User login
Biomarkers may help to predict persistent oligoarticular JIA
Ongoing research in patients with oligoarticular juvenile idiopathic arthritis (JIA) so far suggests that a set of biomarkers in synovial fluid may help to predict which patients may be more likely to stay with persistent oligoarticular disease rather than progress to polyarticular disease, according to new research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year. Identifying biomarkers in synovial fluid or possibly serum could aid families and physicians in being more proactive in treatment protocols, said AnneMarie C. Brescia, MD, of Nemours Children’s Hospital in Wilmington, Del.
“JIA carries the risk of permanent joint damage and disability, which can result when joint involvement evolves from oligoarticular into a polyarticular course, termed extended oligoarticular disease,” Dr. Brescia told attendees. “Since disease progression increases the risk for disability, early prediction of this course is essential.”
This group – those whose oligoarticular disease will begin recruiting joints and ultimately become extended oligoarticular JIA – is “very important because they have been shown to have worse health-related quality of life and greater risk of needing a joint replacement than even polyarticular [JIA],” Dr. Brescia said. “So, our lab has really focused on trying to predict who will fall in this group.”
Melissa Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University in Indianapolis, was not involved in the study but agreed that having highly sensitive and specific biomarkers could be particularly helpful in clinical care.
“Biomarkers can help guide treatment decisions and help physicians and their patients share the decision-making about next choices and when to change,” Dr. Oliver told this news organization. “If a provider and parent know that their child has these markers in their serum or synovial fluid that may predict extension of their disease, then they may be more aggressive upfront with therapy.”
The study aimed to determine whether differential levels of synovial fluid proteins could be used to predict whether JIA would evolve into an extended course before it became clinically evident. Although early aggressive treatment is common with rheumatoid arthritis and can lead to remission, JIA treatment paradigms tend to be more reactive, Dr. Brescia said.
“It would be better to switch to proactive, that if we’re able to predict that this patient may have a more difficult course with extension to polyarticular, we could be prepared, we could inform the parents, and it would just help us have a more proactive approach,” she said.
The researchers used antibody arrays to detect the following inflammatory mediators in blinded samples: CD14, interleukin (IL)-1-alpha, IL-3, IL-5, IL-6, vascular endothelial growth factor (VEGF), and angiogenin. They analyzed 37 samples with persistent disease and 32 samples from disease that had not yet extended but would become extended in that patient. The samples came from patients who were taking no medicines or only NSAIDs. The researchers assessed the sensitivity and specificity of each biomarker. Sensitivity referred the biomarker’s ability to correctly indicate that the sample would extend, and specificity referred to the biomarker’s accuracy in determining that the disease in the sample would remain persistent.
Combining samples from cohorts at Nemours Children’s Health (14 persistent and 7 extended-to-be) and Cincinnati Children’s Hospital (23 persistent and 25 extended-to-be) yielded the following results: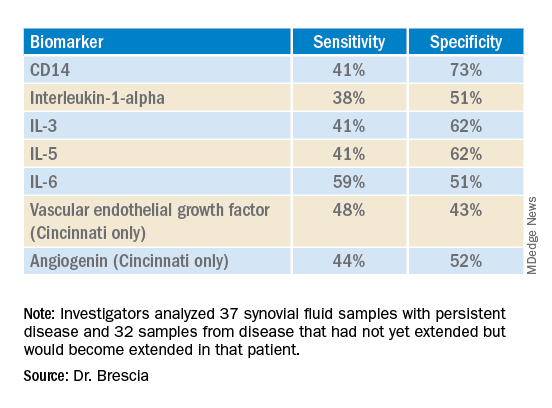
The findings revealed that the selected biomarkers were more accurate at predicting whose disease would remain persistent than predicting those that would extend, Dr. Brescia said. CD14 was the most specific biomarker, and IL-6 was the most sensitive biomarker in both groups.
When the researchers translated the findings from ELISA to the Luminex platform, positive results in synovial fluid for all these biomarkers were also positive in serum samples. Although the differences between persistent and extended-to-be samples did not reach statistical significance using Luminex, the pattern was the same for each biomarker.
“Luminex is more sensitive than ELISA. We believe that conducting an LDA [linear discriminant analysis] using these Luminex measurements will allow us to determine new cutoffs or new protein levels that are appropriate for Luminex to predict who will extend,” Dr. Brescia said. “It’s also our goal to develop a serum panel because ... being able to detect these markers in serum would expand the applicability of these markers to more patients.”
Dr. Brescia then described the group’s work in defining clinically relevant subpopulations of patients based on fibroblast-like synoviocytes (FLS) cells in the synovial intimal lining that produce inflammatory cytokines.
“Our compelling, single-cell, RNA sequencing preliminary data revealing multiple subpopulations within the total FLS population supports our hypothesis that distinct FLS subpopulations correlate with clinical outcome,” said Dr. Brescia. They looked at the percentage of chondrocyte-like, fibroblast-like, and smooth muscle-like subpopulations in samples from patients with oligoarticular JIA, extended-to-be JIA, and polyarticular JIA. Chondrocytes occurred in the largest proportion, and polyarticular JIA FLS had the largest percentage of chondrocytes, compared with the other two subpopulation groups.
“This is a work in progress,” Dr. Brescia said, “so hopefully you’ll hear about it next year.” In response to an attendee’s question, she said she believes identifying reliable biomarkers will eventually lead to refining treatment paradigms.
“I think it will at least change the guidance we can provide parents about making next choices and how quickly to accelerate to those next choices,” Dr. Brescia said. For example, if a child’s serum or synovial fluid has markers that show a very high likelihood of extension, the parent may decide to proceed to the next level medication sooner. “I do think it will push both parents and doctors to be a little more proactive instead of reactive when the poor patient comes back with 13 joints involved when they had just been an oligo for years.”
Dr. Oliver noted the promise of CD14 and IL-6 in potentially predicting which patients’ disease will stay persistent but cautioned that it’s still early in evaluating these biomarkers, especially with the limited patient samples in this study.
“I think these results are promising, and it’s great that there are groups out there working on this,” Dr. Oliver said. “Once we have a reliable, highly sensitive and specific biomarker, that will definitely help providers, parents, and patients be more informed.”
The research was supported by the Open Net Foundation, the Arthritis Foundation, Delaware Community Foundation, the Delaware Clinical and Translational Research (DE-CTR) ACCEL Program, the Nancy Taylor Foundation for Chronic Diseases, and CARRA. Dr. Brescia and Dr. Oliver have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ongoing research in patients with oligoarticular juvenile idiopathic arthritis (JIA) so far suggests that a set of biomarkers in synovial fluid may help to predict which patients may be more likely to stay with persistent oligoarticular disease rather than progress to polyarticular disease, according to new research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year. Identifying biomarkers in synovial fluid or possibly serum could aid families and physicians in being more proactive in treatment protocols, said AnneMarie C. Brescia, MD, of Nemours Children’s Hospital in Wilmington, Del.
“JIA carries the risk of permanent joint damage and disability, which can result when joint involvement evolves from oligoarticular into a polyarticular course, termed extended oligoarticular disease,” Dr. Brescia told attendees. “Since disease progression increases the risk for disability, early prediction of this course is essential.”
This group – those whose oligoarticular disease will begin recruiting joints and ultimately become extended oligoarticular JIA – is “very important because they have been shown to have worse health-related quality of life and greater risk of needing a joint replacement than even polyarticular [JIA],” Dr. Brescia said. “So, our lab has really focused on trying to predict who will fall in this group.”
Melissa Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University in Indianapolis, was not involved in the study but agreed that having highly sensitive and specific biomarkers could be particularly helpful in clinical care.
“Biomarkers can help guide treatment decisions and help physicians and their patients share the decision-making about next choices and when to change,” Dr. Oliver told this news organization. “If a provider and parent know that their child has these markers in their serum or synovial fluid that may predict extension of their disease, then they may be more aggressive upfront with therapy.”
The study aimed to determine whether differential levels of synovial fluid proteins could be used to predict whether JIA would evolve into an extended course before it became clinically evident. Although early aggressive treatment is common with rheumatoid arthritis and can lead to remission, JIA treatment paradigms tend to be more reactive, Dr. Brescia said.
“It would be better to switch to proactive, that if we’re able to predict that this patient may have a more difficult course with extension to polyarticular, we could be prepared, we could inform the parents, and it would just help us have a more proactive approach,” she said.
The researchers used antibody arrays to detect the following inflammatory mediators in blinded samples: CD14, interleukin (IL)-1-alpha, IL-3, IL-5, IL-6, vascular endothelial growth factor (VEGF), and angiogenin. They analyzed 37 samples with persistent disease and 32 samples from disease that had not yet extended but would become extended in that patient. The samples came from patients who were taking no medicines or only NSAIDs. The researchers assessed the sensitivity and specificity of each biomarker. Sensitivity referred the biomarker’s ability to correctly indicate that the sample would extend, and specificity referred to the biomarker’s accuracy in determining that the disease in the sample would remain persistent.
Combining samples from cohorts at Nemours Children’s Health (14 persistent and 7 extended-to-be) and Cincinnati Children’s Hospital (23 persistent and 25 extended-to-be) yielded the following results:
The findings revealed that the selected biomarkers were more accurate at predicting whose disease would remain persistent than predicting those that would extend, Dr. Brescia said. CD14 was the most specific biomarker, and IL-6 was the most sensitive biomarker in both groups.
When the researchers translated the findings from ELISA to the Luminex platform, positive results in synovial fluid for all these biomarkers were also positive in serum samples. Although the differences between persistent and extended-to-be samples did not reach statistical significance using Luminex, the pattern was the same for each biomarker.
“Luminex is more sensitive than ELISA. We believe that conducting an LDA [linear discriminant analysis] using these Luminex measurements will allow us to determine new cutoffs or new protein levels that are appropriate for Luminex to predict who will extend,” Dr. Brescia said. “It’s also our goal to develop a serum panel because ... being able to detect these markers in serum would expand the applicability of these markers to more patients.”
Dr. Brescia then described the group’s work in defining clinically relevant subpopulations of patients based on fibroblast-like synoviocytes (FLS) cells in the synovial intimal lining that produce inflammatory cytokines.
“Our compelling, single-cell, RNA sequencing preliminary data revealing multiple subpopulations within the total FLS population supports our hypothesis that distinct FLS subpopulations correlate with clinical outcome,” said Dr. Brescia. They looked at the percentage of chondrocyte-like, fibroblast-like, and smooth muscle-like subpopulations in samples from patients with oligoarticular JIA, extended-to-be JIA, and polyarticular JIA. Chondrocytes occurred in the largest proportion, and polyarticular JIA FLS had the largest percentage of chondrocytes, compared with the other two subpopulation groups.
“This is a work in progress,” Dr. Brescia said, “so hopefully you’ll hear about it next year.” In response to an attendee’s question, she said she believes identifying reliable biomarkers will eventually lead to refining treatment paradigms.
“I think it will at least change the guidance we can provide parents about making next choices and how quickly to accelerate to those next choices,” Dr. Brescia said. For example, if a child’s serum or synovial fluid has markers that show a very high likelihood of extension, the parent may decide to proceed to the next level medication sooner. “I do think it will push both parents and doctors to be a little more proactive instead of reactive when the poor patient comes back with 13 joints involved when they had just been an oligo for years.”
Dr. Oliver noted the promise of CD14 and IL-6 in potentially predicting which patients’ disease will stay persistent but cautioned that it’s still early in evaluating these biomarkers, especially with the limited patient samples in this study.
“I think these results are promising, and it’s great that there are groups out there working on this,” Dr. Oliver said. “Once we have a reliable, highly sensitive and specific biomarker, that will definitely help providers, parents, and patients be more informed.”
The research was supported by the Open Net Foundation, the Arthritis Foundation, Delaware Community Foundation, the Delaware Clinical and Translational Research (DE-CTR) ACCEL Program, the Nancy Taylor Foundation for Chronic Diseases, and CARRA. Dr. Brescia and Dr. Oliver have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ongoing research in patients with oligoarticular juvenile idiopathic arthritis (JIA) so far suggests that a set of biomarkers in synovial fluid may help to predict which patients may be more likely to stay with persistent oligoarticular disease rather than progress to polyarticular disease, according to new research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year. Identifying biomarkers in synovial fluid or possibly serum could aid families and physicians in being more proactive in treatment protocols, said AnneMarie C. Brescia, MD, of Nemours Children’s Hospital in Wilmington, Del.
“JIA carries the risk of permanent joint damage and disability, which can result when joint involvement evolves from oligoarticular into a polyarticular course, termed extended oligoarticular disease,” Dr. Brescia told attendees. “Since disease progression increases the risk for disability, early prediction of this course is essential.”
This group – those whose oligoarticular disease will begin recruiting joints and ultimately become extended oligoarticular JIA – is “very important because they have been shown to have worse health-related quality of life and greater risk of needing a joint replacement than even polyarticular [JIA],” Dr. Brescia said. “So, our lab has really focused on trying to predict who will fall in this group.”
Melissa Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University in Indianapolis, was not involved in the study but agreed that having highly sensitive and specific biomarkers could be particularly helpful in clinical care.
“Biomarkers can help guide treatment decisions and help physicians and their patients share the decision-making about next choices and when to change,” Dr. Oliver told this news organization. “If a provider and parent know that their child has these markers in their serum or synovial fluid that may predict extension of their disease, then they may be more aggressive upfront with therapy.”
The study aimed to determine whether differential levels of synovial fluid proteins could be used to predict whether JIA would evolve into an extended course before it became clinically evident. Although early aggressive treatment is common with rheumatoid arthritis and can lead to remission, JIA treatment paradigms tend to be more reactive, Dr. Brescia said.
“It would be better to switch to proactive, that if we’re able to predict that this patient may have a more difficult course with extension to polyarticular, we could be prepared, we could inform the parents, and it would just help us have a more proactive approach,” she said.
The researchers used antibody arrays to detect the following inflammatory mediators in blinded samples: CD14, interleukin (IL)-1-alpha, IL-3, IL-5, IL-6, vascular endothelial growth factor (VEGF), and angiogenin. They analyzed 37 samples with persistent disease and 32 samples from disease that had not yet extended but would become extended in that patient. The samples came from patients who were taking no medicines or only NSAIDs. The researchers assessed the sensitivity and specificity of each biomarker. Sensitivity referred the biomarker’s ability to correctly indicate that the sample would extend, and specificity referred to the biomarker’s accuracy in determining that the disease in the sample would remain persistent.
Combining samples from cohorts at Nemours Children’s Health (14 persistent and 7 extended-to-be) and Cincinnati Children’s Hospital (23 persistent and 25 extended-to-be) yielded the following results:
The findings revealed that the selected biomarkers were more accurate at predicting whose disease would remain persistent than predicting those that would extend, Dr. Brescia said. CD14 was the most specific biomarker, and IL-6 was the most sensitive biomarker in both groups.
When the researchers translated the findings from ELISA to the Luminex platform, positive results in synovial fluid for all these biomarkers were also positive in serum samples. Although the differences between persistent and extended-to-be samples did not reach statistical significance using Luminex, the pattern was the same for each biomarker.
“Luminex is more sensitive than ELISA. We believe that conducting an LDA [linear discriminant analysis] using these Luminex measurements will allow us to determine new cutoffs or new protein levels that are appropriate for Luminex to predict who will extend,” Dr. Brescia said. “It’s also our goal to develop a serum panel because ... being able to detect these markers in serum would expand the applicability of these markers to more patients.”
Dr. Brescia then described the group’s work in defining clinically relevant subpopulations of patients based on fibroblast-like synoviocytes (FLS) cells in the synovial intimal lining that produce inflammatory cytokines.
“Our compelling, single-cell, RNA sequencing preliminary data revealing multiple subpopulations within the total FLS population supports our hypothesis that distinct FLS subpopulations correlate with clinical outcome,” said Dr. Brescia. They looked at the percentage of chondrocyte-like, fibroblast-like, and smooth muscle-like subpopulations in samples from patients with oligoarticular JIA, extended-to-be JIA, and polyarticular JIA. Chondrocytes occurred in the largest proportion, and polyarticular JIA FLS had the largest percentage of chondrocytes, compared with the other two subpopulation groups.
“This is a work in progress,” Dr. Brescia said, “so hopefully you’ll hear about it next year.” In response to an attendee’s question, she said she believes identifying reliable biomarkers will eventually lead to refining treatment paradigms.
“I think it will at least change the guidance we can provide parents about making next choices and how quickly to accelerate to those next choices,” Dr. Brescia said. For example, if a child’s serum or synovial fluid has markers that show a very high likelihood of extension, the parent may decide to proceed to the next level medication sooner. “I do think it will push both parents and doctors to be a little more proactive instead of reactive when the poor patient comes back with 13 joints involved when they had just been an oligo for years.”
Dr. Oliver noted the promise of CD14 and IL-6 in potentially predicting which patients’ disease will stay persistent but cautioned that it’s still early in evaluating these biomarkers, especially with the limited patient samples in this study.
“I think these results are promising, and it’s great that there are groups out there working on this,” Dr. Oliver said. “Once we have a reliable, highly sensitive and specific biomarker, that will definitely help providers, parents, and patients be more informed.”
The research was supported by the Open Net Foundation, the Arthritis Foundation, Delaware Community Foundation, the Delaware Clinical and Translational Research (DE-CTR) ACCEL Program, the Nancy Taylor Foundation for Chronic Diseases, and CARRA. Dr. Brescia and Dr. Oliver have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CARRA 2022
Lupus mutation may unlock targeted drugs for patient subset
Scientists have confirmed that a receptor long suspected to be linked to lupus is, in fact, a major driver of the autoimmune disease for at least some subset of patients, according to a study recently published in Nature. Researchers discovered the crucial role of toll-like receptor 7 (TLR7) because of a rare mutation in a pediatric patient with systemic lupus erythematosus (SLE) who had a particularly severe presentation.
“Sometimes it’s valuable to find these very severe cases where there is one mutation that has a strong effect because if we understand how those mutations work, the lessons we learn can generally tell us about disease mechanisms,” explained senior author Carola G. Vinuesa, MD, PhD, of the Centre for Personalised Immunology at Australian National University in Canberra and The Francis Crick Institute in London.

“It’s quite difficult to find one mutation that can alone cause the entire disease,” Dr. Vinuesa added, but what it reveals about how the disease develops may lead to more effective targeted therapies than the immune suppressants most often used to treat lupus currently.
The mutation they found was in the TLR7 gene that encodes the TLR7 protein. TLR7 is a receptor used by immune cells to identify viral RNA so they can fight off viral infections, including COVID-19. But if the body’s own genetic material binds to TLR7 in susceptible individuals, it can lead to an overproduction of type 1 interferons, which are cytokines that trigger or exacerbate the immune reactions that lead to lupus symptoms. The TLR7 gene occurs on the X chromosome, which may explain men’s greater susceptibility to COVID-19 and the greater incidence of lupus in women, who have two X chromosomes instead of the one that men have, Dr. Vinuesa said.
Previous research had shown an association between TLR7 and lupus, but this new study is the first to provide definitive proof that a TLR7 mutation by itself can directly cause human lupus. After discovering the variant in the patient, Dr. Vinuesa’s team used CRISPR to edit the genome of a mouse model and introduce the same mutation the patient had. “And they developed full-blown disease, just with this one single base-pair substitution – 1 letter in the 3 billion letters of the genome,” Dr. Vinuesa said. “It tells us that these receptors are not just there to recognize viral RNA, that in some circumstances, they could be triggered by our own nucleic acids.”
One pathway among many?
The finding does not mean that every lupus patient has this mutation, which remains rare, but suggests that overactivity in this receptor already reported in many lupus patients may be causally related to disease, Dr. Vinuesa said.
Noa Schwartz, MD, an assistant professor of medicine at Albert Einstein College of Medicine, New York, and director of the Montefiore-Einstein Institute for Lupus Care and Research, said in an interview that lupus is thought of as a syndrome, a collection of different but similar diseases that don’t necessarily have a single cause. But finding a single gene mutation that could potentially lead to lupus is an important piece of the puzzle, said Dr. Schwartz, who was not involved in the study. Based on past research in mice models, “we’ve hypothesized that TLR7 is important in humans as well, but this is the last nail in the coffin.”
One of the key questions this finding has prompted is how many patients’ disease results from TLR7 activity. “Because of the evidence from Ignacio Sanz’s group demonstrating TLR7 overactivity in a significant fraction of SLE patients, we believe that it is probably going to be pretty important,” Dr. Vinuesa said. “My feeling is that it is going to be quite a central pathway in lupus pathogenesis, if not the central pathway.”
Dr. Schwartz was more cautious, noting that it is probably important for a subset of patients but may “have a limited effect on the general lupus population.” While it’s not yet clear how large that subset is, it is possible it will include people with cutaneous lupus, those with primarily dermatologic symptoms.
“Hydroxychloroquine works particularly well for cutaneous manifestations of lupus, and one of the ways that works is by inhibiting TLR7 and TLR9, so this [finding] potentially matters for skin disease and lupus, but it’s very early,” Dr. Schwartz said. If it does turn out that TLR7 activity is particularly associated with cutaneous lupus, it may mean therapies with fewer side effects, she said. “Specifically for cutaneous lupus, the concept of suppressing the entire immune system for skin illness sometimes feels, especially to patients, very extreme, so they are [patients] who directed therapy could be so especially relevant for.”
Laura Lewandowski, MD, an assistant clinical investigator and head of the lupus genomics and global health disparities unit at the National Institute of Arthritis and Musculoskeletal and Skin Disease, described this study as particularly remarkable in the way it revealed the mechanism leading to lupus symptoms.
“As whole genome sequencing becomes faster and less expensive, more and more people are employing them in their studies,” most of which report changes in certain genes, Dr. Lewandowski said. “One of the most striking findings about this paper was that they took it to the next step and did a really elegant study on the exact way this gain-of-function TLR7 mutation leads to the autoimmunity that we see in lupus. The detail of mechanism in this paper is really unique.”
A step toward personalized medicine
Dr. Lewandowski is part of a team that recently presented a poster related to genomic sequencing in lupus patients at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance. Her study reported on the whole genome sequencing of patients with childhood-onset SLE who were already enrolled in the CARRA Lupus Registry. Children with lupus may be more likely than adults to have rare genetic variants, so a registry of childhood-onset SLE patients with fully sequenced genomes provides an opportunity to look for single-gene mutations specifically linked to lupus, said Dr. Lewandowski, who has recently begun a research collaboration with Dr. Vinuesa.
“As we move forward and more and more patients are included in these studies, we will understand a little bit more about the genetic architecture of patients who have rare variations leading to disease, or even common variations,” Dr. Lewandowski said about the intersection between her research and Dr. Vinuesa’s study. The more data they gather, the more they can explore the possible interactions of rare and common variants that play a role in SLE as well as what environmental triggers, such as viral infection or pollution exposure, might tip someone into having an autoimmune disease. “We’re just starting to peek under the hood,” Dr. Lewandowski said.
If further research can reveal the relative contribution of genetics to the disease and what those genetic drivers are, it may allow for greater precision in therapies and “ultimately improve the quality of life for our patients, the ultimate goal of all of these studies,” Dr. Lewandowski said.
Drugs that target TLR7 already exist for other indications, and clinical trials have already begun to see if these TLR7 inhibitors benefit lupus patients.
“If the clinical trials work, this will be quite a nice, targeted therapy with potentially much less side effects than other therapies on the market at the moment,” Dr. Vinuesa said. She is cautiously hopeful, saying it’s likely to make an impact on lupus treatment, but it’s too early to say precisely how much.
“It allows us to understand the disease mechanisms a little bit better and to try and assess what percentage of patients’ disease can be explained by overactivity in this receptor,” Dr. Vinuesa said. She thinks it’s possible that TLR7 over activation may be relevant to other systemic autoimmune diseases as well, such as Sjögren’s syndrome, rheumatoid arthritis, or juvenile dermatomyositis, but it will take more studies to find out.
“Right now, we have medicines that broadly inhibit the immune system and aren’t as targeted, but we have a lot more clinical and scientific work to do before we move this field forward for lupus patients,” Dr. Lewandowski said. “This is one case where they were able to find the exact molecular defect, and it’s not the end of the path of precision medicine — it’s the beginning.”
Dr. Vinuesa, Dr. Schwartz, and Dr. Lewandowski reported no disclosures.
A version of this article first appeared on Medscape.com.
Scientists have confirmed that a receptor long suspected to be linked to lupus is, in fact, a major driver of the autoimmune disease for at least some subset of patients, according to a study recently published in Nature. Researchers discovered the crucial role of toll-like receptor 7 (TLR7) because of a rare mutation in a pediatric patient with systemic lupus erythematosus (SLE) who had a particularly severe presentation.
“Sometimes it’s valuable to find these very severe cases where there is one mutation that has a strong effect because if we understand how those mutations work, the lessons we learn can generally tell us about disease mechanisms,” explained senior author Carola G. Vinuesa, MD, PhD, of the Centre for Personalised Immunology at Australian National University in Canberra and The Francis Crick Institute in London.

“It’s quite difficult to find one mutation that can alone cause the entire disease,” Dr. Vinuesa added, but what it reveals about how the disease develops may lead to more effective targeted therapies than the immune suppressants most often used to treat lupus currently.
The mutation they found was in the TLR7 gene that encodes the TLR7 protein. TLR7 is a receptor used by immune cells to identify viral RNA so they can fight off viral infections, including COVID-19. But if the body’s own genetic material binds to TLR7 in susceptible individuals, it can lead to an overproduction of type 1 interferons, which are cytokines that trigger or exacerbate the immune reactions that lead to lupus symptoms. The TLR7 gene occurs on the X chromosome, which may explain men’s greater susceptibility to COVID-19 and the greater incidence of lupus in women, who have two X chromosomes instead of the one that men have, Dr. Vinuesa said.
Previous research had shown an association between TLR7 and lupus, but this new study is the first to provide definitive proof that a TLR7 mutation by itself can directly cause human lupus. After discovering the variant in the patient, Dr. Vinuesa’s team used CRISPR to edit the genome of a mouse model and introduce the same mutation the patient had. “And they developed full-blown disease, just with this one single base-pair substitution – 1 letter in the 3 billion letters of the genome,” Dr. Vinuesa said. “It tells us that these receptors are not just there to recognize viral RNA, that in some circumstances, they could be triggered by our own nucleic acids.”
One pathway among many?
The finding does not mean that every lupus patient has this mutation, which remains rare, but suggests that overactivity in this receptor already reported in many lupus patients may be causally related to disease, Dr. Vinuesa said.
Noa Schwartz, MD, an assistant professor of medicine at Albert Einstein College of Medicine, New York, and director of the Montefiore-Einstein Institute for Lupus Care and Research, said in an interview that lupus is thought of as a syndrome, a collection of different but similar diseases that don’t necessarily have a single cause. But finding a single gene mutation that could potentially lead to lupus is an important piece of the puzzle, said Dr. Schwartz, who was not involved in the study. Based on past research in mice models, “we’ve hypothesized that TLR7 is important in humans as well, but this is the last nail in the coffin.”
One of the key questions this finding has prompted is how many patients’ disease results from TLR7 activity. “Because of the evidence from Ignacio Sanz’s group demonstrating TLR7 overactivity in a significant fraction of SLE patients, we believe that it is probably going to be pretty important,” Dr. Vinuesa said. “My feeling is that it is going to be quite a central pathway in lupus pathogenesis, if not the central pathway.”
Dr. Schwartz was more cautious, noting that it is probably important for a subset of patients but may “have a limited effect on the general lupus population.” While it’s not yet clear how large that subset is, it is possible it will include people with cutaneous lupus, those with primarily dermatologic symptoms.
“Hydroxychloroquine works particularly well for cutaneous manifestations of lupus, and one of the ways that works is by inhibiting TLR7 and TLR9, so this [finding] potentially matters for skin disease and lupus, but it’s very early,” Dr. Schwartz said. If it does turn out that TLR7 activity is particularly associated with cutaneous lupus, it may mean therapies with fewer side effects, she said. “Specifically for cutaneous lupus, the concept of suppressing the entire immune system for skin illness sometimes feels, especially to patients, very extreme, so they are [patients] who directed therapy could be so especially relevant for.”
Laura Lewandowski, MD, an assistant clinical investigator and head of the lupus genomics and global health disparities unit at the National Institute of Arthritis and Musculoskeletal and Skin Disease, described this study as particularly remarkable in the way it revealed the mechanism leading to lupus symptoms.
“As whole genome sequencing becomes faster and less expensive, more and more people are employing them in their studies,” most of which report changes in certain genes, Dr. Lewandowski said. “One of the most striking findings about this paper was that they took it to the next step and did a really elegant study on the exact way this gain-of-function TLR7 mutation leads to the autoimmunity that we see in lupus. The detail of mechanism in this paper is really unique.”
A step toward personalized medicine
Dr. Lewandowski is part of a team that recently presented a poster related to genomic sequencing in lupus patients at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance. Her study reported on the whole genome sequencing of patients with childhood-onset SLE who were already enrolled in the CARRA Lupus Registry. Children with lupus may be more likely than adults to have rare genetic variants, so a registry of childhood-onset SLE patients with fully sequenced genomes provides an opportunity to look for single-gene mutations specifically linked to lupus, said Dr. Lewandowski, who has recently begun a research collaboration with Dr. Vinuesa.
“As we move forward and more and more patients are included in these studies, we will understand a little bit more about the genetic architecture of patients who have rare variations leading to disease, or even common variations,” Dr. Lewandowski said about the intersection between her research and Dr. Vinuesa’s study. The more data they gather, the more they can explore the possible interactions of rare and common variants that play a role in SLE as well as what environmental triggers, such as viral infection or pollution exposure, might tip someone into having an autoimmune disease. “We’re just starting to peek under the hood,” Dr. Lewandowski said.
If further research can reveal the relative contribution of genetics to the disease and what those genetic drivers are, it may allow for greater precision in therapies and “ultimately improve the quality of life for our patients, the ultimate goal of all of these studies,” Dr. Lewandowski said.
Drugs that target TLR7 already exist for other indications, and clinical trials have already begun to see if these TLR7 inhibitors benefit lupus patients.
“If the clinical trials work, this will be quite a nice, targeted therapy with potentially much less side effects than other therapies on the market at the moment,” Dr. Vinuesa said. She is cautiously hopeful, saying it’s likely to make an impact on lupus treatment, but it’s too early to say precisely how much.
“It allows us to understand the disease mechanisms a little bit better and to try and assess what percentage of patients’ disease can be explained by overactivity in this receptor,” Dr. Vinuesa said. She thinks it’s possible that TLR7 over activation may be relevant to other systemic autoimmune diseases as well, such as Sjögren’s syndrome, rheumatoid arthritis, or juvenile dermatomyositis, but it will take more studies to find out.
“Right now, we have medicines that broadly inhibit the immune system and aren’t as targeted, but we have a lot more clinical and scientific work to do before we move this field forward for lupus patients,” Dr. Lewandowski said. “This is one case where they were able to find the exact molecular defect, and it’s not the end of the path of precision medicine — it’s the beginning.”
Dr. Vinuesa, Dr. Schwartz, and Dr. Lewandowski reported no disclosures.
A version of this article first appeared on Medscape.com.
Scientists have confirmed that a receptor long suspected to be linked to lupus is, in fact, a major driver of the autoimmune disease for at least some subset of patients, according to a study recently published in Nature. Researchers discovered the crucial role of toll-like receptor 7 (TLR7) because of a rare mutation in a pediatric patient with systemic lupus erythematosus (SLE) who had a particularly severe presentation.
“Sometimes it’s valuable to find these very severe cases where there is one mutation that has a strong effect because if we understand how those mutations work, the lessons we learn can generally tell us about disease mechanisms,” explained senior author Carola G. Vinuesa, MD, PhD, of the Centre for Personalised Immunology at Australian National University in Canberra and The Francis Crick Institute in London.

“It’s quite difficult to find one mutation that can alone cause the entire disease,” Dr. Vinuesa added, but what it reveals about how the disease develops may lead to more effective targeted therapies than the immune suppressants most often used to treat lupus currently.
The mutation they found was in the TLR7 gene that encodes the TLR7 protein. TLR7 is a receptor used by immune cells to identify viral RNA so they can fight off viral infections, including COVID-19. But if the body’s own genetic material binds to TLR7 in susceptible individuals, it can lead to an overproduction of type 1 interferons, which are cytokines that trigger or exacerbate the immune reactions that lead to lupus symptoms. The TLR7 gene occurs on the X chromosome, which may explain men’s greater susceptibility to COVID-19 and the greater incidence of lupus in women, who have two X chromosomes instead of the one that men have, Dr. Vinuesa said.
Previous research had shown an association between TLR7 and lupus, but this new study is the first to provide definitive proof that a TLR7 mutation by itself can directly cause human lupus. After discovering the variant in the patient, Dr. Vinuesa’s team used CRISPR to edit the genome of a mouse model and introduce the same mutation the patient had. “And they developed full-blown disease, just with this one single base-pair substitution – 1 letter in the 3 billion letters of the genome,” Dr. Vinuesa said. “It tells us that these receptors are not just there to recognize viral RNA, that in some circumstances, they could be triggered by our own nucleic acids.”
One pathway among many?
The finding does not mean that every lupus patient has this mutation, which remains rare, but suggests that overactivity in this receptor already reported in many lupus patients may be causally related to disease, Dr. Vinuesa said.
Noa Schwartz, MD, an assistant professor of medicine at Albert Einstein College of Medicine, New York, and director of the Montefiore-Einstein Institute for Lupus Care and Research, said in an interview that lupus is thought of as a syndrome, a collection of different but similar diseases that don’t necessarily have a single cause. But finding a single gene mutation that could potentially lead to lupus is an important piece of the puzzle, said Dr. Schwartz, who was not involved in the study. Based on past research in mice models, “we’ve hypothesized that TLR7 is important in humans as well, but this is the last nail in the coffin.”
One of the key questions this finding has prompted is how many patients’ disease results from TLR7 activity. “Because of the evidence from Ignacio Sanz’s group demonstrating TLR7 overactivity in a significant fraction of SLE patients, we believe that it is probably going to be pretty important,” Dr. Vinuesa said. “My feeling is that it is going to be quite a central pathway in lupus pathogenesis, if not the central pathway.”
Dr. Schwartz was more cautious, noting that it is probably important for a subset of patients but may “have a limited effect on the general lupus population.” While it’s not yet clear how large that subset is, it is possible it will include people with cutaneous lupus, those with primarily dermatologic symptoms.
“Hydroxychloroquine works particularly well for cutaneous manifestations of lupus, and one of the ways that works is by inhibiting TLR7 and TLR9, so this [finding] potentially matters for skin disease and lupus, but it’s very early,” Dr. Schwartz said. If it does turn out that TLR7 activity is particularly associated with cutaneous lupus, it may mean therapies with fewer side effects, she said. “Specifically for cutaneous lupus, the concept of suppressing the entire immune system for skin illness sometimes feels, especially to patients, very extreme, so they are [patients] who directed therapy could be so especially relevant for.”
Laura Lewandowski, MD, an assistant clinical investigator and head of the lupus genomics and global health disparities unit at the National Institute of Arthritis and Musculoskeletal and Skin Disease, described this study as particularly remarkable in the way it revealed the mechanism leading to lupus symptoms.
“As whole genome sequencing becomes faster and less expensive, more and more people are employing them in their studies,” most of which report changes in certain genes, Dr. Lewandowski said. “One of the most striking findings about this paper was that they took it to the next step and did a really elegant study on the exact way this gain-of-function TLR7 mutation leads to the autoimmunity that we see in lupus. The detail of mechanism in this paper is really unique.”
A step toward personalized medicine
Dr. Lewandowski is part of a team that recently presented a poster related to genomic sequencing in lupus patients at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance. Her study reported on the whole genome sequencing of patients with childhood-onset SLE who were already enrolled in the CARRA Lupus Registry. Children with lupus may be more likely than adults to have rare genetic variants, so a registry of childhood-onset SLE patients with fully sequenced genomes provides an opportunity to look for single-gene mutations specifically linked to lupus, said Dr. Lewandowski, who has recently begun a research collaboration with Dr. Vinuesa.
“As we move forward and more and more patients are included in these studies, we will understand a little bit more about the genetic architecture of patients who have rare variations leading to disease, or even common variations,” Dr. Lewandowski said about the intersection between her research and Dr. Vinuesa’s study. The more data they gather, the more they can explore the possible interactions of rare and common variants that play a role in SLE as well as what environmental triggers, such as viral infection or pollution exposure, might tip someone into having an autoimmune disease. “We’re just starting to peek under the hood,” Dr. Lewandowski said.
If further research can reveal the relative contribution of genetics to the disease and what those genetic drivers are, it may allow for greater precision in therapies and “ultimately improve the quality of life for our patients, the ultimate goal of all of these studies,” Dr. Lewandowski said.
Drugs that target TLR7 already exist for other indications, and clinical trials have already begun to see if these TLR7 inhibitors benefit lupus patients.
“If the clinical trials work, this will be quite a nice, targeted therapy with potentially much less side effects than other therapies on the market at the moment,” Dr. Vinuesa said. She is cautiously hopeful, saying it’s likely to make an impact on lupus treatment, but it’s too early to say precisely how much.
“It allows us to understand the disease mechanisms a little bit better and to try and assess what percentage of patients’ disease can be explained by overactivity in this receptor,” Dr. Vinuesa said. She thinks it’s possible that TLR7 over activation may be relevant to other systemic autoimmune diseases as well, such as Sjögren’s syndrome, rheumatoid arthritis, or juvenile dermatomyositis, but it will take more studies to find out.
“Right now, we have medicines that broadly inhibit the immune system and aren’t as targeted, but we have a lot more clinical and scientific work to do before we move this field forward for lupus patients,” Dr. Lewandowski said. “This is one case where they were able to find the exact molecular defect, and it’s not the end of the path of precision medicine — it’s the beginning.”
Dr. Vinuesa, Dr. Schwartz, and Dr. Lewandowski reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE
Pfizer COVID vaccine performs well in youth with rheumatic diseases
The Pfizer-BioNTech mRNA vaccine (Comirnaty) showed a good safety profile with minimal short-term side effects and no negative impact on disease activity in a cohort of adolescents and young adults with rheumatic diseases, according to research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year.
Only 3% of patients experience a severe transient adverse event, according to Merav Heshin-Bekenstein, MD, of Dana-Dwek Children’s Hospital at the Tel Aviv Sourasky Medical Center in Israel. The findings were published in Rheumatology.
“We found that the mRNA Pfizer vaccine was immunogenic and induced an adequate humoral immune response in adolescent patients,” Dr. Heshin-Bekenstein told CARRA attendees. “It was definitely comparable to healthy controls and practically all patients were seropositive following the second vaccine, except for one patient with long-standing systemic sclerosis.”
The findings were not necessarily surprising but were encouraging to Melissa S. Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University, Indianapolis. Dr. Oliver wasn’t part of the study team.
“We know that the COVID vaccines in healthy adolescents have shown good efficacy with minimal side effects, and it’s good to see that this study showed that in those with rheumatic diseases on immunosuppressive therapy,” Dr. Oliver told this news organization.
Until now, the data on COVID-19 vaccines in teens with rheumatic illnesses has been limited, she said, so “many pediatric rheumatologists only have the data from adult studies to go on or personal experience with their own cohort of patients.”
But the high immunogenicity seen in the study was a pleasant surprise to Beth H. Rutstein, MD, assistant professor of clinical pediatrics in the division of rheumatology at Children’s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania.
“I was both surprised and thrilled with Dr. Heshin-Bekenstein’s findings suggesting near-universal seroconversion for patients with rheumatic disease regardless of underlying diagnosis or immunomodulatory therapy regimen, as much of the adult data has suggested a poorer seroconversion rate” and lower antibody titers in adults with similar illnesses, Dr. Rutstein said in an interview.
The study “provides essential reassurance that vaccination against COVID-19 does not increase the risk of disease flare or worsen disease severity scores,” said Dr. Rutstein, who was not associated with the research. “Rather than speaking purely anecdotally with our patients and their families, we can refer to the science – which is always more reassuring for both our patients and ourselves.”
Study included diverse conditions and therapies
Risk factors for poor outcomes with COVID-19 in children include obesity, cardiovascular disease, chronic lung disease, diabetes, and asthma, Dr. Heshin-Bekenstein told CARRA attendees. Multisystem inflammatory syndrome in children (MIS-C) and long COVID are also potential complications of COVID-19 with less understood risk factors.
Although COVID-19 is most often mild in children, certain severe, systemic rheumatic diseases increase hospitalization risk, including systemic lupus erythematosus (SLE) and vasculitis. Evidence has also shown that COVID-19 infection increases the risk of disease flare in teens with juvenile-onset rheumatic diseases, so it’s “crucial to prevent COVID-19 disease in this population,” Dr. Heshin-Bekenstein said.
Her study therefore aimed to assess the safety and immunogenicity of the Pfizer mRNA vaccine for teens with juvenile-onset rheumatic diseases and those taking immunomodulatory medications. The international prospective multicenter study ran from April to November 2021 at three pediatric rheumatology clinics in Israel and one in Slovenia. Endpoints included short-term side effects, vaccination impact on clinical disease activity, immunogenicity at 2-9 weeks after the second dose, and, secondarily, efficacy against COVID-19 infection.
The 91 participants included adolescents aged 12-18 and young adults aged 18-21. Nearly half of the participants (46%) had juvenile idiopathic arthritis (JIA), and 14% had SLE. Other participants’ conditions included systemic vasculitis, idiopathic uveitis, inflammatory bowel disease–related arthritis, systemic or localized scleroderma, juvenile dermatomyositis, or an autoinflammatory disease. Participants’ mean disease duration was 4.8 years.
The researchers compared the patients with a control group of 40 individuals with similar demographics but without rheumatic disease. The researchers used the LIAISON quantitative assay to assess serum IgG antibody levels against the SARS-CoV-2 spike protein in both groups.
Eight in 10 participants with rheumatic disease were taking an immunomodulatory medication, including a conventional synthetic disease-modifying antirheumatic drug (csDMARD) in 40%, a biologic DMARD in 37%, tumor necrosis factor (TNF) inhibitors in 32%, hydroxychloroquine (HCQ) in 19%, glucocorticoids in 14%, and mycophenolate in 11%. A smaller proportion were on other biologics: JAK inhibitors in 6.6%, anti-CD20 drugs in 4.4%, and an IL-6 inhibitor in 1%.
Side effects similar in both groups
None of the side effects reported by participants were statistically different between those with rheumatic disease and the control group. Localized pain was the most common side effect, reported by 73%-79% of participants after each dose. About twice as many participants with rheumatic disease experienced muscle aches and joint pains, compared with the control group, but the differences were not significant. Fever occurred more often in those with rheumatic disease (6%, five cases) than without (3%, one case). One-third of those with rheumatic disease felt tiredness, compared with 20% of the control group.
None of the healthy controls were hospitalized after vaccination, but three rheumatic patients were, including two after the first dose. Both were 17 years old, had systemic vasculitis with granulomatosis with polyangiitis (GPA), and were taking rituximab (Rituxan). One patient experienced acute onset of chronic renal failure, fever, dehydration, and high C-reactive protein within hours of vaccination. The other experienced new onset of pulmonary hemorrhage a week after vaccination.
In addition, a 14-year-old female with lupus, taking only HCQ, went to the emergency department with fever, headache, vomiting, and joint pain 1 day after the second vaccine dose. She had normal inflammatory markers and no change in disease activity score, and she was discharged with low-dose steroids tapered after 2 weeks.
Immune response high in patients with rheumatic disease
Immunogenicity was similar in both groups, with 97% seropositivity in the rheumatic disease group and 100% in the control group. Average IgG titers were 242 in the rheumatic group and 388 in the control group (P < .0001). Seropositivity was 88% in those taking mycophenolate with another drug (100% with mycophenolate monotherapy), 90% with HCQ, 94% with any csDMARDs and another drug (100% with csDMARD monotherapy), and 100% for all other drugs. During 3 months’ follow-up after vaccination, there were no COVID-19 cases among the participants.
Dr. Heshin-Bekenstein noted that their results showed better immunogenicity in teens, compared with adults, for two specific drugs. Seropositivity in teens taking methotrexate (Rheumatrex, Trexall) or rituximab was 100% in this study, compared with 84% in adults taking methotrexate and 39% in adults taking rituximab in a previous study. However, only three patients in this study were taking rituximab, and only seven were taking methotrexate.
The study’s heterogenous population was both a strength and a weakness of the study. “Due to the diversity of rheumatic diseases and medications included in this cohort, it was not possible to draw significant conclusions regarding the impact of the immunomodulatory medications and type of disease” on titers, Dr. Heshin-Bekenstein told attendees.
Still, “I think as pediatric rheumatologists, we can feel reassured in recommending the COVID-19 vaccine to our patients,” Dr. Oliver said. “I will add that every patient is different, and everyone should have a conversation with their physician about receiving the COVID-19 vaccine.” Dr. Oliver said she discusses vaccination, including COVID vaccination, with every patient, and it’s been challenging to address concerns in the midst of so much misinformation circulating about the vaccine.
These findings do raise questions about whether it’s still necessary to hold immunomodulatory medications to get the vaccine,” Dr. Rutstein said.
“Many families are nervous to pause their medications before and after the vaccine as is currently recommended for many therapies by the American College of Rheumatology, and I do share that concern for some of my patients with more clinically unstable disease, so I try to work with each family to decide on best timing and have delayed or deferred the series until some patients are on a steady dose of a new immunomodulatory medication if it has been recently started,” Dr. Rutstein said. “This is one of the reasons why Dr. Heshin-Bekenstein’s study is so important – we may be holding medications that can be safely continued and even further decrease the risk of disease flare.”
None of the physicians have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Pfizer-BioNTech mRNA vaccine (Comirnaty) showed a good safety profile with minimal short-term side effects and no negative impact on disease activity in a cohort of adolescents and young adults with rheumatic diseases, according to research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year.
Only 3% of patients experience a severe transient adverse event, according to Merav Heshin-Bekenstein, MD, of Dana-Dwek Children’s Hospital at the Tel Aviv Sourasky Medical Center in Israel. The findings were published in Rheumatology.
“We found that the mRNA Pfizer vaccine was immunogenic and induced an adequate humoral immune response in adolescent patients,” Dr. Heshin-Bekenstein told CARRA attendees. “It was definitely comparable to healthy controls and practically all patients were seropositive following the second vaccine, except for one patient with long-standing systemic sclerosis.”
The findings were not necessarily surprising but were encouraging to Melissa S. Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University, Indianapolis. Dr. Oliver wasn’t part of the study team.
“We know that the COVID vaccines in healthy adolescents have shown good efficacy with minimal side effects, and it’s good to see that this study showed that in those with rheumatic diseases on immunosuppressive therapy,” Dr. Oliver told this news organization.
Until now, the data on COVID-19 vaccines in teens with rheumatic illnesses has been limited, she said, so “many pediatric rheumatologists only have the data from adult studies to go on or personal experience with their own cohort of patients.”
But the high immunogenicity seen in the study was a pleasant surprise to Beth H. Rutstein, MD, assistant professor of clinical pediatrics in the division of rheumatology at Children’s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania.
“I was both surprised and thrilled with Dr. Heshin-Bekenstein’s findings suggesting near-universal seroconversion for patients with rheumatic disease regardless of underlying diagnosis or immunomodulatory therapy regimen, as much of the adult data has suggested a poorer seroconversion rate” and lower antibody titers in adults with similar illnesses, Dr. Rutstein said in an interview.
The study “provides essential reassurance that vaccination against COVID-19 does not increase the risk of disease flare or worsen disease severity scores,” said Dr. Rutstein, who was not associated with the research. “Rather than speaking purely anecdotally with our patients and their families, we can refer to the science – which is always more reassuring for both our patients and ourselves.”
Study included diverse conditions and therapies
Risk factors for poor outcomes with COVID-19 in children include obesity, cardiovascular disease, chronic lung disease, diabetes, and asthma, Dr. Heshin-Bekenstein told CARRA attendees. Multisystem inflammatory syndrome in children (MIS-C) and long COVID are also potential complications of COVID-19 with less understood risk factors.
Although COVID-19 is most often mild in children, certain severe, systemic rheumatic diseases increase hospitalization risk, including systemic lupus erythematosus (SLE) and vasculitis. Evidence has also shown that COVID-19 infection increases the risk of disease flare in teens with juvenile-onset rheumatic diseases, so it’s “crucial to prevent COVID-19 disease in this population,” Dr. Heshin-Bekenstein said.
Her study therefore aimed to assess the safety and immunogenicity of the Pfizer mRNA vaccine for teens with juvenile-onset rheumatic diseases and those taking immunomodulatory medications. The international prospective multicenter study ran from April to November 2021 at three pediatric rheumatology clinics in Israel and one in Slovenia. Endpoints included short-term side effects, vaccination impact on clinical disease activity, immunogenicity at 2-9 weeks after the second dose, and, secondarily, efficacy against COVID-19 infection.
The 91 participants included adolescents aged 12-18 and young adults aged 18-21. Nearly half of the participants (46%) had juvenile idiopathic arthritis (JIA), and 14% had SLE. Other participants’ conditions included systemic vasculitis, idiopathic uveitis, inflammatory bowel disease–related arthritis, systemic or localized scleroderma, juvenile dermatomyositis, or an autoinflammatory disease. Participants’ mean disease duration was 4.8 years.
The researchers compared the patients with a control group of 40 individuals with similar demographics but without rheumatic disease. The researchers used the LIAISON quantitative assay to assess serum IgG antibody levels against the SARS-CoV-2 spike protein in both groups.
Eight in 10 participants with rheumatic disease were taking an immunomodulatory medication, including a conventional synthetic disease-modifying antirheumatic drug (csDMARD) in 40%, a biologic DMARD in 37%, tumor necrosis factor (TNF) inhibitors in 32%, hydroxychloroquine (HCQ) in 19%, glucocorticoids in 14%, and mycophenolate in 11%. A smaller proportion were on other biologics: JAK inhibitors in 6.6%, anti-CD20 drugs in 4.4%, and an IL-6 inhibitor in 1%.
Side effects similar in both groups
None of the side effects reported by participants were statistically different between those with rheumatic disease and the control group. Localized pain was the most common side effect, reported by 73%-79% of participants after each dose. About twice as many participants with rheumatic disease experienced muscle aches and joint pains, compared with the control group, but the differences were not significant. Fever occurred more often in those with rheumatic disease (6%, five cases) than without (3%, one case). One-third of those with rheumatic disease felt tiredness, compared with 20% of the control group.
None of the healthy controls were hospitalized after vaccination, but three rheumatic patients were, including two after the first dose. Both were 17 years old, had systemic vasculitis with granulomatosis with polyangiitis (GPA), and were taking rituximab (Rituxan). One patient experienced acute onset of chronic renal failure, fever, dehydration, and high C-reactive protein within hours of vaccination. The other experienced new onset of pulmonary hemorrhage a week after vaccination.
In addition, a 14-year-old female with lupus, taking only HCQ, went to the emergency department with fever, headache, vomiting, and joint pain 1 day after the second vaccine dose. She had normal inflammatory markers and no change in disease activity score, and she was discharged with low-dose steroids tapered after 2 weeks.
Immune response high in patients with rheumatic disease
Immunogenicity was similar in both groups, with 97% seropositivity in the rheumatic disease group and 100% in the control group. Average IgG titers were 242 in the rheumatic group and 388 in the control group (P < .0001). Seropositivity was 88% in those taking mycophenolate with another drug (100% with mycophenolate monotherapy), 90% with HCQ, 94% with any csDMARDs and another drug (100% with csDMARD monotherapy), and 100% for all other drugs. During 3 months’ follow-up after vaccination, there were no COVID-19 cases among the participants.
Dr. Heshin-Bekenstein noted that their results showed better immunogenicity in teens, compared with adults, for two specific drugs. Seropositivity in teens taking methotrexate (Rheumatrex, Trexall) or rituximab was 100% in this study, compared with 84% in adults taking methotrexate and 39% in adults taking rituximab in a previous study. However, only three patients in this study were taking rituximab, and only seven were taking methotrexate.
The study’s heterogenous population was both a strength and a weakness of the study. “Due to the diversity of rheumatic diseases and medications included in this cohort, it was not possible to draw significant conclusions regarding the impact of the immunomodulatory medications and type of disease” on titers, Dr. Heshin-Bekenstein told attendees.
Still, “I think as pediatric rheumatologists, we can feel reassured in recommending the COVID-19 vaccine to our patients,” Dr. Oliver said. “I will add that every patient is different, and everyone should have a conversation with their physician about receiving the COVID-19 vaccine.” Dr. Oliver said she discusses vaccination, including COVID vaccination, with every patient, and it’s been challenging to address concerns in the midst of so much misinformation circulating about the vaccine.
These findings do raise questions about whether it’s still necessary to hold immunomodulatory medications to get the vaccine,” Dr. Rutstein said.
“Many families are nervous to pause their medications before and after the vaccine as is currently recommended for many therapies by the American College of Rheumatology, and I do share that concern for some of my patients with more clinically unstable disease, so I try to work with each family to decide on best timing and have delayed or deferred the series until some patients are on a steady dose of a new immunomodulatory medication if it has been recently started,” Dr. Rutstein said. “This is one of the reasons why Dr. Heshin-Bekenstein’s study is so important – we may be holding medications that can be safely continued and even further decrease the risk of disease flare.”
None of the physicians have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Pfizer-BioNTech mRNA vaccine (Comirnaty) showed a good safety profile with minimal short-term side effects and no negative impact on disease activity in a cohort of adolescents and young adults with rheumatic diseases, according to research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year.
Only 3% of patients experience a severe transient adverse event, according to Merav Heshin-Bekenstein, MD, of Dana-Dwek Children’s Hospital at the Tel Aviv Sourasky Medical Center in Israel. The findings were published in Rheumatology.
“We found that the mRNA Pfizer vaccine was immunogenic and induced an adequate humoral immune response in adolescent patients,” Dr. Heshin-Bekenstein told CARRA attendees. “It was definitely comparable to healthy controls and practically all patients were seropositive following the second vaccine, except for one patient with long-standing systemic sclerosis.”
The findings were not necessarily surprising but were encouraging to Melissa S. Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University, Indianapolis. Dr. Oliver wasn’t part of the study team.
“We know that the COVID vaccines in healthy adolescents have shown good efficacy with minimal side effects, and it’s good to see that this study showed that in those with rheumatic diseases on immunosuppressive therapy,” Dr. Oliver told this news organization.
Until now, the data on COVID-19 vaccines in teens with rheumatic illnesses has been limited, she said, so “many pediatric rheumatologists only have the data from adult studies to go on or personal experience with their own cohort of patients.”
But the high immunogenicity seen in the study was a pleasant surprise to Beth H. Rutstein, MD, assistant professor of clinical pediatrics in the division of rheumatology at Children’s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania.
“I was both surprised and thrilled with Dr. Heshin-Bekenstein’s findings suggesting near-universal seroconversion for patients with rheumatic disease regardless of underlying diagnosis or immunomodulatory therapy regimen, as much of the adult data has suggested a poorer seroconversion rate” and lower antibody titers in adults with similar illnesses, Dr. Rutstein said in an interview.
The study “provides essential reassurance that vaccination against COVID-19 does not increase the risk of disease flare or worsen disease severity scores,” said Dr. Rutstein, who was not associated with the research. “Rather than speaking purely anecdotally with our patients and their families, we can refer to the science – which is always more reassuring for both our patients and ourselves.”
Study included diverse conditions and therapies
Risk factors for poor outcomes with COVID-19 in children include obesity, cardiovascular disease, chronic lung disease, diabetes, and asthma, Dr. Heshin-Bekenstein told CARRA attendees. Multisystem inflammatory syndrome in children (MIS-C) and long COVID are also potential complications of COVID-19 with less understood risk factors.
Although COVID-19 is most often mild in children, certain severe, systemic rheumatic diseases increase hospitalization risk, including systemic lupus erythematosus (SLE) and vasculitis. Evidence has also shown that COVID-19 infection increases the risk of disease flare in teens with juvenile-onset rheumatic diseases, so it’s “crucial to prevent COVID-19 disease in this population,” Dr. Heshin-Bekenstein said.
Her study therefore aimed to assess the safety and immunogenicity of the Pfizer mRNA vaccine for teens with juvenile-onset rheumatic diseases and those taking immunomodulatory medications. The international prospective multicenter study ran from April to November 2021 at three pediatric rheumatology clinics in Israel and one in Slovenia. Endpoints included short-term side effects, vaccination impact on clinical disease activity, immunogenicity at 2-9 weeks after the second dose, and, secondarily, efficacy against COVID-19 infection.
The 91 participants included adolescents aged 12-18 and young adults aged 18-21. Nearly half of the participants (46%) had juvenile idiopathic arthritis (JIA), and 14% had SLE. Other participants’ conditions included systemic vasculitis, idiopathic uveitis, inflammatory bowel disease–related arthritis, systemic or localized scleroderma, juvenile dermatomyositis, or an autoinflammatory disease. Participants’ mean disease duration was 4.8 years.
The researchers compared the patients with a control group of 40 individuals with similar demographics but without rheumatic disease. The researchers used the LIAISON quantitative assay to assess serum IgG antibody levels against the SARS-CoV-2 spike protein in both groups.
Eight in 10 participants with rheumatic disease were taking an immunomodulatory medication, including a conventional synthetic disease-modifying antirheumatic drug (csDMARD) in 40%, a biologic DMARD in 37%, tumor necrosis factor (TNF) inhibitors in 32%, hydroxychloroquine (HCQ) in 19%, glucocorticoids in 14%, and mycophenolate in 11%. A smaller proportion were on other biologics: JAK inhibitors in 6.6%, anti-CD20 drugs in 4.4%, and an IL-6 inhibitor in 1%.
Side effects similar in both groups
None of the side effects reported by participants were statistically different between those with rheumatic disease and the control group. Localized pain was the most common side effect, reported by 73%-79% of participants after each dose. About twice as many participants with rheumatic disease experienced muscle aches and joint pains, compared with the control group, but the differences were not significant. Fever occurred more often in those with rheumatic disease (6%, five cases) than without (3%, one case). One-third of those with rheumatic disease felt tiredness, compared with 20% of the control group.
None of the healthy controls were hospitalized after vaccination, but three rheumatic patients were, including two after the first dose. Both were 17 years old, had systemic vasculitis with granulomatosis with polyangiitis (GPA), and were taking rituximab (Rituxan). One patient experienced acute onset of chronic renal failure, fever, dehydration, and high C-reactive protein within hours of vaccination. The other experienced new onset of pulmonary hemorrhage a week after vaccination.
In addition, a 14-year-old female with lupus, taking only HCQ, went to the emergency department with fever, headache, vomiting, and joint pain 1 day after the second vaccine dose. She had normal inflammatory markers and no change in disease activity score, and she was discharged with low-dose steroids tapered after 2 weeks.
Immune response high in patients with rheumatic disease
Immunogenicity was similar in both groups, with 97% seropositivity in the rheumatic disease group and 100% in the control group. Average IgG titers were 242 in the rheumatic group and 388 in the control group (P < .0001). Seropositivity was 88% in those taking mycophenolate with another drug (100% with mycophenolate monotherapy), 90% with HCQ, 94% with any csDMARDs and another drug (100% with csDMARD monotherapy), and 100% for all other drugs. During 3 months’ follow-up after vaccination, there were no COVID-19 cases among the participants.
Dr. Heshin-Bekenstein noted that their results showed better immunogenicity in teens, compared with adults, for two specific drugs. Seropositivity in teens taking methotrexate (Rheumatrex, Trexall) or rituximab was 100% in this study, compared with 84% in adults taking methotrexate and 39% in adults taking rituximab in a previous study. However, only three patients in this study were taking rituximab, and only seven were taking methotrexate.
The study’s heterogenous population was both a strength and a weakness of the study. “Due to the diversity of rheumatic diseases and medications included in this cohort, it was not possible to draw significant conclusions regarding the impact of the immunomodulatory medications and type of disease” on titers, Dr. Heshin-Bekenstein told attendees.
Still, “I think as pediatric rheumatologists, we can feel reassured in recommending the COVID-19 vaccine to our patients,” Dr. Oliver said. “I will add that every patient is different, and everyone should have a conversation with their physician about receiving the COVID-19 vaccine.” Dr. Oliver said she discusses vaccination, including COVID vaccination, with every patient, and it’s been challenging to address concerns in the midst of so much misinformation circulating about the vaccine.
These findings do raise questions about whether it’s still necessary to hold immunomodulatory medications to get the vaccine,” Dr. Rutstein said.
“Many families are nervous to pause their medications before and after the vaccine as is currently recommended for many therapies by the American College of Rheumatology, and I do share that concern for some of my patients with more clinically unstable disease, so I try to work with each family to decide on best timing and have delayed or deferred the series until some patients are on a steady dose of a new immunomodulatory medication if it has been recently started,” Dr. Rutstein said. “This is one of the reasons why Dr. Heshin-Bekenstein’s study is so important – we may be holding medications that can be safely continued and even further decrease the risk of disease flare.”
None of the physicians have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CARRA 2022
Prior authorizations delay TNF inhibitors for children with JIA
Children with juvenile idiopathic arthritis (JIA) who need a tumor necrosis factor (TNF) inhibitor after failing conventional disease-modifying antirheumatic drug (DMARD) treatment often experience insurance delays before beginning the new drug because of prior authorization denials, according to research presented at the 2022 annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA). The findings were also published as a research letter in JAMA Network Open.
“Prompt escalation to TNF inhibitors is recommended for children with JIA refractory to DMARDs,” author Jordan Roberts, MD, a clinical fellow of the Harvard Medical School Rheumatology Program, Boston, told CARRA attendees. TNF inhibitors are increasingly used as first-line treatment in JIA since growing evidence suggests better outcomes from early treatment with biologics. “Prior authorization requirements that delay TNF inhibitor initiation among children with JIA are common in clinical practice,” Dr. Roberts said, but little evidence exists to understand the extent of this problem and its causes.
The researchers therefore conducted a retrospective cohort study using a search of electronic health records from January 2018 to December 2019 to find all children at a single center with a new diagnosis of nonsystemic JIA. Then the authors pulled the timing of prior authorization requests, approvals, denials, and first TNF inhibitor dose from the medical notes. They also sought out any children who had been recommended a TNF inhibitor but never started one.
The total population included 54 children with an average age of 10 years, about two-thirds of whom had private insurance (63%). The group was predominantly White (63%), although 13% declined to provide race, and 7% were Hispanic. Most subtypes of disease were represented: oligoarticular persistent (28%), oligoarticular extended (2%), polyarticular rheumatoid factor-negative (15%), polyarticular rheumatoid factor-positive (15%), psoriatic arthritis (26%), enthesitis-related arthritis (12%), and undifferentiated arthritis (2%).
The 44 participants with private insurance had an average of two joints with active disease, while the 10 patients with public insurance had an average of four involved joints. Nearly all the patients (91%) had previously taken or were currently taking DMARDs when the prior authorization was submitted, and 61% had received NSAIDs.
All but one of the patients’ insurance plans required a prior authorization. The first prior authorization was denied for about one-third of the public insurance patients (30%) and a quarter of the private insurance patients. About 1 in 5 patients overall (22%) required a written appeal to override the denial, and 4% required peer-to-peer review. Meanwhile, 7% of patients began another medication because of the denial.
It took a median of 3 days for prior authorizations to be approved and a median of 24 days from the time the TNF inhibitor was recommended to the patient receiving the first dose. However, 22% of patients waited at least 2 weeks before the prior authorization was approved, and more than a quarter of the requests took over 30 days before the patient could begin the medication. In the public insurance group in particular, a quarter of children waited at least 19 days for approval and at least 44 days before starting the medication.
In fact, when the researchers looked at the difference in approval time between those who did and did not receive an initial denial, the difference was stark. Median approval time was 16 days when the prior authorization was denied, compared with a median of 5 days when the first prior authorization was approved. Similarly, time to initiation of the drug after recommendation was a median of 35 days for those whose prior authorization was first denied and 17 days for those with an initial approval.
The most common reason for an initial denial was the insurance company requiring a different TNF inhibitor than the one the rheumatologist wanted to prescribe. “These were all children whose rheumatologist has recommended either infliximab or etanercept that were required to use adalimumab instead,” Dr. Roberts said.
The other reasons for initial denial were similarly familiar ones:
- Required submission to another insurer
- Additional documentation required
- Lack of medical necessity
- Prescription was for an indication not approved by the Food and Drug Administration
- Age of patient
- Nonbiologic DMARD required
- NSAID required for step therapy
Only three children who were advised to begin a TNF inhibitor did not do so, including one who was lost to follow-up, one who had injection-related anxiety, and one who had safety concerns about the medication.
“Several children were required to use alternative TNF inhibitors than the one that was recommended due to restricted formularies, which may reduce shared decisionmaking between physicians and families and may not be the optimal clinical choice for an individual child,” Dr. Roberts said in her conclusion. Most children, however, were able to get approval for the TNF inhibitor originally requested, “suggesting that utilization management strategies present barriers to timely care despite appropriate specialty medication requests,” she said. “Therefore, it’s important for us to advocate for access to medications for children with JIA.”
Findings are not surprising
“I have these same experiences at my institution – often insurance will dictate clinical practice, and step therapy is the only option, causing a delay to initiation of TNFi even if we think, as the pediatric rheumatologist, that a child needs this medicine to be initiated on presentation to our clinic,” Nayimisha Balmuri, MD, assistant professor of pediatrics in the division of allergy, immunology, and rheumatology at the Johns Hopkins School of Medicine, Baltimore, told this news organization.
Dr. Balmuri, who was not involved in the study, noted that in her clinic at Johns Hopkins, it is hit or miss if an appeal to insurance companies or to the state (if it is Medicaid coverage) will be successful. “Unfortunately, [we are] mostly unsuccessful, and we have to try another DMARD for 8 to 12 weeks first before trying to get TNFi,” she said.
Dr. Balmuri called for bringing these issues to the attention of state and federal legislators. “It’s so important for us to continue to advocate for our patients at the state and national level! We are the advocates for our patients, and we are uniquely trained to know the best medications to initiate to help patients maximize their chance to reach remission of arthritis. Insurance companies need to hear our voices!”
Dr. Roberts reported grants from CARRA, the Lupus Foundation of America, and the National Institute of Allergy and Infectious Diseases during the conduct of the study.
A version of this article first appeared on Medscape.com.
Children with juvenile idiopathic arthritis (JIA) who need a tumor necrosis factor (TNF) inhibitor after failing conventional disease-modifying antirheumatic drug (DMARD) treatment often experience insurance delays before beginning the new drug because of prior authorization denials, according to research presented at the 2022 annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA). The findings were also published as a research letter in JAMA Network Open.
“Prompt escalation to TNF inhibitors is recommended for children with JIA refractory to DMARDs,” author Jordan Roberts, MD, a clinical fellow of the Harvard Medical School Rheumatology Program, Boston, told CARRA attendees. TNF inhibitors are increasingly used as first-line treatment in JIA since growing evidence suggests better outcomes from early treatment with biologics. “Prior authorization requirements that delay TNF inhibitor initiation among children with JIA are common in clinical practice,” Dr. Roberts said, but little evidence exists to understand the extent of this problem and its causes.
The researchers therefore conducted a retrospective cohort study using a search of electronic health records from January 2018 to December 2019 to find all children at a single center with a new diagnosis of nonsystemic JIA. Then the authors pulled the timing of prior authorization requests, approvals, denials, and first TNF inhibitor dose from the medical notes. They also sought out any children who had been recommended a TNF inhibitor but never started one.
The total population included 54 children with an average age of 10 years, about two-thirds of whom had private insurance (63%). The group was predominantly White (63%), although 13% declined to provide race, and 7% were Hispanic. Most subtypes of disease were represented: oligoarticular persistent (28%), oligoarticular extended (2%), polyarticular rheumatoid factor-negative (15%), polyarticular rheumatoid factor-positive (15%), psoriatic arthritis (26%), enthesitis-related arthritis (12%), and undifferentiated arthritis (2%).
The 44 participants with private insurance had an average of two joints with active disease, while the 10 patients with public insurance had an average of four involved joints. Nearly all the patients (91%) had previously taken or were currently taking DMARDs when the prior authorization was submitted, and 61% had received NSAIDs.
All but one of the patients’ insurance plans required a prior authorization. The first prior authorization was denied for about one-third of the public insurance patients (30%) and a quarter of the private insurance patients. About 1 in 5 patients overall (22%) required a written appeal to override the denial, and 4% required peer-to-peer review. Meanwhile, 7% of patients began another medication because of the denial.
It took a median of 3 days for prior authorizations to be approved and a median of 24 days from the time the TNF inhibitor was recommended to the patient receiving the first dose. However, 22% of patients waited at least 2 weeks before the prior authorization was approved, and more than a quarter of the requests took over 30 days before the patient could begin the medication. In the public insurance group in particular, a quarter of children waited at least 19 days for approval and at least 44 days before starting the medication.
In fact, when the researchers looked at the difference in approval time between those who did and did not receive an initial denial, the difference was stark. Median approval time was 16 days when the prior authorization was denied, compared with a median of 5 days when the first prior authorization was approved. Similarly, time to initiation of the drug after recommendation was a median of 35 days for those whose prior authorization was first denied and 17 days for those with an initial approval.
The most common reason for an initial denial was the insurance company requiring a different TNF inhibitor than the one the rheumatologist wanted to prescribe. “These were all children whose rheumatologist has recommended either infliximab or etanercept that were required to use adalimumab instead,” Dr. Roberts said.
The other reasons for initial denial were similarly familiar ones:
- Required submission to another insurer
- Additional documentation required
- Lack of medical necessity
- Prescription was for an indication not approved by the Food and Drug Administration
- Age of patient
- Nonbiologic DMARD required
- NSAID required for step therapy
Only three children who were advised to begin a TNF inhibitor did not do so, including one who was lost to follow-up, one who had injection-related anxiety, and one who had safety concerns about the medication.
“Several children were required to use alternative TNF inhibitors than the one that was recommended due to restricted formularies, which may reduce shared decisionmaking between physicians and families and may not be the optimal clinical choice for an individual child,” Dr. Roberts said in her conclusion. Most children, however, were able to get approval for the TNF inhibitor originally requested, “suggesting that utilization management strategies present barriers to timely care despite appropriate specialty medication requests,” she said. “Therefore, it’s important for us to advocate for access to medications for children with JIA.”
Findings are not surprising
“I have these same experiences at my institution – often insurance will dictate clinical practice, and step therapy is the only option, causing a delay to initiation of TNFi even if we think, as the pediatric rheumatologist, that a child needs this medicine to be initiated on presentation to our clinic,” Nayimisha Balmuri, MD, assistant professor of pediatrics in the division of allergy, immunology, and rheumatology at the Johns Hopkins School of Medicine, Baltimore, told this news organization.
Dr. Balmuri, who was not involved in the study, noted that in her clinic at Johns Hopkins, it is hit or miss if an appeal to insurance companies or to the state (if it is Medicaid coverage) will be successful. “Unfortunately, [we are] mostly unsuccessful, and we have to try another DMARD for 8 to 12 weeks first before trying to get TNFi,” she said.
Dr. Balmuri called for bringing these issues to the attention of state and federal legislators. “It’s so important for us to continue to advocate for our patients at the state and national level! We are the advocates for our patients, and we are uniquely trained to know the best medications to initiate to help patients maximize their chance to reach remission of arthritis. Insurance companies need to hear our voices!”
Dr. Roberts reported grants from CARRA, the Lupus Foundation of America, and the National Institute of Allergy and Infectious Diseases during the conduct of the study.
A version of this article first appeared on Medscape.com.
Children with juvenile idiopathic arthritis (JIA) who need a tumor necrosis factor (TNF) inhibitor after failing conventional disease-modifying antirheumatic drug (DMARD) treatment often experience insurance delays before beginning the new drug because of prior authorization denials, according to research presented at the 2022 annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA). The findings were also published as a research letter in JAMA Network Open.
“Prompt escalation to TNF inhibitors is recommended for children with JIA refractory to DMARDs,” author Jordan Roberts, MD, a clinical fellow of the Harvard Medical School Rheumatology Program, Boston, told CARRA attendees. TNF inhibitors are increasingly used as first-line treatment in JIA since growing evidence suggests better outcomes from early treatment with biologics. “Prior authorization requirements that delay TNF inhibitor initiation among children with JIA are common in clinical practice,” Dr. Roberts said, but little evidence exists to understand the extent of this problem and its causes.
The researchers therefore conducted a retrospective cohort study using a search of electronic health records from January 2018 to December 2019 to find all children at a single center with a new diagnosis of nonsystemic JIA. Then the authors pulled the timing of prior authorization requests, approvals, denials, and first TNF inhibitor dose from the medical notes. They also sought out any children who had been recommended a TNF inhibitor but never started one.
The total population included 54 children with an average age of 10 years, about two-thirds of whom had private insurance (63%). The group was predominantly White (63%), although 13% declined to provide race, and 7% were Hispanic. Most subtypes of disease were represented: oligoarticular persistent (28%), oligoarticular extended (2%), polyarticular rheumatoid factor-negative (15%), polyarticular rheumatoid factor-positive (15%), psoriatic arthritis (26%), enthesitis-related arthritis (12%), and undifferentiated arthritis (2%).
The 44 participants with private insurance had an average of two joints with active disease, while the 10 patients with public insurance had an average of four involved joints. Nearly all the patients (91%) had previously taken or were currently taking DMARDs when the prior authorization was submitted, and 61% had received NSAIDs.
All but one of the patients’ insurance plans required a prior authorization. The first prior authorization was denied for about one-third of the public insurance patients (30%) and a quarter of the private insurance patients. About 1 in 5 patients overall (22%) required a written appeal to override the denial, and 4% required peer-to-peer review. Meanwhile, 7% of patients began another medication because of the denial.
It took a median of 3 days for prior authorizations to be approved and a median of 24 days from the time the TNF inhibitor was recommended to the patient receiving the first dose. However, 22% of patients waited at least 2 weeks before the prior authorization was approved, and more than a quarter of the requests took over 30 days before the patient could begin the medication. In the public insurance group in particular, a quarter of children waited at least 19 days for approval and at least 44 days before starting the medication.
In fact, when the researchers looked at the difference in approval time between those who did and did not receive an initial denial, the difference was stark. Median approval time was 16 days when the prior authorization was denied, compared with a median of 5 days when the first prior authorization was approved. Similarly, time to initiation of the drug after recommendation was a median of 35 days for those whose prior authorization was first denied and 17 days for those with an initial approval.
The most common reason for an initial denial was the insurance company requiring a different TNF inhibitor than the one the rheumatologist wanted to prescribe. “These were all children whose rheumatologist has recommended either infliximab or etanercept that were required to use adalimumab instead,” Dr. Roberts said.
The other reasons for initial denial were similarly familiar ones:
- Required submission to another insurer
- Additional documentation required
- Lack of medical necessity
- Prescription was for an indication not approved by the Food and Drug Administration
- Age of patient
- Nonbiologic DMARD required
- NSAID required for step therapy
Only three children who were advised to begin a TNF inhibitor did not do so, including one who was lost to follow-up, one who had injection-related anxiety, and one who had safety concerns about the medication.
“Several children were required to use alternative TNF inhibitors than the one that was recommended due to restricted formularies, which may reduce shared decisionmaking between physicians and families and may not be the optimal clinical choice for an individual child,” Dr. Roberts said in her conclusion. Most children, however, were able to get approval for the TNF inhibitor originally requested, “suggesting that utilization management strategies present barriers to timely care despite appropriate specialty medication requests,” she said. “Therefore, it’s important for us to advocate for access to medications for children with JIA.”
Findings are not surprising
“I have these same experiences at my institution – often insurance will dictate clinical practice, and step therapy is the only option, causing a delay to initiation of TNFi even if we think, as the pediatric rheumatologist, that a child needs this medicine to be initiated on presentation to our clinic,” Nayimisha Balmuri, MD, assistant professor of pediatrics in the division of allergy, immunology, and rheumatology at the Johns Hopkins School of Medicine, Baltimore, told this news organization.
Dr. Balmuri, who was not involved in the study, noted that in her clinic at Johns Hopkins, it is hit or miss if an appeal to insurance companies or to the state (if it is Medicaid coverage) will be successful. “Unfortunately, [we are] mostly unsuccessful, and we have to try another DMARD for 8 to 12 weeks first before trying to get TNFi,” she said.
Dr. Balmuri called for bringing these issues to the attention of state and federal legislators. “It’s so important for us to continue to advocate for our patients at the state and national level! We are the advocates for our patients, and we are uniquely trained to know the best medications to initiate to help patients maximize their chance to reach remission of arthritis. Insurance companies need to hear our voices!”
Dr. Roberts reported grants from CARRA, the Lupus Foundation of America, and the National Institute of Allergy and Infectious Diseases during the conduct of the study.
A version of this article first appeared on Medscape.com.




