User login
Natural fertility: When less can be more
As reproductive specialists, part of our obligation is to improve a woman’s or couple’s ability to conceive in the most cost-effective manner, ideally through natural attempts at conception. While assisted reproductive technologies (ART) have provided impressive pregnancy rates across many diagnoses, including unexplained infertility, this advanced procedure comes with a significant financial cost to those without insurance and an emotional burden from the lack of a guaranteed outcome. Infertility procedures have minimal associated but potentially significant risks, most importantly multiple gestations. Contrary to popular belief, ovulation induction with intrauterine insemination (IUI) treatment has a greater risk of high-order multiple gestation when compared with IVF, given the inability of the former to control the number of embryos that may enter and implant in the endometrial cavity and the increased use of single embryo transfers with the latter. The specialist should evaluate the woman or couple for the basic issues of ovulation, tubal, and sperm function, as well as for lifestyle and environmental factors that can impede reproduction. As a result, “one size fits all” should not apply to patients, specifically those with infertility. This month’s column will present the detrimental effect of environmental and lifestyle factors on the goal of enhancing fertility through natural cycles of urine luteinizing-hormone timed intercourse.
Nutrition
Often overlooked in the infertility evaluation, an optimal diet improves fertility for both partners. Processed meat has been associated with reduced sperm quality. In ART, red meat has been associated with decreased embryo blastocyst formation. Lower trans fatty acids and higher omega-3s may improve fecundity. Considered one of the best overall diets, the Mediterranean diet consists of plant-based foods, such as whole grains, vegetables, legumes, fruits, nuts, seeds, herbs, and spices. Olive oil is the main source of added fat whereas fish, seafood, dairy, and poultry should be eaten in moderation. Fatty fish, such as mackerel, herring, sardines, albacore tuna, and salmon, are rich in omega-3 fatty acids, which have been shown to improve fecundity and IVF success, and have a positive association with blastocyst embryo development.1-3
Stress
The emotional effect of an infertility diagnosis has been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities.4 Whether stress causes or is a result of infertility has been a longstanding debate.5 Nevertheless, stress is the number-one reason patients discontinue fertility treatment.6 As fertility specialists, we must be cognizant of the devastation endured by infertility patients and maintain an open dialogue, as well as provide resources for coping strategies and counseling.
One popular method of improving mental health and fertility has been acupuncture. Initial enthusiasm originated from one of the first studies to explore the use of acupuncture during IVF. This was a prospective randomized study that showed treated patients had an approximately 100% improvement in clinical pregnancy rate. Unfortunately, there was no appropriate control group, just untreated controls.7 A subsequent study by the same investigator added a placebo acupuncture control group and did not show a statistically significant increase in pregnancy rates.8 Finally, a meta-analysis and reanalysis did not demonstrate any improvement in pregnancy outcome, whereas three of the studies analyzed suggested a possible reduction in pregnancies; placebo acupuncture was shown to have a higher success rate.9-11 While acupuncture is relatively safe, there appears to be only a placebo effect that may be helpful.
The effect of stress on reproduction has been addressed in one of my previous columns.
Alcohol and caffeine
The damaging effects of alcohol on the fetus during pregnancy are legion – abnormal facial features, microcephaly, low birth weight, hyperactive behavior, vision or hearing deficits, speech and language delays, and intellectual disability. Less known is the amount of alcohol that may have an effect during preconception. One of the first reports on the effect of alcohol on IVF concluded: a 13% decrease in the number of eggs aspirated; a 2.86 times increase in risk of not achieving pregnancy; and a 2.21 times increase in risk of miscarriage. For men, one additional drink per day increased the risk of not achieving a live birth from 2.28 to 8.32 times.12 Subsequent studies demonstrate a 16% reduction in IVF pregnancies in women who have at least four drinks per week; when the couple drank at least four drinks per week, the pregnancy rate decreased by 21%.13
However, a study from Denmark did not demonstrate a negative effect of low to moderate pretreatment amounts of alcohol and caffeine on IVF outcomes.14 Nevertheless, there is evidence that reducing or abstaining from alcohol intake may improve IVF outcomes.15 While there have been reports of higher miscarriage rates from caffeine,16,17 not all reports support a negative association.18
Smoking
The use of tobacco has been estimated to contribute to 13% of female infertility in a dose-response manner, including secondhand smoke. During ART, smoking reduces ovarian response to gonadotropins and decreases IVF success by up to 50%. Discontinuing smoking for 6 months beforehand appears to restore normal outcomes.19-20
The American Society for Reproductive Medicine Practice Committee on smoking provides the following invaluable information to share with patients on the harmful reproductive effects of smoking:21
- Early menopause by accelerating the loss of eggs.
- Higher rates of miscarriage and ectopic pregnancy.
- A decrease in sperm function.
- Possible genetic damage to eggs and sperm.
- Reduced sperm in son from maternal smoking.
Weight and exercise
Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction;22 a lower chance for conception;23 and infertility.24 Obese women have higher rates of miscarriage and recurrent miscarriage, reduced success with ART, an increased number of canceled cycles, and poorer quality oocytes retrieved. During pregnancy, obese women have three to four times higher rates of gestational diabetes and preeclampsia,25 as well as likelihood of having a fetus with macrosomia and birth defects, and a 1.3-2.1 times higher risk of stillbirth.26
Regarding physical activity, the rate of pregnancies (39.0% vs. 16.0%, P = .002) and live births (24.4% vs. 7.4% (P = .004) were higher with regular exercise vs. being sedentary. Obese women who exercised regularly had a live birth rate over threefold higher compared with those who were not active.27 Moderation should be employed given that women who exercise to exhaustion have 2.3 times the odds of fertility problems.28 In men, obesity has been shown to increase estrogens and reduce spermatogenesis. Exercise has improved semen parameters and testosterone. Paternal physical and sedentary activities were not related to clinical pregnancy or live birth rates following infertility treatment.29 As in women, men experience negative effects from high-intensity exercise, including bicycling, which can result in decreased semen parameters, follicle-stimulating hormone, LH, and testosterone levels.30
In couples desiring a more natural approach to infertility, fertility specialists can address environmental and lifestyle factors that may improve reproduction. When natural attempts at conception are not applicable or successful, IUI and ART are appropriate treatment options after considering estimated success rates as well as the physical, emotional, and financial investment of patients.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Wise LA et al. Am J Epidemiol. 2018;187:60-74.
2. Chui Y-H. Hum Reprod. 2018;33:156-65.
3. Ferreira Braga DPA et al. Reprod Biomed Online. 2015;31:30-8.
4. Domar AD et al. J Psychosom Obstet Gynaecol. 1993;14[suppl]:45-52.
5. Trolice MP. J Assist Reprod Genet. 2021 Apr;38[4]:873-5.
6. Gameiro S et al. Hum Reprod Update. 2012;18[6]:652-69.
7. Paulus WE et al. Fertil Steril. 2002;77:721-4.
8. Paulus WE et al. Hum Reprod. 2003;18:S18(abstr).
9. Wing SSE et al. Hum Reprod. 2009;24:341-8.
10. Hong Zheng C et al. Fertil Steril. 2012;97:599-611.
11. Meldrum DR et al. Fertil Steril. 2013;99:1821-4.
12. Klonoff-Cohen H et al. Fertil Steril. 2003;79:330-9.
13. Rossi BV et al. Obstet Gynecol. 2011;117:136-42.
14. Abadia L et al. Hum Reprod. 2017;32:1846-54.
15. Gormack AA et al. Hum Reprod. 2015;30:1617.
16. James JE. BMJ Evid Based Med. 2021;26:114-15.
17. Gaskins AJ et al. Eur J Nutr. 2018 Feb;57:107-17.
18. Machtinger R et al. Fertil Steril. 2017;108:1026-33.
19. Hughes EG et al. Fertil Steril. 1994;62:807.
20. de Ziegler D et al. Fertil Steril. 2013;100:927-8.
21. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2018;110:611-8.
22. Brewer CJ, Balen AH. Reproduction. 2010;140:347-64.
23. Wise LA et al. Hum Reprod. 2010;25:253-64.
24. Silvestris S et al. Reprod Biol Endocrinol. 2018;16[1]:22.
25. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
26. Aune D et al. JAMA. 2014;311:1536-46.
27. Palomba S et al. Reprod Biomed Online. 2014;29:72-9.
28. Gudmundsdottir SL et al. Hum Reprod. 2009;24[12]:3196-204.
29. Gaskins AJ et al. Hum Reprod. 2014;29:2575-82.
30. Wise LA et al. Fertil Steril. 2011;95:1025-30.
As reproductive specialists, part of our obligation is to improve a woman’s or couple’s ability to conceive in the most cost-effective manner, ideally through natural attempts at conception. While assisted reproductive technologies (ART) have provided impressive pregnancy rates across many diagnoses, including unexplained infertility, this advanced procedure comes with a significant financial cost to those without insurance and an emotional burden from the lack of a guaranteed outcome. Infertility procedures have minimal associated but potentially significant risks, most importantly multiple gestations. Contrary to popular belief, ovulation induction with intrauterine insemination (IUI) treatment has a greater risk of high-order multiple gestation when compared with IVF, given the inability of the former to control the number of embryos that may enter and implant in the endometrial cavity and the increased use of single embryo transfers with the latter. The specialist should evaluate the woman or couple for the basic issues of ovulation, tubal, and sperm function, as well as for lifestyle and environmental factors that can impede reproduction. As a result, “one size fits all” should not apply to patients, specifically those with infertility. This month’s column will present the detrimental effect of environmental and lifestyle factors on the goal of enhancing fertility through natural cycles of urine luteinizing-hormone timed intercourse.
Nutrition
Often overlooked in the infertility evaluation, an optimal diet improves fertility for both partners. Processed meat has been associated with reduced sperm quality. In ART, red meat has been associated with decreased embryo blastocyst formation. Lower trans fatty acids and higher omega-3s may improve fecundity. Considered one of the best overall diets, the Mediterranean diet consists of plant-based foods, such as whole grains, vegetables, legumes, fruits, nuts, seeds, herbs, and spices. Olive oil is the main source of added fat whereas fish, seafood, dairy, and poultry should be eaten in moderation. Fatty fish, such as mackerel, herring, sardines, albacore tuna, and salmon, are rich in omega-3 fatty acids, which have been shown to improve fecundity and IVF success, and have a positive association with blastocyst embryo development.1-3
Stress
The emotional effect of an infertility diagnosis has been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities.4 Whether stress causes or is a result of infertility has been a longstanding debate.5 Nevertheless, stress is the number-one reason patients discontinue fertility treatment.6 As fertility specialists, we must be cognizant of the devastation endured by infertility patients and maintain an open dialogue, as well as provide resources for coping strategies and counseling.
One popular method of improving mental health and fertility has been acupuncture. Initial enthusiasm originated from one of the first studies to explore the use of acupuncture during IVF. This was a prospective randomized study that showed treated patients had an approximately 100% improvement in clinical pregnancy rate. Unfortunately, there was no appropriate control group, just untreated controls.7 A subsequent study by the same investigator added a placebo acupuncture control group and did not show a statistically significant increase in pregnancy rates.8 Finally, a meta-analysis and reanalysis did not demonstrate any improvement in pregnancy outcome, whereas three of the studies analyzed suggested a possible reduction in pregnancies; placebo acupuncture was shown to have a higher success rate.9-11 While acupuncture is relatively safe, there appears to be only a placebo effect that may be helpful.
The effect of stress on reproduction has been addressed in one of my previous columns.
Alcohol and caffeine
The damaging effects of alcohol on the fetus during pregnancy are legion – abnormal facial features, microcephaly, low birth weight, hyperactive behavior, vision or hearing deficits, speech and language delays, and intellectual disability. Less known is the amount of alcohol that may have an effect during preconception. One of the first reports on the effect of alcohol on IVF concluded: a 13% decrease in the number of eggs aspirated; a 2.86 times increase in risk of not achieving pregnancy; and a 2.21 times increase in risk of miscarriage. For men, one additional drink per day increased the risk of not achieving a live birth from 2.28 to 8.32 times.12 Subsequent studies demonstrate a 16% reduction in IVF pregnancies in women who have at least four drinks per week; when the couple drank at least four drinks per week, the pregnancy rate decreased by 21%.13
However, a study from Denmark did not demonstrate a negative effect of low to moderate pretreatment amounts of alcohol and caffeine on IVF outcomes.14 Nevertheless, there is evidence that reducing or abstaining from alcohol intake may improve IVF outcomes.15 While there have been reports of higher miscarriage rates from caffeine,16,17 not all reports support a negative association.18
Smoking
The use of tobacco has been estimated to contribute to 13% of female infertility in a dose-response manner, including secondhand smoke. During ART, smoking reduces ovarian response to gonadotropins and decreases IVF success by up to 50%. Discontinuing smoking for 6 months beforehand appears to restore normal outcomes.19-20
The American Society for Reproductive Medicine Practice Committee on smoking provides the following invaluable information to share with patients on the harmful reproductive effects of smoking:21
- Early menopause by accelerating the loss of eggs.
- Higher rates of miscarriage and ectopic pregnancy.
- A decrease in sperm function.
- Possible genetic damage to eggs and sperm.
- Reduced sperm in son from maternal smoking.
Weight and exercise
Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction;22 a lower chance for conception;23 and infertility.24 Obese women have higher rates of miscarriage and recurrent miscarriage, reduced success with ART, an increased number of canceled cycles, and poorer quality oocytes retrieved. During pregnancy, obese women have three to four times higher rates of gestational diabetes and preeclampsia,25 as well as likelihood of having a fetus with macrosomia and birth defects, and a 1.3-2.1 times higher risk of stillbirth.26
Regarding physical activity, the rate of pregnancies (39.0% vs. 16.0%, P = .002) and live births (24.4% vs. 7.4% (P = .004) were higher with regular exercise vs. being sedentary. Obese women who exercised regularly had a live birth rate over threefold higher compared with those who were not active.27 Moderation should be employed given that women who exercise to exhaustion have 2.3 times the odds of fertility problems.28 In men, obesity has been shown to increase estrogens and reduce spermatogenesis. Exercise has improved semen parameters and testosterone. Paternal physical and sedentary activities were not related to clinical pregnancy or live birth rates following infertility treatment.29 As in women, men experience negative effects from high-intensity exercise, including bicycling, which can result in decreased semen parameters, follicle-stimulating hormone, LH, and testosterone levels.30
In couples desiring a more natural approach to infertility, fertility specialists can address environmental and lifestyle factors that may improve reproduction. When natural attempts at conception are not applicable or successful, IUI and ART are appropriate treatment options after considering estimated success rates as well as the physical, emotional, and financial investment of patients.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Wise LA et al. Am J Epidemiol. 2018;187:60-74.
2. Chui Y-H. Hum Reprod. 2018;33:156-65.
3. Ferreira Braga DPA et al. Reprod Biomed Online. 2015;31:30-8.
4. Domar AD et al. J Psychosom Obstet Gynaecol. 1993;14[suppl]:45-52.
5. Trolice MP. J Assist Reprod Genet. 2021 Apr;38[4]:873-5.
6. Gameiro S et al. Hum Reprod Update. 2012;18[6]:652-69.
7. Paulus WE et al. Fertil Steril. 2002;77:721-4.
8. Paulus WE et al. Hum Reprod. 2003;18:S18(abstr).
9. Wing SSE et al. Hum Reprod. 2009;24:341-8.
10. Hong Zheng C et al. Fertil Steril. 2012;97:599-611.
11. Meldrum DR et al. Fertil Steril. 2013;99:1821-4.
12. Klonoff-Cohen H et al. Fertil Steril. 2003;79:330-9.
13. Rossi BV et al. Obstet Gynecol. 2011;117:136-42.
14. Abadia L et al. Hum Reprod. 2017;32:1846-54.
15. Gormack AA et al. Hum Reprod. 2015;30:1617.
16. James JE. BMJ Evid Based Med. 2021;26:114-15.
17. Gaskins AJ et al. Eur J Nutr. 2018 Feb;57:107-17.
18. Machtinger R et al. Fertil Steril. 2017;108:1026-33.
19. Hughes EG et al. Fertil Steril. 1994;62:807.
20. de Ziegler D et al. Fertil Steril. 2013;100:927-8.
21. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2018;110:611-8.
22. Brewer CJ, Balen AH. Reproduction. 2010;140:347-64.
23. Wise LA et al. Hum Reprod. 2010;25:253-64.
24. Silvestris S et al. Reprod Biol Endocrinol. 2018;16[1]:22.
25. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
26. Aune D et al. JAMA. 2014;311:1536-46.
27. Palomba S et al. Reprod Biomed Online. 2014;29:72-9.
28. Gudmundsdottir SL et al. Hum Reprod. 2009;24[12]:3196-204.
29. Gaskins AJ et al. Hum Reprod. 2014;29:2575-82.
30. Wise LA et al. Fertil Steril. 2011;95:1025-30.
As reproductive specialists, part of our obligation is to improve a woman’s or couple’s ability to conceive in the most cost-effective manner, ideally through natural attempts at conception. While assisted reproductive technologies (ART) have provided impressive pregnancy rates across many diagnoses, including unexplained infertility, this advanced procedure comes with a significant financial cost to those without insurance and an emotional burden from the lack of a guaranteed outcome. Infertility procedures have minimal associated but potentially significant risks, most importantly multiple gestations. Contrary to popular belief, ovulation induction with intrauterine insemination (IUI) treatment has a greater risk of high-order multiple gestation when compared with IVF, given the inability of the former to control the number of embryos that may enter and implant in the endometrial cavity and the increased use of single embryo transfers with the latter. The specialist should evaluate the woman or couple for the basic issues of ovulation, tubal, and sperm function, as well as for lifestyle and environmental factors that can impede reproduction. As a result, “one size fits all” should not apply to patients, specifically those with infertility. This month’s column will present the detrimental effect of environmental and lifestyle factors on the goal of enhancing fertility through natural cycles of urine luteinizing-hormone timed intercourse.
Nutrition
Often overlooked in the infertility evaluation, an optimal diet improves fertility for both partners. Processed meat has been associated with reduced sperm quality. In ART, red meat has been associated with decreased embryo blastocyst formation. Lower trans fatty acids and higher omega-3s may improve fecundity. Considered one of the best overall diets, the Mediterranean diet consists of plant-based foods, such as whole grains, vegetables, legumes, fruits, nuts, seeds, herbs, and spices. Olive oil is the main source of added fat whereas fish, seafood, dairy, and poultry should be eaten in moderation. Fatty fish, such as mackerel, herring, sardines, albacore tuna, and salmon, are rich in omega-3 fatty acids, which have been shown to improve fecundity and IVF success, and have a positive association with blastocyst embryo development.1-3
Stress
The emotional effect of an infertility diagnosis has been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities.4 Whether stress causes or is a result of infertility has been a longstanding debate.5 Nevertheless, stress is the number-one reason patients discontinue fertility treatment.6 As fertility specialists, we must be cognizant of the devastation endured by infertility patients and maintain an open dialogue, as well as provide resources for coping strategies and counseling.
One popular method of improving mental health and fertility has been acupuncture. Initial enthusiasm originated from one of the first studies to explore the use of acupuncture during IVF. This was a prospective randomized study that showed treated patients had an approximately 100% improvement in clinical pregnancy rate. Unfortunately, there was no appropriate control group, just untreated controls.7 A subsequent study by the same investigator added a placebo acupuncture control group and did not show a statistically significant increase in pregnancy rates.8 Finally, a meta-analysis and reanalysis did not demonstrate any improvement in pregnancy outcome, whereas three of the studies analyzed suggested a possible reduction in pregnancies; placebo acupuncture was shown to have a higher success rate.9-11 While acupuncture is relatively safe, there appears to be only a placebo effect that may be helpful.
The effect of stress on reproduction has been addressed in one of my previous columns.
Alcohol and caffeine
The damaging effects of alcohol on the fetus during pregnancy are legion – abnormal facial features, microcephaly, low birth weight, hyperactive behavior, vision or hearing deficits, speech and language delays, and intellectual disability. Less known is the amount of alcohol that may have an effect during preconception. One of the first reports on the effect of alcohol on IVF concluded: a 13% decrease in the number of eggs aspirated; a 2.86 times increase in risk of not achieving pregnancy; and a 2.21 times increase in risk of miscarriage. For men, one additional drink per day increased the risk of not achieving a live birth from 2.28 to 8.32 times.12 Subsequent studies demonstrate a 16% reduction in IVF pregnancies in women who have at least four drinks per week; when the couple drank at least four drinks per week, the pregnancy rate decreased by 21%.13
However, a study from Denmark did not demonstrate a negative effect of low to moderate pretreatment amounts of alcohol and caffeine on IVF outcomes.14 Nevertheless, there is evidence that reducing or abstaining from alcohol intake may improve IVF outcomes.15 While there have been reports of higher miscarriage rates from caffeine,16,17 not all reports support a negative association.18
Smoking
The use of tobacco has been estimated to contribute to 13% of female infertility in a dose-response manner, including secondhand smoke. During ART, smoking reduces ovarian response to gonadotropins and decreases IVF success by up to 50%. Discontinuing smoking for 6 months beforehand appears to restore normal outcomes.19-20
The American Society for Reproductive Medicine Practice Committee on smoking provides the following invaluable information to share with patients on the harmful reproductive effects of smoking:21
- Early menopause by accelerating the loss of eggs.
- Higher rates of miscarriage and ectopic pregnancy.
- A decrease in sperm function.
- Possible genetic damage to eggs and sperm.
- Reduced sperm in son from maternal smoking.
Weight and exercise
Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction;22 a lower chance for conception;23 and infertility.24 Obese women have higher rates of miscarriage and recurrent miscarriage, reduced success with ART, an increased number of canceled cycles, and poorer quality oocytes retrieved. During pregnancy, obese women have three to four times higher rates of gestational diabetes and preeclampsia,25 as well as likelihood of having a fetus with macrosomia and birth defects, and a 1.3-2.1 times higher risk of stillbirth.26
Regarding physical activity, the rate of pregnancies (39.0% vs. 16.0%, P = .002) and live births (24.4% vs. 7.4% (P = .004) were higher with regular exercise vs. being sedentary. Obese women who exercised regularly had a live birth rate over threefold higher compared with those who were not active.27 Moderation should be employed given that women who exercise to exhaustion have 2.3 times the odds of fertility problems.28 In men, obesity has been shown to increase estrogens and reduce spermatogenesis. Exercise has improved semen parameters and testosterone. Paternal physical and sedentary activities were not related to clinical pregnancy or live birth rates following infertility treatment.29 As in women, men experience negative effects from high-intensity exercise, including bicycling, which can result in decreased semen parameters, follicle-stimulating hormone, LH, and testosterone levels.30
In couples desiring a more natural approach to infertility, fertility specialists can address environmental and lifestyle factors that may improve reproduction. When natural attempts at conception are not applicable or successful, IUI and ART are appropriate treatment options after considering estimated success rates as well as the physical, emotional, and financial investment of patients.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Wise LA et al. Am J Epidemiol. 2018;187:60-74.
2. Chui Y-H. Hum Reprod. 2018;33:156-65.
3. Ferreira Braga DPA et al. Reprod Biomed Online. 2015;31:30-8.
4. Domar AD et al. J Psychosom Obstet Gynaecol. 1993;14[suppl]:45-52.
5. Trolice MP. J Assist Reprod Genet. 2021 Apr;38[4]:873-5.
6. Gameiro S et al. Hum Reprod Update. 2012;18[6]:652-69.
7. Paulus WE et al. Fertil Steril. 2002;77:721-4.
8. Paulus WE et al. Hum Reprod. 2003;18:S18(abstr).
9. Wing SSE et al. Hum Reprod. 2009;24:341-8.
10. Hong Zheng C et al. Fertil Steril. 2012;97:599-611.
11. Meldrum DR et al. Fertil Steril. 2013;99:1821-4.
12. Klonoff-Cohen H et al. Fertil Steril. 2003;79:330-9.
13. Rossi BV et al. Obstet Gynecol. 2011;117:136-42.
14. Abadia L et al. Hum Reprod. 2017;32:1846-54.
15. Gormack AA et al. Hum Reprod. 2015;30:1617.
16. James JE. BMJ Evid Based Med. 2021;26:114-15.
17. Gaskins AJ et al. Eur J Nutr. 2018 Feb;57:107-17.
18. Machtinger R et al. Fertil Steril. 2017;108:1026-33.
19. Hughes EG et al. Fertil Steril. 1994;62:807.
20. de Ziegler D et al. Fertil Steril. 2013;100:927-8.
21. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2018;110:611-8.
22. Brewer CJ, Balen AH. Reproduction. 2010;140:347-64.
23. Wise LA et al. Hum Reprod. 2010;25:253-64.
24. Silvestris S et al. Reprod Biol Endocrinol. 2018;16[1]:22.
25. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
26. Aune D et al. JAMA. 2014;311:1536-46.
27. Palomba S et al. Reprod Biomed Online. 2014;29:72-9.
28. Gudmundsdottir SL et al. Hum Reprod. 2009;24[12]:3196-204.
29. Gaskins AJ et al. Hum Reprod. 2014;29:2575-82.
30. Wise LA et al. Fertil Steril. 2011;95:1025-30.
Physicians can’t be bystanders in ‘silent scourge’ of medical bullying
Maya Iyer, MD, MEd, experienced bullying as a faculty member, and she sensed that she wasn’t alone. “The best ideas for research often come from individual experiences, in both personal and the professional academic medicine setting,” she said in an interview.
“And I was correct. I was not the only one who experienced bullying. In fact, the most severe bullying experiences among ... women physician leaders occurred when they were in leadership positions,” said Dr. Iyer, a pediatric emergency medicine physician at Nationwide Children’s Hospital in Columbus, Ohio.
She is a coauthor of a study that was published in JAMA Network Open in which investigators surveyed the existence of antibullying policies for faculty at almost 100 U.S. medical schools.
The researchers defined bullying as “a severe form of mistreatment [that] occurs in the medical setting when a power differential allows offenders to consciously target individuals through persistent negative actions to impede the education or career of the target.”
The study included 91 medical schools, of which 4 schools had antibullying policies that included the reporting of procedures. Of the 87 medical schools without antibullying policies, 60 had antiharrassment policies; of those schools, 10 of the schools’ websites cited bullying and antiharassment policies. Five schools required a login to access policies, and one school’s website had a broken webpage link, per the study.
“We need to bring the silent scourge of bullying to the forefront because bullying is causing a brain drain on the medical profession,” said Dr. Iyer. “Bullying has numerous downstream negative effects, including depression, anxiety, burnout stress, decreased patient care satisfaction, increased medical errors, and job attrition.”
She added: “Through bullying, we are losing voices in medicine just at that point in time where we are trying to diversify the workforce to improve representation of all physicians.”
Dr. Iyer’s team sampled the top 25 schools for research and the top 25 schools for primary care. They also took a random sampling from 25 schools for research and a random sampling from top 25 schools for primary care. They assessed antibullying policies, antiharassment policies that mentioned bullying, antiharrassment policies that did not mention bullying, and the absence of policies addressing these issues.
Policy comprehensiveness was another focus for the researchers. They evaluated whether the relevant policies included faculty members and articulated the institution’s commitment to providing a safe and healthy workplace. Other factors included defining bullying and the roles and responsibilities of employees and procedures for reporting bullying.
Physicians can’t be bystanders to bullying
This means transitioning from being a bystander to an upstander.”
She doesn’t let medical schools off the hook, however. Instead, she advocated having institutions “provide safe spaces and opportunities for near-peer mentoring so that targets of bullying can share stories.”
Regarding who is responsible for addressing bullying, Dr. Iyer is emphatic. “I do want to be clear that the onus of disrupting does not fall on the targets. Rather, we need to fix the systems in which such behavior is tolerated.”
Her advice to leaders in academic medicine is to create comprehensive, zero-retaliation bullying policies that include detailed reporting procedures. Dr. Iyer advised leaders to partner with colleagues in human resources, offices of equity, and ombudspersons to develop, implement, and enforce these policies.
The study authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Maya Iyer, MD, MEd, experienced bullying as a faculty member, and she sensed that she wasn’t alone. “The best ideas for research often come from individual experiences, in both personal and the professional academic medicine setting,” she said in an interview.
“And I was correct. I was not the only one who experienced bullying. In fact, the most severe bullying experiences among ... women physician leaders occurred when they were in leadership positions,” said Dr. Iyer, a pediatric emergency medicine physician at Nationwide Children’s Hospital in Columbus, Ohio.
She is a coauthor of a study that was published in JAMA Network Open in which investigators surveyed the existence of antibullying policies for faculty at almost 100 U.S. medical schools.
The researchers defined bullying as “a severe form of mistreatment [that] occurs in the medical setting when a power differential allows offenders to consciously target individuals through persistent negative actions to impede the education or career of the target.”
The study included 91 medical schools, of which 4 schools had antibullying policies that included the reporting of procedures. Of the 87 medical schools without antibullying policies, 60 had antiharrassment policies; of those schools, 10 of the schools’ websites cited bullying and antiharassment policies. Five schools required a login to access policies, and one school’s website had a broken webpage link, per the study.
“We need to bring the silent scourge of bullying to the forefront because bullying is causing a brain drain on the medical profession,” said Dr. Iyer. “Bullying has numerous downstream negative effects, including depression, anxiety, burnout stress, decreased patient care satisfaction, increased medical errors, and job attrition.”
She added: “Through bullying, we are losing voices in medicine just at that point in time where we are trying to diversify the workforce to improve representation of all physicians.”
Dr. Iyer’s team sampled the top 25 schools for research and the top 25 schools for primary care. They also took a random sampling from 25 schools for research and a random sampling from top 25 schools for primary care. They assessed antibullying policies, antiharassment policies that mentioned bullying, antiharrassment policies that did not mention bullying, and the absence of policies addressing these issues.
Policy comprehensiveness was another focus for the researchers. They evaluated whether the relevant policies included faculty members and articulated the institution’s commitment to providing a safe and healthy workplace. Other factors included defining bullying and the roles and responsibilities of employees and procedures for reporting bullying.
Physicians can’t be bystanders to bullying
This means transitioning from being a bystander to an upstander.”
She doesn’t let medical schools off the hook, however. Instead, she advocated having institutions “provide safe spaces and opportunities for near-peer mentoring so that targets of bullying can share stories.”
Regarding who is responsible for addressing bullying, Dr. Iyer is emphatic. “I do want to be clear that the onus of disrupting does not fall on the targets. Rather, we need to fix the systems in which such behavior is tolerated.”
Her advice to leaders in academic medicine is to create comprehensive, zero-retaliation bullying policies that include detailed reporting procedures. Dr. Iyer advised leaders to partner with colleagues in human resources, offices of equity, and ombudspersons to develop, implement, and enforce these policies.
The study authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Maya Iyer, MD, MEd, experienced bullying as a faculty member, and she sensed that she wasn’t alone. “The best ideas for research often come from individual experiences, in both personal and the professional academic medicine setting,” she said in an interview.
“And I was correct. I was not the only one who experienced bullying. In fact, the most severe bullying experiences among ... women physician leaders occurred when they were in leadership positions,” said Dr. Iyer, a pediatric emergency medicine physician at Nationwide Children’s Hospital in Columbus, Ohio.
She is a coauthor of a study that was published in JAMA Network Open in which investigators surveyed the existence of antibullying policies for faculty at almost 100 U.S. medical schools.
The researchers defined bullying as “a severe form of mistreatment [that] occurs in the medical setting when a power differential allows offenders to consciously target individuals through persistent negative actions to impede the education or career of the target.”
The study included 91 medical schools, of which 4 schools had antibullying policies that included the reporting of procedures. Of the 87 medical schools without antibullying policies, 60 had antiharrassment policies; of those schools, 10 of the schools’ websites cited bullying and antiharassment policies. Five schools required a login to access policies, and one school’s website had a broken webpage link, per the study.
“We need to bring the silent scourge of bullying to the forefront because bullying is causing a brain drain on the medical profession,” said Dr. Iyer. “Bullying has numerous downstream negative effects, including depression, anxiety, burnout stress, decreased patient care satisfaction, increased medical errors, and job attrition.”
She added: “Through bullying, we are losing voices in medicine just at that point in time where we are trying to diversify the workforce to improve representation of all physicians.”
Dr. Iyer’s team sampled the top 25 schools for research and the top 25 schools for primary care. They also took a random sampling from 25 schools for research and a random sampling from top 25 schools for primary care. They assessed antibullying policies, antiharassment policies that mentioned bullying, antiharrassment policies that did not mention bullying, and the absence of policies addressing these issues.
Policy comprehensiveness was another focus for the researchers. They evaluated whether the relevant policies included faculty members and articulated the institution’s commitment to providing a safe and healthy workplace. Other factors included defining bullying and the roles and responsibilities of employees and procedures for reporting bullying.
Physicians can’t be bystanders to bullying
This means transitioning from being a bystander to an upstander.”
She doesn’t let medical schools off the hook, however. Instead, she advocated having institutions “provide safe spaces and opportunities for near-peer mentoring so that targets of bullying can share stories.”
Regarding who is responsible for addressing bullying, Dr. Iyer is emphatic. “I do want to be clear that the onus of disrupting does not fall on the targets. Rather, we need to fix the systems in which such behavior is tolerated.”
Her advice to leaders in academic medicine is to create comprehensive, zero-retaliation bullying policies that include detailed reporting procedures. Dr. Iyer advised leaders to partner with colleagues in human resources, offices of equity, and ombudspersons to develop, implement, and enforce these policies.
The study authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Elbow tenderness and swollen joints
The diagnosis for this case is psoriatic arthritis (PsA). The 2018 American College of Rheumatology/National Psoriasis Foundation guidelines offer current treatment recommendations for this condition. For patients with active PsA who are treatment-naive, treatment recommendations are:
- Tumor necrosis factor (TNF) inhibitors are preferred over oral small molecules (OSMs), interleukin (IL)–17 inhibitors, or IL-12/23 inhibitors
- OSMs are recommended over IL-17 inhibitors or IL-12/23 inhibitors
- Methotrexate is recommended over nonsteroidal anti-inflammatory drugs
- IL-17 inhibitors are recommended over IL-12/23 inhibitors
Treatment recommendations for patients with active PsA despite the use of OSMs are:
- Switching to a TNF inhibitor over another OSM, IL-17 or IL-12/23 inhibitors, abatacept, or tofacitinib
- Switching to an IL-17 inhibitor over another OSM, IL-12/23 inhibitor, abatacept, or tofacitinib
- Switching to an IL-12/23 inhibitor over another OSM, abatacept, or tofacitinib.
International groups, such as the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the European League Against Rheumatism (EULAR), have published treatment recommendations as well. These address both PsA and, to a lesser extent, psoriasis. The GRAPPA recommendations consider six domains of involvement (peripheral arthritis, axial disease, enthesitis, dactylitis, skin psoriasis, and nail psoriasis) and use a grid approach to account for various levels of disease activity and severity. The EULAR recommendations use an algorithmic approach that focuses mainly on musculoskeletal manifestations, specifically peripheral arthritis; manifestations, such as dactylitis, enthesitis, and skin and nail involvement, are considered separately.
Psoriasis precedes the onset of PsA in 60%-80% of patients (sometimes by up to 20 years but usually by less than 10 years); but in as many as 15%-20% of patients, arthritis appears before psoriasis. On occasion, arthritis and psoriasis appear simultaneously.
Patients with PsA are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with PsA do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent but rather indicates that a different type of systemic inflammation may be at play for those patients.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The diagnosis for this case is psoriatic arthritis (PsA). The 2018 American College of Rheumatology/National Psoriasis Foundation guidelines offer current treatment recommendations for this condition. For patients with active PsA who are treatment-naive, treatment recommendations are:
- Tumor necrosis factor (TNF) inhibitors are preferred over oral small molecules (OSMs), interleukin (IL)–17 inhibitors, or IL-12/23 inhibitors
- OSMs are recommended over IL-17 inhibitors or IL-12/23 inhibitors
- Methotrexate is recommended over nonsteroidal anti-inflammatory drugs
- IL-17 inhibitors are recommended over IL-12/23 inhibitors
Treatment recommendations for patients with active PsA despite the use of OSMs are:
- Switching to a TNF inhibitor over another OSM, IL-17 or IL-12/23 inhibitors, abatacept, or tofacitinib
- Switching to an IL-17 inhibitor over another OSM, IL-12/23 inhibitor, abatacept, or tofacitinib
- Switching to an IL-12/23 inhibitor over another OSM, abatacept, or tofacitinib.
International groups, such as the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the European League Against Rheumatism (EULAR), have published treatment recommendations as well. These address both PsA and, to a lesser extent, psoriasis. The GRAPPA recommendations consider six domains of involvement (peripheral arthritis, axial disease, enthesitis, dactylitis, skin psoriasis, and nail psoriasis) and use a grid approach to account for various levels of disease activity and severity. The EULAR recommendations use an algorithmic approach that focuses mainly on musculoskeletal manifestations, specifically peripheral arthritis; manifestations, such as dactylitis, enthesitis, and skin and nail involvement, are considered separately.
Psoriasis precedes the onset of PsA in 60%-80% of patients (sometimes by up to 20 years but usually by less than 10 years); but in as many as 15%-20% of patients, arthritis appears before psoriasis. On occasion, arthritis and psoriasis appear simultaneously.
Patients with PsA are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with PsA do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent but rather indicates that a different type of systemic inflammation may be at play for those patients.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The diagnosis for this case is psoriatic arthritis (PsA). The 2018 American College of Rheumatology/National Psoriasis Foundation guidelines offer current treatment recommendations for this condition. For patients with active PsA who are treatment-naive, treatment recommendations are:
- Tumor necrosis factor (TNF) inhibitors are preferred over oral small molecules (OSMs), interleukin (IL)–17 inhibitors, or IL-12/23 inhibitors
- OSMs are recommended over IL-17 inhibitors or IL-12/23 inhibitors
- Methotrexate is recommended over nonsteroidal anti-inflammatory drugs
- IL-17 inhibitors are recommended over IL-12/23 inhibitors
Treatment recommendations for patients with active PsA despite the use of OSMs are:
- Switching to a TNF inhibitor over another OSM, IL-17 or IL-12/23 inhibitors, abatacept, or tofacitinib
- Switching to an IL-17 inhibitor over another OSM, IL-12/23 inhibitor, abatacept, or tofacitinib
- Switching to an IL-12/23 inhibitor over another OSM, abatacept, or tofacitinib.
International groups, such as the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the European League Against Rheumatism (EULAR), have published treatment recommendations as well. These address both PsA and, to a lesser extent, psoriasis. The GRAPPA recommendations consider six domains of involvement (peripheral arthritis, axial disease, enthesitis, dactylitis, skin psoriasis, and nail psoriasis) and use a grid approach to account for various levels of disease activity and severity. The EULAR recommendations use an algorithmic approach that focuses mainly on musculoskeletal manifestations, specifically peripheral arthritis; manifestations, such as dactylitis, enthesitis, and skin and nail involvement, are considered separately.
Psoriasis precedes the onset of PsA in 60%-80% of patients (sometimes by up to 20 years but usually by less than 10 years); but in as many as 15%-20% of patients, arthritis appears before psoriasis. On occasion, arthritis and psoriasis appear simultaneously.
Patients with PsA are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with PsA do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent but rather indicates that a different type of systemic inflammation may be at play for those patients.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
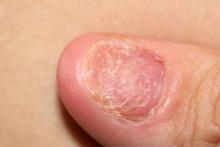
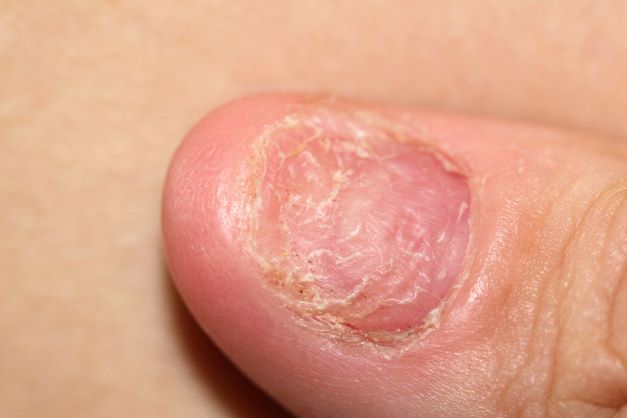
A 45-year-old man presents with complaints of intermittent joint aches to the point that he can no longer golf and has trouble with his handwriting. He has a 6-year history of scalp psoriasis that he has controlled with a salicylic acid shampoo. On physical examination, he has tenderness over both elbows and in his metacarpophalangeal and proximal interphalangeal joints on both hands. Swollen joints are noted in the proximal and distal joints of the right hand. His fingernails show uniform pitting. Neurologic examination shows no sensory deficits or hyperesthesia. Motor examination is unremarkable, as are chest and abdominal findings. Blood pressure is 128/80 mm Hg. Radiographic findings showed periarticular soft-tissue swelling of the distal interphalangeal joints of the right second and fourth fingers and left thumb, although no significant bony abnormalities were observed. There is asymmetric narrowing of the joint space in the interphalangeal joints. Laboratory findings reveal an erythrocyte sedimentation rate of 35 mm/h, negative for rheumatoid factor, negative for antinuclear antibody, and C-reactive protein level of 9 mg/dL.
Around 10% of back pain patients referred by chiropractors have undiagnosed SpA
GHENT, BELGIUM – Over 10% of patients referred by chiropractors to rheumatology had undiagnosed spondyloarthritis, with axial spondyloarthritis being the most common, according to new data. The U.S. study was aimed at understanding what proportion of back pain patients have undiagnosed spondyloarthritis.
The study also found that the most common cause for which patients see chiropractors is neck/cervical pain.
Atul Deodhar, MD, MRCP, rheumatologist and medical director of rheumatology clinics at Oregon Health & Science University, Portland, was senior author of the poster that was presented at the 13th International Congress of Spondyloarthritides.
“In the U.S., many people with back pain go to chiropractors, but many chiropractors are not aware of axial spondyloarthritis [axSpA] terminology, and very little – if anything – is published in chiropractic literature, “ he said in an interview.
He remarked that the study highlighted the need to develop a better strategy to identify undiagnosed patients, because the yield found in their study was poor (13%). “Patient-reported spondyloarthritis criteria are often poor, and do not match rheumatologist-inquired history,” he noted, adding that, “inflammatory back pain is in fact a poor ‘entry point.’ ”
Ulrich Weber, MD, rheumatologist from the Practice Buchsbaum in Schaffhausen, Switzerland, commented on the findings, saying he often receives delayed referrals from chiropractors, so
He added that he welcomed the study but noted, “the criteria used to identify patients in this study are broad and I’d worry that it would inundate our rheumatology practice. There remains a real need for a good method of identifying the patients.”
Referral to rheumatology
Back pain is highly prevalent in the general population, with a global mean lifetime prevalence of 38.9%. Chiropractors treat many patients with back pain of unknown cause.
“In this study, we wanted to see what percentage of patients in chiropractic practice have undiagnosed axial spondyloarthritis, and what are the common complaints. Our hypothesis was that chiropractors may be missing such patients,” Dr. Deodhar explained.
Dr. Deodhar and colleagues recruited chiropractors from four different parts of the city of Portland into the study. “We think Portland, Oregon, is a typical U.S. city, and our results could be generalized. However, this is our impression alone,” he remarked.
Adults, under the age of 45 years who attended a participating chiropractic clinics between November 2020 and November 2021 for chronic back pain and without a prior diagnosis of spondyloarthritis were eligible for inclusion.
If the patient reported at least one feature of spondyloarthritis in the screening questionnaire they were referred to a rheumatologist for a diagnostic assessment. This assessment involved taking history by telephone, both laboratory tests and imaging, and the patients were categorized as radiographic axSpA, nonradiographic axSpA, peripheral SpA, or no SpA.
The screening questionnaire included the following examples: If the patient was under 45 years and had chronic pain in back, hip or buttocks, then they were asked for more information including whether their pain was gradual (insidious) in onset; if the pain started before the age of 40; and if the pain improved with physical activities or movements. Use of drugs was investigated including whether the pain improved significantly with NSAIDs and whether the patient has current or past heel pains, particularly when waking up in the morning. They were also asked if they have experienced skin psoriasis. Other questions were asked about the presence of uveitis, iritis, family history of psoriasis, inflammatory bowel disease, or ankylosing spondylitis, and whether the patient had unexplained joint pains plus joint swelling.
Ten percent of patients referred to rheumatology
A total of 3,103 visits to chiropractor clinics were included, of which 115 patients were referred to a rheumatologist. Eventually, 63 patients were fully assessed by a rheumatologist.
Of those patients who were fully assessed, 12.7% has spondyloarthritis, with one having confirmed radiographic axSpA, five having nonradiographic SpA, and two having peripheral spondyloarthritis or psoriatic arthritis.
Based on the referral questionnaire, all patients reported at least four SpA criteria were met, said Dr. Deodhar.
Of those patients diagnosed with SpA, 14% (1) has elevated C-reactive protein (CRP) level, 14% (1) were HLA-B27 positive, and 14% (1) were identified as having both elevated CRP and HLA-B27 positivity. Sacroiliac joint inflammation was found in 14% (1) on MRI and one had sacroiliac joint inflammation according to modified New York criteria. One (14%) had both sacroiliac joint inflammation on MRI and elevated CRP, and 14% (1) had both sacroiliac joint inflammation and was HLA-B27 positive.
The top complaints reported by patients at chiropractor clinics were neck and cervical spine pain/spasm (16.8%); followed by acute low back pain (11.7%); acute upper back (7.1%); and chronic lower back pain (6.9%).
No patients with more than 10 SpA criteria
The performance of an initial diagnostic assessment based on patient reported SpA criteria, as compared with the outcome of the full diagnosis (by a rheumatologist) showed that patients with one to four SpA criteria had a sensitivity of 0.50 (95% confidence interval, 0.15-0.85), and specificity of 0.73 (95% CI, 0.61-0.84). This increased to sensitivity of 0.60 (95% CI, 0.17-1.03), and specificity of 0.61 (95% CI, 0.44-0.77) when six SpA criteria were present.
Dr. Deodhar said the results supported a need to further develop the chiropractor’s role in identifying the right patients for referral, and that the study showed that a referral strategy is required to find undiagnosed patients with spondyloarthritis from chiropractic offices. “Chiropractors need education for axSpA, when to suspect, and when to refer,” he asserted. “What referral strategy to use is for debate – the ASAS [Assessment in SpondyloArthritis international Society] strategy is too sensitive and not specific enough.”
Dr. Deodhar noted that SPARTAN (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Spondyloarthritis Research & Treatment Network) is working on a referral strategy that is likely to be more specific, and that more data would be forthcoming soon.
Dr. Deodhar declared affiliations with multiple companies involved in the field unrelated to the study. Dr. Weber declared no relevant disclosures.
GHENT, BELGIUM – Over 10% of patients referred by chiropractors to rheumatology had undiagnosed spondyloarthritis, with axial spondyloarthritis being the most common, according to new data. The U.S. study was aimed at understanding what proportion of back pain patients have undiagnosed spondyloarthritis.
The study also found that the most common cause for which patients see chiropractors is neck/cervical pain.
Atul Deodhar, MD, MRCP, rheumatologist and medical director of rheumatology clinics at Oregon Health & Science University, Portland, was senior author of the poster that was presented at the 13th International Congress of Spondyloarthritides.
“In the U.S., many people with back pain go to chiropractors, but many chiropractors are not aware of axial spondyloarthritis [axSpA] terminology, and very little – if anything – is published in chiropractic literature, “ he said in an interview.
He remarked that the study highlighted the need to develop a better strategy to identify undiagnosed patients, because the yield found in their study was poor (13%). “Patient-reported spondyloarthritis criteria are often poor, and do not match rheumatologist-inquired history,” he noted, adding that, “inflammatory back pain is in fact a poor ‘entry point.’ ”
Ulrich Weber, MD, rheumatologist from the Practice Buchsbaum in Schaffhausen, Switzerland, commented on the findings, saying he often receives delayed referrals from chiropractors, so
He added that he welcomed the study but noted, “the criteria used to identify patients in this study are broad and I’d worry that it would inundate our rheumatology practice. There remains a real need for a good method of identifying the patients.”
Referral to rheumatology
Back pain is highly prevalent in the general population, with a global mean lifetime prevalence of 38.9%. Chiropractors treat many patients with back pain of unknown cause.
“In this study, we wanted to see what percentage of patients in chiropractic practice have undiagnosed axial spondyloarthritis, and what are the common complaints. Our hypothesis was that chiropractors may be missing such patients,” Dr. Deodhar explained.
Dr. Deodhar and colleagues recruited chiropractors from four different parts of the city of Portland into the study. “We think Portland, Oregon, is a typical U.S. city, and our results could be generalized. However, this is our impression alone,” he remarked.
Adults, under the age of 45 years who attended a participating chiropractic clinics between November 2020 and November 2021 for chronic back pain and without a prior diagnosis of spondyloarthritis were eligible for inclusion.
If the patient reported at least one feature of spondyloarthritis in the screening questionnaire they were referred to a rheumatologist for a diagnostic assessment. This assessment involved taking history by telephone, both laboratory tests and imaging, and the patients were categorized as radiographic axSpA, nonradiographic axSpA, peripheral SpA, or no SpA.
The screening questionnaire included the following examples: If the patient was under 45 years and had chronic pain in back, hip or buttocks, then they were asked for more information including whether their pain was gradual (insidious) in onset; if the pain started before the age of 40; and if the pain improved with physical activities or movements. Use of drugs was investigated including whether the pain improved significantly with NSAIDs and whether the patient has current or past heel pains, particularly when waking up in the morning. They were also asked if they have experienced skin psoriasis. Other questions were asked about the presence of uveitis, iritis, family history of psoriasis, inflammatory bowel disease, or ankylosing spondylitis, and whether the patient had unexplained joint pains plus joint swelling.
Ten percent of patients referred to rheumatology
A total of 3,103 visits to chiropractor clinics were included, of which 115 patients were referred to a rheumatologist. Eventually, 63 patients were fully assessed by a rheumatologist.
Of those patients who were fully assessed, 12.7% has spondyloarthritis, with one having confirmed radiographic axSpA, five having nonradiographic SpA, and two having peripheral spondyloarthritis or psoriatic arthritis.
Based on the referral questionnaire, all patients reported at least four SpA criteria were met, said Dr. Deodhar.
Of those patients diagnosed with SpA, 14% (1) has elevated C-reactive protein (CRP) level, 14% (1) were HLA-B27 positive, and 14% (1) were identified as having both elevated CRP and HLA-B27 positivity. Sacroiliac joint inflammation was found in 14% (1) on MRI and one had sacroiliac joint inflammation according to modified New York criteria. One (14%) had both sacroiliac joint inflammation on MRI and elevated CRP, and 14% (1) had both sacroiliac joint inflammation and was HLA-B27 positive.
The top complaints reported by patients at chiropractor clinics were neck and cervical spine pain/spasm (16.8%); followed by acute low back pain (11.7%); acute upper back (7.1%); and chronic lower back pain (6.9%).
No patients with more than 10 SpA criteria
The performance of an initial diagnostic assessment based on patient reported SpA criteria, as compared with the outcome of the full diagnosis (by a rheumatologist) showed that patients with one to four SpA criteria had a sensitivity of 0.50 (95% confidence interval, 0.15-0.85), and specificity of 0.73 (95% CI, 0.61-0.84). This increased to sensitivity of 0.60 (95% CI, 0.17-1.03), and specificity of 0.61 (95% CI, 0.44-0.77) when six SpA criteria were present.
Dr. Deodhar said the results supported a need to further develop the chiropractor’s role in identifying the right patients for referral, and that the study showed that a referral strategy is required to find undiagnosed patients with spondyloarthritis from chiropractic offices. “Chiropractors need education for axSpA, when to suspect, and when to refer,” he asserted. “What referral strategy to use is for debate – the ASAS [Assessment in SpondyloArthritis international Society] strategy is too sensitive and not specific enough.”
Dr. Deodhar noted that SPARTAN (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Spondyloarthritis Research & Treatment Network) is working on a referral strategy that is likely to be more specific, and that more data would be forthcoming soon.
Dr. Deodhar declared affiliations with multiple companies involved in the field unrelated to the study. Dr. Weber declared no relevant disclosures.
GHENT, BELGIUM – Over 10% of patients referred by chiropractors to rheumatology had undiagnosed spondyloarthritis, with axial spondyloarthritis being the most common, according to new data. The U.S. study was aimed at understanding what proportion of back pain patients have undiagnosed spondyloarthritis.
The study also found that the most common cause for which patients see chiropractors is neck/cervical pain.
Atul Deodhar, MD, MRCP, rheumatologist and medical director of rheumatology clinics at Oregon Health & Science University, Portland, was senior author of the poster that was presented at the 13th International Congress of Spondyloarthritides.
“In the U.S., many people with back pain go to chiropractors, but many chiropractors are not aware of axial spondyloarthritis [axSpA] terminology, and very little – if anything – is published in chiropractic literature, “ he said in an interview.
He remarked that the study highlighted the need to develop a better strategy to identify undiagnosed patients, because the yield found in their study was poor (13%). “Patient-reported spondyloarthritis criteria are often poor, and do not match rheumatologist-inquired history,” he noted, adding that, “inflammatory back pain is in fact a poor ‘entry point.’ ”
Ulrich Weber, MD, rheumatologist from the Practice Buchsbaum in Schaffhausen, Switzerland, commented on the findings, saying he often receives delayed referrals from chiropractors, so
He added that he welcomed the study but noted, “the criteria used to identify patients in this study are broad and I’d worry that it would inundate our rheumatology practice. There remains a real need for a good method of identifying the patients.”
Referral to rheumatology
Back pain is highly prevalent in the general population, with a global mean lifetime prevalence of 38.9%. Chiropractors treat many patients with back pain of unknown cause.
“In this study, we wanted to see what percentage of patients in chiropractic practice have undiagnosed axial spondyloarthritis, and what are the common complaints. Our hypothesis was that chiropractors may be missing such patients,” Dr. Deodhar explained.
Dr. Deodhar and colleagues recruited chiropractors from four different parts of the city of Portland into the study. “We think Portland, Oregon, is a typical U.S. city, and our results could be generalized. However, this is our impression alone,” he remarked.
Adults, under the age of 45 years who attended a participating chiropractic clinics between November 2020 and November 2021 for chronic back pain and without a prior diagnosis of spondyloarthritis were eligible for inclusion.
If the patient reported at least one feature of spondyloarthritis in the screening questionnaire they were referred to a rheumatologist for a diagnostic assessment. This assessment involved taking history by telephone, both laboratory tests and imaging, and the patients were categorized as radiographic axSpA, nonradiographic axSpA, peripheral SpA, or no SpA.
The screening questionnaire included the following examples: If the patient was under 45 years and had chronic pain in back, hip or buttocks, then they were asked for more information including whether their pain was gradual (insidious) in onset; if the pain started before the age of 40; and if the pain improved with physical activities or movements. Use of drugs was investigated including whether the pain improved significantly with NSAIDs and whether the patient has current or past heel pains, particularly when waking up in the morning. They were also asked if they have experienced skin psoriasis. Other questions were asked about the presence of uveitis, iritis, family history of psoriasis, inflammatory bowel disease, or ankylosing spondylitis, and whether the patient had unexplained joint pains plus joint swelling.
Ten percent of patients referred to rheumatology
A total of 3,103 visits to chiropractor clinics were included, of which 115 patients were referred to a rheumatologist. Eventually, 63 patients were fully assessed by a rheumatologist.
Of those patients who were fully assessed, 12.7% has spondyloarthritis, with one having confirmed radiographic axSpA, five having nonradiographic SpA, and two having peripheral spondyloarthritis or psoriatic arthritis.
Based on the referral questionnaire, all patients reported at least four SpA criteria were met, said Dr. Deodhar.
Of those patients diagnosed with SpA, 14% (1) has elevated C-reactive protein (CRP) level, 14% (1) were HLA-B27 positive, and 14% (1) were identified as having both elevated CRP and HLA-B27 positivity. Sacroiliac joint inflammation was found in 14% (1) on MRI and one had sacroiliac joint inflammation according to modified New York criteria. One (14%) had both sacroiliac joint inflammation on MRI and elevated CRP, and 14% (1) had both sacroiliac joint inflammation and was HLA-B27 positive.
The top complaints reported by patients at chiropractor clinics were neck and cervical spine pain/spasm (16.8%); followed by acute low back pain (11.7%); acute upper back (7.1%); and chronic lower back pain (6.9%).
No patients with more than 10 SpA criteria
The performance of an initial diagnostic assessment based on patient reported SpA criteria, as compared with the outcome of the full diagnosis (by a rheumatologist) showed that patients with one to four SpA criteria had a sensitivity of 0.50 (95% confidence interval, 0.15-0.85), and specificity of 0.73 (95% CI, 0.61-0.84). This increased to sensitivity of 0.60 (95% CI, 0.17-1.03), and specificity of 0.61 (95% CI, 0.44-0.77) when six SpA criteria were present.
Dr. Deodhar said the results supported a need to further develop the chiropractor’s role in identifying the right patients for referral, and that the study showed that a referral strategy is required to find undiagnosed patients with spondyloarthritis from chiropractic offices. “Chiropractors need education for axSpA, when to suspect, and when to refer,” he asserted. “What referral strategy to use is for debate – the ASAS [Assessment in SpondyloArthritis international Society] strategy is too sensitive and not specific enough.”
Dr. Deodhar noted that SPARTAN (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Spondyloarthritis Research & Treatment Network) is working on a referral strategy that is likely to be more specific, and that more data would be forthcoming soon.
Dr. Deodhar declared affiliations with multiple companies involved in the field unrelated to the study. Dr. Weber declared no relevant disclosures.
AT THE 2022 SPA CONGRESS
Being Female: A Serious, Unavoidable Risk Factor for Migraine
Migraine affects more than 1 in 6 US adults. This figure masks the fact that migraine is a predominantly female disorder; compared with men, 3-month migraine prevalence is more than 2-fold higher (21% vs. 10%) in women. Research by the World Health Organization has established migraine as second among the world’s causes of disability, and first among women of reproductive age.
Despite this burden of illness, migraine is often not diagnosed or treated effectively. General lifestyle advice such as maintaining a healthy weight, sleep hygiene, regular meals, regular exercise and hydration, and management of identified predisposing or triggering factors—together with optimization of symptomatic migraine treatment—can benefit all women with migraine. However, specific hormonal events such as menstruation, hormonal contraception, pregnancy, menopause, and hormone replacement therapy have variable, but often predictable, effects on the frequency and severity of migraine. At each of these life stages, there are specific opportunities to intervene and relieve migraine burden.
Menstrual migraine
Menstruation is one of the most significant risk factors for migraine, notably migraine without aura, with increased prevalence during a 5-day perimenstrual window that starts 2 days before the onset of menses and continues through the first 3 days of menstruation.
The International Headache Society (IHS) recognizes 2 types of menstrual migraine. There is menstrually related migraine, which is migraine without aura that regularly occurs on or between day −2 to +3 of menstruation (there is no day 0), with additional attacks of migraine with or without aura at other times of the cycle. There is also pure menstrual migraine, which is migraine without aura that occurs only on or between day −2 to +3, with no attacks at any other time of the cycle.
In women with menstrually related migraine, the diagnosis should only be made if the relationship between migraine and menstruation is greater than a chance association. To confirm this diagnosis, migraine attacks during the day −2 to +3 window must occur in at least 2 of 3 menstrual cycles. Relying on history to confirm the diagnosis can be misleading. Use of a 3-month diary can reveal the predictable patterns associated with menstrual migraine, aiding diagnosis and management.
Menstrual migraine affects 20% to 25% of women with migraine in the general population, and 22% to 70% of women seen in headache clinics. In women diagnosed with menstrual migraine, their perimenstrual attacks have distinctive clinical features which include more associated symptoms, longer duration, greater severity, greater susceptibility to relapse, greater resistance to treatment, and greater disability than migraines occurring at other times during the menstrual cycle.
Symptomatic treatment of perimenstrual attacks of migraine is the same as for treatment of non-menstrual attacks, but due to the longer duration of perimenstrual attacks, treatment usually needs to be repeated on several consecutive days. With respect to prevention, specific consideration should be given to the presence of menstrual disorders, contraceptive requirements, pregnancy wishes, and symptoms of perimenopause.
The 2 established triggers for perimenstrual migraine attacks are prostaglandin release, which also results in dysmenorrhea, and late luteal phase estrogen "withdrawal". There are no investigations to identify the relevant mechanism(s). However, a history of dysmenorrhea is suggestive of a prostaglandin trigger and both migraine and dysmenorrhea can benefit from treatment with prostaglandin inhibitors.
Contraceptive methods can effectively manage both perimenstrual triggers. The European Headache Federation and the European Society of Contraception and Reproductive Health recommend combined hormonal contraception (CHC) in women with menstrual migraine who require contraception or who have additional menstrual disorders that use of CHC would benefit. The desogestrel progestogen-only pill is an alternative option, particularly for women with aura, but bleeding side effects are a common reason for discontinuation.
The principal barriers to effective management of menstrual migraine are lack of awareness and under-diagnosis. Although the IHS criteria facilitate research diagnosis, there continues to be important unmet needs in the clinical management of women with menstrual migraine. Improved awareness by healthcare professionals is critical; women visiting their primary care physician or who are referred to a gynecologist seldom mention migraine unless specifically asked.
Contraception
Most women use contraception at some stage in their lives. Hormonal contraception, particularly CHC, is popular and effective, with additional non-contraceptive benefits.
As with the natural menstrual cycle, estrogen “withdrawal” (in this case the consequence of stopping contraceptive hormones during the hormone-free interval) can trigger migraine without aura. Eliminating the hormone-free interval by taking CHCs continuously, without a break, eliminates the risk of estrogen withdrawal migraine. Further, continuous use of CHC increases contraceptive efficacy and there are no differential safety concerns.
Contraceptive use of CHC is contraindicated in women with migraine aura, since migraine aura and use of ethinylestradiol are independent risk factors for ischemic stroke. Effective contraception need not be compromised since progestogen-only and non-hormonal methods—
several of which are more effective than CHC—are not associated with increased risk.
Despite there being little concern regarding use of CHCs in women with migraine without aura, clinical experience suggests that many women with migraine are denied CHCs. In some cases, this stems from misdiagnosing premonitory migraine symptoms as aura. In other cases, even a clear diagnosis of menstrual migraine without aura can result in CHCs being withheld due to the misconception of risk. To ensure that women receive optimum contraceptive options, contraceptive providers need a better understanding of the Medical Eligibility Criteria for Contraceptive Use as well as simple tools to aid migraine diagnosis.
Pregnancy and breastfeeding
Around 60-70% of pregnant women with migraine experience fewer attacks compared to pre-pregnancy, with improvement more likely in women with a history of menstrual migraine. In contrast, migraine with aura tends to continue to occur throughout pregnancy and postpartum and may start for the first time during this period. Women can be reassured that migraine, both with or without aura, does not have any adverse effect on the outcome of pregnancy. However, women with aura should be monitored during pregnancy since there is an increased risk of comorbid conditions, such as arterial and venous thrombosis, pre-eclampsia, and gestational hypertension.
Healthcare professionals need to be aware of their female patients with migraine who may be planning to conceive so that strategies for treating migraine can be discussed. Most drugs and other teratogens exert their greatest effects on the fetus in the first trimester, often before pregnancy is confirmed. Symptomatic treatment with acetaminophen and metoclopramide is safe. If this is ineffective, sumatriptan is an option. Ergot derivatives are contraindicated. If prophylaxis is considered necessary, propranolol is the safest and most effective option. Valproate is contraindicated for migraine prophylaxis in women of reproductive capacity who are not using adequate contraception due to the increased risk of neural tube defects, cardiac defects, and other developmental effects associated with use of this medication.
Fertility treatment is frequently associated with increased headache and migraine. It is also important to consider that headache can be symptomatic of emotional stress, which would benefit from supportive management.
Breastfeeding generally maintains the benefits of pregnancy on migraine and should be encouraged, where possible. Recent studies suggest that the number of women choosing to breastfeed is rising, but there is also evidence that women with migraine do not initiate breastfeeding or discontinue because of their concerns about taking medication.
Unfortunately, many women and healthcare professionals rely on information in the package inserts, which may not tell the full story. Milk supply can reduce within 48 hours without full and repeated emptying of the breast, so advising a woman to interrupt breastfeeding for even a few days while treating a migraine attack can destroy her milk production. Hence, maintaining breastfeeding during drug treatment is increasingly recommended. Healthcare professionals should be informed about which treatments can safely be used at this time. For acute treatment, acetaminophen and NSAIDS are first-line options and can be combined with metoclopramide. Sumatriptan is a second-line option that allows breastfeeding to continue without the need to ‘pump and dump’.
Menopause and hormone replacement therapy
Despite increased prevalence of menstrual migraine during perimenopause, headache and migraine are under-reported by women with perimenopausal migraine. Management should be directed to treating the menopausal symptoms, which may include hormone replacement therapy (HRT). Studies suggest a significant association between migraine and current use of HRT.
Understanding the effects of different types of HRT is important, as some studies suggest that a history of worsening migraine at menopause is a factor in predicting worsening migraine with HRT. However, the regimen of HRT, route of estrogen, and type of progestogen all have differing effects on migraine. Non-oral routes of continuous estrogen/progestogen are less likely to have a negative effect on migraine than oral formulations, and continuous estrogen/progestogen appears to be better tolerated than cyclical combined HRT. Oral micronized progesterone may directly benefit migraine due to GABAergic effects. Disturbed sleep is both a common migraine trigger and the action of progesterone to restore normal sleep will also benefit migraine.
In contrast to CHCs, physiologic doses of transdermal estradiol and progesterone used in HRT are not associated with increased risk of ischemic stroke and can be used by women with migraine aura.
Conclusion
Many questions related to these topics don't have ready answers—questions like increased prevalence of hormone-related migraine, age of onset of menstrual migraines, and multispecialty treatment of these patients. Research is either limited or yet to be done, and we may not get the answers until healthcare professionals and women are more aware of the hormonal effects of migraine.
The effects of hormonal changes on migraine provide physicians with specific opportunities to identify and manage migraine in women. Under-treatment – in addition to causing unnecessary disability and suffering – is not economically cost-effective in terms of time lost from work and burden placed on the families of these patients. More effective health care would alleviate much of the suffering and therefore reduce both the personal and financial costs of migraine. Ineffective management of migraine in women has significant implications for women, their families, and their employers.
Migraine affects more than 1 in 6 US adults. This figure masks the fact that migraine is a predominantly female disorder; compared with men, 3-month migraine prevalence is more than 2-fold higher (21% vs. 10%) in women. Research by the World Health Organization has established migraine as second among the world’s causes of disability, and first among women of reproductive age.
Despite this burden of illness, migraine is often not diagnosed or treated effectively. General lifestyle advice such as maintaining a healthy weight, sleep hygiene, regular meals, regular exercise and hydration, and management of identified predisposing or triggering factors—together with optimization of symptomatic migraine treatment—can benefit all women with migraine. However, specific hormonal events such as menstruation, hormonal contraception, pregnancy, menopause, and hormone replacement therapy have variable, but often predictable, effects on the frequency and severity of migraine. At each of these life stages, there are specific opportunities to intervene and relieve migraine burden.
Menstrual migraine
Menstruation is one of the most significant risk factors for migraine, notably migraine without aura, with increased prevalence during a 5-day perimenstrual window that starts 2 days before the onset of menses and continues through the first 3 days of menstruation.
The International Headache Society (IHS) recognizes 2 types of menstrual migraine. There is menstrually related migraine, which is migraine without aura that regularly occurs on or between day −2 to +3 of menstruation (there is no day 0), with additional attacks of migraine with or without aura at other times of the cycle. There is also pure menstrual migraine, which is migraine without aura that occurs only on or between day −2 to +3, with no attacks at any other time of the cycle.
In women with menstrually related migraine, the diagnosis should only be made if the relationship between migraine and menstruation is greater than a chance association. To confirm this diagnosis, migraine attacks during the day −2 to +3 window must occur in at least 2 of 3 menstrual cycles. Relying on history to confirm the diagnosis can be misleading. Use of a 3-month diary can reveal the predictable patterns associated with menstrual migraine, aiding diagnosis and management.
Menstrual migraine affects 20% to 25% of women with migraine in the general population, and 22% to 70% of women seen in headache clinics. In women diagnosed with menstrual migraine, their perimenstrual attacks have distinctive clinical features which include more associated symptoms, longer duration, greater severity, greater susceptibility to relapse, greater resistance to treatment, and greater disability than migraines occurring at other times during the menstrual cycle.
Symptomatic treatment of perimenstrual attacks of migraine is the same as for treatment of non-menstrual attacks, but due to the longer duration of perimenstrual attacks, treatment usually needs to be repeated on several consecutive days. With respect to prevention, specific consideration should be given to the presence of menstrual disorders, contraceptive requirements, pregnancy wishes, and symptoms of perimenopause.
The 2 established triggers for perimenstrual migraine attacks are prostaglandin release, which also results in dysmenorrhea, and late luteal phase estrogen "withdrawal". There are no investigations to identify the relevant mechanism(s). However, a history of dysmenorrhea is suggestive of a prostaglandin trigger and both migraine and dysmenorrhea can benefit from treatment with prostaglandin inhibitors.
Contraceptive methods can effectively manage both perimenstrual triggers. The European Headache Federation and the European Society of Contraception and Reproductive Health recommend combined hormonal contraception (CHC) in women with menstrual migraine who require contraception or who have additional menstrual disorders that use of CHC would benefit. The desogestrel progestogen-only pill is an alternative option, particularly for women with aura, but bleeding side effects are a common reason for discontinuation.
The principal barriers to effective management of menstrual migraine are lack of awareness and under-diagnosis. Although the IHS criteria facilitate research diagnosis, there continues to be important unmet needs in the clinical management of women with menstrual migraine. Improved awareness by healthcare professionals is critical; women visiting their primary care physician or who are referred to a gynecologist seldom mention migraine unless specifically asked.
Contraception
Most women use contraception at some stage in their lives. Hormonal contraception, particularly CHC, is popular and effective, with additional non-contraceptive benefits.
As with the natural menstrual cycle, estrogen “withdrawal” (in this case the consequence of stopping contraceptive hormones during the hormone-free interval) can trigger migraine without aura. Eliminating the hormone-free interval by taking CHCs continuously, without a break, eliminates the risk of estrogen withdrawal migraine. Further, continuous use of CHC increases contraceptive efficacy and there are no differential safety concerns.
Contraceptive use of CHC is contraindicated in women with migraine aura, since migraine aura and use of ethinylestradiol are independent risk factors for ischemic stroke. Effective contraception need not be compromised since progestogen-only and non-hormonal methods—
several of which are more effective than CHC—are not associated with increased risk.
Despite there being little concern regarding use of CHCs in women with migraine without aura, clinical experience suggests that many women with migraine are denied CHCs. In some cases, this stems from misdiagnosing premonitory migraine symptoms as aura. In other cases, even a clear diagnosis of menstrual migraine without aura can result in CHCs being withheld due to the misconception of risk. To ensure that women receive optimum contraceptive options, contraceptive providers need a better understanding of the Medical Eligibility Criteria for Contraceptive Use as well as simple tools to aid migraine diagnosis.
Pregnancy and breastfeeding
Around 60-70% of pregnant women with migraine experience fewer attacks compared to pre-pregnancy, with improvement more likely in women with a history of menstrual migraine. In contrast, migraine with aura tends to continue to occur throughout pregnancy and postpartum and may start for the first time during this period. Women can be reassured that migraine, both with or without aura, does not have any adverse effect on the outcome of pregnancy. However, women with aura should be monitored during pregnancy since there is an increased risk of comorbid conditions, such as arterial and venous thrombosis, pre-eclampsia, and gestational hypertension.
Healthcare professionals need to be aware of their female patients with migraine who may be planning to conceive so that strategies for treating migraine can be discussed. Most drugs and other teratogens exert their greatest effects on the fetus in the first trimester, often before pregnancy is confirmed. Symptomatic treatment with acetaminophen and metoclopramide is safe. If this is ineffective, sumatriptan is an option. Ergot derivatives are contraindicated. If prophylaxis is considered necessary, propranolol is the safest and most effective option. Valproate is contraindicated for migraine prophylaxis in women of reproductive capacity who are not using adequate contraception due to the increased risk of neural tube defects, cardiac defects, and other developmental effects associated with use of this medication.
Fertility treatment is frequently associated with increased headache and migraine. It is also important to consider that headache can be symptomatic of emotional stress, which would benefit from supportive management.
Breastfeeding generally maintains the benefits of pregnancy on migraine and should be encouraged, where possible. Recent studies suggest that the number of women choosing to breastfeed is rising, but there is also evidence that women with migraine do not initiate breastfeeding or discontinue because of their concerns about taking medication.
Unfortunately, many women and healthcare professionals rely on information in the package inserts, which may not tell the full story. Milk supply can reduce within 48 hours without full and repeated emptying of the breast, so advising a woman to interrupt breastfeeding for even a few days while treating a migraine attack can destroy her milk production. Hence, maintaining breastfeeding during drug treatment is increasingly recommended. Healthcare professionals should be informed about which treatments can safely be used at this time. For acute treatment, acetaminophen and NSAIDS are first-line options and can be combined with metoclopramide. Sumatriptan is a second-line option that allows breastfeeding to continue without the need to ‘pump and dump’.
Menopause and hormone replacement therapy
Despite increased prevalence of menstrual migraine during perimenopause, headache and migraine are under-reported by women with perimenopausal migraine. Management should be directed to treating the menopausal symptoms, which may include hormone replacement therapy (HRT). Studies suggest a significant association between migraine and current use of HRT.
Understanding the effects of different types of HRT is important, as some studies suggest that a history of worsening migraine at menopause is a factor in predicting worsening migraine with HRT. However, the regimen of HRT, route of estrogen, and type of progestogen all have differing effects on migraine. Non-oral routes of continuous estrogen/progestogen are less likely to have a negative effect on migraine than oral formulations, and continuous estrogen/progestogen appears to be better tolerated than cyclical combined HRT. Oral micronized progesterone may directly benefit migraine due to GABAergic effects. Disturbed sleep is both a common migraine trigger and the action of progesterone to restore normal sleep will also benefit migraine.
In contrast to CHCs, physiologic doses of transdermal estradiol and progesterone used in HRT are not associated with increased risk of ischemic stroke and can be used by women with migraine aura.
Conclusion
Many questions related to these topics don't have ready answers—questions like increased prevalence of hormone-related migraine, age of onset of menstrual migraines, and multispecialty treatment of these patients. Research is either limited or yet to be done, and we may not get the answers until healthcare professionals and women are more aware of the hormonal effects of migraine.
The effects of hormonal changes on migraine provide physicians with specific opportunities to identify and manage migraine in women. Under-treatment – in addition to causing unnecessary disability and suffering – is not economically cost-effective in terms of time lost from work and burden placed on the families of these patients. More effective health care would alleviate much of the suffering and therefore reduce both the personal and financial costs of migraine. Ineffective management of migraine in women has significant implications for women, their families, and their employers.
Migraine affects more than 1 in 6 US adults. This figure masks the fact that migraine is a predominantly female disorder; compared with men, 3-month migraine prevalence is more than 2-fold higher (21% vs. 10%) in women. Research by the World Health Organization has established migraine as second among the world’s causes of disability, and first among women of reproductive age.
Despite this burden of illness, migraine is often not diagnosed or treated effectively. General lifestyle advice such as maintaining a healthy weight, sleep hygiene, regular meals, regular exercise and hydration, and management of identified predisposing or triggering factors—together with optimization of symptomatic migraine treatment—can benefit all women with migraine. However, specific hormonal events such as menstruation, hormonal contraception, pregnancy, menopause, and hormone replacement therapy have variable, but often predictable, effects on the frequency and severity of migraine. At each of these life stages, there are specific opportunities to intervene and relieve migraine burden.
Menstrual migraine
Menstruation is one of the most significant risk factors for migraine, notably migraine without aura, with increased prevalence during a 5-day perimenstrual window that starts 2 days before the onset of menses and continues through the first 3 days of menstruation.
The International Headache Society (IHS) recognizes 2 types of menstrual migraine. There is menstrually related migraine, which is migraine without aura that regularly occurs on or between day −2 to +3 of menstruation (there is no day 0), with additional attacks of migraine with or without aura at other times of the cycle. There is also pure menstrual migraine, which is migraine without aura that occurs only on or between day −2 to +3, with no attacks at any other time of the cycle.
In women with menstrually related migraine, the diagnosis should only be made if the relationship between migraine and menstruation is greater than a chance association. To confirm this diagnosis, migraine attacks during the day −2 to +3 window must occur in at least 2 of 3 menstrual cycles. Relying on history to confirm the diagnosis can be misleading. Use of a 3-month diary can reveal the predictable patterns associated with menstrual migraine, aiding diagnosis and management.
Menstrual migraine affects 20% to 25% of women with migraine in the general population, and 22% to 70% of women seen in headache clinics. In women diagnosed with menstrual migraine, their perimenstrual attacks have distinctive clinical features which include more associated symptoms, longer duration, greater severity, greater susceptibility to relapse, greater resistance to treatment, and greater disability than migraines occurring at other times during the menstrual cycle.
Symptomatic treatment of perimenstrual attacks of migraine is the same as for treatment of non-menstrual attacks, but due to the longer duration of perimenstrual attacks, treatment usually needs to be repeated on several consecutive days. With respect to prevention, specific consideration should be given to the presence of menstrual disorders, contraceptive requirements, pregnancy wishes, and symptoms of perimenopause.
The 2 established triggers for perimenstrual migraine attacks are prostaglandin release, which also results in dysmenorrhea, and late luteal phase estrogen "withdrawal". There are no investigations to identify the relevant mechanism(s). However, a history of dysmenorrhea is suggestive of a prostaglandin trigger and both migraine and dysmenorrhea can benefit from treatment with prostaglandin inhibitors.
Contraceptive methods can effectively manage both perimenstrual triggers. The European Headache Federation and the European Society of Contraception and Reproductive Health recommend combined hormonal contraception (CHC) in women with menstrual migraine who require contraception or who have additional menstrual disorders that use of CHC would benefit. The desogestrel progestogen-only pill is an alternative option, particularly for women with aura, but bleeding side effects are a common reason for discontinuation.
The principal barriers to effective management of menstrual migraine are lack of awareness and under-diagnosis. Although the IHS criteria facilitate research diagnosis, there continues to be important unmet needs in the clinical management of women with menstrual migraine. Improved awareness by healthcare professionals is critical; women visiting their primary care physician or who are referred to a gynecologist seldom mention migraine unless specifically asked.
Contraception
Most women use contraception at some stage in their lives. Hormonal contraception, particularly CHC, is popular and effective, with additional non-contraceptive benefits.
As with the natural menstrual cycle, estrogen “withdrawal” (in this case the consequence of stopping contraceptive hormones during the hormone-free interval) can trigger migraine without aura. Eliminating the hormone-free interval by taking CHCs continuously, without a break, eliminates the risk of estrogen withdrawal migraine. Further, continuous use of CHC increases contraceptive efficacy and there are no differential safety concerns.
Contraceptive use of CHC is contraindicated in women with migraine aura, since migraine aura and use of ethinylestradiol are independent risk factors for ischemic stroke. Effective contraception need not be compromised since progestogen-only and non-hormonal methods—
several of which are more effective than CHC—are not associated with increased risk.
Despite there being little concern regarding use of CHCs in women with migraine without aura, clinical experience suggests that many women with migraine are denied CHCs. In some cases, this stems from misdiagnosing premonitory migraine symptoms as aura. In other cases, even a clear diagnosis of menstrual migraine without aura can result in CHCs being withheld due to the misconception of risk. To ensure that women receive optimum contraceptive options, contraceptive providers need a better understanding of the Medical Eligibility Criteria for Contraceptive Use as well as simple tools to aid migraine diagnosis.
Pregnancy and breastfeeding
Around 60-70% of pregnant women with migraine experience fewer attacks compared to pre-pregnancy, with improvement more likely in women with a history of menstrual migraine. In contrast, migraine with aura tends to continue to occur throughout pregnancy and postpartum and may start for the first time during this period. Women can be reassured that migraine, both with or without aura, does not have any adverse effect on the outcome of pregnancy. However, women with aura should be monitored during pregnancy since there is an increased risk of comorbid conditions, such as arterial and venous thrombosis, pre-eclampsia, and gestational hypertension.
Healthcare professionals need to be aware of their female patients with migraine who may be planning to conceive so that strategies for treating migraine can be discussed. Most drugs and other teratogens exert their greatest effects on the fetus in the first trimester, often before pregnancy is confirmed. Symptomatic treatment with acetaminophen and metoclopramide is safe. If this is ineffective, sumatriptan is an option. Ergot derivatives are contraindicated. If prophylaxis is considered necessary, propranolol is the safest and most effective option. Valproate is contraindicated for migraine prophylaxis in women of reproductive capacity who are not using adequate contraception due to the increased risk of neural tube defects, cardiac defects, and other developmental effects associated with use of this medication.
Fertility treatment is frequently associated with increased headache and migraine. It is also important to consider that headache can be symptomatic of emotional stress, which would benefit from supportive management.
Breastfeeding generally maintains the benefits of pregnancy on migraine and should be encouraged, where possible. Recent studies suggest that the number of women choosing to breastfeed is rising, but there is also evidence that women with migraine do not initiate breastfeeding or discontinue because of their concerns about taking medication.
Unfortunately, many women and healthcare professionals rely on information in the package inserts, which may not tell the full story. Milk supply can reduce within 48 hours without full and repeated emptying of the breast, so advising a woman to interrupt breastfeeding for even a few days while treating a migraine attack can destroy her milk production. Hence, maintaining breastfeeding during drug treatment is increasingly recommended. Healthcare professionals should be informed about which treatments can safely be used at this time. For acute treatment, acetaminophen and NSAIDS are first-line options and can be combined with metoclopramide. Sumatriptan is a second-line option that allows breastfeeding to continue without the need to ‘pump and dump’.
Menopause and hormone replacement therapy
Despite increased prevalence of menstrual migraine during perimenopause, headache and migraine are under-reported by women with perimenopausal migraine. Management should be directed to treating the menopausal symptoms, which may include hormone replacement therapy (HRT). Studies suggest a significant association between migraine and current use of HRT.
Understanding the effects of different types of HRT is important, as some studies suggest that a history of worsening migraine at menopause is a factor in predicting worsening migraine with HRT. However, the regimen of HRT, route of estrogen, and type of progestogen all have differing effects on migraine. Non-oral routes of continuous estrogen/progestogen are less likely to have a negative effect on migraine than oral formulations, and continuous estrogen/progestogen appears to be better tolerated than cyclical combined HRT. Oral micronized progesterone may directly benefit migraine due to GABAergic effects. Disturbed sleep is both a common migraine trigger and the action of progesterone to restore normal sleep will also benefit migraine.
In contrast to CHCs, physiologic doses of transdermal estradiol and progesterone used in HRT are not associated with increased risk of ischemic stroke and can be used by women with migraine aura.
Conclusion
Many questions related to these topics don't have ready answers—questions like increased prevalence of hormone-related migraine, age of onset of menstrual migraines, and multispecialty treatment of these patients. Research is either limited or yet to be done, and we may not get the answers until healthcare professionals and women are more aware of the hormonal effects of migraine.
The effects of hormonal changes on migraine provide physicians with specific opportunities to identify and manage migraine in women. Under-treatment – in addition to causing unnecessary disability and suffering – is not economically cost-effective in terms of time lost from work and burden placed on the families of these patients. More effective health care would alleviate much of the suffering and therefore reduce both the personal and financial costs of migraine. Ineffective management of migraine in women has significant implications for women, their families, and their employers.
Advances in Diabetes and Heart Disease From ESC 2022
Professor Pardeep Jhund, from the University of Glasgow, United Kingdom, discusses highlights in diabetes and heart disease presented at the European Society of Cardiology Congress 2022.
He begins with a Danish study of the causes of excess mortality in individuals who have diabetes but do not have coronary artery disease. This showed that, even in these patients, greater efforts are required to improve outcomes.
A second Danish epidemiologic study examines the prevalence of diabetic neuropathy and cardiovascular outcomes. Worryingly, it found that around half of patients did not have their urinary albumin measured.
Next, Prof Jhund examines a follow-up analysis of the DANISH trial, which showed that implantable cardioverter-defibrillators were effective in reducing mortality, but only in individuals without diabetes.
He moves on to a pooled analysis of DECLARE-TIMI 58 and DAPA-CKD, which revealed that dapagliflozin consistently reduced kidney and cardiovascular events regardless of baseline eGFR and urinary albumin.
Finally, he discusses a large study addressing adherence rates for drugs in type 2 diabetes. The data on more than 38,000 patients showed that initiating therapy with an SGLT2 inhibitor or a GLP-1R agonist was associated with comparably high rates of adherence, which were unaffected by the presence or absence of cardiovascular disease.
--
Professor of Cardiology and Epidemiology, University of Glasgow; Honorary Consultant Cardiologist, Queen Elizabeth University Hospital, Glasgow, Scotland
Pardeep Jhund, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Global Clinical Trial Partners
Serve(d) as a speaker or a member of a speakers bureau for: AstraZeneca; Novartis
Received research grant from: AstraZeneca; Boehringer Ingelheim; Analog Devices
Professor Pardeep Jhund, from the University of Glasgow, United Kingdom, discusses highlights in diabetes and heart disease presented at the European Society of Cardiology Congress 2022.
He begins with a Danish study of the causes of excess mortality in individuals who have diabetes but do not have coronary artery disease. This showed that, even in these patients, greater efforts are required to improve outcomes.
A second Danish epidemiologic study examines the prevalence of diabetic neuropathy and cardiovascular outcomes. Worryingly, it found that around half of patients did not have their urinary albumin measured.
Next, Prof Jhund examines a follow-up analysis of the DANISH trial, which showed that implantable cardioverter-defibrillators were effective in reducing mortality, but only in individuals without diabetes.
He moves on to a pooled analysis of DECLARE-TIMI 58 and DAPA-CKD, which revealed that dapagliflozin consistently reduced kidney and cardiovascular events regardless of baseline eGFR and urinary albumin.
Finally, he discusses a large study addressing adherence rates for drugs in type 2 diabetes. The data on more than 38,000 patients showed that initiating therapy with an SGLT2 inhibitor or a GLP-1R agonist was associated with comparably high rates of adherence, which were unaffected by the presence or absence of cardiovascular disease.
--
Professor of Cardiology and Epidemiology, University of Glasgow; Honorary Consultant Cardiologist, Queen Elizabeth University Hospital, Glasgow, Scotland
Pardeep Jhund, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Global Clinical Trial Partners
Serve(d) as a speaker or a member of a speakers bureau for: AstraZeneca; Novartis
Received research grant from: AstraZeneca; Boehringer Ingelheim; Analog Devices
Professor Pardeep Jhund, from the University of Glasgow, United Kingdom, discusses highlights in diabetes and heart disease presented at the European Society of Cardiology Congress 2022.
He begins with a Danish study of the causes of excess mortality in individuals who have diabetes but do not have coronary artery disease. This showed that, even in these patients, greater efforts are required to improve outcomes.
A second Danish epidemiologic study examines the prevalence of diabetic neuropathy and cardiovascular outcomes. Worryingly, it found that around half of patients did not have their urinary albumin measured.
Next, Prof Jhund examines a follow-up analysis of the DANISH trial, which showed that implantable cardioverter-defibrillators were effective in reducing mortality, but only in individuals without diabetes.
He moves on to a pooled analysis of DECLARE-TIMI 58 and DAPA-CKD, which revealed that dapagliflozin consistently reduced kidney and cardiovascular events regardless of baseline eGFR and urinary albumin.
Finally, he discusses a large study addressing adherence rates for drugs in type 2 diabetes. The data on more than 38,000 patients showed that initiating therapy with an SGLT2 inhibitor or a GLP-1R agonist was associated with comparably high rates of adherence, which were unaffected by the presence or absence of cardiovascular disease.
--
Professor of Cardiology and Epidemiology, University of Glasgow; Honorary Consultant Cardiologist, Queen Elizabeth University Hospital, Glasgow, Scotland
Pardeep Jhund, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Global Clinical Trial Partners
Serve(d) as a speaker or a member of a speakers bureau for: AstraZeneca; Novartis
Received research grant from: AstraZeneca; Boehringer Ingelheim; Analog Devices

What is the best management strategy for complicated appendicitis in pregnancy?
Ashbrook M, et al. Management of complicated appendicitis during pregnancy in the US. JAMA Network Open. 2022;5:e227555. doi:10.1001/jamanetworkopen.2022.7555.
Expert Commentary
Over the last decade, the management of acute appendicitis in the nonpregnant adult has evolved such that some authorities favor first-line nonoperative therapy in the appropriate candidate, including some individuals with complicated appendicitis. While the conventional teaching regarding appendicitis in pregnancy has always been immediate surgery, favorable outcomes from nonoperative management in the nonpregnant population have led to an increasing application of conservative therapy in pregnancy, particularly among patients with uncomplicated appendicitis. However, optimal management of complicated appendicitis in pregnancy is unclear, as the risks of both operative and nonoperative management can be significant.
Details about the study
This retrospective cohort study using data from the National Inpatient Sample (NIS) focuses on outcomes of various management options among pregnant women with complicated appendicitis from January 2003 to September 2015. Complicated appendicitis refers to individuals with appendiceal perforation with peritonitis (a free perforation) or phlegmon/abscess (a walled-off perforation). Women included in the study were identified using ICD-9 codes for both pregnancy and complicated appendicitis; they were categorized into 3 groups: immediate operative management, successful nonoperative management, and failed nonoperative management (defined as surgical intervention >1 day after admission). The clinical and other outcomes of interest included maternal death, preterm labor/delivery or pregnancy loss, amniotic infection, sepsis, pneumonia, antenatal hemorrhage, and premature rupture of membranes. Outcomes included are those that occurred during the hospitalization for appendicitis; outcomes that may have occurred between discharge from the appendicitis hospitalization to the delivery hospitalization are not included in this study.
A total of 8,087 pregnant women with complicated appendicitis were included in this study, of whom 954 (11.8%) had successful nonoperative management, 2,646 (32.7%) had failed nonoperative management, and 4,487 (55.5%) had immediate operative management. First, when comparing successful nonoperative management to immediate operative management, there were no differences in preterm labor/delivery or pregnancy loss, or antenatal hemorrhage; however, successful nonoperative management was also associated with higher risks of maternal infectious complications, including risks of amniotic infection, pneumonia, and sepsis. When comparing failed nonoperative management (women who required surgical intervention during the index hospitalization) to immediate operative management, failed conservative management was associated with higher risks of preterm labor/delivery or pregnancy loss, antenatal hemorrhage, amniotic infection, pneumonia, and sepsis. For every 1 day that surgery was delayed in the group of women who failed nonoperative management, the odds of preterm labor/delivery or pregnancy loss, antenatal hemorrhage, sepsis, amniotic infection, and pneumonia increased.
Study strengths and weaknesses
Database studies have inherent limitations that are overcome with strength in numbers. In this study, our understanding of outcomes associated with management of complicated appendicitis assumes that women were correctly identified as both being pregnant and having complicated appendicitis (as opposed to uncomplicated appendicitis but miscoded). Clinical data that may have led to selection of one management strategy over another, or specific clinical management decisions, are not possible to extract from the NIS. For instance, did nonoperative management systematically include percutaneous guided drainage if an abscess was noted, and appropriately targeted antibiotic therapy? If delayed operative intervention with IV antibiotics to allow for “cooling off” of the abdomen prior to surgery was planned, this strategy would have been included in the failed nonoperative management group, when in fact nonoperative management was never the plan. Whether gestational age (which is not known in this study), or any other clinical data contributed to the initially chosen management strategy is not known.
The treating clinicians, obstetricians and surgeons alike, would like to know the pregnancy outcome when considering the various management strategies for complicated appendicitis. However, this study only provides insight into the outcomes for the hospitalization for appendicitis. Whether or not women categorized as successful nonoperative management go on to require surgery or have preterm labor in the future, or whether women with successful immediate surgical management might be readmitted with complications, is not known. This is a significant limitation of the database, which does not allow for linking of individual hospitalizations, and rather can provide only a snapshot in time.
This study includes a fairly long timespan–2003 to 2015–during which the management of complicated appendicitis was actively evolving. Early in this time frame, nonoperative management outside of pregnancy was uncommon, and nonoperative management may have been even rarer and perhaps reserved for the most ill of pregnant women on presentation (for whom surgery may have been considered too risky without a short time with IV antibiotics to “cool off” the abdomen). As time progressed over the study span, nonoperative management was likely offered with greater frequency and among women with lesser degrees of illness. However, the year of presentation was not controlled for in this study.
Finally, given the differences noted in management strategy by race/ethnicity and type of hospital, it is not clear how this bias influences the findings from this study. ●
Immediate operative intervention for complicated appendicitis in pregnancy remains a mainstay of management. Perinatal risks associated with surgical intervention are low and are comparable in many respects to successful nonoperative intervention. However, characteristics that predict successful nonoperative intervention are not known, and nonoperative therapy still carries higher risks of maternal infectious complications. When nonoperative intervention is the chosen approach in pregnant women with complicated appendicitis, clinicians must maintain a low threshold for conversion to operative management to avoid maternal morbidity. In addition, clinicians must closely monitor women discharged after successful appendicitis treatment for subsequent complications, as the long-term risks of conservative management or delayed operative intervention are not clear.
Ashbrook M, et al. Management of complicated appendicitis during pregnancy in the US. JAMA Network Open. 2022;5:e227555. doi:10.1001/jamanetworkopen.2022.7555.
Expert Commentary
Over the last decade, the management of acute appendicitis in the nonpregnant adult has evolved such that some authorities favor first-line nonoperative therapy in the appropriate candidate, including some individuals with complicated appendicitis. While the conventional teaching regarding appendicitis in pregnancy has always been immediate surgery, favorable outcomes from nonoperative management in the nonpregnant population have led to an increasing application of conservative therapy in pregnancy, particularly among patients with uncomplicated appendicitis. However, optimal management of complicated appendicitis in pregnancy is unclear, as the risks of both operative and nonoperative management can be significant.
Details about the study
This retrospective cohort study using data from the National Inpatient Sample (NIS) focuses on outcomes of various management options among pregnant women with complicated appendicitis from January 2003 to September 2015. Complicated appendicitis refers to individuals with appendiceal perforation with peritonitis (a free perforation) or phlegmon/abscess (a walled-off perforation). Women included in the study were identified using ICD-9 codes for both pregnancy and complicated appendicitis; they were categorized into 3 groups: immediate operative management, successful nonoperative management, and failed nonoperative management (defined as surgical intervention >1 day after admission). The clinical and other outcomes of interest included maternal death, preterm labor/delivery or pregnancy loss, amniotic infection, sepsis, pneumonia, antenatal hemorrhage, and premature rupture of membranes. Outcomes included are those that occurred during the hospitalization for appendicitis; outcomes that may have occurred between discharge from the appendicitis hospitalization to the delivery hospitalization are not included in this study.
A total of 8,087 pregnant women with complicated appendicitis were included in this study, of whom 954 (11.8%) had successful nonoperative management, 2,646 (32.7%) had failed nonoperative management, and 4,487 (55.5%) had immediate operative management. First, when comparing successful nonoperative management to immediate operative management, there were no differences in preterm labor/delivery or pregnancy loss, or antenatal hemorrhage; however, successful nonoperative management was also associated with higher risks of maternal infectious complications, including risks of amniotic infection, pneumonia, and sepsis. When comparing failed nonoperative management (women who required surgical intervention during the index hospitalization) to immediate operative management, failed conservative management was associated with higher risks of preterm labor/delivery or pregnancy loss, antenatal hemorrhage, amniotic infection, pneumonia, and sepsis. For every 1 day that surgery was delayed in the group of women who failed nonoperative management, the odds of preterm labor/delivery or pregnancy loss, antenatal hemorrhage, sepsis, amniotic infection, and pneumonia increased.
Study strengths and weaknesses
Database studies have inherent limitations that are overcome with strength in numbers. In this study, our understanding of outcomes associated with management of complicated appendicitis assumes that women were correctly identified as both being pregnant and having complicated appendicitis (as opposed to uncomplicated appendicitis but miscoded). Clinical data that may have led to selection of one management strategy over another, or specific clinical management decisions, are not possible to extract from the NIS. For instance, did nonoperative management systematically include percutaneous guided drainage if an abscess was noted, and appropriately targeted antibiotic therapy? If delayed operative intervention with IV antibiotics to allow for “cooling off” of the abdomen prior to surgery was planned, this strategy would have been included in the failed nonoperative management group, when in fact nonoperative management was never the plan. Whether gestational age (which is not known in this study), or any other clinical data contributed to the initially chosen management strategy is not known.
The treating clinicians, obstetricians and surgeons alike, would like to know the pregnancy outcome when considering the various management strategies for complicated appendicitis. However, this study only provides insight into the outcomes for the hospitalization for appendicitis. Whether or not women categorized as successful nonoperative management go on to require surgery or have preterm labor in the future, or whether women with successful immediate surgical management might be readmitted with complications, is not known. This is a significant limitation of the database, which does not allow for linking of individual hospitalizations, and rather can provide only a snapshot in time.
This study includes a fairly long timespan–2003 to 2015–during which the management of complicated appendicitis was actively evolving. Early in this time frame, nonoperative management outside of pregnancy was uncommon, and nonoperative management may have been even rarer and perhaps reserved for the most ill of pregnant women on presentation (for whom surgery may have been considered too risky without a short time with IV antibiotics to “cool off” the abdomen). As time progressed over the study span, nonoperative management was likely offered with greater frequency and among women with lesser degrees of illness. However, the year of presentation was not controlled for in this study.
Finally, given the differences noted in management strategy by race/ethnicity and type of hospital, it is not clear how this bias influences the findings from this study. ●
Immediate operative intervention for complicated appendicitis in pregnancy remains a mainstay of management. Perinatal risks associated with surgical intervention are low and are comparable in many respects to successful nonoperative intervention. However, characteristics that predict successful nonoperative intervention are not known, and nonoperative therapy still carries higher risks of maternal infectious complications. When nonoperative intervention is the chosen approach in pregnant women with complicated appendicitis, clinicians must maintain a low threshold for conversion to operative management to avoid maternal morbidity. In addition, clinicians must closely monitor women discharged after successful appendicitis treatment for subsequent complications, as the long-term risks of conservative management or delayed operative intervention are not clear.
Ashbrook M, et al. Management of complicated appendicitis during pregnancy in the US. JAMA Network Open. 2022;5:e227555. doi:10.1001/jamanetworkopen.2022.7555.
Expert Commentary
Over the last decade, the management of acute appendicitis in the nonpregnant adult has evolved such that some authorities favor first-line nonoperative therapy in the appropriate candidate, including some individuals with complicated appendicitis. While the conventional teaching regarding appendicitis in pregnancy has always been immediate surgery, favorable outcomes from nonoperative management in the nonpregnant population have led to an increasing application of conservative therapy in pregnancy, particularly among patients with uncomplicated appendicitis. However, optimal management of complicated appendicitis in pregnancy is unclear, as the risks of both operative and nonoperative management can be significant.
Details about the study
This retrospective cohort study using data from the National Inpatient Sample (NIS) focuses on outcomes of various management options among pregnant women with complicated appendicitis from January 2003 to September 2015. Complicated appendicitis refers to individuals with appendiceal perforation with peritonitis (a free perforation) or phlegmon/abscess (a walled-off perforation). Women included in the study were identified using ICD-9 codes for both pregnancy and complicated appendicitis; they were categorized into 3 groups: immediate operative management, successful nonoperative management, and failed nonoperative management (defined as surgical intervention >1 day after admission). The clinical and other outcomes of interest included maternal death, preterm labor/delivery or pregnancy loss, amniotic infection, sepsis, pneumonia, antenatal hemorrhage, and premature rupture of membranes. Outcomes included are those that occurred during the hospitalization for appendicitis; outcomes that may have occurred between discharge from the appendicitis hospitalization to the delivery hospitalization are not included in this study.
A total of 8,087 pregnant women with complicated appendicitis were included in this study, of whom 954 (11.8%) had successful nonoperative management, 2,646 (32.7%) had failed nonoperative management, and 4,487 (55.5%) had immediate operative management. First, when comparing successful nonoperative management to immediate operative management, there were no differences in preterm labor/delivery or pregnancy loss, or antenatal hemorrhage; however, successful nonoperative management was also associated with higher risks of maternal infectious complications, including risks of amniotic infection, pneumonia, and sepsis. When comparing failed nonoperative management (women who required surgical intervention during the index hospitalization) to immediate operative management, failed conservative management was associated with higher risks of preterm labor/delivery or pregnancy loss, antenatal hemorrhage, amniotic infection, pneumonia, and sepsis. For every 1 day that surgery was delayed in the group of women who failed nonoperative management, the odds of preterm labor/delivery or pregnancy loss, antenatal hemorrhage, sepsis, amniotic infection, and pneumonia increased.
Study strengths and weaknesses
Database studies have inherent limitations that are overcome with strength in numbers. In this study, our understanding of outcomes associated with management of complicated appendicitis assumes that women were correctly identified as both being pregnant and having complicated appendicitis (as opposed to uncomplicated appendicitis but miscoded). Clinical data that may have led to selection of one management strategy over another, or specific clinical management decisions, are not possible to extract from the NIS. For instance, did nonoperative management systematically include percutaneous guided drainage if an abscess was noted, and appropriately targeted antibiotic therapy? If delayed operative intervention with IV antibiotics to allow for “cooling off” of the abdomen prior to surgery was planned, this strategy would have been included in the failed nonoperative management group, when in fact nonoperative management was never the plan. Whether gestational age (which is not known in this study), or any other clinical data contributed to the initially chosen management strategy is not known.
The treating clinicians, obstetricians and surgeons alike, would like to know the pregnancy outcome when considering the various management strategies for complicated appendicitis. However, this study only provides insight into the outcomes for the hospitalization for appendicitis. Whether or not women categorized as successful nonoperative management go on to require surgery or have preterm labor in the future, or whether women with successful immediate surgical management might be readmitted with complications, is not known. This is a significant limitation of the database, which does not allow for linking of individual hospitalizations, and rather can provide only a snapshot in time.
This study includes a fairly long timespan–2003 to 2015–during which the management of complicated appendicitis was actively evolving. Early in this time frame, nonoperative management outside of pregnancy was uncommon, and nonoperative management may have been even rarer and perhaps reserved for the most ill of pregnant women on presentation (for whom surgery may have been considered too risky without a short time with IV antibiotics to “cool off” the abdomen). As time progressed over the study span, nonoperative management was likely offered with greater frequency and among women with lesser degrees of illness. However, the year of presentation was not controlled for in this study.
Finally, given the differences noted in management strategy by race/ethnicity and type of hospital, it is not clear how this bias influences the findings from this study. ●
Immediate operative intervention for complicated appendicitis in pregnancy remains a mainstay of management. Perinatal risks associated with surgical intervention are low and are comparable in many respects to successful nonoperative intervention. However, characteristics that predict successful nonoperative intervention are not known, and nonoperative therapy still carries higher risks of maternal infectious complications. When nonoperative intervention is the chosen approach in pregnant women with complicated appendicitis, clinicians must maintain a low threshold for conversion to operative management to avoid maternal morbidity. In addition, clinicians must closely monitor women discharged after successful appendicitis treatment for subsequent complications, as the long-term risks of conservative management or delayed operative intervention are not clear.
An epidemic of hypertensive disorders of pregnancy
Hypertension in pregnancy is a major challenge in current obstetric practice. Based on an analysis of the National Inpatient Sample, the Centers for Disease Control and Prevention (CDC) recently reported that from 2017 to 2019 the prevalence of hypertensive disorders in pregnancy increased from 13.3% to 15.9% of hospital deliveries.1 During that same time period, the prevalence of pregnancy-associated hypertension, which includes preeclampsia, eclampsia, and gestational hypertension, increased from 10.8% to 13.0%.1 The prevalence of chronic hypertension increased from 2.0% to 2.3%.1 In 2017 and 2019, unspecified maternal hypertension was diagnosed in 0.5% and 0.6% of the sample, respectively.1
Bruno and colleagues reported a 3-fold increase in the prevalence of HDPs from 1989 to 2020, with an acceleration in the rate of increase from 2010 to 2020.2 The increase in prevalence of HDPs may be caused by an increase in the prevalence of advanced maternal age, obesity, and diabetes. Black patients are disproportionately impacted by both pregnancy-associated hypertension and chronic hypertension.1 In 2019, the prevalence of pregnancy-associated hypertension was greater among Black patients (15.6%), than White (12.1%), Hispanic (10.6%), or Asian or Pacific Islander patients (7.7%).1 Similarly, the prevalence of chronic hypertension was greater among Black patients (4.3%) than among White (2.0%), Hispanic (1.5%), or Asian or Pacific Islander patients (1.2%).1 Racial/ethnic differences in HDPs may be influenced by poverty; structural racism; or lack of access to care, diet, and obesity.3,4
HDPs are major contributors to maternal morbidity and mortality. The CDC reported that among maternal deaths occurring during the delivery hospitalization, 32% of the decedents had documented hypertension.1 HDPs are associated with an approximately 2.5-fold increased risk of a severe morbidity, a composite measure that includes blood transfusion, acute kidney injury, disseminated intravascular coagulation, sepsis, shock, and pulmonary edema.5 A history of HDPs is associated with an approximately 67% increase in the lifetime risk of cardiovascular disease, including coronary artery disease, stroke, peripheral vascular disease, and heart failure.6,7
What are the best antihypertensive medications for pregnancy?
All clinicians know that the use of angiotensin-converting-enzyme inhibitors (ACE-Is) and angiotensin-receptor-blockers (ARBs) are contraindicated in pregnancy because they cause major congenital anomalies, with an odds ratio of 1.8 (95% confidence interval [CI], 1.42-2.34), compared with no exposure.8 In addition, ACE-Is and ARBs increase the risk of stillbirth, with an odds ratio of 1.75 (95% CI, 1.21-2.53).8 No increase in congenital anomalies were detected for patients exposed to other antihypertensive medications.8 Prior to attempting conception, patients with chronic hypertension should discontinue ACE-Is and ARBs and initiate an alternative medication.
The most commonly used antihypertensive medications in pregnancy are labetalol, nifedipine, and methyldopa.9 Labetalol blocks the beta-1, beta-2, and alpha-1 adrenergic receptors.10 Nifedipine blocks calcium entry into cells through the L-type calcium channel.11 Methyldopa is a central nervous system alpha-2 adrenergic agonist.12 The dose range for these commonly used medications are labetalol 400 mg to 2,400 mg daily in divided doses every 8 to 12 hours, nifedipine extended-release 30 mg to 120 mg daily, and methyldopa 500 mg to 2 g daily in 2 to 4 divided doses. Some clinicians recommend prescribing divided doses of nifedipine extended release at doses ≥ 60 mg for patients who have bothersome adverse effects, hypotension following a single daily dose, or hypertension between single daily doses. The nifedipine extended release tablets should not be divided. If monotherapy with the maximal daily dose of labetalol does not achieve the blood pressure (BP) target, adding nifedipine as a second agent is an option.9 Similarly, if monotherapy with the maximal daily dose of nifedipine extended release does not achieve the BP target, adding labetalol as a second agent is an option.9
In a network meta-analysis of antihypertensive medications used in pregnancy, that included 61 trials and 6,923 participants, all the medications studied reduced the risk of developing severe hypertension by 30% to 70%.13 Sufficient data was available to also report that labetalol used to treat hypertension in pregnancy reduced the risk of developing proteinuria.13 Given similar efficacy among antihypertensive medications, patient comorbidities may influence the medication choice. For example, labetalol may not be the optimal medication for a patient with poorly controlled asthma due to its ability to cause bronchospasm.14,15 Methyldopa may not be the optimal medication for a patient with depression.16 Based on the available data, labetalol, nifedipine, and methyldopa are the best antihypertensive medications for pregnant patients.
Continue to: What is an optimal BP target when treating chronic hypertension in pregnancy?...
What is an optimal BP target when treating chronic hypertension in pregnancy?
When treating chronic hypertension in pregnant patients, a concern is that reducing maternal BP may decrease uteroplacental perfusion and result in fetal growth restriction. However, a recent trial reported that a BP treatment target < 140/90 mm Hg is associated with better outcomes for both mother and newborn than withholding antihypertension medications. In the trial, 2,408 women with chronic hypertension diagnosed before 20 weeks of gestation were randomly assigned to an active treatment group with prescription of antihypertension medicines to achieve a BP target of < 140/90 mm Hg; or to a control group where no antihypertension or no additional antihypertension treatment was prescribed unless BP was ≥ 160 mm Hg systolic or ≥ 105 mm Hg diastolic.9 The hypertension medications prescribed to the patients in the active treatment group were labetalol (63.2%), nifedipine (33.4%), amlodipine (1.7%), methyldopa (0.5%), hydrochlorothiazide (0.3%), metoprolol (0.2%), and missing/unknown/other (0.7%).9
If a patient in the control group developed severe hypertension, they were started on an antihypertension medicine and the BP treatment target was < 140/90 mm Hg. Compared with the control regimen, active treatment resulted in a significant decrease in the development of preeclampsia (24.4% vs 31.1%; risk ratio [RR], 0.79; 95% CI, 0.69-0.89), severe hypertension (36.1% vs 44.3%; RR, 0.82; 95% CI, 0.74-0.90), preterm birth < 37 weeks’ gestation (27.5% vs 31.4%; RR, 0.87; 95% CI, 0.77-0.99), preterm birth < 35 weeks’ gestation (12.2% vs 16.7%; odds ratio [OR], 0.69; 95% CI, 0.55-0.88), and low birth-weight (< 2,500 g) newborns (19.2% vs 23.1%; RR, 0.83; 95% CI, 0.71-0.97).9 The percentage of small for gestational age birth weight below the 10th percentile was similar in the treatment and control groups, 11.2% and 10.4%, respectively (adjusted RR, 1.04; 95% CI, 0.82-1.31).9 The number of patients who would need to be treated to prevent one primary-outcome event was 15.9 The investigators concluded that for pregnant patients with chronic hypertension, the optimal BP target is < 140/90 mm Hg.9
In pregnant patients with hypertension, BP may decrease immediately after birth. Following birth, BP tends to increase, reaching a peak 3 to 6 days postpartum.17,18 This pattern was observed in patients with and without preeclampsia in the index pregnancy. Among 136 patients without antepartum preeclampsia, the prevalence of a diastolic BP > 89 mm Hg was 5% and 15% on postpartum days 1 and 3, respectively.17 The postpartum rise in BP may be due to mobilization of water from the extravascular to the intravascular space and excretion of total body sodium that accumulated during pregnancy.19 In one study of 998 consecutive singleton cesarean births, 7.7% of the patients with no recorded elevated BP before delivery developed de novo hypertension postpartum.20 Compared with patients without antepartum or new onset postpartum hypertension, the patients who developed postpartum hypertension had a higher body mass index, were more likely to be Black and to have a history of type 2 diabetes. Compared with patients without antepartum or postpartum hypertension, the patients who developed de novo postpartum hypertension, had significantly elevated soluble fms-like tyrosine kinase-1 and significantly decreased placental growth factor, a pattern seen with preeclampsia.20 These results suggest that de novo postpartum hypertension may have molecular causes similar to preeclampsia.20
Postpartum hypertension should be treated with a medication that is thought to be safe for breastfeeding patients, including labetalol, nifedipine, or enalapril.21-23 The relative infant dose of labetalol, nifedipine, and enalapril is approximately 3.6%, ≤ 3.2%, and 1.1%, respectively.24 If the relative infant dose of a medication is < 10% it is generally considered to be compatible with breastfeeding.25
Many obstetricians have seldom prescribed enalapril, an ACE-I. The initial dose of enalapril is 5 mg or 10 mg daily. After initiation of treatment, the dose can be adjusted based on BP measurement. The maximal daily dose is 40 mg daily in one dose or two divided doses. Similar to other hypertension medicines, enalapril therapy may cause hypotension and dizziness. Enalapril should not be used by pregnant patients because it is associated with an increased risk of congenital anomalies and fetal demise.
Does a HDP increase the risk of developing chronic hypertension?
All obstetricians know that a patient with a history of a HDP is at an increased risk for developing chronic hypertension treated with a medication, but the magnitude of the risk is less well known. In a nationwide study in Denmark, the prevalence of chronic hypertension treated with medication 10 years after delivery among patients with a history of a HDP in their first pregnancy, was 14%, 21%, and 32%, if the first pregnancy occurred in the patient’s 20s, 30s, or 40s, respectively.26 The corresponding prevalence of chronic hypertension in patients without a history of a HDP was 4%, 6%, and 11%, if the first pregnancy occurred in the 20s, 30s, or 40s, respectively.26 Maternal age is an important predictor of who will develop chronic hypertension within 10 years following a pregnancy with a HDP.
In modern obstetric practice, the hypertensive disorders of pregnancy are prevalent and associated with increased maternal and newborn morbidity. Appropriate treatment of hypertension with labetalol, nifedipine, or methyldopa improves maternal and newborn health. Available evidence suggests that maintaining BP < 140/90 mm Hg during pregnancy for most patients is a practical goal with significant benefit. A significant public-health concern is that an increase in the prevalence of HDPs will eventually translate into an increase in chronic hypertension and the attendant complications of heart attack, heart failure, stroke, and renal insufficiency. Recognizing the increased prevalence of HDPs, ObGyns will need to alert patients to their long-term health risks and coordinate appropriate follow-up and treatment to optimize the future health of their patients. ●
- Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization-United States, 2017-2019. Morb Mortal Week Report. 2022;71:585-591.
- Bruno AM, Allshouse AA, Metz TD, et al. Trends in hypertensive disorders of pregnancy in the United States from 1989 to 2020. Obstet Gynecol. 2022;140:83-86.
- Doleszar CM, McGrath JJ, Herzig AJM, et al. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol. 2014;33:20-34.
- Centers for Disease Control and Prevention. A Closer Look at African American Men and High Blood Pressure Control; A Review of Psychosocial Factors and Systems-Level Interventions. Atlanta: U.S. Department of Health and Human Services; 2010.
- Boulet SL, Platner M, Joseph NT, et al. Hypertensive disorders of pregnancy, cesarean delivery and severe maternal morbidity in an urban safety-net population. Am J Epidemiol. 2020;189:1502-1511.
- Parikh NI, Gonzalez JM, Andreson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143:e902-e916.
- Okoth K, Chandan JS, Marshall T, et al. Association between the reproductive health of young women and cardiovascular disease later in life: umbrella review. BMJ. 2020;371:m3502.
- Fu J, Tomlinson G, Feig DS. Increased risk of major congenital malformations in early pregnancy uses of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers: a meta-analysis. Diabetes Metab Res Rev. 2021;37:e3453.
- Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781-1792.
- Baum T, Sybertz EJ. Pharmacology of labetalol in experimental animals. Am J Med. 1983;75:15-23.
- Khan KM, Patel JB, Schaefer TJ. StatPearls (Internet). StatPearls Publishing; 2022.
- Gupta M, Khalili. Methyldopa StatPearls (Internet). StatPearls Publishing; 2022.
- Bone JN, Sandhu A, Diablos ED, et al. Oral antihypertensives for non-severe pregnancy hypertension: systematic review, network meta-analysis and trial sequential analysis. Hypertension. 2022;79:614-628.
- Morales DR, Jackson C, Lipworth BJ, et al. Adverse respiratory effects of acute beta-blocker exposure in asthma: a systematic review and meta-analysis of randomized controlled trials. Chest. 2014;145:779-786.
- Huang KY, Tseng PT, Wu YC, et al. Do beta-adrenergic blocking agents increase asthma exacerbation? A network meta-analysis of randomized controlled trials. Sci Rep. 2021;11:452.
- Nayak AS, Nachane HB. Risk analysis of suicidal ideation and postpartum depression with antenatal alpha methyldopa use. Asian J Psychiatry. 2018;38:42-44.
- Walters BNJ, Thompson ME, Lee A, et al. Blood pressure in the puerperium. Clin Sci. 1986;71:589-594.
- Walters BNJ, Walters T. Hypertension in the puerperium. Lancet. 1987;2(8554):330.
- Magee L, von Dadelszen. Prevention and treatment of postpartum hypertension. Cochrane Database Syst Rev. 2013;CD004351.
- Goel A, Maski MR, Bajracharya S, et al. Epidemiology and mechanisms of de novo and persistent hypertension in the postpartum period. Circulation. 2015;132:1726-1733.
- Powles K, Gandhi S. Postpartum hypertension. CMAJ. 2017;189:E913.
- Tosounidou S, Gordon C. Medications in pregnancy and breastfeeding. Best Prac Res Clin Obstet Gynaecol. 2020;64:68-76.
- Anderson PO. Treating hypertension during breastfeeding. Breastfeed Med. 2018;13:95-96.
- Lexicomp web site. https://www.wolterskluwer.com/en/solutions/lexicomp.
- Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343:118-126.
- Behrens I, Basit S, Melbye M, et al. Risk of postpartum hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:j3078.

Hypertension in pregnancy is a major challenge in current obstetric practice. Based on an analysis of the National Inpatient Sample, the Centers for Disease Control and Prevention (CDC) recently reported that from 2017 to 2019 the prevalence of hypertensive disorders in pregnancy increased from 13.3% to 15.9% of hospital deliveries.1 During that same time period, the prevalence of pregnancy-associated hypertension, which includes preeclampsia, eclampsia, and gestational hypertension, increased from 10.8% to 13.0%.1 The prevalence of chronic hypertension increased from 2.0% to 2.3%.1 In 2017 and 2019, unspecified maternal hypertension was diagnosed in 0.5% and 0.6% of the sample, respectively.1
Bruno and colleagues reported a 3-fold increase in the prevalence of HDPs from 1989 to 2020, with an acceleration in the rate of increase from 2010 to 2020.2 The increase in prevalence of HDPs may be caused by an increase in the prevalence of advanced maternal age, obesity, and diabetes. Black patients are disproportionately impacted by both pregnancy-associated hypertension and chronic hypertension.1 In 2019, the prevalence of pregnancy-associated hypertension was greater among Black patients (15.6%), than White (12.1%), Hispanic (10.6%), or Asian or Pacific Islander patients (7.7%).1 Similarly, the prevalence of chronic hypertension was greater among Black patients (4.3%) than among White (2.0%), Hispanic (1.5%), or Asian or Pacific Islander patients (1.2%).1 Racial/ethnic differences in HDPs may be influenced by poverty; structural racism; or lack of access to care, diet, and obesity.3,4
HDPs are major contributors to maternal morbidity and mortality. The CDC reported that among maternal deaths occurring during the delivery hospitalization, 32% of the decedents had documented hypertension.1 HDPs are associated with an approximately 2.5-fold increased risk of a severe morbidity, a composite measure that includes blood transfusion, acute kidney injury, disseminated intravascular coagulation, sepsis, shock, and pulmonary edema.5 A history of HDPs is associated with an approximately 67% increase in the lifetime risk of cardiovascular disease, including coronary artery disease, stroke, peripheral vascular disease, and heart failure.6,7
What are the best antihypertensive medications for pregnancy?
All clinicians know that the use of angiotensin-converting-enzyme inhibitors (ACE-Is) and angiotensin-receptor-blockers (ARBs) are contraindicated in pregnancy because they cause major congenital anomalies, with an odds ratio of 1.8 (95% confidence interval [CI], 1.42-2.34), compared with no exposure.8 In addition, ACE-Is and ARBs increase the risk of stillbirth, with an odds ratio of 1.75 (95% CI, 1.21-2.53).8 No increase in congenital anomalies were detected for patients exposed to other antihypertensive medications.8 Prior to attempting conception, patients with chronic hypertension should discontinue ACE-Is and ARBs and initiate an alternative medication.
The most commonly used antihypertensive medications in pregnancy are labetalol, nifedipine, and methyldopa.9 Labetalol blocks the beta-1, beta-2, and alpha-1 adrenergic receptors.10 Nifedipine blocks calcium entry into cells through the L-type calcium channel.11 Methyldopa is a central nervous system alpha-2 adrenergic agonist.12 The dose range for these commonly used medications are labetalol 400 mg to 2,400 mg daily in divided doses every 8 to 12 hours, nifedipine extended-release 30 mg to 120 mg daily, and methyldopa 500 mg to 2 g daily in 2 to 4 divided doses. Some clinicians recommend prescribing divided doses of nifedipine extended release at doses ≥ 60 mg for patients who have bothersome adverse effects, hypotension following a single daily dose, or hypertension between single daily doses. The nifedipine extended release tablets should not be divided. If monotherapy with the maximal daily dose of labetalol does not achieve the blood pressure (BP) target, adding nifedipine as a second agent is an option.9 Similarly, if monotherapy with the maximal daily dose of nifedipine extended release does not achieve the BP target, adding labetalol as a second agent is an option.9
In a network meta-analysis of antihypertensive medications used in pregnancy, that included 61 trials and 6,923 participants, all the medications studied reduced the risk of developing severe hypertension by 30% to 70%.13 Sufficient data was available to also report that labetalol used to treat hypertension in pregnancy reduced the risk of developing proteinuria.13 Given similar efficacy among antihypertensive medications, patient comorbidities may influence the medication choice. For example, labetalol may not be the optimal medication for a patient with poorly controlled asthma due to its ability to cause bronchospasm.14,15 Methyldopa may not be the optimal medication for a patient with depression.16 Based on the available data, labetalol, nifedipine, and methyldopa are the best antihypertensive medications for pregnant patients.
Continue to: What is an optimal BP target when treating chronic hypertension in pregnancy?...
What is an optimal BP target when treating chronic hypertension in pregnancy?
When treating chronic hypertension in pregnant patients, a concern is that reducing maternal BP may decrease uteroplacental perfusion and result in fetal growth restriction. However, a recent trial reported that a BP treatment target < 140/90 mm Hg is associated with better outcomes for both mother and newborn than withholding antihypertension medications. In the trial, 2,408 women with chronic hypertension diagnosed before 20 weeks of gestation were randomly assigned to an active treatment group with prescription of antihypertension medicines to achieve a BP target of < 140/90 mm Hg; or to a control group where no antihypertension or no additional antihypertension treatment was prescribed unless BP was ≥ 160 mm Hg systolic or ≥ 105 mm Hg diastolic.9 The hypertension medications prescribed to the patients in the active treatment group were labetalol (63.2%), nifedipine (33.4%), amlodipine (1.7%), methyldopa (0.5%), hydrochlorothiazide (0.3%), metoprolol (0.2%), and missing/unknown/other (0.7%).9
If a patient in the control group developed severe hypertension, they were started on an antihypertension medicine and the BP treatment target was < 140/90 mm Hg. Compared with the control regimen, active treatment resulted in a significant decrease in the development of preeclampsia (24.4% vs 31.1%; risk ratio [RR], 0.79; 95% CI, 0.69-0.89), severe hypertension (36.1% vs 44.3%; RR, 0.82; 95% CI, 0.74-0.90), preterm birth < 37 weeks’ gestation (27.5% vs 31.4%; RR, 0.87; 95% CI, 0.77-0.99), preterm birth < 35 weeks’ gestation (12.2% vs 16.7%; odds ratio [OR], 0.69; 95% CI, 0.55-0.88), and low birth-weight (< 2,500 g) newborns (19.2% vs 23.1%; RR, 0.83; 95% CI, 0.71-0.97).9 The percentage of small for gestational age birth weight below the 10th percentile was similar in the treatment and control groups, 11.2% and 10.4%, respectively (adjusted RR, 1.04; 95% CI, 0.82-1.31).9 The number of patients who would need to be treated to prevent one primary-outcome event was 15.9 The investigators concluded that for pregnant patients with chronic hypertension, the optimal BP target is < 140/90 mm Hg.9
In pregnant patients with hypertension, BP may decrease immediately after birth. Following birth, BP tends to increase, reaching a peak 3 to 6 days postpartum.17,18 This pattern was observed in patients with and without preeclampsia in the index pregnancy. Among 136 patients without antepartum preeclampsia, the prevalence of a diastolic BP > 89 mm Hg was 5% and 15% on postpartum days 1 and 3, respectively.17 The postpartum rise in BP may be due to mobilization of water from the extravascular to the intravascular space and excretion of total body sodium that accumulated during pregnancy.19 In one study of 998 consecutive singleton cesarean births, 7.7% of the patients with no recorded elevated BP before delivery developed de novo hypertension postpartum.20 Compared with patients without antepartum or new onset postpartum hypertension, the patients who developed postpartum hypertension had a higher body mass index, were more likely to be Black and to have a history of type 2 diabetes. Compared with patients without antepartum or postpartum hypertension, the patients who developed de novo postpartum hypertension, had significantly elevated soluble fms-like tyrosine kinase-1 and significantly decreased placental growth factor, a pattern seen with preeclampsia.20 These results suggest that de novo postpartum hypertension may have molecular causes similar to preeclampsia.20
Postpartum hypertension should be treated with a medication that is thought to be safe for breastfeeding patients, including labetalol, nifedipine, or enalapril.21-23 The relative infant dose of labetalol, nifedipine, and enalapril is approximately 3.6%, ≤ 3.2%, and 1.1%, respectively.24 If the relative infant dose of a medication is < 10% it is generally considered to be compatible with breastfeeding.25
Many obstetricians have seldom prescribed enalapril, an ACE-I. The initial dose of enalapril is 5 mg or 10 mg daily. After initiation of treatment, the dose can be adjusted based on BP measurement. The maximal daily dose is 40 mg daily in one dose or two divided doses. Similar to other hypertension medicines, enalapril therapy may cause hypotension and dizziness. Enalapril should not be used by pregnant patients because it is associated with an increased risk of congenital anomalies and fetal demise.
Does a HDP increase the risk of developing chronic hypertension?
All obstetricians know that a patient with a history of a HDP is at an increased risk for developing chronic hypertension treated with a medication, but the magnitude of the risk is less well known. In a nationwide study in Denmark, the prevalence of chronic hypertension treated with medication 10 years after delivery among patients with a history of a HDP in their first pregnancy, was 14%, 21%, and 32%, if the first pregnancy occurred in the patient’s 20s, 30s, or 40s, respectively.26 The corresponding prevalence of chronic hypertension in patients without a history of a HDP was 4%, 6%, and 11%, if the first pregnancy occurred in the 20s, 30s, or 40s, respectively.26 Maternal age is an important predictor of who will develop chronic hypertension within 10 years following a pregnancy with a HDP.
In modern obstetric practice, the hypertensive disorders of pregnancy are prevalent and associated with increased maternal and newborn morbidity. Appropriate treatment of hypertension with labetalol, nifedipine, or methyldopa improves maternal and newborn health. Available evidence suggests that maintaining BP < 140/90 mm Hg during pregnancy for most patients is a practical goal with significant benefit. A significant public-health concern is that an increase in the prevalence of HDPs will eventually translate into an increase in chronic hypertension and the attendant complications of heart attack, heart failure, stroke, and renal insufficiency. Recognizing the increased prevalence of HDPs, ObGyns will need to alert patients to their long-term health risks and coordinate appropriate follow-up and treatment to optimize the future health of their patients. ●

Hypertension in pregnancy is a major challenge in current obstetric practice. Based on an analysis of the National Inpatient Sample, the Centers for Disease Control and Prevention (CDC) recently reported that from 2017 to 2019 the prevalence of hypertensive disorders in pregnancy increased from 13.3% to 15.9% of hospital deliveries.1 During that same time period, the prevalence of pregnancy-associated hypertension, which includes preeclampsia, eclampsia, and gestational hypertension, increased from 10.8% to 13.0%.1 The prevalence of chronic hypertension increased from 2.0% to 2.3%.1 In 2017 and 2019, unspecified maternal hypertension was diagnosed in 0.5% and 0.6% of the sample, respectively.1
Bruno and colleagues reported a 3-fold increase in the prevalence of HDPs from 1989 to 2020, with an acceleration in the rate of increase from 2010 to 2020.2 The increase in prevalence of HDPs may be caused by an increase in the prevalence of advanced maternal age, obesity, and diabetes. Black patients are disproportionately impacted by both pregnancy-associated hypertension and chronic hypertension.1 In 2019, the prevalence of pregnancy-associated hypertension was greater among Black patients (15.6%), than White (12.1%), Hispanic (10.6%), or Asian or Pacific Islander patients (7.7%).1 Similarly, the prevalence of chronic hypertension was greater among Black patients (4.3%) than among White (2.0%), Hispanic (1.5%), or Asian or Pacific Islander patients (1.2%).1 Racial/ethnic differences in HDPs may be influenced by poverty; structural racism; or lack of access to care, diet, and obesity.3,4
HDPs are major contributors to maternal morbidity and mortality. The CDC reported that among maternal deaths occurring during the delivery hospitalization, 32% of the decedents had documented hypertension.1 HDPs are associated with an approximately 2.5-fold increased risk of a severe morbidity, a composite measure that includes blood transfusion, acute kidney injury, disseminated intravascular coagulation, sepsis, shock, and pulmonary edema.5 A history of HDPs is associated with an approximately 67% increase in the lifetime risk of cardiovascular disease, including coronary artery disease, stroke, peripheral vascular disease, and heart failure.6,7
What are the best antihypertensive medications for pregnancy?
All clinicians know that the use of angiotensin-converting-enzyme inhibitors (ACE-Is) and angiotensin-receptor-blockers (ARBs) are contraindicated in pregnancy because they cause major congenital anomalies, with an odds ratio of 1.8 (95% confidence interval [CI], 1.42-2.34), compared with no exposure.8 In addition, ACE-Is and ARBs increase the risk of stillbirth, with an odds ratio of 1.75 (95% CI, 1.21-2.53).8 No increase in congenital anomalies were detected for patients exposed to other antihypertensive medications.8 Prior to attempting conception, patients with chronic hypertension should discontinue ACE-Is and ARBs and initiate an alternative medication.
The most commonly used antihypertensive medications in pregnancy are labetalol, nifedipine, and methyldopa.9 Labetalol blocks the beta-1, beta-2, and alpha-1 adrenergic receptors.10 Nifedipine blocks calcium entry into cells through the L-type calcium channel.11 Methyldopa is a central nervous system alpha-2 adrenergic agonist.12 The dose range for these commonly used medications are labetalol 400 mg to 2,400 mg daily in divided doses every 8 to 12 hours, nifedipine extended-release 30 mg to 120 mg daily, and methyldopa 500 mg to 2 g daily in 2 to 4 divided doses. Some clinicians recommend prescribing divided doses of nifedipine extended release at doses ≥ 60 mg for patients who have bothersome adverse effects, hypotension following a single daily dose, or hypertension between single daily doses. The nifedipine extended release tablets should not be divided. If monotherapy with the maximal daily dose of labetalol does not achieve the blood pressure (BP) target, adding nifedipine as a second agent is an option.9 Similarly, if monotherapy with the maximal daily dose of nifedipine extended release does not achieve the BP target, adding labetalol as a second agent is an option.9
In a network meta-analysis of antihypertensive medications used in pregnancy, that included 61 trials and 6,923 participants, all the medications studied reduced the risk of developing severe hypertension by 30% to 70%.13 Sufficient data was available to also report that labetalol used to treat hypertension in pregnancy reduced the risk of developing proteinuria.13 Given similar efficacy among antihypertensive medications, patient comorbidities may influence the medication choice. For example, labetalol may not be the optimal medication for a patient with poorly controlled asthma due to its ability to cause bronchospasm.14,15 Methyldopa may not be the optimal medication for a patient with depression.16 Based on the available data, labetalol, nifedipine, and methyldopa are the best antihypertensive medications for pregnant patients.
Continue to: What is an optimal BP target when treating chronic hypertension in pregnancy?...
What is an optimal BP target when treating chronic hypertension in pregnancy?
When treating chronic hypertension in pregnant patients, a concern is that reducing maternal BP may decrease uteroplacental perfusion and result in fetal growth restriction. However, a recent trial reported that a BP treatment target < 140/90 mm Hg is associated with better outcomes for both mother and newborn than withholding antihypertension medications. In the trial, 2,408 women with chronic hypertension diagnosed before 20 weeks of gestation were randomly assigned to an active treatment group with prescription of antihypertension medicines to achieve a BP target of < 140/90 mm Hg; or to a control group where no antihypertension or no additional antihypertension treatment was prescribed unless BP was ≥ 160 mm Hg systolic or ≥ 105 mm Hg diastolic.9 The hypertension medications prescribed to the patients in the active treatment group were labetalol (63.2%), nifedipine (33.4%), amlodipine (1.7%), methyldopa (0.5%), hydrochlorothiazide (0.3%), metoprolol (0.2%), and missing/unknown/other (0.7%).9
If a patient in the control group developed severe hypertension, they were started on an antihypertension medicine and the BP treatment target was < 140/90 mm Hg. Compared with the control regimen, active treatment resulted in a significant decrease in the development of preeclampsia (24.4% vs 31.1%; risk ratio [RR], 0.79; 95% CI, 0.69-0.89), severe hypertension (36.1% vs 44.3%; RR, 0.82; 95% CI, 0.74-0.90), preterm birth < 37 weeks’ gestation (27.5% vs 31.4%; RR, 0.87; 95% CI, 0.77-0.99), preterm birth < 35 weeks’ gestation (12.2% vs 16.7%; odds ratio [OR], 0.69; 95% CI, 0.55-0.88), and low birth-weight (< 2,500 g) newborns (19.2% vs 23.1%; RR, 0.83; 95% CI, 0.71-0.97).9 The percentage of small for gestational age birth weight below the 10th percentile was similar in the treatment and control groups, 11.2% and 10.4%, respectively (adjusted RR, 1.04; 95% CI, 0.82-1.31).9 The number of patients who would need to be treated to prevent one primary-outcome event was 15.9 The investigators concluded that for pregnant patients with chronic hypertension, the optimal BP target is < 140/90 mm Hg.9
In pregnant patients with hypertension, BP may decrease immediately after birth. Following birth, BP tends to increase, reaching a peak 3 to 6 days postpartum.17,18 This pattern was observed in patients with and without preeclampsia in the index pregnancy. Among 136 patients without antepartum preeclampsia, the prevalence of a diastolic BP > 89 mm Hg was 5% and 15% on postpartum days 1 and 3, respectively.17 The postpartum rise in BP may be due to mobilization of water from the extravascular to the intravascular space and excretion of total body sodium that accumulated during pregnancy.19 In one study of 998 consecutive singleton cesarean births, 7.7% of the patients with no recorded elevated BP before delivery developed de novo hypertension postpartum.20 Compared with patients without antepartum or new onset postpartum hypertension, the patients who developed postpartum hypertension had a higher body mass index, were more likely to be Black and to have a history of type 2 diabetes. Compared with patients without antepartum or postpartum hypertension, the patients who developed de novo postpartum hypertension, had significantly elevated soluble fms-like tyrosine kinase-1 and significantly decreased placental growth factor, a pattern seen with preeclampsia.20 These results suggest that de novo postpartum hypertension may have molecular causes similar to preeclampsia.20
Postpartum hypertension should be treated with a medication that is thought to be safe for breastfeeding patients, including labetalol, nifedipine, or enalapril.21-23 The relative infant dose of labetalol, nifedipine, and enalapril is approximately 3.6%, ≤ 3.2%, and 1.1%, respectively.24 If the relative infant dose of a medication is < 10% it is generally considered to be compatible with breastfeeding.25
Many obstetricians have seldom prescribed enalapril, an ACE-I. The initial dose of enalapril is 5 mg or 10 mg daily. After initiation of treatment, the dose can be adjusted based on BP measurement. The maximal daily dose is 40 mg daily in one dose or two divided doses. Similar to other hypertension medicines, enalapril therapy may cause hypotension and dizziness. Enalapril should not be used by pregnant patients because it is associated with an increased risk of congenital anomalies and fetal demise.
Does a HDP increase the risk of developing chronic hypertension?
All obstetricians know that a patient with a history of a HDP is at an increased risk for developing chronic hypertension treated with a medication, but the magnitude of the risk is less well known. In a nationwide study in Denmark, the prevalence of chronic hypertension treated with medication 10 years after delivery among patients with a history of a HDP in their first pregnancy, was 14%, 21%, and 32%, if the first pregnancy occurred in the patient’s 20s, 30s, or 40s, respectively.26 The corresponding prevalence of chronic hypertension in patients without a history of a HDP was 4%, 6%, and 11%, if the first pregnancy occurred in the 20s, 30s, or 40s, respectively.26 Maternal age is an important predictor of who will develop chronic hypertension within 10 years following a pregnancy with a HDP.
In modern obstetric practice, the hypertensive disorders of pregnancy are prevalent and associated with increased maternal and newborn morbidity. Appropriate treatment of hypertension with labetalol, nifedipine, or methyldopa improves maternal and newborn health. Available evidence suggests that maintaining BP < 140/90 mm Hg during pregnancy for most patients is a practical goal with significant benefit. A significant public-health concern is that an increase in the prevalence of HDPs will eventually translate into an increase in chronic hypertension and the attendant complications of heart attack, heart failure, stroke, and renal insufficiency. Recognizing the increased prevalence of HDPs, ObGyns will need to alert patients to their long-term health risks and coordinate appropriate follow-up and treatment to optimize the future health of their patients. ●
- Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization-United States, 2017-2019. Morb Mortal Week Report. 2022;71:585-591.
- Bruno AM, Allshouse AA, Metz TD, et al. Trends in hypertensive disorders of pregnancy in the United States from 1989 to 2020. Obstet Gynecol. 2022;140:83-86.
- Doleszar CM, McGrath JJ, Herzig AJM, et al. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol. 2014;33:20-34.
- Centers for Disease Control and Prevention. A Closer Look at African American Men and High Blood Pressure Control; A Review of Psychosocial Factors and Systems-Level Interventions. Atlanta: U.S. Department of Health and Human Services; 2010.
- Boulet SL, Platner M, Joseph NT, et al. Hypertensive disorders of pregnancy, cesarean delivery and severe maternal morbidity in an urban safety-net population. Am J Epidemiol. 2020;189:1502-1511.
- Parikh NI, Gonzalez JM, Andreson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143:e902-e916.
- Okoth K, Chandan JS, Marshall T, et al. Association between the reproductive health of young women and cardiovascular disease later in life: umbrella review. BMJ. 2020;371:m3502.
- Fu J, Tomlinson G, Feig DS. Increased risk of major congenital malformations in early pregnancy uses of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers: a meta-analysis. Diabetes Metab Res Rev. 2021;37:e3453.
- Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781-1792.
- Baum T, Sybertz EJ. Pharmacology of labetalol in experimental animals. Am J Med. 1983;75:15-23.
- Khan KM, Patel JB, Schaefer TJ. StatPearls (Internet). StatPearls Publishing; 2022.
- Gupta M, Khalili. Methyldopa StatPearls (Internet). StatPearls Publishing; 2022.
- Bone JN, Sandhu A, Diablos ED, et al. Oral antihypertensives for non-severe pregnancy hypertension: systematic review, network meta-analysis and trial sequential analysis. Hypertension. 2022;79:614-628.
- Morales DR, Jackson C, Lipworth BJ, et al. Adverse respiratory effects of acute beta-blocker exposure in asthma: a systematic review and meta-analysis of randomized controlled trials. Chest. 2014;145:779-786.
- Huang KY, Tseng PT, Wu YC, et al. Do beta-adrenergic blocking agents increase asthma exacerbation? A network meta-analysis of randomized controlled trials. Sci Rep. 2021;11:452.
- Nayak AS, Nachane HB. Risk analysis of suicidal ideation and postpartum depression with antenatal alpha methyldopa use. Asian J Psychiatry. 2018;38:42-44.
- Walters BNJ, Thompson ME, Lee A, et al. Blood pressure in the puerperium. Clin Sci. 1986;71:589-594.
- Walters BNJ, Walters T. Hypertension in the puerperium. Lancet. 1987;2(8554):330.
- Magee L, von Dadelszen. Prevention and treatment of postpartum hypertension. Cochrane Database Syst Rev. 2013;CD004351.
- Goel A, Maski MR, Bajracharya S, et al. Epidemiology and mechanisms of de novo and persistent hypertension in the postpartum period. Circulation. 2015;132:1726-1733.
- Powles K, Gandhi S. Postpartum hypertension. CMAJ. 2017;189:E913.
- Tosounidou S, Gordon C. Medications in pregnancy and breastfeeding. Best Prac Res Clin Obstet Gynaecol. 2020;64:68-76.
- Anderson PO. Treating hypertension during breastfeeding. Breastfeed Med. 2018;13:95-96.
- Lexicomp web site. https://www.wolterskluwer.com/en/solutions/lexicomp.
- Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343:118-126.
- Behrens I, Basit S, Melbye M, et al. Risk of postpartum hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:j3078.
- Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization-United States, 2017-2019. Morb Mortal Week Report. 2022;71:585-591.
- Bruno AM, Allshouse AA, Metz TD, et al. Trends in hypertensive disorders of pregnancy in the United States from 1989 to 2020. Obstet Gynecol. 2022;140:83-86.
- Doleszar CM, McGrath JJ, Herzig AJM, et al. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol. 2014;33:20-34.
- Centers for Disease Control and Prevention. A Closer Look at African American Men and High Blood Pressure Control; A Review of Psychosocial Factors and Systems-Level Interventions. Atlanta: U.S. Department of Health and Human Services; 2010.
- Boulet SL, Platner M, Joseph NT, et al. Hypertensive disorders of pregnancy, cesarean delivery and severe maternal morbidity in an urban safety-net population. Am J Epidemiol. 2020;189:1502-1511.
- Parikh NI, Gonzalez JM, Andreson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143:e902-e916.
- Okoth K, Chandan JS, Marshall T, et al. Association between the reproductive health of young women and cardiovascular disease later in life: umbrella review. BMJ. 2020;371:m3502.
- Fu J, Tomlinson G, Feig DS. Increased risk of major congenital malformations in early pregnancy uses of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers: a meta-analysis. Diabetes Metab Res Rev. 2021;37:e3453.
- Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781-1792.
- Baum T, Sybertz EJ. Pharmacology of labetalol in experimental animals. Am J Med. 1983;75:15-23.
- Khan KM, Patel JB, Schaefer TJ. StatPearls (Internet). StatPearls Publishing; 2022.
- Gupta M, Khalili. Methyldopa StatPearls (Internet). StatPearls Publishing; 2022.
- Bone JN, Sandhu A, Diablos ED, et al. Oral antihypertensives for non-severe pregnancy hypertension: systematic review, network meta-analysis and trial sequential analysis. Hypertension. 2022;79:614-628.
- Morales DR, Jackson C, Lipworth BJ, et al. Adverse respiratory effects of acute beta-blocker exposure in asthma: a systematic review and meta-analysis of randomized controlled trials. Chest. 2014;145:779-786.
- Huang KY, Tseng PT, Wu YC, et al. Do beta-adrenergic blocking agents increase asthma exacerbation? A network meta-analysis of randomized controlled trials. Sci Rep. 2021;11:452.
- Nayak AS, Nachane HB. Risk analysis of suicidal ideation and postpartum depression with antenatal alpha methyldopa use. Asian J Psychiatry. 2018;38:42-44.
- Walters BNJ, Thompson ME, Lee A, et al. Blood pressure in the puerperium. Clin Sci. 1986;71:589-594.
- Walters BNJ, Walters T. Hypertension in the puerperium. Lancet. 1987;2(8554):330.
- Magee L, von Dadelszen. Prevention and treatment of postpartum hypertension. Cochrane Database Syst Rev. 2013;CD004351.
- Goel A, Maski MR, Bajracharya S, et al. Epidemiology and mechanisms of de novo and persistent hypertension in the postpartum period. Circulation. 2015;132:1726-1733.
- Powles K, Gandhi S. Postpartum hypertension. CMAJ. 2017;189:E913.
- Tosounidou S, Gordon C. Medications in pregnancy and breastfeeding. Best Prac Res Clin Obstet Gynaecol. 2020;64:68-76.
- Anderson PO. Treating hypertension during breastfeeding. Breastfeed Med. 2018;13:95-96.
- Lexicomp web site. https://www.wolterskluwer.com/en/solutions/lexicomp.
- Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343:118-126.
- Behrens I, Basit S, Melbye M, et al. Risk of postpartum hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:j3078.
Surgical techniques for excision of juvenile cystic adenomyoma
Experts express caution over type 2 diabetes/tea-drinking claim
type 2 diabetes/tea-drinking claim
A claim that drinking tea might protect people against developing type 2 diabetes has been met with caution from multiple experts ahead of the annual meeting of the European Association for the Study of Diabetes.
The claim is that people who drink four or more cups of tea every day – specifically green, Oolong, or black tea – are 17% less likely to develop type 2 diabetes than those who do not drink tea. Drinking fewer cups of tea per day was not found to confer any benefit.
“Our results are exciting because they suggest that people can do something as simple as drinking four cups of tea a day to potentially lessen their risk of developing type 2 diabetes,” Xiaying Li of Wuhan (China) University of Science and Technology is quoted as saying in an official EASD press release.
“It is possible that particular components in tea, such as polyphenols, may reduce blood glucose levels, but a sufficient amount of these bioactive compounds may be needed to be effective,” Dr. Li added.
“The words ‘suggest’ and ‘potentially’ are crucial here,” said Kevin McConway, PhD, MSc, MBA, emeritus professor of applied statistics at The Open University, said in a separate statement to the press that reeled in Dr. Li’s enthusiasm.
“Tea drinking would only be useful for reducing diabetes risk if the tea drinking causes reductions in risk, that is, if the risk is reduced if you drink the tea and not if you don’t – and this study simply can’t show whether it does this or not,” Dr. Conway stressed.
Naveed Sattar, FMedSci FRCPath FRCPGlas FRSE, professor of metabolic medicine at the University of Glasgow, was also cautiously critical. “There is no good trial evidence whatsoever that the chemicals in tea prevent diabetes,” he observed separately.
“So, I suspect its more about tea being healthier (less calorific) than many alternative drinks or tea drinkers leading healthier lives more generally.”
Dr. Sattar added that it could be that people who drink tea might also be avoiding drinking more harmful sugary drinks and have other health behaviors that might lead them to have a lower risk for type 2 diabetes.
Time for tea?
Dr. Li will present the findings of two analyses on Sept. 21 at the EASD meeting: the first a large observational cohort study and the second an updated systematic review and meta-analysis.
For the cohort study, Dr. Li and her coauthors took data on more than 5,100 adults who had participated in the long-running and ongoing China Health and Nutrition Survey (CHNS). Information on tea drinking behavior was extracted from questionnaires that had been filled out at two time points – 1997 and 2009 – and they determined whether people had developed type 2 diabetes according to American Diabetes Association criteria.
Nearly half, 45.8%, were found to be tea drinkers, and 10% of the population they sampled had developed type 2 diabetes. No association between tea drinking and type 2 diabetes development was found, however, with the hazard ratio comparing tea drinkers and non–tea drinkers sitting firmly at 1.02. Moreover, a sensitivity analysis that excluded participants who had developed type 2 diabetes in the first 3 years of follow-up did not change the result.
Things were slightly different when Dr. Li and associates performed their meta-analysis that involved analyzing data on more than 1 million participants in 19 studies conducted in eight countries that had been published up to September 2021.
Here, they found there was a significant (P < .003) linear association between tea consumption and having type 2 diabetes, with the relative risk of developing type 2 diabetes decreasing by 0.986 for every additional cup of tea that was drunk.
HRs for the development of type 2 diabetes in tea drinkers versus non–tea drinkers were 1.00 for those who drank less than one cup per day, 0.96 for those who had one to two cups, and 0.84 for those who drank four or more cups.
“While more research needs to be done to determine the exact dosage and mechanisms behind these observations, our findings suggest that drinking tea is beneficial in reducing the risk of type 2 diabetes, but only at high doses (at least 4 cups a day)”, said Dr. Li.
Perhaps, “we did not find an association between tea drinking and type 2 diabetes in our cohort study because we did not look at higher tea consumption,” she added.
Tempest in a teacup
“This is large, observational data. It’s not a randomized controlled trial so there’s plenty of room for data to be misunderstood,” warned Matt Sydes, MSc, professor of clinical trials & methodology at the MRC Clinical Trials Unit, University College London.
“Everyone drinks fluids. If there is an effect here (and that’s a big if), it might be not about the tea they drink, but about what they don’t drink. One can’t tell at the moment. It seems unlikely that a large randomized controlled trial could be done to disambiguate” added Dr. Sydes
“Being only a conference abstract, it is difficult to assess the quality of this research,” Baptiste Leurent, PhD, a medical statistician also working at University College London, said. Not only was the cohort study observational, so were all the other studies included in the meta-analysis, he pointed out.
“Therefore, no cause-effect conclusions can be drawn. The association could simply be due to other factors, such as those drinking more tea having a healthier lifestyle. It does not seem that the authors tried to control for confounders, which is usually difficult in meta-analysis,” Dr. Leurent said.
“There is reason to be a bit skeptical at this point; we really need to have the full details to assess it properly,” said Jonathan Cook of the Centre for Statistics in Medicine at the University of Oxford (England). “It’s a fair attempt to look at this, but not cutting edge, [using] fairly standard approaches.”
Similar studies have shown a reduced risk associated with coffee drinking, noted Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University in Birmingham.
“The important take-home message is that lifestyle is important in managing risk of developing type 2 diabetes,” Dr. Mellor said.
“That includes choosing low-calorie drinks including mainly water as well as unsweetened tea and coffee as your drinks of choice as part of a healthy lifestyle.”
The study was funded by the Young Talents Project of Hubei Provincial Health Commission, the Science and Technology Research Key Project of Education Department of Hubei Province, the Sanuo Diabetes Charity Foundation, and the Xiangyang Science and Technology Plan Project, all based in China. Dr. Li had no conflicts of interest to disclose. Dr. McConway is a Trustee and on the advisory committee of The Science Media Centre. Dr. Sattar has consulted for many companies that make diabetes and cardiovascular drugs and has been involved in multiple trials of lifestyle approaches for the prevention and remission of diabetes. Dr. Sydes, Dr. Leurent, Dr. Cook, and Dr. Mellor had no conflicts of interest to report.
A claim that drinking tea might protect people against developing type 2 diabetes has been met with caution from multiple experts ahead of the annual meeting of the European Association for the Study of Diabetes.
The claim is that people who drink four or more cups of tea every day – specifically green, Oolong, or black tea – are 17% less likely to develop type 2 diabetes than those who do not drink tea. Drinking fewer cups of tea per day was not found to confer any benefit.
“Our results are exciting because they suggest that people can do something as simple as drinking four cups of tea a day to potentially lessen their risk of developing type 2 diabetes,” Xiaying Li of Wuhan (China) University of Science and Technology is quoted as saying in an official EASD press release.
“It is possible that particular components in tea, such as polyphenols, may reduce blood glucose levels, but a sufficient amount of these bioactive compounds may be needed to be effective,” Dr. Li added.
“The words ‘suggest’ and ‘potentially’ are crucial here,” said Kevin McConway, PhD, MSc, MBA, emeritus professor of applied statistics at The Open University, said in a separate statement to the press that reeled in Dr. Li’s enthusiasm.
“Tea drinking would only be useful for reducing diabetes risk if the tea drinking causes reductions in risk, that is, if the risk is reduced if you drink the tea and not if you don’t – and this study simply can’t show whether it does this or not,” Dr. Conway stressed.
Naveed Sattar, FMedSci FRCPath FRCPGlas FRSE, professor of metabolic medicine at the University of Glasgow, was also cautiously critical. “There is no good trial evidence whatsoever that the chemicals in tea prevent diabetes,” he observed separately.
“So, I suspect its more about tea being healthier (less calorific) than many alternative drinks or tea drinkers leading healthier lives more generally.”
Dr. Sattar added that it could be that people who drink tea might also be avoiding drinking more harmful sugary drinks and have other health behaviors that might lead them to have a lower risk for type 2 diabetes.
Time for tea?
Dr. Li will present the findings of two analyses on Sept. 21 at the EASD meeting: the first a large observational cohort study and the second an updated systematic review and meta-analysis.
For the cohort study, Dr. Li and her coauthors took data on more than 5,100 adults who had participated in the long-running and ongoing China Health and Nutrition Survey (CHNS). Information on tea drinking behavior was extracted from questionnaires that had been filled out at two time points – 1997 and 2009 – and they determined whether people had developed type 2 diabetes according to American Diabetes Association criteria.
Nearly half, 45.8%, were found to be tea drinkers, and 10% of the population they sampled had developed type 2 diabetes. No association between tea drinking and type 2 diabetes development was found, however, with the hazard ratio comparing tea drinkers and non–tea drinkers sitting firmly at 1.02. Moreover, a sensitivity analysis that excluded participants who had developed type 2 diabetes in the first 3 years of follow-up did not change the result.
Things were slightly different when Dr. Li and associates performed their meta-analysis that involved analyzing data on more than 1 million participants in 19 studies conducted in eight countries that had been published up to September 2021.
Here, they found there was a significant (P < .003) linear association between tea consumption and having type 2 diabetes, with the relative risk of developing type 2 diabetes decreasing by 0.986 for every additional cup of tea that was drunk.
HRs for the development of type 2 diabetes in tea drinkers versus non–tea drinkers were 1.00 for those who drank less than one cup per day, 0.96 for those who had one to two cups, and 0.84 for those who drank four or more cups.
“While more research needs to be done to determine the exact dosage and mechanisms behind these observations, our findings suggest that drinking tea is beneficial in reducing the risk of type 2 diabetes, but only at high doses (at least 4 cups a day)”, said Dr. Li.
Perhaps, “we did not find an association between tea drinking and type 2 diabetes in our cohort study because we did not look at higher tea consumption,” she added.
Tempest in a teacup
“This is large, observational data. It’s not a randomized controlled trial so there’s plenty of room for data to be misunderstood,” warned Matt Sydes, MSc, professor of clinical trials & methodology at the MRC Clinical Trials Unit, University College London.
“Everyone drinks fluids. If there is an effect here (and that’s a big if), it might be not about the tea they drink, but about what they don’t drink. One can’t tell at the moment. It seems unlikely that a large randomized controlled trial could be done to disambiguate” added Dr. Sydes
“Being only a conference abstract, it is difficult to assess the quality of this research,” Baptiste Leurent, PhD, a medical statistician also working at University College London, said. Not only was the cohort study observational, so were all the other studies included in the meta-analysis, he pointed out.
“Therefore, no cause-effect conclusions can be drawn. The association could simply be due to other factors, such as those drinking more tea having a healthier lifestyle. It does not seem that the authors tried to control for confounders, which is usually difficult in meta-analysis,” Dr. Leurent said.
“There is reason to be a bit skeptical at this point; we really need to have the full details to assess it properly,” said Jonathan Cook of the Centre for Statistics in Medicine at the University of Oxford (England). “It’s a fair attempt to look at this, but not cutting edge, [using] fairly standard approaches.”
Similar studies have shown a reduced risk associated with coffee drinking, noted Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University in Birmingham.
“The important take-home message is that lifestyle is important in managing risk of developing type 2 diabetes,” Dr. Mellor said.
“That includes choosing low-calorie drinks including mainly water as well as unsweetened tea and coffee as your drinks of choice as part of a healthy lifestyle.”
The study was funded by the Young Talents Project of Hubei Provincial Health Commission, the Science and Technology Research Key Project of Education Department of Hubei Province, the Sanuo Diabetes Charity Foundation, and the Xiangyang Science and Technology Plan Project, all based in China. Dr. Li had no conflicts of interest to disclose. Dr. McConway is a Trustee and on the advisory committee of The Science Media Centre. Dr. Sattar has consulted for many companies that make diabetes and cardiovascular drugs and has been involved in multiple trials of lifestyle approaches for the prevention and remission of diabetes. Dr. Sydes, Dr. Leurent, Dr. Cook, and Dr. Mellor had no conflicts of interest to report.
A claim that drinking tea might protect people against developing type 2 diabetes has been met with caution from multiple experts ahead of the annual meeting of the European Association for the Study of Diabetes.
The claim is that people who drink four or more cups of tea every day – specifically green, Oolong, or black tea – are 17% less likely to develop type 2 diabetes than those who do not drink tea. Drinking fewer cups of tea per day was not found to confer any benefit.
“Our results are exciting because they suggest that people can do something as simple as drinking four cups of tea a day to potentially lessen their risk of developing type 2 diabetes,” Xiaying Li of Wuhan (China) University of Science and Technology is quoted as saying in an official EASD press release.
“It is possible that particular components in tea, such as polyphenols, may reduce blood glucose levels, but a sufficient amount of these bioactive compounds may be needed to be effective,” Dr. Li added.
“The words ‘suggest’ and ‘potentially’ are crucial here,” said Kevin McConway, PhD, MSc, MBA, emeritus professor of applied statistics at The Open University, said in a separate statement to the press that reeled in Dr. Li’s enthusiasm.
“Tea drinking would only be useful for reducing diabetes risk if the tea drinking causes reductions in risk, that is, if the risk is reduced if you drink the tea and not if you don’t – and this study simply can’t show whether it does this or not,” Dr. Conway stressed.
Naveed Sattar, FMedSci FRCPath FRCPGlas FRSE, professor of metabolic medicine at the University of Glasgow, was also cautiously critical. “There is no good trial evidence whatsoever that the chemicals in tea prevent diabetes,” he observed separately.
“So, I suspect its more about tea being healthier (less calorific) than many alternative drinks or tea drinkers leading healthier lives more generally.”
Dr. Sattar added that it could be that people who drink tea might also be avoiding drinking more harmful sugary drinks and have other health behaviors that might lead them to have a lower risk for type 2 diabetes.
Time for tea?
Dr. Li will present the findings of two analyses on Sept. 21 at the EASD meeting: the first a large observational cohort study and the second an updated systematic review and meta-analysis.
For the cohort study, Dr. Li and her coauthors took data on more than 5,100 adults who had participated in the long-running and ongoing China Health and Nutrition Survey (CHNS). Information on tea drinking behavior was extracted from questionnaires that had been filled out at two time points – 1997 and 2009 – and they determined whether people had developed type 2 diabetes according to American Diabetes Association criteria.
Nearly half, 45.8%, were found to be tea drinkers, and 10% of the population they sampled had developed type 2 diabetes. No association between tea drinking and type 2 diabetes development was found, however, with the hazard ratio comparing tea drinkers and non–tea drinkers sitting firmly at 1.02. Moreover, a sensitivity analysis that excluded participants who had developed type 2 diabetes in the first 3 years of follow-up did not change the result.
Things were slightly different when Dr. Li and associates performed their meta-analysis that involved analyzing data on more than 1 million participants in 19 studies conducted in eight countries that had been published up to September 2021.
Here, they found there was a significant (P < .003) linear association between tea consumption and having type 2 diabetes, with the relative risk of developing type 2 diabetes decreasing by 0.986 for every additional cup of tea that was drunk.
HRs for the development of type 2 diabetes in tea drinkers versus non–tea drinkers were 1.00 for those who drank less than one cup per day, 0.96 for those who had one to two cups, and 0.84 for those who drank four or more cups.
“While more research needs to be done to determine the exact dosage and mechanisms behind these observations, our findings suggest that drinking tea is beneficial in reducing the risk of type 2 diabetes, but only at high doses (at least 4 cups a day)”, said Dr. Li.
Perhaps, “we did not find an association between tea drinking and type 2 diabetes in our cohort study because we did not look at higher tea consumption,” she added.
Tempest in a teacup
“This is large, observational data. It’s not a randomized controlled trial so there’s plenty of room for data to be misunderstood,” warned Matt Sydes, MSc, professor of clinical trials & methodology at the MRC Clinical Trials Unit, University College London.
“Everyone drinks fluids. If there is an effect here (and that’s a big if), it might be not about the tea they drink, but about what they don’t drink. One can’t tell at the moment. It seems unlikely that a large randomized controlled trial could be done to disambiguate” added Dr. Sydes
“Being only a conference abstract, it is difficult to assess the quality of this research,” Baptiste Leurent, PhD, a medical statistician also working at University College London, said. Not only was the cohort study observational, so were all the other studies included in the meta-analysis, he pointed out.
“Therefore, no cause-effect conclusions can be drawn. The association could simply be due to other factors, such as those drinking more tea having a healthier lifestyle. It does not seem that the authors tried to control for confounders, which is usually difficult in meta-analysis,” Dr. Leurent said.
“There is reason to be a bit skeptical at this point; we really need to have the full details to assess it properly,” said Jonathan Cook of the Centre for Statistics in Medicine at the University of Oxford (England). “It’s a fair attempt to look at this, but not cutting edge, [using] fairly standard approaches.”
Similar studies have shown a reduced risk associated with coffee drinking, noted Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University in Birmingham.
“The important take-home message is that lifestyle is important in managing risk of developing type 2 diabetes,” Dr. Mellor said.
“That includes choosing low-calorie drinks including mainly water as well as unsweetened tea and coffee as your drinks of choice as part of a healthy lifestyle.”
The study was funded by the Young Talents Project of Hubei Provincial Health Commission, the Science and Technology Research Key Project of Education Department of Hubei Province, the Sanuo Diabetes Charity Foundation, and the Xiangyang Science and Technology Plan Project, all based in China. Dr. Li had no conflicts of interest to disclose. Dr. McConway is a Trustee and on the advisory committee of The Science Media Centre. Dr. Sattar has consulted for many companies that make diabetes and cardiovascular drugs and has been involved in multiple trials of lifestyle approaches for the prevention and remission of diabetes. Dr. Sydes, Dr. Leurent, Dr. Cook, and Dr. Mellor had no conflicts of interest to report.
type 2 diabetes/tea-drinking claim
type 2 diabetes/tea-drinking claim
FROM EASD 2022