User login
In Case You Missed It: COVID
COVID-19: CPAP has no advantage over conventional oxygen therapy
Key clinical point: Continuous positive airway pressure (CPAP) therapy in patients with severe COVID-19 who are not likely to benefit from invasive mechanical ventilation (IMV) does not confer a survival advantage over oxygen alone.
Major finding: The overall 30-day mortality rate was 75.6% in the oxygen group vs 77.7% in the CPAP group (Pearson’s Chi-square, P = .8).
Study details: The data come from a retrospective multicentre cohort study involving 479 patients with COVID-19 ineligible for IMV from 7 UK hospitals. Patients given CPAP were compared with those receiving oxygen therapy.
Disclosures: L Pearmain is supported by the Medical Research Council, and TW Felton is supported by the National Institute for Health Research Manchester Biomedical Research Centre. AB reported relationships with Fisher and Paykel and Sanofi Genzyme. The remaining authors declared no conflict of interests.
Source: Bradley P et al. EClinicalMedicine. 2021 Sep 8. doi: 10.1016/j.eclinm.2021.101122.
Key clinical point: Continuous positive airway pressure (CPAP) therapy in patients with severe COVID-19 who are not likely to benefit from invasive mechanical ventilation (IMV) does not confer a survival advantage over oxygen alone.
Major finding: The overall 30-day mortality rate was 75.6% in the oxygen group vs 77.7% in the CPAP group (Pearson’s Chi-square, P = .8).
Study details: The data come from a retrospective multicentre cohort study involving 479 patients with COVID-19 ineligible for IMV from 7 UK hospitals. Patients given CPAP were compared with those receiving oxygen therapy.
Disclosures: L Pearmain is supported by the Medical Research Council, and TW Felton is supported by the National Institute for Health Research Manchester Biomedical Research Centre. AB reported relationships with Fisher and Paykel and Sanofi Genzyme. The remaining authors declared no conflict of interests.
Source: Bradley P et al. EClinicalMedicine. 2021 Sep 8. doi: 10.1016/j.eclinm.2021.101122.
Key clinical point: Continuous positive airway pressure (CPAP) therapy in patients with severe COVID-19 who are not likely to benefit from invasive mechanical ventilation (IMV) does not confer a survival advantage over oxygen alone.
Major finding: The overall 30-day mortality rate was 75.6% in the oxygen group vs 77.7% in the CPAP group (Pearson’s Chi-square, P = .8).
Study details: The data come from a retrospective multicentre cohort study involving 479 patients with COVID-19 ineligible for IMV from 7 UK hospitals. Patients given CPAP were compared with those receiving oxygen therapy.
Disclosures: L Pearmain is supported by the Medical Research Council, and TW Felton is supported by the National Institute for Health Research Manchester Biomedical Research Centre. AB reported relationships with Fisher and Paykel and Sanofi Genzyme. The remaining authors declared no conflict of interests.
Source: Bradley P et al. EClinicalMedicine. 2021 Sep 8. doi: 10.1016/j.eclinm.2021.101122.
Does smoking influence COVID-19 outcomes?
Key clinical point: Smokers have a significantly higher risk for COVID-19 related hospital admission and mortality than nonsmokers.
Major finding: Current smokers had an 80% higher risk for hospitalization than never-smokers (odds ratio, 1.80; 95% confidence interval, 1.26-2.29). Smokers who smoked more than 20 cigarettes a day had a 6.11-fold higher risk of dying from COVID-19 than never-smokers.
Study details: The data come from an observational study of 421,469 individuals from the UK Biobank.
Disclosures: The study was supported by individual grants to several authors. PS Tan, CAC Coupland, MR Munafò, J Hippisley-Cox, and A von Ende reported relationships with pharmaceutical companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Clift AK et al. Thorax. 2021 Sep 27. doi: 10.1136/thoraxjnl-2021-217080.
Key clinical point: Smokers have a significantly higher risk for COVID-19 related hospital admission and mortality than nonsmokers.
Major finding: Current smokers had an 80% higher risk for hospitalization than never-smokers (odds ratio, 1.80; 95% confidence interval, 1.26-2.29). Smokers who smoked more than 20 cigarettes a day had a 6.11-fold higher risk of dying from COVID-19 than never-smokers.
Study details: The data come from an observational study of 421,469 individuals from the UK Biobank.
Disclosures: The study was supported by individual grants to several authors. PS Tan, CAC Coupland, MR Munafò, J Hippisley-Cox, and A von Ende reported relationships with pharmaceutical companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Clift AK et al. Thorax. 2021 Sep 27. doi: 10.1136/thoraxjnl-2021-217080.
Key clinical point: Smokers have a significantly higher risk for COVID-19 related hospital admission and mortality than nonsmokers.
Major finding: Current smokers had an 80% higher risk for hospitalization than never-smokers (odds ratio, 1.80; 95% confidence interval, 1.26-2.29). Smokers who smoked more than 20 cigarettes a day had a 6.11-fold higher risk of dying from COVID-19 than never-smokers.
Study details: The data come from an observational study of 421,469 individuals from the UK Biobank.
Disclosures: The study was supported by individual grants to several authors. PS Tan, CAC Coupland, MR Munafò, J Hippisley-Cox, and A von Ende reported relationships with pharmaceutical companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Clift AK et al. Thorax. 2021 Sep 27. doi: 10.1136/thoraxjnl-2021-217080.
Anti-CD20-treated multiple sclerosis patients show robust response to COVID-19 vaccines
Key clinical point: Patients with multiple sclerosis (MS) receiving anti-CD20 (aCD20) treatment demonstrate a robust T-cell response to an mRNA COVID-19 vaccines.
Major finding: Thirty days following the second dose of an mRNA COVID-19 vaccine, 89% of patients with MS had antispike antibodies, and 50% had mounted anti-receptor-binding domain responses.
Study details: A study assessed antibody and T-cell responses in 20 patients with MS receiving aCD20 treatment and 10 healthy controls.
Disclosures: The study was supported by individual grants to several authors. SE Hensley, EJ Wherry, A Sette, ET Luning Prak, D Jacobs, and A Bar-Or reported relationships with pharmaceutical companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Apostolidis SA et al. Nat Med. 2021 Sep 14. doi: 10.1038/s41591-021-01507-2.
Key clinical point: Patients with multiple sclerosis (MS) receiving anti-CD20 (aCD20) treatment demonstrate a robust T-cell response to an mRNA COVID-19 vaccines.
Major finding: Thirty days following the second dose of an mRNA COVID-19 vaccine, 89% of patients with MS had antispike antibodies, and 50% had mounted anti-receptor-binding domain responses.
Study details: A study assessed antibody and T-cell responses in 20 patients with MS receiving aCD20 treatment and 10 healthy controls.
Disclosures: The study was supported by individual grants to several authors. SE Hensley, EJ Wherry, A Sette, ET Luning Prak, D Jacobs, and A Bar-Or reported relationships with pharmaceutical companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Apostolidis SA et al. Nat Med. 2021 Sep 14. doi: 10.1038/s41591-021-01507-2.
Key clinical point: Patients with multiple sclerosis (MS) receiving anti-CD20 (aCD20) treatment demonstrate a robust T-cell response to an mRNA COVID-19 vaccines.
Major finding: Thirty days following the second dose of an mRNA COVID-19 vaccine, 89% of patients with MS had antispike antibodies, and 50% had mounted anti-receptor-binding domain responses.
Study details: A study assessed antibody and T-cell responses in 20 patients with MS receiving aCD20 treatment and 10 healthy controls.
Disclosures: The study was supported by individual grants to several authors. SE Hensley, EJ Wherry, A Sette, ET Luning Prak, D Jacobs, and A Bar-Or reported relationships with pharmaceutical companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Apostolidis SA et al. Nat Med. 2021 Sep 14. doi: 10.1038/s41591-021-01507-2.
Study identifies 3 behaviors that increase COVID-19 risk
Key clinical point: A study has identified at least 3 modifiable behaviors that may increase the personal risk for COVID-19.
Major finding: Higher number of nonhousehold contacts (odds ratio [OR], 1.10 per 10 contacts; P = .024), attending events having at least 10 people (OR, 1.26 per 10 events; P = .007), and visiting restaurants (OR, 1.95 per 10 visits; P less than .001) were associated with a significantly increased risk for incident COVID-19.
Study details: The data come from a prospective cohort study of 28,575 individuals across 99 countries.
Disclosures: The study was supported by grants from the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering to GM Marcus, J Olgin, and M Pletcher. The authors declared no competing interests.
Source: Lin A et al. BMJ Open. 2021 Sep 21. doi: 10.1136/bmjopen-2021-052025.
Key clinical point: A study has identified at least 3 modifiable behaviors that may increase the personal risk for COVID-19.
Major finding: Higher number of nonhousehold contacts (odds ratio [OR], 1.10 per 10 contacts; P = .024), attending events having at least 10 people (OR, 1.26 per 10 events; P = .007), and visiting restaurants (OR, 1.95 per 10 visits; P less than .001) were associated with a significantly increased risk for incident COVID-19.
Study details: The data come from a prospective cohort study of 28,575 individuals across 99 countries.
Disclosures: The study was supported by grants from the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering to GM Marcus, J Olgin, and M Pletcher. The authors declared no competing interests.
Source: Lin A et al. BMJ Open. 2021 Sep 21. doi: 10.1136/bmjopen-2021-052025.
Key clinical point: A study has identified at least 3 modifiable behaviors that may increase the personal risk for COVID-19.
Major finding: Higher number of nonhousehold contacts (odds ratio [OR], 1.10 per 10 contacts; P = .024), attending events having at least 10 people (OR, 1.26 per 10 events; P = .007), and visiting restaurants (OR, 1.95 per 10 visits; P less than .001) were associated with a significantly increased risk for incident COVID-19.
Study details: The data come from a prospective cohort study of 28,575 individuals across 99 countries.
Disclosures: The study was supported by grants from the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering to GM Marcus, J Olgin, and M Pletcher. The authors declared no competing interests.
Source: Lin A et al. BMJ Open. 2021 Sep 21. doi: 10.1136/bmjopen-2021-052025.
COVID-19: Influence of household members' immunity on nonimmune individuals
Key clinical point: The risk of contracting COVID-19 among nonimmune family members decreases as the number of immune family members increases.
Major finding: An inverse dose-response association was seen between the number of immune members in each family and the risk for incident COVID-19 in nonimmune family members. Families with 1 immune member had a 45%-61% lower risk for COVID-19, whereas families with 4 immune members had a 97% lower risk.
Study details: The data come from an analysis of 1,789,728 individuals from 814,806 families, consisting of 2-5 members each.
Disclosures: Information on funding was not available. The authors declared no competing interests.
Source: Nordström P et al. JAMA Intern Med. 2021 Oct 11. doi: 10.1001/jamainternmed.2021.5814.
Key clinical point: The risk of contracting COVID-19 among nonimmune family members decreases as the number of immune family members increases.
Major finding: An inverse dose-response association was seen between the number of immune members in each family and the risk for incident COVID-19 in nonimmune family members. Families with 1 immune member had a 45%-61% lower risk for COVID-19, whereas families with 4 immune members had a 97% lower risk.
Study details: The data come from an analysis of 1,789,728 individuals from 814,806 families, consisting of 2-5 members each.
Disclosures: Information on funding was not available. The authors declared no competing interests.
Source: Nordström P et al. JAMA Intern Med. 2021 Oct 11. doi: 10.1001/jamainternmed.2021.5814.
Key clinical point: The risk of contracting COVID-19 among nonimmune family members decreases as the number of immune family members increases.
Major finding: An inverse dose-response association was seen between the number of immune members in each family and the risk for incident COVID-19 in nonimmune family members. Families with 1 immune member had a 45%-61% lower risk for COVID-19, whereas families with 4 immune members had a 97% lower risk.
Study details: The data come from an analysis of 1,789,728 individuals from 814,806 families, consisting of 2-5 members each.
Disclosures: Information on funding was not available. The authors declared no competing interests.
Source: Nordström P et al. JAMA Intern Med. 2021 Oct 11. doi: 10.1001/jamainternmed.2021.5814.
COVID-19: Early awake proning tied to lower mortality
Key clinical point: In patients receiving high-flow nasal cannula (HFNC) for acute hypoxemic respiratory failure associated with COVID-19, initiating awake prone positioning (proning) soon after HFNC may improve survival.
Major finding: Initiating awake proning within 24 hours of HFNC was associated with lower mortality rates than late proning (26% vs 45%; P = .039).
Study details: The data come from a post hoc analysis of a trial evaluating awake proning in HFNC-treated patients with COVID-19 with respiratory failure (n=125).
Disclosures: The study was supported by the Rice Foundation. R Kaur, DL Vines, JD Scott, MW Trump, and J Li reported relationships with pharmaceutical/medical device companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Kaur R et al. Crit Care. 2021 Sep 17. doi: 10.1186/s13054-021-03761-9.
Key clinical point: In patients receiving high-flow nasal cannula (HFNC) for acute hypoxemic respiratory failure associated with COVID-19, initiating awake prone positioning (proning) soon after HFNC may improve survival.
Major finding: Initiating awake proning within 24 hours of HFNC was associated with lower mortality rates than late proning (26% vs 45%; P = .039).
Study details: The data come from a post hoc analysis of a trial evaluating awake proning in HFNC-treated patients with COVID-19 with respiratory failure (n=125).
Disclosures: The study was supported by the Rice Foundation. R Kaur, DL Vines, JD Scott, MW Trump, and J Li reported relationships with pharmaceutical/medical device companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Kaur R et al. Crit Care. 2021 Sep 17. doi: 10.1186/s13054-021-03761-9.
Key clinical point: In patients receiving high-flow nasal cannula (HFNC) for acute hypoxemic respiratory failure associated with COVID-19, initiating awake prone positioning (proning) soon after HFNC may improve survival.
Major finding: Initiating awake proning within 24 hours of HFNC was associated with lower mortality rates than late proning (26% vs 45%; P = .039).
Study details: The data come from a post hoc analysis of a trial evaluating awake proning in HFNC-treated patients with COVID-19 with respiratory failure (n=125).
Disclosures: The study was supported by the Rice Foundation. R Kaur, DL Vines, JD Scott, MW Trump, and J Li reported relationships with pharmaceutical/medical device companies and/or research organizations. The remaining authors declared no conflict of interests.
Source: Kaur R et al. Crit Care. 2021 Sep 17. doi: 10.1186/s13054-021-03761-9.
Serum albumin may be a prognostic marker for COVID-19
Key clinical point: Serum albumin levels at presentation may be a potential marker for COVID-19 severity and prognosis.
Major finding: Serum albumin of <3.5 g/dL was an independent risk factor for severe infection (adjusted odds ratio [aOR], 2.924; 95% confidence interval [CI], 1.509-5.664) and 30-day mortality (aOR, 2.615; 95% CI, 1.131-6.051).
Study details: A retrospective observational study included 296 patients with COVID-19 from emergency departments of 3 hospitals in Italy.
Disclosures: The study did not receive any funding. The authors declared no competing interests.
Source: Turcato G et al. Emerg Med J. 2021 Sep 21. doi: 10.1136/emermed-2020-210081.
Key clinical point: Serum albumin levels at presentation may be a potential marker for COVID-19 severity and prognosis.
Major finding: Serum albumin of <3.5 g/dL was an independent risk factor for severe infection (adjusted odds ratio [aOR], 2.924; 95% confidence interval [CI], 1.509-5.664) and 30-day mortality (aOR, 2.615; 95% CI, 1.131-6.051).
Study details: A retrospective observational study included 296 patients with COVID-19 from emergency departments of 3 hospitals in Italy.
Disclosures: The study did not receive any funding. The authors declared no competing interests.
Source: Turcato G et al. Emerg Med J. 2021 Sep 21. doi: 10.1136/emermed-2020-210081.
Key clinical point: Serum albumin levels at presentation may be a potential marker for COVID-19 severity and prognosis.
Major finding: Serum albumin of <3.5 g/dL was an independent risk factor for severe infection (adjusted odds ratio [aOR], 2.924; 95% confidence interval [CI], 1.509-5.664) and 30-day mortality (aOR, 2.615; 95% CI, 1.131-6.051).
Study details: A retrospective observational study included 296 patients with COVID-19 from emergency departments of 3 hospitals in Italy.
Disclosures: The study did not receive any funding. The authors declared no competing interests.
Source: Turcato G et al. Emerg Med J. 2021 Sep 21. doi: 10.1136/emermed-2020-210081.
Convalescent plasma not beneficial for critically ill COVID-19 patients
Key clinical point: Critically ill patients with COVID-19 fail to benefit from convalescent plasma.
Major finding: The trial was stopped early after the probability of futility was calculated at 99.4%. Convalescent plasma vs. no plasma groups had 0 vs 3 organ support-free days, 37.3% vs 38.4% in-hospital mortality rates, and 14 vs 14 median days alive and free of organ support.
Study details: The data come from an open-label, randomized component of the ongoing REMAP-CAP trial (n=2,011). Critically ill adults with COVID-19 were randomly assigned to receive convalescent plasma or not.
Disclosures: The study was funded by nonprofits in multiple countries. Several authors reported relationships with pharmaceutical companies and/or research organizations.
Source: Estcourt LJ et al. JAMA. 2021 Oct 4. doi: 10.1001/jama.2021.18178.
Key clinical point: Critically ill patients with COVID-19 fail to benefit from convalescent plasma.
Major finding: The trial was stopped early after the probability of futility was calculated at 99.4%. Convalescent plasma vs. no plasma groups had 0 vs 3 organ support-free days, 37.3% vs 38.4% in-hospital mortality rates, and 14 vs 14 median days alive and free of organ support.
Study details: The data come from an open-label, randomized component of the ongoing REMAP-CAP trial (n=2,011). Critically ill adults with COVID-19 were randomly assigned to receive convalescent plasma or not.
Disclosures: The study was funded by nonprofits in multiple countries. Several authors reported relationships with pharmaceutical companies and/or research organizations.
Source: Estcourt LJ et al. JAMA. 2021 Oct 4. doi: 10.1001/jama.2021.18178.
Key clinical point: Critically ill patients with COVID-19 fail to benefit from convalescent plasma.
Major finding: The trial was stopped early after the probability of futility was calculated at 99.4%. Convalescent plasma vs. no plasma groups had 0 vs 3 organ support-free days, 37.3% vs 38.4% in-hospital mortality rates, and 14 vs 14 median days alive and free of organ support.
Study details: The data come from an open-label, randomized component of the ongoing REMAP-CAP trial (n=2,011). Critically ill adults with COVID-19 were randomly assigned to receive convalescent plasma or not.
Disclosures: The study was funded by nonprofits in multiple countries. Several authors reported relationships with pharmaceutical companies and/or research organizations.
Source: Estcourt LJ et al. JAMA. 2021 Oct 4. doi: 10.1001/jama.2021.18178.
COVID-19-related pulmonary sequelae common in patients with diabetes
Key clinical point: Pulmonary sequelae at 6 months after COVID-19 infection are more common in patients with diabetes or hyperglycemia compared with normoglycemic individuals.
Major finding: At 6 months, residual lung abnormalities on computed tomography were seen in 65.4%, 58.3%, and 36.6% of patients with preexisting diabetes, secondary hyperglycemia, and normoglycemia, respectively.
Study details: The data come from a retrospective study of 141 patients with COVID-19 at 2 hospitals in Wuhan, China.
Disclosures: The research was supported by the National Natural Science Foundation of China, National Key Research and Development Project of China, and Zhejiang University. The authors declared no competing interests.
Source: Li Y et al. Eur J Radiol. 2021 Oct 5. doi: 10.1016/j.ejrad.2021.109997.
Key clinical point: Pulmonary sequelae at 6 months after COVID-19 infection are more common in patients with diabetes or hyperglycemia compared with normoglycemic individuals.
Major finding: At 6 months, residual lung abnormalities on computed tomography were seen in 65.4%, 58.3%, and 36.6% of patients with preexisting diabetes, secondary hyperglycemia, and normoglycemia, respectively.
Study details: The data come from a retrospective study of 141 patients with COVID-19 at 2 hospitals in Wuhan, China.
Disclosures: The research was supported by the National Natural Science Foundation of China, National Key Research and Development Project of China, and Zhejiang University. The authors declared no competing interests.
Source: Li Y et al. Eur J Radiol. 2021 Oct 5. doi: 10.1016/j.ejrad.2021.109997.
Key clinical point: Pulmonary sequelae at 6 months after COVID-19 infection are more common in patients with diabetes or hyperglycemia compared with normoglycemic individuals.
Major finding: At 6 months, residual lung abnormalities on computed tomography were seen in 65.4%, 58.3%, and 36.6% of patients with preexisting diabetes, secondary hyperglycemia, and normoglycemia, respectively.
Study details: The data come from a retrospective study of 141 patients with COVID-19 at 2 hospitals in Wuhan, China.
Disclosures: The research was supported by the National Natural Science Foundation of China, National Key Research and Development Project of China, and Zhejiang University. The authors declared no competing interests.
Source: Li Y et al. Eur J Radiol. 2021 Oct 5. doi: 10.1016/j.ejrad.2021.109997.
Children and COVID: Vaccinations lower than ever as cases continue to drop
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.
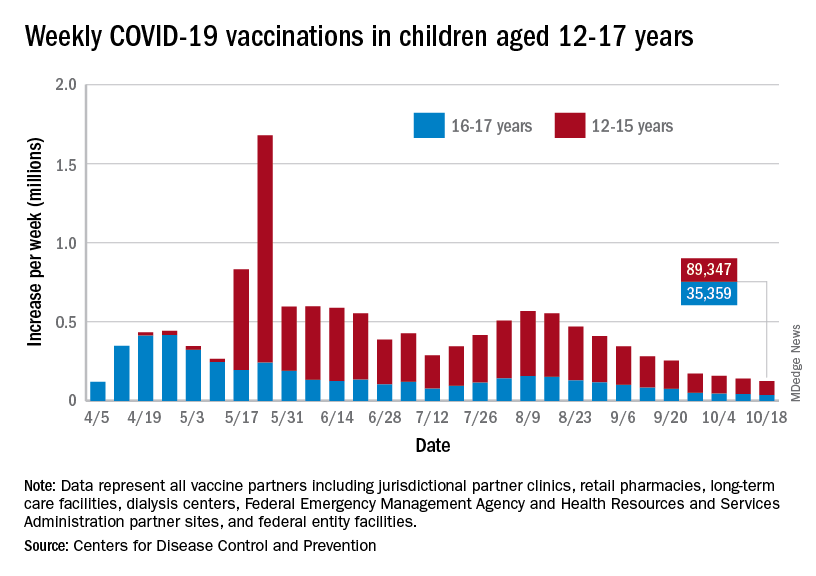
Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.