User login
Novel S. aureus vaccine found safe, immunogenic
SAN DIEGO – A new vaccine was safe and prompted a durable antibody response against pathogenic strains of Staphylococcus aureus, based on findings from a phase I trial conducted in 408 healthy adults.
The investigational three-antigen vaccine, known as SA3Ag, uses antigens that have established pathogenicity, Dr. Michael Nissen reported at IDWeek. "There was a rapid and robust functional antibody response to the vaccine antigens after a single dose and in all age groups," he said.
Analyses further suggested that a second (booster) dose of vaccine had only a modest additional effect on the immune response at 12 months. "High GMTs [geometric mean titers] for each antigen at 6 months, when the booster was administered, probably limited the booster response at this time," speculated Dr. Nissen, who is an associate professor with the Queensland Paediatric Infectious Diseases Laboratory in Brisbane, Australia.
He noted that a similar investigational four-antigen S. aureus vaccine is currently being tested in a pair of clinical trials (NCT 01364571 and NCT 01643941).
"Because of the high health care burden of S. aureus infections, there is an unmet need for an effective S. aureus vaccine," he commented. The new SA3Ag vaccine "has been developed based on known S. aureus virulence factors, namely capsular polysaccharides CP5 and CP8, and clumping factor A." The former are proteins that allow the pathogen to evade phagocytosis, whereas the latter enables it to adhere to host tissues.
Study participants were healthy adults aged 18-85 years. In a first randomization, they were assigned to receive a low, medium, or high dose of the vaccine, or a placebo; in a second randomization 6 months later, the initial vaccine groups were assigned to receive the same dose again (a booster) or a placebo.
Immunogenicity results showed that about 1 month after initial vaccination, there was a rise in titers of functional antibodies against the three vaccine antigens across all dose levels and across all age groups, Dr. Nissen reported.
After a single dose of the vaccine, the titers gradually waned over 12 months, but still remained well above those at baseline and well above those seen with placebo.
The booster dose of vaccine at 6 months only modestly improved on immunogenicity against one of the antigens, clumping factor A.
"Here, there was again a gradual decline in GMTs [of all antigens] through 12 months, but GMTs remained high at 12 months following booster, and again they were above placebo," Dr. Nissen commented. "Therefore, the timing of the booster dose warrants further investigation."
In terms of local reactions to the vaccine, the most common was pain at the injection site. Rates of pain, swelling, and redness all increased with dose, and were all higher after the booster dose than after the initial dose. Local reactions prompted a discontinuation of booster dosing.
In terms of systemic reactions, the overall rate was similar to that with placebo across the three vaccine dose levels. New or worsening muscle pain was more common after the vaccine than after placebo.
Fever developed after vaccination in 1%-2% of participants, regardless of vaccine dose. There were no vaccine-related serious adverse events or deaths during the 12-month study period.
IDWeek is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society
Dr. Nissen disclosed that he has received travel grants from Wyeth and Pfizer, and has been the principal investigator on trials sponsored by Abbott, Baxter, CSL Behring, and other companies.
SAN DIEGO – A new vaccine was safe and prompted a durable antibody response against pathogenic strains of Staphylococcus aureus, based on findings from a phase I trial conducted in 408 healthy adults.
The investigational three-antigen vaccine, known as SA3Ag, uses antigens that have established pathogenicity, Dr. Michael Nissen reported at IDWeek. "There was a rapid and robust functional antibody response to the vaccine antigens after a single dose and in all age groups," he said.
Analyses further suggested that a second (booster) dose of vaccine had only a modest additional effect on the immune response at 12 months. "High GMTs [geometric mean titers] for each antigen at 6 months, when the booster was administered, probably limited the booster response at this time," speculated Dr. Nissen, who is an associate professor with the Queensland Paediatric Infectious Diseases Laboratory in Brisbane, Australia.
He noted that a similar investigational four-antigen S. aureus vaccine is currently being tested in a pair of clinical trials (NCT 01364571 and NCT 01643941).
"Because of the high health care burden of S. aureus infections, there is an unmet need for an effective S. aureus vaccine," he commented. The new SA3Ag vaccine "has been developed based on known S. aureus virulence factors, namely capsular polysaccharides CP5 and CP8, and clumping factor A." The former are proteins that allow the pathogen to evade phagocytosis, whereas the latter enables it to adhere to host tissues.
Study participants were healthy adults aged 18-85 years. In a first randomization, they were assigned to receive a low, medium, or high dose of the vaccine, or a placebo; in a second randomization 6 months later, the initial vaccine groups were assigned to receive the same dose again (a booster) or a placebo.
Immunogenicity results showed that about 1 month after initial vaccination, there was a rise in titers of functional antibodies against the three vaccine antigens across all dose levels and across all age groups, Dr. Nissen reported.
After a single dose of the vaccine, the titers gradually waned over 12 months, but still remained well above those at baseline and well above those seen with placebo.
The booster dose of vaccine at 6 months only modestly improved on immunogenicity against one of the antigens, clumping factor A.
"Here, there was again a gradual decline in GMTs [of all antigens] through 12 months, but GMTs remained high at 12 months following booster, and again they were above placebo," Dr. Nissen commented. "Therefore, the timing of the booster dose warrants further investigation."
In terms of local reactions to the vaccine, the most common was pain at the injection site. Rates of pain, swelling, and redness all increased with dose, and were all higher after the booster dose than after the initial dose. Local reactions prompted a discontinuation of booster dosing.
In terms of systemic reactions, the overall rate was similar to that with placebo across the three vaccine dose levels. New or worsening muscle pain was more common after the vaccine than after placebo.
Fever developed after vaccination in 1%-2% of participants, regardless of vaccine dose. There were no vaccine-related serious adverse events or deaths during the 12-month study period.
IDWeek is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society
Dr. Nissen disclosed that he has received travel grants from Wyeth and Pfizer, and has been the principal investigator on trials sponsored by Abbott, Baxter, CSL Behring, and other companies.
SAN DIEGO – A new vaccine was safe and prompted a durable antibody response against pathogenic strains of Staphylococcus aureus, based on findings from a phase I trial conducted in 408 healthy adults.
The investigational three-antigen vaccine, known as SA3Ag, uses antigens that have established pathogenicity, Dr. Michael Nissen reported at IDWeek. "There was a rapid and robust functional antibody response to the vaccine antigens after a single dose and in all age groups," he said.
Analyses further suggested that a second (booster) dose of vaccine had only a modest additional effect on the immune response at 12 months. "High GMTs [geometric mean titers] for each antigen at 6 months, when the booster was administered, probably limited the booster response at this time," speculated Dr. Nissen, who is an associate professor with the Queensland Paediatric Infectious Diseases Laboratory in Brisbane, Australia.
He noted that a similar investigational four-antigen S. aureus vaccine is currently being tested in a pair of clinical trials (NCT 01364571 and NCT 01643941).
"Because of the high health care burden of S. aureus infections, there is an unmet need for an effective S. aureus vaccine," he commented. The new SA3Ag vaccine "has been developed based on known S. aureus virulence factors, namely capsular polysaccharides CP5 and CP8, and clumping factor A." The former are proteins that allow the pathogen to evade phagocytosis, whereas the latter enables it to adhere to host tissues.
Study participants were healthy adults aged 18-85 years. In a first randomization, they were assigned to receive a low, medium, or high dose of the vaccine, or a placebo; in a second randomization 6 months later, the initial vaccine groups were assigned to receive the same dose again (a booster) or a placebo.
Immunogenicity results showed that about 1 month after initial vaccination, there was a rise in titers of functional antibodies against the three vaccine antigens across all dose levels and across all age groups, Dr. Nissen reported.
After a single dose of the vaccine, the titers gradually waned over 12 months, but still remained well above those at baseline and well above those seen with placebo.
The booster dose of vaccine at 6 months only modestly improved on immunogenicity against one of the antigens, clumping factor A.
"Here, there was again a gradual decline in GMTs [of all antigens] through 12 months, but GMTs remained high at 12 months following booster, and again they were above placebo," Dr. Nissen commented. "Therefore, the timing of the booster dose warrants further investigation."
In terms of local reactions to the vaccine, the most common was pain at the injection site. Rates of pain, swelling, and redness all increased with dose, and were all higher after the booster dose than after the initial dose. Local reactions prompted a discontinuation of booster dosing.
In terms of systemic reactions, the overall rate was similar to that with placebo across the three vaccine dose levels. New or worsening muscle pain was more common after the vaccine than after placebo.
Fever developed after vaccination in 1%-2% of participants, regardless of vaccine dose. There were no vaccine-related serious adverse events or deaths during the 12-month study period.
IDWeek is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society
Dr. Nissen disclosed that he has received travel grants from Wyeth and Pfizer, and has been the principal investigator on trials sponsored by Abbott, Baxter, CSL Behring, and other companies.
AT IDWEEK
Major Finding: The vaccine was highly immunogenic across dose levels and age groups, and was safe and well tolerated, although local reactions prompted a discontinuation of booster dosing.
Data Source: A randomized, placebo-controlled, phase I, first-in-human trial in 408 healthy adults.
Disclosures: Dr. Nissen disclosed that he has received travel grants from Wyeth and Pfizer, and has been the principal investigator on trials sponsored by Abbott, Baxter, CSL Behring, and other companies.
Medication errors prevalent among HIV-positive inpatients
SAN DIEGO – Half of all HIV-positive inpatients experience antiretroviral medication errors during their stay, and these errors often go undetected and uncorrected, suggest a pair of studies reported in poster sessions at IDWeek 2012.
In the first study, a team led by Elizabeth A. Neuner, Pharm.D., of the Cleveland Clinic reviewed the electronic medical records of 162 HIV-positive adults admitted to the hospital during a 10-month period.
During a median hospital stay of 4 days, there were 126 medication errors, for a rate of 1.6 errors per admission. On a per-patient basis, 50% of patients had at least one medication error.
The most common medication errors were major drug interactions (26%), incorrect dosing (20%), and interactions involving contraindicated medications (12%).
Fully 65% of the errors were neither detected nor corrected during the patient’s stay, according to Dr. Neuner. Errors were significantly more likely to be detected and resolved if an infectious disease physician was consulted on the case (47% vs. 15%, P = .002), "so I think getting an ID consult can help with the resolution rate," she commented in an interview.
"We have focused a lot on quality improvement efforts in the hospital to try to reduce the number of errors that happen and also improve the resolution rate," she said. "It has really been a multidisciplinary approach. We have focused on education and transitions of care. We updated our electronic medication files to include common dose buttons and dose frequencies and removed buttons and frequencies that weren’t relevant anymore. And we have been doing some stewardship efforts in collaboration with the infectious disease physicians."
In the second study, a team led by Natasha N. Pettit, Pharm.D., of the University of Chicago began a program to evaluate the highly active antiretroviral therapy (HAART) regimens of HIV-positive inpatients within 12-24 hours of hospital admission or initiation of HAART regimens.
Given their complexity, "HAART regimens have a high potential for drug-drug interactions, adverse drug events, and dosing errors. Missed doses or inadvertent changes in therapy can quickly lead to resistance or toxicity," Dr. Pettit said in an interview.
Among the 155 patients whose regimens were evaluated in a 17-month period, 49% had a regimen containing some kind of error. Of these patients, 47% were errors related to drug dosage and 6% were related to drug interactions.
Protease inhibitors were significantly more often associated with errors, compared with other classes of antiretroviral medications, she said.
Interventions at or prior to the point of order entry, including providing resources to prescribers who may be less familiar with HAART regimens, can give some guidance while physicians are entering orders for these complex regimens, Dr. Pettit said. A process for reconciliation of HAART regimens before orders are placed also is recommended.
IDWeek is the combined annual meeting of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Neuner and Dr. Pettit disclosed no relevant conflicts of interest.
SAN DIEGO – Half of all HIV-positive inpatients experience antiretroviral medication errors during their stay, and these errors often go undetected and uncorrected, suggest a pair of studies reported in poster sessions at IDWeek 2012.
In the first study, a team led by Elizabeth A. Neuner, Pharm.D., of the Cleveland Clinic reviewed the electronic medical records of 162 HIV-positive adults admitted to the hospital during a 10-month period.
During a median hospital stay of 4 days, there were 126 medication errors, for a rate of 1.6 errors per admission. On a per-patient basis, 50% of patients had at least one medication error.
The most common medication errors were major drug interactions (26%), incorrect dosing (20%), and interactions involving contraindicated medications (12%).
Fully 65% of the errors were neither detected nor corrected during the patient’s stay, according to Dr. Neuner. Errors were significantly more likely to be detected and resolved if an infectious disease physician was consulted on the case (47% vs. 15%, P = .002), "so I think getting an ID consult can help with the resolution rate," she commented in an interview.
"We have focused a lot on quality improvement efforts in the hospital to try to reduce the number of errors that happen and also improve the resolution rate," she said. "It has really been a multidisciplinary approach. We have focused on education and transitions of care. We updated our electronic medication files to include common dose buttons and dose frequencies and removed buttons and frequencies that weren’t relevant anymore. And we have been doing some stewardship efforts in collaboration with the infectious disease physicians."
In the second study, a team led by Natasha N. Pettit, Pharm.D., of the University of Chicago began a program to evaluate the highly active antiretroviral therapy (HAART) regimens of HIV-positive inpatients within 12-24 hours of hospital admission or initiation of HAART regimens.
Given their complexity, "HAART regimens have a high potential for drug-drug interactions, adverse drug events, and dosing errors. Missed doses or inadvertent changes in therapy can quickly lead to resistance or toxicity," Dr. Pettit said in an interview.
Among the 155 patients whose regimens were evaluated in a 17-month period, 49% had a regimen containing some kind of error. Of these patients, 47% were errors related to drug dosage and 6% were related to drug interactions.
Protease inhibitors were significantly more often associated with errors, compared with other classes of antiretroviral medications, she said.
Interventions at or prior to the point of order entry, including providing resources to prescribers who may be less familiar with HAART regimens, can give some guidance while physicians are entering orders for these complex regimens, Dr. Pettit said. A process for reconciliation of HAART regimens before orders are placed also is recommended.
IDWeek is the combined annual meeting of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Neuner and Dr. Pettit disclosed no relevant conflicts of interest.
SAN DIEGO – Half of all HIV-positive inpatients experience antiretroviral medication errors during their stay, and these errors often go undetected and uncorrected, suggest a pair of studies reported in poster sessions at IDWeek 2012.
In the first study, a team led by Elizabeth A. Neuner, Pharm.D., of the Cleveland Clinic reviewed the electronic medical records of 162 HIV-positive adults admitted to the hospital during a 10-month period.
During a median hospital stay of 4 days, there were 126 medication errors, for a rate of 1.6 errors per admission. On a per-patient basis, 50% of patients had at least one medication error.
The most common medication errors were major drug interactions (26%), incorrect dosing (20%), and interactions involving contraindicated medications (12%).
Fully 65% of the errors were neither detected nor corrected during the patient’s stay, according to Dr. Neuner. Errors were significantly more likely to be detected and resolved if an infectious disease physician was consulted on the case (47% vs. 15%, P = .002), "so I think getting an ID consult can help with the resolution rate," she commented in an interview.
"We have focused a lot on quality improvement efforts in the hospital to try to reduce the number of errors that happen and also improve the resolution rate," she said. "It has really been a multidisciplinary approach. We have focused on education and transitions of care. We updated our electronic medication files to include common dose buttons and dose frequencies and removed buttons and frequencies that weren’t relevant anymore. And we have been doing some stewardship efforts in collaboration with the infectious disease physicians."
In the second study, a team led by Natasha N. Pettit, Pharm.D., of the University of Chicago began a program to evaluate the highly active antiretroviral therapy (HAART) regimens of HIV-positive inpatients within 12-24 hours of hospital admission or initiation of HAART regimens.
Given their complexity, "HAART regimens have a high potential for drug-drug interactions, adverse drug events, and dosing errors. Missed doses or inadvertent changes in therapy can quickly lead to resistance or toxicity," Dr. Pettit said in an interview.
Among the 155 patients whose regimens were evaluated in a 17-month period, 49% had a regimen containing some kind of error. Of these patients, 47% were errors related to drug dosage and 6% were related to drug interactions.
Protease inhibitors were significantly more often associated with errors, compared with other classes of antiretroviral medications, she said.
Interventions at or prior to the point of order entry, including providing resources to prescribers who may be less familiar with HAART regimens, can give some guidance while physicians are entering orders for these complex regimens, Dr. Pettit said. A process for reconciliation of HAART regimens before orders are placed also is recommended.
IDWeek is the combined annual meeting of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Neuner and Dr. Pettit disclosed no relevant conflicts of interest.
AT IDWEEK 2012
Major Finding: Fully 50% of patients had an error in an antiretroviral regimen or an opportunistic infection medication, and 65% were neither recognized nor corrected by discharge.
Data Source: A retrospective study of 162 HIV-positive inpatients and a prospective study of 155 HIV-positive inpatients.
Disclosures: Dr. Neuner and Dr. Pettit disclosed no relevant conflicts of interest.
PCV13 vaccine benefits extend to nonimmune children
SAN DIEGO – Children who receive just one of the recommended two doses of the 13-valent pneumococcal conjugate vaccine still derive indirect protection, new data suggest.
Colonization rates were similar for immune and nonimmune children, based on surveillance data for the first 2 years after the vaccine’s introduction. The findings come from a study at the Boston Medical Center of 4,338 children under age 60 months. Roughly one-third were considered nonimmune because they did not receive sufficient doses of the vaccine.
Nasopharyngeal colonization with PCV13-unique serotypes fell in the overall population after the introduction of the vaccine. Importantly, colonization declined by at least 50% in the nonimmune group at about 1.5 years after the vaccine was introduced, when roughly 75% of eligible children had received it.
Although it took time for the nonimmune group to catch up with their immune peers, the vaccine reduced serotype-specific colonization in children regardless of their immune status, commented Dr. Stephen I. Pelton, a professor of pediatrics and epidemiology at Boston University. "No change in overall prevalence of pneumococcal colonization is observed," he said at IDWeek.
Introduction of the similar PCV7 vaccine was associated with a greater reduction of cases of pneumococcal disease in nonimmunized children than in their immunized counterparts (MBio. 2011;2:e00309-10). The researchers in that study attributed the shared benefits to the vaccine’s impact in reducing nasopharyngeal carriage and transmission of vaccine serotypes.
The records of children aged 59 months or younger who received care at the Boston Medical Center’s primary care center were reviewed to determine vaccination status. Children under age 12 months were considered immune if they received two doses of PCV13 vaccine, and older children were presumed immune if they received one dose.
Pneumococcal colonization was based on the serotypes of Streptococcus pneumoniae isolates obtained from nasopharyngeal swab samples. Colonization analyses used 25-week rolling intervals, for example, weeks 1-25, weeks 2-26, and so on.
Analyses showed good uptake of the vaccine in all age groups. The percentage of children considered immune rose steadily over the 2-year period and peaked at 80%, Dr. Pelton reported.
On average, 32% of children were considered nonimmune during the study period, but the proportion decreased over time.
The overall prevalence of colonization was essentially stable during the study period, but the prevalence of colonization specifically with PCV13-unique serotypes fell. A distinct seasonal pattern was also evident.
"By week 28, we could already see a difference between the unimmunized group and the immunized group," Dr. Pelton explained. "We saw a rapid increase in PCV13 carriage during the fall, projecting on into the winter season [in the former], whereas we have a blunting in the children who are immunized in terms of the acquisition of PCV13 serotypes."
An indirect effect of the vaccine – a reduction by at least 50% in colonization with PCV13-unique serotypes that persisted among nonimmune children during a comparable season – was achieved at week 81. At that point the prevalence stood at about 3 cases per 100 nonimmune children. This outcome occurred when vaccine uptake reached 75%.
Colonization among nonimmune children fell to the levels seen among immune children at week 52 after the PCV13 vaccine was introduced. By this time, vaccine uptake hit 65%.
The researcher also looked at the impact of a single dose of PCV13 vaccine among children 24-59 months of age during the first half-year after the vaccine’s introduction. Colonization with PCV13-unique serotypes rose during the fall and winter months among children who did not receive any vaccine doses, but the rise was blunted among those who received one dose of vaccine.
The blunting of colonization "strongly suggests to us that a single dose is adequate to prevent colonization with PCV13 serotypes, and we can say that because [the blunting] occurs very early, before we would expect, we were able to observe a significant indirect effect in the population," Dr. Pelton said.
IDWeek is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Pelton disclosed that he has received honoraria and research funding from Merck, GlaxoSmithKline, and Pfizer. The study was funded by the Thrasher Research Foundation and Pfizer.
Nasopharyngeal colonization with PCV13-unique serotypes, Dr. Stephen I. Pelton, pneumococcal colonization, IDWeek, PCV7 vaccine,
SAN DIEGO – Children who receive just one of the recommended two doses of the 13-valent pneumococcal conjugate vaccine still derive indirect protection, new data suggest.
Colonization rates were similar for immune and nonimmune children, based on surveillance data for the first 2 years after the vaccine’s introduction. The findings come from a study at the Boston Medical Center of 4,338 children under age 60 months. Roughly one-third were considered nonimmune because they did not receive sufficient doses of the vaccine.
Nasopharyngeal colonization with PCV13-unique serotypes fell in the overall population after the introduction of the vaccine. Importantly, colonization declined by at least 50% in the nonimmune group at about 1.5 years after the vaccine was introduced, when roughly 75% of eligible children had received it.
Although it took time for the nonimmune group to catch up with their immune peers, the vaccine reduced serotype-specific colonization in children regardless of their immune status, commented Dr. Stephen I. Pelton, a professor of pediatrics and epidemiology at Boston University. "No change in overall prevalence of pneumococcal colonization is observed," he said at IDWeek.
Introduction of the similar PCV7 vaccine was associated with a greater reduction of cases of pneumococcal disease in nonimmunized children than in their immunized counterparts (MBio. 2011;2:e00309-10). The researchers in that study attributed the shared benefits to the vaccine’s impact in reducing nasopharyngeal carriage and transmission of vaccine serotypes.
The records of children aged 59 months or younger who received care at the Boston Medical Center’s primary care center were reviewed to determine vaccination status. Children under age 12 months were considered immune if they received two doses of PCV13 vaccine, and older children were presumed immune if they received one dose.
Pneumococcal colonization was based on the serotypes of Streptococcus pneumoniae isolates obtained from nasopharyngeal swab samples. Colonization analyses used 25-week rolling intervals, for example, weeks 1-25, weeks 2-26, and so on.
Analyses showed good uptake of the vaccine in all age groups. The percentage of children considered immune rose steadily over the 2-year period and peaked at 80%, Dr. Pelton reported.
On average, 32% of children were considered nonimmune during the study period, but the proportion decreased over time.
The overall prevalence of colonization was essentially stable during the study period, but the prevalence of colonization specifically with PCV13-unique serotypes fell. A distinct seasonal pattern was also evident.
"By week 28, we could already see a difference between the unimmunized group and the immunized group," Dr. Pelton explained. "We saw a rapid increase in PCV13 carriage during the fall, projecting on into the winter season [in the former], whereas we have a blunting in the children who are immunized in terms of the acquisition of PCV13 serotypes."
An indirect effect of the vaccine – a reduction by at least 50% in colonization with PCV13-unique serotypes that persisted among nonimmune children during a comparable season – was achieved at week 81. At that point the prevalence stood at about 3 cases per 100 nonimmune children. This outcome occurred when vaccine uptake reached 75%.
Colonization among nonimmune children fell to the levels seen among immune children at week 52 after the PCV13 vaccine was introduced. By this time, vaccine uptake hit 65%.
The researcher also looked at the impact of a single dose of PCV13 vaccine among children 24-59 months of age during the first half-year after the vaccine’s introduction. Colonization with PCV13-unique serotypes rose during the fall and winter months among children who did not receive any vaccine doses, but the rise was blunted among those who received one dose of vaccine.
The blunting of colonization "strongly suggests to us that a single dose is adequate to prevent colonization with PCV13 serotypes, and we can say that because [the blunting] occurs very early, before we would expect, we were able to observe a significant indirect effect in the population," Dr. Pelton said.
IDWeek is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Pelton disclosed that he has received honoraria and research funding from Merck, GlaxoSmithKline, and Pfizer. The study was funded by the Thrasher Research Foundation and Pfizer.
SAN DIEGO – Children who receive just one of the recommended two doses of the 13-valent pneumococcal conjugate vaccine still derive indirect protection, new data suggest.
Colonization rates were similar for immune and nonimmune children, based on surveillance data for the first 2 years after the vaccine’s introduction. The findings come from a study at the Boston Medical Center of 4,338 children under age 60 months. Roughly one-third were considered nonimmune because they did not receive sufficient doses of the vaccine.
Nasopharyngeal colonization with PCV13-unique serotypes fell in the overall population after the introduction of the vaccine. Importantly, colonization declined by at least 50% in the nonimmune group at about 1.5 years after the vaccine was introduced, when roughly 75% of eligible children had received it.
Although it took time for the nonimmune group to catch up with their immune peers, the vaccine reduced serotype-specific colonization in children regardless of their immune status, commented Dr. Stephen I. Pelton, a professor of pediatrics and epidemiology at Boston University. "No change in overall prevalence of pneumococcal colonization is observed," he said at IDWeek.
Introduction of the similar PCV7 vaccine was associated with a greater reduction of cases of pneumococcal disease in nonimmunized children than in their immunized counterparts (MBio. 2011;2:e00309-10). The researchers in that study attributed the shared benefits to the vaccine’s impact in reducing nasopharyngeal carriage and transmission of vaccine serotypes.
The records of children aged 59 months or younger who received care at the Boston Medical Center’s primary care center were reviewed to determine vaccination status. Children under age 12 months were considered immune if they received two doses of PCV13 vaccine, and older children were presumed immune if they received one dose.
Pneumococcal colonization was based on the serotypes of Streptococcus pneumoniae isolates obtained from nasopharyngeal swab samples. Colonization analyses used 25-week rolling intervals, for example, weeks 1-25, weeks 2-26, and so on.
Analyses showed good uptake of the vaccine in all age groups. The percentage of children considered immune rose steadily over the 2-year period and peaked at 80%, Dr. Pelton reported.
On average, 32% of children were considered nonimmune during the study period, but the proportion decreased over time.
The overall prevalence of colonization was essentially stable during the study period, but the prevalence of colonization specifically with PCV13-unique serotypes fell. A distinct seasonal pattern was also evident.
"By week 28, we could already see a difference between the unimmunized group and the immunized group," Dr. Pelton explained. "We saw a rapid increase in PCV13 carriage during the fall, projecting on into the winter season [in the former], whereas we have a blunting in the children who are immunized in terms of the acquisition of PCV13 serotypes."
An indirect effect of the vaccine – a reduction by at least 50% in colonization with PCV13-unique serotypes that persisted among nonimmune children during a comparable season – was achieved at week 81. At that point the prevalence stood at about 3 cases per 100 nonimmune children. This outcome occurred when vaccine uptake reached 75%.
Colonization among nonimmune children fell to the levels seen among immune children at week 52 after the PCV13 vaccine was introduced. By this time, vaccine uptake hit 65%.
The researcher also looked at the impact of a single dose of PCV13 vaccine among children 24-59 months of age during the first half-year after the vaccine’s introduction. Colonization with PCV13-unique serotypes rose during the fall and winter months among children who did not receive any vaccine doses, but the rise was blunted among those who received one dose of vaccine.
The blunting of colonization "strongly suggests to us that a single dose is adequate to prevent colonization with PCV13 serotypes, and we can say that because [the blunting] occurs very early, before we would expect, we were able to observe a significant indirect effect in the population," Dr. Pelton said.
IDWeek is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Pelton disclosed that he has received honoraria and research funding from Merck, GlaxoSmithKline, and Pfizer. The study was funded by the Thrasher Research Foundation and Pfizer.
Nasopharyngeal colonization with PCV13-unique serotypes, Dr. Stephen I. Pelton, pneumococcal colonization, IDWeek, PCV7 vaccine,
Nasopharyngeal colonization with PCV13-unique serotypes, Dr. Stephen I. Pelton, pneumococcal colonization, IDWeek, PCV7 vaccine,
AT IDWEEK
Major Finding: Colonization among nonimmune children fell to the levels seen among immune children at week 52 after the PCV13 vaccine was introduced. By this time, vaccine uptake hit 65%.
Data Source: A single-center surveillance study of 4,338 children under 60 months of age.
Disclosures: Dr. Pelton disclosed that he has received honoraria and research funding from Merck, GlaxoSmithKline, and Pfizer. The study was funded by the Thrasher Research Foundation and Pfizer.
Antibiotic Stewardship Crucial in Special Pediatric Populations
SAN DIEGO – Antibiotic stewardship is important for every patient population, but it’s especially crucial in the care of infants hospitalized in the neonatal intensive care unit and in infants and children with cystic fibrosis, Dr. Lisa Saiman said at IDWeek 2012.
The use of antibiotics in these two pediatric populations "is clearly different," said Dr. Saiman, professor of clinical pediatrics at Columbia University Medical Center, N.Y. "In the NICU, approximately 80% of preterm babies will get IV antibiotics for early-onset sepsis and 20%-30% for late-onset sepsis. In the CF population, everybody gets antibiotics throughout their entire lives. In the NICU, we use relatively few agents because of concerns about toxicity and dosing, while in the CF population the number of agents used is enormous."
Both of these patient populations "have existing networks consisting of care centers of excellence and very motivated providers," noted Dr. Saiman, who is also the hospital epidemiologist for Morgan Stanley Children’s Hospital of New York-Presbyterian. "In the United States, it’s estimated that about 500,000 infants are hospitalized in the neonatal ICU each year, and there are about 30,000 people with CF, making this a rare disease."
In a published study of 323 courses of antibiotics administered in the NICU, Dr. Saiman and her associates found that 35% of infants received at least 1 day of inappropriate antibiotics (Pediatr. Infect. Dis. J. 2009;28:1047-51). The consequences of inappropriate antibiotic use in these two patient populations are potentially dire. In NICU patients, Dr. Saiman said, this practice could result in necrotizing endocarditis, candidemia, nephrotoxicity, ototoxicity, and drug-drug interactions. In CF patients, inappropriate antibiotic use could lead to the emergence of multidrug-resistant strains of Pseudomonas aeruginosa and of other organisms, life-threatening allergies, nephrotoxicity, ototoxicity, hepatotoxicity, phototoxicity, Clostridium difficile infection, and drug-drug interactions.
To optimize antibiotic prescribing practices in these two patient populations, Dr. Saiman advised obtaining "buy-in from key stakeholders from the beginning. Review and implement evidence-based practices to develop local guidelines. Obtain local data and provide feedback to your care teams."
Dr. Saiman disclosed that she served on the advisory boards of Novartis, Vertex, and Insmed. She has also received funding from the CF Foundation.
IDWeek is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
SAN DIEGO – Antibiotic stewardship is important for every patient population, but it’s especially crucial in the care of infants hospitalized in the neonatal intensive care unit and in infants and children with cystic fibrosis, Dr. Lisa Saiman said at IDWeek 2012.
The use of antibiotics in these two pediatric populations "is clearly different," said Dr. Saiman, professor of clinical pediatrics at Columbia University Medical Center, N.Y. "In the NICU, approximately 80% of preterm babies will get IV antibiotics for early-onset sepsis and 20%-30% for late-onset sepsis. In the CF population, everybody gets antibiotics throughout their entire lives. In the NICU, we use relatively few agents because of concerns about toxicity and dosing, while in the CF population the number of agents used is enormous."
Both of these patient populations "have existing networks consisting of care centers of excellence and very motivated providers," noted Dr. Saiman, who is also the hospital epidemiologist for Morgan Stanley Children’s Hospital of New York-Presbyterian. "In the United States, it’s estimated that about 500,000 infants are hospitalized in the neonatal ICU each year, and there are about 30,000 people with CF, making this a rare disease."
In a published study of 323 courses of antibiotics administered in the NICU, Dr. Saiman and her associates found that 35% of infants received at least 1 day of inappropriate antibiotics (Pediatr. Infect. Dis. J. 2009;28:1047-51). The consequences of inappropriate antibiotic use in these two patient populations are potentially dire. In NICU patients, Dr. Saiman said, this practice could result in necrotizing endocarditis, candidemia, nephrotoxicity, ototoxicity, and drug-drug interactions. In CF patients, inappropriate antibiotic use could lead to the emergence of multidrug-resistant strains of Pseudomonas aeruginosa and of other organisms, life-threatening allergies, nephrotoxicity, ototoxicity, hepatotoxicity, phototoxicity, Clostridium difficile infection, and drug-drug interactions.
To optimize antibiotic prescribing practices in these two patient populations, Dr. Saiman advised obtaining "buy-in from key stakeholders from the beginning. Review and implement evidence-based practices to develop local guidelines. Obtain local data and provide feedback to your care teams."
Dr. Saiman disclosed that she served on the advisory boards of Novartis, Vertex, and Insmed. She has also received funding from the CF Foundation.
IDWeek is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
SAN DIEGO – Antibiotic stewardship is important for every patient population, but it’s especially crucial in the care of infants hospitalized in the neonatal intensive care unit and in infants and children with cystic fibrosis, Dr. Lisa Saiman said at IDWeek 2012.
The use of antibiotics in these two pediatric populations "is clearly different," said Dr. Saiman, professor of clinical pediatrics at Columbia University Medical Center, N.Y. "In the NICU, approximately 80% of preterm babies will get IV antibiotics for early-onset sepsis and 20%-30% for late-onset sepsis. In the CF population, everybody gets antibiotics throughout their entire lives. In the NICU, we use relatively few agents because of concerns about toxicity and dosing, while in the CF population the number of agents used is enormous."
Both of these patient populations "have existing networks consisting of care centers of excellence and very motivated providers," noted Dr. Saiman, who is also the hospital epidemiologist for Morgan Stanley Children’s Hospital of New York-Presbyterian. "In the United States, it’s estimated that about 500,000 infants are hospitalized in the neonatal ICU each year, and there are about 30,000 people with CF, making this a rare disease."
In a published study of 323 courses of antibiotics administered in the NICU, Dr. Saiman and her associates found that 35% of infants received at least 1 day of inappropriate antibiotics (Pediatr. Infect. Dis. J. 2009;28:1047-51). The consequences of inappropriate antibiotic use in these two patient populations are potentially dire. In NICU patients, Dr. Saiman said, this practice could result in necrotizing endocarditis, candidemia, nephrotoxicity, ototoxicity, and drug-drug interactions. In CF patients, inappropriate antibiotic use could lead to the emergence of multidrug-resistant strains of Pseudomonas aeruginosa and of other organisms, life-threatening allergies, nephrotoxicity, ototoxicity, hepatotoxicity, phototoxicity, Clostridium difficile infection, and drug-drug interactions.
To optimize antibiotic prescribing practices in these two patient populations, Dr. Saiman advised obtaining "buy-in from key stakeholders from the beginning. Review and implement evidence-based practices to develop local guidelines. Obtain local data and provide feedback to your care teams."
Dr. Saiman disclosed that she served on the advisory boards of Novartis, Vertex, and Insmed. She has also received funding from the CF Foundation.
IDWeek is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
EXPERT ANALYSIS FROM IDWEEK 2012
Gastric Acid Drugs, Antibiotics Tied to Recurrent C. difficile Risk
SAN DIEGO – A variety of factors, some of them modifiable, increase the risk of recurrence of Clostridium difficile infection among inpatients, researchers reported at IDWeek.
The team retrospectively studied 4,200 adult inpatients who had an initial C. difficile infection, defined as a positive toxin assay at hospital admission in the setting of unformed stools. Overall, 10% went on to experience a recurrence within 6 weeks.
Results showed that patients had a more than one-third increase in recurrence risk if they were started on a new gastric acid–suppressing agent during treatment of their initial infection, and a near tripling of the risk if they were started on a high-risk antibiotic after completing treatment.
"Recognizing both modifiable and nonmodifiable recurrent C. difficile infection risk factors may help clinicians tailor the therapy for the initial episode of C. difficile infection more precisely," presenting author Dr. Erik R. Dubberke commented.
"Some characteristics, such as age, were not modifiable, but there were several modifiable exposures, such as gastric acid suppression as well as antimicrobial exposures during as well as after the treatment for the initial C. difficile infection," he said. "Reducing these exposures may help decrease the risk of developing future episodes of C. difficile infection." Dr. Dubberke is with the department of medicine at the Washington University in St. Louis.
Audience members inquired about the finer points of the investigation.
One attendee was "curious" about the elevated risk seen after receipt of intravenous vancomycin, "since this is a drug that doesn’t get into the stool at all." He wondered if perhaps other antibiotics given with vancomycin were actually at play.
Dr. Dubberke noted that multivariate analysis took this into consideration, and receipt of vancomycin after completing treatment was still a risk factor. "There are data to suggest that even intravenous vancomycin – though you don’t get adequate levels to treat C. difficile in the colon – can potentially get into the colon and affect the microbiome. So it’s possible there may be some direct causation," he said. "Conversely ... people who receive intravenous vancomycin tend to get other antibiotics. They also tend to be sicker patients, patients at increased risk for having drug-resistant organisms. So it is possible this is just a marker for recurrent C. difficile based on the patient characteristics."
Regarding whether severity of the initial infection affected recurrence risk, the research did not find an association on multivariate analysis with white count, new-onset renal dysfunction, baseline renal dysfunction, being in the ICU or being transferred to the ICU, or initiation of vasopressors, Dr. Dubberke explained. He speculated that lack of association was due in part to the competing risk of death.
"If a patient is very sick from C. difficile, they may die. Our [group] has demonstrated that you have an increased risk of death even after that initial episode is treated. So maybe that is why were not seeing [severity] as a risk for recurrent C. difficile."
Another attendee asked about the finding of elevated risk associated with gastric acid–suppressing medications. "[Have you] taken any practical steps at your hospital or in your health system to try to modify that risk factor because it’s medication that we clearly use and prescribe, and it’s questionable as to whether these prescriptions actually meet the indications ... " she said.
"We have not done anything in regard to interventions at this point based on the data," Dr. Dubberke replied.
The investigators identified patients having an initial C. difficile infection on admission to Barnes-Jewish Hospital, St. Louis, between 2003 and 2009, and classified those infections according to standard definitions as health care–onset infections; community-onset, health care facility–associated infections; or community-associated infections.
They classified antibiotics received as high risk (clindamycin, cephalosporins, and aminopenicillins), intravenous vancomycin, fluoroquinolones, and low risk (all others).
Study results shows that, compared with their peers who did not have a recurrence, patients who did were older and more likely to have diabetes, had higher levels of comorbidity, were more likely to have a community-onset, health care facility–associated initial infection, and were more likely to have been hospitalized in the past 60 days, Dr. Dubberke reported at the meeting, which is a the combined annual meeting of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society..
In multivariate analyses, patients were significantly more likely to have a recurrence of C. difficile if their initial infection was a community-onset, health care facility–associated infection as compared with a health care–onset infection (hazard ratio, 1.80) or they had at least two hospitalizations in the 60 days before that infection (HR, 1.40). Additionally, risk increased with age (HR, 1.01).
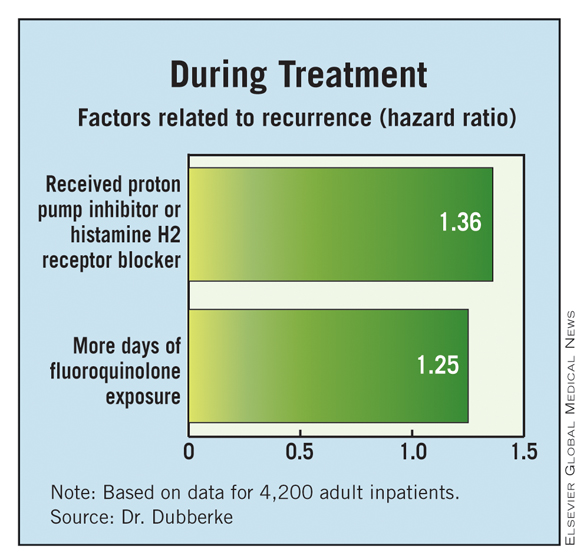
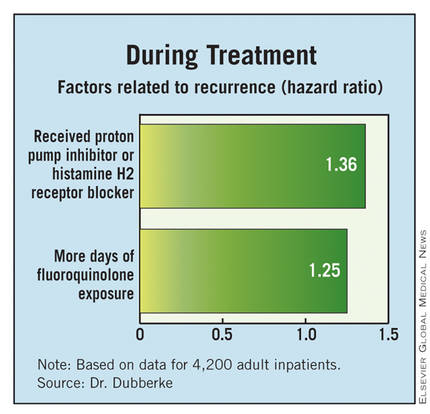
With respect to factors during the treatment of the initial infection, patients were more likely to have a recurrence if they received a new gastric acid–suppressing agent (a proton pump inhibitor or histamine H2 receptor blocker) (HR, 1.36) or had more days of fluoroquinolone exposure (1.24).
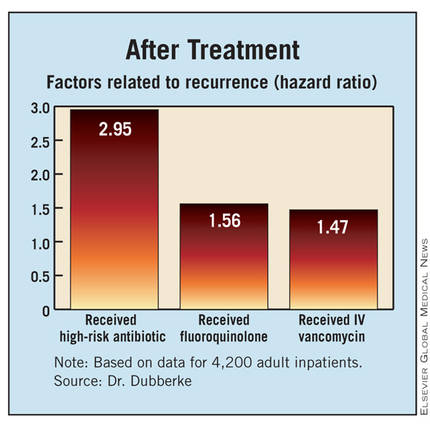
Finally, with respect to factors after completing treatment of the initial infection, patients were more likely to have a recurrence if they received a high-risk antibiotic (HR, 2.95), received a fluoroquinolone (HR, 1.56), or received intravenous vancomycin (HR, 1.45).
Discussing the study’s limitations, Dr. Dubberke noted that the investigators relied on the hospital database to identify cases of C. difficile recurrence. "We likely missed recurrences that were diagnosed and managed as outpatients," he acknowledged. "However, conversely, the recurrences that were identified were presumably more severe in that these patients ended up having another contact with our hospital, either in the emergency room" or through direct admission.
Dr. Dubberke disclosed that he has received research funding from Optimer Pharmaceuticals, ViroPharma, Merck, and Sanofi Pasteur, and that he has been a consultant to Optimer, Merck, Pfizer, and Sanofi Pasteur. The study was supported by Optimer.
SAN DIEGO – A variety of factors, some of them modifiable, increase the risk of recurrence of Clostridium difficile infection among inpatients, researchers reported at IDWeek.
The team retrospectively studied 4,200 adult inpatients who had an initial C. difficile infection, defined as a positive toxin assay at hospital admission in the setting of unformed stools. Overall, 10% went on to experience a recurrence within 6 weeks.
Results showed that patients had a more than one-third increase in recurrence risk if they were started on a new gastric acid–suppressing agent during treatment of their initial infection, and a near tripling of the risk if they were started on a high-risk antibiotic after completing treatment.
"Recognizing both modifiable and nonmodifiable recurrent C. difficile infection risk factors may help clinicians tailor the therapy for the initial episode of C. difficile infection more precisely," presenting author Dr. Erik R. Dubberke commented.
"Some characteristics, such as age, were not modifiable, but there were several modifiable exposures, such as gastric acid suppression as well as antimicrobial exposures during as well as after the treatment for the initial C. difficile infection," he said. "Reducing these exposures may help decrease the risk of developing future episodes of C. difficile infection." Dr. Dubberke is with the department of medicine at the Washington University in St. Louis.
Audience members inquired about the finer points of the investigation.
One attendee was "curious" about the elevated risk seen after receipt of intravenous vancomycin, "since this is a drug that doesn’t get into the stool at all." He wondered if perhaps other antibiotics given with vancomycin were actually at play.
Dr. Dubberke noted that multivariate analysis took this into consideration, and receipt of vancomycin after completing treatment was still a risk factor. "There are data to suggest that even intravenous vancomycin – though you don’t get adequate levels to treat C. difficile in the colon – can potentially get into the colon and affect the microbiome. So it’s possible there may be some direct causation," he said. "Conversely ... people who receive intravenous vancomycin tend to get other antibiotics. They also tend to be sicker patients, patients at increased risk for having drug-resistant organisms. So it is possible this is just a marker for recurrent C. difficile based on the patient characteristics."
Regarding whether severity of the initial infection affected recurrence risk, the research did not find an association on multivariate analysis with white count, new-onset renal dysfunction, baseline renal dysfunction, being in the ICU or being transferred to the ICU, or initiation of vasopressors, Dr. Dubberke explained. He speculated that lack of association was due in part to the competing risk of death.
"If a patient is very sick from C. difficile, they may die. Our [group] has demonstrated that you have an increased risk of death even after that initial episode is treated. So maybe that is why were not seeing [severity] as a risk for recurrent C. difficile."
Another attendee asked about the finding of elevated risk associated with gastric acid–suppressing medications. "[Have you] taken any practical steps at your hospital or in your health system to try to modify that risk factor because it’s medication that we clearly use and prescribe, and it’s questionable as to whether these prescriptions actually meet the indications ... " she said.
"We have not done anything in regard to interventions at this point based on the data," Dr. Dubberke replied.

The investigators identified patients having an initial C. difficile infection on admission to Barnes-Jewish Hospital, St. Louis, between 2003 and 2009, and classified those infections according to standard definitions as health care–onset infections; community-onset, health care facility–associated infections; or community-associated infections.
They classified antibiotics received as high risk (clindamycin, cephalosporins, and aminopenicillins), intravenous vancomycin, fluoroquinolones, and low risk (all others).
Study results shows that, compared with their peers who did not have a recurrence, patients who did were older and more likely to have diabetes, had higher levels of comorbidity, were more likely to have a community-onset, health care facility–associated initial infection, and were more likely to have been hospitalized in the past 60 days, Dr. Dubberke reported at the meeting, which is a the combined annual meeting of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society..
In multivariate analyses, patients were significantly more likely to have a recurrence of C. difficile if their initial infection was a community-onset, health care facility–associated infection as compared with a health care–onset infection (hazard ratio, 1.80) or they had at least two hospitalizations in the 60 days before that infection (HR, 1.40). Additionally, risk increased with age (HR, 1.01).
With respect to factors during the treatment of the initial infection, patients were more likely to have a recurrence if they received a new gastric acid–suppressing agent (a proton pump inhibitor or histamine H2 receptor blocker) (HR, 1.36) or had more days of fluoroquinolone exposure (1.24).

Finally, with respect to factors after completing treatment of the initial infection, patients were more likely to have a recurrence if they received a high-risk antibiotic (HR, 2.95), received a fluoroquinolone (HR, 1.56), or received intravenous vancomycin (HR, 1.45).
Discussing the study’s limitations, Dr. Dubberke noted that the investigators relied on the hospital database to identify cases of C. difficile recurrence. "We likely missed recurrences that were diagnosed and managed as outpatients," he acknowledged. "However, conversely, the recurrences that were identified were presumably more severe in that these patients ended up having another contact with our hospital, either in the emergency room" or through direct admission.
Dr. Dubberke disclosed that he has received research funding from Optimer Pharmaceuticals, ViroPharma, Merck, and Sanofi Pasteur, and that he has been a consultant to Optimer, Merck, Pfizer, and Sanofi Pasteur. The study was supported by Optimer.
SAN DIEGO – A variety of factors, some of them modifiable, increase the risk of recurrence of Clostridium difficile infection among inpatients, researchers reported at IDWeek.
The team retrospectively studied 4,200 adult inpatients who had an initial C. difficile infection, defined as a positive toxin assay at hospital admission in the setting of unformed stools. Overall, 10% went on to experience a recurrence within 6 weeks.
Results showed that patients had a more than one-third increase in recurrence risk if they were started on a new gastric acid–suppressing agent during treatment of their initial infection, and a near tripling of the risk if they were started on a high-risk antibiotic after completing treatment.
"Recognizing both modifiable and nonmodifiable recurrent C. difficile infection risk factors may help clinicians tailor the therapy for the initial episode of C. difficile infection more precisely," presenting author Dr. Erik R. Dubberke commented.
"Some characteristics, such as age, were not modifiable, but there were several modifiable exposures, such as gastric acid suppression as well as antimicrobial exposures during as well as after the treatment for the initial C. difficile infection," he said. "Reducing these exposures may help decrease the risk of developing future episodes of C. difficile infection." Dr. Dubberke is with the department of medicine at the Washington University in St. Louis.
Audience members inquired about the finer points of the investigation.
One attendee was "curious" about the elevated risk seen after receipt of intravenous vancomycin, "since this is a drug that doesn’t get into the stool at all." He wondered if perhaps other antibiotics given with vancomycin were actually at play.
Dr. Dubberke noted that multivariate analysis took this into consideration, and receipt of vancomycin after completing treatment was still a risk factor. "There are data to suggest that even intravenous vancomycin – though you don’t get adequate levels to treat C. difficile in the colon – can potentially get into the colon and affect the microbiome. So it’s possible there may be some direct causation," he said. "Conversely ... people who receive intravenous vancomycin tend to get other antibiotics. They also tend to be sicker patients, patients at increased risk for having drug-resistant organisms. So it is possible this is just a marker for recurrent C. difficile based on the patient characteristics."
Regarding whether severity of the initial infection affected recurrence risk, the research did not find an association on multivariate analysis with white count, new-onset renal dysfunction, baseline renal dysfunction, being in the ICU or being transferred to the ICU, or initiation of vasopressors, Dr. Dubberke explained. He speculated that lack of association was due in part to the competing risk of death.
"If a patient is very sick from C. difficile, they may die. Our [group] has demonstrated that you have an increased risk of death even after that initial episode is treated. So maybe that is why were not seeing [severity] as a risk for recurrent C. difficile."
Another attendee asked about the finding of elevated risk associated with gastric acid–suppressing medications. "[Have you] taken any practical steps at your hospital or in your health system to try to modify that risk factor because it’s medication that we clearly use and prescribe, and it’s questionable as to whether these prescriptions actually meet the indications ... " she said.
"We have not done anything in regard to interventions at this point based on the data," Dr. Dubberke replied.

The investigators identified patients having an initial C. difficile infection on admission to Barnes-Jewish Hospital, St. Louis, between 2003 and 2009, and classified those infections according to standard definitions as health care–onset infections; community-onset, health care facility–associated infections; or community-associated infections.
They classified antibiotics received as high risk (clindamycin, cephalosporins, and aminopenicillins), intravenous vancomycin, fluoroquinolones, and low risk (all others).
Study results shows that, compared with their peers who did not have a recurrence, patients who did were older and more likely to have diabetes, had higher levels of comorbidity, were more likely to have a community-onset, health care facility–associated initial infection, and were more likely to have been hospitalized in the past 60 days, Dr. Dubberke reported at the meeting, which is a the combined annual meeting of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society..
In multivariate analyses, patients were significantly more likely to have a recurrence of C. difficile if their initial infection was a community-onset, health care facility–associated infection as compared with a health care–onset infection (hazard ratio, 1.80) or they had at least two hospitalizations in the 60 days before that infection (HR, 1.40). Additionally, risk increased with age (HR, 1.01).
With respect to factors during the treatment of the initial infection, patients were more likely to have a recurrence if they received a new gastric acid–suppressing agent (a proton pump inhibitor or histamine H2 receptor blocker) (HR, 1.36) or had more days of fluoroquinolone exposure (1.24).

Finally, with respect to factors after completing treatment of the initial infection, patients were more likely to have a recurrence if they received a high-risk antibiotic (HR, 2.95), received a fluoroquinolone (HR, 1.56), or received intravenous vancomycin (HR, 1.45).
Discussing the study’s limitations, Dr. Dubberke noted that the investigators relied on the hospital database to identify cases of C. difficile recurrence. "We likely missed recurrences that were diagnosed and managed as outpatients," he acknowledged. "However, conversely, the recurrences that were identified were presumably more severe in that these patients ended up having another contact with our hospital, either in the emergency room" or through direct admission.
Dr. Dubberke disclosed that he has received research funding from Optimer Pharmaceuticals, ViroPharma, Merck, and Sanofi Pasteur, and that he has been a consultant to Optimer, Merck, Pfizer, and Sanofi Pasteur. The study was supported by Optimer.
AT IDWEEK
Major Finding: Risk factors for recurrence included starting gastric acid suppression during treatment of the initial infection (hazard ratio, 1.36) and receipt of a high-risk antibiotic after completing treatment (HR, 2.95).
Data Source: Results were taken from a retrospective cohort study of 4,200 adults having C. difficile infection on hospital admission
Disclosures: Dr. Dubberke disclosed that he has received research funding from and/or been a consultant for Optimer Pharmaceuticals, ViroPharma, Merck, Pfizer, and Sanofi Pasteur. The study was supported by Optimer.
PCR Bests Nasal Swab in Patients Receiving Anti-MRSA Antibiotics
SAN DIEGO – Polymerase chain reaction testing recovered methicillin-resistant Staphylococcus aureus significantly more frequently than did nasal swab culture in hospitalized patients receiving antibiotics concurrently, a study has shown.
All of the patients studied had a history of MRSA colonization. "Because close to 50% of hospitalized patients receive antibiotics, it’s important to know whether or not your MRSA screening test is going to be accurate in detecting persistent colonization," Dr. Erica S. Shenoy said in an interview during a poster session at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Shenoy of Massachusetts General Hospital’s division of infectious diseases and infection control unit, Boston, and her associates compared the effect of concurrent administration of antibiotics with activity against MRSA on detection from nasal surveillance swabs. The study population included 259 patients who were admitted to Massachusetts General Hospital between Dec. 6, 2010, and Sept. 16, 2011, with a history of MRSA colonization, but no culture more recent than 90 days who underwent simultaneous screening for nasal carriage of MRSA with both culture and commercial PCR testing. Samples obtained within 2 days after administration of select antibiotics were considered to be obtained in the presence of "concurrent antibiotics." The list of select antibiotics included trimethoprim/sulfamethoxazole, mupirocin, ciprofloxacin, clindamycin, daptomycin, doxycycline, levofloxacin, linezolid, nitrofurantoin, quinupristin/dalfopristin, rifampin, tetracycline, tigecycline, and intravenous vancomycin.
Dr. Shenoy reported that 132 of the 259 paired samples were obtained in the presence of antibiotics while the remaining 127 were not. In the absence of antibiotics, the concordance rate between culture and PCR was 94%, with neither test being significantly more likely to yield a positive result. However, in the presence of antibiotics, the concordance rate between paired samples was 91%, with a significantly greater tendency for PCR to yield positive results compared with culture, suggesting to the researchers better performance of PCR testing in the setting of antibiotic exposure.
"These findings are important for clinicians and hospitals interested in an efficient approach to determining colonization status," Dr. Shenoy said. "In populations exposed to antibiotics with activity against MRSA, culture assays may miss true positives."
She emphasized that while PCR is more expensive than nasal swab culture on a per-test basis, the "cost of the capital equipment investment and the personnel time to actually do the swabbing is potentially dwarfed by the downstream impact on the patient and the hospital overall." For example, studies have shown that inpatients on contact precautions "see their doctors fewer times, have more preventable adverse events, and more dissatisfaction with care, and thus we should strive to only place patients on contact precautions if they remain colonized," Dr. Shenoy said. "In an ongoing pilot study at our institution, we’ve transitioned to using PCR to document clearance and discontinue contact precautions in eligible patients."
In their poster, the researchers acknowledged certain limitations of the study, including its single-center design and the fact that "it was not possible to randomize subjects in the study to receipt or nonreceipt of antibiotics, and thus we do not know if subjects in both groups differed on unobservable characteristics."
The study was supported by a 2010 Massachusetts General Hospital Clinical Innovation Award, a National Institute of Allergy and Infectious Diseases Training Grant, the Harvard Catalyst, and Harvard University. Cepheid provided reagents and a loaner GeneXpert for the randomized trial free of charge. Dr. Shenoy said she and her associates had no relevant financial conflicts to disclose.
SAN DIEGO – Polymerase chain reaction testing recovered methicillin-resistant Staphylococcus aureus significantly more frequently than did nasal swab culture in hospitalized patients receiving antibiotics concurrently, a study has shown.
All of the patients studied had a history of MRSA colonization. "Because close to 50% of hospitalized patients receive antibiotics, it’s important to know whether or not your MRSA screening test is going to be accurate in detecting persistent colonization," Dr. Erica S. Shenoy said in an interview during a poster session at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Shenoy of Massachusetts General Hospital’s division of infectious diseases and infection control unit, Boston, and her associates compared the effect of concurrent administration of antibiotics with activity against MRSA on detection from nasal surveillance swabs. The study population included 259 patients who were admitted to Massachusetts General Hospital between Dec. 6, 2010, and Sept. 16, 2011, with a history of MRSA colonization, but no culture more recent than 90 days who underwent simultaneous screening for nasal carriage of MRSA with both culture and commercial PCR testing. Samples obtained within 2 days after administration of select antibiotics were considered to be obtained in the presence of "concurrent antibiotics." The list of select antibiotics included trimethoprim/sulfamethoxazole, mupirocin, ciprofloxacin, clindamycin, daptomycin, doxycycline, levofloxacin, linezolid, nitrofurantoin, quinupristin/dalfopristin, rifampin, tetracycline, tigecycline, and intravenous vancomycin.
Dr. Shenoy reported that 132 of the 259 paired samples were obtained in the presence of antibiotics while the remaining 127 were not. In the absence of antibiotics, the concordance rate between culture and PCR was 94%, with neither test being significantly more likely to yield a positive result. However, in the presence of antibiotics, the concordance rate between paired samples was 91%, with a significantly greater tendency for PCR to yield positive results compared with culture, suggesting to the researchers better performance of PCR testing in the setting of antibiotic exposure.
"These findings are important for clinicians and hospitals interested in an efficient approach to determining colonization status," Dr. Shenoy said. "In populations exposed to antibiotics with activity against MRSA, culture assays may miss true positives."
She emphasized that while PCR is more expensive than nasal swab culture on a per-test basis, the "cost of the capital equipment investment and the personnel time to actually do the swabbing is potentially dwarfed by the downstream impact on the patient and the hospital overall." For example, studies have shown that inpatients on contact precautions "see their doctors fewer times, have more preventable adverse events, and more dissatisfaction with care, and thus we should strive to only place patients on contact precautions if they remain colonized," Dr. Shenoy said. "In an ongoing pilot study at our institution, we’ve transitioned to using PCR to document clearance and discontinue contact precautions in eligible patients."
In their poster, the researchers acknowledged certain limitations of the study, including its single-center design and the fact that "it was not possible to randomize subjects in the study to receipt or nonreceipt of antibiotics, and thus we do not know if subjects in both groups differed on unobservable characteristics."
The study was supported by a 2010 Massachusetts General Hospital Clinical Innovation Award, a National Institute of Allergy and Infectious Diseases Training Grant, the Harvard Catalyst, and Harvard University. Cepheid provided reagents and a loaner GeneXpert for the randomized trial free of charge. Dr. Shenoy said she and her associates had no relevant financial conflicts to disclose.
SAN DIEGO – Polymerase chain reaction testing recovered methicillin-resistant Staphylococcus aureus significantly more frequently than did nasal swab culture in hospitalized patients receiving antibiotics concurrently, a study has shown.
All of the patients studied had a history of MRSA colonization. "Because close to 50% of hospitalized patients receive antibiotics, it’s important to know whether or not your MRSA screening test is going to be accurate in detecting persistent colonization," Dr. Erica S. Shenoy said in an interview during a poster session at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Shenoy of Massachusetts General Hospital’s division of infectious diseases and infection control unit, Boston, and her associates compared the effect of concurrent administration of antibiotics with activity against MRSA on detection from nasal surveillance swabs. The study population included 259 patients who were admitted to Massachusetts General Hospital between Dec. 6, 2010, and Sept. 16, 2011, with a history of MRSA colonization, but no culture more recent than 90 days who underwent simultaneous screening for nasal carriage of MRSA with both culture and commercial PCR testing. Samples obtained within 2 days after administration of select antibiotics were considered to be obtained in the presence of "concurrent antibiotics." The list of select antibiotics included trimethoprim/sulfamethoxazole, mupirocin, ciprofloxacin, clindamycin, daptomycin, doxycycline, levofloxacin, linezolid, nitrofurantoin, quinupristin/dalfopristin, rifampin, tetracycline, tigecycline, and intravenous vancomycin.
Dr. Shenoy reported that 132 of the 259 paired samples were obtained in the presence of antibiotics while the remaining 127 were not. In the absence of antibiotics, the concordance rate between culture and PCR was 94%, with neither test being significantly more likely to yield a positive result. However, in the presence of antibiotics, the concordance rate between paired samples was 91%, with a significantly greater tendency for PCR to yield positive results compared with culture, suggesting to the researchers better performance of PCR testing in the setting of antibiotic exposure.
"These findings are important for clinicians and hospitals interested in an efficient approach to determining colonization status," Dr. Shenoy said. "In populations exposed to antibiotics with activity against MRSA, culture assays may miss true positives."
She emphasized that while PCR is more expensive than nasal swab culture on a per-test basis, the "cost of the capital equipment investment and the personnel time to actually do the swabbing is potentially dwarfed by the downstream impact on the patient and the hospital overall." For example, studies have shown that inpatients on contact precautions "see their doctors fewer times, have more preventable adverse events, and more dissatisfaction with care, and thus we should strive to only place patients on contact precautions if they remain colonized," Dr. Shenoy said. "In an ongoing pilot study at our institution, we’ve transitioned to using PCR to document clearance and discontinue contact precautions in eligible patients."
In their poster, the researchers acknowledged certain limitations of the study, including its single-center design and the fact that "it was not possible to randomize subjects in the study to receipt or nonreceipt of antibiotics, and thus we do not know if subjects in both groups differed on unobservable characteristics."
The study was supported by a 2010 Massachusetts General Hospital Clinical Innovation Award, a National Institute of Allergy and Infectious Diseases Training Grant, the Harvard Catalyst, and Harvard University. Cepheid provided reagents and a loaner GeneXpert for the randomized trial free of charge. Dr. Shenoy said she and her associates had no relevant financial conflicts to disclose.
AT IDWEEK
Infliximab Not Cardioprotective in Kawasaki
SAN DIEGO – Intensifying primary therapy for Kawasaki disease by adding infliximab, an antibody to tumor necrosis factor–alpha, improves certain outcomes but not others, finds a phase III randomized trial reported at IDWeek.
The 196 children studied, all of whom were given intravenous immunoglobulin (IVIG), had a 22% lower erythrocyte sedimentation rate and a 50% or 1-day shorter duration of fever if they also received infliximab. But there was no effect on the rate of treatment resistance (the trial’s primary outcome) or the development of coronary aneurysms.
The findings "really beg the question of what is the role of infliximab in Kawasaki disease," commented Dr. Adriana Tremoulet, a pediatric infectious disease specialist at the Rady Children’s Hospital in San Diego.
"For primary therapy, what I would say is that it’s safe to use. The data do suggest a biological and clinical effect, but there is no reduction in treatment resistance or coronary artery abnormalities that we have proved to date," she said. "In addition, many of you may wonder what we will do about rescue therapy for IVIG resistance. Again, I think we definitely have enough data to say that it’s a safe alternative to a second IVIG [treatment], but that the efficacy is unproved at this time."
In the trial, children aged 4 weeks to 7 years with acute Kawasaki disease were randomized to infliximab (Remicade, 5 mg/kg) or placebo, each followed by IVIG (2 g/kg).
The children had a median age of 2.8 years (10% were younger than 1 year), and 62% were boys. The median duration of illness at trial enrollment was 6 days, according to data reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Results showed that, compared with their counterparts in the placebo group, patients in the infliximab group had less inflammation, as indicated by lower levels of C-reactive protein at 24 hours (4.1 vs. 5.3 mg/dL; P = .01) and a lower absolute neutrophil count (2,271 mm3 vs. 2,706 mm3; P = .001) and erythrocyte sedimentation rate (36 vs. 46; P = .003) at 2 weeks.
On the other hand, the infliximab group had a higher absolute lymphocyte count at 2 weeks (3,630 vs. 2,996; P = .002). "This is something we do see in children as they are going from an acute to a subacute change in their illness," Dr. Tremoulet commented.
The median duration of fever after enrollment was 1 day in the infliximab group, compared with 2 days in the placebo group (P less than .0001). The rate of IVIG infusion reactions, despite premedication, was also lower in the infliximab group (0% vs. 13%).
However, the rate of treatment resistance – defined as presence of fever between 36 hours and 7 days after the end of the first IVIG infusion – did not differ significantly between the groups (11% vs. 11%). And the same was true of the rate of coronary aneurysms detected by echocardiography (4% vs. 6%).
"It was safe to use infliximab even in children less than 1 year of age. Infliximab was tolerated well, even in the infants," Dr. Tremoulet maintained. Rates of serious adverse did not differ between treatment groups.
The investigators are now evaluating levels of various inflammatory cytokines, such as alpha1-antitrypsin and tumor necrosis factor–receptors, to better understand the biological effects of infliximab in this population, she noted.
Dr. Tremoulet disclosed no relevant conflicts of interest. Infliximab was donated by Janssen Biotech, the manufacturer.
SAN DIEGO – Intensifying primary therapy for Kawasaki disease by adding infliximab, an antibody to tumor necrosis factor–alpha, improves certain outcomes but not others, finds a phase III randomized trial reported at IDWeek.
The 196 children studied, all of whom were given intravenous immunoglobulin (IVIG), had a 22% lower erythrocyte sedimentation rate and a 50% or 1-day shorter duration of fever if they also received infliximab. But there was no effect on the rate of treatment resistance (the trial’s primary outcome) or the development of coronary aneurysms.
The findings "really beg the question of what is the role of infliximab in Kawasaki disease," commented Dr. Adriana Tremoulet, a pediatric infectious disease specialist at the Rady Children’s Hospital in San Diego.
"For primary therapy, what I would say is that it’s safe to use. The data do suggest a biological and clinical effect, but there is no reduction in treatment resistance or coronary artery abnormalities that we have proved to date," she said. "In addition, many of you may wonder what we will do about rescue therapy for IVIG resistance. Again, I think we definitely have enough data to say that it’s a safe alternative to a second IVIG [treatment], but that the efficacy is unproved at this time."
In the trial, children aged 4 weeks to 7 years with acute Kawasaki disease were randomized to infliximab (Remicade, 5 mg/kg) or placebo, each followed by IVIG (2 g/kg).
The children had a median age of 2.8 years (10% were younger than 1 year), and 62% were boys. The median duration of illness at trial enrollment was 6 days, according to data reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Results showed that, compared with their counterparts in the placebo group, patients in the infliximab group had less inflammation, as indicated by lower levels of C-reactive protein at 24 hours (4.1 vs. 5.3 mg/dL; P = .01) and a lower absolute neutrophil count (2,271 mm3 vs. 2,706 mm3; P = .001) and erythrocyte sedimentation rate (36 vs. 46; P = .003) at 2 weeks.
On the other hand, the infliximab group had a higher absolute lymphocyte count at 2 weeks (3,630 vs. 2,996; P = .002). "This is something we do see in children as they are going from an acute to a subacute change in their illness," Dr. Tremoulet commented.
The median duration of fever after enrollment was 1 day in the infliximab group, compared with 2 days in the placebo group (P less than .0001). The rate of IVIG infusion reactions, despite premedication, was also lower in the infliximab group (0% vs. 13%).
However, the rate of treatment resistance – defined as presence of fever between 36 hours and 7 days after the end of the first IVIG infusion – did not differ significantly between the groups (11% vs. 11%). And the same was true of the rate of coronary aneurysms detected by echocardiography (4% vs. 6%).
"It was safe to use infliximab even in children less than 1 year of age. Infliximab was tolerated well, even in the infants," Dr. Tremoulet maintained. Rates of serious adverse did not differ between treatment groups.
The investigators are now evaluating levels of various inflammatory cytokines, such as alpha1-antitrypsin and tumor necrosis factor–receptors, to better understand the biological effects of infliximab in this population, she noted.
Dr. Tremoulet disclosed no relevant conflicts of interest. Infliximab was donated by Janssen Biotech, the manufacturer.
SAN DIEGO – Intensifying primary therapy for Kawasaki disease by adding infliximab, an antibody to tumor necrosis factor–alpha, improves certain outcomes but not others, finds a phase III randomized trial reported at IDWeek.
The 196 children studied, all of whom were given intravenous immunoglobulin (IVIG), had a 22% lower erythrocyte sedimentation rate and a 50% or 1-day shorter duration of fever if they also received infliximab. But there was no effect on the rate of treatment resistance (the trial’s primary outcome) or the development of coronary aneurysms.
The findings "really beg the question of what is the role of infliximab in Kawasaki disease," commented Dr. Adriana Tremoulet, a pediatric infectious disease specialist at the Rady Children’s Hospital in San Diego.
"For primary therapy, what I would say is that it’s safe to use. The data do suggest a biological and clinical effect, but there is no reduction in treatment resistance or coronary artery abnormalities that we have proved to date," she said. "In addition, many of you may wonder what we will do about rescue therapy for IVIG resistance. Again, I think we definitely have enough data to say that it’s a safe alternative to a second IVIG [treatment], but that the efficacy is unproved at this time."
In the trial, children aged 4 weeks to 7 years with acute Kawasaki disease were randomized to infliximab (Remicade, 5 mg/kg) or placebo, each followed by IVIG (2 g/kg).
The children had a median age of 2.8 years (10% were younger than 1 year), and 62% were boys. The median duration of illness at trial enrollment was 6 days, according to data reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Results showed that, compared with their counterparts in the placebo group, patients in the infliximab group had less inflammation, as indicated by lower levels of C-reactive protein at 24 hours (4.1 vs. 5.3 mg/dL; P = .01) and a lower absolute neutrophil count (2,271 mm3 vs. 2,706 mm3; P = .001) and erythrocyte sedimentation rate (36 vs. 46; P = .003) at 2 weeks.
On the other hand, the infliximab group had a higher absolute lymphocyte count at 2 weeks (3,630 vs. 2,996; P = .002). "This is something we do see in children as they are going from an acute to a subacute change in their illness," Dr. Tremoulet commented.
The median duration of fever after enrollment was 1 day in the infliximab group, compared with 2 days in the placebo group (P less than .0001). The rate of IVIG infusion reactions, despite premedication, was also lower in the infliximab group (0% vs. 13%).
However, the rate of treatment resistance – defined as presence of fever between 36 hours and 7 days after the end of the first IVIG infusion – did not differ significantly between the groups (11% vs. 11%). And the same was true of the rate of coronary aneurysms detected by echocardiography (4% vs. 6%).
"It was safe to use infliximab even in children less than 1 year of age. Infliximab was tolerated well, even in the infants," Dr. Tremoulet maintained. Rates of serious adverse did not differ between treatment groups.
The investigators are now evaluating levels of various inflammatory cytokines, such as alpha1-antitrypsin and tumor necrosis factor–receptors, to better understand the biological effects of infliximab in this population, she noted.
Dr. Tremoulet disclosed no relevant conflicts of interest. Infliximab was donated by Janssen Biotech, the manufacturer.
AT IDWEEK
Major Finding: Compared with placebo, infliximab reduced the erythrocyte sedimentation rate by 22% and shortened the duration of fever by 50%, but did not alter rates of treatment resistance or development of coronary artery aneurysms.
Data Source: These findings come from a phase III randomized trial among 196 children with acute Kawasaki disease also given IVIG.
Disclosures: Dr. Tremoulet disclosed no relevant conflicts of interest. Infliximab was donated by Janssen Biotech.
Pertussis Immunity Drop Linked to Whole Cell Vaccine Withdrawal
SAN DIEGO – The declining herd immunity to pertussis seen in the United States community may be related to withdrawal of the whole cell vaccine from the market about a decade ago, a study of more than 450,000 vaccinated patients from Kaiser Permanente Medical Center has shown.
Compared with their peers who had received at least one dose of whole cell vaccine, patients who had received only acellular vaccine had at least a tripling of the risk of acquiring the disease, lead author Maxwell Witt reported at IDWeek. The association was still present but weaker among patients who had received a total of six doses versus five.
"Acellular pertussis vaccine offered significantly less protection when compared with the whole cell vaccine," commented Mr. Witt of Kaiser Permanente in San Rafael, Calif. "The risk of pertussis was mitigated, but not eliminated, by a sixth dose of pertussis vaccine, the Tdap [tetanus, diphtheria, and acellular pertussis] vaccine.
"The current generation of children is the first to have been vaccinated solely with the acellular pertussis vaccine," Mr. Witt said. "Our findings would predict a significant population of underprotected children in this group." Recent outbreaks of pertussis in the United States "had peak attack rates among those who are exactly in the same age group," he noted.
"The waning immunity associated with these outbreaks is clearly a call for development of more effective and durable pertussis vaccines," Mr. Witt said. "In the shorter term, strategies to prevent these outbreaks of pertussis could include either earlier or additional booster doses, and targeted vaccination programs to address insufficient immunity in outbreak situations."
The field of pertussis vaccination has undergone transition, with the introduction of the acellular vaccine in 1991 and retirement of the whole cell vaccine in 2001. "In 2010, we saw the largest epidemic of Bordatella pertussis in California in more than 50 years, and that epidemic subsequently spread across the United States," he said at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In a previous study, the investigators noted waning pertussis immunity among preadolescents (with a peak attack rate among 8- to 12-year-olds) but also robust immunity among adolescents (with a sharply lower attack rate among 13-year-olds) (Clin. Infect. Dis. 2010;54:1730-35), suggesting a possible role for the vaccine transition.
In the new study, the investigators used electronic health records to identify vaccines administered and cases of laboratory-confirmed pertussis among Kaiser Permanente members aged 8-20 years in Northern California.
This age range was chosen to include patients who were old enough to have received whole cell vaccine and young enough to have received acellular vaccine, explained Mr. Witt. The search identified 465,059 patients, among whom there were 1,424 cases of pertussis.
A total of 253,005 patients had received all of their vaccine doses at Kaiser Permanente. Analyses restricted to this group showed that among patients who had received five total doses of vaccine, the pertussis attack rate was 786/100,000 for those who had received only acellular vaccine, compared with 92/100,000 for those who had received at least one dose of whole cell vaccine. The difference corresponded to an 8.57-fold higher risk for the former group (P less than .0001).
Similarly, among patients who had received six total doses of vaccine, the attack rate was 378 vs. 106/100,000 for those who received only acellular vaccine compared with those who had received at least one dose of whole cell vaccine. Here, the difference amounted to a smaller but still significant 3.55-fold higher risk.
The study findings were essentially the same when analyses were based on all patients, including those who had received at least some doses of vaccine outside of Kaiser Permanente, with respective 6.76- and 2.46-fold higher risks for patients given only acellular vaccine, depending on the total number of doses.
Mr. Witt disclosed no relevant financial conflicts.
SAN DIEGO – The declining herd immunity to pertussis seen in the United States community may be related to withdrawal of the whole cell vaccine from the market about a decade ago, a study of more than 450,000 vaccinated patients from Kaiser Permanente Medical Center has shown.
Compared with their peers who had received at least one dose of whole cell vaccine, patients who had received only acellular vaccine had at least a tripling of the risk of acquiring the disease, lead author Maxwell Witt reported at IDWeek. The association was still present but weaker among patients who had received a total of six doses versus five.
"Acellular pertussis vaccine offered significantly less protection when compared with the whole cell vaccine," commented Mr. Witt of Kaiser Permanente in San Rafael, Calif. "The risk of pertussis was mitigated, but not eliminated, by a sixth dose of pertussis vaccine, the Tdap [tetanus, diphtheria, and acellular pertussis] vaccine.
"The current generation of children is the first to have been vaccinated solely with the acellular pertussis vaccine," Mr. Witt said. "Our findings would predict a significant population of underprotected children in this group." Recent outbreaks of pertussis in the United States "had peak attack rates among those who are exactly in the same age group," he noted.
"The waning immunity associated with these outbreaks is clearly a call for development of more effective and durable pertussis vaccines," Mr. Witt said. "In the shorter term, strategies to prevent these outbreaks of pertussis could include either earlier or additional booster doses, and targeted vaccination programs to address insufficient immunity in outbreak situations."
The field of pertussis vaccination has undergone transition, with the introduction of the acellular vaccine in 1991 and retirement of the whole cell vaccine in 2001. "In 2010, we saw the largest epidemic of Bordatella pertussis in California in more than 50 years, and that epidemic subsequently spread across the United States," he said at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In a previous study, the investigators noted waning pertussis immunity among preadolescents (with a peak attack rate among 8- to 12-year-olds) but also robust immunity among adolescents (with a sharply lower attack rate among 13-year-olds) (Clin. Infect. Dis. 2010;54:1730-35), suggesting a possible role for the vaccine transition.
In the new study, the investigators used electronic health records to identify vaccines administered and cases of laboratory-confirmed pertussis among Kaiser Permanente members aged 8-20 years in Northern California.
This age range was chosen to include patients who were old enough to have received whole cell vaccine and young enough to have received acellular vaccine, explained Mr. Witt. The search identified 465,059 patients, among whom there were 1,424 cases of pertussis.
A total of 253,005 patients had received all of their vaccine doses at Kaiser Permanente. Analyses restricted to this group showed that among patients who had received five total doses of vaccine, the pertussis attack rate was 786/100,000 for those who had received only acellular vaccine, compared with 92/100,000 for those who had received at least one dose of whole cell vaccine. The difference corresponded to an 8.57-fold higher risk for the former group (P less than .0001).
Similarly, among patients who had received six total doses of vaccine, the attack rate was 378 vs. 106/100,000 for those who received only acellular vaccine compared with those who had received at least one dose of whole cell vaccine. Here, the difference amounted to a smaller but still significant 3.55-fold higher risk.
The study findings were essentially the same when analyses were based on all patients, including those who had received at least some doses of vaccine outside of Kaiser Permanente, with respective 6.76- and 2.46-fold higher risks for patients given only acellular vaccine, depending on the total number of doses.
Mr. Witt disclosed no relevant financial conflicts.
SAN DIEGO – The declining herd immunity to pertussis seen in the United States community may be related to withdrawal of the whole cell vaccine from the market about a decade ago, a study of more than 450,000 vaccinated patients from Kaiser Permanente Medical Center has shown.
Compared with their peers who had received at least one dose of whole cell vaccine, patients who had received only acellular vaccine had at least a tripling of the risk of acquiring the disease, lead author Maxwell Witt reported at IDWeek. The association was still present but weaker among patients who had received a total of six doses versus five.
"Acellular pertussis vaccine offered significantly less protection when compared with the whole cell vaccine," commented Mr. Witt of Kaiser Permanente in San Rafael, Calif. "The risk of pertussis was mitigated, but not eliminated, by a sixth dose of pertussis vaccine, the Tdap [tetanus, diphtheria, and acellular pertussis] vaccine.
"The current generation of children is the first to have been vaccinated solely with the acellular pertussis vaccine," Mr. Witt said. "Our findings would predict a significant population of underprotected children in this group." Recent outbreaks of pertussis in the United States "had peak attack rates among those who are exactly in the same age group," he noted.
"The waning immunity associated with these outbreaks is clearly a call for development of more effective and durable pertussis vaccines," Mr. Witt said. "In the shorter term, strategies to prevent these outbreaks of pertussis could include either earlier or additional booster doses, and targeted vaccination programs to address insufficient immunity in outbreak situations."
The field of pertussis vaccination has undergone transition, with the introduction of the acellular vaccine in 1991 and retirement of the whole cell vaccine in 2001. "In 2010, we saw the largest epidemic of Bordatella pertussis in California in more than 50 years, and that epidemic subsequently spread across the United States," he said at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In a previous study, the investigators noted waning pertussis immunity among preadolescents (with a peak attack rate among 8- to 12-year-olds) but also robust immunity among adolescents (with a sharply lower attack rate among 13-year-olds) (Clin. Infect. Dis. 2010;54:1730-35), suggesting a possible role for the vaccine transition.
In the new study, the investigators used electronic health records to identify vaccines administered and cases of laboratory-confirmed pertussis among Kaiser Permanente members aged 8-20 years in Northern California.
This age range was chosen to include patients who were old enough to have received whole cell vaccine and young enough to have received acellular vaccine, explained Mr. Witt. The search identified 465,059 patients, among whom there were 1,424 cases of pertussis.
A total of 253,005 patients had received all of their vaccine doses at Kaiser Permanente. Analyses restricted to this group showed that among patients who had received five total doses of vaccine, the pertussis attack rate was 786/100,000 for those who had received only acellular vaccine, compared with 92/100,000 for those who had received at least one dose of whole cell vaccine. The difference corresponded to an 8.57-fold higher risk for the former group (P less than .0001).
Similarly, among patients who had received six total doses of vaccine, the attack rate was 378 vs. 106/100,000 for those who received only acellular vaccine compared with those who had received at least one dose of whole cell vaccine. Here, the difference amounted to a smaller but still significant 3.55-fold higher risk.
The study findings were essentially the same when analyses were based on all patients, including those who had received at least some doses of vaccine outside of Kaiser Permanente, with respective 6.76- and 2.46-fold higher risks for patients given only acellular vaccine, depending on the total number of doses.
Mr. Witt disclosed no relevant financial conflicts.
AT IDWEEK
Major Finding: Compared with their peers who had received at least one dose of whole cell vaccine, patients who had received only acellular vaccine had a 3.55- to 8.57-fold higher risk of pertussis.
Data Source: A cross-sectional study of 465,059 vaccinated patients aged 8-20 years from a single health care system
Disclosures: Mr. Witt disclosed no relevant financial conflicts.
Nasal Povidone-Iodine Cuts Postop Infections
SAN DIEGO – Preoperative nasal application of a povidone-iodine solution may be more efficacious than nasal mupirocin for preventing deep surgical site infections caused by Staphylococcus aureus, a study has shown.
Investigators led by Dr. Michael Phillips of the New York University Langone Medical Center conducted a randomized trial, assigning 1,697 patients undergoing arthroplasty or spine fusion surgery evenly to the two treatments. All patients in the study also underwent chlorhexidine cleansing of the skin and received standard antimicrobial prophylaxis.
Trial results, reported at IDWeek, showed that the rate of S. aureus deep surgical site infections was one-sixth as high in the povidone-iodine group as in the mupirocin group. The povidone-iodine group also had a lower rate of adverse events and less often rated their treatment as unpleasant.
"We feel that individuals should consider the use of nasal povidone-iodine as a component of a multifaceted approach to prevent S. aureus infection after high-risk surgery," Dr. Phillips said.
In the trial, adult patients undergoing arthroplasty or spine fusion surgery at the NYU Hospital for Joint Diseases had nasal cultures for S. aureus before surgery and were then assigned to two groups.
In one group, patients were given 2% mupirocin nasal ointment and told to apply it twice daily for the 5 days leading up to surgery. The mupirocin was provided directly to the patients because a survey suggested that some patients were skipping the mupirocin because of its cost, as it is not routinely covered by insurance, he explained at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In the other group, research personnel applied a 5% solution of povidone-iodine to both nostrils in patients up to 2 hours before incision.
Both groups also were given chlorhexidine wipes and told to use them from chin to toes the evening before and again the morning of surgery. Both groups received standard perioperative antimicrobial prophylaxis (cefazolin or clindamycin, plus vancomycin for those having a positive nasal culture for methicillin-resistant S. aureus). Both also had surgical site prepping with 2% chlorhexidine and alcohol.
The patients’ median age was 62 years; 60% were women and 80% were white. Preoperative nasal culture results showed that one in five patients was colonized with S. aureus.
The rate of noncompletion of the protocol was 11% in the mupirocin group and 8% in the povidone-iodine group, Dr. Phillips reported. The leading reason for noncompletion in the mupirocin group was failure to apply mupirocin at least seven times. In the povidone-iodine group, it was failure to use the chlorhexidine wipes.
In intent-to-treat analyses, patients in the nasal povidone-iodine group had a 0.7% rate of any deep surgical site infection at 3 months; those in the mupirocin group had a 1.6% rate. The rate of S. aureus deep surgical site infections was 0.1% and. 0.6%, respectively.
In a univariate analysis, patients had a higher risk of S. aureus deep surgical site infection if they were in the mupirocin group (relative risk, 1.01; P = .04) or were colonized with S. aureus preoperatively (relative risk, 6.79; P = .02).
When patients were stratified by colonization status, there was a trend toward a lower rate of S. aureus deep surgical site infection with povidone-iodine versus mupirocin for those who were colonized (P = .08) but not for those who were not.
Patients in the povidone-iodine group had a lower rate of study drug adverse events overall (2% vs. 9%, P less than .0001). The difference was significant for rhinorrhea, headache, congestion, and pharyngeal pain individually.
In addition, patients in the povidone-iodine group were less likely to report that application of the study medication was unpleasant or very unpleasant (4% vs. 38%).
Dr. Phillips disclosed that he received a research grant from 3M Corp., manufacturer of the povidone-iodine solution. The study was supported by 3M.
SAN DIEGO – Preoperative nasal application of a povidone-iodine solution may be more efficacious than nasal mupirocin for preventing deep surgical site infections caused by Staphylococcus aureus, a study has shown.
Investigators led by Dr. Michael Phillips of the New York University Langone Medical Center conducted a randomized trial, assigning 1,697 patients undergoing arthroplasty or spine fusion surgery evenly to the two treatments. All patients in the study also underwent chlorhexidine cleansing of the skin and received standard antimicrobial prophylaxis.
Trial results, reported at IDWeek, showed that the rate of S. aureus deep surgical site infections was one-sixth as high in the povidone-iodine group as in the mupirocin group. The povidone-iodine group also had a lower rate of adverse events and less often rated their treatment as unpleasant.
"We feel that individuals should consider the use of nasal povidone-iodine as a component of a multifaceted approach to prevent S. aureus infection after high-risk surgery," Dr. Phillips said.
In the trial, adult patients undergoing arthroplasty or spine fusion surgery at the NYU Hospital for Joint Diseases had nasal cultures for S. aureus before surgery and were then assigned to two groups.
In one group, patients were given 2% mupirocin nasal ointment and told to apply it twice daily for the 5 days leading up to surgery. The mupirocin was provided directly to the patients because a survey suggested that some patients were skipping the mupirocin because of its cost, as it is not routinely covered by insurance, he explained at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In the other group, research personnel applied a 5% solution of povidone-iodine to both nostrils in patients up to 2 hours before incision.
Both groups also were given chlorhexidine wipes and told to use them from chin to toes the evening before and again the morning of surgery. Both groups received standard perioperative antimicrobial prophylaxis (cefazolin or clindamycin, plus vancomycin for those having a positive nasal culture for methicillin-resistant S. aureus). Both also had surgical site prepping with 2% chlorhexidine and alcohol.
The patients’ median age was 62 years; 60% were women and 80% were white. Preoperative nasal culture results showed that one in five patients was colonized with S. aureus.
The rate of noncompletion of the protocol was 11% in the mupirocin group and 8% in the povidone-iodine group, Dr. Phillips reported. The leading reason for noncompletion in the mupirocin group was failure to apply mupirocin at least seven times. In the povidone-iodine group, it was failure to use the chlorhexidine wipes.
In intent-to-treat analyses, patients in the nasal povidone-iodine group had a 0.7% rate of any deep surgical site infection at 3 months; those in the mupirocin group had a 1.6% rate. The rate of S. aureus deep surgical site infections was 0.1% and. 0.6%, respectively.
In a univariate analysis, patients had a higher risk of S. aureus deep surgical site infection if they were in the mupirocin group (relative risk, 1.01; P = .04) or were colonized with S. aureus preoperatively (relative risk, 6.79; P = .02).
When patients were stratified by colonization status, there was a trend toward a lower rate of S. aureus deep surgical site infection with povidone-iodine versus mupirocin for those who were colonized (P = .08) but not for those who were not.
Patients in the povidone-iodine group had a lower rate of study drug adverse events overall (2% vs. 9%, P less than .0001). The difference was significant for rhinorrhea, headache, congestion, and pharyngeal pain individually.
In addition, patients in the povidone-iodine group were less likely to report that application of the study medication was unpleasant or very unpleasant (4% vs. 38%).
Dr. Phillips disclosed that he received a research grant from 3M Corp., manufacturer of the povidone-iodine solution. The study was supported by 3M.
SAN DIEGO – Preoperative nasal application of a povidone-iodine solution may be more efficacious than nasal mupirocin for preventing deep surgical site infections caused by Staphylococcus aureus, a study has shown.
Investigators led by Dr. Michael Phillips of the New York University Langone Medical Center conducted a randomized trial, assigning 1,697 patients undergoing arthroplasty or spine fusion surgery evenly to the two treatments. All patients in the study also underwent chlorhexidine cleansing of the skin and received standard antimicrobial prophylaxis.
Trial results, reported at IDWeek, showed that the rate of S. aureus deep surgical site infections was one-sixth as high in the povidone-iodine group as in the mupirocin group. The povidone-iodine group also had a lower rate of adverse events and less often rated their treatment as unpleasant.
"We feel that individuals should consider the use of nasal povidone-iodine as a component of a multifaceted approach to prevent S. aureus infection after high-risk surgery," Dr. Phillips said.
In the trial, adult patients undergoing arthroplasty or spine fusion surgery at the NYU Hospital for Joint Diseases had nasal cultures for S. aureus before surgery and were then assigned to two groups.
In one group, patients were given 2% mupirocin nasal ointment and told to apply it twice daily for the 5 days leading up to surgery. The mupirocin was provided directly to the patients because a survey suggested that some patients were skipping the mupirocin because of its cost, as it is not routinely covered by insurance, he explained at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In the other group, research personnel applied a 5% solution of povidone-iodine to both nostrils in patients up to 2 hours before incision.
Both groups also were given chlorhexidine wipes and told to use them from chin to toes the evening before and again the morning of surgery. Both groups received standard perioperative antimicrobial prophylaxis (cefazolin or clindamycin, plus vancomycin for those having a positive nasal culture for methicillin-resistant S. aureus). Both also had surgical site prepping with 2% chlorhexidine and alcohol.
The patients’ median age was 62 years; 60% were women and 80% were white. Preoperative nasal culture results showed that one in five patients was colonized with S. aureus.
The rate of noncompletion of the protocol was 11% in the mupirocin group and 8% in the povidone-iodine group, Dr. Phillips reported. The leading reason for noncompletion in the mupirocin group was failure to apply mupirocin at least seven times. In the povidone-iodine group, it was failure to use the chlorhexidine wipes.
In intent-to-treat analyses, patients in the nasal povidone-iodine group had a 0.7% rate of any deep surgical site infection at 3 months; those in the mupirocin group had a 1.6% rate. The rate of S. aureus deep surgical site infections was 0.1% and. 0.6%, respectively.
In a univariate analysis, patients had a higher risk of S. aureus deep surgical site infection if they were in the mupirocin group (relative risk, 1.01; P = .04) or were colonized with S. aureus preoperatively (relative risk, 6.79; P = .02).
When patients were stratified by colonization status, there was a trend toward a lower rate of S. aureus deep surgical site infection with povidone-iodine versus mupirocin for those who were colonized (P = .08) but not for those who were not.
Patients in the povidone-iodine group had a lower rate of study drug adverse events overall (2% vs. 9%, P less than .0001). The difference was significant for rhinorrhea, headache, congestion, and pharyngeal pain individually.
In addition, patients in the povidone-iodine group were less likely to report that application of the study medication was unpleasant or very unpleasant (4% vs. 38%).
Dr. Phillips disclosed that he received a research grant from 3M Corp., manufacturer of the povidone-iodine solution. The study was supported by 3M.
AT IDWEEK
Major Finding: S. aureus deep surgical site infections were seen in 0.6% of patients given nasal mupirocin and 0.1% of patients given nasal povidone-iodine solution.
Data Source: Results are from a randomized trial of 1,697 patients undergoing arthroplasty or spine fusion surgery.
Disclosures: Dr. Phillips disclosed that he received a research grant from 3M Corp., which made the povidone-iodine solution. The study was supported by 3M.
Pneumonia Prevalence Highest of Health Care-Associated Infections
SAN DIEGO – The overall prevalence of health care–associated infections among inpatients in the United States stands at 4%, with the most common types of infections being a combination of pneumonia and lower respiratory infections.
Those are key preliminary findings from the Centers for Disease Control and Prevention’s first large-scale health care–associated infection (HAI) prevalence survey in more than 30 years, Dr. Shelley S. Magill reported during IDWeek 2012, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The data "can help us better understand the factors that influence HAI prevalence," said Dr. Magill of the division of health care quality promotion at the Centers for Disease Control and Prevention, Atlanta. "We can also clarify the burden of different HAI types and pathogens across the hospital, which can suggest areas to target for prevention."
The phase 3 survey was conducted in 2011 in 183 hospitals in 10 states: California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee. Dr. Magill reported results from 11,282 patients who were surveyed in the 183 hospitals. Of these, 452 patients had HAIs, for a prevalence of 4%.
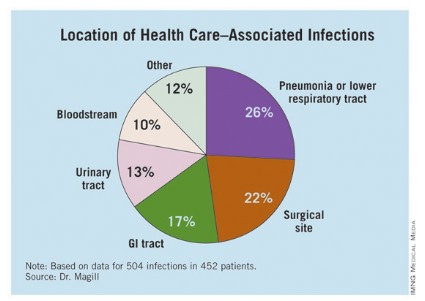
The researchers identified 504 HAIs in the 452 patients. Of these, the highest proportion (26%) were pneumonia or lower respiratory infections. "Of the pneumonia events, 39% were ventilator-associated infections," Dr. Magill said.
Surgical-site infections were the second most common infection type, representing 22% of all HAIs, followed by GI infections (17%), urinary tract infections (13%), and bloodstream infections (10%). "About two-third of UTIs were catheter associated and about 84% of the bloodstream infections were central-line associated," Dr. Magill noted.
Other infections made up the remaining 12% of HAIs.
Dr. Magill also reported that 56% of HAIs were attributed to non-ICU locations in the hospital while 53% were not directly associated with a device or with a procedure.
After multivariable regression analysis, patients with the following factors were at higher risk of having HAIs:
• Older age (risk ratio, 4.60 for patients older than age 77 compared with patients younger than 3 months old).
• Inpatient in a large hospital – defined as having 400 or more beds (RR, 1.24).
• Inpatient in a critical care unit (RR ,1.96 compared with all other units),
• Inpatient for more than 2 weeks at the time of the survey (RR, 26.09 compared with patients in the hospital for 3 days or fewer).
Pathogens were reported for 372 of the 504 HAIs. Clostridium difficile was the most common pathogen, accounting for 12% of all HAIs that were identified. Staphylococcus aureus was the second most common pathogen (11%; about half of these cases were methicillin-resistant S. aureus), followed by Klebsiella pneumoniae and Klebsiella oxytoca (10%).
Dr. Magill acknowledged certain limitations of the survey, including the fact that a small number of patients surveyed in each hospital "make results of limited use to individual facilities," she said. "We also had a relatively small number of hospitals in 10 states participate. This survey method provides a single snapshot of HAIs based on a retrospective review of medical record data, which is sometimes not complete. Finally, we were not able to collect a lot of detailed patient information, such as underlying illnesses and severity of illness."
She and her associates are currently planning a phase 4 survey, anticipated to occur in 2014.
Dr. Magill said she had no relevant financial conflicts to disclose.
SAN DIEGO – The overall prevalence of health care–associated infections among inpatients in the United States stands at 4%, with the most common types of infections being a combination of pneumonia and lower respiratory infections.
Those are key preliminary findings from the Centers for Disease Control and Prevention’s first large-scale health care–associated infection (HAI) prevalence survey in more than 30 years, Dr. Shelley S. Magill reported during IDWeek 2012, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The data "can help us better understand the factors that influence HAI prevalence," said Dr. Magill of the division of health care quality promotion at the Centers for Disease Control and Prevention, Atlanta. "We can also clarify the burden of different HAI types and pathogens across the hospital, which can suggest areas to target for prevention."
The phase 3 survey was conducted in 2011 in 183 hospitals in 10 states: California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee. Dr. Magill reported results from 11,282 patients who were surveyed in the 183 hospitals. Of these, 452 patients had HAIs, for a prevalence of 4%.

The researchers identified 504 HAIs in the 452 patients. Of these, the highest proportion (26%) were pneumonia or lower respiratory infections. "Of the pneumonia events, 39% were ventilator-associated infections," Dr. Magill said.
Surgical-site infections were the second most common infection type, representing 22% of all HAIs, followed by GI infections (17%), urinary tract infections (13%), and bloodstream infections (10%). "About two-third of UTIs were catheter associated and about 84% of the bloodstream infections were central-line associated," Dr. Magill noted.
Other infections made up the remaining 12% of HAIs.
Dr. Magill also reported that 56% of HAIs were attributed to non-ICU locations in the hospital while 53% were not directly associated with a device or with a procedure.
After multivariable regression analysis, patients with the following factors were at higher risk of having HAIs:
• Older age (risk ratio, 4.60 for patients older than age 77 compared with patients younger than 3 months old).
• Inpatient in a large hospital – defined as having 400 or more beds (RR, 1.24).
• Inpatient in a critical care unit (RR ,1.96 compared with all other units),
• Inpatient for more than 2 weeks at the time of the survey (RR, 26.09 compared with patients in the hospital for 3 days or fewer).
Pathogens were reported for 372 of the 504 HAIs. Clostridium difficile was the most common pathogen, accounting for 12% of all HAIs that were identified. Staphylococcus aureus was the second most common pathogen (11%; about half of these cases were methicillin-resistant S. aureus), followed by Klebsiella pneumoniae and Klebsiella oxytoca (10%).
Dr. Magill acknowledged certain limitations of the survey, including the fact that a small number of patients surveyed in each hospital "make results of limited use to individual facilities," she said. "We also had a relatively small number of hospitals in 10 states participate. This survey method provides a single snapshot of HAIs based on a retrospective review of medical record data, which is sometimes not complete. Finally, we were not able to collect a lot of detailed patient information, such as underlying illnesses and severity of illness."
She and her associates are currently planning a phase 4 survey, anticipated to occur in 2014.
Dr. Magill said she had no relevant financial conflicts to disclose.
SAN DIEGO – The overall prevalence of health care–associated infections among inpatients in the United States stands at 4%, with the most common types of infections being a combination of pneumonia and lower respiratory infections.
Those are key preliminary findings from the Centers for Disease Control and Prevention’s first large-scale health care–associated infection (HAI) prevalence survey in more than 30 years, Dr. Shelley S. Magill reported during IDWeek 2012, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The data "can help us better understand the factors that influence HAI prevalence," said Dr. Magill of the division of health care quality promotion at the Centers for Disease Control and Prevention, Atlanta. "We can also clarify the burden of different HAI types and pathogens across the hospital, which can suggest areas to target for prevention."
The phase 3 survey was conducted in 2011 in 183 hospitals in 10 states: California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee. Dr. Magill reported results from 11,282 patients who were surveyed in the 183 hospitals. Of these, 452 patients had HAIs, for a prevalence of 4%.

The researchers identified 504 HAIs in the 452 patients. Of these, the highest proportion (26%) were pneumonia or lower respiratory infections. "Of the pneumonia events, 39% were ventilator-associated infections," Dr. Magill said.
Surgical-site infections were the second most common infection type, representing 22% of all HAIs, followed by GI infections (17%), urinary tract infections (13%), and bloodstream infections (10%). "About two-third of UTIs were catheter associated and about 84% of the bloodstream infections were central-line associated," Dr. Magill noted.
Other infections made up the remaining 12% of HAIs.
Dr. Magill also reported that 56% of HAIs were attributed to non-ICU locations in the hospital while 53% were not directly associated with a device or with a procedure.
After multivariable regression analysis, patients with the following factors were at higher risk of having HAIs:
• Older age (risk ratio, 4.60 for patients older than age 77 compared with patients younger than 3 months old).
• Inpatient in a large hospital – defined as having 400 or more beds (RR, 1.24).
• Inpatient in a critical care unit (RR ,1.96 compared with all other units),
• Inpatient for more than 2 weeks at the time of the survey (RR, 26.09 compared with patients in the hospital for 3 days or fewer).
Pathogens were reported for 372 of the 504 HAIs. Clostridium difficile was the most common pathogen, accounting for 12% of all HAIs that were identified. Staphylococcus aureus was the second most common pathogen (11%; about half of these cases were methicillin-resistant S. aureus), followed by Klebsiella pneumoniae and Klebsiella oxytoca (10%).
Dr. Magill acknowledged certain limitations of the survey, including the fact that a small number of patients surveyed in each hospital "make results of limited use to individual facilities," she said. "We also had a relatively small number of hospitals in 10 states participate. This survey method provides a single snapshot of HAIs based on a retrospective review of medical record data, which is sometimes not complete. Finally, we were not able to collect a lot of detailed patient information, such as underlying illnesses and severity of illness."
She and her associates are currently planning a phase 4 survey, anticipated to occur in 2014.
Dr. Magill said she had no relevant financial conflicts to disclose.
AT IDWEEK 2012
Major Finding: The overall prevalence of health care–associated infections among hospitalized patients nationwide was 4%.
Data Source: Preliminary results were obtained from a 2011 survey of 11,282 inpatients at 183 hospitals located in 10 states.
Disclosures: The study was conducted by the Centers for Disease Control and Prevention. Dr. Magill said she had no relevant financial conflicts to disclose.