User login
For MD-IQ on Family Practice News, but a regular topic for Rheumatology News
Sports Injuries of the Hip in Primary Care
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr Paul Nelson Williams. Paul, how are you feeling about sports injuries?
Paul N. Williams, MD: I’m feeling great, Matt.
Watto: You had a sports injury of the hip. Maybe that’s an overshare, Paul, but we talked about it on a podcast with Dr Carlin Senter (part 1 and part 2).
Williams: I think I’ve shared more than my hip injury, for sure.
Watto: Whenever a patient presented with hip pain, I used to pray it was trochanteric bursitis, which now I know is not really the right thing to think about. Intra-articular hip pain presents as anterior hip pain, usually in the crease of the hip. Depending on the patient’s age and history, the differential for that type of pain includes iliopsoas tendonitis, FAI syndrome, a labral tear, a bone stress injury of the femoral neck, or osteoarthritis.
So, what exactly is FAI and how might we diagnose it?
Williams: FAI is what the cool kids call femoral acetabular impingement, and it’s exactly what it sounds like.
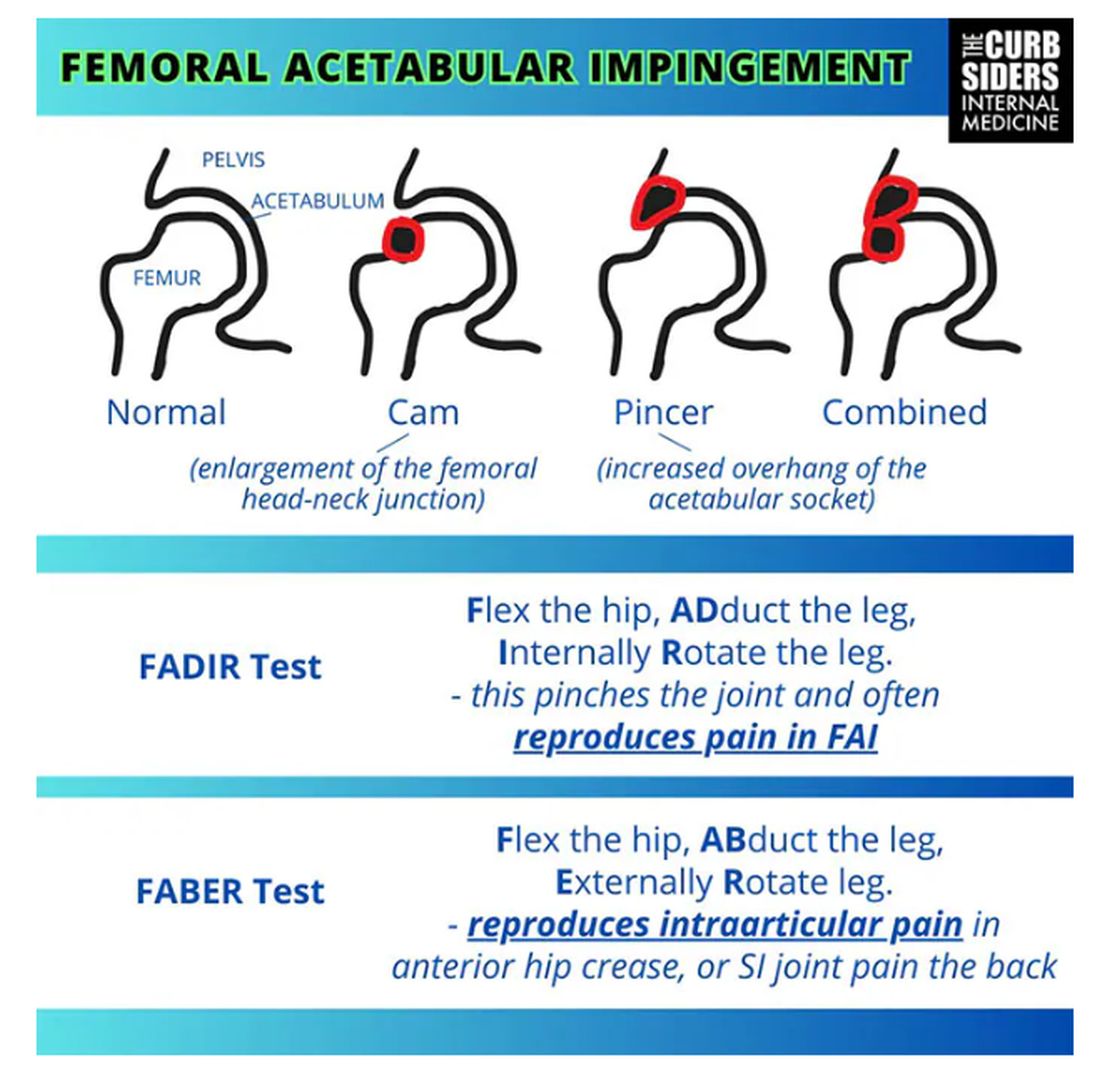
Something is pinching or impinging upon the joint itself and preventing full range of motion. This is a ball-and-socket joint, so it should have tremendous range of motion, able to move in all planes. If it’s impinged, then pain will occur with certain movements. There’s a cam type, which is characterized by enlargement of the femoral head neck junction, or a pincer type, which has more to do with overhang of the acetabulum, and it can also be mixed. In any case, impingement upon the patient’s full range of motion results in pain.
You evaluate this with a couple of tests — the FABER and the FADIR.
The FABER is flexion, abduction, and external rotation, and the FADIR is flexion, adduction, and internal rotation. If you elicit anterior pain with either of those tests, it’s probably one of the intra-articular pathologies, although it is hard to know for sure which one it is because these tests are fairly sensitive but not very specific.
Watto: You can get x-rays to help with the diagnosis. You would order two views of the hip: an AP of the pelvis, which is just a straight-on shot to look for arthritis or fracture. Is there a healthy joint line there? The second is the Dunn view, in which the hip is flexed 90 degrees and abducted about 20 degrees. You are looking for fracture or impingement. You can diagnose FAI based on that view, and you might be able to diagnose a hip stress injury or osteoarthritis.
Unfortunately, you’re not going to see a labral tear, but Dr Senter said that both FAI and labral tears are treated the same way, with physical therapy. Patients with FAI who aren’t getting better might end up going for surgery, so at some point I would refer them to orthopedic surgery. But I feel much more comfortable now diagnosing these conditions with these tests.
Let’s talk a little bit about trochanteric pain syndrome. I used to think it was all bursitis. Why is that not correct?
Williams: It’s nice of you to feign ignorance for the purpose of education. It used to be thought of as bursitis, but these days we know it is probably more likely a tendinopathy.
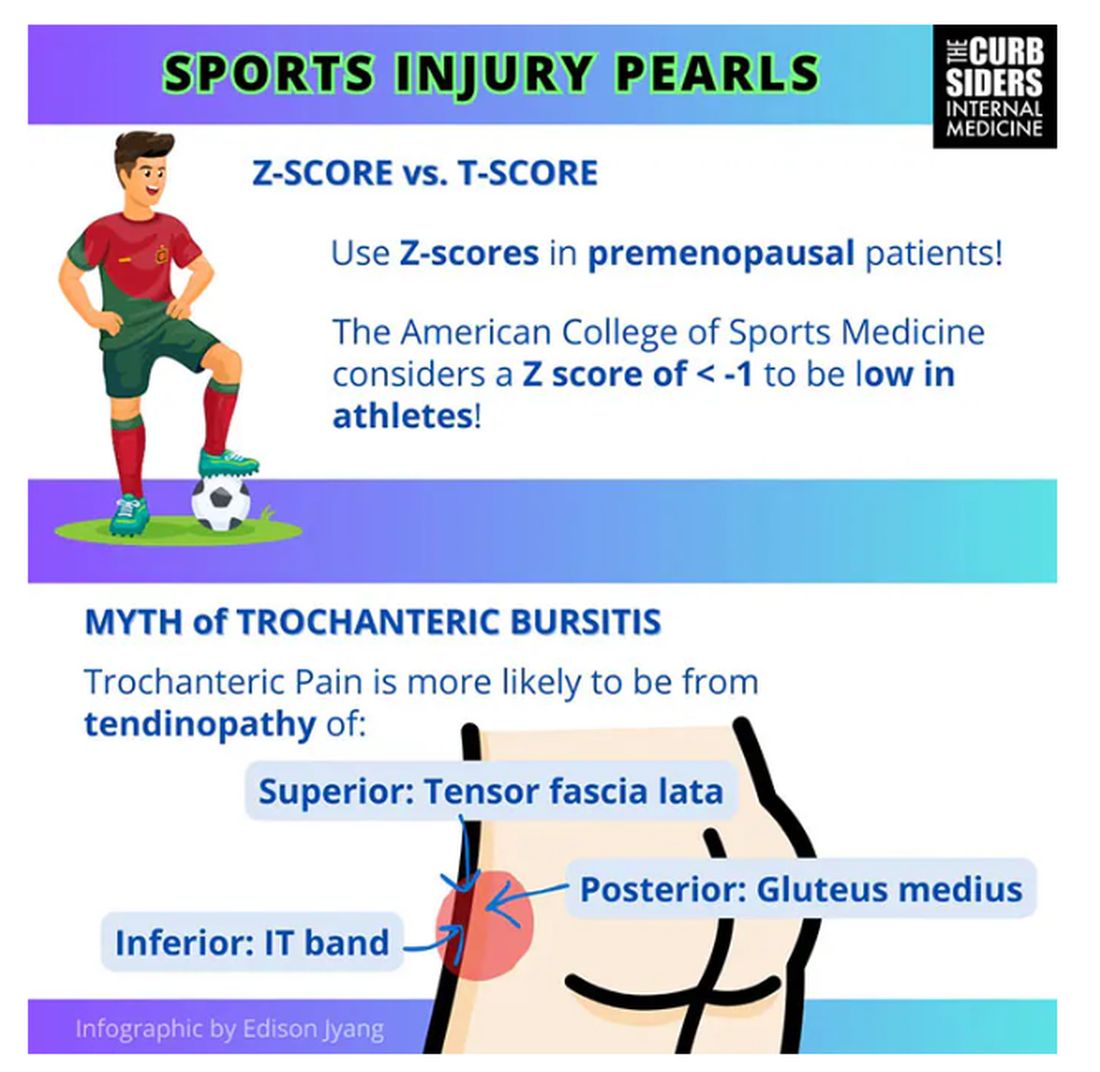
Trochanteric pain syndrome was formerly known as trochanteric bursitis, but the bursa is not typically involved. Trochanteric pain syndrome is a tendinopathy of the surrounding structures: the gluteus medius, the iliotibial band, and the tensor fascia latae. The way these structures relate looks a bit like the face of a clock, as you can see on the infographic. In general, you manage this condition the same way you do with bursitis — physical therapy. You can also give corticosteroid injections. Physical therapy is probably more durable in terms of pain relief and functionality, but in the short term, corticosteroids might provide some degree of analgesia as well.
Watto: The last thing we wanted to mention is bone stress injury, which can occur in high-mileage runners (20 miles or more per week). Patients with bone stress injury need to rest, usually non‒weight bearing, for a period of time.

Treatment of a bone stress fracture depends on which side it’s on (top or bottom). If it’s on the top of the femoral neck (the tension side), it has to be fixed. If it’s on the compression side (the bottom side of the femoral neck), it might be able to be managed conservatively, but many patients are going to need surgery. This is a big deal. But it’s a spectrum; in some cases the bone is merely irritated and unhappy, without a break in the cortex. Those patients might not need surgery.
In patients with a fracture of the femoral neck — especially younger, healthier patients — you should think about getting a bone density test and screening for relative energy deficiency in sport. This used to be called the female athlete triad, which includes disrupted menstrual cycles, being underweight, and fracture. We should be screening patients, asking them in a nonjudgmental way about their relationship with food, to make sure they are getting an appropriate number of calories.
They are actually in an energy deficit. They’re not eating enough to maintain a healthy body with so much activity.
Williams: If you’re interested in this topic, you should refer to the full podcast with Dr Senter which is chock-full of helpful information.
Dr Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, disclosed ties with The Curbsiders.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr Paul Nelson Williams. Paul, how are you feeling about sports injuries?
Paul N. Williams, MD: I’m feeling great, Matt.
Watto: You had a sports injury of the hip. Maybe that’s an overshare, Paul, but we talked about it on a podcast with Dr Carlin Senter (part 1 and part 2).
Williams: I think I’ve shared more than my hip injury, for sure.
Watto: Whenever a patient presented with hip pain, I used to pray it was trochanteric bursitis, which now I know is not really the right thing to think about. Intra-articular hip pain presents as anterior hip pain, usually in the crease of the hip. Depending on the patient’s age and history, the differential for that type of pain includes iliopsoas tendonitis, FAI syndrome, a labral tear, a bone stress injury of the femoral neck, or osteoarthritis.
So, what exactly is FAI and how might we diagnose it?
Williams: FAI is what the cool kids call femoral acetabular impingement, and it’s exactly what it sounds like.

Something is pinching or impinging upon the joint itself and preventing full range of motion. This is a ball-and-socket joint, so it should have tremendous range of motion, able to move in all planes. If it’s impinged, then pain will occur with certain movements. There’s a cam type, which is characterized by enlargement of the femoral head neck junction, or a pincer type, which has more to do with overhang of the acetabulum, and it can also be mixed. In any case, impingement upon the patient’s full range of motion results in pain.
You evaluate this with a couple of tests — the FABER and the FADIR.
The FABER is flexion, abduction, and external rotation, and the FADIR is flexion, adduction, and internal rotation. If you elicit anterior pain with either of those tests, it’s probably one of the intra-articular pathologies, although it is hard to know for sure which one it is because these tests are fairly sensitive but not very specific.
Watto: You can get x-rays to help with the diagnosis. You would order two views of the hip: an AP of the pelvis, which is just a straight-on shot to look for arthritis or fracture. Is there a healthy joint line there? The second is the Dunn view, in which the hip is flexed 90 degrees and abducted about 20 degrees. You are looking for fracture or impingement. You can diagnose FAI based on that view, and you might be able to diagnose a hip stress injury or osteoarthritis.
Unfortunately, you’re not going to see a labral tear, but Dr Senter said that both FAI and labral tears are treated the same way, with physical therapy. Patients with FAI who aren’t getting better might end up going for surgery, so at some point I would refer them to orthopedic surgery. But I feel much more comfortable now diagnosing these conditions with these tests.
Let’s talk a little bit about trochanteric pain syndrome. I used to think it was all bursitis. Why is that not correct?
Williams: It’s nice of you to feign ignorance for the purpose of education. It used to be thought of as bursitis, but these days we know it is probably more likely a tendinopathy.

Trochanteric pain syndrome was formerly known as trochanteric bursitis, but the bursa is not typically involved. Trochanteric pain syndrome is a tendinopathy of the surrounding structures: the gluteus medius, the iliotibial band, and the tensor fascia latae. The way these structures relate looks a bit like the face of a clock, as you can see on the infographic. In general, you manage this condition the same way you do with bursitis — physical therapy. You can also give corticosteroid injections. Physical therapy is probably more durable in terms of pain relief and functionality, but in the short term, corticosteroids might provide some degree of analgesia as well.
Watto: The last thing we wanted to mention is bone stress injury, which can occur in high-mileage runners (20 miles or more per week). Patients with bone stress injury need to rest, usually non‒weight bearing, for a period of time.

Treatment of a bone stress fracture depends on which side it’s on (top or bottom). If it’s on the top of the femoral neck (the tension side), it has to be fixed. If it’s on the compression side (the bottom side of the femoral neck), it might be able to be managed conservatively, but many patients are going to need surgery. This is a big deal. But it’s a spectrum; in some cases the bone is merely irritated and unhappy, without a break in the cortex. Those patients might not need surgery.
In patients with a fracture of the femoral neck — especially younger, healthier patients — you should think about getting a bone density test and screening for relative energy deficiency in sport. This used to be called the female athlete triad, which includes disrupted menstrual cycles, being underweight, and fracture. We should be screening patients, asking them in a nonjudgmental way about their relationship with food, to make sure they are getting an appropriate number of calories.
They are actually in an energy deficit. They’re not eating enough to maintain a healthy body with so much activity.
Williams: If you’re interested in this topic, you should refer to the full podcast with Dr Senter which is chock-full of helpful information.
Dr Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, disclosed ties with The Curbsiders.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr Paul Nelson Williams. Paul, how are you feeling about sports injuries?
Paul N. Williams, MD: I’m feeling great, Matt.
Watto: You had a sports injury of the hip. Maybe that’s an overshare, Paul, but we talked about it on a podcast with Dr Carlin Senter (part 1 and part 2).
Williams: I think I’ve shared more than my hip injury, for sure.
Watto: Whenever a patient presented with hip pain, I used to pray it was trochanteric bursitis, which now I know is not really the right thing to think about. Intra-articular hip pain presents as anterior hip pain, usually in the crease of the hip. Depending on the patient’s age and history, the differential for that type of pain includes iliopsoas tendonitis, FAI syndrome, a labral tear, a bone stress injury of the femoral neck, or osteoarthritis.
So, what exactly is FAI and how might we diagnose it?
Williams: FAI is what the cool kids call femoral acetabular impingement, and it’s exactly what it sounds like.

Something is pinching or impinging upon the joint itself and preventing full range of motion. This is a ball-and-socket joint, so it should have tremendous range of motion, able to move in all planes. If it’s impinged, then pain will occur with certain movements. There’s a cam type, which is characterized by enlargement of the femoral head neck junction, or a pincer type, which has more to do with overhang of the acetabulum, and it can also be mixed. In any case, impingement upon the patient’s full range of motion results in pain.
You evaluate this with a couple of tests — the FABER and the FADIR.
The FABER is flexion, abduction, and external rotation, and the FADIR is flexion, adduction, and internal rotation. If you elicit anterior pain with either of those tests, it’s probably one of the intra-articular pathologies, although it is hard to know for sure which one it is because these tests are fairly sensitive but not very specific.
Watto: You can get x-rays to help with the diagnosis. You would order two views of the hip: an AP of the pelvis, which is just a straight-on shot to look for arthritis or fracture. Is there a healthy joint line there? The second is the Dunn view, in which the hip is flexed 90 degrees and abducted about 20 degrees. You are looking for fracture or impingement. You can diagnose FAI based on that view, and you might be able to diagnose a hip stress injury or osteoarthritis.
Unfortunately, you’re not going to see a labral tear, but Dr Senter said that both FAI and labral tears are treated the same way, with physical therapy. Patients with FAI who aren’t getting better might end up going for surgery, so at some point I would refer them to orthopedic surgery. But I feel much more comfortable now diagnosing these conditions with these tests.
Let’s talk a little bit about trochanteric pain syndrome. I used to think it was all bursitis. Why is that not correct?
Williams: It’s nice of you to feign ignorance for the purpose of education. It used to be thought of as bursitis, but these days we know it is probably more likely a tendinopathy.

Trochanteric pain syndrome was formerly known as trochanteric bursitis, but the bursa is not typically involved. Trochanteric pain syndrome is a tendinopathy of the surrounding structures: the gluteus medius, the iliotibial band, and the tensor fascia latae. The way these structures relate looks a bit like the face of a clock, as you can see on the infographic. In general, you manage this condition the same way you do with bursitis — physical therapy. You can also give corticosteroid injections. Physical therapy is probably more durable in terms of pain relief and functionality, but in the short term, corticosteroids might provide some degree of analgesia as well.
Watto: The last thing we wanted to mention is bone stress injury, which can occur in high-mileage runners (20 miles or more per week). Patients with bone stress injury need to rest, usually non‒weight bearing, for a period of time.

Treatment of a bone stress fracture depends on which side it’s on (top or bottom). If it’s on the top of the femoral neck (the tension side), it has to be fixed. If it’s on the compression side (the bottom side of the femoral neck), it might be able to be managed conservatively, but many patients are going to need surgery. This is a big deal. But it’s a spectrum; in some cases the bone is merely irritated and unhappy, without a break in the cortex. Those patients might not need surgery.
In patients with a fracture of the femoral neck — especially younger, healthier patients — you should think about getting a bone density test and screening for relative energy deficiency in sport. This used to be called the female athlete triad, which includes disrupted menstrual cycles, being underweight, and fracture. We should be screening patients, asking them in a nonjudgmental way about their relationship with food, to make sure they are getting an appropriate number of calories.
They are actually in an energy deficit. They’re not eating enough to maintain a healthy body with so much activity.
Williams: If you’re interested in this topic, you should refer to the full podcast with Dr Senter which is chock-full of helpful information.
Dr Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, disclosed ties with The Curbsiders.
A version of this article appeared on Medscape.com.
Using GLP-1s to Meet BMI Goal for Orthopedic Surgery
The woman, in severe pain from hip and knee osteoarthritis, was confined to a wheelchair and had been told that would likely be for life. To qualify for hip replacement surgery, she needed to lose 100 pounds, a seemingly impossible goal. But she wanted to try.
“We tried a couple of medicines — oral medicines off-label — topiramate, phentermine,” said Leslie Golden, MD, MPH, DABM, a family medicine physician and obesity medicine specialist in Watertown, Wisconsin, 42 miles northeast of Madison.
They weren’t enough. But then Golden turned to glucagon-like peptide 1 (GLP-1) receptor agonists, and they delivered.
“She did lose a significant amount of weight and was able to get the hip replacement,” said Golden.
It took a couple of years. However, seeing her walk into her office, rather than wheel in, “is still one of the joys of my practice,” Golden said. “She’s so grateful. She felt everyone else had written her off.”
As she told Golden: “If I fell and broke my leg today, they would take me to surgery without concern.”
Because her hip replacement was viewed as a nonemergency procedure, the accepted threshold for elective safe surgery was a body mass index (BMI) < 40. That BMI cutoff can vary from provider to provider and medical facility to medical facility but is often required for other surgeries as well, including kidney and lung transplants, gender-affirming surgery, bariatric surgery, hernia surgery, and in vitro fertilization procedures.
She worked with Rajit Chakravarty, MD, an adult reconstructive surgeon who practices in Watertown and nearby Madison, to oversee the weight loss.
High BMIs & Surgery Issues
High BMIs have long been linked with postsurgery complications, poor wound healing, and other issues, although some research now is questioning some of those associations. Even so, surgeons have long stressed weight loss for their patients with obesity before orthopedic and other procedures.
These days, surgeons are more likely to need to have that talk. In the last decade, the age-adjusted prevalence of severe obesity — a BMI of ≥ 40 — has increased from 7.7% to 9.7% of US adults. The number of joint replacements is also rising — more than 700,000 total knee arthroplasty (TKA) and more than 450,000 total hip arthroplasty (THA), according to the American Academy of Orthopaedic Surgeons. As the population ages, those numbers are expected to increase.
Making the GLP-1 Choice
GLP-1s aren’t the only choice, of course. But they’re often more effective, as Golden found, than other medications. And when his patients with obesity are offered bariatric surgery or GLP-1s, “people definitely want to avoid the bariatric surgery,” Chakravarty said.
With the Food and Drug Administration (FDA) approval of semaglutide (Wegovy) in June 2021 for chronic weight management and then tirzepatide (Zepbound) in November 2023, interest has boomed, he said, among his surgery candidates with a high BMI.
The FDA approved Wegovy based on clinical trials, including one in which participants lost an average of 12.4% of initial body weight compared with those on placebo. It approved Zepbound based on clinical trials, including one in which those on Zepbound lost an average of 18% of their body weight, compared with those on placebo.
The wheelchair-bound woman, now 65, began with a BMI of 63, Golden said. She negotiated a cutoff of 45 with the surgeon and got the go-ahead. Currently, her BMI is 36 as she stayed on the medications.
Beyond the benefit of GLP-1s helping patients meet the BMI cutoff, some research finds fewer postoperative infections and readmissions with their use. This study found the medications did lower both, and another found reduced readmissions and complications.
Growing Partnerships, Increasing Success
Helping patients lose weight isn’t just about lowering the BMI, Chakravarty pointed out. The aim is to improve nutritional health — to teach patients how to eat healthfully for their needs, in turn improving other health barometers. Referring them to an obesity medicine physician helps to meet those goals.
When Daniel Wiznia, MD, a Yale Medicine orthopedic surgeon and codirector of the Avascular Necrosis Program, has a patient who must delay a TKA or THA until they meet a BMI cutoff, he refers that patient to the Yale Medicine Center for Weight Management, New Haven, Connecticut, to learn about weight loss, including the options of anti-obesity medications or bariatric surgery.
Taking the GLP-1s can be a game changer, according to Wiznia and John Morton, MD, MPH, FACS, FASMBS, Yale’s medical director of Bariatric Surgery and professor and vice chair of surgery, who is a physician-director of the center. The program includes other options, such as bariatric surgery, and emphasizes diet and other lifestyle measures. GLP-1s give about a 15% weight loss, Morton said, compared with bariatric surgery providing up to 30%.
Sarah Stombaugh, MD, a family medicine and obesity medicine physician in Charlottesville, Virginia, often gets referrals from two orthopedic surgeons in her community. One recent patient in her early 60s had a BMI of 43.2, too high to qualify for the TKA she needed. On GLP-1s, the initial goal was to decrease a weight of 244 to 225, bringing the BMI to 39.9. The woman did that, then kept losing before her surgery was scheduled, getting to a weight of 210 or a BMI of 37 and staying there for 3 months before the surgery.
She had the TKA, and 5 months out, she is doing well, Stombaugh said. “We do medical weight loss primarily with the GLP-1s because they’re simply the best, the most effective,” Stombaugh said. She does occasionally use oral medications such as naltrexone/bupropion (Contrave).
Stombaugh sees the collaborating trend as still evolving. When she attends obesity medicine conferences, not all her colleagues report they are partnering with surgeons. But she predicts the practice will increase, saying the popularization of what she terms the more effective GLP-1 medications Wegovy and Zepbound is driving it. Partnering with the surgeon requires a conversation at the beginning, when the referral is made, about goals. After that, she sees her patient monthly and sends progress notes to the surgeon.
Golden collaborates with three orthopedic groups in her area, primarily for knee and hip surgeries, but has also helped patients meet the BMI cutoff before spine-related surgeries. She is helping a lung transplant patient now. She has seen several patients who must meet BMI requirements before starting in vitro fertilization, due to the need for conscious sedation for egg retrieval. She has had a few patients who had to meet a BMI cutoff for nonemergency hernia repair.
Insurance Issues
Insurance remains an issue for the pricey medications. “Only about a third of patients are routinely covered with insurance,” Morton said.
However, it’s improving, he said. Golden also finds about a third of private payers cover the medication but tries to use manufacturers’ coupons to help defray the costs (from about $1000 or $1400 to about $500 a month). She has sometimes gotten enough samples to get patients to their BMI goal
Morton consulted for Novo Nordisk, Eli Lilly, Olympus, Teleflex, and Johnson & Johnson.
A version of this article appeared on Medscape.com.
The woman, in severe pain from hip and knee osteoarthritis, was confined to a wheelchair and had been told that would likely be for life. To qualify for hip replacement surgery, she needed to lose 100 pounds, a seemingly impossible goal. But she wanted to try.
“We tried a couple of medicines — oral medicines off-label — topiramate, phentermine,” said Leslie Golden, MD, MPH, DABM, a family medicine physician and obesity medicine specialist in Watertown, Wisconsin, 42 miles northeast of Madison.
They weren’t enough. But then Golden turned to glucagon-like peptide 1 (GLP-1) receptor agonists, and they delivered.
“She did lose a significant amount of weight and was able to get the hip replacement,” said Golden.
It took a couple of years. However, seeing her walk into her office, rather than wheel in, “is still one of the joys of my practice,” Golden said. “She’s so grateful. She felt everyone else had written her off.”
As she told Golden: “If I fell and broke my leg today, they would take me to surgery without concern.”
Because her hip replacement was viewed as a nonemergency procedure, the accepted threshold for elective safe surgery was a body mass index (BMI) < 40. That BMI cutoff can vary from provider to provider and medical facility to medical facility but is often required for other surgeries as well, including kidney and lung transplants, gender-affirming surgery, bariatric surgery, hernia surgery, and in vitro fertilization procedures.
She worked with Rajit Chakravarty, MD, an adult reconstructive surgeon who practices in Watertown and nearby Madison, to oversee the weight loss.
High BMIs & Surgery Issues
High BMIs have long been linked with postsurgery complications, poor wound healing, and other issues, although some research now is questioning some of those associations. Even so, surgeons have long stressed weight loss for their patients with obesity before orthopedic and other procedures.
These days, surgeons are more likely to need to have that talk. In the last decade, the age-adjusted prevalence of severe obesity — a BMI of ≥ 40 — has increased from 7.7% to 9.7% of US adults. The number of joint replacements is also rising — more than 700,000 total knee arthroplasty (TKA) and more than 450,000 total hip arthroplasty (THA), according to the American Academy of Orthopaedic Surgeons. As the population ages, those numbers are expected to increase.
Making the GLP-1 Choice
GLP-1s aren’t the only choice, of course. But they’re often more effective, as Golden found, than other medications. And when his patients with obesity are offered bariatric surgery or GLP-1s, “people definitely want to avoid the bariatric surgery,” Chakravarty said.
With the Food and Drug Administration (FDA) approval of semaglutide (Wegovy) in June 2021 for chronic weight management and then tirzepatide (Zepbound) in November 2023, interest has boomed, he said, among his surgery candidates with a high BMI.
The FDA approved Wegovy based on clinical trials, including one in which participants lost an average of 12.4% of initial body weight compared with those on placebo. It approved Zepbound based on clinical trials, including one in which those on Zepbound lost an average of 18% of their body weight, compared with those on placebo.
The wheelchair-bound woman, now 65, began with a BMI of 63, Golden said. She negotiated a cutoff of 45 with the surgeon and got the go-ahead. Currently, her BMI is 36 as she stayed on the medications.
Beyond the benefit of GLP-1s helping patients meet the BMI cutoff, some research finds fewer postoperative infections and readmissions with their use. This study found the medications did lower both, and another found reduced readmissions and complications.
Growing Partnerships, Increasing Success
Helping patients lose weight isn’t just about lowering the BMI, Chakravarty pointed out. The aim is to improve nutritional health — to teach patients how to eat healthfully for their needs, in turn improving other health barometers. Referring them to an obesity medicine physician helps to meet those goals.
When Daniel Wiznia, MD, a Yale Medicine orthopedic surgeon and codirector of the Avascular Necrosis Program, has a patient who must delay a TKA or THA until they meet a BMI cutoff, he refers that patient to the Yale Medicine Center for Weight Management, New Haven, Connecticut, to learn about weight loss, including the options of anti-obesity medications or bariatric surgery.
Taking the GLP-1s can be a game changer, according to Wiznia and John Morton, MD, MPH, FACS, FASMBS, Yale’s medical director of Bariatric Surgery and professor and vice chair of surgery, who is a physician-director of the center. The program includes other options, such as bariatric surgery, and emphasizes diet and other lifestyle measures. GLP-1s give about a 15% weight loss, Morton said, compared with bariatric surgery providing up to 30%.
Sarah Stombaugh, MD, a family medicine and obesity medicine physician in Charlottesville, Virginia, often gets referrals from two orthopedic surgeons in her community. One recent patient in her early 60s had a BMI of 43.2, too high to qualify for the TKA she needed. On GLP-1s, the initial goal was to decrease a weight of 244 to 225, bringing the BMI to 39.9. The woman did that, then kept losing before her surgery was scheduled, getting to a weight of 210 or a BMI of 37 and staying there for 3 months before the surgery.
She had the TKA, and 5 months out, she is doing well, Stombaugh said. “We do medical weight loss primarily with the GLP-1s because they’re simply the best, the most effective,” Stombaugh said. She does occasionally use oral medications such as naltrexone/bupropion (Contrave).
Stombaugh sees the collaborating trend as still evolving. When she attends obesity medicine conferences, not all her colleagues report they are partnering with surgeons. But she predicts the practice will increase, saying the popularization of what she terms the more effective GLP-1 medications Wegovy and Zepbound is driving it. Partnering with the surgeon requires a conversation at the beginning, when the referral is made, about goals. After that, she sees her patient monthly and sends progress notes to the surgeon.
Golden collaborates with three orthopedic groups in her area, primarily for knee and hip surgeries, but has also helped patients meet the BMI cutoff before spine-related surgeries. She is helping a lung transplant patient now. She has seen several patients who must meet BMI requirements before starting in vitro fertilization, due to the need for conscious sedation for egg retrieval. She has had a few patients who had to meet a BMI cutoff for nonemergency hernia repair.
Insurance Issues
Insurance remains an issue for the pricey medications. “Only about a third of patients are routinely covered with insurance,” Morton said.
However, it’s improving, he said. Golden also finds about a third of private payers cover the medication but tries to use manufacturers’ coupons to help defray the costs (from about $1000 or $1400 to about $500 a month). She has sometimes gotten enough samples to get patients to their BMI goal
Morton consulted for Novo Nordisk, Eli Lilly, Olympus, Teleflex, and Johnson & Johnson.
A version of this article appeared on Medscape.com.
The woman, in severe pain from hip and knee osteoarthritis, was confined to a wheelchair and had been told that would likely be for life. To qualify for hip replacement surgery, she needed to lose 100 pounds, a seemingly impossible goal. But she wanted to try.
“We tried a couple of medicines — oral medicines off-label — topiramate, phentermine,” said Leslie Golden, MD, MPH, DABM, a family medicine physician and obesity medicine specialist in Watertown, Wisconsin, 42 miles northeast of Madison.
They weren’t enough. But then Golden turned to glucagon-like peptide 1 (GLP-1) receptor agonists, and they delivered.
“She did lose a significant amount of weight and was able to get the hip replacement,” said Golden.
It took a couple of years. However, seeing her walk into her office, rather than wheel in, “is still one of the joys of my practice,” Golden said. “She’s so grateful. She felt everyone else had written her off.”
As she told Golden: “If I fell and broke my leg today, they would take me to surgery without concern.”
Because her hip replacement was viewed as a nonemergency procedure, the accepted threshold for elective safe surgery was a body mass index (BMI) < 40. That BMI cutoff can vary from provider to provider and medical facility to medical facility but is often required for other surgeries as well, including kidney and lung transplants, gender-affirming surgery, bariatric surgery, hernia surgery, and in vitro fertilization procedures.
She worked with Rajit Chakravarty, MD, an adult reconstructive surgeon who practices in Watertown and nearby Madison, to oversee the weight loss.
High BMIs & Surgery Issues
High BMIs have long been linked with postsurgery complications, poor wound healing, and other issues, although some research now is questioning some of those associations. Even so, surgeons have long stressed weight loss for their patients with obesity before orthopedic and other procedures.
These days, surgeons are more likely to need to have that talk. In the last decade, the age-adjusted prevalence of severe obesity — a BMI of ≥ 40 — has increased from 7.7% to 9.7% of US adults. The number of joint replacements is also rising — more than 700,000 total knee arthroplasty (TKA) and more than 450,000 total hip arthroplasty (THA), according to the American Academy of Orthopaedic Surgeons. As the population ages, those numbers are expected to increase.
Making the GLP-1 Choice
GLP-1s aren’t the only choice, of course. But they’re often more effective, as Golden found, than other medications. And when his patients with obesity are offered bariatric surgery or GLP-1s, “people definitely want to avoid the bariatric surgery,” Chakravarty said.
With the Food and Drug Administration (FDA) approval of semaglutide (Wegovy) in June 2021 for chronic weight management and then tirzepatide (Zepbound) in November 2023, interest has boomed, he said, among his surgery candidates with a high BMI.
The FDA approved Wegovy based on clinical trials, including one in which participants lost an average of 12.4% of initial body weight compared with those on placebo. It approved Zepbound based on clinical trials, including one in which those on Zepbound lost an average of 18% of their body weight, compared with those on placebo.
The wheelchair-bound woman, now 65, began with a BMI of 63, Golden said. She negotiated a cutoff of 45 with the surgeon and got the go-ahead. Currently, her BMI is 36 as she stayed on the medications.
Beyond the benefit of GLP-1s helping patients meet the BMI cutoff, some research finds fewer postoperative infections and readmissions with their use. This study found the medications did lower both, and another found reduced readmissions and complications.
Growing Partnerships, Increasing Success
Helping patients lose weight isn’t just about lowering the BMI, Chakravarty pointed out. The aim is to improve nutritional health — to teach patients how to eat healthfully for their needs, in turn improving other health barometers. Referring them to an obesity medicine physician helps to meet those goals.
When Daniel Wiznia, MD, a Yale Medicine orthopedic surgeon and codirector of the Avascular Necrosis Program, has a patient who must delay a TKA or THA until they meet a BMI cutoff, he refers that patient to the Yale Medicine Center for Weight Management, New Haven, Connecticut, to learn about weight loss, including the options of anti-obesity medications or bariatric surgery.
Taking the GLP-1s can be a game changer, according to Wiznia and John Morton, MD, MPH, FACS, FASMBS, Yale’s medical director of Bariatric Surgery and professor and vice chair of surgery, who is a physician-director of the center. The program includes other options, such as bariatric surgery, and emphasizes diet and other lifestyle measures. GLP-1s give about a 15% weight loss, Morton said, compared with bariatric surgery providing up to 30%.
Sarah Stombaugh, MD, a family medicine and obesity medicine physician in Charlottesville, Virginia, often gets referrals from two orthopedic surgeons in her community. One recent patient in her early 60s had a BMI of 43.2, too high to qualify for the TKA she needed. On GLP-1s, the initial goal was to decrease a weight of 244 to 225, bringing the BMI to 39.9. The woman did that, then kept losing before her surgery was scheduled, getting to a weight of 210 or a BMI of 37 and staying there for 3 months before the surgery.
She had the TKA, and 5 months out, she is doing well, Stombaugh said. “We do medical weight loss primarily with the GLP-1s because they’re simply the best, the most effective,” Stombaugh said. She does occasionally use oral medications such as naltrexone/bupropion (Contrave).
Stombaugh sees the collaborating trend as still evolving. When she attends obesity medicine conferences, not all her colleagues report they are partnering with surgeons. But she predicts the practice will increase, saying the popularization of what she terms the more effective GLP-1 medications Wegovy and Zepbound is driving it. Partnering with the surgeon requires a conversation at the beginning, when the referral is made, about goals. After that, she sees her patient monthly and sends progress notes to the surgeon.
Golden collaborates with three orthopedic groups in her area, primarily for knee and hip surgeries, but has also helped patients meet the BMI cutoff before spine-related surgeries. She is helping a lung transplant patient now. She has seen several patients who must meet BMI requirements before starting in vitro fertilization, due to the need for conscious sedation for egg retrieval. She has had a few patients who had to meet a BMI cutoff for nonemergency hernia repair.
Insurance Issues
Insurance remains an issue for the pricey medications. “Only about a third of patients are routinely covered with insurance,” Morton said.
However, it’s improving, he said. Golden also finds about a third of private payers cover the medication but tries to use manufacturers’ coupons to help defray the costs (from about $1000 or $1400 to about $500 a month). She has sometimes gotten enough samples to get patients to their BMI goal
Morton consulted for Novo Nordisk, Eli Lilly, Olympus, Teleflex, and Johnson & Johnson.
A version of this article appeared on Medscape.com.
New Test’s Utility in Distinguishing OA From Inflammatory Arthritis Questioned
A new diagnostic test can accurately distinguish osteoarthritis (OA) from inflammatory arthritis using two synovial fluid biomarkers, according to research published in the Journal of Orthopaedic Research on December 18, 2024.
However, experts question whether such a test would be useful.
“The need would seem to be fairly limited, mostly those with single joint involvement and a lack of other systemic features to specify a diagnosis, which is not that common, at least in rheumatology, where there are usually features in the history and physical that can clarify the diagnosis,” said Amanda E. Nelson, MD, MSCR, professor of medicine in the Division of Rheumatology, Allergy, and Immunology at the University of North Carolina at Chapel Hill. She was not involved with the research.
The test uses an algorithm that incorporates concentrations of cartilage oligomeric matrix protein (COMP) and interleukin 8 (IL-8) in synovial fluid. The researchers hypothesized that a ratio of the two biomarkers could distinguish between primary OA and other inflammatory arthritic diagnoses.
“Primary OA is unlikely when either COMP concentration or COMP/IL‐8 ratio in the synovial fluid is low since these conditions indicate either lack of cartilage degradation or presence of high inflammation,” wrote Daniel Keter and coauthors at CD Diagnostics, Claymont, Delaware, and CD Laboratories, Towson, Maryland. “In contrast, a high COMP concentration result in combination with high COMP/IL‐8 ratio would be suggestive of low inflammation in the setting of cartilage deterioration, which is indicative of primary OA.”
In patients with OA, synovial fluid can be difficult to aspirate in sufficient amounts for testing, Nelson said.
“If synovial fluid is present and able to be aspirated, it is unclear if this test has any benefit over a simple, standard cell count and crystal assessment, which can also distinguish between osteoarthritis and more inflammatory arthritides,” she said.
Differentiating OA
To test this potential diagnostic algorithm, researchers obtained 171 knee synovial fluid samples from approved clinical remnant sample sources and a biovendor. All samples were annotated with an existing arthritic diagnosis, including 54 with primary OA, 57 with rheumatoid arthritis (RA), 30 with crystal arthritis (CA), and 30 with native septic arthritis (NSA).
Researchers assigned a CA diagnosis based on the presence of monosodium urate or calcium pyrophosphate dehydrate crystals in the synovial fluid, and NSA was determined via the Synovasure Alpha Defensin test. OA was confirmed via radiograph as Kellgren‐Lawrence grades 2‐4 with no other arthritic diagnoses. RA samples were purchased via a biovendor, and researchers were not provided with diagnosis‐confirming data.
All samples were randomized and blinded before testing, and researchers used enzyme-linked immunosorbent assay tests for both COMP and IL-8 biomarkers.
Of the 54 OA samples, 47 tested positive for OA using the COMP + COMP/IL-8 ratio algorithm. Of the 117 samples with inflammatory arthritis, 13 tested positive for OA. Overall, the diagnostic algorithm demonstrated a clinical sensitivity of 87.0% and specificity of 88.9%. The positive predictive value was 78.3%, while the negative predictive value was 93.7%.
Unclear Clinical Need
Nelson noted that while this test aims to differentiate between arthritic diagnoses, patients can also have multiple conditions.
“Many individuals with rheumatoid arthritis will develop osteoarthritis, but they can have both, so a yes/no test is of unclear utility,” she said. OA and calcium pyrophosphate deposition (CPPD) disease can often occur together, “but the driver is really the OA, and the CPPD is present but not actively inflammatory,” she continued. “Septic arthritis should be readily distinguishable by cell count alone [and again, can coexist with any of the other conditions], and a thorough history and physical should be able to differentiate in most cases.”
While these results from this study are “reasonably impressive,” more clinical information is needed to interpret these results, added C. Kent Kwoh, MD, director of the University of Arizona Arthritis Center and professor of medicine and medical imaging at the University of Arizona College of Medicine, Tucson, Arizona.
Because the study is retrospective in nature and researchers obtained specimens from different sources, it was not clear if these patients were being treated when these samples were taken and if their various conditions were controlled or flaring.
“I would say this is a reasonable first step,” Kwoh said. “We would need prospective studies, more clinical characterization, and potentially longitudinal studies to understand when this test may be useful.”
This research was internally funded by Zimmer Biomet. All authors were employees of CD Diagnostics or CD Laboratories, both of which are subsidiaries of Zimmer Biomet. Kwoh reported receiving grants or contracts with AbbVie, Artiva, Eli Lilly and Company, Bristol Myers Squibb, Cumberland, Pfizer, GSK, and Galapagos, and consulting fees from TrialSpark/Formation Bio, Express Scripts, GSK, TLC BioSciences, and AposHealth. He participates on Data Safety Monitoring or Advisory Boards of Moebius Medical, Sun Pharma, Novartis, Xalud, and Kolon TissueGene. Nelson reported no relevant disclosures.
A version of this article appeared on Medscape.com.
A new diagnostic test can accurately distinguish osteoarthritis (OA) from inflammatory arthritis using two synovial fluid biomarkers, according to research published in the Journal of Orthopaedic Research on December 18, 2024.
However, experts question whether such a test would be useful.
“The need would seem to be fairly limited, mostly those with single joint involvement and a lack of other systemic features to specify a diagnosis, which is not that common, at least in rheumatology, where there are usually features in the history and physical that can clarify the diagnosis,” said Amanda E. Nelson, MD, MSCR, professor of medicine in the Division of Rheumatology, Allergy, and Immunology at the University of North Carolina at Chapel Hill. She was not involved with the research.
The test uses an algorithm that incorporates concentrations of cartilage oligomeric matrix protein (COMP) and interleukin 8 (IL-8) in synovial fluid. The researchers hypothesized that a ratio of the two biomarkers could distinguish between primary OA and other inflammatory arthritic diagnoses.
“Primary OA is unlikely when either COMP concentration or COMP/IL‐8 ratio in the synovial fluid is low since these conditions indicate either lack of cartilage degradation or presence of high inflammation,” wrote Daniel Keter and coauthors at CD Diagnostics, Claymont, Delaware, and CD Laboratories, Towson, Maryland. “In contrast, a high COMP concentration result in combination with high COMP/IL‐8 ratio would be suggestive of low inflammation in the setting of cartilage deterioration, which is indicative of primary OA.”
In patients with OA, synovial fluid can be difficult to aspirate in sufficient amounts for testing, Nelson said.
“If synovial fluid is present and able to be aspirated, it is unclear if this test has any benefit over a simple, standard cell count and crystal assessment, which can also distinguish between osteoarthritis and more inflammatory arthritides,” she said.
Differentiating OA
To test this potential diagnostic algorithm, researchers obtained 171 knee synovial fluid samples from approved clinical remnant sample sources and a biovendor. All samples were annotated with an existing arthritic diagnosis, including 54 with primary OA, 57 with rheumatoid arthritis (RA), 30 with crystal arthritis (CA), and 30 with native septic arthritis (NSA).
Researchers assigned a CA diagnosis based on the presence of monosodium urate or calcium pyrophosphate dehydrate crystals in the synovial fluid, and NSA was determined via the Synovasure Alpha Defensin test. OA was confirmed via radiograph as Kellgren‐Lawrence grades 2‐4 with no other arthritic diagnoses. RA samples were purchased via a biovendor, and researchers were not provided with diagnosis‐confirming data.
All samples were randomized and blinded before testing, and researchers used enzyme-linked immunosorbent assay tests for both COMP and IL-8 biomarkers.
Of the 54 OA samples, 47 tested positive for OA using the COMP + COMP/IL-8 ratio algorithm. Of the 117 samples with inflammatory arthritis, 13 tested positive for OA. Overall, the diagnostic algorithm demonstrated a clinical sensitivity of 87.0% and specificity of 88.9%. The positive predictive value was 78.3%, while the negative predictive value was 93.7%.
Unclear Clinical Need
Nelson noted that while this test aims to differentiate between arthritic diagnoses, patients can also have multiple conditions.
“Many individuals with rheumatoid arthritis will develop osteoarthritis, but they can have both, so a yes/no test is of unclear utility,” she said. OA and calcium pyrophosphate deposition (CPPD) disease can often occur together, “but the driver is really the OA, and the CPPD is present but not actively inflammatory,” she continued. “Septic arthritis should be readily distinguishable by cell count alone [and again, can coexist with any of the other conditions], and a thorough history and physical should be able to differentiate in most cases.”
While these results from this study are “reasonably impressive,” more clinical information is needed to interpret these results, added C. Kent Kwoh, MD, director of the University of Arizona Arthritis Center and professor of medicine and medical imaging at the University of Arizona College of Medicine, Tucson, Arizona.
Because the study is retrospective in nature and researchers obtained specimens from different sources, it was not clear if these patients were being treated when these samples were taken and if their various conditions were controlled or flaring.
“I would say this is a reasonable first step,” Kwoh said. “We would need prospective studies, more clinical characterization, and potentially longitudinal studies to understand when this test may be useful.”
This research was internally funded by Zimmer Biomet. All authors were employees of CD Diagnostics or CD Laboratories, both of which are subsidiaries of Zimmer Biomet. Kwoh reported receiving grants or contracts with AbbVie, Artiva, Eli Lilly and Company, Bristol Myers Squibb, Cumberland, Pfizer, GSK, and Galapagos, and consulting fees from TrialSpark/Formation Bio, Express Scripts, GSK, TLC BioSciences, and AposHealth. He participates on Data Safety Monitoring or Advisory Boards of Moebius Medical, Sun Pharma, Novartis, Xalud, and Kolon TissueGene. Nelson reported no relevant disclosures.
A version of this article appeared on Medscape.com.
A new diagnostic test can accurately distinguish osteoarthritis (OA) from inflammatory arthritis using two synovial fluid biomarkers, according to research published in the Journal of Orthopaedic Research on December 18, 2024.
However, experts question whether such a test would be useful.
“The need would seem to be fairly limited, mostly those with single joint involvement and a lack of other systemic features to specify a diagnosis, which is not that common, at least in rheumatology, where there are usually features in the history and physical that can clarify the diagnosis,” said Amanda E. Nelson, MD, MSCR, professor of medicine in the Division of Rheumatology, Allergy, and Immunology at the University of North Carolina at Chapel Hill. She was not involved with the research.
The test uses an algorithm that incorporates concentrations of cartilage oligomeric matrix protein (COMP) and interleukin 8 (IL-8) in synovial fluid. The researchers hypothesized that a ratio of the two biomarkers could distinguish between primary OA and other inflammatory arthritic diagnoses.
“Primary OA is unlikely when either COMP concentration or COMP/IL‐8 ratio in the synovial fluid is low since these conditions indicate either lack of cartilage degradation or presence of high inflammation,” wrote Daniel Keter and coauthors at CD Diagnostics, Claymont, Delaware, and CD Laboratories, Towson, Maryland. “In contrast, a high COMP concentration result in combination with high COMP/IL‐8 ratio would be suggestive of low inflammation in the setting of cartilage deterioration, which is indicative of primary OA.”
In patients with OA, synovial fluid can be difficult to aspirate in sufficient amounts for testing, Nelson said.
“If synovial fluid is present and able to be aspirated, it is unclear if this test has any benefit over a simple, standard cell count and crystal assessment, which can also distinguish between osteoarthritis and more inflammatory arthritides,” she said.
Differentiating OA
To test this potential diagnostic algorithm, researchers obtained 171 knee synovial fluid samples from approved clinical remnant sample sources and a biovendor. All samples were annotated with an existing arthritic diagnosis, including 54 with primary OA, 57 with rheumatoid arthritis (RA), 30 with crystal arthritis (CA), and 30 with native septic arthritis (NSA).
Researchers assigned a CA diagnosis based on the presence of monosodium urate or calcium pyrophosphate dehydrate crystals in the synovial fluid, and NSA was determined via the Synovasure Alpha Defensin test. OA was confirmed via radiograph as Kellgren‐Lawrence grades 2‐4 with no other arthritic diagnoses. RA samples were purchased via a biovendor, and researchers were not provided with diagnosis‐confirming data.
All samples were randomized and blinded before testing, and researchers used enzyme-linked immunosorbent assay tests for both COMP and IL-8 biomarkers.
Of the 54 OA samples, 47 tested positive for OA using the COMP + COMP/IL-8 ratio algorithm. Of the 117 samples with inflammatory arthritis, 13 tested positive for OA. Overall, the diagnostic algorithm demonstrated a clinical sensitivity of 87.0% and specificity of 88.9%. The positive predictive value was 78.3%, while the negative predictive value was 93.7%.
Unclear Clinical Need
Nelson noted that while this test aims to differentiate between arthritic diagnoses, patients can also have multiple conditions.
“Many individuals with rheumatoid arthritis will develop osteoarthritis, but they can have both, so a yes/no test is of unclear utility,” she said. OA and calcium pyrophosphate deposition (CPPD) disease can often occur together, “but the driver is really the OA, and the CPPD is present but not actively inflammatory,” she continued. “Septic arthritis should be readily distinguishable by cell count alone [and again, can coexist with any of the other conditions], and a thorough history and physical should be able to differentiate in most cases.”
While these results from this study are “reasonably impressive,” more clinical information is needed to interpret these results, added C. Kent Kwoh, MD, director of the University of Arizona Arthritis Center and professor of medicine and medical imaging at the University of Arizona College of Medicine, Tucson, Arizona.
Because the study is retrospective in nature and researchers obtained specimens from different sources, it was not clear if these patients were being treated when these samples were taken and if their various conditions were controlled or flaring.
“I would say this is a reasonable first step,” Kwoh said. “We would need prospective studies, more clinical characterization, and potentially longitudinal studies to understand when this test may be useful.”
This research was internally funded by Zimmer Biomet. All authors were employees of CD Diagnostics or CD Laboratories, both of which are subsidiaries of Zimmer Biomet. Kwoh reported receiving grants or contracts with AbbVie, Artiva, Eli Lilly and Company, Bristol Myers Squibb, Cumberland, Pfizer, GSK, and Galapagos, and consulting fees from TrialSpark/Formation Bio, Express Scripts, GSK, TLC BioSciences, and AposHealth. He participates on Data Safety Monitoring or Advisory Boards of Moebius Medical, Sun Pharma, Novartis, Xalud, and Kolon TissueGene. Nelson reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM JOURNAL OF ORTHOPAEDIC RESEARCH
Drugs Targeting Osteoarthritis Pain: What’s in Development?
WASHINGTON — Investigational treatments aimed specifically at reducing pain in knee osteoarthritis (OA) are moving forward in parallel with disease-modifying approaches.
“We still have very few treatments for the pain of osteoarthritis…It worries me that people think the only way forward is structure modification. I think while we’re waiting for some drugs to be structure modifying, we still need more pain relief. About 70% of people can’t tolerate or shouldn’t be on a [nonsteroidal anti-inflammatory drug], and that leaves a large number of people with pain,” Philip Conaghan, MBBS, PhD, Chair of Musculoskeletal Medicine at the University of Leeds in England, said in an interview.
At the annual meeting of the American College of Rheumatology, Conaghan, who is also honorary consultant rheumatologist for the Leeds Teaching Hospitals NHS Trust, presented new data for two novel approaches, both targeting peripheral nociceptive pain signaling.
In a late-breaking poster, he presented phase 2 trial data on RTX-GRT7039 (resiniferatoxin [RTX]), an agonist of the transient receptor potential vanilloid 1 that is a driver of OA pain. The trial investigated the efficacy and safety of a single intra-articular injection of RTX-GRT7039 in people with knee OA.
And separately, in a late-breaking oral abstract session, Conaghan presented phase 2 trial safety and efficacy data for another investigational agent called LEVI-04, a first-in-class neurotrophin receptor fusion protein (p75NTR-Fc) that supplements the endogenous protein and provides analgesia via inhibition of NT-3 activity.
“I think both have potential to provide good pain relief, through slightly different mechanisms,” Conaghan said in an interview.
Asked to comment, session moderator Gregory C. Gardner, MD, emeritus professor in the Division of Rheumatology at the University of Washington, Seattle, said in an interview: “I think the results are really exciting terms of the ability to control pain to a significant degree in patients with osteoarthritis.”
However, Gardner also said, “The molecules can be very expensive ... so who do we give them to? Will insurance companies pay for this simply for OA pain? They improve function ... so clearly, [they] will be a boon to treating osteoarthritis, but do we give them to people with only more advanced forms of osteoarthritis or earlier on?”
Moreover, Gardner said, “One of my concerns about treating osteoarthritis is I don’t want to do too good of a job treating pain in somebody who has a biomechanically abnormal joint. ... You’ve got a knee that’s worn out some of the cartilage, and now you feel like you can go out and play soccer again. That’s not a good thing. That joint will wear out very quickly, even though it doesn’t feel pain.”
Another OA expert, Matlock Jeffries, MD, director of the Arthritis Research Unit at the Oklahoma Medical Research Foundation, Oklahoma City, said in an interview, “I think we don’t focus nearly enough on pain, and that’s [partly] because the [Food and Drug Administration] has defined endpoints for knee OA trials that are radiographic. ... Patients do not care what their joint space narrowing is. They care what their pain is. And joint space changes and pain do not correlate in knee OA. ... About 20% or 30% of patients who have completely normal x-rays have a lot of pain…I hope that we’ll have some new OA pain therapeutics in the future because that’s what patients actually care about.”
But Jeffries noted that it will be very important to ensure that these agents don’t produce significant side effects, as had been seen previously in several large industry-sponsored trials of drugs targeting nerve growth factors.
“The big concern that we have in the field ... is that the nerve growth factor antibody trials were all stopped because there was a low but persistent risk of rapidly progressive OA in a small percent of patients. I think one of the questions in the field is whether targeting other things having to do with OA pain is going to result in similar bad outcomes. I think the answer is probably not, but that’s one thing that people do worry about, and they never really figured out why the [rapidly progressive OA] was happening.”
‘Potential to Provide Meaningful and Sustained Analgesia’
The phase 2 trial of RTX-GRT7039, funded by manufacturer Grünenthal, enrolled 40 patients with a baseline visual analog pain score (VAS) of > 40 mm on motion for average joint pain in the target knee over the past 2 days with or without analgesic medication and Kellgren-Lawrence grades 2-4.
They were randomized to receive a single intra-articular injection of 2 mg or 4 mg RTX-GRT7039 within 1 minute after receiving 5 mL ropivacaine (0.5%) or 4 mg or 8 mg RTX-GRT7039 administered 15 minutes after 5 mL ropivacaine pretreatment, or equivalent placebo treatments plus ropivacaine.
Plasma samples were collected for up to 2 hours, and VAS pain scores were collected for up to 3 hours post injection.
Reductions in VAS scores from baseline in the treated knee were seen in all RTX treatment groups as early as day 8 post injection and were maintained up to 6 months, while no reductions in VAS pain on motion scores were seen in the placebo group.
At 3 months, the absolute baseline-adjusted reductions in VAS scores were similar for RTX 2 mg (–39.75), RTX 4 mg (–40.20), and RTX 8 mg (–30.25), while the reduction in the placebo group was just –8.50. At 6 months, the mean absolute reduction in VAS score was numerically greater in the RTX 2-mg (–46.49), RTX 4-mg (–43.40), and RTX 8-mg (–38.60) groups vs the group that received RTX 4 mg within 1 minute after receiving ropivacaine (–22.00).
At both 3 and 6 months, a higher proportion of patients receiving any dose of RTX-GRT7039 achieved ≥ 50% and ≥ 70% reduction in pain on motion, compared with those who received placebo. All RTX-GRT7039 treatment groups reported a greater improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) total score than the placebo group at both 3 and 6 months.
Rates of treatment-emergent adverse events were similar between the RTX groups (85.7%-90.9%) and placebo (85.7%) and slightly lower in the group that received RTX 4 mg within 1 minute of receiving ropivacaine (60.0%).
There was a trend toward greater procedural/injection site pain in the RTX treatment groups, compared with placebo, most commonly arthralgia (37.5%), headache (17.5%), and back pain (10%). This tended to peak around 0.5 hours post injection and resolve by 1.5-3.0 hours.
No treatment-related serious adverse events occurred, and no treatment-emergent adverse events led to discontinuation or death.
“This early-phase trial indicates that RTX-GRT7039 has the potential to provide meaningful and sustained analgesia for patients with knee OA pain,” Conaghan and colleagues wrote in their poster.
The drug is now being evaluated in three phase 3 trials (NCT05248386, NCT05449132, and NCT05377489).
LEVI-04: Modulation of NT-3 Appears to Work Safely
LEVI-04 was evaluated in a phase 2, 20-week, 13-center (Europe and Hong Kong) randomized, double-blind, placebo-controlled trial in 518 people with knee OA who had WOMAC pain subscale scores ≥ 20, mean average daily pain numeric rating scale score of 4-9, and radiographic Kellgren-Lawrence grade ≥ 2.
They were randomized to a total of five infusions of placebo or 0.3 mg/kg, 0.1 mg/kg, or 2 mg/kg LEVI-04 from baseline through week 16, with safety follow-up to week 30.
The primary endpoint, change in WOMAC pain from baseline to weeks 5 and 17, was met for all three doses. At 17 weeks, those were –2.79, –2.89, and –3.08 for 0.3 mg, 1.0 mg, and 2 mg, respectively, vs –2.28 for placebo (all P < .05).
Secondary endpoints, including WOMAC physical function, WOMAC stiffness, and Patient Global Assessment, and > 50% pain responders, were also all met at weeks 5 and 17. More than 50% of the LEVI-04–treated patients reported ≥ 50% reduction in pain, and > 25% reported ≥ 75% reduction at weeks 5 and 17.
“So, this modulation of NT-3 is working,” Conaghan commented.
There were no increased incidences of severe adverse events, treatment-emergent adverse events, or joint pathologies, including rapidly progressive OA, compared with placebo.
There were more paresthesias reported with the active drug, 2-4 vs 1 with placebo. “That says to me that the drug is working and that it’s having an effect on peripheral nerves, but luckily these were all mild or moderate and didn’t lead to any study withdrawal or discontinuation,” Conaghan said.
Phase 3 trials are in the planning stages, he noted.
Other Approaches to Treating OA Pain
Other approaches to treating OA pain have included methotrexate, for which Conaghan was also a coauthor on one paper that came out earlier in 2024. “This presumably works by treating inflammation, but it’s not clear if that is within-joint inflammation or systemic inflammation,” he said in an interview.
Another approach, using the weight loss drug semaglutide, was presented in April 2024 at the 2024 World Congress on Osteoarthritis annual meeting and published in October 2024 in The New England Journal of Medicine
The trial involving RTX-GRT7039 was funded by Grünenthal, and some study coauthors are employees of the company. The trial involving LEVI-04 was funded by Levicept, and some study coauthors are employees of the company. Conaghan is a consultant and/or speaker for Eli Lilly, Eupraxia Pharmaceuticals, Formation Bio, Galapagos, Genascence, GlaxoSmithKline, Grünenthal, Janssen Pharmaceuticals, Kolon TissueGene, Levicept, Medipost, Moebius, Novartis, Pacira, Sandoz, Stryker Corporation, and Takeda. Gardner and Jeffries had no disclosures.
A version of this article appeared on Medscape.com.
WASHINGTON — Investigational treatments aimed specifically at reducing pain in knee osteoarthritis (OA) are moving forward in parallel with disease-modifying approaches.
“We still have very few treatments for the pain of osteoarthritis…It worries me that people think the only way forward is structure modification. I think while we’re waiting for some drugs to be structure modifying, we still need more pain relief. About 70% of people can’t tolerate or shouldn’t be on a [nonsteroidal anti-inflammatory drug], and that leaves a large number of people with pain,” Philip Conaghan, MBBS, PhD, Chair of Musculoskeletal Medicine at the University of Leeds in England, said in an interview.
At the annual meeting of the American College of Rheumatology, Conaghan, who is also honorary consultant rheumatologist for the Leeds Teaching Hospitals NHS Trust, presented new data for two novel approaches, both targeting peripheral nociceptive pain signaling.
In a late-breaking poster, he presented phase 2 trial data on RTX-GRT7039 (resiniferatoxin [RTX]), an agonist of the transient receptor potential vanilloid 1 that is a driver of OA pain. The trial investigated the efficacy and safety of a single intra-articular injection of RTX-GRT7039 in people with knee OA.
And separately, in a late-breaking oral abstract session, Conaghan presented phase 2 trial safety and efficacy data for another investigational agent called LEVI-04, a first-in-class neurotrophin receptor fusion protein (p75NTR-Fc) that supplements the endogenous protein and provides analgesia via inhibition of NT-3 activity.
“I think both have potential to provide good pain relief, through slightly different mechanisms,” Conaghan said in an interview.
Asked to comment, session moderator Gregory C. Gardner, MD, emeritus professor in the Division of Rheumatology at the University of Washington, Seattle, said in an interview: “I think the results are really exciting terms of the ability to control pain to a significant degree in patients with osteoarthritis.”
However, Gardner also said, “The molecules can be very expensive ... so who do we give them to? Will insurance companies pay for this simply for OA pain? They improve function ... so clearly, [they] will be a boon to treating osteoarthritis, but do we give them to people with only more advanced forms of osteoarthritis or earlier on?”
Moreover, Gardner said, “One of my concerns about treating osteoarthritis is I don’t want to do too good of a job treating pain in somebody who has a biomechanically abnormal joint. ... You’ve got a knee that’s worn out some of the cartilage, and now you feel like you can go out and play soccer again. That’s not a good thing. That joint will wear out very quickly, even though it doesn’t feel pain.”
Another OA expert, Matlock Jeffries, MD, director of the Arthritis Research Unit at the Oklahoma Medical Research Foundation, Oklahoma City, said in an interview, “I think we don’t focus nearly enough on pain, and that’s [partly] because the [Food and Drug Administration] has defined endpoints for knee OA trials that are radiographic. ... Patients do not care what their joint space narrowing is. They care what their pain is. And joint space changes and pain do not correlate in knee OA. ... About 20% or 30% of patients who have completely normal x-rays have a lot of pain…I hope that we’ll have some new OA pain therapeutics in the future because that’s what patients actually care about.”
But Jeffries noted that it will be very important to ensure that these agents don’t produce significant side effects, as had been seen previously in several large industry-sponsored trials of drugs targeting nerve growth factors.
“The big concern that we have in the field ... is that the nerve growth factor antibody trials were all stopped because there was a low but persistent risk of rapidly progressive OA in a small percent of patients. I think one of the questions in the field is whether targeting other things having to do with OA pain is going to result in similar bad outcomes. I think the answer is probably not, but that’s one thing that people do worry about, and they never really figured out why the [rapidly progressive OA] was happening.”
‘Potential to Provide Meaningful and Sustained Analgesia’
The phase 2 trial of RTX-GRT7039, funded by manufacturer Grünenthal, enrolled 40 patients with a baseline visual analog pain score (VAS) of > 40 mm on motion for average joint pain in the target knee over the past 2 days with or without analgesic medication and Kellgren-Lawrence grades 2-4.
They were randomized to receive a single intra-articular injection of 2 mg or 4 mg RTX-GRT7039 within 1 minute after receiving 5 mL ropivacaine (0.5%) or 4 mg or 8 mg RTX-GRT7039 administered 15 minutes after 5 mL ropivacaine pretreatment, or equivalent placebo treatments plus ropivacaine.
Plasma samples were collected for up to 2 hours, and VAS pain scores were collected for up to 3 hours post injection.
Reductions in VAS scores from baseline in the treated knee were seen in all RTX treatment groups as early as day 8 post injection and were maintained up to 6 months, while no reductions in VAS pain on motion scores were seen in the placebo group.
At 3 months, the absolute baseline-adjusted reductions in VAS scores were similar for RTX 2 mg (–39.75), RTX 4 mg (–40.20), and RTX 8 mg (–30.25), while the reduction in the placebo group was just –8.50. At 6 months, the mean absolute reduction in VAS score was numerically greater in the RTX 2-mg (–46.49), RTX 4-mg (–43.40), and RTX 8-mg (–38.60) groups vs the group that received RTX 4 mg within 1 minute after receiving ropivacaine (–22.00).
At both 3 and 6 months, a higher proportion of patients receiving any dose of RTX-GRT7039 achieved ≥ 50% and ≥ 70% reduction in pain on motion, compared with those who received placebo. All RTX-GRT7039 treatment groups reported a greater improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) total score than the placebo group at both 3 and 6 months.
Rates of treatment-emergent adverse events were similar between the RTX groups (85.7%-90.9%) and placebo (85.7%) and slightly lower in the group that received RTX 4 mg within 1 minute of receiving ropivacaine (60.0%).
There was a trend toward greater procedural/injection site pain in the RTX treatment groups, compared with placebo, most commonly arthralgia (37.5%), headache (17.5%), and back pain (10%). This tended to peak around 0.5 hours post injection and resolve by 1.5-3.0 hours.
No treatment-related serious adverse events occurred, and no treatment-emergent adverse events led to discontinuation or death.
“This early-phase trial indicates that RTX-GRT7039 has the potential to provide meaningful and sustained analgesia for patients with knee OA pain,” Conaghan and colleagues wrote in their poster.
The drug is now being evaluated in three phase 3 trials (NCT05248386, NCT05449132, and NCT05377489).
LEVI-04: Modulation of NT-3 Appears to Work Safely
LEVI-04 was evaluated in a phase 2, 20-week, 13-center (Europe and Hong Kong) randomized, double-blind, placebo-controlled trial in 518 people with knee OA who had WOMAC pain subscale scores ≥ 20, mean average daily pain numeric rating scale score of 4-9, and radiographic Kellgren-Lawrence grade ≥ 2.
They were randomized to a total of five infusions of placebo or 0.3 mg/kg, 0.1 mg/kg, or 2 mg/kg LEVI-04 from baseline through week 16, with safety follow-up to week 30.
The primary endpoint, change in WOMAC pain from baseline to weeks 5 and 17, was met for all three doses. At 17 weeks, those were –2.79, –2.89, and –3.08 for 0.3 mg, 1.0 mg, and 2 mg, respectively, vs –2.28 for placebo (all P < .05).
Secondary endpoints, including WOMAC physical function, WOMAC stiffness, and Patient Global Assessment, and > 50% pain responders, were also all met at weeks 5 and 17. More than 50% of the LEVI-04–treated patients reported ≥ 50% reduction in pain, and > 25% reported ≥ 75% reduction at weeks 5 and 17.
“So, this modulation of NT-3 is working,” Conaghan commented.
There were no increased incidences of severe adverse events, treatment-emergent adverse events, or joint pathologies, including rapidly progressive OA, compared with placebo.
There were more paresthesias reported with the active drug, 2-4 vs 1 with placebo. “That says to me that the drug is working and that it’s having an effect on peripheral nerves, but luckily these were all mild or moderate and didn’t lead to any study withdrawal or discontinuation,” Conaghan said.
Phase 3 trials are in the planning stages, he noted.
Other Approaches to Treating OA Pain
Other approaches to treating OA pain have included methotrexate, for which Conaghan was also a coauthor on one paper that came out earlier in 2024. “This presumably works by treating inflammation, but it’s not clear if that is within-joint inflammation or systemic inflammation,” he said in an interview.
Another approach, using the weight loss drug semaglutide, was presented in April 2024 at the 2024 World Congress on Osteoarthritis annual meeting and published in October 2024 in The New England Journal of Medicine
The trial involving RTX-GRT7039 was funded by Grünenthal, and some study coauthors are employees of the company. The trial involving LEVI-04 was funded by Levicept, and some study coauthors are employees of the company. Conaghan is a consultant and/or speaker for Eli Lilly, Eupraxia Pharmaceuticals, Formation Bio, Galapagos, Genascence, GlaxoSmithKline, Grünenthal, Janssen Pharmaceuticals, Kolon TissueGene, Levicept, Medipost, Moebius, Novartis, Pacira, Sandoz, Stryker Corporation, and Takeda. Gardner and Jeffries had no disclosures.
A version of this article appeared on Medscape.com.
WASHINGTON — Investigational treatments aimed specifically at reducing pain in knee osteoarthritis (OA) are moving forward in parallel with disease-modifying approaches.
“We still have very few treatments for the pain of osteoarthritis…It worries me that people think the only way forward is structure modification. I think while we’re waiting for some drugs to be structure modifying, we still need more pain relief. About 70% of people can’t tolerate or shouldn’t be on a [nonsteroidal anti-inflammatory drug], and that leaves a large number of people with pain,” Philip Conaghan, MBBS, PhD, Chair of Musculoskeletal Medicine at the University of Leeds in England, said in an interview.
At the annual meeting of the American College of Rheumatology, Conaghan, who is also honorary consultant rheumatologist for the Leeds Teaching Hospitals NHS Trust, presented new data for two novel approaches, both targeting peripheral nociceptive pain signaling.
In a late-breaking poster, he presented phase 2 trial data on RTX-GRT7039 (resiniferatoxin [RTX]), an agonist of the transient receptor potential vanilloid 1 that is a driver of OA pain. The trial investigated the efficacy and safety of a single intra-articular injection of RTX-GRT7039 in people with knee OA.
And separately, in a late-breaking oral abstract session, Conaghan presented phase 2 trial safety and efficacy data for another investigational agent called LEVI-04, a first-in-class neurotrophin receptor fusion protein (p75NTR-Fc) that supplements the endogenous protein and provides analgesia via inhibition of NT-3 activity.
“I think both have potential to provide good pain relief, through slightly different mechanisms,” Conaghan said in an interview.
Asked to comment, session moderator Gregory C. Gardner, MD, emeritus professor in the Division of Rheumatology at the University of Washington, Seattle, said in an interview: “I think the results are really exciting terms of the ability to control pain to a significant degree in patients with osteoarthritis.”
However, Gardner also said, “The molecules can be very expensive ... so who do we give them to? Will insurance companies pay for this simply for OA pain? They improve function ... so clearly, [they] will be a boon to treating osteoarthritis, but do we give them to people with only more advanced forms of osteoarthritis or earlier on?”
Moreover, Gardner said, “One of my concerns about treating osteoarthritis is I don’t want to do too good of a job treating pain in somebody who has a biomechanically abnormal joint. ... You’ve got a knee that’s worn out some of the cartilage, and now you feel like you can go out and play soccer again. That’s not a good thing. That joint will wear out very quickly, even though it doesn’t feel pain.”
Another OA expert, Matlock Jeffries, MD, director of the Arthritis Research Unit at the Oklahoma Medical Research Foundation, Oklahoma City, said in an interview, “I think we don’t focus nearly enough on pain, and that’s [partly] because the [Food and Drug Administration] has defined endpoints for knee OA trials that are radiographic. ... Patients do not care what their joint space narrowing is. They care what their pain is. And joint space changes and pain do not correlate in knee OA. ... About 20% or 30% of patients who have completely normal x-rays have a lot of pain…I hope that we’ll have some new OA pain therapeutics in the future because that’s what patients actually care about.”
But Jeffries noted that it will be very important to ensure that these agents don’t produce significant side effects, as had been seen previously in several large industry-sponsored trials of drugs targeting nerve growth factors.
“The big concern that we have in the field ... is that the nerve growth factor antibody trials were all stopped because there was a low but persistent risk of rapidly progressive OA in a small percent of patients. I think one of the questions in the field is whether targeting other things having to do with OA pain is going to result in similar bad outcomes. I think the answer is probably not, but that’s one thing that people do worry about, and they never really figured out why the [rapidly progressive OA] was happening.”
‘Potential to Provide Meaningful and Sustained Analgesia’
The phase 2 trial of RTX-GRT7039, funded by manufacturer Grünenthal, enrolled 40 patients with a baseline visual analog pain score (VAS) of > 40 mm on motion for average joint pain in the target knee over the past 2 days with or without analgesic medication and Kellgren-Lawrence grades 2-4.
They were randomized to receive a single intra-articular injection of 2 mg or 4 mg RTX-GRT7039 within 1 minute after receiving 5 mL ropivacaine (0.5%) or 4 mg or 8 mg RTX-GRT7039 administered 15 minutes after 5 mL ropivacaine pretreatment, or equivalent placebo treatments plus ropivacaine.
Plasma samples were collected for up to 2 hours, and VAS pain scores were collected for up to 3 hours post injection.
Reductions in VAS scores from baseline in the treated knee were seen in all RTX treatment groups as early as day 8 post injection and were maintained up to 6 months, while no reductions in VAS pain on motion scores were seen in the placebo group.
At 3 months, the absolute baseline-adjusted reductions in VAS scores were similar for RTX 2 mg (–39.75), RTX 4 mg (–40.20), and RTX 8 mg (–30.25), while the reduction in the placebo group was just –8.50. At 6 months, the mean absolute reduction in VAS score was numerically greater in the RTX 2-mg (–46.49), RTX 4-mg (–43.40), and RTX 8-mg (–38.60) groups vs the group that received RTX 4 mg within 1 minute after receiving ropivacaine (–22.00).
At both 3 and 6 months, a higher proportion of patients receiving any dose of RTX-GRT7039 achieved ≥ 50% and ≥ 70% reduction in pain on motion, compared with those who received placebo. All RTX-GRT7039 treatment groups reported a greater improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) total score than the placebo group at both 3 and 6 months.
Rates of treatment-emergent adverse events were similar between the RTX groups (85.7%-90.9%) and placebo (85.7%) and slightly lower in the group that received RTX 4 mg within 1 minute of receiving ropivacaine (60.0%).
There was a trend toward greater procedural/injection site pain in the RTX treatment groups, compared with placebo, most commonly arthralgia (37.5%), headache (17.5%), and back pain (10%). This tended to peak around 0.5 hours post injection and resolve by 1.5-3.0 hours.
No treatment-related serious adverse events occurred, and no treatment-emergent adverse events led to discontinuation or death.
“This early-phase trial indicates that RTX-GRT7039 has the potential to provide meaningful and sustained analgesia for patients with knee OA pain,” Conaghan and colleagues wrote in their poster.
The drug is now being evaluated in three phase 3 trials (NCT05248386, NCT05449132, and NCT05377489).
LEVI-04: Modulation of NT-3 Appears to Work Safely
LEVI-04 was evaluated in a phase 2, 20-week, 13-center (Europe and Hong Kong) randomized, double-blind, placebo-controlled trial in 518 people with knee OA who had WOMAC pain subscale scores ≥ 20, mean average daily pain numeric rating scale score of 4-9, and radiographic Kellgren-Lawrence grade ≥ 2.
They were randomized to a total of five infusions of placebo or 0.3 mg/kg, 0.1 mg/kg, or 2 mg/kg LEVI-04 from baseline through week 16, with safety follow-up to week 30.
The primary endpoint, change in WOMAC pain from baseline to weeks 5 and 17, was met for all three doses. At 17 weeks, those were –2.79, –2.89, and –3.08 for 0.3 mg, 1.0 mg, and 2 mg, respectively, vs –2.28 for placebo (all P < .05).
Secondary endpoints, including WOMAC physical function, WOMAC stiffness, and Patient Global Assessment, and > 50% pain responders, were also all met at weeks 5 and 17. More than 50% of the LEVI-04–treated patients reported ≥ 50% reduction in pain, and > 25% reported ≥ 75% reduction at weeks 5 and 17.
“So, this modulation of NT-3 is working,” Conaghan commented.
There were no increased incidences of severe adverse events, treatment-emergent adverse events, or joint pathologies, including rapidly progressive OA, compared with placebo.
There were more paresthesias reported with the active drug, 2-4 vs 1 with placebo. “That says to me that the drug is working and that it’s having an effect on peripheral nerves, but luckily these were all mild or moderate and didn’t lead to any study withdrawal or discontinuation,” Conaghan said.
Phase 3 trials are in the planning stages, he noted.
Other Approaches to Treating OA Pain
Other approaches to treating OA pain have included methotrexate, for which Conaghan was also a coauthor on one paper that came out earlier in 2024. “This presumably works by treating inflammation, but it’s not clear if that is within-joint inflammation or systemic inflammation,” he said in an interview.
Another approach, using the weight loss drug semaglutide, was presented in April 2024 at the 2024 World Congress on Osteoarthritis annual meeting and published in October 2024 in The New England Journal of Medicine
The trial involving RTX-GRT7039 was funded by Grünenthal, and some study coauthors are employees of the company. The trial involving LEVI-04 was funded by Levicept, and some study coauthors are employees of the company. Conaghan is a consultant and/or speaker for Eli Lilly, Eupraxia Pharmaceuticals, Formation Bio, Galapagos, Genascence, GlaxoSmithKline, Grünenthal, Janssen Pharmaceuticals, Kolon TissueGene, Levicept, Medipost, Moebius, Novartis, Pacira, Sandoz, Stryker Corporation, and Takeda. Gardner and Jeffries had no disclosures.
A version of this article appeared on Medscape.com.
FROM ACR 2024
What’s the Evidence Behind Popular Supplements in Rheumatology? Experts Weigh in
Many people with rheumatologic diseases try supplements for symptom relief. Here’s what you need to know about some common picks.
Dietary supplements were a $159 billion business in the United States in 2023, and many people with rheumatologic diseases are buying in. Research suggests more than 6 in 10 people with fibromyalgia, nearly 8 in 10 people with Sjögren’s disease, and more than 8 in 10 people with rheumatoid arthritis (RA) take dietary supplements.
Whatever the symptom — pain, swelling, or fatigue — you can probably find a supplement purporting to relieve it. But do these supplements work, and are they safe? A study review in RMD Open comprising 24 systematic reviews and 150 original articles suggests more high-quality research is needed on the effects of dietary supplements on rheumatologic diseases. Most studies have focused on RA or osteoarthritis (OA), where the evidence level is moderate at best.
“The studies in this space are usually not very high quality because there’s no money to support them, among other things, plus the products are disparate,” said Janet Funk, MD, MS, professor in the School of Nutritional Sciences and Wellness at the University of Arizona, Tucson. She recommended brushing up on supplements and finding out what patients are taking so you can offer advice and watch for drug-supplement interactions.
When asked for a medication list, many patients forget to report supplements, Funk said. “You have to prompt them specifically. I think some physicians have very negative views about supplements because so little data is known, and patients might pick up on that and decide not to report their use.” She recommended saying something like: “To give you the best possible care, I want to know everything you’re taking, including supplements. The things I’m prescribing could maybe interact with the things you’re taking, so I want to make sure I know about all of it so that together we can figure out if the combination of things is safe.”
The quality of dietary supplements varies, and they aren’t regulated like drugs by the Food and Drug Administration. Funk recommended selecting products verified by NSF or ConsumerLab. They test supplements to ensure the label reflects what’s inside.
This news organization scoured the literature and asked experts to weigh in on the evidence behind popular supplements in rheumatology today.
The Essential Nutrients
Vitamin supplements are a staple in many homes — but are they helpful? “Individual vitamin supplements will not provide any benefit unless the person is deficient in a specific vitamin or mineral,” according to Elena Philippou, PhD, RD, associate professor of nutrition-dietetics at the University of Nicosia in Cyprus, and Elena Nikiphorou, MBBS, a rheumatologist at King’s College London in England. For some patients, deficiency is a reality. A retrospective cohort study in The Journal of Clinical Medicine found that people with RA were 17% more likely than age-matched control individuals to have nutrient deficiencies, perhaps because symptoms like fatigue, pain, and nausea affect their eating habits. Here’s what the science says about common vitamin supplements.
Vitamin D. This hormone-like vitamin, which attaches to receptors on immune cells to tamp down inflammation, was the most popular dietary supplement among rheumatology patients in a recent study from the United Kingdom. Vitamin D deficiency is common in people with RA, lupus, Sjögren’s disease, ankylosing spondylitis, systemic sclerosis, and fibromyalgia. In some cases, vitamin D levels track with disease activity, research suggests. Corticosteroids can also make vitamin D deficiency more likely. Can supplements help?
In RA, evidence points to small improvements. A systematic review of 11 studies including 3049 patients published in Nutrition Reviews showed that vitamin D supplements significantly reduced patients’ pain and Disease Activity Score in 28 joints (DAS28) using both C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
The research is mixed on the benefits of vitamin D supplementation for fibromyalgia symptoms, according to a study review in SN Comprehensive Clinical Medicine that included two studies and 80 patients on supplementation. However, researchers said it’s still worth discussing the potential benefits of taking vitamin D.
“Vitamin D supplementation is important in the context of various rheumatic diseases to prevent or treat bone disease,” said Philippou and Nikiphorou. “People with rheumatic disease should speak to their healthcare provider and ask to check their blood vitamin D concentration.” The results can help you recommend a dose.
Folate. Patients on methotrexate should take folic acid supplements under the guidance of a healthcare provider, said Philippou and Nikiphorou. The reason: Methotrexate can deplete folic acid levels, increasing the risk for side effects. An analysis of adverse event reports published showed that methotrexate users who took folic acid (or tumor necrosis factor–alpha inhibitors) had a reduced risk for hepatotoxicity or myelosuppression. A commonly recommended dose is 1 mg/d.
Vitamin B12. In a 2024 perspective paper in Rheumatology International, researchers said physicians should assess vitamin B12 levels early in the diagnostic process of rheumatologic diseases. One reason: Many symptoms of pernicious anemia, like fatigue, mimic symptoms of rheumatologic diseases. The gastrointestinal (GI) effects of systemic sclerosis could bring on vitamin B12 deficiency. In a small study in The Journal of Clinical Rheumatology, 44 of 62 patients with systemic sclerosis had low vitamin B12 levels.
Vitamin E. Vitamin E deficiency is rare in healthy adults. However, some medical conditions, like inflammatory bowel disease and malabsorption disorders, can make vitamin E deficiency more likely. In RA, a vitamin E supplement could help reduce joint swelling and sensitivity, according to a systematic review of nine studies including 39,845 patients in The European Journal of Clinical Nutrition. Researchers credit the nutrient’s role in aiding intestinal repair. Use with caution, as this supplement can increase bleeding risk in doses over 1000 mg/d.
Vitamin A. Like vitamin E, vitamin A deficiency is rare in the United States. The risk of oversupplementing is higher than undersupplementing. However, vitamin A deficiency can happen in people with chronic pancreatic, liver, or GI problems. In people with deficiency, a vitamin A supplement can help relieve dry eye symptoms common in Sjögren’s disease, suggests a narrative review published in Nutrients. Vitamin A might help reduce ocular surface changes by supporting the production of proteins that protect the outermost surfaces of the eyes. The recommended daily allowance for vitamin A is 900 μg. High-dose supplements can cause toxicity, resulting in GI symptoms and problems like lethargy, drowsiness, increased intracranial pressure, and skin changes.
The Replacements
These substances are similar to naturally occurring compounds in our bodies. The question is whether ingesting them yields benefits.
Glucosamine and chondroitin. Glucosamine and chondroitin occur naturally in our bodies and help us form and protect connective tissues. In pill form, this combo is the most popular dietary supplement for OA, according to research in the journal Maturitas. But studies of its effectiveness yield mixed results. A systematic review of 25 studies published in Inflammopharmacology showed that, in patients with knee OA, supplementation with about 1500 mg of glucosamine per day reduced tibiofemoral joint space narrowing, while supplementation with about 800 mg/d of chondroitin reduced pain intensity and improved physical function, compared with placebo. The duo of glucosamine and chondroitin did not bring significant benefits, perhaps because more studies are needed. Most side effects were mild, but some literature points to the potential for glucosamine to increase warfarin’s blood-thinning effects.
Omega-3 fatty acids. Fish oil is a top-selling supplement, and it might be helpful in inflammatory rheumatologic diseases. A systematic review of 30 studies including 710 patients published in Arthritis Research & Therapy showed that omega-3 fatty acid supplements can improve pain, swollen and tender joint count, DAS28 scores, and Health Assessment Questionnaire scores in patients with RA, psoriatic arthritis, or ankylosing spondylitis. In patients with lupus, a study review that included five studies and 284 patients in The International Journal of Environmental Research and Public Health suggested omega-3 fatty acid supplements could improve ESR, CRP, disease activity, inflammatory markers, oxidative stress, lipid levels, and endothelial function.
Omega-3 fatty acids have anti-inflammatory effects that might explain their benefits. In patients with RA, for example, fish oil supplementation was associated with elevated blood levels of resolvins and protectins, which help quell inflammation, according to a study in Prostaglandins, Leukotrienes and Essential Fatty Acids.
Philippou and Nikiphorou recommended combining food and supplements: Eat oily fish at least twice a week, regularly consume plant-based sources of omega-3s — like chia seeds, flaxseeds, or walnuts — and consider a daily supplement that contains 2 g of omega-3s from docosahexaenoic acid and eicosapentaenoic acid. Most fish oil side effects are mild, like heartburn and bad breath. Fish oil can have blood-thinning effects at high doses, so special attention is needed for patients on anticoagulants.
Probiotics. Building up the good bacteria in your gut might help you fight the effects of rheumatologic diseases. A systematic review of 80 randomized controlled trials in BMC Medicine suggested that therapies targeting the gut microbiota might improve the symptoms or inflammatory factors in celiac disease, lupus, juvenile idiopathic arthritis, psoriasis, Sjögren’s disease, multiple sclerosis, systemic sclerosis, Crohn’s disease, and ulcerative colitis. Probiotics were also shown to relieve pain in fibromyalgia, but they didn’t affect scores on the Fibromyalgia Impact Questionnaire. Probiotics were not helpful in spondyloarthritis or RA. There were no adverse events. By improving the balance of bacteria in the gut, probiotics might inhibit pro-inflammatory factors and signaling pathways and regulate CD4+ T-cell differentiation, the researchers wrote.
Not all probiotic supplements are created equal. Effects can vary by microorganism and dose. Until more high-quality studies are published, Philippou and Nikiphorou recommend daily consumption of probiotic food sources such as yogurt, kefir, sauerkraut, kimchi, tempeh, miso, and kombucha, along with prebiotic food sources such as bananas, onion, artichokes, asparagus, oats, leeks, and garlic.
Collagen. An increasingly popular supplement for hair, skin, and nails, some collagen peptide or hydrolyzed collagen supplements come with claims about joint health, too. Inside our bodies, collagen helps build joints. As a supplement, the jury is still out. A systematic review of 19 studies in The International Journal of Rheumatic Diseases suggested more research is needed to determine whether collagen supplements are harmful or helpful in OA or RA. Studies haven’t shown adverse events, and doses typically range from 2.5 to 15 g/d.
Coenzyme Q10 (CoQ10). This antioxidant occurs naturally in our cells and is produced through microbial fermentation for use in dietary supplements. A study review of 20 articles including 483 patients in Clinical Nutrition ESPEN concluded that CoQ10 supplementation up to 300 mg/d was beneficial in RA, fibromyalgia, or antiphospholipid syndrome (APS).
In RA, CoQ10 supplementation improved disease activity index, ESR, and cytokine levels and decreased malondialdehyde. CoQ10 might protect against the overproduction of reactive oxygen species that can promote inflammation and joint damage, the researchers said. In fibromyalgia, CoQ10 was linked with improvements in pain, fatigue, sleep, tender points count, mood disorders, and scores on the Fibromyalgia Impact Questionnaire in most of the included studies. CoQ10 might help in fibromyalgia by improving mitochondrial dysfunction. In APS, CoQ10 improved endothelial function and decreased prothrombotic and pro-inflammatory mediators. CoQ10 might change the expression of genes that promote atherosclerosis. A few patients had GI side effects like nausea and diarrhea, but the supplements were generally well tolerated.
Melatonin. Commonly touted as a sleep aid, this hormone has immune and anti-inflammatory activities that could benefit people with rheumatologic diseases. A study review of 13 articles including 533 patients in Clinical Nutrition ESPEN concluded that melatonin can help improve sleep, pain, and mood in fibromyalgia, OA, and osteoporosis but not in RA. Side effects were minimal, but a few people experienced nausea, drowsiness, nightmares, or headaches. Doses of 5-6 mg/d are likely safe for most adults.
The Plant-Derived Antioxidants
Many supplements used in rheumatology are antioxidants derived from herbs, spices, or other plants. When plants encounter stressors, like temperature changes or hungry insects, their secondary metabolism revs up and creates compounds with biological properties. Some of these substances influence inflammatory pathways in the human body, said Luís Silva, PhD, a medicinal chemistry researcher at the Polytechnic Institute of Guarda in Portugal. “If it is possible to reduce these kinds of anti-inflammatory processes, it is also possible that we could help people with inflammatory diseases to a good life, or a better life.”
Turmeric and curcumin. You might see this supplement labeled as turmeric, a golden spice in curry powder, or curcumin, an antioxidant compound known as a curcuminoid in turmeric. Curcuminoids might reduce inflammation by scavenging free radicals and inhibiting enzymes that make prostaglandins, Silva said.
Turmeric is the most popular herbal supplement for people with RA, according to Funk’s research. A study review of six publications including 539 patients in Frontiers in Immunology showed that curcumin supplements improved RA patients’ ESR, DAS, swollen joint count, and tender joint count. Turmeric could help patients with OA, too. Patients with OA who took 1000 mg/d of curcumin improved their pain and function, according to a systematic review including 12 studies and 1438 participants in the journal Nutrients. In lupus, small studies are promising but inconclusive, suggested a study review in Frontiers in Immunology.
Watch patients taking turmeric and methotrexate closely, Funk said. Both have been associated with liver problems. Some users also experience GI symptoms like diarrhea because turmeric doesn’t absorb well in the GI tract.
Milk thistle (silymarin). This flowering plant is often marketed as a liver-supporting supplement, but research also suggests promise in RA and OA. A systematic review of 12 studies in Current Rheumatology Reviews suggested that silymarin supplements might help relieve pain, reduce inflammation, and protect the cartilage matrix, synovial membrane, and cartilage cells in joints. This supplement might help via immunomodulatory, anti-inflammatory, antioxidant, and anti-apoptotic properties, the researchers said. Doses of 250-750 mg appear to be safe. Side effects such as gastroenteritis, diarrhea, bloating, and headache can occur.
Boswellia serrata. Sourced from the resin of a tree that grows in dry, mountainous regions of Asia and Africa, Boswellia serrata can help relieve joint pain and stiffness and improve joint function in OA, suggested a systematic review of seven trials involving 545 patients in BMC Complementary Medicine and Therapies. Users saw benefits when taking 100-250 mg/d for 4 weeks or more. Compounds in Boswellia serrata may inhibit 5-lipoxygenase, an enzyme involved in producing inflammatory leukotrienes. No adverse events were reported. In some studies, users have reported GI side effects.
Ginger. Ginger is a popular herbal supplement among people with RA, Funk’s research suggested. One small clinical trial involving 70 patients with RA in the journal Gene showed that taking 1500 mg/d of ginger for 12 weeks improved their DAS and boosted their expression of FoxP3 genes, which are linked with the function of regulatory T cells. A meta-analysis including three studies with 330 patients taking ginger published in the journal Nutrients suggested ginger can reduce pain and systemic inflammation in people with OA. Preclinical studies suggested phenolic compounds in this spicy root, such as gingerols, reduce inflammation through multiple mechanisms.
Funk’s research revealed wide variation in the quality of ginger supplements, reinforcing the importance of selecting an independently verified product. Research suggested a safe dose is up to 2-2.5 g/kg body weight.
Resveratrol. Found in red grapes and red wine, this compound is particularly good at blocking COX-2 enzymes, an important step in the inflammatory cascade, Silva said. “Because of their chemical structure, they have great affinity to these enzymes to lead to their inhibition,” he said. A study review of five articles including 481 patients in The European Journal of Rheumatology showed that people with OA, RA, or Takayasu arteritis who took 250-1000 mg/d of resveratrol saw improvements in pain, function, disease activity, joint swelling, and inflammation, with no side effects.
Cinnamon. This warming spice is gaining popularity as a supplement, reported the American Botanical Council. Cinnamon is often marketed as lowering blood sugar and supporting bone health. In a small study of 36 women with RA published in The Journal of the American College of Nutrition, participants who consumed 2 g/d of cinnamon powder had reduced DASs along with reduced pain and tender and swollen joint counts. Cinnamon may reduce pain by inhibiting prostaglandin and blunt inflammation by reducing the release of arachidonic acid from cell membranes, according to a study review in Frontiers in Pharmacology. GI problems and allergic reactions are among the most common side effects.
Funk, Nikiphorou, Philippou, and Silva all had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Many people with rheumatologic diseases try supplements for symptom relief. Here’s what you need to know about some common picks.
Dietary supplements were a $159 billion business in the United States in 2023, and many people with rheumatologic diseases are buying in. Research suggests more than 6 in 10 people with fibromyalgia, nearly 8 in 10 people with Sjögren’s disease, and more than 8 in 10 people with rheumatoid arthritis (RA) take dietary supplements.
Whatever the symptom — pain, swelling, or fatigue — you can probably find a supplement purporting to relieve it. But do these supplements work, and are they safe? A study review in RMD Open comprising 24 systematic reviews and 150 original articles suggests more high-quality research is needed on the effects of dietary supplements on rheumatologic diseases. Most studies have focused on RA or osteoarthritis (OA), where the evidence level is moderate at best.
“The studies in this space are usually not very high quality because there’s no money to support them, among other things, plus the products are disparate,” said Janet Funk, MD, MS, professor in the School of Nutritional Sciences and Wellness at the University of Arizona, Tucson. She recommended brushing up on supplements and finding out what patients are taking so you can offer advice and watch for drug-supplement interactions.
When asked for a medication list, many patients forget to report supplements, Funk said. “You have to prompt them specifically. I think some physicians have very negative views about supplements because so little data is known, and patients might pick up on that and decide not to report their use.” She recommended saying something like: “To give you the best possible care, I want to know everything you’re taking, including supplements. The things I’m prescribing could maybe interact with the things you’re taking, so I want to make sure I know about all of it so that together we can figure out if the combination of things is safe.”
The quality of dietary supplements varies, and they aren’t regulated like drugs by the Food and Drug Administration. Funk recommended selecting products verified by NSF or ConsumerLab. They test supplements to ensure the label reflects what’s inside.
This news organization scoured the literature and asked experts to weigh in on the evidence behind popular supplements in rheumatology today.
The Essential Nutrients
Vitamin supplements are a staple in many homes — but are they helpful? “Individual vitamin supplements will not provide any benefit unless the person is deficient in a specific vitamin or mineral,” according to Elena Philippou, PhD, RD, associate professor of nutrition-dietetics at the University of Nicosia in Cyprus, and Elena Nikiphorou, MBBS, a rheumatologist at King’s College London in England. For some patients, deficiency is a reality. A retrospective cohort study in The Journal of Clinical Medicine found that people with RA were 17% more likely than age-matched control individuals to have nutrient deficiencies, perhaps because symptoms like fatigue, pain, and nausea affect their eating habits. Here’s what the science says about common vitamin supplements.
Vitamin D. This hormone-like vitamin, which attaches to receptors on immune cells to tamp down inflammation, was the most popular dietary supplement among rheumatology patients in a recent study from the United Kingdom. Vitamin D deficiency is common in people with RA, lupus, Sjögren’s disease, ankylosing spondylitis, systemic sclerosis, and fibromyalgia. In some cases, vitamin D levels track with disease activity, research suggests. Corticosteroids can also make vitamin D deficiency more likely. Can supplements help?
In RA, evidence points to small improvements. A systematic review of 11 studies including 3049 patients published in Nutrition Reviews showed that vitamin D supplements significantly reduced patients’ pain and Disease Activity Score in 28 joints (DAS28) using both C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
The research is mixed on the benefits of vitamin D supplementation for fibromyalgia symptoms, according to a study review in SN Comprehensive Clinical Medicine that included two studies and 80 patients on supplementation. However, researchers said it’s still worth discussing the potential benefits of taking vitamin D.
“Vitamin D supplementation is important in the context of various rheumatic diseases to prevent or treat bone disease,” said Philippou and Nikiphorou. “People with rheumatic disease should speak to their healthcare provider and ask to check their blood vitamin D concentration.” The results can help you recommend a dose.
Folate. Patients on methotrexate should take folic acid supplements under the guidance of a healthcare provider, said Philippou and Nikiphorou. The reason: Methotrexate can deplete folic acid levels, increasing the risk for side effects. An analysis of adverse event reports published showed that methotrexate users who took folic acid (or tumor necrosis factor–alpha inhibitors) had a reduced risk for hepatotoxicity or myelosuppression. A commonly recommended dose is 1 mg/d.
Vitamin B12. In a 2024 perspective paper in Rheumatology International, researchers said physicians should assess vitamin B12 levels early in the diagnostic process of rheumatologic diseases. One reason: Many symptoms of pernicious anemia, like fatigue, mimic symptoms of rheumatologic diseases. The gastrointestinal (GI) effects of systemic sclerosis could bring on vitamin B12 deficiency. In a small study in The Journal of Clinical Rheumatology, 44 of 62 patients with systemic sclerosis had low vitamin B12 levels.
Vitamin E. Vitamin E deficiency is rare in healthy adults. However, some medical conditions, like inflammatory bowel disease and malabsorption disorders, can make vitamin E deficiency more likely. In RA, a vitamin E supplement could help reduce joint swelling and sensitivity, according to a systematic review of nine studies including 39,845 patients in The European Journal of Clinical Nutrition. Researchers credit the nutrient’s role in aiding intestinal repair. Use with caution, as this supplement can increase bleeding risk in doses over 1000 mg/d.
Vitamin A. Like vitamin E, vitamin A deficiency is rare in the United States. The risk of oversupplementing is higher than undersupplementing. However, vitamin A deficiency can happen in people with chronic pancreatic, liver, or GI problems. In people with deficiency, a vitamin A supplement can help relieve dry eye symptoms common in Sjögren’s disease, suggests a narrative review published in Nutrients. Vitamin A might help reduce ocular surface changes by supporting the production of proteins that protect the outermost surfaces of the eyes. The recommended daily allowance for vitamin A is 900 μg. High-dose supplements can cause toxicity, resulting in GI symptoms and problems like lethargy, drowsiness, increased intracranial pressure, and skin changes.
The Replacements
These substances are similar to naturally occurring compounds in our bodies. The question is whether ingesting them yields benefits.
Glucosamine and chondroitin. Glucosamine and chondroitin occur naturally in our bodies and help us form and protect connective tissues. In pill form, this combo is the most popular dietary supplement for OA, according to research in the journal Maturitas. But studies of its effectiveness yield mixed results. A systematic review of 25 studies published in Inflammopharmacology showed that, in patients with knee OA, supplementation with about 1500 mg of glucosamine per day reduced tibiofemoral joint space narrowing, while supplementation with about 800 mg/d of chondroitin reduced pain intensity and improved physical function, compared with placebo. The duo of glucosamine and chondroitin did not bring significant benefits, perhaps because more studies are needed. Most side effects were mild, but some literature points to the potential for glucosamine to increase warfarin’s blood-thinning effects.
Omega-3 fatty acids. Fish oil is a top-selling supplement, and it might be helpful in inflammatory rheumatologic diseases. A systematic review of 30 studies including 710 patients published in Arthritis Research & Therapy showed that omega-3 fatty acid supplements can improve pain, swollen and tender joint count, DAS28 scores, and Health Assessment Questionnaire scores in patients with RA, psoriatic arthritis, or ankylosing spondylitis. In patients with lupus, a study review that included five studies and 284 patients in The International Journal of Environmental Research and Public Health suggested omega-3 fatty acid supplements could improve ESR, CRP, disease activity, inflammatory markers, oxidative stress, lipid levels, and endothelial function.
Omega-3 fatty acids have anti-inflammatory effects that might explain their benefits. In patients with RA, for example, fish oil supplementation was associated with elevated blood levels of resolvins and protectins, which help quell inflammation, according to a study in Prostaglandins, Leukotrienes and Essential Fatty Acids.
Philippou and Nikiphorou recommended combining food and supplements: Eat oily fish at least twice a week, regularly consume plant-based sources of omega-3s — like chia seeds, flaxseeds, or walnuts — and consider a daily supplement that contains 2 g of omega-3s from docosahexaenoic acid and eicosapentaenoic acid. Most fish oil side effects are mild, like heartburn and bad breath. Fish oil can have blood-thinning effects at high doses, so special attention is needed for patients on anticoagulants.
Probiotics. Building up the good bacteria in your gut might help you fight the effects of rheumatologic diseases. A systematic review of 80 randomized controlled trials in BMC Medicine suggested that therapies targeting the gut microbiota might improve the symptoms or inflammatory factors in celiac disease, lupus, juvenile idiopathic arthritis, psoriasis, Sjögren’s disease, multiple sclerosis, systemic sclerosis, Crohn’s disease, and ulcerative colitis. Probiotics were also shown to relieve pain in fibromyalgia, but they didn’t affect scores on the Fibromyalgia Impact Questionnaire. Probiotics were not helpful in spondyloarthritis or RA. There were no adverse events. By improving the balance of bacteria in the gut, probiotics might inhibit pro-inflammatory factors and signaling pathways and regulate CD4+ T-cell differentiation, the researchers wrote.
Not all probiotic supplements are created equal. Effects can vary by microorganism and dose. Until more high-quality studies are published, Philippou and Nikiphorou recommend daily consumption of probiotic food sources such as yogurt, kefir, sauerkraut, kimchi, tempeh, miso, and kombucha, along with prebiotic food sources such as bananas, onion, artichokes, asparagus, oats, leeks, and garlic.
Collagen. An increasingly popular supplement for hair, skin, and nails, some collagen peptide or hydrolyzed collagen supplements come with claims about joint health, too. Inside our bodies, collagen helps build joints. As a supplement, the jury is still out. A systematic review of 19 studies in The International Journal of Rheumatic Diseases suggested more research is needed to determine whether collagen supplements are harmful or helpful in OA or RA. Studies haven’t shown adverse events, and doses typically range from 2.5 to 15 g/d.
Coenzyme Q10 (CoQ10). This antioxidant occurs naturally in our cells and is produced through microbial fermentation for use in dietary supplements. A study review of 20 articles including 483 patients in Clinical Nutrition ESPEN concluded that CoQ10 supplementation up to 300 mg/d was beneficial in RA, fibromyalgia, or antiphospholipid syndrome (APS).
In RA, CoQ10 supplementation improved disease activity index, ESR, and cytokine levels and decreased malondialdehyde. CoQ10 might protect against the overproduction of reactive oxygen species that can promote inflammation and joint damage, the researchers said. In fibromyalgia, CoQ10 was linked with improvements in pain, fatigue, sleep, tender points count, mood disorders, and scores on the Fibromyalgia Impact Questionnaire in most of the included studies. CoQ10 might help in fibromyalgia by improving mitochondrial dysfunction. In APS, CoQ10 improved endothelial function and decreased prothrombotic and pro-inflammatory mediators. CoQ10 might change the expression of genes that promote atherosclerosis. A few patients had GI side effects like nausea and diarrhea, but the supplements were generally well tolerated.
Melatonin. Commonly touted as a sleep aid, this hormone has immune and anti-inflammatory activities that could benefit people with rheumatologic diseases. A study review of 13 articles including 533 patients in Clinical Nutrition ESPEN concluded that melatonin can help improve sleep, pain, and mood in fibromyalgia, OA, and osteoporosis but not in RA. Side effects were minimal, but a few people experienced nausea, drowsiness, nightmares, or headaches. Doses of 5-6 mg/d are likely safe for most adults.
The Plant-Derived Antioxidants
Many supplements used in rheumatology are antioxidants derived from herbs, spices, or other plants. When plants encounter stressors, like temperature changes or hungry insects, their secondary metabolism revs up and creates compounds with biological properties. Some of these substances influence inflammatory pathways in the human body, said Luís Silva, PhD, a medicinal chemistry researcher at the Polytechnic Institute of Guarda in Portugal. “If it is possible to reduce these kinds of anti-inflammatory processes, it is also possible that we could help people with inflammatory diseases to a good life, or a better life.”
Turmeric and curcumin. You might see this supplement labeled as turmeric, a golden spice in curry powder, or curcumin, an antioxidant compound known as a curcuminoid in turmeric. Curcuminoids might reduce inflammation by scavenging free radicals and inhibiting enzymes that make prostaglandins, Silva said.
Turmeric is the most popular herbal supplement for people with RA, according to Funk’s research. A study review of six publications including 539 patients in Frontiers in Immunology showed that curcumin supplements improved RA patients’ ESR, DAS, swollen joint count, and tender joint count. Turmeric could help patients with OA, too. Patients with OA who took 1000 mg/d of curcumin improved their pain and function, according to a systematic review including 12 studies and 1438 participants in the journal Nutrients. In lupus, small studies are promising but inconclusive, suggested a study review in Frontiers in Immunology.
Watch patients taking turmeric and methotrexate closely, Funk said. Both have been associated with liver problems. Some users also experience GI symptoms like diarrhea because turmeric doesn’t absorb well in the GI tract.
Milk thistle (silymarin). This flowering plant is often marketed as a liver-supporting supplement, but research also suggests promise in RA and OA. A systematic review of 12 studies in Current Rheumatology Reviews suggested that silymarin supplements might help relieve pain, reduce inflammation, and protect the cartilage matrix, synovial membrane, and cartilage cells in joints. This supplement might help via immunomodulatory, anti-inflammatory, antioxidant, and anti-apoptotic properties, the researchers said. Doses of 250-750 mg appear to be safe. Side effects such as gastroenteritis, diarrhea, bloating, and headache can occur.
Boswellia serrata. Sourced from the resin of a tree that grows in dry, mountainous regions of Asia and Africa, Boswellia serrata can help relieve joint pain and stiffness and improve joint function in OA, suggested a systematic review of seven trials involving 545 patients in BMC Complementary Medicine and Therapies. Users saw benefits when taking 100-250 mg/d for 4 weeks or more. Compounds in Boswellia serrata may inhibit 5-lipoxygenase, an enzyme involved in producing inflammatory leukotrienes. No adverse events were reported. In some studies, users have reported GI side effects.
Ginger. Ginger is a popular herbal supplement among people with RA, Funk’s research suggested. One small clinical trial involving 70 patients with RA in the journal Gene showed that taking 1500 mg/d of ginger for 12 weeks improved their DAS and boosted their expression of FoxP3 genes, which are linked with the function of regulatory T cells. A meta-analysis including three studies with 330 patients taking ginger published in the journal Nutrients suggested ginger can reduce pain and systemic inflammation in people with OA. Preclinical studies suggested phenolic compounds in this spicy root, such as gingerols, reduce inflammation through multiple mechanisms.
Funk’s research revealed wide variation in the quality of ginger supplements, reinforcing the importance of selecting an independently verified product. Research suggested a safe dose is up to 2-2.5 g/kg body weight.
Resveratrol. Found in red grapes and red wine, this compound is particularly good at blocking COX-2 enzymes, an important step in the inflammatory cascade, Silva said. “Because of their chemical structure, they have great affinity to these enzymes to lead to their inhibition,” he said. A study review of five articles including 481 patients in The European Journal of Rheumatology showed that people with OA, RA, or Takayasu arteritis who took 250-1000 mg/d of resveratrol saw improvements in pain, function, disease activity, joint swelling, and inflammation, with no side effects.
Cinnamon. This warming spice is gaining popularity as a supplement, reported the American Botanical Council. Cinnamon is often marketed as lowering blood sugar and supporting bone health. In a small study of 36 women with RA published in The Journal of the American College of Nutrition, participants who consumed 2 g/d of cinnamon powder had reduced DASs along with reduced pain and tender and swollen joint counts. Cinnamon may reduce pain by inhibiting prostaglandin and blunt inflammation by reducing the release of arachidonic acid from cell membranes, according to a study review in Frontiers in Pharmacology. GI problems and allergic reactions are among the most common side effects.
Funk, Nikiphorou, Philippou, and Silva all had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Many people with rheumatologic diseases try supplements for symptom relief. Here’s what you need to know about some common picks.
Dietary supplements were a $159 billion business in the United States in 2023, and many people with rheumatologic diseases are buying in. Research suggests more than 6 in 10 people with fibromyalgia, nearly 8 in 10 people with Sjögren’s disease, and more than 8 in 10 people with rheumatoid arthritis (RA) take dietary supplements.
Whatever the symptom — pain, swelling, or fatigue — you can probably find a supplement purporting to relieve it. But do these supplements work, and are they safe? A study review in RMD Open comprising 24 systematic reviews and 150 original articles suggests more high-quality research is needed on the effects of dietary supplements on rheumatologic diseases. Most studies have focused on RA or osteoarthritis (OA), where the evidence level is moderate at best.
“The studies in this space are usually not very high quality because there’s no money to support them, among other things, plus the products are disparate,” said Janet Funk, MD, MS, professor in the School of Nutritional Sciences and Wellness at the University of Arizona, Tucson. She recommended brushing up on supplements and finding out what patients are taking so you can offer advice and watch for drug-supplement interactions.
When asked for a medication list, many patients forget to report supplements, Funk said. “You have to prompt them specifically. I think some physicians have very negative views about supplements because so little data is known, and patients might pick up on that and decide not to report their use.” She recommended saying something like: “To give you the best possible care, I want to know everything you’re taking, including supplements. The things I’m prescribing could maybe interact with the things you’re taking, so I want to make sure I know about all of it so that together we can figure out if the combination of things is safe.”
The quality of dietary supplements varies, and they aren’t regulated like drugs by the Food and Drug Administration. Funk recommended selecting products verified by NSF or ConsumerLab. They test supplements to ensure the label reflects what’s inside.
This news organization scoured the literature and asked experts to weigh in on the evidence behind popular supplements in rheumatology today.
The Essential Nutrients
Vitamin supplements are a staple in many homes — but are they helpful? “Individual vitamin supplements will not provide any benefit unless the person is deficient in a specific vitamin or mineral,” according to Elena Philippou, PhD, RD, associate professor of nutrition-dietetics at the University of Nicosia in Cyprus, and Elena Nikiphorou, MBBS, a rheumatologist at King’s College London in England. For some patients, deficiency is a reality. A retrospective cohort study in The Journal of Clinical Medicine found that people with RA were 17% more likely than age-matched control individuals to have nutrient deficiencies, perhaps because symptoms like fatigue, pain, and nausea affect their eating habits. Here’s what the science says about common vitamin supplements.
Vitamin D. This hormone-like vitamin, which attaches to receptors on immune cells to tamp down inflammation, was the most popular dietary supplement among rheumatology patients in a recent study from the United Kingdom. Vitamin D deficiency is common in people with RA, lupus, Sjögren’s disease, ankylosing spondylitis, systemic sclerosis, and fibromyalgia. In some cases, vitamin D levels track with disease activity, research suggests. Corticosteroids can also make vitamin D deficiency more likely. Can supplements help?
In RA, evidence points to small improvements. A systematic review of 11 studies including 3049 patients published in Nutrition Reviews showed that vitamin D supplements significantly reduced patients’ pain and Disease Activity Score in 28 joints (DAS28) using both C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
The research is mixed on the benefits of vitamin D supplementation for fibromyalgia symptoms, according to a study review in SN Comprehensive Clinical Medicine that included two studies and 80 patients on supplementation. However, researchers said it’s still worth discussing the potential benefits of taking vitamin D.
“Vitamin D supplementation is important in the context of various rheumatic diseases to prevent or treat bone disease,” said Philippou and Nikiphorou. “People with rheumatic disease should speak to their healthcare provider and ask to check their blood vitamin D concentration.” The results can help you recommend a dose.
Folate. Patients on methotrexate should take folic acid supplements under the guidance of a healthcare provider, said Philippou and Nikiphorou. The reason: Methotrexate can deplete folic acid levels, increasing the risk for side effects. An analysis of adverse event reports published showed that methotrexate users who took folic acid (or tumor necrosis factor–alpha inhibitors) had a reduced risk for hepatotoxicity or myelosuppression. A commonly recommended dose is 1 mg/d.
Vitamin B12. In a 2024 perspective paper in Rheumatology International, researchers said physicians should assess vitamin B12 levels early in the diagnostic process of rheumatologic diseases. One reason: Many symptoms of pernicious anemia, like fatigue, mimic symptoms of rheumatologic diseases. The gastrointestinal (GI) effects of systemic sclerosis could bring on vitamin B12 deficiency. In a small study in The Journal of Clinical Rheumatology, 44 of 62 patients with systemic sclerosis had low vitamin B12 levels.
Vitamin E. Vitamin E deficiency is rare in healthy adults. However, some medical conditions, like inflammatory bowel disease and malabsorption disorders, can make vitamin E deficiency more likely. In RA, a vitamin E supplement could help reduce joint swelling and sensitivity, according to a systematic review of nine studies including 39,845 patients in The European Journal of Clinical Nutrition. Researchers credit the nutrient’s role in aiding intestinal repair. Use with caution, as this supplement can increase bleeding risk in doses over 1000 mg/d.
Vitamin A. Like vitamin E, vitamin A deficiency is rare in the United States. The risk of oversupplementing is higher than undersupplementing. However, vitamin A deficiency can happen in people with chronic pancreatic, liver, or GI problems. In people with deficiency, a vitamin A supplement can help relieve dry eye symptoms common in Sjögren’s disease, suggests a narrative review published in Nutrients. Vitamin A might help reduce ocular surface changes by supporting the production of proteins that protect the outermost surfaces of the eyes. The recommended daily allowance for vitamin A is 900 μg. High-dose supplements can cause toxicity, resulting in GI symptoms and problems like lethargy, drowsiness, increased intracranial pressure, and skin changes.
The Replacements
These substances are similar to naturally occurring compounds in our bodies. The question is whether ingesting them yields benefits.
Glucosamine and chondroitin. Glucosamine and chondroitin occur naturally in our bodies and help us form and protect connective tissues. In pill form, this combo is the most popular dietary supplement for OA, according to research in the journal Maturitas. But studies of its effectiveness yield mixed results. A systematic review of 25 studies published in Inflammopharmacology showed that, in patients with knee OA, supplementation with about 1500 mg of glucosamine per day reduced tibiofemoral joint space narrowing, while supplementation with about 800 mg/d of chondroitin reduced pain intensity and improved physical function, compared with placebo. The duo of glucosamine and chondroitin did not bring significant benefits, perhaps because more studies are needed. Most side effects were mild, but some literature points to the potential for glucosamine to increase warfarin’s blood-thinning effects.
Omega-3 fatty acids. Fish oil is a top-selling supplement, and it might be helpful in inflammatory rheumatologic diseases. A systematic review of 30 studies including 710 patients published in Arthritis Research & Therapy showed that omega-3 fatty acid supplements can improve pain, swollen and tender joint count, DAS28 scores, and Health Assessment Questionnaire scores in patients with RA, psoriatic arthritis, or ankylosing spondylitis. In patients with lupus, a study review that included five studies and 284 patients in The International Journal of Environmental Research and Public Health suggested omega-3 fatty acid supplements could improve ESR, CRP, disease activity, inflammatory markers, oxidative stress, lipid levels, and endothelial function.
Omega-3 fatty acids have anti-inflammatory effects that might explain their benefits. In patients with RA, for example, fish oil supplementation was associated with elevated blood levels of resolvins and protectins, which help quell inflammation, according to a study in Prostaglandins, Leukotrienes and Essential Fatty Acids.
Philippou and Nikiphorou recommended combining food and supplements: Eat oily fish at least twice a week, regularly consume plant-based sources of omega-3s — like chia seeds, flaxseeds, or walnuts — and consider a daily supplement that contains 2 g of omega-3s from docosahexaenoic acid and eicosapentaenoic acid. Most fish oil side effects are mild, like heartburn and bad breath. Fish oil can have blood-thinning effects at high doses, so special attention is needed for patients on anticoagulants.
Probiotics. Building up the good bacteria in your gut might help you fight the effects of rheumatologic diseases. A systematic review of 80 randomized controlled trials in BMC Medicine suggested that therapies targeting the gut microbiota might improve the symptoms or inflammatory factors in celiac disease, lupus, juvenile idiopathic arthritis, psoriasis, Sjögren’s disease, multiple sclerosis, systemic sclerosis, Crohn’s disease, and ulcerative colitis. Probiotics were also shown to relieve pain in fibromyalgia, but they didn’t affect scores on the Fibromyalgia Impact Questionnaire. Probiotics were not helpful in spondyloarthritis or RA. There were no adverse events. By improving the balance of bacteria in the gut, probiotics might inhibit pro-inflammatory factors and signaling pathways and regulate CD4+ T-cell differentiation, the researchers wrote.
Not all probiotic supplements are created equal. Effects can vary by microorganism and dose. Until more high-quality studies are published, Philippou and Nikiphorou recommend daily consumption of probiotic food sources such as yogurt, kefir, sauerkraut, kimchi, tempeh, miso, and kombucha, along with prebiotic food sources such as bananas, onion, artichokes, asparagus, oats, leeks, and garlic.
Collagen. An increasingly popular supplement for hair, skin, and nails, some collagen peptide or hydrolyzed collagen supplements come with claims about joint health, too. Inside our bodies, collagen helps build joints. As a supplement, the jury is still out. A systematic review of 19 studies in The International Journal of Rheumatic Diseases suggested more research is needed to determine whether collagen supplements are harmful or helpful in OA or RA. Studies haven’t shown adverse events, and doses typically range from 2.5 to 15 g/d.
Coenzyme Q10 (CoQ10). This antioxidant occurs naturally in our cells and is produced through microbial fermentation for use in dietary supplements. A study review of 20 articles including 483 patients in Clinical Nutrition ESPEN concluded that CoQ10 supplementation up to 300 mg/d was beneficial in RA, fibromyalgia, or antiphospholipid syndrome (APS).
In RA, CoQ10 supplementation improved disease activity index, ESR, and cytokine levels and decreased malondialdehyde. CoQ10 might protect against the overproduction of reactive oxygen species that can promote inflammation and joint damage, the researchers said. In fibromyalgia, CoQ10 was linked with improvements in pain, fatigue, sleep, tender points count, mood disorders, and scores on the Fibromyalgia Impact Questionnaire in most of the included studies. CoQ10 might help in fibromyalgia by improving mitochondrial dysfunction. In APS, CoQ10 improved endothelial function and decreased prothrombotic and pro-inflammatory mediators. CoQ10 might change the expression of genes that promote atherosclerosis. A few patients had GI side effects like nausea and diarrhea, but the supplements were generally well tolerated.
Melatonin. Commonly touted as a sleep aid, this hormone has immune and anti-inflammatory activities that could benefit people with rheumatologic diseases. A study review of 13 articles including 533 patients in Clinical Nutrition ESPEN concluded that melatonin can help improve sleep, pain, and mood in fibromyalgia, OA, and osteoporosis but not in RA. Side effects were minimal, but a few people experienced nausea, drowsiness, nightmares, or headaches. Doses of 5-6 mg/d are likely safe for most adults.
The Plant-Derived Antioxidants
Many supplements used in rheumatology are antioxidants derived from herbs, spices, or other plants. When plants encounter stressors, like temperature changes or hungry insects, their secondary metabolism revs up and creates compounds with biological properties. Some of these substances influence inflammatory pathways in the human body, said Luís Silva, PhD, a medicinal chemistry researcher at the Polytechnic Institute of Guarda in Portugal. “If it is possible to reduce these kinds of anti-inflammatory processes, it is also possible that we could help people with inflammatory diseases to a good life, or a better life.”
Turmeric and curcumin. You might see this supplement labeled as turmeric, a golden spice in curry powder, or curcumin, an antioxidant compound known as a curcuminoid in turmeric. Curcuminoids might reduce inflammation by scavenging free radicals and inhibiting enzymes that make prostaglandins, Silva said.
Turmeric is the most popular herbal supplement for people with RA, according to Funk’s research. A study review of six publications including 539 patients in Frontiers in Immunology showed that curcumin supplements improved RA patients’ ESR, DAS, swollen joint count, and tender joint count. Turmeric could help patients with OA, too. Patients with OA who took 1000 mg/d of curcumin improved their pain and function, according to a systematic review including 12 studies and 1438 participants in the journal Nutrients. In lupus, small studies are promising but inconclusive, suggested a study review in Frontiers in Immunology.
Watch patients taking turmeric and methotrexate closely, Funk said. Both have been associated with liver problems. Some users also experience GI symptoms like diarrhea because turmeric doesn’t absorb well in the GI tract.
Milk thistle (silymarin). This flowering plant is often marketed as a liver-supporting supplement, but research also suggests promise in RA and OA. A systematic review of 12 studies in Current Rheumatology Reviews suggested that silymarin supplements might help relieve pain, reduce inflammation, and protect the cartilage matrix, synovial membrane, and cartilage cells in joints. This supplement might help via immunomodulatory, anti-inflammatory, antioxidant, and anti-apoptotic properties, the researchers said. Doses of 250-750 mg appear to be safe. Side effects such as gastroenteritis, diarrhea, bloating, and headache can occur.
Boswellia serrata. Sourced from the resin of a tree that grows in dry, mountainous regions of Asia and Africa, Boswellia serrata can help relieve joint pain and stiffness and improve joint function in OA, suggested a systematic review of seven trials involving 545 patients in BMC Complementary Medicine and Therapies. Users saw benefits when taking 100-250 mg/d for 4 weeks or more. Compounds in Boswellia serrata may inhibit 5-lipoxygenase, an enzyme involved in producing inflammatory leukotrienes. No adverse events were reported. In some studies, users have reported GI side effects.
Ginger. Ginger is a popular herbal supplement among people with RA, Funk’s research suggested. One small clinical trial involving 70 patients with RA in the journal Gene showed that taking 1500 mg/d of ginger for 12 weeks improved their DAS and boosted their expression of FoxP3 genes, which are linked with the function of regulatory T cells. A meta-analysis including three studies with 330 patients taking ginger published in the journal Nutrients suggested ginger can reduce pain and systemic inflammation in people with OA. Preclinical studies suggested phenolic compounds in this spicy root, such as gingerols, reduce inflammation through multiple mechanisms.
Funk’s research revealed wide variation in the quality of ginger supplements, reinforcing the importance of selecting an independently verified product. Research suggested a safe dose is up to 2-2.5 g/kg body weight.
Resveratrol. Found in red grapes and red wine, this compound is particularly good at blocking COX-2 enzymes, an important step in the inflammatory cascade, Silva said. “Because of their chemical structure, they have great affinity to these enzymes to lead to their inhibition,” he said. A study review of five articles including 481 patients in The European Journal of Rheumatology showed that people with OA, RA, or Takayasu arteritis who took 250-1000 mg/d of resveratrol saw improvements in pain, function, disease activity, joint swelling, and inflammation, with no side effects.
Cinnamon. This warming spice is gaining popularity as a supplement, reported the American Botanical Council. Cinnamon is often marketed as lowering blood sugar and supporting bone health. In a small study of 36 women with RA published in The Journal of the American College of Nutrition, participants who consumed 2 g/d of cinnamon powder had reduced DASs along with reduced pain and tender and swollen joint counts. Cinnamon may reduce pain by inhibiting prostaglandin and blunt inflammation by reducing the release of arachidonic acid from cell membranes, according to a study review in Frontiers in Pharmacology. GI problems and allergic reactions are among the most common side effects.
Funk, Nikiphorou, Philippou, and Silva all had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Cannabis Often Used as a Substitute for Traditional Medications
Nearly two thirds of patients with rheumatic conditions switched to medical cannabis from medications such as nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids, with the substitution being associated with greater self-reported improvement in symptoms than nonsubstitution.
METHODOLOGY:
- Researchers conducted a secondary analysis of a cross-sectional survey to investigate the prevalence of switching to medical cannabis from traditional medications in patients with rheumatic conditions from the United States and Canada.
- The survey included questions on current and past medical cannabis use, sociodemographic characteristics, medication taken and substituted, substance use, and patient-reported outcomes.
- Of the 1727 patients who completed the survey, 763 patients (mean age, 59 years; 84.1% women) reported current use of cannabis and were included in this analysis.
- Participants were asked if they had substituted any medications with medical cannabis and were sub-grouped accordingly.
- They also reported any changes in symptoms after initiating cannabis, the current and anticipated duration of medical cannabis use, methods of ingestion, cannabinoid content, and frequency of use.
TAKEAWAY:
- Overall, 62.5% reported substituting medical cannabis for certain medications, including NSAIDs (54.7%), opioids (48.6%), sleep aids (29.6%), muscle relaxants (25.2%), benzodiazepines (15.5%), and gabapentinoids (10.5%).
- The most common reasons given for substituting medical cannabis were fewer side effects (39%), better symptom control (27%), and fewer adverse effects (12%).
- Participants who substituted medical cannabis reported significant improvements in symptoms such as pain, sleep, joint stiffness, muscle spasms, and inflammation, and in overall health, compared with those who did not substitute it for medications.
- The substitution group was more likely to use inhalation methods (smoking and vaporizing) than the nonsubstitution group; they also used medical cannabis more frequently and preferred products containing delta-9-tetrahydrocannabinol.
IN PRACTICE:
“The changing legal status of cannabis has allowed a greater openness with more people willing to try cannabis for symptom relief. These encouraging results of medication reduction and favorable effect of [medical cannabis] require confirmation with more rigorous methods. At this time, survey information may be seen as a signal for effect, rather than sound evidence that could be applicable to those with musculoskeletal complaints in general,” the authors wrote.
SOURCE:
The study was led by Kevin F. Boehnke, PhD, University of Michigan Medical School, Ann Arbor, and was published online in ACR Open Rheumatology.
LIMITATIONS:
The cross-sectional nature of the study limited the determination of causality between medical cannabis use and symptom improvement. Moreover, the anonymous and self-reported nature of the survey at a single timepoint may have introduced recall bias. The sample predominantly consisted of older, White females, which may have limited the generalizability of the findings to other demographic groups.
DISCLOSURES:
Some authors received grant support from the National Institute on Drug Abuse and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Some others received payments, honoraria, grant funding, consulting fees, and travel support, and reported other ties with pharmaceutical companies and other institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Nearly two thirds of patients with rheumatic conditions switched to medical cannabis from medications such as nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids, with the substitution being associated with greater self-reported improvement in symptoms than nonsubstitution.
METHODOLOGY:
- Researchers conducted a secondary analysis of a cross-sectional survey to investigate the prevalence of switching to medical cannabis from traditional medications in patients with rheumatic conditions from the United States and Canada.
- The survey included questions on current and past medical cannabis use, sociodemographic characteristics, medication taken and substituted, substance use, and patient-reported outcomes.
- Of the 1727 patients who completed the survey, 763 patients (mean age, 59 years; 84.1% women) reported current use of cannabis and were included in this analysis.
- Participants were asked if they had substituted any medications with medical cannabis and were sub-grouped accordingly.
- They also reported any changes in symptoms after initiating cannabis, the current and anticipated duration of medical cannabis use, methods of ingestion, cannabinoid content, and frequency of use.
TAKEAWAY:
- Overall, 62.5% reported substituting medical cannabis for certain medications, including NSAIDs (54.7%), opioids (48.6%), sleep aids (29.6%), muscle relaxants (25.2%), benzodiazepines (15.5%), and gabapentinoids (10.5%).
- The most common reasons given for substituting medical cannabis were fewer side effects (39%), better symptom control (27%), and fewer adverse effects (12%).
- Participants who substituted medical cannabis reported significant improvements in symptoms such as pain, sleep, joint stiffness, muscle spasms, and inflammation, and in overall health, compared with those who did not substitute it for medications.
- The substitution group was more likely to use inhalation methods (smoking and vaporizing) than the nonsubstitution group; they also used medical cannabis more frequently and preferred products containing delta-9-tetrahydrocannabinol.
IN PRACTICE:
“The changing legal status of cannabis has allowed a greater openness with more people willing to try cannabis for symptom relief. These encouraging results of medication reduction and favorable effect of [medical cannabis] require confirmation with more rigorous methods. At this time, survey information may be seen as a signal for effect, rather than sound evidence that could be applicable to those with musculoskeletal complaints in general,” the authors wrote.
SOURCE:
The study was led by Kevin F. Boehnke, PhD, University of Michigan Medical School, Ann Arbor, and was published online in ACR Open Rheumatology.
LIMITATIONS:
The cross-sectional nature of the study limited the determination of causality between medical cannabis use and symptom improvement. Moreover, the anonymous and self-reported nature of the survey at a single timepoint may have introduced recall bias. The sample predominantly consisted of older, White females, which may have limited the generalizability of the findings to other demographic groups.
DISCLOSURES:
Some authors received grant support from the National Institute on Drug Abuse and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Some others received payments, honoraria, grant funding, consulting fees, and travel support, and reported other ties with pharmaceutical companies and other institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Nearly two thirds of patients with rheumatic conditions switched to medical cannabis from medications such as nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids, with the substitution being associated with greater self-reported improvement in symptoms than nonsubstitution.
METHODOLOGY:
- Researchers conducted a secondary analysis of a cross-sectional survey to investigate the prevalence of switching to medical cannabis from traditional medications in patients with rheumatic conditions from the United States and Canada.
- The survey included questions on current and past medical cannabis use, sociodemographic characteristics, medication taken and substituted, substance use, and patient-reported outcomes.
- Of the 1727 patients who completed the survey, 763 patients (mean age, 59 years; 84.1% women) reported current use of cannabis and were included in this analysis.
- Participants were asked if they had substituted any medications with medical cannabis and were sub-grouped accordingly.
- They also reported any changes in symptoms after initiating cannabis, the current and anticipated duration of medical cannabis use, methods of ingestion, cannabinoid content, and frequency of use.
TAKEAWAY:
- Overall, 62.5% reported substituting medical cannabis for certain medications, including NSAIDs (54.7%), opioids (48.6%), sleep aids (29.6%), muscle relaxants (25.2%), benzodiazepines (15.5%), and gabapentinoids (10.5%).
- The most common reasons given for substituting medical cannabis were fewer side effects (39%), better symptom control (27%), and fewer adverse effects (12%).
- Participants who substituted medical cannabis reported significant improvements in symptoms such as pain, sleep, joint stiffness, muscle spasms, and inflammation, and in overall health, compared with those who did not substitute it for medications.
- The substitution group was more likely to use inhalation methods (smoking and vaporizing) than the nonsubstitution group; they also used medical cannabis more frequently and preferred products containing delta-9-tetrahydrocannabinol.
IN PRACTICE:
“The changing legal status of cannabis has allowed a greater openness with more people willing to try cannabis for symptom relief. These encouraging results of medication reduction and favorable effect of [medical cannabis] require confirmation with more rigorous methods. At this time, survey information may be seen as a signal for effect, rather than sound evidence that could be applicable to those with musculoskeletal complaints in general,” the authors wrote.
SOURCE:
The study was led by Kevin F. Boehnke, PhD, University of Michigan Medical School, Ann Arbor, and was published online in ACR Open Rheumatology.
LIMITATIONS:
The cross-sectional nature of the study limited the determination of causality between medical cannabis use and symptom improvement. Moreover, the anonymous and self-reported nature of the survey at a single timepoint may have introduced recall bias. The sample predominantly consisted of older, White females, which may have limited the generalizability of the findings to other demographic groups.
DISCLOSURES:
Some authors received grant support from the National Institute on Drug Abuse and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Some others received payments, honoraria, grant funding, consulting fees, and travel support, and reported other ties with pharmaceutical companies and other institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Total Hip Replacement Superior to Exercise Therapy for Improving Hip Osteoarthritis Pain and Function
For people with severe symptomatic hip osteoarthritis, total hip replacement (THR) alleviates hip pain and improves function much more effectively than a resistance training program supervised by a physiotherapist, according to the results of a randomized controlled clinical trial.
In the PROHIP study, the mean increases in Oxford Hip Scores from baseline to 6 months were 15.9 points for THR and 4.5 points for resistance training. The 11.4-point difference in scores was both statistically and clinically significant, the study’s investigators reported in The New England Journal of Medicine.
“Our results are clear: Surgery is superior to exercise in patients who have hip osteoarthritis and indication for surgery, and now we have finally proven that with the highest level of evidence,” corresponding author Thomas Frydendal, PT, PhD, MSc, told this news organization.
Frydendal, who was involved in the study while working on his PhD at University Hospital of Southern Denmark – Lillebaelt Hospital, Vejle, Denmark, the primary center for the trial, is now a postdoctoral researcher at the Department of Clinical Medicine, Aarhus University, and Department of Orthopedic Surgery, Aarhus University Hospital.
“We believe that our findings are pretty robust,” Frydendal added. “I think if someone in the world conducts a trial similar to ours, they will find fairly close or consistent findings, no matter what type of exercise they choose.”
The PROHIP Study
THR is routinely recommended for the management of severe hip osteoarthritis, but since there are no clinical trial data on the effectiveness of this procedure as compared with first-line treatment such as resistance training, the PROHIP study was conceived.
The trial was conducted at four Danish orthopedic centers and designed as a superiority study, the hypothesis being that THR would be better at alleviating self-reported hip pain and improving hip function than resistance training.
Of a possible 1474 individuals with a clinical suspicion of hip osteoarthritis, 791 were deemed eligible for inclusion in the trial. Inclusion criteria were being aged 50 years or older and having an indication for THR based on the presence of hip pain and clinical and radiographic findings.
However, the majority (86%) declined to enter the study, with almost half (43%) deciding to have a THR and enroll in a parallel observational cohort. This meant that only 110 (14%) individuals agreed to participate and underwent randomization, which does limit the study’s generalizability, the PROHIP investigators acknowledged.
Design and Study Population
The change in Oxford Hip Score from baseline to 6 months was selected as the primary outcome measure based on the findings of a prior qualitative study. This 12-item, patient-reported outcome measure gives a score ranging from 0 to 48, with higher scores indicating less hip pain and better hip function. The estimated minimal clinically important difference is a change of 5 points.
After a baseline assessment, 53 of 109 individuals were randomly assigned to undergo THR and 56 to participate in the resistance training program. Overall, the mean age of participants was 67.6 years, and half were women. The average duration of hip pain was a median of 1.7 years.
The median time to receipt of the allocated treatment was 2.8 months in the THR group and 0.5 months in the resistance training group.
Those allocated to the THR group also underwent a “fast track” program that involved patient education, pain management, and early mobilization.
The resistance training group received 12 weeks of exercise supervised by a physiotherapist and then offered 12 weeks of additional exercise conducted on their own. The physiotherapist-supervised exercise sessions were held twice weekly and lasted for 1 hour. These started with a 10-minute warm-up on a stationary bike, followed by a standard set of resistance-based exercises that included a leg press, hip extension, hip flexion, and hip abduction.
‘Reassuring’ Results
In a comment, consultant orthopedic surgeon Antony Palmer, MA, BMBCh, DPhil, said: “It’s reassuring that patients with advanced symptomatic osteoarthritis do well with hip replacements.”
THR does of course come with the potential risk for complications, but “the rate of these is what you’d expect for that procedure,” Palmer said, who works for the Nuffield Orthopaedic Centre, Oxford University Hospital NHS Foundation Trust, and is a senior clinical research fellow at Oxford University in England.
In the THR arm, there was one case of prosthetic joint infection, one hip dislocation, two revision surgeries, one instance of foot drop, and one case of gastroesophageal reflux. Meanwhile, in the resistance training group, there was one hip dislocation, one pelvic fracture, one case of atrial fibrillation, and one urinary tract and renal infection.
Overall, any serious adverse event was reported in six (12%) of 48 patients in the THR arm vs five (9%) of 55 participants in the resistance training group, of which only one, occurring in the resistance training group, resulted in discontinuation of the program.
Resistance Training Role
A notable finding was that, at 6 months, five (9%) people assigned to the THR arm had not undergone surgery, and 12 (21%) people in the resistance training group had undergone a THR.
This could suggest two things, Palmer suggested in the interview. The first is that there could be a small proportion of people assigned to THR who may not need the operation and do well with exercise therapy. And, conversely, there may be those who would do well having the surgery without first going through the intermediate stage of physical therapy.
It’s a suggestion that “maybe we’ve got to refine that a bit better and identify the patients that really do benefit from physiotherapy and who might not need hip replacement as a result,” Palmer said.
Or in other words, “should all patients undergo a program of physiotherapy before considering surgery?” he added.
Authors’ View
The PROHIP investigators conclude: “These results support current recommendations for the management of hip osteoarthritis and may be used to inform and guide shared decision making in clinical practice.”
Moreover, the results “do not oppose the use of resistance training as initial treatment,” says the authors.
Frydendal highlighted in his interview that nearly three out of four of the patients reported not to have undertaken any type of supervised exercise before entry into the study, which is a first-line, guideline-recommended option.
“If a patient tells me, ‘I haven’t done any exercise previously,’ I’d recommend starting with completing a 6- to 12-week exercise program that is tailored to your individual needs and evaluate your symptoms afterward,” he said.
“But we should refer the patient if our first-line treatment does not offer any improvements in the patient’s symptoms, as surgery with total hip replacement is clearly a really good treatment option,” Frydendal said.
The study was funded by the Danish Rheumatism Association, among other independent bodies. Frydendal and Palmer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For people with severe symptomatic hip osteoarthritis, total hip replacement (THR) alleviates hip pain and improves function much more effectively than a resistance training program supervised by a physiotherapist, according to the results of a randomized controlled clinical trial.
In the PROHIP study, the mean increases in Oxford Hip Scores from baseline to 6 months were 15.9 points for THR and 4.5 points for resistance training. The 11.4-point difference in scores was both statistically and clinically significant, the study’s investigators reported in The New England Journal of Medicine.
“Our results are clear: Surgery is superior to exercise in patients who have hip osteoarthritis and indication for surgery, and now we have finally proven that with the highest level of evidence,” corresponding author Thomas Frydendal, PT, PhD, MSc, told this news organization.
Frydendal, who was involved in the study while working on his PhD at University Hospital of Southern Denmark – Lillebaelt Hospital, Vejle, Denmark, the primary center for the trial, is now a postdoctoral researcher at the Department of Clinical Medicine, Aarhus University, and Department of Orthopedic Surgery, Aarhus University Hospital.
“We believe that our findings are pretty robust,” Frydendal added. “I think if someone in the world conducts a trial similar to ours, they will find fairly close or consistent findings, no matter what type of exercise they choose.”
The PROHIP Study
THR is routinely recommended for the management of severe hip osteoarthritis, but since there are no clinical trial data on the effectiveness of this procedure as compared with first-line treatment such as resistance training, the PROHIP study was conceived.
The trial was conducted at four Danish orthopedic centers and designed as a superiority study, the hypothesis being that THR would be better at alleviating self-reported hip pain and improving hip function than resistance training.
Of a possible 1474 individuals with a clinical suspicion of hip osteoarthritis, 791 were deemed eligible for inclusion in the trial. Inclusion criteria were being aged 50 years or older and having an indication for THR based on the presence of hip pain and clinical and radiographic findings.
However, the majority (86%) declined to enter the study, with almost half (43%) deciding to have a THR and enroll in a parallel observational cohort. This meant that only 110 (14%) individuals agreed to participate and underwent randomization, which does limit the study’s generalizability, the PROHIP investigators acknowledged.
Design and Study Population
The change in Oxford Hip Score from baseline to 6 months was selected as the primary outcome measure based on the findings of a prior qualitative study. This 12-item, patient-reported outcome measure gives a score ranging from 0 to 48, with higher scores indicating less hip pain and better hip function. The estimated minimal clinically important difference is a change of 5 points.
After a baseline assessment, 53 of 109 individuals were randomly assigned to undergo THR and 56 to participate in the resistance training program. Overall, the mean age of participants was 67.6 years, and half were women. The average duration of hip pain was a median of 1.7 years.
The median time to receipt of the allocated treatment was 2.8 months in the THR group and 0.5 months in the resistance training group.
Those allocated to the THR group also underwent a “fast track” program that involved patient education, pain management, and early mobilization.
The resistance training group received 12 weeks of exercise supervised by a physiotherapist and then offered 12 weeks of additional exercise conducted on their own. The physiotherapist-supervised exercise sessions were held twice weekly and lasted for 1 hour. These started with a 10-minute warm-up on a stationary bike, followed by a standard set of resistance-based exercises that included a leg press, hip extension, hip flexion, and hip abduction.
‘Reassuring’ Results
In a comment, consultant orthopedic surgeon Antony Palmer, MA, BMBCh, DPhil, said: “It’s reassuring that patients with advanced symptomatic osteoarthritis do well with hip replacements.”
THR does of course come with the potential risk for complications, but “the rate of these is what you’d expect for that procedure,” Palmer said, who works for the Nuffield Orthopaedic Centre, Oxford University Hospital NHS Foundation Trust, and is a senior clinical research fellow at Oxford University in England.
In the THR arm, there was one case of prosthetic joint infection, one hip dislocation, two revision surgeries, one instance of foot drop, and one case of gastroesophageal reflux. Meanwhile, in the resistance training group, there was one hip dislocation, one pelvic fracture, one case of atrial fibrillation, and one urinary tract and renal infection.
Overall, any serious adverse event was reported in six (12%) of 48 patients in the THR arm vs five (9%) of 55 participants in the resistance training group, of which only one, occurring in the resistance training group, resulted in discontinuation of the program.
Resistance Training Role
A notable finding was that, at 6 months, five (9%) people assigned to the THR arm had not undergone surgery, and 12 (21%) people in the resistance training group had undergone a THR.
This could suggest two things, Palmer suggested in the interview. The first is that there could be a small proportion of people assigned to THR who may not need the operation and do well with exercise therapy. And, conversely, there may be those who would do well having the surgery without first going through the intermediate stage of physical therapy.
It’s a suggestion that “maybe we’ve got to refine that a bit better and identify the patients that really do benefit from physiotherapy and who might not need hip replacement as a result,” Palmer said.
Or in other words, “should all patients undergo a program of physiotherapy before considering surgery?” he added.
Authors’ View
The PROHIP investigators conclude: “These results support current recommendations for the management of hip osteoarthritis and may be used to inform and guide shared decision making in clinical practice.”
Moreover, the results “do not oppose the use of resistance training as initial treatment,” says the authors.
Frydendal highlighted in his interview that nearly three out of four of the patients reported not to have undertaken any type of supervised exercise before entry into the study, which is a first-line, guideline-recommended option.
“If a patient tells me, ‘I haven’t done any exercise previously,’ I’d recommend starting with completing a 6- to 12-week exercise program that is tailored to your individual needs and evaluate your symptoms afterward,” he said.
“But we should refer the patient if our first-line treatment does not offer any improvements in the patient’s symptoms, as surgery with total hip replacement is clearly a really good treatment option,” Frydendal said.
The study was funded by the Danish Rheumatism Association, among other independent bodies. Frydendal and Palmer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For people with severe symptomatic hip osteoarthritis, total hip replacement (THR) alleviates hip pain and improves function much more effectively than a resistance training program supervised by a physiotherapist, according to the results of a randomized controlled clinical trial.
In the PROHIP study, the mean increases in Oxford Hip Scores from baseline to 6 months were 15.9 points for THR and 4.5 points for resistance training. The 11.4-point difference in scores was both statistically and clinically significant, the study’s investigators reported in The New England Journal of Medicine.
“Our results are clear: Surgery is superior to exercise in patients who have hip osteoarthritis and indication for surgery, and now we have finally proven that with the highest level of evidence,” corresponding author Thomas Frydendal, PT, PhD, MSc, told this news organization.
Frydendal, who was involved in the study while working on his PhD at University Hospital of Southern Denmark – Lillebaelt Hospital, Vejle, Denmark, the primary center for the trial, is now a postdoctoral researcher at the Department of Clinical Medicine, Aarhus University, and Department of Orthopedic Surgery, Aarhus University Hospital.
“We believe that our findings are pretty robust,” Frydendal added. “I think if someone in the world conducts a trial similar to ours, they will find fairly close or consistent findings, no matter what type of exercise they choose.”
The PROHIP Study
THR is routinely recommended for the management of severe hip osteoarthritis, but since there are no clinical trial data on the effectiveness of this procedure as compared with first-line treatment such as resistance training, the PROHIP study was conceived.
The trial was conducted at four Danish orthopedic centers and designed as a superiority study, the hypothesis being that THR would be better at alleviating self-reported hip pain and improving hip function than resistance training.
Of a possible 1474 individuals with a clinical suspicion of hip osteoarthritis, 791 were deemed eligible for inclusion in the trial. Inclusion criteria were being aged 50 years or older and having an indication for THR based on the presence of hip pain and clinical and radiographic findings.
However, the majority (86%) declined to enter the study, with almost half (43%) deciding to have a THR and enroll in a parallel observational cohort. This meant that only 110 (14%) individuals agreed to participate and underwent randomization, which does limit the study’s generalizability, the PROHIP investigators acknowledged.
Design and Study Population
The change in Oxford Hip Score from baseline to 6 months was selected as the primary outcome measure based on the findings of a prior qualitative study. This 12-item, patient-reported outcome measure gives a score ranging from 0 to 48, with higher scores indicating less hip pain and better hip function. The estimated minimal clinically important difference is a change of 5 points.
After a baseline assessment, 53 of 109 individuals were randomly assigned to undergo THR and 56 to participate in the resistance training program. Overall, the mean age of participants was 67.6 years, and half were women. The average duration of hip pain was a median of 1.7 years.
The median time to receipt of the allocated treatment was 2.8 months in the THR group and 0.5 months in the resistance training group.
Those allocated to the THR group also underwent a “fast track” program that involved patient education, pain management, and early mobilization.
The resistance training group received 12 weeks of exercise supervised by a physiotherapist and then offered 12 weeks of additional exercise conducted on their own. The physiotherapist-supervised exercise sessions were held twice weekly and lasted for 1 hour. These started with a 10-minute warm-up on a stationary bike, followed by a standard set of resistance-based exercises that included a leg press, hip extension, hip flexion, and hip abduction.
‘Reassuring’ Results
In a comment, consultant orthopedic surgeon Antony Palmer, MA, BMBCh, DPhil, said: “It’s reassuring that patients with advanced symptomatic osteoarthritis do well with hip replacements.”
THR does of course come with the potential risk for complications, but “the rate of these is what you’d expect for that procedure,” Palmer said, who works for the Nuffield Orthopaedic Centre, Oxford University Hospital NHS Foundation Trust, and is a senior clinical research fellow at Oxford University in England.
In the THR arm, there was one case of prosthetic joint infection, one hip dislocation, two revision surgeries, one instance of foot drop, and one case of gastroesophageal reflux. Meanwhile, in the resistance training group, there was one hip dislocation, one pelvic fracture, one case of atrial fibrillation, and one urinary tract and renal infection.
Overall, any serious adverse event was reported in six (12%) of 48 patients in the THR arm vs five (9%) of 55 participants in the resistance training group, of which only one, occurring in the resistance training group, resulted in discontinuation of the program.
Resistance Training Role
A notable finding was that, at 6 months, five (9%) people assigned to the THR arm had not undergone surgery, and 12 (21%) people in the resistance training group had undergone a THR.
This could suggest two things, Palmer suggested in the interview. The first is that there could be a small proportion of people assigned to THR who may not need the operation and do well with exercise therapy. And, conversely, there may be those who would do well having the surgery without first going through the intermediate stage of physical therapy.
It’s a suggestion that “maybe we’ve got to refine that a bit better and identify the patients that really do benefit from physiotherapy and who might not need hip replacement as a result,” Palmer said.
Or in other words, “should all patients undergo a program of physiotherapy before considering surgery?” he added.
Authors’ View
The PROHIP investigators conclude: “These results support current recommendations for the management of hip osteoarthritis and may be used to inform and guide shared decision making in clinical practice.”
Moreover, the results “do not oppose the use of resistance training as initial treatment,” says the authors.
Frydendal highlighted in his interview that nearly three out of four of the patients reported not to have undertaken any type of supervised exercise before entry into the study, which is a first-line, guideline-recommended option.
“If a patient tells me, ‘I haven’t done any exercise previously,’ I’d recommend starting with completing a 6- to 12-week exercise program that is tailored to your individual needs and evaluate your symptoms afterward,” he said.
“But we should refer the patient if our first-line treatment does not offer any improvements in the patient’s symptoms, as surgery with total hip replacement is clearly a really good treatment option,” Frydendal said.
The study was funded by the Danish Rheumatism Association, among other independent bodies. Frydendal and Palmer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Extended-Release Fluticasone Injection Successful in Phase 2 Knee OA Trial
TOPLINE:
The extended-release fluticasone propionate injection (EP-104IAR) significantly reduces knee osteoarthritis (OA) pain over 12 weeks, compared with a vehicle control, with no serious treatment-related adverse events.
METHODOLOGY:
- EP-104IAR utilizes a novel diffusion-based extended-release technology to optimize the action of fluticasone propionate.
- The researchers conducted a phase 2 trial at 12 research sites in Denmark, Poland, and the Czech Republic to assess the clinical efficacy, pharmacokinetics, and safety of EP-104IAR in 318 participants (58% women; 99% White) with a diagnosis of primary knee OA.
- Eligible patients, with a score of at least 4 out of 10 on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain rating scale, were randomly assigned to receive either 25 mg EP-104IAR (n = 163; mean age, 64 years) or a vehicle control (n = 155; mean age, 63.2 years).
- The primary outcome was the between-group difference in the change in the WOMAC pain score from baseline to week 12.
TAKEAWAY:
- The reduction in WOMAC pain scores from baseline to week 12 was significantly higher with EP-104IAR than with a vehicle control (between-group difference, −0.66; P = .0044), with the difference maintained through week 14.
- The treatment resulted in a significant improvement in WOMAC function scores (P = .014) and the area under the curve for changes in the WOMAC pain score (P < .0001) over 12 weeks.
- Treatment-emergent adverse events were noted in 9% of participants in the EP-104IAR group and 7% of participants in the vehicle control group. No serious treatment-related adverse events or discontinuations related to EP-104IAR were reported.
- Fluticasone propionate levels were maintained at around 66% to 33% of peak values between weeks 2 and 24 at near-constant levels. The effects on glucose and cortisol levels were minimal and transient.
IN PRACTICE:
“The results of this trial show that EP-104IAR has the potential for clinically meaningful benefit in reducing knee osteoarthritis pain, addressing a substantial unmet medical need,” the authors wrote. “Additionally, the stable delivery of fluticasone propionate over an extended period with fewer systemic and local side effects than other corticosteroid treatments for knee osteoarthritis support the possibility of bilateral and repeat dosing.”
SOURCE:
The study was led by Amanda Malone, PhD, Eupraxia Pharmaceuticals, Victoria, British Columbia, Canada. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study’s generalizability may be limited because of the predominantly White participant population. The success of masking was not evaluated, and the treatment was administered by an unmasked injector. Efficacy outcomes were patient-reported, with no objective measurement of knee function.
DISCLOSURES:
This study was supported by Eupraxia Pharmaceuticals. Some authors disclosed their employment with Eupraxia Pharmaceuticals or with companies contracted by Eupraxia Pharmaceuticals for clinical research and trial and data management. One author reported serving as a consultant or participating in a speakers’ bureau. Another reported being on the board of directors for Eupraxia Pharmaceuticals and receiving royalties from a medical technology company.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
The extended-release fluticasone propionate injection (EP-104IAR) significantly reduces knee osteoarthritis (OA) pain over 12 weeks, compared with a vehicle control, with no serious treatment-related adverse events.
METHODOLOGY:
- EP-104IAR utilizes a novel diffusion-based extended-release technology to optimize the action of fluticasone propionate.
- The researchers conducted a phase 2 trial at 12 research sites in Denmark, Poland, and the Czech Republic to assess the clinical efficacy, pharmacokinetics, and safety of EP-104IAR in 318 participants (58% women; 99% White) with a diagnosis of primary knee OA.
- Eligible patients, with a score of at least 4 out of 10 on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain rating scale, were randomly assigned to receive either 25 mg EP-104IAR (n = 163; mean age, 64 years) or a vehicle control (n = 155; mean age, 63.2 years).
- The primary outcome was the between-group difference in the change in the WOMAC pain score from baseline to week 12.
TAKEAWAY:
- The reduction in WOMAC pain scores from baseline to week 12 was significantly higher with EP-104IAR than with a vehicle control (between-group difference, −0.66; P = .0044), with the difference maintained through week 14.
- The treatment resulted in a significant improvement in WOMAC function scores (P = .014) and the area under the curve for changes in the WOMAC pain score (P < .0001) over 12 weeks.
- Treatment-emergent adverse events were noted in 9% of participants in the EP-104IAR group and 7% of participants in the vehicle control group. No serious treatment-related adverse events or discontinuations related to EP-104IAR were reported.
- Fluticasone propionate levels were maintained at around 66% to 33% of peak values between weeks 2 and 24 at near-constant levels. The effects on glucose and cortisol levels were minimal and transient.
IN PRACTICE:
“The results of this trial show that EP-104IAR has the potential for clinically meaningful benefit in reducing knee osteoarthritis pain, addressing a substantial unmet medical need,” the authors wrote. “Additionally, the stable delivery of fluticasone propionate over an extended period with fewer systemic and local side effects than other corticosteroid treatments for knee osteoarthritis support the possibility of bilateral and repeat dosing.”
SOURCE:
The study was led by Amanda Malone, PhD, Eupraxia Pharmaceuticals, Victoria, British Columbia, Canada. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study’s generalizability may be limited because of the predominantly White participant population. The success of masking was not evaluated, and the treatment was administered by an unmasked injector. Efficacy outcomes were patient-reported, with no objective measurement of knee function.
DISCLOSURES:
This study was supported by Eupraxia Pharmaceuticals. Some authors disclosed their employment with Eupraxia Pharmaceuticals or with companies contracted by Eupraxia Pharmaceuticals for clinical research and trial and data management. One author reported serving as a consultant or participating in a speakers’ bureau. Another reported being on the board of directors for Eupraxia Pharmaceuticals and receiving royalties from a medical technology company.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
The extended-release fluticasone propionate injection (EP-104IAR) significantly reduces knee osteoarthritis (OA) pain over 12 weeks, compared with a vehicle control, with no serious treatment-related adverse events.
METHODOLOGY:
- EP-104IAR utilizes a novel diffusion-based extended-release technology to optimize the action of fluticasone propionate.
- The researchers conducted a phase 2 trial at 12 research sites in Denmark, Poland, and the Czech Republic to assess the clinical efficacy, pharmacokinetics, and safety of EP-104IAR in 318 participants (58% women; 99% White) with a diagnosis of primary knee OA.
- Eligible patients, with a score of at least 4 out of 10 on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain rating scale, were randomly assigned to receive either 25 mg EP-104IAR (n = 163; mean age, 64 years) or a vehicle control (n = 155; mean age, 63.2 years).
- The primary outcome was the between-group difference in the change in the WOMAC pain score from baseline to week 12.
TAKEAWAY:
- The reduction in WOMAC pain scores from baseline to week 12 was significantly higher with EP-104IAR than with a vehicle control (between-group difference, −0.66; P = .0044), with the difference maintained through week 14.
- The treatment resulted in a significant improvement in WOMAC function scores (P = .014) and the area under the curve for changes in the WOMAC pain score (P < .0001) over 12 weeks.
- Treatment-emergent adverse events were noted in 9% of participants in the EP-104IAR group and 7% of participants in the vehicle control group. No serious treatment-related adverse events or discontinuations related to EP-104IAR were reported.
- Fluticasone propionate levels were maintained at around 66% to 33% of peak values between weeks 2 and 24 at near-constant levels. The effects on glucose and cortisol levels were minimal and transient.
IN PRACTICE:
“The results of this trial show that EP-104IAR has the potential for clinically meaningful benefit in reducing knee osteoarthritis pain, addressing a substantial unmet medical need,” the authors wrote. “Additionally, the stable delivery of fluticasone propionate over an extended period with fewer systemic and local side effects than other corticosteroid treatments for knee osteoarthritis support the possibility of bilateral and repeat dosing.”
SOURCE:
The study was led by Amanda Malone, PhD, Eupraxia Pharmaceuticals, Victoria, British Columbia, Canada. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study’s generalizability may be limited because of the predominantly White participant population. The success of masking was not evaluated, and the treatment was administered by an unmasked injector. Efficacy outcomes were patient-reported, with no objective measurement of knee function.
DISCLOSURES:
This study was supported by Eupraxia Pharmaceuticals. Some authors disclosed their employment with Eupraxia Pharmaceuticals or with companies contracted by Eupraxia Pharmaceuticals for clinical research and trial and data management. One author reported serving as a consultant or participating in a speakers’ bureau. Another reported being on the board of directors for Eupraxia Pharmaceuticals and receiving royalties from a medical technology company.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Clinical Updates on Osteoarthritis of the Hip
As primary care doctors, we diagnosis and treat many patients with osteoarthritis. In fact, according to World Health Organization statistics, approximately 528 million people around the world suffer from some form of this type of arthritis. With the aging of the population and the obesity epidemic, the rate of osteoarthritis has increased 113% since 1990 and is predicted to continue to rise.
While the knee is the most commonly affected joint, osteoarthritis also frequently affects the hands and hips. The American Academy of Orthopaedic Surgeons issued guidelines concerning the management of osteoarthritis of the hip. The clinical guidelines are aimed at orthopedists, but it is important for primary care doctors be aware of them as well since we are the physicians who usually diagnose the disease, manage it in its early stages, and follow the patients with and after the orthopedist has undertaken any procedures.
While the complete set of guidelines is 80 pages long, strong recommendations have been made that everyone should be aware of. The role of non-steroidal anti-inflammatory drugs has been reconfirmed as a modality to improve pain and function. A recommendation against using intra-articular hyaluronic acid in the hip was made as the evidence shows it did not improve pain or function better than placebo. Conversely, intra-articular corticosteroids were shown to improve pain and function in the short-term and many primary care doctors provide this treatment in their practice.
These guidelines do a great job covering the totality of management of osteoarthritis of the hip, from conservation management to surgical and post-surgical treatments. Patients often come to us with their questions so not only is it important to know the evidence for what we do in our practices, we need to know what our orthopedic colleagues are doing. We will be the ones asked to do the pre-operative evaluations on these patients, so we need to understand the procedure and its risks. We will also manage these patients post-operatively and need to be aware of what the evidence shows.
Opioid use is also covered in the guidelines: they recommend against the use of opioids to control pain in these patients. In the age of the opioid epidemic, it is a good reminder to be cautious with these meds. It is also a good time to stress smoking cessation with patients.
The guidelines discuss adverse outcomes in patients with diabetes and/or obesity. As primary care physicians, we need to be aware of those risks and be sure our patients are medically optimized before signing that pre-operative form.
A new feature of these guidelines is a discussion on social detriments to health. This is important for many diseases that we treat and we often don’t realize the impact they can have on a patient’s health and recovery. Even if we know a patient would benefit from physical therapy, it doesn’t help them if the patient has no way to get to the appointment. Some patients have copays for every physical therapy session and just can’t afford it. Knowing what the patient needs medically is not enough. We need to understand how they can access that care. Some patients have no one to help them after hip surgery and may avoid doing it for that reason. As primary care doctors, we should be helping our patients access the care they need.
We need to be able to say that a procedure needs to be delayed in the face of poorly controlled disease, such as diabetes. As the rates of osteoarthritis continue to rise, we need to understand that it is not an inevitable age-based occurrence in a patient’s life but rather an inflammatory disease that causes great pain and dysfunction. Utilizing these guidelines and working with our orthopedic colleagues can help patients decrease pain, improve functioning, and enjoy life again.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She was paid by Pfizer as a consultant on Paxlovid, paid by GlaxoSmithKline as a consultant for the Shingrix vaccine, and is the editor in chief of Physician’s Weekly.
As primary care doctors, we diagnosis and treat many patients with osteoarthritis. In fact, according to World Health Organization statistics, approximately 528 million people around the world suffer from some form of this type of arthritis. With the aging of the population and the obesity epidemic, the rate of osteoarthritis has increased 113% since 1990 and is predicted to continue to rise.
While the knee is the most commonly affected joint, osteoarthritis also frequently affects the hands and hips. The American Academy of Orthopaedic Surgeons issued guidelines concerning the management of osteoarthritis of the hip. The clinical guidelines are aimed at orthopedists, but it is important for primary care doctors be aware of them as well since we are the physicians who usually diagnose the disease, manage it in its early stages, and follow the patients with and after the orthopedist has undertaken any procedures.
While the complete set of guidelines is 80 pages long, strong recommendations have been made that everyone should be aware of. The role of non-steroidal anti-inflammatory drugs has been reconfirmed as a modality to improve pain and function. A recommendation against using intra-articular hyaluronic acid in the hip was made as the evidence shows it did not improve pain or function better than placebo. Conversely, intra-articular corticosteroids were shown to improve pain and function in the short-term and many primary care doctors provide this treatment in their practice.
These guidelines do a great job covering the totality of management of osteoarthritis of the hip, from conservation management to surgical and post-surgical treatments. Patients often come to us with their questions so not only is it important to know the evidence for what we do in our practices, we need to know what our orthopedic colleagues are doing. We will be the ones asked to do the pre-operative evaluations on these patients, so we need to understand the procedure and its risks. We will also manage these patients post-operatively and need to be aware of what the evidence shows.
Opioid use is also covered in the guidelines: they recommend against the use of opioids to control pain in these patients. In the age of the opioid epidemic, it is a good reminder to be cautious with these meds. It is also a good time to stress smoking cessation with patients.
The guidelines discuss adverse outcomes in patients with diabetes and/or obesity. As primary care physicians, we need to be aware of those risks and be sure our patients are medically optimized before signing that pre-operative form.
A new feature of these guidelines is a discussion on social detriments to health. This is important for many diseases that we treat and we often don’t realize the impact they can have on a patient’s health and recovery. Even if we know a patient would benefit from physical therapy, it doesn’t help them if the patient has no way to get to the appointment. Some patients have copays for every physical therapy session and just can’t afford it. Knowing what the patient needs medically is not enough. We need to understand how they can access that care. Some patients have no one to help them after hip surgery and may avoid doing it for that reason. As primary care doctors, we should be helping our patients access the care they need.
We need to be able to say that a procedure needs to be delayed in the face of poorly controlled disease, such as diabetes. As the rates of osteoarthritis continue to rise, we need to understand that it is not an inevitable age-based occurrence in a patient’s life but rather an inflammatory disease that causes great pain and dysfunction. Utilizing these guidelines and working with our orthopedic colleagues can help patients decrease pain, improve functioning, and enjoy life again.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She was paid by Pfizer as a consultant on Paxlovid, paid by GlaxoSmithKline as a consultant for the Shingrix vaccine, and is the editor in chief of Physician’s Weekly.
As primary care doctors, we diagnosis and treat many patients with osteoarthritis. In fact, according to World Health Organization statistics, approximately 528 million people around the world suffer from some form of this type of arthritis. With the aging of the population and the obesity epidemic, the rate of osteoarthritis has increased 113% since 1990 and is predicted to continue to rise.
While the knee is the most commonly affected joint, osteoarthritis also frequently affects the hands and hips. The American Academy of Orthopaedic Surgeons issued guidelines concerning the management of osteoarthritis of the hip. The clinical guidelines are aimed at orthopedists, but it is important for primary care doctors be aware of them as well since we are the physicians who usually diagnose the disease, manage it in its early stages, and follow the patients with and after the orthopedist has undertaken any procedures.
While the complete set of guidelines is 80 pages long, strong recommendations have been made that everyone should be aware of. The role of non-steroidal anti-inflammatory drugs has been reconfirmed as a modality to improve pain and function. A recommendation against using intra-articular hyaluronic acid in the hip was made as the evidence shows it did not improve pain or function better than placebo. Conversely, intra-articular corticosteroids were shown to improve pain and function in the short-term and many primary care doctors provide this treatment in their practice.
These guidelines do a great job covering the totality of management of osteoarthritis of the hip, from conservation management to surgical and post-surgical treatments. Patients often come to us with their questions so not only is it important to know the evidence for what we do in our practices, we need to know what our orthopedic colleagues are doing. We will be the ones asked to do the pre-operative evaluations on these patients, so we need to understand the procedure and its risks. We will also manage these patients post-operatively and need to be aware of what the evidence shows.
Opioid use is also covered in the guidelines: they recommend against the use of opioids to control pain in these patients. In the age of the opioid epidemic, it is a good reminder to be cautious with these meds. It is also a good time to stress smoking cessation with patients.
The guidelines discuss adverse outcomes in patients with diabetes and/or obesity. As primary care physicians, we need to be aware of those risks and be sure our patients are medically optimized before signing that pre-operative form.
A new feature of these guidelines is a discussion on social detriments to health. This is important for many diseases that we treat and we often don’t realize the impact they can have on a patient’s health and recovery. Even if we know a patient would benefit from physical therapy, it doesn’t help them if the patient has no way to get to the appointment. Some patients have copays for every physical therapy session and just can’t afford it. Knowing what the patient needs medically is not enough. We need to understand how they can access that care. Some patients have no one to help them after hip surgery and may avoid doing it for that reason. As primary care doctors, we should be helping our patients access the care they need.
We need to be able to say that a procedure needs to be delayed in the face of poorly controlled disease, such as diabetes. As the rates of osteoarthritis continue to rise, we need to understand that it is not an inevitable age-based occurrence in a patient’s life but rather an inflammatory disease that causes great pain and dysfunction. Utilizing these guidelines and working with our orthopedic colleagues can help patients decrease pain, improve functioning, and enjoy life again.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She was paid by Pfizer as a consultant on Paxlovid, paid by GlaxoSmithKline as a consultant for the Shingrix vaccine, and is the editor in chief of Physician’s Weekly.
MRI-Derived Abdominal Adipose Tissue Linked to Chronic Musculoskeletal Pain
TOPLINE:
MRI-derived abdominal adipose tissue is linked to chronic musculoskeletal pain in multiple sites. The association is stronger in women, suggesting sex differences in fat distribution and hormones.
METHODOLOGY:
- Researchers used data from the UK Biobank, a large population-based cohort study, to investigate the associations between MRI-measured abdominal adipose tissue and chronic musculoskeletal pain.
- A total of 32,409 participants (50.8% women; mean age, 55.0 ± 7.4 years) were included in the analysis, with abdominal MRI scans performed at two imaging visits.
- Pain in the neck/shoulder, back, hip, knee, or “all over the body” was assessed, and participants were categorized based on the number of chronic pain sites.
- Mixed-effects ordinal, multinomial, and logistic regression models were used to analyze the associations between visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and their ratio with chronic pain.
TAKEAWAY:
- According to the authors, there was a dose-response association between VAT, SAT, and their ratio with the number of chronic pain sites in both women and men.
- Higher levels of abdominal adipose tissue were associated with greater odds of reporting chronic pain in both sexes, with effect estimates being relatively larger in women.
- The researchers found that the VAT/SAT ratio was associated with the number of chronic pain sites and chronic pain in both sexes, reflecting differences in fat distribution and hormones.
- The study suggested that excessive abdominal adipose tissue may be involved in the pathogenesis of multisite and widespread chronic musculoskeletal pain.
IN PRACTICE:
“Abdominal adipose tissue was associated with chronic musculoskeletal pain, suggesting that excessive and ectopic fat depositions may be involved in the pathogenesis of multisite and widespread chronic musculoskeletal pain,” wrote the authors of the study.
SOURCE:
This study was led by Zemene Demelash Kifle, University of Tasmania Menzies Institute for Medical Research in Hobart, Australia. It was published online in Regional Anesthesia & Pain Medicine.
LIMITATIONS:
The study’s limitations included the use of a pain questionnaire that did not assess pain severity, which limited the ability to examine the relationship between fat measures and pain severity. Additionally, MRI was conducted on only two occasions, which may have not captured patterns and fluctuations in chronic pain sites. The relatively small size of the imaging sample, compared with the original baseline sample limited the generalizability of the findings. The predominant White ethnicity of participants also limited the generalizability to diverse populations.
DISCLOSURES:
The study was supported by grants from the Australian National Health and Medical Research Council (NHMRC). Mr. Kifle disclosed receiving grants from the Australian NHMRC. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
MRI-derived abdominal adipose tissue is linked to chronic musculoskeletal pain in multiple sites. The association is stronger in women, suggesting sex differences in fat distribution and hormones.
METHODOLOGY:
- Researchers used data from the UK Biobank, a large population-based cohort study, to investigate the associations between MRI-measured abdominal adipose tissue and chronic musculoskeletal pain.
- A total of 32,409 participants (50.8% women; mean age, 55.0 ± 7.4 years) were included in the analysis, with abdominal MRI scans performed at two imaging visits.
- Pain in the neck/shoulder, back, hip, knee, or “all over the body” was assessed, and participants were categorized based on the number of chronic pain sites.
- Mixed-effects ordinal, multinomial, and logistic regression models were used to analyze the associations between visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and their ratio with chronic pain.
TAKEAWAY:
- According to the authors, there was a dose-response association between VAT, SAT, and their ratio with the number of chronic pain sites in both women and men.
- Higher levels of abdominal adipose tissue were associated with greater odds of reporting chronic pain in both sexes, with effect estimates being relatively larger in women.
- The researchers found that the VAT/SAT ratio was associated with the number of chronic pain sites and chronic pain in both sexes, reflecting differences in fat distribution and hormones.
- The study suggested that excessive abdominal adipose tissue may be involved in the pathogenesis of multisite and widespread chronic musculoskeletal pain.
IN PRACTICE:
“Abdominal adipose tissue was associated with chronic musculoskeletal pain, suggesting that excessive and ectopic fat depositions may be involved in the pathogenesis of multisite and widespread chronic musculoskeletal pain,” wrote the authors of the study.
SOURCE:
This study was led by Zemene Demelash Kifle, University of Tasmania Menzies Institute for Medical Research in Hobart, Australia. It was published online in Regional Anesthesia & Pain Medicine.
LIMITATIONS:
The study’s limitations included the use of a pain questionnaire that did not assess pain severity, which limited the ability to examine the relationship between fat measures and pain severity. Additionally, MRI was conducted on only two occasions, which may have not captured patterns and fluctuations in chronic pain sites. The relatively small size of the imaging sample, compared with the original baseline sample limited the generalizability of the findings. The predominant White ethnicity of participants also limited the generalizability to diverse populations.
DISCLOSURES:
The study was supported by grants from the Australian National Health and Medical Research Council (NHMRC). Mr. Kifle disclosed receiving grants from the Australian NHMRC. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
MRI-derived abdominal adipose tissue is linked to chronic musculoskeletal pain in multiple sites. The association is stronger in women, suggesting sex differences in fat distribution and hormones.
METHODOLOGY:
- Researchers used data from the UK Biobank, a large population-based cohort study, to investigate the associations between MRI-measured abdominal adipose tissue and chronic musculoskeletal pain.
- A total of 32,409 participants (50.8% women; mean age, 55.0 ± 7.4 years) were included in the analysis, with abdominal MRI scans performed at two imaging visits.
- Pain in the neck/shoulder, back, hip, knee, or “all over the body” was assessed, and participants were categorized based on the number of chronic pain sites.
- Mixed-effects ordinal, multinomial, and logistic regression models were used to analyze the associations between visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and their ratio with chronic pain.
TAKEAWAY:
- According to the authors, there was a dose-response association between VAT, SAT, and their ratio with the number of chronic pain sites in both women and men.
- Higher levels of abdominal adipose tissue were associated with greater odds of reporting chronic pain in both sexes, with effect estimates being relatively larger in women.
- The researchers found that the VAT/SAT ratio was associated with the number of chronic pain sites and chronic pain in both sexes, reflecting differences in fat distribution and hormones.
- The study suggested that excessive abdominal adipose tissue may be involved in the pathogenesis of multisite and widespread chronic musculoskeletal pain.
IN PRACTICE:
“Abdominal adipose tissue was associated with chronic musculoskeletal pain, suggesting that excessive and ectopic fat depositions may be involved in the pathogenesis of multisite and widespread chronic musculoskeletal pain,” wrote the authors of the study.
SOURCE:
This study was led by Zemene Demelash Kifle, University of Tasmania Menzies Institute for Medical Research in Hobart, Australia. It was published online in Regional Anesthesia & Pain Medicine.
LIMITATIONS:
The study’s limitations included the use of a pain questionnaire that did not assess pain severity, which limited the ability to examine the relationship between fat measures and pain severity. Additionally, MRI was conducted on only two occasions, which may have not captured patterns and fluctuations in chronic pain sites. The relatively small size of the imaging sample, compared with the original baseline sample limited the generalizability of the findings. The predominant White ethnicity of participants also limited the generalizability to diverse populations.
DISCLOSURES:
The study was supported by grants from the Australian National Health and Medical Research Council (NHMRC). Mr. Kifle disclosed receiving grants from the Australian NHMRC. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.











