User login
Impaired kidney function no problem for dabigatran reversal
ORLANDO – Idarucizumab, the reversal agent for the anticoagulant dabigatran, appeared as effective in quickly reversing dabigatran’s effects in patients with severe renal dysfunction as in patients with normally working kidneys, in a post hoc analysis of data collected in the drug’s pivotal trial.
A standard dose of idarucizumab “works just as well in patients with bad kidney function as it does in patients with preserved kidney function,” John W. Eikelboom, MD, said at the annual meeting of the American College of Cardiology. “The time to cessation of bleeding and the degree of normal hemostasis achieved was consistent” across the entire range of renal function examined, from severe renal dysfunction, with a creatinine clearance rate of less than 30 mL/min, to normal function, with an estimated rate of 80 mL/min or greater.
The ability of idarucizumab (Praxbind), conditionally approved by the Food and Drug Administration in 2015 and then fully approved in April 2018, to work in patients with impaired renal function has been an open question and concern because dabigatran (Pradaxa) is excreted renally, so it builds to unusually high levels in patients with poor kidney function. “Plasma dabigatran levels might be sky high, so a standard dose of idarucizumab might not work. That’s been a fear of clinicians,” explained Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont.
To examine whether idarucizumab’s activity varied by renal function he used data from the patients enrolled in the RE-VERSE AD (Reversal Effects of Idarucizumab on Active Dabigatran) study, the pivotal dataset that led to idarucizumab’s U.S. approval. The new, post hoc analysis divided patients into four subgroups based on their kidney function, and focused on the 489 patients for whom renal data were available out of the 503 patients in the study (N Engl J Med. 2017 Aug 3;377[5]:431-41). The subgroups included 91 patients with severe dysfunction with a creatinine clearance rate of less than 30 mL/min; 127 with moderate dysfunction and a clearance rate of 30-49 mL/min; 163 with mild dysfunction and a clearance rate of 50-79 mL/min; and 108 with normal function and a creatinine clearance of at least 80 mL/min.
Patients in the subgroup with severe renal dysfunction had the worst clinical profile overall, and as predicted, had a markedly elevated average plasma level of dabigatran, 231 ng/mL, nearly five times higher than the 47-ng/mL average level in patients with normal renal function.
The ability of a single, standard dose of idarucizumab to reverse the anticoagulant effects of dabigatran were essentially identical across the four strata of renal activity, with 98% of patients in both the severely impaired subgroup and the normal subgroup having 100% reversal within 4 hours of treatment, Dr. Eikelboom reported. Every patient included in the analysis had more than 50% reversal.
The study followed patients to 12-24 hours after they received idarucizumab, and 55% of patients with severe renal dysfunction showed a plasma dabigatran level that crept back toward a clinically meaningful level and so might need a second idarucizumab dose. In contrast, this happened in 8% of patients with normal renal function.
In patients with severe renal dysfunction given idarucizumab, “be alert for a recurrent bleed,” which could require a second dose of idarucizumab, Dr. Eikelboom suggested.
SOURCE: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
ORLANDO – Idarucizumab, the reversal agent for the anticoagulant dabigatran, appeared as effective in quickly reversing dabigatran’s effects in patients with severe renal dysfunction as in patients with normally working kidneys, in a post hoc analysis of data collected in the drug’s pivotal trial.
A standard dose of idarucizumab “works just as well in patients with bad kidney function as it does in patients with preserved kidney function,” John W. Eikelboom, MD, said at the annual meeting of the American College of Cardiology. “The time to cessation of bleeding and the degree of normal hemostasis achieved was consistent” across the entire range of renal function examined, from severe renal dysfunction, with a creatinine clearance rate of less than 30 mL/min, to normal function, with an estimated rate of 80 mL/min or greater.
The ability of idarucizumab (Praxbind), conditionally approved by the Food and Drug Administration in 2015 and then fully approved in April 2018, to work in patients with impaired renal function has been an open question and concern because dabigatran (Pradaxa) is excreted renally, so it builds to unusually high levels in patients with poor kidney function. “Plasma dabigatran levels might be sky high, so a standard dose of idarucizumab might not work. That’s been a fear of clinicians,” explained Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont.
To examine whether idarucizumab’s activity varied by renal function he used data from the patients enrolled in the RE-VERSE AD (Reversal Effects of Idarucizumab on Active Dabigatran) study, the pivotal dataset that led to idarucizumab’s U.S. approval. The new, post hoc analysis divided patients into four subgroups based on their kidney function, and focused on the 489 patients for whom renal data were available out of the 503 patients in the study (N Engl J Med. 2017 Aug 3;377[5]:431-41). The subgroups included 91 patients with severe dysfunction with a creatinine clearance rate of less than 30 mL/min; 127 with moderate dysfunction and a clearance rate of 30-49 mL/min; 163 with mild dysfunction and a clearance rate of 50-79 mL/min; and 108 with normal function and a creatinine clearance of at least 80 mL/min.
Patients in the subgroup with severe renal dysfunction had the worst clinical profile overall, and as predicted, had a markedly elevated average plasma level of dabigatran, 231 ng/mL, nearly five times higher than the 47-ng/mL average level in patients with normal renal function.
The ability of a single, standard dose of idarucizumab to reverse the anticoagulant effects of dabigatran were essentially identical across the four strata of renal activity, with 98% of patients in both the severely impaired subgroup and the normal subgroup having 100% reversal within 4 hours of treatment, Dr. Eikelboom reported. Every patient included in the analysis had more than 50% reversal.
The study followed patients to 12-24 hours after they received idarucizumab, and 55% of patients with severe renal dysfunction showed a plasma dabigatran level that crept back toward a clinically meaningful level and so might need a second idarucizumab dose. In contrast, this happened in 8% of patients with normal renal function.
In patients with severe renal dysfunction given idarucizumab, “be alert for a recurrent bleed,” which could require a second dose of idarucizumab, Dr. Eikelboom suggested.
SOURCE: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
ORLANDO – Idarucizumab, the reversal agent for the anticoagulant dabigatran, appeared as effective in quickly reversing dabigatran’s effects in patients with severe renal dysfunction as in patients with normally working kidneys, in a post hoc analysis of data collected in the drug’s pivotal trial.
A standard dose of idarucizumab “works just as well in patients with bad kidney function as it does in patients with preserved kidney function,” John W. Eikelboom, MD, said at the annual meeting of the American College of Cardiology. “The time to cessation of bleeding and the degree of normal hemostasis achieved was consistent” across the entire range of renal function examined, from severe renal dysfunction, with a creatinine clearance rate of less than 30 mL/min, to normal function, with an estimated rate of 80 mL/min or greater.
The ability of idarucizumab (Praxbind), conditionally approved by the Food and Drug Administration in 2015 and then fully approved in April 2018, to work in patients with impaired renal function has been an open question and concern because dabigatran (Pradaxa) is excreted renally, so it builds to unusually high levels in patients with poor kidney function. “Plasma dabigatran levels might be sky high, so a standard dose of idarucizumab might not work. That’s been a fear of clinicians,” explained Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont.
To examine whether idarucizumab’s activity varied by renal function he used data from the patients enrolled in the RE-VERSE AD (Reversal Effects of Idarucizumab on Active Dabigatran) study, the pivotal dataset that led to idarucizumab’s U.S. approval. The new, post hoc analysis divided patients into four subgroups based on their kidney function, and focused on the 489 patients for whom renal data were available out of the 503 patients in the study (N Engl J Med. 2017 Aug 3;377[5]:431-41). The subgroups included 91 patients with severe dysfunction with a creatinine clearance rate of less than 30 mL/min; 127 with moderate dysfunction and a clearance rate of 30-49 mL/min; 163 with mild dysfunction and a clearance rate of 50-79 mL/min; and 108 with normal function and a creatinine clearance of at least 80 mL/min.
Patients in the subgroup with severe renal dysfunction had the worst clinical profile overall, and as predicted, had a markedly elevated average plasma level of dabigatran, 231 ng/mL, nearly five times higher than the 47-ng/mL average level in patients with normal renal function.
The ability of a single, standard dose of idarucizumab to reverse the anticoagulant effects of dabigatran were essentially identical across the four strata of renal activity, with 98% of patients in both the severely impaired subgroup and the normal subgroup having 100% reversal within 4 hours of treatment, Dr. Eikelboom reported. Every patient included in the analysis had more than 50% reversal.
The study followed patients to 12-24 hours after they received idarucizumab, and 55% of patients with severe renal dysfunction showed a plasma dabigatran level that crept back toward a clinically meaningful level and so might need a second idarucizumab dose. In contrast, this happened in 8% of patients with normal renal function.
In patients with severe renal dysfunction given idarucizumab, “be alert for a recurrent bleed,” which could require a second dose of idarucizumab, Dr. Eikelboom suggested.
SOURCE: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
REPORTING FROM ACC 18
Key clinical point: Renal function had no impact on idarucizumab’s efficacy for dabigatran reversal.
Major finding: Complete dabigatran reversal occurred in 98% of patients with severe renal dysfunction who received idarucizumab.
Study details: Post hoc analysis of data from RE-VERSE AD, idarucizumab’s pivotal trial with 503 patients.
Disclosures: RE-VERSE AD was funded by Boehringer Ingelheim, the company that markets idarucizumab (Praxbind) and dabigatran (Pradaxa). Dr. Eikelboom has been a consultant to and has received research support from Boehringer Ingelheim, as well as from Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Janssen, and Pfizer.
Source: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
VIDEO: Screening ECG patch boosts AF diagnoses ninefold
ORLANDO – An ECG patch worn twice for a total of about 24 days produced a nearly ninefold increase in the number of high-risk people diagnosed with atrial fibrillation, compared with those followed with usual care in a randomized trial with 2,655 people.
During 4 months of follow-up, 1,364 high-risk people assigned to ECG patch screening had a 5.1% rate of new atrial fibrillation (AF) diagnoses, compared with a 0.6% rate among 1,291 controls who wore the patch but were identified with new-onset AF using standard follow-up that did not take the patch data into account. This was a statistically significant difference for the study’s primary endpoint, Steven R. Steinhubl, MD, said at the annual meeting of the American College of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In addition to proving that the ECG patch can better identify asymptomatic people who have AF than conventional means – usually waiting until a stroke occurs or for symptoms to appear – the noninvasive and relatively low-cost patch also gives researchers a new way to try to address the more fundamental medical question created by this line of investigation: How clinically important are relatively brief, asymptomatic episodes of atrial fibrillation, and are patient outcomes improved by treatments begun in this early phase?
The study results “show we can look beyond implantable devices with a less expensive, noninvasive way” to identify patients with asymptomatic AF and determine its natural history and need for intervention, Dr. Steinhubl said in a video interview.
The mSToP (mHealth Screening to Prevent Strokes) trial ran at Scripps and began by identifying more than 359,000 U.S. residents with Aetna health insurance who met the study’s definition of having high AF risk, either by being at least 75 years old, or at least 55 years old and male or at least 65 years old and female. To qualify as high risk those younger than 75 years also had to have at least one clinical risk factor, which could include a prior cerebrovascular event, heart failure, hypertension plus diabetes, obstructive sleep apnea, or any of six other comorbidities. The researchers also excluded potential participants because of several factors, including a history of atrial fibrillation or flutter, current treatment with an anticoagulant, end-stage renal disease, and patients with an implanted pacemaker or defibrillator.
They invited more than 100,000 of these qualifying Aetna beneficiaries to participate, and 2,655 agreed and received by mail a pair of ECG measurement patches (Zio) with instructions to wear one for 2 weeks at the start of the study and to wear the second during the final 2 weeks of the 4-month study period. The participants averaged 73 years of age, and their average CHA2DS2-VASc score was 3.
All patients in the study were told to wear their patches and mail them in, but the researchers used the collected ECG data for diagnosing AF in only the 1,364 patients randomized to the active arm. The ECG findings for the 1,291 controls wasn’t provided to their physicians, and so any new-onset AF had to be found either by symptom onset or incidentally. About one-third of the people assigned to each of the study arms never wore their patches. Those who wore their patches did so for an average of nearly 12 days each. Diagnosis of new-onset AF was based on finding either at least one AF episode recorded by the patches that lasted at least 30 seconds or an AF diagnosis appearing in the patient’s record. The average AF burden – the percentage of time a person with incident AF had an abnormal sinus rhythm – was 0.9%.
Even though many patients did not use their patches, the investigators assessed the primary endpoint of new AF diagnoses during the 4-month study period on an intention-to-treat basis. Their analysis showed an 8.8-fold higher rate of new AF diagnoses among people in the intervention arm whose patch data were used for immediate diagnosis, reported Dr. Steinhubl, an interventional cardiologist and director of digital medicine at the Scripps Translational Science Institute in La Jolla, Ca.
As a secondary endpoint, the researchers merged the entire group of 1,738 participants who had sent in patches with ECG data and compared their 1-year incidence of diagnosed AF against 3,476 matched controls from the Aetna database. After 1 year, the rate of new AF diagnoses was 6.3% in those with patch information and 2.3% among the controls, a threefold difference in diagnosis rates after adjustment for potential confounders.
“The clinical significance of the short AF episodes” manifested by many patch users identified with AF “requires greater clarity, especially in terms of stroke risk,” Dr. Steinhubl said. But he added, “I like to think that, as we learn more, we can look at more than just anticoagulation” as intervention options. For example, if a morbidly obese patient has asymptomatic AF found by patch screening, it might strengthen the case for bariatric surgery if it’s eventually shown that weight loss after bariatric surgery slows AF progression. The same holds true for more aggressive sleep apnea intervention in patients with sleep apnea and asymptomatic AF, as well as for patients with asymptomatic AF and another type of associated comorbidity.
SOURCE: Steinhubl S. ACC 18, Abstract 402-19.
Results from several studies have now shown that some kind of monitoring for AF in asymptomatic, at-risk people results in an increased diagnosis of subclinical AF. Study results also suggest that, in general, people diagnosed with subclinical AF are at a lower risk of stroke than patients with symptomatic AF. As of now, no prospective study has evaluated the efficacy of anticoagulant therapy in people diagnosed with subclinical AF, although such studies are now in progress. Until we have these results, the question of how to manage patients with subclinical AF remains unanswered.
N.A. Mark Estes, MD , is professor of medicine and director of the New England Cardiac Arrhythmia Center at Tufts Medical Center in Boston. He has been a consultant to Boston Scientific, Medtronic, and St. Jude. He made these comments as designated discussant for the mSToPS report.
Results from several studies have now shown that some kind of monitoring for AF in asymptomatic, at-risk people results in an increased diagnosis of subclinical AF. Study results also suggest that, in general, people diagnosed with subclinical AF are at a lower risk of stroke than patients with symptomatic AF. As of now, no prospective study has evaluated the efficacy of anticoagulant therapy in people diagnosed with subclinical AF, although such studies are now in progress. Until we have these results, the question of how to manage patients with subclinical AF remains unanswered.
N.A. Mark Estes, MD , is professor of medicine and director of the New England Cardiac Arrhythmia Center at Tufts Medical Center in Boston. He has been a consultant to Boston Scientific, Medtronic, and St. Jude. He made these comments as designated discussant for the mSToPS report.
Results from several studies have now shown that some kind of monitoring for AF in asymptomatic, at-risk people results in an increased diagnosis of subclinical AF. Study results also suggest that, in general, people diagnosed with subclinical AF are at a lower risk of stroke than patients with symptomatic AF. As of now, no prospective study has evaluated the efficacy of anticoagulant therapy in people diagnosed with subclinical AF, although such studies are now in progress. Until we have these results, the question of how to manage patients with subclinical AF remains unanswered.
N.A. Mark Estes, MD , is professor of medicine and director of the New England Cardiac Arrhythmia Center at Tufts Medical Center in Boston. He has been a consultant to Boston Scientific, Medtronic, and St. Jude. He made these comments as designated discussant for the mSToPS report.
ORLANDO – An ECG patch worn twice for a total of about 24 days produced a nearly ninefold increase in the number of high-risk people diagnosed with atrial fibrillation, compared with those followed with usual care in a randomized trial with 2,655 people.
During 4 months of follow-up, 1,364 high-risk people assigned to ECG patch screening had a 5.1% rate of new atrial fibrillation (AF) diagnoses, compared with a 0.6% rate among 1,291 controls who wore the patch but were identified with new-onset AF using standard follow-up that did not take the patch data into account. This was a statistically significant difference for the study’s primary endpoint, Steven R. Steinhubl, MD, said at the annual meeting of the American College of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In addition to proving that the ECG patch can better identify asymptomatic people who have AF than conventional means – usually waiting until a stroke occurs or for symptoms to appear – the noninvasive and relatively low-cost patch also gives researchers a new way to try to address the more fundamental medical question created by this line of investigation: How clinically important are relatively brief, asymptomatic episodes of atrial fibrillation, and are patient outcomes improved by treatments begun in this early phase?
The study results “show we can look beyond implantable devices with a less expensive, noninvasive way” to identify patients with asymptomatic AF and determine its natural history and need for intervention, Dr. Steinhubl said in a video interview.
The mSToP (mHealth Screening to Prevent Strokes) trial ran at Scripps and began by identifying more than 359,000 U.S. residents with Aetna health insurance who met the study’s definition of having high AF risk, either by being at least 75 years old, or at least 55 years old and male or at least 65 years old and female. To qualify as high risk those younger than 75 years also had to have at least one clinical risk factor, which could include a prior cerebrovascular event, heart failure, hypertension plus diabetes, obstructive sleep apnea, or any of six other comorbidities. The researchers also excluded potential participants because of several factors, including a history of atrial fibrillation or flutter, current treatment with an anticoagulant, end-stage renal disease, and patients with an implanted pacemaker or defibrillator.
They invited more than 100,000 of these qualifying Aetna beneficiaries to participate, and 2,655 agreed and received by mail a pair of ECG measurement patches (Zio) with instructions to wear one for 2 weeks at the start of the study and to wear the second during the final 2 weeks of the 4-month study period. The participants averaged 73 years of age, and their average CHA2DS2-VASc score was 3.
All patients in the study were told to wear their patches and mail them in, but the researchers used the collected ECG data for diagnosing AF in only the 1,364 patients randomized to the active arm. The ECG findings for the 1,291 controls wasn’t provided to their physicians, and so any new-onset AF had to be found either by symptom onset or incidentally. About one-third of the people assigned to each of the study arms never wore their patches. Those who wore their patches did so for an average of nearly 12 days each. Diagnosis of new-onset AF was based on finding either at least one AF episode recorded by the patches that lasted at least 30 seconds or an AF diagnosis appearing in the patient’s record. The average AF burden – the percentage of time a person with incident AF had an abnormal sinus rhythm – was 0.9%.
Even though many patients did not use their patches, the investigators assessed the primary endpoint of new AF diagnoses during the 4-month study period on an intention-to-treat basis. Their analysis showed an 8.8-fold higher rate of new AF diagnoses among people in the intervention arm whose patch data were used for immediate diagnosis, reported Dr. Steinhubl, an interventional cardiologist and director of digital medicine at the Scripps Translational Science Institute in La Jolla, Ca.
As a secondary endpoint, the researchers merged the entire group of 1,738 participants who had sent in patches with ECG data and compared their 1-year incidence of diagnosed AF against 3,476 matched controls from the Aetna database. After 1 year, the rate of new AF diagnoses was 6.3% in those with patch information and 2.3% among the controls, a threefold difference in diagnosis rates after adjustment for potential confounders.
“The clinical significance of the short AF episodes” manifested by many patch users identified with AF “requires greater clarity, especially in terms of stroke risk,” Dr. Steinhubl said. But he added, “I like to think that, as we learn more, we can look at more than just anticoagulation” as intervention options. For example, if a morbidly obese patient has asymptomatic AF found by patch screening, it might strengthen the case for bariatric surgery if it’s eventually shown that weight loss after bariatric surgery slows AF progression. The same holds true for more aggressive sleep apnea intervention in patients with sleep apnea and asymptomatic AF, as well as for patients with asymptomatic AF and another type of associated comorbidity.
SOURCE: Steinhubl S. ACC 18, Abstract 402-19.
ORLANDO – An ECG patch worn twice for a total of about 24 days produced a nearly ninefold increase in the number of high-risk people diagnosed with atrial fibrillation, compared with those followed with usual care in a randomized trial with 2,655 people.
During 4 months of follow-up, 1,364 high-risk people assigned to ECG patch screening had a 5.1% rate of new atrial fibrillation (AF) diagnoses, compared with a 0.6% rate among 1,291 controls who wore the patch but were identified with new-onset AF using standard follow-up that did not take the patch data into account. This was a statistically significant difference for the study’s primary endpoint, Steven R. Steinhubl, MD, said at the annual meeting of the American College of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In addition to proving that the ECG patch can better identify asymptomatic people who have AF than conventional means – usually waiting until a stroke occurs or for symptoms to appear – the noninvasive and relatively low-cost patch also gives researchers a new way to try to address the more fundamental medical question created by this line of investigation: How clinically important are relatively brief, asymptomatic episodes of atrial fibrillation, and are patient outcomes improved by treatments begun in this early phase?
The study results “show we can look beyond implantable devices with a less expensive, noninvasive way” to identify patients with asymptomatic AF and determine its natural history and need for intervention, Dr. Steinhubl said in a video interview.
The mSToP (mHealth Screening to Prevent Strokes) trial ran at Scripps and began by identifying more than 359,000 U.S. residents with Aetna health insurance who met the study’s definition of having high AF risk, either by being at least 75 years old, or at least 55 years old and male or at least 65 years old and female. To qualify as high risk those younger than 75 years also had to have at least one clinical risk factor, which could include a prior cerebrovascular event, heart failure, hypertension plus diabetes, obstructive sleep apnea, or any of six other comorbidities. The researchers also excluded potential participants because of several factors, including a history of atrial fibrillation or flutter, current treatment with an anticoagulant, end-stage renal disease, and patients with an implanted pacemaker or defibrillator.
They invited more than 100,000 of these qualifying Aetna beneficiaries to participate, and 2,655 agreed and received by mail a pair of ECG measurement patches (Zio) with instructions to wear one for 2 weeks at the start of the study and to wear the second during the final 2 weeks of the 4-month study period. The participants averaged 73 years of age, and their average CHA2DS2-VASc score was 3.
All patients in the study were told to wear their patches and mail them in, but the researchers used the collected ECG data for diagnosing AF in only the 1,364 patients randomized to the active arm. The ECG findings for the 1,291 controls wasn’t provided to their physicians, and so any new-onset AF had to be found either by symptom onset or incidentally. About one-third of the people assigned to each of the study arms never wore their patches. Those who wore their patches did so for an average of nearly 12 days each. Diagnosis of new-onset AF was based on finding either at least one AF episode recorded by the patches that lasted at least 30 seconds or an AF diagnosis appearing in the patient’s record. The average AF burden – the percentage of time a person with incident AF had an abnormal sinus rhythm – was 0.9%.
Even though many patients did not use their patches, the investigators assessed the primary endpoint of new AF diagnoses during the 4-month study period on an intention-to-treat basis. Their analysis showed an 8.8-fold higher rate of new AF diagnoses among people in the intervention arm whose patch data were used for immediate diagnosis, reported Dr. Steinhubl, an interventional cardiologist and director of digital medicine at the Scripps Translational Science Institute in La Jolla, Ca.
As a secondary endpoint, the researchers merged the entire group of 1,738 participants who had sent in patches with ECG data and compared their 1-year incidence of diagnosed AF against 3,476 matched controls from the Aetna database. After 1 year, the rate of new AF diagnoses was 6.3% in those with patch information and 2.3% among the controls, a threefold difference in diagnosis rates after adjustment for potential confounders.
“The clinical significance of the short AF episodes” manifested by many patch users identified with AF “requires greater clarity, especially in terms of stroke risk,” Dr. Steinhubl said. But he added, “I like to think that, as we learn more, we can look at more than just anticoagulation” as intervention options. For example, if a morbidly obese patient has asymptomatic AF found by patch screening, it might strengthen the case for bariatric surgery if it’s eventually shown that weight loss after bariatric surgery slows AF progression. The same holds true for more aggressive sleep apnea intervention in patients with sleep apnea and asymptomatic AF, as well as for patients with asymptomatic AF and another type of associated comorbidity.
SOURCE: Steinhubl S. ACC 18, Abstract 402-19.
REPORTING FROM ACC 18
Key clinical point: .
Major finding: After 4 months, new AF diagnoses occurred in 5.1% of patch users and 0.6% of usual-care controls.
Study details: mSToPS, a single-center, randomized study with 2,655 people at high risk for developing AF.
Disclosures: mSToPS received support from Aetna, Janssen, and iRhythm. Dr. Steinhubl has been an advisor to Airstrip, DynoSense, EasyG, FocusMotion, LifeWatch, MyoKardia, Novartis, and Spry Health, he serves on the board of Celes Health, and he has received research support from Janssen and Novartis.
Source: Steinhubl S. ACC 18, Abstract 402-19.
Apixaban prevails in study of 163,000 DOAC users
ORLANDO – Apixaban outperformed both rivaroxaban and dabigatran in a retrospective, observational study of real-world prescribing of direct oral anticoagulants in nearly 163,000 U.S. patients with nonvalvular atrial fibrillation, Steven B. Deitelzweig, MD, reported at the annual meeting of the American College of Cardiology.
This ongoing study, known as ARISTOPHANES (Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients), is the largest real-world analysis of direct oral anticoagulants (DOACs) to date. Unlike most of the previous observational studies of DOACs, which used a single insurance claims database, ARISTOPHANES pools data from Medicare and four large U.S. commercial insurance claims databases that collectively cover more than 180 million Americans.
This was a study of real-world prescribing. Unlike in randomized trials, where everyone is on a standard-dose DOAC, lower-dose therapy was common. It was prescribed for 21% of patients on apixaban, 15% on dabigatran, and 24% on rivaroxaban.
ARISTOPHANES results
The biggest difference in outcome was between patients on apixaban and those on dabigatran. The incidence of stroke/systemic embolism in 27,096 patients on apixaban (Eliquis) was 1.01% in 360 days, for a statistically significant 31% reduction in risk relative to the 1.42% incidence rate in 27,096 extensively matched patients on dabigatran (Pradaxa). The 360-day incidence of major bleeding was 2.7% in the apixaban group and 3.3% in those on dabigatran, for a 23% relative risk reduction. In the apixaban/rivaroxaban comparison, which included 62,619 patients in each group, the incidence of stroke/systemic embolism was 1.21% with apixaban and 1.42% with rivaroxaban, for a 27% relative risk reduction. Major bleeding occurred in 3.1% of the apixaban group and 5.3% of those on rivaroxaban, for a 46% reduction in risk favoring apixaban.
In a comparison of 27,538 patients on dabigatran and an equal number of rivaroxaban, the 360-day cumulative incidence of stroke/systemic embolism was 1.40% with dabigatran versus 1.23% with rivaroxaban, while the major bleeding rate was 3.28% in the dabigatran group and 4.76% with rivaroxaban.
Dr. Deitelzweig was quick to acknowledge the major limitation of ARISTOPHANES.
“Only associations can be drawn from a nonrandomized, retrospective, observational study, not conclusions regarding causality, even though the cohorts were matched using propensity scoring,” he emphasized.
The critique
Session cochair Jeanne E. Poole, MD, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle, commented, “The problem, of course, with using these large databases is that you may not be able to find out important information, such as whether rivaroxaban was being taken appropriately with meals, which is frequently not the case. If it wasn’t, that decreases absorption and efficacy by 40%. That’s a limitation.”
Audience member James A. Reiffel, MD, rose to add that, in his view, another significant limitation of all real-world, observational analyses using claims data is that it’s not possible to know why physicians selected a given drug for a given patient. He used as an example a patient with atrial fibrillation and gastroesophageal reflux disease (GERD).
“People with GERD may be less likely to get dabigatran, and if they’re less likely to get dabigatran with GERD, maybe they’re less likely to get a GI bleed. I don’t know,” said Dr. Reiffel, professor of clinical medicine and director of the electrocardiography laboratory at Columbia University Medical Center, New York.
“We have to take all the real-world analyses with a little grain of salt,” he added.
Dr. Deitelzweig replied, “This study is not meant to be the be-all and end-all.”
That being said, he noted that although randomized trials have shown that DOACs are at least as effective and safe as warfarin for stroke prevention in atrial fibrillation, there have been no randomized, head-to-head clinical trials comparing them, nor are any such studies likely to be done for the foreseeable future. Yet physicians and their patients are hungry for comparative effectiveness data, even if it doesn’t rise to the status of level I evidence.
ARISTOPHANES is sponsored by Bristol-Myers Squibb and Pfizer. Dr. Deitelzweig reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
SOURCE: Deitelzweig SB. ACC 2018.
ORLANDO – Apixaban outperformed both rivaroxaban and dabigatran in a retrospective, observational study of real-world prescribing of direct oral anticoagulants in nearly 163,000 U.S. patients with nonvalvular atrial fibrillation, Steven B. Deitelzweig, MD, reported at the annual meeting of the American College of Cardiology.
This ongoing study, known as ARISTOPHANES (Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients), is the largest real-world analysis of direct oral anticoagulants (DOACs) to date. Unlike most of the previous observational studies of DOACs, which used a single insurance claims database, ARISTOPHANES pools data from Medicare and four large U.S. commercial insurance claims databases that collectively cover more than 180 million Americans.
This was a study of real-world prescribing. Unlike in randomized trials, where everyone is on a standard-dose DOAC, lower-dose therapy was common. It was prescribed for 21% of patients on apixaban, 15% on dabigatran, and 24% on rivaroxaban.
ARISTOPHANES results
The biggest difference in outcome was between patients on apixaban and those on dabigatran. The incidence of stroke/systemic embolism in 27,096 patients on apixaban (Eliquis) was 1.01% in 360 days, for a statistically significant 31% reduction in risk relative to the 1.42% incidence rate in 27,096 extensively matched patients on dabigatran (Pradaxa). The 360-day incidence of major bleeding was 2.7% in the apixaban group and 3.3% in those on dabigatran, for a 23% relative risk reduction. In the apixaban/rivaroxaban comparison, which included 62,619 patients in each group, the incidence of stroke/systemic embolism was 1.21% with apixaban and 1.42% with rivaroxaban, for a 27% relative risk reduction. Major bleeding occurred in 3.1% of the apixaban group and 5.3% of those on rivaroxaban, for a 46% reduction in risk favoring apixaban.
In a comparison of 27,538 patients on dabigatran and an equal number of rivaroxaban, the 360-day cumulative incidence of stroke/systemic embolism was 1.40% with dabigatran versus 1.23% with rivaroxaban, while the major bleeding rate was 3.28% in the dabigatran group and 4.76% with rivaroxaban.
Dr. Deitelzweig was quick to acknowledge the major limitation of ARISTOPHANES.
“Only associations can be drawn from a nonrandomized, retrospective, observational study, not conclusions regarding causality, even though the cohorts were matched using propensity scoring,” he emphasized.
The critique
Session cochair Jeanne E. Poole, MD, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle, commented, “The problem, of course, with using these large databases is that you may not be able to find out important information, such as whether rivaroxaban was being taken appropriately with meals, which is frequently not the case. If it wasn’t, that decreases absorption and efficacy by 40%. That’s a limitation.”
Audience member James A. Reiffel, MD, rose to add that, in his view, another significant limitation of all real-world, observational analyses using claims data is that it’s not possible to know why physicians selected a given drug for a given patient. He used as an example a patient with atrial fibrillation and gastroesophageal reflux disease (GERD).
“People with GERD may be less likely to get dabigatran, and if they’re less likely to get dabigatran with GERD, maybe they’re less likely to get a GI bleed. I don’t know,” said Dr. Reiffel, professor of clinical medicine and director of the electrocardiography laboratory at Columbia University Medical Center, New York.
“We have to take all the real-world analyses with a little grain of salt,” he added.
Dr. Deitelzweig replied, “This study is not meant to be the be-all and end-all.”
That being said, he noted that although randomized trials have shown that DOACs are at least as effective and safe as warfarin for stroke prevention in atrial fibrillation, there have been no randomized, head-to-head clinical trials comparing them, nor are any such studies likely to be done for the foreseeable future. Yet physicians and their patients are hungry for comparative effectiveness data, even if it doesn’t rise to the status of level I evidence.
ARISTOPHANES is sponsored by Bristol-Myers Squibb and Pfizer. Dr. Deitelzweig reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
SOURCE: Deitelzweig SB. ACC 2018.
ORLANDO – Apixaban outperformed both rivaroxaban and dabigatran in a retrospective, observational study of real-world prescribing of direct oral anticoagulants in nearly 163,000 U.S. patients with nonvalvular atrial fibrillation, Steven B. Deitelzweig, MD, reported at the annual meeting of the American College of Cardiology.
This ongoing study, known as ARISTOPHANES (Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients), is the largest real-world analysis of direct oral anticoagulants (DOACs) to date. Unlike most of the previous observational studies of DOACs, which used a single insurance claims database, ARISTOPHANES pools data from Medicare and four large U.S. commercial insurance claims databases that collectively cover more than 180 million Americans.
This was a study of real-world prescribing. Unlike in randomized trials, where everyone is on a standard-dose DOAC, lower-dose therapy was common. It was prescribed for 21% of patients on apixaban, 15% on dabigatran, and 24% on rivaroxaban.
ARISTOPHANES results
The biggest difference in outcome was between patients on apixaban and those on dabigatran. The incidence of stroke/systemic embolism in 27,096 patients on apixaban (Eliquis) was 1.01% in 360 days, for a statistically significant 31% reduction in risk relative to the 1.42% incidence rate in 27,096 extensively matched patients on dabigatran (Pradaxa). The 360-day incidence of major bleeding was 2.7% in the apixaban group and 3.3% in those on dabigatran, for a 23% relative risk reduction. In the apixaban/rivaroxaban comparison, which included 62,619 patients in each group, the incidence of stroke/systemic embolism was 1.21% with apixaban and 1.42% with rivaroxaban, for a 27% relative risk reduction. Major bleeding occurred in 3.1% of the apixaban group and 5.3% of those on rivaroxaban, for a 46% reduction in risk favoring apixaban.
In a comparison of 27,538 patients on dabigatran and an equal number of rivaroxaban, the 360-day cumulative incidence of stroke/systemic embolism was 1.40% with dabigatran versus 1.23% with rivaroxaban, while the major bleeding rate was 3.28% in the dabigatran group and 4.76% with rivaroxaban.
Dr. Deitelzweig was quick to acknowledge the major limitation of ARISTOPHANES.
“Only associations can be drawn from a nonrandomized, retrospective, observational study, not conclusions regarding causality, even though the cohorts were matched using propensity scoring,” he emphasized.
The critique
Session cochair Jeanne E. Poole, MD, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle, commented, “The problem, of course, with using these large databases is that you may not be able to find out important information, such as whether rivaroxaban was being taken appropriately with meals, which is frequently not the case. If it wasn’t, that decreases absorption and efficacy by 40%. That’s a limitation.”
Audience member James A. Reiffel, MD, rose to add that, in his view, another significant limitation of all real-world, observational analyses using claims data is that it’s not possible to know why physicians selected a given drug for a given patient. He used as an example a patient with atrial fibrillation and gastroesophageal reflux disease (GERD).
“People with GERD may be less likely to get dabigatran, and if they’re less likely to get dabigatran with GERD, maybe they’re less likely to get a GI bleed. I don’t know,” said Dr. Reiffel, professor of clinical medicine and director of the electrocardiography laboratory at Columbia University Medical Center, New York.
“We have to take all the real-world analyses with a little grain of salt,” he added.
Dr. Deitelzweig replied, “This study is not meant to be the be-all and end-all.”
That being said, he noted that although randomized trials have shown that DOACs are at least as effective and safe as warfarin for stroke prevention in atrial fibrillation, there have been no randomized, head-to-head clinical trials comparing them, nor are any such studies likely to be done for the foreseeable future. Yet physicians and their patients are hungry for comparative effectiveness data, even if it doesn’t rise to the status of level I evidence.
ARISTOPHANES is sponsored by Bristol-Myers Squibb and Pfizer. Dr. Deitelzweig reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
SOURCE: Deitelzweig SB. ACC 2018.
REPORTING FROM ACC 2018
Key clinical point:
Major finding: The risk of stroke/systemic embolism in apixaban-treated patients was 31% lower than in those on dabigatran and 27% lower than with rivaroxaban.
Study details: This retrospective, observational study based upon claims data included 162,707 propensity score-matched patients with atrial fibrillation on a direct oral anticoagulant for stroke prevention.
Disclosures: The ongoing ARISTOPHANES study is sponsored by Bristol-Myers Squibb and Pfizer. The presenter reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
Source: Deitelzweig SB. ACC 2018.
Common infections are potent risk factor for MI, stroke
ORLANDO – , according to a “big data” registry study from the United Kingdom.
“Our data show infection was just as much a risk factor or more compared with the traditional atherosclerotic cardiovascular disease risk factors,” Paul Carter, MD, said at the annual meeting of the American College of Cardiology.
Dr. Carter of Aston Medical School in Birmingham, England, presented a retrospective analysis from the ACALM (Algorithm for Comorbidities Associated with Length of stay and Mortality) study of administrative data on all of the more than 1.22 million patients admitted to seven U.K. hospitals in 2000-2013. His analysis included all 34,027 adults aged 40 years or older admitted with a urinary tract or respiratory infection on their index hospitalization who had no history of ischemic heart disease or ischemic stroke.
These patients, with a mean age of 73 years, 59% of whom were women, were compared with an equal number of age- and gender-matched adults whose index hospitalization was for reasons other than ischemic heart disease, stroke, urinary tract infection (UTI), or respiratory infection – the two most common infections resulting in hospitalization in the United Kingdom.
Patients with a respiratory infection or UTI had a 9.9% incidence of new-onset ischemic heart disease and a 4.1% rate of ischemic stroke during follow-up starting upon discharge from their index hospitalization, significantly higher than the 5.9% and 1.5% rates in controls. In a multivariate logistic regression analysis adjusted for demographics, standard cardiovascular risk factors, and the top 10 causes of mortality in the United Kingdom, patients with respiratory infection or UTI as their admitting diagnosis had a 1.36-fold increased likelihood of developing ischemic heart disease post discharge and a 2.5-fold greater risk of ischemic stroke than matched controls.
Moreover, mortality following diagnosis of ischemic heart disease was 75.2% in patients whose index hospitalization was for infection, compared with 51.1% in controls who developed ischemic heart disease without a history of hospitalization for infection, for an adjusted 2.98-fold increased likelihood of death. Similarly, mortality after an ischemic stroke was 59.8% in patients with a prior severe infection, compared with 30.8% in controls, which translated to an adjusted 3.1-fold increased risk of death post stroke in patients with a prior hospitalization for infection.
In the multivariate analysis, hospitalization for infection was a stronger risk factor for subsequent ischemic stroke than was atrial fibrillation, heart failure, type 1 or type 2 diabetes, hypertension, or hyperlipidemia. The risk of ischemic heart disease in patients with an infectious disease hospitalization was similar to the risks associated with most of those recognized risk factors.
Two possible mechanisms by which infection might predispose to subsequent ischemic heart disease and stroke are via a direct effect whereby pathogens such as Chlamydia pneumoniae are taken up into arterial plaques, where they cause a local inflammatory response, or an indirect effect in which systemic inflammation primes the atherosclerotic plaque through distribution of inflammatory cytokines, according to Dr. Carter.
He said the ACALM findings are particularly intriguing when considered in the context of the 2017 results of the landmark CANTOS trial, in which canakinumab (Ilaris), a targeted anti-inflammatory agent that inhibits the interleukin-1 beta innate immunity pathway, reduced recurrent ischemic events in post-MI patients who had high systemic inflammation as evidenced by their elevated C-reactive protein level but a normal-range LDL cholesterol (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
“If atherosclerosis is an inflammatory condition, this begs the question of whether other inflammatory conditions, like infection, which induces a large systemic inflammatory response, might drive atherosclerosis,” Dr. Carter commented.
“It’s now very well understood that inflammatory mediators, cells, and processes are involved in every step from the initial endothelial dysfunction that leads to uptake of LDL, inflammatory cells, and monocytes all the way through to plaque progression and rupture, where Th1 cytokines have been implicated in causing that rupture, and ultimately in patient presentation at the hospital,” he added.
He sees the ACALM findings as hypothesis generating, serving to help lay the groundwork for future clinical trials of vaccine or anti-inflammatory antibiotic therapies.
Dr. Carter reported having no financial conflicts related to his presentation.
SOURCE: Carter P. ACC 2018, Abstract 1325M-0.
ORLANDO – , according to a “big data” registry study from the United Kingdom.
“Our data show infection was just as much a risk factor or more compared with the traditional atherosclerotic cardiovascular disease risk factors,” Paul Carter, MD, said at the annual meeting of the American College of Cardiology.
Dr. Carter of Aston Medical School in Birmingham, England, presented a retrospective analysis from the ACALM (Algorithm for Comorbidities Associated with Length of stay and Mortality) study of administrative data on all of the more than 1.22 million patients admitted to seven U.K. hospitals in 2000-2013. His analysis included all 34,027 adults aged 40 years or older admitted with a urinary tract or respiratory infection on their index hospitalization who had no history of ischemic heart disease or ischemic stroke.
These patients, with a mean age of 73 years, 59% of whom were women, were compared with an equal number of age- and gender-matched adults whose index hospitalization was for reasons other than ischemic heart disease, stroke, urinary tract infection (UTI), or respiratory infection – the two most common infections resulting in hospitalization in the United Kingdom.
Patients with a respiratory infection or UTI had a 9.9% incidence of new-onset ischemic heart disease and a 4.1% rate of ischemic stroke during follow-up starting upon discharge from their index hospitalization, significantly higher than the 5.9% and 1.5% rates in controls. In a multivariate logistic regression analysis adjusted for demographics, standard cardiovascular risk factors, and the top 10 causes of mortality in the United Kingdom, patients with respiratory infection or UTI as their admitting diagnosis had a 1.36-fold increased likelihood of developing ischemic heart disease post discharge and a 2.5-fold greater risk of ischemic stroke than matched controls.
Moreover, mortality following diagnosis of ischemic heart disease was 75.2% in patients whose index hospitalization was for infection, compared with 51.1% in controls who developed ischemic heart disease without a history of hospitalization for infection, for an adjusted 2.98-fold increased likelihood of death. Similarly, mortality after an ischemic stroke was 59.8% in patients with a prior severe infection, compared with 30.8% in controls, which translated to an adjusted 3.1-fold increased risk of death post stroke in patients with a prior hospitalization for infection.
In the multivariate analysis, hospitalization for infection was a stronger risk factor for subsequent ischemic stroke than was atrial fibrillation, heart failure, type 1 or type 2 diabetes, hypertension, or hyperlipidemia. The risk of ischemic heart disease in patients with an infectious disease hospitalization was similar to the risks associated with most of those recognized risk factors.
Two possible mechanisms by which infection might predispose to subsequent ischemic heart disease and stroke are via a direct effect whereby pathogens such as Chlamydia pneumoniae are taken up into arterial plaques, where they cause a local inflammatory response, or an indirect effect in which systemic inflammation primes the atherosclerotic plaque through distribution of inflammatory cytokines, according to Dr. Carter.
He said the ACALM findings are particularly intriguing when considered in the context of the 2017 results of the landmark CANTOS trial, in which canakinumab (Ilaris), a targeted anti-inflammatory agent that inhibits the interleukin-1 beta innate immunity pathway, reduced recurrent ischemic events in post-MI patients who had high systemic inflammation as evidenced by their elevated C-reactive protein level but a normal-range LDL cholesterol (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
“If atherosclerosis is an inflammatory condition, this begs the question of whether other inflammatory conditions, like infection, which induces a large systemic inflammatory response, might drive atherosclerosis,” Dr. Carter commented.
“It’s now very well understood that inflammatory mediators, cells, and processes are involved in every step from the initial endothelial dysfunction that leads to uptake of LDL, inflammatory cells, and monocytes all the way through to plaque progression and rupture, where Th1 cytokines have been implicated in causing that rupture, and ultimately in patient presentation at the hospital,” he added.
He sees the ACALM findings as hypothesis generating, serving to help lay the groundwork for future clinical trials of vaccine or anti-inflammatory antibiotic therapies.
Dr. Carter reported having no financial conflicts related to his presentation.
SOURCE: Carter P. ACC 2018, Abstract 1325M-0.
ORLANDO – , according to a “big data” registry study from the United Kingdom.
“Our data show infection was just as much a risk factor or more compared with the traditional atherosclerotic cardiovascular disease risk factors,” Paul Carter, MD, said at the annual meeting of the American College of Cardiology.
Dr. Carter of Aston Medical School in Birmingham, England, presented a retrospective analysis from the ACALM (Algorithm for Comorbidities Associated with Length of stay and Mortality) study of administrative data on all of the more than 1.22 million patients admitted to seven U.K. hospitals in 2000-2013. His analysis included all 34,027 adults aged 40 years or older admitted with a urinary tract or respiratory infection on their index hospitalization who had no history of ischemic heart disease or ischemic stroke.
These patients, with a mean age of 73 years, 59% of whom were women, were compared with an equal number of age- and gender-matched adults whose index hospitalization was for reasons other than ischemic heart disease, stroke, urinary tract infection (UTI), or respiratory infection – the two most common infections resulting in hospitalization in the United Kingdom.
Patients with a respiratory infection or UTI had a 9.9% incidence of new-onset ischemic heart disease and a 4.1% rate of ischemic stroke during follow-up starting upon discharge from their index hospitalization, significantly higher than the 5.9% and 1.5% rates in controls. In a multivariate logistic regression analysis adjusted for demographics, standard cardiovascular risk factors, and the top 10 causes of mortality in the United Kingdom, patients with respiratory infection or UTI as their admitting diagnosis had a 1.36-fold increased likelihood of developing ischemic heart disease post discharge and a 2.5-fold greater risk of ischemic stroke than matched controls.
Moreover, mortality following diagnosis of ischemic heart disease was 75.2% in patients whose index hospitalization was for infection, compared with 51.1% in controls who developed ischemic heart disease without a history of hospitalization for infection, for an adjusted 2.98-fold increased likelihood of death. Similarly, mortality after an ischemic stroke was 59.8% in patients with a prior severe infection, compared with 30.8% in controls, which translated to an adjusted 3.1-fold increased risk of death post stroke in patients with a prior hospitalization for infection.
In the multivariate analysis, hospitalization for infection was a stronger risk factor for subsequent ischemic stroke than was atrial fibrillation, heart failure, type 1 or type 2 diabetes, hypertension, or hyperlipidemia. The risk of ischemic heart disease in patients with an infectious disease hospitalization was similar to the risks associated with most of those recognized risk factors.
Two possible mechanisms by which infection might predispose to subsequent ischemic heart disease and stroke are via a direct effect whereby pathogens such as Chlamydia pneumoniae are taken up into arterial plaques, where they cause a local inflammatory response, or an indirect effect in which systemic inflammation primes the atherosclerotic plaque through distribution of inflammatory cytokines, according to Dr. Carter.
He said the ACALM findings are particularly intriguing when considered in the context of the 2017 results of the landmark CANTOS trial, in which canakinumab (Ilaris), a targeted anti-inflammatory agent that inhibits the interleukin-1 beta innate immunity pathway, reduced recurrent ischemic events in post-MI patients who had high systemic inflammation as evidenced by their elevated C-reactive protein level but a normal-range LDL cholesterol (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
“If atherosclerosis is an inflammatory condition, this begs the question of whether other inflammatory conditions, like infection, which induces a large systemic inflammatory response, might drive atherosclerosis,” Dr. Carter commented.
“It’s now very well understood that inflammatory mediators, cells, and processes are involved in every step from the initial endothelial dysfunction that leads to uptake of LDL, inflammatory cells, and monocytes all the way through to plaque progression and rupture, where Th1 cytokines have been implicated in causing that rupture, and ultimately in patient presentation at the hospital,” he added.
He sees the ACALM findings as hypothesis generating, serving to help lay the groundwork for future clinical trials of vaccine or anti-inflammatory antibiotic therapies.
Dr. Carter reported having no financial conflicts related to his presentation.
SOURCE: Carter P. ACC 2018, Abstract 1325M-0.
REPORTING FROM ACC 2018
Key clinical point: Once patients have been hospitalized for a respiratory infection or UTI, their postdischarge risk of ischemic stroke is 2.5-fold greater than in those without such a history.
Major finding: Patients with a history of hospitalization for UTI or a respiratory infection who later develop ischemic heart disease or stroke have a threefold higher mortality risk than those without such a hospitalization.
Study details: This was a retrospective study of more than 68,000 subjects in the U.K. ACALM registry study.
Disclosures: The study presenter reported having no financial conflicts of interest.
Source: Carter P. ACC 2018, Abstract 1325M-0.
Consider spironolactone in treatment-resistant hypertension
ORLANDO – More than one-third of patients with treatment-resistant hypertension in U.S. cardiology practices are eligible for preferential consideration of spironolactone as their fourth-line agent in accord with the practice-changing findings of the PATHWAY-2 trial, Lauren Thompson, MD, said at the annual meeting of the American College of Cardiology.
She presented a study that harnessed the ACC’s National Cardiovascular Data Registry PINNACLE Registry – the largest observational outpatient cardiovascular registry in the world – to assess the potential impact of PATHWAY-2 on the management of treatment-resistant hypertension (TRH) in U.S. cardiology practices. And as she discovered, the potential implications for daily practice are huge.
Dr. Thompson, a cardiology fellow at the University of Colorado, Denver, identified 19,044 patients in the PINNACLE registry for 2013-2014 with TRH, defined as uncontrolled blood pressure despite use of drugs from three antihypertensive classes. Of these patients, 37% met the PATHWAY-2 enrollment criteria by virtue of already being on an ACE inhibitor or angiotensin receptor blocker, a calcium channel blocker, and a thiazide diuretic, but not spironolactone. This is the large subgroup which, on the basis of PATHWAY-2, should receive serious consideration of spironolactone as the fourth drug.
The most widely prescribed antihypertensive agents in PINNACLE registry patients with TRH were beta-blockers, in 87%; ACE inhibitors, in 72%; calcium channel blockers, in 71%; and thiazide diuretics, in 69%. Of note, 27% of patients with TRH were already on spironolactone.
Audience discussion centered around the uncertainties regarding treatment adherence in patients labeled as having TRH.
“I think sometimes clinicians are afraid to prescribe spironolactone in patients that they think might be nonadherent,” one cardiologist observed.
Dr. Thompson noted that it’s not possible to look at prescription-filling rates in the PINNACLE registry.
“Unfortunately, we can’t exclude white coat hypertension or nonadherence as reasons why patients in PINNACLE end up on multiple antihypertensive medication classes. We can see that a prescription was written, but we have no way to know if it was actually filled or not,” she observed.
Also, since patients in cardiology clinics typically have multiple cardiovascular comorbidities, it’s quite possible that patients with TRH who are on a beta-blocker, for example, might not have received that drug for blood pressure control.
Dr. Thompson’s study was supported by the ACC’s National Cardiovascular Disease Registry. She reported having no financial conflicts of interest.
Source: Thompson L. ACC 18. Abstract 1324M-09.
ORLANDO – More than one-third of patients with treatment-resistant hypertension in U.S. cardiology practices are eligible for preferential consideration of spironolactone as their fourth-line agent in accord with the practice-changing findings of the PATHWAY-2 trial, Lauren Thompson, MD, said at the annual meeting of the American College of Cardiology.
She presented a study that harnessed the ACC’s National Cardiovascular Data Registry PINNACLE Registry – the largest observational outpatient cardiovascular registry in the world – to assess the potential impact of PATHWAY-2 on the management of treatment-resistant hypertension (TRH) in U.S. cardiology practices. And as she discovered, the potential implications for daily practice are huge.
Dr. Thompson, a cardiology fellow at the University of Colorado, Denver, identified 19,044 patients in the PINNACLE registry for 2013-2014 with TRH, defined as uncontrolled blood pressure despite use of drugs from three antihypertensive classes. Of these patients, 37% met the PATHWAY-2 enrollment criteria by virtue of already being on an ACE inhibitor or angiotensin receptor blocker, a calcium channel blocker, and a thiazide diuretic, but not spironolactone. This is the large subgroup which, on the basis of PATHWAY-2, should receive serious consideration of spironolactone as the fourth drug.
The most widely prescribed antihypertensive agents in PINNACLE registry patients with TRH were beta-blockers, in 87%; ACE inhibitors, in 72%; calcium channel blockers, in 71%; and thiazide diuretics, in 69%. Of note, 27% of patients with TRH were already on spironolactone.
Audience discussion centered around the uncertainties regarding treatment adherence in patients labeled as having TRH.
“I think sometimes clinicians are afraid to prescribe spironolactone in patients that they think might be nonadherent,” one cardiologist observed.
Dr. Thompson noted that it’s not possible to look at prescription-filling rates in the PINNACLE registry.
“Unfortunately, we can’t exclude white coat hypertension or nonadherence as reasons why patients in PINNACLE end up on multiple antihypertensive medication classes. We can see that a prescription was written, but we have no way to know if it was actually filled or not,” she observed.
Also, since patients in cardiology clinics typically have multiple cardiovascular comorbidities, it’s quite possible that patients with TRH who are on a beta-blocker, for example, might not have received that drug for blood pressure control.
Dr. Thompson’s study was supported by the ACC’s National Cardiovascular Disease Registry. She reported having no financial conflicts of interest.
Source: Thompson L. ACC 18. Abstract 1324M-09.
ORLANDO – More than one-third of patients with treatment-resistant hypertension in U.S. cardiology practices are eligible for preferential consideration of spironolactone as their fourth-line agent in accord with the practice-changing findings of the PATHWAY-2 trial, Lauren Thompson, MD, said at the annual meeting of the American College of Cardiology.
She presented a study that harnessed the ACC’s National Cardiovascular Data Registry PINNACLE Registry – the largest observational outpatient cardiovascular registry in the world – to assess the potential impact of PATHWAY-2 on the management of treatment-resistant hypertension (TRH) in U.S. cardiology practices. And as she discovered, the potential implications for daily practice are huge.
Dr. Thompson, a cardiology fellow at the University of Colorado, Denver, identified 19,044 patients in the PINNACLE registry for 2013-2014 with TRH, defined as uncontrolled blood pressure despite use of drugs from three antihypertensive classes. Of these patients, 37% met the PATHWAY-2 enrollment criteria by virtue of already being on an ACE inhibitor or angiotensin receptor blocker, a calcium channel blocker, and a thiazide diuretic, but not spironolactone. This is the large subgroup which, on the basis of PATHWAY-2, should receive serious consideration of spironolactone as the fourth drug.
The most widely prescribed antihypertensive agents in PINNACLE registry patients with TRH were beta-blockers, in 87%; ACE inhibitors, in 72%; calcium channel blockers, in 71%; and thiazide diuretics, in 69%. Of note, 27% of patients with TRH were already on spironolactone.
Audience discussion centered around the uncertainties regarding treatment adherence in patients labeled as having TRH.
“I think sometimes clinicians are afraid to prescribe spironolactone in patients that they think might be nonadherent,” one cardiologist observed.
Dr. Thompson noted that it’s not possible to look at prescription-filling rates in the PINNACLE registry.
“Unfortunately, we can’t exclude white coat hypertension or nonadherence as reasons why patients in PINNACLE end up on multiple antihypertensive medication classes. We can see that a prescription was written, but we have no way to know if it was actually filled or not,” she observed.
Also, since patients in cardiology clinics typically have multiple cardiovascular comorbidities, it’s quite possible that patients with TRH who are on a beta-blocker, for example, might not have received that drug for blood pressure control.
Dr. Thompson’s study was supported by the ACC’s National Cardiovascular Disease Registry. She reported having no financial conflicts of interest.
Source: Thompson L. ACC 18. Abstract 1324M-09.
REPORTING FROM ACC 18
Key clinical point:
Major finding: Thirty-seven percent of patients with treatment-resistant hypertension in U.S. cardiology practices could benefit from preferential consideration of spironolactone as their fourth-line antihypertensive agent.
Study details: This retrospective study included more than 19,000 patients with treatment-resistant hypertension in U.S. cardiology practices.
Disclosures: The presenter reported having no financial conflicts regarding her study, supported by the ACC’s National Cardiovascular Data Registry.
Source: Thompson L. ACC 18. Abstract 1324M-09.
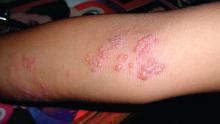
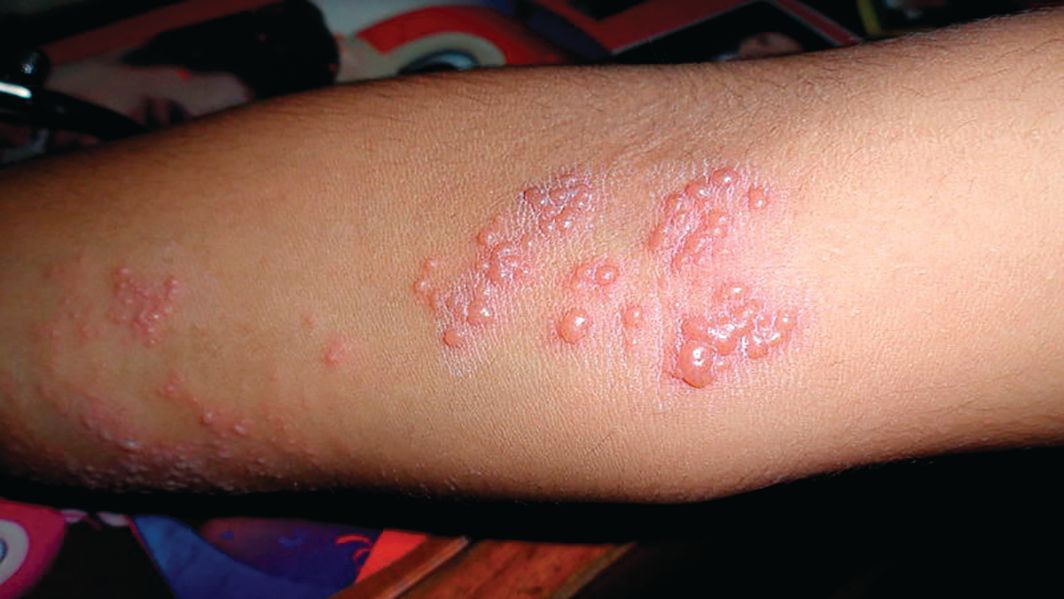
Herpes zoster boosts short-term stroke, TIA risk
ORLANDO – The risk of stroke and TIA – but not of acute MI – is significantly increased during the period surrounding diagnosis of herpes zoster, compared with the rate in matched zoster-free controls, according to a retrospective study of nearly 70,000 U.S. adults. A particularly striking study finding was the marked age disparity in the magnitude of vascular event risk associated with herpes zoster (HZ), with younger adults being at higher risk.
This increased vascular event risk associated with HZ was transitory. During the entire study follow-up period, which stretched from 1 month prior to HZ diagnosis to 12 months afterward, there was no overall increased vascular event risk associated with HZ, noted Dr. Rausch, an infectious diseases specialist who serves as director of clinical and medical affairs for the U.S. zoster program at GlaxoSmithKline in Philadelphia.
She presented a retrospective cohort study of U.S. Medicare and commercial health insurance claims data linked with EHRs for 2007-2014. The study included 23,339 adults diagnosed with HZ and 46,378 controls matched for sociodemographic and cardiovascular risk factors.
During the period from 1 month before to 1 month after HZ diagnosis, the rate of the composite vascular endpoint was 31.4 events per 1,000 person-years in the HZ group versus 24.5 per 1,000 person-years in controls. This difference was driven by a significantly higher rate of stroke/TIA in the HZ group. In contrast, the acute MI rates in the two groups were quite similar, at 6.9 per 1,000 person-years in the HZ group and 7.1 per 1,000 person-years in controls.
Further research is needed in order to shed light on the higher rate of vascular events observed in the younger patients with HZ. One possible explanation for the age-related difference is that HZ’s vascular effects is diluted in older patients, who have a higher burden of cardiovascular risk factors, Dr. Rausch noted.
The Centers for Disease Control and Prevention recommends the use of the two-dose recombinant Shingrix zoster vaccine in adults aged 50 years and older instead of the older, live-virus Zostavax vaccine, which is for adults aged 60 years and older.
Dr. Rausch’s study was sponsored by her employer, GlaxoSmithKline. The findings are supportive of an earlier study by other investigators, who found that the risk of hospitalization for stroke was up to twofold greater within the first 90 days after diagnosis of HZ in patients with rheumatoid arthritis and other autoimmune diseases than the stroke rate 366-730 days after HZ (Arthritis Rheumatol. 2017.69[2]:439-46).
SOURCE: Rausch DA. ACC 18
ORLANDO – The risk of stroke and TIA – but not of acute MI – is significantly increased during the period surrounding diagnosis of herpes zoster, compared with the rate in matched zoster-free controls, according to a retrospective study of nearly 70,000 U.S. adults. A particularly striking study finding was the marked age disparity in the magnitude of vascular event risk associated with herpes zoster (HZ), with younger adults being at higher risk.
This increased vascular event risk associated with HZ was transitory. During the entire study follow-up period, which stretched from 1 month prior to HZ diagnosis to 12 months afterward, there was no overall increased vascular event risk associated with HZ, noted Dr. Rausch, an infectious diseases specialist who serves as director of clinical and medical affairs for the U.S. zoster program at GlaxoSmithKline in Philadelphia.
She presented a retrospective cohort study of U.S. Medicare and commercial health insurance claims data linked with EHRs for 2007-2014. The study included 23,339 adults diagnosed with HZ and 46,378 controls matched for sociodemographic and cardiovascular risk factors.
During the period from 1 month before to 1 month after HZ diagnosis, the rate of the composite vascular endpoint was 31.4 events per 1,000 person-years in the HZ group versus 24.5 per 1,000 person-years in controls. This difference was driven by a significantly higher rate of stroke/TIA in the HZ group. In contrast, the acute MI rates in the two groups were quite similar, at 6.9 per 1,000 person-years in the HZ group and 7.1 per 1,000 person-years in controls.
Further research is needed in order to shed light on the higher rate of vascular events observed in the younger patients with HZ. One possible explanation for the age-related difference is that HZ’s vascular effects is diluted in older patients, who have a higher burden of cardiovascular risk factors, Dr. Rausch noted.
The Centers for Disease Control and Prevention recommends the use of the two-dose recombinant Shingrix zoster vaccine in adults aged 50 years and older instead of the older, live-virus Zostavax vaccine, which is for adults aged 60 years and older.
Dr. Rausch’s study was sponsored by her employer, GlaxoSmithKline. The findings are supportive of an earlier study by other investigators, who found that the risk of hospitalization for stroke was up to twofold greater within the first 90 days after diagnosis of HZ in patients with rheumatoid arthritis and other autoimmune diseases than the stroke rate 366-730 days after HZ (Arthritis Rheumatol. 2017.69[2]:439-46).
SOURCE: Rausch DA. ACC 18
ORLANDO – The risk of stroke and TIA – but not of acute MI – is significantly increased during the period surrounding diagnosis of herpes zoster, compared with the rate in matched zoster-free controls, according to a retrospective study of nearly 70,000 U.S. adults. A particularly striking study finding was the marked age disparity in the magnitude of vascular event risk associated with herpes zoster (HZ), with younger adults being at higher risk.
This increased vascular event risk associated with HZ was transitory. During the entire study follow-up period, which stretched from 1 month prior to HZ diagnosis to 12 months afterward, there was no overall increased vascular event risk associated with HZ, noted Dr. Rausch, an infectious diseases specialist who serves as director of clinical and medical affairs for the U.S. zoster program at GlaxoSmithKline in Philadelphia.
She presented a retrospective cohort study of U.S. Medicare and commercial health insurance claims data linked with EHRs for 2007-2014. The study included 23,339 adults diagnosed with HZ and 46,378 controls matched for sociodemographic and cardiovascular risk factors.
During the period from 1 month before to 1 month after HZ diagnosis, the rate of the composite vascular endpoint was 31.4 events per 1,000 person-years in the HZ group versus 24.5 per 1,000 person-years in controls. This difference was driven by a significantly higher rate of stroke/TIA in the HZ group. In contrast, the acute MI rates in the two groups were quite similar, at 6.9 per 1,000 person-years in the HZ group and 7.1 per 1,000 person-years in controls.
Further research is needed in order to shed light on the higher rate of vascular events observed in the younger patients with HZ. One possible explanation for the age-related difference is that HZ’s vascular effects is diluted in older patients, who have a higher burden of cardiovascular risk factors, Dr. Rausch noted.
The Centers for Disease Control and Prevention recommends the use of the two-dose recombinant Shingrix zoster vaccine in adults aged 50 years and older instead of the older, live-virus Zostavax vaccine, which is for adults aged 60 years and older.
Dr. Rausch’s study was sponsored by her employer, GlaxoSmithKline. The findings are supportive of an earlier study by other investigators, who found that the risk of hospitalization for stroke was up to twofold greater within the first 90 days after diagnosis of HZ in patients with rheumatoid arthritis and other autoimmune diseases than the stroke rate 366-730 days after HZ (Arthritis Rheumatol. 2017.69[2]:439-46).
SOURCE: Rausch DA. ACC 18
REPORTING FROM ACC 2018
Key clinical point: Herpes zoster is associated with significantly increased short-term risk of stroke/TIA.
Major finding: Adults aged 18-49 years were threefold more likely than controls to experience stroke or TIA within a month of herpes zoster diagnosis.
Study details: This retrospective study included 23,339 U.S. adults with herpes zoster and twice as many matched controls.
Disclosures: The study was sponsored by GlaxoSmithKline and presented by a GSK employee.
Source: Rausch DA. ACC 18
VIDEO: Triple-antihypertensive pill nails early therapy
ORLANDO – Hypertensive adults started on a triple-drug, single daily pill regimen as either initial or early treatment had a sharply better rate of reaching their goal blood pressure after 6 months, compared with usual-care controls, in a multicenter, randomized trial with 700 patients.
“Early use of a low-dose, three-in-one blood pressure lowering pill is safe and provides faster and better control of blood pressure compared with usual care,” Ruth Webster, PhD, said at the annual meeting of the American College of Cardiology.
The tested polypill contained half the standard doses of the angiotensin receptor blocker telmisartan (20 mg), the calcium channel blocker amlodipine (2.5 mg), and the diuretic chlorthalidone (12.5 mg). After 6 months on this regimen, 70% of patients were at their goal blood pressure, compared with 55% of the control patients, and patients on the polypill had on average a 10/5 mm Hg greater reduction in their blood pressure than did patients on usual care, reported Dr. Webster, head of research programs at the George Institute for Global Health in Sydney. Rates of total and serious adverse events and withdrawals because of adverse events were similar in the two study arms, and both arms also had nearly identical levels of treatment adherence, about 95%.
“No prior trial has evaluated a triple, low-dose pill for initial or early treatment,” she noted.
“This is a home run,” said Karol E. Watson, MD, professor of medicine and director of the Women’s Cardiovascular Health Center at the University of California, Los Angeles. “In the past, clinicians were told to pick one drug and push it as hard as you could and then maybe think about adding a second drug. Experience has shown that this does not increase efficacy, but it does increase adverse events, so current guidelines say start with two drugs. Now they are showing for the first time that you should start with three drugs. That goes with what we know.”
“Triple-drug therapy for the masses makes complete sense,” especially now that the blood pressure goal for most patients is less than 130/80 mm Hg, said William B. White, MD, professor of medicine and chief of hypertension and clinical pharmacology at the University of Connecticut in Farmington. Plus, “compliance is vastly improved when you use a combination-drug pill,” he noted.
The blood pressure targets that Dr. Webster and her associates used were less than 140/90 mm Hg except in patients with diabetes or chronic kidney disease, who had a target of less than 130/80 mm Hg. At the time researchers designed the trial the generally accepted blood pressure target for antihypertensive treatment was less than 140/90 mm Hg, Dr. Webster noted.
She also stressed that she did not believe the three specific drugs selected for the polypill made a difference. “The specific drugs we used was not that important. We would probably get the same result with different drugs. It’s about the strategy of using triple, low-dose therapy,” Dr. Webster suggested. Dr. Watson agreed.
The TRIUMPH (Triple Pill vs. Usual Care Management for Patients with Mild to Moderate Hypertension) study enrolled patients at 11 hospital outpatient clinics in Sri Lanka. The average age of the patients was 56 years. The average blood pressure was 154/90 mm Hg. About 59% of patients were not on any antihypertensive drug at baseline, with the rest on a single drug. The study protocol excluded patients on two or more drugs at entry. Roughly 30% of enrolled patients had diabetes, and 1%-2% had chronic kidney disease. Their target blood pressure on treatment during the study was less than 130/80 mm Hg.
The study’s primary endpoint was the percentage of patients at their goal blood pressure after 6 months. Patients in the triple-drug polypill group achieved their goal blood pressure 23% more often relative to the control, usual care patients, a statistically significant difference. The between group difference in achievement of goal blood pressure was apparent by the end of the first 6 weeks in the study. Patients in the control arm generally received either one or two drugs during the study, but often at full dose rather than the half doses used in the triple-drug patients. The study’s design specified that patients in the triple-drug arm who were not at their target blood pressure after 6 weeks could, at the discretion of their treating physician, switch to a second formulation that doubled the dosage of each of the three drugs. Patients in the usual care arm could have their treatment adjusted after 6 or 12 weeks as long as they continued to receive either one or two drugs. After 6 weeks, 68% of patients in the triple-drug arm and 44% receiving usual care were at their blood pressure goal. After 12 weeks, the percentages at goal were 73% of patients on the triple-drug pill and 47% on usual care.
Dr. Webster hypothesized that the triple-drug, low-dose strategy for initial or early treatment would surpass usual care not only in low- and middle-income countries, like Sri Lanka, but also in high-income, industrialized countries such as the United States.
TRIUMPH received no commercial funding. Dr. Webster had no disclosures. Dr. Watson has been a consultant to Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, and GlaxoSmithKline. Dr. White has been a consultant to Novartis.
SOURCE: Webster R. ACC 2018. Webster R et al. ACC 18 late breaker.
The TRIUMPH results showed the feasibility and efficacy of achieving good blood pressure control with a single pill containing low doses of three different antihypertensive drugs that are well tolerated and have different mechanisms of action. This strategy avoids the adverse effects from drugs used at their maximum dose.
An attraction of this strategy is how seamless it is for patients. They take a single pill with three drugs, which can enhance compliance and in routine practice can reduce their copay. It’s much easier for patients to take a single pill.
Eileen M. Handberg, PhD , is a research professor of medicine and director of the Clinical Trials Program at the University of Florida in Gainesville. She had no relevant disclosures. She made these comments in an interview.
The TRIUMPH results showed the feasibility and efficacy of achieving good blood pressure control with a single pill containing low doses of three different antihypertensive drugs that are well tolerated and have different mechanisms of action. This strategy avoids the adverse effects from drugs used at their maximum dose.
An attraction of this strategy is how seamless it is for patients. They take a single pill with three drugs, which can enhance compliance and in routine practice can reduce their copay. It’s much easier for patients to take a single pill.
Eileen M. Handberg, PhD , is a research professor of medicine and director of the Clinical Trials Program at the University of Florida in Gainesville. She had no relevant disclosures. She made these comments in an interview.
The TRIUMPH results showed the feasibility and efficacy of achieving good blood pressure control with a single pill containing low doses of three different antihypertensive drugs that are well tolerated and have different mechanisms of action. This strategy avoids the adverse effects from drugs used at their maximum dose.
An attraction of this strategy is how seamless it is for patients. They take a single pill with three drugs, which can enhance compliance and in routine practice can reduce their copay. It’s much easier for patients to take a single pill.
Eileen M. Handberg, PhD , is a research professor of medicine and director of the Clinical Trials Program at the University of Florida in Gainesville. She had no relevant disclosures. She made these comments in an interview.
ORLANDO – Hypertensive adults started on a triple-drug, single daily pill regimen as either initial or early treatment had a sharply better rate of reaching their goal blood pressure after 6 months, compared with usual-care controls, in a multicenter, randomized trial with 700 patients.
“Early use of a low-dose, three-in-one blood pressure lowering pill is safe and provides faster and better control of blood pressure compared with usual care,” Ruth Webster, PhD, said at the annual meeting of the American College of Cardiology.
The tested polypill contained half the standard doses of the angiotensin receptor blocker telmisartan (20 mg), the calcium channel blocker amlodipine (2.5 mg), and the diuretic chlorthalidone (12.5 mg). After 6 months on this regimen, 70% of patients were at their goal blood pressure, compared with 55% of the control patients, and patients on the polypill had on average a 10/5 mm Hg greater reduction in their blood pressure than did patients on usual care, reported Dr. Webster, head of research programs at the George Institute for Global Health in Sydney. Rates of total and serious adverse events and withdrawals because of adverse events were similar in the two study arms, and both arms also had nearly identical levels of treatment adherence, about 95%.
“No prior trial has evaluated a triple, low-dose pill for initial or early treatment,” she noted.
“This is a home run,” said Karol E. Watson, MD, professor of medicine and director of the Women’s Cardiovascular Health Center at the University of California, Los Angeles. “In the past, clinicians were told to pick one drug and push it as hard as you could and then maybe think about adding a second drug. Experience has shown that this does not increase efficacy, but it does increase adverse events, so current guidelines say start with two drugs. Now they are showing for the first time that you should start with three drugs. That goes with what we know.”
“Triple-drug therapy for the masses makes complete sense,” especially now that the blood pressure goal for most patients is less than 130/80 mm Hg, said William B. White, MD, professor of medicine and chief of hypertension and clinical pharmacology at the University of Connecticut in Farmington. Plus, “compliance is vastly improved when you use a combination-drug pill,” he noted.
The blood pressure targets that Dr. Webster and her associates used were less than 140/90 mm Hg except in patients with diabetes or chronic kidney disease, who had a target of less than 130/80 mm Hg. At the time researchers designed the trial the generally accepted blood pressure target for antihypertensive treatment was less than 140/90 mm Hg, Dr. Webster noted.
She also stressed that she did not believe the three specific drugs selected for the polypill made a difference. “The specific drugs we used was not that important. We would probably get the same result with different drugs. It’s about the strategy of using triple, low-dose therapy,” Dr. Webster suggested. Dr. Watson agreed.
The TRIUMPH (Triple Pill vs. Usual Care Management for Patients with Mild to Moderate Hypertension) study enrolled patients at 11 hospital outpatient clinics in Sri Lanka. The average age of the patients was 56 years. The average blood pressure was 154/90 mm Hg. About 59% of patients were not on any antihypertensive drug at baseline, with the rest on a single drug. The study protocol excluded patients on two or more drugs at entry. Roughly 30% of enrolled patients had diabetes, and 1%-2% had chronic kidney disease. Their target blood pressure on treatment during the study was less than 130/80 mm Hg.
The study’s primary endpoint was the percentage of patients at their goal blood pressure after 6 months. Patients in the triple-drug polypill group achieved their goal blood pressure 23% more often relative to the control, usual care patients, a statistically significant difference. The between group difference in achievement of goal blood pressure was apparent by the end of the first 6 weeks in the study. Patients in the control arm generally received either one or two drugs during the study, but often at full dose rather than the half doses used in the triple-drug patients. The study’s design specified that patients in the triple-drug arm who were not at their target blood pressure after 6 weeks could, at the discretion of their treating physician, switch to a second formulation that doubled the dosage of each of the three drugs. Patients in the usual care arm could have their treatment adjusted after 6 or 12 weeks as long as they continued to receive either one or two drugs. After 6 weeks, 68% of patients in the triple-drug arm and 44% receiving usual care were at their blood pressure goal. After 12 weeks, the percentages at goal were 73% of patients on the triple-drug pill and 47% on usual care.
Dr. Webster hypothesized that the triple-drug, low-dose strategy for initial or early treatment would surpass usual care not only in low- and middle-income countries, like Sri Lanka, but also in high-income, industrialized countries such as the United States.
TRIUMPH received no commercial funding. Dr. Webster had no disclosures. Dr. Watson has been a consultant to Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, and GlaxoSmithKline. Dr. White has been a consultant to Novartis.
SOURCE: Webster R. ACC 2018. Webster R et al. ACC 18 late breaker.
ORLANDO – Hypertensive adults started on a triple-drug, single daily pill regimen as either initial or early treatment had a sharply better rate of reaching their goal blood pressure after 6 months, compared with usual-care controls, in a multicenter, randomized trial with 700 patients.
“Early use of a low-dose, three-in-one blood pressure lowering pill is safe and provides faster and better control of blood pressure compared with usual care,” Ruth Webster, PhD, said at the annual meeting of the American College of Cardiology.
The tested polypill contained half the standard doses of the angiotensin receptor blocker telmisartan (20 mg), the calcium channel blocker amlodipine (2.5 mg), and the diuretic chlorthalidone (12.5 mg). After 6 months on this regimen, 70% of patients were at their goal blood pressure, compared with 55% of the control patients, and patients on the polypill had on average a 10/5 mm Hg greater reduction in their blood pressure than did patients on usual care, reported Dr. Webster, head of research programs at the George Institute for Global Health in Sydney. Rates of total and serious adverse events and withdrawals because of adverse events were similar in the two study arms, and both arms also had nearly identical levels of treatment adherence, about 95%.
“No prior trial has evaluated a triple, low-dose pill for initial or early treatment,” she noted.
“This is a home run,” said Karol E. Watson, MD, professor of medicine and director of the Women’s Cardiovascular Health Center at the University of California, Los Angeles. “In the past, clinicians were told to pick one drug and push it as hard as you could and then maybe think about adding a second drug. Experience has shown that this does not increase efficacy, but it does increase adverse events, so current guidelines say start with two drugs. Now they are showing for the first time that you should start with three drugs. That goes with what we know.”
“Triple-drug therapy for the masses makes complete sense,” especially now that the blood pressure goal for most patients is less than 130/80 mm Hg, said William B. White, MD, professor of medicine and chief of hypertension and clinical pharmacology at the University of Connecticut in Farmington. Plus, “compliance is vastly improved when you use a combination-drug pill,” he noted.
The blood pressure targets that Dr. Webster and her associates used were less than 140/90 mm Hg except in patients with diabetes or chronic kidney disease, who had a target of less than 130/80 mm Hg. At the time researchers designed the trial the generally accepted blood pressure target for antihypertensive treatment was less than 140/90 mm Hg, Dr. Webster noted.
She also stressed that she did not believe the three specific drugs selected for the polypill made a difference. “The specific drugs we used was not that important. We would probably get the same result with different drugs. It’s about the strategy of using triple, low-dose therapy,” Dr. Webster suggested. Dr. Watson agreed.
The TRIUMPH (Triple Pill vs. Usual Care Management for Patients with Mild to Moderate Hypertension) study enrolled patients at 11 hospital outpatient clinics in Sri Lanka. The average age of the patients was 56 years. The average blood pressure was 154/90 mm Hg. About 59% of patients were not on any antihypertensive drug at baseline, with the rest on a single drug. The study protocol excluded patients on two or more drugs at entry. Roughly 30% of enrolled patients had diabetes, and 1%-2% had chronic kidney disease. Their target blood pressure on treatment during the study was less than 130/80 mm Hg.
The study’s primary endpoint was the percentage of patients at their goal blood pressure after 6 months. Patients in the triple-drug polypill group achieved their goal blood pressure 23% more often relative to the control, usual care patients, a statistically significant difference. The between group difference in achievement of goal blood pressure was apparent by the end of the first 6 weeks in the study. Patients in the control arm generally received either one or two drugs during the study, but often at full dose rather than the half doses used in the triple-drug patients. The study’s design specified that patients in the triple-drug arm who were not at their target blood pressure after 6 weeks could, at the discretion of their treating physician, switch to a second formulation that doubled the dosage of each of the three drugs. Patients in the usual care arm could have their treatment adjusted after 6 or 12 weeks as long as they continued to receive either one or two drugs. After 6 weeks, 68% of patients in the triple-drug arm and 44% receiving usual care were at their blood pressure goal. After 12 weeks, the percentages at goal were 73% of patients on the triple-drug pill and 47% on usual care.
Dr. Webster hypothesized that the triple-drug, low-dose strategy for initial or early treatment would surpass usual care not only in low- and middle-income countries, like Sri Lanka, but also in high-income, industrialized countries such as the United States.
TRIUMPH received no commercial funding. Dr. Webster had no disclosures. Dr. Watson has been a consultant to Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, and GlaxoSmithKline. Dr. White has been a consultant to Novartis.
SOURCE: Webster R. ACC 2018. Webster R et al. ACC 18 late breaker.
REPORTING FROM ACC 18
Key clinical point: Starting hypertensive patients on a single, triple-drug pill produced excellent control.
Major finding: After 6 months, 70% of patients on the triple-drug pill reached target blood pressure, compared with 55% of control patients.
Study details: TRIUMPH, a multicenter, randomized trial with 700 hypertensive adults.
Disclosures: TRIUMPH received no commercial funding. Dr. Webster had no disclosures. Dr. Watson has been a consultant to Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, and GlaxoSmithKline. Dr. White has been a consultant to Novartis.
Source: Webster R et al. ACC 18 late breaker.
Cardiovascular risk in type 2 diabetes: Patients are often clueless
ORLANDO – What most people with type 2 diabetes don’t know about their cardiovascular risk could get them killed.
A national online survey revealed a surprising lack of awareness among patients with type 2 diabetes and their loved ones regarding the well-established significantly increased risk of cardiovascular mortality associated with the disease.
The same low rate of awareness was present in the general population, as represented by the 1,004 controls who didn’t have type 2 diabetes or know anyone who did, Jonathan Pak, PharmD, reported at the annual meeting of the American College of Cardiology.
“We thought patient awareness would be higher, but some who I think understand this space better are surprised it’s even this high,” Dr. Pak said in an interview.
Putting aside the widespread lack of awareness of cardiovascular disease as the leading cause of death in patients with type 2 diabetes, 52% of the patients with type 2 diabetes and a similar proportion of their loved ones were unaware that type 2 diabetes is associated with any increased risk of cardiovascular disease and other macrovascular events, noted Dr. Pak, director of metabolism at Boehringer Ingelheim in Ridgefield, Conn.
There was a strong whiff of denial in the patients’ attitudes. For example, only 24% of the group with type 2 diabetes rated themselves as “likely” to have an amputation in the future, yet they rated 42% of others with type 2 disease as likely to undergo amputation. The same attitude pertained to the prospect of having an acute MI: It’s the others who are at increased risk, not me.
Encouragingly though, more than 80% of the type 2 diabetes patients who hadn’t realized they were at increased cardiovascular risk indicated that if they truly are at increased risk, they would take preventive measures to reduce that risk, including dietary modification and a conversation with their healthcare provider. In addition, 80% said their motivation in doing so would be to improve their quality of life, and 73% cited a desire to live longer and spend more time with their family, Dr. Pak observed.
“The findings from this survey highlight a huge opportunity to educate and inform, and for patients and their loved ones to act on,” he said.
The For Your Sweetheart survey was supported by Boehringer Ingelheim, Eli Lilly, and the Company Diabetes Alliance.
SOURCE: Pak J. ACC 18.
ORLANDO – What most people with type 2 diabetes don’t know about their cardiovascular risk could get them killed.
A national online survey revealed a surprising lack of awareness among patients with type 2 diabetes and their loved ones regarding the well-established significantly increased risk of cardiovascular mortality associated with the disease.
The same low rate of awareness was present in the general population, as represented by the 1,004 controls who didn’t have type 2 diabetes or know anyone who did, Jonathan Pak, PharmD, reported at the annual meeting of the American College of Cardiology.
“We thought patient awareness would be higher, but some who I think understand this space better are surprised it’s even this high,” Dr. Pak said in an interview.
Putting aside the widespread lack of awareness of cardiovascular disease as the leading cause of death in patients with type 2 diabetes, 52% of the patients with type 2 diabetes and a similar proportion of their loved ones were unaware that type 2 diabetes is associated with any increased risk of cardiovascular disease and other macrovascular events, noted Dr. Pak, director of metabolism at Boehringer Ingelheim in Ridgefield, Conn.
There was a strong whiff of denial in the patients’ attitudes. For example, only 24% of the group with type 2 diabetes rated themselves as “likely” to have an amputation in the future, yet they rated 42% of others with type 2 disease as likely to undergo amputation. The same attitude pertained to the prospect of having an acute MI: It’s the others who are at increased risk, not me.
Encouragingly though, more than 80% of the type 2 diabetes patients who hadn’t realized they were at increased cardiovascular risk indicated that if they truly are at increased risk, they would take preventive measures to reduce that risk, including dietary modification and a conversation with their healthcare provider. In addition, 80% said their motivation in doing so would be to improve their quality of life, and 73% cited a desire to live longer and spend more time with their family, Dr. Pak observed.
“The findings from this survey highlight a huge opportunity to educate and inform, and for patients and their loved ones to act on,” he said.
The For Your Sweetheart survey was supported by Boehringer Ingelheim, Eli Lilly, and the Company Diabetes Alliance.
SOURCE: Pak J. ACC 18.
ORLANDO – What most people with type 2 diabetes don’t know about their cardiovascular risk could get them killed.
A national online survey revealed a surprising lack of awareness among patients with type 2 diabetes and their loved ones regarding the well-established significantly increased risk of cardiovascular mortality associated with the disease.
The same low rate of awareness was present in the general population, as represented by the 1,004 controls who didn’t have type 2 diabetes or know anyone who did, Jonathan Pak, PharmD, reported at the annual meeting of the American College of Cardiology.
“We thought patient awareness would be higher, but some who I think understand this space better are surprised it’s even this high,” Dr. Pak said in an interview.
Putting aside the widespread lack of awareness of cardiovascular disease as the leading cause of death in patients with type 2 diabetes, 52% of the patients with type 2 diabetes and a similar proportion of their loved ones were unaware that type 2 diabetes is associated with any increased risk of cardiovascular disease and other macrovascular events, noted Dr. Pak, director of metabolism at Boehringer Ingelheim in Ridgefield, Conn.
There was a strong whiff of denial in the patients’ attitudes. For example, only 24% of the group with type 2 diabetes rated themselves as “likely” to have an amputation in the future, yet they rated 42% of others with type 2 disease as likely to undergo amputation. The same attitude pertained to the prospect of having an acute MI: It’s the others who are at increased risk, not me.
Encouragingly though, more than 80% of the type 2 diabetes patients who hadn’t realized they were at increased cardiovascular risk indicated that if they truly are at increased risk, they would take preventive measures to reduce that risk, including dietary modification and a conversation with their healthcare provider. In addition, 80% said their motivation in doing so would be to improve their quality of life, and 73% cited a desire to live longer and spend more time with their family, Dr. Pak observed.
“The findings from this survey highlight a huge opportunity to educate and inform, and for patients and their loved ones to act on,” he said.
The For Your Sweetheart survey was supported by Boehringer Ingelheim, Eli Lilly, and the Company Diabetes Alliance.
SOURCE: Pak J. ACC 18.
REPORTING FROM ACC 2018
Key clinical point: What most people with type 2 diabetes don’t know about their cardiovascular risk could get them killed.
Major finding: More than half of patients with type 2 diabetes who participated in an online survey were unaware that their disease places them at increased cardiovascular risk.
Study details: This online, cross-sectional survey included 1,869 respondents.
Disclosures: The For Your Sweetheart survey was supported by Boehringer Ingelheim and Eli Lilly. The presenter is a Boehringer Ingelheim employee.
Source: Pak J. ACC 18.
Permanent His-bundle pacing superior to RV pacing
ORLANDO – Pacing at the bundle of His was associated with significantly reduced morbidity and mortality, compared with right ventricular pacing, over time in a large observational registry of patients needing a permanent pacemaker for bradycardia, Mohamed Abdelrahman, MD, reported at the annual meeting of the American College of Cardiology.
The superiority of His-bundle pacing (HBP) was concentrated in patients who required ventricular pacing more than 20% of the time. This finding is consistent with previous reports that even a modest utilization of ventricular pacing is sufficient to boost the risk of left ventricular dysfunction secondary to electrical and mechanical dyssynchrony, added Dr. Abdelrahman of the Geisinger Heart Institute in Danville, Pa.
The primary study endpoint was a composite of all-cause mortality, heart-failure hospitalization, and biventricular pacing upgrade. During a mean 2 years of follow-up, this endpoint was reached in 25% of the HBP group, compared with 32% of the RVP group, for a significant 29% relative risk reduction. In patients with a ventricular pacing burden greater than 20%, the primary endpoint occurred in 25% of the HBP group and 36% of patients with RVP, for a 35% relative risk reduction. However, in patients who required ventricular pacing less than 20% of the time, there was no significant difference in the primary outcome between the two groups.
Heart failure hospitalization occurred in 12.4% of the HBP group and 17.6% of the RVP patients, for a 37% relative risk reduction. In patients with ventricular pacing more than 20% of the time, the rates were 12.4% and 20.1%, for a 46% relative risk reduction in favor of HBP.
Among patients with a ventricular pacing burden of more than 20%, all-cause mortality occurred in 18% of the HBP group, compared with 23.7% of RVP-treated patients.
One patient in the HBP group required an upgrade to biventricular pacing, as did six patients in the RVP group. Lead revision was necessary in 14 patients in the HBP group, versus 2 in the RVP group. Pericardial effusion within the first month of pacemaker implantation occurred in three patients in the RVP group and did not occur in the HBP group.
Discussant Kristen K. Patton, MD, called the Geisinger work “a really wonderful study,” adding, “It’s incredibly difficult to overstate how excited we are in electrophysiology about His-bundle pacing and what a wonderfully elegant solution this is to the problem of pacing-induced dyssynchrony.
“Is there anything that gives you pause, any patients in whom the increased risk of revisions makes you think, ‘I shouldn’t do this in everyone?’ Because I can tell you, it’s hard not to want to do this in everyone,” said Dr. Patton, professor of medicine at the University of Washington, Seattle.
Dr. Abdelrahman replied that Geisinger electrophysiologists now utilize HBP in all patients who require a permanent pacemaker for bradycardia.
Session chair Martin B. Leon, MD, of Columbia University, New York, had a question: “This is such an important area. Why didn’t you do a randomized trial from the start?”
Dr. Abdelrahman’s senior coinvestigator, Pugazhendhi Vijayaraman, MD, explained: “His-bundle pacing has been around for the last 20 years. It’s had its ups and downs. In the last few years there’s been a groundswell of implanters doing His-bundle pacing. The number of implanters here and around the world is rapidly expanding. So we are ready for a randomized trial, and we’ve applied for funding from the National Institutes of Health. Industry support for this has not been forthcoming because His-bundle pacing does not seem to add to the value of a company’s portfolio, but more to better patient outcomes.”
He emphasized that, of the 14 patients in the HBP group who underwent lead revision, only 2 had absolute lead failure and loss of capture, underscoring the safety of this pacing strategy.
Dr. Abdelrahman reported having no financial conflicts of interest regarding the study.
SOURCE: Abdelrahman M. ACC 18.
ORLANDO – Pacing at the bundle of His was associated with significantly reduced morbidity and mortality, compared with right ventricular pacing, over time in a large observational registry of patients needing a permanent pacemaker for bradycardia, Mohamed Abdelrahman, MD, reported at the annual meeting of the American College of Cardiology.
The superiority of His-bundle pacing (HBP) was concentrated in patients who required ventricular pacing more than 20% of the time. This finding is consistent with previous reports that even a modest utilization of ventricular pacing is sufficient to boost the risk of left ventricular dysfunction secondary to electrical and mechanical dyssynchrony, added Dr. Abdelrahman of the Geisinger Heart Institute in Danville, Pa.
The primary study endpoint was a composite of all-cause mortality, heart-failure hospitalization, and biventricular pacing upgrade. During a mean 2 years of follow-up, this endpoint was reached in 25% of the HBP group, compared with 32% of the RVP group, for a significant 29% relative risk reduction. In patients with a ventricular pacing burden greater than 20%, the primary endpoint occurred in 25% of the HBP group and 36% of patients with RVP, for a 35% relative risk reduction. However, in patients who required ventricular pacing less than 20% of the time, there was no significant difference in the primary outcome between the two groups.
Heart failure hospitalization occurred in 12.4% of the HBP group and 17.6% of the RVP patients, for a 37% relative risk reduction. In patients with ventricular pacing more than 20% of the time, the rates were 12.4% and 20.1%, for a 46% relative risk reduction in favor of HBP.
Among patients with a ventricular pacing burden of more than 20%, all-cause mortality occurred in 18% of the HBP group, compared with 23.7% of RVP-treated patients.
One patient in the HBP group required an upgrade to biventricular pacing, as did six patients in the RVP group. Lead revision was necessary in 14 patients in the HBP group, versus 2 in the RVP group. Pericardial effusion within the first month of pacemaker implantation occurred in three patients in the RVP group and did not occur in the HBP group.
Discussant Kristen K. Patton, MD, called the Geisinger work “a really wonderful study,” adding, “It’s incredibly difficult to overstate how excited we are in electrophysiology about His-bundle pacing and what a wonderfully elegant solution this is to the problem of pacing-induced dyssynchrony.
“Is there anything that gives you pause, any patients in whom the increased risk of revisions makes you think, ‘I shouldn’t do this in everyone?’ Because I can tell you, it’s hard not to want to do this in everyone,” said Dr. Patton, professor of medicine at the University of Washington, Seattle.
Dr. Abdelrahman replied that Geisinger electrophysiologists now utilize HBP in all patients who require a permanent pacemaker for bradycardia.
Session chair Martin B. Leon, MD, of Columbia University, New York, had a question: “This is such an important area. Why didn’t you do a randomized trial from the start?”
Dr. Abdelrahman’s senior coinvestigator, Pugazhendhi Vijayaraman, MD, explained: “His-bundle pacing has been around for the last 20 years. It’s had its ups and downs. In the last few years there’s been a groundswell of implanters doing His-bundle pacing. The number of implanters here and around the world is rapidly expanding. So we are ready for a randomized trial, and we’ve applied for funding from the National Institutes of Health. Industry support for this has not been forthcoming because His-bundle pacing does not seem to add to the value of a company’s portfolio, but more to better patient outcomes.”
He emphasized that, of the 14 patients in the HBP group who underwent lead revision, only 2 had absolute lead failure and loss of capture, underscoring the safety of this pacing strategy.
Dr. Abdelrahman reported having no financial conflicts of interest regarding the study.
SOURCE: Abdelrahman M. ACC 18.
ORLANDO – Pacing at the bundle of His was associated with significantly reduced morbidity and mortality, compared with right ventricular pacing, over time in a large observational registry of patients needing a permanent pacemaker for bradycardia, Mohamed Abdelrahman, MD, reported at the annual meeting of the American College of Cardiology.
The superiority of His-bundle pacing (HBP) was concentrated in patients who required ventricular pacing more than 20% of the time. This finding is consistent with previous reports that even a modest utilization of ventricular pacing is sufficient to boost the risk of left ventricular dysfunction secondary to electrical and mechanical dyssynchrony, added Dr. Abdelrahman of the Geisinger Heart Institute in Danville, Pa.
The primary study endpoint was a composite of all-cause mortality, heart-failure hospitalization, and biventricular pacing upgrade. During a mean 2 years of follow-up, this endpoint was reached in 25% of the HBP group, compared with 32% of the RVP group, for a significant 29% relative risk reduction. In patients with a ventricular pacing burden greater than 20%, the primary endpoint occurred in 25% of the HBP group and 36% of patients with RVP, for a 35% relative risk reduction. However, in patients who required ventricular pacing less than 20% of the time, there was no significant difference in the primary outcome between the two groups.
Heart failure hospitalization occurred in 12.4% of the HBP group and 17.6% of the RVP patients, for a 37% relative risk reduction. In patients with ventricular pacing more than 20% of the time, the rates were 12.4% and 20.1%, for a 46% relative risk reduction in favor of HBP.
Among patients with a ventricular pacing burden of more than 20%, all-cause mortality occurred in 18% of the HBP group, compared with 23.7% of RVP-treated patients.
One patient in the HBP group required an upgrade to biventricular pacing, as did six patients in the RVP group. Lead revision was necessary in 14 patients in the HBP group, versus 2 in the RVP group. Pericardial effusion within the first month of pacemaker implantation occurred in three patients in the RVP group and did not occur in the HBP group.
Discussant Kristen K. Patton, MD, called the Geisinger work “a really wonderful study,” adding, “It’s incredibly difficult to overstate how excited we are in electrophysiology about His-bundle pacing and what a wonderfully elegant solution this is to the problem of pacing-induced dyssynchrony.
“Is there anything that gives you pause, any patients in whom the increased risk of revisions makes you think, ‘I shouldn’t do this in everyone?’ Because I can tell you, it’s hard not to want to do this in everyone,” said Dr. Patton, professor of medicine at the University of Washington, Seattle.
Dr. Abdelrahman replied that Geisinger electrophysiologists now utilize HBP in all patients who require a permanent pacemaker for bradycardia.
Session chair Martin B. Leon, MD, of Columbia University, New York, had a question: “This is such an important area. Why didn’t you do a randomized trial from the start?”
Dr. Abdelrahman’s senior coinvestigator, Pugazhendhi Vijayaraman, MD, explained: “His-bundle pacing has been around for the last 20 years. It’s had its ups and downs. In the last few years there’s been a groundswell of implanters doing His-bundle pacing. The number of implanters here and around the world is rapidly expanding. So we are ready for a randomized trial, and we’ve applied for funding from the National Institutes of Health. Industry support for this has not been forthcoming because His-bundle pacing does not seem to add to the value of a company’s portfolio, but more to better patient outcomes.”
He emphasized that, of the 14 patients in the HBP group who underwent lead revision, only 2 had absolute lead failure and loss of capture, underscoring the safety of this pacing strategy.
Dr. Abdelrahman reported having no financial conflicts of interest regarding the study.
SOURCE: Abdelrahman M. ACC 18.
REPORTING FROM ACC 18
Key clinical point:
Major finding: The combined rate of all-cause mortality, heart-failure hospitalization, and biventricular pacing upgrade during a mean 2 years of follow-up was 25% in patients with His-bundle pacing, compared with 32% with right ventricular pacing.
Study details: This observational registry included 765 consecutive patients who required an initial permanent pacemaker implantation. All those treated at one medical center underwent an attempt at His-bundle pacing, while all those at a closely allied sister medical center received right ventricular pacing.
Disclosures: The study presenter reported having no financial conflicts of interest.
Source: Abdelrahman M. ACC 18.
Statins, ACE inhibitors linked to fetal cardiac anomalies
ORLANDO – Women exposed to statin treatment during the first trimester of pregnancy had a doubled rate of delivering neonates with a cardiac anomaly, and a nearly 400% increased rate of delivering a baby with a ventricular septal defect, compared with infants born to unexposed women in a case-control review of nearly 400,000 U.S. births during 2003-2014.
A similar, parallel analysis of the same cohort also showed that exposure to an ACE inhibitor at any time during pregnancy linked with roughly tripled rates of premature delivery, low birth weight, and neonatal cardiac anomaly, compared with unexposed women, Ming-Sum Lee, MD, and her associates reported in two posters presented at the annual meeting of the American College of Cardiology.
For the statin analysis the researchers reviewed data collected from 379,238 singleton pregnancies delivered during January 2003-December 2014 to women who received their health care from Kaiser Permanente of Southern California. The cohort included 280 women who filled at least one prescription for a statin during their first trimester of pregnancy. Half the women received simvastatin, and 37% received lovastatin. The researchers used propensity score matching to identify 1,160 women with no statin exposure who closely matched 279 of the women with statin exposure.
The review showed a 2.1% incidence of fetal cardiac anomalies in the infants born to the unexposed women and a 5.0% rate among the exposed women; the hazard ratio was 2.5, which was statistically significant. More detailed analysis showed that the increased incidence of cardiac anomalies was primarily caused by ventricular septal defects, which occurred at a 4.3% rate among the infants born to exposed mothers, a rate 370% higher than among the unexposed pregnancies. No other types of cardiac anomaly examined showed a significant increase among the exposed infants.
Assessment of links with ACE-inhibitor use focused on 404 women who had exposure to the drug class at any time during pregnancy. The most commonly used drug was lisinopril, by 98% of the women. The researchers compared these links against all the other women with exposure to an ACE inhibitor who delivered in the database without propensity score matching or in general any adjustment for clinical features or comorbidities. The analysis showed premature birth (less than 37 weeks’ gestational age) occurred at a 24% rate among the ACE inhibitor–exposed infants and 8% of the unexposed; low birth weight (less than 2,500 g) occurred in 15% of the exposed infants and in 5% of those not exposed, and any type of cardiac anomaly occurred in 4.5% of the exposed neonates and in 1.4% of the unexposed.
Dr. Lee and her associates reported the results of one adjusted analysis that factored maternal comorbidities into the calculation of the relative risk for delivering a neonate with any cardiac anomaly. After adjustment, the incremental risk linked with ACE inhibitor exposure any time during gestation was a statistically significant 80% increase.
Dr. Lee had no disclosures.
SOURCES: Hekimian A et al. ACC 18, Poster 1124-366. Chintamaneni S et al. ACC 18, Poster 1124-365.
ORLANDO – Women exposed to statin treatment during the first trimester of pregnancy had a doubled rate of delivering neonates with a cardiac anomaly, and a nearly 400% increased rate of delivering a baby with a ventricular septal defect, compared with infants born to unexposed women in a case-control review of nearly 400,000 U.S. births during 2003-2014.
A similar, parallel analysis of the same cohort also showed that exposure to an ACE inhibitor at any time during pregnancy linked with roughly tripled rates of premature delivery, low birth weight, and neonatal cardiac anomaly, compared with unexposed women, Ming-Sum Lee, MD, and her associates reported in two posters presented at the annual meeting of the American College of Cardiology.
For the statin analysis the researchers reviewed data collected from 379,238 singleton pregnancies delivered during January 2003-December 2014 to women who received their health care from Kaiser Permanente of Southern California. The cohort included 280 women who filled at least one prescription for a statin during their first trimester of pregnancy. Half the women received simvastatin, and 37% received lovastatin. The researchers used propensity score matching to identify 1,160 women with no statin exposure who closely matched 279 of the women with statin exposure.
The review showed a 2.1% incidence of fetal cardiac anomalies in the infants born to the unexposed women and a 5.0% rate among the exposed women; the hazard ratio was 2.5, which was statistically significant. More detailed analysis showed that the increased incidence of cardiac anomalies was primarily caused by ventricular septal defects, which occurred at a 4.3% rate among the infants born to exposed mothers, a rate 370% higher than among the unexposed pregnancies. No other types of cardiac anomaly examined showed a significant increase among the exposed infants.
Assessment of links with ACE-inhibitor use focused on 404 women who had exposure to the drug class at any time during pregnancy. The most commonly used drug was lisinopril, by 98% of the women. The researchers compared these links against all the other women with exposure to an ACE inhibitor who delivered in the database without propensity score matching or in general any adjustment for clinical features or comorbidities. The analysis showed premature birth (less than 37 weeks’ gestational age) occurred at a 24% rate among the ACE inhibitor–exposed infants and 8% of the unexposed; low birth weight (less than 2,500 g) occurred in 15% of the exposed infants and in 5% of those not exposed, and any type of cardiac anomaly occurred in 4.5% of the exposed neonates and in 1.4% of the unexposed.
Dr. Lee and her associates reported the results of one adjusted analysis that factored maternal comorbidities into the calculation of the relative risk for delivering a neonate with any cardiac anomaly. After adjustment, the incremental risk linked with ACE inhibitor exposure any time during gestation was a statistically significant 80% increase.
Dr. Lee had no disclosures.
SOURCES: Hekimian A et al. ACC 18, Poster 1124-366. Chintamaneni S et al. ACC 18, Poster 1124-365.
ORLANDO – Women exposed to statin treatment during the first trimester of pregnancy had a doubled rate of delivering neonates with a cardiac anomaly, and a nearly 400% increased rate of delivering a baby with a ventricular septal defect, compared with infants born to unexposed women in a case-control review of nearly 400,000 U.S. births during 2003-2014.
A similar, parallel analysis of the same cohort also showed that exposure to an ACE inhibitor at any time during pregnancy linked with roughly tripled rates of premature delivery, low birth weight, and neonatal cardiac anomaly, compared with unexposed women, Ming-Sum Lee, MD, and her associates reported in two posters presented at the annual meeting of the American College of Cardiology.
For the statin analysis the researchers reviewed data collected from 379,238 singleton pregnancies delivered during January 2003-December 2014 to women who received their health care from Kaiser Permanente of Southern California. The cohort included 280 women who filled at least one prescription for a statin during their first trimester of pregnancy. Half the women received simvastatin, and 37% received lovastatin. The researchers used propensity score matching to identify 1,160 women with no statin exposure who closely matched 279 of the women with statin exposure.
The review showed a 2.1% incidence of fetal cardiac anomalies in the infants born to the unexposed women and a 5.0% rate among the exposed women; the hazard ratio was 2.5, which was statistically significant. More detailed analysis showed that the increased incidence of cardiac anomalies was primarily caused by ventricular septal defects, which occurred at a 4.3% rate among the infants born to exposed mothers, a rate 370% higher than among the unexposed pregnancies. No other types of cardiac anomaly examined showed a significant increase among the exposed infants.
Assessment of links with ACE-inhibitor use focused on 404 women who had exposure to the drug class at any time during pregnancy. The most commonly used drug was lisinopril, by 98% of the women. The researchers compared these links against all the other women with exposure to an ACE inhibitor who delivered in the database without propensity score matching or in general any adjustment for clinical features or comorbidities. The analysis showed premature birth (less than 37 weeks’ gestational age) occurred at a 24% rate among the ACE inhibitor–exposed infants and 8% of the unexposed; low birth weight (less than 2,500 g) occurred in 15% of the exposed infants and in 5% of those not exposed, and any type of cardiac anomaly occurred in 4.5% of the exposed neonates and in 1.4% of the unexposed.
Dr. Lee and her associates reported the results of one adjusted analysis that factored maternal comorbidities into the calculation of the relative risk for delivering a neonate with any cardiac anomaly. After adjustment, the incremental risk linked with ACE inhibitor exposure any time during gestation was a statistically significant 80% increase.
Dr. Lee had no disclosures.
SOURCES: Hekimian A et al. ACC 18, Poster 1124-366. Chintamaneni S et al. ACC 18, Poster 1124-365.
REPORTING FROM ACC 18
Key clinical point: Fetal exposures to statins or ACE inhibitors link to cardiac anomalies.
Major finding: Ventricular septal defects occurred 370% more often among infants born after first-trimester statin exposure.
Study details: A retrospective review of 379,238 singleton pregnancies delivered at Kaiser Permanente of Southern California during 2003-2014.
Disclosures: Dr. Lee had no disclosures.
Sources: Hekimian A et al. ACC 18, Poster 1124-366. Chintamaneni S et al. ACC 18, Poster 1124-365.