User login
Novel immunostimulant combo shows early efficacy
SAN FRANCISCO – A combination of two novel immune-stimulating agents has shown early evidence of efficacy against malignant melanoma, leiomyosarcoma, and triple-negative breast cancer in a phase 1b, dose-escalating study.
Among 11 evaluable patients enrolled in a trial of NKTR-262, a small molecule agonist of toll-like receptors (TLR) 7/8, and bempegaldesleukin, an interleukin-2 pathway agonist, 2 had a partial response and 3 had stable disease, reported Adi Diab, MD, from the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
Patients tolerated the combination well, and there have been no serious adverse events or dose-limiting toxicities.
“Pharmacodynamic data demonstrate both activation of the systemic adaptive and the local innate immune system, and we have seen early evidence of clinical activity in patients who are refractory to checkpoint inhibitors with immunotherapy regimens,” Dr. Diab said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
NKTR-262 is injected into tumors and is designed to be retained in the tumor microenvironment where it helps to activate antigen-presenting cells, such as dendritic cells, and primes development of new, antigen-specific cytotoxic T cells. Bempegaldesleukin is a cytokine that works within the IL-2 pathway to increase CD8-positive T cells and natural killer (NK) cells in the tumor microenvironment.
The rationale for the combination is that NKTR-262 can activate innate immunity in cells surrounding the tumor microenvironment and activate the machinery of antigen-presenting cells, and bempegaldesleukin can prime and boost a systemic tumor immune response that can ultimately mediate antitumor activity in distant lesions, Dr. Adib said.
In preclinical models, the combination of these agents led to a robust antitumor effect that also involved distant lesions through mediation of the abscopal effect, in which treatment of a tumor activates an immune response against distant tumor cells as well, Dr. Diab said.
The REVEAL study is an ongoing, phase 1b/2 trial looking at the combination in melanoma, Merkel cell carcinoma, triple-negative breast cancer (TNBC), ovarian cancer, renal cell carcinoma, colorectal cancer, urothelial carcinoma, and sarcoma.
The primary goal of the study is to evaluate safety and determine the optimal phase 2 dose of the combination, evaluate biomarkers of response, and assess antitumor activity. As of Jan. 23, 2019, 13 patients were enrolled and evaluable for safety, and 11 were evaluable for the preliminary efficacy analysis.
The most common treatment-related adverse events (TRAEs) with the combination were transient grade 1 or 2 flu-like symptoms, rash, fatigue, pruritus, and nausea. One patients developed grade 3 maculopapular rash and leukocytosis.
Most of the TRAEs are attributable to bempegaldesleukin. There were no immune-mediated AEs and no TRAEs resulted in study discontinuation.
Tumor biopsies obtained 24 hours after injection of NKTR-262 confirmed the activation of TLR 7/8 and robust induction of type 1 interferon, interferon-alpha, and interferon-beta gene-related signatures necessary for optimal antigen presentation.
Dr. Diab noted that in a different trial of bempegaldesleukin monotherapy there was no significant increase in the type 1 interferon gene signature, but the agent did promote activation of the adaptive immune system.
The complementary nature of the two novel agents could also be demonstrated in evaluation of peripheral blood samples, which showed that, although there was no proliferation of T or NK cells following NKTR-262 injection, the addition of bempegaldesleukin resulted in the proliferation of both effector T cells and NK cells to enhance the systemic immune response.
The preliminary efficacy analysis showed that two of five patients with stage IV melanoma who experienced disease progression on prior immune checkpoint inhibitors had partial responses, including one who had a 100% reduction in target lesions and the other with a 50% reduction. In addition, two patients with heavily pretreated leiomyosarcoma had stable disease as the best response, as did the single patient with TNBC.
The maximum tolerated dose of the combination has not been identified, and the investigators are continuing to enroll patients.
The REVEAL study is supported by Nektar Therapeutics. Dr. Diab reported institutional research funding, consulting fees, and advisory board participation from Nektar, Bristol-Myers Squib, Idera Pharmaceuticals, Jounce Therapeutics, and Array BioPharma.
SOURCE: Diab A et al. ASCO-SITC, Abstract 26.
SAN FRANCISCO – A combination of two novel immune-stimulating agents has shown early evidence of efficacy against malignant melanoma, leiomyosarcoma, and triple-negative breast cancer in a phase 1b, dose-escalating study.
Among 11 evaluable patients enrolled in a trial of NKTR-262, a small molecule agonist of toll-like receptors (TLR) 7/8, and bempegaldesleukin, an interleukin-2 pathway agonist, 2 had a partial response and 3 had stable disease, reported Adi Diab, MD, from the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
Patients tolerated the combination well, and there have been no serious adverse events or dose-limiting toxicities.
“Pharmacodynamic data demonstrate both activation of the systemic adaptive and the local innate immune system, and we have seen early evidence of clinical activity in patients who are refractory to checkpoint inhibitors with immunotherapy regimens,” Dr. Diab said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
NKTR-262 is injected into tumors and is designed to be retained in the tumor microenvironment where it helps to activate antigen-presenting cells, such as dendritic cells, and primes development of new, antigen-specific cytotoxic T cells. Bempegaldesleukin is a cytokine that works within the IL-2 pathway to increase CD8-positive T cells and natural killer (NK) cells in the tumor microenvironment.
The rationale for the combination is that NKTR-262 can activate innate immunity in cells surrounding the tumor microenvironment and activate the machinery of antigen-presenting cells, and bempegaldesleukin can prime and boost a systemic tumor immune response that can ultimately mediate antitumor activity in distant lesions, Dr. Adib said.
In preclinical models, the combination of these agents led to a robust antitumor effect that also involved distant lesions through mediation of the abscopal effect, in which treatment of a tumor activates an immune response against distant tumor cells as well, Dr. Diab said.
The REVEAL study is an ongoing, phase 1b/2 trial looking at the combination in melanoma, Merkel cell carcinoma, triple-negative breast cancer (TNBC), ovarian cancer, renal cell carcinoma, colorectal cancer, urothelial carcinoma, and sarcoma.
The primary goal of the study is to evaluate safety and determine the optimal phase 2 dose of the combination, evaluate biomarkers of response, and assess antitumor activity. As of Jan. 23, 2019, 13 patients were enrolled and evaluable for safety, and 11 were evaluable for the preliminary efficacy analysis.
The most common treatment-related adverse events (TRAEs) with the combination were transient grade 1 or 2 flu-like symptoms, rash, fatigue, pruritus, and nausea. One patients developed grade 3 maculopapular rash and leukocytosis.
Most of the TRAEs are attributable to bempegaldesleukin. There were no immune-mediated AEs and no TRAEs resulted in study discontinuation.
Tumor biopsies obtained 24 hours after injection of NKTR-262 confirmed the activation of TLR 7/8 and robust induction of type 1 interferon, interferon-alpha, and interferon-beta gene-related signatures necessary for optimal antigen presentation.
Dr. Diab noted that in a different trial of bempegaldesleukin monotherapy there was no significant increase in the type 1 interferon gene signature, but the agent did promote activation of the adaptive immune system.
The complementary nature of the two novel agents could also be demonstrated in evaluation of peripheral blood samples, which showed that, although there was no proliferation of T or NK cells following NKTR-262 injection, the addition of bempegaldesleukin resulted in the proliferation of both effector T cells and NK cells to enhance the systemic immune response.
The preliminary efficacy analysis showed that two of five patients with stage IV melanoma who experienced disease progression on prior immune checkpoint inhibitors had partial responses, including one who had a 100% reduction in target lesions and the other with a 50% reduction. In addition, two patients with heavily pretreated leiomyosarcoma had stable disease as the best response, as did the single patient with TNBC.
The maximum tolerated dose of the combination has not been identified, and the investigators are continuing to enroll patients.
The REVEAL study is supported by Nektar Therapeutics. Dr. Diab reported institutional research funding, consulting fees, and advisory board participation from Nektar, Bristol-Myers Squib, Idera Pharmaceuticals, Jounce Therapeutics, and Array BioPharma.
SOURCE: Diab A et al. ASCO-SITC, Abstract 26.
SAN FRANCISCO – A combination of two novel immune-stimulating agents has shown early evidence of efficacy against malignant melanoma, leiomyosarcoma, and triple-negative breast cancer in a phase 1b, dose-escalating study.
Among 11 evaluable patients enrolled in a trial of NKTR-262, a small molecule agonist of toll-like receptors (TLR) 7/8, and bempegaldesleukin, an interleukin-2 pathway agonist, 2 had a partial response and 3 had stable disease, reported Adi Diab, MD, from the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
Patients tolerated the combination well, and there have been no serious adverse events or dose-limiting toxicities.
“Pharmacodynamic data demonstrate both activation of the systemic adaptive and the local innate immune system, and we have seen early evidence of clinical activity in patients who are refractory to checkpoint inhibitors with immunotherapy regimens,” Dr. Diab said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
NKTR-262 is injected into tumors and is designed to be retained in the tumor microenvironment where it helps to activate antigen-presenting cells, such as dendritic cells, and primes development of new, antigen-specific cytotoxic T cells. Bempegaldesleukin is a cytokine that works within the IL-2 pathway to increase CD8-positive T cells and natural killer (NK) cells in the tumor microenvironment.
The rationale for the combination is that NKTR-262 can activate innate immunity in cells surrounding the tumor microenvironment and activate the machinery of antigen-presenting cells, and bempegaldesleukin can prime and boost a systemic tumor immune response that can ultimately mediate antitumor activity in distant lesions, Dr. Adib said.
In preclinical models, the combination of these agents led to a robust antitumor effect that also involved distant lesions through mediation of the abscopal effect, in which treatment of a tumor activates an immune response against distant tumor cells as well, Dr. Diab said.
The REVEAL study is an ongoing, phase 1b/2 trial looking at the combination in melanoma, Merkel cell carcinoma, triple-negative breast cancer (TNBC), ovarian cancer, renal cell carcinoma, colorectal cancer, urothelial carcinoma, and sarcoma.
The primary goal of the study is to evaluate safety and determine the optimal phase 2 dose of the combination, evaluate biomarkers of response, and assess antitumor activity. As of Jan. 23, 2019, 13 patients were enrolled and evaluable for safety, and 11 were evaluable for the preliminary efficacy analysis.
The most common treatment-related adverse events (TRAEs) with the combination were transient grade 1 or 2 flu-like symptoms, rash, fatigue, pruritus, and nausea. One patients developed grade 3 maculopapular rash and leukocytosis.
Most of the TRAEs are attributable to bempegaldesleukin. There were no immune-mediated AEs and no TRAEs resulted in study discontinuation.
Tumor biopsies obtained 24 hours after injection of NKTR-262 confirmed the activation of TLR 7/8 and robust induction of type 1 interferon, interferon-alpha, and interferon-beta gene-related signatures necessary for optimal antigen presentation.
Dr. Diab noted that in a different trial of bempegaldesleukin monotherapy there was no significant increase in the type 1 interferon gene signature, but the agent did promote activation of the adaptive immune system.
The complementary nature of the two novel agents could also be demonstrated in evaluation of peripheral blood samples, which showed that, although there was no proliferation of T or NK cells following NKTR-262 injection, the addition of bempegaldesleukin resulted in the proliferation of both effector T cells and NK cells to enhance the systemic immune response.
The preliminary efficacy analysis showed that two of five patients with stage IV melanoma who experienced disease progression on prior immune checkpoint inhibitors had partial responses, including one who had a 100% reduction in target lesions and the other with a 50% reduction. In addition, two patients with heavily pretreated leiomyosarcoma had stable disease as the best response, as did the single patient with TNBC.
The maximum tolerated dose of the combination has not been identified, and the investigators are continuing to enroll patients.
The REVEAL study is supported by Nektar Therapeutics. Dr. Diab reported institutional research funding, consulting fees, and advisory board participation from Nektar, Bristol-Myers Squib, Idera Pharmaceuticals, Jounce Therapeutics, and Array BioPharma.
SOURCE: Diab A et al. ASCO-SITC, Abstract 26.
REPORTING FROM ASCO-SITC
Antibiotics gut checkpoint inhibitor efficacy
SAN FRANCISCO – Antibiotic exposure in the month before cancer immunotherapy starts may hamper the efficacy of immune checkpoint inhibitors, investigators caution.
A prospective study of 196 patients treated with immune checkpoint inhibitors for various cancers showed that the 29 patients who received antibiotics within 30 days of starting immunotherapy had significantly worse overall survival than patients without antibiotic exposure; this effect was seen across cancer types, reported David James Pinato, MD, PhD, from Imperial College London.
In contrast, concurrent antibiotic and checkpoint inhibitor use was not significantly associated with overall survival differences, he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
“I think these data are quite interesting in showing an independent detrimental effect, both on response and survival, in unselected patients treated with immune checkpoint inhibitors in routine clinical practice,” Dr. Pinato said.
The data also suggest “the timing of antibiotic exposure is crucial,” he added. Antibiotic treatment concurrent with immunotherapy did not appear to affect prognosis. Alternatively, prior antibiotic therapy appeared to have “a sort of a priming effect towards the immune system.”
Broad-spectrum antibiotics can affect the diversity of the gut microbiome, which influences mucosal immunity, dendritic cell function, and antigen presentation. Alternatively, enrichment of the microbiome with several bacterial species can enhance the potency of checkpoint inhibitors by facilitating the process of tumor rejection, Dr. Pinato explained.
To see whether antibiotic disruption, or “dysbiosis” of the gut microbiome, could hinder responsiveness to checkpoint inhibitors regardless of the tumor site and whether there were time-dependent effects of antibiotic exposure on response to checkpoint inhibitors, the investigators conducted a prospective, observational study in 196 patients treated with checkpoint inhibitors for non–small cell lung cancer (NSCLC), melanoma, renal cell carcinoma, head and neck cancer, transitional cell carcinoma of the bladder, and other cancers.
The researchers defined prior antibiotic exposure as more than 30 days before the start of checkpoint inhibitor therapy and concurrent exposure as antibiotics begun on the first day of the first cycle of checkpoint inhibitor dosing.
Of the 196 patients, 29 had previously received antibiotics, and 68 received them concurrently. The most frequently prescribed antibiotics were beta-lactam agents given in a single, short course. Other classes of drugs, used in eight or fewer patients each, included quinolones, macrolides, sulfonamides, tetracyclines, aminoglycosides, and nitroimidazole.
Median overall survival for the entire cohort, one of two primary outcomes, was 2 months for patients who had received prior antibiotics and 26 months for patients with no prior exposure. This difference was similar for patients with NSCLC (2.5 vs. 26 months), melanoma (3.9 vs. 14 months), and other cancers combined (1.1 vs. 11.0 months; log-rank P less than .01 for all comparisons).
In multivariate analysis, only response to checkpoints inhibitors (complete vs. partial response, stable disease, or progression) and prior antibiotic exposure were significantly associated with survival. The hazard ratio for survival for patients who had not previously received antibiotics was 3.5 (P less than .001).
In contrast, concurrent antibiotic and checkpoint inhibitor use did not have a significant effect on survival.
An analysis of radiologic responses also showed that patients with prior antibiotic exposure had a significantly higher probability of primary disease progression than those without (81% vs. 44%; P less than .001). There were no associations, however, between specific classes of antibiotics or corticosteroid use.
The findings indicate that “certainly, mechanistic studies are required here, not just to investigate the prognostic role of antibiotic-mediated dysbiosis, but perhaps transform this into an actual driver of antitumor immunity,” Dr. Pinato concluded.
The study was internally supported. Dr. Pinato reported receiving grant funding from Merck and Bristol-Myers Squibb unrelated to the study, as well as honoraria from ViiV Healthcare.
SOURCE: Pinato DJ et al. ASCO-SITC, Abstract 147.
SAN FRANCISCO – Antibiotic exposure in the month before cancer immunotherapy starts may hamper the efficacy of immune checkpoint inhibitors, investigators caution.
A prospective study of 196 patients treated with immune checkpoint inhibitors for various cancers showed that the 29 patients who received antibiotics within 30 days of starting immunotherapy had significantly worse overall survival than patients without antibiotic exposure; this effect was seen across cancer types, reported David James Pinato, MD, PhD, from Imperial College London.
In contrast, concurrent antibiotic and checkpoint inhibitor use was not significantly associated with overall survival differences, he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
“I think these data are quite interesting in showing an independent detrimental effect, both on response and survival, in unselected patients treated with immune checkpoint inhibitors in routine clinical practice,” Dr. Pinato said.
The data also suggest “the timing of antibiotic exposure is crucial,” he added. Antibiotic treatment concurrent with immunotherapy did not appear to affect prognosis. Alternatively, prior antibiotic therapy appeared to have “a sort of a priming effect towards the immune system.”
Broad-spectrum antibiotics can affect the diversity of the gut microbiome, which influences mucosal immunity, dendritic cell function, and antigen presentation. Alternatively, enrichment of the microbiome with several bacterial species can enhance the potency of checkpoint inhibitors by facilitating the process of tumor rejection, Dr. Pinato explained.
To see whether antibiotic disruption, or “dysbiosis” of the gut microbiome, could hinder responsiveness to checkpoint inhibitors regardless of the tumor site and whether there were time-dependent effects of antibiotic exposure on response to checkpoint inhibitors, the investigators conducted a prospective, observational study in 196 patients treated with checkpoint inhibitors for non–small cell lung cancer (NSCLC), melanoma, renal cell carcinoma, head and neck cancer, transitional cell carcinoma of the bladder, and other cancers.
The researchers defined prior antibiotic exposure as more than 30 days before the start of checkpoint inhibitor therapy and concurrent exposure as antibiotics begun on the first day of the first cycle of checkpoint inhibitor dosing.
Of the 196 patients, 29 had previously received antibiotics, and 68 received them concurrently. The most frequently prescribed antibiotics were beta-lactam agents given in a single, short course. Other classes of drugs, used in eight or fewer patients each, included quinolones, macrolides, sulfonamides, tetracyclines, aminoglycosides, and nitroimidazole.
Median overall survival for the entire cohort, one of two primary outcomes, was 2 months for patients who had received prior antibiotics and 26 months for patients with no prior exposure. This difference was similar for patients with NSCLC (2.5 vs. 26 months), melanoma (3.9 vs. 14 months), and other cancers combined (1.1 vs. 11.0 months; log-rank P less than .01 for all comparisons).
In multivariate analysis, only response to checkpoints inhibitors (complete vs. partial response, stable disease, or progression) and prior antibiotic exposure were significantly associated with survival. The hazard ratio for survival for patients who had not previously received antibiotics was 3.5 (P less than .001).
In contrast, concurrent antibiotic and checkpoint inhibitor use did not have a significant effect on survival.
An analysis of radiologic responses also showed that patients with prior antibiotic exposure had a significantly higher probability of primary disease progression than those without (81% vs. 44%; P less than .001). There were no associations, however, between specific classes of antibiotics or corticosteroid use.
The findings indicate that “certainly, mechanistic studies are required here, not just to investigate the prognostic role of antibiotic-mediated dysbiosis, but perhaps transform this into an actual driver of antitumor immunity,” Dr. Pinato concluded.
The study was internally supported. Dr. Pinato reported receiving grant funding from Merck and Bristol-Myers Squibb unrelated to the study, as well as honoraria from ViiV Healthcare.
SOURCE: Pinato DJ et al. ASCO-SITC, Abstract 147.
SAN FRANCISCO – Antibiotic exposure in the month before cancer immunotherapy starts may hamper the efficacy of immune checkpoint inhibitors, investigators caution.
A prospective study of 196 patients treated with immune checkpoint inhibitors for various cancers showed that the 29 patients who received antibiotics within 30 days of starting immunotherapy had significantly worse overall survival than patients without antibiotic exposure; this effect was seen across cancer types, reported David James Pinato, MD, PhD, from Imperial College London.
In contrast, concurrent antibiotic and checkpoint inhibitor use was not significantly associated with overall survival differences, he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
“I think these data are quite interesting in showing an independent detrimental effect, both on response and survival, in unselected patients treated with immune checkpoint inhibitors in routine clinical practice,” Dr. Pinato said.
The data also suggest “the timing of antibiotic exposure is crucial,” he added. Antibiotic treatment concurrent with immunotherapy did not appear to affect prognosis. Alternatively, prior antibiotic therapy appeared to have “a sort of a priming effect towards the immune system.”
Broad-spectrum antibiotics can affect the diversity of the gut microbiome, which influences mucosal immunity, dendritic cell function, and antigen presentation. Alternatively, enrichment of the microbiome with several bacterial species can enhance the potency of checkpoint inhibitors by facilitating the process of tumor rejection, Dr. Pinato explained.
To see whether antibiotic disruption, or “dysbiosis” of the gut microbiome, could hinder responsiveness to checkpoint inhibitors regardless of the tumor site and whether there were time-dependent effects of antibiotic exposure on response to checkpoint inhibitors, the investigators conducted a prospective, observational study in 196 patients treated with checkpoint inhibitors for non–small cell lung cancer (NSCLC), melanoma, renal cell carcinoma, head and neck cancer, transitional cell carcinoma of the bladder, and other cancers.
The researchers defined prior antibiotic exposure as more than 30 days before the start of checkpoint inhibitor therapy and concurrent exposure as antibiotics begun on the first day of the first cycle of checkpoint inhibitor dosing.
Of the 196 patients, 29 had previously received antibiotics, and 68 received them concurrently. The most frequently prescribed antibiotics were beta-lactam agents given in a single, short course. Other classes of drugs, used in eight or fewer patients each, included quinolones, macrolides, sulfonamides, tetracyclines, aminoglycosides, and nitroimidazole.
Median overall survival for the entire cohort, one of two primary outcomes, was 2 months for patients who had received prior antibiotics and 26 months for patients with no prior exposure. This difference was similar for patients with NSCLC (2.5 vs. 26 months), melanoma (3.9 vs. 14 months), and other cancers combined (1.1 vs. 11.0 months; log-rank P less than .01 for all comparisons).
In multivariate analysis, only response to checkpoints inhibitors (complete vs. partial response, stable disease, or progression) and prior antibiotic exposure were significantly associated with survival. The hazard ratio for survival for patients who had not previously received antibiotics was 3.5 (P less than .001).
In contrast, concurrent antibiotic and checkpoint inhibitor use did not have a significant effect on survival.
An analysis of radiologic responses also showed that patients with prior antibiotic exposure had a significantly higher probability of primary disease progression than those without (81% vs. 44%; P less than .001). There were no associations, however, between specific classes of antibiotics or corticosteroid use.
The findings indicate that “certainly, mechanistic studies are required here, not just to investigate the prognostic role of antibiotic-mediated dysbiosis, but perhaps transform this into an actual driver of antitumor immunity,” Dr. Pinato concluded.
The study was internally supported. Dr. Pinato reported receiving grant funding from Merck and Bristol-Myers Squibb unrelated to the study, as well as honoraria from ViiV Healthcare.
SOURCE: Pinato DJ et al. ASCO-SITC, Abstract 147.
REPORTING FROM ASCO-SITC
Pembrolizumab/lenvatinib active against urothelial carcinoma
SAN FRANCISCO – A combination of a targeted therapy and an immune checkpoint inhibitor showed promising activity against advanced urothelial cancer in early data from a phase 1b/2 study.
In a cohort of 20 patients with urothelial carcinoma who were enrolled in a larger clinical trial testing the combination of the tyrosine kinase inhibitor (TKI) lenvatinib (Lenvima) and the checkpoint inhibitor pembrolizumab (Keytruda) against urinary tract and other solid malignancies, 5 had an objective response to the combination, including one complete and four partial responses, for an objective response rate of 25%, reported Nicholas J. Vogelzang, MD, from Comprehensive Cancers Centers of Nevada in Las Vegas.
“This response rate warrants further investigation. The lenvatinib plus pembrolizumab combination will be studied in a phase 3 trial in urothelial carcinoma,” he said at the American Society of Clinical Oncology (ASCO) - Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
Dr. Vogelzang noted that urothelial carcinomas account for more than 90% of all bladder cancers. Pembrolizumab monotherapy is approved for treatment of patients with urothelial carcinoma who are ineligible for cisplatin and whose tumors have a combined positive score (CPS) for programmed death-ligand 1 (PD-L1) of 10 or greater or who are ineligible for platinum-based chemotherapy regimens and, in the second line, for advanced or metastatic urothelial carcinoma.
Lenvatinib, a multikinase inhibitor, is approved as monotherapy for radioiodine-refractory differentiated thyroid cancer, unresectable hepatocellular carcinoma, and in combination with everolimus for advanced renal cell carcinoma (RCC) after one year of antiangiogenic therapy.
Dr. Vogelzang reported results of the urothelial cancer cohort from a multicohort study testing the combination.
Twenty patients with histologically confirmed metastatic urothelial cancer were enrolled. The patients all had no more than two prior systemic regimens, good performance status, and a life expectancy of at least 12 weeks. The patients received oral lenvatinib 20 mg daily and pembrolizumab 200 mg intravenously every 21 days. The median patient age was 72 years. The cohort included 14 men and six women.
The objective response rate (ORR) at 24 weeks, the primary endpoint, was 25%, comprising one complete and four partial responses. Nine patients had stable disease, two had disease progression, and four were not evaluable for efficacy. The results were identical according to immune-related Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and modified RECIST version 1.1. Of the 16 evaluable patients, 12 experienced tumor-size reductions from baseline.
“Although there were five objective responses, there were an additional seven patients or more who had minor regressions of disease – clearly an active regimen,” Dr. Vogelzang said. Four of the patients, including one with a PD-L1–positive tumor and three with PD-L1–negative tumors were still alive, with the longest survival past 80 weeks since the start of therapy. The majority of patients, however, had no objective response or disease progression within about 20 weeks.
After a median follow-up of 11.7 months, the median progression-free survival (PFS) was 5.4 months, and the 12-month PFS rate was 26%.
In all, 18 of the 20 patients (90%) experienced a treatment-related adverse event of any grade, 10 had grade 3 or 4 events, and 6 had serious adverse events including one death from gastrointestinal hemorrhage that Dr. Vogelzang said appeared to be related to lenvatinib. A total of four patients (20%) had a treatment-related event leading to withdrawal or discontinuation, seven had a dose reduction, and 12 had an interruption in therapy, primarily of lenvatinib. The most common toxicities were proteinuria, diarrhea, hypertension, fatigue, hypothyroidism, decreased appetite with nausea, pancreatitis with increased lipase, skin rash, vomiting, and dry mouth.
In addition to the planned phase 3 trial of the combination in urothelial carcinoma, lenvatinib/pembrolizumab is also being studied for the treatment of RCC.
The study was supported by Eisai and Merck Sharp & Dohme. Dr. Vogelzang disclosed financial relationships with Caris Life Sciences, Pfizer, Up to Date, AstraZeneca, MedImmune, and other companies. Five coauthors are employees of Merck or Esai.
SOURCE: Vogelzang NJ et al. ASCO-SITC, Abstract 11.
SAN FRANCISCO – A combination of a targeted therapy and an immune checkpoint inhibitor showed promising activity against advanced urothelial cancer in early data from a phase 1b/2 study.
In a cohort of 20 patients with urothelial carcinoma who were enrolled in a larger clinical trial testing the combination of the tyrosine kinase inhibitor (TKI) lenvatinib (Lenvima) and the checkpoint inhibitor pembrolizumab (Keytruda) against urinary tract and other solid malignancies, 5 had an objective response to the combination, including one complete and four partial responses, for an objective response rate of 25%, reported Nicholas J. Vogelzang, MD, from Comprehensive Cancers Centers of Nevada in Las Vegas.
“This response rate warrants further investigation. The lenvatinib plus pembrolizumab combination will be studied in a phase 3 trial in urothelial carcinoma,” he said at the American Society of Clinical Oncology (ASCO) - Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
Dr. Vogelzang noted that urothelial carcinomas account for more than 90% of all bladder cancers. Pembrolizumab monotherapy is approved for treatment of patients with urothelial carcinoma who are ineligible for cisplatin and whose tumors have a combined positive score (CPS) for programmed death-ligand 1 (PD-L1) of 10 or greater or who are ineligible for platinum-based chemotherapy regimens and, in the second line, for advanced or metastatic urothelial carcinoma.
Lenvatinib, a multikinase inhibitor, is approved as monotherapy for radioiodine-refractory differentiated thyroid cancer, unresectable hepatocellular carcinoma, and in combination with everolimus for advanced renal cell carcinoma (RCC) after one year of antiangiogenic therapy.
Dr. Vogelzang reported results of the urothelial cancer cohort from a multicohort study testing the combination.
Twenty patients with histologically confirmed metastatic urothelial cancer were enrolled. The patients all had no more than two prior systemic regimens, good performance status, and a life expectancy of at least 12 weeks. The patients received oral lenvatinib 20 mg daily and pembrolizumab 200 mg intravenously every 21 days. The median patient age was 72 years. The cohort included 14 men and six women.
The objective response rate (ORR) at 24 weeks, the primary endpoint, was 25%, comprising one complete and four partial responses. Nine patients had stable disease, two had disease progression, and four were not evaluable for efficacy. The results were identical according to immune-related Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and modified RECIST version 1.1. Of the 16 evaluable patients, 12 experienced tumor-size reductions from baseline.
“Although there were five objective responses, there were an additional seven patients or more who had minor regressions of disease – clearly an active regimen,” Dr. Vogelzang said. Four of the patients, including one with a PD-L1–positive tumor and three with PD-L1–negative tumors were still alive, with the longest survival past 80 weeks since the start of therapy. The majority of patients, however, had no objective response or disease progression within about 20 weeks.
After a median follow-up of 11.7 months, the median progression-free survival (PFS) was 5.4 months, and the 12-month PFS rate was 26%.
In all, 18 of the 20 patients (90%) experienced a treatment-related adverse event of any grade, 10 had grade 3 or 4 events, and 6 had serious adverse events including one death from gastrointestinal hemorrhage that Dr. Vogelzang said appeared to be related to lenvatinib. A total of four patients (20%) had a treatment-related event leading to withdrawal or discontinuation, seven had a dose reduction, and 12 had an interruption in therapy, primarily of lenvatinib. The most common toxicities were proteinuria, diarrhea, hypertension, fatigue, hypothyroidism, decreased appetite with nausea, pancreatitis with increased lipase, skin rash, vomiting, and dry mouth.
In addition to the planned phase 3 trial of the combination in urothelial carcinoma, lenvatinib/pembrolizumab is also being studied for the treatment of RCC.
The study was supported by Eisai and Merck Sharp & Dohme. Dr. Vogelzang disclosed financial relationships with Caris Life Sciences, Pfizer, Up to Date, AstraZeneca, MedImmune, and other companies. Five coauthors are employees of Merck or Esai.
SOURCE: Vogelzang NJ et al. ASCO-SITC, Abstract 11.
SAN FRANCISCO – A combination of a targeted therapy and an immune checkpoint inhibitor showed promising activity against advanced urothelial cancer in early data from a phase 1b/2 study.
In a cohort of 20 patients with urothelial carcinoma who were enrolled in a larger clinical trial testing the combination of the tyrosine kinase inhibitor (TKI) lenvatinib (Lenvima) and the checkpoint inhibitor pembrolizumab (Keytruda) against urinary tract and other solid malignancies, 5 had an objective response to the combination, including one complete and four partial responses, for an objective response rate of 25%, reported Nicholas J. Vogelzang, MD, from Comprehensive Cancers Centers of Nevada in Las Vegas.
“This response rate warrants further investigation. The lenvatinib plus pembrolizumab combination will be studied in a phase 3 trial in urothelial carcinoma,” he said at the American Society of Clinical Oncology (ASCO) - Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
Dr. Vogelzang noted that urothelial carcinomas account for more than 90% of all bladder cancers. Pembrolizumab monotherapy is approved for treatment of patients with urothelial carcinoma who are ineligible for cisplatin and whose tumors have a combined positive score (CPS) for programmed death-ligand 1 (PD-L1) of 10 or greater or who are ineligible for platinum-based chemotherapy regimens and, in the second line, for advanced or metastatic urothelial carcinoma.
Lenvatinib, a multikinase inhibitor, is approved as monotherapy for radioiodine-refractory differentiated thyroid cancer, unresectable hepatocellular carcinoma, and in combination with everolimus for advanced renal cell carcinoma (RCC) after one year of antiangiogenic therapy.
Dr. Vogelzang reported results of the urothelial cancer cohort from a multicohort study testing the combination.
Twenty patients with histologically confirmed metastatic urothelial cancer were enrolled. The patients all had no more than two prior systemic regimens, good performance status, and a life expectancy of at least 12 weeks. The patients received oral lenvatinib 20 mg daily and pembrolizumab 200 mg intravenously every 21 days. The median patient age was 72 years. The cohort included 14 men and six women.
The objective response rate (ORR) at 24 weeks, the primary endpoint, was 25%, comprising one complete and four partial responses. Nine patients had stable disease, two had disease progression, and four were not evaluable for efficacy. The results were identical according to immune-related Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and modified RECIST version 1.1. Of the 16 evaluable patients, 12 experienced tumor-size reductions from baseline.
“Although there were five objective responses, there were an additional seven patients or more who had minor regressions of disease – clearly an active regimen,” Dr. Vogelzang said. Four of the patients, including one with a PD-L1–positive tumor and three with PD-L1–negative tumors were still alive, with the longest survival past 80 weeks since the start of therapy. The majority of patients, however, had no objective response or disease progression within about 20 weeks.
After a median follow-up of 11.7 months, the median progression-free survival (PFS) was 5.4 months, and the 12-month PFS rate was 26%.
In all, 18 of the 20 patients (90%) experienced a treatment-related adverse event of any grade, 10 had grade 3 or 4 events, and 6 had serious adverse events including one death from gastrointestinal hemorrhage that Dr. Vogelzang said appeared to be related to lenvatinib. A total of four patients (20%) had a treatment-related event leading to withdrawal or discontinuation, seven had a dose reduction, and 12 had an interruption in therapy, primarily of lenvatinib. The most common toxicities were proteinuria, diarrhea, hypertension, fatigue, hypothyroidism, decreased appetite with nausea, pancreatitis with increased lipase, skin rash, vomiting, and dry mouth.
In addition to the planned phase 3 trial of the combination in urothelial carcinoma, lenvatinib/pembrolizumab is also being studied for the treatment of RCC.
The study was supported by Eisai and Merck Sharp & Dohme. Dr. Vogelzang disclosed financial relationships with Caris Life Sciences, Pfizer, Up to Date, AstraZeneca, MedImmune, and other companies. Five coauthors are employees of Merck or Esai.
SOURCE: Vogelzang NJ et al. ASCO-SITC, Abstract 11.
REPORTING FROM ASCO-SITC
Higher dose of checkpoint inhibitor every 4 weeks feasible in NSCLC
SAN FRANCISCO – For patients with advanced non–small cell lung cancer (NSCLC) who previously had disease control with the checkpoint inhibitor nivolumab (Opdivo), second-line nivolumab at a higher dose every 4 weeks appeared to be comparable in efficacy and safety with standard-dose nivolumab every 2 weeks.
The key word in that last sentence is “appeared,” because the Checkmate 384 trial that was designed to show noninferiority of the every-4-weeks regimen lacked the statistical muscle to get the job done, reported Edward B. Garon, MD, from the University of California, Los Angeles.
“In many respects, extending the dosing frequency of nivolumab fulfills some of the promise of immunotherapy: The idea that we would be able to decrease the medicalization of the lives of our patients. For some people this would lead to them being able to resume a more normal work schedule, and for other people it would allow them to do things for fun, like travel on trips that would take longer than a couple of weeks,” he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
However, because of difficulties in recruitment, the investigators had to stop enrollment early and settle for a sample size of 363 patients, instead of the 600 planned that would be necessary to meet a 10% noninferiority margin and one-sided 95% confidence interval. Thus, the trial analysis can only be reported as descriptive rather than definitive, Dr. Garon acknowledged.
Nivolumab is approved at a fixed dose of 240 mg every 2 weeks for the treatment of multiple tumor types in several different nations, and in the United States and Canada it is approved at a dose of 480 mg every 4 weeks for the treatment of NSCLC.
The CheckMate 384 study enrolled patients with advanced or metastatic NSCLC who had received 3 mg/kg or 240 mg of nivolumab every 2 weeks for up to 1 year. The patients had to have had relatively good performance status (Eastern Cooperative Oncology Group 0-2) and two consecutive assessments of either complete response, partial response, or stable disease.
The patients were stratified by tumor histology (squamous or nonsquamous) and response to prior nivolumab therapy at randomization, and were then randomized to receive nivolumab 240 mg every 2 weeks or 480 mg every 4 weeks until disease progression or unacceptable toxicity for up to 2 years.
Dr. Garon presented an interim analysis including data on 329 of the 363 patients; the final analysis will occur after all patients have had a minimum of 12 months of follow-up. Here, he reported on 6-month progression-free survival, a coprimary endpoint with 12-month PFS.
After a median follow-up of 9.5 months in the Q4-week group and 10.2 months in the Q2-week group, the 6-month PFS rates were identical between the two dosing strategies, at 72%. The median PFS was 12.1 months and 12.2 months, respectively.
“Although the study is no longer formally powered to show noninferiority, there’s certainly nothing in these curves that makes me concerned that this 480 mg every-4-week dose would be inferior,” Dr. Garon said.
There was a slightly higher rate of treatment-related adverse events of any grade in the lower, more frequent dose group: 48% in the Q4-week versus 61% in the Q2-week arm. The respective rates of grade 3 or 4 adverse events were 8% and 12%. Rates of serious adverse events and events leading to treatment discontinuation were similar between the group; there were no treatment-related deaths.
The investigators hypothesize that the higher rate of overall events in the lower-dose group may be attributable to more frequent visits and more opportunities to report adverse events, Dr. Garon said.
“Overall, the clinical data are in agreement with the pharmacokinetic modeling and give further evidence for this 480 mg every 4 week nivolumab dosing option,” he concluded.
The study was supported by Bristol-Myers Squibb. Dr. Garon reported receiving research support from Bristol-Myers Squibb and others and consulting fees from Dracen Pharmaceuticals.
SOURCE: Garon EB et al. ASCO-SITC, Abstract 100.
SAN FRANCISCO – For patients with advanced non–small cell lung cancer (NSCLC) who previously had disease control with the checkpoint inhibitor nivolumab (Opdivo), second-line nivolumab at a higher dose every 4 weeks appeared to be comparable in efficacy and safety with standard-dose nivolumab every 2 weeks.
The key word in that last sentence is “appeared,” because the Checkmate 384 trial that was designed to show noninferiority of the every-4-weeks regimen lacked the statistical muscle to get the job done, reported Edward B. Garon, MD, from the University of California, Los Angeles.
“In many respects, extending the dosing frequency of nivolumab fulfills some of the promise of immunotherapy: The idea that we would be able to decrease the medicalization of the lives of our patients. For some people this would lead to them being able to resume a more normal work schedule, and for other people it would allow them to do things for fun, like travel on trips that would take longer than a couple of weeks,” he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
However, because of difficulties in recruitment, the investigators had to stop enrollment early and settle for a sample size of 363 patients, instead of the 600 planned that would be necessary to meet a 10% noninferiority margin and one-sided 95% confidence interval. Thus, the trial analysis can only be reported as descriptive rather than definitive, Dr. Garon acknowledged.
Nivolumab is approved at a fixed dose of 240 mg every 2 weeks for the treatment of multiple tumor types in several different nations, and in the United States and Canada it is approved at a dose of 480 mg every 4 weeks for the treatment of NSCLC.
The CheckMate 384 study enrolled patients with advanced or metastatic NSCLC who had received 3 mg/kg or 240 mg of nivolumab every 2 weeks for up to 1 year. The patients had to have had relatively good performance status (Eastern Cooperative Oncology Group 0-2) and two consecutive assessments of either complete response, partial response, or stable disease.
The patients were stratified by tumor histology (squamous or nonsquamous) and response to prior nivolumab therapy at randomization, and were then randomized to receive nivolumab 240 mg every 2 weeks or 480 mg every 4 weeks until disease progression or unacceptable toxicity for up to 2 years.
Dr. Garon presented an interim analysis including data on 329 of the 363 patients; the final analysis will occur after all patients have had a minimum of 12 months of follow-up. Here, he reported on 6-month progression-free survival, a coprimary endpoint with 12-month PFS.
After a median follow-up of 9.5 months in the Q4-week group and 10.2 months in the Q2-week group, the 6-month PFS rates were identical between the two dosing strategies, at 72%. The median PFS was 12.1 months and 12.2 months, respectively.
“Although the study is no longer formally powered to show noninferiority, there’s certainly nothing in these curves that makes me concerned that this 480 mg every-4-week dose would be inferior,” Dr. Garon said.
There was a slightly higher rate of treatment-related adverse events of any grade in the lower, more frequent dose group: 48% in the Q4-week versus 61% in the Q2-week arm. The respective rates of grade 3 or 4 adverse events were 8% and 12%. Rates of serious adverse events and events leading to treatment discontinuation were similar between the group; there were no treatment-related deaths.
The investigators hypothesize that the higher rate of overall events in the lower-dose group may be attributable to more frequent visits and more opportunities to report adverse events, Dr. Garon said.
“Overall, the clinical data are in agreement with the pharmacokinetic modeling and give further evidence for this 480 mg every 4 week nivolumab dosing option,” he concluded.
The study was supported by Bristol-Myers Squibb. Dr. Garon reported receiving research support from Bristol-Myers Squibb and others and consulting fees from Dracen Pharmaceuticals.
SOURCE: Garon EB et al. ASCO-SITC, Abstract 100.
SAN FRANCISCO – For patients with advanced non–small cell lung cancer (NSCLC) who previously had disease control with the checkpoint inhibitor nivolumab (Opdivo), second-line nivolumab at a higher dose every 4 weeks appeared to be comparable in efficacy and safety with standard-dose nivolumab every 2 weeks.
The key word in that last sentence is “appeared,” because the Checkmate 384 trial that was designed to show noninferiority of the every-4-weeks regimen lacked the statistical muscle to get the job done, reported Edward B. Garon, MD, from the University of California, Los Angeles.
“In many respects, extending the dosing frequency of nivolumab fulfills some of the promise of immunotherapy: The idea that we would be able to decrease the medicalization of the lives of our patients. For some people this would lead to them being able to resume a more normal work schedule, and for other people it would allow them to do things for fun, like travel on trips that would take longer than a couple of weeks,” he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
However, because of difficulties in recruitment, the investigators had to stop enrollment early and settle for a sample size of 363 patients, instead of the 600 planned that would be necessary to meet a 10% noninferiority margin and one-sided 95% confidence interval. Thus, the trial analysis can only be reported as descriptive rather than definitive, Dr. Garon acknowledged.
Nivolumab is approved at a fixed dose of 240 mg every 2 weeks for the treatment of multiple tumor types in several different nations, and in the United States and Canada it is approved at a dose of 480 mg every 4 weeks for the treatment of NSCLC.
The CheckMate 384 study enrolled patients with advanced or metastatic NSCLC who had received 3 mg/kg or 240 mg of nivolumab every 2 weeks for up to 1 year. The patients had to have had relatively good performance status (Eastern Cooperative Oncology Group 0-2) and two consecutive assessments of either complete response, partial response, or stable disease.
The patients were stratified by tumor histology (squamous or nonsquamous) and response to prior nivolumab therapy at randomization, and were then randomized to receive nivolumab 240 mg every 2 weeks or 480 mg every 4 weeks until disease progression or unacceptable toxicity for up to 2 years.
Dr. Garon presented an interim analysis including data on 329 of the 363 patients; the final analysis will occur after all patients have had a minimum of 12 months of follow-up. Here, he reported on 6-month progression-free survival, a coprimary endpoint with 12-month PFS.
After a median follow-up of 9.5 months in the Q4-week group and 10.2 months in the Q2-week group, the 6-month PFS rates were identical between the two dosing strategies, at 72%. The median PFS was 12.1 months and 12.2 months, respectively.
“Although the study is no longer formally powered to show noninferiority, there’s certainly nothing in these curves that makes me concerned that this 480 mg every-4-week dose would be inferior,” Dr. Garon said.
There was a slightly higher rate of treatment-related adverse events of any grade in the lower, more frequent dose group: 48% in the Q4-week versus 61% in the Q2-week arm. The respective rates of grade 3 or 4 adverse events were 8% and 12%. Rates of serious adverse events and events leading to treatment discontinuation were similar between the group; there were no treatment-related deaths.
The investigators hypothesize that the higher rate of overall events in the lower-dose group may be attributable to more frequent visits and more opportunities to report adverse events, Dr. Garon said.
“Overall, the clinical data are in agreement with the pharmacokinetic modeling and give further evidence for this 480 mg every 4 week nivolumab dosing option,” he concluded.
The study was supported by Bristol-Myers Squibb. Dr. Garon reported receiving research support from Bristol-Myers Squibb and others and consulting fees from Dracen Pharmaceuticals.
SOURCE: Garon EB et al. ASCO-SITC, Abstract 100.
REPORTING FROM ASCO-SITC
Rare, aggressive NSCLC type yields to pembrolizumab
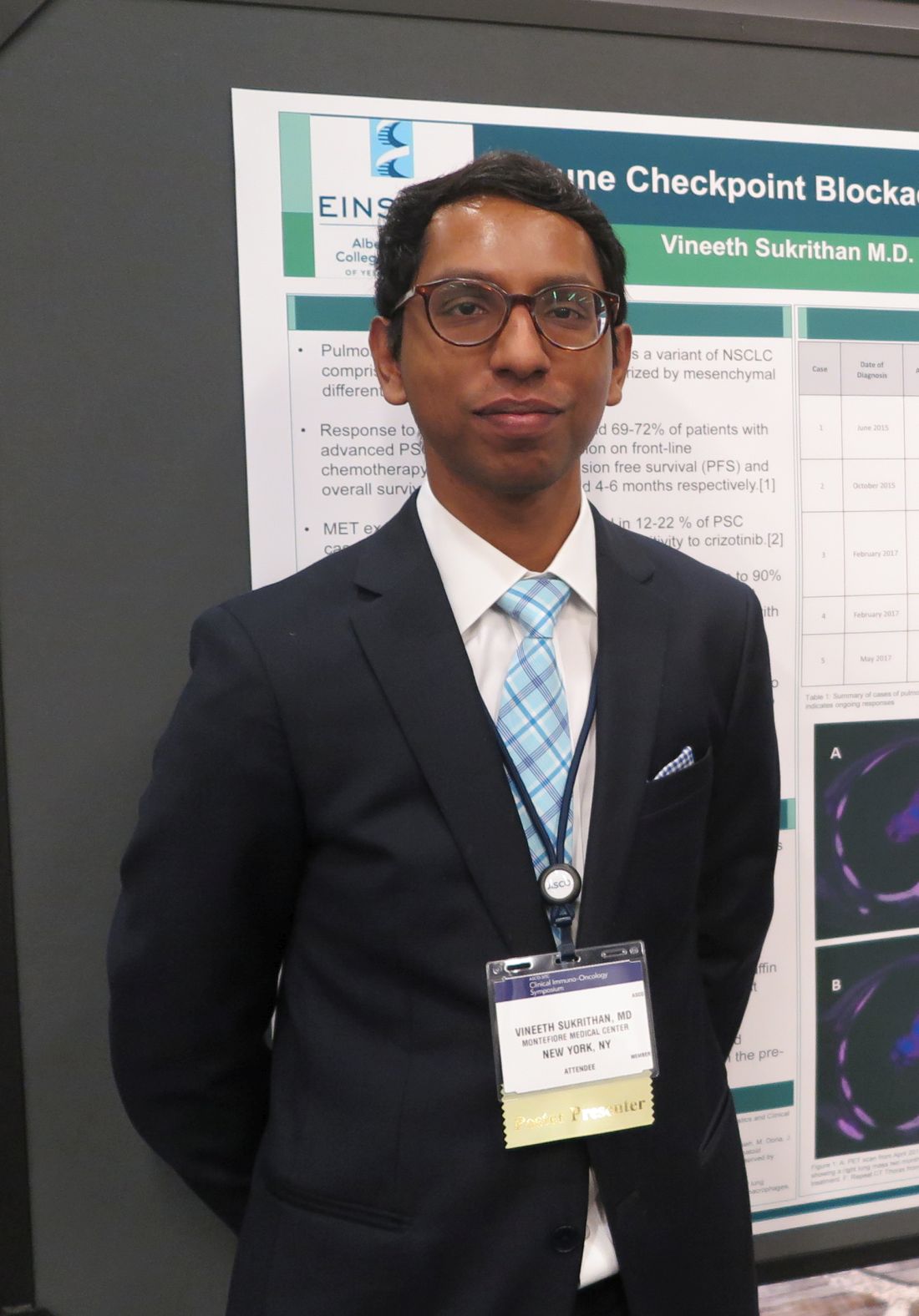
SAN FRANCISCO – The immune checkpoint inhibitor pembrolizumab (Keytruda) was associated in a small case series with remarkable overall and progression-free survival of patients with pulmonary sarcomatoid carcinoma (PSC), a rare variant of non–small cell lung cancer (NSCLC) with a grim prognosis.
Among five patients with PSC, three of whom were treatment naive, none experienced disease progression on pembrolizumab after a median follow-up of 13 months – although one died from a fungal infection unrelated to therapy – with the longest overall survival to date out to 33 months.
In contrast, patients with PSC treated before the advent of immunotherapy had a median progression-free survival of just 2 months and median overall survival a brief 4-6 months, reported Vineeth Sukrithan, MD, and his colleagues at the Albert Einstein College of Medicine, New York.
“It’s a uniquely enriched population of patients with lung cancer that have the best prognosis in terms of benefits from checkpoint inhibitors,” he said in an interview at the ASCO-SITC Clinical Immuno-Oncology Symposium.
PSC, a poorly differentiated subtype of NSCLC, accounts for about 0.3%-1.3% of all cases of lung cancer. It is closely associated with a history of heavy cigarette smoking and is rapidly fatal, with a poor response to conventional chemotherapy, although approximately 20% of patients with PSC have MET exon 14–skipping mutations that are “exquisitely” sensitive to crizotinib (Xalkori), Dr. Sukrithan explained.
PSC tumors are also unique in that they have extraordinarily high levels of programmed death-ligand 1 (PD-L1), the target of immune checkpoint inhibitors, with tumor proportion scores exceeding 90% in some cases.
Additionally, up to 43% of PSC tumors have been found to have a high mutational burden, with more than 10 mutations per megabase, suggesting that these tumors may be especially attractive targets for checkpoint inhibitor therapy, he said.
The investigators retrospectively studied surgical pathology and treatment records for all patients with advanced PSC diagnosed at their center from June 2015 to June 2018 who received pembrolizumab. They performed immunohistochemistry testing on tissue samples from the patients to quantify PD-L1 expression.
They compared the results with a cohort of patients with advanced PSC diagnosed from June 2012 to June 2015, prior to the clinical availability of anti-PD-1/PD-L1 checkpoint inhibitors.
The PD-L1-treated cohort included two men and three women, ranging from 48 to 67 years, with smoking pack-years ranging from 24 to 50. Two of the patients had received prior chemotherapy followed by pembrolizumab, and the remaining three received pembrolizumab monotherapy.
Tumor proportion scores ranged from more than 75% of tumor cells examined in one patient to 100% of cells in another.
The objective response rate to pembrolizumab was 80% consisting of one complete response and three partial responses. The fifth patient continued to have stable disease out to more than 17 months.
“This highly treatment-refractory disease now should be carefully assessed for immuno-oncologic and molecularly targeted options, which are associated with significant improvement in outcomes,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Sukrithan reported having no disclosures.
SOURCE: Sukrithan V et al. ASCO-SITC, Abstract 115.
SAN FRANCISCO – The immune checkpoint inhibitor pembrolizumab (Keytruda) was associated in a small case series with remarkable overall and progression-free survival of patients with pulmonary sarcomatoid carcinoma (PSC), a rare variant of non–small cell lung cancer (NSCLC) with a grim prognosis.
Among five patients with PSC, three of whom were treatment naive, none experienced disease progression on pembrolizumab after a median follow-up of 13 months – although one died from a fungal infection unrelated to therapy – with the longest overall survival to date out to 33 months.
In contrast, patients with PSC treated before the advent of immunotherapy had a median progression-free survival of just 2 months and median overall survival a brief 4-6 months, reported Vineeth Sukrithan, MD, and his colleagues at the Albert Einstein College of Medicine, New York.
“It’s a uniquely enriched population of patients with lung cancer that have the best prognosis in terms of benefits from checkpoint inhibitors,” he said in an interview at the ASCO-SITC Clinical Immuno-Oncology Symposium.
PSC, a poorly differentiated subtype of NSCLC, accounts for about 0.3%-1.3% of all cases of lung cancer. It is closely associated with a history of heavy cigarette smoking and is rapidly fatal, with a poor response to conventional chemotherapy, although approximately 20% of patients with PSC have MET exon 14–skipping mutations that are “exquisitely” sensitive to crizotinib (Xalkori), Dr. Sukrithan explained.
PSC tumors are also unique in that they have extraordinarily high levels of programmed death-ligand 1 (PD-L1), the target of immune checkpoint inhibitors, with tumor proportion scores exceeding 90% in some cases.
Additionally, up to 43% of PSC tumors have been found to have a high mutational burden, with more than 10 mutations per megabase, suggesting that these tumors may be especially attractive targets for checkpoint inhibitor therapy, he said.
The investigators retrospectively studied surgical pathology and treatment records for all patients with advanced PSC diagnosed at their center from June 2015 to June 2018 who received pembrolizumab. They performed immunohistochemistry testing on tissue samples from the patients to quantify PD-L1 expression.
They compared the results with a cohort of patients with advanced PSC diagnosed from June 2012 to June 2015, prior to the clinical availability of anti-PD-1/PD-L1 checkpoint inhibitors.
The PD-L1-treated cohort included two men and three women, ranging from 48 to 67 years, with smoking pack-years ranging from 24 to 50. Two of the patients had received prior chemotherapy followed by pembrolizumab, and the remaining three received pembrolizumab monotherapy.
Tumor proportion scores ranged from more than 75% of tumor cells examined in one patient to 100% of cells in another.
The objective response rate to pembrolizumab was 80% consisting of one complete response and three partial responses. The fifth patient continued to have stable disease out to more than 17 months.
“This highly treatment-refractory disease now should be carefully assessed for immuno-oncologic and molecularly targeted options, which are associated with significant improvement in outcomes,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Sukrithan reported having no disclosures.
SOURCE: Sukrithan V et al. ASCO-SITC, Abstract 115.
SAN FRANCISCO – The immune checkpoint inhibitor pembrolizumab (Keytruda) was associated in a small case series with remarkable overall and progression-free survival of patients with pulmonary sarcomatoid carcinoma (PSC), a rare variant of non–small cell lung cancer (NSCLC) with a grim prognosis.
Among five patients with PSC, three of whom were treatment naive, none experienced disease progression on pembrolizumab after a median follow-up of 13 months – although one died from a fungal infection unrelated to therapy – with the longest overall survival to date out to 33 months.
In contrast, patients with PSC treated before the advent of immunotherapy had a median progression-free survival of just 2 months and median overall survival a brief 4-6 months, reported Vineeth Sukrithan, MD, and his colleagues at the Albert Einstein College of Medicine, New York.
“It’s a uniquely enriched population of patients with lung cancer that have the best prognosis in terms of benefits from checkpoint inhibitors,” he said in an interview at the ASCO-SITC Clinical Immuno-Oncology Symposium.
PSC, a poorly differentiated subtype of NSCLC, accounts for about 0.3%-1.3% of all cases of lung cancer. It is closely associated with a history of heavy cigarette smoking and is rapidly fatal, with a poor response to conventional chemotherapy, although approximately 20% of patients with PSC have MET exon 14–skipping mutations that are “exquisitely” sensitive to crizotinib (Xalkori), Dr. Sukrithan explained.
PSC tumors are also unique in that they have extraordinarily high levels of programmed death-ligand 1 (PD-L1), the target of immune checkpoint inhibitors, with tumor proportion scores exceeding 90% in some cases.
Additionally, up to 43% of PSC tumors have been found to have a high mutational burden, with more than 10 mutations per megabase, suggesting that these tumors may be especially attractive targets for checkpoint inhibitor therapy, he said.
The investigators retrospectively studied surgical pathology and treatment records for all patients with advanced PSC diagnosed at their center from June 2015 to June 2018 who received pembrolizumab. They performed immunohistochemistry testing on tissue samples from the patients to quantify PD-L1 expression.
They compared the results with a cohort of patients with advanced PSC diagnosed from June 2012 to June 2015, prior to the clinical availability of anti-PD-1/PD-L1 checkpoint inhibitors.
The PD-L1-treated cohort included two men and three women, ranging from 48 to 67 years, with smoking pack-years ranging from 24 to 50. Two of the patients had received prior chemotherapy followed by pembrolizumab, and the remaining three received pembrolizumab monotherapy.
Tumor proportion scores ranged from more than 75% of tumor cells examined in one patient to 100% of cells in another.
The objective response rate to pembrolizumab was 80% consisting of one complete response and three partial responses. The fifth patient continued to have stable disease out to more than 17 months.
“This highly treatment-refractory disease now should be carefully assessed for immuno-oncologic and molecularly targeted options, which are associated with significant improvement in outcomes,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Sukrithan reported having no disclosures.
SOURCE: Sukrithan V et al. ASCO-SITC, Abstract 115.
REPORTING FROM ASCO-SITC