prevent her from sharing a bed with her husband. She was raped at age 17 and has suffered nightmares since then. Once or more nightly she dreams of being attacked and awakens in terror, with profuse sweating. She usually has to change her nightclothes and sometimes the sheets.
Her therapist gives Ms. R four rules—the RISC method6—for shifting her dreams from negative to positive:
- Recognize that the dream is not going well.
- Identify what about it is frightening.
- Stop the dream, even if she must force her eyes open.
- Change the action to something positive.
At the third therapy session, Ms. R reports she had a successful dream. She was lying on her back on an open elevator platform. The elevator was rising dangerously high over the cityscape. She realized she was afraid and got up to see what was happening. As she arose, the elevator walls rose up to protect her. The patient says she learned if she “stands up for herself” all would be well.
After two more sessions with successful practice of this skill, she terminates therapy. When called 1 year later, she says she is expecting a child and has only an occasional nightmare, which she feels she can handle.
CLINICAL USES OF DREAM THERAPY
Dream interpretation may help us understand emotional programs that underlie patients’ unsatisfactory waking behavior. For example:
- Victims of posttraumatic stress disorder (PTSD) such as Ms. R may suffer repetitive nightmares with recurrent themes and excessive negative feelings. We can encourage them to shift their dream scripts from negative to positive.7
- Uninsightful, alexythymic patients, who often leave treatment before deriving any benefit, may learn to understand themselves by becoming aware of their dreams.8
- Severely depressed patientsoften have limited dream recall during sleep studies—even when every REM period is interrupted. They may be taught to improve their dream recall.9
Rules for improving dream recall are few, simple, and effective when the sleeper is motivated to remember them (Box). Just as one can learn to awaken before the morning alarm clock goes off, patients can learn to awaken to recall a dream.
After you have enough of a patient’s dreams to work from—20 is a good start—look for repeated dimensions that are the dreams’ building blocks.5 Look for polar opposites—such as safe-at risk, foolish-clever, exposed-hidden, strong-weak, attractive-ugly—that describe major characteristics of the self figure. Each has a positive or negative emotional value that can be explored.
Go to sleep intending to remember a dream as you awaken.
Sleep until you wake up naturally.* Spontaneous awakening is likely to be from REM sleep, which is dominant in the last third of the night.
Once awake, lie perfectly still. Do not jump up or open your eyes. This preserves a REM-like state when attention is focused inward, not on outside stimuli, and motor tone is profoundly reduced.
Rehearse the recalled images, and give the theme a title (“I left my briefcase on the train” or “My husband returned from a trip unexpectedly”), which makes dream details easier to recall.
Write or tape record all that you can remember, noting the date and time of the report.
Add a note about anything the dream brings to mind about your thoughts before sleep.
* To allow spontaneous awakening, practice dream recall when you do not have to wake up to an alarm clock, such as on weekends.
WHAT TURNED US OFF ABOUT DREAMS
Prolonged therapy. Freud’s The Interpretation of Dreams 10 was a major influence on how therapists used dreams to understand their patients in the early 20th century. Freud concluded that dreams —however strange—represent hallucinated fulfillment of repressed early wishes and tie up psychic energy to conceal unacceptable desires.
From this point of view, dreams provide a road map to understand persistent, nonrational, sometimes self-defeating behaviors that bring patients to therapy. The map, however, was more maze than speedway, full of detours and requiring much time to navigate the boulevards of associations that lead from one dream element to the next. Erik Erickson11 suggested that dreams fell into disuse as therapeutic tools in the 1930s because psychoanalysis in general—and dream interpretation in particular—did not fit the American value of “the faster the better.”
In retrospect, the unconscious mind’s defenses may not have been what prolonged efforts to understand dream meaning. Rather, it may have been that the analyst had to work from whatever scraps the patient could remember of past dreams, to say nothing of new ones that occurred since the last appointment. That one dream could occupy many treatment hours did not trouble the Freudians, however, as—they argued—any-thing the patient does remember is proof of its importance.
Sleep research dealt the worst blow to the pursuit of dreams’ meaning and function.12,13 Contrary to Freud’s view, dreams are not elusive if caught in the act. They can be reliably retrieved from REM episodes, which occur three to five times nightly with great regularity.
After REM sleep was found to initiate from the “unthinking pons,” dreams were proclaimed to have no inherent meaning worthy of serious effort. At best, they were explained as the result of random stimuli producing images to which we add meaning as we awaken. Thus, they offer no unique contribution to understanding psychic life. This “activation-synthesis” hypothesis14 wiped out dream research funding.
Medications. The pharmacologic revolution allowed more-rapid relief of anxiety and depression symptoms than did dream interpretation. Clinicians may feel that “we can forget about dreams because we now have better options.” This ignores research showing that psychiatric medication plus psychotherapy is more effective for a longer time than either alone.15
Evidence-based medicine. Dream content is inherently subjective and not open to objective observation, the heart of scientific methods. Only the dreamer can say what was dreamed.
WHAT BROUGHT DREAMS BACK
Better methods and more-sophisticated models renewed dream interpretation as a useful adjunct to other psychotherapies. In addition to research in memory processing, imaging methods and neuropsychological testing have changed our understanding of brain activity during sleep.
Brain imaging. Early sleep study was limited to recordings from the scalp surface. Now positron emission tomography and functional magnetic imagery allow researchers to see changes in brain activity and to study patterns during waking, non-REM sleep, and REM sleep and among clinical groups. Nofzinger et al,16 for example, showed differences in areas of high and low brain activation in persons with major depression, compared with normal controls.
Neuropsychological testing. Solms2 used neuropsychological testing and interviewing to identify waking cognitive deficits and changes in dream experience in persons with brain damage from surgery or accident. Contrary to the “activation-synthesis” model, he concluded that dreams “are both generated and represented by some of the highest mental mechanisms.” He also argued that although dreams often coincide with the REM state, they also occur beyond REM.
COMING SOON: AT-HOME SLEEP MONITORS
In a sleep laboratory, awakening sleepers during REM periods and asking them to tell what they remember is the classic method for examining the relation among a night’s dreams.17-19 This cumbersome and expensive procedure is being simplified for home use.
Computer-linked monitors are being developed that awaken the sleeper after a pre-set number of rapid eye movements and start a tape recorder to which the person can tell his or her dream. This system, which preserves the dream story from memory loss or distortion,20,21 can easily record three or four dreams each night.
Translating this sensory data into verbal reports remains difficult. Although repeated elements give clues to dream structure, a repeated theme within a night might be an artifact induced by waking the sleeper to ask for a report. If a sleeper reports dreaming about being in an accident, for example, he may be influenced to continue that line of thought as he is falling back to sleep. This, in turn, may influence the next dream.
What else can we do? Because dream recall is ephemeral at best, patients may need training before a therapist has a sample large enough to extract repeating elements and be confident of its reliability.
Related resources
- Schneider A, Domhoff GW. Psychology department, University of California, Santa Cruz. Web site for collecting dream reports. www.DreamBank.net.
- American Psychological Association. Dreaming. Quarterly multidisciplinary journal. www.apa.org/journals/drm/.
Disclosures
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pivik R. Tonic states and phasic events in relation to sleep mentation. In: Ellman S, Antrobus J (eds). The mind in sleep. New York: John Wiley and Sons, 1991;214-47.
2. Solms M. The neuropsychology of dreams. Mahwah, NJ: Lawrence Erlbaum Associates; 1997.
3. Cartwright R, Luten A, Young M, et al. Role of REM sleep and dream affect in overnight mood regulation: a study of normals. Psychiatry Res 1998a;81:1-8.
4. Cartwright R, Young M, Mercer P, Bears M. Role of REM sleep and dream variables in the prediction of remission from depression. Psychiatry Res 1998b;80:249-55.
5. Giuditta A, Mandile P, Montagnese P, et al. The role of sleep in memory processing: the sequential hypothesis. In: Maquet P, Smith C, Stickgold R (eds). Sleep and brain plasticity. Oxford, UK: Oxford University Press; 2003.
6. Cartwright R, Lamberg L. Crisis dreaming. New York: Harper Collins; 1993;42-51.
7. Armitage R, Rochlen A, Fitch T, et al. Dream recall and major depression: a preliminary report. Dreaming 1995;5:189-98.
8. Cartwright R, Tipton L, Wicklund J. Focusing on dreams: a preparation program for psychotherapy. Arch Gen Psychiatry 1980;37:275-7.
9. Riemann D, Wiegand M, Majer-Trendal K, et al. Dream recall and dream content in depressive patients, patients with anorexia nervosa and normal controls. In: Koella W, Obal F, Schulz H, Viss P (eds). Sleep ’86. Stuttgart: Gustav Fischer; 1988;373-6.
10. Freud S. The interpretation of dreams. New York: Basic Books; 1955.
11. Erickson E. The dream specimen in psychoanalysis. In: Knight R, Friedman C (eds). Psychoanalytic psychiatry and psychology. New York: International Press; 1954.
12. Aserinsky E, Kleitman N. Regularly occurring periods of eye motility and concomitant phenomena during sleep. Science 1953;118:273.-
13. Dement W, Kleitman N. Relation of eye movements during sleep to dream activity: objective method for the study of dreaming. J Exper Psychol 1957;53:339-46.
14. Hobson JA, McCarley R. The brain as a dream state generator: An activation-synthesis hypothesis of the dream process. Am J Psychiatry 1977;134:1335-48.
15. Weissman M, Klerman G, Prusoff B, et al. Depressed outpatients one year after treatment with drugs and/or interpersonal psychotherapy. Arch Gen Psychiatry 1981;38:51-5.
16. Nofzinger E, Buysse D, Germain A, et al. Increased activation of anterior paralimbic and executive cortex from waking to rapid eye movement sleep in depression. Arch Gen Psychiatry 2004;61:695-702.
17. Rechtschaffen A, Vogel G, Shaikun G. Interrelatedness of mental activity during sleep. Arch Gen Psychiatry 1963;9:536-47.
18. Verdone P. Temporal reference of manifest dream content. Percept Motor Skills 1965;20:1253.-
18. Cartwright R. Night life: explorations in dreaming. Englewood Cliffs, NJ: Prentice-Hall; 1977;18-31.
20. Mamelak A, Hobson JA. Nightcap: a home-based sleep monitoring system. Sleep 1989;12:157-66.
21. Lloyd S, Cartwright R. The collection of home and laboratory dreams by means of an instrumental response technique. Dreaming 1995;5:63-73.
Dreams are a rich resource for understanding how the mind integrates waking experience into older memory networks. Any psychiatrist who doubts dreams’ therapeutic value has probably not attended closely to his or her own dreams or become aware of exciting new evidence.
Recent understandings of how memory is processed during sleep are bringing dreams back into clinical importance. Patients can gather clinically useful data while sleeping—not in laboratories but in their own beds. Detecting and interpreting patterns in that data can help you treat patients not responding adequately to other therapies.
CHARTING DREAM SEQUENCES
The rate at which the eyes move during rapid-eye movement (REM) sleep (Figure) has been associated with memory consolidation. Eye movements increase during REM sleep, and waking performance improves after intensive learning periods. When eye movements are sparse, patients report dreams with less visual imagery and blander emotional content.1
Though all sleep stages contribute to learning and memory, REM sleep appears to allow wider, easier access to memories2 than does slow-wave sleep or waking. In other words, dreams are far from meaningless. They constitute a continuing mental operation that allows us to modify memory networks of emotional importance to us.
Dreams’ emotional tone tends to shift from negative to positive as the night goes on:3
- Dream-to-dream down-regulation of negative feelings is seen when a person’s waking concerns are strong but not overwhelming.
- Conversely, a dream sequence may show no progress within the night4—and the last dream may be as negative as the first—if the person has reached a point of resignation while awake.
This “sequential hypothesis”5 holds that knowledge of dreams as they occur—one after the other within the night—is a valuable resource for observing how a person is relating waking experience to the past. Dreams thus can give the therapist a “heads up” about a patient’s progress in organizing troublesome feelings.
Figure REM sleep: Dreaming’s prime time
CASE EXAMPLE: NIGHTMARES FOR 13 YEARS
Ms. R, a newlywed at age 30, presents for help with repetitive nightmares that prevent her from sharing a bed with her husband. She was raped at age 17 and has suffered nightmares since then. Once or more nightly she dreams of being attacked and awakens in terror, with profuse sweating. She usually has to change her nightclothes and sometimes the sheets.
Her therapist gives Ms. R four rules—the RISC method6—for shifting her dreams from negative to positive:
- Recognize that the dream is not going well.
- Identify what about it is frightening.
- Stop the dream, even if she must force her eyes open.
- Change the action to something positive.
At the third therapy session, Ms. R reports she had a successful dream. She was lying on her back on an open elevator platform. The elevator was rising dangerously high over the cityscape. She realized she was afraid and got up to see what was happening. As she arose, the elevator walls rose up to protect her. The patient says she learned if she “stands up for herself” all would be well.
After two more sessions with successful practice of this skill, she terminates therapy. When called 1 year later, she says she is expecting a child and has only an occasional nightmare, which she feels she can handle.
CLINICAL USES OF DREAM THERAPY
Dream interpretation may help us understand emotional programs that underlie patients’ unsatisfactory waking behavior. For example:
- Victims of posttraumatic stress disorder (PTSD) such as Ms. R may suffer repetitive nightmares with recurrent themes and excessive negative feelings. We can encourage them to shift their dream scripts from negative to positive.7
- Uninsightful, alexythymic patients, who often leave treatment before deriving any benefit, may learn to understand themselves by becoming aware of their dreams.8
- Severely depressed patientsoften have limited dream recall during sleep studies—even when every REM period is interrupted. They may be taught to improve their dream recall.9
Rules for improving dream recall are few, simple, and effective when the sleeper is motivated to remember them (Box). Just as one can learn to awaken before the morning alarm clock goes off, patients can learn to awaken to recall a dream.
After you have enough of a patient’s dreams to work from—20 is a good start—look for repeated dimensions that are the dreams’ building blocks.5 Look for polar opposites—such as safe-at risk, foolish-clever, exposed-hidden, strong-weak, attractive-ugly—that describe major characteristics of the self figure. Each has a positive or negative emotional value that can be explored.
Go to sleep intending to remember a dream as you awaken.
Sleep until you wake up naturally.* Spontaneous awakening is likely to be from REM sleep, which is dominant in the last third of the night.
Once awake, lie perfectly still. Do not jump up or open your eyes. This preserves a REM-like state when attention is focused inward, not on outside stimuli, and motor tone is profoundly reduced.
Rehearse the recalled images, and give the theme a title (“I left my briefcase on the train” or “My husband returned from a trip unexpectedly”), which makes dream details easier to recall.
Write or tape record all that you can remember, noting the date and time of the report.
Add a note about anything the dream brings to mind about your thoughts before sleep.
* To allow spontaneous awakening, practice dream recall when you do not have to wake up to an alarm clock, such as on weekends.
WHAT TURNED US OFF ABOUT DREAMS
Prolonged therapy. Freud’s The Interpretation of Dreams 10 was a major influence on how therapists used dreams to understand their patients in the early 20th century. Freud concluded that dreams —however strange—represent hallucinated fulfillment of repressed early wishes and tie up psychic energy to conceal unacceptable desires.
From this point of view, dreams provide a road map to understand persistent, nonrational, sometimes self-defeating behaviors that bring patients to therapy. The map, however, was more maze than speedway, full of detours and requiring much time to navigate the boulevards of associations that lead from one dream element to the next. Erik Erickson11 suggested that dreams fell into disuse as therapeutic tools in the 1930s because psychoanalysis in general—and dream interpretation in particular—did not fit the American value of “the faster the better.”
In retrospect, the unconscious mind’s defenses may not have been what prolonged efforts to understand dream meaning. Rather, it may have been that the analyst had to work from whatever scraps the patient could remember of past dreams, to say nothing of new ones that occurred since the last appointment. That one dream could occupy many treatment hours did not trouble the Freudians, however, as—they argued—any-thing the patient does remember is proof of its importance.
Sleep research dealt the worst blow to the pursuit of dreams’ meaning and function.12,13 Contrary to Freud’s view, dreams are not elusive if caught in the act. They can be reliably retrieved from REM episodes, which occur three to five times nightly with great regularity.
After REM sleep was found to initiate from the “unthinking pons,” dreams were proclaimed to have no inherent meaning worthy of serious effort. At best, they were explained as the result of random stimuli producing images to which we add meaning as we awaken. Thus, they offer no unique contribution to understanding psychic life. This “activation-synthesis” hypothesis14 wiped out dream research funding.
Medications. The pharmacologic revolution allowed more-rapid relief of anxiety and depression symptoms than did dream interpretation. Clinicians may feel that “we can forget about dreams because we now have better options.” This ignores research showing that psychiatric medication plus psychotherapy is more effective for a longer time than either alone.15
Evidence-based medicine. Dream content is inherently subjective and not open to objective observation, the heart of scientific methods. Only the dreamer can say what was dreamed.
WHAT BROUGHT DREAMS BACK
Better methods and more-sophisticated models renewed dream interpretation as a useful adjunct to other psychotherapies. In addition to research in memory processing, imaging methods and neuropsychological testing have changed our understanding of brain activity during sleep.
Brain imaging. Early sleep study was limited to recordings from the scalp surface. Now positron emission tomography and functional magnetic imagery allow researchers to see changes in brain activity and to study patterns during waking, non-REM sleep, and REM sleep and among clinical groups. Nofzinger et al,16 for example, showed differences in areas of high and low brain activation in persons with major depression, compared with normal controls.
Neuropsychological testing. Solms2 used neuropsychological testing and interviewing to identify waking cognitive deficits and changes in dream experience in persons with brain damage from surgery or accident. Contrary to the “activation-synthesis” model, he concluded that dreams “are both generated and represented by some of the highest mental mechanisms.” He also argued that although dreams often coincide with the REM state, they also occur beyond REM.
COMING SOON: AT-HOME SLEEP MONITORS
In a sleep laboratory, awakening sleepers during REM periods and asking them to tell what they remember is the classic method for examining the relation among a night’s dreams.17-19 This cumbersome and expensive procedure is being simplified for home use.
Computer-linked monitors are being developed that awaken the sleeper after a pre-set number of rapid eye movements and start a tape recorder to which the person can tell his or her dream. This system, which preserves the dream story from memory loss or distortion,20,21 can easily record three or four dreams each night.
Translating this sensory data into verbal reports remains difficult. Although repeated elements give clues to dream structure, a repeated theme within a night might be an artifact induced by waking the sleeper to ask for a report. If a sleeper reports dreaming about being in an accident, for example, he may be influenced to continue that line of thought as he is falling back to sleep. This, in turn, may influence the next dream.
What else can we do? Because dream recall is ephemeral at best, patients may need training before a therapist has a sample large enough to extract repeating elements and be confident of its reliability.
Related resources
- Schneider A, Domhoff GW. Psychology department, University of California, Santa Cruz. Web site for collecting dream reports. www.DreamBank.net.
- American Psychological Association. Dreaming. Quarterly multidisciplinary journal. www.apa.org/journals/drm/.
Disclosures
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dreams are a rich resource for understanding how the mind integrates waking experience into older memory networks. Any psychiatrist who doubts dreams’ therapeutic value has probably not attended closely to his or her own dreams or become aware of exciting new evidence.
Recent understandings of how memory is processed during sleep are bringing dreams back into clinical importance. Patients can gather clinically useful data while sleeping—not in laboratories but in their own beds. Detecting and interpreting patterns in that data can help you treat patients not responding adequately to other therapies.
CHARTING DREAM SEQUENCES
The rate at which the eyes move during rapid-eye movement (REM) sleep (Figure) has been associated with memory consolidation. Eye movements increase during REM sleep, and waking performance improves after intensive learning periods. When eye movements are sparse, patients report dreams with less visual imagery and blander emotional content.1
Though all sleep stages contribute to learning and memory, REM sleep appears to allow wider, easier access to memories2 than does slow-wave sleep or waking. In other words, dreams are far from meaningless. They constitute a continuing mental operation that allows us to modify memory networks of emotional importance to us.
Dreams’ emotional tone tends to shift from negative to positive as the night goes on:3
- Dream-to-dream down-regulation of negative feelings is seen when a person’s waking concerns are strong but not overwhelming.
- Conversely, a dream sequence may show no progress within the night4—and the last dream may be as negative as the first—if the person has reached a point of resignation while awake.
This “sequential hypothesis”5 holds that knowledge of dreams as they occur—one after the other within the night—is a valuable resource for observing how a person is relating waking experience to the past. Dreams thus can give the therapist a “heads up” about a patient’s progress in organizing troublesome feelings.
Figure REM sleep: Dreaming’s prime time
CASE EXAMPLE: NIGHTMARES FOR 13 YEARS
Ms. R, a newlywed at age 30, presents for help with repetitive nightmares that prevent her from sharing a bed with her husband. She was raped at age 17 and has suffered nightmares since then. Once or more nightly she dreams of being attacked and awakens in terror, with profuse sweating. She usually has to change her nightclothes and sometimes the sheets.
Her therapist gives Ms. R four rules—the RISC method6—for shifting her dreams from negative to positive:
- Recognize that the dream is not going well.
- Identify what about it is frightening.
- Stop the dream, even if she must force her eyes open.
- Change the action to something positive.
At the third therapy session, Ms. R reports she had a successful dream. She was lying on her back on an open elevator platform. The elevator was rising dangerously high over the cityscape. She realized she was afraid and got up to see what was happening. As she arose, the elevator walls rose up to protect her. The patient says she learned if she “stands up for herself” all would be well.
After two more sessions with successful practice of this skill, she terminates therapy. When called 1 year later, she says she is expecting a child and has only an occasional nightmare, which she feels she can handle.
CLINICAL USES OF DREAM THERAPY
Dream interpretation may help us understand emotional programs that underlie patients’ unsatisfactory waking behavior. For example:
- Victims of posttraumatic stress disorder (PTSD) such as Ms. R may suffer repetitive nightmares with recurrent themes and excessive negative feelings. We can encourage them to shift their dream scripts from negative to positive.7
- Uninsightful, alexythymic patients, who often leave treatment before deriving any benefit, may learn to understand themselves by becoming aware of their dreams.8
- Severely depressed patientsoften have limited dream recall during sleep studies—even when every REM period is interrupted. They may be taught to improve their dream recall.9
Rules for improving dream recall are few, simple, and effective when the sleeper is motivated to remember them (Box). Just as one can learn to awaken before the morning alarm clock goes off, patients can learn to awaken to recall a dream.
After you have enough of a patient’s dreams to work from—20 is a good start—look for repeated dimensions that are the dreams’ building blocks.5 Look for polar opposites—such as safe-at risk, foolish-clever, exposed-hidden, strong-weak, attractive-ugly—that describe major characteristics of the self figure. Each has a positive or negative emotional value that can be explored.
Go to sleep intending to remember a dream as you awaken.
Sleep until you wake up naturally.* Spontaneous awakening is likely to be from REM sleep, which is dominant in the last third of the night.
Once awake, lie perfectly still. Do not jump up or open your eyes. This preserves a REM-like state when attention is focused inward, not on outside stimuli, and motor tone is profoundly reduced.
Rehearse the recalled images, and give the theme a title (“I left my briefcase on the train” or “My husband returned from a trip unexpectedly”), which makes dream details easier to recall.
Write or tape record all that you can remember, noting the date and time of the report.
Add a note about anything the dream brings to mind about your thoughts before sleep.
* To allow spontaneous awakening, practice dream recall when you do not have to wake up to an alarm clock, such as on weekends.
WHAT TURNED US OFF ABOUT DREAMS
Prolonged therapy. Freud’s The Interpretation of Dreams 10 was a major influence on how therapists used dreams to understand their patients in the early 20th century. Freud concluded that dreams —however strange—represent hallucinated fulfillment of repressed early wishes and tie up psychic energy to conceal unacceptable desires.
From this point of view, dreams provide a road map to understand persistent, nonrational, sometimes self-defeating behaviors that bring patients to therapy. The map, however, was more maze than speedway, full of detours and requiring much time to navigate the boulevards of associations that lead from one dream element to the next. Erik Erickson11 suggested that dreams fell into disuse as therapeutic tools in the 1930s because psychoanalysis in general—and dream interpretation in particular—did not fit the American value of “the faster the better.”
In retrospect, the unconscious mind’s defenses may not have been what prolonged efforts to understand dream meaning. Rather, it may have been that the analyst had to work from whatever scraps the patient could remember of past dreams, to say nothing of new ones that occurred since the last appointment. That one dream could occupy many treatment hours did not trouble the Freudians, however, as—they argued—any-thing the patient does remember is proof of its importance.
Sleep research dealt the worst blow to the pursuit of dreams’ meaning and function.12,13 Contrary to Freud’s view, dreams are not elusive if caught in the act. They can be reliably retrieved from REM episodes, which occur three to five times nightly with great regularity.
After REM sleep was found to initiate from the “unthinking pons,” dreams were proclaimed to have no inherent meaning worthy of serious effort. At best, they were explained as the result of random stimuli producing images to which we add meaning as we awaken. Thus, they offer no unique contribution to understanding psychic life. This “activation-synthesis” hypothesis14 wiped out dream research funding.
Medications. The pharmacologic revolution allowed more-rapid relief of anxiety and depression symptoms than did dream interpretation. Clinicians may feel that “we can forget about dreams because we now have better options.” This ignores research showing that psychiatric medication plus psychotherapy is more effective for a longer time than either alone.15
Evidence-based medicine. Dream content is inherently subjective and not open to objective observation, the heart of scientific methods. Only the dreamer can say what was dreamed.
WHAT BROUGHT DREAMS BACK
Better methods and more-sophisticated models renewed dream interpretation as a useful adjunct to other psychotherapies. In addition to research in memory processing, imaging methods and neuropsychological testing have changed our understanding of brain activity during sleep.
Brain imaging. Early sleep study was limited to recordings from the scalp surface. Now positron emission tomography and functional magnetic imagery allow researchers to see changes in brain activity and to study patterns during waking, non-REM sleep, and REM sleep and among clinical groups. Nofzinger et al,16 for example, showed differences in areas of high and low brain activation in persons with major depression, compared with normal controls.
Neuropsychological testing. Solms2 used neuropsychological testing and interviewing to identify waking cognitive deficits and changes in dream experience in persons with brain damage from surgery or accident. Contrary to the “activation-synthesis” model, he concluded that dreams “are both generated and represented by some of the highest mental mechanisms.” He also argued that although dreams often coincide with the REM state, they also occur beyond REM.
COMING SOON: AT-HOME SLEEP MONITORS
In a sleep laboratory, awakening sleepers during REM periods and asking them to tell what they remember is the classic method for examining the relation among a night’s dreams.17-19 This cumbersome and expensive procedure is being simplified for home use.
Computer-linked monitors are being developed that awaken the sleeper after a pre-set number of rapid eye movements and start a tape recorder to which the person can tell his or her dream. This system, which preserves the dream story from memory loss or distortion,20,21 can easily record three or four dreams each night.
Translating this sensory data into verbal reports remains difficult. Although repeated elements give clues to dream structure, a repeated theme within a night might be an artifact induced by waking the sleeper to ask for a report. If a sleeper reports dreaming about being in an accident, for example, he may be influenced to continue that line of thought as he is falling back to sleep. This, in turn, may influence the next dream.
What else can we do? Because dream recall is ephemeral at best, patients may need training before a therapist has a sample large enough to extract repeating elements and be confident of its reliability.
Related resources
- Schneider A, Domhoff GW. Psychology department, University of California, Santa Cruz. Web site for collecting dream reports. www.DreamBank.net.
- American Psychological Association. Dreaming. Quarterly multidisciplinary journal. www.apa.org/journals/drm/.
Disclosures
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pivik R. Tonic states and phasic events in relation to sleep mentation. In: Ellman S, Antrobus J (eds). The mind in sleep. New York: John Wiley and Sons, 1991;214-47.
2. Solms M. The neuropsychology of dreams. Mahwah, NJ: Lawrence Erlbaum Associates; 1997.
3. Cartwright R, Luten A, Young M, et al. Role of REM sleep and dream affect in overnight mood regulation: a study of normals. Psychiatry Res 1998a;81:1-8.
4. Cartwright R, Young M, Mercer P, Bears M. Role of REM sleep and dream variables in the prediction of remission from depression. Psychiatry Res 1998b;80:249-55.
5. Giuditta A, Mandile P, Montagnese P, et al. The role of sleep in memory processing: the sequential hypothesis. In: Maquet P, Smith C, Stickgold R (eds). Sleep and brain plasticity. Oxford, UK: Oxford University Press; 2003.
6. Cartwright R, Lamberg L. Crisis dreaming. New York: Harper Collins; 1993;42-51.
7. Armitage R, Rochlen A, Fitch T, et al. Dream recall and major depression: a preliminary report. Dreaming 1995;5:189-98.
8. Cartwright R, Tipton L, Wicklund J. Focusing on dreams: a preparation program for psychotherapy. Arch Gen Psychiatry 1980;37:275-7.
9. Riemann D, Wiegand M, Majer-Trendal K, et al. Dream recall and dream content in depressive patients, patients with anorexia nervosa and normal controls. In: Koella W, Obal F, Schulz H, Viss P (eds). Sleep ’86. Stuttgart: Gustav Fischer; 1988;373-6.
10. Freud S. The interpretation of dreams. New York: Basic Books; 1955.
11. Erickson E. The dream specimen in psychoanalysis. In: Knight R, Friedman C (eds). Psychoanalytic psychiatry and psychology. New York: International Press; 1954.
12. Aserinsky E, Kleitman N. Regularly occurring periods of eye motility and concomitant phenomena during sleep. Science 1953;118:273.-
13. Dement W, Kleitman N. Relation of eye movements during sleep to dream activity: objective method for the study of dreaming. J Exper Psychol 1957;53:339-46.
14. Hobson JA, McCarley R. The brain as a dream state generator: An activation-synthesis hypothesis of the dream process. Am J Psychiatry 1977;134:1335-48.
15. Weissman M, Klerman G, Prusoff B, et al. Depressed outpatients one year after treatment with drugs and/or interpersonal psychotherapy. Arch Gen Psychiatry 1981;38:51-5.
16. Nofzinger E, Buysse D, Germain A, et al. Increased activation of anterior paralimbic and executive cortex from waking to rapid eye movement sleep in depression. Arch Gen Psychiatry 2004;61:695-702.
17. Rechtschaffen A, Vogel G, Shaikun G. Interrelatedness of mental activity during sleep. Arch Gen Psychiatry 1963;9:536-47.
18. Verdone P. Temporal reference of manifest dream content. Percept Motor Skills 1965;20:1253.-
18. Cartwright R. Night life: explorations in dreaming. Englewood Cliffs, NJ: Prentice-Hall; 1977;18-31.
20. Mamelak A, Hobson JA. Nightcap: a home-based sleep monitoring system. Sleep 1989;12:157-66.
21. Lloyd S, Cartwright R. The collection of home and laboratory dreams by means of an instrumental response technique. Dreaming 1995;5:63-73.
1. Pivik R. Tonic states and phasic events in relation to sleep mentation. In: Ellman S, Antrobus J (eds). The mind in sleep. New York: John Wiley and Sons, 1991;214-47.
2. Solms M. The neuropsychology of dreams. Mahwah, NJ: Lawrence Erlbaum Associates; 1997.
3. Cartwright R, Luten A, Young M, et al. Role of REM sleep and dream affect in overnight mood regulation: a study of normals. Psychiatry Res 1998a;81:1-8.
4. Cartwright R, Young M, Mercer P, Bears M. Role of REM sleep and dream variables in the prediction of remission from depression. Psychiatry Res 1998b;80:249-55.
5. Giuditta A, Mandile P, Montagnese P, et al. The role of sleep in memory processing: the sequential hypothesis. In: Maquet P, Smith C, Stickgold R (eds). Sleep and brain plasticity. Oxford, UK: Oxford University Press; 2003.
6. Cartwright R, Lamberg L. Crisis dreaming. New York: Harper Collins; 1993;42-51.
7. Armitage R, Rochlen A, Fitch T, et al. Dream recall and major depression: a preliminary report. Dreaming 1995;5:189-98.
8. Cartwright R, Tipton L, Wicklund J. Focusing on dreams: a preparation program for psychotherapy. Arch Gen Psychiatry 1980;37:275-7.
9. Riemann D, Wiegand M, Majer-Trendal K, et al. Dream recall and dream content in depressive patients, patients with anorexia nervosa and normal controls. In: Koella W, Obal F, Schulz H, Viss P (eds). Sleep ’86. Stuttgart: Gustav Fischer; 1988;373-6.
10. Freud S. The interpretation of dreams. New York: Basic Books; 1955.
11. Erickson E. The dream specimen in psychoanalysis. In: Knight R, Friedman C (eds). Psychoanalytic psychiatry and psychology. New York: International Press; 1954.
12. Aserinsky E, Kleitman N. Regularly occurring periods of eye motility and concomitant phenomena during sleep. Science 1953;118:273.-
13. Dement W, Kleitman N. Relation of eye movements during sleep to dream activity: objective method for the study of dreaming. J Exper Psychol 1957;53:339-46.
14. Hobson JA, McCarley R. The brain as a dream state generator: An activation-synthesis hypothesis of the dream process. Am J Psychiatry 1977;134:1335-48.
15. Weissman M, Klerman G, Prusoff B, et al. Depressed outpatients one year after treatment with drugs and/or interpersonal psychotherapy. Arch Gen Psychiatry 1981;38:51-5.
16. Nofzinger E, Buysse D, Germain A, et al. Increased activation of anterior paralimbic and executive cortex from waking to rapid eye movement sleep in depression. Arch Gen Psychiatry 2004;61:695-702.
17. Rechtschaffen A, Vogel G, Shaikun G. Interrelatedness of mental activity during sleep. Arch Gen Psychiatry 1963;9:536-47.
18. Verdone P. Temporal reference of manifest dream content. Percept Motor Skills 1965;20:1253.-
18. Cartwright R. Night life: explorations in dreaming. Englewood Cliffs, NJ: Prentice-Hall; 1977;18-31.
20. Mamelak A, Hobson JA. Nightcap: a home-based sleep monitoring system. Sleep 1989;12:157-66.
21. Lloyd S, Cartwright R. The collection of home and laboratory dreams by means of an instrumental response technique. Dreaming 1995;5:63-73.
When treatment spells trouble
HISTORY: ‘THEY’RE TRYING TO KILL ME’
For the past 7 months Ms. G, age 47, has had worsening paranoid thoughts and sleep disturbances. She sleeps 4 hours or less a night, and her appetite and energy are diminished.
Her mother reports that Ms. G, who lives in an extended-care facility, believes the staff has injected embalming fluid into her body and is plotting to kill her. She says her daughter also has “fits” during which she hears a deafening noise that sounds like a vacuum cleaner, followed by a feeling of being pushed to the ground. Ms. G tells us that someone or something invisible is trying to control her.
Ms. G was diagnosed 2 years ago as having Parkinson’s disease and has chronically high liver transaminase enzymes. She also has moderate mental retardation secondary to cerebral palsy. She fears she will be harmed if she stays at the extendedcare facility, but we find no evidence that she has been abused or mistreated there.
Three months before presenting to us, Ms. G was hospitalized for 3 days to treat symptoms that suggested neuroleptic malignant syndrome (NMS) but were apparently caused by her inadvertently stopping her antiparkinson agents.
One month later, Ms. G was hospitalized again, this time for acute psychosis. Quetiapine, which she had been taking for antiparkinson medication-induced psychosis, was increased from 100 mg nightly to 75 mg bid, with reportedly good effect.
Shortly afterward, however, Ms. G’s paranoia worsened. At the facility, she has called 911 several times to report imagined threats from staff members. After referral from her primary care physician, we evaluate Ms. G and admit her to the adult inpatient psychiatric unit.
At intake, Ms. G is anxious and uncomfortable with notable muscle spasticity and twitching of her arms and legs. Mostly wheelchair-bound, she has longstanding physical abnormalities (shuffling gait; dystonia; drooling; slowed, dysarthric speech) secondary to comorbid Parkinson’s and cerebral palsy. She is agitated at first but grows calmer and cooperative.
Mental status examination shows a disorganized, tangential thought process and evidence of paranoid delusions and auditory hallucinations, but she denies visual hallucinations. She has poor insight into her illness but is oriented to time, place, and person. She can recall two of three objects after 3 minutes of distraction. Attention and concentration are intact.
Ms. G denies depressed mood, anhedonia, mania, or suicidal or homicidal thoughts. Her mother says no stressors other than the imagined threats to her life have affected her daughter.
The patient ’s temperature at admission is 98.0°F, her pulse is 108 beats per minute, and her blood pressure is 150/88 mm Hg. Laboratory workup shows a white blood cell count of 10,100/mm3 (normal range: 4,000 to 10,000/mm3), sodium level of 132 mEq/L (normal range: 135 to 145 mEq/L), and aspartate (AST) and alanine (ALT) transaminase levels of 611 U/L and 79 U/L, respectively (normal range for each: 0 to 35 U/L).
Aside from quetiapine, Ms. G also has been taking carbidopa/levodopa, seven 25/100-mg tablets daily, and pramipexole, 3 mg/d, for parkinsonism; citalopram, 20 mg/d, for depression; trazodone, 300 mg nightly, and lorazepam, 0.5 mg nightly, for insomnia; lopressor, 25 mg every 12 hours, for hypertension; and tolterodine, 1 mg bid, for urinary incontinence.
The authors’ observations
Parkinsonism typically responds to dopaminergic treatment. Excess dopamine agonism is believed to contribute to medication-induced psychosis, a common and often disabling complication of Parkinson’s disease1,2 that often necessitates nursing home placement and may increase mortality.2,3
Paranoia occurs in approximately 8% of patients treated for drug-induced Parkinson’s psychosis, and hallucinations (typically visual) may occur in as many as 30%.2 Quetiapine, 50 to 225 mg/d, is considered a good first-line treatment for psychosis in Parkinson’s, although the agent has been tested for this use only in open-label trials.2,3
Mental retardation and pre-existing parkinsonism, however, may increase Ms. G’s risk for NMS, a rare but potentially fatal reaction to antipsychotics believed to be caused by a sudden D2 dopamine receptor blockade.4,5 Signs include autonomic instability, extrapyramidal symptoms, hyperpyrexia, and altered mental status.
Of 68 patients with NMS studied by Ananth et al,4 13.2% were mentally retarded, and uncontrolled studies6 have proposed mental retardation as a potential risk factor (Table 1). A 2003 case control study6 found a higher incidence of NMS among mentally retarded patients than among nonretarded persons, but the difference was not statistically significant. There are no known links between specific causes of mental retardation and NMS.
Even so, Ms. G’s psychosis is compromising her already diminished quality of life. We will increase her quetiapine dosage slightly and watch for early signs of NMS, including fever, confusion, and increased muscle rigidity.
Table 1
Factors that increase risk of neuroleptic malignant syndrome*
| Abrupt antipsychotic cessation |
| Ambient heat |
| Catatonia |
| Dehydration |
| Exhaustion |
| Genetic predisposition |
| Greater dosage increases |
| Higher neuroleptic doses, especially with typical and atypical IM agents |
| Low serum iron |
| Malnutrition |
| Mental retardation |
| Pre-existing EPS or parkinsonism |
| Previous NMS episode |
| Psychomotor agitation |
| * Infection or concurrent organic brain disease are predisposing factors, but their association with NMS is less clear. |
| EPS: extrapyramidal symptoms |
| Source: References: 4-7, 14-15. |
TREATMENT: MEDICATION CHANGE
Upon admission, quetiapine is increased to 75 mg in the morning and 125 mg at bedtime—still well below the dosage at which quetiapine increases the risk of NMS (Table 2). Trazodone is decreased to 100 mg/d because of quetiapine’s sedating properties. Citalopram and tolterodine are stopped for fear that either agent would aggravate her psychosis. We continue all other drugs as previously prescribed. Her paranoia begins to subside.
Three days later, Ms. G’s is increasingly confused and agitated, and her temperature rises to 101.3°F. Physical exam shows increased muscle rigidity. She is given lorazepam, 1 mg, and transferred to the emergency room for evaluation.
In the ER, Ms. G’s temperature rises to 102.3°F. Other vital signs include:
- heart rate, 112 to 120 beats per minute
- respiratory rate, 18 to 20 breaths per minute
- oxygen saturation, 98% in room air
- blood pressure, 131/61 mm Hg while seated and 92/58 mm Hg while standing.
CNS or systemic infection and myocardial infarction are considered less likely because of her reactive pupils, lack of nuchal rigidity, troponin 3. Additionally, CSF shows normal glucose and protein levels, ALT and AST are 217 and 261 U/L, respectively, and chest x-ray shows no acute cardiopulmonary abnormality.
Ms. G is admitted to the medical intensive care unit and given IV fluids. All psychotropic and antiparkinson medications are stopped for 12 hours. Ms. G is then transferred to the general medical service for continued observation and IV hydration.
Six hours later, lorazepam, 0.5 mg every 8 hours, is resumed to control Ms. G’s anxiety. Carbidopa/levodopa is resumed at the previous dosage; all other medications remain on hold.
Renal damage is not apparent, but repeat chest x-ray taken 2 days after admission to the ER shows right middle lobe pneumonia, which resolved with antibiotics.
Six days after entering the medical unit, Ms. G is no longer agitated or paranoid. She is discharged that day and continued on lorazepam, 1 mg every 8 hours as needed to control her anxiety and prevent paranoia, and carbidopa/levidopa 8-1/2 25/100-mg tablets daily for her parkinsonism. Trazodone, 100 mg nightly, is continued for 3 days to help her sleep, as is amoxicillin/clavulanate, 500 mg every 8 hours, in case an underlying infection exists. Quetiapine, citalopram, and tolterodine are discontinued; all other medications are resumed as previously prescribed.
Table 2
Antipsychotic-related NMS risk increases at these dosages
| Agent | Dosage (mg/d) |
|---|---|
| Aripiprazole | >30 |
| Chlorpromazine | >400 |
| Clozapine | 318+/-299 |
| Olanzapine | 9.7+/-2.3 |
| Quetiapine | 412.5+/-317 |
| Risperidone | 4.3+/-3.1 |
| Ziprasidone | >20 |
| Source: References 4, 6, and 15. | |
The authors’ observations
Ms. G’s NMS symptoms surfaced 3 days after her quetiapine dosage was increased, suggesting that the antipsychotic may have caused this episode.
We ruled out antiparkinson agent withdrawal malignant syndrome—usually caused by abrupt cessation of Parkinson’s medications. Ms. G’s carbidopa/levodopa had not been adjusted before the symptoms emerged, and she did not worsen after the agent was stopped temporarily. Her brief pneumonia episode, however, could have caused symptoms that mimicked this withdrawal syndrome.
Antiparkinson agent withdrawal malignant syndrome symptoms resemble those of NMS.9,10 Worsening parkinsonism, dehydration, and infection increase the risk.10 Some research suggests that leukocytosis or elevated inflammation-related cytokines may accelerate withdrawal syndrome.10
The authors’ observations
Ms. G’s case illustrates the difficulty of treating psychosis in a patient at risk for NMS.
Of the 68 patients in the Ananth et al study with atypical antipsychotic-induced NMS, 11 were rechallenged after an NMS episode with the same agent and 8 were switched to another atypical. NMS recurred in 4 of these 19 patients.4
Ms. G was stable on lorazepam at discharge, but we would consider rechallenging with quetiapine or another antipsychotic if necessary. NMS recurs in 30% to 50%11 of patients after antipsychotic rechallenge, but waiting 2 weeks to resume antipsychotic therapy appears to reduce this risk.12 Benzodiazepines and electroconvulsive therapy are acceptable—though unproven—second-line therapies if antipsychotic rechallenge is deemed too risky,11,13 such as in some patients with a previous severe NMS episode; evidence of stroke, Parkinson’s or other neurodegenerative disease; or multiple acute medical problems.
CONTINUED TREATMENT: A RELAPSE
Three months later, Ms. G is readmitted to the neurology service for 3 weeks after being diagnosed with elevated CK, possibly caused by NMS or rhabdomyolysis secondary to persistent dyskinesia. We believe an inadvertent decrease in her carbidopa/levodopa caused the episode, as she had taken no neuroleptics between hospitalizations.
Ms. G is discharged on quetiapine, 25 mg nightly, along with her other medications. Her current psychiatric and neurologic status is unknown.
The authors’ observations
Detecting NMS symptoms early is critical to preventing mortality. Although NMS risk with atypical and typical antipsychotics is similar,4 fewer deaths from NMS have been reported after use of atypicals (3 deaths among 68 cases) than typical neuroleptics (30% mortality rate in the 1960s and 70s, and 10% mortality from 1980-87).14 Earlier recognition and treatment may be decreasing NMS-related mortality.4
Consider NMS in the differential diagnosis when the patient’s mental status changes.
Related resources
- Emedicine: Neuroleptic malignant syndrome. www.emedicine.com/med/topic2614.htm.
- Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin 2004;22:389-411.
- Susman VL. Clinical management of neuroleptic malignant syndrome. Psychiatr Q 2001;72:325-36.
- Amoxicillin/clavulanate • Augmentin
- Aripiprazole • Abilify
- Carbidopa/levodopa • Sinemet
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Lopressor • Toprol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Pramipexole • Mirapex
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Tolterodine • Detrol
- Trazodone • Desyrel
- Ziprasidone • Geodon
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors wish to thank Robert B. Milstein, MD, PhD, and Benjamin Zigun, MD, JD, for their help in preparing this article for publication.
This project is supported by funds from the Division of State, Community, and Public Health, Bureau of Health Professions (BHPr), Health Resources and Services Administration (HSRA), Department of Health and Human Services (DHHS) under grant number 1 K01 HP 00071-02 and Geriatric Academic Career Award ($58,009). The content and conclusion are those of Dr. Tampi and are not the official position or policy of, nor should be any endorsements be inferred by, the Bureau of Health Professions, HRSA, DHHS or the United States Government.
1. Samii A, Nutt JG, Ransom BR. Parkinson’s disease Lancet 2004;363(9423):1783-93.
2. Reddy S, Factor SA, Molho ES, Feustel PJ. The effect of quetiapine on psychosis and motor function in parkinsonian patients with and without dementia. Movement Disord 2002;17:676-81.
3. Kang GA, Bronstein JM. Psychosis in nursing home patients with Parkinson’s disease. J Am Med Dir Assoc 2004;5:167-73.
4. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65:464-70.
5. Mann SC, Caroff SN, Keck PE, Jr, et al. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, et al. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Association; 2003;1:44.-
6. Viejo LF, Morales V, Punal P, et al. Risk factors in neuroleptic malignant syndrome. A case-control study. Acta Psychiatrica Scandinavica 2003;107:45-9.
7. Adnet P, Lestavel P, Krivosic-Horber R. Neuroleptic malignant syndrome Br J Anaesthesia 2000;85:129-35.
8. Takubo H, Shimoda-Matsubayashi S, Mizuno Y. Serum creatine kinase is elevated in patients with Parkinson’s disease: a case controlled study. Parkinsonism Relat Disord 2003;9 suppl 1:S43-S46.
9. Mizuno Y, Takubo H, Mizuta E, Kuno S. Malignant syndrome in Parkinson’s disease: concept and review of the literature. Parkinsonism Relat Disord 2003;9 suppl 1:S3-S9.
10. Hashimoto T, Tokuda T, Hanyu N, et al. Withdrawal of levodopa and other risk factors for malignant syndrome in Parkinson’s disease. Parkinsonism Relat Disord 2003;9 suppl 1:S25-S30.
11. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin 2004;22:389-411.
12. Rosebush P, Stewart T. A prospective analysis of 24 episodes of neuroleptic malignant syndrome. Am J Psychiatry 1989;146:717-25.
13. Susman VL. Clinical management of neuroleptic malignant syndrome. Psychiatr Q 2001;72:325-36.
14. Caroff S, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
15. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003;64:663-7.
HISTORY: ‘THEY’RE TRYING TO KILL ME’
For the past 7 months Ms. G, age 47, has had worsening paranoid thoughts and sleep disturbances. She sleeps 4 hours or less a night, and her appetite and energy are diminished.
Her mother reports that Ms. G, who lives in an extended-care facility, believes the staff has injected embalming fluid into her body and is plotting to kill her. She says her daughter also has “fits” during which she hears a deafening noise that sounds like a vacuum cleaner, followed by a feeling of being pushed to the ground. Ms. G tells us that someone or something invisible is trying to control her.
Ms. G was diagnosed 2 years ago as having Parkinson’s disease and has chronically high liver transaminase enzymes. She also has moderate mental retardation secondary to cerebral palsy. She fears she will be harmed if she stays at the extendedcare facility, but we find no evidence that she has been abused or mistreated there.
Three months before presenting to us, Ms. G was hospitalized for 3 days to treat symptoms that suggested neuroleptic malignant syndrome (NMS) but were apparently caused by her inadvertently stopping her antiparkinson agents.
One month later, Ms. G was hospitalized again, this time for acute psychosis. Quetiapine, which she had been taking for antiparkinson medication-induced psychosis, was increased from 100 mg nightly to 75 mg bid, with reportedly good effect.
Shortly afterward, however, Ms. G’s paranoia worsened. At the facility, she has called 911 several times to report imagined threats from staff members. After referral from her primary care physician, we evaluate Ms. G and admit her to the adult inpatient psychiatric unit.
At intake, Ms. G is anxious and uncomfortable with notable muscle spasticity and twitching of her arms and legs. Mostly wheelchair-bound, she has longstanding physical abnormalities (shuffling gait; dystonia; drooling; slowed, dysarthric speech) secondary to comorbid Parkinson’s and cerebral palsy. She is agitated at first but grows calmer and cooperative.
Mental status examination shows a disorganized, tangential thought process and evidence of paranoid delusions and auditory hallucinations, but she denies visual hallucinations. She has poor insight into her illness but is oriented to time, place, and person. She can recall two of three objects after 3 minutes of distraction. Attention and concentration are intact.
Ms. G denies depressed mood, anhedonia, mania, or suicidal or homicidal thoughts. Her mother says no stressors other than the imagined threats to her life have affected her daughter.
The patient ’s temperature at admission is 98.0°F, her pulse is 108 beats per minute, and her blood pressure is 150/88 mm Hg. Laboratory workup shows a white blood cell count of 10,100/mm3 (normal range: 4,000 to 10,000/mm3), sodium level of 132 mEq/L (normal range: 135 to 145 mEq/L), and aspartate (AST) and alanine (ALT) transaminase levels of 611 U/L and 79 U/L, respectively (normal range for each: 0 to 35 U/L).
Aside from quetiapine, Ms. G also has been taking carbidopa/levodopa, seven 25/100-mg tablets daily, and pramipexole, 3 mg/d, for parkinsonism; citalopram, 20 mg/d, for depression; trazodone, 300 mg nightly, and lorazepam, 0.5 mg nightly, for insomnia; lopressor, 25 mg every 12 hours, for hypertension; and tolterodine, 1 mg bid, for urinary incontinence.
The authors’ observations
Parkinsonism typically responds to dopaminergic treatment. Excess dopamine agonism is believed to contribute to medication-induced psychosis, a common and often disabling complication of Parkinson’s disease1,2 that often necessitates nursing home placement and may increase mortality.2,3
Paranoia occurs in approximately 8% of patients treated for drug-induced Parkinson’s psychosis, and hallucinations (typically visual) may occur in as many as 30%.2 Quetiapine, 50 to 225 mg/d, is considered a good first-line treatment for psychosis in Parkinson’s, although the agent has been tested for this use only in open-label trials.2,3
Mental retardation and pre-existing parkinsonism, however, may increase Ms. G’s risk for NMS, a rare but potentially fatal reaction to antipsychotics believed to be caused by a sudden D2 dopamine receptor blockade.4,5 Signs include autonomic instability, extrapyramidal symptoms, hyperpyrexia, and altered mental status.
Of 68 patients with NMS studied by Ananth et al,4 13.2% were mentally retarded, and uncontrolled studies6 have proposed mental retardation as a potential risk factor (Table 1). A 2003 case control study6 found a higher incidence of NMS among mentally retarded patients than among nonretarded persons, but the difference was not statistically significant. There are no known links between specific causes of mental retardation and NMS.
Even so, Ms. G’s psychosis is compromising her already diminished quality of life. We will increase her quetiapine dosage slightly and watch for early signs of NMS, including fever, confusion, and increased muscle rigidity.
Table 1
Factors that increase risk of neuroleptic malignant syndrome*
| Abrupt antipsychotic cessation |
| Ambient heat |
| Catatonia |
| Dehydration |
| Exhaustion |
| Genetic predisposition |
| Greater dosage increases |
| Higher neuroleptic doses, especially with typical and atypical IM agents |
| Low serum iron |
| Malnutrition |
| Mental retardation |
| Pre-existing EPS or parkinsonism |
| Previous NMS episode |
| Psychomotor agitation |
| * Infection or concurrent organic brain disease are predisposing factors, but their association with NMS is less clear. |
| EPS: extrapyramidal symptoms |
| Source: References: 4-7, 14-15. |
TREATMENT: MEDICATION CHANGE
Upon admission, quetiapine is increased to 75 mg in the morning and 125 mg at bedtime—still well below the dosage at which quetiapine increases the risk of NMS (Table 2). Trazodone is decreased to 100 mg/d because of quetiapine’s sedating properties. Citalopram and tolterodine are stopped for fear that either agent would aggravate her psychosis. We continue all other drugs as previously prescribed. Her paranoia begins to subside.
Three days later, Ms. G’s is increasingly confused and agitated, and her temperature rises to 101.3°F. Physical exam shows increased muscle rigidity. She is given lorazepam, 1 mg, and transferred to the emergency room for evaluation.
In the ER, Ms. G’s temperature rises to 102.3°F. Other vital signs include:
- heart rate, 112 to 120 beats per minute
- respiratory rate, 18 to 20 breaths per minute
- oxygen saturation, 98% in room air
- blood pressure, 131/61 mm Hg while seated and 92/58 mm Hg while standing.
CNS or systemic infection and myocardial infarction are considered less likely because of her reactive pupils, lack of nuchal rigidity, troponin 3. Additionally, CSF shows normal glucose and protein levels, ALT and AST are 217 and 261 U/L, respectively, and chest x-ray shows no acute cardiopulmonary abnormality.
Ms. G is admitted to the medical intensive care unit and given IV fluids. All psychotropic and antiparkinson medications are stopped for 12 hours. Ms. G is then transferred to the general medical service for continued observation and IV hydration.
Six hours later, lorazepam, 0.5 mg every 8 hours, is resumed to control Ms. G’s anxiety. Carbidopa/levodopa is resumed at the previous dosage; all other medications remain on hold.
Renal damage is not apparent, but repeat chest x-ray taken 2 days after admission to the ER shows right middle lobe pneumonia, which resolved with antibiotics.
Six days after entering the medical unit, Ms. G is no longer agitated or paranoid. She is discharged that day and continued on lorazepam, 1 mg every 8 hours as needed to control her anxiety and prevent paranoia, and carbidopa/levidopa 8-1/2 25/100-mg tablets daily for her parkinsonism. Trazodone, 100 mg nightly, is continued for 3 days to help her sleep, as is amoxicillin/clavulanate, 500 mg every 8 hours, in case an underlying infection exists. Quetiapine, citalopram, and tolterodine are discontinued; all other medications are resumed as previously prescribed.
Table 2
Antipsychotic-related NMS risk increases at these dosages
| Agent | Dosage (mg/d) |
|---|---|
| Aripiprazole | >30 |
| Chlorpromazine | >400 |
| Clozapine | 318+/-299 |
| Olanzapine | 9.7+/-2.3 |
| Quetiapine | 412.5+/-317 |
| Risperidone | 4.3+/-3.1 |
| Ziprasidone | >20 |
| Source: References 4, 6, and 15. | |
The authors’ observations
Ms. G’s NMS symptoms surfaced 3 days after her quetiapine dosage was increased, suggesting that the antipsychotic may have caused this episode.
We ruled out antiparkinson agent withdrawal malignant syndrome—usually caused by abrupt cessation of Parkinson’s medications. Ms. G’s carbidopa/levodopa had not been adjusted before the symptoms emerged, and she did not worsen after the agent was stopped temporarily. Her brief pneumonia episode, however, could have caused symptoms that mimicked this withdrawal syndrome.
Antiparkinson agent withdrawal malignant syndrome symptoms resemble those of NMS.9,10 Worsening parkinsonism, dehydration, and infection increase the risk.10 Some research suggests that leukocytosis or elevated inflammation-related cytokines may accelerate withdrawal syndrome.10
The authors’ observations
Ms. G’s case illustrates the difficulty of treating psychosis in a patient at risk for NMS.
Of the 68 patients in the Ananth et al study with atypical antipsychotic-induced NMS, 11 were rechallenged after an NMS episode with the same agent and 8 were switched to another atypical. NMS recurred in 4 of these 19 patients.4
Ms. G was stable on lorazepam at discharge, but we would consider rechallenging with quetiapine or another antipsychotic if necessary. NMS recurs in 30% to 50%11 of patients after antipsychotic rechallenge, but waiting 2 weeks to resume antipsychotic therapy appears to reduce this risk.12 Benzodiazepines and electroconvulsive therapy are acceptable—though unproven—second-line therapies if antipsychotic rechallenge is deemed too risky,11,13 such as in some patients with a previous severe NMS episode; evidence of stroke, Parkinson’s or other neurodegenerative disease; or multiple acute medical problems.
CONTINUED TREATMENT: A RELAPSE
Three months later, Ms. G is readmitted to the neurology service for 3 weeks after being diagnosed with elevated CK, possibly caused by NMS or rhabdomyolysis secondary to persistent dyskinesia. We believe an inadvertent decrease in her carbidopa/levodopa caused the episode, as she had taken no neuroleptics between hospitalizations.
Ms. G is discharged on quetiapine, 25 mg nightly, along with her other medications. Her current psychiatric and neurologic status is unknown.
The authors’ observations
Detecting NMS symptoms early is critical to preventing mortality. Although NMS risk with atypical and typical antipsychotics is similar,4 fewer deaths from NMS have been reported after use of atypicals (3 deaths among 68 cases) than typical neuroleptics (30% mortality rate in the 1960s and 70s, and 10% mortality from 1980-87).14 Earlier recognition and treatment may be decreasing NMS-related mortality.4
Consider NMS in the differential diagnosis when the patient’s mental status changes.
Related resources
- Emedicine: Neuroleptic malignant syndrome. www.emedicine.com/med/topic2614.htm.
- Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin 2004;22:389-411.
- Susman VL. Clinical management of neuroleptic malignant syndrome. Psychiatr Q 2001;72:325-36.
- Amoxicillin/clavulanate • Augmentin
- Aripiprazole • Abilify
- Carbidopa/levodopa • Sinemet
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Lopressor • Toprol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Pramipexole • Mirapex
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Tolterodine • Detrol
- Trazodone • Desyrel
- Ziprasidone • Geodon
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors wish to thank Robert B. Milstein, MD, PhD, and Benjamin Zigun, MD, JD, for their help in preparing this article for publication.
This project is supported by funds from the Division of State, Community, and Public Health, Bureau of Health Professions (BHPr), Health Resources and Services Administration (HSRA), Department of Health and Human Services (DHHS) under grant number 1 K01 HP 00071-02 and Geriatric Academic Career Award ($58,009). The content and conclusion are those of Dr. Tampi and are not the official position or policy of, nor should be any endorsements be inferred by, the Bureau of Health Professions, HRSA, DHHS or the United States Government.
HISTORY: ‘THEY’RE TRYING TO KILL ME’
For the past 7 months Ms. G, age 47, has had worsening paranoid thoughts and sleep disturbances. She sleeps 4 hours or less a night, and her appetite and energy are diminished.
Her mother reports that Ms. G, who lives in an extended-care facility, believes the staff has injected embalming fluid into her body and is plotting to kill her. She says her daughter also has “fits” during which she hears a deafening noise that sounds like a vacuum cleaner, followed by a feeling of being pushed to the ground. Ms. G tells us that someone or something invisible is trying to control her.
Ms. G was diagnosed 2 years ago as having Parkinson’s disease and has chronically high liver transaminase enzymes. She also has moderate mental retardation secondary to cerebral palsy. She fears she will be harmed if she stays at the extendedcare facility, but we find no evidence that she has been abused or mistreated there.
Three months before presenting to us, Ms. G was hospitalized for 3 days to treat symptoms that suggested neuroleptic malignant syndrome (NMS) but were apparently caused by her inadvertently stopping her antiparkinson agents.
One month later, Ms. G was hospitalized again, this time for acute psychosis. Quetiapine, which she had been taking for antiparkinson medication-induced psychosis, was increased from 100 mg nightly to 75 mg bid, with reportedly good effect.
Shortly afterward, however, Ms. G’s paranoia worsened. At the facility, she has called 911 several times to report imagined threats from staff members. After referral from her primary care physician, we evaluate Ms. G and admit her to the adult inpatient psychiatric unit.
At intake, Ms. G is anxious and uncomfortable with notable muscle spasticity and twitching of her arms and legs. Mostly wheelchair-bound, she has longstanding physical abnormalities (shuffling gait; dystonia; drooling; slowed, dysarthric speech) secondary to comorbid Parkinson’s and cerebral palsy. She is agitated at first but grows calmer and cooperative.
Mental status examination shows a disorganized, tangential thought process and evidence of paranoid delusions and auditory hallucinations, but she denies visual hallucinations. She has poor insight into her illness but is oriented to time, place, and person. She can recall two of three objects after 3 minutes of distraction. Attention and concentration are intact.
Ms. G denies depressed mood, anhedonia, mania, or suicidal or homicidal thoughts. Her mother says no stressors other than the imagined threats to her life have affected her daughter.
The patient ’s temperature at admission is 98.0°F, her pulse is 108 beats per minute, and her blood pressure is 150/88 mm Hg. Laboratory workup shows a white blood cell count of 10,100/mm3 (normal range: 4,000 to 10,000/mm3), sodium level of 132 mEq/L (normal range: 135 to 145 mEq/L), and aspartate (AST) and alanine (ALT) transaminase levels of 611 U/L and 79 U/L, respectively (normal range for each: 0 to 35 U/L).
Aside from quetiapine, Ms. G also has been taking carbidopa/levodopa, seven 25/100-mg tablets daily, and pramipexole, 3 mg/d, for parkinsonism; citalopram, 20 mg/d, for depression; trazodone, 300 mg nightly, and lorazepam, 0.5 mg nightly, for insomnia; lopressor, 25 mg every 12 hours, for hypertension; and tolterodine, 1 mg bid, for urinary incontinence.
The authors’ observations
Parkinsonism typically responds to dopaminergic treatment. Excess dopamine agonism is believed to contribute to medication-induced psychosis, a common and often disabling complication of Parkinson’s disease1,2 that often necessitates nursing home placement and may increase mortality.2,3
Paranoia occurs in approximately 8% of patients treated for drug-induced Parkinson’s psychosis, and hallucinations (typically visual) may occur in as many as 30%.2 Quetiapine, 50 to 225 mg/d, is considered a good first-line treatment for psychosis in Parkinson’s, although the agent has been tested for this use only in open-label trials.2,3
Mental retardation and pre-existing parkinsonism, however, may increase Ms. G’s risk for NMS, a rare but potentially fatal reaction to antipsychotics believed to be caused by a sudden D2 dopamine receptor blockade.4,5 Signs include autonomic instability, extrapyramidal symptoms, hyperpyrexia, and altered mental status.
Of 68 patients with NMS studied by Ananth et al,4 13.2% were mentally retarded, and uncontrolled studies6 have proposed mental retardation as a potential risk factor (Table 1). A 2003 case control study6 found a higher incidence of NMS among mentally retarded patients than among nonretarded persons, but the difference was not statistically significant. There are no known links between specific causes of mental retardation and NMS.
Even so, Ms. G’s psychosis is compromising her already diminished quality of life. We will increase her quetiapine dosage slightly and watch for early signs of NMS, including fever, confusion, and increased muscle rigidity.
Table 1
Factors that increase risk of neuroleptic malignant syndrome*
| Abrupt antipsychotic cessation |
| Ambient heat |
| Catatonia |
| Dehydration |
| Exhaustion |
| Genetic predisposition |
| Greater dosage increases |
| Higher neuroleptic doses, especially with typical and atypical IM agents |
| Low serum iron |
| Malnutrition |
| Mental retardation |
| Pre-existing EPS or parkinsonism |
| Previous NMS episode |
| Psychomotor agitation |
| * Infection or concurrent organic brain disease are predisposing factors, but their association with NMS is less clear. |
| EPS: extrapyramidal symptoms |
| Source: References: 4-7, 14-15. |
TREATMENT: MEDICATION CHANGE
Upon admission, quetiapine is increased to 75 mg in the morning and 125 mg at bedtime—still well below the dosage at which quetiapine increases the risk of NMS (Table 2). Trazodone is decreased to 100 mg/d because of quetiapine’s sedating properties. Citalopram and tolterodine are stopped for fear that either agent would aggravate her psychosis. We continue all other drugs as previously prescribed. Her paranoia begins to subside.
Three days later, Ms. G’s is increasingly confused and agitated, and her temperature rises to 101.3°F. Physical exam shows increased muscle rigidity. She is given lorazepam, 1 mg, and transferred to the emergency room for evaluation.
In the ER, Ms. G’s temperature rises to 102.3°F. Other vital signs include:
- heart rate, 112 to 120 beats per minute
- respiratory rate, 18 to 20 breaths per minute
- oxygen saturation, 98% in room air
- blood pressure, 131/61 mm Hg while seated and 92/58 mm Hg while standing.
CNS or systemic infection and myocardial infarction are considered less likely because of her reactive pupils, lack of nuchal rigidity, troponin 3. Additionally, CSF shows normal glucose and protein levels, ALT and AST are 217 and 261 U/L, respectively, and chest x-ray shows no acute cardiopulmonary abnormality.
Ms. G is admitted to the medical intensive care unit and given IV fluids. All psychotropic and antiparkinson medications are stopped for 12 hours. Ms. G is then transferred to the general medical service for continued observation and IV hydration.
Six hours later, lorazepam, 0.5 mg every 8 hours, is resumed to control Ms. G’s anxiety. Carbidopa/levodopa is resumed at the previous dosage; all other medications remain on hold.
Renal damage is not apparent, but repeat chest x-ray taken 2 days after admission to the ER shows right middle lobe pneumonia, which resolved with antibiotics.
Six days after entering the medical unit, Ms. G is no longer agitated or paranoid. She is discharged that day and continued on lorazepam, 1 mg every 8 hours as needed to control her anxiety and prevent paranoia, and carbidopa/levidopa 8-1/2 25/100-mg tablets daily for her parkinsonism. Trazodone, 100 mg nightly, is continued for 3 days to help her sleep, as is amoxicillin/clavulanate, 500 mg every 8 hours, in case an underlying infection exists. Quetiapine, citalopram, and tolterodine are discontinued; all other medications are resumed as previously prescribed.
Table 2
Antipsychotic-related NMS risk increases at these dosages
| Agent | Dosage (mg/d) |
|---|---|
| Aripiprazole | >30 |
| Chlorpromazine | >400 |
| Clozapine | 318+/-299 |
| Olanzapine | 9.7+/-2.3 |
| Quetiapine | 412.5+/-317 |
| Risperidone | 4.3+/-3.1 |
| Ziprasidone | >20 |
| Source: References 4, 6, and 15. | |
The authors’ observations
Ms. G’s NMS symptoms surfaced 3 days after her quetiapine dosage was increased, suggesting that the antipsychotic may have caused this episode.
We ruled out antiparkinson agent withdrawal malignant syndrome—usually caused by abrupt cessation of Parkinson’s medications. Ms. G’s carbidopa/levodopa had not been adjusted before the symptoms emerged, and she did not worsen after the agent was stopped temporarily. Her brief pneumonia episode, however, could have caused symptoms that mimicked this withdrawal syndrome.
Antiparkinson agent withdrawal malignant syndrome symptoms resemble those of NMS.9,10 Worsening parkinsonism, dehydration, and infection increase the risk.10 Some research suggests that leukocytosis or elevated inflammation-related cytokines may accelerate withdrawal syndrome.10
The authors’ observations
Ms. G’s case illustrates the difficulty of treating psychosis in a patient at risk for NMS.
Of the 68 patients in the Ananth et al study with atypical antipsychotic-induced NMS, 11 were rechallenged after an NMS episode with the same agent and 8 were switched to another atypical. NMS recurred in 4 of these 19 patients.4
Ms. G was stable on lorazepam at discharge, but we would consider rechallenging with quetiapine or another antipsychotic if necessary. NMS recurs in 30% to 50%11 of patients after antipsychotic rechallenge, but waiting 2 weeks to resume antipsychotic therapy appears to reduce this risk.12 Benzodiazepines and electroconvulsive therapy are acceptable—though unproven—second-line therapies if antipsychotic rechallenge is deemed too risky,11,13 such as in some patients with a previous severe NMS episode; evidence of stroke, Parkinson’s or other neurodegenerative disease; or multiple acute medical problems.
CONTINUED TREATMENT: A RELAPSE
Three months later, Ms. G is readmitted to the neurology service for 3 weeks after being diagnosed with elevated CK, possibly caused by NMS or rhabdomyolysis secondary to persistent dyskinesia. We believe an inadvertent decrease in her carbidopa/levodopa caused the episode, as she had taken no neuroleptics between hospitalizations.
Ms. G is discharged on quetiapine, 25 mg nightly, along with her other medications. Her current psychiatric and neurologic status is unknown.
The authors’ observations
Detecting NMS symptoms early is critical to preventing mortality. Although NMS risk with atypical and typical antipsychotics is similar,4 fewer deaths from NMS have been reported after use of atypicals (3 deaths among 68 cases) than typical neuroleptics (30% mortality rate in the 1960s and 70s, and 10% mortality from 1980-87).14 Earlier recognition and treatment may be decreasing NMS-related mortality.4
Consider NMS in the differential diagnosis when the patient’s mental status changes.
Related resources
- Emedicine: Neuroleptic malignant syndrome. www.emedicine.com/med/topic2614.htm.
- Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin 2004;22:389-411.
- Susman VL. Clinical management of neuroleptic malignant syndrome. Psychiatr Q 2001;72:325-36.
- Amoxicillin/clavulanate • Augmentin
- Aripiprazole • Abilify
- Carbidopa/levodopa • Sinemet
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Lopressor • Toprol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Pramipexole • Mirapex
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Tolterodine • Detrol
- Trazodone • Desyrel
- Ziprasidone • Geodon
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors wish to thank Robert B. Milstein, MD, PhD, and Benjamin Zigun, MD, JD, for their help in preparing this article for publication.
This project is supported by funds from the Division of State, Community, and Public Health, Bureau of Health Professions (BHPr), Health Resources and Services Administration (HSRA), Department of Health and Human Services (DHHS) under grant number 1 K01 HP 00071-02 and Geriatric Academic Career Award ($58,009). The content and conclusion are those of Dr. Tampi and are not the official position or policy of, nor should be any endorsements be inferred by, the Bureau of Health Professions, HRSA, DHHS or the United States Government.
1. Samii A, Nutt JG, Ransom BR. Parkinson’s disease Lancet 2004;363(9423):1783-93.
2. Reddy S, Factor SA, Molho ES, Feustel PJ. The effect of quetiapine on psychosis and motor function in parkinsonian patients with and without dementia. Movement Disord 2002;17:676-81.
3. Kang GA, Bronstein JM. Psychosis in nursing home patients with Parkinson’s disease. J Am Med Dir Assoc 2004;5:167-73.
4. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65:464-70.
5. Mann SC, Caroff SN, Keck PE, Jr, et al. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, et al. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Association; 2003;1:44.-
6. Viejo LF, Morales V, Punal P, et al. Risk factors in neuroleptic malignant syndrome. A case-control study. Acta Psychiatrica Scandinavica 2003;107:45-9.
7. Adnet P, Lestavel P, Krivosic-Horber R. Neuroleptic malignant syndrome Br J Anaesthesia 2000;85:129-35.
8. Takubo H, Shimoda-Matsubayashi S, Mizuno Y. Serum creatine kinase is elevated in patients with Parkinson’s disease: a case controlled study. Parkinsonism Relat Disord 2003;9 suppl 1:S43-S46.
9. Mizuno Y, Takubo H, Mizuta E, Kuno S. Malignant syndrome in Parkinson’s disease: concept and review of the literature. Parkinsonism Relat Disord 2003;9 suppl 1:S3-S9.
10. Hashimoto T, Tokuda T, Hanyu N, et al. Withdrawal of levodopa and other risk factors for malignant syndrome in Parkinson’s disease. Parkinsonism Relat Disord 2003;9 suppl 1:S25-S30.
11. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin 2004;22:389-411.
12. Rosebush P, Stewart T. A prospective analysis of 24 episodes of neuroleptic malignant syndrome. Am J Psychiatry 1989;146:717-25.
13. Susman VL. Clinical management of neuroleptic malignant syndrome. Psychiatr Q 2001;72:325-36.
14. Caroff S, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
15. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003;64:663-7.
1. Samii A, Nutt JG, Ransom BR. Parkinson’s disease Lancet 2004;363(9423):1783-93.
2. Reddy S, Factor SA, Molho ES, Feustel PJ. The effect of quetiapine on psychosis and motor function in parkinsonian patients with and without dementia. Movement Disord 2002;17:676-81.
3. Kang GA, Bronstein JM. Psychosis in nursing home patients with Parkinson’s disease. J Am Med Dir Assoc 2004;5:167-73.
4. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65:464-70.
5. Mann SC, Caroff SN, Keck PE, Jr, et al. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, et al. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Association; 2003;1:44.-
6. Viejo LF, Morales V, Punal P, et al. Risk factors in neuroleptic malignant syndrome. A case-control study. Acta Psychiatrica Scandinavica 2003;107:45-9.
7. Adnet P, Lestavel P, Krivosic-Horber R. Neuroleptic malignant syndrome Br J Anaesthesia 2000;85:129-35.
8. Takubo H, Shimoda-Matsubayashi S, Mizuno Y. Serum creatine kinase is elevated in patients with Parkinson’s disease: a case controlled study. Parkinsonism Relat Disord 2003;9 suppl 1:S43-S46.
9. Mizuno Y, Takubo H, Mizuta E, Kuno S. Malignant syndrome in Parkinson’s disease: concept and review of the literature. Parkinsonism Relat Disord 2003;9 suppl 1:S3-S9.
10. Hashimoto T, Tokuda T, Hanyu N, et al. Withdrawal of levodopa and other risk factors for malignant syndrome in Parkinson’s disease. Parkinsonism Relat Disord 2003;9 suppl 1:S25-S30.
11. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin 2004;22:389-411.
12. Rosebush P, Stewart T. A prospective analysis of 24 episodes of neuroleptic malignant syndrome. Am J Psychiatry 1989;146:717-25.
13. Susman VL. Clinical management of neuroleptic malignant syndrome. Psychiatr Q 2001;72:325-36.
14. Caroff S, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
15. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003;64:663-7.
Treating OCD in patients with psychiatric comorbidity
Psychiatric comorbidities complicate the treatment of obsessive-compulsive disorder (OCD) and are much more the rule than the exception in clinical practice (Table 1).1-6 Even so, surprisingly few studies have examined comorbidities’ effects on OCD treatment, and results have been mixed.
For the typical patient with obsessive-compulsive symptoms, we discuss our experience and evidence that supports:
- clinically useful tools to differentiate OCD from other obsessive and anxiety disorders
- how to address comorbidities that pose acute danger or would prevent effective psychotherapy
- how to modify first-line OCD treatments—cognitive behavioral therapy (CBT) and serotonin reuptake inhibitors (SRIs)7-9—to also manage most comorbid disorders.
Table 1
Common psychiatric comorbidities with OCD
| Comorbidities | Estimated prevalence in OCD patients |
|---|---|
| Personality disorders | 63% |
| Major depressive disorder | 28 to 31% |
| Simple phobia | 7 to 48% |
| Social phobia | 11 to 16% |
| Bipolar disorder | 15% |
| Eating disorders | 8 to 13% |
| Alcohol abuse | 8% |
| Panic disorder | 6 to 12% |
| Tourette’s syndrome or tic disorders | 6 to 7% |
| Source: Data from references 1-6 | |
IS OCD PRIMARY?
OCD-like obsessive thoughts or repetitive behaviors may be evident in a number of psychiatric disorders. Distinguishing OCD from masquerading or co-occurring conditions is important because interventions can differ.
Patients with generalized anxiety disorder (GAD), for example, may experience ruminative, anxious thoughts that mimic obsessions. Somatoform conditions such as hypochondriasis or body dysmorphic disorder are characterized by intense preoccupation with illness or appearance, respectively. Repetitive or compulsive behaviors may be seen in impulse control or developmental disorders such as pathologic gambling, trichotillomania, and Asperger’s disorder.
To help differentiate OCD from these conditions, consider the function of a patient’s symptoms. In OCD, obsessions are experienced as ego-dystonic and generally cause great anxiety. OCD patients perform compulsive rituals to alleviate anxiety but do not gain pleasure from their actions. Contrast this with trichotillomania’s repetitive behavior—commonly experienced as pleasurable or gratifying—or with GAD’s ruminative thoughts—seen as ego-syntonic worries about real-life situations.
ASSESSING OCD, COMORBID CONDITIONS
When you suspect psychiatric comorbidity with OCD, an accurate and thorough assessment is key to successful treatment (Table 2).10-14
In specialty OCD clinics, the Structured Clinical Interview for DSM-IV (SCID-IV)15 or Anxiety Disorders Interview Schedule for the DSM-IV (ADIS-IV)10 are routinely given to assess the most common comorbid conditions. In clinical practice, however, these instruments can take up to several hours to perform, especially for patients who meet criteria for several disorders.
An alternative may be the Mini International Neuropsychiatric Interview (MINI).11 The MINI is a short, structured, diagnostic interview for DSM-IV and ICD-10 that takes about 15 minutes and screens for most conditions commonly comorbid with OCD. The MINI provides less-detailed information than the SCID-IV or the ADIS-IV but allows for a quick, accurate diagnosis while using a structured format.
Table 2
Common assessment tools for patients with suspected OCD
| Structured clinical interviews | Time to administer | Use |
|---|---|---|
| Anxiety Disorders Interview Schedule-IV (ADIS-IV) | 2+ hrs | Detailed assessment of anxiety disorders |
| Mini-International Neuropsychiatric Interview (MINI) | 15 to 30 min | Brief screen for diagnosis |
| OCD-specific measures | ||
| Yale-Brown Obsessive Compulsive Scale (YBOCS) | 30 min | Severity and OCD symptom types |
| Obsessive Compulsive Inventory-Revised (OCI-R) | 5 to 10 min | Self-report severity of OCD symptoms |
| Source: Data from references 10-14 | ||
The Yale-Brown Obsessive Compulsive Scale (YBOCS) is widely used.12,13 It includes a checklist of common obsessions and compulsions plus 10 items measuring interference with daily living, distress, resistance, control, and time spent on symptoms. Each item is scored from 0 to 4, for a total score of 0 to 40.
The YBOCS has good reliability and validity, is available in both clinician-rated and self-rated versions, and can be given repeatedly to measure treatment progress. A Children’s Yale-Brown Obsessive-Compulsive Scale (CYBOCS) is useful for patients ages 6 to 17.16
TREATING UNCOMPLICATED OCD
CBT. When OCD is not concurrent with another diagnosis, expert consensus guidelines recommend CBT as first-line treatment.17 Most patients treated with exposure and response prevention (ERP) therapy—the specialized CBT for reducing anxiety that triggers obsessive-compulsive symptoms—report reduced symptoms and often maintain those gains over time.18
In specialty clinics, patients frequently engage in intensive ERP (2 hours per day, 3 to 5 times per week for about 3 weeks). Although studies find excellent outcomes with intensive OCD treatment,18 it is not always practical or indicated (as in patients with moderate symptoms). Less-intensive protocols, such as biweekly sessions, have also shown promise in studies examining how session frequency affects treatment outcome.19
Many studies supporting ERP’s efficacy in OCD have included relatively homogenous samples under well-controlled conditions. Some investigations have also found good effects for ERP when including patients with complex treatment histories, concomitant pharmacotherapy, and comorbid conditions.20
Medication. Functional imaging studies suggest that OCD results from dysregulation in the socalled “OCD circuit”—the orbitofrontal cortex, anterior cingulate, and caudate nucleus. In patients with OCD, metabolic activity in this region is increased at rest relative to controls, increases further with symptoms, and decreases after successful treatment.21 The serotonin hypothesis—which emerged from observation that OCD symptoms responded to serotonergic medications but not to noradrenergic ones—suggests serotonin system dysregulation in patients with OCD.
High dosages of SRIs—selective serotonin reuptake inhibitors or the tricyclic antidepressant clomipramine—are first-line OCD medications (Table 3). Double-blind clinical trials have found clomipramine, fluoxetine, sertraline, paroxetine, fluvoxamine, and citalopram more effective than placebo, and the first five of these drugs are FDA-approved for treating adult OCD.
Table 3
Serotonin reuptake inhibitors indicated for treating OCD*
| Drug | Starting dosage | Target dosage (adults) |
|---|---|---|
| Clomipramine | 25 mg/d | 150 to 200 mg/d |
| Fluoxetine | 20 mg/d | 60 to 80 mg/d |
| Fluvoxamine | 50 mg/d | 150 to 300 mg/d |
| Paroxetine | 20 mg/d | 40 to 60 mg/d |
| Sertraline | 50 mg/d | 150 to 200 mg/d |
| * 10- to 12-week medication trials at target doses; sequential trials may be required to achieve treatment response. | ||
Nonresponse. Patients typically require at least 10 to 12 weeks of treatment at target dosages. Sequential medication trials may be needed to achieve a response. Complete remission is rare, and relapse rates are high when medication is discontinued.22
The up to 40% of patients who do not respond to SRI therapy require alternate strategies:
- Augmenting SRI therapy with a low-dose atypical antipsychotic such as risperidone, 1 to 2 mg bid, or olanzapine, 5 to 10 mg/d, may be effective, even in patients without a comorbid psychotic or tic disorder.23,24 It is worth noting that trials using atypicals as adjunctive therapy for OCD have been brief (12 weeks), and long-term use of these medications carries a risk of metabolic side effects such as weight gain, diabetes, and hyperlipidemia.
- The serotonin-norepinephrine reuptake inhibitor venlafaxine, 225 mg/d or higher, showed efficacy in a naturalistic study of patients who did not respond to SRIs.25
- Augmentation with pindolol, lithium, buspirone, trazodone, tryptophan, or thyroid hormone has shown mixed results.24
FACTORING IN COMORBIDITIES
Acute risk? Conditions that endanger the patient take precedence over OCD treatment. Suicidal risk and self-mutilating behaviors, for instance, must be addressed before a patient can engage in ERP therapy. Active psychosis also would exclude ERP and may be best handled by augmenting SRI therapy with an antipsychotic.17
Interfere with CBT? Exposure therapy can exacerbate symptoms in patients who self-medicate their anxiety with alcohol or other substances. In turn, alcohol or other substance abuse may interfere with habituation by ameliorating the anxiety necessary for effective exposure therapy. Thus, we recommend delaying OCD behavioral treatment until you treat or stabilize these conditions.
Many OCD patients report comorbid depression, which may be secondary to their OCD symptoms and may spontaneously decrease with successful OCD treatment. Patients with mild to moderate depression can usually engage in and benefit from ERP without depressionspecific interventions.
Patients with comorbid depression may not respond to OCD interventions as well as nondepressed OCD patients do.26 For concurrent OCD and major depression, expert consensus guidelines suggest combining CBT with an SRI.17
Less is known about how other comorbidities affect OCD treatment. In one study, patients with comorbid OCD and posttraumatic stress disorder (PTSD) responded poorly to ERP. Exposure therapy reduced OCD symptoms but increased PTSD symptoms in some patients.27 Some Axis II disorders—such as schizotypal, avoidant, paranoid, and borderline personality disorder—have also been found to predict poorer outcome in patients treated with clomipramine.3
Concurrent treatment? In some concomitant conditions, such as PTSD with OCD, preliminary evidence suggests that treatment can or should be simultaneous rather than sequential.27 Likewise, CBT can be used to treat OCD concurrent with other anxiety disorders with only slight modifications, such as:
- constructing exposures for social anxiety disorder patients that, at least initially, minimize extraneous social contact and evaluative fears
- instructing panic disorder patients in anxiety management skills so that exposures do not trigger anxiety attacks and reinforce their fears.
Related resources
- Jenike MA, Baer L, Minichiello WE (eds). Obsessive-compulsive disorders: practical management (3rd ed). New York: Mosby, 1998.
- Clark DA. Cognitive-behavioral therapy for OCD. New York: Guilford Press, 2003.
- Obsessive-Compulsive Foundation. www.ocfoundation.org.
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weissman MM, Bland RC, Canino GJ, et al. The cross national epidemiology of obsessive-compulsive disorder: The Cross National Collaborative Group. J Clin Psychiatry 1994;55(suppl 3):5-10.
2. Overbeek T, Schruers K, Vermetten E, Griez E. Comborbidity of obsessive-compulsive disorder and depression: Prevalence, symptom severity, and treatment effect. J Clin Psychiatry 2002;63(12):1106-12.
3. Baer L, Jenike MA, Black DW, Treece C. Effects of Axis II diagnosis on treatment outcome with clomipramine in 55 patients with obsessive compulsive disorder. Arch Gen Psychiatry 1992;49(11):862-6.
4. Rasmussen SA, Eisen JL. The epidemiology and clinical features of obsessive compulsive disorder. Psychiatr Clin North Am 1992;15(4):743-58.
5. Rubenstein CS, Pigott TA, L’Heureux F, et al. A preliminary investigation of the lifetime prevalence of anorexia and bulimia nervosa in patients with obsessive compulsive disorder. J Clin Psychiatry 1992;53(9):309-14.
6. Perugi G, Akiskal HS, Pfanner C, et al. Clinical impact of bipolar and unipolar affective comorbidity on obsessive compulsive disorder. J Affect Disord 1997;46(1):15-23.
7. March J, Frances A, Kahn D, Carpenter D. Expert consensus guidelines: treatment of obsessive compulsive disorder. J Clin Psychiatry 1997;58(suppl 4):1-72.
8. Foa EB, Liebowitz MR, Kozak MJ, et al. Randomized, placebo-controlled trial of exposure and response prevention, clomipramine, and their combination in the treatment of obsessive compulsive disorder. Am J Psychiatry 2005;162(1):151-61.
9. Kozak MJ, Liebowitz MR, Foa EB. Cognitive behavior therapy and pharmacotherapy for OCD: The NIMH-sponsored collaborative study. In: Goodman W, Rudorfer M, Maser J (eds). Obsessive compulsive disorder: Contemporary issues in treatment. Mahwah, NJ: Lawrence Erlbaum Associates, 2000;501:30.-
10. Brown TA, DiNardo PA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV. New York: Graywind Publications, 1994.
11. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The MiniInternational Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(suppl 20):22-33.
12. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale: I. Development, use, and reliability. Arch Gen Psychiatry 1989;46(11):1006M-11.
13. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale: II.Validity. Arch Gen Psychiatry 1989;46(11):1012-6.
14. Foa EB, Huppert JD, Leiberg S, et al. The Obsessive-Compulsive Inventory: development and validation of a short version. Psychol Assess 2002;14(4):485-96.
15. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition New York: New York State Psychiatric Institute, Biometrics Research Department, 1996.
16. Scahill L, Riddle M, McSwiggin-Hardin M, et al. Children’s Yale-Brown Obsessive-Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry 1997;36(6):844-52.
17. Frances A, Docherty JP, Kahn DA. Treatment of obsessive compulsive disorder. J Clin Psychiatry 1997;58(suppl 4):5-72.
18. Foa EB, Franklin ME. Psychotherapies for obsessive-compulsive disorder: A review. In: Maj M, Sartorius N, Okasha A, Zohar J (eds). Obsessive-Compulsive Disorder New York: Wiley, 2000;93:115.-
19. Abramowitz JS, Foa EB, Franklin ME. Exposure and ritual prevention for obsessive-compulsive disorder: Effect of intensive versus twice weekly sessions. J Consult Clin Psychol 2003;71(2):394-8.
20. Franklin ME, Abramowitz JS, Kozak MJ, et al. Effectiveness of exposure and ritual prevention for obsessive-compulsive disorder: Randomized compared with nonrandomized samples. J Consult Clin Psychol 2000;68(4):594-602.
21. Graybiel A, Rauch SL. Toward a neurobiology of obsessive compulsive disorder. Neuron 2000;28:343-7.
22. Pigott TA, Seay S. Pharmacology of obsessive compulsive disorder: overview and treatment-refractory strategies. In: Goodman MK, Rudorfer MV, Maser JD (eds). Obsessive compulsive disorder: contemporary issues in treatment Mahwah, NJ: Lawrence Erlbaum Associates, 2000;277:302.-
23. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 2000;57(8):794-801.
24. Hollander E, Bienstock CA, Koran LM, et al. Refractory obsessive-compulsive disorder: state-of-the-art treatment. J Clin Psychiatry 2002;63(suppl 6):20-9.
25. Hollander E, Friedberg BS, Wasserman S, et al. Venlafaxine in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry 2003;64(5):546-50.
26. Abramowitz JS, Foa EB. Does major depressive disorder influence outcome of exposure and response prevention for OCD? Behavior Ther 2001;31(4):795-800.
27. Gershuny BS, Baer L, Jenike MA, et al. Comorbid posttraumatic stress disorder: impact on treatment outcome for obsessive-compulsive disorder. Am J Psychiatry 2002;159(5):852-4.
28. Perugi G, Toni C, Frare F, et al. Obsessive-compulsive-bipolar comorbidity: a systematic exploration of clinical features and treatment outcome. J Clin Psychiatry 2002;63(12):1129-34.
Psychiatric comorbidities complicate the treatment of obsessive-compulsive disorder (OCD) and are much more the rule than the exception in clinical practice (Table 1).1-6 Even so, surprisingly few studies have examined comorbidities’ effects on OCD treatment, and results have been mixed.
For the typical patient with obsessive-compulsive symptoms, we discuss our experience and evidence that supports:
- clinically useful tools to differentiate OCD from other obsessive and anxiety disorders
- how to address comorbidities that pose acute danger or would prevent effective psychotherapy
- how to modify first-line OCD treatments—cognitive behavioral therapy (CBT) and serotonin reuptake inhibitors (SRIs)7-9—to also manage most comorbid disorders.
Table 1
Common psychiatric comorbidities with OCD
| Comorbidities | Estimated prevalence in OCD patients |
|---|---|
| Personality disorders | 63% |
| Major depressive disorder | 28 to 31% |
| Simple phobia | 7 to 48% |
| Social phobia | 11 to 16% |
| Bipolar disorder | 15% |
| Eating disorders | 8 to 13% |
| Alcohol abuse | 8% |
| Panic disorder | 6 to 12% |
| Tourette’s syndrome or tic disorders | 6 to 7% |
| Source: Data from references 1-6 | |
IS OCD PRIMARY?
OCD-like obsessive thoughts or repetitive behaviors may be evident in a number of psychiatric disorders. Distinguishing OCD from masquerading or co-occurring conditions is important because interventions can differ.
Patients with generalized anxiety disorder (GAD), for example, may experience ruminative, anxious thoughts that mimic obsessions. Somatoform conditions such as hypochondriasis or body dysmorphic disorder are characterized by intense preoccupation with illness or appearance, respectively. Repetitive or compulsive behaviors may be seen in impulse control or developmental disorders such as pathologic gambling, trichotillomania, and Asperger’s disorder.
To help differentiate OCD from these conditions, consider the function of a patient’s symptoms. In OCD, obsessions are experienced as ego-dystonic and generally cause great anxiety. OCD patients perform compulsive rituals to alleviate anxiety but do not gain pleasure from their actions. Contrast this with trichotillomania’s repetitive behavior—commonly experienced as pleasurable or gratifying—or with GAD’s ruminative thoughts—seen as ego-syntonic worries about real-life situations.
ASSESSING OCD, COMORBID CONDITIONS
When you suspect psychiatric comorbidity with OCD, an accurate and thorough assessment is key to successful treatment (Table 2).10-14
In specialty OCD clinics, the Structured Clinical Interview for DSM-IV (SCID-IV)15 or Anxiety Disorders Interview Schedule for the DSM-IV (ADIS-IV)10 are routinely given to assess the most common comorbid conditions. In clinical practice, however, these instruments can take up to several hours to perform, especially for patients who meet criteria for several disorders.
An alternative may be the Mini International Neuropsychiatric Interview (MINI).11 The MINI is a short, structured, diagnostic interview for DSM-IV and ICD-10 that takes about 15 minutes and screens for most conditions commonly comorbid with OCD. The MINI provides less-detailed information than the SCID-IV or the ADIS-IV but allows for a quick, accurate diagnosis while using a structured format.
Table 2
Common assessment tools for patients with suspected OCD
| Structured clinical interviews | Time to administer | Use |
|---|---|---|
| Anxiety Disorders Interview Schedule-IV (ADIS-IV) | 2+ hrs | Detailed assessment of anxiety disorders |
| Mini-International Neuropsychiatric Interview (MINI) | 15 to 30 min | Brief screen for diagnosis |
| OCD-specific measures | ||
| Yale-Brown Obsessive Compulsive Scale (YBOCS) | 30 min | Severity and OCD symptom types |
| Obsessive Compulsive Inventory-Revised (OCI-R) | 5 to 10 min | Self-report severity of OCD symptoms |
| Source: Data from references 10-14 | ||
The Yale-Brown Obsessive Compulsive Scale (YBOCS) is widely used.12,13 It includes a checklist of common obsessions and compulsions plus 10 items measuring interference with daily living, distress, resistance, control, and time spent on symptoms. Each item is scored from 0 to 4, for a total score of 0 to 40.
The YBOCS has good reliability and validity, is available in both clinician-rated and self-rated versions, and can be given repeatedly to measure treatment progress. A Children’s Yale-Brown Obsessive-Compulsive Scale (CYBOCS) is useful for patients ages 6 to 17.16
TREATING UNCOMPLICATED OCD
CBT. When OCD is not concurrent with another diagnosis, expert consensus guidelines recommend CBT as first-line treatment.17 Most patients treated with exposure and response prevention (ERP) therapy—the specialized CBT for reducing anxiety that triggers obsessive-compulsive symptoms—report reduced symptoms and often maintain those gains over time.18
In specialty clinics, patients frequently engage in intensive ERP (2 hours per day, 3 to 5 times per week for about 3 weeks). Although studies find excellent outcomes with intensive OCD treatment,18 it is not always practical or indicated (as in patients with moderate symptoms). Less-intensive protocols, such as biweekly sessions, have also shown promise in studies examining how session frequency affects treatment outcome.19
Many studies supporting ERP’s efficacy in OCD have included relatively homogenous samples under well-controlled conditions. Some investigations have also found good effects for ERP when including patients with complex treatment histories, concomitant pharmacotherapy, and comorbid conditions.20
Medication. Functional imaging studies suggest that OCD results from dysregulation in the socalled “OCD circuit”—the orbitofrontal cortex, anterior cingulate, and caudate nucleus. In patients with OCD, metabolic activity in this region is increased at rest relative to controls, increases further with symptoms, and decreases after successful treatment.21 The serotonin hypothesis—which emerged from observation that OCD symptoms responded to serotonergic medications but not to noradrenergic ones—suggests serotonin system dysregulation in patients with OCD.
High dosages of SRIs—selective serotonin reuptake inhibitors or the tricyclic antidepressant clomipramine—are first-line OCD medications (Table 3). Double-blind clinical trials have found clomipramine, fluoxetine, sertraline, paroxetine, fluvoxamine, and citalopram more effective than placebo, and the first five of these drugs are FDA-approved for treating adult OCD.
Table 3
Serotonin reuptake inhibitors indicated for treating OCD*
| Drug | Starting dosage | Target dosage (adults) |
|---|---|---|
| Clomipramine | 25 mg/d | 150 to 200 mg/d |
| Fluoxetine | 20 mg/d | 60 to 80 mg/d |
| Fluvoxamine | 50 mg/d | 150 to 300 mg/d |
| Paroxetine | 20 mg/d | 40 to 60 mg/d |
| Sertraline | 50 mg/d | 150 to 200 mg/d |
| * 10- to 12-week medication trials at target doses; sequential trials may be required to achieve treatment response. | ||
Nonresponse. Patients typically require at least 10 to 12 weeks of treatment at target dosages. Sequential medication trials may be needed to achieve a response. Complete remission is rare, and relapse rates are high when medication is discontinued.22
The up to 40% of patients who do not respond to SRI therapy require alternate strategies:
- Augmenting SRI therapy with a low-dose atypical antipsychotic such as risperidone, 1 to 2 mg bid, or olanzapine, 5 to 10 mg/d, may be effective, even in patients without a comorbid psychotic or tic disorder.23,24 It is worth noting that trials using atypicals as adjunctive therapy for OCD have been brief (12 weeks), and long-term use of these medications carries a risk of metabolic side effects such as weight gain, diabetes, and hyperlipidemia.
- The serotonin-norepinephrine reuptake inhibitor venlafaxine, 225 mg/d or higher, showed efficacy in a naturalistic study of patients who did not respond to SRIs.25
- Augmentation with pindolol, lithium, buspirone, trazodone, tryptophan, or thyroid hormone has shown mixed results.24
FACTORING IN COMORBIDITIES
Acute risk? Conditions that endanger the patient take precedence over OCD treatment. Suicidal risk and self-mutilating behaviors, for instance, must be addressed before a patient can engage in ERP therapy. Active psychosis also would exclude ERP and may be best handled by augmenting SRI therapy with an antipsychotic.17
Interfere with CBT? Exposure therapy can exacerbate symptoms in patients who self-medicate their anxiety with alcohol or other substances. In turn, alcohol or other substance abuse may interfere with habituation by ameliorating the anxiety necessary for effective exposure therapy. Thus, we recommend delaying OCD behavioral treatment until you treat or stabilize these conditions.
Many OCD patients report comorbid depression, which may be secondary to their OCD symptoms and may spontaneously decrease with successful OCD treatment. Patients with mild to moderate depression can usually engage in and benefit from ERP without depressionspecific interventions.
Patients with comorbid depression may not respond to OCD interventions as well as nondepressed OCD patients do.26 For concurrent OCD and major depression, expert consensus guidelines suggest combining CBT with an SRI.17
Less is known about how other comorbidities affect OCD treatment. In one study, patients with comorbid OCD and posttraumatic stress disorder (PTSD) responded poorly to ERP. Exposure therapy reduced OCD symptoms but increased PTSD symptoms in some patients.27 Some Axis II disorders—such as schizotypal, avoidant, paranoid, and borderline personality disorder—have also been found to predict poorer outcome in patients treated with clomipramine.3
Concurrent treatment? In some concomitant conditions, such as PTSD with OCD, preliminary evidence suggests that treatment can or should be simultaneous rather than sequential.27 Likewise, CBT can be used to treat OCD concurrent with other anxiety disorders with only slight modifications, such as:
- constructing exposures for social anxiety disorder patients that, at least initially, minimize extraneous social contact and evaluative fears
- instructing panic disorder patients in anxiety management skills so that exposures do not trigger anxiety attacks and reinforce their fears.
Related resources
- Jenike MA, Baer L, Minichiello WE (eds). Obsessive-compulsive disorders: practical management (3rd ed). New York: Mosby, 1998.
- Clark DA. Cognitive-behavioral therapy for OCD. New York: Guilford Press, 2003.
- Obsessive-Compulsive Foundation. www.ocfoundation.org.
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Psychiatric comorbidities complicate the treatment of obsessive-compulsive disorder (OCD) and are much more the rule than the exception in clinical practice (Table 1).1-6 Even so, surprisingly few studies have examined comorbidities’ effects on OCD treatment, and results have been mixed.
For the typical patient with obsessive-compulsive symptoms, we discuss our experience and evidence that supports:
- clinically useful tools to differentiate OCD from other obsessive and anxiety disorders
- how to address comorbidities that pose acute danger or would prevent effective psychotherapy
- how to modify first-line OCD treatments—cognitive behavioral therapy (CBT) and serotonin reuptake inhibitors (SRIs)7-9—to also manage most comorbid disorders.
Table 1
Common psychiatric comorbidities with OCD
| Comorbidities | Estimated prevalence in OCD patients |
|---|---|
| Personality disorders | 63% |
| Major depressive disorder | 28 to 31% |
| Simple phobia | 7 to 48% |
| Social phobia | 11 to 16% |
| Bipolar disorder | 15% |
| Eating disorders | 8 to 13% |
| Alcohol abuse | 8% |
| Panic disorder | 6 to 12% |
| Tourette’s syndrome or tic disorders | 6 to 7% |
| Source: Data from references 1-6 | |
IS OCD PRIMARY?
OCD-like obsessive thoughts or repetitive behaviors may be evident in a number of psychiatric disorders. Distinguishing OCD from masquerading or co-occurring conditions is important because interventions can differ.
Patients with generalized anxiety disorder (GAD), for example, may experience ruminative, anxious thoughts that mimic obsessions. Somatoform conditions such as hypochondriasis or body dysmorphic disorder are characterized by intense preoccupation with illness or appearance, respectively. Repetitive or compulsive behaviors may be seen in impulse control or developmental disorders such as pathologic gambling, trichotillomania, and Asperger’s disorder.
To help differentiate OCD from these conditions, consider the function of a patient’s symptoms. In OCD, obsessions are experienced as ego-dystonic and generally cause great anxiety. OCD patients perform compulsive rituals to alleviate anxiety but do not gain pleasure from their actions. Contrast this with trichotillomania’s repetitive behavior—commonly experienced as pleasurable or gratifying—or with GAD’s ruminative thoughts—seen as ego-syntonic worries about real-life situations.
ASSESSING OCD, COMORBID CONDITIONS
When you suspect psychiatric comorbidity with OCD, an accurate and thorough assessment is key to successful treatment (Table 2).10-14
In specialty OCD clinics, the Structured Clinical Interview for DSM-IV (SCID-IV)15 or Anxiety Disorders Interview Schedule for the DSM-IV (ADIS-IV)10 are routinely given to assess the most common comorbid conditions. In clinical practice, however, these instruments can take up to several hours to perform, especially for patients who meet criteria for several disorders.
An alternative may be the Mini International Neuropsychiatric Interview (MINI).11 The MINI is a short, structured, diagnostic interview for DSM-IV and ICD-10 that takes about 15 minutes and screens for most conditions commonly comorbid with OCD. The MINI provides less-detailed information than the SCID-IV or the ADIS-IV but allows for a quick, accurate diagnosis while using a structured format.
Table 2
Common assessment tools for patients with suspected OCD
| Structured clinical interviews | Time to administer | Use |
|---|---|---|
| Anxiety Disorders Interview Schedule-IV (ADIS-IV) | 2+ hrs | Detailed assessment of anxiety disorders |
| Mini-International Neuropsychiatric Interview (MINI) | 15 to 30 min | Brief screen for diagnosis |
| OCD-specific measures | ||
| Yale-Brown Obsessive Compulsive Scale (YBOCS) | 30 min | Severity and OCD symptom types |
| Obsessive Compulsive Inventory-Revised (OCI-R) | 5 to 10 min | Self-report severity of OCD symptoms |
| Source: Data from references 10-14 | ||
The Yale-Brown Obsessive Compulsive Scale (YBOCS) is widely used.12,13 It includes a checklist of common obsessions and compulsions plus 10 items measuring interference with daily living, distress, resistance, control, and time spent on symptoms. Each item is scored from 0 to 4, for a total score of 0 to 40.
The YBOCS has good reliability and validity, is available in both clinician-rated and self-rated versions, and can be given repeatedly to measure treatment progress. A Children’s Yale-Brown Obsessive-Compulsive Scale (CYBOCS) is useful for patients ages 6 to 17.16
TREATING UNCOMPLICATED OCD
CBT. When OCD is not concurrent with another diagnosis, expert consensus guidelines recommend CBT as first-line treatment.17 Most patients treated with exposure and response prevention (ERP) therapy—the specialized CBT for reducing anxiety that triggers obsessive-compulsive symptoms—report reduced symptoms and often maintain those gains over time.18
In specialty clinics, patients frequently engage in intensive ERP (2 hours per day, 3 to 5 times per week for about 3 weeks). Although studies find excellent outcomes with intensive OCD treatment,18 it is not always practical or indicated (as in patients with moderate symptoms). Less-intensive protocols, such as biweekly sessions, have also shown promise in studies examining how session frequency affects treatment outcome.19
Many studies supporting ERP’s efficacy in OCD have included relatively homogenous samples under well-controlled conditions. Some investigations have also found good effects for ERP when including patients with complex treatment histories, concomitant pharmacotherapy, and comorbid conditions.20
Medication. Functional imaging studies suggest that OCD results from dysregulation in the socalled “OCD circuit”—the orbitofrontal cortex, anterior cingulate, and caudate nucleus. In patients with OCD, metabolic activity in this region is increased at rest relative to controls, increases further with symptoms, and decreases after successful treatment.21 The serotonin hypothesis—which emerged from observation that OCD symptoms responded to serotonergic medications but not to noradrenergic ones—suggests serotonin system dysregulation in patients with OCD.
High dosages of SRIs—selective serotonin reuptake inhibitors or the tricyclic antidepressant clomipramine—are first-line OCD medications (Table 3). Double-blind clinical trials have found clomipramine, fluoxetine, sertraline, paroxetine, fluvoxamine, and citalopram more effective than placebo, and the first five of these drugs are FDA-approved for treating adult OCD.
Table 3
Serotonin reuptake inhibitors indicated for treating OCD*
| Drug | Starting dosage | Target dosage (adults) |
|---|---|---|
| Clomipramine | 25 mg/d | 150 to 200 mg/d |
| Fluoxetine | 20 mg/d | 60 to 80 mg/d |
| Fluvoxamine | 50 mg/d | 150 to 300 mg/d |
| Paroxetine | 20 mg/d | 40 to 60 mg/d |
| Sertraline | 50 mg/d | 150 to 200 mg/d |
| * 10- to 12-week medication trials at target doses; sequential trials may be required to achieve treatment response. | ||
Nonresponse. Patients typically require at least 10 to 12 weeks of treatment at target dosages. Sequential medication trials may be needed to achieve a response. Complete remission is rare, and relapse rates are high when medication is discontinued.22
The up to 40% of patients who do not respond to SRI therapy require alternate strategies:
- Augmenting SRI therapy with a low-dose atypical antipsychotic such as risperidone, 1 to 2 mg bid, or olanzapine, 5 to 10 mg/d, may be effective, even in patients without a comorbid psychotic or tic disorder.23,24 It is worth noting that trials using atypicals as adjunctive therapy for OCD have been brief (12 weeks), and long-term use of these medications carries a risk of metabolic side effects such as weight gain, diabetes, and hyperlipidemia.
- The serotonin-norepinephrine reuptake inhibitor venlafaxine, 225 mg/d or higher, showed efficacy in a naturalistic study of patients who did not respond to SRIs.25
- Augmentation with pindolol, lithium, buspirone, trazodone, tryptophan, or thyroid hormone has shown mixed results.24
FACTORING IN COMORBIDITIES
Acute risk? Conditions that endanger the patient take precedence over OCD treatment. Suicidal risk and self-mutilating behaviors, for instance, must be addressed before a patient can engage in ERP therapy. Active psychosis also would exclude ERP and may be best handled by augmenting SRI therapy with an antipsychotic.17
Interfere with CBT? Exposure therapy can exacerbate symptoms in patients who self-medicate their anxiety with alcohol or other substances. In turn, alcohol or other substance abuse may interfere with habituation by ameliorating the anxiety necessary for effective exposure therapy. Thus, we recommend delaying OCD behavioral treatment until you treat or stabilize these conditions.
Many OCD patients report comorbid depression, which may be secondary to their OCD symptoms and may spontaneously decrease with successful OCD treatment. Patients with mild to moderate depression can usually engage in and benefit from ERP without depressionspecific interventions.
Patients with comorbid depression may not respond to OCD interventions as well as nondepressed OCD patients do.26 For concurrent OCD and major depression, expert consensus guidelines suggest combining CBT with an SRI.17
Less is known about how other comorbidities affect OCD treatment. In one study, patients with comorbid OCD and posttraumatic stress disorder (PTSD) responded poorly to ERP. Exposure therapy reduced OCD symptoms but increased PTSD symptoms in some patients.27 Some Axis II disorders—such as schizotypal, avoidant, paranoid, and borderline personality disorder—have also been found to predict poorer outcome in patients treated with clomipramine.3
Concurrent treatment? In some concomitant conditions, such as PTSD with OCD, preliminary evidence suggests that treatment can or should be simultaneous rather than sequential.27 Likewise, CBT can be used to treat OCD concurrent with other anxiety disorders with only slight modifications, such as:
- constructing exposures for social anxiety disorder patients that, at least initially, minimize extraneous social contact and evaluative fears
- instructing panic disorder patients in anxiety management skills so that exposures do not trigger anxiety attacks and reinforce their fears.
Related resources
- Jenike MA, Baer L, Minichiello WE (eds). Obsessive-compulsive disorders: practical management (3rd ed). New York: Mosby, 1998.
- Clark DA. Cognitive-behavioral therapy for OCD. New York: Guilford Press, 2003.
- Obsessive-Compulsive Foundation. www.ocfoundation.org.
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weissman MM, Bland RC, Canino GJ, et al. The cross national epidemiology of obsessive-compulsive disorder: The Cross National Collaborative Group. J Clin Psychiatry 1994;55(suppl 3):5-10.
2. Overbeek T, Schruers K, Vermetten E, Griez E. Comborbidity of obsessive-compulsive disorder and depression: Prevalence, symptom severity, and treatment effect. J Clin Psychiatry 2002;63(12):1106-12.
3. Baer L, Jenike MA, Black DW, Treece C. Effects of Axis II diagnosis on treatment outcome with clomipramine in 55 patients with obsessive compulsive disorder. Arch Gen Psychiatry 1992;49(11):862-6.
4. Rasmussen SA, Eisen JL. The epidemiology and clinical features of obsessive compulsive disorder. Psychiatr Clin North Am 1992;15(4):743-58.
5. Rubenstein CS, Pigott TA, L’Heureux F, et al. A preliminary investigation of the lifetime prevalence of anorexia and bulimia nervosa in patients with obsessive compulsive disorder. J Clin Psychiatry 1992;53(9):309-14.
6. Perugi G, Akiskal HS, Pfanner C, et al. Clinical impact of bipolar and unipolar affective comorbidity on obsessive compulsive disorder. J Affect Disord 1997;46(1):15-23.
7. March J, Frances A, Kahn D, Carpenter D. Expert consensus guidelines: treatment of obsessive compulsive disorder. J Clin Psychiatry 1997;58(suppl 4):1-72.
8. Foa EB, Liebowitz MR, Kozak MJ, et al. Randomized, placebo-controlled trial of exposure and response prevention, clomipramine, and their combination in the treatment of obsessive compulsive disorder. Am J Psychiatry 2005;162(1):151-61.
9. Kozak MJ, Liebowitz MR, Foa EB. Cognitive behavior therapy and pharmacotherapy for OCD: The NIMH-sponsored collaborative study. In: Goodman W, Rudorfer M, Maser J (eds). Obsessive compulsive disorder: Contemporary issues in treatment. Mahwah, NJ: Lawrence Erlbaum Associates, 2000;501:30.-
10. Brown TA, DiNardo PA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV. New York: Graywind Publications, 1994.
11. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The MiniInternational Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(suppl 20):22-33.
12. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale: I. Development, use, and reliability. Arch Gen Psychiatry 1989;46(11):1006M-11.
13. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale: II.Validity. Arch Gen Psychiatry 1989;46(11):1012-6.
14. Foa EB, Huppert JD, Leiberg S, et al. The Obsessive-Compulsive Inventory: development and validation of a short version. Psychol Assess 2002;14(4):485-96.
15. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition New York: New York State Psychiatric Institute, Biometrics Research Department, 1996.
16. Scahill L, Riddle M, McSwiggin-Hardin M, et al. Children’s Yale-Brown Obsessive-Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry 1997;36(6):844-52.
17. Frances A, Docherty JP, Kahn DA. Treatment of obsessive compulsive disorder. J Clin Psychiatry 1997;58(suppl 4):5-72.
18. Foa EB, Franklin ME. Psychotherapies for obsessive-compulsive disorder: A review. In: Maj M, Sartorius N, Okasha A, Zohar J (eds). Obsessive-Compulsive Disorder New York: Wiley, 2000;93:115.-
19. Abramowitz JS, Foa EB, Franklin ME. Exposure and ritual prevention for obsessive-compulsive disorder: Effect of intensive versus twice weekly sessions. J Consult Clin Psychol 2003;71(2):394-8.
20. Franklin ME, Abramowitz JS, Kozak MJ, et al. Effectiveness of exposure and ritual prevention for obsessive-compulsive disorder: Randomized compared with nonrandomized samples. J Consult Clin Psychol 2000;68(4):594-602.
21. Graybiel A, Rauch SL. Toward a neurobiology of obsessive compulsive disorder. Neuron 2000;28:343-7.
22. Pigott TA, Seay S. Pharmacology of obsessive compulsive disorder: overview and treatment-refractory strategies. In: Goodman MK, Rudorfer MV, Maser JD (eds). Obsessive compulsive disorder: contemporary issues in treatment Mahwah, NJ: Lawrence Erlbaum Associates, 2000;277:302.-
23. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 2000;57(8):794-801.
24. Hollander E, Bienstock CA, Koran LM, et al. Refractory obsessive-compulsive disorder: state-of-the-art treatment. J Clin Psychiatry 2002;63(suppl 6):20-9.
25. Hollander E, Friedberg BS, Wasserman S, et al. Venlafaxine in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry 2003;64(5):546-50.
26. Abramowitz JS, Foa EB. Does major depressive disorder influence outcome of exposure and response prevention for OCD? Behavior Ther 2001;31(4):795-800.
27. Gershuny BS, Baer L, Jenike MA, et al. Comorbid posttraumatic stress disorder: impact on treatment outcome for obsessive-compulsive disorder. Am J Psychiatry 2002;159(5):852-4.
28. Perugi G, Toni C, Frare F, et al. Obsessive-compulsive-bipolar comorbidity: a systematic exploration of clinical features and treatment outcome. J Clin Psychiatry 2002;63(12):1129-34.
1. Weissman MM, Bland RC, Canino GJ, et al. The cross national epidemiology of obsessive-compulsive disorder: The Cross National Collaborative Group. J Clin Psychiatry 1994;55(suppl 3):5-10.
2. Overbeek T, Schruers K, Vermetten E, Griez E. Comborbidity of obsessive-compulsive disorder and depression: Prevalence, symptom severity, and treatment effect. J Clin Psychiatry 2002;63(12):1106-12.
3. Baer L, Jenike MA, Black DW, Treece C. Effects of Axis II diagnosis on treatment outcome with clomipramine in 55 patients with obsessive compulsive disorder. Arch Gen Psychiatry 1992;49(11):862-6.
4. Rasmussen SA, Eisen JL. The epidemiology and clinical features of obsessive compulsive disorder. Psychiatr Clin North Am 1992;15(4):743-58.
5. Rubenstein CS, Pigott TA, L’Heureux F, et al. A preliminary investigation of the lifetime prevalence of anorexia and bulimia nervosa in patients with obsessive compulsive disorder. J Clin Psychiatry 1992;53(9):309-14.
6. Perugi G, Akiskal HS, Pfanner C, et al. Clinical impact of bipolar and unipolar affective comorbidity on obsessive compulsive disorder. J Affect Disord 1997;46(1):15-23.
7. March J, Frances A, Kahn D, Carpenter D. Expert consensus guidelines: treatment of obsessive compulsive disorder. J Clin Psychiatry 1997;58(suppl 4):1-72.
8. Foa EB, Liebowitz MR, Kozak MJ, et al. Randomized, placebo-controlled trial of exposure and response prevention, clomipramine, and their combination in the treatment of obsessive compulsive disorder. Am J Psychiatry 2005;162(1):151-61.
9. Kozak MJ, Liebowitz MR, Foa EB. Cognitive behavior therapy and pharmacotherapy for OCD: The NIMH-sponsored collaborative study. In: Goodman W, Rudorfer M, Maser J (eds). Obsessive compulsive disorder: Contemporary issues in treatment. Mahwah, NJ: Lawrence Erlbaum Associates, 2000;501:30.-
10. Brown TA, DiNardo PA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV. New York: Graywind Publications, 1994.
11. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The MiniInternational Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(suppl 20):22-33.
12. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale: I. Development, use, and reliability. Arch Gen Psychiatry 1989;46(11):1006M-11.
13. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale: II.Validity. Arch Gen Psychiatry 1989;46(11):1012-6.
14. Foa EB, Huppert JD, Leiberg S, et al. The Obsessive-Compulsive Inventory: development and validation of a short version. Psychol Assess 2002;14(4):485-96.
15. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition New York: New York State Psychiatric Institute, Biometrics Research Department, 1996.
16. Scahill L, Riddle M, McSwiggin-Hardin M, et al. Children’s Yale-Brown Obsessive-Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry 1997;36(6):844-52.
17. Frances A, Docherty JP, Kahn DA. Treatment of obsessive compulsive disorder. J Clin Psychiatry 1997;58(suppl 4):5-72.
18. Foa EB, Franklin ME. Psychotherapies for obsessive-compulsive disorder: A review. In: Maj M, Sartorius N, Okasha A, Zohar J (eds). Obsessive-Compulsive Disorder New York: Wiley, 2000;93:115.-
19. Abramowitz JS, Foa EB, Franklin ME. Exposure and ritual prevention for obsessive-compulsive disorder: Effect of intensive versus twice weekly sessions. J Consult Clin Psychol 2003;71(2):394-8.
20. Franklin ME, Abramowitz JS, Kozak MJ, et al. Effectiveness of exposure and ritual prevention for obsessive-compulsive disorder: Randomized compared with nonrandomized samples. J Consult Clin Psychol 2000;68(4):594-602.
21. Graybiel A, Rauch SL. Toward a neurobiology of obsessive compulsive disorder. Neuron 2000;28:343-7.
22. Pigott TA, Seay S. Pharmacology of obsessive compulsive disorder: overview and treatment-refractory strategies. In: Goodman MK, Rudorfer MV, Maser JD (eds). Obsessive compulsive disorder: contemporary issues in treatment Mahwah, NJ: Lawrence Erlbaum Associates, 2000;277:302.-
23. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 2000;57(8):794-801.
24. Hollander E, Bienstock CA, Koran LM, et al. Refractory obsessive-compulsive disorder: state-of-the-art treatment. J Clin Psychiatry 2002;63(suppl 6):20-9.
25. Hollander E, Friedberg BS, Wasserman S, et al. Venlafaxine in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry 2003;64(5):546-50.
26. Abramowitz JS, Foa EB. Does major depressive disorder influence outcome of exposure and response prevention for OCD? Behavior Ther 2001;31(4):795-800.
27. Gershuny BS, Baer L, Jenike MA, et al. Comorbid posttraumatic stress disorder: impact on treatment outcome for obsessive-compulsive disorder. Am J Psychiatry 2002;159(5):852-4.
28. Perugi G, Toni C, Frare F, et al. Obsessive-compulsive-bipolar comorbidity: a systematic exploration of clinical features and treatment outcome. J Clin Psychiatry 2002;63(12):1129-34.
Balanced therapy: How to avoid conflict, help ‘borderline’ patients
Treating borderline personality disorder can seem like a no-win situation. If we try traditional cognitive-behavioral therapy (CBT) and emphasize change, patients feel unheard and invalidated; they may withdraw, quit, or even attack. But if we suggest ways to accept unhappy situations, they may feel we don’t understand their suffering.
A more effective approach is dialectical behavior therapy (DBT), first developed to treat highly suicidal persons with borderline personality disorder and used with other populations that have difficulty regulating their emotions.
This article describes how invalidating environments may damage emotional health and suggests how psychiatrists can use DBT’s methods when treating borderline personality disorder.
BIOLOGY PLUS ENVIRONMENT
For the patient, borderline personality disorder’s behavior clusters (Table 1):
- function to regulate emotions
- or result from emotion dysregulation.
DBT theory identifies emotion dysregulation as the primary deficit in borderline personality disorder. Biologically based emotional vulnerability is seen as interacting with an inability to modulate emotions because of a skills deficit.
Emotional vulnerability. Three characteristics—high sensitivity, high reactivity, and slow return to baseline emotional state—define high emotional vulnerability:
High sensitivity. The person reacts more quickly and to more things than do others in emotion-provoking situations. When walking, for example, they may pass someone who doesn’t say hello. Most people would shrug this off, but persons with high emotional sensitivity may quickly notice, assume there is a problem, feel they have done something wrong, then feel shame and anger.
High reactivity. Their emotional reactions are large, and the high arousal dysregulates cognitive processing.
Slow return to baseline. Events stack up because emotional reactions are long-lasting for persons with high emotional vulnerability. They don’t have time to get over one thing before something else happens.
DBT postulates that, over time, borderline personality disorder results from the transaction of biological emotional vulnerability and an invalidating environment. This therapeutic model asserts that biology and the environment are flexible, and interventions may influence both.
Invalidating environment. DBT acknowledges that invalidation occurs in all environments, even nuturing ones. It becomes detrimental when a vulnerable person is exposed to pervasive invalidation that is not related to the validity of the person’s behavior or to the person’s expressed emotions or thoughts.
An invalidating environment has three characteristic patterns. One is indiscriminate rejection of communication of private experiences and self-generated behaviors.
Case examples. Mary, age 8, says she’s been teased and it hurt her feelings. Her mother tells her she is making too much of the incident. Mary questions herself and searches the social environment for cues about how to respond to similar situations in the future.
Robbie, age 4, completes a drawing and shows it to his father with delight. His father points out some “sloppy” coloring. If his father repeatedly finds fault with his work, Robbie is likely to not show him his work or stop drawing, and his expressions of delight are likely to decrease.
Invalidating environments may also punish emotional displays and intermittently reinforce emotional escalation. Someone may show disapproval for or ignore a person’s genuine sadness or fear but attend to angry outbursts that result when the person feels ignored.
The third invalidating pattern is to oversimplify the ease of problem-solving and meeting goals.
Case example. As a child, when Susan asked for help, her mother would say “just do it,” without considering the skills her daughter needed to accomplish tasks. When Susan became frustrated, her mother demanded that she “just stop cying,” even though no person could modulate his or her emotions that quickly. As an adult, Susan now sets unrealistic goals and expectations for herself and despairs when she is unable to solve problems in her life.
These three invalidating patterns cause persons to search the social environment for cues about how to respond to situations. They may question themselves, their identity, and the appropriateness of any emotional expression. As a result, they may oscillate between emotional inhibition and extreme emotional styles, set unrealistic goals and expectations for themselves, and eventually despair of being able to solve their problems.
Specific to borderline personality disorder is that the environment ignores genuine emotional expression, and the individual’s emotions escalate. This pattern is reinforced when the listener finally rewards emotionally extreme behavior with attention or desired changes.
As the pattern is repeated over time, extreme emotional reactions become the norm rather than the exception, and the emotional chaos can make the person wish to die. Acting on that desire when past expressions of desperation have been ignored or invalidated can provide attention or interventions that would never happen after simple emotional expressions.
Thus, an environment that does not recognize or validate genuine emotional expression can reinforce suicidality.
Table 1
Diagnostic criteria for borderline personality disorder
A pervasive pattern of instability of interpersonal relationships, self-image, and affects, and marked impulsivity beginning by early adulthood and present in a variety of contexts, as indicated by five (or more) of the following:
|
| Source: Adapted and reprinted with permission from the Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Copyright 2000. American Psychiatric Association. |
SOLVING NO-WIN THERAPY
Pitfalls with emphasizing change. Therapy that emphasizes solving problems and getting things to change typically triggers high arousal in persons with borderline personality disorder. Feeling out of control, they respond by trying to get in control, including attempts to control the therapist.
Similarly, they see attempts to get them to change their behavior as invalidating their experiences or, worse, who they are. Intense emotions aroused by the message they hear—that they are the source of their problems—impair learning and intensify their efforts to gain control. In a battle for control, collaboration and therapy cannot occur.
Case example. Ms. K wants you to understand how difficult her life is because of difficulties with her boss. You start talking about what Ms. K can do to change the situation, without acknowledging how difficult it is to deal with her boss.
Ms. K feels upset and says you don’t understand. For her, the interaction has led to emotion dysregulation and impaired cognitive processing.
Pitfalls with emphasizing acceptance. Most persons who come to therapy very distressed want something in their lives to change. If your primary message is acceptance instead of change, they may lose confidence in you.
Case example. Ms. K wants help dealing with her boss, who is making life quite difficult. As her therapist, you respond with warmth and acceptance but offer no suggestions as to how she might change the situation. Ms. K likes the way you listen to her but abandons therapy. after several sessions.
At first, patients with borderline personality disorder may like the warmth of client-centered acceptance approaches. Over time, however, they may feel their therapy sessions are out of control. They may think the therapist doesn’t understand the situation, doesn’t know how to help, or that situations that are troubling them cannot be changed.
Balanced therapy. DBT solves the change-or-acceptance dilemma by attempting to help patients with borderline personality disorder change themselves and their lives while offering strategies for accepting themselves and their situations.1,2 DBT includes problem-solving and acceptance strategies (Table 2).
Table 2
Strategies used in dialectical behavioral therapy
| Structural strategies | Organization of sessions, attending to the treatment hierarchy, reviewing progress, checking on other modes of therapy |
| Problem assessment strategies | Defining problems with specificity, conducting chain analyses, developing and testing hypotheses |
| Problem solving strategies | Providing didactic information, generating and evaluating solutions, teaching skills and coaching on use of skills, generalizing skills to the real-world environment |
| Contingency management | Use of reinforcement, extinction, aversive contingencies, and principles of shaping. |
| Exposure-based procedures | Both formal and informal |
| Cognitive strategies | Contingency clarification, observation and description of cognitions, cognitive modification |
| Validation strategies | Appearing interested, accurately reflecting, correctly articulating things that have not been fully expressed, explaining behavior in terms of learning history or biological factors, acknowledging validity of responses in terms of current events, interacting in a radically genuine manner, communicating belief in the patient |
| Reciprocal communication strategies | Being responsive, expressing warm engagement, being nonjudgmental, using self-disclosure, maintaining a reasonable power equilibrium |
| Irreverent strategies | Engaging in a matter-of-fact manner, directly confronting dysfunctional behavior, using unexpected, irreverent or humorous responses |
| Dialectical strategies | Using a balanced style, balancing acceptance-oriented strategies with change-oriented strategies, magnifying tension, using metaphor, modeling dialectical thinking and behaviors, moving with speed and flow. |
| Case management strategies | Following a model of consultation to the patient when long-term outcome is more important than short-term outcome; intervening in the patient's environment when short-term outcome is more important than long-term outcome |
DBT’S 4 THERAPY STAGES
DBT is a comprehensive treatment. The original outpatient model for borderline personality disorder (Table 3) has been adapted to different settings and applied to other populations.
Outpatients meet weekly in individual psychotherapy and a skills training group.3 Therapists also meet weekly in a consultation team viewed as “therapy for the therapist.”
Between sessions, therapists consult with patients by telephone to:
- decrease suicide crisis behaviors
- increase generalization of behavioral skills
- decrease patients’ feelings of conflict, alienation, or distance with the therapist.
Four stages. DBT follows four stages. For persons with borderline personality disorder, researchers have evaluated the efficacy of stage-1 therapy. Studies on stage-3 DBT have been conducted with nonborderline-personality individuals with eating disorders. The goals at each stage are:
Stage 1. Move from severe behavioral dyscontrol to behavioral control. Decrease suicidal and other life-threatening behaviors and those that interfere with therapy and quality of life. Increase mindfulness, tolerance for distress, interpersonal effectiveness, and emotion regulation.
Stage 2. Move from quiet desperation to emotional experiencing.
Stage 3. Address problems in living, and move toward ordinary happiness/unhappiness.
Stage 4. Move from incompleteness to capacity for joy and freedom.
Seven randomized controlled trials have shown that DBT can be useful in treating borderline personality disorder.4-10 The initial trial by Linehan et al4 included 47 women ages 18 to 45 who met criteria for borderline personality disorder and had at least two parasuicide incidents in the previous 5 years, with one in the previous 8 weeks. Treatment lasted 1 year, and subjects agreed to stop other individual psychotherapy if assigned to DBT.
Subjects were then randomly assigned to either DBT or “treatment as usual” in the community. In the various DBT studies, treatment-as-usual has included community therapists, Department of Veterans Affairs outpatient treatment, client-centered therapy, and treatment by persons identified by their peers as experts in their communities.
Subjects were assessed every 4 months while in treatment and for 1 year thereafter. DBT was more effective than usual treatment in:
- reducing suicide attempts and self-injury
- decreasing premature dropout from therapy
- reducing emergency room admissions and length of psychiatric hospitalization
- reducing drug abuse, depression, hopelessness, and anger.
Table 3
Modes of therapy in outpatient dialectical behavioral therapy
| Therapeutic goals | Mode |
|---|---|
| Improve motivational factors | Individual psychotherapy |
| Enhance capabilities | Skills training |
| Ensure generalization to natural environment | Between-session consultation |
| Enhance therapist capabilities and motivation to treat effectively | Therapist consultation team |
| Structure the environment | Consultation to the patient |
RECOMMENDATIONS
Some psychiatrists may find “borderline patients” frustrating and unpleasant to treat. DBT therapists, however, make two assumptions that can help anyone working with individuals with borderline personality disorder. To avoid falling into the trap of polarization with these patients, assume that:
- they are doing the best they can
- their efforts are insufficient to meet their needs.
They therefore need to do better, and the therapist’s job is to help them do so. Also assume that if you try to help a patient with borderline personality disorder, you will need help, too. We require DBT therapists to participate in consultation teams.
Training. DBT is a comprehensive program that requires familiarity with the manuals mentioned in this article (see Related resources). Some teams have learned DBT through self-study and consultation with other teams.
If you plan to offer DBT to patients with borderline personality disorder, we recommend that you be:
- trained in behavior therapy and CBT
- familiar with research on emotions and processes involved in emotion regulation.
If you have not had CBT training, find a behavior therapist to join your team or get consultation from a behavior therapist.
An intensive training course in DBT—with 2 weeks of instruction and case consultation and several months of consultation with someone well-versed in DBT—is an efficient way to become familiar with the most critical principles of the treatment. If you cannot train toward adherent delivery of individual therapy, we recommend referring patients to someone trained in DBT.
- Lieb K, Zanarini MC, Schmahl C, et al. Borderline personality disorder. Lancet 2004;364(9432):453-61.
- University of Washington, Behavioral Research and Therapy Clinics. www.brtc.psych.washington.edu/frameResearch.htm;
www.brtc.psych.washington.edu/framePublications.htm - Behavioral Tech, LLC. Consultation and training in dialectical behavioral therapy. www.behavioraltech.org.
Disclosures
Dr. DuBose is president and CEO/co-owner of DBT Center of Seattle, PLLC, and a speaker for Behavioral Tech, LLC.
Dr. Linehan is a shareholder in Behavioral Tech Research, Inc., which develops computerized training for DBT, a DBT trainer for Behavioral Tech, LLC, and the author of two books on DBT. She also receives research grants from the National Institute of Mental Health and National Institute on Drug Abuse.
1. Lynch TR, Chapman AL, Rosenthal MZ, et al. Mechanisms of change in dialectical behavior therapy: theoretical and empirical observations. J Clin Psychol 2005 (in press).
2. Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York: Guilford Press, 1993.
3. Linehan MM. Skills training manual for treating borderline personality disorder. New York: Guilford Press, 1993.
4. Linehan MM, Armstrong HE, Suarez A, et al. Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Arch Gen Psychiatry 1991 Dec;48(12):1060-4.
5. Linehan MM, Schmidt HI, Dimeff LA, et al. Dialectical behavior therapy for patients with border-line personality disorder and drug-dependence. Am J Addict 1999;8:279-92.
6. Linehan MM, Dimeff LA, Reynolds SK, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend 2002;67(1):13-26.
7. Turner RM. Naturalistic evaluation of dialectical behavior therapy-oriented treatment for borderline personality disorders. Cognit Behav Pract 2000;7:413-19.
8. Koons CR, Robins CJ, Tweed JL, et al. Efficacy of dialectical behavior therapy in women veterans with borderline personality disorder. Behav Ther 2001;32:371-90.
9. Verheul R, Van Den Bosch LM, Koeter MW, et al. Dialectical behaviour therapy for women with borderline personality disorder: 12-month, randomised clinical trial in The Netherlands. Br J Psychiatry 2003;182:135-40.
10. Linehan MM, Comtois KA, Brown M, et al. DBT versus nonbehavioral treatment by experts in the community: clinical outcomes. Symposium presentation for the Association for Advancement of Behavior Therapy. University of Washington, Reno NV, 2002.
Treating borderline personality disorder can seem like a no-win situation. If we try traditional cognitive-behavioral therapy (CBT) and emphasize change, patients feel unheard and invalidated; they may withdraw, quit, or even attack. But if we suggest ways to accept unhappy situations, they may feel we don’t understand their suffering.
A more effective approach is dialectical behavior therapy (DBT), first developed to treat highly suicidal persons with borderline personality disorder and used with other populations that have difficulty regulating their emotions.
This article describes how invalidating environments may damage emotional health and suggests how psychiatrists can use DBT’s methods when treating borderline personality disorder.
BIOLOGY PLUS ENVIRONMENT
For the patient, borderline personality disorder’s behavior clusters (Table 1):
- function to regulate emotions
- or result from emotion dysregulation.
DBT theory identifies emotion dysregulation as the primary deficit in borderline personality disorder. Biologically based emotional vulnerability is seen as interacting with an inability to modulate emotions because of a skills deficit.
Emotional vulnerability. Three characteristics—high sensitivity, high reactivity, and slow return to baseline emotional state—define high emotional vulnerability:
High sensitivity. The person reacts more quickly and to more things than do others in emotion-provoking situations. When walking, for example, they may pass someone who doesn’t say hello. Most people would shrug this off, but persons with high emotional sensitivity may quickly notice, assume there is a problem, feel they have done something wrong, then feel shame and anger.
High reactivity. Their emotional reactions are large, and the high arousal dysregulates cognitive processing.
Slow return to baseline. Events stack up because emotional reactions are long-lasting for persons with high emotional vulnerability. They don’t have time to get over one thing before something else happens.
DBT postulates that, over time, borderline personality disorder results from the transaction of biological emotional vulnerability and an invalidating environment. This therapeutic model asserts that biology and the environment are flexible, and interventions may influence both.
Invalidating environment. DBT acknowledges that invalidation occurs in all environments, even nuturing ones. It becomes detrimental when a vulnerable person is exposed to pervasive invalidation that is not related to the validity of the person’s behavior or to the person’s expressed emotions or thoughts.
An invalidating environment has three characteristic patterns. One is indiscriminate rejection of communication of private experiences and self-generated behaviors.
Case examples. Mary, age 8, says she’s been teased and it hurt her feelings. Her mother tells her she is making too much of the incident. Mary questions herself and searches the social environment for cues about how to respond to similar situations in the future.
Robbie, age 4, completes a drawing and shows it to his father with delight. His father points out some “sloppy” coloring. If his father repeatedly finds fault with his work, Robbie is likely to not show him his work or stop drawing, and his expressions of delight are likely to decrease.
Invalidating environments may also punish emotional displays and intermittently reinforce emotional escalation. Someone may show disapproval for or ignore a person’s genuine sadness or fear but attend to angry outbursts that result when the person feels ignored.
The third invalidating pattern is to oversimplify the ease of problem-solving and meeting goals.
Case example. As a child, when Susan asked for help, her mother would say “just do it,” without considering the skills her daughter needed to accomplish tasks. When Susan became frustrated, her mother demanded that she “just stop cying,” even though no person could modulate his or her emotions that quickly. As an adult, Susan now sets unrealistic goals and expectations for herself and despairs when she is unable to solve problems in her life.
These three invalidating patterns cause persons to search the social environment for cues about how to respond to situations. They may question themselves, their identity, and the appropriateness of any emotional expression. As a result, they may oscillate between emotional inhibition and extreme emotional styles, set unrealistic goals and expectations for themselves, and eventually despair of being able to solve their problems.
Specific to borderline personality disorder is that the environment ignores genuine emotional expression, and the individual’s emotions escalate. This pattern is reinforced when the listener finally rewards emotionally extreme behavior with attention or desired changes.
As the pattern is repeated over time, extreme emotional reactions become the norm rather than the exception, and the emotional chaos can make the person wish to die. Acting on that desire when past expressions of desperation have been ignored or invalidated can provide attention or interventions that would never happen after simple emotional expressions.
Thus, an environment that does not recognize or validate genuine emotional expression can reinforce suicidality.
Table 1
Diagnostic criteria for borderline personality disorder
A pervasive pattern of instability of interpersonal relationships, self-image, and affects, and marked impulsivity beginning by early adulthood and present in a variety of contexts, as indicated by five (or more) of the following:
|
| Source: Adapted and reprinted with permission from the Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Copyright 2000. American Psychiatric Association. |
SOLVING NO-WIN THERAPY
Pitfalls with emphasizing change. Therapy that emphasizes solving problems and getting things to change typically triggers high arousal in persons with borderline personality disorder. Feeling out of control, they respond by trying to get in control, including attempts to control the therapist.
Similarly, they see attempts to get them to change their behavior as invalidating their experiences or, worse, who they are. Intense emotions aroused by the message they hear—that they are the source of their problems—impair learning and intensify their efforts to gain control. In a battle for control, collaboration and therapy cannot occur.
Case example. Ms. K wants you to understand how difficult her life is because of difficulties with her boss. You start talking about what Ms. K can do to change the situation, without acknowledging how difficult it is to deal with her boss.
Ms. K feels upset and says you don’t understand. For her, the interaction has led to emotion dysregulation and impaired cognitive processing.
Pitfalls with emphasizing acceptance. Most persons who come to therapy very distressed want something in their lives to change. If your primary message is acceptance instead of change, they may lose confidence in you.
Case example. Ms. K wants help dealing with her boss, who is making life quite difficult. As her therapist, you respond with warmth and acceptance but offer no suggestions as to how she might change the situation. Ms. K likes the way you listen to her but abandons therapy. after several sessions.
At first, patients with borderline personality disorder may like the warmth of client-centered acceptance approaches. Over time, however, they may feel their therapy sessions are out of control. They may think the therapist doesn’t understand the situation, doesn’t know how to help, or that situations that are troubling them cannot be changed.
Balanced therapy. DBT solves the change-or-acceptance dilemma by attempting to help patients with borderline personality disorder change themselves and their lives while offering strategies for accepting themselves and their situations.1,2 DBT includes problem-solving and acceptance strategies (Table 2).
Table 2
Strategies used in dialectical behavioral therapy
| Structural strategies | Organization of sessions, attending to the treatment hierarchy, reviewing progress, checking on other modes of therapy |
| Problem assessment strategies | Defining problems with specificity, conducting chain analyses, developing and testing hypotheses |
| Problem solving strategies | Providing didactic information, generating and evaluating solutions, teaching skills and coaching on use of skills, generalizing skills to the real-world environment |
| Contingency management | Use of reinforcement, extinction, aversive contingencies, and principles of shaping. |
| Exposure-based procedures | Both formal and informal |
| Cognitive strategies | Contingency clarification, observation and description of cognitions, cognitive modification |
| Validation strategies | Appearing interested, accurately reflecting, correctly articulating things that have not been fully expressed, explaining behavior in terms of learning history or biological factors, acknowledging validity of responses in terms of current events, interacting in a radically genuine manner, communicating belief in the patient |
| Reciprocal communication strategies | Being responsive, expressing warm engagement, being nonjudgmental, using self-disclosure, maintaining a reasonable power equilibrium |
| Irreverent strategies | Engaging in a matter-of-fact manner, directly confronting dysfunctional behavior, using unexpected, irreverent or humorous responses |
| Dialectical strategies | Using a balanced style, balancing acceptance-oriented strategies with change-oriented strategies, magnifying tension, using metaphor, modeling dialectical thinking and behaviors, moving with speed and flow. |
| Case management strategies | Following a model of consultation to the patient when long-term outcome is more important than short-term outcome; intervening in the patient's environment when short-term outcome is more important than long-term outcome |
DBT’S 4 THERAPY STAGES
DBT is a comprehensive treatment. The original outpatient model for borderline personality disorder (Table 3) has been adapted to different settings and applied to other populations.
Outpatients meet weekly in individual psychotherapy and a skills training group.3 Therapists also meet weekly in a consultation team viewed as “therapy for the therapist.”
Between sessions, therapists consult with patients by telephone to:
- decrease suicide crisis behaviors
- increase generalization of behavioral skills
- decrease patients’ feelings of conflict, alienation, or distance with the therapist.
Four stages. DBT follows four stages. For persons with borderline personality disorder, researchers have evaluated the efficacy of stage-1 therapy. Studies on stage-3 DBT have been conducted with nonborderline-personality individuals with eating disorders. The goals at each stage are:
Stage 1. Move from severe behavioral dyscontrol to behavioral control. Decrease suicidal and other life-threatening behaviors and those that interfere with therapy and quality of life. Increase mindfulness, tolerance for distress, interpersonal effectiveness, and emotion regulation.
Stage 2. Move from quiet desperation to emotional experiencing.
Stage 3. Address problems in living, and move toward ordinary happiness/unhappiness.
Stage 4. Move from incompleteness to capacity for joy and freedom.
Seven randomized controlled trials have shown that DBT can be useful in treating borderline personality disorder.4-10 The initial trial by Linehan et al4 included 47 women ages 18 to 45 who met criteria for borderline personality disorder and had at least two parasuicide incidents in the previous 5 years, with one in the previous 8 weeks. Treatment lasted 1 year, and subjects agreed to stop other individual psychotherapy if assigned to DBT.
Subjects were then randomly assigned to either DBT or “treatment as usual” in the community. In the various DBT studies, treatment-as-usual has included community therapists, Department of Veterans Affairs outpatient treatment, client-centered therapy, and treatment by persons identified by their peers as experts in their communities.
Subjects were assessed every 4 months while in treatment and for 1 year thereafter. DBT was more effective than usual treatment in:
- reducing suicide attempts and self-injury
- decreasing premature dropout from therapy
- reducing emergency room admissions and length of psychiatric hospitalization
- reducing drug abuse, depression, hopelessness, and anger.
Table 3
Modes of therapy in outpatient dialectical behavioral therapy
| Therapeutic goals | Mode |
|---|---|
| Improve motivational factors | Individual psychotherapy |
| Enhance capabilities | Skills training |
| Ensure generalization to natural environment | Between-session consultation |
| Enhance therapist capabilities and motivation to treat effectively | Therapist consultation team |
| Structure the environment | Consultation to the patient |
RECOMMENDATIONS
Some psychiatrists may find “borderline patients” frustrating and unpleasant to treat. DBT therapists, however, make two assumptions that can help anyone working with individuals with borderline personality disorder. To avoid falling into the trap of polarization with these patients, assume that:
- they are doing the best they can
- their efforts are insufficient to meet their needs.
They therefore need to do better, and the therapist’s job is to help them do so. Also assume that if you try to help a patient with borderline personality disorder, you will need help, too. We require DBT therapists to participate in consultation teams.
Training. DBT is a comprehensive program that requires familiarity with the manuals mentioned in this article (see Related resources). Some teams have learned DBT through self-study and consultation with other teams.
If you plan to offer DBT to patients with borderline personality disorder, we recommend that you be:
- trained in behavior therapy and CBT
- familiar with research on emotions and processes involved in emotion regulation.
If you have not had CBT training, find a behavior therapist to join your team or get consultation from a behavior therapist.
An intensive training course in DBT—with 2 weeks of instruction and case consultation and several months of consultation with someone well-versed in DBT—is an efficient way to become familiar with the most critical principles of the treatment. If you cannot train toward adherent delivery of individual therapy, we recommend referring patients to someone trained in DBT.
- Lieb K, Zanarini MC, Schmahl C, et al. Borderline personality disorder. Lancet 2004;364(9432):453-61.
- University of Washington, Behavioral Research and Therapy Clinics. www.brtc.psych.washington.edu/frameResearch.htm;
www.brtc.psych.washington.edu/framePublications.htm - Behavioral Tech, LLC. Consultation and training in dialectical behavioral therapy. www.behavioraltech.org.
Disclosures
Dr. DuBose is president and CEO/co-owner of DBT Center of Seattle, PLLC, and a speaker for Behavioral Tech, LLC.
Dr. Linehan is a shareholder in Behavioral Tech Research, Inc., which develops computerized training for DBT, a DBT trainer for Behavioral Tech, LLC, and the author of two books on DBT. She also receives research grants from the National Institute of Mental Health and National Institute on Drug Abuse.
Treating borderline personality disorder can seem like a no-win situation. If we try traditional cognitive-behavioral therapy (CBT) and emphasize change, patients feel unheard and invalidated; they may withdraw, quit, or even attack. But if we suggest ways to accept unhappy situations, they may feel we don’t understand their suffering.
A more effective approach is dialectical behavior therapy (DBT), first developed to treat highly suicidal persons with borderline personality disorder and used with other populations that have difficulty regulating their emotions.
This article describes how invalidating environments may damage emotional health and suggests how psychiatrists can use DBT’s methods when treating borderline personality disorder.
BIOLOGY PLUS ENVIRONMENT
For the patient, borderline personality disorder’s behavior clusters (Table 1):
- function to regulate emotions
- or result from emotion dysregulation.
DBT theory identifies emotion dysregulation as the primary deficit in borderline personality disorder. Biologically based emotional vulnerability is seen as interacting with an inability to modulate emotions because of a skills deficit.
Emotional vulnerability. Three characteristics—high sensitivity, high reactivity, and slow return to baseline emotional state—define high emotional vulnerability:
High sensitivity. The person reacts more quickly and to more things than do others in emotion-provoking situations. When walking, for example, they may pass someone who doesn’t say hello. Most people would shrug this off, but persons with high emotional sensitivity may quickly notice, assume there is a problem, feel they have done something wrong, then feel shame and anger.
High reactivity. Their emotional reactions are large, and the high arousal dysregulates cognitive processing.
Slow return to baseline. Events stack up because emotional reactions are long-lasting for persons with high emotional vulnerability. They don’t have time to get over one thing before something else happens.
DBT postulates that, over time, borderline personality disorder results from the transaction of biological emotional vulnerability and an invalidating environment. This therapeutic model asserts that biology and the environment are flexible, and interventions may influence both.
Invalidating environment. DBT acknowledges that invalidation occurs in all environments, even nuturing ones. It becomes detrimental when a vulnerable person is exposed to pervasive invalidation that is not related to the validity of the person’s behavior or to the person’s expressed emotions or thoughts.
An invalidating environment has three characteristic patterns. One is indiscriminate rejection of communication of private experiences and self-generated behaviors.
Case examples. Mary, age 8, says she’s been teased and it hurt her feelings. Her mother tells her she is making too much of the incident. Mary questions herself and searches the social environment for cues about how to respond to similar situations in the future.
Robbie, age 4, completes a drawing and shows it to his father with delight. His father points out some “sloppy” coloring. If his father repeatedly finds fault with his work, Robbie is likely to not show him his work or stop drawing, and his expressions of delight are likely to decrease.
Invalidating environments may also punish emotional displays and intermittently reinforce emotional escalation. Someone may show disapproval for or ignore a person’s genuine sadness or fear but attend to angry outbursts that result when the person feels ignored.
The third invalidating pattern is to oversimplify the ease of problem-solving and meeting goals.
Case example. As a child, when Susan asked for help, her mother would say “just do it,” without considering the skills her daughter needed to accomplish tasks. When Susan became frustrated, her mother demanded that she “just stop cying,” even though no person could modulate his or her emotions that quickly. As an adult, Susan now sets unrealistic goals and expectations for herself and despairs when she is unable to solve problems in her life.
These three invalidating patterns cause persons to search the social environment for cues about how to respond to situations. They may question themselves, their identity, and the appropriateness of any emotional expression. As a result, they may oscillate between emotional inhibition and extreme emotional styles, set unrealistic goals and expectations for themselves, and eventually despair of being able to solve their problems.
Specific to borderline personality disorder is that the environment ignores genuine emotional expression, and the individual’s emotions escalate. This pattern is reinforced when the listener finally rewards emotionally extreme behavior with attention or desired changes.
As the pattern is repeated over time, extreme emotional reactions become the norm rather than the exception, and the emotional chaos can make the person wish to die. Acting on that desire when past expressions of desperation have been ignored or invalidated can provide attention or interventions that would never happen after simple emotional expressions.
Thus, an environment that does not recognize or validate genuine emotional expression can reinforce suicidality.
Table 1
Diagnostic criteria for borderline personality disorder
A pervasive pattern of instability of interpersonal relationships, self-image, and affects, and marked impulsivity beginning by early adulthood and present in a variety of contexts, as indicated by five (or more) of the following:
|
| Source: Adapted and reprinted with permission from the Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Copyright 2000. American Psychiatric Association. |
SOLVING NO-WIN THERAPY
Pitfalls with emphasizing change. Therapy that emphasizes solving problems and getting things to change typically triggers high arousal in persons with borderline personality disorder. Feeling out of control, they respond by trying to get in control, including attempts to control the therapist.
Similarly, they see attempts to get them to change their behavior as invalidating their experiences or, worse, who they are. Intense emotions aroused by the message they hear—that they are the source of their problems—impair learning and intensify their efforts to gain control. In a battle for control, collaboration and therapy cannot occur.
Case example. Ms. K wants you to understand how difficult her life is because of difficulties with her boss. You start talking about what Ms. K can do to change the situation, without acknowledging how difficult it is to deal with her boss.
Ms. K feels upset and says you don’t understand. For her, the interaction has led to emotion dysregulation and impaired cognitive processing.
Pitfalls with emphasizing acceptance. Most persons who come to therapy very distressed want something in their lives to change. If your primary message is acceptance instead of change, they may lose confidence in you.
Case example. Ms. K wants help dealing with her boss, who is making life quite difficult. As her therapist, you respond with warmth and acceptance but offer no suggestions as to how she might change the situation. Ms. K likes the way you listen to her but abandons therapy. after several sessions.
At first, patients with borderline personality disorder may like the warmth of client-centered acceptance approaches. Over time, however, they may feel their therapy sessions are out of control. They may think the therapist doesn’t understand the situation, doesn’t know how to help, or that situations that are troubling them cannot be changed.
Balanced therapy. DBT solves the change-or-acceptance dilemma by attempting to help patients with borderline personality disorder change themselves and their lives while offering strategies for accepting themselves and their situations.1,2 DBT includes problem-solving and acceptance strategies (Table 2).
Table 2
Strategies used in dialectical behavioral therapy
| Structural strategies | Organization of sessions, attending to the treatment hierarchy, reviewing progress, checking on other modes of therapy |
| Problem assessment strategies | Defining problems with specificity, conducting chain analyses, developing and testing hypotheses |
| Problem solving strategies | Providing didactic information, generating and evaluating solutions, teaching skills and coaching on use of skills, generalizing skills to the real-world environment |
| Contingency management | Use of reinforcement, extinction, aversive contingencies, and principles of shaping. |
| Exposure-based procedures | Both formal and informal |
| Cognitive strategies | Contingency clarification, observation and description of cognitions, cognitive modification |
| Validation strategies | Appearing interested, accurately reflecting, correctly articulating things that have not been fully expressed, explaining behavior in terms of learning history or biological factors, acknowledging validity of responses in terms of current events, interacting in a radically genuine manner, communicating belief in the patient |
| Reciprocal communication strategies | Being responsive, expressing warm engagement, being nonjudgmental, using self-disclosure, maintaining a reasonable power equilibrium |
| Irreverent strategies | Engaging in a matter-of-fact manner, directly confronting dysfunctional behavior, using unexpected, irreverent or humorous responses |
| Dialectical strategies | Using a balanced style, balancing acceptance-oriented strategies with change-oriented strategies, magnifying tension, using metaphor, modeling dialectical thinking and behaviors, moving with speed and flow. |
| Case management strategies | Following a model of consultation to the patient when long-term outcome is more important than short-term outcome; intervening in the patient's environment when short-term outcome is more important than long-term outcome |
DBT’S 4 THERAPY STAGES
DBT is a comprehensive treatment. The original outpatient model for borderline personality disorder (Table 3) has been adapted to different settings and applied to other populations.
Outpatients meet weekly in individual psychotherapy and a skills training group.3 Therapists also meet weekly in a consultation team viewed as “therapy for the therapist.”
Between sessions, therapists consult with patients by telephone to:
- decrease suicide crisis behaviors
- increase generalization of behavioral skills
- decrease patients’ feelings of conflict, alienation, or distance with the therapist.
Four stages. DBT follows four stages. For persons with borderline personality disorder, researchers have evaluated the efficacy of stage-1 therapy. Studies on stage-3 DBT have been conducted with nonborderline-personality individuals with eating disorders. The goals at each stage are:
Stage 1. Move from severe behavioral dyscontrol to behavioral control. Decrease suicidal and other life-threatening behaviors and those that interfere with therapy and quality of life. Increase mindfulness, tolerance for distress, interpersonal effectiveness, and emotion regulation.
Stage 2. Move from quiet desperation to emotional experiencing.
Stage 3. Address problems in living, and move toward ordinary happiness/unhappiness.
Stage 4. Move from incompleteness to capacity for joy and freedom.
Seven randomized controlled trials have shown that DBT can be useful in treating borderline personality disorder.4-10 The initial trial by Linehan et al4 included 47 women ages 18 to 45 who met criteria for borderline personality disorder and had at least two parasuicide incidents in the previous 5 years, with one in the previous 8 weeks. Treatment lasted 1 year, and subjects agreed to stop other individual psychotherapy if assigned to DBT.
Subjects were then randomly assigned to either DBT or “treatment as usual” in the community. In the various DBT studies, treatment-as-usual has included community therapists, Department of Veterans Affairs outpatient treatment, client-centered therapy, and treatment by persons identified by their peers as experts in their communities.
Subjects were assessed every 4 months while in treatment and for 1 year thereafter. DBT was more effective than usual treatment in:
- reducing suicide attempts and self-injury
- decreasing premature dropout from therapy
- reducing emergency room admissions and length of psychiatric hospitalization
- reducing drug abuse, depression, hopelessness, and anger.
Table 3
Modes of therapy in outpatient dialectical behavioral therapy
| Therapeutic goals | Mode |
|---|---|
| Improve motivational factors | Individual psychotherapy |
| Enhance capabilities | Skills training |
| Ensure generalization to natural environment | Between-session consultation |
| Enhance therapist capabilities and motivation to treat effectively | Therapist consultation team |
| Structure the environment | Consultation to the patient |
RECOMMENDATIONS
Some psychiatrists may find “borderline patients” frustrating and unpleasant to treat. DBT therapists, however, make two assumptions that can help anyone working with individuals with borderline personality disorder. To avoid falling into the trap of polarization with these patients, assume that:
- they are doing the best they can
- their efforts are insufficient to meet their needs.
They therefore need to do better, and the therapist’s job is to help them do so. Also assume that if you try to help a patient with borderline personality disorder, you will need help, too. We require DBT therapists to participate in consultation teams.
Training. DBT is a comprehensive program that requires familiarity with the manuals mentioned in this article (see Related resources). Some teams have learned DBT through self-study and consultation with other teams.
If you plan to offer DBT to patients with borderline personality disorder, we recommend that you be:
- trained in behavior therapy and CBT
- familiar with research on emotions and processes involved in emotion regulation.
If you have not had CBT training, find a behavior therapist to join your team or get consultation from a behavior therapist.
An intensive training course in DBT—with 2 weeks of instruction and case consultation and several months of consultation with someone well-versed in DBT—is an efficient way to become familiar with the most critical principles of the treatment. If you cannot train toward adherent delivery of individual therapy, we recommend referring patients to someone trained in DBT.
- Lieb K, Zanarini MC, Schmahl C, et al. Borderline personality disorder. Lancet 2004;364(9432):453-61.
- University of Washington, Behavioral Research and Therapy Clinics. www.brtc.psych.washington.edu/frameResearch.htm;
www.brtc.psych.washington.edu/framePublications.htm - Behavioral Tech, LLC. Consultation and training in dialectical behavioral therapy. www.behavioraltech.org.
Disclosures
Dr. DuBose is president and CEO/co-owner of DBT Center of Seattle, PLLC, and a speaker for Behavioral Tech, LLC.
Dr. Linehan is a shareholder in Behavioral Tech Research, Inc., which develops computerized training for DBT, a DBT trainer for Behavioral Tech, LLC, and the author of two books on DBT. She also receives research grants from the National Institute of Mental Health and National Institute on Drug Abuse.
1. Lynch TR, Chapman AL, Rosenthal MZ, et al. Mechanisms of change in dialectical behavior therapy: theoretical and empirical observations. J Clin Psychol 2005 (in press).
2. Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York: Guilford Press, 1993.
3. Linehan MM. Skills training manual for treating borderline personality disorder. New York: Guilford Press, 1993.
4. Linehan MM, Armstrong HE, Suarez A, et al. Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Arch Gen Psychiatry 1991 Dec;48(12):1060-4.
5. Linehan MM, Schmidt HI, Dimeff LA, et al. Dialectical behavior therapy for patients with border-line personality disorder and drug-dependence. Am J Addict 1999;8:279-92.
6. Linehan MM, Dimeff LA, Reynolds SK, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend 2002;67(1):13-26.
7. Turner RM. Naturalistic evaluation of dialectical behavior therapy-oriented treatment for borderline personality disorders. Cognit Behav Pract 2000;7:413-19.
8. Koons CR, Robins CJ, Tweed JL, et al. Efficacy of dialectical behavior therapy in women veterans with borderline personality disorder. Behav Ther 2001;32:371-90.
9. Verheul R, Van Den Bosch LM, Koeter MW, et al. Dialectical behaviour therapy for women with borderline personality disorder: 12-month, randomised clinical trial in The Netherlands. Br J Psychiatry 2003;182:135-40.
10. Linehan MM, Comtois KA, Brown M, et al. DBT versus nonbehavioral treatment by experts in the community: clinical outcomes. Symposium presentation for the Association for Advancement of Behavior Therapy. University of Washington, Reno NV, 2002.
1. Lynch TR, Chapman AL, Rosenthal MZ, et al. Mechanisms of change in dialectical behavior therapy: theoretical and empirical observations. J Clin Psychol 2005 (in press).
2. Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York: Guilford Press, 1993.
3. Linehan MM. Skills training manual for treating borderline personality disorder. New York: Guilford Press, 1993.
4. Linehan MM, Armstrong HE, Suarez A, et al. Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Arch Gen Psychiatry 1991 Dec;48(12):1060-4.
5. Linehan MM, Schmidt HI, Dimeff LA, et al. Dialectical behavior therapy for patients with border-line personality disorder and drug-dependence. Am J Addict 1999;8:279-92.
6. Linehan MM, Dimeff LA, Reynolds SK, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend 2002;67(1):13-26.
7. Turner RM. Naturalistic evaluation of dialectical behavior therapy-oriented treatment for borderline personality disorders. Cognit Behav Pract 2000;7:413-19.
8. Koons CR, Robins CJ, Tweed JL, et al. Efficacy of dialectical behavior therapy in women veterans with borderline personality disorder. Behav Ther 2001;32:371-90.
9. Verheul R, Van Den Bosch LM, Koeter MW, et al. Dialectical behaviour therapy for women with borderline personality disorder: 12-month, randomised clinical trial in The Netherlands. Br J Psychiatry 2003;182:135-40.
10. Linehan MM, Comtois KA, Brown M, et al. DBT versus nonbehavioral treatment by experts in the community: clinical outcomes. Symposium presentation for the Association for Advancement of Behavior Therapy. University of Washington, Reno NV, 2002.
Metabolic syndrome: 5 risk factors guide therapy
At what point do the five risk factors that predict type 2 diabetes and cardiovascular disease (CVD) signal metabolic syndrome? When and how often should psychiatrists check for metabolic abnormalities? How can you manage metabolic problems caused by a psychotropic that controls the patient’s psychiatric symptoms?
This article answers those questions by addressing:
- clinical guidelines for diagnosing metabolic syndrome
- suggested intervals for monitoring at-risk patients
- strategies for managing metabolic abnormalities with lifestyle changes or medication.
CASE REPORT: 'FAT' AND FRUSTRATED
Ms. S, age 37, has had bipolar disorder for 10 years. She has tried numerous medications including mood stabilizers, antidepressants, and atypical antipsychotics. The combination of quetiapine, 200 mg bid, and lithium, 300 mg bid, has controlled her symptoms for the past 6 months.
Her weight has increased 40 lbs over the past decade; much of her weight gain has occurred since the birth of her two children, ages 4 and 6. At 5 feet, 3 inches and 170 lbs, she is frustrated over her weight gain, especially on the eve of her 20-year high school reunion. She is convinced that her medications have prevented weight loss.
Her waist, measured at the umbilicus, is 37 inches. Her body mass index (BMI) is 30—indicating clinical obesity—and her blood pressure is in the high normal range (134/80 mm Hg). She has not had gestational diabetes and has not seen a medical doctor since her last pregnancy, but her father has type 2 diabetes and hypertension. She drinks wine occasionally at social events and does not smoke.
The psychiatrist orders a fasting lipid panel and fasting glucose test to further assess her risk of heart disease. Total cholesterol and low-density lipoprotein (LDL) cholesterol are normal. Triglycerides are 125 mg/dL (normal) and her high-density lipoprotein (HDL) is 45 mg/dL—5 mg/dL below normal for a woman her age. Fasting glucose is 86 mg/dL (normal).
The psychiatrist schedules a visit the following month to assess her cardiac and diabetic risk and to discuss weight-loss interventions.
Discussion. In a busy clinical setting, the psychiatrist must accurately gauge Ms. S’ metabolic risk and devise a management strategy. Do her weight and low HDL suggest metabolic syndrome? Is she overeating or making unhealthy dietary choices, or are her psychotropics causing weight gain? Would switching psychotropics lead to bipolar relapse?
IMPLICATIONS OF METABOLIC SYNDROME
Patients with metabolic syndrome are at increased risk for:
In a prospective study that followed 1,209 Finnish men over an average 11.4 years,4 men with metabolic syndrome were more likely than those with no metabolic problems to die from coronary heart disease, CVD, and any cause after adjustment for conventional cardiovascular risk factors. No one in either group had a baseline illness, suggesting that metabolic syndrome increases the risk of CVD or death regardless of whether underlying illness is present.
DEFINING METABOLIC SYNDROME
Metabolic syndrome is not a disease but a constellation of risk factors that provides a definable point of intervention before onset of type 2 diabetes or CVD.
According to the National Cholesterol Education Program—Adult Treatment Panel III (NCEP-ATP III), presence of three of these five criteria suggest metabolic syndrome:
- abdominal obesity
- insulin resistance
- high blood pressure
- elevated triglycerides
- below-normal HDL.
This definition offers a starting point for measuring risk factors in clinical practice and provides a definable target and parameters to avoid (Table 1).5 The guideline is also easy to follow: Waist circumference and blood pressure can be measured within seconds; blood glucose, HDL, and triglycerides can easily be measured before breakfast, after the patient has fasted for at least 6 hours.
Table 1
5 defined risk factors* for metabolic syndrome
| Risk factor | Clinically significant level |
|---|---|
| Abdominal obesity | |
| Men | Waist circumference >40 in (102 cm) |
| Women | Waist circumference >35 in (88 cm) |
| Blood pressure | |
| Systolic | >130 mm Hg |
| Diastolic | >85 mm Hg |
| HDL count | |
| Men | <40 mg/dL (<1.04 mmol/L) |
| Women | <50 mg/dL (<1.30 mmol/L) |
| Fasting glucose | |
| Men, women | >110 mg/dL (>6.11 mmol/L) |
| Triglycerides | |
| Men, women | >150 mg/dL (>1.70 mmol/L) |
| * If 3 risk factors are present, suspect metabolic syndrome | |
| HDL: high-density lipoprotein cholesterol | |
| Source: Adapted from reference 5. | |
MONITORING FREQUENCY
Although no empirical studies have addressed monitoring frequency for metabolic risk factors, several guidelines provide preliminary recommendations. Table 2 summarizes suggested intervals for monitoring weight, lipids, glucose, and waist circumference for patients taking atypical antipsychotics, based on recommendations from the 2004 American Diabetes Association (ADA) and American Psychiatric Association (APA) consensus development conference.6
Because atypicals are associated with serious metabolic risks, screen patients taking these agents for metabolic abnormalities at baseline and at regular intervals. Most guidelines recommend measuring blood pressure, BMI, waist circumference, fasting serum lipids (total, LDL, HDL, and triglycerides) and fasting glucose before starting or switching to an atypical and again 12 weeks later. Established risk for metabolic disturbances or dramatic metabolic changes (such as weight gain ≥7%, waist circumference ≥35 inches in women and ≥40 inches in men, or fasting blood sugars >110 mg/dL) demand more-frequent monitoring (ie, monitor high-risk patients quarterly).
Table 2
Suggested monitoring intervals for patients taking atypical antipsychotics*
| Baseline | 4 weeks | 8 weeks | 12 weeks | Quarterly | Annually | Every 5 years | |
|---|---|---|---|---|---|---|---|
| Personal/family history | X | X | |||||
| Weight (BMI) | X | X | X | X | X | ||
| Waist circumference | X | X | |||||
| Blood pressure | X | X | X | ||||
| Fasting plasma glucose | X | X | X | ||||
| Fasting lipid profile | X | X | X | ||||
| *Clinical status may warrant more-frequent assessments | |||||||
| BMI: Body mass index | |||||||
| Source: Reference 6. | |||||||
MANAGING METABOLIC PROBLEMS
Managing metabolic abnormalities or metabolic syndrome is aimed at preventing type 2 diabetes and CVD. Levels of intervention include:
- weight management, weight control education, and promoting regular exercise and a healthy diet
- switching to a psychotropic that is less likely to cause weight gain, if clinically appropriate
- working with the patient’s primary care physician to manage dyslipidemia, hypertension, obesity, or hyperglycemia.
Weight management. Start by controlling weight and promoting regular exercise and healthy eating. Switching medications—often the first response—may not be the best option, particularly if the offending agent is relieving the patient’s psychiatric symptoms.
Losing weight, increasing exercise, and reducing fat and carbohydrate intake can reverse metabolic syndrome and delay onset of type 2 diabetes and CVD.7 Even a small weight loss, such as 10% of baseline body weight in persons who are overweight (BMI >25) or obese (BMI >30) can significantly reduce the risk of hypertension, hyperlipidemia, hyperglycemia, and death.7
Rather than promoting a single diet, tailor dietary advice to each patient’s metabolic abnormalities (Table 3). Although researchers disagree over whether a low-fat or low-carbohydrate diet produces better results, either diet will work as long as the patient consumes fewer calories than he or she expends. This is because weight loss alone reverses metabolic syndrome.
Likewise, exercise can reverse metabolic syndrome independent of diet change. Regular exercise at modest levels improves HDL,2 triglycerides,17 blood pressure,18 and hyperglycemia.19
In one prospective study,20 621 subjects without chronic disease or injury underwent supervised aerobic training three times weekly for 20 weeks. Participants were told not to otherwise change their health and lifestyle habits.
Of the 105 persons in the cohort who had metabolic syndrome at baseline, 32 (30%) no longer had it after the aerobics program. Among these participants:
- 43% had lower triglycerides than at baseline
- 16% had higher HDL cholesterol
- 38% had lower blood pressure
- 9% had improved fasting glucose
- 28% reduced their waist circumference.
Table 3
Interventions for specific metabolic complications
| Metabolic complication | Nondrug interventions8 | Medications |
|---|---|---|
| Abdominal obesity | Encourage weight loss | Sibutramine*† |
| Increase physical activity | Appetite suppressant | |
| Orlistat*† | ||
| Lipase inhibitor | ||
| Hypertriglyceridemia | Encourage weight loss | Fibrates9* |
| Increase physical activity | Reduce fasting and postprandial triglycerides 20% to 50% | |
| Increase low-glycemic-index food intake | Shift small dense LDL to large buoyant particles | |
| Reduce total carbohydrate intake | Increase HDL particles 10% to 35% | |
| Increase consumption of omega-3 fatty acids | Nicotinic acid10 | |
| Limit alcohol consumption | Reduces triglycerides 20% to 50% | |
| Statins11 | ||
| Reduce fasting and postprandial triglycerides 7% to 30% | ||
| Reduce LDL particles | ||
| Increase HDL particles | ||
| Reduce major coronary vascular events | ||
| Low HDL | Encourage weight loss | Nicotinic acid* |
| Increase physical activity | Increases HDL particles 15% to 35% | |
| Stop smoking | Fibrates9 | |
| Increase monounsaturated fat intake | See above | |
| Statins11 | ||
| See above | ||
| Hypertension | Encourage weight loss | ACE inhibitors* |
| Increase physical activity | May slow progression to diabetes12 | |
| Reduce saturated fat intake | Decrease CVD events13 | |
| Reduce sodium intake | Delay progression of microalbuminuria13 | |
| Limit alcohol consumption | Angiotensin receptor blockers | |
| May improve dyslipidemia associated with metabolic syndrome14 | ||
| Delay progression of microalbuminuria13 | ||
| Hyperglycemia | Encourage weight loss | Metformin,* thiazolidinediones |
| Increase physical activity | Slow progression to diabetes in persons with insulin resistance15,16 (metformin less effective than lifestyle changes)15 | |
| Reduce total carbohydrates | ||
| * Suggested first-line therapy. | ||
| † For patients with BMI 30 kg/m2 | ||
| ACE: Angiotensin-converting enzyme | ||
| CVD: Cardiovascular disease | ||
| HDL: High-density lipoprotein cholesterol | ||
| LDL: Low-density lipoprotein cholesterol | ||
Selling the benefits of exercise and weight loss to a mentally ill patient can be difficult. Attention, memory, and motivation deficits as well as smoking and substance abuse often get in the way.
By teaming up with clinicians with expertise in dieting such as nurses, dietitians, and recreational therapists, psychiatrists can more effectively promote long-term diet, exercise, and lifestyle changes.21
In a prospective 12-month trial,22 20 patients who were taking atypical antipsychotics for schizophrenia or schizoaffective disorder completed a 52-week program that incorporated nutrition, exercise, and behavioral interventions. Twenty similar patients received treatment as usual. Patients in the program saw significant improvements in weight, blood pressure, exercise habits, nutrition, and hemoglobin A1c compared with the treatment-as-usual group.22
Psychiatrists who treat privately insured patients should collaborate with the patient’s primary care physician. Many insurance plans will pay for 1 or 2 personal or group sessions with a dietitian, especially if the patient is diagnosed as being obese (BMI >30). Some large plans, such as Kaiser Permanente, will cover intensive multimodal treatment, especially for patients with a BMI >35. Calculating the patient’s BMI can help you document the need for antiobesity treatment (see Related resources).
Medication. If weight control and exercise do not reduce metabolic risk factors after 3 to 6 months, consider switching to an atypical antipsychotic with a lower propensity for causing metabolic effects.
Which agents most decrease metabolic risk has been debated. Preliminary evidence indicates that switching from other antipsychotics to aripiprazole or ziprasidone may reduce weight and improve cholesterol ratios.23,24 These findings are consistent with the ADA/APA consensus guidelines, which indicate that metabolic risk varies among atypical antipsychotics (Table 4).6
Table 4
Atypical antipsychotics and their propensity for causing metabolic abnormalities
| Drug | Weight gain | Hyperglycemia | Dyslipidemia |
|---|---|---|---|
| Clozapine | High | High | High |
| Olanzapine | High | High | High |
| Risperidone | Medium | Medium | Low |
| Quetiapine | Medium | Medium | High |
| Aripiprazole | Low | Low | Low |
| Ziprasidone | Low | Low | Low |
| Source: Reference 6 | |||
Targeted pharmacotherapy. Wait another 3 to 6 months to see if the medication change and weight loss/exercise interventions reduce metabolic risk factors. If they don’t, work with the patient’s primary care physician to manage hypertension, dyslipidemia, and obesity (Table 3).
Although no agents are approved for treating metabolic syndrome per se, medications targeted at individual symptoms are becoming the standard of care. Controlling blood pressure, HDL, and LDL in patients with metabolic syndrome can reduce risk for coronary heart disease by >50%.25 Insulin-sensitizing agents and metformin in combination with lifestyle changes or used alone have been shown to delay onset of type 2 diabetes (Table 3).
CASE CONTINUED: 10 LBS IN 10 WEEKS
At her follow-up visit, Ms. S and her psychiatrist discuss her increased risk for diabetes and cardiovascular disease. She meets criteria for metabolic syndrome (low HDL, elevated blood pressure, and increased waist circumference).
Ms. S agrees to try a formal diet program with set menus, along with group support at her local community center. She also commits to walking 30 minutes three to four times a week with a target heart rate of 100 beats per minute. Although both quetiapine and lithium carry considerable risk of weight gain, she and her psychiatrist decide to wait at least 3 months before considering a medication change, as she is stable on this combination.
Ms. S schedules a follow-up visit with her primary care physician to ensure that she sticks to her weight loss and exercise programs. In the interim, the primary care physician and psychiatrist agree that her goal will be to lose 10 lbs over 10 weeks.
- National Alliance for the Mentally Ill. Hearts and Minds Program, a booklet and program geared toward raising awareness regarding diabetes, diet, exercise, and smoking. Download at www.nami.org.
- Centers for Disease Control and Prevention: Body mass index formula for adults. http://www.cdc.gov/nccdphp/dnpa/bmi/bmi-adult-formula.htm.
- National Heart, Lung and Blood Institute body mass index calculator. http://www.nhlbisupport.com/bmi/bmicalc.htm.
- Keck PE Jr, Buse JB, Dagago-Jack S, et al. Managing metabolic concerns in patients with severe mental illness. A special report. Postgraduate Med 2003;1-92.
Drug brand names
- Aripiprazole • Abilify
- Clozapine • Clozaril
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Ziprasidone • Geodon
Disclosure
Dr. Bermudes is a speaker for Bristol-Myers Squibb Co. and Pfizer Inc.
1. Haffner SM, Valdez RA, Hazuda HP, et al. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes 1992;41:715-22.
2. Isomma B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001;24:683-9.
3. Trevisan M, Liu J, Bahsas FB, Menotti A. Syndrome X and mortality: a population-based study. Am J Epidemiol 1998;148:958-66.
4. Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002;288:2709-16.
5. National Institutes of Health: Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Cholesterol in Adults (Adult Treatment Panel III). Executive summary of the Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97.
6. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care 2004;27:596-601.
7. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res 1998;6(suppl 2):51S-209S.
8. Darwin D. Metabolic syndrome: time for action. Am Fam Physician 2004;69:2875-82.
9. Maki KC. Fibrates for treatment of the metabolic syndrome. Curr Atheroscler Rep 2004;6:45-51.
10. Boden WE. Therapeutic implications of recent ATP III guidelines and the important role of combination therapy in total dyslipidemia management. Curr Opin Cardiol 2003;18:278-85.
11. Showers JR. Effects of statins on the vasculature: implications for aggressive lipid management in the cardiovascular metabolic syndrome. Am J Cardiol 2003;91(suppl):14B-22B.
12. Yusuf S, Gerstein H, Hoogwerf B, et al. for the HOPE Study Investigators. Ramipril and the development of diabetes. JAMA 2001;286:1882-5.
13. American Diabetes Association. Treatment of hypertension in adults with diabetes. Diabetes Care 2003;26(suppl 1):S80-S82.
14. Derosa G, Cicero AF, Bertone G, et al. Comparison of the effects of telmisartan and nifedipine gastrointestinal therapeutic system on blood pressure control, glucose metabolism, and the lipid profile in patients with type 2 diabetes mellitus and mild hypertension: a 12-month, randomized double-blind study. Clin Ther 2004;26:1228-36.
15. Diabetes prevention program research group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403.
16. Buchanan T, Xiang A, Peters R, et al. Prevention of type 2 diabetes by treatment of insulin resistance: comparison of early vs. late in the TRIPOD study [abstract]. Diabetes 2002;51(suppl 2):A35.-
17. Leon AS, Sanchez O. Meta-analysis of the effects of aerobic exercise training on blood lipids. Circulation 2001;104(suppl II):414-15 (abstract).
18. Fagard RH. Exercise characteristics and blood pressure response to dynamic physical training. Med Sci Sports Exerc 2001;33(6 suppl):S484-S492.
19. Thompson PD, Crouse SF, Goodpaster B, et al. The acute versus the chronic response to exercise. Med Sci Sports Exerc 2001;33(6 suppl):S438-S445.
20. Katzmarzyk PT, Leon AS, Wilmore JH, et al. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study. Med Sci Sports Exerc 2003;35:1703-9.
21. Littrell KH, Hilligoss NM, Kirshner CD, et al. The effects of an educational intervention on antipsychotic-induced weight gain. J Nurs Scholarsh 2003;35:237-41.
22. Menza M, Vreeland B, Minsky S, et al. Managing atypical antipsychotic-associated weight gain: 12-month data on a multimodal weight control program. J Clin Psychiatry 2004;65:471-7.
23. Cohen SA, Fitzgerald BJ, Khan SR, Khan A. The effect of a switch to ziprasidone in an adult population with autistic disorder: chart review and naturalistic, open-label treatment. J Clin Psychiatry 2004;65:110-13.
24. Casey DE, Carson WH, Saha AR, et al. Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology 2003;166:391-9.
25. Wong ND, Pio J, Franklin SS, et al. Preventing coronary events by optimal control of blood pressure and lipids in patients with the metabolic syndrome. Am J Cardiol 2003;91:1421-6.
At what point do the five risk factors that predict type 2 diabetes and cardiovascular disease (CVD) signal metabolic syndrome? When and how often should psychiatrists check for metabolic abnormalities? How can you manage metabolic problems caused by a psychotropic that controls the patient’s psychiatric symptoms?
This article answers those questions by addressing:
- clinical guidelines for diagnosing metabolic syndrome
- suggested intervals for monitoring at-risk patients
- strategies for managing metabolic abnormalities with lifestyle changes or medication.
CASE REPORT: 'FAT' AND FRUSTRATED
Ms. S, age 37, has had bipolar disorder for 10 years. She has tried numerous medications including mood stabilizers, antidepressants, and atypical antipsychotics. The combination of quetiapine, 200 mg bid, and lithium, 300 mg bid, has controlled her symptoms for the past 6 months.
Her weight has increased 40 lbs over the past decade; much of her weight gain has occurred since the birth of her two children, ages 4 and 6. At 5 feet, 3 inches and 170 lbs, she is frustrated over her weight gain, especially on the eve of her 20-year high school reunion. She is convinced that her medications have prevented weight loss.
Her waist, measured at the umbilicus, is 37 inches. Her body mass index (BMI) is 30—indicating clinical obesity—and her blood pressure is in the high normal range (134/80 mm Hg). She has not had gestational diabetes and has not seen a medical doctor since her last pregnancy, but her father has type 2 diabetes and hypertension. She drinks wine occasionally at social events and does not smoke.
The psychiatrist orders a fasting lipid panel and fasting glucose test to further assess her risk of heart disease. Total cholesterol and low-density lipoprotein (LDL) cholesterol are normal. Triglycerides are 125 mg/dL (normal) and her high-density lipoprotein (HDL) is 45 mg/dL—5 mg/dL below normal for a woman her age. Fasting glucose is 86 mg/dL (normal).
The psychiatrist schedules a visit the following month to assess her cardiac and diabetic risk and to discuss weight-loss interventions.
Discussion. In a busy clinical setting, the psychiatrist must accurately gauge Ms. S’ metabolic risk and devise a management strategy. Do her weight and low HDL suggest metabolic syndrome? Is she overeating or making unhealthy dietary choices, or are her psychotropics causing weight gain? Would switching psychotropics lead to bipolar relapse?
IMPLICATIONS OF METABOLIC SYNDROME
Patients with metabolic syndrome are at increased risk for:
In a prospective study that followed 1,209 Finnish men over an average 11.4 years,4 men with metabolic syndrome were more likely than those with no metabolic problems to die from coronary heart disease, CVD, and any cause after adjustment for conventional cardiovascular risk factors. No one in either group had a baseline illness, suggesting that metabolic syndrome increases the risk of CVD or death regardless of whether underlying illness is present.
DEFINING METABOLIC SYNDROME
Metabolic syndrome is not a disease but a constellation of risk factors that provides a definable point of intervention before onset of type 2 diabetes or CVD.
According to the National Cholesterol Education Program—Adult Treatment Panel III (NCEP-ATP III), presence of three of these five criteria suggest metabolic syndrome:
- abdominal obesity
- insulin resistance
- high blood pressure
- elevated triglycerides
- below-normal HDL.
This definition offers a starting point for measuring risk factors in clinical practice and provides a definable target and parameters to avoid (Table 1).5 The guideline is also easy to follow: Waist circumference and blood pressure can be measured within seconds; blood glucose, HDL, and triglycerides can easily be measured before breakfast, after the patient has fasted for at least 6 hours.
Table 1
5 defined risk factors* for metabolic syndrome
| Risk factor | Clinically significant level |
|---|---|
| Abdominal obesity | |
| Men | Waist circumference >40 in (102 cm) |
| Women | Waist circumference >35 in (88 cm) |
| Blood pressure | |
| Systolic | >130 mm Hg |
| Diastolic | >85 mm Hg |
| HDL count | |
| Men | <40 mg/dL (<1.04 mmol/L) |
| Women | <50 mg/dL (<1.30 mmol/L) |
| Fasting glucose | |
| Men, women | >110 mg/dL (>6.11 mmol/L) |
| Triglycerides | |
| Men, women | >150 mg/dL (>1.70 mmol/L) |
| * If 3 risk factors are present, suspect metabolic syndrome | |
| HDL: high-density lipoprotein cholesterol | |
| Source: Adapted from reference 5. | |
MONITORING FREQUENCY
Although no empirical studies have addressed monitoring frequency for metabolic risk factors, several guidelines provide preliminary recommendations. Table 2 summarizes suggested intervals for monitoring weight, lipids, glucose, and waist circumference for patients taking atypical antipsychotics, based on recommendations from the 2004 American Diabetes Association (ADA) and American Psychiatric Association (APA) consensus development conference.6
Because atypicals are associated with serious metabolic risks, screen patients taking these agents for metabolic abnormalities at baseline and at regular intervals. Most guidelines recommend measuring blood pressure, BMI, waist circumference, fasting serum lipids (total, LDL, HDL, and triglycerides) and fasting glucose before starting or switching to an atypical and again 12 weeks later. Established risk for metabolic disturbances or dramatic metabolic changes (such as weight gain ≥7%, waist circumference ≥35 inches in women and ≥40 inches in men, or fasting blood sugars >110 mg/dL) demand more-frequent monitoring (ie, monitor high-risk patients quarterly).
Table 2
Suggested monitoring intervals for patients taking atypical antipsychotics*
| Baseline | 4 weeks | 8 weeks | 12 weeks | Quarterly | Annually | Every 5 years | |
|---|---|---|---|---|---|---|---|
| Personal/family history | X | X | |||||
| Weight (BMI) | X | X | X | X | X | ||
| Waist circumference | X | X | |||||
| Blood pressure | X | X | X | ||||
| Fasting plasma glucose | X | X | X | ||||
| Fasting lipid profile | X | X | X | ||||
| *Clinical status may warrant more-frequent assessments | |||||||
| BMI: Body mass index | |||||||
| Source: Reference 6. | |||||||
MANAGING METABOLIC PROBLEMS
Managing metabolic abnormalities or metabolic syndrome is aimed at preventing type 2 diabetes and CVD. Levels of intervention include:
- weight management, weight control education, and promoting regular exercise and a healthy diet
- switching to a psychotropic that is less likely to cause weight gain, if clinically appropriate
- working with the patient’s primary care physician to manage dyslipidemia, hypertension, obesity, or hyperglycemia.
Weight management. Start by controlling weight and promoting regular exercise and healthy eating. Switching medications—often the first response—may not be the best option, particularly if the offending agent is relieving the patient’s psychiatric symptoms.
Losing weight, increasing exercise, and reducing fat and carbohydrate intake can reverse metabolic syndrome and delay onset of type 2 diabetes and CVD.7 Even a small weight loss, such as 10% of baseline body weight in persons who are overweight (BMI >25) or obese (BMI >30) can significantly reduce the risk of hypertension, hyperlipidemia, hyperglycemia, and death.7
Rather than promoting a single diet, tailor dietary advice to each patient’s metabolic abnormalities (Table 3). Although researchers disagree over whether a low-fat or low-carbohydrate diet produces better results, either diet will work as long as the patient consumes fewer calories than he or she expends. This is because weight loss alone reverses metabolic syndrome.
Likewise, exercise can reverse metabolic syndrome independent of diet change. Regular exercise at modest levels improves HDL,2 triglycerides,17 blood pressure,18 and hyperglycemia.19
In one prospective study,20 621 subjects without chronic disease or injury underwent supervised aerobic training three times weekly for 20 weeks. Participants were told not to otherwise change their health and lifestyle habits.
Of the 105 persons in the cohort who had metabolic syndrome at baseline, 32 (30%) no longer had it after the aerobics program. Among these participants:
- 43% had lower triglycerides than at baseline
- 16% had higher HDL cholesterol
- 38% had lower blood pressure
- 9% had improved fasting glucose
- 28% reduced their waist circumference.
Table 3
Interventions for specific metabolic complications
| Metabolic complication | Nondrug interventions8 | Medications |
|---|---|---|
| Abdominal obesity | Encourage weight loss | Sibutramine*† |
| Increase physical activity | Appetite suppressant | |
| Orlistat*† | ||
| Lipase inhibitor | ||
| Hypertriglyceridemia | Encourage weight loss | Fibrates9* |
| Increase physical activity | Reduce fasting and postprandial triglycerides 20% to 50% | |
| Increase low-glycemic-index food intake | Shift small dense LDL to large buoyant particles | |
| Reduce total carbohydrate intake | Increase HDL particles 10% to 35% | |
| Increase consumption of omega-3 fatty acids | Nicotinic acid10 | |
| Limit alcohol consumption | Reduces triglycerides 20% to 50% | |
| Statins11 | ||
| Reduce fasting and postprandial triglycerides 7% to 30% | ||
| Reduce LDL particles | ||
| Increase HDL particles | ||
| Reduce major coronary vascular events | ||
| Low HDL | Encourage weight loss | Nicotinic acid* |
| Increase physical activity | Increases HDL particles 15% to 35% | |
| Stop smoking | Fibrates9 | |
| Increase monounsaturated fat intake | See above | |
| Statins11 | ||
| See above | ||
| Hypertension | Encourage weight loss | ACE inhibitors* |
| Increase physical activity | May slow progression to diabetes12 | |
| Reduce saturated fat intake | Decrease CVD events13 | |
| Reduce sodium intake | Delay progression of microalbuminuria13 | |
| Limit alcohol consumption | Angiotensin receptor blockers | |
| May improve dyslipidemia associated with metabolic syndrome14 | ||
| Delay progression of microalbuminuria13 | ||
| Hyperglycemia | Encourage weight loss | Metformin,* thiazolidinediones |
| Increase physical activity | Slow progression to diabetes in persons with insulin resistance15,16 (metformin less effective than lifestyle changes)15 | |
| Reduce total carbohydrates | ||
| * Suggested first-line therapy. | ||
| † For patients with BMI 30 kg/m2 | ||
| ACE: Angiotensin-converting enzyme | ||
| CVD: Cardiovascular disease | ||
| HDL: High-density lipoprotein cholesterol | ||
| LDL: Low-density lipoprotein cholesterol | ||
Selling the benefits of exercise and weight loss to a mentally ill patient can be difficult. Attention, memory, and motivation deficits as well as smoking and substance abuse often get in the way.
By teaming up with clinicians with expertise in dieting such as nurses, dietitians, and recreational therapists, psychiatrists can more effectively promote long-term diet, exercise, and lifestyle changes.21
In a prospective 12-month trial,22 20 patients who were taking atypical antipsychotics for schizophrenia or schizoaffective disorder completed a 52-week program that incorporated nutrition, exercise, and behavioral interventions. Twenty similar patients received treatment as usual. Patients in the program saw significant improvements in weight, blood pressure, exercise habits, nutrition, and hemoglobin A1c compared with the treatment-as-usual group.22
Psychiatrists who treat privately insured patients should collaborate with the patient’s primary care physician. Many insurance plans will pay for 1 or 2 personal or group sessions with a dietitian, especially if the patient is diagnosed as being obese (BMI >30). Some large plans, such as Kaiser Permanente, will cover intensive multimodal treatment, especially for patients with a BMI >35. Calculating the patient’s BMI can help you document the need for antiobesity treatment (see Related resources).
Medication. If weight control and exercise do not reduce metabolic risk factors after 3 to 6 months, consider switching to an atypical antipsychotic with a lower propensity for causing metabolic effects.
Which agents most decrease metabolic risk has been debated. Preliminary evidence indicates that switching from other antipsychotics to aripiprazole or ziprasidone may reduce weight and improve cholesterol ratios.23,24 These findings are consistent with the ADA/APA consensus guidelines, which indicate that metabolic risk varies among atypical antipsychotics (Table 4).6
Table 4
Atypical antipsychotics and their propensity for causing metabolic abnormalities
| Drug | Weight gain | Hyperglycemia | Dyslipidemia |
|---|---|---|---|
| Clozapine | High | High | High |
| Olanzapine | High | High | High |
| Risperidone | Medium | Medium | Low |
| Quetiapine | Medium | Medium | High |
| Aripiprazole | Low | Low | Low |
| Ziprasidone | Low | Low | Low |
| Source: Reference 6 | |||
Targeted pharmacotherapy. Wait another 3 to 6 months to see if the medication change and weight loss/exercise interventions reduce metabolic risk factors. If they don’t, work with the patient’s primary care physician to manage hypertension, dyslipidemia, and obesity (Table 3).
Although no agents are approved for treating metabolic syndrome per se, medications targeted at individual symptoms are becoming the standard of care. Controlling blood pressure, HDL, and LDL in patients with metabolic syndrome can reduce risk for coronary heart disease by >50%.25 Insulin-sensitizing agents and metformin in combination with lifestyle changes or used alone have been shown to delay onset of type 2 diabetes (Table 3).
CASE CONTINUED: 10 LBS IN 10 WEEKS
At her follow-up visit, Ms. S and her psychiatrist discuss her increased risk for diabetes and cardiovascular disease. She meets criteria for metabolic syndrome (low HDL, elevated blood pressure, and increased waist circumference).
Ms. S agrees to try a formal diet program with set menus, along with group support at her local community center. She also commits to walking 30 minutes three to four times a week with a target heart rate of 100 beats per minute. Although both quetiapine and lithium carry considerable risk of weight gain, she and her psychiatrist decide to wait at least 3 months before considering a medication change, as she is stable on this combination.
Ms. S schedules a follow-up visit with her primary care physician to ensure that she sticks to her weight loss and exercise programs. In the interim, the primary care physician and psychiatrist agree that her goal will be to lose 10 lbs over 10 weeks.
- National Alliance for the Mentally Ill. Hearts and Minds Program, a booklet and program geared toward raising awareness regarding diabetes, diet, exercise, and smoking. Download at www.nami.org.
- Centers for Disease Control and Prevention: Body mass index formula for adults. http://www.cdc.gov/nccdphp/dnpa/bmi/bmi-adult-formula.htm.
- National Heart, Lung and Blood Institute body mass index calculator. http://www.nhlbisupport.com/bmi/bmicalc.htm.
- Keck PE Jr, Buse JB, Dagago-Jack S, et al. Managing metabolic concerns in patients with severe mental illness. A special report. Postgraduate Med 2003;1-92.
Drug brand names
- Aripiprazole • Abilify
- Clozapine • Clozaril
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Ziprasidone • Geodon
Disclosure
Dr. Bermudes is a speaker for Bristol-Myers Squibb Co. and Pfizer Inc.
At what point do the five risk factors that predict type 2 diabetes and cardiovascular disease (CVD) signal metabolic syndrome? When and how often should psychiatrists check for metabolic abnormalities? How can you manage metabolic problems caused by a psychotropic that controls the patient’s psychiatric symptoms?
This article answers those questions by addressing:
- clinical guidelines for diagnosing metabolic syndrome
- suggested intervals for monitoring at-risk patients
- strategies for managing metabolic abnormalities with lifestyle changes or medication.
CASE REPORT: 'FAT' AND FRUSTRATED
Ms. S, age 37, has had bipolar disorder for 10 years. She has tried numerous medications including mood stabilizers, antidepressants, and atypical antipsychotics. The combination of quetiapine, 200 mg bid, and lithium, 300 mg bid, has controlled her symptoms for the past 6 months.
Her weight has increased 40 lbs over the past decade; much of her weight gain has occurred since the birth of her two children, ages 4 and 6. At 5 feet, 3 inches and 170 lbs, she is frustrated over her weight gain, especially on the eve of her 20-year high school reunion. She is convinced that her medications have prevented weight loss.
Her waist, measured at the umbilicus, is 37 inches. Her body mass index (BMI) is 30—indicating clinical obesity—and her blood pressure is in the high normal range (134/80 mm Hg). She has not had gestational diabetes and has not seen a medical doctor since her last pregnancy, but her father has type 2 diabetes and hypertension. She drinks wine occasionally at social events and does not smoke.
The psychiatrist orders a fasting lipid panel and fasting glucose test to further assess her risk of heart disease. Total cholesterol and low-density lipoprotein (LDL) cholesterol are normal. Triglycerides are 125 mg/dL (normal) and her high-density lipoprotein (HDL) is 45 mg/dL—5 mg/dL below normal for a woman her age. Fasting glucose is 86 mg/dL (normal).
The psychiatrist schedules a visit the following month to assess her cardiac and diabetic risk and to discuss weight-loss interventions.
Discussion. In a busy clinical setting, the psychiatrist must accurately gauge Ms. S’ metabolic risk and devise a management strategy. Do her weight and low HDL suggest metabolic syndrome? Is she overeating or making unhealthy dietary choices, or are her psychotropics causing weight gain? Would switching psychotropics lead to bipolar relapse?
IMPLICATIONS OF METABOLIC SYNDROME
Patients with metabolic syndrome are at increased risk for:
In a prospective study that followed 1,209 Finnish men over an average 11.4 years,4 men with metabolic syndrome were more likely than those with no metabolic problems to die from coronary heart disease, CVD, and any cause after adjustment for conventional cardiovascular risk factors. No one in either group had a baseline illness, suggesting that metabolic syndrome increases the risk of CVD or death regardless of whether underlying illness is present.
DEFINING METABOLIC SYNDROME
Metabolic syndrome is not a disease but a constellation of risk factors that provides a definable point of intervention before onset of type 2 diabetes or CVD.
According to the National Cholesterol Education Program—Adult Treatment Panel III (NCEP-ATP III), presence of three of these five criteria suggest metabolic syndrome:
- abdominal obesity
- insulin resistance
- high blood pressure
- elevated triglycerides
- below-normal HDL.
This definition offers a starting point for measuring risk factors in clinical practice and provides a definable target and parameters to avoid (Table 1).5 The guideline is also easy to follow: Waist circumference and blood pressure can be measured within seconds; blood glucose, HDL, and triglycerides can easily be measured before breakfast, after the patient has fasted for at least 6 hours.
Table 1
5 defined risk factors* for metabolic syndrome
| Risk factor | Clinically significant level |
|---|---|
| Abdominal obesity | |
| Men | Waist circumference >40 in (102 cm) |
| Women | Waist circumference >35 in (88 cm) |
| Blood pressure | |
| Systolic | >130 mm Hg |
| Diastolic | >85 mm Hg |
| HDL count | |
| Men | <40 mg/dL (<1.04 mmol/L) |
| Women | <50 mg/dL (<1.30 mmol/L) |
| Fasting glucose | |
| Men, women | >110 mg/dL (>6.11 mmol/L) |
| Triglycerides | |
| Men, women | >150 mg/dL (>1.70 mmol/L) |
| * If 3 risk factors are present, suspect metabolic syndrome | |
| HDL: high-density lipoprotein cholesterol | |
| Source: Adapted from reference 5. | |
MONITORING FREQUENCY
Although no empirical studies have addressed monitoring frequency for metabolic risk factors, several guidelines provide preliminary recommendations. Table 2 summarizes suggested intervals for monitoring weight, lipids, glucose, and waist circumference for patients taking atypical antipsychotics, based on recommendations from the 2004 American Diabetes Association (ADA) and American Psychiatric Association (APA) consensus development conference.6
Because atypicals are associated with serious metabolic risks, screen patients taking these agents for metabolic abnormalities at baseline and at regular intervals. Most guidelines recommend measuring blood pressure, BMI, waist circumference, fasting serum lipids (total, LDL, HDL, and triglycerides) and fasting glucose before starting or switching to an atypical and again 12 weeks later. Established risk for metabolic disturbances or dramatic metabolic changes (such as weight gain ≥7%, waist circumference ≥35 inches in women and ≥40 inches in men, or fasting blood sugars >110 mg/dL) demand more-frequent monitoring (ie, monitor high-risk patients quarterly).
Table 2
Suggested monitoring intervals for patients taking atypical antipsychotics*
| Baseline | 4 weeks | 8 weeks | 12 weeks | Quarterly | Annually | Every 5 years | |
|---|---|---|---|---|---|---|---|
| Personal/family history | X | X | |||||
| Weight (BMI) | X | X | X | X | X | ||
| Waist circumference | X | X | |||||
| Blood pressure | X | X | X | ||||
| Fasting plasma glucose | X | X | X | ||||
| Fasting lipid profile | X | X | X | ||||
| *Clinical status may warrant more-frequent assessments | |||||||
| BMI: Body mass index | |||||||
| Source: Reference 6. | |||||||
MANAGING METABOLIC PROBLEMS
Managing metabolic abnormalities or metabolic syndrome is aimed at preventing type 2 diabetes and CVD. Levels of intervention include:
- weight management, weight control education, and promoting regular exercise and a healthy diet
- switching to a psychotropic that is less likely to cause weight gain, if clinically appropriate
- working with the patient’s primary care physician to manage dyslipidemia, hypertension, obesity, or hyperglycemia.
Weight management. Start by controlling weight and promoting regular exercise and healthy eating. Switching medications—often the first response—may not be the best option, particularly if the offending agent is relieving the patient’s psychiatric symptoms.
Losing weight, increasing exercise, and reducing fat and carbohydrate intake can reverse metabolic syndrome and delay onset of type 2 diabetes and CVD.7 Even a small weight loss, such as 10% of baseline body weight in persons who are overweight (BMI >25) or obese (BMI >30) can significantly reduce the risk of hypertension, hyperlipidemia, hyperglycemia, and death.7
Rather than promoting a single diet, tailor dietary advice to each patient’s metabolic abnormalities (Table 3). Although researchers disagree over whether a low-fat or low-carbohydrate diet produces better results, either diet will work as long as the patient consumes fewer calories than he or she expends. This is because weight loss alone reverses metabolic syndrome.
Likewise, exercise can reverse metabolic syndrome independent of diet change. Regular exercise at modest levels improves HDL,2 triglycerides,17 blood pressure,18 and hyperglycemia.19
In one prospective study,20 621 subjects without chronic disease or injury underwent supervised aerobic training three times weekly for 20 weeks. Participants were told not to otherwise change their health and lifestyle habits.
Of the 105 persons in the cohort who had metabolic syndrome at baseline, 32 (30%) no longer had it after the aerobics program. Among these participants:
- 43% had lower triglycerides than at baseline
- 16% had higher HDL cholesterol
- 38% had lower blood pressure
- 9% had improved fasting glucose
- 28% reduced their waist circumference.
Table 3
Interventions for specific metabolic complications
| Metabolic complication | Nondrug interventions8 | Medications |
|---|---|---|
| Abdominal obesity | Encourage weight loss | Sibutramine*† |
| Increase physical activity | Appetite suppressant | |
| Orlistat*† | ||
| Lipase inhibitor | ||
| Hypertriglyceridemia | Encourage weight loss | Fibrates9* |
| Increase physical activity | Reduce fasting and postprandial triglycerides 20% to 50% | |
| Increase low-glycemic-index food intake | Shift small dense LDL to large buoyant particles | |
| Reduce total carbohydrate intake | Increase HDL particles 10% to 35% | |
| Increase consumption of omega-3 fatty acids | Nicotinic acid10 | |
| Limit alcohol consumption | Reduces triglycerides 20% to 50% | |
| Statins11 | ||
| Reduce fasting and postprandial triglycerides 7% to 30% | ||
| Reduce LDL particles | ||
| Increase HDL particles | ||
| Reduce major coronary vascular events | ||
| Low HDL | Encourage weight loss | Nicotinic acid* |
| Increase physical activity | Increases HDL particles 15% to 35% | |
| Stop smoking | Fibrates9 | |
| Increase monounsaturated fat intake | See above | |
| Statins11 | ||
| See above | ||
| Hypertension | Encourage weight loss | ACE inhibitors* |
| Increase physical activity | May slow progression to diabetes12 | |
| Reduce saturated fat intake | Decrease CVD events13 | |
| Reduce sodium intake | Delay progression of microalbuminuria13 | |
| Limit alcohol consumption | Angiotensin receptor blockers | |
| May improve dyslipidemia associated with metabolic syndrome14 | ||
| Delay progression of microalbuminuria13 | ||
| Hyperglycemia | Encourage weight loss | Metformin,* thiazolidinediones |
| Increase physical activity | Slow progression to diabetes in persons with insulin resistance15,16 (metformin less effective than lifestyle changes)15 | |
| Reduce total carbohydrates | ||
| * Suggested first-line therapy. | ||
| † For patients with BMI 30 kg/m2 | ||
| ACE: Angiotensin-converting enzyme | ||
| CVD: Cardiovascular disease | ||
| HDL: High-density lipoprotein cholesterol | ||
| LDL: Low-density lipoprotein cholesterol | ||
Selling the benefits of exercise and weight loss to a mentally ill patient can be difficult. Attention, memory, and motivation deficits as well as smoking and substance abuse often get in the way.
By teaming up with clinicians with expertise in dieting such as nurses, dietitians, and recreational therapists, psychiatrists can more effectively promote long-term diet, exercise, and lifestyle changes.21
In a prospective 12-month trial,22 20 patients who were taking atypical antipsychotics for schizophrenia or schizoaffective disorder completed a 52-week program that incorporated nutrition, exercise, and behavioral interventions. Twenty similar patients received treatment as usual. Patients in the program saw significant improvements in weight, blood pressure, exercise habits, nutrition, and hemoglobin A1c compared with the treatment-as-usual group.22
Psychiatrists who treat privately insured patients should collaborate with the patient’s primary care physician. Many insurance plans will pay for 1 or 2 personal or group sessions with a dietitian, especially if the patient is diagnosed as being obese (BMI >30). Some large plans, such as Kaiser Permanente, will cover intensive multimodal treatment, especially for patients with a BMI >35. Calculating the patient’s BMI can help you document the need for antiobesity treatment (see Related resources).
Medication. If weight control and exercise do not reduce metabolic risk factors after 3 to 6 months, consider switching to an atypical antipsychotic with a lower propensity for causing metabolic effects.
Which agents most decrease metabolic risk has been debated. Preliminary evidence indicates that switching from other antipsychotics to aripiprazole or ziprasidone may reduce weight and improve cholesterol ratios.23,24 These findings are consistent with the ADA/APA consensus guidelines, which indicate that metabolic risk varies among atypical antipsychotics (Table 4).6
Table 4
Atypical antipsychotics and their propensity for causing metabolic abnormalities
| Drug | Weight gain | Hyperglycemia | Dyslipidemia |
|---|---|---|---|
| Clozapine | High | High | High |
| Olanzapine | High | High | High |
| Risperidone | Medium | Medium | Low |
| Quetiapine | Medium | Medium | High |
| Aripiprazole | Low | Low | Low |
| Ziprasidone | Low | Low | Low |
| Source: Reference 6 | |||
Targeted pharmacotherapy. Wait another 3 to 6 months to see if the medication change and weight loss/exercise interventions reduce metabolic risk factors. If they don’t, work with the patient’s primary care physician to manage hypertension, dyslipidemia, and obesity (Table 3).
Although no agents are approved for treating metabolic syndrome per se, medications targeted at individual symptoms are becoming the standard of care. Controlling blood pressure, HDL, and LDL in patients with metabolic syndrome can reduce risk for coronary heart disease by >50%.25 Insulin-sensitizing agents and metformin in combination with lifestyle changes or used alone have been shown to delay onset of type 2 diabetes (Table 3).
CASE CONTINUED: 10 LBS IN 10 WEEKS
At her follow-up visit, Ms. S and her psychiatrist discuss her increased risk for diabetes and cardiovascular disease. She meets criteria for metabolic syndrome (low HDL, elevated blood pressure, and increased waist circumference).
Ms. S agrees to try a formal diet program with set menus, along with group support at her local community center. She also commits to walking 30 minutes three to four times a week with a target heart rate of 100 beats per minute. Although both quetiapine and lithium carry considerable risk of weight gain, she and her psychiatrist decide to wait at least 3 months before considering a medication change, as she is stable on this combination.
Ms. S schedules a follow-up visit with her primary care physician to ensure that she sticks to her weight loss and exercise programs. In the interim, the primary care physician and psychiatrist agree that her goal will be to lose 10 lbs over 10 weeks.
- National Alliance for the Mentally Ill. Hearts and Minds Program, a booklet and program geared toward raising awareness regarding diabetes, diet, exercise, and smoking. Download at www.nami.org.
- Centers for Disease Control and Prevention: Body mass index formula for adults. http://www.cdc.gov/nccdphp/dnpa/bmi/bmi-adult-formula.htm.
- National Heart, Lung and Blood Institute body mass index calculator. http://www.nhlbisupport.com/bmi/bmicalc.htm.
- Keck PE Jr, Buse JB, Dagago-Jack S, et al. Managing metabolic concerns in patients with severe mental illness. A special report. Postgraduate Med 2003;1-92.
Drug brand names
- Aripiprazole • Abilify
- Clozapine • Clozaril
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Ziprasidone • Geodon
Disclosure
Dr. Bermudes is a speaker for Bristol-Myers Squibb Co. and Pfizer Inc.
1. Haffner SM, Valdez RA, Hazuda HP, et al. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes 1992;41:715-22.
2. Isomma B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001;24:683-9.
3. Trevisan M, Liu J, Bahsas FB, Menotti A. Syndrome X and mortality: a population-based study. Am J Epidemiol 1998;148:958-66.
4. Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002;288:2709-16.
5. National Institutes of Health: Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Cholesterol in Adults (Adult Treatment Panel III). Executive summary of the Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97.
6. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care 2004;27:596-601.
7. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res 1998;6(suppl 2):51S-209S.
8. Darwin D. Metabolic syndrome: time for action. Am Fam Physician 2004;69:2875-82.
9. Maki KC. Fibrates for treatment of the metabolic syndrome. Curr Atheroscler Rep 2004;6:45-51.
10. Boden WE. Therapeutic implications of recent ATP III guidelines and the important role of combination therapy in total dyslipidemia management. Curr Opin Cardiol 2003;18:278-85.
11. Showers JR. Effects of statins on the vasculature: implications for aggressive lipid management in the cardiovascular metabolic syndrome. Am J Cardiol 2003;91(suppl):14B-22B.
12. Yusuf S, Gerstein H, Hoogwerf B, et al. for the HOPE Study Investigators. Ramipril and the development of diabetes. JAMA 2001;286:1882-5.
13. American Diabetes Association. Treatment of hypertension in adults with diabetes. Diabetes Care 2003;26(suppl 1):S80-S82.
14. Derosa G, Cicero AF, Bertone G, et al. Comparison of the effects of telmisartan and nifedipine gastrointestinal therapeutic system on blood pressure control, glucose metabolism, and the lipid profile in patients with type 2 diabetes mellitus and mild hypertension: a 12-month, randomized double-blind study. Clin Ther 2004;26:1228-36.
15. Diabetes prevention program research group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403.
16. Buchanan T, Xiang A, Peters R, et al. Prevention of type 2 diabetes by treatment of insulin resistance: comparison of early vs. late in the TRIPOD study [abstract]. Diabetes 2002;51(suppl 2):A35.-
17. Leon AS, Sanchez O. Meta-analysis of the effects of aerobic exercise training on blood lipids. Circulation 2001;104(suppl II):414-15 (abstract).
18. Fagard RH. Exercise characteristics and blood pressure response to dynamic physical training. Med Sci Sports Exerc 2001;33(6 suppl):S484-S492.
19. Thompson PD, Crouse SF, Goodpaster B, et al. The acute versus the chronic response to exercise. Med Sci Sports Exerc 2001;33(6 suppl):S438-S445.
20. Katzmarzyk PT, Leon AS, Wilmore JH, et al. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study. Med Sci Sports Exerc 2003;35:1703-9.
21. Littrell KH, Hilligoss NM, Kirshner CD, et al. The effects of an educational intervention on antipsychotic-induced weight gain. J Nurs Scholarsh 2003;35:237-41.
22. Menza M, Vreeland B, Minsky S, et al. Managing atypical antipsychotic-associated weight gain: 12-month data on a multimodal weight control program. J Clin Psychiatry 2004;65:471-7.
23. Cohen SA, Fitzgerald BJ, Khan SR, Khan A. The effect of a switch to ziprasidone in an adult population with autistic disorder: chart review and naturalistic, open-label treatment. J Clin Psychiatry 2004;65:110-13.
24. Casey DE, Carson WH, Saha AR, et al. Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology 2003;166:391-9.
25. Wong ND, Pio J, Franklin SS, et al. Preventing coronary events by optimal control of blood pressure and lipids in patients with the metabolic syndrome. Am J Cardiol 2003;91:1421-6.
1. Haffner SM, Valdez RA, Hazuda HP, et al. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes 1992;41:715-22.
2. Isomma B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001;24:683-9.
3. Trevisan M, Liu J, Bahsas FB, Menotti A. Syndrome X and mortality: a population-based study. Am J Epidemiol 1998;148:958-66.
4. Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002;288:2709-16.
5. National Institutes of Health: Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Cholesterol in Adults (Adult Treatment Panel III). Executive summary of the Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97.
6. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care 2004;27:596-601.
7. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res 1998;6(suppl 2):51S-209S.
8. Darwin D. Metabolic syndrome: time for action. Am Fam Physician 2004;69:2875-82.
9. Maki KC. Fibrates for treatment of the metabolic syndrome. Curr Atheroscler Rep 2004;6:45-51.
10. Boden WE. Therapeutic implications of recent ATP III guidelines and the important role of combination therapy in total dyslipidemia management. Curr Opin Cardiol 2003;18:278-85.
11. Showers JR. Effects of statins on the vasculature: implications for aggressive lipid management in the cardiovascular metabolic syndrome. Am J Cardiol 2003;91(suppl):14B-22B.
12. Yusuf S, Gerstein H, Hoogwerf B, et al. for the HOPE Study Investigators. Ramipril and the development of diabetes. JAMA 2001;286:1882-5.
13. American Diabetes Association. Treatment of hypertension in adults with diabetes. Diabetes Care 2003;26(suppl 1):S80-S82.
14. Derosa G, Cicero AF, Bertone G, et al. Comparison of the effects of telmisartan and nifedipine gastrointestinal therapeutic system on blood pressure control, glucose metabolism, and the lipid profile in patients with type 2 diabetes mellitus and mild hypertension: a 12-month, randomized double-blind study. Clin Ther 2004;26:1228-36.
15. Diabetes prevention program research group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403.
16. Buchanan T, Xiang A, Peters R, et al. Prevention of type 2 diabetes by treatment of insulin resistance: comparison of early vs. late in the TRIPOD study [abstract]. Diabetes 2002;51(suppl 2):A35.-
17. Leon AS, Sanchez O. Meta-analysis of the effects of aerobic exercise training on blood lipids. Circulation 2001;104(suppl II):414-15 (abstract).
18. Fagard RH. Exercise characteristics and blood pressure response to dynamic physical training. Med Sci Sports Exerc 2001;33(6 suppl):S484-S492.
19. Thompson PD, Crouse SF, Goodpaster B, et al. The acute versus the chronic response to exercise. Med Sci Sports Exerc 2001;33(6 suppl):S438-S445.
20. Katzmarzyk PT, Leon AS, Wilmore JH, et al. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study. Med Sci Sports Exerc 2003;35:1703-9.
21. Littrell KH, Hilligoss NM, Kirshner CD, et al. The effects of an educational intervention on antipsychotic-induced weight gain. J Nurs Scholarsh 2003;35:237-41.
22. Menza M, Vreeland B, Minsky S, et al. Managing atypical antipsychotic-associated weight gain: 12-month data on a multimodal weight control program. J Clin Psychiatry 2004;65:471-7.
23. Cohen SA, Fitzgerald BJ, Khan SR, Khan A. The effect of a switch to ziprasidone in an adult population with autistic disorder: chart review and naturalistic, open-label treatment. J Clin Psychiatry 2004;65:110-13.
24. Casey DE, Carson WH, Saha AR, et al. Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology 2003;166:391-9.
25. Wong ND, Pio J, Franklin SS, et al. Preventing coronary events by optimal control of blood pressure and lipids in patients with the metabolic syndrome. Am J Cardiol 2003;91:1421-6.
5 Ways to quiet auditory hallucinations
Cognitive-behavioral therapy (CBT) can help patients cope with auditory hallucinations and reshape delusional beliefs to make the voices less frequent.1 Use the following CBT methods alone or with medication.
1. Engage the patient by showing interest in the voices. Ask: “When did the voices start? Where are they coming from? Can you bring them on or stop them? Do they tell you to do things? What happens when you ignore them?”
2. Normalize the hallucination. List scientifically plausible “reasons for hearing voices,”2 including sleep deprivation, isolation, dehydration and/or starvation, extreme stress, strong thoughts or emotions, fever and illness, and drug/alcohol use.
Ask which reasons might apply. Patients often agree with several explanations and begin questioning their delusional interpretations. Your list should include the possibility that the voices are real, but only if the patient initially believes this.
3. Suggest coping strategies, such as:
- humming or singing a song several times
- listening to music
- reading (forwards and backwards)
- talking with others
- exercise
- ignoring the voices
- medication (important to include).
Ask which methods worked previously and have patients build on that list, if possible.
If a patient hears command hallucinations, assess their acuity and decide whether he or she is likely to act on them before starting CBT.
4. Use in-session voices to teach coping strategies. Ask the patient to hum a song with you (“Happy Birthday” works well). If unsuccessful, try reading a paragraph together forwards or backwards. If the voices stop—even for 2 minutes—tell the patient that he or she has begun to control them.3 Have the patient practice these exercises at home and notice if the voices stop for longer periods.
5. Briefly explain the neurology behind the voices. PET scans have shown that auditory hallucinations activate brain areas that regulate hearing and speaking,4 suggesting that people talk or think to themselves while hearing voices.
When patients ask why they hear strange voices, explain that many voices are buried inside our memory. When people hear voices, the brain’s speech, hearing, and memory centers interact.5
That said, calling auditory hallucinations “voice-thoughts,” rather than “voices,” reduces stigma and reinforces an alternate explanation behind the delusion. As the patient begins to understand that hallucinations are related to dysfunctional thoughts, we can help correct them.
1. Rector NA, Beck AT. A clinical review of cognitive therapy for schizophrenia. Curr Psychiatry Rep. 2002;4:284-292.
2. Kingdon DG, Turkington D. Cognitive-behavioral therapy of schizophrenia. New York: Guilford Press; 1994.
3. Beck AT. E-mail communication.
4. McGuire PK, Shah GMS, Murray RM. Increased blood flow in Broca’s area during auditory hallucinations in schizophrenia. Lancet. 1993;342:703-706.
5. Sosland MD, Deibler MW. Temple University Psychosis Group. 2003.
Cognitive-behavioral therapy (CBT) can help patients cope with auditory hallucinations and reshape delusional beliefs to make the voices less frequent.1 Use the following CBT methods alone or with medication.
1. Engage the patient by showing interest in the voices. Ask: “When did the voices start? Where are they coming from? Can you bring them on or stop them? Do they tell you to do things? What happens when you ignore them?”
2. Normalize the hallucination. List scientifically plausible “reasons for hearing voices,”2 including sleep deprivation, isolation, dehydration and/or starvation, extreme stress, strong thoughts or emotions, fever and illness, and drug/alcohol use.
Ask which reasons might apply. Patients often agree with several explanations and begin questioning their delusional interpretations. Your list should include the possibility that the voices are real, but only if the patient initially believes this.
3. Suggest coping strategies, such as:
- humming or singing a song several times
- listening to music
- reading (forwards and backwards)
- talking with others
- exercise
- ignoring the voices
- medication (important to include).
Ask which methods worked previously and have patients build on that list, if possible.
If a patient hears command hallucinations, assess their acuity and decide whether he or she is likely to act on them before starting CBT.
4. Use in-session voices to teach coping strategies. Ask the patient to hum a song with you (“Happy Birthday” works well). If unsuccessful, try reading a paragraph together forwards or backwards. If the voices stop—even for 2 minutes—tell the patient that he or she has begun to control them.3 Have the patient practice these exercises at home and notice if the voices stop for longer periods.
5. Briefly explain the neurology behind the voices. PET scans have shown that auditory hallucinations activate brain areas that regulate hearing and speaking,4 suggesting that people talk or think to themselves while hearing voices.
When patients ask why they hear strange voices, explain that many voices are buried inside our memory. When people hear voices, the brain’s speech, hearing, and memory centers interact.5
That said, calling auditory hallucinations “voice-thoughts,” rather than “voices,” reduces stigma and reinforces an alternate explanation behind the delusion. As the patient begins to understand that hallucinations are related to dysfunctional thoughts, we can help correct them.
Cognitive-behavioral therapy (CBT) can help patients cope with auditory hallucinations and reshape delusional beliefs to make the voices less frequent.1 Use the following CBT methods alone or with medication.
1. Engage the patient by showing interest in the voices. Ask: “When did the voices start? Where are they coming from? Can you bring them on or stop them? Do they tell you to do things? What happens when you ignore them?”
2. Normalize the hallucination. List scientifically plausible “reasons for hearing voices,”2 including sleep deprivation, isolation, dehydration and/or starvation, extreme stress, strong thoughts or emotions, fever and illness, and drug/alcohol use.
Ask which reasons might apply. Patients often agree with several explanations and begin questioning their delusional interpretations. Your list should include the possibility that the voices are real, but only if the patient initially believes this.
3. Suggest coping strategies, such as:
- humming or singing a song several times
- listening to music
- reading (forwards and backwards)
- talking with others
- exercise
- ignoring the voices
- medication (important to include).
Ask which methods worked previously and have patients build on that list, if possible.
If a patient hears command hallucinations, assess their acuity and decide whether he or she is likely to act on them before starting CBT.
4. Use in-session voices to teach coping strategies. Ask the patient to hum a song with you (“Happy Birthday” works well). If unsuccessful, try reading a paragraph together forwards or backwards. If the voices stop—even for 2 minutes—tell the patient that he or she has begun to control them.3 Have the patient practice these exercises at home and notice if the voices stop for longer periods.
5. Briefly explain the neurology behind the voices. PET scans have shown that auditory hallucinations activate brain areas that regulate hearing and speaking,4 suggesting that people talk or think to themselves while hearing voices.
When patients ask why they hear strange voices, explain that many voices are buried inside our memory. When people hear voices, the brain’s speech, hearing, and memory centers interact.5
That said, calling auditory hallucinations “voice-thoughts,” rather than “voices,” reduces stigma and reinforces an alternate explanation behind the delusion. As the patient begins to understand that hallucinations are related to dysfunctional thoughts, we can help correct them.
1. Rector NA, Beck AT. A clinical review of cognitive therapy for schizophrenia. Curr Psychiatry Rep. 2002;4:284-292.
2. Kingdon DG, Turkington D. Cognitive-behavioral therapy of schizophrenia. New York: Guilford Press; 1994.
3. Beck AT. E-mail communication.
4. McGuire PK, Shah GMS, Murray RM. Increased blood flow in Broca’s area during auditory hallucinations in schizophrenia. Lancet. 1993;342:703-706.
5. Sosland MD, Deibler MW. Temple University Psychosis Group. 2003.
1. Rector NA, Beck AT. A clinical review of cognitive therapy for schizophrenia. Curr Psychiatry Rep. 2002;4:284-292.
2. Kingdon DG, Turkington D. Cognitive-behavioral therapy of schizophrenia. New York: Guilford Press; 1994.
3. Beck AT. E-mail communication.
4. McGuire PK, Shah GMS, Murray RM. Increased blood flow in Broca’s area during auditory hallucinations in schizophrenia. Lancet. 1993;342:703-706.
5. Sosland MD, Deibler MW. Temple University Psychosis Group. 2003.
Helping indigent patients obtain medications
Pharmaceutical companies, through detailers and assistance programs, offer resources for helping our financially strapped patients.
Drug company representatives. Doctors may consider visits from drug reps a waste of time, but we can give the coupons and free drug samples they offer to indigent, disabled, or working-poor patients who often cannot afford the psychotropics they need.
To make meetings with detailers more productive:
- Be clear about your needs, expectations, and how much time you are willing to spend.
- Be frank about whether you want to hear about studies. Tell the detailer that you’ll accept information by request only.
- State specific dates and times you are available.
- Request coupons for patients whose medication samples are likely to be lost or stolen.
Patient assistance programs. Pharmaceutical companies’ assistance programs provide deeply discounted drugs to patients in need (Box). The paperwork to qualify takes minutes for clinician and patient to fill out, and many forms are available online or via fax.
Solvay Pharmaceuticals
800-256-8918
Ortho McNeil Pharmaceutical
800-577-3788
Abbott Laboratories
800-222-6885
Wyeth
800-568-9938
Merck and Co.
800-994-2111
GlaxoSmithKline
800-728-4368
AstraZeneca Pharmaceuticals
800-424-3727; www.astrazeneca-us.com/content/drugAssistance/
Bristol-Myers Squibb Co.
800-736-0003
Novartis Pharmaceuticals Corp.
800-277-2254
Forest Pharmaceuticals
800-851-0758; http://www.forestpharm.com/pap/
Pfizer Inc.
800-707-8990
Cephalon
800-511-2120
Pharmaceutical companies, through detailers and assistance programs, offer resources for helping our financially strapped patients.
Drug company representatives. Doctors may consider visits from drug reps a waste of time, but we can give the coupons and free drug samples they offer to indigent, disabled, or working-poor patients who often cannot afford the psychotropics they need.
To make meetings with detailers more productive:
- Be clear about your needs, expectations, and how much time you are willing to spend.
- Be frank about whether you want to hear about studies. Tell the detailer that you’ll accept information by request only.
- State specific dates and times you are available.
- Request coupons for patients whose medication samples are likely to be lost or stolen.
Patient assistance programs. Pharmaceutical companies’ assistance programs provide deeply discounted drugs to patients in need (Box). The paperwork to qualify takes minutes for clinician and patient to fill out, and many forms are available online or via fax.
Solvay Pharmaceuticals
800-256-8918
Ortho McNeil Pharmaceutical
800-577-3788
Abbott Laboratories
800-222-6885
Wyeth
800-568-9938
Merck and Co.
800-994-2111
GlaxoSmithKline
800-728-4368
AstraZeneca Pharmaceuticals
800-424-3727; www.astrazeneca-us.com/content/drugAssistance/
Bristol-Myers Squibb Co.
800-736-0003
Novartis Pharmaceuticals Corp.
800-277-2254
Forest Pharmaceuticals
800-851-0758; http://www.forestpharm.com/pap/
Pfizer Inc.
800-707-8990
Cephalon
800-511-2120
Pharmaceutical companies, through detailers and assistance programs, offer resources for helping our financially strapped patients.
Drug company representatives. Doctors may consider visits from drug reps a waste of time, but we can give the coupons and free drug samples they offer to indigent, disabled, or working-poor patients who often cannot afford the psychotropics they need.
To make meetings with detailers more productive:
- Be clear about your needs, expectations, and how much time you are willing to spend.
- Be frank about whether you want to hear about studies. Tell the detailer that you’ll accept information by request only.
- State specific dates and times you are available.
- Request coupons for patients whose medication samples are likely to be lost or stolen.
Patient assistance programs. Pharmaceutical companies’ assistance programs provide deeply discounted drugs to patients in need (Box). The paperwork to qualify takes minutes for clinician and patient to fill out, and many forms are available online or via fax.
Solvay Pharmaceuticals
800-256-8918
Ortho McNeil Pharmaceutical
800-577-3788
Abbott Laboratories
800-222-6885
Wyeth
800-568-9938
Merck and Co.
800-994-2111
GlaxoSmithKline
800-728-4368
AstraZeneca Pharmaceuticals
800-424-3727; www.astrazeneca-us.com/content/drugAssistance/
Bristol-Myers Squibb Co.
800-736-0003
Novartis Pharmaceuticals Corp.
800-277-2254
Forest Pharmaceuticals
800-851-0758; http://www.forestpharm.com/pap/
Pfizer Inc.
800-707-8990
Cephalon
800-511-2120
Preventing post-disaster PTSD: Watch for autonomic signs
Posttraumatic stress disorder (PTSD) is underdiagnosed among combat-exposed individuals and overdiagnosed among civilians. An expanded, nondichotomous checklist of emotional and physical signs following a disaster may help address this problem.
PTSD diagnostic criteria shortcomings
Schnurr et al calculated that DSM-IV-TR diagnostic criteria A1 and A2 for PTSD together have a 34% positive predictive value when applied to victims of violent crime.1 Many who meet these criteria may not need intervention, and some interventions—such as critical incident stress debriefing—may be detrimental.2,3
DSM-IV criteria A1 and A2 do not take into account common peritraumatic autonomic activation signs—shortness of breath, tremulousness, racing heart, and sweaty palms/cold sweat—that are part of the human hardwired acute response to threat.4 Last year we published a research checklist of criteria A1 and A2 symptoms plus the four autonomic signs, which we collectively refer to as “criterion A3.”4
A preliminary (tentatively weighted) clinical version of this checklist, the PTSD Criterion A3 Checklist (Table), may be useful for screening persons in the acute aftermath of a disaster. While more research is needed, this version is:
Table
PTSD Criterion A3 Checklist
| Incident: | Total score* (0-15): | ||
|---|---|---|---|
| Time since incident: ________________ | |||
| At the time, did you… | Points for “Yes” answers | ||
| Think… Criterion A1 | That you would be seriously physically injured or killed? | 4 | Total A1 score: |
| That a close family member would be seriously physically injured or killed? | 3 | ||
| That someone else would be killed? | 1 | ||
| Feel… Criterion A2 | Intense fear or fright? | 1 | Total A2 score: |
| Helpless? | 1 | ||
| Horrified? | 1 | ||
| Experience… Criterion A3 (Proposed for DSM-V) | Shortness of breath? | 1 | Total A3 score: |
| Trembling, shaking or buckling knees? | 1 | ||
| Racing/pounding heart? | 1 | ||
| Sweaty palms or other cold sweat? | 1 | ||
| • Consider preventive intervention (eg, propranolol regimen) if total score is 5 or more. | |||
Fear-specific. The checklist includes queries about two peritraumatic, fear-specific signs (tremulousness and sweaty palms/cold sweat) as well as peritraumatic tachycardia and dyspnea.
Brief. This tool takes as little as 2 minutes to administer, thus minimizing the burden on victims in the days or weeks after a mass disaster.
Non-dichotomous but easy to score. One point is scored for each “Yes” answer for 8 of the 10 queries; “Yes” answers to the two other queries are worth 4 and 3 points, respectively. A total score of 5 or more may indicate a need for preventive intervention such as propranolol, 40 mg tid or qid for 7 to 10 days.5,6
Minimizes stigma. Assessing peritraumatic physical signs may help minimize stigma-related bias.4 This is important when screening persons likely to underreport criterion A2 symptoms, including:
- veterans
- military personnel
- firefighters
- police officers
- men in general
- persons from ethnic cultures in which having psychiatric symptoms is viewed as disgraceful.
Easy to remember. After a few administrations, the queries can be easily memorized and incorporated into initial assessments. The four acute autonomic activation signs can be remembered with the acronym “STRS” (shortness of breath, trembling, racing heart, sweaty palms). Consider “A3” a mnemonic for “acute autonomic activation.”
Posttraumatic stress disorder (PTSD) is underdiagnosed among combat-exposed individuals and overdiagnosed among civilians. An expanded, nondichotomous checklist of emotional and physical signs following a disaster may help address this problem.
PTSD diagnostic criteria shortcomings
Schnurr et al calculated that DSM-IV-TR diagnostic criteria A1 and A2 for PTSD together have a 34% positive predictive value when applied to victims of violent crime.1 Many who meet these criteria may not need intervention, and some interventions—such as critical incident stress debriefing—may be detrimental.2,3
DSM-IV criteria A1 and A2 do not take into account common peritraumatic autonomic activation signs—shortness of breath, tremulousness, racing heart, and sweaty palms/cold sweat—that are part of the human hardwired acute response to threat.4 Last year we published a research checklist of criteria A1 and A2 symptoms plus the four autonomic signs, which we collectively refer to as “criterion A3.”4
A preliminary (tentatively weighted) clinical version of this checklist, the PTSD Criterion A3 Checklist (Table), may be useful for screening persons in the acute aftermath of a disaster. While more research is needed, this version is:
Table
PTSD Criterion A3 Checklist
| Incident: | Total score* (0-15): | ||
|---|---|---|---|
| Time since incident: ________________ | |||
| At the time, did you… | Points for “Yes” answers | ||
| Think… Criterion A1 | That you would be seriously physically injured or killed? | 4 | Total A1 score: |
| That a close family member would be seriously physically injured or killed? | 3 | ||
| That someone else would be killed? | 1 | ||
| Feel… Criterion A2 | Intense fear or fright? | 1 | Total A2 score: |
| Helpless? | 1 | ||
| Horrified? | 1 | ||
| Experience… Criterion A3 (Proposed for DSM-V) | Shortness of breath? | 1 | Total A3 score: |
| Trembling, shaking or buckling knees? | 1 | ||
| Racing/pounding heart? | 1 | ||
| Sweaty palms or other cold sweat? | 1 | ||
| • Consider preventive intervention (eg, propranolol regimen) if total score is 5 or more. | |||
Fear-specific. The checklist includes queries about two peritraumatic, fear-specific signs (tremulousness and sweaty palms/cold sweat) as well as peritraumatic tachycardia and dyspnea.
Brief. This tool takes as little as 2 minutes to administer, thus minimizing the burden on victims in the days or weeks after a mass disaster.
Non-dichotomous but easy to score. One point is scored for each “Yes” answer for 8 of the 10 queries; “Yes” answers to the two other queries are worth 4 and 3 points, respectively. A total score of 5 or more may indicate a need for preventive intervention such as propranolol, 40 mg tid or qid for 7 to 10 days.5,6
Minimizes stigma. Assessing peritraumatic physical signs may help minimize stigma-related bias.4 This is important when screening persons likely to underreport criterion A2 symptoms, including:
- veterans
- military personnel
- firefighters
- police officers
- men in general
- persons from ethnic cultures in which having psychiatric symptoms is viewed as disgraceful.
Easy to remember. After a few administrations, the queries can be easily memorized and incorporated into initial assessments. The four acute autonomic activation signs can be remembered with the acronym “STRS” (shortness of breath, trembling, racing heart, sweaty palms). Consider “A3” a mnemonic for “acute autonomic activation.”
Posttraumatic stress disorder (PTSD) is underdiagnosed among combat-exposed individuals and overdiagnosed among civilians. An expanded, nondichotomous checklist of emotional and physical signs following a disaster may help address this problem.
PTSD diagnostic criteria shortcomings
Schnurr et al calculated that DSM-IV-TR diagnostic criteria A1 and A2 for PTSD together have a 34% positive predictive value when applied to victims of violent crime.1 Many who meet these criteria may not need intervention, and some interventions—such as critical incident stress debriefing—may be detrimental.2,3
DSM-IV criteria A1 and A2 do not take into account common peritraumatic autonomic activation signs—shortness of breath, tremulousness, racing heart, and sweaty palms/cold sweat—that are part of the human hardwired acute response to threat.4 Last year we published a research checklist of criteria A1 and A2 symptoms plus the four autonomic signs, which we collectively refer to as “criterion A3.”4
A preliminary (tentatively weighted) clinical version of this checklist, the PTSD Criterion A3 Checklist (Table), may be useful for screening persons in the acute aftermath of a disaster. While more research is needed, this version is:
Table
PTSD Criterion A3 Checklist
| Incident: | Total score* (0-15): | ||
|---|---|---|---|
| Time since incident: ________________ | |||
| At the time, did you… | Points for “Yes” answers | ||
| Think… Criterion A1 | That you would be seriously physically injured or killed? | 4 | Total A1 score: |
| That a close family member would be seriously physically injured or killed? | 3 | ||
| That someone else would be killed? | 1 | ||
| Feel… Criterion A2 | Intense fear or fright? | 1 | Total A2 score: |
| Helpless? | 1 | ||
| Horrified? | 1 | ||
| Experience… Criterion A3 (Proposed for DSM-V) | Shortness of breath? | 1 | Total A3 score: |
| Trembling, shaking or buckling knees? | 1 | ||
| Racing/pounding heart? | 1 | ||
| Sweaty palms or other cold sweat? | 1 | ||
| • Consider preventive intervention (eg, propranolol regimen) if total score is 5 or more. | |||
Fear-specific. The checklist includes queries about two peritraumatic, fear-specific signs (tremulousness and sweaty palms/cold sweat) as well as peritraumatic tachycardia and dyspnea.
Brief. This tool takes as little as 2 minutes to administer, thus minimizing the burden on victims in the days or weeks after a mass disaster.
Non-dichotomous but easy to score. One point is scored for each “Yes” answer for 8 of the 10 queries; “Yes” answers to the two other queries are worth 4 and 3 points, respectively. A total score of 5 or more may indicate a need for preventive intervention such as propranolol, 40 mg tid or qid for 7 to 10 days.5,6
Minimizes stigma. Assessing peritraumatic physical signs may help minimize stigma-related bias.4 This is important when screening persons likely to underreport criterion A2 symptoms, including:
- veterans
- military personnel
- firefighters
- police officers
- men in general
- persons from ethnic cultures in which having psychiatric symptoms is viewed as disgraceful.
Easy to remember. After a few administrations, the queries can be easily memorized and incorporated into initial assessments. The four acute autonomic activation signs can be remembered with the acronym “STRS” (shortness of breath, trembling, racing heart, sweaty palms). Consider “A3” a mnemonic for “acute autonomic activation.”
Psychiatry’s ‘Teammates’
Many thanks for your warm welcome to advanced practice psychiatric nurses (APNs) and for recognizing our need for clinical information (Current Psychiatry, January 2005).
As Dr. Hillard noted, many psychiatrists refuse to accept APNs even though we offer crucial support to psychiatrists, especially to those practicing in rural, underserved areas.
APNs are teammates to—not replacements for—psychiatrists.
For the record, my supervisor—who is also my consulting psychiatrist—is very supportive of my career. I hope that someday many other APNs will be as fortunate as nursing’s relationship with psychiatry evolves.
Cynthia Baugh, CNS
Oklahoma Department of Corrections
McAlester, OK
Many thanks for your warm welcome to advanced practice psychiatric nurses (APNs) and for recognizing our need for clinical information (Current Psychiatry, January 2005).
As Dr. Hillard noted, many psychiatrists refuse to accept APNs even though we offer crucial support to psychiatrists, especially to those practicing in rural, underserved areas.
APNs are teammates to—not replacements for—psychiatrists.
For the record, my supervisor—who is also my consulting psychiatrist—is very supportive of my career. I hope that someday many other APNs will be as fortunate as nursing’s relationship with psychiatry evolves.
Cynthia Baugh, CNS
Oklahoma Department of Corrections
McAlester, OK
Many thanks for your warm welcome to advanced practice psychiatric nurses (APNs) and for recognizing our need for clinical information (Current Psychiatry, January 2005).
As Dr. Hillard noted, many psychiatrists refuse to accept APNs even though we offer crucial support to psychiatrists, especially to those practicing in rural, underserved areas.
APNs are teammates to—not replacements for—psychiatrists.
For the record, my supervisor—who is also my consulting psychiatrist—is very supportive of my career. I hope that someday many other APNs will be as fortunate as nursing’s relationship with psychiatry evolves.
Cynthia Baugh, CNS
Oklahoma Department of Corrections
McAlester, OK
Dementia or depression?
“From Active to Apathetic” (Current Psychiatry, February 2005) reports the decline of a 66-year-old man’s cognitive function as “sudden.”
If the decline was truly sudden, Drs. Rehan Aziz and Rajesh Tampi should have listed many more differential diagnoses than they did. At least major depression or so-called pseudodementia should have been included.
Except for apparent dementia related to some gross organic cerebral lesions, most of the dementias listed in the article would not suggest a pathological process deemed as sudden.
Zhong-Yi Liu, MD
Staff psychiatrist, Ventura County Medical Center
Ventura, CA
Dr. Tampi responds
Dr. Liu makes a very good observation that depression should be among the differential diagnoses for early dementia. We did not consider depression in our patient because:
- Decline was not ‘sudden,’ although the headline used that word. The changes in cognitive functioning, behaviors, and activities of daily living were unfolding for more than a year before the family brought him to our clinic for evaluation. His cognitive deficits were also severe enough to be in the moderate range for a dementia.
- The patient’s flat affect and lack of initiative were consistent with a frontal executive dysfunction as there was no subjective report of depressive symptoms. Also, family members noticed no neurovegetative depressive symptoms.
- The patient was started on bupropion and then was switched to citalopram. Neither medication resolved his apathy.
We included only the most common differential diagnoses for frontotemporal dementia. Including other neurodegenerative conditions would have made our case review very long and confusing.
Rajesh R. Tampi, MD, MS
Assistant professor of psychiatry
Yale University School of Medicine
New Haven, CT
“From Active to Apathetic” (Current Psychiatry, February 2005) reports the decline of a 66-year-old man’s cognitive function as “sudden.”
If the decline was truly sudden, Drs. Rehan Aziz and Rajesh Tampi should have listed many more differential diagnoses than they did. At least major depression or so-called pseudodementia should have been included.
Except for apparent dementia related to some gross organic cerebral lesions, most of the dementias listed in the article would not suggest a pathological process deemed as sudden.
Zhong-Yi Liu, MD
Staff psychiatrist, Ventura County Medical Center
Ventura, CA
Dr. Tampi responds
Dr. Liu makes a very good observation that depression should be among the differential diagnoses for early dementia. We did not consider depression in our patient because:
- Decline was not ‘sudden,’ although the headline used that word. The changes in cognitive functioning, behaviors, and activities of daily living were unfolding for more than a year before the family brought him to our clinic for evaluation. His cognitive deficits were also severe enough to be in the moderate range for a dementia.
- The patient’s flat affect and lack of initiative were consistent with a frontal executive dysfunction as there was no subjective report of depressive symptoms. Also, family members noticed no neurovegetative depressive symptoms.
- The patient was started on bupropion and then was switched to citalopram. Neither medication resolved his apathy.
We included only the most common differential diagnoses for frontotemporal dementia. Including other neurodegenerative conditions would have made our case review very long and confusing.
Rajesh R. Tampi, MD, MS
Assistant professor of psychiatry
Yale University School of Medicine
New Haven, CT
“From Active to Apathetic” (Current Psychiatry, February 2005) reports the decline of a 66-year-old man’s cognitive function as “sudden.”
If the decline was truly sudden, Drs. Rehan Aziz and Rajesh Tampi should have listed many more differential diagnoses than they did. At least major depression or so-called pseudodementia should have been included.
Except for apparent dementia related to some gross organic cerebral lesions, most of the dementias listed in the article would not suggest a pathological process deemed as sudden.
Zhong-Yi Liu, MD
Staff psychiatrist, Ventura County Medical Center
Ventura, CA
Dr. Tampi responds
Dr. Liu makes a very good observation that depression should be among the differential diagnoses for early dementia. We did not consider depression in our patient because:
- Decline was not ‘sudden,’ although the headline used that word. The changes in cognitive functioning, behaviors, and activities of daily living were unfolding for more than a year before the family brought him to our clinic for evaluation. His cognitive deficits were also severe enough to be in the moderate range for a dementia.
- The patient’s flat affect and lack of initiative were consistent with a frontal executive dysfunction as there was no subjective report of depressive symptoms. Also, family members noticed no neurovegetative depressive symptoms.
- The patient was started on bupropion and then was switched to citalopram. Neither medication resolved his apathy.
We included only the most common differential diagnoses for frontotemporal dementia. Including other neurodegenerative conditions would have made our case review very long and confusing.
Rajesh R. Tampi, MD, MS
Assistant professor of psychiatry
Yale University School of Medicine
New Haven, CT