User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Wireless Internet 101
Wireless fidelity, or “Wi-Fi,” is gaining popularity in the medical profession and elsewhere. Some medical professionals are using Wi-Fi’s anytime, anywhere Internet connectivity to access electronic medical information during hospital rounds and to immediately enter demographic information when admitting patients.
What it is-and how it works
Wi-Fi is a certification given by the Wi-Fi Alliance, a nonprofit international trade organization that tests 802.11-based wireless Internet products. The “Wi-Fi Certified” logo signals to purchasers that the product has met rigorous interoperability testing requirements and is compatible with products from different vendors.
Today, the term “Wi-Fi” also commonly describes wireless Internet. Technically speaking, Wi-Fi is the use of radio technology to provide Ethernet connectivity in the unlicensed 2.4 and 5 GHz radio frequencies. By contrast, Internet access provided by wireless modems is based on technology used in cellular phones.
802.11 is the standard protocol ratified by the Institute of Electrical and Electronics Engineers. 802.11b is the most commonly used standard; 802.11a and 802.11g are other options (Table).
Why Wi-Fi?
Wireless Internet access via the 802.11 protocol offers:
- freedom to surf the Internet in your office, back yard, or elsewhere
- the ability to avoid using unsightly wires to connect computers in a local area network (LAN)
- significantly faster access than wireless modems and higher connection speeds than are available via telephone lines or electrical outlets.
Hitting the hot spots
Aside from office and home, Wi-Fi can be used at “hot spots”-public access points at cafes, restaurants, coffee shops, hotels, airports, downtown business districts, malls, and retail stores. Some retailers provide free access to attract business,1 while others pay to partner with wireless Internet service providers such as T-Mobile 2 and Boingo.3
It helps to check online for hot spots before heading out (visit the T- Mobile or Boingo sites or try the Wi-Fi FreeSpot Directory or other Web site guide). Because most network connections are automatic, however, you can turn on your notebook computer anytime and find out in seconds if a wireless Internet service is available. An indication usually appears on the screen if you are connected to a wireless LAN with Windows XP or Mac OS X, but older operating systems may require additional software. A Wi-Fi signal does not guarantee Internet access because many Wi-Fi providers require the user to log in.
An alternative is to look for ‘warchalking’-a series of sidewalk symbols that appear on your screen to indicate nearby wireless access4 (click here to view warchalking symbols). Warchalking has raised legal and moral issues, though to my knowledge this tracking method is seldom used.
Getting started
Several components are necessary for wireless Internet in the home or office. First, broadband Internet access via a cable modem, digital subscriber line (DSL), or satellite must be established. Connecting via a dial-up modem is another option, but its connection rate is too slow to be shared among several computers.
A wireless access point, which serves as the ‘base station,’ is then connected to the modem. Access points often are sold in combination with a built-in router, which delivers network information to the appropriate destination.
Each computer connecting to the access point must have a wireless network adapter. For desktop computers, this can either be a peripheral component interconnect (PCI) card or a Universal Serial Bus (USB) device. Many notebook computers come with a built-in wireless network adapter but can also use a PCI card or USB device.
Once these devices are installed, the wireless network must be set up so that each device can communicate. Most network setups are automatically established and require little user intervention; however, the user must decide which wireless channel to use and whether a security key is required.
Security risks
Wireless network use poses one major drawback: lack of security.
All wireless LANs have built-in wired equivalent privacy security, which uses a security key to limit access. In 2001, researchers at the University of California at Berkeley discovered flaws in the encryption algorithm designed to protect wireless LANs.5 Software has since been designed to exploit this flaw and identify the security key in the wireless traffic, rendering this level of security useless.6
In health care, this risk raises the issue of whether wireless networking is compliant with the Health Insurance Portability and Accountability Act (HIPAA). Medical Records Institute Executive Director C. Peter Waegeman n indicates that access via 802.11b is clearly not HIPAA-compliant7 and that other standards such as 802.11a or 802.11g should be used. Most healthcare systems, however, continue to use 802.11b because it is widely available and economical.
Making your network secure
Although the 802.11b standard is extremely insecure, several practical issues ameliorate the security risk. For one, finding the security key provides access to the wireless network but does not guarantee access to private information. Disabling shared access to network computers offers additional security but will eliminate the benefit of sharing information over a network.
Several hardware and software innovations aimed at increasing remote network security are scheduled to be launched in the coming weeks.8 Until these products reach the mainstream, you can prevent unauthorized network access by:
- Choosing an access point that restricts media access control (MAC). The MAC address is the hardware address of a node in the network, such as a network adapter. By designating which MAC addresses have wireless access, unauthorized access is eliminated.
- Setting up the access point to stop broadcasting its Service Set Identifier (SSID). The SSID is part of the automated connection process that tells network adapters which 802.11b network it is joining. Only authorized users will know the SSID and security key, which are needed to establish a connection.
Internet communications that implement the secure socket layer (SSL) protocol will be encrypted, thus ensuring that the information is sent, unchanged, only to the intended server. Online shopping sites and banks use SSL technology to safeguard sensitive information.
Table
Current Wi-Fi standards
| Standard | Frequency | Theoretical transmission rate/typical rate (megabytes per second) | Range (meters/feet) |
|---|---|---|---|
| 802.11b | 2.4 GHz | 11/4-6 | 30/1000 |
| 802.11a | 5 GHz | 54/20-25 | 25/75 |
| 802.11g (compatible with 802.11b) | 2.4 GHz | 54/6-24 | 30/1000 |
Related Resources
Wi-Fi Alliance: Wi-Fi Overview. Available at: http://www.weca.net/OpenSection/why_Wi-Fi.asp?TID=2. Accessed Nov. 18, 2003
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected].
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
(All URLs accessed Dec. 2, 2003)
1. Wi-Fi FreeSpot Directory. http://www.wififreespot.com/
2. T-Mobile HotSpot. http://www.t-mobile.com/hotspot/default.asp?nav=hm
3. Boingo Wireless: 5,000 HotSpots. http://www.boingo.com
4. Warchalking http://www.warchalking.org
5. Borisov N, Goldberg I, Wagner D. Security of the WEP Algorithm. Available at: http://www.isaac.cs.berkeley.edu/isaac/wep-faq.html
6. AirSnort. http://airsnort.shmoo.com/
7. Wireless networks. Does Wi-Fi Run Afoul of HIPAA? Mobile Health Data Sept. 7, 2003. Available at: http://www.mobilehealthdata.com/article.cfm?articleId=451
8. Nobel C. Wi-Fi to get big extensions. eWeek Dec. 1, 2003. Available at: http://www.eweek.com/article2/0,4149,1400188,00.asp
Wireless fidelity, or “Wi-Fi,” is gaining popularity in the medical profession and elsewhere. Some medical professionals are using Wi-Fi’s anytime, anywhere Internet connectivity to access electronic medical information during hospital rounds and to immediately enter demographic information when admitting patients.
What it is-and how it works
Wi-Fi is a certification given by the Wi-Fi Alliance, a nonprofit international trade organization that tests 802.11-based wireless Internet products. The “Wi-Fi Certified” logo signals to purchasers that the product has met rigorous interoperability testing requirements and is compatible with products from different vendors.
Today, the term “Wi-Fi” also commonly describes wireless Internet. Technically speaking, Wi-Fi is the use of radio technology to provide Ethernet connectivity in the unlicensed 2.4 and 5 GHz radio frequencies. By contrast, Internet access provided by wireless modems is based on technology used in cellular phones.
802.11 is the standard protocol ratified by the Institute of Electrical and Electronics Engineers. 802.11b is the most commonly used standard; 802.11a and 802.11g are other options (Table).
Why Wi-Fi?
Wireless Internet access via the 802.11 protocol offers:
- freedom to surf the Internet in your office, back yard, or elsewhere
- the ability to avoid using unsightly wires to connect computers in a local area network (LAN)
- significantly faster access than wireless modems and higher connection speeds than are available via telephone lines or electrical outlets.
Hitting the hot spots
Aside from office and home, Wi-Fi can be used at “hot spots”-public access points at cafes, restaurants, coffee shops, hotels, airports, downtown business districts, malls, and retail stores. Some retailers provide free access to attract business,1 while others pay to partner with wireless Internet service providers such as T-Mobile 2 and Boingo.3
It helps to check online for hot spots before heading out (visit the T- Mobile or Boingo sites or try the Wi-Fi FreeSpot Directory or other Web site guide). Because most network connections are automatic, however, you can turn on your notebook computer anytime and find out in seconds if a wireless Internet service is available. An indication usually appears on the screen if you are connected to a wireless LAN with Windows XP or Mac OS X, but older operating systems may require additional software. A Wi-Fi signal does not guarantee Internet access because many Wi-Fi providers require the user to log in.
An alternative is to look for ‘warchalking’-a series of sidewalk symbols that appear on your screen to indicate nearby wireless access4 (click here to view warchalking symbols). Warchalking has raised legal and moral issues, though to my knowledge this tracking method is seldom used.
Getting started
Several components are necessary for wireless Internet in the home or office. First, broadband Internet access via a cable modem, digital subscriber line (DSL), or satellite must be established. Connecting via a dial-up modem is another option, but its connection rate is too slow to be shared among several computers.
A wireless access point, which serves as the ‘base station,’ is then connected to the modem. Access points often are sold in combination with a built-in router, which delivers network information to the appropriate destination.
Each computer connecting to the access point must have a wireless network adapter. For desktop computers, this can either be a peripheral component interconnect (PCI) card or a Universal Serial Bus (USB) device. Many notebook computers come with a built-in wireless network adapter but can also use a PCI card or USB device.
Once these devices are installed, the wireless network must be set up so that each device can communicate. Most network setups are automatically established and require little user intervention; however, the user must decide which wireless channel to use and whether a security key is required.
Security risks
Wireless network use poses one major drawback: lack of security.
All wireless LANs have built-in wired equivalent privacy security, which uses a security key to limit access. In 2001, researchers at the University of California at Berkeley discovered flaws in the encryption algorithm designed to protect wireless LANs.5 Software has since been designed to exploit this flaw and identify the security key in the wireless traffic, rendering this level of security useless.6
In health care, this risk raises the issue of whether wireless networking is compliant with the Health Insurance Portability and Accountability Act (HIPAA). Medical Records Institute Executive Director C. Peter Waegeman n indicates that access via 802.11b is clearly not HIPAA-compliant7 and that other standards such as 802.11a or 802.11g should be used. Most healthcare systems, however, continue to use 802.11b because it is widely available and economical.
Making your network secure
Although the 802.11b standard is extremely insecure, several practical issues ameliorate the security risk. For one, finding the security key provides access to the wireless network but does not guarantee access to private information. Disabling shared access to network computers offers additional security but will eliminate the benefit of sharing information over a network.
Several hardware and software innovations aimed at increasing remote network security are scheduled to be launched in the coming weeks.8 Until these products reach the mainstream, you can prevent unauthorized network access by:
- Choosing an access point that restricts media access control (MAC). The MAC address is the hardware address of a node in the network, such as a network adapter. By designating which MAC addresses have wireless access, unauthorized access is eliminated.
- Setting up the access point to stop broadcasting its Service Set Identifier (SSID). The SSID is part of the automated connection process that tells network adapters which 802.11b network it is joining. Only authorized users will know the SSID and security key, which are needed to establish a connection.
Internet communications that implement the secure socket layer (SSL) protocol will be encrypted, thus ensuring that the information is sent, unchanged, only to the intended server. Online shopping sites and banks use SSL technology to safeguard sensitive information.
Table
Current Wi-Fi standards
| Standard | Frequency | Theoretical transmission rate/typical rate (megabytes per second) | Range (meters/feet) |
|---|---|---|---|
| 802.11b | 2.4 GHz | 11/4-6 | 30/1000 |
| 802.11a | 5 GHz | 54/20-25 | 25/75 |
| 802.11g (compatible with 802.11b) | 2.4 GHz | 54/6-24 | 30/1000 |
Related Resources
Wi-Fi Alliance: Wi-Fi Overview. Available at: http://www.weca.net/OpenSection/why_Wi-Fi.asp?TID=2. Accessed Nov. 18, 2003
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected].
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
Wireless fidelity, or “Wi-Fi,” is gaining popularity in the medical profession and elsewhere. Some medical professionals are using Wi-Fi’s anytime, anywhere Internet connectivity to access electronic medical information during hospital rounds and to immediately enter demographic information when admitting patients.
What it is-and how it works
Wi-Fi is a certification given by the Wi-Fi Alliance, a nonprofit international trade organization that tests 802.11-based wireless Internet products. The “Wi-Fi Certified” logo signals to purchasers that the product has met rigorous interoperability testing requirements and is compatible with products from different vendors.
Today, the term “Wi-Fi” also commonly describes wireless Internet. Technically speaking, Wi-Fi is the use of radio technology to provide Ethernet connectivity in the unlicensed 2.4 and 5 GHz radio frequencies. By contrast, Internet access provided by wireless modems is based on technology used in cellular phones.
802.11 is the standard protocol ratified by the Institute of Electrical and Electronics Engineers. 802.11b is the most commonly used standard; 802.11a and 802.11g are other options (Table).
Why Wi-Fi?
Wireless Internet access via the 802.11 protocol offers:
- freedom to surf the Internet in your office, back yard, or elsewhere
- the ability to avoid using unsightly wires to connect computers in a local area network (LAN)
- significantly faster access than wireless modems and higher connection speeds than are available via telephone lines or electrical outlets.
Hitting the hot spots
Aside from office and home, Wi-Fi can be used at “hot spots”-public access points at cafes, restaurants, coffee shops, hotels, airports, downtown business districts, malls, and retail stores. Some retailers provide free access to attract business,1 while others pay to partner with wireless Internet service providers such as T-Mobile 2 and Boingo.3
It helps to check online for hot spots before heading out (visit the T- Mobile or Boingo sites or try the Wi-Fi FreeSpot Directory or other Web site guide). Because most network connections are automatic, however, you can turn on your notebook computer anytime and find out in seconds if a wireless Internet service is available. An indication usually appears on the screen if you are connected to a wireless LAN with Windows XP or Mac OS X, but older operating systems may require additional software. A Wi-Fi signal does not guarantee Internet access because many Wi-Fi providers require the user to log in.
An alternative is to look for ‘warchalking’-a series of sidewalk symbols that appear on your screen to indicate nearby wireless access4 (click here to view warchalking symbols). Warchalking has raised legal and moral issues, though to my knowledge this tracking method is seldom used.
Getting started
Several components are necessary for wireless Internet in the home or office. First, broadband Internet access via a cable modem, digital subscriber line (DSL), or satellite must be established. Connecting via a dial-up modem is another option, but its connection rate is too slow to be shared among several computers.
A wireless access point, which serves as the ‘base station,’ is then connected to the modem. Access points often are sold in combination with a built-in router, which delivers network information to the appropriate destination.
Each computer connecting to the access point must have a wireless network adapter. For desktop computers, this can either be a peripheral component interconnect (PCI) card or a Universal Serial Bus (USB) device. Many notebook computers come with a built-in wireless network adapter but can also use a PCI card or USB device.
Once these devices are installed, the wireless network must be set up so that each device can communicate. Most network setups are automatically established and require little user intervention; however, the user must decide which wireless channel to use and whether a security key is required.
Security risks
Wireless network use poses one major drawback: lack of security.
All wireless LANs have built-in wired equivalent privacy security, which uses a security key to limit access. In 2001, researchers at the University of California at Berkeley discovered flaws in the encryption algorithm designed to protect wireless LANs.5 Software has since been designed to exploit this flaw and identify the security key in the wireless traffic, rendering this level of security useless.6
In health care, this risk raises the issue of whether wireless networking is compliant with the Health Insurance Portability and Accountability Act (HIPAA). Medical Records Institute Executive Director C. Peter Waegeman n indicates that access via 802.11b is clearly not HIPAA-compliant7 and that other standards such as 802.11a or 802.11g should be used. Most healthcare systems, however, continue to use 802.11b because it is widely available and economical.
Making your network secure
Although the 802.11b standard is extremely insecure, several practical issues ameliorate the security risk. For one, finding the security key provides access to the wireless network but does not guarantee access to private information. Disabling shared access to network computers offers additional security but will eliminate the benefit of sharing information over a network.
Several hardware and software innovations aimed at increasing remote network security are scheduled to be launched in the coming weeks.8 Until these products reach the mainstream, you can prevent unauthorized network access by:
- Choosing an access point that restricts media access control (MAC). The MAC address is the hardware address of a node in the network, such as a network adapter. By designating which MAC addresses have wireless access, unauthorized access is eliminated.
- Setting up the access point to stop broadcasting its Service Set Identifier (SSID). The SSID is part of the automated connection process that tells network adapters which 802.11b network it is joining. Only authorized users will know the SSID and security key, which are needed to establish a connection.
Internet communications that implement the secure socket layer (SSL) protocol will be encrypted, thus ensuring that the information is sent, unchanged, only to the intended server. Online shopping sites and banks use SSL technology to safeguard sensitive information.
Table
Current Wi-Fi standards
| Standard | Frequency | Theoretical transmission rate/typical rate (megabytes per second) | Range (meters/feet) |
|---|---|---|---|
| 802.11b | 2.4 GHz | 11/4-6 | 30/1000 |
| 802.11a | 5 GHz | 54/20-25 | 25/75 |
| 802.11g (compatible with 802.11b) | 2.4 GHz | 54/6-24 | 30/1000 |
Related Resources
Wi-Fi Alliance: Wi-Fi Overview. Available at: http://www.weca.net/OpenSection/why_Wi-Fi.asp?TID=2. Accessed Nov. 18, 2003
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected].
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
(All URLs accessed Dec. 2, 2003)
1. Wi-Fi FreeSpot Directory. http://www.wififreespot.com/
2. T-Mobile HotSpot. http://www.t-mobile.com/hotspot/default.asp?nav=hm
3. Boingo Wireless: 5,000 HotSpots. http://www.boingo.com
4. Warchalking http://www.warchalking.org
5. Borisov N, Goldberg I, Wagner D. Security of the WEP Algorithm. Available at: http://www.isaac.cs.berkeley.edu/isaac/wep-faq.html
6. AirSnort. http://airsnort.shmoo.com/
7. Wireless networks. Does Wi-Fi Run Afoul of HIPAA? Mobile Health Data Sept. 7, 2003. Available at: http://www.mobilehealthdata.com/article.cfm?articleId=451
8. Nobel C. Wi-Fi to get big extensions. eWeek Dec. 1, 2003. Available at: http://www.eweek.com/article2/0,4149,1400188,00.asp
(All URLs accessed Dec. 2, 2003)
1. Wi-Fi FreeSpot Directory. http://www.wififreespot.com/
2. T-Mobile HotSpot. http://www.t-mobile.com/hotspot/default.asp?nav=hm
3. Boingo Wireless: 5,000 HotSpots. http://www.boingo.com
4. Warchalking http://www.warchalking.org
5. Borisov N, Goldberg I, Wagner D. Security of the WEP Algorithm. Available at: http://www.isaac.cs.berkeley.edu/isaac/wep-faq.html
6. AirSnort. http://airsnort.shmoo.com/
7. Wireless networks. Does Wi-Fi Run Afoul of HIPAA? Mobile Health Data Sept. 7, 2003. Available at: http://www.mobilehealthdata.com/article.cfm?articleId=451
8. Nobel C. Wi-Fi to get big extensions. eWeek Dec. 1, 2003. Available at: http://www.eweek.com/article2/0,4149,1400188,00.asp
How to remedy excessive salivation in patients taking clozapine
Hypersalivation caused by clozapine can lead to sleep deprivation, salivary gland swelling,1 and aspiration pneumonia.2 Its socially stigmatizing effects can also deter patients with psychotic illnesses from taking clozapine.
It is not clear at what dosage clozapine causes sialorrhea, but the higher the dosage the more severe the problem. Hypersalivation usually resolves with continued clozapine therapy. Until that happens, the following agents may help.
Drug management
- Benztropine, an acetylcholine antagonist used in Parkinson’s disease, can be started at 1 mg at night, when hypersalivation is most troublesome. If needed, increase to 2 mg qhs or 1 mg bid. Benztropine can cause dose-dependent anticholinergic effects ranging from peripheral (dry mouth, blurring of vision, tachycardia, urinary retention, constipation) to central (memory disturbance, restlessness, disorientation, delirium).
- Scopolamine, a transdermal used to prevent motion sickness, significantly reduced disabling hypersalivation in patients who wore a 1-mg patch behind the ear for 72 hours.3 The agent may irritate skin, so reserve it for severe cases.
- Terazocin, an alpha 1 receptor antagonist for hypertension, is effective at 2 mg qhs. Because the agent can cause hypotension, start at 1 mg/d for 1 week, then increase the dosage and monitor blood pressure at each visit.
Atropine, ipratropium bromide, and clonidine also have shown benefit in small studies.4-6
Other strategies
Lowering the clozapine dosage while maintaining its antipsychotic effect may also help reduce salivation. You might also advise the patient to:
- suck or chew sugarless candy or gum to increase swallowing
- place a towel on the pillowcase to prevent soaking the pillow overnight.
Drug brand names
- Benztropine • Cogentin
- Clonidine • Catapres
- Clozapine • Clozaril
- Ipratropium • Atrovent
- Scopolamine • Transderm-Scop
1. Brodkin ES, Pelton GH, Price LH. Treatment of clozapine-induced parotid gland swelling. Am J Psychiatry 1996;153:445.-
2. Hinkes R, Quesada TV, Currier MB, et al. Aspiration pneumonia possibly secondary to clozapine induced sialorrhea. J Clin Psychopharmacol 1996;16:462-3.
3. McKane JP, Hall C, Akram G. Hyoscine patches in clozapine induced hypersalivation. Psychiatr Bull 2001;25:277.-
4. Antonello C, Tessier P. Clozapine and sialorrhea: a new intervention for this bothersome and potentially dangerous side effect. J Psychiatry Neurosci 1999;24:250.
5. Calderon J, Robin E, Sobota WL. Potential use of ipratropium bromide for the treatment of clozapine induced hypersalivation: a preliminary report. Int Clin Psychopharmacol 2000;15:49-52.
6. Grabowski J. Clonidine treatment of clozapine induced hypersalivation. J Clin Psychopharmacol 1992;12:69-70.
Dr. Maju Mathews is a resident, department of psychiatry, Drexel University College of Medicine, Philadelphia, PA.
Dr. Manu Mathews is a staff psychiatrist, East Surrey Hospital, Redhill, UK.
Dr. Joanne Mathews is a staff psychiatrist, West Suffolk Hospital, Bury’s St. Edmonds, UK.
Hypersalivation caused by clozapine can lead to sleep deprivation, salivary gland swelling,1 and aspiration pneumonia.2 Its socially stigmatizing effects can also deter patients with psychotic illnesses from taking clozapine.
It is not clear at what dosage clozapine causes sialorrhea, but the higher the dosage the more severe the problem. Hypersalivation usually resolves with continued clozapine therapy. Until that happens, the following agents may help.
Drug management
- Benztropine, an acetylcholine antagonist used in Parkinson’s disease, can be started at 1 mg at night, when hypersalivation is most troublesome. If needed, increase to 2 mg qhs or 1 mg bid. Benztropine can cause dose-dependent anticholinergic effects ranging from peripheral (dry mouth, blurring of vision, tachycardia, urinary retention, constipation) to central (memory disturbance, restlessness, disorientation, delirium).
- Scopolamine, a transdermal used to prevent motion sickness, significantly reduced disabling hypersalivation in patients who wore a 1-mg patch behind the ear for 72 hours.3 The agent may irritate skin, so reserve it for severe cases.
- Terazocin, an alpha 1 receptor antagonist for hypertension, is effective at 2 mg qhs. Because the agent can cause hypotension, start at 1 mg/d for 1 week, then increase the dosage and monitor blood pressure at each visit.
Atropine, ipratropium bromide, and clonidine also have shown benefit in small studies.4-6
Other strategies
Lowering the clozapine dosage while maintaining its antipsychotic effect may also help reduce salivation. You might also advise the patient to:
- suck or chew sugarless candy or gum to increase swallowing
- place a towel on the pillowcase to prevent soaking the pillow overnight.
Drug brand names
- Benztropine • Cogentin
- Clonidine • Catapres
- Clozapine • Clozaril
- Ipratropium • Atrovent
- Scopolamine • Transderm-Scop
Hypersalivation caused by clozapine can lead to sleep deprivation, salivary gland swelling,1 and aspiration pneumonia.2 Its socially stigmatizing effects can also deter patients with psychotic illnesses from taking clozapine.
It is not clear at what dosage clozapine causes sialorrhea, but the higher the dosage the more severe the problem. Hypersalivation usually resolves with continued clozapine therapy. Until that happens, the following agents may help.
Drug management
- Benztropine, an acetylcholine antagonist used in Parkinson’s disease, can be started at 1 mg at night, when hypersalivation is most troublesome. If needed, increase to 2 mg qhs or 1 mg bid. Benztropine can cause dose-dependent anticholinergic effects ranging from peripheral (dry mouth, blurring of vision, tachycardia, urinary retention, constipation) to central (memory disturbance, restlessness, disorientation, delirium).
- Scopolamine, a transdermal used to prevent motion sickness, significantly reduced disabling hypersalivation in patients who wore a 1-mg patch behind the ear for 72 hours.3 The agent may irritate skin, so reserve it for severe cases.
- Terazocin, an alpha 1 receptor antagonist for hypertension, is effective at 2 mg qhs. Because the agent can cause hypotension, start at 1 mg/d for 1 week, then increase the dosage and monitor blood pressure at each visit.
Atropine, ipratropium bromide, and clonidine also have shown benefit in small studies.4-6
Other strategies
Lowering the clozapine dosage while maintaining its antipsychotic effect may also help reduce salivation. You might also advise the patient to:
- suck or chew sugarless candy or gum to increase swallowing
- place a towel on the pillowcase to prevent soaking the pillow overnight.
Drug brand names
- Benztropine • Cogentin
- Clonidine • Catapres
- Clozapine • Clozaril
- Ipratropium • Atrovent
- Scopolamine • Transderm-Scop
1. Brodkin ES, Pelton GH, Price LH. Treatment of clozapine-induced parotid gland swelling. Am J Psychiatry 1996;153:445.-
2. Hinkes R, Quesada TV, Currier MB, et al. Aspiration pneumonia possibly secondary to clozapine induced sialorrhea. J Clin Psychopharmacol 1996;16:462-3.
3. McKane JP, Hall C, Akram G. Hyoscine patches in clozapine induced hypersalivation. Psychiatr Bull 2001;25:277.-
4. Antonello C, Tessier P. Clozapine and sialorrhea: a new intervention for this bothersome and potentially dangerous side effect. J Psychiatry Neurosci 1999;24:250.
5. Calderon J, Robin E, Sobota WL. Potential use of ipratropium bromide for the treatment of clozapine induced hypersalivation: a preliminary report. Int Clin Psychopharmacol 2000;15:49-52.
6. Grabowski J. Clonidine treatment of clozapine induced hypersalivation. J Clin Psychopharmacol 1992;12:69-70.
Dr. Maju Mathews is a resident, department of psychiatry, Drexel University College of Medicine, Philadelphia, PA.
Dr. Manu Mathews is a staff psychiatrist, East Surrey Hospital, Redhill, UK.
Dr. Joanne Mathews is a staff psychiatrist, West Suffolk Hospital, Bury’s St. Edmonds, UK.
1. Brodkin ES, Pelton GH, Price LH. Treatment of clozapine-induced parotid gland swelling. Am J Psychiatry 1996;153:445.-
2. Hinkes R, Quesada TV, Currier MB, et al. Aspiration pneumonia possibly secondary to clozapine induced sialorrhea. J Clin Psychopharmacol 1996;16:462-3.
3. McKane JP, Hall C, Akram G. Hyoscine patches in clozapine induced hypersalivation. Psychiatr Bull 2001;25:277.-
4. Antonello C, Tessier P. Clozapine and sialorrhea: a new intervention for this bothersome and potentially dangerous side effect. J Psychiatry Neurosci 1999;24:250.
5. Calderon J, Robin E, Sobota WL. Potential use of ipratropium bromide for the treatment of clozapine induced hypersalivation: a preliminary report. Int Clin Psychopharmacol 2000;15:49-52.
6. Grabowski J. Clonidine treatment of clozapine induced hypersalivation. J Clin Psychopharmacol 1992;12:69-70.
Dr. Maju Mathews is a resident, department of psychiatry, Drexel University College of Medicine, Philadelphia, PA.
Dr. Manu Mathews is a staff psychiatrist, East Surrey Hospital, Redhill, UK.
Dr. Joanne Mathews is a staff psychiatrist, West Suffolk Hospital, Bury’s St. Edmonds, UK.
Christmas depression: The data may surprise you
In 1981, I wrote an article called, “Christmas and psychopathology” (Arch Gen Psychiatry 1981;38[Dec]:1377-81). In the Readers’ Guide to Periodical Literature, I found 103 magazine articles across 8 years under “Depression, Mental.” Sixteen dealt with Christmas depression.
This week, I did a Google search for “Christmas” (13,700,000 hits) and “depression” (237,000 hits). This weighty body of Web lore suggests that the popular press assumes many people get depressed before Christmas.
Why is there such an interest in Christmas depression? I think it’s because few of us feel as happy as we think we ought to over the holidays. Given the stresses of shopping, family obligations, and emotional baggage from previous years, I’m surprised that more people do not get depressed.
The fact is, however, that fewer people report to psychiatric emergency rooms just before Christmas than at other times of the year. My study in 1981 and most similar studies show that hospital admissions, suicide attempts and completions, and even letters to advice columnists go down just before Christmas, then go back up immediately afterwards. When you average the pre- and post-holiday statistics, Christmas is not one of the busiest seasons for psychiatric emergencies.
Maybe depression doesn’t increase before Christmas because people use their best coping strategies to get through the holiday. And maybe there is a little Christmas magic after all.
In 1981, I wrote an article called, “Christmas and psychopathology” (Arch Gen Psychiatry 1981;38[Dec]:1377-81). In the Readers’ Guide to Periodical Literature, I found 103 magazine articles across 8 years under “Depression, Mental.” Sixteen dealt with Christmas depression.
This week, I did a Google search for “Christmas” (13,700,000 hits) and “depression” (237,000 hits). This weighty body of Web lore suggests that the popular press assumes many people get depressed before Christmas.
Why is there such an interest in Christmas depression? I think it’s because few of us feel as happy as we think we ought to over the holidays. Given the stresses of shopping, family obligations, and emotional baggage from previous years, I’m surprised that more people do not get depressed.
The fact is, however, that fewer people report to psychiatric emergency rooms just before Christmas than at other times of the year. My study in 1981 and most similar studies show that hospital admissions, suicide attempts and completions, and even letters to advice columnists go down just before Christmas, then go back up immediately afterwards. When you average the pre- and post-holiday statistics, Christmas is not one of the busiest seasons for psychiatric emergencies.
Maybe depression doesn’t increase before Christmas because people use their best coping strategies to get through the holiday. And maybe there is a little Christmas magic after all.
In 1981, I wrote an article called, “Christmas and psychopathology” (Arch Gen Psychiatry 1981;38[Dec]:1377-81). In the Readers’ Guide to Periodical Literature, I found 103 magazine articles across 8 years under “Depression, Mental.” Sixteen dealt with Christmas depression.
This week, I did a Google search for “Christmas” (13,700,000 hits) and “depression” (237,000 hits). This weighty body of Web lore suggests that the popular press assumes many people get depressed before Christmas.
Why is there such an interest in Christmas depression? I think it’s because few of us feel as happy as we think we ought to over the holidays. Given the stresses of shopping, family obligations, and emotional baggage from previous years, I’m surprised that more people do not get depressed.
The fact is, however, that fewer people report to psychiatric emergency rooms just before Christmas than at other times of the year. My study in 1981 and most similar studies show that hospital admissions, suicide attempts and completions, and even letters to advice columnists go down just before Christmas, then go back up immediately afterwards. When you average the pre- and post-holiday statistics, Christmas is not one of the busiest seasons for psychiatric emergencies.
Maybe depression doesn’t increase before Christmas because people use their best coping strategies to get through the holiday. And maybe there is a little Christmas magic after all.
Hypochondriasis prevalence
Suzanne Feinstein, PhD, and Brian Fallon, MD, MPH, note that “In psychiatric or medical clinics, women (have) hypochondriasis three to four times more often than menSeptember 2003).
DSM-IV-TR,2 however, reports equal incidence of hypochondriasis in men and women, as do Tasman, Kay & Lieberman.3
Researchers have distinguished between the incidence and prevalence of hypochondriasis in the general and medical populations. DSM-IV-TR places hypochondriasis prevalence at 1 to 5% in the general population and 2 to 7% in the medical population. Martin & Yutzy’s4 estimate of 3 to 13% prevalence in the medical population jibes with Dr. Feinstein’s and Dr. Fallon’s article.
To my knowledge, however, data on hypochondriasis prevalence among psychiatric outpatients are lacking. Extrapolating this information from the general medical population and implying a similar prevalence among psychiatric outpatients may not be correct.
Jessica Bright, MD
Child psychiatry fellow
Department of psychiatry and human behavior
Thomas Jefferson University, Philadelphia, PA
- Diagnostic and statistical manual of mental disorders (4th ed-text revision). Washington, DC: American Psychiatric Association, 2000.
- Barsky AJ, Wyshak G, Klerman GL, Latham KS. The prevalence of hypochondriasis in medical outpatients. Soc Psychiatry Psychiatr Epidemiol 1990;25(2):89–94.
- Tasman A, Kay J, Lieberman, JA (eds). Psychiatry, Vol. 2 (1st ed). Philadelphia: WB Saunders, 1997.
- Martin RL, Yutzy SH. Somatoform disorders. In: Tasman A, Kay J, Lieberman JA (eds). Psychiatry (1st ed). Philadelphia: WB Saunders, 1997:1144–8.
Suzanne Feinstein, PhD, and Brian Fallon, MD, MPH, note that “In psychiatric or medical clinics, women (have) hypochondriasis three to four times more often than menSeptember 2003).
DSM-IV-TR,2 however, reports equal incidence of hypochondriasis in men and women, as do Tasman, Kay & Lieberman.3
Researchers have distinguished between the incidence and prevalence of hypochondriasis in the general and medical populations. DSM-IV-TR places hypochondriasis prevalence at 1 to 5% in the general population and 2 to 7% in the medical population. Martin & Yutzy’s4 estimate of 3 to 13% prevalence in the medical population jibes with Dr. Feinstein’s and Dr. Fallon’s article.
To my knowledge, however, data on hypochondriasis prevalence among psychiatric outpatients are lacking. Extrapolating this information from the general medical population and implying a similar prevalence among psychiatric outpatients may not be correct.
Jessica Bright, MD
Child psychiatry fellow
Department of psychiatry and human behavior
Thomas Jefferson University, Philadelphia, PA
- Diagnostic and statistical manual of mental disorders (4th ed-text revision). Washington, DC: American Psychiatric Association, 2000.
- Barsky AJ, Wyshak G, Klerman GL, Latham KS. The prevalence of hypochondriasis in medical outpatients. Soc Psychiatry Psychiatr Epidemiol 1990;25(2):89–94.
- Tasman A, Kay J, Lieberman, JA (eds). Psychiatry, Vol. 2 (1st ed). Philadelphia: WB Saunders, 1997.
- Martin RL, Yutzy SH. Somatoform disorders. In: Tasman A, Kay J, Lieberman JA (eds). Psychiatry (1st ed). Philadelphia: WB Saunders, 1997:1144–8.
Suzanne Feinstein, PhD, and Brian Fallon, MD, MPH, note that “In psychiatric or medical clinics, women (have) hypochondriasis three to four times more often than menSeptember 2003).
DSM-IV-TR,2 however, reports equal incidence of hypochondriasis in men and women, as do Tasman, Kay & Lieberman.3
Researchers have distinguished between the incidence and prevalence of hypochondriasis in the general and medical populations. DSM-IV-TR places hypochondriasis prevalence at 1 to 5% in the general population and 2 to 7% in the medical population. Martin & Yutzy’s4 estimate of 3 to 13% prevalence in the medical population jibes with Dr. Feinstein’s and Dr. Fallon’s article.
To my knowledge, however, data on hypochondriasis prevalence among psychiatric outpatients are lacking. Extrapolating this information from the general medical population and implying a similar prevalence among psychiatric outpatients may not be correct.
Jessica Bright, MD
Child psychiatry fellow
Department of psychiatry and human behavior
Thomas Jefferson University, Philadelphia, PA
- Diagnostic and statistical manual of mental disorders (4th ed-text revision). Washington, DC: American Psychiatric Association, 2000.
- Barsky AJ, Wyshak G, Klerman GL, Latham KS. The prevalence of hypochondriasis in medical outpatients. Soc Psychiatry Psychiatr Epidemiol 1990;25(2):89–94.
- Tasman A, Kay J, Lieberman, JA (eds). Psychiatry, Vol. 2 (1st ed). Philadelphia: WB Saunders, 1997.
- Martin RL, Yutzy SH. Somatoform disorders. In: Tasman A, Kay J, Lieberman JA (eds). Psychiatry (1st ed). Philadelphia: WB Saunders, 1997:1144–8.
Using lithium in bipolar disorder
“Tips for Using Lithium in Bipolar Disorder” by West B. Magnon, MD, (Pearls, Current Psychiatry, September 2003) had some “do not try this at home” aspects to it.
For example, when Dr. Magnon said “Lithium, 900 mg/d, works fine,” I believe he was referring to 900 mg/d of lithium carbonate, because 900 mg of lithium is equivalent to 4,791 mg of lithium carbonate, a generally toxic dose. Also, to suggest that one dosage fits all flies in the face of decades of research.
More important, to say that “Gauging lithium blood levels is a waste of time, assuming you have checked for kidney disease” is a dangerous statement. Numerous examples exist of lithium toxicity induced by drug interactions or dehydration in patients without kidney disease.
James W. Jefferson, MD
Distinguished senior scientist, Madison Institute of Medicine
Clinical professor of psychiatry,
University of Wisconsin Medical School
Dr. Magnon responds
Dr. Jefferson’s comments reflect the standard thinking about use of lithium carbonate.
My points are:
- I have not seen these so-called extensive studies about lithium toxicity
- When given in reasonable, effective dosages, the patient responds without toxic effects.
Lithium does have some unpleasant side effects, such as tremor, and weight gain is common in some patients, especially women. Continuous lab testing is just not necessary, however.
West B. Magnon, MD Bradenton, FL
“Tips for Using Lithium in Bipolar Disorder” by West B. Magnon, MD, (Pearls, Current Psychiatry, September 2003) had some “do not try this at home” aspects to it.
For example, when Dr. Magnon said “Lithium, 900 mg/d, works fine,” I believe he was referring to 900 mg/d of lithium carbonate, because 900 mg of lithium is equivalent to 4,791 mg of lithium carbonate, a generally toxic dose. Also, to suggest that one dosage fits all flies in the face of decades of research.
More important, to say that “Gauging lithium blood levels is a waste of time, assuming you have checked for kidney disease” is a dangerous statement. Numerous examples exist of lithium toxicity induced by drug interactions or dehydration in patients without kidney disease.
James W. Jefferson, MD
Distinguished senior scientist, Madison Institute of Medicine
Clinical professor of psychiatry,
University of Wisconsin Medical School
Dr. Magnon responds
Dr. Jefferson’s comments reflect the standard thinking about use of lithium carbonate.
My points are:
- I have not seen these so-called extensive studies about lithium toxicity
- When given in reasonable, effective dosages, the patient responds without toxic effects.
Lithium does have some unpleasant side effects, such as tremor, and weight gain is common in some patients, especially women. Continuous lab testing is just not necessary, however.
West B. Magnon, MD Bradenton, FL
“Tips for Using Lithium in Bipolar Disorder” by West B. Magnon, MD, (Pearls, Current Psychiatry, September 2003) had some “do not try this at home” aspects to it.
For example, when Dr. Magnon said “Lithium, 900 mg/d, works fine,” I believe he was referring to 900 mg/d of lithium carbonate, because 900 mg of lithium is equivalent to 4,791 mg of lithium carbonate, a generally toxic dose. Also, to suggest that one dosage fits all flies in the face of decades of research.
More important, to say that “Gauging lithium blood levels is a waste of time, assuming you have checked for kidney disease” is a dangerous statement. Numerous examples exist of lithium toxicity induced by drug interactions or dehydration in patients without kidney disease.
James W. Jefferson, MD
Distinguished senior scientist, Madison Institute of Medicine
Clinical professor of psychiatry,
University of Wisconsin Medical School
Dr. Magnon responds
Dr. Jefferson’s comments reflect the standard thinking about use of lithium carbonate.
My points are:
- I have not seen these so-called extensive studies about lithium toxicity
- When given in reasonable, effective dosages, the patient responds without toxic effects.
Lithium does have some unpleasant side effects, such as tremor, and weight gain is common in some patients, especially women. Continuous lab testing is just not necessary, however.
West B. Magnon, MD Bradenton, FL
Scheduling: Time to take control
Like many doctors, you often feel the need to be two places at once.
The trick is to avoid scheduling two commitments at the same time. Anyone who has had to manage a busy practice schedule and remember his or her child’s soccer practice knows this can be challenging.
If you’re looking to organize your schedule, the Internet or your personal digital assistant (PDA) may hold the answer. This article will review scheduling solutions and help you decide which is right for your practice.
PDA scheduling-pros and cons
Both Palm and Pocket PC devices offer very good scheduling capabilities; your choice of operating system depends upon whether you need to use Microsoft Outlook. In my view, Palm is simpler and more reliable, whereas I have had more trouble connecting a Pocket PC device to my desktop computer.
Keeping a calendar in a handheld computer can be quite handy. Simply pull out the PDA, click once or twice, and view your daily schedule. Used properly, this can help prevent scheduling conflicts.
This feature, however, may be less helpful if your office staff maintains your schedule. For one, no one else can access the PDA while you’re carrying it. Second, if you do not regularly synchronize your PDA to the office computer, the staffer can make in-office schedule changes that are not recorded on the PDA version, possibly leading to double booking.
To prevent such mixups, assign one office assistant to update your schedule, and allow only that staffer to make changes. Also, be sure to double check the schedule before booking an appointment on your own.
Many major personal information managers such as Microsoft Outlook and Lotus Notes can be synchronized to a handheld. This allows your staff to view and simultaneously edit your schedule in the office, which is then synchronized onto the handheld. What’s more, users who cannot access your computer but have the proper privileges to your information can still connect to your schedule.
If a significant other uses a Palm OS device and you need to coordinate your schedule with his or hers, WeSync provides a solution that synchronizes both schedules via an online server and displays them side by side on your Palm OS PDA. Although WeSync no longer provides tech support for it, the software and service (which PalmOne owns) is still available.
Schedule synchronization between Pocket PC users is tricky: It cannot be done via an Internet server because each device has a one-to-one relationship with Microsoft Outlook, the desktop schedule software.
ManyPartners overcomes this limitation by allowing users to connect two Pocket PC devices to the same desktop scheduling software. Both users then must share information on one profile in Microsoft Outlook and must enter information in a way that avoids confusion, such as “John: doctor’s appointment” and “Mary: lecture on Monday.”)
Making your days colorful
Third-party software can make your calendar easier to use by employing color and icons to designate types of appointments (Table). This software also provides quick access to other information, such as task lists and notes.
For Palm OS devices, products such as Agendus, DateBk5, and Beyond Contacts provide a different and enhanced way to view your data. Similar software for the Pocket PC or Windows Mobile 2003 devices include Ulti-Planner, Pocket Informant, and Agenda Fusion and Agenda Today from DeveloperOne.
Some programs use the PDA’s built-in database while others have a proprietary database. This difference is important because it affects your ability to “beam” information from one device to another. For example, a proprietary database can facilitate data exchange, but only if the user to whom you are sending the data also uses a device with a proprietary database.
Online scheduling
Internet-based scheduling programs can also prevent double-booking. The user enters prospective meeting dates into the online form; the software then checks the dates against the calendar for conflicts and selects the best date. These schedules can also be synchronized to a PDA.
One drawback: changes to the online and PDA schedules must be entered separately, creating the potential for double booking. To avoid this:
- assign one staff member to make schedule changes
- or use a wireless PDA or one with a built-in cellular phone to allow instant schedule updates and prevent miscommunication.
Appointmentquest.com and scheduling.com-two services geared toward health care providers-provide online schedules, can handle multiple service locations and personnel, and will generate reminders for patients. Scheduling.com also can track multiple types of insurance and can provide billing systems with patient registration information to generate statements.
Schedule enhancement software options
| SOLUTION | WEBSITE URL | OS | COST |
|---|---|---|---|
| WeSync | www.wesync.com | Palm | Free |
| Beyond Contacts | www.dataviz.com | Palm | $49.95 |
| Agendus | www.iambic.com | Palm | $25.95 standard $39.95 professional |
| DateBK5 | www.pimlicosoftware.com | Palm | $24.95 |
| Agenda Fusion | www.developerone.com | Pocket PC | $29.95 |
| Pocket Informant | www.pocketinformant.com | Pocket PC | $24.95 |
| Ulti-Planner | www.uglybass.com/ultiplanner | Pocket PC | $14.95 |
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected] .
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
Like many doctors, you often feel the need to be two places at once.
The trick is to avoid scheduling two commitments at the same time. Anyone who has had to manage a busy practice schedule and remember his or her child’s soccer practice knows this can be challenging.
If you’re looking to organize your schedule, the Internet or your personal digital assistant (PDA) may hold the answer. This article will review scheduling solutions and help you decide which is right for your practice.
PDA scheduling-pros and cons
Both Palm and Pocket PC devices offer very good scheduling capabilities; your choice of operating system depends upon whether you need to use Microsoft Outlook. In my view, Palm is simpler and more reliable, whereas I have had more trouble connecting a Pocket PC device to my desktop computer.
Keeping a calendar in a handheld computer can be quite handy. Simply pull out the PDA, click once or twice, and view your daily schedule. Used properly, this can help prevent scheduling conflicts.
This feature, however, may be less helpful if your office staff maintains your schedule. For one, no one else can access the PDA while you’re carrying it. Second, if you do not regularly synchronize your PDA to the office computer, the staffer can make in-office schedule changes that are not recorded on the PDA version, possibly leading to double booking.
To prevent such mixups, assign one office assistant to update your schedule, and allow only that staffer to make changes. Also, be sure to double check the schedule before booking an appointment on your own.
Many major personal information managers such as Microsoft Outlook and Lotus Notes can be synchronized to a handheld. This allows your staff to view and simultaneously edit your schedule in the office, which is then synchronized onto the handheld. What’s more, users who cannot access your computer but have the proper privileges to your information can still connect to your schedule.
If a significant other uses a Palm OS device and you need to coordinate your schedule with his or hers, WeSync provides a solution that synchronizes both schedules via an online server and displays them side by side on your Palm OS PDA. Although WeSync no longer provides tech support for it, the software and service (which PalmOne owns) is still available.
Schedule synchronization between Pocket PC users is tricky: It cannot be done via an Internet server because each device has a one-to-one relationship with Microsoft Outlook, the desktop schedule software.
ManyPartners overcomes this limitation by allowing users to connect two Pocket PC devices to the same desktop scheduling software. Both users then must share information on one profile in Microsoft Outlook and must enter information in a way that avoids confusion, such as “John: doctor’s appointment” and “Mary: lecture on Monday.”)
Making your days colorful
Third-party software can make your calendar easier to use by employing color and icons to designate types of appointments (Table). This software also provides quick access to other information, such as task lists and notes.
For Palm OS devices, products such as Agendus, DateBk5, and Beyond Contacts provide a different and enhanced way to view your data. Similar software for the Pocket PC or Windows Mobile 2003 devices include Ulti-Planner, Pocket Informant, and Agenda Fusion and Agenda Today from DeveloperOne.
Some programs use the PDA’s built-in database while others have a proprietary database. This difference is important because it affects your ability to “beam” information from one device to another. For example, a proprietary database can facilitate data exchange, but only if the user to whom you are sending the data also uses a device with a proprietary database.
Online scheduling
Internet-based scheduling programs can also prevent double-booking. The user enters prospective meeting dates into the online form; the software then checks the dates against the calendar for conflicts and selects the best date. These schedules can also be synchronized to a PDA.
One drawback: changes to the online and PDA schedules must be entered separately, creating the potential for double booking. To avoid this:
- assign one staff member to make schedule changes
- or use a wireless PDA or one with a built-in cellular phone to allow instant schedule updates and prevent miscommunication.
Appointmentquest.com and scheduling.com-two services geared toward health care providers-provide online schedules, can handle multiple service locations and personnel, and will generate reminders for patients. Scheduling.com also can track multiple types of insurance and can provide billing systems with patient registration information to generate statements.
Schedule enhancement software options
| SOLUTION | WEBSITE URL | OS | COST |
|---|---|---|---|
| WeSync | www.wesync.com | Palm | Free |
| Beyond Contacts | www.dataviz.com | Palm | $49.95 |
| Agendus | www.iambic.com | Palm | $25.95 standard $39.95 professional |
| DateBK5 | www.pimlicosoftware.com | Palm | $24.95 |
| Agenda Fusion | www.developerone.com | Pocket PC | $29.95 |
| Pocket Informant | www.pocketinformant.com | Pocket PC | $24.95 |
| Ulti-Planner | www.uglybass.com/ultiplanner | Pocket PC | $14.95 |
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected] .
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
Like many doctors, you often feel the need to be two places at once.
The trick is to avoid scheduling two commitments at the same time. Anyone who has had to manage a busy practice schedule and remember his or her child’s soccer practice knows this can be challenging.
If you’re looking to organize your schedule, the Internet or your personal digital assistant (PDA) may hold the answer. This article will review scheduling solutions and help you decide which is right for your practice.
PDA scheduling-pros and cons
Both Palm and Pocket PC devices offer very good scheduling capabilities; your choice of operating system depends upon whether you need to use Microsoft Outlook. In my view, Palm is simpler and more reliable, whereas I have had more trouble connecting a Pocket PC device to my desktop computer.
Keeping a calendar in a handheld computer can be quite handy. Simply pull out the PDA, click once or twice, and view your daily schedule. Used properly, this can help prevent scheduling conflicts.
This feature, however, may be less helpful if your office staff maintains your schedule. For one, no one else can access the PDA while you’re carrying it. Second, if you do not regularly synchronize your PDA to the office computer, the staffer can make in-office schedule changes that are not recorded on the PDA version, possibly leading to double booking.
To prevent such mixups, assign one office assistant to update your schedule, and allow only that staffer to make changes. Also, be sure to double check the schedule before booking an appointment on your own.
Many major personal information managers such as Microsoft Outlook and Lotus Notes can be synchronized to a handheld. This allows your staff to view and simultaneously edit your schedule in the office, which is then synchronized onto the handheld. What’s more, users who cannot access your computer but have the proper privileges to your information can still connect to your schedule.
If a significant other uses a Palm OS device and you need to coordinate your schedule with his or hers, WeSync provides a solution that synchronizes both schedules via an online server and displays them side by side on your Palm OS PDA. Although WeSync no longer provides tech support for it, the software and service (which PalmOne owns) is still available.
Schedule synchronization between Pocket PC users is tricky: It cannot be done via an Internet server because each device has a one-to-one relationship with Microsoft Outlook, the desktop schedule software.
ManyPartners overcomes this limitation by allowing users to connect two Pocket PC devices to the same desktop scheduling software. Both users then must share information on one profile in Microsoft Outlook and must enter information in a way that avoids confusion, such as “John: doctor’s appointment” and “Mary: lecture on Monday.”)
Making your days colorful
Third-party software can make your calendar easier to use by employing color and icons to designate types of appointments (Table). This software also provides quick access to other information, such as task lists and notes.
For Palm OS devices, products such as Agendus, DateBk5, and Beyond Contacts provide a different and enhanced way to view your data. Similar software for the Pocket PC or Windows Mobile 2003 devices include Ulti-Planner, Pocket Informant, and Agenda Fusion and Agenda Today from DeveloperOne.
Some programs use the PDA’s built-in database while others have a proprietary database. This difference is important because it affects your ability to “beam” information from one device to another. For example, a proprietary database can facilitate data exchange, but only if the user to whom you are sending the data also uses a device with a proprietary database.
Online scheduling
Internet-based scheduling programs can also prevent double-booking. The user enters prospective meeting dates into the online form; the software then checks the dates against the calendar for conflicts and selects the best date. These schedules can also be synchronized to a PDA.
One drawback: changes to the online and PDA schedules must be entered separately, creating the potential for double booking. To avoid this:
- assign one staff member to make schedule changes
- or use a wireless PDA or one with a built-in cellular phone to allow instant schedule updates and prevent miscommunication.
Appointmentquest.com and scheduling.com-two services geared toward health care providers-provide online schedules, can handle multiple service locations and personnel, and will generate reminders for patients. Scheduling.com also can track multiple types of insurance and can provide billing systems with patient registration information to generate statements.
Schedule enhancement software options
| SOLUTION | WEBSITE URL | OS | COST |
|---|---|---|---|
| WeSync | www.wesync.com | Palm | Free |
| Beyond Contacts | www.dataviz.com | Palm | $49.95 |
| Agendus | www.iambic.com | Palm | $25.95 standard $39.95 professional |
| DateBK5 | www.pimlicosoftware.com | Palm | $24.95 |
| Agenda Fusion | www.developerone.com | Pocket PC | $29.95 |
| Pocket Informant | www.pocketinformant.com | Pocket PC | $24.95 |
| Ulti-Planner | www.uglybass.com/ultiplanner | Pocket PC | $14.95 |
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected] .
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
Depressed patients won’t exercise? 7 ways to get them started
Exercise has been shown to significantly reduce depressive symptoms and decrease the chances of relapse.1
If you’re having trouble getting a depressed patient for whom exercise is not contraindicated to take that first step on the road to fitness, the following motivational tips can help:
- Explain how exercise can decrease mild to moderate depressive symptoms and improve overall health.2
- Ask patients to document the time they spend watching television, sitting in traffic, or lying on the couch, and to compare this with time spent doing physical activity. By keeping this record, patients often discover they are not exercising nearly enough.
- Emphasize that yoga, t’ai chi, aerobics, and walking are all effective types of exercise. Patients often associate “exercise” with weightlifting or long-distance running and do not consider less-strenuous options.
- Tell unmotivated patients that getting started is the hardest part. The more a patient exercises, the more motivated he or she will feel.
Keys to safe exercise
Once the patient decides to begin exercising, tell him or her to:
- Start with a light-intensity workout and gradually increase the regimen’s intensity. Encourage the patient to start with at least a once-weekly routine and advise him or her that overexertion can lead to injury.
Have a physical therapist or exercise trainer devise the program. For older or medically ill patients, clearance from an internist or family physician may be necessary.
- Exercise where the patient feels most comfortable. For example, patients who are obese or are self-conscious in public settings may prefer to work out at home.
- Keep exercising regularly and in moderation to guard against exercise addiction, “burnout,” or overtraining. Warn patients that stopping regular exercise can lead to rebound depression, anxiety, and insomnia.3 These pitfalls—which could occur as early as 2 weeks after stopping—can derail a fitness regimen and obstruct future attempts at exercise.
1. Martinsen EW, Hoffart A, Solberg O. Comparing aerobic with nonaerobic forms of exercise in the treatment of clinical depression: a randomized trial. Compr Psychiatry 1989;30:324-31.
2. Penninx BW, Rejeski WJ, Pandya J, et al. Exercise and depressive symptoms. A comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. J Gerontol B Psychol Sci Soc Sci 2002;57:124-32.
3. Morris M, Steinberg H, Sykes EA. Effects of temporary withdrawal from regular running. J Psychosom Res 1990;34:493-500.
Dr. Pillay is assistant neuroscientist at McLean Hospital, Belmont, MA, and an instructor of psychiatry at Harvard Medical School, Boston.
Exercise has been shown to significantly reduce depressive symptoms and decrease the chances of relapse.1
If you’re having trouble getting a depressed patient for whom exercise is not contraindicated to take that first step on the road to fitness, the following motivational tips can help:
- Explain how exercise can decrease mild to moderate depressive symptoms and improve overall health.2
- Ask patients to document the time they spend watching television, sitting in traffic, or lying on the couch, and to compare this with time spent doing physical activity. By keeping this record, patients often discover they are not exercising nearly enough.
- Emphasize that yoga, t’ai chi, aerobics, and walking are all effective types of exercise. Patients often associate “exercise” with weightlifting or long-distance running and do not consider less-strenuous options.
- Tell unmotivated patients that getting started is the hardest part. The more a patient exercises, the more motivated he or she will feel.
Keys to safe exercise
Once the patient decides to begin exercising, tell him or her to:
- Start with a light-intensity workout and gradually increase the regimen’s intensity. Encourage the patient to start with at least a once-weekly routine and advise him or her that overexertion can lead to injury.
Have a physical therapist or exercise trainer devise the program. For older or medically ill patients, clearance from an internist or family physician may be necessary.
- Exercise where the patient feels most comfortable. For example, patients who are obese or are self-conscious in public settings may prefer to work out at home.
- Keep exercising regularly and in moderation to guard against exercise addiction, “burnout,” or overtraining. Warn patients that stopping regular exercise can lead to rebound depression, anxiety, and insomnia.3 These pitfalls—which could occur as early as 2 weeks after stopping—can derail a fitness regimen and obstruct future attempts at exercise.
Exercise has been shown to significantly reduce depressive symptoms and decrease the chances of relapse.1
If you’re having trouble getting a depressed patient for whom exercise is not contraindicated to take that first step on the road to fitness, the following motivational tips can help:
- Explain how exercise can decrease mild to moderate depressive symptoms and improve overall health.2
- Ask patients to document the time they spend watching television, sitting in traffic, or lying on the couch, and to compare this with time spent doing physical activity. By keeping this record, patients often discover they are not exercising nearly enough.
- Emphasize that yoga, t’ai chi, aerobics, and walking are all effective types of exercise. Patients often associate “exercise” with weightlifting or long-distance running and do not consider less-strenuous options.
- Tell unmotivated patients that getting started is the hardest part. The more a patient exercises, the more motivated he or she will feel.
Keys to safe exercise
Once the patient decides to begin exercising, tell him or her to:
- Start with a light-intensity workout and gradually increase the regimen’s intensity. Encourage the patient to start with at least a once-weekly routine and advise him or her that overexertion can lead to injury.
Have a physical therapist or exercise trainer devise the program. For older or medically ill patients, clearance from an internist or family physician may be necessary.
- Exercise where the patient feels most comfortable. For example, patients who are obese or are self-conscious in public settings may prefer to work out at home.
- Keep exercising regularly and in moderation to guard against exercise addiction, “burnout,” or overtraining. Warn patients that stopping regular exercise can lead to rebound depression, anxiety, and insomnia.3 These pitfalls—which could occur as early as 2 weeks after stopping—can derail a fitness regimen and obstruct future attempts at exercise.
1. Martinsen EW, Hoffart A, Solberg O. Comparing aerobic with nonaerobic forms of exercise in the treatment of clinical depression: a randomized trial. Compr Psychiatry 1989;30:324-31.
2. Penninx BW, Rejeski WJ, Pandya J, et al. Exercise and depressive symptoms. A comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. J Gerontol B Psychol Sci Soc Sci 2002;57:124-32.
3. Morris M, Steinberg H, Sykes EA. Effects of temporary withdrawal from regular running. J Psychosom Res 1990;34:493-500.
Dr. Pillay is assistant neuroscientist at McLean Hospital, Belmont, MA, and an instructor of psychiatry at Harvard Medical School, Boston.
1. Martinsen EW, Hoffart A, Solberg O. Comparing aerobic with nonaerobic forms of exercise in the treatment of clinical depression: a randomized trial. Compr Psychiatry 1989;30:324-31.
2. Penninx BW, Rejeski WJ, Pandya J, et al. Exercise and depressive symptoms. A comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. J Gerontol B Psychol Sci Soc Sci 2002;57:124-32.
3. Morris M, Steinberg H, Sykes EA. Effects of temporary withdrawal from regular running. J Psychosom Res 1990;34:493-500.
Dr. Pillay is assistant neuroscientist at McLean Hospital, Belmont, MA, and an instructor of psychiatry at Harvard Medical School, Boston.
Break the ‘fear circuit’ in resistant panic disorder
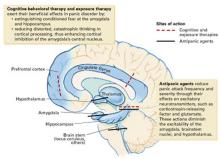
When initial therapy fails to control a patient’s panic attacks, a neuroanatomic model of anxiety disorders may help. This model proposes that panic sufferers have an abnormally sensitive brain “fear circuit.”1 It suggests why both medications and cognitive-behavioral therapy (CBT) are effective for treating panic disorder (PD) and can be used as a guide to more successful treatment.
This article explains the fear circuit model and describes how to determine whether initial drug treatment of panic symptoms has been adequate. It offers evidence-and experience-based dosing ranges, augmentation strategies, tips for antidepressant titration, and solutions to the most common inadequate response problems.
HOW THE FEAR CIRCUIT WORKS
Panic disorder may occur with or without agoraphobia. The diagnosis requires recurrent, unexpected panic attacks (Table 1), with at least one attack followed by 1 month or more of:
- persistent concern about having additional attacks
- worry about the implications of the attack
- or significant change in behavior related to the attack.
Panic disorder is usually accompanied by phobic avoidance and anticipatory anxiety, and it often coexists with other psychiatric disorders. Anxiety disorders may share a common genetic vulnerability. Childhood experiences, gender, and life events may increase or decrease the probability that a biologically vulnerable individual will develop an anxiety disorder or depression.1
Table 1
Panic attacks: The core symptom of panic disorder
| A panic attack is a discrete period of intense fear or discomfort, in which four (or more) of the following symptoms develop abruptly and peak within 10 minutes: |
|
| Source: DSM-IV-TR |
Fear circuit model. PD’s pathophysiology is not completely understood, but evidence suggests that an overactive brain alarm network may increase vulnerability for PD (Box).1,2 Individual patients require different intensities of treatment to normalize their panic symptoms:
Mild to moderate PD (characterized by little or no avoidance and no comorbid disorders) often responds to either medication or CBT. A single intervention—such as using CBT to enhance the cortical inhibitory effects or using medication to reduce the amygdala’s reactivity—may suffice for symptomatic relief.
Severe or complicated PD (characterized by frequent panic attacks, significant agoraphobia, and comorbid anxiety disorders or depression) may require high medication dosages, intense CBT/exposure therapy, or both to normalize more severely disrupted communication among the fear circuit’s components.
ASSESSING TREATMENT OUTCOME
The goal of treatment is remission: a return to functioning without illness-related impairment or loss of quality of life, as if the patient had never been ill. In clinical practice, we can use validated, patient-rated assessment tools to document improvement in panic-related impairment, patient satisfaction, and quality of life—the real targets of treatment. Two useful tools are the Sheehan Disability Scale3 and the Quality of Life Enjoyment and Satisfaction Questionnaire.4
With adequate treatment, achieving remission can take several months or more; without it, remission may never occur. The following guidelines can help ensure that you provide adequate treatment.
What is adequate CBT? When patients’ symptoms fail to respond to CBT, the first step is to examine whether inadequate treatment is the culprit. At least 10 weekly CBT sessions administered by a “qualified professional” has been suggested as an adequate CBT trial for PD.5 Unfortunately, qualified CBT therapists are not always available. If CBT referral is not an option, clinicians can provide patients with at least some elements of CBT, such as education about PD, information resources, and self-exposure instruction as indicated. For more information on CBT for PD, see Related Resources.
What is adequate drug treatment? Noncompliance with medication because a patient fears adverse effects or has insufficient information can easily thwart treatment. Before treatment begins, therefore, it is important to establish your credibility. Provide the patient with information about PD, its treatment options, and what to expect so that he or she can collaborate in treatment (Table 2).
An inherited, abnormally active brain alarm mechanism—or “fear circuit”—may explain panic disorder, according to a theoretical neuroanatomic model.1 Its hub is the central nucleus of the amygdala, which coordinates fear responses via pathways communicating with the hippocampus, thalamus, hypothalamus, brainstem, and cortical processing areas.
The amygdala mediates acute emotional responses, including fear and anxiety. The hypothalamus mediates physiologic changes connected with emotions, such as release of stress hormones and some changes in heart rate. The prefrontal cortex is involved in thinking and memory and may be instrumental in predicting the consequences of rewards or punishments. In vulnerable individuals, defects in coordinating the sensory input among these brain regions may cause the central nucleus to discharge, resulting in a panic attack.
Medication and cognitive-behavioral therapy may reduce fear circuit reactivity and prevent panic attacks by acting at different components of the fear circuit. When the amygdala’s central nucleus no longer overreacts to sensory input, anticipatory anxiety and phobic avoidance usually dissipate over time.2,3 Thus, the fear circuit model integrates the clinical observation that both cognitive-behavioral therapy and medication are effective for treating panic.1
Abnormal interactions among components of this oversensitive fear circuit also may occur in social anxiety disorder, generalized anxiety disorder, posttraumatic stress disorder, and depression.1 In these disorders, communication patterns among the parts of the hypothesized circuit may be disrupted in different ways. The clinical observation that anxious individuals often become depressed when under stress is consistent with this model and with the literature.
Antidepressants are preferred as first-line treatment of PD, even in nondepressed patients. Selective serotonin reuptake inhibitors (SSRIs) are recommended for PD because of their comparable efficacy and tolerability compared with older antipanic agents.6 SSRIs are also effective against other anxiety disorders likely to co-occur with PD.7
Many panic patients are exquisitely sensitive to activation by initial antidepressant dosages. Activation rarely occurs in other disorders, so its appearance suggests that your diagnosis is correct. Clinical strategies to help you manage antidepressant titration are suggested in Table 3.
Table 2
Prescription for success in treating panic disorder
| Relieve patient of perceived burden of being ill Explain the disorder’s familial/genetic origins Describe the fear circuit model Include spouse or significant other in treatment |
| Build patient-physician collaboration Explain potential medication side effects Describe the usual pattern of symptom relief (stop panic attacks → reduce anticipatory anxiety → decrease phobia) Estimate a time frame for improvement Map out next steps if first try is unsuccessful Be available, especially at first |
| Address patient’s long-term medication concerns Discuss safety, long-term efficacy Frame treatment as a pathway to independence from panic attacks Use analogy of diabetes or hypertension to explain that medication is for managing symptoms, rather than a cure Discuss tapering medication after sustained improvement (12 to 18 months) to determine continued need for medication |
In clinical settings, two naturalistic studies suggested that more-favorable outcomes are associated with antipanic medication dosages shown in Table 4 as “possibly effective”—and that most patients with poor medication response received inadequate treatment.8,9Table 4 ’s dosages come from those two studies—published before the efficacy studies of SSRIs in PD—and from later studies of SSRIs and the selective norepinephrine-serotonin reuptake inhibitor (SNRI) venlafaxine.7,8,10
The lower end of the “probably effective” range in Table 4 represents the lowest dose levels generally expected to be effective for PD. Not all agents in the table are FDA-approved for PD, nor are the dosages of approved agents necessarily “within labeling.” Some patients’ symptoms may resolve at higher or lower dosages.
Table 3
Tips to help the patient tolerate antidepressant titration
| Be pre-emptive Before starting therapy, explain that low initial dosing and flexible titration help to control unpleasant but medically safe “jitteriness” known as antidepressant-induced activation Tell the patient that activation rarely occurs in disorders other than PD (“Its appearance suggests that the diagnosis is correct and that we’re likely on the right track”) |
| Be reassuring Tell the patient, “You control the gas peddle—I’ll help you steer” (to an effective dose) |
| Be cautious Start with 25 to 50% of the usual antidepressant initial dosage for depression (Table 4); if too activating, reduce and advance more gradually Activation usually dissipates in 1 to 2 weeks; over time, larger dosage increments are often possible |
| Be attentive Use benzodiazepines or beta blockers as needed to attenuate activation |
Some patients require months to reach and maintain the “probably effective” dosage for at least 6 weeks. Short-term benzodiazepines can be used to control panic symptoms during antidepressant titration, then tapered off.11 We categorize patients who are unable to tolerate an “adequate dose” as not having had a therapeutic trial—not as treatment failures.
No controlled studies of PD have examined the success rate of switching to a second antidepressant after a first one has been ineffective.12 In clinical practice, we may try two different SSRIs and venlafaxine. When switching agents, we usually co-administer the treatments for a few weeks, titrate the second agent upward gradually, then taper and discontinue the first agent over 2 to 4 weeks. We use short-term benzodiazepines as needed.
Partial improvement. Sometimes overall symptoms improve meaningfully, but bothersome panic symptoms remain. Clinical response may improve sufficiently if you raise the medication dosage in increments while monitoring for safety and tolerability. Address medicolegal concerns by documenting in the patient’s chart:
- your rationale for prescribing dosages that exceed FDA guidelines
- that you discussed possible risks versus benefits with the patient, and the patient agrees to the treatment.
When in doubt about using dosages that exceed FDA guidelines for patients with unusually resistant panic symptoms, obtain consultation from an expert or colleague.
Table 4
Recommended drug dosages for panic disorder
| Class/agent | Possibly effective (mg/d) | Probably effective (mg/d) | High dosage (mg/d) | Initial dosage (mg/d) | Confidence level |
|---|---|---|---|---|---|
| SSRIs | |||||
| Citalopram | <20 | 20-60 | >60 | 10 | ++ |
| Escitalopram | <10 | 10-30 | >30 | 5 | ++++ |
| Fluoxetine | <40 | 40-80 | >80 | 10 | ++ |
| Fluvoxamine | <150 | 150-300 | >300 | 25 | ++++ |
| Paroxetine* | <40 | 40-60 | >60 | 5-10 | ++++ |
| Sertraline* | <150 | 150-300 | >300 | 12.5-25 | ++++ |
| SNRI | |||||
| Venlafaxine | <150 | 150-300 | >300 | 18.75-37.5 | ++ |
| Benzodiazepines | |||||
| Alprazolam* | <2 | 2-8 | >8 | 0.5-1.0 | ++++ |
| Clonazepam* | <1 | 2-4 | >4 | 0.25-0.5 | ++++ |
| Tricyclics | |||||
| Clomipramine | <100 | 100-200 | >200 | 10 | ++++ |
| Desipramine | <150 | 150-300 | >300 | 10 | ++ |
| Imipramine | <150 | 150-300 | >300 | 10 | ++++ |
| MAOIs | |||||
| Phenelzine | <45 | 45-90 | >90 | 15 | +++ |
| Tranylcypromine | <30 | 30-70 | >70 | 10 | + |
| Antiepileptics | |||||
| Gabapentin | 100-200 | 600-3,400 | ++ | ||
| Valproate (VPA) | 250-500 | 1,000-2,000 | ++ | ||
| * FDA-approved for treating panic disorder | |||||
| Confidence: | |||||
| + (uncontrolled series) | |||||
| ++ (at least 1 controlled study) | |||||
| +++ (>1 controlled study) | |||||
| ++++ (Unequivocal) | |||||
Using benzodiazepines. As noted above, adjunctive use of benzodiazepines while initiating antidepressant therapy can help extremely anxious or medication-sensitive patients.11 Many clinicians coadminister benzodiazepines with antidepressants over the longer term.7 As a primary treatment, benzodiazepines may be useful for patients who could not tolerate or did not respond to at least two or three antidepressant trials.
Table 5
Solving inadequate response to initial SSRI treatment of panic disorder
| Problem | Differential diagnosis | Suggested solutions |
|---|---|---|
| Persistent panic attacks | Unexpected attacks Inadequate treatment or duration Situational attacks Medical condition Other psychiatric disorder | ≥Threshold dose for 6 weeks Try second SSRI Try venlafaxine CBT/exposure therapy Address specific conditions Rule out social phobia, OCD, PTSD |
| Persistent nonpanic anxiety | Medication-related Activation (SSRI or SNRI) Akathisia from SSRI Comorbid GAD Interdose BZD rebound BZD or alcohol withdrawal Residual anxiety | Adjust dosage, add BZD or beta blocker Adjust dosage, add beta blocker or BZD Increase antidepressant dosage, add BZD Switch to longer-acting agent Assess and treat as indicated Add/increase BZD |
| Residual phobia | Agoraphobia | CBT/exposure, adjust medication |
| Other disorders | Depression Bipolar disorder Personality disorders Medical disorder | Aggressive antidepressant treatment ±BZDs Mood stabilizer and antidepressant ±BZDs Specific psychotherapy Review and modify treatment as indicated |
| Environmental event or stressor(s) | Review work, family events, patient perception of stressor | Family/spouse interview and education Environmental hygiene as indicated Brief adjustment in treatment plan(s) as needed |
| Poor adherence | Drug sexual side effects Inadequate patient or family understanding of panic disorder and its treatment | Try bupropion, sildenafil, amantadine, switch agents Patient/family education Make resource materials available |
| BZD: Benzodiazepine | ||
| CBT: Cognitive-behavioral therapy | ||
| GAD: Generalized anxiety disorder | ||
| OCD: Obsessive-compulsive disorder | ||
| PTSD: Posttraumatic stress disorder | ||
| SNRI: Serotonin-norepinephrine reuptake inhibitor | ||
| SSRI: Selective serotonin reuptake inhibitor | ||
Because benzodiazepine monotherapy does not reliably protect against depression, we advise clinicians to encourage patients to self-monitor and report any signs of emerging depression. Avoid benzodiazepines in patients with a history of alcohol or substance abuse.7
Other agents. Once the mainstay of antipanic treatment, tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) are seldom used today because of their side effects, toxicity in overdose, and—for MAOIs—tyramine-restricted diet. Their usefulness in resistant panic is probably limited to last-ditch efforts.
DISSECTING TREATMENT FAILURE
In uncomplicated PD, lack of improvement after two or more adequate medication trials is unusual. If you observe minimal or no improvement, review carefully for other causes of anxiety or factors that can complicate PD treatment (Table 5).
If no other cause for the persistent symptom(s) is apparent, the fear circuit model may help you decide how to modify or enhance medication treatment, add CBT, or both.
For example:
- If panic attacks persist, advancing the medication dosage (if tolerated and acceptably safe) may help. Consider increasing the dosage, augmenting, or switching to a different agent.
- If persistent attacks are consistently cued to feared situations, try intervening with moreaggressive exposure therapy. Consider whether other disorders such as unrecognized social anxiety disorder, obsessive-compulsive disorder (OCD), or posttraumatic stress disorder (PTSD) may be perpetuating the fearful avoidance.
- If the patient is depressed, consider that depression-related social withdrawal may be causing the avoidance symptoms. Aggressive antidepressant pharmacotherapy is strongly suggested.
AUGMENTATION STRATEGIES
Medication for CBT failure. Only two controlled studies have examined adding an adequate dose of medication after patients failed to respond to exposure/CBT alone:
- One study of 18 hospitalized patients with agoraphobia who failed a course of behavioral psychodynamic therapy reported improvement when clomipramine, 150 mg/d, was given for 3 weeks.13
- In a study of 43 patients who failed initial CBT, greater improvement was reported in patients who received CBT plus paroxetine, 40 mg/d, compared with those who received placebo while continuing CBT.14
Augmentation in drug therapy. Only one controlled study has examined augmentation therapy after lack of response to an SSRI—in this case 8 weeks of fluoxetine after two undefined “antidepressant failures.” When pindolol, 2.5 mg tid, or placebo were added to the fluoxetine therapy, the 13 patients who received pindolol improved clinically and statistically more on several standardized ratings than the 12 who received placebo.15
An 8-week, open-label trial showed beneficial effects of olanzapine, up to 20 mg/d, in patients with well-described treatment-resistant PD.16
Other well-described treatment adjustments reported to benefit nonresponsive PD include:
- Adding fluoxetine to a TCA or adding a TCA to fluoxetine, for TCA/SSRI combination therapy17
- Switching to the selective norepinephrine reuptake inhibitor reboxetine, 2 to 8 mg/d for 6 weeks after inadequate paroxetine or fluoxetine response (average of 8 weeks, maximum dosage 40 mg/d).18 (Note: Reboxetine is not available in the United States.)
- Using open-label gabapentin, 600 to 2,400 mg/d, after two SSRI treatment failures.19
- Adding the dopamine receptor agonist pramipexole, 1.0 to 1.5 mg/d, to various antipanic medications.20
Augmenting an SSRI with pindolol or supplementing unsuccessful behavioral treatment with “probably effective” dosages of paroxetine or clomipramine could be recommended with some confidence, although more definitive studies are needed. As outlined above, some strategies17-20 might be considered if a patient fails to respond to two or more adequate medication trials. Anecdotal reports are difficult to assess but may be clinically useful when other treatment options have been exhausted.
- Barlow DH. Anxiety and its disorders: the nature and treatment of anxiety and panic New York: Guilford Press, 1988.
- Craske MG, DeCola JP, Sachs AD, Pontillo DC. Panic control treatment of agoraphobia. J Anxiety Disord 2003;17:321-33.
- National Institute for Mental Health: Panic Disorder http://www.nimh.nih.gov/publicat/fearandtrauma.cfm
- Anxiety Disorders Association of America http://www.adaa.org/
Drug brand names
- Alprazolam • Xanax
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Imipramine • Tofranil
- Olanzapine • Zyprexa
- Phenelzine • Nardil
- Pindolol • Visken
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Reboxetine • Vestra
- Sertraline • Zoloft
- Tranylcypromine • Parnate
- Venlafaxine • Effexor
Disclosure
Dr. Lydiard receives research support from GlaxoSmithKline, Eli Lilly and Co., Organon, Sanofi-Synthelabo, Cephalon, UCB Pharma, and Merck & Co. and he is a speaker for or consultant to Pfizer Inc., Eli Lilly and Co., Solvay Pharmaceuticals, AstraZeneca Pharmaceuticals, and Forest Pharmaceuticals.
1. Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revisited. Am J Psychiatry 2000;157:493-505.
2. Coplan JD, Lydiard RB. Brain circuits in panic disorder. Biol Psychiatry 1998;44:1264-76.
3. Sheehan DV. The anxiety disease. New York: Charles Scribner and Sons, 1983;151.-
4. Rapaport MH, Wolkow RM, Clary CM. Methodologies and outcomes from the sertraline multicenter flexible-dose trials. Psychopharmacol Bull 1998;34:183-9.
5. Otto MW. Psychosocial approach to treatment-resistant anxiety disorders (presentation). Chantilly, VA: Anxiety Disorders Association of America conference on novel approaches to treatment of refractory anxiety disorders, June 15-16, 2003.
6. Gorman JM, Shear MK, McIntyre JS, Zarin DA. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. Am J Psychiatry 1998;155(May supplement).
7. Lydiard RB, Otto MW, Milrod B. Panic disorder treatment. In: Gabbard, GO (ed). Treatment of psychiatric disorders (3rd ed). Washington, DC: American Psychiatric Press, Inc, 2001;1447-82.
8. Simon NM, Safrens SA, Otto MW, et al. Outcome with pharmacotherapy in a naturalistic study of panic disorder. J Affect Disord 2002;69:201-8.
9. Yonkers KA, Ellison J, Shera D, et al. Description of antipanic therapy in a prospective longitudinal study. J Clin Psychopharmacol 1996;16:223-32.
10. Pollack MH, Worthington JJ, 3rd, Otto MW, et al. Venlafaxine for panic disorder: results from a double-blind, placebo-controlled study. Psychopharmacol Bull 1996;32:667-70.
11. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry 2001;58:681-6.
12. Simon NM. Pharmacological approach to treatment-resistant anxiety disorders (presentation). Chantilly, VA: Anxiety Disorders Association of America conference on novel approaches to treatment of refractory anxiety disorders, June 15-16, 2003.
13. Hoffart A, Due-Madsen J, Lande B, et al. Clomipramine in the treatment of agoraphobic inpatients resistant to behavioral therapy. J Clin Psychiatry 1993;54:481-7.
14. Kampman M, Keijsers GP, Hoogduin CA, Hendriks GJ. A randomized, double-blind, placebo-controlled study of the effects of adjunctive paroxetine in panic disorder patients unsuccessfully treated with cognitive-behavioral therapy alone. J Clin Psychiatry 2002;63:772-7.
15. Hirschmann S, Dannon PN, Iancu I, et al. Pindolol augmentation in patients with treatment-resistant panic disorder: a double-blind, placebo-controlled trial. J Clin Psychopharmacol 2000;20:556-9.
16. Hollifield M, Thompson P, Uhlenluth E. Potential efficacy and safety of olanzapine in refractory panic disorder (presentation). San Francisco: American Psychiatric Association annual meeting, 2003.
17. Tiffon L, Coplan J, Papp L, Gorman J. Augmentation strategies with tricyclic or fluoxetine treatment in seven partially responsive panic disorder patients. J Clin Psychiatry 1994;55:66-9.
18. Dannon PN, Iancu I, Grunhaus L. The efficacy of reboxetine in treatment-refractory patients with panic disorder: an open-label study. Hum Psychopharmacol 2002;17:329-33.
19. Chiu S. Gabapentin treatment response in SSRI-refractory panic disorder (presentation) San Francisco: American Psychiatric Association annual meeting, 2003.
20. Marazziti D, Presta S, Pfanner C, et al. Pramipexole augmentation in panic with agoraphobia. Am J Psychiatry 2001;158:498-9.
When initial therapy fails to control a patient’s panic attacks, a neuroanatomic model of anxiety disorders may help. This model proposes that panic sufferers have an abnormally sensitive brain “fear circuit.”1 It suggests why both medications and cognitive-behavioral therapy (CBT) are effective for treating panic disorder (PD) and can be used as a guide to more successful treatment.
This article explains the fear circuit model and describes how to determine whether initial drug treatment of panic symptoms has been adequate. It offers evidence-and experience-based dosing ranges, augmentation strategies, tips for antidepressant titration, and solutions to the most common inadequate response problems.
HOW THE FEAR CIRCUIT WORKS
Panic disorder may occur with or without agoraphobia. The diagnosis requires recurrent, unexpected panic attacks (Table 1), with at least one attack followed by 1 month or more of:
- persistent concern about having additional attacks
- worry about the implications of the attack
- or significant change in behavior related to the attack.
Panic disorder is usually accompanied by phobic avoidance and anticipatory anxiety, and it often coexists with other psychiatric disorders. Anxiety disorders may share a common genetic vulnerability. Childhood experiences, gender, and life events may increase or decrease the probability that a biologically vulnerable individual will develop an anxiety disorder or depression.1
Table 1
Panic attacks: The core symptom of panic disorder
| A panic attack is a discrete period of intense fear or discomfort, in which four (or more) of the following symptoms develop abruptly and peak within 10 minutes: |
|
| Source: DSM-IV-TR |
Fear circuit model. PD’s pathophysiology is not completely understood, but evidence suggests that an overactive brain alarm network may increase vulnerability for PD (Box).1,2 Individual patients require different intensities of treatment to normalize their panic symptoms:
Mild to moderate PD (characterized by little or no avoidance and no comorbid disorders) often responds to either medication or CBT. A single intervention—such as using CBT to enhance the cortical inhibitory effects or using medication to reduce the amygdala’s reactivity—may suffice for symptomatic relief.
Severe or complicated PD (characterized by frequent panic attacks, significant agoraphobia, and comorbid anxiety disorders or depression) may require high medication dosages, intense CBT/exposure therapy, or both to normalize more severely disrupted communication among the fear circuit’s components.
ASSESSING TREATMENT OUTCOME
The goal of treatment is remission: a return to functioning without illness-related impairment or loss of quality of life, as if the patient had never been ill. In clinical practice, we can use validated, patient-rated assessment tools to document improvement in panic-related impairment, patient satisfaction, and quality of life—the real targets of treatment. Two useful tools are the Sheehan Disability Scale3 and the Quality of Life Enjoyment and Satisfaction Questionnaire.4
With adequate treatment, achieving remission can take several months or more; without it, remission may never occur. The following guidelines can help ensure that you provide adequate treatment.
What is adequate CBT? When patients’ symptoms fail to respond to CBT, the first step is to examine whether inadequate treatment is the culprit. At least 10 weekly CBT sessions administered by a “qualified professional” has been suggested as an adequate CBT trial for PD.5 Unfortunately, qualified CBT therapists are not always available. If CBT referral is not an option, clinicians can provide patients with at least some elements of CBT, such as education about PD, information resources, and self-exposure instruction as indicated. For more information on CBT for PD, see Related Resources.
What is adequate drug treatment? Noncompliance with medication because a patient fears adverse effects or has insufficient information can easily thwart treatment. Before treatment begins, therefore, it is important to establish your credibility. Provide the patient with information about PD, its treatment options, and what to expect so that he or she can collaborate in treatment (Table 2).
An inherited, abnormally active brain alarm mechanism—or “fear circuit”—may explain panic disorder, according to a theoretical neuroanatomic model.1 Its hub is the central nucleus of the amygdala, which coordinates fear responses via pathways communicating with the hippocampus, thalamus, hypothalamus, brainstem, and cortical processing areas.
The amygdala mediates acute emotional responses, including fear and anxiety. The hypothalamus mediates physiologic changes connected with emotions, such as release of stress hormones and some changes in heart rate. The prefrontal cortex is involved in thinking and memory and may be instrumental in predicting the consequences of rewards or punishments. In vulnerable individuals, defects in coordinating the sensory input among these brain regions may cause the central nucleus to discharge, resulting in a panic attack.
Medication and cognitive-behavioral therapy may reduce fear circuit reactivity and prevent panic attacks by acting at different components of the fear circuit. When the amygdala’s central nucleus no longer overreacts to sensory input, anticipatory anxiety and phobic avoidance usually dissipate over time.2,3 Thus, the fear circuit model integrates the clinical observation that both cognitive-behavioral therapy and medication are effective for treating panic.1
Abnormal interactions among components of this oversensitive fear circuit also may occur in social anxiety disorder, generalized anxiety disorder, posttraumatic stress disorder, and depression.1 In these disorders, communication patterns among the parts of the hypothesized circuit may be disrupted in different ways. The clinical observation that anxious individuals often become depressed when under stress is consistent with this model and with the literature.
Antidepressants are preferred as first-line treatment of PD, even in nondepressed patients. Selective serotonin reuptake inhibitors (SSRIs) are recommended for PD because of their comparable efficacy and tolerability compared with older antipanic agents.6 SSRIs are also effective against other anxiety disorders likely to co-occur with PD.7
Many panic patients are exquisitely sensitive to activation by initial antidepressant dosages. Activation rarely occurs in other disorders, so its appearance suggests that your diagnosis is correct. Clinical strategies to help you manage antidepressant titration are suggested in Table 3.
Table 2
Prescription for success in treating panic disorder
| Relieve patient of perceived burden of being ill Explain the disorder’s familial/genetic origins Describe the fear circuit model Include spouse or significant other in treatment |
| Build patient-physician collaboration Explain potential medication side effects Describe the usual pattern of symptom relief (stop panic attacks → reduce anticipatory anxiety → decrease phobia) Estimate a time frame for improvement Map out next steps if first try is unsuccessful Be available, especially at first |
| Address patient’s long-term medication concerns Discuss safety, long-term efficacy Frame treatment as a pathway to independence from panic attacks Use analogy of diabetes or hypertension to explain that medication is for managing symptoms, rather than a cure Discuss tapering medication after sustained improvement (12 to 18 months) to determine continued need for medication |
In clinical settings, two naturalistic studies suggested that more-favorable outcomes are associated with antipanic medication dosages shown in Table 4 as “possibly effective”—and that most patients with poor medication response received inadequate treatment.8,9Table 4 ’s dosages come from those two studies—published before the efficacy studies of SSRIs in PD—and from later studies of SSRIs and the selective norepinephrine-serotonin reuptake inhibitor (SNRI) venlafaxine.7,8,10
The lower end of the “probably effective” range in Table 4 represents the lowest dose levels generally expected to be effective for PD. Not all agents in the table are FDA-approved for PD, nor are the dosages of approved agents necessarily “within labeling.” Some patients’ symptoms may resolve at higher or lower dosages.
Table 3
Tips to help the patient tolerate antidepressant titration
| Be pre-emptive Before starting therapy, explain that low initial dosing and flexible titration help to control unpleasant but medically safe “jitteriness” known as antidepressant-induced activation Tell the patient that activation rarely occurs in disorders other than PD (“Its appearance suggests that the diagnosis is correct and that we’re likely on the right track”) |
| Be reassuring Tell the patient, “You control the gas peddle—I’ll help you steer” (to an effective dose) |
| Be cautious Start with 25 to 50% of the usual antidepressant initial dosage for depression (Table 4); if too activating, reduce and advance more gradually Activation usually dissipates in 1 to 2 weeks; over time, larger dosage increments are often possible |
| Be attentive Use benzodiazepines or beta blockers as needed to attenuate activation |
Some patients require months to reach and maintain the “probably effective” dosage for at least 6 weeks. Short-term benzodiazepines can be used to control panic symptoms during antidepressant titration, then tapered off.11 We categorize patients who are unable to tolerate an “adequate dose” as not having had a therapeutic trial—not as treatment failures.
No controlled studies of PD have examined the success rate of switching to a second antidepressant after a first one has been ineffective.12 In clinical practice, we may try two different SSRIs and venlafaxine. When switching agents, we usually co-administer the treatments for a few weeks, titrate the second agent upward gradually, then taper and discontinue the first agent over 2 to 4 weeks. We use short-term benzodiazepines as needed.
Partial improvement. Sometimes overall symptoms improve meaningfully, but bothersome panic symptoms remain. Clinical response may improve sufficiently if you raise the medication dosage in increments while monitoring for safety and tolerability. Address medicolegal concerns by documenting in the patient’s chart:
- your rationale for prescribing dosages that exceed FDA guidelines
- that you discussed possible risks versus benefits with the patient, and the patient agrees to the treatment.
When in doubt about using dosages that exceed FDA guidelines for patients with unusually resistant panic symptoms, obtain consultation from an expert or colleague.
Table 4
Recommended drug dosages for panic disorder
| Class/agent | Possibly effective (mg/d) | Probably effective (mg/d) | High dosage (mg/d) | Initial dosage (mg/d) | Confidence level |
|---|---|---|---|---|---|
| SSRIs | |||||
| Citalopram | <20 | 20-60 | >60 | 10 | ++ |
| Escitalopram | <10 | 10-30 | >30 | 5 | ++++ |
| Fluoxetine | <40 | 40-80 | >80 | 10 | ++ |
| Fluvoxamine | <150 | 150-300 | >300 | 25 | ++++ |
| Paroxetine* | <40 | 40-60 | >60 | 5-10 | ++++ |
| Sertraline* | <150 | 150-300 | >300 | 12.5-25 | ++++ |
| SNRI | |||||
| Venlafaxine | <150 | 150-300 | >300 | 18.75-37.5 | ++ |
| Benzodiazepines | |||||
| Alprazolam* | <2 | 2-8 | >8 | 0.5-1.0 | ++++ |
| Clonazepam* | <1 | 2-4 | >4 | 0.25-0.5 | ++++ |
| Tricyclics | |||||
| Clomipramine | <100 | 100-200 | >200 | 10 | ++++ |
| Desipramine | <150 | 150-300 | >300 | 10 | ++ |
| Imipramine | <150 | 150-300 | >300 | 10 | ++++ |
| MAOIs | |||||
| Phenelzine | <45 | 45-90 | >90 | 15 | +++ |
| Tranylcypromine | <30 | 30-70 | >70 | 10 | + |
| Antiepileptics | |||||
| Gabapentin | 100-200 | 600-3,400 | ++ | ||
| Valproate (VPA) | 250-500 | 1,000-2,000 | ++ | ||
| * FDA-approved for treating panic disorder | |||||
| Confidence: | |||||
| + (uncontrolled series) | |||||
| ++ (at least 1 controlled study) | |||||
| +++ (>1 controlled study) | |||||
| ++++ (Unequivocal) | |||||
Using benzodiazepines. As noted above, adjunctive use of benzodiazepines while initiating antidepressant therapy can help extremely anxious or medication-sensitive patients.11 Many clinicians coadminister benzodiazepines with antidepressants over the longer term.7 As a primary treatment, benzodiazepines may be useful for patients who could not tolerate or did not respond to at least two or three antidepressant trials.
Table 5
Solving inadequate response to initial SSRI treatment of panic disorder
| Problem | Differential diagnosis | Suggested solutions |
|---|---|---|
| Persistent panic attacks | Unexpected attacks Inadequate treatment or duration Situational attacks Medical condition Other psychiatric disorder | ≥Threshold dose for 6 weeks Try second SSRI Try venlafaxine CBT/exposure therapy Address specific conditions Rule out social phobia, OCD, PTSD |
| Persistent nonpanic anxiety | Medication-related Activation (SSRI or SNRI) Akathisia from SSRI Comorbid GAD Interdose BZD rebound BZD or alcohol withdrawal Residual anxiety | Adjust dosage, add BZD or beta blocker Adjust dosage, add beta blocker or BZD Increase antidepressant dosage, add BZD Switch to longer-acting agent Assess and treat as indicated Add/increase BZD |
| Residual phobia | Agoraphobia | CBT/exposure, adjust medication |
| Other disorders | Depression Bipolar disorder Personality disorders Medical disorder | Aggressive antidepressant treatment ±BZDs Mood stabilizer and antidepressant ±BZDs Specific psychotherapy Review and modify treatment as indicated |
| Environmental event or stressor(s) | Review work, family events, patient perception of stressor | Family/spouse interview and education Environmental hygiene as indicated Brief adjustment in treatment plan(s) as needed |
| Poor adherence | Drug sexual side effects Inadequate patient or family understanding of panic disorder and its treatment | Try bupropion, sildenafil, amantadine, switch agents Patient/family education Make resource materials available |
| BZD: Benzodiazepine | ||
| CBT: Cognitive-behavioral therapy | ||
| GAD: Generalized anxiety disorder | ||
| OCD: Obsessive-compulsive disorder | ||
| PTSD: Posttraumatic stress disorder | ||
| SNRI: Serotonin-norepinephrine reuptake inhibitor | ||
| SSRI: Selective serotonin reuptake inhibitor | ||
Because benzodiazepine monotherapy does not reliably protect against depression, we advise clinicians to encourage patients to self-monitor and report any signs of emerging depression. Avoid benzodiazepines in patients with a history of alcohol or substance abuse.7
Other agents. Once the mainstay of antipanic treatment, tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) are seldom used today because of their side effects, toxicity in overdose, and—for MAOIs—tyramine-restricted diet. Their usefulness in resistant panic is probably limited to last-ditch efforts.
DISSECTING TREATMENT FAILURE
In uncomplicated PD, lack of improvement after two or more adequate medication trials is unusual. If you observe minimal or no improvement, review carefully for other causes of anxiety or factors that can complicate PD treatment (Table 5).
If no other cause for the persistent symptom(s) is apparent, the fear circuit model may help you decide how to modify or enhance medication treatment, add CBT, or both.
For example:
- If panic attacks persist, advancing the medication dosage (if tolerated and acceptably safe) may help. Consider increasing the dosage, augmenting, or switching to a different agent.
- If persistent attacks are consistently cued to feared situations, try intervening with moreaggressive exposure therapy. Consider whether other disorders such as unrecognized social anxiety disorder, obsessive-compulsive disorder (OCD), or posttraumatic stress disorder (PTSD) may be perpetuating the fearful avoidance.
- If the patient is depressed, consider that depression-related social withdrawal may be causing the avoidance symptoms. Aggressive antidepressant pharmacotherapy is strongly suggested.
AUGMENTATION STRATEGIES
Medication for CBT failure. Only two controlled studies have examined adding an adequate dose of medication after patients failed to respond to exposure/CBT alone:
- One study of 18 hospitalized patients with agoraphobia who failed a course of behavioral psychodynamic therapy reported improvement when clomipramine, 150 mg/d, was given for 3 weeks.13
- In a study of 43 patients who failed initial CBT, greater improvement was reported in patients who received CBT plus paroxetine, 40 mg/d, compared with those who received placebo while continuing CBT.14
Augmentation in drug therapy. Only one controlled study has examined augmentation therapy after lack of response to an SSRI—in this case 8 weeks of fluoxetine after two undefined “antidepressant failures.” When pindolol, 2.5 mg tid, or placebo were added to the fluoxetine therapy, the 13 patients who received pindolol improved clinically and statistically more on several standardized ratings than the 12 who received placebo.15
An 8-week, open-label trial showed beneficial effects of olanzapine, up to 20 mg/d, in patients with well-described treatment-resistant PD.16
Other well-described treatment adjustments reported to benefit nonresponsive PD include:
- Adding fluoxetine to a TCA or adding a TCA to fluoxetine, for TCA/SSRI combination therapy17
- Switching to the selective norepinephrine reuptake inhibitor reboxetine, 2 to 8 mg/d for 6 weeks after inadequate paroxetine or fluoxetine response (average of 8 weeks, maximum dosage 40 mg/d).18 (Note: Reboxetine is not available in the United States.)
- Using open-label gabapentin, 600 to 2,400 mg/d, after two SSRI treatment failures.19
- Adding the dopamine receptor agonist pramipexole, 1.0 to 1.5 mg/d, to various antipanic medications.20
Augmenting an SSRI with pindolol or supplementing unsuccessful behavioral treatment with “probably effective” dosages of paroxetine or clomipramine could be recommended with some confidence, although more definitive studies are needed. As outlined above, some strategies17-20 might be considered if a patient fails to respond to two or more adequate medication trials. Anecdotal reports are difficult to assess but may be clinically useful when other treatment options have been exhausted.
- Barlow DH. Anxiety and its disorders: the nature and treatment of anxiety and panic New York: Guilford Press, 1988.
- Craske MG, DeCola JP, Sachs AD, Pontillo DC. Panic control treatment of agoraphobia. J Anxiety Disord 2003;17:321-33.
- National Institute for Mental Health: Panic Disorder http://www.nimh.nih.gov/publicat/fearandtrauma.cfm
- Anxiety Disorders Association of America http://www.adaa.org/
Drug brand names
- Alprazolam • Xanax
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Imipramine • Tofranil
- Olanzapine • Zyprexa
- Phenelzine • Nardil
- Pindolol • Visken
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Reboxetine • Vestra
- Sertraline • Zoloft
- Tranylcypromine • Parnate
- Venlafaxine • Effexor
Disclosure
Dr. Lydiard receives research support from GlaxoSmithKline, Eli Lilly and Co., Organon, Sanofi-Synthelabo, Cephalon, UCB Pharma, and Merck & Co. and he is a speaker for or consultant to Pfizer Inc., Eli Lilly and Co., Solvay Pharmaceuticals, AstraZeneca Pharmaceuticals, and Forest Pharmaceuticals.
When initial therapy fails to control a patient’s panic attacks, a neuroanatomic model of anxiety disorders may help. This model proposes that panic sufferers have an abnormally sensitive brain “fear circuit.”1 It suggests why both medications and cognitive-behavioral therapy (CBT) are effective for treating panic disorder (PD) and can be used as a guide to more successful treatment.
This article explains the fear circuit model and describes how to determine whether initial drug treatment of panic symptoms has been adequate. It offers evidence-and experience-based dosing ranges, augmentation strategies, tips for antidepressant titration, and solutions to the most common inadequate response problems.
HOW THE FEAR CIRCUIT WORKS
Panic disorder may occur with or without agoraphobia. The diagnosis requires recurrent, unexpected panic attacks (Table 1), with at least one attack followed by 1 month or more of:
- persistent concern about having additional attacks
- worry about the implications of the attack
- or significant change in behavior related to the attack.
Panic disorder is usually accompanied by phobic avoidance and anticipatory anxiety, and it often coexists with other psychiatric disorders. Anxiety disorders may share a common genetic vulnerability. Childhood experiences, gender, and life events may increase or decrease the probability that a biologically vulnerable individual will develop an anxiety disorder or depression.1
Table 1
Panic attacks: The core symptom of panic disorder
| A panic attack is a discrete period of intense fear or discomfort, in which four (or more) of the following symptoms develop abruptly and peak within 10 minutes: |
|
| Source: DSM-IV-TR |
Fear circuit model. PD’s pathophysiology is not completely understood, but evidence suggests that an overactive brain alarm network may increase vulnerability for PD (Box).1,2 Individual patients require different intensities of treatment to normalize their panic symptoms:
Mild to moderate PD (characterized by little or no avoidance and no comorbid disorders) often responds to either medication or CBT. A single intervention—such as using CBT to enhance the cortical inhibitory effects or using medication to reduce the amygdala’s reactivity—may suffice for symptomatic relief.
Severe or complicated PD (characterized by frequent panic attacks, significant agoraphobia, and comorbid anxiety disorders or depression) may require high medication dosages, intense CBT/exposure therapy, or both to normalize more severely disrupted communication among the fear circuit’s components.
ASSESSING TREATMENT OUTCOME
The goal of treatment is remission: a return to functioning without illness-related impairment or loss of quality of life, as if the patient had never been ill. In clinical practice, we can use validated, patient-rated assessment tools to document improvement in panic-related impairment, patient satisfaction, and quality of life—the real targets of treatment. Two useful tools are the Sheehan Disability Scale3 and the Quality of Life Enjoyment and Satisfaction Questionnaire.4
With adequate treatment, achieving remission can take several months or more; without it, remission may never occur. The following guidelines can help ensure that you provide adequate treatment.
What is adequate CBT? When patients’ symptoms fail to respond to CBT, the first step is to examine whether inadequate treatment is the culprit. At least 10 weekly CBT sessions administered by a “qualified professional” has been suggested as an adequate CBT trial for PD.5 Unfortunately, qualified CBT therapists are not always available. If CBT referral is not an option, clinicians can provide patients with at least some elements of CBT, such as education about PD, information resources, and self-exposure instruction as indicated. For more information on CBT for PD, see Related Resources.
What is adequate drug treatment? Noncompliance with medication because a patient fears adverse effects or has insufficient information can easily thwart treatment. Before treatment begins, therefore, it is important to establish your credibility. Provide the patient with information about PD, its treatment options, and what to expect so that he or she can collaborate in treatment (Table 2).
An inherited, abnormally active brain alarm mechanism—or “fear circuit”—may explain panic disorder, according to a theoretical neuroanatomic model.1 Its hub is the central nucleus of the amygdala, which coordinates fear responses via pathways communicating with the hippocampus, thalamus, hypothalamus, brainstem, and cortical processing areas.
The amygdala mediates acute emotional responses, including fear and anxiety. The hypothalamus mediates physiologic changes connected with emotions, such as release of stress hormones and some changes in heart rate. The prefrontal cortex is involved in thinking and memory and may be instrumental in predicting the consequences of rewards or punishments. In vulnerable individuals, defects in coordinating the sensory input among these brain regions may cause the central nucleus to discharge, resulting in a panic attack.
Medication and cognitive-behavioral therapy may reduce fear circuit reactivity and prevent panic attacks by acting at different components of the fear circuit. When the amygdala’s central nucleus no longer overreacts to sensory input, anticipatory anxiety and phobic avoidance usually dissipate over time.2,3 Thus, the fear circuit model integrates the clinical observation that both cognitive-behavioral therapy and medication are effective for treating panic.1
Abnormal interactions among components of this oversensitive fear circuit also may occur in social anxiety disorder, generalized anxiety disorder, posttraumatic stress disorder, and depression.1 In these disorders, communication patterns among the parts of the hypothesized circuit may be disrupted in different ways. The clinical observation that anxious individuals often become depressed when under stress is consistent with this model and with the literature.
Antidepressants are preferred as first-line treatment of PD, even in nondepressed patients. Selective serotonin reuptake inhibitors (SSRIs) are recommended for PD because of their comparable efficacy and tolerability compared with older antipanic agents.6 SSRIs are also effective against other anxiety disorders likely to co-occur with PD.7
Many panic patients are exquisitely sensitive to activation by initial antidepressant dosages. Activation rarely occurs in other disorders, so its appearance suggests that your diagnosis is correct. Clinical strategies to help you manage antidepressant titration are suggested in Table 3.
Table 2
Prescription for success in treating panic disorder
| Relieve patient of perceived burden of being ill Explain the disorder’s familial/genetic origins Describe the fear circuit model Include spouse or significant other in treatment |
| Build patient-physician collaboration Explain potential medication side effects Describe the usual pattern of symptom relief (stop panic attacks → reduce anticipatory anxiety → decrease phobia) Estimate a time frame for improvement Map out next steps if first try is unsuccessful Be available, especially at first |
| Address patient’s long-term medication concerns Discuss safety, long-term efficacy Frame treatment as a pathway to independence from panic attacks Use analogy of diabetes or hypertension to explain that medication is for managing symptoms, rather than a cure Discuss tapering medication after sustained improvement (12 to 18 months) to determine continued need for medication |
In clinical settings, two naturalistic studies suggested that more-favorable outcomes are associated with antipanic medication dosages shown in Table 4 as “possibly effective”—and that most patients with poor medication response received inadequate treatment.8,9Table 4 ’s dosages come from those two studies—published before the efficacy studies of SSRIs in PD—and from later studies of SSRIs and the selective norepinephrine-serotonin reuptake inhibitor (SNRI) venlafaxine.7,8,10
The lower end of the “probably effective” range in Table 4 represents the lowest dose levels generally expected to be effective for PD. Not all agents in the table are FDA-approved for PD, nor are the dosages of approved agents necessarily “within labeling.” Some patients’ symptoms may resolve at higher or lower dosages.
Table 3
Tips to help the patient tolerate antidepressant titration
| Be pre-emptive Before starting therapy, explain that low initial dosing and flexible titration help to control unpleasant but medically safe “jitteriness” known as antidepressant-induced activation Tell the patient that activation rarely occurs in disorders other than PD (“Its appearance suggests that the diagnosis is correct and that we’re likely on the right track”) |
| Be reassuring Tell the patient, “You control the gas peddle—I’ll help you steer” (to an effective dose) |
| Be cautious Start with 25 to 50% of the usual antidepressant initial dosage for depression (Table 4); if too activating, reduce and advance more gradually Activation usually dissipates in 1 to 2 weeks; over time, larger dosage increments are often possible |
| Be attentive Use benzodiazepines or beta blockers as needed to attenuate activation |
Some patients require months to reach and maintain the “probably effective” dosage for at least 6 weeks. Short-term benzodiazepines can be used to control panic symptoms during antidepressant titration, then tapered off.11 We categorize patients who are unable to tolerate an “adequate dose” as not having had a therapeutic trial—not as treatment failures.
No controlled studies of PD have examined the success rate of switching to a second antidepressant after a first one has been ineffective.12 In clinical practice, we may try two different SSRIs and venlafaxine. When switching agents, we usually co-administer the treatments for a few weeks, titrate the second agent upward gradually, then taper and discontinue the first agent over 2 to 4 weeks. We use short-term benzodiazepines as needed.
Partial improvement. Sometimes overall symptoms improve meaningfully, but bothersome panic symptoms remain. Clinical response may improve sufficiently if you raise the medication dosage in increments while monitoring for safety and tolerability. Address medicolegal concerns by documenting in the patient’s chart:
- your rationale for prescribing dosages that exceed FDA guidelines
- that you discussed possible risks versus benefits with the patient, and the patient agrees to the treatment.
When in doubt about using dosages that exceed FDA guidelines for patients with unusually resistant panic symptoms, obtain consultation from an expert or colleague.
Table 4
Recommended drug dosages for panic disorder
| Class/agent | Possibly effective (mg/d) | Probably effective (mg/d) | High dosage (mg/d) | Initial dosage (mg/d) | Confidence level |
|---|---|---|---|---|---|
| SSRIs | |||||
| Citalopram | <20 | 20-60 | >60 | 10 | ++ |
| Escitalopram | <10 | 10-30 | >30 | 5 | ++++ |
| Fluoxetine | <40 | 40-80 | >80 | 10 | ++ |
| Fluvoxamine | <150 | 150-300 | >300 | 25 | ++++ |
| Paroxetine* | <40 | 40-60 | >60 | 5-10 | ++++ |
| Sertraline* | <150 | 150-300 | >300 | 12.5-25 | ++++ |
| SNRI | |||||
| Venlafaxine | <150 | 150-300 | >300 | 18.75-37.5 | ++ |
| Benzodiazepines | |||||
| Alprazolam* | <2 | 2-8 | >8 | 0.5-1.0 | ++++ |
| Clonazepam* | <1 | 2-4 | >4 | 0.25-0.5 | ++++ |
| Tricyclics | |||||
| Clomipramine | <100 | 100-200 | >200 | 10 | ++++ |
| Desipramine | <150 | 150-300 | >300 | 10 | ++ |
| Imipramine | <150 | 150-300 | >300 | 10 | ++++ |
| MAOIs | |||||
| Phenelzine | <45 | 45-90 | >90 | 15 | +++ |
| Tranylcypromine | <30 | 30-70 | >70 | 10 | + |
| Antiepileptics | |||||
| Gabapentin | 100-200 | 600-3,400 | ++ | ||
| Valproate (VPA) | 250-500 | 1,000-2,000 | ++ | ||
| * FDA-approved for treating panic disorder | |||||
| Confidence: | |||||
| + (uncontrolled series) | |||||
| ++ (at least 1 controlled study) | |||||
| +++ (>1 controlled study) | |||||
| ++++ (Unequivocal) | |||||
Using benzodiazepines. As noted above, adjunctive use of benzodiazepines while initiating antidepressant therapy can help extremely anxious or medication-sensitive patients.11 Many clinicians coadminister benzodiazepines with antidepressants over the longer term.7 As a primary treatment, benzodiazepines may be useful for patients who could not tolerate or did not respond to at least two or three antidepressant trials.
Table 5
Solving inadequate response to initial SSRI treatment of panic disorder
| Problem | Differential diagnosis | Suggested solutions |
|---|---|---|
| Persistent panic attacks | Unexpected attacks Inadequate treatment or duration Situational attacks Medical condition Other psychiatric disorder | ≥Threshold dose for 6 weeks Try second SSRI Try venlafaxine CBT/exposure therapy Address specific conditions Rule out social phobia, OCD, PTSD |
| Persistent nonpanic anxiety | Medication-related Activation (SSRI or SNRI) Akathisia from SSRI Comorbid GAD Interdose BZD rebound BZD or alcohol withdrawal Residual anxiety | Adjust dosage, add BZD or beta blocker Adjust dosage, add beta blocker or BZD Increase antidepressant dosage, add BZD Switch to longer-acting agent Assess and treat as indicated Add/increase BZD |
| Residual phobia | Agoraphobia | CBT/exposure, adjust medication |
| Other disorders | Depression Bipolar disorder Personality disorders Medical disorder | Aggressive antidepressant treatment ±BZDs Mood stabilizer and antidepressant ±BZDs Specific psychotherapy Review and modify treatment as indicated |
| Environmental event or stressor(s) | Review work, family events, patient perception of stressor | Family/spouse interview and education Environmental hygiene as indicated Brief adjustment in treatment plan(s) as needed |
| Poor adherence | Drug sexual side effects Inadequate patient or family understanding of panic disorder and its treatment | Try bupropion, sildenafil, amantadine, switch agents Patient/family education Make resource materials available |
| BZD: Benzodiazepine | ||
| CBT: Cognitive-behavioral therapy | ||
| GAD: Generalized anxiety disorder | ||
| OCD: Obsessive-compulsive disorder | ||
| PTSD: Posttraumatic stress disorder | ||
| SNRI: Serotonin-norepinephrine reuptake inhibitor | ||
| SSRI: Selective serotonin reuptake inhibitor | ||
Because benzodiazepine monotherapy does not reliably protect against depression, we advise clinicians to encourage patients to self-monitor and report any signs of emerging depression. Avoid benzodiazepines in patients with a history of alcohol or substance abuse.7
Other agents. Once the mainstay of antipanic treatment, tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) are seldom used today because of their side effects, toxicity in overdose, and—for MAOIs—tyramine-restricted diet. Their usefulness in resistant panic is probably limited to last-ditch efforts.
DISSECTING TREATMENT FAILURE
In uncomplicated PD, lack of improvement after two or more adequate medication trials is unusual. If you observe minimal or no improvement, review carefully for other causes of anxiety or factors that can complicate PD treatment (Table 5).
If no other cause for the persistent symptom(s) is apparent, the fear circuit model may help you decide how to modify or enhance medication treatment, add CBT, or both.
For example:
- If panic attacks persist, advancing the medication dosage (if tolerated and acceptably safe) may help. Consider increasing the dosage, augmenting, or switching to a different agent.
- If persistent attacks are consistently cued to feared situations, try intervening with moreaggressive exposure therapy. Consider whether other disorders such as unrecognized social anxiety disorder, obsessive-compulsive disorder (OCD), or posttraumatic stress disorder (PTSD) may be perpetuating the fearful avoidance.
- If the patient is depressed, consider that depression-related social withdrawal may be causing the avoidance symptoms. Aggressive antidepressant pharmacotherapy is strongly suggested.
AUGMENTATION STRATEGIES
Medication for CBT failure. Only two controlled studies have examined adding an adequate dose of medication after patients failed to respond to exposure/CBT alone:
- One study of 18 hospitalized patients with agoraphobia who failed a course of behavioral psychodynamic therapy reported improvement when clomipramine, 150 mg/d, was given for 3 weeks.13
- In a study of 43 patients who failed initial CBT, greater improvement was reported in patients who received CBT plus paroxetine, 40 mg/d, compared with those who received placebo while continuing CBT.14
Augmentation in drug therapy. Only one controlled study has examined augmentation therapy after lack of response to an SSRI—in this case 8 weeks of fluoxetine after two undefined “antidepressant failures.” When pindolol, 2.5 mg tid, or placebo were added to the fluoxetine therapy, the 13 patients who received pindolol improved clinically and statistically more on several standardized ratings than the 12 who received placebo.15
An 8-week, open-label trial showed beneficial effects of olanzapine, up to 20 mg/d, in patients with well-described treatment-resistant PD.16
Other well-described treatment adjustments reported to benefit nonresponsive PD include:
- Adding fluoxetine to a TCA or adding a TCA to fluoxetine, for TCA/SSRI combination therapy17
- Switching to the selective norepinephrine reuptake inhibitor reboxetine, 2 to 8 mg/d for 6 weeks after inadequate paroxetine or fluoxetine response (average of 8 weeks, maximum dosage 40 mg/d).18 (Note: Reboxetine is not available in the United States.)
- Using open-label gabapentin, 600 to 2,400 mg/d, after two SSRI treatment failures.19
- Adding the dopamine receptor agonist pramipexole, 1.0 to 1.5 mg/d, to various antipanic medications.20
Augmenting an SSRI with pindolol or supplementing unsuccessful behavioral treatment with “probably effective” dosages of paroxetine or clomipramine could be recommended with some confidence, although more definitive studies are needed. As outlined above, some strategies17-20 might be considered if a patient fails to respond to two or more adequate medication trials. Anecdotal reports are difficult to assess but may be clinically useful when other treatment options have been exhausted.
- Barlow DH. Anxiety and its disorders: the nature and treatment of anxiety and panic New York: Guilford Press, 1988.
- Craske MG, DeCola JP, Sachs AD, Pontillo DC. Panic control treatment of agoraphobia. J Anxiety Disord 2003;17:321-33.
- National Institute for Mental Health: Panic Disorder http://www.nimh.nih.gov/publicat/fearandtrauma.cfm
- Anxiety Disorders Association of America http://www.adaa.org/
Drug brand names
- Alprazolam • Xanax
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Imipramine • Tofranil
- Olanzapine • Zyprexa
- Phenelzine • Nardil
- Pindolol • Visken
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Reboxetine • Vestra
- Sertraline • Zoloft
- Tranylcypromine • Parnate
- Venlafaxine • Effexor
Disclosure
Dr. Lydiard receives research support from GlaxoSmithKline, Eli Lilly and Co., Organon, Sanofi-Synthelabo, Cephalon, UCB Pharma, and Merck & Co. and he is a speaker for or consultant to Pfizer Inc., Eli Lilly and Co., Solvay Pharmaceuticals, AstraZeneca Pharmaceuticals, and Forest Pharmaceuticals.
1. Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revisited. Am J Psychiatry 2000;157:493-505.
2. Coplan JD, Lydiard RB. Brain circuits in panic disorder. Biol Psychiatry 1998;44:1264-76.
3. Sheehan DV. The anxiety disease. New York: Charles Scribner and Sons, 1983;151.-
4. Rapaport MH, Wolkow RM, Clary CM. Methodologies and outcomes from the sertraline multicenter flexible-dose trials. Psychopharmacol Bull 1998;34:183-9.
5. Otto MW. Psychosocial approach to treatment-resistant anxiety disorders (presentation). Chantilly, VA: Anxiety Disorders Association of America conference on novel approaches to treatment of refractory anxiety disorders, June 15-16, 2003.
6. Gorman JM, Shear MK, McIntyre JS, Zarin DA. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. Am J Psychiatry 1998;155(May supplement).
7. Lydiard RB, Otto MW, Milrod B. Panic disorder treatment. In: Gabbard, GO (ed). Treatment of psychiatric disorders (3rd ed). Washington, DC: American Psychiatric Press, Inc, 2001;1447-82.
8. Simon NM, Safrens SA, Otto MW, et al. Outcome with pharmacotherapy in a naturalistic study of panic disorder. J Affect Disord 2002;69:201-8.
9. Yonkers KA, Ellison J, Shera D, et al. Description of antipanic therapy in a prospective longitudinal study. J Clin Psychopharmacol 1996;16:223-32.
10. Pollack MH, Worthington JJ, 3rd, Otto MW, et al. Venlafaxine for panic disorder: results from a double-blind, placebo-controlled study. Psychopharmacol Bull 1996;32:667-70.
11. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry 2001;58:681-6.
12. Simon NM. Pharmacological approach to treatment-resistant anxiety disorders (presentation). Chantilly, VA: Anxiety Disorders Association of America conference on novel approaches to treatment of refractory anxiety disorders, June 15-16, 2003.
13. Hoffart A, Due-Madsen J, Lande B, et al. Clomipramine in the treatment of agoraphobic inpatients resistant to behavioral therapy. J Clin Psychiatry 1993;54:481-7.
14. Kampman M, Keijsers GP, Hoogduin CA, Hendriks GJ. A randomized, double-blind, placebo-controlled study of the effects of adjunctive paroxetine in panic disorder patients unsuccessfully treated with cognitive-behavioral therapy alone. J Clin Psychiatry 2002;63:772-7.
15. Hirschmann S, Dannon PN, Iancu I, et al. Pindolol augmentation in patients with treatment-resistant panic disorder: a double-blind, placebo-controlled trial. J Clin Psychopharmacol 2000;20:556-9.
16. Hollifield M, Thompson P, Uhlenluth E. Potential efficacy and safety of olanzapine in refractory panic disorder (presentation). San Francisco: American Psychiatric Association annual meeting, 2003.
17. Tiffon L, Coplan J, Papp L, Gorman J. Augmentation strategies with tricyclic or fluoxetine treatment in seven partially responsive panic disorder patients. J Clin Psychiatry 1994;55:66-9.
18. Dannon PN, Iancu I, Grunhaus L. The efficacy of reboxetine in treatment-refractory patients with panic disorder: an open-label study. Hum Psychopharmacol 2002;17:329-33.
19. Chiu S. Gabapentin treatment response in SSRI-refractory panic disorder (presentation) San Francisco: American Psychiatric Association annual meeting, 2003.
20. Marazziti D, Presta S, Pfanner C, et al. Pramipexole augmentation in panic with agoraphobia. Am J Psychiatry 2001;158:498-9.
1. Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revisited. Am J Psychiatry 2000;157:493-505.
2. Coplan JD, Lydiard RB. Brain circuits in panic disorder. Biol Psychiatry 1998;44:1264-76.
3. Sheehan DV. The anxiety disease. New York: Charles Scribner and Sons, 1983;151.-
4. Rapaport MH, Wolkow RM, Clary CM. Methodologies and outcomes from the sertraline multicenter flexible-dose trials. Psychopharmacol Bull 1998;34:183-9.
5. Otto MW. Psychosocial approach to treatment-resistant anxiety disorders (presentation). Chantilly, VA: Anxiety Disorders Association of America conference on novel approaches to treatment of refractory anxiety disorders, June 15-16, 2003.
6. Gorman JM, Shear MK, McIntyre JS, Zarin DA. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. Am J Psychiatry 1998;155(May supplement).
7. Lydiard RB, Otto MW, Milrod B. Panic disorder treatment. In: Gabbard, GO (ed). Treatment of psychiatric disorders (3rd ed). Washington, DC: American Psychiatric Press, Inc, 2001;1447-82.
8. Simon NM, Safrens SA, Otto MW, et al. Outcome with pharmacotherapy in a naturalistic study of panic disorder. J Affect Disord 2002;69:201-8.
9. Yonkers KA, Ellison J, Shera D, et al. Description of antipanic therapy in a prospective longitudinal study. J Clin Psychopharmacol 1996;16:223-32.
10. Pollack MH, Worthington JJ, 3rd, Otto MW, et al. Venlafaxine for panic disorder: results from a double-blind, placebo-controlled study. Psychopharmacol Bull 1996;32:667-70.
11. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry 2001;58:681-6.
12. Simon NM. Pharmacological approach to treatment-resistant anxiety disorders (presentation). Chantilly, VA: Anxiety Disorders Association of America conference on novel approaches to treatment of refractory anxiety disorders, June 15-16, 2003.
13. Hoffart A, Due-Madsen J, Lande B, et al. Clomipramine in the treatment of agoraphobic inpatients resistant to behavioral therapy. J Clin Psychiatry 1993;54:481-7.
14. Kampman M, Keijsers GP, Hoogduin CA, Hendriks GJ. A randomized, double-blind, placebo-controlled study of the effects of adjunctive paroxetine in panic disorder patients unsuccessfully treated with cognitive-behavioral therapy alone. J Clin Psychiatry 2002;63:772-7.
15. Hirschmann S, Dannon PN, Iancu I, et al. Pindolol augmentation in patients with treatment-resistant panic disorder: a double-blind, placebo-controlled trial. J Clin Psychopharmacol 2000;20:556-9.
16. Hollifield M, Thompson P, Uhlenluth E. Potential efficacy and safety of olanzapine in refractory panic disorder (presentation). San Francisco: American Psychiatric Association annual meeting, 2003.
17. Tiffon L, Coplan J, Papp L, Gorman J. Augmentation strategies with tricyclic or fluoxetine treatment in seven partially responsive panic disorder patients. J Clin Psychiatry 1994;55:66-9.
18. Dannon PN, Iancu I, Grunhaus L. The efficacy of reboxetine in treatment-refractory patients with panic disorder: an open-label study. Hum Psychopharmacol 2002;17:329-33.
19. Chiu S. Gabapentin treatment response in SSRI-refractory panic disorder (presentation) San Francisco: American Psychiatric Association annual meeting, 2003.
20. Marazziti D, Presta S, Pfanner C, et al. Pramipexole augmentation in panic with agoraphobia. Am J Psychiatry 2001;158:498-9.
Germ warfare: Arm young patients to fight obsessive-compulsive disorder
Adam, age 10, is extremely distressed at school. Because of obsessional contamination fears, he avoids contact with other children and refuses to eat in the cafeteria. He washes his hands 20 times per day and changes his clothes at least three times daily.
His primary obsessions involve contact with bodily fluids—such as saliva or feces—and excessive concerns that this contamination would cause him serious illness.
Adam’s parents say their son’s worries about dirt and germs began when he entered kindergarten. They sought treatment for him 2 years ago, and he has been receiving outpatient psychotherapy since then. They have brought him to an anxiety disorders specialty clinic for evaluation because his obsessive-compulsive symptoms are worsening,
When treating patients such as Adam, our approach is to use cognitive-behavioral therapy (CBT) and adjunctive drug therapies to relieve their symptoms and help them reclaim their lives. Diagnosis of pediatric OCD is often delayed, and few children receive state-of-the-art treatment.1 The good news, however, is that skillful CBT combined, as needed, with medication is highly effective.
Although family dysfunction does not cause OCD, families affect and are affected by OCD. Control struggles over the child’s rituals are common, as are differences of opinion about how to cope with OCD symptoms. It is important to address these issues early in treatment, as helping the family combat the disorder—rather than each other—is crucial to effective treatment.
Parents need to know that neither they nor the child are to blame. OCD is a neurobehavioral illness, and treatment is most effective when the patient, therapist, and family are aligned to combat it. Families are often entangled in the child’s OCD symptoms, and disentangling them by eliminating their role in ritualizing (such as giving excessive reassurance) is important to address in therapy.
Scaling family involvement is part of the “art” of CBT, and it will remain so until empiric studies determine the family’s role in the treatment plan.2
‘Contaminated’ mother.
Adam becomes distressed when he comes in contact with objects that have been touched by others (such as doorknobs). He is especially anxious when these items are associated with public bathrooms or sick people.
Adam’s mother is a family physician who has daily patient contact. In the last 6 months, Adam has insisted that his mother change her work clothes before she enters his room, touches him, prepares his food, or handles his possessions.
As in Adam’s case, the family often gets caught up in a child or adolescent’s obsessive rituals (Box 1).2 After a detailed discussion with Adam and his parents and because his symptoms were severe, we recommended combined treatment with sertraline and CBT. Adam was willing to consider CBT and medication because he recognized that he was having increasing difficulty doing the things he wanted to do in school and at home.
SNAPSHOT OF PEDIATRIC OCD
Approximately 1 in 200 children and adolescents suffer from clinically significant OCD.3 They experience intrusive thoughts, urges, or images to which they respond with dysphoria-reducing behaviors or rituals.
Common obsessions include:
- fear of dirt or germs
- fear of harm to oneself or someone else
- or a persistent need to complete something “just so.”
Corresponding compulsions include hand washing, checking, and repeating or arranging.
OCD appears more common in boys than in girls. Onset occurs in two modes: first at age 9 for boys and age 12 for girls, followed by a second mode in late adolescence or early adulthood.
Two practice guidelines address OCD in youth: an independent expert consensus guideline4 and the American Academy of Child and Adolescent Psychiatry’s practice parameters for OCD.5
For uncomplicated OCD, these guidelines recommend CBT as first-line treatment. If symptoms do not respond after six to eight sessions, a selective serotonin reuptake inhibitor (SSRI) is added to CBT.
For complicated OCD, medication is considered an appropriate initial treatment. Complicated OCD includes patients who:
- display severe symptoms—such as with scores >30 on the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS)
- or have comorbidity such as depression or panic disorder that is likely to complicate treatment.
KEYS TO SUCCESSFUL TREATMENT
OCD is remarkably resistant to insight-oriented psychotherapy and other nondirective therapies. The benefits of CBT, however, are well-established, with reported response rates of >80% in pilot studies.6,7 Although confirming studies have yet to be conducted, successful CBT for pediatric OCD appears to include four elements (Table 1).
Exposure and response prevention (EX/RP) is central to psychosocial treatment of OCD.7,8 In specialized centers, exposure can be applied intensively (three to five times per week for 3 to 4 weeks).9 In most practices, however, exposure is more gradual (weekly for 12 to 20 weeks). With repeated exposure, the child’s anxiety decreases until he or she no longer fears contact with the targeted stimuli.8,10
Not ‘misbehavior.’ Children—and less commonly adolescents—with this disorder may not view their obsessions as senseless or their compulsions as excessive. Even when insight is clearly present, young OCD patients often hide their symptoms because of embarrassment or fear of being punished for their behavior.
Response predictors. A key to CBT in children or adolescents is that they come to see obsessions and compulsions as symptoms of an illness. The symptoms, therefore, require a skillfully applied “antidote,” as taught by the clinician and implemented by the child, family, and others on the child’s behalf. Besides overt rituals, three response predictors include the patient’s:
- desire to eliminate symptoms
- ability to monitor and report symptoms
- willingness to cooperate with treatment.
Table 1
Pediatric OCD: 4 keys to successful cognitive therapy
| Treat OCD as a neurobehavioral disorder, not a misbehavior |
| Help the child develop a “tool kit” to manage dysphoria and faulty thinking |
| Expose the patient to anxiety-producing stimuli until he or she becomes desensitized and can refrain from the usual compulsive responses (exposure and response prevention) |
| Educate family members and school personnel |
CBT may be difficult with patients younger than age 6 and will invariably involve training the parents to serve as “coaches;” a CBT protocol for patients ages 4 to 6 is under investigation (H. Leonard, personal communication). CBT also can be adapted for patients with intellectual deficits.11
A ‘tool kit.’ Successful exposure therapy for OCD relies on equipping children and adolescents with the knowledge and skills to battle the illness. They often have tried unsuccessfully to resist OCD’s compulsions and must be convinced that EX/RP techniques will work. Using a “tool kit” concept reminds young patients that they have the implements they need to combat OCD (Table 2).
A ‘germ ladder’ and ‘fear thermometer.’
Adam’s tools include a stimulus hierarchy called a “germ ladder,” which the therapist and Adam create collaboratively. It ranks stimuli from low (his own doorknob) to very high (public toilets, sinks, and door handles).
As part of his treatment, Adam begins to touch objects in his room and house while voluntarily refraining from ritualizing. He uses another tool—a fear thermometer—to record his distress level on a scale of 1 to 10 during and after these exposures.
Adam discovers that when he comes into contact with less-threatening items his fear ratings typically return to baseline within 20 to 30 minutes. This insight helps him modify his assessment of the risk they pose.
Table 2
OCD ‘tool box’ can help patients build new behaviors
| Tool | Function |
|---|---|
| Training in exposure response prevention (EX/RP) therapy | Helps patients learn to confront rather than avoid feared stimuli |
| ‘Fear thermometer’ | Enables patients to express the intensity of their distress on a scale of 1 (lowest) to 10 (highest) |
| Positive self-statements | Teaches patients to use statements such as “I can do this” and “I’m the boss of OCD now” to build confidence that they can control their response to feared stimuli |
During office visits, he confronts similar items around the clinic, with the therapist providing encouragement and instruction for additional exposure homework. Eventually Adam works on the clinic’s public bathroom, which he perceived to be relatively clean but less so than his own bathroom. After fear in response to this bathroom is reduced, the therapist and Adam graduate to more-public facilities, such as the bathrooms at Adam’s pool and the local train station.
Exposure therapy. EX/RP is most successful when the child—rather than the therapist—chooses exposure targets from a hierarchy of fears,2 particularly when the list includes behaviors the child is resisting. In a collaborative spirit, the child takes the lead in placing items on the hierarchy and deciding when to confront them.
The therapist and child revise the hierarchy periodically, which demonstrates progress and allows them to add items as the child overcomes fears that cause less distress.
Reducing need for reassurance.
Adam has a habit of repeatedly asking his mother whether contact with particular objects in public is risky. By the third treatment session, he and the therapist agree that he will try to refrain from asking such questions.
His mother, in turn, is asked to reiterate the rationale for response prevention whenever Adam slips. She will offer encouragement and support without answering “OCD’s questions.”
ADJUNCTIVE DRUG THERAPY
While Adam is working with the behavioral therapist to reduce his anxieties and need for reassurance, he is also receiving gradually increasing dosages of sertraline. As discussed, he is considered a candidate for CBT plus medication because of his symptoms are severe. Drug treatment can benefit most pediatric OCD patients.
SSRIs. Two SSRIs are approved for pediatric OCD—fluvoxamine for ages 8 to 18 and sertraline for ages 6 to 18. Most SSRIs are likely effective for OCD in youth (Table 3),12-14 although reports have suggested a link between paroxetine and suicidality in pediatric patients. Other options may be more suitable choices unless further evidence supports the use of paroxetine as a first- or second-line agent for pediatric OCD.
Clomipramine—a nonselective tricyclic—was the first medication studied in treating OCD in children and adolescents. It is now usually considered only after two or three failed SSRI trials because of its potential for cardiac toxicity.15-17
Table 3
Suggested dosages (mg/d) for drug therapy of pediatric OCD
| Drug | Usual starting dosage | Approximate mean dosage* | Typical range | Usual maximum dosage |
|---|---|---|---|---|
| Citalopram | 20 | 40 | 20 to 60 | 80 |
| Clomipramine | 50 | 150 | 200 | 300 |
| Escitalopram | 5 | 10 | 10 to 20 | 30 |
| Fluoxetine | 20 | 40 | 40 to 60 | 80 |
| Fluvoxamine | 50 | 175 | 150 to 250 | 300 |
| Sertraline | 50 | 125 | 150 | 225 |
| *Mean dosage derived from registration trials, expert recommendation, and the authors’ clinical experience | ||||
Dosing. Fixed-dose studies suggest that dosing schedules for OCD are similar to those used for depression. For example, sertraline, 50 mg/d, or fluoxetine, 20 mg/d, are as effective as higher dosages.18
The common misconception that OCD requires higher dosages likely results from:
- increasing the dosage too early in the time-response window for a drug effect to emerge
- giving medication without concomitant exposure therapy.19
Delayed response. Although many patients respond early to an SSRI, others do not respond until 8 or even 12 weeks of treatment at therapeutic dosages. It often takes 3 to 4 weeks for evidence of benefit to emerge, so wait at least 3 weeks between dosage increases. Maintain therapeutic dosages at least 6 to 8 weeks before changing agents or beginning augmentation therapy.
Two-barrel approach.
In treating Adam, we began with sertraline, using a flexible titration schedule keyed to whether he experienced OCD symptom remission.
The starting dosage of 50 mg was titrated to 150 mg over 8 weeks while he was receiving behavioral therapy. We made adjustments with a time-response window of 2 to 3 weeks, allowing us to observe a response to each dosage escalation.
Adam’s OCD symptoms responded well to CBT plus sertraline. The maximum drug effect helped him confront the most difficult EX/RP tasks at the top of his stimulus hierarchy, which he attacked near the end of treatment.
Lessons learned. Multicenter trials have taught important lessons about drug therapy for OCD:
- OCD patients experience little or no placebo effect, unlike patients with depression.
- Clinical effects may appear as early as 2 to 3 weeks after medications are started and plateau at 10 to 12 weeks.
- Partial response is the rule; SSRIs reduce OCD symptoms by about 30%—which correlates with “moderately” to “markedly” improved ratings on patient satisfaction measures.
- Side effects and magnitude of improvement are comparable in pediatric and adult medication trials.
In some children, OCD or tic symptoms arise from or are exacerbated by group A beta hemolytic streptococcal infection (GABHS), which has been labeled pediatric autoimmune neuropsychiatric disorders associated with Streptococcus (PANDAS).21 Obsessive-compulsive symptoms are not uncommon in pediatric patients with Sydenham’s chorea, a neurologic variant of rheumatic fever. OCD is far more common in patients with rheumatic fever when chorea is present.
Acute-onset or dramatic exacerbation of OCD or tic symptoms should prompt investigation of GABHS infection. Immunomodulatory treatments—including antibiotics, plasmapheresis, or IV immunoglobulin G—may benefit some patients.22
MANAGING RESISTANT OCD
Adequate SSRI monotherapy trials fail to relieve OCD symptoms in one-third of patients. Some patients benefit from combination therapy—such as an SSRI plus risperidone—especially when comorbid schizotypal personality disorder or a tic-spectrum disorder is present.
Drug switching or augmentation trials often produce only partial response and cause unnecessary suffering. A more effective strategy for many patients is to augment drug treatment with CBT until symptoms normalize.
On the other hand, augmentation is appropriate when nonresponse or partial response to SSRI monotherapy leaves a patient clinically symptomatic and functionally impaired. Clonazepam, clomipramine, and the amino acid L-tryptophan have been used successfully. Lithium and buspirone also have been tried but seem not to be effective in controlled studies in adults and anecdotal experience in youth.
When augmenting an SSRI, adding clomipramine, 25 to 50 mg/d, is a reasonable choice. However, fluoxetine or paroxetine can inhibit clomipramine metabolism by cytochrome P-450 (CYP) 2D6, with potential for cardiac arrhythmias or seizures. Sertraline or fluvoxamine are less likely to elevate clomipramine levels.20 Fluvoxamine may be the most compatible SSRI with clomipramine because it inhibits CYP 1A2—the enzyme that demethylates clomipramine to its inactive desmethyl metabolite—thereby preserving more of the active parent compound.
Clinical evidence suggests that augmentation’s success may depend in part on a patient’s comorbidities. For example, clonazepam may be particularly helpful for children with comorbid panic symptoms.
MAINTENANCE THERAPY
We typically provide 14 weekly CBT sessions, followed by monthly contacts for a few months to ensure than a patient’s gains are maintained. Standard procedure with drug therapy is to continue maintenance treatment for up to 1 year, although some have suggested continuing maintenance treatment indefinitely.
Related resources
- Chansky TE. Freeing your child from obsessive compulsive disorder: A powerful, practical program for parents of children and adolescents. New York: Crown Publishing Group, 2001.
- March JS, Mulle K. OCD in children and adolescents: A cognitive behavioral treatment manual. New York: Guilford Press, 1998.
- Moritz EK, Jablonsky J. Blink, blink, clop, clop: Why do we do things we can’t stop? An OCD storybook. Newton, MA: Professional Books, Inc, 1998.
- Wagner AP. Up and down the worry hill: A children’s book about obsessive compulsive disorder and its treatment. Rochester, NY: Lighthouse Press, Inc. 2000.
Drug brand names
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertaline • Zoloft
Disclosure
Dr. March receives research support from Pfizer Inc., Eli Lilly and Co., and Wyeth Pharmaceuticals and is a speaker for and/or consultant to Solvay Pharmaceuticals, Pfizer Inc., GlaxoSmithKline, Wyeth Pharmaceuticals, Novartis Pharmaceuticals Corp., and Shire Pharmaceuticals Group.
Dr. Franklin and Dr. Foa report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Preparation of this manuscript was supported in part by National Institute of Mental Health grants 1 K24 MHO1557 and 1 R10 MH55121 to Dr. March and by contributions from the Robert and Sarah Gorrell family and the Lupin Family Foundation.
1. Kendall PC, Southam-Gerow MA. Issues in the transportability of treatment: the case of anxiety disorders in youths. J Consult Clin Psychol 1995;63(5):702-8.
2. March J, Mulle K. OCD in children and adolescents: A cognitive-behavioral treatment manual. New York: Guilford Press, 1998.
3. Flament MF, Whitaker A, Rapoport JL, et al. Obsessive compulsive disorder in adolescence: an epidemiological study. J Am Acad Child Adolesc Psychiatry 1988;27(6):764-71.
4. March J, Frances A, Kahn D, Carpenter D. Expert consensus guidelines: treatment of obsessive-compulsive disorder. J Clin Psychiatry 1997;58(suppl 4):1-72.
5. King R, Leonard H, March J. Practice parameters for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 1998;37(10, suppl):27-45.
6. March JS. Cognitive-behavioral psychotherapy for children and adolescents with OCD: a review and recommendations for treatment. J Am Acad Child Adolesc Psychiatry 1995;34(1):7-18.
7. Franklin ME, Kozak MJ, Cashman LA, et al. Cognitive-behavioral treatment of pediatric obsessive-compulsive disorder: an open clinical trial. J Am Acad Child Adolesc Psychiatry 1998;37(4):412-19.
8. March JS, Mulle K, Herbel B. Behavioral psychotherapy for children and adolescents with obsessive-compulsive disorder: an open trial of a new protocol-driven treatment package. J Am Acad Child Adolesc Psychiatry 1994;33(3):333-41.
9. Franklin ME, Tolin DF, March JS, Foa EB. Treatment of pediatric obsessive-compulsive disorder: A case example of intensive cognitive-behavioral therapy involving exposure and ritual prevention. Cognit Behav Pract 2001;8(4):297-304.
10. March J, Mulle K. Manualized cognitive-behavioral psychotherapy for obsessive-compulsive disorder in childhood: a preliminary single case study. J Anxiety Disord 1995;9(2):175-84.
11. Franklin ME, Rynn M, March JS, Foa EB. Obsessive-compulsive disorder. In: Hersen M (ed). Clinical behavior therapy: adults and children. New York: John Wiley & Sons, 2002;276-303.
12. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA 1998;280(20):1752-6.
13. Riddle MA, Reeve EA, Yaryura-Tobias JA, et al. Fluvoxamine for children and adolescents with obsessive-compulsive disorder: a randomized, controlled, multicenter trial. J Am Acad Child Adolesc Psychiatry 2001;40(2):222-9.
14. Geller DA, Hoog SL, Heiligenstein JH, et al. Fluoxetine treatment for obsessive-compulsive disorder in children and adolescents: a placebo-controlled clinical trial. J Am Acad Child Adolesc Psychiatry 2001;40(7):773-9.
15. Leonard H, March J, Rickler K, Allen A. Pharmacology of the selective serotonin reuptake inhibitors in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(6):725-36.
16. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder—a multicenter trial. J Am Acad Child Adolesc Psychiatry 1992;31(1):45-9.
17. Wilens TE, Biederman J, March JS, et al. Absence of cardiovascular adverse effects of sertraline in children and adolescents. J Am Acad Child Adolesc Psychiatry 1999;38(5):573-7.
18. Greist JH, Jefferson JW, Kobak KA, et al. A 1-year double-blind placebo-controlled fixed dose study of sertraline in the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol 1995;10(2):57-65.
19. Marks IM. Drug versus behavioral treatment of obsessive-compulsive disorder. Biolog Psychiatry 1990;28(12):1072-3.
20. Leonard HL, March J, Rickler KC, Allen AJ. Pharmacology of the selective serotonin reuptake inhibitors in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(6):725-36.
21. Leonard HL, Swedo SE. Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). Int J Neuropsychopharmacol 2001;4(2):191-8.
22. Perlmutter SJ, Leitman SF, Garvey MA, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 1999;354 (9185):1153-8.
Adam, age 10, is extremely distressed at school. Because of obsessional contamination fears, he avoids contact with other children and refuses to eat in the cafeteria. He washes his hands 20 times per day and changes his clothes at least three times daily.
His primary obsessions involve contact with bodily fluids—such as saliva or feces—and excessive concerns that this contamination would cause him serious illness.
Adam’s parents say their son’s worries about dirt and germs began when he entered kindergarten. They sought treatment for him 2 years ago, and he has been receiving outpatient psychotherapy since then. They have brought him to an anxiety disorders specialty clinic for evaluation because his obsessive-compulsive symptoms are worsening,
When treating patients such as Adam, our approach is to use cognitive-behavioral therapy (CBT) and adjunctive drug therapies to relieve their symptoms and help them reclaim their lives. Diagnosis of pediatric OCD is often delayed, and few children receive state-of-the-art treatment.1 The good news, however, is that skillful CBT combined, as needed, with medication is highly effective.
Although family dysfunction does not cause OCD, families affect and are affected by OCD. Control struggles over the child’s rituals are common, as are differences of opinion about how to cope with OCD symptoms. It is important to address these issues early in treatment, as helping the family combat the disorder—rather than each other—is crucial to effective treatment.
Parents need to know that neither they nor the child are to blame. OCD is a neurobehavioral illness, and treatment is most effective when the patient, therapist, and family are aligned to combat it. Families are often entangled in the child’s OCD symptoms, and disentangling them by eliminating their role in ritualizing (such as giving excessive reassurance) is important to address in therapy.
Scaling family involvement is part of the “art” of CBT, and it will remain so until empiric studies determine the family’s role in the treatment plan.2
‘Contaminated’ mother.
Adam becomes distressed when he comes in contact with objects that have been touched by others (such as doorknobs). He is especially anxious when these items are associated with public bathrooms or sick people.
Adam’s mother is a family physician who has daily patient contact. In the last 6 months, Adam has insisted that his mother change her work clothes before she enters his room, touches him, prepares his food, or handles his possessions.
As in Adam’s case, the family often gets caught up in a child or adolescent’s obsessive rituals (Box 1).2 After a detailed discussion with Adam and his parents and because his symptoms were severe, we recommended combined treatment with sertraline and CBT. Adam was willing to consider CBT and medication because he recognized that he was having increasing difficulty doing the things he wanted to do in school and at home.
SNAPSHOT OF PEDIATRIC OCD
Approximately 1 in 200 children and adolescents suffer from clinically significant OCD.3 They experience intrusive thoughts, urges, or images to which they respond with dysphoria-reducing behaviors or rituals.
Common obsessions include:
- fear of dirt or germs
- fear of harm to oneself or someone else
- or a persistent need to complete something “just so.”
Corresponding compulsions include hand washing, checking, and repeating or arranging.
OCD appears more common in boys than in girls. Onset occurs in two modes: first at age 9 for boys and age 12 for girls, followed by a second mode in late adolescence or early adulthood.
Two practice guidelines address OCD in youth: an independent expert consensus guideline4 and the American Academy of Child and Adolescent Psychiatry’s practice parameters for OCD.5
For uncomplicated OCD, these guidelines recommend CBT as first-line treatment. If symptoms do not respond after six to eight sessions, a selective serotonin reuptake inhibitor (SSRI) is added to CBT.
For complicated OCD, medication is considered an appropriate initial treatment. Complicated OCD includes patients who:
- display severe symptoms—such as with scores >30 on the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS)
- or have comorbidity such as depression or panic disorder that is likely to complicate treatment.
KEYS TO SUCCESSFUL TREATMENT
OCD is remarkably resistant to insight-oriented psychotherapy and other nondirective therapies. The benefits of CBT, however, are well-established, with reported response rates of >80% in pilot studies.6,7 Although confirming studies have yet to be conducted, successful CBT for pediatric OCD appears to include four elements (Table 1).
Exposure and response prevention (EX/RP) is central to psychosocial treatment of OCD.7,8 In specialized centers, exposure can be applied intensively (three to five times per week for 3 to 4 weeks).9 In most practices, however, exposure is more gradual (weekly for 12 to 20 weeks). With repeated exposure, the child’s anxiety decreases until he or she no longer fears contact with the targeted stimuli.8,10
Not ‘misbehavior.’ Children—and less commonly adolescents—with this disorder may not view their obsessions as senseless or their compulsions as excessive. Even when insight is clearly present, young OCD patients often hide their symptoms because of embarrassment or fear of being punished for their behavior.
Response predictors. A key to CBT in children or adolescents is that they come to see obsessions and compulsions as symptoms of an illness. The symptoms, therefore, require a skillfully applied “antidote,” as taught by the clinician and implemented by the child, family, and others on the child’s behalf. Besides overt rituals, three response predictors include the patient’s:
- desire to eliminate symptoms
- ability to monitor and report symptoms
- willingness to cooperate with treatment.
Table 1
Pediatric OCD: 4 keys to successful cognitive therapy
| Treat OCD as a neurobehavioral disorder, not a misbehavior |
| Help the child develop a “tool kit” to manage dysphoria and faulty thinking |
| Expose the patient to anxiety-producing stimuli until he or she becomes desensitized and can refrain from the usual compulsive responses (exposure and response prevention) |
| Educate family members and school personnel |
CBT may be difficult with patients younger than age 6 and will invariably involve training the parents to serve as “coaches;” a CBT protocol for patients ages 4 to 6 is under investigation (H. Leonard, personal communication). CBT also can be adapted for patients with intellectual deficits.11
A ‘tool kit.’ Successful exposure therapy for OCD relies on equipping children and adolescents with the knowledge and skills to battle the illness. They often have tried unsuccessfully to resist OCD’s compulsions and must be convinced that EX/RP techniques will work. Using a “tool kit” concept reminds young patients that they have the implements they need to combat OCD (Table 2).
A ‘germ ladder’ and ‘fear thermometer.’
Adam’s tools include a stimulus hierarchy called a “germ ladder,” which the therapist and Adam create collaboratively. It ranks stimuli from low (his own doorknob) to very high (public toilets, sinks, and door handles).
As part of his treatment, Adam begins to touch objects in his room and house while voluntarily refraining from ritualizing. He uses another tool—a fear thermometer—to record his distress level on a scale of 1 to 10 during and after these exposures.
Adam discovers that when he comes into contact with less-threatening items his fear ratings typically return to baseline within 20 to 30 minutes. This insight helps him modify his assessment of the risk they pose.
Table 2
OCD ‘tool box’ can help patients build new behaviors
| Tool | Function |
|---|---|
| Training in exposure response prevention (EX/RP) therapy | Helps patients learn to confront rather than avoid feared stimuli |
| ‘Fear thermometer’ | Enables patients to express the intensity of their distress on a scale of 1 (lowest) to 10 (highest) |
| Positive self-statements | Teaches patients to use statements such as “I can do this” and “I’m the boss of OCD now” to build confidence that they can control their response to feared stimuli |
During office visits, he confronts similar items around the clinic, with the therapist providing encouragement and instruction for additional exposure homework. Eventually Adam works on the clinic’s public bathroom, which he perceived to be relatively clean but less so than his own bathroom. After fear in response to this bathroom is reduced, the therapist and Adam graduate to more-public facilities, such as the bathrooms at Adam’s pool and the local train station.
Exposure therapy. EX/RP is most successful when the child—rather than the therapist—chooses exposure targets from a hierarchy of fears,2 particularly when the list includes behaviors the child is resisting. In a collaborative spirit, the child takes the lead in placing items on the hierarchy and deciding when to confront them.
The therapist and child revise the hierarchy periodically, which demonstrates progress and allows them to add items as the child overcomes fears that cause less distress.
Reducing need for reassurance.
Adam has a habit of repeatedly asking his mother whether contact with particular objects in public is risky. By the third treatment session, he and the therapist agree that he will try to refrain from asking such questions.
His mother, in turn, is asked to reiterate the rationale for response prevention whenever Adam slips. She will offer encouragement and support without answering “OCD’s questions.”
ADJUNCTIVE DRUG THERAPY
While Adam is working with the behavioral therapist to reduce his anxieties and need for reassurance, he is also receiving gradually increasing dosages of sertraline. As discussed, he is considered a candidate for CBT plus medication because of his symptoms are severe. Drug treatment can benefit most pediatric OCD patients.
SSRIs. Two SSRIs are approved for pediatric OCD—fluvoxamine for ages 8 to 18 and sertraline for ages 6 to 18. Most SSRIs are likely effective for OCD in youth (Table 3),12-14 although reports have suggested a link between paroxetine and suicidality in pediatric patients. Other options may be more suitable choices unless further evidence supports the use of paroxetine as a first- or second-line agent for pediatric OCD.
Clomipramine—a nonselective tricyclic—was the first medication studied in treating OCD in children and adolescents. It is now usually considered only after two or three failed SSRI trials because of its potential for cardiac toxicity.15-17
Table 3
Suggested dosages (mg/d) for drug therapy of pediatric OCD
| Drug | Usual starting dosage | Approximate mean dosage* | Typical range | Usual maximum dosage |
|---|---|---|---|---|
| Citalopram | 20 | 40 | 20 to 60 | 80 |
| Clomipramine | 50 | 150 | 200 | 300 |
| Escitalopram | 5 | 10 | 10 to 20 | 30 |
| Fluoxetine | 20 | 40 | 40 to 60 | 80 |
| Fluvoxamine | 50 | 175 | 150 to 250 | 300 |
| Sertraline | 50 | 125 | 150 | 225 |
| *Mean dosage derived from registration trials, expert recommendation, and the authors’ clinical experience | ||||
Dosing. Fixed-dose studies suggest that dosing schedules for OCD are similar to those used for depression. For example, sertraline, 50 mg/d, or fluoxetine, 20 mg/d, are as effective as higher dosages.18
The common misconception that OCD requires higher dosages likely results from:
- increasing the dosage too early in the time-response window for a drug effect to emerge
- giving medication without concomitant exposure therapy.19
Delayed response. Although many patients respond early to an SSRI, others do not respond until 8 or even 12 weeks of treatment at therapeutic dosages. It often takes 3 to 4 weeks for evidence of benefit to emerge, so wait at least 3 weeks between dosage increases. Maintain therapeutic dosages at least 6 to 8 weeks before changing agents or beginning augmentation therapy.
Two-barrel approach.
In treating Adam, we began with sertraline, using a flexible titration schedule keyed to whether he experienced OCD symptom remission.
The starting dosage of 50 mg was titrated to 150 mg over 8 weeks while he was receiving behavioral therapy. We made adjustments with a time-response window of 2 to 3 weeks, allowing us to observe a response to each dosage escalation.
Adam’s OCD symptoms responded well to CBT plus sertraline. The maximum drug effect helped him confront the most difficult EX/RP tasks at the top of his stimulus hierarchy, which he attacked near the end of treatment.
Lessons learned. Multicenter trials have taught important lessons about drug therapy for OCD:
- OCD patients experience little or no placebo effect, unlike patients with depression.
- Clinical effects may appear as early as 2 to 3 weeks after medications are started and plateau at 10 to 12 weeks.
- Partial response is the rule; SSRIs reduce OCD symptoms by about 30%—which correlates with “moderately” to “markedly” improved ratings on patient satisfaction measures.
- Side effects and magnitude of improvement are comparable in pediatric and adult medication trials.
In some children, OCD or tic symptoms arise from or are exacerbated by group A beta hemolytic streptococcal infection (GABHS), which has been labeled pediatric autoimmune neuropsychiatric disorders associated with Streptococcus (PANDAS).21 Obsessive-compulsive symptoms are not uncommon in pediatric patients with Sydenham’s chorea, a neurologic variant of rheumatic fever. OCD is far more common in patients with rheumatic fever when chorea is present.
Acute-onset or dramatic exacerbation of OCD or tic symptoms should prompt investigation of GABHS infection. Immunomodulatory treatments—including antibiotics, plasmapheresis, or IV immunoglobulin G—may benefit some patients.22
MANAGING RESISTANT OCD
Adequate SSRI monotherapy trials fail to relieve OCD symptoms in one-third of patients. Some patients benefit from combination therapy—such as an SSRI plus risperidone—especially when comorbid schizotypal personality disorder or a tic-spectrum disorder is present.
Drug switching or augmentation trials often produce only partial response and cause unnecessary suffering. A more effective strategy for many patients is to augment drug treatment with CBT until symptoms normalize.
On the other hand, augmentation is appropriate when nonresponse or partial response to SSRI monotherapy leaves a patient clinically symptomatic and functionally impaired. Clonazepam, clomipramine, and the amino acid L-tryptophan have been used successfully. Lithium and buspirone also have been tried but seem not to be effective in controlled studies in adults and anecdotal experience in youth.
When augmenting an SSRI, adding clomipramine, 25 to 50 mg/d, is a reasonable choice. However, fluoxetine or paroxetine can inhibit clomipramine metabolism by cytochrome P-450 (CYP) 2D6, with potential for cardiac arrhythmias or seizures. Sertraline or fluvoxamine are less likely to elevate clomipramine levels.20 Fluvoxamine may be the most compatible SSRI with clomipramine because it inhibits CYP 1A2—the enzyme that demethylates clomipramine to its inactive desmethyl metabolite—thereby preserving more of the active parent compound.
Clinical evidence suggests that augmentation’s success may depend in part on a patient’s comorbidities. For example, clonazepam may be particularly helpful for children with comorbid panic symptoms.
MAINTENANCE THERAPY
We typically provide 14 weekly CBT sessions, followed by monthly contacts for a few months to ensure than a patient’s gains are maintained. Standard procedure with drug therapy is to continue maintenance treatment for up to 1 year, although some have suggested continuing maintenance treatment indefinitely.
Related resources
- Chansky TE. Freeing your child from obsessive compulsive disorder: A powerful, practical program for parents of children and adolescents. New York: Crown Publishing Group, 2001.
- March JS, Mulle K. OCD in children and adolescents: A cognitive behavioral treatment manual. New York: Guilford Press, 1998.
- Moritz EK, Jablonsky J. Blink, blink, clop, clop: Why do we do things we can’t stop? An OCD storybook. Newton, MA: Professional Books, Inc, 1998.
- Wagner AP. Up and down the worry hill: A children’s book about obsessive compulsive disorder and its treatment. Rochester, NY: Lighthouse Press, Inc. 2000.
Drug brand names
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertaline • Zoloft
Disclosure
Dr. March receives research support from Pfizer Inc., Eli Lilly and Co., and Wyeth Pharmaceuticals and is a speaker for and/or consultant to Solvay Pharmaceuticals, Pfizer Inc., GlaxoSmithKline, Wyeth Pharmaceuticals, Novartis Pharmaceuticals Corp., and Shire Pharmaceuticals Group.
Dr. Franklin and Dr. Foa report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Preparation of this manuscript was supported in part by National Institute of Mental Health grants 1 K24 MHO1557 and 1 R10 MH55121 to Dr. March and by contributions from the Robert and Sarah Gorrell family and the Lupin Family Foundation.
Adam, age 10, is extremely distressed at school. Because of obsessional contamination fears, he avoids contact with other children and refuses to eat in the cafeteria. He washes his hands 20 times per day and changes his clothes at least three times daily.
His primary obsessions involve contact with bodily fluids—such as saliva or feces—and excessive concerns that this contamination would cause him serious illness.
Adam’s parents say their son’s worries about dirt and germs began when he entered kindergarten. They sought treatment for him 2 years ago, and he has been receiving outpatient psychotherapy since then. They have brought him to an anxiety disorders specialty clinic for evaluation because his obsessive-compulsive symptoms are worsening,
When treating patients such as Adam, our approach is to use cognitive-behavioral therapy (CBT) and adjunctive drug therapies to relieve their symptoms and help them reclaim their lives. Diagnosis of pediatric OCD is often delayed, and few children receive state-of-the-art treatment.1 The good news, however, is that skillful CBT combined, as needed, with medication is highly effective.
Although family dysfunction does not cause OCD, families affect and are affected by OCD. Control struggles over the child’s rituals are common, as are differences of opinion about how to cope with OCD symptoms. It is important to address these issues early in treatment, as helping the family combat the disorder—rather than each other—is crucial to effective treatment.
Parents need to know that neither they nor the child are to blame. OCD is a neurobehavioral illness, and treatment is most effective when the patient, therapist, and family are aligned to combat it. Families are often entangled in the child’s OCD symptoms, and disentangling them by eliminating their role in ritualizing (such as giving excessive reassurance) is important to address in therapy.
Scaling family involvement is part of the “art” of CBT, and it will remain so until empiric studies determine the family’s role in the treatment plan.2
‘Contaminated’ mother.
Adam becomes distressed when he comes in contact with objects that have been touched by others (such as doorknobs). He is especially anxious when these items are associated with public bathrooms or sick people.
Adam’s mother is a family physician who has daily patient contact. In the last 6 months, Adam has insisted that his mother change her work clothes before she enters his room, touches him, prepares his food, or handles his possessions.
As in Adam’s case, the family often gets caught up in a child or adolescent’s obsessive rituals (Box 1).2 After a detailed discussion with Adam and his parents and because his symptoms were severe, we recommended combined treatment with sertraline and CBT. Adam was willing to consider CBT and medication because he recognized that he was having increasing difficulty doing the things he wanted to do in school and at home.
SNAPSHOT OF PEDIATRIC OCD
Approximately 1 in 200 children and adolescents suffer from clinically significant OCD.3 They experience intrusive thoughts, urges, or images to which they respond with dysphoria-reducing behaviors or rituals.
Common obsessions include:
- fear of dirt or germs
- fear of harm to oneself or someone else
- or a persistent need to complete something “just so.”
Corresponding compulsions include hand washing, checking, and repeating or arranging.
OCD appears more common in boys than in girls. Onset occurs in two modes: first at age 9 for boys and age 12 for girls, followed by a second mode in late adolescence or early adulthood.
Two practice guidelines address OCD in youth: an independent expert consensus guideline4 and the American Academy of Child and Adolescent Psychiatry’s practice parameters for OCD.5
For uncomplicated OCD, these guidelines recommend CBT as first-line treatment. If symptoms do not respond after six to eight sessions, a selective serotonin reuptake inhibitor (SSRI) is added to CBT.
For complicated OCD, medication is considered an appropriate initial treatment. Complicated OCD includes patients who:
- display severe symptoms—such as with scores >30 on the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS)
- or have comorbidity such as depression or panic disorder that is likely to complicate treatment.
KEYS TO SUCCESSFUL TREATMENT
OCD is remarkably resistant to insight-oriented psychotherapy and other nondirective therapies. The benefits of CBT, however, are well-established, with reported response rates of >80% in pilot studies.6,7 Although confirming studies have yet to be conducted, successful CBT for pediatric OCD appears to include four elements (Table 1).
Exposure and response prevention (EX/RP) is central to psychosocial treatment of OCD.7,8 In specialized centers, exposure can be applied intensively (three to five times per week for 3 to 4 weeks).9 In most practices, however, exposure is more gradual (weekly for 12 to 20 weeks). With repeated exposure, the child’s anxiety decreases until he or she no longer fears contact with the targeted stimuli.8,10
Not ‘misbehavior.’ Children—and less commonly adolescents—with this disorder may not view their obsessions as senseless or their compulsions as excessive. Even when insight is clearly present, young OCD patients often hide their symptoms because of embarrassment or fear of being punished for their behavior.
Response predictors. A key to CBT in children or adolescents is that they come to see obsessions and compulsions as symptoms of an illness. The symptoms, therefore, require a skillfully applied “antidote,” as taught by the clinician and implemented by the child, family, and others on the child’s behalf. Besides overt rituals, three response predictors include the patient’s:
- desire to eliminate symptoms
- ability to monitor and report symptoms
- willingness to cooperate with treatment.
Table 1
Pediatric OCD: 4 keys to successful cognitive therapy
| Treat OCD as a neurobehavioral disorder, not a misbehavior |
| Help the child develop a “tool kit” to manage dysphoria and faulty thinking |
| Expose the patient to anxiety-producing stimuli until he or she becomes desensitized and can refrain from the usual compulsive responses (exposure and response prevention) |
| Educate family members and school personnel |
CBT may be difficult with patients younger than age 6 and will invariably involve training the parents to serve as “coaches;” a CBT protocol for patients ages 4 to 6 is under investigation (H. Leonard, personal communication). CBT also can be adapted for patients with intellectual deficits.11
A ‘tool kit.’ Successful exposure therapy for OCD relies on equipping children and adolescents with the knowledge and skills to battle the illness. They often have tried unsuccessfully to resist OCD’s compulsions and must be convinced that EX/RP techniques will work. Using a “tool kit” concept reminds young patients that they have the implements they need to combat OCD (Table 2).
A ‘germ ladder’ and ‘fear thermometer.’
Adam’s tools include a stimulus hierarchy called a “germ ladder,” which the therapist and Adam create collaboratively. It ranks stimuli from low (his own doorknob) to very high (public toilets, sinks, and door handles).
As part of his treatment, Adam begins to touch objects in his room and house while voluntarily refraining from ritualizing. He uses another tool—a fear thermometer—to record his distress level on a scale of 1 to 10 during and after these exposures.
Adam discovers that when he comes into contact with less-threatening items his fear ratings typically return to baseline within 20 to 30 minutes. This insight helps him modify his assessment of the risk they pose.
Table 2
OCD ‘tool box’ can help patients build new behaviors
| Tool | Function |
|---|---|
| Training in exposure response prevention (EX/RP) therapy | Helps patients learn to confront rather than avoid feared stimuli |
| ‘Fear thermometer’ | Enables patients to express the intensity of their distress on a scale of 1 (lowest) to 10 (highest) |
| Positive self-statements | Teaches patients to use statements such as “I can do this” and “I’m the boss of OCD now” to build confidence that they can control their response to feared stimuli |
During office visits, he confronts similar items around the clinic, with the therapist providing encouragement and instruction for additional exposure homework. Eventually Adam works on the clinic’s public bathroom, which he perceived to be relatively clean but less so than his own bathroom. After fear in response to this bathroom is reduced, the therapist and Adam graduate to more-public facilities, such as the bathrooms at Adam’s pool and the local train station.
Exposure therapy. EX/RP is most successful when the child—rather than the therapist—chooses exposure targets from a hierarchy of fears,2 particularly when the list includes behaviors the child is resisting. In a collaborative spirit, the child takes the lead in placing items on the hierarchy and deciding when to confront them.
The therapist and child revise the hierarchy periodically, which demonstrates progress and allows them to add items as the child overcomes fears that cause less distress.
Reducing need for reassurance.
Adam has a habit of repeatedly asking his mother whether contact with particular objects in public is risky. By the third treatment session, he and the therapist agree that he will try to refrain from asking such questions.
His mother, in turn, is asked to reiterate the rationale for response prevention whenever Adam slips. She will offer encouragement and support without answering “OCD’s questions.”
ADJUNCTIVE DRUG THERAPY
While Adam is working with the behavioral therapist to reduce his anxieties and need for reassurance, he is also receiving gradually increasing dosages of sertraline. As discussed, he is considered a candidate for CBT plus medication because of his symptoms are severe. Drug treatment can benefit most pediatric OCD patients.
SSRIs. Two SSRIs are approved for pediatric OCD—fluvoxamine for ages 8 to 18 and sertraline for ages 6 to 18. Most SSRIs are likely effective for OCD in youth (Table 3),12-14 although reports have suggested a link between paroxetine and suicidality in pediatric patients. Other options may be more suitable choices unless further evidence supports the use of paroxetine as a first- or second-line agent for pediatric OCD.
Clomipramine—a nonselective tricyclic—was the first medication studied in treating OCD in children and adolescents. It is now usually considered only after two or three failed SSRI trials because of its potential for cardiac toxicity.15-17
Table 3
Suggested dosages (mg/d) for drug therapy of pediatric OCD
| Drug | Usual starting dosage | Approximate mean dosage* | Typical range | Usual maximum dosage |
|---|---|---|---|---|
| Citalopram | 20 | 40 | 20 to 60 | 80 |
| Clomipramine | 50 | 150 | 200 | 300 |
| Escitalopram | 5 | 10 | 10 to 20 | 30 |
| Fluoxetine | 20 | 40 | 40 to 60 | 80 |
| Fluvoxamine | 50 | 175 | 150 to 250 | 300 |
| Sertraline | 50 | 125 | 150 | 225 |
| *Mean dosage derived from registration trials, expert recommendation, and the authors’ clinical experience | ||||
Dosing. Fixed-dose studies suggest that dosing schedules for OCD are similar to those used for depression. For example, sertraline, 50 mg/d, or fluoxetine, 20 mg/d, are as effective as higher dosages.18
The common misconception that OCD requires higher dosages likely results from:
- increasing the dosage too early in the time-response window for a drug effect to emerge
- giving medication without concomitant exposure therapy.19
Delayed response. Although many patients respond early to an SSRI, others do not respond until 8 or even 12 weeks of treatment at therapeutic dosages. It often takes 3 to 4 weeks for evidence of benefit to emerge, so wait at least 3 weeks between dosage increases. Maintain therapeutic dosages at least 6 to 8 weeks before changing agents or beginning augmentation therapy.
Two-barrel approach.
In treating Adam, we began with sertraline, using a flexible titration schedule keyed to whether he experienced OCD symptom remission.
The starting dosage of 50 mg was titrated to 150 mg over 8 weeks while he was receiving behavioral therapy. We made adjustments with a time-response window of 2 to 3 weeks, allowing us to observe a response to each dosage escalation.
Adam’s OCD symptoms responded well to CBT plus sertraline. The maximum drug effect helped him confront the most difficult EX/RP tasks at the top of his stimulus hierarchy, which he attacked near the end of treatment.
Lessons learned. Multicenter trials have taught important lessons about drug therapy for OCD:
- OCD patients experience little or no placebo effect, unlike patients with depression.
- Clinical effects may appear as early as 2 to 3 weeks after medications are started and plateau at 10 to 12 weeks.
- Partial response is the rule; SSRIs reduce OCD symptoms by about 30%—which correlates with “moderately” to “markedly” improved ratings on patient satisfaction measures.
- Side effects and magnitude of improvement are comparable in pediatric and adult medication trials.
In some children, OCD or tic symptoms arise from or are exacerbated by group A beta hemolytic streptococcal infection (GABHS), which has been labeled pediatric autoimmune neuropsychiatric disorders associated with Streptococcus (PANDAS).21 Obsessive-compulsive symptoms are not uncommon in pediatric patients with Sydenham’s chorea, a neurologic variant of rheumatic fever. OCD is far more common in patients with rheumatic fever when chorea is present.
Acute-onset or dramatic exacerbation of OCD or tic symptoms should prompt investigation of GABHS infection. Immunomodulatory treatments—including antibiotics, plasmapheresis, or IV immunoglobulin G—may benefit some patients.22
MANAGING RESISTANT OCD
Adequate SSRI monotherapy trials fail to relieve OCD symptoms in one-third of patients. Some patients benefit from combination therapy—such as an SSRI plus risperidone—especially when comorbid schizotypal personality disorder or a tic-spectrum disorder is present.
Drug switching or augmentation trials often produce only partial response and cause unnecessary suffering. A more effective strategy for many patients is to augment drug treatment with CBT until symptoms normalize.
On the other hand, augmentation is appropriate when nonresponse or partial response to SSRI monotherapy leaves a patient clinically symptomatic and functionally impaired. Clonazepam, clomipramine, and the amino acid L-tryptophan have been used successfully. Lithium and buspirone also have been tried but seem not to be effective in controlled studies in adults and anecdotal experience in youth.
When augmenting an SSRI, adding clomipramine, 25 to 50 mg/d, is a reasonable choice. However, fluoxetine or paroxetine can inhibit clomipramine metabolism by cytochrome P-450 (CYP) 2D6, with potential for cardiac arrhythmias or seizures. Sertraline or fluvoxamine are less likely to elevate clomipramine levels.20 Fluvoxamine may be the most compatible SSRI with clomipramine because it inhibits CYP 1A2—the enzyme that demethylates clomipramine to its inactive desmethyl metabolite—thereby preserving more of the active parent compound.
Clinical evidence suggests that augmentation’s success may depend in part on a patient’s comorbidities. For example, clonazepam may be particularly helpful for children with comorbid panic symptoms.
MAINTENANCE THERAPY
We typically provide 14 weekly CBT sessions, followed by monthly contacts for a few months to ensure than a patient’s gains are maintained. Standard procedure with drug therapy is to continue maintenance treatment for up to 1 year, although some have suggested continuing maintenance treatment indefinitely.
Related resources
- Chansky TE. Freeing your child from obsessive compulsive disorder: A powerful, practical program for parents of children and adolescents. New York: Crown Publishing Group, 2001.
- March JS, Mulle K. OCD in children and adolescents: A cognitive behavioral treatment manual. New York: Guilford Press, 1998.
- Moritz EK, Jablonsky J. Blink, blink, clop, clop: Why do we do things we can’t stop? An OCD storybook. Newton, MA: Professional Books, Inc, 1998.
- Wagner AP. Up and down the worry hill: A children’s book about obsessive compulsive disorder and its treatment. Rochester, NY: Lighthouse Press, Inc. 2000.
Drug brand names
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertaline • Zoloft
Disclosure
Dr. March receives research support from Pfizer Inc., Eli Lilly and Co., and Wyeth Pharmaceuticals and is a speaker for and/or consultant to Solvay Pharmaceuticals, Pfizer Inc., GlaxoSmithKline, Wyeth Pharmaceuticals, Novartis Pharmaceuticals Corp., and Shire Pharmaceuticals Group.
Dr. Franklin and Dr. Foa report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Preparation of this manuscript was supported in part by National Institute of Mental Health grants 1 K24 MHO1557 and 1 R10 MH55121 to Dr. March and by contributions from the Robert and Sarah Gorrell family and the Lupin Family Foundation.
1. Kendall PC, Southam-Gerow MA. Issues in the transportability of treatment: the case of anxiety disorders in youths. J Consult Clin Psychol 1995;63(5):702-8.
2. March J, Mulle K. OCD in children and adolescents: A cognitive-behavioral treatment manual. New York: Guilford Press, 1998.
3. Flament MF, Whitaker A, Rapoport JL, et al. Obsessive compulsive disorder in adolescence: an epidemiological study. J Am Acad Child Adolesc Psychiatry 1988;27(6):764-71.
4. March J, Frances A, Kahn D, Carpenter D. Expert consensus guidelines: treatment of obsessive-compulsive disorder. J Clin Psychiatry 1997;58(suppl 4):1-72.
5. King R, Leonard H, March J. Practice parameters for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 1998;37(10, suppl):27-45.
6. March JS. Cognitive-behavioral psychotherapy for children and adolescents with OCD: a review and recommendations for treatment. J Am Acad Child Adolesc Psychiatry 1995;34(1):7-18.
7. Franklin ME, Kozak MJ, Cashman LA, et al. Cognitive-behavioral treatment of pediatric obsessive-compulsive disorder: an open clinical trial. J Am Acad Child Adolesc Psychiatry 1998;37(4):412-19.
8. March JS, Mulle K, Herbel B. Behavioral psychotherapy for children and adolescents with obsessive-compulsive disorder: an open trial of a new protocol-driven treatment package. J Am Acad Child Adolesc Psychiatry 1994;33(3):333-41.
9. Franklin ME, Tolin DF, March JS, Foa EB. Treatment of pediatric obsessive-compulsive disorder: A case example of intensive cognitive-behavioral therapy involving exposure and ritual prevention. Cognit Behav Pract 2001;8(4):297-304.
10. March J, Mulle K. Manualized cognitive-behavioral psychotherapy for obsessive-compulsive disorder in childhood: a preliminary single case study. J Anxiety Disord 1995;9(2):175-84.
11. Franklin ME, Rynn M, March JS, Foa EB. Obsessive-compulsive disorder. In: Hersen M (ed). Clinical behavior therapy: adults and children. New York: John Wiley & Sons, 2002;276-303.
12. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA 1998;280(20):1752-6.
13. Riddle MA, Reeve EA, Yaryura-Tobias JA, et al. Fluvoxamine for children and adolescents with obsessive-compulsive disorder: a randomized, controlled, multicenter trial. J Am Acad Child Adolesc Psychiatry 2001;40(2):222-9.
14. Geller DA, Hoog SL, Heiligenstein JH, et al. Fluoxetine treatment for obsessive-compulsive disorder in children and adolescents: a placebo-controlled clinical trial. J Am Acad Child Adolesc Psychiatry 2001;40(7):773-9.
15. Leonard H, March J, Rickler K, Allen A. Pharmacology of the selective serotonin reuptake inhibitors in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(6):725-36.
16. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder—a multicenter trial. J Am Acad Child Adolesc Psychiatry 1992;31(1):45-9.
17. Wilens TE, Biederman J, March JS, et al. Absence of cardiovascular adverse effects of sertraline in children and adolescents. J Am Acad Child Adolesc Psychiatry 1999;38(5):573-7.
18. Greist JH, Jefferson JW, Kobak KA, et al. A 1-year double-blind placebo-controlled fixed dose study of sertraline in the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol 1995;10(2):57-65.
19. Marks IM. Drug versus behavioral treatment of obsessive-compulsive disorder. Biolog Psychiatry 1990;28(12):1072-3.
20. Leonard HL, March J, Rickler KC, Allen AJ. Pharmacology of the selective serotonin reuptake inhibitors in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(6):725-36.
21. Leonard HL, Swedo SE. Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). Int J Neuropsychopharmacol 2001;4(2):191-8.
22. Perlmutter SJ, Leitman SF, Garvey MA, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 1999;354 (9185):1153-8.
1. Kendall PC, Southam-Gerow MA. Issues in the transportability of treatment: the case of anxiety disorders in youths. J Consult Clin Psychol 1995;63(5):702-8.
2. March J, Mulle K. OCD in children and adolescents: A cognitive-behavioral treatment manual. New York: Guilford Press, 1998.
3. Flament MF, Whitaker A, Rapoport JL, et al. Obsessive compulsive disorder in adolescence: an epidemiological study. J Am Acad Child Adolesc Psychiatry 1988;27(6):764-71.
4. March J, Frances A, Kahn D, Carpenter D. Expert consensus guidelines: treatment of obsessive-compulsive disorder. J Clin Psychiatry 1997;58(suppl 4):1-72.
5. King R, Leonard H, March J. Practice parameters for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 1998;37(10, suppl):27-45.
6. March JS. Cognitive-behavioral psychotherapy for children and adolescents with OCD: a review and recommendations for treatment. J Am Acad Child Adolesc Psychiatry 1995;34(1):7-18.
7. Franklin ME, Kozak MJ, Cashman LA, et al. Cognitive-behavioral treatment of pediatric obsessive-compulsive disorder: an open clinical trial. J Am Acad Child Adolesc Psychiatry 1998;37(4):412-19.
8. March JS, Mulle K, Herbel B. Behavioral psychotherapy for children and adolescents with obsessive-compulsive disorder: an open trial of a new protocol-driven treatment package. J Am Acad Child Adolesc Psychiatry 1994;33(3):333-41.
9. Franklin ME, Tolin DF, March JS, Foa EB. Treatment of pediatric obsessive-compulsive disorder: A case example of intensive cognitive-behavioral therapy involving exposure and ritual prevention. Cognit Behav Pract 2001;8(4):297-304.
10. March J, Mulle K. Manualized cognitive-behavioral psychotherapy for obsessive-compulsive disorder in childhood: a preliminary single case study. J Anxiety Disord 1995;9(2):175-84.
11. Franklin ME, Rynn M, March JS, Foa EB. Obsessive-compulsive disorder. In: Hersen M (ed). Clinical behavior therapy: adults and children. New York: John Wiley & Sons, 2002;276-303.
12. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA 1998;280(20):1752-6.
13. Riddle MA, Reeve EA, Yaryura-Tobias JA, et al. Fluvoxamine for children and adolescents with obsessive-compulsive disorder: a randomized, controlled, multicenter trial. J Am Acad Child Adolesc Psychiatry 2001;40(2):222-9.
14. Geller DA, Hoog SL, Heiligenstein JH, et al. Fluoxetine treatment for obsessive-compulsive disorder in children and adolescents: a placebo-controlled clinical trial. J Am Acad Child Adolesc Psychiatry 2001;40(7):773-9.
15. Leonard H, March J, Rickler K, Allen A. Pharmacology of the selective serotonin reuptake inhibitors in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(6):725-36.
16. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder—a multicenter trial. J Am Acad Child Adolesc Psychiatry 1992;31(1):45-9.
17. Wilens TE, Biederman J, March JS, et al. Absence of cardiovascular adverse effects of sertraline in children and adolescents. J Am Acad Child Adolesc Psychiatry 1999;38(5):573-7.
18. Greist JH, Jefferson JW, Kobak KA, et al. A 1-year double-blind placebo-controlled fixed dose study of sertraline in the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol 1995;10(2):57-65.
19. Marks IM. Drug versus behavioral treatment of obsessive-compulsive disorder. Biolog Psychiatry 1990;28(12):1072-3.
20. Leonard HL, March J, Rickler KC, Allen AJ. Pharmacology of the selective serotonin reuptake inhibitors in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(6):725-36.
21. Leonard HL, Swedo SE. Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). Int J Neuropsychopharmacol 2001;4(2):191-8.
22. Perlmutter SJ, Leitman SF, Garvey MA, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 1999;354 (9185):1153-8.
The truth about anxiety disorders
Too often, anxiety disorders go unrecognized or undertreated. Worse, many physicians still view an anxiety disorder as a character flaw, mirroring how society sees anxiety. The assumption is, “We all have to deal with frightening stuff. Some can take it, some can’t.”
Yet anxiety disorders are as distinct from everyday anxiety as major depressive disorder is from everyday unhappiness. Further, it is becoming increasingly clear that the consequences of anxiety disorders are serious—and that appropriate treatment can help.
Dr. Bruce Lydiard’s article provides an excellent framework for diagnosis and treatment of panic disorder. Dr. John March’s article on obsessive-compulsive disorder in children and adolescents does the same for another anxiety-related condition.
We as a profession have increased the public’s awareness of major depression; now we need to increase the public’s understanding of anxiety disorders. We need to impress upon the public and upon primary care physicians that anxiety disorders, like depressive disorders, are serious yet treatable illnesses, not character flaws. By getting this message out, we will decrease the stigma of mental illness and remove barriers to the long-await-ed effective treatments that are now becoming available.
Too often, anxiety disorders go unrecognized or undertreated. Worse, many physicians still view an anxiety disorder as a character flaw, mirroring how society sees anxiety. The assumption is, “We all have to deal with frightening stuff. Some can take it, some can’t.”
Yet anxiety disorders are as distinct from everyday anxiety as major depressive disorder is from everyday unhappiness. Further, it is becoming increasingly clear that the consequences of anxiety disorders are serious—and that appropriate treatment can help.
Dr. Bruce Lydiard’s article provides an excellent framework for diagnosis and treatment of panic disorder. Dr. John March’s article on obsessive-compulsive disorder in children and adolescents does the same for another anxiety-related condition.
We as a profession have increased the public’s awareness of major depression; now we need to increase the public’s understanding of anxiety disorders. We need to impress upon the public and upon primary care physicians that anxiety disorders, like depressive disorders, are serious yet treatable illnesses, not character flaws. By getting this message out, we will decrease the stigma of mental illness and remove barriers to the long-await-ed effective treatments that are now becoming available.
Too often, anxiety disorders go unrecognized or undertreated. Worse, many physicians still view an anxiety disorder as a character flaw, mirroring how society sees anxiety. The assumption is, “We all have to deal with frightening stuff. Some can take it, some can’t.”
Yet anxiety disorders are as distinct from everyday anxiety as major depressive disorder is from everyday unhappiness. Further, it is becoming increasingly clear that the consequences of anxiety disorders are serious—and that appropriate treatment can help.
Dr. Bruce Lydiard’s article provides an excellent framework for diagnosis and treatment of panic disorder. Dr. John March’s article on obsessive-compulsive disorder in children and adolescents does the same for another anxiety-related condition.
We as a profession have increased the public’s awareness of major depression; now we need to increase the public’s understanding of anxiety disorders. We need to impress upon the public and upon primary care physicians that anxiety disorders, like depressive disorders, are serious yet treatable illnesses, not character flaws. By getting this message out, we will decrease the stigma of mental illness and remove barriers to the long-await-ed effective treatments that are now becoming available.