User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
In patients with untreated AIDS, monkeypox can be life-threatening
Monkeypox, though often mild, may be severe and even fatal in immunocompromised individuals, particularly those with untreated AIDS, according to a Centers for Disease Control and Prevention study in Morbidity and Mortality Weekly Report.
The study described a group of patients recently treated for severe monkeypox. The majority were Black, HIV positive, and not receiving treatment. Many were also facing homelessness.
The authors urged HIV testing for all sexually active individuals with suspected monkeypox. Early or prolonged monkeypox treatment may be necessary, they concluded.
Coauthor John T. Brooks, MD, called the study “a real call to action.”
“If we want to reduce cases of severe monkeypox, we need to reduce the number of persons with HIV who are undiagnosed and not treated,” said Dr. Brooks, a medical epidemiologist who is chief medical officer of CDC›s multinational monkeypox response. Dr. Brooks also leads the epidemiology research team in CDC’s division of HIV/AIDS prevention.
noted Richard Silvera, MD, MPH, CPH, who is associate program director of the infectious diseases fellowship and assistant professor of medicine (infectious diseases) at the Icahn School of Medicine at Mount Sinai, New York. He was not involved with the study.
“These patients really have not been served by the health care system,” Dr. Silvera said. “Monkeypox is just really taking advantage of that.”
How severe monkeypox can manifest
The authors reported on 57 adults hospitalized with severe monkeypox between Aug. 10 and Sept. 10, 2022, for whose care the providers sought CDC consultation.
The vast majority (95%) were men, their median age was 34 years, and 68% were Black. Nearly one in four were homeless (23%).
Overall, 47 (82%) were HIV positive, of whom just 4 had been receiving antiretroviral therapy (ART). Of 43 for whom CD4 counts were known, 71% had fewer than 50 CD4 cells/mm3.
Clinical signs included severe skin lesions in all patients and severe mucosal lesions in 68%. Other affected organ systems included lungs (21%), eyes (21%), and central nervous system (7%).
Treatments included oral or intravenous tecovirimat (93% and 65%, respectively), vaccinia immune globulin intravenous (VIGIV, 51%), and cidofovir (23%).
Nearly 1 in 3 patients (30%) received care in an ICU; 12 died (21%). Monkeypox was considered the cause or a contributing factor in five of the deaths and not a factor in one death; the remaining six deaths are under investigation.
Case studies
The report included details of three representative cases of the CDC consultations.
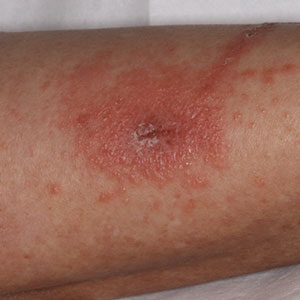
One was a Hispanic man in his 20s with a fever of 102.8° F, a rash including eschars, oral lesions, neck mass, and cervical lymphadenopathy. He had tested positive for monkeypox as an outpatient and upon admission was found to be HIV positive, with a CD4 count of 79 cells/mm3. He experienced a severe and ultimately fatal clinical course that included intubation, refractory hypotension, seizures, renal failure, and cardiac arrest. An autopsy revealed diffuse organ necrosis plus orthopoxvirus and cytomegalovirus.
The second was a Black man in his 30s with untreated AIDS and diffuse rash. He was tested and treated for gonorrhea, chlamydia, and syphilis before phimosis and urinary retention led to admission and a monkeypox diagnosis 4 weeks after his rash began. He was discharged with oral tecovirimat, but his skin lesions developed necrosis and he was readmitted twice, each time with new lesions. His clinical course included methicillin-resistant Staphylococcus aureus bacteremia, atrial fibrillation, eye and ear involvement, a suprapubic catheter, and progressive necrosis of his lesions. As of the CDC report, he was receiving ART and intravenous tecovirimat.
The third patient, a White man in his 40s with untreated AIDS, presented with diffuse rash. He was promptly diagnosed with monkeypox and admitted for pain control. He was discharged with oral tecovirimat and ART, but homelessness and food insecurity jeopardized the absorption of his tecovirimat (which depends on a full fatty meal), and the lesions worsened. Despite readmission and aggressive medical treatment, the patient required finger debridement and a toe amputation. After discharge, he was again readmitted for lesions and pain and, at report publication, remained hospitalized, taking oral tecovirimat and ART.
The patients in the study may not be typical of severe monkeypox cases, wrote the authors reported. Deaths after the study period were not counted.
Fewer cases, some severe
As of Nov. 7, the CDC has confirmed 28,709 monkeypox cases. These have trended downward since August. Most people with recent diagnoses are men who are gay, bisexual, same gender loving, or who have sex with men, and most are Black, according to Brooks.
Dr. Brooks urges clinicians to report suspected monkeypox cases – especially severe ones – to their health departments.
“We don’t have a good bead on exactly how many severe cases there are in the States because of complexities in our surveillance systems,” Dr. Brooks said.
For patients with suspected or confirmed monkeypox, Brooks recommends testing for sexually transmitted infections, including HIV if status is unknown. Patients with HIV should receive prompt ART. For those at risk for severe disease, the authors recommend early treatment for suspected monkeypox, even before results are back. Some patients may benefit from tecovirimat courses lasting beyond 14 days, plus additional antivirals (cidofovir or brincidofovir) and/or VIGIV.
“With severe cases, clinicians may want to consider the value of more than one drug to attack the virus at different stages of its replication cycle,” Dr. Brooks said.
Inequities matter
The authors called on providers to engage communities burdened by HIV and to ensure access to not only monkeypox vaccination, diagnosis, and treatment but also sustained HIV care.
Dr. Silvera added that providers need to tailor care plans to patients’ social determinants of health. For example, he explained, inpatient care for monkeypox could be appropriate for some patients facing homelessness and food insecurity – even if they are able to take tecovirimat orally.
He recommends tapping others’ expertise: “Our social work colleagues are well versed in this.”
“I don’t think these clinicians failed these patients. ... I think everyone made all the right choices medically,” Dr. Silvera added. “I think that the system failed these patients – and we as clinicians are part of those systems. So we also have the power to change those systems. And I think we just need to start opening our eyes to that and [start] to work together towards that goal to take better care of our patients.”
Dr. Brooks reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Monkeypox, though often mild, may be severe and even fatal in immunocompromised individuals, particularly those with untreated AIDS, according to a Centers for Disease Control and Prevention study in Morbidity and Mortality Weekly Report.
The study described a group of patients recently treated for severe monkeypox. The majority were Black, HIV positive, and not receiving treatment. Many were also facing homelessness.
The authors urged HIV testing for all sexually active individuals with suspected monkeypox. Early or prolonged monkeypox treatment may be necessary, they concluded.
Coauthor John T. Brooks, MD, called the study “a real call to action.”
“If we want to reduce cases of severe monkeypox, we need to reduce the number of persons with HIV who are undiagnosed and not treated,” said Dr. Brooks, a medical epidemiologist who is chief medical officer of CDC›s multinational monkeypox response. Dr. Brooks also leads the epidemiology research team in CDC’s division of HIV/AIDS prevention.
noted Richard Silvera, MD, MPH, CPH, who is associate program director of the infectious diseases fellowship and assistant professor of medicine (infectious diseases) at the Icahn School of Medicine at Mount Sinai, New York. He was not involved with the study.
“These patients really have not been served by the health care system,” Dr. Silvera said. “Monkeypox is just really taking advantage of that.”
How severe monkeypox can manifest
The authors reported on 57 adults hospitalized with severe monkeypox between Aug. 10 and Sept. 10, 2022, for whose care the providers sought CDC consultation.
The vast majority (95%) were men, their median age was 34 years, and 68% were Black. Nearly one in four were homeless (23%).
Overall, 47 (82%) were HIV positive, of whom just 4 had been receiving antiretroviral therapy (ART). Of 43 for whom CD4 counts were known, 71% had fewer than 50 CD4 cells/mm3.
Clinical signs included severe skin lesions in all patients and severe mucosal lesions in 68%. Other affected organ systems included lungs (21%), eyes (21%), and central nervous system (7%).
Treatments included oral or intravenous tecovirimat (93% and 65%, respectively), vaccinia immune globulin intravenous (VIGIV, 51%), and cidofovir (23%).
Nearly 1 in 3 patients (30%) received care in an ICU; 12 died (21%). Monkeypox was considered the cause or a contributing factor in five of the deaths and not a factor in one death; the remaining six deaths are under investigation.
Case studies
The report included details of three representative cases of the CDC consultations.
One was a Hispanic man in his 20s with a fever of 102.8° F, a rash including eschars, oral lesions, neck mass, and cervical lymphadenopathy. He had tested positive for monkeypox as an outpatient and upon admission was found to be HIV positive, with a CD4 count of 79 cells/mm3. He experienced a severe and ultimately fatal clinical course that included intubation, refractory hypotension, seizures, renal failure, and cardiac arrest. An autopsy revealed diffuse organ necrosis plus orthopoxvirus and cytomegalovirus.
The second was a Black man in his 30s with untreated AIDS and diffuse rash. He was tested and treated for gonorrhea, chlamydia, and syphilis before phimosis and urinary retention led to admission and a monkeypox diagnosis 4 weeks after his rash began. He was discharged with oral tecovirimat, but his skin lesions developed necrosis and he was readmitted twice, each time with new lesions. His clinical course included methicillin-resistant Staphylococcus aureus bacteremia, atrial fibrillation, eye and ear involvement, a suprapubic catheter, and progressive necrosis of his lesions. As of the CDC report, he was receiving ART and intravenous tecovirimat.
The third patient, a White man in his 40s with untreated AIDS, presented with diffuse rash. He was promptly diagnosed with monkeypox and admitted for pain control. He was discharged with oral tecovirimat and ART, but homelessness and food insecurity jeopardized the absorption of his tecovirimat (which depends on a full fatty meal), and the lesions worsened. Despite readmission and aggressive medical treatment, the patient required finger debridement and a toe amputation. After discharge, he was again readmitted for lesions and pain and, at report publication, remained hospitalized, taking oral tecovirimat and ART.
The patients in the study may not be typical of severe monkeypox cases, wrote the authors reported. Deaths after the study period were not counted.
Fewer cases, some severe
As of Nov. 7, the CDC has confirmed 28,709 monkeypox cases. These have trended downward since August. Most people with recent diagnoses are men who are gay, bisexual, same gender loving, or who have sex with men, and most are Black, according to Brooks.
Dr. Brooks urges clinicians to report suspected monkeypox cases – especially severe ones – to their health departments.
“We don’t have a good bead on exactly how many severe cases there are in the States because of complexities in our surveillance systems,” Dr. Brooks said.
For patients with suspected or confirmed monkeypox, Brooks recommends testing for sexually transmitted infections, including HIV if status is unknown. Patients with HIV should receive prompt ART. For those at risk for severe disease, the authors recommend early treatment for suspected monkeypox, even before results are back. Some patients may benefit from tecovirimat courses lasting beyond 14 days, plus additional antivirals (cidofovir or brincidofovir) and/or VIGIV.
“With severe cases, clinicians may want to consider the value of more than one drug to attack the virus at different stages of its replication cycle,” Dr. Brooks said.
Inequities matter
The authors called on providers to engage communities burdened by HIV and to ensure access to not only monkeypox vaccination, diagnosis, and treatment but also sustained HIV care.
Dr. Silvera added that providers need to tailor care plans to patients’ social determinants of health. For example, he explained, inpatient care for monkeypox could be appropriate for some patients facing homelessness and food insecurity – even if they are able to take tecovirimat orally.
He recommends tapping others’ expertise: “Our social work colleagues are well versed in this.”
“I don’t think these clinicians failed these patients. ... I think everyone made all the right choices medically,” Dr. Silvera added. “I think that the system failed these patients – and we as clinicians are part of those systems. So we also have the power to change those systems. And I think we just need to start opening our eyes to that and [start] to work together towards that goal to take better care of our patients.”
Dr. Brooks reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Monkeypox, though often mild, may be severe and even fatal in immunocompromised individuals, particularly those with untreated AIDS, according to a Centers for Disease Control and Prevention study in Morbidity and Mortality Weekly Report.
The study described a group of patients recently treated for severe monkeypox. The majority were Black, HIV positive, and not receiving treatment. Many were also facing homelessness.
The authors urged HIV testing for all sexually active individuals with suspected monkeypox. Early or prolonged monkeypox treatment may be necessary, they concluded.
Coauthor John T. Brooks, MD, called the study “a real call to action.”
“If we want to reduce cases of severe monkeypox, we need to reduce the number of persons with HIV who are undiagnosed and not treated,” said Dr. Brooks, a medical epidemiologist who is chief medical officer of CDC›s multinational monkeypox response. Dr. Brooks also leads the epidemiology research team in CDC’s division of HIV/AIDS prevention.
noted Richard Silvera, MD, MPH, CPH, who is associate program director of the infectious diseases fellowship and assistant professor of medicine (infectious diseases) at the Icahn School of Medicine at Mount Sinai, New York. He was not involved with the study.
“These patients really have not been served by the health care system,” Dr. Silvera said. “Monkeypox is just really taking advantage of that.”
How severe monkeypox can manifest
The authors reported on 57 adults hospitalized with severe monkeypox between Aug. 10 and Sept. 10, 2022, for whose care the providers sought CDC consultation.
The vast majority (95%) were men, their median age was 34 years, and 68% were Black. Nearly one in four were homeless (23%).
Overall, 47 (82%) were HIV positive, of whom just 4 had been receiving antiretroviral therapy (ART). Of 43 for whom CD4 counts were known, 71% had fewer than 50 CD4 cells/mm3.
Clinical signs included severe skin lesions in all patients and severe mucosal lesions in 68%. Other affected organ systems included lungs (21%), eyes (21%), and central nervous system (7%).
Treatments included oral or intravenous tecovirimat (93% and 65%, respectively), vaccinia immune globulin intravenous (VIGIV, 51%), and cidofovir (23%).
Nearly 1 in 3 patients (30%) received care in an ICU; 12 died (21%). Monkeypox was considered the cause or a contributing factor in five of the deaths and not a factor in one death; the remaining six deaths are under investigation.
Case studies
The report included details of three representative cases of the CDC consultations.
One was a Hispanic man in his 20s with a fever of 102.8° F, a rash including eschars, oral lesions, neck mass, and cervical lymphadenopathy. He had tested positive for monkeypox as an outpatient and upon admission was found to be HIV positive, with a CD4 count of 79 cells/mm3. He experienced a severe and ultimately fatal clinical course that included intubation, refractory hypotension, seizures, renal failure, and cardiac arrest. An autopsy revealed diffuse organ necrosis plus orthopoxvirus and cytomegalovirus.
The second was a Black man in his 30s with untreated AIDS and diffuse rash. He was tested and treated for gonorrhea, chlamydia, and syphilis before phimosis and urinary retention led to admission and a monkeypox diagnosis 4 weeks after his rash began. He was discharged with oral tecovirimat, but his skin lesions developed necrosis and he was readmitted twice, each time with new lesions. His clinical course included methicillin-resistant Staphylococcus aureus bacteremia, atrial fibrillation, eye and ear involvement, a suprapubic catheter, and progressive necrosis of his lesions. As of the CDC report, he was receiving ART and intravenous tecovirimat.
The third patient, a White man in his 40s with untreated AIDS, presented with diffuse rash. He was promptly diagnosed with monkeypox and admitted for pain control. He was discharged with oral tecovirimat and ART, but homelessness and food insecurity jeopardized the absorption of his tecovirimat (which depends on a full fatty meal), and the lesions worsened. Despite readmission and aggressive medical treatment, the patient required finger debridement and a toe amputation. After discharge, he was again readmitted for lesions and pain and, at report publication, remained hospitalized, taking oral tecovirimat and ART.
The patients in the study may not be typical of severe monkeypox cases, wrote the authors reported. Deaths after the study period were not counted.
Fewer cases, some severe
As of Nov. 7, the CDC has confirmed 28,709 monkeypox cases. These have trended downward since August. Most people with recent diagnoses are men who are gay, bisexual, same gender loving, or who have sex with men, and most are Black, according to Brooks.
Dr. Brooks urges clinicians to report suspected monkeypox cases – especially severe ones – to their health departments.
“We don’t have a good bead on exactly how many severe cases there are in the States because of complexities in our surveillance systems,” Dr. Brooks said.
For patients with suspected or confirmed monkeypox, Brooks recommends testing for sexually transmitted infections, including HIV if status is unknown. Patients with HIV should receive prompt ART. For those at risk for severe disease, the authors recommend early treatment for suspected monkeypox, even before results are back. Some patients may benefit from tecovirimat courses lasting beyond 14 days, plus additional antivirals (cidofovir or brincidofovir) and/or VIGIV.
“With severe cases, clinicians may want to consider the value of more than one drug to attack the virus at different stages of its replication cycle,” Dr. Brooks said.
Inequities matter
The authors called on providers to engage communities burdened by HIV and to ensure access to not only monkeypox vaccination, diagnosis, and treatment but also sustained HIV care.
Dr. Silvera added that providers need to tailor care plans to patients’ social determinants of health. For example, he explained, inpatient care for monkeypox could be appropriate for some patients facing homelessness and food insecurity – even if they are able to take tecovirimat orally.
He recommends tapping others’ expertise: “Our social work colleagues are well versed in this.”
“I don’t think these clinicians failed these patients. ... I think everyone made all the right choices medically,” Dr. Silvera added. “I think that the system failed these patients – and we as clinicians are part of those systems. So we also have the power to change those systems. And I think we just need to start opening our eyes to that and [start] to work together towards that goal to take better care of our patients.”
Dr. Brooks reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
Study finds high rate of psychiatric burden in cosmetic dermatology patients
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Have you heard the one about the emergency dept. that called 911?
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.
Medicaid coverage of HPV vaccine in adults: Implications in dermatology
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
FROM JAMA DERMATOLOGY
Physicians react: Climate change and other social issues
Around half of them rated climate change among their five most important issues. Slightly lower percentages of doctors prioritized domestic violence and immigration/refugee policies that highly, and about 40% did so regarding reproductive rights in the United States.
Survey responses and comments left on the Physicians’ Views on Today’s Divisive Social Issues 2022 report provide insights into doctors’ attitudes and thinking about these four social challenges.
Relevance of climate change to health care
In the Medscape report, 61% of physicians described themselves as “very concerned” or “concerned” about climate change, and about 7 in 10 agreed with the statement that it should be a top worldwide priority. “Climate change is the most pressing issue of this century,” a psychiatrist respondent wrote.
What about direct effects on patients’ health? An internist worried that rising temperatures will cause “pathogens to spread and infect disadvantaged people who do not have health access and have immunocompromised conditions.” A family medicine physician predicted “more weather disasters, more asthma, more hormonal changes, and more obesity.”
However, physician viewpoints ran the gamut with an issue that has become politically and emotionally charged. Descriptions such as “overblown,” “hysteria,” “hoax,” and “farce” were used. “Climate change is a natural phenomenon under God’s purview,” an emergency medicine physician said.
And there was some middle-ground thinking. “It’s overstated but quite real,” a pediatrician respondent wrote. Added an ophthalmologist: “It has gone on for ages. We must work to decrease man-made conditions that affect climate change, but it must be done in an intelligent fashion.”
Domestic violence: What physicians can do
About 7 in 10 physicians surveyed by Medscape said they don’t think the United States is adequately tackling domestic violence. “It is underrecognized and ignored,” a psychiatrist respondent argued. The problem is “rampant and unacceptable, pushed into a closet and normalized, with associated shame,” an emergency medicine doctor wrote.
Many respondents noted that physicians are under a mandate to report abuse of or a suspicious injury to a patient. Some shared anecdotes about how they reported action they had taken when they suspected it. “I’ve told patients who may be in dangerous situations that I’m a safe person and provide a safe space,” a radiologist added. An internist said, “I’ve recently started to ask about safety at home during triage on every patient.”
Other doctors bemoaned a lack of adequate education on detecting and managing domestic violence and abuse. “Domestic violence is often not recognized by health care providers,” a psychiatrist respondent observed.
Expanding legal immigration
In the Medscape report, 34% of physicians felt U.S. immigration/refugee policies need to be tougher, while 28% said they are too restrictive, and about a fifth saw them as appropriate.
“As an immigrant, I can tell you that the system is flawed and needs a complete overhaul, which will take a bipartisan effort,” an endocrinologist respondent wrote.
A number of respondents argued that it’s critical to simplify the process of obtaining U.S. citizenship so that fewer will feel forced to enter the country illegally. “For a country that relies very heavily on immigrants to sustain our health care system, we behave like idiots in denying safe harbor,” a nephrologist asserted.
A neurologist concurred. “Legal immigration needs to be encouraged. It should be easier to exchange visitor or student visa to immigrant visa in order to retain talent in the health care and technology fields, which would alleviate the shortage of workers in health care.”
Reproductive rights: No easy answers
Medscape’s survey was conducted before the U.S. Supreme Court in June reversed Roe v. Wade. In the report, 71% of physicians described themselves as very to somewhat concerned about women’s reproductive rights, but their viewpoints became nuanced after that. “There is a big disparity among physicians on this topic,” an oncologist respondent wrote.
At one end of the spectrum, 3% of doctors felt that abortions should never be permitted. “The human baby in the womb is an independent person with the right to life,” a pathologist said. At the other end, nearly one-fourth of physicians believed abortion should be accessible under all circumstances, regardless of trimester or reason. “I am just here to support the woman and make her decision a reality,” an internist said.
While saying an abortion should be granted after “fetal viability” only “in extenuating circumstances,” an ob.gyn. respondent said she is “extremely concerned” about attacks on abortion rights. “Some of us are old enough to remember women coming to the ER in extremis after illegal procedures, prior to Roe v. Wade.”
A version of this article first appeared on Medscape.com.
Around half of them rated climate change among their five most important issues. Slightly lower percentages of doctors prioritized domestic violence and immigration/refugee policies that highly, and about 40% did so regarding reproductive rights in the United States.
Survey responses and comments left on the Physicians’ Views on Today’s Divisive Social Issues 2022 report provide insights into doctors’ attitudes and thinking about these four social challenges.
Relevance of climate change to health care
In the Medscape report, 61% of physicians described themselves as “very concerned” or “concerned” about climate change, and about 7 in 10 agreed with the statement that it should be a top worldwide priority. “Climate change is the most pressing issue of this century,” a psychiatrist respondent wrote.
What about direct effects on patients’ health? An internist worried that rising temperatures will cause “pathogens to spread and infect disadvantaged people who do not have health access and have immunocompromised conditions.” A family medicine physician predicted “more weather disasters, more asthma, more hormonal changes, and more obesity.”
However, physician viewpoints ran the gamut with an issue that has become politically and emotionally charged. Descriptions such as “overblown,” “hysteria,” “hoax,” and “farce” were used. “Climate change is a natural phenomenon under God’s purview,” an emergency medicine physician said.
And there was some middle-ground thinking. “It’s overstated but quite real,” a pediatrician respondent wrote. Added an ophthalmologist: “It has gone on for ages. We must work to decrease man-made conditions that affect climate change, but it must be done in an intelligent fashion.”
Domestic violence: What physicians can do
About 7 in 10 physicians surveyed by Medscape said they don’t think the United States is adequately tackling domestic violence. “It is underrecognized and ignored,” a psychiatrist respondent argued. The problem is “rampant and unacceptable, pushed into a closet and normalized, with associated shame,” an emergency medicine doctor wrote.
Many respondents noted that physicians are under a mandate to report abuse of or a suspicious injury to a patient. Some shared anecdotes about how they reported action they had taken when they suspected it. “I’ve told patients who may be in dangerous situations that I’m a safe person and provide a safe space,” a radiologist added. An internist said, “I’ve recently started to ask about safety at home during triage on every patient.”
Other doctors bemoaned a lack of adequate education on detecting and managing domestic violence and abuse. “Domestic violence is often not recognized by health care providers,” a psychiatrist respondent observed.
Expanding legal immigration
In the Medscape report, 34% of physicians felt U.S. immigration/refugee policies need to be tougher, while 28% said they are too restrictive, and about a fifth saw them as appropriate.
“As an immigrant, I can tell you that the system is flawed and needs a complete overhaul, which will take a bipartisan effort,” an endocrinologist respondent wrote.
A number of respondents argued that it’s critical to simplify the process of obtaining U.S. citizenship so that fewer will feel forced to enter the country illegally. “For a country that relies very heavily on immigrants to sustain our health care system, we behave like idiots in denying safe harbor,” a nephrologist asserted.
A neurologist concurred. “Legal immigration needs to be encouraged. It should be easier to exchange visitor or student visa to immigrant visa in order to retain talent in the health care and technology fields, which would alleviate the shortage of workers in health care.”
Reproductive rights: No easy answers
Medscape’s survey was conducted before the U.S. Supreme Court in June reversed Roe v. Wade. In the report, 71% of physicians described themselves as very to somewhat concerned about women’s reproductive rights, but their viewpoints became nuanced after that. “There is a big disparity among physicians on this topic,” an oncologist respondent wrote.
At one end of the spectrum, 3% of doctors felt that abortions should never be permitted. “The human baby in the womb is an independent person with the right to life,” a pathologist said. At the other end, nearly one-fourth of physicians believed abortion should be accessible under all circumstances, regardless of trimester or reason. “I am just here to support the woman and make her decision a reality,” an internist said.
While saying an abortion should be granted after “fetal viability” only “in extenuating circumstances,” an ob.gyn. respondent said she is “extremely concerned” about attacks on abortion rights. “Some of us are old enough to remember women coming to the ER in extremis after illegal procedures, prior to Roe v. Wade.”
A version of this article first appeared on Medscape.com.
Around half of them rated climate change among their five most important issues. Slightly lower percentages of doctors prioritized domestic violence and immigration/refugee policies that highly, and about 40% did so regarding reproductive rights in the United States.
Survey responses and comments left on the Physicians’ Views on Today’s Divisive Social Issues 2022 report provide insights into doctors’ attitudes and thinking about these four social challenges.
Relevance of climate change to health care
In the Medscape report, 61% of physicians described themselves as “very concerned” or “concerned” about climate change, and about 7 in 10 agreed with the statement that it should be a top worldwide priority. “Climate change is the most pressing issue of this century,” a psychiatrist respondent wrote.
What about direct effects on patients’ health? An internist worried that rising temperatures will cause “pathogens to spread and infect disadvantaged people who do not have health access and have immunocompromised conditions.” A family medicine physician predicted “more weather disasters, more asthma, more hormonal changes, and more obesity.”
However, physician viewpoints ran the gamut with an issue that has become politically and emotionally charged. Descriptions such as “overblown,” “hysteria,” “hoax,” and “farce” were used. “Climate change is a natural phenomenon under God’s purview,” an emergency medicine physician said.
And there was some middle-ground thinking. “It’s overstated but quite real,” a pediatrician respondent wrote. Added an ophthalmologist: “It has gone on for ages. We must work to decrease man-made conditions that affect climate change, but it must be done in an intelligent fashion.”
Domestic violence: What physicians can do
About 7 in 10 physicians surveyed by Medscape said they don’t think the United States is adequately tackling domestic violence. “It is underrecognized and ignored,” a psychiatrist respondent argued. The problem is “rampant and unacceptable, pushed into a closet and normalized, with associated shame,” an emergency medicine doctor wrote.
Many respondents noted that physicians are under a mandate to report abuse of or a suspicious injury to a patient. Some shared anecdotes about how they reported action they had taken when they suspected it. “I’ve told patients who may be in dangerous situations that I’m a safe person and provide a safe space,” a radiologist added. An internist said, “I’ve recently started to ask about safety at home during triage on every patient.”
Other doctors bemoaned a lack of adequate education on detecting and managing domestic violence and abuse. “Domestic violence is often not recognized by health care providers,” a psychiatrist respondent observed.
Expanding legal immigration
In the Medscape report, 34% of physicians felt U.S. immigration/refugee policies need to be tougher, while 28% said they are too restrictive, and about a fifth saw them as appropriate.
“As an immigrant, I can tell you that the system is flawed and needs a complete overhaul, which will take a bipartisan effort,” an endocrinologist respondent wrote.
A number of respondents argued that it’s critical to simplify the process of obtaining U.S. citizenship so that fewer will feel forced to enter the country illegally. “For a country that relies very heavily on immigrants to sustain our health care system, we behave like idiots in denying safe harbor,” a nephrologist asserted.
A neurologist concurred. “Legal immigration needs to be encouraged. It should be easier to exchange visitor or student visa to immigrant visa in order to retain talent in the health care and technology fields, which would alleviate the shortage of workers in health care.”
Reproductive rights: No easy answers
Medscape’s survey was conducted before the U.S. Supreme Court in June reversed Roe v. Wade. In the report, 71% of physicians described themselves as very to somewhat concerned about women’s reproductive rights, but their viewpoints became nuanced after that. “There is a big disparity among physicians on this topic,” an oncologist respondent wrote.
At one end of the spectrum, 3% of doctors felt that abortions should never be permitted. “The human baby in the womb is an independent person with the right to life,” a pathologist said. At the other end, nearly one-fourth of physicians believed abortion should be accessible under all circumstances, regardless of trimester or reason. “I am just here to support the woman and make her decision a reality,” an internist said.
While saying an abortion should be granted after “fetal viability” only “in extenuating circumstances,” an ob.gyn. respondent said she is “extremely concerned” about attacks on abortion rights. “Some of us are old enough to remember women coming to the ER in extremis after illegal procedures, prior to Roe v. Wade.”
A version of this article first appeared on Medscape.com.
The danger when doctors don’t get mental health help
As medical professionals, you’re continually exposed to overwork, burnout, stressful situations, and challenging ethical decisions. Yet seeking help for mental health care may be last on your to-do list – or completely off your radar.
That’s sad and dangerous, since the American College of Emergency Physicians said 300-400 physicians die by suicide each year, and the stigma keeps 69% of female physicians from seeking mental health care, according to a prepandemic study.
In the 2022 Medscape Physician Suicide Report, 11% of female doctors and 9% of male doctors said they have had thoughts of suicide, and 64% experienced colloquial depression (feeling down, sad, or blue).
What’s more, physicians are typically seen as strong and capable and are often put on a pedestal by loved ones, patients, and the public and thought of as superhuman. No wonder it isn’t easy when you need to take time away to decompress and treat your mental well-being.
“There is a real fear for physicians when it comes to getting mental health care,” said Emil Tsai, MD, PhD, MAS, professor at the department of psychiatry and behavioral sciences at the University of California, Los Angeles, and an internationally reputed scientist in neurosciences and brain disorders.
The fear, said Dr. Tsai, comes from the stigma of mental health issues, potential repercussions to employment, and conceivable medical board suspension or revocation of your medical license.
Dr. Tsai said in an interview that to combat anxiety about “punishment” that many physicians fear when seeking care for their mental health, we must allow physicians to take time away from their day-to-day patient care for respite and treatment without reprisal.
Since the medical profession is high stress and has a high depression and suicide rate, finding solutions is imperative. And physicians must feel supported enough to seek treatment when needed. So how can we normalize seeking mental health care among physicians?
Get honest about stress and burnout
The only way to normalize any behavior is to be open and candid, Dr. Tsai said in an interview. The mental health conversation must occur across the board, not just within the medical profession.
“The greatest thing we can do to try and lift the burden that we place on physicians is to be willing to talk and be honest about the stress that physicians deal with and the importance of everyone feeling free to seek treatment and rest to strengthen their mental health,” said Dr. Tsai.
The more we talk about mental health and its treatment, the more we lessen the stigma, said Dr. Tsai. That could be more employer-employee check-ins, counseling as part of physician wellness, and programs structured so as not to construe a penal system.
“Mental health in the medical profession is a big issue and one that has to be met with the same compassion and care as it should be for any patient. We have annual physical checkups. Why don’t we offer annual mental health checkups for all, physicians included?” asked Dr. Tsai.
Evaluate the workload
Elizabeth Lombardo, PhD, psychologist, coach, and global keynote speaker, thinks that health care employers should reexamine their physicians’ workloads to see if they’re contributing to mental health issues.
The conversation on mental health in the workplace shouldn’t be about whether a certain person can handle stressors that are “normal” for health care settings. Instead, workplace managers in health care institutions should redefine workloads to ensure that physicians aren’t too heavily burdened with responsibilities that can cause overwork, burnout, and mental health problems,” she said.
Lessen the stigma
Even when physicians want to seek help for their psychological struggles, they may be weary of how their colleagues would react if they knew.
Raffaello Antonino, MD, clinical director at Therapy Central in London, said several underlying fears may exist at a physician’s core that prevent them from seeking care – being seen as weak, being judged as unfit to practice medicine, and the notion that “something is wrong with them.”
Dr. Antonino said we need to understand that physicians face challenges of bereavement and trauma derived from losing patients and the inability to save someone’s life. “These issues can easily develop into an accumulation of difficult, unprocessed emotions, later arising in symptoms and signs of PTSD, anxiety, and depression.”
Education is the best way to end this stigma, just like with any form of prejudice and stereotypes. For instance, we know that health care professionals are at risk of developing burnout. So, educating physicians on the symptoms and management of burnout and its consequences and prevention strategies is a must.
“Imagine what could happen if there were regular opportunities to work through the day’s events before signing out from a shift. The idea that individual weekly therapy is the only way to relieve mental distress is false,” said Lori McIsaac Bewsher, MSW, RSW, a trauma therapist and owner of a trauma-focused mental health clinic in New Brunswick.
“There are ways of integrating individual care into our doctor’s offices and hospitals that can be brief, effective, and confidential. The best way to introduce these interventions is early and collectively; no one is immune to the potential impact of exposure to trauma. The earlier these interventions can be accessed, the better the outcomes for everyone,” she said.
Dr. Antonino suggests, perhaps in the future, organizations can have “burnout checks” or mental health wellness checks for physicians akin to how we also have quick examinations for various physical ailments. What if physicians regularly answered a 10-question mental health survey as part of a burnout or trauma prevention strategy?
“Theirs is a profession and an identity which is often linked with a sense of strength, leadership and [benevolent] power: adjectives, which on the surface one might see as incompatible with what instead, unfortunately, and wrongly, may be associated with mental health issues,” said Dr. Antonino.
Keep it private
When it comes to removing the stigma from mental health care and treatment for physicians, privacy is top of mind. There needs to be some form of privacy protection for physicians who seek professional help for mental health reasons. Dr. Lombardo said physicians need to have the choice to keep their mental health journeys private. “Ideally, normalization should mean openly conversing about mental health, but for physicians, it can be a matter of life or death for their career, so the choice to remain private is something that should be afforded to them.”
Along those lines, the American Medical Association is pushing for system changes in legislative and regulatory arenas to support the mental health of practicing physicians, residents, and medical students. The organization is also urging health systems and state medical licensing bodies to remove questions on their applications that ask about prior treatment for mental health conditions.
Among many programs across the country, the Foundation of the Pennsylvania Medical Society has also created a Physicians’ Health Program, which provides confidential assessment, counseling, and referral services for physicians with mental health concerns.
“All of these initiatives are important in helping to destigmatize mental health issues among physicians,” said Harold Hong, MD, a board-certified psychiatrist in Raleigh, N.C.
Hail the benefits of treatment
Dr. Hong said to continue to destigmatize mental health among physicians and normalize its treatment, we not only have to emphasize how attending to mental health has individual benefits but also how it helps us help our patients.
“One key aspect that perhaps underpins this issue is the still present separation between mental and physical health, between mind and body, Dr. Hong said in an interview. “Feeling sad or angry or anxious should become a fact of life, a characteristic of being human, just like catching a cold or breaking a leg.”
It’s a normalization that, perhaps more than anything else, can lead the way for improving physicians’ mental health outcomes while also improving them for the rest of society. When society can finally see the health and well-being of someone in both their psychological and physical status, some of the stigmas may dissipate, and perhaps more physicians’ lives can be saved.
A version of this article first appeared on Medscape.com.
As medical professionals, you’re continually exposed to overwork, burnout, stressful situations, and challenging ethical decisions. Yet seeking help for mental health care may be last on your to-do list – or completely off your radar.
That’s sad and dangerous, since the American College of Emergency Physicians said 300-400 physicians die by suicide each year, and the stigma keeps 69% of female physicians from seeking mental health care, according to a prepandemic study.
In the 2022 Medscape Physician Suicide Report, 11% of female doctors and 9% of male doctors said they have had thoughts of suicide, and 64% experienced colloquial depression (feeling down, sad, or blue).
What’s more, physicians are typically seen as strong and capable and are often put on a pedestal by loved ones, patients, and the public and thought of as superhuman. No wonder it isn’t easy when you need to take time away to decompress and treat your mental well-being.
“There is a real fear for physicians when it comes to getting mental health care,” said Emil Tsai, MD, PhD, MAS, professor at the department of psychiatry and behavioral sciences at the University of California, Los Angeles, and an internationally reputed scientist in neurosciences and brain disorders.
The fear, said Dr. Tsai, comes from the stigma of mental health issues, potential repercussions to employment, and conceivable medical board suspension or revocation of your medical license.
Dr. Tsai said in an interview that to combat anxiety about “punishment” that many physicians fear when seeking care for their mental health, we must allow physicians to take time away from their day-to-day patient care for respite and treatment without reprisal.
Since the medical profession is high stress and has a high depression and suicide rate, finding solutions is imperative. And physicians must feel supported enough to seek treatment when needed. So how can we normalize seeking mental health care among physicians?
Get honest about stress and burnout
The only way to normalize any behavior is to be open and candid, Dr. Tsai said in an interview. The mental health conversation must occur across the board, not just within the medical profession.
“The greatest thing we can do to try and lift the burden that we place on physicians is to be willing to talk and be honest about the stress that physicians deal with and the importance of everyone feeling free to seek treatment and rest to strengthen their mental health,” said Dr. Tsai.
The more we talk about mental health and its treatment, the more we lessen the stigma, said Dr. Tsai. That could be more employer-employee check-ins, counseling as part of physician wellness, and programs structured so as not to construe a penal system.
“Mental health in the medical profession is a big issue and one that has to be met with the same compassion and care as it should be for any patient. We have annual physical checkups. Why don’t we offer annual mental health checkups for all, physicians included?” asked Dr. Tsai.
Evaluate the workload
Elizabeth Lombardo, PhD, psychologist, coach, and global keynote speaker, thinks that health care employers should reexamine their physicians’ workloads to see if they’re contributing to mental health issues.
The conversation on mental health in the workplace shouldn’t be about whether a certain person can handle stressors that are “normal” for health care settings. Instead, workplace managers in health care institutions should redefine workloads to ensure that physicians aren’t too heavily burdened with responsibilities that can cause overwork, burnout, and mental health problems,” she said.
Lessen the stigma
Even when physicians want to seek help for their psychological struggles, they may be weary of how their colleagues would react if they knew.
Raffaello Antonino, MD, clinical director at Therapy Central in London, said several underlying fears may exist at a physician’s core that prevent them from seeking care – being seen as weak, being judged as unfit to practice medicine, and the notion that “something is wrong with them.”
Dr. Antonino said we need to understand that physicians face challenges of bereavement and trauma derived from losing patients and the inability to save someone’s life. “These issues can easily develop into an accumulation of difficult, unprocessed emotions, later arising in symptoms and signs of PTSD, anxiety, and depression.”
Education is the best way to end this stigma, just like with any form of prejudice and stereotypes. For instance, we know that health care professionals are at risk of developing burnout. So, educating physicians on the symptoms and management of burnout and its consequences and prevention strategies is a must.
“Imagine what could happen if there were regular opportunities to work through the day’s events before signing out from a shift. The idea that individual weekly therapy is the only way to relieve mental distress is false,” said Lori McIsaac Bewsher, MSW, RSW, a trauma therapist and owner of a trauma-focused mental health clinic in New Brunswick.
“There are ways of integrating individual care into our doctor’s offices and hospitals that can be brief, effective, and confidential. The best way to introduce these interventions is early and collectively; no one is immune to the potential impact of exposure to trauma. The earlier these interventions can be accessed, the better the outcomes for everyone,” she said.
Dr. Antonino suggests, perhaps in the future, organizations can have “burnout checks” or mental health wellness checks for physicians akin to how we also have quick examinations for various physical ailments. What if physicians regularly answered a 10-question mental health survey as part of a burnout or trauma prevention strategy?
“Theirs is a profession and an identity which is often linked with a sense of strength, leadership and [benevolent] power: adjectives, which on the surface one might see as incompatible with what instead, unfortunately, and wrongly, may be associated with mental health issues,” said Dr. Antonino.
Keep it private
When it comes to removing the stigma from mental health care and treatment for physicians, privacy is top of mind. There needs to be some form of privacy protection for physicians who seek professional help for mental health reasons. Dr. Lombardo said physicians need to have the choice to keep their mental health journeys private. “Ideally, normalization should mean openly conversing about mental health, but for physicians, it can be a matter of life or death for their career, so the choice to remain private is something that should be afforded to them.”
Along those lines, the American Medical Association is pushing for system changes in legislative and regulatory arenas to support the mental health of practicing physicians, residents, and medical students. The organization is also urging health systems and state medical licensing bodies to remove questions on their applications that ask about prior treatment for mental health conditions.
Among many programs across the country, the Foundation of the Pennsylvania Medical Society has also created a Physicians’ Health Program, which provides confidential assessment, counseling, and referral services for physicians with mental health concerns.
“All of these initiatives are important in helping to destigmatize mental health issues among physicians,” said Harold Hong, MD, a board-certified psychiatrist in Raleigh, N.C.
Hail the benefits of treatment
Dr. Hong said to continue to destigmatize mental health among physicians and normalize its treatment, we not only have to emphasize how attending to mental health has individual benefits but also how it helps us help our patients.
“One key aspect that perhaps underpins this issue is the still present separation between mental and physical health, between mind and body, Dr. Hong said in an interview. “Feeling sad or angry or anxious should become a fact of life, a characteristic of being human, just like catching a cold or breaking a leg.”
It’s a normalization that, perhaps more than anything else, can lead the way for improving physicians’ mental health outcomes while also improving them for the rest of society. When society can finally see the health and well-being of someone in both their psychological and physical status, some of the stigmas may dissipate, and perhaps more physicians’ lives can be saved.
A version of this article first appeared on Medscape.com.
As medical professionals, you’re continually exposed to overwork, burnout, stressful situations, and challenging ethical decisions. Yet seeking help for mental health care may be last on your to-do list – or completely off your radar.
That’s sad and dangerous, since the American College of Emergency Physicians said 300-400 physicians die by suicide each year, and the stigma keeps 69% of female physicians from seeking mental health care, according to a prepandemic study.
In the 2022 Medscape Physician Suicide Report, 11% of female doctors and 9% of male doctors said they have had thoughts of suicide, and 64% experienced colloquial depression (feeling down, sad, or blue).
What’s more, physicians are typically seen as strong and capable and are often put on a pedestal by loved ones, patients, and the public and thought of as superhuman. No wonder it isn’t easy when you need to take time away to decompress and treat your mental well-being.
“There is a real fear for physicians when it comes to getting mental health care,” said Emil Tsai, MD, PhD, MAS, professor at the department of psychiatry and behavioral sciences at the University of California, Los Angeles, and an internationally reputed scientist in neurosciences and brain disorders.
The fear, said Dr. Tsai, comes from the stigma of mental health issues, potential repercussions to employment, and conceivable medical board suspension or revocation of your medical license.
Dr. Tsai said in an interview that to combat anxiety about “punishment” that many physicians fear when seeking care for their mental health, we must allow physicians to take time away from their day-to-day patient care for respite and treatment without reprisal.
Since the medical profession is high stress and has a high depression and suicide rate, finding solutions is imperative. And physicians must feel supported enough to seek treatment when needed. So how can we normalize seeking mental health care among physicians?
Get honest about stress and burnout
The only way to normalize any behavior is to be open and candid, Dr. Tsai said in an interview. The mental health conversation must occur across the board, not just within the medical profession.
“The greatest thing we can do to try and lift the burden that we place on physicians is to be willing to talk and be honest about the stress that physicians deal with and the importance of everyone feeling free to seek treatment and rest to strengthen their mental health,” said Dr. Tsai.
The more we talk about mental health and its treatment, the more we lessen the stigma, said Dr. Tsai. That could be more employer-employee check-ins, counseling as part of physician wellness, and programs structured so as not to construe a penal system.
“Mental health in the medical profession is a big issue and one that has to be met with the same compassion and care as it should be for any patient. We have annual physical checkups. Why don’t we offer annual mental health checkups for all, physicians included?” asked Dr. Tsai.
Evaluate the workload
Elizabeth Lombardo, PhD, psychologist, coach, and global keynote speaker, thinks that health care employers should reexamine their physicians’ workloads to see if they’re contributing to mental health issues.
The conversation on mental health in the workplace shouldn’t be about whether a certain person can handle stressors that are “normal” for health care settings. Instead, workplace managers in health care institutions should redefine workloads to ensure that physicians aren’t too heavily burdened with responsibilities that can cause overwork, burnout, and mental health problems,” she said.
Lessen the stigma
Even when physicians want to seek help for their psychological struggles, they may be weary of how their colleagues would react if they knew.
Raffaello Antonino, MD, clinical director at Therapy Central in London, said several underlying fears may exist at a physician’s core that prevent them from seeking care – being seen as weak, being judged as unfit to practice medicine, and the notion that “something is wrong with them.”
Dr. Antonino said we need to understand that physicians face challenges of bereavement and trauma derived from losing patients and the inability to save someone’s life. “These issues can easily develop into an accumulation of difficult, unprocessed emotions, later arising in symptoms and signs of PTSD, anxiety, and depression.”
Education is the best way to end this stigma, just like with any form of prejudice and stereotypes. For instance, we know that health care professionals are at risk of developing burnout. So, educating physicians on the symptoms and management of burnout and its consequences and prevention strategies is a must.
“Imagine what could happen if there were regular opportunities to work through the day’s events before signing out from a shift. The idea that individual weekly therapy is the only way to relieve mental distress is false,” said Lori McIsaac Bewsher, MSW, RSW, a trauma therapist and owner of a trauma-focused mental health clinic in New Brunswick.
“There are ways of integrating individual care into our doctor’s offices and hospitals that can be brief, effective, and confidential. The best way to introduce these interventions is early and collectively; no one is immune to the potential impact of exposure to trauma. The earlier these interventions can be accessed, the better the outcomes for everyone,” she said.
Dr. Antonino suggests, perhaps in the future, organizations can have “burnout checks” or mental health wellness checks for physicians akin to how we also have quick examinations for various physical ailments. What if physicians regularly answered a 10-question mental health survey as part of a burnout or trauma prevention strategy?
“Theirs is a profession and an identity which is often linked with a sense of strength, leadership and [benevolent] power: adjectives, which on the surface one might see as incompatible with what instead, unfortunately, and wrongly, may be associated with mental health issues,” said Dr. Antonino.
Keep it private
When it comes to removing the stigma from mental health care and treatment for physicians, privacy is top of mind. There needs to be some form of privacy protection for physicians who seek professional help for mental health reasons. Dr. Lombardo said physicians need to have the choice to keep their mental health journeys private. “Ideally, normalization should mean openly conversing about mental health, but for physicians, it can be a matter of life or death for their career, so the choice to remain private is something that should be afforded to them.”
Along those lines, the American Medical Association is pushing for system changes in legislative and regulatory arenas to support the mental health of practicing physicians, residents, and medical students. The organization is also urging health systems and state medical licensing bodies to remove questions on their applications that ask about prior treatment for mental health conditions.
Among many programs across the country, the Foundation of the Pennsylvania Medical Society has also created a Physicians’ Health Program, which provides confidential assessment, counseling, and referral services for physicians with mental health concerns.
“All of these initiatives are important in helping to destigmatize mental health issues among physicians,” said Harold Hong, MD, a board-certified psychiatrist in Raleigh, N.C.
Hail the benefits of treatment
Dr. Hong said to continue to destigmatize mental health among physicians and normalize its treatment, we not only have to emphasize how attending to mental health has individual benefits but also how it helps us help our patients.
“One key aspect that perhaps underpins this issue is the still present separation between mental and physical health, between mind and body, Dr. Hong said in an interview. “Feeling sad or angry or anxious should become a fact of life, a characteristic of being human, just like catching a cold or breaking a leg.”
It’s a normalization that, perhaps more than anything else, can lead the way for improving physicians’ mental health outcomes while also improving them for the rest of society. When society can finally see the health and well-being of someone in both their psychological and physical status, some of the stigmas may dissipate, and perhaps more physicians’ lives can be saved.
A version of this article first appeared on Medscape.com.
Med students dismayed that residency match process won’t change
– mostly medical students, residents, and fellows – who supported the change.
The program’s decision comes after nearly 3 months of feedback from the public, medical students, and education community. Although about 60% of public respondents believed the change could reduce stress and allow students more time for momentous career decisions, the program’s board of directors decided the disadvantages were “of greater consequence,” according to a Oct. 28 statement.
Those disadvantages included introducing application or interview behaviors that could increase students’ stress; potentially identifying partially matched or unmatched applicants, which could lead to bias; and extending the match process time for those applicants.
In addition, members of 12 medical education and student organizations raised other concerns, such as the proposed change not addressing high application numbers, according to the statement. NRMP has reported record numbers of applicants over the past few years, typically with more applicants than available program slots.
“While the testimony gave nod to the positive aspects of the proposal ... there was substantially more concern voiced about the potential negative consequences identified in the public comments,” NRMP President and CEO Donna L. Lamb, DHSc, MBA, BSN, told this news organization. Some of those issues could not be addressed without further study, so the board decided not to proceed with the proposal, she explained.
The proposal would have separated the Main Residency Match into two phases and replaced the Supplemental Offer and Acceptance Program (SOAP), in which unmatched or partially matched applicants apply for unfilled residency positions. Under the proposed change, each phase would have operated the same way, from rank order lists and using a matching algorithm to a pair of Match Days instead of a single day.
The two-phase process would have given students who didn’t match more time to carefully weigh residency programs – they can apply to up to 45 placements as part of SOAP – that will guide their career path for the next few years, PGY-1 intern Asim A., who asked not to be identified further, told this news organization. The alternative is a hasty decision once students learn which residency spots are available, he added. “Applicants would have breathing room to make a more informed decision.”
Asim, who is Canadian, said he is participating in a transitional year in internal medicine in the hopes of being matched into internal medicine or psychiatry. He said Canada’s two-phase match is a “lot less stressful” than the U.S. system.
Meanwhile, students on Reddit’s medical school community also questioned NRMP’s decision.
“A significant majority of those surveyed thought it would be beneficial. But NRMP decides to not go through with it,” one Reddit user wrote. Another posted, “The one thing that could have improved the match and they chose not to do it.”
Others supported the decision to retain a 1-day match.
“I think this was the right call,” Bryan Carmody, MD, an outspoken medical education blogger, tweeted after learning of NRMP’s decision. Dr. Carmody, a pediatric nephrologist, previously expressed to this news organization misgivings about whether the two-phase match would make it difficult for programs to thoroughly review candidates and vice versa. He was concerned that it would compress the interview season and pressure programs to rapidly review applicants and conduct interviews.
More than 8,000 people responded to the public survey that began in August and ran for a month. Nearly two-thirds of the respondents (60%) were students, residents, or fellows. About 25% included faculty, program directors, and staff. Among the survey findings, respondents were equally divided between whether the two-phase match would be modestly advantageous (30%) or significantly advantageous (30%) compared to 20% who viewed it as modestly or significantly disadvantageous.
The NRMP said it would continue engaging with the community through focus groups and other means to improve the match experience and transition to residency.
“It is important to remember that a proposal is just that,” Dr. Lamb told this news orgnization, “an opportunity to discuss the pros and cons of an idea or framework ... and to mitigate unwanted consequences determined to be detrimental to learners and programs.”
The NRMP will involve the community in future discussions “to continue to give learners a voice,” she said.
A version of this article first appeared on Medscape.com.
– mostly medical students, residents, and fellows – who supported the change.
The program’s decision comes after nearly 3 months of feedback from the public, medical students, and education community. Although about 60% of public respondents believed the change could reduce stress and allow students more time for momentous career decisions, the program’s board of directors decided the disadvantages were “of greater consequence,” according to a Oct. 28 statement.
Those disadvantages included introducing application or interview behaviors that could increase students’ stress; potentially identifying partially matched or unmatched applicants, which could lead to bias; and extending the match process time for those applicants.
In addition, members of 12 medical education and student organizations raised other concerns, such as the proposed change not addressing high application numbers, according to the statement. NRMP has reported record numbers of applicants over the past few years, typically with more applicants than available program slots.
“While the testimony gave nod to the positive aspects of the proposal ... there was substantially more concern voiced about the potential negative consequences identified in the public comments,” NRMP President and CEO Donna L. Lamb, DHSc, MBA, BSN, told this news organization. Some of those issues could not be addressed without further study, so the board decided not to proceed with the proposal, she explained.
The proposal would have separated the Main Residency Match into two phases and replaced the Supplemental Offer and Acceptance Program (SOAP), in which unmatched or partially matched applicants apply for unfilled residency positions. Under the proposed change, each phase would have operated the same way, from rank order lists and using a matching algorithm to a pair of Match Days instead of a single day.
The two-phase process would have given students who didn’t match more time to carefully weigh residency programs – they can apply to up to 45 placements as part of SOAP – that will guide their career path for the next few years, PGY-1 intern Asim A., who asked not to be identified further, told this news organization. The alternative is a hasty decision once students learn which residency spots are available, he added. “Applicants would have breathing room to make a more informed decision.”
Asim, who is Canadian, said he is participating in a transitional year in internal medicine in the hopes of being matched into internal medicine or psychiatry. He said Canada’s two-phase match is a “lot less stressful” than the U.S. system.
Meanwhile, students on Reddit’s medical school community also questioned NRMP’s decision.
“A significant majority of those surveyed thought it would be beneficial. But NRMP decides to not go through with it,” one Reddit user wrote. Another posted, “The one thing that could have improved the match and they chose not to do it.”
Others supported the decision to retain a 1-day match.
“I think this was the right call,” Bryan Carmody, MD, an outspoken medical education blogger, tweeted after learning of NRMP’s decision. Dr. Carmody, a pediatric nephrologist, previously expressed to this news organization misgivings about whether the two-phase match would make it difficult for programs to thoroughly review candidates and vice versa. He was concerned that it would compress the interview season and pressure programs to rapidly review applicants and conduct interviews.
More than 8,000 people responded to the public survey that began in August and ran for a month. Nearly two-thirds of the respondents (60%) were students, residents, or fellows. About 25% included faculty, program directors, and staff. Among the survey findings, respondents were equally divided between whether the two-phase match would be modestly advantageous (30%) or significantly advantageous (30%) compared to 20% who viewed it as modestly or significantly disadvantageous.
The NRMP said it would continue engaging with the community through focus groups and other means to improve the match experience and transition to residency.
“It is important to remember that a proposal is just that,” Dr. Lamb told this news orgnization, “an opportunity to discuss the pros and cons of an idea or framework ... and to mitigate unwanted consequences determined to be detrimental to learners and programs.”
The NRMP will involve the community in future discussions “to continue to give learners a voice,” she said.
A version of this article first appeared on Medscape.com.
– mostly medical students, residents, and fellows – who supported the change.
The program’s decision comes after nearly 3 months of feedback from the public, medical students, and education community. Although about 60% of public respondents believed the change could reduce stress and allow students more time for momentous career decisions, the program’s board of directors decided the disadvantages were “of greater consequence,” according to a Oct. 28 statement.
Those disadvantages included introducing application or interview behaviors that could increase students’ stress; potentially identifying partially matched or unmatched applicants, which could lead to bias; and extending the match process time for those applicants.
In addition, members of 12 medical education and student organizations raised other concerns, such as the proposed change not addressing high application numbers, according to the statement. NRMP has reported record numbers of applicants over the past few years, typically with more applicants than available program slots.
“While the testimony gave nod to the positive aspects of the proposal ... there was substantially more concern voiced about the potential negative consequences identified in the public comments,” NRMP President and CEO Donna L. Lamb, DHSc, MBA, BSN, told this news organization. Some of those issues could not be addressed without further study, so the board decided not to proceed with the proposal, she explained.
The proposal would have separated the Main Residency Match into two phases and replaced the Supplemental Offer and Acceptance Program (SOAP), in which unmatched or partially matched applicants apply for unfilled residency positions. Under the proposed change, each phase would have operated the same way, from rank order lists and using a matching algorithm to a pair of Match Days instead of a single day.
The two-phase process would have given students who didn’t match more time to carefully weigh residency programs – they can apply to up to 45 placements as part of SOAP – that will guide their career path for the next few years, PGY-1 intern Asim A., who asked not to be identified further, told this news organization. The alternative is a hasty decision once students learn which residency spots are available, he added. “Applicants would have breathing room to make a more informed decision.”
Asim, who is Canadian, said he is participating in a transitional year in internal medicine in the hopes of being matched into internal medicine or psychiatry. He said Canada’s two-phase match is a “lot less stressful” than the U.S. system.
Meanwhile, students on Reddit’s medical school community also questioned NRMP’s decision.
“A significant majority of those surveyed thought it would be beneficial. But NRMP decides to not go through with it,” one Reddit user wrote. Another posted, “The one thing that could have improved the match and they chose not to do it.”
Others supported the decision to retain a 1-day match.
“I think this was the right call,” Bryan Carmody, MD, an outspoken medical education blogger, tweeted after learning of NRMP’s decision. Dr. Carmody, a pediatric nephrologist, previously expressed to this news organization misgivings about whether the two-phase match would make it difficult for programs to thoroughly review candidates and vice versa. He was concerned that it would compress the interview season and pressure programs to rapidly review applicants and conduct interviews.
More than 8,000 people responded to the public survey that began in August and ran for a month. Nearly two-thirds of the respondents (60%) were students, residents, or fellows. About 25% included faculty, program directors, and staff. Among the survey findings, respondents were equally divided between whether the two-phase match would be modestly advantageous (30%) or significantly advantageous (30%) compared to 20% who viewed it as modestly or significantly disadvantageous.
The NRMP said it would continue engaging with the community through focus groups and other means to improve the match experience and transition to residency.
“It is important to remember that a proposal is just that,” Dr. Lamb told this news orgnization, “an opportunity to discuss the pros and cons of an idea or framework ... and to mitigate unwanted consequences determined to be detrimental to learners and programs.”
The NRMP will involve the community in future discussions “to continue to give learners a voice,” she said.
A version of this article first appeared on Medscape.com.
Lego introduces first character with vitiligo
The
The character appears with the customizable array of players to assemble for a table football team.
It’s the latest representation of the disease as toymakers diversify their lines.
In May 2022, Mattel released a Ken doll with vitiligo after a Barbie with vitiligo was released in 2020. Rainbow High and other toy makers also have character versions.
The Lego addition follows a big summer medically for vitiligo as the first treatment was approved for repigmentation. In July, a cream formulation of ruxolitinib (Opzelura), a Janus kinase inhibitor, became the first repigmentation treatment approved by the Food and Drug Administration for nonsegmental vitiligo, the most common form of the disease.
Vitiligo is estimated to affect 1.9 million–2.8 million adults in the United States and more than 100 million people worldwide. It cuts across races and genders and can be psychologically painful for many who live with it.
John E. Harris, MD, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester, wrote about the Lego character in his blog “Speaking of Vitiligo ...” saying: “I could not be more excited. This new minifigure also serves as a way to educate both children and adults who are not familiar with vitiligo about the disease.”
He noted that until recently vitiligo representation in kids’ toys has been limited. “By adding diversity such as representations of vitiligo in toys, it can help remove stigmas associated with vitiligo and give children more options that they can relate to.”
Erika Page of Richmond, Va., who founded and edits the vitiligo blog “Living Dappled,” told this news organization she was thrilled to see the new Lego character.
“Growing up I didn’t know anyone who looked like me, let alone a toy or a character,” she said. The message the representations send is important not just for the kids but for the parents of kids with vitiligo who want to help their kids in any way they can.
Ms. Page was diagnosed with vitiligo at age 7 and struggled emotionally in her high school and college years when she often looked in the mirror, saw “giraffe-like” spots, and cried. Over time she lost 100% of her pigment to the condition and today at age 33, lives with universal vitiligo or overall very pale skin.
She founded the Living Dappled blog 6 years ago to help people with the disease feel less alone. The Lego character will also help with that, she said.
“Growing up with vitiligo was so isolating and you felt so different,” Ms. Page said. “Today we see billboards and models and dolls and now Legos that look like us. I hope this is a first of many to come for Lego.”
Dr. Harris and Ms. Page declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The
The character appears with the customizable array of players to assemble for a table football team.
It’s the latest representation of the disease as toymakers diversify their lines.
In May 2022, Mattel released a Ken doll with vitiligo after a Barbie with vitiligo was released in 2020. Rainbow High and other toy makers also have character versions.
The Lego addition follows a big summer medically for vitiligo as the first treatment was approved for repigmentation. In July, a cream formulation of ruxolitinib (Opzelura), a Janus kinase inhibitor, became the first repigmentation treatment approved by the Food and Drug Administration for nonsegmental vitiligo, the most common form of the disease.
Vitiligo is estimated to affect 1.9 million–2.8 million adults in the United States and more than 100 million people worldwide. It cuts across races and genders and can be psychologically painful for many who live with it.
John E. Harris, MD, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester, wrote about the Lego character in his blog “Speaking of Vitiligo ...” saying: “I could not be more excited. This new minifigure also serves as a way to educate both children and adults who are not familiar with vitiligo about the disease.”
He noted that until recently vitiligo representation in kids’ toys has been limited. “By adding diversity such as representations of vitiligo in toys, it can help remove stigmas associated with vitiligo and give children more options that they can relate to.”
Erika Page of Richmond, Va., who founded and edits the vitiligo blog “Living Dappled,” told this news organization she was thrilled to see the new Lego character.
“Growing up I didn’t know anyone who looked like me, let alone a toy or a character,” she said. The message the representations send is important not just for the kids but for the parents of kids with vitiligo who want to help their kids in any way they can.
Ms. Page was diagnosed with vitiligo at age 7 and struggled emotionally in her high school and college years when she often looked in the mirror, saw “giraffe-like” spots, and cried. Over time she lost 100% of her pigment to the condition and today at age 33, lives with universal vitiligo or overall very pale skin.
She founded the Living Dappled blog 6 years ago to help people with the disease feel less alone. The Lego character will also help with that, she said.
“Growing up with vitiligo was so isolating and you felt so different,” Ms. Page said. “Today we see billboards and models and dolls and now Legos that look like us. I hope this is a first of many to come for Lego.”
Dr. Harris and Ms. Page declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The
The character appears with the customizable array of players to assemble for a table football team.
It’s the latest representation of the disease as toymakers diversify their lines.
In May 2022, Mattel released a Ken doll with vitiligo after a Barbie with vitiligo was released in 2020. Rainbow High and other toy makers also have character versions.
The Lego addition follows a big summer medically for vitiligo as the first treatment was approved for repigmentation. In July, a cream formulation of ruxolitinib (Opzelura), a Janus kinase inhibitor, became the first repigmentation treatment approved by the Food and Drug Administration for nonsegmental vitiligo, the most common form of the disease.
Vitiligo is estimated to affect 1.9 million–2.8 million adults in the United States and more than 100 million people worldwide. It cuts across races and genders and can be psychologically painful for many who live with it.
John E. Harris, MD, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester, wrote about the Lego character in his blog “Speaking of Vitiligo ...” saying: “I could not be more excited. This new minifigure also serves as a way to educate both children and adults who are not familiar with vitiligo about the disease.”
He noted that until recently vitiligo representation in kids’ toys has been limited. “By adding diversity such as representations of vitiligo in toys, it can help remove stigmas associated with vitiligo and give children more options that they can relate to.”
Erika Page of Richmond, Va., who founded and edits the vitiligo blog “Living Dappled,” told this news organization she was thrilled to see the new Lego character.
“Growing up I didn’t know anyone who looked like me, let alone a toy or a character,” she said. The message the representations send is important not just for the kids but for the parents of kids with vitiligo who want to help their kids in any way they can.
Ms. Page was diagnosed with vitiligo at age 7 and struggled emotionally in her high school and college years when she often looked in the mirror, saw “giraffe-like” spots, and cried. Over time she lost 100% of her pigment to the condition and today at age 33, lives with universal vitiligo or overall very pale skin.
She founded the Living Dappled blog 6 years ago to help people with the disease feel less alone. The Lego character will also help with that, she said.
“Growing up with vitiligo was so isolating and you felt so different,” Ms. Page said. “Today we see billboards and models and dolls and now Legos that look like us. I hope this is a first of many to come for Lego.”
Dr. Harris and Ms. Page declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practical pearls guide treatment of psoriasis in tricky areas
LAS VEGAS – With the right regimen, a majority of patients with psoriasis can achieve at least a Psoriasis Area and Severity Index (PASI) 75 score, Jennifer Soung, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
The array of treatment options includes mainstay topicals, new nonsteroidals, traditional oral systemics, new oral systemics, biologics, and light therapy, said Dr. Soung, director of clinical research at Harbor-UCLA Medical Center, Santa Ana, Calif. However, she said.
For these areas, make sure the diagnosis of psoriasis is correct, to avoid wasting time on the wrong course of treatment, Dr. Soung emphasized.
Scalp strategies
The scalp is often the first area of the body affected by psoriasis, and patients with severe scalp psoriasis may have minimal plaques on the body, Dr. Soung said. However, a differential diagnosis should include seborrheic dermatitis, she noted.
For most cases of scalp psoriasis, “start with localized topical treatment,” such as vitamin D and corticosteroid combination therapy, or excimer laser, Dr. Soung advised.
Systemic treatments with demonstrated effectiveness on scalp psoriasis in post hoc analyses of patients with moderate to severe plaque psoriasis include adalimumab, etanercept, ixekizumab, and secukinumab. Studies specifically focused on treatment of scalp psoriasis have shown success with secukinumab and apremilast, she noted.
Roflumilast foam, 0.3%, is in development and is an emerging option for scalp psoriasis. (A cream formulation of roflumilast, a topical phosphodiesterase 4 inhibitor, was approved for treatment of plaque psoriasis in July 2022.) A phase 2b study of roflumilast foam showed that approximately one-third of patients with scalp psoriasis achieved a status of clear based on scalp-investigator global assessment, compared with approximately 3% of those on vehicle, and similar results were seen in a recently completed phase 3 trial for scalp and body psoriasis, she added.
Facial psoriasis
Patients with facial psoriasis tend to be younger, and they may have more severe disease overall, Dr. Soung said. Given the sensitivity of facial skin, “it is nice to have a nonsteroidal option,” she noted. Current novel nonsteroidal therapies include a cream formulation of tapinarof, an aryl hydrocarbon receptor agonist, which was approved earlier this year for plaque psoriasis in adults, and roflumilast cream. Vitamin D and topical calcineurin inhibitors are options as well, she said.
Intertriginous tricks
Intertriginous (inverse) psoriasis is distinct from other areas in that the plaques are usually smooth and well-demarcated, with little or no scaling, Dr. Soung said. Erosions or signs of maceration may be present. The prevalence of inverse psoriasis is approximately 30%, but the prevalence on external genitalia is 80%, she noted. For inverse psoriasis, topical corticosteroids can result in adverse events such as poor wound healing and skin fragility, and some patients resist the idea of a steroid and don’t adhere to the medication, she added. Dr. Soung recommended topical corticosteroids for the short term, and topical calcineurin inhibitors or calcipotriol for the long term.
New topical options for inverse psoriasis include tapinarof and roflumilast, Dr. Soung said. For tapinarof, the phase 3 PSOARING program included assessment of tolerability in sensitive skin areas and found little to no irritation. Similarly, treatment with roflumilast cream was effective and well tolerated by patients with intertriginous plaque psoriasis in the DERMIS-1 and DERMIS-2 studies, she said.
Genital psoriasis
Ask patients with psoriasis about genital psoriasis, because they often are too embarrassed to provide that information on their own, said Dr. Soung. In fact, 63% of patients with psoriasis report ever experiencing genital psoriasis, but it often goes undiagnosed and undertreated, which has a significant impact on patient quality of life and sexual health.
A differential diagnosis of genital psoriasis should include dermatitis, tinea or candidiasis, and even squamous cell carcinoma, she noted. Other considerations include fixed drug eruption, lichen nitidus, lichen sclerosus, and scabies.
Dr. Soung’s first line of treatment for genital psoriasis is low-potency topical corticosteroids for 2-4 weeks. If long-term topical therapy is needed, alternatives include calcineurin inhibitors and vitamin D analogs, she said. The new topicals roflumilast and tapinarof are options as well, she said.
For those patients with severe and extensive genital psoriasis, consider systemic therapy, possibly with ixekizumab or secukinumab, she added. Patients with moderate to severe genital psoriasis treated with apremilast have shown improvement at week 16, in an ongoing clinical trial, she noted.
Palmoplantar involvement
For patients with palmar plantar psoriasis, “don’t underestimate the impact on quality of life,” said Dr. Soung. Approximately 12%-16% of patients with psoriasis report palmoplantar involvement, she noted.
Palmoplantar psoriasis can be stubborn, and many patients will need combination therapy with topicals and systemics, she said. “I am very curious about how well our new topical nonsteroidals will work in these areas,” she added.
Dr. Soung starts patients with palmoplantar psoriasis with a “potent to super-potent” twice daily topical corticosteroid, with or without occlusion. Her first-line systemic therapy is acitretin, 10-50 mg daily. However, keep in mind that acitretin is contraindicated in pregnancy, and also may cause side effects including cheilitis, alopecia, and peeling skin, she cautioned.
During the question and answer session, Dr. Soung was asked whether she routinely biopsies patients with palmoplantar psoriasis. “Not always,” was her answer. Instead, she looks for clues elsewhere on the body to confirm the diagnosis.
Nail know-how
Approximately 23%-27% of patients with psoriasis experience nail involvement, said Dr. Soung. Nail psoriasis can appear on the nail plate as pitting, onycholysis, or subungual hyperkeratosis, or in the nail bed as splinter hemorrhages or oil spots, she said.
For patients with psoriasis of the nails only, Dr. Soung described the use of high-potency topical corticosteroids, with or without calcipotriol. In her experience, she said that intralesional steroids for nail psoriasis are torturous to patients. For patients who have failed topical therapy or have psoriasis in other areas, with or without psoriatic arthritis, she advised the use of either IL-17 antagonists (secukinumab, ixekizumab, brodalumab) or IL-23 antagonists (risankizumab, guselkumab).
Dr. Soung disclosed serving as a consultant or advisor for Arcutis, Bristol Myers Squibb Company, Dermavant, and Novartis. She also disclosed serving as a speaker or member of the speakers’ bureau for AbbVie, Amgen, Arcutis, Bristol Myers Squibb Company, Celgene, Leo Pharma, Eli Lilly, Novartis, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi, as well as research funding from AbbVie, Amgen, Arcutis, Castle Biosciences, Dermavant, KoBio, Kyowa Kirin, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – With the right regimen, a majority of patients with psoriasis can achieve at least a Psoriasis Area and Severity Index (PASI) 75 score, Jennifer Soung, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
The array of treatment options includes mainstay topicals, new nonsteroidals, traditional oral systemics, new oral systemics, biologics, and light therapy, said Dr. Soung, director of clinical research at Harbor-UCLA Medical Center, Santa Ana, Calif. However, she said.
For these areas, make sure the diagnosis of psoriasis is correct, to avoid wasting time on the wrong course of treatment, Dr. Soung emphasized.
Scalp strategies
The scalp is often the first area of the body affected by psoriasis, and patients with severe scalp psoriasis may have minimal plaques on the body, Dr. Soung said. However, a differential diagnosis should include seborrheic dermatitis, she noted.
For most cases of scalp psoriasis, “start with localized topical treatment,” such as vitamin D and corticosteroid combination therapy, or excimer laser, Dr. Soung advised.
Systemic treatments with demonstrated effectiveness on scalp psoriasis in post hoc analyses of patients with moderate to severe plaque psoriasis include adalimumab, etanercept, ixekizumab, and secukinumab. Studies specifically focused on treatment of scalp psoriasis have shown success with secukinumab and apremilast, she noted.
Roflumilast foam, 0.3%, is in development and is an emerging option for scalp psoriasis. (A cream formulation of roflumilast, a topical phosphodiesterase 4 inhibitor, was approved for treatment of plaque psoriasis in July 2022.) A phase 2b study of roflumilast foam showed that approximately one-third of patients with scalp psoriasis achieved a status of clear based on scalp-investigator global assessment, compared with approximately 3% of those on vehicle, and similar results were seen in a recently completed phase 3 trial for scalp and body psoriasis, she added.
Facial psoriasis
Patients with facial psoriasis tend to be younger, and they may have more severe disease overall, Dr. Soung said. Given the sensitivity of facial skin, “it is nice to have a nonsteroidal option,” she noted. Current novel nonsteroidal therapies include a cream formulation of tapinarof, an aryl hydrocarbon receptor agonist, which was approved earlier this year for plaque psoriasis in adults, and roflumilast cream. Vitamin D and topical calcineurin inhibitors are options as well, she said.
Intertriginous tricks
Intertriginous (inverse) psoriasis is distinct from other areas in that the plaques are usually smooth and well-demarcated, with little or no scaling, Dr. Soung said. Erosions or signs of maceration may be present. The prevalence of inverse psoriasis is approximately 30%, but the prevalence on external genitalia is 80%, she noted. For inverse psoriasis, topical corticosteroids can result in adverse events such as poor wound healing and skin fragility, and some patients resist the idea of a steroid and don’t adhere to the medication, she added. Dr. Soung recommended topical corticosteroids for the short term, and topical calcineurin inhibitors or calcipotriol for the long term.
New topical options for inverse psoriasis include tapinarof and roflumilast, Dr. Soung said. For tapinarof, the phase 3 PSOARING program included assessment of tolerability in sensitive skin areas and found little to no irritation. Similarly, treatment with roflumilast cream was effective and well tolerated by patients with intertriginous plaque psoriasis in the DERMIS-1 and DERMIS-2 studies, she said.
Genital psoriasis
Ask patients with psoriasis about genital psoriasis, because they often are too embarrassed to provide that information on their own, said Dr. Soung. In fact, 63% of patients with psoriasis report ever experiencing genital psoriasis, but it often goes undiagnosed and undertreated, which has a significant impact on patient quality of life and sexual health.
A differential diagnosis of genital psoriasis should include dermatitis, tinea or candidiasis, and even squamous cell carcinoma, she noted. Other considerations include fixed drug eruption, lichen nitidus, lichen sclerosus, and scabies.
Dr. Soung’s first line of treatment for genital psoriasis is low-potency topical corticosteroids for 2-4 weeks. If long-term topical therapy is needed, alternatives include calcineurin inhibitors and vitamin D analogs, she said. The new topicals roflumilast and tapinarof are options as well, she said.
For those patients with severe and extensive genital psoriasis, consider systemic therapy, possibly with ixekizumab or secukinumab, she added. Patients with moderate to severe genital psoriasis treated with apremilast have shown improvement at week 16, in an ongoing clinical trial, she noted.
Palmoplantar involvement
For patients with palmar plantar psoriasis, “don’t underestimate the impact on quality of life,” said Dr. Soung. Approximately 12%-16% of patients with psoriasis report palmoplantar involvement, she noted.
Palmoplantar psoriasis can be stubborn, and many patients will need combination therapy with topicals and systemics, she said. “I am very curious about how well our new topical nonsteroidals will work in these areas,” she added.
Dr. Soung starts patients with palmoplantar psoriasis with a “potent to super-potent” twice daily topical corticosteroid, with or without occlusion. Her first-line systemic therapy is acitretin, 10-50 mg daily. However, keep in mind that acitretin is contraindicated in pregnancy, and also may cause side effects including cheilitis, alopecia, and peeling skin, she cautioned.
During the question and answer session, Dr. Soung was asked whether she routinely biopsies patients with palmoplantar psoriasis. “Not always,” was her answer. Instead, she looks for clues elsewhere on the body to confirm the diagnosis.
Nail know-how
Approximately 23%-27% of patients with psoriasis experience nail involvement, said Dr. Soung. Nail psoriasis can appear on the nail plate as pitting, onycholysis, or subungual hyperkeratosis, or in the nail bed as splinter hemorrhages or oil spots, she said.
For patients with psoriasis of the nails only, Dr. Soung described the use of high-potency topical corticosteroids, with or without calcipotriol. In her experience, she said that intralesional steroids for nail psoriasis are torturous to patients. For patients who have failed topical therapy or have psoriasis in other areas, with or without psoriatic arthritis, she advised the use of either IL-17 antagonists (secukinumab, ixekizumab, brodalumab) or IL-23 antagonists (risankizumab, guselkumab).
Dr. Soung disclosed serving as a consultant or advisor for Arcutis, Bristol Myers Squibb Company, Dermavant, and Novartis. She also disclosed serving as a speaker or member of the speakers’ bureau for AbbVie, Amgen, Arcutis, Bristol Myers Squibb Company, Celgene, Leo Pharma, Eli Lilly, Novartis, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi, as well as research funding from AbbVie, Amgen, Arcutis, Castle Biosciences, Dermavant, KoBio, Kyowa Kirin, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – With the right regimen, a majority of patients with psoriasis can achieve at least a Psoriasis Area and Severity Index (PASI) 75 score, Jennifer Soung, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
The array of treatment options includes mainstay topicals, new nonsteroidals, traditional oral systemics, new oral systemics, biologics, and light therapy, said Dr. Soung, director of clinical research at Harbor-UCLA Medical Center, Santa Ana, Calif. However, she said.
For these areas, make sure the diagnosis of psoriasis is correct, to avoid wasting time on the wrong course of treatment, Dr. Soung emphasized.
Scalp strategies
The scalp is often the first area of the body affected by psoriasis, and patients with severe scalp psoriasis may have minimal plaques on the body, Dr. Soung said. However, a differential diagnosis should include seborrheic dermatitis, she noted.
For most cases of scalp psoriasis, “start with localized topical treatment,” such as vitamin D and corticosteroid combination therapy, or excimer laser, Dr. Soung advised.
Systemic treatments with demonstrated effectiveness on scalp psoriasis in post hoc analyses of patients with moderate to severe plaque psoriasis include adalimumab, etanercept, ixekizumab, and secukinumab. Studies specifically focused on treatment of scalp psoriasis have shown success with secukinumab and apremilast, she noted.
Roflumilast foam, 0.3%, is in development and is an emerging option for scalp psoriasis. (A cream formulation of roflumilast, a topical phosphodiesterase 4 inhibitor, was approved for treatment of plaque psoriasis in July 2022.) A phase 2b study of roflumilast foam showed that approximately one-third of patients with scalp psoriasis achieved a status of clear based on scalp-investigator global assessment, compared with approximately 3% of those on vehicle, and similar results were seen in a recently completed phase 3 trial for scalp and body psoriasis, she added.
Facial psoriasis
Patients with facial psoriasis tend to be younger, and they may have more severe disease overall, Dr. Soung said. Given the sensitivity of facial skin, “it is nice to have a nonsteroidal option,” she noted. Current novel nonsteroidal therapies include a cream formulation of tapinarof, an aryl hydrocarbon receptor agonist, which was approved earlier this year for plaque psoriasis in adults, and roflumilast cream. Vitamin D and topical calcineurin inhibitors are options as well, she said.
Intertriginous tricks
Intertriginous (inverse) psoriasis is distinct from other areas in that the plaques are usually smooth and well-demarcated, with little or no scaling, Dr. Soung said. Erosions or signs of maceration may be present. The prevalence of inverse psoriasis is approximately 30%, but the prevalence on external genitalia is 80%, she noted. For inverse psoriasis, topical corticosteroids can result in adverse events such as poor wound healing and skin fragility, and some patients resist the idea of a steroid and don’t adhere to the medication, she added. Dr. Soung recommended topical corticosteroids for the short term, and topical calcineurin inhibitors or calcipotriol for the long term.
New topical options for inverse psoriasis include tapinarof and roflumilast, Dr. Soung said. For tapinarof, the phase 3 PSOARING program included assessment of tolerability in sensitive skin areas and found little to no irritation. Similarly, treatment with roflumilast cream was effective and well tolerated by patients with intertriginous plaque psoriasis in the DERMIS-1 and DERMIS-2 studies, she said.
Genital psoriasis
Ask patients with psoriasis about genital psoriasis, because they often are too embarrassed to provide that information on their own, said Dr. Soung. In fact, 63% of patients with psoriasis report ever experiencing genital psoriasis, but it often goes undiagnosed and undertreated, which has a significant impact on patient quality of life and sexual health.
A differential diagnosis of genital psoriasis should include dermatitis, tinea or candidiasis, and even squamous cell carcinoma, she noted. Other considerations include fixed drug eruption, lichen nitidus, lichen sclerosus, and scabies.
Dr. Soung’s first line of treatment for genital psoriasis is low-potency topical corticosteroids for 2-4 weeks. If long-term topical therapy is needed, alternatives include calcineurin inhibitors and vitamin D analogs, she said. The new topicals roflumilast and tapinarof are options as well, she said.
For those patients with severe and extensive genital psoriasis, consider systemic therapy, possibly with ixekizumab or secukinumab, she added. Patients with moderate to severe genital psoriasis treated with apremilast have shown improvement at week 16, in an ongoing clinical trial, she noted.
Palmoplantar involvement
For patients with palmar plantar psoriasis, “don’t underestimate the impact on quality of life,” said Dr. Soung. Approximately 12%-16% of patients with psoriasis report palmoplantar involvement, she noted.
Palmoplantar psoriasis can be stubborn, and many patients will need combination therapy with topicals and systemics, she said. “I am very curious about how well our new topical nonsteroidals will work in these areas,” she added.
Dr. Soung starts patients with palmoplantar psoriasis with a “potent to super-potent” twice daily topical corticosteroid, with or without occlusion. Her first-line systemic therapy is acitretin, 10-50 mg daily. However, keep in mind that acitretin is contraindicated in pregnancy, and also may cause side effects including cheilitis, alopecia, and peeling skin, she cautioned.
During the question and answer session, Dr. Soung was asked whether she routinely biopsies patients with palmoplantar psoriasis. “Not always,” was her answer. Instead, she looks for clues elsewhere on the body to confirm the diagnosis.
Nail know-how
Approximately 23%-27% of patients with psoriasis experience nail involvement, said Dr. Soung. Nail psoriasis can appear on the nail plate as pitting, onycholysis, or subungual hyperkeratosis, or in the nail bed as splinter hemorrhages or oil spots, she said.
For patients with psoriasis of the nails only, Dr. Soung described the use of high-potency topical corticosteroids, with or without calcipotriol. In her experience, she said that intralesional steroids for nail psoriasis are torturous to patients. For patients who have failed topical therapy or have psoriasis in other areas, with or without psoriatic arthritis, she advised the use of either IL-17 antagonists (secukinumab, ixekizumab, brodalumab) or IL-23 antagonists (risankizumab, guselkumab).
Dr. Soung disclosed serving as a consultant or advisor for Arcutis, Bristol Myers Squibb Company, Dermavant, and Novartis. She also disclosed serving as a speaker or member of the speakers’ bureau for AbbVie, Amgen, Arcutis, Bristol Myers Squibb Company, Celgene, Leo Pharma, Eli Lilly, Novartis, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi, as well as research funding from AbbVie, Amgen, Arcutis, Castle Biosciences, Dermavant, KoBio, Kyowa Kirin, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Botanical Briefs: Toxicodendron Dermatitis
Reactions to poison ivy, poison oak, and poison sumac, which affect 10 to 50 million Americans a year,1 are classified as Toxicodendron dermatitis; 50% to 75% of US adults are clinically sensitive to these plants.2 Furthermore, people of all ethnicities, skin types, and ages residing in most US geographical regions are at risk.3 Allergenicity is caused by urushiol, which is found in members of the Anacardiaceae family.4 Once absorbed, urushiol causes a type IV hypersensitivity reaction in those who are susceptible.5
Cutaneous Manifestations
Toxicodendron dermatitis presents with an acute eczematous eruption characterized by streaks of intensely pruritic and erythematous papules and vesicles (Figure 1). Areas of involvement are characterized by sharp margins that follow the pattern of contact made by the plant’s leaves, berries, stems, and vines.6 The fluid content of the vesicles is not antigenic and cannot cause subsequent transmission to oneself or others.3 A person with prior contact to the plant who becomes sensitized develops an eruption 24 to 48 hours after subsequent contact with the plant; peak severity manifests 1 to 14 days later.7
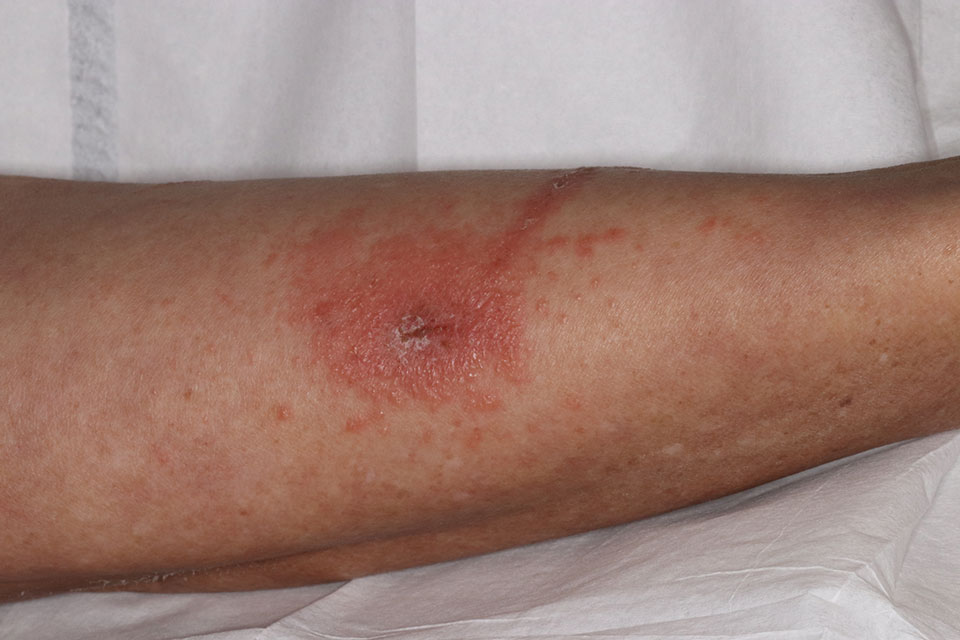
When left untreated, the eruption can last 3 weeks. If the plant is burned, urushiol can be aerosolized in smoke, causing respiratory tract inflammation and generalized dermatitis, which has been reported among wildland firefighters.2 Long-term complications from an outbreak are limited but can include postinflammatory hyperpigmentation and secondary bacterial infection.8 Rare reports of nephrotic syndrome also have appeared in the literature.9 Toxicodendron dermatitis can present distinctively as so-called black dot dermatitis.6
Nomenclature
Poison ivy, poison oak, and poison sumac are members of the family Anacardiaceae and genus Toxicodendron,6 derived from the Greek words toxikos (poison) and dendron (tree).10
Distribution
Toxicodendron plants characteristically are found in various regions of the United States. Poison ivy is the most common and is comprised of 2 species: Toxicodendron rydbergii and Toxicodendron radicans. Toxicodendron rydbergii is a nonclimbing dwarf shrub typically found in the northern and western United States. Toxicodendron radicans is a climbing vine found in the eastern United States. Poison oak also is comprised of 2 species—Toxicodendron toxicarium and Toxicodendron diversilobum—and is more common in the western United States. Poison sumac (also known as Toxicodendron vernix) is a small shrub that grows in moist swampy areas. It has a predilection for marshes of the eastern and southeastern United States.6,11
Identifying Features
Educating patients on how to identify poison ivy can play a key role in avoidance, which is the most important step in preventing Toxicodendron dermatitis. A challenge in identification of poison ivy is the plant’s variable appearance; it grows as a small shrub, low-lying vine, or vine that climbs other trees.
As the vine matures, it develops tiny, rough, “hairy” rootlets—hence the saying, “Hairy vine, no friend of mine!” Rootlets help the plant attach to trees growing near a water source. Vines can reach a diameter of 3 inches. From mature vines, solitary stems extend 1 to 2 inches with 3 characteristic leaves at the terminus (Figure 2), prompting another classic saying, “Leaves of 3, let it be!”12

Poison oak is characterized by 3 to 5 leaflets. Poison sumac has 7 to 13 pointed, smooth-edged leaves.6
Dermatitis-Inducing Plant Parts
The primary allergenic component of Toxicodendron plants is urushiol, a resinous sap found in stems, roots, leaves, and skins of the fruits. These components must be damaged or bruised to release the allergen; slight contact with an uninjured plant part might not lead to harm.2,13 Some common forms of transmission include skin contact, ingestion, inhalation of smoke from burning plants, and contact with skin through contaminated items, such as clothing, animals, and tools.14
Allergens
The catecholic ring and aliphatic chain of the urushiol molecule are allergenic.15 The degree of saturation and length of the side chains vary with different catechols. Urushiol displays cross-reactivity with poison ivy, poison oak, and poison sumac. Urushiol from these plants differs only slightly in structure; therefore, sensitization to one causes sensitization to all. There also is cross-reactivity between different members of the Anacardiaceae family, including Anacardium occidentale (tropical cashew nut), Mangifera indica (tropical mango tree), Ginkgo biloba (ginkgo tree), and Semecarpus anacardium (Indian marking nut tree).12
Poison ivy, poison oak, and poison sumac cause allergic contact dermatitis as a type IV hypersensitivity reaction. First, urushiol binds and penetrates the skin, where it is oxidized to quinone intermediates and bound to haptens. Then, the intermediates bind surface proteins on antigen-presenting cells, specifically Langerhans cells in the epidermis and dermis.5
Presentation of nonpeptide antigens, such as urushiol, to T cells requires expression of langerin (also known as CD207) and CD1a.16 Langerin is a C-type lectin that causes formation of Birbeck granules; CD1a is a major histocompatibility complex class I molecule found in Birbeck granules.5,17 After Langerhans cells internalize and process the urushiol self-hapten neoantigen, it is presented to CD4+ T cells.6 These cells then expand to form circulating activated T-effector and T-memory lymphocytes.18
The molecular link that occurs between the hapten and carrier protein determines the response. When linked by an amino nucleophile, selective induction of T-effector cells ensues, resulting in allergic contact dermatitis. When linked by a sulfhydryl bond, selective induction of suppressor cells occurs, resulting in a reduced allergic contact dermatitis response.19 In the case of activation of T-effector cells, a cell-mediated cytotoxic immune response is generated that destroys epidermal cells and dermal vasculature.2 The incidence and intensity of poison ivy sensitivity decline proportionally with age and the absence of continued exposure.20
Preventive Action—Patients should be counseled that if contact between plant and skin occurs, it is important to remove contaminated clothing or objects and wash them with soap to prevent additional exposure.14,21 Areas of the skin that made contact with the plant should be washed with water as soon as possible; after 30 minutes, urushiol has sufficiently penetrated to cause a reaction.2 Forceful unidirectional washing with a damp washcloth and liquid dishwashing soap is recommended.22
Several barrier creams are commercially available to help prevent absorption or to deactivate the urushiol antigen. These products are used widely by forestry workers and wildland firefighters.23 One such barrier cream is bentoquatam (sold as various trade names), an organoclay compound made of quaternium-18 bentonite that interferes with absorption of the allergen by acting as a physical blocker.24
Treatment
After Toxicodendron dermatitis develops, several treatments are available to help manage symptoms. Calamine lotion can be used to help dry weeping lesions.25,26 Topical steroids can be used to help control pruritus and alleviate inflammation. High-potency topical corticosteroids such as clobetasol and mid-potency steroids such as triamcinolone can be used. Topical anesthetics (eg, benzocaine, pramoxine, benzyl alcohol) might provide symptomatic relief.27,28
Oral antihistamines can allow for better sleep by providing sedation but do not target the pruritus of poison ivy dermatitis, which is not histamine mediated.29,30 Systemic corticosteroids usually are considered in more severe dermatitis—when 20% or more of the body surface area is involved; blistering and itching are severe; or the face, hands, or genitalia are involved.31,32
Clinical Uses
Therapeutic uses for poison ivy have been explored extensively. In 1892, Dakin33 reported that ingestion of leaves by Native Americans reduced the incidence and severity of skin lesions after contact with poison ivy. Consumption of poison ivy was further studied by Epstein and colleagues34 in 1974; they concluded that ingestion of a large amount of urushiol over a period of 3 months or longer may help with hyposensitization—but not complete desensitization—to contact with poison ivy. However, the risk for adverse effects is thought to outweigh benefits because ingestion can cause perianal dermatitis, mucocutaneous sequelae, and systemic contact dermatitis.2
Although the use of Toxicodendron plants in modern-day medicine is limited, development of a vaccine (immunotherapy) against Toxicodendron dermatitis offers an exciting opportunity for further research.
- Pariser DM, Ceilley RI, Lefkovits AM, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Cruse JM, Lewis RE. Atlas of Immunology. CRC Press; 2004.
- Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71-81. doi:10.1016/s1074-7613(00)80160-0
- Marks JG. Poison ivy and poison oak allergic contact dermatitis. J Allergy Clin Immunol. 1989;9:497-506.
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Rytand DA. Fatal anuria, the nephrotic syndrome and glomerular nephritis as sequels of the dermatitis of poison oak. Am J Med. 1948;5:548-560. doi:10.1016/0002-9343(48)90105-3
- Gledhill D. The Names of Plants. Cambridge University Press; 2008.
- American Academy of Dermatology Association. Poison ivy, oak, and sumac: how to treat the rash. Accessed October 19, 2022. https://www.aad.org/public/everyday-care/itchy-skin/poison-ivy/treat-rash
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 suppl 1):S29-S34.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers Medical Publishers; 2016.
- Fisher AA, Mitchell JC. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 4th ed. Williams and Wilkins; 1995:461-523.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701-708. doi:10.1172/JCI19655
- Hanau D, Fabre M, Schmitt DA, et al. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis “non-classical” major histocompatibility complex class Imolecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987;84:2901-2905. doi:10.1073/pnas.84.9.2901
- Gayer KD, Burnett JW. Toxicodendron dermatitis. Cutis. 1988;42:99-100.
- Dunn IS, Liberato DJ, Castagnoli N, et al. Contact sensitivity to urushiol: role of covalent bond formation. Cell Immunol. 1982;74:220-233. doi:10.1016/0008-8749(82)90023-5
- Kligman AM. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958;77:149-180. doi:10.1001/archderm.1958.01560020001001
- Derraik JGB. Heracleum mantegazzianum and Toxicodendron succedaneum: plants of human health significance in New Zealand and the National Pest Plant Accord. N Z Med J. 2007;120:U2657.
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2018;81:E25. doi:10.1016/j.jaad.2017.12.081
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? Dermatitis. 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Marks JG Jr, Fowler JF Jr, Sheretz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216. doi:10.1016/0190-9622(95)90237-6
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Williford PM, Sheretz EF. Poison ivy dermatitis. nuances in treatment. Arch Fam Med. 1995;3:184.
- Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566. doi:10.1016/S1081-1206(10)61535-9
- Stephanides SL, Moore C. Toxicodendron poisoning treatment & management. Medscape. Updated June 13, 2022. Accessed October 19, 2022. https://emedicine.medscape.com/article/817671-treatment#d11
- Munday J, Bloomfield R, Goldman M, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology. 2002;205:40-45. doi:10.1159/000063138
- Yosipovitch G, Fleischer A. Itch associated with skin disease: advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4:617-622. doi:10.2165/00128071-200304090-00004
- Li LY, Cruz PD Jr. Allergic contact dermatitis: pathophysiology applied to future therapy. Dermatol Ther. 2004;17:219-223. doi:10.1111/j.1396-0296.2004.04023.x
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (Rhus)? J Fam Pract. 2006;55:166-167.
- Dakin R. Remarks on a cutaneous affection, produced by certain poisonous vegetables. Am J Med Sci. 1829;4:98-100.
- Epstein WL, Baer H, Dawson CR, et al. Poison oak hyposensitization. evaluation of purified urushiol. Arch Dermatol. 1974;109:356-360.
Reactions to poison ivy, poison oak, and poison sumac, which affect 10 to 50 million Americans a year,1 are classified as Toxicodendron dermatitis; 50% to 75% of US adults are clinically sensitive to these plants.2 Furthermore, people of all ethnicities, skin types, and ages residing in most US geographical regions are at risk.3 Allergenicity is caused by urushiol, which is found in members of the Anacardiaceae family.4 Once absorbed, urushiol causes a type IV hypersensitivity reaction in those who are susceptible.5
Cutaneous Manifestations
Toxicodendron dermatitis presents with an acute eczematous eruption characterized by streaks of intensely pruritic and erythematous papules and vesicles (Figure 1). Areas of involvement are characterized by sharp margins that follow the pattern of contact made by the plant’s leaves, berries, stems, and vines.6 The fluid content of the vesicles is not antigenic and cannot cause subsequent transmission to oneself or others.3 A person with prior contact to the plant who becomes sensitized develops an eruption 24 to 48 hours after subsequent contact with the plant; peak severity manifests 1 to 14 days later.7

When left untreated, the eruption can last 3 weeks. If the plant is burned, urushiol can be aerosolized in smoke, causing respiratory tract inflammation and generalized dermatitis, which has been reported among wildland firefighters.2 Long-term complications from an outbreak are limited but can include postinflammatory hyperpigmentation and secondary bacterial infection.8 Rare reports of nephrotic syndrome also have appeared in the literature.9 Toxicodendron dermatitis can present distinctively as so-called black dot dermatitis.6
Nomenclature
Poison ivy, poison oak, and poison sumac are members of the family Anacardiaceae and genus Toxicodendron,6 derived from the Greek words toxikos (poison) and dendron (tree).10
Distribution
Toxicodendron plants characteristically are found in various regions of the United States. Poison ivy is the most common and is comprised of 2 species: Toxicodendron rydbergii and Toxicodendron radicans. Toxicodendron rydbergii is a nonclimbing dwarf shrub typically found in the northern and western United States. Toxicodendron radicans is a climbing vine found in the eastern United States. Poison oak also is comprised of 2 species—Toxicodendron toxicarium and Toxicodendron diversilobum—and is more common in the western United States. Poison sumac (also known as Toxicodendron vernix) is a small shrub that grows in moist swampy areas. It has a predilection for marshes of the eastern and southeastern United States.6,11
Identifying Features
Educating patients on how to identify poison ivy can play a key role in avoidance, which is the most important step in preventing Toxicodendron dermatitis. A challenge in identification of poison ivy is the plant’s variable appearance; it grows as a small shrub, low-lying vine, or vine that climbs other trees.
As the vine matures, it develops tiny, rough, “hairy” rootlets—hence the saying, “Hairy vine, no friend of mine!” Rootlets help the plant attach to trees growing near a water source. Vines can reach a diameter of 3 inches. From mature vines, solitary stems extend 1 to 2 inches with 3 characteristic leaves at the terminus (Figure 2), prompting another classic saying, “Leaves of 3, let it be!”12

Poison oak is characterized by 3 to 5 leaflets. Poison sumac has 7 to 13 pointed, smooth-edged leaves.6
Dermatitis-Inducing Plant Parts
The primary allergenic component of Toxicodendron plants is urushiol, a resinous sap found in stems, roots, leaves, and skins of the fruits. These components must be damaged or bruised to release the allergen; slight contact with an uninjured plant part might not lead to harm.2,13 Some common forms of transmission include skin contact, ingestion, inhalation of smoke from burning plants, and contact with skin through contaminated items, such as clothing, animals, and tools.14
Allergens
The catecholic ring and aliphatic chain of the urushiol molecule are allergenic.15 The degree of saturation and length of the side chains vary with different catechols. Urushiol displays cross-reactivity with poison ivy, poison oak, and poison sumac. Urushiol from these plants differs only slightly in structure; therefore, sensitization to one causes sensitization to all. There also is cross-reactivity between different members of the Anacardiaceae family, including Anacardium occidentale (tropical cashew nut), Mangifera indica (tropical mango tree), Ginkgo biloba (ginkgo tree), and Semecarpus anacardium (Indian marking nut tree).12
Poison ivy, poison oak, and poison sumac cause allergic contact dermatitis as a type IV hypersensitivity reaction. First, urushiol binds and penetrates the skin, where it is oxidized to quinone intermediates and bound to haptens. Then, the intermediates bind surface proteins on antigen-presenting cells, specifically Langerhans cells in the epidermis and dermis.5
Presentation of nonpeptide antigens, such as urushiol, to T cells requires expression of langerin (also known as CD207) and CD1a.16 Langerin is a C-type lectin that causes formation of Birbeck granules; CD1a is a major histocompatibility complex class I molecule found in Birbeck granules.5,17 After Langerhans cells internalize and process the urushiol self-hapten neoantigen, it is presented to CD4+ T cells.6 These cells then expand to form circulating activated T-effector and T-memory lymphocytes.18
The molecular link that occurs between the hapten and carrier protein determines the response. When linked by an amino nucleophile, selective induction of T-effector cells ensues, resulting in allergic contact dermatitis. When linked by a sulfhydryl bond, selective induction of suppressor cells occurs, resulting in a reduced allergic contact dermatitis response.19 In the case of activation of T-effector cells, a cell-mediated cytotoxic immune response is generated that destroys epidermal cells and dermal vasculature.2 The incidence and intensity of poison ivy sensitivity decline proportionally with age and the absence of continued exposure.20
Preventive Action—Patients should be counseled that if contact between plant and skin occurs, it is important to remove contaminated clothing or objects and wash them with soap to prevent additional exposure.14,21 Areas of the skin that made contact with the plant should be washed with water as soon as possible; after 30 minutes, urushiol has sufficiently penetrated to cause a reaction.2 Forceful unidirectional washing with a damp washcloth and liquid dishwashing soap is recommended.22
Several barrier creams are commercially available to help prevent absorption or to deactivate the urushiol antigen. These products are used widely by forestry workers and wildland firefighters.23 One such barrier cream is bentoquatam (sold as various trade names), an organoclay compound made of quaternium-18 bentonite that interferes with absorption of the allergen by acting as a physical blocker.24
Treatment
After Toxicodendron dermatitis develops, several treatments are available to help manage symptoms. Calamine lotion can be used to help dry weeping lesions.25,26 Topical steroids can be used to help control pruritus and alleviate inflammation. High-potency topical corticosteroids such as clobetasol and mid-potency steroids such as triamcinolone can be used. Topical anesthetics (eg, benzocaine, pramoxine, benzyl alcohol) might provide symptomatic relief.27,28
Oral antihistamines can allow for better sleep by providing sedation but do not target the pruritus of poison ivy dermatitis, which is not histamine mediated.29,30 Systemic corticosteroids usually are considered in more severe dermatitis—when 20% or more of the body surface area is involved; blistering and itching are severe; or the face, hands, or genitalia are involved.31,32
Clinical Uses
Therapeutic uses for poison ivy have been explored extensively. In 1892, Dakin33 reported that ingestion of leaves by Native Americans reduced the incidence and severity of skin lesions after contact with poison ivy. Consumption of poison ivy was further studied by Epstein and colleagues34 in 1974; they concluded that ingestion of a large amount of urushiol over a period of 3 months or longer may help with hyposensitization—but not complete desensitization—to contact with poison ivy. However, the risk for adverse effects is thought to outweigh benefits because ingestion can cause perianal dermatitis, mucocutaneous sequelae, and systemic contact dermatitis.2
Although the use of Toxicodendron plants in modern-day medicine is limited, development of a vaccine (immunotherapy) against Toxicodendron dermatitis offers an exciting opportunity for further research.
Reactions to poison ivy, poison oak, and poison sumac, which affect 10 to 50 million Americans a year,1 are classified as Toxicodendron dermatitis; 50% to 75% of US adults are clinically sensitive to these plants.2 Furthermore, people of all ethnicities, skin types, and ages residing in most US geographical regions are at risk.3 Allergenicity is caused by urushiol, which is found in members of the Anacardiaceae family.4 Once absorbed, urushiol causes a type IV hypersensitivity reaction in those who are susceptible.5
Cutaneous Manifestations
Toxicodendron dermatitis presents with an acute eczematous eruption characterized by streaks of intensely pruritic and erythematous papules and vesicles (Figure 1). Areas of involvement are characterized by sharp margins that follow the pattern of contact made by the plant’s leaves, berries, stems, and vines.6 The fluid content of the vesicles is not antigenic and cannot cause subsequent transmission to oneself or others.3 A person with prior contact to the plant who becomes sensitized develops an eruption 24 to 48 hours after subsequent contact with the plant; peak severity manifests 1 to 14 days later.7

When left untreated, the eruption can last 3 weeks. If the plant is burned, urushiol can be aerosolized in smoke, causing respiratory tract inflammation and generalized dermatitis, which has been reported among wildland firefighters.2 Long-term complications from an outbreak are limited but can include postinflammatory hyperpigmentation and secondary bacterial infection.8 Rare reports of nephrotic syndrome also have appeared in the literature.9 Toxicodendron dermatitis can present distinctively as so-called black dot dermatitis.6
Nomenclature
Poison ivy, poison oak, and poison sumac are members of the family Anacardiaceae and genus Toxicodendron,6 derived from the Greek words toxikos (poison) and dendron (tree).10
Distribution
Toxicodendron plants characteristically are found in various regions of the United States. Poison ivy is the most common and is comprised of 2 species: Toxicodendron rydbergii and Toxicodendron radicans. Toxicodendron rydbergii is a nonclimbing dwarf shrub typically found in the northern and western United States. Toxicodendron radicans is a climbing vine found in the eastern United States. Poison oak also is comprised of 2 species—Toxicodendron toxicarium and Toxicodendron diversilobum—and is more common in the western United States. Poison sumac (also known as Toxicodendron vernix) is a small shrub that grows in moist swampy areas. It has a predilection for marshes of the eastern and southeastern United States.6,11
Identifying Features
Educating patients on how to identify poison ivy can play a key role in avoidance, which is the most important step in preventing Toxicodendron dermatitis. A challenge in identification of poison ivy is the plant’s variable appearance; it grows as a small shrub, low-lying vine, or vine that climbs other trees.
As the vine matures, it develops tiny, rough, “hairy” rootlets—hence the saying, “Hairy vine, no friend of mine!” Rootlets help the plant attach to trees growing near a water source. Vines can reach a diameter of 3 inches. From mature vines, solitary stems extend 1 to 2 inches with 3 characteristic leaves at the terminus (Figure 2), prompting another classic saying, “Leaves of 3, let it be!”12

Poison oak is characterized by 3 to 5 leaflets. Poison sumac has 7 to 13 pointed, smooth-edged leaves.6
Dermatitis-Inducing Plant Parts
The primary allergenic component of Toxicodendron plants is urushiol, a resinous sap found in stems, roots, leaves, and skins of the fruits. These components must be damaged or bruised to release the allergen; slight contact with an uninjured plant part might not lead to harm.2,13 Some common forms of transmission include skin contact, ingestion, inhalation of smoke from burning plants, and contact with skin through contaminated items, such as clothing, animals, and tools.14
Allergens
The catecholic ring and aliphatic chain of the urushiol molecule are allergenic.15 The degree of saturation and length of the side chains vary with different catechols. Urushiol displays cross-reactivity with poison ivy, poison oak, and poison sumac. Urushiol from these plants differs only slightly in structure; therefore, sensitization to one causes sensitization to all. There also is cross-reactivity between different members of the Anacardiaceae family, including Anacardium occidentale (tropical cashew nut), Mangifera indica (tropical mango tree), Ginkgo biloba (ginkgo tree), and Semecarpus anacardium (Indian marking nut tree).12
Poison ivy, poison oak, and poison sumac cause allergic contact dermatitis as a type IV hypersensitivity reaction. First, urushiol binds and penetrates the skin, where it is oxidized to quinone intermediates and bound to haptens. Then, the intermediates bind surface proteins on antigen-presenting cells, specifically Langerhans cells in the epidermis and dermis.5
Presentation of nonpeptide antigens, such as urushiol, to T cells requires expression of langerin (also known as CD207) and CD1a.16 Langerin is a C-type lectin that causes formation of Birbeck granules; CD1a is a major histocompatibility complex class I molecule found in Birbeck granules.5,17 After Langerhans cells internalize and process the urushiol self-hapten neoantigen, it is presented to CD4+ T cells.6 These cells then expand to form circulating activated T-effector and T-memory lymphocytes.18
The molecular link that occurs between the hapten and carrier protein determines the response. When linked by an amino nucleophile, selective induction of T-effector cells ensues, resulting in allergic contact dermatitis. When linked by a sulfhydryl bond, selective induction of suppressor cells occurs, resulting in a reduced allergic contact dermatitis response.19 In the case of activation of T-effector cells, a cell-mediated cytotoxic immune response is generated that destroys epidermal cells and dermal vasculature.2 The incidence and intensity of poison ivy sensitivity decline proportionally with age and the absence of continued exposure.20
Preventive Action—Patients should be counseled that if contact between plant and skin occurs, it is important to remove contaminated clothing or objects and wash them with soap to prevent additional exposure.14,21 Areas of the skin that made contact with the plant should be washed with water as soon as possible; after 30 minutes, urushiol has sufficiently penetrated to cause a reaction.2 Forceful unidirectional washing with a damp washcloth and liquid dishwashing soap is recommended.22
Several barrier creams are commercially available to help prevent absorption or to deactivate the urushiol antigen. These products are used widely by forestry workers and wildland firefighters.23 One such barrier cream is bentoquatam (sold as various trade names), an organoclay compound made of quaternium-18 bentonite that interferes with absorption of the allergen by acting as a physical blocker.24
Treatment
After Toxicodendron dermatitis develops, several treatments are available to help manage symptoms. Calamine lotion can be used to help dry weeping lesions.25,26 Topical steroids can be used to help control pruritus and alleviate inflammation. High-potency topical corticosteroids such as clobetasol and mid-potency steroids such as triamcinolone can be used. Topical anesthetics (eg, benzocaine, pramoxine, benzyl alcohol) might provide symptomatic relief.27,28
Oral antihistamines can allow for better sleep by providing sedation but do not target the pruritus of poison ivy dermatitis, which is not histamine mediated.29,30 Systemic corticosteroids usually are considered in more severe dermatitis—when 20% or more of the body surface area is involved; blistering and itching are severe; or the face, hands, or genitalia are involved.31,32
Clinical Uses
Therapeutic uses for poison ivy have been explored extensively. In 1892, Dakin33 reported that ingestion of leaves by Native Americans reduced the incidence and severity of skin lesions after contact with poison ivy. Consumption of poison ivy was further studied by Epstein and colleagues34 in 1974; they concluded that ingestion of a large amount of urushiol over a period of 3 months or longer may help with hyposensitization—but not complete desensitization—to contact with poison ivy. However, the risk for adverse effects is thought to outweigh benefits because ingestion can cause perianal dermatitis, mucocutaneous sequelae, and systemic contact dermatitis.2
Although the use of Toxicodendron plants in modern-day medicine is limited, development of a vaccine (immunotherapy) against Toxicodendron dermatitis offers an exciting opportunity for further research.
- Pariser DM, Ceilley RI, Lefkovits AM, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Cruse JM, Lewis RE. Atlas of Immunology. CRC Press; 2004.
- Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71-81. doi:10.1016/s1074-7613(00)80160-0
- Marks JG. Poison ivy and poison oak allergic contact dermatitis. J Allergy Clin Immunol. 1989;9:497-506.
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Rytand DA. Fatal anuria, the nephrotic syndrome and glomerular nephritis as sequels of the dermatitis of poison oak. Am J Med. 1948;5:548-560. doi:10.1016/0002-9343(48)90105-3
- Gledhill D. The Names of Plants. Cambridge University Press; 2008.
- American Academy of Dermatology Association. Poison ivy, oak, and sumac: how to treat the rash. Accessed October 19, 2022. https://www.aad.org/public/everyday-care/itchy-skin/poison-ivy/treat-rash
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 suppl 1):S29-S34.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers Medical Publishers; 2016.
- Fisher AA, Mitchell JC. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 4th ed. Williams and Wilkins; 1995:461-523.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701-708. doi:10.1172/JCI19655
- Hanau D, Fabre M, Schmitt DA, et al. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis “non-classical” major histocompatibility complex class Imolecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987;84:2901-2905. doi:10.1073/pnas.84.9.2901
- Gayer KD, Burnett JW. Toxicodendron dermatitis. Cutis. 1988;42:99-100.
- Dunn IS, Liberato DJ, Castagnoli N, et al. Contact sensitivity to urushiol: role of covalent bond formation. Cell Immunol. 1982;74:220-233. doi:10.1016/0008-8749(82)90023-5
- Kligman AM. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958;77:149-180. doi:10.1001/archderm.1958.01560020001001
- Derraik JGB. Heracleum mantegazzianum and Toxicodendron succedaneum: plants of human health significance in New Zealand and the National Pest Plant Accord. N Z Med J. 2007;120:U2657.
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2018;81:E25. doi:10.1016/j.jaad.2017.12.081
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? Dermatitis. 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Marks JG Jr, Fowler JF Jr, Sheretz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216. doi:10.1016/0190-9622(95)90237-6
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Williford PM, Sheretz EF. Poison ivy dermatitis. nuances in treatment. Arch Fam Med. 1995;3:184.
- Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566. doi:10.1016/S1081-1206(10)61535-9
- Stephanides SL, Moore C. Toxicodendron poisoning treatment & management. Medscape. Updated June 13, 2022. Accessed October 19, 2022. https://emedicine.medscape.com/article/817671-treatment#d11
- Munday J, Bloomfield R, Goldman M, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology. 2002;205:40-45. doi:10.1159/000063138
- Yosipovitch G, Fleischer A. Itch associated with skin disease: advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4:617-622. doi:10.2165/00128071-200304090-00004
- Li LY, Cruz PD Jr. Allergic contact dermatitis: pathophysiology applied to future therapy. Dermatol Ther. 2004;17:219-223. doi:10.1111/j.1396-0296.2004.04023.x
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (Rhus)? J Fam Pract. 2006;55:166-167.
- Dakin R. Remarks on a cutaneous affection, produced by certain poisonous vegetables. Am J Med Sci. 1829;4:98-100.
- Epstein WL, Baer H, Dawson CR, et al. Poison oak hyposensitization. evaluation of purified urushiol. Arch Dermatol. 1974;109:356-360.
- Pariser DM, Ceilley RI, Lefkovits AM, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Cruse JM, Lewis RE. Atlas of Immunology. CRC Press; 2004.
- Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71-81. doi:10.1016/s1074-7613(00)80160-0
- Marks JG. Poison ivy and poison oak allergic contact dermatitis. J Allergy Clin Immunol. 1989;9:497-506.
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Rytand DA. Fatal anuria, the nephrotic syndrome and glomerular nephritis as sequels of the dermatitis of poison oak. Am J Med. 1948;5:548-560. doi:10.1016/0002-9343(48)90105-3
- Gledhill D. The Names of Plants. Cambridge University Press; 2008.
- American Academy of Dermatology Association. Poison ivy, oak, and sumac: how to treat the rash. Accessed October 19, 2022. https://www.aad.org/public/everyday-care/itchy-skin/poison-ivy/treat-rash
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 suppl 1):S29-S34.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers Medical Publishers; 2016.
- Fisher AA, Mitchell JC. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 4th ed. Williams and Wilkins; 1995:461-523.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701-708. doi:10.1172/JCI19655
- Hanau D, Fabre M, Schmitt DA, et al. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis “non-classical” major histocompatibility complex class Imolecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987;84:2901-2905. doi:10.1073/pnas.84.9.2901
- Gayer KD, Burnett JW. Toxicodendron dermatitis. Cutis. 1988;42:99-100.
- Dunn IS, Liberato DJ, Castagnoli N, et al. Contact sensitivity to urushiol: role of covalent bond formation. Cell Immunol. 1982;74:220-233. doi:10.1016/0008-8749(82)90023-5
- Kligman AM. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958;77:149-180. doi:10.1001/archderm.1958.01560020001001
- Derraik JGB. Heracleum mantegazzianum and Toxicodendron succedaneum: plants of human health significance in New Zealand and the National Pest Plant Accord. N Z Med J. 2007;120:U2657.
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2018;81:E25. doi:10.1016/j.jaad.2017.12.081
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? Dermatitis. 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Marks JG Jr, Fowler JF Jr, Sheretz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216. doi:10.1016/0190-9622(95)90237-6
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Williford PM, Sheretz EF. Poison ivy dermatitis. nuances in treatment. Arch Fam Med. 1995;3:184.
- Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566. doi:10.1016/S1081-1206(10)61535-9
- Stephanides SL, Moore C. Toxicodendron poisoning treatment & management. Medscape. Updated June 13, 2022. Accessed October 19, 2022. https://emedicine.medscape.com/article/817671-treatment#d11
- Munday J, Bloomfield R, Goldman M, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology. 2002;205:40-45. doi:10.1159/000063138
- Yosipovitch G, Fleischer A. Itch associated with skin disease: advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4:617-622. doi:10.2165/00128071-200304090-00004
- Li LY, Cruz PD Jr. Allergic contact dermatitis: pathophysiology applied to future therapy. Dermatol Ther. 2004;17:219-223. doi:10.1111/j.1396-0296.2004.04023.x
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (Rhus)? J Fam Pract. 2006;55:166-167.
- Dakin R. Remarks on a cutaneous affection, produced by certain poisonous vegetables. Am J Med Sci. 1829;4:98-100.
- Epstein WL, Baer H, Dawson CR, et al. Poison oak hyposensitization. evaluation of purified urushiol. Arch Dermatol. 1974;109:356-360.
Practice Points
- Toxicodendron dermatitis is a pruritic vesicular eruption in areas of contact with the plant.
- Identification and avoidance are primary methods of preventing Toxicodendron dermatitis.