User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Recovery from schizophrenia: Fact or fiction?
Is it realistic for patients with schizophrenia to believe they can recover? Recent observational studies show that some do,1 even though all DSM editions have defined schizophrenia as a chronic disease with a poor outcome.2
Our understanding of schizophrenia is changing as we gain new insights into:
- mechanism of recovery
- efficacy of combined psychotherapeutic, psychosocial, and drug therapies for sustaining remission and recovery
- the value of long-term aftercare. This article examines evidence on:
- achieving recovery from schizophrenia
- factors associated with remission
- treatments that may help prevent relapse and lead to stable, lasting recovery.
What is ‘recovery’?
Diagnostic criteria. Recovery from schizophrenia has social, occupational, symptomatic, and psychostructural dimensions. For clinical practice, Liberman et al3 developed a useful set of 10 criteria for recovery (Table 1) by analyzing the literature and cases of 23 schizophrenia patients who returned to work or school with their symptoms under control.
Table 1
Recovery from schizophrenia: 10 clinical criteria
| Criteria | Characteristics of recovered patients* |
|---|---|
| Family relationships | 70% reported good or very good family relationships |
| Substance abuse | None reported illicit drug use in the past year, and two reported occasional alcohol consumption |
| Duration of untreated psychosis | Only 13% reported >1 year delay between symptom onset and treatment |
| Initial response to medication | 87% reported effective symptom control with their first antipsychotic medication |
| Adherence to treatment | All reported adherence to psychiatric care and medication regimens |
| Supportive therapy | 91% reported ongoing psychotherapy, and 78% reported that accessible and supportive psychiatrists and therapists aided their recovery |
| Cognitive abilities | Normal or near-normal functioning on tests of flexibility in solving problems, verbal working memory, and perceptual skills |
| Social skills | None showed more than very mild negative symptoms |
| Personal history | 70% graduated from college, 13% completed 2 years of college, and 3 of remaining 4 worked full time before becoming ill |
| Access to care | 91% received antipsychotics and psychotherapy, 48% social skills training, 57% family participation, 26% vocational rehabilitation, and 61% self-help groups |
| * Based on a study of 23 schizophrenia patients who returned to work or school with their symptoms under control. | |
| Source: Reference 3 | |
Recovery is not a smooth, linear progression. Even when patients attain remission, they often find it hard to make up for “lost life” during years of disability.4 Recovery also can be defined as social, emotional, and biological maturation. This definition considers recovery not as an end-state or cure but as a process of personal growth.5
Several groups proposed recently that recovery from schizophrenia includes four processes:
- finding hope
- re-establishing identity
- taking responsibility for recovery
- finding meaning6 and “getting on with life”7 (Box).
Long-tem vs short-term
Recovery has been studied in many populations, but the evidence is difficult to compare. Data quality is compromised by poorly-defined cohorts, weak study designs, and lack of clear definitions of recovery and its diagnostic criteria. Moreover, empirical evidence is lacking on recovery’s multidimensional nature, including psychosocial, biochemical, genetic, environmental, cultural, and ethnic correlates.
Long-term recovery. Recently, three studies of American populations diagnosed with schizophrenia detected trends toward long-term (>5 years) recovery.
U.S. populations. Modestin et al8 in 2003 re-evaluated diagnoses of 208 patients in Swiss psychiatrist Manfred Bleuler’s influential 1972 study on schizophrenia’s long-term course. Using DSM-III-R, DSM-IV, and International Statistical Classification of Diseases and Related Health Problems (ICD-10) criteria, the authors excluded about 30% of the original patients (most rediagnosed with schizoaffective disorder). Among those remaining, 12% to 15% showed long-term recovery and one-half had an undulating course with remissions.
In 1997, Stephens et al9 examined hospital records from 1913 to 1940 of 484 patients, mean age 27, hospitalized with schizophrenia. Using >5 years of follow-up data and DSM-IV criteria, the authors rated 13% as recovered and 58% unimproved.
Also in 1997, Harrow et al10 evaluated 74 patients diagnosed with schizophrenia by DSM-IV criteria at 2, 4.5, and 7.5 years. In this longitudinal study, one-third (32%) showed complete remission at one follow-up session, compared with 5% at all three evaluations.
This study suggested that schizophrenia patients show relatively poor functioning, compared with other psychotic patients. Over time, however, the likelihood of long-term remission appeared to increase. A similar pattern was seen in a sample of 658 Americans age >65 with schizophrenia diagnosed by DSM-III criteria. As these patients aged, 15% developed long-lasting remission.11
Elsewhere, empirical findings across 15 years from three Norwegian studies indicate that lasting recovery from schizophrenia—with symptom improvement and psychosocial adjustment—is rare (3% to 5% of patients).3 Similarly, only 4% of a Scandinavian sample of 301 patients attained complete, long-term remission during 3 to 39 years of follow-up.12
Across cultures, an international study13 evaluated 15- and 25-year outcomes in 1,633 patients diagnosed with schizophrenia. Approximately 50% had favorable outcomes—stable work, independent from support, no imprisonment, no substance abuse, no rehospitalization, improved social life—but heterogeneity was marked.
Ms J, 48, is in recovery from schizophrenia. She has a stable job as a Web designer, is married, and has learned to build and maintain social relationships. Much of her life, however, has been very different.
At age 15 she was diagnosed with schizophrenia, paranoid psychotic type, with occasional comorbid bipolar symptoms. Over the next 20 years, she was admitted to psychiatric hospitals six times for treatment. At age 36, she was hospitalized with psychosis, depressive symptoms, and insomnia. At that point, she was taking carbamazepine, 500 mg/d, for mood stabilization, and haloperidol, 50 mg/d.
Changing medications. Her psychiatriststarted olanzapine, 5 mg/d, and tapered off haloperidol, which appeared to be gradually becoming less effective while causing mood-related side effects. Ms. J’s psychosis persisted, however, with no response to olanzapine.
Her psychiatrist then tapered carbamazepine to 175 mg/d while starting lamotrigine, 150 to 300 mg/d. The rationale for switching mood stabilizers was that lamotrigine may be more effective than carbamazepine in controlling mixed bipolar states, provide a greater antidepressant effect, and cause fewer side effects.
Intensive treatment. Within 10 days, Ms J’s thought form and composition improved, and her psychiatrist immediately started psychotherapy and psychosocial guidance. Carbamazepine was withdrawn 3 months later, but Ms. J remained on olanzapine, 5 mg/d, and lamotrigine, 300 mg/d. With these medications, the paranoid psychosis went into remission.
After 5 months of intensive treatment, Ms. J was discharged. Outpatient treatment included weekly psychotherapy plus psychosocial guidance and social and coping training 6 times per month. These therapies —along with olanzapine, 5 mg/d, and carbamazepine, 300 mg/d—continue today.
Ms. J’s mental and emotional condition stabilized, and her cognitive abilities improved. Education and therapy helped reduce stress within her family. She has not been rehospitalized or suffered a serious relapse in 12 years.
Table 2
Psychosocial interventions for patients in recovery from schizophrenia*
|
| * Psychosocial interventions are most effective when combined with antipsychotic therapy and individualized psychotherapy |
Short-term course predicted long-term outcome, and local environment played a significant role in determining symptoms and social disability. The authors concluded that adequate early treatment and an optimum environment might lead to favorable long-term outcome.
In the United Kingdom, 14% of a sample of patients diagnosed by ICD-10 criteria achieved remission across a mean 8.5 years.14 In a study of Czechoslovakian patients (70 men, 50 women) with early-onset schizophrenia diagnosed by DSM-III-R criteria, 10% recovered during 13 to 42 years of follow-up.15
Short-term recovery. The McLean-Harvard first-episode project examined outcomes 6 months after schizophrenia diagnosis in 102 patients (55 men, 47 women). Sixty-five percent attained syndromal recovery (significant reduction of diagnostic features), whereas only 33% achieved functional recovery (increased social-emotional, vocational, and coping abilities).16
In Japan, 62 patients (33 men, 29 women; mean age 25) were followed for 13 years after a first hospitalization for schizophrenia. The authors reported an undulating course with recovery or a mild end-state in 53%, and a simple course of recovery and a moderate or severe end-state in 28%.17
Conclusions. The evidence suggests that early and lasting treatment of schizophrenic symptoms—even in recovered patients—might prevent frequent rehospitalizations. Thus, patients with schizophrenia must be followed carefully during and after recovery. Health care professionals, colleagues, friends, and relatives can help patients sustain recovery by watching for the earliest signs of deterioration and intervening before relapse occurs.
Strategies for recovery
Therapeutic factors. Many studies suggest psychosocial interventions (Table 2), psychotherapy, and medication are most effective in combination for stabilizing patients with schizophrenia and continuing their recovery. Other patient factors that may contribute to recovery include:
- quality of relationships with family, friends, and professional caregivers
- ability and motivation to use resources and take responsibility for one’s life
- spiritual and religious activities
- awareness that recovery is possible.
Sells et al18 noted that attempting to make new contacts outside of their former spheres (“positive withdrawal”) may allow schizophrenia patients to reconsider and ultimately recover a durable sense of self.
We at the W. Kahn Institute19 find that all these treatment strategies may be useful and even necessary to continue and stabilize recovery from schizophrenia. We feel they merit the attention of all professionals involved in recovered patients’ aftercare and guidance.
Table 3
Suggested antipsychotic dosages during schizophrenia recovery*
| Drug | Dosage (mg/d) | Potential side effects | Positive effects |
|---|---|---|---|
| Aripiprazole | 10 to 30 | Headache, anxiety, insomnia, lightheadedness | Reduced positive, negative symptoms |
| Clozapine | 300 to 900 | Withdrawal, blunted emotions, seizures, lack of motivation | Reduced positive symptoms |
| Haloperidol | 30 to 100 | Tardive dyskinesia, parkinsonian symptoms, insomnia, depressive reactions, confusion, drowsiness, hypertension | Reduced mania, hyperactivity, agitation |
| Olanzapine | 5 to 10 | Drowsiness, agitation, weight gain, involuntary movements, restlessness | Reduced positive, negative symptoms |
| Quetiapine | 25 to 100 | Dizziness, hypotension, increased cholesterol, weight gain | Reduced positive symptoms |
| Risperidone | 2.5 to 5 | Anxiety, nervousness, back pain, bleeding, dizziness, irregular blood pressure | Reduced positive, negative symptoms |
| Ziprasidone | 10 to 200 | Heart-rhythm irregularity, loss of consciousness, restlessness, weakness, drowsiness | Reduced positive symptoms |
| * Dosages are individualized and may vary among patients and situations, but most will be gradually reduced to minimum levels during remission. | |||
Social/vocational network. Family, friends, neighbors, and social workers play an important role in the patient’s development during recovery. They provide positive stimulation (such as physical activities and social or vocational engagements) and support.
To equip the patient’s network for this responsible task, provide them with training (such as in acceptance, empathy, feedback, and communication), education, and guidance. Support groups can enhance the social networks of patients whose own networks are too small to prevent social isolation or overburdening of members.
Vocational training and mediation also may be stabilizing. Religious activities are central to self-understanding and recovery for many psychiatric patients and may improve outcomes.20
Patient skills. To achieve stabilization and continue their recovery, patients must develop social interaction skills and coping strategies. Conversation training, for example, seems to help improve social interaction. Patients in remission must learn to:
- find or create low-stress, positively stimulating environments in which their recovery can flourish
- tolerate discomfort and stress
- overcome internalized stigma about recovery.
Patients also need to learn when and how to withdraw from hectic, stressful environments and from people who are overly emotional, patronizing, or hold unrealistic expectations about them.
Pharmacotherapy. Sound pharmacotherapy underlies rehabilitation and psychosocial treatment of patients in remission from schizophrenia.21 Healthy neurobiological functioning and equilibrium may help normalize social-emotional behavior and create opportunities to improve all life dimensions via psychotherapy, psychosocial guidance, education, and training.
Dosages often can be reduced during recovery, titrating gradually downward to reduce the risk of relapse. Suggested antipsychotic dosages during recovery are listed in Table 3. Be patient and consistent when adjusting dosages, guided by information in package inserts, from clinical trials, and in recent articles on specific medications. When introducing a medication, start with the lowest dosage and increase in small steps until symptoms are reduced and side effects are minimal.
Discontinuing antipsychotics. Is it therapeutically reasonable to discontinue antipsychotics after recovery? Probably not.
Relapse rates in unmedicated patients with schizophrenia appear extremely high—perhaps 8 or 9 out of 10 cases—even during remission. By comparison, relapse rates appear very low—perhaps 3 or 4 out of 10 cases—for remitted patients who remain on antipsychotics. Atypical antipsychotics or low doses of conventional agents are generally well-tolerated and safe in the long term.22
Patient monitoring. Patients in remission from schizophrenia benefit from:
- 24-hour phone lines to call for guidance, treatment, and quick interventions
- central, alert treatment coordination among psychiatrists, psychologists, social workers, therapists, vocational experts, and activity counselors.
Psychiatrists in solo or small-group settings may need to seek out these resources in their communities. The goal of this team approach—in cooperation with the patient’s social network—is to help the patient develop employment and social activities appropriate to his or her needs and capabilities.
Related resources
- Ascher-Svanuw H, Kraus A. Psychoeducational groups for patients with schizophrenia: a guide for practitioners. Gaithersburg, MD: Aspen Publishers, 1991.
- Suzuki T, Uchida H, Tanaka KF, et al. Revising polypharmacy to a single antipsychotic regimen for patients with chronic schizophrenia. Int J Neuropsychopharmacol 2004;7(2):133-42.
- American Psychiatric Association. Schizophrenia (patient/family information). www.psych.org/public_info/schizo.cfm
- Schizophrenia.com. Nonprofit Web community providing information, support, and education. www.schizophrenia.com
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Davidson L. Living outside mental illness: qualitative studies of recovery in schizophrenia. New York: New York University Press, 2003.
2. Kruger A. Schizophrenia: Recovery and hope. Psychiatr Rehabil J 2000;24(1):29-37.
3. Liberman RP, Kopelowicz A, Ventura J, Gutkind D. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry 2002;14(4):256-72.
4. Torgalsboen AK, Rund BR. Lessons learned from three studies of recovery from schizophrenia. Int Rev Psychiatry 2002;14(4):312-17.
5. Boone EC. A qualitative investigation of the process of recovery in people experiencing schizophrenia. Dissert Abstr Int 1996;57 (5-B):3402.-
6. Andresen R, Oades L, Caputi P. The experience of recovery from schizophrenia: towards an empirically validated stage model. Aust NZ J Psychiatry 2003;37(5):586-94.
7. Noordsy D, Torrey W, Mueser K, et al. Recovery from severe mental illness: an interpersonal and functional outcome definition. Int Rev Psychiatry 2002;14(4):318-26.
8. Modestin J, Huber A, Satirli E, et al. Long-term course of schizophrenic illness: Bleuler’s study reconsidered. Am J Psychiatry 2003;160(12):2202-8.
9. Stephens JH, Richard P, McHugh PR. Long-term follow-up of patients hospitalized for schizophrenia, 1913 to1940. J Nerv Ment Dis 1997;185(12):715-21.
10. Harrow M, Sands JR, Silverstein ML, Goldberg JF. Course and outcome for schizophrenia versus other psychotic patients: a longitudinal study. Schizophr Bull 1997;23:287-303.
11. Craig TJ, Bregman Z. Late-onset schizophrenia-like illness. J Am Geriatr Soc 1988;36(2):104-7.
12. Opjordsmoen S. Long-term course and outcome in unipolar affective and schizoaffective psychoses. Acta Psychiatr Scand 1989;79(4):317-26.
13. Harris G, Hopper K, Craig T, et al. Recovery from psychotic illness: a 15- and 25-year international follow up study. Br J Psychiatry 2001;178:506-17.
14. Kiriakakis V, Bhatia KP, Quinn NP, Marsden CD. The natural history of tardive dystonia. A long-term follow-up study of 107 cases. Brain 1998;121(Pt 11):2053-66.
15. Mala E. Early onset schizophrenia. Cesk Slov Psychiatry 1993;89(5):259-71.
16. Tohen M, Strakowski SM, Zarate C, et al. The McLean-Harvard first episode project: 6-month symptomatic and functional outcome in affective and non-affective psychosis. Biol Psychiatry 2000;48(6):467-76.
17. Kobayashi T. Course types of first admission schizophrenia: A mean 13-year follow back study. Seishin Igaku Clin Psychiatry 2002;44(2):161-8.
18. Sells DJ, Stayner DA, Davidson L. Recovering the self in schizophrenia: An integrative review of qualitative studies. Psychiatr Q. 2004;75(1):87-97.
19. Martens WHJ, Kahn W, Oppenheimer C. Predictors and prevalence of recovery in schizophrenia. W. Kahn Institute of Theoretical Psychiatry and Neuroscience (WKITPN) Publication 36, 2001(5):113-26.
20. Fallot RD. The place of spirituality and religion in mental health services. In: Lamb HR (ed). Best of new directions for mental health services, 1979-2001. San Francisco: Jossey Bass, 2001:79-88.
21. Kane JM. Long-term treatment of schizophrenia: Moving from a relapse-prevention model to a recovery model. J Clin Psychiatry. 2003;64(11):1384-5.
22. Tauscher-Wisniewski S, Zipursky RB. The role of maintenance pharmacotherapy in achieving recovery from a first episode of schizophrenia. Int Rev Psychiatry. 2002;14(4):284-92.
Is it realistic for patients with schizophrenia to believe they can recover? Recent observational studies show that some do,1 even though all DSM editions have defined schizophrenia as a chronic disease with a poor outcome.2
Our understanding of schizophrenia is changing as we gain new insights into:
- mechanism of recovery
- efficacy of combined psychotherapeutic, psychosocial, and drug therapies for sustaining remission and recovery
- the value of long-term aftercare. This article examines evidence on:
- achieving recovery from schizophrenia
- factors associated with remission
- treatments that may help prevent relapse and lead to stable, lasting recovery.
What is ‘recovery’?
Diagnostic criteria. Recovery from schizophrenia has social, occupational, symptomatic, and psychostructural dimensions. For clinical practice, Liberman et al3 developed a useful set of 10 criteria for recovery (Table 1) by analyzing the literature and cases of 23 schizophrenia patients who returned to work or school with their symptoms under control.
Table 1
Recovery from schizophrenia: 10 clinical criteria
| Criteria | Characteristics of recovered patients* |
|---|---|
| Family relationships | 70% reported good or very good family relationships |
| Substance abuse | None reported illicit drug use in the past year, and two reported occasional alcohol consumption |
| Duration of untreated psychosis | Only 13% reported >1 year delay between symptom onset and treatment |
| Initial response to medication | 87% reported effective symptom control with their first antipsychotic medication |
| Adherence to treatment | All reported adherence to psychiatric care and medication regimens |
| Supportive therapy | 91% reported ongoing psychotherapy, and 78% reported that accessible and supportive psychiatrists and therapists aided their recovery |
| Cognitive abilities | Normal or near-normal functioning on tests of flexibility in solving problems, verbal working memory, and perceptual skills |
| Social skills | None showed more than very mild negative symptoms |
| Personal history | 70% graduated from college, 13% completed 2 years of college, and 3 of remaining 4 worked full time before becoming ill |
| Access to care | 91% received antipsychotics and psychotherapy, 48% social skills training, 57% family participation, 26% vocational rehabilitation, and 61% self-help groups |
| * Based on a study of 23 schizophrenia patients who returned to work or school with their symptoms under control. | |
| Source: Reference 3 | |
Recovery is not a smooth, linear progression. Even when patients attain remission, they often find it hard to make up for “lost life” during years of disability.4 Recovery also can be defined as social, emotional, and biological maturation. This definition considers recovery not as an end-state or cure but as a process of personal growth.5
Several groups proposed recently that recovery from schizophrenia includes four processes:
- finding hope
- re-establishing identity
- taking responsibility for recovery
- finding meaning6 and “getting on with life”7 (Box).
Long-tem vs short-term
Recovery has been studied in many populations, but the evidence is difficult to compare. Data quality is compromised by poorly-defined cohorts, weak study designs, and lack of clear definitions of recovery and its diagnostic criteria. Moreover, empirical evidence is lacking on recovery’s multidimensional nature, including psychosocial, biochemical, genetic, environmental, cultural, and ethnic correlates.
Long-term recovery. Recently, three studies of American populations diagnosed with schizophrenia detected trends toward long-term (>5 years) recovery.
U.S. populations. Modestin et al8 in 2003 re-evaluated diagnoses of 208 patients in Swiss psychiatrist Manfred Bleuler’s influential 1972 study on schizophrenia’s long-term course. Using DSM-III-R, DSM-IV, and International Statistical Classification of Diseases and Related Health Problems (ICD-10) criteria, the authors excluded about 30% of the original patients (most rediagnosed with schizoaffective disorder). Among those remaining, 12% to 15% showed long-term recovery and one-half had an undulating course with remissions.
In 1997, Stephens et al9 examined hospital records from 1913 to 1940 of 484 patients, mean age 27, hospitalized with schizophrenia. Using >5 years of follow-up data and DSM-IV criteria, the authors rated 13% as recovered and 58% unimproved.
Also in 1997, Harrow et al10 evaluated 74 patients diagnosed with schizophrenia by DSM-IV criteria at 2, 4.5, and 7.5 years. In this longitudinal study, one-third (32%) showed complete remission at one follow-up session, compared with 5% at all three evaluations.
This study suggested that schizophrenia patients show relatively poor functioning, compared with other psychotic patients. Over time, however, the likelihood of long-term remission appeared to increase. A similar pattern was seen in a sample of 658 Americans age >65 with schizophrenia diagnosed by DSM-III criteria. As these patients aged, 15% developed long-lasting remission.11
Elsewhere, empirical findings across 15 years from three Norwegian studies indicate that lasting recovery from schizophrenia—with symptom improvement and psychosocial adjustment—is rare (3% to 5% of patients).3 Similarly, only 4% of a Scandinavian sample of 301 patients attained complete, long-term remission during 3 to 39 years of follow-up.12
Across cultures, an international study13 evaluated 15- and 25-year outcomes in 1,633 patients diagnosed with schizophrenia. Approximately 50% had favorable outcomes—stable work, independent from support, no imprisonment, no substance abuse, no rehospitalization, improved social life—but heterogeneity was marked.
Ms J, 48, is in recovery from schizophrenia. She has a stable job as a Web designer, is married, and has learned to build and maintain social relationships. Much of her life, however, has been very different.
At age 15 she was diagnosed with schizophrenia, paranoid psychotic type, with occasional comorbid bipolar symptoms. Over the next 20 years, she was admitted to psychiatric hospitals six times for treatment. At age 36, she was hospitalized with psychosis, depressive symptoms, and insomnia. At that point, she was taking carbamazepine, 500 mg/d, for mood stabilization, and haloperidol, 50 mg/d.
Changing medications. Her psychiatriststarted olanzapine, 5 mg/d, and tapered off haloperidol, which appeared to be gradually becoming less effective while causing mood-related side effects. Ms. J’s psychosis persisted, however, with no response to olanzapine.
Her psychiatrist then tapered carbamazepine to 175 mg/d while starting lamotrigine, 150 to 300 mg/d. The rationale for switching mood stabilizers was that lamotrigine may be more effective than carbamazepine in controlling mixed bipolar states, provide a greater antidepressant effect, and cause fewer side effects.
Intensive treatment. Within 10 days, Ms J’s thought form and composition improved, and her psychiatrist immediately started psychotherapy and psychosocial guidance. Carbamazepine was withdrawn 3 months later, but Ms. J remained on olanzapine, 5 mg/d, and lamotrigine, 300 mg/d. With these medications, the paranoid psychosis went into remission.
After 5 months of intensive treatment, Ms. J was discharged. Outpatient treatment included weekly psychotherapy plus psychosocial guidance and social and coping training 6 times per month. These therapies —along with olanzapine, 5 mg/d, and carbamazepine, 300 mg/d—continue today.
Ms. J’s mental and emotional condition stabilized, and her cognitive abilities improved. Education and therapy helped reduce stress within her family. She has not been rehospitalized or suffered a serious relapse in 12 years.
Table 2
Psychosocial interventions for patients in recovery from schizophrenia*
|
| * Psychosocial interventions are most effective when combined with antipsychotic therapy and individualized psychotherapy |
Short-term course predicted long-term outcome, and local environment played a significant role in determining symptoms and social disability. The authors concluded that adequate early treatment and an optimum environment might lead to favorable long-term outcome.
In the United Kingdom, 14% of a sample of patients diagnosed by ICD-10 criteria achieved remission across a mean 8.5 years.14 In a study of Czechoslovakian patients (70 men, 50 women) with early-onset schizophrenia diagnosed by DSM-III-R criteria, 10% recovered during 13 to 42 years of follow-up.15
Short-term recovery. The McLean-Harvard first-episode project examined outcomes 6 months after schizophrenia diagnosis in 102 patients (55 men, 47 women). Sixty-five percent attained syndromal recovery (significant reduction of diagnostic features), whereas only 33% achieved functional recovery (increased social-emotional, vocational, and coping abilities).16
In Japan, 62 patients (33 men, 29 women; mean age 25) were followed for 13 years after a first hospitalization for schizophrenia. The authors reported an undulating course with recovery or a mild end-state in 53%, and a simple course of recovery and a moderate or severe end-state in 28%.17
Conclusions. The evidence suggests that early and lasting treatment of schizophrenic symptoms—even in recovered patients—might prevent frequent rehospitalizations. Thus, patients with schizophrenia must be followed carefully during and after recovery. Health care professionals, colleagues, friends, and relatives can help patients sustain recovery by watching for the earliest signs of deterioration and intervening before relapse occurs.
Strategies for recovery
Therapeutic factors. Many studies suggest psychosocial interventions (Table 2), psychotherapy, and medication are most effective in combination for stabilizing patients with schizophrenia and continuing their recovery. Other patient factors that may contribute to recovery include:
- quality of relationships with family, friends, and professional caregivers
- ability and motivation to use resources and take responsibility for one’s life
- spiritual and religious activities
- awareness that recovery is possible.
Sells et al18 noted that attempting to make new contacts outside of their former spheres (“positive withdrawal”) may allow schizophrenia patients to reconsider and ultimately recover a durable sense of self.
We at the W. Kahn Institute19 find that all these treatment strategies may be useful and even necessary to continue and stabilize recovery from schizophrenia. We feel they merit the attention of all professionals involved in recovered patients’ aftercare and guidance.
Table 3
Suggested antipsychotic dosages during schizophrenia recovery*
| Drug | Dosage (mg/d) | Potential side effects | Positive effects |
|---|---|---|---|
| Aripiprazole | 10 to 30 | Headache, anxiety, insomnia, lightheadedness | Reduced positive, negative symptoms |
| Clozapine | 300 to 900 | Withdrawal, blunted emotions, seizures, lack of motivation | Reduced positive symptoms |
| Haloperidol | 30 to 100 | Tardive dyskinesia, parkinsonian symptoms, insomnia, depressive reactions, confusion, drowsiness, hypertension | Reduced mania, hyperactivity, agitation |
| Olanzapine | 5 to 10 | Drowsiness, agitation, weight gain, involuntary movements, restlessness | Reduced positive, negative symptoms |
| Quetiapine | 25 to 100 | Dizziness, hypotension, increased cholesterol, weight gain | Reduced positive symptoms |
| Risperidone | 2.5 to 5 | Anxiety, nervousness, back pain, bleeding, dizziness, irregular blood pressure | Reduced positive, negative symptoms |
| Ziprasidone | 10 to 200 | Heart-rhythm irregularity, loss of consciousness, restlessness, weakness, drowsiness | Reduced positive symptoms |
| * Dosages are individualized and may vary among patients and situations, but most will be gradually reduced to minimum levels during remission. | |||
Social/vocational network. Family, friends, neighbors, and social workers play an important role in the patient’s development during recovery. They provide positive stimulation (such as physical activities and social or vocational engagements) and support.
To equip the patient’s network for this responsible task, provide them with training (such as in acceptance, empathy, feedback, and communication), education, and guidance. Support groups can enhance the social networks of patients whose own networks are too small to prevent social isolation or overburdening of members.
Vocational training and mediation also may be stabilizing. Religious activities are central to self-understanding and recovery for many psychiatric patients and may improve outcomes.20
Patient skills. To achieve stabilization and continue their recovery, patients must develop social interaction skills and coping strategies. Conversation training, for example, seems to help improve social interaction. Patients in remission must learn to:
- find or create low-stress, positively stimulating environments in which their recovery can flourish
- tolerate discomfort and stress
- overcome internalized stigma about recovery.
Patients also need to learn when and how to withdraw from hectic, stressful environments and from people who are overly emotional, patronizing, or hold unrealistic expectations about them.
Pharmacotherapy. Sound pharmacotherapy underlies rehabilitation and psychosocial treatment of patients in remission from schizophrenia.21 Healthy neurobiological functioning and equilibrium may help normalize social-emotional behavior and create opportunities to improve all life dimensions via psychotherapy, psychosocial guidance, education, and training.
Dosages often can be reduced during recovery, titrating gradually downward to reduce the risk of relapse. Suggested antipsychotic dosages during recovery are listed in Table 3. Be patient and consistent when adjusting dosages, guided by information in package inserts, from clinical trials, and in recent articles on specific medications. When introducing a medication, start with the lowest dosage and increase in small steps until symptoms are reduced and side effects are minimal.
Discontinuing antipsychotics. Is it therapeutically reasonable to discontinue antipsychotics after recovery? Probably not.
Relapse rates in unmedicated patients with schizophrenia appear extremely high—perhaps 8 or 9 out of 10 cases—even during remission. By comparison, relapse rates appear very low—perhaps 3 or 4 out of 10 cases—for remitted patients who remain on antipsychotics. Atypical antipsychotics or low doses of conventional agents are generally well-tolerated and safe in the long term.22
Patient monitoring. Patients in remission from schizophrenia benefit from:
- 24-hour phone lines to call for guidance, treatment, and quick interventions
- central, alert treatment coordination among psychiatrists, psychologists, social workers, therapists, vocational experts, and activity counselors.
Psychiatrists in solo or small-group settings may need to seek out these resources in their communities. The goal of this team approach—in cooperation with the patient’s social network—is to help the patient develop employment and social activities appropriate to his or her needs and capabilities.
Related resources
- Ascher-Svanuw H, Kraus A. Psychoeducational groups for patients with schizophrenia: a guide for practitioners. Gaithersburg, MD: Aspen Publishers, 1991.
- Suzuki T, Uchida H, Tanaka KF, et al. Revising polypharmacy to a single antipsychotic regimen for patients with chronic schizophrenia. Int J Neuropsychopharmacol 2004;7(2):133-42.
- American Psychiatric Association. Schizophrenia (patient/family information). www.psych.org/public_info/schizo.cfm
- Schizophrenia.com. Nonprofit Web community providing information, support, and education. www.schizophrenia.com
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Is it realistic for patients with schizophrenia to believe they can recover? Recent observational studies show that some do,1 even though all DSM editions have defined schizophrenia as a chronic disease with a poor outcome.2
Our understanding of schizophrenia is changing as we gain new insights into:
- mechanism of recovery
- efficacy of combined psychotherapeutic, psychosocial, and drug therapies for sustaining remission and recovery
- the value of long-term aftercare. This article examines evidence on:
- achieving recovery from schizophrenia
- factors associated with remission
- treatments that may help prevent relapse and lead to stable, lasting recovery.
What is ‘recovery’?
Diagnostic criteria. Recovery from schizophrenia has social, occupational, symptomatic, and psychostructural dimensions. For clinical practice, Liberman et al3 developed a useful set of 10 criteria for recovery (Table 1) by analyzing the literature and cases of 23 schizophrenia patients who returned to work or school with their symptoms under control.
Table 1
Recovery from schizophrenia: 10 clinical criteria
| Criteria | Characteristics of recovered patients* |
|---|---|
| Family relationships | 70% reported good or very good family relationships |
| Substance abuse | None reported illicit drug use in the past year, and two reported occasional alcohol consumption |
| Duration of untreated psychosis | Only 13% reported >1 year delay between symptom onset and treatment |
| Initial response to medication | 87% reported effective symptom control with their first antipsychotic medication |
| Adherence to treatment | All reported adherence to psychiatric care and medication regimens |
| Supportive therapy | 91% reported ongoing psychotherapy, and 78% reported that accessible and supportive psychiatrists and therapists aided their recovery |
| Cognitive abilities | Normal or near-normal functioning on tests of flexibility in solving problems, verbal working memory, and perceptual skills |
| Social skills | None showed more than very mild negative symptoms |
| Personal history | 70% graduated from college, 13% completed 2 years of college, and 3 of remaining 4 worked full time before becoming ill |
| Access to care | 91% received antipsychotics and psychotherapy, 48% social skills training, 57% family participation, 26% vocational rehabilitation, and 61% self-help groups |
| * Based on a study of 23 schizophrenia patients who returned to work or school with their symptoms under control. | |
| Source: Reference 3 | |
Recovery is not a smooth, linear progression. Even when patients attain remission, they often find it hard to make up for “lost life” during years of disability.4 Recovery also can be defined as social, emotional, and biological maturation. This definition considers recovery not as an end-state or cure but as a process of personal growth.5
Several groups proposed recently that recovery from schizophrenia includes four processes:
- finding hope
- re-establishing identity
- taking responsibility for recovery
- finding meaning6 and “getting on with life”7 (Box).
Long-tem vs short-term
Recovery has been studied in many populations, but the evidence is difficult to compare. Data quality is compromised by poorly-defined cohorts, weak study designs, and lack of clear definitions of recovery and its diagnostic criteria. Moreover, empirical evidence is lacking on recovery’s multidimensional nature, including psychosocial, biochemical, genetic, environmental, cultural, and ethnic correlates.
Long-term recovery. Recently, three studies of American populations diagnosed with schizophrenia detected trends toward long-term (>5 years) recovery.
U.S. populations. Modestin et al8 in 2003 re-evaluated diagnoses of 208 patients in Swiss psychiatrist Manfred Bleuler’s influential 1972 study on schizophrenia’s long-term course. Using DSM-III-R, DSM-IV, and International Statistical Classification of Diseases and Related Health Problems (ICD-10) criteria, the authors excluded about 30% of the original patients (most rediagnosed with schizoaffective disorder). Among those remaining, 12% to 15% showed long-term recovery and one-half had an undulating course with remissions.
In 1997, Stephens et al9 examined hospital records from 1913 to 1940 of 484 patients, mean age 27, hospitalized with schizophrenia. Using >5 years of follow-up data and DSM-IV criteria, the authors rated 13% as recovered and 58% unimproved.
Also in 1997, Harrow et al10 evaluated 74 patients diagnosed with schizophrenia by DSM-IV criteria at 2, 4.5, and 7.5 years. In this longitudinal study, one-third (32%) showed complete remission at one follow-up session, compared with 5% at all three evaluations.
This study suggested that schizophrenia patients show relatively poor functioning, compared with other psychotic patients. Over time, however, the likelihood of long-term remission appeared to increase. A similar pattern was seen in a sample of 658 Americans age >65 with schizophrenia diagnosed by DSM-III criteria. As these patients aged, 15% developed long-lasting remission.11
Elsewhere, empirical findings across 15 years from three Norwegian studies indicate that lasting recovery from schizophrenia—with symptom improvement and psychosocial adjustment—is rare (3% to 5% of patients).3 Similarly, only 4% of a Scandinavian sample of 301 patients attained complete, long-term remission during 3 to 39 years of follow-up.12
Across cultures, an international study13 evaluated 15- and 25-year outcomes in 1,633 patients diagnosed with schizophrenia. Approximately 50% had favorable outcomes—stable work, independent from support, no imprisonment, no substance abuse, no rehospitalization, improved social life—but heterogeneity was marked.
Ms J, 48, is in recovery from schizophrenia. She has a stable job as a Web designer, is married, and has learned to build and maintain social relationships. Much of her life, however, has been very different.
At age 15 she was diagnosed with schizophrenia, paranoid psychotic type, with occasional comorbid bipolar symptoms. Over the next 20 years, she was admitted to psychiatric hospitals six times for treatment. At age 36, she was hospitalized with psychosis, depressive symptoms, and insomnia. At that point, she was taking carbamazepine, 500 mg/d, for mood stabilization, and haloperidol, 50 mg/d.
Changing medications. Her psychiatriststarted olanzapine, 5 mg/d, and tapered off haloperidol, which appeared to be gradually becoming less effective while causing mood-related side effects. Ms. J’s psychosis persisted, however, with no response to olanzapine.
Her psychiatrist then tapered carbamazepine to 175 mg/d while starting lamotrigine, 150 to 300 mg/d. The rationale for switching mood stabilizers was that lamotrigine may be more effective than carbamazepine in controlling mixed bipolar states, provide a greater antidepressant effect, and cause fewer side effects.
Intensive treatment. Within 10 days, Ms J’s thought form and composition improved, and her psychiatrist immediately started psychotherapy and psychosocial guidance. Carbamazepine was withdrawn 3 months later, but Ms. J remained on olanzapine, 5 mg/d, and lamotrigine, 300 mg/d. With these medications, the paranoid psychosis went into remission.
After 5 months of intensive treatment, Ms. J was discharged. Outpatient treatment included weekly psychotherapy plus psychosocial guidance and social and coping training 6 times per month. These therapies —along with olanzapine, 5 mg/d, and carbamazepine, 300 mg/d—continue today.
Ms. J’s mental and emotional condition stabilized, and her cognitive abilities improved. Education and therapy helped reduce stress within her family. She has not been rehospitalized or suffered a serious relapse in 12 years.
Table 2
Psychosocial interventions for patients in recovery from schizophrenia*
|
| * Psychosocial interventions are most effective when combined with antipsychotic therapy and individualized psychotherapy |
Short-term course predicted long-term outcome, and local environment played a significant role in determining symptoms and social disability. The authors concluded that adequate early treatment and an optimum environment might lead to favorable long-term outcome.
In the United Kingdom, 14% of a sample of patients diagnosed by ICD-10 criteria achieved remission across a mean 8.5 years.14 In a study of Czechoslovakian patients (70 men, 50 women) with early-onset schizophrenia diagnosed by DSM-III-R criteria, 10% recovered during 13 to 42 years of follow-up.15
Short-term recovery. The McLean-Harvard first-episode project examined outcomes 6 months after schizophrenia diagnosis in 102 patients (55 men, 47 women). Sixty-five percent attained syndromal recovery (significant reduction of diagnostic features), whereas only 33% achieved functional recovery (increased social-emotional, vocational, and coping abilities).16
In Japan, 62 patients (33 men, 29 women; mean age 25) were followed for 13 years after a first hospitalization for schizophrenia. The authors reported an undulating course with recovery or a mild end-state in 53%, and a simple course of recovery and a moderate or severe end-state in 28%.17
Conclusions. The evidence suggests that early and lasting treatment of schizophrenic symptoms—even in recovered patients—might prevent frequent rehospitalizations. Thus, patients with schizophrenia must be followed carefully during and after recovery. Health care professionals, colleagues, friends, and relatives can help patients sustain recovery by watching for the earliest signs of deterioration and intervening before relapse occurs.
Strategies for recovery
Therapeutic factors. Many studies suggest psychosocial interventions (Table 2), psychotherapy, and medication are most effective in combination for stabilizing patients with schizophrenia and continuing their recovery. Other patient factors that may contribute to recovery include:
- quality of relationships with family, friends, and professional caregivers
- ability and motivation to use resources and take responsibility for one’s life
- spiritual and religious activities
- awareness that recovery is possible.
Sells et al18 noted that attempting to make new contacts outside of their former spheres (“positive withdrawal”) may allow schizophrenia patients to reconsider and ultimately recover a durable sense of self.
We at the W. Kahn Institute19 find that all these treatment strategies may be useful and even necessary to continue and stabilize recovery from schizophrenia. We feel they merit the attention of all professionals involved in recovered patients’ aftercare and guidance.
Table 3
Suggested antipsychotic dosages during schizophrenia recovery*
| Drug | Dosage (mg/d) | Potential side effects | Positive effects |
|---|---|---|---|
| Aripiprazole | 10 to 30 | Headache, anxiety, insomnia, lightheadedness | Reduced positive, negative symptoms |
| Clozapine | 300 to 900 | Withdrawal, blunted emotions, seizures, lack of motivation | Reduced positive symptoms |
| Haloperidol | 30 to 100 | Tardive dyskinesia, parkinsonian symptoms, insomnia, depressive reactions, confusion, drowsiness, hypertension | Reduced mania, hyperactivity, agitation |
| Olanzapine | 5 to 10 | Drowsiness, agitation, weight gain, involuntary movements, restlessness | Reduced positive, negative symptoms |
| Quetiapine | 25 to 100 | Dizziness, hypotension, increased cholesterol, weight gain | Reduced positive symptoms |
| Risperidone | 2.5 to 5 | Anxiety, nervousness, back pain, bleeding, dizziness, irregular blood pressure | Reduced positive, negative symptoms |
| Ziprasidone | 10 to 200 | Heart-rhythm irregularity, loss of consciousness, restlessness, weakness, drowsiness | Reduced positive symptoms |
| * Dosages are individualized and may vary among patients and situations, but most will be gradually reduced to minimum levels during remission. | |||
Social/vocational network. Family, friends, neighbors, and social workers play an important role in the patient’s development during recovery. They provide positive stimulation (such as physical activities and social or vocational engagements) and support.
To equip the patient’s network for this responsible task, provide them with training (such as in acceptance, empathy, feedback, and communication), education, and guidance. Support groups can enhance the social networks of patients whose own networks are too small to prevent social isolation or overburdening of members.
Vocational training and mediation also may be stabilizing. Religious activities are central to self-understanding and recovery for many psychiatric patients and may improve outcomes.20
Patient skills. To achieve stabilization and continue their recovery, patients must develop social interaction skills and coping strategies. Conversation training, for example, seems to help improve social interaction. Patients in remission must learn to:
- find or create low-stress, positively stimulating environments in which their recovery can flourish
- tolerate discomfort and stress
- overcome internalized stigma about recovery.
Patients also need to learn when and how to withdraw from hectic, stressful environments and from people who are overly emotional, patronizing, or hold unrealistic expectations about them.
Pharmacotherapy. Sound pharmacotherapy underlies rehabilitation and psychosocial treatment of patients in remission from schizophrenia.21 Healthy neurobiological functioning and equilibrium may help normalize social-emotional behavior and create opportunities to improve all life dimensions via psychotherapy, psychosocial guidance, education, and training.
Dosages often can be reduced during recovery, titrating gradually downward to reduce the risk of relapse. Suggested antipsychotic dosages during recovery are listed in Table 3. Be patient and consistent when adjusting dosages, guided by information in package inserts, from clinical trials, and in recent articles on specific medications. When introducing a medication, start with the lowest dosage and increase in small steps until symptoms are reduced and side effects are minimal.
Discontinuing antipsychotics. Is it therapeutically reasonable to discontinue antipsychotics after recovery? Probably not.
Relapse rates in unmedicated patients with schizophrenia appear extremely high—perhaps 8 or 9 out of 10 cases—even during remission. By comparison, relapse rates appear very low—perhaps 3 or 4 out of 10 cases—for remitted patients who remain on antipsychotics. Atypical antipsychotics or low doses of conventional agents are generally well-tolerated and safe in the long term.22
Patient monitoring. Patients in remission from schizophrenia benefit from:
- 24-hour phone lines to call for guidance, treatment, and quick interventions
- central, alert treatment coordination among psychiatrists, psychologists, social workers, therapists, vocational experts, and activity counselors.
Psychiatrists in solo or small-group settings may need to seek out these resources in their communities. The goal of this team approach—in cooperation with the patient’s social network—is to help the patient develop employment and social activities appropriate to his or her needs and capabilities.
Related resources
- Ascher-Svanuw H, Kraus A. Psychoeducational groups for patients with schizophrenia: a guide for practitioners. Gaithersburg, MD: Aspen Publishers, 1991.
- Suzuki T, Uchida H, Tanaka KF, et al. Revising polypharmacy to a single antipsychotic regimen for patients with chronic schizophrenia. Int J Neuropsychopharmacol 2004;7(2):133-42.
- American Psychiatric Association. Schizophrenia (patient/family information). www.psych.org/public_info/schizo.cfm
- Schizophrenia.com. Nonprofit Web community providing information, support, and education. www.schizophrenia.com
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Davidson L. Living outside mental illness: qualitative studies of recovery in schizophrenia. New York: New York University Press, 2003.
2. Kruger A. Schizophrenia: Recovery and hope. Psychiatr Rehabil J 2000;24(1):29-37.
3. Liberman RP, Kopelowicz A, Ventura J, Gutkind D. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry 2002;14(4):256-72.
4. Torgalsboen AK, Rund BR. Lessons learned from three studies of recovery from schizophrenia. Int Rev Psychiatry 2002;14(4):312-17.
5. Boone EC. A qualitative investigation of the process of recovery in people experiencing schizophrenia. Dissert Abstr Int 1996;57 (5-B):3402.-
6. Andresen R, Oades L, Caputi P. The experience of recovery from schizophrenia: towards an empirically validated stage model. Aust NZ J Psychiatry 2003;37(5):586-94.
7. Noordsy D, Torrey W, Mueser K, et al. Recovery from severe mental illness: an interpersonal and functional outcome definition. Int Rev Psychiatry 2002;14(4):318-26.
8. Modestin J, Huber A, Satirli E, et al. Long-term course of schizophrenic illness: Bleuler’s study reconsidered. Am J Psychiatry 2003;160(12):2202-8.
9. Stephens JH, Richard P, McHugh PR. Long-term follow-up of patients hospitalized for schizophrenia, 1913 to1940. J Nerv Ment Dis 1997;185(12):715-21.
10. Harrow M, Sands JR, Silverstein ML, Goldberg JF. Course and outcome for schizophrenia versus other psychotic patients: a longitudinal study. Schizophr Bull 1997;23:287-303.
11. Craig TJ, Bregman Z. Late-onset schizophrenia-like illness. J Am Geriatr Soc 1988;36(2):104-7.
12. Opjordsmoen S. Long-term course and outcome in unipolar affective and schizoaffective psychoses. Acta Psychiatr Scand 1989;79(4):317-26.
13. Harris G, Hopper K, Craig T, et al. Recovery from psychotic illness: a 15- and 25-year international follow up study. Br J Psychiatry 2001;178:506-17.
14. Kiriakakis V, Bhatia KP, Quinn NP, Marsden CD. The natural history of tardive dystonia. A long-term follow-up study of 107 cases. Brain 1998;121(Pt 11):2053-66.
15. Mala E. Early onset schizophrenia. Cesk Slov Psychiatry 1993;89(5):259-71.
16. Tohen M, Strakowski SM, Zarate C, et al. The McLean-Harvard first episode project: 6-month symptomatic and functional outcome in affective and non-affective psychosis. Biol Psychiatry 2000;48(6):467-76.
17. Kobayashi T. Course types of first admission schizophrenia: A mean 13-year follow back study. Seishin Igaku Clin Psychiatry 2002;44(2):161-8.
18. Sells DJ, Stayner DA, Davidson L. Recovering the self in schizophrenia: An integrative review of qualitative studies. Psychiatr Q. 2004;75(1):87-97.
19. Martens WHJ, Kahn W, Oppenheimer C. Predictors and prevalence of recovery in schizophrenia. W. Kahn Institute of Theoretical Psychiatry and Neuroscience (WKITPN) Publication 36, 2001(5):113-26.
20. Fallot RD. The place of spirituality and religion in mental health services. In: Lamb HR (ed). Best of new directions for mental health services, 1979-2001. San Francisco: Jossey Bass, 2001:79-88.
21. Kane JM. Long-term treatment of schizophrenia: Moving from a relapse-prevention model to a recovery model. J Clin Psychiatry. 2003;64(11):1384-5.
22. Tauscher-Wisniewski S, Zipursky RB. The role of maintenance pharmacotherapy in achieving recovery from a first episode of schizophrenia. Int Rev Psychiatry. 2002;14(4):284-92.
1. Davidson L. Living outside mental illness: qualitative studies of recovery in schizophrenia. New York: New York University Press, 2003.
2. Kruger A. Schizophrenia: Recovery and hope. Psychiatr Rehabil J 2000;24(1):29-37.
3. Liberman RP, Kopelowicz A, Ventura J, Gutkind D. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry 2002;14(4):256-72.
4. Torgalsboen AK, Rund BR. Lessons learned from three studies of recovery from schizophrenia. Int Rev Psychiatry 2002;14(4):312-17.
5. Boone EC. A qualitative investigation of the process of recovery in people experiencing schizophrenia. Dissert Abstr Int 1996;57 (5-B):3402.-
6. Andresen R, Oades L, Caputi P. The experience of recovery from schizophrenia: towards an empirically validated stage model. Aust NZ J Psychiatry 2003;37(5):586-94.
7. Noordsy D, Torrey W, Mueser K, et al. Recovery from severe mental illness: an interpersonal and functional outcome definition. Int Rev Psychiatry 2002;14(4):318-26.
8. Modestin J, Huber A, Satirli E, et al. Long-term course of schizophrenic illness: Bleuler’s study reconsidered. Am J Psychiatry 2003;160(12):2202-8.
9. Stephens JH, Richard P, McHugh PR. Long-term follow-up of patients hospitalized for schizophrenia, 1913 to1940. J Nerv Ment Dis 1997;185(12):715-21.
10. Harrow M, Sands JR, Silverstein ML, Goldberg JF. Course and outcome for schizophrenia versus other psychotic patients: a longitudinal study. Schizophr Bull 1997;23:287-303.
11. Craig TJ, Bregman Z. Late-onset schizophrenia-like illness. J Am Geriatr Soc 1988;36(2):104-7.
12. Opjordsmoen S. Long-term course and outcome in unipolar affective and schizoaffective psychoses. Acta Psychiatr Scand 1989;79(4):317-26.
13. Harris G, Hopper K, Craig T, et al. Recovery from psychotic illness: a 15- and 25-year international follow up study. Br J Psychiatry 2001;178:506-17.
14. Kiriakakis V, Bhatia KP, Quinn NP, Marsden CD. The natural history of tardive dystonia. A long-term follow-up study of 107 cases. Brain 1998;121(Pt 11):2053-66.
15. Mala E. Early onset schizophrenia. Cesk Slov Psychiatry 1993;89(5):259-71.
16. Tohen M, Strakowski SM, Zarate C, et al. The McLean-Harvard first episode project: 6-month symptomatic and functional outcome in affective and non-affective psychosis. Biol Psychiatry 2000;48(6):467-76.
17. Kobayashi T. Course types of first admission schizophrenia: A mean 13-year follow back study. Seishin Igaku Clin Psychiatry 2002;44(2):161-8.
18. Sells DJ, Stayner DA, Davidson L. Recovering the self in schizophrenia: An integrative review of qualitative studies. Psychiatr Q. 2004;75(1):87-97.
19. Martens WHJ, Kahn W, Oppenheimer C. Predictors and prevalence of recovery in schizophrenia. W. Kahn Institute of Theoretical Psychiatry and Neuroscience (WKITPN) Publication 36, 2001(5):113-26.
20. Fallot RD. The place of spirituality and religion in mental health services. In: Lamb HR (ed). Best of new directions for mental health services, 1979-2001. San Francisco: Jossey Bass, 2001:79-88.
21. Kane JM. Long-term treatment of schizophrenia: Moving from a relapse-prevention model to a recovery model. J Clin Psychiatry. 2003;64(11):1384-5.
22. Tauscher-Wisniewski S, Zipursky RB. The role of maintenance pharmacotherapy in achieving recovery from a first episode of schizophrenia. Int Rev Psychiatry. 2002;14(4):284-92.
To upgrade or not to upgrade?
Personal digital assistants (PDA) are in a state of flux. Thanks to a flurry of hardware and operating system improvements over the last 18 months, PDAs that were cutting-edge last year pale in comparison to newer models.
Should you buy a new PDA now, or stick with your current model and wait for still more innovations? This article can help you decide.
Why upgrade?
Pros. Today’s PDAs are more versatile and intuitive. For example, many combination PDA/mobile phones have newer operating systems, more memory, and greater software compatibility than earlier devices.
A frequent PDA user who craves more speed or added features might want to upgrade now. Likewise, users who are constantly on the road might want a new combination PDA/global positioning system.
Cons. PDA operating systems are updated frequently, reflecting continuing improvements in handheld hardware. Microsoft late last year upgraded its Pocket PC operating system and changed its name to Windows Mobile.
Although frequent users will rejoice in the opportunity to do more, others might not want to spend $300 or more to get the latest features—only to see their new device become outmoded in a few months.
Hardware advances
Processors. The processor, the heart of a PDA, has also seen much change. Newer Palm and Pocket PC devices are based on the strongARM microprocessor produced by Intel under the Xscale brand. Each new processor has more speed, better multimedia, an improved camera interface, and lower power consumption than previous processors.
Smartphones, which reached the market in 2002, are geared to physicians who rely mostly on email and calendar functions and have little need for mobile medical information.
A Windows Mobile smartphone typically looks like a mobile phone but has basic Pocket PC capabilities, such as viewing mail, a calendar, to-do list, and notes.
Windows Mobile smartphones, however, are compatible only with smartphone-specific programs, not with general Pocket PC software. This means that drug reference guides, DSM-IV-TR, and other commonly used medical programs cannot be viewed on a smartphone. If you love the idea of a smartphone but want a specialized program, check out www.smartphone.net.
Pocket PC phone. By contrast, the Pocket PC phone looks and works more like a PDA than a phone. Because it is connected to the cellular network, the device has wider Internet access than does a WiFi-enabled Pocket PC3. At 3.5-by-3.5 inches, the screen size is about twice that of the smartphone’s screen. The device also is compatible with all Pocket PC software.
Pocket PC phones typically have more main memory than smartphones. Smartphones are limited to secure digital-based external memory, but Pocket PC phones have infrared and other connectivity options.
Palm-based smartphones can run most Palm software, depending upon operating system compatibility. Previous Palm smartphones were more limited because of an older operating system and lack of external memory cards.
The palmOne Treo 600, based on the new Palm Version 5 operating system, offers an external memory slot, built-in digital camera, and text messaging. palmOne offers a GSM (global system for mobile communication) protocol version for the T-Mobile, Cingular, and AT&T networks, and a CDMA (code-division multiple access) protocol version for the Sprint network.
Operating system improvements
Palm Source has released version 6 of its operating system—code named “Cobalt”—to hardware developers. This versatile new version—with higher resolution, Word and JPEG file support, simultaneous multiple communications, and other features—could reach the market around the winter holiday.
Microsoft has released Windows Mobile 2003 Second Edition. With certain devices, the operating system will help users read text or browse the Web by using more of the screen. With larger screen resolutions packed into a smaller area, fonts are smaller and hard to read. Windows Mobile 2003 Second Edition provides additional font-size controls to compensate for this change.
The new Windows Mobile version also features a start menu that displays frequently used applications, and WiFi security is improved compared with the previous version.
The future
Tiquit, OQO, and FlipStart plan to release fully operable handheld computers later this year.
These new devices will run on Windows XP and other operating systems and will feature full central processing units, hard disks, liquid crystal display panels, USB connectivity, and built-in QWERTY keyboards. It remains to be seen if these computers will supersede Palm and Windows Mobile PDAs.
Sony’s new Vaio U70 handheld computer, recently released in Japan, is available in the United States via specialty retailers such as dynamism.com.
Personal digital assistants (PDA) are in a state of flux. Thanks to a flurry of hardware and operating system improvements over the last 18 months, PDAs that were cutting-edge last year pale in comparison to newer models.
Should you buy a new PDA now, or stick with your current model and wait for still more innovations? This article can help you decide.
Why upgrade?
Pros. Today’s PDAs are more versatile and intuitive. For example, many combination PDA/mobile phones have newer operating systems, more memory, and greater software compatibility than earlier devices.
A frequent PDA user who craves more speed or added features might want to upgrade now. Likewise, users who are constantly on the road might want a new combination PDA/global positioning system.
Cons. PDA operating systems are updated frequently, reflecting continuing improvements in handheld hardware. Microsoft late last year upgraded its Pocket PC operating system and changed its name to Windows Mobile.
Although frequent users will rejoice in the opportunity to do more, others might not want to spend $300 or more to get the latest features—only to see their new device become outmoded in a few months.
Hardware advances
Processors. The processor, the heart of a PDA, has also seen much change. Newer Palm and Pocket PC devices are based on the strongARM microprocessor produced by Intel under the Xscale brand. Each new processor has more speed, better multimedia, an improved camera interface, and lower power consumption than previous processors.
Smartphones, which reached the market in 2002, are geared to physicians who rely mostly on email and calendar functions and have little need for mobile medical information.
A Windows Mobile smartphone typically looks like a mobile phone but has basic Pocket PC capabilities, such as viewing mail, a calendar, to-do list, and notes.
Windows Mobile smartphones, however, are compatible only with smartphone-specific programs, not with general Pocket PC software. This means that drug reference guides, DSM-IV-TR, and other commonly used medical programs cannot be viewed on a smartphone. If you love the idea of a smartphone but want a specialized program, check out www.smartphone.net.
Pocket PC phone. By contrast, the Pocket PC phone looks and works more like a PDA than a phone. Because it is connected to the cellular network, the device has wider Internet access than does a WiFi-enabled Pocket PC3. At 3.5-by-3.5 inches, the screen size is about twice that of the smartphone’s screen. The device also is compatible with all Pocket PC software.
Pocket PC phones typically have more main memory than smartphones. Smartphones are limited to secure digital-based external memory, but Pocket PC phones have infrared and other connectivity options.
Palm-based smartphones can run most Palm software, depending upon operating system compatibility. Previous Palm smartphones were more limited because of an older operating system and lack of external memory cards.
The palmOne Treo 600, based on the new Palm Version 5 operating system, offers an external memory slot, built-in digital camera, and text messaging. palmOne offers a GSM (global system for mobile communication) protocol version for the T-Mobile, Cingular, and AT&T networks, and a CDMA (code-division multiple access) protocol version for the Sprint network.
Operating system improvements
Palm Source has released version 6 of its operating system—code named “Cobalt”—to hardware developers. This versatile new version—with higher resolution, Word and JPEG file support, simultaneous multiple communications, and other features—could reach the market around the winter holiday.
Microsoft has released Windows Mobile 2003 Second Edition. With certain devices, the operating system will help users read text or browse the Web by using more of the screen. With larger screen resolutions packed into a smaller area, fonts are smaller and hard to read. Windows Mobile 2003 Second Edition provides additional font-size controls to compensate for this change.
The new Windows Mobile version also features a start menu that displays frequently used applications, and WiFi security is improved compared with the previous version.
The future
Tiquit, OQO, and FlipStart plan to release fully operable handheld computers later this year.
These new devices will run on Windows XP and other operating systems and will feature full central processing units, hard disks, liquid crystal display panels, USB connectivity, and built-in QWERTY keyboards. It remains to be seen if these computers will supersede Palm and Windows Mobile PDAs.
Sony’s new Vaio U70 handheld computer, recently released in Japan, is available in the United States via specialty retailers such as dynamism.com.
Personal digital assistants (PDA) are in a state of flux. Thanks to a flurry of hardware and operating system improvements over the last 18 months, PDAs that were cutting-edge last year pale in comparison to newer models.
Should you buy a new PDA now, or stick with your current model and wait for still more innovations? This article can help you decide.
Why upgrade?
Pros. Today’s PDAs are more versatile and intuitive. For example, many combination PDA/mobile phones have newer operating systems, more memory, and greater software compatibility than earlier devices.
A frequent PDA user who craves more speed or added features might want to upgrade now. Likewise, users who are constantly on the road might want a new combination PDA/global positioning system.
Cons. PDA operating systems are updated frequently, reflecting continuing improvements in handheld hardware. Microsoft late last year upgraded its Pocket PC operating system and changed its name to Windows Mobile.
Although frequent users will rejoice in the opportunity to do more, others might not want to spend $300 or more to get the latest features—only to see their new device become outmoded in a few months.
Hardware advances
Processors. The processor, the heart of a PDA, has also seen much change. Newer Palm and Pocket PC devices are based on the strongARM microprocessor produced by Intel under the Xscale brand. Each new processor has more speed, better multimedia, an improved camera interface, and lower power consumption than previous processors.
Smartphones, which reached the market in 2002, are geared to physicians who rely mostly on email and calendar functions and have little need for mobile medical information.
A Windows Mobile smartphone typically looks like a mobile phone but has basic Pocket PC capabilities, such as viewing mail, a calendar, to-do list, and notes.
Windows Mobile smartphones, however, are compatible only with smartphone-specific programs, not with general Pocket PC software. This means that drug reference guides, DSM-IV-TR, and other commonly used medical programs cannot be viewed on a smartphone. If you love the idea of a smartphone but want a specialized program, check out www.smartphone.net.
Pocket PC phone. By contrast, the Pocket PC phone looks and works more like a PDA than a phone. Because it is connected to the cellular network, the device has wider Internet access than does a WiFi-enabled Pocket PC3. At 3.5-by-3.5 inches, the screen size is about twice that of the smartphone’s screen. The device also is compatible with all Pocket PC software.
Pocket PC phones typically have more main memory than smartphones. Smartphones are limited to secure digital-based external memory, but Pocket PC phones have infrared and other connectivity options.
Palm-based smartphones can run most Palm software, depending upon operating system compatibility. Previous Palm smartphones were more limited because of an older operating system and lack of external memory cards.
The palmOne Treo 600, based on the new Palm Version 5 operating system, offers an external memory slot, built-in digital camera, and text messaging. palmOne offers a GSM (global system for mobile communication) protocol version for the T-Mobile, Cingular, and AT&T networks, and a CDMA (code-division multiple access) protocol version for the Sprint network.
Operating system improvements
Palm Source has released version 6 of its operating system—code named “Cobalt”—to hardware developers. This versatile new version—with higher resolution, Word and JPEG file support, simultaneous multiple communications, and other features—could reach the market around the winter holiday.
Microsoft has released Windows Mobile 2003 Second Edition. With certain devices, the operating system will help users read text or browse the Web by using more of the screen. With larger screen resolutions packed into a smaller area, fonts are smaller and hard to read. Windows Mobile 2003 Second Edition provides additional font-size controls to compensate for this change.
The new Windows Mobile version also features a start menu that displays frequently used applications, and WiFi security is improved compared with the previous version.
The future
Tiquit, OQO, and FlipStart plan to release fully operable handheld computers later this year.
These new devices will run on Windows XP and other operating systems and will feature full central processing units, hard disks, liquid crystal display panels, USB connectivity, and built-in QWERTY keyboards. It remains to be seen if these computers will supersede Palm and Windows Mobile PDAs.
Sony’s new Vaio U70 handheld computer, recently released in Japan, is available in the United States via specialty retailers such as dynamism.com.
Bipolar depression dilemma: Continue antidepressants after remission—or not?
Psychiatrists in the trenches are not alone in being unsure how to treat breakthrough bipolar depression. No panelist or other expert attending a recent American College of Neuropsychopharmacology symposium could definitively recommend:
- when to add an antidepressant to mood-stabilizer therapy
- whether to discontinue antidepressants after bipolar depression is stabilized.
Until controlled trials address these issues, we must use limited evidence to treat patients with bipolar depression. To help you meet this challenge, this article offers provisional suggestions based on recent published and unpublished reports.
Evidence for continuing
No published, randomized studies have examined how long to continue antidepressants after bipolar depression has stabilized. Until recently, conventional wisdom has been to discontinue antidepressants as soon as possible because of worries about antidepressant-induced mania. Then two case-controlled studies—one retrospective and one prospective—challenged that assumption.
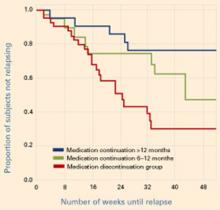
Figure Reduced relapse rates in patients whose antidepressants were continued
In a prospective case-controlled study, patients with bipolar disorder who continued antidepressant treatment for 6 to 12 months or >12 months after depressive episode remission had lower relapse rates than those who discontinued antidepressants within 6 months.
Source: Reprinted with permission from reference 2. Copyright 2003. American Psychiatric AssociationUsing similar methodologies, Altshuler et al1,2 examined bipolar patients who remained in remission for 6 weeks on mood stabilizers plus adjunctive antidepressants. Those who continued the antidepressants were less likely to relapse into depression (without an increase in manic episodes) than those whose antidepressants were discontinued (Figure).
Relapse rates in the first study1 were 35% at 1 year in those who continued antidepressants and 68% in those who did not. In the second study,2 36% and 70% of patients, respectively, had relapsed at 1 year. In the latter study, those who continued to take antidepressants for at least 6 months—instead of at least 12 months—had intermediate depressive relapse rates (53% for 6 months vs. 24% for 12 months).
When interpreting these data, keep several caveats in mind. For one, patients were not randomly assigned to continue or discontinue antidepressants but were designated by patient and clinician choice. When comparing the two patient groups, however, the authors found no inherent differences in illness characteristics that might have biased the results.
More importantly, few patients initially treated with antidepressant augmentation responded well and remained in remission. In the prospective study, 549 of 1,078 patients in the Stanley Foundation Bipolar Network received an antidepressant for breakthrough depressive symptoms.2 Only 189 remained on antidepressants for 2 months, and only 84 (15%) of the original population remained in remission for 2 months.
Summary. A small subgroup of patients appears to respond well to antidepressants and sustains this response for 6 to 8 weeks. For this subset, continuing antidepressant therapy would appear to be an appropriate strategy, based on:
- a significantly reduced risk for depressive relapse
- no increased risk of switching to mania, which is the usual reason to terminate antidepressants early.
Subsequent evidence
One needs to assess these observations in light of an interim analysis reported halfway through a small, open, randomized study. Ghaemi et al3 found no significant difference in outcomes—regardless of rapid-cycling status—among 14 bipolar patients who remained on antidepressants and 19 who discontinued across an average 60 and 50 weeks, respectively.
Table
Evidence on continuing antidepressants in breakthrough bipolar depression
| Study | Design | Results |
|---|---|---|
| Altshuler et al 1 | Retrospective, case-control, 39 patients (1 year) | Relapse rates: 35% in patients who continued antidepressants after mood stabilization and and 68% in those who did not; study included no rapid-cyclers |
| Altshuler et al 2 | Prospective, case control 84 patients (1 year) | Relapse rates: 36% in patients who continued antidepressants after mood stabilization and 70% in those who did not; study included no rapid cyclers |
| Ghaemi et al 3 (unpublished) | Open, randomized 33 patients (approx. 1 yr) | Relapse rate: 50% within 20 weeks, whether or not patients continued antidepressants; one-third of patients were rapid cyclers |
A survival analysis initially suggested some benefit for continuing antidepressants, but this disappeared when the authors adjusted for potential confounding factors—which was not done in the Altshuler et al studies. Patients receiving short-term or long-term antidepressants had a similar number of depressive episodes per year (1.00 vs. 0.75 episodes/year, respectively). For some reason, nonrapid-cycling patients showed greater depressive morbidity than those with rapid cycling.
Rapid cyclers comprised 30% of patients who continued antidepressants and 37% of those who discontinued. This may explain the 50% relapse rate within 20 weeks in both groups. By comparison, the Altshuler et al studies included no rapid cyclers.1,2 More details on this unpublished data are expected.
Are antidepressants the answer?
The high relapse rate in patients taking mood stabilizers—with or without an antidepressant—and the Ghaemi et al data3 suggest that we need alternate antidepressant approaches to bipolar depression. Potential regimens—anticonvulsants, atypical antipsychotics, and other agents—merit further study. So far the evidence is mixed, and the most effective approaches are not well-delineated.
Anticonvulsants. Sachs et al4 reported that adding lamotrigine or lamotrigine plus an antidepressant to mood stabilizer therapy did not appear more effective in treating bipolar depression than simply maintaining the mood stabilizers.
Similarly, a small randomized, double-blind study in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) was stopped early because of low total response. Adjunctive lamotrigine (24%) and inositol (17%) appeared more effective than risperidone (5%) for refractory bipolar depression.
Antipsychotics. In an 8-week randomized trial by Tohen et al,5 833 patients with moderate to severe bipolar I depression were treated with olanzapine, olanzapine plus fluoxetine, or placebo. Core depression symptoms improved significantly more with olanzapine alone or with fluoxetine compared with placebo, as measured by mean changes in the Montgomery-Asberg Depression Rating Scale.
In a 6-month open trial, 192 patients whose bipolar I depression remitted with any of the three treatments6 continued olanzapine and then, if needed after the first week, the olanzapine/fluoxetine combination (OFC). Nearly two-thirds of patients (62%) remained without a depressive recurrence, and 94% remained without a manic recurrence while taking the OFC. These unpublished data indicate that the OFC provided greater prophylactic antimanic than antidepressant effects.
Calabrese et al7 recently presented unpublished data comparing the effects of quetiapine monotherapy, 300 or 600 mg/d, with placebo in patients with bipolar depression. Beginning in the first week of treatment, both quetiapine dosages produced significantly greater antidepressant, antianxiety, and anti-insomnia effects than placebo (P < 0.001). Remission rates with both dosages were >50%.
Summary. These first controlled studies of atypical antipsychotics in bipolar depression suggest that this drug class may have antidepressant as well as their demonstrated antimanic effects.
Recommendations—for now
Controlled clinical trials have not been conducted, and the evidence on using adjunctive antidepressants in bipolar depression is ambiguous.8-11 As a result, it is unclear when or how long to use antidepressants, and many of the inferences and suggestions in this paper remain highly provisional.
Initiating antidepressants. My personal treatment guidelines—and those of many other clinicians—are to use the unimodal antidepressants to augment mood stabilizers in bipolar patients experiencing breakthrough depression, as long as they have not had:
- ultra-rapid cycling (>4 episodes/week)
- antidepressant-induced cycle acceleration
- or multiple episodes of antidepressant-induced mania, despite co-treatment with mood stabilizers.
If any of these variables is present, I would instead add another mood stabilizer or an atypical antipsychotic.
Continuing antidepressants. If the patient remains stable for 2 months after the antidepressant is added—with no depression or manic occurrence—I would continue the antidepressant indefinitely, based on the Altshuler et al data.12
Mood charting.I also recommend that clinicians help patients develop an individual method for mood charting, such as that used in the National Institute of Mental Health Life Chart Method (NIMH-LCM)12,13 or the STEP-BD program.14 Goals of the 5-year STEP-BD are to:
- determine the most effective treatments and relapse prevention strategies for bipolar disorder
- evaluate the psychotropic benefit of anticonvulsants, atypical antipsychotics, cholinesterase inhibitors, and neurotransmitter precursors
- determine the benefit of psychotropic combinations.
Mood charts can provide a retrospective and prospective overview of a patient’s illness course and response to medications. Compared with patient recall, mood charts help clinicians evaluate more precisely the risk of switching and the risks and benefits of starting, continuing, or discontinuing antidepressants. Mood charts may be your most effective tool for managing a patient’s bipolar depression and achieving and maintaining long-term remission.
Summary. In the absence of consensus or guidelines for treating bipolar depression, I suggest:
- a conservative approach—ie, no changes in medication—when the patient remains well on a given regimen
- an aggressive—if not radical—series of treatments and revisions when the illness course remains problematic.
Many medication classes and adjunctive strategies are available for treating bipolar illness.15-18 I recommend that you continue to systematically explore adjunctive treatments and combinations until the patient improves or—even better—attains remission. Good response can be achieved—even in treatment-refractory patients ill for long periods or with recurrent bipolar depression—although complex combination therapy is often required.
Related resources
- National Institute of Mental Health-Life Chart Method. Retrospective and prospective mood-tracking charts for patients with bipolar disorder. www.bipolarnews.org
- Systematic Treatment Enhancement Program for Bipolar Depression (STEP-BD). National Institute of Mental Health. www.stepbd.org/research/
Drug brand names
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosure
Dr. Post is a consultant to Abbott Laboratories, Astra-Zeneca Pharmaceuticals, Bristol-Myers Squibb Co., Elan Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., Shire Pharmaceuticals, and UCB Pharma.
1. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-16.
2. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003;160:1252-62.
3. Ghaemi S, El-Mallakh R, Baldassano CF, et al. Antidepressant treatment in bipolar depression: Long-term outcome (abstract). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
4. Sachs GS. Mood stabilizers alone vs.mood stabilizers plus antidepressant in bipolar depression (symposium). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
5. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60:1079-88.
6. Tohen M, Vieta E, Ketter TA, et al. Acute and long-term efficacy of olanzapine and olanzapine/fluoxetine combination for bipolar depression (abstract). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
7. Calabrese JR, Macfadden W, McCoy R, et al. Double-blind, placebo-controlled study of quetiapine in bipolar depression (abstract). New York: American Psychiatric Association annual meeting, 2004.
8. Post RM, Denicoff KD, Leverich GS, et al. Presentations of depression in bipolar illness. Clin Neurosci Res 2002a;2:142-57.
9. Post RM, Leverich GS, Nolen WA, et al. A re-evaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2003a;5:396-406.
10. Post RM, Speer AM, Leverich GS. Bipolar illness: Which critical treatment issues need studying? Clinical Approaches in Bipolar Disorders 2003b;2:24-30.
11. Goodwin GM. Bipolar disorder: is psychiatry’s Cinderella starting to get out a little more? Acta Neuropsychiatry 2000;12:105.-
12. Leverich GS, Post RM. Life charting the course of bipolar disorder. Current Review of Mood and Anxiety Disorders 1996;1:48-61.
13. Leverich GS, Post RM. Life charting of affective disorders. CNS Spectrums 1998;3:21-37.
14. Sachs GS, Thase ME, Otto MW, et al. Rationale, design, and methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biol Psychiatry 2003;53:1028-42.
15. Post RM, Speer AM, Obrocea GV, Leverich GS. Acute and prophylactic effects of anticonvulsants in bipolar depression. Clin Neurosci Res 2002;2:228-51.
16. Post RM, Speer AM. A brief history of anticonvulsant use in affective disorders. In: Trimble MR, Schmitz B (eds). Seizures, affective disorders and anticonvulsant drugs Surrey, UK: Clarius Press 2002;53:81.-
17. Post RM, Speer AM, Leverich GS. Complex combination therapy: the evolution toward rational polypharmacy in lithium-resistant bipolar illness, In: Akiskal H, Tohen M (eds). 50 years: the psychopharmacology of bipolar illness London: John Wiley & Sons Ltd. 2004 (in press).
18. Post RM, Altshuler LL. Mood disorders: treatment of bipolar disorders. Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry (8th ed) New York: Lippincott Williams & Wilkins, 2004 (in press).
Psychiatrists in the trenches are not alone in being unsure how to treat breakthrough bipolar depression. No panelist or other expert attending a recent American College of Neuropsychopharmacology symposium could definitively recommend:
- when to add an antidepressant to mood-stabilizer therapy
- whether to discontinue antidepressants after bipolar depression is stabilized.
Until controlled trials address these issues, we must use limited evidence to treat patients with bipolar depression. To help you meet this challenge, this article offers provisional suggestions based on recent published and unpublished reports.
Evidence for continuing
No published, randomized studies have examined how long to continue antidepressants after bipolar depression has stabilized. Until recently, conventional wisdom has been to discontinue antidepressants as soon as possible because of worries about antidepressant-induced mania. Then two case-controlled studies—one retrospective and one prospective—challenged that assumption.
Figure Reduced relapse rates in patients whose antidepressants were continued
In a prospective case-controlled study, patients with bipolar disorder who continued antidepressant treatment for 6 to 12 months or >12 months after depressive episode remission had lower relapse rates than those who discontinued antidepressants within 6 months.
Source: Reprinted with permission from reference 2. Copyright 2003. American Psychiatric AssociationUsing similar methodologies, Altshuler et al1,2 examined bipolar patients who remained in remission for 6 weeks on mood stabilizers plus adjunctive antidepressants. Those who continued the antidepressants were less likely to relapse into depression (without an increase in manic episodes) than those whose antidepressants were discontinued (Figure).
Relapse rates in the first study1 were 35% at 1 year in those who continued antidepressants and 68% in those who did not. In the second study,2 36% and 70% of patients, respectively, had relapsed at 1 year. In the latter study, those who continued to take antidepressants for at least 6 months—instead of at least 12 months—had intermediate depressive relapse rates (53% for 6 months vs. 24% for 12 months).
When interpreting these data, keep several caveats in mind. For one, patients were not randomly assigned to continue or discontinue antidepressants but were designated by patient and clinician choice. When comparing the two patient groups, however, the authors found no inherent differences in illness characteristics that might have biased the results.
More importantly, few patients initially treated with antidepressant augmentation responded well and remained in remission. In the prospective study, 549 of 1,078 patients in the Stanley Foundation Bipolar Network received an antidepressant for breakthrough depressive symptoms.2 Only 189 remained on antidepressants for 2 months, and only 84 (15%) of the original population remained in remission for 2 months.
Summary. A small subgroup of patients appears to respond well to antidepressants and sustains this response for 6 to 8 weeks. For this subset, continuing antidepressant therapy would appear to be an appropriate strategy, based on:
- a significantly reduced risk for depressive relapse
- no increased risk of switching to mania, which is the usual reason to terminate antidepressants early.
Subsequent evidence
One needs to assess these observations in light of an interim analysis reported halfway through a small, open, randomized study. Ghaemi et al3 found no significant difference in outcomes—regardless of rapid-cycling status—among 14 bipolar patients who remained on antidepressants and 19 who discontinued across an average 60 and 50 weeks, respectively.
Table
Evidence on continuing antidepressants in breakthrough bipolar depression
| Study | Design | Results |
|---|---|---|
| Altshuler et al 1 | Retrospective, case-control, 39 patients (1 year) | Relapse rates: 35% in patients who continued antidepressants after mood stabilization and and 68% in those who did not; study included no rapid-cyclers |
| Altshuler et al 2 | Prospective, case control 84 patients (1 year) | Relapse rates: 36% in patients who continued antidepressants after mood stabilization and 70% in those who did not; study included no rapid cyclers |
| Ghaemi et al 3 (unpublished) | Open, randomized 33 patients (approx. 1 yr) | Relapse rate: 50% within 20 weeks, whether or not patients continued antidepressants; one-third of patients were rapid cyclers |
A survival analysis initially suggested some benefit for continuing antidepressants, but this disappeared when the authors adjusted for potential confounding factors—which was not done in the Altshuler et al studies. Patients receiving short-term or long-term antidepressants had a similar number of depressive episodes per year (1.00 vs. 0.75 episodes/year, respectively). For some reason, nonrapid-cycling patients showed greater depressive morbidity than those with rapid cycling.
Rapid cyclers comprised 30% of patients who continued antidepressants and 37% of those who discontinued. This may explain the 50% relapse rate within 20 weeks in both groups. By comparison, the Altshuler et al studies included no rapid cyclers.1,2 More details on this unpublished data are expected.
Are antidepressants the answer?
The high relapse rate in patients taking mood stabilizers—with or without an antidepressant—and the Ghaemi et al data3 suggest that we need alternate antidepressant approaches to bipolar depression. Potential regimens—anticonvulsants, atypical antipsychotics, and other agents—merit further study. So far the evidence is mixed, and the most effective approaches are not well-delineated.
Anticonvulsants. Sachs et al4 reported that adding lamotrigine or lamotrigine plus an antidepressant to mood stabilizer therapy did not appear more effective in treating bipolar depression than simply maintaining the mood stabilizers.
Similarly, a small randomized, double-blind study in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) was stopped early because of low total response. Adjunctive lamotrigine (24%) and inositol (17%) appeared more effective than risperidone (5%) for refractory bipolar depression.
Antipsychotics. In an 8-week randomized trial by Tohen et al,5 833 patients with moderate to severe bipolar I depression were treated with olanzapine, olanzapine plus fluoxetine, or placebo. Core depression symptoms improved significantly more with olanzapine alone or with fluoxetine compared with placebo, as measured by mean changes in the Montgomery-Asberg Depression Rating Scale.
In a 6-month open trial, 192 patients whose bipolar I depression remitted with any of the three treatments6 continued olanzapine and then, if needed after the first week, the olanzapine/fluoxetine combination (OFC). Nearly two-thirds of patients (62%) remained without a depressive recurrence, and 94% remained without a manic recurrence while taking the OFC. These unpublished data indicate that the OFC provided greater prophylactic antimanic than antidepressant effects.
Calabrese et al7 recently presented unpublished data comparing the effects of quetiapine monotherapy, 300 or 600 mg/d, with placebo in patients with bipolar depression. Beginning in the first week of treatment, both quetiapine dosages produced significantly greater antidepressant, antianxiety, and anti-insomnia effects than placebo (P < 0.001). Remission rates with both dosages were >50%.
Summary. These first controlled studies of atypical antipsychotics in bipolar depression suggest that this drug class may have antidepressant as well as their demonstrated antimanic effects.
Recommendations—for now
Controlled clinical trials have not been conducted, and the evidence on using adjunctive antidepressants in bipolar depression is ambiguous.8-11 As a result, it is unclear when or how long to use antidepressants, and many of the inferences and suggestions in this paper remain highly provisional.
Initiating antidepressants. My personal treatment guidelines—and those of many other clinicians—are to use the unimodal antidepressants to augment mood stabilizers in bipolar patients experiencing breakthrough depression, as long as they have not had:
- ultra-rapid cycling (>4 episodes/week)
- antidepressant-induced cycle acceleration
- or multiple episodes of antidepressant-induced mania, despite co-treatment with mood stabilizers.
If any of these variables is present, I would instead add another mood stabilizer or an atypical antipsychotic.
Continuing antidepressants. If the patient remains stable for 2 months after the antidepressant is added—with no depression or manic occurrence—I would continue the antidepressant indefinitely, based on the Altshuler et al data.12
Mood charting.I also recommend that clinicians help patients develop an individual method for mood charting, such as that used in the National Institute of Mental Health Life Chart Method (NIMH-LCM)12,13 or the STEP-BD program.14 Goals of the 5-year STEP-BD are to:
- determine the most effective treatments and relapse prevention strategies for bipolar disorder
- evaluate the psychotropic benefit of anticonvulsants, atypical antipsychotics, cholinesterase inhibitors, and neurotransmitter precursors
- determine the benefit of psychotropic combinations.
Mood charts can provide a retrospective and prospective overview of a patient’s illness course and response to medications. Compared with patient recall, mood charts help clinicians evaluate more precisely the risk of switching and the risks and benefits of starting, continuing, or discontinuing antidepressants. Mood charts may be your most effective tool for managing a patient’s bipolar depression and achieving and maintaining long-term remission.
Summary. In the absence of consensus or guidelines for treating bipolar depression, I suggest:
- a conservative approach—ie, no changes in medication—when the patient remains well on a given regimen
- an aggressive—if not radical—series of treatments and revisions when the illness course remains problematic.
Many medication classes and adjunctive strategies are available for treating bipolar illness.15-18 I recommend that you continue to systematically explore adjunctive treatments and combinations until the patient improves or—even better—attains remission. Good response can be achieved—even in treatment-refractory patients ill for long periods or with recurrent bipolar depression—although complex combination therapy is often required.
Related resources
- National Institute of Mental Health-Life Chart Method. Retrospective and prospective mood-tracking charts for patients with bipolar disorder. www.bipolarnews.org
- Systematic Treatment Enhancement Program for Bipolar Depression (STEP-BD). National Institute of Mental Health. www.stepbd.org/research/
Drug brand names
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosure
Dr. Post is a consultant to Abbott Laboratories, Astra-Zeneca Pharmaceuticals, Bristol-Myers Squibb Co., Elan Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., Shire Pharmaceuticals, and UCB Pharma.
Psychiatrists in the trenches are not alone in being unsure how to treat breakthrough bipolar depression. No panelist or other expert attending a recent American College of Neuropsychopharmacology symposium could definitively recommend:
- when to add an antidepressant to mood-stabilizer therapy
- whether to discontinue antidepressants after bipolar depression is stabilized.
Until controlled trials address these issues, we must use limited evidence to treat patients with bipolar depression. To help you meet this challenge, this article offers provisional suggestions based on recent published and unpublished reports.
Evidence for continuing
No published, randomized studies have examined how long to continue antidepressants after bipolar depression has stabilized. Until recently, conventional wisdom has been to discontinue antidepressants as soon as possible because of worries about antidepressant-induced mania. Then two case-controlled studies—one retrospective and one prospective—challenged that assumption.
Figure Reduced relapse rates in patients whose antidepressants were continued
In a prospective case-controlled study, patients with bipolar disorder who continued antidepressant treatment for 6 to 12 months or >12 months after depressive episode remission had lower relapse rates than those who discontinued antidepressants within 6 months.
Source: Reprinted with permission from reference 2. Copyright 2003. American Psychiatric AssociationUsing similar methodologies, Altshuler et al1,2 examined bipolar patients who remained in remission for 6 weeks on mood stabilizers plus adjunctive antidepressants. Those who continued the antidepressants were less likely to relapse into depression (without an increase in manic episodes) than those whose antidepressants were discontinued (Figure).
Relapse rates in the first study1 were 35% at 1 year in those who continued antidepressants and 68% in those who did not. In the second study,2 36% and 70% of patients, respectively, had relapsed at 1 year. In the latter study, those who continued to take antidepressants for at least 6 months—instead of at least 12 months—had intermediate depressive relapse rates (53% for 6 months vs. 24% for 12 months).
When interpreting these data, keep several caveats in mind. For one, patients were not randomly assigned to continue or discontinue antidepressants but were designated by patient and clinician choice. When comparing the two patient groups, however, the authors found no inherent differences in illness characteristics that might have biased the results.
More importantly, few patients initially treated with antidepressant augmentation responded well and remained in remission. In the prospective study, 549 of 1,078 patients in the Stanley Foundation Bipolar Network received an antidepressant for breakthrough depressive symptoms.2 Only 189 remained on antidepressants for 2 months, and only 84 (15%) of the original population remained in remission for 2 months.
Summary. A small subgroup of patients appears to respond well to antidepressants and sustains this response for 6 to 8 weeks. For this subset, continuing antidepressant therapy would appear to be an appropriate strategy, based on:
- a significantly reduced risk for depressive relapse
- no increased risk of switching to mania, which is the usual reason to terminate antidepressants early.
Subsequent evidence
One needs to assess these observations in light of an interim analysis reported halfway through a small, open, randomized study. Ghaemi et al3 found no significant difference in outcomes—regardless of rapid-cycling status—among 14 bipolar patients who remained on antidepressants and 19 who discontinued across an average 60 and 50 weeks, respectively.
Table
Evidence on continuing antidepressants in breakthrough bipolar depression
| Study | Design | Results |
|---|---|---|
| Altshuler et al 1 | Retrospective, case-control, 39 patients (1 year) | Relapse rates: 35% in patients who continued antidepressants after mood stabilization and and 68% in those who did not; study included no rapid-cyclers |
| Altshuler et al 2 | Prospective, case control 84 patients (1 year) | Relapse rates: 36% in patients who continued antidepressants after mood stabilization and 70% in those who did not; study included no rapid cyclers |
| Ghaemi et al 3 (unpublished) | Open, randomized 33 patients (approx. 1 yr) | Relapse rate: 50% within 20 weeks, whether or not patients continued antidepressants; one-third of patients were rapid cyclers |
A survival analysis initially suggested some benefit for continuing antidepressants, but this disappeared when the authors adjusted for potential confounding factors—which was not done in the Altshuler et al studies. Patients receiving short-term or long-term antidepressants had a similar number of depressive episodes per year (1.00 vs. 0.75 episodes/year, respectively). For some reason, nonrapid-cycling patients showed greater depressive morbidity than those with rapid cycling.
Rapid cyclers comprised 30% of patients who continued antidepressants and 37% of those who discontinued. This may explain the 50% relapse rate within 20 weeks in both groups. By comparison, the Altshuler et al studies included no rapid cyclers.1,2 More details on this unpublished data are expected.
Are antidepressants the answer?
The high relapse rate in patients taking mood stabilizers—with or without an antidepressant—and the Ghaemi et al data3 suggest that we need alternate antidepressant approaches to bipolar depression. Potential regimens—anticonvulsants, atypical antipsychotics, and other agents—merit further study. So far the evidence is mixed, and the most effective approaches are not well-delineated.
Anticonvulsants. Sachs et al4 reported that adding lamotrigine or lamotrigine plus an antidepressant to mood stabilizer therapy did not appear more effective in treating bipolar depression than simply maintaining the mood stabilizers.
Similarly, a small randomized, double-blind study in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) was stopped early because of low total response. Adjunctive lamotrigine (24%) and inositol (17%) appeared more effective than risperidone (5%) for refractory bipolar depression.
Antipsychotics. In an 8-week randomized trial by Tohen et al,5 833 patients with moderate to severe bipolar I depression were treated with olanzapine, olanzapine plus fluoxetine, or placebo. Core depression symptoms improved significantly more with olanzapine alone or with fluoxetine compared with placebo, as measured by mean changes in the Montgomery-Asberg Depression Rating Scale.
In a 6-month open trial, 192 patients whose bipolar I depression remitted with any of the three treatments6 continued olanzapine and then, if needed after the first week, the olanzapine/fluoxetine combination (OFC). Nearly two-thirds of patients (62%) remained without a depressive recurrence, and 94% remained without a manic recurrence while taking the OFC. These unpublished data indicate that the OFC provided greater prophylactic antimanic than antidepressant effects.
Calabrese et al7 recently presented unpublished data comparing the effects of quetiapine monotherapy, 300 or 600 mg/d, with placebo in patients with bipolar depression. Beginning in the first week of treatment, both quetiapine dosages produced significantly greater antidepressant, antianxiety, and anti-insomnia effects than placebo (P < 0.001). Remission rates with both dosages were >50%.
Summary. These first controlled studies of atypical antipsychotics in bipolar depression suggest that this drug class may have antidepressant as well as their demonstrated antimanic effects.
Recommendations—for now
Controlled clinical trials have not been conducted, and the evidence on using adjunctive antidepressants in bipolar depression is ambiguous.8-11 As a result, it is unclear when or how long to use antidepressants, and many of the inferences and suggestions in this paper remain highly provisional.
Initiating antidepressants. My personal treatment guidelines—and those of many other clinicians—are to use the unimodal antidepressants to augment mood stabilizers in bipolar patients experiencing breakthrough depression, as long as they have not had:
- ultra-rapid cycling (>4 episodes/week)
- antidepressant-induced cycle acceleration
- or multiple episodes of antidepressant-induced mania, despite co-treatment with mood stabilizers.
If any of these variables is present, I would instead add another mood stabilizer or an atypical antipsychotic.
Continuing antidepressants. If the patient remains stable for 2 months after the antidepressant is added—with no depression or manic occurrence—I would continue the antidepressant indefinitely, based on the Altshuler et al data.12
Mood charting.I also recommend that clinicians help patients develop an individual method for mood charting, such as that used in the National Institute of Mental Health Life Chart Method (NIMH-LCM)12,13 or the STEP-BD program.14 Goals of the 5-year STEP-BD are to:
- determine the most effective treatments and relapse prevention strategies for bipolar disorder
- evaluate the psychotropic benefit of anticonvulsants, atypical antipsychotics, cholinesterase inhibitors, and neurotransmitter precursors
- determine the benefit of psychotropic combinations.
Mood charts can provide a retrospective and prospective overview of a patient’s illness course and response to medications. Compared with patient recall, mood charts help clinicians evaluate more precisely the risk of switching and the risks and benefits of starting, continuing, or discontinuing antidepressants. Mood charts may be your most effective tool for managing a patient’s bipolar depression and achieving and maintaining long-term remission.
Summary. In the absence of consensus or guidelines for treating bipolar depression, I suggest:
- a conservative approach—ie, no changes in medication—when the patient remains well on a given regimen
- an aggressive—if not radical—series of treatments and revisions when the illness course remains problematic.
Many medication classes and adjunctive strategies are available for treating bipolar illness.15-18 I recommend that you continue to systematically explore adjunctive treatments and combinations until the patient improves or—even better—attains remission. Good response can be achieved—even in treatment-refractory patients ill for long periods or with recurrent bipolar depression—although complex combination therapy is often required.
Related resources
- National Institute of Mental Health-Life Chart Method. Retrospective and prospective mood-tracking charts for patients with bipolar disorder. www.bipolarnews.org
- Systematic Treatment Enhancement Program for Bipolar Depression (STEP-BD). National Institute of Mental Health. www.stepbd.org/research/
Drug brand names
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosure
Dr. Post is a consultant to Abbott Laboratories, Astra-Zeneca Pharmaceuticals, Bristol-Myers Squibb Co., Elan Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., Shire Pharmaceuticals, and UCB Pharma.
1. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-16.
2. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003;160:1252-62.
3. Ghaemi S, El-Mallakh R, Baldassano CF, et al. Antidepressant treatment in bipolar depression: Long-term outcome (abstract). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
4. Sachs GS. Mood stabilizers alone vs.mood stabilizers plus antidepressant in bipolar depression (symposium). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
5. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60:1079-88.
6. Tohen M, Vieta E, Ketter TA, et al. Acute and long-term efficacy of olanzapine and olanzapine/fluoxetine combination for bipolar depression (abstract). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
7. Calabrese JR, Macfadden W, McCoy R, et al. Double-blind, placebo-controlled study of quetiapine in bipolar depression (abstract). New York: American Psychiatric Association annual meeting, 2004.
8. Post RM, Denicoff KD, Leverich GS, et al. Presentations of depression in bipolar illness. Clin Neurosci Res 2002a;2:142-57.
9. Post RM, Leverich GS, Nolen WA, et al. A re-evaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2003a;5:396-406.
10. Post RM, Speer AM, Leverich GS. Bipolar illness: Which critical treatment issues need studying? Clinical Approaches in Bipolar Disorders 2003b;2:24-30.
11. Goodwin GM. Bipolar disorder: is psychiatry’s Cinderella starting to get out a little more? Acta Neuropsychiatry 2000;12:105.-
12. Leverich GS, Post RM. Life charting the course of bipolar disorder. Current Review of Mood and Anxiety Disorders 1996;1:48-61.
13. Leverich GS, Post RM. Life charting of affective disorders. CNS Spectrums 1998;3:21-37.
14. Sachs GS, Thase ME, Otto MW, et al. Rationale, design, and methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biol Psychiatry 2003;53:1028-42.
15. Post RM, Speer AM, Obrocea GV, Leverich GS. Acute and prophylactic effects of anticonvulsants in bipolar depression. Clin Neurosci Res 2002;2:228-51.
16. Post RM, Speer AM. A brief history of anticonvulsant use in affective disorders. In: Trimble MR, Schmitz B (eds). Seizures, affective disorders and anticonvulsant drugs Surrey, UK: Clarius Press 2002;53:81.-
17. Post RM, Speer AM, Leverich GS. Complex combination therapy: the evolution toward rational polypharmacy in lithium-resistant bipolar illness, In: Akiskal H, Tohen M (eds). 50 years: the psychopharmacology of bipolar illness London: John Wiley & Sons Ltd. 2004 (in press).
18. Post RM, Altshuler LL. Mood disorders: treatment of bipolar disorders. Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry (8th ed) New York: Lippincott Williams & Wilkins, 2004 (in press).
1. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-16.
2. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003;160:1252-62.
3. Ghaemi S, El-Mallakh R, Baldassano CF, et al. Antidepressant treatment in bipolar depression: Long-term outcome (abstract). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
4. Sachs GS. Mood stabilizers alone vs.mood stabilizers plus antidepressant in bipolar depression (symposium). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
5. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60:1079-88.
6. Tohen M, Vieta E, Ketter TA, et al. Acute and long-term efficacy of olanzapine and olanzapine/fluoxetine combination for bipolar depression (abstract). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
7. Calabrese JR, Macfadden W, McCoy R, et al. Double-blind, placebo-controlled study of quetiapine in bipolar depression (abstract). New York: American Psychiatric Association annual meeting, 2004.
8. Post RM, Denicoff KD, Leverich GS, et al. Presentations of depression in bipolar illness. Clin Neurosci Res 2002a;2:142-57.
9. Post RM, Leverich GS, Nolen WA, et al. A re-evaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2003a;5:396-406.
10. Post RM, Speer AM, Leverich GS. Bipolar illness: Which critical treatment issues need studying? Clinical Approaches in Bipolar Disorders 2003b;2:24-30.
11. Goodwin GM. Bipolar disorder: is psychiatry’s Cinderella starting to get out a little more? Acta Neuropsychiatry 2000;12:105.-
12. Leverich GS, Post RM. Life charting the course of bipolar disorder. Current Review of Mood and Anxiety Disorders 1996;1:48-61.
13. Leverich GS, Post RM. Life charting of affective disorders. CNS Spectrums 1998;3:21-37.
14. Sachs GS, Thase ME, Otto MW, et al. Rationale, design, and methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biol Psychiatry 2003;53:1028-42.
15. Post RM, Speer AM, Obrocea GV, Leverich GS. Acute and prophylactic effects of anticonvulsants in bipolar depression. Clin Neurosci Res 2002;2:228-51.
16. Post RM, Speer AM. A brief history of anticonvulsant use in affective disorders. In: Trimble MR, Schmitz B (eds). Seizures, affective disorders and anticonvulsant drugs Surrey, UK: Clarius Press 2002;53:81.-
17. Post RM, Speer AM, Leverich GS. Complex combination therapy: the evolution toward rational polypharmacy in lithium-resistant bipolar illness, In: Akiskal H, Tohen M (eds). 50 years: the psychopharmacology of bipolar illness London: John Wiley & Sons Ltd. 2004 (in press).
18. Post RM, Altshuler LL. Mood disorders: treatment of bipolar disorders. Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry (8th ed) New York: Lippincott Williams & Wilkins, 2004 (in press).
‘Prescribing’ psychotherapy as if it were medication
Early in training, psychiatry residents learn to formulate specific medication plans, but then add the vague, “I would recommend psychotherapy as well.” To help them understand each psychotherapy’s features and clinical applications, tell them to prescribe psychotherapy as if it were medication.
Like pharmacotherapy, psychotherapy has numerous forms, indications, and contraindications. It can be categorized by:
- theoretical orientation (psychodynamic, cognitive-behavioral, interpersonal)
- treatment duration (time-limited, open-ended)
- number of persons in attendance (individual, couples, family, group).
Teach residents to prescribe psychotherapy in a specific dose and frequency to address target symptoms. A sample treatment plan for a patient with major depressive disorder is shown in the Table.
Table
Sample treatment plan for major depressive disorder
| Therapy | Type of intervention | Specific intervention | Starting dosage, frequency | Target symptoms | Side effects |
|---|---|---|---|---|---|
| Pharmacotherapy | SSRI | Sertraline | 50 mg/d | Depressed mood, anhedonia, sleep disturbance | Nausea, diarrhea, sexual dysfunction |
| Psychotherapy | Individual | Cognitive-behavioral | 50 minutes weekly | Trauma, loss, low self-esteem | Anxiety, anger, grief |
Urge residents to prescribe psychotherapy “off-label” if it might help. For example, some clinicians offered cognitive-behavioral therapy (CBT) to patients with schizophrenia before CBT gained wider acceptance for that disorder.
Finally—like any treatment—psychotherapy may be associated with side effects, including anxiety, anger, and grief. Encourage residents to review these risks with their patients before beginning psychotherapy.
Choosing a psychotherapy type
Psychotherapy may be prescribed alone or with pharmacotherapy, as clinically indicated. When choosing a particular psychotherapy, research supports use of:
- behavior therapy, cognitive therapy, and CBT for depression, certain anxiety disorders (such as obsessive-compulsive disorder), and other mental disorders (substance use disorders, eating disorders, chronic pain syndromes)
- dialectical behavior therapy for reducing self-injurious behavior and hospitalizations in borderline personality disorder
- interpersonal psychotherapy for depression.
Some research supports using psychodynamic psychotherapy to treat severe, chronic personality disorders, but the nature of this therapy makes controlled studies difficult. Similarly, though no published data have shown supportive psychotherapy to be effective—in general or for specific disorders—lack of evidence does not necessarily correlate with lack of efficacy.
A model that’s easy to learn
Once residents become familiar with this model, it is remarkable to see the sophistication with which they incorporate specific psychotherapeutic recommendations into their treatment plans.
Use of this model need not be restricted to residents, however. A good model helps all clinicians sharpen their skills and improve the care they provide.
Related resources
- Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry (4th ed). Washington, DC: American Psychiatric Publishing, 2003.
- Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry (7th ed). New York: Lippincott Williams & Wilkins, 1999.
Drug brand names
- Sertraline • Zoloft
Dr. Campbell is assistant professor, department of psychiatry, Case Western Reserve University School of Medicine, Cleveland, OH, and is clinical director, division of ambulatory care, department of psychiatry, University Hospitals of Cleveland.
Early in training, psychiatry residents learn to formulate specific medication plans, but then add the vague, “I would recommend psychotherapy as well.” To help them understand each psychotherapy’s features and clinical applications, tell them to prescribe psychotherapy as if it were medication.
Like pharmacotherapy, psychotherapy has numerous forms, indications, and contraindications. It can be categorized by:
- theoretical orientation (psychodynamic, cognitive-behavioral, interpersonal)
- treatment duration (time-limited, open-ended)
- number of persons in attendance (individual, couples, family, group).
Teach residents to prescribe psychotherapy in a specific dose and frequency to address target symptoms. A sample treatment plan for a patient with major depressive disorder is shown in the Table.
Table
Sample treatment plan for major depressive disorder
| Therapy | Type of intervention | Specific intervention | Starting dosage, frequency | Target symptoms | Side effects |
|---|---|---|---|---|---|
| Pharmacotherapy | SSRI | Sertraline | 50 mg/d | Depressed mood, anhedonia, sleep disturbance | Nausea, diarrhea, sexual dysfunction |
| Psychotherapy | Individual | Cognitive-behavioral | 50 minutes weekly | Trauma, loss, low self-esteem | Anxiety, anger, grief |
Urge residents to prescribe psychotherapy “off-label” if it might help. For example, some clinicians offered cognitive-behavioral therapy (CBT) to patients with schizophrenia before CBT gained wider acceptance for that disorder.
Finally—like any treatment—psychotherapy may be associated with side effects, including anxiety, anger, and grief. Encourage residents to review these risks with their patients before beginning psychotherapy.
Choosing a psychotherapy type
Psychotherapy may be prescribed alone or with pharmacotherapy, as clinically indicated. When choosing a particular psychotherapy, research supports use of:
- behavior therapy, cognitive therapy, and CBT for depression, certain anxiety disorders (such as obsessive-compulsive disorder), and other mental disorders (substance use disorders, eating disorders, chronic pain syndromes)
- dialectical behavior therapy for reducing self-injurious behavior and hospitalizations in borderline personality disorder
- interpersonal psychotherapy for depression.
Some research supports using psychodynamic psychotherapy to treat severe, chronic personality disorders, but the nature of this therapy makes controlled studies difficult. Similarly, though no published data have shown supportive psychotherapy to be effective—in general or for specific disorders—lack of evidence does not necessarily correlate with lack of efficacy.
A model that’s easy to learn
Once residents become familiar with this model, it is remarkable to see the sophistication with which they incorporate specific psychotherapeutic recommendations into their treatment plans.
Use of this model need not be restricted to residents, however. A good model helps all clinicians sharpen their skills and improve the care they provide.
Related resources
- Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry (4th ed). Washington, DC: American Psychiatric Publishing, 2003.
- Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry (7th ed). New York: Lippincott Williams & Wilkins, 1999.
Drug brand names
- Sertraline • Zoloft
Early in training, psychiatry residents learn to formulate specific medication plans, but then add the vague, “I would recommend psychotherapy as well.” To help them understand each psychotherapy’s features and clinical applications, tell them to prescribe psychotherapy as if it were medication.
Like pharmacotherapy, psychotherapy has numerous forms, indications, and contraindications. It can be categorized by:
- theoretical orientation (psychodynamic, cognitive-behavioral, interpersonal)
- treatment duration (time-limited, open-ended)
- number of persons in attendance (individual, couples, family, group).
Teach residents to prescribe psychotherapy in a specific dose and frequency to address target symptoms. A sample treatment plan for a patient with major depressive disorder is shown in the Table.
Table
Sample treatment plan for major depressive disorder
| Therapy | Type of intervention | Specific intervention | Starting dosage, frequency | Target symptoms | Side effects |
|---|---|---|---|---|---|
| Pharmacotherapy | SSRI | Sertraline | 50 mg/d | Depressed mood, anhedonia, sleep disturbance | Nausea, diarrhea, sexual dysfunction |
| Psychotherapy | Individual | Cognitive-behavioral | 50 minutes weekly | Trauma, loss, low self-esteem | Anxiety, anger, grief |
Urge residents to prescribe psychotherapy “off-label” if it might help. For example, some clinicians offered cognitive-behavioral therapy (CBT) to patients with schizophrenia before CBT gained wider acceptance for that disorder.
Finally—like any treatment—psychotherapy may be associated with side effects, including anxiety, anger, and grief. Encourage residents to review these risks with their patients before beginning psychotherapy.
Choosing a psychotherapy type
Psychotherapy may be prescribed alone or with pharmacotherapy, as clinically indicated. When choosing a particular psychotherapy, research supports use of:
- behavior therapy, cognitive therapy, and CBT for depression, certain anxiety disorders (such as obsessive-compulsive disorder), and other mental disorders (substance use disorders, eating disorders, chronic pain syndromes)
- dialectical behavior therapy for reducing self-injurious behavior and hospitalizations in borderline personality disorder
- interpersonal psychotherapy for depression.
Some research supports using psychodynamic psychotherapy to treat severe, chronic personality disorders, but the nature of this therapy makes controlled studies difficult. Similarly, though no published data have shown supportive psychotherapy to be effective—in general or for specific disorders—lack of evidence does not necessarily correlate with lack of efficacy.
A model that’s easy to learn
Once residents become familiar with this model, it is remarkable to see the sophistication with which they incorporate specific psychotherapeutic recommendations into their treatment plans.
Use of this model need not be restricted to residents, however. A good model helps all clinicians sharpen their skills and improve the care they provide.
Related resources
- Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry (4th ed). Washington, DC: American Psychiatric Publishing, 2003.
- Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry (7th ed). New York: Lippincott Williams & Wilkins, 1999.
Drug brand names
- Sertraline • Zoloft
Dr. Campbell is assistant professor, department of psychiatry, Case Western Reserve University School of Medicine, Cleveland, OH, and is clinical director, division of ambulatory care, department of psychiatry, University Hospitals of Cleveland.
Dr. Campbell is assistant professor, department of psychiatry, Case Western Reserve University School of Medicine, Cleveland, OH, and is clinical director, division of ambulatory care, department of psychiatry, University Hospitals of Cleveland.
Delusions: How cognitive therapy helps patients let go
Patients with psychosis often hold on to delusional beliefs while on medication. Learning more about these beliefs through cognitive therapy can improve drug efficacy, engagement, and coping skills for any chronically or acutely psychotic patient who is willing to discuss his or her delusions.
Start by asking these five questions:
1. How strong is your belief? When starting therapy, ask the patient to rate the certainty of his belief from 0 to 100%. A patient who remains 100% certain over several visits probably will not respond to treatment.
Then engage the patient by suggesting that together you’ll view the belief as a scientist or detective would, carefully evaluating all evidence before reaching a conclusion.
2. How long have you had this belief? Some patients say they have always held a specific belief. Beliefs that have lasted years may be harder to change than more-recently adopted ones.
Looking back, some patients acknowledge initial doubts and recall considering alternate beliefs, which the physician can help strengthen.
3. How has the belief affected your life? Have the patient write down the advantages and disadvantages of his delusional thinking;1 seeing the consequences in writing may discourage the belief. For example, a patient might stop thinking he is a prophet if he realizes the belief could lead to alienation and hospitalization.
4. Until now, how have you coped with negative aspects of this belief (such as ‘death threats’)? For 10 years, one patient believed gangsters were trying to kill him. We asked how he survived attempts on his life. He identified activities and situations in which he felt safer, such as being with his parents, playing basketball with others, and visiting the doctor. We encouraged him to spend more time in these situations. He acknowledged his role in improving his sense of safety and felt empowered to confront the delusion.
5. What if the delusion is/is not true? Asking this question may uncover other dysfunctional thinking that can be addressed in therapy.
Have the patient rate certainty at each visit, and document changes in score. Patients with delusions usually are relieved that they are not being judged and that their beliefs are not invalidated. They often start questioning their delusions and develop coping skills to deal with them.
Some patients feel depressed after abandoning a delusion that once shielded them from low self-esteem (eg, “the FBI is after me because I’m important”). Steer patients toward various activities and have them rate their enjoyment and mastery of them. This will help them find alternate beliefs.
Reference
1. Rector N, Beck A. Cognitive therapy for schizophrenia: from conceptualization to intervention. Can J Psychiatry 2002;47:39-48.
Dr. Pinninti is assistant professor of psychiatry, School of Osteopathic Medicine, University of Medicine and Dentistry of New Jersey, and is medical director, Steininger Behavioral Care Services, Cherry Hill, NJ.
Dr. Sosland is a child and adolescent psychiatry fellow, Jefferson Medical College, Thomas Jefferson University, Philadelphia, PA.
Patients with psychosis often hold on to delusional beliefs while on medication. Learning more about these beliefs through cognitive therapy can improve drug efficacy, engagement, and coping skills for any chronically or acutely psychotic patient who is willing to discuss his or her delusions.
Start by asking these five questions:
1. How strong is your belief? When starting therapy, ask the patient to rate the certainty of his belief from 0 to 100%. A patient who remains 100% certain over several visits probably will not respond to treatment.
Then engage the patient by suggesting that together you’ll view the belief as a scientist or detective would, carefully evaluating all evidence before reaching a conclusion.
2. How long have you had this belief? Some patients say they have always held a specific belief. Beliefs that have lasted years may be harder to change than more-recently adopted ones.
Looking back, some patients acknowledge initial doubts and recall considering alternate beliefs, which the physician can help strengthen.
3. How has the belief affected your life? Have the patient write down the advantages and disadvantages of his delusional thinking;1 seeing the consequences in writing may discourage the belief. For example, a patient might stop thinking he is a prophet if he realizes the belief could lead to alienation and hospitalization.
4. Until now, how have you coped with negative aspects of this belief (such as ‘death threats’)? For 10 years, one patient believed gangsters were trying to kill him. We asked how he survived attempts on his life. He identified activities and situations in which he felt safer, such as being with his parents, playing basketball with others, and visiting the doctor. We encouraged him to spend more time in these situations. He acknowledged his role in improving his sense of safety and felt empowered to confront the delusion.
5. What if the delusion is/is not true? Asking this question may uncover other dysfunctional thinking that can be addressed in therapy.
Have the patient rate certainty at each visit, and document changes in score. Patients with delusions usually are relieved that they are not being judged and that their beliefs are not invalidated. They often start questioning their delusions and develop coping skills to deal with them.
Some patients feel depressed after abandoning a delusion that once shielded them from low self-esteem (eg, “the FBI is after me because I’m important”). Steer patients toward various activities and have them rate their enjoyment and mastery of them. This will help them find alternate beliefs.
Patients with psychosis often hold on to delusional beliefs while on medication. Learning more about these beliefs through cognitive therapy can improve drug efficacy, engagement, and coping skills for any chronically or acutely psychotic patient who is willing to discuss his or her delusions.
Start by asking these five questions:
1. How strong is your belief? When starting therapy, ask the patient to rate the certainty of his belief from 0 to 100%. A patient who remains 100% certain over several visits probably will not respond to treatment.
Then engage the patient by suggesting that together you’ll view the belief as a scientist or detective would, carefully evaluating all evidence before reaching a conclusion.
2. How long have you had this belief? Some patients say they have always held a specific belief. Beliefs that have lasted years may be harder to change than more-recently adopted ones.
Looking back, some patients acknowledge initial doubts and recall considering alternate beliefs, which the physician can help strengthen.
3. How has the belief affected your life? Have the patient write down the advantages and disadvantages of his delusional thinking;1 seeing the consequences in writing may discourage the belief. For example, a patient might stop thinking he is a prophet if he realizes the belief could lead to alienation and hospitalization.
4. Until now, how have you coped with negative aspects of this belief (such as ‘death threats’)? For 10 years, one patient believed gangsters were trying to kill him. We asked how he survived attempts on his life. He identified activities and situations in which he felt safer, such as being with his parents, playing basketball with others, and visiting the doctor. We encouraged him to spend more time in these situations. He acknowledged his role in improving his sense of safety and felt empowered to confront the delusion.
5. What if the delusion is/is not true? Asking this question may uncover other dysfunctional thinking that can be addressed in therapy.
Have the patient rate certainty at each visit, and document changes in score. Patients with delusions usually are relieved that they are not being judged and that their beliefs are not invalidated. They often start questioning their delusions and develop coping skills to deal with them.
Some patients feel depressed after abandoning a delusion that once shielded them from low self-esteem (eg, “the FBI is after me because I’m important”). Steer patients toward various activities and have them rate their enjoyment and mastery of them. This will help them find alternate beliefs.
Reference
1. Rector N, Beck A. Cognitive therapy for schizophrenia: from conceptualization to intervention. Can J Psychiatry 2002;47:39-48.
Dr. Pinninti is assistant professor of psychiatry, School of Osteopathic Medicine, University of Medicine and Dentistry of New Jersey, and is medical director, Steininger Behavioral Care Services, Cherry Hill, NJ.
Dr. Sosland is a child and adolescent psychiatry fellow, Jefferson Medical College, Thomas Jefferson University, Philadelphia, PA.
Reference
1. Rector N, Beck A. Cognitive therapy for schizophrenia: from conceptualization to intervention. Can J Psychiatry 2002;47:39-48.
Dr. Pinninti is assistant professor of psychiatry, School of Osteopathic Medicine, University of Medicine and Dentistry of New Jersey, and is medical director, Steininger Behavioral Care Services, Cherry Hill, NJ.
Dr. Sosland is a child and adolescent psychiatry fellow, Jefferson Medical College, Thomas Jefferson University, Philadelphia, PA.
Suicide risk assessment: Questions that reveal what you really need to know
You can make more-informed decisions about a patient’s acute suicide risk—such as over the phone at 3 AM—if you know what to ask the psychiatry resident or crisis worker. For suicide risk assessment—especially when you have not seen the patient—you need specific, high-yield questions to draw out danger signals from large amounts of data.
We are not suggesting that a short list of questions is sufficient for this extremely difficult task. Rather—because we recognize its complexity—we offer the questions we find most useful when evaluating patients with suicidal behaviors.
American Psychiatric Association practice guidelines1 provide a comprehensive discussion of assessing suicide risk. In addition, we teach clinicians we supervise to probe for high-risk and less-commonly explored “protective” factors.
High-risk factors
Mental health clinicians are more experienced in probing for high-risk factors than for protective factors. Because population studies offer limited help (Box 1),2 we ask clinicians these questions to evaluate the seriousness of a suicide attempt:
- Most studies of suicide risk factors focus on medium- to long-range risk
- Population-based risk factors (such as being Caucasian, over age 65, or depressed) apply to so many patients that their clinical usefulness is limited1
- Population-based risk factors often have high sensitivity but low specificity (recent loss is an important risk factor for attempting suicide, for example, but very few persons with a recent loss attempt suicide)
- In an acute situation, the positive predictive value of suicide risk factors—alone or in combination—is not known
Table
3 important questions to ask in overdose cases
|
What method was used? Methods other than taking an overdose or cutting the wrists may be more dangerous.
What amount was used? (in overdose or poisoning cases)
What treatment was needed? If the patient took an overdose of opioids and needs intubation, this indicates a relatively serious attempt. On the other hand, the psychiatric seriousness of an acetaminophen overdose depends on whether the patient realized the danger in taking high doses of acetaminophen. Additional questions can help determine the seriousness of suicide attempts by overdose (Table).
Was the attempt impulsive or planned? Planned attempts tend to be more serious.
What is the ‘risk-rescue ratio’? The potential lethality of the attempt and the likelihood of being saved must be evaluated together. Where did the attempt occur? In a setting where others were likely to intervene? Was the patient alone? Attempts in the presence of others may be considered less alarming.
What did the patient do immediately afterward? Did he tell anyone? How did he get to the hospital? Did he seek help on his own? Who called the ambulance?
How does the patient feel about the attempt now? Is she glad or disappointed she didn’t die? Does she regret the attempt?
Have there been past attempts? Does the patient have a history of suicide attempts or significant selfmutilation? If so, what was the most serious incident? Past attempts tend to predict future attempts.
Other considerations for patients who have attempted suicide and those who have not but are being evaluated for possible suicide risk include:
Mental state. To estimate a patient’s mental state and depth of negative affect, without having seen her yourself, three helpful questions are:
- Does she still look upset, depressed, or angry? Anger and agitation tend to increase risk.
- Can she smile or relax, even briefly?
- Does she feel things are likely to improve?
Access to firearms. Suicide by firearms accounts for 55% of all suicides.3 Does the patient have access to a gun and bullets? If so, get details. Does he now keep the gun on his person instead of in a locked cabinet, as he did earlier? We find that questions about guns are all too frequently omitted.
Contract for safety. Can the patient reliably contract not to harm himself and to call for help in a crisis? Although contracts for safety have limited value—as will be discussed—a patient’s refusal to contract for safety may indicate a higher suicide risk.
Some patients may refuse to contract for safety in order to be hospitalized for other reasons. They may say they can’t be sure what they will do if not admitted or declare that the clinician will be blamed for their death.
Protective factors
A patient’s high-risk clinical features must be balanced against factors that may reduce suicide risk.
How much social support? Can family or friends constantly stay with the patient, watch him closely, and get help if the situation worsens? This is the simplest and most relevant method to assess the availability of protective support.
How much mental health support? Does the patient have a good relationship with a psychiatrist or therapist who can see the patient soon?
Have circumstances changed? Has the stressor that led to suicidal behavior resolved, at least in part? For example, if a patient’s fight with her boyfriend led to her taking an overdose, have they made up?
Four common myths. Clinicians assessing patients for acute suicide risk often overestimate the protective value of some factors. They may tell you:
- The patient only tried to harm himself while he was intoxicated. He’s not intoxicated now and therefore is not at high risk. The patient will likely get intoxicated again, despite his protestations to the contrary. Substance intoxication and withdrawal tend to worsen depression and diminish inhibitions, making suicide more—not less—likely.
- The patient contracts for safety. A contract for safety may have some value,4 but its clinical and legal merits in suicide risk assessment are overestimated.5 We are concerned about how often we see clinicians judge that a contract for safety overrides numerous high-risk factors.
- The patient was only trying to get attention. It is difficult for mental health professionals—and even for patients—to reliably ascertain what motivates someone to attempt suicide. Multiple motivations and ambivalence are common.
- The patient is ‘just a borderline.’ Because patients with borderline personality disorder tend to make repeated suicide gestures, clinicians may not take their suicide attempts seriously. This statement reveals ignorance about the suffering of persons with borderline personality disorder; their rate of completed suicide is approximately 10%.6
- When a suicide assessment is inconclusive, draw two columns on a sheet of paper. List the patient’s high-risk factors on one side and protective factors on the other
- Seeing the information in black and white often helps clarify the assessment
- Consider both the number of factors in each column and your clinical sense of each factor’s importance and intensity. Place a check mark next to particularly important factors
- This balance sheet can remind you of further questions to ask and often reveals that either the high-risk or protective factors far outweigh the others in number and/or intensity
Practical advice
Distinguish short-term vs long-term risk. Based on the questions above, we often conclude that a patient is at high long-term risk of suicide, but the immediate risk is much lower. Acute hospitalization is unlikely to alleviate the long-term risk (though sometimes is the only way to get the patient into psychiatric treatment).
Consider the source. Never disregard the “gut feeling” of the person who interviewed the patient, but also factor in your assessment of that clinician’s judgment. Sometimes inexperienced staffs’ intuitions may derive more from countertransference than from objective assessment.
Write it down. In cases where suicide risk seems unclear, it may help to list a patient’s risk and protective factors (Box 2). We have found this technique to be a useful teaching tool as well.
Be flexible. Because no method for assessing shortterm suicide risk is foolproof, be ready to re-evaluate your assessment and—if you are unsure—to take action to protect the patient.
1. American Psychiatric Association. Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry. 2003;160(11 suppl):1-60.
2. Fawcett J. Suicide risk factors in depressive disorders and in panic disorder. J Clin Psychiatry. 1992;53(suppl):9-13.
3. National Institute of Mental Health. Suicide facts. Available at: http://www.nimh.nih.gov/SuicidePrevention/suifact.cfm. Accessed June 3, 2004.
4. Stanford EJ, Goetz RR, Bloom JD. The no harm contract in the emergency assessment of suicidal risk. J Clin Psychiatry 1994;55(8):344-8.
5. Simon RI. The suicide prevention contract: clinical, legal, and risk management issues. J Am Acad Psychiatry Law 1999;27(3):445-50.
6. Paris J. Chronic suicidality among patients with borderline personality disorder. Psychiatr Serv 2002;53(6):738-42.
You can make more-informed decisions about a patient’s acute suicide risk—such as over the phone at 3 AM—if you know what to ask the psychiatry resident or crisis worker. For suicide risk assessment—especially when you have not seen the patient—you need specific, high-yield questions to draw out danger signals from large amounts of data.
We are not suggesting that a short list of questions is sufficient for this extremely difficult task. Rather—because we recognize its complexity—we offer the questions we find most useful when evaluating patients with suicidal behaviors.
American Psychiatric Association practice guidelines1 provide a comprehensive discussion of assessing suicide risk. In addition, we teach clinicians we supervise to probe for high-risk and less-commonly explored “protective” factors.
High-risk factors
Mental health clinicians are more experienced in probing for high-risk factors than for protective factors. Because population studies offer limited help (Box 1),2 we ask clinicians these questions to evaluate the seriousness of a suicide attempt:
- Most studies of suicide risk factors focus on medium- to long-range risk
- Population-based risk factors (such as being Caucasian, over age 65, or depressed) apply to so many patients that their clinical usefulness is limited1
- Population-based risk factors often have high sensitivity but low specificity (recent loss is an important risk factor for attempting suicide, for example, but very few persons with a recent loss attempt suicide)
- In an acute situation, the positive predictive value of suicide risk factors—alone or in combination—is not known
Table
3 important questions to ask in overdose cases
|
What method was used? Methods other than taking an overdose or cutting the wrists may be more dangerous.
What amount was used? (in overdose or poisoning cases)
What treatment was needed? If the patient took an overdose of opioids and needs intubation, this indicates a relatively serious attempt. On the other hand, the psychiatric seriousness of an acetaminophen overdose depends on whether the patient realized the danger in taking high doses of acetaminophen. Additional questions can help determine the seriousness of suicide attempts by overdose (Table).
Was the attempt impulsive or planned? Planned attempts tend to be more serious.
What is the ‘risk-rescue ratio’? The potential lethality of the attempt and the likelihood of being saved must be evaluated together. Where did the attempt occur? In a setting where others were likely to intervene? Was the patient alone? Attempts in the presence of others may be considered less alarming.
What did the patient do immediately afterward? Did he tell anyone? How did he get to the hospital? Did he seek help on his own? Who called the ambulance?
How does the patient feel about the attempt now? Is she glad or disappointed she didn’t die? Does she regret the attempt?
Have there been past attempts? Does the patient have a history of suicide attempts or significant selfmutilation? If so, what was the most serious incident? Past attempts tend to predict future attempts.
Other considerations for patients who have attempted suicide and those who have not but are being evaluated for possible suicide risk include:
Mental state. To estimate a patient’s mental state and depth of negative affect, without having seen her yourself, three helpful questions are:
- Does she still look upset, depressed, or angry? Anger and agitation tend to increase risk.
- Can she smile or relax, even briefly?
- Does she feel things are likely to improve?
Access to firearms. Suicide by firearms accounts for 55% of all suicides.3 Does the patient have access to a gun and bullets? If so, get details. Does he now keep the gun on his person instead of in a locked cabinet, as he did earlier? We find that questions about guns are all too frequently omitted.
Contract for safety. Can the patient reliably contract not to harm himself and to call for help in a crisis? Although contracts for safety have limited value—as will be discussed—a patient’s refusal to contract for safety may indicate a higher suicide risk.
Some patients may refuse to contract for safety in order to be hospitalized for other reasons. They may say they can’t be sure what they will do if not admitted or declare that the clinician will be blamed for their death.
Protective factors
A patient’s high-risk clinical features must be balanced against factors that may reduce suicide risk.
How much social support? Can family or friends constantly stay with the patient, watch him closely, and get help if the situation worsens? This is the simplest and most relevant method to assess the availability of protective support.
How much mental health support? Does the patient have a good relationship with a psychiatrist or therapist who can see the patient soon?
Have circumstances changed? Has the stressor that led to suicidal behavior resolved, at least in part? For example, if a patient’s fight with her boyfriend led to her taking an overdose, have they made up?
Four common myths. Clinicians assessing patients for acute suicide risk often overestimate the protective value of some factors. They may tell you:
- The patient only tried to harm himself while he was intoxicated. He’s not intoxicated now and therefore is not at high risk. The patient will likely get intoxicated again, despite his protestations to the contrary. Substance intoxication and withdrawal tend to worsen depression and diminish inhibitions, making suicide more—not less—likely.
- The patient contracts for safety. A contract for safety may have some value,4 but its clinical and legal merits in suicide risk assessment are overestimated.5 We are concerned about how often we see clinicians judge that a contract for safety overrides numerous high-risk factors.
- The patient was only trying to get attention. It is difficult for mental health professionals—and even for patients—to reliably ascertain what motivates someone to attempt suicide. Multiple motivations and ambivalence are common.
- The patient is ‘just a borderline.’ Because patients with borderline personality disorder tend to make repeated suicide gestures, clinicians may not take their suicide attempts seriously. This statement reveals ignorance about the suffering of persons with borderline personality disorder; their rate of completed suicide is approximately 10%.6
- When a suicide assessment is inconclusive, draw two columns on a sheet of paper. List the patient’s high-risk factors on one side and protective factors on the other
- Seeing the information in black and white often helps clarify the assessment
- Consider both the number of factors in each column and your clinical sense of each factor’s importance and intensity. Place a check mark next to particularly important factors
- This balance sheet can remind you of further questions to ask and often reveals that either the high-risk or protective factors far outweigh the others in number and/or intensity
Practical advice
Distinguish short-term vs long-term risk. Based on the questions above, we often conclude that a patient is at high long-term risk of suicide, but the immediate risk is much lower. Acute hospitalization is unlikely to alleviate the long-term risk (though sometimes is the only way to get the patient into psychiatric treatment).
Consider the source. Never disregard the “gut feeling” of the person who interviewed the patient, but also factor in your assessment of that clinician’s judgment. Sometimes inexperienced staffs’ intuitions may derive more from countertransference than from objective assessment.
Write it down. In cases where suicide risk seems unclear, it may help to list a patient’s risk and protective factors (Box 2). We have found this technique to be a useful teaching tool as well.
Be flexible. Because no method for assessing shortterm suicide risk is foolproof, be ready to re-evaluate your assessment and—if you are unsure—to take action to protect the patient.
You can make more-informed decisions about a patient’s acute suicide risk—such as over the phone at 3 AM—if you know what to ask the psychiatry resident or crisis worker. For suicide risk assessment—especially when you have not seen the patient—you need specific, high-yield questions to draw out danger signals from large amounts of data.
We are not suggesting that a short list of questions is sufficient for this extremely difficult task. Rather—because we recognize its complexity—we offer the questions we find most useful when evaluating patients with suicidal behaviors.
American Psychiatric Association practice guidelines1 provide a comprehensive discussion of assessing suicide risk. In addition, we teach clinicians we supervise to probe for high-risk and less-commonly explored “protective” factors.
High-risk factors
Mental health clinicians are more experienced in probing for high-risk factors than for protective factors. Because population studies offer limited help (Box 1),2 we ask clinicians these questions to evaluate the seriousness of a suicide attempt:
- Most studies of suicide risk factors focus on medium- to long-range risk
- Population-based risk factors (such as being Caucasian, over age 65, or depressed) apply to so many patients that their clinical usefulness is limited1
- Population-based risk factors often have high sensitivity but low specificity (recent loss is an important risk factor for attempting suicide, for example, but very few persons with a recent loss attempt suicide)
- In an acute situation, the positive predictive value of suicide risk factors—alone or in combination—is not known
Table
3 important questions to ask in overdose cases
|
What method was used? Methods other than taking an overdose or cutting the wrists may be more dangerous.
What amount was used? (in overdose or poisoning cases)
What treatment was needed? If the patient took an overdose of opioids and needs intubation, this indicates a relatively serious attempt. On the other hand, the psychiatric seriousness of an acetaminophen overdose depends on whether the patient realized the danger in taking high doses of acetaminophen. Additional questions can help determine the seriousness of suicide attempts by overdose (Table).
Was the attempt impulsive or planned? Planned attempts tend to be more serious.
What is the ‘risk-rescue ratio’? The potential lethality of the attempt and the likelihood of being saved must be evaluated together. Where did the attempt occur? In a setting where others were likely to intervene? Was the patient alone? Attempts in the presence of others may be considered less alarming.
What did the patient do immediately afterward? Did he tell anyone? How did he get to the hospital? Did he seek help on his own? Who called the ambulance?
How does the patient feel about the attempt now? Is she glad or disappointed she didn’t die? Does she regret the attempt?
Have there been past attempts? Does the patient have a history of suicide attempts or significant selfmutilation? If so, what was the most serious incident? Past attempts tend to predict future attempts.
Other considerations for patients who have attempted suicide and those who have not but are being evaluated for possible suicide risk include:
Mental state. To estimate a patient’s mental state and depth of negative affect, without having seen her yourself, three helpful questions are:
- Does she still look upset, depressed, or angry? Anger and agitation tend to increase risk.
- Can she smile or relax, even briefly?
- Does she feel things are likely to improve?
Access to firearms. Suicide by firearms accounts for 55% of all suicides.3 Does the patient have access to a gun and bullets? If so, get details. Does he now keep the gun on his person instead of in a locked cabinet, as he did earlier? We find that questions about guns are all too frequently omitted.
Contract for safety. Can the patient reliably contract not to harm himself and to call for help in a crisis? Although contracts for safety have limited value—as will be discussed—a patient’s refusal to contract for safety may indicate a higher suicide risk.
Some patients may refuse to contract for safety in order to be hospitalized for other reasons. They may say they can’t be sure what they will do if not admitted or declare that the clinician will be blamed for their death.
Protective factors
A patient’s high-risk clinical features must be balanced against factors that may reduce suicide risk.
How much social support? Can family or friends constantly stay with the patient, watch him closely, and get help if the situation worsens? This is the simplest and most relevant method to assess the availability of protective support.
How much mental health support? Does the patient have a good relationship with a psychiatrist or therapist who can see the patient soon?
Have circumstances changed? Has the stressor that led to suicidal behavior resolved, at least in part? For example, if a patient’s fight with her boyfriend led to her taking an overdose, have they made up?
Four common myths. Clinicians assessing patients for acute suicide risk often overestimate the protective value of some factors. They may tell you:
- The patient only tried to harm himself while he was intoxicated. He’s not intoxicated now and therefore is not at high risk. The patient will likely get intoxicated again, despite his protestations to the contrary. Substance intoxication and withdrawal tend to worsen depression and diminish inhibitions, making suicide more—not less—likely.
- The patient contracts for safety. A contract for safety may have some value,4 but its clinical and legal merits in suicide risk assessment are overestimated.5 We are concerned about how often we see clinicians judge that a contract for safety overrides numerous high-risk factors.
- The patient was only trying to get attention. It is difficult for mental health professionals—and even for patients—to reliably ascertain what motivates someone to attempt suicide. Multiple motivations and ambivalence are common.
- The patient is ‘just a borderline.’ Because patients with borderline personality disorder tend to make repeated suicide gestures, clinicians may not take their suicide attempts seriously. This statement reveals ignorance about the suffering of persons with borderline personality disorder; their rate of completed suicide is approximately 10%.6
- When a suicide assessment is inconclusive, draw two columns on a sheet of paper. List the patient’s high-risk factors on one side and protective factors on the other
- Seeing the information in black and white often helps clarify the assessment
- Consider both the number of factors in each column and your clinical sense of each factor’s importance and intensity. Place a check mark next to particularly important factors
- This balance sheet can remind you of further questions to ask and often reveals that either the high-risk or protective factors far outweigh the others in number and/or intensity
Practical advice
Distinguish short-term vs long-term risk. Based on the questions above, we often conclude that a patient is at high long-term risk of suicide, but the immediate risk is much lower. Acute hospitalization is unlikely to alleviate the long-term risk (though sometimes is the only way to get the patient into psychiatric treatment).
Consider the source. Never disregard the “gut feeling” of the person who interviewed the patient, but also factor in your assessment of that clinician’s judgment. Sometimes inexperienced staffs’ intuitions may derive more from countertransference than from objective assessment.
Write it down. In cases where suicide risk seems unclear, it may help to list a patient’s risk and protective factors (Box 2). We have found this technique to be a useful teaching tool as well.
Be flexible. Because no method for assessing shortterm suicide risk is foolproof, be ready to re-evaluate your assessment and—if you are unsure—to take action to protect the patient.
1. American Psychiatric Association. Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry. 2003;160(11 suppl):1-60.
2. Fawcett J. Suicide risk factors in depressive disorders and in panic disorder. J Clin Psychiatry. 1992;53(suppl):9-13.
3. National Institute of Mental Health. Suicide facts. Available at: http://www.nimh.nih.gov/SuicidePrevention/suifact.cfm. Accessed June 3, 2004.
4. Stanford EJ, Goetz RR, Bloom JD. The no harm contract in the emergency assessment of suicidal risk. J Clin Psychiatry 1994;55(8):344-8.
5. Simon RI. The suicide prevention contract: clinical, legal, and risk management issues. J Am Acad Psychiatry Law 1999;27(3):445-50.
6. Paris J. Chronic suicidality among patients with borderline personality disorder. Psychiatr Serv 2002;53(6):738-42.
1. American Psychiatric Association. Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry. 2003;160(11 suppl):1-60.
2. Fawcett J. Suicide risk factors in depressive disorders and in panic disorder. J Clin Psychiatry. 1992;53(suppl):9-13.
3. National Institute of Mental Health. Suicide facts. Available at: http://www.nimh.nih.gov/SuicidePrevention/suifact.cfm. Accessed June 3, 2004.
4. Stanford EJ, Goetz RR, Bloom JD. The no harm contract in the emergency assessment of suicidal risk. J Clin Psychiatry 1994;55(8):344-8.
5. Simon RI. The suicide prevention contract: clinical, legal, and risk management issues. J Am Acad Psychiatry Law 1999;27(3):445-50.
6. Paris J. Chronic suicidality among patients with borderline personality disorder. Psychiatr Serv 2002;53(6):738-42.
A wise aproach to bipolar depression
Most of us care for patients with bipolar disorder and face this problem regularly: We start an antidepressant for breakthrough depressive symptoms, and the patient responds. How long do we continue the antidepressant?
In this issue, Robert M. Post, MD, head of the Bipolar Collaborative Network, summarizes the published literature—and some very recent unpublished reports—related to this question and makes systematic recommendations. I won’t attempt to summarize his carefully considered conclusions, but I would like to highlight his two-pronged” approach:
- conservative treatment—no change in medication—when the patient remains well
- aggressive—if not radical—treatment when the illness course remains problematic.
I cannot help but reflect that these recommendations—wise advice for many clinical problems—echo the Hippocratic principles of “first, do no harm” and “extreme illnesses require extreme remedies.” Although, thank goodness, today’s medical treatments bear no resemblance to those used in Hippocrates’ time, our wisdom still bears a resemblance to his.
Conscientious physicians aspire to keep up with the latest literature while growing in professional wisdom. Helping us with those challenging tasks is Current Psychiatry’s goal.
Most of us care for patients with bipolar disorder and face this problem regularly: We start an antidepressant for breakthrough depressive symptoms, and the patient responds. How long do we continue the antidepressant?
In this issue, Robert M. Post, MD, head of the Bipolar Collaborative Network, summarizes the published literature—and some very recent unpublished reports—related to this question and makes systematic recommendations. I won’t attempt to summarize his carefully considered conclusions, but I would like to highlight his two-pronged” approach:
- conservative treatment—no change in medication—when the patient remains well
- aggressive—if not radical—treatment when the illness course remains problematic.
I cannot help but reflect that these recommendations—wise advice for many clinical problems—echo the Hippocratic principles of “first, do no harm” and “extreme illnesses require extreme remedies.” Although, thank goodness, today’s medical treatments bear no resemblance to those used in Hippocrates’ time, our wisdom still bears a resemblance to his.
Conscientious physicians aspire to keep up with the latest literature while growing in professional wisdom. Helping us with those challenging tasks is Current Psychiatry’s goal.
Most of us care for patients with bipolar disorder and face this problem regularly: We start an antidepressant for breakthrough depressive symptoms, and the patient responds. How long do we continue the antidepressant?
In this issue, Robert M. Post, MD, head of the Bipolar Collaborative Network, summarizes the published literature—and some very recent unpublished reports—related to this question and makes systematic recommendations. I won’t attempt to summarize his carefully considered conclusions, but I would like to highlight his two-pronged” approach:
- conservative treatment—no change in medication—when the patient remains well
- aggressive—if not radical—treatment when the illness course remains problematic.
I cannot help but reflect that these recommendations—wise advice for many clinical problems—echo the Hippocratic principles of “first, do no harm” and “extreme illnesses require extreme remedies.” Although, thank goodness, today’s medical treatments bear no resemblance to those used in Hippocrates’ time, our wisdom still bears a resemblance to his.
Conscientious physicians aspire to keep up with the latest literature while growing in professional wisdom. Helping us with those challenging tasks is Current Psychiatry’s goal.
The ‘show-off’ who couldn’t walk
Presentation: 3 o’clock comes early
Miss T, age 9, presents with decreased sensation and motility in both legs. She cannot walk or stand.
Three days before, Miss T said she was consistently answering the teacher’s questions correctly at school. Because of this, a classmate teased her by calling her a “show off.” Soon after, Miss T began feeling weak and nauseous. The school nurse got a blood sugar reading of 68 mg/dL, slightly below the low-normal range.
Miss T’s mother arrived and convinced her to eat crackers and drink juice. Her blood sugar rose to 139 mg/dL and she began to feel better. When Miss T tried to stand, however, her legs and feet felt weak. She had trouble standing without support and needed help walking even a short distance. The mother brought Miss T home from school early.
Over the next 2 days, Miss T’s lower-extremity symptoms worsened. Her mother brought her to the pediatric emergency room.
Initial physical exam showed normal vital signs with no gross abnormalities. Neurologic exam revealed no lower-extremity masses, lesions, or deformities. Laboratory tests were normal. Cranial nerves were grossly intact. Right and left upper and lower extremities exhibited good tone, normal reflexes, and good strength against resistance. Miss T, however, said she could not feel sharp or dull objects against her lower legs.
ER pediatricians then called on the child psychiatry department to evaluate Miss T for possible psychiatric causes.
At intake, Miss T sits with her legs dangling from a stretcher. She is pleasant, articulate, and well-mannered. She spontaneously moves both legs and does not seem distressed when asked about her sudden disability. Her mood is euthymic, but she reports that constant teasing at school sometimes causes intense stress. She says that her sister sometimes “gets mean” with her but does not elaborate. She adds that she is sometimes sad because her parents recently separated, but she denies resultant emotional effects.
No suicidal/homicidal ideations or psychotic symptoms are present. Miss T’s thought process is logical and goal-directed. She is alert and oriented with good memory, language, concentration, and impulse control.
Neither Miss T nor her family has a significant psychiatric or medical history. Miss T has not been taking medications or over-the-counter supplements. Upon questioning, the mother denies that her daughter has been physically or sexually abused.
Despite a lack of positive neurologic findings, the neurology team recommends admission to rule out unseen medical problems.
The authors’ observations
Medical diagnosis.1,2 In Guillain-Barré syndrome, an infection usually precedes symptom onset, and maximum weakness is seen within 7 to 10 days. Respiration may be compromised, and weakness tends to spread throughout the body. Miss T’s symptoms came on more rapidly, her breathing was normal, and weakness was confined to her legs and feet.
In neuromuscular junction disorders such as myasthenia gravis and Lambert-Eaton syndrome, symptoms are not as acute, fluctuate throughout the day, and usually worsen with exertion. By contrast, Miss T’s symptoms steadily worsened without provocation.
Muscular dystrophy and myopathy were ruled out because of Miss T’s rapid symptom onset and lack of prior health problems. Dystrophy usually is seen in early childhood, affects the hip and girdle muscles, and progresses slowly. Myopathy symptoms usually are chronic and progressive, and associated medical disorders overshadow the muscle disease.
Patients with intracranial lesions may present with:
- symptoms of diffuse cerebral disease, such as mental impairment, headache, or seizures
- focal neurologic signs, such as aphasia or hemiparesis
- evidence of increased intracranial pressure, such as headache, vomiting, drowsiness, or papilledema.
Miss T had none of these.
Patients with spinal cord tumors usually have radicular pain, sensory/motor involvement, sphincteric dysfunction, and percussible back tenderness. Symptoms develop over weeks to months.
Psychiatric diagnosis. Factitious disorder, malingering, somatization disorder, and conversion disorder (Box) also were considered:
- In factitious disorder, the patient exacerbates his or her symptoms to assume the sick role.
- Malingering patients have external motivations behind symptom fabrication.
- Somatization disorder involves multiple organ systems, and patients often are preoccupied with their symptoms.
Miss T’s complaints were not consciously induced, external motivations were absent, a single organ system (musculoskeletal) was involved, and she appeared largely untroubled by her deficit.
Her presentation most closely fit the diagnosis of conversion disorder. Patients with this disorder complain of symptoms or deficits affecting voluntary muscles or of sensory function deficits that suggest a neurologic or medical condition. The symptoms’ temporal relationship to a stressful event suggests psychological factors. Symptoms:
- are not intentionally produced
- cannot be attributed to an organic cause
- cause significant functional impairment
- are not limited to pain or sexual dysfunction
- cannot be explained by another mental disorder.
Treatment: A miraculous recovery
The pediatrics, child psychiatry, pediatric neurology, and physical medicine/rehabilitation departments treated Miss T. No organic cause of her symptoms was found; results of an MRI with contrast, EEG, and repeated lab tests were negative.
On day 3, Miss T started taking small steps on her own. Two days later, she walked without assistance; discharge was considered.
The hospital’s social services department, however, discovered that the state child welfare agency had investigated Miss T’s family for alleged child abuse/neglect years before but found no evidence.
Also, a school social worker had recently visited Miss T’s family after receiving a complaint that an older sibling was allegedly hitting the younger ones. The social worker noticed that the kitchen door was padlocked; she speculated that the family was struggling financially and did not want the children to eat all the food. No other evidence of child maltreatment was found and the investigation was stopped. None of Miss T’s lab results indicated malnutrition.
The girl was discharged after the mother agreed to allow a home health aide to monitor the children’s well-being and a psychologist to perform neuropsychological tests on Miss T. Follow-up out-patient visits with the pediatric neurology, general pediatrics, and child psychiatry departments were also required.
Conversion disorder each year accounts for approximately 22 psychiatric cases per 100,000 overall.3 In the hospital setting, 5% to 14% of medical inpatient referrals for psychiatric evaluation result in conversion disorder diagnosis.3
Conversion disorder is seen in men and women but is more common in young women. Symptoms can occur at any age but are rare in children age < 7 and probably do not occur in children age < 4.3
Prevalence is higher in rural areas and among undereducated and low-income persons.3,4 Researchers also suggest that family history of conversion disorder contributes to symptom onset in offspring.4
The authors’ observations
Once we learned Miss T’s family had been investigated for child neglect, we had to find out if her symptoms were an expression of maltreatment or were caused by psychological stressors at school. Definitive maltreatment never surfaced, and school stress was determined to be a minor factor.
Neglected children exhibit characteristics at different ages that might contribute to conversion symptom development (Table). Most notably, such children have trouble understanding appropriate affective responses to interpersonal situations. As a result, they may express distress in unconventional ways.3
Table
Psychopathology of the neglected child
| Age | Developmental difficulties |
|---|---|
| 1 year | Insecure attachments |
| 2 years | Easily frustrated |
| 3 years | Low self-esteem/self-assertion Impaired flexibility, self-control compared with similarly aged healthy children Difficulty dealing with frustration Lack of persistence, enthusiasm when performing educational tasks |
| Preschool (4-6 years) | Overly dependent Lack of enthusiasm in preschool environment |
| Elementary school (6-12 years) | Attention problems Low self-assertion, self-esteem Withdrawal behaviors, dysphoric affect Social isolation |
| General | Trouble understanding appropriate affective responses to interpersonal situations Limited social problem-solving skills |
| Source: adapted from reference 4 | |
Little empirical evidence supports the link between childhood maltreatment and conversion disorder. In one study:5
- Adults with conversion disorder (mean age 37.6) reported a higher incidence of physical and sexual abuse, more types of physical abuse, sexual abuse of longer duration, and more-frequent incestuous episodes than did adults with affective disorder.
- Among patients with conversion disorder, having a mother with recurrent illness, nervousness or depression, or who abuses alcohol or sedatives was associated with higher dissociative and somatoform scale scores.
- Physical abuse was associated with increased conversion symptoms.
The authors concluded that childhood trauma is a distinct and predictive—though not necessary—feature of conversion disorder.5
The authors’ observations
Psychotherapy and attention to socio-cultural beliefs may enable the patient to “give up” the conversion symptom.3 Several factors determine choices of psychotherapies, although elements of all approaches are commonly used:
- CBT and behavioral therapy have roles in treating acute symptoms.
- Supportive therapy and hypnotherapy are recommended for treating rare, longstanding conversion symptoms (4 to 6 months duration).
- Psychodynamic therapy can help patients who are introspective, can remember details about their past, and are willing to participate in longer-term therapy.
Cognitive-behavioral therapy. Behavior is shaped by what we learn from the environment. Conversion behavior can be reinforced by others who help maintain the symptoms. Behavioral therapy and CBT are aimed at modifying behaviors via desensitization and by increasing the patient’s understanding of his or her physical capacities.
Hypnosis gives patients a medium to recall experiences and feelings they cannot consciously bring up in treatment. Symptom exploration and reduction are broad goals.
Psychodynamic therapy aims to resolve unconscious conflict after a traumatic event. A patient who develops lower-extremity paralysis or sensory problems after having been chastised for running away might benefit from this model, for example.
Supportive psychotherapy emphasizes reassurance and education. For Miss T, that would mean reassurance that her paralysis will improve, with education about conversion disorder and how difficult life events can cause similar symptoms.
Sociocultural considerations. Cultural beliefs inhibit some people’s emotions and may predispose them to conversion symptoms.
No empirical evidence indicates that medication improves conversion disorder. Anecdotal reports cite positive response to older antipsychotics, lithium, and electroconvulsive therapy.6
Patients with conversion disorder, however, tend to develop mood and/or anxiety symptoms later, and psychotropics may help treat these comorbidities. Follow the patient while symptoms are present.7 Comorbid symptoms’ severity, response, and presentation dictate follow-up frequency.
Prognosis. Children with conversion disorder generally have good outcomes,5 particularly those with good premorbid function who are diagnosed early.7 Time from symptom onset to diagnosis ranges from weeks to 1 year, and most cases resolve within 3 months of diagnosis. Symptom recurrence is rare but may indicate emerging polysymptomatic somatization disorder.5
Treatment: Reassurance and support
Miss T responded well to supportive psychotherapy and reassurance from hospital staff. No psychiatric screening tests were done, but child psychiatrists saw Miss T several times daily, and she exhibited no other psychiatric symptoms. We have no information on follow-up treatment, which occurred outside the hospital.
Related resources
- Academy of Psychosomatic Medicine. www.apm.org
- Weiner J, Dulcan M. Textbook of child and adolescent psychiatry (3rd ed). Washington, DC: American Psychiatric Publishing, 2003.
1. Fauci AS, Braunwald E, Hauser SL, et al. Harrison’s principles of medicine: companion handbook (14th ed). New York: McGraw Hill, 1999.
2. Adams R, Victor M. Companion to principles of neurology. New York: McGraw Hill, 1991.
3. Schwartz A, Calhoun A. Treatment of conversion disorder in an African-American Christian woman: cultural and social considerations. Am J Psychiatry 2001;158:1385-91.
4. Lewis M. Child and adolescent psychiatry—a comprehensive textbook (3rd ed). Baltimore: Lippincott Williams and Wilkins, 2002.
5. Roelofs K, Keijsers GP, Hoogduin KA, et al. Childhood abuse in patients with conversion disorder. Am J Psychiatry 2002;159:1908-13.
6. Hales R, Yudofsky S. Essentials of clinical psychiatry(2nd ed). Washington, DC: American Psychiatric Publishing, 2004.
7. Pehlivanturk B, Unal F. Conversion disorder in children and adolescents: a 4-year follow-up study. J Psychosom Res 2002;52:187-91.
Presentation: 3 o’clock comes early
Miss T, age 9, presents with decreased sensation and motility in both legs. She cannot walk or stand.
Three days before, Miss T said she was consistently answering the teacher’s questions correctly at school. Because of this, a classmate teased her by calling her a “show off.” Soon after, Miss T began feeling weak and nauseous. The school nurse got a blood sugar reading of 68 mg/dL, slightly below the low-normal range.
Miss T’s mother arrived and convinced her to eat crackers and drink juice. Her blood sugar rose to 139 mg/dL and she began to feel better. When Miss T tried to stand, however, her legs and feet felt weak. She had trouble standing without support and needed help walking even a short distance. The mother brought Miss T home from school early.
Over the next 2 days, Miss T’s lower-extremity symptoms worsened. Her mother brought her to the pediatric emergency room.
Initial physical exam showed normal vital signs with no gross abnormalities. Neurologic exam revealed no lower-extremity masses, lesions, or deformities. Laboratory tests were normal. Cranial nerves were grossly intact. Right and left upper and lower extremities exhibited good tone, normal reflexes, and good strength against resistance. Miss T, however, said she could not feel sharp or dull objects against her lower legs.
ER pediatricians then called on the child psychiatry department to evaluate Miss T for possible psychiatric causes.
At intake, Miss T sits with her legs dangling from a stretcher. She is pleasant, articulate, and well-mannered. She spontaneously moves both legs and does not seem distressed when asked about her sudden disability. Her mood is euthymic, but she reports that constant teasing at school sometimes causes intense stress. She says that her sister sometimes “gets mean” with her but does not elaborate. She adds that she is sometimes sad because her parents recently separated, but she denies resultant emotional effects.
No suicidal/homicidal ideations or psychotic symptoms are present. Miss T’s thought process is logical and goal-directed. She is alert and oriented with good memory, language, concentration, and impulse control.
Neither Miss T nor her family has a significant psychiatric or medical history. Miss T has not been taking medications or over-the-counter supplements. Upon questioning, the mother denies that her daughter has been physically or sexually abused.
Despite a lack of positive neurologic findings, the neurology team recommends admission to rule out unseen medical problems.
The authors’ observations
Medical diagnosis.1,2 In Guillain-Barré syndrome, an infection usually precedes symptom onset, and maximum weakness is seen within 7 to 10 days. Respiration may be compromised, and weakness tends to spread throughout the body. Miss T’s symptoms came on more rapidly, her breathing was normal, and weakness was confined to her legs and feet.
In neuromuscular junction disorders such as myasthenia gravis and Lambert-Eaton syndrome, symptoms are not as acute, fluctuate throughout the day, and usually worsen with exertion. By contrast, Miss T’s symptoms steadily worsened without provocation.
Muscular dystrophy and myopathy were ruled out because of Miss T’s rapid symptom onset and lack of prior health problems. Dystrophy usually is seen in early childhood, affects the hip and girdle muscles, and progresses slowly. Myopathy symptoms usually are chronic and progressive, and associated medical disorders overshadow the muscle disease.
Patients with intracranial lesions may present with:
- symptoms of diffuse cerebral disease, such as mental impairment, headache, or seizures
- focal neurologic signs, such as aphasia or hemiparesis
- evidence of increased intracranial pressure, such as headache, vomiting, drowsiness, or papilledema.
Miss T had none of these.
Patients with spinal cord tumors usually have radicular pain, sensory/motor involvement, sphincteric dysfunction, and percussible back tenderness. Symptoms develop over weeks to months.
Psychiatric diagnosis. Factitious disorder, malingering, somatization disorder, and conversion disorder (Box) also were considered:
- In factitious disorder, the patient exacerbates his or her symptoms to assume the sick role.
- Malingering patients have external motivations behind symptom fabrication.
- Somatization disorder involves multiple organ systems, and patients often are preoccupied with their symptoms.
Miss T’s complaints were not consciously induced, external motivations were absent, a single organ system (musculoskeletal) was involved, and she appeared largely untroubled by her deficit.
Her presentation most closely fit the diagnosis of conversion disorder. Patients with this disorder complain of symptoms or deficits affecting voluntary muscles or of sensory function deficits that suggest a neurologic or medical condition. The symptoms’ temporal relationship to a stressful event suggests psychological factors. Symptoms:
- are not intentionally produced
- cannot be attributed to an organic cause
- cause significant functional impairment
- are not limited to pain or sexual dysfunction
- cannot be explained by another mental disorder.
Treatment: A miraculous recovery
The pediatrics, child psychiatry, pediatric neurology, and physical medicine/rehabilitation departments treated Miss T. No organic cause of her symptoms was found; results of an MRI with contrast, EEG, and repeated lab tests were negative.
On day 3, Miss T started taking small steps on her own. Two days later, she walked without assistance; discharge was considered.
The hospital’s social services department, however, discovered that the state child welfare agency had investigated Miss T’s family for alleged child abuse/neglect years before but found no evidence.
Also, a school social worker had recently visited Miss T’s family after receiving a complaint that an older sibling was allegedly hitting the younger ones. The social worker noticed that the kitchen door was padlocked; she speculated that the family was struggling financially and did not want the children to eat all the food. No other evidence of child maltreatment was found and the investigation was stopped. None of Miss T’s lab results indicated malnutrition.
The girl was discharged after the mother agreed to allow a home health aide to monitor the children’s well-being and a psychologist to perform neuropsychological tests on Miss T. Follow-up out-patient visits with the pediatric neurology, general pediatrics, and child psychiatry departments were also required.
Conversion disorder each year accounts for approximately 22 psychiatric cases per 100,000 overall.3 In the hospital setting, 5% to 14% of medical inpatient referrals for psychiatric evaluation result in conversion disorder diagnosis.3
Conversion disorder is seen in men and women but is more common in young women. Symptoms can occur at any age but are rare in children age < 7 and probably do not occur in children age < 4.3
Prevalence is higher in rural areas and among undereducated and low-income persons.3,4 Researchers also suggest that family history of conversion disorder contributes to symptom onset in offspring.4
The authors’ observations
Once we learned Miss T’s family had been investigated for child neglect, we had to find out if her symptoms were an expression of maltreatment or were caused by psychological stressors at school. Definitive maltreatment never surfaced, and school stress was determined to be a minor factor.
Neglected children exhibit characteristics at different ages that might contribute to conversion symptom development (Table). Most notably, such children have trouble understanding appropriate affective responses to interpersonal situations. As a result, they may express distress in unconventional ways.3
Table
Psychopathology of the neglected child
| Age | Developmental difficulties |
|---|---|
| 1 year | Insecure attachments |
| 2 years | Easily frustrated |
| 3 years | Low self-esteem/self-assertion Impaired flexibility, self-control compared with similarly aged healthy children Difficulty dealing with frustration Lack of persistence, enthusiasm when performing educational tasks |
| Preschool (4-6 years) | Overly dependent Lack of enthusiasm in preschool environment |
| Elementary school (6-12 years) | Attention problems Low self-assertion, self-esteem Withdrawal behaviors, dysphoric affect Social isolation |
| General | Trouble understanding appropriate affective responses to interpersonal situations Limited social problem-solving skills |
| Source: adapted from reference 4 | |
Little empirical evidence supports the link between childhood maltreatment and conversion disorder. In one study:5
- Adults with conversion disorder (mean age 37.6) reported a higher incidence of physical and sexual abuse, more types of physical abuse, sexual abuse of longer duration, and more-frequent incestuous episodes than did adults with affective disorder.
- Among patients with conversion disorder, having a mother with recurrent illness, nervousness or depression, or who abuses alcohol or sedatives was associated with higher dissociative and somatoform scale scores.
- Physical abuse was associated with increased conversion symptoms.
The authors concluded that childhood trauma is a distinct and predictive—though not necessary—feature of conversion disorder.5
The authors’ observations
Psychotherapy and attention to socio-cultural beliefs may enable the patient to “give up” the conversion symptom.3 Several factors determine choices of psychotherapies, although elements of all approaches are commonly used:
- CBT and behavioral therapy have roles in treating acute symptoms.
- Supportive therapy and hypnotherapy are recommended for treating rare, longstanding conversion symptoms (4 to 6 months duration).
- Psychodynamic therapy can help patients who are introspective, can remember details about their past, and are willing to participate in longer-term therapy.
Cognitive-behavioral therapy. Behavior is shaped by what we learn from the environment. Conversion behavior can be reinforced by others who help maintain the symptoms. Behavioral therapy and CBT are aimed at modifying behaviors via desensitization and by increasing the patient’s understanding of his or her physical capacities.
Hypnosis gives patients a medium to recall experiences and feelings they cannot consciously bring up in treatment. Symptom exploration and reduction are broad goals.
Psychodynamic therapy aims to resolve unconscious conflict after a traumatic event. A patient who develops lower-extremity paralysis or sensory problems after having been chastised for running away might benefit from this model, for example.
Supportive psychotherapy emphasizes reassurance and education. For Miss T, that would mean reassurance that her paralysis will improve, with education about conversion disorder and how difficult life events can cause similar symptoms.
Sociocultural considerations. Cultural beliefs inhibit some people’s emotions and may predispose them to conversion symptoms.
No empirical evidence indicates that medication improves conversion disorder. Anecdotal reports cite positive response to older antipsychotics, lithium, and electroconvulsive therapy.6
Patients with conversion disorder, however, tend to develop mood and/or anxiety symptoms later, and psychotropics may help treat these comorbidities. Follow the patient while symptoms are present.7 Comorbid symptoms’ severity, response, and presentation dictate follow-up frequency.
Prognosis. Children with conversion disorder generally have good outcomes,5 particularly those with good premorbid function who are diagnosed early.7 Time from symptom onset to diagnosis ranges from weeks to 1 year, and most cases resolve within 3 months of diagnosis. Symptom recurrence is rare but may indicate emerging polysymptomatic somatization disorder.5
Treatment: Reassurance and support
Miss T responded well to supportive psychotherapy and reassurance from hospital staff. No psychiatric screening tests were done, but child psychiatrists saw Miss T several times daily, and she exhibited no other psychiatric symptoms. We have no information on follow-up treatment, which occurred outside the hospital.
Related resources
- Academy of Psychosomatic Medicine. www.apm.org
- Weiner J, Dulcan M. Textbook of child and adolescent psychiatry (3rd ed). Washington, DC: American Psychiatric Publishing, 2003.
Presentation: 3 o’clock comes early
Miss T, age 9, presents with decreased sensation and motility in both legs. She cannot walk or stand.
Three days before, Miss T said she was consistently answering the teacher’s questions correctly at school. Because of this, a classmate teased her by calling her a “show off.” Soon after, Miss T began feeling weak and nauseous. The school nurse got a blood sugar reading of 68 mg/dL, slightly below the low-normal range.
Miss T’s mother arrived and convinced her to eat crackers and drink juice. Her blood sugar rose to 139 mg/dL and she began to feel better. When Miss T tried to stand, however, her legs and feet felt weak. She had trouble standing without support and needed help walking even a short distance. The mother brought Miss T home from school early.
Over the next 2 days, Miss T’s lower-extremity symptoms worsened. Her mother brought her to the pediatric emergency room.
Initial physical exam showed normal vital signs with no gross abnormalities. Neurologic exam revealed no lower-extremity masses, lesions, or deformities. Laboratory tests were normal. Cranial nerves were grossly intact. Right and left upper and lower extremities exhibited good tone, normal reflexes, and good strength against resistance. Miss T, however, said she could not feel sharp or dull objects against her lower legs.
ER pediatricians then called on the child psychiatry department to evaluate Miss T for possible psychiatric causes.
At intake, Miss T sits with her legs dangling from a stretcher. She is pleasant, articulate, and well-mannered. She spontaneously moves both legs and does not seem distressed when asked about her sudden disability. Her mood is euthymic, but she reports that constant teasing at school sometimes causes intense stress. She says that her sister sometimes “gets mean” with her but does not elaborate. She adds that she is sometimes sad because her parents recently separated, but she denies resultant emotional effects.
No suicidal/homicidal ideations or psychotic symptoms are present. Miss T’s thought process is logical and goal-directed. She is alert and oriented with good memory, language, concentration, and impulse control.
Neither Miss T nor her family has a significant psychiatric or medical history. Miss T has not been taking medications or over-the-counter supplements. Upon questioning, the mother denies that her daughter has been physically or sexually abused.
Despite a lack of positive neurologic findings, the neurology team recommends admission to rule out unseen medical problems.
The authors’ observations
Medical diagnosis.1,2 In Guillain-Barré syndrome, an infection usually precedes symptom onset, and maximum weakness is seen within 7 to 10 days. Respiration may be compromised, and weakness tends to spread throughout the body. Miss T’s symptoms came on more rapidly, her breathing was normal, and weakness was confined to her legs and feet.
In neuromuscular junction disorders such as myasthenia gravis and Lambert-Eaton syndrome, symptoms are not as acute, fluctuate throughout the day, and usually worsen with exertion. By contrast, Miss T’s symptoms steadily worsened without provocation.
Muscular dystrophy and myopathy were ruled out because of Miss T’s rapid symptom onset and lack of prior health problems. Dystrophy usually is seen in early childhood, affects the hip and girdle muscles, and progresses slowly. Myopathy symptoms usually are chronic and progressive, and associated medical disorders overshadow the muscle disease.
Patients with intracranial lesions may present with:
- symptoms of diffuse cerebral disease, such as mental impairment, headache, or seizures
- focal neurologic signs, such as aphasia or hemiparesis
- evidence of increased intracranial pressure, such as headache, vomiting, drowsiness, or papilledema.
Miss T had none of these.
Patients with spinal cord tumors usually have radicular pain, sensory/motor involvement, sphincteric dysfunction, and percussible back tenderness. Symptoms develop over weeks to months.
Psychiatric diagnosis. Factitious disorder, malingering, somatization disorder, and conversion disorder (Box) also were considered:
- In factitious disorder, the patient exacerbates his or her symptoms to assume the sick role.
- Malingering patients have external motivations behind symptom fabrication.
- Somatization disorder involves multiple organ systems, and patients often are preoccupied with their symptoms.
Miss T’s complaints were not consciously induced, external motivations were absent, a single organ system (musculoskeletal) was involved, and she appeared largely untroubled by her deficit.
Her presentation most closely fit the diagnosis of conversion disorder. Patients with this disorder complain of symptoms or deficits affecting voluntary muscles or of sensory function deficits that suggest a neurologic or medical condition. The symptoms’ temporal relationship to a stressful event suggests psychological factors. Symptoms:
- are not intentionally produced
- cannot be attributed to an organic cause
- cause significant functional impairment
- are not limited to pain or sexual dysfunction
- cannot be explained by another mental disorder.
Treatment: A miraculous recovery
The pediatrics, child psychiatry, pediatric neurology, and physical medicine/rehabilitation departments treated Miss T. No organic cause of her symptoms was found; results of an MRI with contrast, EEG, and repeated lab tests were negative.
On day 3, Miss T started taking small steps on her own. Two days later, she walked without assistance; discharge was considered.
The hospital’s social services department, however, discovered that the state child welfare agency had investigated Miss T’s family for alleged child abuse/neglect years before but found no evidence.
Also, a school social worker had recently visited Miss T’s family after receiving a complaint that an older sibling was allegedly hitting the younger ones. The social worker noticed that the kitchen door was padlocked; she speculated that the family was struggling financially and did not want the children to eat all the food. No other evidence of child maltreatment was found and the investigation was stopped. None of Miss T’s lab results indicated malnutrition.
The girl was discharged after the mother agreed to allow a home health aide to monitor the children’s well-being and a psychologist to perform neuropsychological tests on Miss T. Follow-up out-patient visits with the pediatric neurology, general pediatrics, and child psychiatry departments were also required.
Conversion disorder each year accounts for approximately 22 psychiatric cases per 100,000 overall.3 In the hospital setting, 5% to 14% of medical inpatient referrals for psychiatric evaluation result in conversion disorder diagnosis.3
Conversion disorder is seen in men and women but is more common in young women. Symptoms can occur at any age but are rare in children age < 7 and probably do not occur in children age < 4.3
Prevalence is higher in rural areas and among undereducated and low-income persons.3,4 Researchers also suggest that family history of conversion disorder contributes to symptom onset in offspring.4
The authors’ observations
Once we learned Miss T’s family had been investigated for child neglect, we had to find out if her symptoms were an expression of maltreatment or were caused by psychological stressors at school. Definitive maltreatment never surfaced, and school stress was determined to be a minor factor.
Neglected children exhibit characteristics at different ages that might contribute to conversion symptom development (Table). Most notably, such children have trouble understanding appropriate affective responses to interpersonal situations. As a result, they may express distress in unconventional ways.3
Table
Psychopathology of the neglected child
| Age | Developmental difficulties |
|---|---|
| 1 year | Insecure attachments |
| 2 years | Easily frustrated |
| 3 years | Low self-esteem/self-assertion Impaired flexibility, self-control compared with similarly aged healthy children Difficulty dealing with frustration Lack of persistence, enthusiasm when performing educational tasks |
| Preschool (4-6 years) | Overly dependent Lack of enthusiasm in preschool environment |
| Elementary school (6-12 years) | Attention problems Low self-assertion, self-esteem Withdrawal behaviors, dysphoric affect Social isolation |
| General | Trouble understanding appropriate affective responses to interpersonal situations Limited social problem-solving skills |
| Source: adapted from reference 4 | |
Little empirical evidence supports the link between childhood maltreatment and conversion disorder. In one study:5
- Adults with conversion disorder (mean age 37.6) reported a higher incidence of physical and sexual abuse, more types of physical abuse, sexual abuse of longer duration, and more-frequent incestuous episodes than did adults with affective disorder.
- Among patients with conversion disorder, having a mother with recurrent illness, nervousness or depression, or who abuses alcohol or sedatives was associated with higher dissociative and somatoform scale scores.
- Physical abuse was associated with increased conversion symptoms.
The authors concluded that childhood trauma is a distinct and predictive—though not necessary—feature of conversion disorder.5
The authors’ observations
Psychotherapy and attention to socio-cultural beliefs may enable the patient to “give up” the conversion symptom.3 Several factors determine choices of psychotherapies, although elements of all approaches are commonly used:
- CBT and behavioral therapy have roles in treating acute symptoms.
- Supportive therapy and hypnotherapy are recommended for treating rare, longstanding conversion symptoms (4 to 6 months duration).
- Psychodynamic therapy can help patients who are introspective, can remember details about their past, and are willing to participate in longer-term therapy.
Cognitive-behavioral therapy. Behavior is shaped by what we learn from the environment. Conversion behavior can be reinforced by others who help maintain the symptoms. Behavioral therapy and CBT are aimed at modifying behaviors via desensitization and by increasing the patient’s understanding of his or her physical capacities.
Hypnosis gives patients a medium to recall experiences and feelings they cannot consciously bring up in treatment. Symptom exploration and reduction are broad goals.
Psychodynamic therapy aims to resolve unconscious conflict after a traumatic event. A patient who develops lower-extremity paralysis or sensory problems after having been chastised for running away might benefit from this model, for example.
Supportive psychotherapy emphasizes reassurance and education. For Miss T, that would mean reassurance that her paralysis will improve, with education about conversion disorder and how difficult life events can cause similar symptoms.
Sociocultural considerations. Cultural beliefs inhibit some people’s emotions and may predispose them to conversion symptoms.
No empirical evidence indicates that medication improves conversion disorder. Anecdotal reports cite positive response to older antipsychotics, lithium, and electroconvulsive therapy.6
Patients with conversion disorder, however, tend to develop mood and/or anxiety symptoms later, and psychotropics may help treat these comorbidities. Follow the patient while symptoms are present.7 Comorbid symptoms’ severity, response, and presentation dictate follow-up frequency.
Prognosis. Children with conversion disorder generally have good outcomes,5 particularly those with good premorbid function who are diagnosed early.7 Time from symptom onset to diagnosis ranges from weeks to 1 year, and most cases resolve within 3 months of diagnosis. Symptom recurrence is rare but may indicate emerging polysymptomatic somatization disorder.5
Treatment: Reassurance and support
Miss T responded well to supportive psychotherapy and reassurance from hospital staff. No psychiatric screening tests were done, but child psychiatrists saw Miss T several times daily, and she exhibited no other psychiatric symptoms. We have no information on follow-up treatment, which occurred outside the hospital.
Related resources
- Academy of Psychosomatic Medicine. www.apm.org
- Weiner J, Dulcan M. Textbook of child and adolescent psychiatry (3rd ed). Washington, DC: American Psychiatric Publishing, 2003.
1. Fauci AS, Braunwald E, Hauser SL, et al. Harrison’s principles of medicine: companion handbook (14th ed). New York: McGraw Hill, 1999.
2. Adams R, Victor M. Companion to principles of neurology. New York: McGraw Hill, 1991.
3. Schwartz A, Calhoun A. Treatment of conversion disorder in an African-American Christian woman: cultural and social considerations. Am J Psychiatry 2001;158:1385-91.
4. Lewis M. Child and adolescent psychiatry—a comprehensive textbook (3rd ed). Baltimore: Lippincott Williams and Wilkins, 2002.
5. Roelofs K, Keijsers GP, Hoogduin KA, et al. Childhood abuse in patients with conversion disorder. Am J Psychiatry 2002;159:1908-13.
6. Hales R, Yudofsky S. Essentials of clinical psychiatry(2nd ed). Washington, DC: American Psychiatric Publishing, 2004.
7. Pehlivanturk B, Unal F. Conversion disorder in children and adolescents: a 4-year follow-up study. J Psychosom Res 2002;52:187-91.
1. Fauci AS, Braunwald E, Hauser SL, et al. Harrison’s principles of medicine: companion handbook (14th ed). New York: McGraw Hill, 1999.
2. Adams R, Victor M. Companion to principles of neurology. New York: McGraw Hill, 1991.
3. Schwartz A, Calhoun A. Treatment of conversion disorder in an African-American Christian woman: cultural and social considerations. Am J Psychiatry 2001;158:1385-91.
4. Lewis M. Child and adolescent psychiatry—a comprehensive textbook (3rd ed). Baltimore: Lippincott Williams and Wilkins, 2002.
5. Roelofs K, Keijsers GP, Hoogduin KA, et al. Childhood abuse in patients with conversion disorder. Am J Psychiatry 2002;159:1908-13.
6. Hales R, Yudofsky S. Essentials of clinical psychiatry(2nd ed). Washington, DC: American Psychiatric Publishing, 2004.
7. Pehlivanturk B, Unal F. Conversion disorder in children and adolescents: a 4-year follow-up study. J Psychosom Res 2002;52:187-91.
Is your patient’s dizziness psychogenic?
Dizziness is common among patients age 65 and older, and more than one-third have a psychiatric disorder that is caused by or is causing their dizziness.1
When older patients present with dizziness, psychiatrists may be asked to alleviate the psychological symptoms and help identify the underlying disease state.2
More than 60 medical and psychiatric disorders and many medications can cause dizziness. To help you sort through the possibilities, we offer:
- six diagnostic questions to rule out underlying medical problems
- lists of commonly used psychotropics and other drugs that may cause dizziness
- advice on treating depression, anxiety, and panic disorder in an older patient with dizziness while avoiding side effects and drug interactions.
Table 1
Four types of dizziness and their usual causes
| Vertigo Benign positional vertigo CNS cause—tumor, demyelination, neurodegenerative disorders Labyrinthitis Meniere’s disease Peripheral vestibulopathy (in 50% of cases) Vestibular neuronitis |
| Presyncope Arrhythmias Carotid sinus disease Hypoglycemia Neurocardiogenic syncope Organic heart disease Orthostatic hypotension Seizures Situational Transient ischemic attacks |
| Disequilibrium Balance and gait disorder Mixed CNS diseases (ischemic, degenerative) Neurodegenerative CNS disorders Presbystasis Sensorimotor dysfunction |
| Psychogenic lightheadedness Agoraphobia Anxiety Depression Panic disorder Hyperventilation |
| Source: Adapted from reference 6 |
Many causes of dizziness
The term “dizziness” is hard to define because of its nonspecific and variable symptom description, multiple causes, and lack of clear diagnostic and management guidelines. In clinical use, dizziness encompasses abnormal sensations relating to perception of the body’s relationship to space.
Some researchers believe dizziness is a distinct geriatric syndrome because numerous factors related to aging cause dizziness,2 including physiologic changes (presbystasis), accumulated impairment, disease states, and interactions between multiple medications.
Anxiety, somatization, panic disorder, and depression cause dizziness in the elderly, as do:
- peripheral vestibular disorders
- brainstem cerebrovascular accident
- diabetes mellitus
- neurologic disorders such as Parkinson’s disease
- and cardiovascular disorders.
Selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants also have been shown to cause dizziness, as have numerous nonpsychotropic agents.
Recognizing patterns, testing hypotheses, and extending the diagnostic process over time can help you differentiate psychogenic from medicationinduced or neurologic dizziness.3 Because the presentation is so complex and the differential diagnosis so broad, algorithmic diagnosis is less effective than a flexible clinical approach that allows for uncertainty in evaluating initial symptoms.
Determining the cause
A thorough patient history and physical examination can uncover a cause of dizziness in 75% of cases.4 Look for duration of dizziness symptoms; history of heart disease, diabetes or other illnesses; family history of psychiatric disorders; and other illnesses among family members.
Ask the following six complaint-specific questions to help you narrow the differential diagnosis and rule out nonpsychiatric causes.5
1. WHAT TYPE OF DIZZINESS DOES THE PATIENT HAVE?
Four categories—vertigo, presyncope, disequilibrium, and lightheadedness—are used to classify dizziness (Table 1).6
Vertigo is a sense that the body or environment is Patients may feel as if the floor is tilting, sinking, rising or veering sideways, or they may feel pulled to one side.
Vertigo is commonly caused by peripheral vestibular disorders—including benign positional vertigo, Meniere’s disease, labyrinthitis, and vestibular neuronitis—and central vestibular disorders associated with cerebrovascular disease, tumors, demyelinating diseases, migraines, seizures, multiple sclerosis and other CNS diseases. Acute-onset vertigo and neurologic signs suggest brainstem infarction.
Nystagmus is usually present, horizontal, and may be rotational at times. A vertical-beating nystagmus points to a probable CNS cause and requires urgent neuroimaging and referral to a neurologist or otolaryngologist.
Presyncope describes near-fainting. A dimming of vision and roaring in the ears may precede presyncope.
Depending on its cause, presyncope may occur regardless of position or only when upright. Common causes include orthostatic hypotension, neurocardiogenic syncope, organic heart disease, arrhythmias, carotid sinus disease, seizures, hypoglycemia, and transient ischemic attacks.
Abrupt presyncopal attacks that occur regardless of position suggest a cardiovascular cause. If onset is gradual and not improved by lying down, suspect a cerebral metabolic cause such as hypoglycemia.
Syncope, like presyncope, often is traced to an underlying cardiovascular disease. Dizziness and syncope often coexist, and both can be multifactorial. Dizziness may precede or follow syncopal episodes.
Differentiating syncope and dizziness is important because many underlying causes of syncope can be fatal. By contrast, dizziness symptoms are usually benign and self-limiting.7
A thorough history is critical to distinguishing dizziness from presyncope. Assess medication effects—especially CNS-acting medications, cardiovascular drugs, antihypertensives, antibiotics, and over-the-counter medications such as dextromethorphan and acetaminophen compounds. Also check for dehydration.
Disequilibrium disorder signifies unsteadiness or a loss of balance primarily involving the lower extremities. Symptoms are evoked by walking or standing and relieved by sitting or lying down. Gait is abnormal and balance is compromised without abnormal head sensations.
Common causes include balance and gait disorders, sensorimotor dysfunction, presbystasis, neurodegenerative CNS disorders, and mixed ischemic and degenerative CNS diseases.
Vague lightheadedness is often associated with somatic symptoms such as headache. Some patients describe a floating sensation.
Lightheadedness is frequently associated with anxiety, panic disorder, depression, and somatization. Hyperventilation and agoraphobia are other common causes.
Multiple symptoms, multiple types. Classifying an older patient’s dizziness can be challenging because many patients report symptoms that suggest two or more subtypes.2 Also, patients often have trouble describing their dizziness symptoms, sometimes using terms such as “giddiness,” “wooziness,” or “confusion.”
To help patients explain dizziness symptoms more accurately, ask specific questions such as:
- Do you at times feel like you’re about to faint?
- Do you feel as if the room is moving?
- Do you sometimes feel as though you’re going to fall?
Table 2
Psychotropics that may cause dizziness
| Anti-Alzheimer’s medications Memantine, rivastigmine, tacrine |
| Anticonvulsants Phenytoin |
| Antidepressants Monoamine oxidase inhibitors (phenelzine, selegiline) Selective serotonin reuptake inhibitors (all) Tricyclics (amitriptyline, imipramine, nortriptyline, trazodone) Others (bupropion, buspirone, mirtazapine, nefazodone, venlafaxine) |
| Antipsychotics Typicals (chlorpromazine, fluphenazine, perphenazine, prochlorperazine, thioridazine, trifluoperazine) Atypicals (all except olanzapine) |
| Anxiolytics Alprazolam, chlordiazepoxide, clonazepam, diazepam, lorazepam, oxazepam |
| Hypnotics Estazolam, flurazepam, quazepam, temazepam, triazolam, zolpidem |
| Mood stabilizers Carbamazepine, divalproex/valproic acid, gabapentin, lamotrigine, oxcarbazepine |
| Source: Clinical Pharmacology version 2.11. Tampa, FL: Gold Standard MultiMedia, 2004. |
2. HOW DO DIZZINESS SYMPTOMS RELATE TO POSITION OR MOTION?
By reproducing dizziness symptoms, some quick-maneuver tests can help patients describe their symptoms and may reveal a medical cause.
Dix-Hallpike maneuver.3 Move the patient rapidly from a seated to prone position with the head below the horizontal plane and turned 45 degrees for 10 seconds; then have the patient sit up. Repeat with the head turned to the other side. If dizziness does not occur within a few seconds after each test, rule out benign positional vertigo.
Seated head turn, or head-thrust test, measures qualitative vestibular function.8 Move the head rapidly by 45 degrees in a brief, small-amplitude thrust to one side while the patient focuses on your nose; this gauges vestibularocular control. Repeat the test in the other direction. A refixation corrective saccade, occurring as the patient tries to fixate on the target, indicates a possible vestibular disorder.
‘Get-Up and Go’ test, which takes less than 10 seconds, measures balance in older patents.9 Have the patient stand up, walk 10 feet, turn around, walk back, and sit down. Watch for staggering, unsteadiness, and use of hands to balance. Onset of symptoms suggests dizziness brought on during activities of daily living and provides information on how dizziness is affecting the patient’s ability to function.
Romberg test. Have the patient stand with heels together, first with eyes open and then closed. Vision and proprioceptive signals are used to compensate for vestibular loss. Thus, a balance disturbance with eyes closed suggests vestibular or spinal proprioceptive problems and may predict risk of falls caused by inability to compensate.8
3. WHAT IS THE COURSE OF DIZZINESS?
Differentiating acute, sudden-onset dizziness from chronic, gradual-onset dizziness can help uncover the problem’s cause and seriousness. The latter often has a psychological cause or may point to vestibular or minor cardiovascular problems. Tinetti et al2 identified anxiety or depressive symptoms as risk factors among community-based older persons who reported dizziness episodes lasting 1 month.
Table 3
Recommended SSRI starting dosages for older patients
| SSRI | Starting dosage (mg/d) | Maximum dosage (mg/d) |
|---|---|---|
| Citalopram | 10 to 20 | 30 |
| Escitalopram | 10 | 10 |
| Fluoxetine* | 5 to 10 | 60 |
| Paroxetine | 5 | 40 |
| Sertraline | 25 to 50 | 200 |
| * Most patients will not need more than 20 mg/d. Dosages 40 mg/d should be divided into twice-daily doses. | ||
| Source: Adapted from Reuben DB, Herr K, Pacala JT, et al. Geriatrics at your fingertips (5th ed). Malden, MA: Blackwell Publishing, 2003:47. | ||
An acute presentation can suggest a panic disorder or acute anxiety state, but first rule out serious conditions such as acute myocardial infarction, arrhythmias, acute infections, GI bleeding, and carbon monoxide poisoning.
Also ask about:
- exacerbating and relieving factors. For example, positional changes, exercise or other physical activity, eating, or missing a meal can trigger presyncope. Also find out about situations that may bring on anxiety, panic, or phobia. Onset of dizziness following these situations may suggest psychogenesis.
- recent falls and injuries. Recurrent falls with presyncope suggest a probable orthostatic or cardiovascular diagnosis in older adults.
4. ANY PAST MEDICAL PROBLEMS?
Ask disease-specific questions. For example:
- Tinnitus or hearing loss could point to a vestibular disorder.
- Metabolic and cardiovascular disorders such as diabetes, ischemic heart disease, postural hypotension, and seizures can result in presyncope.
- Orthostasis, coronary ischemic events, hypoglycemia, and transient ischemic attacks may cause dizziness.
5. IS DIZZINESS RECURRENT?
Panic disorder, anxiety disorders, phobia, and psychogenic hyperventilation are commonly associated with chronic, recurrent dizziness episodes.
6. WHAT MEDICATIONS IS THE PATIENT TAKING?
All psychotropics are suspect when a patient presents with dizziness. When dizziness occurs after a dose or start of therapy, evaluate response to the medication and consider reducing the dosage or changing the medication. If symptoms persist, refer the patient back to the primary care physician to investigate for other causes of dizziness.
Psychotropics that may cause dizziness are listed in Table 2, For a list of other medications associated with dizziness, see this article at www.currentpsychiatry.com.
If the above strategies do not reveal a physical cause of dizziness despite multiple physical complaints, consider examining the patient for depression, anxiety, or panic disorder.
Treating a psychiatric cause
If dizziness is found to be psychogenic and the symptoms impede daily activities or contribute to functional decline, treat the psychiatric disorder but carefully weigh the risks and benefits of drug treatment.
Although SSRIs may cause dizziness, these agents are recommended first-line treatment for depression, anxiety, and/or phobia in older patients with dizziness because of their relative lack of anticholinergic action and side effects compared with other antidepressants or anxiolytics.
Coexisting medical symptoms may dictate choice of agent. For example, consider a sedating SSRI for a patient with sleep disturbances caused by dizziness or the psychiatric disorder; choose a nonsedating SSRI if the patient is sleeping normally.
Because SSRIs may cause weight loss, avoid giving them to patients with weight loss associated with dizziness or an underlying psychiatric illness. Mirtazapine, which is associated with weight gain, may offset weight loss. Start mirtazapine at 15 mg at bedtime for older patients.
Start low and go slow when prescribing an SSRI to an older patient. Dosing strategies applicable to younger patients should not be extrapolated to older patients, especially those with dizziness.
We have found that older patients respond well to minimum or below-normal SSRI dosages (Table 3). Titrate very slowly and instruct patients to report dizziness. Reduce the dosage if dizziness emerges.
If the patient does not respond to an SSRI or mirtazapine, consider a serotonin and norepinephrine reuptake inhibitor, which also has favorable anticholinergic and side-effect profiles.
Related resources
- WebMD Health—Dizziness: lightheadedness and vertigo. http://my.webMD.com/hw/health_guide_atoz/hw88500.asp.
- Sloane PD. Clinical research and geriatric dizziness: The blind men and the elephant. J Am Geriatr Soc 1999;47:113-14.
- Kroenke K, Hoffman RM, Einstadter D. How common are various forms of dizziness? A critical review. South Med J 2000;93:160-7.
Drug brand names
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Carbamazepine • Tegretol
- Chlordiazepoxide • Librium
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clonazepam • Klonopin
- Diazepam • Valium
- Divalproex/valproic acid • Depakote
- Escitalopram • Lexapro
- Estazolam • ProSom
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Flurazepam • Dalmane
- Gabapentin • Neurontin
- Imipramine • Tofranil
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Memantine • Namenda
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Oxazepam • Serax
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Phenelzine • Nardil
- Phenytoin • Dilantin
- Prochlorperazine • Compazine
- Quazepam • Doral
- Rivastigmine • Exelon
- Selegiline • Eldepryl
- Sertraline • Zoloft
- Tacrine • Cognex
- Temazepam • Restoril
- Thioridazine • Mellaril
- Trazodone • Desyrel
- Triazolam • Halcion
- Trifluoperazine • Vesprin
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Robert Cluxton, PharmD, University of Cincinnati College of Pharmacy, for helping to prepare this manuscript for publication.
1. Sloane PD, Hartman M, Mitchell CM. Psychological factors associated with chronic dizziness in patients aged 60 and older. J Am Geriatr Soc 1994;42:847-52.
2. Tinetti ME, Williams CS, Gill TM. Dizziness among older adults: a possible geriatric syndrome. Ann Intern Med 2000;132:337-44.
3. Sloane PD, Coeytaux RR, Beck RS, Dallara J. Dizziness: state of the science. Ann Intern Med 2001;134(9 pt 2):823-32.
4. Hoffman RM, Einstadter D, Kroenke K. Evaluating dizziness. Am J Med 1999;107:468-78.
5. Drachman DA. A 69-year-old man with chronic dizziness. JAMA 1998;280:2111-18.
6. Drachman DA, Hart CW. An approach to the dizzy patient. Neurology 1972;22:323-34.
7. Kapoor WN. Syncope. N Engl J Med 2000;343:1856-62.
8. Baloh RW. Hearing and equilibrium. In: Goldman L, Ansiello D (eds). Cecil textbook of medicine (22nd ed). Philadelphia: Saunders 2004;2436-42.
9. Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil 1986;67:387-9.
Dizziness is common among patients age 65 and older, and more than one-third have a psychiatric disorder that is caused by or is causing their dizziness.1
When older patients present with dizziness, psychiatrists may be asked to alleviate the psychological symptoms and help identify the underlying disease state.2
More than 60 medical and psychiatric disorders and many medications can cause dizziness. To help you sort through the possibilities, we offer:
- six diagnostic questions to rule out underlying medical problems
- lists of commonly used psychotropics and other drugs that may cause dizziness
- advice on treating depression, anxiety, and panic disorder in an older patient with dizziness while avoiding side effects and drug interactions.
Table 1
Four types of dizziness and their usual causes
| Vertigo Benign positional vertigo CNS cause—tumor, demyelination, neurodegenerative disorders Labyrinthitis Meniere’s disease Peripheral vestibulopathy (in 50% of cases) Vestibular neuronitis |
| Presyncope Arrhythmias Carotid sinus disease Hypoglycemia Neurocardiogenic syncope Organic heart disease Orthostatic hypotension Seizures Situational Transient ischemic attacks |
| Disequilibrium Balance and gait disorder Mixed CNS diseases (ischemic, degenerative) Neurodegenerative CNS disorders Presbystasis Sensorimotor dysfunction |
| Psychogenic lightheadedness Agoraphobia Anxiety Depression Panic disorder Hyperventilation |
| Source: Adapted from reference 6 |
Many causes of dizziness
The term “dizziness” is hard to define because of its nonspecific and variable symptom description, multiple causes, and lack of clear diagnostic and management guidelines. In clinical use, dizziness encompasses abnormal sensations relating to perception of the body’s relationship to space.
Some researchers believe dizziness is a distinct geriatric syndrome because numerous factors related to aging cause dizziness,2 including physiologic changes (presbystasis), accumulated impairment, disease states, and interactions between multiple medications.
Anxiety, somatization, panic disorder, and depression cause dizziness in the elderly, as do:
- peripheral vestibular disorders
- brainstem cerebrovascular accident
- diabetes mellitus
- neurologic disorders such as Parkinson’s disease
- and cardiovascular disorders.
Selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants also have been shown to cause dizziness, as have numerous nonpsychotropic agents.
Recognizing patterns, testing hypotheses, and extending the diagnostic process over time can help you differentiate psychogenic from medicationinduced or neurologic dizziness.3 Because the presentation is so complex and the differential diagnosis so broad, algorithmic diagnosis is less effective than a flexible clinical approach that allows for uncertainty in evaluating initial symptoms.
Determining the cause
A thorough patient history and physical examination can uncover a cause of dizziness in 75% of cases.4 Look for duration of dizziness symptoms; history of heart disease, diabetes or other illnesses; family history of psychiatric disorders; and other illnesses among family members.
Ask the following six complaint-specific questions to help you narrow the differential diagnosis and rule out nonpsychiatric causes.5
1. WHAT TYPE OF DIZZINESS DOES THE PATIENT HAVE?
Four categories—vertigo, presyncope, disequilibrium, and lightheadedness—are used to classify dizziness (Table 1).6
Vertigo is a sense that the body or environment is Patients may feel as if the floor is tilting, sinking, rising or veering sideways, or they may feel pulled to one side.
Vertigo is commonly caused by peripheral vestibular disorders—including benign positional vertigo, Meniere’s disease, labyrinthitis, and vestibular neuronitis—and central vestibular disorders associated with cerebrovascular disease, tumors, demyelinating diseases, migraines, seizures, multiple sclerosis and other CNS diseases. Acute-onset vertigo and neurologic signs suggest brainstem infarction.
Nystagmus is usually present, horizontal, and may be rotational at times. A vertical-beating nystagmus points to a probable CNS cause and requires urgent neuroimaging and referral to a neurologist or otolaryngologist.
Presyncope describes near-fainting. A dimming of vision and roaring in the ears may precede presyncope.
Depending on its cause, presyncope may occur regardless of position or only when upright. Common causes include orthostatic hypotension, neurocardiogenic syncope, organic heart disease, arrhythmias, carotid sinus disease, seizures, hypoglycemia, and transient ischemic attacks.
Abrupt presyncopal attacks that occur regardless of position suggest a cardiovascular cause. If onset is gradual and not improved by lying down, suspect a cerebral metabolic cause such as hypoglycemia.
Syncope, like presyncope, often is traced to an underlying cardiovascular disease. Dizziness and syncope often coexist, and both can be multifactorial. Dizziness may precede or follow syncopal episodes.
Differentiating syncope and dizziness is important because many underlying causes of syncope can be fatal. By contrast, dizziness symptoms are usually benign and self-limiting.7
A thorough history is critical to distinguishing dizziness from presyncope. Assess medication effects—especially CNS-acting medications, cardiovascular drugs, antihypertensives, antibiotics, and over-the-counter medications such as dextromethorphan and acetaminophen compounds. Also check for dehydration.
Disequilibrium disorder signifies unsteadiness or a loss of balance primarily involving the lower extremities. Symptoms are evoked by walking or standing and relieved by sitting or lying down. Gait is abnormal and balance is compromised without abnormal head sensations.
Common causes include balance and gait disorders, sensorimotor dysfunction, presbystasis, neurodegenerative CNS disorders, and mixed ischemic and degenerative CNS diseases.
Vague lightheadedness is often associated with somatic symptoms such as headache. Some patients describe a floating sensation.
Lightheadedness is frequently associated with anxiety, panic disorder, depression, and somatization. Hyperventilation and agoraphobia are other common causes.
Multiple symptoms, multiple types. Classifying an older patient’s dizziness can be challenging because many patients report symptoms that suggest two or more subtypes.2 Also, patients often have trouble describing their dizziness symptoms, sometimes using terms such as “giddiness,” “wooziness,” or “confusion.”
To help patients explain dizziness symptoms more accurately, ask specific questions such as:
- Do you at times feel like you’re about to faint?
- Do you feel as if the room is moving?
- Do you sometimes feel as though you’re going to fall?
Table 2
Psychotropics that may cause dizziness
| Anti-Alzheimer’s medications Memantine, rivastigmine, tacrine |
| Anticonvulsants Phenytoin |
| Antidepressants Monoamine oxidase inhibitors (phenelzine, selegiline) Selective serotonin reuptake inhibitors (all) Tricyclics (amitriptyline, imipramine, nortriptyline, trazodone) Others (bupropion, buspirone, mirtazapine, nefazodone, venlafaxine) |
| Antipsychotics Typicals (chlorpromazine, fluphenazine, perphenazine, prochlorperazine, thioridazine, trifluoperazine) Atypicals (all except olanzapine) |
| Anxiolytics Alprazolam, chlordiazepoxide, clonazepam, diazepam, lorazepam, oxazepam |
| Hypnotics Estazolam, flurazepam, quazepam, temazepam, triazolam, zolpidem |
| Mood stabilizers Carbamazepine, divalproex/valproic acid, gabapentin, lamotrigine, oxcarbazepine |
| Source: Clinical Pharmacology version 2.11. Tampa, FL: Gold Standard MultiMedia, 2004. |
2. HOW DO DIZZINESS SYMPTOMS RELATE TO POSITION OR MOTION?
By reproducing dizziness symptoms, some quick-maneuver tests can help patients describe their symptoms and may reveal a medical cause.
Dix-Hallpike maneuver.3 Move the patient rapidly from a seated to prone position with the head below the horizontal plane and turned 45 degrees for 10 seconds; then have the patient sit up. Repeat with the head turned to the other side. If dizziness does not occur within a few seconds after each test, rule out benign positional vertigo.
Seated head turn, or head-thrust test, measures qualitative vestibular function.8 Move the head rapidly by 45 degrees in a brief, small-amplitude thrust to one side while the patient focuses on your nose; this gauges vestibularocular control. Repeat the test in the other direction. A refixation corrective saccade, occurring as the patient tries to fixate on the target, indicates a possible vestibular disorder.
‘Get-Up and Go’ test, which takes less than 10 seconds, measures balance in older patents.9 Have the patient stand up, walk 10 feet, turn around, walk back, and sit down. Watch for staggering, unsteadiness, and use of hands to balance. Onset of symptoms suggests dizziness brought on during activities of daily living and provides information on how dizziness is affecting the patient’s ability to function.
Romberg test. Have the patient stand with heels together, first with eyes open and then closed. Vision and proprioceptive signals are used to compensate for vestibular loss. Thus, a balance disturbance with eyes closed suggests vestibular or spinal proprioceptive problems and may predict risk of falls caused by inability to compensate.8
3. WHAT IS THE COURSE OF DIZZINESS?
Differentiating acute, sudden-onset dizziness from chronic, gradual-onset dizziness can help uncover the problem’s cause and seriousness. The latter often has a psychological cause or may point to vestibular or minor cardiovascular problems. Tinetti et al2 identified anxiety or depressive symptoms as risk factors among community-based older persons who reported dizziness episodes lasting 1 month.
Table 3
Recommended SSRI starting dosages for older patients
| SSRI | Starting dosage (mg/d) | Maximum dosage (mg/d) |
|---|---|---|
| Citalopram | 10 to 20 | 30 |
| Escitalopram | 10 | 10 |
| Fluoxetine* | 5 to 10 | 60 |
| Paroxetine | 5 | 40 |
| Sertraline | 25 to 50 | 200 |
| * Most patients will not need more than 20 mg/d. Dosages 40 mg/d should be divided into twice-daily doses. | ||
| Source: Adapted from Reuben DB, Herr K, Pacala JT, et al. Geriatrics at your fingertips (5th ed). Malden, MA: Blackwell Publishing, 2003:47. | ||
An acute presentation can suggest a panic disorder or acute anxiety state, but first rule out serious conditions such as acute myocardial infarction, arrhythmias, acute infections, GI bleeding, and carbon monoxide poisoning.
Also ask about:
- exacerbating and relieving factors. For example, positional changes, exercise or other physical activity, eating, or missing a meal can trigger presyncope. Also find out about situations that may bring on anxiety, panic, or phobia. Onset of dizziness following these situations may suggest psychogenesis.
- recent falls and injuries. Recurrent falls with presyncope suggest a probable orthostatic or cardiovascular diagnosis in older adults.
4. ANY PAST MEDICAL PROBLEMS?
Ask disease-specific questions. For example:
- Tinnitus or hearing loss could point to a vestibular disorder.
- Metabolic and cardiovascular disorders such as diabetes, ischemic heart disease, postural hypotension, and seizures can result in presyncope.
- Orthostasis, coronary ischemic events, hypoglycemia, and transient ischemic attacks may cause dizziness.
5. IS DIZZINESS RECURRENT?
Panic disorder, anxiety disorders, phobia, and psychogenic hyperventilation are commonly associated with chronic, recurrent dizziness episodes.
6. WHAT MEDICATIONS IS THE PATIENT TAKING?
All psychotropics are suspect when a patient presents with dizziness. When dizziness occurs after a dose or start of therapy, evaluate response to the medication and consider reducing the dosage or changing the medication. If symptoms persist, refer the patient back to the primary care physician to investigate for other causes of dizziness.
Psychotropics that may cause dizziness are listed in Table 2, For a list of other medications associated with dizziness, see this article at www.currentpsychiatry.com.
If the above strategies do not reveal a physical cause of dizziness despite multiple physical complaints, consider examining the patient for depression, anxiety, or panic disorder.
Treating a psychiatric cause
If dizziness is found to be psychogenic and the symptoms impede daily activities or contribute to functional decline, treat the psychiatric disorder but carefully weigh the risks and benefits of drug treatment.
Although SSRIs may cause dizziness, these agents are recommended first-line treatment for depression, anxiety, and/or phobia in older patients with dizziness because of their relative lack of anticholinergic action and side effects compared with other antidepressants or anxiolytics.
Coexisting medical symptoms may dictate choice of agent. For example, consider a sedating SSRI for a patient with sleep disturbances caused by dizziness or the psychiatric disorder; choose a nonsedating SSRI if the patient is sleeping normally.
Because SSRIs may cause weight loss, avoid giving them to patients with weight loss associated with dizziness or an underlying psychiatric illness. Mirtazapine, which is associated with weight gain, may offset weight loss. Start mirtazapine at 15 mg at bedtime for older patients.
Start low and go slow when prescribing an SSRI to an older patient. Dosing strategies applicable to younger patients should not be extrapolated to older patients, especially those with dizziness.
We have found that older patients respond well to minimum or below-normal SSRI dosages (Table 3). Titrate very slowly and instruct patients to report dizziness. Reduce the dosage if dizziness emerges.
If the patient does not respond to an SSRI or mirtazapine, consider a serotonin and norepinephrine reuptake inhibitor, which also has favorable anticholinergic and side-effect profiles.
Related resources
- WebMD Health—Dizziness: lightheadedness and vertigo. http://my.webMD.com/hw/health_guide_atoz/hw88500.asp.
- Sloane PD. Clinical research and geriatric dizziness: The blind men and the elephant. J Am Geriatr Soc 1999;47:113-14.
- Kroenke K, Hoffman RM, Einstadter D. How common are various forms of dizziness? A critical review. South Med J 2000;93:160-7.
Drug brand names
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Carbamazepine • Tegretol
- Chlordiazepoxide • Librium
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clonazepam • Klonopin
- Diazepam • Valium
- Divalproex/valproic acid • Depakote
- Escitalopram • Lexapro
- Estazolam • ProSom
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Flurazepam • Dalmane
- Gabapentin • Neurontin
- Imipramine • Tofranil
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Memantine • Namenda
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Oxazepam • Serax
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Phenelzine • Nardil
- Phenytoin • Dilantin
- Prochlorperazine • Compazine
- Quazepam • Doral
- Rivastigmine • Exelon
- Selegiline • Eldepryl
- Sertraline • Zoloft
- Tacrine • Cognex
- Temazepam • Restoril
- Thioridazine • Mellaril
- Trazodone • Desyrel
- Triazolam • Halcion
- Trifluoperazine • Vesprin
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Robert Cluxton, PharmD, University of Cincinnati College of Pharmacy, for helping to prepare this manuscript for publication.
Dizziness is common among patients age 65 and older, and more than one-third have a psychiatric disorder that is caused by or is causing their dizziness.1
When older patients present with dizziness, psychiatrists may be asked to alleviate the psychological symptoms and help identify the underlying disease state.2
More than 60 medical and psychiatric disorders and many medications can cause dizziness. To help you sort through the possibilities, we offer:
- six diagnostic questions to rule out underlying medical problems
- lists of commonly used psychotropics and other drugs that may cause dizziness
- advice on treating depression, anxiety, and panic disorder in an older patient with dizziness while avoiding side effects and drug interactions.
Table 1
Four types of dizziness and their usual causes
| Vertigo Benign positional vertigo CNS cause—tumor, demyelination, neurodegenerative disorders Labyrinthitis Meniere’s disease Peripheral vestibulopathy (in 50% of cases) Vestibular neuronitis |
| Presyncope Arrhythmias Carotid sinus disease Hypoglycemia Neurocardiogenic syncope Organic heart disease Orthostatic hypotension Seizures Situational Transient ischemic attacks |
| Disequilibrium Balance and gait disorder Mixed CNS diseases (ischemic, degenerative) Neurodegenerative CNS disorders Presbystasis Sensorimotor dysfunction |
| Psychogenic lightheadedness Agoraphobia Anxiety Depression Panic disorder Hyperventilation |
| Source: Adapted from reference 6 |
Many causes of dizziness
The term “dizziness” is hard to define because of its nonspecific and variable symptom description, multiple causes, and lack of clear diagnostic and management guidelines. In clinical use, dizziness encompasses abnormal sensations relating to perception of the body’s relationship to space.
Some researchers believe dizziness is a distinct geriatric syndrome because numerous factors related to aging cause dizziness,2 including physiologic changes (presbystasis), accumulated impairment, disease states, and interactions between multiple medications.
Anxiety, somatization, panic disorder, and depression cause dizziness in the elderly, as do:
- peripheral vestibular disorders
- brainstem cerebrovascular accident
- diabetes mellitus
- neurologic disorders such as Parkinson’s disease
- and cardiovascular disorders.
Selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants also have been shown to cause dizziness, as have numerous nonpsychotropic agents.
Recognizing patterns, testing hypotheses, and extending the diagnostic process over time can help you differentiate psychogenic from medicationinduced or neurologic dizziness.3 Because the presentation is so complex and the differential diagnosis so broad, algorithmic diagnosis is less effective than a flexible clinical approach that allows for uncertainty in evaluating initial symptoms.
Determining the cause
A thorough patient history and physical examination can uncover a cause of dizziness in 75% of cases.4 Look for duration of dizziness symptoms; history of heart disease, diabetes or other illnesses; family history of psychiatric disorders; and other illnesses among family members.
Ask the following six complaint-specific questions to help you narrow the differential diagnosis and rule out nonpsychiatric causes.5
1. WHAT TYPE OF DIZZINESS DOES THE PATIENT HAVE?
Four categories—vertigo, presyncope, disequilibrium, and lightheadedness—are used to classify dizziness (Table 1).6
Vertigo is a sense that the body or environment is Patients may feel as if the floor is tilting, sinking, rising or veering sideways, or they may feel pulled to one side.
Vertigo is commonly caused by peripheral vestibular disorders—including benign positional vertigo, Meniere’s disease, labyrinthitis, and vestibular neuronitis—and central vestibular disorders associated with cerebrovascular disease, tumors, demyelinating diseases, migraines, seizures, multiple sclerosis and other CNS diseases. Acute-onset vertigo and neurologic signs suggest brainstem infarction.
Nystagmus is usually present, horizontal, and may be rotational at times. A vertical-beating nystagmus points to a probable CNS cause and requires urgent neuroimaging and referral to a neurologist or otolaryngologist.
Presyncope describes near-fainting. A dimming of vision and roaring in the ears may precede presyncope.
Depending on its cause, presyncope may occur regardless of position or only when upright. Common causes include orthostatic hypotension, neurocardiogenic syncope, organic heart disease, arrhythmias, carotid sinus disease, seizures, hypoglycemia, and transient ischemic attacks.
Abrupt presyncopal attacks that occur regardless of position suggest a cardiovascular cause. If onset is gradual and not improved by lying down, suspect a cerebral metabolic cause such as hypoglycemia.
Syncope, like presyncope, often is traced to an underlying cardiovascular disease. Dizziness and syncope often coexist, and both can be multifactorial. Dizziness may precede or follow syncopal episodes.
Differentiating syncope and dizziness is important because many underlying causes of syncope can be fatal. By contrast, dizziness symptoms are usually benign and self-limiting.7
A thorough history is critical to distinguishing dizziness from presyncope. Assess medication effects—especially CNS-acting medications, cardiovascular drugs, antihypertensives, antibiotics, and over-the-counter medications such as dextromethorphan and acetaminophen compounds. Also check for dehydration.
Disequilibrium disorder signifies unsteadiness or a loss of balance primarily involving the lower extremities. Symptoms are evoked by walking or standing and relieved by sitting or lying down. Gait is abnormal and balance is compromised without abnormal head sensations.
Common causes include balance and gait disorders, sensorimotor dysfunction, presbystasis, neurodegenerative CNS disorders, and mixed ischemic and degenerative CNS diseases.
Vague lightheadedness is often associated with somatic symptoms such as headache. Some patients describe a floating sensation.
Lightheadedness is frequently associated with anxiety, panic disorder, depression, and somatization. Hyperventilation and agoraphobia are other common causes.
Multiple symptoms, multiple types. Classifying an older patient’s dizziness can be challenging because many patients report symptoms that suggest two or more subtypes.2 Also, patients often have trouble describing their dizziness symptoms, sometimes using terms such as “giddiness,” “wooziness,” or “confusion.”
To help patients explain dizziness symptoms more accurately, ask specific questions such as:
- Do you at times feel like you’re about to faint?
- Do you feel as if the room is moving?
- Do you sometimes feel as though you’re going to fall?
Table 2
Psychotropics that may cause dizziness
| Anti-Alzheimer’s medications Memantine, rivastigmine, tacrine |
| Anticonvulsants Phenytoin |
| Antidepressants Monoamine oxidase inhibitors (phenelzine, selegiline) Selective serotonin reuptake inhibitors (all) Tricyclics (amitriptyline, imipramine, nortriptyline, trazodone) Others (bupropion, buspirone, mirtazapine, nefazodone, venlafaxine) |
| Antipsychotics Typicals (chlorpromazine, fluphenazine, perphenazine, prochlorperazine, thioridazine, trifluoperazine) Atypicals (all except olanzapine) |
| Anxiolytics Alprazolam, chlordiazepoxide, clonazepam, diazepam, lorazepam, oxazepam |
| Hypnotics Estazolam, flurazepam, quazepam, temazepam, triazolam, zolpidem |
| Mood stabilizers Carbamazepine, divalproex/valproic acid, gabapentin, lamotrigine, oxcarbazepine |
| Source: Clinical Pharmacology version 2.11. Tampa, FL: Gold Standard MultiMedia, 2004. |
2. HOW DO DIZZINESS SYMPTOMS RELATE TO POSITION OR MOTION?
By reproducing dizziness symptoms, some quick-maneuver tests can help patients describe their symptoms and may reveal a medical cause.
Dix-Hallpike maneuver.3 Move the patient rapidly from a seated to prone position with the head below the horizontal plane and turned 45 degrees for 10 seconds; then have the patient sit up. Repeat with the head turned to the other side. If dizziness does not occur within a few seconds after each test, rule out benign positional vertigo.
Seated head turn, or head-thrust test, measures qualitative vestibular function.8 Move the head rapidly by 45 degrees in a brief, small-amplitude thrust to one side while the patient focuses on your nose; this gauges vestibularocular control. Repeat the test in the other direction. A refixation corrective saccade, occurring as the patient tries to fixate on the target, indicates a possible vestibular disorder.
‘Get-Up and Go’ test, which takes less than 10 seconds, measures balance in older patents.9 Have the patient stand up, walk 10 feet, turn around, walk back, and sit down. Watch for staggering, unsteadiness, and use of hands to balance. Onset of symptoms suggests dizziness brought on during activities of daily living and provides information on how dizziness is affecting the patient’s ability to function.
Romberg test. Have the patient stand with heels together, first with eyes open and then closed. Vision and proprioceptive signals are used to compensate for vestibular loss. Thus, a balance disturbance with eyes closed suggests vestibular or spinal proprioceptive problems and may predict risk of falls caused by inability to compensate.8
3. WHAT IS THE COURSE OF DIZZINESS?
Differentiating acute, sudden-onset dizziness from chronic, gradual-onset dizziness can help uncover the problem’s cause and seriousness. The latter often has a psychological cause or may point to vestibular or minor cardiovascular problems. Tinetti et al2 identified anxiety or depressive symptoms as risk factors among community-based older persons who reported dizziness episodes lasting 1 month.
Table 3
Recommended SSRI starting dosages for older patients
| SSRI | Starting dosage (mg/d) | Maximum dosage (mg/d) |
|---|---|---|
| Citalopram | 10 to 20 | 30 |
| Escitalopram | 10 | 10 |
| Fluoxetine* | 5 to 10 | 60 |
| Paroxetine | 5 | 40 |
| Sertraline | 25 to 50 | 200 |
| * Most patients will not need more than 20 mg/d. Dosages 40 mg/d should be divided into twice-daily doses. | ||
| Source: Adapted from Reuben DB, Herr K, Pacala JT, et al. Geriatrics at your fingertips (5th ed). Malden, MA: Blackwell Publishing, 2003:47. | ||
An acute presentation can suggest a panic disorder or acute anxiety state, but first rule out serious conditions such as acute myocardial infarction, arrhythmias, acute infections, GI bleeding, and carbon monoxide poisoning.
Also ask about:
- exacerbating and relieving factors. For example, positional changes, exercise or other physical activity, eating, or missing a meal can trigger presyncope. Also find out about situations that may bring on anxiety, panic, or phobia. Onset of dizziness following these situations may suggest psychogenesis.
- recent falls and injuries. Recurrent falls with presyncope suggest a probable orthostatic or cardiovascular diagnosis in older adults.
4. ANY PAST MEDICAL PROBLEMS?
Ask disease-specific questions. For example:
- Tinnitus or hearing loss could point to a vestibular disorder.
- Metabolic and cardiovascular disorders such as diabetes, ischemic heart disease, postural hypotension, and seizures can result in presyncope.
- Orthostasis, coronary ischemic events, hypoglycemia, and transient ischemic attacks may cause dizziness.
5. IS DIZZINESS RECURRENT?
Panic disorder, anxiety disorders, phobia, and psychogenic hyperventilation are commonly associated with chronic, recurrent dizziness episodes.
6. WHAT MEDICATIONS IS THE PATIENT TAKING?
All psychotropics are suspect when a patient presents with dizziness. When dizziness occurs after a dose or start of therapy, evaluate response to the medication and consider reducing the dosage or changing the medication. If symptoms persist, refer the patient back to the primary care physician to investigate for other causes of dizziness.
Psychotropics that may cause dizziness are listed in Table 2, For a list of other medications associated with dizziness, see this article at www.currentpsychiatry.com.
If the above strategies do not reveal a physical cause of dizziness despite multiple physical complaints, consider examining the patient for depression, anxiety, or panic disorder.
Treating a psychiatric cause
If dizziness is found to be psychogenic and the symptoms impede daily activities or contribute to functional decline, treat the psychiatric disorder but carefully weigh the risks and benefits of drug treatment.
Although SSRIs may cause dizziness, these agents are recommended first-line treatment for depression, anxiety, and/or phobia in older patients with dizziness because of their relative lack of anticholinergic action and side effects compared with other antidepressants or anxiolytics.
Coexisting medical symptoms may dictate choice of agent. For example, consider a sedating SSRI for a patient with sleep disturbances caused by dizziness or the psychiatric disorder; choose a nonsedating SSRI if the patient is sleeping normally.
Because SSRIs may cause weight loss, avoid giving them to patients with weight loss associated with dizziness or an underlying psychiatric illness. Mirtazapine, which is associated with weight gain, may offset weight loss. Start mirtazapine at 15 mg at bedtime for older patients.
Start low and go slow when prescribing an SSRI to an older patient. Dosing strategies applicable to younger patients should not be extrapolated to older patients, especially those with dizziness.
We have found that older patients respond well to minimum or below-normal SSRI dosages (Table 3). Titrate very slowly and instruct patients to report dizziness. Reduce the dosage if dizziness emerges.
If the patient does not respond to an SSRI or mirtazapine, consider a serotonin and norepinephrine reuptake inhibitor, which also has favorable anticholinergic and side-effect profiles.
Related resources
- WebMD Health—Dizziness: lightheadedness and vertigo. http://my.webMD.com/hw/health_guide_atoz/hw88500.asp.
- Sloane PD. Clinical research and geriatric dizziness: The blind men and the elephant. J Am Geriatr Soc 1999;47:113-14.
- Kroenke K, Hoffman RM, Einstadter D. How common are various forms of dizziness? A critical review. South Med J 2000;93:160-7.
Drug brand names
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Carbamazepine • Tegretol
- Chlordiazepoxide • Librium
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clonazepam • Klonopin
- Diazepam • Valium
- Divalproex/valproic acid • Depakote
- Escitalopram • Lexapro
- Estazolam • ProSom
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Flurazepam • Dalmane
- Gabapentin • Neurontin
- Imipramine • Tofranil
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Memantine • Namenda
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Oxazepam • Serax
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Phenelzine • Nardil
- Phenytoin • Dilantin
- Prochlorperazine • Compazine
- Quazepam • Doral
- Rivastigmine • Exelon
- Selegiline • Eldepryl
- Sertraline • Zoloft
- Tacrine • Cognex
- Temazepam • Restoril
- Thioridazine • Mellaril
- Trazodone • Desyrel
- Triazolam • Halcion
- Trifluoperazine • Vesprin
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Robert Cluxton, PharmD, University of Cincinnati College of Pharmacy, for helping to prepare this manuscript for publication.
1. Sloane PD, Hartman M, Mitchell CM. Psychological factors associated with chronic dizziness in patients aged 60 and older. J Am Geriatr Soc 1994;42:847-52.
2. Tinetti ME, Williams CS, Gill TM. Dizziness among older adults: a possible geriatric syndrome. Ann Intern Med 2000;132:337-44.
3. Sloane PD, Coeytaux RR, Beck RS, Dallara J. Dizziness: state of the science. Ann Intern Med 2001;134(9 pt 2):823-32.
4. Hoffman RM, Einstadter D, Kroenke K. Evaluating dizziness. Am J Med 1999;107:468-78.
5. Drachman DA. A 69-year-old man with chronic dizziness. JAMA 1998;280:2111-18.
6. Drachman DA, Hart CW. An approach to the dizzy patient. Neurology 1972;22:323-34.
7. Kapoor WN. Syncope. N Engl J Med 2000;343:1856-62.
8. Baloh RW. Hearing and equilibrium. In: Goldman L, Ansiello D (eds). Cecil textbook of medicine (22nd ed). Philadelphia: Saunders 2004;2436-42.
9. Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil 1986;67:387-9.
1. Sloane PD, Hartman M, Mitchell CM. Psychological factors associated with chronic dizziness in patients aged 60 and older. J Am Geriatr Soc 1994;42:847-52.
2. Tinetti ME, Williams CS, Gill TM. Dizziness among older adults: a possible geriatric syndrome. Ann Intern Med 2000;132:337-44.
3. Sloane PD, Coeytaux RR, Beck RS, Dallara J. Dizziness: state of the science. Ann Intern Med 2001;134(9 pt 2):823-32.
4. Hoffman RM, Einstadter D, Kroenke K. Evaluating dizziness. Am J Med 1999;107:468-78.
5. Drachman DA. A 69-year-old man with chronic dizziness. JAMA 1998;280:2111-18.
6. Drachman DA, Hart CW. An approach to the dizzy patient. Neurology 1972;22:323-34.
7. Kapoor WN. Syncope. N Engl J Med 2000;343:1856-62.
8. Baloh RW. Hearing and equilibrium. In: Goldman L, Ansiello D (eds). Cecil textbook of medicine (22nd ed). Philadelphia: Saunders 2004;2436-42.
9. Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil 1986;67:387-9.
Post-stroke depression: Rapid action helps restore lost function
Patients with post-stroke depression (PSD) pose many clinical dilemmas: Is their depression a psychological reaction or a biological event? Are antidepressants effective for either type? Should antidepressants be given prophylactically after a stroke, even if the patient is not depressed?
Although the answers are not clear, this article describes a practical approach to stroke patients referred for psychiatric evaluation, including:
- strategies to distinguish reactive from endogenous depression
- issues that guide antidepressant selection
- benefits and risks of using medication to prevent depression after an acute stroke.
Reactive or endogenous depression?
Each year 500,000 to 600,000 Americans suffer strokes.1 Depression is the most common emotional sequela, reported in up to 40% of survivors within several months of an acute stroke.
Figure Possible mechanism for endogenous post-stroke depression
Anterior cerebrovascular lesions may block serotonergic and noradrenergic projections into the superficial cortex. The closer the lesion to the nuceli, the greater the pathway interruption, and the more severe the depression may be. Drawing represents nuclei in the brainstem, slightly enlarged, with their projections greatly simplified.
Source: Reference 8
Illustration for Current Psychiatry by Marcia Hartsock, CMIPSD is characterized as reactive (related to physical and psychosocial losses of stroke) or endogenous (a biologic consequence of stroke).
Reactive depression. Patients exhibit a constellation of emotional symptoms while attempting to cope with a new physical or cognitive deficit. This “catastrophic reaction”2 includes anxiety, crying, aggressive behavior, cursing, refusal, displacement, renouncement, and sometimes compensatory boasting.
In 62 stroke patients evaluated with the Catastrophic Reaction Scale:
- approximately 20% had a catastrophic reaction
- the reaction was significantly associated with major depression.
Anterior subcortical damage may be the common mechanism underlying both catastrophic reaction and major depression in stroke patients.3
Post-stroke emotional lability resembles PSD. This “pathologic emotion” or “emotional incontinence” can manifest as sudden, frequent, easily-provoked episodes of crying that are generally mood-congruent. Affected patients may also respond to nonemotional events with outbursts of pathologic crying or laughing.
The pathogenesis of post-stroke emotional lability is unclear, although biogenic amines may play a role. In a 6-week, double-blind trial, 28 patients with post-stroke pathologic laughter and crying were treated with nortriptyline or placebo. Symptoms improved significantly more with nortriptyline in both depressed and nondepressed patients, indicating that the response was not related simply to improved depressive symptoms.4
Endogenous depression. Robinson et al5 propose a neuroanatomic PSD model. They contend that major—but not minor—PSD correlates with the stroke lesion’s proximity to the left anterior frontal pole or underlying basal ganglia. Other investigators, however, question this anatomic distinction between major and minor PSD.
For example, Gainotti et al6 used their own Post-Stroke Depression Rating Scale to compare stroke patients without depression, with minor depression, or with major depressive disorder (MDD) and a group of nonstroke patients with functional MDD. They found that:
- the phenomenology of patients with major PSD was more similar to that of patients with minor PSD than to that of patients with functional major depression
- major and minor PSD were much more likely to be associated with reactive depression than with the endogenous form.
Other researchers disputed the neuroanatomic model after failing to confirm a correlation between PSD and the location of lesions in the left hemisphere.7
Most recently, a meta-analysis by Narushima et al8 suggested a moderately strong correlation between depressive symptom severity and the distance between the anterior border of a left-hemispheric lesion and the frontal pole during the first 6 months following a stroke. This group hypothesizes that more-anterior lesions interrupt the brain’s serotonergic and noradrenergic pathways (Figure) at a site closer to their origin—before they branch posteriorly into the superficial cortex. This interruption presumably increases serotonin and noradrenergic depletion, which is manifest as depression.
A common denominator? Two other recent studies suggest that depression may be a significant independent risk factor for stroke:
- A prospective study has assessed stroke risk factors in 2,800 Australians since 1988. Depression has been a significant stroke risk factor in men and women ages 70 and older.9
- A population-based study showed that depression predicted stroke across all strata in a cohort of 6,000 men and women ages 25 to 74. Subjects were stroke-free at enrollment and followed for up to 22 years.10
Table 1
How to evaluate a patient for post-stroke depression (PSD)
|
These observations—plus the fact that depression is a common sequela of stroke—suggest that stroke and depression may result from a common pathophysiology. Some evidence suggests that depression is a cerebrovascular disease. For example, stroke and depression are both associated with increased platelet reactivity (stickiness). However, platelet reactivity does not appear greater in patients who develop depression after a stroke than in those who do not.11
Table 2
Diagnostic criteria for mood disorder due to stroke*
|
| Specify type: |
| With depressive features: if the predominant mood is depressed but the full criteria are not met for a major depressive episode. |
| With major-depressive-like episode: if the full criteria are met (except criterion D) for a major depressive episode. |
| *DSM-IV-TR diagnostic criteria for mood disorder due to a general medical condition |
| Source: Adapted and reprinted with permission from the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision. Copyright 2000. American Psychiatric Association. |
A study of antidepressants’ effects on platelet dysfunction found that the selective serotonin reuptake inhibitors (SSRIs) paroxetine and sertraline may reverse platelet abnormalities, whereas the tricyclic nortriptyline does not.12
Summary. A biopsychosocial model helps explain the pathogenesis PSD, which may present as:
- a syndrome similar to MDD
- a relatively minor depression similar to dysthymic disorder
- a discrete phenomenon such as catastrophic reaction and emotional lability.
Evaluating patients for PSD
In the absence of PSD diagnostic guidelines, Table 1 provides a clinically useful approach. A comprehensive workup may suggest whether an individual’s depressive symptoms are endogenous or reactive, although this distinction may be subtle. Gathering information from other physicians, the patient, family, and caregivers may require more than one session.
Diagnostic criteria. Consult DSM-IV criteria for “mood disorder due to general medical condition” as they pertain to patients “with depressive features” (minor depression) or “with major depression-like episodes” (Table 2). In the initial evaluation, do not rely exclusively on instruments such as the Hamilton Depression Rating Scale for measuring depression, as patients with emotional lability may not meet the cut-off scores for depression. These scales may help monitor progress later during therapy.
Risk factors. Interview the patient, family, and caregivers to determine if the patient has possible risk factors for PSD:
- history of stroke
- personal or family history of depression
- loss of social activities
- major life event within 6 months of stroke
- cognitive impairment 1 month post-stroke.
Determine whether the patient’s stroke was precipitated by cocaine or other drug abuse. If so, address the cause.
Medical comorbidities. Consider the effect of medical comorbidities such as Parkinson’s disease, heart disease, or diabetes on the patient’s mood. Also rule out other metabolic causes of depression such as thyroid abnormalities, medication side effects, and vitamin B12 deficiency.
Table 3
Treating post-stroke depression with antidepressants
| TCAs | SSRIs | |
|---|---|---|
| Efficacy | Proven in double-blind, placebo-controlled trials One study found nortriptyline more effective than fluoxetine | Greater anxiolytic effect than TCAs (?) Overall less efficacious than TCAs (?) |
| Side effects | Alpha-adrenergic blockade, anticholinergic, antihistaminic, and cardiac effects | Drug interactions related to cytochrome P-450 isoenzyme inhibition Fluoxetine may prolong bleeding time |
| Overdose risk | Potentially fatal | Safe in overdose |
| Onset of action | Slower than SSRIs (?) | More rapid than TCAs (?) |
| Cost | Less expensive than SSRIs | More expensive than TCAs |
| (?) Not supported by controlled clinical trials | ||
| TCAs: Tricyclic antidepressants | ||
| SSRIs: Selective serotonin reuptake inhibitors | ||
Cognitive changes. Quantify any cognitive deficits with neuropsychological testing, such as the Mini-Mental State Examination. Also learn all you can about the patient’s premorbid personality for comparison with post-stroke behavior. Ask the nursing or rehabilitation staff about inpatients’ motivation and participation in care.
Social support. Determine if caregivers can provide transportation, medication monitoring, and other social support.
Treatment options
PSD calls for rapid, comprehensive treatment with antidepressants, psychotherapy, and help reintegrating into the community. Untreated PSD is associated with increased morbidity and mortality, whereas effective treatment improves functional outcomes.13
Antidepressants. Tricyclic antidepressants (desipramine, imipramine, and nortriptyline), SSRIs (citalopram and fluoxetine), and trazodone have been used to treat depression in stroke patients (Table 3) 14 Controlled studies suggest that:
- >60% of patients with PSD respond to medication
- they tolerate antidepressants well
- no antidepressant class has a distinct therapeutic advantage over others.
Antidepressants in other classes—such as venlafaxine, bupropion, and mirtazapine—have not been studied in patients with PSD.
Without strong evidence to guide the initial antidepressant choice, a pragmatic approach is to start with one or two agents with which you are most familiar. Consider side effects, potential drug-drug interactions, cost, and available formulations. A patient with post-stroke swallowing difficulties, for example, may benefit from a liquid form.
We do not have good data regarding the optimum starting dose and duration of therapy for any antidepressants in PSD. To minimize side effects, I recommend starting with low dosages, such as:
- fluoxetine, 10 mg/d
- sertraline, 25 to 50 mg/d
- nortriptyline, 10 to 25 mg/d.
Increase dosages gradually, watching for side effects and symptom improvement.
If treatment is effective, continue the antidepressant for at least 1 year. In patients with a history of depression, continue treatment longer to prevent depressive relapse.
Small, double-blind, controlled trials have used citalopram, fluoxetine, nortriptyline, or sertraline to treat post-stroke emotional lability.14 Compared with placebo, these agents all significantly reduced post-stroke emotionalism.
Psychostimulants such as methylphenidate and dextroamphetamine might be an effective alternative to antidepressants.15 They have a morerapid onset of action, better tolerability, and may be more effective in alleviating post-stroke apathy. Disadvantages include risks for tolerance, dependence, and psychiatric side effects.
As with antidepressants, start low and go slow to minimize side effects. You could start methylphenidate at 5 mg in the morning and increase to 20 to 30 mg/d before you decide—based on response—to continue or discontinue. After the dosage is stabilized, you could switch to a controlled-release formulation.
ECT. Although no controlled trials of electroconvulsive therapy for PSD have been reported, ECT can be an effective option for patients with treatment-resistant depression. Some retrospective studies16 have shown a good response among patients with PSD, although ECT may worsen stroke-related cognitive deficits.
rTMS. University of Iowa researchers recently completed a double-blind controlled trial evaluating the efficacy of repetitive transcranial magnetic stimulation (rTMS) in PSD. R.G. Robinson, MD (personal communication, 2003), reported that preliminary results are encouraging.
Psychotherapy. Cognitive-behavioral therapy—used alone patients or combined with medication—has yielded good results in some PSD studies. Controlled trials17,18 have shown that individual counseling, occupational therapy, leisure activities, and social work improve all aspects of PSD except mood.
Can be prevented?
Many patients who are not depressed during an initial post-stroke evaluation develop depression within 2 years.19 This raises the question: Can PSD be prevented by treating stroke patients prophylactically?
In a 12-week, randomized, double-blind trial,20 48 nondepressed post-stroke patients were treated with nortriptyline, fluoxetine, or placebo. Dosages were:
- nortriptyline—25 mg/d in week 1; 50 mg/d in weeks 2 and 3; 75 mg/d in weeks 4 to 6, and 100 mg/d in weeks 7 to 12
- fluoxetine—10 mg/d in weeks 1 to 3; 20 mg/d in weeks 4 to 6; 30 mg/d in weeks 7 to 9, and 40 mg/d in weeks 10 to 12.
After 3 months, the two antidepressants appeared comparable in efficacy and significantly more effective than placebo in preventing depression. Patients who had taken nortriptyline were more likely to develop depression during the subsequent 6 months than were patients in the other two groups, and their symptoms were more severe. However, the authors noted many study limitations, including small sample size and loss of some patients to follow-up.
In a randomized, double-blind study, prophylaxis with mianserin (not available in the United States) did not prevent post-stroke depression.21 For 1 year, 100 patients presenting with acute ischemic stroke received mianserin, 60 mg/d, or placebo. Monitoring at 2, 6, 12, and 18 months found no differences between the two groups with respect to PSD onset.
Recommendation. As with most aspects of PSD treatment, the issue of prophylaxis remains unsettled. I do not routinely start antidepressants in nondepressed stroke patients because evidence of benefit is lacking and any added medications increase the risk of side effects and drug-drug interactions.
Related resources
- National Institute of Neurological Disorders and Stroke. Stroke information page www.ninds.nih.gov/health_and_medical/disorders/stroke.htm
- Robinson RG. The clinical neuropsychiatry of stroke: cognitive, behavioral, and emotional disorders following vascular brain injury. New York: Cambridge University Press, 1998.
Drug brand names
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrin
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Methylphenidate • Concerta, Ritalin
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Trazodone • Desyrel
- Venlafaxine • Effexor
Disclosure
Dr. Fozdar is a speaker for Eli Lilly and Company.
1. Heart and stroke statistical update. Dallas: American Heart Association, 2002.
2. Goldstein K. The organism:a holistic approach to biology derived from pathological data in man. New York: American Book Co, 1939.
3. Starkstein SE, Fedoroff JP, Price TR, et al. Catastrophic reaction after cerebrovascular lesions: frequency, correlates, and validation of a scale. J Neuropsychiatry Clin Neurosci 1993;5(2):189-94.
4. Robinson RG, Parikh RM, Lipsey JR, et al. Pathological laughing and crying following stroke: Validation of measurement scale and double-blind treatment study. Am J Psychiatry 1993;150:286-93.
5. Robinson RG, Kubos KL, Starr LB, et al. Mood changes in stroke patients: relationship to lesion location. Compr Psychiatry 1983;24(6):555-66.
6. Gainotti G, Azzoni A, Razzano C, et al. The Post-Stroke Depression Rating Scale: a test specifically devised to investigate affective disorders of stroke patients. J Clin Exp Neuropsychol 1997;19(3):340-56.
7. Carson AJ, MacHale S, Allen K, et al. Depression after stroke and lesion location: a systematic review. Lancet 2000;356(9224):122-6.
8. Narushima K, Kosier JT, Robinson RG. A reappraisal of poststroke depression, intra- and inter-hemispheric lesion location using meta-analysis. J Neuropsychiatry Clin Neurosci 2003;15(4):422-30.
9. Simons LA, McCallum J, Friedlander Y, Simons J. Risk factors for ischemic stroke: Dubbo Study of the elderly. Stroke 1998;29(7):1341-6.
10. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med 2000;62(4):463-71.
11. Cassidy EM, Walsh MT, O’Connor R, et al. Platelet surface glycoprotein expression in post-stroke depression: a preliminary study. Psychiatry Res 2003;118(2):175-81.
12. Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol 2000;20(2):137-40.
13. Everson SA, Roberts RE, Goldberg DE, Kaplan GA. Depressive symptoms and increased risk of stroke mortality over a 29-year period. Arch Intern Med 1998;158(10):1133-8.
14. Turner-Stokes L, Hassan N. Depression after stroke: a review of the evidence base to inform the development of an integrated care pathway. Part 2: Treatment alternatives. Clin Rehabil 2002;16(3):248-60.
15. Whyte EM, Mulsant BH. Post-stroke depression: epidemiology, pathophysiology, and biologic treatment. Biol Psychiatry 2002;52:253-64.
16. Currier M, Murray G, Welsh C. Electroconvulsive therapy for poststroke depressed geriatric patients. J Neuropsychiatry Clin Neurosci 1992;4(2):140-4.
17. Kemp BJ, Corgiat M, Gill C. Effects of brief cognitive behavioral group psychotherapy in older persons with and without disabling illness. Behav Health Ageing 1992;2:21-8.
18. Lincoln NB, Flannaghan T, Sutcliffe L, Rother L. Evaluation of cognitive behavioural treatment for depression after stroke: a pilot study. Clin Rehabil 1997;11(2):114-22.
19. Astrom M, Adolfsson R, Asplund K. Major depression in stroke patients: a 13-year longitudinal study. Stroke 1993;24:976-82.
20. Narushima K, Kosier JT, Robinson RG. Preventing poststroke depression: a 12-week double-blind randomized treatment trial and 21-month follow-up. J Nerv Ment Dis 2002;190(5):296-303.
21. Palomaki H, Kaste M, Berg A, et al. Prevention of poststroke depression: 1 year randomised placebo controlled double blind trial of mianserin with 6 month follow up after therapy. J Neurol Neurosurg Psychiatry 1999;66(4):490-4.
Patients with post-stroke depression (PSD) pose many clinical dilemmas: Is their depression a psychological reaction or a biological event? Are antidepressants effective for either type? Should antidepressants be given prophylactically after a stroke, even if the patient is not depressed?
Although the answers are not clear, this article describes a practical approach to stroke patients referred for psychiatric evaluation, including:
- strategies to distinguish reactive from endogenous depression
- issues that guide antidepressant selection
- benefits and risks of using medication to prevent depression after an acute stroke.
Reactive or endogenous depression?
Each year 500,000 to 600,000 Americans suffer strokes.1 Depression is the most common emotional sequela, reported in up to 40% of survivors within several months of an acute stroke.
Figure Possible mechanism for endogenous post-stroke depression
Anterior cerebrovascular lesions may block serotonergic and noradrenergic projections into the superficial cortex. The closer the lesion to the nuceli, the greater the pathway interruption, and the more severe the depression may be. Drawing represents nuclei in the brainstem, slightly enlarged, with their projections greatly simplified.
Source: Reference 8
Illustration for Current Psychiatry by Marcia Hartsock, CMIPSD is characterized as reactive (related to physical and psychosocial losses of stroke) or endogenous (a biologic consequence of stroke).
Reactive depression. Patients exhibit a constellation of emotional symptoms while attempting to cope with a new physical or cognitive deficit. This “catastrophic reaction”2 includes anxiety, crying, aggressive behavior, cursing, refusal, displacement, renouncement, and sometimes compensatory boasting.
In 62 stroke patients evaluated with the Catastrophic Reaction Scale:
- approximately 20% had a catastrophic reaction
- the reaction was significantly associated with major depression.
Anterior subcortical damage may be the common mechanism underlying both catastrophic reaction and major depression in stroke patients.3
Post-stroke emotional lability resembles PSD. This “pathologic emotion” or “emotional incontinence” can manifest as sudden, frequent, easily-provoked episodes of crying that are generally mood-congruent. Affected patients may also respond to nonemotional events with outbursts of pathologic crying or laughing.
The pathogenesis of post-stroke emotional lability is unclear, although biogenic amines may play a role. In a 6-week, double-blind trial, 28 patients with post-stroke pathologic laughter and crying were treated with nortriptyline or placebo. Symptoms improved significantly more with nortriptyline in both depressed and nondepressed patients, indicating that the response was not related simply to improved depressive symptoms.4
Endogenous depression. Robinson et al5 propose a neuroanatomic PSD model. They contend that major—but not minor—PSD correlates with the stroke lesion’s proximity to the left anterior frontal pole or underlying basal ganglia. Other investigators, however, question this anatomic distinction between major and minor PSD.
For example, Gainotti et al6 used their own Post-Stroke Depression Rating Scale to compare stroke patients without depression, with minor depression, or with major depressive disorder (MDD) and a group of nonstroke patients with functional MDD. They found that:
- the phenomenology of patients with major PSD was more similar to that of patients with minor PSD than to that of patients with functional major depression
- major and minor PSD were much more likely to be associated with reactive depression than with the endogenous form.
Other researchers disputed the neuroanatomic model after failing to confirm a correlation between PSD and the location of lesions in the left hemisphere.7
Most recently, a meta-analysis by Narushima et al8 suggested a moderately strong correlation between depressive symptom severity and the distance between the anterior border of a left-hemispheric lesion and the frontal pole during the first 6 months following a stroke. This group hypothesizes that more-anterior lesions interrupt the brain’s serotonergic and noradrenergic pathways (Figure) at a site closer to their origin—before they branch posteriorly into the superficial cortex. This interruption presumably increases serotonin and noradrenergic depletion, which is manifest as depression.
A common denominator? Two other recent studies suggest that depression may be a significant independent risk factor for stroke:
- A prospective study has assessed stroke risk factors in 2,800 Australians since 1988. Depression has been a significant stroke risk factor in men and women ages 70 and older.9
- A population-based study showed that depression predicted stroke across all strata in a cohort of 6,000 men and women ages 25 to 74. Subjects were stroke-free at enrollment and followed for up to 22 years.10
Table 1
How to evaluate a patient for post-stroke depression (PSD)
|
These observations—plus the fact that depression is a common sequela of stroke—suggest that stroke and depression may result from a common pathophysiology. Some evidence suggests that depression is a cerebrovascular disease. For example, stroke and depression are both associated with increased platelet reactivity (stickiness). However, platelet reactivity does not appear greater in patients who develop depression after a stroke than in those who do not.11
Table 2
Diagnostic criteria for mood disorder due to stroke*
|
| Specify type: |
| With depressive features: if the predominant mood is depressed but the full criteria are not met for a major depressive episode. |
| With major-depressive-like episode: if the full criteria are met (except criterion D) for a major depressive episode. |
| *DSM-IV-TR diagnostic criteria for mood disorder due to a general medical condition |
| Source: Adapted and reprinted with permission from the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision. Copyright 2000. American Psychiatric Association. |
A study of antidepressants’ effects on platelet dysfunction found that the selective serotonin reuptake inhibitors (SSRIs) paroxetine and sertraline may reverse platelet abnormalities, whereas the tricyclic nortriptyline does not.12
Summary. A biopsychosocial model helps explain the pathogenesis PSD, which may present as:
- a syndrome similar to MDD
- a relatively minor depression similar to dysthymic disorder
- a discrete phenomenon such as catastrophic reaction and emotional lability.
Evaluating patients for PSD
In the absence of PSD diagnostic guidelines, Table 1 provides a clinically useful approach. A comprehensive workup may suggest whether an individual’s depressive symptoms are endogenous or reactive, although this distinction may be subtle. Gathering information from other physicians, the patient, family, and caregivers may require more than one session.
Diagnostic criteria. Consult DSM-IV criteria for “mood disorder due to general medical condition” as they pertain to patients “with depressive features” (minor depression) or “with major depression-like episodes” (Table 2). In the initial evaluation, do not rely exclusively on instruments such as the Hamilton Depression Rating Scale for measuring depression, as patients with emotional lability may not meet the cut-off scores for depression. These scales may help monitor progress later during therapy.
Risk factors. Interview the patient, family, and caregivers to determine if the patient has possible risk factors for PSD:
- history of stroke
- personal or family history of depression
- loss of social activities
- major life event within 6 months of stroke
- cognitive impairment 1 month post-stroke.
Determine whether the patient’s stroke was precipitated by cocaine or other drug abuse. If so, address the cause.
Medical comorbidities. Consider the effect of medical comorbidities such as Parkinson’s disease, heart disease, or diabetes on the patient’s mood. Also rule out other metabolic causes of depression such as thyroid abnormalities, medication side effects, and vitamin B12 deficiency.
Table 3
Treating post-stroke depression with antidepressants
| TCAs | SSRIs | |
|---|---|---|
| Efficacy | Proven in double-blind, placebo-controlled trials One study found nortriptyline more effective than fluoxetine | Greater anxiolytic effect than TCAs (?) Overall less efficacious than TCAs (?) |
| Side effects | Alpha-adrenergic blockade, anticholinergic, antihistaminic, and cardiac effects | Drug interactions related to cytochrome P-450 isoenzyme inhibition Fluoxetine may prolong bleeding time |
| Overdose risk | Potentially fatal | Safe in overdose |
| Onset of action | Slower than SSRIs (?) | More rapid than TCAs (?) |
| Cost | Less expensive than SSRIs | More expensive than TCAs |
| (?) Not supported by controlled clinical trials | ||
| TCAs: Tricyclic antidepressants | ||
| SSRIs: Selective serotonin reuptake inhibitors | ||
Cognitive changes. Quantify any cognitive deficits with neuropsychological testing, such as the Mini-Mental State Examination. Also learn all you can about the patient’s premorbid personality for comparison with post-stroke behavior. Ask the nursing or rehabilitation staff about inpatients’ motivation and participation in care.
Social support. Determine if caregivers can provide transportation, medication monitoring, and other social support.
Treatment options
PSD calls for rapid, comprehensive treatment with antidepressants, psychotherapy, and help reintegrating into the community. Untreated PSD is associated with increased morbidity and mortality, whereas effective treatment improves functional outcomes.13
Antidepressants. Tricyclic antidepressants (desipramine, imipramine, and nortriptyline), SSRIs (citalopram and fluoxetine), and trazodone have been used to treat depression in stroke patients (Table 3) 14 Controlled studies suggest that:
- >60% of patients with PSD respond to medication
- they tolerate antidepressants well
- no antidepressant class has a distinct therapeutic advantage over others.
Antidepressants in other classes—such as venlafaxine, bupropion, and mirtazapine—have not been studied in patients with PSD.
Without strong evidence to guide the initial antidepressant choice, a pragmatic approach is to start with one or two agents with which you are most familiar. Consider side effects, potential drug-drug interactions, cost, and available formulations. A patient with post-stroke swallowing difficulties, for example, may benefit from a liquid form.
We do not have good data regarding the optimum starting dose and duration of therapy for any antidepressants in PSD. To minimize side effects, I recommend starting with low dosages, such as:
- fluoxetine, 10 mg/d
- sertraline, 25 to 50 mg/d
- nortriptyline, 10 to 25 mg/d.
Increase dosages gradually, watching for side effects and symptom improvement.
If treatment is effective, continue the antidepressant for at least 1 year. In patients with a history of depression, continue treatment longer to prevent depressive relapse.
Small, double-blind, controlled trials have used citalopram, fluoxetine, nortriptyline, or sertraline to treat post-stroke emotional lability.14 Compared with placebo, these agents all significantly reduced post-stroke emotionalism.
Psychostimulants such as methylphenidate and dextroamphetamine might be an effective alternative to antidepressants.15 They have a morerapid onset of action, better tolerability, and may be more effective in alleviating post-stroke apathy. Disadvantages include risks for tolerance, dependence, and psychiatric side effects.
As with antidepressants, start low and go slow to minimize side effects. You could start methylphenidate at 5 mg in the morning and increase to 20 to 30 mg/d before you decide—based on response—to continue or discontinue. After the dosage is stabilized, you could switch to a controlled-release formulation.
ECT. Although no controlled trials of electroconvulsive therapy for PSD have been reported, ECT can be an effective option for patients with treatment-resistant depression. Some retrospective studies16 have shown a good response among patients with PSD, although ECT may worsen stroke-related cognitive deficits.
rTMS. University of Iowa researchers recently completed a double-blind controlled trial evaluating the efficacy of repetitive transcranial magnetic stimulation (rTMS) in PSD. R.G. Robinson, MD (personal communication, 2003), reported that preliminary results are encouraging.
Psychotherapy. Cognitive-behavioral therapy—used alone patients or combined with medication—has yielded good results in some PSD studies. Controlled trials17,18 have shown that individual counseling, occupational therapy, leisure activities, and social work improve all aspects of PSD except mood.
Can be prevented?
Many patients who are not depressed during an initial post-stroke evaluation develop depression within 2 years.19 This raises the question: Can PSD be prevented by treating stroke patients prophylactically?
In a 12-week, randomized, double-blind trial,20 48 nondepressed post-stroke patients were treated with nortriptyline, fluoxetine, or placebo. Dosages were:
- nortriptyline—25 mg/d in week 1; 50 mg/d in weeks 2 and 3; 75 mg/d in weeks 4 to 6, and 100 mg/d in weeks 7 to 12
- fluoxetine—10 mg/d in weeks 1 to 3; 20 mg/d in weeks 4 to 6; 30 mg/d in weeks 7 to 9, and 40 mg/d in weeks 10 to 12.
After 3 months, the two antidepressants appeared comparable in efficacy and significantly more effective than placebo in preventing depression. Patients who had taken nortriptyline were more likely to develop depression during the subsequent 6 months than were patients in the other two groups, and their symptoms were more severe. However, the authors noted many study limitations, including small sample size and loss of some patients to follow-up.
In a randomized, double-blind study, prophylaxis with mianserin (not available in the United States) did not prevent post-stroke depression.21 For 1 year, 100 patients presenting with acute ischemic stroke received mianserin, 60 mg/d, or placebo. Monitoring at 2, 6, 12, and 18 months found no differences between the two groups with respect to PSD onset.
Recommendation. As with most aspects of PSD treatment, the issue of prophylaxis remains unsettled. I do not routinely start antidepressants in nondepressed stroke patients because evidence of benefit is lacking and any added medications increase the risk of side effects and drug-drug interactions.
Related resources
- National Institute of Neurological Disorders and Stroke. Stroke information page www.ninds.nih.gov/health_and_medical/disorders/stroke.htm
- Robinson RG. The clinical neuropsychiatry of stroke: cognitive, behavioral, and emotional disorders following vascular brain injury. New York: Cambridge University Press, 1998.
Drug brand names
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrin
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Methylphenidate • Concerta, Ritalin
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Trazodone • Desyrel
- Venlafaxine • Effexor
Disclosure
Dr. Fozdar is a speaker for Eli Lilly and Company.
Patients with post-stroke depression (PSD) pose many clinical dilemmas: Is their depression a psychological reaction or a biological event? Are antidepressants effective for either type? Should antidepressants be given prophylactically after a stroke, even if the patient is not depressed?
Although the answers are not clear, this article describes a practical approach to stroke patients referred for psychiatric evaluation, including:
- strategies to distinguish reactive from endogenous depression
- issues that guide antidepressant selection
- benefits and risks of using medication to prevent depression after an acute stroke.
Reactive or endogenous depression?
Each year 500,000 to 600,000 Americans suffer strokes.1 Depression is the most common emotional sequela, reported in up to 40% of survivors within several months of an acute stroke.
Figure Possible mechanism for endogenous post-stroke depression
Anterior cerebrovascular lesions may block serotonergic and noradrenergic projections into the superficial cortex. The closer the lesion to the nuceli, the greater the pathway interruption, and the more severe the depression may be. Drawing represents nuclei in the brainstem, slightly enlarged, with their projections greatly simplified.
Source: Reference 8
Illustration for Current Psychiatry by Marcia Hartsock, CMIPSD is characterized as reactive (related to physical and psychosocial losses of stroke) or endogenous (a biologic consequence of stroke).
Reactive depression. Patients exhibit a constellation of emotional symptoms while attempting to cope with a new physical or cognitive deficit. This “catastrophic reaction”2 includes anxiety, crying, aggressive behavior, cursing, refusal, displacement, renouncement, and sometimes compensatory boasting.
In 62 stroke patients evaluated with the Catastrophic Reaction Scale:
- approximately 20% had a catastrophic reaction
- the reaction was significantly associated with major depression.
Anterior subcortical damage may be the common mechanism underlying both catastrophic reaction and major depression in stroke patients.3
Post-stroke emotional lability resembles PSD. This “pathologic emotion” or “emotional incontinence” can manifest as sudden, frequent, easily-provoked episodes of crying that are generally mood-congruent. Affected patients may also respond to nonemotional events with outbursts of pathologic crying or laughing.
The pathogenesis of post-stroke emotional lability is unclear, although biogenic amines may play a role. In a 6-week, double-blind trial, 28 patients with post-stroke pathologic laughter and crying were treated with nortriptyline or placebo. Symptoms improved significantly more with nortriptyline in both depressed and nondepressed patients, indicating that the response was not related simply to improved depressive symptoms.4
Endogenous depression. Robinson et al5 propose a neuroanatomic PSD model. They contend that major—but not minor—PSD correlates with the stroke lesion’s proximity to the left anterior frontal pole or underlying basal ganglia. Other investigators, however, question this anatomic distinction between major and minor PSD.
For example, Gainotti et al6 used their own Post-Stroke Depression Rating Scale to compare stroke patients without depression, with minor depression, or with major depressive disorder (MDD) and a group of nonstroke patients with functional MDD. They found that:
- the phenomenology of patients with major PSD was more similar to that of patients with minor PSD than to that of patients with functional major depression
- major and minor PSD were much more likely to be associated with reactive depression than with the endogenous form.
Other researchers disputed the neuroanatomic model after failing to confirm a correlation between PSD and the location of lesions in the left hemisphere.7
Most recently, a meta-analysis by Narushima et al8 suggested a moderately strong correlation between depressive symptom severity and the distance between the anterior border of a left-hemispheric lesion and the frontal pole during the first 6 months following a stroke. This group hypothesizes that more-anterior lesions interrupt the brain’s serotonergic and noradrenergic pathways (Figure) at a site closer to their origin—before they branch posteriorly into the superficial cortex. This interruption presumably increases serotonin and noradrenergic depletion, which is manifest as depression.
A common denominator? Two other recent studies suggest that depression may be a significant independent risk factor for stroke:
- A prospective study has assessed stroke risk factors in 2,800 Australians since 1988. Depression has been a significant stroke risk factor in men and women ages 70 and older.9
- A population-based study showed that depression predicted stroke across all strata in a cohort of 6,000 men and women ages 25 to 74. Subjects were stroke-free at enrollment and followed for up to 22 years.10
Table 1
How to evaluate a patient for post-stroke depression (PSD)
|
These observations—plus the fact that depression is a common sequela of stroke—suggest that stroke and depression may result from a common pathophysiology. Some evidence suggests that depression is a cerebrovascular disease. For example, stroke and depression are both associated with increased platelet reactivity (stickiness). However, platelet reactivity does not appear greater in patients who develop depression after a stroke than in those who do not.11
Table 2
Diagnostic criteria for mood disorder due to stroke*
|
| Specify type: |
| With depressive features: if the predominant mood is depressed but the full criteria are not met for a major depressive episode. |
| With major-depressive-like episode: if the full criteria are met (except criterion D) for a major depressive episode. |
| *DSM-IV-TR diagnostic criteria for mood disorder due to a general medical condition |
| Source: Adapted and reprinted with permission from the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision. Copyright 2000. American Psychiatric Association. |
A study of antidepressants’ effects on platelet dysfunction found that the selective serotonin reuptake inhibitors (SSRIs) paroxetine and sertraline may reverse platelet abnormalities, whereas the tricyclic nortriptyline does not.12
Summary. A biopsychosocial model helps explain the pathogenesis PSD, which may present as:
- a syndrome similar to MDD
- a relatively minor depression similar to dysthymic disorder
- a discrete phenomenon such as catastrophic reaction and emotional lability.
Evaluating patients for PSD
In the absence of PSD diagnostic guidelines, Table 1 provides a clinically useful approach. A comprehensive workup may suggest whether an individual’s depressive symptoms are endogenous or reactive, although this distinction may be subtle. Gathering information from other physicians, the patient, family, and caregivers may require more than one session.
Diagnostic criteria. Consult DSM-IV criteria for “mood disorder due to general medical condition” as they pertain to patients “with depressive features” (minor depression) or “with major depression-like episodes” (Table 2). In the initial evaluation, do not rely exclusively on instruments such as the Hamilton Depression Rating Scale for measuring depression, as patients with emotional lability may not meet the cut-off scores for depression. These scales may help monitor progress later during therapy.
Risk factors. Interview the patient, family, and caregivers to determine if the patient has possible risk factors for PSD:
- history of stroke
- personal or family history of depression
- loss of social activities
- major life event within 6 months of stroke
- cognitive impairment 1 month post-stroke.
Determine whether the patient’s stroke was precipitated by cocaine or other drug abuse. If so, address the cause.
Medical comorbidities. Consider the effect of medical comorbidities such as Parkinson’s disease, heart disease, or diabetes on the patient’s mood. Also rule out other metabolic causes of depression such as thyroid abnormalities, medication side effects, and vitamin B12 deficiency.
Table 3
Treating post-stroke depression with antidepressants
| TCAs | SSRIs | |
|---|---|---|
| Efficacy | Proven in double-blind, placebo-controlled trials One study found nortriptyline more effective than fluoxetine | Greater anxiolytic effect than TCAs (?) Overall less efficacious than TCAs (?) |
| Side effects | Alpha-adrenergic blockade, anticholinergic, antihistaminic, and cardiac effects | Drug interactions related to cytochrome P-450 isoenzyme inhibition Fluoxetine may prolong bleeding time |
| Overdose risk | Potentially fatal | Safe in overdose |
| Onset of action | Slower than SSRIs (?) | More rapid than TCAs (?) |
| Cost | Less expensive than SSRIs | More expensive than TCAs |
| (?) Not supported by controlled clinical trials | ||
| TCAs: Tricyclic antidepressants | ||
| SSRIs: Selective serotonin reuptake inhibitors | ||
Cognitive changes. Quantify any cognitive deficits with neuropsychological testing, such as the Mini-Mental State Examination. Also learn all you can about the patient’s premorbid personality for comparison with post-stroke behavior. Ask the nursing or rehabilitation staff about inpatients’ motivation and participation in care.
Social support. Determine if caregivers can provide transportation, medication monitoring, and other social support.
Treatment options
PSD calls for rapid, comprehensive treatment with antidepressants, psychotherapy, and help reintegrating into the community. Untreated PSD is associated with increased morbidity and mortality, whereas effective treatment improves functional outcomes.13
Antidepressants. Tricyclic antidepressants (desipramine, imipramine, and nortriptyline), SSRIs (citalopram and fluoxetine), and trazodone have been used to treat depression in stroke patients (Table 3) 14 Controlled studies suggest that:
- >60% of patients with PSD respond to medication
- they tolerate antidepressants well
- no antidepressant class has a distinct therapeutic advantage over others.
Antidepressants in other classes—such as venlafaxine, bupropion, and mirtazapine—have not been studied in patients with PSD.
Without strong evidence to guide the initial antidepressant choice, a pragmatic approach is to start with one or two agents with which you are most familiar. Consider side effects, potential drug-drug interactions, cost, and available formulations. A patient with post-stroke swallowing difficulties, for example, may benefit from a liquid form.
We do not have good data regarding the optimum starting dose and duration of therapy for any antidepressants in PSD. To minimize side effects, I recommend starting with low dosages, such as:
- fluoxetine, 10 mg/d
- sertraline, 25 to 50 mg/d
- nortriptyline, 10 to 25 mg/d.
Increase dosages gradually, watching for side effects and symptom improvement.
If treatment is effective, continue the antidepressant for at least 1 year. In patients with a history of depression, continue treatment longer to prevent depressive relapse.
Small, double-blind, controlled trials have used citalopram, fluoxetine, nortriptyline, or sertraline to treat post-stroke emotional lability.14 Compared with placebo, these agents all significantly reduced post-stroke emotionalism.
Psychostimulants such as methylphenidate and dextroamphetamine might be an effective alternative to antidepressants.15 They have a morerapid onset of action, better tolerability, and may be more effective in alleviating post-stroke apathy. Disadvantages include risks for tolerance, dependence, and psychiatric side effects.
As with antidepressants, start low and go slow to minimize side effects. You could start methylphenidate at 5 mg in the morning and increase to 20 to 30 mg/d before you decide—based on response—to continue or discontinue. After the dosage is stabilized, you could switch to a controlled-release formulation.
ECT. Although no controlled trials of electroconvulsive therapy for PSD have been reported, ECT can be an effective option for patients with treatment-resistant depression. Some retrospective studies16 have shown a good response among patients with PSD, although ECT may worsen stroke-related cognitive deficits.
rTMS. University of Iowa researchers recently completed a double-blind controlled trial evaluating the efficacy of repetitive transcranial magnetic stimulation (rTMS) in PSD. R.G. Robinson, MD (personal communication, 2003), reported that preliminary results are encouraging.
Psychotherapy. Cognitive-behavioral therapy—used alone patients or combined with medication—has yielded good results in some PSD studies. Controlled trials17,18 have shown that individual counseling, occupational therapy, leisure activities, and social work improve all aspects of PSD except mood.
Can be prevented?
Many patients who are not depressed during an initial post-stroke evaluation develop depression within 2 years.19 This raises the question: Can PSD be prevented by treating stroke patients prophylactically?
In a 12-week, randomized, double-blind trial,20 48 nondepressed post-stroke patients were treated with nortriptyline, fluoxetine, or placebo. Dosages were:
- nortriptyline—25 mg/d in week 1; 50 mg/d in weeks 2 and 3; 75 mg/d in weeks 4 to 6, and 100 mg/d in weeks 7 to 12
- fluoxetine—10 mg/d in weeks 1 to 3; 20 mg/d in weeks 4 to 6; 30 mg/d in weeks 7 to 9, and 40 mg/d in weeks 10 to 12.
After 3 months, the two antidepressants appeared comparable in efficacy and significantly more effective than placebo in preventing depression. Patients who had taken nortriptyline were more likely to develop depression during the subsequent 6 months than were patients in the other two groups, and their symptoms were more severe. However, the authors noted many study limitations, including small sample size and loss of some patients to follow-up.
In a randomized, double-blind study, prophylaxis with mianserin (not available in the United States) did not prevent post-stroke depression.21 For 1 year, 100 patients presenting with acute ischemic stroke received mianserin, 60 mg/d, or placebo. Monitoring at 2, 6, 12, and 18 months found no differences between the two groups with respect to PSD onset.
Recommendation. As with most aspects of PSD treatment, the issue of prophylaxis remains unsettled. I do not routinely start antidepressants in nondepressed stroke patients because evidence of benefit is lacking and any added medications increase the risk of side effects and drug-drug interactions.
Related resources
- National Institute of Neurological Disorders and Stroke. Stroke information page www.ninds.nih.gov/health_and_medical/disorders/stroke.htm
- Robinson RG. The clinical neuropsychiatry of stroke: cognitive, behavioral, and emotional disorders following vascular brain injury. New York: Cambridge University Press, 1998.
Drug brand names
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrin
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Methylphenidate • Concerta, Ritalin
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Trazodone • Desyrel
- Venlafaxine • Effexor
Disclosure
Dr. Fozdar is a speaker for Eli Lilly and Company.
1. Heart and stroke statistical update. Dallas: American Heart Association, 2002.
2. Goldstein K. The organism:a holistic approach to biology derived from pathological data in man. New York: American Book Co, 1939.
3. Starkstein SE, Fedoroff JP, Price TR, et al. Catastrophic reaction after cerebrovascular lesions: frequency, correlates, and validation of a scale. J Neuropsychiatry Clin Neurosci 1993;5(2):189-94.
4. Robinson RG, Parikh RM, Lipsey JR, et al. Pathological laughing and crying following stroke: Validation of measurement scale and double-blind treatment study. Am J Psychiatry 1993;150:286-93.
5. Robinson RG, Kubos KL, Starr LB, et al. Mood changes in stroke patients: relationship to lesion location. Compr Psychiatry 1983;24(6):555-66.
6. Gainotti G, Azzoni A, Razzano C, et al. The Post-Stroke Depression Rating Scale: a test specifically devised to investigate affective disorders of stroke patients. J Clin Exp Neuropsychol 1997;19(3):340-56.
7. Carson AJ, MacHale S, Allen K, et al. Depression after stroke and lesion location: a systematic review. Lancet 2000;356(9224):122-6.
8. Narushima K, Kosier JT, Robinson RG. A reappraisal of poststroke depression, intra- and inter-hemispheric lesion location using meta-analysis. J Neuropsychiatry Clin Neurosci 2003;15(4):422-30.
9. Simons LA, McCallum J, Friedlander Y, Simons J. Risk factors for ischemic stroke: Dubbo Study of the elderly. Stroke 1998;29(7):1341-6.
10. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med 2000;62(4):463-71.
11. Cassidy EM, Walsh MT, O’Connor R, et al. Platelet surface glycoprotein expression in post-stroke depression: a preliminary study. Psychiatry Res 2003;118(2):175-81.
12. Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol 2000;20(2):137-40.
13. Everson SA, Roberts RE, Goldberg DE, Kaplan GA. Depressive symptoms and increased risk of stroke mortality over a 29-year period. Arch Intern Med 1998;158(10):1133-8.
14. Turner-Stokes L, Hassan N. Depression after stroke: a review of the evidence base to inform the development of an integrated care pathway. Part 2: Treatment alternatives. Clin Rehabil 2002;16(3):248-60.
15. Whyte EM, Mulsant BH. Post-stroke depression: epidemiology, pathophysiology, and biologic treatment. Biol Psychiatry 2002;52:253-64.
16. Currier M, Murray G, Welsh C. Electroconvulsive therapy for poststroke depressed geriatric patients. J Neuropsychiatry Clin Neurosci 1992;4(2):140-4.
17. Kemp BJ, Corgiat M, Gill C. Effects of brief cognitive behavioral group psychotherapy in older persons with and without disabling illness. Behav Health Ageing 1992;2:21-8.
18. Lincoln NB, Flannaghan T, Sutcliffe L, Rother L. Evaluation of cognitive behavioural treatment for depression after stroke: a pilot study. Clin Rehabil 1997;11(2):114-22.
19. Astrom M, Adolfsson R, Asplund K. Major depression in stroke patients: a 13-year longitudinal study. Stroke 1993;24:976-82.
20. Narushima K, Kosier JT, Robinson RG. Preventing poststroke depression: a 12-week double-blind randomized treatment trial and 21-month follow-up. J Nerv Ment Dis 2002;190(5):296-303.
21. Palomaki H, Kaste M, Berg A, et al. Prevention of poststroke depression: 1 year randomised placebo controlled double blind trial of mianserin with 6 month follow up after therapy. J Neurol Neurosurg Psychiatry 1999;66(4):490-4.
1. Heart and stroke statistical update. Dallas: American Heart Association, 2002.
2. Goldstein K. The organism:a holistic approach to biology derived from pathological data in man. New York: American Book Co, 1939.
3. Starkstein SE, Fedoroff JP, Price TR, et al. Catastrophic reaction after cerebrovascular lesions: frequency, correlates, and validation of a scale. J Neuropsychiatry Clin Neurosci 1993;5(2):189-94.
4. Robinson RG, Parikh RM, Lipsey JR, et al. Pathological laughing and crying following stroke: Validation of measurement scale and double-blind treatment study. Am J Psychiatry 1993;150:286-93.
5. Robinson RG, Kubos KL, Starr LB, et al. Mood changes in stroke patients: relationship to lesion location. Compr Psychiatry 1983;24(6):555-66.
6. Gainotti G, Azzoni A, Razzano C, et al. The Post-Stroke Depression Rating Scale: a test specifically devised to investigate affective disorders of stroke patients. J Clin Exp Neuropsychol 1997;19(3):340-56.
7. Carson AJ, MacHale S, Allen K, et al. Depression after stroke and lesion location: a systematic review. Lancet 2000;356(9224):122-6.
8. Narushima K, Kosier JT, Robinson RG. A reappraisal of poststroke depression, intra- and inter-hemispheric lesion location using meta-analysis. J Neuropsychiatry Clin Neurosci 2003;15(4):422-30.
9. Simons LA, McCallum J, Friedlander Y, Simons J. Risk factors for ischemic stroke: Dubbo Study of the elderly. Stroke 1998;29(7):1341-6.
10. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med 2000;62(4):463-71.
11. Cassidy EM, Walsh MT, O’Connor R, et al. Platelet surface glycoprotein expression in post-stroke depression: a preliminary study. Psychiatry Res 2003;118(2):175-81.
12. Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol 2000;20(2):137-40.
13. Everson SA, Roberts RE, Goldberg DE, Kaplan GA. Depressive symptoms and increased risk of stroke mortality over a 29-year period. Arch Intern Med 1998;158(10):1133-8.
14. Turner-Stokes L, Hassan N. Depression after stroke: a review of the evidence base to inform the development of an integrated care pathway. Part 2: Treatment alternatives. Clin Rehabil 2002;16(3):248-60.
15. Whyte EM, Mulsant BH. Post-stroke depression: epidemiology, pathophysiology, and biologic treatment. Biol Psychiatry 2002;52:253-64.
16. Currier M, Murray G, Welsh C. Electroconvulsive therapy for poststroke depressed geriatric patients. J Neuropsychiatry Clin Neurosci 1992;4(2):140-4.
17. Kemp BJ, Corgiat M, Gill C. Effects of brief cognitive behavioral group psychotherapy in older persons with and without disabling illness. Behav Health Ageing 1992;2:21-8.
18. Lincoln NB, Flannaghan T, Sutcliffe L, Rother L. Evaluation of cognitive behavioural treatment for depression after stroke: a pilot study. Clin Rehabil 1997;11(2):114-22.
19. Astrom M, Adolfsson R, Asplund K. Major depression in stroke patients: a 13-year longitudinal study. Stroke 1993;24:976-82.
20. Narushima K, Kosier JT, Robinson RG. Preventing poststroke depression: a 12-week double-blind randomized treatment trial and 21-month follow-up. J Nerv Ment Dis 2002;190(5):296-303.
21. Palomaki H, Kaste M, Berg A, et al. Prevention of poststroke depression: 1 year randomised placebo controlled double blind trial of mianserin with 6 month follow up after therapy. J Neurol Neurosurg Psychiatry 1999;66(4):490-4.