User login
Increased risk of second cancers in mycosis fungoides
LA JOLLA, CALIF. – A retrospective study suggests patients with mycosis fungoides (MF) have an increased risk of developing hematologic and solid tumor malignancies.

Researchers found the risk of second malignancy was highest among MF patients aged 30 to 50 years and patients who had tumor stage or advanced stage MF.
The increased risk was present during the entire period after MF diagnosis, but it was greatest in the first 6 months after diagnosis and roughly a dozen years later.
Amrita Goyal, MD, of the University of Minnesota in Minneapolis, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The researchers first assessed the risk of second malignancy in 172 MF patients treated at UMN from 2005 to 2017, comparing this cohort to a control group of 172 patients with seborrheic dermatitis.
Second malignancies occurred in 24 MF patients and three controls, which was a significant difference (P = .0045). The most common second malignancies among the MF patients were melanoma (n = 4), prostate cancer (n = 3), and renal cell carcinoma (n = 3).
Further analyses revealed that MF patients were more likely to develop a second malignancy if they had tumor stage disease (P = .0024) or stage IIB or higher disease (P = .03).
To corroborate and expand upon these results, Dr. Goyal and her colleagues analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database on patients diagnosed with MF from 2000 to 2014.
Among the 6,196 MF patients in this cohort, there were 514 second cancers.
“We found that MF patients were, overall, 10 times more likely to develop a second malignancy [compared with the general population],” Dr. Goyal said.
Specifically, the standardized incidence ratio was 10.15 for all malignancies, 7.33 for solid tumors, and 41.72 for hematologic malignancies.
Standardized incidence ratios for individual malignancies were:
- 69.8 for Hodgkin lymphoma.
- 46.5 for non-Hodgkin lymphoma.
- 8.6 for leukemia.
- 7.2 for melanoma.
- 6.2 for lung cancer.
- 7.9 for female breast cancer.
- 5.2 for colon cancer.
- 4.1 for prostate cancer.
- 3.9 for renal cell carcinoma.
- 3.8 for pancreatic cancer.
- 3.6 for bladder cancer.
“We found there is an increased risk [of second malignancy] during the first 6 months after diagnosis of MF, likely related to patients being in contact with the health care system more,” Dr. Goyal said. “Over time, patients have about a 7- to 10-fold increased risk over baseline, until they reach about 12 or 13 years after diagnosis, at which point, there is an increase in risk.”
The researchers found the greatest risk of second malignancy was among patients aged 30 to 50 years, although there was an increased risk for all age groups.
“The reason we think patients are experiencing an increased risk of cancers is we believe this may be due to immune suppression secondary to the mycosis fungoides, although further studies need to be performed to determine if that’s accurate,” Dr. Goyal said.
To that end, she and her colleagues are planning gene expression studies in patients from the UMN cohort. The researchers plan to examine genes involved in the pathogenesis of second malignancies and MF progression in tissue samples from 36 MF patients, 12 who developed second malignancies and 24 who did not.
The current research was funded by the American Society of Hematology. Dr. Goyal reported having no relevant financial disclosures. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – A retrospective study suggests patients with mycosis fungoides (MF) have an increased risk of developing hematologic and solid tumor malignancies.

Researchers found the risk of second malignancy was highest among MF patients aged 30 to 50 years and patients who had tumor stage or advanced stage MF.
The increased risk was present during the entire period after MF diagnosis, but it was greatest in the first 6 months after diagnosis and roughly a dozen years later.
Amrita Goyal, MD, of the University of Minnesota in Minneapolis, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The researchers first assessed the risk of second malignancy in 172 MF patients treated at UMN from 2005 to 2017, comparing this cohort to a control group of 172 patients with seborrheic dermatitis.
Second malignancies occurred in 24 MF patients and three controls, which was a significant difference (P = .0045). The most common second malignancies among the MF patients were melanoma (n = 4), prostate cancer (n = 3), and renal cell carcinoma (n = 3).
Further analyses revealed that MF patients were more likely to develop a second malignancy if they had tumor stage disease (P = .0024) or stage IIB or higher disease (P = .03).
To corroborate and expand upon these results, Dr. Goyal and her colleagues analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database on patients diagnosed with MF from 2000 to 2014.
Among the 6,196 MF patients in this cohort, there were 514 second cancers.
“We found that MF patients were, overall, 10 times more likely to develop a second malignancy [compared with the general population],” Dr. Goyal said.
Specifically, the standardized incidence ratio was 10.15 for all malignancies, 7.33 for solid tumors, and 41.72 for hematologic malignancies.
Standardized incidence ratios for individual malignancies were:
- 69.8 for Hodgkin lymphoma.
- 46.5 for non-Hodgkin lymphoma.
- 8.6 for leukemia.
- 7.2 for melanoma.
- 6.2 for lung cancer.
- 7.9 for female breast cancer.
- 5.2 for colon cancer.
- 4.1 for prostate cancer.
- 3.9 for renal cell carcinoma.
- 3.8 for pancreatic cancer.
- 3.6 for bladder cancer.
“We found there is an increased risk [of second malignancy] during the first 6 months after diagnosis of MF, likely related to patients being in contact with the health care system more,” Dr. Goyal said. “Over time, patients have about a 7- to 10-fold increased risk over baseline, until they reach about 12 or 13 years after diagnosis, at which point, there is an increase in risk.”
The researchers found the greatest risk of second malignancy was among patients aged 30 to 50 years, although there was an increased risk for all age groups.
“The reason we think patients are experiencing an increased risk of cancers is we believe this may be due to immune suppression secondary to the mycosis fungoides, although further studies need to be performed to determine if that’s accurate,” Dr. Goyal said.
To that end, she and her colleagues are planning gene expression studies in patients from the UMN cohort. The researchers plan to examine genes involved in the pathogenesis of second malignancies and MF progression in tissue samples from 36 MF patients, 12 who developed second malignancies and 24 who did not.
The current research was funded by the American Society of Hematology. Dr. Goyal reported having no relevant financial disclosures. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – A retrospective study suggests patients with mycosis fungoides (MF) have an increased risk of developing hematologic and solid tumor malignancies.

Researchers found the risk of second malignancy was highest among MF patients aged 30 to 50 years and patients who had tumor stage or advanced stage MF.
The increased risk was present during the entire period after MF diagnosis, but it was greatest in the first 6 months after diagnosis and roughly a dozen years later.
Amrita Goyal, MD, of the University of Minnesota in Minneapolis, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The researchers first assessed the risk of second malignancy in 172 MF patients treated at UMN from 2005 to 2017, comparing this cohort to a control group of 172 patients with seborrheic dermatitis.
Second malignancies occurred in 24 MF patients and three controls, which was a significant difference (P = .0045). The most common second malignancies among the MF patients were melanoma (n = 4), prostate cancer (n = 3), and renal cell carcinoma (n = 3).
Further analyses revealed that MF patients were more likely to develop a second malignancy if they had tumor stage disease (P = .0024) or stage IIB or higher disease (P = .03).
To corroborate and expand upon these results, Dr. Goyal and her colleagues analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database on patients diagnosed with MF from 2000 to 2014.
Among the 6,196 MF patients in this cohort, there were 514 second cancers.
“We found that MF patients were, overall, 10 times more likely to develop a second malignancy [compared with the general population],” Dr. Goyal said.
Specifically, the standardized incidence ratio was 10.15 for all malignancies, 7.33 for solid tumors, and 41.72 for hematologic malignancies.
Standardized incidence ratios for individual malignancies were:
- 69.8 for Hodgkin lymphoma.
- 46.5 for non-Hodgkin lymphoma.
- 8.6 for leukemia.
- 7.2 for melanoma.
- 6.2 for lung cancer.
- 7.9 for female breast cancer.
- 5.2 for colon cancer.
- 4.1 for prostate cancer.
- 3.9 for renal cell carcinoma.
- 3.8 for pancreatic cancer.
- 3.6 for bladder cancer.
“We found there is an increased risk [of second malignancy] during the first 6 months after diagnosis of MF, likely related to patients being in contact with the health care system more,” Dr. Goyal said. “Over time, patients have about a 7- to 10-fold increased risk over baseline, until they reach about 12 or 13 years after diagnosis, at which point, there is an increase in risk.”
The researchers found the greatest risk of second malignancy was among patients aged 30 to 50 years, although there was an increased risk for all age groups.
“The reason we think patients are experiencing an increased risk of cancers is we believe this may be due to immune suppression secondary to the mycosis fungoides, although further studies need to be performed to determine if that’s accurate,” Dr. Goyal said.
To that end, she and her colleagues are planning gene expression studies in patients from the UMN cohort. The researchers plan to examine genes involved in the pathogenesis of second malignancies and MF progression in tissue samples from 36 MF patients, 12 who developed second malignancies and 24 who did not.
The current research was funded by the American Society of Hematology. Dr. Goyal reported having no relevant financial disclosures. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: In a cohort of MF patients from the SEER database, the standardized incidence ratio was 10.15 for all malignancies, 7.33 for solid tumors, and 41.72 for hematologic malignancies.
Study details: Retrospective study of 6,196 MF patients from the SEER database, and a single-center cohort of 172 MF patients who were matched to 172 patients with seborrheic dermatitis.
Disclosures: This research was funded by the American Society of Hematology. Dr. Goyal reported having no relevant financial disclosures.
Cobomarsen shows early promise for treating ATLL
LA JOLLA, CALIF. – Phase 1 results suggest cobomarsen is well tolerated and can maintain or improve responses in patients with previously treated adult T-cell leukemia/lymphoma (ATLL).
Five of eight ATLL patients studied experienced disease stabilization or improvement while receiving cobomarsen (MRG-106), an inhibitor of microRNA-155.
There were no grade 3/4 adverse events (AEs) or serious AEs related to cobomarsen in these patients.
Francine Foss, MD, of Yale Cancer Center in New Haven, Conn., and her colleagues presented these results at the annual T-cell Lymphoma Forum.
In this ongoing trial (NCT02580552), researchers are evaluating cobomarsen in patients with B- and T-cell lymphomas, including mycosis fungoides and ATLL.
Results are available for eight patients with previously treated ATLL. These patients had received a median of 4 (range, 1-10) prior systemic therapies, and they had a median age of 51 years (range, 40-68).
The patients received three loading doses of cobomarsen during the first week of cycle 1, followed by weekly dosing. All patients have received cobomarsen as a 600 mg intravenous infusion. They can remain on cobomarsen until they progress, experience clinically significant side effects, cannot tolerate the drug, or the trial is terminated.
The researchers have measured efficacy at least monthly by monitoring tumor cell burden in the peripheral blood and lymph nodes, as well as evaluating changes in skin involvement.
Stabilization and response
“Initially, we saw some very good responses in patients who had escalating disease. In other words, their disease was progressing after conventional chemotherapy,” Dr. Foss said. “They went on this microRNA, [and] their disease stabilized and then regressed. We saw, subsequently, in another three or four patients, the same pattern of activity.”
In all, five patients achieved or maintained a response while on cobomarsen. All five were still receiving the drug at the data cutoff on Dec. 13, 2018.
Two of these patients had acute disease and were in partial response (PR) at baseline. These patients had received cobomarsen for 87 days and 401 days as of the data cutoff.
The other three patients still receiving cobomarsen at the cutoff had lymphomatous disease. At baseline, two of the patients were in PR and one had stable disease.
The two patients in PR at baseline had received cobomarsen for 80 days and 366 days at the data cutoff. The patient with stable disease had received the drug for 161 days.
Progression and withdrawal
There were three patients who withdrew from the study because of disease progression. Two of these patients were relapsing with significant skin involvement at baseline.
One of the patients discontinued cobomarsen after 23 days of treatment. The other patient received cobomarsen for 91 days and left the study, then re-enrolled and received cobomarsen for another 42 days before withdrawing from the study again.
The third patient had relapsed lymphomatous disease at baseline. This patient had a mixed response to cobomarsen, with some nodes decreasing in size and others increasing. She discontinued cobomarsen after 9 days.
“It’s still early on in our experience with ATLL, so we don’t really know yet who the patient is that’s going to respond – what are the clinical features that would predict response in these patients,” Dr. Foss said. “And we’re still really trying to understand how we give the drug to these patients, for how long, and whether or not we can change the dosing interval. But, nevertheless, we have some very interesting data.”
Safety
There were no dose-limiting toxicities, AE-related discontinuations, treatment-related grade 3/4 AEs, or new opportunistic infections observed.
“[I] have to say, in using this drug now for over a year in two of my patients – and that’s with weekly administration – we really haven’t seen anything as far as adverse events,” Dr. Foss said.
She noted that one patient has reported transient diarrhea after dosing.
Two serious AEs – febrile neutropenia and pyrexia – occurred in one patient, but neither of these events were considered related to cobomarsen. The AEs occurred after the patient had stopped cobomarsen, and both events resolved.
There were no on-treatment deaths. One patient (the one who received cobomarsen for 9 days) died from disease progression approximately 2 months after stopping cobomarsen and while on a different therapy.
Dr. Foss said, based on their results, she and her colleagues are hoping to accrue more ATLL patients in this trial.
The trial is sponsored by miRagen Therapeutics. Dr. Foss is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Phase 1 results suggest cobomarsen is well tolerated and can maintain or improve responses in patients with previously treated adult T-cell leukemia/lymphoma (ATLL).
Five of eight ATLL patients studied experienced disease stabilization or improvement while receiving cobomarsen (MRG-106), an inhibitor of microRNA-155.
There were no grade 3/4 adverse events (AEs) or serious AEs related to cobomarsen in these patients.
Francine Foss, MD, of Yale Cancer Center in New Haven, Conn., and her colleagues presented these results at the annual T-cell Lymphoma Forum.
In this ongoing trial (NCT02580552), researchers are evaluating cobomarsen in patients with B- and T-cell lymphomas, including mycosis fungoides and ATLL.
Results are available for eight patients with previously treated ATLL. These patients had received a median of 4 (range, 1-10) prior systemic therapies, and they had a median age of 51 years (range, 40-68).
The patients received three loading doses of cobomarsen during the first week of cycle 1, followed by weekly dosing. All patients have received cobomarsen as a 600 mg intravenous infusion. They can remain on cobomarsen until they progress, experience clinically significant side effects, cannot tolerate the drug, or the trial is terminated.
The researchers have measured efficacy at least monthly by monitoring tumor cell burden in the peripheral blood and lymph nodes, as well as evaluating changes in skin involvement.
Stabilization and response
“Initially, we saw some very good responses in patients who had escalating disease. In other words, their disease was progressing after conventional chemotherapy,” Dr. Foss said. “They went on this microRNA, [and] their disease stabilized and then regressed. We saw, subsequently, in another three or four patients, the same pattern of activity.”
In all, five patients achieved or maintained a response while on cobomarsen. All five were still receiving the drug at the data cutoff on Dec. 13, 2018.
Two of these patients had acute disease and were in partial response (PR) at baseline. These patients had received cobomarsen for 87 days and 401 days as of the data cutoff.
The other three patients still receiving cobomarsen at the cutoff had lymphomatous disease. At baseline, two of the patients were in PR and one had stable disease.
The two patients in PR at baseline had received cobomarsen for 80 days and 366 days at the data cutoff. The patient with stable disease had received the drug for 161 days.
Progression and withdrawal
There were three patients who withdrew from the study because of disease progression. Two of these patients were relapsing with significant skin involvement at baseline.
One of the patients discontinued cobomarsen after 23 days of treatment. The other patient received cobomarsen for 91 days and left the study, then re-enrolled and received cobomarsen for another 42 days before withdrawing from the study again.
The third patient had relapsed lymphomatous disease at baseline. This patient had a mixed response to cobomarsen, with some nodes decreasing in size and others increasing. She discontinued cobomarsen after 9 days.
“It’s still early on in our experience with ATLL, so we don’t really know yet who the patient is that’s going to respond – what are the clinical features that would predict response in these patients,” Dr. Foss said. “And we’re still really trying to understand how we give the drug to these patients, for how long, and whether or not we can change the dosing interval. But, nevertheless, we have some very interesting data.”
Safety
There were no dose-limiting toxicities, AE-related discontinuations, treatment-related grade 3/4 AEs, or new opportunistic infections observed.
“[I] have to say, in using this drug now for over a year in two of my patients – and that’s with weekly administration – we really haven’t seen anything as far as adverse events,” Dr. Foss said.
She noted that one patient has reported transient diarrhea after dosing.
Two serious AEs – febrile neutropenia and pyrexia – occurred in one patient, but neither of these events were considered related to cobomarsen. The AEs occurred after the patient had stopped cobomarsen, and both events resolved.
There were no on-treatment deaths. One patient (the one who received cobomarsen for 9 days) died from disease progression approximately 2 months after stopping cobomarsen and while on a different therapy.
Dr. Foss said, based on their results, she and her colleagues are hoping to accrue more ATLL patients in this trial.
The trial is sponsored by miRagen Therapeutics. Dr. Foss is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Phase 1 results suggest cobomarsen is well tolerated and can maintain or improve responses in patients with previously treated adult T-cell leukemia/lymphoma (ATLL).
Five of eight ATLL patients studied experienced disease stabilization or improvement while receiving cobomarsen (MRG-106), an inhibitor of microRNA-155.
There were no grade 3/4 adverse events (AEs) or serious AEs related to cobomarsen in these patients.
Francine Foss, MD, of Yale Cancer Center in New Haven, Conn., and her colleagues presented these results at the annual T-cell Lymphoma Forum.
In this ongoing trial (NCT02580552), researchers are evaluating cobomarsen in patients with B- and T-cell lymphomas, including mycosis fungoides and ATLL.
Results are available for eight patients with previously treated ATLL. These patients had received a median of 4 (range, 1-10) prior systemic therapies, and they had a median age of 51 years (range, 40-68).
The patients received three loading doses of cobomarsen during the first week of cycle 1, followed by weekly dosing. All patients have received cobomarsen as a 600 mg intravenous infusion. They can remain on cobomarsen until they progress, experience clinically significant side effects, cannot tolerate the drug, or the trial is terminated.
The researchers have measured efficacy at least monthly by monitoring tumor cell burden in the peripheral blood and lymph nodes, as well as evaluating changes in skin involvement.
Stabilization and response
“Initially, we saw some very good responses in patients who had escalating disease. In other words, their disease was progressing after conventional chemotherapy,” Dr. Foss said. “They went on this microRNA, [and] their disease stabilized and then regressed. We saw, subsequently, in another three or four patients, the same pattern of activity.”
In all, five patients achieved or maintained a response while on cobomarsen. All five were still receiving the drug at the data cutoff on Dec. 13, 2018.
Two of these patients had acute disease and were in partial response (PR) at baseline. These patients had received cobomarsen for 87 days and 401 days as of the data cutoff.
The other three patients still receiving cobomarsen at the cutoff had lymphomatous disease. At baseline, two of the patients were in PR and one had stable disease.
The two patients in PR at baseline had received cobomarsen for 80 days and 366 days at the data cutoff. The patient with stable disease had received the drug for 161 days.
Progression and withdrawal
There were three patients who withdrew from the study because of disease progression. Two of these patients were relapsing with significant skin involvement at baseline.
One of the patients discontinued cobomarsen after 23 days of treatment. The other patient received cobomarsen for 91 days and left the study, then re-enrolled and received cobomarsen for another 42 days before withdrawing from the study again.
The third patient had relapsed lymphomatous disease at baseline. This patient had a mixed response to cobomarsen, with some nodes decreasing in size and others increasing. She discontinued cobomarsen after 9 days.
“It’s still early on in our experience with ATLL, so we don’t really know yet who the patient is that’s going to respond – what are the clinical features that would predict response in these patients,” Dr. Foss said. “And we’re still really trying to understand how we give the drug to these patients, for how long, and whether or not we can change the dosing interval. But, nevertheless, we have some very interesting data.”
Safety
There were no dose-limiting toxicities, AE-related discontinuations, treatment-related grade 3/4 AEs, or new opportunistic infections observed.
“[I] have to say, in using this drug now for over a year in two of my patients – and that’s with weekly administration – we really haven’t seen anything as far as adverse events,” Dr. Foss said.
She noted that one patient has reported transient diarrhea after dosing.
Two serious AEs – febrile neutropenia and pyrexia – occurred in one patient, but neither of these events were considered related to cobomarsen. The AEs occurred after the patient had stopped cobomarsen, and both events resolved.
There were no on-treatment deaths. One patient (the one who received cobomarsen for 9 days) died from disease progression approximately 2 months after stopping cobomarsen and while on a different therapy.
Dr. Foss said, based on their results, she and her colleagues are hoping to accrue more ATLL patients in this trial.
The trial is sponsored by miRagen Therapeutics. Dr. Foss is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: Five of eight ATLL patients studied experienced disease stabilization or improvement while receiving cobomarsen.
Study details: Phase 1 trial including eight ATLL patients.
Disclosures: The trial is sponsored by miRagen Therapeutics.
Cerdulatinib yields ‘encouraging’ results in CTCL, PTCL
LA JOLLA, CALIF. – The spleen tyrosine kinase/Janus kinase inhibitor cerdulatinib has demonstrated activity against relapsed and refractory T-cell lymphomas.

In a phase 2 trial, cerdulatinib produced responses in 34% of patients with peripheral T-cell lymphoma (PTCL) and 26% of those with cutaneous T-cell lymphoma (CTCL).
The best responders were patients with angioimmunoblastic T-cell lymphoma, half of whom achieved a complete response (CR).
The most common grade 3 or higher adverse events (AEs) were amylase increase and lipase increase. However, these increases resolved with dose reduction or interruption, and there were no cases of clinical pancreatitis.
“The data is very encouraging,” said Tatyana Feldman, MD, of the John Theurer Cancer Center in Hackensack, N.J.
Dr. Feldman and her colleagues previously presented results from the phase 2 trial of cerdulatinib (NCT01994382) at the 2018 annual congress of the European Hematology Association.
Dr. Feldman and her colleagues presented data from expansion cohorts of the ongoing trial at the annual T-cell Lymphoma Forum. The cohorts included patients with PTCL or CTCL who had received at least one prior systemic therapy.
PTCL cohort
The 45 PTCL patients had a median age of 65 years (range, 21-84). They had received a median of 3 (range, 1-12) prior therapeutic regimens, 51% were refractory to their last therapy, and 27% had undergone stem cell transplant (SCT).
The patients received cerdulatinib at 30 mg orally twice a day until progression or intolerance, and 41 patients were evaluable for response.
The overall response rate was 34% (n = 14). Eleven patients had a CR, three had a partial response (PR), and nine had stable disease.
Responses according to subtype were as follows:
- 7 CRs and 1 PR in angioimmunoblastic T-cell lymphoma.
- 2 CRs in PTCL not otherwise specified.
- 1 CR in gamma-delta T-cell lymphoma.
- 1 PR in ALK-negative anaplastic large-cell lymphoma.
- 1 CR and 1 PR in adult T-cell leukemia/lymphoma.
Eight responders have remained on cerdulatinib for anywhere from 3 months to more than 12 months. Five patients have had a response lasting at least 6 months. One patient went on to SCT after achieving a CR.
The most common grade 3 or higher AEs observed in PTCL patients were amylase increase (n = 8), lipase increase (n = 6), pneumonia/lung infection (n = 5), neutropenia (n = 4), diarrhea (n = 4), febrile neutropenia (n = 4), abdominal pain (n = 4), sepsis/bacteremia (n = 3), anemia (n = 3), fatigue (n = 2), and pain (n = 1).
There were two grade 5 AEs – acute respiratory distress syndrome and pneumonia.
CTCL cohort
The 29 CTCL patients had a median age of 62 years (range, 24-79). They had received a median of 4 (range, 1-13) prior therapies, 55% were refractory to their last therapy, and 3% had undergone SCT.
The patients received cerdulatinib at 30 mg orally twice a day until progression or intolerance, and 27 were evaluable for response.
The overall response rate was 26% (n = 7). Two patients achieved a CR, five achieved a PR, and nine had stable disease. Responses occurred in mycosis fungoides and Sézary syndrome.
Eleven of 23 patients (48%) achieved at least a 50% reduction in skin lesions, and the researchers observed rapid improvements in pruritus.
“I saw patients who would take the first pill, and they would call me and say, ‘I no longer itch,’ ” Dr. Feldman said.
The most common grade 3 or higher AEs in CTCL patients were lipase increase (n = 11), amylase increase (n = 5), sepsis/bacteremia (n = 3), pain (n = 2), fatigue (n = 1), neutropenia (n = 1), and diarrhea (n = 1).
“It’s a very well-tolerated drug,” Dr. Feldman said, adding that there were “really no severe side effects which would prohibit the use of the drug.”
She noted that cerdulatinib’s “favorable” side effect profile might make it a promising candidate for use in combination regimens.
“I think it will be possible to combine it with other drugs in development in T-cell lymphoma. … immunological checkpoint inhibitors, epigenetic modulators such as HDAC [histone deacetylase] inhibitors, methylating agents, and PI3 kinase inhibitors,” Dr. Feldman said.
She reported having no disclosures relevant to this study. The trial is sponsored by Portola Pharmaceuticals.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – The spleen tyrosine kinase/Janus kinase inhibitor cerdulatinib has demonstrated activity against relapsed and refractory T-cell lymphomas.

In a phase 2 trial, cerdulatinib produced responses in 34% of patients with peripheral T-cell lymphoma (PTCL) and 26% of those with cutaneous T-cell lymphoma (CTCL).
The best responders were patients with angioimmunoblastic T-cell lymphoma, half of whom achieved a complete response (CR).
The most common grade 3 or higher adverse events (AEs) were amylase increase and lipase increase. However, these increases resolved with dose reduction or interruption, and there were no cases of clinical pancreatitis.
“The data is very encouraging,” said Tatyana Feldman, MD, of the John Theurer Cancer Center in Hackensack, N.J.
Dr. Feldman and her colleagues previously presented results from the phase 2 trial of cerdulatinib (NCT01994382) at the 2018 annual congress of the European Hematology Association.
Dr. Feldman and her colleagues presented data from expansion cohorts of the ongoing trial at the annual T-cell Lymphoma Forum. The cohorts included patients with PTCL or CTCL who had received at least one prior systemic therapy.
PTCL cohort
The 45 PTCL patients had a median age of 65 years (range, 21-84). They had received a median of 3 (range, 1-12) prior therapeutic regimens, 51% were refractory to their last therapy, and 27% had undergone stem cell transplant (SCT).
The patients received cerdulatinib at 30 mg orally twice a day until progression or intolerance, and 41 patients were evaluable for response.
The overall response rate was 34% (n = 14). Eleven patients had a CR, three had a partial response (PR), and nine had stable disease.
Responses according to subtype were as follows:
- 7 CRs and 1 PR in angioimmunoblastic T-cell lymphoma.
- 2 CRs in PTCL not otherwise specified.
- 1 CR in gamma-delta T-cell lymphoma.
- 1 PR in ALK-negative anaplastic large-cell lymphoma.
- 1 CR and 1 PR in adult T-cell leukemia/lymphoma.
Eight responders have remained on cerdulatinib for anywhere from 3 months to more than 12 months. Five patients have had a response lasting at least 6 months. One patient went on to SCT after achieving a CR.
The most common grade 3 or higher AEs observed in PTCL patients were amylase increase (n = 8), lipase increase (n = 6), pneumonia/lung infection (n = 5), neutropenia (n = 4), diarrhea (n = 4), febrile neutropenia (n = 4), abdominal pain (n = 4), sepsis/bacteremia (n = 3), anemia (n = 3), fatigue (n = 2), and pain (n = 1).
There were two grade 5 AEs – acute respiratory distress syndrome and pneumonia.
CTCL cohort
The 29 CTCL patients had a median age of 62 years (range, 24-79). They had received a median of 4 (range, 1-13) prior therapies, 55% were refractory to their last therapy, and 3% had undergone SCT.
The patients received cerdulatinib at 30 mg orally twice a day until progression or intolerance, and 27 were evaluable for response.
The overall response rate was 26% (n = 7). Two patients achieved a CR, five achieved a PR, and nine had stable disease. Responses occurred in mycosis fungoides and Sézary syndrome.
Eleven of 23 patients (48%) achieved at least a 50% reduction in skin lesions, and the researchers observed rapid improvements in pruritus.
“I saw patients who would take the first pill, and they would call me and say, ‘I no longer itch,’ ” Dr. Feldman said.
The most common grade 3 or higher AEs in CTCL patients were lipase increase (n = 11), amylase increase (n = 5), sepsis/bacteremia (n = 3), pain (n = 2), fatigue (n = 1), neutropenia (n = 1), and diarrhea (n = 1).
“It’s a very well-tolerated drug,” Dr. Feldman said, adding that there were “really no severe side effects which would prohibit the use of the drug.”
She noted that cerdulatinib’s “favorable” side effect profile might make it a promising candidate for use in combination regimens.
“I think it will be possible to combine it with other drugs in development in T-cell lymphoma. … immunological checkpoint inhibitors, epigenetic modulators such as HDAC [histone deacetylase] inhibitors, methylating agents, and PI3 kinase inhibitors,” Dr. Feldman said.
She reported having no disclosures relevant to this study. The trial is sponsored by Portola Pharmaceuticals.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – The spleen tyrosine kinase/Janus kinase inhibitor cerdulatinib has demonstrated activity against relapsed and refractory T-cell lymphomas.

In a phase 2 trial, cerdulatinib produced responses in 34% of patients with peripheral T-cell lymphoma (PTCL) and 26% of those with cutaneous T-cell lymphoma (CTCL).
The best responders were patients with angioimmunoblastic T-cell lymphoma, half of whom achieved a complete response (CR).
The most common grade 3 or higher adverse events (AEs) were amylase increase and lipase increase. However, these increases resolved with dose reduction or interruption, and there were no cases of clinical pancreatitis.
“The data is very encouraging,” said Tatyana Feldman, MD, of the John Theurer Cancer Center in Hackensack, N.J.
Dr. Feldman and her colleagues previously presented results from the phase 2 trial of cerdulatinib (NCT01994382) at the 2018 annual congress of the European Hematology Association.
Dr. Feldman and her colleagues presented data from expansion cohorts of the ongoing trial at the annual T-cell Lymphoma Forum. The cohorts included patients with PTCL or CTCL who had received at least one prior systemic therapy.
PTCL cohort
The 45 PTCL patients had a median age of 65 years (range, 21-84). They had received a median of 3 (range, 1-12) prior therapeutic regimens, 51% were refractory to their last therapy, and 27% had undergone stem cell transplant (SCT).
The patients received cerdulatinib at 30 mg orally twice a day until progression or intolerance, and 41 patients were evaluable for response.
The overall response rate was 34% (n = 14). Eleven patients had a CR, three had a partial response (PR), and nine had stable disease.
Responses according to subtype were as follows:
- 7 CRs and 1 PR in angioimmunoblastic T-cell lymphoma.
- 2 CRs in PTCL not otherwise specified.
- 1 CR in gamma-delta T-cell lymphoma.
- 1 PR in ALK-negative anaplastic large-cell lymphoma.
- 1 CR and 1 PR in adult T-cell leukemia/lymphoma.
Eight responders have remained on cerdulatinib for anywhere from 3 months to more than 12 months. Five patients have had a response lasting at least 6 months. One patient went on to SCT after achieving a CR.
The most common grade 3 or higher AEs observed in PTCL patients were amylase increase (n = 8), lipase increase (n = 6), pneumonia/lung infection (n = 5), neutropenia (n = 4), diarrhea (n = 4), febrile neutropenia (n = 4), abdominal pain (n = 4), sepsis/bacteremia (n = 3), anemia (n = 3), fatigue (n = 2), and pain (n = 1).
There were two grade 5 AEs – acute respiratory distress syndrome and pneumonia.
CTCL cohort
The 29 CTCL patients had a median age of 62 years (range, 24-79). They had received a median of 4 (range, 1-13) prior therapies, 55% were refractory to their last therapy, and 3% had undergone SCT.
The patients received cerdulatinib at 30 mg orally twice a day until progression or intolerance, and 27 were evaluable for response.
The overall response rate was 26% (n = 7). Two patients achieved a CR, five achieved a PR, and nine had stable disease. Responses occurred in mycosis fungoides and Sézary syndrome.
Eleven of 23 patients (48%) achieved at least a 50% reduction in skin lesions, and the researchers observed rapid improvements in pruritus.
“I saw patients who would take the first pill, and they would call me and say, ‘I no longer itch,’ ” Dr. Feldman said.
The most common grade 3 or higher AEs in CTCL patients were lipase increase (n = 11), amylase increase (n = 5), sepsis/bacteremia (n = 3), pain (n = 2), fatigue (n = 1), neutropenia (n = 1), and diarrhea (n = 1).
“It’s a very well-tolerated drug,” Dr. Feldman said, adding that there were “really no severe side effects which would prohibit the use of the drug.”
She noted that cerdulatinib’s “favorable” side effect profile might make it a promising candidate for use in combination regimens.
“I think it will be possible to combine it with other drugs in development in T-cell lymphoma. … immunological checkpoint inhibitors, epigenetic modulators such as HDAC [histone deacetylase] inhibitors, methylating agents, and PI3 kinase inhibitors,” Dr. Feldman said.
She reported having no disclosures relevant to this study. The trial is sponsored by Portola Pharmaceuticals.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: The overall response rate was 34% in patients with peripheral T-cell lymphoma (PTCL) and 26% in patients with cutaneous T-cell lymphoma (CTCL).
Study details: Expansion cohorts of a phase 2 trial including 45 PTCL patients and 29 CTCL patients
Disclosures: The study was funded by Portola Pharmaceuticals. The investigator reported having no relevant conflicts.
Combo emerges as bridge to transplant in rel/ref PTCL
LA JOLLA, CALIF. – The combination of duvelisib and romidepsin is active and can provide a bridge to transplant in relapsed or refractory peripheral T-cell lymphoma (PTCL), according to researchers.

In a phase 1 trial, duvelisib plus romidepsin produced an overall response rate (ORR) of 59% in patients with PTCL. Sixteen patients achieved a response, nine had a complete response (CR), and six complete responders went on to transplant.
“So we think that you can achieve remission deep enough to then move on to a potentially curative approach,” said study investigator Neha Mehta-Shah, MD, of Washington University in St. Louis.
She and her colleagues evaluated romidepsin plus duvelisib, as well as bortezomib plus duvelisib, in a phase 1 trial (NCT02783625) of patients with relapsed or refractory PTCL or cutaneous T-cell lymphoma (CTCL).
Dr. Mehta-Shah presented the results at the annual T-cell Lymphoma Forum.
She reported results in 80 patients – 51 with PTCL and 29 with CTCL. The patients’ median age was 64 years (range, 28-83), and 57% of the study population were men. Patients had received a median of 3 (range, 1-16) prior therapies, and 16% had received a prior transplant.
Treatment
Dr. Mehta-Shah noted that patients and providers could choose whether patients would receive romidepsin or bortezomib.
Patients in the romidepsin arm received romidepsin at 10 mg/m2 on days 1, 8, and 15 of each 28-day cycle. Patients in the bortezomib arm received bortezomib at 1 mg/m2 on days 1, 4, 8, and 11 of each cycle.
Duvelisib dosing was escalated, so patients received duvelisib at 25 mg, 50 mg, or 75 mg twice daily.
In the bortezomib arm, there was one dose-limiting toxicity – grade 3 neutropenia – in a patient who received duvelisib at the 25-mg dose. There were no dose-limiting toxicities in the romidepsin arm.
The researchers determined that the maximum tolerated dose (MTD) of duvelisib was 75 mg twice daily in the romidepsin arm and 25 mg twice daily in the bortezomib arm.
Lead-in phase
The study also had a lead-in phase during which patients could receive single-agent duvelisib.
“Because the original phase 1 study of duvelisib did not collect as many prospective tumor biopsies or on-treatment biopsies, we built into this study a lead-in phase so that we could characterize on-treatment biopsies to better understand mechanisms of response or resistance,” Dr. Mehta-Shah said.
Patients and providers could choose to be part of the lead-in phase, she noted. Patients who did not achieve a CR during this phase went on to receive either combination therapy, which was predetermined before the monotherapy began.
There were 14 patients who received duvelisib monotherapy at 75 mg twice daily. Four of them achieved a CR, and three had a partial response (PR). Ten patients went on to receive romidepsin as well. One of them achieved a CR, and three had a PR.
There were 12 patients who received duvelisib monotherapy at 25 mg twice daily. Three of them achieved a CR, and two had a PR. Nine patients went on to receive bortezomib as well. This combination produced one CR and two PRs.
Efficacy with romidepsin
Among all evaluable PTCL patients in the romidepsin arm, the ORR was 59% (16/27), and the CR rate was 33% (9/27).
Responses occurred in seven patients with PTCL not otherwise specified (NOS), six with angioimmunoblastic T-cell lymphoma (AITL), one with hepatosplenic T-cell lymphoma, one with aggressive epidermotropic CD8+ T-cell lymphoma, and one with primary cutaneous PTCL.
CRs occurred in five patients with AITL and four with PTCL-NOS. Six patients who achieved a CR went on to transplant.
Among evaluable CTCL patients in the romidepsin arm, the ORR was 45% (5/11), and there were no CRs. Responses occurred in three patients with mycosis fungoides and two with Sézary syndrome.
The median progression-free survival was 5.41 months in CTCL patients and 6.72 months in PTCL patients.
Efficacy with bortezomib
Among evaluable PTCL patients in the bortezomib arm, the ORR was 44% (7/16), and the CR rate was 25% (4/16).
Responses occurred in three patients with AITL and four with PTCL-NOS. CRs occurred in two patients with each subtype.
Among evaluable CTCL patients in the bortezomib arm, the ORR was 27% (4/15), and there were no CRs. Responses occurred in one patient with mycosis fungoides and three with Sézary syndrome. One CTCL patient went on to transplant.
The median progression-free survival was 4.56 months among CTCL patients and 4.39 months in PTCL patients.
Safety
Dr. Mehta-Shah said both combinations were considered safe and well tolerated. However, there was a grade 5 adverse event (AE) – Stevens-Johnson syndrome – that occurred in the bortezomib arm and was considered possibly related to treatment.
Grade 3/4 AEs observed in the 31 patients treated at the MTD in the romidepsin arm were transaminase increase (n = 7), diarrhea (n = 6), hyponatremia (n = 4), neutrophil count decrease (n = 10), and platelet count decrease (n = 3).
Grade 3/4 AEs observed in the 23 patients treated at the MTD in the bortezomib arm were transaminase increase (n = 2) and neutrophil count decrease (n = 5).
Grade 3/4 transaminitis seemed to be more common among patients who received duvelisib alone during the lead-in phase, Dr. Mehta-Shah said.
Among patients treated at the MTD in the romidepsin arm, grade 3/4 transaminitis occurred in four patients treated during the lead-in phase and three who began receiving romidepsin and duvelisib together. In the bortezomib arm, grade 3/4 transaminitis occurred in two patients treated at the MTD, both of whom received duvelisib alone during the lead-in phase.
Based on these results, Dr. Mehta-Shah and her colleagues are planning to expand the romidepsin arm to an additional 25 patients. By testing the combination in more patients, the researchers hope to better understand the occurrence of transaminitis and assess the durability of response.
This study is supported by Verastem. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – The combination of duvelisib and romidepsin is active and can provide a bridge to transplant in relapsed or refractory peripheral T-cell lymphoma (PTCL), according to researchers.

In a phase 1 trial, duvelisib plus romidepsin produced an overall response rate (ORR) of 59% in patients with PTCL. Sixteen patients achieved a response, nine had a complete response (CR), and six complete responders went on to transplant.
“So we think that you can achieve remission deep enough to then move on to a potentially curative approach,” said study investigator Neha Mehta-Shah, MD, of Washington University in St. Louis.
She and her colleagues evaluated romidepsin plus duvelisib, as well as bortezomib plus duvelisib, in a phase 1 trial (NCT02783625) of patients with relapsed or refractory PTCL or cutaneous T-cell lymphoma (CTCL).
Dr. Mehta-Shah presented the results at the annual T-cell Lymphoma Forum.
She reported results in 80 patients – 51 with PTCL and 29 with CTCL. The patients’ median age was 64 years (range, 28-83), and 57% of the study population were men. Patients had received a median of 3 (range, 1-16) prior therapies, and 16% had received a prior transplant.
Treatment
Dr. Mehta-Shah noted that patients and providers could choose whether patients would receive romidepsin or bortezomib.
Patients in the romidepsin arm received romidepsin at 10 mg/m2 on days 1, 8, and 15 of each 28-day cycle. Patients in the bortezomib arm received bortezomib at 1 mg/m2 on days 1, 4, 8, and 11 of each cycle.
Duvelisib dosing was escalated, so patients received duvelisib at 25 mg, 50 mg, or 75 mg twice daily.
In the bortezomib arm, there was one dose-limiting toxicity – grade 3 neutropenia – in a patient who received duvelisib at the 25-mg dose. There were no dose-limiting toxicities in the romidepsin arm.
The researchers determined that the maximum tolerated dose (MTD) of duvelisib was 75 mg twice daily in the romidepsin arm and 25 mg twice daily in the bortezomib arm.
Lead-in phase
The study also had a lead-in phase during which patients could receive single-agent duvelisib.
“Because the original phase 1 study of duvelisib did not collect as many prospective tumor biopsies or on-treatment biopsies, we built into this study a lead-in phase so that we could characterize on-treatment biopsies to better understand mechanisms of response or resistance,” Dr. Mehta-Shah said.
Patients and providers could choose to be part of the lead-in phase, she noted. Patients who did not achieve a CR during this phase went on to receive either combination therapy, which was predetermined before the monotherapy began.
There were 14 patients who received duvelisib monotherapy at 75 mg twice daily. Four of them achieved a CR, and three had a partial response (PR). Ten patients went on to receive romidepsin as well. One of them achieved a CR, and three had a PR.
There were 12 patients who received duvelisib monotherapy at 25 mg twice daily. Three of them achieved a CR, and two had a PR. Nine patients went on to receive bortezomib as well. This combination produced one CR and two PRs.
Efficacy with romidepsin
Among all evaluable PTCL patients in the romidepsin arm, the ORR was 59% (16/27), and the CR rate was 33% (9/27).
Responses occurred in seven patients with PTCL not otherwise specified (NOS), six with angioimmunoblastic T-cell lymphoma (AITL), one with hepatosplenic T-cell lymphoma, one with aggressive epidermotropic CD8+ T-cell lymphoma, and one with primary cutaneous PTCL.
CRs occurred in five patients with AITL and four with PTCL-NOS. Six patients who achieved a CR went on to transplant.
Among evaluable CTCL patients in the romidepsin arm, the ORR was 45% (5/11), and there were no CRs. Responses occurred in three patients with mycosis fungoides and two with Sézary syndrome.
The median progression-free survival was 5.41 months in CTCL patients and 6.72 months in PTCL patients.
Efficacy with bortezomib
Among evaluable PTCL patients in the bortezomib arm, the ORR was 44% (7/16), and the CR rate was 25% (4/16).
Responses occurred in three patients with AITL and four with PTCL-NOS. CRs occurred in two patients with each subtype.
Among evaluable CTCL patients in the bortezomib arm, the ORR was 27% (4/15), and there were no CRs. Responses occurred in one patient with mycosis fungoides and three with Sézary syndrome. One CTCL patient went on to transplant.
The median progression-free survival was 4.56 months among CTCL patients and 4.39 months in PTCL patients.
Safety
Dr. Mehta-Shah said both combinations were considered safe and well tolerated. However, there was a grade 5 adverse event (AE) – Stevens-Johnson syndrome – that occurred in the bortezomib arm and was considered possibly related to treatment.
Grade 3/4 AEs observed in the 31 patients treated at the MTD in the romidepsin arm were transaminase increase (n = 7), diarrhea (n = 6), hyponatremia (n = 4), neutrophil count decrease (n = 10), and platelet count decrease (n = 3).
Grade 3/4 AEs observed in the 23 patients treated at the MTD in the bortezomib arm were transaminase increase (n = 2) and neutrophil count decrease (n = 5).
Grade 3/4 transaminitis seemed to be more common among patients who received duvelisib alone during the lead-in phase, Dr. Mehta-Shah said.
Among patients treated at the MTD in the romidepsin arm, grade 3/4 transaminitis occurred in four patients treated during the lead-in phase and three who began receiving romidepsin and duvelisib together. In the bortezomib arm, grade 3/4 transaminitis occurred in two patients treated at the MTD, both of whom received duvelisib alone during the lead-in phase.
Based on these results, Dr. Mehta-Shah and her colleagues are planning to expand the romidepsin arm to an additional 25 patients. By testing the combination in more patients, the researchers hope to better understand the occurrence of transaminitis and assess the durability of response.
This study is supported by Verastem. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – The combination of duvelisib and romidepsin is active and can provide a bridge to transplant in relapsed or refractory peripheral T-cell lymphoma (PTCL), according to researchers.

In a phase 1 trial, duvelisib plus romidepsin produced an overall response rate (ORR) of 59% in patients with PTCL. Sixteen patients achieved a response, nine had a complete response (CR), and six complete responders went on to transplant.
“So we think that you can achieve remission deep enough to then move on to a potentially curative approach,” said study investigator Neha Mehta-Shah, MD, of Washington University in St. Louis.
She and her colleagues evaluated romidepsin plus duvelisib, as well as bortezomib plus duvelisib, in a phase 1 trial (NCT02783625) of patients with relapsed or refractory PTCL or cutaneous T-cell lymphoma (CTCL).
Dr. Mehta-Shah presented the results at the annual T-cell Lymphoma Forum.
She reported results in 80 patients – 51 with PTCL and 29 with CTCL. The patients’ median age was 64 years (range, 28-83), and 57% of the study population were men. Patients had received a median of 3 (range, 1-16) prior therapies, and 16% had received a prior transplant.
Treatment
Dr. Mehta-Shah noted that patients and providers could choose whether patients would receive romidepsin or bortezomib.
Patients in the romidepsin arm received romidepsin at 10 mg/m2 on days 1, 8, and 15 of each 28-day cycle. Patients in the bortezomib arm received bortezomib at 1 mg/m2 on days 1, 4, 8, and 11 of each cycle.
Duvelisib dosing was escalated, so patients received duvelisib at 25 mg, 50 mg, or 75 mg twice daily.
In the bortezomib arm, there was one dose-limiting toxicity – grade 3 neutropenia – in a patient who received duvelisib at the 25-mg dose. There were no dose-limiting toxicities in the romidepsin arm.
The researchers determined that the maximum tolerated dose (MTD) of duvelisib was 75 mg twice daily in the romidepsin arm and 25 mg twice daily in the bortezomib arm.
Lead-in phase
The study also had a lead-in phase during which patients could receive single-agent duvelisib.
“Because the original phase 1 study of duvelisib did not collect as many prospective tumor biopsies or on-treatment biopsies, we built into this study a lead-in phase so that we could characterize on-treatment biopsies to better understand mechanisms of response or resistance,” Dr. Mehta-Shah said.
Patients and providers could choose to be part of the lead-in phase, she noted. Patients who did not achieve a CR during this phase went on to receive either combination therapy, which was predetermined before the monotherapy began.
There were 14 patients who received duvelisib monotherapy at 75 mg twice daily. Four of them achieved a CR, and three had a partial response (PR). Ten patients went on to receive romidepsin as well. One of them achieved a CR, and three had a PR.
There were 12 patients who received duvelisib monotherapy at 25 mg twice daily. Three of them achieved a CR, and two had a PR. Nine patients went on to receive bortezomib as well. This combination produced one CR and two PRs.
Efficacy with romidepsin
Among all evaluable PTCL patients in the romidepsin arm, the ORR was 59% (16/27), and the CR rate was 33% (9/27).
Responses occurred in seven patients with PTCL not otherwise specified (NOS), six with angioimmunoblastic T-cell lymphoma (AITL), one with hepatosplenic T-cell lymphoma, one with aggressive epidermotropic CD8+ T-cell lymphoma, and one with primary cutaneous PTCL.
CRs occurred in five patients with AITL and four with PTCL-NOS. Six patients who achieved a CR went on to transplant.
Among evaluable CTCL patients in the romidepsin arm, the ORR was 45% (5/11), and there were no CRs. Responses occurred in three patients with mycosis fungoides and two with Sézary syndrome.
The median progression-free survival was 5.41 months in CTCL patients and 6.72 months in PTCL patients.
Efficacy with bortezomib
Among evaluable PTCL patients in the bortezomib arm, the ORR was 44% (7/16), and the CR rate was 25% (4/16).
Responses occurred in three patients with AITL and four with PTCL-NOS. CRs occurred in two patients with each subtype.
Among evaluable CTCL patients in the bortezomib arm, the ORR was 27% (4/15), and there were no CRs. Responses occurred in one patient with mycosis fungoides and three with Sézary syndrome. One CTCL patient went on to transplant.
The median progression-free survival was 4.56 months among CTCL patients and 4.39 months in PTCL patients.
Safety
Dr. Mehta-Shah said both combinations were considered safe and well tolerated. However, there was a grade 5 adverse event (AE) – Stevens-Johnson syndrome – that occurred in the bortezomib arm and was considered possibly related to treatment.
Grade 3/4 AEs observed in the 31 patients treated at the MTD in the romidepsin arm were transaminase increase (n = 7), diarrhea (n = 6), hyponatremia (n = 4), neutrophil count decrease (n = 10), and platelet count decrease (n = 3).
Grade 3/4 AEs observed in the 23 patients treated at the MTD in the bortezomib arm were transaminase increase (n = 2) and neutrophil count decrease (n = 5).
Grade 3/4 transaminitis seemed to be more common among patients who received duvelisib alone during the lead-in phase, Dr. Mehta-Shah said.
Among patients treated at the MTD in the romidepsin arm, grade 3/4 transaminitis occurred in four patients treated during the lead-in phase and three who began receiving romidepsin and duvelisib together. In the bortezomib arm, grade 3/4 transaminitis occurred in two patients treated at the MTD, both of whom received duvelisib alone during the lead-in phase.
Based on these results, Dr. Mehta-Shah and her colleagues are planning to expand the romidepsin arm to an additional 25 patients. By testing the combination in more patients, the researchers hope to better understand the occurrence of transaminitis and assess the durability of response.
This study is supported by Verastem. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: The overall response rate was 59%, and six of nine complete responders went on to transplant.
Study details: Phase 1 trial of 80 patients that included 27 evaluable PTCL patients who received romidepsin and duvelisib.
Disclosures: The study is supported by Verastem. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
Applying ECHELON-2 results to clinical practice
LA JOLLA, CALIF. – Results from the ECHELON-2 trial led to the U.S. approval of brentuximab vedotin (BV) in combination with cyclophosphamide, doxorubicin, and prednisone (CHP), but there are still questions about how to apply the trial results to practice.

At the annual T-cell Lymphoma Forum, trial investigators and other physicians debated the best use of this combination.
BV-CHP is approved to treat patients with previously untreated systemic anaplastic large-cell lymphoma (sALCL) or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma (AITL) and PTCL not otherwise specified (NOS).
Patients who received BV-CHP in ECHELON-2 had superior progression-free survival (PFS) and overall survival (OS) compared to patients who received cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
These results were initially presented at the 2018 annual meeting of the American Society of Hematology and simultaneously published in The Lancet (2019 Jan 19;393[10168]:229-40).
ECHELON-2 investigator Owen O’Connor, MD, PhD, of Columbia University Medical Center in New York, also presented details on the trial at the T-cell Lymphoma Forum. His presentation was followed by a discussion with meeting attendees about applying the trial results to clinical practice.
CD30 expression
One of the issues discussed was the importance of CD30 expression in deciding which patients should receive BV.
For a patient to be eligible for ECHELON-2, the diagnostic biopsy had to show at least 10% of the neoplastic cells were CD30-positive. However, the Food and Drug Administration (FDA) has not made a similar requirement for prescribing BV. PTCL patients with any level of CD30 expression are eligible for treatment with BV-CHP, according to the FDA.
“[I]t’s still a matter of great debate and controversy as to whether we have good enough data to suggest that there’s a threshold effect with regard to the expression of CD30 and responsiveness or sensitivity to brentuximab vedotin,” Dr. O’Connor said.
“This has been an issue from the very first day with this drug, which is, ‘Just how much CD30 do you need to get a response?’ I can’t speak on behalf of the FDA, but I think they are not absolutely convinced that there’s a threshold. They take [CD30-] positive as ‘good enough’ across the board.”
“The FDA has said, ‘The data we’ve seen says there’s a lot of heterogeneity [with biopsies].’ You may do a biopsy and find 30% [of cells are CD30-positive], and you may do another biopsy [in the same patient] and find less than 10%. I don’t think the regulatory agencies are convinced that a single biopsy looking at CD30 ... is representative of the entire tumor burden.”
Andrei Shustov, MD, an ECHELON-2 investigator from the University of Washington in Seattle, questioned whether CD30 expression should be considered when deciding on the use of BV in PTCL.
“Is CD30 staining relevant at all, or should we default back to studies, say, in colon cancer where we didn’t even care about EGFR because we might be missing it by current techniques?” Dr. Shustov asked. “Should we even worry about CD30 expression ... because we cannot reliably detect low levels of CD30?”
Some attendees echoed this sentiment, questioning the utility of assessing CD30 expression. Other attendees said they would defer to the trial data and only treat patients with BV-CHP if they had at least 10% CD30.
PTCL subtypes
Meeting attendees also discussed the value of BV in different PTCL subtypes.
At the request of European regulatory agencies, ECHELON-2 was largely focused on patients with sALCL. They made up 70% of the total trial population, while 16% of patients had PTCL-NOS, 12% had AITL, and a small number of patients had other subtypes. These numbers meant ECHELON-2 was not powered to determine differences in OS or PFS in non-sALCL subtypes.
As a result, some attendees expressed concerns about using BV-CHP to treat PTCL-NOS or AITL. They argued that it wasn’t clear whether patients with these subtypes would derive more benefit from BV-CHP, CHOP, or CHOP plus etoposide (CHOEP).
Other attendees said they would feel comfortable using BV-CHP in patients with PTCL-NOS or AITL based on ECHELON-2 results.
CHOP vs. CHOEP
The use of CHOP in ECHELON-2 was another point of discussion. Some attendees said CHOEP should have been used as the comparator instead.
A few individuals mentioned retrospective data suggesting CHOEP may confer a benefit over CHOP in PTCL (Blood. 2010 Nov 4;116[18]:3418-25).
Marek Trneny, MD, of Charles University General Hospital in Prague, referenced new data from the Czech National Lymphoma Registry, which showed that patients newly diagnosed with PTCL had superior PFS and OS when they received CHOEP rather than CHOP.
Based on these findings, Dr. Trneny said he would consider treating CD30-positive PTCL patients with CHOEP plus BV rather than BV-CHP.
However, most other attendees said they would not consider adding BV to CHOEP due to the absence of clinical trial data supporting this approach.
Some attendees did say they would use CHOEP instead of BV-CHP, particularly in patients with PTCL-NOS or AITL and in patients with CD30 expression below 10%.
ECHELON-2 was funded by Seattle Genetics and Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical Company.
Dr. O’Connor and Dr. Shustov were investigators on ECHELON-2. Dr. O’Connor is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Results from the ECHELON-2 trial led to the U.S. approval of brentuximab vedotin (BV) in combination with cyclophosphamide, doxorubicin, and prednisone (CHP), but there are still questions about how to apply the trial results to practice.

At the annual T-cell Lymphoma Forum, trial investigators and other physicians debated the best use of this combination.
BV-CHP is approved to treat patients with previously untreated systemic anaplastic large-cell lymphoma (sALCL) or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma (AITL) and PTCL not otherwise specified (NOS).
Patients who received BV-CHP in ECHELON-2 had superior progression-free survival (PFS) and overall survival (OS) compared to patients who received cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
These results were initially presented at the 2018 annual meeting of the American Society of Hematology and simultaneously published in The Lancet (2019 Jan 19;393[10168]:229-40).
ECHELON-2 investigator Owen O’Connor, MD, PhD, of Columbia University Medical Center in New York, also presented details on the trial at the T-cell Lymphoma Forum. His presentation was followed by a discussion with meeting attendees about applying the trial results to clinical practice.
CD30 expression
One of the issues discussed was the importance of CD30 expression in deciding which patients should receive BV.
For a patient to be eligible for ECHELON-2, the diagnostic biopsy had to show at least 10% of the neoplastic cells were CD30-positive. However, the Food and Drug Administration (FDA) has not made a similar requirement for prescribing BV. PTCL patients with any level of CD30 expression are eligible for treatment with BV-CHP, according to the FDA.
“[I]t’s still a matter of great debate and controversy as to whether we have good enough data to suggest that there’s a threshold effect with regard to the expression of CD30 and responsiveness or sensitivity to brentuximab vedotin,” Dr. O’Connor said.
“This has been an issue from the very first day with this drug, which is, ‘Just how much CD30 do you need to get a response?’ I can’t speak on behalf of the FDA, but I think they are not absolutely convinced that there’s a threshold. They take [CD30-] positive as ‘good enough’ across the board.”
“The FDA has said, ‘The data we’ve seen says there’s a lot of heterogeneity [with biopsies].’ You may do a biopsy and find 30% [of cells are CD30-positive], and you may do another biopsy [in the same patient] and find less than 10%. I don’t think the regulatory agencies are convinced that a single biopsy looking at CD30 ... is representative of the entire tumor burden.”
Andrei Shustov, MD, an ECHELON-2 investigator from the University of Washington in Seattle, questioned whether CD30 expression should be considered when deciding on the use of BV in PTCL.
“Is CD30 staining relevant at all, or should we default back to studies, say, in colon cancer where we didn’t even care about EGFR because we might be missing it by current techniques?” Dr. Shustov asked. “Should we even worry about CD30 expression ... because we cannot reliably detect low levels of CD30?”
Some attendees echoed this sentiment, questioning the utility of assessing CD30 expression. Other attendees said they would defer to the trial data and only treat patients with BV-CHP if they had at least 10% CD30.
PTCL subtypes
Meeting attendees also discussed the value of BV in different PTCL subtypes.
At the request of European regulatory agencies, ECHELON-2 was largely focused on patients with sALCL. They made up 70% of the total trial population, while 16% of patients had PTCL-NOS, 12% had AITL, and a small number of patients had other subtypes. These numbers meant ECHELON-2 was not powered to determine differences in OS or PFS in non-sALCL subtypes.
As a result, some attendees expressed concerns about using BV-CHP to treat PTCL-NOS or AITL. They argued that it wasn’t clear whether patients with these subtypes would derive more benefit from BV-CHP, CHOP, or CHOP plus etoposide (CHOEP).
Other attendees said they would feel comfortable using BV-CHP in patients with PTCL-NOS or AITL based on ECHELON-2 results.
CHOP vs. CHOEP
The use of CHOP in ECHELON-2 was another point of discussion. Some attendees said CHOEP should have been used as the comparator instead.
A few individuals mentioned retrospective data suggesting CHOEP may confer a benefit over CHOP in PTCL (Blood. 2010 Nov 4;116[18]:3418-25).
Marek Trneny, MD, of Charles University General Hospital in Prague, referenced new data from the Czech National Lymphoma Registry, which showed that patients newly diagnosed with PTCL had superior PFS and OS when they received CHOEP rather than CHOP.
Based on these findings, Dr. Trneny said he would consider treating CD30-positive PTCL patients with CHOEP plus BV rather than BV-CHP.
However, most other attendees said they would not consider adding BV to CHOEP due to the absence of clinical trial data supporting this approach.
Some attendees did say they would use CHOEP instead of BV-CHP, particularly in patients with PTCL-NOS or AITL and in patients with CD30 expression below 10%.
ECHELON-2 was funded by Seattle Genetics and Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical Company.
Dr. O’Connor and Dr. Shustov were investigators on ECHELON-2. Dr. O’Connor is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Results from the ECHELON-2 trial led to the U.S. approval of brentuximab vedotin (BV) in combination with cyclophosphamide, doxorubicin, and prednisone (CHP), but there are still questions about how to apply the trial results to practice.

At the annual T-cell Lymphoma Forum, trial investigators and other physicians debated the best use of this combination.
BV-CHP is approved to treat patients with previously untreated systemic anaplastic large-cell lymphoma (sALCL) or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma (AITL) and PTCL not otherwise specified (NOS).
Patients who received BV-CHP in ECHELON-2 had superior progression-free survival (PFS) and overall survival (OS) compared to patients who received cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
These results were initially presented at the 2018 annual meeting of the American Society of Hematology and simultaneously published in The Lancet (2019 Jan 19;393[10168]:229-40).
ECHELON-2 investigator Owen O’Connor, MD, PhD, of Columbia University Medical Center in New York, also presented details on the trial at the T-cell Lymphoma Forum. His presentation was followed by a discussion with meeting attendees about applying the trial results to clinical practice.
CD30 expression
One of the issues discussed was the importance of CD30 expression in deciding which patients should receive BV.
For a patient to be eligible for ECHELON-2, the diagnostic biopsy had to show at least 10% of the neoplastic cells were CD30-positive. However, the Food and Drug Administration (FDA) has not made a similar requirement for prescribing BV. PTCL patients with any level of CD30 expression are eligible for treatment with BV-CHP, according to the FDA.
“[I]t’s still a matter of great debate and controversy as to whether we have good enough data to suggest that there’s a threshold effect with regard to the expression of CD30 and responsiveness or sensitivity to brentuximab vedotin,” Dr. O’Connor said.
“This has been an issue from the very first day with this drug, which is, ‘Just how much CD30 do you need to get a response?’ I can’t speak on behalf of the FDA, but I think they are not absolutely convinced that there’s a threshold. They take [CD30-] positive as ‘good enough’ across the board.”
“The FDA has said, ‘The data we’ve seen says there’s a lot of heterogeneity [with biopsies].’ You may do a biopsy and find 30% [of cells are CD30-positive], and you may do another biopsy [in the same patient] and find less than 10%. I don’t think the regulatory agencies are convinced that a single biopsy looking at CD30 ... is representative of the entire tumor burden.”
Andrei Shustov, MD, an ECHELON-2 investigator from the University of Washington in Seattle, questioned whether CD30 expression should be considered when deciding on the use of BV in PTCL.
“Is CD30 staining relevant at all, or should we default back to studies, say, in colon cancer where we didn’t even care about EGFR because we might be missing it by current techniques?” Dr. Shustov asked. “Should we even worry about CD30 expression ... because we cannot reliably detect low levels of CD30?”
Some attendees echoed this sentiment, questioning the utility of assessing CD30 expression. Other attendees said they would defer to the trial data and only treat patients with BV-CHP if they had at least 10% CD30.
PTCL subtypes
Meeting attendees also discussed the value of BV in different PTCL subtypes.
At the request of European regulatory agencies, ECHELON-2 was largely focused on patients with sALCL. They made up 70% of the total trial population, while 16% of patients had PTCL-NOS, 12% had AITL, and a small number of patients had other subtypes. These numbers meant ECHELON-2 was not powered to determine differences in OS or PFS in non-sALCL subtypes.
As a result, some attendees expressed concerns about using BV-CHP to treat PTCL-NOS or AITL. They argued that it wasn’t clear whether patients with these subtypes would derive more benefit from BV-CHP, CHOP, or CHOP plus etoposide (CHOEP).
Other attendees said they would feel comfortable using BV-CHP in patients with PTCL-NOS or AITL based on ECHELON-2 results.
CHOP vs. CHOEP
The use of CHOP in ECHELON-2 was another point of discussion. Some attendees said CHOEP should have been used as the comparator instead.
A few individuals mentioned retrospective data suggesting CHOEP may confer a benefit over CHOP in PTCL (Blood. 2010 Nov 4;116[18]:3418-25).
Marek Trneny, MD, of Charles University General Hospital in Prague, referenced new data from the Czech National Lymphoma Registry, which showed that patients newly diagnosed with PTCL had superior PFS and OS when they received CHOEP rather than CHOP.
Based on these findings, Dr. Trneny said he would consider treating CD30-positive PTCL patients with CHOEP plus BV rather than BV-CHP.
However, most other attendees said they would not consider adding BV to CHOEP due to the absence of clinical trial data supporting this approach.
Some attendees did say they would use CHOEP instead of BV-CHP, particularly in patients with PTCL-NOS or AITL and in patients with CD30 expression below 10%.
ECHELON-2 was funded by Seattle Genetics and Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical Company.
Dr. O’Connor and Dr. Shustov were investigators on ECHELON-2. Dr. O’Connor is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
EXPERT ANALYSIS FROM TCLF 2019
Combo treatment may improve quality of life in CTCL
LA JOLLA, CALIF. — Treatment with brentuximab vedotin (BV) and lenalidomide (len) may improve quality of life (QOL) for patients with cutaneous T-cell lymphoma (CTCL), according to the principal investigator of a phase 2 trial.

In this small trial, most CTCL patients experienced relief from pruritus after one cycle of treatment with BV-len.
Investigators also observed durable responses to the combination, although two patients experienced tumor flare prior to response.
“Because of the tumor flare, we decreased the dose of lenalidomide ... and, since then, it has not been a major problem,” said Basem M. William, MD, principal investigator of the trial and a professor at Ohio State University in Columbus.
“We’re trying to be more reassuring to patients that, if they experience a little bit of tumor flare, as long as it’s not dangerous or life-threatening, if they can hold on with the treatment, this might translate to a later durable response.”
Dr. William and his colleagues presented results from this ongoing, phase 2 trial (NCT03409432) at the annual T-cell Lymphoma Forum.
Thus far, the investigators have treated 12 patients with relapsed or refractory CTCL or peripheral T-cell lymphoma (PTCL). The CTCL patients had received at least two lines of skin-directed therapy or one line of systemic therapy, and the PTCL patients had received at least one line of systemic therapy.
Dr. William and his colleagues reported results for 10 patients. Six patients had mycosis fungoides (MF), two had Sézary syndrome (SS), one had CD30+ lymphoproliferative disorder, and one had systemic anaplastic large-cell lymphoma (ALCL).
The patients’ median age was 59 (range, 49-74), there were nine males, and patients had received a median of 2 (range, 1-10) prior therapies.
The first seven patients received BV at 1.2 mg/kg and len at 20 mg daily every 3 weeks. However, after the investigators observed tumor flare in two patients, the dose of len was lowered to 10 mg.
Safety
The investigators said all adverse events (AEs) were reversible by stopping therapy, there were no grade 4 AEs, and none of the patients had grade 3 or higher neuropathy.
“We have not seen an excess of neuropathy, which is very important because both brentuximab and lenalidomide are known to cause neuropathy,” Dr. William said. “So we were fairly concerned that there would be a synergistic neurotoxic effect, which we don’t want, but we haven’t seen that.”
The most common treatment-related AE was neutropenia. Grade 3 neutropenia occurred in four patients.
Other grade 3 AEs, which occurred in patients on the 20 mg dose of len, were thrombocytopenia (n = 1), dyspnea (n = 1), vertigo (n = 1), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome (n = 1), and tumor flare (n = 1).
Three patients discontinued treatment because of AEs — thrombocytopenia, tumor flare, and DRESS syndrome.
Tumor flare and response
“We did see tumor flare in two initial patients treated with the higher dose of lenalidomide, and we had to remove them from the study for their safety,” Dr. William said. “One of them had a full-blown DRESS syndrome. For their safety, we did have to remove them, but both did experience durable remissions after.”
One of the patients with tumor flare, who had MF, didn’t require treatment for 6 months after going off study. The other patient, who had SS, cleared the clone from his blood but developed DRESS syndrome.
In all, three patients achieved a response to treatment. The ALCL patient had a complete response, and two MF patients achieved a partial response.
Two MF patients and one SS patient had stable disease. The remaining four patients — two with MF, one with SS, and one with lymphoproliferative disorder — progressed.
QOL
The investigators used the Skindex-16 to assess the effect of treatment on QOL.
Five of six evaluable patients with CTCL had a 50% or greater reduction in their Skindex-16 scores after two cycles of treatment. In fact, most patients had relief from pruritus after one cycle, Dr. William said.
“Patients with cutaneous T-cell lymphoma, their biggest problem is with the symptom burden, with pruritus,” he said. “They’re really miserable from all the itching they have. They cannot sleep at night. So we’re fairly excited that most of the patients we’ve treated so far had relief from pruritus just after one cycle.”
Dr. William said he and his colleagues are excited about the overall results they have observed with BV-len, although it’s “still pretty early” in the trial. The investigators are planning to enroll a total of 42 patients and may open the trial at a second center.
The study is sponsored by Ohio State University and the lenalidomide is provided by Celgene. Dr. William reported relationships with miRagen Therapeutics, GuidePoint, Kyowa Kirin, and Celgene.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Treatment with brentuximab vedotin (BV) and lenalidomide (len) may improve quality of life (QOL) for patients with cutaneous T-cell lymphoma (CTCL), according to the principal investigator of a phase 2 trial.

In this small trial, most CTCL patients experienced relief from pruritus after one cycle of treatment with BV-len.
Investigators also observed durable responses to the combination, although two patients experienced tumor flare prior to response.
“Because of the tumor flare, we decreased the dose of lenalidomide ... and, since then, it has not been a major problem,” said Basem M. William, MD, principal investigator of the trial and a professor at Ohio State University in Columbus.
“We’re trying to be more reassuring to patients that, if they experience a little bit of tumor flare, as long as it’s not dangerous or life-threatening, if they can hold on with the treatment, this might translate to a later durable response.”
Dr. William and his colleagues presented results from this ongoing, phase 2 trial (NCT03409432) at the annual T-cell Lymphoma Forum.
Thus far, the investigators have treated 12 patients with relapsed or refractory CTCL or peripheral T-cell lymphoma (PTCL). The CTCL patients had received at least two lines of skin-directed therapy or one line of systemic therapy, and the PTCL patients had received at least one line of systemic therapy.
Dr. William and his colleagues reported results for 10 patients. Six patients had mycosis fungoides (MF), two had Sézary syndrome (SS), one had CD30+ lymphoproliferative disorder, and one had systemic anaplastic large-cell lymphoma (ALCL).
The patients’ median age was 59 (range, 49-74), there were nine males, and patients had received a median of 2 (range, 1-10) prior therapies.
The first seven patients received BV at 1.2 mg/kg and len at 20 mg daily every 3 weeks. However, after the investigators observed tumor flare in two patients, the dose of len was lowered to 10 mg.
Safety
The investigators said all adverse events (AEs) were reversible by stopping therapy, there were no grade 4 AEs, and none of the patients had grade 3 or higher neuropathy.
“We have not seen an excess of neuropathy, which is very important because both brentuximab and lenalidomide are known to cause neuropathy,” Dr. William said. “So we were fairly concerned that there would be a synergistic neurotoxic effect, which we don’t want, but we haven’t seen that.”
The most common treatment-related AE was neutropenia. Grade 3 neutropenia occurred in four patients.
Other grade 3 AEs, which occurred in patients on the 20 mg dose of len, were thrombocytopenia (n = 1), dyspnea (n = 1), vertigo (n = 1), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome (n = 1), and tumor flare (n = 1).
Three patients discontinued treatment because of AEs — thrombocytopenia, tumor flare, and DRESS syndrome.
Tumor flare and response
“We did see tumor flare in two initial patients treated with the higher dose of lenalidomide, and we had to remove them from the study for their safety,” Dr. William said. “One of them had a full-blown DRESS syndrome. For their safety, we did have to remove them, but both did experience durable remissions after.”
One of the patients with tumor flare, who had MF, didn’t require treatment for 6 months after going off study. The other patient, who had SS, cleared the clone from his blood but developed DRESS syndrome.
In all, three patients achieved a response to treatment. The ALCL patient had a complete response, and two MF patients achieved a partial response.
Two MF patients and one SS patient had stable disease. The remaining four patients — two with MF, one with SS, and one with lymphoproliferative disorder — progressed.
QOL
The investigators used the Skindex-16 to assess the effect of treatment on QOL.
Five of six evaluable patients with CTCL had a 50% or greater reduction in their Skindex-16 scores after two cycles of treatment. In fact, most patients had relief from pruritus after one cycle, Dr. William said.
“Patients with cutaneous T-cell lymphoma, their biggest problem is with the symptom burden, with pruritus,” he said. “They’re really miserable from all the itching they have. They cannot sleep at night. So we’re fairly excited that most of the patients we’ve treated so far had relief from pruritus just after one cycle.”
Dr. William said he and his colleagues are excited about the overall results they have observed with BV-len, although it’s “still pretty early” in the trial. The investigators are planning to enroll a total of 42 patients and may open the trial at a second center.
The study is sponsored by Ohio State University and the lenalidomide is provided by Celgene. Dr. William reported relationships with miRagen Therapeutics, GuidePoint, Kyowa Kirin, and Celgene.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Treatment with brentuximab vedotin (BV) and lenalidomide (len) may improve quality of life (QOL) for patients with cutaneous T-cell lymphoma (CTCL), according to the principal investigator of a phase 2 trial.

In this small trial, most CTCL patients experienced relief from pruritus after one cycle of treatment with BV-len.
Investigators also observed durable responses to the combination, although two patients experienced tumor flare prior to response.
“Because of the tumor flare, we decreased the dose of lenalidomide ... and, since then, it has not been a major problem,” said Basem M. William, MD, principal investigator of the trial and a professor at Ohio State University in Columbus.
“We’re trying to be more reassuring to patients that, if they experience a little bit of tumor flare, as long as it’s not dangerous or life-threatening, if they can hold on with the treatment, this might translate to a later durable response.”
Dr. William and his colleagues presented results from this ongoing, phase 2 trial (NCT03409432) at the annual T-cell Lymphoma Forum.
Thus far, the investigators have treated 12 patients with relapsed or refractory CTCL or peripheral T-cell lymphoma (PTCL). The CTCL patients had received at least two lines of skin-directed therapy or one line of systemic therapy, and the PTCL patients had received at least one line of systemic therapy.
Dr. William and his colleagues reported results for 10 patients. Six patients had mycosis fungoides (MF), two had Sézary syndrome (SS), one had CD30+ lymphoproliferative disorder, and one had systemic anaplastic large-cell lymphoma (ALCL).
The patients’ median age was 59 (range, 49-74), there were nine males, and patients had received a median of 2 (range, 1-10) prior therapies.
The first seven patients received BV at 1.2 mg/kg and len at 20 mg daily every 3 weeks. However, after the investigators observed tumor flare in two patients, the dose of len was lowered to 10 mg.
Safety
The investigators said all adverse events (AEs) were reversible by stopping therapy, there were no grade 4 AEs, and none of the patients had grade 3 or higher neuropathy.
“We have not seen an excess of neuropathy, which is very important because both brentuximab and lenalidomide are known to cause neuropathy,” Dr. William said. “So we were fairly concerned that there would be a synergistic neurotoxic effect, which we don’t want, but we haven’t seen that.”
The most common treatment-related AE was neutropenia. Grade 3 neutropenia occurred in four patients.
Other grade 3 AEs, which occurred in patients on the 20 mg dose of len, were thrombocytopenia (n = 1), dyspnea (n = 1), vertigo (n = 1), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome (n = 1), and tumor flare (n = 1).
Three patients discontinued treatment because of AEs — thrombocytopenia, tumor flare, and DRESS syndrome.
Tumor flare and response
“We did see tumor flare in two initial patients treated with the higher dose of lenalidomide, and we had to remove them from the study for their safety,” Dr. William said. “One of them had a full-blown DRESS syndrome. For their safety, we did have to remove them, but both did experience durable remissions after.”
One of the patients with tumor flare, who had MF, didn’t require treatment for 6 months after going off study. The other patient, who had SS, cleared the clone from his blood but developed DRESS syndrome.
In all, three patients achieved a response to treatment. The ALCL patient had a complete response, and two MF patients achieved a partial response.
Two MF patients and one SS patient had stable disease. The remaining four patients — two with MF, one with SS, and one with lymphoproliferative disorder — progressed.
QOL
The investigators used the Skindex-16 to assess the effect of treatment on QOL.
Five of six evaluable patients with CTCL had a 50% or greater reduction in their Skindex-16 scores after two cycles of treatment. In fact, most patients had relief from pruritus after one cycle, Dr. William said.
“Patients with cutaneous T-cell lymphoma, their biggest problem is with the symptom burden, with pruritus,” he said. “They’re really miserable from all the itching they have. They cannot sleep at night. So we’re fairly excited that most of the patients we’ve treated so far had relief from pruritus just after one cycle.”
Dr. William said he and his colleagues are excited about the overall results they have observed with BV-len, although it’s “still pretty early” in the trial. The investigators are planning to enroll a total of 42 patients and may open the trial at a second center.
The study is sponsored by Ohio State University and the lenalidomide is provided by Celgene. Dr. William reported relationships with miRagen Therapeutics, GuidePoint, Kyowa Kirin, and Celgene.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: Five of six evaluable CTCL patients had a 50% or greater reduction in their Skindex-16 scores after two cycles of treatment.
Study details: A phase 2 study with results reported for 10 patients.
Disclosures: The study is sponsored by Ohio State University and the lenalidomide is provided by Celgene. The principal investigator reported relationships with miRagen Therapeutics, GuidePoint, Kyowa Kirin, and Celgene.
Are single agents better than chemo for relapsed/refractory PTCL?
LA JOLLA, CALIF. — Results from the COMPLETE registry suggest newer single agents may be more effective than combination chemotherapy for patients with relapsed/refractory peripheral T-cell lymphoma (PTCL).
Complete response (CR) rates and median survival times were significantly better among patients who received single agents than among those who received combination therapy.
Although researchers don’t know what is driving these differences in outcomes, they did find that outcomes were best among patients who received single-agent brentuximab vedotin (BV), and a disproportionate number of patients received BV.
The researchers also found that patients who received single-agent therapy were more likely to proceed to stem cell transplant.
Therefore, it’s still unclear if single-agent treatment is superior to combination therapy for relapsed/refractory PTCL, according to Robert Stuver, MD, of Beth Israel Deaconess Medical Center in Boston.
Dr. Stuver presented data from the COMPLETE (Comprehensive Oncology Measures for Peripheral T-cell Lymphoma Treatment) registry at the annual T-cell Lymphoma Forum.
The registry (NCT01110733) enrolled patients newly diagnosed with PTCL. Dr. Stuver presented results among patients who had relapsed after, or were refractory to, upfront therapy and went on to receive single-agent therapy or any combination regimen excluding those single agents. Outcome data were collected for 5 years or until death.
Patients and treatment
There were 26 patients in the combination treatment group — 10 with PTCL not otherwise specified (NOS), 6 with angioimmunoblastic T-cell lymphoma (AITL), 5 with natural killer T-cell lymphoma (NKTL), 3 with anaplastic large-cell lymphoma (ALCL), 1 with enteropathy-associated T-cell lymphoma (EATL), and 1 with hepatosplenic T-cell lymphoma (HSTCL).
Patients in the combination group received gemcitabine-based therapy (n = 10), ifosfamide-based therapy (n = 7), platinum-based therapy (n = 4), CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)-like therapy (n = 1), DHAP (dexamethasone, high-dose cytarabine, and cisplatin; n = 1), and other combinations (n = 3).
There were 31 patients in the single-agent group – 13 with PTCL-NOS, 7 with ALCL, 5 with AITL, 2 with EATL, 2 with NKTL, and 2 with HSTCL.
These patients were treated with BV (n = 12), romidepsin (n = 8), pralatrexate (n = 5), alisertib (n = 3), bendamustine (n = 1), denileukin diftitox (n = 1), and lenalidomide (n = 1).
Response
The CR rates were significantly higher among patients who received single-agent treatment than among those who received combination therapy — 41.4% (12/31) and 19.2% (5/26), respectively (P = .02). The partial response rates were 17.2% (5/31) and 26.9% (7/26), respectively. Rates of stable disease were 3.4% (1/31) and 30.8% (8/26), respectively.
Complete responders in the single-agent arm were treated with BV (n = 7), romidepsin (n = 2), pralatrexate (n = 1), alisertib (n = 1), and bendamustine (n = 1). Four of the patients treated with BV had ALCL.
“We had an enrichment of patients treated with brentuximab,” Dr. Stuver said. “So the obvious question this begs is, ‘Are the favorable results that were seen for single agents over combination therapy solely due to patients treated with brentuximab?’ ”
To investigate, Dr. Stuver and his colleagues compared responses among patients who received BV with patients who received other single agents or combination therapies.
The CR rate was 58.3% (7/12) among BV recipients, 29.4% (5/17) among patients who received other single agents, and 19.2% (5/26) among patients who received combination therapy.
“The takeaway here is that, when you do divide the single-agent group into BV and other single agents, you’re seeing that BV is doing much better than every other group,” Dr. Stuver said. “And all the other single agents are doing somewhat similarly to the combination group, although there’s still a 10% difference, 29% versus 19%.”
Survival
The median progression-free survival (PFS) and overall survival (OS) were significantly better among patients who received single-agent therapy. The median PFS was 11.7 months in the single-agent group and 6.7 months in the combination group (P = .0197). The median OS was 38.9 months and 17.1 months, respectively (P = .0170).
A factor that may have affected survival is that patients were more likely to undergo stem cell transplant after single-agent therapy (25.8%, 8/31), compared with those who had received combination therapy (7.7%, 2/26).
Another factor that may have affected the survival differences is the enrichment of patients treated with BV.
The researchers found the median PFS was 11.9 months among BV recipients, 10.4 months among patients who received other single agents, and 6.7 months in the combination-therapy group. The median OS was 44.5 months, 19.1 months, and 17.1 months, respectively.
Dr. Stuver said these results suggest there is a role for single agents as first retreatment in the salvage setting. However, this analysis was limited by the small sample size and the enrichment of patients treated with BV.
Larger, randomized studies are needed to identify the “truly superior” treatment strategy for relapsed/refractory PTCL, Dr. Stuver said.
The COMPLETE registry is sponsored by Spectrum Pharmaceuticals. Dr. Stuver did not declare any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Results from the COMPLETE registry suggest newer single agents may be more effective than combination chemotherapy for patients with relapsed/refractory peripheral T-cell lymphoma (PTCL).
Complete response (CR) rates and median survival times were significantly better among patients who received single agents than among those who received combination therapy.
Although researchers don’t know what is driving these differences in outcomes, they did find that outcomes were best among patients who received single-agent brentuximab vedotin (BV), and a disproportionate number of patients received BV.
The researchers also found that patients who received single-agent therapy were more likely to proceed to stem cell transplant.
Therefore, it’s still unclear if single-agent treatment is superior to combination therapy for relapsed/refractory PTCL, according to Robert Stuver, MD, of Beth Israel Deaconess Medical Center in Boston.
Dr. Stuver presented data from the COMPLETE (Comprehensive Oncology Measures for Peripheral T-cell Lymphoma Treatment) registry at the annual T-cell Lymphoma Forum.
The registry (NCT01110733) enrolled patients newly diagnosed with PTCL. Dr. Stuver presented results among patients who had relapsed after, or were refractory to, upfront therapy and went on to receive single-agent therapy or any combination regimen excluding those single agents. Outcome data were collected for 5 years or until death.
Patients and treatment
There were 26 patients in the combination treatment group — 10 with PTCL not otherwise specified (NOS), 6 with angioimmunoblastic T-cell lymphoma (AITL), 5 with natural killer T-cell lymphoma (NKTL), 3 with anaplastic large-cell lymphoma (ALCL), 1 with enteropathy-associated T-cell lymphoma (EATL), and 1 with hepatosplenic T-cell lymphoma (HSTCL).
Patients in the combination group received gemcitabine-based therapy (n = 10), ifosfamide-based therapy (n = 7), platinum-based therapy (n = 4), CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)-like therapy (n = 1), DHAP (dexamethasone, high-dose cytarabine, and cisplatin; n = 1), and other combinations (n = 3).
There were 31 patients in the single-agent group – 13 with PTCL-NOS, 7 with ALCL, 5 with AITL, 2 with EATL, 2 with NKTL, and 2 with HSTCL.
These patients were treated with BV (n = 12), romidepsin (n = 8), pralatrexate (n = 5), alisertib (n = 3), bendamustine (n = 1), denileukin diftitox (n = 1), and lenalidomide (n = 1).
Response
The CR rates were significantly higher among patients who received single-agent treatment than among those who received combination therapy — 41.4% (12/31) and 19.2% (5/26), respectively (P = .02). The partial response rates were 17.2% (5/31) and 26.9% (7/26), respectively. Rates of stable disease were 3.4% (1/31) and 30.8% (8/26), respectively.
Complete responders in the single-agent arm were treated with BV (n = 7), romidepsin (n = 2), pralatrexate (n = 1), alisertib (n = 1), and bendamustine (n = 1). Four of the patients treated with BV had ALCL.
“We had an enrichment of patients treated with brentuximab,” Dr. Stuver said. “So the obvious question this begs is, ‘Are the favorable results that were seen for single agents over combination therapy solely due to patients treated with brentuximab?’ ”
To investigate, Dr. Stuver and his colleagues compared responses among patients who received BV with patients who received other single agents or combination therapies.
The CR rate was 58.3% (7/12) among BV recipients, 29.4% (5/17) among patients who received other single agents, and 19.2% (5/26) among patients who received combination therapy.
“The takeaway here is that, when you do divide the single-agent group into BV and other single agents, you’re seeing that BV is doing much better than every other group,” Dr. Stuver said. “And all the other single agents are doing somewhat similarly to the combination group, although there’s still a 10% difference, 29% versus 19%.”
Survival
The median progression-free survival (PFS) and overall survival (OS) were significantly better among patients who received single-agent therapy. The median PFS was 11.7 months in the single-agent group and 6.7 months in the combination group (P = .0197). The median OS was 38.9 months and 17.1 months, respectively (P = .0170).
A factor that may have affected survival is that patients were more likely to undergo stem cell transplant after single-agent therapy (25.8%, 8/31), compared with those who had received combination therapy (7.7%, 2/26).
Another factor that may have affected the survival differences is the enrichment of patients treated with BV.
The researchers found the median PFS was 11.9 months among BV recipients, 10.4 months among patients who received other single agents, and 6.7 months in the combination-therapy group. The median OS was 44.5 months, 19.1 months, and 17.1 months, respectively.
Dr. Stuver said these results suggest there is a role for single agents as first retreatment in the salvage setting. However, this analysis was limited by the small sample size and the enrichment of patients treated with BV.
Larger, randomized studies are needed to identify the “truly superior” treatment strategy for relapsed/refractory PTCL, Dr. Stuver said.
The COMPLETE registry is sponsored by Spectrum Pharmaceuticals. Dr. Stuver did not declare any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Results from the COMPLETE registry suggest newer single agents may be more effective than combination chemotherapy for patients with relapsed/refractory peripheral T-cell lymphoma (PTCL).
Complete response (CR) rates and median survival times were significantly better among patients who received single agents than among those who received combination therapy.
Although researchers don’t know what is driving these differences in outcomes, they did find that outcomes were best among patients who received single-agent brentuximab vedotin (BV), and a disproportionate number of patients received BV.
The researchers also found that patients who received single-agent therapy were more likely to proceed to stem cell transplant.
Therefore, it’s still unclear if single-agent treatment is superior to combination therapy for relapsed/refractory PTCL, according to Robert Stuver, MD, of Beth Israel Deaconess Medical Center in Boston.
Dr. Stuver presented data from the COMPLETE (Comprehensive Oncology Measures for Peripheral T-cell Lymphoma Treatment) registry at the annual T-cell Lymphoma Forum.
The registry (NCT01110733) enrolled patients newly diagnosed with PTCL. Dr. Stuver presented results among patients who had relapsed after, or were refractory to, upfront therapy and went on to receive single-agent therapy or any combination regimen excluding those single agents. Outcome data were collected for 5 years or until death.
Patients and treatment
There were 26 patients in the combination treatment group — 10 with PTCL not otherwise specified (NOS), 6 with angioimmunoblastic T-cell lymphoma (AITL), 5 with natural killer T-cell lymphoma (NKTL), 3 with anaplastic large-cell lymphoma (ALCL), 1 with enteropathy-associated T-cell lymphoma (EATL), and 1 with hepatosplenic T-cell lymphoma (HSTCL).
Patients in the combination group received gemcitabine-based therapy (n = 10), ifosfamide-based therapy (n = 7), platinum-based therapy (n = 4), CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)-like therapy (n = 1), DHAP (dexamethasone, high-dose cytarabine, and cisplatin; n = 1), and other combinations (n = 3).
There were 31 patients in the single-agent group – 13 with PTCL-NOS, 7 with ALCL, 5 with AITL, 2 with EATL, 2 with NKTL, and 2 with HSTCL.
These patients were treated with BV (n = 12), romidepsin (n = 8), pralatrexate (n = 5), alisertib (n = 3), bendamustine (n = 1), denileukin diftitox (n = 1), and lenalidomide (n = 1).
Response
The CR rates were significantly higher among patients who received single-agent treatment than among those who received combination therapy — 41.4% (12/31) and 19.2% (5/26), respectively (P = .02). The partial response rates were 17.2% (5/31) and 26.9% (7/26), respectively. Rates of stable disease were 3.4% (1/31) and 30.8% (8/26), respectively.
Complete responders in the single-agent arm were treated with BV (n = 7), romidepsin (n = 2), pralatrexate (n = 1), alisertib (n = 1), and bendamustine (n = 1). Four of the patients treated with BV had ALCL.
“We had an enrichment of patients treated with brentuximab,” Dr. Stuver said. “So the obvious question this begs is, ‘Are the favorable results that were seen for single agents over combination therapy solely due to patients treated with brentuximab?’ ”
To investigate, Dr. Stuver and his colleagues compared responses among patients who received BV with patients who received other single agents or combination therapies.
The CR rate was 58.3% (7/12) among BV recipients, 29.4% (5/17) among patients who received other single agents, and 19.2% (5/26) among patients who received combination therapy.
“The takeaway here is that, when you do divide the single-agent group into BV and other single agents, you’re seeing that BV is doing much better than every other group,” Dr. Stuver said. “And all the other single agents are doing somewhat similarly to the combination group, although there’s still a 10% difference, 29% versus 19%.”
Survival
The median progression-free survival (PFS) and overall survival (OS) were significantly better among patients who received single-agent therapy. The median PFS was 11.7 months in the single-agent group and 6.7 months in the combination group (P = .0197). The median OS was 38.9 months and 17.1 months, respectively (P = .0170).
A factor that may have affected survival is that patients were more likely to undergo stem cell transplant after single-agent therapy (25.8%, 8/31), compared with those who had received combination therapy (7.7%, 2/26).
Another factor that may have affected the survival differences is the enrichment of patients treated with BV.
The researchers found the median PFS was 11.9 months among BV recipients, 10.4 months among patients who received other single agents, and 6.7 months in the combination-therapy group. The median OS was 44.5 months, 19.1 months, and 17.1 months, respectively.
Dr. Stuver said these results suggest there is a role for single agents as first retreatment in the salvage setting. However, this analysis was limited by the small sample size and the enrichment of patients treated with BV.
Larger, randomized studies are needed to identify the “truly superior” treatment strategy for relapsed/refractory PTCL, Dr. Stuver said.
The COMPLETE registry is sponsored by Spectrum Pharmaceuticals. Dr. Stuver did not declare any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: The complete response rate was significantly higher among patients who received single-agent treatment than it was among those who received combination therapy – 41.4% and 19.2%, respectively (P = .02).
Study details: Analysis of 57 patients with relapsed/refractory PTCL in the COMPLETE registry.
Disclosures: The COMPLETE registry is sponsored by Spectrum Pharmaceuticals. Dr. Stuver did not declare any conflicts of interest.
Four-drug combo shows durable responses in relapsed/refractory lymphomas
LA JOLLA, CALIF. — Results of a phase 1 trial suggest a four-drug combination can produce durable responses in patients with relapsed or refractory T- and B-cell lymphomas.
Seven of 15 patients responded to treatment with romidepsin, gemcitabine, oxaliplatin, and dexamethasone, including six patients who achieved a complete response (CR).
The median duration of response was 8.5 months, and three patients had responses lasting more than 24 months.
Patients with angioimmunoblastic T-cell lymphoma (AITL) in particular responded well to the combination.
Neha Mehta-Shah, MD, of Washington University in St. Louis, and her colleagues presented these results in a poster at the annual T-cell Lymphoma Forum.
“[I]t was thought that the addition of histone deacetylase inhibitors to traditional platinum-based chemotherapies, which tend to cause DNA damage, would increase the response of platinum-based therapies,” Dr. Shah said.
With that in mind, she and her colleagues added romidepsin to gemcitabine, oxaliplatin, and dexamethasone and evaluated this combination in patients with relapsed/refractory lymphomas.
The trial (NCT02181218) enrolled 15 patients — 6 with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 6 with diffuse large B-cell lymphoma (DLBCL), and 3 with AITL.
The patients’ median age was 66 (range, 55-83), and they had received a median of 2 (range, 1-4) prior therapies.
The researchers tested three dose levels of romidepsin — 8 mg/m2, 10 mg/m2, and 12 mg/m2 — given on day 2 of a 21-day cycle. The study originally included romidepsin on day 8 as well. However, the researchers discontinued the day 8 dose after patients developed grade 4 thrombocytopenia.
Patients also received gemcitabine at 1,000 mg/m2 (day 1), oxaliplatin at 100 mg/m2 (day 1), and dexamethasone at 20 mg (days 1-4). All patients received pegfilgrastim at 6 mg (day 3) as well.
The patients could receive up to eight cycles of treatment if they had stable disease or better and did not experience significant toxicity.
Safety
There was one dose-limiting toxicity (DLT) — pneumonia — at the 8 mg/m2 dose of romidepsin (given on days 2 and 8). There was one DLT — bleeding — at the 10 mg/m2 dose (day 2 only).
Two patients experienced DLTs — neutropenic fever and grade 4 thrombocytopenia — at the 12 mg/m2 dose (day 2 only).
Based on these events, 10 mg/m2 was considered the maximum-tolerated dose of romidepsin.
The most common adverse events (AEs) in this trial were thrombocytopenia (n = 13), electrolyte abnormalities (n = 12), liver function abnormalities (n = 10), anemia (n = 9), neutropenia (n = 8), fatigue (n = 7), nausea (n = 7), and creatinine increase (n = 5).
Grade 3/4 AEs included thrombocytopenia (n = 13), neutropenia (n = 5), anemia (n = 3), hyperglycemia (n = 2), hyperuricemia (n = 2), febrile neutropenia (n = 1), tumor lysis syndrome (n = 1), vomiting (n = 1), peripheral sensory neuropathy (n = 1), pneumonia (n = 1), sepsis (n = 1), bleeding (n = 1), and elevated troponin (n = 1).
Serious AEs requiring hospitalization included pneumonia (n = 1), nausea and vomiting (n = 1), tumor lysis syndrome (n = 1), and complications of disease progression (n = 4).
Efficacy
The overall response rate was 47% (7/15). CRs occurred in all three patients with AITL and two patients with DLBCL. One patient with PTCL-NOS had a CR, and one had a partial response.
The median duration of response was 8.5 months (range, 1.2-36.6 months). Four patients remain in CR — two with AITL, one with PTCL-NOS, and one with DLBCL.
Dr. Shah noted that the CRs in the AITL patients “have been quite prolonged.” One patient had a CR lasting 27 months, and another had a CR lasting 29 months.
Dr. Shah said these results are particularly exciting because patients discontinued study treatment after eight cycles or two cycles after they achieved a CR.
“[S]ome of these patients remained in remission for 2 years without any therapy thereafter, which is quite impressive in a population where the median survival — for patients with relapsed/refractory AITL — is thought to be 6-10 months,” Dr. Shah said.
She noted that this study is ongoing with an expansion cohort of patients with T-cell lymphomas.
This research was supported by Celgene. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Results of a phase 1 trial suggest a four-drug combination can produce durable responses in patients with relapsed or refractory T- and B-cell lymphomas.
Seven of 15 patients responded to treatment with romidepsin, gemcitabine, oxaliplatin, and dexamethasone, including six patients who achieved a complete response (CR).
The median duration of response was 8.5 months, and three patients had responses lasting more than 24 months.
Patients with angioimmunoblastic T-cell lymphoma (AITL) in particular responded well to the combination.
Neha Mehta-Shah, MD, of Washington University in St. Louis, and her colleagues presented these results in a poster at the annual T-cell Lymphoma Forum.
“[I]t was thought that the addition of histone deacetylase inhibitors to traditional platinum-based chemotherapies, which tend to cause DNA damage, would increase the response of platinum-based therapies,” Dr. Shah said.
With that in mind, she and her colleagues added romidepsin to gemcitabine, oxaliplatin, and dexamethasone and evaluated this combination in patients with relapsed/refractory lymphomas.
The trial (NCT02181218) enrolled 15 patients — 6 with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 6 with diffuse large B-cell lymphoma (DLBCL), and 3 with AITL.
The patients’ median age was 66 (range, 55-83), and they had received a median of 2 (range, 1-4) prior therapies.
The researchers tested three dose levels of romidepsin — 8 mg/m2, 10 mg/m2, and 12 mg/m2 — given on day 2 of a 21-day cycle. The study originally included romidepsin on day 8 as well. However, the researchers discontinued the day 8 dose after patients developed grade 4 thrombocytopenia.
Patients also received gemcitabine at 1,000 mg/m2 (day 1), oxaliplatin at 100 mg/m2 (day 1), and dexamethasone at 20 mg (days 1-4). All patients received pegfilgrastim at 6 mg (day 3) as well.
The patients could receive up to eight cycles of treatment if they had stable disease or better and did not experience significant toxicity.
Safety
There was one dose-limiting toxicity (DLT) — pneumonia — at the 8 mg/m2 dose of romidepsin (given on days 2 and 8). There was one DLT — bleeding — at the 10 mg/m2 dose (day 2 only).
Two patients experienced DLTs — neutropenic fever and grade 4 thrombocytopenia — at the 12 mg/m2 dose (day 2 only).
Based on these events, 10 mg/m2 was considered the maximum-tolerated dose of romidepsin.
The most common adverse events (AEs) in this trial were thrombocytopenia (n = 13), electrolyte abnormalities (n = 12), liver function abnormalities (n = 10), anemia (n = 9), neutropenia (n = 8), fatigue (n = 7), nausea (n = 7), and creatinine increase (n = 5).
Grade 3/4 AEs included thrombocytopenia (n = 13), neutropenia (n = 5), anemia (n = 3), hyperglycemia (n = 2), hyperuricemia (n = 2), febrile neutropenia (n = 1), tumor lysis syndrome (n = 1), vomiting (n = 1), peripheral sensory neuropathy (n = 1), pneumonia (n = 1), sepsis (n = 1), bleeding (n = 1), and elevated troponin (n = 1).
Serious AEs requiring hospitalization included pneumonia (n = 1), nausea and vomiting (n = 1), tumor lysis syndrome (n = 1), and complications of disease progression (n = 4).
Efficacy
The overall response rate was 47% (7/15). CRs occurred in all three patients with AITL and two patients with DLBCL. One patient with PTCL-NOS had a CR, and one had a partial response.
The median duration of response was 8.5 months (range, 1.2-36.6 months). Four patients remain in CR — two with AITL, one with PTCL-NOS, and one with DLBCL.
Dr. Shah noted that the CRs in the AITL patients “have been quite prolonged.” One patient had a CR lasting 27 months, and another had a CR lasting 29 months.
Dr. Shah said these results are particularly exciting because patients discontinued study treatment after eight cycles or two cycles after they achieved a CR.
“[S]ome of these patients remained in remission for 2 years without any therapy thereafter, which is quite impressive in a population where the median survival — for patients with relapsed/refractory AITL — is thought to be 6-10 months,” Dr. Shah said.
She noted that this study is ongoing with an expansion cohort of patients with T-cell lymphomas.
This research was supported by Celgene. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Results of a phase 1 trial suggest a four-drug combination can produce durable responses in patients with relapsed or refractory T- and B-cell lymphomas.
Seven of 15 patients responded to treatment with romidepsin, gemcitabine, oxaliplatin, and dexamethasone, including six patients who achieved a complete response (CR).
The median duration of response was 8.5 months, and three patients had responses lasting more than 24 months.
Patients with angioimmunoblastic T-cell lymphoma (AITL) in particular responded well to the combination.
Neha Mehta-Shah, MD, of Washington University in St. Louis, and her colleagues presented these results in a poster at the annual T-cell Lymphoma Forum.
“[I]t was thought that the addition of histone deacetylase inhibitors to traditional platinum-based chemotherapies, which tend to cause DNA damage, would increase the response of platinum-based therapies,” Dr. Shah said.
With that in mind, she and her colleagues added romidepsin to gemcitabine, oxaliplatin, and dexamethasone and evaluated this combination in patients with relapsed/refractory lymphomas.
The trial (NCT02181218) enrolled 15 patients — 6 with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 6 with diffuse large B-cell lymphoma (DLBCL), and 3 with AITL.
The patients’ median age was 66 (range, 55-83), and they had received a median of 2 (range, 1-4) prior therapies.
The researchers tested three dose levels of romidepsin — 8 mg/m2, 10 mg/m2, and 12 mg/m2 — given on day 2 of a 21-day cycle. The study originally included romidepsin on day 8 as well. However, the researchers discontinued the day 8 dose after patients developed grade 4 thrombocytopenia.
Patients also received gemcitabine at 1,000 mg/m2 (day 1), oxaliplatin at 100 mg/m2 (day 1), and dexamethasone at 20 mg (days 1-4). All patients received pegfilgrastim at 6 mg (day 3) as well.
The patients could receive up to eight cycles of treatment if they had stable disease or better and did not experience significant toxicity.
Safety
There was one dose-limiting toxicity (DLT) — pneumonia — at the 8 mg/m2 dose of romidepsin (given on days 2 and 8). There was one DLT — bleeding — at the 10 mg/m2 dose (day 2 only).
Two patients experienced DLTs — neutropenic fever and grade 4 thrombocytopenia — at the 12 mg/m2 dose (day 2 only).
Based on these events, 10 mg/m2 was considered the maximum-tolerated dose of romidepsin.
The most common adverse events (AEs) in this trial were thrombocytopenia (n = 13), electrolyte abnormalities (n = 12), liver function abnormalities (n = 10), anemia (n = 9), neutropenia (n = 8), fatigue (n = 7), nausea (n = 7), and creatinine increase (n = 5).
Grade 3/4 AEs included thrombocytopenia (n = 13), neutropenia (n = 5), anemia (n = 3), hyperglycemia (n = 2), hyperuricemia (n = 2), febrile neutropenia (n = 1), tumor lysis syndrome (n = 1), vomiting (n = 1), peripheral sensory neuropathy (n = 1), pneumonia (n = 1), sepsis (n = 1), bleeding (n = 1), and elevated troponin (n = 1).
Serious AEs requiring hospitalization included pneumonia (n = 1), nausea and vomiting (n = 1), tumor lysis syndrome (n = 1), and complications of disease progression (n = 4).
Efficacy
The overall response rate was 47% (7/15). CRs occurred in all three patients with AITL and two patients with DLBCL. One patient with PTCL-NOS had a CR, and one had a partial response.
The median duration of response was 8.5 months (range, 1.2-36.6 months). Four patients remain in CR — two with AITL, one with PTCL-NOS, and one with DLBCL.
Dr. Shah noted that the CRs in the AITL patients “have been quite prolonged.” One patient had a CR lasting 27 months, and another had a CR lasting 29 months.
Dr. Shah said these results are particularly exciting because patients discontinued study treatment after eight cycles or two cycles after they achieved a CR.
“[S]ome of these patients remained in remission for 2 years without any therapy thereafter, which is quite impressive in a population where the median survival — for patients with relapsed/refractory AITL — is thought to be 6-10 months,” Dr. Shah said.
She noted that this study is ongoing with an expansion cohort of patients with T-cell lymphomas.
This research was supported by Celgene. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: Seven patients responded, and three patients had responses lasting more than 24 months.
Study details: Phase 1 trial of 15 patients.
Disclosures: This research was supported by Celgene. The presenter reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
Long-term mogamulizumab appears safe, effective in CTCL
LA JOLLA, CALIF. — Prolonged exposure to mogamulizumab can improve responses without compromising safety in patients with cutaneous T-cell lymphoma (CTCL), according to a post hoc analysis of the MAVORIC trial.
Investigators found that exposure to mogamulizumab correlated with response. The highest response rate — 75.6% — was observed in patients exposed to the drug for at least 351 days, and the lowest — 1.9% — was observed in patients exposed to mogamulizumab for less than 72 days.
On the other hand, rates of adverse events (AEs) were similar regardless of how long patients were treated with mogamulizumab.
Youn H. Kim, MD, of Stanford Cancer Institute at Stanford (Calif.) University, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The phase 3 MAVORIC trial (NCT01728805) included 372 adults with CTCL who had failed at least one systemic therapy. The patients were randomized to treatment with mogamulizumab or vorinostat.
Results from this comparison were previously reported at the 10th annual T-cell Lymphoma Forum.
At this year’s meeting, Dr. Kim and her colleagues reported results in 184 patients who were randomized to mogamulizumab — 105 of whom had mycosis fungoides (MF) and 79 of whom had Sézary syndrome (SS).
Patients were exposed to mogamulizumab for a mean of 275.2 days and a median of 170.0 days (range, 1-1,617 days).
The investigators divided patients into the following quartiles according to mogamulizumab exposure:
- Less than 72 days — 52 patients (28%)
- 72-170 days — 40 patients (22%)
- 171-351 days — 47 patients (26%)
- More than 351 days — 45 patients (24%).
Patients exposed to mogamulizumab for longer were more likely to have SS, stage III/IV disease, blood involvement, and a performance status of 0.
Dr. Kim said the SS patients “benefited a lot” from mogamulizumab and therefore remained on treatment longer.
Response
As expected, patients exposed to mogamulizumab for the longest period had the highest global response rates. Confirmed response rates according to drug exposure were as follows:
- Less than 72 days: 1.9% overall, 0% for SS, and 2.9% for MF
- 72-170 days: 10% overall, 18.8% for SS, and 4.2% for MF
- 171-351 days: 29.8% overall, 36.4% for SS, and 24% for MF
- More than 351 days: 75.6% overall, 83.3% for SS, and 66.7% for MF.
In addition, rates of complete response (CR) and partial response (PR) tended to increase with mogamulizumab exposure. Rates of CR, PR, and stable disease (SD) according to exposure time were as follows:
- Less than 72 days: 0% CR, 7.7% PR, and 38.5% SD
- 72-170 days: 2.5% CR, 20% PR, and 62.5% SD
- 171-351 days: 2.1% CR, 34% PR, and 57.4% SD
- More than 351 days: 6.7% CR, 71.1% PR, and 17.8% SD.
Safety
“The percentage of patients reporting adverse events was not different in the long-term treatment-exposure patients, compared to the short-term,” Dr. Kim said.
Percentages of treatment-emergent AEs (TEAEs) and serious AEs (SAEs) according to mogamulizumab exposure were as follows:
- Less than 72 days: 26.6% TEAEs and 6.5% SAEs
- 72-170 days: 18.5% TEAEs and 3.3% SAEs
- 171-351 days: 23.4% TEAEs and 6.0% SAEs
- More than 351 days: 21.7% TEAEs and 4.3% SAEs.
“The majority of the grade 3 events occurred in the first two quartiles, not later, which is important to show,” Dr. Kim said.
Most grade 3 AEs occurred within 170 days of treatment initiation, and the median time to a grade 3 or higher AE was 109 days.
The most common treatment-related AEs in the longest exposure cohort were drug eruption (20.0%), thrombocytopenia (11.1%), stomatitis (8.9%), and anemia (8.9%).
Of all patients in this analysis, 45 experienced drug eruption, which was defined as a skin rash possibly, probably, or definitely related to the study drug.
Nine drug eruption events were grade 3, and the rest were grade 1 or 2. The median time to drug eruption was 107 days.
While drug eruption “didn’t show up early,” there is no cumulative risk with longer exposure to mogamulizumab, Dr. Kim said. Likewise, she said, autoimmune AEs were not dose-cumulative events.
There were two patients with definite autoimmune disease — a 65-year-old man with Miller Fisher syndrome (occurring 199 days after mogamulizumab initiation) and a 40-year-old woman with myositis (151 days) and myocarditis (310 days).
The investigators also identified three patients with possible autoimmune disease, including:
- Pneumonitis (310 days) in a 74-year-old woman
- Polymyalgia rheumatica (209 days) and myopathy (not available) in an 84-year-old man
- Hepatitis (144 days), pneumonitis (about 174 days), and polymyositis (about 174 days) in a 73-year-old man.
Dr. Kim and her colleagues said these data suggest prolonged treatment with mogamulizumab is not associated with an increased safety risk in patients with MF or SS. And the manageable safety profile of mogamulizumab meant that patients who derived a clinical benefit could remain on the drug for an extended period of time.
The MAVORIC trial was sponsored by Kyowa Hakko Kirin Pharma. Dr. Kim reported relationships with Merck, Portola Pharmaceuticals, Soligenix, Takeda, TetraLogic Pharmaceuticals, Kyowa Kirin, Seattle Genetics, Medivir, Neumedicines, Eisai, Innate Pharma, Galderma, Miragen Therapeutics, Forty Seven, and Horizon Pharma. Her coinvestigators reported relationships with several companies.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Prolonged exposure to mogamulizumab can improve responses without compromising safety in patients with cutaneous T-cell lymphoma (CTCL), according to a post hoc analysis of the MAVORIC trial.
Investigators found that exposure to mogamulizumab correlated with response. The highest response rate — 75.6% — was observed in patients exposed to the drug for at least 351 days, and the lowest — 1.9% — was observed in patients exposed to mogamulizumab for less than 72 days.
On the other hand, rates of adverse events (AEs) were similar regardless of how long patients were treated with mogamulizumab.
Youn H. Kim, MD, of Stanford Cancer Institute at Stanford (Calif.) University, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The phase 3 MAVORIC trial (NCT01728805) included 372 adults with CTCL who had failed at least one systemic therapy. The patients were randomized to treatment with mogamulizumab or vorinostat.
Results from this comparison were previously reported at the 10th annual T-cell Lymphoma Forum.
At this year’s meeting, Dr. Kim and her colleagues reported results in 184 patients who were randomized to mogamulizumab — 105 of whom had mycosis fungoides (MF) and 79 of whom had Sézary syndrome (SS).
Patients were exposed to mogamulizumab for a mean of 275.2 days and a median of 170.0 days (range, 1-1,617 days).
The investigators divided patients into the following quartiles according to mogamulizumab exposure:
- Less than 72 days — 52 patients (28%)
- 72-170 days — 40 patients (22%)
- 171-351 days — 47 patients (26%)
- More than 351 days — 45 patients (24%).
Patients exposed to mogamulizumab for longer were more likely to have SS, stage III/IV disease, blood involvement, and a performance status of 0.
Dr. Kim said the SS patients “benefited a lot” from mogamulizumab and therefore remained on treatment longer.
Response
As expected, patients exposed to mogamulizumab for the longest period had the highest global response rates. Confirmed response rates according to drug exposure were as follows:
- Less than 72 days: 1.9% overall, 0% for SS, and 2.9% for MF
- 72-170 days: 10% overall, 18.8% for SS, and 4.2% for MF
- 171-351 days: 29.8% overall, 36.4% for SS, and 24% for MF
- More than 351 days: 75.6% overall, 83.3% for SS, and 66.7% for MF.
In addition, rates of complete response (CR) and partial response (PR) tended to increase with mogamulizumab exposure. Rates of CR, PR, and stable disease (SD) according to exposure time were as follows:
- Less than 72 days: 0% CR, 7.7% PR, and 38.5% SD
- 72-170 days: 2.5% CR, 20% PR, and 62.5% SD
- 171-351 days: 2.1% CR, 34% PR, and 57.4% SD
- More than 351 days: 6.7% CR, 71.1% PR, and 17.8% SD.
Safety
“The percentage of patients reporting adverse events was not different in the long-term treatment-exposure patients, compared to the short-term,” Dr. Kim said.
Percentages of treatment-emergent AEs (TEAEs) and serious AEs (SAEs) according to mogamulizumab exposure were as follows:
- Less than 72 days: 26.6% TEAEs and 6.5% SAEs
- 72-170 days: 18.5% TEAEs and 3.3% SAEs
- 171-351 days: 23.4% TEAEs and 6.0% SAEs
- More than 351 days: 21.7% TEAEs and 4.3% SAEs.
“The majority of the grade 3 events occurred in the first two quartiles, not later, which is important to show,” Dr. Kim said.
Most grade 3 AEs occurred within 170 days of treatment initiation, and the median time to a grade 3 or higher AE was 109 days.
The most common treatment-related AEs in the longest exposure cohort were drug eruption (20.0%), thrombocytopenia (11.1%), stomatitis (8.9%), and anemia (8.9%).
Of all patients in this analysis, 45 experienced drug eruption, which was defined as a skin rash possibly, probably, or definitely related to the study drug.
Nine drug eruption events were grade 3, and the rest were grade 1 or 2. The median time to drug eruption was 107 days.
While drug eruption “didn’t show up early,” there is no cumulative risk with longer exposure to mogamulizumab, Dr. Kim said. Likewise, she said, autoimmune AEs were not dose-cumulative events.
There were two patients with definite autoimmune disease — a 65-year-old man with Miller Fisher syndrome (occurring 199 days after mogamulizumab initiation) and a 40-year-old woman with myositis (151 days) and myocarditis (310 days).
The investigators also identified three patients with possible autoimmune disease, including:
- Pneumonitis (310 days) in a 74-year-old woman
- Polymyalgia rheumatica (209 days) and myopathy (not available) in an 84-year-old man
- Hepatitis (144 days), pneumonitis (about 174 days), and polymyositis (about 174 days) in a 73-year-old man.
Dr. Kim and her colleagues said these data suggest prolonged treatment with mogamulizumab is not associated with an increased safety risk in patients with MF or SS. And the manageable safety profile of mogamulizumab meant that patients who derived a clinical benefit could remain on the drug for an extended period of time.
The MAVORIC trial was sponsored by Kyowa Hakko Kirin Pharma. Dr. Kim reported relationships with Merck, Portola Pharmaceuticals, Soligenix, Takeda, TetraLogic Pharmaceuticals, Kyowa Kirin, Seattle Genetics, Medivir, Neumedicines, Eisai, Innate Pharma, Galderma, Miragen Therapeutics, Forty Seven, and Horizon Pharma. Her coinvestigators reported relationships with several companies.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Prolonged exposure to mogamulizumab can improve responses without compromising safety in patients with cutaneous T-cell lymphoma (CTCL), according to a post hoc analysis of the MAVORIC trial.
Investigators found that exposure to mogamulizumab correlated with response. The highest response rate — 75.6% — was observed in patients exposed to the drug for at least 351 days, and the lowest — 1.9% — was observed in patients exposed to mogamulizumab for less than 72 days.
On the other hand, rates of adverse events (AEs) were similar regardless of how long patients were treated with mogamulizumab.
Youn H. Kim, MD, of Stanford Cancer Institute at Stanford (Calif.) University, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The phase 3 MAVORIC trial (NCT01728805) included 372 adults with CTCL who had failed at least one systemic therapy. The patients were randomized to treatment with mogamulizumab or vorinostat.
Results from this comparison were previously reported at the 10th annual T-cell Lymphoma Forum.
At this year’s meeting, Dr. Kim and her colleagues reported results in 184 patients who were randomized to mogamulizumab — 105 of whom had mycosis fungoides (MF) and 79 of whom had Sézary syndrome (SS).
Patients were exposed to mogamulizumab for a mean of 275.2 days and a median of 170.0 days (range, 1-1,617 days).
The investigators divided patients into the following quartiles according to mogamulizumab exposure:
- Less than 72 days — 52 patients (28%)
- 72-170 days — 40 patients (22%)
- 171-351 days — 47 patients (26%)
- More than 351 days — 45 patients (24%).
Patients exposed to mogamulizumab for longer were more likely to have SS, stage III/IV disease, blood involvement, and a performance status of 0.
Dr. Kim said the SS patients “benefited a lot” from mogamulizumab and therefore remained on treatment longer.
Response
As expected, patients exposed to mogamulizumab for the longest period had the highest global response rates. Confirmed response rates according to drug exposure were as follows:
- Less than 72 days: 1.9% overall, 0% for SS, and 2.9% for MF
- 72-170 days: 10% overall, 18.8% for SS, and 4.2% for MF
- 171-351 days: 29.8% overall, 36.4% for SS, and 24% for MF
- More than 351 days: 75.6% overall, 83.3% for SS, and 66.7% for MF.
In addition, rates of complete response (CR) and partial response (PR) tended to increase with mogamulizumab exposure. Rates of CR, PR, and stable disease (SD) according to exposure time were as follows:
- Less than 72 days: 0% CR, 7.7% PR, and 38.5% SD
- 72-170 days: 2.5% CR, 20% PR, and 62.5% SD
- 171-351 days: 2.1% CR, 34% PR, and 57.4% SD
- More than 351 days: 6.7% CR, 71.1% PR, and 17.8% SD.
Safety
“The percentage of patients reporting adverse events was not different in the long-term treatment-exposure patients, compared to the short-term,” Dr. Kim said.
Percentages of treatment-emergent AEs (TEAEs) and serious AEs (SAEs) according to mogamulizumab exposure were as follows:
- Less than 72 days: 26.6% TEAEs and 6.5% SAEs
- 72-170 days: 18.5% TEAEs and 3.3% SAEs
- 171-351 days: 23.4% TEAEs and 6.0% SAEs
- More than 351 days: 21.7% TEAEs and 4.3% SAEs.
“The majority of the grade 3 events occurred in the first two quartiles, not later, which is important to show,” Dr. Kim said.
Most grade 3 AEs occurred within 170 days of treatment initiation, and the median time to a grade 3 or higher AE was 109 days.
The most common treatment-related AEs in the longest exposure cohort were drug eruption (20.0%), thrombocytopenia (11.1%), stomatitis (8.9%), and anemia (8.9%).
Of all patients in this analysis, 45 experienced drug eruption, which was defined as a skin rash possibly, probably, or definitely related to the study drug.
Nine drug eruption events were grade 3, and the rest were grade 1 or 2. The median time to drug eruption was 107 days.
While drug eruption “didn’t show up early,” there is no cumulative risk with longer exposure to mogamulizumab, Dr. Kim said. Likewise, she said, autoimmune AEs were not dose-cumulative events.
There were two patients with definite autoimmune disease — a 65-year-old man with Miller Fisher syndrome (occurring 199 days after mogamulizumab initiation) and a 40-year-old woman with myositis (151 days) and myocarditis (310 days).
The investigators also identified three patients with possible autoimmune disease, including:
- Pneumonitis (310 days) in a 74-year-old woman
- Polymyalgia rheumatica (209 days) and myopathy (not available) in an 84-year-old man
- Hepatitis (144 days), pneumonitis (about 174 days), and polymyositis (about 174 days) in a 73-year-old man.
Dr. Kim and her colleagues said these data suggest prolonged treatment with mogamulizumab is not associated with an increased safety risk in patients with MF or SS. And the manageable safety profile of mogamulizumab meant that patients who derived a clinical benefit could remain on the drug for an extended period of time.
The MAVORIC trial was sponsored by Kyowa Hakko Kirin Pharma. Dr. Kim reported relationships with Merck, Portola Pharmaceuticals, Soligenix, Takeda, TetraLogic Pharmaceuticals, Kyowa Kirin, Seattle Genetics, Medivir, Neumedicines, Eisai, Innate Pharma, Galderma, Miragen Therapeutics, Forty Seven, and Horizon Pharma. Her coinvestigators reported relationships with several companies.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: The highest response rate – 75.6% – was observed in patients exposed to mogamulizumab for at least 351 days.
Study details: A post hoc analysis of the MAVORIC trial, including 184 patients treated with mogamulizumab.
Disclosures: The MAVORIC trial was sponsored by Kyowa Hakko Kirin Pharma. Investigators disclosed relationships with several companies.
Chidamide may be more effective in PTCL than previously thought
LA JOLLA, CALIF. – Real-world data suggest chidamide may be more effective against relapsed or refractory peripheral T-cell lymphoma (PTCL) than a pivotal study indicated.
Single-agent chidamide produced an overall response rate of 47.0% in a real-world study of more than 1,000 patients, compared with the 28.0% overall response rate that was observed in the phase 2 study of chidamide (Ann Oncol. 2015 Aug;26[8]:1766-71).
Yuqin Song, MD, PhD, of Peking University Cancer Hospital and Institute in Beijing, China, presented data from the real-world study at the annual T-cell Lymphoma Forum.
Dr. Song said this study is the largest cohort of real-world patients with relapsed or refractory PTCL. She and her colleagues analyzed data on 1,064 patients treated at 216 sites across China between February 2015 and December 2017.
The patients had a median age of 54 years, 63.9% were male, and 88.1% had stage III-IV disease.
Disease subtypes included PTCL not otherwise specified (NOS, 38.0%), angioimmunoblastic T-cell lymphoma (AITL, 29.1%), extranodal natural killer T-cell lymphoma (ENKTL, 13.4%), anaplastic large-cell lymphoma (ALCL, 9.1%), and others (10.3%), including cutaneous T-cell lymphoma (CTCL).
Fifty-two percent of patients (n = 553) received chidamide as a single agent, and 48% (n = 511) received the drug with other agents. The most common treatment regimens combined with chidamide were the following
- Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP, 20.7%).
- Gemcitabine, dexamethasone, and cisplatin (GDP, 11.8%).
- Etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH, 9.8%).
- Patients with ENKTL received chidamide with L-asparaginase (35.4%) or without it (64.5%).
The median follow-up was 4.9 months (range, 0-36.2 months). Across disease subtypes, the overall response rate was 47.0% with single-agent chidamide and 65.4% when chidamide was given in combination with other agents (P less than .01).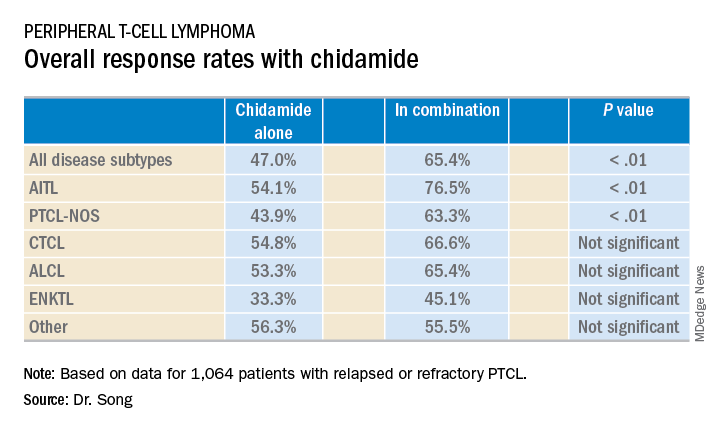
The median overall survival was 400 days for all patients, 342 days for patients treated with chidamide alone, and 457 days for patients who received combination therapy. The 1-year overall survival rates were 52%, 48%, and 56%, respectively.
Dr. Song said these data verify the efficacy of chidamide as a single agent and suggest chidamide might lead to improved survival in refractory or relapsed PTCLs.
Chidamide was generally well tolerated in this study, Dr. Song said. There were no unexpected adverse events (AEs) and most were grade 1 or 2.
The most common AEs (of any grade) observed with single-agent chidamide were neutropenia (42.9%), thrombocytopenia (40.5%), fatigue (38.3%), anemia (31.6%), and nausea/vomiting (21.0%).
The most common AEs observed with chidamide in combination were neutropenia (61.4%), thrombocytopenia (58.5%), fatigue (56.2%), anemia (54.2%), nausea/vomiting (30.7%), and fever (22.1%).
This study was supported by the Union for China Lymphoma Investigators and the Chinese Society of Clinical Oncology. Dr. Song did not disclose any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Real-world data suggest chidamide may be more effective against relapsed or refractory peripheral T-cell lymphoma (PTCL) than a pivotal study indicated.
Single-agent chidamide produced an overall response rate of 47.0% in a real-world study of more than 1,000 patients, compared with the 28.0% overall response rate that was observed in the phase 2 study of chidamide (Ann Oncol. 2015 Aug;26[8]:1766-71).
Yuqin Song, MD, PhD, of Peking University Cancer Hospital and Institute in Beijing, China, presented data from the real-world study at the annual T-cell Lymphoma Forum.
Dr. Song said this study is the largest cohort of real-world patients with relapsed or refractory PTCL. She and her colleagues analyzed data on 1,064 patients treated at 216 sites across China between February 2015 and December 2017.
The patients had a median age of 54 years, 63.9% were male, and 88.1% had stage III-IV disease.
Disease subtypes included PTCL not otherwise specified (NOS, 38.0%), angioimmunoblastic T-cell lymphoma (AITL, 29.1%), extranodal natural killer T-cell lymphoma (ENKTL, 13.4%), anaplastic large-cell lymphoma (ALCL, 9.1%), and others (10.3%), including cutaneous T-cell lymphoma (CTCL).
Fifty-two percent of patients (n = 553) received chidamide as a single agent, and 48% (n = 511) received the drug with other agents. The most common treatment regimens combined with chidamide were the following
- Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP, 20.7%).
- Gemcitabine, dexamethasone, and cisplatin (GDP, 11.8%).
- Etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH, 9.8%).
- Patients with ENKTL received chidamide with L-asparaginase (35.4%) or without it (64.5%).
The median follow-up was 4.9 months (range, 0-36.2 months). Across disease subtypes, the overall response rate was 47.0% with single-agent chidamide and 65.4% when chidamide was given in combination with other agents (P less than .01).
The median overall survival was 400 days for all patients, 342 days for patients treated with chidamide alone, and 457 days for patients who received combination therapy. The 1-year overall survival rates were 52%, 48%, and 56%, respectively.
Dr. Song said these data verify the efficacy of chidamide as a single agent and suggest chidamide might lead to improved survival in refractory or relapsed PTCLs.
Chidamide was generally well tolerated in this study, Dr. Song said. There were no unexpected adverse events (AEs) and most were grade 1 or 2.
The most common AEs (of any grade) observed with single-agent chidamide were neutropenia (42.9%), thrombocytopenia (40.5%), fatigue (38.3%), anemia (31.6%), and nausea/vomiting (21.0%).
The most common AEs observed with chidamide in combination were neutropenia (61.4%), thrombocytopenia (58.5%), fatigue (56.2%), anemia (54.2%), nausea/vomiting (30.7%), and fever (22.1%).
This study was supported by the Union for China Lymphoma Investigators and the Chinese Society of Clinical Oncology. Dr. Song did not disclose any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Real-world data suggest chidamide may be more effective against relapsed or refractory peripheral T-cell lymphoma (PTCL) than a pivotal study indicated.
Single-agent chidamide produced an overall response rate of 47.0% in a real-world study of more than 1,000 patients, compared with the 28.0% overall response rate that was observed in the phase 2 study of chidamide (Ann Oncol. 2015 Aug;26[8]:1766-71).
Yuqin Song, MD, PhD, of Peking University Cancer Hospital and Institute in Beijing, China, presented data from the real-world study at the annual T-cell Lymphoma Forum.
Dr. Song said this study is the largest cohort of real-world patients with relapsed or refractory PTCL. She and her colleagues analyzed data on 1,064 patients treated at 216 sites across China between February 2015 and December 2017.
The patients had a median age of 54 years, 63.9% were male, and 88.1% had stage III-IV disease.
Disease subtypes included PTCL not otherwise specified (NOS, 38.0%), angioimmunoblastic T-cell lymphoma (AITL, 29.1%), extranodal natural killer T-cell lymphoma (ENKTL, 13.4%), anaplastic large-cell lymphoma (ALCL, 9.1%), and others (10.3%), including cutaneous T-cell lymphoma (CTCL).
Fifty-two percent of patients (n = 553) received chidamide as a single agent, and 48% (n = 511) received the drug with other agents. The most common treatment regimens combined with chidamide were the following
- Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP, 20.7%).
- Gemcitabine, dexamethasone, and cisplatin (GDP, 11.8%).
- Etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH, 9.8%).
- Patients with ENKTL received chidamide with L-asparaginase (35.4%) or without it (64.5%).
The median follow-up was 4.9 months (range, 0-36.2 months). Across disease subtypes, the overall response rate was 47.0% with single-agent chidamide and 65.4% when chidamide was given in combination with other agents (P less than .01).
The median overall survival was 400 days for all patients, 342 days for patients treated with chidamide alone, and 457 days for patients who received combination therapy. The 1-year overall survival rates were 52%, 48%, and 56%, respectively.
Dr. Song said these data verify the efficacy of chidamide as a single agent and suggest chidamide might lead to improved survival in refractory or relapsed PTCLs.
Chidamide was generally well tolerated in this study, Dr. Song said. There were no unexpected adverse events (AEs) and most were grade 1 or 2.
The most common AEs (of any grade) observed with single-agent chidamide were neutropenia (42.9%), thrombocytopenia (40.5%), fatigue (38.3%), anemia (31.6%), and nausea/vomiting (21.0%).
The most common AEs observed with chidamide in combination were neutropenia (61.4%), thrombocytopenia (58.5%), fatigue (56.2%), anemia (54.2%), nausea/vomiting (30.7%), and fever (22.1%).
This study was supported by the Union for China Lymphoma Investigators and the Chinese Society of Clinical Oncology. Dr. Song did not disclose any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: Single-agent chidamide had an overall response rate of 47.0% among relapsed/refractory PTCL patients, compared with 65.4% when used in combination with other agents (P less than .01).
Study details: A real-world cohort of 1,064 relapsed/refractory PTCL patients treated at 216 sites across China between February 2015 and December 2017.
Disclosures: The study was supported by the Union for China Lymphoma Investigators and the Chinese Society of Clinical Oncology. Dr. Song did not disclose any conflicts of interest.