User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Disaster Preparedness in Dermatology Residency Programs
In an age of changing climate and emerging global pandemics, the ability of residency programs to prepare for and adapt to potential disasters may be paramount in preserving the training of physicians. The current literature regarding residency program disaster preparedness, which focuses predominantly on hurricanes and COVID-19,1-8 is lacking in recommendations specific to dermatology residency programs. Likewise, the Accreditation Council for Graduate Medical Education (ACGME) guidelines9 do not address dermatology-specific concerns in disaster preparedness or response. Herein, we propose recommendations to mitigate the impact of various types of disasters on dermatology residency programs and their trainees with regard to resident safety and wellness, resident education, and patient care (Table).
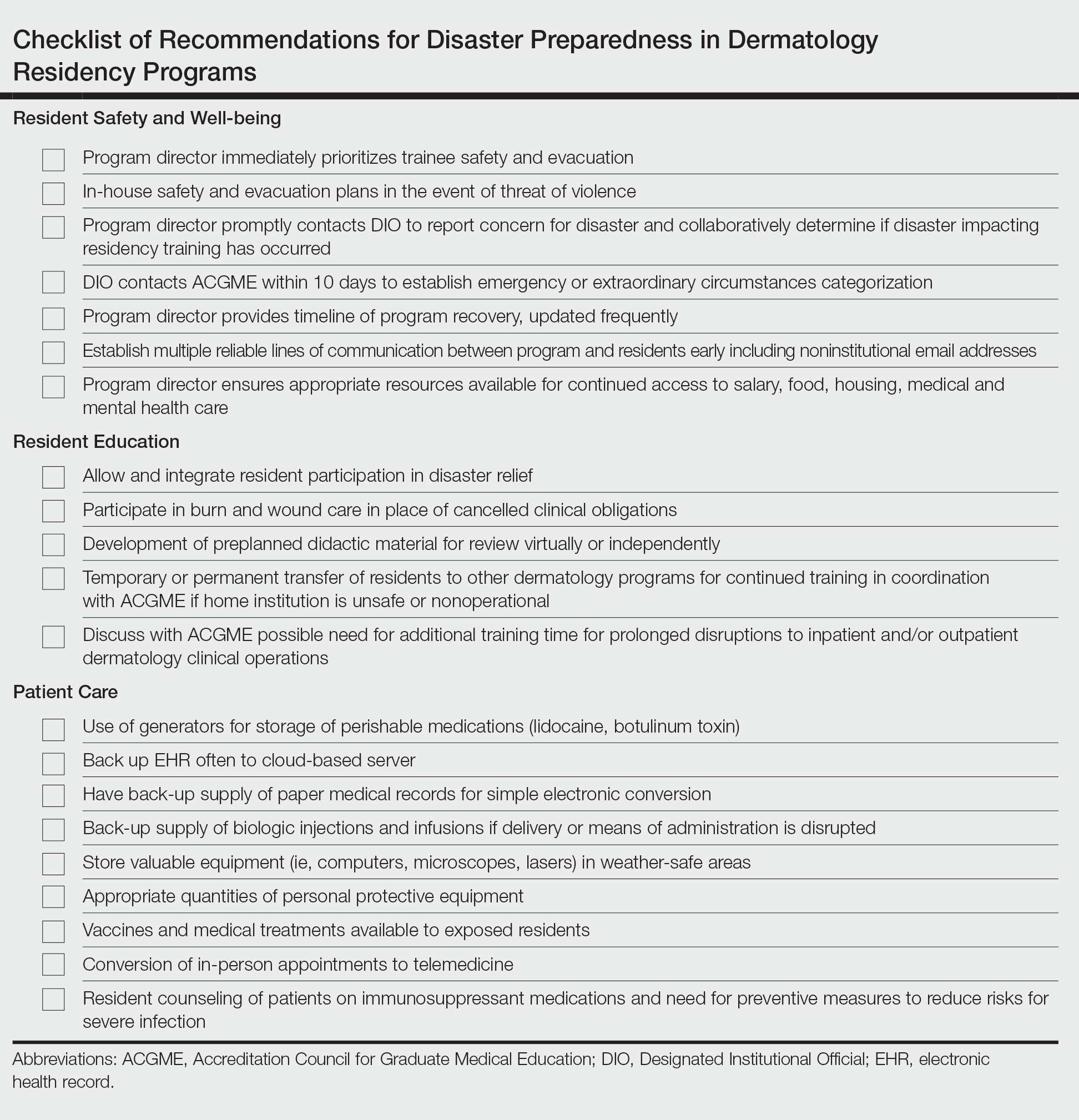
Resident Safety and Wellness
Role of the Program Director—The role of the program director is critical, serving as a figure of structure and reassurance.4,7,10 Once concern of disaster arises, the program director should contact the Designated Institutional Official (DIO) to express concerns about possible disruptions to resident training. The DIO should then contact the ACGME within 10 days to report the disaster and submit a request for emergency (eg, pandemic) or extraordinary circumstances (eg, natural disaster) categorization.4,9 Program directors should promptly prepare plans for program reconfiguration and resident transfers in alignment with ACGME requirements to maintain evaluation and completion of core competencies of training during disasters.9 Program directors should prioritize the safety of trainees during the immediate threat with clear guidelines on sheltering, evacuations, or quarantines; a timeline of program recovery based on communication with residents, faculty, and administration should then be established.10,11
Communication—Establishing a strong line of communication between program directors and residents is paramount. Collection of emergency noninstitutional contact information, establishment of a centralized website for information dissemination, use of noninstitutional email and proxy servers outside of the location of impact, social media updates, on-site use of 2-way radios, and program-wide conference calls when possible should be strongly considered as part of the disaster response.2-4,12,13
Resident Accommodations and Mental Health—If training is disrupted, residents should be reassured of continued access to salary, housing, food, or other resources as necessary.3,4,11 There should be clear contingency plans if residents need to leave the program for extended periods of time due to injury, illness, or personal circumstances. Although relevant in all types of disasters, resident mental health and response to trauma also must be addressed. Access to counseling, morale-building opportunities (eg, resident social events), and screening for depression or posttraumatic stress disorder may help promote well-being among residents following traumatic events.14
Resident Education
Participation in Disaster Relief—Residents may seek to aid in the disaster response, which may prove challenging in the setting of programs with high patient volume.4 In coordination with the ACGME and graduate medical education governing bodies, program directors should consider how residents may fulfill dermatology training requirements in conjunction with disaster relief efforts, such as working in an inpatient setting or providing wound care.10
Continued Didactic Education—The use of online learning and conference calls for continuing the dermatology curriculum is an efficient means to maintaining resident education when meeting in person poses risks to residents.15 Projections of microscopy images, clinical photographs, or other instructional materials allow for continued instruction on resident examination, histopathology, and diagnostic skills.
Continued Clinical Training—If the home institution cannot support the operation of dermatology clinics, residents should be guaranteed continued training at other institutions. Agreements with other dermatology programs, community hospitals, or private dermatology practices should be established in advance, with consideration given to the number of residents a program can support, funding transfers, and credentialing requirements.2,4,5
Prolonged Disruptions—Nonessential departments of medical institutions may cease to function during war or mass casualty disasters, and it may be unsafe to send dermatology residents to other institutions or clinical areas. If the threat is prolonged, programs may need to consider allowing current residents a longer duration of training despite potential overlap with incoming dermatology residents.7
Patient Care
Disruptions to Clinic Operations—Regarding threats of violence, dangerous exposures, or natural disasters, there should be clear guidelines on sheltering in the clinical setting or stabilizing patients during a procedure.11 Equipment used by residents such as laptops, microscopes, and treatment devices (eg, lasers) should be stored in weather-safe locations that would not be notably impacted by moisture or structural damage to the clinic building. If electricity or internet access are compromised, paper medical records should be available to residents to continue clinical operations. Electronic health records used by residents should regularly be backed up on remote servers or cloud storage to allow continued access to patient health information if on-site servers are not functional.12 If disruptions are prolonged, residency program administration should coordinate with the institution to ensure there is adequate supply and storage of medications (eg, lidocaine, botulinum toxin) as well as a continued means of delivering biologic medications to patients and an ability to obtain laboratory or dermatopathology services.
In-Person Appointments vs Telemedicine—There are benefits to both residency training and patient care when physicians are able to perform in-person examinations, biopsies, and in-office treatments.16 Programs should ensure an adequate supply of personal protective equipment to continue in-office appointments, vaccinations, and medical care if a resident or other members of the team are exposed to an infectious disease.7 If in-person appointments are limited or impossible, telemedicine capabilities may still allow residents to meet program requirements.7,10,15 However, reduced patient volume due to decreased elective visits or procedures may complicate the fulfillment of clinical requirements, which may need to be adjusted in the wake of a disaster.7
Use of Immunosuppressive Therapies—Residency programs should address the risks of prescribing immunosuppressive therapies (eg, biologics) during an infectious threat with their residents and encourage trainees to counsel patients on the importance of preventative measures to reduce risks for severe infection.17
Final Thoughts
- Davis W. Hurricane Katrina: the challenge to graduate medical education. Ochsner J. 2006;6:39.
- Cefalu CA, Schwartz RS. Salvaging a geriatric medicine academic program in disaster mode—the LSU training program post-Katrina.J Natl Med Assoc. 2007;99:590-596.
- Ayyala R. Lessons from Katrina: a program director’s perspective. Ophthalmology. 2007;114:1425-1426.
- Wiese JG. Leadership in graduate medical education: eleven steps instrumental in recovering residency programs after a disaster. Am J Med Sci. 2008;336:168-173.
- Griffies WS. Post-Katrina stabilization of the LSU/Ochsner Psychiatry Residency Program: caveats for disaster preparedness. Acad Psychiatry. 2009;33:418-422.
- Kearns DG, Chat VS, Uppal S, et al. Applying to dermatology residency during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:1214-1215.
- Matthews JB, Blair PG, Ellison EC, et al. Checklist framework for surgical education disaster plans. J Am Coll Surg. 2021;233:557-563.
- Litchman GH, Marson JW, Rigel DS. The continuing impact of COVID-19 on dermatology practice: office workflow, economics, and future implications. J Am Acad Dermatol. 2021;84:576-579.
- Accreditation Council for Graduate Medical Education. Sponsoring institution emergency categorization. Accessed October 20, 2022. https://www.acgme.org/covid-19/sponsoring-institution-emergency-categorization/
- Li YM, Galimberti F, Abrouk M, et al. US dermatology resident responses about the COVID-19 pandemic: results from a nationwide survey. South Med J. 2020;113:462-465.
- Newman B, Gallion C. Hurricane Harvey: firsthand perspectives for disaster preparedness in graduate medical education. Acad Med. 2019;94:1267-1269.
- Pero CD, Pou AM, Arriaga MA, et al. Post-Katrina: study in crisis-related program adaptability. Otolaryngol Head Neck Surg. 2008;138:394-397.
- Hattaway R, Singh N, Rais-Bahrami S, et al. Adaptations of dermatology residency programs to changes in medical education amid the COVID-19 pandemic: virtual opportunities and social media. SKIN. 2021;5:94-100.
- Hillier K, Paskaradevan J, Wilkes JK, et al. Disaster plans: resident involvement and well-being during Hurricane Harvey. J Grad Med Educ. 2019;11:129-131.
- Samimi S, Choi J, Rosman IS, et al. Impact of COVID-19 on dermatology residency. Dermatol Clin. 2021;39:609-618.
- Bastola M, Locatis C, Fontelo P. Diagnostic reliability of in-person versus remote dermatology: a meta-analysis. Telemed J E Health. 2021;27:247-250.
- Bashyam AM, Feldman SR. Should patients stop their biologic treatment during the COVID-19 pandemic? J Dermatolog Treat. 2020;31:317-318.
In an age of changing climate and emerging global pandemics, the ability of residency programs to prepare for and adapt to potential disasters may be paramount in preserving the training of physicians. The current literature regarding residency program disaster preparedness, which focuses predominantly on hurricanes and COVID-19,1-8 is lacking in recommendations specific to dermatology residency programs. Likewise, the Accreditation Council for Graduate Medical Education (ACGME) guidelines9 do not address dermatology-specific concerns in disaster preparedness or response. Herein, we propose recommendations to mitigate the impact of various types of disasters on dermatology residency programs and their trainees with regard to resident safety and wellness, resident education, and patient care (Table).

Resident Safety and Wellness
Role of the Program Director—The role of the program director is critical, serving as a figure of structure and reassurance.4,7,10 Once concern of disaster arises, the program director should contact the Designated Institutional Official (DIO) to express concerns about possible disruptions to resident training. The DIO should then contact the ACGME within 10 days to report the disaster and submit a request for emergency (eg, pandemic) or extraordinary circumstances (eg, natural disaster) categorization.4,9 Program directors should promptly prepare plans for program reconfiguration and resident transfers in alignment with ACGME requirements to maintain evaluation and completion of core competencies of training during disasters.9 Program directors should prioritize the safety of trainees during the immediate threat with clear guidelines on sheltering, evacuations, or quarantines; a timeline of program recovery based on communication with residents, faculty, and administration should then be established.10,11
Communication—Establishing a strong line of communication between program directors and residents is paramount. Collection of emergency noninstitutional contact information, establishment of a centralized website for information dissemination, use of noninstitutional email and proxy servers outside of the location of impact, social media updates, on-site use of 2-way radios, and program-wide conference calls when possible should be strongly considered as part of the disaster response.2-4,12,13
Resident Accommodations and Mental Health—If training is disrupted, residents should be reassured of continued access to salary, housing, food, or other resources as necessary.3,4,11 There should be clear contingency plans if residents need to leave the program for extended periods of time due to injury, illness, or personal circumstances. Although relevant in all types of disasters, resident mental health and response to trauma also must be addressed. Access to counseling, morale-building opportunities (eg, resident social events), and screening for depression or posttraumatic stress disorder may help promote well-being among residents following traumatic events.14
Resident Education
Participation in Disaster Relief—Residents may seek to aid in the disaster response, which may prove challenging in the setting of programs with high patient volume.4 In coordination with the ACGME and graduate medical education governing bodies, program directors should consider how residents may fulfill dermatology training requirements in conjunction with disaster relief efforts, such as working in an inpatient setting or providing wound care.10
Continued Didactic Education—The use of online learning and conference calls for continuing the dermatology curriculum is an efficient means to maintaining resident education when meeting in person poses risks to residents.15 Projections of microscopy images, clinical photographs, or other instructional materials allow for continued instruction on resident examination, histopathology, and diagnostic skills.
Continued Clinical Training—If the home institution cannot support the operation of dermatology clinics, residents should be guaranteed continued training at other institutions. Agreements with other dermatology programs, community hospitals, or private dermatology practices should be established in advance, with consideration given to the number of residents a program can support, funding transfers, and credentialing requirements.2,4,5
Prolonged Disruptions—Nonessential departments of medical institutions may cease to function during war or mass casualty disasters, and it may be unsafe to send dermatology residents to other institutions or clinical areas. If the threat is prolonged, programs may need to consider allowing current residents a longer duration of training despite potential overlap with incoming dermatology residents.7
Patient Care
Disruptions to Clinic Operations—Regarding threats of violence, dangerous exposures, or natural disasters, there should be clear guidelines on sheltering in the clinical setting or stabilizing patients during a procedure.11 Equipment used by residents such as laptops, microscopes, and treatment devices (eg, lasers) should be stored in weather-safe locations that would not be notably impacted by moisture or structural damage to the clinic building. If electricity or internet access are compromised, paper medical records should be available to residents to continue clinical operations. Electronic health records used by residents should regularly be backed up on remote servers or cloud storage to allow continued access to patient health information if on-site servers are not functional.12 If disruptions are prolonged, residency program administration should coordinate with the institution to ensure there is adequate supply and storage of medications (eg, lidocaine, botulinum toxin) as well as a continued means of delivering biologic medications to patients and an ability to obtain laboratory or dermatopathology services.
In-Person Appointments vs Telemedicine—There are benefits to both residency training and patient care when physicians are able to perform in-person examinations, biopsies, and in-office treatments.16 Programs should ensure an adequate supply of personal protective equipment to continue in-office appointments, vaccinations, and medical care if a resident or other members of the team are exposed to an infectious disease.7 If in-person appointments are limited or impossible, telemedicine capabilities may still allow residents to meet program requirements.7,10,15 However, reduced patient volume due to decreased elective visits or procedures may complicate the fulfillment of clinical requirements, which may need to be adjusted in the wake of a disaster.7
Use of Immunosuppressive Therapies—Residency programs should address the risks of prescribing immunosuppressive therapies (eg, biologics) during an infectious threat with their residents and encourage trainees to counsel patients on the importance of preventative measures to reduce risks for severe infection.17
Final Thoughts
In an age of changing climate and emerging global pandemics, the ability of residency programs to prepare for and adapt to potential disasters may be paramount in preserving the training of physicians. The current literature regarding residency program disaster preparedness, which focuses predominantly on hurricanes and COVID-19,1-8 is lacking in recommendations specific to dermatology residency programs. Likewise, the Accreditation Council for Graduate Medical Education (ACGME) guidelines9 do not address dermatology-specific concerns in disaster preparedness or response. Herein, we propose recommendations to mitigate the impact of various types of disasters on dermatology residency programs and their trainees with regard to resident safety and wellness, resident education, and patient care (Table).

Resident Safety and Wellness
Role of the Program Director—The role of the program director is critical, serving as a figure of structure and reassurance.4,7,10 Once concern of disaster arises, the program director should contact the Designated Institutional Official (DIO) to express concerns about possible disruptions to resident training. The DIO should then contact the ACGME within 10 days to report the disaster and submit a request for emergency (eg, pandemic) or extraordinary circumstances (eg, natural disaster) categorization.4,9 Program directors should promptly prepare plans for program reconfiguration and resident transfers in alignment with ACGME requirements to maintain evaluation and completion of core competencies of training during disasters.9 Program directors should prioritize the safety of trainees during the immediate threat with clear guidelines on sheltering, evacuations, or quarantines; a timeline of program recovery based on communication with residents, faculty, and administration should then be established.10,11
Communication—Establishing a strong line of communication between program directors and residents is paramount. Collection of emergency noninstitutional contact information, establishment of a centralized website for information dissemination, use of noninstitutional email and proxy servers outside of the location of impact, social media updates, on-site use of 2-way radios, and program-wide conference calls when possible should be strongly considered as part of the disaster response.2-4,12,13
Resident Accommodations and Mental Health—If training is disrupted, residents should be reassured of continued access to salary, housing, food, or other resources as necessary.3,4,11 There should be clear contingency plans if residents need to leave the program for extended periods of time due to injury, illness, or personal circumstances. Although relevant in all types of disasters, resident mental health and response to trauma also must be addressed. Access to counseling, morale-building opportunities (eg, resident social events), and screening for depression or posttraumatic stress disorder may help promote well-being among residents following traumatic events.14
Resident Education
Participation in Disaster Relief—Residents may seek to aid in the disaster response, which may prove challenging in the setting of programs with high patient volume.4 In coordination with the ACGME and graduate medical education governing bodies, program directors should consider how residents may fulfill dermatology training requirements in conjunction with disaster relief efforts, such as working in an inpatient setting or providing wound care.10
Continued Didactic Education—The use of online learning and conference calls for continuing the dermatology curriculum is an efficient means to maintaining resident education when meeting in person poses risks to residents.15 Projections of microscopy images, clinical photographs, or other instructional materials allow for continued instruction on resident examination, histopathology, and diagnostic skills.
Continued Clinical Training—If the home institution cannot support the operation of dermatology clinics, residents should be guaranteed continued training at other institutions. Agreements with other dermatology programs, community hospitals, or private dermatology practices should be established in advance, with consideration given to the number of residents a program can support, funding transfers, and credentialing requirements.2,4,5
Prolonged Disruptions—Nonessential departments of medical institutions may cease to function during war or mass casualty disasters, and it may be unsafe to send dermatology residents to other institutions or clinical areas. If the threat is prolonged, programs may need to consider allowing current residents a longer duration of training despite potential overlap with incoming dermatology residents.7
Patient Care
Disruptions to Clinic Operations—Regarding threats of violence, dangerous exposures, or natural disasters, there should be clear guidelines on sheltering in the clinical setting or stabilizing patients during a procedure.11 Equipment used by residents such as laptops, microscopes, and treatment devices (eg, lasers) should be stored in weather-safe locations that would not be notably impacted by moisture or structural damage to the clinic building. If electricity or internet access are compromised, paper medical records should be available to residents to continue clinical operations. Electronic health records used by residents should regularly be backed up on remote servers or cloud storage to allow continued access to patient health information if on-site servers are not functional.12 If disruptions are prolonged, residency program administration should coordinate with the institution to ensure there is adequate supply and storage of medications (eg, lidocaine, botulinum toxin) as well as a continued means of delivering biologic medications to patients and an ability to obtain laboratory or dermatopathology services.
In-Person Appointments vs Telemedicine—There are benefits to both residency training and patient care when physicians are able to perform in-person examinations, biopsies, and in-office treatments.16 Programs should ensure an adequate supply of personal protective equipment to continue in-office appointments, vaccinations, and medical care if a resident or other members of the team are exposed to an infectious disease.7 If in-person appointments are limited or impossible, telemedicine capabilities may still allow residents to meet program requirements.7,10,15 However, reduced patient volume due to decreased elective visits or procedures may complicate the fulfillment of clinical requirements, which may need to be adjusted in the wake of a disaster.7
Use of Immunosuppressive Therapies—Residency programs should address the risks of prescribing immunosuppressive therapies (eg, biologics) during an infectious threat with their residents and encourage trainees to counsel patients on the importance of preventative measures to reduce risks for severe infection.17
Final Thoughts
- Davis W. Hurricane Katrina: the challenge to graduate medical education. Ochsner J. 2006;6:39.
- Cefalu CA, Schwartz RS. Salvaging a geriatric medicine academic program in disaster mode—the LSU training program post-Katrina.J Natl Med Assoc. 2007;99:590-596.
- Ayyala R. Lessons from Katrina: a program director’s perspective. Ophthalmology. 2007;114:1425-1426.
- Wiese JG. Leadership in graduate medical education: eleven steps instrumental in recovering residency programs after a disaster. Am J Med Sci. 2008;336:168-173.
- Griffies WS. Post-Katrina stabilization of the LSU/Ochsner Psychiatry Residency Program: caveats for disaster preparedness. Acad Psychiatry. 2009;33:418-422.
- Kearns DG, Chat VS, Uppal S, et al. Applying to dermatology residency during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:1214-1215.
- Matthews JB, Blair PG, Ellison EC, et al. Checklist framework for surgical education disaster plans. J Am Coll Surg. 2021;233:557-563.
- Litchman GH, Marson JW, Rigel DS. The continuing impact of COVID-19 on dermatology practice: office workflow, economics, and future implications. J Am Acad Dermatol. 2021;84:576-579.
- Accreditation Council for Graduate Medical Education. Sponsoring institution emergency categorization. Accessed October 20, 2022. https://www.acgme.org/covid-19/sponsoring-institution-emergency-categorization/
- Li YM, Galimberti F, Abrouk M, et al. US dermatology resident responses about the COVID-19 pandemic: results from a nationwide survey. South Med J. 2020;113:462-465.
- Newman B, Gallion C. Hurricane Harvey: firsthand perspectives for disaster preparedness in graduate medical education. Acad Med. 2019;94:1267-1269.
- Pero CD, Pou AM, Arriaga MA, et al. Post-Katrina: study in crisis-related program adaptability. Otolaryngol Head Neck Surg. 2008;138:394-397.
- Hattaway R, Singh N, Rais-Bahrami S, et al. Adaptations of dermatology residency programs to changes in medical education amid the COVID-19 pandemic: virtual opportunities and social media. SKIN. 2021;5:94-100.
- Hillier K, Paskaradevan J, Wilkes JK, et al. Disaster plans: resident involvement and well-being during Hurricane Harvey. J Grad Med Educ. 2019;11:129-131.
- Samimi S, Choi J, Rosman IS, et al. Impact of COVID-19 on dermatology residency. Dermatol Clin. 2021;39:609-618.
- Bastola M, Locatis C, Fontelo P. Diagnostic reliability of in-person versus remote dermatology: a meta-analysis. Telemed J E Health. 2021;27:247-250.
- Bashyam AM, Feldman SR. Should patients stop their biologic treatment during the COVID-19 pandemic? J Dermatolog Treat. 2020;31:317-318.
- Davis W. Hurricane Katrina: the challenge to graduate medical education. Ochsner J. 2006;6:39.
- Cefalu CA, Schwartz RS. Salvaging a geriatric medicine academic program in disaster mode—the LSU training program post-Katrina.J Natl Med Assoc. 2007;99:590-596.
- Ayyala R. Lessons from Katrina: a program director’s perspective. Ophthalmology. 2007;114:1425-1426.
- Wiese JG. Leadership in graduate medical education: eleven steps instrumental in recovering residency programs after a disaster. Am J Med Sci. 2008;336:168-173.
- Griffies WS. Post-Katrina stabilization of the LSU/Ochsner Psychiatry Residency Program: caveats for disaster preparedness. Acad Psychiatry. 2009;33:418-422.
- Kearns DG, Chat VS, Uppal S, et al. Applying to dermatology residency during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:1214-1215.
- Matthews JB, Blair PG, Ellison EC, et al. Checklist framework for surgical education disaster plans. J Am Coll Surg. 2021;233:557-563.
- Litchman GH, Marson JW, Rigel DS. The continuing impact of COVID-19 on dermatology practice: office workflow, economics, and future implications. J Am Acad Dermatol. 2021;84:576-579.
- Accreditation Council for Graduate Medical Education. Sponsoring institution emergency categorization. Accessed October 20, 2022. https://www.acgme.org/covid-19/sponsoring-institution-emergency-categorization/
- Li YM, Galimberti F, Abrouk M, et al. US dermatology resident responses about the COVID-19 pandemic: results from a nationwide survey. South Med J. 2020;113:462-465.
- Newman B, Gallion C. Hurricane Harvey: firsthand perspectives for disaster preparedness in graduate medical education. Acad Med. 2019;94:1267-1269.
- Pero CD, Pou AM, Arriaga MA, et al. Post-Katrina: study in crisis-related program adaptability. Otolaryngol Head Neck Surg. 2008;138:394-397.
- Hattaway R, Singh N, Rais-Bahrami S, et al. Adaptations of dermatology residency programs to changes in medical education amid the COVID-19 pandemic: virtual opportunities and social media. SKIN. 2021;5:94-100.
- Hillier K, Paskaradevan J, Wilkes JK, et al. Disaster plans: resident involvement and well-being during Hurricane Harvey. J Grad Med Educ. 2019;11:129-131.
- Samimi S, Choi J, Rosman IS, et al. Impact of COVID-19 on dermatology residency. Dermatol Clin. 2021;39:609-618.
- Bastola M, Locatis C, Fontelo P. Diagnostic reliability of in-person versus remote dermatology: a meta-analysis. Telemed J E Health. 2021;27:247-250.
- Bashyam AM, Feldman SR. Should patients stop their biologic treatment during the COVID-19 pandemic? J Dermatolog Treat. 2020;31:317-318.
Practice Points
- Dermatology residency programs should prioritize the development of disaster preparedness plans prior to the onset of disasters.
- Comprehensive disaster preparedness addresses many possible disruptions to dermatology resident training and clinic operations, including natural and manmade disasters and threats of widespread infectious disease.
- Safety being paramount, dermatology residency programs may be tasked with maintaining resident wellness, continuing resident education—potentially in unconventional ways—and adapting clinical operations to continue patient care.
Simplify Postoperative Self-removal of Bandages for Isolated Patients With Limited Range of Motion Using Pull Tabs
Practice Gap
A male patient presented with 2 concerning lesions, which histopathology revealed were invasive squamous cell carcinoma (SCC) on the right medial chest and SCC in situ on the right upper scapular region. Both were treated with wide local excision; margins were clear in our office the same day.
This case highlighted a practice gap in postoperative care. Two factors posed a challenge to proper postoperative wound care for our patient:
• Because of the high risk of transmission of SARS-CoV-2, the patient hoped to limit exposure by avoiding an office visit to remove the bandage.
• The patient did not have someone at home to serve as an immediate support system, which made it impossible for him to rely on others for postoperative wound care.
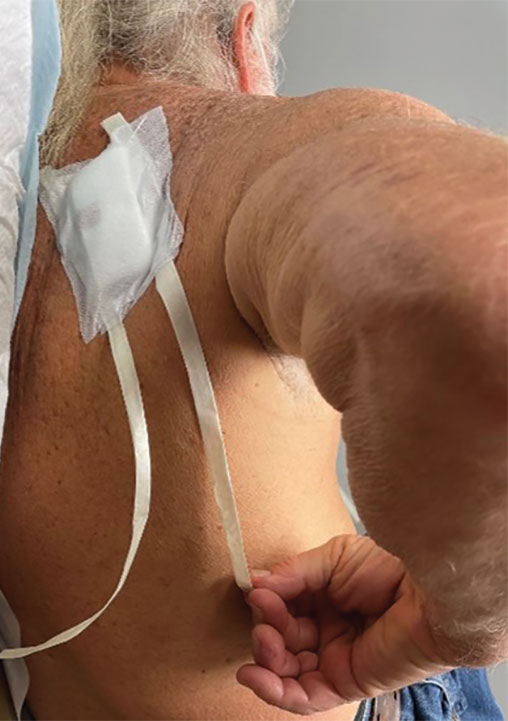
Previously, the patient had to ask a friend to remove a bandage for melanoma in situ on the inner aspect of the left upper arm. Therefore, after this procedure, the patient asked if the bandage could be fashioned in a manner that would allow him to remove it without assistance (Figure 1).
Technique
In constructing a bandage that is easier to remove, some necessary pressure that is provided by the bandage often is sacrificed by making it looser. Considering that our patient had moderate bleeding during the procedure—in part because he took low-dose aspirin (81 mg/d)—it was important to maintain firm pressure under the bandage postoperatively to help prevent untoward bleeding. Furthermore, because of the location of the treated site and the patient’s limited range of motion, it was not feasible for him to reach the area on the scapula and remove the bandage.1
For easy self-removal, we designed a bandage with a pull tab that was within the patient’s reach. Suitable materials for the pull tab bandage included surgical tape, bandaging tape with adequate stretch, sterile nonadhesive gauze, fenestrated surgical gauze, and a topical emollient such as petroleum jelly or antibacterial ointment.
To clean the site and decrease the amount of oil that would reduce the effectiveness of the adhesive, the wound was prepared with 70% alcohol. The site was then treated with petroleum jelly.
Next, we designed 2 pull tab bandage prototypes that allowed easy self-removal. For both prototypes, sterile nonadhesive gauze was applied to the wound along with folded and fenestrated gauze, which provided pressure. We used prototype #1 in our patient, and prototype #2 was demonstrated as an option.
Prototype #1—We created 2 tabs—each 2-feet long—using bandaging tape that was folded on itself once horizontally (Figure 2). The tabs were aligned on either side of the wound, the tops of which sat approximately 2 inches above the top of the first layer of adhesive bandage. An initial layer of adhesive surgical dressing was applied to cover the wound; 1 inch of the dressing was left exposed on the top of each tab. In addition, there were 2 “feet” running on the bottom.
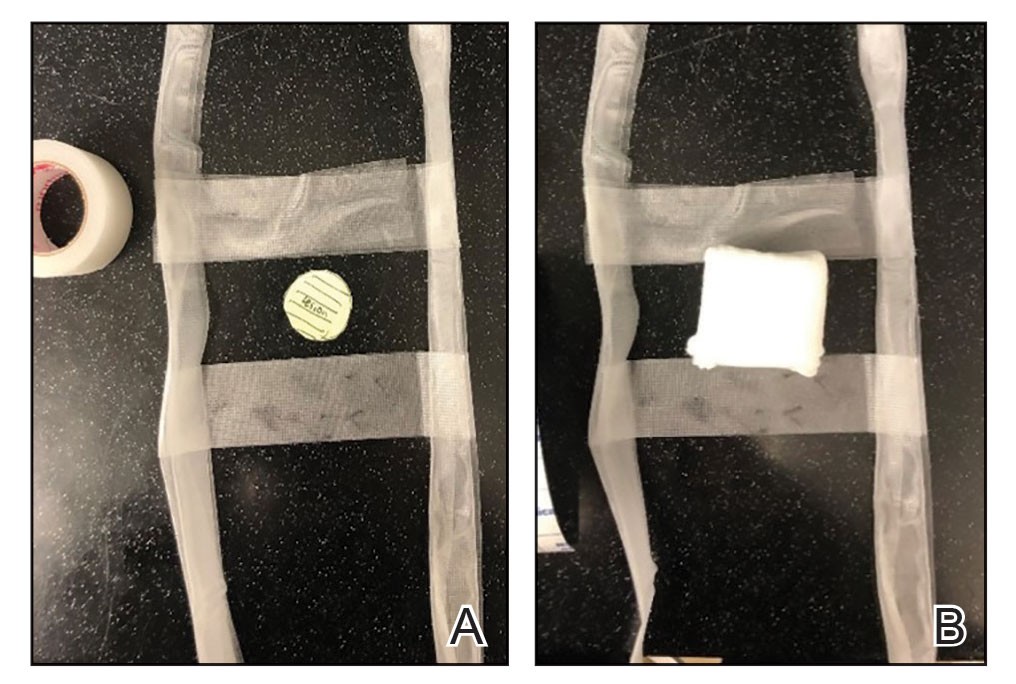
The tops of the tabs were folded back over the adhesive tape, creating a type of “hook.” An additional final layer of adhesive tape was applied to ensure adequate pressure on the surgical site.
The patient was instructed to remove the bandage 2 days after the procedure. The outcome was qualified through a 3-day postoperative telephone call. The patient was asked about postoperative pain and his level of satisfaction with treatment. He was asked if he had any changes such as bleeding, swelling, signs of infection, or increased pain in the days after surgery or perceived postoperative complications, such as irritation. We asked the patient about the relative ease of removing the bandage and if removal was painful. He reported that the bandage was easy to remove, and that doing so was not painful; furthermore, he did not have problems with the bandage or healing and did not experience any medical changes. He found the bandage to be comfortable. The patient stated that the hanging feet of the prototype #1 bandage were not bothersome and were sturdy for the time that the bandage was on.
Prototype #2—We prepared a bandage using surgical packing as the tab (Figure 3). The packing was slowly placed around the site, which was already covered with nonadhesive gauze and fenestrated surgical gauze, with adequate spacing between each loop (for a total of 3 loops), 1 of which crossed over the third loop so that the adhesive bandaging tape could be removed easily. This allowed for a single tab that could be removed by a single pull. A final layer of adhesive tape was applied to ensure adequate pressure, similar to prototype #1. The same postoperative protocol was employed to provide a consistent standard of care. We recommend use of this prototype when surgical tape is not available, and surgical packing can be used as a substitute.

Practice Implications
Patients have a better appreciation for avoiding excess visits to medical offices due to the COVID-19 pandemic. The risk for exposure to SARS-CoV-2 infection is greater when patients who lack a support system must return to the office for aftercare or to have a bandage removed. Although protection offered by the COVID-19 vaccine alleviates concern, many patients have realized the benefits of only visiting medical offices in person when necessary.
The concept of pull tab bandages that can be removed by the patient at home has other applications. For example, patients who travel a long distance to see their physician will benefit from easier aftercare and avoid additional follow-up visits when provided with a self-removable bandage.
- Stathokostas, L, McDonald MW, Little RMD, et al. Flexibility of older adults aged 55-86 years and the influence of physical activity. J Aging Res. 2013;2013:1-8. doi:10.1155/2013/743843
Practice Gap
A male patient presented with 2 concerning lesions, which histopathology revealed were invasive squamous cell carcinoma (SCC) on the right medial chest and SCC in situ on the right upper scapular region. Both were treated with wide local excision; margins were clear in our office the same day.
This case highlighted a practice gap in postoperative care. Two factors posed a challenge to proper postoperative wound care for our patient:
• Because of the high risk of transmission of SARS-CoV-2, the patient hoped to limit exposure by avoiding an office visit to remove the bandage.
• The patient did not have someone at home to serve as an immediate support system, which made it impossible for him to rely on others for postoperative wound care.
Previously, the patient had to ask a friend to remove a bandage for melanoma in situ on the inner aspect of the left upper arm. Therefore, after this procedure, the patient asked if the bandage could be fashioned in a manner that would allow him to remove it without assistance (Figure 1).

Technique
In constructing a bandage that is easier to remove, some necessary pressure that is provided by the bandage often is sacrificed by making it looser. Considering that our patient had moderate bleeding during the procedure—in part because he took low-dose aspirin (81 mg/d)—it was important to maintain firm pressure under the bandage postoperatively to help prevent untoward bleeding. Furthermore, because of the location of the treated site and the patient’s limited range of motion, it was not feasible for him to reach the area on the scapula and remove the bandage.1
For easy self-removal, we designed a bandage with a pull tab that was within the patient’s reach. Suitable materials for the pull tab bandage included surgical tape, bandaging tape with adequate stretch, sterile nonadhesive gauze, fenestrated surgical gauze, and a topical emollient such as petroleum jelly or antibacterial ointment.
To clean the site and decrease the amount of oil that would reduce the effectiveness of the adhesive, the wound was prepared with 70% alcohol. The site was then treated with petroleum jelly.
Next, we designed 2 pull tab bandage prototypes that allowed easy self-removal. For both prototypes, sterile nonadhesive gauze was applied to the wound along with folded and fenestrated gauze, which provided pressure. We used prototype #1 in our patient, and prototype #2 was demonstrated as an option.
Prototype #1—We created 2 tabs—each 2-feet long—using bandaging tape that was folded on itself once horizontally (Figure 2). The tabs were aligned on either side of the wound, the tops of which sat approximately 2 inches above the top of the first layer of adhesive bandage. An initial layer of adhesive surgical dressing was applied to cover the wound; 1 inch of the dressing was left exposed on the top of each tab. In addition, there were 2 “feet” running on the bottom.

The tops of the tabs were folded back over the adhesive tape, creating a type of “hook.” An additional final layer of adhesive tape was applied to ensure adequate pressure on the surgical site.
The patient was instructed to remove the bandage 2 days after the procedure. The outcome was qualified through a 3-day postoperative telephone call. The patient was asked about postoperative pain and his level of satisfaction with treatment. He was asked if he had any changes such as bleeding, swelling, signs of infection, or increased pain in the days after surgery or perceived postoperative complications, such as irritation. We asked the patient about the relative ease of removing the bandage and if removal was painful. He reported that the bandage was easy to remove, and that doing so was not painful; furthermore, he did not have problems with the bandage or healing and did not experience any medical changes. He found the bandage to be comfortable. The patient stated that the hanging feet of the prototype #1 bandage were not bothersome and were sturdy for the time that the bandage was on.
Prototype #2—We prepared a bandage using surgical packing as the tab (Figure 3). The packing was slowly placed around the site, which was already covered with nonadhesive gauze and fenestrated surgical gauze, with adequate spacing between each loop (for a total of 3 loops), 1 of which crossed over the third loop so that the adhesive bandaging tape could be removed easily. This allowed for a single tab that could be removed by a single pull. A final layer of adhesive tape was applied to ensure adequate pressure, similar to prototype #1. The same postoperative protocol was employed to provide a consistent standard of care. We recommend use of this prototype when surgical tape is not available, and surgical packing can be used as a substitute.

Practice Implications
Patients have a better appreciation for avoiding excess visits to medical offices due to the COVID-19 pandemic. The risk for exposure to SARS-CoV-2 infection is greater when patients who lack a support system must return to the office for aftercare or to have a bandage removed. Although protection offered by the COVID-19 vaccine alleviates concern, many patients have realized the benefits of only visiting medical offices in person when necessary.
The concept of pull tab bandages that can be removed by the patient at home has other applications. For example, patients who travel a long distance to see their physician will benefit from easier aftercare and avoid additional follow-up visits when provided with a self-removable bandage.
Practice Gap
A male patient presented with 2 concerning lesions, which histopathology revealed were invasive squamous cell carcinoma (SCC) on the right medial chest and SCC in situ on the right upper scapular region. Both were treated with wide local excision; margins were clear in our office the same day.
This case highlighted a practice gap in postoperative care. Two factors posed a challenge to proper postoperative wound care for our patient:
• Because of the high risk of transmission of SARS-CoV-2, the patient hoped to limit exposure by avoiding an office visit to remove the bandage.
• The patient did not have someone at home to serve as an immediate support system, which made it impossible for him to rely on others for postoperative wound care.
Previously, the patient had to ask a friend to remove a bandage for melanoma in situ on the inner aspect of the left upper arm. Therefore, after this procedure, the patient asked if the bandage could be fashioned in a manner that would allow him to remove it without assistance (Figure 1).

Technique
In constructing a bandage that is easier to remove, some necessary pressure that is provided by the bandage often is sacrificed by making it looser. Considering that our patient had moderate bleeding during the procedure—in part because he took low-dose aspirin (81 mg/d)—it was important to maintain firm pressure under the bandage postoperatively to help prevent untoward bleeding. Furthermore, because of the location of the treated site and the patient’s limited range of motion, it was not feasible for him to reach the area on the scapula and remove the bandage.1
For easy self-removal, we designed a bandage with a pull tab that was within the patient’s reach. Suitable materials for the pull tab bandage included surgical tape, bandaging tape with adequate stretch, sterile nonadhesive gauze, fenestrated surgical gauze, and a topical emollient such as petroleum jelly or antibacterial ointment.
To clean the site and decrease the amount of oil that would reduce the effectiveness of the adhesive, the wound was prepared with 70% alcohol. The site was then treated with petroleum jelly.
Next, we designed 2 pull tab bandage prototypes that allowed easy self-removal. For both prototypes, sterile nonadhesive gauze was applied to the wound along with folded and fenestrated gauze, which provided pressure. We used prototype #1 in our patient, and prototype #2 was demonstrated as an option.
Prototype #1—We created 2 tabs—each 2-feet long—using bandaging tape that was folded on itself once horizontally (Figure 2). The tabs were aligned on either side of the wound, the tops of which sat approximately 2 inches above the top of the first layer of adhesive bandage. An initial layer of adhesive surgical dressing was applied to cover the wound; 1 inch of the dressing was left exposed on the top of each tab. In addition, there were 2 “feet” running on the bottom.

The tops of the tabs were folded back over the adhesive tape, creating a type of “hook.” An additional final layer of adhesive tape was applied to ensure adequate pressure on the surgical site.
The patient was instructed to remove the bandage 2 days after the procedure. The outcome was qualified through a 3-day postoperative telephone call. The patient was asked about postoperative pain and his level of satisfaction with treatment. He was asked if he had any changes such as bleeding, swelling, signs of infection, or increased pain in the days after surgery or perceived postoperative complications, such as irritation. We asked the patient about the relative ease of removing the bandage and if removal was painful. He reported that the bandage was easy to remove, and that doing so was not painful; furthermore, he did not have problems with the bandage or healing and did not experience any medical changes. He found the bandage to be comfortable. The patient stated that the hanging feet of the prototype #1 bandage were not bothersome and were sturdy for the time that the bandage was on.
Prototype #2—We prepared a bandage using surgical packing as the tab (Figure 3). The packing was slowly placed around the site, which was already covered with nonadhesive gauze and fenestrated surgical gauze, with adequate spacing between each loop (for a total of 3 loops), 1 of which crossed over the third loop so that the adhesive bandaging tape could be removed easily. This allowed for a single tab that could be removed by a single pull. A final layer of adhesive tape was applied to ensure adequate pressure, similar to prototype #1. The same postoperative protocol was employed to provide a consistent standard of care. We recommend use of this prototype when surgical tape is not available, and surgical packing can be used as a substitute.

Practice Implications
Patients have a better appreciation for avoiding excess visits to medical offices due to the COVID-19 pandemic. The risk for exposure to SARS-CoV-2 infection is greater when patients who lack a support system must return to the office for aftercare or to have a bandage removed. Although protection offered by the COVID-19 vaccine alleviates concern, many patients have realized the benefits of only visiting medical offices in person when necessary.
The concept of pull tab bandages that can be removed by the patient at home has other applications. For example, patients who travel a long distance to see their physician will benefit from easier aftercare and avoid additional follow-up visits when provided with a self-removable bandage.
- Stathokostas, L, McDonald MW, Little RMD, et al. Flexibility of older adults aged 55-86 years and the influence of physical activity. J Aging Res. 2013;2013:1-8. doi:10.1155/2013/743843
- Stathokostas, L, McDonald MW, Little RMD, et al. Flexibility of older adults aged 55-86 years and the influence of physical activity. J Aging Res. 2013;2013:1-8. doi:10.1155/2013/743843
What does it take for men to embrace cosmetic treatments?
with the same gusto as women.
However, this could be changing as millennials – who tend to be more proactive about efforts to prevent skin aging – are getting older.
At a virtual course on laser and aesthetic skin therapy, Dr. Carruthers referred to the results of an online survey of 600 men aged 30-65 years conducted by Jared Jagdeo, MD, and colleagues in 2016, to gauge attitudes toward age-related changes of their facial features and their preferences for prioritizing treatment. The top five barriers to treatment cited by the respondents were: “I don’t think I need it yet” (47%); “concerned about safety/side effects” (46%); “concerned about injecting a foreign substance into my body” (45%); “cost” (42%), and “concerned my face won’t look natural” (41%).
“Since then, millennials took over as the largest portion of our workforce in North America,” said Dr. Carruthers who, with her husband, Alastair Carruthers, MD, pioneered the cosmetic use of onabotulinumtoxinA (Botox). “Millennials are interested in how they look and how to keep their aesthetic the best it can possibly be,” she said, so there may be “a generational aspect to this.”
Another factor that may affect the uptake of cosmetic procedures among men is the number of hours they spend gazing at their own image on a computer screen. Since the beginning of the COVID-19 pandemic, men have spent an increasing number of hours on video-conferencing calls via Zoom and other platforms, causing them to rethink how they view their appearance, Dr. Carruthers added. “Zoom dysmorphia” is the term that describes the phenomenon that developed during the pandemic where more patients expressed a desire to make changes to their appearance, including nose jobs and smoothing out forehead wrinkles.
“When we’re on a Zoom call, we’re spending 40% of our time looking at ourselves,” said Dr. Carruthers, clinical professor of ophthalmology and visual sciences at the University of British Columbia in Vancouver. “This would hint that the looking glass is not as powerful as the computer screen to motivate men” to pursue aesthetic treatments.
According to data from the American Society of Plastic Surgeons, the top 5 cosmetic surgical procedures performed in men in 2020 were nose shaping, eyelid surgery, cheek implants, liposuction, and ear surgery. The top 5 minimally-invasive procedures in men were botulinum toxin type A, followed by laser skin resurfacing, laser hair removal, soft tissue fillers, and microdermabrasion.
Why might men consider an injectable instead of surgery? Dr. Carruthers asked. “According to the 2016 survey by Dr. Jagdeo and colleagues, it’s to appear more youthful and to appear good for their age.”
From a clinical standpoint, success comes from understanding the subtle differences between treating men and women, she added.
In a 2022 article about optimizing skin tightening in aesthetics in men, Christian A. Albornoz, MD, and colleagues noted that in contrast to women, men “tend to have higher levels of collagen density and greater skin thickness, but these begin to decrease earlier on. They can also more frequently have severe photodamage”.
In another article published in 2018, Terrence Keaney, MD, and colleagues reviewed the objective data available on male aging and aesthetics. They stated that a “communication gap exists for men, as evidenced by the lack of information available online or by word of mouth about injectable treatments” and concluded that “educating men about available aesthetic treatments and about the safety and side effects associated with each treatment, as well as addressing concerns about their treatment results looking natural, are key considerations.”
That sentiment resonates with Dr. Carruthers. Part of the reason why men have not sought cosmetic treatments along with their female partners and friends seeking cosmetic treatments “is that they haven’t had anything in their cup,” she said. “Maybe this is something we need to think about, to try and help men come in and enjoy the positive benefits of aesthetic, noninvasive cosmetic treatments.”
The course was sponsored by Harvard Medical School, Massachusetts General Hospital, and Wellman Center for Photomedicine.
Dr. Carruthers disclosed that she is a consultant and researcher for Alastin, Appiell, Allergan Aesthetics, Avari Medical, Bonti, Evolus, Fount Bio, Jeune Aesthetics, Merz, and Revance Biopharma.
with the same gusto as women.
However, this could be changing as millennials – who tend to be more proactive about efforts to prevent skin aging – are getting older.
At a virtual course on laser and aesthetic skin therapy, Dr. Carruthers referred to the results of an online survey of 600 men aged 30-65 years conducted by Jared Jagdeo, MD, and colleagues in 2016, to gauge attitudes toward age-related changes of their facial features and their preferences for prioritizing treatment. The top five barriers to treatment cited by the respondents were: “I don’t think I need it yet” (47%); “concerned about safety/side effects” (46%); “concerned about injecting a foreign substance into my body” (45%); “cost” (42%), and “concerned my face won’t look natural” (41%).
“Since then, millennials took over as the largest portion of our workforce in North America,” said Dr. Carruthers who, with her husband, Alastair Carruthers, MD, pioneered the cosmetic use of onabotulinumtoxinA (Botox). “Millennials are interested in how they look and how to keep their aesthetic the best it can possibly be,” she said, so there may be “a generational aspect to this.”
Another factor that may affect the uptake of cosmetic procedures among men is the number of hours they spend gazing at their own image on a computer screen. Since the beginning of the COVID-19 pandemic, men have spent an increasing number of hours on video-conferencing calls via Zoom and other platforms, causing them to rethink how they view their appearance, Dr. Carruthers added. “Zoom dysmorphia” is the term that describes the phenomenon that developed during the pandemic where more patients expressed a desire to make changes to their appearance, including nose jobs and smoothing out forehead wrinkles.
“When we’re on a Zoom call, we’re spending 40% of our time looking at ourselves,” said Dr. Carruthers, clinical professor of ophthalmology and visual sciences at the University of British Columbia in Vancouver. “This would hint that the looking glass is not as powerful as the computer screen to motivate men” to pursue aesthetic treatments.
According to data from the American Society of Plastic Surgeons, the top 5 cosmetic surgical procedures performed in men in 2020 were nose shaping, eyelid surgery, cheek implants, liposuction, and ear surgery. The top 5 minimally-invasive procedures in men were botulinum toxin type A, followed by laser skin resurfacing, laser hair removal, soft tissue fillers, and microdermabrasion.
Why might men consider an injectable instead of surgery? Dr. Carruthers asked. “According to the 2016 survey by Dr. Jagdeo and colleagues, it’s to appear more youthful and to appear good for their age.”
From a clinical standpoint, success comes from understanding the subtle differences between treating men and women, she added.
In a 2022 article about optimizing skin tightening in aesthetics in men, Christian A. Albornoz, MD, and colleagues noted that in contrast to women, men “tend to have higher levels of collagen density and greater skin thickness, but these begin to decrease earlier on. They can also more frequently have severe photodamage”.
In another article published in 2018, Terrence Keaney, MD, and colleagues reviewed the objective data available on male aging and aesthetics. They stated that a “communication gap exists for men, as evidenced by the lack of information available online or by word of mouth about injectable treatments” and concluded that “educating men about available aesthetic treatments and about the safety and side effects associated with each treatment, as well as addressing concerns about their treatment results looking natural, are key considerations.”
That sentiment resonates with Dr. Carruthers. Part of the reason why men have not sought cosmetic treatments along with their female partners and friends seeking cosmetic treatments “is that they haven’t had anything in their cup,” she said. “Maybe this is something we need to think about, to try and help men come in and enjoy the positive benefits of aesthetic, noninvasive cosmetic treatments.”
The course was sponsored by Harvard Medical School, Massachusetts General Hospital, and Wellman Center for Photomedicine.
Dr. Carruthers disclosed that she is a consultant and researcher for Alastin, Appiell, Allergan Aesthetics, Avari Medical, Bonti, Evolus, Fount Bio, Jeune Aesthetics, Merz, and Revance Biopharma.
with the same gusto as women.
However, this could be changing as millennials – who tend to be more proactive about efforts to prevent skin aging – are getting older.
At a virtual course on laser and aesthetic skin therapy, Dr. Carruthers referred to the results of an online survey of 600 men aged 30-65 years conducted by Jared Jagdeo, MD, and colleagues in 2016, to gauge attitudes toward age-related changes of their facial features and their preferences for prioritizing treatment. The top five barriers to treatment cited by the respondents were: “I don’t think I need it yet” (47%); “concerned about safety/side effects” (46%); “concerned about injecting a foreign substance into my body” (45%); “cost” (42%), and “concerned my face won’t look natural” (41%).
“Since then, millennials took over as the largest portion of our workforce in North America,” said Dr. Carruthers who, with her husband, Alastair Carruthers, MD, pioneered the cosmetic use of onabotulinumtoxinA (Botox). “Millennials are interested in how they look and how to keep their aesthetic the best it can possibly be,” she said, so there may be “a generational aspect to this.”
Another factor that may affect the uptake of cosmetic procedures among men is the number of hours they spend gazing at their own image on a computer screen. Since the beginning of the COVID-19 pandemic, men have spent an increasing number of hours on video-conferencing calls via Zoom and other platforms, causing them to rethink how they view their appearance, Dr. Carruthers added. “Zoom dysmorphia” is the term that describes the phenomenon that developed during the pandemic where more patients expressed a desire to make changes to their appearance, including nose jobs and smoothing out forehead wrinkles.
“When we’re on a Zoom call, we’re spending 40% of our time looking at ourselves,” said Dr. Carruthers, clinical professor of ophthalmology and visual sciences at the University of British Columbia in Vancouver. “This would hint that the looking glass is not as powerful as the computer screen to motivate men” to pursue aesthetic treatments.
According to data from the American Society of Plastic Surgeons, the top 5 cosmetic surgical procedures performed in men in 2020 were nose shaping, eyelid surgery, cheek implants, liposuction, and ear surgery. The top 5 minimally-invasive procedures in men were botulinum toxin type A, followed by laser skin resurfacing, laser hair removal, soft tissue fillers, and microdermabrasion.
Why might men consider an injectable instead of surgery? Dr. Carruthers asked. “According to the 2016 survey by Dr. Jagdeo and colleagues, it’s to appear more youthful and to appear good for their age.”
From a clinical standpoint, success comes from understanding the subtle differences between treating men and women, she added.
In a 2022 article about optimizing skin tightening in aesthetics in men, Christian A. Albornoz, MD, and colleagues noted that in contrast to women, men “tend to have higher levels of collagen density and greater skin thickness, but these begin to decrease earlier on. They can also more frequently have severe photodamage”.
In another article published in 2018, Terrence Keaney, MD, and colleagues reviewed the objective data available on male aging and aesthetics. They stated that a “communication gap exists for men, as evidenced by the lack of information available online or by word of mouth about injectable treatments” and concluded that “educating men about available aesthetic treatments and about the safety and side effects associated with each treatment, as well as addressing concerns about their treatment results looking natural, are key considerations.”
That sentiment resonates with Dr. Carruthers. Part of the reason why men have not sought cosmetic treatments along with their female partners and friends seeking cosmetic treatments “is that they haven’t had anything in their cup,” she said. “Maybe this is something we need to think about, to try and help men come in and enjoy the positive benefits of aesthetic, noninvasive cosmetic treatments.”
The course was sponsored by Harvard Medical School, Massachusetts General Hospital, and Wellman Center for Photomedicine.
Dr. Carruthers disclosed that she is a consultant and researcher for Alastin, Appiell, Allergan Aesthetics, Avari Medical, Bonti, Evolus, Fount Bio, Jeune Aesthetics, Merz, and Revance Biopharma.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
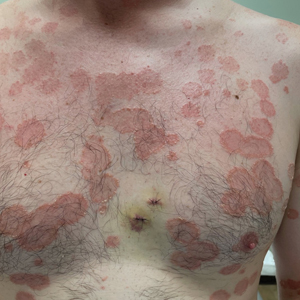
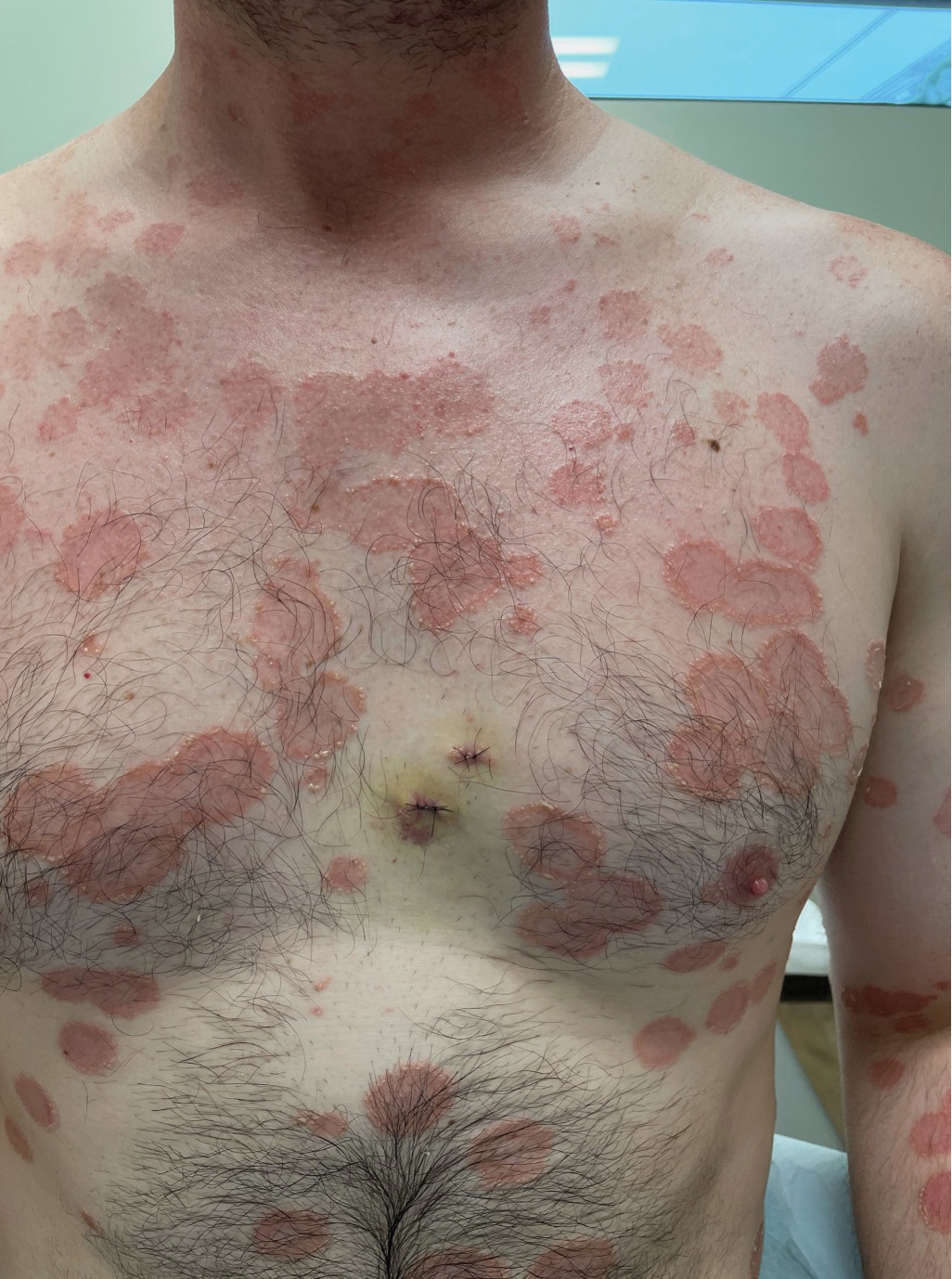
Painful and Pruritic Eruptions on the Entire Body
The Diagnosis: IgA Pemphigus
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.

IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
The Diagnosis: IgA Pemphigus
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.

IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
The Diagnosis: IgA Pemphigus
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.

IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
A 36-year-old man presented with painful tender blisters and rashes on the entire body, including the ears and tongue. The rash began as a few pinpointed red dots on the abdomen, which subsequently increased in size and spread over the last week. He initially felt red and flushed and noticed new lesions appearing throughout the day. He did not attempt any specific treatment for these lesions. The patient tested positive for COVID-19 four months prior to the skin eruption. He denied systemic symptoms, smoking, or recent travel. He had no history of skin cancer, skin disorders, HIV, or hepatitis. He had no known medication allergies. Physical examination revealed multiple disseminated pustules on the ears, superficial ulcerations on the tongue, and blisters on the right lip. Few lesions were tender to the touch and drained clear fluid. Bacterial, viral, HIV, herpes, and rapid plasma reagin culture and laboratory screenings were negative. He was started on valaciclovir and cephalexin; however, no improvement was noticed. Punch biopsies were taken from the blisters on the chest and perilesional area.
HPV vaccine effectiveness dependent on age at receipt
The effectiveness of the human papillomavirus (HPV) vaccine against HPV types 16 and 18 is highly dependent on the age at which it is given. Prevalence rates have been shown to be significantly lower among girls who are vaccinated at the recommended ages of 9-12 years, compared with those who are vaccinated after their sexual debut, data from the National Health and Nutrition Examination Survey (NHANES) indicate.
“HPV vaccination does not have any therapeutic effect on HPV infections already acquired, which is more likely to explain the difference in prevalence between predebut versus postdebut recipients than a lower immune response [among older recipients],” lead study author Didem Egemen, PhD, National Cancer Institute, Rockville, Md., told this news organization in an email.
“Still, among older females, the immune response of the vaccine is likely to still be quite strong, and we would encourage vaccination [of female patients] if unvaccinated, as our paper showed that vaccination post debut will still reduce HPV 16/18 prevalence by half,” she added.
The research letter was published online in JAMA Network Open.
National sample evaluated
Using data from NHANES, a biennial, cross-sectional sample (cycles 2011 through 2018), the researchers identified female persons who were aged 26 years or younger in 2006, when HPV vaccination was introduced, and who were eligible for routine vaccination or “catch-up” vaccination (given between the ages of 13 and 26 years), as per recommendations from the Advisory Committee on Immunization Practices. The investigators then compared the prevalence of HPV types 16 and 18 among unvaccinated female patients, female patients who had been vaccinated prior to their sexual debut (predebut group), and those who had been vaccinated after their sexual debut (postdebut group).
They also estimated vaccine uptake among those who were eligible for routine vaccination, as well as the proportion of vaccinated female patients with respect to racial and ethnic subgroups.
In the overall cohort, the prevalence of HPV types 16 and 18 decreased by 6% (95% confidence interval, 4%-7%) in the unvaccinated group to 3% (95% CI, 1%-6%) in the postdebut group and to less than 1% (95% CI, <1%-1%) in the predebut group, Dr. Egemen and colleagues report.
In real percentages, the prevalence of HPV 16 and 18 was 89% lower in the predebut group (P < .001) but only 41% lower in the postdebut group (P = .29) compared with unvaccinated female patients. And compared with female patients who were vaccinated after their sexual debut, the prevalence of HPV 16 and 18 was reduced by 82% among those who had received the vaccine at the recommended ages of 9-12 years (P = .08).
In the current study, Dr. Egeman acknowledged that only 38% of ever-eligible female patients received the vaccine, although the prevalence increased to 56% when only female patients who were eligible for routine vaccination were taken into account. On the other hand, only 21% (95% CI, 14%-28%) of female patients eligible for routine vaccination received their first dose by age 12 years.
Indeed, the mean age on receipt of the first vaccination dose was 14.5 years (95% CI, 14.1-14.8 years), the authors note, and only 59% of girls received their first dose prior to their sexual debut. Additionally, among routine vaccination–eligible girls aged 12 years or younger in 2006, 33% were vaccinated before and 23% after their sexual debut, and the rest were not vaccinated.
Interestingly, differences in the age at which the HPV vaccine was received by race and ethnicity were negligible, the investigators point out.
Vaccination rates increasing
Asked to comment on the findings, Rebecca Perkins, MD, professor of obstetrics and gynecology at Boston University, Boston Medical Center, pointed out that the investigators evaluated data from 2011 to 2018. “We know that HPV vaccination rates have increased over that period and continue to increase,” she emphasized in an email to this news organization.
Physicians also know that more persons are being vaccinated between the ages of 9 and 12 than was the case at the beginning of this study. “This is good news,” she said, “as it means that more adolescents now in 2022 are benefiting fully from vaccination than they were in 2011,” she added.
At the same time, Dr. Perkins acknowledged that many persons are still missing out on the chance to receive the vaccine on time – which means they are missing out on the chance to prevent cancer.
“Making sure that all adolescents receive vaccination between the ages of 9 to 12 has the potential to prevent up to 40,000 cancers every year in the U.S., [including] the most common HPV-related cancers, such as cervical cancer in women and tongue and tonsillar cancer in men,” Dr. Perkins noted.
“Thus, it’s critical that doctors and parents get the message that you can’t vaccinate too early, only too late,” she emphasized.
Dr. Edgman and Dr. Perkins report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The effectiveness of the human papillomavirus (HPV) vaccine against HPV types 16 and 18 is highly dependent on the age at which it is given. Prevalence rates have been shown to be significantly lower among girls who are vaccinated at the recommended ages of 9-12 years, compared with those who are vaccinated after their sexual debut, data from the National Health and Nutrition Examination Survey (NHANES) indicate.
“HPV vaccination does not have any therapeutic effect on HPV infections already acquired, which is more likely to explain the difference in prevalence between predebut versus postdebut recipients than a lower immune response [among older recipients],” lead study author Didem Egemen, PhD, National Cancer Institute, Rockville, Md., told this news organization in an email.
“Still, among older females, the immune response of the vaccine is likely to still be quite strong, and we would encourage vaccination [of female patients] if unvaccinated, as our paper showed that vaccination post debut will still reduce HPV 16/18 prevalence by half,” she added.
The research letter was published online in JAMA Network Open.
National sample evaluated
Using data from NHANES, a biennial, cross-sectional sample (cycles 2011 through 2018), the researchers identified female persons who were aged 26 years or younger in 2006, when HPV vaccination was introduced, and who were eligible for routine vaccination or “catch-up” vaccination (given between the ages of 13 and 26 years), as per recommendations from the Advisory Committee on Immunization Practices. The investigators then compared the prevalence of HPV types 16 and 18 among unvaccinated female patients, female patients who had been vaccinated prior to their sexual debut (predebut group), and those who had been vaccinated after their sexual debut (postdebut group).
They also estimated vaccine uptake among those who were eligible for routine vaccination, as well as the proportion of vaccinated female patients with respect to racial and ethnic subgroups.
In the overall cohort, the prevalence of HPV types 16 and 18 decreased by 6% (95% confidence interval, 4%-7%) in the unvaccinated group to 3% (95% CI, 1%-6%) in the postdebut group and to less than 1% (95% CI, <1%-1%) in the predebut group, Dr. Egemen and colleagues report.
In real percentages, the prevalence of HPV 16 and 18 was 89% lower in the predebut group (P < .001) but only 41% lower in the postdebut group (P = .29) compared with unvaccinated female patients. And compared with female patients who were vaccinated after their sexual debut, the prevalence of HPV 16 and 18 was reduced by 82% among those who had received the vaccine at the recommended ages of 9-12 years (P = .08).
In the current study, Dr. Egeman acknowledged that only 38% of ever-eligible female patients received the vaccine, although the prevalence increased to 56% when only female patients who were eligible for routine vaccination were taken into account. On the other hand, only 21% (95% CI, 14%-28%) of female patients eligible for routine vaccination received their first dose by age 12 years.
Indeed, the mean age on receipt of the first vaccination dose was 14.5 years (95% CI, 14.1-14.8 years), the authors note, and only 59% of girls received their first dose prior to their sexual debut. Additionally, among routine vaccination–eligible girls aged 12 years or younger in 2006, 33% were vaccinated before and 23% after their sexual debut, and the rest were not vaccinated.
Interestingly, differences in the age at which the HPV vaccine was received by race and ethnicity were negligible, the investigators point out.
Vaccination rates increasing
Asked to comment on the findings, Rebecca Perkins, MD, professor of obstetrics and gynecology at Boston University, Boston Medical Center, pointed out that the investigators evaluated data from 2011 to 2018. “We know that HPV vaccination rates have increased over that period and continue to increase,” she emphasized in an email to this news organization.
Physicians also know that more persons are being vaccinated between the ages of 9 and 12 than was the case at the beginning of this study. “This is good news,” she said, “as it means that more adolescents now in 2022 are benefiting fully from vaccination than they were in 2011,” she added.
At the same time, Dr. Perkins acknowledged that many persons are still missing out on the chance to receive the vaccine on time – which means they are missing out on the chance to prevent cancer.
“Making sure that all adolescents receive vaccination between the ages of 9 to 12 has the potential to prevent up to 40,000 cancers every year in the U.S., [including] the most common HPV-related cancers, such as cervical cancer in women and tongue and tonsillar cancer in men,” Dr. Perkins noted.
“Thus, it’s critical that doctors and parents get the message that you can’t vaccinate too early, only too late,” she emphasized.
Dr. Edgman and Dr. Perkins report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The effectiveness of the human papillomavirus (HPV) vaccine against HPV types 16 and 18 is highly dependent on the age at which it is given. Prevalence rates have been shown to be significantly lower among girls who are vaccinated at the recommended ages of 9-12 years, compared with those who are vaccinated after their sexual debut, data from the National Health and Nutrition Examination Survey (NHANES) indicate.
“HPV vaccination does not have any therapeutic effect on HPV infections already acquired, which is more likely to explain the difference in prevalence between predebut versus postdebut recipients than a lower immune response [among older recipients],” lead study author Didem Egemen, PhD, National Cancer Institute, Rockville, Md., told this news organization in an email.
“Still, among older females, the immune response of the vaccine is likely to still be quite strong, and we would encourage vaccination [of female patients] if unvaccinated, as our paper showed that vaccination post debut will still reduce HPV 16/18 prevalence by half,” she added.
The research letter was published online in JAMA Network Open.
National sample evaluated
Using data from NHANES, a biennial, cross-sectional sample (cycles 2011 through 2018), the researchers identified female persons who were aged 26 years or younger in 2006, when HPV vaccination was introduced, and who were eligible for routine vaccination or “catch-up” vaccination (given between the ages of 13 and 26 years), as per recommendations from the Advisory Committee on Immunization Practices. The investigators then compared the prevalence of HPV types 16 and 18 among unvaccinated female patients, female patients who had been vaccinated prior to their sexual debut (predebut group), and those who had been vaccinated after their sexual debut (postdebut group).
They also estimated vaccine uptake among those who were eligible for routine vaccination, as well as the proportion of vaccinated female patients with respect to racial and ethnic subgroups.
In the overall cohort, the prevalence of HPV types 16 and 18 decreased by 6% (95% confidence interval, 4%-7%) in the unvaccinated group to 3% (95% CI, 1%-6%) in the postdebut group and to less than 1% (95% CI, <1%-1%) in the predebut group, Dr. Egemen and colleagues report.
In real percentages, the prevalence of HPV 16 and 18 was 89% lower in the predebut group (P < .001) but only 41% lower in the postdebut group (P = .29) compared with unvaccinated female patients. And compared with female patients who were vaccinated after their sexual debut, the prevalence of HPV 16 and 18 was reduced by 82% among those who had received the vaccine at the recommended ages of 9-12 years (P = .08).
In the current study, Dr. Egeman acknowledged that only 38% of ever-eligible female patients received the vaccine, although the prevalence increased to 56% when only female patients who were eligible for routine vaccination were taken into account. On the other hand, only 21% (95% CI, 14%-28%) of female patients eligible for routine vaccination received their first dose by age 12 years.
Indeed, the mean age on receipt of the first vaccination dose was 14.5 years (95% CI, 14.1-14.8 years), the authors note, and only 59% of girls received their first dose prior to their sexual debut. Additionally, among routine vaccination–eligible girls aged 12 years or younger in 2006, 33% were vaccinated before and 23% after their sexual debut, and the rest were not vaccinated.
Interestingly, differences in the age at which the HPV vaccine was received by race and ethnicity were negligible, the investigators point out.
Vaccination rates increasing
Asked to comment on the findings, Rebecca Perkins, MD, professor of obstetrics and gynecology at Boston University, Boston Medical Center, pointed out that the investigators evaluated data from 2011 to 2018. “We know that HPV vaccination rates have increased over that period and continue to increase,” she emphasized in an email to this news organization.
Physicians also know that more persons are being vaccinated between the ages of 9 and 12 than was the case at the beginning of this study. “This is good news,” she said, “as it means that more adolescents now in 2022 are benefiting fully from vaccination than they were in 2011,” she added.
At the same time, Dr. Perkins acknowledged that many persons are still missing out on the chance to receive the vaccine on time – which means they are missing out on the chance to prevent cancer.
“Making sure that all adolescents receive vaccination between the ages of 9 to 12 has the potential to prevent up to 40,000 cancers every year in the U.S., [including] the most common HPV-related cancers, such as cervical cancer in women and tongue and tonsillar cancer in men,” Dr. Perkins noted.
“Thus, it’s critical that doctors and parents get the message that you can’t vaccinate too early, only too late,” she emphasized.
Dr. Edgman and Dr. Perkins report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Academic dermatology: Gender diversity advances as some gaps persist
, according to a recent cross-sectional study.
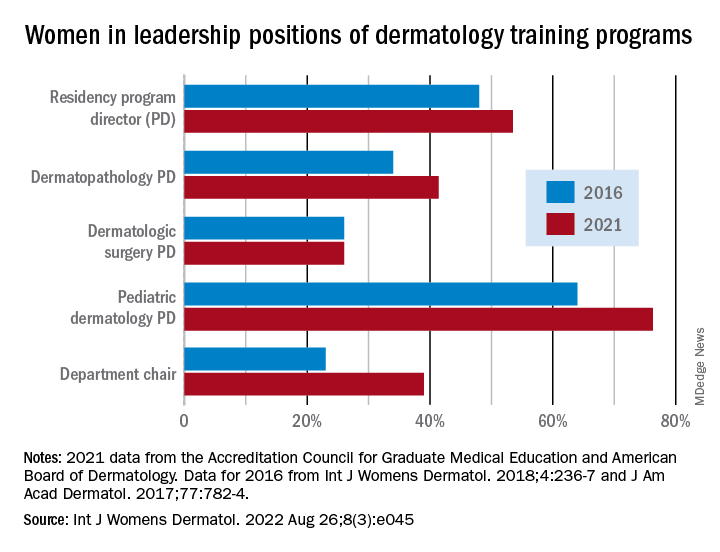
Although women made up more than half of the dermatology residency program directors (53.5%), associate directors (62.6%), and assistant directors (58.3%) in 2021, those numbers fall short of women’s majority (65% in 2018) among the trainees themselves, Yasmine Abushukur of Oakland University in Rochester, Mich., and associates said in a research letter.
Advancements were “made in gender diversity within academic dermatology from 2016 to 2021, [but] women remain underrepresented, particularly in leadership of dermatopathology and dermatologic surgery fellowships,” the investigators wrote.
Data gathered from 142 dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education show that progress has been made since 2016, at least among program directors (PDs), of whom 48% were women, according to a previous study. Data on associate and assistant PDs from 2016 were not available to Ms. Abushukur and associates.
At the fellowship program level, women made gains as PDs in dermatopathology (34% in 2016 and 41% in 2021) and pediatric dermatology (64% in 2016 and 76% in 2021), but not in dermatologic surgery, where the proportion held at 26% over the study period. “This disparity is reflective of the general trend in surgery and pathology leadership nationally,” the researchers noted.
Taking a couple of steps up the ladder of authority shows that 39% of dermatology chairs were women in 2021, compared with 23% in 2016. A study published in 2016 demonstrated decreased diversity among academic faculty members as faculty rank increased, and “our data mirror this sentiment by demonstrating a majority of women in assistant and associate PD positions, with a minority of women chairs,” they wrote.
The investigators said that they had no conflicts of interest and no outside funding. Ms. Abushukur’s coauthors were from the departments of dermatology at the Henry Ford Health System, Detroit, and Wayne State University, Dearborn, Mich.
, according to a recent cross-sectional study.

Although women made up more than half of the dermatology residency program directors (53.5%), associate directors (62.6%), and assistant directors (58.3%) in 2021, those numbers fall short of women’s majority (65% in 2018) among the trainees themselves, Yasmine Abushukur of Oakland University in Rochester, Mich., and associates said in a research letter.
Advancements were “made in gender diversity within academic dermatology from 2016 to 2021, [but] women remain underrepresented, particularly in leadership of dermatopathology and dermatologic surgery fellowships,” the investigators wrote.
Data gathered from 142 dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education show that progress has been made since 2016, at least among program directors (PDs), of whom 48% were women, according to a previous study. Data on associate and assistant PDs from 2016 were not available to Ms. Abushukur and associates.
At the fellowship program level, women made gains as PDs in dermatopathology (34% in 2016 and 41% in 2021) and pediatric dermatology (64% in 2016 and 76% in 2021), but not in dermatologic surgery, where the proportion held at 26% over the study period. “This disparity is reflective of the general trend in surgery and pathology leadership nationally,” the researchers noted.
Taking a couple of steps up the ladder of authority shows that 39% of dermatology chairs were women in 2021, compared with 23% in 2016. A study published in 2016 demonstrated decreased diversity among academic faculty members as faculty rank increased, and “our data mirror this sentiment by demonstrating a majority of women in assistant and associate PD positions, with a minority of women chairs,” they wrote.
The investigators said that they had no conflicts of interest and no outside funding. Ms. Abushukur’s coauthors were from the departments of dermatology at the Henry Ford Health System, Detroit, and Wayne State University, Dearborn, Mich.
, according to a recent cross-sectional study.

Although women made up more than half of the dermatology residency program directors (53.5%), associate directors (62.6%), and assistant directors (58.3%) in 2021, those numbers fall short of women’s majority (65% in 2018) among the trainees themselves, Yasmine Abushukur of Oakland University in Rochester, Mich., and associates said in a research letter.
Advancements were “made in gender diversity within academic dermatology from 2016 to 2021, [but] women remain underrepresented, particularly in leadership of dermatopathology and dermatologic surgery fellowships,” the investigators wrote.
Data gathered from 142 dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education show that progress has been made since 2016, at least among program directors (PDs), of whom 48% were women, according to a previous study. Data on associate and assistant PDs from 2016 were not available to Ms. Abushukur and associates.
At the fellowship program level, women made gains as PDs in dermatopathology (34% in 2016 and 41% in 2021) and pediatric dermatology (64% in 2016 and 76% in 2021), but not in dermatologic surgery, where the proportion held at 26% over the study period. “This disparity is reflective of the general trend in surgery and pathology leadership nationally,” the researchers noted.
Taking a couple of steps up the ladder of authority shows that 39% of dermatology chairs were women in 2021, compared with 23% in 2016. A study published in 2016 demonstrated decreased diversity among academic faculty members as faculty rank increased, and “our data mirror this sentiment by demonstrating a majority of women in assistant and associate PD positions, with a minority of women chairs,” they wrote.
The investigators said that they had no conflicts of interest and no outside funding. Ms. Abushukur’s coauthors were from the departments of dermatology at the Henry Ford Health System, Detroit, and Wayne State University, Dearborn, Mich.
FROM INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
Surgical site dressing turns blue when it needs changing
Surgical site infections are one of the top causes of postoperative morbidity and death worldwide, but there is little agreement and much debate over the most effective wound dressing to improve outcomes and reduce the health care burden.
Recent clinical trials have indicated that transparent, semiocclusive films have advantages over gauze held by adhesive tape.
But current transparent film bandages may become dislodged during activities such as showering, say authors of a pilot study published in the Journal of Wound Care. Patients may not realize the bandage has been disrupted, which can lead to infection.
.
“Clinicians, patients, and caregivers are alerted to the loss of dressing integrity and can replace the dressing when any portion of the perimeter changes to a blue [color],” the authors explain. “In addition, the dressing turns blue when the central pad is saturated with fluid, allowing the patient or provider to change the dressing.”
DSD is indicated for wounds that have low levels of exudate.
Two transparent film dressings compared
Researchers recruited 20 patients from the general population in Pittsburgh, for a small pilot study to test DSD against a comparator film dressing (3M Tegaderm + Pad). The volunteers received “a small stipend,” according to the paper.
A 1.5-centimeter incision was made in both forearms of each volunteer. The forearms were randomized regarding which got which bandage. Both bandages have been cleared by the U.S. Food and Drug Administration as nonsignificant-risk devices.
Volunteers were instructed to wear the dressing and continue their typical activities of daily living.
The average age of the volunteers was 52 years (range, 20-80 years). Among the 20 volunteers, 11 reported no comorbidities, and 45% reported at least one comorbidity.
Most of the volunteers favored DSD over the comparator in a postoperative survey – 75% to 25%, according to the report.
The wear time between the two transparent dressings across all subjects was 1.4 days. There was no difference in wear time, logged by the volunteers, between the two groups.
There were no infectious complications, the paper states.
The maker, DrySee (Houston), which holds three patents on the product, supported the research with an unrestricted grant.
DrySee CEO Brad Greer told this news organization, “With DrySee, you know when to change your dressing. All other dressings look the same wet, saturated, or dry.”
He said the study confirms what they have seen in practice, adding that the product is unique.
“No one else in the world has this technology,” Mr. Greer said.
Surgeons want to see more data
Heather Evans, MD, a general surgeon with the Medical University of South Carolina, MUSC Health, Charleston, who was not involved with the study, praised the color-indicator design and said she liked the bandage’s narrow indication for low-exudate wounds.
She said in an interview, “It’s a lot to put on a layperson to suddenly know how to take care of wounds when you leave the hospital.”
Giving them the confidence that their wound is safe if the blue doesn’t appear “is a really cool concept,” she said.
She said that, although the volunteers included some elderly people and people with conditions such as diabetes that could affect wound healing, the bandage needs to be tested with a bigger trial to see if it is effective outside controlled conditions.
She also said that some occlusive dressings will be more durable and stay on days longer than DSD or the comparator, which may affect the choice for some.
“The average length of dressing time in this study was less than 2 days,” she pointed out.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health Bedford, who was not involved with the study, agreed that any surgical or wound dressing, including transparent films, can become dislodged, and said, “This type of product has promise but this is a small pilot study. I would want to see results from a trial of actual surgical patients to see if this type of dressing did indeed decrease post-op infections compared to standard dressing materials.”
Not all are convinced either that there is a need to be filled or that DSD will be the right solution.
Therese Duane, MD, a general surgeon with Texas Health Harris Methodist Fort Worth, who was not part of the study, said in an interview that she “has no issues with the current products.”
She added that more information is needed before considering DSD a better solution, including animal studies and use “on very sick patients.”
“Twenty volunteers with cuts on their arm is barely a start for comparison,” she said.
The authors, led by Kristy Breisinger, a research analyst with the SerenaGroup Research Foundation in Cambridge, Mass., acknowledged the limitations, including the small sample size and that the trial was conducted at only one institution. Additionally, the analysis is based on descriptive statistics.
They write that the trial design was chosen “to simulate a real-world setting that is not always achievable in animal studies.”
The research was sponsored by an unrestricted grant from the maker of DSD, DrySee Inc., in Houston.
Mr. Greer is DrySee’s CEO. The authors and Dr. Duane, Dr. Rickert, and Dr. Evans declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Surgical site infections are one of the top causes of postoperative morbidity and death worldwide, but there is little agreement and much debate over the most effective wound dressing to improve outcomes and reduce the health care burden.
Recent clinical trials have indicated that transparent, semiocclusive films have advantages over gauze held by adhesive tape.
But current transparent film bandages may become dislodged during activities such as showering, say authors of a pilot study published in the Journal of Wound Care. Patients may not realize the bandage has been disrupted, which can lead to infection.
.
“Clinicians, patients, and caregivers are alerted to the loss of dressing integrity and can replace the dressing when any portion of the perimeter changes to a blue [color],” the authors explain. “In addition, the dressing turns blue when the central pad is saturated with fluid, allowing the patient or provider to change the dressing.”
DSD is indicated for wounds that have low levels of exudate.
Two transparent film dressings compared
Researchers recruited 20 patients from the general population in Pittsburgh, for a small pilot study to test DSD against a comparator film dressing (3M Tegaderm + Pad). The volunteers received “a small stipend,” according to the paper.
A 1.5-centimeter incision was made in both forearms of each volunteer. The forearms were randomized regarding which got which bandage. Both bandages have been cleared by the U.S. Food and Drug Administration as nonsignificant-risk devices.
Volunteers were instructed to wear the dressing and continue their typical activities of daily living.
The average age of the volunteers was 52 years (range, 20-80 years). Among the 20 volunteers, 11 reported no comorbidities, and 45% reported at least one comorbidity.
Most of the volunteers favored DSD over the comparator in a postoperative survey – 75% to 25%, according to the report.
The wear time between the two transparent dressings across all subjects was 1.4 days. There was no difference in wear time, logged by the volunteers, between the two groups.
There were no infectious complications, the paper states.
The maker, DrySee (Houston), which holds three patents on the product, supported the research with an unrestricted grant.
DrySee CEO Brad Greer told this news organization, “With DrySee, you know when to change your dressing. All other dressings look the same wet, saturated, or dry.”
He said the study confirms what they have seen in practice, adding that the product is unique.
“No one else in the world has this technology,” Mr. Greer said.
Surgeons want to see more data
Heather Evans, MD, a general surgeon with the Medical University of South Carolina, MUSC Health, Charleston, who was not involved with the study, praised the color-indicator design and said she liked the bandage’s narrow indication for low-exudate wounds.
She said in an interview, “It’s a lot to put on a layperson to suddenly know how to take care of wounds when you leave the hospital.”
Giving them the confidence that their wound is safe if the blue doesn’t appear “is a really cool concept,” she said.
She said that, although the volunteers included some elderly people and people with conditions such as diabetes that could affect wound healing, the bandage needs to be tested with a bigger trial to see if it is effective outside controlled conditions.
She also said that some occlusive dressings will be more durable and stay on days longer than DSD or the comparator, which may affect the choice for some.
“The average length of dressing time in this study was less than 2 days,” she pointed out.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health Bedford, who was not involved with the study, agreed that any surgical or wound dressing, including transparent films, can become dislodged, and said, “This type of product has promise but this is a small pilot study. I would want to see results from a trial of actual surgical patients to see if this type of dressing did indeed decrease post-op infections compared to standard dressing materials.”
Not all are convinced either that there is a need to be filled or that DSD will be the right solution.
Therese Duane, MD, a general surgeon with Texas Health Harris Methodist Fort Worth, who was not part of the study, said in an interview that she “has no issues with the current products.”
She added that more information is needed before considering DSD a better solution, including animal studies and use “on very sick patients.”
“Twenty volunteers with cuts on their arm is barely a start for comparison,” she said.
The authors, led by Kristy Breisinger, a research analyst with the SerenaGroup Research Foundation in Cambridge, Mass., acknowledged the limitations, including the small sample size and that the trial was conducted at only one institution. Additionally, the analysis is based on descriptive statistics.
They write that the trial design was chosen “to simulate a real-world setting that is not always achievable in animal studies.”
The research was sponsored by an unrestricted grant from the maker of DSD, DrySee Inc., in Houston.
Mr. Greer is DrySee’s CEO. The authors and Dr. Duane, Dr. Rickert, and Dr. Evans declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Surgical site infections are one of the top causes of postoperative morbidity and death worldwide, but there is little agreement and much debate over the most effective wound dressing to improve outcomes and reduce the health care burden.
Recent clinical trials have indicated that transparent, semiocclusive films have advantages over gauze held by adhesive tape.
But current transparent film bandages may become dislodged during activities such as showering, say authors of a pilot study published in the Journal of Wound Care. Patients may not realize the bandage has been disrupted, which can lead to infection.
.
“Clinicians, patients, and caregivers are alerted to the loss of dressing integrity and can replace the dressing when any portion of the perimeter changes to a blue [color],” the authors explain. “In addition, the dressing turns blue when the central pad is saturated with fluid, allowing the patient or provider to change the dressing.”
DSD is indicated for wounds that have low levels of exudate.
Two transparent film dressings compared
Researchers recruited 20 patients from the general population in Pittsburgh, for a small pilot study to test DSD against a comparator film dressing (3M Tegaderm + Pad). The volunteers received “a small stipend,” according to the paper.
A 1.5-centimeter incision was made in both forearms of each volunteer. The forearms were randomized regarding which got which bandage. Both bandages have been cleared by the U.S. Food and Drug Administration as nonsignificant-risk devices.
Volunteers were instructed to wear the dressing and continue their typical activities of daily living.
The average age of the volunteers was 52 years (range, 20-80 years). Among the 20 volunteers, 11 reported no comorbidities, and 45% reported at least one comorbidity.
Most of the volunteers favored DSD over the comparator in a postoperative survey – 75% to 25%, according to the report.
The wear time between the two transparent dressings across all subjects was 1.4 days. There was no difference in wear time, logged by the volunteers, between the two groups.
There were no infectious complications, the paper states.
The maker, DrySee (Houston), which holds three patents on the product, supported the research with an unrestricted grant.
DrySee CEO Brad Greer told this news organization, “With DrySee, you know when to change your dressing. All other dressings look the same wet, saturated, or dry.”
He said the study confirms what they have seen in practice, adding that the product is unique.
“No one else in the world has this technology,” Mr. Greer said.
Surgeons want to see more data
Heather Evans, MD, a general surgeon with the Medical University of South Carolina, MUSC Health, Charleston, who was not involved with the study, praised the color-indicator design and said she liked the bandage’s narrow indication for low-exudate wounds.
She said in an interview, “It’s a lot to put on a layperson to suddenly know how to take care of wounds when you leave the hospital.”
Giving them the confidence that their wound is safe if the blue doesn’t appear “is a really cool concept,” she said.
She said that, although the volunteers included some elderly people and people with conditions such as diabetes that could affect wound healing, the bandage needs to be tested with a bigger trial to see if it is effective outside controlled conditions.
She also said that some occlusive dressings will be more durable and stay on days longer than DSD or the comparator, which may affect the choice for some.
“The average length of dressing time in this study was less than 2 days,” she pointed out.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health Bedford, who was not involved with the study, agreed that any surgical or wound dressing, including transparent films, can become dislodged, and said, “This type of product has promise but this is a small pilot study. I would want to see results from a trial of actual surgical patients to see if this type of dressing did indeed decrease post-op infections compared to standard dressing materials.”
Not all are convinced either that there is a need to be filled or that DSD will be the right solution.
Therese Duane, MD, a general surgeon with Texas Health Harris Methodist Fort Worth, who was not part of the study, said in an interview that she “has no issues with the current products.”
She added that more information is needed before considering DSD a better solution, including animal studies and use “on very sick patients.”
“Twenty volunteers with cuts on their arm is barely a start for comparison,” she said.
The authors, led by Kristy Breisinger, a research analyst with the SerenaGroup Research Foundation in Cambridge, Mass., acknowledged the limitations, including the small sample size and that the trial was conducted at only one institution. Additionally, the analysis is based on descriptive statistics.
They write that the trial design was chosen “to simulate a real-world setting that is not always achievable in animal studies.”
The research was sponsored by an unrestricted grant from the maker of DSD, DrySee Inc., in Houston.
Mr. Greer is DrySee’s CEO. The authors and Dr. Duane, Dr. Rickert, and Dr. Evans declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF WOUND CARE
A cost-effective de-escalation strategy in advanced melanoma
Response-adapted , an economic analysis found.
“The rising costs of cancer therapies are becoming untenable for both patients and payers, and there is both clinical and economic benefit to finding less expensive treatment alternatives,” Wolfgang Kunz, MD, University Hospital, Ludwig Maximilian University of Munich, told this news organization.
This economic analysis “highlights that leveraging modern diagnostic capabilities can do just that: Pairing drug regimens with CT-image analysis to optimize dosages can reduce health care costs and improve clinical outcomes,” Dr. Kunz said.
The study was published online in JAMA Dermatology.
While the use of immunotherapies over the past decade has improved the prognosis for patients with advanced melanoma, these drugs come with a hefty price tag.
One potential way to help reduce costs: de-escalate therapy. The ADAPT-IT trial demonstrated similar progression-free and overall survival among patients who received response-adapted ipilimumab discontinuation and those who received standard of care.
In the current analysis, Dr. Kunz and colleagues wanted to understand whether this response-adapted approach was also cost effective.
The team applied economic modeling to data from the ADAPT-IT trial as well as CheckMate 067, in which patients received standard of care four doses of combination ipilimumab-nivolumab followed by nivolumab monotherapy. In the ADAPT-IT trial, patients also initially received the immunotherapy combination but had CT scans to determine their response after two doses; if they responded, patients discontinued ipilimumab and continued with nivolumab monotherapy.
Overall, ADAPT-IT showed that responders could forgo the additional two doses of ipilimumab plus nivolumab while maintaining similar progression-free survival and overall survival seen at 18 months in the CheckMate 067 trial.
The current economic analysis, based on 41 patients from ADAPT-IT and 314 from CheckMate 067, showed a potential reduction in health care costs of $19,891 per patient with the response-adapted approach.
Response-adapted treatment was the cost-effective option in 94% of simulated scenarios.
When extrapolated to 2019 incidence rates of distant melanoma cases, yearly national savings could reach about $58 million.
“In the relatively small space of immunotherapies in advanced melanoma, we hope this analysis motivates clinicians to consider response-adapted treatment,” Dr. Kunz told this news organization.
“On the larger scale, this analysis serves as a stepping stone to more response-guided treatment protocols,” Dr. Kunz added. “With drug costs rising and imaging capabilities growing, more frequent image-guided adjustments are a perfect fit into the personalized care model.”
When applying the cost savings noted in this analysis across all treated patients, “the economic impact may be profound,” said Joseph Skitzki, MD, surgical oncologist, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., who wasn’t involved in the study. The “financial toxicity of cancer care is increasingly recognized as a potential barrier to optimal outcomes and any measures to mitigate cost may be impactful.”
However, Dr. Skitzki said several caveats need to be considered.
One is that the data included from ADAPT-IT only included 41 patients, compared with 314 patients from CheckMate 067.
“It is possible that a larger real-world study utilizing the ADAPT-IT protocol may not be as favorable in terms of outcomes and could lessen the economic impact of de-escalation, although any form of de-escalation is likely to have a cost benefit,” Dr. Skitzki said in an interview.
A real-world response–adapted de-escalation clinical trial, with an emphasis on costs and a benchmark of similar progression-free and overall survival, should be conducted before the de-escalated option becomes “practice changing,” Dr. Skitzki said.
Jeffrey Weber, MD, PhD, deputy director, Perlmutter Cancer Center, NYU Langone Health, New York, also urged caution in interpreting the results.
“I would not base treatment decisions on a small sampling of 41 patients in the absence of a randomized comparison,” Dr. Weber told this news organization. “Without a proper comparison, I would not advocate using only two doses of ipilimumab-nivolumab to make decisions on treatment.”
Dr. Skitzki added that, while “studies like this one are desperately needed to lessen the economic impact of new and emerging combination immunotherapies,” there is likely also a “disincentive for pharmaceutical companies to conduct this type of research.”
This research had no specific funding. Dr. Kunz and Dr. Skitzki reported no relevant conflicts of interest. Dr. Weber disclosed relationships with Merck, Genentech, AstraZeneca, Pfizer, Regeneron, and GSK, among others, and holds equity in Cytomx, Biond, NexImmune, and Immunomax.
A version of this article first appeared on Medscape.com.
Response-adapted , an economic analysis found.
“The rising costs of cancer therapies are becoming untenable for both patients and payers, and there is both clinical and economic benefit to finding less expensive treatment alternatives,” Wolfgang Kunz, MD, University Hospital, Ludwig Maximilian University of Munich, told this news organization.
This economic analysis “highlights that leveraging modern diagnostic capabilities can do just that: Pairing drug regimens with CT-image analysis to optimize dosages can reduce health care costs and improve clinical outcomes,” Dr. Kunz said.
The study was published online in JAMA Dermatology.
While the use of immunotherapies over the past decade has improved the prognosis for patients with advanced melanoma, these drugs come with a hefty price tag.
One potential way to help reduce costs: de-escalate therapy. The ADAPT-IT trial demonstrated similar progression-free and overall survival among patients who received response-adapted ipilimumab discontinuation and those who received standard of care.
In the current analysis, Dr. Kunz and colleagues wanted to understand whether this response-adapted approach was also cost effective.
The team applied economic modeling to data from the ADAPT-IT trial as well as CheckMate 067, in which patients received standard of care four doses of combination ipilimumab-nivolumab followed by nivolumab monotherapy. In the ADAPT-IT trial, patients also initially received the immunotherapy combination but had CT scans to determine their response after two doses; if they responded, patients discontinued ipilimumab and continued with nivolumab monotherapy.
Overall, ADAPT-IT showed that responders could forgo the additional two doses of ipilimumab plus nivolumab while maintaining similar progression-free survival and overall survival seen at 18 months in the CheckMate 067 trial.
The current economic analysis, based on 41 patients from ADAPT-IT and 314 from CheckMate 067, showed a potential reduction in health care costs of $19,891 per patient with the response-adapted approach.
Response-adapted treatment was the cost-effective option in 94% of simulated scenarios.
When extrapolated to 2019 incidence rates of distant melanoma cases, yearly national savings could reach about $58 million.
“In the relatively small space of immunotherapies in advanced melanoma, we hope this analysis motivates clinicians to consider response-adapted treatment,” Dr. Kunz told this news organization.
“On the larger scale, this analysis serves as a stepping stone to more response-guided treatment protocols,” Dr. Kunz added. “With drug costs rising and imaging capabilities growing, more frequent image-guided adjustments are a perfect fit into the personalized care model.”
When applying the cost savings noted in this analysis across all treated patients, “the economic impact may be profound,” said Joseph Skitzki, MD, surgical oncologist, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., who wasn’t involved in the study. The “financial toxicity of cancer care is increasingly recognized as a potential barrier to optimal outcomes and any measures to mitigate cost may be impactful.”
However, Dr. Skitzki said several caveats need to be considered.
One is that the data included from ADAPT-IT only included 41 patients, compared with 314 patients from CheckMate 067.
“It is possible that a larger real-world study utilizing the ADAPT-IT protocol may not be as favorable in terms of outcomes and could lessen the economic impact of de-escalation, although any form of de-escalation is likely to have a cost benefit,” Dr. Skitzki said in an interview.
A real-world response–adapted de-escalation clinical trial, with an emphasis on costs and a benchmark of similar progression-free and overall survival, should be conducted before the de-escalated option becomes “practice changing,” Dr. Skitzki said.
Jeffrey Weber, MD, PhD, deputy director, Perlmutter Cancer Center, NYU Langone Health, New York, also urged caution in interpreting the results.
“I would not base treatment decisions on a small sampling of 41 patients in the absence of a randomized comparison,” Dr. Weber told this news organization. “Without a proper comparison, I would not advocate using only two doses of ipilimumab-nivolumab to make decisions on treatment.”
Dr. Skitzki added that, while “studies like this one are desperately needed to lessen the economic impact of new and emerging combination immunotherapies,” there is likely also a “disincentive for pharmaceutical companies to conduct this type of research.”
This research had no specific funding. Dr. Kunz and Dr. Skitzki reported no relevant conflicts of interest. Dr. Weber disclosed relationships with Merck, Genentech, AstraZeneca, Pfizer, Regeneron, and GSK, among others, and holds equity in Cytomx, Biond, NexImmune, and Immunomax.
A version of this article first appeared on Medscape.com.
Response-adapted , an economic analysis found.
“The rising costs of cancer therapies are becoming untenable for both patients and payers, and there is both clinical and economic benefit to finding less expensive treatment alternatives,” Wolfgang Kunz, MD, University Hospital, Ludwig Maximilian University of Munich, told this news organization.
This economic analysis “highlights that leveraging modern diagnostic capabilities can do just that: Pairing drug regimens with CT-image analysis to optimize dosages can reduce health care costs and improve clinical outcomes,” Dr. Kunz said.
The study was published online in JAMA Dermatology.
While the use of immunotherapies over the past decade has improved the prognosis for patients with advanced melanoma, these drugs come with a hefty price tag.
One potential way to help reduce costs: de-escalate therapy. The ADAPT-IT trial demonstrated similar progression-free and overall survival among patients who received response-adapted ipilimumab discontinuation and those who received standard of care.
In the current analysis, Dr. Kunz and colleagues wanted to understand whether this response-adapted approach was also cost effective.
The team applied economic modeling to data from the ADAPT-IT trial as well as CheckMate 067, in which patients received standard of care four doses of combination ipilimumab-nivolumab followed by nivolumab monotherapy. In the ADAPT-IT trial, patients also initially received the immunotherapy combination but had CT scans to determine their response after two doses; if they responded, patients discontinued ipilimumab and continued with nivolumab monotherapy.
Overall, ADAPT-IT showed that responders could forgo the additional two doses of ipilimumab plus nivolumab while maintaining similar progression-free survival and overall survival seen at 18 months in the CheckMate 067 trial.
The current economic analysis, based on 41 patients from ADAPT-IT and 314 from CheckMate 067, showed a potential reduction in health care costs of $19,891 per patient with the response-adapted approach.
Response-adapted treatment was the cost-effective option in 94% of simulated scenarios.
When extrapolated to 2019 incidence rates of distant melanoma cases, yearly national savings could reach about $58 million.
“In the relatively small space of immunotherapies in advanced melanoma, we hope this analysis motivates clinicians to consider response-adapted treatment,” Dr. Kunz told this news organization.
“On the larger scale, this analysis serves as a stepping stone to more response-guided treatment protocols,” Dr. Kunz added. “With drug costs rising and imaging capabilities growing, more frequent image-guided adjustments are a perfect fit into the personalized care model.”
When applying the cost savings noted in this analysis across all treated patients, “the economic impact may be profound,” said Joseph Skitzki, MD, surgical oncologist, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., who wasn’t involved in the study. The “financial toxicity of cancer care is increasingly recognized as a potential barrier to optimal outcomes and any measures to mitigate cost may be impactful.”
However, Dr. Skitzki said several caveats need to be considered.
One is that the data included from ADAPT-IT only included 41 patients, compared with 314 patients from CheckMate 067.
“It is possible that a larger real-world study utilizing the ADAPT-IT protocol may not be as favorable in terms of outcomes and could lessen the economic impact of de-escalation, although any form of de-escalation is likely to have a cost benefit,” Dr. Skitzki said in an interview.
A real-world response–adapted de-escalation clinical trial, with an emphasis on costs and a benchmark of similar progression-free and overall survival, should be conducted before the de-escalated option becomes “practice changing,” Dr. Skitzki said.
Jeffrey Weber, MD, PhD, deputy director, Perlmutter Cancer Center, NYU Langone Health, New York, also urged caution in interpreting the results.
“I would not base treatment decisions on a small sampling of 41 patients in the absence of a randomized comparison,” Dr. Weber told this news organization. “Without a proper comparison, I would not advocate using only two doses of ipilimumab-nivolumab to make decisions on treatment.”
Dr. Skitzki added that, while “studies like this one are desperately needed to lessen the economic impact of new and emerging combination immunotherapies,” there is likely also a “disincentive for pharmaceutical companies to conduct this type of research.”
This research had no specific funding. Dr. Kunz and Dr. Skitzki reported no relevant conflicts of interest. Dr. Weber disclosed relationships with Merck, Genentech, AstraZeneca, Pfizer, Regeneron, and GSK, among others, and holds equity in Cytomx, Biond, NexImmune, and Immunomax.
A version of this article first appeared on Medscape.com.
New Medicare physician fee schedule leaves docs fuming over pay cuts
The rule also seeks to ease financial and administrative burdens on accountable care organizations (ACOs).
But physician groups’ initial reactions centered on what the American Medical Association describes as a “damaging across-the-board reduction” of 4.4% in a base calculation, known as a conversion factor.
The reduction is only one of the current threats to physician’s finances, Jack Resneck Jr, MD, AMA’s president, said in a statement. Medicare payment rates also fail to account for inflation in practice costs and COVID-related challenges. Physician’s Medicare payments could be cut by nearly 8.5% in 2023, factoring in other budget cuts, Dr. Resneck said in the statement.
That “would severely impede patient access to care due to the forced closure of physician practices and put further strain on those that remained open during the pandemic,” he said.
A key driver of these cuts is a law that was intended to resolve budget battles between Congress and physicians, while also transitioning Medicare away from fee-for-service payments and pegging reimbursement to judgments about value of care provided. The Centers for Medicare & Medicaid Services thus had little choice about cuts mandated by the Medicare Access and CHIP Reauthorization Act (MACRA) of 2015.
For AMA and other physician groups, the finalization of the Medicare rule served as a rallying point to build support for pending legislation intended to stave off at least some payment cuts.
Federal officials should act soon to block the expected cuts before this season of Congress ends in January, said Anders Gilberg, senior vice president for government affairs at the Medical Group Management Association, in a statement.
“This cannot wait until next Congress – there are claims-processing implications for retroactively applying these policies,” Mr. Gilberg said.
He said MGMA would work with Congress and CMS “to mitigate these cuts and develop sustainable payment policies to allow physician practices to focus on treating patients instead of scrambling to keep their doors open.”
Chronic budget battles
Once seen as a promising resolution to chronic annual budget battles between physicians and Medicare, MACRA has proven a near-universal disappointment. A federal advisory commission in 2018 recommended that Congress scrap MACRA’s Merit-based Incentive Payment System (MIPS) and replace it with a new approach for attempting to tie reimbursement to judgments about the quality of medical care.
MACRA replaced an earlier budgeting approach on Medicare physician pay, known as the sustainable growth rate (SGR). Physician groups successfully lobbied Congress for many years to block threatened Medicare payment cuts. Between 2003 and April 2014, Congress passed 17 laws overriding the cuts to physician pay that the lawmakers earlier mandated through the SGR.
A similar pattern has emerged as Congress now acts on short-term fixes to stave off MACRA-mandated cuts. A law passed last December postponed cuts in physician pay from MACRA and federal budget laws.
And more than 70 members of the House support a bill (HR 8800) intended to block a slated 4.4% MACRA-related cut in physician pay for 2023. Two physicians, Rep. Ami Bera, MD, (D-CA) and Rep. Larry Bucshon (R-IN) sponsored the bill.
Among the groups backing the bill are the AMA, American Academy of Family Physicians, and American College of Physicians. The lawmakers may try to attach this bill to a large spending measure, known as an omnibus, that Congress will try to clear in December to avoid a partial government shutdown.
In a statement, Tochi Iroku-Malize, MD, MPH, MBA, the president of AAFP, urged Congress to factor in inflation in setting physician reimbursement and to reconsider Medicare’s approach to paying physicians.
“It’s past time to end the untenable physician payment cuts – which have now become an annual threat to the stability of physician practices – caused by Medicare budget neutrality requirements and the ongoing freeze in annual payment updates,” Dr. Iroku-Malize said.
Congress also needs to retool its approach to alternative payment models (APMs) intended to improve the quality of patient care, Dr. Iroku-Malize said.
“Physicians in APMs are better equipped to address unmet social needs and provide other enhanced services that are not supported by fee-for-service payment rates,” Dr. Iroku-Malize said. “However, insufficient Medicare fee-for-service payment rates, inadequate support, and burdensome timelines are undermining the move to value-based care and exacerbating our nation’s underinvestment in primary care.”
Policy changes
But the new rule did have some good news for family physicians, Dr. Iroku-Malize told this news organization in an email.
CMS said it will pay psychologists and social workers to help manage behavioral health needs as part of the primary care team, in addition to their own services. This change will give primary care practices more flexibility to coordinate with behavioral health professionals, Dr. Iroku-Malize noted.
“We know that primary care physicians are the first point of contact for many patients, and behavioral health integration increases critical access to mental health care, decreases stigma for patients, and can prevent more severe medical and behavioral health events,” she wrote.
CMS also eased a supervision requirement for nonphysicians providing behavioral health services.
It intends to allow certain health professionals to provide this care without requiring that a supervising physician or nurse practitioner be physically on site. This shift from direct supervision to what’s called general supervision applies to marriage and family therapists, licensed professional counselors, addiction counselors, certified peer recovery specialists, and behavioral health specialists, CMS said.
Other major policy changes include:
Medicare will pay for telehealth opioid treatment programs allowing patients to initiate treatment with buprenorphine. CMS also clarified that certain programs can bill for opioid use disorder treatment services provided through mobile units, such as vans.
Medicare enrollees may see audiologists for nonacute hearing conditions without an order from a physician or nurse practitioner. The policy is meant to allow audiologists to examine patients to prescribe, fit, or change hearing aids, or to provide hearing tests unrelated to disequilibrium.
CMS created new reimbursement codes for chronic pain management and treatment services to encourage clinicians to see patients with this condition. The codes also are meant to encourage practitioners already treating Medicare patients with chronic pain to spend more time helping them manage their condition “within a trusting, supportive, and ongoing care partnership,” CMS said.
CMS also made changes to the Medicare Shared Savings Program (MSSP) intended to reduce administrative burdens and offer more financial support to practices involved in ACOs. These steps include expanding opportunities for certain low-revenue ACOs to share in savings even if they do not meet a target rate.
A version of this article first appeared on Medscape.com.
The rule also seeks to ease financial and administrative burdens on accountable care organizations (ACOs).
But physician groups’ initial reactions centered on what the American Medical Association describes as a “damaging across-the-board reduction” of 4.4% in a base calculation, known as a conversion factor.
The reduction is only one of the current threats to physician’s finances, Jack Resneck Jr, MD, AMA’s president, said in a statement. Medicare payment rates also fail to account for inflation in practice costs and COVID-related challenges. Physician’s Medicare payments could be cut by nearly 8.5% in 2023, factoring in other budget cuts, Dr. Resneck said in the statement.
That “would severely impede patient access to care due to the forced closure of physician practices and put further strain on those that remained open during the pandemic,” he said.
A key driver of these cuts is a law that was intended to resolve budget battles between Congress and physicians, while also transitioning Medicare away from fee-for-service payments and pegging reimbursement to judgments about value of care provided. The Centers for Medicare & Medicaid Services thus had little choice about cuts mandated by the Medicare Access and CHIP Reauthorization Act (MACRA) of 2015.
For AMA and other physician groups, the finalization of the Medicare rule served as a rallying point to build support for pending legislation intended to stave off at least some payment cuts.
Federal officials should act soon to block the expected cuts before this season of Congress ends in January, said Anders Gilberg, senior vice president for government affairs at the Medical Group Management Association, in a statement.
“This cannot wait until next Congress – there are claims-processing implications for retroactively applying these policies,” Mr. Gilberg said.
He said MGMA would work with Congress and CMS “to mitigate these cuts and develop sustainable payment policies to allow physician practices to focus on treating patients instead of scrambling to keep their doors open.”
Chronic budget battles
Once seen as a promising resolution to chronic annual budget battles between physicians and Medicare, MACRA has proven a near-universal disappointment. A federal advisory commission in 2018 recommended that Congress scrap MACRA’s Merit-based Incentive Payment System (MIPS) and replace it with a new approach for attempting to tie reimbursement to judgments about the quality of medical care.
MACRA replaced an earlier budgeting approach on Medicare physician pay, known as the sustainable growth rate (SGR). Physician groups successfully lobbied Congress for many years to block threatened Medicare payment cuts. Between 2003 and April 2014, Congress passed 17 laws overriding the cuts to physician pay that the lawmakers earlier mandated through the SGR.
A similar pattern has emerged as Congress now acts on short-term fixes to stave off MACRA-mandated cuts. A law passed last December postponed cuts in physician pay from MACRA and federal budget laws.
And more than 70 members of the House support a bill (HR 8800) intended to block a slated 4.4% MACRA-related cut in physician pay for 2023. Two physicians, Rep. Ami Bera, MD, (D-CA) and Rep. Larry Bucshon (R-IN) sponsored the bill.
Among the groups backing the bill are the AMA, American Academy of Family Physicians, and American College of Physicians. The lawmakers may try to attach this bill to a large spending measure, known as an omnibus, that Congress will try to clear in December to avoid a partial government shutdown.
In a statement, Tochi Iroku-Malize, MD, MPH, MBA, the president of AAFP, urged Congress to factor in inflation in setting physician reimbursement and to reconsider Medicare’s approach to paying physicians.
“It’s past time to end the untenable physician payment cuts – which have now become an annual threat to the stability of physician practices – caused by Medicare budget neutrality requirements and the ongoing freeze in annual payment updates,” Dr. Iroku-Malize said.
Congress also needs to retool its approach to alternative payment models (APMs) intended to improve the quality of patient care, Dr. Iroku-Malize said.
“Physicians in APMs are better equipped to address unmet social needs and provide other enhanced services that are not supported by fee-for-service payment rates,” Dr. Iroku-Malize said. “However, insufficient Medicare fee-for-service payment rates, inadequate support, and burdensome timelines are undermining the move to value-based care and exacerbating our nation’s underinvestment in primary care.”
Policy changes
But the new rule did have some good news for family physicians, Dr. Iroku-Malize told this news organization in an email.
CMS said it will pay psychologists and social workers to help manage behavioral health needs as part of the primary care team, in addition to their own services. This change will give primary care practices more flexibility to coordinate with behavioral health professionals, Dr. Iroku-Malize noted.
“We know that primary care physicians are the first point of contact for many patients, and behavioral health integration increases critical access to mental health care, decreases stigma for patients, and can prevent more severe medical and behavioral health events,” she wrote.
CMS also eased a supervision requirement for nonphysicians providing behavioral health services.
It intends to allow certain health professionals to provide this care without requiring that a supervising physician or nurse practitioner be physically on site. This shift from direct supervision to what’s called general supervision applies to marriage and family therapists, licensed professional counselors, addiction counselors, certified peer recovery specialists, and behavioral health specialists, CMS said.
Other major policy changes include:
Medicare will pay for telehealth opioid treatment programs allowing patients to initiate treatment with buprenorphine. CMS also clarified that certain programs can bill for opioid use disorder treatment services provided through mobile units, such as vans.
Medicare enrollees may see audiologists for nonacute hearing conditions without an order from a physician or nurse practitioner. The policy is meant to allow audiologists to examine patients to prescribe, fit, or change hearing aids, or to provide hearing tests unrelated to disequilibrium.
CMS created new reimbursement codes for chronic pain management and treatment services to encourage clinicians to see patients with this condition. The codes also are meant to encourage practitioners already treating Medicare patients with chronic pain to spend more time helping them manage their condition “within a trusting, supportive, and ongoing care partnership,” CMS said.
CMS also made changes to the Medicare Shared Savings Program (MSSP) intended to reduce administrative burdens and offer more financial support to practices involved in ACOs. These steps include expanding opportunities for certain low-revenue ACOs to share in savings even if they do not meet a target rate.
A version of this article first appeared on Medscape.com.
The rule also seeks to ease financial and administrative burdens on accountable care organizations (ACOs).
But physician groups’ initial reactions centered on what the American Medical Association describes as a “damaging across-the-board reduction” of 4.4% in a base calculation, known as a conversion factor.
The reduction is only one of the current threats to physician’s finances, Jack Resneck Jr, MD, AMA’s president, said in a statement. Medicare payment rates also fail to account for inflation in practice costs and COVID-related challenges. Physician’s Medicare payments could be cut by nearly 8.5% in 2023, factoring in other budget cuts, Dr. Resneck said in the statement.
That “would severely impede patient access to care due to the forced closure of physician practices and put further strain on those that remained open during the pandemic,” he said.
A key driver of these cuts is a law that was intended to resolve budget battles between Congress and physicians, while also transitioning Medicare away from fee-for-service payments and pegging reimbursement to judgments about value of care provided. The Centers for Medicare & Medicaid Services thus had little choice about cuts mandated by the Medicare Access and CHIP Reauthorization Act (MACRA) of 2015.
For AMA and other physician groups, the finalization of the Medicare rule served as a rallying point to build support for pending legislation intended to stave off at least some payment cuts.
Federal officials should act soon to block the expected cuts before this season of Congress ends in January, said Anders Gilberg, senior vice president for government affairs at the Medical Group Management Association, in a statement.
“This cannot wait until next Congress – there are claims-processing implications for retroactively applying these policies,” Mr. Gilberg said.
He said MGMA would work with Congress and CMS “to mitigate these cuts and develop sustainable payment policies to allow physician practices to focus on treating patients instead of scrambling to keep their doors open.”
Chronic budget battles
Once seen as a promising resolution to chronic annual budget battles between physicians and Medicare, MACRA has proven a near-universal disappointment. A federal advisory commission in 2018 recommended that Congress scrap MACRA’s Merit-based Incentive Payment System (MIPS) and replace it with a new approach for attempting to tie reimbursement to judgments about the quality of medical care.
MACRA replaced an earlier budgeting approach on Medicare physician pay, known as the sustainable growth rate (SGR). Physician groups successfully lobbied Congress for many years to block threatened Medicare payment cuts. Between 2003 and April 2014, Congress passed 17 laws overriding the cuts to physician pay that the lawmakers earlier mandated through the SGR.
A similar pattern has emerged as Congress now acts on short-term fixes to stave off MACRA-mandated cuts. A law passed last December postponed cuts in physician pay from MACRA and federal budget laws.
And more than 70 members of the House support a bill (HR 8800) intended to block a slated 4.4% MACRA-related cut in physician pay for 2023. Two physicians, Rep. Ami Bera, MD, (D-CA) and Rep. Larry Bucshon (R-IN) sponsored the bill.
Among the groups backing the bill are the AMA, American Academy of Family Physicians, and American College of Physicians. The lawmakers may try to attach this bill to a large spending measure, known as an omnibus, that Congress will try to clear in December to avoid a partial government shutdown.
In a statement, Tochi Iroku-Malize, MD, MPH, MBA, the president of AAFP, urged Congress to factor in inflation in setting physician reimbursement and to reconsider Medicare’s approach to paying physicians.
“It’s past time to end the untenable physician payment cuts – which have now become an annual threat to the stability of physician practices – caused by Medicare budget neutrality requirements and the ongoing freeze in annual payment updates,” Dr. Iroku-Malize said.
Congress also needs to retool its approach to alternative payment models (APMs) intended to improve the quality of patient care, Dr. Iroku-Malize said.
“Physicians in APMs are better equipped to address unmet social needs and provide other enhanced services that are not supported by fee-for-service payment rates,” Dr. Iroku-Malize said. “However, insufficient Medicare fee-for-service payment rates, inadequate support, and burdensome timelines are undermining the move to value-based care and exacerbating our nation’s underinvestment in primary care.”
Policy changes
But the new rule did have some good news for family physicians, Dr. Iroku-Malize told this news organization in an email.
CMS said it will pay psychologists and social workers to help manage behavioral health needs as part of the primary care team, in addition to their own services. This change will give primary care practices more flexibility to coordinate with behavioral health professionals, Dr. Iroku-Malize noted.
“We know that primary care physicians are the first point of contact for many patients, and behavioral health integration increases critical access to mental health care, decreases stigma for patients, and can prevent more severe medical and behavioral health events,” she wrote.
CMS also eased a supervision requirement for nonphysicians providing behavioral health services.
It intends to allow certain health professionals to provide this care without requiring that a supervising physician or nurse practitioner be physically on site. This shift from direct supervision to what’s called general supervision applies to marriage and family therapists, licensed professional counselors, addiction counselors, certified peer recovery specialists, and behavioral health specialists, CMS said.
Other major policy changes include:
Medicare will pay for telehealth opioid treatment programs allowing patients to initiate treatment with buprenorphine. CMS also clarified that certain programs can bill for opioid use disorder treatment services provided through mobile units, such as vans.
Medicare enrollees may see audiologists for nonacute hearing conditions without an order from a physician or nurse practitioner. The policy is meant to allow audiologists to examine patients to prescribe, fit, or change hearing aids, or to provide hearing tests unrelated to disequilibrium.
CMS created new reimbursement codes for chronic pain management and treatment services to encourage clinicians to see patients with this condition. The codes also are meant to encourage practitioners already treating Medicare patients with chronic pain to spend more time helping them manage their condition “within a trusting, supportive, and ongoing care partnership,” CMS said.
CMS also made changes to the Medicare Shared Savings Program (MSSP) intended to reduce administrative burdens and offer more financial support to practices involved in ACOs. These steps include expanding opportunities for certain low-revenue ACOs to share in savings even if they do not meet a target rate.
A version of this article first appeared on Medscape.com.
Education about OTC tools key for patients with acne and rosacea
LAS VEGAS – , Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical Center, New Brunswick, N.J., said in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
In some cases, the use of good-quality over-the -counter skin care products can improve acne without prescription treatment, said Dr. Baldwin, who is medical director of the Acne Treatment and Research Center, New York. Good skin care can enhance the effects of prescription medication by decreasing side effects such as inflammation, pain, and erythema, and improving compliance; and use of OTC products has not been shown to interfere with the efficacy of prescription products, she noted.
However, patient education about OTC products is key, she said. In particular, “cleansers are a double-edged sword,” Dr. Baldwin emphasized.
Cleansing is important to preserve barrier function, but “there is a risk of skin damage” if cleansers are too harsh, she said. The goal is to remove dirt, oils, and bacteria without disrupting the lipids, proteins, and normal flora that keep skin healthy, and to avoid altering pH, she added.
Key considerations for OTC cleansers include surfactants, pH, and patient preferences, Dr. Baldwin said.
Surfactants, the main components of OTC cleansers, can do more harm than good in some cases. Surfactants break down impurities on the skin surface, but not all are created equal, and some may cause skin irritation, she explained.
Surfactants fall into four categories: nonionic (no charge), anionic (negative charge), cationic (positive charge), and amphoteric (dual charge). Of these, cationic surfactants have the highest level of antimicrobial activity.
Many patients with acne seek out antibacterial cleansers, but many of these products have a high pH, which can inhibit healthy skin function and promote inflammation, Dr. Baldwin noted.
The right OTC skin care products can normalize pH, which promotes repair of the skin barrier and reduces inflammation, she said. While some products are labeled as “gentle,” they may have a high pH, and many products don’t list a pH, Dr. Baldwin pointed out. Many antibacterial products have pH levels in the 10-12 range, while true soaps fall in the 9-10 range, and hydrating liquid cleansers often land in the 5-7 range, she said.
“Most of our patients don’t know what ingredients to look for” in a cleanser, she noted. However, data show that a majority of patients prefer a foaming cleanser, enjoy the face-washing experience – and wash their faces at least twice a day, with a range of products including bath soap, said Dr. Baldwin. Consequently, “educate your patient about moisturizing,” she advised.
For patients with greasy or oily skin, Dr. Baldwin recommends lipid-free foaming cleansers, such as those with ceramides or glycerin. For patients with dry, irritated acne, she advises once-daily washing only, without cleansing devices, which includes washcloths, she said. Look for hydrating cleansers that are nonfoaming or slightly foaming for these patients, she added.
Another tip for patients is to remind them that “sebum is not a moisturizer,” said Dr. Baldwin. Acne patients may still need moisturizers, especially if they experience dry skin as a side effect of their acne medication, but finding the right fit can be a challenge requiring some trial and error, she noted.
OTC products for rosacea
Dr. Baldwin also addressed the use of OTC products for patients with rosacea. For cleansers, she recommends the same hydrating, nonfoaming categories as for her acne patients, with a once-daily, no-device regimen. She advises rosacea patients to avoid pure humectants for moisturizing and noted that silicone-based products are often the least irritating.
Seek moisturizers with ceramides, hyaluronic acid, glycerin, or niacinamide, she said. Data have shown that effective moisturization improves the ability of patients with rosacea to use and adhere to their prescription medications, Dr. Baldwin emphasized. Moisturizers also can make the medication more effective by enhancing the penetration of products such as azelaic acid, she added.
No acne or rosacea visit is complete until overall skin care has been discussed, Dr. Baldwin said.
Dr. Baldwin disclosed serving as a consultant or adviser for Almirall, EPI Health, Galderma, La Roche Posay, Ortho Dermatologics, Sun, and Vyne; and serving as a speaker or member of the speakers’ bureau for Almirall, Galderma, La Roche Posay, Ortho Dermatologics, and Sun. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical Center, New Brunswick, N.J., said in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
In some cases, the use of good-quality over-the -counter skin care products can improve acne without prescription treatment, said Dr. Baldwin, who is medical director of the Acne Treatment and Research Center, New York. Good skin care can enhance the effects of prescription medication by decreasing side effects such as inflammation, pain, and erythema, and improving compliance; and use of OTC products has not been shown to interfere with the efficacy of prescription products, she noted.
However, patient education about OTC products is key, she said. In particular, “cleansers are a double-edged sword,” Dr. Baldwin emphasized.
Cleansing is important to preserve barrier function, but “there is a risk of skin damage” if cleansers are too harsh, she said. The goal is to remove dirt, oils, and bacteria without disrupting the lipids, proteins, and normal flora that keep skin healthy, and to avoid altering pH, she added.
Key considerations for OTC cleansers include surfactants, pH, and patient preferences, Dr. Baldwin said.
Surfactants, the main components of OTC cleansers, can do more harm than good in some cases. Surfactants break down impurities on the skin surface, but not all are created equal, and some may cause skin irritation, she explained.
Surfactants fall into four categories: nonionic (no charge), anionic (negative charge), cationic (positive charge), and amphoteric (dual charge). Of these, cationic surfactants have the highest level of antimicrobial activity.
Many patients with acne seek out antibacterial cleansers, but many of these products have a high pH, which can inhibit healthy skin function and promote inflammation, Dr. Baldwin noted.
The right OTC skin care products can normalize pH, which promotes repair of the skin barrier and reduces inflammation, she said. While some products are labeled as “gentle,” they may have a high pH, and many products don’t list a pH, Dr. Baldwin pointed out. Many antibacterial products have pH levels in the 10-12 range, while true soaps fall in the 9-10 range, and hydrating liquid cleansers often land in the 5-7 range, she said.
“Most of our patients don’t know what ingredients to look for” in a cleanser, she noted. However, data show that a majority of patients prefer a foaming cleanser, enjoy the face-washing experience – and wash their faces at least twice a day, with a range of products including bath soap, said Dr. Baldwin. Consequently, “educate your patient about moisturizing,” she advised.
For patients with greasy or oily skin, Dr. Baldwin recommends lipid-free foaming cleansers, such as those with ceramides or glycerin. For patients with dry, irritated acne, she advises once-daily washing only, without cleansing devices, which includes washcloths, she said. Look for hydrating cleansers that are nonfoaming or slightly foaming for these patients, she added.
Another tip for patients is to remind them that “sebum is not a moisturizer,” said Dr. Baldwin. Acne patients may still need moisturizers, especially if they experience dry skin as a side effect of their acne medication, but finding the right fit can be a challenge requiring some trial and error, she noted.
OTC products for rosacea
Dr. Baldwin also addressed the use of OTC products for patients with rosacea. For cleansers, she recommends the same hydrating, nonfoaming categories as for her acne patients, with a once-daily, no-device regimen. She advises rosacea patients to avoid pure humectants for moisturizing and noted that silicone-based products are often the least irritating.
Seek moisturizers with ceramides, hyaluronic acid, glycerin, or niacinamide, she said. Data have shown that effective moisturization improves the ability of patients with rosacea to use and adhere to their prescription medications, Dr. Baldwin emphasized. Moisturizers also can make the medication more effective by enhancing the penetration of products such as azelaic acid, she added.
No acne or rosacea visit is complete until overall skin care has been discussed, Dr. Baldwin said.
Dr. Baldwin disclosed serving as a consultant or adviser for Almirall, EPI Health, Galderma, La Roche Posay, Ortho Dermatologics, Sun, and Vyne; and serving as a speaker or member of the speakers’ bureau for Almirall, Galderma, La Roche Posay, Ortho Dermatologics, and Sun. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical Center, New Brunswick, N.J., said in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
In some cases, the use of good-quality over-the -counter skin care products can improve acne without prescription treatment, said Dr. Baldwin, who is medical director of the Acne Treatment and Research Center, New York. Good skin care can enhance the effects of prescription medication by decreasing side effects such as inflammation, pain, and erythema, and improving compliance; and use of OTC products has not been shown to interfere with the efficacy of prescription products, she noted.
However, patient education about OTC products is key, she said. In particular, “cleansers are a double-edged sword,” Dr. Baldwin emphasized.
Cleansing is important to preserve barrier function, but “there is a risk of skin damage” if cleansers are too harsh, she said. The goal is to remove dirt, oils, and bacteria without disrupting the lipids, proteins, and normal flora that keep skin healthy, and to avoid altering pH, she added.
Key considerations for OTC cleansers include surfactants, pH, and patient preferences, Dr. Baldwin said.
Surfactants, the main components of OTC cleansers, can do more harm than good in some cases. Surfactants break down impurities on the skin surface, but not all are created equal, and some may cause skin irritation, she explained.
Surfactants fall into four categories: nonionic (no charge), anionic (negative charge), cationic (positive charge), and amphoteric (dual charge). Of these, cationic surfactants have the highest level of antimicrobial activity.
Many patients with acne seek out antibacterial cleansers, but many of these products have a high pH, which can inhibit healthy skin function and promote inflammation, Dr. Baldwin noted.
The right OTC skin care products can normalize pH, which promotes repair of the skin barrier and reduces inflammation, she said. While some products are labeled as “gentle,” they may have a high pH, and many products don’t list a pH, Dr. Baldwin pointed out. Many antibacterial products have pH levels in the 10-12 range, while true soaps fall in the 9-10 range, and hydrating liquid cleansers often land in the 5-7 range, she said.
“Most of our patients don’t know what ingredients to look for” in a cleanser, she noted. However, data show that a majority of patients prefer a foaming cleanser, enjoy the face-washing experience – and wash their faces at least twice a day, with a range of products including bath soap, said Dr. Baldwin. Consequently, “educate your patient about moisturizing,” she advised.
For patients with greasy or oily skin, Dr. Baldwin recommends lipid-free foaming cleansers, such as those with ceramides or glycerin. For patients with dry, irritated acne, she advises once-daily washing only, without cleansing devices, which includes washcloths, she said. Look for hydrating cleansers that are nonfoaming or slightly foaming for these patients, she added.
Another tip for patients is to remind them that “sebum is not a moisturizer,” said Dr. Baldwin. Acne patients may still need moisturizers, especially if they experience dry skin as a side effect of their acne medication, but finding the right fit can be a challenge requiring some trial and error, she noted.
OTC products for rosacea
Dr. Baldwin also addressed the use of OTC products for patients with rosacea. For cleansers, she recommends the same hydrating, nonfoaming categories as for her acne patients, with a once-daily, no-device regimen. She advises rosacea patients to avoid pure humectants for moisturizing and noted that silicone-based products are often the least irritating.
Seek moisturizers with ceramides, hyaluronic acid, glycerin, or niacinamide, she said. Data have shown that effective moisturization improves the ability of patients with rosacea to use and adhere to their prescription medications, Dr. Baldwin emphasized. Moisturizers also can make the medication more effective by enhancing the penetration of products such as azelaic acid, she added.
No acne or rosacea visit is complete until overall skin care has been discussed, Dr. Baldwin said.
Dr. Baldwin disclosed serving as a consultant or adviser for Almirall, EPI Health, Galderma, La Roche Posay, Ortho Dermatologics, Sun, and Vyne; and serving as a speaker or member of the speakers’ bureau for Almirall, Galderma, La Roche Posay, Ortho Dermatologics, and Sun. MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY