User login
Out-of-state telehealth visits could help more patients if restrictions eased: Study
About 5% of traditional Medicare patients who had telehealth visits were seen virtually by out-of-state clinicians in the first half of 2021, according to a new study in JAMA Health Forum.
Since then, however, many states have restored restrictions that prevent physicians who are licensed in one state from having telehealth visits with patients unless they’re licensed in the state where the patients live.
This is not fair to many people who live in areas near state borders, the authors argued. For those patients, it is much more convenient to see their primary care physician in a virtual visit from home than to travel to the doctor’s office in another state. This convenience is enjoyed by most patients who reside elsewhere in their state because they’re seeing physicians who are licensed there.
Moreover, the paper said, patients who live in rural areas and in counties with relatively few physicians per capita would also benefit from relaxed telemedicine restrictions.
Using Medicare claims data, the researchers examined the characteristics of out-of-state (OOS) telemedicine visits for the 6 months from January to June 2021. They chose that period for two reasons: by then, health care had stabilized after the chaotic early phase of the pandemic, and in most states, the relaxation of licensing rules for OOS telehealth had not yet lapsed. Earlier periods of time were also used for certain types of comparisons.
Among fee-for-service Medicare beneficiaries, the number of OOS telemedicine visits peaked at 451,086 in April 2020 and slowly fell to 175,545 in June 2021, according to the study. The fraction of OOS telehealth visits among all virtual visits was 4.5% in April 2020 and increased to 5.6% by June 2021.
Staying close to home
Of all beneficiaries with a telemedicine visit in the study period, 33% lived within 15 miles of a state border. That cohort accounted for 57.2% of all OOS telemedicine visits.
The highest rates of OOS telehealth visits were seen in the District of Columbia (38.5%), Wyoming (25.6%), and North Dakota (21.1%). California (1%), Texas (2%), and Massachusetts (2.1%) had the lowest rates.
Though intuitive in retrospect, the correlation of OOS telemedicine use with proximity to state borders was one of the study’s most important findings, lead author Ateev Mehrotra, MD, a professor at Harvard Medical School, Boston, said in an interview. “It makes sense,” he said. “If you’re in D.C. and you need a cardiologist, you don’t think: ‘I’ll stay in D.C.’ No, Maryland is right there, so you might use a Maryland cardiologist. Now you’re out of state, even though that office might be only half a mile away from you.”
Similar dynamics, he noted, are seen in many metropolitan areas that border on other states, such as Cincinnati; Philadelphia; and Portland, Ore.
This finding lines up with another result of the study: The majority of patients who had OOS telemedicine visits had previously seen in person the doctor who conducted the virtual visit.
Across all OOS telemedicine visits in the first half of 2021, the researchers observed a prior in-person visit between March 2019 and the date of the virtual visit with the same patient and the same clinician in 62.8% of those visits. Across all in-state telehealth visits, 75.8% of them were made by patients who had seen the same clinician in person since March 2019. This preponderance of virtual visits to clinicians whom the patients had already seen in person reflects the fact that, during the pandemic, most physicians began conducting telehealth visits with their own patients, Dr. Mehrotra said.
It also lays to rest the concern that some states have had about allowing OOS telemedicine visits to physicians not licensed in those states, he added. “They think that all these docs from far away are going to start taking care of patients they don’t even know. But our study shows that isn’t the case. Most of the time, doctors are seeing a patient who’s switching over from in-person visits to out-of-state telemedicine.”
More specialty care sought
The dominant conditions that patients presented with were the same in OOS telemedicine and within-state virtual visits. However, the use of OOS telemedicine was higher for some types of specialized care.
For example, the rate of OOS telemedicine use, compared with all telemedicine use, was highest for cancer care (9.8%). Drilling down to more specific conditions, the top three in OOS telemedicine visits were assessment of organ transplant (13%); male reproductive cancers, such as prostate cancer (11.3%); and graft-related issues (10.2%).
The specialty trend was also evident in the types of OOS clinicians from whom Medicare patients sought virtual care. The rates of OOS telemedicine use as a percentage of all telemedicine use in particular specialties were highest for uncommon specialties, such as hematology/oncology, rheumatology, urology, medical oncology, and orthopedic surgery (8.5%). There was less use of OOS telemedicine as a percentage of all telemedicine among more common medical specialties (6.4%), mental health specialties (4.4%), and primary care (4.4%).
Despite its relatively low showing in this category, however, behavioral health was the leading condition treated in both within-state and OOS telemedicine visits, accounting for 30.7% and 25.8%, respectively, of those encounters.
States backslide on OOS telehealth
Since the end of the study period, over half of the states have restored some or all of the restrictions on OOS telemedicine that they had lifted during the pandemic.
According to Dr. Mehrotra, 22 states have some kind of regulation in place to allow an OOS clinician to conduct telehealth visits without being licensed in the state. This varies all the way from complete reciprocity with other states’ licenses to “emergency” telemedicine licenses. The other 28 states and Washington, D.C., require an OOS telemedicine practitioner to get a state license.
Various proposals have been floated to ameliorate this situation, the JAMA paper noted. These proposals include an expansion of the Interstate Medical Licensure Compact that the Federation of State Medical Boards organized in 2014. Since the pact became effective in 2014, at least 35 states and the District of Columbia have joined it. Those states have made it simpler for physicians to gain licensure in states other than their original state of licensure. However, Mehrotra said, it’s still not easy, and not many physicians have taken advantage of it.
One new wrinkle has emerged in this policy debate as a result of the Supreme Court decision overturning Roe v. Wade, he noted. Because people are using OOS telemedicine visits to get prescriptions to abort their fetuses, “that has changed the enthusiasm level for it among many states,” he said.
Dr. Mehrotra reported personal fees from the Pew Charitable Trust, Sanofi Pasteur, and Black Opal Ventures outside the submitted work. One coauthor reported receiving grants from Patient-Centered Outcomes Research, National Institute on Aging, Roundtrip, Independence Blue Cross; personal fees or salary from RAND Corporation from Verily Life Sciences; and that the American Telemedicine Association covered a conference fee. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
About 5% of traditional Medicare patients who had telehealth visits were seen virtually by out-of-state clinicians in the first half of 2021, according to a new study in JAMA Health Forum.
Since then, however, many states have restored restrictions that prevent physicians who are licensed in one state from having telehealth visits with patients unless they’re licensed in the state where the patients live.
This is not fair to many people who live in areas near state borders, the authors argued. For those patients, it is much more convenient to see their primary care physician in a virtual visit from home than to travel to the doctor’s office in another state. This convenience is enjoyed by most patients who reside elsewhere in their state because they’re seeing physicians who are licensed there.
Moreover, the paper said, patients who live in rural areas and in counties with relatively few physicians per capita would also benefit from relaxed telemedicine restrictions.
Using Medicare claims data, the researchers examined the characteristics of out-of-state (OOS) telemedicine visits for the 6 months from January to June 2021. They chose that period for two reasons: by then, health care had stabilized after the chaotic early phase of the pandemic, and in most states, the relaxation of licensing rules for OOS telehealth had not yet lapsed. Earlier periods of time were also used for certain types of comparisons.
Among fee-for-service Medicare beneficiaries, the number of OOS telemedicine visits peaked at 451,086 in April 2020 and slowly fell to 175,545 in June 2021, according to the study. The fraction of OOS telehealth visits among all virtual visits was 4.5% in April 2020 and increased to 5.6% by June 2021.
Staying close to home
Of all beneficiaries with a telemedicine visit in the study period, 33% lived within 15 miles of a state border. That cohort accounted for 57.2% of all OOS telemedicine visits.
The highest rates of OOS telehealth visits were seen in the District of Columbia (38.5%), Wyoming (25.6%), and North Dakota (21.1%). California (1%), Texas (2%), and Massachusetts (2.1%) had the lowest rates.
Though intuitive in retrospect, the correlation of OOS telemedicine use with proximity to state borders was one of the study’s most important findings, lead author Ateev Mehrotra, MD, a professor at Harvard Medical School, Boston, said in an interview. “It makes sense,” he said. “If you’re in D.C. and you need a cardiologist, you don’t think: ‘I’ll stay in D.C.’ No, Maryland is right there, so you might use a Maryland cardiologist. Now you’re out of state, even though that office might be only half a mile away from you.”
Similar dynamics, he noted, are seen in many metropolitan areas that border on other states, such as Cincinnati; Philadelphia; and Portland, Ore.
This finding lines up with another result of the study: The majority of patients who had OOS telemedicine visits had previously seen in person the doctor who conducted the virtual visit.
Across all OOS telemedicine visits in the first half of 2021, the researchers observed a prior in-person visit between March 2019 and the date of the virtual visit with the same patient and the same clinician in 62.8% of those visits. Across all in-state telehealth visits, 75.8% of them were made by patients who had seen the same clinician in person since March 2019. This preponderance of virtual visits to clinicians whom the patients had already seen in person reflects the fact that, during the pandemic, most physicians began conducting telehealth visits with their own patients, Dr. Mehrotra said.
It also lays to rest the concern that some states have had about allowing OOS telemedicine visits to physicians not licensed in those states, he added. “They think that all these docs from far away are going to start taking care of patients they don’t even know. But our study shows that isn’t the case. Most of the time, doctors are seeing a patient who’s switching over from in-person visits to out-of-state telemedicine.”
More specialty care sought
The dominant conditions that patients presented with were the same in OOS telemedicine and within-state virtual visits. However, the use of OOS telemedicine was higher for some types of specialized care.
For example, the rate of OOS telemedicine use, compared with all telemedicine use, was highest for cancer care (9.8%). Drilling down to more specific conditions, the top three in OOS telemedicine visits were assessment of organ transplant (13%); male reproductive cancers, such as prostate cancer (11.3%); and graft-related issues (10.2%).
The specialty trend was also evident in the types of OOS clinicians from whom Medicare patients sought virtual care. The rates of OOS telemedicine use as a percentage of all telemedicine use in particular specialties were highest for uncommon specialties, such as hematology/oncology, rheumatology, urology, medical oncology, and orthopedic surgery (8.5%). There was less use of OOS telemedicine as a percentage of all telemedicine among more common medical specialties (6.4%), mental health specialties (4.4%), and primary care (4.4%).
Despite its relatively low showing in this category, however, behavioral health was the leading condition treated in both within-state and OOS telemedicine visits, accounting for 30.7% and 25.8%, respectively, of those encounters.
States backslide on OOS telehealth
Since the end of the study period, over half of the states have restored some or all of the restrictions on OOS telemedicine that they had lifted during the pandemic.
According to Dr. Mehrotra, 22 states have some kind of regulation in place to allow an OOS clinician to conduct telehealth visits without being licensed in the state. This varies all the way from complete reciprocity with other states’ licenses to “emergency” telemedicine licenses. The other 28 states and Washington, D.C., require an OOS telemedicine practitioner to get a state license.
Various proposals have been floated to ameliorate this situation, the JAMA paper noted. These proposals include an expansion of the Interstate Medical Licensure Compact that the Federation of State Medical Boards organized in 2014. Since the pact became effective in 2014, at least 35 states and the District of Columbia have joined it. Those states have made it simpler for physicians to gain licensure in states other than their original state of licensure. However, Mehrotra said, it’s still not easy, and not many physicians have taken advantage of it.
One new wrinkle has emerged in this policy debate as a result of the Supreme Court decision overturning Roe v. Wade, he noted. Because people are using OOS telemedicine visits to get prescriptions to abort their fetuses, “that has changed the enthusiasm level for it among many states,” he said.
Dr. Mehrotra reported personal fees from the Pew Charitable Trust, Sanofi Pasteur, and Black Opal Ventures outside the submitted work. One coauthor reported receiving grants from Patient-Centered Outcomes Research, National Institute on Aging, Roundtrip, Independence Blue Cross; personal fees or salary from RAND Corporation from Verily Life Sciences; and that the American Telemedicine Association covered a conference fee. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
About 5% of traditional Medicare patients who had telehealth visits were seen virtually by out-of-state clinicians in the first half of 2021, according to a new study in JAMA Health Forum.
Since then, however, many states have restored restrictions that prevent physicians who are licensed in one state from having telehealth visits with patients unless they’re licensed in the state where the patients live.
This is not fair to many people who live in areas near state borders, the authors argued. For those patients, it is much more convenient to see their primary care physician in a virtual visit from home than to travel to the doctor’s office in another state. This convenience is enjoyed by most patients who reside elsewhere in their state because they’re seeing physicians who are licensed there.
Moreover, the paper said, patients who live in rural areas and in counties with relatively few physicians per capita would also benefit from relaxed telemedicine restrictions.
Using Medicare claims data, the researchers examined the characteristics of out-of-state (OOS) telemedicine visits for the 6 months from January to June 2021. They chose that period for two reasons: by then, health care had stabilized after the chaotic early phase of the pandemic, and in most states, the relaxation of licensing rules for OOS telehealth had not yet lapsed. Earlier periods of time were also used for certain types of comparisons.
Among fee-for-service Medicare beneficiaries, the number of OOS telemedicine visits peaked at 451,086 in April 2020 and slowly fell to 175,545 in June 2021, according to the study. The fraction of OOS telehealth visits among all virtual visits was 4.5% in April 2020 and increased to 5.6% by June 2021.
Staying close to home
Of all beneficiaries with a telemedicine visit in the study period, 33% lived within 15 miles of a state border. That cohort accounted for 57.2% of all OOS telemedicine visits.
The highest rates of OOS telehealth visits were seen in the District of Columbia (38.5%), Wyoming (25.6%), and North Dakota (21.1%). California (1%), Texas (2%), and Massachusetts (2.1%) had the lowest rates.
Though intuitive in retrospect, the correlation of OOS telemedicine use with proximity to state borders was one of the study’s most important findings, lead author Ateev Mehrotra, MD, a professor at Harvard Medical School, Boston, said in an interview. “It makes sense,” he said. “If you’re in D.C. and you need a cardiologist, you don’t think: ‘I’ll stay in D.C.’ No, Maryland is right there, so you might use a Maryland cardiologist. Now you’re out of state, even though that office might be only half a mile away from you.”
Similar dynamics, he noted, are seen in many metropolitan areas that border on other states, such as Cincinnati; Philadelphia; and Portland, Ore.
This finding lines up with another result of the study: The majority of patients who had OOS telemedicine visits had previously seen in person the doctor who conducted the virtual visit.
Across all OOS telemedicine visits in the first half of 2021, the researchers observed a prior in-person visit between March 2019 and the date of the virtual visit with the same patient and the same clinician in 62.8% of those visits. Across all in-state telehealth visits, 75.8% of them were made by patients who had seen the same clinician in person since March 2019. This preponderance of virtual visits to clinicians whom the patients had already seen in person reflects the fact that, during the pandemic, most physicians began conducting telehealth visits with their own patients, Dr. Mehrotra said.
It also lays to rest the concern that some states have had about allowing OOS telemedicine visits to physicians not licensed in those states, he added. “They think that all these docs from far away are going to start taking care of patients they don’t even know. But our study shows that isn’t the case. Most of the time, doctors are seeing a patient who’s switching over from in-person visits to out-of-state telemedicine.”
More specialty care sought
The dominant conditions that patients presented with were the same in OOS telemedicine and within-state virtual visits. However, the use of OOS telemedicine was higher for some types of specialized care.
For example, the rate of OOS telemedicine use, compared with all telemedicine use, was highest for cancer care (9.8%). Drilling down to more specific conditions, the top three in OOS telemedicine visits were assessment of organ transplant (13%); male reproductive cancers, such as prostate cancer (11.3%); and graft-related issues (10.2%).
The specialty trend was also evident in the types of OOS clinicians from whom Medicare patients sought virtual care. The rates of OOS telemedicine use as a percentage of all telemedicine use in particular specialties were highest for uncommon specialties, such as hematology/oncology, rheumatology, urology, medical oncology, and orthopedic surgery (8.5%). There was less use of OOS telemedicine as a percentage of all telemedicine among more common medical specialties (6.4%), mental health specialties (4.4%), and primary care (4.4%).
Despite its relatively low showing in this category, however, behavioral health was the leading condition treated in both within-state and OOS telemedicine visits, accounting for 30.7% and 25.8%, respectively, of those encounters.
States backslide on OOS telehealth
Since the end of the study period, over half of the states have restored some or all of the restrictions on OOS telemedicine that they had lifted during the pandemic.
According to Dr. Mehrotra, 22 states have some kind of regulation in place to allow an OOS clinician to conduct telehealth visits without being licensed in the state. This varies all the way from complete reciprocity with other states’ licenses to “emergency” telemedicine licenses. The other 28 states and Washington, D.C., require an OOS telemedicine practitioner to get a state license.
Various proposals have been floated to ameliorate this situation, the JAMA paper noted. These proposals include an expansion of the Interstate Medical Licensure Compact that the Federation of State Medical Boards organized in 2014. Since the pact became effective in 2014, at least 35 states and the District of Columbia have joined it. Those states have made it simpler for physicians to gain licensure in states other than their original state of licensure. However, Mehrotra said, it’s still not easy, and not many physicians have taken advantage of it.
One new wrinkle has emerged in this policy debate as a result of the Supreme Court decision overturning Roe v. Wade, he noted. Because people are using OOS telemedicine visits to get prescriptions to abort their fetuses, “that has changed the enthusiasm level for it among many states,” he said.
Dr. Mehrotra reported personal fees from the Pew Charitable Trust, Sanofi Pasteur, and Black Opal Ventures outside the submitted work. One coauthor reported receiving grants from Patient-Centered Outcomes Research, National Institute on Aging, Roundtrip, Independence Blue Cross; personal fees or salary from RAND Corporation from Verily Life Sciences; and that the American Telemedicine Association covered a conference fee. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA HEALTH FORUM
Despite benefits, extended-interval pembro uptake remains low
In April 2020, the Food and Drug Administration approved extended dosing for standalone pembrolizumab – 400 mg every 6 weeks instead of the standard dosing of 200 mg every 3 weeks. The shift came, in part, to reduce patient health care encounters during the early days of the COVID-19 pandemic, but also because fewer infusions save patients time and out-of-pocket costs and reduce the burden on the health care system.
The FDA deemed this move safe after pharmacologic studies and a small melanoma study found that responses and adverse events were equivalent in comparison with standard dosing.
Given the benefits, one would expect “brisk adoption” of extended-interval dosing, Garth Strohbehn, MD, an oncologist at the VA Medical Center in Ann Arbor, Mich., and colleagues wrote in a recent report in JAMA Oncology.
However, when the team reviewed data on 835 veterans from the Veterans Health Administration who began taking single-agent pembrolizumab between April 1, 2020, and July 1, 2021, only about one-third received extended-interval dosing.
Between April and January 2021, use of extended-interval dosing rose steadily to about 35% of patients but then hovered in that range through August 2021.
Among the patients, age, sex, Charlson comorbidity index, and pembrolizumab indications were well balanced between the standard-dosing and the extended-interval dosing groups.
Notably, Dr. Strohbehn and colleagues also found no difference in time-to-treatment discontinuation between patients receiving extended dosing in comparison with patients receiving standard dosing, which is “a real-world measure of clinical effectiveness,” the team said.
And there was no difference in immune-related side effects between the two regimens, as assessed by incident levothyroxine and prednisone prescriptions.
The real-world near equivalence of extended and standard dosing intervals that was demonstrated in the study is “reassuring” and helps make the case for considering it “as a best practice” for single-agent pembrolizumab, the investigators wrote.
Dr. Strohbehn remained somewhat puzzled by the low uptake of the extended-dosing option.
“I was frankly surprised by the small number of patients who received the extended-interval regimen,” Dr. Strohbehn said in an interview.
“Admittedly, there are patients who would prefer to receive standard-interval therapy, and that preference should of course be accommodated whenever possible, but in my experience, those numbers are small,” at least in the VA system, he noted.
In addition, the authors noted, there is no direct financial incentive for more frequent dosing in the VA system.
It’s possible that low uptake could stem from clinicians’ doubts about switching to an extended-interval dose, given that the FDA’s approval was based largely on a study of 44 patients with melanoma in a single-arm trial.
If that is indeed the case, the new findings – which represent the first health system–level, real-world comparative effectiveness data for standard vs. extended-interval pembrolizumab – should help address these concerns, the team said.
“This observational dataset lends further credence to [the dosing] regimens being clinically equivalent,” said Zachery Reichert, MD, PhD, a urologic oncologist at the University of Michigan, Ann Arbor, who was not involved in the study.
To address the issue, Dr. Strohbehn and his team suggested “clinical guideline promotion to overcome some of the barriers to the adoption of extended-interval pembrolizumab.”
Dr. Riechert suggested further validation of equivalent outcomes for the two regimens, more advocacy to encourage patients to ask about the 6-week option, as well as incentives from insurers to adopt it.
Dr. Strohbehn added that the situation highlights a broader issue in oncology, namely that many drugs “end up on the market with dosing regimens that haven’t necessarily been optimized.”
Across the world, investigators are conducting clinical trials “to identify the minimum dosages, frequencies, and durations patients need in order to achieve their best outcome,” Dr. Strohbehn said. In oncology, much of this effort is being led by Project Optimus, from the FDA’s Oncology Center of Excellence, he said.
The study was funded by the VA National Oncology Program. Dr. Reichert and Dr. Strohbehn have disclosed no relevant financial relationships. One investigator has received grants from Novartis, Bristol-Myers Squibb, Regeneron, and Genentech.
A version of this article first appeared on Medscape.com.
In April 2020, the Food and Drug Administration approved extended dosing for standalone pembrolizumab – 400 mg every 6 weeks instead of the standard dosing of 200 mg every 3 weeks. The shift came, in part, to reduce patient health care encounters during the early days of the COVID-19 pandemic, but also because fewer infusions save patients time and out-of-pocket costs and reduce the burden on the health care system.
The FDA deemed this move safe after pharmacologic studies and a small melanoma study found that responses and adverse events were equivalent in comparison with standard dosing.
Given the benefits, one would expect “brisk adoption” of extended-interval dosing, Garth Strohbehn, MD, an oncologist at the VA Medical Center in Ann Arbor, Mich., and colleagues wrote in a recent report in JAMA Oncology.
However, when the team reviewed data on 835 veterans from the Veterans Health Administration who began taking single-agent pembrolizumab between April 1, 2020, and July 1, 2021, only about one-third received extended-interval dosing.
Between April and January 2021, use of extended-interval dosing rose steadily to about 35% of patients but then hovered in that range through August 2021.
Among the patients, age, sex, Charlson comorbidity index, and pembrolizumab indications were well balanced between the standard-dosing and the extended-interval dosing groups.
Notably, Dr. Strohbehn and colleagues also found no difference in time-to-treatment discontinuation between patients receiving extended dosing in comparison with patients receiving standard dosing, which is “a real-world measure of clinical effectiveness,” the team said.
And there was no difference in immune-related side effects between the two regimens, as assessed by incident levothyroxine and prednisone prescriptions.
The real-world near equivalence of extended and standard dosing intervals that was demonstrated in the study is “reassuring” and helps make the case for considering it “as a best practice” for single-agent pembrolizumab, the investigators wrote.
Dr. Strohbehn remained somewhat puzzled by the low uptake of the extended-dosing option.
“I was frankly surprised by the small number of patients who received the extended-interval regimen,” Dr. Strohbehn said in an interview.
“Admittedly, there are patients who would prefer to receive standard-interval therapy, and that preference should of course be accommodated whenever possible, but in my experience, those numbers are small,” at least in the VA system, he noted.
In addition, the authors noted, there is no direct financial incentive for more frequent dosing in the VA system.
It’s possible that low uptake could stem from clinicians’ doubts about switching to an extended-interval dose, given that the FDA’s approval was based largely on a study of 44 patients with melanoma in a single-arm trial.
If that is indeed the case, the new findings – which represent the first health system–level, real-world comparative effectiveness data for standard vs. extended-interval pembrolizumab – should help address these concerns, the team said.
“This observational dataset lends further credence to [the dosing] regimens being clinically equivalent,” said Zachery Reichert, MD, PhD, a urologic oncologist at the University of Michigan, Ann Arbor, who was not involved in the study.
To address the issue, Dr. Strohbehn and his team suggested “clinical guideline promotion to overcome some of the barriers to the adoption of extended-interval pembrolizumab.”
Dr. Riechert suggested further validation of equivalent outcomes for the two regimens, more advocacy to encourage patients to ask about the 6-week option, as well as incentives from insurers to adopt it.
Dr. Strohbehn added that the situation highlights a broader issue in oncology, namely that many drugs “end up on the market with dosing regimens that haven’t necessarily been optimized.”
Across the world, investigators are conducting clinical trials “to identify the minimum dosages, frequencies, and durations patients need in order to achieve their best outcome,” Dr. Strohbehn said. In oncology, much of this effort is being led by Project Optimus, from the FDA’s Oncology Center of Excellence, he said.
The study was funded by the VA National Oncology Program. Dr. Reichert and Dr. Strohbehn have disclosed no relevant financial relationships. One investigator has received grants from Novartis, Bristol-Myers Squibb, Regeneron, and Genentech.
A version of this article first appeared on Medscape.com.
In April 2020, the Food and Drug Administration approved extended dosing for standalone pembrolizumab – 400 mg every 6 weeks instead of the standard dosing of 200 mg every 3 weeks. The shift came, in part, to reduce patient health care encounters during the early days of the COVID-19 pandemic, but also because fewer infusions save patients time and out-of-pocket costs and reduce the burden on the health care system.
The FDA deemed this move safe after pharmacologic studies and a small melanoma study found that responses and adverse events were equivalent in comparison with standard dosing.
Given the benefits, one would expect “brisk adoption” of extended-interval dosing, Garth Strohbehn, MD, an oncologist at the VA Medical Center in Ann Arbor, Mich., and colleagues wrote in a recent report in JAMA Oncology.
However, when the team reviewed data on 835 veterans from the Veterans Health Administration who began taking single-agent pembrolizumab between April 1, 2020, and July 1, 2021, only about one-third received extended-interval dosing.
Between April and January 2021, use of extended-interval dosing rose steadily to about 35% of patients but then hovered in that range through August 2021.
Among the patients, age, sex, Charlson comorbidity index, and pembrolizumab indications were well balanced between the standard-dosing and the extended-interval dosing groups.
Notably, Dr. Strohbehn and colleagues also found no difference in time-to-treatment discontinuation between patients receiving extended dosing in comparison with patients receiving standard dosing, which is “a real-world measure of clinical effectiveness,” the team said.
And there was no difference in immune-related side effects between the two regimens, as assessed by incident levothyroxine and prednisone prescriptions.
The real-world near equivalence of extended and standard dosing intervals that was demonstrated in the study is “reassuring” and helps make the case for considering it “as a best practice” for single-agent pembrolizumab, the investigators wrote.
Dr. Strohbehn remained somewhat puzzled by the low uptake of the extended-dosing option.
“I was frankly surprised by the small number of patients who received the extended-interval regimen,” Dr. Strohbehn said in an interview.
“Admittedly, there are patients who would prefer to receive standard-interval therapy, and that preference should of course be accommodated whenever possible, but in my experience, those numbers are small,” at least in the VA system, he noted.
In addition, the authors noted, there is no direct financial incentive for more frequent dosing in the VA system.
It’s possible that low uptake could stem from clinicians’ doubts about switching to an extended-interval dose, given that the FDA’s approval was based largely on a study of 44 patients with melanoma in a single-arm trial.
If that is indeed the case, the new findings – which represent the first health system–level, real-world comparative effectiveness data for standard vs. extended-interval pembrolizumab – should help address these concerns, the team said.
“This observational dataset lends further credence to [the dosing] regimens being clinically equivalent,” said Zachery Reichert, MD, PhD, a urologic oncologist at the University of Michigan, Ann Arbor, who was not involved in the study.
To address the issue, Dr. Strohbehn and his team suggested “clinical guideline promotion to overcome some of the barriers to the adoption of extended-interval pembrolizumab.”
Dr. Riechert suggested further validation of equivalent outcomes for the two regimens, more advocacy to encourage patients to ask about the 6-week option, as well as incentives from insurers to adopt it.
Dr. Strohbehn added that the situation highlights a broader issue in oncology, namely that many drugs “end up on the market with dosing regimens that haven’t necessarily been optimized.”
Across the world, investigators are conducting clinical trials “to identify the minimum dosages, frequencies, and durations patients need in order to achieve their best outcome,” Dr. Strohbehn said. In oncology, much of this effort is being led by Project Optimus, from the FDA’s Oncology Center of Excellence, he said.
The study was funded by the VA National Oncology Program. Dr. Reichert and Dr. Strohbehn have disclosed no relevant financial relationships. One investigator has received grants from Novartis, Bristol-Myers Squibb, Regeneron, and Genentech.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
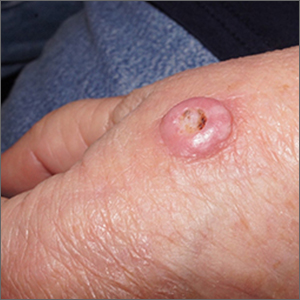
Fast growing hand lesion
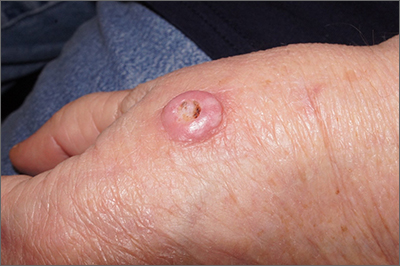
A scoop shave biopsy at the lower edge of the lesion revealed that this was a well-differentiated squamous cell carcinoma.
Squamous cell carcinoma is the second most common cancer in the United States and the most common skin cancer in Black people.1 A patient’s age and their accumulated UV radiation from sun exposure or artificial tanning is a major contributing factor. Lesions may manifest as precancers characterized as rough pink or brown papules with a sandpaper-like texture on sun-exposed skin. These lesions may clear spontaneously or develop into invasive disease, as occurred in this case.
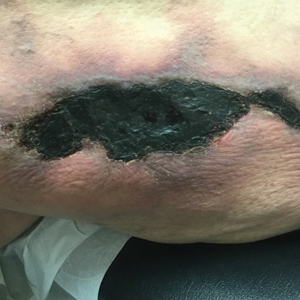
Surgical treatment is often curative. Fusiform excision and Mohs micrographic surgery are 2 common options. More advanced squamous cell carcinomas that are large or found to have poorly differentiated cells or large perineural invasion carry a risk of metastasis.
In elderly patients, optimal treatment isn’t always straightforward.1 Nonsurgical options include radiation and intralesional chemotherapy. These nonsurgical choices may seem less aggressive, but total inconvenience, wound care, and discomfort can be equal to or worse than a single session of curative surgery.
This patient’s lesion was excised with a 5-mm margin. The patient tolerated an in-office procedure lasting about 45 minutes but would have struggled with a longer session with Mohs microsurgery. The postoperative period required limiting full use of his left hand for about 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178. 2. Renzi M Jr, Schimmel J, Decker A, et al. Management of skin cancer in the elderly. Dermatol Clin. 2019;37:279-286. doi: 10.1016/j.det.2019.02.003

A scoop shave biopsy at the lower edge of the lesion revealed that this was a well-differentiated squamous cell carcinoma.
Squamous cell carcinoma is the second most common cancer in the United States and the most common skin cancer in Black people.1 A patient’s age and their accumulated UV radiation from sun exposure or artificial tanning is a major contributing factor. Lesions may manifest as precancers characterized as rough pink or brown papules with a sandpaper-like texture on sun-exposed skin. These lesions may clear spontaneously or develop into invasive disease, as occurred in this case.
Surgical treatment is often curative. Fusiform excision and Mohs micrographic surgery are 2 common options. More advanced squamous cell carcinomas that are large or found to have poorly differentiated cells or large perineural invasion carry a risk of metastasis.
In elderly patients, optimal treatment isn’t always straightforward.1 Nonsurgical options include radiation and intralesional chemotherapy. These nonsurgical choices may seem less aggressive, but total inconvenience, wound care, and discomfort can be equal to or worse than a single session of curative surgery.
This patient’s lesion was excised with a 5-mm margin. The patient tolerated an in-office procedure lasting about 45 minutes but would have struggled with a longer session with Mohs microsurgery. The postoperative period required limiting full use of his left hand for about 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A scoop shave biopsy at the lower edge of the lesion revealed that this was a well-differentiated squamous cell carcinoma.
Squamous cell carcinoma is the second most common cancer in the United States and the most common skin cancer in Black people.1 A patient’s age and their accumulated UV radiation from sun exposure or artificial tanning is a major contributing factor. Lesions may manifest as precancers characterized as rough pink or brown papules with a sandpaper-like texture on sun-exposed skin. These lesions may clear spontaneously or develop into invasive disease, as occurred in this case.
Surgical treatment is often curative. Fusiform excision and Mohs micrographic surgery are 2 common options. More advanced squamous cell carcinomas that are large or found to have poorly differentiated cells or large perineural invasion carry a risk of metastasis.
In elderly patients, optimal treatment isn’t always straightforward.1 Nonsurgical options include radiation and intralesional chemotherapy. These nonsurgical choices may seem less aggressive, but total inconvenience, wound care, and discomfort can be equal to or worse than a single session of curative surgery.
This patient’s lesion was excised with a 5-mm margin. The patient tolerated an in-office procedure lasting about 45 minutes but would have struggled with a longer session with Mohs microsurgery. The postoperative period required limiting full use of his left hand for about 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178. 2. Renzi M Jr, Schimmel J, Decker A, et al. Management of skin cancer in the elderly. Dermatol Clin. 2019;37:279-286. doi: 10.1016/j.det.2019.02.003
1. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178. 2. Renzi M Jr, Schimmel J, Decker A, et al. Management of skin cancer in the elderly. Dermatol Clin. 2019;37:279-286. doi: 10.1016/j.det.2019.02.003
How well are we doing with adolescent vaccination?
Every year, the Centers for Disease Control and Prevention (CDC) conducts a national survey to provide an estimate of vaccination rates among adolescents ages 13 to 17 years. The results for 2021, published recently, illustrate the progress that we’ve made and the areas in which improvement is still needed; notably, human papillomavirus (HPV) vaccine is an example of both.1
First, what’s recommended? The CDC recommends the following vaccines at age 11 to 12 years: tetanus, diphtheria, and acellular pertussis vaccine (Tdap); HPV vaccine series (2 doses if the first dose is received prior to age 15 years; 3 doses if the first dose is received at age 15 years or older); and quadrivalent meningococcal conjugate vaccine (MenACWY). A second (booster) dose of MenACWY is recommended at age 16 years. Adolescents should also receive an annual influenza vaccine and a COVID-19 vaccine series.2
For adolescents not fully vaccinated in childhood, catch-up vaccination is recommended for hepatitis A (HepA); hepatitis B (HepB); measles, mumps, and rubella (MMR); and varicella (VAR).2
How are we doing? In 2021, 89.6% of adolescents had received ≥ 1 Tdap dose and 89.0% had received ≥ 1 MenACWY dose; both these rates remained stable from the year before. For HPV vaccine, 76.9% had received ≥ 1 dose (an increase of 1.8 percentage points from 2020); 61.7% were HPV vaccine “up to date” (an increase of 3.1 percentage points). The teen HPV vaccination rate has increased slowly but progressively since the first recommendation for routine HPV vaccination was made for females in 2006 and for males in 2011.1
Among those age 17 years, coverage with ≥ 2 MenACWY doses was 60.0% (an increase of 5.6 percentage points from 2020). Coverage was 85% for ≥ 2 HepA doses (an increase of 2.9 percentage points from 2020) and remained stable at > 90% for each of the following: ≥ 2 doses of MMR, ≥ 3 doses of HepB, and both VAR doses.1
Keeping the momentum. As a country, we continue to make progress at increasing vaccination rates among US adolescents—but there is still plenty of room for improvement. Family physicians should check vaccine status at each clinical encounter and encourage parents and caregivers to schedule future wellness and vaccine visits for these young patients. This may be especially important among adolescents who were due for and missed a vaccination during the COVID-19 pandemic.
1. Pingali C, Yankey D, Elam-Evans LD, et al. National vaccination coverage among adolescents aged 13-17 years—National Immunization Survey-Teen, United States, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:1101-1108.
2. Wodi AP, Murthy N, Bernstein H, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:234-237.
Every year, the Centers for Disease Control and Prevention (CDC) conducts a national survey to provide an estimate of vaccination rates among adolescents ages 13 to 17 years. The results for 2021, published recently, illustrate the progress that we’ve made and the areas in which improvement is still needed; notably, human papillomavirus (HPV) vaccine is an example of both.1
First, what’s recommended? The CDC recommends the following vaccines at age 11 to 12 years: tetanus, diphtheria, and acellular pertussis vaccine (Tdap); HPV vaccine series (2 doses if the first dose is received prior to age 15 years; 3 doses if the first dose is received at age 15 years or older); and quadrivalent meningococcal conjugate vaccine (MenACWY). A second (booster) dose of MenACWY is recommended at age 16 years. Adolescents should also receive an annual influenza vaccine and a COVID-19 vaccine series.2
For adolescents not fully vaccinated in childhood, catch-up vaccination is recommended for hepatitis A (HepA); hepatitis B (HepB); measles, mumps, and rubella (MMR); and varicella (VAR).2
How are we doing? In 2021, 89.6% of adolescents had received ≥ 1 Tdap dose and 89.0% had received ≥ 1 MenACWY dose; both these rates remained stable from the year before. For HPV vaccine, 76.9% had received ≥ 1 dose (an increase of 1.8 percentage points from 2020); 61.7% were HPV vaccine “up to date” (an increase of 3.1 percentage points). The teen HPV vaccination rate has increased slowly but progressively since the first recommendation for routine HPV vaccination was made for females in 2006 and for males in 2011.1
Among those age 17 years, coverage with ≥ 2 MenACWY doses was 60.0% (an increase of 5.6 percentage points from 2020). Coverage was 85% for ≥ 2 HepA doses (an increase of 2.9 percentage points from 2020) and remained stable at > 90% for each of the following: ≥ 2 doses of MMR, ≥ 3 doses of HepB, and both VAR doses.1
Keeping the momentum. As a country, we continue to make progress at increasing vaccination rates among US adolescents—but there is still plenty of room for improvement. Family physicians should check vaccine status at each clinical encounter and encourage parents and caregivers to schedule future wellness and vaccine visits for these young patients. This may be especially important among adolescents who were due for and missed a vaccination during the COVID-19 pandemic.
Every year, the Centers for Disease Control and Prevention (CDC) conducts a national survey to provide an estimate of vaccination rates among adolescents ages 13 to 17 years. The results for 2021, published recently, illustrate the progress that we’ve made and the areas in which improvement is still needed; notably, human papillomavirus (HPV) vaccine is an example of both.1
First, what’s recommended? The CDC recommends the following vaccines at age 11 to 12 years: tetanus, diphtheria, and acellular pertussis vaccine (Tdap); HPV vaccine series (2 doses if the first dose is received prior to age 15 years; 3 doses if the first dose is received at age 15 years or older); and quadrivalent meningococcal conjugate vaccine (MenACWY). A second (booster) dose of MenACWY is recommended at age 16 years. Adolescents should also receive an annual influenza vaccine and a COVID-19 vaccine series.2
For adolescents not fully vaccinated in childhood, catch-up vaccination is recommended for hepatitis A (HepA); hepatitis B (HepB); measles, mumps, and rubella (MMR); and varicella (VAR).2
How are we doing? In 2021, 89.6% of adolescents had received ≥ 1 Tdap dose and 89.0% had received ≥ 1 MenACWY dose; both these rates remained stable from the year before. For HPV vaccine, 76.9% had received ≥ 1 dose (an increase of 1.8 percentage points from 2020); 61.7% were HPV vaccine “up to date” (an increase of 3.1 percentage points). The teen HPV vaccination rate has increased slowly but progressively since the first recommendation for routine HPV vaccination was made for females in 2006 and for males in 2011.1
Among those age 17 years, coverage with ≥ 2 MenACWY doses was 60.0% (an increase of 5.6 percentage points from 2020). Coverage was 85% for ≥ 2 HepA doses (an increase of 2.9 percentage points from 2020) and remained stable at > 90% for each of the following: ≥ 2 doses of MMR, ≥ 3 doses of HepB, and both VAR doses.1
Keeping the momentum. As a country, we continue to make progress at increasing vaccination rates among US adolescents—but there is still plenty of room for improvement. Family physicians should check vaccine status at each clinical encounter and encourage parents and caregivers to schedule future wellness and vaccine visits for these young patients. This may be especially important among adolescents who were due for and missed a vaccination during the COVID-19 pandemic.
1. Pingali C, Yankey D, Elam-Evans LD, et al. National vaccination coverage among adolescents aged 13-17 years—National Immunization Survey-Teen, United States, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:1101-1108.
2. Wodi AP, Murthy N, Bernstein H, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:234-237.
1. Pingali C, Yankey D, Elam-Evans LD, et al. National vaccination coverage among adolescents aged 13-17 years—National Immunization Survey-Teen, United States, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:1101-1108.
2. Wodi AP, Murthy N, Bernstein H, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:234-237.
Britain’s hard lessons from handing elder care over to private equity
Domestic and global private equity investors had supercharged the company’s growth, betting that the rising needs of aging Britons would yield big returns.
Within weeks, the Four Seasons brand may be finished.
Christie & Co., a commercial real estate broker, splashed a summer sale across its website that signaled the demise: The last 111 Four Seasons facilities in England, Scotland, and Jersey were on the market. Already sold were its 29 homes in Northern Ireland.
Four Seasons collapsed after years of private equity investors rolling in one after another to buy its business, sell its real estate, and at times wrest multimillion-dollar profits through complex debt schemes – until the last big equity fund, Terra Firma, which in 2012 paid about $1.3 billion for the company, was caught short.
In a country where government health care is a right, the Four Seasons story exemplifies the high-stakes rise – and, ultimately, fall – of private equity investment in health and social services. Hanging over society’s most vulnerable patients, these heavily leveraged deals failed to account for the cost of their care. Private equity firms are known for making a profit on quick-turnaround investments.
“People often say: ‘Why have American investors, as well as professional investors here and in other countries, poured so much into this sector?’ I think they were dazzled by the potential of the demographics,” said Nick Hood, an analyst at Opus Restructuring & Insolvency in London, which advises care homes – the British equivalent of U.S. nursing homes or assisted living facilities. They “saw the baby boomers aging and thought there would be infinite demands.”
What they missed, Mr. Hood said, “was that about half of all the residents in U.K. homes are funded by the government in one way or another. They aren’t private pay – and they’ve got no money.”
Residents as ‘revenue streams’
As in the United States, long-term care homes in Britain serve a mixed market of public- and private-pay residents, and those whose balance sheets rest heavily on government payments are stressed even in better economic times. Andrew Dobbie, a community officer for Unison, a union that represents care home workers, said private equity investors often see homes like Four Seasons as having “two revenue streams, the properties themselves and the residents,” with efficiencies to exploit.
But investors don’t always understand what caregivers do, he said, or that older residents require more time than spreadsheets have calculated. “That’s a problem when you are looking at operating care homes,” Mr. Dobbie said. “Care workers need to have soft skills to work with a vulnerable group of people. It’s not the same skills as stocking shelves in a supermarket.”
A recent study, funded in part by Unison and conducted by University of Surrey researchers, found big changes in the quality of care after private equity investments. More than a dozen staff members, who weren’t identified by name or facility, said companies were “cutting corners” to curb costs because their priority was profit. Staffers said “these changes meant residents sometimes went without the appropriate care, timely medication or sufficient sanitary supplies.”
In August, the House of Commons received a sobering account: The number of adults 65 and older who will need care is speedily rising, estimated to go from 3.5 million in 2018 to 5.2 million in 2038. Yet workers at care homes are among the lowest paid in health care.
“The covid-19 pandemic shone a light on the adult social care sector,” according to the parliamentary report, which noted that “many frustrated and burnt out care workers left” for better-paying jobs. The report’s advice in a year of soaring inflation and energy costs? The government should add “at least £7 billion a year” – more than $8 billion – or risk deterioration of care.
Britain’s care homes are separate from the much-lauded National Health Service, funded by the government. Care homes rely on support from local authorities, akin to counties in the United States. But they have seen a sharp drop in funding from the British government, which cut a third of its payments in the past decade. When the pandemic hit, the differences were apparent: Care home workers were not afforded masks, gloves, or gowns to shield them from the deadly virus.
Years ago, care homes were largely run by families or local entities. In the 1990s, the government promoted privatization, triggering investments and consolidations. Today, private equity firms own three of the country’s five biggest care home providers.
Chris Thomas, a research fellow at the Institute for Public Policy Research, said investors benefited from scant financial oversight. “The accounting practices are horrendously complicated and meant to be complicated,” he said. Local authorities try “to regulate more, but they don’t have the expertise.”
The financial shuffle
At Four Seasons, the speed of change was dizzying. From 2004 to 2017, big money came and went, with revenue at times threaded through multiple offshore vehicles. Among the groups that owned Four Seasons, in part or in its entirety: British private equity firm Alchemy Partners; Allianz Capital Partners, a German private equity firm; Three Delta, an investment fund backed by Qatar; the American hedge fund Monarch Alternative Capital; and Terra Firma, the British private equity group that wallowed in debt demands. H/2 Capital Partners, a hedge fund in Connecticut, was Four Seasons’ main creditor and took over. By 2019, Four Seasons was managed by insolvency experts.
Pressed on whether Four Seasons would exist in any form after the current sale of its property and businesses, MHP Communications, representing the company, said in an email: “It is too early in the process to speculate about the future of the brand.”
Vivek Kotecha, an accountant who has examined the Four Seasons financial shuffle and coauthored the Unison report, said private equity investment – in homes for older residents and, increasingly, in facilities for troubled children – is now part of the financial mainstream. The consulting firm McKinsey in 2022estimated that private markets manage nearly $10 trillion in assets, making them a dominant force in global markets.
“What you find in America with private equity is much the same here,” said Mr. Kotecha, the founder of Trinava Consulting in London. “They are often the same firms, doing the same things.” What was remarkable about Four Seasons was the enormous liability from high-yield bonds that underpinned the deal – one equaling $514 million at 8.75% interest and another for $277 million at 12.75% interest.
Guy Hands, the high-flying British founder of Terra Firma, bought Four Seasons in 2012, soon after losing an epic court battle with Citigroup over the purchase price of the music company EMI Group. Terra Firma acquired the care homes and then a gardening business with more than 100 stores. Neither proved easy, or good, bets. Hands, a Londoner who moved offshore to Guernsey, declined through a representative to discuss Four Seasons.
Mr. Kotecha, however, helped the BBC try to make sense of Four Seasons’ holdings by tracking financial filings. It was “the most complicated spreadsheet I’ve ever seen,” Mr. Kotecha said. “I think there were more subsidiaries involved in Four Seasons’ care homes than there were with General Motors in Europe.”
As Britain’s small homes were swept up in consolidations, some financial practices were dubious. At times, businesses sold the buildings as lease-back deals – not a problem at first – that, after multiple purchases, left operators paying rent with heavy interest that sapped operating budgets. By 2020, some care homes were estimated to be spending as much as 16% of their bed fees on debt payments, according to parliamentary testimony this year.
How could that happen? In part, for-profit providers – backed by private-equity groups and other corporations – had subsidiaries of their parent companies act as lender, setting the rates.
Britain’s elder care was unrecognizable within a generation. By 2022, private-equity companies alone accounted for 55,000 beds, or about 12.6% of the total for-profit care beds for older people in the United Kingdom, according to LaingBuisson, a health care consultancy. LaingBuisson calculated that the average residential care home fee as of February 2022 was about $44,700 a year; the average nursing home fee was $62,275 a year.
From 1980 to 2018, the number of residential care beds provided by local authorities fell 88% – from 141,719 to 17,100, according to the nonprofit Centre for Health and the Public Interest. Independent operators – nonprofits and for-profits – moved in, it said, controlling 243,000 beds by 2018. Nursing homes saw a similar shift: Private providers accounted for 194,100 beds in 2018, compared with 25,500 decades earlier.
Beyond government control
British lawmakers in the winter of 2021-2022 tried – and failed – to bolster financial reporting rules for care homes, including banning the use of government funds to pay off debt.
“I don’t have a problem with offshore companies that make profits if they offer good services. I don’t have a problem with private equity and hedge funds who deliver good returns to their shareholders,” Ros Altmann, a Conservative Party member in the House of Lords and a pension expert, said in a February debate. “I do have a problem if those companies are taking advantage of some of the most vulnerable people in our society without oversight, without controls.”
She cited Four Seasons as an example of how regulators “have no control over the financial models that are used.” Ms. Altmann warned that economic headwinds could worsen matters: “We now have very heavily debt-laden [homes] in an environment where interest rates are heading upward.”
In August, the Bank of England raised borrowing rates. It now forecasts double-digit inflation – as much as 11% – through 2023.
And that leaves care home owner Robert Kilgour pensive about whether government grasps the risks and possibilities that the sector is facing. “It’s a struggle, and it’s becoming more of a struggle,” he said. A global energy crisis is the latest unexpected emergency. Mr. Kilgour said he recently signed electricity contracts, for April 2023, at rates that will rise by 200%. That means an extra $2,400 a day in utility costs for his homes.
Mr. Kilgour founded Four Seasons, opening its first home, in Fife, Scotland, in 1989. His ambition for its growth was modest: “Ten by 2000.” That changed in 1999 when Alchemy swooped in to expand nationally. Mr. Kilgour had left Four Seasons by 2004, turning to other ventures.
Still, he saw opportunity in elder care and opened Renaissance Care, which now operates 16 homes with 750 beds in Scotland. “I missed it,” he said in an interview in London. “It’s people and it’s property, and I like that.”
“People asked me if I had any regrets about selling to private equity. Well, no, the people I dealt with were very fair, very straight. There were no shenanigans,” Mr. Kilgour said, noting that Alchemy made money but invested as well.
Mr. Kilgour said the pandemic motivated him to improve his business. He is spending millions on new LED lighting and boilers, as well as training staffers on digital record-keeping, all to winnow costs. He increased hourly wages by 5%, but employees have suggested other ways to retain staff: shorter shifts and workdays that fit school schedules or allow them to care for their own older relatives.
Debates over whether the government should move back into elder care make little sense to Mr. Kilgour. Britain has had private care for decades, and he doesn’t see that changing. Instead, operators need help balancing private and publicly funded beds “so you have a blended rate for care and some certainty in the business.”
Consolidations are slowing, he said, which might be part of a long-overdue reckoning. “The idea of 200, 300, 400 care homes – that big is good and big is best – those days are gone,” Mr. Kilgour said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Domestic and global private equity investors had supercharged the company’s growth, betting that the rising needs of aging Britons would yield big returns.
Within weeks, the Four Seasons brand may be finished.
Christie & Co., a commercial real estate broker, splashed a summer sale across its website that signaled the demise: The last 111 Four Seasons facilities in England, Scotland, and Jersey were on the market. Already sold were its 29 homes in Northern Ireland.
Four Seasons collapsed after years of private equity investors rolling in one after another to buy its business, sell its real estate, and at times wrest multimillion-dollar profits through complex debt schemes – until the last big equity fund, Terra Firma, which in 2012 paid about $1.3 billion for the company, was caught short.
In a country where government health care is a right, the Four Seasons story exemplifies the high-stakes rise – and, ultimately, fall – of private equity investment in health and social services. Hanging over society’s most vulnerable patients, these heavily leveraged deals failed to account for the cost of their care. Private equity firms are known for making a profit on quick-turnaround investments.
“People often say: ‘Why have American investors, as well as professional investors here and in other countries, poured so much into this sector?’ I think they were dazzled by the potential of the demographics,” said Nick Hood, an analyst at Opus Restructuring & Insolvency in London, which advises care homes – the British equivalent of U.S. nursing homes or assisted living facilities. They “saw the baby boomers aging and thought there would be infinite demands.”
What they missed, Mr. Hood said, “was that about half of all the residents in U.K. homes are funded by the government in one way or another. They aren’t private pay – and they’ve got no money.”
Residents as ‘revenue streams’
As in the United States, long-term care homes in Britain serve a mixed market of public- and private-pay residents, and those whose balance sheets rest heavily on government payments are stressed even in better economic times. Andrew Dobbie, a community officer for Unison, a union that represents care home workers, said private equity investors often see homes like Four Seasons as having “two revenue streams, the properties themselves and the residents,” with efficiencies to exploit.
But investors don’t always understand what caregivers do, he said, or that older residents require more time than spreadsheets have calculated. “That’s a problem when you are looking at operating care homes,” Mr. Dobbie said. “Care workers need to have soft skills to work with a vulnerable group of people. It’s not the same skills as stocking shelves in a supermarket.”
A recent study, funded in part by Unison and conducted by University of Surrey researchers, found big changes in the quality of care after private equity investments. More than a dozen staff members, who weren’t identified by name or facility, said companies were “cutting corners” to curb costs because their priority was profit. Staffers said “these changes meant residents sometimes went without the appropriate care, timely medication or sufficient sanitary supplies.”
In August, the House of Commons received a sobering account: The number of adults 65 and older who will need care is speedily rising, estimated to go from 3.5 million in 2018 to 5.2 million in 2038. Yet workers at care homes are among the lowest paid in health care.
“The covid-19 pandemic shone a light on the adult social care sector,” according to the parliamentary report, which noted that “many frustrated and burnt out care workers left” for better-paying jobs. The report’s advice in a year of soaring inflation and energy costs? The government should add “at least £7 billion a year” – more than $8 billion – or risk deterioration of care.
Britain’s care homes are separate from the much-lauded National Health Service, funded by the government. Care homes rely on support from local authorities, akin to counties in the United States. But they have seen a sharp drop in funding from the British government, which cut a third of its payments in the past decade. When the pandemic hit, the differences were apparent: Care home workers were not afforded masks, gloves, or gowns to shield them from the deadly virus.
Years ago, care homes were largely run by families or local entities. In the 1990s, the government promoted privatization, triggering investments and consolidations. Today, private equity firms own three of the country’s five biggest care home providers.
Chris Thomas, a research fellow at the Institute for Public Policy Research, said investors benefited from scant financial oversight. “The accounting practices are horrendously complicated and meant to be complicated,” he said. Local authorities try “to regulate more, but they don’t have the expertise.”
The financial shuffle
At Four Seasons, the speed of change was dizzying. From 2004 to 2017, big money came and went, with revenue at times threaded through multiple offshore vehicles. Among the groups that owned Four Seasons, in part or in its entirety: British private equity firm Alchemy Partners; Allianz Capital Partners, a German private equity firm; Three Delta, an investment fund backed by Qatar; the American hedge fund Monarch Alternative Capital; and Terra Firma, the British private equity group that wallowed in debt demands. H/2 Capital Partners, a hedge fund in Connecticut, was Four Seasons’ main creditor and took over. By 2019, Four Seasons was managed by insolvency experts.
Pressed on whether Four Seasons would exist in any form after the current sale of its property and businesses, MHP Communications, representing the company, said in an email: “It is too early in the process to speculate about the future of the brand.”
Vivek Kotecha, an accountant who has examined the Four Seasons financial shuffle and coauthored the Unison report, said private equity investment – in homes for older residents and, increasingly, in facilities for troubled children – is now part of the financial mainstream. The consulting firm McKinsey in 2022estimated that private markets manage nearly $10 trillion in assets, making them a dominant force in global markets.
“What you find in America with private equity is much the same here,” said Mr. Kotecha, the founder of Trinava Consulting in London. “They are often the same firms, doing the same things.” What was remarkable about Four Seasons was the enormous liability from high-yield bonds that underpinned the deal – one equaling $514 million at 8.75% interest and another for $277 million at 12.75% interest.
Guy Hands, the high-flying British founder of Terra Firma, bought Four Seasons in 2012, soon after losing an epic court battle with Citigroup over the purchase price of the music company EMI Group. Terra Firma acquired the care homes and then a gardening business with more than 100 stores. Neither proved easy, or good, bets. Hands, a Londoner who moved offshore to Guernsey, declined through a representative to discuss Four Seasons.
Mr. Kotecha, however, helped the BBC try to make sense of Four Seasons’ holdings by tracking financial filings. It was “the most complicated spreadsheet I’ve ever seen,” Mr. Kotecha said. “I think there were more subsidiaries involved in Four Seasons’ care homes than there were with General Motors in Europe.”
As Britain’s small homes were swept up in consolidations, some financial practices were dubious. At times, businesses sold the buildings as lease-back deals – not a problem at first – that, after multiple purchases, left operators paying rent with heavy interest that sapped operating budgets. By 2020, some care homes were estimated to be spending as much as 16% of their bed fees on debt payments, according to parliamentary testimony this year.
How could that happen? In part, for-profit providers – backed by private-equity groups and other corporations – had subsidiaries of their parent companies act as lender, setting the rates.
Britain’s elder care was unrecognizable within a generation. By 2022, private-equity companies alone accounted for 55,000 beds, or about 12.6% of the total for-profit care beds for older people in the United Kingdom, according to LaingBuisson, a health care consultancy. LaingBuisson calculated that the average residential care home fee as of February 2022 was about $44,700 a year; the average nursing home fee was $62,275 a year.
From 1980 to 2018, the number of residential care beds provided by local authorities fell 88% – from 141,719 to 17,100, according to the nonprofit Centre for Health and the Public Interest. Independent operators – nonprofits and for-profits – moved in, it said, controlling 243,000 beds by 2018. Nursing homes saw a similar shift: Private providers accounted for 194,100 beds in 2018, compared with 25,500 decades earlier.
Beyond government control
British lawmakers in the winter of 2021-2022 tried – and failed – to bolster financial reporting rules for care homes, including banning the use of government funds to pay off debt.
“I don’t have a problem with offshore companies that make profits if they offer good services. I don’t have a problem with private equity and hedge funds who deliver good returns to their shareholders,” Ros Altmann, a Conservative Party member in the House of Lords and a pension expert, said in a February debate. “I do have a problem if those companies are taking advantage of some of the most vulnerable people in our society without oversight, without controls.”
She cited Four Seasons as an example of how regulators “have no control over the financial models that are used.” Ms. Altmann warned that economic headwinds could worsen matters: “We now have very heavily debt-laden [homes] in an environment where interest rates are heading upward.”
In August, the Bank of England raised borrowing rates. It now forecasts double-digit inflation – as much as 11% – through 2023.
And that leaves care home owner Robert Kilgour pensive about whether government grasps the risks and possibilities that the sector is facing. “It’s a struggle, and it’s becoming more of a struggle,” he said. A global energy crisis is the latest unexpected emergency. Mr. Kilgour said he recently signed electricity contracts, for April 2023, at rates that will rise by 200%. That means an extra $2,400 a day in utility costs for his homes.
Mr. Kilgour founded Four Seasons, opening its first home, in Fife, Scotland, in 1989. His ambition for its growth was modest: “Ten by 2000.” That changed in 1999 when Alchemy swooped in to expand nationally. Mr. Kilgour had left Four Seasons by 2004, turning to other ventures.
Still, he saw opportunity in elder care and opened Renaissance Care, which now operates 16 homes with 750 beds in Scotland. “I missed it,” he said in an interview in London. “It’s people and it’s property, and I like that.”
“People asked me if I had any regrets about selling to private equity. Well, no, the people I dealt with were very fair, very straight. There were no shenanigans,” Mr. Kilgour said, noting that Alchemy made money but invested as well.
Mr. Kilgour said the pandemic motivated him to improve his business. He is spending millions on new LED lighting and boilers, as well as training staffers on digital record-keeping, all to winnow costs. He increased hourly wages by 5%, but employees have suggested other ways to retain staff: shorter shifts and workdays that fit school schedules or allow them to care for their own older relatives.
Debates over whether the government should move back into elder care make little sense to Mr. Kilgour. Britain has had private care for decades, and he doesn’t see that changing. Instead, operators need help balancing private and publicly funded beds “so you have a blended rate for care and some certainty in the business.”
Consolidations are slowing, he said, which might be part of a long-overdue reckoning. “The idea of 200, 300, 400 care homes – that big is good and big is best – those days are gone,” Mr. Kilgour said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Domestic and global private equity investors had supercharged the company’s growth, betting that the rising needs of aging Britons would yield big returns.
Within weeks, the Four Seasons brand may be finished.
Christie & Co., a commercial real estate broker, splashed a summer sale across its website that signaled the demise: The last 111 Four Seasons facilities in England, Scotland, and Jersey were on the market. Already sold were its 29 homes in Northern Ireland.
Four Seasons collapsed after years of private equity investors rolling in one after another to buy its business, sell its real estate, and at times wrest multimillion-dollar profits through complex debt schemes – until the last big equity fund, Terra Firma, which in 2012 paid about $1.3 billion for the company, was caught short.
In a country where government health care is a right, the Four Seasons story exemplifies the high-stakes rise – and, ultimately, fall – of private equity investment in health and social services. Hanging over society’s most vulnerable patients, these heavily leveraged deals failed to account for the cost of their care. Private equity firms are known for making a profit on quick-turnaround investments.
“People often say: ‘Why have American investors, as well as professional investors here and in other countries, poured so much into this sector?’ I think they were dazzled by the potential of the demographics,” said Nick Hood, an analyst at Opus Restructuring & Insolvency in London, which advises care homes – the British equivalent of U.S. nursing homes or assisted living facilities. They “saw the baby boomers aging and thought there would be infinite demands.”
What they missed, Mr. Hood said, “was that about half of all the residents in U.K. homes are funded by the government in one way or another. They aren’t private pay – and they’ve got no money.”
Residents as ‘revenue streams’
As in the United States, long-term care homes in Britain serve a mixed market of public- and private-pay residents, and those whose balance sheets rest heavily on government payments are stressed even in better economic times. Andrew Dobbie, a community officer for Unison, a union that represents care home workers, said private equity investors often see homes like Four Seasons as having “two revenue streams, the properties themselves and the residents,” with efficiencies to exploit.
But investors don’t always understand what caregivers do, he said, or that older residents require more time than spreadsheets have calculated. “That’s a problem when you are looking at operating care homes,” Mr. Dobbie said. “Care workers need to have soft skills to work with a vulnerable group of people. It’s not the same skills as stocking shelves in a supermarket.”
A recent study, funded in part by Unison and conducted by University of Surrey researchers, found big changes in the quality of care after private equity investments. More than a dozen staff members, who weren’t identified by name or facility, said companies were “cutting corners” to curb costs because their priority was profit. Staffers said “these changes meant residents sometimes went without the appropriate care, timely medication or sufficient sanitary supplies.”
In August, the House of Commons received a sobering account: The number of adults 65 and older who will need care is speedily rising, estimated to go from 3.5 million in 2018 to 5.2 million in 2038. Yet workers at care homes are among the lowest paid in health care.
“The covid-19 pandemic shone a light on the adult social care sector,” according to the parliamentary report, which noted that “many frustrated and burnt out care workers left” for better-paying jobs. The report’s advice in a year of soaring inflation and energy costs? The government should add “at least £7 billion a year” – more than $8 billion – or risk deterioration of care.
Britain’s care homes are separate from the much-lauded National Health Service, funded by the government. Care homes rely on support from local authorities, akin to counties in the United States. But they have seen a sharp drop in funding from the British government, which cut a third of its payments in the past decade. When the pandemic hit, the differences were apparent: Care home workers were not afforded masks, gloves, or gowns to shield them from the deadly virus.
Years ago, care homes were largely run by families or local entities. In the 1990s, the government promoted privatization, triggering investments and consolidations. Today, private equity firms own three of the country’s five biggest care home providers.
Chris Thomas, a research fellow at the Institute for Public Policy Research, said investors benefited from scant financial oversight. “The accounting practices are horrendously complicated and meant to be complicated,” he said. Local authorities try “to regulate more, but they don’t have the expertise.”
The financial shuffle
At Four Seasons, the speed of change was dizzying. From 2004 to 2017, big money came and went, with revenue at times threaded through multiple offshore vehicles. Among the groups that owned Four Seasons, in part or in its entirety: British private equity firm Alchemy Partners; Allianz Capital Partners, a German private equity firm; Three Delta, an investment fund backed by Qatar; the American hedge fund Monarch Alternative Capital; and Terra Firma, the British private equity group that wallowed in debt demands. H/2 Capital Partners, a hedge fund in Connecticut, was Four Seasons’ main creditor and took over. By 2019, Four Seasons was managed by insolvency experts.
Pressed on whether Four Seasons would exist in any form after the current sale of its property and businesses, MHP Communications, representing the company, said in an email: “It is too early in the process to speculate about the future of the brand.”
Vivek Kotecha, an accountant who has examined the Four Seasons financial shuffle and coauthored the Unison report, said private equity investment – in homes for older residents and, increasingly, in facilities for troubled children – is now part of the financial mainstream. The consulting firm McKinsey in 2022estimated that private markets manage nearly $10 trillion in assets, making them a dominant force in global markets.
“What you find in America with private equity is much the same here,” said Mr. Kotecha, the founder of Trinava Consulting in London. “They are often the same firms, doing the same things.” What was remarkable about Four Seasons was the enormous liability from high-yield bonds that underpinned the deal – one equaling $514 million at 8.75% interest and another for $277 million at 12.75% interest.
Guy Hands, the high-flying British founder of Terra Firma, bought Four Seasons in 2012, soon after losing an epic court battle with Citigroup over the purchase price of the music company EMI Group. Terra Firma acquired the care homes and then a gardening business with more than 100 stores. Neither proved easy, or good, bets. Hands, a Londoner who moved offshore to Guernsey, declined through a representative to discuss Four Seasons.
Mr. Kotecha, however, helped the BBC try to make sense of Four Seasons’ holdings by tracking financial filings. It was “the most complicated spreadsheet I’ve ever seen,” Mr. Kotecha said. “I think there were more subsidiaries involved in Four Seasons’ care homes than there were with General Motors in Europe.”
As Britain’s small homes were swept up in consolidations, some financial practices were dubious. At times, businesses sold the buildings as lease-back deals – not a problem at first – that, after multiple purchases, left operators paying rent with heavy interest that sapped operating budgets. By 2020, some care homes were estimated to be spending as much as 16% of their bed fees on debt payments, according to parliamentary testimony this year.
How could that happen? In part, for-profit providers – backed by private-equity groups and other corporations – had subsidiaries of their parent companies act as lender, setting the rates.
Britain’s elder care was unrecognizable within a generation. By 2022, private-equity companies alone accounted for 55,000 beds, or about 12.6% of the total for-profit care beds for older people in the United Kingdom, according to LaingBuisson, a health care consultancy. LaingBuisson calculated that the average residential care home fee as of February 2022 was about $44,700 a year; the average nursing home fee was $62,275 a year.
From 1980 to 2018, the number of residential care beds provided by local authorities fell 88% – from 141,719 to 17,100, according to the nonprofit Centre for Health and the Public Interest. Independent operators – nonprofits and for-profits – moved in, it said, controlling 243,000 beds by 2018. Nursing homes saw a similar shift: Private providers accounted for 194,100 beds in 2018, compared with 25,500 decades earlier.
Beyond government control
British lawmakers in the winter of 2021-2022 tried – and failed – to bolster financial reporting rules for care homes, including banning the use of government funds to pay off debt.
“I don’t have a problem with offshore companies that make profits if they offer good services. I don’t have a problem with private equity and hedge funds who deliver good returns to their shareholders,” Ros Altmann, a Conservative Party member in the House of Lords and a pension expert, said in a February debate. “I do have a problem if those companies are taking advantage of some of the most vulnerable people in our society without oversight, without controls.”
She cited Four Seasons as an example of how regulators “have no control over the financial models that are used.” Ms. Altmann warned that economic headwinds could worsen matters: “We now have very heavily debt-laden [homes] in an environment where interest rates are heading upward.”
In August, the Bank of England raised borrowing rates. It now forecasts double-digit inflation – as much as 11% – through 2023.
And that leaves care home owner Robert Kilgour pensive about whether government grasps the risks and possibilities that the sector is facing. “It’s a struggle, and it’s becoming more of a struggle,” he said. A global energy crisis is the latest unexpected emergency. Mr. Kilgour said he recently signed electricity contracts, for April 2023, at rates that will rise by 200%. That means an extra $2,400 a day in utility costs for his homes.
Mr. Kilgour founded Four Seasons, opening its first home, in Fife, Scotland, in 1989. His ambition for its growth was modest: “Ten by 2000.” That changed in 1999 when Alchemy swooped in to expand nationally. Mr. Kilgour had left Four Seasons by 2004, turning to other ventures.
Still, he saw opportunity in elder care and opened Renaissance Care, which now operates 16 homes with 750 beds in Scotland. “I missed it,” he said in an interview in London. “It’s people and it’s property, and I like that.”
“People asked me if I had any regrets about selling to private equity. Well, no, the people I dealt with were very fair, very straight. There were no shenanigans,” Mr. Kilgour said, noting that Alchemy made money but invested as well.
Mr. Kilgour said the pandemic motivated him to improve his business. He is spending millions on new LED lighting and boilers, as well as training staffers on digital record-keeping, all to winnow costs. He increased hourly wages by 5%, but employees have suggested other ways to retain staff: shorter shifts and workdays that fit school schedules or allow them to care for their own older relatives.
Debates over whether the government should move back into elder care make little sense to Mr. Kilgour. Britain has had private care for decades, and he doesn’t see that changing. Instead, operators need help balancing private and publicly funded beds “so you have a blended rate for care and some certainty in the business.”
Consolidations are slowing, he said, which might be part of a long-overdue reckoning. “The idea of 200, 300, 400 care homes – that big is good and big is best – those days are gone,” Mr. Kilgour said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Heart failure drug a new treatment option for alcoholism?
(AUD), new research suggests.
Researchers at the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and Yale University, New Haven, Conn., investigated the impact of spironolactone on AUD.
Initially, they studied rodents and found that spironolactone reduced binge drinking in mice and reduced self-administration of alcohol in rats without adversely affecting food or water intake or causing motor or coordination problems.
They also analyzed electronic health records of patients drawn from the United States Veterans Affairs health care system to explore potential changes in alcohol use after spironolactone treatment was initiated for other conditions and found a significant link between spironolactone treatment and reduction in self-reported alcohol consumption, with the largest effects observed among those who reported hazardous/heavy episodic alcohol use prior to starting spironolactone treatment.
“Combining findings across three species and different types of research studies, and then seeing similarities in these data, gives us confidence that we are onto something potentially important scientifically and clinically,” senior coauthor Lorenzo Leggio, MD, PhD, senior investigator in the Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, a joint NIDA and NIAAA laboratory, said in a news release.
The study was published online in Molecular Psychiatry.
There is a “critical need to increase the armamentarium of pharmacotherapies to treat individuals with AUD,” the authors note, adding that neuroendocrine systems involved in alcohol craving and drinking “offer promising pharmacologic targets in this regard.”
“Both our team and others have observed that patients with AUD often present with changes in peripheral hormones, including aldosterone, which plays a key role in regulating blood pressure and electrolytes,” Dr. Leggio said in an interview.
Spironolactone is a nonselective mineralocorticoid receptor (MT) antagonist. In studies in animal models, investigators said they found “an inverse correlation between alcohol drinking and the expression of the MR in the amygdala, a key brain region in the development and maintenance of AUD and addiction in general.”
Taken together, this led them to hypothesize that blocking the MR, which is the mechanism of action of spironolactone, “could be a novel pharmacotherapeutic approach for AUD,” he said.
Previous research by the same group of researchers suggested spironolactone “may be a potential new medication to treat patients with AUD.” The present study expanded on those findings and consisted of a three-part investigation.
In the current study, the investigators tested different dosages of spironolactone on binge-like alcohol consumption in male and female mice and assessed food and water intake, blood alcohol levels, motor coordination, and spontaneous locomotion.
They then tested the effects of different dosages of spironolactone injections on operant alcohol self-administration in alcohol-dependent and nondependent male and female rats, also testing blood alcohol levels and motor coordination.
Finally, they analyzed health records of veterans to examine the association between at least 60 continuous days of spironolactone treatment and self-reported alcohol consumption (measured by the Alcohol Use Disorders Identification Test-Consumption [AUDIT-C]).
Each of the spironolactone-exposed patients was matched using propensity scores with up to five unexposed patients who had reported alcohol consumption in the 2 years prior to the index date.
The final analysis included a matched cohort of 10,726 spironolactone-exposed individuals who were matched to 34,461 unexposed individuals.
New targets
Spironolactone reduced alcohol intake in mice drinking a sweetened alcohol solution; a 2-way ANOVA revealed a main effect of dose (F 4,52 = 9.09; P < .0001) and sex, with female mice drinking more alcohol, compared to male mice (F 1,13 = 6.05; P = .02).
Post hoc comparisons showed that spironolactone at doses of 50, 100, and 200 mg/kg significantly reduced alcohol intake (P values = .007, .002, and .0001, respectively).
In mice drinking an unsweetened alcohol solution, the 2-way repeated measures ANOVA similarly found a main effect of dose (F 4,52 = 5.77; P = .0006), but not of sex (F 1,13 = 1.41; P = .25).
Spironolactone had no effect on the mice’s intake of a sweet solution without alcohol and had no impact on the consumption of food and water or on locomotion and coordination.
In rats, a 2-way ANOVA revealed a significant spironolactone effect of dose (F 3,66 = 43.95; P < .001), with a post hoc test indicating that spironolactone at 25, 50, and 75 mg/kg reduced alcohol self-administration in alcohol-dependent and nondependent rats (all P values = .0001).
In humans, among the exposed individuals in the matched cohort, 25%, 57%, and 18% received daily doses of spironolactone of less than 25 mg/day, 25-49 mg/day, and 50 mg/day or higher, respectively, with a median follow-up time of 542 (interquartile range, 337-730) days.
The AUDIT-C scores decreased during the study period in both treatment groups, with a larger decrease in average AUDIT-C scores among the exposed vs. unexposed individuals.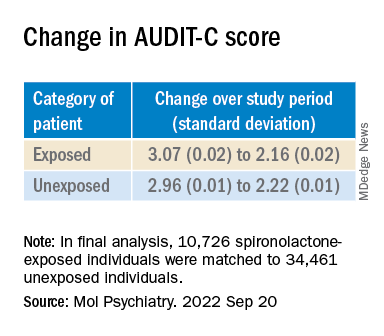
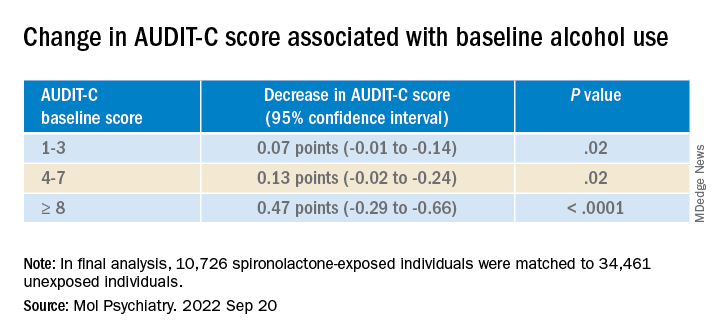
“These are very exciting times because, thanks to the progress in the addiction biomedical research field, we are increasing our understanding of the mechanisms how some people develop AUD; hence we can use this knowledge to identify new targets.” The current study “is an example of these ongoing efforts,” said Dr. Leggio.
“It is important to note that [these results] are important but preliminary.” At this juncture, “it would be too premature to think about prescribing spironolactone to treat AUD,” he added.
Exciting findings
Commenting on the study, Joyce Besheer, PhD, professor, department of psychiatry and Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, called the study an “elegant demonstration of translational science.”
“While clinical trials will be needed to determine whether this medication is effective at reducing drinking in patients with AUD, these findings are exciting as they suggest that spironolactone may be a promising compound and new treatment options for AUD are much needed,” said Dr. Besheer, who was not involved with the current study.
Dr. Leggio agreed. “We now need prospective, placebo-controlled studies to assess the potential safety and efficacy of spironolactone in people with AUD,” he said.
This work was supported by the National Institutes of Health and the NIAAA. Dr. Leggio, study coauthors, and Dr. Besheer declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AUD), new research suggests.
Researchers at the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and Yale University, New Haven, Conn., investigated the impact of spironolactone on AUD.
Initially, they studied rodents and found that spironolactone reduced binge drinking in mice and reduced self-administration of alcohol in rats without adversely affecting food or water intake or causing motor or coordination problems.
They also analyzed electronic health records of patients drawn from the United States Veterans Affairs health care system to explore potential changes in alcohol use after spironolactone treatment was initiated for other conditions and found a significant link between spironolactone treatment and reduction in self-reported alcohol consumption, with the largest effects observed among those who reported hazardous/heavy episodic alcohol use prior to starting spironolactone treatment.
“Combining findings across three species and different types of research studies, and then seeing similarities in these data, gives us confidence that we are onto something potentially important scientifically and clinically,” senior coauthor Lorenzo Leggio, MD, PhD, senior investigator in the Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, a joint NIDA and NIAAA laboratory, said in a news release.
The study was published online in Molecular Psychiatry.
There is a “critical need to increase the armamentarium of pharmacotherapies to treat individuals with AUD,” the authors note, adding that neuroendocrine systems involved in alcohol craving and drinking “offer promising pharmacologic targets in this regard.”
“Both our team and others have observed that patients with AUD often present with changes in peripheral hormones, including aldosterone, which plays a key role in regulating blood pressure and electrolytes,” Dr. Leggio said in an interview.
Spironolactone is a nonselective mineralocorticoid receptor (MT) antagonist. In studies in animal models, investigators said they found “an inverse correlation between alcohol drinking and the expression of the MR in the amygdala, a key brain region in the development and maintenance of AUD and addiction in general.”
Taken together, this led them to hypothesize that blocking the MR, which is the mechanism of action of spironolactone, “could be a novel pharmacotherapeutic approach for AUD,” he said.
Previous research by the same group of researchers suggested spironolactone “may be a potential new medication to treat patients with AUD.” The present study expanded on those findings and consisted of a three-part investigation.
In the current study, the investigators tested different dosages of spironolactone on binge-like alcohol consumption in male and female mice and assessed food and water intake, blood alcohol levels, motor coordination, and spontaneous locomotion.
They then tested the effects of different dosages of spironolactone injections on operant alcohol self-administration in alcohol-dependent and nondependent male and female rats, also testing blood alcohol levels and motor coordination.
Finally, they analyzed health records of veterans to examine the association between at least 60 continuous days of spironolactone treatment and self-reported alcohol consumption (measured by the Alcohol Use Disorders Identification Test-Consumption [AUDIT-C]).
Each of the spironolactone-exposed patients was matched using propensity scores with up to five unexposed patients who had reported alcohol consumption in the 2 years prior to the index date.
The final analysis included a matched cohort of 10,726 spironolactone-exposed individuals who were matched to 34,461 unexposed individuals.
New targets
Spironolactone reduced alcohol intake in mice drinking a sweetened alcohol solution; a 2-way ANOVA revealed a main effect of dose (F 4,52 = 9.09; P < .0001) and sex, with female mice drinking more alcohol, compared to male mice (F 1,13 = 6.05; P = .02).
Post hoc comparisons showed that spironolactone at doses of 50, 100, and 200 mg/kg significantly reduced alcohol intake (P values = .007, .002, and .0001, respectively).
In mice drinking an unsweetened alcohol solution, the 2-way repeated measures ANOVA similarly found a main effect of dose (F 4,52 = 5.77; P = .0006), but not of sex (F 1,13 = 1.41; P = .25).
Spironolactone had no effect on the mice’s intake of a sweet solution without alcohol and had no impact on the consumption of food and water or on locomotion and coordination.
In rats, a 2-way ANOVA revealed a significant spironolactone effect of dose (F 3,66 = 43.95; P < .001), with a post hoc test indicating that spironolactone at 25, 50, and 75 mg/kg reduced alcohol self-administration in alcohol-dependent and nondependent rats (all P values = .0001).
In humans, among the exposed individuals in the matched cohort, 25%, 57%, and 18% received daily doses of spironolactone of less than 25 mg/day, 25-49 mg/day, and 50 mg/day or higher, respectively, with a median follow-up time of 542 (interquartile range, 337-730) days.
The AUDIT-C scores decreased during the study period in both treatment groups, with a larger decrease in average AUDIT-C scores among the exposed vs. unexposed individuals.

“These are very exciting times because, thanks to the progress in the addiction biomedical research field, we are increasing our understanding of the mechanisms how some people develop AUD; hence we can use this knowledge to identify new targets.” The current study “is an example of these ongoing efforts,” said Dr. Leggio.
“It is important to note that [these results] are important but preliminary.” At this juncture, “it would be too premature to think about prescribing spironolactone to treat AUD,” he added.
Exciting findings
Commenting on the study, Joyce Besheer, PhD, professor, department of psychiatry and Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, called the study an “elegant demonstration of translational science.”
“While clinical trials will be needed to determine whether this medication is effective at reducing drinking in patients with AUD, these findings are exciting as they suggest that spironolactone may be a promising compound and new treatment options for AUD are much needed,” said Dr. Besheer, who was not involved with the current study.
Dr. Leggio agreed. “We now need prospective, placebo-controlled studies to assess the potential safety and efficacy of spironolactone in people with AUD,” he said.
This work was supported by the National Institutes of Health and the NIAAA. Dr. Leggio, study coauthors, and Dr. Besheer declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AUD), new research suggests.
Researchers at the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and Yale University, New Haven, Conn., investigated the impact of spironolactone on AUD.
Initially, they studied rodents and found that spironolactone reduced binge drinking in mice and reduced self-administration of alcohol in rats without adversely affecting food or water intake or causing motor or coordination problems.
They also analyzed electronic health records of patients drawn from the United States Veterans Affairs health care system to explore potential changes in alcohol use after spironolactone treatment was initiated for other conditions and found a significant link between spironolactone treatment and reduction in self-reported alcohol consumption, with the largest effects observed among those who reported hazardous/heavy episodic alcohol use prior to starting spironolactone treatment.
“Combining findings across three species and different types of research studies, and then seeing similarities in these data, gives us confidence that we are onto something potentially important scientifically and clinically,” senior coauthor Lorenzo Leggio, MD, PhD, senior investigator in the Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, a joint NIDA and NIAAA laboratory, said in a news release.
The study was published online in Molecular Psychiatry.
There is a “critical need to increase the armamentarium of pharmacotherapies to treat individuals with AUD,” the authors note, adding that neuroendocrine systems involved in alcohol craving and drinking “offer promising pharmacologic targets in this regard.”
“Both our team and others have observed that patients with AUD often present with changes in peripheral hormones, including aldosterone, which plays a key role in regulating blood pressure and electrolytes,” Dr. Leggio said in an interview.
Spironolactone is a nonselective mineralocorticoid receptor (MT) antagonist. In studies in animal models, investigators said they found “an inverse correlation between alcohol drinking and the expression of the MR in the amygdala, a key brain region in the development and maintenance of AUD and addiction in general.”
Taken together, this led them to hypothesize that blocking the MR, which is the mechanism of action of spironolactone, “could be a novel pharmacotherapeutic approach for AUD,” he said.
Previous research by the same group of researchers suggested spironolactone “may be a potential new medication to treat patients with AUD.” The present study expanded on those findings and consisted of a three-part investigation.
In the current study, the investigators tested different dosages of spironolactone on binge-like alcohol consumption in male and female mice and assessed food and water intake, blood alcohol levels, motor coordination, and spontaneous locomotion.
They then tested the effects of different dosages of spironolactone injections on operant alcohol self-administration in alcohol-dependent and nondependent male and female rats, also testing blood alcohol levels and motor coordination.
Finally, they analyzed health records of veterans to examine the association between at least 60 continuous days of spironolactone treatment and self-reported alcohol consumption (measured by the Alcohol Use Disorders Identification Test-Consumption [AUDIT-C]).
Each of the spironolactone-exposed patients was matched using propensity scores with up to five unexposed patients who had reported alcohol consumption in the 2 years prior to the index date.
The final analysis included a matched cohort of 10,726 spironolactone-exposed individuals who were matched to 34,461 unexposed individuals.
New targets
Spironolactone reduced alcohol intake in mice drinking a sweetened alcohol solution; a 2-way ANOVA revealed a main effect of dose (F 4,52 = 9.09; P < .0001) and sex, with female mice drinking more alcohol, compared to male mice (F 1,13 = 6.05; P = .02).
Post hoc comparisons showed that spironolactone at doses of 50, 100, and 200 mg/kg significantly reduced alcohol intake (P values = .007, .002, and .0001, respectively).
In mice drinking an unsweetened alcohol solution, the 2-way repeated measures ANOVA similarly found a main effect of dose (F 4,52 = 5.77; P = .0006), but not of sex (F 1,13 = 1.41; P = .25).
Spironolactone had no effect on the mice’s intake of a sweet solution without alcohol and had no impact on the consumption of food and water or on locomotion and coordination.
In rats, a 2-way ANOVA revealed a significant spironolactone effect of dose (F 3,66 = 43.95; P < .001), with a post hoc test indicating that spironolactone at 25, 50, and 75 mg/kg reduced alcohol self-administration in alcohol-dependent and nondependent rats (all P values = .0001).
In humans, among the exposed individuals in the matched cohort, 25%, 57%, and 18% received daily doses of spironolactone of less than 25 mg/day, 25-49 mg/day, and 50 mg/day or higher, respectively, with a median follow-up time of 542 (interquartile range, 337-730) days.
The AUDIT-C scores decreased during the study period in both treatment groups, with a larger decrease in average AUDIT-C scores among the exposed vs. unexposed individuals.

“These are very exciting times because, thanks to the progress in the addiction biomedical research field, we are increasing our understanding of the mechanisms how some people develop AUD; hence we can use this knowledge to identify new targets.” The current study “is an example of these ongoing efforts,” said Dr. Leggio.
“It is important to note that [these results] are important but preliminary.” At this juncture, “it would be too premature to think about prescribing spironolactone to treat AUD,” he added.
Exciting findings
Commenting on the study, Joyce Besheer, PhD, professor, department of psychiatry and Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, called the study an “elegant demonstration of translational science.”
“While clinical trials will be needed to determine whether this medication is effective at reducing drinking in patients with AUD, these findings are exciting as they suggest that spironolactone may be a promising compound and new treatment options for AUD are much needed,” said Dr. Besheer, who was not involved with the current study.
Dr. Leggio agreed. “We now need prospective, placebo-controlled studies to assess the potential safety and efficacy of spironolactone in people with AUD,” he said.
This work was supported by the National Institutes of Health and the NIAAA. Dr. Leggio, study coauthors, and Dr. Besheer declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MOLECULAR PSYCHIATRY
Coffee linked to reduced cardiovascular disease and mortality risk
Drinking two to three daily cups of – ground, instant, or decaffeinated – is associated with significant reductions in new cardiovascular disease (CVD) and mortality risk, compared with avoiding coffee, a new analysis of the prospective UK Biobank suggests.
Ground and instant coffee, but not decaffeinated coffee, also was associated with reduced risk of new-onset arrhythmia, including atrial fibrillation.
“Our study is the first to look at differences in coffee subtypes to tease out important differences which may explain some of the mechanisms through which coffee works,” Peter M. Kistler, MD, of the Alfred Hospital and Baker Heart and Diabetes Institute, Melbourne, Australia, told this news organization.
“Daily coffee intake should not be discouraged by physicians but rather considered part of a healthy diet,” Dr. Kistler said.
“This study supports that coffee is safe and even potentially beneficial, which is consistent with most of the prior evidence,” Carl “Chip” Lavie, MD, who wasn’t involved in the study, told this news organization.
“We do not prescribe coffee to patients, but for the majority who like coffee, they can be encouraged it is fine to take a few cups daily,” said Dr. Lavie, with the Ochsner Heart and Vascular Institute in New Orleans.
The study was published online in the European Journal of Preventive Cardiology.
Clear cardiovascular benefits
A total of 449,563 UK Biobank participants (median age 58 years; 55% women), who were free of arrhythmias or other CVD at baseline, reported in questionnaires their level of daily coffee intake and preferred type of coffee.
During more than 12.5 years of follow-up, 27,809 participants (6.2%) died.
Drinking one to five cups per day of ground or instant coffee (but not decaffeinated coffee) was associated with a significant reduction in incident arrhythmia. The lowest risk was with four to five cups per day for ground coffee (hazard ratio [HR] 0.83; 95% confidence interval [CI], 0.76-0.91; P < .0001) and two to three cups per day for instant coffee (HR, 0.88; 95% CI, 0.85-0.92; P < .0001).
Habitual coffee drinking of up to five cups perday was also associated with significant reductions in the risk of incident CVD, when compared with nondrinkers.
Significant reductions in the risk of incident coronary heart disease (CHD) were associated with habitual coffee intake of up to five cups per day, with the lowest risk for CHD observed in those who consumed two to three cups per day (HR 0.89; 95% CI, 0.86-0.91; P < .0001).
Coffee consumption at all levels was linked to significant reduction in the risk of congestive cardiac failure (CCF) and ischemic stroke. The lowest risks were observed in those who consumed two to three cups per day, with HR, 0.83 (95% CI, 0.79-0.87; P < .0001) for CCF and HR, 0.84 (95% CI, 0.78-0.90; P < .0001) for ischemic stroke.
Death from any cause was significantly reduced for all coffee subtypes, with the greatest risk reduction seen with two to three cups per day for decaffeinated (HR, 0.86; 95% CI, 0.81-0.91; P < .0001); ground (HR, 0.73; 95% CI, 0.69-0.78; P < .0001); and instant coffee (HR, 0.89; 95% CI, 0.86-0.93; P < .0001).
“Coffee consumption is associated with cardiovascular benefits and should not empirically be discontinued in those with underlying heart rhythm disorders or cardiovascular disease,” Dr. Kistler told this news organization.
Plausible mechanisms
There are a number of proposed mechanisms to explain the benefits of coffee on CVD.
“Caffeine has antiarrhythmic properties through adenosine A1 and A2A receptor inhibition, hence the difference in effects of decaf vs. full-strength coffee on heart rhythm disorders,” Dr. Kistler explained.
Coffee has vasodilatory effects and coffee also contains antioxidant polyphenols, which reduce oxidative stress and modulate metabolism.
“The explanation for improved survival with habitual coffee consumption remains unclear,” Dr. Kistler said.
“Putative mechanisms include improved endothelial function, circulating antioxidants, improved insulin sensitivity, and reduced inflammation. Another potential mechanism includes the beneficial effects of coffee on metabolic syndrome,” he said.
“Caffeine has a role in weight loss through inhibition of gut fatty acid absorption and increase in basal metabolic rate. Furthermore, coffee has been associated with a significantly lower incidence of type 2 diabetes mellitus,” Dr. Kistler added.
Direction of relationship unclear
Charlotte Mills, PhD, University of Reading, England, said this study “adds to the body of evidence from observational trials associating moderate coffee consumption with cardioprotection, which looks promising.”
However, with the observational design, it’s unclear “which direction the relationship goes – for example, does coffee make you healthy or do inherently healthier people consume coffee? Randomized controlled trials are needed to fully understand the relationship between coffee and health before recommendations can be made,” Dr. Mills told the UK nonprofit Science Media Centre.
Annette Creedon, PhD, nutrition scientist with the British Nutrition Foundation, said it’s possible that respondents over- or underestimated the amount of coffee that they were consuming at the start of the study when they self-reported their intake.
“It is therefore difficult to determine whether the outcomes can be directly associated with the behaviors in coffee consumption reported at the start of the study,” she told the Science Media Centre.
The study had no funding. Dr. Kistler has received funding from Abbott Medical for consultancy and speaking engagements and fellowship support from Biosense Webster. Dr. Lavie has no relevant disclosures. Dr. Mills has worked in collaboration with Nestle on research relating to coffee and health funded by UKRI. Dr. Creedon has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Drinking two to three daily cups of – ground, instant, or decaffeinated – is associated with significant reductions in new cardiovascular disease (CVD) and mortality risk, compared with avoiding coffee, a new analysis of the prospective UK Biobank suggests.
Ground and instant coffee, but not decaffeinated coffee, also was associated with reduced risk of new-onset arrhythmia, including atrial fibrillation.
“Our study is the first to look at differences in coffee subtypes to tease out important differences which may explain some of the mechanisms through which coffee works,” Peter M. Kistler, MD, of the Alfred Hospital and Baker Heart and Diabetes Institute, Melbourne, Australia, told this news organization.
“Daily coffee intake should not be discouraged by physicians but rather considered part of a healthy diet,” Dr. Kistler said.
“This study supports that coffee is safe and even potentially beneficial, which is consistent with most of the prior evidence,” Carl “Chip” Lavie, MD, who wasn’t involved in the study, told this news organization.
“We do not prescribe coffee to patients, but for the majority who like coffee, they can be encouraged it is fine to take a few cups daily,” said Dr. Lavie, with the Ochsner Heart and Vascular Institute in New Orleans.
The study was published online in the European Journal of Preventive Cardiology.
Clear cardiovascular benefits
A total of 449,563 UK Biobank participants (median age 58 years; 55% women), who were free of arrhythmias or other CVD at baseline, reported in questionnaires their level of daily coffee intake and preferred type of coffee.
During more than 12.5 years of follow-up, 27,809 participants (6.2%) died.
Drinking one to five cups per day of ground or instant coffee (but not decaffeinated coffee) was associated with a significant reduction in incident arrhythmia. The lowest risk was with four to five cups per day for ground coffee (hazard ratio [HR] 0.83; 95% confidence interval [CI], 0.76-0.91; P < .0001) and two to three cups per day for instant coffee (HR, 0.88; 95% CI, 0.85-0.92; P < .0001).
Habitual coffee drinking of up to five cups perday was also associated with significant reductions in the risk of incident CVD, when compared with nondrinkers.
Significant reductions in the risk of incident coronary heart disease (CHD) were associated with habitual coffee intake of up to five cups per day, with the lowest risk for CHD observed in those who consumed two to three cups per day (HR 0.89; 95% CI, 0.86-0.91; P < .0001).
Coffee consumption at all levels was linked to significant reduction in the risk of congestive cardiac failure (CCF) and ischemic stroke. The lowest risks were observed in those who consumed two to three cups per day, with HR, 0.83 (95% CI, 0.79-0.87; P < .0001) for CCF and HR, 0.84 (95% CI, 0.78-0.90; P < .0001) for ischemic stroke.
Death from any cause was significantly reduced for all coffee subtypes, with the greatest risk reduction seen with two to three cups per day for decaffeinated (HR, 0.86; 95% CI, 0.81-0.91; P < .0001); ground (HR, 0.73; 95% CI, 0.69-0.78; P < .0001); and instant coffee (HR, 0.89; 95% CI, 0.86-0.93; P < .0001).
“Coffee consumption is associated with cardiovascular benefits and should not empirically be discontinued in those with underlying heart rhythm disorders or cardiovascular disease,” Dr. Kistler told this news organization.
Plausible mechanisms
There are a number of proposed mechanisms to explain the benefits of coffee on CVD.
“Caffeine has antiarrhythmic properties through adenosine A1 and A2A receptor inhibition, hence the difference in effects of decaf vs. full-strength coffee on heart rhythm disorders,” Dr. Kistler explained.
Coffee has vasodilatory effects and coffee also contains antioxidant polyphenols, which reduce oxidative stress and modulate metabolism.
“The explanation for improved survival with habitual coffee consumption remains unclear,” Dr. Kistler said.
“Putative mechanisms include improved endothelial function, circulating antioxidants, improved insulin sensitivity, and reduced inflammation. Another potential mechanism includes the beneficial effects of coffee on metabolic syndrome,” he said.
“Caffeine has a role in weight loss through inhibition of gut fatty acid absorption and increase in basal metabolic rate. Furthermore, coffee has been associated with a significantly lower incidence of type 2 diabetes mellitus,” Dr. Kistler added.
Direction of relationship unclear
Charlotte Mills, PhD, University of Reading, England, said this study “adds to the body of evidence from observational trials associating moderate coffee consumption with cardioprotection, which looks promising.”
However, with the observational design, it’s unclear “which direction the relationship goes – for example, does coffee make you healthy or do inherently healthier people consume coffee? Randomized controlled trials are needed to fully understand the relationship between coffee and health before recommendations can be made,” Dr. Mills told the UK nonprofit Science Media Centre.
Annette Creedon, PhD, nutrition scientist with the British Nutrition Foundation, said it’s possible that respondents over- or underestimated the amount of coffee that they were consuming at the start of the study when they self-reported their intake.
“It is therefore difficult to determine whether the outcomes can be directly associated with the behaviors in coffee consumption reported at the start of the study,” she told the Science Media Centre.
The study had no funding. Dr. Kistler has received funding from Abbott Medical for consultancy and speaking engagements and fellowship support from Biosense Webster. Dr. Lavie has no relevant disclosures. Dr. Mills has worked in collaboration with Nestle on research relating to coffee and health funded by UKRI. Dr. Creedon has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Drinking two to three daily cups of – ground, instant, or decaffeinated – is associated with significant reductions in new cardiovascular disease (CVD) and mortality risk, compared with avoiding coffee, a new analysis of the prospective UK Biobank suggests.
Ground and instant coffee, but not decaffeinated coffee, also was associated with reduced risk of new-onset arrhythmia, including atrial fibrillation.
“Our study is the first to look at differences in coffee subtypes to tease out important differences which may explain some of the mechanisms through which coffee works,” Peter M. Kistler, MD, of the Alfred Hospital and Baker Heart and Diabetes Institute, Melbourne, Australia, told this news organization.
“Daily coffee intake should not be discouraged by physicians but rather considered part of a healthy diet,” Dr. Kistler said.
“This study supports that coffee is safe and even potentially beneficial, which is consistent with most of the prior evidence,” Carl “Chip” Lavie, MD, who wasn’t involved in the study, told this news organization.
“We do not prescribe coffee to patients, but for the majority who like coffee, they can be encouraged it is fine to take a few cups daily,” said Dr. Lavie, with the Ochsner Heart and Vascular Institute in New Orleans.
The study was published online in the European Journal of Preventive Cardiology.
Clear cardiovascular benefits
A total of 449,563 UK Biobank participants (median age 58 years; 55% women), who were free of arrhythmias or other CVD at baseline, reported in questionnaires their level of daily coffee intake and preferred type of coffee.
During more than 12.5 years of follow-up, 27,809 participants (6.2%) died.
Drinking one to five cups per day of ground or instant coffee (but not decaffeinated coffee) was associated with a significant reduction in incident arrhythmia. The lowest risk was with four to five cups per day for ground coffee (hazard ratio [HR] 0.83; 95% confidence interval [CI], 0.76-0.91; P < .0001) and two to three cups per day for instant coffee (HR, 0.88; 95% CI, 0.85-0.92; P < .0001).
Habitual coffee drinking of up to five cups perday was also associated with significant reductions in the risk of incident CVD, when compared with nondrinkers.
Significant reductions in the risk of incident coronary heart disease (CHD) were associated with habitual coffee intake of up to five cups per day, with the lowest risk for CHD observed in those who consumed two to three cups per day (HR 0.89; 95% CI, 0.86-0.91; P < .0001).
Coffee consumption at all levels was linked to significant reduction in the risk of congestive cardiac failure (CCF) and ischemic stroke. The lowest risks were observed in those who consumed two to three cups per day, with HR, 0.83 (95% CI, 0.79-0.87; P < .0001) for CCF and HR, 0.84 (95% CI, 0.78-0.90; P < .0001) for ischemic stroke.
Death from any cause was significantly reduced for all coffee subtypes, with the greatest risk reduction seen with two to three cups per day for decaffeinated (HR, 0.86; 95% CI, 0.81-0.91; P < .0001); ground (HR, 0.73; 95% CI, 0.69-0.78; P < .0001); and instant coffee (HR, 0.89; 95% CI, 0.86-0.93; P < .0001).
“Coffee consumption is associated with cardiovascular benefits and should not empirically be discontinued in those with underlying heart rhythm disorders or cardiovascular disease,” Dr. Kistler told this news organization.
Plausible mechanisms
There are a number of proposed mechanisms to explain the benefits of coffee on CVD.
“Caffeine has antiarrhythmic properties through adenosine A1 and A2A receptor inhibition, hence the difference in effects of decaf vs. full-strength coffee on heart rhythm disorders,” Dr. Kistler explained.
Coffee has vasodilatory effects and coffee also contains antioxidant polyphenols, which reduce oxidative stress and modulate metabolism.
“The explanation for improved survival with habitual coffee consumption remains unclear,” Dr. Kistler said.
“Putative mechanisms include improved endothelial function, circulating antioxidants, improved insulin sensitivity, and reduced inflammation. Another potential mechanism includes the beneficial effects of coffee on metabolic syndrome,” he said.
“Caffeine has a role in weight loss through inhibition of gut fatty acid absorption and increase in basal metabolic rate. Furthermore, coffee has been associated with a significantly lower incidence of type 2 diabetes mellitus,” Dr. Kistler added.
Direction of relationship unclear
Charlotte Mills, PhD, University of Reading, England, said this study “adds to the body of evidence from observational trials associating moderate coffee consumption with cardioprotection, which looks promising.”
However, with the observational design, it’s unclear “which direction the relationship goes – for example, does coffee make you healthy or do inherently healthier people consume coffee? Randomized controlled trials are needed to fully understand the relationship between coffee and health before recommendations can be made,” Dr. Mills told the UK nonprofit Science Media Centre.
Annette Creedon, PhD, nutrition scientist with the British Nutrition Foundation, said it’s possible that respondents over- or underestimated the amount of coffee that they were consuming at the start of the study when they self-reported their intake.
“It is therefore difficult to determine whether the outcomes can be directly associated with the behaviors in coffee consumption reported at the start of the study,” she told the Science Media Centre.
The study had no funding. Dr. Kistler has received funding from Abbott Medical for consultancy and speaking engagements and fellowship support from Biosense Webster. Dr. Lavie has no relevant disclosures. Dr. Mills has worked in collaboration with Nestle on research relating to coffee and health funded by UKRI. Dr. Creedon has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
Newer 3D lung models starting to remake research
Pulmonologist-scientist Veena B. Antony, MD, professor of medicine at the University of Alabama in Birmingham, grows “pulmospheres” in her lab. The tiny spheres, about 1 mL in diameter, contain cells representing all of the cell types in a lung struck with pulmonary fibrosis.
They are a three-dimensional model of idiopathic pulmonary fibrosis (IPF) that can be used to study the behavior of invasive myofibroblasts and to predict in vivo responsiveness to antifibrotic drugs;
“The utility is extensive, including looking at the impact of early-life exposures on mid-life lung disease. We can ask all kinds of questions and answer them much faster, and with more accuracy, than with any 2D model,” said Dr. Antony, also professor of environmental health sciences and director of UAB’s program for environmental and translational medicine.
“The future of 3D modeling of the lung will happen step by step ... but we’re right at the edge of a prime explosion of information coming from these models, in all kinds of lung diseases,” she said.
Two-dimensional model systems – mainly monolayer cell cultures where cells adhere to and grow on a plate – cannot approximate the variety of cell types and architecture found in tissue, nor can they recapitulate cell-cell communication, biochemical cues, and other factors that are key to lung development and the pathogenesis of disease.
Dr. Antony’s pulmospheres resemble what have come to be known as organoids – 3D tissue cultures emanating from induced pluripotent stem cells (iPSC) or adult stem cells, in which multiple cell types self-organize, usually while suspended in natural or synthetic extracellular matrix (with or without a scaffold of some kind).
Lung-on-a-chip
In lung-on-a-chip (LOC) models, multiple cell types are seeded into miniature chambers, or “chips,” that contain networks of microfabricated channels designed to deliver and remove fluids, chemical cues, oxygen, and biomechanical forces. LOCs and other organs-on-chips – also called tissues-on-chips – can be continuously perfused and are highly structured and precisely controlled.
It’s the organs-on-chip model – or potential fusions of the organoid and organs-on-chip models – that will likely impact drug development. Almost 9 out of 10 investigational drugs fail in clinical trials – approximately 60% because of lack of efficacy and 30% because of toxicity. More reliable and predictive preclinical investigation is key, said Danilo A. Tagle, PhD, director of the Office of Special Initiatives in the National Center for Advancing Translational Sciences, of the National Institutes of Health.
“We have so many candidate drugs that go through preclinical safety testing, and that do relatively well in animal studies of efficacy, but then fail in clinical trials,” Dr. Tagle said. “We need better preclinical models.”
In its 10 years of life, the Tissue Chip for Drug Screening Program led by the NCATS – and funded by the NIH and Defense Advanced Research Projects Agency – has shown that organs-on-chips can be used to model disease and to predict both the safety and efficacy of clinical compounds, he said.
Lung organoids
Dr. Antony’s pulmospheres emanate not from stem cells but from primary tissue obtained from diseased lung. “We reconstitute the lung cells in single-cell suspensions, and then we allow them to come back together to form lung tissue,” she said. The pulmospheres take about 3 days to grow.
In a study published 5 years ago of pulmospheres of 20 patients with IPF and 9 control subjects, Dr. Antony and colleagues quantitated invasiveness and found “remarkable” differences in the invasiveness of IPF pulmospheres following exposure to the Food and Drug Administration–approved antifibrotic drugs nintedanib and pirfenidone. Some pulmospheres responded to one or the other drug, some to both, and two to neither – findings that Dr. Antony said offer hope for the goals of personalizing therapy and assessing new drugs.
Moreover, clinical disease progression correlated with invasiveness of the pulmospheres, showing that the organoid-like structures “do give us a model that [reflects] what’s happening in the clinical setting,” she said. (Lung tissue for the study was obtained via video-assisted thoracic surgery biopsy of IPF patients and from failed donor lung explants, but bronchoscopic forceps biopsies have become a useful method for obtaining tissue.)
The pulmospheres are not yet in clinical use, Dr. Antony said, but her lab is testing other fibrosis modifiers and continuing to use the model as a research tool.
One state to the east, at Vanderbilt University, Nashville, Tenn., Amanda Linkous, PhD, grows “branching lung organoids” and brain organoids to study the biology of small cell lung cancer (SCLC).
“We want to understand how [SCLC] cells change in the primary organ site, compared with metastatic sites like the brain. ... Are different transcription factors expressed [for instance] depending on where the tumor is growing?” said Dr. Linkous, scientific center manager of the National Cancer Institute’s Center for Systems Biology of SCLC at Vanderbilt. “Then we hope to start drug screening within the next year.”
Her lung organoids take shape from either human embryonic stem cells or iPSCs. Within commercially available media, the cells mature through several stages of differentiation, forming definitive endoderm, anterior foregut endoderm, and then circular lung bud structures – the latter of which are then placed into droplets of Matrigel, an extracellular matrix gel.
“In the Matrigel droplets, the lung bud cells will develop proximal and distal-like branching structures that express things like EPCAM, MUC1, SOX2, SOX9, and NKX2.1 – key markers that you should see in a more mature lung microenvironment,” she said. Tumor cells from established SCLC cell lines will then easily invade the branching lung organoid.
Dr. Linkous said she has found her organoid models highly reproducible and values their long-lasting nature – especially for future drug screening. “We can keep organoids going for months at a time,” said Dr. Linkous, a research associate professor in Vanderbilt’s department of biochemistry.
Like Dr. Antony, she envisions personalizing treatment in the future. “SCLC is a very heterogeneous tumor with many different cell types, so what works for one patient may not work well at all for another patient,” she said.
As recently as 5 years ago, “many in the cancer field would have been resistant to moving away from mouse models,” Dr. Linkous noted. “But preclinical studies in mice often don’t pan out in the clinic ... so we’re moving toward a human microenvironment to study human disease.”
The greatest challenge, Dr. Linkous and Dr. Antony said, lies in integrating both vascular blood flow and air into these models. “We just don’t have that combination as of yet,” Dr. Antony said.
LOC models
One of the first LOC models – and a galvanizing event for organs-on-chips more broadly – was a 1- to 2-cm–long model of the alveolar-capillary interface developed at the Wyss Institute for Biologically Inspired Engineering at Harvard Medical School, Boston.
Microchannels ran alongside a porous membrane coated with extracellular matrix, with alveolar cells seeded on one side and lung endothelial cells on the other side. When a vacuum was applied rhythmically to the channels, the cell-lined membrane stretched and relaxed, mimicking breathing movements.
Lead investigator Dongeun (Dan) Huh, PhD, then a postdoctoral student working with Donald E. Ingber, MD, PhD, founding director of the institute, ran tests showing that the model could reproduce organ-level responses to bacteria and inflammatory cytokines, as well as to silica nanoparticles. The widely cited paper was published in 2010 (Science. 2010;328[5986]:1662-8), and was followed by another study published in 2012 (Sci Transl Med. 2012;4[159]:159ra147) that used the LOC device to reproduce drug toxicity–induced pulmonary edema. “Here we were demonstrating for the first time that we could use the lung-on-chip to model human lung disease,” said Dr. Huh, who started his own lab at the University of Pennsylvania, Philadelphia, in 2013.
Since then, “as a field we’ve come a long way in modeling the complexity of human lung tissues ... with more advanced devices that can be used to mimic different parts of the lung and different processes, like immune responses in asthma and viral infections,” said Dr. Huh, “and with several studies using primary human cells taken from lung disease patients.”
Among Dr. Huh’s latest devices, built with NIH funding, is an asthma-on-a-chip device. Lung cells isolated from asthma patients are grown in a microfabricated device to create multilayered airway tissue, with airspace, that contains a fully differentiated epithelium and a vascularized stroma. “We can compress the entire engineered area of asthmatic human tissue in a lateral direction to mimic bronchoconstriction that happens during an asthma attack,” he said.
A paper soon to be published will describe how “abnormal pathophysiologic compressive forces due to bronchoconstriction in asthmatic lungs can make the lungs fibrotic, and how those mechanical forces also can induce increased vascularity,” said Dr. Huh, associate professor in the university’s department of bioengineering. “The increased vascular density can also change the phenotype of blood vessels in asthmatic airways.”
Dr. Huh also has an $8.3 million contract with the government’s Biomedical Advanced Research and Development Authority to study how chlorine gas damages lung tissues and identify biomarkers of chlorine gas–induced lung injury, with the goal of developing therapeutics.
Dr. Ingber and associates have developed a device modeling cystic fibrosis (CF). The chip is lined with primary human CF bronchial epithelial cells grown under an air-liquid interface and interfaced with primary lung microvascular endothelium that are exposed to fluid flow.
The chip reproduced, “with high fidelity, many of the structural, biochemical, and pathophysiological features of the human CF lung airway and its response to pathogens and circulating immune cells in vitro,” Dr. Ingber and colleagues reported (J Cyst Fibros. 2022;21:605-15).
Government investment in tissue chips
Efforts to commercialize organs-on-chip platforms and translate them for nonengineers have also picked in recent years. Several companies in the United States (including Emulate, a Wyss start-up) and in Europe now offer microengineered lung tissue models that can be used for research and drug testing. And some large pharmaceutical companies, said Dr. Tagle, have begun integrating tissue chip technology into their drug development programs.
The FDA, meanwhile, “has come to embrace the technology and see its promise,” Dr. Tagle said. An FDA pilot program announced in 2021 – called ISTAND (Innovative Science and Technology Approaches for New Drugs) – allows for tissue chip data to be submitted, as standalone data, for some drug applications.
The first 5 years of the government’s Tissue Chip for Drug Screening Program focused on safety and toxicity, and it “was successful in that model organ systems were able to capture the human response that [had been missed in] animal models,” he said.
For example, when a liver-tissue model was used to test several compounds that had passed animal testing for toxicity/safety but then failed in human clinical trials – killing some of the participants – the model showed a 100% sensitivity and a 87% specificity in predicting the human response, said Dr. Tagle, who recently coauthored a review on the future of organs-on-chips (Nature Reviews I Drug Discovery. 2021;20:345-61).
The second 5 years of the program, currently winding down, have focused on efficacy – the ability of organs-on-chip models to recreate the pathophysiology of chronic obstructive pulmonary disease, influenza, and other diseases, so that potential drugs can be assessed. In 2020, with extra support from the Coronavirus Aid, Relief, and Economic Security Act, NCATS funded academic labs to use organs-on-chip technology to evaluate SARS-CoV-2 and potential therapeutics.
Dr. Ingbar was one of the grantees. His team screened a number of FDA-approved drugs for potential repurposing using a bronchial-airway-on-a-chip and compared results with 2D model systems (Nat Biomed Eng. 2021;5:815-29). Amodiaquine inhibited infection in the 3D model and is now in phase 2 COVID trials. Several other drugs showed effectiveness in a 2D model but not in the chip.
Now, in a next phase of study at NCATS, coined Clinical Trials on a Chip, the center has awarded $35.5 million for investigators to test candidate therapies, often in parallel to ongoing clinical trials. The hope is that organs-on-chips can improve clinical trial design, from enrollment criteria and patient stratification to endpoints and the use of biomarkers. And in his lab, Dr. Huh is now engineering a shift to “organoids-on-a-chip” that combines the best features of each approach. “The idea,” he said, “is to grow organoids, and maintain the organoids in the microengineered systems where we can control their environment better ... and apply cues to allow them to develop into even more realistic tissues.”
Drs. Antony, Linkous, and Tagle reported no relevant disclosures. Dr. Huh is a co-founder of Vivodyne Inc, and owns shares in Vivodyne Inc. and Emulate Inc.
Pulmonologist-scientist Veena B. Antony, MD, professor of medicine at the University of Alabama in Birmingham, grows “pulmospheres” in her lab. The tiny spheres, about 1 mL in diameter, contain cells representing all of the cell types in a lung struck with pulmonary fibrosis.
They are a three-dimensional model of idiopathic pulmonary fibrosis (IPF) that can be used to study the behavior of invasive myofibroblasts and to predict in vivo responsiveness to antifibrotic drugs;
“The utility is extensive, including looking at the impact of early-life exposures on mid-life lung disease. We can ask all kinds of questions and answer them much faster, and with more accuracy, than with any 2D model,” said Dr. Antony, also professor of environmental health sciences and director of UAB’s program for environmental and translational medicine.
“The future of 3D modeling of the lung will happen step by step ... but we’re right at the edge of a prime explosion of information coming from these models, in all kinds of lung diseases,” she said.
Two-dimensional model systems – mainly monolayer cell cultures where cells adhere to and grow on a plate – cannot approximate the variety of cell types and architecture found in tissue, nor can they recapitulate cell-cell communication, biochemical cues, and other factors that are key to lung development and the pathogenesis of disease.
Dr. Antony’s pulmospheres resemble what have come to be known as organoids – 3D tissue cultures emanating from induced pluripotent stem cells (iPSC) or adult stem cells, in which multiple cell types self-organize, usually while suspended in natural or synthetic extracellular matrix (with or without a scaffold of some kind).
Lung-on-a-chip
In lung-on-a-chip (LOC) models, multiple cell types are seeded into miniature chambers, or “chips,” that contain networks of microfabricated channels designed to deliver and remove fluids, chemical cues, oxygen, and biomechanical forces. LOCs and other organs-on-chips – also called tissues-on-chips – can be continuously perfused and are highly structured and precisely controlled.
It’s the organs-on-chip model – or potential fusions of the organoid and organs-on-chip models – that will likely impact drug development. Almost 9 out of 10 investigational drugs fail in clinical trials – approximately 60% because of lack of efficacy and 30% because of toxicity. More reliable and predictive preclinical investigation is key, said Danilo A. Tagle, PhD, director of the Office of Special Initiatives in the National Center for Advancing Translational Sciences, of the National Institutes of Health.
“We have so many candidate drugs that go through preclinical safety testing, and that do relatively well in animal studies of efficacy, but then fail in clinical trials,” Dr. Tagle said. “We need better preclinical models.”
In its 10 years of life, the Tissue Chip for Drug Screening Program led by the NCATS – and funded by the NIH and Defense Advanced Research Projects Agency – has shown that organs-on-chips can be used to model disease and to predict both the safety and efficacy of clinical compounds, he said.
Lung organoids
Dr. Antony’s pulmospheres emanate not from stem cells but from primary tissue obtained from diseased lung. “We reconstitute the lung cells in single-cell suspensions, and then we allow them to come back together to form lung tissue,” she said. The pulmospheres take about 3 days to grow.
In a study published 5 years ago of pulmospheres of 20 patients with IPF and 9 control subjects, Dr. Antony and colleagues quantitated invasiveness and found “remarkable” differences in the invasiveness of IPF pulmospheres following exposure to the Food and Drug Administration–approved antifibrotic drugs nintedanib and pirfenidone. Some pulmospheres responded to one or the other drug, some to both, and two to neither – findings that Dr. Antony said offer hope for the goals of personalizing therapy and assessing new drugs.
Moreover, clinical disease progression correlated with invasiveness of the pulmospheres, showing that the organoid-like structures “do give us a model that [reflects] what’s happening in the clinical setting,” she said. (Lung tissue for the study was obtained via video-assisted thoracic surgery biopsy of IPF patients and from failed donor lung explants, but bronchoscopic forceps biopsies have become a useful method for obtaining tissue.)
The pulmospheres are not yet in clinical use, Dr. Antony said, but her lab is testing other fibrosis modifiers and continuing to use the model as a research tool.
One state to the east, at Vanderbilt University, Nashville, Tenn., Amanda Linkous, PhD, grows “branching lung organoids” and brain organoids to study the biology of small cell lung cancer (SCLC).
“We want to understand how [SCLC] cells change in the primary organ site, compared with metastatic sites like the brain. ... Are different transcription factors expressed [for instance] depending on where the tumor is growing?” said Dr. Linkous, scientific center manager of the National Cancer Institute’s Center for Systems Biology of SCLC at Vanderbilt. “Then we hope to start drug screening within the next year.”
Her lung organoids take shape from either human embryonic stem cells or iPSCs. Within commercially available media, the cells mature through several stages of differentiation, forming definitive endoderm, anterior foregut endoderm, and then circular lung bud structures – the latter of which are then placed into droplets of Matrigel, an extracellular matrix gel.
“In the Matrigel droplets, the lung bud cells will develop proximal and distal-like branching structures that express things like EPCAM, MUC1, SOX2, SOX9, and NKX2.1 – key markers that you should see in a more mature lung microenvironment,” she said. Tumor cells from established SCLC cell lines will then easily invade the branching lung organoid.
Dr. Linkous said she has found her organoid models highly reproducible and values their long-lasting nature – especially for future drug screening. “We can keep organoids going for months at a time,” said Dr. Linkous, a research associate professor in Vanderbilt’s department of biochemistry.
Like Dr. Antony, she envisions personalizing treatment in the future. “SCLC is a very heterogeneous tumor with many different cell types, so what works for one patient may not work well at all for another patient,” she said.
As recently as 5 years ago, “many in the cancer field would have been resistant to moving away from mouse models,” Dr. Linkous noted. “But preclinical studies in mice often don’t pan out in the clinic ... so we’re moving toward a human microenvironment to study human disease.”
The greatest challenge, Dr. Linkous and Dr. Antony said, lies in integrating both vascular blood flow and air into these models. “We just don’t have that combination as of yet,” Dr. Antony said.
LOC models
One of the first LOC models – and a galvanizing event for organs-on-chips more broadly – was a 1- to 2-cm–long model of the alveolar-capillary interface developed at the Wyss Institute for Biologically Inspired Engineering at Harvard Medical School, Boston.
Microchannels ran alongside a porous membrane coated with extracellular matrix, with alveolar cells seeded on one side and lung endothelial cells on the other side. When a vacuum was applied rhythmically to the channels, the cell-lined membrane stretched and relaxed, mimicking breathing movements.
Lead investigator Dongeun (Dan) Huh, PhD, then a postdoctoral student working with Donald E. Ingber, MD, PhD, founding director of the institute, ran tests showing that the model could reproduce organ-level responses to bacteria and inflammatory cytokines, as well as to silica nanoparticles. The widely cited paper was published in 2010 (Science. 2010;328[5986]:1662-8), and was followed by another study published in 2012 (Sci Transl Med. 2012;4[159]:159ra147) that used the LOC device to reproduce drug toxicity–induced pulmonary edema. “Here we were demonstrating for the first time that we could use the lung-on-chip to model human lung disease,” said Dr. Huh, who started his own lab at the University of Pennsylvania, Philadelphia, in 2013.
Since then, “as a field we’ve come a long way in modeling the complexity of human lung tissues ... with more advanced devices that can be used to mimic different parts of the lung and different processes, like immune responses in asthma and viral infections,” said Dr. Huh, “and with several studies using primary human cells taken from lung disease patients.”
Among Dr. Huh’s latest devices, built with NIH funding, is an asthma-on-a-chip device. Lung cells isolated from asthma patients are grown in a microfabricated device to create multilayered airway tissue, with airspace, that contains a fully differentiated epithelium and a vascularized stroma. “We can compress the entire engineered area of asthmatic human tissue in a lateral direction to mimic bronchoconstriction that happens during an asthma attack,” he said.
A paper soon to be published will describe how “abnormal pathophysiologic compressive forces due to bronchoconstriction in asthmatic lungs can make the lungs fibrotic, and how those mechanical forces also can induce increased vascularity,” said Dr. Huh, associate professor in the university’s department of bioengineering. “The increased vascular density can also change the phenotype of blood vessels in asthmatic airways.”
Dr. Huh also has an $8.3 million contract with the government’s Biomedical Advanced Research and Development Authority to study how chlorine gas damages lung tissues and identify biomarkers of chlorine gas–induced lung injury, with the goal of developing therapeutics.
Dr. Ingber and associates have developed a device modeling cystic fibrosis (CF). The chip is lined with primary human CF bronchial epithelial cells grown under an air-liquid interface and interfaced with primary lung microvascular endothelium that are exposed to fluid flow.
The chip reproduced, “with high fidelity, many of the structural, biochemical, and pathophysiological features of the human CF lung airway and its response to pathogens and circulating immune cells in vitro,” Dr. Ingber and colleagues reported (J Cyst Fibros. 2022;21:605-15).
Government investment in tissue chips
Efforts to commercialize organs-on-chip platforms and translate them for nonengineers have also picked in recent years. Several companies in the United States (including Emulate, a Wyss start-up) and in Europe now offer microengineered lung tissue models that can be used for research and drug testing. And some large pharmaceutical companies, said Dr. Tagle, have begun integrating tissue chip technology into their drug development programs.
The FDA, meanwhile, “has come to embrace the technology and see its promise,” Dr. Tagle said. An FDA pilot program announced in 2021 – called ISTAND (Innovative Science and Technology Approaches for New Drugs) – allows for tissue chip data to be submitted, as standalone data, for some drug applications.
The first 5 years of the government’s Tissue Chip for Drug Screening Program focused on safety and toxicity, and it “was successful in that model organ systems were able to capture the human response that [had been missed in] animal models,” he said.
For example, when a liver-tissue model was used to test several compounds that had passed animal testing for toxicity/safety but then failed in human clinical trials – killing some of the participants – the model showed a 100% sensitivity and a 87% specificity in predicting the human response, said Dr. Tagle, who recently coauthored a review on the future of organs-on-chips (Nature Reviews I Drug Discovery. 2021;20:345-61).
The second 5 years of the program, currently winding down, have focused on efficacy – the ability of organs-on-chip models to recreate the pathophysiology of chronic obstructive pulmonary disease, influenza, and other diseases, so that potential drugs can be assessed. In 2020, with extra support from the Coronavirus Aid, Relief, and Economic Security Act, NCATS funded academic labs to use organs-on-chip technology to evaluate SARS-CoV-2 and potential therapeutics.
Dr. Ingbar was one of the grantees. His team screened a number of FDA-approved drugs for potential repurposing using a bronchial-airway-on-a-chip and compared results with 2D model systems (Nat Biomed Eng. 2021;5:815-29). Amodiaquine inhibited infection in the 3D model and is now in phase 2 COVID trials. Several other drugs showed effectiveness in a 2D model but not in the chip.
Now, in a next phase of study at NCATS, coined Clinical Trials on a Chip, the center has awarded $35.5 million for investigators to test candidate therapies, often in parallel to ongoing clinical trials. The hope is that organs-on-chips can improve clinical trial design, from enrollment criteria and patient stratification to endpoints and the use of biomarkers. And in his lab, Dr. Huh is now engineering a shift to “organoids-on-a-chip” that combines the best features of each approach. “The idea,” he said, “is to grow organoids, and maintain the organoids in the microengineered systems where we can control their environment better ... and apply cues to allow them to develop into even more realistic tissues.”
Drs. Antony, Linkous, and Tagle reported no relevant disclosures. Dr. Huh is a co-founder of Vivodyne Inc, and owns shares in Vivodyne Inc. and Emulate Inc.
Pulmonologist-scientist Veena B. Antony, MD, professor of medicine at the University of Alabama in Birmingham, grows “pulmospheres” in her lab. The tiny spheres, about 1 mL in diameter, contain cells representing all of the cell types in a lung struck with pulmonary fibrosis.
They are a three-dimensional model of idiopathic pulmonary fibrosis (IPF) that can be used to study the behavior of invasive myofibroblasts and to predict in vivo responsiveness to antifibrotic drugs;
“The utility is extensive, including looking at the impact of early-life exposures on mid-life lung disease. We can ask all kinds of questions and answer them much faster, and with more accuracy, than with any 2D model,” said Dr. Antony, also professor of environmental health sciences and director of UAB’s program for environmental and translational medicine.
“The future of 3D modeling of the lung will happen step by step ... but we’re right at the edge of a prime explosion of information coming from these models, in all kinds of lung diseases,” she said.
Two-dimensional model systems – mainly monolayer cell cultures where cells adhere to and grow on a plate – cannot approximate the variety of cell types and architecture found in tissue, nor can they recapitulate cell-cell communication, biochemical cues, and other factors that are key to lung development and the pathogenesis of disease.
Dr. Antony’s pulmospheres resemble what have come to be known as organoids – 3D tissue cultures emanating from induced pluripotent stem cells (iPSC) or adult stem cells, in which multiple cell types self-organize, usually while suspended in natural or synthetic extracellular matrix (with or without a scaffold of some kind).
Lung-on-a-chip
In lung-on-a-chip (LOC) models, multiple cell types are seeded into miniature chambers, or “chips,” that contain networks of microfabricated channels designed to deliver and remove fluids, chemical cues, oxygen, and biomechanical forces. LOCs and other organs-on-chips – also called tissues-on-chips – can be continuously perfused and are highly structured and precisely controlled.
It’s the organs-on-chip model – or potential fusions of the organoid and organs-on-chip models – that will likely impact drug development. Almost 9 out of 10 investigational drugs fail in clinical trials – approximately 60% because of lack of efficacy and 30% because of toxicity. More reliable and predictive preclinical investigation is key, said Danilo A. Tagle, PhD, director of the Office of Special Initiatives in the National Center for Advancing Translational Sciences, of the National Institutes of Health.
“We have so many candidate drugs that go through preclinical safety testing, and that do relatively well in animal studies of efficacy, but then fail in clinical trials,” Dr. Tagle said. “We need better preclinical models.”
In its 10 years of life, the Tissue Chip for Drug Screening Program led by the NCATS – and funded by the NIH and Defense Advanced Research Projects Agency – has shown that organs-on-chips can be used to model disease and to predict both the safety and efficacy of clinical compounds, he said.
Lung organoids
Dr. Antony’s pulmospheres emanate not from stem cells but from primary tissue obtained from diseased lung. “We reconstitute the lung cells in single-cell suspensions, and then we allow them to come back together to form lung tissue,” she said. The pulmospheres take about 3 days to grow.
In a study published 5 years ago of pulmospheres of 20 patients with IPF and 9 control subjects, Dr. Antony and colleagues quantitated invasiveness and found “remarkable” differences in the invasiveness of IPF pulmospheres following exposure to the Food and Drug Administration–approved antifibrotic drugs nintedanib and pirfenidone. Some pulmospheres responded to one or the other drug, some to both, and two to neither – findings that Dr. Antony said offer hope for the goals of personalizing therapy and assessing new drugs.
Moreover, clinical disease progression correlated with invasiveness of the pulmospheres, showing that the organoid-like structures “do give us a model that [reflects] what’s happening in the clinical setting,” she said. (Lung tissue for the study was obtained via video-assisted thoracic surgery biopsy of IPF patients and from failed donor lung explants, but bronchoscopic forceps biopsies have become a useful method for obtaining tissue.)
The pulmospheres are not yet in clinical use, Dr. Antony said, but her lab is testing other fibrosis modifiers and continuing to use the model as a research tool.
One state to the east, at Vanderbilt University, Nashville, Tenn., Amanda Linkous, PhD, grows “branching lung organoids” and brain organoids to study the biology of small cell lung cancer (SCLC).
“We want to understand how [SCLC] cells change in the primary organ site, compared with metastatic sites like the brain. ... Are different transcription factors expressed [for instance] depending on where the tumor is growing?” said Dr. Linkous, scientific center manager of the National Cancer Institute’s Center for Systems Biology of SCLC at Vanderbilt. “Then we hope to start drug screening within the next year.”
Her lung organoids take shape from either human embryonic stem cells or iPSCs. Within commercially available media, the cells mature through several stages of differentiation, forming definitive endoderm, anterior foregut endoderm, and then circular lung bud structures – the latter of which are then placed into droplets of Matrigel, an extracellular matrix gel.
“In the Matrigel droplets, the lung bud cells will develop proximal and distal-like branching structures that express things like EPCAM, MUC1, SOX2, SOX9, and NKX2.1 – key markers that you should see in a more mature lung microenvironment,” she said. Tumor cells from established SCLC cell lines will then easily invade the branching lung organoid.
Dr. Linkous said she has found her organoid models highly reproducible and values their long-lasting nature – especially for future drug screening. “We can keep organoids going for months at a time,” said Dr. Linkous, a research associate professor in Vanderbilt’s department of biochemistry.
Like Dr. Antony, she envisions personalizing treatment in the future. “SCLC is a very heterogeneous tumor with many different cell types, so what works for one patient may not work well at all for another patient,” she said.
As recently as 5 years ago, “many in the cancer field would have been resistant to moving away from mouse models,” Dr. Linkous noted. “But preclinical studies in mice often don’t pan out in the clinic ... so we’re moving toward a human microenvironment to study human disease.”
The greatest challenge, Dr. Linkous and Dr. Antony said, lies in integrating both vascular blood flow and air into these models. “We just don’t have that combination as of yet,” Dr. Antony said.
LOC models
One of the first LOC models – and a galvanizing event for organs-on-chips more broadly – was a 1- to 2-cm–long model of the alveolar-capillary interface developed at the Wyss Institute for Biologically Inspired Engineering at Harvard Medical School, Boston.
Microchannels ran alongside a porous membrane coated with extracellular matrix, with alveolar cells seeded on one side and lung endothelial cells on the other side. When a vacuum was applied rhythmically to the channels, the cell-lined membrane stretched and relaxed, mimicking breathing movements.
Lead investigator Dongeun (Dan) Huh, PhD, then a postdoctoral student working with Donald E. Ingber, MD, PhD, founding director of the institute, ran tests showing that the model could reproduce organ-level responses to bacteria and inflammatory cytokines, as well as to silica nanoparticles. The widely cited paper was published in 2010 (Science. 2010;328[5986]:1662-8), and was followed by another study published in 2012 (Sci Transl Med. 2012;4[159]:159ra147) that used the LOC device to reproduce drug toxicity–induced pulmonary edema. “Here we were demonstrating for the first time that we could use the lung-on-chip to model human lung disease,” said Dr. Huh, who started his own lab at the University of Pennsylvania, Philadelphia, in 2013.
Since then, “as a field we’ve come a long way in modeling the complexity of human lung tissues ... with more advanced devices that can be used to mimic different parts of the lung and different processes, like immune responses in asthma and viral infections,” said Dr. Huh, “and with several studies using primary human cells taken from lung disease patients.”
Among Dr. Huh’s latest devices, built with NIH funding, is an asthma-on-a-chip device. Lung cells isolated from asthma patients are grown in a microfabricated device to create multilayered airway tissue, with airspace, that contains a fully differentiated epithelium and a vascularized stroma. “We can compress the entire engineered area of asthmatic human tissue in a lateral direction to mimic bronchoconstriction that happens during an asthma attack,” he said.
A paper soon to be published will describe how “abnormal pathophysiologic compressive forces due to bronchoconstriction in asthmatic lungs can make the lungs fibrotic, and how those mechanical forces also can induce increased vascularity,” said Dr. Huh, associate professor in the university’s department of bioengineering. “The increased vascular density can also change the phenotype of blood vessels in asthmatic airways.”
Dr. Huh also has an $8.3 million contract with the government’s Biomedical Advanced Research and Development Authority to study how chlorine gas damages lung tissues and identify biomarkers of chlorine gas–induced lung injury, with the goal of developing therapeutics.
Dr. Ingber and associates have developed a device modeling cystic fibrosis (CF). The chip is lined with primary human CF bronchial epithelial cells grown under an air-liquid interface and interfaced with primary lung microvascular endothelium that are exposed to fluid flow.
The chip reproduced, “with high fidelity, many of the structural, biochemical, and pathophysiological features of the human CF lung airway and its response to pathogens and circulating immune cells in vitro,” Dr. Ingber and colleagues reported (J Cyst Fibros. 2022;21:605-15).
Government investment in tissue chips
Efforts to commercialize organs-on-chip platforms and translate them for nonengineers have also picked in recent years. Several companies in the United States (including Emulate, a Wyss start-up) and in Europe now offer microengineered lung tissue models that can be used for research and drug testing. And some large pharmaceutical companies, said Dr. Tagle, have begun integrating tissue chip technology into their drug development programs.
The FDA, meanwhile, “has come to embrace the technology and see its promise,” Dr. Tagle said. An FDA pilot program announced in 2021 – called ISTAND (Innovative Science and Technology Approaches for New Drugs) – allows for tissue chip data to be submitted, as standalone data, for some drug applications.
The first 5 years of the government’s Tissue Chip for Drug Screening Program focused on safety and toxicity, and it “was successful in that model organ systems were able to capture the human response that [had been missed in] animal models,” he said.
For example, when a liver-tissue model was used to test several compounds that had passed animal testing for toxicity/safety but then failed in human clinical trials – killing some of the participants – the model showed a 100% sensitivity and a 87% specificity in predicting the human response, said Dr. Tagle, who recently coauthored a review on the future of organs-on-chips (Nature Reviews I Drug Discovery. 2021;20:345-61).
The second 5 years of the program, currently winding down, have focused on efficacy – the ability of organs-on-chip models to recreate the pathophysiology of chronic obstructive pulmonary disease, influenza, and other diseases, so that potential drugs can be assessed. In 2020, with extra support from the Coronavirus Aid, Relief, and Economic Security Act, NCATS funded academic labs to use organs-on-chip technology to evaluate SARS-CoV-2 and potential therapeutics.
Dr. Ingbar was one of the grantees. His team screened a number of FDA-approved drugs for potential repurposing using a bronchial-airway-on-a-chip and compared results with 2D model systems (Nat Biomed Eng. 2021;5:815-29). Amodiaquine inhibited infection in the 3D model and is now in phase 2 COVID trials. Several other drugs showed effectiveness in a 2D model but not in the chip.
Now, in a next phase of study at NCATS, coined Clinical Trials on a Chip, the center has awarded $35.5 million for investigators to test candidate therapies, often in parallel to ongoing clinical trials. The hope is that organs-on-chips can improve clinical trial design, from enrollment criteria and patient stratification to endpoints and the use of biomarkers. And in his lab, Dr. Huh is now engineering a shift to “organoids-on-a-chip” that combines the best features of each approach. “The idea,” he said, “is to grow organoids, and maintain the organoids in the microengineered systems where we can control their environment better ... and apply cues to allow them to develop into even more realistic tissues.”
Drs. Antony, Linkous, and Tagle reported no relevant disclosures. Dr. Huh is a co-founder of Vivodyne Inc, and owns shares in Vivodyne Inc. and Emulate Inc.
Tender Nonhealing Lesion on the Leg
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
A 50-year-old woman presented to our dermatology clinic with an exquisitely tender, nonhealing lesion on the left leg of 2 weeks’ duration that began as a small red-purplish spot. She applied a triple antibiotic ointment and wrapped the area with gauze daily but reported that it continued to enlarge and darken in color before forming a “scab.” She noted occasional seropurulent discharge and denied any trauma or new exposures to the area. She was seen at a local emergency department 3 days prior to presentation and was prescribed oral clindamycin for suspected cellulitis, but she denied any improvement with the initiation of antibiotics. Her medical history was notable for obesity, depression, hypothyroidism, and liver disease secondary to alcohol use disorder. She reported that she drank a pint of vodka daily. Her medications included pantoprazole, spironolactone, bumetanide, citalopram, levothyroxine, naltrexone, tramadol, and a multivitamin. Physical examination revealed violaceous mottling with areas of superficial erythema and ulceration with necrotic eschars on the proximal left thigh that were extremely painful. A biopsy was obtained for confirmation of diagnosis, but the patient died before the results were returned.
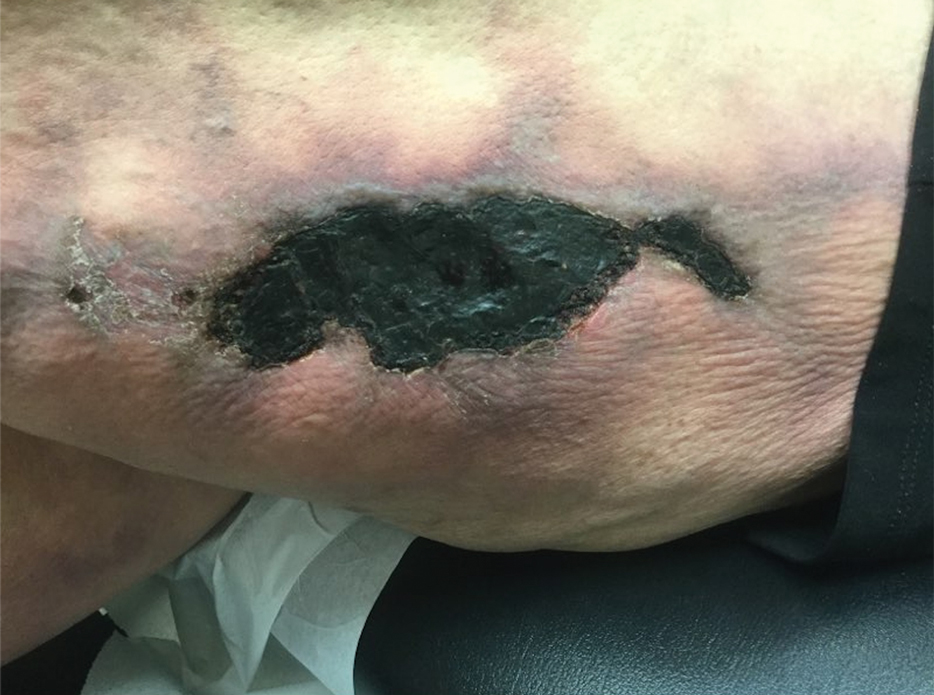
Increasing primary care doctors’ knowledge of IPF could speed up diagnoses, suggests white paper
The nonspecific nature of the symptoms of idiopathic pulmonary fibrosis (IPF) especially in early stages, and the relative rarity of IPF compared with other conditions that have similar symptoms, may contribute to a delay in diagnosis in the primary care setting, wrote Daniel F. Dilling, MD, of Loyola University Chicago, Maywood, Ill., and colleagues in Chest: Clinical Perspectives (Dilling et al. State of Practice: Factors Driving Diagnostic Delays in Idiopathic Pulmonary Fibrosis. Chest. 2022).
“We have learned over and over again through research, and also through talking with our own patients with IPF, that there is often a long lag between the first signs of the disease and a diagnosis of IPF,” corresponding author Dr. Dilling said in an interview.
“Even some pulmonary specialists can be uncertain about how to approach the diagnosis when a CT scan or other test first suggests the possibility; this can cost a patient precious time, as being on drug therapy earlier can result in preservation of lung function,” he said. “By sounding the alarm bell with this paper, we hope to promote awareness and education/training within the primary care community as well as the pulmonary community, and also to make all of them aware of the possibility of referral to specialty ILD [interstitial lung disease] centers when desired and possible,” he added.
The researchers conducted a pair of online surveys to inform the development of improving education on IPF among primary care providers.
In the white paper, which can be accessed online, the authors reported results of the surveys. One included 100 general pulmonologists and the other included 306 primary care physicians (156 practiced family physicians and 150 practiced general internal medicine). The data were collected between April 11, 2022, and May 16, 2022. Participants were asked to respond to a patient case scenario of a 55-year-old woman with nonspecific symptoms such as shortness of breath on moderate exertion, cough, exhaustion, and trouble sleeping.
The PCPs were most likely to evaluate the patient for a cardiac condition (46%), 25% would evaluate for chronic obstructive pulmonary disease (COPD), and 23% for asthma. More than half (58%) ranked progressive fibrosing ILD as one of their bottom two diagnoses.
A total of 87% of PCPs said they would begin a diagnostic workup to evaluate symptoms if the patient had no preexisting respiratory disease, compared with 61% for patients with a respiratory diagnosis.
Although 93% of PCPs cited a chest x-ray as part of the initial patient workup, fewer than half said they would order an echocardiogram, spirometry, or pulmonary function test (PFT), and 11% said they would include diffusion capacity testing in the initial workup.
In addition, PCPs were less likely to ask patients about issues that might prompt an IPF diagnosis, such as exposures to agents through work, hobbies, the environment, or comorbidities.
In the pulmonology survey, more than 75% of respondents cited patient history, high-resolution tomography scan, serologic testing, and review for autoimmune disease symptoms as first steps in a diagnostic response to patients with suspected IPF.
Differences between PCPs’ and pulmonolgists’ responses
Both PCPs and pulmonologists responded to several questions to assess knowledge and opinion gaps related to IPF. Overall, pulmonologists were more likely than PCPs to cite both imaging and testing issues and waiting 6-8 weeks after symptom onset before imaging as contributing factors to diagnostic delays.
PCPs more often expressed beliefs that delayed diagnosis had little impact on a patient with IPF, and that the treatments may be worse than the disease.
Dr. Dilling said he was not surprised by the survey findings, as similar clues about the underdiagnosis of IPF have surfaced in prior studies.
“We need to get the word out to primary care physicians, to pulmonary physicians, and even to the public, that idiopathic pulmonary fibrosis and other forms of interstitial lung disease are out there and prevalent, and that making the right diagnosis in a timely way can lead to better outcomes for patients,” he said.
The take-home message for primary care is to think outside the COPD box, said Dr. Dilling. “Just because someone has shortness of breath or cough and used to smoke does not automatically mean that they have COPD,” he emphasized. “Listen carefully for crackles (rales) on exam. Get spirometry or PFTs before you secure the diagnosis of COPD, or else you will be missing all of your cases of pulmonary fibrosis; think of pulmonary fibrosis and use imaging to help guide your diagnosis,” he said.
The authors suggested several education goals for PCPs, including establishing the importance of early evaluation, outlining the correct approach to a patient workup, encouraging prompt referral, and empowering PCPs as part of the team approach to IPF patients’ care. For pulmonologists, only 11% of those surveyed said they were aware of the latest developments in antifibrotic research, and education efforts might include information about drug pipelines and clinical trials, as well as technology.
Looking ahead, “We need to better understand how to find the pulmonary fibrosis in the community,” Dr. Dilling said. This understanding may come in part from greater education and awareness, he noted. However, eventually there may be ways to enhance the reading of PFTs and of CT scans through artificial intelligence technologies that would not only prompt clinicians to recognize what they are seeing, but would prompt them to refer and send the patient on the correct diagnostic path as soon as possible, he added.
Key message: Include ILD in differential diagnosis of patients with shortness of breath and/or cough
Advances in diagnostics and therapies for interstitial lung disease can take time to be absorbed and adopted, and patients with ILD and pulmonologists caring for ILD, specifically IPF, continue to report delays in diagnosis and therapy, said Krishna Thavarajah, MD, a pulmonologist at Henry Ford Hospital, Detroit, Mich., in an interview.
The current study findings of the time to diagnosis and the approach to patient workups echo her own clinical experience, Dr. Thavarajah said. “There is a delay in IPF diagnosis as physicians look to more common diagnoses, such as cardiac disease or chronic obstructive pulmonary disease, prior to pursuit of additional workup, and the attitude toward treatment has, in some ways, lagged behind advances in therapy, including timing and feasibility of therapy for IPF,” she said.
The key message for primary care physicians is to include ILD in the differential diagnosis of patients with shortness of breath and/or cough, especially if the initial cardiac and pulmonary test (meaning at least a chest x-ray and pulmonary function tests, including a diffusion capacity) are not pointing to an alternative cause within 3 months of presentation, Dr. Thavarajah said.
Once IPF is diagnosed, primary care clinicians should know that there are FDA-approved therapies that improve survival, said Dr. Thavarajah. “There are identifiable and treatable comorbid conditions,” she added. “The statement of ‘time lost is lung lost’ sums up the care of an IPF patient; partnerships between primary care clinicians, pulmonologists, and referral centers can provide the patient multiple levels of support with quality-of-life interventions, treatments, and also clinical trials, delivered by a team of providers,” she said.
In the wake of the current study, more research is needed with outcome studies regarding educational interventions targeting primary care and pulmonologists on appropriate workup, timing of workup, and current therapy for IPF patients, she added.
The white paper received no outside funding. The authors and Dr. Thavarajah had no financial conflicts to disclose.
The nonspecific nature of the symptoms of idiopathic pulmonary fibrosis (IPF) especially in early stages, and the relative rarity of IPF compared with other conditions that have similar symptoms, may contribute to a delay in diagnosis in the primary care setting, wrote Daniel F. Dilling, MD, of Loyola University Chicago, Maywood, Ill., and colleagues in Chest: Clinical Perspectives (Dilling et al. State of Practice: Factors Driving Diagnostic Delays in Idiopathic Pulmonary Fibrosis. Chest. 2022).
“We have learned over and over again through research, and also through talking with our own patients with IPF, that there is often a long lag between the first signs of the disease and a diagnosis of IPF,” corresponding author Dr. Dilling said in an interview.
“Even some pulmonary specialists can be uncertain about how to approach the diagnosis when a CT scan or other test first suggests the possibility; this can cost a patient precious time, as being on drug therapy earlier can result in preservation of lung function,” he said. “By sounding the alarm bell with this paper, we hope to promote awareness and education/training within the primary care community as well as the pulmonary community, and also to make all of them aware of the possibility of referral to specialty ILD [interstitial lung disease] centers when desired and possible,” he added.
The researchers conducted a pair of online surveys to inform the development of improving education on IPF among primary care providers.
In the white paper, which can be accessed online, the authors reported results of the surveys. One included 100 general pulmonologists and the other included 306 primary care physicians (156 practiced family physicians and 150 practiced general internal medicine). The data were collected between April 11, 2022, and May 16, 2022. Participants were asked to respond to a patient case scenario of a 55-year-old woman with nonspecific symptoms such as shortness of breath on moderate exertion, cough, exhaustion, and trouble sleeping.
The PCPs were most likely to evaluate the patient for a cardiac condition (46%), 25% would evaluate for chronic obstructive pulmonary disease (COPD), and 23% for asthma. More than half (58%) ranked progressive fibrosing ILD as one of their bottom two diagnoses.
A total of 87% of PCPs said they would begin a diagnostic workup to evaluate symptoms if the patient had no preexisting respiratory disease, compared with 61% for patients with a respiratory diagnosis.
Although 93% of PCPs cited a chest x-ray as part of the initial patient workup, fewer than half said they would order an echocardiogram, spirometry, or pulmonary function test (PFT), and 11% said they would include diffusion capacity testing in the initial workup.
In addition, PCPs were less likely to ask patients about issues that might prompt an IPF diagnosis, such as exposures to agents through work, hobbies, the environment, or comorbidities.
In the pulmonology survey, more than 75% of respondents cited patient history, high-resolution tomography scan, serologic testing, and review for autoimmune disease symptoms as first steps in a diagnostic response to patients with suspected IPF.
Differences between PCPs’ and pulmonolgists’ responses
Both PCPs and pulmonologists responded to several questions to assess knowledge and opinion gaps related to IPF. Overall, pulmonologists were more likely than PCPs to cite both imaging and testing issues and waiting 6-8 weeks after symptom onset before imaging as contributing factors to diagnostic delays.
PCPs more often expressed beliefs that delayed diagnosis had little impact on a patient with IPF, and that the treatments may be worse than the disease.
Dr. Dilling said he was not surprised by the survey findings, as similar clues about the underdiagnosis of IPF have surfaced in prior studies.
“We need to get the word out to primary care physicians, to pulmonary physicians, and even to the public, that idiopathic pulmonary fibrosis and other forms of interstitial lung disease are out there and prevalent, and that making the right diagnosis in a timely way can lead to better outcomes for patients,” he said.
The take-home message for primary care is to think outside the COPD box, said Dr. Dilling. “Just because someone has shortness of breath or cough and used to smoke does not automatically mean that they have COPD,” he emphasized. “Listen carefully for crackles (rales) on exam. Get spirometry or PFTs before you secure the diagnosis of COPD, or else you will be missing all of your cases of pulmonary fibrosis; think of pulmonary fibrosis and use imaging to help guide your diagnosis,” he said.
The authors suggested several education goals for PCPs, including establishing the importance of early evaluation, outlining the correct approach to a patient workup, encouraging prompt referral, and empowering PCPs as part of the team approach to IPF patients’ care. For pulmonologists, only 11% of those surveyed said they were aware of the latest developments in antifibrotic research, and education efforts might include information about drug pipelines and clinical trials, as well as technology.
Looking ahead, “We need to better understand how to find the pulmonary fibrosis in the community,” Dr. Dilling said. This understanding may come in part from greater education and awareness, he noted. However, eventually there may be ways to enhance the reading of PFTs and of CT scans through artificial intelligence technologies that would not only prompt clinicians to recognize what they are seeing, but would prompt them to refer and send the patient on the correct diagnostic path as soon as possible, he added.
Key message: Include ILD in differential diagnosis of patients with shortness of breath and/or cough
Advances in diagnostics and therapies for interstitial lung disease can take time to be absorbed and adopted, and patients with ILD and pulmonologists caring for ILD, specifically IPF, continue to report delays in diagnosis and therapy, said Krishna Thavarajah, MD, a pulmonologist at Henry Ford Hospital, Detroit, Mich., in an interview.
The current study findings of the time to diagnosis and the approach to patient workups echo her own clinical experience, Dr. Thavarajah said. “There is a delay in IPF diagnosis as physicians look to more common diagnoses, such as cardiac disease or chronic obstructive pulmonary disease, prior to pursuit of additional workup, and the attitude toward treatment has, in some ways, lagged behind advances in therapy, including timing and feasibility of therapy for IPF,” she said.
The key message for primary care physicians is to include ILD in the differential diagnosis of patients with shortness of breath and/or cough, especially if the initial cardiac and pulmonary test (meaning at least a chest x-ray and pulmonary function tests, including a diffusion capacity) are not pointing to an alternative cause within 3 months of presentation, Dr. Thavarajah said.
Once IPF is diagnosed, primary care clinicians should know that there are FDA-approved therapies that improve survival, said Dr. Thavarajah. “There are identifiable and treatable comorbid conditions,” she added. “The statement of ‘time lost is lung lost’ sums up the care of an IPF patient; partnerships between primary care clinicians, pulmonologists, and referral centers can provide the patient multiple levels of support with quality-of-life interventions, treatments, and also clinical trials, delivered by a team of providers,” she said.
In the wake of the current study, more research is needed with outcome studies regarding educational interventions targeting primary care and pulmonologists on appropriate workup, timing of workup, and current therapy for IPF patients, she added.
The white paper received no outside funding. The authors and Dr. Thavarajah had no financial conflicts to disclose.
The nonspecific nature of the symptoms of idiopathic pulmonary fibrosis (IPF) especially in early stages, and the relative rarity of IPF compared with other conditions that have similar symptoms, may contribute to a delay in diagnosis in the primary care setting, wrote Daniel F. Dilling, MD, of Loyola University Chicago, Maywood, Ill., and colleagues in Chest: Clinical Perspectives (Dilling et al. State of Practice: Factors Driving Diagnostic Delays in Idiopathic Pulmonary Fibrosis. Chest. 2022).
“We have learned over and over again through research, and also through talking with our own patients with IPF, that there is often a long lag between the first signs of the disease and a diagnosis of IPF,” corresponding author Dr. Dilling said in an interview.
“Even some pulmonary specialists can be uncertain about how to approach the diagnosis when a CT scan or other test first suggests the possibility; this can cost a patient precious time, as being on drug therapy earlier can result in preservation of lung function,” he said. “By sounding the alarm bell with this paper, we hope to promote awareness and education/training within the primary care community as well as the pulmonary community, and also to make all of them aware of the possibility of referral to specialty ILD [interstitial lung disease] centers when desired and possible,” he added.
The researchers conducted a pair of online surveys to inform the development of improving education on IPF among primary care providers.
In the white paper, which can be accessed online, the authors reported results of the surveys. One included 100 general pulmonologists and the other included 306 primary care physicians (156 practiced family physicians and 150 practiced general internal medicine). The data were collected between April 11, 2022, and May 16, 2022. Participants were asked to respond to a patient case scenario of a 55-year-old woman with nonspecific symptoms such as shortness of breath on moderate exertion, cough, exhaustion, and trouble sleeping.
The PCPs were most likely to evaluate the patient for a cardiac condition (46%), 25% would evaluate for chronic obstructive pulmonary disease (COPD), and 23% for asthma. More than half (58%) ranked progressive fibrosing ILD as one of their bottom two diagnoses.
A total of 87% of PCPs said they would begin a diagnostic workup to evaluate symptoms if the patient had no preexisting respiratory disease, compared with 61% for patients with a respiratory diagnosis.
Although 93% of PCPs cited a chest x-ray as part of the initial patient workup, fewer than half said they would order an echocardiogram, spirometry, or pulmonary function test (PFT), and 11% said they would include diffusion capacity testing in the initial workup.
In addition, PCPs were less likely to ask patients about issues that might prompt an IPF diagnosis, such as exposures to agents through work, hobbies, the environment, or comorbidities.
In the pulmonology survey, more than 75% of respondents cited patient history, high-resolution tomography scan, serologic testing, and review for autoimmune disease symptoms as first steps in a diagnostic response to patients with suspected IPF.
Differences between PCPs’ and pulmonolgists’ responses
Both PCPs and pulmonologists responded to several questions to assess knowledge and opinion gaps related to IPF. Overall, pulmonologists were more likely than PCPs to cite both imaging and testing issues and waiting 6-8 weeks after symptom onset before imaging as contributing factors to diagnostic delays.
PCPs more often expressed beliefs that delayed diagnosis had little impact on a patient with IPF, and that the treatments may be worse than the disease.
Dr. Dilling said he was not surprised by the survey findings, as similar clues about the underdiagnosis of IPF have surfaced in prior studies.
“We need to get the word out to primary care physicians, to pulmonary physicians, and even to the public, that idiopathic pulmonary fibrosis and other forms of interstitial lung disease are out there and prevalent, and that making the right diagnosis in a timely way can lead to better outcomes for patients,” he said.
The take-home message for primary care is to think outside the COPD box, said Dr. Dilling. “Just because someone has shortness of breath or cough and used to smoke does not automatically mean that they have COPD,” he emphasized. “Listen carefully for crackles (rales) on exam. Get spirometry or PFTs before you secure the diagnosis of COPD, or else you will be missing all of your cases of pulmonary fibrosis; think of pulmonary fibrosis and use imaging to help guide your diagnosis,” he said.
The authors suggested several education goals for PCPs, including establishing the importance of early evaluation, outlining the correct approach to a patient workup, encouraging prompt referral, and empowering PCPs as part of the team approach to IPF patients’ care. For pulmonologists, only 11% of those surveyed said they were aware of the latest developments in antifibrotic research, and education efforts might include information about drug pipelines and clinical trials, as well as technology.
Looking ahead, “We need to better understand how to find the pulmonary fibrosis in the community,” Dr. Dilling said. This understanding may come in part from greater education and awareness, he noted. However, eventually there may be ways to enhance the reading of PFTs and of CT scans through artificial intelligence technologies that would not only prompt clinicians to recognize what they are seeing, but would prompt them to refer and send the patient on the correct diagnostic path as soon as possible, he added.
Key message: Include ILD in differential diagnosis of patients with shortness of breath and/or cough
Advances in diagnostics and therapies for interstitial lung disease can take time to be absorbed and adopted, and patients with ILD and pulmonologists caring for ILD, specifically IPF, continue to report delays in diagnosis and therapy, said Krishna Thavarajah, MD, a pulmonologist at Henry Ford Hospital, Detroit, Mich., in an interview.
The current study findings of the time to diagnosis and the approach to patient workups echo her own clinical experience, Dr. Thavarajah said. “There is a delay in IPF diagnosis as physicians look to more common diagnoses, such as cardiac disease or chronic obstructive pulmonary disease, prior to pursuit of additional workup, and the attitude toward treatment has, in some ways, lagged behind advances in therapy, including timing and feasibility of therapy for IPF,” she said.
The key message for primary care physicians is to include ILD in the differential diagnosis of patients with shortness of breath and/or cough, especially if the initial cardiac and pulmonary test (meaning at least a chest x-ray and pulmonary function tests, including a diffusion capacity) are not pointing to an alternative cause within 3 months of presentation, Dr. Thavarajah said.
Once IPF is diagnosed, primary care clinicians should know that there are FDA-approved therapies that improve survival, said Dr. Thavarajah. “There are identifiable and treatable comorbid conditions,” she added. “The statement of ‘time lost is lung lost’ sums up the care of an IPF patient; partnerships between primary care clinicians, pulmonologists, and referral centers can provide the patient multiple levels of support with quality-of-life interventions, treatments, and also clinical trials, delivered by a team of providers,” she said.
In the wake of the current study, more research is needed with outcome studies regarding educational interventions targeting primary care and pulmonologists on appropriate workup, timing of workup, and current therapy for IPF patients, she added.
The white paper received no outside funding. The authors and Dr. Thavarajah had no financial conflicts to disclose.
FROM CHEST CLINICAL PERSPECTIVES