User login
Adding bevacizumab to chemo prolongs life in advanced cervical cancer
CHICAGO – Adding the antiangiogenic agent bevacizumab to chemotherapy improves overall survival in women who have advanced cervical cancer, according to findings of a randomized phase III trial of the Gynecologic Oncology Group.
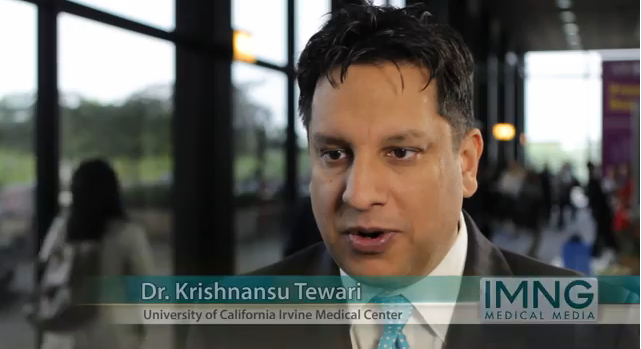
In the trial – Gynecologic Oncology Group (GOG) protocol 240 – women who received bevacizumab (Avastin) in addition to chemotherapy lived about 4 months longer than their counterparts who received chemotherapy alone, lead investigator Dr. Krishnansu S. Tewari reported in the plenary session at the annual meeting of the American Society of Clinical Oncology.
The adverse effects seen were largely consistent with previous experience in other cancers with bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor.
"This study met its primary endpoint," said Dr. Tewari, a professor of obstetrics and gynecology at the University of California, Irvine. "Bevacizumab is the first molecularly targeted agent to improve overall survival in a gynecologic cancer."
"Moving forward, the incorporation of anti-VEGF therapy into primary treatment for locally advanced disease should be considered ..." he said. Also, "these data open up doors to study other classes of antiangiogenesis agents – both VEGF-dependent molecules as well as non–VEGF-dependent molecules."
Invited discussant Dr. Gottfried E. Konecny of the University of California, Los Angeles, commented, "Progression-free and overall survival improvements through bevacizumab in advanced cervical cancer as shown in the GOG 240 study are clinically meaningful. Adverse events and toxicities are manageable and, I believe, class specific."
In his opinion, as only about a fifth of the patients had metastatic disease, it is uncertain whether the drug benefits that population. And the data suggest bevacizumab may have less benefit in patients with adenocarcinoma tumors as compared with squamous tumors.
For future research, "most important is the identification and validation of response predictors," according to Dr. Konecny.
"Extending the global reach of antiangiogenic therapies for advanced cervical cancer is critical," he concluded, noting that the countries with highest incidence and mortality rates for this disease also have the lowest annual health care spending. "I think GOG 240 can be seen as a practice-changing study. But we urgently need to develop antiangiogenic treatment strategies that will benefit the global population."
The trial was open to women who had chemotherapy-naive, recurrent, persistent, or metastatic cervical cancer. They were randomized in dual factorial design according to chemotherapy doublet (cisplatin plus paclitaxel vs. topotecan plus paclitaxel) and receipt of bevacizumab (yes vs. no), which was provided by Roche/Genentech, manufacturer of the recombinant monoclonal antibody. Treatment was on an open-label basis, and crossover was not allowed.
Bevacizumab is approved by the Food and Drug Administration for selected indications in colorectal, renal, and lung cancer, and in glioblastoma.
An interim analysis concluded that the topotecan-paclitaxel regimen was not superior (or inferior) to the cisplatin-paclitaxel regimen that has been the standard in this setting, Dr. Tewari reported.
With longer follow-up, to a median of 20.8 months, women who received added bevacizumab had a longer median overall survival than did their counterparts who received chemotherapy alone (17.0 vs. 13.3 months; hazard ratio, 0.71; P = .0035).
Bevacizumab conferred a significant survival benefit when added to cisplatin-paclitaxel chemotherapy (HR, 0.68; P = .03) but not when added to topotecan-paclitaxel chemotherapy (HR, 0.74; P = .09).
"The effect of bevacizumab was sustained with multiple prognostic factors, including when the disease was in the previously irradiated pelvis," Dr. Tewari noted when reporting subgroup analyses.
Women who received added bevacizumab also had a longer median progression-free survival (8.2 vs. 5.9 months; HR, 0.67; P = .0002) and a higher response rate (48% vs. 36%, P = .008).
Adding bevacizumab led to significantly higher rates of grade 3/4 gastrointestinal fistula (3% vs. 0%), genitourinary fistula (2% vs. 0%), and thromboembolism (8% vs. 1%); grade 2 or higher hypertension (25% vs. 2%); and grade 4 or higher neutropenia (35% vs. 26%).
"We saw no new adverse events in this patient population, which includes patients who had been heavily irradiated," Dr. Tewari commented. There were four deaths each in the groups treated with and without bevacizumab.
Addition of bevacizumab did not compromise health-related quality of life as assessed with the FACT(Functional Assessment of Cancer Therapy) – Cervical Cancer Trial Outcome Index.
Dr. Tewari disclosed no relevant conflicts of interest. Dr. Konecny disclosed that he receives honoraria from Genentech and Sanofi, and research funding from Amgen and Pfizer.
CHICAGO – Adding the antiangiogenic agent bevacizumab to chemotherapy improves overall survival in women who have advanced cervical cancer, according to findings of a randomized phase III trial of the Gynecologic Oncology Group.
In the trial – Gynecologic Oncology Group (GOG) protocol 240 – women who received bevacizumab (Avastin) in addition to chemotherapy lived about 4 months longer than their counterparts who received chemotherapy alone, lead investigator Dr. Krishnansu S. Tewari reported in the plenary session at the annual meeting of the American Society of Clinical Oncology.
The adverse effects seen were largely consistent with previous experience in other cancers with bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor.
"This study met its primary endpoint," said Dr. Tewari, a professor of obstetrics and gynecology at the University of California, Irvine. "Bevacizumab is the first molecularly targeted agent to improve overall survival in a gynecologic cancer."
"Moving forward, the incorporation of anti-VEGF therapy into primary treatment for locally advanced disease should be considered ..." he said. Also, "these data open up doors to study other classes of antiangiogenesis agents – both VEGF-dependent molecules as well as non–VEGF-dependent molecules."
Invited discussant Dr. Gottfried E. Konecny of the University of California, Los Angeles, commented, "Progression-free and overall survival improvements through bevacizumab in advanced cervical cancer as shown in the GOG 240 study are clinically meaningful. Adverse events and toxicities are manageable and, I believe, class specific."
In his opinion, as only about a fifth of the patients had metastatic disease, it is uncertain whether the drug benefits that population. And the data suggest bevacizumab may have less benefit in patients with adenocarcinoma tumors as compared with squamous tumors.
For future research, "most important is the identification and validation of response predictors," according to Dr. Konecny.
"Extending the global reach of antiangiogenic therapies for advanced cervical cancer is critical," he concluded, noting that the countries with highest incidence and mortality rates for this disease also have the lowest annual health care spending. "I think GOG 240 can be seen as a practice-changing study. But we urgently need to develop antiangiogenic treatment strategies that will benefit the global population."
The trial was open to women who had chemotherapy-naive, recurrent, persistent, or metastatic cervical cancer. They were randomized in dual factorial design according to chemotherapy doublet (cisplatin plus paclitaxel vs. topotecan plus paclitaxel) and receipt of bevacizumab (yes vs. no), which was provided by Roche/Genentech, manufacturer of the recombinant monoclonal antibody. Treatment was on an open-label basis, and crossover was not allowed.
Bevacizumab is approved by the Food and Drug Administration for selected indications in colorectal, renal, and lung cancer, and in glioblastoma.
An interim analysis concluded that the topotecan-paclitaxel regimen was not superior (or inferior) to the cisplatin-paclitaxel regimen that has been the standard in this setting, Dr. Tewari reported.
With longer follow-up, to a median of 20.8 months, women who received added bevacizumab had a longer median overall survival than did their counterparts who received chemotherapy alone (17.0 vs. 13.3 months; hazard ratio, 0.71; P = .0035).
Bevacizumab conferred a significant survival benefit when added to cisplatin-paclitaxel chemotherapy (HR, 0.68; P = .03) but not when added to topotecan-paclitaxel chemotherapy (HR, 0.74; P = .09).
"The effect of bevacizumab was sustained with multiple prognostic factors, including when the disease was in the previously irradiated pelvis," Dr. Tewari noted when reporting subgroup analyses.
Women who received added bevacizumab also had a longer median progression-free survival (8.2 vs. 5.9 months; HR, 0.67; P = .0002) and a higher response rate (48% vs. 36%, P = .008).
Adding bevacizumab led to significantly higher rates of grade 3/4 gastrointestinal fistula (3% vs. 0%), genitourinary fistula (2% vs. 0%), and thromboembolism (8% vs. 1%); grade 2 or higher hypertension (25% vs. 2%); and grade 4 or higher neutropenia (35% vs. 26%).
"We saw no new adverse events in this patient population, which includes patients who had been heavily irradiated," Dr. Tewari commented. There were four deaths each in the groups treated with and without bevacizumab.
Addition of bevacizumab did not compromise health-related quality of life as assessed with the FACT(Functional Assessment of Cancer Therapy) – Cervical Cancer Trial Outcome Index.
Dr. Tewari disclosed no relevant conflicts of interest. Dr. Konecny disclosed that he receives honoraria from Genentech and Sanofi, and research funding from Amgen and Pfizer.
CHICAGO – Adding the antiangiogenic agent bevacizumab to chemotherapy improves overall survival in women who have advanced cervical cancer, according to findings of a randomized phase III trial of the Gynecologic Oncology Group.
In the trial – Gynecologic Oncology Group (GOG) protocol 240 – women who received bevacizumab (Avastin) in addition to chemotherapy lived about 4 months longer than their counterparts who received chemotherapy alone, lead investigator Dr. Krishnansu S. Tewari reported in the plenary session at the annual meeting of the American Society of Clinical Oncology.
The adverse effects seen were largely consistent with previous experience in other cancers with bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor.
"This study met its primary endpoint," said Dr. Tewari, a professor of obstetrics and gynecology at the University of California, Irvine. "Bevacizumab is the first molecularly targeted agent to improve overall survival in a gynecologic cancer."
"Moving forward, the incorporation of anti-VEGF therapy into primary treatment for locally advanced disease should be considered ..." he said. Also, "these data open up doors to study other classes of antiangiogenesis agents – both VEGF-dependent molecules as well as non–VEGF-dependent molecules."
Invited discussant Dr. Gottfried E. Konecny of the University of California, Los Angeles, commented, "Progression-free and overall survival improvements through bevacizumab in advanced cervical cancer as shown in the GOG 240 study are clinically meaningful. Adverse events and toxicities are manageable and, I believe, class specific."
In his opinion, as only about a fifth of the patients had metastatic disease, it is uncertain whether the drug benefits that population. And the data suggest bevacizumab may have less benefit in patients with adenocarcinoma tumors as compared with squamous tumors.
For future research, "most important is the identification and validation of response predictors," according to Dr. Konecny.
"Extending the global reach of antiangiogenic therapies for advanced cervical cancer is critical," he concluded, noting that the countries with highest incidence and mortality rates for this disease also have the lowest annual health care spending. "I think GOG 240 can be seen as a practice-changing study. But we urgently need to develop antiangiogenic treatment strategies that will benefit the global population."
The trial was open to women who had chemotherapy-naive, recurrent, persistent, or metastatic cervical cancer. They were randomized in dual factorial design according to chemotherapy doublet (cisplatin plus paclitaxel vs. topotecan plus paclitaxel) and receipt of bevacizumab (yes vs. no), which was provided by Roche/Genentech, manufacturer of the recombinant monoclonal antibody. Treatment was on an open-label basis, and crossover was not allowed.
Bevacizumab is approved by the Food and Drug Administration for selected indications in colorectal, renal, and lung cancer, and in glioblastoma.
An interim analysis concluded that the topotecan-paclitaxel regimen was not superior (or inferior) to the cisplatin-paclitaxel regimen that has been the standard in this setting, Dr. Tewari reported.
With longer follow-up, to a median of 20.8 months, women who received added bevacizumab had a longer median overall survival than did their counterparts who received chemotherapy alone (17.0 vs. 13.3 months; hazard ratio, 0.71; P = .0035).
Bevacizumab conferred a significant survival benefit when added to cisplatin-paclitaxel chemotherapy (HR, 0.68; P = .03) but not when added to topotecan-paclitaxel chemotherapy (HR, 0.74; P = .09).
"The effect of bevacizumab was sustained with multiple prognostic factors, including when the disease was in the previously irradiated pelvis," Dr. Tewari noted when reporting subgroup analyses.
Women who received added bevacizumab also had a longer median progression-free survival (8.2 vs. 5.9 months; HR, 0.67; P = .0002) and a higher response rate (48% vs. 36%, P = .008).
Adding bevacizumab led to significantly higher rates of grade 3/4 gastrointestinal fistula (3% vs. 0%), genitourinary fistula (2% vs. 0%), and thromboembolism (8% vs. 1%); grade 2 or higher hypertension (25% vs. 2%); and grade 4 or higher neutropenia (35% vs. 26%).
"We saw no new adverse events in this patient population, which includes patients who had been heavily irradiated," Dr. Tewari commented. There were four deaths each in the groups treated with and without bevacizumab.
Addition of bevacizumab did not compromise health-related quality of life as assessed with the FACT(Functional Assessment of Cancer Therapy) – Cervical Cancer Trial Outcome Index.
Dr. Tewari disclosed no relevant conflicts of interest. Dr. Konecny disclosed that he receives honoraria from Genentech and Sanofi, and research funding from Amgen and Pfizer.
AT THE ASCO ANNUAL MEETING 2013
Major Finding: Median overall survival was 17.0 months with bevacizumab plus chemotherapy vs. 13.3 months with chemotherapy alone (P = .0035).
Data Source: A randomized phase III trial (GOG 240) among 452 women with recurrent, persistent, or metastatic cervical cancer.
Disclosures: Dr. Tewari disclosed no relevant conflicts of interest. Roche/Genentech provided bevacizumab for the trial. Dr. Konecny disclosed that he receives honoraria from Genentech and Sanofi, and research funding from Amgen and Pfizer.
Nivolumab activity is durable in advanced melanoma
CHICAGO – Nivolumab, an investigational PD-1 inhibitor, shrank tumors by at least 30% in 33 of 107 pretreated patients with metastatic melanoma, based on results from an expanded phase I trial reported at the annual meeting of the American Society of Clinical Oncology.
An additional 11% of patients had prolonged stable disease or non-conventional, immune-related response profiles.
The findings build on favorable initial results reported at last year’s annual meeting for nivolumab in melanoma, renal cell carcinoma, and nonsmall cell lung cancer. The latest results in melanoma patients at follow-ups as long as 2 years indicate no new safety signals with nivolumab, which was associated with a 21% rate of grade 3-4 events. The rate of severe immune-related adverse events was 5%, and there were no cases of grade 3 or higher pneumonitis.
Median overall survival was nearly 17 months with nivolumab, with a 2-year survival rate of 43%. Median overall survival with vemurafenib (Zelboraf) is 16 months and with ipilimumab (Yervoy) is 10 months, with 2-year survival rates of 24% to 33% with ipilimumab, according to Dr. Mario Sznol, who presented the results from the phase I trial.
"We’re very excited that there is potential for even more activity [with nivolumab] in combination with other drugs," said Dr. Sznol, professor of medicine (medical oncology) at the Yale Cancer Center in New Haven, Conn.
Responses were seen at all five dose levels tested (0.1, 0.3, 1, 3, and 10 mg/kg), with a 41% objective response rate at the 3 mg/kg dose, which has been selected for evaluation in phase III studies.
Median overall survival across all doses of nivolumab was 16.8 months. It reached 20.3 months for the 3 mg/kg dose. The 1-year survival rate was 62% and the 2-year survival rate, 43%.
Patients in the nivolumab trial were representative of typical patients with advanced melanoma, Dr. Sznol said. All patients in the study had disease that worsened despite prior standard systemic therapies, 25% of them had three or more prior therapies and 63% had two or more prior therapies.
All had ECOG performance standards of 0 or 1. Patients received up to 12 cycles of treatment, with four doses of nivolumab per cycle, until discontinuation criteria were met.
Response has persisted after stopping treatment in 17 of 33 patients, with 12 of the 17 continuing to respond for at least 4 months, Dr. Sznol said.
The overall objective response rate included partial and complete responses. Dr. Sznol acknowledged that just one patient had a verified complete response to nivolumab and four others had near complete responses at 2 years. Historical response rates to immunotherapy drugs are 5%-10% in advanced melanoma, he noted, which is lower than the 30% response seen in these pretreated patients.
"I have seen a few relapses after 2 years of response, but some patients continue to do well at 4 years. One patient who has been off nivolumab for 2 years continues to do well at over 4 years," Dr. Sznol commented during a question and answer session.
To define the best candidates for nivolumab, molecular markers need to be identified to predict probable response, he added. As nivolumab is a PD-1 inhibitor, one potential marker is the protein PD-L1 on the surface of tumor cells, which is being studied in several other clinical trials.
The research was supported by Bristol-Myers Squibb. Dr. Sznol disclosed that he serves in a consultant or advisory role with Bristol-Myers Squibb.
CHICAGO – Nivolumab, an investigational PD-1 inhibitor, shrank tumors by at least 30% in 33 of 107 pretreated patients with metastatic melanoma, based on results from an expanded phase I trial reported at the annual meeting of the American Society of Clinical Oncology.
An additional 11% of patients had prolonged stable disease or non-conventional, immune-related response profiles.
The findings build on favorable initial results reported at last year’s annual meeting for nivolumab in melanoma, renal cell carcinoma, and nonsmall cell lung cancer. The latest results in melanoma patients at follow-ups as long as 2 years indicate no new safety signals with nivolumab, which was associated with a 21% rate of grade 3-4 events. The rate of severe immune-related adverse events was 5%, and there were no cases of grade 3 or higher pneumonitis.
Median overall survival was nearly 17 months with nivolumab, with a 2-year survival rate of 43%. Median overall survival with vemurafenib (Zelboraf) is 16 months and with ipilimumab (Yervoy) is 10 months, with 2-year survival rates of 24% to 33% with ipilimumab, according to Dr. Mario Sznol, who presented the results from the phase I trial.
"We’re very excited that there is potential for even more activity [with nivolumab] in combination with other drugs," said Dr. Sznol, professor of medicine (medical oncology) at the Yale Cancer Center in New Haven, Conn.
Responses were seen at all five dose levels tested (0.1, 0.3, 1, 3, and 10 mg/kg), with a 41% objective response rate at the 3 mg/kg dose, which has been selected for evaluation in phase III studies.
Median overall survival across all doses of nivolumab was 16.8 months. It reached 20.3 months for the 3 mg/kg dose. The 1-year survival rate was 62% and the 2-year survival rate, 43%.
Patients in the nivolumab trial were representative of typical patients with advanced melanoma, Dr. Sznol said. All patients in the study had disease that worsened despite prior standard systemic therapies, 25% of them had three or more prior therapies and 63% had two or more prior therapies.
All had ECOG performance standards of 0 or 1. Patients received up to 12 cycles of treatment, with four doses of nivolumab per cycle, until discontinuation criteria were met.
Response has persisted after stopping treatment in 17 of 33 patients, with 12 of the 17 continuing to respond for at least 4 months, Dr. Sznol said.
The overall objective response rate included partial and complete responses. Dr. Sznol acknowledged that just one patient had a verified complete response to nivolumab and four others had near complete responses at 2 years. Historical response rates to immunotherapy drugs are 5%-10% in advanced melanoma, he noted, which is lower than the 30% response seen in these pretreated patients.
"I have seen a few relapses after 2 years of response, but some patients continue to do well at 4 years. One patient who has been off nivolumab for 2 years continues to do well at over 4 years," Dr. Sznol commented during a question and answer session.
To define the best candidates for nivolumab, molecular markers need to be identified to predict probable response, he added. As nivolumab is a PD-1 inhibitor, one potential marker is the protein PD-L1 on the surface of tumor cells, which is being studied in several other clinical trials.
The research was supported by Bristol-Myers Squibb. Dr. Sznol disclosed that he serves in a consultant or advisory role with Bristol-Myers Squibb.
CHICAGO – Nivolumab, an investigational PD-1 inhibitor, shrank tumors by at least 30% in 33 of 107 pretreated patients with metastatic melanoma, based on results from an expanded phase I trial reported at the annual meeting of the American Society of Clinical Oncology.
An additional 11% of patients had prolonged stable disease or non-conventional, immune-related response profiles.
The findings build on favorable initial results reported at last year’s annual meeting for nivolumab in melanoma, renal cell carcinoma, and nonsmall cell lung cancer. The latest results in melanoma patients at follow-ups as long as 2 years indicate no new safety signals with nivolumab, which was associated with a 21% rate of grade 3-4 events. The rate of severe immune-related adverse events was 5%, and there were no cases of grade 3 or higher pneumonitis.
Median overall survival was nearly 17 months with nivolumab, with a 2-year survival rate of 43%. Median overall survival with vemurafenib (Zelboraf) is 16 months and with ipilimumab (Yervoy) is 10 months, with 2-year survival rates of 24% to 33% with ipilimumab, according to Dr. Mario Sznol, who presented the results from the phase I trial.
"We’re very excited that there is potential for even more activity [with nivolumab] in combination with other drugs," said Dr. Sznol, professor of medicine (medical oncology) at the Yale Cancer Center in New Haven, Conn.
Responses were seen at all five dose levels tested (0.1, 0.3, 1, 3, and 10 mg/kg), with a 41% objective response rate at the 3 mg/kg dose, which has been selected for evaluation in phase III studies.
Median overall survival across all doses of nivolumab was 16.8 months. It reached 20.3 months for the 3 mg/kg dose. The 1-year survival rate was 62% and the 2-year survival rate, 43%.
Patients in the nivolumab trial were representative of typical patients with advanced melanoma, Dr. Sznol said. All patients in the study had disease that worsened despite prior standard systemic therapies, 25% of them had three or more prior therapies and 63% had two or more prior therapies.
All had ECOG performance standards of 0 or 1. Patients received up to 12 cycles of treatment, with four doses of nivolumab per cycle, until discontinuation criteria were met.
Response has persisted after stopping treatment in 17 of 33 patients, with 12 of the 17 continuing to respond for at least 4 months, Dr. Sznol said.
The overall objective response rate included partial and complete responses. Dr. Sznol acknowledged that just one patient had a verified complete response to nivolumab and four others had near complete responses at 2 years. Historical response rates to immunotherapy drugs are 5%-10% in advanced melanoma, he noted, which is lower than the 30% response seen in these pretreated patients.
"I have seen a few relapses after 2 years of response, but some patients continue to do well at 4 years. One patient who has been off nivolumab for 2 years continues to do well at over 4 years," Dr. Sznol commented during a question and answer session.
To define the best candidates for nivolumab, molecular markers need to be identified to predict probable response, he added. As nivolumab is a PD-1 inhibitor, one potential marker is the protein PD-L1 on the surface of tumor cells, which is being studied in several other clinical trials.
The research was supported by Bristol-Myers Squibb. Dr. Sznol disclosed that he serves in a consultant or advisory role with Bristol-Myers Squibb.
AT THE ASCO ANNUAL MEETING 2013
Major finding: Median overall survival across all doses of nivolumab was 16.8 months. It reached 20.3 months for the 3 mg/kg dose that will be used in phase III studies.
Data source: An expanded phase I study of 107 pretreated patients with metastatic melanoma.
Disclosures: The research was supported by Bristol-Myers Squibb. Dr. Sznol disclosed that he serves in a consultant or advisory role with Bristol-Myers Squibb.
Cervical cancer screening with acetic acid saves lives
CHICAGO — A simple approach to cervical cancer screening that can be easily implemented in resource-strapped settings could save tens of thousands of lives worldwide each year, researchers reported at the annual meeting of the American Society of Clinical Oncology.
A team led by Dr. Surendra Srinivas Shastri, a professor of preventive oncology at Tata Memorial Center in Mumbai, India, conducted a cluster-randomized trial of visual inspection with acetic acid (VIA) among more than 150,000 women living in some of India’s poorest areas.
Main results showed that women who had four rounds of biennial screening with VIA delivered by trained health workers were almost a third less likely to die from cervical cancer than peers who were not screened, which is usual care in India given the lack of infrastructure and resources for country-wide Pap screening.
Of note, the health workers were women, who were drawn mainly from the local community and who had a 10th grade education, according Dr. Shastri. Such professionals can reach remote and rural areas of the country, he said. Additionally, the VIA technique does not require a laboratory and provides immediate results.
"It is widely implementable in the lowest-resource settings in India or wherever," he said in a press briefing. "If implemented nationally in India, it would prevent around 22,000 cervical cancer deaths in the country. And if taken globally in middle-income and low-resource countries, we could prevent around 72,000 deaths in developing countries annually."
On the basis of the trial’s results, India is preparing to implement the VIA program nationally. He said that training of health workers, using a train-the-trainers approach, and a quality control process could be completed within 6 months.
Women receiving a cancer diagnosis through the VIA program will have access to treatment, assured Dr. Rajendra A. Badwe, one of the trial’s co-investigators and director of the Tata Memorial Center.
"There is a cafeteria choice" in India, he explained. "Individuals can have free treatment, individuals can pay for treatment. And treatment is currently available in 34 regional cancer centers run by the government of India. The way I see it, for any screening trial, if there is something done [to identify cancer], we need to be proactively with them to see that they reach the closest treatment facility... [Treatment] will be made available – no question."
The trial has established that VIA is "a good alternative" to Pap screening in limited-resource settings, according to Electra D. Paskett, Ph.D., co-program leader of the Cancer Control Program in the Comprehensive Cancer Center at the Ohio State University in Columbus and an ASCO spokesperson. And there may well be a role for VIA screening in some settings in developed countries.
"From my perspective, it could be an alternative ... in more resource-rich countries where there are these pockets of high cervical cancer incidence and mortality that don’t have access to the same treatment resources that the rest of the country does," she said in the press briefing. "And we do have those pockets here in the United States."
The trial was conducted in 20 impoverished areas of Mumbai that were assigned evenly to VIA screening and control groups. The target population was women aged 35 to 64 years who had no prior history of cancer.
Women in both groups received cancer education. In the VIA group, women underwent four rounds of biennial screening; those with positive results were referred to a health facility for definitive diagnosis. In the control group, women were asked to report to the health workers any signs or symptoms of cervical cancer; those doing so were similarly referred to a health facility. All women receiving a cancer diagnosis were offered free treatment.
Analyses were based on 75,360 women in the VIA screening group and 76,178 women in the control group.
"The screening participation rate was 89% – huge for a country like India and most other cases," Dr. Shastri commented. Participation in education in the control group was also high, at 91%. Overall, 86% of women with a confirmed case of cervical cancer received treatment.
The screening and control groups did not differ significantly with respect to the incidence of invasive cervical cancer (26.7 vs. 27.5 per 100,000), suggesting that screening did not lead to overdiagnosis.
"Overdiagnosis is a huge problem in all screening programs and all screening trials," Dr. Shastri noted. "There was almost zero overdiagnosis in this trial. In fact, what little overdiagnosis we had, that ceased by the eighth year."
In intention-to-treat analysis, screening with VIA resulted in a 31% reduction in the cervical cancer–specific death rates (11.1 vs. 16.2 deaths per 100,000; mortality rate ratio, 0.69, P = .003).
Screened women also had a 7% reduction in overall mortality stemming from earlier diagnosis, although this difference was not statistically significant.
Dr. Shastri disclosed no relevant conflicts of interest. Dr. Badwe disclosed no relevant conflicts of interest.
CHICAGO — A simple approach to cervical cancer screening that can be easily implemented in resource-strapped settings could save tens of thousands of lives worldwide each year, researchers reported at the annual meeting of the American Society of Clinical Oncology.
A team led by Dr. Surendra Srinivas Shastri, a professor of preventive oncology at Tata Memorial Center in Mumbai, India, conducted a cluster-randomized trial of visual inspection with acetic acid (VIA) among more than 150,000 women living in some of India’s poorest areas.
Main results showed that women who had four rounds of biennial screening with VIA delivered by trained health workers were almost a third less likely to die from cervical cancer than peers who were not screened, which is usual care in India given the lack of infrastructure and resources for country-wide Pap screening.
Of note, the health workers were women, who were drawn mainly from the local community and who had a 10th grade education, according Dr. Shastri. Such professionals can reach remote and rural areas of the country, he said. Additionally, the VIA technique does not require a laboratory and provides immediate results.
"It is widely implementable in the lowest-resource settings in India or wherever," he said in a press briefing. "If implemented nationally in India, it would prevent around 22,000 cervical cancer deaths in the country. And if taken globally in middle-income and low-resource countries, we could prevent around 72,000 deaths in developing countries annually."
On the basis of the trial’s results, India is preparing to implement the VIA program nationally. He said that training of health workers, using a train-the-trainers approach, and a quality control process could be completed within 6 months.
Women receiving a cancer diagnosis through the VIA program will have access to treatment, assured Dr. Rajendra A. Badwe, one of the trial’s co-investigators and director of the Tata Memorial Center.
"There is a cafeteria choice" in India, he explained. "Individuals can have free treatment, individuals can pay for treatment. And treatment is currently available in 34 regional cancer centers run by the government of India. The way I see it, for any screening trial, if there is something done [to identify cancer], we need to be proactively with them to see that they reach the closest treatment facility... [Treatment] will be made available – no question."
The trial has established that VIA is "a good alternative" to Pap screening in limited-resource settings, according to Electra D. Paskett, Ph.D., co-program leader of the Cancer Control Program in the Comprehensive Cancer Center at the Ohio State University in Columbus and an ASCO spokesperson. And there may well be a role for VIA screening in some settings in developed countries.
"From my perspective, it could be an alternative ... in more resource-rich countries where there are these pockets of high cervical cancer incidence and mortality that don’t have access to the same treatment resources that the rest of the country does," she said in the press briefing. "And we do have those pockets here in the United States."
The trial was conducted in 20 impoverished areas of Mumbai that were assigned evenly to VIA screening and control groups. The target population was women aged 35 to 64 years who had no prior history of cancer.
Women in both groups received cancer education. In the VIA group, women underwent four rounds of biennial screening; those with positive results were referred to a health facility for definitive diagnosis. In the control group, women were asked to report to the health workers any signs or symptoms of cervical cancer; those doing so were similarly referred to a health facility. All women receiving a cancer diagnosis were offered free treatment.
Analyses were based on 75,360 women in the VIA screening group and 76,178 women in the control group.
"The screening participation rate was 89% – huge for a country like India and most other cases," Dr. Shastri commented. Participation in education in the control group was also high, at 91%. Overall, 86% of women with a confirmed case of cervical cancer received treatment.
The screening and control groups did not differ significantly with respect to the incidence of invasive cervical cancer (26.7 vs. 27.5 per 100,000), suggesting that screening did not lead to overdiagnosis.
"Overdiagnosis is a huge problem in all screening programs and all screening trials," Dr. Shastri noted. "There was almost zero overdiagnosis in this trial. In fact, what little overdiagnosis we had, that ceased by the eighth year."
In intention-to-treat analysis, screening with VIA resulted in a 31% reduction in the cervical cancer–specific death rates (11.1 vs. 16.2 deaths per 100,000; mortality rate ratio, 0.69, P = .003).
Screened women also had a 7% reduction in overall mortality stemming from earlier diagnosis, although this difference was not statistically significant.
Dr. Shastri disclosed no relevant conflicts of interest. Dr. Badwe disclosed no relevant conflicts of interest.
CHICAGO — A simple approach to cervical cancer screening that can be easily implemented in resource-strapped settings could save tens of thousands of lives worldwide each year, researchers reported at the annual meeting of the American Society of Clinical Oncology.
A team led by Dr. Surendra Srinivas Shastri, a professor of preventive oncology at Tata Memorial Center in Mumbai, India, conducted a cluster-randomized trial of visual inspection with acetic acid (VIA) among more than 150,000 women living in some of India’s poorest areas.
Main results showed that women who had four rounds of biennial screening with VIA delivered by trained health workers were almost a third less likely to die from cervical cancer than peers who were not screened, which is usual care in India given the lack of infrastructure and resources for country-wide Pap screening.
Of note, the health workers were women, who were drawn mainly from the local community and who had a 10th grade education, according Dr. Shastri. Such professionals can reach remote and rural areas of the country, he said. Additionally, the VIA technique does not require a laboratory and provides immediate results.
"It is widely implementable in the lowest-resource settings in India or wherever," he said in a press briefing. "If implemented nationally in India, it would prevent around 22,000 cervical cancer deaths in the country. And if taken globally in middle-income and low-resource countries, we could prevent around 72,000 deaths in developing countries annually."
On the basis of the trial’s results, India is preparing to implement the VIA program nationally. He said that training of health workers, using a train-the-trainers approach, and a quality control process could be completed within 6 months.
Women receiving a cancer diagnosis through the VIA program will have access to treatment, assured Dr. Rajendra A. Badwe, one of the trial’s co-investigators and director of the Tata Memorial Center.
"There is a cafeteria choice" in India, he explained. "Individuals can have free treatment, individuals can pay for treatment. And treatment is currently available in 34 regional cancer centers run by the government of India. The way I see it, for any screening trial, if there is something done [to identify cancer], we need to be proactively with them to see that they reach the closest treatment facility... [Treatment] will be made available – no question."
The trial has established that VIA is "a good alternative" to Pap screening in limited-resource settings, according to Electra D. Paskett, Ph.D., co-program leader of the Cancer Control Program in the Comprehensive Cancer Center at the Ohio State University in Columbus and an ASCO spokesperson. And there may well be a role for VIA screening in some settings in developed countries.
"From my perspective, it could be an alternative ... in more resource-rich countries where there are these pockets of high cervical cancer incidence and mortality that don’t have access to the same treatment resources that the rest of the country does," she said in the press briefing. "And we do have those pockets here in the United States."
The trial was conducted in 20 impoverished areas of Mumbai that were assigned evenly to VIA screening and control groups. The target population was women aged 35 to 64 years who had no prior history of cancer.
Women in both groups received cancer education. In the VIA group, women underwent four rounds of biennial screening; those with positive results were referred to a health facility for definitive diagnosis. In the control group, women were asked to report to the health workers any signs or symptoms of cervical cancer; those doing so were similarly referred to a health facility. All women receiving a cancer diagnosis were offered free treatment.
Analyses were based on 75,360 women in the VIA screening group and 76,178 women in the control group.
"The screening participation rate was 89% – huge for a country like India and most other cases," Dr. Shastri commented. Participation in education in the control group was also high, at 91%. Overall, 86% of women with a confirmed case of cervical cancer received treatment.
The screening and control groups did not differ significantly with respect to the incidence of invasive cervical cancer (26.7 vs. 27.5 per 100,000), suggesting that screening did not lead to overdiagnosis.
"Overdiagnosis is a huge problem in all screening programs and all screening trials," Dr. Shastri noted. "There was almost zero overdiagnosis in this trial. In fact, what little overdiagnosis we had, that ceased by the eighth year."
In intention-to-treat analysis, screening with VIA resulted in a 31% reduction in the cervical cancer–specific death rates (11.1 vs. 16.2 deaths per 100,000; mortality rate ratio, 0.69, P = .003).
Screened women also had a 7% reduction in overall mortality stemming from earlier diagnosis, although this difference was not statistically significant.
Dr. Shastri disclosed no relevant conflicts of interest. Dr. Badwe disclosed no relevant conflicts of interest.
AT THE ASCO ANNUAL MEETING 2013
Major finding: Compared with usual care (no screening), biennial visual inspection of the cervix with acetic acid delivered by health workers reduced cervical cancer mortality by 31%.
Data source: A cluster-randomized trial involving 151,538 women from 20 impoverished areas in India
Disclosures: Dr. Shastri disclosed no relevant conflicts of interest.
Bevacizumab plus irinotecan beats temozolomide in stalling glioblastoma
CHICAGO – The combination of bevacizumab and irinotecan far outshone tenozolomide in delaying disease progression among patients with MGMT-unmethylated glioblastoma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
In the phase II GLARIUS trial, 79.6% of patients treated with bevacizumab (Avastin) and irinotecan (Camptosar) were free of progression at 6 months, compared with 41.3% of patients randomized to receive temozolmide (Temodar) (P less than .0001), reported Dr. Ulrich Herrlinger from the department of neurology at University Clinic of Bonn, Germany.
Progression-free survival at 6 months (PFS-6) was the study's primary endpoint.
A preliminary analysis also hinted at a potential overall survival advantage for the bevacizumab-irinotecan (BEV/IRI) combination. After nearly 50% of patients in each arm had died, median overall survival was 16.6 months for the BEV/IRI group, compared with 14.8 months for the temozolomide group (hazard ratio, 0.60; P = .031).
"Obviously we did not harm our patients by omitting temozolomide and choosing something different, BEV/IRI, for treating these patients," Dr. Herrlinger said.
The combination is a promising alternative to temozolomide therapy in patients with with MGMT (O6-methylguanine-DNA methyltransferase)-unmethylated glioblastoma, he said. About 55%-65% of newly diagnosed glioblastomas are not methylated by MGMT, a DNA repair enzyme, and these have a worse prognosis than those in which MGMT promotes methylation, according to Dr. Herrlinger.
Investigators at 22 centers in Germany tested patients with glioblastoma for MGMT status, and randomized a total of 182 patients with newly diagnosed, histologically confirmed MGMT-unmethylated glioblastoma. Of these, 170 received at least one course of drug therapy and were evaluable for response; these patients were included in the analysis.
All patients received 60 Gy localized radiation in 30 fragments of 2 Gy each. They were randomized 2:1 to BEV-IRI (116 patients) or temozolomide (54 patients).
The experimental arm received bevacizumab 10 mg/kg every 2 weeks during radiotherapy followed by maintenance bevacizumab at the same dose and irinotecan 125 mg/m2 every 2 weeks without or with enzyme-inducing antiepileptic drugs at a dose of 340 mg/m2. The standard therapy arm was given temozolomide 75 mg/m2 daily during radiotherapy, followed by 6 courses of temozolomide 150-200 mg/m2 for 5 days every 4 weeks.
In addition to the advantage in progression-free survival at 6 months, median progression-free survival also was longer with BEV/IRI: 9.74 months vs. 6 months in patients treated with temozolomide (HR 0.30, P less than .0001).
In addition patients on the combination used fewer mean daily steroids than patients on temozolomide.
The safety analysis showed that grade 3 or 4 vascular disorders – including deep vein thrombosis, pulmonary embolism, and hypertension – occurred in 10.9% of patients on BEV/IRI, compared with 3.6% of those on temozolomide. The combination was also associated with more grade 3 or 4 diarrhea and nausea, wound infections, and proteinuria. However, hematotoxicity was higher among patients on temozolomide, occurring in 14.8%, compared with 1.7% of patients on BEV/IRI.
"I think it’s important to recognize that there is a [bevacizumab] toxicity signal," said Dr Albert Lai, a neuro-oncologist at the University of California, Los Angeles, who was the invited discussant.
Dr. Lai commented that the overall survival signal seen by the GLARIUS investigators may have been affected by an optional crossover to BEV/IRI after disease progression on temozolomide. Of the 54 patients in the temozolomide arm, 29 crossed over to BEV/IRI.
The GLARIUS trial was sponsored by Hoffman-La Roche. Dr. Herrlinger disclosed being a consultant and speaker and receiving research support from the company. Dr. Lai disclosed serving as a consultant and receiving research funding from Genentech/Roche.
CHICAGO – The combination of bevacizumab and irinotecan far outshone tenozolomide in delaying disease progression among patients with MGMT-unmethylated glioblastoma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
In the phase II GLARIUS trial, 79.6% of patients treated with bevacizumab (Avastin) and irinotecan (Camptosar) were free of progression at 6 months, compared with 41.3% of patients randomized to receive temozolmide (Temodar) (P less than .0001), reported Dr. Ulrich Herrlinger from the department of neurology at University Clinic of Bonn, Germany.
Progression-free survival at 6 months (PFS-6) was the study's primary endpoint.
A preliminary analysis also hinted at a potential overall survival advantage for the bevacizumab-irinotecan (BEV/IRI) combination. After nearly 50% of patients in each arm had died, median overall survival was 16.6 months for the BEV/IRI group, compared with 14.8 months for the temozolomide group (hazard ratio, 0.60; P = .031).
"Obviously we did not harm our patients by omitting temozolomide and choosing something different, BEV/IRI, for treating these patients," Dr. Herrlinger said.
The combination is a promising alternative to temozolomide therapy in patients with with MGMT (O6-methylguanine-DNA methyltransferase)-unmethylated glioblastoma, he said. About 55%-65% of newly diagnosed glioblastomas are not methylated by MGMT, a DNA repair enzyme, and these have a worse prognosis than those in which MGMT promotes methylation, according to Dr. Herrlinger.
Investigators at 22 centers in Germany tested patients with glioblastoma for MGMT status, and randomized a total of 182 patients with newly diagnosed, histologically confirmed MGMT-unmethylated glioblastoma. Of these, 170 received at least one course of drug therapy and were evaluable for response; these patients were included in the analysis.
All patients received 60 Gy localized radiation in 30 fragments of 2 Gy each. They were randomized 2:1 to BEV-IRI (116 patients) or temozolomide (54 patients).
The experimental arm received bevacizumab 10 mg/kg every 2 weeks during radiotherapy followed by maintenance bevacizumab at the same dose and irinotecan 125 mg/m2 every 2 weeks without or with enzyme-inducing antiepileptic drugs at a dose of 340 mg/m2. The standard therapy arm was given temozolomide 75 mg/m2 daily during radiotherapy, followed by 6 courses of temozolomide 150-200 mg/m2 for 5 days every 4 weeks.
In addition to the advantage in progression-free survival at 6 months, median progression-free survival also was longer with BEV/IRI: 9.74 months vs. 6 months in patients treated with temozolomide (HR 0.30, P less than .0001).
In addition patients on the combination used fewer mean daily steroids than patients on temozolomide.
The safety analysis showed that grade 3 or 4 vascular disorders – including deep vein thrombosis, pulmonary embolism, and hypertension – occurred in 10.9% of patients on BEV/IRI, compared with 3.6% of those on temozolomide. The combination was also associated with more grade 3 or 4 diarrhea and nausea, wound infections, and proteinuria. However, hematotoxicity was higher among patients on temozolomide, occurring in 14.8%, compared with 1.7% of patients on BEV/IRI.
"I think it’s important to recognize that there is a [bevacizumab] toxicity signal," said Dr Albert Lai, a neuro-oncologist at the University of California, Los Angeles, who was the invited discussant.
Dr. Lai commented that the overall survival signal seen by the GLARIUS investigators may have been affected by an optional crossover to BEV/IRI after disease progression on temozolomide. Of the 54 patients in the temozolomide arm, 29 crossed over to BEV/IRI.
The GLARIUS trial was sponsored by Hoffman-La Roche. Dr. Herrlinger disclosed being a consultant and speaker and receiving research support from the company. Dr. Lai disclosed serving as a consultant and receiving research funding from Genentech/Roche.
CHICAGO – The combination of bevacizumab and irinotecan far outshone tenozolomide in delaying disease progression among patients with MGMT-unmethylated glioblastoma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
In the phase II GLARIUS trial, 79.6% of patients treated with bevacizumab (Avastin) and irinotecan (Camptosar) were free of progression at 6 months, compared with 41.3% of patients randomized to receive temozolmide (Temodar) (P less than .0001), reported Dr. Ulrich Herrlinger from the department of neurology at University Clinic of Bonn, Germany.
Progression-free survival at 6 months (PFS-6) was the study's primary endpoint.
A preliminary analysis also hinted at a potential overall survival advantage for the bevacizumab-irinotecan (BEV/IRI) combination. After nearly 50% of patients in each arm had died, median overall survival was 16.6 months for the BEV/IRI group, compared with 14.8 months for the temozolomide group (hazard ratio, 0.60; P = .031).
"Obviously we did not harm our patients by omitting temozolomide and choosing something different, BEV/IRI, for treating these patients," Dr. Herrlinger said.
The combination is a promising alternative to temozolomide therapy in patients with with MGMT (O6-methylguanine-DNA methyltransferase)-unmethylated glioblastoma, he said. About 55%-65% of newly diagnosed glioblastomas are not methylated by MGMT, a DNA repair enzyme, and these have a worse prognosis than those in which MGMT promotes methylation, according to Dr. Herrlinger.
Investigators at 22 centers in Germany tested patients with glioblastoma for MGMT status, and randomized a total of 182 patients with newly diagnosed, histologically confirmed MGMT-unmethylated glioblastoma. Of these, 170 received at least one course of drug therapy and were evaluable for response; these patients were included in the analysis.
All patients received 60 Gy localized radiation in 30 fragments of 2 Gy each. They were randomized 2:1 to BEV-IRI (116 patients) or temozolomide (54 patients).
The experimental arm received bevacizumab 10 mg/kg every 2 weeks during radiotherapy followed by maintenance bevacizumab at the same dose and irinotecan 125 mg/m2 every 2 weeks without or with enzyme-inducing antiepileptic drugs at a dose of 340 mg/m2. The standard therapy arm was given temozolomide 75 mg/m2 daily during radiotherapy, followed by 6 courses of temozolomide 150-200 mg/m2 for 5 days every 4 weeks.
In addition to the advantage in progression-free survival at 6 months, median progression-free survival also was longer with BEV/IRI: 9.74 months vs. 6 months in patients treated with temozolomide (HR 0.30, P less than .0001).
In addition patients on the combination used fewer mean daily steroids than patients on temozolomide.
The safety analysis showed that grade 3 or 4 vascular disorders – including deep vein thrombosis, pulmonary embolism, and hypertension – occurred in 10.9% of patients on BEV/IRI, compared with 3.6% of those on temozolomide. The combination was also associated with more grade 3 or 4 diarrhea and nausea, wound infections, and proteinuria. However, hematotoxicity was higher among patients on temozolomide, occurring in 14.8%, compared with 1.7% of patients on BEV/IRI.
"I think it’s important to recognize that there is a [bevacizumab] toxicity signal," said Dr Albert Lai, a neuro-oncologist at the University of California, Los Angeles, who was the invited discussant.
Dr. Lai commented that the overall survival signal seen by the GLARIUS investigators may have been affected by an optional crossover to BEV/IRI after disease progression on temozolomide. Of the 54 patients in the temozolomide arm, 29 crossed over to BEV/IRI.
The GLARIUS trial was sponsored by Hoffman-La Roche. Dr. Herrlinger disclosed being a consultant and speaker and receiving research support from the company. Dr. Lai disclosed serving as a consultant and receiving research funding from Genentech/Roche.
AT ASCO ANNUAL MEETING 2013
Major finding: The 6-month progression-free survival 6 months after randomization was 79.6% for patients treated with bevacizumab and irinotecan vs. 41.3% with temozolomide.
Data source: Randomized controlled trial in 170 patients with MGMT-unmethylated glioblastoma from 22 centers in Germany.
Disclosures: The GLARIUS trial was sponsored by Hoffman-La Roche. Dr. Herrlinger disclosed being a consultant and speaker and receiving research support from the company. Dr. Lai disclosed serving as a consultant and receiving research funding from Genentech/Roche.