User login
Bedside Tools to ID Severe C. difficile Fall Short
SAN FRANCISCO – A side-by-side comparison of three bedside tools used to identify severe cases of Clostridium difficile infection yielded no clear winner, a reminder that judgment at diagnosis is still the clinician’s best bet.
Criteria from the Infectious Diseases Society of America were more sensitive but the least specific than both the Hines Veterans Affairs (VA) and the ATLAS severity scoring systems, Thien-Ly Doan, Pharm.D. explained in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
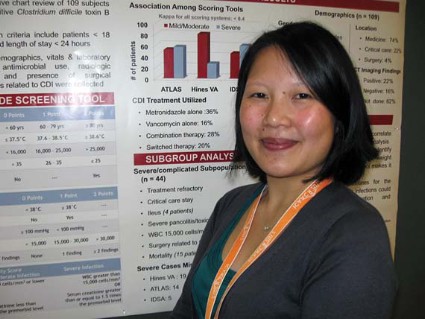
The Hines VA system for stratifying patients missed 19 of 44 severe/complicated cases of C. difficile infection. The ATLAS scoring system (which incorporates five parameters: age, temperature, leukocytosis, albumin, and systemic concomitant antibiotic use) missed 14 of the 44 cases in a retrospective chart review of 109 patients hospitalized for more than a day with confirmed C. difficile infection.
The IDSA guidelines missed only 5 of the 44 severe/complicated infections, but they cast such a wide net that anyone with a white count above 15,000 cells/mm3 or an elevated creatinine (1.5 times or greater than the premorbid level) is considered to have severe C. difficile infection, she said.
Use of the IDSA guidelines could increase unnecessary use of vancomycin instead of metronidazole, said Dr. Doan, a clinical coordinator at Long Island Jewish Medical Center, New Hyde Park, N.Y.
The IDSA criteria suggested that nearly 60% of the 109 patients had severe infection. However, the 44 severe/complicated C. difficile patients comprised just 40% of the study population. They were defined in the study as patients who were in critical care or whose infections were refractory to treatment and who had ileus, severe pancolitis/toxic megacolon, a WBC of 15,000 cells/mL with hypotension, surgery related to C. difficile infection, or who had died from infection.
Dr. Doan and her associates compared the three stratification systems in evaluating the charts of adults with C. difficile infection at the medical center, who had a mean age of 71 years. A total of 74% of patients were on the medicine service, 22% were in critical care, and 4% were on the surgical service; 34% were female.
The Centers for Disease Control and Prevention also offer severity criteria, but these require the observation of clinical end points and thus are ineffective for assessing patients at initial presentation, she said in a poster presentation at the meeting, sponsored by the American Society for Microbiology.
The Hines VA scoring system, in addition to missing the most severe cases, also gives a great deal of weight to diagnostic imaging, which "makes it impractical at our institution," she said. The Hines VA tool incorporates temperature, the presence of ileus, systolic blood pressure, leukocytosis, and abnormal CT findings to stratify patients by severity.
"We’re going to continue relying on the clinician’s assessment at the bedside at the time of diagnosis to evaluate whether cases are severe or not severe, and not use any of these tools that are available," Dr. Doan said.
A good bedside tool sure would be nice, though, to have a good, objective way of identifying severe C. difficile infection, she added. In a large health system, order sets could be developed based on the tool’s findings "so that everybody would be on the same page in terms of treatment," she said. None of the current tools are good enough for that.
Severe cases of C. difficile are on the rise because of increasing prevalence of the hypervirulent NAP1/BI/027 strain, she noted.
A number of clinicians at the meeting approached her with their own versions of bedside tools for identifying severe C. difficile infection, which Dr. Doan and her associates may evaluate next. They also may compare the tools on different subpopulations of patients with severe infection, such as only patients whose death or surgery was related to C. difficile infection.
Dr. Doan reported having no financial disclosures.
Reported mortality from Clostridium difficile infection (CDI) in the United States has increased dramatically in recent years (Emerg. Infect. Dis. 2007;13: 1417-9). Current guidelines call for the use of oral vancomy-cin as first-line therapy in severe CDI while metronidazole may be used in milder disease (Infect. Control Hosp. Epidemiol. 2010;31:431-55). Thus, it becomes important for therapy to identify those with potentially severe CDI early in their clinical course. However, a systematic review published in 2012 that specifically looked at clinical prediction rules (CPRs) for poor outcomes in CDI concluded that the available tools are inadequate for the task (PLoS One 2012;7:e30258).
The study by Dr. Doan and colleagues assessed the utility of bedside severity-of-illness tools in the treatment of patients with CDI. This was a retrospective chart review of 109 patients hospitalized for more than a day with confirmed CDI. Three CPRs were assessed: The Hines VA system , ; the ATLAS scoring system; and the Infectious Diseases Society of America (IDSA) guidelines. . Sensitivity in detecting severe outcomes of CDI were 57%, 68%, and 89%, respectively. However, the most sensitive CPR, the IDSA guideline, showed poor specificity because it categorized 60% of all subjects as severe. Thus, the IDSA guideline will encourage more widespread use of oral vancomycin in CDI.
Therefore, we lack a risk-scoring system for severe CDI that is easy to use, sensitive, specific, and validated. Such a prediction tool is essential to allow us to follow the current CDI treatment guidelines.
CIARAN P. KELLY, M.D., is director of gastroenterology training and is medical director of the Celiac Center at Beth Israel Deaconess Medical Center, Boston. SAURABH SETHI, M.D., is a fellow in gastroenterology and hepatology at Beth Israe Deaconess. Dr. Kelly reported serving as a consultant or scientific advisor for, being a member of an advisory board for, or receiving research support from many companies developing drugs for C. difficile. Dr. Sethi had no relevant financial disclosures.
Reported mortality from Clostridium difficile infection (CDI) in the United States has increased dramatically in recent years (Emerg. Infect. Dis. 2007;13: 1417-9). Current guidelines call for the use of oral vancomy-cin as first-line therapy in severe CDI while metronidazole may be used in milder disease (Infect. Control Hosp. Epidemiol. 2010;31:431-55). Thus, it becomes important for therapy to identify those with potentially severe CDI early in their clinical course. However, a systematic review published in 2012 that specifically looked at clinical prediction rules (CPRs) for poor outcomes in CDI concluded that the available tools are inadequate for the task (PLoS One 2012;7:e30258).
The study by Dr. Doan and colleagues assessed the utility of bedside severity-of-illness tools in the treatment of patients with CDI. This was a retrospective chart review of 109 patients hospitalized for more than a day with confirmed CDI. Three CPRs were assessed: The Hines VA system , ; the ATLAS scoring system; and the Infectious Diseases Society of America (IDSA) guidelines. . Sensitivity in detecting severe outcomes of CDI were 57%, 68%, and 89%, respectively. However, the most sensitive CPR, the IDSA guideline, showed poor specificity because it categorized 60% of all subjects as severe. Thus, the IDSA guideline will encourage more widespread use of oral vancomycin in CDI.
Therefore, we lack a risk-scoring system for severe CDI that is easy to use, sensitive, specific, and validated. Such a prediction tool is essential to allow us to follow the current CDI treatment guidelines.
CIARAN P. KELLY, M.D., is director of gastroenterology training and is medical director of the Celiac Center at Beth Israel Deaconess Medical Center, Boston. SAURABH SETHI, M.D., is a fellow in gastroenterology and hepatology at Beth Israe Deaconess. Dr. Kelly reported serving as a consultant or scientific advisor for, being a member of an advisory board for, or receiving research support from many companies developing drugs for C. difficile. Dr. Sethi had no relevant financial disclosures.
Reported mortality from Clostridium difficile infection (CDI) in the United States has increased dramatically in recent years (Emerg. Infect. Dis. 2007;13: 1417-9). Current guidelines call for the use of oral vancomy-cin as first-line therapy in severe CDI while metronidazole may be used in milder disease (Infect. Control Hosp. Epidemiol. 2010;31:431-55). Thus, it becomes important for therapy to identify those with potentially severe CDI early in their clinical course. However, a systematic review published in 2012 that specifically looked at clinical prediction rules (CPRs) for poor outcomes in CDI concluded that the available tools are inadequate for the task (PLoS One 2012;7:e30258).
The study by Dr. Doan and colleagues assessed the utility of bedside severity-of-illness tools in the treatment of patients with CDI. This was a retrospective chart review of 109 patients hospitalized for more than a day with confirmed CDI. Three CPRs were assessed: The Hines VA system , ; the ATLAS scoring system; and the Infectious Diseases Society of America (IDSA) guidelines. . Sensitivity in detecting severe outcomes of CDI were 57%, 68%, and 89%, respectively. However, the most sensitive CPR, the IDSA guideline, showed poor specificity because it categorized 60% of all subjects as severe. Thus, the IDSA guideline will encourage more widespread use of oral vancomycin in CDI.
Therefore, we lack a risk-scoring system for severe CDI that is easy to use, sensitive, specific, and validated. Such a prediction tool is essential to allow us to follow the current CDI treatment guidelines.
CIARAN P. KELLY, M.D., is director of gastroenterology training and is medical director of the Celiac Center at Beth Israel Deaconess Medical Center, Boston. SAURABH SETHI, M.D., is a fellow in gastroenterology and hepatology at Beth Israe Deaconess. Dr. Kelly reported serving as a consultant or scientific advisor for, being a member of an advisory board for, or receiving research support from many companies developing drugs for C. difficile. Dr. Sethi had no relevant financial disclosures.
SAN FRANCISCO – A side-by-side comparison of three bedside tools used to identify severe cases of Clostridium difficile infection yielded no clear winner, a reminder that judgment at diagnosis is still the clinician’s best bet.
Criteria from the Infectious Diseases Society of America were more sensitive but the least specific than both the Hines Veterans Affairs (VA) and the ATLAS severity scoring systems, Thien-Ly Doan, Pharm.D. explained in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The Hines VA system for stratifying patients missed 19 of 44 severe/complicated cases of C. difficile infection. The ATLAS scoring system (which incorporates five parameters: age, temperature, leukocytosis, albumin, and systemic concomitant antibiotic use) missed 14 of the 44 cases in a retrospective chart review of 109 patients hospitalized for more than a day with confirmed C. difficile infection.
The IDSA guidelines missed only 5 of the 44 severe/complicated infections, but they cast such a wide net that anyone with a white count above 15,000 cells/mm3 or an elevated creatinine (1.5 times or greater than the premorbid level) is considered to have severe C. difficile infection, she said.
Use of the IDSA guidelines could increase unnecessary use of vancomycin instead of metronidazole, said Dr. Doan, a clinical coordinator at Long Island Jewish Medical Center, New Hyde Park, N.Y.
The IDSA criteria suggested that nearly 60% of the 109 patients had severe infection. However, the 44 severe/complicated C. difficile patients comprised just 40% of the study population. They were defined in the study as patients who were in critical care or whose infections were refractory to treatment and who had ileus, severe pancolitis/toxic megacolon, a WBC of 15,000 cells/mL with hypotension, surgery related to C. difficile infection, or who had died from infection.
Dr. Doan and her associates compared the three stratification systems in evaluating the charts of adults with C. difficile infection at the medical center, who had a mean age of 71 years. A total of 74% of patients were on the medicine service, 22% were in critical care, and 4% were on the surgical service; 34% were female.
The Centers for Disease Control and Prevention also offer severity criteria, but these require the observation of clinical end points and thus are ineffective for assessing patients at initial presentation, she said in a poster presentation at the meeting, sponsored by the American Society for Microbiology.
The Hines VA scoring system, in addition to missing the most severe cases, also gives a great deal of weight to diagnostic imaging, which "makes it impractical at our institution," she said. The Hines VA tool incorporates temperature, the presence of ileus, systolic blood pressure, leukocytosis, and abnormal CT findings to stratify patients by severity.
"We’re going to continue relying on the clinician’s assessment at the bedside at the time of diagnosis to evaluate whether cases are severe or not severe, and not use any of these tools that are available," Dr. Doan said.
A good bedside tool sure would be nice, though, to have a good, objective way of identifying severe C. difficile infection, she added. In a large health system, order sets could be developed based on the tool’s findings "so that everybody would be on the same page in terms of treatment," she said. None of the current tools are good enough for that.
Severe cases of C. difficile are on the rise because of increasing prevalence of the hypervirulent NAP1/BI/027 strain, she noted.
A number of clinicians at the meeting approached her with their own versions of bedside tools for identifying severe C. difficile infection, which Dr. Doan and her associates may evaluate next. They also may compare the tools on different subpopulations of patients with severe infection, such as only patients whose death or surgery was related to C. difficile infection.
Dr. Doan reported having no financial disclosures.
SAN FRANCISCO – A side-by-side comparison of three bedside tools used to identify severe cases of Clostridium difficile infection yielded no clear winner, a reminder that judgment at diagnosis is still the clinician’s best bet.
Criteria from the Infectious Diseases Society of America were more sensitive but the least specific than both the Hines Veterans Affairs (VA) and the ATLAS severity scoring systems, Thien-Ly Doan, Pharm.D. explained in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The Hines VA system for stratifying patients missed 19 of 44 severe/complicated cases of C. difficile infection. The ATLAS scoring system (which incorporates five parameters: age, temperature, leukocytosis, albumin, and systemic concomitant antibiotic use) missed 14 of the 44 cases in a retrospective chart review of 109 patients hospitalized for more than a day with confirmed C. difficile infection.
The IDSA guidelines missed only 5 of the 44 severe/complicated infections, but they cast such a wide net that anyone with a white count above 15,000 cells/mm3 or an elevated creatinine (1.5 times or greater than the premorbid level) is considered to have severe C. difficile infection, she said.
Use of the IDSA guidelines could increase unnecessary use of vancomycin instead of metronidazole, said Dr. Doan, a clinical coordinator at Long Island Jewish Medical Center, New Hyde Park, N.Y.
The IDSA criteria suggested that nearly 60% of the 109 patients had severe infection. However, the 44 severe/complicated C. difficile patients comprised just 40% of the study population. They were defined in the study as patients who were in critical care or whose infections were refractory to treatment and who had ileus, severe pancolitis/toxic megacolon, a WBC of 15,000 cells/mL with hypotension, surgery related to C. difficile infection, or who had died from infection.
Dr. Doan and her associates compared the three stratification systems in evaluating the charts of adults with C. difficile infection at the medical center, who had a mean age of 71 years. A total of 74% of patients were on the medicine service, 22% were in critical care, and 4% were on the surgical service; 34% were female.
The Centers for Disease Control and Prevention also offer severity criteria, but these require the observation of clinical end points and thus are ineffective for assessing patients at initial presentation, she said in a poster presentation at the meeting, sponsored by the American Society for Microbiology.
The Hines VA scoring system, in addition to missing the most severe cases, also gives a great deal of weight to diagnostic imaging, which "makes it impractical at our institution," she said. The Hines VA tool incorporates temperature, the presence of ileus, systolic blood pressure, leukocytosis, and abnormal CT findings to stratify patients by severity.
"We’re going to continue relying on the clinician’s assessment at the bedside at the time of diagnosis to evaluate whether cases are severe or not severe, and not use any of these tools that are available," Dr. Doan said.
A good bedside tool sure would be nice, though, to have a good, objective way of identifying severe C. difficile infection, she added. In a large health system, order sets could be developed based on the tool’s findings "so that everybody would be on the same page in terms of treatment," she said. None of the current tools are good enough for that.
Severe cases of C. difficile are on the rise because of increasing prevalence of the hypervirulent NAP1/BI/027 strain, she noted.
A number of clinicians at the meeting approached her with their own versions of bedside tools for identifying severe C. difficile infection, which Dr. Doan and her associates may evaluate next. They also may compare the tools on different subpopulations of patients with severe infection, such as only patients whose death or surgery was related to C. difficile infection.
Dr. Doan reported having no financial disclosures.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Ten States Dealing With H3N2 Outbreak
SAN FRANCISCO – The H3N2 virus could sicken 500-900 people in the United States by the end of 2012, surveillance studies by the Centers for Disease Control and Prevention suggest.
"Cooler weather is approaching, and it is likely that additional cases of H3N2v will be identified in the coming weeks," said Lyn Finelli, Dr.PH., chief of surveillance and outbreak response in the influenza division at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta.
Between December 2005 and June of 2012, there have been 36 human cases of swine influenza A virus infection identified, Dr. Finelli said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "Identification has increased since 2009" as novel influenza A became a reportable disease in 2007, better diagnostics became available at state health departments, and there has been greater awareness due to the 2009 pandemic H1N1 virus.
In 2011, public health officials in the United States identified 12 cases of H3N2v with the pandemic M gene from the 2009 pandemic H1N1 virus. Six were associated with exposure at agricultural fairs or farms and six were cases of human to human transmission.
This subtype continues to spike in prevalence. Between July 1 and Sept. 10, 2012, 302 cases of H3N2v infection with the M gene have been confirmed in the United States. The M gene "was thought to contribute to increased transmissibility of the pandemic H1N1 virus," Dr. Finelli noted. "In two animal studies it was shown to increase transmissibility for pandemic H1N1 ... Various serologic studies to date suggest that children under 12 have very little protection against this virus."
An outbreak of H3N2v is occurring in 10 states, and 16 patients have been hospitalized. Indiana has the most confirmed cases, followed by Ohio and Wisconsin. "Ohio also has a large number of probable cases, many of whom are rapid test positive but who have not been tested with PCR, so that state probably has more cases than Indiana at this point," Dr. Finelli said.
The mean age of the 302 cases is 8 years, with a range between 4 months and 74 years, and the incubation period is 2-3 days. "The secondary attack rate is low," she said. "We only have 10 probable cases of human to human transmission. The duration of illness is 3-4 days and the period of infectiousness is unknown. We don’t have enough secondary transmission to tell."
Of the 16 patients who have been hospitalized with H3N2v, 14 (87%) were 0-17 years of age and the remaining 2 were at least 18 years of age. The most common underlying condition is being 5 years of age or younger (38%), followed by asthma (19%), cancer or immune suppression (19%), and neurological disorder (13%). One patient died (7%).
Dr. Finelli and her associates have exposure data on 203 cases. Of these, 198 (98%) had either direct or indirect swine contact, or attended a state or county fair.
Antiviral treatment with oral oseltamivir or inhaled zanamivir is encouraged as soon as possible for patients with suspected H3N2v virus infection, especially hospitalized patients or patients with severe or progressive illness, she said. "All non–high-risk outpatients without underlying medical conditions can be started within 48 hours of illness onset."
"Surveillance guidance for state and local public health has focused on increasing collection of PCR quality specimens from patients presenting with influenza-like illness in high risk groups, such as outbreaks in child care and school settings or in populations where confirmed H3N2v cases have occurred," Dr. Finelli said.
Two candidate H3N2v vaccines have been identified and have been sent to the World Health Organization, the Food and Drug Administration, and vaccine manufacturers, she said. Clinical trials using the National Institutes of Health’s Vaccine and Therapeutics Evaluation Unit are now under way.
The conference was sponsored by the American Society for Microbiology. Dr. Finelli said that she had no relevant financial disclosures to make.
SAN FRANCISCO – The H3N2 virus could sicken 500-900 people in the United States by the end of 2012, surveillance studies by the Centers for Disease Control and Prevention suggest.
"Cooler weather is approaching, and it is likely that additional cases of H3N2v will be identified in the coming weeks," said Lyn Finelli, Dr.PH., chief of surveillance and outbreak response in the influenza division at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta.
Between December 2005 and June of 2012, there have been 36 human cases of swine influenza A virus infection identified, Dr. Finelli said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "Identification has increased since 2009" as novel influenza A became a reportable disease in 2007, better diagnostics became available at state health departments, and there has been greater awareness due to the 2009 pandemic H1N1 virus.
In 2011, public health officials in the United States identified 12 cases of H3N2v with the pandemic M gene from the 2009 pandemic H1N1 virus. Six were associated with exposure at agricultural fairs or farms and six were cases of human to human transmission.
This subtype continues to spike in prevalence. Between July 1 and Sept. 10, 2012, 302 cases of H3N2v infection with the M gene have been confirmed in the United States. The M gene "was thought to contribute to increased transmissibility of the pandemic H1N1 virus," Dr. Finelli noted. "In two animal studies it was shown to increase transmissibility for pandemic H1N1 ... Various serologic studies to date suggest that children under 12 have very little protection against this virus."
An outbreak of H3N2v is occurring in 10 states, and 16 patients have been hospitalized. Indiana has the most confirmed cases, followed by Ohio and Wisconsin. "Ohio also has a large number of probable cases, many of whom are rapid test positive but who have not been tested with PCR, so that state probably has more cases than Indiana at this point," Dr. Finelli said.
The mean age of the 302 cases is 8 years, with a range between 4 months and 74 years, and the incubation period is 2-3 days. "The secondary attack rate is low," she said. "We only have 10 probable cases of human to human transmission. The duration of illness is 3-4 days and the period of infectiousness is unknown. We don’t have enough secondary transmission to tell."
Of the 16 patients who have been hospitalized with H3N2v, 14 (87%) were 0-17 years of age and the remaining 2 were at least 18 years of age. The most common underlying condition is being 5 years of age or younger (38%), followed by asthma (19%), cancer or immune suppression (19%), and neurological disorder (13%). One patient died (7%).
Dr. Finelli and her associates have exposure data on 203 cases. Of these, 198 (98%) had either direct or indirect swine contact, or attended a state or county fair.
Antiviral treatment with oral oseltamivir or inhaled zanamivir is encouraged as soon as possible for patients with suspected H3N2v virus infection, especially hospitalized patients or patients with severe or progressive illness, she said. "All non–high-risk outpatients without underlying medical conditions can be started within 48 hours of illness onset."
"Surveillance guidance for state and local public health has focused on increasing collection of PCR quality specimens from patients presenting with influenza-like illness in high risk groups, such as outbreaks in child care and school settings or in populations where confirmed H3N2v cases have occurred," Dr. Finelli said.
Two candidate H3N2v vaccines have been identified and have been sent to the World Health Organization, the Food and Drug Administration, and vaccine manufacturers, she said. Clinical trials using the National Institutes of Health’s Vaccine and Therapeutics Evaluation Unit are now under way.
The conference was sponsored by the American Society for Microbiology. Dr. Finelli said that she had no relevant financial disclosures to make.
SAN FRANCISCO – The H3N2 virus could sicken 500-900 people in the United States by the end of 2012, surveillance studies by the Centers for Disease Control and Prevention suggest.
"Cooler weather is approaching, and it is likely that additional cases of H3N2v will be identified in the coming weeks," said Lyn Finelli, Dr.PH., chief of surveillance and outbreak response in the influenza division at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta.
Between December 2005 and June of 2012, there have been 36 human cases of swine influenza A virus infection identified, Dr. Finelli said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "Identification has increased since 2009" as novel influenza A became a reportable disease in 2007, better diagnostics became available at state health departments, and there has been greater awareness due to the 2009 pandemic H1N1 virus.
In 2011, public health officials in the United States identified 12 cases of H3N2v with the pandemic M gene from the 2009 pandemic H1N1 virus. Six were associated with exposure at agricultural fairs or farms and six were cases of human to human transmission.
This subtype continues to spike in prevalence. Between July 1 and Sept. 10, 2012, 302 cases of H3N2v infection with the M gene have been confirmed in the United States. The M gene "was thought to contribute to increased transmissibility of the pandemic H1N1 virus," Dr. Finelli noted. "In two animal studies it was shown to increase transmissibility for pandemic H1N1 ... Various serologic studies to date suggest that children under 12 have very little protection against this virus."
An outbreak of H3N2v is occurring in 10 states, and 16 patients have been hospitalized. Indiana has the most confirmed cases, followed by Ohio and Wisconsin. "Ohio also has a large number of probable cases, many of whom are rapid test positive but who have not been tested with PCR, so that state probably has more cases than Indiana at this point," Dr. Finelli said.
The mean age of the 302 cases is 8 years, with a range between 4 months and 74 years, and the incubation period is 2-3 days. "The secondary attack rate is low," she said. "We only have 10 probable cases of human to human transmission. The duration of illness is 3-4 days and the period of infectiousness is unknown. We don’t have enough secondary transmission to tell."
Of the 16 patients who have been hospitalized with H3N2v, 14 (87%) were 0-17 years of age and the remaining 2 were at least 18 years of age. The most common underlying condition is being 5 years of age or younger (38%), followed by asthma (19%), cancer or immune suppression (19%), and neurological disorder (13%). One patient died (7%).
Dr. Finelli and her associates have exposure data on 203 cases. Of these, 198 (98%) had either direct or indirect swine contact, or attended a state or county fair.
Antiviral treatment with oral oseltamivir or inhaled zanamivir is encouraged as soon as possible for patients with suspected H3N2v virus infection, especially hospitalized patients or patients with severe or progressive illness, she said. "All non–high-risk outpatients without underlying medical conditions can be started within 48 hours of illness onset."
"Surveillance guidance for state and local public health has focused on increasing collection of PCR quality specimens from patients presenting with influenza-like illness in high risk groups, such as outbreaks in child care and school settings or in populations where confirmed H3N2v cases have occurred," Dr. Finelli said.
Two candidate H3N2v vaccines have been identified and have been sent to the World Health Organization, the Food and Drug Administration, and vaccine manufacturers, she said. Clinical trials using the National Institutes of Health’s Vaccine and Therapeutics Evaluation Unit are now under way.
The conference was sponsored by the American Society for Microbiology. Dr. Finelli said that she had no relevant financial disclosures to make.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
HPV Vaccination Held Cost Effective for Boys
SAN FRANCISCO – So few girls are receiving the human papillomavirus vaccine that vaccinating boys is becoming cost effective, according to Dr. Janet A. Englund.
That’s an important message from recent data on human papillomavirus (HPV) vaccination rates and cost-effectiveness studies, Dr. Englund said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Federal data show "quite low uptake in girls" in each of the past 3 years, "which makes it important to push for increased vaccination of boys," not only to protect males against genital warts and HPV-associated cancers but to increase protection for girls, she said.
The percentage of U.S. adolescents who received at least one dose of HPV vaccine increased from 69% in 2010 to 78% in 2011, but the proportion of U.S. girls who got all three recommended doses was only 32% in 2010 and 35% in 2011 (MMWR 2012;61:671-7).
That’s "disappointing" compared with larger increases in the use of other vaccines in U.S. girls in the same time period and in comparison with HPV vaccination coverage in other countries, said Dr. Englund, a specialist in infectious diseases at Seattle Children’s Hospital and professor of pediatrics at the University of Washington, Seattle. She was a member of the Advisory Committee on Immunization Practices (ACIP) during much of the time HPV vaccine issues were discussed.
School-based vaccination programs have produced HPV vaccination series coverage rates of 56%-85% in Canadian provinces, 80%-90% in the United Kingdom, and greater than 90% in Australia. The United States does not have a school-based vaccination program. Four years after the HPV vaccination program was started in Australia, genital warts have nearly disappeared in young women and men, one study found (Sex Transm. Infect. 2011;87:544-7).
In late 2011, ACIP recommended routine use of HPV vaccine in boys aged 11-12 years. Studies have shown that immunogenicity after the quadrivalent vaccine series is more than doubled if males get vaccinated at ages 9-15 years compared with 16-26 years, she said at the meeting, sponsored by the American Society for Microbiology. For both boys and girls, the vaccine is most effective if given prior to becoming sexually active.
In males, pivotal trials found efficacy rates for the quadrivalent HPV vaccine to be 89% in previously uninfected males and 67% regardless of infection status at baseline.
"These vaccines remain quite expensive," but they are cost effective in the United States, she said.
Analyses presented to ACIP in 2009 suggest that the HPV vaccine’s cost per quality-adjusted life year (QALY) gained for 12-year-old girls ranges from $3,000 to $45,000, depending on the model used in the analysis, Dr. Englund said.
If just the prevention of cervical disease is considered, the cost of adding HPV vaccinations for males seems too costly, adding $115,400-$182,400 in cost per QALY. But if the vaccine’s benefits in reducing vulvar and vaginal cancers, genital warts, anal cancer, recurrent respiratory papillomatosis, oropharyngeal cancer, and penile cancer are also considered, the cost per QALY when vaccinating males drops to $24,400-$42,700. In the United States, costs below $50,000 per QALY are generally considered to be cost effective, Dr. Englund said.
Other analyses suggest that vaccinating boys against HPV is cost effective when the immunization rate for girls is low, "which it is in the United States," she said. "Although coverage in females is increasing, we believe that male vaccination would remain cost effective into the foreseeable future." When female coverage reaches high levels, male vaccination likely would not be cost effective.
Between 2004 and 2007, invasive cancers associated with HPV types 16 and 18 were diagnosed in more than 11,000 men and more than 20,000 women in the United States, data presented to ACIP in 2011 showed.
One study that enrolled freshmen at the University of Washington found approximately a 50% cumulative incidence of HPV in males during the next 24 months, she added.
The most common adverse events in males after vaccination are injection-site reactions. Dr. Englund said that serious adverse events have been minimized in her office by having the adolescents sit down for half an hour, or at least 15 minutes, before leaving the office.
Dr. Englund has been a consultant for GlaxoSmithKline (which markets an HPV vaccine) and Novavax and has received research support from Novartis and Chimerix.
Advisory Committee on Vaccination Practices, ACIP,
SAN FRANCISCO – So few girls are receiving the human papillomavirus vaccine that vaccinating boys is becoming cost effective, according to Dr. Janet A. Englund.
That’s an important message from recent data on human papillomavirus (HPV) vaccination rates and cost-effectiveness studies, Dr. Englund said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Federal data show "quite low uptake in girls" in each of the past 3 years, "which makes it important to push for increased vaccination of boys," not only to protect males against genital warts and HPV-associated cancers but to increase protection for girls, she said.
The percentage of U.S. adolescents who received at least one dose of HPV vaccine increased from 69% in 2010 to 78% in 2011, but the proportion of U.S. girls who got all three recommended doses was only 32% in 2010 and 35% in 2011 (MMWR 2012;61:671-7).
That’s "disappointing" compared with larger increases in the use of other vaccines in U.S. girls in the same time period and in comparison with HPV vaccination coverage in other countries, said Dr. Englund, a specialist in infectious diseases at Seattle Children’s Hospital and professor of pediatrics at the University of Washington, Seattle. She was a member of the Advisory Committee on Immunization Practices (ACIP) during much of the time HPV vaccine issues were discussed.
School-based vaccination programs have produced HPV vaccination series coverage rates of 56%-85% in Canadian provinces, 80%-90% in the United Kingdom, and greater than 90% in Australia. The United States does not have a school-based vaccination program. Four years after the HPV vaccination program was started in Australia, genital warts have nearly disappeared in young women and men, one study found (Sex Transm. Infect. 2011;87:544-7).
In late 2011, ACIP recommended routine use of HPV vaccine in boys aged 11-12 years. Studies have shown that immunogenicity after the quadrivalent vaccine series is more than doubled if males get vaccinated at ages 9-15 years compared with 16-26 years, she said at the meeting, sponsored by the American Society for Microbiology. For both boys and girls, the vaccine is most effective if given prior to becoming sexually active.
In males, pivotal trials found efficacy rates for the quadrivalent HPV vaccine to be 89% in previously uninfected males and 67% regardless of infection status at baseline.
"These vaccines remain quite expensive," but they are cost effective in the United States, she said.
Analyses presented to ACIP in 2009 suggest that the HPV vaccine’s cost per quality-adjusted life year (QALY) gained for 12-year-old girls ranges from $3,000 to $45,000, depending on the model used in the analysis, Dr. Englund said.
If just the prevention of cervical disease is considered, the cost of adding HPV vaccinations for males seems too costly, adding $115,400-$182,400 in cost per QALY. But if the vaccine’s benefits in reducing vulvar and vaginal cancers, genital warts, anal cancer, recurrent respiratory papillomatosis, oropharyngeal cancer, and penile cancer are also considered, the cost per QALY when vaccinating males drops to $24,400-$42,700. In the United States, costs below $50,000 per QALY are generally considered to be cost effective, Dr. Englund said.
Other analyses suggest that vaccinating boys against HPV is cost effective when the immunization rate for girls is low, "which it is in the United States," she said. "Although coverage in females is increasing, we believe that male vaccination would remain cost effective into the foreseeable future." When female coverage reaches high levels, male vaccination likely would not be cost effective.
Between 2004 and 2007, invasive cancers associated with HPV types 16 and 18 were diagnosed in more than 11,000 men and more than 20,000 women in the United States, data presented to ACIP in 2011 showed.
One study that enrolled freshmen at the University of Washington found approximately a 50% cumulative incidence of HPV in males during the next 24 months, she added.
The most common adverse events in males after vaccination are injection-site reactions. Dr. Englund said that serious adverse events have been minimized in her office by having the adolescents sit down for half an hour, or at least 15 minutes, before leaving the office.
Dr. Englund has been a consultant for GlaxoSmithKline (which markets an HPV vaccine) and Novavax and has received research support from Novartis and Chimerix.
SAN FRANCISCO – So few girls are receiving the human papillomavirus vaccine that vaccinating boys is becoming cost effective, according to Dr. Janet A. Englund.
That’s an important message from recent data on human papillomavirus (HPV) vaccination rates and cost-effectiveness studies, Dr. Englund said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Federal data show "quite low uptake in girls" in each of the past 3 years, "which makes it important to push for increased vaccination of boys," not only to protect males against genital warts and HPV-associated cancers but to increase protection for girls, she said.
The percentage of U.S. adolescents who received at least one dose of HPV vaccine increased from 69% in 2010 to 78% in 2011, but the proportion of U.S. girls who got all three recommended doses was only 32% in 2010 and 35% in 2011 (MMWR 2012;61:671-7).
That’s "disappointing" compared with larger increases in the use of other vaccines in U.S. girls in the same time period and in comparison with HPV vaccination coverage in other countries, said Dr. Englund, a specialist in infectious diseases at Seattle Children’s Hospital and professor of pediatrics at the University of Washington, Seattle. She was a member of the Advisory Committee on Immunization Practices (ACIP) during much of the time HPV vaccine issues were discussed.
School-based vaccination programs have produced HPV vaccination series coverage rates of 56%-85% in Canadian provinces, 80%-90% in the United Kingdom, and greater than 90% in Australia. The United States does not have a school-based vaccination program. Four years after the HPV vaccination program was started in Australia, genital warts have nearly disappeared in young women and men, one study found (Sex Transm. Infect. 2011;87:544-7).
In late 2011, ACIP recommended routine use of HPV vaccine in boys aged 11-12 years. Studies have shown that immunogenicity after the quadrivalent vaccine series is more than doubled if males get vaccinated at ages 9-15 years compared with 16-26 years, she said at the meeting, sponsored by the American Society for Microbiology. For both boys and girls, the vaccine is most effective if given prior to becoming sexually active.
In males, pivotal trials found efficacy rates for the quadrivalent HPV vaccine to be 89% in previously uninfected males and 67% regardless of infection status at baseline.
"These vaccines remain quite expensive," but they are cost effective in the United States, she said.
Analyses presented to ACIP in 2009 suggest that the HPV vaccine’s cost per quality-adjusted life year (QALY) gained for 12-year-old girls ranges from $3,000 to $45,000, depending on the model used in the analysis, Dr. Englund said.
If just the prevention of cervical disease is considered, the cost of adding HPV vaccinations for males seems too costly, adding $115,400-$182,400 in cost per QALY. But if the vaccine’s benefits in reducing vulvar and vaginal cancers, genital warts, anal cancer, recurrent respiratory papillomatosis, oropharyngeal cancer, and penile cancer are also considered, the cost per QALY when vaccinating males drops to $24,400-$42,700. In the United States, costs below $50,000 per QALY are generally considered to be cost effective, Dr. Englund said.
Other analyses suggest that vaccinating boys against HPV is cost effective when the immunization rate for girls is low, "which it is in the United States," she said. "Although coverage in females is increasing, we believe that male vaccination would remain cost effective into the foreseeable future." When female coverage reaches high levels, male vaccination likely would not be cost effective.
Between 2004 and 2007, invasive cancers associated with HPV types 16 and 18 were diagnosed in more than 11,000 men and more than 20,000 women in the United States, data presented to ACIP in 2011 showed.
One study that enrolled freshmen at the University of Washington found approximately a 50% cumulative incidence of HPV in males during the next 24 months, she added.
The most common adverse events in males after vaccination are injection-site reactions. Dr. Englund said that serious adverse events have been minimized in her office by having the adolescents sit down for half an hour, or at least 15 minutes, before leaving the office.
Dr. Englund has been a consultant for GlaxoSmithKline (which markets an HPV vaccine) and Novavax and has received research support from Novartis and Chimerix.
Advisory Committee on Vaccination Practices, ACIP,
Advisory Committee on Vaccination Practices, ACIP,
EXPERT ANALYSIS FROM THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
H5N1 Called an Entrenched Threat to Human Health
SAN FRANCISCO – The pathogenic avian influenza A(H5N1) virus remains entrenched in poultry in many countries and is unlikely to be eradicated, according to Dr. Malik Peiris.
"Over the past 15 years the virus has spread through Asia and to part of Africa. Even this year there have been poultry outbreaks in about nine countries, especially in Egypt but also in Asia and Indonesia," said Dr. Peiris, director of the Center of Influenza Research at the University of Hong Kong. "That, I think, is the real cause for concern."
So far, human disease has been uncommon, but the potential for human exposure to H5N1 is massive, he said. The reasons for viral spread are multifactorial, including the prevalence of backyard flocks of poultry and game birds, which are extremely common in parts of Asia, and the fact that the virus can infect ducks. "These ducks are moved from paddy field to paddy field," he said. "They graze on fallen rice in these paddy fields, and they move the virus without any ill effect to themselves. Live poultry markets are also a reservoir and amplifier. Some lineages of this virus can be moved long distances through migration of wild birds, but it is not clear whether wild birds are a true reservoir of this virus."
To date, Dr. Peiris said, 608 human cases of H5N1 infection have been reported from 15 countries in Asia and Africa. Of these, 359 (59%) have been fatal. The incubation period is 2-3 days, and the virus presents as severe viral pneumonia. "It’s rapidly progressing in previously healthy younger persons," he said. "It’s not the type of pneumonia [caused by] complications of influenza that you see with typical seasonal flu, which is at the extremes of age and is often associated with secondary bacterial superinfection. These are perfectly healthy people."
Virus clades from Indonesia seem to carry the greatest severity, Dr. Peiris said, followed by clades from the Middle East and those from other parts of Asia. There appears to be lower mortality among affected children under age 5 and among patients who receive oseltamivir treatment within 2 days of symptom onset.
"The virus strains are generally sensitive to oseltamivir, though different clades have a different range of sensitivity," Dr. Peiris said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "There are cases where antiviral resistance has been detected, with adverse outcomes."
According to World Health Organization guidelines published in 2007, "modified regimens of oseltamivir treatment, including twofold higher dosage, longer duration, and, possibly, combination therapy with amantadine or rimantadine (in countries where A(H5N1) viruses are likely to be susceptible to amantadines), may be considered on a case by case basis, especially in patients with pneumonia or progressive disease."
According to Dr. Peiris, data on this approach "are limited, but observational data suggest that a higher dose of oseltamivir is not associated with lower mortality."
While some experts argue that H5N1 viruses are inherently unable to transmit from human to human, two recent studies of ferrets suggest that airborne transmission is possible (Science 2012;336:1534-41 and Nature 2012;486:420-8). "While combinations of mutations are required for acquisition of mammalian transmissibility, some of these are individually present in some field isolates of H5N1 viruses, highlighting the need for enhanced and continued vigilance," Dr. Peiris noted.
The H5N1 virus "has diversified genetically and antigenically over the years," he said. "This is the challenge that we face in developing a vaccine for H5N1."
The conference was sponsored by the American Society for Microbiology. Dr. Peiris disclosed that he is a scientific adviser for Crucell and is a consultant for GlaxoSmithKline.
SAN FRANCISCO – The pathogenic avian influenza A(H5N1) virus remains entrenched in poultry in many countries and is unlikely to be eradicated, according to Dr. Malik Peiris.
"Over the past 15 years the virus has spread through Asia and to part of Africa. Even this year there have been poultry outbreaks in about nine countries, especially in Egypt but also in Asia and Indonesia," said Dr. Peiris, director of the Center of Influenza Research at the University of Hong Kong. "That, I think, is the real cause for concern."
So far, human disease has been uncommon, but the potential for human exposure to H5N1 is massive, he said. The reasons for viral spread are multifactorial, including the prevalence of backyard flocks of poultry and game birds, which are extremely common in parts of Asia, and the fact that the virus can infect ducks. "These ducks are moved from paddy field to paddy field," he said. "They graze on fallen rice in these paddy fields, and they move the virus without any ill effect to themselves. Live poultry markets are also a reservoir and amplifier. Some lineages of this virus can be moved long distances through migration of wild birds, but it is not clear whether wild birds are a true reservoir of this virus."
To date, Dr. Peiris said, 608 human cases of H5N1 infection have been reported from 15 countries in Asia and Africa. Of these, 359 (59%) have been fatal. The incubation period is 2-3 days, and the virus presents as severe viral pneumonia. "It’s rapidly progressing in previously healthy younger persons," he said. "It’s not the type of pneumonia [caused by] complications of influenza that you see with typical seasonal flu, which is at the extremes of age and is often associated with secondary bacterial superinfection. These are perfectly healthy people."
Virus clades from Indonesia seem to carry the greatest severity, Dr. Peiris said, followed by clades from the Middle East and those from other parts of Asia. There appears to be lower mortality among affected children under age 5 and among patients who receive oseltamivir treatment within 2 days of symptom onset.
"The virus strains are generally sensitive to oseltamivir, though different clades have a different range of sensitivity," Dr. Peiris said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "There are cases where antiviral resistance has been detected, with adverse outcomes."
According to World Health Organization guidelines published in 2007, "modified regimens of oseltamivir treatment, including twofold higher dosage, longer duration, and, possibly, combination therapy with amantadine or rimantadine (in countries where A(H5N1) viruses are likely to be susceptible to amantadines), may be considered on a case by case basis, especially in patients with pneumonia or progressive disease."
According to Dr. Peiris, data on this approach "are limited, but observational data suggest that a higher dose of oseltamivir is not associated with lower mortality."
While some experts argue that H5N1 viruses are inherently unable to transmit from human to human, two recent studies of ferrets suggest that airborne transmission is possible (Science 2012;336:1534-41 and Nature 2012;486:420-8). "While combinations of mutations are required for acquisition of mammalian transmissibility, some of these are individually present in some field isolates of H5N1 viruses, highlighting the need for enhanced and continued vigilance," Dr. Peiris noted.
The H5N1 virus "has diversified genetically and antigenically over the years," he said. "This is the challenge that we face in developing a vaccine for H5N1."
The conference was sponsored by the American Society for Microbiology. Dr. Peiris disclosed that he is a scientific adviser for Crucell and is a consultant for GlaxoSmithKline.
SAN FRANCISCO – The pathogenic avian influenza A(H5N1) virus remains entrenched in poultry in many countries and is unlikely to be eradicated, according to Dr. Malik Peiris.
"Over the past 15 years the virus has spread through Asia and to part of Africa. Even this year there have been poultry outbreaks in about nine countries, especially in Egypt but also in Asia and Indonesia," said Dr. Peiris, director of the Center of Influenza Research at the University of Hong Kong. "That, I think, is the real cause for concern."
So far, human disease has been uncommon, but the potential for human exposure to H5N1 is massive, he said. The reasons for viral spread are multifactorial, including the prevalence of backyard flocks of poultry and game birds, which are extremely common in parts of Asia, and the fact that the virus can infect ducks. "These ducks are moved from paddy field to paddy field," he said. "They graze on fallen rice in these paddy fields, and they move the virus without any ill effect to themselves. Live poultry markets are also a reservoir and amplifier. Some lineages of this virus can be moved long distances through migration of wild birds, but it is not clear whether wild birds are a true reservoir of this virus."
To date, Dr. Peiris said, 608 human cases of H5N1 infection have been reported from 15 countries in Asia and Africa. Of these, 359 (59%) have been fatal. The incubation period is 2-3 days, and the virus presents as severe viral pneumonia. "It’s rapidly progressing in previously healthy younger persons," he said. "It’s not the type of pneumonia [caused by] complications of influenza that you see with typical seasonal flu, which is at the extremes of age and is often associated with secondary bacterial superinfection. These are perfectly healthy people."
Virus clades from Indonesia seem to carry the greatest severity, Dr. Peiris said, followed by clades from the Middle East and those from other parts of Asia. There appears to be lower mortality among affected children under age 5 and among patients who receive oseltamivir treatment within 2 days of symptom onset.
"The virus strains are generally sensitive to oseltamivir, though different clades have a different range of sensitivity," Dr. Peiris said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "There are cases where antiviral resistance has been detected, with adverse outcomes."
According to World Health Organization guidelines published in 2007, "modified regimens of oseltamivir treatment, including twofold higher dosage, longer duration, and, possibly, combination therapy with amantadine or rimantadine (in countries where A(H5N1) viruses are likely to be susceptible to amantadines), may be considered on a case by case basis, especially in patients with pneumonia or progressive disease."
According to Dr. Peiris, data on this approach "are limited, but observational data suggest that a higher dose of oseltamivir is not associated with lower mortality."
While some experts argue that H5N1 viruses are inherently unable to transmit from human to human, two recent studies of ferrets suggest that airborne transmission is possible (Science 2012;336:1534-41 and Nature 2012;486:420-8). "While combinations of mutations are required for acquisition of mammalian transmissibility, some of these are individually present in some field isolates of H5N1 viruses, highlighting the need for enhanced and continued vigilance," Dr. Peiris noted.
The H5N1 virus "has diversified genetically and antigenically over the years," he said. "This is the challenge that we face in developing a vaccine for H5N1."
The conference was sponsored by the American Society for Microbiology. Dr. Peiris disclosed that he is a scientific adviser for Crucell and is a consultant for GlaxoSmithKline.
EXPERT ANALYSIS FROM THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Higher Dose for Severe C. diff Speeds Response
SAN FRANCISCO – Two small studies suggest that treating severe Clostridium difficile infection with a higher initial dose of vancomycin may work better than the recommended dose of 125 mg every six hours.
The most recent study, presented in a poster at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy, surprised the investigators.
"Pharmacodynamically, the concentrations in stool of the standard dose of 125 mg are about 500-1,000 times greater than the MIC [minimum inhibitory concentration]," Yleana T. Garcia, Pharm.D., said in an interview. "So, we have enough concentration in the stool. We wanted to see that we have similar outcomes with patients who are treated with standard doses as with higher doses."
Instead, the retrospective review of 62 patients with severe diarrhea who received oral vancomycin for at least 3 days found that symptoms resolved significantly quicker in 19 patients who got 250 mg every 6 hours compared with 43 patients who got 125 mg every 6 hours, she and her associates reported.
Symptoms resolved by day 3 in nine patients (47%) on the high dose and six patients (15%) on the conventional dose, said Dr. Garcia, a palliative care fellow at the James J. Peters Veterans Affairs Medical Center, Bronx, N.Y.
There also were statistically nonsignificant trends toward a higher likelihood of clinical cure, shorter length of stay, and reduced risk of recurrence in patients with the higher dose. An increased death rate in the higher-dose group also was not statistically significant, and might be due to greater severity of illness at baseline in patients who got the higher dose of vancomycin, she said at the meeting, sponsored by the American Society for Microbiology.
The findings support those of a small prospective study that analyzed levels of vancomycin in feces collected from 15 patients with presumed or confirmed C. difficile infection. Drug concentrations were high in patients who got 250 or 500 mg q.i.d. but were inadequate in at least one patient on the first day of treatment with 125 mg q.i.d. (BMC Infect. Dis. 2010;10:363).
"Higher doses like 250-500 mg may be warranted to reach adequate concentrations in the stool in the first 24-48 hours," Dr. Garcia said. "I’m not saying to use 250 for the whole treatment course, but there may be a role for a loading dose of 250 mg q6 for the first 24-48 hours, and then switching to 125 mg q6 for the remainder of the treatment course. We know that 125 does have adequate fecal concentration; it just may not be adequate on day 1."
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends treating severe C. difficile infection with oral vancomycin 125 mg every 6 hours or using 500 mg every 6 hours for patients with severe disease complicated by ileus, megacolon, or hypotension (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
Severe C. difficile infection generally is defined as the presence of the organism plus leukocytosis with a white blood cell count of 15,000 cells/microL or greater, or a serum creatinine level at least 1.5 times baseline. The study reviewed records of patients who received vancomycin for these indications or hypotension, shock, ileus, megacolon, or evidence of colitis. The study excluded patients who were treated with any other medication besides metronidazole.
The study is continuing in order to increase the number of patients reviewed and the power of the findings.
Dr. Garcia reported having no financial disclosures.
SAN FRANCISCO – Two small studies suggest that treating severe Clostridium difficile infection with a higher initial dose of vancomycin may work better than the recommended dose of 125 mg every six hours.
The most recent study, presented in a poster at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy, surprised the investigators.
"Pharmacodynamically, the concentrations in stool of the standard dose of 125 mg are about 500-1,000 times greater than the MIC [minimum inhibitory concentration]," Yleana T. Garcia, Pharm.D., said in an interview. "So, we have enough concentration in the stool. We wanted to see that we have similar outcomes with patients who are treated with standard doses as with higher doses."
Instead, the retrospective review of 62 patients with severe diarrhea who received oral vancomycin for at least 3 days found that symptoms resolved significantly quicker in 19 patients who got 250 mg every 6 hours compared with 43 patients who got 125 mg every 6 hours, she and her associates reported.
Symptoms resolved by day 3 in nine patients (47%) on the high dose and six patients (15%) on the conventional dose, said Dr. Garcia, a palliative care fellow at the James J. Peters Veterans Affairs Medical Center, Bronx, N.Y.
There also were statistically nonsignificant trends toward a higher likelihood of clinical cure, shorter length of stay, and reduced risk of recurrence in patients with the higher dose. An increased death rate in the higher-dose group also was not statistically significant, and might be due to greater severity of illness at baseline in patients who got the higher dose of vancomycin, she said at the meeting, sponsored by the American Society for Microbiology.
The findings support those of a small prospective study that analyzed levels of vancomycin in feces collected from 15 patients with presumed or confirmed C. difficile infection. Drug concentrations were high in patients who got 250 or 500 mg q.i.d. but were inadequate in at least one patient on the first day of treatment with 125 mg q.i.d. (BMC Infect. Dis. 2010;10:363).
"Higher doses like 250-500 mg may be warranted to reach adequate concentrations in the stool in the first 24-48 hours," Dr. Garcia said. "I’m not saying to use 250 for the whole treatment course, but there may be a role for a loading dose of 250 mg q6 for the first 24-48 hours, and then switching to 125 mg q6 for the remainder of the treatment course. We know that 125 does have adequate fecal concentration; it just may not be adequate on day 1."
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends treating severe C. difficile infection with oral vancomycin 125 mg every 6 hours or using 500 mg every 6 hours for patients with severe disease complicated by ileus, megacolon, or hypotension (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
Severe C. difficile infection generally is defined as the presence of the organism plus leukocytosis with a white blood cell count of 15,000 cells/microL or greater, or a serum creatinine level at least 1.5 times baseline. The study reviewed records of patients who received vancomycin for these indications or hypotension, shock, ileus, megacolon, or evidence of colitis. The study excluded patients who were treated with any other medication besides metronidazole.
The study is continuing in order to increase the number of patients reviewed and the power of the findings.
Dr. Garcia reported having no financial disclosures.
SAN FRANCISCO – Two small studies suggest that treating severe Clostridium difficile infection with a higher initial dose of vancomycin may work better than the recommended dose of 125 mg every six hours.
The most recent study, presented in a poster at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy, surprised the investigators.
"Pharmacodynamically, the concentrations in stool of the standard dose of 125 mg are about 500-1,000 times greater than the MIC [minimum inhibitory concentration]," Yleana T. Garcia, Pharm.D., said in an interview. "So, we have enough concentration in the stool. We wanted to see that we have similar outcomes with patients who are treated with standard doses as with higher doses."
Instead, the retrospective review of 62 patients with severe diarrhea who received oral vancomycin for at least 3 days found that symptoms resolved significantly quicker in 19 patients who got 250 mg every 6 hours compared with 43 patients who got 125 mg every 6 hours, she and her associates reported.
Symptoms resolved by day 3 in nine patients (47%) on the high dose and six patients (15%) on the conventional dose, said Dr. Garcia, a palliative care fellow at the James J. Peters Veterans Affairs Medical Center, Bronx, N.Y.
There also were statistically nonsignificant trends toward a higher likelihood of clinical cure, shorter length of stay, and reduced risk of recurrence in patients with the higher dose. An increased death rate in the higher-dose group also was not statistically significant, and might be due to greater severity of illness at baseline in patients who got the higher dose of vancomycin, she said at the meeting, sponsored by the American Society for Microbiology.
The findings support those of a small prospective study that analyzed levels of vancomycin in feces collected from 15 patients with presumed or confirmed C. difficile infection. Drug concentrations were high in patients who got 250 or 500 mg q.i.d. but were inadequate in at least one patient on the first day of treatment with 125 mg q.i.d. (BMC Infect. Dis. 2010;10:363).
"Higher doses like 250-500 mg may be warranted to reach adequate concentrations in the stool in the first 24-48 hours," Dr. Garcia said. "I’m not saying to use 250 for the whole treatment course, but there may be a role for a loading dose of 250 mg q6 for the first 24-48 hours, and then switching to 125 mg q6 for the remainder of the treatment course. We know that 125 does have adequate fecal concentration; it just may not be adequate on day 1."
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends treating severe C. difficile infection with oral vancomycin 125 mg every 6 hours or using 500 mg every 6 hours for patients with severe disease complicated by ileus, megacolon, or hypotension (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
Severe C. difficile infection generally is defined as the presence of the organism plus leukocytosis with a white blood cell count of 15,000 cells/microL or greater, or a serum creatinine level at least 1.5 times baseline. The study reviewed records of patients who received vancomycin for these indications or hypotension, shock, ileus, megacolon, or evidence of colitis. The study excluded patients who were treated with any other medication besides metronidazole.
The study is continuing in order to increase the number of patients reviewed and the power of the findings.
Dr. Garcia reported having no financial disclosures.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Three Degrees Help Drive Antibiotic Decision in Cellulitis
SAN FRANCISCO – A greater than 3° C difference in skin temperature between affected and unaffected limbs in cellulitis – measured using inexpensive, handheld, infrared laser thermometers – was found to signal the need for hospital admission for intravenous antibiotics.
Skin temperature changes in cellulitis had never been quantified, said Dr. Michael Montalto. "We’ve never had a concept in absolute terms of the differences we feel as clinicians every day. [Our study gives] an idea of the kind of scale that might cause you to think the patient needs to have an admission for IV therapy. At least in our study, if the temperature difference [between the affected and unaffected limb] was above 3 °C, those people were getting IV therapy," he said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Of 63 cellulitis patients who were admitted to the hospital for intravenous antibiotic therapy, lesions were on average 3.4 °C warmer (95% confidence interval, 3.0-3.9) than the corresponding location on the unaffected limb. The difference dropped to an average 2.1° C warmer (95% CI, 1.7-2.6) at discharge after a mean stay of 5 days, the investigators found.
Patients in the study, who were 50 years old on average, had mostly lower-limb cellulitis; just over half were men. Nurses took the limb temperatures to keep researchers blinded to the results until the study’s end. Skin temperatures did not correlate with blood pressure, core temperature, or other variables.
Dr. Montalto and his colleagues found that the warmest point on limbs affected by cellulitis dropped from an average of 34.4 °C on the day of admission for intravenous antibiotics to 32° C when patients were well enough to be discharged on oral antibiotics, a statistically significant difference (95% CI, 1.9-3.0).
Furthermore, the results also suggested a role for laser thermometers – which can cost less than $50 at electronic stores and until now have been used mostly for industrial purposes – to measure severity and treatment response in cellulitis, said Dr. Montalto, a hospitalist at Epworth Hospital and Royal Melbourne Hospital. The devices emit two beams that are focused into one dot on the skin, at which point the temperature is read from a screen. The process is quick and painless.
The thermometers are "another tool to use for tricky patients when you are wondering whether or not they are getting better," he said. Current measures – white cell counts, erythema, fever, and skin color, among others – are not specific enough, he said.
The next step in the project is to see if skin temperature helps identify the causative organism in cellulitis, which remains unknown in many cases. Methicillin-resistant Staphylococcus aureus (MRSA) cellulitis, for instance, may project a higher temperature than other types of cellulitis.
"We often have people presenting from nursing homes who don’t have a wound. They just have a big, fat, painful, red leg with nothing to swab. You’ve got no way of determining what the organism is except trial and error. If we could show that the temperature profile helps with that," and, thus, appropriate antibiotic selection, it would be a significant advance, Dr. Montalto said at the meeting, which was sponsored by the American Society for Microbiology.
True to the point, 12 patients (19%) had positive swabs in the study, mostly for staphylococci, but a few MRSA and gram-negative bacteria also showed up.
Dr. Montalto said that he had no relevant financial disclosures.
SAN FRANCISCO – A greater than 3° C difference in skin temperature between affected and unaffected limbs in cellulitis – measured using inexpensive, handheld, infrared laser thermometers – was found to signal the need for hospital admission for intravenous antibiotics.
Skin temperature changes in cellulitis had never been quantified, said Dr. Michael Montalto. "We’ve never had a concept in absolute terms of the differences we feel as clinicians every day. [Our study gives] an idea of the kind of scale that might cause you to think the patient needs to have an admission for IV therapy. At least in our study, if the temperature difference [between the affected and unaffected limb] was above 3 °C, those people were getting IV therapy," he said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Of 63 cellulitis patients who were admitted to the hospital for intravenous antibiotic therapy, lesions were on average 3.4 °C warmer (95% confidence interval, 3.0-3.9) than the corresponding location on the unaffected limb. The difference dropped to an average 2.1° C warmer (95% CI, 1.7-2.6) at discharge after a mean stay of 5 days, the investigators found.
Patients in the study, who were 50 years old on average, had mostly lower-limb cellulitis; just over half were men. Nurses took the limb temperatures to keep researchers blinded to the results until the study’s end. Skin temperatures did not correlate with blood pressure, core temperature, or other variables.
Dr. Montalto and his colleagues found that the warmest point on limbs affected by cellulitis dropped from an average of 34.4 °C on the day of admission for intravenous antibiotics to 32° C when patients were well enough to be discharged on oral antibiotics, a statistically significant difference (95% CI, 1.9-3.0).
Furthermore, the results also suggested a role for laser thermometers – which can cost less than $50 at electronic stores and until now have been used mostly for industrial purposes – to measure severity and treatment response in cellulitis, said Dr. Montalto, a hospitalist at Epworth Hospital and Royal Melbourne Hospital. The devices emit two beams that are focused into one dot on the skin, at which point the temperature is read from a screen. The process is quick and painless.
The thermometers are "another tool to use for tricky patients when you are wondering whether or not they are getting better," he said. Current measures – white cell counts, erythema, fever, and skin color, among others – are not specific enough, he said.
The next step in the project is to see if skin temperature helps identify the causative organism in cellulitis, which remains unknown in many cases. Methicillin-resistant Staphylococcus aureus (MRSA) cellulitis, for instance, may project a higher temperature than other types of cellulitis.
"We often have people presenting from nursing homes who don’t have a wound. They just have a big, fat, painful, red leg with nothing to swab. You’ve got no way of determining what the organism is except trial and error. If we could show that the temperature profile helps with that," and, thus, appropriate antibiotic selection, it would be a significant advance, Dr. Montalto said at the meeting, which was sponsored by the American Society for Microbiology.
True to the point, 12 patients (19%) had positive swabs in the study, mostly for staphylococci, but a few MRSA and gram-negative bacteria also showed up.
Dr. Montalto said that he had no relevant financial disclosures.
SAN FRANCISCO – A greater than 3° C difference in skin temperature between affected and unaffected limbs in cellulitis – measured using inexpensive, handheld, infrared laser thermometers – was found to signal the need for hospital admission for intravenous antibiotics.
Skin temperature changes in cellulitis had never been quantified, said Dr. Michael Montalto. "We’ve never had a concept in absolute terms of the differences we feel as clinicians every day. [Our study gives] an idea of the kind of scale that might cause you to think the patient needs to have an admission for IV therapy. At least in our study, if the temperature difference [between the affected and unaffected limb] was above 3 °C, those people were getting IV therapy," he said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Of 63 cellulitis patients who were admitted to the hospital for intravenous antibiotic therapy, lesions were on average 3.4 °C warmer (95% confidence interval, 3.0-3.9) than the corresponding location on the unaffected limb. The difference dropped to an average 2.1° C warmer (95% CI, 1.7-2.6) at discharge after a mean stay of 5 days, the investigators found.
Patients in the study, who were 50 years old on average, had mostly lower-limb cellulitis; just over half were men. Nurses took the limb temperatures to keep researchers blinded to the results until the study’s end. Skin temperatures did not correlate with blood pressure, core temperature, or other variables.
Dr. Montalto and his colleagues found that the warmest point on limbs affected by cellulitis dropped from an average of 34.4 °C on the day of admission for intravenous antibiotics to 32° C when patients were well enough to be discharged on oral antibiotics, a statistically significant difference (95% CI, 1.9-3.0).
Furthermore, the results also suggested a role for laser thermometers – which can cost less than $50 at electronic stores and until now have been used mostly for industrial purposes – to measure severity and treatment response in cellulitis, said Dr. Montalto, a hospitalist at Epworth Hospital and Royal Melbourne Hospital. The devices emit two beams that are focused into one dot on the skin, at which point the temperature is read from a screen. The process is quick and painless.
The thermometers are "another tool to use for tricky patients when you are wondering whether or not they are getting better," he said. Current measures – white cell counts, erythema, fever, and skin color, among others – are not specific enough, he said.
The next step in the project is to see if skin temperature helps identify the causative organism in cellulitis, which remains unknown in many cases. Methicillin-resistant Staphylococcus aureus (MRSA) cellulitis, for instance, may project a higher temperature than other types of cellulitis.
"We often have people presenting from nursing homes who don’t have a wound. They just have a big, fat, painful, red leg with nothing to swab. You’ve got no way of determining what the organism is except trial and error. If we could show that the temperature profile helps with that," and, thus, appropriate antibiotic selection, it would be a significant advance, Dr. Montalto said at the meeting, which was sponsored by the American Society for Microbiology.
True to the point, 12 patients (19%) had positive swabs in the study, mostly for staphylococci, but a few MRSA and gram-negative bacteria also showed up.
Dr. Montalto said that he had no relevant financial disclosures.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Major Finding: Of 63 cellulitis patients who were admitted to the hospital for intravenous antibiotic therapy, lesions were on average 3.4 °C warmer (95% CI, 3.0-3.9) than the corresponding location on the unaffected limb.
Data Source: The data are from a prospective, blinded cohort study.
Disclosures: Dr. Montalto said that he had no relevant financial disclosures.
Linezolid Bests Vancomycin in Obese MRSA Pneumonia Patients
SAN FRANCISCO – Linezolid works better than vancomycin in obese patients with MRSA pneumonia, according to an industry-supported analysis.
Clinical success – ICU or hospital discharge by day 14 in the absence of death, therapy change, or intubation – was more likely among 49 patients with body-mass-indices of 30 or more treated with 600 mg of linezolid IV or orally every 12 hours, the standard dose, than among 740 treated with standard dosing of vancomycin (HR 1.77, 95% CI 1.18-2.64). The findings come from a national retrospective cohort analysis of Veterans Affairs hospital data. The study was funded in part by Pfizer, which markets linezolid as Zyvox.
"Clinical success rates were higher [in obese patients] with linezolid. We don’t know exactly why," lead investigator Aisling Caffrey, Ph.D., assistant professor of pharmacoepidemiology at the University of Rhode Island College of Pharmacy in Kingston, R.I., said at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
Maybe it was because vancomycin dosing is, in part, weight based and perhaps problematic for obese patients. Clinicians may be reluctant to exceed standard dosing even if BMIs suggest it, due to toxicity concerns; it’s unclear if patients in the study received adequate doses.
Linezolid "is a little more straightforward. Maybe obese patients were more likely to get the right dose," Dr. Caffrey said.
She and her coinvestigators hope to look further into the antibiotic treatment of MRSA pneumonia in the overweight population.
The investigation was a subgroup analysis of a larger MRSA pneumonia comparison study that found nonobese patients treated with linezolid were less likely to have 30-day hospital readmissions (HR 0.60, 95% CI 0.37-0.97). There were no other outcome differences between the drugs. Patients in the study were treated for at least 3 days.
The conference was sponsored by the American Society for Microbiology. Pfizer and the Department of Veterans Affairs funded the study. Two of the researchers were Pfizer employees. Dr. Caffrey\'s research is funded in part by Pfizer.
SAN FRANCISCO – Linezolid works better than vancomycin in obese patients with MRSA pneumonia, according to an industry-supported analysis.
Clinical success – ICU or hospital discharge by day 14 in the absence of death, therapy change, or intubation – was more likely among 49 patients with body-mass-indices of 30 or more treated with 600 mg of linezolid IV or orally every 12 hours, the standard dose, than among 740 treated with standard dosing of vancomycin (HR 1.77, 95% CI 1.18-2.64). The findings come from a national retrospective cohort analysis of Veterans Affairs hospital data. The study was funded in part by Pfizer, which markets linezolid as Zyvox.
"Clinical success rates were higher [in obese patients] with linezolid. We don’t know exactly why," lead investigator Aisling Caffrey, Ph.D., assistant professor of pharmacoepidemiology at the University of Rhode Island College of Pharmacy in Kingston, R.I., said at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
Maybe it was because vancomycin dosing is, in part, weight based and perhaps problematic for obese patients. Clinicians may be reluctant to exceed standard dosing even if BMIs suggest it, due to toxicity concerns; it’s unclear if patients in the study received adequate doses.
Linezolid "is a little more straightforward. Maybe obese patients were more likely to get the right dose," Dr. Caffrey said.
She and her coinvestigators hope to look further into the antibiotic treatment of MRSA pneumonia in the overweight population.
The investigation was a subgroup analysis of a larger MRSA pneumonia comparison study that found nonobese patients treated with linezolid were less likely to have 30-day hospital readmissions (HR 0.60, 95% CI 0.37-0.97). There were no other outcome differences between the drugs. Patients in the study were treated for at least 3 days.
The conference was sponsored by the American Society for Microbiology. Pfizer and the Department of Veterans Affairs funded the study. Two of the researchers were Pfizer employees. Dr. Caffrey\'s research is funded in part by Pfizer.
SAN FRANCISCO – Linezolid works better than vancomycin in obese patients with MRSA pneumonia, according to an industry-supported analysis.
Clinical success – ICU or hospital discharge by day 14 in the absence of death, therapy change, or intubation – was more likely among 49 patients with body-mass-indices of 30 or more treated with 600 mg of linezolid IV or orally every 12 hours, the standard dose, than among 740 treated with standard dosing of vancomycin (HR 1.77, 95% CI 1.18-2.64). The findings come from a national retrospective cohort analysis of Veterans Affairs hospital data. The study was funded in part by Pfizer, which markets linezolid as Zyvox.
"Clinical success rates were higher [in obese patients] with linezolid. We don’t know exactly why," lead investigator Aisling Caffrey, Ph.D., assistant professor of pharmacoepidemiology at the University of Rhode Island College of Pharmacy in Kingston, R.I., said at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
Maybe it was because vancomycin dosing is, in part, weight based and perhaps problematic for obese patients. Clinicians may be reluctant to exceed standard dosing even if BMIs suggest it, due to toxicity concerns; it’s unclear if patients in the study received adequate doses.
Linezolid "is a little more straightforward. Maybe obese patients were more likely to get the right dose," Dr. Caffrey said.
She and her coinvestigators hope to look further into the antibiotic treatment of MRSA pneumonia in the overweight population.
The investigation was a subgroup analysis of a larger MRSA pneumonia comparison study that found nonobese patients treated with linezolid were less likely to have 30-day hospital readmissions (HR 0.60, 95% CI 0.37-0.97). There were no other outcome differences between the drugs. Patients in the study were treated for at least 3 days.
The conference was sponsored by the American Society for Microbiology. Pfizer and the Department of Veterans Affairs funded the study. Two of the researchers were Pfizer employees. Dr. Caffrey\'s research is funded in part by Pfizer.
AT THE INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Major Finding: Clinical success – ICU or hospital discharge by day 14 in the absence of death, therapy change, or intubation – is more likely when obese patients with MRSA pneumonia are treated with linezolid instead of vancomycin (HR 1.77, 95% CI 1.18-2.64).
Data Source: Researchers conducted a national, retrospective analysis of Veterans Affairs data.
Disclosures: Pfizer and the U.S. Department of Veterans Affairs funded the study. Two of the researchers were Pfizer employees. Dr. Caffrey’s research is funded in part by Pfizer.
UV Light Beat Bleach for C. difficile Decontamination
SAN FRANCISCO – The M.D. Anderson Cancer Center is abandoning bleach for cleaning hospital rooms exposed to Clostridium difficile in favor of a new machine that kills the organism using ultraviolet light.
The machine reduced C. difficile counts as much as, or more than, bleach cleaning in a preliminary prospective trial in 30 hospital rooms previously occupied by patients infected with C. difficile. The machine is a bit more expensive than bleach at a cost of approximately $82,000 (or $3,000-$4,000 per month to lease), but it avoids damage to materials and the toxic environment for workers caused by the use of bleach or other corrosive chemicals, Dr. Shashank S. Ghantoji said in an interview at a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Bleach treatment reduced the average number of colony-forming units of C. difficile from 2.39 before cleaning to 0.71, a 70% reduction in the contamination level. Treatment with the Pulsed Xenon UV machine (PX-UV) reduced the average number of colony-forming units from 22.97 to 1.10, a 95% reduction.
The postcleaning contamination levels were not statistically different between the bleach and PX-UV rooms, Dr. Ghantoji and his associates found. However, PX-UV decontamination is faster than using bleach, Dr. Ghantoji said. "It takes at least 45 minutes to clean a room with bleach, and it’s not good for the patients or the health care professionals," plus admissions staff usually are clamoring for the room to be ready as soon as possible, he said. Cleaning a room using the PX-UV method takes perhaps 15 minutes.
The PX-UV machine has been available for some time, but its adoption depends on how proactive hospital infection control teams are, he added. He said he is aware of at least two medical centers beyond M.D. Anderson that are also using the machine.
In the study, 298 samples were taken before and after cleaning from high-touch surfaces – the bathroom handrail, the bed control panel, the bed rail, the top of the bedside table, and the IV pole control panel or other equipment control panel – and analyzed for C. difficile endospores. Fifteen rooms were cleaned by the conventional method using a 1:10 solution of sodium hypochlorite (bleach), and 15 underwent a visual, nonbleach cleaning of surfaces followed by 15 minutes of treatment with the PX-UV.
With the PX-UV method, housekeeping workers clean the bathroom and place the remote-operated PX-UV in the bathroom with the door shut while they finish cleaning the rest of the room. Then the machine is placed on each side of the bed for 4 minutes of operation with workers gone. Sensors stop the machine if any movement is detected.
It works by emitting ultraviolet C light, which kills C. difficile. And here’s a bonus – it also kills vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus, Dr. Ghantoji of the M.D. Anderson Cancer Center, Houston, said at the meeting, sponsored by the American Society for Microbiology.
"The PX-UV method may be a promising alternative to the current standard of decontamination, bleach," he said. Future studies should look at whether the PX-UV method decreases not just endospore counts but transmission of C. difficile, he added.
C. difficile causes more than 300,000 health care–associated infections each year in the United States, incurring $2,500-$3,500 in costs per infection aside from any surgical costs, he estimated. Current guidelines recommend that rooms previously occupied by patients infected with C. difficile be cleaned with a disinfectant registered with the Environmental Protection Agency as effective against the organism.
Xenex Healthcare Services, which markets the PX-UV machine, funded the study, and two of the investigators are employees of the company. Dr. Ghantoji reported having no other relevant financial disclosures.
SAN FRANCISCO – The M.D. Anderson Cancer Center is abandoning bleach for cleaning hospital rooms exposed to Clostridium difficile in favor of a new machine that kills the organism using ultraviolet light.
The machine reduced C. difficile counts as much as, or more than, bleach cleaning in a preliminary prospective trial in 30 hospital rooms previously occupied by patients infected with C. difficile. The machine is a bit more expensive than bleach at a cost of approximately $82,000 (or $3,000-$4,000 per month to lease), but it avoids damage to materials and the toxic environment for workers caused by the use of bleach or other corrosive chemicals, Dr. Shashank S. Ghantoji said in an interview at a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Bleach treatment reduced the average number of colony-forming units of C. difficile from 2.39 before cleaning to 0.71, a 70% reduction in the contamination level. Treatment with the Pulsed Xenon UV machine (PX-UV) reduced the average number of colony-forming units from 22.97 to 1.10, a 95% reduction.
The postcleaning contamination levels were not statistically different between the bleach and PX-UV rooms, Dr. Ghantoji and his associates found. However, PX-UV decontamination is faster than using bleach, Dr. Ghantoji said. "It takes at least 45 minutes to clean a room with bleach, and it’s not good for the patients or the health care professionals," plus admissions staff usually are clamoring for the room to be ready as soon as possible, he said. Cleaning a room using the PX-UV method takes perhaps 15 minutes.
The PX-UV machine has been available for some time, but its adoption depends on how proactive hospital infection control teams are, he added. He said he is aware of at least two medical centers beyond M.D. Anderson that are also using the machine.
In the study, 298 samples were taken before and after cleaning from high-touch surfaces – the bathroom handrail, the bed control panel, the bed rail, the top of the bedside table, and the IV pole control panel or other equipment control panel – and analyzed for C. difficile endospores. Fifteen rooms were cleaned by the conventional method using a 1:10 solution of sodium hypochlorite (bleach), and 15 underwent a visual, nonbleach cleaning of surfaces followed by 15 minutes of treatment with the PX-UV.
With the PX-UV method, housekeeping workers clean the bathroom and place the remote-operated PX-UV in the bathroom with the door shut while they finish cleaning the rest of the room. Then the machine is placed on each side of the bed for 4 minutes of operation with workers gone. Sensors stop the machine if any movement is detected.
It works by emitting ultraviolet C light, which kills C. difficile. And here’s a bonus – it also kills vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus, Dr. Ghantoji of the M.D. Anderson Cancer Center, Houston, said at the meeting, sponsored by the American Society for Microbiology.
"The PX-UV method may be a promising alternative to the current standard of decontamination, bleach," he said. Future studies should look at whether the PX-UV method decreases not just endospore counts but transmission of C. difficile, he added.
C. difficile causes more than 300,000 health care–associated infections each year in the United States, incurring $2,500-$3,500 in costs per infection aside from any surgical costs, he estimated. Current guidelines recommend that rooms previously occupied by patients infected with C. difficile be cleaned with a disinfectant registered with the Environmental Protection Agency as effective against the organism.
Xenex Healthcare Services, which markets the PX-UV machine, funded the study, and two of the investigators are employees of the company. Dr. Ghantoji reported having no other relevant financial disclosures.
SAN FRANCISCO – The M.D. Anderson Cancer Center is abandoning bleach for cleaning hospital rooms exposed to Clostridium difficile in favor of a new machine that kills the organism using ultraviolet light.
The machine reduced C. difficile counts as much as, or more than, bleach cleaning in a preliminary prospective trial in 30 hospital rooms previously occupied by patients infected with C. difficile. The machine is a bit more expensive than bleach at a cost of approximately $82,000 (or $3,000-$4,000 per month to lease), but it avoids damage to materials and the toxic environment for workers caused by the use of bleach or other corrosive chemicals, Dr. Shashank S. Ghantoji said in an interview at a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Bleach treatment reduced the average number of colony-forming units of C. difficile from 2.39 before cleaning to 0.71, a 70% reduction in the contamination level. Treatment with the Pulsed Xenon UV machine (PX-UV) reduced the average number of colony-forming units from 22.97 to 1.10, a 95% reduction.
The postcleaning contamination levels were not statistically different between the bleach and PX-UV rooms, Dr. Ghantoji and his associates found. However, PX-UV decontamination is faster than using bleach, Dr. Ghantoji said. "It takes at least 45 minutes to clean a room with bleach, and it’s not good for the patients or the health care professionals," plus admissions staff usually are clamoring for the room to be ready as soon as possible, he said. Cleaning a room using the PX-UV method takes perhaps 15 minutes.
The PX-UV machine has been available for some time, but its adoption depends on how proactive hospital infection control teams are, he added. He said he is aware of at least two medical centers beyond M.D. Anderson that are also using the machine.
In the study, 298 samples were taken before and after cleaning from high-touch surfaces – the bathroom handrail, the bed control panel, the bed rail, the top of the bedside table, and the IV pole control panel or other equipment control panel – and analyzed for C. difficile endospores. Fifteen rooms were cleaned by the conventional method using a 1:10 solution of sodium hypochlorite (bleach), and 15 underwent a visual, nonbleach cleaning of surfaces followed by 15 minutes of treatment with the PX-UV.
With the PX-UV method, housekeeping workers clean the bathroom and place the remote-operated PX-UV in the bathroom with the door shut while they finish cleaning the rest of the room. Then the machine is placed on each side of the bed for 4 minutes of operation with workers gone. Sensors stop the machine if any movement is detected.
It works by emitting ultraviolet C light, which kills C. difficile. And here’s a bonus – it also kills vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus, Dr. Ghantoji of the M.D. Anderson Cancer Center, Houston, said at the meeting, sponsored by the American Society for Microbiology.
"The PX-UV method may be a promising alternative to the current standard of decontamination, bleach," he said. Future studies should look at whether the PX-UV method decreases not just endospore counts but transmission of C. difficile, he added.
C. difficile causes more than 300,000 health care–associated infections each year in the United States, incurring $2,500-$3,500 in costs per infection aside from any surgical costs, he estimated. Current guidelines recommend that rooms previously occupied by patients infected with C. difficile be cleaned with a disinfectant registered with the Environmental Protection Agency as effective against the organism.
Xenex Healthcare Services, which markets the PX-UV machine, funded the study, and two of the investigators are employees of the company. Dr. Ghantoji reported having no other relevant financial disclosures.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Major Finding: Bleach killed 70% of C. difficile spores in hospital rooms compared with 95% decontamination using nonbleach cleaning plus UV light treatment. The difference was not statistically significant.
Data Source: A prospective comparison was performed of the two cleaning methods in 30 rooms after discharge of patients infected with C. difficile.
Disclosures: Xenex Healthcare Services, which markets the PX-UV machine, funded the study, and two of the investigators are employees of the company. Dr. Ghantoji reported having no other relevant financial disclosures.
Linezolid May Predict MRSA Pneumonia Treatment Success
SAN FRANCISCO – In a national cohort of VA patients with MRSA pneumonia, treatment with linezolid was the only modifiable variable in predicting clinical success.
"Pneumonia is the No. 1 cause of infectious disease–related deaths in the United States yet there are limited treatment options for pneumonia caused by MRSA," Aisling R. Caffrey, Ph.D., said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "Identification of independent predictors of clinical success can optimize patient care."
In an effort to identify independent predictors of clinical success in MRSA pneumonia, Dr. Caffrey, assistant professor of pharmacoepidemiology at the University of Rhode Island College of Pharmacy, and her associates conducted a retrospective cohort study of VA hospital admissions between January 2002 and September 2010 with diagnosis codes for MRSA and pneumonia. They used pharmacy records to identify initiation of linezolid or vancomycin during admission, with at least 3 days of therapy as dosed per protocol.
Patients who died or were discharged within 3 days of treatment initiation with either agent were excluded from the study, as were those whose treatment was initiated in a nursing home and those who were exposed to more than 2 consecutive days of antibiotic therapy with MRSA activity within 3 days prior to initiation of linezolid or vancomycin or during treatment with either agent.
Clinical success was defined as discharge from the hospital or intensive care unit by day 14 after treatment initiation, in the absence of death, therapy change, or intubation. Nonsuccess was defined as therapy change, intubation, discharge, and readmission, or death between treatment initiation and day 14. They also investigated numerous potential predictors of clinical success, including treatment with linezolid or vancomycin, demographics and admission characteristics, and comorbidities and medical history.
Dr. Caffrey reported data from 231 patients who received linezolid and 3,501 patients who received vancomycin. Their mean age was 70 years and most (98%) were male. Predictors of clinical success included treatment with linezolid (OR 1.53) and having a previous complication of an implant or graft (OR 1.55). Factors associated with nonsuccess included dialysis (OR 0.54), intravenous line (OR 0.76), having three or more inpatient procedures (OR 0.53), inpatient surgery (OR 0.48), urinary tract infection (0.82), previous coagulopathy (0.74), previous endocarditis (0.24), and previous amputation procedure (OR 0.72).
Dr. Caffrey acknowledged certain limitations of the study, including the reliance on diagnostic codes to ascertain the number of MRSA pneumonia cases. "We’re probably only capturing 20%-40% of MRSA diagnoses by using the diagnosis codes," she said at the meeting, which was sponsored by the American Society for Microbiology.
Low generalizability of the findings to other patient populations is another limitation: "The VA is the largest integrated health care system in the United States but it [consists of] mainly older white males with a lot of comorbidities," she explained.
She concluded that patients with MRSA pneumonia "are often complex, and identifying predictors of success is useful in maximizing clinical decision making."
The study was supported by the Department of Veterans Affairs and Pfizer.
SAN FRANCISCO – In a national cohort of VA patients with MRSA pneumonia, treatment with linezolid was the only modifiable variable in predicting clinical success.
"Pneumonia is the No. 1 cause of infectious disease–related deaths in the United States yet there are limited treatment options for pneumonia caused by MRSA," Aisling R. Caffrey, Ph.D., said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "Identification of independent predictors of clinical success can optimize patient care."
In an effort to identify independent predictors of clinical success in MRSA pneumonia, Dr. Caffrey, assistant professor of pharmacoepidemiology at the University of Rhode Island College of Pharmacy, and her associates conducted a retrospective cohort study of VA hospital admissions between January 2002 and September 2010 with diagnosis codes for MRSA and pneumonia. They used pharmacy records to identify initiation of linezolid or vancomycin during admission, with at least 3 days of therapy as dosed per protocol.
Patients who died or were discharged within 3 days of treatment initiation with either agent were excluded from the study, as were those whose treatment was initiated in a nursing home and those who were exposed to more than 2 consecutive days of antibiotic therapy with MRSA activity within 3 days prior to initiation of linezolid or vancomycin or during treatment with either agent.
Clinical success was defined as discharge from the hospital or intensive care unit by day 14 after treatment initiation, in the absence of death, therapy change, or intubation. Nonsuccess was defined as therapy change, intubation, discharge, and readmission, or death between treatment initiation and day 14. They also investigated numerous potential predictors of clinical success, including treatment with linezolid or vancomycin, demographics and admission characteristics, and comorbidities and medical history.
Dr. Caffrey reported data from 231 patients who received linezolid and 3,501 patients who received vancomycin. Their mean age was 70 years and most (98%) were male. Predictors of clinical success included treatment with linezolid (OR 1.53) and having a previous complication of an implant or graft (OR 1.55). Factors associated with nonsuccess included dialysis (OR 0.54), intravenous line (OR 0.76), having three or more inpatient procedures (OR 0.53), inpatient surgery (OR 0.48), urinary tract infection (0.82), previous coagulopathy (0.74), previous endocarditis (0.24), and previous amputation procedure (OR 0.72).
Dr. Caffrey acknowledged certain limitations of the study, including the reliance on diagnostic codes to ascertain the number of MRSA pneumonia cases. "We’re probably only capturing 20%-40% of MRSA diagnoses by using the diagnosis codes," she said at the meeting, which was sponsored by the American Society for Microbiology.
Low generalizability of the findings to other patient populations is another limitation: "The VA is the largest integrated health care system in the United States but it [consists of] mainly older white males with a lot of comorbidities," she explained.
She concluded that patients with MRSA pneumonia "are often complex, and identifying predictors of success is useful in maximizing clinical decision making."
The study was supported by the Department of Veterans Affairs and Pfizer.
SAN FRANCISCO – In a national cohort of VA patients with MRSA pneumonia, treatment with linezolid was the only modifiable variable in predicting clinical success.
"Pneumonia is the No. 1 cause of infectious disease–related deaths in the United States yet there are limited treatment options for pneumonia caused by MRSA," Aisling R. Caffrey, Ph.D., said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "Identification of independent predictors of clinical success can optimize patient care."
In an effort to identify independent predictors of clinical success in MRSA pneumonia, Dr. Caffrey, assistant professor of pharmacoepidemiology at the University of Rhode Island College of Pharmacy, and her associates conducted a retrospective cohort study of VA hospital admissions between January 2002 and September 2010 with diagnosis codes for MRSA and pneumonia. They used pharmacy records to identify initiation of linezolid or vancomycin during admission, with at least 3 days of therapy as dosed per protocol.
Patients who died or were discharged within 3 days of treatment initiation with either agent were excluded from the study, as were those whose treatment was initiated in a nursing home and those who were exposed to more than 2 consecutive days of antibiotic therapy with MRSA activity within 3 days prior to initiation of linezolid or vancomycin or during treatment with either agent.
Clinical success was defined as discharge from the hospital or intensive care unit by day 14 after treatment initiation, in the absence of death, therapy change, or intubation. Nonsuccess was defined as therapy change, intubation, discharge, and readmission, or death between treatment initiation and day 14. They also investigated numerous potential predictors of clinical success, including treatment with linezolid or vancomycin, demographics and admission characteristics, and comorbidities and medical history.
Dr. Caffrey reported data from 231 patients who received linezolid and 3,501 patients who received vancomycin. Their mean age was 70 years and most (98%) were male. Predictors of clinical success included treatment with linezolid (OR 1.53) and having a previous complication of an implant or graft (OR 1.55). Factors associated with nonsuccess included dialysis (OR 0.54), intravenous line (OR 0.76), having three or more inpatient procedures (OR 0.53), inpatient surgery (OR 0.48), urinary tract infection (0.82), previous coagulopathy (0.74), previous endocarditis (0.24), and previous amputation procedure (OR 0.72).
Dr. Caffrey acknowledged certain limitations of the study, including the reliance on diagnostic codes to ascertain the number of MRSA pneumonia cases. "We’re probably only capturing 20%-40% of MRSA diagnoses by using the diagnosis codes," she said at the meeting, which was sponsored by the American Society for Microbiology.
Low generalizability of the findings to other patient populations is another limitation: "The VA is the largest integrated health care system in the United States but it [consists of] mainly older white males with a lot of comorbidities," she explained.
She concluded that patients with MRSA pneumonia "are often complex, and identifying predictors of success is useful in maximizing clinical decision making."
The study was supported by the Department of Veterans Affairs and Pfizer.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Major Finding: Treatment with linezolid (OR 1.53) and having a previous complication of an implant or graft (OR 1.55) are independent predictors of clinical success in patients with MRSA pneumonia.
Data Source: Findings are from a study of 231 patients who received linezolid and 3,501 patients who received vancomycin during Veterans Affairs hospital admission for MRSA pneumonia between January 2002 and September 2010.
Disclosures: The study was supported by the Department of Veterans Affairs and Pfizer.
Analysis Supports First-Line Daptomycin Against Catheter-Related Bacteremia
SAN FRANCISCO – Daptomycin demonstrated an overall clinical success rate of 83% when used as a first-line treatment of catheter-related bacteremia, according to analysis of a large European database.
"In Europe, we have seen daptomycin being used as more of a salvage therapy for catheter-related bacteremia," study investigator Mike Allen, Ph.D., said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "This is our first opportunity with a reasonable number of patients to ask how it performs as a first-line treatment."
Dr. Allen, senior medical development adviser at Novartis Pharmaceuticals UK, and his associates reported results from 487 patients in the EUCORE (European Cubicin Outcomes Registry and Experience) trial, an ongoing noninterventional multicenter study designed to assess the characteristics and clinical outcomes of patients with catheter-related bacteremia who were treated with at least one dose of daptomycin (Cubicin). Investigators followed protocol criteria and were asked to rate outcomes following daptomycin therapy as improved, failure, or nonevaluable. An outcome of cured or improved was considered a clinical success.
Of the 487 patients, 148 received daptomycin as a first-line therapy. Of these, 59% were male and 34% were aged 65 years or older. The majority (97%) had significant underlying diseases, particularly cardiovascular disease (44%) and oncologic disease (28%).
"This registry includes all comers, so what we have is a snapshot of clinical practice," Dr. Allen said. "It gives us a far better feel for how the drug is actually used. We don’t have the caveats of a clinical trial whereby we’re limited to a certain patient population."
Samples for microbiologic analysis were available for 134 patients, with 117 (87%) yielding positive culture. The two most common pathogens were coagulase-negative staphylococci (55%) and Staphylococcus aureus (27%), followed by enterococci (10%) and other pathogens.
The most frequent initial dose of daptomycin was 6 mg/kg and the median duration of therapy was 10 days. A majority of patients met the definition of clinical success (57% cured, 26% improved). Success rates reached 79% against methicillin-resistant S. aureus and S. epidermidis and 87% against other coagulase-negative staphylococci.
Among all 148 patients who received daptomycin as first-line therapy, 16 (11%) experienced mild to severe adverse events regardless of relationship to daptomycin treatment, including infections and renal and urinary disorders urinary disorders, and two of skin and subcutaneous tissue disorders.
The study was funded by Novartis. Dr. Allen is employed by Novartis Pharmaceuticals UK, which markets daptomycin in the EU.
The meeting was sponsored by the American Society for Microbiology.
SAN FRANCISCO – Daptomycin demonstrated an overall clinical success rate of 83% when used as a first-line treatment of catheter-related bacteremia, according to analysis of a large European database.
"In Europe, we have seen daptomycin being used as more of a salvage therapy for catheter-related bacteremia," study investigator Mike Allen, Ph.D., said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "This is our first opportunity with a reasonable number of patients to ask how it performs as a first-line treatment."
Dr. Allen, senior medical development adviser at Novartis Pharmaceuticals UK, and his associates reported results from 487 patients in the EUCORE (European Cubicin Outcomes Registry and Experience) trial, an ongoing noninterventional multicenter study designed to assess the characteristics and clinical outcomes of patients with catheter-related bacteremia who were treated with at least one dose of daptomycin (Cubicin). Investigators followed protocol criteria and were asked to rate outcomes following daptomycin therapy as improved, failure, or nonevaluable. An outcome of cured or improved was considered a clinical success.
Of the 487 patients, 148 received daptomycin as a first-line therapy. Of these, 59% were male and 34% were aged 65 years or older. The majority (97%) had significant underlying diseases, particularly cardiovascular disease (44%) and oncologic disease (28%).
"This registry includes all comers, so what we have is a snapshot of clinical practice," Dr. Allen said. "It gives us a far better feel for how the drug is actually used. We don’t have the caveats of a clinical trial whereby we’re limited to a certain patient population."
Samples for microbiologic analysis were available for 134 patients, with 117 (87%) yielding positive culture. The two most common pathogens were coagulase-negative staphylococci (55%) and Staphylococcus aureus (27%), followed by enterococci (10%) and other pathogens.
The most frequent initial dose of daptomycin was 6 mg/kg and the median duration of therapy was 10 days. A majority of patients met the definition of clinical success (57% cured, 26% improved). Success rates reached 79% against methicillin-resistant S. aureus and S. epidermidis and 87% against other coagulase-negative staphylococci.
Among all 148 patients who received daptomycin as first-line therapy, 16 (11%) experienced mild to severe adverse events regardless of relationship to daptomycin treatment, including infections and renal and urinary disorders urinary disorders, and two of skin and subcutaneous tissue disorders.
The study was funded by Novartis. Dr. Allen is employed by Novartis Pharmaceuticals UK, which markets daptomycin in the EU.
The meeting was sponsored by the American Society for Microbiology.
SAN FRANCISCO – Daptomycin demonstrated an overall clinical success rate of 83% when used as a first-line treatment of catheter-related bacteremia, according to analysis of a large European database.
"In Europe, we have seen daptomycin being used as more of a salvage therapy for catheter-related bacteremia," study investigator Mike Allen, Ph.D., said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "This is our first opportunity with a reasonable number of patients to ask how it performs as a first-line treatment."
Dr. Allen, senior medical development adviser at Novartis Pharmaceuticals UK, and his associates reported results from 487 patients in the EUCORE (European Cubicin Outcomes Registry and Experience) trial, an ongoing noninterventional multicenter study designed to assess the characteristics and clinical outcomes of patients with catheter-related bacteremia who were treated with at least one dose of daptomycin (Cubicin). Investigators followed protocol criteria and were asked to rate outcomes following daptomycin therapy as improved, failure, or nonevaluable. An outcome of cured or improved was considered a clinical success.
Of the 487 patients, 148 received daptomycin as a first-line therapy. Of these, 59% were male and 34% were aged 65 years or older. The majority (97%) had significant underlying diseases, particularly cardiovascular disease (44%) and oncologic disease (28%).
"This registry includes all comers, so what we have is a snapshot of clinical practice," Dr. Allen said. "It gives us a far better feel for how the drug is actually used. We don’t have the caveats of a clinical trial whereby we’re limited to a certain patient population."
Samples for microbiologic analysis were available for 134 patients, with 117 (87%) yielding positive culture. The two most common pathogens were coagulase-negative staphylococci (55%) and Staphylococcus aureus (27%), followed by enterococci (10%) and other pathogens.
The most frequent initial dose of daptomycin was 6 mg/kg and the median duration of therapy was 10 days. A majority of patients met the definition of clinical success (57% cured, 26% improved). Success rates reached 79% against methicillin-resistant S. aureus and S. epidermidis and 87% against other coagulase-negative staphylococci.
Among all 148 patients who received daptomycin as first-line therapy, 16 (11%) experienced mild to severe adverse events regardless of relationship to daptomycin treatment, including infections and renal and urinary disorders urinary disorders, and two of skin and subcutaneous tissue disorders.
The study was funded by Novartis. Dr. Allen is employed by Novartis Pharmaceuticals UK, which markets daptomycin in the EU.
The meeting was sponsored by the American Society for Microbiology.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Major Finding: Daptomycin was successful in 83% of patients who received it as a first-line treatment for catheter-related bacteremia (57% cured, 26% improved).
Data Source: Results were taken from a multicenter analysis of 148 patients enrolled in the ongoing EUCORE (European Cubicin Outcomes Registry and Experience) study.
Disclosures: The investigator is employed by Novartis Pharmaceuticals UK, which markets daptomycin and funded the study.