User login
Inhibitor promotes chemosensitization in CLL

PHILADELPHIA—A DNA-dependent protein kinase (DNA-PK) inhibitor can sensitize chronic lymphocytic leukemia (CLL) cells to chemotherapy, according to
preclinical research.
The inhibitor, NDD0004, sensitized CLL cells—even those from patients with high-risk cytogenetics—to treatment with mitoxantrone.
However, not all CLL samples were sensitive to treatment, so researchers are now trying to determine which patients might derive benefit from DNA-PK inhibitors.
Gesa Junge, a PhD student at Newcastle University in the UK, and her colleagues conducted this research and presented the results at the AACR Annual Meeting 2015 (abstract 3624*). The work was supported by AstraZeneca.
The researchers’ goal was to validate that DNA-PK inhibition is a valid approach to chemosensitization in CLL. So the team tested NU7441—a compound that inhibits DNA-PK and PI3 kinase—and NDD0004—a more selective DNA-PK inhibitor.
The team isolated CLL cells from patients’ peripheral blood, cultured the cells, and treated them with mitoxantrone and/or 1μM of NDD0004 or 1μM of NU7441.
Junge and her colleagues found that NDD0004 sensitized cells to mitoxantrone more effectively than NU7441. Sensitization was 202-fold higher with NDD004 plus mitoxantrone than with mitoxantrone alone and 69-fold higher with NU7441 plus mitoxantrone than with mitoxantrone alone (P=0.02).
However, sensitization varied between CLL samples, and the researchers have yet to determine why. Their experiments showed that variability was not a result of DNA-PK levels.
Still, the team found that CLL cells from patients with poor prognostic markers were sensitive to DNA-PK inhibition.
Sensitization with NU7441 plus mitoxantrone was 69-fold higher than mitoxantrone alone in CLL samples with del(13q), 25-fold higher in samples with del(11q), 12-fold higher in samples with TP53 mutation, and 16-fold higher in samples with ATM dysfunction.
Sensitization with NDD0004 plus mitoxantrone was 201-fold higher than mitoxantrone alone in CLL samples with del(13q), 314-fold higher in samples with del(11q), 27-fold higher in samples with TP53 mutation, and 18-fold higher in samples with ATM dysfunction.
To confirm that sensitization was a result of DNA-PK inhibition, Junge and her colleagues tested NDD0004 in an isogenic pair of DNA-PK-deficient and DNA-PK-proficient HCT116 cells. They found that HCT116 cells lacking DNA-PK were not sensitive to NDD0004, but cells with DNA-PK were sensitive.
The researchers also investigated the mechanism of NDD0004. Their results suggest the drug works by inhibiting the repair of DNA double-strand breaks.
“What we think is happening is that we are inducing DNA damage with mitoxantrone, and that gets repaired by 24 hours,” Junge said. “But if the DNA-PK inhibitor is there, the damage persists, and that seems to translate quite nicely into an apoptosis response.”
To further this research, Junge and her colleagues are hoping to identify biomarkers that can help them determine which CLL patients are likely to respond to DNA-PK inhibitors. ![]()
*Information in the abstract differs from that presented at the meeting.

PHILADELPHIA—A DNA-dependent protein kinase (DNA-PK) inhibitor can sensitize chronic lymphocytic leukemia (CLL) cells to chemotherapy, according to
preclinical research.
The inhibitor, NDD0004, sensitized CLL cells—even those from patients with high-risk cytogenetics—to treatment with mitoxantrone.
However, not all CLL samples were sensitive to treatment, so researchers are now trying to determine which patients might derive benefit from DNA-PK inhibitors.
Gesa Junge, a PhD student at Newcastle University in the UK, and her colleagues conducted this research and presented the results at the AACR Annual Meeting 2015 (abstract 3624*). The work was supported by AstraZeneca.
The researchers’ goal was to validate that DNA-PK inhibition is a valid approach to chemosensitization in CLL. So the team tested NU7441—a compound that inhibits DNA-PK and PI3 kinase—and NDD0004—a more selective DNA-PK inhibitor.
The team isolated CLL cells from patients’ peripheral blood, cultured the cells, and treated them with mitoxantrone and/or 1μM of NDD0004 or 1μM of NU7441.
Junge and her colleagues found that NDD0004 sensitized cells to mitoxantrone more effectively than NU7441. Sensitization was 202-fold higher with NDD004 plus mitoxantrone than with mitoxantrone alone and 69-fold higher with NU7441 plus mitoxantrone than with mitoxantrone alone (P=0.02).
However, sensitization varied between CLL samples, and the researchers have yet to determine why. Their experiments showed that variability was not a result of DNA-PK levels.
Still, the team found that CLL cells from patients with poor prognostic markers were sensitive to DNA-PK inhibition.
Sensitization with NU7441 plus mitoxantrone was 69-fold higher than mitoxantrone alone in CLL samples with del(13q), 25-fold higher in samples with del(11q), 12-fold higher in samples with TP53 mutation, and 16-fold higher in samples with ATM dysfunction.
Sensitization with NDD0004 plus mitoxantrone was 201-fold higher than mitoxantrone alone in CLL samples with del(13q), 314-fold higher in samples with del(11q), 27-fold higher in samples with TP53 mutation, and 18-fold higher in samples with ATM dysfunction.
To confirm that sensitization was a result of DNA-PK inhibition, Junge and her colleagues tested NDD0004 in an isogenic pair of DNA-PK-deficient and DNA-PK-proficient HCT116 cells. They found that HCT116 cells lacking DNA-PK were not sensitive to NDD0004, but cells with DNA-PK were sensitive.
The researchers also investigated the mechanism of NDD0004. Their results suggest the drug works by inhibiting the repair of DNA double-strand breaks.
“What we think is happening is that we are inducing DNA damage with mitoxantrone, and that gets repaired by 24 hours,” Junge said. “But if the DNA-PK inhibitor is there, the damage persists, and that seems to translate quite nicely into an apoptosis response.”
To further this research, Junge and her colleagues are hoping to identify biomarkers that can help them determine which CLL patients are likely to respond to DNA-PK inhibitors. ![]()
*Information in the abstract differs from that presented at the meeting.

PHILADELPHIA—A DNA-dependent protein kinase (DNA-PK) inhibitor can sensitize chronic lymphocytic leukemia (CLL) cells to chemotherapy, according to
preclinical research.
The inhibitor, NDD0004, sensitized CLL cells—even those from patients with high-risk cytogenetics—to treatment with mitoxantrone.
However, not all CLL samples were sensitive to treatment, so researchers are now trying to determine which patients might derive benefit from DNA-PK inhibitors.
Gesa Junge, a PhD student at Newcastle University in the UK, and her colleagues conducted this research and presented the results at the AACR Annual Meeting 2015 (abstract 3624*). The work was supported by AstraZeneca.
The researchers’ goal was to validate that DNA-PK inhibition is a valid approach to chemosensitization in CLL. So the team tested NU7441—a compound that inhibits DNA-PK and PI3 kinase—and NDD0004—a more selective DNA-PK inhibitor.
The team isolated CLL cells from patients’ peripheral blood, cultured the cells, and treated them with mitoxantrone and/or 1μM of NDD0004 or 1μM of NU7441.
Junge and her colleagues found that NDD0004 sensitized cells to mitoxantrone more effectively than NU7441. Sensitization was 202-fold higher with NDD004 plus mitoxantrone than with mitoxantrone alone and 69-fold higher with NU7441 plus mitoxantrone than with mitoxantrone alone (P=0.02).
However, sensitization varied between CLL samples, and the researchers have yet to determine why. Their experiments showed that variability was not a result of DNA-PK levels.
Still, the team found that CLL cells from patients with poor prognostic markers were sensitive to DNA-PK inhibition.
Sensitization with NU7441 plus mitoxantrone was 69-fold higher than mitoxantrone alone in CLL samples with del(13q), 25-fold higher in samples with del(11q), 12-fold higher in samples with TP53 mutation, and 16-fold higher in samples with ATM dysfunction.
Sensitization with NDD0004 plus mitoxantrone was 201-fold higher than mitoxantrone alone in CLL samples with del(13q), 314-fold higher in samples with del(11q), 27-fold higher in samples with TP53 mutation, and 18-fold higher in samples with ATM dysfunction.
To confirm that sensitization was a result of DNA-PK inhibition, Junge and her colleagues tested NDD0004 in an isogenic pair of DNA-PK-deficient and DNA-PK-proficient HCT116 cells. They found that HCT116 cells lacking DNA-PK were not sensitive to NDD0004, but cells with DNA-PK were sensitive.
The researchers also investigated the mechanism of NDD0004. Their results suggest the drug works by inhibiting the repair of DNA double-strand breaks.
“What we think is happening is that we are inducing DNA damage with mitoxantrone, and that gets repaired by 24 hours,” Junge said. “But if the DNA-PK inhibitor is there, the damage persists, and that seems to translate quite nicely into an apoptosis response.”
To further this research, Junge and her colleagues are hoping to identify biomarkers that can help them determine which CLL patients are likely to respond to DNA-PK inhibitors. ![]()
*Information in the abstract differs from that presented at the meeting.
Study helps explain how drug fights DLBCL
PHILADELPHIA—The tumor microenvironment may play a key role in treatment with CUDC-427, according to researchers.
Their experiments showed that certain diffuse large B-cell lymphoma (DLBCL) cell lines were sensitive to CUDC-427, and others were not.
However, co-culturing with stromal cells or TNF family ligands made resistant cell lines sensitive to CUDC-427.
And mice bearing cells that resisted CUDC-427 in vitro responded very well to treatment, experiencing complete tumor regression.
Ze Tian, PhD, and her colleagues from Curis, Inc. (the company developing CUDC-427) presented these findings at the AACR Annual Meeting 2015 (abstract 5502).
CUDC-427 is an inhibitor of apoptosis (IAP) antagonist that is in early stage clinical testing in patients with solid tumors and lymphomas.
For the current research, Dr Tian and her colleagues first evaluated the effects of CUDC-427 against a range of hematologic malignancies in vitro. They tested the drug in activated B-cell-like (ABC) DLBCL, germinal center B-cell-like (GCB) DLBCL, other non-Hodgkin lymphomas, Hodgkin lymphoma, multiple myeloma, and various leukemia cell lines.
DLBCL cells (both ABC and GCB) proved the most sensitive to treatment, and CUDC-427 induced apoptosis in these cells. However, certain DLBCL cell lines, such as Karpas 422, were not sensitive to treatment.
The researchers found they could remedy that in two ways. The presence of stromal cells in culture sensitized resistant DLBCL cells to treatment, as did TNF family ligands (TNFα or TRAIL). In previous research, TNF family ligands were shown to synergize with IAP antagonists.
The investigators then analyzed CUDC-427’s mechanism of action. In the sensitive WSU-DLCL2 cell line, the drug worked by activating caspases 3, 8, and 9 by inhibiting cIAP1 and XIAP, as well as activating the non-canonical NF-ĸB pathway and inducing TNFα.
In the resistant Karpas 422 cell line, there was no caspase activity following CUDC-427 treatment. However, when the researchers co-cultured the cell line with stromal cells, they saw caspase activity.
“Because of this finding, we think that the microenvironment may play a role in CUDC-427 treatment,” Dr Tian said.
So the investigators went on to test CUDC-427 in mouse models. The drug inhibited tumor growth by 94% in the WSU-DLCL2 xenograft model. But CUDC-427 induced complete tumor regression in the Karpas 422 xenograft model.
To further investigate the interaction between the tumor microenvironment and CUDC-427, the researchers tested the drug in the A20 B-cell lymphoma mouse syngeneic model.
They found that CUDC-427 induced tumor stasis in this fast-growing lymphoma. They believe this may be due, in part, to the high levels of TRAIL in this model.
Dr Tian and her colleagues said the interaction between CUDC-427 and TNF family ligands or stromal cells warrants further analysis. And this research supports additional investigation to improve outcomes in patients with DLBCL. ![]()
PHILADELPHIA—The tumor microenvironment may play a key role in treatment with CUDC-427, according to researchers.
Their experiments showed that certain diffuse large B-cell lymphoma (DLBCL) cell lines were sensitive to CUDC-427, and others were not.
However, co-culturing with stromal cells or TNF family ligands made resistant cell lines sensitive to CUDC-427.
And mice bearing cells that resisted CUDC-427 in vitro responded very well to treatment, experiencing complete tumor regression.
Ze Tian, PhD, and her colleagues from Curis, Inc. (the company developing CUDC-427) presented these findings at the AACR Annual Meeting 2015 (abstract 5502).
CUDC-427 is an inhibitor of apoptosis (IAP) antagonist that is in early stage clinical testing in patients with solid tumors and lymphomas.
For the current research, Dr Tian and her colleagues first evaluated the effects of CUDC-427 against a range of hematologic malignancies in vitro. They tested the drug in activated B-cell-like (ABC) DLBCL, germinal center B-cell-like (GCB) DLBCL, other non-Hodgkin lymphomas, Hodgkin lymphoma, multiple myeloma, and various leukemia cell lines.
DLBCL cells (both ABC and GCB) proved the most sensitive to treatment, and CUDC-427 induced apoptosis in these cells. However, certain DLBCL cell lines, such as Karpas 422, were not sensitive to treatment.
The researchers found they could remedy that in two ways. The presence of stromal cells in culture sensitized resistant DLBCL cells to treatment, as did TNF family ligands (TNFα or TRAIL). In previous research, TNF family ligands were shown to synergize with IAP antagonists.
The investigators then analyzed CUDC-427’s mechanism of action. In the sensitive WSU-DLCL2 cell line, the drug worked by activating caspases 3, 8, and 9 by inhibiting cIAP1 and XIAP, as well as activating the non-canonical NF-ĸB pathway and inducing TNFα.
In the resistant Karpas 422 cell line, there was no caspase activity following CUDC-427 treatment. However, when the researchers co-cultured the cell line with stromal cells, they saw caspase activity.
“Because of this finding, we think that the microenvironment may play a role in CUDC-427 treatment,” Dr Tian said.
So the investigators went on to test CUDC-427 in mouse models. The drug inhibited tumor growth by 94% in the WSU-DLCL2 xenograft model. But CUDC-427 induced complete tumor regression in the Karpas 422 xenograft model.
To further investigate the interaction between the tumor microenvironment and CUDC-427, the researchers tested the drug in the A20 B-cell lymphoma mouse syngeneic model.
They found that CUDC-427 induced tumor stasis in this fast-growing lymphoma. They believe this may be due, in part, to the high levels of TRAIL in this model.
Dr Tian and her colleagues said the interaction between CUDC-427 and TNF family ligands or stromal cells warrants further analysis. And this research supports additional investigation to improve outcomes in patients with DLBCL. ![]()
PHILADELPHIA—The tumor microenvironment may play a key role in treatment with CUDC-427, according to researchers.
Their experiments showed that certain diffuse large B-cell lymphoma (DLBCL) cell lines were sensitive to CUDC-427, and others were not.
However, co-culturing with stromal cells or TNF family ligands made resistant cell lines sensitive to CUDC-427.
And mice bearing cells that resisted CUDC-427 in vitro responded very well to treatment, experiencing complete tumor regression.
Ze Tian, PhD, and her colleagues from Curis, Inc. (the company developing CUDC-427) presented these findings at the AACR Annual Meeting 2015 (abstract 5502).
CUDC-427 is an inhibitor of apoptosis (IAP) antagonist that is in early stage clinical testing in patients with solid tumors and lymphomas.
For the current research, Dr Tian and her colleagues first evaluated the effects of CUDC-427 against a range of hematologic malignancies in vitro. They tested the drug in activated B-cell-like (ABC) DLBCL, germinal center B-cell-like (GCB) DLBCL, other non-Hodgkin lymphomas, Hodgkin lymphoma, multiple myeloma, and various leukemia cell lines.
DLBCL cells (both ABC and GCB) proved the most sensitive to treatment, and CUDC-427 induced apoptosis in these cells. However, certain DLBCL cell lines, such as Karpas 422, were not sensitive to treatment.
The researchers found they could remedy that in two ways. The presence of stromal cells in culture sensitized resistant DLBCL cells to treatment, as did TNF family ligands (TNFα or TRAIL). In previous research, TNF family ligands were shown to synergize with IAP antagonists.
The investigators then analyzed CUDC-427’s mechanism of action. In the sensitive WSU-DLCL2 cell line, the drug worked by activating caspases 3, 8, and 9 by inhibiting cIAP1 and XIAP, as well as activating the non-canonical NF-ĸB pathway and inducing TNFα.
In the resistant Karpas 422 cell line, there was no caspase activity following CUDC-427 treatment. However, when the researchers co-cultured the cell line with stromal cells, they saw caspase activity.
“Because of this finding, we think that the microenvironment may play a role in CUDC-427 treatment,” Dr Tian said.
So the investigators went on to test CUDC-427 in mouse models. The drug inhibited tumor growth by 94% in the WSU-DLCL2 xenograft model. But CUDC-427 induced complete tumor regression in the Karpas 422 xenograft model.
To further investigate the interaction between the tumor microenvironment and CUDC-427, the researchers tested the drug in the A20 B-cell lymphoma mouse syngeneic model.
They found that CUDC-427 induced tumor stasis in this fast-growing lymphoma. They believe this may be due, in part, to the high levels of TRAIL in this model.
Dr Tian and her colleagues said the interaction between CUDC-427 and TNF family ligands or stromal cells warrants further analysis. And this research supports additional investigation to improve outcomes in patients with DLBCL. ![]()
AACR: HPV vaccine may protect some women with prior exposure
The human papillomavirus vaccine may afford protection to some women with prior HPV exposure, based on the results of a post-hoc analysis of the HPV Vaccine Trial in Costa Rica.
At the 4-year follow-up visit, multi-site efficacy against HPV16/18 DNA at the cervical, anal, or oral region was, as expected, the strongest at 84% among HPV-naive women who received the vaccine, but significant multi-site efficacy of 58% also was seen among women with evidence of HPV exposure prior to vaccination. Vaccine efficacy was nonsignificant at 25% in women with active cervical infection at vaccination, indicating that the vaccine is not therapeutic, study author Daniel C. Beachler, Ph.D., reported at the annual meeting of the American Association for Cancer Research.
The findings support current U.S. guidelines from the Centers for Disease Control and Prevention recommending routine vaccination for those aged 11-12 years, and vaccination through age 26 for those not previously vaccinated. HPV vaccination is known to be highly effective at cervical, anal, and oral regions in HPV-naive individuals and to have reduced impact in women aged 18-25.
What’s unknown is the combined multi-site vaccine efficacy on an individual basis, and whether HPV vaccine protects noninfected sites against HPV infection, Dr. Beachler, a postdoctoral fellow in the Infections and Immunoepidemiology Branch of the National Cancer Institute, said.
The phase III, National Cancer Institute HPV Vaccine Trial in Costa Rica enrolled 7,466 women, aged 18-25 years, over 6 months and randomly assigned them to one of three doses of the HPV16/18 vaccine (Cervarix) or to a hepatitis A vaccine (Havrix). Cervical samples were collected at every annual visit. Oral and anal samples were collected at only the 4-year follow-up visit. A total of 4,186 women contributed samples for all three sites and were included in the analysis.
Samples were tested for alpha mucosal HPV DNA types utilizing the SPF10 PCR-DEIA-LiPA25 system, version 1. An HPV event was defined as a woman with prevalent HPV16/18 DNA at the cervical, anal, or oral regions.
HPV naive was defined as HPV 16/18 seronegative and cervical HPV 16/18 DNA negative, prior infection was defined as HPV 16/18 antibody positive and cervical HPV 16/18 DNA negative, and active infection was defined as cervical HPV16/18 DNA positive.
Among all 4,186 women, multi-site vaccine efficacy at the 4-year visit was 65%, Dr. Beachler said.
HPV vaccinated women had fewer concordant infections, with vaccine efficacy reaching 91% for protection of at least two or three sites.
Indeed, only 7% of HPV 16/18-infected women in the vaccine arm had that same HPV type at two or more anatomic sites, compared with 30% of HPV 16/18-infected women in the control arm (P value < .01), again “giving support that the vaccine may provide some protection against uninfected sites in previously exposed women,” Dr. Beachler said.
He pointed out that the one-time sampling of oral and anal HPV and misclassification in assays could have affected the results. If complete information were available, vaccine efficacy would likely have been closer to 100% in the HPV-naive subgroup, he said.
Follow-up is continuing in the trial to determine the duration of protection, but there is still strong evidence of protection at 9 years, suggesting a booster dose is not necessary, Dr. Beachler said.
Further research of HPV infection outside the cervix, however, is very much needed.
“We know that only a certain percentage, 60%-70% of people who are infected, actually develop a serological response. We don’t even really know if an oral HPV infection or an anal HPV infection produces a serological response. We really need to understand HPV outside the cervix,” he said.
The National Cancer Institute funded the study. GlaxoSmithKline provided vaccines and support for regulatory aspects of the trial. Dr. Beachler declared no financial disclosures.
The human papillomavirus vaccine may afford protection to some women with prior HPV exposure, based on the results of a post-hoc analysis of the HPV Vaccine Trial in Costa Rica.
At the 4-year follow-up visit, multi-site efficacy against HPV16/18 DNA at the cervical, anal, or oral region was, as expected, the strongest at 84% among HPV-naive women who received the vaccine, but significant multi-site efficacy of 58% also was seen among women with evidence of HPV exposure prior to vaccination. Vaccine efficacy was nonsignificant at 25% in women with active cervical infection at vaccination, indicating that the vaccine is not therapeutic, study author Daniel C. Beachler, Ph.D., reported at the annual meeting of the American Association for Cancer Research.
The findings support current U.S. guidelines from the Centers for Disease Control and Prevention recommending routine vaccination for those aged 11-12 years, and vaccination through age 26 for those not previously vaccinated. HPV vaccination is known to be highly effective at cervical, anal, and oral regions in HPV-naive individuals and to have reduced impact in women aged 18-25.
What’s unknown is the combined multi-site vaccine efficacy on an individual basis, and whether HPV vaccine protects noninfected sites against HPV infection, Dr. Beachler, a postdoctoral fellow in the Infections and Immunoepidemiology Branch of the National Cancer Institute, said.
The phase III, National Cancer Institute HPV Vaccine Trial in Costa Rica enrolled 7,466 women, aged 18-25 years, over 6 months and randomly assigned them to one of three doses of the HPV16/18 vaccine (Cervarix) or to a hepatitis A vaccine (Havrix). Cervical samples were collected at every annual visit. Oral and anal samples were collected at only the 4-year follow-up visit. A total of 4,186 women contributed samples for all three sites and were included in the analysis.
Samples were tested for alpha mucosal HPV DNA types utilizing the SPF10 PCR-DEIA-LiPA25 system, version 1. An HPV event was defined as a woman with prevalent HPV16/18 DNA at the cervical, anal, or oral regions.
HPV naive was defined as HPV 16/18 seronegative and cervical HPV 16/18 DNA negative, prior infection was defined as HPV 16/18 antibody positive and cervical HPV 16/18 DNA negative, and active infection was defined as cervical HPV16/18 DNA positive.
Among all 4,186 women, multi-site vaccine efficacy at the 4-year visit was 65%, Dr. Beachler said.
HPV vaccinated women had fewer concordant infections, with vaccine efficacy reaching 91% for protection of at least two or three sites.
Indeed, only 7% of HPV 16/18-infected women in the vaccine arm had that same HPV type at two or more anatomic sites, compared with 30% of HPV 16/18-infected women in the control arm (P value < .01), again “giving support that the vaccine may provide some protection against uninfected sites in previously exposed women,” Dr. Beachler said.
He pointed out that the one-time sampling of oral and anal HPV and misclassification in assays could have affected the results. If complete information were available, vaccine efficacy would likely have been closer to 100% in the HPV-naive subgroup, he said.
Follow-up is continuing in the trial to determine the duration of protection, but there is still strong evidence of protection at 9 years, suggesting a booster dose is not necessary, Dr. Beachler said.
Further research of HPV infection outside the cervix, however, is very much needed.
“We know that only a certain percentage, 60%-70% of people who are infected, actually develop a serological response. We don’t even really know if an oral HPV infection or an anal HPV infection produces a serological response. We really need to understand HPV outside the cervix,” he said.
The National Cancer Institute funded the study. GlaxoSmithKline provided vaccines and support for regulatory aspects of the trial. Dr. Beachler declared no financial disclosures.
The human papillomavirus vaccine may afford protection to some women with prior HPV exposure, based on the results of a post-hoc analysis of the HPV Vaccine Trial in Costa Rica.
At the 4-year follow-up visit, multi-site efficacy against HPV16/18 DNA at the cervical, anal, or oral region was, as expected, the strongest at 84% among HPV-naive women who received the vaccine, but significant multi-site efficacy of 58% also was seen among women with evidence of HPV exposure prior to vaccination. Vaccine efficacy was nonsignificant at 25% in women with active cervical infection at vaccination, indicating that the vaccine is not therapeutic, study author Daniel C. Beachler, Ph.D., reported at the annual meeting of the American Association for Cancer Research.
The findings support current U.S. guidelines from the Centers for Disease Control and Prevention recommending routine vaccination for those aged 11-12 years, and vaccination through age 26 for those not previously vaccinated. HPV vaccination is known to be highly effective at cervical, anal, and oral regions in HPV-naive individuals and to have reduced impact in women aged 18-25.
What’s unknown is the combined multi-site vaccine efficacy on an individual basis, and whether HPV vaccine protects noninfected sites against HPV infection, Dr. Beachler, a postdoctoral fellow in the Infections and Immunoepidemiology Branch of the National Cancer Institute, said.
The phase III, National Cancer Institute HPV Vaccine Trial in Costa Rica enrolled 7,466 women, aged 18-25 years, over 6 months and randomly assigned them to one of three doses of the HPV16/18 vaccine (Cervarix) or to a hepatitis A vaccine (Havrix). Cervical samples were collected at every annual visit. Oral and anal samples were collected at only the 4-year follow-up visit. A total of 4,186 women contributed samples for all three sites and were included in the analysis.
Samples were tested for alpha mucosal HPV DNA types utilizing the SPF10 PCR-DEIA-LiPA25 system, version 1. An HPV event was defined as a woman with prevalent HPV16/18 DNA at the cervical, anal, or oral regions.
HPV naive was defined as HPV 16/18 seronegative and cervical HPV 16/18 DNA negative, prior infection was defined as HPV 16/18 antibody positive and cervical HPV 16/18 DNA negative, and active infection was defined as cervical HPV16/18 DNA positive.
Among all 4,186 women, multi-site vaccine efficacy at the 4-year visit was 65%, Dr. Beachler said.
HPV vaccinated women had fewer concordant infections, with vaccine efficacy reaching 91% for protection of at least two or three sites.
Indeed, only 7% of HPV 16/18-infected women in the vaccine arm had that same HPV type at two or more anatomic sites, compared with 30% of HPV 16/18-infected women in the control arm (P value < .01), again “giving support that the vaccine may provide some protection against uninfected sites in previously exposed women,” Dr. Beachler said.
He pointed out that the one-time sampling of oral and anal HPV and misclassification in assays could have affected the results. If complete information were available, vaccine efficacy would likely have been closer to 100% in the HPV-naive subgroup, he said.
Follow-up is continuing in the trial to determine the duration of protection, but there is still strong evidence of protection at 9 years, suggesting a booster dose is not necessary, Dr. Beachler said.
Further research of HPV infection outside the cervix, however, is very much needed.
“We know that only a certain percentage, 60%-70% of people who are infected, actually develop a serological response. We don’t even really know if an oral HPV infection or an anal HPV infection produces a serological response. We really need to understand HPV outside the cervix,” he said.
The National Cancer Institute funded the study. GlaxoSmithKline provided vaccines and support for regulatory aspects of the trial. Dr. Beachler declared no financial disclosures.
FROM THE AACR ANNUAL MEETING
Key clinical point: HPV vaccination provided strong protection at multiple anatomic sites in women without prior HPV exposure, and may still offer protection in those with prior exposure.
Major finding: Multi-site efficacy against HPV16/18 was 84% in HPV-naive women, and 58% in women with prior exposure.
Data source: Post-hoc analysis of 4,186 women in the HPV Vaccine Trial in Costa Rica.
Disclosures: The National Cancer Institute funded the study. GlaxoSmithKline provided vaccines and support for regulatory aspects of the trial. Dr. Beachler declared no financial disclosures.
Inhibitor may benefit certain ALL patients
PHILADELPHIA—Results of preclinical research suggest the BCL-2 inhibitor ABT-199 (venetoclax) may be effective in certain pediatric patients with acute lymphoblastic leukemia (ALL).
In xenograft models of various ALL subtypes, ABT-199 produced an objective response rate below 30%.
However, additional analyses unearthed information that could potentially help us identify which ALL patients might respond to the drug.
Santi Suryani, PhD, of the Children’s Cancer Institute in Sydney, New South Wales, Australia, and her colleagues presented this research at the AACR Annual Meeting 2015 (abstract 3276*). The work was supported by AbbVie, one of the companies developing ABT-199.
Dr Suryani and her colleagues decided to investigate ABT-199 in pediatric ALL after observing mixed results with the BCL-2/BCL-W/BCL-XL inhibitor ABT-263 (navitoclax).
ABT-263 delayed ALL progression in nearly all of the xenograft models the team tested and produced a 61% response rate. However, the drug also induced BCL-XL-mediated thrombocytopenia.
As ABT-199 doesn’t target BCL-XL, the researchers thought the drug might produce similar responses as ABT-263 without inducing thrombocytopenia.
“When ABT-199 came into the picture, we were very excited,” Dr Suryani said. “We thought, ‘This is a wonder drug. This will cure pediatric ALL.’”
To test this hypothesis, the team compared ABT-199 (100 mg/kg x 21 days) and vehicle control in 19 pediatric ALL patient-derived xenografts, including infant mixed-lineage leukemia (MLL) ALL (n=4), B-cell precursor (BCP) ALL (n=5), BCP-ALL categorized as Ph-like (n=4), T-cell ALL (n=4), and early T-cell precursor (ETP) ALL (n=2).
ABT-199 significantly delayed progression in 12 xenografts (63%) for periods ranging from 0.4 days to 28 days. And the drug produced objective responses in 5 xenografts (26%).
Responses occurred in MLL-ALL, BCP-ALL, and Ph-like BCP ALL, but not T-cell ALL or ETP-ALL. Complete responses were seen in MLL-ALL (n=1) and BCP-ALL (n=2), and partial responses occurred in MLL-ALL (n=1) and Ph-like BCP-ALL (n=1).
As the response rate with ABT-263 was more than double that of ABT-199 (61% vs 26%), the researchers found the results with ABT-199 “a little bit disappointing,” according to Dr Suryani.
“But we thought, ‘That’s okay. That already tells us the science behind it—that pediatric ALL is probably more BCL-XL-dependent, rather than BCL-2-dependent,’” she said. “We wondered if there was any way we could come up with a predictive biomarker so we could select patients who will benefit from this treatment.”
With that in mind, the researchers evaluated the link between protein expression and response. They looked at BCL-2 and BCL-XL, as well as a range of other proteins, including BCL-W, MCL1, BAK1, and BAX, among others.
And they found that high BCL-XL and low BCL-2 expression were significantly associated with ABT-199 resistance.
The researchers are still investigating ways to guide treatment with ABT-199 in ALL. They are also hoping to improve responses by administering the drug in combination with other agents. ![]()
*Information in the abstract differs from that presented at the meeting.
PHILADELPHIA—Results of preclinical research suggest the BCL-2 inhibitor ABT-199 (venetoclax) may be effective in certain pediatric patients with acute lymphoblastic leukemia (ALL).
In xenograft models of various ALL subtypes, ABT-199 produced an objective response rate below 30%.
However, additional analyses unearthed information that could potentially help us identify which ALL patients might respond to the drug.
Santi Suryani, PhD, of the Children’s Cancer Institute in Sydney, New South Wales, Australia, and her colleagues presented this research at the AACR Annual Meeting 2015 (abstract 3276*). The work was supported by AbbVie, one of the companies developing ABT-199.
Dr Suryani and her colleagues decided to investigate ABT-199 in pediatric ALL after observing mixed results with the BCL-2/BCL-W/BCL-XL inhibitor ABT-263 (navitoclax).
ABT-263 delayed ALL progression in nearly all of the xenograft models the team tested and produced a 61% response rate. However, the drug also induced BCL-XL-mediated thrombocytopenia.
As ABT-199 doesn’t target BCL-XL, the researchers thought the drug might produce similar responses as ABT-263 without inducing thrombocytopenia.
“When ABT-199 came into the picture, we were very excited,” Dr Suryani said. “We thought, ‘This is a wonder drug. This will cure pediatric ALL.’”
To test this hypothesis, the team compared ABT-199 (100 mg/kg x 21 days) and vehicle control in 19 pediatric ALL patient-derived xenografts, including infant mixed-lineage leukemia (MLL) ALL (n=4), B-cell precursor (BCP) ALL (n=5), BCP-ALL categorized as Ph-like (n=4), T-cell ALL (n=4), and early T-cell precursor (ETP) ALL (n=2).
ABT-199 significantly delayed progression in 12 xenografts (63%) for periods ranging from 0.4 days to 28 days. And the drug produced objective responses in 5 xenografts (26%).
Responses occurred in MLL-ALL, BCP-ALL, and Ph-like BCP ALL, but not T-cell ALL or ETP-ALL. Complete responses were seen in MLL-ALL (n=1) and BCP-ALL (n=2), and partial responses occurred in MLL-ALL (n=1) and Ph-like BCP-ALL (n=1).
As the response rate with ABT-263 was more than double that of ABT-199 (61% vs 26%), the researchers found the results with ABT-199 “a little bit disappointing,” according to Dr Suryani.
“But we thought, ‘That’s okay. That already tells us the science behind it—that pediatric ALL is probably more BCL-XL-dependent, rather than BCL-2-dependent,’” she said. “We wondered if there was any way we could come up with a predictive biomarker so we could select patients who will benefit from this treatment.”
With that in mind, the researchers evaluated the link between protein expression and response. They looked at BCL-2 and BCL-XL, as well as a range of other proteins, including BCL-W, MCL1, BAK1, and BAX, among others.
And they found that high BCL-XL and low BCL-2 expression were significantly associated with ABT-199 resistance.
The researchers are still investigating ways to guide treatment with ABT-199 in ALL. They are also hoping to improve responses by administering the drug in combination with other agents. ![]()
*Information in the abstract differs from that presented at the meeting.
PHILADELPHIA—Results of preclinical research suggest the BCL-2 inhibitor ABT-199 (venetoclax) may be effective in certain pediatric patients with acute lymphoblastic leukemia (ALL).
In xenograft models of various ALL subtypes, ABT-199 produced an objective response rate below 30%.
However, additional analyses unearthed information that could potentially help us identify which ALL patients might respond to the drug.
Santi Suryani, PhD, of the Children’s Cancer Institute in Sydney, New South Wales, Australia, and her colleagues presented this research at the AACR Annual Meeting 2015 (abstract 3276*). The work was supported by AbbVie, one of the companies developing ABT-199.
Dr Suryani and her colleagues decided to investigate ABT-199 in pediatric ALL after observing mixed results with the BCL-2/BCL-W/BCL-XL inhibitor ABT-263 (navitoclax).
ABT-263 delayed ALL progression in nearly all of the xenograft models the team tested and produced a 61% response rate. However, the drug also induced BCL-XL-mediated thrombocytopenia.
As ABT-199 doesn’t target BCL-XL, the researchers thought the drug might produce similar responses as ABT-263 without inducing thrombocytopenia.
“When ABT-199 came into the picture, we were very excited,” Dr Suryani said. “We thought, ‘This is a wonder drug. This will cure pediatric ALL.’”
To test this hypothesis, the team compared ABT-199 (100 mg/kg x 21 days) and vehicle control in 19 pediatric ALL patient-derived xenografts, including infant mixed-lineage leukemia (MLL) ALL (n=4), B-cell precursor (BCP) ALL (n=5), BCP-ALL categorized as Ph-like (n=4), T-cell ALL (n=4), and early T-cell precursor (ETP) ALL (n=2).
ABT-199 significantly delayed progression in 12 xenografts (63%) for periods ranging from 0.4 days to 28 days. And the drug produced objective responses in 5 xenografts (26%).
Responses occurred in MLL-ALL, BCP-ALL, and Ph-like BCP ALL, but not T-cell ALL or ETP-ALL. Complete responses were seen in MLL-ALL (n=1) and BCP-ALL (n=2), and partial responses occurred in MLL-ALL (n=1) and Ph-like BCP-ALL (n=1).
As the response rate with ABT-263 was more than double that of ABT-199 (61% vs 26%), the researchers found the results with ABT-199 “a little bit disappointing,” according to Dr Suryani.
“But we thought, ‘That’s okay. That already tells us the science behind it—that pediatric ALL is probably more BCL-XL-dependent, rather than BCL-2-dependent,’” she said. “We wondered if there was any way we could come up with a predictive biomarker so we could select patients who will benefit from this treatment.”
With that in mind, the researchers evaluated the link between protein expression and response. They looked at BCL-2 and BCL-XL, as well as a range of other proteins, including BCL-W, MCL1, BAK1, and BAX, among others.
And they found that high BCL-XL and low BCL-2 expression were significantly associated with ABT-199 resistance.
The researchers are still investigating ways to guide treatment with ABT-199 in ALL. They are also hoping to improve responses by administering the drug in combination with other agents. ![]()
*Information in the abstract differs from that presented at the meeting.
Agent preferentially targets FLT3-ITD AML

PHILADELPHIA—Preclinical research suggests a novel agent has preferential activity in acute myeloid leukemia (AML) with FMS-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) mutations.
The agent, VNLG-152, proved more cytotoxic in AML cell lines and patient samples with FLT3-ITD than in samples and cell lines with wild-type FLT3.
Exactly how and why this occurs remains somewhat of a mystery, however.
Sheetal Karne, MD, of the University of Maryland School of Medicine in Baltimore, and her colleagues detailed this mystery in a poster presentation at the AACR Annual Meeting 2015 (abstract 5408*).
Dr Karne noted that VNLG-152 targets translation by promoting the degradation of MAPK-interacting kinases (Mnks).
“[VNLG-152] has been previously published as functioning in Mnk degradation, which has been shown in triple-negative breast cancer and prostate cancer—in vivo and in vitro,” she said. “Our hypothesis was that, since [the drug] worked via decreasing translation, it would function in leukemia cells and, specifically, in leukemic cells with ITD mutations.”
So the investigators tested VNLG-152 in samples from AML patients, as well as both murine and human cell lines. They found that VNLG-152 was more cytotoxic in the presence of FLT3-ITD mutations, as evidenced by low micromolar IC50 concentrations.
The IC50 concentration was 3.4 μM in Ba/F3-ITD cells and 5.8 μM in Ba/F3-WT cells, which are murine cells transfected with human FLT3-ITD and wild-type FLT3, respectively. Similarly, the IC50 concentration was 1.8 μM in 32D-ITD cells and 18.2 μM in 32D-WT cells.
In the human FLT3-ITD AML cell lines MV4-11 and MOLM-14, IC50 concentrations were 2.3 μM and 4.2 μM, respectively. But concentrations were greater than 10 µM in the wild-type FLT3 human cell lines HL60 and U937.
In patient samples, the IC50 concentration was 1.0 μM in FLT3-ITD AML and 7.5 μM in AML with wild-type FLT3.
In additional tests with murine cell lines, the investigators found that VNLG-152 inhibits the growth of Ba/F3-ITD and Ba/F3-WT cells. But the drug induces apoptosis in these cell lines only when given in high concentrations.
Looking into the mechanism of VNLG-152, the investigators found that the drug decreased Mnk-1 expression in Ba/F3-ITD and Ba/F3-WT cell lines.
“We saw that VNLG-152 worked via degradation of Mnk, but it was the same in both wild-type and ITD, so we’re still looking for an explanation as to what caused this difference,” Dr Karne said.
She and her colleagues believe the Mnk degradation inhibits the phosphorylation of eukaryotic translation initiation factor 4E (eIF4E), a downstream target of FLT3-ITD. But they are still investigating that possibility.
The team is also hoping to test VNLG-152 in combination with other drugs, such as FLT3 inhibitors or chemotherapeutic agents. ![]()
*Information in the abstract differs from that presented at the meeting.

PHILADELPHIA—Preclinical research suggests a novel agent has preferential activity in acute myeloid leukemia (AML) with FMS-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) mutations.
The agent, VNLG-152, proved more cytotoxic in AML cell lines and patient samples with FLT3-ITD than in samples and cell lines with wild-type FLT3.
Exactly how and why this occurs remains somewhat of a mystery, however.
Sheetal Karne, MD, of the University of Maryland School of Medicine in Baltimore, and her colleagues detailed this mystery in a poster presentation at the AACR Annual Meeting 2015 (abstract 5408*).
Dr Karne noted that VNLG-152 targets translation by promoting the degradation of MAPK-interacting kinases (Mnks).
“[VNLG-152] has been previously published as functioning in Mnk degradation, which has been shown in triple-negative breast cancer and prostate cancer—in vivo and in vitro,” she said. “Our hypothesis was that, since [the drug] worked via decreasing translation, it would function in leukemia cells and, specifically, in leukemic cells with ITD mutations.”
So the investigators tested VNLG-152 in samples from AML patients, as well as both murine and human cell lines. They found that VNLG-152 was more cytotoxic in the presence of FLT3-ITD mutations, as evidenced by low micromolar IC50 concentrations.
The IC50 concentration was 3.4 μM in Ba/F3-ITD cells and 5.8 μM in Ba/F3-WT cells, which are murine cells transfected with human FLT3-ITD and wild-type FLT3, respectively. Similarly, the IC50 concentration was 1.8 μM in 32D-ITD cells and 18.2 μM in 32D-WT cells.
In the human FLT3-ITD AML cell lines MV4-11 and MOLM-14, IC50 concentrations were 2.3 μM and 4.2 μM, respectively. But concentrations were greater than 10 µM in the wild-type FLT3 human cell lines HL60 and U937.
In patient samples, the IC50 concentration was 1.0 μM in FLT3-ITD AML and 7.5 μM in AML with wild-type FLT3.
In additional tests with murine cell lines, the investigators found that VNLG-152 inhibits the growth of Ba/F3-ITD and Ba/F3-WT cells. But the drug induces apoptosis in these cell lines only when given in high concentrations.
Looking into the mechanism of VNLG-152, the investigators found that the drug decreased Mnk-1 expression in Ba/F3-ITD and Ba/F3-WT cell lines.
“We saw that VNLG-152 worked via degradation of Mnk, but it was the same in both wild-type and ITD, so we’re still looking for an explanation as to what caused this difference,” Dr Karne said.
She and her colleagues believe the Mnk degradation inhibits the phosphorylation of eukaryotic translation initiation factor 4E (eIF4E), a downstream target of FLT3-ITD. But they are still investigating that possibility.
The team is also hoping to test VNLG-152 in combination with other drugs, such as FLT3 inhibitors or chemotherapeutic agents. ![]()
*Information in the abstract differs from that presented at the meeting.

PHILADELPHIA—Preclinical research suggests a novel agent has preferential activity in acute myeloid leukemia (AML) with FMS-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) mutations.
The agent, VNLG-152, proved more cytotoxic in AML cell lines and patient samples with FLT3-ITD than in samples and cell lines with wild-type FLT3.
Exactly how and why this occurs remains somewhat of a mystery, however.
Sheetal Karne, MD, of the University of Maryland School of Medicine in Baltimore, and her colleagues detailed this mystery in a poster presentation at the AACR Annual Meeting 2015 (abstract 5408*).
Dr Karne noted that VNLG-152 targets translation by promoting the degradation of MAPK-interacting kinases (Mnks).
“[VNLG-152] has been previously published as functioning in Mnk degradation, which has been shown in triple-negative breast cancer and prostate cancer—in vivo and in vitro,” she said. “Our hypothesis was that, since [the drug] worked via decreasing translation, it would function in leukemia cells and, specifically, in leukemic cells with ITD mutations.”
So the investigators tested VNLG-152 in samples from AML patients, as well as both murine and human cell lines. They found that VNLG-152 was more cytotoxic in the presence of FLT3-ITD mutations, as evidenced by low micromolar IC50 concentrations.
The IC50 concentration was 3.4 μM in Ba/F3-ITD cells and 5.8 μM in Ba/F3-WT cells, which are murine cells transfected with human FLT3-ITD and wild-type FLT3, respectively. Similarly, the IC50 concentration was 1.8 μM in 32D-ITD cells and 18.2 μM in 32D-WT cells.
In the human FLT3-ITD AML cell lines MV4-11 and MOLM-14, IC50 concentrations were 2.3 μM and 4.2 μM, respectively. But concentrations were greater than 10 µM in the wild-type FLT3 human cell lines HL60 and U937.
In patient samples, the IC50 concentration was 1.0 μM in FLT3-ITD AML and 7.5 μM in AML with wild-type FLT3.
In additional tests with murine cell lines, the investigators found that VNLG-152 inhibits the growth of Ba/F3-ITD and Ba/F3-WT cells. But the drug induces apoptosis in these cell lines only when given in high concentrations.
Looking into the mechanism of VNLG-152, the investigators found that the drug decreased Mnk-1 expression in Ba/F3-ITD and Ba/F3-WT cell lines.
“We saw that VNLG-152 worked via degradation of Mnk, but it was the same in both wild-type and ITD, so we’re still looking for an explanation as to what caused this difference,” Dr Karne said.
She and her colleagues believe the Mnk degradation inhibits the phosphorylation of eukaryotic translation initiation factor 4E (eIF4E), a downstream target of FLT3-ITD. But they are still investigating that possibility.
The team is also hoping to test VNLG-152 in combination with other drugs, such as FLT3 inhibitors or chemotherapeutic agents. ![]()
*Information in the abstract differs from that presented at the meeting.
Defining the role of TAMs in DLBCL
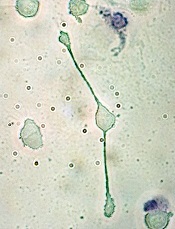
pseudopodia to engulf particles
PHILADELPHIA—New research suggests the prognostic value of tumor-associated macrophages (TAMs) is disease-specific as well as treatment-specific.
Investigators set out to determine if TAMs have a negative prognostic impact in diffuse large B-cell lymphoma (DLBCL), as previous studies produced conflicting results.
The team found that higher TAM levels are associated with worse survival in DLBCL, but only in patients who do not receive rituximab. The drug can overcome the poor prognosis TAMs confer in DLBCL.
Eri Matsuki, MD, PhD, of Memorial Sloan-Kettering Cancer Center in New York, New York, and her colleagues presented these findings in a poster at the AACR Annual Meeting 2015 (abstract 2371*).
To ascertain the role of TAMs in DLBCL, the investigators analyzed specimens from 103 DLBCL patients, 61 of whom received rituximab, 33 who did not, and 9 whose rituximab status was unknown. The team first looked at the expression of CD163 as a marker of TAMs.
“CD163 is more specific to M2-type macrophages, which have pro-tumor effects, compared to a more pan-macrophage marker which is widely used—CD68,” Dr Matsuki noted.
She and her colleagues found that a high level of CD163-positive cells (more than 150) was significantly associated with advanced-stage disease (P=0.016), non-GCB DLBCL (P=0.0071), and higher expression of c-Myc (P=0.0022).
“Our interpretation of that at this point is that, the more aggressive the tumor, the more likely that it would induce macrophages into the tissue,” Dr Matsuki said.
The investigators then assessed the impact of rituximab use. Among patients who didn’t receive rituximab, having a high level of CD163-positive cells (more than 150) was associated with inferior overall survival (OS, P=0.022). However, if patients did receive rituximab, there was no significant difference in OS.
Dr Matsuki said this supports previous studies showing that the prognostic effect of TAMs diminishes with rituximab use, as well as the in vitro finding that M2 macrophages exhibit increased phagocytosis of rituximab-opsonized tumor cells.
“So overall, what we’re seeing is that . . . the negative influence of TAMs can be overcome with rituximab use,” she summarized.
Dr Matsuki and her colleagues also looked at the patients’ lymphocyte-to-monocyte-ratio (LMR) because TAMs partly arise from peripheral blood monocytes.
The team found that having an LMR higher than 2.77 was significantly associated with superior OS (P=0.03) in patients who did not receive rituximab. And there was a trend toward improved OS with a higher LMR in patients who did receive the drug (P=0.07).
The investigators believe the differences they observed in the prognostic value of CD163 and LMR could be explained by the fact that TAMs are derived from both circulating monocytes and resident macrophages.
Dr Matsuki said this research has improved her group’s understanding of DLBCL, but they are still working to identify additional biomarkers associated with prognosis in this disease. ![]()
*Information in the abstract differs from that presented at the meeting.

pseudopodia to engulf particles
PHILADELPHIA—New research suggests the prognostic value of tumor-associated macrophages (TAMs) is disease-specific as well as treatment-specific.
Investigators set out to determine if TAMs have a negative prognostic impact in diffuse large B-cell lymphoma (DLBCL), as previous studies produced conflicting results.
The team found that higher TAM levels are associated with worse survival in DLBCL, but only in patients who do not receive rituximab. The drug can overcome the poor prognosis TAMs confer in DLBCL.
Eri Matsuki, MD, PhD, of Memorial Sloan-Kettering Cancer Center in New York, New York, and her colleagues presented these findings in a poster at the AACR Annual Meeting 2015 (abstract 2371*).
To ascertain the role of TAMs in DLBCL, the investigators analyzed specimens from 103 DLBCL patients, 61 of whom received rituximab, 33 who did not, and 9 whose rituximab status was unknown. The team first looked at the expression of CD163 as a marker of TAMs.
“CD163 is more specific to M2-type macrophages, which have pro-tumor effects, compared to a more pan-macrophage marker which is widely used—CD68,” Dr Matsuki noted.
She and her colleagues found that a high level of CD163-positive cells (more than 150) was significantly associated with advanced-stage disease (P=0.016), non-GCB DLBCL (P=0.0071), and higher expression of c-Myc (P=0.0022).
“Our interpretation of that at this point is that, the more aggressive the tumor, the more likely that it would induce macrophages into the tissue,” Dr Matsuki said.
The investigators then assessed the impact of rituximab use. Among patients who didn’t receive rituximab, having a high level of CD163-positive cells (more than 150) was associated with inferior overall survival (OS, P=0.022). However, if patients did receive rituximab, there was no significant difference in OS.
Dr Matsuki said this supports previous studies showing that the prognostic effect of TAMs diminishes with rituximab use, as well as the in vitro finding that M2 macrophages exhibit increased phagocytosis of rituximab-opsonized tumor cells.
“So overall, what we’re seeing is that . . . the negative influence of TAMs can be overcome with rituximab use,” she summarized.
Dr Matsuki and her colleagues also looked at the patients’ lymphocyte-to-monocyte-ratio (LMR) because TAMs partly arise from peripheral blood monocytes.
The team found that having an LMR higher than 2.77 was significantly associated with superior OS (P=0.03) in patients who did not receive rituximab. And there was a trend toward improved OS with a higher LMR in patients who did receive the drug (P=0.07).
The investigators believe the differences they observed in the prognostic value of CD163 and LMR could be explained by the fact that TAMs are derived from both circulating monocytes and resident macrophages.
Dr Matsuki said this research has improved her group’s understanding of DLBCL, but they are still working to identify additional biomarkers associated with prognosis in this disease. ![]()
*Information in the abstract differs from that presented at the meeting.

pseudopodia to engulf particles
PHILADELPHIA—New research suggests the prognostic value of tumor-associated macrophages (TAMs) is disease-specific as well as treatment-specific.
Investigators set out to determine if TAMs have a negative prognostic impact in diffuse large B-cell lymphoma (DLBCL), as previous studies produced conflicting results.
The team found that higher TAM levels are associated with worse survival in DLBCL, but only in patients who do not receive rituximab. The drug can overcome the poor prognosis TAMs confer in DLBCL.
Eri Matsuki, MD, PhD, of Memorial Sloan-Kettering Cancer Center in New York, New York, and her colleagues presented these findings in a poster at the AACR Annual Meeting 2015 (abstract 2371*).
To ascertain the role of TAMs in DLBCL, the investigators analyzed specimens from 103 DLBCL patients, 61 of whom received rituximab, 33 who did not, and 9 whose rituximab status was unknown. The team first looked at the expression of CD163 as a marker of TAMs.
“CD163 is more specific to M2-type macrophages, which have pro-tumor effects, compared to a more pan-macrophage marker which is widely used—CD68,” Dr Matsuki noted.
She and her colleagues found that a high level of CD163-positive cells (more than 150) was significantly associated with advanced-stage disease (P=0.016), non-GCB DLBCL (P=0.0071), and higher expression of c-Myc (P=0.0022).
“Our interpretation of that at this point is that, the more aggressive the tumor, the more likely that it would induce macrophages into the tissue,” Dr Matsuki said.
The investigators then assessed the impact of rituximab use. Among patients who didn’t receive rituximab, having a high level of CD163-positive cells (more than 150) was associated with inferior overall survival (OS, P=0.022). However, if patients did receive rituximab, there was no significant difference in OS.
Dr Matsuki said this supports previous studies showing that the prognostic effect of TAMs diminishes with rituximab use, as well as the in vitro finding that M2 macrophages exhibit increased phagocytosis of rituximab-opsonized tumor cells.
“So overall, what we’re seeing is that . . . the negative influence of TAMs can be overcome with rituximab use,” she summarized.
Dr Matsuki and her colleagues also looked at the patients’ lymphocyte-to-monocyte-ratio (LMR) because TAMs partly arise from peripheral blood monocytes.
The team found that having an LMR higher than 2.77 was significantly associated with superior OS (P=0.03) in patients who did not receive rituximab. And there was a trend toward improved OS with a higher LMR in patients who did receive the drug (P=0.07).
The investigators believe the differences they observed in the prognostic value of CD163 and LMR could be explained by the fact that TAMs are derived from both circulating monocytes and resident macrophages.
Dr Matsuki said this research has improved her group’s understanding of DLBCL, but they are still working to identify additional biomarkers associated with prognosis in this disease. ![]()
*Information in the abstract differs from that presented at the meeting.
AACR: IMCgp100 activity eyed in ocular melanoma
The first-in-class immunotherapy IMCgp100 was active in late-stage melanoma, particularly ocular melanoma, in an ongoing, phase I/IIa trial.
Four of 14 patients treated with a weekly regimen in the phase IIa portion of the trial had a partial or complete response by RECIST 1.1, including two partial responses persisting more than 18 months.
Responses were observed across a variety of anatomical sites including liver and lung, and in patients refractory to ipilimumab (Yervoy) and pembrolizumab (Keytruda), “suggesting efficacy beyond the use of emerging and licensed treatments for melanoma,” principal investigator Dr. Mark Middleton said at the annual meeting of the American Association for Cancer Research.
The two shorter-lived responses – a partial response for 5.9 months and a complete response for 4.7 months – were in patients with ocular melanoma. One had been treated with surgery only, but the other had significant prior therapy and progressed through surgery, chemotherapy, radiation, and ipilimumab, he said. A third uveal melanoma patient left the trial after receiving what was later determined to be a nontolerated dose.
Progression-free survival in the two ocular melanoma responders was 9 months and 12 months, which bears up very well, compared with chemotherapy and data reported last year with MEK inhibition (JAMA 2014;311:2397-2405), said Dr. Middleton of the University of Oxford, England.
Invited discussant Dr. Antoni Ribas of the University of California Los Angeles Jonsson Comprehensive Cancer Center, commented that the responses were impressive.
“Anyone who’s treated ocular melanoma knows that if there’s a bad disease out there, this is one,” Dr. Ribas said. “It’s the subtype of melanoma that would not respond to any of the other immunotherapies and now we’re seeing responses here.”
The study enrolled patients with stage IV or unresectable stage III melanoma who were HLA-A2 (human leukocyte antigen-A2) positive and had no standard therapeutic options or had an appropriate window between alternative therapeutic options.
Patients had an ECOG (Eastern Cooperative Oncology Group) performance status of 0 or 1, three-fourths had M1c disease, and 16 had prior exposure to immunotherapy.
The phase I portion of the trial was designed to determine the maximum tolerated dose (MTD) of IMCgp100, with results reported at last year’s AACR.
IMCgp100 was dosed using a weekly regimen or a high-intensity regimen consisting of four consecutive daily doses in 3-week cycles. The maximum tolerated dose (MTD) for weekly administration was 600 nanograms/kilogram, which was converted to a flat dose of 50 mcg. Dose escalation of the high-intensity regimen is ongoing.
IMCgp100 contains an enhanced T-cell receptor and an anti-CD3 antibody fragment and is designed to redirect T cells to kill gp100-positive melanoma cells.
There is no clear correlation as yet between gp100 expression as measured by immunohistochemistry in the tumors because one patient who measured negative has a response at one of the lower dose levels, Dr. Middleton said.
“This isn’t a CLIA [Clinical Laboratory Improvement Amendments]-validated test, so the value of this, in particular, when taking relatively small samples is uncertain, and our working hypothesis has to be that gp100 expression is required in order to see an effective therapeutic response, albeit the ability to measure it remains uncertain,” he said.
Four dose-limiting toxicities, predominantly grade 3 or 4 hypotension, have been reported and emerged first at the 405 mg/kg dose. Most events resolved within 1-3 days.
One grade 4 hypotension was associated with associated acute respiratory distress syndrome and severe dyspnea in a patient on the MTD and resolved after intensive supportive care. With additional experience managing the potential for hypotension, however, there has been no significant hypotension at the MTD in the expansion phase, Dr. Middleton observed.
A phase 1b/II trial is being planned to evaluate IMCgp100 in combination with the anti–programmed death ligand-1 antibody MEDI4736 and/or the anti-CTLA-4 antibody tremelimumab in metastatic melanoma, according to a recent joint statement from Immunocore, developer of IMCgp100, and MedImmune.
On Twitter @pwendl
The first-in-class immunotherapy IMCgp100 was active in late-stage melanoma, particularly ocular melanoma, in an ongoing, phase I/IIa trial.
Four of 14 patients treated with a weekly regimen in the phase IIa portion of the trial had a partial or complete response by RECIST 1.1, including two partial responses persisting more than 18 months.
Responses were observed across a variety of anatomical sites including liver and lung, and in patients refractory to ipilimumab (Yervoy) and pembrolizumab (Keytruda), “suggesting efficacy beyond the use of emerging and licensed treatments for melanoma,” principal investigator Dr. Mark Middleton said at the annual meeting of the American Association for Cancer Research.
The two shorter-lived responses – a partial response for 5.9 months and a complete response for 4.7 months – were in patients with ocular melanoma. One had been treated with surgery only, but the other had significant prior therapy and progressed through surgery, chemotherapy, radiation, and ipilimumab, he said. A third uveal melanoma patient left the trial after receiving what was later determined to be a nontolerated dose.
Progression-free survival in the two ocular melanoma responders was 9 months and 12 months, which bears up very well, compared with chemotherapy and data reported last year with MEK inhibition (JAMA 2014;311:2397-2405), said Dr. Middleton of the University of Oxford, England.
Invited discussant Dr. Antoni Ribas of the University of California Los Angeles Jonsson Comprehensive Cancer Center, commented that the responses were impressive.
“Anyone who’s treated ocular melanoma knows that if there’s a bad disease out there, this is one,” Dr. Ribas said. “It’s the subtype of melanoma that would not respond to any of the other immunotherapies and now we’re seeing responses here.”
The study enrolled patients with stage IV or unresectable stage III melanoma who were HLA-A2 (human leukocyte antigen-A2) positive and had no standard therapeutic options or had an appropriate window between alternative therapeutic options.
Patients had an ECOG (Eastern Cooperative Oncology Group) performance status of 0 or 1, three-fourths had M1c disease, and 16 had prior exposure to immunotherapy.
The phase I portion of the trial was designed to determine the maximum tolerated dose (MTD) of IMCgp100, with results reported at last year’s AACR.
IMCgp100 was dosed using a weekly regimen or a high-intensity regimen consisting of four consecutive daily doses in 3-week cycles. The maximum tolerated dose (MTD) for weekly administration was 600 nanograms/kilogram, which was converted to a flat dose of 50 mcg. Dose escalation of the high-intensity regimen is ongoing.
IMCgp100 contains an enhanced T-cell receptor and an anti-CD3 antibody fragment and is designed to redirect T cells to kill gp100-positive melanoma cells.
There is no clear correlation as yet between gp100 expression as measured by immunohistochemistry in the tumors because one patient who measured negative has a response at one of the lower dose levels, Dr. Middleton said.
“This isn’t a CLIA [Clinical Laboratory Improvement Amendments]-validated test, so the value of this, in particular, when taking relatively small samples is uncertain, and our working hypothesis has to be that gp100 expression is required in order to see an effective therapeutic response, albeit the ability to measure it remains uncertain,” he said.
Four dose-limiting toxicities, predominantly grade 3 or 4 hypotension, have been reported and emerged first at the 405 mg/kg dose. Most events resolved within 1-3 days.
One grade 4 hypotension was associated with associated acute respiratory distress syndrome and severe dyspnea in a patient on the MTD and resolved after intensive supportive care. With additional experience managing the potential for hypotension, however, there has been no significant hypotension at the MTD in the expansion phase, Dr. Middleton observed.
A phase 1b/II trial is being planned to evaluate IMCgp100 in combination with the anti–programmed death ligand-1 antibody MEDI4736 and/or the anti-CTLA-4 antibody tremelimumab in metastatic melanoma, according to a recent joint statement from Immunocore, developer of IMCgp100, and MedImmune.
On Twitter @pwendl
The first-in-class immunotherapy IMCgp100 was active in late-stage melanoma, particularly ocular melanoma, in an ongoing, phase I/IIa trial.
Four of 14 patients treated with a weekly regimen in the phase IIa portion of the trial had a partial or complete response by RECIST 1.1, including two partial responses persisting more than 18 months.
Responses were observed across a variety of anatomical sites including liver and lung, and in patients refractory to ipilimumab (Yervoy) and pembrolizumab (Keytruda), “suggesting efficacy beyond the use of emerging and licensed treatments for melanoma,” principal investigator Dr. Mark Middleton said at the annual meeting of the American Association for Cancer Research.
The two shorter-lived responses – a partial response for 5.9 months and a complete response for 4.7 months – were in patients with ocular melanoma. One had been treated with surgery only, but the other had significant prior therapy and progressed through surgery, chemotherapy, radiation, and ipilimumab, he said. A third uveal melanoma patient left the trial after receiving what was later determined to be a nontolerated dose.
Progression-free survival in the two ocular melanoma responders was 9 months and 12 months, which bears up very well, compared with chemotherapy and data reported last year with MEK inhibition (JAMA 2014;311:2397-2405), said Dr. Middleton of the University of Oxford, England.
Invited discussant Dr. Antoni Ribas of the University of California Los Angeles Jonsson Comprehensive Cancer Center, commented that the responses were impressive.
“Anyone who’s treated ocular melanoma knows that if there’s a bad disease out there, this is one,” Dr. Ribas said. “It’s the subtype of melanoma that would not respond to any of the other immunotherapies and now we’re seeing responses here.”
The study enrolled patients with stage IV or unresectable stage III melanoma who were HLA-A2 (human leukocyte antigen-A2) positive and had no standard therapeutic options or had an appropriate window between alternative therapeutic options.
Patients had an ECOG (Eastern Cooperative Oncology Group) performance status of 0 or 1, three-fourths had M1c disease, and 16 had prior exposure to immunotherapy.
The phase I portion of the trial was designed to determine the maximum tolerated dose (MTD) of IMCgp100, with results reported at last year’s AACR.
IMCgp100 was dosed using a weekly regimen or a high-intensity regimen consisting of four consecutive daily doses in 3-week cycles. The maximum tolerated dose (MTD) for weekly administration was 600 nanograms/kilogram, which was converted to a flat dose of 50 mcg. Dose escalation of the high-intensity regimen is ongoing.
IMCgp100 contains an enhanced T-cell receptor and an anti-CD3 antibody fragment and is designed to redirect T cells to kill gp100-positive melanoma cells.
There is no clear correlation as yet between gp100 expression as measured by immunohistochemistry in the tumors because one patient who measured negative has a response at one of the lower dose levels, Dr. Middleton said.
“This isn’t a CLIA [Clinical Laboratory Improvement Amendments]-validated test, so the value of this, in particular, when taking relatively small samples is uncertain, and our working hypothesis has to be that gp100 expression is required in order to see an effective therapeutic response, albeit the ability to measure it remains uncertain,” he said.
Four dose-limiting toxicities, predominantly grade 3 or 4 hypotension, have been reported and emerged first at the 405 mg/kg dose. Most events resolved within 1-3 days.
One grade 4 hypotension was associated with associated acute respiratory distress syndrome and severe dyspnea in a patient on the MTD and resolved after intensive supportive care. With additional experience managing the potential for hypotension, however, there has been no significant hypotension at the MTD in the expansion phase, Dr. Middleton observed.
A phase 1b/II trial is being planned to evaluate IMCgp100 in combination with the anti–programmed death ligand-1 antibody MEDI4736 and/or the anti-CTLA-4 antibody tremelimumab in metastatic melanoma, according to a recent joint statement from Immunocore, developer of IMCgp100, and MedImmune.
On Twitter @pwendl
FROM THE AACR ANNUAL MEETING
Key clinical point: IMCgp100 is clinically active in advanced melanoma, including ocular melanoma and patients refractory to immunotherapy.
Major finding: Four of 14 patients responded to IMCgp100.
Data source: Phase I/IIa trial in 17 patients with late-stage melanoma.
Disclosures: The study was funded by Immunocore. Dr. Middleton reported consulting for Amgen, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Millenium, and Roche, and research support to his institution from several drug companies including Immuocore. Dr. Ribas reported stock in Kite Pharma.
AACR: Targeted combo active in triple-negative breast cancer, ovarian cancer
Combining the poly(ADP-ribose) polymerase inhibitor olaparib and the investigational P13K inhibitor BKM 120 was safe and active in triple-negative breast cancer and ovarian cancer in a phase I trial.
Patients with both BRCA-mutant and BRCA-wildtype breast cancer responded to the combination. One patient with germline BRCA-wildtype triple-negative breast cancer (TNBC) and lung metastases had a partial response and remained on treatment for 20 cycles, or nearly 2 years, study author Dr. Ursula Matulonis reported at the annual meeting of the American Association for Cancer Research.
Rationale for the study lay in data from mouse models showing that combination olaparib and BKM120 was more effective than either drug alone in BRCA-mutant breast cancer and BRCA-wildtype TNBC. Similarities also exist between high-grade serous ovarian cancer and TNBC, including an association with germline BRCA mutations, sensitivity to platinum agents, and high copy number alteration rates, she said in a press briefing at the meeting.
Olaparib (Lynparza), a PARP (poly [ADP-ribose] polymerase) inhibitor, was approved in the United States in December 2014 for treating BRCA-positive advanced ovarian cancer.
The phase I, dose-escalation study enrolled 70 patients with a diagnosis of recurrent high-grade serous ovarian cancer or TNBC but also allowed documented germline BRCA mutation carriers regardless of histology.
The histology was high-grade serous in 90% of the 46 ovarian cancer patients, while 2% had high-grade endometrioid disease, 4% carcinosarcoma, and 4% poorly differentiated carcinoma. Most of the 24 breast cancer patients (63%) had TNBC, while 29% had estrogen receptor–positive/progesterone receptor–positive disease and 8% had ER+/PR– breast cancer.
Germline BRCA mutations were present in 77% of ovarian and 58% of breast cancer patients. The median age in the two groups was 60 years and 47.5 years, respectively. Prior PARP or P13kinase pathway inhibitors were allowed during dose escalation.
Among ovarian cancer patients, 12 (26%) had a partial response and 22 (48%) had stable disease. Responses were similar in the breast cancer group, with 5 (21%) partial responses and 12 (50%) patients with stable disease, said Dr. Matulonis of the Dana-Farber Cancer Center and Harvard Medical School, both in Boston.
Ten dosing regimens were evaluated in the study, beginning with an initial dose of BKM120 60 mg once daily and olaparib 100 mg twice daily, both given orally on a continuous basis. This elicited two dose-limiting toxicities (DLTs) – grade 3 hyperglycemia and grade 3 transaminitis – and prompted the investigators to back down to dose levels of 40 mg and 50 mg, respectively.
No DLTs occurred until dosing reached BKM120 60 mg and olaparib 300 mg, at which point one grade-4 transaminitis and one grade-3 depression were reported in cycle 2, she said. Dose levels of 50 mg and 300 mg, respectively, were selected for the expansion cohort.
As for why the two DLTs occurred at the initial dose but the same doses were later used without incident, Dr. Matulonis said that one of the patients with a DLT fell out of well-controlled diabetes and the other had liver metastases that accelerated during treatment.
Overall, the most common nonhematologic toxicities of any grade were nausea (79.4%), fatigue (66%), and hyperglycemia (40%). Related hematologic toxicities of any grade were anemia in 23.5%, neutropenia in 12%, and thrombocytopenia and leukopenia, both in 10% of patients.
“Combinations of biologic agents will require establishment of target patient populations using biomarkers in order to predict sensitivity as well as determine mechanisms of resistance,” Dr. Matulonis concluded.
Next-generation sequencing is ongoing for BKM120/olaparib patients and mechanisms of response and resistance are being studied in human ovarian mouse models, she added.
The study was funded by Stand Up to Cancer, the Kathryn Fox Samway Foundation, and participating centers. Olaparib was provided by AstraZeneca and BKM120 by Novartis. Dr. Matulonis reported research funding from AstraZeneca, as well as renumeration for attending a speaker’s bureau.
On Twitter @pwendl
Combining the poly(ADP-ribose) polymerase inhibitor olaparib and the investigational P13K inhibitor BKM 120 was safe and active in triple-negative breast cancer and ovarian cancer in a phase I trial.
Patients with both BRCA-mutant and BRCA-wildtype breast cancer responded to the combination. One patient with germline BRCA-wildtype triple-negative breast cancer (TNBC) and lung metastases had a partial response and remained on treatment for 20 cycles, or nearly 2 years, study author Dr. Ursula Matulonis reported at the annual meeting of the American Association for Cancer Research.
Rationale for the study lay in data from mouse models showing that combination olaparib and BKM120 was more effective than either drug alone in BRCA-mutant breast cancer and BRCA-wildtype TNBC. Similarities also exist between high-grade serous ovarian cancer and TNBC, including an association with germline BRCA mutations, sensitivity to platinum agents, and high copy number alteration rates, she said in a press briefing at the meeting.
Olaparib (Lynparza), a PARP (poly [ADP-ribose] polymerase) inhibitor, was approved in the United States in December 2014 for treating BRCA-positive advanced ovarian cancer.
The phase I, dose-escalation study enrolled 70 patients with a diagnosis of recurrent high-grade serous ovarian cancer or TNBC but also allowed documented germline BRCA mutation carriers regardless of histology.
The histology was high-grade serous in 90% of the 46 ovarian cancer patients, while 2% had high-grade endometrioid disease, 4% carcinosarcoma, and 4% poorly differentiated carcinoma. Most of the 24 breast cancer patients (63%) had TNBC, while 29% had estrogen receptor–positive/progesterone receptor–positive disease and 8% had ER+/PR– breast cancer.
Germline BRCA mutations were present in 77% of ovarian and 58% of breast cancer patients. The median age in the two groups was 60 years and 47.5 years, respectively. Prior PARP or P13kinase pathway inhibitors were allowed during dose escalation.
Among ovarian cancer patients, 12 (26%) had a partial response and 22 (48%) had stable disease. Responses were similar in the breast cancer group, with 5 (21%) partial responses and 12 (50%) patients with stable disease, said Dr. Matulonis of the Dana-Farber Cancer Center and Harvard Medical School, both in Boston.
Ten dosing regimens were evaluated in the study, beginning with an initial dose of BKM120 60 mg once daily and olaparib 100 mg twice daily, both given orally on a continuous basis. This elicited two dose-limiting toxicities (DLTs) – grade 3 hyperglycemia and grade 3 transaminitis – and prompted the investigators to back down to dose levels of 40 mg and 50 mg, respectively.
No DLTs occurred until dosing reached BKM120 60 mg and olaparib 300 mg, at which point one grade-4 transaminitis and one grade-3 depression were reported in cycle 2, she said. Dose levels of 50 mg and 300 mg, respectively, were selected for the expansion cohort.
As for why the two DLTs occurred at the initial dose but the same doses were later used without incident, Dr. Matulonis said that one of the patients with a DLT fell out of well-controlled diabetes and the other had liver metastases that accelerated during treatment.
Overall, the most common nonhematologic toxicities of any grade were nausea (79.4%), fatigue (66%), and hyperglycemia (40%). Related hematologic toxicities of any grade were anemia in 23.5%, neutropenia in 12%, and thrombocytopenia and leukopenia, both in 10% of patients.
“Combinations of biologic agents will require establishment of target patient populations using biomarkers in order to predict sensitivity as well as determine mechanisms of resistance,” Dr. Matulonis concluded.
Next-generation sequencing is ongoing for BKM120/olaparib patients and mechanisms of response and resistance are being studied in human ovarian mouse models, she added.
The study was funded by Stand Up to Cancer, the Kathryn Fox Samway Foundation, and participating centers. Olaparib was provided by AstraZeneca and BKM120 by Novartis. Dr. Matulonis reported research funding from AstraZeneca, as well as renumeration for attending a speaker’s bureau.
On Twitter @pwendl
Combining the poly(ADP-ribose) polymerase inhibitor olaparib and the investigational P13K inhibitor BKM 120 was safe and active in triple-negative breast cancer and ovarian cancer in a phase I trial.
Patients with both BRCA-mutant and BRCA-wildtype breast cancer responded to the combination. One patient with germline BRCA-wildtype triple-negative breast cancer (TNBC) and lung metastases had a partial response and remained on treatment for 20 cycles, or nearly 2 years, study author Dr. Ursula Matulonis reported at the annual meeting of the American Association for Cancer Research.
Rationale for the study lay in data from mouse models showing that combination olaparib and BKM120 was more effective than either drug alone in BRCA-mutant breast cancer and BRCA-wildtype TNBC. Similarities also exist between high-grade serous ovarian cancer and TNBC, including an association with germline BRCA mutations, sensitivity to platinum agents, and high copy number alteration rates, she said in a press briefing at the meeting.
Olaparib (Lynparza), a PARP (poly [ADP-ribose] polymerase) inhibitor, was approved in the United States in December 2014 for treating BRCA-positive advanced ovarian cancer.
The phase I, dose-escalation study enrolled 70 patients with a diagnosis of recurrent high-grade serous ovarian cancer or TNBC but also allowed documented germline BRCA mutation carriers regardless of histology.
The histology was high-grade serous in 90% of the 46 ovarian cancer patients, while 2% had high-grade endometrioid disease, 4% carcinosarcoma, and 4% poorly differentiated carcinoma. Most of the 24 breast cancer patients (63%) had TNBC, while 29% had estrogen receptor–positive/progesterone receptor–positive disease and 8% had ER+/PR– breast cancer.
Germline BRCA mutations were present in 77% of ovarian and 58% of breast cancer patients. The median age in the two groups was 60 years and 47.5 years, respectively. Prior PARP or P13kinase pathway inhibitors were allowed during dose escalation.
Among ovarian cancer patients, 12 (26%) had a partial response and 22 (48%) had stable disease. Responses were similar in the breast cancer group, with 5 (21%) partial responses and 12 (50%) patients with stable disease, said Dr. Matulonis of the Dana-Farber Cancer Center and Harvard Medical School, both in Boston.
Ten dosing regimens were evaluated in the study, beginning with an initial dose of BKM120 60 mg once daily and olaparib 100 mg twice daily, both given orally on a continuous basis. This elicited two dose-limiting toxicities (DLTs) – grade 3 hyperglycemia and grade 3 transaminitis – and prompted the investigators to back down to dose levels of 40 mg and 50 mg, respectively.
No DLTs occurred until dosing reached BKM120 60 mg and olaparib 300 mg, at which point one grade-4 transaminitis and one grade-3 depression were reported in cycle 2, she said. Dose levels of 50 mg and 300 mg, respectively, were selected for the expansion cohort.
As for why the two DLTs occurred at the initial dose but the same doses were later used without incident, Dr. Matulonis said that one of the patients with a DLT fell out of well-controlled diabetes and the other had liver metastases that accelerated during treatment.
Overall, the most common nonhematologic toxicities of any grade were nausea (79.4%), fatigue (66%), and hyperglycemia (40%). Related hematologic toxicities of any grade were anemia in 23.5%, neutropenia in 12%, and thrombocytopenia and leukopenia, both in 10% of patients.
“Combinations of biologic agents will require establishment of target patient populations using biomarkers in order to predict sensitivity as well as determine mechanisms of resistance,” Dr. Matulonis concluded.
Next-generation sequencing is ongoing for BKM120/olaparib patients and mechanisms of response and resistance are being studied in human ovarian mouse models, she added.
The study was funded by Stand Up to Cancer, the Kathryn Fox Samway Foundation, and participating centers. Olaparib was provided by AstraZeneca and BKM120 by Novartis. Dr. Matulonis reported research funding from AstraZeneca, as well as renumeration for attending a speaker’s bureau.
On Twitter @pwendl
FROM THE AACR ANNUAL MEETING
Key clinical point: Combining the PARP inhibitor olaparib and the investigational P13K inhibitor BKM 120 is safe and active in triple-negative breast cancer and ovarian cancer in early studies.
Major finding: Partial responses occurred in 26% of patients with ovarian cancer and 21% with breast cancer.
Data source: Phase I study in 70 women with ovarian cancer or breast cancer.
Disclosures: The study was funded by Stand Up to Cancer, the Kathryn Fox Samway Foundation, and participating centers. Olaparib was provided by AstraZeneca and BKM120 by Novartis. Dr. Matulonis reported research funding from AstraZeneca, as well as renumeration for attending a speaker’s bureau.
Susceptibility to 2nd cancers in WM/LPL survivors

PHILADELPHIA—A retrospective study has revealed factors that appear to influence a person’s susceptibility to Waldenström’s macroglobulinemia (WM)/lymphoplasmacytic lymphoma (LPL) and other malignancies.
Study investigators looked at patients diagnosed with WM or LPL over a 20-year period and found about a 50% excess of second primary cancers in this population.
The patients had a significantly increased risk of multiple hematologic and solid tumor malignancies, and a few of these malignancies had shared susceptibility factors with WM/LPL.
The investigators believe that identifying these factors may prove useful for determining genetic susceptibility to WM/LPL.
Mary L. McMaster, MD, of the National Cancer Institute in Bethesda, Maryland, and her colleagues presented these findings at the AACR Annual Meeting 2015 (abstract 3709).
The team used data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SSER) database to evaluate the risk of subsequent primary cancer in 3825 patients diagnosed with WM (n=2163) or LPL (n=1662) from 1992 to 2011. The patients’ median age was 70, most of them were male (n=2221), and most were white (n=3153).
Dr McMaster said she and her colleagues looked at both WM and LPL in this study because SEER does not include information about immunoglobulin subtype, which makes it difficult to identify all WM cases with absolute certainty.
“[D]epending on what information a pathologist has when they review a bone marrow biopsy, for example, they may or may not know whether there’s IgM present,” Dr McMaster said. “So you may have a diagnosis of LPL and not have the information required to make the diagnosis of WM. For that reason, we combined both entities for this study.”
Dr McMaster and her colleagues calculated the observed-to-expected standardized incidence ratios (SIRs) for invasive cancers. After adjusting for multiple comparisons, the team found that survivors of WM/LPL had a significantly increased risk of developing a second primary malignancy (SIR=1.49).
This increased risk was seen for males and females and persisted throughout follow-up. The risk was higher for patients younger than 65 years of age (SIR=1.95).
Hematologic malignancies
WM/LPL survivors had a significantly increased risk of several hematologic malignancies. The SIR was 4.09 for all hematologic malignancies, 4.29 for lymphomas, and 3.16 for leukemias.
Dr McMaster pointed out that several lymphoma subtypes can have lymphoplasmacytic differentiation, the most common being marginal zone lymphoma. And this could potentially result in misclassification.
“So we actually ran the study with and without marginal zone lymphoma and saw no difference in the results,” she said. “So we don’t think misclassification accounts for the majority of what we’re seeing.”
The investigators found that WM/LPL survivors had the highest risk of developing Burkitt lymphoma (SIR=13.45), followed by Hodgkin lymphoma (SIR=9.80), T-cell non-Hodgkin lymphoma (SIR=6.62), mantle cell lymphoma (SIR=5.37), diffuse large B-cell lymphoma (DLBCL, SIR=4.76), multiple myeloma (SIR=4.40), any non-Hodgkin lymphoma (SIR=4.08), and acute myeloid leukemia (AML, SIR=3.27).
“Waldenström’s is known to transform, on occasion, to DLBCL,” Dr McMaster said. “So that may well account for the excess of DLBCL that we see in this population.”
She also noted that, prior to the early 2000s, WM was typically treated with alkylating agents. And alkylating agents have been linked to an increased risk of AML.
In this population, the risk of AML peaked 5 to 10 years after WM/LPL diagnosis and was only present in patients treated prior to 2002. This suggests the AML observed in this study was likely treatment-related.
Dr McMaster and her colleagues also found that WM/LPL survivors did not have a significantly increased risk of developing acute lymphocytic leukemia (SIR=0), hairy cell leukemia (SIR=0), chronic lymphocytic leukemia/small lymphocytic lymphoma (SIR=0.97), or follicular lymphoma (SIR=2.25).
Solid tumors
WM/LPL survivors did have a significantly increased risk of certain solid tumor malignancies. The overall SIR for solid tumors was 1.21.
The risk was significant for non-epithelial skin cancers (SIR=5.15), thyroid cancers (SIR=3.13), melanoma (SIR=1.72), and cancers of the lung and bronchus (SIR=1.44) or respiratory system (SIR=1.42).
“Melanoma has an immunological basis, as does Waldenström’s, so we think there may be some shared etiology there,” Dr McMaster said.
She also noted that a strong risk factor for thyroid cancer, particularly papillary thyroid cancer, is a history of autoimmune thyroid disease.
“Autoimmune disease of any sort is a risk factor for Waldenström’s macroglobulinemia,” she said. “So again, we think there might be a basis for shared susceptibility there.”
Dr McMaster said this research suggests that multiple primary cancers may occur in a single individual because of shared genetic susceptibility, shared environmental exposures, treatment effects, or chance. She believes future research will show that both genetic and environmental factors contribute to WM.
Investigators are currently conducting whole-exome sequencing studies and genome-wide association studies in patients with familial and spontaneous WM, with the hopes of identifying genes that contribute to WM susceptibility. ![]()

PHILADELPHIA—A retrospective study has revealed factors that appear to influence a person’s susceptibility to Waldenström’s macroglobulinemia (WM)/lymphoplasmacytic lymphoma (LPL) and other malignancies.
Study investigators looked at patients diagnosed with WM or LPL over a 20-year period and found about a 50% excess of second primary cancers in this population.
The patients had a significantly increased risk of multiple hematologic and solid tumor malignancies, and a few of these malignancies had shared susceptibility factors with WM/LPL.
The investigators believe that identifying these factors may prove useful for determining genetic susceptibility to WM/LPL.
Mary L. McMaster, MD, of the National Cancer Institute in Bethesda, Maryland, and her colleagues presented these findings at the AACR Annual Meeting 2015 (abstract 3709).
The team used data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SSER) database to evaluate the risk of subsequent primary cancer in 3825 patients diagnosed with WM (n=2163) or LPL (n=1662) from 1992 to 2011. The patients’ median age was 70, most of them were male (n=2221), and most were white (n=3153).
Dr McMaster said she and her colleagues looked at both WM and LPL in this study because SEER does not include information about immunoglobulin subtype, which makes it difficult to identify all WM cases with absolute certainty.
“[D]epending on what information a pathologist has when they review a bone marrow biopsy, for example, they may or may not know whether there’s IgM present,” Dr McMaster said. “So you may have a diagnosis of LPL and not have the information required to make the diagnosis of WM. For that reason, we combined both entities for this study.”
Dr McMaster and her colleagues calculated the observed-to-expected standardized incidence ratios (SIRs) for invasive cancers. After adjusting for multiple comparisons, the team found that survivors of WM/LPL had a significantly increased risk of developing a second primary malignancy (SIR=1.49).
This increased risk was seen for males and females and persisted throughout follow-up. The risk was higher for patients younger than 65 years of age (SIR=1.95).
Hematologic malignancies
WM/LPL survivors had a significantly increased risk of several hematologic malignancies. The SIR was 4.09 for all hematologic malignancies, 4.29 for lymphomas, and 3.16 for leukemias.
Dr McMaster pointed out that several lymphoma subtypes can have lymphoplasmacytic differentiation, the most common being marginal zone lymphoma. And this could potentially result in misclassification.
“So we actually ran the study with and without marginal zone lymphoma and saw no difference in the results,” she said. “So we don’t think misclassification accounts for the majority of what we’re seeing.”
The investigators found that WM/LPL survivors had the highest risk of developing Burkitt lymphoma (SIR=13.45), followed by Hodgkin lymphoma (SIR=9.80), T-cell non-Hodgkin lymphoma (SIR=6.62), mantle cell lymphoma (SIR=5.37), diffuse large B-cell lymphoma (DLBCL, SIR=4.76), multiple myeloma (SIR=4.40), any non-Hodgkin lymphoma (SIR=4.08), and acute myeloid leukemia (AML, SIR=3.27).
“Waldenström’s is known to transform, on occasion, to DLBCL,” Dr McMaster said. “So that may well account for the excess of DLBCL that we see in this population.”
She also noted that, prior to the early 2000s, WM was typically treated with alkylating agents. And alkylating agents have been linked to an increased risk of AML.
In this population, the risk of AML peaked 5 to 10 years after WM/LPL diagnosis and was only present in patients treated prior to 2002. This suggests the AML observed in this study was likely treatment-related.
Dr McMaster and her colleagues also found that WM/LPL survivors did not have a significantly increased risk of developing acute lymphocytic leukemia (SIR=0), hairy cell leukemia (SIR=0), chronic lymphocytic leukemia/small lymphocytic lymphoma (SIR=0.97), or follicular lymphoma (SIR=2.25).
Solid tumors
WM/LPL survivors did have a significantly increased risk of certain solid tumor malignancies. The overall SIR for solid tumors was 1.21.
The risk was significant for non-epithelial skin cancers (SIR=5.15), thyroid cancers (SIR=3.13), melanoma (SIR=1.72), and cancers of the lung and bronchus (SIR=1.44) or respiratory system (SIR=1.42).
“Melanoma has an immunological basis, as does Waldenström’s, so we think there may be some shared etiology there,” Dr McMaster said.
She also noted that a strong risk factor for thyroid cancer, particularly papillary thyroid cancer, is a history of autoimmune thyroid disease.
“Autoimmune disease of any sort is a risk factor for Waldenström’s macroglobulinemia,” she said. “So again, we think there might be a basis for shared susceptibility there.”
Dr McMaster said this research suggests that multiple primary cancers may occur in a single individual because of shared genetic susceptibility, shared environmental exposures, treatment effects, or chance. She believes future research will show that both genetic and environmental factors contribute to WM.
Investigators are currently conducting whole-exome sequencing studies and genome-wide association studies in patients with familial and spontaneous WM, with the hopes of identifying genes that contribute to WM susceptibility. ![]()

PHILADELPHIA—A retrospective study has revealed factors that appear to influence a person’s susceptibility to Waldenström’s macroglobulinemia (WM)/lymphoplasmacytic lymphoma (LPL) and other malignancies.
Study investigators looked at patients diagnosed with WM or LPL over a 20-year period and found about a 50% excess of second primary cancers in this population.
The patients had a significantly increased risk of multiple hematologic and solid tumor malignancies, and a few of these malignancies had shared susceptibility factors with WM/LPL.
The investigators believe that identifying these factors may prove useful for determining genetic susceptibility to WM/LPL.
Mary L. McMaster, MD, of the National Cancer Institute in Bethesda, Maryland, and her colleagues presented these findings at the AACR Annual Meeting 2015 (abstract 3709).
The team used data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SSER) database to evaluate the risk of subsequent primary cancer in 3825 patients diagnosed with WM (n=2163) or LPL (n=1662) from 1992 to 2011. The patients’ median age was 70, most of them were male (n=2221), and most were white (n=3153).
Dr McMaster said she and her colleagues looked at both WM and LPL in this study because SEER does not include information about immunoglobulin subtype, which makes it difficult to identify all WM cases with absolute certainty.
“[D]epending on what information a pathologist has when they review a bone marrow biopsy, for example, they may or may not know whether there’s IgM present,” Dr McMaster said. “So you may have a diagnosis of LPL and not have the information required to make the diagnosis of WM. For that reason, we combined both entities for this study.”
Dr McMaster and her colleagues calculated the observed-to-expected standardized incidence ratios (SIRs) for invasive cancers. After adjusting for multiple comparisons, the team found that survivors of WM/LPL had a significantly increased risk of developing a second primary malignancy (SIR=1.49).
This increased risk was seen for males and females and persisted throughout follow-up. The risk was higher for patients younger than 65 years of age (SIR=1.95).
Hematologic malignancies
WM/LPL survivors had a significantly increased risk of several hematologic malignancies. The SIR was 4.09 for all hematologic malignancies, 4.29 for lymphomas, and 3.16 for leukemias.
Dr McMaster pointed out that several lymphoma subtypes can have lymphoplasmacytic differentiation, the most common being marginal zone lymphoma. And this could potentially result in misclassification.
“So we actually ran the study with and without marginal zone lymphoma and saw no difference in the results,” she said. “So we don’t think misclassification accounts for the majority of what we’re seeing.”
The investigators found that WM/LPL survivors had the highest risk of developing Burkitt lymphoma (SIR=13.45), followed by Hodgkin lymphoma (SIR=9.80), T-cell non-Hodgkin lymphoma (SIR=6.62), mantle cell lymphoma (SIR=5.37), diffuse large B-cell lymphoma (DLBCL, SIR=4.76), multiple myeloma (SIR=4.40), any non-Hodgkin lymphoma (SIR=4.08), and acute myeloid leukemia (AML, SIR=3.27).
“Waldenström’s is known to transform, on occasion, to DLBCL,” Dr McMaster said. “So that may well account for the excess of DLBCL that we see in this population.”
She also noted that, prior to the early 2000s, WM was typically treated with alkylating agents. And alkylating agents have been linked to an increased risk of AML.
In this population, the risk of AML peaked 5 to 10 years after WM/LPL diagnosis and was only present in patients treated prior to 2002. This suggests the AML observed in this study was likely treatment-related.
Dr McMaster and her colleagues also found that WM/LPL survivors did not have a significantly increased risk of developing acute lymphocytic leukemia (SIR=0), hairy cell leukemia (SIR=0), chronic lymphocytic leukemia/small lymphocytic lymphoma (SIR=0.97), or follicular lymphoma (SIR=2.25).
Solid tumors
WM/LPL survivors did have a significantly increased risk of certain solid tumor malignancies. The overall SIR for solid tumors was 1.21.
The risk was significant for non-epithelial skin cancers (SIR=5.15), thyroid cancers (SIR=3.13), melanoma (SIR=1.72), and cancers of the lung and bronchus (SIR=1.44) or respiratory system (SIR=1.42).
“Melanoma has an immunological basis, as does Waldenström’s, so we think there may be some shared etiology there,” Dr McMaster said.
She also noted that a strong risk factor for thyroid cancer, particularly papillary thyroid cancer, is a history of autoimmune thyroid disease.
“Autoimmune disease of any sort is a risk factor for Waldenström’s macroglobulinemia,” she said. “So again, we think there might be a basis for shared susceptibility there.”
Dr McMaster said this research suggests that multiple primary cancers may occur in a single individual because of shared genetic susceptibility, shared environmental exposures, treatment effects, or chance. She believes future research will show that both genetic and environmental factors contribute to WM.
Investigators are currently conducting whole-exome sequencing studies and genome-wide association studies in patients with familial and spontaneous WM, with the hopes of identifying genes that contribute to WM susceptibility. ![]()
AACR: Biomarker of response to olaparib found
A phase II study evaluating single-agent olaparib in metastatic, castration-resistant prostate cancer offers the first evidence that genetic testing could be used to select patients for treatment.
The overall response rate was 86.7% in a subgroup of men (14/16) identified as harboring genomic defects in DNA repair genes and 32.7% among all evaluable patients (16/49).
Median radiological progression-free survival was estimated at 13.2 months in men with mutations and 2.7 months in those without mutations (hazard ratio, 0.21; P < .001), Dr. Joaquin Mateo of the Institute of Cancer Research and the Royal Marsden NHS Trust, London, reported at the annual meeting of the American Association for Cancer Research.
“We hope that this is a step towards molecular stratification of treatment for prostate cancer. We are a year behind breast cancer or colorectal cancer investigators,” he said during a press briefing at the meeting.
Olaparib (Lynparza), a PARP (poly ADP-ribose polymerase) inhibitor ,was approved in the United States in December 2014 for treating BRCA-positive advanced ovarian cancer.
Most of the genomic aberrations in patients with prostate cancer occurred in BRCA2 and ATM, although biallelic loss of other relevant genes, including members of the Fanconi Anemia complementation group and CHEK2, were also observed. Research presented at the meeting suggests that BRCA2 is present in about 12% of prostate tumors and ATM in about 7%, Dr. Mateo told reporters.
The first phase of the study, referred to as TOPARP-A, enrolled 50 men from 7 U.K. centers who had metastatic, castration-resistant prostate cancer after 1-2 lines of taxane chemotherapy and a circulating tumor cell (CTC) count of at least 5 cells/7.5 mL of blood at screening. All patients had received prior docetaxel (Taxotere), 58% cabazitaxel (Jevtana), 96% abiraterone (Zytiga), 28% enzalutamide (Xtandi), and 26% palliative radiotherapy. The median baseline prostate specific antigen (PSA) was 349.5 mcg/L and median age was 67.5 years.
Patients received olaparib 400 mg twice daily continuously in a 28-day cycle. Response rate, the study’s primary endpoint, was defined as objective response by RECIST 1.1 and/or PSA decline of at least 50% and/or CTC count decline from at least 5 cells to less than 5 cells/7.5 mL blood. One patient was not evaluable for response.
Genomic defects in DNA repair genes, somatic and germline, were identified via next-generation sequencing and other studies of fresh tumor samples, taken before and while on olaparib.
Among the 16 unselected patients with responses, 6 had radiological responses and 11 had biochemical responses. Four of these responses have lasted more than 1 year, Dr. Mateo said.
Among those with genomic testing results, seven patients had a BRCA2 alteration and all responded to olaparib. Four of the five men with ATM truncating mutations responded.
PTEN loss and ERG rearrangements were not associated with response, he said.
The biomarker panel had a high specificity of 94% and sensitivity of 87.5%.
Consistent with previous olaparib studies, the most common grade 3 or higher adverse events were anemia (20%) and fatigue (12%), with 13% of patients requiring a dose reduction.
The next phase of the study, TOPARP-B, will validate the biomarker panel by enrolling only patients who screen positive for the DNA repair mutations linked to response in TOPARP-A, said Dr. Mateo, noting that samples can be turned around in 7-10 days.
Press briefing moderator Dr. William Nelson, director of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University in Baltimore, said newer drugs such as abiraterone and enzalutamide have given clinicians more mileage in treating castration-resistant prostate cancer by better targeting the androgen-receptor, but that the current results with olaparib will likely prompt more drug development off the androgen receptor.
The Cancer Research UK Clinical Trials Awards and Advisory Committee, SU2C, PCF, the Experimental Cancer Medicine Center, and the U.K. National Institute for Health Research Biomedical Research Center funded the study. Dr. Mateo disclosed no financial conflicts. Two coauthors reported serving as advisors to AstraZeneca
On Twitter @pwendl
A phase II study evaluating single-agent olaparib in metastatic, castration-resistant prostate cancer offers the first evidence that genetic testing could be used to select patients for treatment.
The overall response rate was 86.7% in a subgroup of men (14/16) identified as harboring genomic defects in DNA repair genes and 32.7% among all evaluable patients (16/49).
Median radiological progression-free survival was estimated at 13.2 months in men with mutations and 2.7 months in those without mutations (hazard ratio, 0.21; P < .001), Dr. Joaquin Mateo of the Institute of Cancer Research and the Royal Marsden NHS Trust, London, reported at the annual meeting of the American Association for Cancer Research.
“We hope that this is a step towards molecular stratification of treatment for prostate cancer. We are a year behind breast cancer or colorectal cancer investigators,” he said during a press briefing at the meeting.
Olaparib (Lynparza), a PARP (poly ADP-ribose polymerase) inhibitor ,was approved in the United States in December 2014 for treating BRCA-positive advanced ovarian cancer.
Most of the genomic aberrations in patients with prostate cancer occurred in BRCA2 and ATM, although biallelic loss of other relevant genes, including members of the Fanconi Anemia complementation group and CHEK2, were also observed. Research presented at the meeting suggests that BRCA2 is present in about 12% of prostate tumors and ATM in about 7%, Dr. Mateo told reporters.
The first phase of the study, referred to as TOPARP-A, enrolled 50 men from 7 U.K. centers who had metastatic, castration-resistant prostate cancer after 1-2 lines of taxane chemotherapy and a circulating tumor cell (CTC) count of at least 5 cells/7.5 mL of blood at screening. All patients had received prior docetaxel (Taxotere), 58% cabazitaxel (Jevtana), 96% abiraterone (Zytiga), 28% enzalutamide (Xtandi), and 26% palliative radiotherapy. The median baseline prostate specific antigen (PSA) was 349.5 mcg/L and median age was 67.5 years.
Patients received olaparib 400 mg twice daily continuously in a 28-day cycle. Response rate, the study’s primary endpoint, was defined as objective response by RECIST 1.1 and/or PSA decline of at least 50% and/or CTC count decline from at least 5 cells to less than 5 cells/7.5 mL blood. One patient was not evaluable for response.
Genomic defects in DNA repair genes, somatic and germline, were identified via next-generation sequencing and other studies of fresh tumor samples, taken before and while on olaparib.
Among the 16 unselected patients with responses, 6 had radiological responses and 11 had biochemical responses. Four of these responses have lasted more than 1 year, Dr. Mateo said.
Among those with genomic testing results, seven patients had a BRCA2 alteration and all responded to olaparib. Four of the five men with ATM truncating mutations responded.
PTEN loss and ERG rearrangements were not associated with response, he said.
The biomarker panel had a high specificity of 94% and sensitivity of 87.5%.
Consistent with previous olaparib studies, the most common grade 3 or higher adverse events were anemia (20%) and fatigue (12%), with 13% of patients requiring a dose reduction.
The next phase of the study, TOPARP-B, will validate the biomarker panel by enrolling only patients who screen positive for the DNA repair mutations linked to response in TOPARP-A, said Dr. Mateo, noting that samples can be turned around in 7-10 days.
Press briefing moderator Dr. William Nelson, director of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University in Baltimore, said newer drugs such as abiraterone and enzalutamide have given clinicians more mileage in treating castration-resistant prostate cancer by better targeting the androgen-receptor, but that the current results with olaparib will likely prompt more drug development off the androgen receptor.
The Cancer Research UK Clinical Trials Awards and Advisory Committee, SU2C, PCF, the Experimental Cancer Medicine Center, and the U.K. National Institute for Health Research Biomedical Research Center funded the study. Dr. Mateo disclosed no financial conflicts. Two coauthors reported serving as advisors to AstraZeneca
On Twitter @pwendl
A phase II study evaluating single-agent olaparib in metastatic, castration-resistant prostate cancer offers the first evidence that genetic testing could be used to select patients for treatment.
The overall response rate was 86.7% in a subgroup of men (14/16) identified as harboring genomic defects in DNA repair genes and 32.7% among all evaluable patients (16/49).
Median radiological progression-free survival was estimated at 13.2 months in men with mutations and 2.7 months in those without mutations (hazard ratio, 0.21; P < .001), Dr. Joaquin Mateo of the Institute of Cancer Research and the Royal Marsden NHS Trust, London, reported at the annual meeting of the American Association for Cancer Research.
“We hope that this is a step towards molecular stratification of treatment for prostate cancer. We are a year behind breast cancer or colorectal cancer investigators,” he said during a press briefing at the meeting.
Olaparib (Lynparza), a PARP (poly ADP-ribose polymerase) inhibitor ,was approved in the United States in December 2014 for treating BRCA-positive advanced ovarian cancer.
Most of the genomic aberrations in patients with prostate cancer occurred in BRCA2 and ATM, although biallelic loss of other relevant genes, including members of the Fanconi Anemia complementation group and CHEK2, were also observed. Research presented at the meeting suggests that BRCA2 is present in about 12% of prostate tumors and ATM in about 7%, Dr. Mateo told reporters.
The first phase of the study, referred to as TOPARP-A, enrolled 50 men from 7 U.K. centers who had metastatic, castration-resistant prostate cancer after 1-2 lines of taxane chemotherapy and a circulating tumor cell (CTC) count of at least 5 cells/7.5 mL of blood at screening. All patients had received prior docetaxel (Taxotere), 58% cabazitaxel (Jevtana), 96% abiraterone (Zytiga), 28% enzalutamide (Xtandi), and 26% palliative radiotherapy. The median baseline prostate specific antigen (PSA) was 349.5 mcg/L and median age was 67.5 years.
Patients received olaparib 400 mg twice daily continuously in a 28-day cycle. Response rate, the study’s primary endpoint, was defined as objective response by RECIST 1.1 and/or PSA decline of at least 50% and/or CTC count decline from at least 5 cells to less than 5 cells/7.5 mL blood. One patient was not evaluable for response.
Genomic defects in DNA repair genes, somatic and germline, were identified via next-generation sequencing and other studies of fresh tumor samples, taken before and while on olaparib.
Among the 16 unselected patients with responses, 6 had radiological responses and 11 had biochemical responses. Four of these responses have lasted more than 1 year, Dr. Mateo said.
Among those with genomic testing results, seven patients had a BRCA2 alteration and all responded to olaparib. Four of the five men with ATM truncating mutations responded.
PTEN loss and ERG rearrangements were not associated with response, he said.
The biomarker panel had a high specificity of 94% and sensitivity of 87.5%.
Consistent with previous olaparib studies, the most common grade 3 or higher adverse events were anemia (20%) and fatigue (12%), with 13% of patients requiring a dose reduction.
The next phase of the study, TOPARP-B, will validate the biomarker panel by enrolling only patients who screen positive for the DNA repair mutations linked to response in TOPARP-A, said Dr. Mateo, noting that samples can be turned around in 7-10 days.
Press briefing moderator Dr. William Nelson, director of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University in Baltimore, said newer drugs such as abiraterone and enzalutamide have given clinicians more mileage in treating castration-resistant prostate cancer by better targeting the androgen-receptor, but that the current results with olaparib will likely prompt more drug development off the androgen receptor.
The Cancer Research UK Clinical Trials Awards and Advisory Committee, SU2C, PCF, the Experimental Cancer Medicine Center, and the U.K. National Institute for Health Research Biomedical Research Center funded the study. Dr. Mateo disclosed no financial conflicts. Two coauthors reported serving as advisors to AstraZeneca
On Twitter @pwendl
FROM THE AACR ANNUAL MEETING
Key clinical point: Genomic defects, most commonly in BRCA2 and ATM, were associated with olaparib response in metastatic castration-resistant prostate cancer, representing the first step toward genetic treatment stratification of this cancer.
Major finding: The response rate among biomarker-positive men was 86.7% vs. 32.7% overall.
Data source: Phase II open-label, single-arm study of 49 men with metastatic, castration-resistant prostate cancer.
Disclosures: The Cancer Research UK Clinical Trials Awards and Advisory Committee, SU2C, PCF, the Experimental Cancer Medicine Center, and the U.K. National Institute for Health Research Biomedical Research Center funded the study. Dr. Mateo disclosed no financial conflicts. Two coauthors reported serving as advisors to AstraZeneca.





