User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Taking psychiatry’s changing image personally
Notice anything different about my picture this month? Yes, my beard is gone after 28 years. I grew it between medical school and psychiatry residency to look older. Recently, I reached what author Malcolm Gladwell coined a “tipping point” and got rid of it to look younger.
I also grew my beard (a goatee, actually) to project my vision of a psychiatrist—a nonconformist, an intellectual. Sigmund Freud had a beard, of course, and I suspect that because of him the percentage of hirsute faces is much higher in psychiatry than in other medical specialties.
I went to college in the ‘60s and early ‘70s, so the beard helped me resist feeling that I had “gone establishment” when I became a doctor. But now I am more secure in my psychiatric identity and have come to terms with being part of established medicine. I am president of my university’s multispecialty practice group, and without the beard I look more like my clean-shaven colleagues from other departments. Maybe shaving will help secure my identity as a “real” doctor, despite decades of not doing physical exams.
Are beards becoming less common among psychiatrists? My impression is yes, but my only evidence comes from counting beards in the University of Cincinnati psychiatry department’s annual photos. In 1990, 27% of men on the faculty sported facial hair, and the percentage fell to 8.8% by 2001.
As psychiatry becomes more mainstream, maybe our appearances are becoming more mainstream, too. Or maybe I am indulging in that psychiatric temptation to over-generalize from a single case report.
Notice anything different about my picture this month? Yes, my beard is gone after 28 years. I grew it between medical school and psychiatry residency to look older. Recently, I reached what author Malcolm Gladwell coined a “tipping point” and got rid of it to look younger.
I also grew my beard (a goatee, actually) to project my vision of a psychiatrist—a nonconformist, an intellectual. Sigmund Freud had a beard, of course, and I suspect that because of him the percentage of hirsute faces is much higher in psychiatry than in other medical specialties.
I went to college in the ‘60s and early ‘70s, so the beard helped me resist feeling that I had “gone establishment” when I became a doctor. But now I am more secure in my psychiatric identity and have come to terms with being part of established medicine. I am president of my university’s multispecialty practice group, and without the beard I look more like my clean-shaven colleagues from other departments. Maybe shaving will help secure my identity as a “real” doctor, despite decades of not doing physical exams.
Are beards becoming less common among psychiatrists? My impression is yes, but my only evidence comes from counting beards in the University of Cincinnati psychiatry department’s annual photos. In 1990, 27% of men on the faculty sported facial hair, and the percentage fell to 8.8% by 2001.
As psychiatry becomes more mainstream, maybe our appearances are becoming more mainstream, too. Or maybe I am indulging in that psychiatric temptation to over-generalize from a single case report.
Notice anything different about my picture this month? Yes, my beard is gone after 28 years. I grew it between medical school and psychiatry residency to look older. Recently, I reached what author Malcolm Gladwell coined a “tipping point” and got rid of it to look younger.
I also grew my beard (a goatee, actually) to project my vision of a psychiatrist—a nonconformist, an intellectual. Sigmund Freud had a beard, of course, and I suspect that because of him the percentage of hirsute faces is much higher in psychiatry than in other medical specialties.
I went to college in the ‘60s and early ‘70s, so the beard helped me resist feeling that I had “gone establishment” when I became a doctor. But now I am more secure in my psychiatric identity and have come to terms with being part of established medicine. I am president of my university’s multispecialty practice group, and without the beard I look more like my clean-shaven colleagues from other departments. Maybe shaving will help secure my identity as a “real” doctor, despite decades of not doing physical exams.
Are beards becoming less common among psychiatrists? My impression is yes, but my only evidence comes from counting beards in the University of Cincinnati psychiatry department’s annual photos. In 1990, 27% of men on the faculty sported facial hair, and the percentage fell to 8.8% by 2001.
As psychiatry becomes more mainstream, maybe our appearances are becoming more mainstream, too. Or maybe I am indulging in that psychiatric temptation to over-generalize from a single case report.
Using IM antipsychotics: Lessons from clinical practice
Knowing how to use IM risperidone—and other long-acting atypicals that are likely to be approved—will enable you to help your patients benefit from reliable antipsychotic dosing. Long-acting antipsychotics address the challenge that makes schizophrenia particularly difficult to treat: medication nonadherence because of psychotic illness’ effect on insight, reality testing, and motivation.1,2
Too few schizophrenia patients in the United States—perhaps <5% of appropriate candidates—receive depot antipsychotics.1 We believe these agents provide the best delivery system to our patients and welcome IM risperidone’s approval3
This article shares what we have learned from research and clinical practice about using injectable antipsychotics, with a focus on how to effectively use long-acting IM risperidone.
CONVENTIONAL ANTIPSYCHOTICS
Once seen as an improvement over oral conventional antipsychotics, IM agents were relegated over time to a means of coercion (as in, “If you don’t take your medicine orally, we’ll force you to take a shot.”). Oral atypical antipsychotics, with improved side-effect profiles and possibly reduced relapse risk, also discouraged psychiatrists from using long-acting conventional antipsychotics as first-line medication.4
Available agents. Fluphenazine and haloperidol—the two long-acting conventional antipsychotics available in the United States (Table 1)—are esterified to a fatty acid (oil) to create an IM injectable prodrug. They can be given in gluteal or deltoid injection, although doses >2 cc should be given in the gluteus.
Hydrolysis releases the active drug, usually within 3 days. This interval allows loading doses to reach therapeutic blood levels rapidly when the goal is to stabilize patients in the hospital or during a short-term crisis stay. Disadvantages include:
- pain and lasting reactions at the injection site
- risk of extrapyramidal symptoms (EPS), neuroleptic malignant syndrome, and tardive dyskinesia.5
Table 1
Administering long-acting injectable antipsychotics
| Name | Preparation | Dose range | Interval | Injection site | Comment |
|---|---|---|---|---|---|
| Fluphenazine | 25 mg/mL | 5 to 75 mg each injection | Every 1 to 2 weeks | Deltoid or gluteal | Site reaction common |
| Haloperidol | 50 or 100 mg/mL | 25 to 200 mg each injection | Every 2 to 4 weeks | Deltoid or gluteal | Site reaction common |
| Risperidone | 25, 37.5, or 50 mg in prefilled bottles | 25 to 50 mg each injection | Every 2 weeks | Gluteal only | Requires reconstitution, proprietary kit |
Depot administration. Fluphenazine depot is commonly given every 2 weeks, starting with 25 mg, but a weekly or monthly interval is not rare. The dose range is broad because the drug can be given in fine gradations from as low as 2.5 mg (0.1 cc) to 75 mg (3 cc). Thus, you can individually titrate it by varying the dose and/or interval.
Because haloperidol is usually given monthly and thus requires less-frequent dosing, it tends to be used more often than fluphenazine. Haloperidol can be given in shorter intervals but is rarely used at intervals >4 weeks. Usual dosing is 50 to 100 mg per shot but can range from small amounts to hundreds of milligrams.
Transition from oral to IM. Switching from an oral antipsychotic to a long-acting medication is straightforward. As long as test doses or history predetermine that patients have no untoward effects from fluphenazine or haloperidol, the first injection can be given and the oral agent maintained for 3 to 5 days.
Monitor for dystonias and other emergent EPS. Some practitioners pretreat with anticholinergics to avoid these neurologic side effects. If you can monitor the patient over the first week, you can often avoid pretreatment and add side-effect medication as needed.
LONG-ACTING IM RISPERIDONE
For technical and approval reasons, it took nearly a decade for a long-acting atypical to be developed and approved. Because risperidone could not easily be attached to an oil, the solution to making risperidone long-acting was to use microspheres.6
Microspheres are best conceptualized as a solid sphere of dissolvable suture-like material (glycolide-lactide polymers) embedded with risperidone bits. The microspheres are packaged dry and reconstituted at the clinic with aqueous diluent at the time of medication. Once reconstituted, it forms a suspension of microspheres in water.
‘Snow in a snow globe.’ Reconstituted long-acting risperidone appears like snow in a snow globe. With shaking, the microspheres become suspended but quickly settle in the bottle or syringe. Shake to resuspend the microspheres if you are giving an injection more than 2 minutes after the initial reconstitution. Reconstituted microspheres can be given up to 6 hours after hydration.
Transfer the medication to the syringe via the proprietary exchange system, and use the specialized needle to inject the medication into the gluteal region. Once injected, the microspheres swell with water from local muscle, then break down.
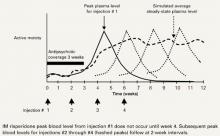
Delayed action. The microspheres begin releasing risperidone in 3 to 4 weeks. Therapeutic levels last approximately 2 weeks until the microspheres gradually convert to carbon dioxide and water. This delayed action requires coverage with oral or other depot medication. Coverage is no longer needed after the medication reaches a steady state (Figure).
Tolerability. As with any long-acting antipsychotic, establish riperidone’s tolerability by history or test dosing. No set number of doses will ensure that a patient won’t have an allergic reaction, but we usually recommend several days of oral dosing before the first injection.
Dosing interval. The approved dosing interval of every 2 weeks should work for most patients. Longer intervals are being studied but are not approved practice.
From oral to IM. Risperidone’s manufacturer recommends at least 3 weeks of coverage by another agent when transitioning from oral to IM to ensure that long-acting risperidone reaches a therapeutic level before being used alone.7 We use even longer coverage—4 to 6 weeks if feasible and acceptable to the patient. We worry more about under-medication than about possible overmedication caused by the overlap. We also consider other factors (Table 2).8
Knowing your patient’s history is critical to ensuring a safe transition. Imagine two patients who are stable on the same antipsychotic dose. When under-medicated during a dose switch, the first recedes into his room and is isolative, whereas the second hits his mother. The first patient will more likely tolerate a quick switch without untoward consequences; the second will need a longer and slower overlap to prevent a recurrence of violent behavior.
Figure Long-acting IM risperidone: Steady-state blood levels by 4th dose
Source: Adapted and reprinted with permission of Robert Lasser from a poster presented at the American Psychiatric Nurses Association annual meeting, Dallas, TX, 2002.Table 2
Switching from another antipsychotic to long-acting IM risperidone
|
DOSING IM RISPERIDONE
Choosing a dose of long-acting risperidone can be difficult. Several points of reference are helpful, but no formula exists. The long-acting form of any antipsychotic behaves differently from its oral counterpart. The maximum daily blood level is lower and the daily trough higher, so that blood levels are governed in a more narrow range. This allows fewer dose- or blood level-related side effects and steadier blood levels.
Dosing equivalents. Long-acting IM risperidone’s preclinical pharmacokinetics data suggested that 25 mg every 2 weeks is equivalent to 2 mg oral per day, 50 mg to 4 mg/d, and 75 mg to 6 mg/d.3 In clinical practice, we find equivalency is broader and represents a range of values. Thus, the 25-mg injection is equivalent to 2 to 4 mg of oral risperidone. Because oral risperidone’s average dose is slightly greater than 4 mg/d, the 25-mg injection should work for most patients.
Side effects. With IM risperidone, the maximum blood level is approximately 30% lower than with the oral dose, so dose-related EPS and prolactin elevation may be less than would be expected for the oral dose range.9 Individual sensitivities do exist, however. We have had some patients experience EPS at low doses and many others not experience EPS at high doses.
Recommendations. If evidence suggests that the patient might be stable on oral doses of 2 to 4 mg/d, start with the 25-mg, every 2-week injection. A recent study in which patients switched directly to long-acting risperidone, without intervening oral risperidone, supports this approach.10
Monitor the patient’s symptoms, being aware that the medication does not begin to take effect until 3 weeks after the first injection. That implies that the second injection is given before you know the efficacy of the first.
We try to maintain the patient on this regimen for several weeks, using oral supplementation if necessary and practical. If the patient requires supplementation throughout the four-injection, 2-month trial, we then:
- increase the dose to 37.5 mg
- repeat the 2-month trial with supplementation as necessary
- and, again, if the patient requires supplementation for the entire 2 months, increase to the 50-mg dose.
This plan requires patience by both clinician and patient but reduces overmedication.
SPECIAL CIRCUMSTANCES
Missed doses. Patients who are stable on a long-acting IM risperidone dose should be maintained indefinitely. As we have all experienced, however, indefinitely is rarely forever. Patients may need periodic dose adjustments or miss doses for a variety of reasons.
We find that patients who are up to a week overdue for an injection are still stable. When a patient arrives more than a week late, check for symptom worsening to determine if you need to supplement the injection with oral medication. If the patient has missed several months, you probably need to restart the initial process.
Inpatient use. Long-acting IM risperidone has been studied in hospitalized patients.11 Getting approval for inpatient use may be difficult, however, if inpatient services and the outpatient pharmacy are on separate budgets.
Though IM risperidone may not take effect until several weeks after a patient’s discharge, starting inpatient treatment may be appropriate. At our institution, we developed tenets for reviewing each case (Table 3).
Before we give any injection, we require that the system for outpatient injections—including place and payment source—be in place. Without these precautions, you may find you are unable to give the next injection at the proper interval.
Table 3
Tenets for using long-acting IM risperidone for inpatients
| If the patient… | Then… |
|---|---|
| is receiving IM risperidone on admission and you decide to continue it | give the dose at the appropriate time if patient will not be discharged within 3 days of the injection appointment |
| is to be started on IM risperidone and to be discharged in <3 days | start treatment as an outpatient |
| is committed to the hospital for >3 weeks | start IM risperidone in the hospital |
| is admitted to be started on IM risperidone because starting as an outpatient has failed | start IM risperidone in the hospital |
Related resources
- Keith SJ, Pani L, Nick B, et al. Practical application of pharmacotherapy with long-acting risperidone for patients with schizophrenia. Psychiatr Serv. 2004;55(9):997-1005.
- Risperdal Consta. Risperidone long-acting injection. Janssen Pharmaceutica. Available at www.risperdalconsta.com. Accessed Feb. 25, 2005.
Drug brand names
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Risperidone (long-acting) • Risperdal Consta
Disclosure
Dr. Lauriello receives grant/research support from AstraZeneca Pharmaceuticals, Janssen Pharmaceutica, and Eli Lilly and Co. He is a consultant to or speaker for Eli Lilly and Co., Janssen Pharmaceutica, Pfizer Inc., and Bristol-Myers Squibb Co.
Dr. Keith is a consultant to or speaker for Bristol-Myers Squibb Co., Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., and Pfizer Inc.
1. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry 2003;64(11):1308-15.
2. Dolder CR, Lacro JP, Dunn LB, et al. Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry 2002;159(1):103-8.
3. Kane JM, Eerdekens M, Lindenmayer JP, et al. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry 2003;160(6):1125-32.
4. Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 2002;346(1):16-22.
5. Tandon R, Jibson MD. Extrapyramidal side effects of antipsychotic treatment: scope of problem and impact on outcome. Ann Clin Psychiatry 2002;14(2):123-9.
6. Ramstack M, Grandolfi G, Mannaert E, et al. Long-acting risperidone: prolonged-release injectable delivery of risperidone using medisorb microsphere technology. Biol Psychiatry 2003;53(suppl 89):204.-
7. Janssen-Cilag Ltd. Product information for Risperdal Consta. Available at: http://www.janssencilag.co.uk (go to products page). Accessed February 3, 2005.
8. Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry 2003;64(10):1250-7.
9. Eerdekens M, Van Hove I, Remmerie B, Mannaert E. Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res 2004;70(1):91-100.
10. Lindenmayer JP, Eerdekens E, Berry SA, Eerdekens M. Safety and efficacy of long-acting risperidone in schizophrenia: a 12-week, multicenter, open-label study in stable patients switched from typical and atypical oral antipsychotics. J Clin Psychiatry 2004;65(8):1084-9.
11. Lauriello J, McEvoy JP, Rodriguez S, et al. Long-acting risperidone vs. placebo in the treatment of hospital inpatients with schizophrenia. Schizophr Res 2005;72(2-3):249-58.
Knowing how to use IM risperidone—and other long-acting atypicals that are likely to be approved—will enable you to help your patients benefit from reliable antipsychotic dosing. Long-acting antipsychotics address the challenge that makes schizophrenia particularly difficult to treat: medication nonadherence because of psychotic illness’ effect on insight, reality testing, and motivation.1,2
Too few schizophrenia patients in the United States—perhaps <5% of appropriate candidates—receive depot antipsychotics.1 We believe these agents provide the best delivery system to our patients and welcome IM risperidone’s approval3
This article shares what we have learned from research and clinical practice about using injectable antipsychotics, with a focus on how to effectively use long-acting IM risperidone.
CONVENTIONAL ANTIPSYCHOTICS
Once seen as an improvement over oral conventional antipsychotics, IM agents were relegated over time to a means of coercion (as in, “If you don’t take your medicine orally, we’ll force you to take a shot.”). Oral atypical antipsychotics, with improved side-effect profiles and possibly reduced relapse risk, also discouraged psychiatrists from using long-acting conventional antipsychotics as first-line medication.4
Available agents. Fluphenazine and haloperidol—the two long-acting conventional antipsychotics available in the United States (Table 1)—are esterified to a fatty acid (oil) to create an IM injectable prodrug. They can be given in gluteal or deltoid injection, although doses >2 cc should be given in the gluteus.
Hydrolysis releases the active drug, usually within 3 days. This interval allows loading doses to reach therapeutic blood levels rapidly when the goal is to stabilize patients in the hospital or during a short-term crisis stay. Disadvantages include:
- pain and lasting reactions at the injection site
- risk of extrapyramidal symptoms (EPS), neuroleptic malignant syndrome, and tardive dyskinesia.5
Table 1
Administering long-acting injectable antipsychotics
| Name | Preparation | Dose range | Interval | Injection site | Comment |
|---|---|---|---|---|---|
| Fluphenazine | 25 mg/mL | 5 to 75 mg each injection | Every 1 to 2 weeks | Deltoid or gluteal | Site reaction common |
| Haloperidol | 50 or 100 mg/mL | 25 to 200 mg each injection | Every 2 to 4 weeks | Deltoid or gluteal | Site reaction common |
| Risperidone | 25, 37.5, or 50 mg in prefilled bottles | 25 to 50 mg each injection | Every 2 weeks | Gluteal only | Requires reconstitution, proprietary kit |
Depot administration. Fluphenazine depot is commonly given every 2 weeks, starting with 25 mg, but a weekly or monthly interval is not rare. The dose range is broad because the drug can be given in fine gradations from as low as 2.5 mg (0.1 cc) to 75 mg (3 cc). Thus, you can individually titrate it by varying the dose and/or interval.
Because haloperidol is usually given monthly and thus requires less-frequent dosing, it tends to be used more often than fluphenazine. Haloperidol can be given in shorter intervals but is rarely used at intervals >4 weeks. Usual dosing is 50 to 100 mg per shot but can range from small amounts to hundreds of milligrams.
Transition from oral to IM. Switching from an oral antipsychotic to a long-acting medication is straightforward. As long as test doses or history predetermine that patients have no untoward effects from fluphenazine or haloperidol, the first injection can be given and the oral agent maintained for 3 to 5 days.
Monitor for dystonias and other emergent EPS. Some practitioners pretreat with anticholinergics to avoid these neurologic side effects. If you can monitor the patient over the first week, you can often avoid pretreatment and add side-effect medication as needed.
LONG-ACTING IM RISPERIDONE
For technical and approval reasons, it took nearly a decade for a long-acting atypical to be developed and approved. Because risperidone could not easily be attached to an oil, the solution to making risperidone long-acting was to use microspheres.6
Microspheres are best conceptualized as a solid sphere of dissolvable suture-like material (glycolide-lactide polymers) embedded with risperidone bits. The microspheres are packaged dry and reconstituted at the clinic with aqueous diluent at the time of medication. Once reconstituted, it forms a suspension of microspheres in water.
‘Snow in a snow globe.’ Reconstituted long-acting risperidone appears like snow in a snow globe. With shaking, the microspheres become suspended but quickly settle in the bottle or syringe. Shake to resuspend the microspheres if you are giving an injection more than 2 minutes after the initial reconstitution. Reconstituted microspheres can be given up to 6 hours after hydration.
Transfer the medication to the syringe via the proprietary exchange system, and use the specialized needle to inject the medication into the gluteal region. Once injected, the microspheres swell with water from local muscle, then break down.
Delayed action. The microspheres begin releasing risperidone in 3 to 4 weeks. Therapeutic levels last approximately 2 weeks until the microspheres gradually convert to carbon dioxide and water. This delayed action requires coverage with oral or other depot medication. Coverage is no longer needed after the medication reaches a steady state (Figure).
Tolerability. As with any long-acting antipsychotic, establish riperidone’s tolerability by history or test dosing. No set number of doses will ensure that a patient won’t have an allergic reaction, but we usually recommend several days of oral dosing before the first injection.
Dosing interval. The approved dosing interval of every 2 weeks should work for most patients. Longer intervals are being studied but are not approved practice.
From oral to IM. Risperidone’s manufacturer recommends at least 3 weeks of coverage by another agent when transitioning from oral to IM to ensure that long-acting risperidone reaches a therapeutic level before being used alone.7 We use even longer coverage—4 to 6 weeks if feasible and acceptable to the patient. We worry more about under-medication than about possible overmedication caused by the overlap. We also consider other factors (Table 2).8
Knowing your patient’s history is critical to ensuring a safe transition. Imagine two patients who are stable on the same antipsychotic dose. When under-medicated during a dose switch, the first recedes into his room and is isolative, whereas the second hits his mother. The first patient will more likely tolerate a quick switch without untoward consequences; the second will need a longer and slower overlap to prevent a recurrence of violent behavior.
Figure Long-acting IM risperidone: Steady-state blood levels by 4th dose
Source: Adapted and reprinted with permission of Robert Lasser from a poster presented at the American Psychiatric Nurses Association annual meeting, Dallas, TX, 2002.Table 2
Switching from another antipsychotic to long-acting IM risperidone
|
DOSING IM RISPERIDONE
Choosing a dose of long-acting risperidone can be difficult. Several points of reference are helpful, but no formula exists. The long-acting form of any antipsychotic behaves differently from its oral counterpart. The maximum daily blood level is lower and the daily trough higher, so that blood levels are governed in a more narrow range. This allows fewer dose- or blood level-related side effects and steadier blood levels.
Dosing equivalents. Long-acting IM risperidone’s preclinical pharmacokinetics data suggested that 25 mg every 2 weeks is equivalent to 2 mg oral per day, 50 mg to 4 mg/d, and 75 mg to 6 mg/d.3 In clinical practice, we find equivalency is broader and represents a range of values. Thus, the 25-mg injection is equivalent to 2 to 4 mg of oral risperidone. Because oral risperidone’s average dose is slightly greater than 4 mg/d, the 25-mg injection should work for most patients.
Side effects. With IM risperidone, the maximum blood level is approximately 30% lower than with the oral dose, so dose-related EPS and prolactin elevation may be less than would be expected for the oral dose range.9 Individual sensitivities do exist, however. We have had some patients experience EPS at low doses and many others not experience EPS at high doses.
Recommendations. If evidence suggests that the patient might be stable on oral doses of 2 to 4 mg/d, start with the 25-mg, every 2-week injection. A recent study in which patients switched directly to long-acting risperidone, without intervening oral risperidone, supports this approach.10
Monitor the patient’s symptoms, being aware that the medication does not begin to take effect until 3 weeks after the first injection. That implies that the second injection is given before you know the efficacy of the first.
We try to maintain the patient on this regimen for several weeks, using oral supplementation if necessary and practical. If the patient requires supplementation throughout the four-injection, 2-month trial, we then:
- increase the dose to 37.5 mg
- repeat the 2-month trial with supplementation as necessary
- and, again, if the patient requires supplementation for the entire 2 months, increase to the 50-mg dose.
This plan requires patience by both clinician and patient but reduces overmedication.
SPECIAL CIRCUMSTANCES
Missed doses. Patients who are stable on a long-acting IM risperidone dose should be maintained indefinitely. As we have all experienced, however, indefinitely is rarely forever. Patients may need periodic dose adjustments or miss doses for a variety of reasons.
We find that patients who are up to a week overdue for an injection are still stable. When a patient arrives more than a week late, check for symptom worsening to determine if you need to supplement the injection with oral medication. If the patient has missed several months, you probably need to restart the initial process.
Inpatient use. Long-acting IM risperidone has been studied in hospitalized patients.11 Getting approval for inpatient use may be difficult, however, if inpatient services and the outpatient pharmacy are on separate budgets.
Though IM risperidone may not take effect until several weeks after a patient’s discharge, starting inpatient treatment may be appropriate. At our institution, we developed tenets for reviewing each case (Table 3).
Before we give any injection, we require that the system for outpatient injections—including place and payment source—be in place. Without these precautions, you may find you are unable to give the next injection at the proper interval.
Table 3
Tenets for using long-acting IM risperidone for inpatients
| If the patient… | Then… |
|---|---|
| is receiving IM risperidone on admission and you decide to continue it | give the dose at the appropriate time if patient will not be discharged within 3 days of the injection appointment |
| is to be started on IM risperidone and to be discharged in <3 days | start treatment as an outpatient |
| is committed to the hospital for >3 weeks | start IM risperidone in the hospital |
| is admitted to be started on IM risperidone because starting as an outpatient has failed | start IM risperidone in the hospital |
Related resources
- Keith SJ, Pani L, Nick B, et al. Practical application of pharmacotherapy with long-acting risperidone for patients with schizophrenia. Psychiatr Serv. 2004;55(9):997-1005.
- Risperdal Consta. Risperidone long-acting injection. Janssen Pharmaceutica. Available at www.risperdalconsta.com. Accessed Feb. 25, 2005.
Drug brand names
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Risperidone (long-acting) • Risperdal Consta
Disclosure
Dr. Lauriello receives grant/research support from AstraZeneca Pharmaceuticals, Janssen Pharmaceutica, and Eli Lilly and Co. He is a consultant to or speaker for Eli Lilly and Co., Janssen Pharmaceutica, Pfizer Inc., and Bristol-Myers Squibb Co.
Dr. Keith is a consultant to or speaker for Bristol-Myers Squibb Co., Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., and Pfizer Inc.
Knowing how to use IM risperidone—and other long-acting atypicals that are likely to be approved—will enable you to help your patients benefit from reliable antipsychotic dosing. Long-acting antipsychotics address the challenge that makes schizophrenia particularly difficult to treat: medication nonadherence because of psychotic illness’ effect on insight, reality testing, and motivation.1,2
Too few schizophrenia patients in the United States—perhaps <5% of appropriate candidates—receive depot antipsychotics.1 We believe these agents provide the best delivery system to our patients and welcome IM risperidone’s approval3
This article shares what we have learned from research and clinical practice about using injectable antipsychotics, with a focus on how to effectively use long-acting IM risperidone.
CONVENTIONAL ANTIPSYCHOTICS
Once seen as an improvement over oral conventional antipsychotics, IM agents were relegated over time to a means of coercion (as in, “If you don’t take your medicine orally, we’ll force you to take a shot.”). Oral atypical antipsychotics, with improved side-effect profiles and possibly reduced relapse risk, also discouraged psychiatrists from using long-acting conventional antipsychotics as first-line medication.4
Available agents. Fluphenazine and haloperidol—the two long-acting conventional antipsychotics available in the United States (Table 1)—are esterified to a fatty acid (oil) to create an IM injectable prodrug. They can be given in gluteal or deltoid injection, although doses >2 cc should be given in the gluteus.
Hydrolysis releases the active drug, usually within 3 days. This interval allows loading doses to reach therapeutic blood levels rapidly when the goal is to stabilize patients in the hospital or during a short-term crisis stay. Disadvantages include:
- pain and lasting reactions at the injection site
- risk of extrapyramidal symptoms (EPS), neuroleptic malignant syndrome, and tardive dyskinesia.5
Table 1
Administering long-acting injectable antipsychotics
| Name | Preparation | Dose range | Interval | Injection site | Comment |
|---|---|---|---|---|---|
| Fluphenazine | 25 mg/mL | 5 to 75 mg each injection | Every 1 to 2 weeks | Deltoid or gluteal | Site reaction common |
| Haloperidol | 50 or 100 mg/mL | 25 to 200 mg each injection | Every 2 to 4 weeks | Deltoid or gluteal | Site reaction common |
| Risperidone | 25, 37.5, or 50 mg in prefilled bottles | 25 to 50 mg each injection | Every 2 weeks | Gluteal only | Requires reconstitution, proprietary kit |
Depot administration. Fluphenazine depot is commonly given every 2 weeks, starting with 25 mg, but a weekly or monthly interval is not rare. The dose range is broad because the drug can be given in fine gradations from as low as 2.5 mg (0.1 cc) to 75 mg (3 cc). Thus, you can individually titrate it by varying the dose and/or interval.
Because haloperidol is usually given monthly and thus requires less-frequent dosing, it tends to be used more often than fluphenazine. Haloperidol can be given in shorter intervals but is rarely used at intervals >4 weeks. Usual dosing is 50 to 100 mg per shot but can range from small amounts to hundreds of milligrams.
Transition from oral to IM. Switching from an oral antipsychotic to a long-acting medication is straightforward. As long as test doses or history predetermine that patients have no untoward effects from fluphenazine or haloperidol, the first injection can be given and the oral agent maintained for 3 to 5 days.
Monitor for dystonias and other emergent EPS. Some practitioners pretreat with anticholinergics to avoid these neurologic side effects. If you can monitor the patient over the first week, you can often avoid pretreatment and add side-effect medication as needed.
LONG-ACTING IM RISPERIDONE
For technical and approval reasons, it took nearly a decade for a long-acting atypical to be developed and approved. Because risperidone could not easily be attached to an oil, the solution to making risperidone long-acting was to use microspheres.6
Microspheres are best conceptualized as a solid sphere of dissolvable suture-like material (glycolide-lactide polymers) embedded with risperidone bits. The microspheres are packaged dry and reconstituted at the clinic with aqueous diluent at the time of medication. Once reconstituted, it forms a suspension of microspheres in water.
‘Snow in a snow globe.’ Reconstituted long-acting risperidone appears like snow in a snow globe. With shaking, the microspheres become suspended but quickly settle in the bottle or syringe. Shake to resuspend the microspheres if you are giving an injection more than 2 minutes after the initial reconstitution. Reconstituted microspheres can be given up to 6 hours after hydration.
Transfer the medication to the syringe via the proprietary exchange system, and use the specialized needle to inject the medication into the gluteal region. Once injected, the microspheres swell with water from local muscle, then break down.
Delayed action. The microspheres begin releasing risperidone in 3 to 4 weeks. Therapeutic levels last approximately 2 weeks until the microspheres gradually convert to carbon dioxide and water. This delayed action requires coverage with oral or other depot medication. Coverage is no longer needed after the medication reaches a steady state (Figure).
Tolerability. As with any long-acting antipsychotic, establish riperidone’s tolerability by history or test dosing. No set number of doses will ensure that a patient won’t have an allergic reaction, but we usually recommend several days of oral dosing before the first injection.
Dosing interval. The approved dosing interval of every 2 weeks should work for most patients. Longer intervals are being studied but are not approved practice.
From oral to IM. Risperidone’s manufacturer recommends at least 3 weeks of coverage by another agent when transitioning from oral to IM to ensure that long-acting risperidone reaches a therapeutic level before being used alone.7 We use even longer coverage—4 to 6 weeks if feasible and acceptable to the patient. We worry more about under-medication than about possible overmedication caused by the overlap. We also consider other factors (Table 2).8
Knowing your patient’s history is critical to ensuring a safe transition. Imagine two patients who are stable on the same antipsychotic dose. When under-medicated during a dose switch, the first recedes into his room and is isolative, whereas the second hits his mother. The first patient will more likely tolerate a quick switch without untoward consequences; the second will need a longer and slower overlap to prevent a recurrence of violent behavior.
Figure Long-acting IM risperidone: Steady-state blood levels by 4th dose
Source: Adapted and reprinted with permission of Robert Lasser from a poster presented at the American Psychiatric Nurses Association annual meeting, Dallas, TX, 2002.Table 2
Switching from another antipsychotic to long-acting IM risperidone
|
DOSING IM RISPERIDONE
Choosing a dose of long-acting risperidone can be difficult. Several points of reference are helpful, but no formula exists. The long-acting form of any antipsychotic behaves differently from its oral counterpart. The maximum daily blood level is lower and the daily trough higher, so that blood levels are governed in a more narrow range. This allows fewer dose- or blood level-related side effects and steadier blood levels.
Dosing equivalents. Long-acting IM risperidone’s preclinical pharmacokinetics data suggested that 25 mg every 2 weeks is equivalent to 2 mg oral per day, 50 mg to 4 mg/d, and 75 mg to 6 mg/d.3 In clinical practice, we find equivalency is broader and represents a range of values. Thus, the 25-mg injection is equivalent to 2 to 4 mg of oral risperidone. Because oral risperidone’s average dose is slightly greater than 4 mg/d, the 25-mg injection should work for most patients.
Side effects. With IM risperidone, the maximum blood level is approximately 30% lower than with the oral dose, so dose-related EPS and prolactin elevation may be less than would be expected for the oral dose range.9 Individual sensitivities do exist, however. We have had some patients experience EPS at low doses and many others not experience EPS at high doses.
Recommendations. If evidence suggests that the patient might be stable on oral doses of 2 to 4 mg/d, start with the 25-mg, every 2-week injection. A recent study in which patients switched directly to long-acting risperidone, without intervening oral risperidone, supports this approach.10
Monitor the patient’s symptoms, being aware that the medication does not begin to take effect until 3 weeks after the first injection. That implies that the second injection is given before you know the efficacy of the first.
We try to maintain the patient on this regimen for several weeks, using oral supplementation if necessary and practical. If the patient requires supplementation throughout the four-injection, 2-month trial, we then:
- increase the dose to 37.5 mg
- repeat the 2-month trial with supplementation as necessary
- and, again, if the patient requires supplementation for the entire 2 months, increase to the 50-mg dose.
This plan requires patience by both clinician and patient but reduces overmedication.
SPECIAL CIRCUMSTANCES
Missed doses. Patients who are stable on a long-acting IM risperidone dose should be maintained indefinitely. As we have all experienced, however, indefinitely is rarely forever. Patients may need periodic dose adjustments or miss doses for a variety of reasons.
We find that patients who are up to a week overdue for an injection are still stable. When a patient arrives more than a week late, check for symptom worsening to determine if you need to supplement the injection with oral medication. If the patient has missed several months, you probably need to restart the initial process.
Inpatient use. Long-acting IM risperidone has been studied in hospitalized patients.11 Getting approval for inpatient use may be difficult, however, if inpatient services and the outpatient pharmacy are on separate budgets.
Though IM risperidone may not take effect until several weeks after a patient’s discharge, starting inpatient treatment may be appropriate. At our institution, we developed tenets for reviewing each case (Table 3).
Before we give any injection, we require that the system for outpatient injections—including place and payment source—be in place. Without these precautions, you may find you are unable to give the next injection at the proper interval.
Table 3
Tenets for using long-acting IM risperidone for inpatients
| If the patient… | Then… |
|---|---|
| is receiving IM risperidone on admission and you decide to continue it | give the dose at the appropriate time if patient will not be discharged within 3 days of the injection appointment |
| is to be started on IM risperidone and to be discharged in <3 days | start treatment as an outpatient |
| is committed to the hospital for >3 weeks | start IM risperidone in the hospital |
| is admitted to be started on IM risperidone because starting as an outpatient has failed | start IM risperidone in the hospital |
Related resources
- Keith SJ, Pani L, Nick B, et al. Practical application of pharmacotherapy with long-acting risperidone for patients with schizophrenia. Psychiatr Serv. 2004;55(9):997-1005.
- Risperdal Consta. Risperidone long-acting injection. Janssen Pharmaceutica. Available at www.risperdalconsta.com. Accessed Feb. 25, 2005.
Drug brand names
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Risperidone (long-acting) • Risperdal Consta
Disclosure
Dr. Lauriello receives grant/research support from AstraZeneca Pharmaceuticals, Janssen Pharmaceutica, and Eli Lilly and Co. He is a consultant to or speaker for Eli Lilly and Co., Janssen Pharmaceutica, Pfizer Inc., and Bristol-Myers Squibb Co.
Dr. Keith is a consultant to or speaker for Bristol-Myers Squibb Co., Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., and Pfizer Inc.
1. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry 2003;64(11):1308-15.
2. Dolder CR, Lacro JP, Dunn LB, et al. Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry 2002;159(1):103-8.
3. Kane JM, Eerdekens M, Lindenmayer JP, et al. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry 2003;160(6):1125-32.
4. Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 2002;346(1):16-22.
5. Tandon R, Jibson MD. Extrapyramidal side effects of antipsychotic treatment: scope of problem and impact on outcome. Ann Clin Psychiatry 2002;14(2):123-9.
6. Ramstack M, Grandolfi G, Mannaert E, et al. Long-acting risperidone: prolonged-release injectable delivery of risperidone using medisorb microsphere technology. Biol Psychiatry 2003;53(suppl 89):204.-
7. Janssen-Cilag Ltd. Product information for Risperdal Consta. Available at: http://www.janssencilag.co.uk (go to products page). Accessed February 3, 2005.
8. Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry 2003;64(10):1250-7.
9. Eerdekens M, Van Hove I, Remmerie B, Mannaert E. Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res 2004;70(1):91-100.
10. Lindenmayer JP, Eerdekens E, Berry SA, Eerdekens M. Safety and efficacy of long-acting risperidone in schizophrenia: a 12-week, multicenter, open-label study in stable patients switched from typical and atypical oral antipsychotics. J Clin Psychiatry 2004;65(8):1084-9.
11. Lauriello J, McEvoy JP, Rodriguez S, et al. Long-acting risperidone vs. placebo in the treatment of hospital inpatients with schizophrenia. Schizophr Res 2005;72(2-3):249-58.
1. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry 2003;64(11):1308-15.
2. Dolder CR, Lacro JP, Dunn LB, et al. Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry 2002;159(1):103-8.
3. Kane JM, Eerdekens M, Lindenmayer JP, et al. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry 2003;160(6):1125-32.
4. Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 2002;346(1):16-22.
5. Tandon R, Jibson MD. Extrapyramidal side effects of antipsychotic treatment: scope of problem and impact on outcome. Ann Clin Psychiatry 2002;14(2):123-9.
6. Ramstack M, Grandolfi G, Mannaert E, et al. Long-acting risperidone: prolonged-release injectable delivery of risperidone using medisorb microsphere technology. Biol Psychiatry 2003;53(suppl 89):204.-
7. Janssen-Cilag Ltd. Product information for Risperdal Consta. Available at: http://www.janssencilag.co.uk (go to products page). Accessed February 3, 2005.
8. Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry 2003;64(10):1250-7.
9. Eerdekens M, Van Hove I, Remmerie B, Mannaert E. Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res 2004;70(1):91-100.
10. Lindenmayer JP, Eerdekens E, Berry SA, Eerdekens M. Safety and efficacy of long-acting risperidone in schizophrenia: a 12-week, multicenter, open-label study in stable patients switched from typical and atypical oral antipsychotics. J Clin Psychiatry 2004;65(8):1084-9.
11. Lauriello J, McEvoy JP, Rodriguez S, et al. Long-acting risperidone vs. placebo in the treatment of hospital inpatients with schizophrenia. Schizophr Res 2005;72(2-3):249-58.
What’s the best treatment for comorbid ADHD/bipolar mania?
Comorbid attention-deficit/hyperactivity disorder (ADHD) is nearly universal in youths with bipolar disorder (BPD),1 and comorbid mania has been noted in 16% of children with ADHD.2 Choosing medication for these complex patients is difficult because psychostimulants may worsen mania and mood stabilizers may not resolve ADHD symptoms. Yet, very little information exists on combining psychostimulants with mood stabilizers or atypical antipsychotics.
This article offers evidence to help you decide:
- which to treat first—ADHD or BPD
- how to individualize combination therapy.
CHALLENGES OF COMORBIDITY
Differential diagnosis. ADHD and bipolar disorder (BPD) symptoms overlap, and experts disagree on which symptoms indicate co-existing ADHD and BPD. Multiple daily mood swings and irritability are commonly found in prepubertal BPD.3 Recent reviews address differential diagnosis and specific assessment tools;3-5 after careful evaluation, then focus on treatment.
Treating comorbid ADHD and BPD usually requires more than one medication, and use of multiple drugs in children and adolescents is becoming increasingly common.6,7
PSYCHOSTIMULANTS AND MOOD STABILIZERS
Small, uncontrolled studies of children and adolescents with comorbid ADHD and BPD have shown that treatment with a mood stabilizer and a psychostimulant can control both sets of symptoms. For example:
- Lithium (serum levels 0.7 to 1.1 mEq/L) plus methylphenidate (10 to 20 mg/d) improved attention and hyperactivity symptoms more effectively than either agent alone in 7 children (6 boys, 1 girl) ages 6 to 10 hospitalized with disruptive behavioral disorders and BPD or major depression.8
- A retrospective analysis of 38 children (ages 3 to 16; 84% male) with BPD found that ADHD symptoms were 7.5 times more likely to improve if mood was stabilized before rather than after ADHD treatment with tricyclic antidepressants.9
The efficacy of combining a mood stabilizer and psychostimulant has been confirmed by only one controlled study—a randomized, placebo-controlled trial of mixed amphetamine salts in divalproex-treated patients.10 Forty patients (ages 6 to 17; 83% male) with BPD and ADHD received open-label divalproex (median dosage 750 mg/d) for 8 weeks. Thirty patients whose manic symptoms were significantly reduced entered a 4-week, double-blind, crossover trial of mixed amphetamine salts, 10 mg/d, or placebo.
Following this double-blind phase, 23 patients received open-label divalproex plus mixed amphetamine salts for 12 weeks. The Young Mania Rating Scale and Clinical Global Impression-Improvement scale were used to assess manic and ADHD symptoms during all three study phases.
Manic symptoms in patients treated with divalproex monotherapy improved significantly, but ADHD symptoms did not. ADHD symptoms improved more with divalproex plus mixed amphetamine salts than with divalproex plus placebo. One patient experienced manic symptom exacerbation with combination therapy.
PSYCHOSTIMULANTS AND ANTIPSYCHOTICS
Combinations of psychostimulants and atypical antipsychotics are commonly used in children and adolescents with comorbid psychiatric and behavioral disorders, such as ADHD and disruptive behavioral disorders (oppositional defiant disorder, conduct disorder). In 78 children ages 5 to 12 (83% male) with comorbid ADHD and a disruptive behavioral disorder, disruptive behavior and hyperactivity improved significantly with risperidone alone or with a psychostimulant.11
Combined psychostimulant/atypical antipsychotic therapy may help youths with comorbid ADHD and Tourette syndrome. Methylphenidate can reduce ADHD symptoms without exacerbating tics,12 and risperidone can treat tic disorders, even in patients with comorbid ADHD.13,14 No controlled trials have examined psychostimulant and atypical antipsychotic combinations in these patients, however.
Atypical antipsychotics have been shown to be effective in treating adult BPD, and limited data suggest the same to be true in pediatric patients. Olanzapine, quetiapine, and risperidone have been shown to reduce manic symptoms in children and adolescents (Table 1).15-17 Atypical antipsychotics, however, have been associated with metabolic side effects, including weight gain, hyperglycemia, hyperlipidemia, and hyperprolactinemia.
To date, no study has systematically evaluated combination psychostimulant and atypical antipsychotic treatment in comorbid ADHD and BPD. In the olanzapine and risperidone studies,15,17 concomitant psychostimulant use was permitted and did not affect manic symptom response.
Table 1
Atypical antipsychotic studies in pediatric bipolar disorder
| Drug and mean dosage | Study design | Sample characteristics | Efficacy measures | Results |
|---|---|---|---|---|
| Olanzapine15 9.6±4.3 mg/d | 8-week, open-label monotherapy | 23 patients, mean age 10±3 yrs, 57% male | ≥30% decrease on YMRS | Response rate 61% |
| Quetiapine16 432 mg/d | 6-week, randomized, placebo-controlled, adjunctive (+DVP) | 30 patients, mean age 14±2 yrs, 53% male | ≥50% decrease on YMRS | Response rates: DVP + placebo 53% DVP + quetiapine 87% |
| Risperidone17 1.7±1.3 mg/d | Retrospective, adjunctive | 28 patients, mean age 10±4 yrs, 97% male | ≤2 on CGI-I | Response rate 82% |
| CGI-I: Clinical Global Impressions-Improvement scale | ||||
| DVP: divalproex | ||||
| YMRS: Young Mania Rating Scale | ||||
WHICH COMBINATION?
Which combination treatment—psychostimulant plus mood stabilizer, psychostimulant plus atypical antipsychotic, or psychostimulant plus both mood stabilizer and atypical antipsychotic—is most appropriate for a child or adolescent with comorbid ADHD and BPD? Recommended treatment strategies are based on studies of pediatric and adult BPD and expert consensus.18,19
Consider the type of bipolar episode (Table 2).
For initial treatment of youths with BPD manic or mixed without psychosis, recent guidelines by Kowatch et al suggest using mood-stabilizer or atypical antipsychotic monotherapy. Youths who are more severely ill or present with psychosis may respond more favorably to a mood stabilizer plus an atypical antipsychotic.16,19
Individual patient traits will also determine whether a mood stabilizer or atypical antipsychotic is used and which agent within either medication class is chosen. For example:
- If the patient is aggressive, risperidone may reduce aggression and manic symptoms. Among the atypicals, risperidone has the most evidence suggesting efficacy for aggressive behaviors in youths across psychiatric conditions.20
- If an atypical antipsychotic is warranted and the patient’s weight is an issue, ziprasidone or aripiprazole would be preferred. These agents are considered weight-neutral compared with other atypicals.20
Other factors to consider include medication side effects, interactions, adherence, and cost.
Table 2
Mood stabilizer, atypical antipsychotic, or both with ADHD therapy?
| Type of bipolar episode | Recommended psychotropics |
|---|---|
| Manic or mixed episode with psychosis | Mood stabilizer + atypical antipsychotic |
| Manic or mixed episode without psychosis | Mood stabilizer or atypical antipsychotic monotherapy first
|
| Prominent irritability without psychosis | Atypical antipsychotic |
| Source: Adapted from references 18 and 19 | |
WHICH TO TREAT FIRST?
If the child or adolescent with comorbid ADHD and BPD has acute manic symptoms, available data and expert opinion recommend starting treatment with a mood stabilizer or atypical antipsychotic.9,19,21 If ADHD symptoms persist after mood stabilization, a psychostimulant trial is warranted.
In practice, however, youngsters usually present with ADHD symptoms first. Psychostimulant treatment is initiated, ADHD symptoms are controlled, and the child’s academic and social functioning improve. Bipolar symptoms emerge later, often heralded by a depressive or mixed episode. Is it necessary to discontinue the psychostimulant and risk worsening ADHD symptoms before starting a mood stabilizer or atypical antipsychotic?
Clinical lore and one case report suggest that psychostimulants may destabilize mood.22,23 A 10-year-old boy with severe hyperactivity and family history of BPD experienced manic symptoms—rapid and pressured speech, grandiose delusions of identity, and tangentiality of thought processes—during methylphenidate treatment.22
Conversely, an analysis24 of children ages 7 to 10 from the National Institute of Mental Health Multimodal Treatment Study of Children with ADHD contradicts these assumptions. Although a clinical diagnosis of BPD was not assigned, 29 children (83% male) met the Diagnostic Interview Schedule for Children proxy for mania, 32 (88% male) met the Child Behavior Checklist proxy, and 7 met both proxies for mania.
The first month of methylphenidate treatment did not increase irritability, mood symptoms, or mania in the 54 children with ADHD and manic symptoms, compared with children with ADHD alone. The authors concluded that clinicians should not categorically avoid using stimulants in children with ADHD and some manic symptoms.
In a study by Pavuluri et al18 of pediatric bipolar type I disorder, 17 patients (mean age 11±4 years) received mood stabilizers—following a drug therapy algorithm that included risperidone—and typically received a psychostimulant after mood stabilization. This group was compared with 17 patients receiving “treatment as usual.”
The usual-treatment group remained on psychostimulant therapy after BPD intervention with a mood stabilizer and was less likely to receive an atypical antipsychotic. The algorithm treatment group showed better outcomes overall, specifically for mania and aggression.
Clearly, more studies are needed to determine the optimum treatment sequence with psychostimulants and mood stabilizers in youths with comorbid ADHD and BPD. With either approach, routinely monitor patients treated with psychostimulants for emerging or worsening bipolar symptoms.
LESSONS FROM CLINICAL EXPERIENCE
Nonstimulants. Using psychostimulants is appropriate for ADHD in patients with stable bipolar symptoms. Evidence for using nonstimulants such as clonidine, guanfacine, or atomoxetine is less clear.
In a naturalistic study of 153 children and adolescent outpatients treated with atomoxetine, 51 (33%) experienced irritability, aggression, mania, or hypomania. Of these patients, 31 (61%) had a family history of a mood disorder, and 41 (80%) had a personal history of mood symptoms.25 Although these findings suggest that atomoxetine may be associated with mood exacerbation and hypomania, additional data are needed to determine whether atomoxetine may be used for ADHD symptoms in youths with comorbid BPD.
Atypical antipsychotics. Mood stabilization—particularly with atypical antipsychotics—often can address comorbid disruptive behaviors and aggressive symptoms. Combinations of atypical antipsychotics with psychostimulants are largely devoid of drug-drug interactions and metabolic interference, making them uncomplicated to use.
Though published studies of pediatric BPD have focused on three atypical antipsychotics—olanzapine, quetiapine, and risperidone—any agent in this class can be used in this population, with the choice often depending on how side effects are likely to affect individual patients (Table 3 ).
Pharmacologic attributes may also determine which atypical antipsychotic is used. For example, ziprasidone’s serotonergic profile—with serotonin-1A receptor agonism and serotonin-1D antagonism—may make it useful for patients with mixed states and bipolar depression.26 Aripiprazole offers potential synergism of dopamine agonism with psychostimulant therapy, which could be useful for treating both disruptive behaviors and ADHD.
Table 3
Using atypical antipsychotics to treat comorbid ADHD/bipolar disorder
| Drug | Target dosage (mg/d) | Side effects | Useful in… |
|---|---|---|---|
| Aripiprazole | 10 to 15 | Nausea, vomiting | Comorbid disruptive behavioral disorders, maintenance stabilization |
| Olanzapine | 10 to 20 | Weight gain, hyperlipidemia, hyperglycemia, sedation | Maintenance stabilization |
| Quetiapine | 400 to 600 | Weight gain, sedation | Mixed states, bipolar depression |
| Risperidone | 1 to 2 | Weight gain, hyperprolactinemia, extrapyramidal symptoms | Comorbid disruptive behavioral disorders, including aggression |
| Ziprasidone | 80 to 120 | Cardiac abnormalities, akathisia | Mixed states, bipolar depression |
Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org
- Children and Adults with Attention-Deficit Hyperactivity Disorder (CHADD). www.chadd.org
- Findling RF, Kowatch RA, Post RM. P ediatric bipolar disorders: a handbook for clinicians. London: Martin Dunitz Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Aripiprazole • Abilify
- Divalproex • Depakote
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Patel is a consultant to Eli Lilly and Co. and a speaker for Eli Lilly and Co. and Pfizer Inc.
Dr. Sallee receives research support from Otsuka America Pharmaceutical, Pfizer Inc., and Bristol-Myers Squibb Co. and is a consultant or speaker for Eli Lilly and Co, Otsuka America Pharmaceutical, and Pfizer Inc.
1. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry 1998;37(2):171-8.
2. Wozniak J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34(7):867-76.
3. Kowatch RA, DelBello MP. Pediatric bipolar disorder: mood swings, irritability are diagnostic cues. Current Psychiatry 2003;2(3):40-7.
4. Quinn CA, Fristad MA. Defining and identifying early onset bipolar spectrum disorder. Curr Psychiatry Rep 2004;6(2):101-7.
5. Bhatara VS, Feil M, Hoagwood K, et al. Trends in combined pharmacotherapy with stimulants for children. Psychiatr Serv 2002;53(3):244.-
6. Zito JM, Safer DJ, dosReis S, et al. Psychotherapeutic medication patterns for youths with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med 1999;153(12):1257-63.
7. Wolf DV, Wagner KD. Bipolar disorder in children and adolescents. CNS Spectr 2003;8(12):954-9.
8. Carlson GA, Rapport MD, Kelly KL, Pataki CS. The effects of methylphenidate and lithium on attention and activity level. J Am Acad Child Adolesc Psychiatry 1992;31(2):262-70.
9. Biederman J, Mick E, Prince J, et al. Systematic chart review of the pharmacologic treatment of comorbid attention deficit hyperactivity disorder in youth with bipolar disorder. J Child Adolesc Psychopharmacol 1999;9(4):247-56.
10. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
11. Aman MG, Binder C, Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behavioral disorders, and subaverage IQ. J Child Adolesc Psychopharmacology 2004;14(2):243-54.
12. Gadow KD, Sverd J, Sprafkin J, et al. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry 1999;56(4):330-6.
13. Gaffney GR, Perry PJ, Lund BC, et al. Risperidone versus clonidine in the treatment of children and adolescents with Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 2002;41(3):330-6.
14. Lombroso PJ, Scahill L, King RA, et al. Risperidone treatment of children and adolescents with chronic tic disorders: a preliminary report. J Am Acad Child Adolesc Psychiatry 1995;34(9):1147-52.
15. Frazier JA, Biederman J, Tohen M, et al. A prospective open-label treatment trial of olanzapine monotherapy in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol 2001;11(3):239-50.
16. DelBello MP, Schwiers ML, Rosenberg HL, Strakowski SM. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry 2002;41(10):1216-23.
17. Frazier JA, Meyer MC, Biederman J, et al. Risperidone treatment for juvenile bipolar disorder: a retrospective chart review. J Am Acad Child Adolesc Psychiatry 1999;38(8):960-5.
18. Pavuluri MN, Henry DB, Devineni B, et al. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2004;43(7):859-67.
19. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44(3):213-35.
20. Findling RL, McNamara NK. Atypical antipsychotics in the treatment of children and adolescents: clinical applications. J Clin Psychiatry 2004;65(suppl 6):30-44.
21. Kowatch RA, Sethuraman G, Hume JH, et al. Combination pharmacotherapy in children and adolescents with bipolar disorder. Biol Psychiatry 2003;53(11):978-84.
22. Koehler-Troy C, Strober M, Malenbaum R. Methylphenidate-induced mania in a prepubertal child. J Clin Psychiatry 1986;47(11):566-7.
23. Craney J, Geller B. Clinical implications of antidepressant and stimulant use on switching from depression to mania in children. J Child Adolesc Psychopharmacol 2003;13(2):201-4.
24. Galanter CA, Carlson GA, Jensen PS, et al. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. J Child Adolesc Psychopharmacol 2003;13(2):123-36.
25. Henderson TA, Hartman K. Aggression, mania, and hypomania induction associated with atomoxetine. Pediatrics 2004;114(3):895-6.
26. Sallee FR, Gilbert DL, Vinks AA, et al. Pharmacodynamics of ziprasidone in children and adolescents: impact on dopamine transmission. J Am Acad Child Adolesc Psychiatry 2003;42(8):902-7.
Comorbid attention-deficit/hyperactivity disorder (ADHD) is nearly universal in youths with bipolar disorder (BPD),1 and comorbid mania has been noted in 16% of children with ADHD.2 Choosing medication for these complex patients is difficult because psychostimulants may worsen mania and mood stabilizers may not resolve ADHD symptoms. Yet, very little information exists on combining psychostimulants with mood stabilizers or atypical antipsychotics.
This article offers evidence to help you decide:
- which to treat first—ADHD or BPD
- how to individualize combination therapy.
CHALLENGES OF COMORBIDITY
Differential diagnosis. ADHD and bipolar disorder (BPD) symptoms overlap, and experts disagree on which symptoms indicate co-existing ADHD and BPD. Multiple daily mood swings and irritability are commonly found in prepubertal BPD.3 Recent reviews address differential diagnosis and specific assessment tools;3-5 after careful evaluation, then focus on treatment.
Treating comorbid ADHD and BPD usually requires more than one medication, and use of multiple drugs in children and adolescents is becoming increasingly common.6,7
PSYCHOSTIMULANTS AND MOOD STABILIZERS
Small, uncontrolled studies of children and adolescents with comorbid ADHD and BPD have shown that treatment with a mood stabilizer and a psychostimulant can control both sets of symptoms. For example:
- Lithium (serum levels 0.7 to 1.1 mEq/L) plus methylphenidate (10 to 20 mg/d) improved attention and hyperactivity symptoms more effectively than either agent alone in 7 children (6 boys, 1 girl) ages 6 to 10 hospitalized with disruptive behavioral disorders and BPD or major depression.8
- A retrospective analysis of 38 children (ages 3 to 16; 84% male) with BPD found that ADHD symptoms were 7.5 times more likely to improve if mood was stabilized before rather than after ADHD treatment with tricyclic antidepressants.9
The efficacy of combining a mood stabilizer and psychostimulant has been confirmed by only one controlled study—a randomized, placebo-controlled trial of mixed amphetamine salts in divalproex-treated patients.10 Forty patients (ages 6 to 17; 83% male) with BPD and ADHD received open-label divalproex (median dosage 750 mg/d) for 8 weeks. Thirty patients whose manic symptoms were significantly reduced entered a 4-week, double-blind, crossover trial of mixed amphetamine salts, 10 mg/d, or placebo.
Following this double-blind phase, 23 patients received open-label divalproex plus mixed amphetamine salts for 12 weeks. The Young Mania Rating Scale and Clinical Global Impression-Improvement scale were used to assess manic and ADHD symptoms during all three study phases.
Manic symptoms in patients treated with divalproex monotherapy improved significantly, but ADHD symptoms did not. ADHD symptoms improved more with divalproex plus mixed amphetamine salts than with divalproex plus placebo. One patient experienced manic symptom exacerbation with combination therapy.
PSYCHOSTIMULANTS AND ANTIPSYCHOTICS
Combinations of psychostimulants and atypical antipsychotics are commonly used in children and adolescents with comorbid psychiatric and behavioral disorders, such as ADHD and disruptive behavioral disorders (oppositional defiant disorder, conduct disorder). In 78 children ages 5 to 12 (83% male) with comorbid ADHD and a disruptive behavioral disorder, disruptive behavior and hyperactivity improved significantly with risperidone alone or with a psychostimulant.11
Combined psychostimulant/atypical antipsychotic therapy may help youths with comorbid ADHD and Tourette syndrome. Methylphenidate can reduce ADHD symptoms without exacerbating tics,12 and risperidone can treat tic disorders, even in patients with comorbid ADHD.13,14 No controlled trials have examined psychostimulant and atypical antipsychotic combinations in these patients, however.
Atypical antipsychotics have been shown to be effective in treating adult BPD, and limited data suggest the same to be true in pediatric patients. Olanzapine, quetiapine, and risperidone have been shown to reduce manic symptoms in children and adolescents (Table 1).15-17 Atypical antipsychotics, however, have been associated with metabolic side effects, including weight gain, hyperglycemia, hyperlipidemia, and hyperprolactinemia.
To date, no study has systematically evaluated combination psychostimulant and atypical antipsychotic treatment in comorbid ADHD and BPD. In the olanzapine and risperidone studies,15,17 concomitant psychostimulant use was permitted and did not affect manic symptom response.
Table 1
Atypical antipsychotic studies in pediatric bipolar disorder
| Drug and mean dosage | Study design | Sample characteristics | Efficacy measures | Results |
|---|---|---|---|---|
| Olanzapine15 9.6±4.3 mg/d | 8-week, open-label monotherapy | 23 patients, mean age 10±3 yrs, 57% male | ≥30% decrease on YMRS | Response rate 61% |
| Quetiapine16 432 mg/d | 6-week, randomized, placebo-controlled, adjunctive (+DVP) | 30 patients, mean age 14±2 yrs, 53% male | ≥50% decrease on YMRS | Response rates: DVP + placebo 53% DVP + quetiapine 87% |
| Risperidone17 1.7±1.3 mg/d | Retrospective, adjunctive | 28 patients, mean age 10±4 yrs, 97% male | ≤2 on CGI-I | Response rate 82% |
| CGI-I: Clinical Global Impressions-Improvement scale | ||||
| DVP: divalproex | ||||
| YMRS: Young Mania Rating Scale | ||||
WHICH COMBINATION?
Which combination treatment—psychostimulant plus mood stabilizer, psychostimulant plus atypical antipsychotic, or psychostimulant plus both mood stabilizer and atypical antipsychotic—is most appropriate for a child or adolescent with comorbid ADHD and BPD? Recommended treatment strategies are based on studies of pediatric and adult BPD and expert consensus.18,19
Consider the type of bipolar episode (Table 2).
For initial treatment of youths with BPD manic or mixed without psychosis, recent guidelines by Kowatch et al suggest using mood-stabilizer or atypical antipsychotic monotherapy. Youths who are more severely ill or present with psychosis may respond more favorably to a mood stabilizer plus an atypical antipsychotic.16,19
Individual patient traits will also determine whether a mood stabilizer or atypical antipsychotic is used and which agent within either medication class is chosen. For example:
- If the patient is aggressive, risperidone may reduce aggression and manic symptoms. Among the atypicals, risperidone has the most evidence suggesting efficacy for aggressive behaviors in youths across psychiatric conditions.20
- If an atypical antipsychotic is warranted and the patient’s weight is an issue, ziprasidone or aripiprazole would be preferred. These agents are considered weight-neutral compared with other atypicals.20
Other factors to consider include medication side effects, interactions, adherence, and cost.
Table 2
Mood stabilizer, atypical antipsychotic, or both with ADHD therapy?
| Type of bipolar episode | Recommended psychotropics |
|---|---|
| Manic or mixed episode with psychosis | Mood stabilizer + atypical antipsychotic |
| Manic or mixed episode without psychosis | Mood stabilizer or atypical antipsychotic monotherapy first
|
| Prominent irritability without psychosis | Atypical antipsychotic |
| Source: Adapted from references 18 and 19 | |
WHICH TO TREAT FIRST?
If the child or adolescent with comorbid ADHD and BPD has acute manic symptoms, available data and expert opinion recommend starting treatment with a mood stabilizer or atypical antipsychotic.9,19,21 If ADHD symptoms persist after mood stabilization, a psychostimulant trial is warranted.
In practice, however, youngsters usually present with ADHD symptoms first. Psychostimulant treatment is initiated, ADHD symptoms are controlled, and the child’s academic and social functioning improve. Bipolar symptoms emerge later, often heralded by a depressive or mixed episode. Is it necessary to discontinue the psychostimulant and risk worsening ADHD symptoms before starting a mood stabilizer or atypical antipsychotic?
Clinical lore and one case report suggest that psychostimulants may destabilize mood.22,23 A 10-year-old boy with severe hyperactivity and family history of BPD experienced manic symptoms—rapid and pressured speech, grandiose delusions of identity, and tangentiality of thought processes—during methylphenidate treatment.22
Conversely, an analysis24 of children ages 7 to 10 from the National Institute of Mental Health Multimodal Treatment Study of Children with ADHD contradicts these assumptions. Although a clinical diagnosis of BPD was not assigned, 29 children (83% male) met the Diagnostic Interview Schedule for Children proxy for mania, 32 (88% male) met the Child Behavior Checklist proxy, and 7 met both proxies for mania.
The first month of methylphenidate treatment did not increase irritability, mood symptoms, or mania in the 54 children with ADHD and manic symptoms, compared with children with ADHD alone. The authors concluded that clinicians should not categorically avoid using stimulants in children with ADHD and some manic symptoms.
In a study by Pavuluri et al18 of pediatric bipolar type I disorder, 17 patients (mean age 11±4 years) received mood stabilizers—following a drug therapy algorithm that included risperidone—and typically received a psychostimulant after mood stabilization. This group was compared with 17 patients receiving “treatment as usual.”
The usual-treatment group remained on psychostimulant therapy after BPD intervention with a mood stabilizer and was less likely to receive an atypical antipsychotic. The algorithm treatment group showed better outcomes overall, specifically for mania and aggression.
Clearly, more studies are needed to determine the optimum treatment sequence with psychostimulants and mood stabilizers in youths with comorbid ADHD and BPD. With either approach, routinely monitor patients treated with psychostimulants for emerging or worsening bipolar symptoms.
LESSONS FROM CLINICAL EXPERIENCE
Nonstimulants. Using psychostimulants is appropriate for ADHD in patients with stable bipolar symptoms. Evidence for using nonstimulants such as clonidine, guanfacine, or atomoxetine is less clear.
In a naturalistic study of 153 children and adolescent outpatients treated with atomoxetine, 51 (33%) experienced irritability, aggression, mania, or hypomania. Of these patients, 31 (61%) had a family history of a mood disorder, and 41 (80%) had a personal history of mood symptoms.25 Although these findings suggest that atomoxetine may be associated with mood exacerbation and hypomania, additional data are needed to determine whether atomoxetine may be used for ADHD symptoms in youths with comorbid BPD.
Atypical antipsychotics. Mood stabilization—particularly with atypical antipsychotics—often can address comorbid disruptive behaviors and aggressive symptoms. Combinations of atypical antipsychotics with psychostimulants are largely devoid of drug-drug interactions and metabolic interference, making them uncomplicated to use.
Though published studies of pediatric BPD have focused on three atypical antipsychotics—olanzapine, quetiapine, and risperidone—any agent in this class can be used in this population, with the choice often depending on how side effects are likely to affect individual patients (Table 3 ).
Pharmacologic attributes may also determine which atypical antipsychotic is used. For example, ziprasidone’s serotonergic profile—with serotonin-1A receptor agonism and serotonin-1D antagonism—may make it useful for patients with mixed states and bipolar depression.26 Aripiprazole offers potential synergism of dopamine agonism with psychostimulant therapy, which could be useful for treating both disruptive behaviors and ADHD.
Table 3
Using atypical antipsychotics to treat comorbid ADHD/bipolar disorder
| Drug | Target dosage (mg/d) | Side effects | Useful in… |
|---|---|---|---|
| Aripiprazole | 10 to 15 | Nausea, vomiting | Comorbid disruptive behavioral disorders, maintenance stabilization |
| Olanzapine | 10 to 20 | Weight gain, hyperlipidemia, hyperglycemia, sedation | Maintenance stabilization |
| Quetiapine | 400 to 600 | Weight gain, sedation | Mixed states, bipolar depression |
| Risperidone | 1 to 2 | Weight gain, hyperprolactinemia, extrapyramidal symptoms | Comorbid disruptive behavioral disorders, including aggression |
| Ziprasidone | 80 to 120 | Cardiac abnormalities, akathisia | Mixed states, bipolar depression |
Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org
- Children and Adults with Attention-Deficit Hyperactivity Disorder (CHADD). www.chadd.org
- Findling RF, Kowatch RA, Post RM. P ediatric bipolar disorders: a handbook for clinicians. London: Martin Dunitz Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Aripiprazole • Abilify
- Divalproex • Depakote
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Patel is a consultant to Eli Lilly and Co. and a speaker for Eli Lilly and Co. and Pfizer Inc.
Dr. Sallee receives research support from Otsuka America Pharmaceutical, Pfizer Inc., and Bristol-Myers Squibb Co. and is a consultant or speaker for Eli Lilly and Co, Otsuka America Pharmaceutical, and Pfizer Inc.
Comorbid attention-deficit/hyperactivity disorder (ADHD) is nearly universal in youths with bipolar disorder (BPD),1 and comorbid mania has been noted in 16% of children with ADHD.2 Choosing medication for these complex patients is difficult because psychostimulants may worsen mania and mood stabilizers may not resolve ADHD symptoms. Yet, very little information exists on combining psychostimulants with mood stabilizers or atypical antipsychotics.
This article offers evidence to help you decide:
- which to treat first—ADHD or BPD
- how to individualize combination therapy.
CHALLENGES OF COMORBIDITY
Differential diagnosis. ADHD and bipolar disorder (BPD) symptoms overlap, and experts disagree on which symptoms indicate co-existing ADHD and BPD. Multiple daily mood swings and irritability are commonly found in prepubertal BPD.3 Recent reviews address differential diagnosis and specific assessment tools;3-5 after careful evaluation, then focus on treatment.
Treating comorbid ADHD and BPD usually requires more than one medication, and use of multiple drugs in children and adolescents is becoming increasingly common.6,7
PSYCHOSTIMULANTS AND MOOD STABILIZERS
Small, uncontrolled studies of children and adolescents with comorbid ADHD and BPD have shown that treatment with a mood stabilizer and a psychostimulant can control both sets of symptoms. For example:
- Lithium (serum levels 0.7 to 1.1 mEq/L) plus methylphenidate (10 to 20 mg/d) improved attention and hyperactivity symptoms more effectively than either agent alone in 7 children (6 boys, 1 girl) ages 6 to 10 hospitalized with disruptive behavioral disorders and BPD or major depression.8
- A retrospective analysis of 38 children (ages 3 to 16; 84% male) with BPD found that ADHD symptoms were 7.5 times more likely to improve if mood was stabilized before rather than after ADHD treatment with tricyclic antidepressants.9
The efficacy of combining a mood stabilizer and psychostimulant has been confirmed by only one controlled study—a randomized, placebo-controlled trial of mixed amphetamine salts in divalproex-treated patients.10 Forty patients (ages 6 to 17; 83% male) with BPD and ADHD received open-label divalproex (median dosage 750 mg/d) for 8 weeks. Thirty patients whose manic symptoms were significantly reduced entered a 4-week, double-blind, crossover trial of mixed amphetamine salts, 10 mg/d, or placebo.
Following this double-blind phase, 23 patients received open-label divalproex plus mixed amphetamine salts for 12 weeks. The Young Mania Rating Scale and Clinical Global Impression-Improvement scale were used to assess manic and ADHD symptoms during all three study phases.
Manic symptoms in patients treated with divalproex monotherapy improved significantly, but ADHD symptoms did not. ADHD symptoms improved more with divalproex plus mixed amphetamine salts than with divalproex plus placebo. One patient experienced manic symptom exacerbation with combination therapy.
PSYCHOSTIMULANTS AND ANTIPSYCHOTICS
Combinations of psychostimulants and atypical antipsychotics are commonly used in children and adolescents with comorbid psychiatric and behavioral disorders, such as ADHD and disruptive behavioral disorders (oppositional defiant disorder, conduct disorder). In 78 children ages 5 to 12 (83% male) with comorbid ADHD and a disruptive behavioral disorder, disruptive behavior and hyperactivity improved significantly with risperidone alone or with a psychostimulant.11
Combined psychostimulant/atypical antipsychotic therapy may help youths with comorbid ADHD and Tourette syndrome. Methylphenidate can reduce ADHD symptoms without exacerbating tics,12 and risperidone can treat tic disorders, even in patients with comorbid ADHD.13,14 No controlled trials have examined psychostimulant and atypical antipsychotic combinations in these patients, however.
Atypical antipsychotics have been shown to be effective in treating adult BPD, and limited data suggest the same to be true in pediatric patients. Olanzapine, quetiapine, and risperidone have been shown to reduce manic symptoms in children and adolescents (Table 1).15-17 Atypical antipsychotics, however, have been associated with metabolic side effects, including weight gain, hyperglycemia, hyperlipidemia, and hyperprolactinemia.
To date, no study has systematically evaluated combination psychostimulant and atypical antipsychotic treatment in comorbid ADHD and BPD. In the olanzapine and risperidone studies,15,17 concomitant psychostimulant use was permitted and did not affect manic symptom response.
Table 1
Atypical antipsychotic studies in pediatric bipolar disorder
| Drug and mean dosage | Study design | Sample characteristics | Efficacy measures | Results |
|---|---|---|---|---|
| Olanzapine15 9.6±4.3 mg/d | 8-week, open-label monotherapy | 23 patients, mean age 10±3 yrs, 57% male | ≥30% decrease on YMRS | Response rate 61% |
| Quetiapine16 432 mg/d | 6-week, randomized, placebo-controlled, adjunctive (+DVP) | 30 patients, mean age 14±2 yrs, 53% male | ≥50% decrease on YMRS | Response rates: DVP + placebo 53% DVP + quetiapine 87% |
| Risperidone17 1.7±1.3 mg/d | Retrospective, adjunctive | 28 patients, mean age 10±4 yrs, 97% male | ≤2 on CGI-I | Response rate 82% |
| CGI-I: Clinical Global Impressions-Improvement scale | ||||
| DVP: divalproex | ||||
| YMRS: Young Mania Rating Scale | ||||
WHICH COMBINATION?
Which combination treatment—psychostimulant plus mood stabilizer, psychostimulant plus atypical antipsychotic, or psychostimulant plus both mood stabilizer and atypical antipsychotic—is most appropriate for a child or adolescent with comorbid ADHD and BPD? Recommended treatment strategies are based on studies of pediatric and adult BPD and expert consensus.18,19
Consider the type of bipolar episode (Table 2).
For initial treatment of youths with BPD manic or mixed without psychosis, recent guidelines by Kowatch et al suggest using mood-stabilizer or atypical antipsychotic monotherapy. Youths who are more severely ill or present with psychosis may respond more favorably to a mood stabilizer plus an atypical antipsychotic.16,19
Individual patient traits will also determine whether a mood stabilizer or atypical antipsychotic is used and which agent within either medication class is chosen. For example:
- If the patient is aggressive, risperidone may reduce aggression and manic symptoms. Among the atypicals, risperidone has the most evidence suggesting efficacy for aggressive behaviors in youths across psychiatric conditions.20
- If an atypical antipsychotic is warranted and the patient’s weight is an issue, ziprasidone or aripiprazole would be preferred. These agents are considered weight-neutral compared with other atypicals.20
Other factors to consider include medication side effects, interactions, adherence, and cost.
Table 2
Mood stabilizer, atypical antipsychotic, or both with ADHD therapy?
| Type of bipolar episode | Recommended psychotropics |
|---|---|
| Manic or mixed episode with psychosis | Mood stabilizer + atypical antipsychotic |
| Manic or mixed episode without psychosis | Mood stabilizer or atypical antipsychotic monotherapy first
|
| Prominent irritability without psychosis | Atypical antipsychotic |
| Source: Adapted from references 18 and 19 | |
WHICH TO TREAT FIRST?
If the child or adolescent with comorbid ADHD and BPD has acute manic symptoms, available data and expert opinion recommend starting treatment with a mood stabilizer or atypical antipsychotic.9,19,21 If ADHD symptoms persist after mood stabilization, a psychostimulant trial is warranted.
In practice, however, youngsters usually present with ADHD symptoms first. Psychostimulant treatment is initiated, ADHD symptoms are controlled, and the child’s academic and social functioning improve. Bipolar symptoms emerge later, often heralded by a depressive or mixed episode. Is it necessary to discontinue the psychostimulant and risk worsening ADHD symptoms before starting a mood stabilizer or atypical antipsychotic?
Clinical lore and one case report suggest that psychostimulants may destabilize mood.22,23 A 10-year-old boy with severe hyperactivity and family history of BPD experienced manic symptoms—rapid and pressured speech, grandiose delusions of identity, and tangentiality of thought processes—during methylphenidate treatment.22
Conversely, an analysis24 of children ages 7 to 10 from the National Institute of Mental Health Multimodal Treatment Study of Children with ADHD contradicts these assumptions. Although a clinical diagnosis of BPD was not assigned, 29 children (83% male) met the Diagnostic Interview Schedule for Children proxy for mania, 32 (88% male) met the Child Behavior Checklist proxy, and 7 met both proxies for mania.
The first month of methylphenidate treatment did not increase irritability, mood symptoms, or mania in the 54 children with ADHD and manic symptoms, compared with children with ADHD alone. The authors concluded that clinicians should not categorically avoid using stimulants in children with ADHD and some manic symptoms.
In a study by Pavuluri et al18 of pediatric bipolar type I disorder, 17 patients (mean age 11±4 years) received mood stabilizers—following a drug therapy algorithm that included risperidone—and typically received a psychostimulant after mood stabilization. This group was compared with 17 patients receiving “treatment as usual.”
The usual-treatment group remained on psychostimulant therapy after BPD intervention with a mood stabilizer and was less likely to receive an atypical antipsychotic. The algorithm treatment group showed better outcomes overall, specifically for mania and aggression.
Clearly, more studies are needed to determine the optimum treatment sequence with psychostimulants and mood stabilizers in youths with comorbid ADHD and BPD. With either approach, routinely monitor patients treated with psychostimulants for emerging or worsening bipolar symptoms.
LESSONS FROM CLINICAL EXPERIENCE
Nonstimulants. Using psychostimulants is appropriate for ADHD in patients with stable bipolar symptoms. Evidence for using nonstimulants such as clonidine, guanfacine, or atomoxetine is less clear.
In a naturalistic study of 153 children and adolescent outpatients treated with atomoxetine, 51 (33%) experienced irritability, aggression, mania, or hypomania. Of these patients, 31 (61%) had a family history of a mood disorder, and 41 (80%) had a personal history of mood symptoms.25 Although these findings suggest that atomoxetine may be associated with mood exacerbation and hypomania, additional data are needed to determine whether atomoxetine may be used for ADHD symptoms in youths with comorbid BPD.
Atypical antipsychotics. Mood stabilization—particularly with atypical antipsychotics—often can address comorbid disruptive behaviors and aggressive symptoms. Combinations of atypical antipsychotics with psychostimulants are largely devoid of drug-drug interactions and metabolic interference, making them uncomplicated to use.
Though published studies of pediatric BPD have focused on three atypical antipsychotics—olanzapine, quetiapine, and risperidone—any agent in this class can be used in this population, with the choice often depending on how side effects are likely to affect individual patients (Table 3 ).
Pharmacologic attributes may also determine which atypical antipsychotic is used. For example, ziprasidone’s serotonergic profile—with serotonin-1A receptor agonism and serotonin-1D antagonism—may make it useful for patients with mixed states and bipolar depression.26 Aripiprazole offers potential synergism of dopamine agonism with psychostimulant therapy, which could be useful for treating both disruptive behaviors and ADHD.
Table 3
Using atypical antipsychotics to treat comorbid ADHD/bipolar disorder
| Drug | Target dosage (mg/d) | Side effects | Useful in… |
|---|---|---|---|
| Aripiprazole | 10 to 15 | Nausea, vomiting | Comorbid disruptive behavioral disorders, maintenance stabilization |
| Olanzapine | 10 to 20 | Weight gain, hyperlipidemia, hyperglycemia, sedation | Maintenance stabilization |
| Quetiapine | 400 to 600 | Weight gain, sedation | Mixed states, bipolar depression |
| Risperidone | 1 to 2 | Weight gain, hyperprolactinemia, extrapyramidal symptoms | Comorbid disruptive behavioral disorders, including aggression |
| Ziprasidone | 80 to 120 | Cardiac abnormalities, akathisia | Mixed states, bipolar depression |
Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org
- Children and Adults with Attention-Deficit Hyperactivity Disorder (CHADD). www.chadd.org
- Findling RF, Kowatch RA, Post RM. P ediatric bipolar disorders: a handbook for clinicians. London: Martin Dunitz Press, 2002.
Drug brand names
- Atomoxetine • Strattera
- Aripiprazole • Abilify
- Divalproex • Depakote
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Patel is a consultant to Eli Lilly and Co. and a speaker for Eli Lilly and Co. and Pfizer Inc.
Dr. Sallee receives research support from Otsuka America Pharmaceutical, Pfizer Inc., and Bristol-Myers Squibb Co. and is a consultant or speaker for Eli Lilly and Co, Otsuka America Pharmaceutical, and Pfizer Inc.
1. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry 1998;37(2):171-8.
2. Wozniak J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34(7):867-76.
3. Kowatch RA, DelBello MP. Pediatric bipolar disorder: mood swings, irritability are diagnostic cues. Current Psychiatry 2003;2(3):40-7.
4. Quinn CA, Fristad MA. Defining and identifying early onset bipolar spectrum disorder. Curr Psychiatry Rep 2004;6(2):101-7.
5. Bhatara VS, Feil M, Hoagwood K, et al. Trends in combined pharmacotherapy with stimulants for children. Psychiatr Serv 2002;53(3):244.-
6. Zito JM, Safer DJ, dosReis S, et al. Psychotherapeutic medication patterns for youths with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med 1999;153(12):1257-63.
7. Wolf DV, Wagner KD. Bipolar disorder in children and adolescents. CNS Spectr 2003;8(12):954-9.
8. Carlson GA, Rapport MD, Kelly KL, Pataki CS. The effects of methylphenidate and lithium on attention and activity level. J Am Acad Child Adolesc Psychiatry 1992;31(2):262-70.
9. Biederman J, Mick E, Prince J, et al. Systematic chart review of the pharmacologic treatment of comorbid attention deficit hyperactivity disorder in youth with bipolar disorder. J Child Adolesc Psychopharmacol 1999;9(4):247-56.
10. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
11. Aman MG, Binder C, Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behavioral disorders, and subaverage IQ. J Child Adolesc Psychopharmacology 2004;14(2):243-54.
12. Gadow KD, Sverd J, Sprafkin J, et al. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry 1999;56(4):330-6.
13. Gaffney GR, Perry PJ, Lund BC, et al. Risperidone versus clonidine in the treatment of children and adolescents with Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 2002;41(3):330-6.
14. Lombroso PJ, Scahill L, King RA, et al. Risperidone treatment of children and adolescents with chronic tic disorders: a preliminary report. J Am Acad Child Adolesc Psychiatry 1995;34(9):1147-52.
15. Frazier JA, Biederman J, Tohen M, et al. A prospective open-label treatment trial of olanzapine monotherapy in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol 2001;11(3):239-50.
16. DelBello MP, Schwiers ML, Rosenberg HL, Strakowski SM. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry 2002;41(10):1216-23.
17. Frazier JA, Meyer MC, Biederman J, et al. Risperidone treatment for juvenile bipolar disorder: a retrospective chart review. J Am Acad Child Adolesc Psychiatry 1999;38(8):960-5.
18. Pavuluri MN, Henry DB, Devineni B, et al. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2004;43(7):859-67.
19. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44(3):213-35.
20. Findling RL, McNamara NK. Atypical antipsychotics in the treatment of children and adolescents: clinical applications. J Clin Psychiatry 2004;65(suppl 6):30-44.
21. Kowatch RA, Sethuraman G, Hume JH, et al. Combination pharmacotherapy in children and adolescents with bipolar disorder. Biol Psychiatry 2003;53(11):978-84.
22. Koehler-Troy C, Strober M, Malenbaum R. Methylphenidate-induced mania in a prepubertal child. J Clin Psychiatry 1986;47(11):566-7.
23. Craney J, Geller B. Clinical implications of antidepressant and stimulant use on switching from depression to mania in children. J Child Adolesc Psychopharmacol 2003;13(2):201-4.
24. Galanter CA, Carlson GA, Jensen PS, et al. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. J Child Adolesc Psychopharmacol 2003;13(2):123-36.
25. Henderson TA, Hartman K. Aggression, mania, and hypomania induction associated with atomoxetine. Pediatrics 2004;114(3):895-6.
26. Sallee FR, Gilbert DL, Vinks AA, et al. Pharmacodynamics of ziprasidone in children and adolescents: impact on dopamine transmission. J Am Acad Child Adolesc Psychiatry 2003;42(8):902-7.
1. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry 1998;37(2):171-8.
2. Wozniak J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34(7):867-76.
3. Kowatch RA, DelBello MP. Pediatric bipolar disorder: mood swings, irritability are diagnostic cues. Current Psychiatry 2003;2(3):40-7.
4. Quinn CA, Fristad MA. Defining and identifying early onset bipolar spectrum disorder. Curr Psychiatry Rep 2004;6(2):101-7.
5. Bhatara VS, Feil M, Hoagwood K, et al. Trends in combined pharmacotherapy with stimulants for children. Psychiatr Serv 2002;53(3):244.-
6. Zito JM, Safer DJ, dosReis S, et al. Psychotherapeutic medication patterns for youths with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med 1999;153(12):1257-63.
7. Wolf DV, Wagner KD. Bipolar disorder in children and adolescents. CNS Spectr 2003;8(12):954-9.
8. Carlson GA, Rapport MD, Kelly KL, Pataki CS. The effects of methylphenidate and lithium on attention and activity level. J Am Acad Child Adolesc Psychiatry 1992;31(2):262-70.
9. Biederman J, Mick E, Prince J, et al. Systematic chart review of the pharmacologic treatment of comorbid attention deficit hyperactivity disorder in youth with bipolar disorder. J Child Adolesc Psychopharmacol 1999;9(4):247-56.
10. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
11. Aman MG, Binder C, Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behavioral disorders, and subaverage IQ. J Child Adolesc Psychopharmacology 2004;14(2):243-54.
12. Gadow KD, Sverd J, Sprafkin J, et al. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry 1999;56(4):330-6.
13. Gaffney GR, Perry PJ, Lund BC, et al. Risperidone versus clonidine in the treatment of children and adolescents with Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 2002;41(3):330-6.
14. Lombroso PJ, Scahill L, King RA, et al. Risperidone treatment of children and adolescents with chronic tic disorders: a preliminary report. J Am Acad Child Adolesc Psychiatry 1995;34(9):1147-52.
15. Frazier JA, Biederman J, Tohen M, et al. A prospective open-label treatment trial of olanzapine monotherapy in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol 2001;11(3):239-50.
16. DelBello MP, Schwiers ML, Rosenberg HL, Strakowski SM. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry 2002;41(10):1216-23.
17. Frazier JA, Meyer MC, Biederman J, et al. Risperidone treatment for juvenile bipolar disorder: a retrospective chart review. J Am Acad Child Adolesc Psychiatry 1999;38(8):960-5.
18. Pavuluri MN, Henry DB, Devineni B, et al. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2004;43(7):859-67.
19. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44(3):213-35.
20. Findling RL, McNamara NK. Atypical antipsychotics in the treatment of children and adolescents: clinical applications. J Clin Psychiatry 2004;65(suppl 6):30-44.
21. Kowatch RA, Sethuraman G, Hume JH, et al. Combination pharmacotherapy in children and adolescents with bipolar disorder. Biol Psychiatry 2003;53(11):978-84.
22. Koehler-Troy C, Strober M, Malenbaum R. Methylphenidate-induced mania in a prepubertal child. J Clin Psychiatry 1986;47(11):566-7.
23. Craney J, Geller B. Clinical implications of antidepressant and stimulant use on switching from depression to mania in children. J Child Adolesc Psychopharmacol 2003;13(2):201-4.
24. Galanter CA, Carlson GA, Jensen PS, et al. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. J Child Adolesc Psychopharmacol 2003;13(2):123-36.
25. Henderson TA, Hartman K. Aggression, mania, and hypomania induction associated with atomoxetine. Pediatrics 2004;114(3):895-6.
26. Sallee FR, Gilbert DL, Vinks AA, et al. Pharmacodynamics of ziprasidone in children and adolescents: impact on dopamine transmission. J Am Acad Child Adolesc Psychiatry 2003;42(8):902-7.
Printing on the go
You need a printout now, but you’re at a meeting, in a hotel, or at the hospital. What do you do?
Printers have become more compact and versatile, and numerous remote printing solutions exist with more on the way. This article reviews the options to help you print anytime, anywhere.
Portable printers
HP and Canon make inkjet printers that weigh approximately 4 lbs but are portable and compatible with most systems. These printers can be connected to your notebook via a USB or parallel port cable, or wirelessly with Bluetooth or infrared. Built with high-resolution inkjet nozzles, portable printers provide sharp printouts and can even print photos (although this can quickly drain their small ink cartridges).
By contrast, the Psyber Psychiatry, December 2003). WiFi makes printing from your hotel room relatively easy.
Psyber Psychiatry, December 2004).
First, make sure the program you used to create the document is compatible with the business center’s computers. Because most business centers use the free Adobe document reader, your best bet is to convert the file to Adobe PDF, which maintains its format. Use PDFCreator or PrimoPDF to convert the file; both are free and work on Windows computers. Mac users can download the Mac-Net Freeware PDF file creator.
The future
Imagine a pocket-size device that prints onto a blank page as you move it across.
PrintDreams is developing such a device using its random-movement printing technology (RMPT). PrintDreams reports that the scanning device can print any document with 100% accuracy, though it seems best suited to text. The device is still a year or two from reaching the mainstream; PrintDreams is licensing its technology to other printer manufacturers.
Also, don’t be surprised if cellular phones one day have the capability to print e-mail attachments using Bluetooth or general packet radio service (GPRS), a very fast data transfer protocol on a GSM network.
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
You need a printout now, but you’re at a meeting, in a hotel, or at the hospital. What do you do?
Printers have become more compact and versatile, and numerous remote printing solutions exist with more on the way. This article reviews the options to help you print anytime, anywhere.
Portable printers
HP and Canon make inkjet printers that weigh approximately 4 lbs but are portable and compatible with most systems. These printers can be connected to your notebook via a USB or parallel port cable, or wirelessly with Bluetooth or infrared. Built with high-resolution inkjet nozzles, portable printers provide sharp printouts and can even print photos (although this can quickly drain their small ink cartridges).
By contrast, the Psyber Psychiatry, December 2003). WiFi makes printing from your hotel room relatively easy.
Psyber Psychiatry, December 2004).
First, make sure the program you used to create the document is compatible with the business center’s computers. Because most business centers use the free Adobe document reader, your best bet is to convert the file to Adobe PDF, which maintains its format. Use PDFCreator or PrimoPDF to convert the file; both are free and work on Windows computers. Mac users can download the Mac-Net Freeware PDF file creator.
The future
Imagine a pocket-size device that prints onto a blank page as you move it across.
PrintDreams is developing such a device using its random-movement printing technology (RMPT). PrintDreams reports that the scanning device can print any document with 100% accuracy, though it seems best suited to text. The device is still a year or two from reaching the mainstream; PrintDreams is licensing its technology to other printer manufacturers.
Also, don’t be surprised if cellular phones one day have the capability to print e-mail attachments using Bluetooth or general packet radio service (GPRS), a very fast data transfer protocol on a GSM network.
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
You need a printout now, but you’re at a meeting, in a hotel, or at the hospital. What do you do?
Printers have become more compact and versatile, and numerous remote printing solutions exist with more on the way. This article reviews the options to help you print anytime, anywhere.
Portable printers
HP and Canon make inkjet printers that weigh approximately 4 lbs but are portable and compatible with most systems. These printers can be connected to your notebook via a USB or parallel port cable, or wirelessly with Bluetooth or infrared. Built with high-resolution inkjet nozzles, portable printers provide sharp printouts and can even print photos (although this can quickly drain their small ink cartridges).
By contrast, the Psyber Psychiatry, December 2003). WiFi makes printing from your hotel room relatively easy.
Psyber Psychiatry, December 2004).
First, make sure the program you used to create the document is compatible with the business center’s computers. Because most business centers use the free Adobe document reader, your best bet is to convert the file to Adobe PDF, which maintains its format. Use PDFCreator or PrimoPDF to convert the file; both are free and work on Windows computers. Mac users can download the Mac-Net Freeware PDF file creator.
The future
Imagine a pocket-size device that prints onto a blank page as you move it across.
PrintDreams is developing such a device using its random-movement printing technology (RMPT). PrintDreams reports that the scanning device can print any document with 100% accuracy, though it seems best suited to text. The device is still a year or two from reaching the mainstream; PrintDreams is licensing its technology to other printer manufacturers.
Also, don’t be surprised if cellular phones one day have the capability to print e-mail attachments using Bluetooth or general packet radio service (GPRS), a very fast data transfer protocol on a GSM network.
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
Treating persistent catatonia when benzodiazepines fail
Many catatonia cases respond to benzodiazepines—especially lorazepam—but up to 30% do not. Electroconvulsive therapy (ECT) can be effective, but what’s the next step when ECT is unavailable or inappropriate for your patient?
To help you solve this dilemma, we describe our diagnosis and treatment decisions for a patient we call Mr. C. We explain how our process was guided by recent understandings of an abnormal neural circuit that appears to cause catatonia’s complex motor and behavioral symptoms.
This article describes that neurologic pathology and answers common questions about the clinical workup and treatment of catatonia.
CASE: TROUBLE IN TV LAND
Mr. C, age 69, caused a disturbance at a local TV station, demanding that they broadcast a manuscript he had written. Police took him to a local hospital, where he was stabilized and then transferred to a neuropsychiatric hospital for evaluation.
The psychiatric interview revealed that he had developed insomnia, excessive activity, and delusional thinking 2 weeks before admission. His medical history included coronary artery disease (CAD), hypertension, and hypothyroidism. Medications included thyroid hormone replacement therapy, furosemide, potassium, ranitidine, simvastatin, metoprolol, and lisinopril. CAD treatment included stent placement and nitroglycerin as needed.
He had been hospitalized in his 30s and treated with ECT for what he called “bad thoughts.” He said he improved after 1 month and had no subsequent psychiatric history. He denied drug or alcohol abuse.
Shortly after admission, he refused to eat or drink and after 1 week became dehydrated. He also showed mutism, immobility, and stupor. He was transferred to the medical service for IV rehydration.
MANY SCENARIOS AND SIGNS
Mr. C’s symptoms suggest possible catatonia, a neuropsychiatric syndrome of motor dysregulation found in up to 10% of acutely ill psychiatric inpatients.1,2 A movement disorder,1,2 catatonia occurs with general medical conditions and psychiatric disorders (Table 1).
Pathophysiology. Catatonic signs develop when aberrant signals from neurochemical abnormalities trigger a neural circuit that affects the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Box).3-5
Presentation. A focused exam is required because patients with catatonia often do not provide a comprehensive or reliable history.2 They show mutism, characteristic postures, rigidity, aberrant speech, negativism, and stereotyped behaviors.1,2 They may present in an excited or retarded state:
- Excited patients may injure themselves or others and develop hyperthermia, tachycardia, and elevated blood pressure from excessive motor activity.
- Patients in a retarded state may present with bradykinesia and poor self-care. They may be unresponsive to external stimuli, develop catatonic stupor, and refuse to eat or drink.
Mr. C’s earlier insomnia, excessive activity, and delusional thinking (such as the TV station incident) may have signaled an excited catatonia. On admission to the medical service, however, he presented in a retarded state.
Signs. Part of the challenge with detecting catatonia’s signs is that there are so many; some rating scales list more than 20. Not all signs need to be present to make the diagnosis, however, and if you find one, others usually turn up in the examination.
A mnemonic from the Bush-Francis Catatonia Screening Instrument (Table 2) represents diagnostic signs in patients with the excited or retarded forms.2 We recommend that you review an authoritative text (see Related resources) to understand catatonia’s psychopathology.2
Table 1
Common diagnoses of patients with catatonia
| Psychiatric |
|
| Organic |
|
Catatonia is caused by neurochemical abnormalities including low GABA activity in the frontal cortex, low dopamine (D2) activity in the basal ganglia, high glutamate—N-methyl-D-aspartate (NMDA)—activity in the parietal cortex, or a combination of these.3-5 Catatonic signs occur when these neurochemical changes cause aberrant signals and trigger a neural circuit affecting the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Figure).
Posturing occurs when the aberrant signal reaches the posterior parietal lobe. Patients’ bizarre and mundane postures in catatonia are maintained by “anosognosia of position.” For example, an individual does not know the position of rest for his arm, and it remains in an unusual position as if at rest.3
The PP goes on to influence the supplemental motor area (SMA), causing bradykinesia, rigidity, and other motor phenomena that catatonia shares with Parkinson’s disease. The SMA feeds back to the medial orbital gyrus, completing the neural circuit.3
Regions such as the anterior cingulate area (ACA) and amygdala (1AMG) — also may be recruited into the expanded circuit. ACA recruitment may cause akinetic mutism, and fear is a symptom of AMG recruitment. If the anterior hypothalamus is affected, malignant catatonia or neuroleptic malignant syndrome may occur.3,5
This neural loop demonstrates an integrated model of psychosis. It may help explain why catatonia responds to treatment with lorazepam, ECT, and other agents such as antipsychotics and NMDA antagonists.
Illustration for CURRENT PSYCHIATRY by Marcia Hartsock, CMI
Table 2
WIRED `N MIRED: Mnemonic for detecting catatonia
| Waxy flexibility/catalepsy |
| Immobility/stupor |
| Refusal to eat or drink |
| Excitement |
| Deadpan staring |
| Negativism/negative symptoms |
| Mutism |
| Impulsivity |
| Rigidity |
| Echolalia/echopraxia |
| Direct observation |
CASE CONTINUED: MAKING THE DIAGNOSIS
In the medical unit, Mr. C was found to be in a catatonic stupor, with immobility, mutism (monosyllabic speech), catalepsy, intermittent waxy flexibility, withdrawal (refusal to eat and drink), automatic obedience, and mitgehen (exaggerated movements in response to light finger pressure, despite instructions to stay still). ECT workup was started, along with a trial of lorazepam, 1 mg tid.
Laboratory studies revealed high BUN/creatinine (80/2.0) that returned to normal range (BUN 7 to 21 mg/dL; creatinine 0.5 to 1.2 mg/dL) after 3 days of hydration. Because of Mr. C’s earlier excited symptoms and delusional thinking, we considered a diagnosis of bipolar disorder with catatonia. However, his symptoms did not improve with a trial of valproic acid (serum level 64 mcg/mL).
Head CT showed generalized atrophy and EEG showed delta slowing. Single-photo emission computer tomography (SPECT) showed areas of decreased perfusion in the cortex, with no perfusion in the left posterior parietal area (PP).
Mental status exam found Mr. C disoriented with poor short-term memory and unable to complete the Mini Mental State Examination (MMSE). His Bush-Francis Catatonia Rating Scale score was 28 and included many catatonic signs that would not be seen a patient with simple dehydration.
The workup supported a diagnosis of catatonia due to general medical condition (vascular dementia) and ruled out schizophrenia with catatonic features, bipolar disorder, or major depression with catatonia.
EVALUATION AND DIAGNOSIS
Medical causes. A careful history and thorough physical examination are essential for making an accurate diagnosis and ruling out medical conditions that could present with or mimic catatonia (Table 3). Medications that can induce catatonia include antipsychotics, corticosteroids, and disulfiram at therapeutic doses. Drug abuse (such as with phencyclidine), use of the general anesthetic ketamine, and benzodiazepine withdrawal may also lead to catatonia.
Head CT or MRI is indicated for patients being considered for ECT or for localizing neurologic findings. EEG can be useful when patients present with features of seizure activity—such as tongue biting, incontinence, or stupor—or with catatonia as a manifestation of delirium or dementia.
A history of head injury or neurologic disease warrants further neurologic investigation. Also consider a neurology consult when the patient has prolonged stupor or does not respond to initial drug therapy.
Psychiatric causes. The clinical setting may suggest the most likely primary psychiatric disorders to consider, such as:
- bipolar or major depression in acute inpatient psychiatric units
- autism and pervasive developmental disorders (PDD) in pediatric or PDD units
- catatonic schizophrenia in chronic psychotic patients
- somatoform or factitious disorders in forensic settings.
These generalizations are not clinically exclusive, of course, but may provide a starting point for the treatment team confronted with limited history and exam information.
Table 3
Catatonia workup: Recommended lab tests
| Test | Recommendation |
|---|---|
| Complete blood count with WBC differential | Look for leukocytosis |
| Serum chemistries | Look for electrolyte imbalances |
| Serum iron | May be low in NMS |
| Serum creatine kinase | If NMS is suspected |
| Brain MRI or CT | If structural lesion is suspected |
| Electroencephalography | If seizure disorder or brain abnormality is suspected |
| Lumbar puncture | If encephalitis or meningits is suspected |
| NMS: neuroleptic malignant syndrome | |
Initial treatment. Catatonia related to medical and psychiatric causes has been shown to respond to lorazepam and to ECT.6,7 Lorazepam is preferred because of its specificity for the GABAa receptor and ease of administration (oral, IM, or IV). Other agents that act on GABA—including amobarbital and zolpidem—have also been used. Catatonia’s hallmark features such as mutism and immobility have been shown to respond to lorazepam.8,9
ECT is a first-line treatment for catatonia with life-threatening conditions and should be considered for refractory cases.
Lorazepam. The starting dosage is usually 1 mg tid for healthy adults; 0.5 mg tid can be used for children and the elderly. Observe the patient for improvement in catatonic signs after the first dose and before giving the second. Dosages of up to 16 mg/d have been used.
In many cases, lorazepam can be tapered off after adequate treatment of the primary psychiatric condition. In severe cases, however—such as when patients refuse to eat or drink—lorazepam may be continued for as long as 1 year. Weigh the risk of benzodiazepine tolerance, dependence, and misuse versus the possibility of relapse and rehospitalization.
Medical catatonias and neuroleptic malignant syndrome (NMS) have responded favorably to ECT.8 Addressing the medical cause itself usually does not resolve catatonia, with the possible exception of seizure-induced (“ictal”) catatonia, which may respond to anticonvulsants and lorazepam.6,7
ECT. An ECT workup can begin as soon as a patient presents with catatonia. If lorazepam produces no response within 24 hours, consider ECT.
CASE CONTINUED: PERSISTENT SYMPTOMS
After three 1-mg doses of lorazepam, Mr. C became more alert and oriented but his catatonia symptoms persisted, as indicated by a Bush-Francis score of 23, significant grasp reflex, and gegenhalten (automatic rather than willful resistance to passive limb movement in proportion to the strength of the stimulus). An attempt to gradually increase lorazepam to 2 mg tid produced delirium. He remained confused even when lorazepam was reduced to 0.5 mg tid, so the drug was discontinued.
Mr. C’s neurologist added amantadine, 100 mg tid, and carbidopa/levodopa, 10/100 mg tid, to treat his parkinsonian rigidity.
WHAT NEXT? OTHER OPTIONS
Antipsychotics have been investigated as a possible treatment for catatonia. The literature suggests that conventional antipsychotics may cause catatonia and atypical antipsychotics may improve it. Conventional antipsychotics are best avoided in catatonia because they:
- appear less effective than other treatments in resolving catatonic symptoms8,10
- are associated with catatonic-like side effects, such as rigidity, akinesia, and staring10
- appear to increase NMS risk in patients with catatonic symptoms.11,12
Atypicals appear more effective in treating catatonia and less likely to cause NMS. Case reports13,14 indicate many of these agents can be effective and well tolerated in treating catatonic symptoms, although this was not the case for Mr. C.
Anticonvulsants such as valproate15 and carbamazepine, 600 to 1200 mg/d,16 may take longer to work than lorazepam but may be options for patients who do not respond to benzodiazepines.8,9
Amantadine, an N-methyl-D-aspartate (NMDA) antagonist, has been used with some success for catatonia that does not unrespond to lorazepam.17 However, amantidine’s dopamine agonist activity could worsen underlying psychosis.
Memantine—another NMDA antagonist—differs from amantadine despite having a similar chemical structure. Memantine is a noncompetitive antagonist at the NMDA receptor, without affinity for dopamine, norepinephrine, serotonin, or muscarinic receptors.18
Although no published data support using memantine in patients with catatonia, it might be considered for those who are not candidates for lorazepam or ECT. For instance, a double-blind, placebo-controlled study found that lorazepam was not effective for catatonic schizophrenia.19 We have found memantine to help in some patients with catatonic schizophrenia.
CASE CONTINUED: TRIAL OF MEMANTINE
Mr. C remained in a catatonic stupor, but we decided against ECT because he resumed eating and drinking and was not medically at risk. Quetiapine, 100 to 300 mg/d, was tried to address his dementia symptoms, confusion, and poor mentation. This trial was discontinued after Mr. C fell and was readmitted to the medical unit. We then added memantine, 5 mg bid.
In the first week after beginning memantine, Mr. C’s MMSE score was 21, consistent with vascular dementia, but he remained immobile and staring. Motor signs also persisted, including automatic obedience, ambitendency, and a grasp reflex.
The next week, we increased memantine to 10 mg bid. Mr. C was oriented to person, place, and time, and his affect was blunted. His MMSE score increased to 25, showing improved cognition and memory. His Bush-Francis scale score was 6, showing reduced catatonic signs, with remaining mild immobility, bradykinesia, speech-prompt mutism, staring, and grasp reflex.
He maintained this improvement on carbidopa/levodopa, 10/100 tid; amantadine, 100 mg tid; and memantine, 10 mg bid, and was discharged from the nursing home unit.
IMPROVEMENT WITH MEMANTINE
Memantine may reduce excess glutamate at the NMDA receptor in the parietal-SMA-frontal cortical circuit. It may help to increase GABA and dopamine, which are deficient in catatonia. Our patient with vascular dementia had a severe ischemic deficit in the posterior parietal area, as seen on SPECT.
Amantadine, another NMDA receptor antagonist, acts on dopamine neurons and may have anticholinergic-like side effects, whereas memantine does not. Although both drugs share antagonism at the NMDA glutamate receptor, noncompetitive binding is weak for amantadine and moderate for memantine. Memantine has some serotonin (5-HT3) antagonism, but neither agent has direct GABA activity.
Memantine can improve function in vascular dementia.20 Thus, Mr. C’s improvement may have been caused by the drug’s effect on his vascular dementia, the primary neuropsychiatric illness. However, his catatonic signs improved without antipsychotics, cholinesterase inhibitors, benzodiazepines, or ECT. No anticoagulation treatment or cerebral perfusion procedures account for his improved mental status.
CASE CONCLUSION
Mr. C went to live with his son’s family. Although he has problems with calculation, he shows good selfcare. When asked why he did not respond during his catatonic stupor, Mr. C stated that he believed the physician was an Internal Revenue Service agent asking him about serious tax problems. Upon reflection, he said he no longer believes this.
- Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press, 2003.
- Caroff SN, Mann SC, Francis A, Fricchione GE. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing, 2004.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003.
- Neuroleptic Malignant Syndrome Information Service. www.NMSIS.org.
Drug brand names
- Amantadine • Symmetrel
- Amobarbital • Amytal sodium
- Carbamazepine • Carbatrol, Equetro
- Carbidopa/levodopa • Sinemet
- Disulfiram • Antabuse
- Divalproex • Depakote
- Furosemide • Lasix
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Memantine • Namenda
- Metoprolol • Lopressor
- Ranitidine • Zantac
- Simvastatin • Zocor
- Valproic acid • Depakene
- Zolpidem • Ambien
Disclosure
Dr. Carroll and Dr. Hawkins are speakers for Forest Laboratories. The other authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Dr. Niraj Ahuja, consultant psychiatrist and honorary clinical lecturer (psychiatry), Newcastle, North Tyneside and Northumberland Mental Health Trust, UK, for assistance with the figure.
1. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry 2003;160:1233-41.
2. Bush G, Fink M, Petrides G, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
3. Northoff G. What catatonia can tell us about “top-down” modulation:” a neuropsychiatric hypothesis. Brain Behav Sci 2002;25:555-604.
4. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums 2000;5(7):26-33.
5. Carroll BT. Catatonia is the rosetta stone of psychosis (poster presentation). New York: American Psychiatric Association annual meeting, 2004.
6. Barnes MP, Saunders M, Walls TJ, et al. The syndrome of Karl Ludwig Kahlbaum. J Neurol Neurosurg Psychiatry 1986;49:991-6.
7. Carroll BT, Anfinson TJ, Kennedy JC, et al. Catatonic disorder due to general medical conditions. J Neuropsychiatry Clin Neurosci 1994;6:122-33.
8. Hawkins JM, Archer KJ, Strakowski SM, Keck PE. Somatic treatments of catatonia. Int J Psychiatry Med 1995;25:345-69.
9. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
10. Dose M. Neuroleptic-induced pseudo-catatonia. Pharmacopsychiatry 2001;34:262-4.
11. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. Arch Gen Psychiatry 1989;46:914-18.
12. White DAC. 17 catatonic patients diagnosed as neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
13. Levy WO, Nunez CY. Use of ziprasidone to treat bipolar-associated catatonia. Bipolar Disord 2004;6:166-7.
14. Hesslinger B, Walden J, Normann C. Acute and long-term treatment of catatonia with risperidone. Pharmacopsychiatry 2001;34:25-6.
15. Kruger S, Braunig P. Intravenous valproic acid in the treatment of severe catatonia. J Neuropsychiatry Clin Neurosci 2001;13:303-4.
16. Kritzinger PR, Jordaan GP. Catatonia: an open prospective series with carbamazepine. Int J Neuropsychopharmacol 2001;4:251-7.
17. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
18. Namenda (memantine) Package labeling. Forest Laboratories, 2004.
19. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: a random, double-blind, placebo-controlled, cross-over study. Psychopharmacol 1999;142:393-8.
20. Mobius HJ. Pharmacologic rationale for memantine in chronic cerebral hypoperfusion, especially vascular dementia. Alz Dis Assoc Disord 1999;13(suppl 3):172-8.
Many catatonia cases respond to benzodiazepines—especially lorazepam—but up to 30% do not. Electroconvulsive therapy (ECT) can be effective, but what’s the next step when ECT is unavailable or inappropriate for your patient?
To help you solve this dilemma, we describe our diagnosis and treatment decisions for a patient we call Mr. C. We explain how our process was guided by recent understandings of an abnormal neural circuit that appears to cause catatonia’s complex motor and behavioral symptoms.
This article describes that neurologic pathology and answers common questions about the clinical workup and treatment of catatonia.
CASE: TROUBLE IN TV LAND
Mr. C, age 69, caused a disturbance at a local TV station, demanding that they broadcast a manuscript he had written. Police took him to a local hospital, where he was stabilized and then transferred to a neuropsychiatric hospital for evaluation.
The psychiatric interview revealed that he had developed insomnia, excessive activity, and delusional thinking 2 weeks before admission. His medical history included coronary artery disease (CAD), hypertension, and hypothyroidism. Medications included thyroid hormone replacement therapy, furosemide, potassium, ranitidine, simvastatin, metoprolol, and lisinopril. CAD treatment included stent placement and nitroglycerin as needed.
He had been hospitalized in his 30s and treated with ECT for what he called “bad thoughts.” He said he improved after 1 month and had no subsequent psychiatric history. He denied drug or alcohol abuse.
Shortly after admission, he refused to eat or drink and after 1 week became dehydrated. He also showed mutism, immobility, and stupor. He was transferred to the medical service for IV rehydration.
MANY SCENARIOS AND SIGNS
Mr. C’s symptoms suggest possible catatonia, a neuropsychiatric syndrome of motor dysregulation found in up to 10% of acutely ill psychiatric inpatients.1,2 A movement disorder,1,2 catatonia occurs with general medical conditions and psychiatric disorders (Table 1).
Pathophysiology. Catatonic signs develop when aberrant signals from neurochemical abnormalities trigger a neural circuit that affects the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Box).3-5
Presentation. A focused exam is required because patients with catatonia often do not provide a comprehensive or reliable history.2 They show mutism, characteristic postures, rigidity, aberrant speech, negativism, and stereotyped behaviors.1,2 They may present in an excited or retarded state:
- Excited patients may injure themselves or others and develop hyperthermia, tachycardia, and elevated blood pressure from excessive motor activity.
- Patients in a retarded state may present with bradykinesia and poor self-care. They may be unresponsive to external stimuli, develop catatonic stupor, and refuse to eat or drink.
Mr. C’s earlier insomnia, excessive activity, and delusional thinking (such as the TV station incident) may have signaled an excited catatonia. On admission to the medical service, however, he presented in a retarded state.
Signs. Part of the challenge with detecting catatonia’s signs is that there are so many; some rating scales list more than 20. Not all signs need to be present to make the diagnosis, however, and if you find one, others usually turn up in the examination.
A mnemonic from the Bush-Francis Catatonia Screening Instrument (Table 2) represents diagnostic signs in patients with the excited or retarded forms.2 We recommend that you review an authoritative text (see Related resources) to understand catatonia’s psychopathology.2
Table 1
Common diagnoses of patients with catatonia
| Psychiatric |
|
| Organic |
|
Catatonia is caused by neurochemical abnormalities including low GABA activity in the frontal cortex, low dopamine (D2) activity in the basal ganglia, high glutamate—N-methyl-D-aspartate (NMDA)—activity in the parietal cortex, or a combination of these.3-5 Catatonic signs occur when these neurochemical changes cause aberrant signals and trigger a neural circuit affecting the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Figure).
Posturing occurs when the aberrant signal reaches the posterior parietal lobe. Patients’ bizarre and mundane postures in catatonia are maintained by “anosognosia of position.” For example, an individual does not know the position of rest for his arm, and it remains in an unusual position as if at rest.3
The PP goes on to influence the supplemental motor area (SMA), causing bradykinesia, rigidity, and other motor phenomena that catatonia shares with Parkinson’s disease. The SMA feeds back to the medial orbital gyrus, completing the neural circuit.3
Regions such as the anterior cingulate area (ACA) and amygdala (1AMG) — also may be recruited into the expanded circuit. ACA recruitment may cause akinetic mutism, and fear is a symptom of AMG recruitment. If the anterior hypothalamus is affected, malignant catatonia or neuroleptic malignant syndrome may occur.3,5
This neural loop demonstrates an integrated model of psychosis. It may help explain why catatonia responds to treatment with lorazepam, ECT, and other agents such as antipsychotics and NMDA antagonists.
Illustration for CURRENT PSYCHIATRY by Marcia Hartsock, CMI
Table 2
WIRED `N MIRED: Mnemonic for detecting catatonia
| Waxy flexibility/catalepsy |
| Immobility/stupor |
| Refusal to eat or drink |
| Excitement |
| Deadpan staring |
| Negativism/negative symptoms |
| Mutism |
| Impulsivity |
| Rigidity |
| Echolalia/echopraxia |
| Direct observation |
CASE CONTINUED: MAKING THE DIAGNOSIS
In the medical unit, Mr. C was found to be in a catatonic stupor, with immobility, mutism (monosyllabic speech), catalepsy, intermittent waxy flexibility, withdrawal (refusal to eat and drink), automatic obedience, and mitgehen (exaggerated movements in response to light finger pressure, despite instructions to stay still). ECT workup was started, along with a trial of lorazepam, 1 mg tid.
Laboratory studies revealed high BUN/creatinine (80/2.0) that returned to normal range (BUN 7 to 21 mg/dL; creatinine 0.5 to 1.2 mg/dL) after 3 days of hydration. Because of Mr. C’s earlier excited symptoms and delusional thinking, we considered a diagnosis of bipolar disorder with catatonia. However, his symptoms did not improve with a trial of valproic acid (serum level 64 mcg/mL).
Head CT showed generalized atrophy and EEG showed delta slowing. Single-photo emission computer tomography (SPECT) showed areas of decreased perfusion in the cortex, with no perfusion in the left posterior parietal area (PP).
Mental status exam found Mr. C disoriented with poor short-term memory and unable to complete the Mini Mental State Examination (MMSE). His Bush-Francis Catatonia Rating Scale score was 28 and included many catatonic signs that would not be seen a patient with simple dehydration.
The workup supported a diagnosis of catatonia due to general medical condition (vascular dementia) and ruled out schizophrenia with catatonic features, bipolar disorder, or major depression with catatonia.
EVALUATION AND DIAGNOSIS
Medical causes. A careful history and thorough physical examination are essential for making an accurate diagnosis and ruling out medical conditions that could present with or mimic catatonia (Table 3). Medications that can induce catatonia include antipsychotics, corticosteroids, and disulfiram at therapeutic doses. Drug abuse (such as with phencyclidine), use of the general anesthetic ketamine, and benzodiazepine withdrawal may also lead to catatonia.
Head CT or MRI is indicated for patients being considered for ECT or for localizing neurologic findings. EEG can be useful when patients present with features of seizure activity—such as tongue biting, incontinence, or stupor—or with catatonia as a manifestation of delirium or dementia.
A history of head injury or neurologic disease warrants further neurologic investigation. Also consider a neurology consult when the patient has prolonged stupor or does not respond to initial drug therapy.
Psychiatric causes. The clinical setting may suggest the most likely primary psychiatric disorders to consider, such as:
- bipolar or major depression in acute inpatient psychiatric units
- autism and pervasive developmental disorders (PDD) in pediatric or PDD units
- catatonic schizophrenia in chronic psychotic patients
- somatoform or factitious disorders in forensic settings.
These generalizations are not clinically exclusive, of course, but may provide a starting point for the treatment team confronted with limited history and exam information.
Table 3
Catatonia workup: Recommended lab tests
| Test | Recommendation |
|---|---|
| Complete blood count with WBC differential | Look for leukocytosis |
| Serum chemistries | Look for electrolyte imbalances |
| Serum iron | May be low in NMS |
| Serum creatine kinase | If NMS is suspected |
| Brain MRI or CT | If structural lesion is suspected |
| Electroencephalography | If seizure disorder or brain abnormality is suspected |
| Lumbar puncture | If encephalitis or meningits is suspected |
| NMS: neuroleptic malignant syndrome | |
Initial treatment. Catatonia related to medical and psychiatric causes has been shown to respond to lorazepam and to ECT.6,7 Lorazepam is preferred because of its specificity for the GABAa receptor and ease of administration (oral, IM, or IV). Other agents that act on GABA—including amobarbital and zolpidem—have also been used. Catatonia’s hallmark features such as mutism and immobility have been shown to respond to lorazepam.8,9
ECT is a first-line treatment for catatonia with life-threatening conditions and should be considered for refractory cases.
Lorazepam. The starting dosage is usually 1 mg tid for healthy adults; 0.5 mg tid can be used for children and the elderly. Observe the patient for improvement in catatonic signs after the first dose and before giving the second. Dosages of up to 16 mg/d have been used.
In many cases, lorazepam can be tapered off after adequate treatment of the primary psychiatric condition. In severe cases, however—such as when patients refuse to eat or drink—lorazepam may be continued for as long as 1 year. Weigh the risk of benzodiazepine tolerance, dependence, and misuse versus the possibility of relapse and rehospitalization.
Medical catatonias and neuroleptic malignant syndrome (NMS) have responded favorably to ECT.8 Addressing the medical cause itself usually does not resolve catatonia, with the possible exception of seizure-induced (“ictal”) catatonia, which may respond to anticonvulsants and lorazepam.6,7
ECT. An ECT workup can begin as soon as a patient presents with catatonia. If lorazepam produces no response within 24 hours, consider ECT.
CASE CONTINUED: PERSISTENT SYMPTOMS
After three 1-mg doses of lorazepam, Mr. C became more alert and oriented but his catatonia symptoms persisted, as indicated by a Bush-Francis score of 23, significant grasp reflex, and gegenhalten (automatic rather than willful resistance to passive limb movement in proportion to the strength of the stimulus). An attempt to gradually increase lorazepam to 2 mg tid produced delirium. He remained confused even when lorazepam was reduced to 0.5 mg tid, so the drug was discontinued.
Mr. C’s neurologist added amantadine, 100 mg tid, and carbidopa/levodopa, 10/100 mg tid, to treat his parkinsonian rigidity.
WHAT NEXT? OTHER OPTIONS
Antipsychotics have been investigated as a possible treatment for catatonia. The literature suggests that conventional antipsychotics may cause catatonia and atypical antipsychotics may improve it. Conventional antipsychotics are best avoided in catatonia because they:
- appear less effective than other treatments in resolving catatonic symptoms8,10
- are associated with catatonic-like side effects, such as rigidity, akinesia, and staring10
- appear to increase NMS risk in patients with catatonic symptoms.11,12
Atypicals appear more effective in treating catatonia and less likely to cause NMS. Case reports13,14 indicate many of these agents can be effective and well tolerated in treating catatonic symptoms, although this was not the case for Mr. C.
Anticonvulsants such as valproate15 and carbamazepine, 600 to 1200 mg/d,16 may take longer to work than lorazepam but may be options for patients who do not respond to benzodiazepines.8,9
Amantadine, an N-methyl-D-aspartate (NMDA) antagonist, has been used with some success for catatonia that does not unrespond to lorazepam.17 However, amantidine’s dopamine agonist activity could worsen underlying psychosis.
Memantine—another NMDA antagonist—differs from amantadine despite having a similar chemical structure. Memantine is a noncompetitive antagonist at the NMDA receptor, without affinity for dopamine, norepinephrine, serotonin, or muscarinic receptors.18
Although no published data support using memantine in patients with catatonia, it might be considered for those who are not candidates for lorazepam or ECT. For instance, a double-blind, placebo-controlled study found that lorazepam was not effective for catatonic schizophrenia.19 We have found memantine to help in some patients with catatonic schizophrenia.
CASE CONTINUED: TRIAL OF MEMANTINE
Mr. C remained in a catatonic stupor, but we decided against ECT because he resumed eating and drinking and was not medically at risk. Quetiapine, 100 to 300 mg/d, was tried to address his dementia symptoms, confusion, and poor mentation. This trial was discontinued after Mr. C fell and was readmitted to the medical unit. We then added memantine, 5 mg bid.
In the first week after beginning memantine, Mr. C’s MMSE score was 21, consistent with vascular dementia, but he remained immobile and staring. Motor signs also persisted, including automatic obedience, ambitendency, and a grasp reflex.
The next week, we increased memantine to 10 mg bid. Mr. C was oriented to person, place, and time, and his affect was blunted. His MMSE score increased to 25, showing improved cognition and memory. His Bush-Francis scale score was 6, showing reduced catatonic signs, with remaining mild immobility, bradykinesia, speech-prompt mutism, staring, and grasp reflex.
He maintained this improvement on carbidopa/levodopa, 10/100 tid; amantadine, 100 mg tid; and memantine, 10 mg bid, and was discharged from the nursing home unit.
IMPROVEMENT WITH MEMANTINE
Memantine may reduce excess glutamate at the NMDA receptor in the parietal-SMA-frontal cortical circuit. It may help to increase GABA and dopamine, which are deficient in catatonia. Our patient with vascular dementia had a severe ischemic deficit in the posterior parietal area, as seen on SPECT.
Amantadine, another NMDA receptor antagonist, acts on dopamine neurons and may have anticholinergic-like side effects, whereas memantine does not. Although both drugs share antagonism at the NMDA glutamate receptor, noncompetitive binding is weak for amantadine and moderate for memantine. Memantine has some serotonin (5-HT3) antagonism, but neither agent has direct GABA activity.
Memantine can improve function in vascular dementia.20 Thus, Mr. C’s improvement may have been caused by the drug’s effect on his vascular dementia, the primary neuropsychiatric illness. However, his catatonic signs improved without antipsychotics, cholinesterase inhibitors, benzodiazepines, or ECT. No anticoagulation treatment or cerebral perfusion procedures account for his improved mental status.
CASE CONCLUSION
Mr. C went to live with his son’s family. Although he has problems with calculation, he shows good selfcare. When asked why he did not respond during his catatonic stupor, Mr. C stated that he believed the physician was an Internal Revenue Service agent asking him about serious tax problems. Upon reflection, he said he no longer believes this.
- Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press, 2003.
- Caroff SN, Mann SC, Francis A, Fricchione GE. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing, 2004.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003.
- Neuroleptic Malignant Syndrome Information Service. www.NMSIS.org.
Drug brand names
- Amantadine • Symmetrel
- Amobarbital • Amytal sodium
- Carbamazepine • Carbatrol, Equetro
- Carbidopa/levodopa • Sinemet
- Disulfiram • Antabuse
- Divalproex • Depakote
- Furosemide • Lasix
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Memantine • Namenda
- Metoprolol • Lopressor
- Ranitidine • Zantac
- Simvastatin • Zocor
- Valproic acid • Depakene
- Zolpidem • Ambien
Disclosure
Dr. Carroll and Dr. Hawkins are speakers for Forest Laboratories. The other authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Dr. Niraj Ahuja, consultant psychiatrist and honorary clinical lecturer (psychiatry), Newcastle, North Tyneside and Northumberland Mental Health Trust, UK, for assistance with the figure.
Many catatonia cases respond to benzodiazepines—especially lorazepam—but up to 30% do not. Electroconvulsive therapy (ECT) can be effective, but what’s the next step when ECT is unavailable or inappropriate for your patient?
To help you solve this dilemma, we describe our diagnosis and treatment decisions for a patient we call Mr. C. We explain how our process was guided by recent understandings of an abnormal neural circuit that appears to cause catatonia’s complex motor and behavioral symptoms.
This article describes that neurologic pathology and answers common questions about the clinical workup and treatment of catatonia.
CASE: TROUBLE IN TV LAND
Mr. C, age 69, caused a disturbance at a local TV station, demanding that they broadcast a manuscript he had written. Police took him to a local hospital, where he was stabilized and then transferred to a neuropsychiatric hospital for evaluation.
The psychiatric interview revealed that he had developed insomnia, excessive activity, and delusional thinking 2 weeks before admission. His medical history included coronary artery disease (CAD), hypertension, and hypothyroidism. Medications included thyroid hormone replacement therapy, furosemide, potassium, ranitidine, simvastatin, metoprolol, and lisinopril. CAD treatment included stent placement and nitroglycerin as needed.
He had been hospitalized in his 30s and treated with ECT for what he called “bad thoughts.” He said he improved after 1 month and had no subsequent psychiatric history. He denied drug or alcohol abuse.
Shortly after admission, he refused to eat or drink and after 1 week became dehydrated. He also showed mutism, immobility, and stupor. He was transferred to the medical service for IV rehydration.
MANY SCENARIOS AND SIGNS
Mr. C’s symptoms suggest possible catatonia, a neuropsychiatric syndrome of motor dysregulation found in up to 10% of acutely ill psychiatric inpatients.1,2 A movement disorder,1,2 catatonia occurs with general medical conditions and psychiatric disorders (Table 1).
Pathophysiology. Catatonic signs develop when aberrant signals from neurochemical abnormalities trigger a neural circuit that affects the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Box).3-5
Presentation. A focused exam is required because patients with catatonia often do not provide a comprehensive or reliable history.2 They show mutism, characteristic postures, rigidity, aberrant speech, negativism, and stereotyped behaviors.1,2 They may present in an excited or retarded state:
- Excited patients may injure themselves or others and develop hyperthermia, tachycardia, and elevated blood pressure from excessive motor activity.
- Patients in a retarded state may present with bradykinesia and poor self-care. They may be unresponsive to external stimuli, develop catatonic stupor, and refuse to eat or drink.
Mr. C’s earlier insomnia, excessive activity, and delusional thinking (such as the TV station incident) may have signaled an excited catatonia. On admission to the medical service, however, he presented in a retarded state.
Signs. Part of the challenge with detecting catatonia’s signs is that there are so many; some rating scales list more than 20. Not all signs need to be present to make the diagnosis, however, and if you find one, others usually turn up in the examination.
A mnemonic from the Bush-Francis Catatonia Screening Instrument (Table 2) represents diagnostic signs in patients with the excited or retarded forms.2 We recommend that you review an authoritative text (see Related resources) to understand catatonia’s psychopathology.2
Table 1
Common diagnoses of patients with catatonia
| Psychiatric |
|
| Organic |
|
Catatonia is caused by neurochemical abnormalities including low GABA activity in the frontal cortex, low dopamine (D2) activity in the basal ganglia, high glutamate—N-methyl-D-aspartate (NMDA)—activity in the parietal cortex, or a combination of these.3-5 Catatonic signs occur when these neurochemical changes cause aberrant signals and trigger a neural circuit affecting the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Figure).
Posturing occurs when the aberrant signal reaches the posterior parietal lobe. Patients’ bizarre and mundane postures in catatonia are maintained by “anosognosia of position.” For example, an individual does not know the position of rest for his arm, and it remains in an unusual position as if at rest.3
The PP goes on to influence the supplemental motor area (SMA), causing bradykinesia, rigidity, and other motor phenomena that catatonia shares with Parkinson’s disease. The SMA feeds back to the medial orbital gyrus, completing the neural circuit.3
Regions such as the anterior cingulate area (ACA) and amygdala (1AMG) — also may be recruited into the expanded circuit. ACA recruitment may cause akinetic mutism, and fear is a symptom of AMG recruitment. If the anterior hypothalamus is affected, malignant catatonia or neuroleptic malignant syndrome may occur.3,5
This neural loop demonstrates an integrated model of psychosis. It may help explain why catatonia responds to treatment with lorazepam, ECT, and other agents such as antipsychotics and NMDA antagonists.
Illustration for CURRENT PSYCHIATRY by Marcia Hartsock, CMI
Table 2
WIRED `N MIRED: Mnemonic for detecting catatonia
| Waxy flexibility/catalepsy |
| Immobility/stupor |
| Refusal to eat or drink |
| Excitement |
| Deadpan staring |
| Negativism/negative symptoms |
| Mutism |
| Impulsivity |
| Rigidity |
| Echolalia/echopraxia |
| Direct observation |
CASE CONTINUED: MAKING THE DIAGNOSIS
In the medical unit, Mr. C was found to be in a catatonic stupor, with immobility, mutism (monosyllabic speech), catalepsy, intermittent waxy flexibility, withdrawal (refusal to eat and drink), automatic obedience, and mitgehen (exaggerated movements in response to light finger pressure, despite instructions to stay still). ECT workup was started, along with a trial of lorazepam, 1 mg tid.
Laboratory studies revealed high BUN/creatinine (80/2.0) that returned to normal range (BUN 7 to 21 mg/dL; creatinine 0.5 to 1.2 mg/dL) after 3 days of hydration. Because of Mr. C’s earlier excited symptoms and delusional thinking, we considered a diagnosis of bipolar disorder with catatonia. However, his symptoms did not improve with a trial of valproic acid (serum level 64 mcg/mL).
Head CT showed generalized atrophy and EEG showed delta slowing. Single-photo emission computer tomography (SPECT) showed areas of decreased perfusion in the cortex, with no perfusion in the left posterior parietal area (PP).
Mental status exam found Mr. C disoriented with poor short-term memory and unable to complete the Mini Mental State Examination (MMSE). His Bush-Francis Catatonia Rating Scale score was 28 and included many catatonic signs that would not be seen a patient with simple dehydration.
The workup supported a diagnosis of catatonia due to general medical condition (vascular dementia) and ruled out schizophrenia with catatonic features, bipolar disorder, or major depression with catatonia.
EVALUATION AND DIAGNOSIS
Medical causes. A careful history and thorough physical examination are essential for making an accurate diagnosis and ruling out medical conditions that could present with or mimic catatonia (Table 3). Medications that can induce catatonia include antipsychotics, corticosteroids, and disulfiram at therapeutic doses. Drug abuse (such as with phencyclidine), use of the general anesthetic ketamine, and benzodiazepine withdrawal may also lead to catatonia.
Head CT or MRI is indicated for patients being considered for ECT or for localizing neurologic findings. EEG can be useful when patients present with features of seizure activity—such as tongue biting, incontinence, or stupor—or with catatonia as a manifestation of delirium or dementia.
A history of head injury or neurologic disease warrants further neurologic investigation. Also consider a neurology consult when the patient has prolonged stupor or does not respond to initial drug therapy.
Psychiatric causes. The clinical setting may suggest the most likely primary psychiatric disorders to consider, such as:
- bipolar or major depression in acute inpatient psychiatric units
- autism and pervasive developmental disorders (PDD) in pediatric or PDD units
- catatonic schizophrenia in chronic psychotic patients
- somatoform or factitious disorders in forensic settings.
These generalizations are not clinically exclusive, of course, but may provide a starting point for the treatment team confronted with limited history and exam information.
Table 3
Catatonia workup: Recommended lab tests
| Test | Recommendation |
|---|---|
| Complete blood count with WBC differential | Look for leukocytosis |
| Serum chemistries | Look for electrolyte imbalances |
| Serum iron | May be low in NMS |
| Serum creatine kinase | If NMS is suspected |
| Brain MRI or CT | If structural lesion is suspected |
| Electroencephalography | If seizure disorder or brain abnormality is suspected |
| Lumbar puncture | If encephalitis or meningits is suspected |
| NMS: neuroleptic malignant syndrome | |
Initial treatment. Catatonia related to medical and psychiatric causes has been shown to respond to lorazepam and to ECT.6,7 Lorazepam is preferred because of its specificity for the GABAa receptor and ease of administration (oral, IM, or IV). Other agents that act on GABA—including amobarbital and zolpidem—have also been used. Catatonia’s hallmark features such as mutism and immobility have been shown to respond to lorazepam.8,9
ECT is a first-line treatment for catatonia with life-threatening conditions and should be considered for refractory cases.
Lorazepam. The starting dosage is usually 1 mg tid for healthy adults; 0.5 mg tid can be used for children and the elderly. Observe the patient for improvement in catatonic signs after the first dose and before giving the second. Dosages of up to 16 mg/d have been used.
In many cases, lorazepam can be tapered off after adequate treatment of the primary psychiatric condition. In severe cases, however—such as when patients refuse to eat or drink—lorazepam may be continued for as long as 1 year. Weigh the risk of benzodiazepine tolerance, dependence, and misuse versus the possibility of relapse and rehospitalization.
Medical catatonias and neuroleptic malignant syndrome (NMS) have responded favorably to ECT.8 Addressing the medical cause itself usually does not resolve catatonia, with the possible exception of seizure-induced (“ictal”) catatonia, which may respond to anticonvulsants and lorazepam.6,7
ECT. An ECT workup can begin as soon as a patient presents with catatonia. If lorazepam produces no response within 24 hours, consider ECT.
CASE CONTINUED: PERSISTENT SYMPTOMS
After three 1-mg doses of lorazepam, Mr. C became more alert and oriented but his catatonia symptoms persisted, as indicated by a Bush-Francis score of 23, significant grasp reflex, and gegenhalten (automatic rather than willful resistance to passive limb movement in proportion to the strength of the stimulus). An attempt to gradually increase lorazepam to 2 mg tid produced delirium. He remained confused even when lorazepam was reduced to 0.5 mg tid, so the drug was discontinued.
Mr. C’s neurologist added amantadine, 100 mg tid, and carbidopa/levodopa, 10/100 mg tid, to treat his parkinsonian rigidity.
WHAT NEXT? OTHER OPTIONS
Antipsychotics have been investigated as a possible treatment for catatonia. The literature suggests that conventional antipsychotics may cause catatonia and atypical antipsychotics may improve it. Conventional antipsychotics are best avoided in catatonia because they:
- appear less effective than other treatments in resolving catatonic symptoms8,10
- are associated with catatonic-like side effects, such as rigidity, akinesia, and staring10
- appear to increase NMS risk in patients with catatonic symptoms.11,12
Atypicals appear more effective in treating catatonia and less likely to cause NMS. Case reports13,14 indicate many of these agents can be effective and well tolerated in treating catatonic symptoms, although this was not the case for Mr. C.
Anticonvulsants such as valproate15 and carbamazepine, 600 to 1200 mg/d,16 may take longer to work than lorazepam but may be options for patients who do not respond to benzodiazepines.8,9
Amantadine, an N-methyl-D-aspartate (NMDA) antagonist, has been used with some success for catatonia that does not unrespond to lorazepam.17 However, amantidine’s dopamine agonist activity could worsen underlying psychosis.
Memantine—another NMDA antagonist—differs from amantadine despite having a similar chemical structure. Memantine is a noncompetitive antagonist at the NMDA receptor, without affinity for dopamine, norepinephrine, serotonin, or muscarinic receptors.18
Although no published data support using memantine in patients with catatonia, it might be considered for those who are not candidates for lorazepam or ECT. For instance, a double-blind, placebo-controlled study found that lorazepam was not effective for catatonic schizophrenia.19 We have found memantine to help in some patients with catatonic schizophrenia.
CASE CONTINUED: TRIAL OF MEMANTINE
Mr. C remained in a catatonic stupor, but we decided against ECT because he resumed eating and drinking and was not medically at risk. Quetiapine, 100 to 300 mg/d, was tried to address his dementia symptoms, confusion, and poor mentation. This trial was discontinued after Mr. C fell and was readmitted to the medical unit. We then added memantine, 5 mg bid.
In the first week after beginning memantine, Mr. C’s MMSE score was 21, consistent with vascular dementia, but he remained immobile and staring. Motor signs also persisted, including automatic obedience, ambitendency, and a grasp reflex.
The next week, we increased memantine to 10 mg bid. Mr. C was oriented to person, place, and time, and his affect was blunted. His MMSE score increased to 25, showing improved cognition and memory. His Bush-Francis scale score was 6, showing reduced catatonic signs, with remaining mild immobility, bradykinesia, speech-prompt mutism, staring, and grasp reflex.
He maintained this improvement on carbidopa/levodopa, 10/100 tid; amantadine, 100 mg tid; and memantine, 10 mg bid, and was discharged from the nursing home unit.
IMPROVEMENT WITH MEMANTINE
Memantine may reduce excess glutamate at the NMDA receptor in the parietal-SMA-frontal cortical circuit. It may help to increase GABA and dopamine, which are deficient in catatonia. Our patient with vascular dementia had a severe ischemic deficit in the posterior parietal area, as seen on SPECT.
Amantadine, another NMDA receptor antagonist, acts on dopamine neurons and may have anticholinergic-like side effects, whereas memantine does not. Although both drugs share antagonism at the NMDA glutamate receptor, noncompetitive binding is weak for amantadine and moderate for memantine. Memantine has some serotonin (5-HT3) antagonism, but neither agent has direct GABA activity.
Memantine can improve function in vascular dementia.20 Thus, Mr. C’s improvement may have been caused by the drug’s effect on his vascular dementia, the primary neuropsychiatric illness. However, his catatonic signs improved without antipsychotics, cholinesterase inhibitors, benzodiazepines, or ECT. No anticoagulation treatment or cerebral perfusion procedures account for his improved mental status.
CASE CONCLUSION
Mr. C went to live with his son’s family. Although he has problems with calculation, he shows good selfcare. When asked why he did not respond during his catatonic stupor, Mr. C stated that he believed the physician was an Internal Revenue Service agent asking him about serious tax problems. Upon reflection, he said he no longer believes this.
- Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press, 2003.
- Caroff SN, Mann SC, Francis A, Fricchione GE. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing, 2004.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003.
- Neuroleptic Malignant Syndrome Information Service. www.NMSIS.org.
Drug brand names
- Amantadine • Symmetrel
- Amobarbital • Amytal sodium
- Carbamazepine • Carbatrol, Equetro
- Carbidopa/levodopa • Sinemet
- Disulfiram • Antabuse
- Divalproex • Depakote
- Furosemide • Lasix
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Memantine • Namenda
- Metoprolol • Lopressor
- Ranitidine • Zantac
- Simvastatin • Zocor
- Valproic acid • Depakene
- Zolpidem • Ambien
Disclosure
Dr. Carroll and Dr. Hawkins are speakers for Forest Laboratories. The other authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Dr. Niraj Ahuja, consultant psychiatrist and honorary clinical lecturer (psychiatry), Newcastle, North Tyneside and Northumberland Mental Health Trust, UK, for assistance with the figure.
1. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry 2003;160:1233-41.
2. Bush G, Fink M, Petrides G, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
3. Northoff G. What catatonia can tell us about “top-down” modulation:” a neuropsychiatric hypothesis. Brain Behav Sci 2002;25:555-604.
4. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums 2000;5(7):26-33.
5. Carroll BT. Catatonia is the rosetta stone of psychosis (poster presentation). New York: American Psychiatric Association annual meeting, 2004.
6. Barnes MP, Saunders M, Walls TJ, et al. The syndrome of Karl Ludwig Kahlbaum. J Neurol Neurosurg Psychiatry 1986;49:991-6.
7. Carroll BT, Anfinson TJ, Kennedy JC, et al. Catatonic disorder due to general medical conditions. J Neuropsychiatry Clin Neurosci 1994;6:122-33.
8. Hawkins JM, Archer KJ, Strakowski SM, Keck PE. Somatic treatments of catatonia. Int J Psychiatry Med 1995;25:345-69.
9. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
10. Dose M. Neuroleptic-induced pseudo-catatonia. Pharmacopsychiatry 2001;34:262-4.
11. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. Arch Gen Psychiatry 1989;46:914-18.
12. White DAC. 17 catatonic patients diagnosed as neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
13. Levy WO, Nunez CY. Use of ziprasidone to treat bipolar-associated catatonia. Bipolar Disord 2004;6:166-7.
14. Hesslinger B, Walden J, Normann C. Acute and long-term treatment of catatonia with risperidone. Pharmacopsychiatry 2001;34:25-6.
15. Kruger S, Braunig P. Intravenous valproic acid in the treatment of severe catatonia. J Neuropsychiatry Clin Neurosci 2001;13:303-4.
16. Kritzinger PR, Jordaan GP. Catatonia: an open prospective series with carbamazepine. Int J Neuropsychopharmacol 2001;4:251-7.
17. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
18. Namenda (memantine) Package labeling. Forest Laboratories, 2004.
19. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: a random, double-blind, placebo-controlled, cross-over study. Psychopharmacol 1999;142:393-8.
20. Mobius HJ. Pharmacologic rationale for memantine in chronic cerebral hypoperfusion, especially vascular dementia. Alz Dis Assoc Disord 1999;13(suppl 3):172-8.
1. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry 2003;160:1233-41.
2. Bush G, Fink M, Petrides G, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
3. Northoff G. What catatonia can tell us about “top-down” modulation:” a neuropsychiatric hypothesis. Brain Behav Sci 2002;25:555-604.
4. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums 2000;5(7):26-33.
5. Carroll BT. Catatonia is the rosetta stone of psychosis (poster presentation). New York: American Psychiatric Association annual meeting, 2004.
6. Barnes MP, Saunders M, Walls TJ, et al. The syndrome of Karl Ludwig Kahlbaum. J Neurol Neurosurg Psychiatry 1986;49:991-6.
7. Carroll BT, Anfinson TJ, Kennedy JC, et al. Catatonic disorder due to general medical conditions. J Neuropsychiatry Clin Neurosci 1994;6:122-33.
8. Hawkins JM, Archer KJ, Strakowski SM, Keck PE. Somatic treatments of catatonia. Int J Psychiatry Med 1995;25:345-69.
9. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
10. Dose M. Neuroleptic-induced pseudo-catatonia. Pharmacopsychiatry 2001;34:262-4.
11. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. Arch Gen Psychiatry 1989;46:914-18.
12. White DAC. 17 catatonic patients diagnosed as neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
13. Levy WO, Nunez CY. Use of ziprasidone to treat bipolar-associated catatonia. Bipolar Disord 2004;6:166-7.
14. Hesslinger B, Walden J, Normann C. Acute and long-term treatment of catatonia with risperidone. Pharmacopsychiatry 2001;34:25-6.
15. Kruger S, Braunig P. Intravenous valproic acid in the treatment of severe catatonia. J Neuropsychiatry Clin Neurosci 2001;13:303-4.
16. Kritzinger PR, Jordaan GP. Catatonia: an open prospective series with carbamazepine. Int J Neuropsychopharmacol 2001;4:251-7.
17. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
18. Namenda (memantine) Package labeling. Forest Laboratories, 2004.
19. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: a random, double-blind, placebo-controlled, cross-over study. Psychopharmacol 1999;142:393-8.
20. Mobius HJ. Pharmacologic rationale for memantine in chronic cerebral hypoperfusion, especially vascular dementia. Alz Dis Assoc Disord 1999;13(suppl 3):172-8.
Blame the brain for promiscuity?
Why do some people stay married for decades while others jump from one relationship to the next? If humans are like rodents, recent research suggests that neuropeptides may help forge the ties that bind.
FOLLOWING VOLE BEHAVIOR
The vole, which inhabits many grasslands in the United States, is a rodent resembling a mouse but related to the lemming. Males in some vole species (such as meadow voles) show no partner preference whereas males in other species prefer one partner, share the same nest with her, and help care for their offspring.1
The neuropeptides oxytocin and arginine vasopressin are mediators of pair bonding. The brain of a prairie vole—a species in which males tend to bond with female partners—has more vasopressin receptors than that of the solitary meadow vole (Figure 1).
Figure 1 More vasopressin in bonding voles
Ventral forebrain autoradiograms show greater vasopressin (VP) expression in prairie voles (top) than meadow voles.
Reprinted with permission. © 2004, Nature Publishing Group.Lim et al, however, found that meadow voles were more likely to bond with a single female after the males’ vasopressin receptors were increased.2 The researchers isolated and replicated the gene sequence responsible for the vasopressin receptor in the prairie vole. Then, using a viral vector, they injected the gene into the ventral pallidum of 11 male meadow voles. Eleven other voles received placebo.
Two weeks later, each sexually naïve male vole was housed for 24 hours with a sexually receptive female vole. Then, each male was placed for 3 hours in a three-chamber apparatus with the partner female in one chamber and a novel female in another. The time spent huddling with each female was recorded.
Across 3 hours, the placebo group voles spent 10 to 15 minutes with either female—normal behavior for this species. By contrast, the voles that received the vasopressin receptors spent approximately 40 minutes with their partners but only about 5 minutes with the novel voles, thus showing more affiliative behavior and a clear preference for their mates (Figure 2). The findings suggest that the researchers may have produced a profound change in social behavior by altering one gene.
IMPLICATIONS FOR HUMANS
How this research relates to humans is unknown, as there is no sound evidence of a link between vole and human pair bonding. Likewise, the influence of the higher cortical areas in orchestrating human behaviors cannot be underestimated.
Neuroimaging, however, has shown that brain regions rich with oxytocin and vasopressin receptors are activated while a person views pictures of loved ones.3 Additionally, mens’ vasopressin levels have been shown to increase when they are sexually aroused.4 Whether these findings one day lead to a medicine that promotes monogamous behavior in men remains to be seen.5
Figure 2 Male meadow voles’ interactions with females after vasopressin or placebo treatment
Source: Adapted from reference 2
1. Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci 2004;7:1048-54.
2. Lim MM, Wang Z, Olazabal DE, et al. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 2004;429(6993):754-7.
3. Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage 2004;21:1155-66.
4. Murphy MR, Seckl JR, Burton S, et al. Changes in oxytocin and vasopressin secretion during sexual activity in men. J Clin Endocrinol Metab 1987;65:738-41.
5. Konner M. The ties that bind. Nature 2004;429(6993):705.-
Why do some people stay married for decades while others jump from one relationship to the next? If humans are like rodents, recent research suggests that neuropeptides may help forge the ties that bind.
FOLLOWING VOLE BEHAVIOR
The vole, which inhabits many grasslands in the United States, is a rodent resembling a mouse but related to the lemming. Males in some vole species (such as meadow voles) show no partner preference whereas males in other species prefer one partner, share the same nest with her, and help care for their offspring.1
The neuropeptides oxytocin and arginine vasopressin are mediators of pair bonding. The brain of a prairie vole—a species in which males tend to bond with female partners—has more vasopressin receptors than that of the solitary meadow vole (Figure 1).
Figure 1 More vasopressin in bonding voles
Ventral forebrain autoradiograms show greater vasopressin (VP) expression in prairie voles (top) than meadow voles.
Reprinted with permission. © 2004, Nature Publishing Group.Lim et al, however, found that meadow voles were more likely to bond with a single female after the males’ vasopressin receptors were increased.2 The researchers isolated and replicated the gene sequence responsible for the vasopressin receptor in the prairie vole. Then, using a viral vector, they injected the gene into the ventral pallidum of 11 male meadow voles. Eleven other voles received placebo.
Two weeks later, each sexually naïve male vole was housed for 24 hours with a sexually receptive female vole. Then, each male was placed for 3 hours in a three-chamber apparatus with the partner female in one chamber and a novel female in another. The time spent huddling with each female was recorded.
Across 3 hours, the placebo group voles spent 10 to 15 minutes with either female—normal behavior for this species. By contrast, the voles that received the vasopressin receptors spent approximately 40 minutes with their partners but only about 5 minutes with the novel voles, thus showing more affiliative behavior and a clear preference for their mates (Figure 2). The findings suggest that the researchers may have produced a profound change in social behavior by altering one gene.
IMPLICATIONS FOR HUMANS
How this research relates to humans is unknown, as there is no sound evidence of a link between vole and human pair bonding. Likewise, the influence of the higher cortical areas in orchestrating human behaviors cannot be underestimated.
Neuroimaging, however, has shown that brain regions rich with oxytocin and vasopressin receptors are activated while a person views pictures of loved ones.3 Additionally, mens’ vasopressin levels have been shown to increase when they are sexually aroused.4 Whether these findings one day lead to a medicine that promotes monogamous behavior in men remains to be seen.5
Figure 2 Male meadow voles’ interactions with females after vasopressin or placebo treatment
Source: Adapted from reference 2
Why do some people stay married for decades while others jump from one relationship to the next? If humans are like rodents, recent research suggests that neuropeptides may help forge the ties that bind.
FOLLOWING VOLE BEHAVIOR
The vole, which inhabits many grasslands in the United States, is a rodent resembling a mouse but related to the lemming. Males in some vole species (such as meadow voles) show no partner preference whereas males in other species prefer one partner, share the same nest with her, and help care for their offspring.1
The neuropeptides oxytocin and arginine vasopressin are mediators of pair bonding. The brain of a prairie vole—a species in which males tend to bond with female partners—has more vasopressin receptors than that of the solitary meadow vole (Figure 1).
Figure 1 More vasopressin in bonding voles
Ventral forebrain autoradiograms show greater vasopressin (VP) expression in prairie voles (top) than meadow voles.
Reprinted with permission. © 2004, Nature Publishing Group.Lim et al, however, found that meadow voles were more likely to bond with a single female after the males’ vasopressin receptors were increased.2 The researchers isolated and replicated the gene sequence responsible for the vasopressin receptor in the prairie vole. Then, using a viral vector, they injected the gene into the ventral pallidum of 11 male meadow voles. Eleven other voles received placebo.
Two weeks later, each sexually naïve male vole was housed for 24 hours with a sexually receptive female vole. Then, each male was placed for 3 hours in a three-chamber apparatus with the partner female in one chamber and a novel female in another. The time spent huddling with each female was recorded.
Across 3 hours, the placebo group voles spent 10 to 15 minutes with either female—normal behavior for this species. By contrast, the voles that received the vasopressin receptors spent approximately 40 minutes with their partners but only about 5 minutes with the novel voles, thus showing more affiliative behavior and a clear preference for their mates (Figure 2). The findings suggest that the researchers may have produced a profound change in social behavior by altering one gene.
IMPLICATIONS FOR HUMANS
How this research relates to humans is unknown, as there is no sound evidence of a link between vole and human pair bonding. Likewise, the influence of the higher cortical areas in orchestrating human behaviors cannot be underestimated.
Neuroimaging, however, has shown that brain regions rich with oxytocin and vasopressin receptors are activated while a person views pictures of loved ones.3 Additionally, mens’ vasopressin levels have been shown to increase when they are sexually aroused.4 Whether these findings one day lead to a medicine that promotes monogamous behavior in men remains to be seen.5
Figure 2 Male meadow voles’ interactions with females after vasopressin or placebo treatment
Source: Adapted from reference 2
1. Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci 2004;7:1048-54.
2. Lim MM, Wang Z, Olazabal DE, et al. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 2004;429(6993):754-7.
3. Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage 2004;21:1155-66.
4. Murphy MR, Seckl JR, Burton S, et al. Changes in oxytocin and vasopressin secretion during sexual activity in men. J Clin Endocrinol Metab 1987;65:738-41.
5. Konner M. The ties that bind. Nature 2004;429(6993):705.-
1. Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci 2004;7:1048-54.
2. Lim MM, Wang Z, Olazabal DE, et al. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 2004;429(6993):754-7.
3. Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage 2004;21:1155-66.
4. Murphy MR, Seckl JR, Burton S, et al. Changes in oxytocin and vasopressin secretion during sexual activity in men. J Clin Endocrinol Metab 1987;65:738-41.
5. Konner M. The ties that bind. Nature 2004;429(6993):705.-
Correcting steroid data
I wish to correct a mistake in our article on anabolicandrogenic steroids (AAS) (Current Psychiatry, December 2004).
When describing the Malone et al study, we incorrectly cited a statistic on the association between AAS use and attempted suicide. The sentence should have read: “In a field study of 77 steroid users (71 male and 6 female), 3 (3.9%) reported they attempted suicide during AAS withdrawal.”
Harrison G. Pope, Jr., MD
Professor of psychiatry,
Harvard Medical School
I wish to correct a mistake in our article on anabolicandrogenic steroids (AAS) (Current Psychiatry, December 2004).
When describing the Malone et al study, we incorrectly cited a statistic on the association between AAS use and attempted suicide. The sentence should have read: “In a field study of 77 steroid users (71 male and 6 female), 3 (3.9%) reported they attempted suicide during AAS withdrawal.”
Harrison G. Pope, Jr., MD
Professor of psychiatry,
Harvard Medical School
I wish to correct a mistake in our article on anabolicandrogenic steroids (AAS) (Current Psychiatry, December 2004).
When describing the Malone et al study, we incorrectly cited a statistic on the association between AAS use and attempted suicide. The sentence should have read: “In a field study of 77 steroid users (71 male and 6 female), 3 (3.9%) reported they attempted suicide during AAS withdrawal.”
Harrison G. Pope, Jr., MD
Professor of psychiatry,
Harvard Medical School
How psychiatrists are helping our troops
Psychiatrists have a long and respected history of service during wartime. Through their work as clinicians, prevention specialists, and commanders in combat areas, psychiatrists contribute to the emotional well-being of service members—and to the success of military operations.
Clinicians. Psychiatrists are crucial to restoring and maintaining the emotional health of service members in Iraq and Afghanistan. With a limited formulary of psychotropics, psychiatrists are seeing many men and women suffering from sleep deprivation, acute anxiety, and depressive symptoms. Aside from the challenges of deployment in a hostile environment, many service members face marital, family, and financial troubles that have followed them from home.
When a psychotropic is not indicated, such as when a service member is needed back at the front line, psychiatrists perform brief psychotherapeutic interventions such as relaxation training and cognitive therapy.
Prevention specialists. Psychiatrists help maintain fighting strength by providing mental illness prevention services. By identifying service members at risk for emotional problems, the psychiatrist can intervene and provide rudimentary interventions. If a higher level of care is needed, the psychiatrist can oversee the service member’s evacuation to a combat support hospital or to the mental health division.
Prevention specialists also facilitate debriefings after traumatic events. When necessary, the psychiatrist can help normalize feelings and challenge distressing beliefs after combat casualties.
Commanders. Like other military officers, psychiatrists advance through the ranks and serve in positions of authority, such as treatment and prevention team leaders, medical detachment commanders, and brigade surgeons. Commanding psychiatrists also help draft medical evacuation procedures and determine locations for medical treatment facilities.
Every day, psychiatrists put themselves in harms way to meet the mental health needs of service members overseas. There is much to learn from their unique experiences and they should be commended for adding continued respect to the field of psychiatry.
CPT Bret A. Moore, PsyD
Clinical psychologist, 85th Medical Team
Combat Stress Control
Fort Hood, TX
Dr. Moore notes that the views expressed in this letter do not reflect the official policy or position of the Department of the Army, Department of Defense, or the United States government.
Psychiatrists have a long and respected history of service during wartime. Through their work as clinicians, prevention specialists, and commanders in combat areas, psychiatrists contribute to the emotional well-being of service members—and to the success of military operations.
Clinicians. Psychiatrists are crucial to restoring and maintaining the emotional health of service members in Iraq and Afghanistan. With a limited formulary of psychotropics, psychiatrists are seeing many men and women suffering from sleep deprivation, acute anxiety, and depressive symptoms. Aside from the challenges of deployment in a hostile environment, many service members face marital, family, and financial troubles that have followed them from home.
When a psychotropic is not indicated, such as when a service member is needed back at the front line, psychiatrists perform brief psychotherapeutic interventions such as relaxation training and cognitive therapy.
Prevention specialists. Psychiatrists help maintain fighting strength by providing mental illness prevention services. By identifying service members at risk for emotional problems, the psychiatrist can intervene and provide rudimentary interventions. If a higher level of care is needed, the psychiatrist can oversee the service member’s evacuation to a combat support hospital or to the mental health division.
Prevention specialists also facilitate debriefings after traumatic events. When necessary, the psychiatrist can help normalize feelings and challenge distressing beliefs after combat casualties.
Commanders. Like other military officers, psychiatrists advance through the ranks and serve in positions of authority, such as treatment and prevention team leaders, medical detachment commanders, and brigade surgeons. Commanding psychiatrists also help draft medical evacuation procedures and determine locations for medical treatment facilities.
Every day, psychiatrists put themselves in harms way to meet the mental health needs of service members overseas. There is much to learn from their unique experiences and they should be commended for adding continued respect to the field of psychiatry.
CPT Bret A. Moore, PsyD
Clinical psychologist, 85th Medical Team
Combat Stress Control
Fort Hood, TX
Dr. Moore notes that the views expressed in this letter do not reflect the official policy or position of the Department of the Army, Department of Defense, or the United States government.
Psychiatrists have a long and respected history of service during wartime. Through their work as clinicians, prevention specialists, and commanders in combat areas, psychiatrists contribute to the emotional well-being of service members—and to the success of military operations.
Clinicians. Psychiatrists are crucial to restoring and maintaining the emotional health of service members in Iraq and Afghanistan. With a limited formulary of psychotropics, psychiatrists are seeing many men and women suffering from sleep deprivation, acute anxiety, and depressive symptoms. Aside from the challenges of deployment in a hostile environment, many service members face marital, family, and financial troubles that have followed them from home.
When a psychotropic is not indicated, such as when a service member is needed back at the front line, psychiatrists perform brief psychotherapeutic interventions such as relaxation training and cognitive therapy.
Prevention specialists. Psychiatrists help maintain fighting strength by providing mental illness prevention services. By identifying service members at risk for emotional problems, the psychiatrist can intervene and provide rudimentary interventions. If a higher level of care is needed, the psychiatrist can oversee the service member’s evacuation to a combat support hospital or to the mental health division.
Prevention specialists also facilitate debriefings after traumatic events. When necessary, the psychiatrist can help normalize feelings and challenge distressing beliefs after combat casualties.
Commanders. Like other military officers, psychiatrists advance through the ranks and serve in positions of authority, such as treatment and prevention team leaders, medical detachment commanders, and brigade surgeons. Commanding psychiatrists also help draft medical evacuation procedures and determine locations for medical treatment facilities.
Every day, psychiatrists put themselves in harms way to meet the mental health needs of service members overseas. There is much to learn from their unique experiences and they should be commended for adding continued respect to the field of psychiatry.
CPT Bret A. Moore, PsyD
Clinical psychologist, 85th Medical Team
Combat Stress Control
Fort Hood, TX
Dr. Moore notes that the views expressed in this letter do not reflect the official policy or position of the Department of the Army, Department of Defense, or the United States government.
Recovered memories, hypnosis, and dissociation
Dr. Grant writes, “Recovered memory, hypnosis, and multiple personality disorder lack a clear standard of care and are potential legal minefields for any clinician” (“Malpractice Verdicts”).
This misinformation has done the trauma profession—and your readers—an injustice. The American Medical Association and American Psychiatric Association both sanction the use of hypnosis, the oldest Western version of psychotherapy. Furthermore:
- All memories are recovered memories.
- “Multiple personality disorder” is now called “dissociative identity disorder.”
At least six scientific organizations over the past decade have published treatment guidelines for dissociative identity disorder, recovered memories, and use of hypnosis.1-7 When the guidelines are followed, these areas are not dangerous.
Dr. Grant’s article could prompt clinicians to avoid effective and safe treatments. We have treated dissociative identity disorder and recovered memories for 23 years and 30 years, respectively, without touching a legal land mine.
Elizabeth S. Bowman, MD
Editor emerita, Journal of Trauma and Dissociation
Adjunct professor, Department of neurology
Indiana University, Indianapolis
David Spiegel, MD
Jack, Lulu and Sam Willson
Professor in the School of Medicine
Associate chair of psychiatry & behavioral sciences
Stanford University School of Medicine
Stanford, CA
- Roth S, Friedman MJ (eds). Childhood trauma remembered. A report on the current scientific knowledge base and its applications. International Society for Traumatic Stress Studies; 1997. Available at: www.istss.org/publications/ChildhoodTraumaRemembered.pdf. Accessed Feb. 7, 2005.
- American Medical Association. Memories of childhood abuse. Report of the Council on Scientific Affairs. (CSA Report 5-A-94). Available at: www.amaassn.org/apps/pf_new/pf_online?f_n=resultLink&doc=policyfiles/HnE/H-515.973.HTM. Accessed Feb. 7, 2005.
- American Psychiatric Association Fact Sheet: Therapies focused on memories of childhood physical and sexual abuse. Available at: www.psych.org/public_info/memorieschildphysexualabuse.pdf. Accessed Jan. 28, 2005.
- Recovered memories. The report of the working party of the British Psychological Society. In: Pezdek K, Banks W (eds). The recovered memory/false memory debate. New York: Academic Press; 1996.
- International Society for the Study of Dissociation Standards of Practice Committee. Guidelines for treating dissociative identity disorder (multiple personality disorder) in adults (1997). J Trauma Dissoc 2000;1:115-34.
- International Society for the Study of Dissociation Task Force on Children and Adolescents. Guidelines for the evaluation and treatment of dissociative symptoms in children and adolescents. J Trauma Dissoc 2004;5:119-50.
- American Society of Clinical Hypnosis, Hypnosis and Memory Committee. Clinical hypnosis and memory: guidelines for clinicians and for forensic hypnosis. Chicago: American Society for Clinical Hypnosis Press; 1995.
Dr. Grant responds
I appreciate the above comments and respect the authors’ clinical and research perspectives.
To clarify, I don’t want to suggest that clinicians avoid effective treatments for any psychiatric difficulty, such as dissociative identity disorder or childhood sexual abuse. These treatments by experienced clinicians can be beneficial.
My intention, however, was to convey the need for caution when using hypnosis for recovered memories of childhood sexual abuse, as this may represent complex legal issues.
Jon E. Grant, JD, MD, MPH
Assistant professor of psychiatry and human behavior
Brown Medical School, Providence, RI
Dr. Grant writes, “Recovered memory, hypnosis, and multiple personality disorder lack a clear standard of care and are potential legal minefields for any clinician” (“Malpractice Verdicts”).
This misinformation has done the trauma profession—and your readers—an injustice. The American Medical Association and American Psychiatric Association both sanction the use of hypnosis, the oldest Western version of psychotherapy. Furthermore:
- All memories are recovered memories.
- “Multiple personality disorder” is now called “dissociative identity disorder.”
At least six scientific organizations over the past decade have published treatment guidelines for dissociative identity disorder, recovered memories, and use of hypnosis.1-7 When the guidelines are followed, these areas are not dangerous.
Dr. Grant’s article could prompt clinicians to avoid effective and safe treatments. We have treated dissociative identity disorder and recovered memories for 23 years and 30 years, respectively, without touching a legal land mine.
Elizabeth S. Bowman, MD
Editor emerita, Journal of Trauma and Dissociation
Adjunct professor, Department of neurology
Indiana University, Indianapolis
David Spiegel, MD
Jack, Lulu and Sam Willson
Professor in the School of Medicine
Associate chair of psychiatry & behavioral sciences
Stanford University School of Medicine
Stanford, CA
- Roth S, Friedman MJ (eds). Childhood trauma remembered. A report on the current scientific knowledge base and its applications. International Society for Traumatic Stress Studies; 1997. Available at: www.istss.org/publications/ChildhoodTraumaRemembered.pdf. Accessed Feb. 7, 2005.
- American Medical Association. Memories of childhood abuse. Report of the Council on Scientific Affairs. (CSA Report 5-A-94). Available at: www.amaassn.org/apps/pf_new/pf_online?f_n=resultLink&doc=policyfiles/HnE/H-515.973.HTM. Accessed Feb. 7, 2005.
- American Psychiatric Association Fact Sheet: Therapies focused on memories of childhood physical and sexual abuse. Available at: www.psych.org/public_info/memorieschildphysexualabuse.pdf. Accessed Jan. 28, 2005.
- Recovered memories. The report of the working party of the British Psychological Society. In: Pezdek K, Banks W (eds). The recovered memory/false memory debate. New York: Academic Press; 1996.
- International Society for the Study of Dissociation Standards of Practice Committee. Guidelines for treating dissociative identity disorder (multiple personality disorder) in adults (1997). J Trauma Dissoc 2000;1:115-34.
- International Society for the Study of Dissociation Task Force on Children and Adolescents. Guidelines for the evaluation and treatment of dissociative symptoms in children and adolescents. J Trauma Dissoc 2004;5:119-50.
- American Society of Clinical Hypnosis, Hypnosis and Memory Committee. Clinical hypnosis and memory: guidelines for clinicians and for forensic hypnosis. Chicago: American Society for Clinical Hypnosis Press; 1995.
Dr. Grant responds
I appreciate the above comments and respect the authors’ clinical and research perspectives.
To clarify, I don’t want to suggest that clinicians avoid effective treatments for any psychiatric difficulty, such as dissociative identity disorder or childhood sexual abuse. These treatments by experienced clinicians can be beneficial.
My intention, however, was to convey the need for caution when using hypnosis for recovered memories of childhood sexual abuse, as this may represent complex legal issues.
Jon E. Grant, JD, MD, MPH
Assistant professor of psychiatry and human behavior
Brown Medical School, Providence, RI
Dr. Grant writes, “Recovered memory, hypnosis, and multiple personality disorder lack a clear standard of care and are potential legal minefields for any clinician” (“Malpractice Verdicts”).
This misinformation has done the trauma profession—and your readers—an injustice. The American Medical Association and American Psychiatric Association both sanction the use of hypnosis, the oldest Western version of psychotherapy. Furthermore:
- All memories are recovered memories.
- “Multiple personality disorder” is now called “dissociative identity disorder.”
At least six scientific organizations over the past decade have published treatment guidelines for dissociative identity disorder, recovered memories, and use of hypnosis.1-7 When the guidelines are followed, these areas are not dangerous.
Dr. Grant’s article could prompt clinicians to avoid effective and safe treatments. We have treated dissociative identity disorder and recovered memories for 23 years and 30 years, respectively, without touching a legal land mine.
Elizabeth S. Bowman, MD
Editor emerita, Journal of Trauma and Dissociation
Adjunct professor, Department of neurology
Indiana University, Indianapolis
David Spiegel, MD
Jack, Lulu and Sam Willson
Professor in the School of Medicine
Associate chair of psychiatry & behavioral sciences
Stanford University School of Medicine
Stanford, CA
- Roth S, Friedman MJ (eds). Childhood trauma remembered. A report on the current scientific knowledge base and its applications. International Society for Traumatic Stress Studies; 1997. Available at: www.istss.org/publications/ChildhoodTraumaRemembered.pdf. Accessed Feb. 7, 2005.
- American Medical Association. Memories of childhood abuse. Report of the Council on Scientific Affairs. (CSA Report 5-A-94). Available at: www.amaassn.org/apps/pf_new/pf_online?f_n=resultLink&doc=policyfiles/HnE/H-515.973.HTM. Accessed Feb. 7, 2005.
- American Psychiatric Association Fact Sheet: Therapies focused on memories of childhood physical and sexual abuse. Available at: www.psych.org/public_info/memorieschildphysexualabuse.pdf. Accessed Jan. 28, 2005.
- Recovered memories. The report of the working party of the British Psychological Society. In: Pezdek K, Banks W (eds). The recovered memory/false memory debate. New York: Academic Press; 1996.
- International Society for the Study of Dissociation Standards of Practice Committee. Guidelines for treating dissociative identity disorder (multiple personality disorder) in adults (1997). J Trauma Dissoc 2000;1:115-34.
- International Society for the Study of Dissociation Task Force on Children and Adolescents. Guidelines for the evaluation and treatment of dissociative symptoms in children and adolescents. J Trauma Dissoc 2004;5:119-50.
- American Society of Clinical Hypnosis, Hypnosis and Memory Committee. Clinical hypnosis and memory: guidelines for clinicians and for forensic hypnosis. Chicago: American Society for Clinical Hypnosis Press; 1995.
Dr. Grant responds
I appreciate the above comments and respect the authors’ clinical and research perspectives.
To clarify, I don’t want to suggest that clinicians avoid effective treatments for any psychiatric difficulty, such as dissociative identity disorder or childhood sexual abuse. These treatments by experienced clinicians can be beneficial.
My intention, however, was to convey the need for caution when using hypnosis for recovered memories of childhood sexual abuse, as this may represent complex legal issues.
Jon E. Grant, JD, MD, MPH
Assistant professor of psychiatry and human behavior
Brown Medical School, Providence, RI
Dealing with dissociation
In “Malpractice Verdicts” (Current Psychiatry, January 2005) Dr. Jon Grant offers valuable suggestions on how to proceed with caution when assessing risk or ordering involuntary admissions.
Concerning his statement that there is no standard of care for dissociative identity disorder, the International Society for the Study of Dissociation has developed standards for assessing and treating dissociation. These guidelines are available at www.issd.org.
Fran S. Waters, DCSW, LMFT
President, International Society for the Study of Dissociation
Marquette, MI
In “Malpractice Verdicts” (Current Psychiatry, January 2005) Dr. Jon Grant offers valuable suggestions on how to proceed with caution when assessing risk or ordering involuntary admissions.
Concerning his statement that there is no standard of care for dissociative identity disorder, the International Society for the Study of Dissociation has developed standards for assessing and treating dissociation. These guidelines are available at www.issd.org.
Fran S. Waters, DCSW, LMFT
President, International Society for the Study of Dissociation
Marquette, MI
In “Malpractice Verdicts” (Current Psychiatry, January 2005) Dr. Jon Grant offers valuable suggestions on how to proceed with caution when assessing risk or ordering involuntary admissions.
Concerning his statement that there is no standard of care for dissociative identity disorder, the International Society for the Study of Dissociation has developed standards for assessing and treating dissociation. These guidelines are available at www.issd.org.
Fran S. Waters, DCSW, LMFT
President, International Society for the Study of Dissociation
Marquette, MI