User login
Heightened Emphasis on Sex-specific Cardiovascular Risk Factors
SNOWMASS, COLO. – Achieving continued reductions in cardiovascular deaths in U.S. women will require that physicians make greater use of sex-specific risk factors that aren’t incorporated in the ACC/AHA atherosclerotic cardiovascular disease risk score, Dr. Jennifer H. Mieres asserted at the Annual Cardiovascular Conference at Snowmass.
In the 13-year period beginning in 2000, with the launch of a national initiative to boost the research focus on cardiovascular disease in women, the annual number of women dying from cardiovascular disease has dropped by roughly 30%. That’s a steeper decline than in men. One of the keys to further reductions in women is more widespread physician evaluation of sex-specific risk factors – such as a history of elevated blood pressure in pregnancy, polycystic ovarian syndrome, or radiation therapy for breast cancer – as part of routine cardiovascular risk assessment in women, said Dr. Mieres, senior vice president office of community and public health at Hofstra Northwell in Hempstead, N.Y.
Hypertension in pregnancy as a harbinger of premature cardiovascular disease and other chronic diseases has been a topic of particularly fruitful research in the past few years.
“The ongoing hypothesis is that pregnancy is a sort of stress test. Pregnancy-related complications indicate an inability to adequately adapt to the physiologic stress of pregnancy and thus reveal the presence of underlying susceptibility to ischemic heart disease,” according to the cardiologist.
She cited a landmark prospective study of 10,314 women born in Northern Finland in 1966 and followed for an average of more than 39 years after a singleton pregnancy. The investigators showed that any elevation in blood pressure during pregnancy, including isolated systolic or diastolic hypertension that resolved during or shortly after pregnancy, was associated with increased future risks of various forms of cardiovascular disease.
For example, de novo gestational hypertension without proteinuria was associated with significantly increased risks of subsequent ischemic cerebrovascular disease, chronic kidney disease, diabetes, ischemic heart disease, acute MI, chronic hypertension, and heart failure. The MIs that occurred in Finns with a history of gestational hypertension were more serious, too, with an associated threefold greater risk of being fatal than MIs in women who had been normotensive in pregnancy (Circulation. 2013 Feb 12;127[6]:681-90).
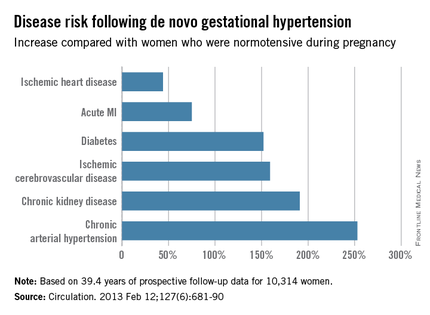
New-onset isolated systolic or diastolic hypertension emerged during pregnancy in about 17% of the Finnish women. Roughly 30% of them had a cardiovascular event before their late 60s. This translated to a 14%-18% greater risk than in women who remained normotensive in pregnancy.
The highest risk of all in the Finnish study was seen in women with preeclampsia/eclampsia superimposed on a background of chronic hypertension. They had a 3.18-fold greater risk of subsequent MI than did women who were normotensive in pregnancy, a 3.32-fold increased risk of heart failure, and a 2.22-fold greater risk of developing diabetes.
In addition to the growing appreciation that it’s important to consider sex-specific cardiovascular risk factors, recent evidence shows that many of the traditional risk factors are stronger predictors of ischemic heart disease in women than men. These include diabetes, smoking, obesity, and hypertension, Dr. Mieres observed.
For example, a recent meta-analysis of 26 studies including more than 214,000 subjects concluded that women with type 1 diabetes had a 2.5-fold greater risk of incident coronary heart disease than did men with type 1 diabetes. The women with type 1 diabetes also had an 86% greater risk of fatal cardiovascular diseases, a 44% increase in the risk of fatal kidney disease, a 37% greater risk of stroke, and a 37% increase in all-cause mortality relative to type 1 diabetic men (Lancet Diabetes Endocrinol. 2015 Mar;3[3]:198-206).
A wealth of accumulating data indicates that type 2 diabetes, too, is a much stronger risk factor for cardiovascular diseases in women than in men. The evidence prompted a recent formal scientific statement to that effect by the American Heart Association (Circulation. 2015 Dec 22;132[25]:2424-47).
Dr. Mieres reported having no financial conflicts of interest regarding her presentation.
SNOWMASS, COLO. – Achieving continued reductions in cardiovascular deaths in U.S. women will require that physicians make greater use of sex-specific risk factors that aren’t incorporated in the ACC/AHA atherosclerotic cardiovascular disease risk score, Dr. Jennifer H. Mieres asserted at the Annual Cardiovascular Conference at Snowmass.
In the 13-year period beginning in 2000, with the launch of a national initiative to boost the research focus on cardiovascular disease in women, the annual number of women dying from cardiovascular disease has dropped by roughly 30%. That’s a steeper decline than in men. One of the keys to further reductions in women is more widespread physician evaluation of sex-specific risk factors – such as a history of elevated blood pressure in pregnancy, polycystic ovarian syndrome, or radiation therapy for breast cancer – as part of routine cardiovascular risk assessment in women, said Dr. Mieres, senior vice president office of community and public health at Hofstra Northwell in Hempstead, N.Y.
Hypertension in pregnancy as a harbinger of premature cardiovascular disease and other chronic diseases has been a topic of particularly fruitful research in the past few years.
“The ongoing hypothesis is that pregnancy is a sort of stress test. Pregnancy-related complications indicate an inability to adequately adapt to the physiologic stress of pregnancy and thus reveal the presence of underlying susceptibility to ischemic heart disease,” according to the cardiologist.
She cited a landmark prospective study of 10,314 women born in Northern Finland in 1966 and followed for an average of more than 39 years after a singleton pregnancy. The investigators showed that any elevation in blood pressure during pregnancy, including isolated systolic or diastolic hypertension that resolved during or shortly after pregnancy, was associated with increased future risks of various forms of cardiovascular disease.
For example, de novo gestational hypertension without proteinuria was associated with significantly increased risks of subsequent ischemic cerebrovascular disease, chronic kidney disease, diabetes, ischemic heart disease, acute MI, chronic hypertension, and heart failure. The MIs that occurred in Finns with a history of gestational hypertension were more serious, too, with an associated threefold greater risk of being fatal than MIs in women who had been normotensive in pregnancy (Circulation. 2013 Feb 12;127[6]:681-90).

New-onset isolated systolic or diastolic hypertension emerged during pregnancy in about 17% of the Finnish women. Roughly 30% of them had a cardiovascular event before their late 60s. This translated to a 14%-18% greater risk than in women who remained normotensive in pregnancy.
The highest risk of all in the Finnish study was seen in women with preeclampsia/eclampsia superimposed on a background of chronic hypertension. They had a 3.18-fold greater risk of subsequent MI than did women who were normotensive in pregnancy, a 3.32-fold increased risk of heart failure, and a 2.22-fold greater risk of developing diabetes.
In addition to the growing appreciation that it’s important to consider sex-specific cardiovascular risk factors, recent evidence shows that many of the traditional risk factors are stronger predictors of ischemic heart disease in women than men. These include diabetes, smoking, obesity, and hypertension, Dr. Mieres observed.
For example, a recent meta-analysis of 26 studies including more than 214,000 subjects concluded that women with type 1 diabetes had a 2.5-fold greater risk of incident coronary heart disease than did men with type 1 diabetes. The women with type 1 diabetes also had an 86% greater risk of fatal cardiovascular diseases, a 44% increase in the risk of fatal kidney disease, a 37% greater risk of stroke, and a 37% increase in all-cause mortality relative to type 1 diabetic men (Lancet Diabetes Endocrinol. 2015 Mar;3[3]:198-206).
A wealth of accumulating data indicates that type 2 diabetes, too, is a much stronger risk factor for cardiovascular diseases in women than in men. The evidence prompted a recent formal scientific statement to that effect by the American Heart Association (Circulation. 2015 Dec 22;132[25]:2424-47).
Dr. Mieres reported having no financial conflicts of interest regarding her presentation.
SNOWMASS, COLO. – Achieving continued reductions in cardiovascular deaths in U.S. women will require that physicians make greater use of sex-specific risk factors that aren’t incorporated in the ACC/AHA atherosclerotic cardiovascular disease risk score, Dr. Jennifer H. Mieres asserted at the Annual Cardiovascular Conference at Snowmass.
In the 13-year period beginning in 2000, with the launch of a national initiative to boost the research focus on cardiovascular disease in women, the annual number of women dying from cardiovascular disease has dropped by roughly 30%. That’s a steeper decline than in men. One of the keys to further reductions in women is more widespread physician evaluation of sex-specific risk factors – such as a history of elevated blood pressure in pregnancy, polycystic ovarian syndrome, or radiation therapy for breast cancer – as part of routine cardiovascular risk assessment in women, said Dr. Mieres, senior vice president office of community and public health at Hofstra Northwell in Hempstead, N.Y.
Hypertension in pregnancy as a harbinger of premature cardiovascular disease and other chronic diseases has been a topic of particularly fruitful research in the past few years.
“The ongoing hypothesis is that pregnancy is a sort of stress test. Pregnancy-related complications indicate an inability to adequately adapt to the physiologic stress of pregnancy and thus reveal the presence of underlying susceptibility to ischemic heart disease,” according to the cardiologist.
She cited a landmark prospective study of 10,314 women born in Northern Finland in 1966 and followed for an average of more than 39 years after a singleton pregnancy. The investigators showed that any elevation in blood pressure during pregnancy, including isolated systolic or diastolic hypertension that resolved during or shortly after pregnancy, was associated with increased future risks of various forms of cardiovascular disease.
For example, de novo gestational hypertension without proteinuria was associated with significantly increased risks of subsequent ischemic cerebrovascular disease, chronic kidney disease, diabetes, ischemic heart disease, acute MI, chronic hypertension, and heart failure. The MIs that occurred in Finns with a history of gestational hypertension were more serious, too, with an associated threefold greater risk of being fatal than MIs in women who had been normotensive in pregnancy (Circulation. 2013 Feb 12;127[6]:681-90).

New-onset isolated systolic or diastolic hypertension emerged during pregnancy in about 17% of the Finnish women. Roughly 30% of them had a cardiovascular event before their late 60s. This translated to a 14%-18% greater risk than in women who remained normotensive in pregnancy.
The highest risk of all in the Finnish study was seen in women with preeclampsia/eclampsia superimposed on a background of chronic hypertension. They had a 3.18-fold greater risk of subsequent MI than did women who were normotensive in pregnancy, a 3.32-fold increased risk of heart failure, and a 2.22-fold greater risk of developing diabetes.
In addition to the growing appreciation that it’s important to consider sex-specific cardiovascular risk factors, recent evidence shows that many of the traditional risk factors are stronger predictors of ischemic heart disease in women than men. These include diabetes, smoking, obesity, and hypertension, Dr. Mieres observed.
For example, a recent meta-analysis of 26 studies including more than 214,000 subjects concluded that women with type 1 diabetes had a 2.5-fold greater risk of incident coronary heart disease than did men with type 1 diabetes. The women with type 1 diabetes also had an 86% greater risk of fatal cardiovascular diseases, a 44% increase in the risk of fatal kidney disease, a 37% greater risk of stroke, and a 37% increase in all-cause mortality relative to type 1 diabetic men (Lancet Diabetes Endocrinol. 2015 Mar;3[3]:198-206).
A wealth of accumulating data indicates that type 2 diabetes, too, is a much stronger risk factor for cardiovascular diseases in women than in men. The evidence prompted a recent formal scientific statement to that effect by the American Heart Association (Circulation. 2015 Dec 22;132[25]:2424-47).
Dr. Mieres reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Heightened emphasis on sex-specific cardiovascular risk factors
SNOWMASS, COLO. – Achieving continued reductions in cardiovascular deaths in U.S. women will require that physicians make greater use of sex-specific risk factors that aren’t incorporated in the ACC/AHA atherosclerotic cardiovascular disease risk score, Dr. Jennifer H. Mieres asserted at the Annual Cardiovascular Conference at Snowmass.
In the 13-year period beginning in 2000, with the launch of a national initiative to boost the research focus on cardiovascular disease in women, the annual number of women dying from cardiovascular disease has dropped by roughly 30%. That’s a steeper decline than in men. One of the keys to further reductions in women is more widespread physician evaluation of sex-specific risk factors – such as a history of elevated blood pressure in pregnancy, polycystic ovarian syndrome, or radiation therapy for breast cancer – as part of routine cardiovascular risk assessment in women, said Dr. Mieres, senior vice president office of community and public health at Hofstra Northwell in Hempstead, N.Y.
Hypertension in pregnancy as a harbinger of premature cardiovascular disease and other chronic diseases has been a topic of particularly fruitful research in the past few years.
“The ongoing hypothesis is that pregnancy is a sort of stress test. Pregnancy-related complications indicate an inability to adequately adapt to the physiologic stress of pregnancy and thus reveal the presence of underlying susceptibility to ischemic heart disease,” according to the cardiologist.
She cited a landmark prospective study of 10,314 women born in Northern Finland in 1966 and followed for an average of more than 39 years after a singleton pregnancy. The investigators showed that any elevation in blood pressure during pregnancy, including isolated systolic or diastolic hypertension that resolved during or shortly after pregnancy, was associated with increased future risks of various forms of cardiovascular disease.
For example, de novo gestational hypertension without proteinuria was associated with significantly increased risks of subsequent ischemic cerebrovascular disease, chronic kidney disease, diabetes, ischemic heart disease, acute MI, chronic hypertension, and heart failure. The MIs that occurred in Finns with a history of gestational hypertension were more serious, too, with an associated threefold greater risk of being fatal than MIs in women who had been normotensive in pregnancy (Circulation. 2013 Feb 12;127[6]:681-90).

New-onset isolated systolic or diastolic hypertension emerged during pregnancy in about 17% of the Finnish women. Roughly 30% of them had a cardiovascular event before their late 60s. This translated to a 14%-18% greater risk than in women who remained normotensive in pregnancy.
The highest risk of all in the Finnish study was seen in women with preeclampsia/eclampsia superimposed on a background of chronic hypertension. They had a 3.18-fold greater risk of subsequent MI than did women who were normotensive in pregnancy, a 3.32-fold increased risk of heart failure, and a 2.22-fold greater risk of developing diabetes.
In addition to the growing appreciation that it’s important to consider sex-specific cardiovascular risk factors, recent evidence shows that many of the traditional risk factors are stronger predictors of ischemic heart disease in women than men. These include diabetes, smoking, obesity, and hypertension, Dr. Mieres observed.
For example, a recent meta-analysis of 26 studies including more than 214,000 subjects concluded that women with type 1 diabetes had a 2.5-fold greater risk of incident coronary heart disease than did men with type 1 diabetes. The women with type 1 diabetes also had an 86% greater risk of fatal cardiovascular diseases, a 44% increase in the risk of fatal kidney disease, a 37% greater risk of stroke, and a 37% increase in all-cause mortality relative to type 1 diabetic men (Lancet Diabetes Endocrinol. 2015 Mar;3[3]:198-206).
A wealth of accumulating data indicates that type 2 diabetes, too, is a much stronger risk factor for cardiovascular diseases in women than in men. The evidence prompted a recent formal scientific statement to that effect by the American Heart Association (Circulation. 2015 Dec 22;132[25]:2424-47).
Dr. Mieres reported having no financial conflicts of interest regarding her presentation.
SNOWMASS, COLO. – Achieving continued reductions in cardiovascular deaths in U.S. women will require that physicians make greater use of sex-specific risk factors that aren’t incorporated in the ACC/AHA atherosclerotic cardiovascular disease risk score, Dr. Jennifer H. Mieres asserted at the Annual Cardiovascular Conference at Snowmass.
In the 13-year period beginning in 2000, with the launch of a national initiative to boost the research focus on cardiovascular disease in women, the annual number of women dying from cardiovascular disease has dropped by roughly 30%. That’s a steeper decline than in men. One of the keys to further reductions in women is more widespread physician evaluation of sex-specific risk factors – such as a history of elevated blood pressure in pregnancy, polycystic ovarian syndrome, or radiation therapy for breast cancer – as part of routine cardiovascular risk assessment in women, said Dr. Mieres, senior vice president office of community and public health at Hofstra Northwell in Hempstead, N.Y.
Hypertension in pregnancy as a harbinger of premature cardiovascular disease and other chronic diseases has been a topic of particularly fruitful research in the past few years.
“The ongoing hypothesis is that pregnancy is a sort of stress test. Pregnancy-related complications indicate an inability to adequately adapt to the physiologic stress of pregnancy and thus reveal the presence of underlying susceptibility to ischemic heart disease,” according to the cardiologist.
She cited a landmark prospective study of 10,314 women born in Northern Finland in 1966 and followed for an average of more than 39 years after a singleton pregnancy. The investigators showed that any elevation in blood pressure during pregnancy, including isolated systolic or diastolic hypertension that resolved during or shortly after pregnancy, was associated with increased future risks of various forms of cardiovascular disease.
For example, de novo gestational hypertension without proteinuria was associated with significantly increased risks of subsequent ischemic cerebrovascular disease, chronic kidney disease, diabetes, ischemic heart disease, acute MI, chronic hypertension, and heart failure. The MIs that occurred in Finns with a history of gestational hypertension were more serious, too, with an associated threefold greater risk of being fatal than MIs in women who had been normotensive in pregnancy (Circulation. 2013 Feb 12;127[6]:681-90).

New-onset isolated systolic or diastolic hypertension emerged during pregnancy in about 17% of the Finnish women. Roughly 30% of them had a cardiovascular event before their late 60s. This translated to a 14%-18% greater risk than in women who remained normotensive in pregnancy.
The highest risk of all in the Finnish study was seen in women with preeclampsia/eclampsia superimposed on a background of chronic hypertension. They had a 3.18-fold greater risk of subsequent MI than did women who were normotensive in pregnancy, a 3.32-fold increased risk of heart failure, and a 2.22-fold greater risk of developing diabetes.
In addition to the growing appreciation that it’s important to consider sex-specific cardiovascular risk factors, recent evidence shows that many of the traditional risk factors are stronger predictors of ischemic heart disease in women than men. These include diabetes, smoking, obesity, and hypertension, Dr. Mieres observed.
For example, a recent meta-analysis of 26 studies including more than 214,000 subjects concluded that women with type 1 diabetes had a 2.5-fold greater risk of incident coronary heart disease than did men with type 1 diabetes. The women with type 1 diabetes also had an 86% greater risk of fatal cardiovascular diseases, a 44% increase in the risk of fatal kidney disease, a 37% greater risk of stroke, and a 37% increase in all-cause mortality relative to type 1 diabetic men (Lancet Diabetes Endocrinol. 2015 Mar;3[3]:198-206).
A wealth of accumulating data indicates that type 2 diabetes, too, is a much stronger risk factor for cardiovascular diseases in women than in men. The evidence prompted a recent formal scientific statement to that effect by the American Heart Association (Circulation. 2015 Dec 22;132[25]:2424-47).
Dr. Mieres reported having no financial conflicts of interest regarding her presentation.
SNOWMASS, COLO. – Achieving continued reductions in cardiovascular deaths in U.S. women will require that physicians make greater use of sex-specific risk factors that aren’t incorporated in the ACC/AHA atherosclerotic cardiovascular disease risk score, Dr. Jennifer H. Mieres asserted at the Annual Cardiovascular Conference at Snowmass.
In the 13-year period beginning in 2000, with the launch of a national initiative to boost the research focus on cardiovascular disease in women, the annual number of women dying from cardiovascular disease has dropped by roughly 30%. That’s a steeper decline than in men. One of the keys to further reductions in women is more widespread physician evaluation of sex-specific risk factors – such as a history of elevated blood pressure in pregnancy, polycystic ovarian syndrome, or radiation therapy for breast cancer – as part of routine cardiovascular risk assessment in women, said Dr. Mieres, senior vice president office of community and public health at Hofstra Northwell in Hempstead, N.Y.
Hypertension in pregnancy as a harbinger of premature cardiovascular disease and other chronic diseases has been a topic of particularly fruitful research in the past few years.
“The ongoing hypothesis is that pregnancy is a sort of stress test. Pregnancy-related complications indicate an inability to adequately adapt to the physiologic stress of pregnancy and thus reveal the presence of underlying susceptibility to ischemic heart disease,” according to the cardiologist.
She cited a landmark prospective study of 10,314 women born in Northern Finland in 1966 and followed for an average of more than 39 years after a singleton pregnancy. The investigators showed that any elevation in blood pressure during pregnancy, including isolated systolic or diastolic hypertension that resolved during or shortly after pregnancy, was associated with increased future risks of various forms of cardiovascular disease.
For example, de novo gestational hypertension without proteinuria was associated with significantly increased risks of subsequent ischemic cerebrovascular disease, chronic kidney disease, diabetes, ischemic heart disease, acute MI, chronic hypertension, and heart failure. The MIs that occurred in Finns with a history of gestational hypertension were more serious, too, with an associated threefold greater risk of being fatal than MIs in women who had been normotensive in pregnancy (Circulation. 2013 Feb 12;127[6]:681-90).

New-onset isolated systolic or diastolic hypertension emerged during pregnancy in about 17% of the Finnish women. Roughly 30% of them had a cardiovascular event before their late 60s. This translated to a 14%-18% greater risk than in women who remained normotensive in pregnancy.
The highest risk of all in the Finnish study was seen in women with preeclampsia/eclampsia superimposed on a background of chronic hypertension. They had a 3.18-fold greater risk of subsequent MI than did women who were normotensive in pregnancy, a 3.32-fold increased risk of heart failure, and a 2.22-fold greater risk of developing diabetes.
In addition to the growing appreciation that it’s important to consider sex-specific cardiovascular risk factors, recent evidence shows that many of the traditional risk factors are stronger predictors of ischemic heart disease in women than men. These include diabetes, smoking, obesity, and hypertension, Dr. Mieres observed.
For example, a recent meta-analysis of 26 studies including more than 214,000 subjects concluded that women with type 1 diabetes had a 2.5-fold greater risk of incident coronary heart disease than did men with type 1 diabetes. The women with type 1 diabetes also had an 86% greater risk of fatal cardiovascular diseases, a 44% increase in the risk of fatal kidney disease, a 37% greater risk of stroke, and a 37% increase in all-cause mortality relative to type 1 diabetic men (Lancet Diabetes Endocrinol. 2015 Mar;3[3]:198-206).
A wealth of accumulating data indicates that type 2 diabetes, too, is a much stronger risk factor for cardiovascular diseases in women than in men. The evidence prompted a recent formal scientific statement to that effect by the American Heart Association (Circulation. 2015 Dec 22;132[25]:2424-47).
Dr. Mieres reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Ischemic mitral regurgitation: valve repair vs. replacement
SNOWMASS, COLO. – A clear message from the first-ever randomized trial of surgical mitral valve repair versus replacement for patients with severe ischemic mitral regurgitation is that replacement should be utilized more liberally, Dr. Michael J. Mack said at the Annual Cardiovascular Conference at Snowmass.
The results of prosthetic valve implantation proved far more durable than repair. At 2 years of follow-up in this 251-patient multicenter trial conducted by the Cardiothoracic Surgical Trials Network (CSTN), the incidence of recurrent moderate or severe mitral regurgitation was just 3.8% in the valve replacement group, compared with 58.8% with repair via restrictive annuloplasty. As a result, the repair group had significantly more heart failure–related adverse events and cardiovascular hospitalizations and a lower rate of clinically meaningful improvement in quality of life scores, noted Dr. Mack, an investigator in the trial and medical director of the Baylor Health Care System in Plano, Tex.
“I think surgical mitral valve replacement has had a bad name over the years, and one of the reasons is because of the worse left ventricular function afterwards. However, that was a casualty of excising the mitral valve and the subvalvular apparatus, causing atrial-ventricular disconnection. We’ve gotten smarter about this. The techniques we now use are valve sparing,” the cardiothoracic surgeon said.
He was quick to add, however, that the CSTN study results are by no means the death knell for restrictive mitral annuloplasty. Indeed, participants in the mitral valve repair group who didn’t develop recurrent regurgitation actually experienced significant positive reverse remodeling as reflected by improvement in their left ventricular end-systolic volume index, the primary endpoint of the study (N Engl J Med. 2016;374:344-35).
The key to successful outcomes in mitral valve repair is to save the procedure for patients who are unlikely to develop recurrent regurgitation. And a substudy of the CTSN trial led by Dr. Irving L. Kron, professor of surgery at the University of Virginia, Charlottesville, provides practical guidance on that score. The investigators conducted a logistic regression analysis of the mitral valve repair group’s baseline echocardiographic and clinical characteristics and identified a collection of strong predictors of recurrent regurgitation within 2 years (J Thorac Cardiovasc Surg. 2015 Mar;149[3]:752-61).
“The bottom line is, the more tethering you have of the mitral valve leaflets, the more likely you are to have recurrent mitral regurgitation after mitral valve annuloplasty,” Dr. Mack said.
The predictors of recurrent regurgitation included a coaptation depth greater than 10 mm, a posterior leaflet angle in excess of 45 degrees, a distal anterior leaflet angle greater than 25 degrees, inferior basal aneurysm, mitral annular calcification, and a left ventricular end diastolic diameter greater than 65 mm, as well as other indices of advanced left ventricular remodeling.
No or only mild annular dilation, as occurs, for example, in patients whose mitral regurgitation is caused by atrial fibrillation, is another independent predictor of recurrent regurgitation post repair.
“Shrinking the annulus isn’t going to make a difference if the annulus wasn’t dilated to begin with,” the surgeon observed. “If surgery is performed, we now know those patients who are most likely to recur – and they should have mitral valve replacement. If those factors are not present, then repair is still a viable option,” according to Dr. Mack.
That being said, it’s still not known whether correcting severe ischemic mitral regurgitation prolongs life or improves quality of life long term, compared with guideline-directed medical therapy, he stressed.
“Secondary mitral regurgitation is a disease of the left ventricle, not the mitral valve. So it’s possible that mitral regurgitation reduction has no benefit because the regurgitation is a surrogate marker not causally related to outcome. I don’t think so, but it is a possibility,” Dr. Mack conceded.
This is a clinically important unresolved question because secondary mitral regurgitation is extremely common. In a retrospective echocardiographic study of 558 heart failure patients with a left ventricular ejection fraction of 35% or less and class III-IV symptoms, 90% of them had some degree of mitral regurgitation (J Card Fail. 2004 Aug;10[4]:285-91).
Together with Columbia University cardiologist Dr. Gregg W. Stone, Dr. Mack is coprincipal investigator of the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) trial, which is expected to provide an answer to this key question. The multicenter U.S. study involves a planned 420 patients with severely symptomatic secondary mitral regurgitation who are deemed at prohibitive risk for surgery. They are to be randomized to guideline-directed medical therapy with or without transcatheter mitral valve repair using the MitraClip device. Enrollment should be completed by May, with initial results available in late 2017.
Dr. Mack reported receiving research grants from Abbott Vascular, which is sponsoring the COAPT trial, as well as from Edwards Lifesciences.
SNOWMASS, COLO. – A clear message from the first-ever randomized trial of surgical mitral valve repair versus replacement for patients with severe ischemic mitral regurgitation is that replacement should be utilized more liberally, Dr. Michael J. Mack said at the Annual Cardiovascular Conference at Snowmass.
The results of prosthetic valve implantation proved far more durable than repair. At 2 years of follow-up in this 251-patient multicenter trial conducted by the Cardiothoracic Surgical Trials Network (CSTN), the incidence of recurrent moderate or severe mitral regurgitation was just 3.8% in the valve replacement group, compared with 58.8% with repair via restrictive annuloplasty. As a result, the repair group had significantly more heart failure–related adverse events and cardiovascular hospitalizations and a lower rate of clinically meaningful improvement in quality of life scores, noted Dr. Mack, an investigator in the trial and medical director of the Baylor Health Care System in Plano, Tex.
“I think surgical mitral valve replacement has had a bad name over the years, and one of the reasons is because of the worse left ventricular function afterwards. However, that was a casualty of excising the mitral valve and the subvalvular apparatus, causing atrial-ventricular disconnection. We’ve gotten smarter about this. The techniques we now use are valve sparing,” the cardiothoracic surgeon said.
He was quick to add, however, that the CSTN study results are by no means the death knell for restrictive mitral annuloplasty. Indeed, participants in the mitral valve repair group who didn’t develop recurrent regurgitation actually experienced significant positive reverse remodeling as reflected by improvement in their left ventricular end-systolic volume index, the primary endpoint of the study (N Engl J Med. 2016;374:344-35).
The key to successful outcomes in mitral valve repair is to save the procedure for patients who are unlikely to develop recurrent regurgitation. And a substudy of the CTSN trial led by Dr. Irving L. Kron, professor of surgery at the University of Virginia, Charlottesville, provides practical guidance on that score. The investigators conducted a logistic regression analysis of the mitral valve repair group’s baseline echocardiographic and clinical characteristics and identified a collection of strong predictors of recurrent regurgitation within 2 years (J Thorac Cardiovasc Surg. 2015 Mar;149[3]:752-61).
“The bottom line is, the more tethering you have of the mitral valve leaflets, the more likely you are to have recurrent mitral regurgitation after mitral valve annuloplasty,” Dr. Mack said.
The predictors of recurrent regurgitation included a coaptation depth greater than 10 mm, a posterior leaflet angle in excess of 45 degrees, a distal anterior leaflet angle greater than 25 degrees, inferior basal aneurysm, mitral annular calcification, and a left ventricular end diastolic diameter greater than 65 mm, as well as other indices of advanced left ventricular remodeling.
No or only mild annular dilation, as occurs, for example, in patients whose mitral regurgitation is caused by atrial fibrillation, is another independent predictor of recurrent regurgitation post repair.
“Shrinking the annulus isn’t going to make a difference if the annulus wasn’t dilated to begin with,” the surgeon observed. “If surgery is performed, we now know those patients who are most likely to recur – and they should have mitral valve replacement. If those factors are not present, then repair is still a viable option,” according to Dr. Mack.
That being said, it’s still not known whether correcting severe ischemic mitral regurgitation prolongs life or improves quality of life long term, compared with guideline-directed medical therapy, he stressed.
“Secondary mitral regurgitation is a disease of the left ventricle, not the mitral valve. So it’s possible that mitral regurgitation reduction has no benefit because the regurgitation is a surrogate marker not causally related to outcome. I don’t think so, but it is a possibility,” Dr. Mack conceded.
This is a clinically important unresolved question because secondary mitral regurgitation is extremely common. In a retrospective echocardiographic study of 558 heart failure patients with a left ventricular ejection fraction of 35% or less and class III-IV symptoms, 90% of them had some degree of mitral regurgitation (J Card Fail. 2004 Aug;10[4]:285-91).
Together with Columbia University cardiologist Dr. Gregg W. Stone, Dr. Mack is coprincipal investigator of the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) trial, which is expected to provide an answer to this key question. The multicenter U.S. study involves a planned 420 patients with severely symptomatic secondary mitral regurgitation who are deemed at prohibitive risk for surgery. They are to be randomized to guideline-directed medical therapy with or without transcatheter mitral valve repair using the MitraClip device. Enrollment should be completed by May, with initial results available in late 2017.
Dr. Mack reported receiving research grants from Abbott Vascular, which is sponsoring the COAPT trial, as well as from Edwards Lifesciences.
SNOWMASS, COLO. – A clear message from the first-ever randomized trial of surgical mitral valve repair versus replacement for patients with severe ischemic mitral regurgitation is that replacement should be utilized more liberally, Dr. Michael J. Mack said at the Annual Cardiovascular Conference at Snowmass.
The results of prosthetic valve implantation proved far more durable than repair. At 2 years of follow-up in this 251-patient multicenter trial conducted by the Cardiothoracic Surgical Trials Network (CSTN), the incidence of recurrent moderate or severe mitral regurgitation was just 3.8% in the valve replacement group, compared with 58.8% with repair via restrictive annuloplasty. As a result, the repair group had significantly more heart failure–related adverse events and cardiovascular hospitalizations and a lower rate of clinically meaningful improvement in quality of life scores, noted Dr. Mack, an investigator in the trial and medical director of the Baylor Health Care System in Plano, Tex.
“I think surgical mitral valve replacement has had a bad name over the years, and one of the reasons is because of the worse left ventricular function afterwards. However, that was a casualty of excising the mitral valve and the subvalvular apparatus, causing atrial-ventricular disconnection. We’ve gotten smarter about this. The techniques we now use are valve sparing,” the cardiothoracic surgeon said.
He was quick to add, however, that the CSTN study results are by no means the death knell for restrictive mitral annuloplasty. Indeed, participants in the mitral valve repair group who didn’t develop recurrent regurgitation actually experienced significant positive reverse remodeling as reflected by improvement in their left ventricular end-systolic volume index, the primary endpoint of the study (N Engl J Med. 2016;374:344-35).
The key to successful outcomes in mitral valve repair is to save the procedure for patients who are unlikely to develop recurrent regurgitation. And a substudy of the CTSN trial led by Dr. Irving L. Kron, professor of surgery at the University of Virginia, Charlottesville, provides practical guidance on that score. The investigators conducted a logistic regression analysis of the mitral valve repair group’s baseline echocardiographic and clinical characteristics and identified a collection of strong predictors of recurrent regurgitation within 2 years (J Thorac Cardiovasc Surg. 2015 Mar;149[3]:752-61).
“The bottom line is, the more tethering you have of the mitral valve leaflets, the more likely you are to have recurrent mitral regurgitation after mitral valve annuloplasty,” Dr. Mack said.
The predictors of recurrent regurgitation included a coaptation depth greater than 10 mm, a posterior leaflet angle in excess of 45 degrees, a distal anterior leaflet angle greater than 25 degrees, inferior basal aneurysm, mitral annular calcification, and a left ventricular end diastolic diameter greater than 65 mm, as well as other indices of advanced left ventricular remodeling.
No or only mild annular dilation, as occurs, for example, in patients whose mitral regurgitation is caused by atrial fibrillation, is another independent predictor of recurrent regurgitation post repair.
“Shrinking the annulus isn’t going to make a difference if the annulus wasn’t dilated to begin with,” the surgeon observed. “If surgery is performed, we now know those patients who are most likely to recur – and they should have mitral valve replacement. If those factors are not present, then repair is still a viable option,” according to Dr. Mack.
That being said, it’s still not known whether correcting severe ischemic mitral regurgitation prolongs life or improves quality of life long term, compared with guideline-directed medical therapy, he stressed.
“Secondary mitral regurgitation is a disease of the left ventricle, not the mitral valve. So it’s possible that mitral regurgitation reduction has no benefit because the regurgitation is a surrogate marker not causally related to outcome. I don’t think so, but it is a possibility,” Dr. Mack conceded.
This is a clinically important unresolved question because secondary mitral regurgitation is extremely common. In a retrospective echocardiographic study of 558 heart failure patients with a left ventricular ejection fraction of 35% or less and class III-IV symptoms, 90% of them had some degree of mitral regurgitation (J Card Fail. 2004 Aug;10[4]:285-91).
Together with Columbia University cardiologist Dr. Gregg W. Stone, Dr. Mack is coprincipal investigator of the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) trial, which is expected to provide an answer to this key question. The multicenter U.S. study involves a planned 420 patients with severely symptomatic secondary mitral regurgitation who are deemed at prohibitive risk for surgery. They are to be randomized to guideline-directed medical therapy with or without transcatheter mitral valve repair using the MitraClip device. Enrollment should be completed by May, with initial results available in late 2017.
Dr. Mack reported receiving research grants from Abbott Vascular, which is sponsoring the COAPT trial, as well as from Edwards Lifesciences.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
How to use two new game-changing heart failure drugs
SNOWMASS, COLO. – Ivabradine and sacubitril/valsartan are paradigm-changing drugs approved last year for the treatment of heart failure with reduced ejection fraction – and it’s entirely reasonable to begin using them now in the appropriate patients, Dr. Akshay S. Desai said at the Annual Cardiovascular Conference at Snowmass.
The impressive positive results seen in the pivotal trials for these novel agents – the SHIFT trial for ivabradine (Corlanor) and PARADIGM-HF for sacubitril/valsartan (Entresto) – have rocked the heart failure world.
The studies showed that, in the right patients, these two medications improve heart failure morbidity and mortality significantly beyond what’s achievable with the current gold standard, guideline-directed medical therapy. That’s exciting because even though great therapeutic strides have been made during the past 15 years, symptomatic patients with heart failure with reduced ejection fraction (HFrEF) treated with optimal guideline-directed pharmacotherapy still have substantial residual risk for heart failure hospitalization and death, noted Dr. Desai, director of heart failure disease management at Brigham and Women’s Hospital in Boston.
The U.S. heart failure guidelines panel hasn’t yet addressed the use of either of these recently approved drugs, but Dr. Desai provided his best sense of the data and how he thinks physicians might start using them now.
Ivabradine and sacubitril/valsartan are first-in-class agents with novel mechanisms of action. Ivabradine’s demonstrated safety and efficacy in the SHIFT trial confirmed the hypothesis that elevated heart rate is a legitimate therapeutic target in HFrEF.
Sacubitril/valsartan, an angiotensin II receptor/neprilysin inhibitor formerly known as LCZ696, provides what is to date a unique ability to enhance the activity of endogenous vasoactive peptides, including natriuretic peptides, bradykinin, substance P, adrenomedullin, and calcitonin gene–related peptide. These peptides are antifibrotic, antihypertrophic, and they promote vasodilation and diuresis, thus counteracting the adverse effects of neurohormonal activation. But in HFrEF, these vasoactive peptides are less active and patients are less sensitive to them.
Ivabradine
This selective sinus node inhibitor decreases heart rate and has essentially no other effects. The drug has been available for years in Europe, and the European Society of Cardiology (ESC) has had sufficient time to integrate ivabradine into its guidelines for pharmacotherapy in HFrEF.
The ESC treatment algorithm for HFrEF (Eur Heart J. 2012 Jul;33[14]:1787-847) is built upon a foundation of thiazide diuretics to relieve signs and symptoms of congestion along with a beta-blocker and an ACE inhibitor or angiotensin receptor blocker (ARB). In a patient who still has New York Heart Association class II-IV symptoms after those drugs are titrated to guideline-recommended target levels or maximally tolerated doses, a mineralocorticoid receptor antagonist – either spironolactone or eplerenone – is added. And, in a patient who still remains symptomatic, has a left ventricular ejection fraction of 35% or less, is in sinus rhythm, and has a heart rate of 70 beats per minute or more, it’s time to consider adding ivabradine.
“This is how our own guidelines may elect to incorporate ivabradine, but of course, we don’t know yet,” Dr. Desai observed.
In the randomized, double-blind SHIFT trial involving 6,558 HFrEF patients who fit the description of ivabradine candidates described in the ESC guidelines, those who received ivabradine titrated to a maximum of 7.5 mg twice daily experienced a 26% reduction in hospital admissions for worsening heart failure, compared with placebo, a 26% reduction in deaths from heart failure, and fewer adverse events than the control group (Lancet. 2010 Sep 11;376[9744]:875-85).
The important question is who should get ivabradine and who should just get a little more beta-blocker in order to slow the heart rate. The fact is, many heart failure patients simply can’t tolerate the guideline-recommended target dose of beta-blocker therapy, which is 12.5 mg twice daily of carvedilol or its equivalent. Indeed, only 26% of SHIFT participants were able to do so.
“My interpretation of the SHIFT trial is that the goal is to reduce heart rate by any means necessary; preferentially, with a beta-blocker, and with ivabradine as an adjunct in patients who can’t get to target doses,” the cardiologist said.
Sacubitril/valsartan
In the landmark double-blind, 8,442-patient PARADIGM-HF trial, the group randomized to sacubitril/valsartan had a 20% reduction in the primary endpoint of cardiovascular death or heart failure hospitalization over 27 months of follow-up, compared with controls on enalapril at the guideline-recommended dose of 10 mg twice a day. The number needed to treat (NNT) was 21. Moreover, all-cause mortality was reduced by 16% (N Engl J Med. 2014 Sep 11;37[11]:993-1004).
In a recent follow-up cause of death analysis, Dr. Desai and his coinvestigators reported that 81% of all deaths in PARADIGM-HF were cardiovascular in nature. The NNT for sacubitril/valsartan in order to prevent one cardiovascular death was 32. The risk of sudden cardiac death was reduced by 80%, while the risk of death due to worsening heart failure was decreased by 21% (Eur Heart J 2015 Aug 7;36[30]:1990-7).
In another secondary analysis from the PARADIGM-HF investigators, the use of the angiotensin receptor/neprilysin inhibitor was shown to prevent clinical progression of surviving patients with heart failure much more effectively than enalapril. The sacubitril/valsartan group was 34% less likely to have an emergency department visit for worsening heart failure, 18% less likely to require intensive care, and 22% less likely to receive an implantable heart failure device or undergo cardiac transplantation. The reduction in the rate of heart failure hospitalization became significant within the first 30 days (Circulation. 2015 Jan 6;131[1]:54-61).
Moreover, the absolute benefit of sacubitril/valsartan in PARADIGM-HF was consistent across the full spectrum of patient risk (J Am Coll Cardiol. 2015 Nov 10;66[19]:2059-71).
To put this into perspective, Dr. Desai continued, for every 1,000 HFrEF patients switched from an ACE inhibitor or ARB to sacubitril/valsartan, the absolute benefit over the course of 27 months includes 31 fewer cardiovascular deaths, 28 fewer hospitalizations for heart failure, and 37 fewer hospitalizations for any reason.
“This is potent therapy for patients with HFrEF who have the right phenotype,” he observed.
While substitution of sacubitril/valsartan for an ACE inhibitor or ARB may be appropriate in many patients with chronic HFrEF who continue to have NYHA Class II-IV symptoms on guideline-directed medical therapy, several caveats apply, according to Dr. Desai.
It’s important to be aware of the PARADIGM-HF eligibility criteria, because it’s only in patients who fit that profile that sacubitril/valsartan provides evidence-based therapy. There are as yet no data to support the drug’s use in patients with new-onset HFrEF, acute decompensated HFrEF, in patients who are immediately post-MI, or in those with advanced chronic kidney disease, he emphasized.
“I think you have to be mindful of eligibility because the label that’s applied to this drug is basically ‘patients with HFrEF who are treated with guideline-directed medical therapy.’ There’s no specific requirement that you follow the detailed eligibility criteria of the PARADIGM-HF trial, but you should realize that the drug is known to be effective only in patients who fit the PARADIGM-HF eligibility profile,” he said.
Dr. Desai gave a few clinical pearls for prescribing sacubitril/valsartan. For most patients, the initial recommended dose is 49/51 mg twice daily. In those with low baseline blood pressure and tenuous hemodynamics, it’s appropriate to initiate therapy at 24/26 mg BID. It’s important to halt ACE inhibitor therapy 36 hours prior to starting sacubitril/valsartan so as to avoid overlap and consequent increased risk of angioedema. And while serum n-terminal prohormone brain natriuretic peptide (NT-proBNP) remains a useful biomarker to monitor heart rate severity and response to treatment while a patient is on sacubitril/valsartan, BNP is not because serum levels of that biomarker rise with neprilysin inhibition.
Dr. Desai reported receiving research support from Novartis and St. Jude Medical and serving as a consultant to those companies as well as Merck and Relypsa.
SNOWMASS, COLO. – Ivabradine and sacubitril/valsartan are paradigm-changing drugs approved last year for the treatment of heart failure with reduced ejection fraction – and it’s entirely reasonable to begin using them now in the appropriate patients, Dr. Akshay S. Desai said at the Annual Cardiovascular Conference at Snowmass.
The impressive positive results seen in the pivotal trials for these novel agents – the SHIFT trial for ivabradine (Corlanor) and PARADIGM-HF for sacubitril/valsartan (Entresto) – have rocked the heart failure world.
The studies showed that, in the right patients, these two medications improve heart failure morbidity and mortality significantly beyond what’s achievable with the current gold standard, guideline-directed medical therapy. That’s exciting because even though great therapeutic strides have been made during the past 15 years, symptomatic patients with heart failure with reduced ejection fraction (HFrEF) treated with optimal guideline-directed pharmacotherapy still have substantial residual risk for heart failure hospitalization and death, noted Dr. Desai, director of heart failure disease management at Brigham and Women’s Hospital in Boston.
The U.S. heart failure guidelines panel hasn’t yet addressed the use of either of these recently approved drugs, but Dr. Desai provided his best sense of the data and how he thinks physicians might start using them now.
Ivabradine and sacubitril/valsartan are first-in-class agents with novel mechanisms of action. Ivabradine’s demonstrated safety and efficacy in the SHIFT trial confirmed the hypothesis that elevated heart rate is a legitimate therapeutic target in HFrEF.
Sacubitril/valsartan, an angiotensin II receptor/neprilysin inhibitor formerly known as LCZ696, provides what is to date a unique ability to enhance the activity of endogenous vasoactive peptides, including natriuretic peptides, bradykinin, substance P, adrenomedullin, and calcitonin gene–related peptide. These peptides are antifibrotic, antihypertrophic, and they promote vasodilation and diuresis, thus counteracting the adverse effects of neurohormonal activation. But in HFrEF, these vasoactive peptides are less active and patients are less sensitive to them.
Ivabradine
This selective sinus node inhibitor decreases heart rate and has essentially no other effects. The drug has been available for years in Europe, and the European Society of Cardiology (ESC) has had sufficient time to integrate ivabradine into its guidelines for pharmacotherapy in HFrEF.
The ESC treatment algorithm for HFrEF (Eur Heart J. 2012 Jul;33[14]:1787-847) is built upon a foundation of thiazide diuretics to relieve signs and symptoms of congestion along with a beta-blocker and an ACE inhibitor or angiotensin receptor blocker (ARB). In a patient who still has New York Heart Association class II-IV symptoms after those drugs are titrated to guideline-recommended target levels or maximally tolerated doses, a mineralocorticoid receptor antagonist – either spironolactone or eplerenone – is added. And, in a patient who still remains symptomatic, has a left ventricular ejection fraction of 35% or less, is in sinus rhythm, and has a heart rate of 70 beats per minute or more, it’s time to consider adding ivabradine.
“This is how our own guidelines may elect to incorporate ivabradine, but of course, we don’t know yet,” Dr. Desai observed.
In the randomized, double-blind SHIFT trial involving 6,558 HFrEF patients who fit the description of ivabradine candidates described in the ESC guidelines, those who received ivabradine titrated to a maximum of 7.5 mg twice daily experienced a 26% reduction in hospital admissions for worsening heart failure, compared with placebo, a 26% reduction in deaths from heart failure, and fewer adverse events than the control group (Lancet. 2010 Sep 11;376[9744]:875-85).
The important question is who should get ivabradine and who should just get a little more beta-blocker in order to slow the heart rate. The fact is, many heart failure patients simply can’t tolerate the guideline-recommended target dose of beta-blocker therapy, which is 12.5 mg twice daily of carvedilol or its equivalent. Indeed, only 26% of SHIFT participants were able to do so.
“My interpretation of the SHIFT trial is that the goal is to reduce heart rate by any means necessary; preferentially, with a beta-blocker, and with ivabradine as an adjunct in patients who can’t get to target doses,” the cardiologist said.
Sacubitril/valsartan
In the landmark double-blind, 8,442-patient PARADIGM-HF trial, the group randomized to sacubitril/valsartan had a 20% reduction in the primary endpoint of cardiovascular death or heart failure hospitalization over 27 months of follow-up, compared with controls on enalapril at the guideline-recommended dose of 10 mg twice a day. The number needed to treat (NNT) was 21. Moreover, all-cause mortality was reduced by 16% (N Engl J Med. 2014 Sep 11;37[11]:993-1004).
In a recent follow-up cause of death analysis, Dr. Desai and his coinvestigators reported that 81% of all deaths in PARADIGM-HF were cardiovascular in nature. The NNT for sacubitril/valsartan in order to prevent one cardiovascular death was 32. The risk of sudden cardiac death was reduced by 80%, while the risk of death due to worsening heart failure was decreased by 21% (Eur Heart J 2015 Aug 7;36[30]:1990-7).
In another secondary analysis from the PARADIGM-HF investigators, the use of the angiotensin receptor/neprilysin inhibitor was shown to prevent clinical progression of surviving patients with heart failure much more effectively than enalapril. The sacubitril/valsartan group was 34% less likely to have an emergency department visit for worsening heart failure, 18% less likely to require intensive care, and 22% less likely to receive an implantable heart failure device or undergo cardiac transplantation. The reduction in the rate of heart failure hospitalization became significant within the first 30 days (Circulation. 2015 Jan 6;131[1]:54-61).
Moreover, the absolute benefit of sacubitril/valsartan in PARADIGM-HF was consistent across the full spectrum of patient risk (J Am Coll Cardiol. 2015 Nov 10;66[19]:2059-71).
To put this into perspective, Dr. Desai continued, for every 1,000 HFrEF patients switched from an ACE inhibitor or ARB to sacubitril/valsartan, the absolute benefit over the course of 27 months includes 31 fewer cardiovascular deaths, 28 fewer hospitalizations for heart failure, and 37 fewer hospitalizations for any reason.
“This is potent therapy for patients with HFrEF who have the right phenotype,” he observed.
While substitution of sacubitril/valsartan for an ACE inhibitor or ARB may be appropriate in many patients with chronic HFrEF who continue to have NYHA Class II-IV symptoms on guideline-directed medical therapy, several caveats apply, according to Dr. Desai.
It’s important to be aware of the PARADIGM-HF eligibility criteria, because it’s only in patients who fit that profile that sacubitril/valsartan provides evidence-based therapy. There are as yet no data to support the drug’s use in patients with new-onset HFrEF, acute decompensated HFrEF, in patients who are immediately post-MI, or in those with advanced chronic kidney disease, he emphasized.
“I think you have to be mindful of eligibility because the label that’s applied to this drug is basically ‘patients with HFrEF who are treated with guideline-directed medical therapy.’ There’s no specific requirement that you follow the detailed eligibility criteria of the PARADIGM-HF trial, but you should realize that the drug is known to be effective only in patients who fit the PARADIGM-HF eligibility profile,” he said.
Dr. Desai gave a few clinical pearls for prescribing sacubitril/valsartan. For most patients, the initial recommended dose is 49/51 mg twice daily. In those with low baseline blood pressure and tenuous hemodynamics, it’s appropriate to initiate therapy at 24/26 mg BID. It’s important to halt ACE inhibitor therapy 36 hours prior to starting sacubitril/valsartan so as to avoid overlap and consequent increased risk of angioedema. And while serum n-terminal prohormone brain natriuretic peptide (NT-proBNP) remains a useful biomarker to monitor heart rate severity and response to treatment while a patient is on sacubitril/valsartan, BNP is not because serum levels of that biomarker rise with neprilysin inhibition.
Dr. Desai reported receiving research support from Novartis and St. Jude Medical and serving as a consultant to those companies as well as Merck and Relypsa.
SNOWMASS, COLO. – Ivabradine and sacubitril/valsartan are paradigm-changing drugs approved last year for the treatment of heart failure with reduced ejection fraction – and it’s entirely reasonable to begin using them now in the appropriate patients, Dr. Akshay S. Desai said at the Annual Cardiovascular Conference at Snowmass.
The impressive positive results seen in the pivotal trials for these novel agents – the SHIFT trial for ivabradine (Corlanor) and PARADIGM-HF for sacubitril/valsartan (Entresto) – have rocked the heart failure world.
The studies showed that, in the right patients, these two medications improve heart failure morbidity and mortality significantly beyond what’s achievable with the current gold standard, guideline-directed medical therapy. That’s exciting because even though great therapeutic strides have been made during the past 15 years, symptomatic patients with heart failure with reduced ejection fraction (HFrEF) treated with optimal guideline-directed pharmacotherapy still have substantial residual risk for heart failure hospitalization and death, noted Dr. Desai, director of heart failure disease management at Brigham and Women’s Hospital in Boston.
The U.S. heart failure guidelines panel hasn’t yet addressed the use of either of these recently approved drugs, but Dr. Desai provided his best sense of the data and how he thinks physicians might start using them now.
Ivabradine and sacubitril/valsartan are first-in-class agents with novel mechanisms of action. Ivabradine’s demonstrated safety and efficacy in the SHIFT trial confirmed the hypothesis that elevated heart rate is a legitimate therapeutic target in HFrEF.
Sacubitril/valsartan, an angiotensin II receptor/neprilysin inhibitor formerly known as LCZ696, provides what is to date a unique ability to enhance the activity of endogenous vasoactive peptides, including natriuretic peptides, bradykinin, substance P, adrenomedullin, and calcitonin gene–related peptide. These peptides are antifibrotic, antihypertrophic, and they promote vasodilation and diuresis, thus counteracting the adverse effects of neurohormonal activation. But in HFrEF, these vasoactive peptides are less active and patients are less sensitive to them.
Ivabradine
This selective sinus node inhibitor decreases heart rate and has essentially no other effects. The drug has been available for years in Europe, and the European Society of Cardiology (ESC) has had sufficient time to integrate ivabradine into its guidelines for pharmacotherapy in HFrEF.
The ESC treatment algorithm for HFrEF (Eur Heart J. 2012 Jul;33[14]:1787-847) is built upon a foundation of thiazide diuretics to relieve signs and symptoms of congestion along with a beta-blocker and an ACE inhibitor or angiotensin receptor blocker (ARB). In a patient who still has New York Heart Association class II-IV symptoms after those drugs are titrated to guideline-recommended target levels or maximally tolerated doses, a mineralocorticoid receptor antagonist – either spironolactone or eplerenone – is added. And, in a patient who still remains symptomatic, has a left ventricular ejection fraction of 35% or less, is in sinus rhythm, and has a heart rate of 70 beats per minute or more, it’s time to consider adding ivabradine.
“This is how our own guidelines may elect to incorporate ivabradine, but of course, we don’t know yet,” Dr. Desai observed.
In the randomized, double-blind SHIFT trial involving 6,558 HFrEF patients who fit the description of ivabradine candidates described in the ESC guidelines, those who received ivabradine titrated to a maximum of 7.5 mg twice daily experienced a 26% reduction in hospital admissions for worsening heart failure, compared with placebo, a 26% reduction in deaths from heart failure, and fewer adverse events than the control group (Lancet. 2010 Sep 11;376[9744]:875-85).
The important question is who should get ivabradine and who should just get a little more beta-blocker in order to slow the heart rate. The fact is, many heart failure patients simply can’t tolerate the guideline-recommended target dose of beta-blocker therapy, which is 12.5 mg twice daily of carvedilol or its equivalent. Indeed, only 26% of SHIFT participants were able to do so.
“My interpretation of the SHIFT trial is that the goal is to reduce heart rate by any means necessary; preferentially, with a beta-blocker, and with ivabradine as an adjunct in patients who can’t get to target doses,” the cardiologist said.
Sacubitril/valsartan
In the landmark double-blind, 8,442-patient PARADIGM-HF trial, the group randomized to sacubitril/valsartan had a 20% reduction in the primary endpoint of cardiovascular death or heart failure hospitalization over 27 months of follow-up, compared with controls on enalapril at the guideline-recommended dose of 10 mg twice a day. The number needed to treat (NNT) was 21. Moreover, all-cause mortality was reduced by 16% (N Engl J Med. 2014 Sep 11;37[11]:993-1004).
In a recent follow-up cause of death analysis, Dr. Desai and his coinvestigators reported that 81% of all deaths in PARADIGM-HF were cardiovascular in nature. The NNT for sacubitril/valsartan in order to prevent one cardiovascular death was 32. The risk of sudden cardiac death was reduced by 80%, while the risk of death due to worsening heart failure was decreased by 21% (Eur Heart J 2015 Aug 7;36[30]:1990-7).
In another secondary analysis from the PARADIGM-HF investigators, the use of the angiotensin receptor/neprilysin inhibitor was shown to prevent clinical progression of surviving patients with heart failure much more effectively than enalapril. The sacubitril/valsartan group was 34% less likely to have an emergency department visit for worsening heart failure, 18% less likely to require intensive care, and 22% less likely to receive an implantable heart failure device or undergo cardiac transplantation. The reduction in the rate of heart failure hospitalization became significant within the first 30 days (Circulation. 2015 Jan 6;131[1]:54-61).
Moreover, the absolute benefit of sacubitril/valsartan in PARADIGM-HF was consistent across the full spectrum of patient risk (J Am Coll Cardiol. 2015 Nov 10;66[19]:2059-71).
To put this into perspective, Dr. Desai continued, for every 1,000 HFrEF patients switched from an ACE inhibitor or ARB to sacubitril/valsartan, the absolute benefit over the course of 27 months includes 31 fewer cardiovascular deaths, 28 fewer hospitalizations for heart failure, and 37 fewer hospitalizations for any reason.
“This is potent therapy for patients with HFrEF who have the right phenotype,” he observed.
While substitution of sacubitril/valsartan for an ACE inhibitor or ARB may be appropriate in many patients with chronic HFrEF who continue to have NYHA Class II-IV symptoms on guideline-directed medical therapy, several caveats apply, according to Dr. Desai.
It’s important to be aware of the PARADIGM-HF eligibility criteria, because it’s only in patients who fit that profile that sacubitril/valsartan provides evidence-based therapy. There are as yet no data to support the drug’s use in patients with new-onset HFrEF, acute decompensated HFrEF, in patients who are immediately post-MI, or in those with advanced chronic kidney disease, he emphasized.
“I think you have to be mindful of eligibility because the label that’s applied to this drug is basically ‘patients with HFrEF who are treated with guideline-directed medical therapy.’ There’s no specific requirement that you follow the detailed eligibility criteria of the PARADIGM-HF trial, but you should realize that the drug is known to be effective only in patients who fit the PARADIGM-HF eligibility profile,” he said.
Dr. Desai gave a few clinical pearls for prescribing sacubitril/valsartan. For most patients, the initial recommended dose is 49/51 mg twice daily. In those with low baseline blood pressure and tenuous hemodynamics, it’s appropriate to initiate therapy at 24/26 mg BID. It’s important to halt ACE inhibitor therapy 36 hours prior to starting sacubitril/valsartan so as to avoid overlap and consequent increased risk of angioedema. And while serum n-terminal prohormone brain natriuretic peptide (NT-proBNP) remains a useful biomarker to monitor heart rate severity and response to treatment while a patient is on sacubitril/valsartan, BNP is not because serum levels of that biomarker rise with neprilysin inhibition.
Dr. Desai reported receiving research support from Novartis and St. Jude Medical and serving as a consultant to those companies as well as Merck and Relypsa.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Warfarin is best for anticoagulation in prosthetic heart valve pregnancies
SNOWMASS, COLO. – How would you manage anticoagulation in a newly pregnant 23-year-old with a mechanical heart valve who has been on warfarin at 3 mg/day?
A) Weight-adjusted low-molecular-weight heparin during the first trimester, then warfarin in the second and third until switching to unfractionated heparin for delivery.
B) Low-molecular-weight heparin throughout pregnancy.
C) Warfarin throughout pregnancy.
D) Unfractionated heparin in the first trimester, warfarin in the second and third until returning to unfractionated heparin peridelivery.
The correct answer, according to both the ACC/AHA guidelines (Circulation. 2014 Jun 10;129[23]:e521-643) and European Society of Cardiology guidelines (Eur Heart J. 2011 Dec;32[24]:3147-97), is C in women who are on 5 mg/day of warfarin or less.
“Oral anticoagulants throughout pregnancy are much better for the mother, and this is where the guidelines have moved,” Dr. Carole A. Warnes said at the Annual Cardiovascular Conference at Snowmass.
Both sets of guidelines give a class I recommendation to warfarin during the second and third trimesters, because the risk of warfarin embryopathy is confined to weeks 6-12. During the first trimester, warfarin at 5 mg/day or less gets a class IIa rating – making it preferable to unfractionated or low-molecular-weight heparin – because heparin is a far less effective anticoagulant. Plus, multiple small studies indicate the risk of embryopathy is low – roughly 1%-2% – when the mother is on warfarin at 5 mg/day or less.
In a woman on more than 5 mg/day of warfarin, the risk of warfarin embryopathy is about 6%, so the guidelines recommend replacing the drug with heparin during weeks 6-12.
“It’s not a walk in the park,” said Dr. Warnes, director of the Snowmass conference and professor of medicine at the Mayo Clinic in Rochester, Minn.
The major concern in using heparin for anticoagulation in pregnancy is valve thrombosis. It doubles the risk.
“Pregnancy is the most prothrombotic state there is,” she said. “It’s not like managing a patient through a hip replacement or prostate surgery. Women with a mechanical prosthetic valve should be managed by a heart valve team with expertise in treatment during pregnancy.”
The alternatives to warfarin are adjusted-dose unfractionated heparin, which must be given in a continuous intravenous infusion with meticulous monitoring of activated partial thromboplastin time, or twice-daily low-molecular-weight heparin with dose adjustment by weight and maintenance of a target anti–Factor Xa level of 1.0-1.2 IU/mL.
“If you use low-molecular-weight heparin, you’re going to be seeing that patient every week to monitor anti–Factor Xa 4-6 hours post injection. You’ll find it’s not that easy to stay in the sweet spot, with excellent anticoagulation without an increased risk of maternal thromboembolism, or at the other extreme, fetal bleeding. What might look initially as a relatively easy strategy with a lot of appeal turns out to entail considerable risk,” Dr. Warnes said.
This was underscored in a cautionary report by highly experienced University of Toronto investigators. In their series of 23 pregnancies in 17 women with mechanical heart valves on low-molecular-weight heparin throughout pregnancy with careful monitoring, there was one maternal thromboembolic event resulting in maternal and fetal death despite a documented therapeutic anti–Factor Xa level (Am J Cardiol. 2009 Nov 1;104[9]:1259-63).
Although warfarin is clearly the better anticoagulant for the mother, the fetus pays the price. This was highlighted in a recent report from the ESC Registry of Pregnancy and Cardiac Disease (ROPAC) that compared pregnancy outcomes in 212 patients with a mechanical heart valve, 134 with a tissue valve, and 2,620 women without a prosthetic heart valve. Use of warfarin or another vitamin K antagonist in the first trimester was associated with a higher rate of miscarriage than heparin – 28.6% vs. 9.2% – as well as a 7.1% incidence of late fetal death, compared with just 0.7% with heparin.
On the other hand, the mechanical valve thrombosis rate was 4.7%, with half of those serious events occurring during the first trimester in patients after they’d been switched to heparin (Circulation. 2015 Jul 14;132[2]:132-42).
Hemorrhagic events occurred in 23.1% of mothers with a mechanical heart valve, 5.1% of those with a bioprosthetic valve, and 4.9% of patients without a prosthetic valve. A point worth incorporating into prepregnancy patient counseling, Dr. Warnes noted, is that only 58% of ROPAC participants with a mechanical heart valve had an uncomplicated pregnancy with a live birth, in contrast to 79% of those with a tissue valve and 78% of controls.
Because warfarin crosses the placenta, and it takes about a week for the fetus to eliminate the drug following maternal discontinuation, the guidelines recommend stopping warfarin at about week 36 and changing to a continuous infusion of dose-adjusted unfractionated heparin peridelivery. The heparin should be stopped for as short a time as possible before delivery and resumed 6-12 hours post delivery in order to protect against valve thrombosis.
Of course, opting for a bioprosthetic rather than a mechanical heart valve avoids all these difficult anticoagulation-related issues. But it poses a different serious problem: The younger the patient at the time of tissue valve implantation, the greater the risk of rapid calcification and structural valve deterioration. Indeed, among patients who are age 16-39 when they receive a bioprosthetic valve, the rate of structural valve deterioration is 50% at 10 years and 90% at 15 years.
“There is no ideal valve prosthesis. If you elect a tissue prosthesis, you have to discuss the risk of reoperation in that young woman,” Dr. Warnes advised.
Recent data from the Society of Thoracic Surgeons database indicate the mortality associated with redo elective aortic valve replacement in a 35-year-old woman with no comorbidities averages 1.63%, with a 2% mortality rate for redo mitral valve replacement.
Dr. Warnes reported having no financial conflicts regarding her presentation.
SNOWMASS, COLO. – How would you manage anticoagulation in a newly pregnant 23-year-old with a mechanical heart valve who has been on warfarin at 3 mg/day?
A) Weight-adjusted low-molecular-weight heparin during the first trimester, then warfarin in the second and third until switching to unfractionated heparin for delivery.
B) Low-molecular-weight heparin throughout pregnancy.
C) Warfarin throughout pregnancy.
D) Unfractionated heparin in the first trimester, warfarin in the second and third until returning to unfractionated heparin peridelivery.
The correct answer, according to both the ACC/AHA guidelines (Circulation. 2014 Jun 10;129[23]:e521-643) and European Society of Cardiology guidelines (Eur Heart J. 2011 Dec;32[24]:3147-97), is C in women who are on 5 mg/day of warfarin or less.
“Oral anticoagulants throughout pregnancy are much better for the mother, and this is where the guidelines have moved,” Dr. Carole A. Warnes said at the Annual Cardiovascular Conference at Snowmass.
Both sets of guidelines give a class I recommendation to warfarin during the second and third trimesters, because the risk of warfarin embryopathy is confined to weeks 6-12. During the first trimester, warfarin at 5 mg/day or less gets a class IIa rating – making it preferable to unfractionated or low-molecular-weight heparin – because heparin is a far less effective anticoagulant. Plus, multiple small studies indicate the risk of embryopathy is low – roughly 1%-2% – when the mother is on warfarin at 5 mg/day or less.
In a woman on more than 5 mg/day of warfarin, the risk of warfarin embryopathy is about 6%, so the guidelines recommend replacing the drug with heparin during weeks 6-12.
“It’s not a walk in the park,” said Dr. Warnes, director of the Snowmass conference and professor of medicine at the Mayo Clinic in Rochester, Minn.
The major concern in using heparin for anticoagulation in pregnancy is valve thrombosis. It doubles the risk.
“Pregnancy is the most prothrombotic state there is,” she said. “It’s not like managing a patient through a hip replacement or prostate surgery. Women with a mechanical prosthetic valve should be managed by a heart valve team with expertise in treatment during pregnancy.”
The alternatives to warfarin are adjusted-dose unfractionated heparin, which must be given in a continuous intravenous infusion with meticulous monitoring of activated partial thromboplastin time, or twice-daily low-molecular-weight heparin with dose adjustment by weight and maintenance of a target anti–Factor Xa level of 1.0-1.2 IU/mL.
“If you use low-molecular-weight heparin, you’re going to be seeing that patient every week to monitor anti–Factor Xa 4-6 hours post injection. You’ll find it’s not that easy to stay in the sweet spot, with excellent anticoagulation without an increased risk of maternal thromboembolism, or at the other extreme, fetal bleeding. What might look initially as a relatively easy strategy with a lot of appeal turns out to entail considerable risk,” Dr. Warnes said.
This was underscored in a cautionary report by highly experienced University of Toronto investigators. In their series of 23 pregnancies in 17 women with mechanical heart valves on low-molecular-weight heparin throughout pregnancy with careful monitoring, there was one maternal thromboembolic event resulting in maternal and fetal death despite a documented therapeutic anti–Factor Xa level (Am J Cardiol. 2009 Nov 1;104[9]:1259-63).
Although warfarin is clearly the better anticoagulant for the mother, the fetus pays the price. This was highlighted in a recent report from the ESC Registry of Pregnancy and Cardiac Disease (ROPAC) that compared pregnancy outcomes in 212 patients with a mechanical heart valve, 134 with a tissue valve, and 2,620 women without a prosthetic heart valve. Use of warfarin or another vitamin K antagonist in the first trimester was associated with a higher rate of miscarriage than heparin – 28.6% vs. 9.2% – as well as a 7.1% incidence of late fetal death, compared with just 0.7% with heparin.
On the other hand, the mechanical valve thrombosis rate was 4.7%, with half of those serious events occurring during the first trimester in patients after they’d been switched to heparin (Circulation. 2015 Jul 14;132[2]:132-42).
Hemorrhagic events occurred in 23.1% of mothers with a mechanical heart valve, 5.1% of those with a bioprosthetic valve, and 4.9% of patients without a prosthetic valve. A point worth incorporating into prepregnancy patient counseling, Dr. Warnes noted, is that only 58% of ROPAC participants with a mechanical heart valve had an uncomplicated pregnancy with a live birth, in contrast to 79% of those with a tissue valve and 78% of controls.
Because warfarin crosses the placenta, and it takes about a week for the fetus to eliminate the drug following maternal discontinuation, the guidelines recommend stopping warfarin at about week 36 and changing to a continuous infusion of dose-adjusted unfractionated heparin peridelivery. The heparin should be stopped for as short a time as possible before delivery and resumed 6-12 hours post delivery in order to protect against valve thrombosis.
Of course, opting for a bioprosthetic rather than a mechanical heart valve avoids all these difficult anticoagulation-related issues. But it poses a different serious problem: The younger the patient at the time of tissue valve implantation, the greater the risk of rapid calcification and structural valve deterioration. Indeed, among patients who are age 16-39 when they receive a bioprosthetic valve, the rate of structural valve deterioration is 50% at 10 years and 90% at 15 years.
“There is no ideal valve prosthesis. If you elect a tissue prosthesis, you have to discuss the risk of reoperation in that young woman,” Dr. Warnes advised.
Recent data from the Society of Thoracic Surgeons database indicate the mortality associated with redo elective aortic valve replacement in a 35-year-old woman with no comorbidities averages 1.63%, with a 2% mortality rate for redo mitral valve replacement.
Dr. Warnes reported having no financial conflicts regarding her presentation.
SNOWMASS, COLO. – How would you manage anticoagulation in a newly pregnant 23-year-old with a mechanical heart valve who has been on warfarin at 3 mg/day?
A) Weight-adjusted low-molecular-weight heparin during the first trimester, then warfarin in the second and third until switching to unfractionated heparin for delivery.
B) Low-molecular-weight heparin throughout pregnancy.
C) Warfarin throughout pregnancy.
D) Unfractionated heparin in the first trimester, warfarin in the second and third until returning to unfractionated heparin peridelivery.
The correct answer, according to both the ACC/AHA guidelines (Circulation. 2014 Jun 10;129[23]:e521-643) and European Society of Cardiology guidelines (Eur Heart J. 2011 Dec;32[24]:3147-97), is C in women who are on 5 mg/day of warfarin or less.
“Oral anticoagulants throughout pregnancy are much better for the mother, and this is where the guidelines have moved,” Dr. Carole A. Warnes said at the Annual Cardiovascular Conference at Snowmass.
Both sets of guidelines give a class I recommendation to warfarin during the second and third trimesters, because the risk of warfarin embryopathy is confined to weeks 6-12. During the first trimester, warfarin at 5 mg/day or less gets a class IIa rating – making it preferable to unfractionated or low-molecular-weight heparin – because heparin is a far less effective anticoagulant. Plus, multiple small studies indicate the risk of embryopathy is low – roughly 1%-2% – when the mother is on warfarin at 5 mg/day or less.
In a woman on more than 5 mg/day of warfarin, the risk of warfarin embryopathy is about 6%, so the guidelines recommend replacing the drug with heparin during weeks 6-12.
“It’s not a walk in the park,” said Dr. Warnes, director of the Snowmass conference and professor of medicine at the Mayo Clinic in Rochester, Minn.
The major concern in using heparin for anticoagulation in pregnancy is valve thrombosis. It doubles the risk.
“Pregnancy is the most prothrombotic state there is,” she said. “It’s not like managing a patient through a hip replacement or prostate surgery. Women with a mechanical prosthetic valve should be managed by a heart valve team with expertise in treatment during pregnancy.”
The alternatives to warfarin are adjusted-dose unfractionated heparin, which must be given in a continuous intravenous infusion with meticulous monitoring of activated partial thromboplastin time, or twice-daily low-molecular-weight heparin with dose adjustment by weight and maintenance of a target anti–Factor Xa level of 1.0-1.2 IU/mL.
“If you use low-molecular-weight heparin, you’re going to be seeing that patient every week to monitor anti–Factor Xa 4-6 hours post injection. You’ll find it’s not that easy to stay in the sweet spot, with excellent anticoagulation without an increased risk of maternal thromboembolism, or at the other extreme, fetal bleeding. What might look initially as a relatively easy strategy with a lot of appeal turns out to entail considerable risk,” Dr. Warnes said.
This was underscored in a cautionary report by highly experienced University of Toronto investigators. In their series of 23 pregnancies in 17 women with mechanical heart valves on low-molecular-weight heparin throughout pregnancy with careful monitoring, there was one maternal thromboembolic event resulting in maternal and fetal death despite a documented therapeutic anti–Factor Xa level (Am J Cardiol. 2009 Nov 1;104[9]:1259-63).
Although warfarin is clearly the better anticoagulant for the mother, the fetus pays the price. This was highlighted in a recent report from the ESC Registry of Pregnancy and Cardiac Disease (ROPAC) that compared pregnancy outcomes in 212 patients with a mechanical heart valve, 134 with a tissue valve, and 2,620 women without a prosthetic heart valve. Use of warfarin or another vitamin K antagonist in the first trimester was associated with a higher rate of miscarriage than heparin – 28.6% vs. 9.2% – as well as a 7.1% incidence of late fetal death, compared with just 0.7% with heparin.
On the other hand, the mechanical valve thrombosis rate was 4.7%, with half of those serious events occurring during the first trimester in patients after they’d been switched to heparin (Circulation. 2015 Jul 14;132[2]:132-42).
Hemorrhagic events occurred in 23.1% of mothers with a mechanical heart valve, 5.1% of those with a bioprosthetic valve, and 4.9% of patients without a prosthetic valve. A point worth incorporating into prepregnancy patient counseling, Dr. Warnes noted, is that only 58% of ROPAC participants with a mechanical heart valve had an uncomplicated pregnancy with a live birth, in contrast to 79% of those with a tissue valve and 78% of controls.
Because warfarin crosses the placenta, and it takes about a week for the fetus to eliminate the drug following maternal discontinuation, the guidelines recommend stopping warfarin at about week 36 and changing to a continuous infusion of dose-adjusted unfractionated heparin peridelivery. The heparin should be stopped for as short a time as possible before delivery and resumed 6-12 hours post delivery in order to protect against valve thrombosis.
Of course, opting for a bioprosthetic rather than a mechanical heart valve avoids all these difficult anticoagulation-related issues. But it poses a different serious problem: The younger the patient at the time of tissue valve implantation, the greater the risk of rapid calcification and structural valve deterioration. Indeed, among patients who are age 16-39 when they receive a bioprosthetic valve, the rate of structural valve deterioration is 50% at 10 years and 90% at 15 years.
“There is no ideal valve prosthesis. If you elect a tissue prosthesis, you have to discuss the risk of reoperation in that young woman,” Dr. Warnes advised.
Recent data from the Society of Thoracic Surgeons database indicate the mortality associated with redo elective aortic valve replacement in a 35-year-old woman with no comorbidities averages 1.63%, with a 2% mortality rate for redo mitral valve replacement.
Dr. Warnes reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Hypertrophic cardiomyopathy: Who should get an ICD?
SNOWMASS, COLO. – Since the 2011 release of the current American College of Cardiology/American Heart Association guidelines on hypertrophic cardiomyopathy, several new evidence-based tools have emerged as being helpful in decision making regarding which patients should receive an implantable cardioverter-defibrillator (ICD) for primary prevention of sudden cardiac death, Dr. Rick A. Nishimura said at the annual Cardiovascular Conference at Snowmass.
These three tools – gadolinium-enhanced cardiovascular magnetic resonance imaging, a novel European risk score calculator, and a new appreciation of the importance of age-related risk – are most useful in the many cases of hypertrophic cardiomyopathy (HCM) where the cardiologist is on the fence regarding ICD placement because the patient doesn’t clearly meet the conventional major criteria for an ICD, according to Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Nishimura, a member of the writing panel for the current guidelines (Circulation. 2011 Dec 13;124[24]:2761-96), predicted these tools will be incorporated into the next iteration of the HCM guidelines.
Notably absent from Dr. Nishimura’s list of useful tools was genetic testing for assessment of SCD risk in a patient with HCM.
“You should not spend $6,000 to do a genetic study to try to predict who’s at risk for sudden death. It turns out that most mutations are neither inherently benign nor malignant. High-risk mutations come from high-risk families, so you can do just as well by taking a family history,” according to the cardiologist.
Dr. Nishimura explained that the clinical dilemma in trying to evaluate SCD risk in a patient who presents with HCM is that the overall risk is quite low – probably 1% or less per year in the total HCM population – yet HCM is the number-one cause of SCD in younger patients. And it can occur unpredictably years or decades after diagnosis of HCM.
While ICDs are of proven effectiveness in preventing SCD in patients with HCM, reliance solely upon the conventional risk predictors to identify those who should get a device is clearly inadequate. Those criteria have a positive predictive accuracy of less than 15%; in other words, roughly 85% of HCM patients who get an ICD never receive an appropriate, life-saving shock, Dr. Nishimura said.
“We have a lot of work left to do in order to better identify these patients. In our own data from the Mayo Clinic, 20%-25% of patients have inappropriate ICD shocks despite efforts to program the device to prevent such shocks. That’s especially common in younger, active patients with HCM, and when it occurs patients find it absolutely devastating,” according to the cardiologist.
As stated in the current guidelines, the established SCD risk factors that provide a strong indication for an ICD in a patient with HCM are prior documented cardiac arrest, ventricular fibrillation, or hemodynamically significant ventricular tachycardia. Additionally, risk factors which, in Dr. Nishimura’s view, probably warrant insertion of an ICD and, at the very least should trigger a physician-patient discussion about the risks and benefits of preventive device therapy, include a family history of HCM-related sudden death in a first-degree relative, massive left ventricular (LV) hypertrophy as defined by a maximum wall thickness of at least 30 mm, and recent unexplained syncope inconsistent with neurocardiogenic origin.
Less potent risk predictors where savvy clinical judgment becomes imperative include nonsustained ventricular tachycardia on 24-hour Holter monitoring, a hypotensive blood pressure response to exercise, and an increased LV wall thickness in a younger patient that doesn’t rise to the 30-mm standard. These are situations where gadolinium-enhanced MRI, consideration of patient age, and the European risk scoring system can help in the decision-making process, he said.
Gadolinium-enhanced MRI: Contrast-enhanced cardiovascular MRI with late gadolinium enhancement has emerged as a reliable marker of the myocyte disarray and interstitial fibrosis which serves as a substrate for ventricular arrhythmias. In a recent study of 1,293 HCM patients followed for a median of 3.3 years, the incidence of SCD events increased progressively with the extent of late gadolinium enhancement. Extensive late gadolinium enhancement, defined as involving at least 15% of LV mass, was associated with a doubled risk of SCD events in patients otherwise considered at low risk (Circulation. 2014 Aug 5;130[6]:484-95).
“This is probably going to become one of the key markers that can help you when you’re on the fence as to whether or not to put in an ICD. We’re getting MRIs with gadolinium now in all of our HCM patients. What matters is not gadolinium enhancement at the insertion of the left ventricle into the septum – a lot of people have that – but diffuse gadolinium enhancement throughout the septum,” Dr. Nishimura said.
Because SCD risk increases linearly with greater maximal LV wall thickness, gadolinium-enhanced MRI is particularly helpful in assessing risk in a younger patient with a maximal LV wall thickness of, say, 26 mm, he added.
Age: A study by led by Dr. Barry J. Maron, the cochair of the 2011 guideline committee and director of the HCM center at the Minneapolis Heart Institute, provides a new understanding that prophylactic ICD implantation is not warranted in patients with HCM who present at age 60 or older. In their study of 428 consecutive patients presenting with HCM at age 60 or above, the investigators found during 5.8 years of follow-up that the incidence of arrhythmic sudden death events was just 0.2% per year (Circulation. 2013 Feb 5;127[5]:585-93).
“They’ve shown that if you look at patients age 60 or above who have HCM, the risk of sudden cardiac death is almost nonexistent. That’s incredibly important to remember. Sudden death is something that’s going to happen in the younger population, under age 30,” Dr. Nishimura emphasized.
European SCD risk prediction tool: This tool was hailed as a major advance in the current European Society of Cardiology guidelines on HCM (Eur Heart J. 2014;35:2733-2779). The tool was incorporated into the guidelines. It is also available as a smartphone app.
The risk prediction tool (Eur Heart J. 2014 Aug 7;35[30]:2010-20) is a complex equation that incorporates seven predictive factors: age, maximal LV wall thickness, left atrial diameter, LV outflow tract gradient, family history of SCD, nonsustained VT, and unexplained syncope. After input on these seven factors, the equation spits out an individual’s estimated 5-year SCD risk. Based on the study of 3,675 consecutive HCM patients with a median 5.7 years of follow-up that was used to develop the risk equation, the current ESC guidelines state that an ICD is not warranted in HCM patients with a 5-year risk below 4%, device implantation should be considered in those whose risk is 4%-6%, and an ICD should be even more strongly considered in patients with a 5-year risk in excess of 6%.
“A lot of people across the pond are using this risk score. But there are some problems with it,” according to Dr. Nishimura.
In his view, it “doesn’t make much sense” to include left ventricular outflow tract gradient or left atrial diameter in the risk equation. Nor is unexplained syncope carefully defined. Also, the equation would be improved by incorporation of late gadolinium enhancement on MRI, left ventricular dysfunction, and presence or absence of apical aneurysm as predictive variables. But on the plus side, the European equation treats maximal LV wall thickness as a continuous variable, which is more appropriate than the single 30-mm cutoff used in the ACC/AHA guidelines.
The biggest limitation of the European prognostic score, however, is that it hasn’t yet been validated in an independent patient cohort, Dr. Nishimura said. He noted that when Dr. Maron and coworkers recently applied the European SCD risk equation retrospectively to 1,629 consecutive U.S. patients with HCM, the investigators concluded that the risk equation proved unreliable for prediction of future SCD events. Fifty-nine percent of patients who got an appropriate ICD shock or experienced SCD were misclassified as low risk and hence would not have received an ICD under the European guidelines (Am J Cardiol. 2015 Sep 1;116[5]:757-64).
Nonetheless, because of the limited predictive accuracy of today’s standard methods of assessing SCD risk, Dr. Nishimura considers application of the European risk score to be “reasonable” in HCM patients who don’t have any of the strong indications for an ICD.
“If it comes up with an estimated 5-year risk greater than 6%, I think it’s very reasonable to consider implantation of an ICD,” he said.
Dr. Nishimura observed that in addition to assessing SCD risk, cardiologists have two other separate essential tasks when a patient presents with HCM. One is to screen and counsel the first-degree relatives. The other is to determine whether a left ventricular outflow tract obstruction is present in a symptomatic patient and, if so, to improve symptoms by treating the associated hemodynamic abnormalities medically and if need be by septal ablation or septal myectomy.
He reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – Since the 2011 release of the current American College of Cardiology/American Heart Association guidelines on hypertrophic cardiomyopathy, several new evidence-based tools have emerged as being helpful in decision making regarding which patients should receive an implantable cardioverter-defibrillator (ICD) for primary prevention of sudden cardiac death, Dr. Rick A. Nishimura said at the annual Cardiovascular Conference at Snowmass.
These three tools – gadolinium-enhanced cardiovascular magnetic resonance imaging, a novel European risk score calculator, and a new appreciation of the importance of age-related risk – are most useful in the many cases of hypertrophic cardiomyopathy (HCM) where the cardiologist is on the fence regarding ICD placement because the patient doesn’t clearly meet the conventional major criteria for an ICD, according to Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Nishimura, a member of the writing panel for the current guidelines (Circulation. 2011 Dec 13;124[24]:2761-96), predicted these tools will be incorporated into the next iteration of the HCM guidelines.
Notably absent from Dr. Nishimura’s list of useful tools was genetic testing for assessment of SCD risk in a patient with HCM.
“You should not spend $6,000 to do a genetic study to try to predict who’s at risk for sudden death. It turns out that most mutations are neither inherently benign nor malignant. High-risk mutations come from high-risk families, so you can do just as well by taking a family history,” according to the cardiologist.
Dr. Nishimura explained that the clinical dilemma in trying to evaluate SCD risk in a patient who presents with HCM is that the overall risk is quite low – probably 1% or less per year in the total HCM population – yet HCM is the number-one cause of SCD in younger patients. And it can occur unpredictably years or decades after diagnosis of HCM.
While ICDs are of proven effectiveness in preventing SCD in patients with HCM, reliance solely upon the conventional risk predictors to identify those who should get a device is clearly inadequate. Those criteria have a positive predictive accuracy of less than 15%; in other words, roughly 85% of HCM patients who get an ICD never receive an appropriate, life-saving shock, Dr. Nishimura said.
“We have a lot of work left to do in order to better identify these patients. In our own data from the Mayo Clinic, 20%-25% of patients have inappropriate ICD shocks despite efforts to program the device to prevent such shocks. That’s especially common in younger, active patients with HCM, and when it occurs patients find it absolutely devastating,” according to the cardiologist.
As stated in the current guidelines, the established SCD risk factors that provide a strong indication for an ICD in a patient with HCM are prior documented cardiac arrest, ventricular fibrillation, or hemodynamically significant ventricular tachycardia. Additionally, risk factors which, in Dr. Nishimura’s view, probably warrant insertion of an ICD and, at the very least should trigger a physician-patient discussion about the risks and benefits of preventive device therapy, include a family history of HCM-related sudden death in a first-degree relative, massive left ventricular (LV) hypertrophy as defined by a maximum wall thickness of at least 30 mm, and recent unexplained syncope inconsistent with neurocardiogenic origin.
Less potent risk predictors where savvy clinical judgment becomes imperative include nonsustained ventricular tachycardia on 24-hour Holter monitoring, a hypotensive blood pressure response to exercise, and an increased LV wall thickness in a younger patient that doesn’t rise to the 30-mm standard. These are situations where gadolinium-enhanced MRI, consideration of patient age, and the European risk scoring system can help in the decision-making process, he said.
Gadolinium-enhanced MRI: Contrast-enhanced cardiovascular MRI with late gadolinium enhancement has emerged as a reliable marker of the myocyte disarray and interstitial fibrosis which serves as a substrate for ventricular arrhythmias. In a recent study of 1,293 HCM patients followed for a median of 3.3 years, the incidence of SCD events increased progressively with the extent of late gadolinium enhancement. Extensive late gadolinium enhancement, defined as involving at least 15% of LV mass, was associated with a doubled risk of SCD events in patients otherwise considered at low risk (Circulation. 2014 Aug 5;130[6]:484-95).
“This is probably going to become one of the key markers that can help you when you’re on the fence as to whether or not to put in an ICD. We’re getting MRIs with gadolinium now in all of our HCM patients. What matters is not gadolinium enhancement at the insertion of the left ventricle into the septum – a lot of people have that – but diffuse gadolinium enhancement throughout the septum,” Dr. Nishimura said.
Because SCD risk increases linearly with greater maximal LV wall thickness, gadolinium-enhanced MRI is particularly helpful in assessing risk in a younger patient with a maximal LV wall thickness of, say, 26 mm, he added.
Age: A study by led by Dr. Barry J. Maron, the cochair of the 2011 guideline committee and director of the HCM center at the Minneapolis Heart Institute, provides a new understanding that prophylactic ICD implantation is not warranted in patients with HCM who present at age 60 or older. In their study of 428 consecutive patients presenting with HCM at age 60 or above, the investigators found during 5.8 years of follow-up that the incidence of arrhythmic sudden death events was just 0.2% per year (Circulation. 2013 Feb 5;127[5]:585-93).
“They’ve shown that if you look at patients age 60 or above who have HCM, the risk of sudden cardiac death is almost nonexistent. That’s incredibly important to remember. Sudden death is something that’s going to happen in the younger population, under age 30,” Dr. Nishimura emphasized.
European SCD risk prediction tool: This tool was hailed as a major advance in the current European Society of Cardiology guidelines on HCM (Eur Heart J. 2014;35:2733-2779). The tool was incorporated into the guidelines. It is also available as a smartphone app.
The risk prediction tool (Eur Heart J. 2014 Aug 7;35[30]:2010-20) is a complex equation that incorporates seven predictive factors: age, maximal LV wall thickness, left atrial diameter, LV outflow tract gradient, family history of SCD, nonsustained VT, and unexplained syncope. After input on these seven factors, the equation spits out an individual’s estimated 5-year SCD risk. Based on the study of 3,675 consecutive HCM patients with a median 5.7 years of follow-up that was used to develop the risk equation, the current ESC guidelines state that an ICD is not warranted in HCM patients with a 5-year risk below 4%, device implantation should be considered in those whose risk is 4%-6%, and an ICD should be even more strongly considered in patients with a 5-year risk in excess of 6%.
“A lot of people across the pond are using this risk score. But there are some problems with it,” according to Dr. Nishimura.
In his view, it “doesn’t make much sense” to include left ventricular outflow tract gradient or left atrial diameter in the risk equation. Nor is unexplained syncope carefully defined. Also, the equation would be improved by incorporation of late gadolinium enhancement on MRI, left ventricular dysfunction, and presence or absence of apical aneurysm as predictive variables. But on the plus side, the European equation treats maximal LV wall thickness as a continuous variable, which is more appropriate than the single 30-mm cutoff used in the ACC/AHA guidelines.
The biggest limitation of the European prognostic score, however, is that it hasn’t yet been validated in an independent patient cohort, Dr. Nishimura said. He noted that when Dr. Maron and coworkers recently applied the European SCD risk equation retrospectively to 1,629 consecutive U.S. patients with HCM, the investigators concluded that the risk equation proved unreliable for prediction of future SCD events. Fifty-nine percent of patients who got an appropriate ICD shock or experienced SCD were misclassified as low risk and hence would not have received an ICD under the European guidelines (Am J Cardiol. 2015 Sep 1;116[5]:757-64).
Nonetheless, because of the limited predictive accuracy of today’s standard methods of assessing SCD risk, Dr. Nishimura considers application of the European risk score to be “reasonable” in HCM patients who don’t have any of the strong indications for an ICD.
“If it comes up with an estimated 5-year risk greater than 6%, I think it’s very reasonable to consider implantation of an ICD,” he said.
Dr. Nishimura observed that in addition to assessing SCD risk, cardiologists have two other separate essential tasks when a patient presents with HCM. One is to screen and counsel the first-degree relatives. The other is to determine whether a left ventricular outflow tract obstruction is present in a symptomatic patient and, if so, to improve symptoms by treating the associated hemodynamic abnormalities medically and if need be by septal ablation or septal myectomy.
He reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – Since the 2011 release of the current American College of Cardiology/American Heart Association guidelines on hypertrophic cardiomyopathy, several new evidence-based tools have emerged as being helpful in decision making regarding which patients should receive an implantable cardioverter-defibrillator (ICD) for primary prevention of sudden cardiac death, Dr. Rick A. Nishimura said at the annual Cardiovascular Conference at Snowmass.
These three tools – gadolinium-enhanced cardiovascular magnetic resonance imaging, a novel European risk score calculator, and a new appreciation of the importance of age-related risk – are most useful in the many cases of hypertrophic cardiomyopathy (HCM) where the cardiologist is on the fence regarding ICD placement because the patient doesn’t clearly meet the conventional major criteria for an ICD, according to Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Nishimura, a member of the writing panel for the current guidelines (Circulation. 2011 Dec 13;124[24]:2761-96), predicted these tools will be incorporated into the next iteration of the HCM guidelines.
Notably absent from Dr. Nishimura’s list of useful tools was genetic testing for assessment of SCD risk in a patient with HCM.
“You should not spend $6,000 to do a genetic study to try to predict who’s at risk for sudden death. It turns out that most mutations are neither inherently benign nor malignant. High-risk mutations come from high-risk families, so you can do just as well by taking a family history,” according to the cardiologist.
Dr. Nishimura explained that the clinical dilemma in trying to evaluate SCD risk in a patient who presents with HCM is that the overall risk is quite low – probably 1% or less per year in the total HCM population – yet HCM is the number-one cause of SCD in younger patients. And it can occur unpredictably years or decades after diagnosis of HCM.
While ICDs are of proven effectiveness in preventing SCD in patients with HCM, reliance solely upon the conventional risk predictors to identify those who should get a device is clearly inadequate. Those criteria have a positive predictive accuracy of less than 15%; in other words, roughly 85% of HCM patients who get an ICD never receive an appropriate, life-saving shock, Dr. Nishimura said.
“We have a lot of work left to do in order to better identify these patients. In our own data from the Mayo Clinic, 20%-25% of patients have inappropriate ICD shocks despite efforts to program the device to prevent such shocks. That’s especially common in younger, active patients with HCM, and when it occurs patients find it absolutely devastating,” according to the cardiologist.
As stated in the current guidelines, the established SCD risk factors that provide a strong indication for an ICD in a patient with HCM are prior documented cardiac arrest, ventricular fibrillation, or hemodynamically significant ventricular tachycardia. Additionally, risk factors which, in Dr. Nishimura’s view, probably warrant insertion of an ICD and, at the very least should trigger a physician-patient discussion about the risks and benefits of preventive device therapy, include a family history of HCM-related sudden death in a first-degree relative, massive left ventricular (LV) hypertrophy as defined by a maximum wall thickness of at least 30 mm, and recent unexplained syncope inconsistent with neurocardiogenic origin.
Less potent risk predictors where savvy clinical judgment becomes imperative include nonsustained ventricular tachycardia on 24-hour Holter monitoring, a hypotensive blood pressure response to exercise, and an increased LV wall thickness in a younger patient that doesn’t rise to the 30-mm standard. These are situations where gadolinium-enhanced MRI, consideration of patient age, and the European risk scoring system can help in the decision-making process, he said.
Gadolinium-enhanced MRI: Contrast-enhanced cardiovascular MRI with late gadolinium enhancement has emerged as a reliable marker of the myocyte disarray and interstitial fibrosis which serves as a substrate for ventricular arrhythmias. In a recent study of 1,293 HCM patients followed for a median of 3.3 years, the incidence of SCD events increased progressively with the extent of late gadolinium enhancement. Extensive late gadolinium enhancement, defined as involving at least 15% of LV mass, was associated with a doubled risk of SCD events in patients otherwise considered at low risk (Circulation. 2014 Aug 5;130[6]:484-95).
“This is probably going to become one of the key markers that can help you when you’re on the fence as to whether or not to put in an ICD. We’re getting MRIs with gadolinium now in all of our HCM patients. What matters is not gadolinium enhancement at the insertion of the left ventricle into the septum – a lot of people have that – but diffuse gadolinium enhancement throughout the septum,” Dr. Nishimura said.
Because SCD risk increases linearly with greater maximal LV wall thickness, gadolinium-enhanced MRI is particularly helpful in assessing risk in a younger patient with a maximal LV wall thickness of, say, 26 mm, he added.
Age: A study by led by Dr. Barry J. Maron, the cochair of the 2011 guideline committee and director of the HCM center at the Minneapolis Heart Institute, provides a new understanding that prophylactic ICD implantation is not warranted in patients with HCM who present at age 60 or older. In their study of 428 consecutive patients presenting with HCM at age 60 or above, the investigators found during 5.8 years of follow-up that the incidence of arrhythmic sudden death events was just 0.2% per year (Circulation. 2013 Feb 5;127[5]:585-93).
“They’ve shown that if you look at patients age 60 or above who have HCM, the risk of sudden cardiac death is almost nonexistent. That’s incredibly important to remember. Sudden death is something that’s going to happen in the younger population, under age 30,” Dr. Nishimura emphasized.
European SCD risk prediction tool: This tool was hailed as a major advance in the current European Society of Cardiology guidelines on HCM (Eur Heart J. 2014;35:2733-2779). The tool was incorporated into the guidelines. It is also available as a smartphone app.
The risk prediction tool (Eur Heart J. 2014 Aug 7;35[30]:2010-20) is a complex equation that incorporates seven predictive factors: age, maximal LV wall thickness, left atrial diameter, LV outflow tract gradient, family history of SCD, nonsustained VT, and unexplained syncope. After input on these seven factors, the equation spits out an individual’s estimated 5-year SCD risk. Based on the study of 3,675 consecutive HCM patients with a median 5.7 years of follow-up that was used to develop the risk equation, the current ESC guidelines state that an ICD is not warranted in HCM patients with a 5-year risk below 4%, device implantation should be considered in those whose risk is 4%-6%, and an ICD should be even more strongly considered in patients with a 5-year risk in excess of 6%.
“A lot of people across the pond are using this risk score. But there are some problems with it,” according to Dr. Nishimura.
In his view, it “doesn’t make much sense” to include left ventricular outflow tract gradient or left atrial diameter in the risk equation. Nor is unexplained syncope carefully defined. Also, the equation would be improved by incorporation of late gadolinium enhancement on MRI, left ventricular dysfunction, and presence or absence of apical aneurysm as predictive variables. But on the plus side, the European equation treats maximal LV wall thickness as a continuous variable, which is more appropriate than the single 30-mm cutoff used in the ACC/AHA guidelines.
The biggest limitation of the European prognostic score, however, is that it hasn’t yet been validated in an independent patient cohort, Dr. Nishimura said. He noted that when Dr. Maron and coworkers recently applied the European SCD risk equation retrospectively to 1,629 consecutive U.S. patients with HCM, the investigators concluded that the risk equation proved unreliable for prediction of future SCD events. Fifty-nine percent of patients who got an appropriate ICD shock or experienced SCD were misclassified as low risk and hence would not have received an ICD under the European guidelines (Am J Cardiol. 2015 Sep 1;116[5]:757-64).
Nonetheless, because of the limited predictive accuracy of today’s standard methods of assessing SCD risk, Dr. Nishimura considers application of the European risk score to be “reasonable” in HCM patients who don’t have any of the strong indications for an ICD.
“If it comes up with an estimated 5-year risk greater than 6%, I think it’s very reasonable to consider implantation of an ICD,” he said.
Dr. Nishimura observed that in addition to assessing SCD risk, cardiologists have two other separate essential tasks when a patient presents with HCM. One is to screen and counsel the first-degree relatives. The other is to determine whether a left ventricular outflow tract obstruction is present in a symptomatic patient and, if so, to improve symptoms by treating the associated hemodynamic abnormalities medically and if need be by septal ablation or septal myectomy.
He reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Experts say abandon aspirin for stroke prevention in atrial fib
SNOWMASS, COLO. – It’s time to eliminate the practice of prescribing aspirin for stroke prevention in patients with atrial fibrillation and a CHA2DS2-VASc score of 1, two eminent cardiologists agreed at the Annual Cardiovascular Conference at Snowmass.
“The European guidelines have done away with aspirin for stroke prevention in atrial fibrillation. It barely made it into our current U.S. guidelines. I don’t think aspirin should be in there and I don’t think it will be there in the next guidelines. The role of aspirin will fall away,” predicted Dr. Bernard J. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
“It’s not that aspirin is less effective than the oral anticoagulants, it’s that there’s no role for it. There are no good data to support aspirin in the prevention of stroke in atrial fibrillation,” he declared.
Dr. N.A. Mark Estes III agreed the aspirin evidence is seriously flawed.
“The use of aspirin has probably been misguided, based upon a single trial which showed a profound effect and was probably just an anomaly,” according to Dr. Estes, a past president of the Heart Rhythm Society who is professor of medicine and director of the New England Cardiac Arrhythmia Center at Tufts University, Boston.
The sole positive clinical trial of aspirin versus placebo, the 25-year-old Stroke Prevention in Atrial Fibrillation (SPAF) study (Circulation. 1991 Aug;84[2]:527-39), found an unrealistically high stroke protection benefit for aspirin, a result made implausible by multiple other randomized trials showing no benefit, the cardiologists agreed.
“In our current guidelines for atrial fibrillation (Circulation. 2014 Dec 2;130[23]:2071-104), aspirin can be considered as a Class IIb level of evidence C recommendation in patients with a CHA2DS2-VASc of 1. But I would just take it off of your clinical armamentarium because the best available data indicates that it doesn’t prevent strokes. I’m certainly not using it in my patients. Increasingly in my patients with a CHA2DS2-VASc of 1, I’m discussing the risks and benefits of a NOAC [novel oral anticoagulant],” Dr. Estes said.
Dr. Gersh was also critical of another common practice in stroke prevention in atrial fibrillation: concomitant use of aspirin with an oral anticoagulant.
“We use too much aspirin in patients on oral anticoagulation. Aspirin is perhaps the major cause of bleeding in patients on an oral anticoagulant. Other than in people with a drug-eluting stent, there’s no role at all for aspirin in stroke prevention,” he asserted.
He was coauthor of an analysis of 7,347 participants in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) who were on an oral anticoagulant. Fully 35% of them were also on aspirin. In a multivariate analysis, concomitant aspirin and oral anticoagulation was independently associated with a 53% increased risk of major bleeding and a 52% increase in hospitalization for bleeding, compared with atrial fibrillation patients on an oral anticoagulant alone (Circulation. 2013 Aug 13;128[7]:721-8).
Moreover, the widespread use of dual therapy in this real-world registry didn’t appear to be rational. Thirty-nine percent of those on aspirin plus an oral anticoagulant had no history of atherosclerotic disease, the presence of which would be an indication for considering aspirin. And 17% of dual therapy patients had an elevated Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) risk score of 5 or more, making dual therapy particularly risky.
This clinically important interaction between aspirin and oral anticoagulation was recently underscored in an analysis of rivaroxaban-treated patients in the ROCKET AF trial, Dr. Gersh observed. Long-term use of aspirin at entry into this pivotal randomized trial of rivaroxaban (Xarelto) versus warfarin in patients with atrial fibrillation proved to be an independent predictor of a 47% increase in the risk of gastrointestinal bleeding, compared with patients on rivaroxaban alone (J Am Coll Cardiol. 2015 Dec 1;66[21]:2271-81).
He added that there is no evidence that combining aspirin and oral anticoagulation enhances stroke prevention beyond the marked benefit achieved with oral anticoagulation alone.
Dr. Gersh reported serving on the leadership of the ORBIT-AF Registry, which was sponsored by Janssen Pharmaceuticals. Dr. Estes reported having no financial conflicts relevant to this discussion.
SNOWMASS, COLO. – It’s time to eliminate the practice of prescribing aspirin for stroke prevention in patients with atrial fibrillation and a CHA2DS2-VASc score of 1, two eminent cardiologists agreed at the Annual Cardiovascular Conference at Snowmass.
“The European guidelines have done away with aspirin for stroke prevention in atrial fibrillation. It barely made it into our current U.S. guidelines. I don’t think aspirin should be in there and I don’t think it will be there in the next guidelines. The role of aspirin will fall away,” predicted Dr. Bernard J. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
“It’s not that aspirin is less effective than the oral anticoagulants, it’s that there’s no role for it. There are no good data to support aspirin in the prevention of stroke in atrial fibrillation,” he declared.
Dr. N.A. Mark Estes III agreed the aspirin evidence is seriously flawed.
“The use of aspirin has probably been misguided, based upon a single trial which showed a profound effect and was probably just an anomaly,” according to Dr. Estes, a past president of the Heart Rhythm Society who is professor of medicine and director of the New England Cardiac Arrhythmia Center at Tufts University, Boston.
The sole positive clinical trial of aspirin versus placebo, the 25-year-old Stroke Prevention in Atrial Fibrillation (SPAF) study (Circulation. 1991 Aug;84[2]:527-39), found an unrealistically high stroke protection benefit for aspirin, a result made implausible by multiple other randomized trials showing no benefit, the cardiologists agreed.
“In our current guidelines for atrial fibrillation (Circulation. 2014 Dec 2;130[23]:2071-104), aspirin can be considered as a Class IIb level of evidence C recommendation in patients with a CHA2DS2-VASc of 1. But I would just take it off of your clinical armamentarium because the best available data indicates that it doesn’t prevent strokes. I’m certainly not using it in my patients. Increasingly in my patients with a CHA2DS2-VASc of 1, I’m discussing the risks and benefits of a NOAC [novel oral anticoagulant],” Dr. Estes said.
Dr. Gersh was also critical of another common practice in stroke prevention in atrial fibrillation: concomitant use of aspirin with an oral anticoagulant.
“We use too much aspirin in patients on oral anticoagulation. Aspirin is perhaps the major cause of bleeding in patients on an oral anticoagulant. Other than in people with a drug-eluting stent, there’s no role at all for aspirin in stroke prevention,” he asserted.
He was coauthor of an analysis of 7,347 participants in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) who were on an oral anticoagulant. Fully 35% of them were also on aspirin. In a multivariate analysis, concomitant aspirin and oral anticoagulation was independently associated with a 53% increased risk of major bleeding and a 52% increase in hospitalization for bleeding, compared with atrial fibrillation patients on an oral anticoagulant alone (Circulation. 2013 Aug 13;128[7]:721-8).
Moreover, the widespread use of dual therapy in this real-world registry didn’t appear to be rational. Thirty-nine percent of those on aspirin plus an oral anticoagulant had no history of atherosclerotic disease, the presence of which would be an indication for considering aspirin. And 17% of dual therapy patients had an elevated Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) risk score of 5 or more, making dual therapy particularly risky.
This clinically important interaction between aspirin and oral anticoagulation was recently underscored in an analysis of rivaroxaban-treated patients in the ROCKET AF trial, Dr. Gersh observed. Long-term use of aspirin at entry into this pivotal randomized trial of rivaroxaban (Xarelto) versus warfarin in patients with atrial fibrillation proved to be an independent predictor of a 47% increase in the risk of gastrointestinal bleeding, compared with patients on rivaroxaban alone (J Am Coll Cardiol. 2015 Dec 1;66[21]:2271-81).
He added that there is no evidence that combining aspirin and oral anticoagulation enhances stroke prevention beyond the marked benefit achieved with oral anticoagulation alone.
Dr. Gersh reported serving on the leadership of the ORBIT-AF Registry, which was sponsored by Janssen Pharmaceuticals. Dr. Estes reported having no financial conflicts relevant to this discussion.
SNOWMASS, COLO. – It’s time to eliminate the practice of prescribing aspirin for stroke prevention in patients with atrial fibrillation and a CHA2DS2-VASc score of 1, two eminent cardiologists agreed at the Annual Cardiovascular Conference at Snowmass.
“The European guidelines have done away with aspirin for stroke prevention in atrial fibrillation. It barely made it into our current U.S. guidelines. I don’t think aspirin should be in there and I don’t think it will be there in the next guidelines. The role of aspirin will fall away,” predicted Dr. Bernard J. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
“It’s not that aspirin is less effective than the oral anticoagulants, it’s that there’s no role for it. There are no good data to support aspirin in the prevention of stroke in atrial fibrillation,” he declared.
Dr. N.A. Mark Estes III agreed the aspirin evidence is seriously flawed.
“The use of aspirin has probably been misguided, based upon a single trial which showed a profound effect and was probably just an anomaly,” according to Dr. Estes, a past president of the Heart Rhythm Society who is professor of medicine and director of the New England Cardiac Arrhythmia Center at Tufts University, Boston.
The sole positive clinical trial of aspirin versus placebo, the 25-year-old Stroke Prevention in Atrial Fibrillation (SPAF) study (Circulation. 1991 Aug;84[2]:527-39), found an unrealistically high stroke protection benefit for aspirin, a result made implausible by multiple other randomized trials showing no benefit, the cardiologists agreed.
“In our current guidelines for atrial fibrillation (Circulation. 2014 Dec 2;130[23]:2071-104), aspirin can be considered as a Class IIb level of evidence C recommendation in patients with a CHA2DS2-VASc of 1. But I would just take it off of your clinical armamentarium because the best available data indicates that it doesn’t prevent strokes. I’m certainly not using it in my patients. Increasingly in my patients with a CHA2DS2-VASc of 1, I’m discussing the risks and benefits of a NOAC [novel oral anticoagulant],” Dr. Estes said.
Dr. Gersh was also critical of another common practice in stroke prevention in atrial fibrillation: concomitant use of aspirin with an oral anticoagulant.
“We use too much aspirin in patients on oral anticoagulation. Aspirin is perhaps the major cause of bleeding in patients on an oral anticoagulant. Other than in people with a drug-eluting stent, there’s no role at all for aspirin in stroke prevention,” he asserted.
He was coauthor of an analysis of 7,347 participants in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) who were on an oral anticoagulant. Fully 35% of them were also on aspirin. In a multivariate analysis, concomitant aspirin and oral anticoagulation was independently associated with a 53% increased risk of major bleeding and a 52% increase in hospitalization for bleeding, compared with atrial fibrillation patients on an oral anticoagulant alone (Circulation. 2013 Aug 13;128[7]:721-8).
Moreover, the widespread use of dual therapy in this real-world registry didn’t appear to be rational. Thirty-nine percent of those on aspirin plus an oral anticoagulant had no history of atherosclerotic disease, the presence of which would be an indication for considering aspirin. And 17% of dual therapy patients had an elevated Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) risk score of 5 or more, making dual therapy particularly risky.
This clinically important interaction between aspirin and oral anticoagulation was recently underscored in an analysis of rivaroxaban-treated patients in the ROCKET AF trial, Dr. Gersh observed. Long-term use of aspirin at entry into this pivotal randomized trial of rivaroxaban (Xarelto) versus warfarin in patients with atrial fibrillation proved to be an independent predictor of a 47% increase in the risk of gastrointestinal bleeding, compared with patients on rivaroxaban alone (J Am Coll Cardiol. 2015 Dec 1;66[21]:2271-81).
He added that there is no evidence that combining aspirin and oral anticoagulation enhances stroke prevention beyond the marked benefit achieved with oral anticoagulation alone.
Dr. Gersh reported serving on the leadership of the ORBIT-AF Registry, which was sponsored by Janssen Pharmaceuticals. Dr. Estes reported having no financial conflicts relevant to this discussion.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS














