User login
Ixekizumab psoriasis outcomes, sliced and diced
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
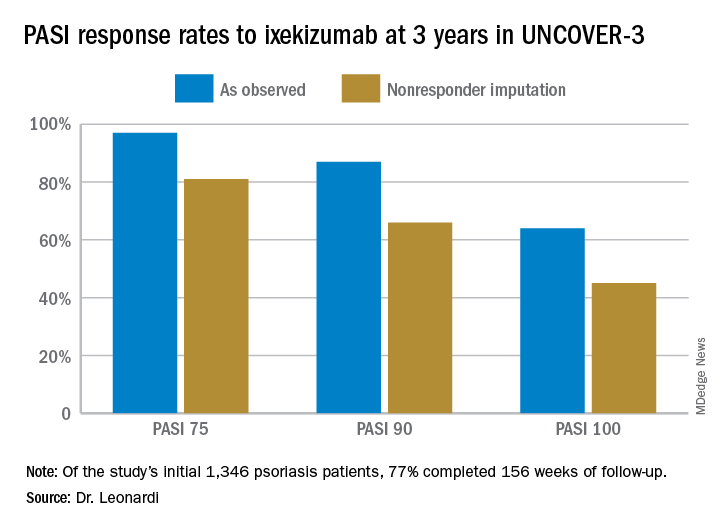
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.

However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.

However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Simple treatments can address bleeding in dermatologic surgery
WAIKOLOA, HAWAII – “Nothing can ruin your day more than a lot of bleeding,” said Mohs surgeon Daniel Siegel, MD, clinical professor of dermatology at the State University of New York Downstate Medical Center, New York.

“Part of planning for surgery is to prevent that sort of problem,” and fortunately, and some are very inexpensive. There’s even a “fancy form of potato starch” that can be left in the wound and sewn over, he said in an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
During the interview, Dr. Siegel described several options and how to use them. They are good products to have on hand in the clinic, just in case, he said.
SDEF/Global Academy for Medical Education and this news organization and are owned by the same parent company.
WAIKOLOA, HAWAII – “Nothing can ruin your day more than a lot of bleeding,” said Mohs surgeon Daniel Siegel, MD, clinical professor of dermatology at the State University of New York Downstate Medical Center, New York.

“Part of planning for surgery is to prevent that sort of problem,” and fortunately, and some are very inexpensive. There’s even a “fancy form of potato starch” that can be left in the wound and sewn over, he said in an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
During the interview, Dr. Siegel described several options and how to use them. They are good products to have on hand in the clinic, just in case, he said.
SDEF/Global Academy for Medical Education and this news organization and are owned by the same parent company.
WAIKOLOA, HAWAII – “Nothing can ruin your day more than a lot of bleeding,” said Mohs surgeon Daniel Siegel, MD, clinical professor of dermatology at the State University of New York Downstate Medical Center, New York.

“Part of planning for surgery is to prevent that sort of problem,” and fortunately, and some are very inexpensive. There’s even a “fancy form of potato starch” that can be left in the wound and sewn over, he said in an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
During the interview, Dr. Siegel described several options and how to use them. They are good products to have on hand in the clinic, just in case, he said.
SDEF/Global Academy for Medical Education and this news organization and are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Dupilumab conjunctivitis does not always require an ophthalmologist referral
WAIKOLOA, HAWAII – Since its approval in 2017, dupilumab (Dupixent) has proven to be a solid addition to the atopic dermatitis (AD) armamentarium.

About 80% to 85% of patients treated with the biologic will achieve a 50% reduction in their Eczema Area and Severity Index score, and some will go on to a 90% reduction, according to Jonathan Silverberg, MD, PhD, of the department of dermatology, Northwestern University, Chicago.
But Dr. Silverberg has seen it in his own practice and said it can be hard to know whether or not to refer to ophthalmology. “We’re often left with this conundrum of ... ‘Is it a side effect of the medication, or is it just because they happen to have hay fever or keratoconjunctivitis or other ophthalmic comorbidities?’ And it’s not always easy to sort out.”
In an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation, he offered his advice on managing a patient who develops conjunctivitis during dupilumab treatment, including his treatment tips for when it is safe to handle in the dermatology clinic.
Dr. Silverberg, who was an investigator in the dupilumab phase 3 trials, said that, while dupilumab is the only systemic agent approved by the Food and Drug Administration for treating AD, and more are on the way for AD, there will always still be a role for traditional immunosuppressives. He explained why in the interview and why he favors methotrexate when old school options are in order.
This news organization and SDEF/Global Academy for Medical Education are owned by the same parent company.
WAIKOLOA, HAWAII – Since its approval in 2017, dupilumab (Dupixent) has proven to be a solid addition to the atopic dermatitis (AD) armamentarium.

About 80% to 85% of patients treated with the biologic will achieve a 50% reduction in their Eczema Area and Severity Index score, and some will go on to a 90% reduction, according to Jonathan Silverberg, MD, PhD, of the department of dermatology, Northwestern University, Chicago.
But Dr. Silverberg has seen it in his own practice and said it can be hard to know whether or not to refer to ophthalmology. “We’re often left with this conundrum of ... ‘Is it a side effect of the medication, or is it just because they happen to have hay fever or keratoconjunctivitis or other ophthalmic comorbidities?’ And it’s not always easy to sort out.”
In an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation, he offered his advice on managing a patient who develops conjunctivitis during dupilumab treatment, including his treatment tips for when it is safe to handle in the dermatology clinic.
Dr. Silverberg, who was an investigator in the dupilumab phase 3 trials, said that, while dupilumab is the only systemic agent approved by the Food and Drug Administration for treating AD, and more are on the way for AD, there will always still be a role for traditional immunosuppressives. He explained why in the interview and why he favors methotrexate when old school options are in order.
This news organization and SDEF/Global Academy for Medical Education are owned by the same parent company.
WAIKOLOA, HAWAII – Since its approval in 2017, dupilumab (Dupixent) has proven to be a solid addition to the atopic dermatitis (AD) armamentarium.

About 80% to 85% of patients treated with the biologic will achieve a 50% reduction in their Eczema Area and Severity Index score, and some will go on to a 90% reduction, according to Jonathan Silverberg, MD, PhD, of the department of dermatology, Northwestern University, Chicago.
But Dr. Silverberg has seen it in his own practice and said it can be hard to know whether or not to refer to ophthalmology. “We’re often left with this conundrum of ... ‘Is it a side effect of the medication, or is it just because they happen to have hay fever or keratoconjunctivitis or other ophthalmic comorbidities?’ And it’s not always easy to sort out.”
In an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation, he offered his advice on managing a patient who develops conjunctivitis during dupilumab treatment, including his treatment tips for when it is safe to handle in the dermatology clinic.
Dr. Silverberg, who was an investigator in the dupilumab phase 3 trials, said that, while dupilumab is the only systemic agent approved by the Food and Drug Administration for treating AD, and more are on the way for AD, there will always still be a role for traditional immunosuppressives. He explained why in the interview and why he favors methotrexate when old school options are in order.
This news organization and SDEF/Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
What’s new with adalimumab? Plenty
WAIKOLOA, HAWAII – A flurry of recent impressive , identifies a simple biomarker predictive of the likelihood of a favorable PASI 75 response, and highlights a disconnect in psoriatic arthritis (PsA) patients between clinical response as reflected in disease activity and radiographic progression of joint disease, according to Kristina C. Duffin, MD.
Also, a new citrate-free version of adalimumab (Humira) is available. It requires a new prescription, and an additional prior authorization is mandated by some insurers. But this is a welcome innovation for patients bothered by significant burning and stinging with their injections of classic adalimumab, Dr. Duffin, cochair of the department of dermatology at the University of Utah, Salt Lake City, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
New long-term safety data
Adalimumab is a market leader in biologic therapy for psoriasis. But the long-term experience with biologics in dermatology is still relatively limited, so the recent publication of two large studies providing encouraging evidence of the long-term safety of adalimumab is noteworthy.
Craig L. Leonardi, MD, of Saint Louis University, St. Louis, Mo., was first author of an analysis of long-term safety data from 18 clinical trials in adults with moderate to severe plaque psoriasis. The key takeaway, in Dr. Duffin’s view, was that the rate of adverse events, including serious infections and malignancies other than nonmelanoma skin cancer, remained stable over time out to 240 weeks of follow-up in patients on continuous treatment, with no new safety signals emerging (Br J Dermatol. 2019 Jan;180[1]:76-85).
However, randomized clinical trials often paint an overly rosy safety picture because of their strict inclusion and exclusion criteria.
“We single out patients for clinical trials because they’re especially healthy. That doesn’t happen in real-world registries,” she noted.
That’s why a systematic review of adalimumab’s safety performance in 10 real-world registries of adalimumab-treated psoriasis patients is particularly informative. The registries included in the systematic review, led by Bruce E. Strober, MD, PhD, professor of dermatology at the University of Connecticut, Farmington, didn’t all measure the same outcomes. But the three registries that documented major adverse cardiovascular events showed rates of less than 0.1 to less than 1 per 100 patient-years. Rates of malignancies other than nonmelanoma skin cancer were consistently in the 0.3-0.6 events per 100 patient-years range, similar to what has been reported in studies of other systemic psoriasis therapies, biologic as well as nonbiologic (J Eur Acad Dermatol Venereol. 2018 Dec;32[12]:2126-33).
Overall infection rates reported in the real-world registries ranged from 7.7 to 14.7 events per 100 patient-years, which is actually considerably lower than in the clinical trials. Rates of serious infections ranged from less than one up to two events per 100 patient-years, with the most common ones being cellulitis and pneumonia, consistent with the randomized trial experience.
Predicting response to adalimumab
A prospective, multicenter, observational cohort study of 544 psoriasis patients on adalimumab monotherapy conducted by U.K. investigators concluded that a patient’s serum drug level is the single most important predictor of treatment response. A cut point of 3.2 mcg/mL, which is considered the minimal effective circulating drug level, was associated with a 65% probability of a 75% improvement in Psoriasis Area and Severity Index from baseline, or PASI 75 response. The higher the serum drug level, the greater the likelihood of a PASI 75 response, up to a serum level of 7 mcg/mL, which was associated with an 81% probability of achieving PASI 75. Beyond 7 mcg/mL, however, the relationship with treatment response plateaued. Importantly, drug levels measured early on – at 1-12 weeks into therapy – were predictive of response 6 months later. So were steady-state levels (J Invest Dermatol. 2019 Jan;139[1]:115-23).
This is clinically useful information, Dr. Duffin observed.
“I’m hoping we’re going to see more real-world use of checking drug levels,” she said.
Indeed, even though the approved dosing of adalimumab for psoriasis is 40 mg by subcutaneous injection every 2 weeks, the new American Academy of Dermatology/National Psoriasis Foundation joint guidelines for treatment of psoriasis with biologics declare that “a maintenance dose of adalimumab at 40 mg/week is recommended for better disease control in some patients” (J Am Acad Dermatol. 2019 Feb 7. doi: 10.1016/j.jaad.2018.11.057. [Epub ahead of print]).
The new guidelines provide support for dermatologists who decide weekly therapy is best for a given patient, and adalimumab drug levels could prove useful in identifying the patient subgroup likely to benefit.
Dr. Duffin is often consulted by other physicians as to whether they should check for neutralizing antibodies in patients who appear to be losing therapeutic efficacy on a given biologic. She’s not a fan of the practice.
“There are commercial assays out there, but it’s very hard to interpret them because we don’t really know if they’re truly measuring neutralizing antibodies. And the cost is not insignificant; it can be hundreds of dollars,” she noted.
She believes a straightforward measurement of the serum biologic level is a better strategy.
“It makes sense: This is an indirect way of determining if there’s been neutralization of the drug, rather than trying to check the antibody that’s doing it, which is fraught with problems,” Dr. Duffin said.
Radiographic progression and clinical PsA activity on adalimumab don’t always correlate
A post hoc analysis of the randomized, double-blind, placebo-controlled ADEPT trial in PsA patients demonstrated that inhibition of radiographic progression as measured by change in modified total Sharp score from baseline through 24 weeks of adalimumab therapy was greater than expected based upon control of clinical disease activity (Rheumatology [Oxford]. 2019 Jan 3. doi: 10.1093/rheumatology/key417. [Epub ahead of print]).
One implication of the disconnect between radiographic progression and clinical disease documented in this study is that a dermatologist shouldn’t be too quick to change from adalimumab to another biologic just because a patient with PsA reports continued but bearable joint pain. And the converse is also true.
“I think that we as dermatologists probably shouldn’t be reassured when a patient says, ‘My joints feel great!” That’s because you may not necessarily be able to predict lack of progression in Sharp score based upon clinical response,” Dr. Duffin cautioned. “I think you should still have a rheumatologist check in with the patient and do x-rays periodically. The rheumatologist I work with does that, usually about on a yearly basis.”
Another key finding in the ADEPT analysis was that concomitant methotrexate had no added effect in terms of preventing joint destruction. This underscores the prescience of the first-ever collaborative American College of Rheumatology/National Psoriasis Foundation guidelines for the treatment of PsA (Arthritis Care Res (Hoboken). 2019 Jan;71[1]:2-29).
The new guidelines recommend that, in a psoriasis patient with confirmed PsA, the first-line treatment is a tumor necrosis factor (TNF) inhibitor. Agents from this class are preferred over other biologics because they are backed by a larger body of data regarding inhibition of joint disease progression. If the patient fails on the first TNF inhibitor prescribed, second-line therapy is another TNF inhibitor. So is third-line therapy.
Adalimumab citrate free
Not only does this new iteration of adalimumab do away with citrate as a buffer because it can cause pain and burning, it also utilizes a thinner 29-gauge needle rather than the standard 27-gauge. And the needle cover isn’t made with natural rubber latex. Also, both the pen and prefilled syringe contain half the volume of liquid, compared with the classic version of the biologic, so it’s 40 mg of drug in 0.4 mL rather than in 0.8 mL.
The packaging of adalimumab citrate free is different. It comes in a blue box to distinguish the product from the classic version.
Dr. Duffin reported receiving research grants from and serving as a consultant to AbbVie, which markets adalimumab, as well as close to a dozen other pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – A flurry of recent impressive , identifies a simple biomarker predictive of the likelihood of a favorable PASI 75 response, and highlights a disconnect in psoriatic arthritis (PsA) patients between clinical response as reflected in disease activity and radiographic progression of joint disease, according to Kristina C. Duffin, MD.
Also, a new citrate-free version of adalimumab (Humira) is available. It requires a new prescription, and an additional prior authorization is mandated by some insurers. But this is a welcome innovation for patients bothered by significant burning and stinging with their injections of classic adalimumab, Dr. Duffin, cochair of the department of dermatology at the University of Utah, Salt Lake City, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
New long-term safety data
Adalimumab is a market leader in biologic therapy for psoriasis. But the long-term experience with biologics in dermatology is still relatively limited, so the recent publication of two large studies providing encouraging evidence of the long-term safety of adalimumab is noteworthy.
Craig L. Leonardi, MD, of Saint Louis University, St. Louis, Mo., was first author of an analysis of long-term safety data from 18 clinical trials in adults with moderate to severe plaque psoriasis. The key takeaway, in Dr. Duffin’s view, was that the rate of adverse events, including serious infections and malignancies other than nonmelanoma skin cancer, remained stable over time out to 240 weeks of follow-up in patients on continuous treatment, with no new safety signals emerging (Br J Dermatol. 2019 Jan;180[1]:76-85).
However, randomized clinical trials often paint an overly rosy safety picture because of their strict inclusion and exclusion criteria.
“We single out patients for clinical trials because they’re especially healthy. That doesn’t happen in real-world registries,” she noted.
That’s why a systematic review of adalimumab’s safety performance in 10 real-world registries of adalimumab-treated psoriasis patients is particularly informative. The registries included in the systematic review, led by Bruce E. Strober, MD, PhD, professor of dermatology at the University of Connecticut, Farmington, didn’t all measure the same outcomes. But the three registries that documented major adverse cardiovascular events showed rates of less than 0.1 to less than 1 per 100 patient-years. Rates of malignancies other than nonmelanoma skin cancer were consistently in the 0.3-0.6 events per 100 patient-years range, similar to what has been reported in studies of other systemic psoriasis therapies, biologic as well as nonbiologic (J Eur Acad Dermatol Venereol. 2018 Dec;32[12]:2126-33).
Overall infection rates reported in the real-world registries ranged from 7.7 to 14.7 events per 100 patient-years, which is actually considerably lower than in the clinical trials. Rates of serious infections ranged from less than one up to two events per 100 patient-years, with the most common ones being cellulitis and pneumonia, consistent with the randomized trial experience.
Predicting response to adalimumab
A prospective, multicenter, observational cohort study of 544 psoriasis patients on adalimumab monotherapy conducted by U.K. investigators concluded that a patient’s serum drug level is the single most important predictor of treatment response. A cut point of 3.2 mcg/mL, which is considered the minimal effective circulating drug level, was associated with a 65% probability of a 75% improvement in Psoriasis Area and Severity Index from baseline, or PASI 75 response. The higher the serum drug level, the greater the likelihood of a PASI 75 response, up to a serum level of 7 mcg/mL, which was associated with an 81% probability of achieving PASI 75. Beyond 7 mcg/mL, however, the relationship with treatment response plateaued. Importantly, drug levels measured early on – at 1-12 weeks into therapy – were predictive of response 6 months later. So were steady-state levels (J Invest Dermatol. 2019 Jan;139[1]:115-23).
This is clinically useful information, Dr. Duffin observed.
“I’m hoping we’re going to see more real-world use of checking drug levels,” she said.
Indeed, even though the approved dosing of adalimumab for psoriasis is 40 mg by subcutaneous injection every 2 weeks, the new American Academy of Dermatology/National Psoriasis Foundation joint guidelines for treatment of psoriasis with biologics declare that “a maintenance dose of adalimumab at 40 mg/week is recommended for better disease control in some patients” (J Am Acad Dermatol. 2019 Feb 7. doi: 10.1016/j.jaad.2018.11.057. [Epub ahead of print]).
The new guidelines provide support for dermatologists who decide weekly therapy is best for a given patient, and adalimumab drug levels could prove useful in identifying the patient subgroup likely to benefit.
Dr. Duffin is often consulted by other physicians as to whether they should check for neutralizing antibodies in patients who appear to be losing therapeutic efficacy on a given biologic. She’s not a fan of the practice.
“There are commercial assays out there, but it’s very hard to interpret them because we don’t really know if they’re truly measuring neutralizing antibodies. And the cost is not insignificant; it can be hundreds of dollars,” she noted.
She believes a straightforward measurement of the serum biologic level is a better strategy.
“It makes sense: This is an indirect way of determining if there’s been neutralization of the drug, rather than trying to check the antibody that’s doing it, which is fraught with problems,” Dr. Duffin said.
Radiographic progression and clinical PsA activity on adalimumab don’t always correlate
A post hoc analysis of the randomized, double-blind, placebo-controlled ADEPT trial in PsA patients demonstrated that inhibition of radiographic progression as measured by change in modified total Sharp score from baseline through 24 weeks of adalimumab therapy was greater than expected based upon control of clinical disease activity (Rheumatology [Oxford]. 2019 Jan 3. doi: 10.1093/rheumatology/key417. [Epub ahead of print]).
One implication of the disconnect between radiographic progression and clinical disease documented in this study is that a dermatologist shouldn’t be too quick to change from adalimumab to another biologic just because a patient with PsA reports continued but bearable joint pain. And the converse is also true.
“I think that we as dermatologists probably shouldn’t be reassured when a patient says, ‘My joints feel great!” That’s because you may not necessarily be able to predict lack of progression in Sharp score based upon clinical response,” Dr. Duffin cautioned. “I think you should still have a rheumatologist check in with the patient and do x-rays periodically. The rheumatologist I work with does that, usually about on a yearly basis.”
Another key finding in the ADEPT analysis was that concomitant methotrexate had no added effect in terms of preventing joint destruction. This underscores the prescience of the first-ever collaborative American College of Rheumatology/National Psoriasis Foundation guidelines for the treatment of PsA (Arthritis Care Res (Hoboken). 2019 Jan;71[1]:2-29).
The new guidelines recommend that, in a psoriasis patient with confirmed PsA, the first-line treatment is a tumor necrosis factor (TNF) inhibitor. Agents from this class are preferred over other biologics because they are backed by a larger body of data regarding inhibition of joint disease progression. If the patient fails on the first TNF inhibitor prescribed, second-line therapy is another TNF inhibitor. So is third-line therapy.
Adalimumab citrate free
Not only does this new iteration of adalimumab do away with citrate as a buffer because it can cause pain and burning, it also utilizes a thinner 29-gauge needle rather than the standard 27-gauge. And the needle cover isn’t made with natural rubber latex. Also, both the pen and prefilled syringe contain half the volume of liquid, compared with the classic version of the biologic, so it’s 40 mg of drug in 0.4 mL rather than in 0.8 mL.
The packaging of adalimumab citrate free is different. It comes in a blue box to distinguish the product from the classic version.
Dr. Duffin reported receiving research grants from and serving as a consultant to AbbVie, which markets adalimumab, as well as close to a dozen other pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – A flurry of recent impressive , identifies a simple biomarker predictive of the likelihood of a favorable PASI 75 response, and highlights a disconnect in psoriatic arthritis (PsA) patients between clinical response as reflected in disease activity and radiographic progression of joint disease, according to Kristina C. Duffin, MD.
Also, a new citrate-free version of adalimumab (Humira) is available. It requires a new prescription, and an additional prior authorization is mandated by some insurers. But this is a welcome innovation for patients bothered by significant burning and stinging with their injections of classic adalimumab, Dr. Duffin, cochair of the department of dermatology at the University of Utah, Salt Lake City, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
New long-term safety data
Adalimumab is a market leader in biologic therapy for psoriasis. But the long-term experience with biologics in dermatology is still relatively limited, so the recent publication of two large studies providing encouraging evidence of the long-term safety of adalimumab is noteworthy.
Craig L. Leonardi, MD, of Saint Louis University, St. Louis, Mo., was first author of an analysis of long-term safety data from 18 clinical trials in adults with moderate to severe plaque psoriasis. The key takeaway, in Dr. Duffin’s view, was that the rate of adverse events, including serious infections and malignancies other than nonmelanoma skin cancer, remained stable over time out to 240 weeks of follow-up in patients on continuous treatment, with no new safety signals emerging (Br J Dermatol. 2019 Jan;180[1]:76-85).
However, randomized clinical trials often paint an overly rosy safety picture because of their strict inclusion and exclusion criteria.
“We single out patients for clinical trials because they’re especially healthy. That doesn’t happen in real-world registries,” she noted.
That’s why a systematic review of adalimumab’s safety performance in 10 real-world registries of adalimumab-treated psoriasis patients is particularly informative. The registries included in the systematic review, led by Bruce E. Strober, MD, PhD, professor of dermatology at the University of Connecticut, Farmington, didn’t all measure the same outcomes. But the three registries that documented major adverse cardiovascular events showed rates of less than 0.1 to less than 1 per 100 patient-years. Rates of malignancies other than nonmelanoma skin cancer were consistently in the 0.3-0.6 events per 100 patient-years range, similar to what has been reported in studies of other systemic psoriasis therapies, biologic as well as nonbiologic (J Eur Acad Dermatol Venereol. 2018 Dec;32[12]:2126-33).
Overall infection rates reported in the real-world registries ranged from 7.7 to 14.7 events per 100 patient-years, which is actually considerably lower than in the clinical trials. Rates of serious infections ranged from less than one up to two events per 100 patient-years, with the most common ones being cellulitis and pneumonia, consistent with the randomized trial experience.
Predicting response to adalimumab
A prospective, multicenter, observational cohort study of 544 psoriasis patients on adalimumab monotherapy conducted by U.K. investigators concluded that a patient’s serum drug level is the single most important predictor of treatment response. A cut point of 3.2 mcg/mL, which is considered the minimal effective circulating drug level, was associated with a 65% probability of a 75% improvement in Psoriasis Area and Severity Index from baseline, or PASI 75 response. The higher the serum drug level, the greater the likelihood of a PASI 75 response, up to a serum level of 7 mcg/mL, which was associated with an 81% probability of achieving PASI 75. Beyond 7 mcg/mL, however, the relationship with treatment response plateaued. Importantly, drug levels measured early on – at 1-12 weeks into therapy – were predictive of response 6 months later. So were steady-state levels (J Invest Dermatol. 2019 Jan;139[1]:115-23).
This is clinically useful information, Dr. Duffin observed.
“I’m hoping we’re going to see more real-world use of checking drug levels,” she said.
Indeed, even though the approved dosing of adalimumab for psoriasis is 40 mg by subcutaneous injection every 2 weeks, the new American Academy of Dermatology/National Psoriasis Foundation joint guidelines for treatment of psoriasis with biologics declare that “a maintenance dose of adalimumab at 40 mg/week is recommended for better disease control in some patients” (J Am Acad Dermatol. 2019 Feb 7. doi: 10.1016/j.jaad.2018.11.057. [Epub ahead of print]).
The new guidelines provide support for dermatologists who decide weekly therapy is best for a given patient, and adalimumab drug levels could prove useful in identifying the patient subgroup likely to benefit.
Dr. Duffin is often consulted by other physicians as to whether they should check for neutralizing antibodies in patients who appear to be losing therapeutic efficacy on a given biologic. She’s not a fan of the practice.
“There are commercial assays out there, but it’s very hard to interpret them because we don’t really know if they’re truly measuring neutralizing antibodies. And the cost is not insignificant; it can be hundreds of dollars,” she noted.
She believes a straightforward measurement of the serum biologic level is a better strategy.
“It makes sense: This is an indirect way of determining if there’s been neutralization of the drug, rather than trying to check the antibody that’s doing it, which is fraught with problems,” Dr. Duffin said.
Radiographic progression and clinical PsA activity on adalimumab don’t always correlate
A post hoc analysis of the randomized, double-blind, placebo-controlled ADEPT trial in PsA patients demonstrated that inhibition of radiographic progression as measured by change in modified total Sharp score from baseline through 24 weeks of adalimumab therapy was greater than expected based upon control of clinical disease activity (Rheumatology [Oxford]. 2019 Jan 3. doi: 10.1093/rheumatology/key417. [Epub ahead of print]).
One implication of the disconnect between radiographic progression and clinical disease documented in this study is that a dermatologist shouldn’t be too quick to change from adalimumab to another biologic just because a patient with PsA reports continued but bearable joint pain. And the converse is also true.
“I think that we as dermatologists probably shouldn’t be reassured when a patient says, ‘My joints feel great!” That’s because you may not necessarily be able to predict lack of progression in Sharp score based upon clinical response,” Dr. Duffin cautioned. “I think you should still have a rheumatologist check in with the patient and do x-rays periodically. The rheumatologist I work with does that, usually about on a yearly basis.”
Another key finding in the ADEPT analysis was that concomitant methotrexate had no added effect in terms of preventing joint destruction. This underscores the prescience of the first-ever collaborative American College of Rheumatology/National Psoriasis Foundation guidelines for the treatment of PsA (Arthritis Care Res (Hoboken). 2019 Jan;71[1]:2-29).
The new guidelines recommend that, in a psoriasis patient with confirmed PsA, the first-line treatment is a tumor necrosis factor (TNF) inhibitor. Agents from this class are preferred over other biologics because they are backed by a larger body of data regarding inhibition of joint disease progression. If the patient fails on the first TNF inhibitor prescribed, second-line therapy is another TNF inhibitor. So is third-line therapy.
Adalimumab citrate free
Not only does this new iteration of adalimumab do away with citrate as a buffer because it can cause pain and burning, it also utilizes a thinner 29-gauge needle rather than the standard 27-gauge. And the needle cover isn’t made with natural rubber latex. Also, both the pen and prefilled syringe contain half the volume of liquid, compared with the classic version of the biologic, so it’s 40 mg of drug in 0.4 mL rather than in 0.8 mL.
The packaging of adalimumab citrate free is different. It comes in a blue box to distinguish the product from the classic version.
Dr. Duffin reported receiving research grants from and serving as a consultant to AbbVie, which markets adalimumab, as well as close to a dozen other pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Don’t miss early joint involvement in psoriasis
WAIKOLOA, HAWAII – advised Alan Menter, MD.
About a third of patients with psoriasis will go on to develop joint involvement, and about half of those will go on to develop permanent joint destruction “if left untreated,” he noted. But early joint involvement has to be caught first, and dermatologists aren’t doing a very good job at early detection, according to Dr. Menter, clinical professor of dermatology at the University of Texas, Dallas.
The consequences, including arthritis mutilans, can be devastating. “It’s vitally important for us to prevent any permanent joint disease by” picking it up early, he said. “Our job as dermatologists is to diagnose it early.”
It’s not hard to do, just a few extra questions and a few extra steps on the physical exam, which takes a minute or two during each visit with psoriasis patients, are needed, he said.
Dr. Menter reviewed questions to ask patients, and explained how to examine patients for joint involvement and alter treatment when it’s found, in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – advised Alan Menter, MD.
About a third of patients with psoriasis will go on to develop joint involvement, and about half of those will go on to develop permanent joint destruction “if left untreated,” he noted. But early joint involvement has to be caught first, and dermatologists aren’t doing a very good job at early detection, according to Dr. Menter, clinical professor of dermatology at the University of Texas, Dallas.
The consequences, including arthritis mutilans, can be devastating. “It’s vitally important for us to prevent any permanent joint disease by” picking it up early, he said. “Our job as dermatologists is to diagnose it early.”
It’s not hard to do, just a few extra questions and a few extra steps on the physical exam, which takes a minute or two during each visit with psoriasis patients, are needed, he said.
Dr. Menter reviewed questions to ask patients, and explained how to examine patients for joint involvement and alter treatment when it’s found, in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – advised Alan Menter, MD.
About a third of patients with psoriasis will go on to develop joint involvement, and about half of those will go on to develop permanent joint destruction “if left untreated,” he noted. But early joint involvement has to be caught first, and dermatologists aren’t doing a very good job at early detection, according to Dr. Menter, clinical professor of dermatology at the University of Texas, Dallas.
The consequences, including arthritis mutilans, can be devastating. “It’s vitally important for us to prevent any permanent joint disease by” picking it up early, he said. “Our job as dermatologists is to diagnose it early.”
It’s not hard to do, just a few extra questions and a few extra steps on the physical exam, which takes a minute or two during each visit with psoriasis patients, are needed, he said.
Dr. Menter reviewed questions to ask patients, and explained how to examine patients for joint involvement and alter treatment when it’s found, in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Onychomycosis that fails terbinafine probably isn’t T. rubrum
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Tropical travelers’ top dermatologic infestations
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
Cutaneous larva migrans
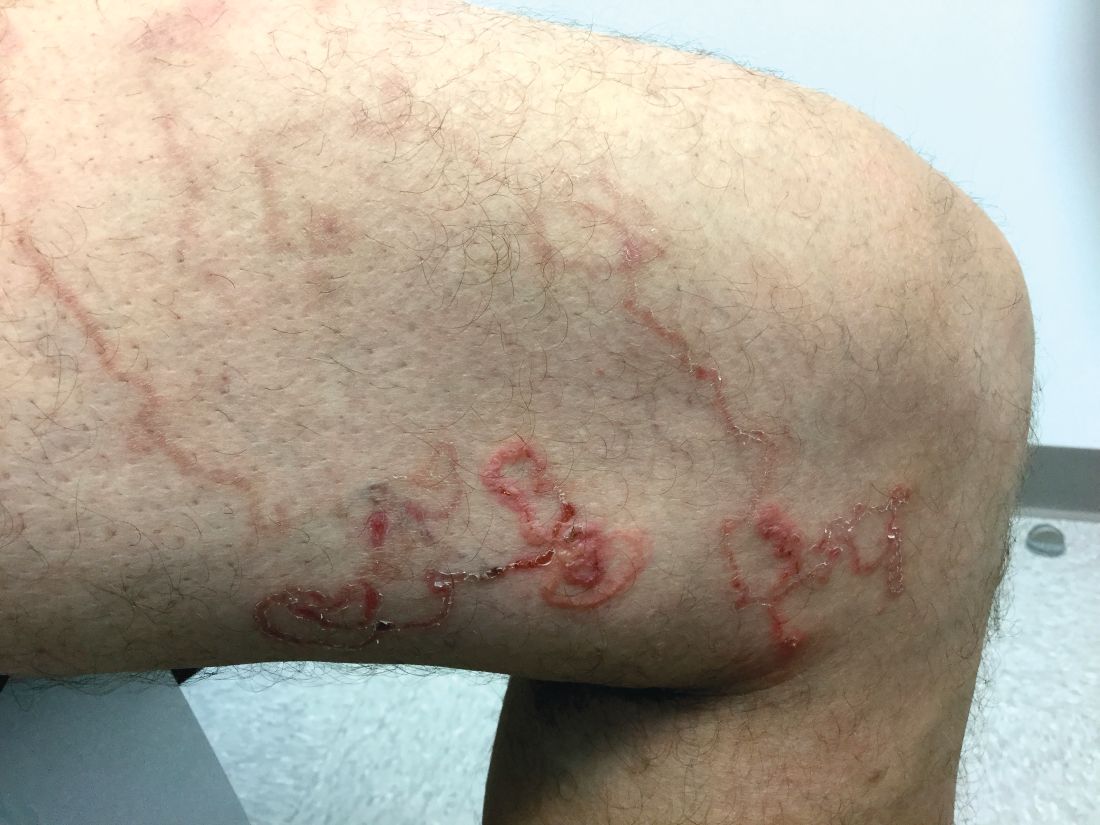
This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
Cutaneous larva migrans

This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
Cutaneous larva migrans

This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
How to surf the rosacea treatment algorithm
WAIKOLOA, HAWAII – according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.

Papules and pustules need an oral or topical anti-inflammatory drug. Background erythema requires an alpha adrenergic agonist. Telangiectasia is best handled by a laser device, and if a patient has a phyma, “you’ve got to use a surgical approach,” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. It sounds simple, but there are decisions to be made about what drugs and formulations to use, and when, and when to combine them.
In an interview, Dr. Stein Gold shared her approach to treatment, along with the latest on using ivermectin and brimonidine together, plus her thoughts on new medications under development and the role of the Demodex mite in rosacea.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.

Papules and pustules need an oral or topical anti-inflammatory drug. Background erythema requires an alpha adrenergic agonist. Telangiectasia is best handled by a laser device, and if a patient has a phyma, “you’ve got to use a surgical approach,” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. It sounds simple, but there are decisions to be made about what drugs and formulations to use, and when, and when to combine them.
In an interview, Dr. Stein Gold shared her approach to treatment, along with the latest on using ivermectin and brimonidine together, plus her thoughts on new medications under development and the role of the Demodex mite in rosacea.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.

Papules and pustules need an oral or topical anti-inflammatory drug. Background erythema requires an alpha adrenergic agonist. Telangiectasia is best handled by a laser device, and if a patient has a phyma, “you’ve got to use a surgical approach,” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. It sounds simple, but there are decisions to be made about what drugs and formulations to use, and when, and when to combine them.
In an interview, Dr. Stein Gold shared her approach to treatment, along with the latest on using ivermectin and brimonidine together, plus her thoughts on new medications under development and the role of the Demodex mite in rosacea.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Don’t fear spironolactone, isotretinoin, OCs for acne
WAIKOLOA, HAWAII – There’s really , of the department of dermatology at the University of Alabama at Birmingham.
There have been concerns with all three in the past, but most of the worries have been recently laid to rest.
The news hasn’t reached everyone, though, so, by and large, they are “tools I think we are not using enough of,” Dr. Harper said in an interview. With isotretinoin, for instance, it really isn’t necessary to do blood work for lipids and liver function every month, a daunting prospect for patients; baseline testing with a repeat at 2 months is sufficient, as long as there’s no dose escalation and results are acceptable, with the exception of a monthly pregnancy test for women, she noted. Meanwhile, there’s no evidence of a link with inflammatory bowel disease, and wound healing isn’t as much of an issue as once thought.
It’s the same story with spironolactone. Hyperkalemia is a long-standing concern, but it turns out that “in healthy young women taking spironolactone for acne, we don’t need to be checking potassium.” As far as breast cancer goes, the potential risk with spironolactone hasn’t panned out in the literature, and there may not be “a link at all,” Dr. Harper said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
There are caveats, of course. Hormonal treatments shouldn’t be used in young women until they’ve established their menstrual cycle. OCs should not be used in smokers, or people who have hypertension or migraines, among other conditions. Also, elevated triglycerides remain a concern with isotretinoin. “The number I would want people to remember is 500 [mg/dL],” the threshold when triglycerides become a problem.
In the interview, Dr. Harper explained the new thinking on these three options, and shared her treatment tips, including what to do if patients’ triglycerides hit the 500 mg/dL mark.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – There’s really , of the department of dermatology at the University of Alabama at Birmingham.
There have been concerns with all three in the past, but most of the worries have been recently laid to rest.
The news hasn’t reached everyone, though, so, by and large, they are “tools I think we are not using enough of,” Dr. Harper said in an interview. With isotretinoin, for instance, it really isn’t necessary to do blood work for lipids and liver function every month, a daunting prospect for patients; baseline testing with a repeat at 2 months is sufficient, as long as there’s no dose escalation and results are acceptable, with the exception of a monthly pregnancy test for women, she noted. Meanwhile, there’s no evidence of a link with inflammatory bowel disease, and wound healing isn’t as much of an issue as once thought.
It’s the same story with spironolactone. Hyperkalemia is a long-standing concern, but it turns out that “in healthy young women taking spironolactone for acne, we don’t need to be checking potassium.” As far as breast cancer goes, the potential risk with spironolactone hasn’t panned out in the literature, and there may not be “a link at all,” Dr. Harper said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
There are caveats, of course. Hormonal treatments shouldn’t be used in young women until they’ve established their menstrual cycle. OCs should not be used in smokers, or people who have hypertension or migraines, among other conditions. Also, elevated triglycerides remain a concern with isotretinoin. “The number I would want people to remember is 500 [mg/dL],” the threshold when triglycerides become a problem.
In the interview, Dr. Harper explained the new thinking on these three options, and shared her treatment tips, including what to do if patients’ triglycerides hit the 500 mg/dL mark.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – There’s really , of the department of dermatology at the University of Alabama at Birmingham.
There have been concerns with all three in the past, but most of the worries have been recently laid to rest.
The news hasn’t reached everyone, though, so, by and large, they are “tools I think we are not using enough of,” Dr. Harper said in an interview. With isotretinoin, for instance, it really isn’t necessary to do blood work for lipids and liver function every month, a daunting prospect for patients; baseline testing with a repeat at 2 months is sufficient, as long as there’s no dose escalation and results are acceptable, with the exception of a monthly pregnancy test for women, she noted. Meanwhile, there’s no evidence of a link with inflammatory bowel disease, and wound healing isn’t as much of an issue as once thought.
It’s the same story with spironolactone. Hyperkalemia is a long-standing concern, but it turns out that “in healthy young women taking spironolactone for acne, we don’t need to be checking potassium.” As far as breast cancer goes, the potential risk with spironolactone hasn’t panned out in the literature, and there may not be “a link at all,” Dr. Harper said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
There are caveats, of course. Hormonal treatments shouldn’t be used in young women until they’ve established their menstrual cycle. OCs should not be used in smokers, or people who have hypertension or migraines, among other conditions. Also, elevated triglycerides remain a concern with isotretinoin. “The number I would want people to remember is 500 [mg/dL],” the threshold when triglycerides become a problem.
In the interview, Dr. Harper explained the new thinking on these three options, and shared her treatment tips, including what to do if patients’ triglycerides hit the 500 mg/dL mark.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR










