User login
Point/Counterpoint: Ordering molecular profiling for patients with gastrointestinal cancers: Are we ready?
Yes: Both patients and science can benefit from genomic analysis of tumors.
Use of molecular profiling of gastrointestinal tumors is ready for prime time. My contention is that both patients and science can benefit from genomic analysis of tumors.
Genomic analysis is now feasible in clinical practice because next-generation sequencing panels have been made available to us now by the enormous drop in the cost. The current cost of about $5,000-$6,000 per test for a comprehensive genomic sequencing is well within the range of feasibility, and we expect it to become less expensive.
The technology is robust, reliable, and flexible. It allows for broad sequencing of the whole expressed panel or targeted sequencing in an area of interest. And data suggest that the technology has sensitivity for DNA alterations exceeding 95% (Nat. Biotechnol. 2013;31:1023-31).
We can find actionable mutations. Our group sequenced tumors from 101 patients with colorectal cancer and found that 42% could have an approved or investigational therapy that was directed by the assay. This frequency reassuringly mirrors that of larger databases, and actionable mutations are similarly common in other gastrointestinal malignancies.
We can’t really recommend who should be tested. Testing is an option, but it is not currently standard of care, it’s not validated yet to contribute to the overall benefit of patients. So it may be better to consider whom not to test: patients who don’t need a treatment option or who are too sick to receive the treatment, and possibly those who have tumors that rarely yield positive findings.
A key question is whether there is benefit to patients. Perhaps the main benefit is that genomic analysis may make them eligible for investigational therapies. Your patients with GI cancers may be candidates for enrollment in several ongoing trials, such as FOCUS4, MODUL, and Signature, and several forthcoming trials, such as MATCH and ASSIGN.
It is not yet clear whether patients derive benefit in terms of tumor response. Response is currently anecdotal; we really don’t have the denominators to quantify the level of benefit here. And off-label use of drugs can be hard to negotiate with both companies and insurance, which may affect applicability to individual patients.
Science can benefit from performing genomic analysis for several reasons. Such analysis tests whether knowing the genomic sequence can define useful therapies. We have good responses in uncommon diseases and in subsets of common diseases, but we need to know if defining the genomic aberrations can make us better at treating the diseases in question by virtue of the drugs and what we know of the pathways. Findings will also help us determine how this approach of genomic-informed therapy selection fits into the context of other high-priority therapeutic research in this field.
Dr. Peter J. O’Dwyer is director of the developmental therapeutics program, Abramson Cancer Center, and professor of medicine, University of Pennsylvania, Philadelphia. Dr. O’Dwyer disclosed that he owns stock in TetraLogic; has a consulting or advisory role with Genentech; receives research funding from Bristol-Myers Squibb, Pfizer, Novartis, Genentech, Mirati Therapeutics, Celgene, GlaxoSmithKline, and BBI; and provides expert testimony for Eli Lilly.
No: A variety of technologic, clinical, financial, and logistic factors must be addressed first.
Genomic analysis could help meet the great need for better treatments for our patients with gastrointestinal cancers. But we are not ready for routine molecular profiling of tumors.
There are many assay platform limitations, such as variability in sensitivity and specificity, lack of rigorous analytic validation of some, and questions about selection of the genes on a platform. And tumors are heterogeneous and complex. Depending on where you biopsy from a particular lump and whether it’s a metastasis or primary, the gene expression profile may be very different. Also, gastrointestinal tumors commonly have multiple mutations that might be viewed as drivers.
We simply don’t know the drivers from the bystanders, and we don’t have the evidence linking drugs to these drivers. The best level of evidence is an FDA-approved drug-target interaction or dyad. But we don’t yet have that in most of the cases we face. Progress has been so slow in developing biomarkers because historically, they have been poorly valued.
Investigational drugs are not widely available. Clinical trial sites are often limited, and patients have to travel to them. Activation of studies with low expected accrual because they accept only very rare molecularly defined subsets of patients is costly, and getting compassionate access to a new drug in development is logistically complicated.
It isn’t practical to screen many patients to perhaps help a few when the evidence is simply not there; in a recent phase I trial, only 2% of 1,283 patients studied had a mutation identified, were matched to a treatment, and had an antitumor response (Clin. Cancer Research 2012;18:6373-83). At this time, genomic analysis requires fresh biopsies, so we are subjecting patients to extra biopsies, and there is a delay in decision making of weeks to months. Also, this requires a large infrastructure involving pathologists, informatics support, clinical geneticists, and bioethicists.
There is no mechanism to pay for drugs for off-label use. Payers are increasingly scrutinizing such use of expensive targeted agents, and costs are falling on our patients. Recommending cancer drugs with high coinsurance, high copays may simply not be ethical without strong evidence of benefit.
Cancer drug approval today is typically based on organ of origin and not genomics. The old paradigm that we are living with is histology based and requires large phase III trials to provide the level of evidence that will lead to a licensing approval. We are pressured to embrace an emerging paradigm when we make genome-based decisions using small studies looking for big effect sizes and accepting registry data.
We really don’t have a standard statistical approach to interpreting our current evidence; for example, what is enough evidence to lead us to conclude that a particular mutation should receive a particular therapy? And finally, there can be many unintended negative consequences of routine genomic analysis for our patients. We don’t want to give them false hope or subject them to the risks of needless biopsies and the financial burden of therapies that are not destined to help them. And we need to be prepared to address all mutations identified in the proper ethical fashion.
Dr. Neal J. Meropol is division chief, hematology and oncology, University Hospitals Case Medical Center and Case Western Reserve University, Cleveland; associate director for clinical research, Case Comprehensive Cancer Center and associate director for clinical programs, University Hospitals Seidman Cancer Center; and the Dr. Lester E. Coleman Jr. Endowed Chair in Cancer Research and Therapeutics at Case Western Reserve University. Dr. Meropol disclosed that he has a consulting or advisory role with BioMotiv and has a relevant patent (Methods of Therapy for Cancers Characterized by Overexpression of HER2 Receptor Protein).
Yes: Both patients and science can benefit from genomic analysis of tumors.
Use of molecular profiling of gastrointestinal tumors is ready for prime time. My contention is that both patients and science can benefit from genomic analysis of tumors.
Genomic analysis is now feasible in clinical practice because next-generation sequencing panels have been made available to us now by the enormous drop in the cost. The current cost of about $5,000-$6,000 per test for a comprehensive genomic sequencing is well within the range of feasibility, and we expect it to become less expensive.
The technology is robust, reliable, and flexible. It allows for broad sequencing of the whole expressed panel or targeted sequencing in an area of interest. And data suggest that the technology has sensitivity for DNA alterations exceeding 95% (Nat. Biotechnol. 2013;31:1023-31).
We can find actionable mutations. Our group sequenced tumors from 101 patients with colorectal cancer and found that 42% could have an approved or investigational therapy that was directed by the assay. This frequency reassuringly mirrors that of larger databases, and actionable mutations are similarly common in other gastrointestinal malignancies.
We can’t really recommend who should be tested. Testing is an option, but it is not currently standard of care, it’s not validated yet to contribute to the overall benefit of patients. So it may be better to consider whom not to test: patients who don’t need a treatment option or who are too sick to receive the treatment, and possibly those who have tumors that rarely yield positive findings.
A key question is whether there is benefit to patients. Perhaps the main benefit is that genomic analysis may make them eligible for investigational therapies. Your patients with GI cancers may be candidates for enrollment in several ongoing trials, such as FOCUS4, MODUL, and Signature, and several forthcoming trials, such as MATCH and ASSIGN.
It is not yet clear whether patients derive benefit in terms of tumor response. Response is currently anecdotal; we really don’t have the denominators to quantify the level of benefit here. And off-label use of drugs can be hard to negotiate with both companies and insurance, which may affect applicability to individual patients.
Science can benefit from performing genomic analysis for several reasons. Such analysis tests whether knowing the genomic sequence can define useful therapies. We have good responses in uncommon diseases and in subsets of common diseases, but we need to know if defining the genomic aberrations can make us better at treating the diseases in question by virtue of the drugs and what we know of the pathways. Findings will also help us determine how this approach of genomic-informed therapy selection fits into the context of other high-priority therapeutic research in this field.
Dr. Peter J. O’Dwyer is director of the developmental therapeutics program, Abramson Cancer Center, and professor of medicine, University of Pennsylvania, Philadelphia. Dr. O’Dwyer disclosed that he owns stock in TetraLogic; has a consulting or advisory role with Genentech; receives research funding from Bristol-Myers Squibb, Pfizer, Novartis, Genentech, Mirati Therapeutics, Celgene, GlaxoSmithKline, and BBI; and provides expert testimony for Eli Lilly.
No: A variety of technologic, clinical, financial, and logistic factors must be addressed first.
Genomic analysis could help meet the great need for better treatments for our patients with gastrointestinal cancers. But we are not ready for routine molecular profiling of tumors.
There are many assay platform limitations, such as variability in sensitivity and specificity, lack of rigorous analytic validation of some, and questions about selection of the genes on a platform. And tumors are heterogeneous and complex. Depending on where you biopsy from a particular lump and whether it’s a metastasis or primary, the gene expression profile may be very different. Also, gastrointestinal tumors commonly have multiple mutations that might be viewed as drivers.
We simply don’t know the drivers from the bystanders, and we don’t have the evidence linking drugs to these drivers. The best level of evidence is an FDA-approved drug-target interaction or dyad. But we don’t yet have that in most of the cases we face. Progress has been so slow in developing biomarkers because historically, they have been poorly valued.
Investigational drugs are not widely available. Clinical trial sites are often limited, and patients have to travel to them. Activation of studies with low expected accrual because they accept only very rare molecularly defined subsets of patients is costly, and getting compassionate access to a new drug in development is logistically complicated.
It isn’t practical to screen many patients to perhaps help a few when the evidence is simply not there; in a recent phase I trial, only 2% of 1,283 patients studied had a mutation identified, were matched to a treatment, and had an antitumor response (Clin. Cancer Research 2012;18:6373-83). At this time, genomic analysis requires fresh biopsies, so we are subjecting patients to extra biopsies, and there is a delay in decision making of weeks to months. Also, this requires a large infrastructure involving pathologists, informatics support, clinical geneticists, and bioethicists.
There is no mechanism to pay for drugs for off-label use. Payers are increasingly scrutinizing such use of expensive targeted agents, and costs are falling on our patients. Recommending cancer drugs with high coinsurance, high copays may simply not be ethical without strong evidence of benefit.
Cancer drug approval today is typically based on organ of origin and not genomics. The old paradigm that we are living with is histology based and requires large phase III trials to provide the level of evidence that will lead to a licensing approval. We are pressured to embrace an emerging paradigm when we make genome-based decisions using small studies looking for big effect sizes and accepting registry data.
We really don’t have a standard statistical approach to interpreting our current evidence; for example, what is enough evidence to lead us to conclude that a particular mutation should receive a particular therapy? And finally, there can be many unintended negative consequences of routine genomic analysis for our patients. We don’t want to give them false hope or subject them to the risks of needless biopsies and the financial burden of therapies that are not destined to help them. And we need to be prepared to address all mutations identified in the proper ethical fashion.
Dr. Neal J. Meropol is division chief, hematology and oncology, University Hospitals Case Medical Center and Case Western Reserve University, Cleveland; associate director for clinical research, Case Comprehensive Cancer Center and associate director for clinical programs, University Hospitals Seidman Cancer Center; and the Dr. Lester E. Coleman Jr. Endowed Chair in Cancer Research and Therapeutics at Case Western Reserve University. Dr. Meropol disclosed that he has a consulting or advisory role with BioMotiv and has a relevant patent (Methods of Therapy for Cancers Characterized by Overexpression of HER2 Receptor Protein).
Yes: Both patients and science can benefit from genomic analysis of tumors.
Use of molecular profiling of gastrointestinal tumors is ready for prime time. My contention is that both patients and science can benefit from genomic analysis of tumors.
Genomic analysis is now feasible in clinical practice because next-generation sequencing panels have been made available to us now by the enormous drop in the cost. The current cost of about $5,000-$6,000 per test for a comprehensive genomic sequencing is well within the range of feasibility, and we expect it to become less expensive.
The technology is robust, reliable, and flexible. It allows for broad sequencing of the whole expressed panel or targeted sequencing in an area of interest. And data suggest that the technology has sensitivity for DNA alterations exceeding 95% (Nat. Biotechnol. 2013;31:1023-31).
We can find actionable mutations. Our group sequenced tumors from 101 patients with colorectal cancer and found that 42% could have an approved or investigational therapy that was directed by the assay. This frequency reassuringly mirrors that of larger databases, and actionable mutations are similarly common in other gastrointestinal malignancies.
We can’t really recommend who should be tested. Testing is an option, but it is not currently standard of care, it’s not validated yet to contribute to the overall benefit of patients. So it may be better to consider whom not to test: patients who don’t need a treatment option or who are too sick to receive the treatment, and possibly those who have tumors that rarely yield positive findings.
A key question is whether there is benefit to patients. Perhaps the main benefit is that genomic analysis may make them eligible for investigational therapies. Your patients with GI cancers may be candidates for enrollment in several ongoing trials, such as FOCUS4, MODUL, and Signature, and several forthcoming trials, such as MATCH and ASSIGN.
It is not yet clear whether patients derive benefit in terms of tumor response. Response is currently anecdotal; we really don’t have the denominators to quantify the level of benefit here. And off-label use of drugs can be hard to negotiate with both companies and insurance, which may affect applicability to individual patients.
Science can benefit from performing genomic analysis for several reasons. Such analysis tests whether knowing the genomic sequence can define useful therapies. We have good responses in uncommon diseases and in subsets of common diseases, but we need to know if defining the genomic aberrations can make us better at treating the diseases in question by virtue of the drugs and what we know of the pathways. Findings will also help us determine how this approach of genomic-informed therapy selection fits into the context of other high-priority therapeutic research in this field.
Dr. Peter J. O’Dwyer is director of the developmental therapeutics program, Abramson Cancer Center, and professor of medicine, University of Pennsylvania, Philadelphia. Dr. O’Dwyer disclosed that he owns stock in TetraLogic; has a consulting or advisory role with Genentech; receives research funding from Bristol-Myers Squibb, Pfizer, Novartis, Genentech, Mirati Therapeutics, Celgene, GlaxoSmithKline, and BBI; and provides expert testimony for Eli Lilly.
No: A variety of technologic, clinical, financial, and logistic factors must be addressed first.
Genomic analysis could help meet the great need for better treatments for our patients with gastrointestinal cancers. But we are not ready for routine molecular profiling of tumors.
There are many assay platform limitations, such as variability in sensitivity and specificity, lack of rigorous analytic validation of some, and questions about selection of the genes on a platform. And tumors are heterogeneous and complex. Depending on where you biopsy from a particular lump and whether it’s a metastasis or primary, the gene expression profile may be very different. Also, gastrointestinal tumors commonly have multiple mutations that might be viewed as drivers.
We simply don’t know the drivers from the bystanders, and we don’t have the evidence linking drugs to these drivers. The best level of evidence is an FDA-approved drug-target interaction or dyad. But we don’t yet have that in most of the cases we face. Progress has been so slow in developing biomarkers because historically, they have been poorly valued.
Investigational drugs are not widely available. Clinical trial sites are often limited, and patients have to travel to them. Activation of studies with low expected accrual because they accept only very rare molecularly defined subsets of patients is costly, and getting compassionate access to a new drug in development is logistically complicated.
It isn’t practical to screen many patients to perhaps help a few when the evidence is simply not there; in a recent phase I trial, only 2% of 1,283 patients studied had a mutation identified, were matched to a treatment, and had an antitumor response (Clin. Cancer Research 2012;18:6373-83). At this time, genomic analysis requires fresh biopsies, so we are subjecting patients to extra biopsies, and there is a delay in decision making of weeks to months. Also, this requires a large infrastructure involving pathologists, informatics support, clinical geneticists, and bioethicists.
There is no mechanism to pay for drugs for off-label use. Payers are increasingly scrutinizing such use of expensive targeted agents, and costs are falling on our patients. Recommending cancer drugs with high coinsurance, high copays may simply not be ethical without strong evidence of benefit.
Cancer drug approval today is typically based on organ of origin and not genomics. The old paradigm that we are living with is histology based and requires large phase III trials to provide the level of evidence that will lead to a licensing approval. We are pressured to embrace an emerging paradigm when we make genome-based decisions using small studies looking for big effect sizes and accepting registry data.
We really don’t have a standard statistical approach to interpreting our current evidence; for example, what is enough evidence to lead us to conclude that a particular mutation should receive a particular therapy? And finally, there can be many unintended negative consequences of routine genomic analysis for our patients. We don’t want to give them false hope or subject them to the risks of needless biopsies and the financial burden of therapies that are not destined to help them. And we need to be prepared to address all mutations identified in the proper ethical fashion.
Dr. Neal J. Meropol is division chief, hematology and oncology, University Hospitals Case Medical Center and Case Western Reserve University, Cleveland; associate director for clinical research, Case Comprehensive Cancer Center and associate director for clinical programs, University Hospitals Seidman Cancer Center; and the Dr. Lester E. Coleman Jr. Endowed Chair in Cancer Research and Therapeutics at Case Western Reserve University. Dr. Meropol disclosed that he has a consulting or advisory role with BioMotiv and has a relevant patent (Methods of Therapy for Cancers Characterized by Overexpression of HER2 Receptor Protein).
THE GI CANCERS SYMPOSIUM
Jury still out on survival benefit of resecting primary in mCRC
SAN FRANCISCO – Resecting the primary tumor in patients with metastatic colon or colorectal cancer may prolong survival. But then again, it may not.
This was the overarching take-home message from a trio of cohort studies presented at the Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology. Results were reported in a poster session.
“Whereas surgery is the primary treatment of localized colorectal cancer, resection of the primary tumor in patients with incurable metastatic disease is usually recommended for palliative purposes to manage obstruction, perforation, or bleeding,” Dr. Shahid Ahmed, lead investigator of one of the studies, noted in comments provided by e-mail. “The role of surgical resection of the primary tumor in patients with newly diagnosed incurable stage IV colorectal cancer remains controversial.”
In earlier research, he and colleagues found a survival benefit of primary resection among Canadian patients whose cancer was diagnosed between 1992 and 2005 (Cancer 2014;120:683-91). But the majority did not receive systemic therapy, and those who did were often given older regimens.
In a new study aimed at testing the association in the contemporary treatment era, the researchers analyzed data from 569 patients with stage IV colorectal cancer diagnosed between 2006 and 2010 who had a median follow-up of 11 months. Overall, 55% had resection of the primary tumor.
Among the 57% of patients who received systemic therapy, 91% received FOLFIRI or FOLFOX, 65% received bevacizumab (Avastin), and 10% received cetuximab (Erbitux) or panitumumab (Vectibix), according to Dr. Ahmed, professor of medicine, University of Saskatchewan, Canada.
Results for the entire cohort showed that median overall survival was 18 months in patients who had resection of their primary versus 4 months in those who did not (multivariate hazard ratio, 0.44; P less than .001).
Among the subgroup of patients who received chemotherapy, median survival was 27 months with primary resection versus 14 months without it (P less than .0001). And among the subgroup that specifically received FOLFIRI or FOLFOX and a biologic agent, it was 35 months with primary resection and 23 months without it (P less than .001).
“Surgical resection of primary tumor improves survival of patients with stage IV colorectal cancer, independent of other prognostic variables including age, performance status, comorbid illness, and chemotherapy,” maintained Dr. Ahmed. “The current study validates our findings and supports surgical resection of primary tumor in patients with stage IV colorectal cancer who are treated with modern chemotherapy and biologics.
“A well-designed prospective randomized trial is warranted to confirm the survival benefit conferred by the primary tumor resection,” he added, noting that two such trials in Europe – SYNCHRONOUS and CAIRO4 – are underway.
“If the magnitude of survival benefit is confirmed in these future randomized studies, surgical resection of the primary tumor could potentially be a more cost-effective intervention compared with novel systemic therapy in the management of metastatic colorectal cancer,” he concluded.
In a second study, Dr. Aaron Lewis, a surgical oncology fellow at the City of Hope, Duarte, Calif., and colleagues analyzed data from patients with stage IV colon cancer in the Surveillance, Epidemiology, and End Results (SEER) database for the years 1998 through 2011. They excluded those who died within 30 days of diagnosis or had resection of metastases. Overall, 70% of the 28,068 included patients had resection of their primary.
In multivariate analyses, patients who underwent resection had half the risk of death when compared with peers who did not have this surgery (hazard ratio, 0.49), reported Dr. Lewis.
Findings were essentially the same when the analysis was repeated in a subset of matched patients: Median survival was 17 months with resection versus 9 months without it (hazard ratio, 0.48; P less than .0001). Estimated 3-year survival was 23% and 6%, respectively.
“There are limitations, factors that we couldn’t completely control for. For example, there is no chemotherapy data in the SEER database. We didn’t know the timing of surgery in relation to chemotherapy. And we didn’t know whether these patients were asymptomatic or symptomatic,” Dr. Lewis noted in an interview. “But analysis of this huge group of patients in the United States that are getting treated shows that there is a survival benefit.”
Possible reasons why surgery might prolong life in this setting are unknown but may include the effects of tumor debulking or some enhancement of the immune response, he proposed.
To definitively confirm a survival benefit, a randomized controlled trial is needed, he agreed. “This seems to be a popular question in the literature in the last couple of years, so maybe somebody will be willing to take it on.”
In a third study, a team led by Dr. Zeinab Alawadi, a surgeon and postdoctoral fellow at the University of Texas MD Anderson Cancer Center, Houston, analyzed data from 14,399 patients in the National Cancer Data Base. They had been diagnosed with stage IV colon cancer between 2003 and 2005. The researchers excluded patients who had nonelective resection or surgery at other sites, such as metastasectomy.
The primary tumor was resected in 55% of all patients studied and in 74% of patients included in a 1-year landmark analysis done to account for early deaths related to comorbidity or disease burden, reported Dr. Alawadi.
In the entire cohort, primary resection conferred a significant survival benefit after standard multivariate adjustment (hazard ratio, 0.39) that persisted after propensity score weighting to account for treatment selection bias (hazard ratio, 0.41). The benefit was also significant, but much attenuated, in an instrumental variable analysis, another method for accounting for treatment selection bias (relative mortality rate, 0.88).
In the 1-year landmark population, primary resection conferred a smaller significant survival benefit after standard multivariate adjustment (hazard ratio, 0.60) that persisted after propensity score weighting (hazard ratio, 0.59). But there was no longer a significant benefit in the instrumental variable analysis here.
“Among the entire cohort of patients with stage 4 colon cancer, primary tumor resection offered no survival benefit over systemic chemotherapy alone when the [instrumental variable] method was applied at the 1 year landmark,” the investigators write.
“Subject to selection and survivor treatment bias, standard regression analysis may overestimate the benefit of [primary tumor resection],” they concluded.
SAN FRANCISCO – Resecting the primary tumor in patients with metastatic colon or colorectal cancer may prolong survival. But then again, it may not.
This was the overarching take-home message from a trio of cohort studies presented at the Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology. Results were reported in a poster session.
“Whereas surgery is the primary treatment of localized colorectal cancer, resection of the primary tumor in patients with incurable metastatic disease is usually recommended for palliative purposes to manage obstruction, perforation, or bleeding,” Dr. Shahid Ahmed, lead investigator of one of the studies, noted in comments provided by e-mail. “The role of surgical resection of the primary tumor in patients with newly diagnosed incurable stage IV colorectal cancer remains controversial.”
In earlier research, he and colleagues found a survival benefit of primary resection among Canadian patients whose cancer was diagnosed between 1992 and 2005 (Cancer 2014;120:683-91). But the majority did not receive systemic therapy, and those who did were often given older regimens.
In a new study aimed at testing the association in the contemporary treatment era, the researchers analyzed data from 569 patients with stage IV colorectal cancer diagnosed between 2006 and 2010 who had a median follow-up of 11 months. Overall, 55% had resection of the primary tumor.
Among the 57% of patients who received systemic therapy, 91% received FOLFIRI or FOLFOX, 65% received bevacizumab (Avastin), and 10% received cetuximab (Erbitux) or panitumumab (Vectibix), according to Dr. Ahmed, professor of medicine, University of Saskatchewan, Canada.
Results for the entire cohort showed that median overall survival was 18 months in patients who had resection of their primary versus 4 months in those who did not (multivariate hazard ratio, 0.44; P less than .001).
Among the subgroup of patients who received chemotherapy, median survival was 27 months with primary resection versus 14 months without it (P less than .0001). And among the subgroup that specifically received FOLFIRI or FOLFOX and a biologic agent, it was 35 months with primary resection and 23 months without it (P less than .001).
“Surgical resection of primary tumor improves survival of patients with stage IV colorectal cancer, independent of other prognostic variables including age, performance status, comorbid illness, and chemotherapy,” maintained Dr. Ahmed. “The current study validates our findings and supports surgical resection of primary tumor in patients with stage IV colorectal cancer who are treated with modern chemotherapy and biologics.
“A well-designed prospective randomized trial is warranted to confirm the survival benefit conferred by the primary tumor resection,” he added, noting that two such trials in Europe – SYNCHRONOUS and CAIRO4 – are underway.
“If the magnitude of survival benefit is confirmed in these future randomized studies, surgical resection of the primary tumor could potentially be a more cost-effective intervention compared with novel systemic therapy in the management of metastatic colorectal cancer,” he concluded.
In a second study, Dr. Aaron Lewis, a surgical oncology fellow at the City of Hope, Duarte, Calif., and colleagues analyzed data from patients with stage IV colon cancer in the Surveillance, Epidemiology, and End Results (SEER) database for the years 1998 through 2011. They excluded those who died within 30 days of diagnosis or had resection of metastases. Overall, 70% of the 28,068 included patients had resection of their primary.
In multivariate analyses, patients who underwent resection had half the risk of death when compared with peers who did not have this surgery (hazard ratio, 0.49), reported Dr. Lewis.
Findings were essentially the same when the analysis was repeated in a subset of matched patients: Median survival was 17 months with resection versus 9 months without it (hazard ratio, 0.48; P less than .0001). Estimated 3-year survival was 23% and 6%, respectively.
“There are limitations, factors that we couldn’t completely control for. For example, there is no chemotherapy data in the SEER database. We didn’t know the timing of surgery in relation to chemotherapy. And we didn’t know whether these patients were asymptomatic or symptomatic,” Dr. Lewis noted in an interview. “But analysis of this huge group of patients in the United States that are getting treated shows that there is a survival benefit.”
Possible reasons why surgery might prolong life in this setting are unknown but may include the effects of tumor debulking or some enhancement of the immune response, he proposed.
To definitively confirm a survival benefit, a randomized controlled trial is needed, he agreed. “This seems to be a popular question in the literature in the last couple of years, so maybe somebody will be willing to take it on.”
In a third study, a team led by Dr. Zeinab Alawadi, a surgeon and postdoctoral fellow at the University of Texas MD Anderson Cancer Center, Houston, analyzed data from 14,399 patients in the National Cancer Data Base. They had been diagnosed with stage IV colon cancer between 2003 and 2005. The researchers excluded patients who had nonelective resection or surgery at other sites, such as metastasectomy.
The primary tumor was resected in 55% of all patients studied and in 74% of patients included in a 1-year landmark analysis done to account for early deaths related to comorbidity or disease burden, reported Dr. Alawadi.
In the entire cohort, primary resection conferred a significant survival benefit after standard multivariate adjustment (hazard ratio, 0.39) that persisted after propensity score weighting to account for treatment selection bias (hazard ratio, 0.41). The benefit was also significant, but much attenuated, in an instrumental variable analysis, another method for accounting for treatment selection bias (relative mortality rate, 0.88).
In the 1-year landmark population, primary resection conferred a smaller significant survival benefit after standard multivariate adjustment (hazard ratio, 0.60) that persisted after propensity score weighting (hazard ratio, 0.59). But there was no longer a significant benefit in the instrumental variable analysis here.
“Among the entire cohort of patients with stage 4 colon cancer, primary tumor resection offered no survival benefit over systemic chemotherapy alone when the [instrumental variable] method was applied at the 1 year landmark,” the investigators write.
“Subject to selection and survivor treatment bias, standard regression analysis may overestimate the benefit of [primary tumor resection],” they concluded.
SAN FRANCISCO – Resecting the primary tumor in patients with metastatic colon or colorectal cancer may prolong survival. But then again, it may not.
This was the overarching take-home message from a trio of cohort studies presented at the Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology. Results were reported in a poster session.
“Whereas surgery is the primary treatment of localized colorectal cancer, resection of the primary tumor in patients with incurable metastatic disease is usually recommended for palliative purposes to manage obstruction, perforation, or bleeding,” Dr. Shahid Ahmed, lead investigator of one of the studies, noted in comments provided by e-mail. “The role of surgical resection of the primary tumor in patients with newly diagnosed incurable stage IV colorectal cancer remains controversial.”
In earlier research, he and colleagues found a survival benefit of primary resection among Canadian patients whose cancer was diagnosed between 1992 and 2005 (Cancer 2014;120:683-91). But the majority did not receive systemic therapy, and those who did were often given older regimens.
In a new study aimed at testing the association in the contemporary treatment era, the researchers analyzed data from 569 patients with stage IV colorectal cancer diagnosed between 2006 and 2010 who had a median follow-up of 11 months. Overall, 55% had resection of the primary tumor.
Among the 57% of patients who received systemic therapy, 91% received FOLFIRI or FOLFOX, 65% received bevacizumab (Avastin), and 10% received cetuximab (Erbitux) or panitumumab (Vectibix), according to Dr. Ahmed, professor of medicine, University of Saskatchewan, Canada.
Results for the entire cohort showed that median overall survival was 18 months in patients who had resection of their primary versus 4 months in those who did not (multivariate hazard ratio, 0.44; P less than .001).
Among the subgroup of patients who received chemotherapy, median survival was 27 months with primary resection versus 14 months without it (P less than .0001). And among the subgroup that specifically received FOLFIRI or FOLFOX and a biologic agent, it was 35 months with primary resection and 23 months without it (P less than .001).
“Surgical resection of primary tumor improves survival of patients with stage IV colorectal cancer, independent of other prognostic variables including age, performance status, comorbid illness, and chemotherapy,” maintained Dr. Ahmed. “The current study validates our findings and supports surgical resection of primary tumor in patients with stage IV colorectal cancer who are treated with modern chemotherapy and biologics.
“A well-designed prospective randomized trial is warranted to confirm the survival benefit conferred by the primary tumor resection,” he added, noting that two such trials in Europe – SYNCHRONOUS and CAIRO4 – are underway.
“If the magnitude of survival benefit is confirmed in these future randomized studies, surgical resection of the primary tumor could potentially be a more cost-effective intervention compared with novel systemic therapy in the management of metastatic colorectal cancer,” he concluded.
In a second study, Dr. Aaron Lewis, a surgical oncology fellow at the City of Hope, Duarte, Calif., and colleagues analyzed data from patients with stage IV colon cancer in the Surveillance, Epidemiology, and End Results (SEER) database for the years 1998 through 2011. They excluded those who died within 30 days of diagnosis or had resection of metastases. Overall, 70% of the 28,068 included patients had resection of their primary.
In multivariate analyses, patients who underwent resection had half the risk of death when compared with peers who did not have this surgery (hazard ratio, 0.49), reported Dr. Lewis.
Findings were essentially the same when the analysis was repeated in a subset of matched patients: Median survival was 17 months with resection versus 9 months without it (hazard ratio, 0.48; P less than .0001). Estimated 3-year survival was 23% and 6%, respectively.
“There are limitations, factors that we couldn’t completely control for. For example, there is no chemotherapy data in the SEER database. We didn’t know the timing of surgery in relation to chemotherapy. And we didn’t know whether these patients were asymptomatic or symptomatic,” Dr. Lewis noted in an interview. “But analysis of this huge group of patients in the United States that are getting treated shows that there is a survival benefit.”
Possible reasons why surgery might prolong life in this setting are unknown but may include the effects of tumor debulking or some enhancement of the immune response, he proposed.
To definitively confirm a survival benefit, a randomized controlled trial is needed, he agreed. “This seems to be a popular question in the literature in the last couple of years, so maybe somebody will be willing to take it on.”
In a third study, a team led by Dr. Zeinab Alawadi, a surgeon and postdoctoral fellow at the University of Texas MD Anderson Cancer Center, Houston, analyzed data from 14,399 patients in the National Cancer Data Base. They had been diagnosed with stage IV colon cancer between 2003 and 2005. The researchers excluded patients who had nonelective resection or surgery at other sites, such as metastasectomy.
The primary tumor was resected in 55% of all patients studied and in 74% of patients included in a 1-year landmark analysis done to account for early deaths related to comorbidity or disease burden, reported Dr. Alawadi.
In the entire cohort, primary resection conferred a significant survival benefit after standard multivariate adjustment (hazard ratio, 0.39) that persisted after propensity score weighting to account for treatment selection bias (hazard ratio, 0.41). The benefit was also significant, but much attenuated, in an instrumental variable analysis, another method for accounting for treatment selection bias (relative mortality rate, 0.88).
In the 1-year landmark population, primary resection conferred a smaller significant survival benefit after standard multivariate adjustment (hazard ratio, 0.60) that persisted after propensity score weighting (hazard ratio, 0.59). But there was no longer a significant benefit in the instrumental variable analysis here.
“Among the entire cohort of patients with stage 4 colon cancer, primary tumor resection offered no survival benefit over systemic chemotherapy alone when the [instrumental variable] method was applied at the 1 year landmark,” the investigators write.
“Subject to selection and survivor treatment bias, standard regression analysis may overestimate the benefit of [primary tumor resection],” they concluded.
AT THE GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point: Data are mixed regarding an overall survival benefit of resecting the primary tumor.
Major finding: Two studies found a halving of the risk of death, whereas one study found lesser or even no benefit.
Data source: A trio of cohort studies in 569 patients, 28,068 patients, and 14,399 patients with metastatic colon or colorectal cancer.
Disclosures: Dr. Ahmed, Dr. Lewis, and Dr. Alawadi disclosed that they had no conflicts of interest.
New immunomodulator shows promise in pancreatic cancer
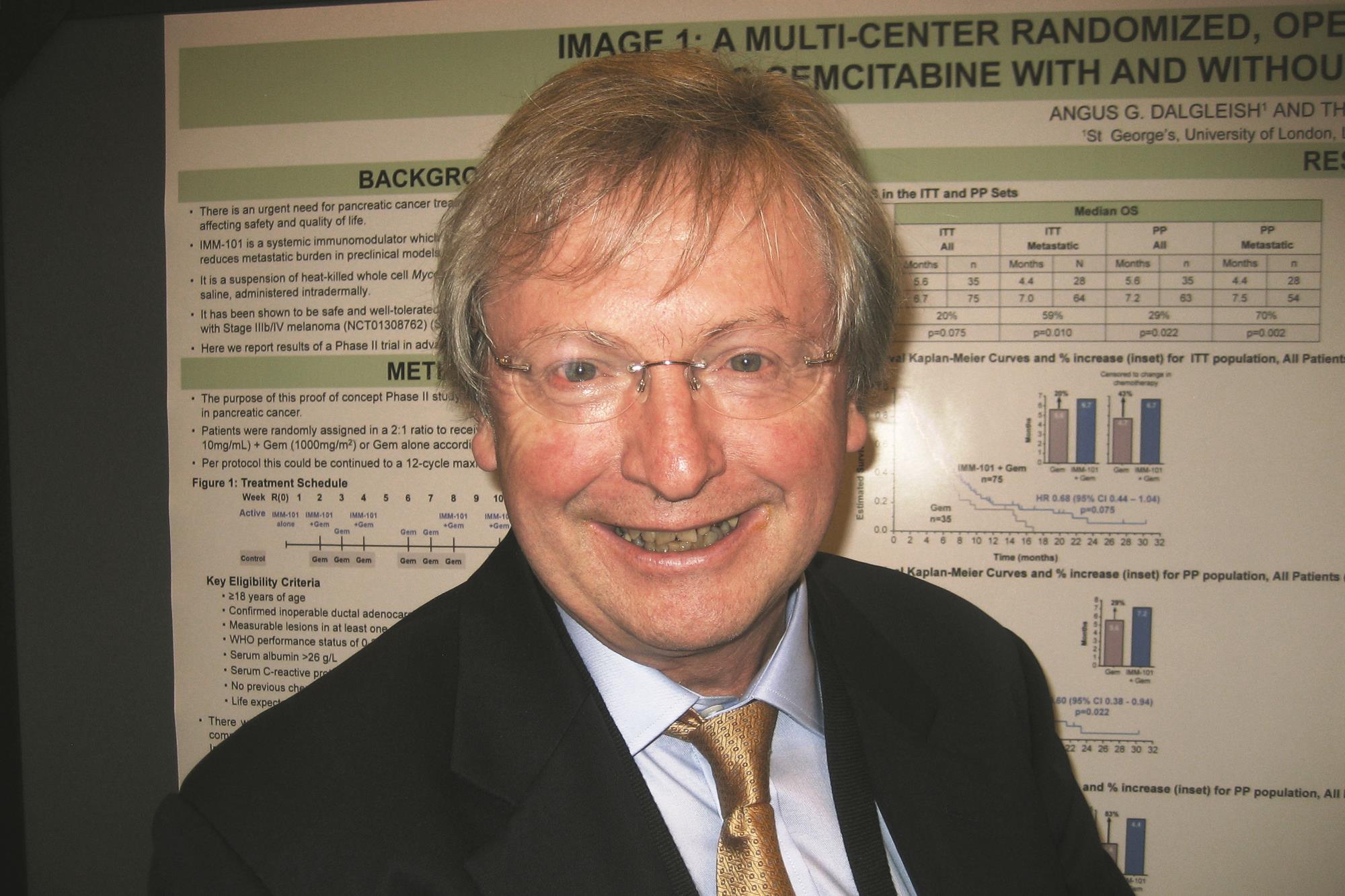
SAN FRANCISCO – An investigational immunomodulator, IMM-101, improves outcomes in patients who have advanced pancreatic cancer when combined with chemotherapy, with minimal increase in toxicity, suggest data from an open-label, phase II trial conducted in Europe.
Results reported in a poster session at the annual Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology showed that median progression-free survival was about 1 month longer for patients given IMM-101 plus gemcitabine than for patients given gemcitabine alone; the gain exceeded 2 months among the subgroup having metastatic disease. The combination was associated with slightly higher rates of certain toxicities that seemed to stem from being on gemcitabine for longer.
“I think IMM-101 brings to the field something that’s missing,” lead investigator Dr. Angus G. Dalgleish, a professor and medical oncologist at St. George’s University of London, commented in an interview. “Everybody has been doing doublets, triplets, etcetera. All they do is guarantee increased toxicity and, in some cases, misery to the patient, whereas this allows you to start treatment and stabilize [the cancer].”
“This shows that actually boosting the immune response in a nonspecific manner before we do anything else may well become the gold standard when you add in any other treatment,” he added. “And I think it’s a non–chemo specific thing and a non–tumor specific thing, because we have done all the preliminary work in melanoma. We’ve also got very encouraging data in prostate and are starting prostate studies this year. And I would like to see it go into a whole host of ones where chemo needs a nonspecific boost, like ovarian [cancer].”
Of note, the trial opened just before FOLFIRINOX (irinotecan plus 5-fluorouracil plus leucovorin plus oxaliplatin) became available to patients in Europe, and many of the more fit eligible patients opted for that therapy instead. “I think this makes the survival benefit even more remarkable,” Dr. Dalgleish stated.
IMM-101 is a heat-killed whole-cell vaccine that is given intradermally and induces protective CD8 T-cell responses. “Although we had good data on this agent for melanoma, we noticed that it sort of boosted the immune system in all cancers [in which] we could show you get immune suppression,” he noted. Additionally, preclinical data hinted at synergy with gemcitabine.
The trial, known as IMAGE 1 (Immune Modulation And Gemcitabine Evaluation 1) and sponsored by manufacturer Immodulon Therapeutics, enrolled 110 patients with stage III or IV pancreatic cancer who had not previously received any chemotherapy. The patients were randomized 2:1 to receive gemcitabine (Gemzar) either with or without IMM-101.
Intention-to-treat analyses showed that median overall survival, the primary endpoint, was 6.7 months with IMM-101 and 5.6 months without it in the entire trial population (hazard ratio, 0.68; P = .075). The difference was significant among the subset of patients having metastatic disease (7.0 vs. 4.4 months; HR, 0.54; P = .010).
“You are getting a quick effect here, 2-4 months,” Dr. Dalgleish pointed out. “I’ve done several vaccine studies where we don’t even get the split [in survival curves] for 12 months. ... So that’s absolutely staggering that we get the split so early on.”
“In the metastatic group, the survival advantage is really quite dramatic,” he added. “And unlike many other studies that we have been involved in, you are getting this tail. ... these patients are still continuing to survive.”
One patient given the combination experienced progression and was then given nab-paclitaxel (Abraxane), and had a complete response to that agent, according to Dr. Dalgleish. He speculated that it may have been due to the immune priming by IMM-101.
In per protocol analyses, the survival benefit was significant in both the entire population and the patients with metastatic disease.
Patients in the IMM-101 group also had superior progression-free survival, seen in the entire trial population (4.4 vs. 2.4 months; HR, 0.51; P = .003) and in the subgroup with metastases (4.4 vs. 2.3 months; HR, 0.40; P less than .001).
Apart from injection site reactions in some patients, IMM-101 was well tolerated, according to Dr. Dalgleish.
“When you add gemcitabine plus ‘x’ – Iressa [gefitinib], capecitabine [Xeloda], Abraxane, etcetera – you get a benefit but at great cost,” he commented. In this trial, toxicity “is slightly worse in the combination arm, but the side effects are all those associated with the chemotherapy and the disease – nothing to do with the immune modulator, which we have given to hundreds of patients before. And that would appear to reflect the fact that those patients have been on gemcitabine for longer than the ones on gemcitabine alone.”
Quality of life data are still being analyzed, according to Dr. Dalgleish. “But ... from my personal experience, the patients do feel better on the vaccine as [it has] a kind of overall boosting effect.”
The investigators plan to take IMM-101 forward in clinical trials, he said. “But I think in the States, they might want to do that with Abraxane first line.” In the United Kingdom, the National Institute for Health and Care Excellence (NICE) recently declined Abraxane, meaning cost won’t be reimbursed if it is used, Dr. Dalgleish said. “So we may well be able to do the same study in Europe in order to get approval, although personally, I’d be very keen for conditional approval on this [trial] in the U.K. because it has a clear benefit without any toxicity. And there is a system where you can get a phase 4 approval and have postapproval monitoring.”
Dr. Dalgleish disclosed that he has a consulting or advisory role with and receives research funding from Immodulon Therapeutics.
SAN FRANCISCO – An investigational immunomodulator, IMM-101, improves outcomes in patients who have advanced pancreatic cancer when combined with chemotherapy, with minimal increase in toxicity, suggest data from an open-label, phase II trial conducted in Europe.
Results reported in a poster session at the annual Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology showed that median progression-free survival was about 1 month longer for patients given IMM-101 plus gemcitabine than for patients given gemcitabine alone; the gain exceeded 2 months among the subgroup having metastatic disease. The combination was associated with slightly higher rates of certain toxicities that seemed to stem from being on gemcitabine for longer.
“I think IMM-101 brings to the field something that’s missing,” lead investigator Dr. Angus G. Dalgleish, a professor and medical oncologist at St. George’s University of London, commented in an interview. “Everybody has been doing doublets, triplets, etcetera. All they do is guarantee increased toxicity and, in some cases, misery to the patient, whereas this allows you to start treatment and stabilize [the cancer].”
“This shows that actually boosting the immune response in a nonspecific manner before we do anything else may well become the gold standard when you add in any other treatment,” he added. “And I think it’s a non–chemo specific thing and a non–tumor specific thing, because we have done all the preliminary work in melanoma. We’ve also got very encouraging data in prostate and are starting prostate studies this year. And I would like to see it go into a whole host of ones where chemo needs a nonspecific boost, like ovarian [cancer].”
Of note, the trial opened just before FOLFIRINOX (irinotecan plus 5-fluorouracil plus leucovorin plus oxaliplatin) became available to patients in Europe, and many of the more fit eligible patients opted for that therapy instead. “I think this makes the survival benefit even more remarkable,” Dr. Dalgleish stated.
IMM-101 is a heat-killed whole-cell vaccine that is given intradermally and induces protective CD8 T-cell responses. “Although we had good data on this agent for melanoma, we noticed that it sort of boosted the immune system in all cancers [in which] we could show you get immune suppression,” he noted. Additionally, preclinical data hinted at synergy with gemcitabine.
The trial, known as IMAGE 1 (Immune Modulation And Gemcitabine Evaluation 1) and sponsored by manufacturer Immodulon Therapeutics, enrolled 110 patients with stage III or IV pancreatic cancer who had not previously received any chemotherapy. The patients were randomized 2:1 to receive gemcitabine (Gemzar) either with or without IMM-101.
Intention-to-treat analyses showed that median overall survival, the primary endpoint, was 6.7 months with IMM-101 and 5.6 months without it in the entire trial population (hazard ratio, 0.68; P = .075). The difference was significant among the subset of patients having metastatic disease (7.0 vs. 4.4 months; HR, 0.54; P = .010).
“You are getting a quick effect here, 2-4 months,” Dr. Dalgleish pointed out. “I’ve done several vaccine studies where we don’t even get the split [in survival curves] for 12 months. ... So that’s absolutely staggering that we get the split so early on.”
“In the metastatic group, the survival advantage is really quite dramatic,” he added. “And unlike many other studies that we have been involved in, you are getting this tail. ... these patients are still continuing to survive.”
One patient given the combination experienced progression and was then given nab-paclitaxel (Abraxane), and had a complete response to that agent, according to Dr. Dalgleish. He speculated that it may have been due to the immune priming by IMM-101.
In per protocol analyses, the survival benefit was significant in both the entire population and the patients with metastatic disease.
Patients in the IMM-101 group also had superior progression-free survival, seen in the entire trial population (4.4 vs. 2.4 months; HR, 0.51; P = .003) and in the subgroup with metastases (4.4 vs. 2.3 months; HR, 0.40; P less than .001).
Apart from injection site reactions in some patients, IMM-101 was well tolerated, according to Dr. Dalgleish.
“When you add gemcitabine plus ‘x’ – Iressa [gefitinib], capecitabine [Xeloda], Abraxane, etcetera – you get a benefit but at great cost,” he commented. In this trial, toxicity “is slightly worse in the combination arm, but the side effects are all those associated with the chemotherapy and the disease – nothing to do with the immune modulator, which we have given to hundreds of patients before. And that would appear to reflect the fact that those patients have been on gemcitabine for longer than the ones on gemcitabine alone.”
Quality of life data are still being analyzed, according to Dr. Dalgleish. “But ... from my personal experience, the patients do feel better on the vaccine as [it has] a kind of overall boosting effect.”
The investigators plan to take IMM-101 forward in clinical trials, he said. “But I think in the States, they might want to do that with Abraxane first line.” In the United Kingdom, the National Institute for Health and Care Excellence (NICE) recently declined Abraxane, meaning cost won’t be reimbursed if it is used, Dr. Dalgleish said. “So we may well be able to do the same study in Europe in order to get approval, although personally, I’d be very keen for conditional approval on this [trial] in the U.K. because it has a clear benefit without any toxicity. And there is a system where you can get a phase 4 approval and have postapproval monitoring.”
Dr. Dalgleish disclosed that he has a consulting or advisory role with and receives research funding from Immodulon Therapeutics.
SAN FRANCISCO – An investigational immunomodulator, IMM-101, improves outcomes in patients who have advanced pancreatic cancer when combined with chemotherapy, with minimal increase in toxicity, suggest data from an open-label, phase II trial conducted in Europe.
Results reported in a poster session at the annual Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology showed that median progression-free survival was about 1 month longer for patients given IMM-101 plus gemcitabine than for patients given gemcitabine alone; the gain exceeded 2 months among the subgroup having metastatic disease. The combination was associated with slightly higher rates of certain toxicities that seemed to stem from being on gemcitabine for longer.
“I think IMM-101 brings to the field something that’s missing,” lead investigator Dr. Angus G. Dalgleish, a professor and medical oncologist at St. George’s University of London, commented in an interview. “Everybody has been doing doublets, triplets, etcetera. All they do is guarantee increased toxicity and, in some cases, misery to the patient, whereas this allows you to start treatment and stabilize [the cancer].”
“This shows that actually boosting the immune response in a nonspecific manner before we do anything else may well become the gold standard when you add in any other treatment,” he added. “And I think it’s a non–chemo specific thing and a non–tumor specific thing, because we have done all the preliminary work in melanoma. We’ve also got very encouraging data in prostate and are starting prostate studies this year. And I would like to see it go into a whole host of ones where chemo needs a nonspecific boost, like ovarian [cancer].”
Of note, the trial opened just before FOLFIRINOX (irinotecan plus 5-fluorouracil plus leucovorin plus oxaliplatin) became available to patients in Europe, and many of the more fit eligible patients opted for that therapy instead. “I think this makes the survival benefit even more remarkable,” Dr. Dalgleish stated.
IMM-101 is a heat-killed whole-cell vaccine that is given intradermally and induces protective CD8 T-cell responses. “Although we had good data on this agent for melanoma, we noticed that it sort of boosted the immune system in all cancers [in which] we could show you get immune suppression,” he noted. Additionally, preclinical data hinted at synergy with gemcitabine.
The trial, known as IMAGE 1 (Immune Modulation And Gemcitabine Evaluation 1) and sponsored by manufacturer Immodulon Therapeutics, enrolled 110 patients with stage III or IV pancreatic cancer who had not previously received any chemotherapy. The patients were randomized 2:1 to receive gemcitabine (Gemzar) either with or without IMM-101.
Intention-to-treat analyses showed that median overall survival, the primary endpoint, was 6.7 months with IMM-101 and 5.6 months without it in the entire trial population (hazard ratio, 0.68; P = .075). The difference was significant among the subset of patients having metastatic disease (7.0 vs. 4.4 months; HR, 0.54; P = .010).
“You are getting a quick effect here, 2-4 months,” Dr. Dalgleish pointed out. “I’ve done several vaccine studies where we don’t even get the split [in survival curves] for 12 months. ... So that’s absolutely staggering that we get the split so early on.”
“In the metastatic group, the survival advantage is really quite dramatic,” he added. “And unlike many other studies that we have been involved in, you are getting this tail. ... these patients are still continuing to survive.”
One patient given the combination experienced progression and was then given nab-paclitaxel (Abraxane), and had a complete response to that agent, according to Dr. Dalgleish. He speculated that it may have been due to the immune priming by IMM-101.
In per protocol analyses, the survival benefit was significant in both the entire population and the patients with metastatic disease.
Patients in the IMM-101 group also had superior progression-free survival, seen in the entire trial population (4.4 vs. 2.4 months; HR, 0.51; P = .003) and in the subgroup with metastases (4.4 vs. 2.3 months; HR, 0.40; P less than .001).
Apart from injection site reactions in some patients, IMM-101 was well tolerated, according to Dr. Dalgleish.
“When you add gemcitabine plus ‘x’ – Iressa [gefitinib], capecitabine [Xeloda], Abraxane, etcetera – you get a benefit but at great cost,” he commented. In this trial, toxicity “is slightly worse in the combination arm, but the side effects are all those associated with the chemotherapy and the disease – nothing to do with the immune modulator, which we have given to hundreds of patients before. And that would appear to reflect the fact that those patients have been on gemcitabine for longer than the ones on gemcitabine alone.”
Quality of life data are still being analyzed, according to Dr. Dalgleish. “But ... from my personal experience, the patients do feel better on the vaccine as [it has] a kind of overall boosting effect.”
The investigators plan to take IMM-101 forward in clinical trials, he said. “But I think in the States, they might want to do that with Abraxane first line.” In the United Kingdom, the National Institute for Health and Care Excellence (NICE) recently declined Abraxane, meaning cost won’t be reimbursed if it is used, Dr. Dalgleish said. “So we may well be able to do the same study in Europe in order to get approval, although personally, I’d be very keen for conditional approval on this [trial] in the U.K. because it has a clear benefit without any toxicity. And there is a system where you can get a phase 4 approval and have postapproval monitoring.”
Dr. Dalgleish disclosed that he has a consulting or advisory role with and receives research funding from Immodulon Therapeutics.
AT THE GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point: IMM-101 appears safe and efficacious when added to gemcitabine as first-line therapy.
Major finding: Median overall survival was 6.7 months with IMM-101 and 5.6 months without it.
Data source: A randomized, open-label, phase II trial among 110 patients with advanced pancreatic cancer.
Disclosures: Dr. Dalgleish disclosed that he has a consulting or advisory role with and receives research funding from Immodulon Therapeutics. The trial was sponsored by Immodulon Therapeutics.
Older age doesn’t preclude use of ramucirumab for gastric cancers
SAN FRANCISCO – The efficacy and safety of the antiangiogenic antibody ramucirumab in older patients with advanced esophagogastric cancers are similar to those seen in younger peers, a subgroup analysis of the phase III RAINBOW trial showed.
The trial tested ramucirumab (Cyramza) against placebo, each combined with paclitaxel, as second-line therapy among 665 patients with locally advanced or metastatic gastric and gastroesophageal junction adenocarcinoma. The antibody targets vascular endothelial growth factor receptor–2 (VEGFR-2) and is approved by the U.S. Food and Drug Administration for the treatment of esophagogastric cancers, as well as non–small cell lung cancer.
Overall, 37% of the patients studied were aged 65 years or older, according to data reported in a poster session at the at the annual Gastrointestinal Cancers Symposium sponsored by the American Society of Clinical Oncology.
The results showed that with the addition of ramucirumab versus placebo, these older patients had a 2.0-month overall survival gain that was similar to the 2.2-month survival gain seen in younger patients. The safety profile with added ramucirumab was much the same as well, except that grade 3 or higher neutropenia was more common among the older group.
“We saw very similar results” by age group, lead investigator Dr. Kei Muro, an oncologist at the Aichi Cancer Center Hospital in Nagoya, Japan, said in an interview. “Neutropenia is relatively higher in [the older group] and careful management is needed, but very similar efficacy and manageable toxicity were shown in this trial.”
The older patients in the trial, which was sponsored by manufacturer Eli Lilly, were fairly healthy other than having cancer, meeting eligibility criteria such as good performance status and adequate organ function, he said. Thus, they may not necessarily reflect patients seen in routine care.
“Ramucirumab for gastric cancer is feasible and effective in the second-line setting of advanced gastric cancer,” Dr. Muro concluded. “Ramucirumab use in all ages in the second-line setting for gastric cancer can be recommended.”
Intention-to-treat analyses showed that relative to placebo, ramucirumab prolonged median overall survival similarly among patients aged 65 years and older (10.7 vs. 8.7 months; hazard ratio, 0.86; P = .34) and among younger patients (9.3 vs. 7.1 months; HR, 0.75; P = .01).
Findings were much the same for median progression-free survival, with a benefit seen in the older age group (4.6 vs. 2.9 months; HR, 0.67; P = .0066) roughly matching that in the younger one (4.3 vs. 2.8 months; HR, 0.57; P less than .0001), reported Dr. Muro, who disclosed that he receives research funding from Eli Lilly, as well as Bristol-Myers Squibb and Merck.
Relative to placebo, ramucirumab was associated with a higher rate of grade 3 or worse neutropenia in both age groups. Among patients aged 65 years or older, the rate was 49% with the drug versus 24% with placebo; among younger patients, it was 36% and 16%, respectively.
In the ramucirumab group, the rates of most grade 3 or worse adverse events possibly related to VEGF inhibition, such as bleeding and proteinuria, were generally similar for the two age groups. Hypertension of these grades was seen in 19% of the older group, compared with 12% of the younger one.
SAN FRANCISCO – The efficacy and safety of the antiangiogenic antibody ramucirumab in older patients with advanced esophagogastric cancers are similar to those seen in younger peers, a subgroup analysis of the phase III RAINBOW trial showed.
The trial tested ramucirumab (Cyramza) against placebo, each combined with paclitaxel, as second-line therapy among 665 patients with locally advanced or metastatic gastric and gastroesophageal junction adenocarcinoma. The antibody targets vascular endothelial growth factor receptor–2 (VEGFR-2) and is approved by the U.S. Food and Drug Administration for the treatment of esophagogastric cancers, as well as non–small cell lung cancer.
Overall, 37% of the patients studied were aged 65 years or older, according to data reported in a poster session at the at the annual Gastrointestinal Cancers Symposium sponsored by the American Society of Clinical Oncology.
The results showed that with the addition of ramucirumab versus placebo, these older patients had a 2.0-month overall survival gain that was similar to the 2.2-month survival gain seen in younger patients. The safety profile with added ramucirumab was much the same as well, except that grade 3 or higher neutropenia was more common among the older group.
“We saw very similar results” by age group, lead investigator Dr. Kei Muro, an oncologist at the Aichi Cancer Center Hospital in Nagoya, Japan, said in an interview. “Neutropenia is relatively higher in [the older group] and careful management is needed, but very similar efficacy and manageable toxicity were shown in this trial.”
The older patients in the trial, which was sponsored by manufacturer Eli Lilly, were fairly healthy other than having cancer, meeting eligibility criteria such as good performance status and adequate organ function, he said. Thus, they may not necessarily reflect patients seen in routine care.
“Ramucirumab for gastric cancer is feasible and effective in the second-line setting of advanced gastric cancer,” Dr. Muro concluded. “Ramucirumab use in all ages in the second-line setting for gastric cancer can be recommended.”
Intention-to-treat analyses showed that relative to placebo, ramucirumab prolonged median overall survival similarly among patients aged 65 years and older (10.7 vs. 8.7 months; hazard ratio, 0.86; P = .34) and among younger patients (9.3 vs. 7.1 months; HR, 0.75; P = .01).
Findings were much the same for median progression-free survival, with a benefit seen in the older age group (4.6 vs. 2.9 months; HR, 0.67; P = .0066) roughly matching that in the younger one (4.3 vs. 2.8 months; HR, 0.57; P less than .0001), reported Dr. Muro, who disclosed that he receives research funding from Eli Lilly, as well as Bristol-Myers Squibb and Merck.
Relative to placebo, ramucirumab was associated with a higher rate of grade 3 or worse neutropenia in both age groups. Among patients aged 65 years or older, the rate was 49% with the drug versus 24% with placebo; among younger patients, it was 36% and 16%, respectively.
In the ramucirumab group, the rates of most grade 3 or worse adverse events possibly related to VEGF inhibition, such as bleeding and proteinuria, were generally similar for the two age groups. Hypertension of these grades was seen in 19% of the older group, compared with 12% of the younger one.
SAN FRANCISCO – The efficacy and safety of the antiangiogenic antibody ramucirumab in older patients with advanced esophagogastric cancers are similar to those seen in younger peers, a subgroup analysis of the phase III RAINBOW trial showed.
The trial tested ramucirumab (Cyramza) against placebo, each combined with paclitaxel, as second-line therapy among 665 patients with locally advanced or metastatic gastric and gastroesophageal junction adenocarcinoma. The antibody targets vascular endothelial growth factor receptor–2 (VEGFR-2) and is approved by the U.S. Food and Drug Administration for the treatment of esophagogastric cancers, as well as non–small cell lung cancer.
Overall, 37% of the patients studied were aged 65 years or older, according to data reported in a poster session at the at the annual Gastrointestinal Cancers Symposium sponsored by the American Society of Clinical Oncology.
The results showed that with the addition of ramucirumab versus placebo, these older patients had a 2.0-month overall survival gain that was similar to the 2.2-month survival gain seen in younger patients. The safety profile with added ramucirumab was much the same as well, except that grade 3 or higher neutropenia was more common among the older group.
“We saw very similar results” by age group, lead investigator Dr. Kei Muro, an oncologist at the Aichi Cancer Center Hospital in Nagoya, Japan, said in an interview. “Neutropenia is relatively higher in [the older group] and careful management is needed, but very similar efficacy and manageable toxicity were shown in this trial.”
The older patients in the trial, which was sponsored by manufacturer Eli Lilly, were fairly healthy other than having cancer, meeting eligibility criteria such as good performance status and adequate organ function, he said. Thus, they may not necessarily reflect patients seen in routine care.
“Ramucirumab for gastric cancer is feasible and effective in the second-line setting of advanced gastric cancer,” Dr. Muro concluded. “Ramucirumab use in all ages in the second-line setting for gastric cancer can be recommended.”
Intention-to-treat analyses showed that relative to placebo, ramucirumab prolonged median overall survival similarly among patients aged 65 years and older (10.7 vs. 8.7 months; hazard ratio, 0.86; P = .34) and among younger patients (9.3 vs. 7.1 months; HR, 0.75; P = .01).
Findings were much the same for median progression-free survival, with a benefit seen in the older age group (4.6 vs. 2.9 months; HR, 0.67; P = .0066) roughly matching that in the younger one (4.3 vs. 2.8 months; HR, 0.57; P less than .0001), reported Dr. Muro, who disclosed that he receives research funding from Eli Lilly, as well as Bristol-Myers Squibb and Merck.
Relative to placebo, ramucirumab was associated with a higher rate of grade 3 or worse neutropenia in both age groups. Among patients aged 65 years or older, the rate was 49% with the drug versus 24% with placebo; among younger patients, it was 36% and 16%, respectively.
In the ramucirumab group, the rates of most grade 3 or worse adverse events possibly related to VEGF inhibition, such as bleeding and proteinuria, were generally similar for the two age groups. Hypertension of these grades was seen in 19% of the older group, compared with 12% of the younger one.
AT THE GASTROINTESTINAL CANCERS SYMPOSIUM
(Cyramza)
Survival seen in mCRC trials has limited generalizability to real world
SAN FRANCISCO – Survival benefits seen with first-line therapy for metastatic colorectal cancer in clinical trials have limited generalizability to patients treated in real-world practice, suggests a study reported at the 2015 Gastrointestinal Cancers Symposium.
Investigators compared 16,614 patients from 31 randomized phase III trials of first-line therapy published between 2005 and 2010 with similar matched patients from the Surveillance, Epidemiology, and End Results (SEER) database.
Findings showed that the patients on trials as a whole had a median overall survival of 18.9 months and 1- and 2-year overall survival rates of 70% and 36%, respectively, Ziwei Wang reported in a poster session.
Relative to their real-world counterparts, trial patients had better median overall survival in 95% of trial arms (average difference, 5.4 months), better 1-year overall survival in 94% of trial arms (average difference, 16.7%), and better 2-year overall survival in 71% of trial arms (average difference, 7.2%) (P less than .0001 for each).
“When patients go to their physicians, they want to know what’s the prognosis, what benefit does the drug that you are giving me translate into, how does that help my overall survival,” Ms. Wang commented in an interview. “And most physicians get their information just from the clinical trial data that comes out.”
Taken together, the study’s findings are “a good message to physicians just to be mindful that these differences do exist and not to just draw from clinical trials to inform your patients,” she said. “You can sort of think about what you are telling them to let them know that the information you are giving them is coming from trials that might not be reflective of what we see in the general population.”
The survival gaps likely stem in part from the often-strict eligibility criteria used in clinical trials, according to Ms. Wang, who is a medical student at the University of California, San Diego.
“When you are designing clinical trials, you have a set of criteria that a patient has to meet before they are even allowed to enroll, so already, you might kind of be drawing from an unrepresentative sample when you start these trials,” she elaborated. “And so we think that a lot of that plays into why you see these differences between what the trial concludes and what SEER shows.”
A previous similar study compared survival among patients with a variety of cancers treated in versus out of clinical trials (J. Natl. Cancer Inst. 2014 [doi:10.1093/jnci/dju002]), Ms. Wang noted at the meeting cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology.
“They found that initially, the clinical trial patients did better, but after 5 years or so, it kind of evened out,” she said. “What we found was up until 5 years and even longer, you do see a difference.”
SAN FRANCISCO – Survival benefits seen with first-line therapy for metastatic colorectal cancer in clinical trials have limited generalizability to patients treated in real-world practice, suggests a study reported at the 2015 Gastrointestinal Cancers Symposium.
Investigators compared 16,614 patients from 31 randomized phase III trials of first-line therapy published between 2005 and 2010 with similar matched patients from the Surveillance, Epidemiology, and End Results (SEER) database.
Findings showed that the patients on trials as a whole had a median overall survival of 18.9 months and 1- and 2-year overall survival rates of 70% and 36%, respectively, Ziwei Wang reported in a poster session.
Relative to their real-world counterparts, trial patients had better median overall survival in 95% of trial arms (average difference, 5.4 months), better 1-year overall survival in 94% of trial arms (average difference, 16.7%), and better 2-year overall survival in 71% of trial arms (average difference, 7.2%) (P less than .0001 for each).
“When patients go to their physicians, they want to know what’s the prognosis, what benefit does the drug that you are giving me translate into, how does that help my overall survival,” Ms. Wang commented in an interview. “And most physicians get their information just from the clinical trial data that comes out.”
Taken together, the study’s findings are “a good message to physicians just to be mindful that these differences do exist and not to just draw from clinical trials to inform your patients,” she said. “You can sort of think about what you are telling them to let them know that the information you are giving them is coming from trials that might not be reflective of what we see in the general population.”
The survival gaps likely stem in part from the often-strict eligibility criteria used in clinical trials, according to Ms. Wang, who is a medical student at the University of California, San Diego.
“When you are designing clinical trials, you have a set of criteria that a patient has to meet before they are even allowed to enroll, so already, you might kind of be drawing from an unrepresentative sample when you start these trials,” she elaborated. “And so we think that a lot of that plays into why you see these differences between what the trial concludes and what SEER shows.”
A previous similar study compared survival among patients with a variety of cancers treated in versus out of clinical trials (J. Natl. Cancer Inst. 2014 [doi:10.1093/jnci/dju002]), Ms. Wang noted at the meeting cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology.
“They found that initially, the clinical trial patients did better, but after 5 years or so, it kind of evened out,” she said. “What we found was up until 5 years and even longer, you do see a difference.”
SAN FRANCISCO – Survival benefits seen with first-line therapy for metastatic colorectal cancer in clinical trials have limited generalizability to patients treated in real-world practice, suggests a study reported at the 2015 Gastrointestinal Cancers Symposium.
Investigators compared 16,614 patients from 31 randomized phase III trials of first-line therapy published between 2005 and 2010 with similar matched patients from the Surveillance, Epidemiology, and End Results (SEER) database.
Findings showed that the patients on trials as a whole had a median overall survival of 18.9 months and 1- and 2-year overall survival rates of 70% and 36%, respectively, Ziwei Wang reported in a poster session.
Relative to their real-world counterparts, trial patients had better median overall survival in 95% of trial arms (average difference, 5.4 months), better 1-year overall survival in 94% of trial arms (average difference, 16.7%), and better 2-year overall survival in 71% of trial arms (average difference, 7.2%) (P less than .0001 for each).
“When patients go to their physicians, they want to know what’s the prognosis, what benefit does the drug that you are giving me translate into, how does that help my overall survival,” Ms. Wang commented in an interview. “And most physicians get their information just from the clinical trial data that comes out.”
Taken together, the study’s findings are “a good message to physicians just to be mindful that these differences do exist and not to just draw from clinical trials to inform your patients,” she said. “You can sort of think about what you are telling them to let them know that the information you are giving them is coming from trials that might not be reflective of what we see in the general population.”
The survival gaps likely stem in part from the often-strict eligibility criteria used in clinical trials, according to Ms. Wang, who is a medical student at the University of California, San Diego.
“When you are designing clinical trials, you have a set of criteria that a patient has to meet before they are even allowed to enroll, so already, you might kind of be drawing from an unrepresentative sample when you start these trials,” she elaborated. “And so we think that a lot of that plays into why you see these differences between what the trial concludes and what SEER shows.”
A previous similar study compared survival among patients with a variety of cancers treated in versus out of clinical trials (J. Natl. Cancer Inst. 2014 [doi:10.1093/jnci/dju002]), Ms. Wang noted at the meeting cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology.
“They found that initially, the clinical trial patients did better, but after 5 years or so, it kind of evened out,” she said. “What we found was up until 5 years and even longer, you do see a difference.”
AT THE 2015 GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point: Survival of patients with metastatic colorectal cancer in the real world falls short of that in clinical trials.
Major finding: Median overall survival in the general population was 5.4 months shorter than that in the trial population.
Data source: An observational study comparing 16,614 trial patients receiving first-line therapy for mCRC on trials with matched patients from SEER.
Disclosures: Ms. Wang disclosed that she had no relevant conflicts of interest.
AFP may help guide ramucirumab therapy in advanced HCC
SAN FRANCISCO – Baseline levels of alpha-fetoprotein (AFP) may help identify patients with advanced hepatocellular carcinoma (HCC) who are most likely to benefit from treatment with the antiangiogenic agent ramucirumab, suggests a study reported at the 2015 Gastrointestinal Cancers Symposium.
Researchers led by Dr. Andrew X. Zhu of Harvard Medical School and director of Liver Cancer Research at the Massachusetts General Hospital, both in Boston, performed subgroup analyses of data from the randomized phase III REACH trial. The trial pitted ramucirumab (Cyramza) against placebo as second-line therapy in 544 patients with advanced HCC who had previously received sorafenib.
Ramucirumab is an antibody to the vascular endothelial growth factor receptor 2 (VEGFR2). It is currently approved by the U.S. Food and Drug Administration for the treatment of esophagogastric cancers and non–small cell lung cancer.
The trial, which was sponsored by Eli Lilly, failed to meet its primary endpoint of significantly improving overall survival (Ann. Oncol. 2014;25 [doi:10.1093/annonc/mdu438.16]), according to Dr. Zhu.
But the new, prespecified subgroup analyses showed that patients having a baseline AFP level of 400 ng/mL or higher – typically a poor prognostic factor – had a one-third reduction in the risk of death if given ramucirumab instead of placebo (median survival, 7.8 vs. 4.2 months; hazard ratio, 0.67; P = .0059), he reported at the meeting cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology.
The findings were similar in post hoc analyses among patients who met an alternate definition of elevated AFP level of at least 1.5 times the upper limit of normal (median survival, 8.6 vs. 5.7 months; HR, 0.75; P = .0088).
Additionally, threshold analyses indicated that absolute benefit increased with AFP level in the trial population as a whole; however, patients having levels as low as approximately 10 ng/mL still derived some benefit.
“Further analysis from the REACH study has identified AFP as a potential marker for selecting patients who may benefit from ramucirumab treatment in advanced HCC,” Dr. Zhu commented. “A robust and clinically meaningful improvement in overall survival was observed in the population with an elevated AFP level above 400 ng/mL.”
Session cochair W. Ray Kim, chief of the division of gastroenterology and hepatology, department of medicine, Stanford (Calif.) University, expressed some skepticism about the new data, however, noting the lack of ramucirumab benefit in the trial population overall but the presence of benefit down to normal AFP levels in the post hoc threshold analysis. “The two results don’t seem to be congruent with each other,” Dr. Kim commented.
Various methods can be used to model the baseline AFP threshold to predict the likely benefit, Dr. Zhu replied. “Suffice it to say that if you do different modeling, the results may be slightly different. I think that probably the 400 ng/mL still gives us the confidence as best as we could. But there is no doubt in my mind that between the upper limit of normal and 400 ng/mL, there will be patients who are also likely to benefit. And I think the cutoff for this will continue to be an issue.”
A session attendee asked, “What is the underlying biology, why [do patients with] higher AFP levels respond to antiangiogenesis treatment?”
“This is obviously a very critical question that we are actively pursuing,” Dr. Zhu replied. “We think that there may be some underlying link for those patients with high AFP with increased angiogenesis. And whether this is purely driven by VEGF or VEGFR2, I don’t think that the literature is actually very clear. There is definitely mounting evidence suggesting the high-AFP group is really a unique subgroup of patients with a unique genetic signature. But how this interlink is playing, I think at this point we don’t have the clear answer.”
Ramucirumab was similarly well tolerated in patients with AFP levels of 400 ng/mL or higher, as compared with counterparts having lower levels, reported Dr. Zhu, who disclosed that he has a consulting or advisory role with Celgene, Daiichi Sankyo, Eisai, Exelixis, and Sanofi. Notably, the rate of grade 3 or worse liver injury or failure was 22% with ramucirumab vs. 31% with placebo in the high-AFP group.
“Further investigation of the relationship between baseline AFP levels and angiogenesis inhibition is currently ongoing to better understand the mechanism of ramucirumab action in HCC,” Dr. Zhu said. “Whether there will be a posttreatment response in AFP and whether the posttreatment response will predict the outcome, we are currently analyzing that.”The researchers are additionally evaluating serum biomarkers of angiogenesis. “Unfortunately, we don’t have the archived tissue that may actually allow us to do some more detailed investigation. But I think we will present the circulating angiogenesis-related biomarkers to see whether there will be an additional biomarker that will actually segregate with the elevated AFP,” he concluded.
SAN FRANCISCO – Baseline levels of alpha-fetoprotein (AFP) may help identify patients with advanced hepatocellular carcinoma (HCC) who are most likely to benefit from treatment with the antiangiogenic agent ramucirumab, suggests a study reported at the 2015 Gastrointestinal Cancers Symposium.
Researchers led by Dr. Andrew X. Zhu of Harvard Medical School and director of Liver Cancer Research at the Massachusetts General Hospital, both in Boston, performed subgroup analyses of data from the randomized phase III REACH trial. The trial pitted ramucirumab (Cyramza) against placebo as second-line therapy in 544 patients with advanced HCC who had previously received sorafenib.
Ramucirumab is an antibody to the vascular endothelial growth factor receptor 2 (VEGFR2). It is currently approved by the U.S. Food and Drug Administration for the treatment of esophagogastric cancers and non–small cell lung cancer.
The trial, which was sponsored by Eli Lilly, failed to meet its primary endpoint of significantly improving overall survival (Ann. Oncol. 2014;25 [doi:10.1093/annonc/mdu438.16]), according to Dr. Zhu.
But the new, prespecified subgroup analyses showed that patients having a baseline AFP level of 400 ng/mL or higher – typically a poor prognostic factor – had a one-third reduction in the risk of death if given ramucirumab instead of placebo (median survival, 7.8 vs. 4.2 months; hazard ratio, 0.67; P = .0059), he reported at the meeting cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology.
The findings were similar in post hoc analyses among patients who met an alternate definition of elevated AFP level of at least 1.5 times the upper limit of normal (median survival, 8.6 vs. 5.7 months; HR, 0.75; P = .0088).
Additionally, threshold analyses indicated that absolute benefit increased with AFP level in the trial population as a whole; however, patients having levels as low as approximately 10 ng/mL still derived some benefit.
“Further analysis from the REACH study has identified AFP as a potential marker for selecting patients who may benefit from ramucirumab treatment in advanced HCC,” Dr. Zhu commented. “A robust and clinically meaningful improvement in overall survival was observed in the population with an elevated AFP level above 400 ng/mL.”
Session cochair W. Ray Kim, chief of the division of gastroenterology and hepatology, department of medicine, Stanford (Calif.) University, expressed some skepticism about the new data, however, noting the lack of ramucirumab benefit in the trial population overall but the presence of benefit down to normal AFP levels in the post hoc threshold analysis. “The two results don’t seem to be congruent with each other,” Dr. Kim commented.
Various methods can be used to model the baseline AFP threshold to predict the likely benefit, Dr. Zhu replied. “Suffice it to say that if you do different modeling, the results may be slightly different. I think that probably the 400 ng/mL still gives us the confidence as best as we could. But there is no doubt in my mind that between the upper limit of normal and 400 ng/mL, there will be patients who are also likely to benefit. And I think the cutoff for this will continue to be an issue.”
A session attendee asked, “What is the underlying biology, why [do patients with] higher AFP levels respond to antiangiogenesis treatment?”
“This is obviously a very critical question that we are actively pursuing,” Dr. Zhu replied. “We think that there may be some underlying link for those patients with high AFP with increased angiogenesis. And whether this is purely driven by VEGF or VEGFR2, I don’t think that the literature is actually very clear. There is definitely mounting evidence suggesting the high-AFP group is really a unique subgroup of patients with a unique genetic signature. But how this interlink is playing, I think at this point we don’t have the clear answer.”
Ramucirumab was similarly well tolerated in patients with AFP levels of 400 ng/mL or higher, as compared with counterparts having lower levels, reported Dr. Zhu, who disclosed that he has a consulting or advisory role with Celgene, Daiichi Sankyo, Eisai, Exelixis, and Sanofi. Notably, the rate of grade 3 or worse liver injury or failure was 22% with ramucirumab vs. 31% with placebo in the high-AFP group.
“Further investigation of the relationship between baseline AFP levels and angiogenesis inhibition is currently ongoing to better understand the mechanism of ramucirumab action in HCC,” Dr. Zhu said. “Whether there will be a posttreatment response in AFP and whether the posttreatment response will predict the outcome, we are currently analyzing that.”The researchers are additionally evaluating serum biomarkers of angiogenesis. “Unfortunately, we don’t have the archived tissue that may actually allow us to do some more detailed investigation. But I think we will present the circulating angiogenesis-related biomarkers to see whether there will be an additional biomarker that will actually segregate with the elevated AFP,” he concluded.
SAN FRANCISCO – Baseline levels of alpha-fetoprotein (AFP) may help identify patients with advanced hepatocellular carcinoma (HCC) who are most likely to benefit from treatment with the antiangiogenic agent ramucirumab, suggests a study reported at the 2015 Gastrointestinal Cancers Symposium.
Researchers led by Dr. Andrew X. Zhu of Harvard Medical School and director of Liver Cancer Research at the Massachusetts General Hospital, both in Boston, performed subgroup analyses of data from the randomized phase III REACH trial. The trial pitted ramucirumab (Cyramza) against placebo as second-line therapy in 544 patients with advanced HCC who had previously received sorafenib.
Ramucirumab is an antibody to the vascular endothelial growth factor receptor 2 (VEGFR2). It is currently approved by the U.S. Food and Drug Administration for the treatment of esophagogastric cancers and non–small cell lung cancer.
The trial, which was sponsored by Eli Lilly, failed to meet its primary endpoint of significantly improving overall survival (Ann. Oncol. 2014;25 [doi:10.1093/annonc/mdu438.16]), according to Dr. Zhu.
But the new, prespecified subgroup analyses showed that patients having a baseline AFP level of 400 ng/mL or higher – typically a poor prognostic factor – had a one-third reduction in the risk of death if given ramucirumab instead of placebo (median survival, 7.8 vs. 4.2 months; hazard ratio, 0.67; P = .0059), he reported at the meeting cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology.
The findings were similar in post hoc analyses among patients who met an alternate definition of elevated AFP level of at least 1.5 times the upper limit of normal (median survival, 8.6 vs. 5.7 months; HR, 0.75; P = .0088).
Additionally, threshold analyses indicated that absolute benefit increased with AFP level in the trial population as a whole; however, patients having levels as low as approximately 10 ng/mL still derived some benefit.
“Further analysis from the REACH study has identified AFP as a potential marker for selecting patients who may benefit from ramucirumab treatment in advanced HCC,” Dr. Zhu commented. “A robust and clinically meaningful improvement in overall survival was observed in the population with an elevated AFP level above 400 ng/mL.”
Session cochair W. Ray Kim, chief of the division of gastroenterology and hepatology, department of medicine, Stanford (Calif.) University, expressed some skepticism about the new data, however, noting the lack of ramucirumab benefit in the trial population overall but the presence of benefit down to normal AFP levels in the post hoc threshold analysis. “The two results don’t seem to be congruent with each other,” Dr. Kim commented.
Various methods can be used to model the baseline AFP threshold to predict the likely benefit, Dr. Zhu replied. “Suffice it to say that if you do different modeling, the results may be slightly different. I think that probably the 400 ng/mL still gives us the confidence as best as we could. But there is no doubt in my mind that between the upper limit of normal and 400 ng/mL, there will be patients who are also likely to benefit. And I think the cutoff for this will continue to be an issue.”
A session attendee asked, “What is the underlying biology, why [do patients with] higher AFP levels respond to antiangiogenesis treatment?”
“This is obviously a very critical question that we are actively pursuing,” Dr. Zhu replied. “We think that there may be some underlying link for those patients with high AFP with increased angiogenesis. And whether this is purely driven by VEGF or VEGFR2, I don’t think that the literature is actually very clear. There is definitely mounting evidence suggesting the high-AFP group is really a unique subgroup of patients with a unique genetic signature. But how this interlink is playing, I think at this point we don’t have the clear answer.”
Ramucirumab was similarly well tolerated in patients with AFP levels of 400 ng/mL or higher, as compared with counterparts having lower levels, reported Dr. Zhu, who disclosed that he has a consulting or advisory role with Celgene, Daiichi Sankyo, Eisai, Exelixis, and Sanofi. Notably, the rate of grade 3 or worse liver injury or failure was 22% with ramucirumab vs. 31% with placebo in the high-AFP group.
“Further investigation of the relationship between baseline AFP levels and angiogenesis inhibition is currently ongoing to better understand the mechanism of ramucirumab action in HCC,” Dr. Zhu said. “Whether there will be a posttreatment response in AFP and whether the posttreatment response will predict the outcome, we are currently analyzing that.”The researchers are additionally evaluating serum biomarkers of angiogenesis. “Unfortunately, we don’t have the archived tissue that may actually allow us to do some more detailed investigation. But I think we will present the circulating angiogenesis-related biomarkers to see whether there will be an additional biomarker that will actually segregate with the elevated AFP,” he concluded.
AT THE 2015 GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point: Pretreatment AFP levels may be a biomarker for ramucirumab benefit.
Major finding: Patients with AFP levels of 400 ng/mL or higher were 33% less likely to die if given ramucirumab instead of placebo.
Data source: A subgroup analysis of a randomized phase III trial in 544 patients with advanced HCC.
Disclosures: Dr. Zhu disclosed that he has a consulting or advisory role with Celgene, Daiichi Sankyo, Eisai, Exelixis, and Sanofi. The trial was sponsored by Eli Lilly.
Statins found to have a survival benefit in colorectal cancer
SAN FRANCISCO – Statin use confers a survival benefit in patients with colorectal cancer, suggests a systematic review and meta-analysis presented at the annual Gastrointestinal Cancers Symposium.
Investigators analyzed data from seven observational studies having a total of 64,773 patients with this cancer, a fifth of whom were using statins. Results showed that statin users had a nearly 30% reduction in the adjusted risk of all-cause mortality relative to nonusers, Dr. Arjun Gupta, the lead investigator, reported in a poster session at the meeting cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology.
“There is a lot of interest in non–cytotoxic chemotherapy drugs that can be used to treat cancer,” he said in an interview. “Thirty percent is a very, very significant number – we use chemotherapy drugs even if they have just a 10% survival benefit. So I am hopeful that this will help people.”
Accumulating preclinical data suggest that statins have anticancer actions, inhibiting cell proliferation and angiogenesis, and inducing apoptosis, according to Dr. Gupta, who is a resident in internal medicine at the University of Texas Southwestern Medical Center, Dallas. Previous studies have shown use to be associated with a decreased risk of developing colorectal cancer, but its impact on established disease has not been well assessed on a large scale.
“This is all observational data. There is no randomized controlled trial that’s been done,” he acknowledged. “But these data certainly seem to point out that this is something we can do. It’s a cheap and easy, safe drug which people take for years on end without any complications.”
At present, statins are approved by the Food and Drug Administration only for lipid-lowering indications, he noted. However, patients with colorectal cancer often have cardiovascular risk factors that make them candidates for statins.
“No one really gets a statin right now for colon cancer. We are just sort of lucky, maybe, that so many people with colon cancer have high cholesterol and are getting these drugs,” Dr. Gupta said. “But hopefully, my dream is 5 years down the line, it will be prescribed to people for this indication.”
Study results showed that in multivariate analyses, patients using statins were 26% less likely to die of any cause (hazard ratio, 0.74). Findings were similar whether they had colon cancer (hazard ratio, 0.79) or rectal cancer (hazard ratio, 0.63).
When analyses were restricted to studies that adjusted for concomitant use of aspirin and nonsteroidal anti-inflammatory drugs, statin users had significantly reduced risks of both all-cause mortality (hazard ratio, 0.74) and colorectal cancer–specific mortality (HR, 0.76), reported Dr. Gupta.
Patients who started using the medications after their cancer diagnosis had a significantly reduced risk of colorectal cancer-specific mortality (HR, 0.70) but not all-cause mortality.
Dr. Gupta disclosed that he had no relevant conflicts of interest.
SAN FRANCISCO – Statin use confers a survival benefit in patients with colorectal cancer, suggests a systematic review and meta-analysis presented at the annual Gastrointestinal Cancers Symposium.
Investigators analyzed data from seven observational studies having a total of 64,773 patients with this cancer, a fifth of whom were using statins. Results showed that statin users had a nearly 30% reduction in the adjusted risk of all-cause mortality relative to nonusers, Dr. Arjun Gupta, the lead investigator, reported in a poster session at the meeting cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology.
“There is a lot of interest in non–cytotoxic chemotherapy drugs that can be used to treat cancer,” he said in an interview. “Thirty percent is a very, very significant number – we use chemotherapy drugs even if they have just a 10% survival benefit. So I am hopeful that this will help people.”
Accumulating preclinical data suggest that statins have anticancer actions, inhibiting cell proliferation and angiogenesis, and inducing apoptosis, according to Dr. Gupta, who is a resident in internal medicine at the University of Texas Southwestern Medical Center, Dallas. Previous studies have shown use to be associated with a decreased risk of developing colorectal cancer, but its impact on established disease has not been well assessed on a large scale.
“This is all observational data. There is no randomized controlled trial that’s been done,” he acknowledged. “But these data certainly seem to point out that this is something we can do. It’s a cheap and easy, safe drug which people take for years on end without any complications.”
At present, statins are approved by the Food and Drug Administration only for lipid-lowering indications, he noted. However, patients with colorectal cancer often have cardiovascular risk factors that make them candidates for statins.
“No one really gets a statin right now for colon cancer. We are just sort of lucky, maybe, that so many people with colon cancer have high cholesterol and are getting these drugs,” Dr. Gupta said. “But hopefully, my dream is 5 years down the line, it will be prescribed to people for this indication.”
Study results showed that in multivariate analyses, patients using statins were 26% less likely to die of any cause (hazard ratio, 0.74). Findings were similar whether they had colon cancer (hazard ratio, 0.79) or rectal cancer (hazard ratio, 0.63).
When analyses were restricted to studies that adjusted for concomitant use of aspirin and nonsteroidal anti-inflammatory drugs, statin users had significantly reduced risks of both all-cause mortality (hazard ratio, 0.74) and colorectal cancer–specific mortality (HR, 0.76), reported Dr. Gupta.
Patients who started using the medications after their cancer diagnosis had a significantly reduced risk of colorectal cancer-specific mortality (HR, 0.70) but not all-cause mortality.
Dr. Gupta disclosed that he had no relevant conflicts of interest.
SAN FRANCISCO – Statin use confers a survival benefit in patients with colorectal cancer, suggests a systematic review and meta-analysis presented at the annual Gastrointestinal Cancers Symposium.
Investigators analyzed data from seven observational studies having a total of 64,773 patients with this cancer, a fifth of whom were using statins. Results showed that statin users had a nearly 30% reduction in the adjusted risk of all-cause mortality relative to nonusers, Dr. Arjun Gupta, the lead investigator, reported in a poster session at the meeting cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology.
“There is a lot of interest in non–cytotoxic chemotherapy drugs that can be used to treat cancer,” he said in an interview. “Thirty percent is a very, very significant number – we use chemotherapy drugs even if they have just a 10% survival benefit. So I am hopeful that this will help people.”
Accumulating preclinical data suggest that statins have anticancer actions, inhibiting cell proliferation and angiogenesis, and inducing apoptosis, according to Dr. Gupta, who is a resident in internal medicine at the University of Texas Southwestern Medical Center, Dallas. Previous studies have shown use to be associated with a decreased risk of developing colorectal cancer, but its impact on established disease has not been well assessed on a large scale.
“This is all observational data. There is no randomized controlled trial that’s been done,” he acknowledged. “But these data certainly seem to point out that this is something we can do. It’s a cheap and easy, safe drug which people take for years on end without any complications.”
At present, statins are approved by the Food and Drug Administration only for lipid-lowering indications, he noted. However, patients with colorectal cancer often have cardiovascular risk factors that make them candidates for statins.
“No one really gets a statin right now for colon cancer. We are just sort of lucky, maybe, that so many people with colon cancer have high cholesterol and are getting these drugs,” Dr. Gupta said. “But hopefully, my dream is 5 years down the line, it will be prescribed to people for this indication.”
Study results showed that in multivariate analyses, patients using statins were 26% less likely to die of any cause (hazard ratio, 0.74). Findings were similar whether they had colon cancer (hazard ratio, 0.79) or rectal cancer (hazard ratio, 0.63).
When analyses were restricted to studies that adjusted for concomitant use of aspirin and nonsteroidal anti-inflammatory drugs, statin users had significantly reduced risks of both all-cause mortality (hazard ratio, 0.74) and colorectal cancer–specific mortality (HR, 0.76), reported Dr. Gupta.
Patients who started using the medications after their cancer diagnosis had a significantly reduced risk of colorectal cancer-specific mortality (HR, 0.70) but not all-cause mortality.
Dr. Gupta disclosed that he had no relevant conflicts of interest.
Key clinical point: Patients with colorectal cancer who take statins are less likely to die.
Major finding: Relative to nonusers, statin users had a 26% reduction in the adjusted risk of all-cause mortality.
Data source: A systematic review and meta-analysis of seven observational studies having a total of 64,773 patients.
Disclosures: Dr. Gupta disclosed that he had no relevant conflicts of interest.
Increased industry funding and reliance on PFS as endpoint biggest shifts in mCRC trials since 1980
SAN FRANCISCO – None of the phase III randomized controlled trials in metastatic colorectal cancer conducted during 1980-1989 used progression-free survival as their endpoint, whereas approximately 40% of those conducted during 2010-2014 did, according to an analysis reported at the 2015 Gastrointestinal Cancers Symposium, sponsored by the American Society of Clinical Oncology.
Investigators led by Dr. Sunil Parimi, a fellow at the Tom Baker Cancer Centre, University of Calgary, Alberta, studied the characteristics of 192 phase III randomized controlled trials in metastatic colorectal cancer (mCRC) conducted between 1980 and 2014 and reported in an abstract or published article. To assess temporal trends, they split the trials into decades.
The use of overall survival as an endpoint peaked at 35% of trials in 2000-2009 and fell thereafter. None of the trials conducted in the first decade used progression-free survival (PFS) as their endpoint, whereas approximately 40% of those conducted in 2010-2014 did.
This trend “is one of the most surprising and interesting changes to me that has occurred over the decades,” Dr. Parimi commented in an interview. “Before, we used to use hard endpoints such as overall survival and quality of life, especially from the metastatic standpoint, and now that’s gradually started to taper off and surrogate endpoints have started to take the lead, progression-free survival in particular.”
Its use has likely contributed to increases in the proportions of trials meeting their primary endpoint (from 11% to 42%) and reporting in their conclusions that their results were positive (from 33% to 53%), “either warranting further study or endorsing a drug to be used in the general population,” he added.
Yet, PFS has not been validated as a surrogate for overall survival in this context, according to Dr. Parimi. “It is definitely still controversial whether or not we are looking at the outcomes that really matter in the mCRC population, or is it just something that’s more attainable,” he elaborated. “The proponents of it would say, it’s more difficult to find overall survival with later-line therapy, with cross-over trial designs, which are all new advents. So that’s why, partially at least, there is more of a dependence on progression-free survival as an endpoint.”
The proportion of trials relying on industry funding rose from 1990 onward, from roughly 10% to 60%. There were also increases during the study period in the median number of agents used in investigational arms (from 1.5 to 2.5) and in the share of trials evaluating targeted therapies (from 0% to 68%), Dr. Parimi reported in a poster session.
The proportion of the trials that were published more than doubled, going from 39% of those conducted during 1980-1989 to 82% of those conducted during 2000-2014.
A variety of factors may be contributing to the observed trends, he speculated. “It’s pressure from various angles that’s driving people to come up with a positive study, quote unquote, regardless of what the outcome is. The intention behind doing this study was just to raise awareness of what the shifts are and then subsequently, we can debate the ramifications or the implications of those shifts.”
Although median progression-free and overall survival on the trials increased during the study period, the absolute magnitude of benefit derived from the investigational therapy relative to the control therapy did not increase significantly, hovering at about a 1-month gain for each endpoint across decades.
“The improvements that we make are always small amounts – there have not been any big explosions,” Dr. Parimi commented. “There are a number of reasons why that may be. But maybe it’s because we have come up with so many therapeutics, and so much research has been put into this already that we just are not making those dramatic findings.”
The study’s results have important implications when it comes to allocation of research funding, according to Dr. Parimi, who disclosed that he had no relevant conflicts of interest.
“I think that if we did fewer studies that had a higher standard of efficacy or outcome, we’d save more money on the drug-development process, and we could shift it to areas where it was truly needed. And I actually think that we can accelerate drug development in that way, as opposed to coming out with multiple bodies of literature that disperse the amount of funds that we use for different projects, and drugs which may or may not have true hard-outcome benefits, at least in the eyes of many,” he concluded. “So if we can allow for that paradigm shift, or maybe rather go back to that, fewer trials with more quality would definitely be advantageous.”
SAN FRANCISCO – None of the phase III randomized controlled trials in metastatic colorectal cancer conducted during 1980-1989 used progression-free survival as their endpoint, whereas approximately 40% of those conducted during 2010-2014 did, according to an analysis reported at the 2015 Gastrointestinal Cancers Symposium, sponsored by the American Society of Clinical Oncology.
Investigators led by Dr. Sunil Parimi, a fellow at the Tom Baker Cancer Centre, University of Calgary, Alberta, studied the characteristics of 192 phase III randomized controlled trials in metastatic colorectal cancer (mCRC) conducted between 1980 and 2014 and reported in an abstract or published article. To assess temporal trends, they split the trials into decades.
The use of overall survival as an endpoint peaked at 35% of trials in 2000-2009 and fell thereafter. None of the trials conducted in the first decade used progression-free survival (PFS) as their endpoint, whereas approximately 40% of those conducted in 2010-2014 did.
This trend “is one of the most surprising and interesting changes to me that has occurred over the decades,” Dr. Parimi commented in an interview. “Before, we used to use hard endpoints such as overall survival and quality of life, especially from the metastatic standpoint, and now that’s gradually started to taper off and surrogate endpoints have started to take the lead, progression-free survival in particular.”
Its use has likely contributed to increases in the proportions of trials meeting their primary endpoint (from 11% to 42%) and reporting in their conclusions that their results were positive (from 33% to 53%), “either warranting further study or endorsing a drug to be used in the general population,” he added.
Yet, PFS has not been validated as a surrogate for overall survival in this context, according to Dr. Parimi. “It is definitely still controversial whether or not we are looking at the outcomes that really matter in the mCRC population, or is it just something that’s more attainable,” he elaborated. “The proponents of it would say, it’s more difficult to find overall survival with later-line therapy, with cross-over trial designs, which are all new advents. So that’s why, partially at least, there is more of a dependence on progression-free survival as an endpoint.”
The proportion of trials relying on industry funding rose from 1990 onward, from roughly 10% to 60%. There were also increases during the study period in the median number of agents used in investigational arms (from 1.5 to 2.5) and in the share of trials evaluating targeted therapies (from 0% to 68%), Dr. Parimi reported in a poster session.
The proportion of the trials that were published more than doubled, going from 39% of those conducted during 1980-1989 to 82% of those conducted during 2000-2014.
A variety of factors may be contributing to the observed trends, he speculated. “It’s pressure from various angles that’s driving people to come up with a positive study, quote unquote, regardless of what the outcome is. The intention behind doing this study was just to raise awareness of what the shifts are and then subsequently, we can debate the ramifications or the implications of those shifts.”
Although median progression-free and overall survival on the trials increased during the study period, the absolute magnitude of benefit derived from the investigational therapy relative to the control therapy did not increase significantly, hovering at about a 1-month gain for each endpoint across decades.
“The improvements that we make are always small amounts – there have not been any big explosions,” Dr. Parimi commented. “There are a number of reasons why that may be. But maybe it’s because we have come up with so many therapeutics, and so much research has been put into this already that we just are not making those dramatic findings.”
The study’s results have important implications when it comes to allocation of research funding, according to Dr. Parimi, who disclosed that he had no relevant conflicts of interest.
“I think that if we did fewer studies that had a higher standard of efficacy or outcome, we’d save more money on the drug-development process, and we could shift it to areas where it was truly needed. And I actually think that we can accelerate drug development in that way, as opposed to coming out with multiple bodies of literature that disperse the amount of funds that we use for different projects, and drugs which may or may not have true hard-outcome benefits, at least in the eyes of many,” he concluded. “So if we can allow for that paradigm shift, or maybe rather go back to that, fewer trials with more quality would definitely be advantageous.”
SAN FRANCISCO – None of the phase III randomized controlled trials in metastatic colorectal cancer conducted during 1980-1989 used progression-free survival as their endpoint, whereas approximately 40% of those conducted during 2010-2014 did, according to an analysis reported at the 2015 Gastrointestinal Cancers Symposium, sponsored by the American Society of Clinical Oncology.
Investigators led by Dr. Sunil Parimi, a fellow at the Tom Baker Cancer Centre, University of Calgary, Alberta, studied the characteristics of 192 phase III randomized controlled trials in metastatic colorectal cancer (mCRC) conducted between 1980 and 2014 and reported in an abstract or published article. To assess temporal trends, they split the trials into decades.
The use of overall survival as an endpoint peaked at 35% of trials in 2000-2009 and fell thereafter. None of the trials conducted in the first decade used progression-free survival (PFS) as their endpoint, whereas approximately 40% of those conducted in 2010-2014 did.
This trend “is one of the most surprising and interesting changes to me that has occurred over the decades,” Dr. Parimi commented in an interview. “Before, we used to use hard endpoints such as overall survival and quality of life, especially from the metastatic standpoint, and now that’s gradually started to taper off and surrogate endpoints have started to take the lead, progression-free survival in particular.”
Its use has likely contributed to increases in the proportions of trials meeting their primary endpoint (from 11% to 42%) and reporting in their conclusions that their results were positive (from 33% to 53%), “either warranting further study or endorsing a drug to be used in the general population,” he added.
Yet, PFS has not been validated as a surrogate for overall survival in this context, according to Dr. Parimi. “It is definitely still controversial whether or not we are looking at the outcomes that really matter in the mCRC population, or is it just something that’s more attainable,” he elaborated. “The proponents of it would say, it’s more difficult to find overall survival with later-line therapy, with cross-over trial designs, which are all new advents. So that’s why, partially at least, there is more of a dependence on progression-free survival as an endpoint.”
The proportion of trials relying on industry funding rose from 1990 onward, from roughly 10% to 60%. There were also increases during the study period in the median number of agents used in investigational arms (from 1.5 to 2.5) and in the share of trials evaluating targeted therapies (from 0% to 68%), Dr. Parimi reported in a poster session.
The proportion of the trials that were published more than doubled, going from 39% of those conducted during 1980-1989 to 82% of those conducted during 2000-2014.
A variety of factors may be contributing to the observed trends, he speculated. “It’s pressure from various angles that’s driving people to come up with a positive study, quote unquote, regardless of what the outcome is. The intention behind doing this study was just to raise awareness of what the shifts are and then subsequently, we can debate the ramifications or the implications of those shifts.”
Although median progression-free and overall survival on the trials increased during the study period, the absolute magnitude of benefit derived from the investigational therapy relative to the control therapy did not increase significantly, hovering at about a 1-month gain for each endpoint across decades.
“The improvements that we make are always small amounts – there have not been any big explosions,” Dr. Parimi commented. “There are a number of reasons why that may be. But maybe it’s because we have come up with so many therapeutics, and so much research has been put into this already that we just are not making those dramatic findings.”
The study’s results have important implications when it comes to allocation of research funding, according to Dr. Parimi, who disclosed that he had no relevant conflicts of interest.
“I think that if we did fewer studies that had a higher standard of efficacy or outcome, we’d save more money on the drug-development process, and we could shift it to areas where it was truly needed. And I actually think that we can accelerate drug development in that way, as opposed to coming out with multiple bodies of literature that disperse the amount of funds that we use for different projects, and drugs which may or may not have true hard-outcome benefits, at least in the eyes of many,” he concluded. “So if we can allow for that paradigm shift, or maybe rather go back to that, fewer trials with more quality would definitely be advantageous.”
AT THE 2015 GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point: Characteristics of phase III trials in metastatic colorectal cancer have shifted over the past 3 decades, particularly an increase in PFS as an endpoint instead of overall survival.
Major finding: Over time, more trials relied on industry funding (60% vs. 10%), used PFS as their primary endpoint (40% vs. 0%), and reported positive results in their conclusion (53% vs. 33%).
Data source: An analysis of 192 phase III trials in mCRC conducted between 1980 and 2014.
Disclosures: Dr. Parimi disclosed that he had no relevant conflicts of interest.
Laparoscopic distal gastrectomy gains clout
SAN FRANCISCO – Patients with early gastric cancer have less postoperative morbidity if they undergo laparoscopic instead of open distal gastrectomy, according to safety results of a phase III trial presented at the at the annual Gastrointestinal Cancers Symposium sponsored by the American Society of Clinical Oncology.
The trial – the first in a series by the Korean Laparo-endoscopic Gastrointestinal Surgery Study Group (KLASS-01) – was conducted among 1,416 patients with clinical stage I disease in Korea, where the proportion of esophagogastric cancers caught in early stages has increased with the introduction of a national screening program. The patients were randomized evenly to laparoscopic or open surgery.
Main results showed mortality was similarly low for the open and laparoscopic approaches. But the laparoscopic group had half the rate of wound complications and, in multivariate analysis, a 41% lower risk of postoperative morbidity.
“Laparoscopy-assisted distal gastrectomy for patients with clinical stage I gastric cancer is safe and has a benefit of lower occurrence of wound complications compared with conventional open surgery,” concluded first author Dr. Hyuk-Joon Lee, a gastrointestinal surgeon at the Seoul National University Hospital, Korea.
Invited discussant Dr. Michael Kent of Harvard Medical School, director of minimally invasive thoracic surgery at Beth Israel Deaconess Medical Center, Boston, said, “It is clear that the KLASS study is the largest by far to evaluate laparoscopic gastrectomy.”
A similar trial in the setting of early colorectal cancer (N. Engl. J. Med. 2004;350:2050-9) led to rapid uptake of laparoscopic resection for those patients at high-volume centers, he noted. “Although we still await survival data from the KLASS study, I anticipate that laparoscopic gastrectomy will likewise become the preferred approach in high-volume centers, especially in countries such as Korea with a national screening program.
“However, I do not think that open gastrectomy is an operation of the past,” he added. “For one, the benefits have not been shown yet in advanced gastric cancer. Also, in low-volume centers, the expertise in laparoscopic surgery may not be sufficient to warrant this approach. I should also add that body habitus may render this operation more difficult in obese patients.”
Dr. Kent pointed out that patients with T1a disease have yet another option, endoscopic resection, which has been found to yield good results at least in a single-center retrospective study (Surg. Endosc. 2013;27:4250-8). “In regards to future clinical trials, I believe it would be important to determine which patients require surgical as opposed to an endoscopic resection,” he concluded.
A previously reported interim analysis of the KLASS-01 trial showed no significant difference in morbidity and mortality between the laparoscopic and open groups, allowing the trial to continue (Ann. Surg. 2010;251:417-20).
Eighty percent of patients on the trial had T1 disease. Within this subset, about 60% had T1a and 40% had T1b disease, Dr. Lee said.
In modified intention-to-treat analyses, patients in the laparoscopic group had a longer operation time (185 vs. 146 minutes) and fewer lymph nodes retrieved (41 vs. 43). But they had a lower estimated blood loss (119 vs. 194 mL) and shorter hospital stay (7.2 vs. 8.0 days).
The rate of surgical mortality was less than 1% and the rate of reoperation was about 1%, with no significant differences between groups.
Patients in the laparoscopic group had lower 30-day rates of postoperative morbidity (13.7% vs. 18.9%, P = .009), and the benefit of the less invasive approach remained significant after multivariate adjustment (odds ratio, 0.59; P = .001). Wound complications were half as common with laparoscopy (3.6% vs. 7.0%, P = .005).
Data on 5-year overall survival, the primary endpoint of the KLASS-01 trial, are expected later this year.
Dr. Kent asked, “In your country, for those patients with clinical T1a disease, how is a decision made regarding therapy?”
“I think if we can accurately diagnose the T stage as well as the N stage, we can surely adapt the endoscopic treatment for all the T1a cancer patients,” Dr. Lee replied. Such diagnosis has proved challenging, he added. Endoscopic resection is usually reserved for patients with tumors less than 2 cm in diameter showing differentiated histology and not invading the mucosa. “We have to move to the more accurate diagnosis of the TNM stage,” he concluded.
SAN FRANCISCO – Patients with early gastric cancer have less postoperative morbidity if they undergo laparoscopic instead of open distal gastrectomy, according to safety results of a phase III trial presented at the at the annual Gastrointestinal Cancers Symposium sponsored by the American Society of Clinical Oncology.
The trial – the first in a series by the Korean Laparo-endoscopic Gastrointestinal Surgery Study Group (KLASS-01) – was conducted among 1,416 patients with clinical stage I disease in Korea, where the proportion of esophagogastric cancers caught in early stages has increased with the introduction of a national screening program. The patients were randomized evenly to laparoscopic or open surgery.
Main results showed mortality was similarly low for the open and laparoscopic approaches. But the laparoscopic group had half the rate of wound complications and, in multivariate analysis, a 41% lower risk of postoperative morbidity.
“Laparoscopy-assisted distal gastrectomy for patients with clinical stage I gastric cancer is safe and has a benefit of lower occurrence of wound complications compared with conventional open surgery,” concluded first author Dr. Hyuk-Joon Lee, a gastrointestinal surgeon at the Seoul National University Hospital, Korea.
Invited discussant Dr. Michael Kent of Harvard Medical School, director of minimally invasive thoracic surgery at Beth Israel Deaconess Medical Center, Boston, said, “It is clear that the KLASS study is the largest by far to evaluate laparoscopic gastrectomy.”
A similar trial in the setting of early colorectal cancer (N. Engl. J. Med. 2004;350:2050-9) led to rapid uptake of laparoscopic resection for those patients at high-volume centers, he noted. “Although we still await survival data from the KLASS study, I anticipate that laparoscopic gastrectomy will likewise become the preferred approach in high-volume centers, especially in countries such as Korea with a national screening program.
“However, I do not think that open gastrectomy is an operation of the past,” he added. “For one, the benefits have not been shown yet in advanced gastric cancer. Also, in low-volume centers, the expertise in laparoscopic surgery may not be sufficient to warrant this approach. I should also add that body habitus may render this operation more difficult in obese patients.”
Dr. Kent pointed out that patients with T1a disease have yet another option, endoscopic resection, which has been found to yield good results at least in a single-center retrospective study (Surg. Endosc. 2013;27:4250-8). “In regards to future clinical trials, I believe it would be important to determine which patients require surgical as opposed to an endoscopic resection,” he concluded.
A previously reported interim analysis of the KLASS-01 trial showed no significant difference in morbidity and mortality between the laparoscopic and open groups, allowing the trial to continue (Ann. Surg. 2010;251:417-20).
Eighty percent of patients on the trial had T1 disease. Within this subset, about 60% had T1a and 40% had T1b disease, Dr. Lee said.
In modified intention-to-treat analyses, patients in the laparoscopic group had a longer operation time (185 vs. 146 minutes) and fewer lymph nodes retrieved (41 vs. 43). But they had a lower estimated blood loss (119 vs. 194 mL) and shorter hospital stay (7.2 vs. 8.0 days).
The rate of surgical mortality was less than 1% and the rate of reoperation was about 1%, with no significant differences between groups.
Patients in the laparoscopic group had lower 30-day rates of postoperative morbidity (13.7% vs. 18.9%, P = .009), and the benefit of the less invasive approach remained significant after multivariate adjustment (odds ratio, 0.59; P = .001). Wound complications were half as common with laparoscopy (3.6% vs. 7.0%, P = .005).
Data on 5-year overall survival, the primary endpoint of the KLASS-01 trial, are expected later this year.
Dr. Kent asked, “In your country, for those patients with clinical T1a disease, how is a decision made regarding therapy?”
“I think if we can accurately diagnose the T stage as well as the N stage, we can surely adapt the endoscopic treatment for all the T1a cancer patients,” Dr. Lee replied. Such diagnosis has proved challenging, he added. Endoscopic resection is usually reserved for patients with tumors less than 2 cm in diameter showing differentiated histology and not invading the mucosa. “We have to move to the more accurate diagnosis of the TNM stage,” he concluded.
SAN FRANCISCO – Patients with early gastric cancer have less postoperative morbidity if they undergo laparoscopic instead of open distal gastrectomy, according to safety results of a phase III trial presented at the at the annual Gastrointestinal Cancers Symposium sponsored by the American Society of Clinical Oncology.
The trial – the first in a series by the Korean Laparo-endoscopic Gastrointestinal Surgery Study Group (KLASS-01) – was conducted among 1,416 patients with clinical stage I disease in Korea, where the proportion of esophagogastric cancers caught in early stages has increased with the introduction of a national screening program. The patients were randomized evenly to laparoscopic or open surgery.
Main results showed mortality was similarly low for the open and laparoscopic approaches. But the laparoscopic group had half the rate of wound complications and, in multivariate analysis, a 41% lower risk of postoperative morbidity.
“Laparoscopy-assisted distal gastrectomy for patients with clinical stage I gastric cancer is safe and has a benefit of lower occurrence of wound complications compared with conventional open surgery,” concluded first author Dr. Hyuk-Joon Lee, a gastrointestinal surgeon at the Seoul National University Hospital, Korea.
Invited discussant Dr. Michael Kent of Harvard Medical School, director of minimally invasive thoracic surgery at Beth Israel Deaconess Medical Center, Boston, said, “It is clear that the KLASS study is the largest by far to evaluate laparoscopic gastrectomy.”
A similar trial in the setting of early colorectal cancer (N. Engl. J. Med. 2004;350:2050-9) led to rapid uptake of laparoscopic resection for those patients at high-volume centers, he noted. “Although we still await survival data from the KLASS study, I anticipate that laparoscopic gastrectomy will likewise become the preferred approach in high-volume centers, especially in countries such as Korea with a national screening program.
“However, I do not think that open gastrectomy is an operation of the past,” he added. “For one, the benefits have not been shown yet in advanced gastric cancer. Also, in low-volume centers, the expertise in laparoscopic surgery may not be sufficient to warrant this approach. I should also add that body habitus may render this operation more difficult in obese patients.”
Dr. Kent pointed out that patients with T1a disease have yet another option, endoscopic resection, which has been found to yield good results at least in a single-center retrospective study (Surg. Endosc. 2013;27:4250-8). “In regards to future clinical trials, I believe it would be important to determine which patients require surgical as opposed to an endoscopic resection,” he concluded.
A previously reported interim analysis of the KLASS-01 trial showed no significant difference in morbidity and mortality between the laparoscopic and open groups, allowing the trial to continue (Ann. Surg. 2010;251:417-20).
Eighty percent of patients on the trial had T1 disease. Within this subset, about 60% had T1a and 40% had T1b disease, Dr. Lee said.
In modified intention-to-treat analyses, patients in the laparoscopic group had a longer operation time (185 vs. 146 minutes) and fewer lymph nodes retrieved (41 vs. 43). But they had a lower estimated blood loss (119 vs. 194 mL) and shorter hospital stay (7.2 vs. 8.0 days).
The rate of surgical mortality was less than 1% and the rate of reoperation was about 1%, with no significant differences between groups.
Patients in the laparoscopic group had lower 30-day rates of postoperative morbidity (13.7% vs. 18.9%, P = .009), and the benefit of the less invasive approach remained significant after multivariate adjustment (odds ratio, 0.59; P = .001). Wound complications were half as common with laparoscopy (3.6% vs. 7.0%, P = .005).
Data on 5-year overall survival, the primary endpoint of the KLASS-01 trial, are expected later this year.
Dr. Kent asked, “In your country, for those patients with clinical T1a disease, how is a decision made regarding therapy?”
“I think if we can accurately diagnose the T stage as well as the N stage, we can surely adapt the endoscopic treatment for all the T1a cancer patients,” Dr. Lee replied. Such diagnosis has proved challenging, he added. Endoscopic resection is usually reserved for patients with tumors less than 2 cm in diameter showing differentiated histology and not invading the mucosa. “We have to move to the more accurate diagnosis of the TNM stage,” he concluded.
AT THE GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point: Laparoscopic distal gastrectomy has less morbidity than does open distal gastrectomy.
Major finding: Patients in the laparoscopic group had a 41% lower adjusted risk of 30-day postoperative morbidity.
Data source: A randomized phase III trial of 1,416 patients with clinical stage I gastric cancer.
Disclosures: Dr. Lee disclosed that he had no conflicts of interest.
Liposome-encapsulated irinotecan performs well in pancreatic cancer
SAN FRANCISCO – A nanoliposomal formulation of irinotecan, MM-398, prolongs survival for patients with metastatic pancreatic cancer by nearly 2 months when combined with conventional chemotherapy drugs, according to an expanded analysis of the NAPOLI-1 trial. Results were reported at the annual Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology.
“This study’s intention-to-treat analysis demonstrated a statistically significant increase in overall survival with the MM-398 plus 5-FU/LV combination … over 5-FU/LV,” said Dr. Li-Tzong Chen of the National Institute of Cancer Research, Tainan, Taiwan. “The safety profile suggests the combination is associated with a manageable toxicity profile.”
In contrast, analyses did not show any significant survival advantage of monotherapy with MM-398 (also known as nal-IRI), which achieves extended circulation of irinotecan in the blood relative to the conventional formulation.
As a result of accumulated data, MM-398 in combination with 5-FU/LV has received fast-track status from the Food and Drug Administration.
“The survival was similar to previous reports of FOLFIRI in second-line pancreas cancer,” said invited discussant Dr. Laura W. Goff, assistant professor of medicine (hematology/oncology) at the Vanderbilt-Ingram Cancer Center in Nashville, Tenn. “MM-398 appears to have activity in combination with 5-FU/LV in previously treated pancreas cancer.”
Session attendee Dr. Hanna Sanoff of the UNC Lineberger Comprehensive Cancer Center, Chapel Hill, N.C., said, “I’d like to ask a little bit of a provocative question if I could. [Dr. Goff] showed us data that FOLFIRI actually looked pretty similar to nal-irinotecan and fluorouracil, so do you think that this warrants FDA approval in the absence of an appropriate comparator with the existing cheaper drug?”
“At the current time, no, I would not approve FDA approval,” Dr. Goff replied. “But I think it would be interesting to see where that would shake out. Obviously, we see this in pancreas cancer as well as in other regimens, there is not as much interest in investigating the standard already-approved therapies. I don’t know if MM-398 with 5FU is significantly different than FOLFIRI. But I don’t have a lot of clinical experience with it.”
In additional comments provided in an interview, Dr. Goff said, “I am always happy to have more options in pancreas cancer … Figuring out what to do with [MM-398] is going to be the hard part, but I think it will be exciting to see how it translates into other regimens, possibly even in combination with oxaliplatin; I think the company has that planned. And certainly seeing where this has a role, either in front line or in previously treated gemcitabine patients, is going to be important.”
“It will require bigger studies to sort out exactly how efficacious it is and where the best place to use it is going to be, but it’s exciting to have agents to explore,” she concluded.
The international randomized phase III trial, sponsored by Merrimack Pharmaceuticals, enrolled 417 patients with metastatic pancreatic cancer who had previously received gemcitabine-based therapy. They were randomized to receive the investigational agent MM-398 alone, 5-fluorouracil (5-FU) plus leucovorin (LV) alone, or the combination.
Intention-to-treat analysis showed that median overall survival was 4.2 months with 5-FU/LV alone but 6.1 months with MM-398 plus 5-FU/LV (hazard ratio, 0.57; P = .0009), Dr. Chen reported.
Findings were similar across subgroups and in the per-protocol population, defined as patients who received at least 80% of the dose intensity of the protocol-defined treatment during the first 6 weeks of treatment (8.9 vs. 5.1 months; hazard ratio, 0.47; P = .0018).
In addition, the MM-398 plus 5-FU/LV combination was associated with a better median progression-free survival (3.1 vs. 1.5 months, P = .0001), overall response rate (16% vs. 1%, P less than .001), and cancer antigen 19-9 (CA19-9) response rate (36% vs. 12%, P = .0009).
The combination did lead to higher rates of dose reductions and dose delays, but treatment discontinuations were generally similar at 11% with the combination and 8% with 5-FU/LV alone.
Patients in the combination group were more likely to experience various grade 3 or worse toxicities, such as fatigue (14% vs. 4%), diarrhea (13% vs. 5%), and a reduction in neutrophil count (20% vs. 2%).
SAN FRANCISCO – A nanoliposomal formulation of irinotecan, MM-398, prolongs survival for patients with metastatic pancreatic cancer by nearly 2 months when combined with conventional chemotherapy drugs, according to an expanded analysis of the NAPOLI-1 trial. Results were reported at the annual Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology.
“This study’s intention-to-treat analysis demonstrated a statistically significant increase in overall survival with the MM-398 plus 5-FU/LV combination … over 5-FU/LV,” said Dr. Li-Tzong Chen of the National Institute of Cancer Research, Tainan, Taiwan. “The safety profile suggests the combination is associated with a manageable toxicity profile.”
In contrast, analyses did not show any significant survival advantage of monotherapy with MM-398 (also known as nal-IRI), which achieves extended circulation of irinotecan in the blood relative to the conventional formulation.
As a result of accumulated data, MM-398 in combination with 5-FU/LV has received fast-track status from the Food and Drug Administration.
“The survival was similar to previous reports of FOLFIRI in second-line pancreas cancer,” said invited discussant Dr. Laura W. Goff, assistant professor of medicine (hematology/oncology) at the Vanderbilt-Ingram Cancer Center in Nashville, Tenn. “MM-398 appears to have activity in combination with 5-FU/LV in previously treated pancreas cancer.”
Session attendee Dr. Hanna Sanoff of the UNC Lineberger Comprehensive Cancer Center, Chapel Hill, N.C., said, “I’d like to ask a little bit of a provocative question if I could. [Dr. Goff] showed us data that FOLFIRI actually looked pretty similar to nal-irinotecan and fluorouracil, so do you think that this warrants FDA approval in the absence of an appropriate comparator with the existing cheaper drug?”
“At the current time, no, I would not approve FDA approval,” Dr. Goff replied. “But I think it would be interesting to see where that would shake out. Obviously, we see this in pancreas cancer as well as in other regimens, there is not as much interest in investigating the standard already-approved therapies. I don’t know if MM-398 with 5FU is significantly different than FOLFIRI. But I don’t have a lot of clinical experience with it.”
In additional comments provided in an interview, Dr. Goff said, “I am always happy to have more options in pancreas cancer … Figuring out what to do with [MM-398] is going to be the hard part, but I think it will be exciting to see how it translates into other regimens, possibly even in combination with oxaliplatin; I think the company has that planned. And certainly seeing where this has a role, either in front line or in previously treated gemcitabine patients, is going to be important.”
“It will require bigger studies to sort out exactly how efficacious it is and where the best place to use it is going to be, but it’s exciting to have agents to explore,” she concluded.
The international randomized phase III trial, sponsored by Merrimack Pharmaceuticals, enrolled 417 patients with metastatic pancreatic cancer who had previously received gemcitabine-based therapy. They were randomized to receive the investigational agent MM-398 alone, 5-fluorouracil (5-FU) plus leucovorin (LV) alone, or the combination.
Intention-to-treat analysis showed that median overall survival was 4.2 months with 5-FU/LV alone but 6.1 months with MM-398 plus 5-FU/LV (hazard ratio, 0.57; P = .0009), Dr. Chen reported.
Findings were similar across subgroups and in the per-protocol population, defined as patients who received at least 80% of the dose intensity of the protocol-defined treatment during the first 6 weeks of treatment (8.9 vs. 5.1 months; hazard ratio, 0.47; P = .0018).
In addition, the MM-398 plus 5-FU/LV combination was associated with a better median progression-free survival (3.1 vs. 1.5 months, P = .0001), overall response rate (16% vs. 1%, P less than .001), and cancer antigen 19-9 (CA19-9) response rate (36% vs. 12%, P = .0009).
The combination did lead to higher rates of dose reductions and dose delays, but treatment discontinuations were generally similar at 11% with the combination and 8% with 5-FU/LV alone.
Patients in the combination group were more likely to experience various grade 3 or worse toxicities, such as fatigue (14% vs. 4%), diarrhea (13% vs. 5%), and a reduction in neutrophil count (20% vs. 2%).
SAN FRANCISCO – A nanoliposomal formulation of irinotecan, MM-398, prolongs survival for patients with metastatic pancreatic cancer by nearly 2 months when combined with conventional chemotherapy drugs, according to an expanded analysis of the NAPOLI-1 trial. Results were reported at the annual Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology.
“This study’s intention-to-treat analysis demonstrated a statistically significant increase in overall survival with the MM-398 plus 5-FU/LV combination … over 5-FU/LV,” said Dr. Li-Tzong Chen of the National Institute of Cancer Research, Tainan, Taiwan. “The safety profile suggests the combination is associated with a manageable toxicity profile.”
In contrast, analyses did not show any significant survival advantage of monotherapy with MM-398 (also known as nal-IRI), which achieves extended circulation of irinotecan in the blood relative to the conventional formulation.
As a result of accumulated data, MM-398 in combination with 5-FU/LV has received fast-track status from the Food and Drug Administration.
“The survival was similar to previous reports of FOLFIRI in second-line pancreas cancer,” said invited discussant Dr. Laura W. Goff, assistant professor of medicine (hematology/oncology) at the Vanderbilt-Ingram Cancer Center in Nashville, Tenn. “MM-398 appears to have activity in combination with 5-FU/LV in previously treated pancreas cancer.”
Session attendee Dr. Hanna Sanoff of the UNC Lineberger Comprehensive Cancer Center, Chapel Hill, N.C., said, “I’d like to ask a little bit of a provocative question if I could. [Dr. Goff] showed us data that FOLFIRI actually looked pretty similar to nal-irinotecan and fluorouracil, so do you think that this warrants FDA approval in the absence of an appropriate comparator with the existing cheaper drug?”
“At the current time, no, I would not approve FDA approval,” Dr. Goff replied. “But I think it would be interesting to see where that would shake out. Obviously, we see this in pancreas cancer as well as in other regimens, there is not as much interest in investigating the standard already-approved therapies. I don’t know if MM-398 with 5FU is significantly different than FOLFIRI. But I don’t have a lot of clinical experience with it.”
In additional comments provided in an interview, Dr. Goff said, “I am always happy to have more options in pancreas cancer … Figuring out what to do with [MM-398] is going to be the hard part, but I think it will be exciting to see how it translates into other regimens, possibly even in combination with oxaliplatin; I think the company has that planned. And certainly seeing where this has a role, either in front line or in previously treated gemcitabine patients, is going to be important.”
“It will require bigger studies to sort out exactly how efficacious it is and where the best place to use it is going to be, but it’s exciting to have agents to explore,” she concluded.
The international randomized phase III trial, sponsored by Merrimack Pharmaceuticals, enrolled 417 patients with metastatic pancreatic cancer who had previously received gemcitabine-based therapy. They were randomized to receive the investigational agent MM-398 alone, 5-fluorouracil (5-FU) plus leucovorin (LV) alone, or the combination.
Intention-to-treat analysis showed that median overall survival was 4.2 months with 5-FU/LV alone but 6.1 months with MM-398 plus 5-FU/LV (hazard ratio, 0.57; P = .0009), Dr. Chen reported.
Findings were similar across subgroups and in the per-protocol population, defined as patients who received at least 80% of the dose intensity of the protocol-defined treatment during the first 6 weeks of treatment (8.9 vs. 5.1 months; hazard ratio, 0.47; P = .0018).
In addition, the MM-398 plus 5-FU/LV combination was associated with a better median progression-free survival (3.1 vs. 1.5 months, P = .0001), overall response rate (16% vs. 1%, P less than .001), and cancer antigen 19-9 (CA19-9) response rate (36% vs. 12%, P = .0009).
The combination did lead to higher rates of dose reductions and dose delays, but treatment discontinuations were generally similar at 11% with the combination and 8% with 5-FU/LV alone.
Patients in the combination group were more likely to experience various grade 3 or worse toxicities, such as fatigue (14% vs. 4%), diarrhea (13% vs. 5%), and a reduction in neutrophil count (20% vs. 2%).
AT THE GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point: The combination of MM-398 plus 5-FU/LV was superior to 5-FU/LV alone.
Major finding: Median overall survival was 6.1 months with the combination versus 4.2 months with 5-FU/LV alone.
Data source: A randomized phase III trial among 417 patients with gemcitabine-treated metastatic pancreatic cancer.
Disclosures: Dr. Chen disclosed ties with PharmaEngine. The trial was sponsored by Merrimack Pharmaceuticals.