User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Treatment-resistant schizophrenia: What role for mood stabilizers?
In patients with treatment-resistant schizophrenia, lithium and anticonvulsants have become such common adjuncts to antipsychotics that 50% of inpatients may be receiving them.1 Evidence supporting this practice is mixed:
- Initial case reports and open-label studies that showed benefit have not always been followed by randomized clinical trials.
- Lack of clear benefit also has been described (Table 1).
This article examines the extent of this prescribing pattern, its evidence base, and mechanisms of action that may help explain why some adjunctive mood stabilizers are more effective than others for treatment-resistant schizophrenia.
EXTENT OF USE
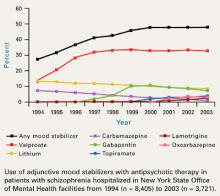
For 5 years, the rate at which inpatients with schizophrenia received adjunctive mood stabilizers has held steady at approximately 50% in New York State Office of Mental Health (NYSOMH) facilities (Figure1). These facilities provide intermediate and long-term care for the seriously, persistently mentally ill. Adjunctive mood stabilizers might not be used as often for outpatients or for inpatients treated in short-stay facilities.
Table 1
Evidence for adjunctive use of lithium or anticonvulsants for treating schizophrenia
| Agent | Case reports and open studies | Randomized, double-blind trials | Benefit? |
|---|---|---|---|
| Lithium | Yes (many) | Yes (several, +/-) | Probably not |
| Carbamazepine | Yes (many) | Yes (several; small total sample) | Limited |
| Valproate | Yes (many) | Yes | Yes |
| Gabapentin | Yes (very few, +/-) | None | Probably not |
| Lamotrigine | Yes (few, +/-) | Yes (2, +) | Yes |
| Topiramate | Yes (very few, +/-) | Yes (1, +/-) | Probably not |
| Oxcarbazepine | Yes (very few, +/-) | None | Probably not |
| + = positive results | |||
| - = negative results | |||
| +/- = both positive and negative results | |||
Valproate is the most commonly used anticonvulsant, with one out of three patients with schizophrenia receiving it. Adjunctive gabapentin use is declining, probably because of inadequate efficacy—as will be discussed later. Use of adjunctive lamotrigine is expected to increase as more data become available on its usefulness in treatment-resistant schizophrenia.
Clinicians are using substantial dosages of adjunctive mood stabilizers. During first-quarter 2004, average daily dosages for 4,788 NYSOMH patients (80% with schizophrenia or schizoaffective disorder) receiving antipsychotics were:
- valproate, 1639 mg (n = 1921)
- gabapentin, 1524 mg (n = 303)
- oxcarbazepine, 1226 mg (n = 201)
- carbamazepine, 908 mg (n = 112)
- lithium, 894 mg (n = 715)
- topiramate, 234 mg (n = 269)
- lamotrigine, 204 mg (n = 231).2
Mood-stabilizer combinations were also used. Approximately one-half of patients receiving adjunctive mood stabilizers—with the exception of valproate—were receiving more than one. In patients receiving valproate, the rate of mood-stabilizer co-prescribing was about 25%.2
WHAT IS THE EVIDENCE?
Evidence supporting the use of adjunctive lithium or anticonvulsants to treat schizophrenia varies in quality and quantity (Table 1). Case reports and open studies offer the weakest evidence but can spur double-blind, randomized clinical trials (RCTs). Unfortunately, RCTs are not often done, and the published studies usually suffer from methodologic flaws such as:
- inadequate number of subjects (insufficient statistical power to detect differences)
- lack of control of confounds such as mood symptoms (seen when studies include patients with schizoaffective disorder)
- inadequate duration
- inappropriate target populations (patients with acute exacerbations of schizophrenia instead of persistent symptoms in treatment-resistant schizophrenia).
Because controlled trials of the use of adjunctive mood stabilizers are relatively scarce, clinical practice has transcended clinical research. Clinicians need effective regimens for treatment-resistant schizophrenia, and mood-stabilizer augmentation helps some patients.
Lithium is perhaps the best-known mood stabilizer. Although early studies showed adjunctive lithium useful in treating schizophrenia, later and better-designed trials did not. The authors of a recent meta-analysis of randomized clinical trials (n = 611 in 20 studies) concluded that despite some evidence supporting the efficacy of lithium augmentation, overall results were inconclusive. A large trial would be required to detect a small benefit in patients with schizophrenia who lack affective symptoms.3
Carbamazepine use among patients with schizophrenia is declining, primarily because this drug induces its own metabolism and can require frequent dose adjustments. Adjunctive carbamazepine has been used to manage persistent aggressive behavior in patients with schizophrenia and schizoaffective disorder. Evidence comes primarily from small trials or case reports (Table 2),4-8 but results of a larger clinical trial (n = 162) by Okuma et al6 are also available
In the Okuma report—a double-blind, placebo-controlled trial of carbamazepine in patients with DSM-III schizophrenia or schizoaffective disorder—carbamazepine did not significantly improve patients’ total Brief Psychiatric Rating Scale (BPRS) scores. Compared with placebo, however, some benefit with carbamazepine did emerge in measures of suspiciousness, uncooperativeness, and excitement.
A systematic review and meta-analysis (n = 283 in 10 studies) detected a trend toward reduced psychopathology with carbamazepine augmentation for schizophrenia. BPRS scores declined by 20% and 35% in the six trials (n = 147) for which data were available (P = 0.08 and 0.09, respectively).9 Because the double-blind trial by Okuma et al6 was not randomized, it was not included in this meta-analysis.9
Figure 1 10-year trend in use of adjunctive mood stabilizers for schizophrenia
Valproate. Among the anticonvulsants, the greatest body of evidence supports the use of valproate in patients with schizophrenia,10 although a recent meta-analysis (n = 378 in 5 studies) indicates inconsistent beneficial effects.11
Initial double-blind, RCTs of adjunctive valproate in patients with schizophrenia were limited in size and failed to show benefit with adjunctive valproate12-15 (Table 2). A more recent study16 showed that adjunctive valproate affects acute psychotic symptoms rather than mood. This study, however, did not answer whether adjunctive valproate would help patients with persistent symptoms of schizophrenia.
Table 2
Double-blind studies of adjunctive carbamazepine in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Neppe (1983) | 11 | 42 | Crossover | Mixed, 8 with schizophrenia | “Overall clinical rating” improved |
| Dose et al (1987) | 22 | 28 | HAL + CBZ vs HAL + placebo | Schizophrenia or schizoaffective disorder | No difference on BPRS |
| Okuma et al (1989) | 162 | 28 | NL + CBZ vs NL + placebo | Schizophrenia or schizoaffective disorder | No difference on BPRS; possible improvement in suspiciousness, uncooperativeness, and excitement |
| Nachshoni et al (1994) | 28 | 49 | NL + CBZ vs NL + placebo | “Residual schizophrenia with negative symptoms” | No difference on BPRS or SANS |
| Simhandl et al (1996) | 42 | 42 | NL + CBZ vs NL + lithium vs NL + placebo | Schizophrenia(treatment- nonresponsive) | No difference on BPRS; CGI improved from baseline in groups receiving CBZ and lithium |
| n = number of subjects | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| CGI = Clinical Global Impression scale | |||||
| CBZ = carbamazepine | |||||
| HAL = haloperidol | |||||
| NL = neuroleptic (first-generation antipsychotic) | |||||
| SANS = Scale for the Assessment of Negative Symptoms | |||||
In this large (n = 249), multicenter, randomized, double-blind trial, hospitalized patients with an acute exacerbation of schizophrenia received olanzapine or risperidone plus divalproex or placebo for 28 days. Patients with schizoaffective disorder and treatment-resistant schizophrenia were excluded.
By day 6, dosages reached 6 mg/d for risperidone and 15 mg/d for olanzapine. Divalproex was started at 15 mg/kg and titrated to a maximum of 30 mg/kg by day 14. Mean divalproex dosage was approximately 2300 mg/d (mean plasma level approximately 100 mg/mL).
Positive and Negative Syndrome Scale (PANSS) total scores improved significantly in patients receiving adjunctive divalproex compared with those receiving antipsychotic monotherapy, and significant differences occurred as early as day 3. The major effect was seen on schizophrenia’s positive symptoms. A post-hoc analysis17 also showed greater reductions in hostility (as measured by the hostility item in the PANSS Positive Subscale) in patients receiving adjunctive divalproex compared with antipsychotic monotherapy. This effect was independent of the effect on positive symptoms or sedation.
A large, multi-site, 84-day acute schizophrenia RCT similar to the 28-day trial—but using extended-release divalproex—is being conducted. An extended-release preparation may be particularly helpful in encouraging medication adherence for patients taking complicated medication regimens.
Table 3
Double-blind studies of adjunctive valproate in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Ko et al (1985) | 6 | 28 | Crossover | Neurolepticresistant patients with chronic schizophrenia, not exacerbation | No valproate effect noted |
| Fisk and York (1987) | 62 | 42 | Antipsychotic + valproate vs antipsychotic + placebo | Chronic psychosis and tardive dyskinesia | No differences in mental state and behavior, as measured by“ Krawiecka scale”* |
| Dose et al (1998) | 42 | 28 | HAL + valproate vs HAL + placebo | Acute, nonmanic schizophrenic or schizoaffective psychosis | No difference on BPRS; possible effect on “hostile belligerence” |
| Wassef et al (2000) | 12 | 21 | HAL + valproate vs HAL + placebo | Acute exacerbation of chronic schizophrenia | CGI and SANS scores improved significantly, but BPRS scores did not |
| Casey et al (2003) | 249 | 28 | RIS + valproate vs OLZ + valproate vs RIS + placebo vs OLZ + placebo | Acute exacerbation of schizophrenia | PANSS scores improved |
| * Krawiecka M, Goldberg D, Vaughan M. A standardized psychiatric assessment scale for rating chronic psychotic patients. Acta Psychiatr Scand 1977;55(4):299-308. | |||||
| n = number of subjects | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| CGI = Clinical Global Impression scale | |||||
| HAL = haloperidol | |||||
| OLZ = olanzapine | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
| RIS = risperidone | |||||
| SANS = Scale for the Assessment of Negative Symptoms | |||||
In a recent large (n = 10,262), retrospective, pharmacoepidemiologic analysis,18 valproate augmentation led to longer persistence of treatment than did the strategy of switching antipsychotics. Average valproate dosages were small, however (<425 mg/d), as were antipsychotic dosages (risperidone <1.7 mg/d, quetiapine <120 mg/d, and olanzapine <7.5 mg/d). Patients’ diagnostic categories were not available. One interpretation of this study is that valproate augmentation would be more successful than switching antipsychotics, assuming that treatment persistence can be viewed as a positive outcome.
Table 4
Double-blind studies of adjunctive lamotrigine in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Tiihonen et al (2003) | 34 | 84 | Crossover; clozapine with or without lamotrigine | Clozapine-resistant male inpatients with chronic schizophrenia, not exacerbation | BPRS, PANSS positive, and PANSS general psychopathology symptom scores improved Negative symptoms did not improve |
| Kremer et al (2004) | 38 | 70 | Antipsychotic* + lamotrigine vs antipsychotic* + placebo | Treatment- resistant inpatients with schizophrenia | Completers’ PANSS positive, general psychopathology and total symptom scores improved No difference in negative symptoms or total BPRS scores No difference with intent-to-treat analyses |
| n = number of patients | |||||
| * First- or second-generation antipsychotic | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
Lamotrigine is the only other anticonvulsant for which published, double-blind, randomized evidence of use in patients with schizophrenia is available (Table 4).19,20 Adjunctive lamotrigine may be effective in managing treatment-resistant schizophrenia, as was shown in a small (n = 34), double-blind, placebo-controlled, crossover trial.19 Hospitalized patients whose symptoms were inadequately controlled with clozapine monotherapy received lamotrigine, 200 mg/d, for up to 12 weeks. Adjunctive lamotrigine improved positive but not negative symptoms.
Similar results were seen in treatment-resistant inpatients with schizophrenia (n = 38) in a 10-week, double-blind, parallel group trial by Kremer et al.20 Adjunctive lamotrigine improved PANSS positive, general psychopathology, and total symptom scores in the 31 patients who completed the trial. No differences were seen, however, in negative symptoms, total BPRS scores, or in the intent-to-treat analysis. These results have spurred the launch of a large, multi-site, RCT of adjunctive lamotrigine in patients with schizophrenia who have responded inadequately to antipsychotics alone.
Topiramate, one of the few psychotropics associated with weight loss, has attracted interest as an adjunct to second-generation antipsychotics to address weight gain. Although case reports have shown benefit,21 one showed deterioration in both positive and negative symptoms when topiramate was added to second-generation antipsychotics.22
Table 5
Double-blind study of adjunctive topiramate in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Tiihonen (2004)* | 26 | 84 | Crossover; SGA plus topiramate or placebo | Treatment- resistant male inpatients with chronic schizophrenia | PANSS general scores improved No difference in total PANSS, PANSS positive, or PANSS negative scores |
| * 2004 Collegium Internationale Neuro-Psychopharmacologicum (CINP) presentation, and personal communication (6/22/04) | |||||
| n = number of patients | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
| SGA = Second-generation antipsychotic (patients were taking clozapine, olanzapine, or quetiapine) | |||||
An unpublished, randomized, crossover trial compared second-generation antipsychotics plus topiramate or placebo in 26 male inpatients with chronic schizophrenia. With adjunctive topiramate, the authors found a statistically significant improvement in the PANSS general psychopathology subscale but not in PANSS total, positive subscale, or negative subscale scores (Table 5) (Tiihonen J, personal communication 6/22/04).
Other agents. Very little information—all uncontrolled—supports adjunctive use of gabapentin or oxcarbazepine for patients with schizophrenia.23-28 Of concern are reports of patients suffering worsening of psychosis with gabapentin25 or of dysphoria and irritability with oxcarbazepine (attributed to a pharmacokinetic interaction).26
Conclusion. More trials are needed to examine the use of adjunctive mood stabilizers in patients with schizophrenia—particularly in those with chronic symptoms. Although mood stabilizers are widely used in this population, important questions remain unanswered, including:
- characteristics of patients likely to require adjunctive treatment
- how long treatment should continue.
MECHANISMS OF ACTION
Unlike antipsychotics, mood stabilizers do not exert their therapeutic effects by acting directly on dopamine (D2) receptors. Differences in mechanism of action among the anticonvulsants may help explain why some—such as valproate and lamotrigine—have been useful for bipolar disorder or schizophrenia and others—such as gabapentin—have not.29
One possibility is that anticonvulsants that affect voltage-gated sodium channels—such as valproate, lamotrigine, carbamazepine and oxcarbazepine—may be most useful for patients with bipolar disorder or schizophrenia. On the other hand, agents that affect voltage-gated calcium channels—such as gabapentin—may be efficacious as anticonvulsants but not as efficacious for bipolar disorder or schizophrenia.
Ketter et al30 proposed an anticonvulsant classification system based on predominant psychotropic profiles:
- the “GABA-ergic” group predominantly potentiates the inhibitory neurotransmitter GABA, resulting in sedation, fatigue, cognitive slowing, and weight gain, as well as possible anxiolytic and antimanic effects
- the “anti-glutamate” group predominantly attenuates glutamate excitatory neurotransmission and is associated with activation, weight loss, and possibly anxiogenic and antidepressant effects.
In the GABA-ergic group are anticonvulsants such as barbiturates, benzodiazepines, valproate, gabapentin, tiagabine, and vigabatrin. The antiglutamate group includes agents such as felbamate and lamotrigine. A “mixed” category includes anticonvulsants with GABA-ergic and anti-glutaminergic actions such as topiramate, which has sedating and weight-loss properties.
Because GABA appears to modulate dopamine neurotransmission,31 this may explain valproate’s role as an adjunctive agent for schizophrenia. Similarly, mechanisms related to Nmethyl-D-aspartate (NMDA) and non-NMDA glutamate receptor function may explain lamotrigine’s usefulness in this setting.19,20
SUMMARY
Clinicians resort to combination therapies when monotherapies fail to adequately control symptoms or maintain response. Co-prescribing of anticonvulsants with antipsychotics for inpatients with schizophrenia is common practice in New York State and most likely elsewhere. In general, antipsychotics’ and mood stabilizers’ different—and perhaps complementary—mechanisms of action explain the synergism between them. Mechanisms of action also may explain why some anticonvulsants help in schizophrenia (or bipolar disorder) whereas others do not.
Evidence for using adjunctive anticonvulsants is variable. The strongest data support using valproate (and perhaps lamotrigine), followed by carbamazepine and then topiramate. Gabapentin and oxcarbazepine have only anecdotal evidence, some of it negative. Well-designed, randomized clinical trials with the appropriate populations are needed.
Related resources
- Stahl SM. Essential psychopharmacology of antipsychotics and mood stabilizers. New York: Cambridge University Press, 2002.
- Harvard Medical School Department of Psychiatry’s psychopharmacology algorithm project. Osser DN, Patterson RD. Consultant for the pharmacotherapy of schizophrenia. Available at http://mhc.com/Algorithms/. Accessed Nov. 5, 2004.
Drug brand names
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Lithobid, Eskalith
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Topiramate • Topamax
- Valproate (valproic acid, divalproex sodium) • Depakene, Depakote
Disclosure
Dr. Citrome receives research grants/contracts from Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly & Co., Janssen Pharmaceutica, and Pfizer Inc. He is a consultant to and/or speaker for Bristol-Myers Squibb Co., Eli Lilly & Co., Pfizer Inc., Abbott Laboratories, AstraZeneca Pharmaceuticals, and Novartis Pharmaceuticals Corp.
Acknowledgment
Adapted from Citrome L. “Antipsychotic polypharmacy versus augmentation with anticonvulsants: the U.S. perspective” (presentation). Paris: Collegium Internationale Neuro-Psychopharmacologicum (CINP), June 2004 [abstract in Int J Neuropsychopharmacol. 2004; 7(suppl 1):S69], and from Citrome L. “Mood-stabilizer use in schizophrenia: 1994-2002” (NR350) (poster). New York: American Psychiatric Association annual meeting, May 2004.
1. Citrome L, Jaffe A, Levine J. Datapoints - mood stabilizers: utilization trends in patients diagnosed with schizophrenia 1994-2001. Psychiatr Serv 2002;53(10):1212.-
2. Citrome L. Antipsychotic polypharmacy versus augmentation with anticonvulsants: the U.S.perspective (presentation). Paris: Collegium Internationale Neuro-Psychopharmacologicum(CINP), June 2004 [abstract in Int J Neuropsychopharmacol 2004;7(suppl 1):S69].
3. Leucht S, Kissling W, McGrath J. Lithium for schizophrenia revisited: a systematic review and meta-analysis of randomized clinical trials. J Clin Psychiatry 2004;65(2):177-86.
4. Neppe VM. Carbamazepine as adjunctive treatment in nonepileptic chronic inpatients with EEG temporal lobe abnormalities. J Clin Psychiatry 1983;44:326-31.
5. Dose M, Apelt S, Emrich HM. Carbamazepine as an adjunct of antipsychotic therapy. Psychiatry Res 1987;22:303-10.
6. Okuma T, Yamashita I, Takahashi R, et al. A double-blind study of adjunctive carbamazepine versus placebo on excited states of schizophrenic and schizoaffective disorders. Acta Psychiatr Scand 1989;80:250-9.
7. Nachshoni T, Levin Y, Levy A, et al. A double-blind trial of carbamazepine in negative symptom schizophrenia. Biol Psychiatry 1994;35(1):22-26.
8. Simhandl C, Meszaros K, Denk E, et al. Adjunctive carbamazepine or lithium carbonate in therapy-resistant chronic schizophrenia. Can J Psychiatry 1996;41(5):317.-
9. Leucht S, McGrath J, White P, et al. Carbamazepine augmentation for schizophrenia: how good is the evidence? J Clin Psychiatry 2002;63(3):218-24.
10. Citrome L. Schizophrenia and valproate. Psychopharmacol Bull 2003;37(suppl 2):74-88.
11. Basan A, Kissling W, Leucht S. Valproate as an adjunct to antipsychotics for schizophrenia: a systematic review of randomized trials. Schizophr Res 2004;70(1):33-7.
12. Ko GN, Korpi ER, Freed WJ, et al. Effect of valproic acid on behavior and plasma amino acid concentrations in chronic schizophrenia patients. Biol Psychiatry 1985;20:209-15.
13. Dose M, Hellweg R, Yassouridis A, et al. Combined treatment of schizophrenic psychoses with haloperidol and valproate. Pharmacopsychiatry 1998;31(4):122-5.
14. Fisk GG, York SM. The effect of sodium valproate on tardive dyskinesia—revisited. Br J Psychiatry 1987;150:542-6.
15. Wassef AA, Dott SG, Harris A, et al. Randomized, placebo-controlled pilot study of divalproex sodium in the treatment of acute exacerbations of chronic schizophrenia. J Clin Psychopharmacol 2000;20(3):357-361.
16. Casey DE, Daniel DG, Wassef AA, et al. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacol 2003;28(1):182-92.
17. Citrome L, Casey DE, Daniel DG, et al. Effects of adjunctive valproate on hostility in patients with schizophrenia receiving olanzapine or risperidone: a double-blind multi-center study. Psychiatr Serv 2004;55(3):290-4.
18. Cramer JA, Sernyak M. Results of a naturalistic study of treatment options: switching atypical antipsychotic drugs or augmenting with valproate. Clin Ther 2004;26(6):905-14.
19. Tiihonen J, Hallikainen T, Ryynanen OP, et al. Lamotrigine in treatment-resistant schizophrenia: a randomized placebo-controlled trial. Biol Psychiatry 2003;54(11):1241-8.
20. Kremer I, Vass A, Gurelik I, et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol Psychiatry 2004;56(6):441-6.
21. Drapalski AL, Rosse RB, Peebles RR, et al. Topiramate improves deficit symptoms in a patient with schizophrenia when added to a stable regimen of antipsychotic medication. Clin Neuropharmacol 2001;24:290-4.
22. Millson RC, Owen JA, Lorberg GW, Tackaberry L. Topiramate for refractory schizophrenia. Am J Psychiatry 2002;159(4):675.-
23. Chouinard G, Beauclair L, Belanger MC. Gabapentin: long term antianxiety and hypnotic effects in psychiatric patients with comorbid anxiety-related disorders. Can J Psychiatry 1998;43:305.-
24. Megna JL, Devitt PJ, Sauro MD, Dewan MJ. Gabapentin’s effect on agitation in severely and persistently mentally ill patients. Ann Pharmacother 2002;35:12-16.
25. Jablonowski K, Margolese HC, Chouinard G. Gabapentin-induced paradoxical exacerbation of psychosis in a patient with schizophrenia. Can J Psychiatry 2002;47(10):975-6.
26. Baird P. The interactive metabolism effect of oxcarbazepine coadministered with tricyclic antidepressant therapy for OCD symptoms. J Clin Psychopharmacol 2003;23(4):419.-
27. Centorrino F, Albert MJ, Berry JM, et al. Oxcarbazepine: clinical experience with hospitalized psychiatric patients. Bipolar Disord 2003;5(5):370-4.
28. Leweke FM, Gerth CW, Koethe D, et al. Oxcarbazepine as an adjunct for schizophrenia. Am J Psychiatry 2004;161(6):1130-1.
29. Stahl SM. Psychopharmacology of anticonvulsants: do all anticonvulsants have the same mechanism of action? J Clin Psychiatry 2004;65(2):149-50.
30. Ketter TA, Wong PW. The emerging differential roles of GABAergic and antiglutaminergic agents in bipolar disorders. J Clin Psychiatry 2003;64(suppl 3):15-20.
31. Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol 2003;23(6):601-40.
In patients with treatment-resistant schizophrenia, lithium and anticonvulsants have become such common adjuncts to antipsychotics that 50% of inpatients may be receiving them.1 Evidence supporting this practice is mixed:
- Initial case reports and open-label studies that showed benefit have not always been followed by randomized clinical trials.
- Lack of clear benefit also has been described (Table 1).
This article examines the extent of this prescribing pattern, its evidence base, and mechanisms of action that may help explain why some adjunctive mood stabilizers are more effective than others for treatment-resistant schizophrenia.
EXTENT OF USE
For 5 years, the rate at which inpatients with schizophrenia received adjunctive mood stabilizers has held steady at approximately 50% in New York State Office of Mental Health (NYSOMH) facilities (Figure1). These facilities provide intermediate and long-term care for the seriously, persistently mentally ill. Adjunctive mood stabilizers might not be used as often for outpatients or for inpatients treated in short-stay facilities.
Table 1
Evidence for adjunctive use of lithium or anticonvulsants for treating schizophrenia
| Agent | Case reports and open studies | Randomized, double-blind trials | Benefit? |
|---|---|---|---|
| Lithium | Yes (many) | Yes (several, +/-) | Probably not |
| Carbamazepine | Yes (many) | Yes (several; small total sample) | Limited |
| Valproate | Yes (many) | Yes | Yes |
| Gabapentin | Yes (very few, +/-) | None | Probably not |
| Lamotrigine | Yes (few, +/-) | Yes (2, +) | Yes |
| Topiramate | Yes (very few, +/-) | Yes (1, +/-) | Probably not |
| Oxcarbazepine | Yes (very few, +/-) | None | Probably not |
| + = positive results | |||
| - = negative results | |||
| +/- = both positive and negative results | |||
Valproate is the most commonly used anticonvulsant, with one out of three patients with schizophrenia receiving it. Adjunctive gabapentin use is declining, probably because of inadequate efficacy—as will be discussed later. Use of adjunctive lamotrigine is expected to increase as more data become available on its usefulness in treatment-resistant schizophrenia.
Clinicians are using substantial dosages of adjunctive mood stabilizers. During first-quarter 2004, average daily dosages for 4,788 NYSOMH patients (80% with schizophrenia or schizoaffective disorder) receiving antipsychotics were:
- valproate, 1639 mg (n = 1921)
- gabapentin, 1524 mg (n = 303)
- oxcarbazepine, 1226 mg (n = 201)
- carbamazepine, 908 mg (n = 112)
- lithium, 894 mg (n = 715)
- topiramate, 234 mg (n = 269)
- lamotrigine, 204 mg (n = 231).2
Mood-stabilizer combinations were also used. Approximately one-half of patients receiving adjunctive mood stabilizers—with the exception of valproate—were receiving more than one. In patients receiving valproate, the rate of mood-stabilizer co-prescribing was about 25%.2
WHAT IS THE EVIDENCE?
Evidence supporting the use of adjunctive lithium or anticonvulsants to treat schizophrenia varies in quality and quantity (Table 1). Case reports and open studies offer the weakest evidence but can spur double-blind, randomized clinical trials (RCTs). Unfortunately, RCTs are not often done, and the published studies usually suffer from methodologic flaws such as:
- inadequate number of subjects (insufficient statistical power to detect differences)
- lack of control of confounds such as mood symptoms (seen when studies include patients with schizoaffective disorder)
- inadequate duration
- inappropriate target populations (patients with acute exacerbations of schizophrenia instead of persistent symptoms in treatment-resistant schizophrenia).
Because controlled trials of the use of adjunctive mood stabilizers are relatively scarce, clinical practice has transcended clinical research. Clinicians need effective regimens for treatment-resistant schizophrenia, and mood-stabilizer augmentation helps some patients.
Lithium is perhaps the best-known mood stabilizer. Although early studies showed adjunctive lithium useful in treating schizophrenia, later and better-designed trials did not. The authors of a recent meta-analysis of randomized clinical trials (n = 611 in 20 studies) concluded that despite some evidence supporting the efficacy of lithium augmentation, overall results were inconclusive. A large trial would be required to detect a small benefit in patients with schizophrenia who lack affective symptoms.3
Carbamazepine use among patients with schizophrenia is declining, primarily because this drug induces its own metabolism and can require frequent dose adjustments. Adjunctive carbamazepine has been used to manage persistent aggressive behavior in patients with schizophrenia and schizoaffective disorder. Evidence comes primarily from small trials or case reports (Table 2),4-8 but results of a larger clinical trial (n = 162) by Okuma et al6 are also available
In the Okuma report—a double-blind, placebo-controlled trial of carbamazepine in patients with DSM-III schizophrenia or schizoaffective disorder—carbamazepine did not significantly improve patients’ total Brief Psychiatric Rating Scale (BPRS) scores. Compared with placebo, however, some benefit with carbamazepine did emerge in measures of suspiciousness, uncooperativeness, and excitement.
A systematic review and meta-analysis (n = 283 in 10 studies) detected a trend toward reduced psychopathology with carbamazepine augmentation for schizophrenia. BPRS scores declined by 20% and 35% in the six trials (n = 147) for which data were available (P = 0.08 and 0.09, respectively).9 Because the double-blind trial by Okuma et al6 was not randomized, it was not included in this meta-analysis.9
Figure 1 10-year trend in use of adjunctive mood stabilizers for schizophrenia
Valproate. Among the anticonvulsants, the greatest body of evidence supports the use of valproate in patients with schizophrenia,10 although a recent meta-analysis (n = 378 in 5 studies) indicates inconsistent beneficial effects.11
Initial double-blind, RCTs of adjunctive valproate in patients with schizophrenia were limited in size and failed to show benefit with adjunctive valproate12-15 (Table 2). A more recent study16 showed that adjunctive valproate affects acute psychotic symptoms rather than mood. This study, however, did not answer whether adjunctive valproate would help patients with persistent symptoms of schizophrenia.
Table 2
Double-blind studies of adjunctive carbamazepine in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Neppe (1983) | 11 | 42 | Crossover | Mixed, 8 with schizophrenia | “Overall clinical rating” improved |
| Dose et al (1987) | 22 | 28 | HAL + CBZ vs HAL + placebo | Schizophrenia or schizoaffective disorder | No difference on BPRS |
| Okuma et al (1989) | 162 | 28 | NL + CBZ vs NL + placebo | Schizophrenia or schizoaffective disorder | No difference on BPRS; possible improvement in suspiciousness, uncooperativeness, and excitement |
| Nachshoni et al (1994) | 28 | 49 | NL + CBZ vs NL + placebo | “Residual schizophrenia with negative symptoms” | No difference on BPRS or SANS |
| Simhandl et al (1996) | 42 | 42 | NL + CBZ vs NL + lithium vs NL + placebo | Schizophrenia(treatment- nonresponsive) | No difference on BPRS; CGI improved from baseline in groups receiving CBZ and lithium |
| n = number of subjects | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| CGI = Clinical Global Impression scale | |||||
| CBZ = carbamazepine | |||||
| HAL = haloperidol | |||||
| NL = neuroleptic (first-generation antipsychotic) | |||||
| SANS = Scale for the Assessment of Negative Symptoms | |||||
In this large (n = 249), multicenter, randomized, double-blind trial, hospitalized patients with an acute exacerbation of schizophrenia received olanzapine or risperidone plus divalproex or placebo for 28 days. Patients with schizoaffective disorder and treatment-resistant schizophrenia were excluded.
By day 6, dosages reached 6 mg/d for risperidone and 15 mg/d for olanzapine. Divalproex was started at 15 mg/kg and titrated to a maximum of 30 mg/kg by day 14. Mean divalproex dosage was approximately 2300 mg/d (mean plasma level approximately 100 mg/mL).
Positive and Negative Syndrome Scale (PANSS) total scores improved significantly in patients receiving adjunctive divalproex compared with those receiving antipsychotic monotherapy, and significant differences occurred as early as day 3. The major effect was seen on schizophrenia’s positive symptoms. A post-hoc analysis17 also showed greater reductions in hostility (as measured by the hostility item in the PANSS Positive Subscale) in patients receiving adjunctive divalproex compared with antipsychotic monotherapy. This effect was independent of the effect on positive symptoms or sedation.
A large, multi-site, 84-day acute schizophrenia RCT similar to the 28-day trial—but using extended-release divalproex—is being conducted. An extended-release preparation may be particularly helpful in encouraging medication adherence for patients taking complicated medication regimens.
Table 3
Double-blind studies of adjunctive valproate in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Ko et al (1985) | 6 | 28 | Crossover | Neurolepticresistant patients with chronic schizophrenia, not exacerbation | No valproate effect noted |
| Fisk and York (1987) | 62 | 42 | Antipsychotic + valproate vs antipsychotic + placebo | Chronic psychosis and tardive dyskinesia | No differences in mental state and behavior, as measured by“ Krawiecka scale”* |
| Dose et al (1998) | 42 | 28 | HAL + valproate vs HAL + placebo | Acute, nonmanic schizophrenic or schizoaffective psychosis | No difference on BPRS; possible effect on “hostile belligerence” |
| Wassef et al (2000) | 12 | 21 | HAL + valproate vs HAL + placebo | Acute exacerbation of chronic schizophrenia | CGI and SANS scores improved significantly, but BPRS scores did not |
| Casey et al (2003) | 249 | 28 | RIS + valproate vs OLZ + valproate vs RIS + placebo vs OLZ + placebo | Acute exacerbation of schizophrenia | PANSS scores improved |
| * Krawiecka M, Goldberg D, Vaughan M. A standardized psychiatric assessment scale for rating chronic psychotic patients. Acta Psychiatr Scand 1977;55(4):299-308. | |||||
| n = number of subjects | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| CGI = Clinical Global Impression scale | |||||
| HAL = haloperidol | |||||
| OLZ = olanzapine | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
| RIS = risperidone | |||||
| SANS = Scale for the Assessment of Negative Symptoms | |||||
In a recent large (n = 10,262), retrospective, pharmacoepidemiologic analysis,18 valproate augmentation led to longer persistence of treatment than did the strategy of switching antipsychotics. Average valproate dosages were small, however (<425 mg/d), as were antipsychotic dosages (risperidone <1.7 mg/d, quetiapine <120 mg/d, and olanzapine <7.5 mg/d). Patients’ diagnostic categories were not available. One interpretation of this study is that valproate augmentation would be more successful than switching antipsychotics, assuming that treatment persistence can be viewed as a positive outcome.
Table 4
Double-blind studies of adjunctive lamotrigine in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Tiihonen et al (2003) | 34 | 84 | Crossover; clozapine with or without lamotrigine | Clozapine-resistant male inpatients with chronic schizophrenia, not exacerbation | BPRS, PANSS positive, and PANSS general psychopathology symptom scores improved Negative symptoms did not improve |
| Kremer et al (2004) | 38 | 70 | Antipsychotic* + lamotrigine vs antipsychotic* + placebo | Treatment- resistant inpatients with schizophrenia | Completers’ PANSS positive, general psychopathology and total symptom scores improved No difference in negative symptoms or total BPRS scores No difference with intent-to-treat analyses |
| n = number of patients | |||||
| * First- or second-generation antipsychotic | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
Lamotrigine is the only other anticonvulsant for which published, double-blind, randomized evidence of use in patients with schizophrenia is available (Table 4).19,20 Adjunctive lamotrigine may be effective in managing treatment-resistant schizophrenia, as was shown in a small (n = 34), double-blind, placebo-controlled, crossover trial.19 Hospitalized patients whose symptoms were inadequately controlled with clozapine monotherapy received lamotrigine, 200 mg/d, for up to 12 weeks. Adjunctive lamotrigine improved positive but not negative symptoms.
Similar results were seen in treatment-resistant inpatients with schizophrenia (n = 38) in a 10-week, double-blind, parallel group trial by Kremer et al.20 Adjunctive lamotrigine improved PANSS positive, general psychopathology, and total symptom scores in the 31 patients who completed the trial. No differences were seen, however, in negative symptoms, total BPRS scores, or in the intent-to-treat analysis. These results have spurred the launch of a large, multi-site, RCT of adjunctive lamotrigine in patients with schizophrenia who have responded inadequately to antipsychotics alone.
Topiramate, one of the few psychotropics associated with weight loss, has attracted interest as an adjunct to second-generation antipsychotics to address weight gain. Although case reports have shown benefit,21 one showed deterioration in both positive and negative symptoms when topiramate was added to second-generation antipsychotics.22
Table 5
Double-blind study of adjunctive topiramate in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Tiihonen (2004)* | 26 | 84 | Crossover; SGA plus topiramate or placebo | Treatment- resistant male inpatients with chronic schizophrenia | PANSS general scores improved No difference in total PANSS, PANSS positive, or PANSS negative scores |
| * 2004 Collegium Internationale Neuro-Psychopharmacologicum (CINP) presentation, and personal communication (6/22/04) | |||||
| n = number of patients | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
| SGA = Second-generation antipsychotic (patients were taking clozapine, olanzapine, or quetiapine) | |||||
An unpublished, randomized, crossover trial compared second-generation antipsychotics plus topiramate or placebo in 26 male inpatients with chronic schizophrenia. With adjunctive topiramate, the authors found a statistically significant improvement in the PANSS general psychopathology subscale but not in PANSS total, positive subscale, or negative subscale scores (Table 5) (Tiihonen J, personal communication 6/22/04).
Other agents. Very little information—all uncontrolled—supports adjunctive use of gabapentin or oxcarbazepine for patients with schizophrenia.23-28 Of concern are reports of patients suffering worsening of psychosis with gabapentin25 or of dysphoria and irritability with oxcarbazepine (attributed to a pharmacokinetic interaction).26
Conclusion. More trials are needed to examine the use of adjunctive mood stabilizers in patients with schizophrenia—particularly in those with chronic symptoms. Although mood stabilizers are widely used in this population, important questions remain unanswered, including:
- characteristics of patients likely to require adjunctive treatment
- how long treatment should continue.
MECHANISMS OF ACTION
Unlike antipsychotics, mood stabilizers do not exert their therapeutic effects by acting directly on dopamine (D2) receptors. Differences in mechanism of action among the anticonvulsants may help explain why some—such as valproate and lamotrigine—have been useful for bipolar disorder or schizophrenia and others—such as gabapentin—have not.29
One possibility is that anticonvulsants that affect voltage-gated sodium channels—such as valproate, lamotrigine, carbamazepine and oxcarbazepine—may be most useful for patients with bipolar disorder or schizophrenia. On the other hand, agents that affect voltage-gated calcium channels—such as gabapentin—may be efficacious as anticonvulsants but not as efficacious for bipolar disorder or schizophrenia.
Ketter et al30 proposed an anticonvulsant classification system based on predominant psychotropic profiles:
- the “GABA-ergic” group predominantly potentiates the inhibitory neurotransmitter GABA, resulting in sedation, fatigue, cognitive slowing, and weight gain, as well as possible anxiolytic and antimanic effects
- the “anti-glutamate” group predominantly attenuates glutamate excitatory neurotransmission and is associated with activation, weight loss, and possibly anxiogenic and antidepressant effects.
In the GABA-ergic group are anticonvulsants such as barbiturates, benzodiazepines, valproate, gabapentin, tiagabine, and vigabatrin. The antiglutamate group includes agents such as felbamate and lamotrigine. A “mixed” category includes anticonvulsants with GABA-ergic and anti-glutaminergic actions such as topiramate, which has sedating and weight-loss properties.
Because GABA appears to modulate dopamine neurotransmission,31 this may explain valproate’s role as an adjunctive agent for schizophrenia. Similarly, mechanisms related to Nmethyl-D-aspartate (NMDA) and non-NMDA glutamate receptor function may explain lamotrigine’s usefulness in this setting.19,20
SUMMARY
Clinicians resort to combination therapies when monotherapies fail to adequately control symptoms or maintain response. Co-prescribing of anticonvulsants with antipsychotics for inpatients with schizophrenia is common practice in New York State and most likely elsewhere. In general, antipsychotics’ and mood stabilizers’ different—and perhaps complementary—mechanisms of action explain the synergism between them. Mechanisms of action also may explain why some anticonvulsants help in schizophrenia (or bipolar disorder) whereas others do not.
Evidence for using adjunctive anticonvulsants is variable. The strongest data support using valproate (and perhaps lamotrigine), followed by carbamazepine and then topiramate. Gabapentin and oxcarbazepine have only anecdotal evidence, some of it negative. Well-designed, randomized clinical trials with the appropriate populations are needed.
Related resources
- Stahl SM. Essential psychopharmacology of antipsychotics and mood stabilizers. New York: Cambridge University Press, 2002.
- Harvard Medical School Department of Psychiatry’s psychopharmacology algorithm project. Osser DN, Patterson RD. Consultant for the pharmacotherapy of schizophrenia. Available at http://mhc.com/Algorithms/. Accessed Nov. 5, 2004.
Drug brand names
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Lithobid, Eskalith
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Topiramate • Topamax
- Valproate (valproic acid, divalproex sodium) • Depakene, Depakote
Disclosure
Dr. Citrome receives research grants/contracts from Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly & Co., Janssen Pharmaceutica, and Pfizer Inc. He is a consultant to and/or speaker for Bristol-Myers Squibb Co., Eli Lilly & Co., Pfizer Inc., Abbott Laboratories, AstraZeneca Pharmaceuticals, and Novartis Pharmaceuticals Corp.
Acknowledgment
Adapted from Citrome L. “Antipsychotic polypharmacy versus augmentation with anticonvulsants: the U.S. perspective” (presentation). Paris: Collegium Internationale Neuro-Psychopharmacologicum (CINP), June 2004 [abstract in Int J Neuropsychopharmacol. 2004; 7(suppl 1):S69], and from Citrome L. “Mood-stabilizer use in schizophrenia: 1994-2002” (NR350) (poster). New York: American Psychiatric Association annual meeting, May 2004.
In patients with treatment-resistant schizophrenia, lithium and anticonvulsants have become such common adjuncts to antipsychotics that 50% of inpatients may be receiving them.1 Evidence supporting this practice is mixed:
- Initial case reports and open-label studies that showed benefit have not always been followed by randomized clinical trials.
- Lack of clear benefit also has been described (Table 1).
This article examines the extent of this prescribing pattern, its evidence base, and mechanisms of action that may help explain why some adjunctive mood stabilizers are more effective than others for treatment-resistant schizophrenia.
EXTENT OF USE
For 5 years, the rate at which inpatients with schizophrenia received adjunctive mood stabilizers has held steady at approximately 50% in New York State Office of Mental Health (NYSOMH) facilities (Figure1). These facilities provide intermediate and long-term care for the seriously, persistently mentally ill. Adjunctive mood stabilizers might not be used as often for outpatients or for inpatients treated in short-stay facilities.
Table 1
Evidence for adjunctive use of lithium or anticonvulsants for treating schizophrenia
| Agent | Case reports and open studies | Randomized, double-blind trials | Benefit? |
|---|---|---|---|
| Lithium | Yes (many) | Yes (several, +/-) | Probably not |
| Carbamazepine | Yes (many) | Yes (several; small total sample) | Limited |
| Valproate | Yes (many) | Yes | Yes |
| Gabapentin | Yes (very few, +/-) | None | Probably not |
| Lamotrigine | Yes (few, +/-) | Yes (2, +) | Yes |
| Topiramate | Yes (very few, +/-) | Yes (1, +/-) | Probably not |
| Oxcarbazepine | Yes (very few, +/-) | None | Probably not |
| + = positive results | |||
| - = negative results | |||
| +/- = both positive and negative results | |||
Valproate is the most commonly used anticonvulsant, with one out of three patients with schizophrenia receiving it. Adjunctive gabapentin use is declining, probably because of inadequate efficacy—as will be discussed later. Use of adjunctive lamotrigine is expected to increase as more data become available on its usefulness in treatment-resistant schizophrenia.
Clinicians are using substantial dosages of adjunctive mood stabilizers. During first-quarter 2004, average daily dosages for 4,788 NYSOMH patients (80% with schizophrenia or schizoaffective disorder) receiving antipsychotics were:
- valproate, 1639 mg (n = 1921)
- gabapentin, 1524 mg (n = 303)
- oxcarbazepine, 1226 mg (n = 201)
- carbamazepine, 908 mg (n = 112)
- lithium, 894 mg (n = 715)
- topiramate, 234 mg (n = 269)
- lamotrigine, 204 mg (n = 231).2
Mood-stabilizer combinations were also used. Approximately one-half of patients receiving adjunctive mood stabilizers—with the exception of valproate—were receiving more than one. In patients receiving valproate, the rate of mood-stabilizer co-prescribing was about 25%.2
WHAT IS THE EVIDENCE?
Evidence supporting the use of adjunctive lithium or anticonvulsants to treat schizophrenia varies in quality and quantity (Table 1). Case reports and open studies offer the weakest evidence but can spur double-blind, randomized clinical trials (RCTs). Unfortunately, RCTs are not often done, and the published studies usually suffer from methodologic flaws such as:
- inadequate number of subjects (insufficient statistical power to detect differences)
- lack of control of confounds such as mood symptoms (seen when studies include patients with schizoaffective disorder)
- inadequate duration
- inappropriate target populations (patients with acute exacerbations of schizophrenia instead of persistent symptoms in treatment-resistant schizophrenia).
Because controlled trials of the use of adjunctive mood stabilizers are relatively scarce, clinical practice has transcended clinical research. Clinicians need effective regimens for treatment-resistant schizophrenia, and mood-stabilizer augmentation helps some patients.
Lithium is perhaps the best-known mood stabilizer. Although early studies showed adjunctive lithium useful in treating schizophrenia, later and better-designed trials did not. The authors of a recent meta-analysis of randomized clinical trials (n = 611 in 20 studies) concluded that despite some evidence supporting the efficacy of lithium augmentation, overall results were inconclusive. A large trial would be required to detect a small benefit in patients with schizophrenia who lack affective symptoms.3
Carbamazepine use among patients with schizophrenia is declining, primarily because this drug induces its own metabolism and can require frequent dose adjustments. Adjunctive carbamazepine has been used to manage persistent aggressive behavior in patients with schizophrenia and schizoaffective disorder. Evidence comes primarily from small trials or case reports (Table 2),4-8 but results of a larger clinical trial (n = 162) by Okuma et al6 are also available
In the Okuma report—a double-blind, placebo-controlled trial of carbamazepine in patients with DSM-III schizophrenia or schizoaffective disorder—carbamazepine did not significantly improve patients’ total Brief Psychiatric Rating Scale (BPRS) scores. Compared with placebo, however, some benefit with carbamazepine did emerge in measures of suspiciousness, uncooperativeness, and excitement.
A systematic review and meta-analysis (n = 283 in 10 studies) detected a trend toward reduced psychopathology with carbamazepine augmentation for schizophrenia. BPRS scores declined by 20% and 35% in the six trials (n = 147) for which data were available (P = 0.08 and 0.09, respectively).9 Because the double-blind trial by Okuma et al6 was not randomized, it was not included in this meta-analysis.9
Figure 1 10-year trend in use of adjunctive mood stabilizers for schizophrenia
Valproate. Among the anticonvulsants, the greatest body of evidence supports the use of valproate in patients with schizophrenia,10 although a recent meta-analysis (n = 378 in 5 studies) indicates inconsistent beneficial effects.11
Initial double-blind, RCTs of adjunctive valproate in patients with schizophrenia were limited in size and failed to show benefit with adjunctive valproate12-15 (Table 2). A more recent study16 showed that adjunctive valproate affects acute psychotic symptoms rather than mood. This study, however, did not answer whether adjunctive valproate would help patients with persistent symptoms of schizophrenia.
Table 2
Double-blind studies of adjunctive carbamazepine in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Neppe (1983) | 11 | 42 | Crossover | Mixed, 8 with schizophrenia | “Overall clinical rating” improved |
| Dose et al (1987) | 22 | 28 | HAL + CBZ vs HAL + placebo | Schizophrenia or schizoaffective disorder | No difference on BPRS |
| Okuma et al (1989) | 162 | 28 | NL + CBZ vs NL + placebo | Schizophrenia or schizoaffective disorder | No difference on BPRS; possible improvement in suspiciousness, uncooperativeness, and excitement |
| Nachshoni et al (1994) | 28 | 49 | NL + CBZ vs NL + placebo | “Residual schizophrenia with negative symptoms” | No difference on BPRS or SANS |
| Simhandl et al (1996) | 42 | 42 | NL + CBZ vs NL + lithium vs NL + placebo | Schizophrenia(treatment- nonresponsive) | No difference on BPRS; CGI improved from baseline in groups receiving CBZ and lithium |
| n = number of subjects | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| CGI = Clinical Global Impression scale | |||||
| CBZ = carbamazepine | |||||
| HAL = haloperidol | |||||
| NL = neuroleptic (first-generation antipsychotic) | |||||
| SANS = Scale for the Assessment of Negative Symptoms | |||||
In this large (n = 249), multicenter, randomized, double-blind trial, hospitalized patients with an acute exacerbation of schizophrenia received olanzapine or risperidone plus divalproex or placebo for 28 days. Patients with schizoaffective disorder and treatment-resistant schizophrenia were excluded.
By day 6, dosages reached 6 mg/d for risperidone and 15 mg/d for olanzapine. Divalproex was started at 15 mg/kg and titrated to a maximum of 30 mg/kg by day 14. Mean divalproex dosage was approximately 2300 mg/d (mean plasma level approximately 100 mg/mL).
Positive and Negative Syndrome Scale (PANSS) total scores improved significantly in patients receiving adjunctive divalproex compared with those receiving antipsychotic monotherapy, and significant differences occurred as early as day 3. The major effect was seen on schizophrenia’s positive symptoms. A post-hoc analysis17 also showed greater reductions in hostility (as measured by the hostility item in the PANSS Positive Subscale) in patients receiving adjunctive divalproex compared with antipsychotic monotherapy. This effect was independent of the effect on positive symptoms or sedation.
A large, multi-site, 84-day acute schizophrenia RCT similar to the 28-day trial—but using extended-release divalproex—is being conducted. An extended-release preparation may be particularly helpful in encouraging medication adherence for patients taking complicated medication regimens.
Table 3
Double-blind studies of adjunctive valproate in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Ko et al (1985) | 6 | 28 | Crossover | Neurolepticresistant patients with chronic schizophrenia, not exacerbation | No valproate effect noted |
| Fisk and York (1987) | 62 | 42 | Antipsychotic + valproate vs antipsychotic + placebo | Chronic psychosis and tardive dyskinesia | No differences in mental state and behavior, as measured by“ Krawiecka scale”* |
| Dose et al (1998) | 42 | 28 | HAL + valproate vs HAL + placebo | Acute, nonmanic schizophrenic or schizoaffective psychosis | No difference on BPRS; possible effect on “hostile belligerence” |
| Wassef et al (2000) | 12 | 21 | HAL + valproate vs HAL + placebo | Acute exacerbation of chronic schizophrenia | CGI and SANS scores improved significantly, but BPRS scores did not |
| Casey et al (2003) | 249 | 28 | RIS + valproate vs OLZ + valproate vs RIS + placebo vs OLZ + placebo | Acute exacerbation of schizophrenia | PANSS scores improved |
| * Krawiecka M, Goldberg D, Vaughan M. A standardized psychiatric assessment scale for rating chronic psychotic patients. Acta Psychiatr Scand 1977;55(4):299-308. | |||||
| n = number of subjects | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| CGI = Clinical Global Impression scale | |||||
| HAL = haloperidol | |||||
| OLZ = olanzapine | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
| RIS = risperidone | |||||
| SANS = Scale for the Assessment of Negative Symptoms | |||||
In a recent large (n = 10,262), retrospective, pharmacoepidemiologic analysis,18 valproate augmentation led to longer persistence of treatment than did the strategy of switching antipsychotics. Average valproate dosages were small, however (<425 mg/d), as were antipsychotic dosages (risperidone <1.7 mg/d, quetiapine <120 mg/d, and olanzapine <7.5 mg/d). Patients’ diagnostic categories were not available. One interpretation of this study is that valproate augmentation would be more successful than switching antipsychotics, assuming that treatment persistence can be viewed as a positive outcome.
Table 4
Double-blind studies of adjunctive lamotrigine in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Tiihonen et al (2003) | 34 | 84 | Crossover; clozapine with or without lamotrigine | Clozapine-resistant male inpatients with chronic schizophrenia, not exacerbation | BPRS, PANSS positive, and PANSS general psychopathology symptom scores improved Negative symptoms did not improve |
| Kremer et al (2004) | 38 | 70 | Antipsychotic* + lamotrigine vs antipsychotic* + placebo | Treatment- resistant inpatients with schizophrenia | Completers’ PANSS positive, general psychopathology and total symptom scores improved No difference in negative symptoms or total BPRS scores No difference with intent-to-treat analyses |
| n = number of patients | |||||
| * First- or second-generation antipsychotic | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
Lamotrigine is the only other anticonvulsant for which published, double-blind, randomized evidence of use in patients with schizophrenia is available (Table 4).19,20 Adjunctive lamotrigine may be effective in managing treatment-resistant schizophrenia, as was shown in a small (n = 34), double-blind, placebo-controlled, crossover trial.19 Hospitalized patients whose symptoms were inadequately controlled with clozapine monotherapy received lamotrigine, 200 mg/d, for up to 12 weeks. Adjunctive lamotrigine improved positive but not negative symptoms.
Similar results were seen in treatment-resistant inpatients with schizophrenia (n = 38) in a 10-week, double-blind, parallel group trial by Kremer et al.20 Adjunctive lamotrigine improved PANSS positive, general psychopathology, and total symptom scores in the 31 patients who completed the trial. No differences were seen, however, in negative symptoms, total BPRS scores, or in the intent-to-treat analysis. These results have spurred the launch of a large, multi-site, RCT of adjunctive lamotrigine in patients with schizophrenia who have responded inadequately to antipsychotics alone.
Topiramate, one of the few psychotropics associated with weight loss, has attracted interest as an adjunct to second-generation antipsychotics to address weight gain. Although case reports have shown benefit,21 one showed deterioration in both positive and negative symptoms when topiramate was added to second-generation antipsychotics.22
Table 5
Double-blind study of adjunctive topiramate in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Tiihonen (2004)* | 26 | 84 | Crossover; SGA plus topiramate or placebo | Treatment- resistant male inpatients with chronic schizophrenia | PANSS general scores improved No difference in total PANSS, PANSS positive, or PANSS negative scores |
| * 2004 Collegium Internationale Neuro-Psychopharmacologicum (CINP) presentation, and personal communication (6/22/04) | |||||
| n = number of patients | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
| SGA = Second-generation antipsychotic (patients were taking clozapine, olanzapine, or quetiapine) | |||||
An unpublished, randomized, crossover trial compared second-generation antipsychotics plus topiramate or placebo in 26 male inpatients with chronic schizophrenia. With adjunctive topiramate, the authors found a statistically significant improvement in the PANSS general psychopathology subscale but not in PANSS total, positive subscale, or negative subscale scores (Table 5) (Tiihonen J, personal communication 6/22/04).
Other agents. Very little information—all uncontrolled—supports adjunctive use of gabapentin or oxcarbazepine for patients with schizophrenia.23-28 Of concern are reports of patients suffering worsening of psychosis with gabapentin25 or of dysphoria and irritability with oxcarbazepine (attributed to a pharmacokinetic interaction).26
Conclusion. More trials are needed to examine the use of adjunctive mood stabilizers in patients with schizophrenia—particularly in those with chronic symptoms. Although mood stabilizers are widely used in this population, important questions remain unanswered, including:
- characteristics of patients likely to require adjunctive treatment
- how long treatment should continue.
MECHANISMS OF ACTION
Unlike antipsychotics, mood stabilizers do not exert their therapeutic effects by acting directly on dopamine (D2) receptors. Differences in mechanism of action among the anticonvulsants may help explain why some—such as valproate and lamotrigine—have been useful for bipolar disorder or schizophrenia and others—such as gabapentin—have not.29
One possibility is that anticonvulsants that affect voltage-gated sodium channels—such as valproate, lamotrigine, carbamazepine and oxcarbazepine—may be most useful for patients with bipolar disorder or schizophrenia. On the other hand, agents that affect voltage-gated calcium channels—such as gabapentin—may be efficacious as anticonvulsants but not as efficacious for bipolar disorder or schizophrenia.
Ketter et al30 proposed an anticonvulsant classification system based on predominant psychotropic profiles:
- the “GABA-ergic” group predominantly potentiates the inhibitory neurotransmitter GABA, resulting in sedation, fatigue, cognitive slowing, and weight gain, as well as possible anxiolytic and antimanic effects
- the “anti-glutamate” group predominantly attenuates glutamate excitatory neurotransmission and is associated with activation, weight loss, and possibly anxiogenic and antidepressant effects.
In the GABA-ergic group are anticonvulsants such as barbiturates, benzodiazepines, valproate, gabapentin, tiagabine, and vigabatrin. The antiglutamate group includes agents such as felbamate and lamotrigine. A “mixed” category includes anticonvulsants with GABA-ergic and anti-glutaminergic actions such as topiramate, which has sedating and weight-loss properties.
Because GABA appears to modulate dopamine neurotransmission,31 this may explain valproate’s role as an adjunctive agent for schizophrenia. Similarly, mechanisms related to Nmethyl-D-aspartate (NMDA) and non-NMDA glutamate receptor function may explain lamotrigine’s usefulness in this setting.19,20
SUMMARY
Clinicians resort to combination therapies when monotherapies fail to adequately control symptoms or maintain response. Co-prescribing of anticonvulsants with antipsychotics for inpatients with schizophrenia is common practice in New York State and most likely elsewhere. In general, antipsychotics’ and mood stabilizers’ different—and perhaps complementary—mechanisms of action explain the synergism between them. Mechanisms of action also may explain why some anticonvulsants help in schizophrenia (or bipolar disorder) whereas others do not.
Evidence for using adjunctive anticonvulsants is variable. The strongest data support using valproate (and perhaps lamotrigine), followed by carbamazepine and then topiramate. Gabapentin and oxcarbazepine have only anecdotal evidence, some of it negative. Well-designed, randomized clinical trials with the appropriate populations are needed.
Related resources
- Stahl SM. Essential psychopharmacology of antipsychotics and mood stabilizers. New York: Cambridge University Press, 2002.
- Harvard Medical School Department of Psychiatry’s psychopharmacology algorithm project. Osser DN, Patterson RD. Consultant for the pharmacotherapy of schizophrenia. Available at http://mhc.com/Algorithms/. Accessed Nov. 5, 2004.
Drug brand names
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Lithobid, Eskalith
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Topiramate • Topamax
- Valproate (valproic acid, divalproex sodium) • Depakene, Depakote
Disclosure
Dr. Citrome receives research grants/contracts from Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly & Co., Janssen Pharmaceutica, and Pfizer Inc. He is a consultant to and/or speaker for Bristol-Myers Squibb Co., Eli Lilly & Co., Pfizer Inc., Abbott Laboratories, AstraZeneca Pharmaceuticals, and Novartis Pharmaceuticals Corp.
Acknowledgment
Adapted from Citrome L. “Antipsychotic polypharmacy versus augmentation with anticonvulsants: the U.S. perspective” (presentation). Paris: Collegium Internationale Neuro-Psychopharmacologicum (CINP), June 2004 [abstract in Int J Neuropsychopharmacol. 2004; 7(suppl 1):S69], and from Citrome L. “Mood-stabilizer use in schizophrenia: 1994-2002” (NR350) (poster). New York: American Psychiatric Association annual meeting, May 2004.
1. Citrome L, Jaffe A, Levine J. Datapoints - mood stabilizers: utilization trends in patients diagnosed with schizophrenia 1994-2001. Psychiatr Serv 2002;53(10):1212.-
2. Citrome L. Antipsychotic polypharmacy versus augmentation with anticonvulsants: the U.S.perspective (presentation). Paris: Collegium Internationale Neuro-Psychopharmacologicum(CINP), June 2004 [abstract in Int J Neuropsychopharmacol 2004;7(suppl 1):S69].
3. Leucht S, Kissling W, McGrath J. Lithium for schizophrenia revisited: a systematic review and meta-analysis of randomized clinical trials. J Clin Psychiatry 2004;65(2):177-86.
4. Neppe VM. Carbamazepine as adjunctive treatment in nonepileptic chronic inpatients with EEG temporal lobe abnormalities. J Clin Psychiatry 1983;44:326-31.
5. Dose M, Apelt S, Emrich HM. Carbamazepine as an adjunct of antipsychotic therapy. Psychiatry Res 1987;22:303-10.
6. Okuma T, Yamashita I, Takahashi R, et al. A double-blind study of adjunctive carbamazepine versus placebo on excited states of schizophrenic and schizoaffective disorders. Acta Psychiatr Scand 1989;80:250-9.
7. Nachshoni T, Levin Y, Levy A, et al. A double-blind trial of carbamazepine in negative symptom schizophrenia. Biol Psychiatry 1994;35(1):22-26.
8. Simhandl C, Meszaros K, Denk E, et al. Adjunctive carbamazepine or lithium carbonate in therapy-resistant chronic schizophrenia. Can J Psychiatry 1996;41(5):317.-
9. Leucht S, McGrath J, White P, et al. Carbamazepine augmentation for schizophrenia: how good is the evidence? J Clin Psychiatry 2002;63(3):218-24.
10. Citrome L. Schizophrenia and valproate. Psychopharmacol Bull 2003;37(suppl 2):74-88.
11. Basan A, Kissling W, Leucht S. Valproate as an adjunct to antipsychotics for schizophrenia: a systematic review of randomized trials. Schizophr Res 2004;70(1):33-7.
12. Ko GN, Korpi ER, Freed WJ, et al. Effect of valproic acid on behavior and plasma amino acid concentrations in chronic schizophrenia patients. Biol Psychiatry 1985;20:209-15.
13. Dose M, Hellweg R, Yassouridis A, et al. Combined treatment of schizophrenic psychoses with haloperidol and valproate. Pharmacopsychiatry 1998;31(4):122-5.
14. Fisk GG, York SM. The effect of sodium valproate on tardive dyskinesia—revisited. Br J Psychiatry 1987;150:542-6.
15. Wassef AA, Dott SG, Harris A, et al. Randomized, placebo-controlled pilot study of divalproex sodium in the treatment of acute exacerbations of chronic schizophrenia. J Clin Psychopharmacol 2000;20(3):357-361.
16. Casey DE, Daniel DG, Wassef AA, et al. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacol 2003;28(1):182-92.
17. Citrome L, Casey DE, Daniel DG, et al. Effects of adjunctive valproate on hostility in patients with schizophrenia receiving olanzapine or risperidone: a double-blind multi-center study. Psychiatr Serv 2004;55(3):290-4.
18. Cramer JA, Sernyak M. Results of a naturalistic study of treatment options: switching atypical antipsychotic drugs or augmenting with valproate. Clin Ther 2004;26(6):905-14.
19. Tiihonen J, Hallikainen T, Ryynanen OP, et al. Lamotrigine in treatment-resistant schizophrenia: a randomized placebo-controlled trial. Biol Psychiatry 2003;54(11):1241-8.
20. Kremer I, Vass A, Gurelik I, et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol Psychiatry 2004;56(6):441-6.
21. Drapalski AL, Rosse RB, Peebles RR, et al. Topiramate improves deficit symptoms in a patient with schizophrenia when added to a stable regimen of antipsychotic medication. Clin Neuropharmacol 2001;24:290-4.
22. Millson RC, Owen JA, Lorberg GW, Tackaberry L. Topiramate for refractory schizophrenia. Am J Psychiatry 2002;159(4):675.-
23. Chouinard G, Beauclair L, Belanger MC. Gabapentin: long term antianxiety and hypnotic effects in psychiatric patients with comorbid anxiety-related disorders. Can J Psychiatry 1998;43:305.-
24. Megna JL, Devitt PJ, Sauro MD, Dewan MJ. Gabapentin’s effect on agitation in severely and persistently mentally ill patients. Ann Pharmacother 2002;35:12-16.
25. Jablonowski K, Margolese HC, Chouinard G. Gabapentin-induced paradoxical exacerbation of psychosis in a patient with schizophrenia. Can J Psychiatry 2002;47(10):975-6.
26. Baird P. The interactive metabolism effect of oxcarbazepine coadministered with tricyclic antidepressant therapy for OCD symptoms. J Clin Psychopharmacol 2003;23(4):419.-
27. Centorrino F, Albert MJ, Berry JM, et al. Oxcarbazepine: clinical experience with hospitalized psychiatric patients. Bipolar Disord 2003;5(5):370-4.
28. Leweke FM, Gerth CW, Koethe D, et al. Oxcarbazepine as an adjunct for schizophrenia. Am J Psychiatry 2004;161(6):1130-1.
29. Stahl SM. Psychopharmacology of anticonvulsants: do all anticonvulsants have the same mechanism of action? J Clin Psychiatry 2004;65(2):149-50.
30. Ketter TA, Wong PW. The emerging differential roles of GABAergic and antiglutaminergic agents in bipolar disorders. J Clin Psychiatry 2003;64(suppl 3):15-20.
31. Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol 2003;23(6):601-40.
1. Citrome L, Jaffe A, Levine J. Datapoints - mood stabilizers: utilization trends in patients diagnosed with schizophrenia 1994-2001. Psychiatr Serv 2002;53(10):1212.-
2. Citrome L. Antipsychotic polypharmacy versus augmentation with anticonvulsants: the U.S.perspective (presentation). Paris: Collegium Internationale Neuro-Psychopharmacologicum(CINP), June 2004 [abstract in Int J Neuropsychopharmacol 2004;7(suppl 1):S69].
3. Leucht S, Kissling W, McGrath J. Lithium for schizophrenia revisited: a systematic review and meta-analysis of randomized clinical trials. J Clin Psychiatry 2004;65(2):177-86.
4. Neppe VM. Carbamazepine as adjunctive treatment in nonepileptic chronic inpatients with EEG temporal lobe abnormalities. J Clin Psychiatry 1983;44:326-31.
5. Dose M, Apelt S, Emrich HM. Carbamazepine as an adjunct of antipsychotic therapy. Psychiatry Res 1987;22:303-10.
6. Okuma T, Yamashita I, Takahashi R, et al. A double-blind study of adjunctive carbamazepine versus placebo on excited states of schizophrenic and schizoaffective disorders. Acta Psychiatr Scand 1989;80:250-9.
7. Nachshoni T, Levin Y, Levy A, et al. A double-blind trial of carbamazepine in negative symptom schizophrenia. Biol Psychiatry 1994;35(1):22-26.
8. Simhandl C, Meszaros K, Denk E, et al. Adjunctive carbamazepine or lithium carbonate in therapy-resistant chronic schizophrenia. Can J Psychiatry 1996;41(5):317.-
9. Leucht S, McGrath J, White P, et al. Carbamazepine augmentation for schizophrenia: how good is the evidence? J Clin Psychiatry 2002;63(3):218-24.
10. Citrome L. Schizophrenia and valproate. Psychopharmacol Bull 2003;37(suppl 2):74-88.
11. Basan A, Kissling W, Leucht S. Valproate as an adjunct to antipsychotics for schizophrenia: a systematic review of randomized trials. Schizophr Res 2004;70(1):33-7.
12. Ko GN, Korpi ER, Freed WJ, et al. Effect of valproic acid on behavior and plasma amino acid concentrations in chronic schizophrenia patients. Biol Psychiatry 1985;20:209-15.
13. Dose M, Hellweg R, Yassouridis A, et al. Combined treatment of schizophrenic psychoses with haloperidol and valproate. Pharmacopsychiatry 1998;31(4):122-5.
14. Fisk GG, York SM. The effect of sodium valproate on tardive dyskinesia—revisited. Br J Psychiatry 1987;150:542-6.
15. Wassef AA, Dott SG, Harris A, et al. Randomized, placebo-controlled pilot study of divalproex sodium in the treatment of acute exacerbations of chronic schizophrenia. J Clin Psychopharmacol 2000;20(3):357-361.
16. Casey DE, Daniel DG, Wassef AA, et al. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacol 2003;28(1):182-92.
17. Citrome L, Casey DE, Daniel DG, et al. Effects of adjunctive valproate on hostility in patients with schizophrenia receiving olanzapine or risperidone: a double-blind multi-center study. Psychiatr Serv 2004;55(3):290-4.
18. Cramer JA, Sernyak M. Results of a naturalistic study of treatment options: switching atypical antipsychotic drugs or augmenting with valproate. Clin Ther 2004;26(6):905-14.
19. Tiihonen J, Hallikainen T, Ryynanen OP, et al. Lamotrigine in treatment-resistant schizophrenia: a randomized placebo-controlled trial. Biol Psychiatry 2003;54(11):1241-8.
20. Kremer I, Vass A, Gurelik I, et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol Psychiatry 2004;56(6):441-6.
21. Drapalski AL, Rosse RB, Peebles RR, et al. Topiramate improves deficit symptoms in a patient with schizophrenia when added to a stable regimen of antipsychotic medication. Clin Neuropharmacol 2001;24:290-4.
22. Millson RC, Owen JA, Lorberg GW, Tackaberry L. Topiramate for refractory schizophrenia. Am J Psychiatry 2002;159(4):675.-
23. Chouinard G, Beauclair L, Belanger MC. Gabapentin: long term antianxiety and hypnotic effects in psychiatric patients with comorbid anxiety-related disorders. Can J Psychiatry 1998;43:305.-
24. Megna JL, Devitt PJ, Sauro MD, Dewan MJ. Gabapentin’s effect on agitation in severely and persistently mentally ill patients. Ann Pharmacother 2002;35:12-16.
25. Jablonowski K, Margolese HC, Chouinard G. Gabapentin-induced paradoxical exacerbation of psychosis in a patient with schizophrenia. Can J Psychiatry 2002;47(10):975-6.
26. Baird P. The interactive metabolism effect of oxcarbazepine coadministered with tricyclic antidepressant therapy for OCD symptoms. J Clin Psychopharmacol 2003;23(4):419.-
27. Centorrino F, Albert MJ, Berry JM, et al. Oxcarbazepine: clinical experience with hospitalized psychiatric patients. Bipolar Disord 2003;5(5):370-4.
28. Leweke FM, Gerth CW, Koethe D, et al. Oxcarbazepine as an adjunct for schizophrenia. Am J Psychiatry 2004;161(6):1130-1.
29. Stahl SM. Psychopharmacology of anticonvulsants: do all anticonvulsants have the same mechanism of action? J Clin Psychiatry 2004;65(2):149-50.
30. Ketter TA, Wong PW. The emerging differential roles of GABAergic and antiglutaminergic agents in bipolar disorders. J Clin Psychiatry 2003;64(suppl 3):15-20.
31. Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol 2003;23(6):601-40.
Bodybuilding’s dark side: Clues to anabolic steroid use
Anabolic steroid use by athletes and body-builders has captured public attention but remains poorly understood by most physicians. This is not surprising because users of anabolic-androgenic steroids (AAS):
- rarely seek treatment or disclose their drug use
- frequently distrust professionals.
If you are a clinician who regularly sees male adolescents and young men, you need to become familiar with—and watch for—this often-secret form of substance abuse. This article provides the groundwork for that understanding, starting with the story of “Aaron”—a composite patient whose case represents experiences and verbatim quotes from AAS users known to the authors.
CASE REPORT: ‘I FEEL INVINCIBLE’
At his first visit, Aaron, age 18, told the psychiatrist he had no complaints but was coming to please his parents. “I have a lot of arguments with my Dad,” he said, “and they keep thinking something’s wrong with me.”
The patient was very muscular and dressed in baggy sweats that masked his body proportions. He was appropriately groomed and darkly tanned but displayed some acne. The clinician guessed he weighed about 175 lbs and stood at about 65 inches, with very low body fat. Although superficially confident, he seemed restless, somewhat anxious, and guarded as the interview progressed.
Aaron admitted he experienced prominent mood swings. During rage outbursts, he had damaged objects and put his fist through the wall. “There’s holes all over the wall of my room,” he joked.
He also had assaulted a motorist in a traffic altercation, then left the scene. “Did you hurt him?” the clinician asked. Somewhat sheepishly, Aaron responded, “Well, I bought the newspaper and kept checking the obituaries for about 2 weeks afterwards.”
He spoke with pride about his weightlifting, which was the focus of his life. He revealed that he was preparing for a body-building contest in 2 months. The psychiatrist asked him about use of supplements—protein shakes, creatine, and “andro” (androstenedione)—all of which Aaron acknowledged. The psychiatrist then gently asked about anabolic steroid use (Box 1).
Initially, Aaron strongly denied using AAS. The psychiatrist persisted, pointing out that no information would be disclosed to his parents, and asked again using colloquial terms from the AAS subculture: “Anybody who is prepping for an untested contest in a couple of months is going to be on a cycle. Come on, what are you taking?”
Anabolic-androgenic steroids (AAS) are hormones that include testosterone—nature’s own AAS—and more than 100 synthetically developed testosterone relatives. All AAS possess anabolic (muscle-building) and androgenic (masculinizing) properties; no known compound can produce one of these effects without the other.
Because of their masculinizing effects, AAS are rarely used by women—and even then in much lower doses than those used by men. Thus, this article focuses on evaluating and treating male adolescents and men.
AAS are not:
- Corticosteroids (such as cortisol) are often called “steroids” but possess no muscle-building properties. Corticosteroids’ prominent but idiosyncratic psychiatric effects are usually seen in consultation-liaison settings where patients have been prescribed these drugs, rather than among substance abusers.
- Androstenedione (“andro”) and its relatives are adrenal steroids that are weakly metabolized into testosterone or other AAS. These substances were sold legally without prescription in the United States for many years but were banned by federal law in October 2004. Their anabolic and psychiatric effects are much weaker than those of AAS.
- Athletic “supplements” with names designed to sound like AAS (such as beginning with “Ana…”) or supplements claimed to be “testosterone-releasers” or the like. If sold legally in supplement stores, an athletic supplement is not an AAS. Psychiatric effects are extremely unlikely.
Eventually it emerged that Aaron had taken five 8- to 20-week AAS “cycles” (courses), during which he had “stacked” (combined) various “injectables” such as IM testosterone and “orals” such as methyltestosterone (Table 1). His current cycle included:
- a blend of testosterone esters (Sustanon), 500 mg IM once a week
- boldenone (Equipoise), a veterinary AAS normally used for horses, 200 mg IM per week
- oxymetholone (Anadrol), 50 mg orally per day.
Table 1
Commonly used anabolic-androgenic steroids
| ‘Injectables’ (usually administered only by injection) | |
| Boldenone (Equipoise)* | |
| Methenolone (Primobolan depot) | |
| Nandrolone (Deca-Durabolin, Durabolin, Laurabolin, others) | |
| Stanozolol (Winstrol-V)* | |
| Testosterone esters (Depo-testosterone, Sten, Sustanon, others) | |
| Trenbolone (Finajet, Parabolan) | |
| ‘Orals’ | |
| Methandienone (formerly called methandrostenolone) (Dianabol, others) | |
| Methenolone (Primobolan) | |
| Methyltestosterone (Android, others) | |
| Mibolerone (Checque Drops)* | |
| Oxandrolone (Anavar, Lipidex) | |
| Oxmetholone (Anadrol, Anapolon) | |
| Stanoxolol (Stromba, Winstrol) | |
| Other anabolic substances sold on the ‘black market’ | |
| Human growth hormone (HGH) | Possesses anabolic properties |
| Extremely expensive | |
| Almost impossible to detect by testing | |
| Lacks androgenic effects | |
| Psychiatric effects appear negligible | |
| Large doses can cause acromegaly | |
| Clenbuterol | Beta-adrenergic agonist with stimulant and anabolic properties |
| Used less commonly than AAS | |
| Lacks androgenic effects and assists fat loss | |
| Can produce psychiatric effects similar to those of amphetamine abuse (rare, in the authors’ experience) | |
| Human chorionic gonadotropin (HCG) | Stimulates testes to produce more testosterone, creating an AAS effect |
| Most commonly used near the end of an AAS “cycle” to “jump-start” the hypothalamic-pituitary-testicular axis and minimize AAS withdrawal | |
| * Veterinary preparation | |
| AAS: anabolic-androgenic steroid | |
His friends had taught him to self-inject AAS at age 15; he admitted that he was also occasionally self-injecting the opioid analgesic nalbuphine intravenously because of “pain in my ‘delts’ from military presses.”
During his cycles, Aaron experienced hypomanic symptoms, including euphoria, prominent irritability, increased libido, decreased need for sleep, and grandiosity. “I feel invincible,” he said. His aggressive outbursts had worsened with increasing AAS doses; in addition to attacking the motorist, he also had been physically violent with his girlfriend. “She’s scared of me when I’m on juice,” he conceded.
During the withdrawal phase after stopping each cycle, Aaron described prominent depression with anhedonia, hypersomnia, loss of libido, and suicidal ideation. “I once almost jumped off a bridge after my fourth cycle,” he admitted. “I couldn’t wait to get on my next cycle to feel good again.” His depressions were also characterized by body-image obsessions; he would regularly spend at least 1 hour a day examining his musculature in a mirror, and sometimes refused to go out in public because he “was getting too small.”
Perhaps most disturbing was his increasing use of opioids. In addition to self-injecting nalbuphine, he also ingested oral opioids such as oxycodone almost daily. He mentioned that several of his friends in the gym had progressed from injecting nalbuphine to injecting morphine or heroin, and he knew two bodybuilders who had died from apparently unintentional opioid overdoses.
Aaron said his parents, teachers, and non-bodybuilding friends were unaware of this history. He claimed his parents were proud that their son had apparently eschewed drugs and alcohol to pursue a healthy athletic lifestyle.
RECOGNIZING AAS USE
In our experience with treating substance abusers, we find that AAS users may be the least likely to disclose their drug use to clinicians. In a recent study,1 20 of 36 AAS users (56%) reported they had never revealed their AAS use to any physician. When asked to rate their trust in sources of information about AAS, 17 of 42 AAS users (40%) said they trusted information from their drug dealers at least as much as information from any physician they had seen.
Some expressed contempt for physicians as “geeks” or “pencil-necks” who could not comprehend the body-building lifestyle. They gave doctors high marks on knowledge of tobacco, alcohol, and ordinary “street drugs” but much lower ratings on AAS knowledge. Other investigators have shown that many clinicians are unfamiliar with AAS.2,3
AAS users embrace these beliefs for two other reasons. First, to admit to AAS use is to admit that one’s muscularity and physical prowess is the result of taking a drug; there is no comparable motivation to withhold information about, say, one’s use of marijuana or cocaine.
Second, AAS users are much less likely than other substance abusers to view their behavior as pathologic. We have argued that our culture is partially to blame.4 Americans pay to watch 300-lb football linemen and AAS-using movie stars. Makers of cars, computers, and electronics do not hesitate to advertise their products as “on steroids,” but they would never claim their products were “on cocaine.” In this climate, it is easy to forget that AAS use is an illicit substance abuse.
Formula: FFMI = (W x (100 - BF)/100)H2 + 6.1 x (1.8 - H)
W = weight in kilograms
BF = body fat percentage
H = height in meters
Obtain height in meters and weight in kilograms. Ideally, measure body fat using calipers, electrical impedance, or some other method. Alternately, estimate body fat by visual inspection:
- 20% = average 30-year-old American man
- 10% = quite lean
- 5% = approaching lowest body fat normally attainable
FFMI values for American men:
20 = average
22 = visibly muscular
25 = approximate maximum attainable by a lean individual without using drugs
Example 1
Young male weightlifter is 69 inches tall, weighs 175 lbs, and is moderately lean, with body fat of 10%; he denies AAS use
H = 69 inches x .0254 meters/inch = 1.75 m
H2= 1.75 x 1.75 = 3.06 m2
W = 175 pounds x 0.454 kilograms/lb = 79.5 kg
Therefore, FFMI = (79.5 x (100-10)/100)/3.06 + 6.1 x (1.8 - 1.75) = 23.7
This degree of muscularity can be attained without using AAS
Example 2
Young male weightlifter is 66 inches tall, weighs 175 lbs, and is very lean, with body fat of 6%; he also denies AAS use
H = 66 inches x .0254 meters/inch = 1.68 m
H2= 1.68 x 1.68 = 2.82 m2
W = 175 pounds x 0.454 kilograms/lb = 79.5 kg
Therefore, FFMI = (79.5 x (100-6)/100)2.82 + 6.1 x (1.8 - 1.68) = 27.2
This level of muscularity is extremely unlikely without drugs. Patient is almost certainly lying and should be gently confronted, especially if other symptoms (Table 2) suggest AAS use
Table 2
Clues to possible AAS use in men
| Muscularity |
| Estimated fat-free mass index (FFMI) >26 (see Box 2) |
| Recent rapid muscle gains (>8 lb/month) |
| Striae over pectoralis muscles caused by rapid hypertrophy of underlying muscle |
| Other physical signs |
| Acne |
| Gynecomastia |
| Testicular atrophy |
| Psychiatric signs |
| Uncharacteristically aggressive behavior |
Uncharacteristic hypomanic symptoms
|
Uncharacteristic depressive symptoms
|
To overcome these treatment obstacles, we recommend that you:
- Become as knowledgeable about AAS use as you are about other forms of substance abuse (see Related resources).
- Approach AAS users as you would any other substance abusers—as individuals at risk for potentially serious medical and psychiatric consequences.
- Maintain a high index of suspicion when evaluating any muscular young male patient, even if he initially denies AAS use.
AAS use can often be suspected by looking at the patient as he walks in the door. Using what we call the “fat-free mass index” (FFMI) to calculate muscularity (Box 2), we have shown that a lean man can achieve only a certain amount of muscularity without using drugs.5 Although this finding needs to be replicated elsewhere, in our experience a man is almost certainly lying if he:
- is relatively lean (with approximately 10% body fat)
- displays an FFMI >26
- and claims he has not used drugs.
If a patient has an elevated FFMI and other cues suggesting AAS use (Table 2), gently but persistently question him if he denies using these drugs.
TREATING AAS-ASSOCIATED SYNDROMES
When you have established a history of AAS use, you will be far better prepared to anticipate and possibly treat its associated syndromes. Discussion of these effects is beyond the scope of this paper; for details, see reviews of AAS-associated medical effects,3,6 psychiatric effects,6,7 and general treatment principles.8 We focus here on the four scenarios clinicians encounter most often in practice and offer some pragmatic suggestions.
Forensic cases. AAS users almost never voluntarily seek help to stop their drug use. Such a request would be somewhat analogous to a girl with anorexia nervosa voluntarily asking for help to gain weight. We are unaware of any rehabilitation centers, clinics, 12-step programs, or the like for AAS users—there is no demand for them.
Thus, an AAS user may first come to clinical attention through legal channels. For example, if an AAS user committed a violent crime while experiencing hypomanic effects from these drugs, he might be required to undergo random urine testing as a condition of probation. This may be reasonable, provided that the tests are unannounced and urine is always collected under direct observation.
Monitoring clinicians may serve as little better than policemen, although sometimes it is possible to forge an alliance with the patient.
Depression. Exogenous AAS administration suppresses endogenous testosterone production through feedback mechanisms involving the hypothalamic-pituitary-testicular axis.3,6 Thus, during a long cycle, the user’s testes may shrink to half their normal size and stop producing testosterone and spermatozoa.
If the user then stops AAS rapidly, he may plunge into a profoundly hypogonadal state associated with symptoms of major depression. In a field study of 77 steroid users (71 male and 6 female), 6 (7.8%) reported they attempted suicide during AAS withdrawal.9 Depression associated with AAS withdrawal may prompt users to resume AAS quickly, triggering a syndrome of AAS dependence.6,10,11
Fortunately, AAS-withdrawal depression is usually self-limited and responds—in our experience and that of others12—to standard antidepressants. We recommend aggressively treating such depressions, as doing so may prevent resumption of AAS use and eventual AAS dependence.
Body-image disorders. AAS users often report body-image disorders, especially muscle dysmorphia—a form of body dysmorphic disorder where individuals become preoccupied with the belief that they are not adequately muscular.13,14 Anxieties about muscularity are a risk factor for subsequent AAS use15 and a major contributor to AAS dependence.8,11
Body dysmorphic disorder responds to pharmacologic and cognitive-behavioral interventions.3,16 Young men showing pathologic concerns about their muscularity or displaying related body-image pathology may benefit from prompt treatment before they are tempted to use AAS.
Progression to opioid dependence. An ominous development among American17 and British18 AAS users is a growing tendency to use opioids. In two studies of individuals with opioid dependence,19,20 7% to 9% reported beginning as AAS users, then learning about opioids from fellow bodybuilders and often buying their first illicit opioids from the person who had sold them AAS. Most learned as teenagers to use needles to inject AAS intramuscularly, so beginning to using opioids intravenously was only a small step.
In the last 5 years, we have become anecdotally aware of numerous AAS users who developed heroin addiction requiring repeated inpatient detoxification or who died of unintentional opioid overdoses. We suspect this phenomenon is under-recognized and urge clinicians to watch for it.
- Pope HG Jr, Brower KJ. Anabolic-androgenic steroid abuse. In: Sadock BJ, Sadock VA (eds). Comprehensive textbook of psychiatry (8th ed). Philadelphia: Lippincott Williams & Wilkins (in press).
- Yesalis CE (ed). Anabolic steroids in sport and exercise (2nd ed). Champaign, IL: Human Kinetics, 2000.
- The Taylor Hooton Foundation. Started by the father of a high school athlete who committed suicide during a depressive episode apparently precipitated by AAS withdrawal. Includes links to related Web sites. http://www.taylorhooton.org/about.asp. Accessed Nov. 10, 2004.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pope HG, Jr, Kanayama G, Ionescu-Pioggia M, Hudson JI. Anabolic steroid users’ attitudes towards physicians. Addiction 2004;99:1189-94.
2. Dawson RT. Drugs in sport—the role of the physician. J Endocrinol 2001;170:55-61.
3. Kutscher EC, Lund BC, Perry PJ. Anabolic steroids: a review for the clinician. Sports Med 2002;32:285-96.
4. Pope HG, Jr, Phillips KA, Olivardia R. The Adonis complex: the secret crisis of male body obsession. New York: Free Press, 2000.
5. Kouri EM, Pope HG, Jr, Katz DL, Oliva PS. Fat-free mass index in users and non-users of anabolic-androgenic steroids. Clin J Sport Med 1995;5:223-8.
6. Brower KJ. Anabolic steroid abuse and dependence. Curr Psychiatry Rep 2002;4:377-83.
7. Pope HG, Jr, Katz DL. Psychiatric effects of exogenous anabolic-androgenic steroids. In: Wolkowitz OM, Rothschild AJ (eds). Psychoneuroendocrinology: the scientific basis of clinical practice. Washington. DC: American Psychiatric Publishing, 2003;331-58.
8. Pope HG, Jr, Brower KJ. Anabolic-androgenic steroids. In: Galanter M, Kleber HD (eds). American Psychiatric Publishing textbook of substance abuse treatment (3rd ed). Washington DC: American Psychiatric Publishing, 2004;257-64.
9. Malone DA, Jr, Dimeff R, Lombardo JA, Sample BRH. Psychiatric effects and psychoactive substance use in anabolic-androgenic steroid users. Clin J Sports Med 1995;5:25-31
10. Kashkin KB, Kleber HD. Hooked on hormones? An anabolic steroid addiction hypothesis. JAMA 1989;262:3166-70.
11. Brower KJ, Eliopulos GA, Blow FC, et al. Evidence for physical and psychological dependence on anabolic androgenic steroids in eight weight lifters. Am J Psychiatry. 1990;147(4):510-2.
12. Malone DA, Jr, Dimeff RJ. The use of fluoxetine in depression associated with anabolic steroid withdrawal: a case series. J Clin Psychiatry. 1992;53:130-2.
13. Pope HG, Jr, Gruber AJ, Choi PY. Muscle dysmorphia: an underrecognized form of body dysmorphic disorder. Psychosomatics 1997;38:548-57.
14. Olivardia R, Pope HG, Jr, Hudson JI. ‘Muscle dysmorphia’ in male weightlifters: a case-control study. Am J Psychiatry 2000;157:1291-6.
15. Kanayama G, Pope HG, Jr, Cohane G, Hudson JI. Risk factors for anabolic-androgenic steroid use among weightlifters: a case-control study. Drug Alcohol Depend 2003;71:77-86.
16. Phillips KA. Pharmacologic treatment of body dysmorphic disorder: a review of empirical data and a proposed treatment algorithm. Psychiatr Clin North Am 2000;7:59-82.
17. Wines JD, Jr, Gruber AJ, Pope HG, Jr, Lukas SE. Nalbuphine hydrochloride dependence in anabolic steroid users. Am J Addictions 1999;8:161-4.
18. McBride AJ, Williamson K, Petersen T. Three cases of nalbuphine hydrochloride dependence associated with anabolic steroid abuse. Br J Sports Med 1996;30:69-70.
19. Kanayama G, Cohane G, Weiss RD, Pope HG, Jr. Past anabolic-androgenic steroid use among men admitted for substance abuse treatment: an underrecognized problem? J Clin Psychiatry. 2003;64:156-60.
20. Arvary D, Pope HG, Jr. Anabolic steroids: a possible gateway to opioid dependence. N Engl J Med 2000;342:1532.-
Anabolic steroid use by athletes and body-builders has captured public attention but remains poorly understood by most physicians. This is not surprising because users of anabolic-androgenic steroids (AAS):
- rarely seek treatment or disclose their drug use
- frequently distrust professionals.
If you are a clinician who regularly sees male adolescents and young men, you need to become familiar with—and watch for—this often-secret form of substance abuse. This article provides the groundwork for that understanding, starting with the story of “Aaron”—a composite patient whose case represents experiences and verbatim quotes from AAS users known to the authors.
CASE REPORT: ‘I FEEL INVINCIBLE’
At his first visit, Aaron, age 18, told the psychiatrist he had no complaints but was coming to please his parents. “I have a lot of arguments with my Dad,” he said, “and they keep thinking something’s wrong with me.”
The patient was very muscular and dressed in baggy sweats that masked his body proportions. He was appropriately groomed and darkly tanned but displayed some acne. The clinician guessed he weighed about 175 lbs and stood at about 65 inches, with very low body fat. Although superficially confident, he seemed restless, somewhat anxious, and guarded as the interview progressed.
Aaron admitted he experienced prominent mood swings. During rage outbursts, he had damaged objects and put his fist through the wall. “There’s holes all over the wall of my room,” he joked.
He also had assaulted a motorist in a traffic altercation, then left the scene. “Did you hurt him?” the clinician asked. Somewhat sheepishly, Aaron responded, “Well, I bought the newspaper and kept checking the obituaries for about 2 weeks afterwards.”
He spoke with pride about his weightlifting, which was the focus of his life. He revealed that he was preparing for a body-building contest in 2 months. The psychiatrist asked him about use of supplements—protein shakes, creatine, and “andro” (androstenedione)—all of which Aaron acknowledged. The psychiatrist then gently asked about anabolic steroid use (Box 1).
Initially, Aaron strongly denied using AAS. The psychiatrist persisted, pointing out that no information would be disclosed to his parents, and asked again using colloquial terms from the AAS subculture: “Anybody who is prepping for an untested contest in a couple of months is going to be on a cycle. Come on, what are you taking?”
Anabolic-androgenic steroids (AAS) are hormones that include testosterone—nature’s own AAS—and more than 100 synthetically developed testosterone relatives. All AAS possess anabolic (muscle-building) and androgenic (masculinizing) properties; no known compound can produce one of these effects without the other.
Because of their masculinizing effects, AAS are rarely used by women—and even then in much lower doses than those used by men. Thus, this article focuses on evaluating and treating male adolescents and men.
AAS are not:
- Corticosteroids (such as cortisol) are often called “steroids” but possess no muscle-building properties. Corticosteroids’ prominent but idiosyncratic psychiatric effects are usually seen in consultation-liaison settings where patients have been prescribed these drugs, rather than among substance abusers.
- Androstenedione (“andro”) and its relatives are adrenal steroids that are weakly metabolized into testosterone or other AAS. These substances were sold legally without prescription in the United States for many years but were banned by federal law in October 2004. Their anabolic and psychiatric effects are much weaker than those of AAS.
- Athletic “supplements” with names designed to sound like AAS (such as beginning with “Ana…”) or supplements claimed to be “testosterone-releasers” or the like. If sold legally in supplement stores, an athletic supplement is not an AAS. Psychiatric effects are extremely unlikely.
Eventually it emerged that Aaron had taken five 8- to 20-week AAS “cycles” (courses), during which he had “stacked” (combined) various “injectables” such as IM testosterone and “orals” such as methyltestosterone (Table 1). His current cycle included:
- a blend of testosterone esters (Sustanon), 500 mg IM once a week
- boldenone (Equipoise), a veterinary AAS normally used for horses, 200 mg IM per week
- oxymetholone (Anadrol), 50 mg orally per day.
Table 1
Commonly used anabolic-androgenic steroids
| ‘Injectables’ (usually administered only by injection) | |
| Boldenone (Equipoise)* | |
| Methenolone (Primobolan depot) | |
| Nandrolone (Deca-Durabolin, Durabolin, Laurabolin, others) | |
| Stanozolol (Winstrol-V)* | |
| Testosterone esters (Depo-testosterone, Sten, Sustanon, others) | |
| Trenbolone (Finajet, Parabolan) | |
| ‘Orals’ | |
| Methandienone (formerly called methandrostenolone) (Dianabol, others) | |
| Methenolone (Primobolan) | |
| Methyltestosterone (Android, others) | |
| Mibolerone (Checque Drops)* | |
| Oxandrolone (Anavar, Lipidex) | |
| Oxmetholone (Anadrol, Anapolon) | |
| Stanoxolol (Stromba, Winstrol) | |
| Other anabolic substances sold on the ‘black market’ | |
| Human growth hormone (HGH) | Possesses anabolic properties |
| Extremely expensive | |
| Almost impossible to detect by testing | |
| Lacks androgenic effects | |
| Psychiatric effects appear negligible | |
| Large doses can cause acromegaly | |
| Clenbuterol | Beta-adrenergic agonist with stimulant and anabolic properties |
| Used less commonly than AAS | |
| Lacks androgenic effects and assists fat loss | |
| Can produce psychiatric effects similar to those of amphetamine abuse (rare, in the authors’ experience) | |
| Human chorionic gonadotropin (HCG) | Stimulates testes to produce more testosterone, creating an AAS effect |
| Most commonly used near the end of an AAS “cycle” to “jump-start” the hypothalamic-pituitary-testicular axis and minimize AAS withdrawal | |
| * Veterinary preparation | |
| AAS: anabolic-androgenic steroid | |
His friends had taught him to self-inject AAS at age 15; he admitted that he was also occasionally self-injecting the opioid analgesic nalbuphine intravenously because of “pain in my ‘delts’ from military presses.”
During his cycles, Aaron experienced hypomanic symptoms, including euphoria, prominent irritability, increased libido, decreased need for sleep, and grandiosity. “I feel invincible,” he said. His aggressive outbursts had worsened with increasing AAS doses; in addition to attacking the motorist, he also had been physically violent with his girlfriend. “She’s scared of me when I’m on juice,” he conceded.
During the withdrawal phase after stopping each cycle, Aaron described prominent depression with anhedonia, hypersomnia, loss of libido, and suicidal ideation. “I once almost jumped off a bridge after my fourth cycle,” he admitted. “I couldn’t wait to get on my next cycle to feel good again.” His depressions were also characterized by body-image obsessions; he would regularly spend at least 1 hour a day examining his musculature in a mirror, and sometimes refused to go out in public because he “was getting too small.”
Perhaps most disturbing was his increasing use of opioids. In addition to self-injecting nalbuphine, he also ingested oral opioids such as oxycodone almost daily. He mentioned that several of his friends in the gym had progressed from injecting nalbuphine to injecting morphine or heroin, and he knew two bodybuilders who had died from apparently unintentional opioid overdoses.
Aaron said his parents, teachers, and non-bodybuilding friends were unaware of this history. He claimed his parents were proud that their son had apparently eschewed drugs and alcohol to pursue a healthy athletic lifestyle.
RECOGNIZING AAS USE
In our experience with treating substance abusers, we find that AAS users may be the least likely to disclose their drug use to clinicians. In a recent study,1 20 of 36 AAS users (56%) reported they had never revealed their AAS use to any physician. When asked to rate their trust in sources of information about AAS, 17 of 42 AAS users (40%) said they trusted information from their drug dealers at least as much as information from any physician they had seen.
Some expressed contempt for physicians as “geeks” or “pencil-necks” who could not comprehend the body-building lifestyle. They gave doctors high marks on knowledge of tobacco, alcohol, and ordinary “street drugs” but much lower ratings on AAS knowledge. Other investigators have shown that many clinicians are unfamiliar with AAS.2,3
AAS users embrace these beliefs for two other reasons. First, to admit to AAS use is to admit that one’s muscularity and physical prowess is the result of taking a drug; there is no comparable motivation to withhold information about, say, one’s use of marijuana or cocaine.
Second, AAS users are much less likely than other substance abusers to view their behavior as pathologic. We have argued that our culture is partially to blame.4 Americans pay to watch 300-lb football linemen and AAS-using movie stars. Makers of cars, computers, and electronics do not hesitate to advertise their products as “on steroids,” but they would never claim their products were “on cocaine.” In this climate, it is easy to forget that AAS use is an illicit substance abuse.
Formula: FFMI = (W x (100 - BF)/100)H2 + 6.1 x (1.8 - H)
W = weight in kilograms
BF = body fat percentage
H = height in meters
Obtain height in meters and weight in kilograms. Ideally, measure body fat using calipers, electrical impedance, or some other method. Alternately, estimate body fat by visual inspection:
- 20% = average 30-year-old American man
- 10% = quite lean
- 5% = approaching lowest body fat normally attainable
FFMI values for American men:
20 = average
22 = visibly muscular
25 = approximate maximum attainable by a lean individual without using drugs
Example 1
Young male weightlifter is 69 inches tall, weighs 175 lbs, and is moderately lean, with body fat of 10%; he denies AAS use
H = 69 inches x .0254 meters/inch = 1.75 m
H2= 1.75 x 1.75 = 3.06 m2
W = 175 pounds x 0.454 kilograms/lb = 79.5 kg
Therefore, FFMI = (79.5 x (100-10)/100)/3.06 + 6.1 x (1.8 - 1.75) = 23.7
This degree of muscularity can be attained without using AAS
Example 2
Young male weightlifter is 66 inches tall, weighs 175 lbs, and is very lean, with body fat of 6%; he also denies AAS use
H = 66 inches x .0254 meters/inch = 1.68 m
H2= 1.68 x 1.68 = 2.82 m2
W = 175 pounds x 0.454 kilograms/lb = 79.5 kg
Therefore, FFMI = (79.5 x (100-6)/100)2.82 + 6.1 x (1.8 - 1.68) = 27.2
This level of muscularity is extremely unlikely without drugs. Patient is almost certainly lying and should be gently confronted, especially if other symptoms (Table 2) suggest AAS use
Table 2
Clues to possible AAS use in men
| Muscularity |
| Estimated fat-free mass index (FFMI) >26 (see Box 2) |
| Recent rapid muscle gains (>8 lb/month) |
| Striae over pectoralis muscles caused by rapid hypertrophy of underlying muscle |
| Other physical signs |
| Acne |
| Gynecomastia |
| Testicular atrophy |
| Psychiatric signs |
| Uncharacteristically aggressive behavior |
Uncharacteristic hypomanic symptoms
|
Uncharacteristic depressive symptoms
|
To overcome these treatment obstacles, we recommend that you:
- Become as knowledgeable about AAS use as you are about other forms of substance abuse (see Related resources).
- Approach AAS users as you would any other substance abusers—as individuals at risk for potentially serious medical and psychiatric consequences.
- Maintain a high index of suspicion when evaluating any muscular young male patient, even if he initially denies AAS use.
AAS use can often be suspected by looking at the patient as he walks in the door. Using what we call the “fat-free mass index” (FFMI) to calculate muscularity (Box 2), we have shown that a lean man can achieve only a certain amount of muscularity without using drugs.5 Although this finding needs to be replicated elsewhere, in our experience a man is almost certainly lying if he:
- is relatively lean (with approximately 10% body fat)
- displays an FFMI >26
- and claims he has not used drugs.
If a patient has an elevated FFMI and other cues suggesting AAS use (Table 2), gently but persistently question him if he denies using these drugs.
TREATING AAS-ASSOCIATED SYNDROMES
When you have established a history of AAS use, you will be far better prepared to anticipate and possibly treat its associated syndromes. Discussion of these effects is beyond the scope of this paper; for details, see reviews of AAS-associated medical effects,3,6 psychiatric effects,6,7 and general treatment principles.8 We focus here on the four scenarios clinicians encounter most often in practice and offer some pragmatic suggestions.
Forensic cases. AAS users almost never voluntarily seek help to stop their drug use. Such a request would be somewhat analogous to a girl with anorexia nervosa voluntarily asking for help to gain weight. We are unaware of any rehabilitation centers, clinics, 12-step programs, or the like for AAS users—there is no demand for them.
Thus, an AAS user may first come to clinical attention through legal channels. For example, if an AAS user committed a violent crime while experiencing hypomanic effects from these drugs, he might be required to undergo random urine testing as a condition of probation. This may be reasonable, provided that the tests are unannounced and urine is always collected under direct observation.
Monitoring clinicians may serve as little better than policemen, although sometimes it is possible to forge an alliance with the patient.
Depression. Exogenous AAS administration suppresses endogenous testosterone production through feedback mechanisms involving the hypothalamic-pituitary-testicular axis.3,6 Thus, during a long cycle, the user’s testes may shrink to half their normal size and stop producing testosterone and spermatozoa.
If the user then stops AAS rapidly, he may plunge into a profoundly hypogonadal state associated with symptoms of major depression. In a field study of 77 steroid users (71 male and 6 female), 6 (7.8%) reported they attempted suicide during AAS withdrawal.9 Depression associated with AAS withdrawal may prompt users to resume AAS quickly, triggering a syndrome of AAS dependence.6,10,11
Fortunately, AAS-withdrawal depression is usually self-limited and responds—in our experience and that of others12—to standard antidepressants. We recommend aggressively treating such depressions, as doing so may prevent resumption of AAS use and eventual AAS dependence.
Body-image disorders. AAS users often report body-image disorders, especially muscle dysmorphia—a form of body dysmorphic disorder where individuals become preoccupied with the belief that they are not adequately muscular.13,14 Anxieties about muscularity are a risk factor for subsequent AAS use15 and a major contributor to AAS dependence.8,11
Body dysmorphic disorder responds to pharmacologic and cognitive-behavioral interventions.3,16 Young men showing pathologic concerns about their muscularity or displaying related body-image pathology may benefit from prompt treatment before they are tempted to use AAS.
Progression to opioid dependence. An ominous development among American17 and British18 AAS users is a growing tendency to use opioids. In two studies of individuals with opioid dependence,19,20 7% to 9% reported beginning as AAS users, then learning about opioids from fellow bodybuilders and often buying their first illicit opioids from the person who had sold them AAS. Most learned as teenagers to use needles to inject AAS intramuscularly, so beginning to using opioids intravenously was only a small step.
In the last 5 years, we have become anecdotally aware of numerous AAS users who developed heroin addiction requiring repeated inpatient detoxification or who died of unintentional opioid overdoses. We suspect this phenomenon is under-recognized and urge clinicians to watch for it.
- Pope HG Jr, Brower KJ. Anabolic-androgenic steroid abuse. In: Sadock BJ, Sadock VA (eds). Comprehensive textbook of psychiatry (8th ed). Philadelphia: Lippincott Williams & Wilkins (in press).
- Yesalis CE (ed). Anabolic steroids in sport and exercise (2nd ed). Champaign, IL: Human Kinetics, 2000.
- The Taylor Hooton Foundation. Started by the father of a high school athlete who committed suicide during a depressive episode apparently precipitated by AAS withdrawal. Includes links to related Web sites. http://www.taylorhooton.org/about.asp. Accessed Nov. 10, 2004.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Anabolic steroid use by athletes and body-builders has captured public attention but remains poorly understood by most physicians. This is not surprising because users of anabolic-androgenic steroids (AAS):
- rarely seek treatment or disclose their drug use
- frequently distrust professionals.
If you are a clinician who regularly sees male adolescents and young men, you need to become familiar with—and watch for—this often-secret form of substance abuse. This article provides the groundwork for that understanding, starting with the story of “Aaron”—a composite patient whose case represents experiences and verbatim quotes from AAS users known to the authors.
CASE REPORT: ‘I FEEL INVINCIBLE’
At his first visit, Aaron, age 18, told the psychiatrist he had no complaints but was coming to please his parents. “I have a lot of arguments with my Dad,” he said, “and they keep thinking something’s wrong with me.”
The patient was very muscular and dressed in baggy sweats that masked his body proportions. He was appropriately groomed and darkly tanned but displayed some acne. The clinician guessed he weighed about 175 lbs and stood at about 65 inches, with very low body fat. Although superficially confident, he seemed restless, somewhat anxious, and guarded as the interview progressed.
Aaron admitted he experienced prominent mood swings. During rage outbursts, he had damaged objects and put his fist through the wall. “There’s holes all over the wall of my room,” he joked.
He also had assaulted a motorist in a traffic altercation, then left the scene. “Did you hurt him?” the clinician asked. Somewhat sheepishly, Aaron responded, “Well, I bought the newspaper and kept checking the obituaries for about 2 weeks afterwards.”
He spoke with pride about his weightlifting, which was the focus of his life. He revealed that he was preparing for a body-building contest in 2 months. The psychiatrist asked him about use of supplements—protein shakes, creatine, and “andro” (androstenedione)—all of which Aaron acknowledged. The psychiatrist then gently asked about anabolic steroid use (Box 1).
Initially, Aaron strongly denied using AAS. The psychiatrist persisted, pointing out that no information would be disclosed to his parents, and asked again using colloquial terms from the AAS subculture: “Anybody who is prepping for an untested contest in a couple of months is going to be on a cycle. Come on, what are you taking?”
Anabolic-androgenic steroids (AAS) are hormones that include testosterone—nature’s own AAS—and more than 100 synthetically developed testosterone relatives. All AAS possess anabolic (muscle-building) and androgenic (masculinizing) properties; no known compound can produce one of these effects without the other.
Because of their masculinizing effects, AAS are rarely used by women—and even then in much lower doses than those used by men. Thus, this article focuses on evaluating and treating male adolescents and men.
AAS are not:
- Corticosteroids (such as cortisol) are often called “steroids” but possess no muscle-building properties. Corticosteroids’ prominent but idiosyncratic psychiatric effects are usually seen in consultation-liaison settings where patients have been prescribed these drugs, rather than among substance abusers.
- Androstenedione (“andro”) and its relatives are adrenal steroids that are weakly metabolized into testosterone or other AAS. These substances were sold legally without prescription in the United States for many years but were banned by federal law in October 2004. Their anabolic and psychiatric effects are much weaker than those of AAS.
- Athletic “supplements” with names designed to sound like AAS (such as beginning with “Ana…”) or supplements claimed to be “testosterone-releasers” or the like. If sold legally in supplement stores, an athletic supplement is not an AAS. Psychiatric effects are extremely unlikely.
Eventually it emerged that Aaron had taken five 8- to 20-week AAS “cycles” (courses), during which he had “stacked” (combined) various “injectables” such as IM testosterone and “orals” such as methyltestosterone (Table 1). His current cycle included:
- a blend of testosterone esters (Sustanon), 500 mg IM once a week
- boldenone (Equipoise), a veterinary AAS normally used for horses, 200 mg IM per week
- oxymetholone (Anadrol), 50 mg orally per day.
Table 1
Commonly used anabolic-androgenic steroids
| ‘Injectables’ (usually administered only by injection) | |
| Boldenone (Equipoise)* | |
| Methenolone (Primobolan depot) | |
| Nandrolone (Deca-Durabolin, Durabolin, Laurabolin, others) | |
| Stanozolol (Winstrol-V)* | |
| Testosterone esters (Depo-testosterone, Sten, Sustanon, others) | |
| Trenbolone (Finajet, Parabolan) | |
| ‘Orals’ | |
| Methandienone (formerly called methandrostenolone) (Dianabol, others) | |
| Methenolone (Primobolan) | |
| Methyltestosterone (Android, others) | |
| Mibolerone (Checque Drops)* | |
| Oxandrolone (Anavar, Lipidex) | |
| Oxmetholone (Anadrol, Anapolon) | |
| Stanoxolol (Stromba, Winstrol) | |
| Other anabolic substances sold on the ‘black market’ | |
| Human growth hormone (HGH) | Possesses anabolic properties |
| Extremely expensive | |
| Almost impossible to detect by testing | |
| Lacks androgenic effects | |
| Psychiatric effects appear negligible | |
| Large doses can cause acromegaly | |
| Clenbuterol | Beta-adrenergic agonist with stimulant and anabolic properties |
| Used less commonly than AAS | |
| Lacks androgenic effects and assists fat loss | |
| Can produce psychiatric effects similar to those of amphetamine abuse (rare, in the authors’ experience) | |
| Human chorionic gonadotropin (HCG) | Stimulates testes to produce more testosterone, creating an AAS effect |
| Most commonly used near the end of an AAS “cycle” to “jump-start” the hypothalamic-pituitary-testicular axis and minimize AAS withdrawal | |
| * Veterinary preparation | |
| AAS: anabolic-androgenic steroid | |
His friends had taught him to self-inject AAS at age 15; he admitted that he was also occasionally self-injecting the opioid analgesic nalbuphine intravenously because of “pain in my ‘delts’ from military presses.”
During his cycles, Aaron experienced hypomanic symptoms, including euphoria, prominent irritability, increased libido, decreased need for sleep, and grandiosity. “I feel invincible,” he said. His aggressive outbursts had worsened with increasing AAS doses; in addition to attacking the motorist, he also had been physically violent with his girlfriend. “She’s scared of me when I’m on juice,” he conceded.
During the withdrawal phase after stopping each cycle, Aaron described prominent depression with anhedonia, hypersomnia, loss of libido, and suicidal ideation. “I once almost jumped off a bridge after my fourth cycle,” he admitted. “I couldn’t wait to get on my next cycle to feel good again.” His depressions were also characterized by body-image obsessions; he would regularly spend at least 1 hour a day examining his musculature in a mirror, and sometimes refused to go out in public because he “was getting too small.”
Perhaps most disturbing was his increasing use of opioids. In addition to self-injecting nalbuphine, he also ingested oral opioids such as oxycodone almost daily. He mentioned that several of his friends in the gym had progressed from injecting nalbuphine to injecting morphine or heroin, and he knew two bodybuilders who had died from apparently unintentional opioid overdoses.
Aaron said his parents, teachers, and non-bodybuilding friends were unaware of this history. He claimed his parents were proud that their son had apparently eschewed drugs and alcohol to pursue a healthy athletic lifestyle.
RECOGNIZING AAS USE
In our experience with treating substance abusers, we find that AAS users may be the least likely to disclose their drug use to clinicians. In a recent study,1 20 of 36 AAS users (56%) reported they had never revealed their AAS use to any physician. When asked to rate their trust in sources of information about AAS, 17 of 42 AAS users (40%) said they trusted information from their drug dealers at least as much as information from any physician they had seen.
Some expressed contempt for physicians as “geeks” or “pencil-necks” who could not comprehend the body-building lifestyle. They gave doctors high marks on knowledge of tobacco, alcohol, and ordinary “street drugs” but much lower ratings on AAS knowledge. Other investigators have shown that many clinicians are unfamiliar with AAS.2,3
AAS users embrace these beliefs for two other reasons. First, to admit to AAS use is to admit that one’s muscularity and physical prowess is the result of taking a drug; there is no comparable motivation to withhold information about, say, one’s use of marijuana or cocaine.
Second, AAS users are much less likely than other substance abusers to view their behavior as pathologic. We have argued that our culture is partially to blame.4 Americans pay to watch 300-lb football linemen and AAS-using movie stars. Makers of cars, computers, and electronics do not hesitate to advertise their products as “on steroids,” but they would never claim their products were “on cocaine.” In this climate, it is easy to forget that AAS use is an illicit substance abuse.
Formula: FFMI = (W x (100 - BF)/100)H2 + 6.1 x (1.8 - H)
W = weight in kilograms
BF = body fat percentage
H = height in meters
Obtain height in meters and weight in kilograms. Ideally, measure body fat using calipers, electrical impedance, or some other method. Alternately, estimate body fat by visual inspection:
- 20% = average 30-year-old American man
- 10% = quite lean
- 5% = approaching lowest body fat normally attainable
FFMI values for American men:
20 = average
22 = visibly muscular
25 = approximate maximum attainable by a lean individual without using drugs
Example 1
Young male weightlifter is 69 inches tall, weighs 175 lbs, and is moderately lean, with body fat of 10%; he denies AAS use
H = 69 inches x .0254 meters/inch = 1.75 m
H2= 1.75 x 1.75 = 3.06 m2
W = 175 pounds x 0.454 kilograms/lb = 79.5 kg
Therefore, FFMI = (79.5 x (100-10)/100)/3.06 + 6.1 x (1.8 - 1.75) = 23.7
This degree of muscularity can be attained without using AAS
Example 2
Young male weightlifter is 66 inches tall, weighs 175 lbs, and is very lean, with body fat of 6%; he also denies AAS use
H = 66 inches x .0254 meters/inch = 1.68 m
H2= 1.68 x 1.68 = 2.82 m2
W = 175 pounds x 0.454 kilograms/lb = 79.5 kg
Therefore, FFMI = (79.5 x (100-6)/100)2.82 + 6.1 x (1.8 - 1.68) = 27.2
This level of muscularity is extremely unlikely without drugs. Patient is almost certainly lying and should be gently confronted, especially if other symptoms (Table 2) suggest AAS use
Table 2
Clues to possible AAS use in men
| Muscularity |
| Estimated fat-free mass index (FFMI) >26 (see Box 2) |
| Recent rapid muscle gains (>8 lb/month) |
| Striae over pectoralis muscles caused by rapid hypertrophy of underlying muscle |
| Other physical signs |
| Acne |
| Gynecomastia |
| Testicular atrophy |
| Psychiatric signs |
| Uncharacteristically aggressive behavior |
Uncharacteristic hypomanic symptoms
|
Uncharacteristic depressive symptoms
|
To overcome these treatment obstacles, we recommend that you:
- Become as knowledgeable about AAS use as you are about other forms of substance abuse (see Related resources).
- Approach AAS users as you would any other substance abusers—as individuals at risk for potentially serious medical and psychiatric consequences.
- Maintain a high index of suspicion when evaluating any muscular young male patient, even if he initially denies AAS use.
AAS use can often be suspected by looking at the patient as he walks in the door. Using what we call the “fat-free mass index” (FFMI) to calculate muscularity (Box 2), we have shown that a lean man can achieve only a certain amount of muscularity without using drugs.5 Although this finding needs to be replicated elsewhere, in our experience a man is almost certainly lying if he:
- is relatively lean (with approximately 10% body fat)
- displays an FFMI >26
- and claims he has not used drugs.
If a patient has an elevated FFMI and other cues suggesting AAS use (Table 2), gently but persistently question him if he denies using these drugs.
TREATING AAS-ASSOCIATED SYNDROMES
When you have established a history of AAS use, you will be far better prepared to anticipate and possibly treat its associated syndromes. Discussion of these effects is beyond the scope of this paper; for details, see reviews of AAS-associated medical effects,3,6 psychiatric effects,6,7 and general treatment principles.8 We focus here on the four scenarios clinicians encounter most often in practice and offer some pragmatic suggestions.
Forensic cases. AAS users almost never voluntarily seek help to stop their drug use. Such a request would be somewhat analogous to a girl with anorexia nervosa voluntarily asking for help to gain weight. We are unaware of any rehabilitation centers, clinics, 12-step programs, or the like for AAS users—there is no demand for them.
Thus, an AAS user may first come to clinical attention through legal channels. For example, if an AAS user committed a violent crime while experiencing hypomanic effects from these drugs, he might be required to undergo random urine testing as a condition of probation. This may be reasonable, provided that the tests are unannounced and urine is always collected under direct observation.
Monitoring clinicians may serve as little better than policemen, although sometimes it is possible to forge an alliance with the patient.
Depression. Exogenous AAS administration suppresses endogenous testosterone production through feedback mechanisms involving the hypothalamic-pituitary-testicular axis.3,6 Thus, during a long cycle, the user’s testes may shrink to half their normal size and stop producing testosterone and spermatozoa.
If the user then stops AAS rapidly, he may plunge into a profoundly hypogonadal state associated with symptoms of major depression. In a field study of 77 steroid users (71 male and 6 female), 6 (7.8%) reported they attempted suicide during AAS withdrawal.9 Depression associated with AAS withdrawal may prompt users to resume AAS quickly, triggering a syndrome of AAS dependence.6,10,11
Fortunately, AAS-withdrawal depression is usually self-limited and responds—in our experience and that of others12—to standard antidepressants. We recommend aggressively treating such depressions, as doing so may prevent resumption of AAS use and eventual AAS dependence.
Body-image disorders. AAS users often report body-image disorders, especially muscle dysmorphia—a form of body dysmorphic disorder where individuals become preoccupied with the belief that they are not adequately muscular.13,14 Anxieties about muscularity are a risk factor for subsequent AAS use15 and a major contributor to AAS dependence.8,11
Body dysmorphic disorder responds to pharmacologic and cognitive-behavioral interventions.3,16 Young men showing pathologic concerns about their muscularity or displaying related body-image pathology may benefit from prompt treatment before they are tempted to use AAS.
Progression to opioid dependence. An ominous development among American17 and British18 AAS users is a growing tendency to use opioids. In two studies of individuals with opioid dependence,19,20 7% to 9% reported beginning as AAS users, then learning about opioids from fellow bodybuilders and often buying their first illicit opioids from the person who had sold them AAS. Most learned as teenagers to use needles to inject AAS intramuscularly, so beginning to using opioids intravenously was only a small step.
In the last 5 years, we have become anecdotally aware of numerous AAS users who developed heroin addiction requiring repeated inpatient detoxification or who died of unintentional opioid overdoses. We suspect this phenomenon is under-recognized and urge clinicians to watch for it.
- Pope HG Jr, Brower KJ. Anabolic-androgenic steroid abuse. In: Sadock BJ, Sadock VA (eds). Comprehensive textbook of psychiatry (8th ed). Philadelphia: Lippincott Williams & Wilkins (in press).
- Yesalis CE (ed). Anabolic steroids in sport and exercise (2nd ed). Champaign, IL: Human Kinetics, 2000.
- The Taylor Hooton Foundation. Started by the father of a high school athlete who committed suicide during a depressive episode apparently precipitated by AAS withdrawal. Includes links to related Web sites. http://www.taylorhooton.org/about.asp. Accessed Nov. 10, 2004.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pope HG, Jr, Kanayama G, Ionescu-Pioggia M, Hudson JI. Anabolic steroid users’ attitudes towards physicians. Addiction 2004;99:1189-94.
2. Dawson RT. Drugs in sport—the role of the physician. J Endocrinol 2001;170:55-61.
3. Kutscher EC, Lund BC, Perry PJ. Anabolic steroids: a review for the clinician. Sports Med 2002;32:285-96.
4. Pope HG, Jr, Phillips KA, Olivardia R. The Adonis complex: the secret crisis of male body obsession. New York: Free Press, 2000.
5. Kouri EM, Pope HG, Jr, Katz DL, Oliva PS. Fat-free mass index in users and non-users of anabolic-androgenic steroids. Clin J Sport Med 1995;5:223-8.
6. Brower KJ. Anabolic steroid abuse and dependence. Curr Psychiatry Rep 2002;4:377-83.
7. Pope HG, Jr, Katz DL. Psychiatric effects of exogenous anabolic-androgenic steroids. In: Wolkowitz OM, Rothschild AJ (eds). Psychoneuroendocrinology: the scientific basis of clinical practice. Washington. DC: American Psychiatric Publishing, 2003;331-58.
8. Pope HG, Jr, Brower KJ. Anabolic-androgenic steroids. In: Galanter M, Kleber HD (eds). American Psychiatric Publishing textbook of substance abuse treatment (3rd ed). Washington DC: American Psychiatric Publishing, 2004;257-64.
9. Malone DA, Jr, Dimeff R, Lombardo JA, Sample BRH. Psychiatric effects and psychoactive substance use in anabolic-androgenic steroid users. Clin J Sports Med 1995;5:25-31
10. Kashkin KB, Kleber HD. Hooked on hormones? An anabolic steroid addiction hypothesis. JAMA 1989;262:3166-70.
11. Brower KJ, Eliopulos GA, Blow FC, et al. Evidence for physical and psychological dependence on anabolic androgenic steroids in eight weight lifters. Am J Psychiatry. 1990;147(4):510-2.
12. Malone DA, Jr, Dimeff RJ. The use of fluoxetine in depression associated with anabolic steroid withdrawal: a case series. J Clin Psychiatry. 1992;53:130-2.
13. Pope HG, Jr, Gruber AJ, Choi PY. Muscle dysmorphia: an underrecognized form of body dysmorphic disorder. Psychosomatics 1997;38:548-57.
14. Olivardia R, Pope HG, Jr, Hudson JI. ‘Muscle dysmorphia’ in male weightlifters: a case-control study. Am J Psychiatry 2000;157:1291-6.
15. Kanayama G, Pope HG, Jr, Cohane G, Hudson JI. Risk factors for anabolic-androgenic steroid use among weightlifters: a case-control study. Drug Alcohol Depend 2003;71:77-86.
16. Phillips KA. Pharmacologic treatment of body dysmorphic disorder: a review of empirical data and a proposed treatment algorithm. Psychiatr Clin North Am 2000;7:59-82.
17. Wines JD, Jr, Gruber AJ, Pope HG, Jr, Lukas SE. Nalbuphine hydrochloride dependence in anabolic steroid users. Am J Addictions 1999;8:161-4.
18. McBride AJ, Williamson K, Petersen T. Three cases of nalbuphine hydrochloride dependence associated with anabolic steroid abuse. Br J Sports Med 1996;30:69-70.
19. Kanayama G, Cohane G, Weiss RD, Pope HG, Jr. Past anabolic-androgenic steroid use among men admitted for substance abuse treatment: an underrecognized problem? J Clin Psychiatry. 2003;64:156-60.
20. Arvary D, Pope HG, Jr. Anabolic steroids: a possible gateway to opioid dependence. N Engl J Med 2000;342:1532.-
1. Pope HG, Jr, Kanayama G, Ionescu-Pioggia M, Hudson JI. Anabolic steroid users’ attitudes towards physicians. Addiction 2004;99:1189-94.
2. Dawson RT. Drugs in sport—the role of the physician. J Endocrinol 2001;170:55-61.
3. Kutscher EC, Lund BC, Perry PJ. Anabolic steroids: a review for the clinician. Sports Med 2002;32:285-96.
4. Pope HG, Jr, Phillips KA, Olivardia R. The Adonis complex: the secret crisis of male body obsession. New York: Free Press, 2000.
5. Kouri EM, Pope HG, Jr, Katz DL, Oliva PS. Fat-free mass index in users and non-users of anabolic-androgenic steroids. Clin J Sport Med 1995;5:223-8.
6. Brower KJ. Anabolic steroid abuse and dependence. Curr Psychiatry Rep 2002;4:377-83.
7. Pope HG, Jr, Katz DL. Psychiatric effects of exogenous anabolic-androgenic steroids. In: Wolkowitz OM, Rothschild AJ (eds). Psychoneuroendocrinology: the scientific basis of clinical practice. Washington. DC: American Psychiatric Publishing, 2003;331-58.
8. Pope HG, Jr, Brower KJ. Anabolic-androgenic steroids. In: Galanter M, Kleber HD (eds). American Psychiatric Publishing textbook of substance abuse treatment (3rd ed). Washington DC: American Psychiatric Publishing, 2004;257-64.
9. Malone DA, Jr, Dimeff R, Lombardo JA, Sample BRH. Psychiatric effects and psychoactive substance use in anabolic-androgenic steroid users. Clin J Sports Med 1995;5:25-31
10. Kashkin KB, Kleber HD. Hooked on hormones? An anabolic steroid addiction hypothesis. JAMA 1989;262:3166-70.
11. Brower KJ, Eliopulos GA, Blow FC, et al. Evidence for physical and psychological dependence on anabolic androgenic steroids in eight weight lifters. Am J Psychiatry. 1990;147(4):510-2.
12. Malone DA, Jr, Dimeff RJ. The use of fluoxetine in depression associated with anabolic steroid withdrawal: a case series. J Clin Psychiatry. 1992;53:130-2.
13. Pope HG, Jr, Gruber AJ, Choi PY. Muscle dysmorphia: an underrecognized form of body dysmorphic disorder. Psychosomatics 1997;38:548-57.
14. Olivardia R, Pope HG, Jr, Hudson JI. ‘Muscle dysmorphia’ in male weightlifters: a case-control study. Am J Psychiatry 2000;157:1291-6.
15. Kanayama G, Pope HG, Jr, Cohane G, Hudson JI. Risk factors for anabolic-androgenic steroid use among weightlifters: a case-control study. Drug Alcohol Depend 2003;71:77-86.
16. Phillips KA. Pharmacologic treatment of body dysmorphic disorder: a review of empirical data and a proposed treatment algorithm. Psychiatr Clin North Am 2000;7:59-82.
17. Wines JD, Jr, Gruber AJ, Pope HG, Jr, Lukas SE. Nalbuphine hydrochloride dependence in anabolic steroid users. Am J Addictions 1999;8:161-4.
18. McBride AJ, Williamson K, Petersen T. Three cases of nalbuphine hydrochloride dependence associated with anabolic steroid abuse. Br J Sports Med 1996;30:69-70.
19. Kanayama G, Cohane G, Weiss RD, Pope HG, Jr. Past anabolic-androgenic steroid use among men admitted for substance abuse treatment: an underrecognized problem? J Clin Psychiatry. 2003;64:156-60.
20. Arvary D, Pope HG, Jr. Anabolic steroids: a possible gateway to opioid dependence. N Engl J Med 2000;342:1532.-
A holiday wish: More security and accessibility
It’s the holiday season. You’ve got better things to think about than your computers’ security and data accessibility.
Many “tech toys” can help secure your computers and let you August 2004.)
Still, notebook computers are easy to steal, and desktop computers can be stolen or infiltrated. To secure your data, you need encryption to supplement password-restricted access. Also, you should regularly save and copy data in an alternate location.
Solutions. The Authenex HDLock is a universal serial bus (USB) key that encrypts your hard drive’s contents. The device uses “two-factor authentication,” a security method that blocks access until you provide something you have (the key) and something you know (the password). Without both the key and password, encrypted data cannot be accessed. If you lose the key or forget the password, an online support section provides a one-time password.
If more than one person needs access, the Silex Technology FUS-200N USB fingerprint reader is more appropriate. This device and its accompanying software:
- provide secured access for multiple users
- encrypt files and folders
- and force users to show their fingerprints before allowing access to selected programs, such as your medical records program.
The FUS-200N is more accurate than other fingerprint recognition devices because it uses electricity rather than light patterns to record fingerprints.
Another option, the DiskOnKey Classic 2.0 USB flash drive, includes a small built-in microprocessor that lets you run your electronic medical records program or other applications from the key. CapMed uses this key to allow consumers to store their medical information on the Personal HealthKey device.
See Table 1 for more information on these security enhancement programs.
Problem. When shuttling between home and office, it makes sense to carry files on a USB flash drive. These devices are great for transporting documents because the computer sees a flash drive as just another disk drive.
Flash drives, however, are easily lost—and no psychiatrist wants to be sued for losing sensitive information.
Solutions. Some USB flash drives, such as the Sony Micro Vault, offer password protection for files and folders. Another option, the Trek Thumbdrive Touch, has a built-in fingerprint reader to guard your data (Table 2).
If you already have a favorite USB flash drive, add quick- and easy-to-use encryption software to your home and office computers. AxCrypt, a free Windows-compatible program, lets you encrypt and decrypt files with a simple right-mouse click. A similar program, Fairly Good Privacy, is Mac OS-compatible (Table 1).
Problem. You need to access critical files in both your office and home computer from either location.
Solutions. Internet-based synchronization services can simultaneously update files on both computers (Table 3). fusionOne Plus, for example, keeps files, contacts, e-mail, and calendars in sync. Contacts and calendars can also be accessed and synchronized via some mobile phones. What’s more, FusionOne Plus provides an online backup copy of your files.
LogMeIn provides free secured remote access to your office files from any computer. After your install a server program on the host computer, you can run programs and open files via a Web browser. The LogMeIn Pro version, which costs $12.95 per month, adds the ability to synchronize computers, transfer files, and distribute them over the Internet.
GoToMyPC allows you to access files and programs on a host computer and also allows Pocket PC PDA viewing as well as remote printing, but it does not provide file synchronization.
For direct computer-to-computer connection, pcAnywhere and RealVNC allow you to access files and programs on the host computer. Unlike GoToMyPC and LogMeIn, however, these programs do not offer additional password protection by verifying the user’s account.
The $200 PC Anywhere program will encrypt data between computers, whereas the free RealVNC program only provides password security. PC Anywhere synchronizes file, whereas RealVNC only helps you run the remote computer. RealVNC has enterprise versions and is developing a personal version with additional features.
Online storage can help you avoid synchronization issues. Xdrive has desktop software that creates a virtual drive for storage. When starting your computer, the software will automatically connect via the Internet to your Xdrive account. You can also change the settings of your electronic medical records program or document editing software to save data only to this Internet drive. This way, your data will always be backed up to a safe location even if your home or office computer is stolen.
Table 1
Programs, services that enhance data security
| Product | URL | Cost | Requirements |
|---|---|---|---|
| AxCrypt | http://axcrypt.sourceforge.net/ | Free | Windows 95/98/ME/NT/2K/XP |
| DiskOnKey Classic 2.0 | http://www.diskonkey.com/prod_dok2.asp | $99.50 (256 MB), $159.90 (512MB), $329.90 (1GB) | Windows 98 SE, NT 4.0, 2000, ME, XP Mac OS: 9.x, 10.0.x, 10.1.x, 10.2.x, Linux 2.4.x |
| Fairly Good Privacy | http://www.securemac.com/fgp.php | Free | Mac OS System 7 and later |
| FUS-200N | http://www.silexamerica.com/us/products/fingerprint/fus200n.html | $149 | Windows XP, 2000 |
| HDLock | http://www.authenex.com/products_hdlock.cfm | $79.95 | Windows XP, 2000 |
Table 2
Secure flash drives
| Product | URL | Cost | Requirements |
|---|---|---|---|
| Micro Vault | http://www.sonystyle.com | $45.99 (256 MB) | Windows 98, 2000, ME, XP and MAC OS 9.0 and higher |
| Thumbdrive Touch | http://www.thumbdrive.com/touch.htm | $69 (16 MB), $299 (256 MB) | Mac OS 8.6 and above, Windows 98, 2000 and ME |
Table 3
Programs, services that facilitate remote data access
| Product | URL | Cost | Requirements |
|---|---|---|---|
| fusionOne Plus | http://store.yahoo.com/fusionone/ | $69.96/yr | Microsoft Windows 95/98/NT/2000/ME/XP |
| GoToMyPC | https://www.gotomypc.com/ | $19.95/month or $179.40/yr | Microsoft Windows 95/98/NT/2000/ME/XP |
| LogMeIn | https://secure.logmein.com/go.asp?page=home | Free | Windows 2000, XP, or Server 2003 |
| LogMeIn Pro | https://secure.logmein.com/go.asp?page=home | $12.95/month | Windows 2000, XP, and Server 2003 |
| pcAnywhere | http://www.symantec.com | $199.95 | Windows® XP Home/XP Pro/ 2000/NT 4/Me/98 |
| RealVNC free edition | http://www.realvnc.com | Free | Windows 9x/2000/ NT/XP, Linux, Mac OSX |
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
poll here
It’s the holiday season. You’ve got better things to think about than your computers’ security and data accessibility.
Many “tech toys” can help secure your computers and let you August 2004.)
Still, notebook computers are easy to steal, and desktop computers can be stolen or infiltrated. To secure your data, you need encryption to supplement password-restricted access. Also, you should regularly save and copy data in an alternate location.
Solutions. The Authenex HDLock is a universal serial bus (USB) key that encrypts your hard drive’s contents. The device uses “two-factor authentication,” a security method that blocks access until you provide something you have (the key) and something you know (the password). Without both the key and password, encrypted data cannot be accessed. If you lose the key or forget the password, an online support section provides a one-time password.
If more than one person needs access, the Silex Technology FUS-200N USB fingerprint reader is more appropriate. This device and its accompanying software:
- provide secured access for multiple users
- encrypt files and folders
- and force users to show their fingerprints before allowing access to selected programs, such as your medical records program.
The FUS-200N is more accurate than other fingerprint recognition devices because it uses electricity rather than light patterns to record fingerprints.
Another option, the DiskOnKey Classic 2.0 USB flash drive, includes a small built-in microprocessor that lets you run your electronic medical records program or other applications from the key. CapMed uses this key to allow consumers to store their medical information on the Personal HealthKey device.
See Table 1 for more information on these security enhancement programs.
Problem. When shuttling between home and office, it makes sense to carry files on a USB flash drive. These devices are great for transporting documents because the computer sees a flash drive as just another disk drive.
Flash drives, however, are easily lost—and no psychiatrist wants to be sued for losing sensitive information.
Solutions. Some USB flash drives, such as the Sony Micro Vault, offer password protection for files and folders. Another option, the Trek Thumbdrive Touch, has a built-in fingerprint reader to guard your data (Table 2).
If you already have a favorite USB flash drive, add quick- and easy-to-use encryption software to your home and office computers. AxCrypt, a free Windows-compatible program, lets you encrypt and decrypt files with a simple right-mouse click. A similar program, Fairly Good Privacy, is Mac OS-compatible (Table 1).
Problem. You need to access critical files in both your office and home computer from either location.
Solutions. Internet-based synchronization services can simultaneously update files on both computers (Table 3). fusionOne Plus, for example, keeps files, contacts, e-mail, and calendars in sync. Contacts and calendars can also be accessed and synchronized via some mobile phones. What’s more, FusionOne Plus provides an online backup copy of your files.
LogMeIn provides free secured remote access to your office files from any computer. After your install a server program on the host computer, you can run programs and open files via a Web browser. The LogMeIn Pro version, which costs $12.95 per month, adds the ability to synchronize computers, transfer files, and distribute them over the Internet.
GoToMyPC allows you to access files and programs on a host computer and also allows Pocket PC PDA viewing as well as remote printing, but it does not provide file synchronization.
For direct computer-to-computer connection, pcAnywhere and RealVNC allow you to access files and programs on the host computer. Unlike GoToMyPC and LogMeIn, however, these programs do not offer additional password protection by verifying the user’s account.
The $200 PC Anywhere program will encrypt data between computers, whereas the free RealVNC program only provides password security. PC Anywhere synchronizes file, whereas RealVNC only helps you run the remote computer. RealVNC has enterprise versions and is developing a personal version with additional features.
Online storage can help you avoid synchronization issues. Xdrive has desktop software that creates a virtual drive for storage. When starting your computer, the software will automatically connect via the Internet to your Xdrive account. You can also change the settings of your electronic medical records program or document editing software to save data only to this Internet drive. This way, your data will always be backed up to a safe location even if your home or office computer is stolen.
Table 1
Programs, services that enhance data security
| Product | URL | Cost | Requirements |
|---|---|---|---|
| AxCrypt | http://axcrypt.sourceforge.net/ | Free | Windows 95/98/ME/NT/2K/XP |
| DiskOnKey Classic 2.0 | http://www.diskonkey.com/prod_dok2.asp | $99.50 (256 MB), $159.90 (512MB), $329.90 (1GB) | Windows 98 SE, NT 4.0, 2000, ME, XP Mac OS: 9.x, 10.0.x, 10.1.x, 10.2.x, Linux 2.4.x |
| Fairly Good Privacy | http://www.securemac.com/fgp.php | Free | Mac OS System 7 and later |
| FUS-200N | http://www.silexamerica.com/us/products/fingerprint/fus200n.html | $149 | Windows XP, 2000 |
| HDLock | http://www.authenex.com/products_hdlock.cfm | $79.95 | Windows XP, 2000 |
Table 2
Secure flash drives
| Product | URL | Cost | Requirements |
|---|---|---|---|
| Micro Vault | http://www.sonystyle.com | $45.99 (256 MB) | Windows 98, 2000, ME, XP and MAC OS 9.0 and higher |
| Thumbdrive Touch | http://www.thumbdrive.com/touch.htm | $69 (16 MB), $299 (256 MB) | Mac OS 8.6 and above, Windows 98, 2000 and ME |
Table 3
Programs, services that facilitate remote data access
| Product | URL | Cost | Requirements |
|---|---|---|---|
| fusionOne Plus | http://store.yahoo.com/fusionone/ | $69.96/yr | Microsoft Windows 95/98/NT/2000/ME/XP |
| GoToMyPC | https://www.gotomypc.com/ | $19.95/month or $179.40/yr | Microsoft Windows 95/98/NT/2000/ME/XP |
| LogMeIn | https://secure.logmein.com/go.asp?page=home | Free | Windows 2000, XP, or Server 2003 |
| LogMeIn Pro | https://secure.logmein.com/go.asp?page=home | $12.95/month | Windows 2000, XP, and Server 2003 |
| pcAnywhere | http://www.symantec.com | $199.95 | Windows® XP Home/XP Pro/ 2000/NT 4/Me/98 |
| RealVNC free edition | http://www.realvnc.com | Free | Windows 9x/2000/ NT/XP, Linux, Mac OSX |
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
poll here
It’s the holiday season. You’ve got better things to think about than your computers’ security and data accessibility.
Many “tech toys” can help secure your computers and let you August 2004.)
Still, notebook computers are easy to steal, and desktop computers can be stolen or infiltrated. To secure your data, you need encryption to supplement password-restricted access. Also, you should regularly save and copy data in an alternate location.
Solutions. The Authenex HDLock is a universal serial bus (USB) key that encrypts your hard drive’s contents. The device uses “two-factor authentication,” a security method that blocks access until you provide something you have (the key) and something you know (the password). Without both the key and password, encrypted data cannot be accessed. If you lose the key or forget the password, an online support section provides a one-time password.
If more than one person needs access, the Silex Technology FUS-200N USB fingerprint reader is more appropriate. This device and its accompanying software:
- provide secured access for multiple users
- encrypt files and folders
- and force users to show their fingerprints before allowing access to selected programs, such as your medical records program.
The FUS-200N is more accurate than other fingerprint recognition devices because it uses electricity rather than light patterns to record fingerprints.
Another option, the DiskOnKey Classic 2.0 USB flash drive, includes a small built-in microprocessor that lets you run your electronic medical records program or other applications from the key. CapMed uses this key to allow consumers to store their medical information on the Personal HealthKey device.
See Table 1 for more information on these security enhancement programs.
Problem. When shuttling between home and office, it makes sense to carry files on a USB flash drive. These devices are great for transporting documents because the computer sees a flash drive as just another disk drive.
Flash drives, however, are easily lost—and no psychiatrist wants to be sued for losing sensitive information.
Solutions. Some USB flash drives, such as the Sony Micro Vault, offer password protection for files and folders. Another option, the Trek Thumbdrive Touch, has a built-in fingerprint reader to guard your data (Table 2).
If you already have a favorite USB flash drive, add quick- and easy-to-use encryption software to your home and office computers. AxCrypt, a free Windows-compatible program, lets you encrypt and decrypt files with a simple right-mouse click. A similar program, Fairly Good Privacy, is Mac OS-compatible (Table 1).
Problem. You need to access critical files in both your office and home computer from either location.
Solutions. Internet-based synchronization services can simultaneously update files on both computers (Table 3). fusionOne Plus, for example, keeps files, contacts, e-mail, and calendars in sync. Contacts and calendars can also be accessed and synchronized via some mobile phones. What’s more, FusionOne Plus provides an online backup copy of your files.
LogMeIn provides free secured remote access to your office files from any computer. After your install a server program on the host computer, you can run programs and open files via a Web browser. The LogMeIn Pro version, which costs $12.95 per month, adds the ability to synchronize computers, transfer files, and distribute them over the Internet.
GoToMyPC allows you to access files and programs on a host computer and also allows Pocket PC PDA viewing as well as remote printing, but it does not provide file synchronization.
For direct computer-to-computer connection, pcAnywhere and RealVNC allow you to access files and programs on the host computer. Unlike GoToMyPC and LogMeIn, however, these programs do not offer additional password protection by verifying the user’s account.
The $200 PC Anywhere program will encrypt data between computers, whereas the free RealVNC program only provides password security. PC Anywhere synchronizes file, whereas RealVNC only helps you run the remote computer. RealVNC has enterprise versions and is developing a personal version with additional features.
Online storage can help you avoid synchronization issues. Xdrive has desktop software that creates a virtual drive for storage. When starting your computer, the software will automatically connect via the Internet to your Xdrive account. You can also change the settings of your electronic medical records program or document editing software to save data only to this Internet drive. This way, your data will always be backed up to a safe location even if your home or office computer is stolen.
Table 1
Programs, services that enhance data security
| Product | URL | Cost | Requirements |
|---|---|---|---|
| AxCrypt | http://axcrypt.sourceforge.net/ | Free | Windows 95/98/ME/NT/2K/XP |
| DiskOnKey Classic 2.0 | http://www.diskonkey.com/prod_dok2.asp | $99.50 (256 MB), $159.90 (512MB), $329.90 (1GB) | Windows 98 SE, NT 4.0, 2000, ME, XP Mac OS: 9.x, 10.0.x, 10.1.x, 10.2.x, Linux 2.4.x |
| Fairly Good Privacy | http://www.securemac.com/fgp.php | Free | Mac OS System 7 and later |
| FUS-200N | http://www.silexamerica.com/us/products/fingerprint/fus200n.html | $149 | Windows XP, 2000 |
| HDLock | http://www.authenex.com/products_hdlock.cfm | $79.95 | Windows XP, 2000 |
Table 2
Secure flash drives
| Product | URL | Cost | Requirements |
|---|---|---|---|
| Micro Vault | http://www.sonystyle.com | $45.99 (256 MB) | Windows 98, 2000, ME, XP and MAC OS 9.0 and higher |
| Thumbdrive Touch | http://www.thumbdrive.com/touch.htm | $69 (16 MB), $299 (256 MB) | Mac OS 8.6 and above, Windows 98, 2000 and ME |
Table 3
Programs, services that facilitate remote data access
| Product | URL | Cost | Requirements |
|---|---|---|---|
| fusionOne Plus | http://store.yahoo.com/fusionone/ | $69.96/yr | Microsoft Windows 95/98/NT/2000/ME/XP |
| GoToMyPC | https://www.gotomypc.com/ | $19.95/month or $179.40/yr | Microsoft Windows 95/98/NT/2000/ME/XP |
| LogMeIn | https://secure.logmein.com/go.asp?page=home | Free | Windows 2000, XP, or Server 2003 |
| LogMeIn Pro | https://secure.logmein.com/go.asp?page=home | $12.95/month | Windows 2000, XP, and Server 2003 |
| pcAnywhere | http://www.symantec.com | $199.95 | Windows® XP Home/XP Pro/ 2000/NT 4/Me/98 |
| RealVNC free edition | http://www.realvnc.com | Free | Windows 9x/2000/ NT/XP, Linux, Mac OSX |
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
poll here
Bipolar MANIAS: Life events help confirm the diagnosis
Can knowing a patient’s environmental stressors and family history help us more quickly diagnose bipolar mania?
Kessing et al1 studied patients who were diagnosed as having mania or a mixed episode during their first psychiatric hospitalization. They found that certain life events were associated with these diagnoses, reinforcing the belief that environment to some extent influences psychiatric illness.
Although more research is needed, this finding may help psychiatrists reach a diagnosis of bipolar mania when the clinical course is unclear. Life events that may contribute to bipolar mania are remembered with the mnemonic MANIAS:
- Marital status change. The patient recently was married, divorced, or lost a significant other to death.
- Family Admission. The patient’s mother, father, or sibling was hospitalized at some point for a psychiatric disorder. It does not seem to matter whether the patient remembers the family member’s hospitalization.
- No work. The patient is unemployed.
- Inability to work. The patient is disabled or collects disability benefits.
- Abstaining from relationships. The patient does not have a significant other.
- Suicide was completed by the patient’s mother, father, or sibling. It does not matter how long ago or at what point in the patient’s life the suicide happened.
1. Kessing LV, Agerbo E, Mortensen PB. Major stressful life events and other risk factors for first admission with mania. Bipolar Disord 2004;6(2):122-9.
Dr. Wilson is a fellow, division of child and adolescent psychiatry, department of psychiatry, Louisiana State University Health Sciences Center, New Orleans.
Can knowing a patient’s environmental stressors and family history help us more quickly diagnose bipolar mania?
Kessing et al1 studied patients who were diagnosed as having mania or a mixed episode during their first psychiatric hospitalization. They found that certain life events were associated with these diagnoses, reinforcing the belief that environment to some extent influences psychiatric illness.
Although more research is needed, this finding may help psychiatrists reach a diagnosis of bipolar mania when the clinical course is unclear. Life events that may contribute to bipolar mania are remembered with the mnemonic MANIAS:
- Marital status change. The patient recently was married, divorced, or lost a significant other to death.
- Family Admission. The patient’s mother, father, or sibling was hospitalized at some point for a psychiatric disorder. It does not seem to matter whether the patient remembers the family member’s hospitalization.
- No work. The patient is unemployed.
- Inability to work. The patient is disabled or collects disability benefits.
- Abstaining from relationships. The patient does not have a significant other.
- Suicide was completed by the patient’s mother, father, or sibling. It does not matter how long ago or at what point in the patient’s life the suicide happened.
Can knowing a patient’s environmental stressors and family history help us more quickly diagnose bipolar mania?
Kessing et al1 studied patients who were diagnosed as having mania or a mixed episode during their first psychiatric hospitalization. They found that certain life events were associated with these diagnoses, reinforcing the belief that environment to some extent influences psychiatric illness.
Although more research is needed, this finding may help psychiatrists reach a diagnosis of bipolar mania when the clinical course is unclear. Life events that may contribute to bipolar mania are remembered with the mnemonic MANIAS:
- Marital status change. The patient recently was married, divorced, or lost a significant other to death.
- Family Admission. The patient’s mother, father, or sibling was hospitalized at some point for a psychiatric disorder. It does not seem to matter whether the patient remembers the family member’s hospitalization.
- No work. The patient is unemployed.
- Inability to work. The patient is disabled or collects disability benefits.
- Abstaining from relationships. The patient does not have a significant other.
- Suicide was completed by the patient’s mother, father, or sibling. It does not matter how long ago or at what point in the patient’s life the suicide happened.
1. Kessing LV, Agerbo E, Mortensen PB. Major stressful life events and other risk factors for first admission with mania. Bipolar Disord 2004;6(2):122-9.
Dr. Wilson is a fellow, division of child and adolescent psychiatry, department of psychiatry, Louisiana State University Health Sciences Center, New Orleans.
1. Kessing LV, Agerbo E, Mortensen PB. Major stressful life events and other risk factors for first admission with mania. Bipolar Disord 2004;6(2):122-9.
Dr. Wilson is a fellow, division of child and adolescent psychiatry, department of psychiatry, Louisiana State University Health Sciences Center, New Orleans.
Lab tests in psychiatry
“Prudent prescribing: Intelligent use of lab tests and other diagnostics” (Current Psychiatry, October 2004) effectively reviewed key principles for using diagnostic and monitoring tools in psychiatry.
At a time when psychologists in some states have gained or may soon gain prescribing privileges, we as psychiatrists must embrace our medical training and apply our understanding of laboratory testing and diagnostic studies to our patients’ advantage. Outpatient laboratory testing is often difficult to coordinate, mostly because we traditionally have saved such tests for rare and unusual cases. As our use of such testing expands, we will more efficiently coordinate sample collection and report results to provide safe, effective medical care.
Also, more laboratory tests will soon be available. For example, the FDA recently approved a carbohydrate-deficient transferrin test to help clinicians detect or monitor an alcohol use disorder.
Regular and substantial alcohol use affects liver metabolism and transferrin synthesis, causing an abnormal, carbohydrate-deficient form of transferrin to be released into the bloodstream. Abnormal transferrin is measured as a percentage of overall transferrin in the bloodstream (%CDT). Any increase in %CDT above the normal range indicates a clinically significant increase in drinking. We consider 2.6 the upper limit of normal.
The test detects %CDT elevations in patients who have consumed 60 to 80 grams of alcohol (4 to 6 standard drinks) daily for 2 to 3 weeks. The test offers 90% to 95% specificity, with false elevations coming from inborn errors of glycoprotein metabolism (1% to 2% of the population) and in some cases from severe liver disease. Sensitivity is approximately 60% but improves significantly when the test is correlated with gamma-glutamyltransferase (GGT) testing and other serum markers.
Jeffrey S. Cluver, MD
Assistant professor of psychiatry
Ralph H. Johnson Veterans Affairs
Medical Center Medical University of South Carolina,
Charleston
- Anton RF, Lieber C, Tabakoff B, for the CDTect Study Group. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multisite study. Alcohol Clin Exp Res 2002;26:1215–22.
- Conigrave KM, Degenhardt LJ, Whitfield JB, et al, for the WHO/ ISBRA Study Group. CDT, GGT, and AST as markers of alcohol use: the WHO/ISBRA Collaborative Project. Alcohol Clin Exp Res 2002;26:332–9.
“Prudent prescribing: Intelligent use of lab tests and other diagnostics” (Current Psychiatry, October 2004) effectively reviewed key principles for using diagnostic and monitoring tools in psychiatry.
At a time when psychologists in some states have gained or may soon gain prescribing privileges, we as psychiatrists must embrace our medical training and apply our understanding of laboratory testing and diagnostic studies to our patients’ advantage. Outpatient laboratory testing is often difficult to coordinate, mostly because we traditionally have saved such tests for rare and unusual cases. As our use of such testing expands, we will more efficiently coordinate sample collection and report results to provide safe, effective medical care.
Also, more laboratory tests will soon be available. For example, the FDA recently approved a carbohydrate-deficient transferrin test to help clinicians detect or monitor an alcohol use disorder.
Regular and substantial alcohol use affects liver metabolism and transferrin synthesis, causing an abnormal, carbohydrate-deficient form of transferrin to be released into the bloodstream. Abnormal transferrin is measured as a percentage of overall transferrin in the bloodstream (%CDT). Any increase in %CDT above the normal range indicates a clinically significant increase in drinking. We consider 2.6 the upper limit of normal.
The test detects %CDT elevations in patients who have consumed 60 to 80 grams of alcohol (4 to 6 standard drinks) daily for 2 to 3 weeks. The test offers 90% to 95% specificity, with false elevations coming from inborn errors of glycoprotein metabolism (1% to 2% of the population) and in some cases from severe liver disease. Sensitivity is approximately 60% but improves significantly when the test is correlated with gamma-glutamyltransferase (GGT) testing and other serum markers.
Jeffrey S. Cluver, MD
Assistant professor of psychiatry
Ralph H. Johnson Veterans Affairs
Medical Center Medical University of South Carolina,
Charleston
- Anton RF, Lieber C, Tabakoff B, for the CDTect Study Group. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multisite study. Alcohol Clin Exp Res 2002;26:1215–22.
- Conigrave KM, Degenhardt LJ, Whitfield JB, et al, for the WHO/ ISBRA Study Group. CDT, GGT, and AST as markers of alcohol use: the WHO/ISBRA Collaborative Project. Alcohol Clin Exp Res 2002;26:332–9.
“Prudent prescribing: Intelligent use of lab tests and other diagnostics” (Current Psychiatry, October 2004) effectively reviewed key principles for using diagnostic and monitoring tools in psychiatry.
At a time when psychologists in some states have gained or may soon gain prescribing privileges, we as psychiatrists must embrace our medical training and apply our understanding of laboratory testing and diagnostic studies to our patients’ advantage. Outpatient laboratory testing is often difficult to coordinate, mostly because we traditionally have saved such tests for rare and unusual cases. As our use of such testing expands, we will more efficiently coordinate sample collection and report results to provide safe, effective medical care.
Also, more laboratory tests will soon be available. For example, the FDA recently approved a carbohydrate-deficient transferrin test to help clinicians detect or monitor an alcohol use disorder.
Regular and substantial alcohol use affects liver metabolism and transferrin synthesis, causing an abnormal, carbohydrate-deficient form of transferrin to be released into the bloodstream. Abnormal transferrin is measured as a percentage of overall transferrin in the bloodstream (%CDT). Any increase in %CDT above the normal range indicates a clinically significant increase in drinking. We consider 2.6 the upper limit of normal.
The test detects %CDT elevations in patients who have consumed 60 to 80 grams of alcohol (4 to 6 standard drinks) daily for 2 to 3 weeks. The test offers 90% to 95% specificity, with false elevations coming from inborn errors of glycoprotein metabolism (1% to 2% of the population) and in some cases from severe liver disease. Sensitivity is approximately 60% but improves significantly when the test is correlated with gamma-glutamyltransferase (GGT) testing and other serum markers.
Jeffrey S. Cluver, MD
Assistant professor of psychiatry
Ralph H. Johnson Veterans Affairs
Medical Center Medical University of South Carolina,
Charleston
- Anton RF, Lieber C, Tabakoff B, for the CDTect Study Group. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multisite study. Alcohol Clin Exp Res 2002;26:1215–22.
- Conigrave KM, Degenhardt LJ, Whitfield JB, et al, for the WHO/ ISBRA Study Group. CDT, GGT, and AST as markers of alcohol use: the WHO/ISBRA Collaborative Project. Alcohol Clin Exp Res 2002;26:332–9.
What would Confucius say about mood stabilizers?
When Confucius was asked what he would do first should he become minister of the Kingdom of Wei, he replied: “What is necessary is to rectify names.” He further stated, “If names be not correct, language is not in accordance with the truth of things. If language be not in accordance with the truth of things, affairs cannot be carried on to success” (from The Analects of Confucius, James R. Ware translation, 1980: book 13, verse 3).
I am reminded of this aphorism by Dr. Leslie Citrome’s article, “Treatment-resistant schizophrenia: What role for mood stabilizers?”. This article highlights several psychotropic classes whose names badly need rectification, including “mood stabilizers,” “anticonvulsants,” and “antipsychotics.”
A compound’s first use tends to give it its functional name. Sodium valproate was first used to treat seizures, so it is called an anticonvulsant. Lithium was first used to stabilize mood, so it is called a mood stabilizer. Sometimes valproate is now called a mood stabilizer as well. As Dr. Citrome demonstrates, both compounds also may have efficacy as adjuncts in treating schizophrenia. So do we call them antipsychotics, too?
Each compound has a variety of effects, of course, and we start to diverge from the “truth of things” when we get locked into thinking of a compound in just one way. We also make it less likely that affairs can “be carried on to success” for our patients.
When Confucius was asked what he would do first should he become minister of the Kingdom of Wei, he replied: “What is necessary is to rectify names.” He further stated, “If names be not correct, language is not in accordance with the truth of things. If language be not in accordance with the truth of things, affairs cannot be carried on to success” (from The Analects of Confucius, James R. Ware translation, 1980: book 13, verse 3).
I am reminded of this aphorism by Dr. Leslie Citrome’s article, “Treatment-resistant schizophrenia: What role for mood stabilizers?”. This article highlights several psychotropic classes whose names badly need rectification, including “mood stabilizers,” “anticonvulsants,” and “antipsychotics.”
A compound’s first use tends to give it its functional name. Sodium valproate was first used to treat seizures, so it is called an anticonvulsant. Lithium was first used to stabilize mood, so it is called a mood stabilizer. Sometimes valproate is now called a mood stabilizer as well. As Dr. Citrome demonstrates, both compounds also may have efficacy as adjuncts in treating schizophrenia. So do we call them antipsychotics, too?
Each compound has a variety of effects, of course, and we start to diverge from the “truth of things” when we get locked into thinking of a compound in just one way. We also make it less likely that affairs can “be carried on to success” for our patients.
When Confucius was asked what he would do first should he become minister of the Kingdom of Wei, he replied: “What is necessary is to rectify names.” He further stated, “If names be not correct, language is not in accordance with the truth of things. If language be not in accordance with the truth of things, affairs cannot be carried on to success” (from The Analects of Confucius, James R. Ware translation, 1980: book 13, verse 3).
I am reminded of this aphorism by Dr. Leslie Citrome’s article, “Treatment-resistant schizophrenia: What role for mood stabilizers?”. This article highlights several psychotropic classes whose names badly need rectification, including “mood stabilizers,” “anticonvulsants,” and “antipsychotics.”
A compound’s first use tends to give it its functional name. Sodium valproate was first used to treat seizures, so it is called an anticonvulsant. Lithium was first used to stabilize mood, so it is called a mood stabilizer. Sometimes valproate is now called a mood stabilizer as well. As Dr. Citrome demonstrates, both compounds also may have efficacy as adjuncts in treating schizophrenia. So do we call them antipsychotics, too?
Each compound has a variety of effects, of course, and we start to diverge from the “truth of things” when we get locked into thinking of a compound in just one way. We also make it less likely that affairs can “be carried on to success” for our patients.
Commentary: When medical illness complicates schizophrenia and bipolar disorder
Patients with schizophrenia or bipolar disorder face a higher risk of premature death, compared with the general population. Their overall mortality rate is elevated not only by higher suicide rates but also by higher rates of medical comorbidities, including obesity, diabetes mellitus, cardiovascular and pulmonary diseases, HIV infection, and cancer.1,2
Schizophrenia and bipolar disorder are also frequently complicated by psychiatric comorbidities including alcohol and substance use disorders (such as nicotine dependence), anxiety disorders, and eating disorders (such as binge eating).3,4 Even so, the impact of psychiatric comorbidity on medical morbidity and mortality has not been adequately investigated and has—until rather recently—received little attention from clinical researchers.
Within the past 5 years or so, epidemiologic and population studies have shown increased morbidity and mortality from medical illnesses in patients with serious and persistent mental illness.5,6 These findings have sparked renewed interest in behavioral, biological, and psychosocial factors associated with schizophrenia and bipolar disorder that may contribute to comorbid medical illnesses. These factors include:
- negative and depressive symptoms
- physical inactivity and poor diet
- hypothalamic-pituitary-adrenal axis dysregulation associated with acute psychotic and affective episodes
- amotivation
- social isolation
- limited access to primary and preventive health care.7
The degree to which these factors may elevate medical comorbidity rates in patients with Commentary these serious psychiatric illnesses has not been well-studied or described.
TREATMENT IMPLICATIONS
These observations raise concerns about safe and effective use of medications by patients with schizophrenia or bipolar disorder as well as medical illness. Issues for clinicians to consider include:
- possible pharmacokinetic and pharmacodynamic interactions in patients taking concomitant medications for psychiatric and medical illnesses
- potential beneficial and adverse effects of psychotropics on medical illnesses
- and—conversely—potential beneficial and adverse effects on mood and psychotic disorders of medications used to treat medical illnesses.
Being aware of metabolic effects when prescribing psychotropics is not a new idea. Lithium, for example, has long been known to cause weight gain, suppress thyroid hormone, and interact with thiazide diuretics when used to treat bipolar disorder. Thus, therapeutic blood monitoring is recommended for patients receiving acute and maintenance lithium therapy.
More recently, atypical antipsychotics such as olanzapine and clozapine (and risperidone and quetiapine to a lesser extent) have been shown to produce substantial weight gain and other metabolic effects that increase the risk of diabetes and cardiovascular disease.7 As a result, the American Diabetes Association—along with the American Psychiatric Association and other groups—now recommends that psychiatrists and other physicians monitor patients’ body weight and body mass index, vital signs, serum glucose, and lipids when prescribing these agents.7-11 Careful monitoring should improve the medical care of patients with schizophrenia and bipolar disorder and help protect them from the medical risks associated with overweight and obesity.
Similar precautions are needed when schizophrenia and bipolar disorder co-occur with other medical illnesses treated by complex pharmacologic regimens, such as pulmonary disease, cancer, and HIV. Three points to keep in mind are:
- careful selection of treatments for co-occurring medical illnesses, considering potential effects on the primary psychiatric disorder
- the impact of psychotropics on patients’ medical illnesses
- and the potential for drug interactions.
1. Kilbourne AM, Cornelius JR, Han X, et al. Burden of general medical conditions among individuals with bipolar disorder. Bipolar Disord 2004;6:368-73.
2. Meyer JM, Nasrallah HA. Medical illness and schizophrenia Washington, DC: American Psychiatric Press, Inc, 2003.
3. McElroy SL, Altshuler LL, Suppes T, et al. Axis I psychiatric comorbidity and its relationship with historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry 2001;158:420-6.
4. Green AI, Canuso CM, Brenner MJ, et al. Detection and management of comorbidity in patients with schizophrenia. Psychiatr Clin North Am 2003;26:115-39.
5. Buda M, Tsuang MT, Fleming JA. Causes of death in DSM-III schizophrenics and other psychotics (atypical group). Comparison with the general population. Arch Gen Psychiatry 1988;45:283-5.
6. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 2001;58:844-50.
7. Keck PE, Jr, Buse JB, Dagago-Jack S, et al. Managing metabolic concerns in patients with severe mental illness: a special report (Postgrad Med). Minneapolis, MN: McGraw-Hill, 2003.
8. American Diabetes Association. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care 2004;27:596-601.
9. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004;161:1334-9.
10. Chue P, Kovacs CS. Safety and tolerability of atypical antipsychotics in patients with bipolar disorder: prevalence, monitoring and management. Bipolar Disord 2003;5(suppl 2):62-79.
11. Nasrallah HA, Newcomer JW. Atypical antipsychotics and metabolic dysregulation. Evaluating the risk/benefit equation and improving standard of care. J Clin Psychopharmacol 2004;24(suppl 2):S7-S14.
Patients with schizophrenia or bipolar disorder face a higher risk of premature death, compared with the general population. Their overall mortality rate is elevated not only by higher suicide rates but also by higher rates of medical comorbidities, including obesity, diabetes mellitus, cardiovascular and pulmonary diseases, HIV infection, and cancer.1,2
Schizophrenia and bipolar disorder are also frequently complicated by psychiatric comorbidities including alcohol and substance use disorders (such as nicotine dependence), anxiety disorders, and eating disorders (such as binge eating).3,4 Even so, the impact of psychiatric comorbidity on medical morbidity and mortality has not been adequately investigated and has—until rather recently—received little attention from clinical researchers.
Within the past 5 years or so, epidemiologic and population studies have shown increased morbidity and mortality from medical illnesses in patients with serious and persistent mental illness.5,6 These findings have sparked renewed interest in behavioral, biological, and psychosocial factors associated with schizophrenia and bipolar disorder that may contribute to comorbid medical illnesses. These factors include:
- negative and depressive symptoms
- physical inactivity and poor diet
- hypothalamic-pituitary-adrenal axis dysregulation associated with acute psychotic and affective episodes
- amotivation
- social isolation
- limited access to primary and preventive health care.7
The degree to which these factors may elevate medical comorbidity rates in patients with Commentary these serious psychiatric illnesses has not been well-studied or described.
TREATMENT IMPLICATIONS
These observations raise concerns about safe and effective use of medications by patients with schizophrenia or bipolar disorder as well as medical illness. Issues for clinicians to consider include:
- possible pharmacokinetic and pharmacodynamic interactions in patients taking concomitant medications for psychiatric and medical illnesses
- potential beneficial and adverse effects of psychotropics on medical illnesses
- and—conversely—potential beneficial and adverse effects on mood and psychotic disorders of medications used to treat medical illnesses.
Being aware of metabolic effects when prescribing psychotropics is not a new idea. Lithium, for example, has long been known to cause weight gain, suppress thyroid hormone, and interact with thiazide diuretics when used to treat bipolar disorder. Thus, therapeutic blood monitoring is recommended for patients receiving acute and maintenance lithium therapy.
More recently, atypical antipsychotics such as olanzapine and clozapine (and risperidone and quetiapine to a lesser extent) have been shown to produce substantial weight gain and other metabolic effects that increase the risk of diabetes and cardiovascular disease.7 As a result, the American Diabetes Association—along with the American Psychiatric Association and other groups—now recommends that psychiatrists and other physicians monitor patients’ body weight and body mass index, vital signs, serum glucose, and lipids when prescribing these agents.7-11 Careful monitoring should improve the medical care of patients with schizophrenia and bipolar disorder and help protect them from the medical risks associated with overweight and obesity.
Similar precautions are needed when schizophrenia and bipolar disorder co-occur with other medical illnesses treated by complex pharmacologic regimens, such as pulmonary disease, cancer, and HIV. Three points to keep in mind are:
- careful selection of treatments for co-occurring medical illnesses, considering potential effects on the primary psychiatric disorder
- the impact of psychotropics on patients’ medical illnesses
- and the potential for drug interactions.
Patients with schizophrenia or bipolar disorder face a higher risk of premature death, compared with the general population. Their overall mortality rate is elevated not only by higher suicide rates but also by higher rates of medical comorbidities, including obesity, diabetes mellitus, cardiovascular and pulmonary diseases, HIV infection, and cancer.1,2
Schizophrenia and bipolar disorder are also frequently complicated by psychiatric comorbidities including alcohol and substance use disorders (such as nicotine dependence), anxiety disorders, and eating disorders (such as binge eating).3,4 Even so, the impact of psychiatric comorbidity on medical morbidity and mortality has not been adequately investigated and has—until rather recently—received little attention from clinical researchers.
Within the past 5 years or so, epidemiologic and population studies have shown increased morbidity and mortality from medical illnesses in patients with serious and persistent mental illness.5,6 These findings have sparked renewed interest in behavioral, biological, and psychosocial factors associated with schizophrenia and bipolar disorder that may contribute to comorbid medical illnesses. These factors include:
- negative and depressive symptoms
- physical inactivity and poor diet
- hypothalamic-pituitary-adrenal axis dysregulation associated with acute psychotic and affective episodes
- amotivation
- social isolation
- limited access to primary and preventive health care.7
The degree to which these factors may elevate medical comorbidity rates in patients with Commentary these serious psychiatric illnesses has not been well-studied or described.
TREATMENT IMPLICATIONS
These observations raise concerns about safe and effective use of medications by patients with schizophrenia or bipolar disorder as well as medical illness. Issues for clinicians to consider include:
- possible pharmacokinetic and pharmacodynamic interactions in patients taking concomitant medications for psychiatric and medical illnesses
- potential beneficial and adverse effects of psychotropics on medical illnesses
- and—conversely—potential beneficial and adverse effects on mood and psychotic disorders of medications used to treat medical illnesses.
Being aware of metabolic effects when prescribing psychotropics is not a new idea. Lithium, for example, has long been known to cause weight gain, suppress thyroid hormone, and interact with thiazide diuretics when used to treat bipolar disorder. Thus, therapeutic blood monitoring is recommended for patients receiving acute and maintenance lithium therapy.
More recently, atypical antipsychotics such as olanzapine and clozapine (and risperidone and quetiapine to a lesser extent) have been shown to produce substantial weight gain and other metabolic effects that increase the risk of diabetes and cardiovascular disease.7 As a result, the American Diabetes Association—along with the American Psychiatric Association and other groups—now recommends that psychiatrists and other physicians monitor patients’ body weight and body mass index, vital signs, serum glucose, and lipids when prescribing these agents.7-11 Careful monitoring should improve the medical care of patients with schizophrenia and bipolar disorder and help protect them from the medical risks associated with overweight and obesity.
Similar precautions are needed when schizophrenia and bipolar disorder co-occur with other medical illnesses treated by complex pharmacologic regimens, such as pulmonary disease, cancer, and HIV. Three points to keep in mind are:
- careful selection of treatments for co-occurring medical illnesses, considering potential effects on the primary psychiatric disorder
- the impact of psychotropics on patients’ medical illnesses
- and the potential for drug interactions.
1. Kilbourne AM, Cornelius JR, Han X, et al. Burden of general medical conditions among individuals with bipolar disorder. Bipolar Disord 2004;6:368-73.
2. Meyer JM, Nasrallah HA. Medical illness and schizophrenia Washington, DC: American Psychiatric Press, Inc, 2003.
3. McElroy SL, Altshuler LL, Suppes T, et al. Axis I psychiatric comorbidity and its relationship with historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry 2001;158:420-6.
4. Green AI, Canuso CM, Brenner MJ, et al. Detection and management of comorbidity in patients with schizophrenia. Psychiatr Clin North Am 2003;26:115-39.
5. Buda M, Tsuang MT, Fleming JA. Causes of death in DSM-III schizophrenics and other psychotics (atypical group). Comparison with the general population. Arch Gen Psychiatry 1988;45:283-5.
6. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 2001;58:844-50.
7. Keck PE, Jr, Buse JB, Dagago-Jack S, et al. Managing metabolic concerns in patients with severe mental illness: a special report (Postgrad Med). Minneapolis, MN: McGraw-Hill, 2003.
8. American Diabetes Association. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care 2004;27:596-601.
9. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004;161:1334-9.
10. Chue P, Kovacs CS. Safety and tolerability of atypical antipsychotics in patients with bipolar disorder: prevalence, monitoring and management. Bipolar Disord 2003;5(suppl 2):62-79.
11. Nasrallah HA, Newcomer JW. Atypical antipsychotics and metabolic dysregulation. Evaluating the risk/benefit equation and improving standard of care. J Clin Psychopharmacol 2004;24(suppl 2):S7-S14.
1. Kilbourne AM, Cornelius JR, Han X, et al. Burden of general medical conditions among individuals with bipolar disorder. Bipolar Disord 2004;6:368-73.
2. Meyer JM, Nasrallah HA. Medical illness and schizophrenia Washington, DC: American Psychiatric Press, Inc, 2003.
3. McElroy SL, Altshuler LL, Suppes T, et al. Axis I psychiatric comorbidity and its relationship with historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry 2001;158:420-6.
4. Green AI, Canuso CM, Brenner MJ, et al. Detection and management of comorbidity in patients with schizophrenia. Psychiatr Clin North Am 2003;26:115-39.
5. Buda M, Tsuang MT, Fleming JA. Causes of death in DSM-III schizophrenics and other psychotics (atypical group). Comparison with the general population. Arch Gen Psychiatry 1988;45:283-5.
6. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 2001;58:844-50.
7. Keck PE, Jr, Buse JB, Dagago-Jack S, et al. Managing metabolic concerns in patients with severe mental illness: a special report (Postgrad Med). Minneapolis, MN: McGraw-Hill, 2003.
8. American Diabetes Association. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care 2004;27:596-601.
9. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004;161:1334-9.
10. Chue P, Kovacs CS. Safety and tolerability of atypical antipsychotics in patients with bipolar disorder: prevalence, monitoring and management. Bipolar Disord 2003;5(suppl 2):62-79.
11. Nasrallah HA, Newcomer JW. Atypical antipsychotics and metabolic dysregulation. Evaluating the risk/benefit equation and improving standard of care. J Clin Psychopharmacol 2004;24(suppl 2):S7-S14.
Sex and antidepressants: When to switch drugs or try an antidote
Antidepressants’ sexual side effects can often be managed—while preserving the antidepressant effect—by altering dosages, switching to another drug class, or adding an “antidote.” Understanding the benefits and risks of each strategy can help you:
- base treatment choices on your patient’s history and side-effect experience
- improve long-term compliance with antidepressant regimens.
EFFECTS VARY BY ANTIDEPRESSANT CLASS
Antidepressants may affect one or more phases of sexual functioning:
- desire (libido)
- arousal (erection or vaginal lubrication)
- orgasm/ejaculation.
Sexual symptoms linked to antidepressants range from diminished interest/arousal and delayed orgasm to heightened sexual functioning (Table 1). Resulting sexual dysfunction can impair quality of life and intimate relationships and discourage patients from taking antidepressants (Box) 1,2
Table 1
Sexual side effects linked to antidepressants
| Most common effects | Shown by these drugs |
|---|---|
| Decreased desire | TCAs, MAOIs, SSRIs |
| Delayed or absent ejaculation/orgasm | TCAs, MAOIs, SSRIs |
| Impaired erection | TCAs, MAOIs, SSRIs |
| Less common effects | |
| Increased desire | Bupropion |
| Spontaneous/prolonged erections | SSRIs, CMI,bupropion, trazodone, nefazodone |
| Premature/retrograde/painful ejaculations | TCAs, trazodone, nefazodone |
| Priapism | SSRIs, CMI, bupropion, trazodone, nefazodone |
| Spontaneous orgasms (associated with yawning) | SSRIs, CMI, bupropion |
| Altered sexual sensation and sensitivity | SSRIs, CMI, bupropion |
| TCAs: tricyclics | |
| MAOIs: monoamine oxidase inhibitors | |
| SSRIs: selective serotonin reuptake inhibitors | |
| CMI: clomipramine | |
Although most reports have focused on SSRIs, all antidepressant classes have been associated with sexual dysfunction, with prevalence likely influenced by differences in neurotransmitter modulation (Table 2).1,3,4 The highest rates of sexual side effects have been reported with SSRIs, certain tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs).
A recent study reported similarly high rates with mirtazapine, but its small sample size limits conclusions about side effect prevalence with this drug.1 Other studies have found significantly lower rates with bupropion and nefazodone.
TCAs’ sexual side-effect rates and types depend on how much each drug inhibits serotonin reuptake. Clomipramine appears to have the highest rates of sexual dysfunction—particularly anorgasmia—probably because it inhibits the serotonin transporter more than do other TCAs.5 In TCAs with lesser effects on serotonergic neurotransmission, alpha-adrenergic and cholinergic receptor blockade may cause sexual side effects—particularly erectile dysfunction (ED).
Cholinergic agonists such as bethanechol, 10 to 50 mg/d, may reverse sexual dysfunction caused by anticholinergic effects.6 Cyproheptadine—a nonselective serotonin receptor antagonist—has also shown benefit at 4 to 12 mg/d in treating TCA-related sexual side effects.7
MAOIs. Sexual side effects appear to be more prevalent with MAOIs than with TCAs,4 perhaps similar to the rate seen with SSRIs. MAOIs directly increase serotonergic neurotransmission, and their substantial alpha-adrenergic antagonist effects may also produce sexual side effects.
Waiting for symptoms to subside may be appropriate, as anorgasmia caused by MAOIs may remit spontaneously. Sildenafil8 and cyproheptadine9 may reverse MAOI sexual side effects, although serious toxicity has been reported in a patient taking cyproheptadine and an MAOI.10
SSRIs. Increased serotonergic neurotransmission is widely believed to cause SSRI sexual side effects. Resulting secondary effects—such as inhibited central dopamine release, increased prolactin secretion, and inhibited nitric oxide synthesis—may also play important roles.
In general, SSRIs appear to alter sexual functioning in 40% to 60% of patients—both men and women. Anorgasmia is the most commonly reported sexual symptom.
Although all SSRIs are associated with sexual dysfunction, some studies have found higher rates with paroxetine. One study associated paroxetine with significantly higher rates of ED compared with other SSRIs. The authors attributed this finding to paroxetine’s greater anticholinergic effects or to its directly decreasing nitric oxide synthesis.3
SSRI MANAGEMENT STRATEGIES
Waiting. The simplest, safest way to manage SSRI-related sexual dysfunction is to wait and see if side effects resolve spontaneously. Sexual side effects improve without treatment in approximately 20% of cases,3 although improvement is often incomplete. Moreover, several months may pass before symptoms diminish adequately, making this strategy impractical for patients with substantial sexual dysfunction.
Dosing changes. Because SSRIs’ sexual side effects appear to be dose-related,11 carefully reducing the dosage may reduce sexual dysfunction without compromising antidepressant efficacy. This strategy is most likely to sustain remission when you avoid dosages that have proven ineffective. For example, consider a patient who achieves remission of depressive symptoms when fluoxetine is increased from 20 to 40 mg/d. If sexual side effects emerge at 40 mg/d, relapse may be less likely at 30 mg/d than at 20 mg/d.
Sexual side effects are common in patients taking selective serotonin reuptake inhibitors.1 Sexual side effects diminish patients’ quality of life and significantly decrease adherence to antidepressant regimens,2 which in turn diminishes depression treatment efficacy.
Hidden problem. Drug-related sexual side effects often go undetected because:
- patients are too embarrassed to discuss sexual problems with their physicians
- onset is often later and more insidious than that of other antidepressant side effects
- they may be difficult to distinguish from pre-existing sexual dysfunction caused by depression, other medical reasons, or psychosocial factors
- physicians often fail to educate patients about them.
Clinical tips. These problems point out the importance of obtaining a sexual history before starting antidepressant therapy, educating patients about the potential for sexual side effects with antidepressants (including when they occur and what may be done to manage them), and directly asking patients about specific sexual side effects at follow-up visits.
Other strategies that lessen sexual side effects for some patients include:
- dividing the dosage
- delaying dosing until after sexual activity
- allowing 2- to 3-day “drug holidays” over weekends, when sexual activity is more likely to occur.12
Drug holidays probably would not help patients taking fluoxetine, as plasma concentrations would not drop sufficiently in 2 to 3 days to alleviate sexual side effects. Also, drug holidays are presumably safest for patients who are in maintenance treatment, are asymptomatic, and have no history of rapid symptom recurrence or withdrawal effects when discontinuing SSRIs.12
Switching medications. When sexual side effects do not resolve spontaneously or with dose reduction, consider switching to an antidepressant with a lower incidence of sexual dysfunction.
Table 2
Prevalence of antidepressant sexual side effects
| SSRIs | % of patients affected |
|---|---|
| Citalopram | 38 to 731,3 |
| Fluoxetine | 36 to 581,3 |
| Fluvoxamine | 623 |
| Paroxetine | 42 to 711,3 |
| Sertraline | 40 to 631,3 |
| Other antidepressants | |
| Bupropion | 20 to 241 |
| Mirtazapine | 24 to 401,3 |
| MAO inhibitors | 404 |
| Nefazodone | 8 to 291,3 |
| Tricyclics (excluding clomipramine) | 30 4 |
| Venlafaxine | 40 to 671,3 |
| SSRI: selective serotonin reuptake inhibitor | |
Bupropion has been shown to improve sexual functioning in patients treated for depression. One study reported improved sexual functioning in patients with SSRI-induced sexual side effects who were switched to bupropion.13 Similar studies have shown benefits with substituting nefazodone or mirtazapine for an SSRI.
These uncontrolled studies suggest that switching some patients to a non-SSRI antidepressant may diminish sexual side effects while continuing antidepressant efficacy. Bupropion or nefazodone may be more effective for this purpose, as mirtazapine showed a high rate of sexual side effects in a large observational study.1
Use caution when switching from an SSRI to nefazodone, as cytochrome P-450 2D6 isoenzyme inhibition may increase levels of mCPP—a nefazodone metabolite with anxiogenic properties. To avoid this interaction, taper the SSRI before starting nefazodone.
Switching medications may not be ideal for patients with an unacceptable depression relapse risk, characterized by severe dysfunction, suicidal ideation, or past treatment resistance.
USING AN ANTIDOTE
Adding a second medication to antidepressant therapy is another strategy to consider. An antidote seems most practical when:
- a patient clearly benefits from an antidepressant regimen
- the risk of losing efficacy with a new medication is high
- reducing the dosage or waiting for sexual dysfunction to resolve spontaneously are impractical or have failed.
Most reports of sexual side effect antidotes have been open-label trials of drugs thought to:
- improve some aspect of sexual functioning as with dopamine or noradrenergic agonists)
- or block antidepressant mechanisms suspected of contributing to sexual side effects (as with serotonin receptor antagonists or cholinergic agonists).
Unfortunately, controlled trials with many of these strategies have been less than promising (Table 3).5,14-28 Several trials reported high placebo-response rates—which may complicate assessment of any sexual side effect treatment—and most produced negative results. Two notable exceptions have been sildenafil and bupropion.
Sildenafil, a phosphodiesterase-5 inhibitor, showed greater benefit than placebo in a prospective trial of 90 depressed men (mean age 45) diagnosed with sexual dysfunction caused by an SSRI.28 The men took sildenafil, 50 to 100 mg, 1 hour before sexual activity.
After 6 weeks, 55% of sildenafil-treated patients were rated as much/very much improved on the Clinical Global Impression Scale adapted for Sexual Function, compared with 4% of those taking placebo, a statistically significant difference. Measures used to assess sexual function showed that arousal, erectile function, and orgasm improved significantly, with a lesser effect on desire. This suggests that adjunctive sildenafil reduces SSRIs’ sexual side effects, and this benefit may extend beyond improving ED.
Table 3
Evidence for antidotes used to treat antidepressant sexual side effects
| Drug/dosage Dopaminergic agents | Study designs and outcomes |
|---|---|
| Amantadine, 100 to 400 mg/d14 | Open-label (+) |
| Placebo-controlled (−) | |
| Bupropion SR, 75 to 300 mg/d15 | Open-label (+) |
| Placebo-controlled (+) | |
| Ephedrine16 | Placebo-controlled (−) |
| Methylphenidate, 10 to 30 mg/d14 | Open-label (+) |
| Pramipexole, 0.125 to 2.0 mg/d18 | Open-label (+) |
| Ropinirole, 1 to 4 mg/d19 | Open-label (+) |
| 5-HT antagonists | |
| Cyproheptadine, 2 to 16 mg/d20 | Open-label (+) |
| Granisetron, 1 to 1.5 mg/d21 | Open-label (+) |
| Placebo-controlled (−) | |
| Mianserin, 30 mg/d22 | Open-label (+) |
| Mirtazapine, 15 to 45 mg/d23 | Open-label (+) |
| Placebo-controlled (−) | |
| Nefazodone, 50 to 150 mg/d24 | Open-label (+) |
| Noradrenergic agent | |
| Yohimbine, 5.4 mg/d25 | Open-label (+) |
| Placebo-controlled (−) | |
| Others | |
| Bethanechol, 10 to 50 mg/d6 | Open-label (+) |
| Buspirone, 15 to 60 mg/d26 | Placebo-controlled (+)(−) |
| Ginkgo biloba, 60 to 240 mg/d27 | Open-label (+) |
| Placebo-controlled (−) | |
| Sildenafil, 25 to 200 mg28 | Open-label (+) |
| Placebo-controlled: (+) | |
| (+) = evidence supports effectiveness | |
| (−) = evidence does not support effectiveness | |
| Source: Prepared from references 6 and 14-28. | |
Sildenafil improves peripheral vasodilatation due to smooth muscle relaxation caused by enhanced nitric oxide release. Other sexual side effects—such as delayed orgasm/ejaculation—may improve because of indirect effects of increased penile and clitoral blood flow caused by vasodilatation.29
Sildenafil treatment was well-tolerated; the most common side effects were headache (40.5%), flushing (16.7%), dyspepsia (7.1%), nasal congestion (11.9%), and transient visual disturbances (11.9%).
Bupropion has also shown therapeutic efficacy for SSRI-related sexual dysfunction in a 4-week, placebo-controlled trial of 55 patients (mean age 39) diagnosed with SSRI-induced sexual dysfunction.15 Compared with the placebo group, those receiving add-on bupropion SR, 150 mg bid, improved significantly more in sexual desire and frequency of sexual activity, as measured by the Changes in Sexual Functioning Questionnaire.
Measures of arousal, orgasm, and global sexual functioning did not differ significantly between the two groups. Bupropion added to SSRI treatment was well-tolerated; most-commonly reported side effects were irritability (12%), dry mouth (12%), and headache (15%).
Other ED treatments. Two additional phosphodiesterase-5 inhibitors have become available in the past year. Like sildenafil, tadalafil and vardenafil are indicated for treating ED. They may be useful as alternatives for patients who do not respond to or tolerate sildenafil, although no published studies have examined their use in antidepressant-induced sexual dysfunction.
Recommendation. Based on the evidence, it seems reasonable to start with bupropion or sildenafil when considering an antidote for sexual side effects caused by SSRIs or other medications with strong serotonergic effects. Determining which agent would be “first-line” depends on patient factors, as summarized in Table 130,31For example:
- Bupropion has been reported to augment SSRIs’ antidepressant effects32 and thus may provide added benefit in patients with residual depressive symptoms.
- Bupropion is more effective than sildenafil for improving sexual desire and thus would be preferred for patients in whom this sexual dysfunction symptom is prominent.
- Sildenafil appears to be more effective than bupropion for improving overall sexual satisfaction for men experiencing substantial erectile dysfunction.
Table 4
Bupropion vs. sildenafil as antidote therapy for antidepressant sexual side effects
| Bupropion |
| Possible advantages |
| May reduce residual depressive symptoms, if present32 |
| Appears to improve sexual desire15 |
| Possible disadvantages |
| Daily dosing may increase side-effect risk, but less effective when taken as needed30 |
| Less-clear benefits for arousal and orgasm-related symptoms |
| Sildenafil |
| Possible advantages |
| Can be taken as needed as opposed to daily31 |
| Benefits for arousal and orgasm-related symptoms demonstrated in men31 |
| Possible disadvantages |
| Benefit less-proven for women than for men |
| Reduced sexual spontaneity |
| Unclear benefits for sexual desire31 |
Contraindicated in patients taking organic nitrates because of potentiation of hypotensive effects. Caution advised in patients with:
|
| Source: Prepared from references 15 and 30-32. |
Related resources
- Worthington JJ 3rd, Peters PM. Treatment of antidepressant-induced sexual dysfunction. Drugs Today (Barc) 2003;39(11):887-96.
- Montgomery SA, Baldwin DS, Riley A. Antidepressant medications: a review of the evidence for drug-induced sexual dysfunction. J Affect Disord 2002;69(1-3):119-40.
Drug brand names
- Amantadine • Symmetrel
- Bethanechol • Duvoid, Urecholine, Urabeth
- Bupropion SR • Wellbutrin SR
- Buspirone • Buspar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Cyproheptadine • Periactin
- Fluoxetine • Prozac
- Granisetron • Kytril
- Methyphenidate • Ritalin
- Mianserin • Bolvidon, Norval
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Ropinirole • Requip
- Sertraline • Zoloft
- Sildenafil • Viagra
- Tadalafil • Cialis
- Vardenafil • Levitra
- Venlafaxine • Effexor
Disclosure
Dr. Nelson receives research support from Eli Lilly and Co. and Forest Laboratories and is a speaker for Pfizer Inc. and Wyeth Pharmaceuticals.
1. Clayton AH, Pradko JF, Croft HA, et al. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry 2002;63(4):357-66.
2. Worthington JJ, 3rd, Peters PM. Treatment of antidepressant-induced sexual dysfunction. Drugs Today (Barc) 2003;39(11):887-96.
3. Montejo AL, Llorca G, Izquierdo JA, Rico-Villademoros F. Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients.Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. J Clin Psychiatry 2001;62(suppl 3):10-21.
4. Harrison WM, Rabkin JG, Ehrhardt AA, et al. Effects of antidepressant medication on sexual function: a controlled study. J Clin Psychopharmacol 1986;6(3):144-9.
5. Monteiro WO, Noshirvani HF, Marks IM, Lelliott PT. Anorgasmia from clomipramine in obsessive-compulsive disorder. A controlled trial. Br J Psychiatry 1987;151:107-12.
6. Gross MD. Reversal by bethanechol of sexual dysfunction caused by anticholinergic antidepressants. Am J Psychiatry 1982;139(9):1193-4.
7. Sovner R. Treatment of tricyclic antidepressant-induced orgasmic inhibition with cyproheptadine. J Clin Psychopharmacol 1984;4(3):169.-
8. Gupta S, Masand P, Ashton AK, Berry SL. Phenelzine-induced sexual dysfunction treated with sildenafil. J Sex Marital Ther 1999;25(2):131-5.
9. Decastro RM. Reversal of MAOI-induced anorgasmia with cyproheptadine. Am J Psychiatry 1985;142(6):783.-
10. Kahn DA. Possible toxic interaction between cyproheptadine and phenelzine. Am J Psychiatry 1987;144(9):1242-3.
11. Montejo-Gonzalez AL, Llorca G, Izquierdo JA, et al. SSRI-induced sexual dysfunction: fluoxetine, paroxetine, sertraline, and fluvoxamine in a prospective, multicenter, and descriptive clinical study of 344 patients. J Sex Marital Ther 1997;23:176-94.
12. Rothschild AJ. Selective serotonin reuptake inhibitor-induced sexual dysfunction: efficacy of a drug holiday. Am J Psychiatry 1995;152(10):1514-16.
13. Clayton AH, McGarvey EL, Abouesh AI, Pinkerton RC. Substitution of an SSRI with bupropion sustained release following SSRI-induced sexual dysfunction. J Clin Psychiatry 2001;62(3):185-90.
14. Shrivastava RK. Amantadine in the treatment of sexual dysfunction associated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol 1995;15:83-84.
15. Clayton AH, Warnock JK, Kornstein SG, et al. A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry, 2004;65(1):62-7.
16. Meston CM. A randomized, placebo-controlled, crossover study of ephedrine for SSRI-induced female sexual dysfunction. J Sex Marital Ther 2004;30(2):57-68.
17. Roeloffs C, Bartlik B, Kaplan PM, Kocsis JH. Methylphenidate and SSRI-induced sexual side effects. J Clin Psychiatry 1996;57(11):548.-
18. DeBattista C, Solvason HB, Breen JA, Schatzberg AF. Pramipexole augmentation of a selective serotonin reuptake inhibitor in the treatment of depression. J Clin Psychopharmacol 2000;20(2):274-5.
19. Worthington JJ, 3rd, Simon NM, Korbly NB, et al. Ropinirole for antidepressant-induced sexual dysfunction. Int Clin Psychopharmacol 2002;17(6):307-10.
20. Aizenberg D, Zemishlany Z, Weizman A. Cyproheptadine treatment of sexual dysfunction induced by serotonin reuptake inhibitors. Clin Neuropharmacol 1995;18:320-4.
21. Nelson EB, Keck PE, Jr, McElroy SL. Resolution of fluoxetine-induced sexual dysfunction with the 5-HT 3 antagonist granisetron (letter). J Clin Psychiatry 1997;58:496-7.
22. Aizenberg D, Naor S, Zemishlany Z, Weizman A. The serotonin antagonist mianserin for treatment of serotonin reuptake inhibitor-induced sexual dysfunction in women: an open-label add-on study. Clin Neuropharmacol 1999;22:347-50.
23. Farah A. Relief of SSRI-induced sexual dysfunction with mirtazapine treatment. J Clin Psychiatry 1999;60:260-1.
24. Reynolds RD. Sertraline-induced anorgasmia treated with intermittent nefazodone. J Clin Psychiatry 1997;58:89.-
25. Jacobsen F. Fluoxetine-induced sexual dysfunction and an open trial of yohimbine. J Clin Psychiatry 1992;53:119-22.
26. Michelson D, Bancroft J, Targum S, et al. Female sexual dysfunction associated with antidepressant administration: a randomized, placebo-controlled study of pharmacologic intervention. Am J Psychiatry 2000;157(2):239-43.
27. Kang BJ, Lee SJ, Kim MD, Cho MJ. A placebo-controlled, double-blind trial of Ginkgo biloba for antidepressant-induced sexual dysfunction. Hum Psychopharmacol 2002;17(6):279-84.
28. Nurnberg HG, Hensley PL. Sildenafil citrate for the management of antidepressant-associated erectile dysfunction. J Clin Psychiatry 2003;64(suppl 10):20-5.
29. Zajecka J. Strategies for the treatment of antidepressant-related sexual dysfunction. J Clin Psychiatry 2001;62(suppl 3):35-43.
30. DeBattista C, Solvason HB, Poirier J, et al. A prospective trial of bupropion SR augmentation of partial and non-responders to serotonergic antidepressants. J Clin Psychopharmacol 2003;23:27-30.
31. Ashton AK, Rosen RC. Bupropion as an antidote for serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry 1998;59:112-15.
32. Nurnberg HG, Gelenberg A, Hargreave TB, et al. Efficacy of sildenafil citrate for the treatment of erectile dysfunction in men taking serotonin reuptake inhibitors. Am J Psychiatry 2001;158:1926-8.
Antidepressants’ sexual side effects can often be managed—while preserving the antidepressant effect—by altering dosages, switching to another drug class, or adding an “antidote.” Understanding the benefits and risks of each strategy can help you:
- base treatment choices on your patient’s history and side-effect experience
- improve long-term compliance with antidepressant regimens.
EFFECTS VARY BY ANTIDEPRESSANT CLASS
Antidepressants may affect one or more phases of sexual functioning:
- desire (libido)
- arousal (erection or vaginal lubrication)
- orgasm/ejaculation.
Sexual symptoms linked to antidepressants range from diminished interest/arousal and delayed orgasm to heightened sexual functioning (Table 1). Resulting sexual dysfunction can impair quality of life and intimate relationships and discourage patients from taking antidepressants (Box) 1,2
Table 1
Sexual side effects linked to antidepressants
| Most common effects | Shown by these drugs |
|---|---|
| Decreased desire | TCAs, MAOIs, SSRIs |
| Delayed or absent ejaculation/orgasm | TCAs, MAOIs, SSRIs |
| Impaired erection | TCAs, MAOIs, SSRIs |
| Less common effects | |
| Increased desire | Bupropion |
| Spontaneous/prolonged erections | SSRIs, CMI,bupropion, trazodone, nefazodone |
| Premature/retrograde/painful ejaculations | TCAs, trazodone, nefazodone |
| Priapism | SSRIs, CMI, bupropion, trazodone, nefazodone |
| Spontaneous orgasms (associated with yawning) | SSRIs, CMI, bupropion |
| Altered sexual sensation and sensitivity | SSRIs, CMI, bupropion |
| TCAs: tricyclics | |
| MAOIs: monoamine oxidase inhibitors | |
| SSRIs: selective serotonin reuptake inhibitors | |
| CMI: clomipramine | |
Although most reports have focused on SSRIs, all antidepressant classes have been associated with sexual dysfunction, with prevalence likely influenced by differences in neurotransmitter modulation (Table 2).1,3,4 The highest rates of sexual side effects have been reported with SSRIs, certain tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs).
A recent study reported similarly high rates with mirtazapine, but its small sample size limits conclusions about side effect prevalence with this drug.1 Other studies have found significantly lower rates with bupropion and nefazodone.
TCAs’ sexual side-effect rates and types depend on how much each drug inhibits serotonin reuptake. Clomipramine appears to have the highest rates of sexual dysfunction—particularly anorgasmia—probably because it inhibits the serotonin transporter more than do other TCAs.5 In TCAs with lesser effects on serotonergic neurotransmission, alpha-adrenergic and cholinergic receptor blockade may cause sexual side effects—particularly erectile dysfunction (ED).
Cholinergic agonists such as bethanechol, 10 to 50 mg/d, may reverse sexual dysfunction caused by anticholinergic effects.6 Cyproheptadine—a nonselective serotonin receptor antagonist—has also shown benefit at 4 to 12 mg/d in treating TCA-related sexual side effects.7
MAOIs. Sexual side effects appear to be more prevalent with MAOIs than with TCAs,4 perhaps similar to the rate seen with SSRIs. MAOIs directly increase serotonergic neurotransmission, and their substantial alpha-adrenergic antagonist effects may also produce sexual side effects.
Waiting for symptoms to subside may be appropriate, as anorgasmia caused by MAOIs may remit spontaneously. Sildenafil8 and cyproheptadine9 may reverse MAOI sexual side effects, although serious toxicity has been reported in a patient taking cyproheptadine and an MAOI.10
SSRIs. Increased serotonergic neurotransmission is widely believed to cause SSRI sexual side effects. Resulting secondary effects—such as inhibited central dopamine release, increased prolactin secretion, and inhibited nitric oxide synthesis—may also play important roles.
In general, SSRIs appear to alter sexual functioning in 40% to 60% of patients—both men and women. Anorgasmia is the most commonly reported sexual symptom.
Although all SSRIs are associated with sexual dysfunction, some studies have found higher rates with paroxetine. One study associated paroxetine with significantly higher rates of ED compared with other SSRIs. The authors attributed this finding to paroxetine’s greater anticholinergic effects or to its directly decreasing nitric oxide synthesis.3
SSRI MANAGEMENT STRATEGIES
Waiting. The simplest, safest way to manage SSRI-related sexual dysfunction is to wait and see if side effects resolve spontaneously. Sexual side effects improve without treatment in approximately 20% of cases,3 although improvement is often incomplete. Moreover, several months may pass before symptoms diminish adequately, making this strategy impractical for patients with substantial sexual dysfunction.
Dosing changes. Because SSRIs’ sexual side effects appear to be dose-related,11 carefully reducing the dosage may reduce sexual dysfunction without compromising antidepressant efficacy. This strategy is most likely to sustain remission when you avoid dosages that have proven ineffective. For example, consider a patient who achieves remission of depressive symptoms when fluoxetine is increased from 20 to 40 mg/d. If sexual side effects emerge at 40 mg/d, relapse may be less likely at 30 mg/d than at 20 mg/d.
Sexual side effects are common in patients taking selective serotonin reuptake inhibitors.1 Sexual side effects diminish patients’ quality of life and significantly decrease adherence to antidepressant regimens,2 which in turn diminishes depression treatment efficacy.
Hidden problem. Drug-related sexual side effects often go undetected because:
- patients are too embarrassed to discuss sexual problems with their physicians
- onset is often later and more insidious than that of other antidepressant side effects
- they may be difficult to distinguish from pre-existing sexual dysfunction caused by depression, other medical reasons, or psychosocial factors
- physicians often fail to educate patients about them.
Clinical tips. These problems point out the importance of obtaining a sexual history before starting antidepressant therapy, educating patients about the potential for sexual side effects with antidepressants (including when they occur and what may be done to manage them), and directly asking patients about specific sexual side effects at follow-up visits.
Other strategies that lessen sexual side effects for some patients include:
- dividing the dosage
- delaying dosing until after sexual activity
- allowing 2- to 3-day “drug holidays” over weekends, when sexual activity is more likely to occur.12
Drug holidays probably would not help patients taking fluoxetine, as plasma concentrations would not drop sufficiently in 2 to 3 days to alleviate sexual side effects. Also, drug holidays are presumably safest for patients who are in maintenance treatment, are asymptomatic, and have no history of rapid symptom recurrence or withdrawal effects when discontinuing SSRIs.12
Switching medications. When sexual side effects do not resolve spontaneously or with dose reduction, consider switching to an antidepressant with a lower incidence of sexual dysfunction.
Table 2
Prevalence of antidepressant sexual side effects
| SSRIs | % of patients affected |
|---|---|
| Citalopram | 38 to 731,3 |
| Fluoxetine | 36 to 581,3 |
| Fluvoxamine | 623 |
| Paroxetine | 42 to 711,3 |
| Sertraline | 40 to 631,3 |
| Other antidepressants | |
| Bupropion | 20 to 241 |
| Mirtazapine | 24 to 401,3 |
| MAO inhibitors | 404 |
| Nefazodone | 8 to 291,3 |
| Tricyclics (excluding clomipramine) | 30 4 |
| Venlafaxine | 40 to 671,3 |
| SSRI: selective serotonin reuptake inhibitor | |
Bupropion has been shown to improve sexual functioning in patients treated for depression. One study reported improved sexual functioning in patients with SSRI-induced sexual side effects who were switched to bupropion.13 Similar studies have shown benefits with substituting nefazodone or mirtazapine for an SSRI.
These uncontrolled studies suggest that switching some patients to a non-SSRI antidepressant may diminish sexual side effects while continuing antidepressant efficacy. Bupropion or nefazodone may be more effective for this purpose, as mirtazapine showed a high rate of sexual side effects in a large observational study.1
Use caution when switching from an SSRI to nefazodone, as cytochrome P-450 2D6 isoenzyme inhibition may increase levels of mCPP—a nefazodone metabolite with anxiogenic properties. To avoid this interaction, taper the SSRI before starting nefazodone.
Switching medications may not be ideal for patients with an unacceptable depression relapse risk, characterized by severe dysfunction, suicidal ideation, or past treatment resistance.
USING AN ANTIDOTE
Adding a second medication to antidepressant therapy is another strategy to consider. An antidote seems most practical when:
- a patient clearly benefits from an antidepressant regimen
- the risk of losing efficacy with a new medication is high
- reducing the dosage or waiting for sexual dysfunction to resolve spontaneously are impractical or have failed.
Most reports of sexual side effect antidotes have been open-label trials of drugs thought to:
- improve some aspect of sexual functioning as with dopamine or noradrenergic agonists)
- or block antidepressant mechanisms suspected of contributing to sexual side effects (as with serotonin receptor antagonists or cholinergic agonists).
Unfortunately, controlled trials with many of these strategies have been less than promising (Table 3).5,14-28 Several trials reported high placebo-response rates—which may complicate assessment of any sexual side effect treatment—and most produced negative results. Two notable exceptions have been sildenafil and bupropion.
Sildenafil, a phosphodiesterase-5 inhibitor, showed greater benefit than placebo in a prospective trial of 90 depressed men (mean age 45) diagnosed with sexual dysfunction caused by an SSRI.28 The men took sildenafil, 50 to 100 mg, 1 hour before sexual activity.
After 6 weeks, 55% of sildenafil-treated patients were rated as much/very much improved on the Clinical Global Impression Scale adapted for Sexual Function, compared with 4% of those taking placebo, a statistically significant difference. Measures used to assess sexual function showed that arousal, erectile function, and orgasm improved significantly, with a lesser effect on desire. This suggests that adjunctive sildenafil reduces SSRIs’ sexual side effects, and this benefit may extend beyond improving ED.
Table 3
Evidence for antidotes used to treat antidepressant sexual side effects
| Drug/dosage Dopaminergic agents | Study designs and outcomes |
|---|---|
| Amantadine, 100 to 400 mg/d14 | Open-label (+) |
| Placebo-controlled (−) | |
| Bupropion SR, 75 to 300 mg/d15 | Open-label (+) |
| Placebo-controlled (+) | |
| Ephedrine16 | Placebo-controlled (−) |
| Methylphenidate, 10 to 30 mg/d14 | Open-label (+) |
| Pramipexole, 0.125 to 2.0 mg/d18 | Open-label (+) |
| Ropinirole, 1 to 4 mg/d19 | Open-label (+) |
| 5-HT antagonists | |
| Cyproheptadine, 2 to 16 mg/d20 | Open-label (+) |
| Granisetron, 1 to 1.5 mg/d21 | Open-label (+) |
| Placebo-controlled (−) | |
| Mianserin, 30 mg/d22 | Open-label (+) |
| Mirtazapine, 15 to 45 mg/d23 | Open-label (+) |
| Placebo-controlled (−) | |
| Nefazodone, 50 to 150 mg/d24 | Open-label (+) |
| Noradrenergic agent | |
| Yohimbine, 5.4 mg/d25 | Open-label (+) |
| Placebo-controlled (−) | |
| Others | |
| Bethanechol, 10 to 50 mg/d6 | Open-label (+) |
| Buspirone, 15 to 60 mg/d26 | Placebo-controlled (+)(−) |
| Ginkgo biloba, 60 to 240 mg/d27 | Open-label (+) |
| Placebo-controlled (−) | |
| Sildenafil, 25 to 200 mg28 | Open-label (+) |
| Placebo-controlled: (+) | |
| (+) = evidence supports effectiveness | |
| (−) = evidence does not support effectiveness | |
| Source: Prepared from references 6 and 14-28. | |
Sildenafil improves peripheral vasodilatation due to smooth muscle relaxation caused by enhanced nitric oxide release. Other sexual side effects—such as delayed orgasm/ejaculation—may improve because of indirect effects of increased penile and clitoral blood flow caused by vasodilatation.29
Sildenafil treatment was well-tolerated; the most common side effects were headache (40.5%), flushing (16.7%), dyspepsia (7.1%), nasal congestion (11.9%), and transient visual disturbances (11.9%).
Bupropion has also shown therapeutic efficacy for SSRI-related sexual dysfunction in a 4-week, placebo-controlled trial of 55 patients (mean age 39) diagnosed with SSRI-induced sexual dysfunction.15 Compared with the placebo group, those receiving add-on bupropion SR, 150 mg bid, improved significantly more in sexual desire and frequency of sexual activity, as measured by the Changes in Sexual Functioning Questionnaire.
Measures of arousal, orgasm, and global sexual functioning did not differ significantly between the two groups. Bupropion added to SSRI treatment was well-tolerated; most-commonly reported side effects were irritability (12%), dry mouth (12%), and headache (15%).
Other ED treatments. Two additional phosphodiesterase-5 inhibitors have become available in the past year. Like sildenafil, tadalafil and vardenafil are indicated for treating ED. They may be useful as alternatives for patients who do not respond to or tolerate sildenafil, although no published studies have examined their use in antidepressant-induced sexual dysfunction.
Recommendation. Based on the evidence, it seems reasonable to start with bupropion or sildenafil when considering an antidote for sexual side effects caused by SSRIs or other medications with strong serotonergic effects. Determining which agent would be “first-line” depends on patient factors, as summarized in Table 130,31For example:
- Bupropion has been reported to augment SSRIs’ antidepressant effects32 and thus may provide added benefit in patients with residual depressive symptoms.
- Bupropion is more effective than sildenafil for improving sexual desire and thus would be preferred for patients in whom this sexual dysfunction symptom is prominent.
- Sildenafil appears to be more effective than bupropion for improving overall sexual satisfaction for men experiencing substantial erectile dysfunction.
Table 4
Bupropion vs. sildenafil as antidote therapy for antidepressant sexual side effects
| Bupropion |
| Possible advantages |
| May reduce residual depressive symptoms, if present32 |
| Appears to improve sexual desire15 |
| Possible disadvantages |
| Daily dosing may increase side-effect risk, but less effective when taken as needed30 |
| Less-clear benefits for arousal and orgasm-related symptoms |
| Sildenafil |
| Possible advantages |
| Can be taken as needed as opposed to daily31 |
| Benefits for arousal and orgasm-related symptoms demonstrated in men31 |
| Possible disadvantages |
| Benefit less-proven for women than for men |
| Reduced sexual spontaneity |
| Unclear benefits for sexual desire31 |
Contraindicated in patients taking organic nitrates because of potentiation of hypotensive effects. Caution advised in patients with:
|
| Source: Prepared from references 15 and 30-32. |
Related resources
- Worthington JJ 3rd, Peters PM. Treatment of antidepressant-induced sexual dysfunction. Drugs Today (Barc) 2003;39(11):887-96.
- Montgomery SA, Baldwin DS, Riley A. Antidepressant medications: a review of the evidence for drug-induced sexual dysfunction. J Affect Disord 2002;69(1-3):119-40.
Drug brand names
- Amantadine • Symmetrel
- Bethanechol • Duvoid, Urecholine, Urabeth
- Bupropion SR • Wellbutrin SR
- Buspirone • Buspar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Cyproheptadine • Periactin
- Fluoxetine • Prozac
- Granisetron • Kytril
- Methyphenidate • Ritalin
- Mianserin • Bolvidon, Norval
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Ropinirole • Requip
- Sertraline • Zoloft
- Sildenafil • Viagra
- Tadalafil • Cialis
- Vardenafil • Levitra
- Venlafaxine • Effexor
Disclosure
Dr. Nelson receives research support from Eli Lilly and Co. and Forest Laboratories and is a speaker for Pfizer Inc. and Wyeth Pharmaceuticals.
Antidepressants’ sexual side effects can often be managed—while preserving the antidepressant effect—by altering dosages, switching to another drug class, or adding an “antidote.” Understanding the benefits and risks of each strategy can help you:
- base treatment choices on your patient’s history and side-effect experience
- improve long-term compliance with antidepressant regimens.
EFFECTS VARY BY ANTIDEPRESSANT CLASS
Antidepressants may affect one or more phases of sexual functioning:
- desire (libido)
- arousal (erection or vaginal lubrication)
- orgasm/ejaculation.
Sexual symptoms linked to antidepressants range from diminished interest/arousal and delayed orgasm to heightened sexual functioning (Table 1). Resulting sexual dysfunction can impair quality of life and intimate relationships and discourage patients from taking antidepressants (Box) 1,2
Table 1
Sexual side effects linked to antidepressants
| Most common effects | Shown by these drugs |
|---|---|
| Decreased desire | TCAs, MAOIs, SSRIs |
| Delayed or absent ejaculation/orgasm | TCAs, MAOIs, SSRIs |
| Impaired erection | TCAs, MAOIs, SSRIs |
| Less common effects | |
| Increased desire | Bupropion |
| Spontaneous/prolonged erections | SSRIs, CMI,bupropion, trazodone, nefazodone |
| Premature/retrograde/painful ejaculations | TCAs, trazodone, nefazodone |
| Priapism | SSRIs, CMI, bupropion, trazodone, nefazodone |
| Spontaneous orgasms (associated with yawning) | SSRIs, CMI, bupropion |
| Altered sexual sensation and sensitivity | SSRIs, CMI, bupropion |
| TCAs: tricyclics | |
| MAOIs: monoamine oxidase inhibitors | |
| SSRIs: selective serotonin reuptake inhibitors | |
| CMI: clomipramine | |
Although most reports have focused on SSRIs, all antidepressant classes have been associated with sexual dysfunction, with prevalence likely influenced by differences in neurotransmitter modulation (Table 2).1,3,4 The highest rates of sexual side effects have been reported with SSRIs, certain tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs).
A recent study reported similarly high rates with mirtazapine, but its small sample size limits conclusions about side effect prevalence with this drug.1 Other studies have found significantly lower rates with bupropion and nefazodone.
TCAs’ sexual side-effect rates and types depend on how much each drug inhibits serotonin reuptake. Clomipramine appears to have the highest rates of sexual dysfunction—particularly anorgasmia—probably because it inhibits the serotonin transporter more than do other TCAs.5 In TCAs with lesser effects on serotonergic neurotransmission, alpha-adrenergic and cholinergic receptor blockade may cause sexual side effects—particularly erectile dysfunction (ED).
Cholinergic agonists such as bethanechol, 10 to 50 mg/d, may reverse sexual dysfunction caused by anticholinergic effects.6 Cyproheptadine—a nonselective serotonin receptor antagonist—has also shown benefit at 4 to 12 mg/d in treating TCA-related sexual side effects.7
MAOIs. Sexual side effects appear to be more prevalent with MAOIs than with TCAs,4 perhaps similar to the rate seen with SSRIs. MAOIs directly increase serotonergic neurotransmission, and their substantial alpha-adrenergic antagonist effects may also produce sexual side effects.
Waiting for symptoms to subside may be appropriate, as anorgasmia caused by MAOIs may remit spontaneously. Sildenafil8 and cyproheptadine9 may reverse MAOI sexual side effects, although serious toxicity has been reported in a patient taking cyproheptadine and an MAOI.10
SSRIs. Increased serotonergic neurotransmission is widely believed to cause SSRI sexual side effects. Resulting secondary effects—such as inhibited central dopamine release, increased prolactin secretion, and inhibited nitric oxide synthesis—may also play important roles.
In general, SSRIs appear to alter sexual functioning in 40% to 60% of patients—both men and women. Anorgasmia is the most commonly reported sexual symptom.
Although all SSRIs are associated with sexual dysfunction, some studies have found higher rates with paroxetine. One study associated paroxetine with significantly higher rates of ED compared with other SSRIs. The authors attributed this finding to paroxetine’s greater anticholinergic effects or to its directly decreasing nitric oxide synthesis.3
SSRI MANAGEMENT STRATEGIES
Waiting. The simplest, safest way to manage SSRI-related sexual dysfunction is to wait and see if side effects resolve spontaneously. Sexual side effects improve without treatment in approximately 20% of cases,3 although improvement is often incomplete. Moreover, several months may pass before symptoms diminish adequately, making this strategy impractical for patients with substantial sexual dysfunction.
Dosing changes. Because SSRIs’ sexual side effects appear to be dose-related,11 carefully reducing the dosage may reduce sexual dysfunction without compromising antidepressant efficacy. This strategy is most likely to sustain remission when you avoid dosages that have proven ineffective. For example, consider a patient who achieves remission of depressive symptoms when fluoxetine is increased from 20 to 40 mg/d. If sexual side effects emerge at 40 mg/d, relapse may be less likely at 30 mg/d than at 20 mg/d.
Sexual side effects are common in patients taking selective serotonin reuptake inhibitors.1 Sexual side effects diminish patients’ quality of life and significantly decrease adherence to antidepressant regimens,2 which in turn diminishes depression treatment efficacy.
Hidden problem. Drug-related sexual side effects often go undetected because:
- patients are too embarrassed to discuss sexual problems with their physicians
- onset is often later and more insidious than that of other antidepressant side effects
- they may be difficult to distinguish from pre-existing sexual dysfunction caused by depression, other medical reasons, or psychosocial factors
- physicians often fail to educate patients about them.
Clinical tips. These problems point out the importance of obtaining a sexual history before starting antidepressant therapy, educating patients about the potential for sexual side effects with antidepressants (including when they occur and what may be done to manage them), and directly asking patients about specific sexual side effects at follow-up visits.
Other strategies that lessen sexual side effects for some patients include:
- dividing the dosage
- delaying dosing until after sexual activity
- allowing 2- to 3-day “drug holidays” over weekends, when sexual activity is more likely to occur.12
Drug holidays probably would not help patients taking fluoxetine, as plasma concentrations would not drop sufficiently in 2 to 3 days to alleviate sexual side effects. Also, drug holidays are presumably safest for patients who are in maintenance treatment, are asymptomatic, and have no history of rapid symptom recurrence or withdrawal effects when discontinuing SSRIs.12
Switching medications. When sexual side effects do not resolve spontaneously or with dose reduction, consider switching to an antidepressant with a lower incidence of sexual dysfunction.
Table 2
Prevalence of antidepressant sexual side effects
| SSRIs | % of patients affected |
|---|---|
| Citalopram | 38 to 731,3 |
| Fluoxetine | 36 to 581,3 |
| Fluvoxamine | 623 |
| Paroxetine | 42 to 711,3 |
| Sertraline | 40 to 631,3 |
| Other antidepressants | |
| Bupropion | 20 to 241 |
| Mirtazapine | 24 to 401,3 |
| MAO inhibitors | 404 |
| Nefazodone | 8 to 291,3 |
| Tricyclics (excluding clomipramine) | 30 4 |
| Venlafaxine | 40 to 671,3 |
| SSRI: selective serotonin reuptake inhibitor | |
Bupropion has been shown to improve sexual functioning in patients treated for depression. One study reported improved sexual functioning in patients with SSRI-induced sexual side effects who were switched to bupropion.13 Similar studies have shown benefits with substituting nefazodone or mirtazapine for an SSRI.
These uncontrolled studies suggest that switching some patients to a non-SSRI antidepressant may diminish sexual side effects while continuing antidepressant efficacy. Bupropion or nefazodone may be more effective for this purpose, as mirtazapine showed a high rate of sexual side effects in a large observational study.1
Use caution when switching from an SSRI to nefazodone, as cytochrome P-450 2D6 isoenzyme inhibition may increase levels of mCPP—a nefazodone metabolite with anxiogenic properties. To avoid this interaction, taper the SSRI before starting nefazodone.
Switching medications may not be ideal for patients with an unacceptable depression relapse risk, characterized by severe dysfunction, suicidal ideation, or past treatment resistance.
USING AN ANTIDOTE
Adding a second medication to antidepressant therapy is another strategy to consider. An antidote seems most practical when:
- a patient clearly benefits from an antidepressant regimen
- the risk of losing efficacy with a new medication is high
- reducing the dosage or waiting for sexual dysfunction to resolve spontaneously are impractical or have failed.
Most reports of sexual side effect antidotes have been open-label trials of drugs thought to:
- improve some aspect of sexual functioning as with dopamine or noradrenergic agonists)
- or block antidepressant mechanisms suspected of contributing to sexual side effects (as with serotonin receptor antagonists or cholinergic agonists).
Unfortunately, controlled trials with many of these strategies have been less than promising (Table 3).5,14-28 Several trials reported high placebo-response rates—which may complicate assessment of any sexual side effect treatment—and most produced negative results. Two notable exceptions have been sildenafil and bupropion.
Sildenafil, a phosphodiesterase-5 inhibitor, showed greater benefit than placebo in a prospective trial of 90 depressed men (mean age 45) diagnosed with sexual dysfunction caused by an SSRI.28 The men took sildenafil, 50 to 100 mg, 1 hour before sexual activity.
After 6 weeks, 55% of sildenafil-treated patients were rated as much/very much improved on the Clinical Global Impression Scale adapted for Sexual Function, compared with 4% of those taking placebo, a statistically significant difference. Measures used to assess sexual function showed that arousal, erectile function, and orgasm improved significantly, with a lesser effect on desire. This suggests that adjunctive sildenafil reduces SSRIs’ sexual side effects, and this benefit may extend beyond improving ED.
Table 3
Evidence for antidotes used to treat antidepressant sexual side effects
| Drug/dosage Dopaminergic agents | Study designs and outcomes |
|---|---|
| Amantadine, 100 to 400 mg/d14 | Open-label (+) |
| Placebo-controlled (−) | |
| Bupropion SR, 75 to 300 mg/d15 | Open-label (+) |
| Placebo-controlled (+) | |
| Ephedrine16 | Placebo-controlled (−) |
| Methylphenidate, 10 to 30 mg/d14 | Open-label (+) |
| Pramipexole, 0.125 to 2.0 mg/d18 | Open-label (+) |
| Ropinirole, 1 to 4 mg/d19 | Open-label (+) |
| 5-HT antagonists | |
| Cyproheptadine, 2 to 16 mg/d20 | Open-label (+) |
| Granisetron, 1 to 1.5 mg/d21 | Open-label (+) |
| Placebo-controlled (−) | |
| Mianserin, 30 mg/d22 | Open-label (+) |
| Mirtazapine, 15 to 45 mg/d23 | Open-label (+) |
| Placebo-controlled (−) | |
| Nefazodone, 50 to 150 mg/d24 | Open-label (+) |
| Noradrenergic agent | |
| Yohimbine, 5.4 mg/d25 | Open-label (+) |
| Placebo-controlled (−) | |
| Others | |
| Bethanechol, 10 to 50 mg/d6 | Open-label (+) |
| Buspirone, 15 to 60 mg/d26 | Placebo-controlled (+)(−) |
| Ginkgo biloba, 60 to 240 mg/d27 | Open-label (+) |
| Placebo-controlled (−) | |
| Sildenafil, 25 to 200 mg28 | Open-label (+) |
| Placebo-controlled: (+) | |
| (+) = evidence supports effectiveness | |
| (−) = evidence does not support effectiveness | |
| Source: Prepared from references 6 and 14-28. | |
Sildenafil improves peripheral vasodilatation due to smooth muscle relaxation caused by enhanced nitric oxide release. Other sexual side effects—such as delayed orgasm/ejaculation—may improve because of indirect effects of increased penile and clitoral blood flow caused by vasodilatation.29
Sildenafil treatment was well-tolerated; the most common side effects were headache (40.5%), flushing (16.7%), dyspepsia (7.1%), nasal congestion (11.9%), and transient visual disturbances (11.9%).
Bupropion has also shown therapeutic efficacy for SSRI-related sexual dysfunction in a 4-week, placebo-controlled trial of 55 patients (mean age 39) diagnosed with SSRI-induced sexual dysfunction.15 Compared with the placebo group, those receiving add-on bupropion SR, 150 mg bid, improved significantly more in sexual desire and frequency of sexual activity, as measured by the Changes in Sexual Functioning Questionnaire.
Measures of arousal, orgasm, and global sexual functioning did not differ significantly between the two groups. Bupropion added to SSRI treatment was well-tolerated; most-commonly reported side effects were irritability (12%), dry mouth (12%), and headache (15%).
Other ED treatments. Two additional phosphodiesterase-5 inhibitors have become available in the past year. Like sildenafil, tadalafil and vardenafil are indicated for treating ED. They may be useful as alternatives for patients who do not respond to or tolerate sildenafil, although no published studies have examined their use in antidepressant-induced sexual dysfunction.
Recommendation. Based on the evidence, it seems reasonable to start with bupropion or sildenafil when considering an antidote for sexual side effects caused by SSRIs or other medications with strong serotonergic effects. Determining which agent would be “first-line” depends on patient factors, as summarized in Table 130,31For example:
- Bupropion has been reported to augment SSRIs’ antidepressant effects32 and thus may provide added benefit in patients with residual depressive symptoms.
- Bupropion is more effective than sildenafil for improving sexual desire and thus would be preferred for patients in whom this sexual dysfunction symptom is prominent.
- Sildenafil appears to be more effective than bupropion for improving overall sexual satisfaction for men experiencing substantial erectile dysfunction.
Table 4
Bupropion vs. sildenafil as antidote therapy for antidepressant sexual side effects
| Bupropion |
| Possible advantages |
| May reduce residual depressive symptoms, if present32 |
| Appears to improve sexual desire15 |
| Possible disadvantages |
| Daily dosing may increase side-effect risk, but less effective when taken as needed30 |
| Less-clear benefits for arousal and orgasm-related symptoms |
| Sildenafil |
| Possible advantages |
| Can be taken as needed as opposed to daily31 |
| Benefits for arousal and orgasm-related symptoms demonstrated in men31 |
| Possible disadvantages |
| Benefit less-proven for women than for men |
| Reduced sexual spontaneity |
| Unclear benefits for sexual desire31 |
Contraindicated in patients taking organic nitrates because of potentiation of hypotensive effects. Caution advised in patients with:
|
| Source: Prepared from references 15 and 30-32. |
Related resources
- Worthington JJ 3rd, Peters PM. Treatment of antidepressant-induced sexual dysfunction. Drugs Today (Barc) 2003;39(11):887-96.
- Montgomery SA, Baldwin DS, Riley A. Antidepressant medications: a review of the evidence for drug-induced sexual dysfunction. J Affect Disord 2002;69(1-3):119-40.
Drug brand names
- Amantadine • Symmetrel
- Bethanechol • Duvoid, Urecholine, Urabeth
- Bupropion SR • Wellbutrin SR
- Buspirone • Buspar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Cyproheptadine • Periactin
- Fluoxetine • Prozac
- Granisetron • Kytril
- Methyphenidate • Ritalin
- Mianserin • Bolvidon, Norval
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Ropinirole • Requip
- Sertraline • Zoloft
- Sildenafil • Viagra
- Tadalafil • Cialis
- Vardenafil • Levitra
- Venlafaxine • Effexor
Disclosure
Dr. Nelson receives research support from Eli Lilly and Co. and Forest Laboratories and is a speaker for Pfizer Inc. and Wyeth Pharmaceuticals.
1. Clayton AH, Pradko JF, Croft HA, et al. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry 2002;63(4):357-66.
2. Worthington JJ, 3rd, Peters PM. Treatment of antidepressant-induced sexual dysfunction. Drugs Today (Barc) 2003;39(11):887-96.
3. Montejo AL, Llorca G, Izquierdo JA, Rico-Villademoros F. Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients.Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. J Clin Psychiatry 2001;62(suppl 3):10-21.
4. Harrison WM, Rabkin JG, Ehrhardt AA, et al. Effects of antidepressant medication on sexual function: a controlled study. J Clin Psychopharmacol 1986;6(3):144-9.
5. Monteiro WO, Noshirvani HF, Marks IM, Lelliott PT. Anorgasmia from clomipramine in obsessive-compulsive disorder. A controlled trial. Br J Psychiatry 1987;151:107-12.
6. Gross MD. Reversal by bethanechol of sexual dysfunction caused by anticholinergic antidepressants. Am J Psychiatry 1982;139(9):1193-4.
7. Sovner R. Treatment of tricyclic antidepressant-induced orgasmic inhibition with cyproheptadine. J Clin Psychopharmacol 1984;4(3):169.-
8. Gupta S, Masand P, Ashton AK, Berry SL. Phenelzine-induced sexual dysfunction treated with sildenafil. J Sex Marital Ther 1999;25(2):131-5.
9. Decastro RM. Reversal of MAOI-induced anorgasmia with cyproheptadine. Am J Psychiatry 1985;142(6):783.-
10. Kahn DA. Possible toxic interaction between cyproheptadine and phenelzine. Am J Psychiatry 1987;144(9):1242-3.
11. Montejo-Gonzalez AL, Llorca G, Izquierdo JA, et al. SSRI-induced sexual dysfunction: fluoxetine, paroxetine, sertraline, and fluvoxamine in a prospective, multicenter, and descriptive clinical study of 344 patients. J Sex Marital Ther 1997;23:176-94.
12. Rothschild AJ. Selective serotonin reuptake inhibitor-induced sexual dysfunction: efficacy of a drug holiday. Am J Psychiatry 1995;152(10):1514-16.
13. Clayton AH, McGarvey EL, Abouesh AI, Pinkerton RC. Substitution of an SSRI with bupropion sustained release following SSRI-induced sexual dysfunction. J Clin Psychiatry 2001;62(3):185-90.
14. Shrivastava RK. Amantadine in the treatment of sexual dysfunction associated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol 1995;15:83-84.
15. Clayton AH, Warnock JK, Kornstein SG, et al. A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry, 2004;65(1):62-7.
16. Meston CM. A randomized, placebo-controlled, crossover study of ephedrine for SSRI-induced female sexual dysfunction. J Sex Marital Ther 2004;30(2):57-68.
17. Roeloffs C, Bartlik B, Kaplan PM, Kocsis JH. Methylphenidate and SSRI-induced sexual side effects. J Clin Psychiatry 1996;57(11):548.-
18. DeBattista C, Solvason HB, Breen JA, Schatzberg AF. Pramipexole augmentation of a selective serotonin reuptake inhibitor in the treatment of depression. J Clin Psychopharmacol 2000;20(2):274-5.
19. Worthington JJ, 3rd, Simon NM, Korbly NB, et al. Ropinirole for antidepressant-induced sexual dysfunction. Int Clin Psychopharmacol 2002;17(6):307-10.
20. Aizenberg D, Zemishlany Z, Weizman A. Cyproheptadine treatment of sexual dysfunction induced by serotonin reuptake inhibitors. Clin Neuropharmacol 1995;18:320-4.
21. Nelson EB, Keck PE, Jr, McElroy SL. Resolution of fluoxetine-induced sexual dysfunction with the 5-HT 3 antagonist granisetron (letter). J Clin Psychiatry 1997;58:496-7.
22. Aizenberg D, Naor S, Zemishlany Z, Weizman A. The serotonin antagonist mianserin for treatment of serotonin reuptake inhibitor-induced sexual dysfunction in women: an open-label add-on study. Clin Neuropharmacol 1999;22:347-50.
23. Farah A. Relief of SSRI-induced sexual dysfunction with mirtazapine treatment. J Clin Psychiatry 1999;60:260-1.
24. Reynolds RD. Sertraline-induced anorgasmia treated with intermittent nefazodone. J Clin Psychiatry 1997;58:89.-
25. Jacobsen F. Fluoxetine-induced sexual dysfunction and an open trial of yohimbine. J Clin Psychiatry 1992;53:119-22.
26. Michelson D, Bancroft J, Targum S, et al. Female sexual dysfunction associated with antidepressant administration: a randomized, placebo-controlled study of pharmacologic intervention. Am J Psychiatry 2000;157(2):239-43.
27. Kang BJ, Lee SJ, Kim MD, Cho MJ. A placebo-controlled, double-blind trial of Ginkgo biloba for antidepressant-induced sexual dysfunction. Hum Psychopharmacol 2002;17(6):279-84.
28. Nurnberg HG, Hensley PL. Sildenafil citrate for the management of antidepressant-associated erectile dysfunction. J Clin Psychiatry 2003;64(suppl 10):20-5.
29. Zajecka J. Strategies for the treatment of antidepressant-related sexual dysfunction. J Clin Psychiatry 2001;62(suppl 3):35-43.
30. DeBattista C, Solvason HB, Poirier J, et al. A prospective trial of bupropion SR augmentation of partial and non-responders to serotonergic antidepressants. J Clin Psychopharmacol 2003;23:27-30.
31. Ashton AK, Rosen RC. Bupropion as an antidote for serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry 1998;59:112-15.
32. Nurnberg HG, Gelenberg A, Hargreave TB, et al. Efficacy of sildenafil citrate for the treatment of erectile dysfunction in men taking serotonin reuptake inhibitors. Am J Psychiatry 2001;158:1926-8.
1. Clayton AH, Pradko JF, Croft HA, et al. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry 2002;63(4):357-66.
2. Worthington JJ, 3rd, Peters PM. Treatment of antidepressant-induced sexual dysfunction. Drugs Today (Barc) 2003;39(11):887-96.
3. Montejo AL, Llorca G, Izquierdo JA, Rico-Villademoros F. Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients.Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. J Clin Psychiatry 2001;62(suppl 3):10-21.
4. Harrison WM, Rabkin JG, Ehrhardt AA, et al. Effects of antidepressant medication on sexual function: a controlled study. J Clin Psychopharmacol 1986;6(3):144-9.
5. Monteiro WO, Noshirvani HF, Marks IM, Lelliott PT. Anorgasmia from clomipramine in obsessive-compulsive disorder. A controlled trial. Br J Psychiatry 1987;151:107-12.
6. Gross MD. Reversal by bethanechol of sexual dysfunction caused by anticholinergic antidepressants. Am J Psychiatry 1982;139(9):1193-4.
7. Sovner R. Treatment of tricyclic antidepressant-induced orgasmic inhibition with cyproheptadine. J Clin Psychopharmacol 1984;4(3):169.-
8. Gupta S, Masand P, Ashton AK, Berry SL. Phenelzine-induced sexual dysfunction treated with sildenafil. J Sex Marital Ther 1999;25(2):131-5.
9. Decastro RM. Reversal of MAOI-induced anorgasmia with cyproheptadine. Am J Psychiatry 1985;142(6):783.-
10. Kahn DA. Possible toxic interaction between cyproheptadine and phenelzine. Am J Psychiatry 1987;144(9):1242-3.
11. Montejo-Gonzalez AL, Llorca G, Izquierdo JA, et al. SSRI-induced sexual dysfunction: fluoxetine, paroxetine, sertraline, and fluvoxamine in a prospective, multicenter, and descriptive clinical study of 344 patients. J Sex Marital Ther 1997;23:176-94.
12. Rothschild AJ. Selective serotonin reuptake inhibitor-induced sexual dysfunction: efficacy of a drug holiday. Am J Psychiatry 1995;152(10):1514-16.
13. Clayton AH, McGarvey EL, Abouesh AI, Pinkerton RC. Substitution of an SSRI with bupropion sustained release following SSRI-induced sexual dysfunction. J Clin Psychiatry 2001;62(3):185-90.
14. Shrivastava RK. Amantadine in the treatment of sexual dysfunction associated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol 1995;15:83-84.
15. Clayton AH, Warnock JK, Kornstein SG, et al. A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry, 2004;65(1):62-7.
16. Meston CM. A randomized, placebo-controlled, crossover study of ephedrine for SSRI-induced female sexual dysfunction. J Sex Marital Ther 2004;30(2):57-68.
17. Roeloffs C, Bartlik B, Kaplan PM, Kocsis JH. Methylphenidate and SSRI-induced sexual side effects. J Clin Psychiatry 1996;57(11):548.-
18. DeBattista C, Solvason HB, Breen JA, Schatzberg AF. Pramipexole augmentation of a selective serotonin reuptake inhibitor in the treatment of depression. J Clin Psychopharmacol 2000;20(2):274-5.
19. Worthington JJ, 3rd, Simon NM, Korbly NB, et al. Ropinirole for antidepressant-induced sexual dysfunction. Int Clin Psychopharmacol 2002;17(6):307-10.
20. Aizenberg D, Zemishlany Z, Weizman A. Cyproheptadine treatment of sexual dysfunction induced by serotonin reuptake inhibitors. Clin Neuropharmacol 1995;18:320-4.
21. Nelson EB, Keck PE, Jr, McElroy SL. Resolution of fluoxetine-induced sexual dysfunction with the 5-HT 3 antagonist granisetron (letter). J Clin Psychiatry 1997;58:496-7.
22. Aizenberg D, Naor S, Zemishlany Z, Weizman A. The serotonin antagonist mianserin for treatment of serotonin reuptake inhibitor-induced sexual dysfunction in women: an open-label add-on study. Clin Neuropharmacol 1999;22:347-50.
23. Farah A. Relief of SSRI-induced sexual dysfunction with mirtazapine treatment. J Clin Psychiatry 1999;60:260-1.
24. Reynolds RD. Sertraline-induced anorgasmia treated with intermittent nefazodone. J Clin Psychiatry 1997;58:89.-
25. Jacobsen F. Fluoxetine-induced sexual dysfunction and an open trial of yohimbine. J Clin Psychiatry 1992;53:119-22.
26. Michelson D, Bancroft J, Targum S, et al. Female sexual dysfunction associated with antidepressant administration: a randomized, placebo-controlled study of pharmacologic intervention. Am J Psychiatry 2000;157(2):239-43.
27. Kang BJ, Lee SJ, Kim MD, Cho MJ. A placebo-controlled, double-blind trial of Ginkgo biloba for antidepressant-induced sexual dysfunction. Hum Psychopharmacol 2002;17(6):279-84.
28. Nurnberg HG, Hensley PL. Sildenafil citrate for the management of antidepressant-associated erectile dysfunction. J Clin Psychiatry 2003;64(suppl 10):20-5.
29. Zajecka J. Strategies for the treatment of antidepressant-related sexual dysfunction. J Clin Psychiatry 2001;62(suppl 3):35-43.
30. DeBattista C, Solvason HB, Poirier J, et al. A prospective trial of bupropion SR augmentation of partial and non-responders to serotonergic antidepressants. J Clin Psychopharmacol 2003;23:27-30.
31. Ashton AK, Rosen RC. Bupropion as an antidote for serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry 1998;59:112-15.
32. Nurnberg HG, Gelenberg A, Hargreave TB, et al. Efficacy of sildenafil citrate for the treatment of erectile dysfunction in men taking serotonin reuptake inhibitors. Am J Psychiatry 2001;158:1926-8.
Blocking the ‘munchies’ receptor: A novel approach to obesity
Marijuana has long been known to stimulate appetite, particularly for sweet foods.1 The naughty boys in my fraternity called it “the munchies;” the professionals call it hyperphagia. Cannabinoid receptor (CB1) stimulation by marijuana’s main active component—9-THC—is believed to induce this behavior. Clinicians have successfully used this effect to treat AIDS-related wasting syndrome and other anorexic conditions.2
CB1 is widely expressed throughout the brain and seems to inhibit release of various neurotransmitters.3 How this effect leads to increased appetite is unclear, but it may result from a decrease in the appetite-suppressing effects of hormones such as leptin. In other words, tweaking the CB1 receptor may take the “brakes” off appetite.
Some researchers have speculated that if stimulating CB1 triggers appetite, blocking the receptor might inhibit it (Figure 1).
THE ‘MUNCHIES’ IN MICE
Rimonabant (SR141716), an experimental agent, is a potent and selective CB1 antagonist.
Ravinet Trillou et al fed mice a high-fat diet known to induce obesity.4 The mice were randomized to receive rimonabant or placebo while maintained on the highly palatable diet. The authors asked: Would rimonabant help the mice lose weight even when they could eat as much delicious fatty food as they wanted?
Figure 1 Blocking CB1 may prevent weight gain
Δ9-THC activates the cannabinoid receptor (CB1), stimulating appetite and leading to weight gain in mice (left). When the same receptor is blocked, appetite is controlled (right).
Source: Illustration for CURRENT PSYCHIATRY by Marcia HartsockRimonabant induced a sustained body weight reduction of approximately 20% in the treatment group compared with the placebo group across 5 weeks (Figure 2). Estimated fat stores among the treatment group were depleted by slightly more than 50%.
The authors noted that the mice in the treatment group had decreased their food intake, but the decrease was not sufficient to explain the weight loss. They speculate that rimonabant could activate metabolic processes and decrease intake.
RIMONABANT’S ROLE IN PSYCHIATRY
Phase III human trials of rimonabant are under way for obesity as well as smoking cessation.5 In uncontrolled studies, rimonabant has been shown to help people avoid weight gain while quitting smoking.5
If rimonabant shows effectiveness in controlled trials and is safe in humans, it could be most valuable. Obesity in industrial countries is epidemic and causes serious secondary morbidity, including diabetes, arthritis, and hypertension. Rimonabant, if approved by the FDA, could reach the market by early 2006.6
It is unknown whether rimonabant’s metabolic effects could offset those of many psychotropics. As psychiatrists, we often must stop an effective antipsychotic or antidepressant because it is causing significant weight gain. A treatment that would prevent medication-induced weight gain could improve patient compliance and, ultimately, outcomes.
MANAGING SCHIZOPHRENIA
Some evidence also suggests that rimonabant may offer additional benefits for patients with schizophrenia beyond weight reduction or smoking cessation.
Figure 2 Rimonabant’s effects on weight in mice on a high-fat diet
Source: Adapted from reference 4.Leweke et al found increased endogenous cannabinoids in the CSF of patients with schizophrenia, suggesting that a cannabinoid signaling imbalance may contribute to the disorder’s pathogenesis.7 However, 72 patients with schizophrenia or schizoaffective disorder who took rimonabant for 6 weeks showed no improvement compared with a placebo group.8
1. Abel EL. Cannabis: effects on hunger and thirst. Behav Biol 1975;15:255-81.
2. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage 1995;10:89-97.
3. Iversen L. Cannabis and the brain. Brain 2003;126:1252-70.
4. Ravinet Trillou C, Arnone M, Delgorge C, et al. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 2003;284:R345-53.
5. Fernandez JR, Allison DB. Rimonabant Sanofi-Synthelabo. Curr Opin Investig Drugs 2004;5:430-5.
6. The Website for the Drug Development Industry. Acomplia (rimonabant)—investigational agent for the management of obesity. London: SPGMedia. Available at: http://www. drugdevelopment-technology.com/projects/rimonabant/. Accessed Oct. 14, 2004.
7. Leweke FM, Giuffrida A, Wurster U, et al. Elevated endogenous cannabinoids in schizophrenia. Neuroreport 1999;10:1665-9.
8. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry 2004;161:975-84.
Marijuana has long been known to stimulate appetite, particularly for sweet foods.1 The naughty boys in my fraternity called it “the munchies;” the professionals call it hyperphagia. Cannabinoid receptor (CB1) stimulation by marijuana’s main active component—9-THC—is believed to induce this behavior. Clinicians have successfully used this effect to treat AIDS-related wasting syndrome and other anorexic conditions.2
CB1 is widely expressed throughout the brain and seems to inhibit release of various neurotransmitters.3 How this effect leads to increased appetite is unclear, but it may result from a decrease in the appetite-suppressing effects of hormones such as leptin. In other words, tweaking the CB1 receptor may take the “brakes” off appetite.
Some researchers have speculated that if stimulating CB1 triggers appetite, blocking the receptor might inhibit it (Figure 1).
THE ‘MUNCHIES’ IN MICE
Rimonabant (SR141716), an experimental agent, is a potent and selective CB1 antagonist.
Ravinet Trillou et al fed mice a high-fat diet known to induce obesity.4 The mice were randomized to receive rimonabant or placebo while maintained on the highly palatable diet. The authors asked: Would rimonabant help the mice lose weight even when they could eat as much delicious fatty food as they wanted?
Figure 1 Blocking CB1 may prevent weight gain
Δ9-THC activates the cannabinoid receptor (CB1), stimulating appetite and leading to weight gain in mice (left). When the same receptor is blocked, appetite is controlled (right).
Source: Illustration for CURRENT PSYCHIATRY by Marcia HartsockRimonabant induced a sustained body weight reduction of approximately 20% in the treatment group compared with the placebo group across 5 weeks (Figure 2). Estimated fat stores among the treatment group were depleted by slightly more than 50%.
The authors noted that the mice in the treatment group had decreased their food intake, but the decrease was not sufficient to explain the weight loss. They speculate that rimonabant could activate metabolic processes and decrease intake.
RIMONABANT’S ROLE IN PSYCHIATRY
Phase III human trials of rimonabant are under way for obesity as well as smoking cessation.5 In uncontrolled studies, rimonabant has been shown to help people avoid weight gain while quitting smoking.5
If rimonabant shows effectiveness in controlled trials and is safe in humans, it could be most valuable. Obesity in industrial countries is epidemic and causes serious secondary morbidity, including diabetes, arthritis, and hypertension. Rimonabant, if approved by the FDA, could reach the market by early 2006.6
It is unknown whether rimonabant’s metabolic effects could offset those of many psychotropics. As psychiatrists, we often must stop an effective antipsychotic or antidepressant because it is causing significant weight gain. A treatment that would prevent medication-induced weight gain could improve patient compliance and, ultimately, outcomes.
MANAGING SCHIZOPHRENIA
Some evidence also suggests that rimonabant may offer additional benefits for patients with schizophrenia beyond weight reduction or smoking cessation.
Figure 2 Rimonabant’s effects on weight in mice on a high-fat diet
Source: Adapted from reference 4.Leweke et al found increased endogenous cannabinoids in the CSF of patients with schizophrenia, suggesting that a cannabinoid signaling imbalance may contribute to the disorder’s pathogenesis.7 However, 72 patients with schizophrenia or schizoaffective disorder who took rimonabant for 6 weeks showed no improvement compared with a placebo group.8
Marijuana has long been known to stimulate appetite, particularly for sweet foods.1 The naughty boys in my fraternity called it “the munchies;” the professionals call it hyperphagia. Cannabinoid receptor (CB1) stimulation by marijuana’s main active component—9-THC—is believed to induce this behavior. Clinicians have successfully used this effect to treat AIDS-related wasting syndrome and other anorexic conditions.2
CB1 is widely expressed throughout the brain and seems to inhibit release of various neurotransmitters.3 How this effect leads to increased appetite is unclear, but it may result from a decrease in the appetite-suppressing effects of hormones such as leptin. In other words, tweaking the CB1 receptor may take the “brakes” off appetite.
Some researchers have speculated that if stimulating CB1 triggers appetite, blocking the receptor might inhibit it (Figure 1).
THE ‘MUNCHIES’ IN MICE
Rimonabant (SR141716), an experimental agent, is a potent and selective CB1 antagonist.
Ravinet Trillou et al fed mice a high-fat diet known to induce obesity.4 The mice were randomized to receive rimonabant or placebo while maintained on the highly palatable diet. The authors asked: Would rimonabant help the mice lose weight even when they could eat as much delicious fatty food as they wanted?
Figure 1 Blocking CB1 may prevent weight gain
Δ9-THC activates the cannabinoid receptor (CB1), stimulating appetite and leading to weight gain in mice (left). When the same receptor is blocked, appetite is controlled (right).
Source: Illustration for CURRENT PSYCHIATRY by Marcia HartsockRimonabant induced a sustained body weight reduction of approximately 20% in the treatment group compared with the placebo group across 5 weeks (Figure 2). Estimated fat stores among the treatment group were depleted by slightly more than 50%.
The authors noted that the mice in the treatment group had decreased their food intake, but the decrease was not sufficient to explain the weight loss. They speculate that rimonabant could activate metabolic processes and decrease intake.
RIMONABANT’S ROLE IN PSYCHIATRY
Phase III human trials of rimonabant are under way for obesity as well as smoking cessation.5 In uncontrolled studies, rimonabant has been shown to help people avoid weight gain while quitting smoking.5
If rimonabant shows effectiveness in controlled trials and is safe in humans, it could be most valuable. Obesity in industrial countries is epidemic and causes serious secondary morbidity, including diabetes, arthritis, and hypertension. Rimonabant, if approved by the FDA, could reach the market by early 2006.6
It is unknown whether rimonabant’s metabolic effects could offset those of many psychotropics. As psychiatrists, we often must stop an effective antipsychotic or antidepressant because it is causing significant weight gain. A treatment that would prevent medication-induced weight gain could improve patient compliance and, ultimately, outcomes.
MANAGING SCHIZOPHRENIA
Some evidence also suggests that rimonabant may offer additional benefits for patients with schizophrenia beyond weight reduction or smoking cessation.
Figure 2 Rimonabant’s effects on weight in mice on a high-fat diet
Source: Adapted from reference 4.Leweke et al found increased endogenous cannabinoids in the CSF of patients with schizophrenia, suggesting that a cannabinoid signaling imbalance may contribute to the disorder’s pathogenesis.7 However, 72 patients with schizophrenia or schizoaffective disorder who took rimonabant for 6 weeks showed no improvement compared with a placebo group.8
1. Abel EL. Cannabis: effects on hunger and thirst. Behav Biol 1975;15:255-81.
2. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage 1995;10:89-97.
3. Iversen L. Cannabis and the brain. Brain 2003;126:1252-70.
4. Ravinet Trillou C, Arnone M, Delgorge C, et al. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 2003;284:R345-53.
5. Fernandez JR, Allison DB. Rimonabant Sanofi-Synthelabo. Curr Opin Investig Drugs 2004;5:430-5.
6. The Website for the Drug Development Industry. Acomplia (rimonabant)—investigational agent for the management of obesity. London: SPGMedia. Available at: http://www. drugdevelopment-technology.com/projects/rimonabant/. Accessed Oct. 14, 2004.
7. Leweke FM, Giuffrida A, Wurster U, et al. Elevated endogenous cannabinoids in schizophrenia. Neuroreport 1999;10:1665-9.
8. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry 2004;161:975-84.
1. Abel EL. Cannabis: effects on hunger and thirst. Behav Biol 1975;15:255-81.
2. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage 1995;10:89-97.
3. Iversen L. Cannabis and the brain. Brain 2003;126:1252-70.
4. Ravinet Trillou C, Arnone M, Delgorge C, et al. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 2003;284:R345-53.
5. Fernandez JR, Allison DB. Rimonabant Sanofi-Synthelabo. Curr Opin Investig Drugs 2004;5:430-5.
6. The Website for the Drug Development Industry. Acomplia (rimonabant)—investigational agent for the management of obesity. London: SPGMedia. Available at: http://www. drugdevelopment-technology.com/projects/rimonabant/. Accessed Oct. 14, 2004.
7. Leweke FM, Giuffrida A, Wurster U, et al. Elevated endogenous cannabinoids in schizophrenia. Neuroreport 1999;10:1665-9.
8. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry 2004;161:975-84.
A Divine delusion
“Delusions: How cognitive therapy helps patients let go” (Pearls, July 2004) reminded me of a patient who once told me and my colleagues that he was God.
“That is very good news,” I responded. “We’re all so glad that you have finally come to us. We’ve waited for you for a long time.”
The patient was pleased with my greeting. Then I added, “We have a lot of sick patients in this hospital. We hope you can help them.” He was puzzled, as he had not expected that responsibilities come with exalted status.
A few days later, after he had begun taking an antipsychotic, I asked about his identity. He now told me he was the “Son of God.” We talked a few days later, and he said, “I am the son of God. Aren’t we all God’s children?”
I was pleased to see his mental health improve but disappointed to learn we would receive no supernatural help with treating our patients.
Yehuda Sherman, MD
Lafayette, CA
“Delusions: How cognitive therapy helps patients let go” (Pearls, July 2004) reminded me of a patient who once told me and my colleagues that he was God.
“That is very good news,” I responded. “We’re all so glad that you have finally come to us. We’ve waited for you for a long time.”
The patient was pleased with my greeting. Then I added, “We have a lot of sick patients in this hospital. We hope you can help them.” He was puzzled, as he had not expected that responsibilities come with exalted status.
A few days later, after he had begun taking an antipsychotic, I asked about his identity. He now told me he was the “Son of God.” We talked a few days later, and he said, “I am the son of God. Aren’t we all God’s children?”
I was pleased to see his mental health improve but disappointed to learn we would receive no supernatural help with treating our patients.
Yehuda Sherman, MD
Lafayette, CA
“Delusions: How cognitive therapy helps patients let go” (Pearls, July 2004) reminded me of a patient who once told me and my colleagues that he was God.
“That is very good news,” I responded. “We’re all so glad that you have finally come to us. We’ve waited for you for a long time.”
The patient was pleased with my greeting. Then I added, “We have a lot of sick patients in this hospital. We hope you can help them.” He was puzzled, as he had not expected that responsibilities come with exalted status.
A few days later, after he had begun taking an antipsychotic, I asked about his identity. He now told me he was the “Son of God.” We talked a few days later, and he said, “I am the son of God. Aren’t we all God’s children?”
I was pleased to see his mental health improve but disappointed to learn we would receive no supernatural help with treating our patients.
Yehuda Sherman, MD
Lafayette, CA