User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Herbal hazards: Which psychotropics interact with four common supplements
What do you tell your patients who are self-medicating with herbal remedies? Can dietary supplements safely improve mood disorders, insomnia, and other psychiatric complaints?
Evidence is limited on complementary and alternative medicines (CAMs), their active components, pharmacokinetics/dynamics, adverse effects, drug interactions, and therapeutic outcomes. Based on our review of trial data, case reports, and an NIH National Center for Complementary and Alternative Medicine (NCCAM) survey,1 we offer information to help you:
- identify patients using dietary supplements
- avoid serious interactions with common psychotropics
- counsel patients on the efficacy and safety of ginkgo biloba, St. John’s wort, kava kava, and valerian (Table 1).
DON’T BE AFRAID TO ASK
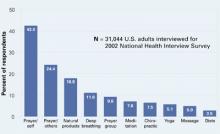
One-third of Americans who took part in the NCCAM survey reported using CAM. After prayer—the number-one CAM—respondents said they most often used natural products such as herbals, botanicals, nutraceuticals, phytomedicinals, and dietary supplements (Figure).1
Table 1
4 herbal supplements with purported psychotropic effects
| Herb | Promoted use | Safety | Recommendation |
|---|---|---|---|
| Ginkgo biloba | Dementia, memory | Bleeding complications, drug interactions a concern | Some data support a trial in dementia; beware of safety concerns |
| St. John’s wort | Depression | Substantial drug interactions | Use not recommended because of wide-ranging drug interactions |
| Kava kava | Anxiety | Hepatotoxicity risk, drug interactions; off market in Europe and Canada | Use not recommended because of safety issues |
| Valerian | Insomnia | Limited data available | Benign (?); monitor for possible adverse events or drug interactions |
Patients tend to use CAM to treat chronic medical conditions such as back pain, depression, and anxiety.1 Although CAM use is common, only 38% of patients say they disclose using CAM to their physicians.2 These use and disclosure patterns are similar in psychiatry.3
Herbal products may produce symptoms that mimic those of mental illnesses, such as psychosis and mania, complicating diagnosis and treatment. Abruptly stopping some herbs can produce withdrawal symptoms similar to those seen with benzodiazepine and antidepressant cessation. Supplements also can inhibit or augment prescribed psychotropics’ effects, and notable consequences include the potential for serotonin syndrome with use of St. John’s wort.
The key to learning about a patient’s use of dietary supplements is to ask. Patients commonly say they do not tell their doctors about using dietary supplements because “it wasn’t important for the doctor to know” or “the doctor never asked.”4,5 Ten tips for discussing nutritional supplements with patients are shown in the Box.
Buyer—and psychiatrist—beware. Because of dietary supplements’ regulatory status, physicians and patients need to learn as much as they can about these products’ documented safety and efficacy. The Dietary Supplement Health and Education Act passed by Congress in 1994 does not require manufacturers to prove their products are safe or effective before marketing them. They also can make claims that suggest uses in physical/emotional structure and function, such as “helps maintain a healthy emotional outlook.”
The FDA bears the burden of proof regarding safety and can remove a dietary supplement from the market only after receiving documented adverse event information from the public.6 The FDA has confiscated herbal products containing prescription drugs and misidentified herbal components.
GINKGO BILOBA FOR DEMENTIA
Ginkgo biloba was the third most commonly used herbal product (21%) in the NCCAM survey. Ginkgo is promoted primarily for dementia, cerebrovascular dysfunction, and memory enhancement. The standardized ginkgo extract (EGb 761) contains several components to which its pharmacologic activity has been attributed. Its constituents are thought to act primarily through anticoagulant effects by inhibiting platelet-activating factor and cyclic GMP phosphodiesterase. They also act through membrane stabilization, antioxidant properties, free-radical scavenging, and inhibition of beta-amyloid deposition.7
Efficacy. In patients with dementia, clinical trials using EGb 761 have shown small improvements in or maintenance of cognitive and social functioning, compared with placebo.8,9 The clinical significance of these findings is unclear, however. Ginkgo’s usefulness in enhancing memory is less certain. Controlled clinical trials are evaluating ginkgo’s efficacy in various conditions.10
Adverse effects. Bleeding complications are the primary concern with ginkgo biloba use, and caution is urged when it is taken concomitantly with aspirin or other antithrombotic drugs. Patients receiving warfarin should not take ginkgo because of the combined antiplatelet effects and ginkgo’s inhibition of warfarin metabolism and elimination.
Drug-herb interactions. One report showed that EGb 761 is a strong inhibitor of the cytochrome P-450 2C9 enzyme system (CYP 2C9).11 Other identified inhibitors of CYP 2C9 are fluvoxamine (strong inhibitor), amiodarone, cimetidine, fluoxetine, and omeprazole. Drugs that serve as substrates for that system and can have decreased clearance include phenytoin, warfarin, amitriptyline, and diazepam;12 use caution, therefore, when you co-administer these drugs with ginkgo.
Figure 10 CAM therapies patients report using most often
CAM: Complimentary and alternative medicines
Source: Barnes T, Powell-Griner E, McFann K, Nahin R. Complementary and alternative medicine use among adults: United States, 2002. Atlanta: Centers for Disease Control and Prevention, May 27, 2004: Advance Data Report #343 (available at http://www.cdc.gov/nchs/data/ad/ad343.pdf) Dosage. The usual dosage for standardized ginkgo extract is 120 to 240 mg/d, given in divided doses. Improvements associated with ginkgo use usually are seen within 4 to 12 weeks after starting therapy.7 Consider discontinuing therapy if you see no results after that time. Little is known about ginkgo’s effect after 1 year of use.
Recommendation. Limited data show a slight benefit in treating dementia by slowing cognitive and behavioral decline, but ginkgo biloba cannot be recommended as a first-line treatment until further controlled trials are available.
- Broach the subject without being judgmental; nutritional supplement use is tied to a patient’s health beliefs
- Ask specifically about use of herbs, supplements, teas, elixirs, vitamins, etc., and document in the medical record at each visit
- Include in appointment reminders a request that patients bring all medicines, herbs, and supplements to appointments
- Segue into a discussion of supplements by noting their use by other patients with similar diagnoses
- Learn about and provide objective information on products
- Suggest use of single-ingredient products because:
- Suggest a symptom diary and a plan to discontinue a supplement if desired results are not seen
- Report suspected adverse events or drug interactions to the FDA
- Choose your battles carefully; testimonials and the placebo effect can strongly influence patients’ desire to continue using dietary supplements
- Remember: Patient safety is paramount
ST. JOHN’S WORT FOR DEPRESSION
St. John’s wort (Hypericum perforatum), used to treat mild-to-moderate depression and anxiety,13 is one of the most-recognized herbal remedies. It accounted for 12% of the natural products used in the NCCAM survey. Several clinical trials assessing St. John’s wort are in progress and include placebo-controlled evaluations in obsessive-compulsive disorder and social phobia.14
As with ginkgo, St. John’s wort has many proposed active constituents; most preparations are standardized based on a hypericin content of 0.3%. Its mechanism of action in depression is unclear but laboratory models hint that it may be related to very mild inhibition of:
- monoamine oxidase (MAO)
- catechol-O-methyltransferase(COMT)
- selective serotonin reuptake
- interleukin-6 release (thereby increasing corticotropin-releasing hormone levels)
- norepinephrine uptake.15
Efficacy. St. John’s wort has been studied in mild, moderate, and major depression and compared with placebo and prescription therapies. In treating mild and moderate depression, St. John’s wort has been more effective than placebo and equivalent to tricyclic antidepressants.16,17 Criticisms of these trials include lack of product standardization, lack of comparison with standard antidepressants at appropriate dosages, and small sample sizes. Larger trials comparing St. John’s wort with placebo and sertraline in treating major depression showed no difference in effect among the three.18,19
Adverse effects are generally infrequent and include insomnia, anxiety, GI upset, and photosensitivity reactions.20 St. John’s wort can induce hypomania and mania in patients with bipolar disorder and cause psychosis in schizophrenic patients.13
Drug-herb interactions. Of greatest concern with St. John’s wort use is the remarkable number of drug-herb interactions that have been identified (Table 2 and Table 3).13,21 The primary mechanisms appear to be substantial induction of CYP 3A4, induction of P-glycoprotein mediated drug elimination, and—to a lesser extent—induction of other CYP isoenzymes.22 Interactions resulting in serotonin syndrome have been documented, with restlessness, sweating, and agitation.23
The 3A4 isoenzyme metabolizes most drugs processed via the CYP system.12 Severe interactions seen with St. John’s wort include:
- reduced cyclosporine levels, resulting in heart transplant rejection in two patients
- reduced antiretroviral levels in HIV patients
- pregnancy in women taking oral contraceptives.
Enzyme induction may persist for as long as 14 days after patients stop taking St. John’s wort.
Dosage. The recommended St. John’s wort dosage (using standardized 0.3% hypericin content) is 300 mg 2 or 3 times daily. Dosages of 1,200 to 1,800 mg/d have been used.13,18 Benefits may not be seen for 2 to 3 weeks, and experience with use beyond 8 weeks in mild-to-moderate depression is very limited.
Recommendation. Advise patients to taper off St. John’s wort when stopping therapy to decrease the risk of withdrawal symptoms such as confusion, headache, nausea, insomnia, and fatigue.21 Given the high risk for drug interactions associated with St. John’s wort, we do not recommend its use in patients receiving any other medications.
KAVA KAVA AND LIVER TOXICITY
Kava kava (Piper methysticum) is used by some patients to treat anxiety and insomnia. Compared with other nutritional supplements, kava is less commonly used—by only 6.6% of adults using nutritional supplements,1—and it is not being evaluated in NIH-sponsored trials.
The active-ingredient content varies considerably in kava root, so extracts are standardized to contain 70% kava-lactones (WS 1490). Although its exact mechanism is unclear, kava appears to:
- alter the limbic system
- inhibit monoamine oxidase type B (MAO-B)
- increase the number of gamma-aminobutyric acid (GABA) binding sites
- relax skeletal muscle
- produce anesthesia.24
Table 2
Documented interactions with St. John’s wort
| Alprazolam ↓ | Nevirapine ↓ |
| Amitriptyline ↓ | Oral contraceptives ↓ |
| Buspirone (ss) | Paroxetine (ss) |
| Cyclosporine ↓ | Sertraline (ss, mania) |
| Digoxin ↓ | Simvastatin ↓ |
| Fexofenadine ↓ | Tacrolimus ↓ |
| Indinavir ↓ | Theophylline ↓ |
| Irinotecan ↓ | Tyramine-containing |
| Methadone ↓ | foods (MAO-I reaction) |
| Midazolam ↓ | Venlafaxine (ss) |
| Nefazodone (ss) | Warfarin ↓ |
| ↓= decreased levels/effectiveness | |
| ss = serotonin syndrome | |
| Source: Adapted from reference 21 | |
Table 3
Select potential interactions with St. John’s wort
| Cannabinoids ↓ | Fentanyl ↓ |
| Cocaine ↓ | Temazepam ↓ |
| Diazepam ↓ | Triazolam ↓ |
| Donepezil ↓ | |
| ↓= decreased levels/effectiveness | |
| Source: Adapted from reference 12 | |
Efficacy. A meta-analysis of studies found kava more effective than placebo in treating anxiety,25 although most studies suffer from poor design and/or small sample size.
Adverse effects. Reports have associated kava use with hepatic toxicity, liver failure requiring liver transplantation, and death. The European Union and Canada have banned kava sales, and the FDA issued a consumer advisory noting kava’s risks. Emerging information indicates that kava inhibits virtually all CYP-450 enzymes, which would increase levels of and potential adverse effects from any medications taken with kava.26
Other common adverse effects include GI upset, enlarged pupils, extrapyramidal side effects, and dizziness.24,27
Dosage. Kava is usually given at 100 mg (70 mg of kavalactones) three times daily. Urge patients to avoid driving when taking kava because of its side effects. Anxiety symptoms may improve with 1 to 8 weeks of therapy, but adverse hepatic effects can occur within 3 to 4 weeks of starting kava use.
Recommendation. Avoid kava kava use because of substantial risk of hepatotoxicity and drug interactions.
VALERIAN FOR INSOMNIA, ANXIETY
Valerian (Valeriana officinalis) is promoted as a sedative/hypnotic and anxiolytic. The prevalence of valerian use (5.9%) mirrors that of kava.1 An NCCAM study is enrolling patients to evaluate valerian’s effectiveness in treating Parkinson’s disease-related sleep disturbances.
Efficacy. Information on valerian’s mechanism of action and clinical effectiveness is quite limited. In animal studies, its components produced direct sedative effects and inhibited CNS catabolism of GABA.28 Results are mixed in humans with insomnia; some studies have found reduced sleep latency and improved sleep quality with valerian use, whereas others found no improvements.29 Limited, small evaluations suggest that valerian may be useful in treating anxiety.
Adverse effects. The FDA categorizes valerian as an approved food additive, so it is considered safe in usual amounts found in food. FDA lists no amount that it considers safe in food, however, and the federal code covering valerian states that only enough needed to impart the desired flavor should be used.
When taken in therapeutic amounts, valerian’s most common adverse effects are headache and residual morning drowsiness. Because of the herb’s sedative effects, urge caution if patients drive while using it. On discontinuation, withdrawal symptoms similar to those seen with benzodiazepine withdrawal—anxiety, headache, emotional lability—have been reported.
Dosage. For insomnia, valerian is taken 2 hours to 30 minutes before bedtime; doses start at 300 to 400 mg and increase to 600 to 900 mg. Recommended doses vary, as standardization is less common with valerian than with other herbals. Continuous treatment seems more effective than as-needed dosing, as valerian may take up to 4 weeks to improve insomnia.
Recommendation. Well-controlled trials are lacking, and safety data at therapeutic doses are limited. Monitor patients using valerian for adverse effects and drug interactions.
- Natural Medicines Comprehensive Database.www.naturaldatabase.com
- National Center for Complementary and Alternative Medicine. http://nccam.nih.gov
- FDA MedWatch Adverse Event Reporting Program. www.fda.gov/medwatch/how.htm
- ConsumerLab.com. Independent testing of dietary supplements. www.consumerlab.com
Drug brand names
- Alprazolam • Xanax
- Amiodarone • Cordarone, Pacerone
- Amitriptyline • Elavil
- Buspirone • BuSpar
- Cimetidine • Tagamet
- Cyclosporine • various
- Diazepam • Valium
- Digoxin • Lanoxin
- Donepezil • Aricept
- Fentanyl • Duragesic
- Fexofenadine • Allegra
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Indinavir • Crixivan
- Irinotecan • Camptosar
- Methadone • various
- Midazolam • Versed
- Nefazodone • Serzone
- Nevirapine • Viramune
- Omeprazole • Prilosec
- Paroxetine • Paxil
- Phenytoin • Dilantin
- Sertraline • Zoloft
- Simvastatin • Zocor
- Tacrolimus • Prograf
- Temazepam • Restoril
- Theophylline • various
- Triazolam • Halcion
- Venlafaxine • Effexor
- Warfarin • Coumadin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Advance data from vital and health statistics; no 343. Hyattsville, MD: National Center for Health Statistics, 2004.
2. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. Results of a follow-up survey. JAMA 1998;280:1569-75.
3. Matthews SC, Camacho A, Lawson K, Dimsdale JE. Use of herbal medications among 200 psychiatric outpatients: prevalence, patterns of use, and potential dangers. Gen Hosp Psychiatry 2003;25:24-6.
4. Eisenberg DM. Advising patients who seek alternative medical therapies. Ann Intern Med 1997;127:61-9.
5. Grant KL. Patient education and herbal dietary supplements. Am J Health-Syst Pharm 2000;57:1997-2003.
6. Harris IM. Regulatory and ethical issues with dietary supplements. Pharmacotherapy 2000;20:1295-1302.
7. Sierpina VS, Wollschlaeger B, Blumenthal M. Ginkgo biloba. Am Fam Physician 2003;68:923-6.
8. LeBars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of ginkgo biloba for dementia. JAMA 1997;278:1327-32.
9. Oken BS, Storzbach DM, Kaye JA. The efficacy of ginkgo biloba on cognitive function in Alzheimer’s disease. Arch Neurol 1998;55:1409-15.
10. Ginkgo biloba clinical trials National Center for Complementary and Alternative Medicine.National Institutes of Health. http://nccam.nih.gov/clinicaltrials/ginkgo.htm. Accessed Dec. 9, 2004.
11. Gaudineau C, Beckerman R, Welbourn S, Auclair K. Inhibition of human P450 enzymes by multiple constituents of the ginkgo biloba extract. Biochem Biophys Res Commun 2004;318:1072-8.
12. Michalets EL. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy 1998;18:84-112.
13. Pepping J. St.John’s wort: hypericum perforatum. Am J HealthSyst Pharm 1999;56:329-30.
14. St John’s wort (hypericum) clinical trials. National Center for Complementary and Alternative Medicine. National Institutes of Health. http://nccam.nih.gov/clinicaltrials/stjohnswort/index.htm. Accessed Dec. 9, 2004.
15. Bennett DA, Phun L, Polk JF, et al. Neuropharmacology of St.John’s wort (hypericum). Ann Pharmacother 1998;32:1201-8.
16. Gaster B, Holroyd J. St.John’s wort for depression: a systematic review. Arch Intern Med 2000;160:152-6.
17. Linde K, Ramirez G, Mulrow CD, et al. St.John’s wort for depression—an overview and meta-analysis of randomized clinical trials. BMJ 1996;313:253-8.
18. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St.John’s wort in major depression: a randomized controlled trial. JAMA 2001;285:1978-86.
19. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St.John’s wort) in major depressive disorder: a randomized controlled trial. JAMA 2002;287:1807-14.
20. Beckman SE, Sommi RW, Switzer J. Consumer use of St.John’s wort: a survey on effectiveness, safety, and tolerability. Pharmacotherapy 2000;20:568-74.
21. Izzo AA. Drug interactions with St.John’s wort (hypericum perforatum): a review of the clinical evidence. Int J Clin Pharmacol Ther 2004;42:139-48.
22. Markowitz JS, Donovan JL, DeVane CL, et al. Effect of St.John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA 2003;290:1500-4.
23. Sternbach H. Serotonin syndrome: how to avoid, identify, and treat dangerous drug reactions. Current Psychiatry 2003;2(5):14-24.
24. Pepping J. Kava: Piper methysticum. Am J Health-Syst Pharm. 11999;56:957-60.
25. Pittler MH, Ernst E. Efficacy of kava extract for treating anxiety: systematic review and meta-analysis. J Clin Psychopharmacol 2000;20:84-9.
26. Matthews JM, Etheridge AS, Black SR. Inhibition of human cytochrome P450 activities by kava extract and kavalactones. Drug Metab Dispos 2002;30:1153-7.
27. Jellin JM, Gregory P, Batz F, et al. Kava Pharmacist’s Letter/Prescriber’s Letter Natural Medicines Comprehensive Database (3rd ed). Stockton, CA: Therapeutic Research Faculty, 2000;625:7.-
28. Hadley S, Petry JJ. Valerian. Am Fam Physician 2003;67:1755-8.
29. Plushner SL. Valerian: Valeriana officinalis. Am J Health-Syst Pharm 2000;57:328-35.
What do you tell your patients who are self-medicating with herbal remedies? Can dietary supplements safely improve mood disorders, insomnia, and other psychiatric complaints?
Evidence is limited on complementary and alternative medicines (CAMs), their active components, pharmacokinetics/dynamics, adverse effects, drug interactions, and therapeutic outcomes. Based on our review of trial data, case reports, and an NIH National Center for Complementary and Alternative Medicine (NCCAM) survey,1 we offer information to help you:
- identify patients using dietary supplements
- avoid serious interactions with common psychotropics
- counsel patients on the efficacy and safety of ginkgo biloba, St. John’s wort, kava kava, and valerian (Table 1).
DON’T BE AFRAID TO ASK
One-third of Americans who took part in the NCCAM survey reported using CAM. After prayer—the number-one CAM—respondents said they most often used natural products such as herbals, botanicals, nutraceuticals, phytomedicinals, and dietary supplements (Figure).1
Table 1
4 herbal supplements with purported psychotropic effects
| Herb | Promoted use | Safety | Recommendation |
|---|---|---|---|
| Ginkgo biloba | Dementia, memory | Bleeding complications, drug interactions a concern | Some data support a trial in dementia; beware of safety concerns |
| St. John’s wort | Depression | Substantial drug interactions | Use not recommended because of wide-ranging drug interactions |
| Kava kava | Anxiety | Hepatotoxicity risk, drug interactions; off market in Europe and Canada | Use not recommended because of safety issues |
| Valerian | Insomnia | Limited data available | Benign (?); monitor for possible adverse events or drug interactions |
Patients tend to use CAM to treat chronic medical conditions such as back pain, depression, and anxiety.1 Although CAM use is common, only 38% of patients say they disclose using CAM to their physicians.2 These use and disclosure patterns are similar in psychiatry.3
Herbal products may produce symptoms that mimic those of mental illnesses, such as psychosis and mania, complicating diagnosis and treatment. Abruptly stopping some herbs can produce withdrawal symptoms similar to those seen with benzodiazepine and antidepressant cessation. Supplements also can inhibit or augment prescribed psychotropics’ effects, and notable consequences include the potential for serotonin syndrome with use of St. John’s wort.
The key to learning about a patient’s use of dietary supplements is to ask. Patients commonly say they do not tell their doctors about using dietary supplements because “it wasn’t important for the doctor to know” or “the doctor never asked.”4,5 Ten tips for discussing nutritional supplements with patients are shown in the Box.
Buyer—and psychiatrist—beware. Because of dietary supplements’ regulatory status, physicians and patients need to learn as much as they can about these products’ documented safety and efficacy. The Dietary Supplement Health and Education Act passed by Congress in 1994 does not require manufacturers to prove their products are safe or effective before marketing them. They also can make claims that suggest uses in physical/emotional structure and function, such as “helps maintain a healthy emotional outlook.”
The FDA bears the burden of proof regarding safety and can remove a dietary supplement from the market only after receiving documented adverse event information from the public.6 The FDA has confiscated herbal products containing prescription drugs and misidentified herbal components.
GINKGO BILOBA FOR DEMENTIA
Ginkgo biloba was the third most commonly used herbal product (21%) in the NCCAM survey. Ginkgo is promoted primarily for dementia, cerebrovascular dysfunction, and memory enhancement. The standardized ginkgo extract (EGb 761) contains several components to which its pharmacologic activity has been attributed. Its constituents are thought to act primarily through anticoagulant effects by inhibiting platelet-activating factor and cyclic GMP phosphodiesterase. They also act through membrane stabilization, antioxidant properties, free-radical scavenging, and inhibition of beta-amyloid deposition.7
Efficacy. In patients with dementia, clinical trials using EGb 761 have shown small improvements in or maintenance of cognitive and social functioning, compared with placebo.8,9 The clinical significance of these findings is unclear, however. Ginkgo’s usefulness in enhancing memory is less certain. Controlled clinical trials are evaluating ginkgo’s efficacy in various conditions.10
Adverse effects. Bleeding complications are the primary concern with ginkgo biloba use, and caution is urged when it is taken concomitantly with aspirin or other antithrombotic drugs. Patients receiving warfarin should not take ginkgo because of the combined antiplatelet effects and ginkgo’s inhibition of warfarin metabolism and elimination.
Drug-herb interactions. One report showed that EGb 761 is a strong inhibitor of the cytochrome P-450 2C9 enzyme system (CYP 2C9).11 Other identified inhibitors of CYP 2C9 are fluvoxamine (strong inhibitor), amiodarone, cimetidine, fluoxetine, and omeprazole. Drugs that serve as substrates for that system and can have decreased clearance include phenytoin, warfarin, amitriptyline, and diazepam;12 use caution, therefore, when you co-administer these drugs with ginkgo.
Figure 10 CAM therapies patients report using most often
CAM: Complimentary and alternative medicines
Source: Barnes T, Powell-Griner E, McFann K, Nahin R. Complementary and alternative medicine use among adults: United States, 2002. Atlanta: Centers for Disease Control and Prevention, May 27, 2004: Advance Data Report #343 (available at http://www.cdc.gov/nchs/data/ad/ad343.pdf) Dosage. The usual dosage for standardized ginkgo extract is 120 to 240 mg/d, given in divided doses. Improvements associated with ginkgo use usually are seen within 4 to 12 weeks after starting therapy.7 Consider discontinuing therapy if you see no results after that time. Little is known about ginkgo’s effect after 1 year of use.
Recommendation. Limited data show a slight benefit in treating dementia by slowing cognitive and behavioral decline, but ginkgo biloba cannot be recommended as a first-line treatment until further controlled trials are available.
- Broach the subject without being judgmental; nutritional supplement use is tied to a patient’s health beliefs
- Ask specifically about use of herbs, supplements, teas, elixirs, vitamins, etc., and document in the medical record at each visit
- Include in appointment reminders a request that patients bring all medicines, herbs, and supplements to appointments
- Segue into a discussion of supplements by noting their use by other patients with similar diagnoses
- Learn about and provide objective information on products
- Suggest use of single-ingredient products because:
- Suggest a symptom diary and a plan to discontinue a supplement if desired results are not seen
- Report suspected adverse events or drug interactions to the FDA
- Choose your battles carefully; testimonials and the placebo effect can strongly influence patients’ desire to continue using dietary supplements
- Remember: Patient safety is paramount
ST. JOHN’S WORT FOR DEPRESSION
St. John’s wort (Hypericum perforatum), used to treat mild-to-moderate depression and anxiety,13 is one of the most-recognized herbal remedies. It accounted for 12% of the natural products used in the NCCAM survey. Several clinical trials assessing St. John’s wort are in progress and include placebo-controlled evaluations in obsessive-compulsive disorder and social phobia.14
As with ginkgo, St. John’s wort has many proposed active constituents; most preparations are standardized based on a hypericin content of 0.3%. Its mechanism of action in depression is unclear but laboratory models hint that it may be related to very mild inhibition of:
- monoamine oxidase (MAO)
- catechol-O-methyltransferase(COMT)
- selective serotonin reuptake
- interleukin-6 release (thereby increasing corticotropin-releasing hormone levels)
- norepinephrine uptake.15
Efficacy. St. John’s wort has been studied in mild, moderate, and major depression and compared with placebo and prescription therapies. In treating mild and moderate depression, St. John’s wort has been more effective than placebo and equivalent to tricyclic antidepressants.16,17 Criticisms of these trials include lack of product standardization, lack of comparison with standard antidepressants at appropriate dosages, and small sample sizes. Larger trials comparing St. John’s wort with placebo and sertraline in treating major depression showed no difference in effect among the three.18,19
Adverse effects are generally infrequent and include insomnia, anxiety, GI upset, and photosensitivity reactions.20 St. John’s wort can induce hypomania and mania in patients with bipolar disorder and cause psychosis in schizophrenic patients.13
Drug-herb interactions. Of greatest concern with St. John’s wort use is the remarkable number of drug-herb interactions that have been identified (Table 2 and Table 3).13,21 The primary mechanisms appear to be substantial induction of CYP 3A4, induction of P-glycoprotein mediated drug elimination, and—to a lesser extent—induction of other CYP isoenzymes.22 Interactions resulting in serotonin syndrome have been documented, with restlessness, sweating, and agitation.23
The 3A4 isoenzyme metabolizes most drugs processed via the CYP system.12 Severe interactions seen with St. John’s wort include:
- reduced cyclosporine levels, resulting in heart transplant rejection in two patients
- reduced antiretroviral levels in HIV patients
- pregnancy in women taking oral contraceptives.
Enzyme induction may persist for as long as 14 days after patients stop taking St. John’s wort.
Dosage. The recommended St. John’s wort dosage (using standardized 0.3% hypericin content) is 300 mg 2 or 3 times daily. Dosages of 1,200 to 1,800 mg/d have been used.13,18 Benefits may not be seen for 2 to 3 weeks, and experience with use beyond 8 weeks in mild-to-moderate depression is very limited.
Recommendation. Advise patients to taper off St. John’s wort when stopping therapy to decrease the risk of withdrawal symptoms such as confusion, headache, nausea, insomnia, and fatigue.21 Given the high risk for drug interactions associated with St. John’s wort, we do not recommend its use in patients receiving any other medications.
KAVA KAVA AND LIVER TOXICITY
Kava kava (Piper methysticum) is used by some patients to treat anxiety and insomnia. Compared with other nutritional supplements, kava is less commonly used—by only 6.6% of adults using nutritional supplements,1—and it is not being evaluated in NIH-sponsored trials.
The active-ingredient content varies considerably in kava root, so extracts are standardized to contain 70% kava-lactones (WS 1490). Although its exact mechanism is unclear, kava appears to:
- alter the limbic system
- inhibit monoamine oxidase type B (MAO-B)
- increase the number of gamma-aminobutyric acid (GABA) binding sites
- relax skeletal muscle
- produce anesthesia.24
Table 2
Documented interactions with St. John’s wort
| Alprazolam ↓ | Nevirapine ↓ |
| Amitriptyline ↓ | Oral contraceptives ↓ |
| Buspirone (ss) | Paroxetine (ss) |
| Cyclosporine ↓ | Sertraline (ss, mania) |
| Digoxin ↓ | Simvastatin ↓ |
| Fexofenadine ↓ | Tacrolimus ↓ |
| Indinavir ↓ | Theophylline ↓ |
| Irinotecan ↓ | Tyramine-containing |
| Methadone ↓ | foods (MAO-I reaction) |
| Midazolam ↓ | Venlafaxine (ss) |
| Nefazodone (ss) | Warfarin ↓ |
| ↓= decreased levels/effectiveness | |
| ss = serotonin syndrome | |
| Source: Adapted from reference 21 | |
Table 3
Select potential interactions with St. John’s wort
| Cannabinoids ↓ | Fentanyl ↓ |
| Cocaine ↓ | Temazepam ↓ |
| Diazepam ↓ | Triazolam ↓ |
| Donepezil ↓ | |
| ↓= decreased levels/effectiveness | |
| Source: Adapted from reference 12 | |
Efficacy. A meta-analysis of studies found kava more effective than placebo in treating anxiety,25 although most studies suffer from poor design and/or small sample size.
Adverse effects. Reports have associated kava use with hepatic toxicity, liver failure requiring liver transplantation, and death. The European Union and Canada have banned kava sales, and the FDA issued a consumer advisory noting kava’s risks. Emerging information indicates that kava inhibits virtually all CYP-450 enzymes, which would increase levels of and potential adverse effects from any medications taken with kava.26
Other common adverse effects include GI upset, enlarged pupils, extrapyramidal side effects, and dizziness.24,27
Dosage. Kava is usually given at 100 mg (70 mg of kavalactones) three times daily. Urge patients to avoid driving when taking kava because of its side effects. Anxiety symptoms may improve with 1 to 8 weeks of therapy, but adverse hepatic effects can occur within 3 to 4 weeks of starting kava use.
Recommendation. Avoid kava kava use because of substantial risk of hepatotoxicity and drug interactions.
VALERIAN FOR INSOMNIA, ANXIETY
Valerian (Valeriana officinalis) is promoted as a sedative/hypnotic and anxiolytic. The prevalence of valerian use (5.9%) mirrors that of kava.1 An NCCAM study is enrolling patients to evaluate valerian’s effectiveness in treating Parkinson’s disease-related sleep disturbances.
Efficacy. Information on valerian’s mechanism of action and clinical effectiveness is quite limited. In animal studies, its components produced direct sedative effects and inhibited CNS catabolism of GABA.28 Results are mixed in humans with insomnia; some studies have found reduced sleep latency and improved sleep quality with valerian use, whereas others found no improvements.29 Limited, small evaluations suggest that valerian may be useful in treating anxiety.
Adverse effects. The FDA categorizes valerian as an approved food additive, so it is considered safe in usual amounts found in food. FDA lists no amount that it considers safe in food, however, and the federal code covering valerian states that only enough needed to impart the desired flavor should be used.
When taken in therapeutic amounts, valerian’s most common adverse effects are headache and residual morning drowsiness. Because of the herb’s sedative effects, urge caution if patients drive while using it. On discontinuation, withdrawal symptoms similar to those seen with benzodiazepine withdrawal—anxiety, headache, emotional lability—have been reported.
Dosage. For insomnia, valerian is taken 2 hours to 30 minutes before bedtime; doses start at 300 to 400 mg and increase to 600 to 900 mg. Recommended doses vary, as standardization is less common with valerian than with other herbals. Continuous treatment seems more effective than as-needed dosing, as valerian may take up to 4 weeks to improve insomnia.
Recommendation. Well-controlled trials are lacking, and safety data at therapeutic doses are limited. Monitor patients using valerian for adverse effects and drug interactions.
- Natural Medicines Comprehensive Database.www.naturaldatabase.com
- National Center for Complementary and Alternative Medicine. http://nccam.nih.gov
- FDA MedWatch Adverse Event Reporting Program. www.fda.gov/medwatch/how.htm
- ConsumerLab.com. Independent testing of dietary supplements. www.consumerlab.com
Drug brand names
- Alprazolam • Xanax
- Amiodarone • Cordarone, Pacerone
- Amitriptyline • Elavil
- Buspirone • BuSpar
- Cimetidine • Tagamet
- Cyclosporine • various
- Diazepam • Valium
- Digoxin • Lanoxin
- Donepezil • Aricept
- Fentanyl • Duragesic
- Fexofenadine • Allegra
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Indinavir • Crixivan
- Irinotecan • Camptosar
- Methadone • various
- Midazolam • Versed
- Nefazodone • Serzone
- Nevirapine • Viramune
- Omeprazole • Prilosec
- Paroxetine • Paxil
- Phenytoin • Dilantin
- Sertraline • Zoloft
- Simvastatin • Zocor
- Tacrolimus • Prograf
- Temazepam • Restoril
- Theophylline • various
- Triazolam • Halcion
- Venlafaxine • Effexor
- Warfarin • Coumadin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
What do you tell your patients who are self-medicating with herbal remedies? Can dietary supplements safely improve mood disorders, insomnia, and other psychiatric complaints?
Evidence is limited on complementary and alternative medicines (CAMs), their active components, pharmacokinetics/dynamics, adverse effects, drug interactions, and therapeutic outcomes. Based on our review of trial data, case reports, and an NIH National Center for Complementary and Alternative Medicine (NCCAM) survey,1 we offer information to help you:
- identify patients using dietary supplements
- avoid serious interactions with common psychotropics
- counsel patients on the efficacy and safety of ginkgo biloba, St. John’s wort, kava kava, and valerian (Table 1).
DON’T BE AFRAID TO ASK
One-third of Americans who took part in the NCCAM survey reported using CAM. After prayer—the number-one CAM—respondents said they most often used natural products such as herbals, botanicals, nutraceuticals, phytomedicinals, and dietary supplements (Figure).1
Table 1
4 herbal supplements with purported psychotropic effects
| Herb | Promoted use | Safety | Recommendation |
|---|---|---|---|
| Ginkgo biloba | Dementia, memory | Bleeding complications, drug interactions a concern | Some data support a trial in dementia; beware of safety concerns |
| St. John’s wort | Depression | Substantial drug interactions | Use not recommended because of wide-ranging drug interactions |
| Kava kava | Anxiety | Hepatotoxicity risk, drug interactions; off market in Europe and Canada | Use not recommended because of safety issues |
| Valerian | Insomnia | Limited data available | Benign (?); monitor for possible adverse events or drug interactions |
Patients tend to use CAM to treat chronic medical conditions such as back pain, depression, and anxiety.1 Although CAM use is common, only 38% of patients say they disclose using CAM to their physicians.2 These use and disclosure patterns are similar in psychiatry.3
Herbal products may produce symptoms that mimic those of mental illnesses, such as psychosis and mania, complicating diagnosis and treatment. Abruptly stopping some herbs can produce withdrawal symptoms similar to those seen with benzodiazepine and antidepressant cessation. Supplements also can inhibit or augment prescribed psychotropics’ effects, and notable consequences include the potential for serotonin syndrome with use of St. John’s wort.
The key to learning about a patient’s use of dietary supplements is to ask. Patients commonly say they do not tell their doctors about using dietary supplements because “it wasn’t important for the doctor to know” or “the doctor never asked.”4,5 Ten tips for discussing nutritional supplements with patients are shown in the Box.
Buyer—and psychiatrist—beware. Because of dietary supplements’ regulatory status, physicians and patients need to learn as much as they can about these products’ documented safety and efficacy. The Dietary Supplement Health and Education Act passed by Congress in 1994 does not require manufacturers to prove their products are safe or effective before marketing them. They also can make claims that suggest uses in physical/emotional structure and function, such as “helps maintain a healthy emotional outlook.”
The FDA bears the burden of proof regarding safety and can remove a dietary supplement from the market only after receiving documented adverse event information from the public.6 The FDA has confiscated herbal products containing prescription drugs and misidentified herbal components.
GINKGO BILOBA FOR DEMENTIA
Ginkgo biloba was the third most commonly used herbal product (21%) in the NCCAM survey. Ginkgo is promoted primarily for dementia, cerebrovascular dysfunction, and memory enhancement. The standardized ginkgo extract (EGb 761) contains several components to which its pharmacologic activity has been attributed. Its constituents are thought to act primarily through anticoagulant effects by inhibiting platelet-activating factor and cyclic GMP phosphodiesterase. They also act through membrane stabilization, antioxidant properties, free-radical scavenging, and inhibition of beta-amyloid deposition.7
Efficacy. In patients with dementia, clinical trials using EGb 761 have shown small improvements in or maintenance of cognitive and social functioning, compared with placebo.8,9 The clinical significance of these findings is unclear, however. Ginkgo’s usefulness in enhancing memory is less certain. Controlled clinical trials are evaluating ginkgo’s efficacy in various conditions.10
Adverse effects. Bleeding complications are the primary concern with ginkgo biloba use, and caution is urged when it is taken concomitantly with aspirin or other antithrombotic drugs. Patients receiving warfarin should not take ginkgo because of the combined antiplatelet effects and ginkgo’s inhibition of warfarin metabolism and elimination.
Drug-herb interactions. One report showed that EGb 761 is a strong inhibitor of the cytochrome P-450 2C9 enzyme system (CYP 2C9).11 Other identified inhibitors of CYP 2C9 are fluvoxamine (strong inhibitor), amiodarone, cimetidine, fluoxetine, and omeprazole. Drugs that serve as substrates for that system and can have decreased clearance include phenytoin, warfarin, amitriptyline, and diazepam;12 use caution, therefore, when you co-administer these drugs with ginkgo.
Figure 10 CAM therapies patients report using most often
CAM: Complimentary and alternative medicines
Source: Barnes T, Powell-Griner E, McFann K, Nahin R. Complementary and alternative medicine use among adults: United States, 2002. Atlanta: Centers for Disease Control and Prevention, May 27, 2004: Advance Data Report #343 (available at http://www.cdc.gov/nchs/data/ad/ad343.pdf) Dosage. The usual dosage for standardized ginkgo extract is 120 to 240 mg/d, given in divided doses. Improvements associated with ginkgo use usually are seen within 4 to 12 weeks after starting therapy.7 Consider discontinuing therapy if you see no results after that time. Little is known about ginkgo’s effect after 1 year of use.
Recommendation. Limited data show a slight benefit in treating dementia by slowing cognitive and behavioral decline, but ginkgo biloba cannot be recommended as a first-line treatment until further controlled trials are available.
- Broach the subject without being judgmental; nutritional supplement use is tied to a patient’s health beliefs
- Ask specifically about use of herbs, supplements, teas, elixirs, vitamins, etc., and document in the medical record at each visit
- Include in appointment reminders a request that patients bring all medicines, herbs, and supplements to appointments
- Segue into a discussion of supplements by noting their use by other patients with similar diagnoses
- Learn about and provide objective information on products
- Suggest use of single-ingredient products because:
- Suggest a symptom diary and a plan to discontinue a supplement if desired results are not seen
- Report suspected adverse events or drug interactions to the FDA
- Choose your battles carefully; testimonials and the placebo effect can strongly influence patients’ desire to continue using dietary supplements
- Remember: Patient safety is paramount
ST. JOHN’S WORT FOR DEPRESSION
St. John’s wort (Hypericum perforatum), used to treat mild-to-moderate depression and anxiety,13 is one of the most-recognized herbal remedies. It accounted for 12% of the natural products used in the NCCAM survey. Several clinical trials assessing St. John’s wort are in progress and include placebo-controlled evaluations in obsessive-compulsive disorder and social phobia.14
As with ginkgo, St. John’s wort has many proposed active constituents; most preparations are standardized based on a hypericin content of 0.3%. Its mechanism of action in depression is unclear but laboratory models hint that it may be related to very mild inhibition of:
- monoamine oxidase (MAO)
- catechol-O-methyltransferase(COMT)
- selective serotonin reuptake
- interleukin-6 release (thereby increasing corticotropin-releasing hormone levels)
- norepinephrine uptake.15
Efficacy. St. John’s wort has been studied in mild, moderate, and major depression and compared with placebo and prescription therapies. In treating mild and moderate depression, St. John’s wort has been more effective than placebo and equivalent to tricyclic antidepressants.16,17 Criticisms of these trials include lack of product standardization, lack of comparison with standard antidepressants at appropriate dosages, and small sample sizes. Larger trials comparing St. John’s wort with placebo and sertraline in treating major depression showed no difference in effect among the three.18,19
Adverse effects are generally infrequent and include insomnia, anxiety, GI upset, and photosensitivity reactions.20 St. John’s wort can induce hypomania and mania in patients with bipolar disorder and cause psychosis in schizophrenic patients.13
Drug-herb interactions. Of greatest concern with St. John’s wort use is the remarkable number of drug-herb interactions that have been identified (Table 2 and Table 3).13,21 The primary mechanisms appear to be substantial induction of CYP 3A4, induction of P-glycoprotein mediated drug elimination, and—to a lesser extent—induction of other CYP isoenzymes.22 Interactions resulting in serotonin syndrome have been documented, with restlessness, sweating, and agitation.23
The 3A4 isoenzyme metabolizes most drugs processed via the CYP system.12 Severe interactions seen with St. John’s wort include:
- reduced cyclosporine levels, resulting in heart transplant rejection in two patients
- reduced antiretroviral levels in HIV patients
- pregnancy in women taking oral contraceptives.
Enzyme induction may persist for as long as 14 days after patients stop taking St. John’s wort.
Dosage. The recommended St. John’s wort dosage (using standardized 0.3% hypericin content) is 300 mg 2 or 3 times daily. Dosages of 1,200 to 1,800 mg/d have been used.13,18 Benefits may not be seen for 2 to 3 weeks, and experience with use beyond 8 weeks in mild-to-moderate depression is very limited.
Recommendation. Advise patients to taper off St. John’s wort when stopping therapy to decrease the risk of withdrawal symptoms such as confusion, headache, nausea, insomnia, and fatigue.21 Given the high risk for drug interactions associated with St. John’s wort, we do not recommend its use in patients receiving any other medications.
KAVA KAVA AND LIVER TOXICITY
Kava kava (Piper methysticum) is used by some patients to treat anxiety and insomnia. Compared with other nutritional supplements, kava is less commonly used—by only 6.6% of adults using nutritional supplements,1—and it is not being evaluated in NIH-sponsored trials.
The active-ingredient content varies considerably in kava root, so extracts are standardized to contain 70% kava-lactones (WS 1490). Although its exact mechanism is unclear, kava appears to:
- alter the limbic system
- inhibit monoamine oxidase type B (MAO-B)
- increase the number of gamma-aminobutyric acid (GABA) binding sites
- relax skeletal muscle
- produce anesthesia.24
Table 2
Documented interactions with St. John’s wort
| Alprazolam ↓ | Nevirapine ↓ |
| Amitriptyline ↓ | Oral contraceptives ↓ |
| Buspirone (ss) | Paroxetine (ss) |
| Cyclosporine ↓ | Sertraline (ss, mania) |
| Digoxin ↓ | Simvastatin ↓ |
| Fexofenadine ↓ | Tacrolimus ↓ |
| Indinavir ↓ | Theophylline ↓ |
| Irinotecan ↓ | Tyramine-containing |
| Methadone ↓ | foods (MAO-I reaction) |
| Midazolam ↓ | Venlafaxine (ss) |
| Nefazodone (ss) | Warfarin ↓ |
| ↓= decreased levels/effectiveness | |
| ss = serotonin syndrome | |
| Source: Adapted from reference 21 | |
Table 3
Select potential interactions with St. John’s wort
| Cannabinoids ↓ | Fentanyl ↓ |
| Cocaine ↓ | Temazepam ↓ |
| Diazepam ↓ | Triazolam ↓ |
| Donepezil ↓ | |
| ↓= decreased levels/effectiveness | |
| Source: Adapted from reference 12 | |
Efficacy. A meta-analysis of studies found kava more effective than placebo in treating anxiety,25 although most studies suffer from poor design and/or small sample size.
Adverse effects. Reports have associated kava use with hepatic toxicity, liver failure requiring liver transplantation, and death. The European Union and Canada have banned kava sales, and the FDA issued a consumer advisory noting kava’s risks. Emerging information indicates that kava inhibits virtually all CYP-450 enzymes, which would increase levels of and potential adverse effects from any medications taken with kava.26
Other common adverse effects include GI upset, enlarged pupils, extrapyramidal side effects, and dizziness.24,27
Dosage. Kava is usually given at 100 mg (70 mg of kavalactones) three times daily. Urge patients to avoid driving when taking kava because of its side effects. Anxiety symptoms may improve with 1 to 8 weeks of therapy, but adverse hepatic effects can occur within 3 to 4 weeks of starting kava use.
Recommendation. Avoid kava kava use because of substantial risk of hepatotoxicity and drug interactions.
VALERIAN FOR INSOMNIA, ANXIETY
Valerian (Valeriana officinalis) is promoted as a sedative/hypnotic and anxiolytic. The prevalence of valerian use (5.9%) mirrors that of kava.1 An NCCAM study is enrolling patients to evaluate valerian’s effectiveness in treating Parkinson’s disease-related sleep disturbances.
Efficacy. Information on valerian’s mechanism of action and clinical effectiveness is quite limited. In animal studies, its components produced direct sedative effects and inhibited CNS catabolism of GABA.28 Results are mixed in humans with insomnia; some studies have found reduced sleep latency and improved sleep quality with valerian use, whereas others found no improvements.29 Limited, small evaluations suggest that valerian may be useful in treating anxiety.
Adverse effects. The FDA categorizes valerian as an approved food additive, so it is considered safe in usual amounts found in food. FDA lists no amount that it considers safe in food, however, and the federal code covering valerian states that only enough needed to impart the desired flavor should be used.
When taken in therapeutic amounts, valerian’s most common adverse effects are headache and residual morning drowsiness. Because of the herb’s sedative effects, urge caution if patients drive while using it. On discontinuation, withdrawal symptoms similar to those seen with benzodiazepine withdrawal—anxiety, headache, emotional lability—have been reported.
Dosage. For insomnia, valerian is taken 2 hours to 30 minutes before bedtime; doses start at 300 to 400 mg and increase to 600 to 900 mg. Recommended doses vary, as standardization is less common with valerian than with other herbals. Continuous treatment seems more effective than as-needed dosing, as valerian may take up to 4 weeks to improve insomnia.
Recommendation. Well-controlled trials are lacking, and safety data at therapeutic doses are limited. Monitor patients using valerian for adverse effects and drug interactions.
- Natural Medicines Comprehensive Database.www.naturaldatabase.com
- National Center for Complementary and Alternative Medicine. http://nccam.nih.gov
- FDA MedWatch Adverse Event Reporting Program. www.fda.gov/medwatch/how.htm
- ConsumerLab.com. Independent testing of dietary supplements. www.consumerlab.com
Drug brand names
- Alprazolam • Xanax
- Amiodarone • Cordarone, Pacerone
- Amitriptyline • Elavil
- Buspirone • BuSpar
- Cimetidine • Tagamet
- Cyclosporine • various
- Diazepam • Valium
- Digoxin • Lanoxin
- Donepezil • Aricept
- Fentanyl • Duragesic
- Fexofenadine • Allegra
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Indinavir • Crixivan
- Irinotecan • Camptosar
- Methadone • various
- Midazolam • Versed
- Nefazodone • Serzone
- Nevirapine • Viramune
- Omeprazole • Prilosec
- Paroxetine • Paxil
- Phenytoin • Dilantin
- Sertraline • Zoloft
- Simvastatin • Zocor
- Tacrolimus • Prograf
- Temazepam • Restoril
- Theophylline • various
- Triazolam • Halcion
- Venlafaxine • Effexor
- Warfarin • Coumadin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Advance data from vital and health statistics; no 343. Hyattsville, MD: National Center for Health Statistics, 2004.
2. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. Results of a follow-up survey. JAMA 1998;280:1569-75.
3. Matthews SC, Camacho A, Lawson K, Dimsdale JE. Use of herbal medications among 200 psychiatric outpatients: prevalence, patterns of use, and potential dangers. Gen Hosp Psychiatry 2003;25:24-6.
4. Eisenberg DM. Advising patients who seek alternative medical therapies. Ann Intern Med 1997;127:61-9.
5. Grant KL. Patient education and herbal dietary supplements. Am J Health-Syst Pharm 2000;57:1997-2003.
6. Harris IM. Regulatory and ethical issues with dietary supplements. Pharmacotherapy 2000;20:1295-1302.
7. Sierpina VS, Wollschlaeger B, Blumenthal M. Ginkgo biloba. Am Fam Physician 2003;68:923-6.
8. LeBars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of ginkgo biloba for dementia. JAMA 1997;278:1327-32.
9. Oken BS, Storzbach DM, Kaye JA. The efficacy of ginkgo biloba on cognitive function in Alzheimer’s disease. Arch Neurol 1998;55:1409-15.
10. Ginkgo biloba clinical trials National Center for Complementary and Alternative Medicine.National Institutes of Health. http://nccam.nih.gov/clinicaltrials/ginkgo.htm. Accessed Dec. 9, 2004.
11. Gaudineau C, Beckerman R, Welbourn S, Auclair K. Inhibition of human P450 enzymes by multiple constituents of the ginkgo biloba extract. Biochem Biophys Res Commun 2004;318:1072-8.
12. Michalets EL. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy 1998;18:84-112.
13. Pepping J. St.John’s wort: hypericum perforatum. Am J HealthSyst Pharm 1999;56:329-30.
14. St John’s wort (hypericum) clinical trials. National Center for Complementary and Alternative Medicine. National Institutes of Health. http://nccam.nih.gov/clinicaltrials/stjohnswort/index.htm. Accessed Dec. 9, 2004.
15. Bennett DA, Phun L, Polk JF, et al. Neuropharmacology of St.John’s wort (hypericum). Ann Pharmacother 1998;32:1201-8.
16. Gaster B, Holroyd J. St.John’s wort for depression: a systematic review. Arch Intern Med 2000;160:152-6.
17. Linde K, Ramirez G, Mulrow CD, et al. St.John’s wort for depression—an overview and meta-analysis of randomized clinical trials. BMJ 1996;313:253-8.
18. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St.John’s wort in major depression: a randomized controlled trial. JAMA 2001;285:1978-86.
19. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St.John’s wort) in major depressive disorder: a randomized controlled trial. JAMA 2002;287:1807-14.
20. Beckman SE, Sommi RW, Switzer J. Consumer use of St.John’s wort: a survey on effectiveness, safety, and tolerability. Pharmacotherapy 2000;20:568-74.
21. Izzo AA. Drug interactions with St.John’s wort (hypericum perforatum): a review of the clinical evidence. Int J Clin Pharmacol Ther 2004;42:139-48.
22. Markowitz JS, Donovan JL, DeVane CL, et al. Effect of St.John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA 2003;290:1500-4.
23. Sternbach H. Serotonin syndrome: how to avoid, identify, and treat dangerous drug reactions. Current Psychiatry 2003;2(5):14-24.
24. Pepping J. Kava: Piper methysticum. Am J Health-Syst Pharm. 11999;56:957-60.
25. Pittler MH, Ernst E. Efficacy of kava extract for treating anxiety: systematic review and meta-analysis. J Clin Psychopharmacol 2000;20:84-9.
26. Matthews JM, Etheridge AS, Black SR. Inhibition of human cytochrome P450 activities by kava extract and kavalactones. Drug Metab Dispos 2002;30:1153-7.
27. Jellin JM, Gregory P, Batz F, et al. Kava Pharmacist’s Letter/Prescriber’s Letter Natural Medicines Comprehensive Database (3rd ed). Stockton, CA: Therapeutic Research Faculty, 2000;625:7.-
28. Hadley S, Petry JJ. Valerian. Am Fam Physician 2003;67:1755-8.
29. Plushner SL. Valerian: Valeriana officinalis. Am J Health-Syst Pharm 2000;57:328-35.
1. Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Advance data from vital and health statistics; no 343. Hyattsville, MD: National Center for Health Statistics, 2004.
2. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. Results of a follow-up survey. JAMA 1998;280:1569-75.
3. Matthews SC, Camacho A, Lawson K, Dimsdale JE. Use of herbal medications among 200 psychiatric outpatients: prevalence, patterns of use, and potential dangers. Gen Hosp Psychiatry 2003;25:24-6.
4. Eisenberg DM. Advising patients who seek alternative medical therapies. Ann Intern Med 1997;127:61-9.
5. Grant KL. Patient education and herbal dietary supplements. Am J Health-Syst Pharm 2000;57:1997-2003.
6. Harris IM. Regulatory and ethical issues with dietary supplements. Pharmacotherapy 2000;20:1295-1302.
7. Sierpina VS, Wollschlaeger B, Blumenthal M. Ginkgo biloba. Am Fam Physician 2003;68:923-6.
8. LeBars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of ginkgo biloba for dementia. JAMA 1997;278:1327-32.
9. Oken BS, Storzbach DM, Kaye JA. The efficacy of ginkgo biloba on cognitive function in Alzheimer’s disease. Arch Neurol 1998;55:1409-15.
10. Ginkgo biloba clinical trials National Center for Complementary and Alternative Medicine.National Institutes of Health. http://nccam.nih.gov/clinicaltrials/ginkgo.htm. Accessed Dec. 9, 2004.
11. Gaudineau C, Beckerman R, Welbourn S, Auclair K. Inhibition of human P450 enzymes by multiple constituents of the ginkgo biloba extract. Biochem Biophys Res Commun 2004;318:1072-8.
12. Michalets EL. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy 1998;18:84-112.
13. Pepping J. St.John’s wort: hypericum perforatum. Am J HealthSyst Pharm 1999;56:329-30.
14. St John’s wort (hypericum) clinical trials. National Center for Complementary and Alternative Medicine. National Institutes of Health. http://nccam.nih.gov/clinicaltrials/stjohnswort/index.htm. Accessed Dec. 9, 2004.
15. Bennett DA, Phun L, Polk JF, et al. Neuropharmacology of St.John’s wort (hypericum). Ann Pharmacother 1998;32:1201-8.
16. Gaster B, Holroyd J. St.John’s wort for depression: a systematic review. Arch Intern Med 2000;160:152-6.
17. Linde K, Ramirez G, Mulrow CD, et al. St.John’s wort for depression—an overview and meta-analysis of randomized clinical trials. BMJ 1996;313:253-8.
18. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St.John’s wort in major depression: a randomized controlled trial. JAMA 2001;285:1978-86.
19. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St.John’s wort) in major depressive disorder: a randomized controlled trial. JAMA 2002;287:1807-14.
20. Beckman SE, Sommi RW, Switzer J. Consumer use of St.John’s wort: a survey on effectiveness, safety, and tolerability. Pharmacotherapy 2000;20:568-74.
21. Izzo AA. Drug interactions with St.John’s wort (hypericum perforatum): a review of the clinical evidence. Int J Clin Pharmacol Ther 2004;42:139-48.
22. Markowitz JS, Donovan JL, DeVane CL, et al. Effect of St.John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA 2003;290:1500-4.
23. Sternbach H. Serotonin syndrome: how to avoid, identify, and treat dangerous drug reactions. Current Psychiatry 2003;2(5):14-24.
24. Pepping J. Kava: Piper methysticum. Am J Health-Syst Pharm. 11999;56:957-60.
25. Pittler MH, Ernst E. Efficacy of kava extract for treating anxiety: systematic review and meta-analysis. J Clin Psychopharmacol 2000;20:84-9.
26. Matthews JM, Etheridge AS, Black SR. Inhibition of human cytochrome P450 activities by kava extract and kavalactones. Drug Metab Dispos 2002;30:1153-7.
27. Jellin JM, Gregory P, Batz F, et al. Kava Pharmacist’s Letter/Prescriber’s Letter Natural Medicines Comprehensive Database (3rd ed). Stockton, CA: Therapeutic Research Faculty, 2000;625:7.-
28. Hadley S, Petry JJ. Valerian. Am Fam Physician 2003;67:1755-8.
29. Plushner SL. Valerian: Valeriana officinalis. Am J Health-Syst Pharm 2000;57:328-35.
E-mailing on the run
Many physicians communicate with patients or colleagues via e-mail but lose this connectivity when they travel. If you find traditional home and office e-mail accounts are no longer enough, several e-mail access options can help you stay connected anytime.
How e-mail works
Typical home e-mail accounts-known as post office protocol (POP) accounts-use a client-server access method. The client-such as Outlook Express, Eudora, or Netscape Mail-checks the server for mail, which is then downloaded onto the home computer. Once downloaded, the message is gone from the server.
By contrast, an Internet message access protocol (IMAP) account offers more capabilities, such as allowing users to store e-mail on the server and organize mail into folders.December 2003). Also, some public locations such as New York’s Bryant Park offer free wireless Internet as a public service.2 Personal digital assistants or notebook computers with wireless capability, such as the Tungsten TC or the Toshiba e800, are best suited to this type of access.
Dial-up Internet service is possible over your cell phone. Most mobile phone carriers charge extra for data transmission, and you will need a specific cable to connect your phone to your computer. Bluetooth wireless technology can eliminate the need for cables but beware: Data transfer is much slower with Bluetooth than with other methods.
Third-generation networks (3G)-higher-speed protocols that allow faster data transmission for multimedia-have been touted as the next best service from mobile phone providers. Wireless devices such as the Palm Treo or the Research in Motion BlackBerry are specifically designed for this type of access. Monthly data service costs approximately $30 for unlimited downloads or less when bundled with a voice plan. Access is limited to the cellular coverage area, however.
Wireless access protocol, an alternative to 3G, lets you access e-mail via your mobile phone. This service, available from mobile service providers for an additional monthly fee (about $10), lets you read mail on the phone screen, but there are several drawbacks:
- Some people may find the text too small to read.
- Text entry via the telephone keypad can be difficult. You either tap a key multiple times to select letters or use word prediction based on letters entered.
- Not all phones available for each carrier can perform this function.
Wireless access protocol is well suited to reading e-mails. To compose an e-mail, however, you need to choose letters by clicking on numbers, which can be very difficult.
Many physicians communicate with patients or colleagues via e-mail but lose this connectivity when they travel. If you find traditional home and office e-mail accounts are no longer enough, several e-mail access options can help you stay connected anytime.
How e-mail works
Typical home e-mail accounts-known as post office protocol (POP) accounts-use a client-server access method. The client-such as Outlook Express, Eudora, or Netscape Mail-checks the server for mail, which is then downloaded onto the home computer. Once downloaded, the message is gone from the server.
By contrast, an Internet message access protocol (IMAP) account offers more capabilities, such as allowing users to store e-mail on the server and organize mail into folders.December 2003). Also, some public locations such as New York’s Bryant Park offer free wireless Internet as a public service.2 Personal digital assistants or notebook computers with wireless capability, such as the Tungsten TC or the Toshiba e800, are best suited to this type of access.
Dial-up Internet service is possible over your cell phone. Most mobile phone carriers charge extra for data transmission, and you will need a specific cable to connect your phone to your computer. Bluetooth wireless technology can eliminate the need for cables but beware: Data transfer is much slower with Bluetooth than with other methods.
Third-generation networks (3G)-higher-speed protocols that allow faster data transmission for multimedia-have been touted as the next best service from mobile phone providers. Wireless devices such as the Palm Treo or the Research in Motion BlackBerry are specifically designed for this type of access. Monthly data service costs approximately $30 for unlimited downloads or less when bundled with a voice plan. Access is limited to the cellular coverage area, however.
Wireless access protocol, an alternative to 3G, lets you access e-mail via your mobile phone. This service, available from mobile service providers for an additional monthly fee (about $10), lets you read mail on the phone screen, but there are several drawbacks:
- Some people may find the text too small to read.
- Text entry via the telephone keypad can be difficult. You either tap a key multiple times to select letters or use word prediction based on letters entered.
- Not all phones available for each carrier can perform this function.
Wireless access protocol is well suited to reading e-mails. To compose an e-mail, however, you need to choose letters by clicking on numbers, which can be very difficult.
Many physicians communicate with patients or colleagues via e-mail but lose this connectivity when they travel. If you find traditional home and office e-mail accounts are no longer enough, several e-mail access options can help you stay connected anytime.
How e-mail works
Typical home e-mail accounts-known as post office protocol (POP) accounts-use a client-server access method. The client-such as Outlook Express, Eudora, or Netscape Mail-checks the server for mail, which is then downloaded onto the home computer. Once downloaded, the message is gone from the server.
By contrast, an Internet message access protocol (IMAP) account offers more capabilities, such as allowing users to store e-mail on the server and organize mail into folders.December 2003). Also, some public locations such as New York’s Bryant Park offer free wireless Internet as a public service.2 Personal digital assistants or notebook computers with wireless capability, such as the Tungsten TC or the Toshiba e800, are best suited to this type of access.
Dial-up Internet service is possible over your cell phone. Most mobile phone carriers charge extra for data transmission, and you will need a specific cable to connect your phone to your computer. Bluetooth wireless technology can eliminate the need for cables but beware: Data transfer is much slower with Bluetooth than with other methods.
Third-generation networks (3G)-higher-speed protocols that allow faster data transmission for multimedia-have been touted as the next best service from mobile phone providers. Wireless devices such as the Palm Treo or the Research in Motion BlackBerry are specifically designed for this type of access. Monthly data service costs approximately $30 for unlimited downloads or less when bundled with a voice plan. Access is limited to the cellular coverage area, however.
Wireless access protocol, an alternative to 3G, lets you access e-mail via your mobile phone. This service, available from mobile service providers for an additional monthly fee (about $10), lets you read mail on the phone screen, but there are several drawbacks:
- Some people may find the text too small to read.
- Text entry via the telephone keypad can be difficult. You either tap a key multiple times to select letters or use word prediction based on letters entered.
- Not all phones available for each carrier can perform this function.
Wireless access protocol is well suited to reading e-mails. To compose an e-mail, however, you need to choose letters by clicking on numbers, which can be very difficult.
No ‘super-sizing’: Help kids on psychotropics avoid weight gain
Pediatric overweight and obesity can cause serious health problems later on. Use of psychotropics associated with potential weight gain compounds this risk.
Convincing youths to exercise and eat healthier foods can help them maintain a normal weight for their age and gender (see “Choose precise BMI charts to track youths’ weight gain,” (Current Psychiatry, October 2004,).
Cutting calories
Discuss the fat and calorie content of popular high-fat foods with the parents, who can then encourage their child to make more-informed dietary choices. Explain, for example, that it takes all day to burn the calories in two doughnuts, one fast-food bacon cheeseburger, or one extra-large serving of fast-food french fries.
A table listing portions of high-fat foods equaling 500 Kcal—the amount of energy a typical youth burns in 1 day through brisk walking—accompanies this article at www.currentpsychiatry.com.
Increasing exercise
Physical activity declines substantially during adolescence.1 In youths with chronic mental illness, reduced activity may contribute more than increased caloric intake to overweight/obesity.
Tell youths that they can lose 1 lb of body fat per week with normal walking and can lose more weight by simply taking a 30-minute walk each day. Alternately, each of the following activities burns as many Kcal as a 30-minute walk:
- jumping rope 11 minutes
- jogging 13 minutes
- swimming 19 minutes
- moderate cycling 24 minutes
- mowing lawn 30 minutes.
The pedometer is attached to the belt near the buckle and records a step every time the hip drops. Pedometers measure activity by steps per day; they cannot gauge activity on a bicycle.
Assuming that a youth burns 40 Kcal per 1,000 steps, a child will burn 480 to 640 Kcal and an adolescent will burn 440 to 480 Kcal per day through brisk walking. Burning 500 Kcal/day translates to 3,500 Kcal—the equivalent of 1 lb of body fat—across 1 week.
Encouraging exercise. It is harder to promote exercise to a chronically mentally ill youth than to a youth who is not mentally ill. Work with the patient’s family, case manager, school system, teacher, nurse, and/or family doctor or pediatrician to plan an exercise regimen.
Reference
1. Kimm SYS, Glynn NW, Kriska AM, et al. Decline in physical activity in black girls and white girls during adolescence. N Engl J Med 2002;347:709-15.
The authors are faculty members in the department of psychiatry, Medical College of Virginia, Virginia Commonwealth University, Richmond.
Pediatric overweight and obesity can cause serious health problems later on. Use of psychotropics associated with potential weight gain compounds this risk.
Convincing youths to exercise and eat healthier foods can help them maintain a normal weight for their age and gender (see “Choose precise BMI charts to track youths’ weight gain,” (Current Psychiatry, October 2004,).
Cutting calories
Discuss the fat and calorie content of popular high-fat foods with the parents, who can then encourage their child to make more-informed dietary choices. Explain, for example, that it takes all day to burn the calories in two doughnuts, one fast-food bacon cheeseburger, or one extra-large serving of fast-food french fries.
A table listing portions of high-fat foods equaling 500 Kcal—the amount of energy a typical youth burns in 1 day through brisk walking—accompanies this article at www.currentpsychiatry.com.
Increasing exercise
Physical activity declines substantially during adolescence.1 In youths with chronic mental illness, reduced activity may contribute more than increased caloric intake to overweight/obesity.
Tell youths that they can lose 1 lb of body fat per week with normal walking and can lose more weight by simply taking a 30-minute walk each day. Alternately, each of the following activities burns as many Kcal as a 30-minute walk:
- jumping rope 11 minutes
- jogging 13 minutes
- swimming 19 minutes
- moderate cycling 24 minutes
- mowing lawn 30 minutes.
The pedometer is attached to the belt near the buckle and records a step every time the hip drops. Pedometers measure activity by steps per day; they cannot gauge activity on a bicycle.
Assuming that a youth burns 40 Kcal per 1,000 steps, a child will burn 480 to 640 Kcal and an adolescent will burn 440 to 480 Kcal per day through brisk walking. Burning 500 Kcal/day translates to 3,500 Kcal—the equivalent of 1 lb of body fat—across 1 week.
Encouraging exercise. It is harder to promote exercise to a chronically mentally ill youth than to a youth who is not mentally ill. Work with the patient’s family, case manager, school system, teacher, nurse, and/or family doctor or pediatrician to plan an exercise regimen.
Pediatric overweight and obesity can cause serious health problems later on. Use of psychotropics associated with potential weight gain compounds this risk.
Convincing youths to exercise and eat healthier foods can help them maintain a normal weight for their age and gender (see “Choose precise BMI charts to track youths’ weight gain,” (Current Psychiatry, October 2004,).
Cutting calories
Discuss the fat and calorie content of popular high-fat foods with the parents, who can then encourage their child to make more-informed dietary choices. Explain, for example, that it takes all day to burn the calories in two doughnuts, one fast-food bacon cheeseburger, or one extra-large serving of fast-food french fries.
A table listing portions of high-fat foods equaling 500 Kcal—the amount of energy a typical youth burns in 1 day through brisk walking—accompanies this article at www.currentpsychiatry.com.
Increasing exercise
Physical activity declines substantially during adolescence.1 In youths with chronic mental illness, reduced activity may contribute more than increased caloric intake to overweight/obesity.
Tell youths that they can lose 1 lb of body fat per week with normal walking and can lose more weight by simply taking a 30-minute walk each day. Alternately, each of the following activities burns as many Kcal as a 30-minute walk:
- jumping rope 11 minutes
- jogging 13 minutes
- swimming 19 minutes
- moderate cycling 24 minutes
- mowing lawn 30 minutes.
The pedometer is attached to the belt near the buckle and records a step every time the hip drops. Pedometers measure activity by steps per day; they cannot gauge activity on a bicycle.
Assuming that a youth burns 40 Kcal per 1,000 steps, a child will burn 480 to 640 Kcal and an adolescent will burn 440 to 480 Kcal per day through brisk walking. Burning 500 Kcal/day translates to 3,500 Kcal—the equivalent of 1 lb of body fat—across 1 week.
Encouraging exercise. It is harder to promote exercise to a chronically mentally ill youth than to a youth who is not mentally ill. Work with the patient’s family, case manager, school system, teacher, nurse, and/or family doctor or pediatrician to plan an exercise regimen.
Reference
1. Kimm SYS, Glynn NW, Kriska AM, et al. Decline in physical activity in black girls and white girls during adolescence. N Engl J Med 2002;347:709-15.
The authors are faculty members in the department of psychiatry, Medical College of Virginia, Virginia Commonwealth University, Richmond.
Reference
1. Kimm SYS, Glynn NW, Kriska AM, et al. Decline in physical activity in black girls and white girls during adolescence. N Engl J Med 2002;347:709-15.
The authors are faculty members in the department of psychiatry, Medical College of Virginia, Virginia Commonwealth University, Richmond.
Involuntary commitment, ‘false’ memories
Self-proclaimed ‘exorcist’ claims he was improperly committed
Court of claims (NY)
In response to a 911 call, police arrested a man who, the caller said, was trying to choke and stab an individual. Upon his arrest, the man claimed that he was an exorcist. He justified his attack by alleging that his victim was a medium for demons and spirits.
The suspect was taken to a psychiatric hospital; staff decided that he endangered himself and others and should be committed. During his commitment, he was restrained and forcibly given medication.
The man later sued the hospital for intentional infliction of emotional distress and malpractice. He charged that the hospital denied him the right to a court hearing after he was admitted.
The court dismissed the case. The court noted that the plaintiff did not present expert testimony to support his emotional distress claim.
Dr. Grant’s observations
Involuntary commitment. Although the standards for involuntary commitment vary from state to state, some general principles apply.
A patient who endangers himself or others may be held for varying periods until a court hearing can be arranged. State law determines how long someone can be held before a court-ordered commitment. Although the patient has a right to a court hearing, the state is not obligated to conduct that hearing sooner than is determined by state law. Clinicians need to learn the laws governing involuntary commitment in the states in which they practice.
Patients who are involuntarily committed are not required to accept treatment, however. Competent adults generally must give informed consent to treatment, but this rule is usually suspended in an emergency. When a patient is a danger to self or others, that person can be restrained and medicated against his or her will for as long as the emergency lasts. In such cases, the clinician should clearly document:
- indications for using restraint and forced medication (include a detailed assessment of the patient’s dangerous behaviors)
- the patient’s response to previous behavioral approaches or treatments
- grounds for believing that the patient’s refusal of other interventions is clearly a product of the illness.
Patient: psychiatrists planted false memories, gave wrong diagnosis
Green County (WI) circuit court
A 55-year-old woman was seen in a hospital clinic’s weight-loss program and developed anxiety symptoms as she reached normal weight. Her psychologist assigned her to read a book about surviving incest, which focused on repressed memories that surface during recovery. The woman then received hypnosis from a psychiatrist who was not trained as a hypnotist.
During the hypnosis sessions, the patient reported “remembering” past instances of abuse that she had not previously recalled. The psychiatrist guided her to relive or reenact one event, in which she reported remembering being anally raped. The patient became more depressed and required hospitalization. Another psychiatrist, who took over the case when the first psychiatrist left the clinic, diagnosed the patient as having multiple personality disorder.
The patient later questioned the diagnosis and came to believe that her treatment had been inappropriate and that the memories had been planted. The patient, once a registered nurse, is now disabled.
In court, the jury heard:
- charges of negligence against the treating physicians on behalf of the woman and her son, who was briefly treated by the original psychiatrist
- charges that neither the psychiatrists nor the clinic obtained informed consent before treating the woman or her son.
- The jury decided for the physician, clinic, and hospital on all charges.
Dr. Grant’s observations
This case involves several complex and controversial areas in psychiatry: recovered memory, multiple personality disorder, and use of hypnosis. Although the jury found for the physician, clinic, and hospital, these areas provide fertile ground for lawsuits, many of which are successful.
The case involved two distinct legal causes of action:
- negligent care
- lack of informed consent.
Recovered memory. The veracity of recovered memory has been vigorously debated.1 Because the credibility of recovered memory cannot be established, the clinician should clearly state in the chart that the past incident the patient reports during therapy may not have happened. The clinician also must avoid imposing his or her beliefs on the patient (such as assuming that patients with eating disorders have been sexually abused) or advocating for action on the patient’s part.
Hypnosis used to recover memories of abuse may be particularly complex legally.2 A clinician using hypnosis may jeopardize therapeutic disinterest by interjecting suggestions—often without realizing that he or she is doing so.
To avoid negligence claims, clinicians should stay within their areas of competence when treating patients. If hypnosis is deemed clinically necessary, a clinician not trained in hypnosis should refer the patient to a certified clinical hypnotist.
Multiple personality disorder is included in DSM-IV-TR as dissociative identity disorder, but approximately one-third of psychiatrists question whether this is a legitimate diagnosis.3 Clearly documenting the basis for this—or any—diagnosis may help the clinician avoid a lawsuit or defend against a negligence charge.
Informed consent. Failure to inform patients about the risks associated with recovered memories is one of the most common allegations against clinicians in recovered memory cases.
Canterbury v. Spence, the landmark case of informed consent, offers some guidance. The court found that the clinician must provide reasonable disclosure of:
- therapy alternatives open to the patient
- goals expected to be achieved
- the risks involved with recovering memories.4
Some have proposed that clinicians should disclose the risk of recovering false memories of sexual and physical abuse before starting treatment.5 The clinician should then clearly document this disclosure.
1. Pope HG, Jr. Psychology astray: Fallacies in studies of repressed memory and childhood trauma. Boca Raton, FL: Upton Books, 1997.
2. Borawick v. Shay. 68 F3d 597 (2d Cir. 1995).
3. Pope HG, Jr, Oliva PS, Hudson JI, et al. Attitudes toward DSM-IV dissociative disorders diagnoses among board-certified American psychiatrists. Am J Psychiatry 1999;156:321-3.
4. Canterbury v. Spence. 464 F2d 775 (DC Cir 1972).
5. Cannell J, Hudson JI, Pope HG, Jr. Standards for informed consent in recovered memory therapy. J Am Acad Psychiatry Law 2001;29:138-47.
Self-proclaimed ‘exorcist’ claims he was improperly committed
Court of claims (NY)
In response to a 911 call, police arrested a man who, the caller said, was trying to choke and stab an individual. Upon his arrest, the man claimed that he was an exorcist. He justified his attack by alleging that his victim was a medium for demons and spirits.
The suspect was taken to a psychiatric hospital; staff decided that he endangered himself and others and should be committed. During his commitment, he was restrained and forcibly given medication.
The man later sued the hospital for intentional infliction of emotional distress and malpractice. He charged that the hospital denied him the right to a court hearing after he was admitted.
The court dismissed the case. The court noted that the plaintiff did not present expert testimony to support his emotional distress claim.
Dr. Grant’s observations
Involuntary commitment. Although the standards for involuntary commitment vary from state to state, some general principles apply.
A patient who endangers himself or others may be held for varying periods until a court hearing can be arranged. State law determines how long someone can be held before a court-ordered commitment. Although the patient has a right to a court hearing, the state is not obligated to conduct that hearing sooner than is determined by state law. Clinicians need to learn the laws governing involuntary commitment in the states in which they practice.
Patients who are involuntarily committed are not required to accept treatment, however. Competent adults generally must give informed consent to treatment, but this rule is usually suspended in an emergency. When a patient is a danger to self or others, that person can be restrained and medicated against his or her will for as long as the emergency lasts. In such cases, the clinician should clearly document:
- indications for using restraint and forced medication (include a detailed assessment of the patient’s dangerous behaviors)
- the patient’s response to previous behavioral approaches or treatments
- grounds for believing that the patient’s refusal of other interventions is clearly a product of the illness.
Patient: psychiatrists planted false memories, gave wrong diagnosis
Green County (WI) circuit court
A 55-year-old woman was seen in a hospital clinic’s weight-loss program and developed anxiety symptoms as she reached normal weight. Her psychologist assigned her to read a book about surviving incest, which focused on repressed memories that surface during recovery. The woman then received hypnosis from a psychiatrist who was not trained as a hypnotist.
During the hypnosis sessions, the patient reported “remembering” past instances of abuse that she had not previously recalled. The psychiatrist guided her to relive or reenact one event, in which she reported remembering being anally raped. The patient became more depressed and required hospitalization. Another psychiatrist, who took over the case when the first psychiatrist left the clinic, diagnosed the patient as having multiple personality disorder.
The patient later questioned the diagnosis and came to believe that her treatment had been inappropriate and that the memories had been planted. The patient, once a registered nurse, is now disabled.
In court, the jury heard:
- charges of negligence against the treating physicians on behalf of the woman and her son, who was briefly treated by the original psychiatrist
- charges that neither the psychiatrists nor the clinic obtained informed consent before treating the woman or her son.
- The jury decided for the physician, clinic, and hospital on all charges.
Dr. Grant’s observations
This case involves several complex and controversial areas in psychiatry: recovered memory, multiple personality disorder, and use of hypnosis. Although the jury found for the physician, clinic, and hospital, these areas provide fertile ground for lawsuits, many of which are successful.
The case involved two distinct legal causes of action:
- negligent care
- lack of informed consent.
Recovered memory. The veracity of recovered memory has been vigorously debated.1 Because the credibility of recovered memory cannot be established, the clinician should clearly state in the chart that the past incident the patient reports during therapy may not have happened. The clinician also must avoid imposing his or her beliefs on the patient (such as assuming that patients with eating disorders have been sexually abused) or advocating for action on the patient’s part.
Hypnosis used to recover memories of abuse may be particularly complex legally.2 A clinician using hypnosis may jeopardize therapeutic disinterest by interjecting suggestions—often without realizing that he or she is doing so.
To avoid negligence claims, clinicians should stay within their areas of competence when treating patients. If hypnosis is deemed clinically necessary, a clinician not trained in hypnosis should refer the patient to a certified clinical hypnotist.
Multiple personality disorder is included in DSM-IV-TR as dissociative identity disorder, but approximately one-third of psychiatrists question whether this is a legitimate diagnosis.3 Clearly documenting the basis for this—or any—diagnosis may help the clinician avoid a lawsuit or defend against a negligence charge.
Informed consent. Failure to inform patients about the risks associated with recovered memories is one of the most common allegations against clinicians in recovered memory cases.
Canterbury v. Spence, the landmark case of informed consent, offers some guidance. The court found that the clinician must provide reasonable disclosure of:
- therapy alternatives open to the patient
- goals expected to be achieved
- the risks involved with recovering memories.4
Some have proposed that clinicians should disclose the risk of recovering false memories of sexual and physical abuse before starting treatment.5 The clinician should then clearly document this disclosure.
Self-proclaimed ‘exorcist’ claims he was improperly committed
Court of claims (NY)
In response to a 911 call, police arrested a man who, the caller said, was trying to choke and stab an individual. Upon his arrest, the man claimed that he was an exorcist. He justified his attack by alleging that his victim was a medium for demons and spirits.
The suspect was taken to a psychiatric hospital; staff decided that he endangered himself and others and should be committed. During his commitment, he was restrained and forcibly given medication.
The man later sued the hospital for intentional infliction of emotional distress and malpractice. He charged that the hospital denied him the right to a court hearing after he was admitted.
The court dismissed the case. The court noted that the plaintiff did not present expert testimony to support his emotional distress claim.
Dr. Grant’s observations
Involuntary commitment. Although the standards for involuntary commitment vary from state to state, some general principles apply.
A patient who endangers himself or others may be held for varying periods until a court hearing can be arranged. State law determines how long someone can be held before a court-ordered commitment. Although the patient has a right to a court hearing, the state is not obligated to conduct that hearing sooner than is determined by state law. Clinicians need to learn the laws governing involuntary commitment in the states in which they practice.
Patients who are involuntarily committed are not required to accept treatment, however. Competent adults generally must give informed consent to treatment, but this rule is usually suspended in an emergency. When a patient is a danger to self or others, that person can be restrained and medicated against his or her will for as long as the emergency lasts. In such cases, the clinician should clearly document:
- indications for using restraint and forced medication (include a detailed assessment of the patient’s dangerous behaviors)
- the patient’s response to previous behavioral approaches or treatments
- grounds for believing that the patient’s refusal of other interventions is clearly a product of the illness.
Patient: psychiatrists planted false memories, gave wrong diagnosis
Green County (WI) circuit court
A 55-year-old woman was seen in a hospital clinic’s weight-loss program and developed anxiety symptoms as she reached normal weight. Her psychologist assigned her to read a book about surviving incest, which focused on repressed memories that surface during recovery. The woman then received hypnosis from a psychiatrist who was not trained as a hypnotist.
During the hypnosis sessions, the patient reported “remembering” past instances of abuse that she had not previously recalled. The psychiatrist guided her to relive or reenact one event, in which she reported remembering being anally raped. The patient became more depressed and required hospitalization. Another psychiatrist, who took over the case when the first psychiatrist left the clinic, diagnosed the patient as having multiple personality disorder.
The patient later questioned the diagnosis and came to believe that her treatment had been inappropriate and that the memories had been planted. The patient, once a registered nurse, is now disabled.
In court, the jury heard:
- charges of negligence against the treating physicians on behalf of the woman and her son, who was briefly treated by the original psychiatrist
- charges that neither the psychiatrists nor the clinic obtained informed consent before treating the woman or her son.
- The jury decided for the physician, clinic, and hospital on all charges.
Dr. Grant’s observations
This case involves several complex and controversial areas in psychiatry: recovered memory, multiple personality disorder, and use of hypnosis. Although the jury found for the physician, clinic, and hospital, these areas provide fertile ground for lawsuits, many of which are successful.
The case involved two distinct legal causes of action:
- negligent care
- lack of informed consent.
Recovered memory. The veracity of recovered memory has been vigorously debated.1 Because the credibility of recovered memory cannot be established, the clinician should clearly state in the chart that the past incident the patient reports during therapy may not have happened. The clinician also must avoid imposing his or her beliefs on the patient (such as assuming that patients with eating disorders have been sexually abused) or advocating for action on the patient’s part.
Hypnosis used to recover memories of abuse may be particularly complex legally.2 A clinician using hypnosis may jeopardize therapeutic disinterest by interjecting suggestions—often without realizing that he or she is doing so.
To avoid negligence claims, clinicians should stay within their areas of competence when treating patients. If hypnosis is deemed clinically necessary, a clinician not trained in hypnosis should refer the patient to a certified clinical hypnotist.
Multiple personality disorder is included in DSM-IV-TR as dissociative identity disorder, but approximately one-third of psychiatrists question whether this is a legitimate diagnosis.3 Clearly documenting the basis for this—or any—diagnosis may help the clinician avoid a lawsuit or defend against a negligence charge.
Informed consent. Failure to inform patients about the risks associated with recovered memories is one of the most common allegations against clinicians in recovered memory cases.
Canterbury v. Spence, the landmark case of informed consent, offers some guidance. The court found that the clinician must provide reasonable disclosure of:
- therapy alternatives open to the patient
- goals expected to be achieved
- the risks involved with recovering memories.4
Some have proposed that clinicians should disclose the risk of recovering false memories of sexual and physical abuse before starting treatment.5 The clinician should then clearly document this disclosure.
1. Pope HG, Jr. Psychology astray: Fallacies in studies of repressed memory and childhood trauma. Boca Raton, FL: Upton Books, 1997.
2. Borawick v. Shay. 68 F3d 597 (2d Cir. 1995).
3. Pope HG, Jr, Oliva PS, Hudson JI, et al. Attitudes toward DSM-IV dissociative disorders diagnoses among board-certified American psychiatrists. Am J Psychiatry 1999;156:321-3.
4. Canterbury v. Spence. 464 F2d 775 (DC Cir 1972).
5. Cannell J, Hudson JI, Pope HG, Jr. Standards for informed consent in recovered memory therapy. J Am Acad Psychiatry Law 2001;29:138-47.
1. Pope HG, Jr. Psychology astray: Fallacies in studies of repressed memory and childhood trauma. Boca Raton, FL: Upton Books, 1997.
2. Borawick v. Shay. 68 F3d 597 (2d Cir. 1995).
3. Pope HG, Jr, Oliva PS, Hudson JI, et al. Attitudes toward DSM-IV dissociative disorders diagnoses among board-certified American psychiatrists. Am J Psychiatry 1999;156:321-3.
4. Canterbury v. Spence. 464 F2d 775 (DC Cir 1972).
5. Cannell J, Hudson JI, Pope HG, Jr. Standards for informed consent in recovered memory therapy. J Am Acad Psychiatry Law 2001;29:138-47.
Welcome, advanced practice psychiatric nurses
Starting with this issue, Current Psychiatry is being sent to more than 3,000 advanced practice psychiatric nurses, who join the 37,000 psychiatrists who already receive it. The same “news you can use” that has made Current Psychiatry the most highly read journal among psychiatrists should help psychiatric nurses as well.
Advanced practice nurses have prescriptive authority in 49 states, and many prescribe on their own signatures. Laws governing their prescribing vary from state to state, as do their titles, including nurse practitioner (NP), clinical nurse specialist (CNS), advanced practitioner of nursing (APN), and perhaps a dozen others. All are registered nurses who have completed the additional graduate-level education and training required to diagnose and treat psychiatric disorders. [For more information, see the special report, “The Role of Advanced Practice Psychiatric Nurses,” at www.currentpsychiatry.com.]
In the past, organized psychiatry has systematically opposed nurses prescribing, and I believe that opposition was a bad thing. Nurses and physicians have been partners in caring for patients with mental illness for more than a century. We are members of distinct health professions but share the same goal: to ensure that our patients receive the best care.
Both professional groups have the necessary biologically-based training to appropriately prescribe the wonderful—and potentially dangerous—medications used in psychiatric practice. Both groups are committed to a program of lifelong learning, and both need unbiased, clinically relevant sources of therapeutic information—such as Current Psychiatry.
We are all in this together. Welcome, welcome.
Starting with this issue, Current Psychiatry is being sent to more than 3,000 advanced practice psychiatric nurses, who join the 37,000 psychiatrists who already receive it. The same “news you can use” that has made Current Psychiatry the most highly read journal among psychiatrists should help psychiatric nurses as well.
Advanced practice nurses have prescriptive authority in 49 states, and many prescribe on their own signatures. Laws governing their prescribing vary from state to state, as do their titles, including nurse practitioner (NP), clinical nurse specialist (CNS), advanced practitioner of nursing (APN), and perhaps a dozen others. All are registered nurses who have completed the additional graduate-level education and training required to diagnose and treat psychiatric disorders. [For more information, see the special report, “The Role of Advanced Practice Psychiatric Nurses,” at www.currentpsychiatry.com.]
In the past, organized psychiatry has systematically opposed nurses prescribing, and I believe that opposition was a bad thing. Nurses and physicians have been partners in caring for patients with mental illness for more than a century. We are members of distinct health professions but share the same goal: to ensure that our patients receive the best care.
Both professional groups have the necessary biologically-based training to appropriately prescribe the wonderful—and potentially dangerous—medications used in psychiatric practice. Both groups are committed to a program of lifelong learning, and both need unbiased, clinically relevant sources of therapeutic information—such as Current Psychiatry.
We are all in this together. Welcome, welcome.
Starting with this issue, Current Psychiatry is being sent to more than 3,000 advanced practice psychiatric nurses, who join the 37,000 psychiatrists who already receive it. The same “news you can use” that has made Current Psychiatry the most highly read journal among psychiatrists should help psychiatric nurses as well.
Advanced practice nurses have prescriptive authority in 49 states, and many prescribe on their own signatures. Laws governing their prescribing vary from state to state, as do their titles, including nurse practitioner (NP), clinical nurse specialist (CNS), advanced practitioner of nursing (APN), and perhaps a dozen others. All are registered nurses who have completed the additional graduate-level education and training required to diagnose and treat psychiatric disorders. [For more information, see the special report, “The Role of Advanced Practice Psychiatric Nurses,” at www.currentpsychiatry.com.]
In the past, organized psychiatry has systematically opposed nurses prescribing, and I believe that opposition was a bad thing. Nurses and physicians have been partners in caring for patients with mental illness for more than a century. We are members of distinct health professions but share the same goal: to ensure that our patients receive the best care.
Both professional groups have the necessary biologically-based training to appropriately prescribe the wonderful—and potentially dangerous—medications used in psychiatric practice. Both groups are committed to a program of lifelong learning, and both need unbiased, clinically relevant sources of therapeutic information—such as Current Psychiatry.
We are all in this together. Welcome, welcome.
Delirium: Apply the ‘4 Ps’ for comprehensive treatment
Four principles of treating delirium can help protect medical/surgical patients at risk for morbidity and functional decline. These principals—which I call the “four Ps”—are prompt identification, protection, pragmatic intervention, and pharmacotherapy.
This article describes an up-to-date, “four-Ps” approach to treating delirium—including use of antipsychotics and supportive care—and offers evidence and case reports to address these clinical questions:
- What causes delirium?
- Does delirium worsen prognosis?
- Can delirium be prevented?
FOUR ‘Ps’ FOR TREATING DELIRIUM
When a patient’s mental status changes dramatically (Box 1),1 identifying potential delirium causes requires careful medical, psychiatric, and neurologic assessment. Assimilating this information is as essential to positive outcomes as are intensive nursing care and appropriate interventions.
- Disturbance of consciousness (i.e. reduced clarity of awareness of the environment) with reduced ability to focus, sustain, or shift attention
- A change in cognition (such as memory deficit, disorientation, language disturbance) or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia
- The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of a 24-hour period
- There is evidence from the history, physical examination, or laboratory findings that the disturbance is caused by the direct physiologic consequences of a general medical condition
Source: Reprinted with permission from the Diagnostic and statistical manual of mental disorders (4th ed., text rev). Copyright 2000. American Psychiatric Publishing.
Prompt identification. Delirium often goes unrecognized, delaying treatment. Easily administered rating scales—such as the Delirium Rating Scale (DRS)2 and the Confusion Assessment Method (CAM)3 —can help detect emerging symptoms.
Patient protection. Provide intensive nursing care—often one-to-one observation and containment—and, where possible, enlist the family in reassuring and calming the patient. Restraints may be needed to safeguard against injury and to prevent the patient from removing or dislocating monitoring equipment and IV access.
Pragmatic intervention. With medical colleagues, begin treating biochemical and physiologic abnormalities that are the most likely and most remediable contributors (Box 2).2,4-6 Review the patient’s medications and discontinue or replace any that may be causing delirium.
Pharmacotherapy. Based on clinical studies, antipsychotics appear to possess antidelirium properties and may be considered as one part of a patient’s treatment plan. Interpreting these studies is complicated, however, by delirium’s complexity, numerous causes, and presumed mechanisms, as well as the transience of some forms. For ethical reasons, no placebo-controlled studies of delirium treatment have been done.
EVIDENCE ON ANTIPSYCHOTICS
Haloperidol has been the drug of choice for managing delirium because it is less likely to cause hypotension and sedation than other neuroleptics. Optimum haloperidol dosing in delirium has not been established, but the usual range is 2 to 6 mg every 4 to 6 hours, depending on the patient’s age and delirium severity.
Instances of QTc interval prolongation have been reported with high-dose IV haloperidol (> 100 mg). This life-threatening effect—which can induce torsades de pointes dysrhythmia, ventricular tachycardias, and fibrillation—is very rare, quite variable, and unpredictable. It probably is a function of total dose and neuroleptic administration rate.
Atypical antipsychotics share haloperidol’s advantages over first-generation neuroleptics, with lower potential for dystonic reactions, parkinsonian side effects, and tardive dyskinesia. Preliminary evidence suggests that atypicals may be safe and effective in treating delirium, although no randomized controlled trials have been done and accurate dose-response curves have not been established. Low to modest dosages have been used in case series.
Risperidone. Two prospective, open-label trials—each with 10 patients—suggest that low-dose risperidone is effective for treating delirium:
- In one trial, risperidone given at an average dosage of 1.7 mg/d was effective in 80% of patients with delirium, and one patient responded to 0.5 mg/d. Some patients experienced sleepiness or mild drug-induced parkinsonism.7
- In the other trial, risperidone was started at 0.5 mg twice daily, with additional doses allowed on day 1 for cognitive and behavioral symptoms. This dosage was maintained until DRS scores declined to ≤12, then was reduced by 50% and continued until day 6. Mean maintenance dosage was 0.75 mg/d. Two patients discontinued risperidone because of sedation or hypotension.8
At least 10% to 30% of hospitalized medically ill patients develop delirium, and rates approach 40% after age 65.4 Especially in older patients, delirium is a risk factor for:
- prolonged hospital stays
- increased morbidity and mortality
- increased functional decline and need for custodial care after hospital discharge.2
Risk factors. Prospectively identified risk factors for delirium include pre-existing dementia; age >65 years; serious medical illness; alcohol/sedative withdrawal; abnormal serum sodium, potassium, or blood glucose levels; vision or hearing impairment; hypoxia; malnutrition; and fever. Medication—particularly anticholinergic drugs—is one of the most common delirium triggers in susceptible patients.5
The most common underlying disorders that increase delirium risk in older patients are hip fracture, dementia, infections, and cerebrovascular events.6
In a larger prospective study, 64 patients (mean age 67) with delirium were treated with risperidone, given at a mean dose of 2.6 +/- 1.7 mg/d at day 3. This dosage was effective in 90% of patients and significantly improved all symptoms, as measured with scales including the DRS. Two patients (3%) experienced adverse effects.9
No significant differences in response frequency were seen in a 7-day, double-blind comparison of flexibly-dosed risperidone (starting at 0.5 mg bid) and haloperidol (starting at 0.75 mg bid) in 28 patients with delirium. Symptom severity decreased for each group, as measured with the Memorial Delirium Assessment Scale. One patient receiving haloperidol experienced mild akathisia, but no others reported clinically significant side effects.10
Quetiapine. In a retrospective review, the charts of 11 patients who received quetiapine for delirium were compared with those of 11 similar patients treated with haloperidol. DRS scores improved by >50% in 10 of 11 patients in both groups, with similar onset of effect, treatment duration, and overall clinical improvement.11 Small prospective trials with flexible dosing schedules have reported similar results.12,13
In a study of 12 older hospitalized patients with delirium, quetiapine at a mean dosage of 93.75 +/-23.31 mg/d was associated with significant DRS score improvements. Interestingly, patients’ Mini-Mental State Examination and Clock-Drawing Test scores continued to improve 3 months after their delirium symptoms stabilized.14
Olanzapine. In a prospective trial, hospitalized patients with delirium were randomly assigned to receive enteral olanzapine or haloperidol. Delirium symptoms decreased across 5 days in both groups, and clinical improvement was similar. Some patients receiving haloperidol reported extrapyramidal symptoms, whereas those receiving olanzapine reported no adverse effects.15
Parenteral forms of some atypicals (aripiprazole, olanzapine, and ziprasidone) have become available and may increase this class’ usefulness in treating delirium.
Other drugs. Benzodiazepines appear ineffective and generally play only an adjunctive role in treating delirium. An exception may be delirium induced by acute alcohol or benzodiazepine withdrawal. Sedating antidepressants have been used as hypnotics in patients with delirium, but supporting evidence is lacking.
Other drug classes—general anesthetics, narcotics, cholinomimetics—may help manage the dangerously hyperactive delirious patient, but the literature contains no systematic analyses.
WHAT CAUSES DELIRIUM?
Delirium’s pathophysiology is not completely understood, although most authors believe several mechanisms are involved.
The brain’s exclusively oxidative metabolism and its systems’ hierarchical vulnerability to substrate deficiency—as might occur in even transient hypoxia or hypotension—appear to play important roles. Factors such as fever and stress that increase metabolic demand on the brain intensify the effects of oxygen deficiency or circulatory compromise.
At least three molecular mechanisms have been proposed for delirium, including cholinergic transmission disruption, monoaminergic dysfunction, and cytokine release ( Box 3).16-19 These mechanisms may interact, cascading into a common final pathway that results in delirium.
Features not considered essential to delirium’s diagnosis—such as visual hallucinations or aggressive behaviors—indicate that additional cortical and subcortical systems are involved.
CASE REPORT: DRUG-DRUG INTERACTION
Three days after hip replacement surgery, Mr. S, age 64, becomes confused, distractible, and combative. He is alert one minute and somnolent the next. His arms and legs jerk involuntarily, and his muscle tone is diffusely increased. He talks with absent friends and family as though they are present in his hospital room. His body temperature and blood pressure fluctuate widely, despite no evidence of infection.
Cholinergic transmission disruption
The greater a medication’s anticholinergic activity, the greater its risk of causing delirium. Combining drugs with anticholinergic effects—such as theophylline, warfarin, or codeine (Table19)—compounds the delirium risk.
Acetylcholine-secreting neurons—widely if sparsely distributed throughout the brain—affect arousal, attention, memory, and sleep regulation. Acetylcholine is produced by oxidative metabolism and thus is vulnerable to physiologic disturbances that increase oxygen demand or disrupt oxygen supply.
Anticholinergic poisoning and abuse of anticholinergic substances are known to cause acute delirium—a finding that supports the key role of acetylcholine in maintaining alertness and concentration. Agents that enhance cholinergic transmission—such as the cholinesterase inhibitor physostigmine—can effectively treat drug-induced delirium.
Monoaminergic dysfunction
The principal monoamines of dopamine, serotonin, and norepinephrine help sustain attention, regulate the sleep-wake cycle, inhibit affective responses, and modulate aggressive and impulsive behaviors. Treating patients with dopamine and serotonin agonists can cause psychotic symptoms.
Glutamate—a monoamine neurotransmitter with excitatory properties—is released during metabolic stress and likely contributes to the psychotic features sometimes seen in delirium.
Cytokine release
Infection in a distant organ, such as gallbladder or kidney, is known to cause delirium. Cytokines such as interleukins and interferon-alpha are polypeptides secreted by macrophagesin response to tissue injury. They easily cross the blood-brain barrier and stimulate glial cells to release more cytokines, which interfere with neurotransmitter synthesis and transmission.
Table
Drugs whose anticholinergic effects may increase the risk of delirium
| Drug | Anticholinergic level* |
|---|---|
| Cimetidine | 0.86 |
| Prednisolone | 0.55 |
| Theophylline | 0.44 |
| Digoxin | 0.25 |
| Lanoxin | 0.25 |
| Nifedipine | 0.22 |
| Ranitidine | 0.22 |
| Furosemide | 0.22 |
| Isosorbide | 0.15 |
| Warfarin | 0.12 |
| Dipyridamole | 0.11 |
| Codeine | 0.11 |
| * ng/mL in atropine equivalents | |
| Source: Adapted from reference 19. | |
For several years, Mr. S has been taking the monoamine oxidase inhibitor (MAOI) phenelzine, 30 mg/d, for depression maintenance treatment. On admission, he insisted that the MAOI be continued during hospitalization because it had relieved his severe depressions.
Within 24 hours of surgery, he was given the skeletal muscle relaxant cyclobenzaprine, 5 mg tid, for painful muscle spasms in the operated hip. When this brought little relief, the dosage was increased to 10 mg tid. Delirium and autonomic instability developed approximately 4 hours after the first 10-mg dose and gradually worsened.
The two drugs are discontinued, and Mr S. gradually recovers after several days of physiologic support, protection, and sedation in the intensive-care unit.
Discussion. Mr. S developed serotonin syndrome from a drug-drug interaction. Phenelzine inhibited serotonin metabolism, and cyclobenzaprine—a drug chemically similar to tricyclic antidepressants—inhibited serotonin reuptake, resulting in substantially increased CNS serotonergic activity.20 Serotonin syndrome symptoms include delirium, autonomic dysfunction, and neurologic signs such as myoclonus and rigidity when patients are taking drugs that enhance serotonergic transmission.
DOES DELIRIUM WORSEN PROGNOSIS?
In the largest study of delirium in older patients, Inouye et al21 examined outcomes of 727 consecutive patients age 65 and older with various medical diagnoses who were admitted to three teaching hospitals. Delirium was diagnosed in 88 patients (12%) at admission.
Within 3 months of hospital discharge, 165 (25%) of 663 patients had died or been newly admitted to a nursing home. After the authors controlled the data for age, gender, dementia, illness severity, and functional status, they found that delirium:
- tripled the likelihood of nursing home placement at hospital discharge and after 3 months (adjusted odds ratio [OR] for delirium 3.0)
- more than doubled the likelihood of death or new nursing home placement at discharge (OR for delirium 2.1) and after 3 months (OR for delirium 2.6).
They concluded that delirium was a significant predictor of functional decline at hospital discharge and also at follow-up in older patients.
Interestingly, although these authors did not find a statistically significant association between delirium and death alone, the risk of death was particularly strong for patients who were not demented (OR for delirium, 3.77). Similarly, Rabins and Folstein22 found higher mortality rates in medically ill patients diagnosed with delirium on hospital admission than in demented, cognitively intact, or depressed patients. After 1 year, the death rate remained higher in those who had been delirious than in those with dementia.
In a 12-month observational study comparing 243 older medical inpatients with delirium and 118 controls without delirium, McCusker et al23 found that:
- patients with delirium were twice as likely to die within 12 months as those without delirium
- the greater severity of delirium symptoms, the higher the risk of death in patients with delirium but without dementia.
In a recent study, some of the same investigators found that delirium symptoms—especially inattention, disorientation, and impaired memory—persisted for 12 months after hospital discharge in medical inpatients age 65 and older with or without dementia. Mean numbers of delirium symptoms at diagnosis and 12-month follow-up, respectively, were:
- 4.5 and 3.5 in patients with dementia
- 3.4 and 2.2 in patients without dementia.24
CASE REPORT: DELIRIUM AS PROGNOSTIC SIGN
Mrs. W, age 70, is hospitalized for treatment of anemia and dehydration after falling at home. She has metastatic adenocarcinoma of the colon and is hypernatremic and hypotensive on admission.
Within 24 hours, she becomes floridly delirious, despite transfusion of two units of packed red cells and IV fluid replacement. She receives IM haloperidol to reduce the agitation and counteract delirium. Head CT reveals mild, diffuse cerebral atrophy but no metastasis or subdural hematoma.
Although aggressive treatment corrects her electrolyte disturbance and dehydration and restores normal vital signs, the delirium does not resolve. She is discharged to a nursing home, where she is discovered dead in bed 1 week later.
Discussion. Delirium independently increases the risk of death during hospitalization and thereafter, particularly in older patients. As in the case of Mrs. W, delirium is a common preterminal event in cancer patients.25
Evidence suggests that delirium is a marker for declining functional status and of relatively poor outcomes in older patients. In patients who are hospitalized, however, the relative effects of comorbid medical and neurologic conditions on prognosis are difficult to differentiate from the effects of delirium.
CAN DELIRIUM BE PREVENTED?
Researchers at Yale University examined whether a multicomponent, nonpharmacologic intervention could reduce delirium incidence and episode duration in 852 at-risk hospitalized medical patients age 70 and older.26 Patients were randomly assigned to intervention or usual care and then observed daily until discharge. Interventions included protocols for orientation, mobilization, sleep hygiene, and sensory enhancement, as well as prompt treatment of dehydration.
Delirium occurred in 10% of the intervention group and in 15% of the usual-care group (matched odds ratio 0.6). Total days with delirium (105 vs. 161; P = 0.02) and total episodes (62 vs. 90; P = 0.03) were significantly lower in the intervention group. A potential source of bias in this study was a lack of randomization in assigning patients to intervention or usual care. Follow-up studies found that:
- The intervention increased health care costs for patients at high risk for delirium but had no significant effect on overall costs for patients at intermediate risk.27
- Delirium risk decreased the most (89%) in older patients who were most adherent to the intervention protocols during hospitalization.28
- Among the 705 patients who survived at least 6 months after discharge, those who had been in the intervention and usual-care groups showed similar functional and cognitive status and rates of depression, delirium, nursing home placement, and rehospitalization.29
CASE REPORT: A SUCCESSFUL INTERVENTION
Mr. A, age 66, who has moderate-to-severe chronic obstructive pulmonary disease, is hospitalized for surgery to remove a suspicious lung nodule. Two years ago, he experienced delirium following a transurethral prostatectomy. His hemoglobin is 9.1 g/dL (normal, 11.5 to 14 g/dL), defined as anemia related to chronic disease.
Because of his history of postoperative delirium, the hospital staff initiates preventive measures. Before surgery, he is given two units of blood for anemia. To assist with orientation, he and his family receive information about delirium, and his hearing aid—which has been malfunctioning—is readjusted to improve his auditory acuity. During surgery, his oxygen saturation and blood pressure are monitored scrupulously.
Afterward, no mental status changes are observed, and Mr. A recovers uneventfully. The surgery revealed a benign granuloma.
Discussion. Surgical patients such as Mr. A—particularly those with hemoglobin <10 g/dL—face a higher risk for delirium than medical patients do. The reason, although undetermined, may be related to unavoidable tissue injury and hemorrhage associated with surgery.30
Nonpharmacologic intervention shows promise in preventing delirium, but more evidence is needed to develop simpler, less-costly strategies for at-risk hospitalized patients and to preserve their functional status after discharge.
Related resources
- Cook IA. Guideline Watch. Practice guideline for the treatment of patients with delirium. American Psychiatric Association, August 2004. www.psych.org/psych_pract/treatg/pg/prac_guide.cfm (scroll down to “Delirium” under topic list). Accessed Dec. 14, 2004.
Drug brand names
- Aripiprazole • Abilify
- Cyclobenzapine • Flexeril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Phenylzine • Nardil
- Physostigmine • Antilirium
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Warfarin • Coumadin
- Ziprasidone • Geodon
Disclosures
Dr. O’Connor reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th edition, text rev. Washington, DC: American Psychiatric Association, 2000.
2. Trzepacz PT, Mulsant BH, Amanda Dew M, et al. Is delirium different when it occurs with dementia? A study using the delirium rating scale. J Neuropsychiatry Clin Neurosci 1998;10:199-204.
3. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996;275:852-7.
4. Liptzin B. Clinical diagnosis and management of delirium. In: Stoudemire A, Fogel BS, Greenberg DB (eds). Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000;581-96.
5. Bourgeois JA, Seaman JS, Servis M. Delirium, dementia, and amnestic disorders. In: Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry (4th ed). Washington, DC: American Psychiatric Publishing, 2003;270.-
6. Rahkonen T, Makela H, Paanila S, et al. Delirium in elderly people without severe predisposing disorders: etiology and 1-year prognosis after discharge. Int Psychogeriatr 2000;12(4):473-81.
7. Horikawa N, Yamazaki T, Miyamoto K, et al. Treatment of delirium with risperidone: results of a prospective open trial with 10 patients. Gen Hosp Psychiatry 2003;25(4):289-92.
8. Mittal D, Jimerson NA, Neely EP, et al. Risperidone in the treatment of delirium: results from a prospective open-label trial. J Clin Psychiatry 2004;65(5):662-7.
9. Parellada E, Baeza I, de Pablo J, Martinez G. Risperidone in the treatment of patients with delirium. J Clin Psychiatry 2004;65(3):348-53.
10. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics 2004;45:297-301.
11. Schwartz TL, Masand PS. Treatment of delirium with quetiapine. Prim Care Companion J Clin Psychiatry 2000;2(1):10-12.
12. Sasaki Y, Matsuyama T, Inoue S, et al. A prospective, open-label, flexible-dose study of quetiapine in the treatment of delirium. J Clin Psychiatry 2003;64(11):1316-21.
13. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol 2004;19(2):125-7.
14. Kim KY, Bader GM, Kotlyar V, Gropper D. Treatment of delirium in older adults with quetiapine. J Geriatr Psychiatry Neurol 2003;16(1):29-31.
15. Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med 2004;30(3):444-9.
16. Van der Mast RC. Pathophysiology of delirium. J Geriatr Psychiatry Neurol 1998;11:138-45.
17. Trzepacz PT. Update on the neuropathogenesis of delirium. Dement Geriatr Cogn Disord 1999;10:330-4.
18. Mussi C, Ferrari R, Ascari S, et al. Importance of serum anticholinergic activity in the assessment of elderly patients with delirium. J Geriatr Psychiatry Neurol 1999;12:82-6.
19. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing risk of delirium.[see comment]. Am J Psychiatry 1992;149:1393-4.
20. Keck PE, Jr, Arnold LM. The serotonin syndrome. Psychiatr Ann 2000;30:333-43.
21. Inouye SK, Rushing JT, Foreman MD, et al. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998;13:234-42.
22. Rabins PV, Folstein MF. Delirium and dementia: diagnostic criteria and fatality rates. Br J Psychiatry 1982;140:149-53.
23. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med 2002;162(4):457-63.
24. McCusker J, Cole M, Dendukuri N, et al. The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med 2003;18(9):696-704.
25. Greenberg DB. Preventing delirium at the end of life: lessons from recent research. Primary Care Companion J Clin Psychiatry 2003;5:62-7.
26. Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340:669-76.
27. Rizzo JA, Bogardus ST, Jr, Leo-Summers L, et al. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value? Med Care 2001;39(7):740-52.
28. Inouye SK, Bogardus ST, Jr, Williams CS, et al. The role of adherence on the effectiveness of nonpharmacologic interventions: evidence from the delirium prevention trial. Arch Intern Med 2003;163(8):958-64.
29. Bogardus ST, Jr, Desai MM, Williams CS, et al. The effects of a targeted multicomponent delirium intervention on postdischarge outcomes for hospitalized older adults. Am J Med 2003;114(5):383-90.
30. Marcantonio ER, Goldman L, Orav EJ, et al. The association of intraoperative factors with the development of postoperative delirium. Am J Med 1998;105(5):380-4.
Four principles of treating delirium can help protect medical/surgical patients at risk for morbidity and functional decline. These principals—which I call the “four Ps”—are prompt identification, protection, pragmatic intervention, and pharmacotherapy.
This article describes an up-to-date, “four-Ps” approach to treating delirium—including use of antipsychotics and supportive care—and offers evidence and case reports to address these clinical questions:
- What causes delirium?
- Does delirium worsen prognosis?
- Can delirium be prevented?
FOUR ‘Ps’ FOR TREATING DELIRIUM
When a patient’s mental status changes dramatically (Box 1),1 identifying potential delirium causes requires careful medical, psychiatric, and neurologic assessment. Assimilating this information is as essential to positive outcomes as are intensive nursing care and appropriate interventions.
- Disturbance of consciousness (i.e. reduced clarity of awareness of the environment) with reduced ability to focus, sustain, or shift attention
- A change in cognition (such as memory deficit, disorientation, language disturbance) or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia
- The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of a 24-hour period
- There is evidence from the history, physical examination, or laboratory findings that the disturbance is caused by the direct physiologic consequences of a general medical condition
Source: Reprinted with permission from the Diagnostic and statistical manual of mental disorders (4th ed., text rev). Copyright 2000. American Psychiatric Publishing.
Prompt identification. Delirium often goes unrecognized, delaying treatment. Easily administered rating scales—such as the Delirium Rating Scale (DRS)2 and the Confusion Assessment Method (CAM)3 —can help detect emerging symptoms.
Patient protection. Provide intensive nursing care—often one-to-one observation and containment—and, where possible, enlist the family in reassuring and calming the patient. Restraints may be needed to safeguard against injury and to prevent the patient from removing or dislocating monitoring equipment and IV access.
Pragmatic intervention. With medical colleagues, begin treating biochemical and physiologic abnormalities that are the most likely and most remediable contributors (Box 2).2,4-6 Review the patient’s medications and discontinue or replace any that may be causing delirium.
Pharmacotherapy. Based on clinical studies, antipsychotics appear to possess antidelirium properties and may be considered as one part of a patient’s treatment plan. Interpreting these studies is complicated, however, by delirium’s complexity, numerous causes, and presumed mechanisms, as well as the transience of some forms. For ethical reasons, no placebo-controlled studies of delirium treatment have been done.
EVIDENCE ON ANTIPSYCHOTICS
Haloperidol has been the drug of choice for managing delirium because it is less likely to cause hypotension and sedation than other neuroleptics. Optimum haloperidol dosing in delirium has not been established, but the usual range is 2 to 6 mg every 4 to 6 hours, depending on the patient’s age and delirium severity.
Instances of QTc interval prolongation have been reported with high-dose IV haloperidol (> 100 mg). This life-threatening effect—which can induce torsades de pointes dysrhythmia, ventricular tachycardias, and fibrillation—is very rare, quite variable, and unpredictable. It probably is a function of total dose and neuroleptic administration rate.
Atypical antipsychotics share haloperidol’s advantages over first-generation neuroleptics, with lower potential for dystonic reactions, parkinsonian side effects, and tardive dyskinesia. Preliminary evidence suggests that atypicals may be safe and effective in treating delirium, although no randomized controlled trials have been done and accurate dose-response curves have not been established. Low to modest dosages have been used in case series.
Risperidone. Two prospective, open-label trials—each with 10 patients—suggest that low-dose risperidone is effective for treating delirium:
- In one trial, risperidone given at an average dosage of 1.7 mg/d was effective in 80% of patients with delirium, and one patient responded to 0.5 mg/d. Some patients experienced sleepiness or mild drug-induced parkinsonism.7
- In the other trial, risperidone was started at 0.5 mg twice daily, with additional doses allowed on day 1 for cognitive and behavioral symptoms. This dosage was maintained until DRS scores declined to ≤12, then was reduced by 50% and continued until day 6. Mean maintenance dosage was 0.75 mg/d. Two patients discontinued risperidone because of sedation or hypotension.8
At least 10% to 30% of hospitalized medically ill patients develop delirium, and rates approach 40% after age 65.4 Especially in older patients, delirium is a risk factor for:
- prolonged hospital stays
- increased morbidity and mortality
- increased functional decline and need for custodial care after hospital discharge.2
Risk factors. Prospectively identified risk factors for delirium include pre-existing dementia; age >65 years; serious medical illness; alcohol/sedative withdrawal; abnormal serum sodium, potassium, or blood glucose levels; vision or hearing impairment; hypoxia; malnutrition; and fever. Medication—particularly anticholinergic drugs—is one of the most common delirium triggers in susceptible patients.5
The most common underlying disorders that increase delirium risk in older patients are hip fracture, dementia, infections, and cerebrovascular events.6
In a larger prospective study, 64 patients (mean age 67) with delirium were treated with risperidone, given at a mean dose of 2.6 +/- 1.7 mg/d at day 3. This dosage was effective in 90% of patients and significantly improved all symptoms, as measured with scales including the DRS. Two patients (3%) experienced adverse effects.9
No significant differences in response frequency were seen in a 7-day, double-blind comparison of flexibly-dosed risperidone (starting at 0.5 mg bid) and haloperidol (starting at 0.75 mg bid) in 28 patients with delirium. Symptom severity decreased for each group, as measured with the Memorial Delirium Assessment Scale. One patient receiving haloperidol experienced mild akathisia, but no others reported clinically significant side effects.10
Quetiapine. In a retrospective review, the charts of 11 patients who received quetiapine for delirium were compared with those of 11 similar patients treated with haloperidol. DRS scores improved by >50% in 10 of 11 patients in both groups, with similar onset of effect, treatment duration, and overall clinical improvement.11 Small prospective trials with flexible dosing schedules have reported similar results.12,13
In a study of 12 older hospitalized patients with delirium, quetiapine at a mean dosage of 93.75 +/-23.31 mg/d was associated with significant DRS score improvements. Interestingly, patients’ Mini-Mental State Examination and Clock-Drawing Test scores continued to improve 3 months after their delirium symptoms stabilized.14
Olanzapine. In a prospective trial, hospitalized patients with delirium were randomly assigned to receive enteral olanzapine or haloperidol. Delirium symptoms decreased across 5 days in both groups, and clinical improvement was similar. Some patients receiving haloperidol reported extrapyramidal symptoms, whereas those receiving olanzapine reported no adverse effects.15
Parenteral forms of some atypicals (aripiprazole, olanzapine, and ziprasidone) have become available and may increase this class’ usefulness in treating delirium.
Other drugs. Benzodiazepines appear ineffective and generally play only an adjunctive role in treating delirium. An exception may be delirium induced by acute alcohol or benzodiazepine withdrawal. Sedating antidepressants have been used as hypnotics in patients with delirium, but supporting evidence is lacking.
Other drug classes—general anesthetics, narcotics, cholinomimetics—may help manage the dangerously hyperactive delirious patient, but the literature contains no systematic analyses.
WHAT CAUSES DELIRIUM?
Delirium’s pathophysiology is not completely understood, although most authors believe several mechanisms are involved.
The brain’s exclusively oxidative metabolism and its systems’ hierarchical vulnerability to substrate deficiency—as might occur in even transient hypoxia or hypotension—appear to play important roles. Factors such as fever and stress that increase metabolic demand on the brain intensify the effects of oxygen deficiency or circulatory compromise.
At least three molecular mechanisms have been proposed for delirium, including cholinergic transmission disruption, monoaminergic dysfunction, and cytokine release ( Box 3).16-19 These mechanisms may interact, cascading into a common final pathway that results in delirium.
Features not considered essential to delirium’s diagnosis—such as visual hallucinations or aggressive behaviors—indicate that additional cortical and subcortical systems are involved.
CASE REPORT: DRUG-DRUG INTERACTION
Three days after hip replacement surgery, Mr. S, age 64, becomes confused, distractible, and combative. He is alert one minute and somnolent the next. His arms and legs jerk involuntarily, and his muscle tone is diffusely increased. He talks with absent friends and family as though they are present in his hospital room. His body temperature and blood pressure fluctuate widely, despite no evidence of infection.
Cholinergic transmission disruption
The greater a medication’s anticholinergic activity, the greater its risk of causing delirium. Combining drugs with anticholinergic effects—such as theophylline, warfarin, or codeine (Table19)—compounds the delirium risk.
Acetylcholine-secreting neurons—widely if sparsely distributed throughout the brain—affect arousal, attention, memory, and sleep regulation. Acetylcholine is produced by oxidative metabolism and thus is vulnerable to physiologic disturbances that increase oxygen demand or disrupt oxygen supply.
Anticholinergic poisoning and abuse of anticholinergic substances are known to cause acute delirium—a finding that supports the key role of acetylcholine in maintaining alertness and concentration. Agents that enhance cholinergic transmission—such as the cholinesterase inhibitor physostigmine—can effectively treat drug-induced delirium.
Monoaminergic dysfunction
The principal monoamines of dopamine, serotonin, and norepinephrine help sustain attention, regulate the sleep-wake cycle, inhibit affective responses, and modulate aggressive and impulsive behaviors. Treating patients with dopamine and serotonin agonists can cause psychotic symptoms.
Glutamate—a monoamine neurotransmitter with excitatory properties—is released during metabolic stress and likely contributes to the psychotic features sometimes seen in delirium.
Cytokine release
Infection in a distant organ, such as gallbladder or kidney, is known to cause delirium. Cytokines such as interleukins and interferon-alpha are polypeptides secreted by macrophagesin response to tissue injury. They easily cross the blood-brain barrier and stimulate glial cells to release more cytokines, which interfere with neurotransmitter synthesis and transmission.
Table
Drugs whose anticholinergic effects may increase the risk of delirium
| Drug | Anticholinergic level* |
|---|---|
| Cimetidine | 0.86 |
| Prednisolone | 0.55 |
| Theophylline | 0.44 |
| Digoxin | 0.25 |
| Lanoxin | 0.25 |
| Nifedipine | 0.22 |
| Ranitidine | 0.22 |
| Furosemide | 0.22 |
| Isosorbide | 0.15 |
| Warfarin | 0.12 |
| Dipyridamole | 0.11 |
| Codeine | 0.11 |
| * ng/mL in atropine equivalents | |
| Source: Adapted from reference 19. | |
For several years, Mr. S has been taking the monoamine oxidase inhibitor (MAOI) phenelzine, 30 mg/d, for depression maintenance treatment. On admission, he insisted that the MAOI be continued during hospitalization because it had relieved his severe depressions.
Within 24 hours of surgery, he was given the skeletal muscle relaxant cyclobenzaprine, 5 mg tid, for painful muscle spasms in the operated hip. When this brought little relief, the dosage was increased to 10 mg tid. Delirium and autonomic instability developed approximately 4 hours after the first 10-mg dose and gradually worsened.
The two drugs are discontinued, and Mr S. gradually recovers after several days of physiologic support, protection, and sedation in the intensive-care unit.
Discussion. Mr. S developed serotonin syndrome from a drug-drug interaction. Phenelzine inhibited serotonin metabolism, and cyclobenzaprine—a drug chemically similar to tricyclic antidepressants—inhibited serotonin reuptake, resulting in substantially increased CNS serotonergic activity.20 Serotonin syndrome symptoms include delirium, autonomic dysfunction, and neurologic signs such as myoclonus and rigidity when patients are taking drugs that enhance serotonergic transmission.
DOES DELIRIUM WORSEN PROGNOSIS?
In the largest study of delirium in older patients, Inouye et al21 examined outcomes of 727 consecutive patients age 65 and older with various medical diagnoses who were admitted to three teaching hospitals. Delirium was diagnosed in 88 patients (12%) at admission.
Within 3 months of hospital discharge, 165 (25%) of 663 patients had died or been newly admitted to a nursing home. After the authors controlled the data for age, gender, dementia, illness severity, and functional status, they found that delirium:
- tripled the likelihood of nursing home placement at hospital discharge and after 3 months (adjusted odds ratio [OR] for delirium 3.0)
- more than doubled the likelihood of death or new nursing home placement at discharge (OR for delirium 2.1) and after 3 months (OR for delirium 2.6).
They concluded that delirium was a significant predictor of functional decline at hospital discharge and also at follow-up in older patients.
Interestingly, although these authors did not find a statistically significant association between delirium and death alone, the risk of death was particularly strong for patients who were not demented (OR for delirium, 3.77). Similarly, Rabins and Folstein22 found higher mortality rates in medically ill patients diagnosed with delirium on hospital admission than in demented, cognitively intact, or depressed patients. After 1 year, the death rate remained higher in those who had been delirious than in those with dementia.
In a 12-month observational study comparing 243 older medical inpatients with delirium and 118 controls without delirium, McCusker et al23 found that:
- patients with delirium were twice as likely to die within 12 months as those without delirium
- the greater severity of delirium symptoms, the higher the risk of death in patients with delirium but without dementia.
In a recent study, some of the same investigators found that delirium symptoms—especially inattention, disorientation, and impaired memory—persisted for 12 months after hospital discharge in medical inpatients age 65 and older with or without dementia. Mean numbers of delirium symptoms at diagnosis and 12-month follow-up, respectively, were:
- 4.5 and 3.5 in patients with dementia
- 3.4 and 2.2 in patients without dementia.24
CASE REPORT: DELIRIUM AS PROGNOSTIC SIGN
Mrs. W, age 70, is hospitalized for treatment of anemia and dehydration after falling at home. She has metastatic adenocarcinoma of the colon and is hypernatremic and hypotensive on admission.
Within 24 hours, she becomes floridly delirious, despite transfusion of two units of packed red cells and IV fluid replacement. She receives IM haloperidol to reduce the agitation and counteract delirium. Head CT reveals mild, diffuse cerebral atrophy but no metastasis or subdural hematoma.
Although aggressive treatment corrects her electrolyte disturbance and dehydration and restores normal vital signs, the delirium does not resolve. She is discharged to a nursing home, where she is discovered dead in bed 1 week later.
Discussion. Delirium independently increases the risk of death during hospitalization and thereafter, particularly in older patients. As in the case of Mrs. W, delirium is a common preterminal event in cancer patients.25
Evidence suggests that delirium is a marker for declining functional status and of relatively poor outcomes in older patients. In patients who are hospitalized, however, the relative effects of comorbid medical and neurologic conditions on prognosis are difficult to differentiate from the effects of delirium.
CAN DELIRIUM BE PREVENTED?
Researchers at Yale University examined whether a multicomponent, nonpharmacologic intervention could reduce delirium incidence and episode duration in 852 at-risk hospitalized medical patients age 70 and older.26 Patients were randomly assigned to intervention or usual care and then observed daily until discharge. Interventions included protocols for orientation, mobilization, sleep hygiene, and sensory enhancement, as well as prompt treatment of dehydration.
Delirium occurred in 10% of the intervention group and in 15% of the usual-care group (matched odds ratio 0.6). Total days with delirium (105 vs. 161; P = 0.02) and total episodes (62 vs. 90; P = 0.03) were significantly lower in the intervention group. A potential source of bias in this study was a lack of randomization in assigning patients to intervention or usual care. Follow-up studies found that:
- The intervention increased health care costs for patients at high risk for delirium but had no significant effect on overall costs for patients at intermediate risk.27
- Delirium risk decreased the most (89%) in older patients who were most adherent to the intervention protocols during hospitalization.28
- Among the 705 patients who survived at least 6 months after discharge, those who had been in the intervention and usual-care groups showed similar functional and cognitive status and rates of depression, delirium, nursing home placement, and rehospitalization.29
CASE REPORT: A SUCCESSFUL INTERVENTION
Mr. A, age 66, who has moderate-to-severe chronic obstructive pulmonary disease, is hospitalized for surgery to remove a suspicious lung nodule. Two years ago, he experienced delirium following a transurethral prostatectomy. His hemoglobin is 9.1 g/dL (normal, 11.5 to 14 g/dL), defined as anemia related to chronic disease.
Because of his history of postoperative delirium, the hospital staff initiates preventive measures. Before surgery, he is given two units of blood for anemia. To assist with orientation, he and his family receive information about delirium, and his hearing aid—which has been malfunctioning—is readjusted to improve his auditory acuity. During surgery, his oxygen saturation and blood pressure are monitored scrupulously.
Afterward, no mental status changes are observed, and Mr. A recovers uneventfully. The surgery revealed a benign granuloma.
Discussion. Surgical patients such as Mr. A—particularly those with hemoglobin <10 g/dL—face a higher risk for delirium than medical patients do. The reason, although undetermined, may be related to unavoidable tissue injury and hemorrhage associated with surgery.30
Nonpharmacologic intervention shows promise in preventing delirium, but more evidence is needed to develop simpler, less-costly strategies for at-risk hospitalized patients and to preserve their functional status after discharge.
Related resources
- Cook IA. Guideline Watch. Practice guideline for the treatment of patients with delirium. American Psychiatric Association, August 2004. www.psych.org/psych_pract/treatg/pg/prac_guide.cfm (scroll down to “Delirium” under topic list). Accessed Dec. 14, 2004.
Drug brand names
- Aripiprazole • Abilify
- Cyclobenzapine • Flexeril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Phenylzine • Nardil
- Physostigmine • Antilirium
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Warfarin • Coumadin
- Ziprasidone • Geodon
Disclosures
Dr. O’Connor reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Four principles of treating delirium can help protect medical/surgical patients at risk for morbidity and functional decline. These principals—which I call the “four Ps”—are prompt identification, protection, pragmatic intervention, and pharmacotherapy.
This article describes an up-to-date, “four-Ps” approach to treating delirium—including use of antipsychotics and supportive care—and offers evidence and case reports to address these clinical questions:
- What causes delirium?
- Does delirium worsen prognosis?
- Can delirium be prevented?
FOUR ‘Ps’ FOR TREATING DELIRIUM
When a patient’s mental status changes dramatically (Box 1),1 identifying potential delirium causes requires careful medical, psychiatric, and neurologic assessment. Assimilating this information is as essential to positive outcomes as are intensive nursing care and appropriate interventions.
- Disturbance of consciousness (i.e. reduced clarity of awareness of the environment) with reduced ability to focus, sustain, or shift attention
- A change in cognition (such as memory deficit, disorientation, language disturbance) or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia
- The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of a 24-hour period
- There is evidence from the history, physical examination, or laboratory findings that the disturbance is caused by the direct physiologic consequences of a general medical condition
Source: Reprinted with permission from the Diagnostic and statistical manual of mental disorders (4th ed., text rev). Copyright 2000. American Psychiatric Publishing.
Prompt identification. Delirium often goes unrecognized, delaying treatment. Easily administered rating scales—such as the Delirium Rating Scale (DRS)2 and the Confusion Assessment Method (CAM)3 —can help detect emerging symptoms.
Patient protection. Provide intensive nursing care—often one-to-one observation and containment—and, where possible, enlist the family in reassuring and calming the patient. Restraints may be needed to safeguard against injury and to prevent the patient from removing or dislocating monitoring equipment and IV access.
Pragmatic intervention. With medical colleagues, begin treating biochemical and physiologic abnormalities that are the most likely and most remediable contributors (Box 2).2,4-6 Review the patient’s medications and discontinue or replace any that may be causing delirium.
Pharmacotherapy. Based on clinical studies, antipsychotics appear to possess antidelirium properties and may be considered as one part of a patient’s treatment plan. Interpreting these studies is complicated, however, by delirium’s complexity, numerous causes, and presumed mechanisms, as well as the transience of some forms. For ethical reasons, no placebo-controlled studies of delirium treatment have been done.
EVIDENCE ON ANTIPSYCHOTICS
Haloperidol has been the drug of choice for managing delirium because it is less likely to cause hypotension and sedation than other neuroleptics. Optimum haloperidol dosing in delirium has not been established, but the usual range is 2 to 6 mg every 4 to 6 hours, depending on the patient’s age and delirium severity.
Instances of QTc interval prolongation have been reported with high-dose IV haloperidol (> 100 mg). This life-threatening effect—which can induce torsades de pointes dysrhythmia, ventricular tachycardias, and fibrillation—is very rare, quite variable, and unpredictable. It probably is a function of total dose and neuroleptic administration rate.
Atypical antipsychotics share haloperidol’s advantages over first-generation neuroleptics, with lower potential for dystonic reactions, parkinsonian side effects, and tardive dyskinesia. Preliminary evidence suggests that atypicals may be safe and effective in treating delirium, although no randomized controlled trials have been done and accurate dose-response curves have not been established. Low to modest dosages have been used in case series.
Risperidone. Two prospective, open-label trials—each with 10 patients—suggest that low-dose risperidone is effective for treating delirium:
- In one trial, risperidone given at an average dosage of 1.7 mg/d was effective in 80% of patients with delirium, and one patient responded to 0.5 mg/d. Some patients experienced sleepiness or mild drug-induced parkinsonism.7
- In the other trial, risperidone was started at 0.5 mg twice daily, with additional doses allowed on day 1 for cognitive and behavioral symptoms. This dosage was maintained until DRS scores declined to ≤12, then was reduced by 50% and continued until day 6. Mean maintenance dosage was 0.75 mg/d. Two patients discontinued risperidone because of sedation or hypotension.8
At least 10% to 30% of hospitalized medically ill patients develop delirium, and rates approach 40% after age 65.4 Especially in older patients, delirium is a risk factor for:
- prolonged hospital stays
- increased morbidity and mortality
- increased functional decline and need for custodial care after hospital discharge.2
Risk factors. Prospectively identified risk factors for delirium include pre-existing dementia; age >65 years; serious medical illness; alcohol/sedative withdrawal; abnormal serum sodium, potassium, or blood glucose levels; vision or hearing impairment; hypoxia; malnutrition; and fever. Medication—particularly anticholinergic drugs—is one of the most common delirium triggers in susceptible patients.5
The most common underlying disorders that increase delirium risk in older patients are hip fracture, dementia, infections, and cerebrovascular events.6
In a larger prospective study, 64 patients (mean age 67) with delirium were treated with risperidone, given at a mean dose of 2.6 +/- 1.7 mg/d at day 3. This dosage was effective in 90% of patients and significantly improved all symptoms, as measured with scales including the DRS. Two patients (3%) experienced adverse effects.9
No significant differences in response frequency were seen in a 7-day, double-blind comparison of flexibly-dosed risperidone (starting at 0.5 mg bid) and haloperidol (starting at 0.75 mg bid) in 28 patients with delirium. Symptom severity decreased for each group, as measured with the Memorial Delirium Assessment Scale. One patient receiving haloperidol experienced mild akathisia, but no others reported clinically significant side effects.10
Quetiapine. In a retrospective review, the charts of 11 patients who received quetiapine for delirium were compared with those of 11 similar patients treated with haloperidol. DRS scores improved by >50% in 10 of 11 patients in both groups, with similar onset of effect, treatment duration, and overall clinical improvement.11 Small prospective trials with flexible dosing schedules have reported similar results.12,13
In a study of 12 older hospitalized patients with delirium, quetiapine at a mean dosage of 93.75 +/-23.31 mg/d was associated with significant DRS score improvements. Interestingly, patients’ Mini-Mental State Examination and Clock-Drawing Test scores continued to improve 3 months after their delirium symptoms stabilized.14
Olanzapine. In a prospective trial, hospitalized patients with delirium were randomly assigned to receive enteral olanzapine or haloperidol. Delirium symptoms decreased across 5 days in both groups, and clinical improvement was similar. Some patients receiving haloperidol reported extrapyramidal symptoms, whereas those receiving olanzapine reported no adverse effects.15
Parenteral forms of some atypicals (aripiprazole, olanzapine, and ziprasidone) have become available and may increase this class’ usefulness in treating delirium.
Other drugs. Benzodiazepines appear ineffective and generally play only an adjunctive role in treating delirium. An exception may be delirium induced by acute alcohol or benzodiazepine withdrawal. Sedating antidepressants have been used as hypnotics in patients with delirium, but supporting evidence is lacking.
Other drug classes—general anesthetics, narcotics, cholinomimetics—may help manage the dangerously hyperactive delirious patient, but the literature contains no systematic analyses.
WHAT CAUSES DELIRIUM?
Delirium’s pathophysiology is not completely understood, although most authors believe several mechanisms are involved.
The brain’s exclusively oxidative metabolism and its systems’ hierarchical vulnerability to substrate deficiency—as might occur in even transient hypoxia or hypotension—appear to play important roles. Factors such as fever and stress that increase metabolic demand on the brain intensify the effects of oxygen deficiency or circulatory compromise.
At least three molecular mechanisms have been proposed for delirium, including cholinergic transmission disruption, monoaminergic dysfunction, and cytokine release ( Box 3).16-19 These mechanisms may interact, cascading into a common final pathway that results in delirium.
Features not considered essential to delirium’s diagnosis—such as visual hallucinations or aggressive behaviors—indicate that additional cortical and subcortical systems are involved.
CASE REPORT: DRUG-DRUG INTERACTION
Three days after hip replacement surgery, Mr. S, age 64, becomes confused, distractible, and combative. He is alert one minute and somnolent the next. His arms and legs jerk involuntarily, and his muscle tone is diffusely increased. He talks with absent friends and family as though they are present in his hospital room. His body temperature and blood pressure fluctuate widely, despite no evidence of infection.
Cholinergic transmission disruption
The greater a medication’s anticholinergic activity, the greater its risk of causing delirium. Combining drugs with anticholinergic effects—such as theophylline, warfarin, or codeine (Table19)—compounds the delirium risk.
Acetylcholine-secreting neurons—widely if sparsely distributed throughout the brain—affect arousal, attention, memory, and sleep regulation. Acetylcholine is produced by oxidative metabolism and thus is vulnerable to physiologic disturbances that increase oxygen demand or disrupt oxygen supply.
Anticholinergic poisoning and abuse of anticholinergic substances are known to cause acute delirium—a finding that supports the key role of acetylcholine in maintaining alertness and concentration. Agents that enhance cholinergic transmission—such as the cholinesterase inhibitor physostigmine—can effectively treat drug-induced delirium.
Monoaminergic dysfunction
The principal monoamines of dopamine, serotonin, and norepinephrine help sustain attention, regulate the sleep-wake cycle, inhibit affective responses, and modulate aggressive and impulsive behaviors. Treating patients with dopamine and serotonin agonists can cause psychotic symptoms.
Glutamate—a monoamine neurotransmitter with excitatory properties—is released during metabolic stress and likely contributes to the psychotic features sometimes seen in delirium.
Cytokine release
Infection in a distant organ, such as gallbladder or kidney, is known to cause delirium. Cytokines such as interleukins and interferon-alpha are polypeptides secreted by macrophagesin response to tissue injury. They easily cross the blood-brain barrier and stimulate glial cells to release more cytokines, which interfere with neurotransmitter synthesis and transmission.
Table
Drugs whose anticholinergic effects may increase the risk of delirium
| Drug | Anticholinergic level* |
|---|---|
| Cimetidine | 0.86 |
| Prednisolone | 0.55 |
| Theophylline | 0.44 |
| Digoxin | 0.25 |
| Lanoxin | 0.25 |
| Nifedipine | 0.22 |
| Ranitidine | 0.22 |
| Furosemide | 0.22 |
| Isosorbide | 0.15 |
| Warfarin | 0.12 |
| Dipyridamole | 0.11 |
| Codeine | 0.11 |
| * ng/mL in atropine equivalents | |
| Source: Adapted from reference 19. | |
For several years, Mr. S has been taking the monoamine oxidase inhibitor (MAOI) phenelzine, 30 mg/d, for depression maintenance treatment. On admission, he insisted that the MAOI be continued during hospitalization because it had relieved his severe depressions.
Within 24 hours of surgery, he was given the skeletal muscle relaxant cyclobenzaprine, 5 mg tid, for painful muscle spasms in the operated hip. When this brought little relief, the dosage was increased to 10 mg tid. Delirium and autonomic instability developed approximately 4 hours after the first 10-mg dose and gradually worsened.
The two drugs are discontinued, and Mr S. gradually recovers after several days of physiologic support, protection, and sedation in the intensive-care unit.
Discussion. Mr. S developed serotonin syndrome from a drug-drug interaction. Phenelzine inhibited serotonin metabolism, and cyclobenzaprine—a drug chemically similar to tricyclic antidepressants—inhibited serotonin reuptake, resulting in substantially increased CNS serotonergic activity.20 Serotonin syndrome symptoms include delirium, autonomic dysfunction, and neurologic signs such as myoclonus and rigidity when patients are taking drugs that enhance serotonergic transmission.
DOES DELIRIUM WORSEN PROGNOSIS?
In the largest study of delirium in older patients, Inouye et al21 examined outcomes of 727 consecutive patients age 65 and older with various medical diagnoses who were admitted to three teaching hospitals. Delirium was diagnosed in 88 patients (12%) at admission.
Within 3 months of hospital discharge, 165 (25%) of 663 patients had died or been newly admitted to a nursing home. After the authors controlled the data for age, gender, dementia, illness severity, and functional status, they found that delirium:
- tripled the likelihood of nursing home placement at hospital discharge and after 3 months (adjusted odds ratio [OR] for delirium 3.0)
- more than doubled the likelihood of death or new nursing home placement at discharge (OR for delirium 2.1) and after 3 months (OR for delirium 2.6).
They concluded that delirium was a significant predictor of functional decline at hospital discharge and also at follow-up in older patients.
Interestingly, although these authors did not find a statistically significant association between delirium and death alone, the risk of death was particularly strong for patients who were not demented (OR for delirium, 3.77). Similarly, Rabins and Folstein22 found higher mortality rates in medically ill patients diagnosed with delirium on hospital admission than in demented, cognitively intact, or depressed patients. After 1 year, the death rate remained higher in those who had been delirious than in those with dementia.
In a 12-month observational study comparing 243 older medical inpatients with delirium and 118 controls without delirium, McCusker et al23 found that:
- patients with delirium were twice as likely to die within 12 months as those without delirium
- the greater severity of delirium symptoms, the higher the risk of death in patients with delirium but without dementia.
In a recent study, some of the same investigators found that delirium symptoms—especially inattention, disorientation, and impaired memory—persisted for 12 months after hospital discharge in medical inpatients age 65 and older with or without dementia. Mean numbers of delirium symptoms at diagnosis and 12-month follow-up, respectively, were:
- 4.5 and 3.5 in patients with dementia
- 3.4 and 2.2 in patients without dementia.24
CASE REPORT: DELIRIUM AS PROGNOSTIC SIGN
Mrs. W, age 70, is hospitalized for treatment of anemia and dehydration after falling at home. She has metastatic adenocarcinoma of the colon and is hypernatremic and hypotensive on admission.
Within 24 hours, she becomes floridly delirious, despite transfusion of two units of packed red cells and IV fluid replacement. She receives IM haloperidol to reduce the agitation and counteract delirium. Head CT reveals mild, diffuse cerebral atrophy but no metastasis or subdural hematoma.
Although aggressive treatment corrects her electrolyte disturbance and dehydration and restores normal vital signs, the delirium does not resolve. She is discharged to a nursing home, where she is discovered dead in bed 1 week later.
Discussion. Delirium independently increases the risk of death during hospitalization and thereafter, particularly in older patients. As in the case of Mrs. W, delirium is a common preterminal event in cancer patients.25
Evidence suggests that delirium is a marker for declining functional status and of relatively poor outcomes in older patients. In patients who are hospitalized, however, the relative effects of comorbid medical and neurologic conditions on prognosis are difficult to differentiate from the effects of delirium.
CAN DELIRIUM BE PREVENTED?
Researchers at Yale University examined whether a multicomponent, nonpharmacologic intervention could reduce delirium incidence and episode duration in 852 at-risk hospitalized medical patients age 70 and older.26 Patients were randomly assigned to intervention or usual care and then observed daily until discharge. Interventions included protocols for orientation, mobilization, sleep hygiene, and sensory enhancement, as well as prompt treatment of dehydration.
Delirium occurred in 10% of the intervention group and in 15% of the usual-care group (matched odds ratio 0.6). Total days with delirium (105 vs. 161; P = 0.02) and total episodes (62 vs. 90; P = 0.03) were significantly lower in the intervention group. A potential source of bias in this study was a lack of randomization in assigning patients to intervention or usual care. Follow-up studies found that:
- The intervention increased health care costs for patients at high risk for delirium but had no significant effect on overall costs for patients at intermediate risk.27
- Delirium risk decreased the most (89%) in older patients who were most adherent to the intervention protocols during hospitalization.28
- Among the 705 patients who survived at least 6 months after discharge, those who had been in the intervention and usual-care groups showed similar functional and cognitive status and rates of depression, delirium, nursing home placement, and rehospitalization.29
CASE REPORT: A SUCCESSFUL INTERVENTION
Mr. A, age 66, who has moderate-to-severe chronic obstructive pulmonary disease, is hospitalized for surgery to remove a suspicious lung nodule. Two years ago, he experienced delirium following a transurethral prostatectomy. His hemoglobin is 9.1 g/dL (normal, 11.5 to 14 g/dL), defined as anemia related to chronic disease.
Because of his history of postoperative delirium, the hospital staff initiates preventive measures. Before surgery, he is given two units of blood for anemia. To assist with orientation, he and his family receive information about delirium, and his hearing aid—which has been malfunctioning—is readjusted to improve his auditory acuity. During surgery, his oxygen saturation and blood pressure are monitored scrupulously.
Afterward, no mental status changes are observed, and Mr. A recovers uneventfully. The surgery revealed a benign granuloma.
Discussion. Surgical patients such as Mr. A—particularly those with hemoglobin <10 g/dL—face a higher risk for delirium than medical patients do. The reason, although undetermined, may be related to unavoidable tissue injury and hemorrhage associated with surgery.30
Nonpharmacologic intervention shows promise in preventing delirium, but more evidence is needed to develop simpler, less-costly strategies for at-risk hospitalized patients and to preserve their functional status after discharge.
Related resources
- Cook IA. Guideline Watch. Practice guideline for the treatment of patients with delirium. American Psychiatric Association, August 2004. www.psych.org/psych_pract/treatg/pg/prac_guide.cfm (scroll down to “Delirium” under topic list). Accessed Dec. 14, 2004.
Drug brand names
- Aripiprazole • Abilify
- Cyclobenzapine • Flexeril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Phenylzine • Nardil
- Physostigmine • Antilirium
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Warfarin • Coumadin
- Ziprasidone • Geodon
Disclosures
Dr. O’Connor reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th edition, text rev. Washington, DC: American Psychiatric Association, 2000.
2. Trzepacz PT, Mulsant BH, Amanda Dew M, et al. Is delirium different when it occurs with dementia? A study using the delirium rating scale. J Neuropsychiatry Clin Neurosci 1998;10:199-204.
3. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996;275:852-7.
4. Liptzin B. Clinical diagnosis and management of delirium. In: Stoudemire A, Fogel BS, Greenberg DB (eds). Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000;581-96.
5. Bourgeois JA, Seaman JS, Servis M. Delirium, dementia, and amnestic disorders. In: Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry (4th ed). Washington, DC: American Psychiatric Publishing, 2003;270.-
6. Rahkonen T, Makela H, Paanila S, et al. Delirium in elderly people without severe predisposing disorders: etiology and 1-year prognosis after discharge. Int Psychogeriatr 2000;12(4):473-81.
7. Horikawa N, Yamazaki T, Miyamoto K, et al. Treatment of delirium with risperidone: results of a prospective open trial with 10 patients. Gen Hosp Psychiatry 2003;25(4):289-92.
8. Mittal D, Jimerson NA, Neely EP, et al. Risperidone in the treatment of delirium: results from a prospective open-label trial. J Clin Psychiatry 2004;65(5):662-7.
9. Parellada E, Baeza I, de Pablo J, Martinez G. Risperidone in the treatment of patients with delirium. J Clin Psychiatry 2004;65(3):348-53.
10. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics 2004;45:297-301.
11. Schwartz TL, Masand PS. Treatment of delirium with quetiapine. Prim Care Companion J Clin Psychiatry 2000;2(1):10-12.
12. Sasaki Y, Matsuyama T, Inoue S, et al. A prospective, open-label, flexible-dose study of quetiapine in the treatment of delirium. J Clin Psychiatry 2003;64(11):1316-21.
13. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol 2004;19(2):125-7.
14. Kim KY, Bader GM, Kotlyar V, Gropper D. Treatment of delirium in older adults with quetiapine. J Geriatr Psychiatry Neurol 2003;16(1):29-31.
15. Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med 2004;30(3):444-9.
16. Van der Mast RC. Pathophysiology of delirium. J Geriatr Psychiatry Neurol 1998;11:138-45.
17. Trzepacz PT. Update on the neuropathogenesis of delirium. Dement Geriatr Cogn Disord 1999;10:330-4.
18. Mussi C, Ferrari R, Ascari S, et al. Importance of serum anticholinergic activity in the assessment of elderly patients with delirium. J Geriatr Psychiatry Neurol 1999;12:82-6.
19. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing risk of delirium.[see comment]. Am J Psychiatry 1992;149:1393-4.
20. Keck PE, Jr, Arnold LM. The serotonin syndrome. Psychiatr Ann 2000;30:333-43.
21. Inouye SK, Rushing JT, Foreman MD, et al. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998;13:234-42.
22. Rabins PV, Folstein MF. Delirium and dementia: diagnostic criteria and fatality rates. Br J Psychiatry 1982;140:149-53.
23. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med 2002;162(4):457-63.
24. McCusker J, Cole M, Dendukuri N, et al. The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med 2003;18(9):696-704.
25. Greenberg DB. Preventing delirium at the end of life: lessons from recent research. Primary Care Companion J Clin Psychiatry 2003;5:62-7.
26. Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340:669-76.
27. Rizzo JA, Bogardus ST, Jr, Leo-Summers L, et al. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value? Med Care 2001;39(7):740-52.
28. Inouye SK, Bogardus ST, Jr, Williams CS, et al. The role of adherence on the effectiveness of nonpharmacologic interventions: evidence from the delirium prevention trial. Arch Intern Med 2003;163(8):958-64.
29. Bogardus ST, Jr, Desai MM, Williams CS, et al. The effects of a targeted multicomponent delirium intervention on postdischarge outcomes for hospitalized older adults. Am J Med 2003;114(5):383-90.
30. Marcantonio ER, Goldman L, Orav EJ, et al. The association of intraoperative factors with the development of postoperative delirium. Am J Med 1998;105(5):380-4.
1. Diagnostic and statistical manual of mental disorders, 4th edition, text rev. Washington, DC: American Psychiatric Association, 2000.
2. Trzepacz PT, Mulsant BH, Amanda Dew M, et al. Is delirium different when it occurs with dementia? A study using the delirium rating scale. J Neuropsychiatry Clin Neurosci 1998;10:199-204.
3. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996;275:852-7.
4. Liptzin B. Clinical diagnosis and management of delirium. In: Stoudemire A, Fogel BS, Greenberg DB (eds). Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000;581-96.
5. Bourgeois JA, Seaman JS, Servis M. Delirium, dementia, and amnestic disorders. In: Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry (4th ed). Washington, DC: American Psychiatric Publishing, 2003;270.-
6. Rahkonen T, Makela H, Paanila S, et al. Delirium in elderly people without severe predisposing disorders: etiology and 1-year prognosis after discharge. Int Psychogeriatr 2000;12(4):473-81.
7. Horikawa N, Yamazaki T, Miyamoto K, et al. Treatment of delirium with risperidone: results of a prospective open trial with 10 patients. Gen Hosp Psychiatry 2003;25(4):289-92.
8. Mittal D, Jimerson NA, Neely EP, et al. Risperidone in the treatment of delirium: results from a prospective open-label trial. J Clin Psychiatry 2004;65(5):662-7.
9. Parellada E, Baeza I, de Pablo J, Martinez G. Risperidone in the treatment of patients with delirium. J Clin Psychiatry 2004;65(3):348-53.
10. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics 2004;45:297-301.
11. Schwartz TL, Masand PS. Treatment of delirium with quetiapine. Prim Care Companion J Clin Psychiatry 2000;2(1):10-12.
12. Sasaki Y, Matsuyama T, Inoue S, et al. A prospective, open-label, flexible-dose study of quetiapine in the treatment of delirium. J Clin Psychiatry 2003;64(11):1316-21.
13. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol 2004;19(2):125-7.
14. Kim KY, Bader GM, Kotlyar V, Gropper D. Treatment of delirium in older adults with quetiapine. J Geriatr Psychiatry Neurol 2003;16(1):29-31.
15. Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med 2004;30(3):444-9.
16. Van der Mast RC. Pathophysiology of delirium. J Geriatr Psychiatry Neurol 1998;11:138-45.
17. Trzepacz PT. Update on the neuropathogenesis of delirium. Dement Geriatr Cogn Disord 1999;10:330-4.
18. Mussi C, Ferrari R, Ascari S, et al. Importance of serum anticholinergic activity in the assessment of elderly patients with delirium. J Geriatr Psychiatry Neurol 1999;12:82-6.
19. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing risk of delirium.[see comment]. Am J Psychiatry 1992;149:1393-4.
20. Keck PE, Jr, Arnold LM. The serotonin syndrome. Psychiatr Ann 2000;30:333-43.
21. Inouye SK, Rushing JT, Foreman MD, et al. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998;13:234-42.
22. Rabins PV, Folstein MF. Delirium and dementia: diagnostic criteria and fatality rates. Br J Psychiatry 1982;140:149-53.
23. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med 2002;162(4):457-63.
24. McCusker J, Cole M, Dendukuri N, et al. The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med 2003;18(9):696-704.
25. Greenberg DB. Preventing delirium at the end of life: lessons from recent research. Primary Care Companion J Clin Psychiatry 2003;5:62-7.
26. Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340:669-76.
27. Rizzo JA, Bogardus ST, Jr, Leo-Summers L, et al. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value? Med Care 2001;39(7):740-52.
28. Inouye SK, Bogardus ST, Jr, Williams CS, et al. The role of adherence on the effectiveness of nonpharmacologic interventions: evidence from the delirium prevention trial. Arch Intern Med 2003;163(8):958-64.
29. Bogardus ST, Jr, Desai MM, Williams CS, et al. The effects of a targeted multicomponent delirium intervention on postdischarge outcomes for hospitalized older adults. Am J Med 2003;114(5):383-90.
30. Marcantonio ER, Goldman L, Orav EJ, et al. The association of intraoperative factors with the development of postoperative delirium. Am J Med 1998;105(5):380-4.
5-minute first aid for psychosis
Adding just 5 to 10 minutes of psychotherapy to medication monitoring visits can help patients overcome hallucinations, delusions, and other psychotic symptoms. Targeted cognitive-behavioral therapy (CBT) can:
- prevent crisis visits and hospitalizations
- improve long-term medication and treatment adherence
- enhance the therapeutic alliance.
Treatment goals for patients with chronic mental illness are changing as clinicians, patients, and families aspire for more than improved symptoms ( Box ).1-14 This article describes brief interventions to target medication nonadherence and positive and negative symptoms in patients with schizophrenia, schizoaffective disorder, bipolar disorder, major depressive disorder, and other chronic disorders.
CASE: VOICES FROM THE PAST
Ms. W, age 45, is seen every 6 to 8 weeks in an outpatient medication management clinic for symptoms of schizoaffective disorder, depressed type; posttraumatic stress disorder; and generalized anxiety disorder. She has a history of severe abuse by her father, self-mutilation in response to anxiety and stress, and repeated hospitalizations following visits to her mother.
She recently visited her mother again and saw her father as well. The trip led to increased symptoms of intrusive traumatic memories, thoughts of suicide with plans to overdose, visual hallucinations of her father, and increased auditory hallucinations with derogatory content.
Goals of the first therapy session after Ms. W’s trip home were to reduce her suicidal thoughts and prevent hospitalization. We encouraged her to list her positive qualities, accomplishments, important relationships, religious beliefs, goals, and dreams. She then wrote all these reasons to live on a cue card. Reading the card twice in the session stopped her suicidal thoughts, and she expressed some hope.
We encouraged her to read the card whenever suicidal ideas became strong. We scheduled her next visit 1 week later, and she contracted not to attempt suicide during that time.
DEVELOPING AN ALLIANCE
To develop an alliance with psychotic patients such as Ms. W, the first task is to help them leave each session feeling understood, validated, and enjoying the therapist’s company. This alone provides a powerful counterbalance to the isolation, demoralization, and hopelessness they bring to therapy.
Pharmacologic and psychosocial interventions are changing treatment goals for patients with serious mental illness from improved symptoms to functional recovery, improved quality of life, and reintegration into the community.1,2 Patients, families and clinicians increasingly view self-determination, independence, and recovery as realistic treatment goals.3,4
Medication limits. Drugs are crucial to managing psychotic symptoms but inadequate for achieving recovery:
- many patients with positive psychotic symptoms respond only partially or not at all5
- functional improvement does not always follow symptomatic improvement6
- medication nonadherence remains high, leading to repeated relapses.7
Dual-therapy benefits. A combination of antipsychotics and psychotherapy has been found to increase the chances of recovery in schizophrenia.8 Psychotherapy is also highly valued by patients and their families:
- In patient satisfaction studies, 72% to 90% of participants with psychotic disorders said individual psychotherapy improved their lives.9,10
- In a survey of 3,099 National Alliance for the Mentally Ill family members, 88% rated psychotherapy as having some (53%) or considerable (35%) value.11
Access problems. Despite psychotherapy’s benefits, access is extremely limited. In one survey, only 7.3% of patients with nonaffective psychosis received at least “minimally adequate” care (four or more medication visits that did not include psychotherapy).12 Incorporating therapeutic techniques into medication monitoring clinics is one way to improve access to therapy for patients with serious mental illnesses.
Keep it brief. Psychotherapy in medication clinics differs from traditional models’ 15- to 45-minute sessions.13 Patients with psychotic illness prefer brief interventions; a study of 212 patients found that 85% of those with schizophrenia preferred sessions:
- less often than once a week
- that focus on solving practical problems.14
5 steps in effective cognitive-behavioral interventions
|
In normalization, the stress vulnerability model is used to explain psychosis to the patient. Psychotic symptoms are emphasized as something normal people can experience in extreme situations, such as:
- hallucinations in states of sleep deprivation or medical and drug-induced states
- paranoia as error in thinking in states of heightened vigilance and perceived threat.15
Universality is the understanding that many people have experiences similar to the patient’s.
In a collaborative therapeutic alliance, the patient is not a passive recipient but an active collaborator in therapy. He or she contributes to decisions—such as the length of therapy and topics to be discussed—and gives feedback on interventions and therapist style.
Focusing on life goals makes therapy meaningful to the patient.
Set priorities. Because only one or two therapeutic interventions can be tried during a medication-monitoring visit, problems need to be prioritized. As with Ms. W, the first visit’s goal was crisis intervention: to reduce suicidal thoughts and prevent hospitalization. Table 1 offers a framework for effective therapeutic interventions.
Save time by giving patients out-of-session assignments, which:
- collect important information to review with patients during the next monitoring session
- help empower patients to manage their symptoms.
IMPROVING ADHERENCE
Medication nonadherence and partial adherence can result from:
- illness-related factors such as lack of insight
- patient-related factors such as attitudes and beliefs about medication
- treatment factors such as side effects
- physician-related factors such as showing an authoritarian attitude toward patients
- system-related factors such as treatment access problems.
Table 2
Interventions to improve patient medication adherence
| Issue | Intervention |
|---|---|
| Assessing medication adherence and beliefs |
|
| Dysfunctional beliefs about medication (“Taking it means I am weak.” “It can turn me into a zombie.” “I will be dependent on medication.”) |
|
| Lack of insight (“I do not need medication”) |
|
| Forgetting to take medication |
|
| Lack of a shared understanding of the illness between patient and physician |
|
CASE: NOT REALLY HER FATHER
By the second session 1 week later, Ms. W’s suicidal thoughts had become infrequent and mild, and she was using the coping card as needed. This visit focused on visual hallucinations associated with anxiety about facing her father. We encouraged her to describe the hallucinations in great detail, and she realized that she visualized her father as he had looked 20 years ago, not as he looks today. Her anxiety decreased as she considered that she might be seeing not him but an image. Her homework assignment was to closely observe the hallucinations. Because she was more stable, the next visit was scheduled in 2 weeks.
By the third session, she reported that the visual hallucinations had disappeared, and the focusing technique had helped her. She continued to hear voices, however, particularly in the evening when she was alone and anxious or depressed. With prompting, she identified activities she could engage in at night, such as calling her mother and praying with her mother on the phone. This reduced her loneliness and helped her relax.
Table 3
Interventions to manage auditory and visual hallucinations
| Problem | Intervention |
|---|---|
| Acting on hallucinations | Ask questions such as: |
| |
| Tell patient, “It is not the voices themselves but the thoughts in your mind in response to the voices that determine whether or not you follow them” | |
| List thoughts patient generates when choosing not to follow voice commands and encourage patient to read the list when hearing voices | |
| Triggers of negative emotions that cause voices | Ask questions such as: |
| |
| Identify techniques to deal with triggers and rate their effectiveness | |
| Dysfunctional beliefs that voices cannot be controlled or are prophetic | When voices are strong, coach patient to rate them on a scale of 0 to 10, try different distraction techniques, and rate them again |
| Encourage patient to write down what the voices say and whether their prophecies come true; reviewing the record in subsequent session shows voices are not prophetic | |
| Voices during the session | Hum a familiar tune with patient |
| Ask patient to read out loud | |
| Visual hallucinations | Encourage patient to examine details of what they see; this alone can make hallucinations disappear |
| Encourage patient to try to make hallucinations funny, such as making the image’s nose long (personal communication: e-mail Morton Sosland MD) |
One month later, the voices had diminished greatly, and Ms. W returned to her regular medication monitoring appointments of every 6 to 8 weeks.
MANAGING POSITIVE SYMPTOMS
In serious mental illnesses such as schizophrenia, the most common hallucinations are auditory ( Table 3 ),19 followed by visual and other types.20 Sometimes patients view hallucinations as helpful, providing reassurance, advice, or companionship. The content may be an expression of the patient’s own beliefs.
Table 4
Interventions to help patients examine common delusions
| Symptom | Questions to ask the patient |
|---|---|
| Behaviors of acting on delusions |
|
| Delusion with changing conviction |
|
| Delusion with complete conviction |
|
| Addressing underlying beliefs |
|
| Delusion associated with lack of real world knowledge | Provide real-world knowledge. For example, for delusion that people can read a patient’s mind, inform patient that scientific experiments have shown that no one can read complex thoughts of others |
| Delusion involving physician | For example, say, “It is normal for you to sometimes question my intentions and believe that I am part of the conspiracy. I can assure you that is not the case. Anytime you have those doubts I would like the opportunity to clarify those for you. Can I rely on you to bring those doubts to my attention?” |
| When patient’s body language or behavior changes, ask if patient is suspicious and paranoid about you | |
| Behavioral experiment for delusions | For example, a patient believed people parking cars on his street would break into his apartment. Homework was designed with two columns on a paper, one for him to check when someone parked and the other if they broke in. Next visit, patient returned with no checks in the break-ins column |
- Are the hallucinations distressing, and does the patient want them to stop?
- What triggers them (usually depressed mood, anxiety, anger, or boredom)?
- What coping mechanisms has the patient used, and how effective have they been?
- What is the source of the patient’s distress?
Table 5
Interventions for managing schizophrenia’s negative symptoms
| Symptom | Intervention |
|---|---|
| Anergia/anhedonia |
|
| Impaired attention |
|
| Alogia |
|
Never dispute a patient’s delusional beliefs. Maintain an attitude of benevolent curiosity to elicit the reasoning processes by which he or she came to believe the delusions. By encouraging the patient to become curious about the experience, you can create a chink of insight and help the him or her achieve important goals despite disturbing sensory experiences and beliefs.
Thought disorder can be addressed by gently pointing out that you are having trouble understanding the patient’s speech. Ask if other people whom the patient trusts have commented on his or her speech.
Because thought disorder worsens the longer a patient talks, suggest a 5-sentence rule during sessions. You and the patient try to speak no more than 5 sentences at a time before pausing to let the other person speak. Encourage the patient to monitor your speech and to indicate when you violate the rule. Monitoring your speech helps patients start monitoring their own.
Thought disorder worsens when patients experience negative emotions such as anxiety. When this occurs, move the discussion to a neutral topic or encourage deep regular breathing for 2 minutes to reduce anxiety.
MANAGING NEGATIVE SYMPTOMS
Negative symptoms of schizophrenia ( Table 5 ) overlap with depression and with medication side effects. Anhedonia and social withdrawal, for example, may reflect a patient’s depression and demoralization, rather than just schizophrenia’s biological core symptoms.
Similarly, limited facial expression may be caused by drug side effects, rather than absence of affect. Negative symptoms also can occur in the absence of depression or side effects, such as when a patient’s automatic thoughts related to expectations of failure lead to lack of motivation.
Negative symptoms usually bother patients much less than positive symptoms do. Thus, enlisting family members to help patients monitor and deal with negative symptoms can be very useful.
CASE SUMMARY
Ms. W’s stress-related psychotic symptoms resolved to baseline with cognitive therapy done in a regular medication management clinic. Throughout this episode, her medication dosages remained unchanged. The interventions added about 10 minutes to sessions, effectively dealt with her symptom exacerbation, and prevented hospitalization.
Psychotropics remain a critical component of treating psychotic disorders, and psychotherapy can also be very helpful. But in the many situations when psychotherapy is not available, brief psychotherapeutic techniques can:
- increase patient and family satisfaction
- enhance the therapeutic alliance
- improve medication adherence
- promote recovery.
1. American Psychiatric Association. Work group on Schizophrenia. Practice guidelines for the treatment of patients with schizophrenia. Am J Psychiatry 2004;161:29(suppl):26-7.
2. Kane JM. Long-term treatment of schizophrenia: moving from a relapse-prevention model to a recovery model. J Clin Psychiatry 2004;64(11):1384-5.
3. Coursey RD, Alford J, Safarjan B. Significant advances in understanding and treating serious mental lllness. Prof Psychol Res Pract 1997;28(3):205-16.
4. Cunningham R. In my own voice: how early intervention led to great success. NAMI Voice 2004;1:1-5.
5. Conley RR, Buchanan RW. Evaluation of treatment-resistant schizophrenia. Schizophr Bull 1997;23:663-74.
6. Harvey PD, Green M, Keefe RS, Velligan DI. Cognitive functioning in schizophrenia: a consensus statement on its role in the definition and evaluation of effective treatments for the illness. J Clin Psychiatry 2004;65(3):361-72.
7. Bridge JA, Barbe RP. Reducing hospital readmission in depression and schizophrenia: current evidence. Curr Opin Psychiatry 2004;17(6):505-11.
8. Lieberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry 2002;14(4):256-72.
9. Leggatt M. Schizophrenia: the consumer’s viewpoint. In: Burrows GD, Norman TR, Rubinstein G (eds). Handbook of studies on schizophrenia, vol 2. New York: Elsevier Science Publishers, 1986;143-53.
10. Coursey RD, Keller AB, Farrell EW. Individual psychotherapy and persons with serious mental illness: the client’s perspective. Schizophr Bull 1995;21:283-301.
11. Hatfield A, Gearon J, Coursey R. Family members’ ratings of the use and value of mental health services: results of a national NAMI survey. Psychiatr Serv 1996;47:825-31.
12. Wang PS, Demler O, Kessler RC. Adequacy of treatment for serious mental illness in the United States. Am J Public Health 2002;92(1):92-8.
13. Rector N, Beck A. CBT for schizophrenia. Can J Psychiatry 2002;47(1):39-48.
14. Coursey RD, Keller A, Farrell EW. Individual psychotherapy and serious mental illness: the clients’ perspective. Schizophr Bull 1995;21:283-301.
15. Kingdon DG, Turkington D. Explanations of schizophrenia. In: Kingdon DG, Turkington D (eds). Cognitive-behavioral therapy of schizophrenia. New York: Guilford Press, 1994;9.-
16. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:361-9.
17. Velligan DI, Bow-Thomas CC, Huntzinger C, et al. Randomized controlled trial of the use of compensatory strategies to enhance adaptive functioning in outpatients with schizophrenia. Am J Psychiatry 2000;157:1317-23.
18. Gilmer T, Dolder C, Lacro J, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry 2004;161(4):692-99.
19. Romme MAJ. Hearing voices. Schizophr Bull 1989;15:209-16.
20. Andreasen NC, Flaum M. Schizophrenia: the characteristic symptoms. Schizophr Bull 1991;17(1):27-49.
Adding just 5 to 10 minutes of psychotherapy to medication monitoring visits can help patients overcome hallucinations, delusions, and other psychotic symptoms. Targeted cognitive-behavioral therapy (CBT) can:
- prevent crisis visits and hospitalizations
- improve long-term medication and treatment adherence
- enhance the therapeutic alliance.
Treatment goals for patients with chronic mental illness are changing as clinicians, patients, and families aspire for more than improved symptoms ( Box ).1-14 This article describes brief interventions to target medication nonadherence and positive and negative symptoms in patients with schizophrenia, schizoaffective disorder, bipolar disorder, major depressive disorder, and other chronic disorders.
CASE: VOICES FROM THE PAST
Ms. W, age 45, is seen every 6 to 8 weeks in an outpatient medication management clinic for symptoms of schizoaffective disorder, depressed type; posttraumatic stress disorder; and generalized anxiety disorder. She has a history of severe abuse by her father, self-mutilation in response to anxiety and stress, and repeated hospitalizations following visits to her mother.
She recently visited her mother again and saw her father as well. The trip led to increased symptoms of intrusive traumatic memories, thoughts of suicide with plans to overdose, visual hallucinations of her father, and increased auditory hallucinations with derogatory content.
Goals of the first therapy session after Ms. W’s trip home were to reduce her suicidal thoughts and prevent hospitalization. We encouraged her to list her positive qualities, accomplishments, important relationships, religious beliefs, goals, and dreams. She then wrote all these reasons to live on a cue card. Reading the card twice in the session stopped her suicidal thoughts, and she expressed some hope.
We encouraged her to read the card whenever suicidal ideas became strong. We scheduled her next visit 1 week later, and she contracted not to attempt suicide during that time.
DEVELOPING AN ALLIANCE
To develop an alliance with psychotic patients such as Ms. W, the first task is to help them leave each session feeling understood, validated, and enjoying the therapist’s company. This alone provides a powerful counterbalance to the isolation, demoralization, and hopelessness they bring to therapy.
Pharmacologic and psychosocial interventions are changing treatment goals for patients with serious mental illness from improved symptoms to functional recovery, improved quality of life, and reintegration into the community.1,2 Patients, families and clinicians increasingly view self-determination, independence, and recovery as realistic treatment goals.3,4
Medication limits. Drugs are crucial to managing psychotic symptoms but inadequate for achieving recovery:
- many patients with positive psychotic symptoms respond only partially or not at all5
- functional improvement does not always follow symptomatic improvement6
- medication nonadherence remains high, leading to repeated relapses.7
Dual-therapy benefits. A combination of antipsychotics and psychotherapy has been found to increase the chances of recovery in schizophrenia.8 Psychotherapy is also highly valued by patients and their families:
- In patient satisfaction studies, 72% to 90% of participants with psychotic disorders said individual psychotherapy improved their lives.9,10
- In a survey of 3,099 National Alliance for the Mentally Ill family members, 88% rated psychotherapy as having some (53%) or considerable (35%) value.11
Access problems. Despite psychotherapy’s benefits, access is extremely limited. In one survey, only 7.3% of patients with nonaffective psychosis received at least “minimally adequate” care (four or more medication visits that did not include psychotherapy).12 Incorporating therapeutic techniques into medication monitoring clinics is one way to improve access to therapy for patients with serious mental illnesses.
Keep it brief. Psychotherapy in medication clinics differs from traditional models’ 15- to 45-minute sessions.13 Patients with psychotic illness prefer brief interventions; a study of 212 patients found that 85% of those with schizophrenia preferred sessions:
- less often than once a week
- that focus on solving practical problems.14
5 steps in effective cognitive-behavioral interventions
|
In normalization, the stress vulnerability model is used to explain psychosis to the patient. Psychotic symptoms are emphasized as something normal people can experience in extreme situations, such as:
- hallucinations in states of sleep deprivation or medical and drug-induced states
- paranoia as error in thinking in states of heightened vigilance and perceived threat.15
Universality is the understanding that many people have experiences similar to the patient’s.
In a collaborative therapeutic alliance, the patient is not a passive recipient but an active collaborator in therapy. He or she contributes to decisions—such as the length of therapy and topics to be discussed—and gives feedback on interventions and therapist style.
Focusing on life goals makes therapy meaningful to the patient.
Set priorities. Because only one or two therapeutic interventions can be tried during a medication-monitoring visit, problems need to be prioritized. As with Ms. W, the first visit’s goal was crisis intervention: to reduce suicidal thoughts and prevent hospitalization. Table 1 offers a framework for effective therapeutic interventions.
Save time by giving patients out-of-session assignments, which:
- collect important information to review with patients during the next monitoring session
- help empower patients to manage their symptoms.
IMPROVING ADHERENCE
Medication nonadherence and partial adherence can result from:
- illness-related factors such as lack of insight
- patient-related factors such as attitudes and beliefs about medication
- treatment factors such as side effects
- physician-related factors such as showing an authoritarian attitude toward patients
- system-related factors such as treatment access problems.
Table 2
Interventions to improve patient medication adherence
| Issue | Intervention |
|---|---|
| Assessing medication adherence and beliefs |
|
| Dysfunctional beliefs about medication (“Taking it means I am weak.” “It can turn me into a zombie.” “I will be dependent on medication.”) |
|
| Lack of insight (“I do not need medication”) |
|
| Forgetting to take medication |
|
| Lack of a shared understanding of the illness between patient and physician |
|
CASE: NOT REALLY HER FATHER
By the second session 1 week later, Ms. W’s suicidal thoughts had become infrequent and mild, and she was using the coping card as needed. This visit focused on visual hallucinations associated with anxiety about facing her father. We encouraged her to describe the hallucinations in great detail, and she realized that she visualized her father as he had looked 20 years ago, not as he looks today. Her anxiety decreased as she considered that she might be seeing not him but an image. Her homework assignment was to closely observe the hallucinations. Because she was more stable, the next visit was scheduled in 2 weeks.
By the third session, she reported that the visual hallucinations had disappeared, and the focusing technique had helped her. She continued to hear voices, however, particularly in the evening when she was alone and anxious or depressed. With prompting, she identified activities she could engage in at night, such as calling her mother and praying with her mother on the phone. This reduced her loneliness and helped her relax.
Table 3
Interventions to manage auditory and visual hallucinations
| Problem | Intervention |
|---|---|
| Acting on hallucinations | Ask questions such as: |
| |
| Tell patient, “It is not the voices themselves but the thoughts in your mind in response to the voices that determine whether or not you follow them” | |
| List thoughts patient generates when choosing not to follow voice commands and encourage patient to read the list when hearing voices | |
| Triggers of negative emotions that cause voices | Ask questions such as: |
| |
| Identify techniques to deal with triggers and rate their effectiveness | |
| Dysfunctional beliefs that voices cannot be controlled or are prophetic | When voices are strong, coach patient to rate them on a scale of 0 to 10, try different distraction techniques, and rate them again |
| Encourage patient to write down what the voices say and whether their prophecies come true; reviewing the record in subsequent session shows voices are not prophetic | |
| Voices during the session | Hum a familiar tune with patient |
| Ask patient to read out loud | |
| Visual hallucinations | Encourage patient to examine details of what they see; this alone can make hallucinations disappear |
| Encourage patient to try to make hallucinations funny, such as making the image’s nose long (personal communication: e-mail Morton Sosland MD) |
One month later, the voices had diminished greatly, and Ms. W returned to her regular medication monitoring appointments of every 6 to 8 weeks.
MANAGING POSITIVE SYMPTOMS
In serious mental illnesses such as schizophrenia, the most common hallucinations are auditory ( Table 3 ),19 followed by visual and other types.20 Sometimes patients view hallucinations as helpful, providing reassurance, advice, or companionship. The content may be an expression of the patient’s own beliefs.
Table 4
Interventions to help patients examine common delusions
| Symptom | Questions to ask the patient |
|---|---|
| Behaviors of acting on delusions |
|
| Delusion with changing conviction |
|
| Delusion with complete conviction |
|
| Addressing underlying beliefs |
|
| Delusion associated with lack of real world knowledge | Provide real-world knowledge. For example, for delusion that people can read a patient’s mind, inform patient that scientific experiments have shown that no one can read complex thoughts of others |
| Delusion involving physician | For example, say, “It is normal for you to sometimes question my intentions and believe that I am part of the conspiracy. I can assure you that is not the case. Anytime you have those doubts I would like the opportunity to clarify those for you. Can I rely on you to bring those doubts to my attention?” |
| When patient’s body language or behavior changes, ask if patient is suspicious and paranoid about you | |
| Behavioral experiment for delusions | For example, a patient believed people parking cars on his street would break into his apartment. Homework was designed with two columns on a paper, one for him to check when someone parked and the other if they broke in. Next visit, patient returned with no checks in the break-ins column |
- Are the hallucinations distressing, and does the patient want them to stop?
- What triggers them (usually depressed mood, anxiety, anger, or boredom)?
- What coping mechanisms has the patient used, and how effective have they been?
- What is the source of the patient’s distress?
Table 5
Interventions for managing schizophrenia’s negative symptoms
| Symptom | Intervention |
|---|---|
| Anergia/anhedonia |
|
| Impaired attention |
|
| Alogia |
|
Never dispute a patient’s delusional beliefs. Maintain an attitude of benevolent curiosity to elicit the reasoning processes by which he or she came to believe the delusions. By encouraging the patient to become curious about the experience, you can create a chink of insight and help the him or her achieve important goals despite disturbing sensory experiences and beliefs.
Thought disorder can be addressed by gently pointing out that you are having trouble understanding the patient’s speech. Ask if other people whom the patient trusts have commented on his or her speech.
Because thought disorder worsens the longer a patient talks, suggest a 5-sentence rule during sessions. You and the patient try to speak no more than 5 sentences at a time before pausing to let the other person speak. Encourage the patient to monitor your speech and to indicate when you violate the rule. Monitoring your speech helps patients start monitoring their own.
Thought disorder worsens when patients experience negative emotions such as anxiety. When this occurs, move the discussion to a neutral topic or encourage deep regular breathing for 2 minutes to reduce anxiety.
MANAGING NEGATIVE SYMPTOMS
Negative symptoms of schizophrenia ( Table 5 ) overlap with depression and with medication side effects. Anhedonia and social withdrawal, for example, may reflect a patient’s depression and demoralization, rather than just schizophrenia’s biological core symptoms.
Similarly, limited facial expression may be caused by drug side effects, rather than absence of affect. Negative symptoms also can occur in the absence of depression or side effects, such as when a patient’s automatic thoughts related to expectations of failure lead to lack of motivation.
Negative symptoms usually bother patients much less than positive symptoms do. Thus, enlisting family members to help patients monitor and deal with negative symptoms can be very useful.
CASE SUMMARY
Ms. W’s stress-related psychotic symptoms resolved to baseline with cognitive therapy done in a regular medication management clinic. Throughout this episode, her medication dosages remained unchanged. The interventions added about 10 minutes to sessions, effectively dealt with her symptom exacerbation, and prevented hospitalization.
Psychotropics remain a critical component of treating psychotic disorders, and psychotherapy can also be very helpful. But in the many situations when psychotherapy is not available, brief psychotherapeutic techniques can:
- increase patient and family satisfaction
- enhance the therapeutic alliance
- improve medication adherence
- promote recovery.
Adding just 5 to 10 minutes of psychotherapy to medication monitoring visits can help patients overcome hallucinations, delusions, and other psychotic symptoms. Targeted cognitive-behavioral therapy (CBT) can:
- prevent crisis visits and hospitalizations
- improve long-term medication and treatment adherence
- enhance the therapeutic alliance.
Treatment goals for patients with chronic mental illness are changing as clinicians, patients, and families aspire for more than improved symptoms ( Box ).1-14 This article describes brief interventions to target medication nonadherence and positive and negative symptoms in patients with schizophrenia, schizoaffective disorder, bipolar disorder, major depressive disorder, and other chronic disorders.
CASE: VOICES FROM THE PAST
Ms. W, age 45, is seen every 6 to 8 weeks in an outpatient medication management clinic for symptoms of schizoaffective disorder, depressed type; posttraumatic stress disorder; and generalized anxiety disorder. She has a history of severe abuse by her father, self-mutilation in response to anxiety and stress, and repeated hospitalizations following visits to her mother.
She recently visited her mother again and saw her father as well. The trip led to increased symptoms of intrusive traumatic memories, thoughts of suicide with plans to overdose, visual hallucinations of her father, and increased auditory hallucinations with derogatory content.
Goals of the first therapy session after Ms. W’s trip home were to reduce her suicidal thoughts and prevent hospitalization. We encouraged her to list her positive qualities, accomplishments, important relationships, religious beliefs, goals, and dreams. She then wrote all these reasons to live on a cue card. Reading the card twice in the session stopped her suicidal thoughts, and she expressed some hope.
We encouraged her to read the card whenever suicidal ideas became strong. We scheduled her next visit 1 week later, and she contracted not to attempt suicide during that time.
DEVELOPING AN ALLIANCE
To develop an alliance with psychotic patients such as Ms. W, the first task is to help them leave each session feeling understood, validated, and enjoying the therapist’s company. This alone provides a powerful counterbalance to the isolation, demoralization, and hopelessness they bring to therapy.
Pharmacologic and psychosocial interventions are changing treatment goals for patients with serious mental illness from improved symptoms to functional recovery, improved quality of life, and reintegration into the community.1,2 Patients, families and clinicians increasingly view self-determination, independence, and recovery as realistic treatment goals.3,4
Medication limits. Drugs are crucial to managing psychotic symptoms but inadequate for achieving recovery:
- many patients with positive psychotic symptoms respond only partially or not at all5
- functional improvement does not always follow symptomatic improvement6
- medication nonadherence remains high, leading to repeated relapses.7
Dual-therapy benefits. A combination of antipsychotics and psychotherapy has been found to increase the chances of recovery in schizophrenia.8 Psychotherapy is also highly valued by patients and their families:
- In patient satisfaction studies, 72% to 90% of participants with psychotic disorders said individual psychotherapy improved their lives.9,10
- In a survey of 3,099 National Alliance for the Mentally Ill family members, 88% rated psychotherapy as having some (53%) or considerable (35%) value.11
Access problems. Despite psychotherapy’s benefits, access is extremely limited. In one survey, only 7.3% of patients with nonaffective psychosis received at least “minimally adequate” care (four or more medication visits that did not include psychotherapy).12 Incorporating therapeutic techniques into medication monitoring clinics is one way to improve access to therapy for patients with serious mental illnesses.
Keep it brief. Psychotherapy in medication clinics differs from traditional models’ 15- to 45-minute sessions.13 Patients with psychotic illness prefer brief interventions; a study of 212 patients found that 85% of those with schizophrenia preferred sessions:
- less often than once a week
- that focus on solving practical problems.14
5 steps in effective cognitive-behavioral interventions
|
In normalization, the stress vulnerability model is used to explain psychosis to the patient. Psychotic symptoms are emphasized as something normal people can experience in extreme situations, such as:
- hallucinations in states of sleep deprivation or medical and drug-induced states
- paranoia as error in thinking in states of heightened vigilance and perceived threat.15
Universality is the understanding that many people have experiences similar to the patient’s.
In a collaborative therapeutic alliance, the patient is not a passive recipient but an active collaborator in therapy. He or she contributes to decisions—such as the length of therapy and topics to be discussed—and gives feedback on interventions and therapist style.
Focusing on life goals makes therapy meaningful to the patient.
Set priorities. Because only one or two therapeutic interventions can be tried during a medication-monitoring visit, problems need to be prioritized. As with Ms. W, the first visit’s goal was crisis intervention: to reduce suicidal thoughts and prevent hospitalization. Table 1 offers a framework for effective therapeutic interventions.
Save time by giving patients out-of-session assignments, which:
- collect important information to review with patients during the next monitoring session
- help empower patients to manage their symptoms.
IMPROVING ADHERENCE
Medication nonadherence and partial adherence can result from:
- illness-related factors such as lack of insight
- patient-related factors such as attitudes and beliefs about medication
- treatment factors such as side effects
- physician-related factors such as showing an authoritarian attitude toward patients
- system-related factors such as treatment access problems.
Table 2
Interventions to improve patient medication adherence
| Issue | Intervention |
|---|---|
| Assessing medication adherence and beliefs |
|
| Dysfunctional beliefs about medication (“Taking it means I am weak.” “It can turn me into a zombie.” “I will be dependent on medication.”) |
|
| Lack of insight (“I do not need medication”) |
|
| Forgetting to take medication |
|
| Lack of a shared understanding of the illness between patient and physician |
|
CASE: NOT REALLY HER FATHER
By the second session 1 week later, Ms. W’s suicidal thoughts had become infrequent and mild, and she was using the coping card as needed. This visit focused on visual hallucinations associated with anxiety about facing her father. We encouraged her to describe the hallucinations in great detail, and she realized that she visualized her father as he had looked 20 years ago, not as he looks today. Her anxiety decreased as she considered that she might be seeing not him but an image. Her homework assignment was to closely observe the hallucinations. Because she was more stable, the next visit was scheduled in 2 weeks.
By the third session, she reported that the visual hallucinations had disappeared, and the focusing technique had helped her. She continued to hear voices, however, particularly in the evening when she was alone and anxious or depressed. With prompting, she identified activities she could engage in at night, such as calling her mother and praying with her mother on the phone. This reduced her loneliness and helped her relax.
Table 3
Interventions to manage auditory and visual hallucinations
| Problem | Intervention |
|---|---|
| Acting on hallucinations | Ask questions such as: |
| |
| Tell patient, “It is not the voices themselves but the thoughts in your mind in response to the voices that determine whether or not you follow them” | |
| List thoughts patient generates when choosing not to follow voice commands and encourage patient to read the list when hearing voices | |
| Triggers of negative emotions that cause voices | Ask questions such as: |
| |
| Identify techniques to deal with triggers and rate their effectiveness | |
| Dysfunctional beliefs that voices cannot be controlled or are prophetic | When voices are strong, coach patient to rate them on a scale of 0 to 10, try different distraction techniques, and rate them again |
| Encourage patient to write down what the voices say and whether their prophecies come true; reviewing the record in subsequent session shows voices are not prophetic | |
| Voices during the session | Hum a familiar tune with patient |
| Ask patient to read out loud | |
| Visual hallucinations | Encourage patient to examine details of what they see; this alone can make hallucinations disappear |
| Encourage patient to try to make hallucinations funny, such as making the image’s nose long (personal communication: e-mail Morton Sosland MD) |
One month later, the voices had diminished greatly, and Ms. W returned to her regular medication monitoring appointments of every 6 to 8 weeks.
MANAGING POSITIVE SYMPTOMS
In serious mental illnesses such as schizophrenia, the most common hallucinations are auditory ( Table 3 ),19 followed by visual and other types.20 Sometimes patients view hallucinations as helpful, providing reassurance, advice, or companionship. The content may be an expression of the patient’s own beliefs.
Table 4
Interventions to help patients examine common delusions
| Symptom | Questions to ask the patient |
|---|---|
| Behaviors of acting on delusions |
|
| Delusion with changing conviction |
|
| Delusion with complete conviction |
|
| Addressing underlying beliefs |
|
| Delusion associated with lack of real world knowledge | Provide real-world knowledge. For example, for delusion that people can read a patient’s mind, inform patient that scientific experiments have shown that no one can read complex thoughts of others |
| Delusion involving physician | For example, say, “It is normal for you to sometimes question my intentions and believe that I am part of the conspiracy. I can assure you that is not the case. Anytime you have those doubts I would like the opportunity to clarify those for you. Can I rely on you to bring those doubts to my attention?” |
| When patient’s body language or behavior changes, ask if patient is suspicious and paranoid about you | |
| Behavioral experiment for delusions | For example, a patient believed people parking cars on his street would break into his apartment. Homework was designed with two columns on a paper, one for him to check when someone parked and the other if they broke in. Next visit, patient returned with no checks in the break-ins column |
- Are the hallucinations distressing, and does the patient want them to stop?
- What triggers them (usually depressed mood, anxiety, anger, or boredom)?
- What coping mechanisms has the patient used, and how effective have they been?
- What is the source of the patient’s distress?
Table 5
Interventions for managing schizophrenia’s negative symptoms
| Symptom | Intervention |
|---|---|
| Anergia/anhedonia |
|
| Impaired attention |
|
| Alogia |
|
Never dispute a patient’s delusional beliefs. Maintain an attitude of benevolent curiosity to elicit the reasoning processes by which he or she came to believe the delusions. By encouraging the patient to become curious about the experience, you can create a chink of insight and help the him or her achieve important goals despite disturbing sensory experiences and beliefs.
Thought disorder can be addressed by gently pointing out that you are having trouble understanding the patient’s speech. Ask if other people whom the patient trusts have commented on his or her speech.
Because thought disorder worsens the longer a patient talks, suggest a 5-sentence rule during sessions. You and the patient try to speak no more than 5 sentences at a time before pausing to let the other person speak. Encourage the patient to monitor your speech and to indicate when you violate the rule. Monitoring your speech helps patients start monitoring their own.
Thought disorder worsens when patients experience negative emotions such as anxiety. When this occurs, move the discussion to a neutral topic or encourage deep regular breathing for 2 minutes to reduce anxiety.
MANAGING NEGATIVE SYMPTOMS
Negative symptoms of schizophrenia ( Table 5 ) overlap with depression and with medication side effects. Anhedonia and social withdrawal, for example, may reflect a patient’s depression and demoralization, rather than just schizophrenia’s biological core symptoms.
Similarly, limited facial expression may be caused by drug side effects, rather than absence of affect. Negative symptoms also can occur in the absence of depression or side effects, such as when a patient’s automatic thoughts related to expectations of failure lead to lack of motivation.
Negative symptoms usually bother patients much less than positive symptoms do. Thus, enlisting family members to help patients monitor and deal with negative symptoms can be very useful.
CASE SUMMARY
Ms. W’s stress-related psychotic symptoms resolved to baseline with cognitive therapy done in a regular medication management clinic. Throughout this episode, her medication dosages remained unchanged. The interventions added about 10 minutes to sessions, effectively dealt with her symptom exacerbation, and prevented hospitalization.
Psychotropics remain a critical component of treating psychotic disorders, and psychotherapy can also be very helpful. But in the many situations when psychotherapy is not available, brief psychotherapeutic techniques can:
- increase patient and family satisfaction
- enhance the therapeutic alliance
- improve medication adherence
- promote recovery.
1. American Psychiatric Association. Work group on Schizophrenia. Practice guidelines for the treatment of patients with schizophrenia. Am J Psychiatry 2004;161:29(suppl):26-7.
2. Kane JM. Long-term treatment of schizophrenia: moving from a relapse-prevention model to a recovery model. J Clin Psychiatry 2004;64(11):1384-5.
3. Coursey RD, Alford J, Safarjan B. Significant advances in understanding and treating serious mental lllness. Prof Psychol Res Pract 1997;28(3):205-16.
4. Cunningham R. In my own voice: how early intervention led to great success. NAMI Voice 2004;1:1-5.
5. Conley RR, Buchanan RW. Evaluation of treatment-resistant schizophrenia. Schizophr Bull 1997;23:663-74.
6. Harvey PD, Green M, Keefe RS, Velligan DI. Cognitive functioning in schizophrenia: a consensus statement on its role in the definition and evaluation of effective treatments for the illness. J Clin Psychiatry 2004;65(3):361-72.
7. Bridge JA, Barbe RP. Reducing hospital readmission in depression and schizophrenia: current evidence. Curr Opin Psychiatry 2004;17(6):505-11.
8. Lieberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry 2002;14(4):256-72.
9. Leggatt M. Schizophrenia: the consumer’s viewpoint. In: Burrows GD, Norman TR, Rubinstein G (eds). Handbook of studies on schizophrenia, vol 2. New York: Elsevier Science Publishers, 1986;143-53.
10. Coursey RD, Keller AB, Farrell EW. Individual psychotherapy and persons with serious mental illness: the client’s perspective. Schizophr Bull 1995;21:283-301.
11. Hatfield A, Gearon J, Coursey R. Family members’ ratings of the use and value of mental health services: results of a national NAMI survey. Psychiatr Serv 1996;47:825-31.
12. Wang PS, Demler O, Kessler RC. Adequacy of treatment for serious mental illness in the United States. Am J Public Health 2002;92(1):92-8.
13. Rector N, Beck A. CBT for schizophrenia. Can J Psychiatry 2002;47(1):39-48.
14. Coursey RD, Keller A, Farrell EW. Individual psychotherapy and serious mental illness: the clients’ perspective. Schizophr Bull 1995;21:283-301.
15. Kingdon DG, Turkington D. Explanations of schizophrenia. In: Kingdon DG, Turkington D (eds). Cognitive-behavioral therapy of schizophrenia. New York: Guilford Press, 1994;9.-
16. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:361-9.
17. Velligan DI, Bow-Thomas CC, Huntzinger C, et al. Randomized controlled trial of the use of compensatory strategies to enhance adaptive functioning in outpatients with schizophrenia. Am J Psychiatry 2000;157:1317-23.
18. Gilmer T, Dolder C, Lacro J, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry 2004;161(4):692-99.
19. Romme MAJ. Hearing voices. Schizophr Bull 1989;15:209-16.
20. Andreasen NC, Flaum M. Schizophrenia: the characteristic symptoms. Schizophr Bull 1991;17(1):27-49.
1. American Psychiatric Association. Work group on Schizophrenia. Practice guidelines for the treatment of patients with schizophrenia. Am J Psychiatry 2004;161:29(suppl):26-7.
2. Kane JM. Long-term treatment of schizophrenia: moving from a relapse-prevention model to a recovery model. J Clin Psychiatry 2004;64(11):1384-5.
3. Coursey RD, Alford J, Safarjan B. Significant advances in understanding and treating serious mental lllness. Prof Psychol Res Pract 1997;28(3):205-16.
4. Cunningham R. In my own voice: how early intervention led to great success. NAMI Voice 2004;1:1-5.
5. Conley RR, Buchanan RW. Evaluation of treatment-resistant schizophrenia. Schizophr Bull 1997;23:663-74.
6. Harvey PD, Green M, Keefe RS, Velligan DI. Cognitive functioning in schizophrenia: a consensus statement on its role in the definition and evaluation of effective treatments for the illness. J Clin Psychiatry 2004;65(3):361-72.
7. Bridge JA, Barbe RP. Reducing hospital readmission in depression and schizophrenia: current evidence. Curr Opin Psychiatry 2004;17(6):505-11.
8. Lieberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry 2002;14(4):256-72.
9. Leggatt M. Schizophrenia: the consumer’s viewpoint. In: Burrows GD, Norman TR, Rubinstein G (eds). Handbook of studies on schizophrenia, vol 2. New York: Elsevier Science Publishers, 1986;143-53.
10. Coursey RD, Keller AB, Farrell EW. Individual psychotherapy and persons with serious mental illness: the client’s perspective. Schizophr Bull 1995;21:283-301.
11. Hatfield A, Gearon J, Coursey R. Family members’ ratings of the use and value of mental health services: results of a national NAMI survey. Psychiatr Serv 1996;47:825-31.
12. Wang PS, Demler O, Kessler RC. Adequacy of treatment for serious mental illness in the United States. Am J Public Health 2002;92(1):92-8.
13. Rector N, Beck A. CBT for schizophrenia. Can J Psychiatry 2002;47(1):39-48.
14. Coursey RD, Keller A, Farrell EW. Individual psychotherapy and serious mental illness: the clients’ perspective. Schizophr Bull 1995;21:283-301.
15. Kingdon DG, Turkington D. Explanations of schizophrenia. In: Kingdon DG, Turkington D (eds). Cognitive-behavioral therapy of schizophrenia. New York: Guilford Press, 1994;9.-
16. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:361-9.
17. Velligan DI, Bow-Thomas CC, Huntzinger C, et al. Randomized controlled trial of the use of compensatory strategies to enhance adaptive functioning in outpatients with schizophrenia. Am J Psychiatry 2000;157:1317-23.
18. Gilmer T, Dolder C, Lacro J, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry 2004;161(4):692-99.
19. Romme MAJ. Hearing voices. Schizophr Bull 1989;15:209-16.
20. Andreasen NC, Flaum M. Schizophrenia: the characteristic symptoms. Schizophr Bull 1991;17(1):27-49.
Something in the air
HISTORY: WINTER WOES
Mrs. A, age 64, lives alone in an old farmhouse. For approximately 8 months, she had complained of depressed mood, decreased interest, difficulty sleeping, low energy, decreased concentration, and feelings of hopelessness. She met DSM-IV-TR criteria for major depressive disorder with underlying anxiety.
Mrs. A also reported having sinus headaches throughout the fall and winter. Blood chemistry, CBC with differential, thyroid profile including T4& TSH, urine drug screen, urine analysis, and ECG results were normal.
In April, Mrs. A was enrolled in an outpatient study of depression relapse prevention treatment. After taking the active study drug for 2 months, she reported continued low mood, low energy, difficulty concentrating, poor sleep and worsening headaches. Because her depression did not improve sufficiently, she was dropped from the study.
In July, Mrs. A saw a psychiatrist and was started on sertraline, 50 mg/d. By November, the dosage had been increased to 150 mg/d. At this time, she reported unsteadiness, dizziness, frequent falls, and intolerable headaches in addition to her depressive symptoms. She was referred to a neurologist to rule out a neurologic disorder.
Table 1
Symptoms that suggest major depression and/or chronic CO poisoning
| Symptom | Major depression | Chronic low-level CO poisoning |
|---|---|---|
| Depressed mood | + | + |
| Diminished interest | + | - |
| Weight loss | + | - |
| Decreased appetite | + | - |
| Difficulty sleeping | + | + |
| Diminished concentration | + | + |
| Suicidal thoughts | + | - |
| Fatigue, weakness | + | + |
| Headaches | + | + |
| Palpitations | + | + |
| Shortness of breath | + | + |
| Nausea | + | + |
| Abdominal pain | + | + |
| Vomiting | + | + |
| Diarrhea | + | + |
| Confusion | - | + |
| Diminished cognitive function | + | + |
| Sexual dysfunction | + | - |
| + = suggests disorder | ||
| - = does not suggest disorder | ||
| CO = Carbon monoxide | ||
| Source: Diagnostic and Statistical Manual of Mental Disorders (4th ed, rev). | ||
| Copyright 2000. American Psychiatric Association; and Tierney LM, McPhee SJ, Papadakis MA (eds). Current Medical Diagnosis and Treatment. New York: McGraw Hill, 2003. | ||
The authors’ observations
Chronic fatigue syndrome is characterized by severe unexplained fatigue that persists for >6 months. The new-onset fatigue is not abated with rest. Other symptoms include impaired memory or concentration, sore throat, tender lymph nodes, muscle pain, headaches, pain in several joints, and disturbed sleep.1
Mrs. A, however, never complained of sore throat or joint or muscle pain, and her laboratory findings were normal.
Seasonal affective disorder (SAD) is characterized by a temporal relationship between onset of depressive symptoms and a particular time of year (eg, symptoms emerge each winter) for at least 2 years. Full remission also occurs at a characteristic time (eg, each summer).2
Mrs. A’s headaches, frequent falls, dizziness, and difficulties with balance do not suggest SAD. Also, these symptoms have not persisted long enough for an SAD diagnosis.
Thyroid disorder. Hypothyroidism symptoms—particularly low mood, decreased energy, fatigue, psychomotor retardation, and lack of motivation—can mimic depression. Mrs. A’s T4 and TSH readings were normal, however.
Metabolic dysfunction. Symptoms secondary to decreased serum concentrations of sodium, potassium, magnesium, or calcium can mimic depression, but blood tests showed Mrs. A has normal electrolyte levels.
Brain tumor. Patients with a brain tumor can present with mood symptoms, psychosis, headaches, mania, cognitive impairments, seizure problems, and other symptoms depending on the tumor’s size and location.
FURTHER TREATMENT: SUDDEN RELIEF
By late November Mrs. A’s fatigue, once present only mornings, plagued her throughout the day. We considered changing antidepressants because of her complaints and sertraline’s lack of efficacy.
The following month, however, Mrs. A told us that her fatigue and headaches were gone. Mood, sleep, and concentration were also improved. Her Hamilton Rating Scale for Depression score had improved from 21 when she entered the study—indicating moderate severity—to 6, indicating remission. Her neurologic referral was cancelled.
Mrs. A then mentioned that her home’s water heater had been malfunctioning for several months. She said she could not afford to get it repaired during the summer but finally hired plumbers to fix it in late November.
After working all day in Mrs. A’s basement, two workers suffered acute headaches and nausea. The symptoms prompted the workers to search the basement for a carbon monoxide leak; they found a small leak in the water heater, which they replaced.
The next morning, Mrs. A said, her headache disappeared. Her other symptoms were gone within 4 days.
The authors’ observations
The sudden disappearance of Mrs. A’s symptoms after her water heater was replaced and emergence of severe physical symptoms in the two plumbers suggest carbon monoxide (CO) poisoning, a common and potentially lethal medical problem.
Low-level CO poisoning usually results from repeated exposure to incomplete combustion in a defective heating appliance, such as a water heater (Box 1).3,4 Symptoms usually surface in the winter, when heating appliance use peaks and windows are left closed, allowing indoor CO to accumulate in high concentrations.7
Carbon monoxide (CO) poisoning is preventable yet causes more than 2,000 deaths each year in the United States.3,5 CO poisoning may result from intentional or accidental exposure to motor vehicle exhaust, malfunctioning home heating systems, and improperly vented combustion appliances.
Indoor heating systems account for about 75% of CO poisoning-related deaths.5 Fatal CO exposure has also been attributed to charcoal grills/burning charcoal, gas water heaters, camp stoves, lanterns, kitchen gas ranges/ovens, and other fuel-burning products.5
Although most states do not require residential use of CO detectors, clinicians should encourage patients to install at least one CO detector near their beds.5,6
Whereas severe, acute CO poisoning typically is detected immediately after exposure, symptoms of chronic low-level CO exposure are easily mistaken for a primary depressive (Table 1) or other neuropsychiatric disorder—or overlooked altogether. Some cases persist for months before CO exposure is diagnosed. Clinicians often give unnecessary—sometimes costly—medical treatment while ignoring the underlying poisoning.
Mechanism of action. CO binds with hemoglobin (with an affinity >200 times that of oxygen) to form carboxyhemoglobin (COHb), which causes cellular anoxia by blocking transport of oxygen to the tissues, including the brain.4,6,8
CO poisoning symptoms vary depending on COHb concentration (Table 2). COHb >5% in a symptomatic nonsmoker may indicate chronic low-level CO poisoning and require further evaluation.9 Levels >10% are common in heavy smokers (2 to 4 packs/day). It should be noted that Mrs. A does not smoke.
Presentation. Patients with chronic low-level CO poisoning often present with vague, nonspecific symptoms, such as weakness and fatigue, abdominal pain, nausea, vomiting, diarrhea, decreased concentration, diminished cognitive abilities, persistent headaches, and trouble sleeping.4,8,10,11 Patients age >65 especially may present with multiple cognitive and somatic complaints that suggest Parkinson’s disease, chronic fatigue syndrome, dementia, or—in Mrs. A’s case—depression.5,10,12
Table 2
Signs, symptoms of CO poisoning that emerge at different carboxyhemoglobin levels
| Carboxyhemoglobin level (% HgB) | Signs, symptoms |
|---|---|
| 5-10 % | Exacerbates angina in some patients with heart disease |
| 10-20 % | Mild headache, breathlessness on exertion |
| 20-30 % | Throbbing headache, irritability, mental status changes, fatigue |
| 30-40 % | Severe headache, weakness, nausea, dizziness, visual problems, confusion |
| 40-50% | Increased confusion, hallucinations, severe ataxia, rapid breathing |
| 50-60 % | Syncope or coma with convulsions, tachycardia with weak pulse |
| 60-70 % | Deep coma, incontinence |
| 70-80% | Profound coma, depressed respiration, absent reflexes |
| >80 % | Rapid death from respiratory arrest |
| Source: Adapted from Gilman AG, Rall TW, Nies AS, Taylor P (eds). Goodman and Gilman’s the pharmacological basis of therapeutics (8th ed). New York: Pergamon Press, 1990. | |
Health effects of CO exposure range from subtle cardiovascular and neurobehavioral sequelae at low concentrations to loss of consciousness and death after acute exposure to higher concentrations.3,5
Hypoxia of the brain and other organs resulting from low-level CO poisoning can cause a range of physiologic effects, including mental status changes.10,11 Low-level CO exposure is particularly dangerous to pregnant women and to patients with a pre-existing ischemic illness.
Pregnancy. Chronic low-level CO exposure during pregnancy can harm the fetus, leading to low birth weight, short neonatal length, prematurity, perinatal death, and increased risk of developmental dysfunction.13
Ischemic illnesses. Because COHb cannot transport oxygen, the tissues that demand the most oxygen—such as the brain, heart, and skeletal muscles—are most affected. Because cardiac muscles extract approximately 75% of available oxygen from blood, patients with cardiac and pulmonary ischemic illnesses face a high risk for tissue injury with CO poisoning. At COHb levels >10%, patients with pre-existing cardiac disease experience increased severity and duration of angina; concentrations >15% place them at risk of myocardial infarction.6
Length of recovery from chronic CO exposure varies widely depending on severity of exposure and the patient’s general health.3,5 CO has a 4- to 6-hour half-life and is excreted via the lungs fairly rapidly, so recovery can be swift once CO exposure is stopped. Emergency room referral depends upon severity of symptoms and CO exposure duration and nature (accidental or intentional).
The authors’ observations
CO poisoning can lead to long-term mental status changes. In a 3-year follow-up of patients repeatedly exposed to low CO levels:
- 43% developed neurologic sequelae including memory impairment
- 33% experienced personality changes including irritability, verbal aggression, violence and impulsivity, moodiness, distractibility, and sexual promiscuity
- 11% suffered gross neuropsychological effects, including psychosis, disorientation, and blindness.4
Primary care physicians and psychiatrists should monitor patients who have recovered from CO poisoning for symptoms of these disorders.
DETECTING CHRONIC CO EXPOSURE
Mrs. A’s case illustrates the seriousness and diagnostic complexity of chronic low-level CO exposure in older patients, especially during the fall and winter with increased home heating appliance use.7 CO exposure was not considered as a cause of Mrs. A’s symptoms until heating contractors found the water heater leak.
Watch for patients whose neuropsychiatric symptoms do not respond to treatment. Ask them about possible environmental, seasonal, or diurnal variations in symptoms. Also ask if the patient’s home heating system or water heater is ≥10 years old or has been malfunctioning (Box 2).
Checking COHb blood levels is the simplest way to confirm CO poisoning.6,14
- Is your home heating system or water heater 10 or more years old or malfunctioning?
- Do you use a gas range or stove for supplemental heat?
- Do symptoms improve or worsen in certain environments or at a certain time of day?
- Have fireplace flues and/or chimney vents been checked within the past year?
- Has another household member—including a pet—also been ill?
- Is a family member who remains at home persistently ill, whereas others who leave periodically improve?
- Do symptoms improve or worsen during certain months or seasons?
FOLLOW-UP: ANXIOUS MOMENTS
Mrs. A’s depressive symptoms, headaches, dizziness, and balance problems have not returned. Her underlying anxiety symptoms worsened, however, when the psychiatrist tried to taper sertraline. She was diagnosed with generalized anxiety disorder and continued on sertraline, 100 mg/d.
The psychiatrist sees her every 4 to 6 weeks, and she routinely sees her primary care physician. No long-term effects of CO poisoning have been found.
Related resources
- U.S. Centers for Disease Control and Prevention. Enter “carbon monoxide poisoning” in search field. http://www.cdc.gov.
- Kao LW, Nanagas KA. Carbon monoxide poisoning. Emerg Med Clin North America 2004;22:985-1018.
Drug brand names
- Sertraline • Zoloft
Disclosure
Drs. Khan and D’Empaire report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Preskorn has been a speaker for, consultant to, or principal investigator for several antidepressant manufacturers, including Pfizer Inc.
1. Sadock BJ, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry (9th ed). Philadelphia, PA: Lippincott Williams & Wilkins, 2003:662.
2. Diagnostic and statistical manual of mental disorders (4th ed. rev). Washington, DC: American Psychiatric Association, 2000.
3. Mott JA, Wolfe MI, Alverson CJ, et al. National vehicle emissions policies and practices and declining US carbon monoxide-related mortality. JAMA 2002;288:988-95.
4. Thorpe M. Chronic carbon monoxide poisoning. Can J Psychiatry 1994;39:59-61.
5. Knobeloch L, Jackson R. Recognition of chronic carbon monoxide poisoning. WMJ 1999;98(6):26-9.
6. Turner M, Hamilton-Farrell MR, Clark RJ. Carbon monoxide poisoning: an update. J Accid Emerg Med 1999;16:92-6.
7. Unintentional carbon monoxide poisoning following winter storm—Washington January 1993. MMWR. 1993;42:109-11.
8. Wright J. Chronic and occult carbon monoxide poisoning: we don’t know what we’re missing. Emer Med J 2002;19:386-90.
9. Wald N, Idle M, Smith PG. Carboxyhaemoglobin levels in smokers of filter and plain cigarettes. Lancet 1977;1:110-12.
10. Raub JA, Benignus VA. Carbon monoxide and the nervous system. Neurosci Biobehav Rev 2002;26:925-40.
11. Ryan CM. Memory disturbances following chronic, low-level carbon monoxide exposure. Arch Clin Neuropsychol 1990;5:59-67.
12. Webb CJ, 2nd, Vaitkevicius PV. Dementia with a seasonal onset secondary to carbon monoxide poisoning. J Am Geriatr Soc 1997;45:1281-2.
13. Farrow JR, Davis GJ, Roy TM, et al. Fetal death due to nonlethal maternal carbon monoxide poisoning. J Forens Sci 1990;35:1448-52.
14. Vreman HJ, Mahoney JJ, Stevenson DK. Carbon monoxide and carboxyhemoglobin. Adv Pediatr 1995;42:303-34.
HISTORY: WINTER WOES
Mrs. A, age 64, lives alone in an old farmhouse. For approximately 8 months, she had complained of depressed mood, decreased interest, difficulty sleeping, low energy, decreased concentration, and feelings of hopelessness. She met DSM-IV-TR criteria for major depressive disorder with underlying anxiety.
Mrs. A also reported having sinus headaches throughout the fall and winter. Blood chemistry, CBC with differential, thyroid profile including T4& TSH, urine drug screen, urine analysis, and ECG results were normal.
In April, Mrs. A was enrolled in an outpatient study of depression relapse prevention treatment. After taking the active study drug for 2 months, she reported continued low mood, low energy, difficulty concentrating, poor sleep and worsening headaches. Because her depression did not improve sufficiently, she was dropped from the study.
In July, Mrs. A saw a psychiatrist and was started on sertraline, 50 mg/d. By November, the dosage had been increased to 150 mg/d. At this time, she reported unsteadiness, dizziness, frequent falls, and intolerable headaches in addition to her depressive symptoms. She was referred to a neurologist to rule out a neurologic disorder.
Table 1
Symptoms that suggest major depression and/or chronic CO poisoning
| Symptom | Major depression | Chronic low-level CO poisoning |
|---|---|---|
| Depressed mood | + | + |
| Diminished interest | + | - |
| Weight loss | + | - |
| Decreased appetite | + | - |
| Difficulty sleeping | + | + |
| Diminished concentration | + | + |
| Suicidal thoughts | + | - |
| Fatigue, weakness | + | + |
| Headaches | + | + |
| Palpitations | + | + |
| Shortness of breath | + | + |
| Nausea | + | + |
| Abdominal pain | + | + |
| Vomiting | + | + |
| Diarrhea | + | + |
| Confusion | - | + |
| Diminished cognitive function | + | + |
| Sexual dysfunction | + | - |
| + = suggests disorder | ||
| - = does not suggest disorder | ||
| CO = Carbon monoxide | ||
| Source: Diagnostic and Statistical Manual of Mental Disorders (4th ed, rev). | ||
| Copyright 2000. American Psychiatric Association; and Tierney LM, McPhee SJ, Papadakis MA (eds). Current Medical Diagnosis and Treatment. New York: McGraw Hill, 2003. | ||
The authors’ observations
Chronic fatigue syndrome is characterized by severe unexplained fatigue that persists for >6 months. The new-onset fatigue is not abated with rest. Other symptoms include impaired memory or concentration, sore throat, tender lymph nodes, muscle pain, headaches, pain in several joints, and disturbed sleep.1
Mrs. A, however, never complained of sore throat or joint or muscle pain, and her laboratory findings were normal.
Seasonal affective disorder (SAD) is characterized by a temporal relationship between onset of depressive symptoms and a particular time of year (eg, symptoms emerge each winter) for at least 2 years. Full remission also occurs at a characteristic time (eg, each summer).2
Mrs. A’s headaches, frequent falls, dizziness, and difficulties with balance do not suggest SAD. Also, these symptoms have not persisted long enough for an SAD diagnosis.
Thyroid disorder. Hypothyroidism symptoms—particularly low mood, decreased energy, fatigue, psychomotor retardation, and lack of motivation—can mimic depression. Mrs. A’s T4 and TSH readings were normal, however.
Metabolic dysfunction. Symptoms secondary to decreased serum concentrations of sodium, potassium, magnesium, or calcium can mimic depression, but blood tests showed Mrs. A has normal electrolyte levels.
Brain tumor. Patients with a brain tumor can present with mood symptoms, psychosis, headaches, mania, cognitive impairments, seizure problems, and other symptoms depending on the tumor’s size and location.
FURTHER TREATMENT: SUDDEN RELIEF
By late November Mrs. A’s fatigue, once present only mornings, plagued her throughout the day. We considered changing antidepressants because of her complaints and sertraline’s lack of efficacy.
The following month, however, Mrs. A told us that her fatigue and headaches were gone. Mood, sleep, and concentration were also improved. Her Hamilton Rating Scale for Depression score had improved from 21 when she entered the study—indicating moderate severity—to 6, indicating remission. Her neurologic referral was cancelled.
Mrs. A then mentioned that her home’s water heater had been malfunctioning for several months. She said she could not afford to get it repaired during the summer but finally hired plumbers to fix it in late November.
After working all day in Mrs. A’s basement, two workers suffered acute headaches and nausea. The symptoms prompted the workers to search the basement for a carbon monoxide leak; they found a small leak in the water heater, which they replaced.
The next morning, Mrs. A said, her headache disappeared. Her other symptoms were gone within 4 days.
The authors’ observations
The sudden disappearance of Mrs. A’s symptoms after her water heater was replaced and emergence of severe physical symptoms in the two plumbers suggest carbon monoxide (CO) poisoning, a common and potentially lethal medical problem.
Low-level CO poisoning usually results from repeated exposure to incomplete combustion in a defective heating appliance, such as a water heater (Box 1).3,4 Symptoms usually surface in the winter, when heating appliance use peaks and windows are left closed, allowing indoor CO to accumulate in high concentrations.7
Carbon monoxide (CO) poisoning is preventable yet causes more than 2,000 deaths each year in the United States.3,5 CO poisoning may result from intentional or accidental exposure to motor vehicle exhaust, malfunctioning home heating systems, and improperly vented combustion appliances.
Indoor heating systems account for about 75% of CO poisoning-related deaths.5 Fatal CO exposure has also been attributed to charcoal grills/burning charcoal, gas water heaters, camp stoves, lanterns, kitchen gas ranges/ovens, and other fuel-burning products.5
Although most states do not require residential use of CO detectors, clinicians should encourage patients to install at least one CO detector near their beds.5,6
Whereas severe, acute CO poisoning typically is detected immediately after exposure, symptoms of chronic low-level CO exposure are easily mistaken for a primary depressive (Table 1) or other neuropsychiatric disorder—or overlooked altogether. Some cases persist for months before CO exposure is diagnosed. Clinicians often give unnecessary—sometimes costly—medical treatment while ignoring the underlying poisoning.
Mechanism of action. CO binds with hemoglobin (with an affinity >200 times that of oxygen) to form carboxyhemoglobin (COHb), which causes cellular anoxia by blocking transport of oxygen to the tissues, including the brain.4,6,8
CO poisoning symptoms vary depending on COHb concentration (Table 2). COHb >5% in a symptomatic nonsmoker may indicate chronic low-level CO poisoning and require further evaluation.9 Levels >10% are common in heavy smokers (2 to 4 packs/day). It should be noted that Mrs. A does not smoke.
Presentation. Patients with chronic low-level CO poisoning often present with vague, nonspecific symptoms, such as weakness and fatigue, abdominal pain, nausea, vomiting, diarrhea, decreased concentration, diminished cognitive abilities, persistent headaches, and trouble sleeping.4,8,10,11 Patients age >65 especially may present with multiple cognitive and somatic complaints that suggest Parkinson’s disease, chronic fatigue syndrome, dementia, or—in Mrs. A’s case—depression.5,10,12
Table 2
Signs, symptoms of CO poisoning that emerge at different carboxyhemoglobin levels
| Carboxyhemoglobin level (% HgB) | Signs, symptoms |
|---|---|
| 5-10 % | Exacerbates angina in some patients with heart disease |
| 10-20 % | Mild headache, breathlessness on exertion |
| 20-30 % | Throbbing headache, irritability, mental status changes, fatigue |
| 30-40 % | Severe headache, weakness, nausea, dizziness, visual problems, confusion |
| 40-50% | Increased confusion, hallucinations, severe ataxia, rapid breathing |
| 50-60 % | Syncope or coma with convulsions, tachycardia with weak pulse |
| 60-70 % | Deep coma, incontinence |
| 70-80% | Profound coma, depressed respiration, absent reflexes |
| >80 % | Rapid death from respiratory arrest |
| Source: Adapted from Gilman AG, Rall TW, Nies AS, Taylor P (eds). Goodman and Gilman’s the pharmacological basis of therapeutics (8th ed). New York: Pergamon Press, 1990. | |
Health effects of CO exposure range from subtle cardiovascular and neurobehavioral sequelae at low concentrations to loss of consciousness and death after acute exposure to higher concentrations.3,5
Hypoxia of the brain and other organs resulting from low-level CO poisoning can cause a range of physiologic effects, including mental status changes.10,11 Low-level CO exposure is particularly dangerous to pregnant women and to patients with a pre-existing ischemic illness.
Pregnancy. Chronic low-level CO exposure during pregnancy can harm the fetus, leading to low birth weight, short neonatal length, prematurity, perinatal death, and increased risk of developmental dysfunction.13
Ischemic illnesses. Because COHb cannot transport oxygen, the tissues that demand the most oxygen—such as the brain, heart, and skeletal muscles—are most affected. Because cardiac muscles extract approximately 75% of available oxygen from blood, patients with cardiac and pulmonary ischemic illnesses face a high risk for tissue injury with CO poisoning. At COHb levels >10%, patients with pre-existing cardiac disease experience increased severity and duration of angina; concentrations >15% place them at risk of myocardial infarction.6
Length of recovery from chronic CO exposure varies widely depending on severity of exposure and the patient’s general health.3,5 CO has a 4- to 6-hour half-life and is excreted via the lungs fairly rapidly, so recovery can be swift once CO exposure is stopped. Emergency room referral depends upon severity of symptoms and CO exposure duration and nature (accidental or intentional).
The authors’ observations
CO poisoning can lead to long-term mental status changes. In a 3-year follow-up of patients repeatedly exposed to low CO levels:
- 43% developed neurologic sequelae including memory impairment
- 33% experienced personality changes including irritability, verbal aggression, violence and impulsivity, moodiness, distractibility, and sexual promiscuity
- 11% suffered gross neuropsychological effects, including psychosis, disorientation, and blindness.4
Primary care physicians and psychiatrists should monitor patients who have recovered from CO poisoning for symptoms of these disorders.
DETECTING CHRONIC CO EXPOSURE
Mrs. A’s case illustrates the seriousness and diagnostic complexity of chronic low-level CO exposure in older patients, especially during the fall and winter with increased home heating appliance use.7 CO exposure was not considered as a cause of Mrs. A’s symptoms until heating contractors found the water heater leak.
Watch for patients whose neuropsychiatric symptoms do not respond to treatment. Ask them about possible environmental, seasonal, or diurnal variations in symptoms. Also ask if the patient’s home heating system or water heater is ≥10 years old or has been malfunctioning (Box 2).
Checking COHb blood levels is the simplest way to confirm CO poisoning.6,14
- Is your home heating system or water heater 10 or more years old or malfunctioning?
- Do you use a gas range or stove for supplemental heat?
- Do symptoms improve or worsen in certain environments or at a certain time of day?
- Have fireplace flues and/or chimney vents been checked within the past year?
- Has another household member—including a pet—also been ill?
- Is a family member who remains at home persistently ill, whereas others who leave periodically improve?
- Do symptoms improve or worsen during certain months or seasons?
FOLLOW-UP: ANXIOUS MOMENTS
Mrs. A’s depressive symptoms, headaches, dizziness, and balance problems have not returned. Her underlying anxiety symptoms worsened, however, when the psychiatrist tried to taper sertraline. She was diagnosed with generalized anxiety disorder and continued on sertraline, 100 mg/d.
The psychiatrist sees her every 4 to 6 weeks, and she routinely sees her primary care physician. No long-term effects of CO poisoning have been found.
Related resources
- U.S. Centers for Disease Control and Prevention. Enter “carbon monoxide poisoning” in search field. http://www.cdc.gov.
- Kao LW, Nanagas KA. Carbon monoxide poisoning. Emerg Med Clin North America 2004;22:985-1018.
Drug brand names
- Sertraline • Zoloft
Disclosure
Drs. Khan and D’Empaire report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Preskorn has been a speaker for, consultant to, or principal investigator for several antidepressant manufacturers, including Pfizer Inc.
HISTORY: WINTER WOES
Mrs. A, age 64, lives alone in an old farmhouse. For approximately 8 months, she had complained of depressed mood, decreased interest, difficulty sleeping, low energy, decreased concentration, and feelings of hopelessness. She met DSM-IV-TR criteria for major depressive disorder with underlying anxiety.
Mrs. A also reported having sinus headaches throughout the fall and winter. Blood chemistry, CBC with differential, thyroid profile including T4& TSH, urine drug screen, urine analysis, and ECG results were normal.
In April, Mrs. A was enrolled in an outpatient study of depression relapse prevention treatment. After taking the active study drug for 2 months, she reported continued low mood, low energy, difficulty concentrating, poor sleep and worsening headaches. Because her depression did not improve sufficiently, she was dropped from the study.
In July, Mrs. A saw a psychiatrist and was started on sertraline, 50 mg/d. By November, the dosage had been increased to 150 mg/d. At this time, she reported unsteadiness, dizziness, frequent falls, and intolerable headaches in addition to her depressive symptoms. She was referred to a neurologist to rule out a neurologic disorder.
Table 1
Symptoms that suggest major depression and/or chronic CO poisoning
| Symptom | Major depression | Chronic low-level CO poisoning |
|---|---|---|
| Depressed mood | + | + |
| Diminished interest | + | - |
| Weight loss | + | - |
| Decreased appetite | + | - |
| Difficulty sleeping | + | + |
| Diminished concentration | + | + |
| Suicidal thoughts | + | - |
| Fatigue, weakness | + | + |
| Headaches | + | + |
| Palpitations | + | + |
| Shortness of breath | + | + |
| Nausea | + | + |
| Abdominal pain | + | + |
| Vomiting | + | + |
| Diarrhea | + | + |
| Confusion | - | + |
| Diminished cognitive function | + | + |
| Sexual dysfunction | + | - |
| + = suggests disorder | ||
| - = does not suggest disorder | ||
| CO = Carbon monoxide | ||
| Source: Diagnostic and Statistical Manual of Mental Disorders (4th ed, rev). | ||
| Copyright 2000. American Psychiatric Association; and Tierney LM, McPhee SJ, Papadakis MA (eds). Current Medical Diagnosis and Treatment. New York: McGraw Hill, 2003. | ||
The authors’ observations
Chronic fatigue syndrome is characterized by severe unexplained fatigue that persists for >6 months. The new-onset fatigue is not abated with rest. Other symptoms include impaired memory or concentration, sore throat, tender lymph nodes, muscle pain, headaches, pain in several joints, and disturbed sleep.1
Mrs. A, however, never complained of sore throat or joint or muscle pain, and her laboratory findings were normal.
Seasonal affective disorder (SAD) is characterized by a temporal relationship between onset of depressive symptoms and a particular time of year (eg, symptoms emerge each winter) for at least 2 years. Full remission also occurs at a characteristic time (eg, each summer).2
Mrs. A’s headaches, frequent falls, dizziness, and difficulties with balance do not suggest SAD. Also, these symptoms have not persisted long enough for an SAD diagnosis.
Thyroid disorder. Hypothyroidism symptoms—particularly low mood, decreased energy, fatigue, psychomotor retardation, and lack of motivation—can mimic depression. Mrs. A’s T4 and TSH readings were normal, however.
Metabolic dysfunction. Symptoms secondary to decreased serum concentrations of sodium, potassium, magnesium, or calcium can mimic depression, but blood tests showed Mrs. A has normal electrolyte levels.
Brain tumor. Patients with a brain tumor can present with mood symptoms, psychosis, headaches, mania, cognitive impairments, seizure problems, and other symptoms depending on the tumor’s size and location.
FURTHER TREATMENT: SUDDEN RELIEF
By late November Mrs. A’s fatigue, once present only mornings, plagued her throughout the day. We considered changing antidepressants because of her complaints and sertraline’s lack of efficacy.
The following month, however, Mrs. A told us that her fatigue and headaches were gone. Mood, sleep, and concentration were also improved. Her Hamilton Rating Scale for Depression score had improved from 21 when she entered the study—indicating moderate severity—to 6, indicating remission. Her neurologic referral was cancelled.
Mrs. A then mentioned that her home’s water heater had been malfunctioning for several months. She said she could not afford to get it repaired during the summer but finally hired plumbers to fix it in late November.
After working all day in Mrs. A’s basement, two workers suffered acute headaches and nausea. The symptoms prompted the workers to search the basement for a carbon monoxide leak; they found a small leak in the water heater, which they replaced.
The next morning, Mrs. A said, her headache disappeared. Her other symptoms were gone within 4 days.
The authors’ observations
The sudden disappearance of Mrs. A’s symptoms after her water heater was replaced and emergence of severe physical symptoms in the two plumbers suggest carbon monoxide (CO) poisoning, a common and potentially lethal medical problem.
Low-level CO poisoning usually results from repeated exposure to incomplete combustion in a defective heating appliance, such as a water heater (Box 1).3,4 Symptoms usually surface in the winter, when heating appliance use peaks and windows are left closed, allowing indoor CO to accumulate in high concentrations.7
Carbon monoxide (CO) poisoning is preventable yet causes more than 2,000 deaths each year in the United States.3,5 CO poisoning may result from intentional or accidental exposure to motor vehicle exhaust, malfunctioning home heating systems, and improperly vented combustion appliances.
Indoor heating systems account for about 75% of CO poisoning-related deaths.5 Fatal CO exposure has also been attributed to charcoal grills/burning charcoal, gas water heaters, camp stoves, lanterns, kitchen gas ranges/ovens, and other fuel-burning products.5
Although most states do not require residential use of CO detectors, clinicians should encourage patients to install at least one CO detector near their beds.5,6
Whereas severe, acute CO poisoning typically is detected immediately after exposure, symptoms of chronic low-level CO exposure are easily mistaken for a primary depressive (Table 1) or other neuropsychiatric disorder—or overlooked altogether. Some cases persist for months before CO exposure is diagnosed. Clinicians often give unnecessary—sometimes costly—medical treatment while ignoring the underlying poisoning.
Mechanism of action. CO binds with hemoglobin (with an affinity >200 times that of oxygen) to form carboxyhemoglobin (COHb), which causes cellular anoxia by blocking transport of oxygen to the tissues, including the brain.4,6,8
CO poisoning symptoms vary depending on COHb concentration (Table 2). COHb >5% in a symptomatic nonsmoker may indicate chronic low-level CO poisoning and require further evaluation.9 Levels >10% are common in heavy smokers (2 to 4 packs/day). It should be noted that Mrs. A does not smoke.
Presentation. Patients with chronic low-level CO poisoning often present with vague, nonspecific symptoms, such as weakness and fatigue, abdominal pain, nausea, vomiting, diarrhea, decreased concentration, diminished cognitive abilities, persistent headaches, and trouble sleeping.4,8,10,11 Patients age >65 especially may present with multiple cognitive and somatic complaints that suggest Parkinson’s disease, chronic fatigue syndrome, dementia, or—in Mrs. A’s case—depression.5,10,12
Table 2
Signs, symptoms of CO poisoning that emerge at different carboxyhemoglobin levels
| Carboxyhemoglobin level (% HgB) | Signs, symptoms |
|---|---|
| 5-10 % | Exacerbates angina in some patients with heart disease |
| 10-20 % | Mild headache, breathlessness on exertion |
| 20-30 % | Throbbing headache, irritability, mental status changes, fatigue |
| 30-40 % | Severe headache, weakness, nausea, dizziness, visual problems, confusion |
| 40-50% | Increased confusion, hallucinations, severe ataxia, rapid breathing |
| 50-60 % | Syncope or coma with convulsions, tachycardia with weak pulse |
| 60-70 % | Deep coma, incontinence |
| 70-80% | Profound coma, depressed respiration, absent reflexes |
| >80 % | Rapid death from respiratory arrest |
| Source: Adapted from Gilman AG, Rall TW, Nies AS, Taylor P (eds). Goodman and Gilman’s the pharmacological basis of therapeutics (8th ed). New York: Pergamon Press, 1990. | |
Health effects of CO exposure range from subtle cardiovascular and neurobehavioral sequelae at low concentrations to loss of consciousness and death after acute exposure to higher concentrations.3,5
Hypoxia of the brain and other organs resulting from low-level CO poisoning can cause a range of physiologic effects, including mental status changes.10,11 Low-level CO exposure is particularly dangerous to pregnant women and to patients with a pre-existing ischemic illness.
Pregnancy. Chronic low-level CO exposure during pregnancy can harm the fetus, leading to low birth weight, short neonatal length, prematurity, perinatal death, and increased risk of developmental dysfunction.13
Ischemic illnesses. Because COHb cannot transport oxygen, the tissues that demand the most oxygen—such as the brain, heart, and skeletal muscles—are most affected. Because cardiac muscles extract approximately 75% of available oxygen from blood, patients with cardiac and pulmonary ischemic illnesses face a high risk for tissue injury with CO poisoning. At COHb levels >10%, patients with pre-existing cardiac disease experience increased severity and duration of angina; concentrations >15% place them at risk of myocardial infarction.6
Length of recovery from chronic CO exposure varies widely depending on severity of exposure and the patient’s general health.3,5 CO has a 4- to 6-hour half-life and is excreted via the lungs fairly rapidly, so recovery can be swift once CO exposure is stopped. Emergency room referral depends upon severity of symptoms and CO exposure duration and nature (accidental or intentional).
The authors’ observations
CO poisoning can lead to long-term mental status changes. In a 3-year follow-up of patients repeatedly exposed to low CO levels:
- 43% developed neurologic sequelae including memory impairment
- 33% experienced personality changes including irritability, verbal aggression, violence and impulsivity, moodiness, distractibility, and sexual promiscuity
- 11% suffered gross neuropsychological effects, including psychosis, disorientation, and blindness.4
Primary care physicians and psychiatrists should monitor patients who have recovered from CO poisoning for symptoms of these disorders.
DETECTING CHRONIC CO EXPOSURE
Mrs. A’s case illustrates the seriousness and diagnostic complexity of chronic low-level CO exposure in older patients, especially during the fall and winter with increased home heating appliance use.7 CO exposure was not considered as a cause of Mrs. A’s symptoms until heating contractors found the water heater leak.
Watch for patients whose neuropsychiatric symptoms do not respond to treatment. Ask them about possible environmental, seasonal, or diurnal variations in symptoms. Also ask if the patient’s home heating system or water heater is ≥10 years old or has been malfunctioning (Box 2).
Checking COHb blood levels is the simplest way to confirm CO poisoning.6,14
- Is your home heating system or water heater 10 or more years old or malfunctioning?
- Do you use a gas range or stove for supplemental heat?
- Do symptoms improve or worsen in certain environments or at a certain time of day?
- Have fireplace flues and/or chimney vents been checked within the past year?
- Has another household member—including a pet—also been ill?
- Is a family member who remains at home persistently ill, whereas others who leave periodically improve?
- Do symptoms improve or worsen during certain months or seasons?
FOLLOW-UP: ANXIOUS MOMENTS
Mrs. A’s depressive symptoms, headaches, dizziness, and balance problems have not returned. Her underlying anxiety symptoms worsened, however, when the psychiatrist tried to taper sertraline. She was diagnosed with generalized anxiety disorder and continued on sertraline, 100 mg/d.
The psychiatrist sees her every 4 to 6 weeks, and she routinely sees her primary care physician. No long-term effects of CO poisoning have been found.
Related resources
- U.S. Centers for Disease Control and Prevention. Enter “carbon monoxide poisoning” in search field. http://www.cdc.gov.
- Kao LW, Nanagas KA. Carbon monoxide poisoning. Emerg Med Clin North America 2004;22:985-1018.
Drug brand names
- Sertraline • Zoloft
Disclosure
Drs. Khan and D’Empaire report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Preskorn has been a speaker for, consultant to, or principal investigator for several antidepressant manufacturers, including Pfizer Inc.
1. Sadock BJ, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry (9th ed). Philadelphia, PA: Lippincott Williams & Wilkins, 2003:662.
2. Diagnostic and statistical manual of mental disorders (4th ed. rev). Washington, DC: American Psychiatric Association, 2000.
3. Mott JA, Wolfe MI, Alverson CJ, et al. National vehicle emissions policies and practices and declining US carbon monoxide-related mortality. JAMA 2002;288:988-95.
4. Thorpe M. Chronic carbon monoxide poisoning. Can J Psychiatry 1994;39:59-61.
5. Knobeloch L, Jackson R. Recognition of chronic carbon monoxide poisoning. WMJ 1999;98(6):26-9.
6. Turner M, Hamilton-Farrell MR, Clark RJ. Carbon monoxide poisoning: an update. J Accid Emerg Med 1999;16:92-6.
7. Unintentional carbon monoxide poisoning following winter storm—Washington January 1993. MMWR. 1993;42:109-11.
8. Wright J. Chronic and occult carbon monoxide poisoning: we don’t know what we’re missing. Emer Med J 2002;19:386-90.
9. Wald N, Idle M, Smith PG. Carboxyhaemoglobin levels in smokers of filter and plain cigarettes. Lancet 1977;1:110-12.
10. Raub JA, Benignus VA. Carbon monoxide and the nervous system. Neurosci Biobehav Rev 2002;26:925-40.
11. Ryan CM. Memory disturbances following chronic, low-level carbon monoxide exposure. Arch Clin Neuropsychol 1990;5:59-67.
12. Webb CJ, 2nd, Vaitkevicius PV. Dementia with a seasonal onset secondary to carbon monoxide poisoning. J Am Geriatr Soc 1997;45:1281-2.
13. Farrow JR, Davis GJ, Roy TM, et al. Fetal death due to nonlethal maternal carbon monoxide poisoning. J Forens Sci 1990;35:1448-52.
14. Vreman HJ, Mahoney JJ, Stevenson DK. Carbon monoxide and carboxyhemoglobin. Adv Pediatr 1995;42:303-34.
1. Sadock BJ, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry (9th ed). Philadelphia, PA: Lippincott Williams & Wilkins, 2003:662.
2. Diagnostic and statistical manual of mental disorders (4th ed. rev). Washington, DC: American Psychiatric Association, 2000.
3. Mott JA, Wolfe MI, Alverson CJ, et al. National vehicle emissions policies and practices and declining US carbon monoxide-related mortality. JAMA 2002;288:988-95.
4. Thorpe M. Chronic carbon monoxide poisoning. Can J Psychiatry 1994;39:59-61.
5. Knobeloch L, Jackson R. Recognition of chronic carbon monoxide poisoning. WMJ 1999;98(6):26-9.
6. Turner M, Hamilton-Farrell MR, Clark RJ. Carbon monoxide poisoning: an update. J Accid Emerg Med 1999;16:92-6.
7. Unintentional carbon monoxide poisoning following winter storm—Washington January 1993. MMWR. 1993;42:109-11.
8. Wright J. Chronic and occult carbon monoxide poisoning: we don’t know what we’re missing. Emer Med J 2002;19:386-90.
9. Wald N, Idle M, Smith PG. Carboxyhaemoglobin levels in smokers of filter and plain cigarettes. Lancet 1977;1:110-12.
10. Raub JA, Benignus VA. Carbon monoxide and the nervous system. Neurosci Biobehav Rev 2002;26:925-40.
11. Ryan CM. Memory disturbances following chronic, low-level carbon monoxide exposure. Arch Clin Neuropsychol 1990;5:59-67.
12. Webb CJ, 2nd, Vaitkevicius PV. Dementia with a seasonal onset secondary to carbon monoxide poisoning. J Am Geriatr Soc 1997;45:1281-2.
13. Farrow JR, Davis GJ, Roy TM, et al. Fetal death due to nonlethal maternal carbon monoxide poisoning. J Forens Sci 1990;35:1448-52.
14. Vreman HJ, Mahoney JJ, Stevenson DK. Carbon monoxide and carboxyhemoglobin. Adv Pediatr 1995;42:303-34.
Help children and teens stop impulsive hair pulling
Trichotillomania (TTM) is distressing to pediatric patients who pull their hair and to their parents who feel helpless to stop the destructive behavior. Hair-pulling with psychiatric comorbidity requires comprehensive assessment and treatment, but we have found that cognitive-behavioral therapy (CBT) alone can help children and adolescents with uncomplicated TTM.
This article describes a typical patient with adolescent-onset mild-to-moderate TTM and the three-step CBT approach—awareness training, stimulus control, and habit reversal—that we find effective in reducing pediatric hair pulling.
Jane, age 12, was referred to our clinic by her primary doctor after an 8-week trial of fluoxetine, 80 mg/d, failed to stop her hair pulling. Jane, who is right-handed, has been pulling her hair for 2 years, mostly in the right front scalp. Her shame over the hair loss makes her reluctant to participate in social activities. A dermatologist found no medical cause for her behavior, such as alopecia or folliculitis.
Jane’s parents say she has no history of a major mood disorder or anxiety. Her hair pulling causes significant “tension and stress” for all family members.
WHY DO PATIENTS PULL HAIR?
Cognitive-behavioral theory suggests that chronic TTM originates as a normal response to stress that often escapes personal and social awareness but gradually increases in frequency and severity (Box).1-8 Thus, hair pulling becomes associated with internal and external cues through conditioning and is maintained primarily by positive reinforcement. Hair-pulling urges that are reinforced by pulling intensify the need to pull, perpetuating the behavioral cycle.
A genetic link? Familial research has associated TTM with increased rates of obsessive-compulsive disorder (OCD) or other excessive habits—such as nail biting or skin picking—among first-degree relatives.6,9,10 Neuroimaging of persons with TTM has shown hyperactivity in the left cerebellum and right superior parietal lobe11 as well as possible structural abnormalities in the left putamen,12 left inferior frontal gyrus, and right cluneal cortex.13
These findings do not necessarily indicate pre-existing brain pathology, however. Perhaps TTM leads to changes in brain structure or function, or both TTM and the brain abnormalities may be caused by another as-yet-unknown variable.
Decreased pain sensitivity. Patients with TTM often report that hair pulling is not painful,2 though we suspect that persons without TTM would disagree and derive no pleasure from it. Changes in pain sensitivity may influence the reinforcing quality of pulling behavior. One possible mechanism for such alterations is upregulation of the endogenous opioid system; some intriguing evidence suggests that opioid receptor antagonists such as naltrexone may reduce pulling.14
Trichotillomania (TTM) is an impulse control disorder characterized by repetitive hair pulling,1 which typically emerges during adolescence. In a large clinical sample of adult hair pullers, mean age of onset was 13.2 Very-early onset (before age 5) may be a more benign form of TTM that tends to abate spontaneously and requires little or no therapeutic intervention.3
Despite the absence of body hair in prepubertal children, their pulling patterns are consistent with those of adults. The scalp is the most common pulling site, followed by eyelashes and eyebrows.4
Psychiatric comorbidity. In two studies evaluating psychiatric comorbidity in pediatric clinical samples, 60% to 70% of children and teens with TTM had at least one comorbid axis I disorder.5,6 Disruptive behavior disorders were most common in one study,6 whereas overanxious disorder was most common in the other.5 In a large clinical sample of adults with TTM, 51% met criteria for comorbid depression.2
Early identification and treatment of TTM are recommended because of the disorder’s distressing nature and social stigma. Early interventions also may help prevent later adult psychiatric comorbidity and functional impairment, although no studies have been done to demonstrate this benefit.7,8
Pain tolerance at the preferred pulling site has not been studied, however. For patients who feel pain from hair pulling, the pain itself may reinforce the behavior by distracting the individual from negative emotional or physiologic states.15
CASE CONTINUED: COUNTING THE WAYS
Jane and her parents agree that she pulls her hair 5 to 8 times daily, one hair at a time with her right index finger and thumb while doing homework or watching TV. The trigger, she says, is “an itch” on her scalp; “sometimes pulling relieves the itch.” She fails to resist pulling her hair 9 out of 10 times.
Table 1
Defining hair pulling: What to ask the pediatric patient
| Response description | How many times do you pull your hair each day? |
| How many hairs do you pull each time? | |
| From what body areas do you pull hair? | |
| What are all the steps involved in pulling (Touching the head before pulling? Pulling one hair at a time with the thumb and index finger)? | |
| Response detection | Under what circumstances do you sense the urge to pull? |
| How strong is the urge on a scale of 1 to 10, with 10 being the greatest intensity you ever felt? | |
| How do you try to resist and overcome the urge to pull? | |
| Precursors | External cues (Do you pull when you look at yourself in a mirror?) |
| Internal cues (Do you pull when you are nervous?) | |
| High-risk situations | What are you usually doing when you get the urge to pull? (reading, talking on the telephone, watching TV, using a computer, etc.) |
| Consequences that reinforce the behavior | Do you pull to reduce physical sensations (such as itching) at the site of pulling? |
| Does pulling relieve sadness or worry about problems at home or in school? | |
| Do you pull to create a more even hairline? |
Psychiatric comorbidity is common—if not the norm—in adults with TTM. Although axis I comorbidity is also seen in children and adolescents, their hair pulling is frequently uncomplicated. Jane meets criteria for TTM, as determined by the Trichotillomania Diagnostic Interview,16 but her history does not support a comorbid disorder. After discussing the diagnosis with Jane and her parents, the psychiatrist begins treatment with CBT alone.
MEDICATION OR CBT?
SSRIs. Literature on TTM pharmacotherapy is very limited and equivocal. Medications that have helped adults with TTM have been described,17 but the lack of a single, randomized, controlled trial in pediatric TTM severely limits treatment recommendations for children.
Selective serotonin reuptake inhibitors (SSRIs) have shown efficacy for treating anger and other impulse control problems but not for TTM. Some practitioners use SSRIs for TTM because of the belief that TTM is a variant of OCD. However, TTM may be maintained by positive reinforcement rather than compulsive tendencies and thus may not respond to SSRIs.
CBT. Evidence on CBT justifies cautious recommendations for pediatric TTM. In randomized trials, CBT reduced hair pulling in adults and was more effective than SSRIs or placebo.18,19
REDUCING THE URGE
Obtain detailed information about a child or adolescent’s hair-pulling episodes (Table 1), as recognizing triggers and reactions is vital to effective CBT. Explain to the patient that:
- the pleasure or satisfaction she derives from pulling reinforces the urge to pull
- she can reduce the urge by learning and using awareness training, stimulus control, and habit reversal (Table 2).
Awareness training involves patient self-monitoring to gain awareness of urges to pull and of pulling behavior. The child must become alert to every hair pulled and to response precursors, such as placing her hand on her head. For a patient such as Jane, a useful technique is to post reminders on the TV and school notebook and in the bedroom and bathroom—wherever pulling typically occurs.
A “PULLING CALENDAR”
Jane begins a daily “pulling calendar” in which she records each time she pulls a hair while watching TV or doing homework. She is asked to include the total number of hairs pulled and the intensity of the “itch to pull” on a scale of 1 to 10.
Stimulus control. Most patients can identify high-risk situations, such as time in the bathroom, talking on the phone, watching TV, driving, reading, or while falling asleep. Boredom, frustration, anxiety, and sadness may serve as pulling cues.
With stimulus control, the patient tries to reduce her ability to freely engage in pulling behavior in high-risk situations. For instance, you might encourage a child who pulls hairs while doing homework to stick Band-Aid®-type adhesive strips on her thumb and index finger tips before she starts studying as an impediment to gripping hairs. Such “speed bumps” may allow her to delay pulling and reach for tools that assist in habit reversal.
TREATMENT THAT APPEALS
Jane agrees to apply adhesive strips to her fingers and understands why. Because she is a fan of Peter Pan, we place Peter Pan stickers on her books and notebooks and on the TV remote control as reminders not to pull.
Table 2
CBT strategies to reduce the hair-pulling urge
| Awareness training | Increases patient’s awareness of pulling |
| Stimulus control | Establishes an environment less conducive to pulling |
| Habit reversal/ response | Patient develops alternate activities that provide competing positive reinforcement comparable to that gained from pulling |
Habit reversal and competing response procedures provide pleasurable physical stimulation as an alternative to pulling. The most effective methods engage the same motions as used in hair pulling. Examples include sculpting with clay, hulling sunflower seeds, and playing with Koosh® balls—small rubbery balls filled with a jellylike plasma and covered with hundreds of soft “tentacles.”
‘CALMER, HAPPIER’
We explain habit reversal to Jane and instruct her to use the Koosh ball a few times a day. She enjoys pulling its rubber strands, an action that uses the same muscles as hair pulling. Because she will need Koosh balls during all identified high-risk situations, we instruct her to buy one for her book bag and to leave one near the couch where she watches TV.
Over time, Jane reports a gradual decrease of hair pulling with the use of awareness training and stimulus control techniques. Using the Koosh ball (habit reversal) helps her improve. By the 10th week, Jane and her parents report a 70% decrease in hair pulling, based on the pulling calendar entries and other objective evidence of treatment response. All report feeling “calmer and happier.”
CONCLUSION
Cognitive and behavioral strategies are useful and safe for treating pediatric TTM. Enlisting the parents and patient in identifying problem situations and applying creative solutions may increase the chances of success.
Follow-up is important for maintaining new cognitive and behavioral patterns. We recommend that you see patients monthly for at least 3 months, depending on how the patient feels about additional sessions. We encourage families to call and report on progress or relapses. Booster CBT sessions can help deal with setbacks.
Related resources
- Trichotillomania Learning Center, Inc.; devoted to improving TTM understanding and providing access to treatments and support groups. www.trich.org. Accessed Sept. 17, 2004.
- Golomb RG, Vavrichek SM. The hair pulling “habit” and you: how to solve the trichotillomania puzzle (rev ed). Silver Spring, MD: Writer’s Cooperative of Greater Washington; 2000. Book for children and teenagers.
Drug brand names
- Fluoxetine • Prozac
- Naltrexone • Depade, ReVia
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Preparation of this article was supported in part by a grant from the National Institute of Mental Health (MH61457).
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association Press; 2000;674-7.
2. Christenson GA, Mackenzie TB, Mitchell JE. Characteristics of 60 adult chronic hairpullers. Am J Psychiatry 1991;148:365-70.
3. Swedo SE, Leonard HL. Trichotillomania: an obsessive compulsive spectrum disorder? Psychiatr Clin North Am 1992;15:777-90.
4. Reeve E. Hair pulling in children and adolescents. In: Stein DJ, Christenson GA, Hollander E, eds. Trichotillomania. Washington, DC: American Psychiatric Association Press, 1999;201-24.
5. Reeve EA, Bernstein GA, Christenson GA. Clinical characteristics and psychiatric comorbidity in children with trichotillomania. J Am Acad Child Adolesc Psychiatry 1992;31:132-8.
6. King RA, Scahill L, Vitulano LA, et al. Childhood trichotillomania: clinical phenomenology, comorbidity, and family genetics. J Am Acad Child Adolesc Psychiatry 1995;34:1451-9.
7. Franklin ME, Bux DA, Foa EB. Pediatric trichotillomania: conceptualization and treatment implications. In: Orvashel H, Faust J, Hersen M, eds. Handbook of conceptualization and treatment of child psychopathology. Oxford, UK: Elsevier Science; 2001;379-98.
8. Keuthen NJ, Franklin ME. Trichotillomania: psychopathology and treatment development [presentation]. Reno, NV: Association for the Advancement of Behavior Therapy annual meeting, 2002.
9. Bienvenu OJ, Samuels JF, Riddle MA, et al. The relationship of obsessive-compulsive disorder to possible spectrum disorders: results from a family study. Biol Psychiatry 2000;48:287-93.
10. Lenane MC, Swedo SE, Rapoport JL, et al. Rates of obsessive compulsive disorder in first degree relatives of patients with trichotillomania: a research note. J Child Psychol Psychiatry 1992;33:925-33.
11. Swedo SE, Rapoport JL, Leonard HL, et al. Regional cerebral glucose metabolism of women in trichotillomania. Arch Gen Psychiatry 1991;48:828-33.
12. O’Sullivan RL, Rauch SL, Breiter HC, et al. Reduced basal ganglia volumes in trichotillomania measured via morphometric magnetic resonance imaging. Biol Psychiatry 1997;42:39-45.
13. Grachev ID. MRI-based morphometric topographic parcellation of human neocortex in trichotillomania. Psychiatry Clin Neurosci 1997;51:315-21.
14. Carrion VG. Naltrexone for the treatment of trichotillomania: a case report. J Clin Psychopharmacol 1995;15:444-5.
15. Christenson GA, Mansueto CS. Trichotillomania: descriptive characteristics and phenomenology. In: Stein DJ, Christenson GA, Hollander E, eds. Trichotillomania. Washington, DC: American Psychiatric Press, 1999;1-41.
16. Rothbaum BO, Ninan PT. The assessment of trichotillomania. Behav Res Ther 1994;32(6):651-62.
17. Lundt LP. Trichotillomania: a heads-up on severe cases. Current Psychiatry 2004;3(5):89-105.
18. Ninan PT, Rothbaum BO, Marsteller FA, et al. A placebo-controlled trial of cognitive-behavioral therapy and clomipramine in trichotillomania. J Clin Psychiatry. 2000;61:47-50.
19. Azrin NH, Nunn RG, Frantz SE. Treatment of hairpulling (trichotillomania): a comparative study of habit reversal and negative practice training. J Behav Ther Exp Psychiatry 1980;11:13-20.
Trichotillomania (TTM) is distressing to pediatric patients who pull their hair and to their parents who feel helpless to stop the destructive behavior. Hair-pulling with psychiatric comorbidity requires comprehensive assessment and treatment, but we have found that cognitive-behavioral therapy (CBT) alone can help children and adolescents with uncomplicated TTM.
This article describes a typical patient with adolescent-onset mild-to-moderate TTM and the three-step CBT approach—awareness training, stimulus control, and habit reversal—that we find effective in reducing pediatric hair pulling.
Jane, age 12, was referred to our clinic by her primary doctor after an 8-week trial of fluoxetine, 80 mg/d, failed to stop her hair pulling. Jane, who is right-handed, has been pulling her hair for 2 years, mostly in the right front scalp. Her shame over the hair loss makes her reluctant to participate in social activities. A dermatologist found no medical cause for her behavior, such as alopecia or folliculitis.
Jane’s parents say she has no history of a major mood disorder or anxiety. Her hair pulling causes significant “tension and stress” for all family members.
WHY DO PATIENTS PULL HAIR?
Cognitive-behavioral theory suggests that chronic TTM originates as a normal response to stress that often escapes personal and social awareness but gradually increases in frequency and severity (Box).1-8 Thus, hair pulling becomes associated with internal and external cues through conditioning and is maintained primarily by positive reinforcement. Hair-pulling urges that are reinforced by pulling intensify the need to pull, perpetuating the behavioral cycle.
A genetic link? Familial research has associated TTM with increased rates of obsessive-compulsive disorder (OCD) or other excessive habits—such as nail biting or skin picking—among first-degree relatives.6,9,10 Neuroimaging of persons with TTM has shown hyperactivity in the left cerebellum and right superior parietal lobe11 as well as possible structural abnormalities in the left putamen,12 left inferior frontal gyrus, and right cluneal cortex.13
These findings do not necessarily indicate pre-existing brain pathology, however. Perhaps TTM leads to changes in brain structure or function, or both TTM and the brain abnormalities may be caused by another as-yet-unknown variable.
Decreased pain sensitivity. Patients with TTM often report that hair pulling is not painful,2 though we suspect that persons without TTM would disagree and derive no pleasure from it. Changes in pain sensitivity may influence the reinforcing quality of pulling behavior. One possible mechanism for such alterations is upregulation of the endogenous opioid system; some intriguing evidence suggests that opioid receptor antagonists such as naltrexone may reduce pulling.14
Trichotillomania (TTM) is an impulse control disorder characterized by repetitive hair pulling,1 which typically emerges during adolescence. In a large clinical sample of adult hair pullers, mean age of onset was 13.2 Very-early onset (before age 5) may be a more benign form of TTM that tends to abate spontaneously and requires little or no therapeutic intervention.3
Despite the absence of body hair in prepubertal children, their pulling patterns are consistent with those of adults. The scalp is the most common pulling site, followed by eyelashes and eyebrows.4
Psychiatric comorbidity. In two studies evaluating psychiatric comorbidity in pediatric clinical samples, 60% to 70% of children and teens with TTM had at least one comorbid axis I disorder.5,6 Disruptive behavior disorders were most common in one study,6 whereas overanxious disorder was most common in the other.5 In a large clinical sample of adults with TTM, 51% met criteria for comorbid depression.2
Early identification and treatment of TTM are recommended because of the disorder’s distressing nature and social stigma. Early interventions also may help prevent later adult psychiatric comorbidity and functional impairment, although no studies have been done to demonstrate this benefit.7,8
Pain tolerance at the preferred pulling site has not been studied, however. For patients who feel pain from hair pulling, the pain itself may reinforce the behavior by distracting the individual from negative emotional or physiologic states.15
CASE CONTINUED: COUNTING THE WAYS
Jane and her parents agree that she pulls her hair 5 to 8 times daily, one hair at a time with her right index finger and thumb while doing homework or watching TV. The trigger, she says, is “an itch” on her scalp; “sometimes pulling relieves the itch.” She fails to resist pulling her hair 9 out of 10 times.
Table 1
Defining hair pulling: What to ask the pediatric patient
| Response description | How many times do you pull your hair each day? |
| How many hairs do you pull each time? | |
| From what body areas do you pull hair? | |
| What are all the steps involved in pulling (Touching the head before pulling? Pulling one hair at a time with the thumb and index finger)? | |
| Response detection | Under what circumstances do you sense the urge to pull? |
| How strong is the urge on a scale of 1 to 10, with 10 being the greatest intensity you ever felt? | |
| How do you try to resist and overcome the urge to pull? | |
| Precursors | External cues (Do you pull when you look at yourself in a mirror?) |
| Internal cues (Do you pull when you are nervous?) | |
| High-risk situations | What are you usually doing when you get the urge to pull? (reading, talking on the telephone, watching TV, using a computer, etc.) |
| Consequences that reinforce the behavior | Do you pull to reduce physical sensations (such as itching) at the site of pulling? |
| Does pulling relieve sadness or worry about problems at home or in school? | |
| Do you pull to create a more even hairline? |
Psychiatric comorbidity is common—if not the norm—in adults with TTM. Although axis I comorbidity is also seen in children and adolescents, their hair pulling is frequently uncomplicated. Jane meets criteria for TTM, as determined by the Trichotillomania Diagnostic Interview,16 but her history does not support a comorbid disorder. After discussing the diagnosis with Jane and her parents, the psychiatrist begins treatment with CBT alone.
MEDICATION OR CBT?
SSRIs. Literature on TTM pharmacotherapy is very limited and equivocal. Medications that have helped adults with TTM have been described,17 but the lack of a single, randomized, controlled trial in pediatric TTM severely limits treatment recommendations for children.
Selective serotonin reuptake inhibitors (SSRIs) have shown efficacy for treating anger and other impulse control problems but not for TTM. Some practitioners use SSRIs for TTM because of the belief that TTM is a variant of OCD. However, TTM may be maintained by positive reinforcement rather than compulsive tendencies and thus may not respond to SSRIs.
CBT. Evidence on CBT justifies cautious recommendations for pediatric TTM. In randomized trials, CBT reduced hair pulling in adults and was more effective than SSRIs or placebo.18,19
REDUCING THE URGE
Obtain detailed information about a child or adolescent’s hair-pulling episodes (Table 1), as recognizing triggers and reactions is vital to effective CBT. Explain to the patient that:
- the pleasure or satisfaction she derives from pulling reinforces the urge to pull
- she can reduce the urge by learning and using awareness training, stimulus control, and habit reversal (Table 2).
Awareness training involves patient self-monitoring to gain awareness of urges to pull and of pulling behavior. The child must become alert to every hair pulled and to response precursors, such as placing her hand on her head. For a patient such as Jane, a useful technique is to post reminders on the TV and school notebook and in the bedroom and bathroom—wherever pulling typically occurs.
A “PULLING CALENDAR”
Jane begins a daily “pulling calendar” in which she records each time she pulls a hair while watching TV or doing homework. She is asked to include the total number of hairs pulled and the intensity of the “itch to pull” on a scale of 1 to 10.
Stimulus control. Most patients can identify high-risk situations, such as time in the bathroom, talking on the phone, watching TV, driving, reading, or while falling asleep. Boredom, frustration, anxiety, and sadness may serve as pulling cues.
With stimulus control, the patient tries to reduce her ability to freely engage in pulling behavior in high-risk situations. For instance, you might encourage a child who pulls hairs while doing homework to stick Band-Aid®-type adhesive strips on her thumb and index finger tips before she starts studying as an impediment to gripping hairs. Such “speed bumps” may allow her to delay pulling and reach for tools that assist in habit reversal.
TREATMENT THAT APPEALS
Jane agrees to apply adhesive strips to her fingers and understands why. Because she is a fan of Peter Pan, we place Peter Pan stickers on her books and notebooks and on the TV remote control as reminders not to pull.
Table 2
CBT strategies to reduce the hair-pulling urge
| Awareness training | Increases patient’s awareness of pulling |
| Stimulus control | Establishes an environment less conducive to pulling |
| Habit reversal/ response | Patient develops alternate activities that provide competing positive reinforcement comparable to that gained from pulling |
Habit reversal and competing response procedures provide pleasurable physical stimulation as an alternative to pulling. The most effective methods engage the same motions as used in hair pulling. Examples include sculpting with clay, hulling sunflower seeds, and playing with Koosh® balls—small rubbery balls filled with a jellylike plasma and covered with hundreds of soft “tentacles.”
‘CALMER, HAPPIER’
We explain habit reversal to Jane and instruct her to use the Koosh ball a few times a day. She enjoys pulling its rubber strands, an action that uses the same muscles as hair pulling. Because she will need Koosh balls during all identified high-risk situations, we instruct her to buy one for her book bag and to leave one near the couch where she watches TV.
Over time, Jane reports a gradual decrease of hair pulling with the use of awareness training and stimulus control techniques. Using the Koosh ball (habit reversal) helps her improve. By the 10th week, Jane and her parents report a 70% decrease in hair pulling, based on the pulling calendar entries and other objective evidence of treatment response. All report feeling “calmer and happier.”
CONCLUSION
Cognitive and behavioral strategies are useful and safe for treating pediatric TTM. Enlisting the parents and patient in identifying problem situations and applying creative solutions may increase the chances of success.
Follow-up is important for maintaining new cognitive and behavioral patterns. We recommend that you see patients monthly for at least 3 months, depending on how the patient feels about additional sessions. We encourage families to call and report on progress or relapses. Booster CBT sessions can help deal with setbacks.
Related resources
- Trichotillomania Learning Center, Inc.; devoted to improving TTM understanding and providing access to treatments and support groups. www.trich.org. Accessed Sept. 17, 2004.
- Golomb RG, Vavrichek SM. The hair pulling “habit” and you: how to solve the trichotillomania puzzle (rev ed). Silver Spring, MD: Writer’s Cooperative of Greater Washington; 2000. Book for children and teenagers.
Drug brand names
- Fluoxetine • Prozac
- Naltrexone • Depade, ReVia
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Preparation of this article was supported in part by a grant from the National Institute of Mental Health (MH61457).
Trichotillomania (TTM) is distressing to pediatric patients who pull their hair and to their parents who feel helpless to stop the destructive behavior. Hair-pulling with psychiatric comorbidity requires comprehensive assessment and treatment, but we have found that cognitive-behavioral therapy (CBT) alone can help children and adolescents with uncomplicated TTM.
This article describes a typical patient with adolescent-onset mild-to-moderate TTM and the three-step CBT approach—awareness training, stimulus control, and habit reversal—that we find effective in reducing pediatric hair pulling.
Jane, age 12, was referred to our clinic by her primary doctor after an 8-week trial of fluoxetine, 80 mg/d, failed to stop her hair pulling. Jane, who is right-handed, has been pulling her hair for 2 years, mostly in the right front scalp. Her shame over the hair loss makes her reluctant to participate in social activities. A dermatologist found no medical cause for her behavior, such as alopecia or folliculitis.
Jane’s parents say she has no history of a major mood disorder or anxiety. Her hair pulling causes significant “tension and stress” for all family members.
WHY DO PATIENTS PULL HAIR?
Cognitive-behavioral theory suggests that chronic TTM originates as a normal response to stress that often escapes personal and social awareness but gradually increases in frequency and severity (Box).1-8 Thus, hair pulling becomes associated with internal and external cues through conditioning and is maintained primarily by positive reinforcement. Hair-pulling urges that are reinforced by pulling intensify the need to pull, perpetuating the behavioral cycle.
A genetic link? Familial research has associated TTM with increased rates of obsessive-compulsive disorder (OCD) or other excessive habits—such as nail biting or skin picking—among first-degree relatives.6,9,10 Neuroimaging of persons with TTM has shown hyperactivity in the left cerebellum and right superior parietal lobe11 as well as possible structural abnormalities in the left putamen,12 left inferior frontal gyrus, and right cluneal cortex.13
These findings do not necessarily indicate pre-existing brain pathology, however. Perhaps TTM leads to changes in brain structure or function, or both TTM and the brain abnormalities may be caused by another as-yet-unknown variable.
Decreased pain sensitivity. Patients with TTM often report that hair pulling is not painful,2 though we suspect that persons without TTM would disagree and derive no pleasure from it. Changes in pain sensitivity may influence the reinforcing quality of pulling behavior. One possible mechanism for such alterations is upregulation of the endogenous opioid system; some intriguing evidence suggests that opioid receptor antagonists such as naltrexone may reduce pulling.14
Trichotillomania (TTM) is an impulse control disorder characterized by repetitive hair pulling,1 which typically emerges during adolescence. In a large clinical sample of adult hair pullers, mean age of onset was 13.2 Very-early onset (before age 5) may be a more benign form of TTM that tends to abate spontaneously and requires little or no therapeutic intervention.3
Despite the absence of body hair in prepubertal children, their pulling patterns are consistent with those of adults. The scalp is the most common pulling site, followed by eyelashes and eyebrows.4
Psychiatric comorbidity. In two studies evaluating psychiatric comorbidity in pediatric clinical samples, 60% to 70% of children and teens with TTM had at least one comorbid axis I disorder.5,6 Disruptive behavior disorders were most common in one study,6 whereas overanxious disorder was most common in the other.5 In a large clinical sample of adults with TTM, 51% met criteria for comorbid depression.2
Early identification and treatment of TTM are recommended because of the disorder’s distressing nature and social stigma. Early interventions also may help prevent later adult psychiatric comorbidity and functional impairment, although no studies have been done to demonstrate this benefit.7,8
Pain tolerance at the preferred pulling site has not been studied, however. For patients who feel pain from hair pulling, the pain itself may reinforce the behavior by distracting the individual from negative emotional or physiologic states.15
CASE CONTINUED: COUNTING THE WAYS
Jane and her parents agree that she pulls her hair 5 to 8 times daily, one hair at a time with her right index finger and thumb while doing homework or watching TV. The trigger, she says, is “an itch” on her scalp; “sometimes pulling relieves the itch.” She fails to resist pulling her hair 9 out of 10 times.
Table 1
Defining hair pulling: What to ask the pediatric patient
| Response description | How many times do you pull your hair each day? |
| How many hairs do you pull each time? | |
| From what body areas do you pull hair? | |
| What are all the steps involved in pulling (Touching the head before pulling? Pulling one hair at a time with the thumb and index finger)? | |
| Response detection | Under what circumstances do you sense the urge to pull? |
| How strong is the urge on a scale of 1 to 10, with 10 being the greatest intensity you ever felt? | |
| How do you try to resist and overcome the urge to pull? | |
| Precursors | External cues (Do you pull when you look at yourself in a mirror?) |
| Internal cues (Do you pull when you are nervous?) | |
| High-risk situations | What are you usually doing when you get the urge to pull? (reading, talking on the telephone, watching TV, using a computer, etc.) |
| Consequences that reinforce the behavior | Do you pull to reduce physical sensations (such as itching) at the site of pulling? |
| Does pulling relieve sadness or worry about problems at home or in school? | |
| Do you pull to create a more even hairline? |
Psychiatric comorbidity is common—if not the norm—in adults with TTM. Although axis I comorbidity is also seen in children and adolescents, their hair pulling is frequently uncomplicated. Jane meets criteria for TTM, as determined by the Trichotillomania Diagnostic Interview,16 but her history does not support a comorbid disorder. After discussing the diagnosis with Jane and her parents, the psychiatrist begins treatment with CBT alone.
MEDICATION OR CBT?
SSRIs. Literature on TTM pharmacotherapy is very limited and equivocal. Medications that have helped adults with TTM have been described,17 but the lack of a single, randomized, controlled trial in pediatric TTM severely limits treatment recommendations for children.
Selective serotonin reuptake inhibitors (SSRIs) have shown efficacy for treating anger and other impulse control problems but not for TTM. Some practitioners use SSRIs for TTM because of the belief that TTM is a variant of OCD. However, TTM may be maintained by positive reinforcement rather than compulsive tendencies and thus may not respond to SSRIs.
CBT. Evidence on CBT justifies cautious recommendations for pediatric TTM. In randomized trials, CBT reduced hair pulling in adults and was more effective than SSRIs or placebo.18,19
REDUCING THE URGE
Obtain detailed information about a child or adolescent’s hair-pulling episodes (Table 1), as recognizing triggers and reactions is vital to effective CBT. Explain to the patient that:
- the pleasure or satisfaction she derives from pulling reinforces the urge to pull
- she can reduce the urge by learning and using awareness training, stimulus control, and habit reversal (Table 2).
Awareness training involves patient self-monitoring to gain awareness of urges to pull and of pulling behavior. The child must become alert to every hair pulled and to response precursors, such as placing her hand on her head. For a patient such as Jane, a useful technique is to post reminders on the TV and school notebook and in the bedroom and bathroom—wherever pulling typically occurs.
A “PULLING CALENDAR”
Jane begins a daily “pulling calendar” in which she records each time she pulls a hair while watching TV or doing homework. She is asked to include the total number of hairs pulled and the intensity of the “itch to pull” on a scale of 1 to 10.
Stimulus control. Most patients can identify high-risk situations, such as time in the bathroom, talking on the phone, watching TV, driving, reading, or while falling asleep. Boredom, frustration, anxiety, and sadness may serve as pulling cues.
With stimulus control, the patient tries to reduce her ability to freely engage in pulling behavior in high-risk situations. For instance, you might encourage a child who pulls hairs while doing homework to stick Band-Aid®-type adhesive strips on her thumb and index finger tips before she starts studying as an impediment to gripping hairs. Such “speed bumps” may allow her to delay pulling and reach for tools that assist in habit reversal.
TREATMENT THAT APPEALS
Jane agrees to apply adhesive strips to her fingers and understands why. Because she is a fan of Peter Pan, we place Peter Pan stickers on her books and notebooks and on the TV remote control as reminders not to pull.
Table 2
CBT strategies to reduce the hair-pulling urge
| Awareness training | Increases patient’s awareness of pulling |
| Stimulus control | Establishes an environment less conducive to pulling |
| Habit reversal/ response | Patient develops alternate activities that provide competing positive reinforcement comparable to that gained from pulling |
Habit reversal and competing response procedures provide pleasurable physical stimulation as an alternative to pulling. The most effective methods engage the same motions as used in hair pulling. Examples include sculpting with clay, hulling sunflower seeds, and playing with Koosh® balls—small rubbery balls filled with a jellylike plasma and covered with hundreds of soft “tentacles.”
‘CALMER, HAPPIER’
We explain habit reversal to Jane and instruct her to use the Koosh ball a few times a day. She enjoys pulling its rubber strands, an action that uses the same muscles as hair pulling. Because she will need Koosh balls during all identified high-risk situations, we instruct her to buy one for her book bag and to leave one near the couch where she watches TV.
Over time, Jane reports a gradual decrease of hair pulling with the use of awareness training and stimulus control techniques. Using the Koosh ball (habit reversal) helps her improve. By the 10th week, Jane and her parents report a 70% decrease in hair pulling, based on the pulling calendar entries and other objective evidence of treatment response. All report feeling “calmer and happier.”
CONCLUSION
Cognitive and behavioral strategies are useful and safe for treating pediatric TTM. Enlisting the parents and patient in identifying problem situations and applying creative solutions may increase the chances of success.
Follow-up is important for maintaining new cognitive and behavioral patterns. We recommend that you see patients monthly for at least 3 months, depending on how the patient feels about additional sessions. We encourage families to call and report on progress or relapses. Booster CBT sessions can help deal with setbacks.
Related resources
- Trichotillomania Learning Center, Inc.; devoted to improving TTM understanding and providing access to treatments and support groups. www.trich.org. Accessed Sept. 17, 2004.
- Golomb RG, Vavrichek SM. The hair pulling “habit” and you: how to solve the trichotillomania puzzle (rev ed). Silver Spring, MD: Writer’s Cooperative of Greater Washington; 2000. Book for children and teenagers.
Drug brand names
- Fluoxetine • Prozac
- Naltrexone • Depade, ReVia
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Preparation of this article was supported in part by a grant from the National Institute of Mental Health (MH61457).
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association Press; 2000;674-7.
2. Christenson GA, Mackenzie TB, Mitchell JE. Characteristics of 60 adult chronic hairpullers. Am J Psychiatry 1991;148:365-70.
3. Swedo SE, Leonard HL. Trichotillomania: an obsessive compulsive spectrum disorder? Psychiatr Clin North Am 1992;15:777-90.
4. Reeve E. Hair pulling in children and adolescents. In: Stein DJ, Christenson GA, Hollander E, eds. Trichotillomania. Washington, DC: American Psychiatric Association Press, 1999;201-24.
5. Reeve EA, Bernstein GA, Christenson GA. Clinical characteristics and psychiatric comorbidity in children with trichotillomania. J Am Acad Child Adolesc Psychiatry 1992;31:132-8.
6. King RA, Scahill L, Vitulano LA, et al. Childhood trichotillomania: clinical phenomenology, comorbidity, and family genetics. J Am Acad Child Adolesc Psychiatry 1995;34:1451-9.
7. Franklin ME, Bux DA, Foa EB. Pediatric trichotillomania: conceptualization and treatment implications. In: Orvashel H, Faust J, Hersen M, eds. Handbook of conceptualization and treatment of child psychopathology. Oxford, UK: Elsevier Science; 2001;379-98.
8. Keuthen NJ, Franklin ME. Trichotillomania: psychopathology and treatment development [presentation]. Reno, NV: Association for the Advancement of Behavior Therapy annual meeting, 2002.
9. Bienvenu OJ, Samuels JF, Riddle MA, et al. The relationship of obsessive-compulsive disorder to possible spectrum disorders: results from a family study. Biol Psychiatry 2000;48:287-93.
10. Lenane MC, Swedo SE, Rapoport JL, et al. Rates of obsessive compulsive disorder in first degree relatives of patients with trichotillomania: a research note. J Child Psychol Psychiatry 1992;33:925-33.
11. Swedo SE, Rapoport JL, Leonard HL, et al. Regional cerebral glucose metabolism of women in trichotillomania. Arch Gen Psychiatry 1991;48:828-33.
12. O’Sullivan RL, Rauch SL, Breiter HC, et al. Reduced basal ganglia volumes in trichotillomania measured via morphometric magnetic resonance imaging. Biol Psychiatry 1997;42:39-45.
13. Grachev ID. MRI-based morphometric topographic parcellation of human neocortex in trichotillomania. Psychiatry Clin Neurosci 1997;51:315-21.
14. Carrion VG. Naltrexone for the treatment of trichotillomania: a case report. J Clin Psychopharmacol 1995;15:444-5.
15. Christenson GA, Mansueto CS. Trichotillomania: descriptive characteristics and phenomenology. In: Stein DJ, Christenson GA, Hollander E, eds. Trichotillomania. Washington, DC: American Psychiatric Press, 1999;1-41.
16. Rothbaum BO, Ninan PT. The assessment of trichotillomania. Behav Res Ther 1994;32(6):651-62.
17. Lundt LP. Trichotillomania: a heads-up on severe cases. Current Psychiatry 2004;3(5):89-105.
18. Ninan PT, Rothbaum BO, Marsteller FA, et al. A placebo-controlled trial of cognitive-behavioral therapy and clomipramine in trichotillomania. J Clin Psychiatry. 2000;61:47-50.
19. Azrin NH, Nunn RG, Frantz SE. Treatment of hairpulling (trichotillomania): a comparative study of habit reversal and negative practice training. J Behav Ther Exp Psychiatry 1980;11:13-20.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association Press; 2000;674-7.
2. Christenson GA, Mackenzie TB, Mitchell JE. Characteristics of 60 adult chronic hairpullers. Am J Psychiatry 1991;148:365-70.
3. Swedo SE, Leonard HL. Trichotillomania: an obsessive compulsive spectrum disorder? Psychiatr Clin North Am 1992;15:777-90.
4. Reeve E. Hair pulling in children and adolescents. In: Stein DJ, Christenson GA, Hollander E, eds. Trichotillomania. Washington, DC: American Psychiatric Association Press, 1999;201-24.
5. Reeve EA, Bernstein GA, Christenson GA. Clinical characteristics and psychiatric comorbidity in children with trichotillomania. J Am Acad Child Adolesc Psychiatry 1992;31:132-8.
6. King RA, Scahill L, Vitulano LA, et al. Childhood trichotillomania: clinical phenomenology, comorbidity, and family genetics. J Am Acad Child Adolesc Psychiatry 1995;34:1451-9.
7. Franklin ME, Bux DA, Foa EB. Pediatric trichotillomania: conceptualization and treatment implications. In: Orvashel H, Faust J, Hersen M, eds. Handbook of conceptualization and treatment of child psychopathology. Oxford, UK: Elsevier Science; 2001;379-98.
8. Keuthen NJ, Franklin ME. Trichotillomania: psychopathology and treatment development [presentation]. Reno, NV: Association for the Advancement of Behavior Therapy annual meeting, 2002.
9. Bienvenu OJ, Samuels JF, Riddle MA, et al. The relationship of obsessive-compulsive disorder to possible spectrum disorders: results from a family study. Biol Psychiatry 2000;48:287-93.
10. Lenane MC, Swedo SE, Rapoport JL, et al. Rates of obsessive compulsive disorder in first degree relatives of patients with trichotillomania: a research note. J Child Psychol Psychiatry 1992;33:925-33.
11. Swedo SE, Rapoport JL, Leonard HL, et al. Regional cerebral glucose metabolism of women in trichotillomania. Arch Gen Psychiatry 1991;48:828-33.
12. O’Sullivan RL, Rauch SL, Breiter HC, et al. Reduced basal ganglia volumes in trichotillomania measured via morphometric magnetic resonance imaging. Biol Psychiatry 1997;42:39-45.
13. Grachev ID. MRI-based morphometric topographic parcellation of human neocortex in trichotillomania. Psychiatry Clin Neurosci 1997;51:315-21.
14. Carrion VG. Naltrexone for the treatment of trichotillomania: a case report. J Clin Psychopharmacol 1995;15:444-5.
15. Christenson GA, Mansueto CS. Trichotillomania: descriptive characteristics and phenomenology. In: Stein DJ, Christenson GA, Hollander E, eds. Trichotillomania. Washington, DC: American Psychiatric Press, 1999;1-41.
16. Rothbaum BO, Ninan PT. The assessment of trichotillomania. Behav Res Ther 1994;32(6):651-62.
17. Lundt LP. Trichotillomania: a heads-up on severe cases. Current Psychiatry 2004;3(5):89-105.
18. Ninan PT, Rothbaum BO, Marsteller FA, et al. A placebo-controlled trial of cognitive-behavioral therapy and clomipramine in trichotillomania. J Clin Psychiatry. 2000;61:47-50.
19. Azrin NH, Nunn RG, Frantz SE. Treatment of hairpulling (trichotillomania): a comparative study of habit reversal and negative practice training. J Behav Ther Exp Psychiatry 1980;11:13-20.
Identify and manage 2 common non-Alzheimer’s dementias
Primary care doctors refer patients with dementia to psychiatrists when the diagnosis or disease course is unclear. Psychiatrists thus must often discern non-Alzheimer’s dementias— particularly the vascular and Lewy body types— from Alzheimer’s dementia. This article describes:
- features that distinguish vascular, Lewy body, and Alzheimer’s dementias
- cognitive and medical tests to help determine dementia type and facilitate treatment
- risk factors that promote cognitive and functional decline
- strategies for using medication while minimizing side-effect risks.
CASE REPORT: DISRUPTIVE IN DAY CARE
Ms. Z, age 82, is referred to a psychiatrist after numerous failed attempts by her primary care physician to stop her medical and psychiatric deterioration.
Table 1
Estimated dementia type prevalence among patients with dementia
| Dementia type | Prevalence |
|---|---|
| Alzheimer's | 65% |
| Lewy body | 0-15% |
| Vascular | 10-15% |
| Mixed | 10-15% |
| Source: reference 1 | |
The patient was functioning well at home until 6 months ago, when her husband’s death triggered a dramatic functional decline. She has Parkinson’s disease and has had dementia symptoms for 3 years, but family members do not recall a dementia diagnosis.
Ms. Z has become increasingly disruptive in day care; she yelled at and slapped a staff member during one episode. Her son is concerned that additional outbursts will prompt her dismissal. Her Mini-Mental State Examination (MMSE) score is 19, indicating moderate dementia.
Donepezil, 10 mg/d across 2 years, has not slowed Ms. Z’s memory decline. Carbidopa/levadopa, 25/250 mg tid over 1 year, has not improved her Parkinson’s symptoms. Risperidone, 0.5 mg bid, caused marked sedation and unsteady gait and was stopped after 4 weeks. She also is taking hydrocodone/acetaminophen, 5/500 mg/d for osteoarthritis, and lisinopril/hydrochlorothiazide, 10/12.5 mg/d for hypertension.
Discussion. As with Ms. Z, a significant other can mask a dementia patient’s cognitive deficits, but these deficits become apparent after the partner dies. Family members then discover that a parent or sibling cannot function independently.
Treatment should target Ms. Z’s aggression to allow her to stay in day care and her son to care for her at home. Determining the dementia type is crucial to planning treatment and preserving function.
WHICH DEMENTIA IS WHICH?
Non-Alzheimer’s dementias account for up to 35% of dementia cases (Table 1).1 The pathologic correlations separating Alzheimer’s, vascular, and Lewy body dementias are often confusing:
- Beta-amyloid plaques are common in Alzheimer’s and Lewy body dementias, although neurofibrillary tangles are much less common in the Lewy body type.
- Synaptic cholinergic deficiencies are seen in Alzheimer’s and vascular dementias.
- Hypertension and hyperlipidemia—both traditional vascular risk factors—also appear to contribute to Alzheimer’s dementia.
Vascular dementia. Large, single-vessel hemispheric infarcts cause substantial damage, whereas multiple small vascular lesions (such as lacunae or mini-infarcts) can have more-subtle effects when strategically located, such as in the basal ganglia, hippocampus, or thalamus. These smaller lesions can disrupt frontal cortical-subcortical neural pathways and contribute to difficulties with executive functions (judgment, insight), emotional control, and behavior.
Lesions from a cerebrovascular accident, however, do not necessarily cause dementia, and the mechanism by which lesions cause dementia is not fully understood. Post-stroke dementia sometimes is progressive, suggesting a degenerative rather than vascular cause.
Lewy body dementia is associated with Parkinson’s disease, as Lewy body inclusion deposits are common to both disorders. The deposits typically appear in the cerebral cortex in Lewy body dementia but not in Parkinson’s.
Amyloid protein deposits alter the clinical presentation. Patients with these lesions have fewer visual hallucinations and motor problems, making diagnosis more difficult.
Lewy body dementia, like all major dementias, usually surfaces after age 75. Its clinical course generally is considered worse than that of Alzheimer’s dementia, but these two dementia types do not differ substantially in age of onset, age of death, or survival rates.
Table 2
Clinical features that characterize Lewy body dementia
| Central | Progressive cognitive decline that interferes with normal social and occupational function; deficits on tests of attention, frontal-subcortical skills, and visuospatial ability can be especially prominent |
| Core | Two of three needed for probable diagnosis:
|
| Supportive | Repeated falls Syncope Transient loss of consciousness REM sleep behavior disorder Systematized delusions Hallucinations in other modalities Neuroleptic sensitivity Depression |
| Features less likely to be present | History of stroke Another physical illness or brain disorder that interferes with cognitive performance |
| Source: reference 6 | |
FEATURES OF VASCULAR DEMENTIA
Onset can be gradual but is more often sudden— usually occurring shortly after an ischemic stroke. Disease progression can be gradual or dramatic, depending on the vascular event type. Cognitive and physical decline in vascular dementia usually is stepwise over time, whereas decline in Alzheimer’s dementia is more gradual with progressive severity.
Patients with vascular dementia classically present with memory loss temporally associated with other typical stroke stigmata. Brain imaging often uncovers evidence of stroke that is otherwise not clinically evident.
CNS manifestations of vascular dementia often include memory loss, emotional lability (including depression), and executive-task dysfunction. Patients usually have atrial fibrillation or vascular risk factors, including diabetes mellitus, hypertension, hyperlipidemia, obesity, or tobacco use. Patients with previous stroke, coronary artery disease, or peripheral vascular disease are at increased risk.
Vascular dementia is categorized by stroke type:
Embolic infarct. Emboli, typically cardiac in origin, can occlude small or large cerebral arteries, resulting in correspondingly sized infarcts. Atrial fibrillation can promote areas in the atria with relatively low flow turbulence. Blood clots can form that eventually embolize via the carotid arteries. Multiple emboli can occur, causing progressive dementia.
Cerebral hemorrhage —small or large—can be devastating. Hypertension is the major risk factor for this form of stroke.
Multi-infarct dementia. Multiple cerebral blood vessel infarcts classically lead to stepwise functional decline after each event. Multiple small infarcts can occur in various brain regions, including the cortex and basal ganglia. Binswanger’s disease, a variant of vascular dementia in which incomplete ischemia is limited to the hemispheric white matter, tends to be fairly progressive.2
Small-vessel disease. Reduced blood flow and tissue perfusion can cause small-vessel disease. Often the ischemia is “silent,” detectable only on MRI or CT. The infarcts typically cause lacunar lesions, nerve demyelination, and gliosis.3 These can occur to some extent in nondemented patients but become significant with more-extensive disease.
FEATURES OF LEWY BODY DEMENTIA
As with all dementias, permanent memory loss must be present to diagnose this dementia sub-type. Overall cognitive deficits may be more prominent than memory loss, however. The patient may have trouble performing cognitive tasks that employ visuospatial abilities, executive functions, and attention. Neuropsychiatric symptoms that overlap with Alzheimer’s dementia include apathy, anxiety, agitation, depression, anhedonia, and paranoia.
The presence of visual hallucinations, fluctuating cognition, or extrapyramidal symptoms (EPS) distinguish Lewy body from Alzheimer’s dementia.
Visual hallucinations are prominent in Lewy body dementia and often prompt psychiatric referral (Table 2). They usually surface early in the disease course and tend to persist. Other sensory hallucinations also can occur.
The hallucinations often are detailed and vivid and the patient may be aware they are occurring, especially if the dementia is not advanced. Treatment might not be necessary for mild hallucinations, which can concern the caregiver more than the patient.
Antipsychotics paradoxically worsen hallucinations in Lewy body dementia, and many patients present to psychiatrists after failing an empiric trial. A failed antipsychotic course in a patient diagnosed with Alzheimer’s dementia could indicate that the diagnosis is incorrect.
Fluctuating cognition occurs in 50% to 75% of Lewy body cases. Alertness, attention, and concentration are variable and can cycle within hours to weeks. The patient often is fairly interactive and social for a time, then has periods of diminished function and being “out of it.” Some patients have recurrent delirium and undergo multiple workups in search of a cause.
EPS. As many as 75% of Lewy body patients have parkinsonian motor features.4 Because these features are not essential to the diagnosis, their absence is the most common reason Lewy body dementia goes unrecognized.1
Motor involvement varies and can be worsened by antipsychotics. Overuse of antipsychotics in Alzheimer’s or vascular dementia also can cause motor symptoms that mimic Lewy body features.
EPS orientation tends to be axial, showing less facial expressivity and more postural imbalance. Peripheral signs such as tremor and extremity rigidity tend to be less dominant.
MAKING THE DIAGNOSIS
Vascular and Lewy body dementia diagnoses are primarily based on clinical features and findings. Memory loss is necessary for either diagnosis.
Vascular dementia. Most consensus criteria require presence of dementia, physical or radiologic signs of a stroke, and a temporal relationship between the stroke and the dementia for a vascular dementia diagnosis.
Hachinski’s “ischemia scale” can help differentiate multi-infarct from Alzheimer’s dementia.5 Cases are scored on a 0-to-9 scale, with point values for abrupt onset; stepwise course; history of stroke; and presence of somatic complaints, emotional lability, hypertension, and focal neurologic signs. A score ≥4 suggests vascular dementia.
The scale, however, does not account for imaging studies, vascular risk factors other than hypertension, or repeated silent strokes that can cause symptoms. Also, some patients who score below the cutoff have strategic infarct dementias.
Lewy body dementia. Clinical consensus guidelines developed by McKeith et al6 can help clinicians recognize and categorize this dementia type (Table 2). Several studies of diagnostic criteria have shown very good specificity but variable sensitivity.7 Because no standard imaging modalities or serum markers exist, presence of progressive memory loss, fluctuating cognition, visual hallucinations, and EPS should drive the diagnosis.
Lewy body dementia is commonly misdiagnosed as Parkinson’s dementia. The two types are readily differentiated by onset of memory loss, which emerges late in Parkinson’s dementia but is early and prominent in Lewy body dementia.
CASE CONTINUED: HISTORY LEADS TO DIAGNOSIS
Ms. Z was diagnosed as having Lewy body dementia, as her cognitive decline clearly preceded her motor deficits. Further questioning revealed fluctuating attention levels and a history of visual hallucinations.
TESTING PATIENT FUNCTION
Neuropsychiatric tests. DSM-IV recommends testing memory, orientation, language, praxis, constructional ability, and executive control function in patients with dementia. Numerous tests can aid in diagnosis, but they generally are too lengthy to be practical. The MMSE takes 5 to 10 minutes, but it might miss mild memory loss or executive dysfunction.
Giving a quick clock-drawing test in tandem with the MMSE can help measure basic executive control and constructional ability. Also, patients with Lewy body or vascular dementia often are more proficient than patients with Alzheimer’s dementia on verbal memory tests but less proficient on visuospatial performance. Consider referring clinically challenging patients for more-extensive neuropsychiatric testing.
Lab tests. Blood tests including TSH and B12/folate screens are usually performed but rarely positive. Rapid plasma reagin testing for syphilis is no longer recommended unless syphilis is suspected.
Table 3
Potential cognitive side effects associated with psychotropic classes*
| Drug class | Potential cognitive side effects |
|---|---|
| Antidepressants Tricyclics, SSRIs, SNRIs | Confusion, sedation, falls |
| Antihistamines | Confusion, sedation, dizziness |
| Antipsychotics | Sedation, fatigue, anxiety |
| Antispasmodics | Confusion, sedation |
| Benzodiazepines | Sedation, confusion, ataxia, depression |
| Opioids | Sedation, confusion, dizziness |
| Sleep-promoting agents | Amnesia, confusion, ataxia |
| * Not all agents in each class are associated with listed side effects | |
| SSRIs: Selective serotonin reuptake inhibitors | |
| SNRIs: Serotonin-norepinephrine reuptake inhibitors | |
Radiologic imaging. Radiologic imaging (MRI or CT) can show infarcts in vascular dementia and can rule out:
- a brain tumor
- a subdural hemorrhage after recent head trauma
- or normal-pressure hydrocephalus in patients with dementia, gait instability, and/or urinary incontinence.
Brain imaging in Lewy body dementia can show hippocampal preservation8 but is not specific and does not significantly support the diagnosis. Specialized tests such as single-photon emission computed tomography or positron-emission tomography show occipital hypoperfusion9 but are expensive, not sufficiently specific, and do not add substantial value over clinical criteria.
MANAGING SYMPTOMS
Medication may be necessary if the patient is frequently and significantly agitated. Consider prescribing a selective serotonin reuptake inhibitor, an anticonvulsant such as divalproex or carbamazepine as a mood stabilizer, or a short-acting benzodiazepine. Start low and titrate slowly if needed.
Find out if the patient is taking medications that may be causing bothersome side effects. Avoid agents with potential cognitive or anticholinergic effects (Table 3); the latter can cause confusion, sedation, and falls in the elderly.
Cholinesterase inhibitors, FDA-approved for use in Alzheimer’s dementia, have been shown to reduce cognitive and global functioning decline in vascular dementia.10 A cholinergic deficit present in vascular dementia may explain the drugs’ effectiveness. Donepezil, galantamine, and rivastigmine have all shown positive effects on cognition.
Because patients with Lewy body hallucinations have greater synaptic acetylcholine deficits, cholinesterase inhibitors tend to be more effective in Lewy body dementia than in other dementia subtypes. In small open-label studies, patients taking cholinesterase inhibitors for Lewy body dementia have shown sustained improvements (up to 96 months) in cognition and behavior. Wild et al,11 however, concluded that the evidence supporting use of these agents—specifically rivastigmine—is weak.
Also, cholinesterase inhibitors offer fairly modest effectiveness, do not work for all patients, and do not prevent cognitive decline even when taken regularly. Because cholinesterase inhibitors are costly and most Medicare patients lack prescription medication coverage, an initial short (6-month) trial is recommended. Re-evaluate the patient periodically by using caregiver reports, caregiver assessment scales, and basic cognitive testing.
Cholinesterase inhibitor dosing is the same for vascular and Lewy body dementia as it is for Alzheimer’s disease. Tell patients to take the agents with food to minimize potential intestinal side effects.
Memantine. In European studies, memantine has shown positive effects on cognition and function in vascular dementia. Memantine, a N-methyl-D-aspartate receptor antagonist, is FDA-approved for moderate to severe Alzheimer’s dementia.12
DELAYING DECLINE
Controlling risk factors. Controlling vascular risk factors—especially high blood pressure—is the most effective way to prevent or treat vascular dementia. In primary prevention studies, patients with good hypertension and hyperlipidemia control developed dementia more slowly than did nontreated cohorts.
In patients with coronary artery disease, statins have been shown to lower cholesterol and stabilize pre-existing plaques in the arterial wall, reducing the risk of plaque rupture. Low-density lipoproteincholesterol goals vary according to vascular risk factors but should be <100 mg/dL for patients with vascular dementia, who are at highest risk. Blood pressure goals are ≤140 mm Hg (systolic) and ≤90 mm Hg (diastolic).
Glycemic control (fasting blood glucose <110 mg/dL) and smoking cessation can also reduce the risk of further vascular events. Most patients should be taking an antiplatelet medication, preferably aspirin, to reduce clotting risk.
Although Lewy body dementia has no known risk factors other than age, research will determine whether vascular or other factors contribute to its development.
CASE CONTINUED: TARGETING AGGRESSION
Ms. Z was given divalproex, 250 mg bid, to reduce her frequent aggression. Her visual hallucinations were considered mild and not problematic and therefore were not treated. She responded well to the medication, allowing her to remain in day care and avoid nursing home placement.
Related resources
- Alzheimer’s Association. http://www.alz.org
- American Geriatrics Society. http://www.americangeriatrics.org
Drug brand names
- Carbamazepine • Tegretol, others
- Carbidopa/Levodopa • Various
- Divalproex • Depakote
- Donepezil • Aricept
- Galantamine • Reminyl
- Hydrocodone/acetaminophen • Vicodin, others
- Lisinopril/hydrochlorothiazide • Prinzide, Zestoretic
- Memantine • Namenda
- Risperdone • Risperdal
- Rivastigmine • Exelon
Disclosure
Dr. Bartz is a speaker for Forest Pharmaceuticals and Novartis Pharmaceuticals Corp.
1. McKeith JG, Ballard CG, Perry RH, et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology 2000;54:1050-8.
2. Roman GC, Erkinjuntti T, Wallin A, et al. Subcortical ischemic vascular dementia. Lancet Neurol 2002;17:426-36.
3. Pohjasraara T, Mantyla R, Ylikoski MA, et al. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. Stroke 2000;31:2952-7.
4. Del Ser T, McKeith I, Anand R, et al. Dementia with Lewy bodies: findings from an international multicenter study. Int J Geriatr Psychiatry 2000;15:1034-45.
5. Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol 1975;32:632-7.
6. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47:1113-24.
7. Luis CA, Barker WW, Gajaraj K, et al. Sensitivity and specificity of three clinical criteria for dementia with Lewy bodies in an autopsy-verified sample. Int J Geriatr Psychiatry 1999;14:526-33.
8. Barber R, Ballard C, McKeith IG, et al. MRI volumetric study of dementia with Lewy bodies: a comparison with AD and vascular dementia. Neurology 2000;54:1304-9.
9. Lobotesis K, Fenwick JD, Phipps A, et al. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology 2001;56:643-9.
10. Pratt RD, Perdomo CA. Results of clinical studies with donepezil in vascular dementia. Am J Geriatr Psychiatry 2002;10(suppl 1):88-9.
11. Wild R, Pettit T, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database Syst Rev 2003;3:CD003672.-
12. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 2004;291:317-24.
Primary care doctors refer patients with dementia to psychiatrists when the diagnosis or disease course is unclear. Psychiatrists thus must often discern non-Alzheimer’s dementias— particularly the vascular and Lewy body types— from Alzheimer’s dementia. This article describes:
- features that distinguish vascular, Lewy body, and Alzheimer’s dementias
- cognitive and medical tests to help determine dementia type and facilitate treatment
- risk factors that promote cognitive and functional decline
- strategies for using medication while minimizing side-effect risks.
CASE REPORT: DISRUPTIVE IN DAY CARE
Ms. Z, age 82, is referred to a psychiatrist after numerous failed attempts by her primary care physician to stop her medical and psychiatric deterioration.
Table 1
Estimated dementia type prevalence among patients with dementia
| Dementia type | Prevalence |
|---|---|
| Alzheimer's | 65% |
| Lewy body | 0-15% |
| Vascular | 10-15% |
| Mixed | 10-15% |
| Source: reference 1 | |
The patient was functioning well at home until 6 months ago, when her husband’s death triggered a dramatic functional decline. She has Parkinson’s disease and has had dementia symptoms for 3 years, but family members do not recall a dementia diagnosis.
Ms. Z has become increasingly disruptive in day care; she yelled at and slapped a staff member during one episode. Her son is concerned that additional outbursts will prompt her dismissal. Her Mini-Mental State Examination (MMSE) score is 19, indicating moderate dementia.
Donepezil, 10 mg/d across 2 years, has not slowed Ms. Z’s memory decline. Carbidopa/levadopa, 25/250 mg tid over 1 year, has not improved her Parkinson’s symptoms. Risperidone, 0.5 mg bid, caused marked sedation and unsteady gait and was stopped after 4 weeks. She also is taking hydrocodone/acetaminophen, 5/500 mg/d for osteoarthritis, and lisinopril/hydrochlorothiazide, 10/12.5 mg/d for hypertension.
Discussion. As with Ms. Z, a significant other can mask a dementia patient’s cognitive deficits, but these deficits become apparent after the partner dies. Family members then discover that a parent or sibling cannot function independently.
Treatment should target Ms. Z’s aggression to allow her to stay in day care and her son to care for her at home. Determining the dementia type is crucial to planning treatment and preserving function.
WHICH DEMENTIA IS WHICH?
Non-Alzheimer’s dementias account for up to 35% of dementia cases (Table 1).1 The pathologic correlations separating Alzheimer’s, vascular, and Lewy body dementias are often confusing:
- Beta-amyloid plaques are common in Alzheimer’s and Lewy body dementias, although neurofibrillary tangles are much less common in the Lewy body type.
- Synaptic cholinergic deficiencies are seen in Alzheimer’s and vascular dementias.
- Hypertension and hyperlipidemia—both traditional vascular risk factors—also appear to contribute to Alzheimer’s dementia.
Vascular dementia. Large, single-vessel hemispheric infarcts cause substantial damage, whereas multiple small vascular lesions (such as lacunae or mini-infarcts) can have more-subtle effects when strategically located, such as in the basal ganglia, hippocampus, or thalamus. These smaller lesions can disrupt frontal cortical-subcortical neural pathways and contribute to difficulties with executive functions (judgment, insight), emotional control, and behavior.
Lesions from a cerebrovascular accident, however, do not necessarily cause dementia, and the mechanism by which lesions cause dementia is not fully understood. Post-stroke dementia sometimes is progressive, suggesting a degenerative rather than vascular cause.
Lewy body dementia is associated with Parkinson’s disease, as Lewy body inclusion deposits are common to both disorders. The deposits typically appear in the cerebral cortex in Lewy body dementia but not in Parkinson’s.
Amyloid protein deposits alter the clinical presentation. Patients with these lesions have fewer visual hallucinations and motor problems, making diagnosis more difficult.
Lewy body dementia, like all major dementias, usually surfaces after age 75. Its clinical course generally is considered worse than that of Alzheimer’s dementia, but these two dementia types do not differ substantially in age of onset, age of death, or survival rates.
Table 2
Clinical features that characterize Lewy body dementia
| Central | Progressive cognitive decline that interferes with normal social and occupational function; deficits on tests of attention, frontal-subcortical skills, and visuospatial ability can be especially prominent |
| Core | Two of three needed for probable diagnosis:
|
| Supportive | Repeated falls Syncope Transient loss of consciousness REM sleep behavior disorder Systematized delusions Hallucinations in other modalities Neuroleptic sensitivity Depression |
| Features less likely to be present | History of stroke Another physical illness or brain disorder that interferes with cognitive performance |
| Source: reference 6 | |
FEATURES OF VASCULAR DEMENTIA
Onset can be gradual but is more often sudden— usually occurring shortly after an ischemic stroke. Disease progression can be gradual or dramatic, depending on the vascular event type. Cognitive and physical decline in vascular dementia usually is stepwise over time, whereas decline in Alzheimer’s dementia is more gradual with progressive severity.
Patients with vascular dementia classically present with memory loss temporally associated with other typical stroke stigmata. Brain imaging often uncovers evidence of stroke that is otherwise not clinically evident.
CNS manifestations of vascular dementia often include memory loss, emotional lability (including depression), and executive-task dysfunction. Patients usually have atrial fibrillation or vascular risk factors, including diabetes mellitus, hypertension, hyperlipidemia, obesity, or tobacco use. Patients with previous stroke, coronary artery disease, or peripheral vascular disease are at increased risk.
Vascular dementia is categorized by stroke type:
Embolic infarct. Emboli, typically cardiac in origin, can occlude small or large cerebral arteries, resulting in correspondingly sized infarcts. Atrial fibrillation can promote areas in the atria with relatively low flow turbulence. Blood clots can form that eventually embolize via the carotid arteries. Multiple emboli can occur, causing progressive dementia.
Cerebral hemorrhage —small or large—can be devastating. Hypertension is the major risk factor for this form of stroke.
Multi-infarct dementia. Multiple cerebral blood vessel infarcts classically lead to stepwise functional decline after each event. Multiple small infarcts can occur in various brain regions, including the cortex and basal ganglia. Binswanger’s disease, a variant of vascular dementia in which incomplete ischemia is limited to the hemispheric white matter, tends to be fairly progressive.2
Small-vessel disease. Reduced blood flow and tissue perfusion can cause small-vessel disease. Often the ischemia is “silent,” detectable only on MRI or CT. The infarcts typically cause lacunar lesions, nerve demyelination, and gliosis.3 These can occur to some extent in nondemented patients but become significant with more-extensive disease.
FEATURES OF LEWY BODY DEMENTIA
As with all dementias, permanent memory loss must be present to diagnose this dementia sub-type. Overall cognitive deficits may be more prominent than memory loss, however. The patient may have trouble performing cognitive tasks that employ visuospatial abilities, executive functions, and attention. Neuropsychiatric symptoms that overlap with Alzheimer’s dementia include apathy, anxiety, agitation, depression, anhedonia, and paranoia.
The presence of visual hallucinations, fluctuating cognition, or extrapyramidal symptoms (EPS) distinguish Lewy body from Alzheimer’s dementia.
Visual hallucinations are prominent in Lewy body dementia and often prompt psychiatric referral (Table 2). They usually surface early in the disease course and tend to persist. Other sensory hallucinations also can occur.
The hallucinations often are detailed and vivid and the patient may be aware they are occurring, especially if the dementia is not advanced. Treatment might not be necessary for mild hallucinations, which can concern the caregiver more than the patient.
Antipsychotics paradoxically worsen hallucinations in Lewy body dementia, and many patients present to psychiatrists after failing an empiric trial. A failed antipsychotic course in a patient diagnosed with Alzheimer’s dementia could indicate that the diagnosis is incorrect.
Fluctuating cognition occurs in 50% to 75% of Lewy body cases. Alertness, attention, and concentration are variable and can cycle within hours to weeks. The patient often is fairly interactive and social for a time, then has periods of diminished function and being “out of it.” Some patients have recurrent delirium and undergo multiple workups in search of a cause.
EPS. As many as 75% of Lewy body patients have parkinsonian motor features.4 Because these features are not essential to the diagnosis, their absence is the most common reason Lewy body dementia goes unrecognized.1
Motor involvement varies and can be worsened by antipsychotics. Overuse of antipsychotics in Alzheimer’s or vascular dementia also can cause motor symptoms that mimic Lewy body features.
EPS orientation tends to be axial, showing less facial expressivity and more postural imbalance. Peripheral signs such as tremor and extremity rigidity tend to be less dominant.
MAKING THE DIAGNOSIS
Vascular and Lewy body dementia diagnoses are primarily based on clinical features and findings. Memory loss is necessary for either diagnosis.
Vascular dementia. Most consensus criteria require presence of dementia, physical or radiologic signs of a stroke, and a temporal relationship between the stroke and the dementia for a vascular dementia diagnosis.
Hachinski’s “ischemia scale” can help differentiate multi-infarct from Alzheimer’s dementia.5 Cases are scored on a 0-to-9 scale, with point values for abrupt onset; stepwise course; history of stroke; and presence of somatic complaints, emotional lability, hypertension, and focal neurologic signs. A score ≥4 suggests vascular dementia.
The scale, however, does not account for imaging studies, vascular risk factors other than hypertension, or repeated silent strokes that can cause symptoms. Also, some patients who score below the cutoff have strategic infarct dementias.
Lewy body dementia. Clinical consensus guidelines developed by McKeith et al6 can help clinicians recognize and categorize this dementia type (Table 2). Several studies of diagnostic criteria have shown very good specificity but variable sensitivity.7 Because no standard imaging modalities or serum markers exist, presence of progressive memory loss, fluctuating cognition, visual hallucinations, and EPS should drive the diagnosis.
Lewy body dementia is commonly misdiagnosed as Parkinson’s dementia. The two types are readily differentiated by onset of memory loss, which emerges late in Parkinson’s dementia but is early and prominent in Lewy body dementia.
CASE CONTINUED: HISTORY LEADS TO DIAGNOSIS
Ms. Z was diagnosed as having Lewy body dementia, as her cognitive decline clearly preceded her motor deficits. Further questioning revealed fluctuating attention levels and a history of visual hallucinations.
TESTING PATIENT FUNCTION
Neuropsychiatric tests. DSM-IV recommends testing memory, orientation, language, praxis, constructional ability, and executive control function in patients with dementia. Numerous tests can aid in diagnosis, but they generally are too lengthy to be practical. The MMSE takes 5 to 10 minutes, but it might miss mild memory loss or executive dysfunction.
Giving a quick clock-drawing test in tandem with the MMSE can help measure basic executive control and constructional ability. Also, patients with Lewy body or vascular dementia often are more proficient than patients with Alzheimer’s dementia on verbal memory tests but less proficient on visuospatial performance. Consider referring clinically challenging patients for more-extensive neuropsychiatric testing.
Lab tests. Blood tests including TSH and B12/folate screens are usually performed but rarely positive. Rapid plasma reagin testing for syphilis is no longer recommended unless syphilis is suspected.
Table 3
Potential cognitive side effects associated with psychotropic classes*
| Drug class | Potential cognitive side effects |
|---|---|
| Antidepressants Tricyclics, SSRIs, SNRIs | Confusion, sedation, falls |
| Antihistamines | Confusion, sedation, dizziness |
| Antipsychotics | Sedation, fatigue, anxiety |
| Antispasmodics | Confusion, sedation |
| Benzodiazepines | Sedation, confusion, ataxia, depression |
| Opioids | Sedation, confusion, dizziness |
| Sleep-promoting agents | Amnesia, confusion, ataxia |
| * Not all agents in each class are associated with listed side effects | |
| SSRIs: Selective serotonin reuptake inhibitors | |
| SNRIs: Serotonin-norepinephrine reuptake inhibitors | |
Radiologic imaging. Radiologic imaging (MRI or CT) can show infarcts in vascular dementia and can rule out:
- a brain tumor
- a subdural hemorrhage after recent head trauma
- or normal-pressure hydrocephalus in patients with dementia, gait instability, and/or urinary incontinence.
Brain imaging in Lewy body dementia can show hippocampal preservation8 but is not specific and does not significantly support the diagnosis. Specialized tests such as single-photon emission computed tomography or positron-emission tomography show occipital hypoperfusion9 but are expensive, not sufficiently specific, and do not add substantial value over clinical criteria.
MANAGING SYMPTOMS
Medication may be necessary if the patient is frequently and significantly agitated. Consider prescribing a selective serotonin reuptake inhibitor, an anticonvulsant such as divalproex or carbamazepine as a mood stabilizer, or a short-acting benzodiazepine. Start low and titrate slowly if needed.
Find out if the patient is taking medications that may be causing bothersome side effects. Avoid agents with potential cognitive or anticholinergic effects (Table 3); the latter can cause confusion, sedation, and falls in the elderly.
Cholinesterase inhibitors, FDA-approved for use in Alzheimer’s dementia, have been shown to reduce cognitive and global functioning decline in vascular dementia.10 A cholinergic deficit present in vascular dementia may explain the drugs’ effectiveness. Donepezil, galantamine, and rivastigmine have all shown positive effects on cognition.
Because patients with Lewy body hallucinations have greater synaptic acetylcholine deficits, cholinesterase inhibitors tend to be more effective in Lewy body dementia than in other dementia subtypes. In small open-label studies, patients taking cholinesterase inhibitors for Lewy body dementia have shown sustained improvements (up to 96 months) in cognition and behavior. Wild et al,11 however, concluded that the evidence supporting use of these agents—specifically rivastigmine—is weak.
Also, cholinesterase inhibitors offer fairly modest effectiveness, do not work for all patients, and do not prevent cognitive decline even when taken regularly. Because cholinesterase inhibitors are costly and most Medicare patients lack prescription medication coverage, an initial short (6-month) trial is recommended. Re-evaluate the patient periodically by using caregiver reports, caregiver assessment scales, and basic cognitive testing.
Cholinesterase inhibitor dosing is the same for vascular and Lewy body dementia as it is for Alzheimer’s disease. Tell patients to take the agents with food to minimize potential intestinal side effects.
Memantine. In European studies, memantine has shown positive effects on cognition and function in vascular dementia. Memantine, a N-methyl-D-aspartate receptor antagonist, is FDA-approved for moderate to severe Alzheimer’s dementia.12
DELAYING DECLINE
Controlling risk factors. Controlling vascular risk factors—especially high blood pressure—is the most effective way to prevent or treat vascular dementia. In primary prevention studies, patients with good hypertension and hyperlipidemia control developed dementia more slowly than did nontreated cohorts.
In patients with coronary artery disease, statins have been shown to lower cholesterol and stabilize pre-existing plaques in the arterial wall, reducing the risk of plaque rupture. Low-density lipoproteincholesterol goals vary according to vascular risk factors but should be <100 mg/dL for patients with vascular dementia, who are at highest risk. Blood pressure goals are ≤140 mm Hg (systolic) and ≤90 mm Hg (diastolic).
Glycemic control (fasting blood glucose <110 mg/dL) and smoking cessation can also reduce the risk of further vascular events. Most patients should be taking an antiplatelet medication, preferably aspirin, to reduce clotting risk.
Although Lewy body dementia has no known risk factors other than age, research will determine whether vascular or other factors contribute to its development.
CASE CONTINUED: TARGETING AGGRESSION
Ms. Z was given divalproex, 250 mg bid, to reduce her frequent aggression. Her visual hallucinations were considered mild and not problematic and therefore were not treated. She responded well to the medication, allowing her to remain in day care and avoid nursing home placement.
Related resources
- Alzheimer’s Association. http://www.alz.org
- American Geriatrics Society. http://www.americangeriatrics.org
Drug brand names
- Carbamazepine • Tegretol, others
- Carbidopa/Levodopa • Various
- Divalproex • Depakote
- Donepezil • Aricept
- Galantamine • Reminyl
- Hydrocodone/acetaminophen • Vicodin, others
- Lisinopril/hydrochlorothiazide • Prinzide, Zestoretic
- Memantine • Namenda
- Risperdone • Risperdal
- Rivastigmine • Exelon
Disclosure
Dr. Bartz is a speaker for Forest Pharmaceuticals and Novartis Pharmaceuticals Corp.
Primary care doctors refer patients with dementia to psychiatrists when the diagnosis or disease course is unclear. Psychiatrists thus must often discern non-Alzheimer’s dementias— particularly the vascular and Lewy body types— from Alzheimer’s dementia. This article describes:
- features that distinguish vascular, Lewy body, and Alzheimer’s dementias
- cognitive and medical tests to help determine dementia type and facilitate treatment
- risk factors that promote cognitive and functional decline
- strategies for using medication while minimizing side-effect risks.
CASE REPORT: DISRUPTIVE IN DAY CARE
Ms. Z, age 82, is referred to a psychiatrist after numerous failed attempts by her primary care physician to stop her medical and psychiatric deterioration.
Table 1
Estimated dementia type prevalence among patients with dementia
| Dementia type | Prevalence |
|---|---|
| Alzheimer's | 65% |
| Lewy body | 0-15% |
| Vascular | 10-15% |
| Mixed | 10-15% |
| Source: reference 1 | |
The patient was functioning well at home until 6 months ago, when her husband’s death triggered a dramatic functional decline. She has Parkinson’s disease and has had dementia symptoms for 3 years, but family members do not recall a dementia diagnosis.
Ms. Z has become increasingly disruptive in day care; she yelled at and slapped a staff member during one episode. Her son is concerned that additional outbursts will prompt her dismissal. Her Mini-Mental State Examination (MMSE) score is 19, indicating moderate dementia.
Donepezil, 10 mg/d across 2 years, has not slowed Ms. Z’s memory decline. Carbidopa/levadopa, 25/250 mg tid over 1 year, has not improved her Parkinson’s symptoms. Risperidone, 0.5 mg bid, caused marked sedation and unsteady gait and was stopped after 4 weeks. She also is taking hydrocodone/acetaminophen, 5/500 mg/d for osteoarthritis, and lisinopril/hydrochlorothiazide, 10/12.5 mg/d for hypertension.
Discussion. As with Ms. Z, a significant other can mask a dementia patient’s cognitive deficits, but these deficits become apparent after the partner dies. Family members then discover that a parent or sibling cannot function independently.
Treatment should target Ms. Z’s aggression to allow her to stay in day care and her son to care for her at home. Determining the dementia type is crucial to planning treatment and preserving function.
WHICH DEMENTIA IS WHICH?
Non-Alzheimer’s dementias account for up to 35% of dementia cases (Table 1).1 The pathologic correlations separating Alzheimer’s, vascular, and Lewy body dementias are often confusing:
- Beta-amyloid plaques are common in Alzheimer’s and Lewy body dementias, although neurofibrillary tangles are much less common in the Lewy body type.
- Synaptic cholinergic deficiencies are seen in Alzheimer’s and vascular dementias.
- Hypertension and hyperlipidemia—both traditional vascular risk factors—also appear to contribute to Alzheimer’s dementia.
Vascular dementia. Large, single-vessel hemispheric infarcts cause substantial damage, whereas multiple small vascular lesions (such as lacunae or mini-infarcts) can have more-subtle effects when strategically located, such as in the basal ganglia, hippocampus, or thalamus. These smaller lesions can disrupt frontal cortical-subcortical neural pathways and contribute to difficulties with executive functions (judgment, insight), emotional control, and behavior.
Lesions from a cerebrovascular accident, however, do not necessarily cause dementia, and the mechanism by which lesions cause dementia is not fully understood. Post-stroke dementia sometimes is progressive, suggesting a degenerative rather than vascular cause.
Lewy body dementia is associated with Parkinson’s disease, as Lewy body inclusion deposits are common to both disorders. The deposits typically appear in the cerebral cortex in Lewy body dementia but not in Parkinson’s.
Amyloid protein deposits alter the clinical presentation. Patients with these lesions have fewer visual hallucinations and motor problems, making diagnosis more difficult.
Lewy body dementia, like all major dementias, usually surfaces after age 75. Its clinical course generally is considered worse than that of Alzheimer’s dementia, but these two dementia types do not differ substantially in age of onset, age of death, or survival rates.
Table 2
Clinical features that characterize Lewy body dementia
| Central | Progressive cognitive decline that interferes with normal social and occupational function; deficits on tests of attention, frontal-subcortical skills, and visuospatial ability can be especially prominent |
| Core | Two of three needed for probable diagnosis:
|
| Supportive | Repeated falls Syncope Transient loss of consciousness REM sleep behavior disorder Systematized delusions Hallucinations in other modalities Neuroleptic sensitivity Depression |
| Features less likely to be present | History of stroke Another physical illness or brain disorder that interferes with cognitive performance |
| Source: reference 6 | |
FEATURES OF VASCULAR DEMENTIA
Onset can be gradual but is more often sudden— usually occurring shortly after an ischemic stroke. Disease progression can be gradual or dramatic, depending on the vascular event type. Cognitive and physical decline in vascular dementia usually is stepwise over time, whereas decline in Alzheimer’s dementia is more gradual with progressive severity.
Patients with vascular dementia classically present with memory loss temporally associated with other typical stroke stigmata. Brain imaging often uncovers evidence of stroke that is otherwise not clinically evident.
CNS manifestations of vascular dementia often include memory loss, emotional lability (including depression), and executive-task dysfunction. Patients usually have atrial fibrillation or vascular risk factors, including diabetes mellitus, hypertension, hyperlipidemia, obesity, or tobacco use. Patients with previous stroke, coronary artery disease, or peripheral vascular disease are at increased risk.
Vascular dementia is categorized by stroke type:
Embolic infarct. Emboli, typically cardiac in origin, can occlude small or large cerebral arteries, resulting in correspondingly sized infarcts. Atrial fibrillation can promote areas in the atria with relatively low flow turbulence. Blood clots can form that eventually embolize via the carotid arteries. Multiple emboli can occur, causing progressive dementia.
Cerebral hemorrhage —small or large—can be devastating. Hypertension is the major risk factor for this form of stroke.
Multi-infarct dementia. Multiple cerebral blood vessel infarcts classically lead to stepwise functional decline after each event. Multiple small infarcts can occur in various brain regions, including the cortex and basal ganglia. Binswanger’s disease, a variant of vascular dementia in which incomplete ischemia is limited to the hemispheric white matter, tends to be fairly progressive.2
Small-vessel disease. Reduced blood flow and tissue perfusion can cause small-vessel disease. Often the ischemia is “silent,” detectable only on MRI or CT. The infarcts typically cause lacunar lesions, nerve demyelination, and gliosis.3 These can occur to some extent in nondemented patients but become significant with more-extensive disease.
FEATURES OF LEWY BODY DEMENTIA
As with all dementias, permanent memory loss must be present to diagnose this dementia sub-type. Overall cognitive deficits may be more prominent than memory loss, however. The patient may have trouble performing cognitive tasks that employ visuospatial abilities, executive functions, and attention. Neuropsychiatric symptoms that overlap with Alzheimer’s dementia include apathy, anxiety, agitation, depression, anhedonia, and paranoia.
The presence of visual hallucinations, fluctuating cognition, or extrapyramidal symptoms (EPS) distinguish Lewy body from Alzheimer’s dementia.
Visual hallucinations are prominent in Lewy body dementia and often prompt psychiatric referral (Table 2). They usually surface early in the disease course and tend to persist. Other sensory hallucinations also can occur.
The hallucinations often are detailed and vivid and the patient may be aware they are occurring, especially if the dementia is not advanced. Treatment might not be necessary for mild hallucinations, which can concern the caregiver more than the patient.
Antipsychotics paradoxically worsen hallucinations in Lewy body dementia, and many patients present to psychiatrists after failing an empiric trial. A failed antipsychotic course in a patient diagnosed with Alzheimer’s dementia could indicate that the diagnosis is incorrect.
Fluctuating cognition occurs in 50% to 75% of Lewy body cases. Alertness, attention, and concentration are variable and can cycle within hours to weeks. The patient often is fairly interactive and social for a time, then has periods of diminished function and being “out of it.” Some patients have recurrent delirium and undergo multiple workups in search of a cause.
EPS. As many as 75% of Lewy body patients have parkinsonian motor features.4 Because these features are not essential to the diagnosis, their absence is the most common reason Lewy body dementia goes unrecognized.1
Motor involvement varies and can be worsened by antipsychotics. Overuse of antipsychotics in Alzheimer’s or vascular dementia also can cause motor symptoms that mimic Lewy body features.
EPS orientation tends to be axial, showing less facial expressivity and more postural imbalance. Peripheral signs such as tremor and extremity rigidity tend to be less dominant.
MAKING THE DIAGNOSIS
Vascular and Lewy body dementia diagnoses are primarily based on clinical features and findings. Memory loss is necessary for either diagnosis.
Vascular dementia. Most consensus criteria require presence of dementia, physical or radiologic signs of a stroke, and a temporal relationship between the stroke and the dementia for a vascular dementia diagnosis.
Hachinski’s “ischemia scale” can help differentiate multi-infarct from Alzheimer’s dementia.5 Cases are scored on a 0-to-9 scale, with point values for abrupt onset; stepwise course; history of stroke; and presence of somatic complaints, emotional lability, hypertension, and focal neurologic signs. A score ≥4 suggests vascular dementia.
The scale, however, does not account for imaging studies, vascular risk factors other than hypertension, or repeated silent strokes that can cause symptoms. Also, some patients who score below the cutoff have strategic infarct dementias.
Lewy body dementia. Clinical consensus guidelines developed by McKeith et al6 can help clinicians recognize and categorize this dementia type (Table 2). Several studies of diagnostic criteria have shown very good specificity but variable sensitivity.7 Because no standard imaging modalities or serum markers exist, presence of progressive memory loss, fluctuating cognition, visual hallucinations, and EPS should drive the diagnosis.
Lewy body dementia is commonly misdiagnosed as Parkinson’s dementia. The two types are readily differentiated by onset of memory loss, which emerges late in Parkinson’s dementia but is early and prominent in Lewy body dementia.
CASE CONTINUED: HISTORY LEADS TO DIAGNOSIS
Ms. Z was diagnosed as having Lewy body dementia, as her cognitive decline clearly preceded her motor deficits. Further questioning revealed fluctuating attention levels and a history of visual hallucinations.
TESTING PATIENT FUNCTION
Neuropsychiatric tests. DSM-IV recommends testing memory, orientation, language, praxis, constructional ability, and executive control function in patients with dementia. Numerous tests can aid in diagnosis, but they generally are too lengthy to be practical. The MMSE takes 5 to 10 minutes, but it might miss mild memory loss or executive dysfunction.
Giving a quick clock-drawing test in tandem with the MMSE can help measure basic executive control and constructional ability. Also, patients with Lewy body or vascular dementia often are more proficient than patients with Alzheimer’s dementia on verbal memory tests but less proficient on visuospatial performance. Consider referring clinically challenging patients for more-extensive neuropsychiatric testing.
Lab tests. Blood tests including TSH and B12/folate screens are usually performed but rarely positive. Rapid plasma reagin testing for syphilis is no longer recommended unless syphilis is suspected.
Table 3
Potential cognitive side effects associated with psychotropic classes*
| Drug class | Potential cognitive side effects |
|---|---|
| Antidepressants Tricyclics, SSRIs, SNRIs | Confusion, sedation, falls |
| Antihistamines | Confusion, sedation, dizziness |
| Antipsychotics | Sedation, fatigue, anxiety |
| Antispasmodics | Confusion, sedation |
| Benzodiazepines | Sedation, confusion, ataxia, depression |
| Opioids | Sedation, confusion, dizziness |
| Sleep-promoting agents | Amnesia, confusion, ataxia |
| * Not all agents in each class are associated with listed side effects | |
| SSRIs: Selective serotonin reuptake inhibitors | |
| SNRIs: Serotonin-norepinephrine reuptake inhibitors | |
Radiologic imaging. Radiologic imaging (MRI or CT) can show infarcts in vascular dementia and can rule out:
- a brain tumor
- a subdural hemorrhage after recent head trauma
- or normal-pressure hydrocephalus in patients with dementia, gait instability, and/or urinary incontinence.
Brain imaging in Lewy body dementia can show hippocampal preservation8 but is not specific and does not significantly support the diagnosis. Specialized tests such as single-photon emission computed tomography or positron-emission tomography show occipital hypoperfusion9 but are expensive, not sufficiently specific, and do not add substantial value over clinical criteria.
MANAGING SYMPTOMS
Medication may be necessary if the patient is frequently and significantly agitated. Consider prescribing a selective serotonin reuptake inhibitor, an anticonvulsant such as divalproex or carbamazepine as a mood stabilizer, or a short-acting benzodiazepine. Start low and titrate slowly if needed.
Find out if the patient is taking medications that may be causing bothersome side effects. Avoid agents with potential cognitive or anticholinergic effects (Table 3); the latter can cause confusion, sedation, and falls in the elderly.
Cholinesterase inhibitors, FDA-approved for use in Alzheimer’s dementia, have been shown to reduce cognitive and global functioning decline in vascular dementia.10 A cholinergic deficit present in vascular dementia may explain the drugs’ effectiveness. Donepezil, galantamine, and rivastigmine have all shown positive effects on cognition.
Because patients with Lewy body hallucinations have greater synaptic acetylcholine deficits, cholinesterase inhibitors tend to be more effective in Lewy body dementia than in other dementia subtypes. In small open-label studies, patients taking cholinesterase inhibitors for Lewy body dementia have shown sustained improvements (up to 96 months) in cognition and behavior. Wild et al,11 however, concluded that the evidence supporting use of these agents—specifically rivastigmine—is weak.
Also, cholinesterase inhibitors offer fairly modest effectiveness, do not work for all patients, and do not prevent cognitive decline even when taken regularly. Because cholinesterase inhibitors are costly and most Medicare patients lack prescription medication coverage, an initial short (6-month) trial is recommended. Re-evaluate the patient periodically by using caregiver reports, caregiver assessment scales, and basic cognitive testing.
Cholinesterase inhibitor dosing is the same for vascular and Lewy body dementia as it is for Alzheimer’s disease. Tell patients to take the agents with food to minimize potential intestinal side effects.
Memantine. In European studies, memantine has shown positive effects on cognition and function in vascular dementia. Memantine, a N-methyl-D-aspartate receptor antagonist, is FDA-approved for moderate to severe Alzheimer’s dementia.12
DELAYING DECLINE
Controlling risk factors. Controlling vascular risk factors—especially high blood pressure—is the most effective way to prevent or treat vascular dementia. In primary prevention studies, patients with good hypertension and hyperlipidemia control developed dementia more slowly than did nontreated cohorts.
In patients with coronary artery disease, statins have been shown to lower cholesterol and stabilize pre-existing plaques in the arterial wall, reducing the risk of plaque rupture. Low-density lipoproteincholesterol goals vary according to vascular risk factors but should be <100 mg/dL for patients with vascular dementia, who are at highest risk. Blood pressure goals are ≤140 mm Hg (systolic) and ≤90 mm Hg (diastolic).
Glycemic control (fasting blood glucose <110 mg/dL) and smoking cessation can also reduce the risk of further vascular events. Most patients should be taking an antiplatelet medication, preferably aspirin, to reduce clotting risk.
Although Lewy body dementia has no known risk factors other than age, research will determine whether vascular or other factors contribute to its development.
CASE CONTINUED: TARGETING AGGRESSION
Ms. Z was given divalproex, 250 mg bid, to reduce her frequent aggression. Her visual hallucinations were considered mild and not problematic and therefore were not treated. She responded well to the medication, allowing her to remain in day care and avoid nursing home placement.
Related resources
- Alzheimer’s Association. http://www.alz.org
- American Geriatrics Society. http://www.americangeriatrics.org
Drug brand names
- Carbamazepine • Tegretol, others
- Carbidopa/Levodopa • Various
- Divalproex • Depakote
- Donepezil • Aricept
- Galantamine • Reminyl
- Hydrocodone/acetaminophen • Vicodin, others
- Lisinopril/hydrochlorothiazide • Prinzide, Zestoretic
- Memantine • Namenda
- Risperdone • Risperdal
- Rivastigmine • Exelon
Disclosure
Dr. Bartz is a speaker for Forest Pharmaceuticals and Novartis Pharmaceuticals Corp.
1. McKeith JG, Ballard CG, Perry RH, et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology 2000;54:1050-8.
2. Roman GC, Erkinjuntti T, Wallin A, et al. Subcortical ischemic vascular dementia. Lancet Neurol 2002;17:426-36.
3. Pohjasraara T, Mantyla R, Ylikoski MA, et al. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. Stroke 2000;31:2952-7.
4. Del Ser T, McKeith I, Anand R, et al. Dementia with Lewy bodies: findings from an international multicenter study. Int J Geriatr Psychiatry 2000;15:1034-45.
5. Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol 1975;32:632-7.
6. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47:1113-24.
7. Luis CA, Barker WW, Gajaraj K, et al. Sensitivity and specificity of three clinical criteria for dementia with Lewy bodies in an autopsy-verified sample. Int J Geriatr Psychiatry 1999;14:526-33.
8. Barber R, Ballard C, McKeith IG, et al. MRI volumetric study of dementia with Lewy bodies: a comparison with AD and vascular dementia. Neurology 2000;54:1304-9.
9. Lobotesis K, Fenwick JD, Phipps A, et al. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology 2001;56:643-9.
10. Pratt RD, Perdomo CA. Results of clinical studies with donepezil in vascular dementia. Am J Geriatr Psychiatry 2002;10(suppl 1):88-9.
11. Wild R, Pettit T, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database Syst Rev 2003;3:CD003672.-
12. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 2004;291:317-24.
1. McKeith JG, Ballard CG, Perry RH, et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology 2000;54:1050-8.
2. Roman GC, Erkinjuntti T, Wallin A, et al. Subcortical ischemic vascular dementia. Lancet Neurol 2002;17:426-36.
3. Pohjasraara T, Mantyla R, Ylikoski MA, et al. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. Stroke 2000;31:2952-7.
4. Del Ser T, McKeith I, Anand R, et al. Dementia with Lewy bodies: findings from an international multicenter study. Int J Geriatr Psychiatry 2000;15:1034-45.
5. Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol 1975;32:632-7.
6. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47:1113-24.
7. Luis CA, Barker WW, Gajaraj K, et al. Sensitivity and specificity of three clinical criteria for dementia with Lewy bodies in an autopsy-verified sample. Int J Geriatr Psychiatry 1999;14:526-33.
8. Barber R, Ballard C, McKeith IG, et al. MRI volumetric study of dementia with Lewy bodies: a comparison with AD and vascular dementia. Neurology 2000;54:1304-9.
9. Lobotesis K, Fenwick JD, Phipps A, et al. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology 2001;56:643-9.
10. Pratt RD, Perdomo CA. Results of clinical studies with donepezil in vascular dementia. Am J Geriatr Psychiatry 2002;10(suppl 1):88-9.
11. Wild R, Pettit T, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database Syst Rev 2003;3:CD003672.-
12. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 2004;291:317-24.