User login
Antibiotic Resistance May Drive Cystitis Rx Changes
SAN FRANCISCO – Concerns about antibiotic resistance may be driving more physicians to prescribe second-line antibiotics for uncomplicated cystitis in women, a new study indicates.
Between 1998 and 2009, outpatient prescribing of first-line antibiotics for uncomplicated cystitis in women remained constant – yet the number of quinolone prescriptions grew significantly, results from an analysis of national data demonstrated.
The findings "reinforce what our gut instincts have been saying about prescribing for cystitis: Physicians are having more concerns about resistance and are therefore going to the second-line agents like quinolones, cephalosporins, and other medications," Jessina C. McGregor, Ph.D., said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. McGregor and her associates analyzed data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, which are nationally representative surveys of ambulatory and emergency department visits. They focused their study on women over age 17, and used linear tests to analyze trends in rates of visits for cystitis and related antibiotic prescribing patterns.
An estimated 124,978,631 visits among adult women were attributable to cystitis between 1998 and 2009, reported Dr. McGregor of the department of pharmacy practice at Oregon State University, Portland. The mean age of patients for these visits was 50 years, 83% were white, 69% presented to a physician’s office for their cystitis, and 48% had private insurance.
The annual rates of cystitis visits were stable between 1998 and 2009, with an average of 195 visits per 1,000 women. Nearly three-quarters of women (71%) received an antibiotic prescription during the study period.
Prescriptions for the first-line urinary anti-infectives trimethoprim/sulfamethoxazole, trimethoprim, fosfomycin, and nitrofurantoin remained constant (P = .10). But prescriptions for quinolones grew by 10%, and prescriptions for cephalosporins grew by 3%, which were statistically significant increases (P less than .01 and P = .02, respectively).
The findings "correlate with other data showing increasing rates of uropathogen resistance," the researchers wrote. "Further research is needed to evaluate empiric antibiotic prescribing in this setting to ensure prudent antibiotic use and limit the spread of resistance."
The conference was sponsored by the American Society for Microbiology.
Dr. McGregor reported that the National Institutes of Health and the National Center for Research Resources supported the study.
SAN FRANCISCO – Concerns about antibiotic resistance may be driving more physicians to prescribe second-line antibiotics for uncomplicated cystitis in women, a new study indicates.
Between 1998 and 2009, outpatient prescribing of first-line antibiotics for uncomplicated cystitis in women remained constant – yet the number of quinolone prescriptions grew significantly, results from an analysis of national data demonstrated.
The findings "reinforce what our gut instincts have been saying about prescribing for cystitis: Physicians are having more concerns about resistance and are therefore going to the second-line agents like quinolones, cephalosporins, and other medications," Jessina C. McGregor, Ph.D., said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. McGregor and her associates analyzed data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, which are nationally representative surveys of ambulatory and emergency department visits. They focused their study on women over age 17, and used linear tests to analyze trends in rates of visits for cystitis and related antibiotic prescribing patterns.
An estimated 124,978,631 visits among adult women were attributable to cystitis between 1998 and 2009, reported Dr. McGregor of the department of pharmacy practice at Oregon State University, Portland. The mean age of patients for these visits was 50 years, 83% were white, 69% presented to a physician’s office for their cystitis, and 48% had private insurance.
The annual rates of cystitis visits were stable between 1998 and 2009, with an average of 195 visits per 1,000 women. Nearly three-quarters of women (71%) received an antibiotic prescription during the study period.
Prescriptions for the first-line urinary anti-infectives trimethoprim/sulfamethoxazole, trimethoprim, fosfomycin, and nitrofurantoin remained constant (P = .10). But prescriptions for quinolones grew by 10%, and prescriptions for cephalosporins grew by 3%, which were statistically significant increases (P less than .01 and P = .02, respectively).
The findings "correlate with other data showing increasing rates of uropathogen resistance," the researchers wrote. "Further research is needed to evaluate empiric antibiotic prescribing in this setting to ensure prudent antibiotic use and limit the spread of resistance."
The conference was sponsored by the American Society for Microbiology.
Dr. McGregor reported that the National Institutes of Health and the National Center for Research Resources supported the study.
SAN FRANCISCO – Concerns about antibiotic resistance may be driving more physicians to prescribe second-line antibiotics for uncomplicated cystitis in women, a new study indicates.
Between 1998 and 2009, outpatient prescribing of first-line antibiotics for uncomplicated cystitis in women remained constant – yet the number of quinolone prescriptions grew significantly, results from an analysis of national data demonstrated.
The findings "reinforce what our gut instincts have been saying about prescribing for cystitis: Physicians are having more concerns about resistance and are therefore going to the second-line agents like quinolones, cephalosporins, and other medications," Jessina C. McGregor, Ph.D., said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. McGregor and her associates analyzed data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, which are nationally representative surveys of ambulatory and emergency department visits. They focused their study on women over age 17, and used linear tests to analyze trends in rates of visits for cystitis and related antibiotic prescribing patterns.
An estimated 124,978,631 visits among adult women were attributable to cystitis between 1998 and 2009, reported Dr. McGregor of the department of pharmacy practice at Oregon State University, Portland. The mean age of patients for these visits was 50 years, 83% were white, 69% presented to a physician’s office for their cystitis, and 48% had private insurance.
The annual rates of cystitis visits were stable between 1998 and 2009, with an average of 195 visits per 1,000 women. Nearly three-quarters of women (71%) received an antibiotic prescription during the study period.
Prescriptions for the first-line urinary anti-infectives trimethoprim/sulfamethoxazole, trimethoprim, fosfomycin, and nitrofurantoin remained constant (P = .10). But prescriptions for quinolones grew by 10%, and prescriptions for cephalosporins grew by 3%, which were statistically significant increases (P less than .01 and P = .02, respectively).
The findings "correlate with other data showing increasing rates of uropathogen resistance," the researchers wrote. "Further research is needed to evaluate empiric antibiotic prescribing in this setting to ensure prudent antibiotic use and limit the spread of resistance."
The conference was sponsored by the American Society for Microbiology.
Dr. McGregor reported that the National Institutes of Health and the National Center for Research Resources supported the study.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Major Finding: Between 1998 and 2009, prescriptions for the first-line urinary anti-infectives trimethoprim/ sulfamethoxazole, trimethoprim, fosfomycin, and nitrofurantoin for uncomplicated cystitis in women remained constant (P = .10); yet prescriptions for quinolones grew by 10% and prescriptions for cephalosporins grew by 3% (P less than .01 and P = .02, respectively).
Data Source: This was an analysis of data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey related to an estimated 124,978,631 visits for cystitis over the 10-year period.
Disclosures: Dr. McGregor reported that the National Institutes of Health and the National Center for Research Resources supported the study.
Two Drugs Not Better Than One for C. Difficile
SAN FRANCISCO – If a patient with Clostridium difficile infection is not responding to single-drug treatment, it’s better to switch drugs than to add a second one, a retrospective study suggests.
The chart review encompassed 248 patients at one institution who received at least 24 hours of monotherapy or combination drug therapy for C. difficile infection from 2008 to 2010. After adjustment for confounding factors, the study showed no significant difference in the time to resolution of diarrhea in patients treated with metronidazole, vancomycin, rifaximin, or nitazoxanide alone, compared with patients who got combinations of these drugs, Jessica C. Njoku, Pharm.D., and her associates reported.
The 39 patients who got combination therapy (16%) also showed no significant differences in clinical cure rate, length of hospital stay, or mortality, compared with patients on monotherapy, Dr. Njoku said in a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Diarrhea resolved in the monotherapy group in a mean of 5 days, compared with 10 days in the combination therapy group, a difference that seemed to favor monotherapy but was not statistically significant after adjustment for age, severity of C. difficile infection, immunocompetence, and whether it was a first episode or first recurrence, said Dr. Njoku, currently of Baylor University Medical Center, Dallas. She led the study while she was a fellow at the University of Nebraska Medical Center, Omaha.
The findings suggest that switching drugs instead of adding a drug is the best strategy for a patient who is not responding to initial monotherapy because adding a drug wastes resources, she said in an interview.
The study is one of the first to compare combination therapy to monotherapy for C. difficile infection, Dr. Njoku said. She and her associates conducted the study because clinicians were using drug combinations despite advice from an antimicrobial stewardship team against the practice. "We needed evidence," she said at the meeting, which was sponsored by the American Society for Microbiology.
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends oral metronidazole for mild to moderate C. difficile infections or oral vancomycin for severe infection. For C. difficile infection with severe complications, including ileus or toxic megacolon, expert opinion in the guidelines suggests treating with oral and IV vancomycin with the option of a vancomycin enema (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
"In clinical practice, we don’t follow the guidelines to the letter," she noted. Her records review found that some patients in each category of severity received monotherapy and some were treated with drug combinations.
The C. difficile infection was a first episode in 90% of patients and a first recurrence in 10%. A first episode of infection was significantly more common in the monotherapy group (93%), compared with the combination therapy group (29 [74%] of 39 patients).
The monotherapy group also was significantly more likely to have mild to moderate infection (39%), compared with the combination group (3 patients [ 8%]). Patients with severe infection or severe complications were less common in the monotherapy group (55% vs. 6%, respectively) compared with patients in the combination group, 27 (69%) of whom had severe infection and 9 (23%) of whom had severe complications.
Cure, defined as the resolution of diarrhea (no more than two stools per day) by day 10, was achieved in 74% of patients on monotherapy and in 56% on combination therapy. The mean length of hospitalization was 16 days for monotherapy and 13 days for combination therapy. Eight percent of patients on monotherapy died, compared with 18% of patients on combination therapy. These differences between groups were not statistically significant in multivariate analyses.
Although the findings showed there was no benefit from combination therapy, this was a relatively small retrospective study at a single center, Dr. Njoku acknowledged. A larger, prospective, randomized trial comparing monotherapy with drug combinations is warranted, she said.
Patients in the study were 1 year of age or older, were positive for C. difficile toxin, and had symptoms of the infection. The study excluded patients who were pregnant or had C. difficile infection beyond a first recurrence. Exclusion criteria also cited patients with ileostomy and patients who had other enteric pathogens implicated in infectious diarrhea that were isolated in stool samples.
Dr. Njoku reported having no financial disclosures.
SAN FRANCISCO – If a patient with Clostridium difficile infection is not responding to single-drug treatment, it’s better to switch drugs than to add a second one, a retrospective study suggests.
The chart review encompassed 248 patients at one institution who received at least 24 hours of monotherapy or combination drug therapy for C. difficile infection from 2008 to 2010. After adjustment for confounding factors, the study showed no significant difference in the time to resolution of diarrhea in patients treated with metronidazole, vancomycin, rifaximin, or nitazoxanide alone, compared with patients who got combinations of these drugs, Jessica C. Njoku, Pharm.D., and her associates reported.
The 39 patients who got combination therapy (16%) also showed no significant differences in clinical cure rate, length of hospital stay, or mortality, compared with patients on monotherapy, Dr. Njoku said in a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Diarrhea resolved in the monotherapy group in a mean of 5 days, compared with 10 days in the combination therapy group, a difference that seemed to favor monotherapy but was not statistically significant after adjustment for age, severity of C. difficile infection, immunocompetence, and whether it was a first episode or first recurrence, said Dr. Njoku, currently of Baylor University Medical Center, Dallas. She led the study while she was a fellow at the University of Nebraska Medical Center, Omaha.
The findings suggest that switching drugs instead of adding a drug is the best strategy for a patient who is not responding to initial monotherapy because adding a drug wastes resources, she said in an interview.
The study is one of the first to compare combination therapy to monotherapy for C. difficile infection, Dr. Njoku said. She and her associates conducted the study because clinicians were using drug combinations despite advice from an antimicrobial stewardship team against the practice. "We needed evidence," she said at the meeting, which was sponsored by the American Society for Microbiology.
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends oral metronidazole for mild to moderate C. difficile infections or oral vancomycin for severe infection. For C. difficile infection with severe complications, including ileus or toxic megacolon, expert opinion in the guidelines suggests treating with oral and IV vancomycin with the option of a vancomycin enema (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
"In clinical practice, we don’t follow the guidelines to the letter," she noted. Her records review found that some patients in each category of severity received monotherapy and some were treated with drug combinations.
The C. difficile infection was a first episode in 90% of patients and a first recurrence in 10%. A first episode of infection was significantly more common in the monotherapy group (93%), compared with the combination therapy group (29 [74%] of 39 patients).
The monotherapy group also was significantly more likely to have mild to moderate infection (39%), compared with the combination group (3 patients [ 8%]). Patients with severe infection or severe complications were less common in the monotherapy group (55% vs. 6%, respectively) compared with patients in the combination group, 27 (69%) of whom had severe infection and 9 (23%) of whom had severe complications.
Cure, defined as the resolution of diarrhea (no more than two stools per day) by day 10, was achieved in 74% of patients on monotherapy and in 56% on combination therapy. The mean length of hospitalization was 16 days for monotherapy and 13 days for combination therapy. Eight percent of patients on monotherapy died, compared with 18% of patients on combination therapy. These differences between groups were not statistically significant in multivariate analyses.
Although the findings showed there was no benefit from combination therapy, this was a relatively small retrospective study at a single center, Dr. Njoku acknowledged. A larger, prospective, randomized trial comparing monotherapy with drug combinations is warranted, she said.
Patients in the study were 1 year of age or older, were positive for C. difficile toxin, and had symptoms of the infection. The study excluded patients who were pregnant or had C. difficile infection beyond a first recurrence. Exclusion criteria also cited patients with ileostomy and patients who had other enteric pathogens implicated in infectious diarrhea that were isolated in stool samples.
Dr. Njoku reported having no financial disclosures.
SAN FRANCISCO – If a patient with Clostridium difficile infection is not responding to single-drug treatment, it’s better to switch drugs than to add a second one, a retrospective study suggests.
The chart review encompassed 248 patients at one institution who received at least 24 hours of monotherapy or combination drug therapy for C. difficile infection from 2008 to 2010. After adjustment for confounding factors, the study showed no significant difference in the time to resolution of diarrhea in patients treated with metronidazole, vancomycin, rifaximin, or nitazoxanide alone, compared with patients who got combinations of these drugs, Jessica C. Njoku, Pharm.D., and her associates reported.
The 39 patients who got combination therapy (16%) also showed no significant differences in clinical cure rate, length of hospital stay, or mortality, compared with patients on monotherapy, Dr. Njoku said in a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Diarrhea resolved in the monotherapy group in a mean of 5 days, compared with 10 days in the combination therapy group, a difference that seemed to favor monotherapy but was not statistically significant after adjustment for age, severity of C. difficile infection, immunocompetence, and whether it was a first episode or first recurrence, said Dr. Njoku, currently of Baylor University Medical Center, Dallas. She led the study while she was a fellow at the University of Nebraska Medical Center, Omaha.
The findings suggest that switching drugs instead of adding a drug is the best strategy for a patient who is not responding to initial monotherapy because adding a drug wastes resources, she said in an interview.
The study is one of the first to compare combination therapy to monotherapy for C. difficile infection, Dr. Njoku said. She and her associates conducted the study because clinicians were using drug combinations despite advice from an antimicrobial stewardship team against the practice. "We needed evidence," she said at the meeting, which was sponsored by the American Society for Microbiology.
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends oral metronidazole for mild to moderate C. difficile infections or oral vancomycin for severe infection. For C. difficile infection with severe complications, including ileus or toxic megacolon, expert opinion in the guidelines suggests treating with oral and IV vancomycin with the option of a vancomycin enema (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
"In clinical practice, we don’t follow the guidelines to the letter," she noted. Her records review found that some patients in each category of severity received monotherapy and some were treated with drug combinations.
The C. difficile infection was a first episode in 90% of patients and a first recurrence in 10%. A first episode of infection was significantly more common in the monotherapy group (93%), compared with the combination therapy group (29 [74%] of 39 patients).
The monotherapy group also was significantly more likely to have mild to moderate infection (39%), compared with the combination group (3 patients [ 8%]). Patients with severe infection or severe complications were less common in the monotherapy group (55% vs. 6%, respectively) compared with patients in the combination group, 27 (69%) of whom had severe infection and 9 (23%) of whom had severe complications.
Cure, defined as the resolution of diarrhea (no more than two stools per day) by day 10, was achieved in 74% of patients on monotherapy and in 56% on combination therapy. The mean length of hospitalization was 16 days for monotherapy and 13 days for combination therapy. Eight percent of patients on monotherapy died, compared with 18% of patients on combination therapy. These differences between groups were not statistically significant in multivariate analyses.
Although the findings showed there was no benefit from combination therapy, this was a relatively small retrospective study at a single center, Dr. Njoku acknowledged. A larger, prospective, randomized trial comparing monotherapy with drug combinations is warranted, she said.
Patients in the study were 1 year of age or older, were positive for C. difficile toxin, and had symptoms of the infection. The study excluded patients who were pregnant or had C. difficile infection beyond a first recurrence. Exclusion criteria also cited patients with ileostomy and patients who had other enteric pathogens implicated in infectious diarrhea that were isolated in stool samples.
Dr. Njoku reported having no financial disclosures.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Major Finding: C. difficile–associated diarrhea resolved after a mean of 5 days of single-drug treatment or 10 days of combination drug treatment, a difference that was not statistically significant after adjustment for the effects of other factors.
Data Source: Chart review of 248 patients treated at one institution.
Disclosures: Dr. Njoku reported having no financial disclosures.
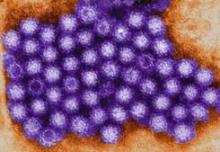
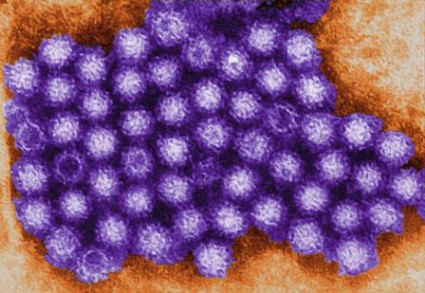
Noroviruses Affecting Diverse Populations
SAN FRANCISCO – As the availability of more sensitive diagnostic methods such as reverse transcription-polymerase chain reaction testing become more widespread, noroviruses are increasingly being recognized as important enteric pathogens in diverse populations.
"In the past, much of our knowledge about noroviruses has been hindered because we don’t have a good animal model or method for culturing norovirus," Dr. Hoonmo L. Koo said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "The majority of our current understanding has come from studying human outbreaks, volunteer challenge studies, and evaluating surrogate caliciviruses such as feline and murine caliciviruses, which don’t cause human infection but can be cultured."
Noroviruses (NoVs) are classified into five genetic groups based on their RNA capsid sequences, with genogroup I (GI) and genogroup II (GII) NoVs causing the most human NoV infections. GII.4 NoV strains are the predominant circulating genotype in the United States and worldwide, said Dr. Koo of Baylor College of Medicine, Houston. As reverse transcription-polymerase chain reaction testing (RT-PCR) has become more available in recent years, NoVs "are now recognized as the most definable common cause of acute nonbacterial gastroenteritis worldwide," he said. "They account for approximately half of all food-borne illness in the United States.
"Each year, NoVs cause about 21 million cases of infection in the United States. They occur throughout the year, but they peak in the winter season."
NoV outbreaks are common in children, travelers, restaurant patrons, military personnel, patients, and health care staff at hospitals, nursing homes, and other medical facilities. Dr. Koo and his associates conducted an 8.5-year surveillance study at Texas Children’s Hospital (TCH) investigating NoV, rotavirus (RV), and adenovirus prevalence at the facility before and after introduction of the RV vaccine in 2006. The study evaluated 8,173 stool samples from inpatients and outpatients at TCH from February 2002 to June 2010. The samples were evaluated for RV by antigen detection or electron microscopy and adenoviruses by electron microscopy. In addition, a subset of 3,222 stools were evaluated for NoV by RT-PCR (J. Ped. Infect. Dis. 2012 Aug. 3 [doi:10.1093/jpids/pis070]).
"We found that RV prevalence decreased significantly after the introduction of the RV vaccine in 2006," Dr. Koo said at the meeting, which was sponsored by the American Society for Microbiology. "In more recent years, it decreased from about 9% in 2007 to 3% in 2010." At the same time, he continued, "NoV prevalence increased in 2004 and was consistently between 11% and 17% from 2004 to 2010. There was no significant increase in NoV prevalence after the RV vaccine was introduced in 2006."
The researchers concluded that NoVs have emerged as the most common viral gastroenteritis pathogen at TCH, which is one of the largest pediatric hospitals in the United States. "We believe that as RV prevalence continues to decline with vaccination, NoVs will soon eclipse rotaviruses as the most important cause of pediatric gastroenteritis in the United States and other countries where the RV vaccine is successfully administered," Dr. Koo said.
In a separate study, he and his associates evaluated stools from 571 international travelers who acquired diarrhea in Guatemala, India, and Mexico (J. Clin. Microbiol. 2010;48:1673-6). NoVs were identified in 10% of cases of travelers’ diarrhea, and overall were the second most common pathogen following diarrheagenic Escherichia coli. "We concluded that NoVs are important pathogens of travelers’ diarrhea in multiple developing regions of the world," Dr. Koo said. "However, there was significant variation evident in the prevalence of NoV diarrhea and in the predominant genogroup infecting the travelers, depending on the specific geographic location and time period we looked at."
Immunocompromised patients also have been affected by NoVs. One report described 12 hematopoietic stem cell transplant patients with NoV gastroenteritis who were hospitalized for a median of 73 days (Clin. Infect. Dis. 2009;49:1069-71). Half of the patients required supplemental feeding with enteral or parenteral nutrition, and two patients died: one secondary to malnutrition and chronic NoV gastroenteritis. "Future areas of NoV study include further defining the burden of disease in pediatric and immunocompromised populations," Dr. Koo said. "We need development of sensitive diagnostic assays that can be used by clinical laboratories. Unfortunately, the ELISA assay that we have now for NoV detection is relatively insensitive, and most clinical laboratories cannot perform RT-PCR. We need effective therapeutic agents to be developed, and we need an effective NoV vaccine."
Results from a recent randomized, double-blind, placebo-controlled trial of an investigational NoV vaccine found a significantly lower frequency of viral gastroenteritis among vaccine recipients, compared with placebo recipients (37% vs. 69%, respectively; P = .006). It was found to be safe and well tolerated, and no severe adverse events were reported (N. Engl. J. Med. 2011;365:2178-87). "The vaccine was also found to be immunogenic, with 70% of vaccine recipients producing a fourfold rise in serum total antibody and serum IgA," Dr. Koo said. "However, there are significant challenges to the development of NoV vaccines. In the NEJM study, the frequency and magnitude of serum antibody response with the vaccine was lower than what’s been observed with natural infection. Previous studies have shown that acquired immunity with natural infection may be short-lived: less than 2 years. So how long will the vaccine protection last?"
Another challenge, he said, is that antibodies are only cross-protective within the same genogroup. "Future NoV vaccines will need to be composed of both GI and GII virus–like particles (VLPs)," he said. "Finally, significant antigenic diversity of noroviruses and the constant antigenic drift may require active surveillance similar to what we have for influenza viruses, where we are constantly surveying what strains are in the community and changing the composition of vaccine VLP strains to represent these important community strains."
Dr. Koo disclosed that he has received research support from the National Institute of Diabetes and Digestive and Kidney Diseases, and the Baylor College of Medicine Center for Globalization.
SAN FRANCISCO – As the availability of more sensitive diagnostic methods such as reverse transcription-polymerase chain reaction testing become more widespread, noroviruses are increasingly being recognized as important enteric pathogens in diverse populations.
"In the past, much of our knowledge about noroviruses has been hindered because we don’t have a good animal model or method for culturing norovirus," Dr. Hoonmo L. Koo said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "The majority of our current understanding has come from studying human outbreaks, volunteer challenge studies, and evaluating surrogate caliciviruses such as feline and murine caliciviruses, which don’t cause human infection but can be cultured."
Noroviruses (NoVs) are classified into five genetic groups based on their RNA capsid sequences, with genogroup I (GI) and genogroup II (GII) NoVs causing the most human NoV infections. GII.4 NoV strains are the predominant circulating genotype in the United States and worldwide, said Dr. Koo of Baylor College of Medicine, Houston. As reverse transcription-polymerase chain reaction testing (RT-PCR) has become more available in recent years, NoVs "are now recognized as the most definable common cause of acute nonbacterial gastroenteritis worldwide," he said. "They account for approximately half of all food-borne illness in the United States.
"Each year, NoVs cause about 21 million cases of infection in the United States. They occur throughout the year, but they peak in the winter season."
NoV outbreaks are common in children, travelers, restaurant patrons, military personnel, patients, and health care staff at hospitals, nursing homes, and other medical facilities. Dr. Koo and his associates conducted an 8.5-year surveillance study at Texas Children’s Hospital (TCH) investigating NoV, rotavirus (RV), and adenovirus prevalence at the facility before and after introduction of the RV vaccine in 2006. The study evaluated 8,173 stool samples from inpatients and outpatients at TCH from February 2002 to June 2010. The samples were evaluated for RV by antigen detection or electron microscopy and adenoviruses by electron microscopy. In addition, a subset of 3,222 stools were evaluated for NoV by RT-PCR (J. Ped. Infect. Dis. 2012 Aug. 3 [doi:10.1093/jpids/pis070]).
"We found that RV prevalence decreased significantly after the introduction of the RV vaccine in 2006," Dr. Koo said at the meeting, which was sponsored by the American Society for Microbiology. "In more recent years, it decreased from about 9% in 2007 to 3% in 2010." At the same time, he continued, "NoV prevalence increased in 2004 and was consistently between 11% and 17% from 2004 to 2010. There was no significant increase in NoV prevalence after the RV vaccine was introduced in 2006."
The researchers concluded that NoVs have emerged as the most common viral gastroenteritis pathogen at TCH, which is one of the largest pediatric hospitals in the United States. "We believe that as RV prevalence continues to decline with vaccination, NoVs will soon eclipse rotaviruses as the most important cause of pediatric gastroenteritis in the United States and other countries where the RV vaccine is successfully administered," Dr. Koo said.
In a separate study, he and his associates evaluated stools from 571 international travelers who acquired diarrhea in Guatemala, India, and Mexico (J. Clin. Microbiol. 2010;48:1673-6). NoVs were identified in 10% of cases of travelers’ diarrhea, and overall were the second most common pathogen following diarrheagenic Escherichia coli. "We concluded that NoVs are important pathogens of travelers’ diarrhea in multiple developing regions of the world," Dr. Koo said. "However, there was significant variation evident in the prevalence of NoV diarrhea and in the predominant genogroup infecting the travelers, depending on the specific geographic location and time period we looked at."
Immunocompromised patients also have been affected by NoVs. One report described 12 hematopoietic stem cell transplant patients with NoV gastroenteritis who were hospitalized for a median of 73 days (Clin. Infect. Dis. 2009;49:1069-71). Half of the patients required supplemental feeding with enteral or parenteral nutrition, and two patients died: one secondary to malnutrition and chronic NoV gastroenteritis. "Future areas of NoV study include further defining the burden of disease in pediatric and immunocompromised populations," Dr. Koo said. "We need development of sensitive diagnostic assays that can be used by clinical laboratories. Unfortunately, the ELISA assay that we have now for NoV detection is relatively insensitive, and most clinical laboratories cannot perform RT-PCR. We need effective therapeutic agents to be developed, and we need an effective NoV vaccine."
Results from a recent randomized, double-blind, placebo-controlled trial of an investigational NoV vaccine found a significantly lower frequency of viral gastroenteritis among vaccine recipients, compared with placebo recipients (37% vs. 69%, respectively; P = .006). It was found to be safe and well tolerated, and no severe adverse events were reported (N. Engl. J. Med. 2011;365:2178-87). "The vaccine was also found to be immunogenic, with 70% of vaccine recipients producing a fourfold rise in serum total antibody and serum IgA," Dr. Koo said. "However, there are significant challenges to the development of NoV vaccines. In the NEJM study, the frequency and magnitude of serum antibody response with the vaccine was lower than what’s been observed with natural infection. Previous studies have shown that acquired immunity with natural infection may be short-lived: less than 2 years. So how long will the vaccine protection last?"
Another challenge, he said, is that antibodies are only cross-protective within the same genogroup. "Future NoV vaccines will need to be composed of both GI and GII virus–like particles (VLPs)," he said. "Finally, significant antigenic diversity of noroviruses and the constant antigenic drift may require active surveillance similar to what we have for influenza viruses, where we are constantly surveying what strains are in the community and changing the composition of vaccine VLP strains to represent these important community strains."
Dr. Koo disclosed that he has received research support from the National Institute of Diabetes and Digestive and Kidney Diseases, and the Baylor College of Medicine Center for Globalization.
SAN FRANCISCO – As the availability of more sensitive diagnostic methods such as reverse transcription-polymerase chain reaction testing become more widespread, noroviruses are increasingly being recognized as important enteric pathogens in diverse populations.
"In the past, much of our knowledge about noroviruses has been hindered because we don’t have a good animal model or method for culturing norovirus," Dr. Hoonmo L. Koo said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. "The majority of our current understanding has come from studying human outbreaks, volunteer challenge studies, and evaluating surrogate caliciviruses such as feline and murine caliciviruses, which don’t cause human infection but can be cultured."
Noroviruses (NoVs) are classified into five genetic groups based on their RNA capsid sequences, with genogroup I (GI) and genogroup II (GII) NoVs causing the most human NoV infections. GII.4 NoV strains are the predominant circulating genotype in the United States and worldwide, said Dr. Koo of Baylor College of Medicine, Houston. As reverse transcription-polymerase chain reaction testing (RT-PCR) has become more available in recent years, NoVs "are now recognized as the most definable common cause of acute nonbacterial gastroenteritis worldwide," he said. "They account for approximately half of all food-borne illness in the United States.
"Each year, NoVs cause about 21 million cases of infection in the United States. They occur throughout the year, but they peak in the winter season."
NoV outbreaks are common in children, travelers, restaurant patrons, military personnel, patients, and health care staff at hospitals, nursing homes, and other medical facilities. Dr. Koo and his associates conducted an 8.5-year surveillance study at Texas Children’s Hospital (TCH) investigating NoV, rotavirus (RV), and adenovirus prevalence at the facility before and after introduction of the RV vaccine in 2006. The study evaluated 8,173 stool samples from inpatients and outpatients at TCH from February 2002 to June 2010. The samples were evaluated for RV by antigen detection or electron microscopy and adenoviruses by electron microscopy. In addition, a subset of 3,222 stools were evaluated for NoV by RT-PCR (J. Ped. Infect. Dis. 2012 Aug. 3 [doi:10.1093/jpids/pis070]).
"We found that RV prevalence decreased significantly after the introduction of the RV vaccine in 2006," Dr. Koo said at the meeting, which was sponsored by the American Society for Microbiology. "In more recent years, it decreased from about 9% in 2007 to 3% in 2010." At the same time, he continued, "NoV prevalence increased in 2004 and was consistently between 11% and 17% from 2004 to 2010. There was no significant increase in NoV prevalence after the RV vaccine was introduced in 2006."
The researchers concluded that NoVs have emerged as the most common viral gastroenteritis pathogen at TCH, which is one of the largest pediatric hospitals in the United States. "We believe that as RV prevalence continues to decline with vaccination, NoVs will soon eclipse rotaviruses as the most important cause of pediatric gastroenteritis in the United States and other countries where the RV vaccine is successfully administered," Dr. Koo said.
In a separate study, he and his associates evaluated stools from 571 international travelers who acquired diarrhea in Guatemala, India, and Mexico (J. Clin. Microbiol. 2010;48:1673-6). NoVs were identified in 10% of cases of travelers’ diarrhea, and overall were the second most common pathogen following diarrheagenic Escherichia coli. "We concluded that NoVs are important pathogens of travelers’ diarrhea in multiple developing regions of the world," Dr. Koo said. "However, there was significant variation evident in the prevalence of NoV diarrhea and in the predominant genogroup infecting the travelers, depending on the specific geographic location and time period we looked at."
Immunocompromised patients also have been affected by NoVs. One report described 12 hematopoietic stem cell transplant patients with NoV gastroenteritis who were hospitalized for a median of 73 days (Clin. Infect. Dis. 2009;49:1069-71). Half of the patients required supplemental feeding with enteral or parenteral nutrition, and two patients died: one secondary to malnutrition and chronic NoV gastroenteritis. "Future areas of NoV study include further defining the burden of disease in pediatric and immunocompromised populations," Dr. Koo said. "We need development of sensitive diagnostic assays that can be used by clinical laboratories. Unfortunately, the ELISA assay that we have now for NoV detection is relatively insensitive, and most clinical laboratories cannot perform RT-PCR. We need effective therapeutic agents to be developed, and we need an effective NoV vaccine."
Results from a recent randomized, double-blind, placebo-controlled trial of an investigational NoV vaccine found a significantly lower frequency of viral gastroenteritis among vaccine recipients, compared with placebo recipients (37% vs. 69%, respectively; P = .006). It was found to be safe and well tolerated, and no severe adverse events were reported (N. Engl. J. Med. 2011;365:2178-87). "The vaccine was also found to be immunogenic, with 70% of vaccine recipients producing a fourfold rise in serum total antibody and serum IgA," Dr. Koo said. "However, there are significant challenges to the development of NoV vaccines. In the NEJM study, the frequency and magnitude of serum antibody response with the vaccine was lower than what’s been observed with natural infection. Previous studies have shown that acquired immunity with natural infection may be short-lived: less than 2 years. So how long will the vaccine protection last?"
Another challenge, he said, is that antibodies are only cross-protective within the same genogroup. "Future NoV vaccines will need to be composed of both GI and GII virus–like particles (VLPs)," he said. "Finally, significant antigenic diversity of noroviruses and the constant antigenic drift may require active surveillance similar to what we have for influenza viruses, where we are constantly surveying what strains are in the community and changing the composition of vaccine VLP strains to represent these important community strains."
Dr. Koo disclosed that he has received research support from the National Institute of Diabetes and Digestive and Kidney Diseases, and the Baylor College of Medicine Center for Globalization.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Promising C. difficile Antibiotic in Pipeline
SAN FRANCISCO – Those desperate for new treatments for Clostridium difficile infection may want to keep an eye on the experimental oral antibiotic cadazolid, which looked promising in an early-phase trial, according to Daniela Baldoni, Pharm.D.
Cadazolid produced low systemic exposure with high concentrations at the desired site – the colon – and was well tolerated in 64 healthy men who received up to 3,000 mg b.i.d. for 10 days, she reported in a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. Dr. Baldoni is employed by Actelion Pharmaceuticals, the company that is developing cadazolid.
The study randomized nonsmoking men to either the drug (single or multiple doses of cadazolid) or placebo. All subjects remained in the clinic for observation for 120 hours after the last dose.
In the single-dose group, 30 fasting subjects received a single dose of 30, 100, 300, 1,000, or 3,000 mg cadazolid and 10 subjects received matching placebo. After a wash-out period of 8-15 days, the six subjects who had taken 300 mg received a second dose of 300 mg after eating instead of after fasting. In the multiple-dose group, 18 subjects took 300, 1,000, or 3,000 mg of cadazolid twice a day and 6 received matching placebo for 10 days.
Taking cadazolid with food appeared to increase the rate and extent of drug absorption by two- to fivefold. Blood samples showed low systemic exposure after single or multiple doses, with a minor, twofold increase in cadazolid in plasma after 10 days for all doses in the twice-a-day group, Dr. Baldoni reported at the meeting, sponsored by the American Society for Microbiology.
Negligible recovery of unchanged cadazolid in urine samples and the fact that most of the dose was recovered in feces suggests that treatment produced high concentrations at the colon. In fecal samples collected during days 6 and 10 of treatment, the mean cumulative recovery of unchanged cadazolid in feces was between 87% and 94%.
There were no significant changes in vital signs, ECG results, or laboratory parameters compared with baseline measurements, and no drug-related serious adverse events. All subjects completed the study except one man in the 100-mg single-dose subgroup who withdrew consent for reasons unrelated to adverse events.
The dose or duration of treatment did not seem to affect the number of adverse events.
In the single-dose group, 8 of 30 subjects on cadazolid (27%) and 4 of 10 on placebo (40%) reported adverse events, most commonly headache and diarrhea. Four subjects on cadazolid (13%) and two on placebo (20%) reported headache, and three subjects on cadazolid (10%) and one on placebo (10%) reported diarrhea.
In the twice-a-day dosing group, 7 of 18 subjects on cadazolid (39%) and 1 of 6 subjects on placebo (17%) reported adverse events, most commonly headache in 5 subjects on cadazolid (28%) and 1 subject on placebo (17%).
Subjects were aged 45-60 years and had a body mass index of 18-32 kg/m2.
C. difficile is the most common cause of antibiotic-associated infectious diarrhea, especially among the elderly. The mainstays of treating C. difficile–associated infection, metronidazole or vancomycin, fail in approximately 20%-45% of patients.
Cadazolid is in the oxazolidinone class of antibiotics. Its mechanism of action consists mainly of bacterial protein-synthesis inhibition.
Dr. Baldoni and most of her coinvestigators are employees of Actelion Pharmaceuticals, which funded the study.
SAN FRANCISCO – Those desperate for new treatments for Clostridium difficile infection may want to keep an eye on the experimental oral antibiotic cadazolid, which looked promising in an early-phase trial, according to Daniela Baldoni, Pharm.D.
Cadazolid produced low systemic exposure with high concentrations at the desired site – the colon – and was well tolerated in 64 healthy men who received up to 3,000 mg b.i.d. for 10 days, she reported in a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. Dr. Baldoni is employed by Actelion Pharmaceuticals, the company that is developing cadazolid.
The study randomized nonsmoking men to either the drug (single or multiple doses of cadazolid) or placebo. All subjects remained in the clinic for observation for 120 hours after the last dose.
In the single-dose group, 30 fasting subjects received a single dose of 30, 100, 300, 1,000, or 3,000 mg cadazolid and 10 subjects received matching placebo. After a wash-out period of 8-15 days, the six subjects who had taken 300 mg received a second dose of 300 mg after eating instead of after fasting. In the multiple-dose group, 18 subjects took 300, 1,000, or 3,000 mg of cadazolid twice a day and 6 received matching placebo for 10 days.
Taking cadazolid with food appeared to increase the rate and extent of drug absorption by two- to fivefold. Blood samples showed low systemic exposure after single or multiple doses, with a minor, twofold increase in cadazolid in plasma after 10 days for all doses in the twice-a-day group, Dr. Baldoni reported at the meeting, sponsored by the American Society for Microbiology.
Negligible recovery of unchanged cadazolid in urine samples and the fact that most of the dose was recovered in feces suggests that treatment produced high concentrations at the colon. In fecal samples collected during days 6 and 10 of treatment, the mean cumulative recovery of unchanged cadazolid in feces was between 87% and 94%.
There were no significant changes in vital signs, ECG results, or laboratory parameters compared with baseline measurements, and no drug-related serious adverse events. All subjects completed the study except one man in the 100-mg single-dose subgroup who withdrew consent for reasons unrelated to adverse events.
The dose or duration of treatment did not seem to affect the number of adverse events.
In the single-dose group, 8 of 30 subjects on cadazolid (27%) and 4 of 10 on placebo (40%) reported adverse events, most commonly headache and diarrhea. Four subjects on cadazolid (13%) and two on placebo (20%) reported headache, and three subjects on cadazolid (10%) and one on placebo (10%) reported diarrhea.
In the twice-a-day dosing group, 7 of 18 subjects on cadazolid (39%) and 1 of 6 subjects on placebo (17%) reported adverse events, most commonly headache in 5 subjects on cadazolid (28%) and 1 subject on placebo (17%).
Subjects were aged 45-60 years and had a body mass index of 18-32 kg/m2.
C. difficile is the most common cause of antibiotic-associated infectious diarrhea, especially among the elderly. The mainstays of treating C. difficile–associated infection, metronidazole or vancomycin, fail in approximately 20%-45% of patients.
Cadazolid is in the oxazolidinone class of antibiotics. Its mechanism of action consists mainly of bacterial protein-synthesis inhibition.
Dr. Baldoni and most of her coinvestigators are employees of Actelion Pharmaceuticals, which funded the study.
SAN FRANCISCO – Those desperate for new treatments for Clostridium difficile infection may want to keep an eye on the experimental oral antibiotic cadazolid, which looked promising in an early-phase trial, according to Daniela Baldoni, Pharm.D.
Cadazolid produced low systemic exposure with high concentrations at the desired site – the colon – and was well tolerated in 64 healthy men who received up to 3,000 mg b.i.d. for 10 days, she reported in a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. Dr. Baldoni is employed by Actelion Pharmaceuticals, the company that is developing cadazolid.
The study randomized nonsmoking men to either the drug (single or multiple doses of cadazolid) or placebo. All subjects remained in the clinic for observation for 120 hours after the last dose.
In the single-dose group, 30 fasting subjects received a single dose of 30, 100, 300, 1,000, or 3,000 mg cadazolid and 10 subjects received matching placebo. After a wash-out period of 8-15 days, the six subjects who had taken 300 mg received a second dose of 300 mg after eating instead of after fasting. In the multiple-dose group, 18 subjects took 300, 1,000, or 3,000 mg of cadazolid twice a day and 6 received matching placebo for 10 days.
Taking cadazolid with food appeared to increase the rate and extent of drug absorption by two- to fivefold. Blood samples showed low systemic exposure after single or multiple doses, with a minor, twofold increase in cadazolid in plasma after 10 days for all doses in the twice-a-day group, Dr. Baldoni reported at the meeting, sponsored by the American Society for Microbiology.
Negligible recovery of unchanged cadazolid in urine samples and the fact that most of the dose was recovered in feces suggests that treatment produced high concentrations at the colon. In fecal samples collected during days 6 and 10 of treatment, the mean cumulative recovery of unchanged cadazolid in feces was between 87% and 94%.
There were no significant changes in vital signs, ECG results, or laboratory parameters compared with baseline measurements, and no drug-related serious adverse events. All subjects completed the study except one man in the 100-mg single-dose subgroup who withdrew consent for reasons unrelated to adverse events.
The dose or duration of treatment did not seem to affect the number of adverse events.
In the single-dose group, 8 of 30 subjects on cadazolid (27%) and 4 of 10 on placebo (40%) reported adverse events, most commonly headache and diarrhea. Four subjects on cadazolid (13%) and two on placebo (20%) reported headache, and three subjects on cadazolid (10%) and one on placebo (10%) reported diarrhea.
In the twice-a-day dosing group, 7 of 18 subjects on cadazolid (39%) and 1 of 6 subjects on placebo (17%) reported adverse events, most commonly headache in 5 subjects on cadazolid (28%) and 1 subject on placebo (17%).
Subjects were aged 45-60 years and had a body mass index of 18-32 kg/m2.
C. difficile is the most common cause of antibiotic-associated infectious diarrhea, especially among the elderly. The mainstays of treating C. difficile–associated infection, metronidazole or vancomycin, fail in approximately 20%-45% of patients.
Cadazolid is in the oxazolidinone class of antibiotics. Its mechanism of action consists mainly of bacterial protein-synthesis inhibition.
Dr. Baldoni and most of her coinvestigators are employees of Actelion Pharmaceuticals, which funded the study.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Major Finding: The experimental antibiotic cadazolid concentrated in feces with low systemic exposure and few side effects after single doses or twice-a-day dosing for 10 days.
Data Source: Data are from a randomized, placebo-controlled study in 64 healthy, nonsmoking men.
Disclosures: Dr. Baldoni and most of her coinvestigators are employees of Actelion Pharmaceuticals, which funded the study.
Rifampin Combinations May Protect Against C. difficile Colitis
SAN FRANCISCO – The addition of rifampin to long-term antibiotic regimens significantly lowers the risk for Clostridium difficile–associated colitis in patients being treated for osteoarticular infections.
The retrospective cohort study comprised 393 treatment episodes – 55% of them for infections subsequent to arthroplasty – among patients admitted to a Swiss orthopedic surgery unit from 1996 to 2012. The ribotype 027 was not endemic in the region.
The 42% of patients who were treated with combination therapy that included oral rifampin (600 mg/day) were significantly less like to develop C. difficile colitis despite being on the antibiotics for a median of 63 days (ranging from 20-294 days), Caroline Landelle, Pharm.D., Ph.D. and her associates reported at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
Rifampin use was inversely associated with C. difficile colitis, with an adjusted hazard ratio of 0.18, Dr. Landelle reported in a poster presentation.
In general, longer duration of antibiotics treatment was associated with C. difficile colitis, with an adjusted hazard ratio of 1.01, said Dr. Landelle of the University of Geneva.
Factors not associated with colitis risk (either positively or negatively) included the use of antianaerobic antibiotics (in 38% of patients), treatment with intravenous vancomycin (in 45%, for a median of 13 days), age, or sex.
Fourteen patients (4%) developed symptomatic C. difficile colitis after a median of 14 days of antibiotic treatment (ranging from 8-193 days). Of these, six patients were on vancomycin (43%) and two patients were receiving rifampin (14%) prior to development of the colitis, Dr. Landelle reported at the meeting, sponsored by the American Society for Microbiology.
"C. difficile colitis was rare despite long-term antibiotic use among patients with osteoarticular infection. In contrast to intravenous vancomycin, combination antibiotic therapy with oral rifampin might protect against C. difficile–associated colitis," the investigators suggested.
The cohort had a median age of 69 years; 41% was female, and 31% of patients were immunosuppressed.
Of the 401 microorganisms isolated from the osteoarticular infections, 32% were methicillin-sensitive Staphylococcus aureus, 16% were methicillin-resistant S. aureus, 17% each were coagulase-negative Staphylococcus or gram-negative bacilli, and 18% were other organisms.
The investigators hypothesized that combination therapy with rifampin or derivative medications might protect against C. difficile colitis because very few cases have been reported of rifampin-associated pseudomembranous colitis, and patients undergoing treatment with long-term antibiotic combination therapy for osteoarticular infections rarely develop symptomatic C. difficile–associated disease.
Rifaximin and other antibiotics belonging to the macrocyclic family are being investigated as alternative therapies for C. difficile–associated disease, Dr. Landelle said. Some studies have shown high rates of C. difficile resistance to rifampin in Europe, but the rate of resistance at her institution was low. In Switzerland, rifamycin antibiotics have been used for decades, but rifaximin is not licensed for the treatment of hepatic encephalopathy, diarrhea, and traveler’s diarrhea or for prophylaxis in patients undergoing GI surgery, which may explain the low rate of resistance there.
The study excluded patients with prior episodes of C. difficile–associated disease, patients being treated with metronidazole, and patients with septic arthritis.
Dr. Landelle did not provide her financial disclosures.
SAN FRANCISCO – The addition of rifampin to long-term antibiotic regimens significantly lowers the risk for Clostridium difficile–associated colitis in patients being treated for osteoarticular infections.
The retrospective cohort study comprised 393 treatment episodes – 55% of them for infections subsequent to arthroplasty – among patients admitted to a Swiss orthopedic surgery unit from 1996 to 2012. The ribotype 027 was not endemic in the region.
The 42% of patients who were treated with combination therapy that included oral rifampin (600 mg/day) were significantly less like to develop C. difficile colitis despite being on the antibiotics for a median of 63 days (ranging from 20-294 days), Caroline Landelle, Pharm.D., Ph.D. and her associates reported at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
Rifampin use was inversely associated with C. difficile colitis, with an adjusted hazard ratio of 0.18, Dr. Landelle reported in a poster presentation.
In general, longer duration of antibiotics treatment was associated with C. difficile colitis, with an adjusted hazard ratio of 1.01, said Dr. Landelle of the University of Geneva.
Factors not associated with colitis risk (either positively or negatively) included the use of antianaerobic antibiotics (in 38% of patients), treatment with intravenous vancomycin (in 45%, for a median of 13 days), age, or sex.
Fourteen patients (4%) developed symptomatic C. difficile colitis after a median of 14 days of antibiotic treatment (ranging from 8-193 days). Of these, six patients were on vancomycin (43%) and two patients were receiving rifampin (14%) prior to development of the colitis, Dr. Landelle reported at the meeting, sponsored by the American Society for Microbiology.
"C. difficile colitis was rare despite long-term antibiotic use among patients with osteoarticular infection. In contrast to intravenous vancomycin, combination antibiotic therapy with oral rifampin might protect against C. difficile–associated colitis," the investigators suggested.
The cohort had a median age of 69 years; 41% was female, and 31% of patients were immunosuppressed.
Of the 401 microorganisms isolated from the osteoarticular infections, 32% were methicillin-sensitive Staphylococcus aureus, 16% were methicillin-resistant S. aureus, 17% each were coagulase-negative Staphylococcus or gram-negative bacilli, and 18% were other organisms.
The investigators hypothesized that combination therapy with rifampin or derivative medications might protect against C. difficile colitis because very few cases have been reported of rifampin-associated pseudomembranous colitis, and patients undergoing treatment with long-term antibiotic combination therapy for osteoarticular infections rarely develop symptomatic C. difficile–associated disease.
Rifaximin and other antibiotics belonging to the macrocyclic family are being investigated as alternative therapies for C. difficile–associated disease, Dr. Landelle said. Some studies have shown high rates of C. difficile resistance to rifampin in Europe, but the rate of resistance at her institution was low. In Switzerland, rifamycin antibiotics have been used for decades, but rifaximin is not licensed for the treatment of hepatic encephalopathy, diarrhea, and traveler’s diarrhea or for prophylaxis in patients undergoing GI surgery, which may explain the low rate of resistance there.
The study excluded patients with prior episodes of C. difficile–associated disease, patients being treated with metronidazole, and patients with septic arthritis.
Dr. Landelle did not provide her financial disclosures.
SAN FRANCISCO – The addition of rifampin to long-term antibiotic regimens significantly lowers the risk for Clostridium difficile–associated colitis in patients being treated for osteoarticular infections.
The retrospective cohort study comprised 393 treatment episodes – 55% of them for infections subsequent to arthroplasty – among patients admitted to a Swiss orthopedic surgery unit from 1996 to 2012. The ribotype 027 was not endemic in the region.
The 42% of patients who were treated with combination therapy that included oral rifampin (600 mg/day) were significantly less like to develop C. difficile colitis despite being on the antibiotics for a median of 63 days (ranging from 20-294 days), Caroline Landelle, Pharm.D., Ph.D. and her associates reported at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
Rifampin use was inversely associated with C. difficile colitis, with an adjusted hazard ratio of 0.18, Dr. Landelle reported in a poster presentation.
In general, longer duration of antibiotics treatment was associated with C. difficile colitis, with an adjusted hazard ratio of 1.01, said Dr. Landelle of the University of Geneva.
Factors not associated with colitis risk (either positively or negatively) included the use of antianaerobic antibiotics (in 38% of patients), treatment with intravenous vancomycin (in 45%, for a median of 13 days), age, or sex.
Fourteen patients (4%) developed symptomatic C. difficile colitis after a median of 14 days of antibiotic treatment (ranging from 8-193 days). Of these, six patients were on vancomycin (43%) and two patients were receiving rifampin (14%) prior to development of the colitis, Dr. Landelle reported at the meeting, sponsored by the American Society for Microbiology.
"C. difficile colitis was rare despite long-term antibiotic use among patients with osteoarticular infection. In contrast to intravenous vancomycin, combination antibiotic therapy with oral rifampin might protect against C. difficile–associated colitis," the investigators suggested.
The cohort had a median age of 69 years; 41% was female, and 31% of patients were immunosuppressed.
Of the 401 microorganisms isolated from the osteoarticular infections, 32% were methicillin-sensitive Staphylococcus aureus, 16% were methicillin-resistant S. aureus, 17% each were coagulase-negative Staphylococcus or gram-negative bacilli, and 18% were other organisms.
The investigators hypothesized that combination therapy with rifampin or derivative medications might protect against C. difficile colitis because very few cases have been reported of rifampin-associated pseudomembranous colitis, and patients undergoing treatment with long-term antibiotic combination therapy for osteoarticular infections rarely develop symptomatic C. difficile–associated disease.
Rifaximin and other antibiotics belonging to the macrocyclic family are being investigated as alternative therapies for C. difficile–associated disease, Dr. Landelle said. Some studies have shown high rates of C. difficile resistance to rifampin in Europe, but the rate of resistance at her institution was low. In Switzerland, rifamycin antibiotics have been used for decades, but rifaximin is not licensed for the treatment of hepatic encephalopathy, diarrhea, and traveler’s diarrhea or for prophylaxis in patients undergoing GI surgery, which may explain the low rate of resistance there.
The study excluded patients with prior episodes of C. difficile–associated disease, patients being treated with metronidazole, and patients with septic arthritis.
Dr. Landelle did not provide her financial disclosures.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Major Finding: Among 14 patients who developed C. difficile colitis during long-term antibiotic treatment for osteoarticular infections, 2 were on combination regimens including rifampin (14%), compared with 6 who were on vancomycin (43%).
Data Source: Results were taken from a Swiss retrospective cohort study of 393 treatment episodes for osteoarticular infections at one institution, 42% of which used combination rifampin regimens and 45% of which used vancomycin.
Disclosures: Dr. Landelle did not provide her financial disclosures.
Good Hand-Hygiene Practices Taking Hold Worldwide
SAN FRANCISCO – Good hand-hygiene practices at health care facilities appear to be taking hold worldwide, results from a large World Health Organization survey suggest.
"Health care facilities around the world have taken seriously the need for improving hand-hygiene practices at the point of care," Dr. Benedetta Allegranzi said in an interview before a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. These efforts must be sustained, she urged.
Dr. Allegranzi, a scientist in WHO’s World Alliance for Patient Safety in Geneva, reported results from the WHO Hand Hygiene Self-Assessment Framework (HHSF), a validated questionnaire that includes 27 indicators that support the five key components of the WHO hand hygiene–improvement strategy (namely system change, training and education, evaluation and performance feedback, reminders in the workplace, and institutional safety climate). The study is the first effort to provide a snapshot of hand-hygiene practices and promotion worldwide.
The survey’s maximum possible overall score is 500 points. Based on its overall score, each health facility was assigned to one of four levels of progress: inadequate (0-125 points), basic (126-250 points), intermediate (251-375 points), or advanced (376-500 points).
Of the 9,032 health care facilities invited to participate, 2,119 facilities from 69 countries completed the survey, for a response rate of 25%. Nearly three-quarters of facilities (70%) were registered with the WHO SAVE LIVES: Clean Your Hands initiative, and 74% were involved in a national hand-hygiene campaign.
Nearly half of responding health care facilities (48%) delivered acute care and 48% delivered a mix of acute and long-term care. The overall score was a mean of 292.5 (intermediate level). Most facilities scored in the intermediate level (41%) or the advanced level (24%), Dr. Allegranzi said.
The researchers observed significant differences between regions, with the lowest overall score in Africa (mean, 218.5) and the highest in Western Pacific (mean, 351.8; P less than .001). The highest mean scores were observed in the HHSF sections regarding availability of facilities for hand hygiene (78.1), use of reminders (63.9) and staff education (61.4).
A majority of facilities (90%) reported having alcohol-based handrub available, 98% reported training staff on hand-hygiene best practices, and 92% reported displaying hand-hygiene posters in the wards (92%). However, "response to some specific questions indicate that substantial improvement is needed in the area of monitoring and feedback on hand-hygiene activities [a very important element of improvement strategies] and for the establishment of a comprehensive patient safety climate [well known to facilitate health care workers’ behavioral change]," Dr. Allegranzi said at the meeting, which was sponsored by the American Society for Microbiology.
Participating in the WHO global campaign for hand-hygiene promotion or in a national campaign and having dedicated infection control professionals "are factors independently associated by a good level of progress at the facility level," she added. "Each health care worker should show personal accountability and commitment by practicing optimal hand hygiene to reduce the transmission of harmful pathogens during care delivery."
She acknowledged that the study is limited by selection bias and by the fact that several countries "are not adequately represented because an insufficient number of facilities submitted their results."
Dr. Allegranzi said that she had no relevant financial conflicts to disclose.
SAN FRANCISCO – Good hand-hygiene practices at health care facilities appear to be taking hold worldwide, results from a large World Health Organization survey suggest.
"Health care facilities around the world have taken seriously the need for improving hand-hygiene practices at the point of care," Dr. Benedetta Allegranzi said in an interview before a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. These efforts must be sustained, she urged.
Dr. Allegranzi, a scientist in WHO’s World Alliance for Patient Safety in Geneva, reported results from the WHO Hand Hygiene Self-Assessment Framework (HHSF), a validated questionnaire that includes 27 indicators that support the five key components of the WHO hand hygiene–improvement strategy (namely system change, training and education, evaluation and performance feedback, reminders in the workplace, and institutional safety climate). The study is the first effort to provide a snapshot of hand-hygiene practices and promotion worldwide.
The survey’s maximum possible overall score is 500 points. Based on its overall score, each health facility was assigned to one of four levels of progress: inadequate (0-125 points), basic (126-250 points), intermediate (251-375 points), or advanced (376-500 points).
Of the 9,032 health care facilities invited to participate, 2,119 facilities from 69 countries completed the survey, for a response rate of 25%. Nearly three-quarters of facilities (70%) were registered with the WHO SAVE LIVES: Clean Your Hands initiative, and 74% were involved in a national hand-hygiene campaign.
Nearly half of responding health care facilities (48%) delivered acute care and 48% delivered a mix of acute and long-term care. The overall score was a mean of 292.5 (intermediate level). Most facilities scored in the intermediate level (41%) or the advanced level (24%), Dr. Allegranzi said.
The researchers observed significant differences between regions, with the lowest overall score in Africa (mean, 218.5) and the highest in Western Pacific (mean, 351.8; P less than .001). The highest mean scores were observed in the HHSF sections regarding availability of facilities for hand hygiene (78.1), use of reminders (63.9) and staff education (61.4).
A majority of facilities (90%) reported having alcohol-based handrub available, 98% reported training staff on hand-hygiene best practices, and 92% reported displaying hand-hygiene posters in the wards (92%). However, "response to some specific questions indicate that substantial improvement is needed in the area of monitoring and feedback on hand-hygiene activities [a very important element of improvement strategies] and for the establishment of a comprehensive patient safety climate [well known to facilitate health care workers’ behavioral change]," Dr. Allegranzi said at the meeting, which was sponsored by the American Society for Microbiology.
Participating in the WHO global campaign for hand-hygiene promotion or in a national campaign and having dedicated infection control professionals "are factors independently associated by a good level of progress at the facility level," she added. "Each health care worker should show personal accountability and commitment by practicing optimal hand hygiene to reduce the transmission of harmful pathogens during care delivery."
She acknowledged that the study is limited by selection bias and by the fact that several countries "are not adequately represented because an insufficient number of facilities submitted their results."
Dr. Allegranzi said that she had no relevant financial conflicts to disclose.
SAN FRANCISCO – Good hand-hygiene practices at health care facilities appear to be taking hold worldwide, results from a large World Health Organization survey suggest.
"Health care facilities around the world have taken seriously the need for improving hand-hygiene practices at the point of care," Dr. Benedetta Allegranzi said in an interview before a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. These efforts must be sustained, she urged.
Dr. Allegranzi, a scientist in WHO’s World Alliance for Patient Safety in Geneva, reported results from the WHO Hand Hygiene Self-Assessment Framework (HHSF), a validated questionnaire that includes 27 indicators that support the five key components of the WHO hand hygiene–improvement strategy (namely system change, training and education, evaluation and performance feedback, reminders in the workplace, and institutional safety climate). The study is the first effort to provide a snapshot of hand-hygiene practices and promotion worldwide.
The survey’s maximum possible overall score is 500 points. Based on its overall score, each health facility was assigned to one of four levels of progress: inadequate (0-125 points), basic (126-250 points), intermediate (251-375 points), or advanced (376-500 points).
Of the 9,032 health care facilities invited to participate, 2,119 facilities from 69 countries completed the survey, for a response rate of 25%. Nearly three-quarters of facilities (70%) were registered with the WHO SAVE LIVES: Clean Your Hands initiative, and 74% were involved in a national hand-hygiene campaign.
Nearly half of responding health care facilities (48%) delivered acute care and 48% delivered a mix of acute and long-term care. The overall score was a mean of 292.5 (intermediate level). Most facilities scored in the intermediate level (41%) or the advanced level (24%), Dr. Allegranzi said.
The researchers observed significant differences between regions, with the lowest overall score in Africa (mean, 218.5) and the highest in Western Pacific (mean, 351.8; P less than .001). The highest mean scores were observed in the HHSF sections regarding availability of facilities for hand hygiene (78.1), use of reminders (63.9) and staff education (61.4).
A majority of facilities (90%) reported having alcohol-based handrub available, 98% reported training staff on hand-hygiene best practices, and 92% reported displaying hand-hygiene posters in the wards (92%). However, "response to some specific questions indicate that substantial improvement is needed in the area of monitoring and feedback on hand-hygiene activities [a very important element of improvement strategies] and for the establishment of a comprehensive patient safety climate [well known to facilitate health care workers’ behavioral change]," Dr. Allegranzi said at the meeting, which was sponsored by the American Society for Microbiology.
Participating in the WHO global campaign for hand-hygiene promotion or in a national campaign and having dedicated infection control professionals "are factors independently associated by a good level of progress at the facility level," she added. "Each health care worker should show personal accountability and commitment by practicing optimal hand hygiene to reduce the transmission of harmful pathogens during care delivery."
She acknowledged that the study is limited by selection bias and by the fact that several countries "are not adequately represented because an insufficient number of facilities submitted their results."
Dr. Allegranzi said that she had no relevant financial conflicts to disclose.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Major Finding: A majority of health care facilities (90%) reported having alcohol-based handrub available, 98% reported training staff on hand-hygiene best practices, and 92% reported displaying hand-hygiene posters in the wards (92%).
Data Source: The World Health Organization survey of hand hygiene practices was completed by 2,119 health care facilities in 69 countries.
Disclosures: Dr. Allegranzi said that she had no relevant financial disclosures to make.
IV Ampicillin Found Effective for VRE UTI
SAN FRANCISCO – Intravenous ampicillin is an effective first-line treatment for urinary tract infections caused by vancomycin-resistant enterococci, according to a review from Shands Hospital at the University of Florida in Gainesville.
Although linezolid and daptomycin are sometimes used elsewhere to treat these infections, the hospital staff decided in 2000 to use intravenous ampicillin as the vancomycin-resistant enterococci (VRE) UTI treatment of choice. To prevent resistance, staff there wanted to keep newer, more powerful antibiotics in reserve for more complex infections, Shands infectious disease specialist Ken Klinker, Pharm.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The hospital staff reasoned that the high urine concentrations achieved by ampicillin would overwhelm VRE despite possible resistance to the antibiotic, Dr. Klinker said. The approach led to clinical cures in 64 of 70 patients (91%) and microbiologic cures in 40 of the 46 (87%) who had repeat urine cultures.
Patients received a median ampicillin dose of 4 g/day for an average of 4.25 days. When possible, they were then switched to oral ampicillin or amoxicillin.
Ampicillin "seems to work for us and has for many years," said coinvestigator and pharmacist Sam Borgert, also an infectious disease specialist at Shands. "Ampicillin should be considered a viable treatment option for patients with VRE UTI," the investigators concluded when presenting their findings.
Most of the patients had grown out at least 105 CFU/mL on urine culture and were clinically symptomatic. Enterococcus faecium was the predominant pathogen, and it tested almost uniformly resistant to ampicillin.
The median age in the study was 60.5 years and median Charlson Comorbidity Index score was 6. Just over half of the participants were women.
A total of 48 patients had Foley catheters at baseline. Thirty-three of the 34 (97%) who had their catheter exchanged or removed cleared the infection. The cure rate was 57% (8 of 14) among patients who retained their catheter.
"We can’t discern between drug therapy and catheter removal in terms of what drove the results. It’s probably a combination of both," Dr. Klinker said at the conference, which was sponsored by the American Society for Microbiology.
The few patients who failed ampicillin responded to alternate VRE therapies.
Dr. Klinker and Mr. Borgert said that they have no relevant disclosures.
SAN FRANCISCO – Intravenous ampicillin is an effective first-line treatment for urinary tract infections caused by vancomycin-resistant enterococci, according to a review from Shands Hospital at the University of Florida in Gainesville.
Although linezolid and daptomycin are sometimes used elsewhere to treat these infections, the hospital staff decided in 2000 to use intravenous ampicillin as the vancomycin-resistant enterococci (VRE) UTI treatment of choice. To prevent resistance, staff there wanted to keep newer, more powerful antibiotics in reserve for more complex infections, Shands infectious disease specialist Ken Klinker, Pharm.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The hospital staff reasoned that the high urine concentrations achieved by ampicillin would overwhelm VRE despite possible resistance to the antibiotic, Dr. Klinker said. The approach led to clinical cures in 64 of 70 patients (91%) and microbiologic cures in 40 of the 46 (87%) who had repeat urine cultures.
Patients received a median ampicillin dose of 4 g/day for an average of 4.25 days. When possible, they were then switched to oral ampicillin or amoxicillin.
Ampicillin "seems to work for us and has for many years," said coinvestigator and pharmacist Sam Borgert, also an infectious disease specialist at Shands. "Ampicillin should be considered a viable treatment option for patients with VRE UTI," the investigators concluded when presenting their findings.
Most of the patients had grown out at least 105 CFU/mL on urine culture and were clinically symptomatic. Enterococcus faecium was the predominant pathogen, and it tested almost uniformly resistant to ampicillin.
The median age in the study was 60.5 years and median Charlson Comorbidity Index score was 6. Just over half of the participants were women.
A total of 48 patients had Foley catheters at baseline. Thirty-three of the 34 (97%) who had their catheter exchanged or removed cleared the infection. The cure rate was 57% (8 of 14) among patients who retained their catheter.
"We can’t discern between drug therapy and catheter removal in terms of what drove the results. It’s probably a combination of both," Dr. Klinker said at the conference, which was sponsored by the American Society for Microbiology.
The few patients who failed ampicillin responded to alternate VRE therapies.
Dr. Klinker and Mr. Borgert said that they have no relevant disclosures.
SAN FRANCISCO – Intravenous ampicillin is an effective first-line treatment for urinary tract infections caused by vancomycin-resistant enterococci, according to a review from Shands Hospital at the University of Florida in Gainesville.
Although linezolid and daptomycin are sometimes used elsewhere to treat these infections, the hospital staff decided in 2000 to use intravenous ampicillin as the vancomycin-resistant enterococci (VRE) UTI treatment of choice. To prevent resistance, staff there wanted to keep newer, more powerful antibiotics in reserve for more complex infections, Shands infectious disease specialist Ken Klinker, Pharm.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The hospital staff reasoned that the high urine concentrations achieved by ampicillin would overwhelm VRE despite possible resistance to the antibiotic, Dr. Klinker said. The approach led to clinical cures in 64 of 70 patients (91%) and microbiologic cures in 40 of the 46 (87%) who had repeat urine cultures.
Patients received a median ampicillin dose of 4 g/day for an average of 4.25 days. When possible, they were then switched to oral ampicillin or amoxicillin.
Ampicillin "seems to work for us and has for many years," said coinvestigator and pharmacist Sam Borgert, also an infectious disease specialist at Shands. "Ampicillin should be considered a viable treatment option for patients with VRE UTI," the investigators concluded when presenting their findings.
Most of the patients had grown out at least 105 CFU/mL on urine culture and were clinically symptomatic. Enterococcus faecium was the predominant pathogen, and it tested almost uniformly resistant to ampicillin.
The median age in the study was 60.5 years and median Charlson Comorbidity Index score was 6. Just over half of the participants were women.
A total of 48 patients had Foley catheters at baseline. Thirty-three of the 34 (97%) who had their catheter exchanged or removed cleared the infection. The cure rate was 57% (8 of 14) among patients who retained their catheter.
"We can’t discern between drug therapy and catheter removal in terms of what drove the results. It’s probably a combination of both," Dr. Klinker said at the conference, which was sponsored by the American Society for Microbiology.
The few patients who failed ampicillin responded to alternate VRE therapies.
Dr. Klinker and Mr. Borgert said that they have no relevant disclosures.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Major Finding: Intravenous ampicillin cures 90% of VRE urinary tract infections.
Data Source: The review involved 70 VRE UTI cases.
Disclosures: The investigators said that they have no relevant disclosures.
Star Trek Tricorders Coming to Medicine
Attention Trekkies – the tricorder is here! Well, almost. The healthy possibility of this development has both doctors and Star Trek fans excited. (Admit it – there are more than a few of you who qualify as both.)
I’m talking about the all-in-one, supersmart device that doctors on the fictional U.S.S. Enterprise and subsequent starships would simply wave over a patient’s body to obtain an instant diagnosis. The tricorder is possible today, thanks to advances in the miniaturization and portability of standard medical testing platforms, portability of devices, self-testing by patients, and informatics.
The first real iteration of the device won’t be quite that simple, but "the tricorder is coming very soon," Dr. Franklin R. Cockerill III said in a plenary session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC).
In recognition that this is possible, the communications corporation Qualcomm is offering a $10 million prize in a Tricorder X-Prize Contest to the team that builds the best device that weighs less than 5 pounds, can be used by patients, and screens for melanoma and diagnoses anemia, urinary tract infection, diabetes, strep throat, sleep apnea, chronic obstructive pulmonary disease, metabolic abnormalities, hypo- or hyperthyroidism, and the absence of these diseases.
"You may be very skeptical about this, but it’s possible," said Dr. Cockerill, professor of microbiology and medicine and chair of the department of laboratory medicine and pathology at the Mayo Clinic, Rochester, Minn.
His team at the clinic has been working on tricorder concepts. Ever-smaller computer chips can analyze large amounts of data collected into a tricorder from biosensors and patient-collected specimens, transcript information by smartphones, and use very little energy in doing so.
Even tricorderlike polymerase chain reaction (PCR) testing is feasible, performed by patients using disposable PCR units, he said at the meeting, sponsored by the American Society for Microbiology. Theoretically, 30 cycles of PCR testing could be done in 30 seconds using an AA battery, lyophilized reagents, and self-collected specimens in a disposable device.
He and his associates showed in a recent study that nasal swabs collected by patients were as good as swabbing by health care workers for PCR to detect influenza A and B virus (Mayo Clin. Proc. 2012:87:548-54).
For more than a decade, patients in the Mayo health plan with suspected group A streptococcus infection have complained of the hassle of getting into an office for a health care provider to collect a swab specimen, after which PCR results are available via an 800 phone number within 6-8 hours and they can pick up a medication, if needed, at a prearranged pharmacy. Patient self-sampling is the next step.
"This sort of streamlining of delivery of patient care is where we’re going to go in iterative phases to eventually a tricorder," he said. "I think we’ll see that many physicians are going to be managers over testing, some of which is done by the patients. You will move that patient forward in a more efficient way toward intervention if they require it."
Already, physicians are being "pummeled" with more and more data points, laboratory information, and vital signs without the ability to react in dynamic ways, he added. How to manage all this? Dr. Cockerill predicted that tricorderlike tools will enable real-time integrative interpretation of data at the bedside.
He described a theoretical scenario: Data from automated surveillance of a patient in the ICU will be interpreted in real time. Tricorderlike tools might report that at 8:32 a.m. the patient has a 77% chance of having sepsis, a 79% chance of pneumonia, and only a 13% chance of pulmonary embolus. The device then suggests that the probabilities be better defined by assessing for small molecules 257, 396, and 422 (which are being studied by mass spectrometry as diagnostic adjuncts in trials now, he noted). The presence of small molecule 257 might prompt the device to revise the risk of sepsis to 97%.
For the moment, it’s a scenario he simply made up, and it may seem "heretical" to some of his infectious disease colleagues, but "I think this is what’s coming," Dr. Cockerill said.
Medicine isn’t the only field getting in on the tricorder game. After all, in Star Trek the tricorder was used for much more. A nonmedical tricorder prototype collects and analyzes atmospheric and spatial data, Reuters reports.
Although in the early phases, tricorders are coming. Once they’re everywhere, can Star Trek’s transporters be far behind? Beam me up, Scottie!
Dr. Cockerill reported relationships with nanoMR and Roche, whose products were not discussed in his talk.
–Sherry Boschert
Attention Trekkies – the tricorder is here! Well, almost. The healthy possibility of this development has both doctors and Star Trek fans excited. (Admit it – there are more than a few of you who qualify as both.)
I’m talking about the all-in-one, supersmart device that doctors on the fictional U.S.S. Enterprise and subsequent starships would simply wave over a patient’s body to obtain an instant diagnosis. The tricorder is possible today, thanks to advances in the miniaturization and portability of standard medical testing platforms, portability of devices, self-testing by patients, and informatics.
The first real iteration of the device won’t be quite that simple, but "the tricorder is coming very soon," Dr. Franklin R. Cockerill III said in a plenary session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC).
In recognition that this is possible, the communications corporation Qualcomm is offering a $10 million prize in a Tricorder X-Prize Contest to the team that builds the best device that weighs less than 5 pounds, can be used by patients, and screens for melanoma and diagnoses anemia, urinary tract infection, diabetes, strep throat, sleep apnea, chronic obstructive pulmonary disease, metabolic abnormalities, hypo- or hyperthyroidism, and the absence of these diseases.
"You may be very skeptical about this, but it’s possible," said Dr. Cockerill, professor of microbiology and medicine and chair of the department of laboratory medicine and pathology at the Mayo Clinic, Rochester, Minn.
His team at the clinic has been working on tricorder concepts. Ever-smaller computer chips can analyze large amounts of data collected into a tricorder from biosensors and patient-collected specimens, transcript information by smartphones, and use very little energy in doing so.
Even tricorderlike polymerase chain reaction (PCR) testing is feasible, performed by patients using disposable PCR units, he said at the meeting, sponsored by the American Society for Microbiology. Theoretically, 30 cycles of PCR testing could be done in 30 seconds using an AA battery, lyophilized reagents, and self-collected specimens in a disposable device.
He and his associates showed in a recent study that nasal swabs collected by patients were as good as swabbing by health care workers for PCR to detect influenza A and B virus (Mayo Clin. Proc. 2012:87:548-54).
For more than a decade, patients in the Mayo health plan with suspected group A streptococcus infection have complained of the hassle of getting into an office for a health care provider to collect a swab specimen, after which PCR results are available via an 800 phone number within 6-8 hours and they can pick up a medication, if needed, at a prearranged pharmacy. Patient self-sampling is the next step.
"This sort of streamlining of delivery of patient care is where we’re going to go in iterative phases to eventually a tricorder," he said. "I think we’ll see that many physicians are going to be managers over testing, some of which is done by the patients. You will move that patient forward in a more efficient way toward intervention if they require it."
Already, physicians are being "pummeled" with more and more data points, laboratory information, and vital signs without the ability to react in dynamic ways, he added. How to manage all this? Dr. Cockerill predicted that tricorderlike tools will enable real-time integrative interpretation of data at the bedside.
He described a theoretical scenario: Data from automated surveillance of a patient in the ICU will be interpreted in real time. Tricorderlike tools might report that at 8:32 a.m. the patient has a 77% chance of having sepsis, a 79% chance of pneumonia, and only a 13% chance of pulmonary embolus. The device then suggests that the probabilities be better defined by assessing for small molecules 257, 396, and 422 (which are being studied by mass spectrometry as diagnostic adjuncts in trials now, he noted). The presence of small molecule 257 might prompt the device to revise the risk of sepsis to 97%.
For the moment, it’s a scenario he simply made up, and it may seem "heretical" to some of his infectious disease colleagues, but "I think this is what’s coming," Dr. Cockerill said.
Medicine isn’t the only field getting in on the tricorder game. After all, in Star Trek the tricorder was used for much more. A nonmedical tricorder prototype collects and analyzes atmospheric and spatial data, Reuters reports.
Although in the early phases, tricorders are coming. Once they’re everywhere, can Star Trek’s transporters be far behind? Beam me up, Scottie!
Dr. Cockerill reported relationships with nanoMR and Roche, whose products were not discussed in his talk.
–Sherry Boschert
Attention Trekkies – the tricorder is here! Well, almost. The healthy possibility of this development has both doctors and Star Trek fans excited. (Admit it – there are more than a few of you who qualify as both.)
I’m talking about the all-in-one, supersmart device that doctors on the fictional U.S.S. Enterprise and subsequent starships would simply wave over a patient’s body to obtain an instant diagnosis. The tricorder is possible today, thanks to advances in the miniaturization and portability of standard medical testing platforms, portability of devices, self-testing by patients, and informatics.
The first real iteration of the device won’t be quite that simple, but "the tricorder is coming very soon," Dr. Franklin R. Cockerill III said in a plenary session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC).
In recognition that this is possible, the communications corporation Qualcomm is offering a $10 million prize in a Tricorder X-Prize Contest to the team that builds the best device that weighs less than 5 pounds, can be used by patients, and screens for melanoma and diagnoses anemia, urinary tract infection, diabetes, strep throat, sleep apnea, chronic obstructive pulmonary disease, metabolic abnormalities, hypo- or hyperthyroidism, and the absence of these diseases.
"You may be very skeptical about this, but it’s possible," said Dr. Cockerill, professor of microbiology and medicine and chair of the department of laboratory medicine and pathology at the Mayo Clinic, Rochester, Minn.
His team at the clinic has been working on tricorder concepts. Ever-smaller computer chips can analyze large amounts of data collected into a tricorder from biosensors and patient-collected specimens, transcript information by smartphones, and use very little energy in doing so.
Even tricorderlike polymerase chain reaction (PCR) testing is feasible, performed by patients using disposable PCR units, he said at the meeting, sponsored by the American Society for Microbiology. Theoretically, 30 cycles of PCR testing could be done in 30 seconds using an AA battery, lyophilized reagents, and self-collected specimens in a disposable device.
He and his associates showed in a recent study that nasal swabs collected by patients were as good as swabbing by health care workers for PCR to detect influenza A and B virus (Mayo Clin. Proc. 2012:87:548-54).
For more than a decade, patients in the Mayo health plan with suspected group A streptococcus infection have complained of the hassle of getting into an office for a health care provider to collect a swab specimen, after which PCR results are available via an 800 phone number within 6-8 hours and they can pick up a medication, if needed, at a prearranged pharmacy. Patient self-sampling is the next step.
"This sort of streamlining of delivery of patient care is where we’re going to go in iterative phases to eventually a tricorder," he said. "I think we’ll see that many physicians are going to be managers over testing, some of which is done by the patients. You will move that patient forward in a more efficient way toward intervention if they require it."
Already, physicians are being "pummeled" with more and more data points, laboratory information, and vital signs without the ability to react in dynamic ways, he added. How to manage all this? Dr. Cockerill predicted that tricorderlike tools will enable real-time integrative interpretation of data at the bedside.
He described a theoretical scenario: Data from automated surveillance of a patient in the ICU will be interpreted in real time. Tricorderlike tools might report that at 8:32 a.m. the patient has a 77% chance of having sepsis, a 79% chance of pneumonia, and only a 13% chance of pulmonary embolus. The device then suggests that the probabilities be better defined by assessing for small molecules 257, 396, and 422 (which are being studied by mass spectrometry as diagnostic adjuncts in trials now, he noted). The presence of small molecule 257 might prompt the device to revise the risk of sepsis to 97%.
For the moment, it’s a scenario he simply made up, and it may seem "heretical" to some of his infectious disease colleagues, but "I think this is what’s coming," Dr. Cockerill said.
Medicine isn’t the only field getting in on the tricorder game. After all, in Star Trek the tricorder was used for much more. A nonmedical tricorder prototype collects and analyzes atmospheric and spatial data, Reuters reports.
Although in the early phases, tricorders are coming. Once they’re everywhere, can Star Trek’s transporters be far behind? Beam me up, Scottie!
Dr. Cockerill reported relationships with nanoMR and Roche, whose products were not discussed in his talk.
–Sherry Boschert