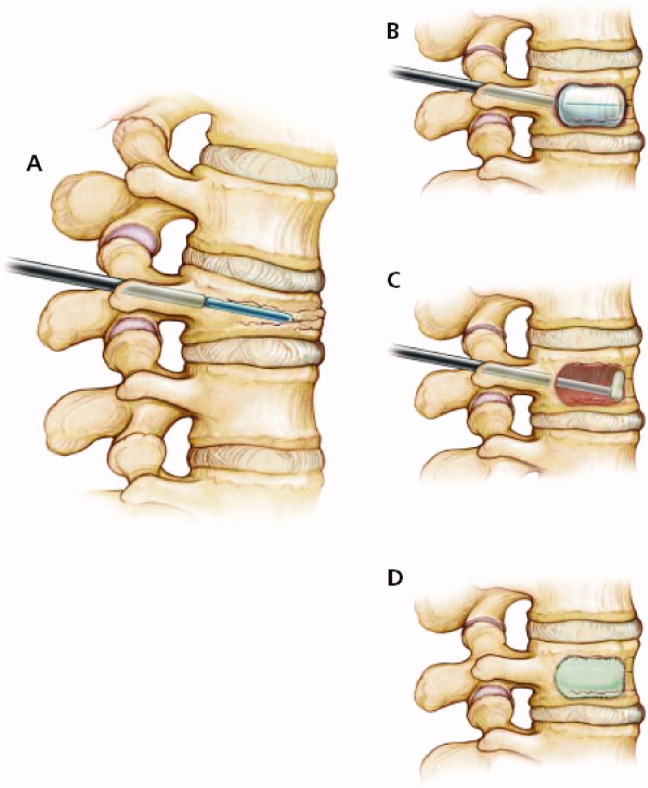
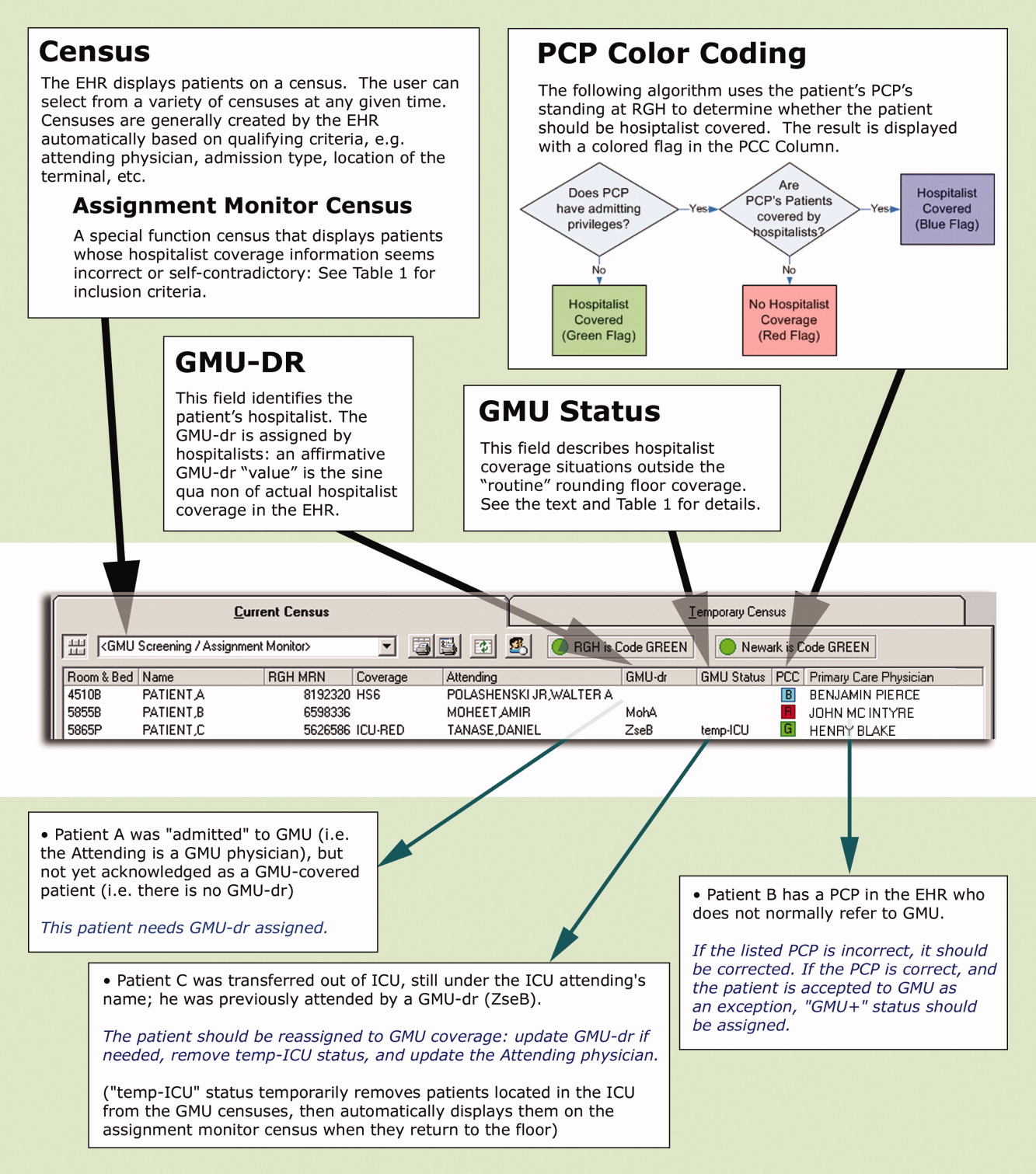
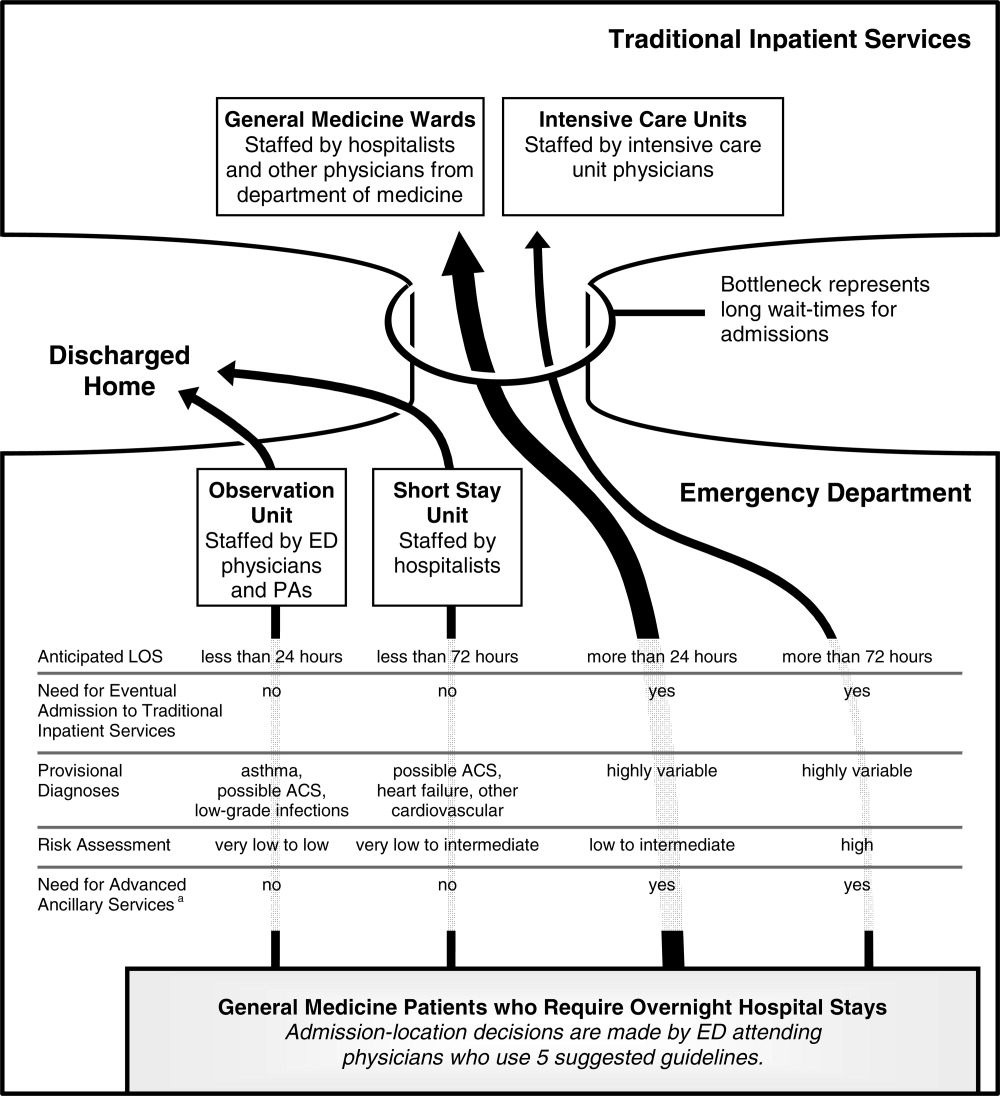
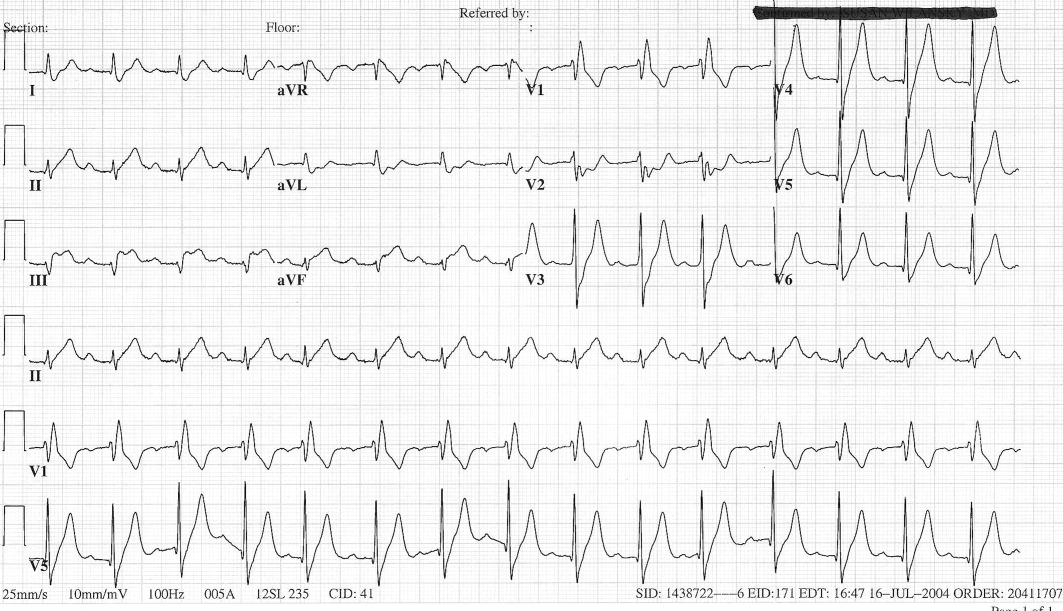
User login
New Tools of the Trade
Andrew Fishmann, MD, FCCP, FACP, listened for more than an hour as an award-winning hospitalist talked about strategies to improve communication between physicians and administrators. Brian Bossard, MD, FACP, FHM, gleaned lessons from case studies of how two HM groups (HMGs) navigated the adolescent years. And dozens more hospitalists sat still for the bulk of an hour as they were given a nuts-and-bolts tutorial of HM finances.
Welcome to SHM’s new practice management track, a three-day series of training sessions that debuted at HM09 and focuses on the inner workings of HMGs—from startup to coding and billing to expansion. “There’s always a quality track, there’s always a clinical track, and there’s always a leadership track,” says Kimberly Dickinson, vice president of operations for Cogent Healthcare in Brentwood, Tenn., and an SHM Career Satisfaction Task Force member. “This was trying to bring together some of the nonclinical aspects.”
The experiment drew positive reviews, if rooms crowded with physicians—some lined up along the walls—are any indication. Dr. Fishmann, a Cogent co-founder who is practicing as a hospitalist with California Lung Associates in Los Angeles, says the courses give younger hospitalists different perspectives. “It’s taking doctors a little out of the hospital and putting them in the boardroom,” he says. “You don’t have a choice. … You need to be involved and have more input.”
The practice management courses are structured to give a broad overview of nearly every facet of opening and operating an HMG. One popular course, which ran nearly 15 minutes long because of participant questions, focused on managing HMG growth. Another session spent more than an hour taking physicians through comanagement issues that arise during collaborations with surgeons.
Real-Life Experiences
Most of the courses were led by familiar SHM leaders. But several attendees said they enjoyed sessions that were led by rank-and-file hospitalists and administrators who live the front-line struggles every day.
The “Case Studies in Managing Program Growth” course featured detailed explanations of the growing pains of HM programs in Michigan and Massachusetts. In the former case, Carole Montgomery, MD, talked about how the Michigan Medical PC group she helped start in western Michigan struggled to formalize certain procedures when it signed its first contract with a hospital in 2002. The group doubled its hospitalist roster and instantly went from an informal HM practice in which everyone knew each other and had relatively similar opinions to a business in which it was unclear who would make major decisions.
In response, Dr. Montgomery’s group crafted a mission statement, created a hospitalist executive committee to make routine operational decisions, and changed how it negotiated contracts with hospitals. Soon after, the group fired its first physician. Last year, the group instituted “internal governance guidelines” to make management decisions clearer. Dr. Montgomery says each of the developments taught her that solving major issues takes patience and a willingness to continually adapt. “I thought I was done each time,” she says. “Now I realize it’s an interactive process.”
Results-Oriented
Peter Short, MD, FAAP, CPE, medical director of Northeast Hospital Corp., shared his recent struggles to hire one hospitalist and expand night coverage. Hospitalists in Dr. Short’s service, who practice at Beverly Hospital in Beverly, Mass., resisted the change at first, not wanting to add additional night-shift responsibility. Dr. Short also spoke about financial concerns to the group and the hospital. After explaining the pros and cons of hiring a sixth rounder, the hospitalists embraced the idea. So far, hospital administrators have had zero complaints, as the first three months of data show a reduction in length of stay by 0.3 days and an average cost decrease of roughly $500 per case.
Dr. Short also explained an unexpected byproduct of the hiring: Night work proved so popular that the hospitalists have demanded that they receive a specified number of overnight shifts a year. “They went from not wanting it to fighting for it,” Dr. Short says proudly. “There is no one-size-fits-all for hospital programs.”
Dr. Bossard, who founded Inpatient Physician Associates in Lincoln, Neb., says the types of detail-oriented presentations made by Drs. Montgomery and Short are useful if physicians learn lessons they can take home and adapt to their practice. “If it’s a lot of smoke without an action item or a bullet point we can latch on to,” he says, “it’s not as helpful.”
Richard Quinn is a freelance writer based in New Jersey.
Andrew Fishmann, MD, FCCP, FACP, listened for more than an hour as an award-winning hospitalist talked about strategies to improve communication between physicians and administrators. Brian Bossard, MD, FACP, FHM, gleaned lessons from case studies of how two HM groups (HMGs) navigated the adolescent years. And dozens more hospitalists sat still for the bulk of an hour as they were given a nuts-and-bolts tutorial of HM finances.
Welcome to SHM’s new practice management track, a three-day series of training sessions that debuted at HM09 and focuses on the inner workings of HMGs—from startup to coding and billing to expansion. “There’s always a quality track, there’s always a clinical track, and there’s always a leadership track,” says Kimberly Dickinson, vice president of operations for Cogent Healthcare in Brentwood, Tenn., and an SHM Career Satisfaction Task Force member. “This was trying to bring together some of the nonclinical aspects.”
The experiment drew positive reviews, if rooms crowded with physicians—some lined up along the walls—are any indication. Dr. Fishmann, a Cogent co-founder who is practicing as a hospitalist with California Lung Associates in Los Angeles, says the courses give younger hospitalists different perspectives. “It’s taking doctors a little out of the hospital and putting them in the boardroom,” he says. “You don’t have a choice. … You need to be involved and have more input.”
The practice management courses are structured to give a broad overview of nearly every facet of opening and operating an HMG. One popular course, which ran nearly 15 minutes long because of participant questions, focused on managing HMG growth. Another session spent more than an hour taking physicians through comanagement issues that arise during collaborations with surgeons.
Real-Life Experiences
Most of the courses were led by familiar SHM leaders. But several attendees said they enjoyed sessions that were led by rank-and-file hospitalists and administrators who live the front-line struggles every day.
The “Case Studies in Managing Program Growth” course featured detailed explanations of the growing pains of HM programs in Michigan and Massachusetts. In the former case, Carole Montgomery, MD, talked about how the Michigan Medical PC group she helped start in western Michigan struggled to formalize certain procedures when it signed its first contract with a hospital in 2002. The group doubled its hospitalist roster and instantly went from an informal HM practice in which everyone knew each other and had relatively similar opinions to a business in which it was unclear who would make major decisions.
In response, Dr. Montgomery’s group crafted a mission statement, created a hospitalist executive committee to make routine operational decisions, and changed how it negotiated contracts with hospitals. Soon after, the group fired its first physician. Last year, the group instituted “internal governance guidelines” to make management decisions clearer. Dr. Montgomery says each of the developments taught her that solving major issues takes patience and a willingness to continually adapt. “I thought I was done each time,” she says. “Now I realize it’s an interactive process.”
Results-Oriented
Peter Short, MD, FAAP, CPE, medical director of Northeast Hospital Corp., shared his recent struggles to hire one hospitalist and expand night coverage. Hospitalists in Dr. Short’s service, who practice at Beverly Hospital in Beverly, Mass., resisted the change at first, not wanting to add additional night-shift responsibility. Dr. Short also spoke about financial concerns to the group and the hospital. After explaining the pros and cons of hiring a sixth rounder, the hospitalists embraced the idea. So far, hospital administrators have had zero complaints, as the first three months of data show a reduction in length of stay by 0.3 days and an average cost decrease of roughly $500 per case.
Dr. Short also explained an unexpected byproduct of the hiring: Night work proved so popular that the hospitalists have demanded that they receive a specified number of overnight shifts a year. “They went from not wanting it to fighting for it,” Dr. Short says proudly. “There is no one-size-fits-all for hospital programs.”
Dr. Bossard, who founded Inpatient Physician Associates in Lincoln, Neb., says the types of detail-oriented presentations made by Drs. Montgomery and Short are useful if physicians learn lessons they can take home and adapt to their practice. “If it’s a lot of smoke without an action item or a bullet point we can latch on to,” he says, “it’s not as helpful.”
Richard Quinn is a freelance writer based in New Jersey.
Andrew Fishmann, MD, FCCP, FACP, listened for more than an hour as an award-winning hospitalist talked about strategies to improve communication between physicians and administrators. Brian Bossard, MD, FACP, FHM, gleaned lessons from case studies of how two HM groups (HMGs) navigated the adolescent years. And dozens more hospitalists sat still for the bulk of an hour as they were given a nuts-and-bolts tutorial of HM finances.
Welcome to SHM’s new practice management track, a three-day series of training sessions that debuted at HM09 and focuses on the inner workings of HMGs—from startup to coding and billing to expansion. “There’s always a quality track, there’s always a clinical track, and there’s always a leadership track,” says Kimberly Dickinson, vice president of operations for Cogent Healthcare in Brentwood, Tenn., and an SHM Career Satisfaction Task Force member. “This was trying to bring together some of the nonclinical aspects.”
The experiment drew positive reviews, if rooms crowded with physicians—some lined up along the walls—are any indication. Dr. Fishmann, a Cogent co-founder who is practicing as a hospitalist with California Lung Associates in Los Angeles, says the courses give younger hospitalists different perspectives. “It’s taking doctors a little out of the hospital and putting them in the boardroom,” he says. “You don’t have a choice. … You need to be involved and have more input.”
The practice management courses are structured to give a broad overview of nearly every facet of opening and operating an HMG. One popular course, which ran nearly 15 minutes long because of participant questions, focused on managing HMG growth. Another session spent more than an hour taking physicians through comanagement issues that arise during collaborations with surgeons.
Real-Life Experiences
Most of the courses were led by familiar SHM leaders. But several attendees said they enjoyed sessions that were led by rank-and-file hospitalists and administrators who live the front-line struggles every day.
The “Case Studies in Managing Program Growth” course featured detailed explanations of the growing pains of HM programs in Michigan and Massachusetts. In the former case, Carole Montgomery, MD, talked about how the Michigan Medical PC group she helped start in western Michigan struggled to formalize certain procedures when it signed its first contract with a hospital in 2002. The group doubled its hospitalist roster and instantly went from an informal HM practice in which everyone knew each other and had relatively similar opinions to a business in which it was unclear who would make major decisions.
In response, Dr. Montgomery’s group crafted a mission statement, created a hospitalist executive committee to make routine operational decisions, and changed how it negotiated contracts with hospitals. Soon after, the group fired its first physician. Last year, the group instituted “internal governance guidelines” to make management decisions clearer. Dr. Montgomery says each of the developments taught her that solving major issues takes patience and a willingness to continually adapt. “I thought I was done each time,” she says. “Now I realize it’s an interactive process.”
Results-Oriented
Peter Short, MD, FAAP, CPE, medical director of Northeast Hospital Corp., shared his recent struggles to hire one hospitalist and expand night coverage. Hospitalists in Dr. Short’s service, who practice at Beverly Hospital in Beverly, Mass., resisted the change at first, not wanting to add additional night-shift responsibility. Dr. Short also spoke about financial concerns to the group and the hospital. After explaining the pros and cons of hiring a sixth rounder, the hospitalists embraced the idea. So far, hospital administrators have had zero complaints, as the first three months of data show a reduction in length of stay by 0.3 days and an average cost decrease of roughly $500 per case.
Dr. Short also explained an unexpected byproduct of the hiring: Night work proved so popular that the hospitalists have demanded that they receive a specified number of overnight shifts a year. “They went from not wanting it to fighting for it,” Dr. Short says proudly. “There is no one-size-fits-all for hospital programs.”
Dr. Bossard, who founded Inpatient Physician Associates in Lincoln, Neb., says the types of detail-oriented presentations made by Drs. Montgomery and Short are useful if physicians learn lessons they can take home and adapt to their practice. “If it’s a lot of smoke without an action item or a bullet point we can latch on to,” he says, “it’s not as helpful.”
Richard Quinn is a freelance writer based in New Jersey.
Reporting Hospital Quality
Acknowledging striking deficiencies in the quality and safety of healthcare, the Institute of Medicine, policy makers, and payors have called for transformation of the US healthcare system.1 Public reporting of hospital performance is one key strategy for accelerating improvement2 and may improve quality in several ways. First, feedback about performance relative to peers may stimulate quality improvement activities by appealing to professionalism. Second, the desire to preserve one's reputation by not appearing on a list of poor performers may be a powerful incentive. Finally, patients and referring providers could use reports to select high‐quality hospitals, thereby shifting care from low‐quality to high‐quality hospitals and stimulating quality improvement efforts to maintain or enhance market share.
Almost 20 years after New York and Pennsylvania began reporting cardiac surgery outcomes,3 the evidence that public reporting improves healthcare quality is equivocal.4 Moreover, stakeholders have embraced public reporting to differing degrees. Public reporting does lead to greater engagement in quality improvement activities,58 and additional financial incentives provide modest incremental benefits.9 Purchasers, too, are starting to pay attention.10 In New York State, payors appear to contract more with high‐quality surgeons and avoid poorly performing outliers.11 Some payors are creating tiered systems, assigning higher patient copayments for hospitals with poor quality metrics. These new systems have not been rigorously studied and should raise concern among hospitals.12
In contrast to hospitals and payors, patients have been slow to embrace public reporting. In a survey of coronary artery bypass graft (CABG) patients in Pennsylvania, only 2% said that public reporting of mortality rates affected their decision making.13 Eight years later, only 11% of patients sought information about hospitals before deciding on elective major surgery,14 although a majority of patients in both studies expressed interest in the information. It is not clear whether recent proliferation of information on the internet will change patient behavior, but to date public reporting appears not to effect market share.5, 15, 16
Barriers to patients' use of public reporting include difficulty accessing the information, lack of trust, information that is not salient, and data that are difficult to interpret.17 In the absence of consensus on what or how to report, a growing number of organizations, including state and federal government, accrediting bodies, private foundations, and for‐profit companies report a variety of measures relating to structure, processes, and outcomes. Although these sites purport to target consumers, they sometimes offer conflicting information18 and are not easily interpreted by lay readers.19
To realize the benefits of public reporting, and minimize the unintended consequences, rating systems must report salient information in a way that is comprehensible to patients and trusted by the doctors who advise them. At the same time, they should be fair to hospitals and offer useful data for quality improvement. We offer 10 recommendations for improving the public reporting of healthcare quality information: 5 describing what to report and 5 detailing how it should be reported (Figure 1). We also examine 3 leading performance reporting programs to see how well they implement these recommendations.

Recommendations to Make Data Salient for Patients
1. Prioritize Elective Procedures
Hospital quality is not uniform across conditions.2 For data to be salient, then, it should be disease‐specific and focus on common elective procedures, for which consumer choice is possible. Table 1 compares 3 popular reporting services. Hospital Compare, produced by the Center for Medicare Services (CMS, US Department of Health and Human Services, Washington, DC), provides process of care measures for 4 conditions, 3 of which are not elective. The fourth, surgical infection prevention, contains 5 measures3 related to perioperative antibiotics and 2 related to thromboembolism prophylaxisfor all surgical cases. Recently, more conditions have been added, but reports are limited to the number of cases and mean Medicare charge. By year 2011, however, Hospital Compare will offer many new measures, including rates of central line infection, ventilator‐associated pneumonia, and surgical site infection. HealthGrades, a private company, offers comparative mortality rates on over 30 diagnoses, of which 15 can be considered elective, at least some of the time. Only the Leapfrog group, an industry consortium, focuses exclusively on elective procedures, offering volume measures on 7 and outcome measures on 2.
| Rule | Hospital Compare | HealthGrades | Leapfrog | |||
|---|---|---|---|---|---|---|
| ||||||
| 1. Prioritize elective procedures | Yes | 22/28 at least partially elective | Yes | 15/31 at least partially elective | Yes | 7/8 elective |
| 2. Include quality of life and outcome data, if possible | Yes | Mortality for AMI and CHF | Yes | Mortality or complications* | Yes | Outcomes for CABG, PCI, and AVR |
| 3. Include standardized patient satisfaction and service measures | Yes | HCAHPS | No | No | ||
| 4. Offer composite measures that are weighted and evidence‐based | No | No | Specialty excellence award, not evidence‐based | No | ||
| 5. Costs comparisons should include patient prices | Yes | Average Medicare payment | Yes | Charges, health plan and Medicare costs available for a fee | No | |
| 6. Adjust outcomes for severity and risk | Yes | Methodology published on website | Yes | Methodology not public | Yes | Various methodologies published or referenced on website |
| 7. Identify differences not due to chance | Yes | Compares mortality to national mean | Yes | Compares mortality or complications to mean | Yes | Compares mortality to national mean |
| 8. Standardize reporting periods | October 2005 to September 2006 | 2004‐2006 | 12‐24 months, ending 12/31/07 or 6/30/08 | |||
| 9. Avoid use of nonvalidated administrative data | Yes | None used | No | Uses PSIs for safety rating | Yes | None used |
| 10. Utilization rates should be evidence‐based | No | Surgical case volume of Medicare patients | No | Includes Caesarian‐section rates | Yes | Some case volume rates are evidence‐based |
2. Include Quality of Life and Outcome Data
Outcomes are more valuable to patients than process measures, but the risk adjustment needed to compare outcomes requires considerable effort. So far, public reporting of risk‐adjusted outcomes has been limited almost exclusively to mortality. Yet a patient contemplating knee replacement surgery would find no meaningful difference in mortalitythere were only 510 deaths nationally in year 200620but might be interested in whether patients return to full mobility after surgery, and all patients should compare rates of nosocomial infections. For some low‐risk procedures, HealthGrades Inc. (Golden, CO) includes a composite measure of major complications, including complication of an orthopedic implant, stroke, cardiac arrest, excessive bleeding, and some types of infection; CMS will soon add rates of infection and readmission.
3. Include Measures of Patient Experience, Such as Satisfaction and Service Measures
Beyond outcomes, patients want to know about the experience of others.21 Satisfaction surveys should be standardized and made disease‐specific, since patients' experiences may differ between the cardiology suite and the delivery unit. Questions could address the attentiveness of the nursing staff, how well privacy was respected, how easy it was to deal with insurance issues, whether patients were promptly informed of test results, and whether the care team answered questions fully. Medicare has begun reporting patient satisfaction using the Hospital Consumer Assessment of Healthcare Providers (HCAHPS) survey on Hospital Compare, but the data are not disease‐specific and audit a very small number of patients from each institution. Other services are unlikely to perform their own surveys, as multiple surveys would prove burdensome. Social networking sites that allow patients to post their own personal reviews of hospitals and doctors offer an additional if less reliable dimension to traditional public reporting. Such sites are already transforming the market for other industries, such as travel.22
4. Offer Composite Measures That Are Weighted and Evidence‐Based
Interpreting multiple measures, some of which are more important than others, and some of which have better evidence than others, is difficult for health care providers and may be impossible for patients. Is it more important to get aspirin on arrival or at discharge? Also, how does a patient weigh a 1% difference in the number of heart attack patients who get aspirin on arrival against a 14% difference in those who are offered smoking cessation? Because patients may be overwhelmed by data,23 public reports should include evidence‐based, weighted measures of overall care for a given condition, with higher weights attached to those process measures most likely to have clinical benefit, and careful attention to visual representations that convey relative differences.19, 23 More sophisticated measures should be developed to guard against overuse. For example, while hospitals should be rewarded for providing vaccination, they should be penalized for vaccinating the same patient twice.
None of the services we examined provides weighted outcomes. Leapfrog (The Leapfrog Group, Washington, DC) offers a composite snapshot containing 9 pie charts, divided into 4 leaps. The 6 pies representing high‐risk procedures are of equal size, even though 2 of these, esophagectomy and pancreatic resection represent very rare surgeries, even at major medical centers. From a visual perspective, however, these are equivalent to having computerized physician order entry and full‐time intensive care unit staffing, which affect thousands more patients. Similarly, in determining pay‐for‐performance measures, CMS created a composite based on the total number of opportunities of all interventions, weighting all measures equally. Because no validated weighting measures exist, future research will be necessary to achieve this goal. Also, none of the evidence‐based measures contained safeguards against overtreatment.
5. Cost Comparisons Should Include Patient Prices
In an era of patient copayments and deductibles, consumers are increasingly aware of costs. For patients with very high deductible plans or no health insurance, hospital fees are a common cause of bankruptcy.24 Several public reporting agencies, including Hospital Compare and HealthGrades have incorporated Medicare costs into their reported measures, but these have little connection to what patients actually pay. Health sites aimed at consumers should publish the average patient copayment.
Recommendations to Ensure That Data Reflects Hospital Quality
6. Adjust Outcomes for Severity and Risk
Not all bypass operations are the same and not all patients are at equal risk. More difficult operations (eg, CABG for a patient with a previous bypass) will have more complications; similarly, patients with serious comorbidities will experience worse outcomes. Since hospitals which specialize in a procedure will attract complicated cases and higher risk patients, it is important to adjust outcomes to account for these differences. Otherwise, hospitals and surgeons may be discouraged from taking difficult cases. Outside of cardiac surgery, most risk adjustment systems use administrative claims data but vary dramatically in the numbers of variables considered and the underlying proprietary models, which are often criticized as being black boxes that yield discordant results.25 Thus, a hospital's mortality may appear below expected by 1 system and above expected by another. Instead, risk adjustment systems should include clinical data abstracted from patient records using standardized data definitions. Although costly to collect, clinical data offer more predictive information than do administrative data. For example, for heart failure patients undergoing CABG, the ejection fraction predicts mortality better than many stable comorbid diagnoses. A single transparent risk‐adjustment system should be recognized as the industry standard. The American College of Surgeons' standardized risk‐adjusted outcome reporting for the National Surgical Quality Improvement Program (NSQIP) is a good example of such an effort.
7. Identify Differences Not Due to Chance
As a result of random variation, during any period, some hospitals will appear better than average and others worse. Statistical tests should be employed to identify hospitals that differ from the mean, and to allow consumers to compare 2 hospitals directly, with appropriate caveats when the hospitals serve very different patient populations. Medicare's mortality rating system for myocardial infarction identifies only 17 hospitals in the nation as better than average and 7 as worse, out of 4,500 institutions. HealthGrades compares hospitals' actual mortality or complication rates to their predicted rates based on disease‐specific logistic regression models and reports whether the hospital is statistically better or worse than predicted. Hospitals are not compared directly to one another. Given the rarity of mortality in most procedures, other outcome measures will be necessary to distinguish among hospitals.26
8. Standardize Reporting Periods
In a world of continuous quality improvement, public reporting should represent a hospital's recent performance, but reporting periods also need to be long enough to provide a stable estimate of infrequent events, especially at low‐volume institutions. In contrast, the lag time between the end of the reporting period and public availability should be kept to a minimum. We found that reporting periods varied from 1 to 3 years, and did not always cover the same years for all conditions, even on the same website. Some data were 3 years old. Patients will have a hard time making decisions on data that is 1 year old, and hospitals will have little incentive to make improvements that will not be acknowledged for years.
9. Avoid Use of Nonvalidated Administrative Data
Administrative data collected for billing purposes, unlike most clinical data, are already in electronic format, and can inexpensively produce quality rankings using validated models.27 In contrast, screening tools, such as the Agency for Healthcare Research and Quality's patient safety indicators (PSIs), were designed to identify potential quality problems, such as postoperative deep vein thrombosis, for internal quality improvement. Cases identified by the PSI software require additional chart review,28, 29 and should not be used as quality indicators. Even so, HealthGrades reports PSIs and some insurers use them in pay‐for‐performance initiatives. Improvements in PSIs, including present‐on‐admission coding, may increase accuracy,30 but these measures need to be validated before they can be adopted for public reporting.
10. Utilization Rates Should Be Evidence‐Based
Although utilization rates for most procedures vary as much as 2‐fold by state or institution, there is little evidence for a best rate. Nevertheless, HealthGrades reports utilization rates for several obstetrical procedures. At present, there are no standards for these, and it is possible that utilization could be too low in some places. Further research is needed; until then, utilization should not purport to measure quality.
Discussion
The growing commitment to making hospital performance data public could transform the quality and safety of care in the US, introducing competition on quality and price and fostering informed consumer choice. To date, the promise of public reporting remains only partially fulfilled. Few hospitals have done more than comply with regulatory mandates and payer incentives, and consumers have failed to respond. To capture the full benefits of public reporting, we have made 10 recommendations to benefit patients and better engage hospitals. We suggest that reporting be patient‐centered, with an emphasis on making the data useful, meaningful, important, interpretable, and relevant. At the same time, hospitals, which are being judged on their performance, should have a level playing field, with measures that are timely, consistent, severity‐adjusted, evidence‐based, and which foster good clinical care. Of the 3 services we examined, Hospital Compare came closest to meeting these recommendations.
Although this blueprint for public reporting is easy to draft, it is challenging to implement. In particular, some of our suggestions, such as the one regarding risk adjustment, may not currently be feasible, because the complexity and cost of collecting clinical data, even in the era of electronic medical records, may be prohibitive. Until such data are readily available, it may be preferable to report nothing at all, rather than report data that are misleading. In the rush to make hospitals accountable, enthusiasm has often outstripped science,31 and several measures have had to be revised for unintended consequences.32
Any initiative to improve public reporting should have the buy‐in of all stakeholders, but particularly hospitals, which stand to benefit in several ways. By receiving regular feedback, they can focus on improving care, becoming better organizations. These improvements may be rewarded through direct compensation (pay‐for‐performance), decreased costs from complications, or increased market share. Hospitals will be more engaged if the data reflect actual quality, are adequately adjusted for severity, and acknowledge the role of chance. Otherwise, they will merely comply, or worse, look for opportunities to game the system. To succeed, public reporting needs to involve hospitals in establishing standards for reporting and validation, as well as auditing procedures to prevent fraud.33 The Hospital Quality Alliance (HQA, Washington, DC), a first step in this direction, at present has few measures. NSQIP (American College of Surgeons, Chicago, IL) is perhaps a better example of hospitals cooperating to set measurement standards to promote best‐practices. Public release of NSQIP data might accelerate progress. Alternatively, the National Quality Forum (NQF, Washington, DC) could expand its role from endorsing quality measures to include standardizing the way these measures are used in public reporting.
Still, if you build it, will they come? To date, public reporting has not been embraced by the public, despite its stated interest in the information. Several explanations could be offered. First, we may be presenting the wrong data. Process measures and mortality rates are important but represent abstract concepts for most patients. Surveys tell us that patients value most the experiences of other patients.14, 21 They want to know whether their pain will be controlled, whether the doctor will listen to them, whether the nurse will come when they call. The recent advent of the HCAHPS survey (AHRQ, Washington, DC) is another positive step. Stratifying the results by diagnosis and adding a few diagnosis‐specific questions would make HCAHPS even more valuable. Second, the data may not be readily available. Although most public reporting is done on the web, older patients who are deciding about hospitals may not have Internet access. Some reports are still proprietary, and cost could present another obstacle. Finally, even if freely‐available and patient‐centered, the results may not be interpretable by physicians, let alone patients.34
If public reporting is to succeed, it will require measures that better reflect patients' concerns. In order to collect the massive amounts of data required and present them in a timely fashion, better electronic record systems will be necessary. But these are no panacea; others have noted that the Department of Veterans Affairs, a leader in electronic records, still invests considerable time and money to review charts for NSQIP.35 Given the value that Americans place on transparency in other facets of their lives, it is clear that public reporting is here to stay. While much progress has been made over the past 5 years, additional research is needed to better measure quality from the patient's perspective, and to determine how this information can be used to help guide decision‐making, and to reward hospitals for offering the highest‐quality care.
Acknowledgements
The authors thank Kenneth Flax for his help with an earlier version of this manuscript.
- Committee on Quality of Health Care in America IoM.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,,,.Care in U.S. hospitals: the Hospital Quality Alliance program.N Engl J Med.2005;353(3):265–274.
- .Achieving and sustaining improved quality: lessons from New York state and cardiac surgery.Health Aff. 20022002;21(4):40–51.
- ,,,,.Systematic review: the evidence that publishing patient care performance data improves quality of care.Ann Intern Med.2008;148(2):111–123.
- ,,.Hospital performance reports: impact on quality, market share, and reputation.Health Aff (Millwood).2005;24(4):1150–1160.
- ,,.Does publicizing hospital performance stimulate quality improvement efforts?Health Aff (Millwood).2003;22(2):84–94.
- ,,,,.Improving the outcomes of coronary artery bypass surgery in New York State.JAMA.1994;271(10):761–766.
- ,,.Declines in hospital mortality associated with a regional initiative to measure hospital performance.Am J Med Qual.1997;12(2):103–112.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
- ,,,,,.Do quality report cards play a role in HMOs' contracting practices? Evidence from New York State.Health Serv Res.2000;35(1 Pt 2):319–332.
- ,,,.Quality of cardiac surgeons and managed care contracting practices.Health Serv Res.2002;37(5):1129–1144.
- ,,,,,.Using performance data to identify preferred hospitals.Health Serv Res.2007;42(6 Pt 1):2109–2119; discussion 2294–2323.
- ,.Use of public performance reports: a survey of patients undergoing cardiac surgery.JAMA.1998;279(20):1638–1642.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,,,,.The effect of publicly reporting hospital performance on market share and risk‐adjusted mortality at high‐mortality hospitals.Med Care.2003;41(6):729–740.
- ,.The predictive accuracy of the New York State coronary artery bypass surgery report‐card system.Health Aff (Millwood).2006;25(3):844–855.
- ,.Publicly disclosed information about the quality of health care: response of the US public.Qual Saf Health Care.2001;10(2):96–103.
- ,,,,.Choosing the best hospital: the limitations of public reporting of hospital quality.Health Aff (Millwood).2008;27(6):1680–1687.
- ,.Will quality report cards help consumers?Health Aff (Millwood).1997;16(3):218–228.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. Available at: http://hcupnet.ahrq.gov. Accessed January 2009.
- ,,.Recovering from cardiac surgery: what patients want you to know.Am J Crit Care.2002;11(4):333–343.
- Trip Advisor. Available at: http://www.tripadvisor.com. Accessed January 2009.
- ,,,,.Less is more in presenting quality information to consumers.Med Care Res Rev.2007;64(2):169–190.
- ,,,.MarketWatch: illness and injury as contributors to bankruptcy.Health Aff (Millwood)2005;(Suppl Web Exclusives):W5‐63–W5‐73.
- .The Lake Wobegon effect: when all the patients are sicker.Am J Med Qual.2006;21(6):365–366.
- ,,.Surgical mortality as an indicator of hospital quality: the problem with small sample size.JAMA.2004;292(7):847–851.
- ,,, et al.An administrative claims model suitable for profiling hospital performance based on 30‐day mortality rates among patients with heart failure.Circulation.2006;113(13):1693–1701.
- ,,,.Can administrative data be used to compare postoperative complication rates across hospitals?Med Care.2002;40(10):856–867.
- ,,,,.Impact of diagnosis‐timing indicators on measures of safety, comorbidity, and case mix groupings from administrative data sources.Med Care.2007;45(8):781–788.
- ,,,,.Do the AHRQ patient safety indicators flag conditions that are present at the time of hospital admission?Med Care.2008;46(5):516–522.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357(6):608–613.
- ,,,.Public reporting of antibiotic timing in patients with pneumonia: lessons from a flawed performance measure.Ann Intern Med.2008;149(1):29–32.
- ,,.The GAAP in quality measurement and reporting.JAMA.2007;298(15):1800–1802.
- ,,,.Consumer competencies and the use of comparative quality information: it isn't just about literacy.Med Care Res Rev.2007;64(4):379–394.
- .Performance measurement in search of a path.N Engl J Med.2007;356(9):951–953.
Acknowledging striking deficiencies in the quality and safety of healthcare, the Institute of Medicine, policy makers, and payors have called for transformation of the US healthcare system.1 Public reporting of hospital performance is one key strategy for accelerating improvement2 and may improve quality in several ways. First, feedback about performance relative to peers may stimulate quality improvement activities by appealing to professionalism. Second, the desire to preserve one's reputation by not appearing on a list of poor performers may be a powerful incentive. Finally, patients and referring providers could use reports to select high‐quality hospitals, thereby shifting care from low‐quality to high‐quality hospitals and stimulating quality improvement efforts to maintain or enhance market share.
Almost 20 years after New York and Pennsylvania began reporting cardiac surgery outcomes,3 the evidence that public reporting improves healthcare quality is equivocal.4 Moreover, stakeholders have embraced public reporting to differing degrees. Public reporting does lead to greater engagement in quality improvement activities,58 and additional financial incentives provide modest incremental benefits.9 Purchasers, too, are starting to pay attention.10 In New York State, payors appear to contract more with high‐quality surgeons and avoid poorly performing outliers.11 Some payors are creating tiered systems, assigning higher patient copayments for hospitals with poor quality metrics. These new systems have not been rigorously studied and should raise concern among hospitals.12
In contrast to hospitals and payors, patients have been slow to embrace public reporting. In a survey of coronary artery bypass graft (CABG) patients in Pennsylvania, only 2% said that public reporting of mortality rates affected their decision making.13 Eight years later, only 11% of patients sought information about hospitals before deciding on elective major surgery,14 although a majority of patients in both studies expressed interest in the information. It is not clear whether recent proliferation of information on the internet will change patient behavior, but to date public reporting appears not to effect market share.5, 15, 16
Barriers to patients' use of public reporting include difficulty accessing the information, lack of trust, information that is not salient, and data that are difficult to interpret.17 In the absence of consensus on what or how to report, a growing number of organizations, including state and federal government, accrediting bodies, private foundations, and for‐profit companies report a variety of measures relating to structure, processes, and outcomes. Although these sites purport to target consumers, they sometimes offer conflicting information18 and are not easily interpreted by lay readers.19
To realize the benefits of public reporting, and minimize the unintended consequences, rating systems must report salient information in a way that is comprehensible to patients and trusted by the doctors who advise them. At the same time, they should be fair to hospitals and offer useful data for quality improvement. We offer 10 recommendations for improving the public reporting of healthcare quality information: 5 describing what to report and 5 detailing how it should be reported (Figure 1). We also examine 3 leading performance reporting programs to see how well they implement these recommendations.

Recommendations to Make Data Salient for Patients
1. Prioritize Elective Procedures
Hospital quality is not uniform across conditions.2 For data to be salient, then, it should be disease‐specific and focus on common elective procedures, for which consumer choice is possible. Table 1 compares 3 popular reporting services. Hospital Compare, produced by the Center for Medicare Services (CMS, US Department of Health and Human Services, Washington, DC), provides process of care measures for 4 conditions, 3 of which are not elective. The fourth, surgical infection prevention, contains 5 measures3 related to perioperative antibiotics and 2 related to thromboembolism prophylaxisfor all surgical cases. Recently, more conditions have been added, but reports are limited to the number of cases and mean Medicare charge. By year 2011, however, Hospital Compare will offer many new measures, including rates of central line infection, ventilator‐associated pneumonia, and surgical site infection. HealthGrades, a private company, offers comparative mortality rates on over 30 diagnoses, of which 15 can be considered elective, at least some of the time. Only the Leapfrog group, an industry consortium, focuses exclusively on elective procedures, offering volume measures on 7 and outcome measures on 2.
| Rule | Hospital Compare | HealthGrades | Leapfrog | |||
|---|---|---|---|---|---|---|
| ||||||
| 1. Prioritize elective procedures | Yes | 22/28 at least partially elective | Yes | 15/31 at least partially elective | Yes | 7/8 elective |
| 2. Include quality of life and outcome data, if possible | Yes | Mortality for AMI and CHF | Yes | Mortality or complications* | Yes | Outcomes for CABG, PCI, and AVR |
| 3. Include standardized patient satisfaction and service measures | Yes | HCAHPS | No | No | ||
| 4. Offer composite measures that are weighted and evidence‐based | No | No | Specialty excellence award, not evidence‐based | No | ||
| 5. Costs comparisons should include patient prices | Yes | Average Medicare payment | Yes | Charges, health plan and Medicare costs available for a fee | No | |
| 6. Adjust outcomes for severity and risk | Yes | Methodology published on website | Yes | Methodology not public | Yes | Various methodologies published or referenced on website |
| 7. Identify differences not due to chance | Yes | Compares mortality to national mean | Yes | Compares mortality or complications to mean | Yes | Compares mortality to national mean |
| 8. Standardize reporting periods | October 2005 to September 2006 | 2004‐2006 | 12‐24 months, ending 12/31/07 or 6/30/08 | |||
| 9. Avoid use of nonvalidated administrative data | Yes | None used | No | Uses PSIs for safety rating | Yes | None used |
| 10. Utilization rates should be evidence‐based | No | Surgical case volume of Medicare patients | No | Includes Caesarian‐section rates | Yes | Some case volume rates are evidence‐based |
2. Include Quality of Life and Outcome Data
Outcomes are more valuable to patients than process measures, but the risk adjustment needed to compare outcomes requires considerable effort. So far, public reporting of risk‐adjusted outcomes has been limited almost exclusively to mortality. Yet a patient contemplating knee replacement surgery would find no meaningful difference in mortalitythere were only 510 deaths nationally in year 200620but might be interested in whether patients return to full mobility after surgery, and all patients should compare rates of nosocomial infections. For some low‐risk procedures, HealthGrades Inc. (Golden, CO) includes a composite measure of major complications, including complication of an orthopedic implant, stroke, cardiac arrest, excessive bleeding, and some types of infection; CMS will soon add rates of infection and readmission.
3. Include Measures of Patient Experience, Such as Satisfaction and Service Measures
Beyond outcomes, patients want to know about the experience of others.21 Satisfaction surveys should be standardized and made disease‐specific, since patients' experiences may differ between the cardiology suite and the delivery unit. Questions could address the attentiveness of the nursing staff, how well privacy was respected, how easy it was to deal with insurance issues, whether patients were promptly informed of test results, and whether the care team answered questions fully. Medicare has begun reporting patient satisfaction using the Hospital Consumer Assessment of Healthcare Providers (HCAHPS) survey on Hospital Compare, but the data are not disease‐specific and audit a very small number of patients from each institution. Other services are unlikely to perform their own surveys, as multiple surveys would prove burdensome. Social networking sites that allow patients to post their own personal reviews of hospitals and doctors offer an additional if less reliable dimension to traditional public reporting. Such sites are already transforming the market for other industries, such as travel.22
4. Offer Composite Measures That Are Weighted and Evidence‐Based
Interpreting multiple measures, some of which are more important than others, and some of which have better evidence than others, is difficult for health care providers and may be impossible for patients. Is it more important to get aspirin on arrival or at discharge? Also, how does a patient weigh a 1% difference in the number of heart attack patients who get aspirin on arrival against a 14% difference in those who are offered smoking cessation? Because patients may be overwhelmed by data,23 public reports should include evidence‐based, weighted measures of overall care for a given condition, with higher weights attached to those process measures most likely to have clinical benefit, and careful attention to visual representations that convey relative differences.19, 23 More sophisticated measures should be developed to guard against overuse. For example, while hospitals should be rewarded for providing vaccination, they should be penalized for vaccinating the same patient twice.
None of the services we examined provides weighted outcomes. Leapfrog (The Leapfrog Group, Washington, DC) offers a composite snapshot containing 9 pie charts, divided into 4 leaps. The 6 pies representing high‐risk procedures are of equal size, even though 2 of these, esophagectomy and pancreatic resection represent very rare surgeries, even at major medical centers. From a visual perspective, however, these are equivalent to having computerized physician order entry and full‐time intensive care unit staffing, which affect thousands more patients. Similarly, in determining pay‐for‐performance measures, CMS created a composite based on the total number of opportunities of all interventions, weighting all measures equally. Because no validated weighting measures exist, future research will be necessary to achieve this goal. Also, none of the evidence‐based measures contained safeguards against overtreatment.
5. Cost Comparisons Should Include Patient Prices
In an era of patient copayments and deductibles, consumers are increasingly aware of costs. For patients with very high deductible plans or no health insurance, hospital fees are a common cause of bankruptcy.24 Several public reporting agencies, including Hospital Compare and HealthGrades have incorporated Medicare costs into their reported measures, but these have little connection to what patients actually pay. Health sites aimed at consumers should publish the average patient copayment.
Recommendations to Ensure That Data Reflects Hospital Quality
6. Adjust Outcomes for Severity and Risk
Not all bypass operations are the same and not all patients are at equal risk. More difficult operations (eg, CABG for a patient with a previous bypass) will have more complications; similarly, patients with serious comorbidities will experience worse outcomes. Since hospitals which specialize in a procedure will attract complicated cases and higher risk patients, it is important to adjust outcomes to account for these differences. Otherwise, hospitals and surgeons may be discouraged from taking difficult cases. Outside of cardiac surgery, most risk adjustment systems use administrative claims data but vary dramatically in the numbers of variables considered and the underlying proprietary models, which are often criticized as being black boxes that yield discordant results.25 Thus, a hospital's mortality may appear below expected by 1 system and above expected by another. Instead, risk adjustment systems should include clinical data abstracted from patient records using standardized data definitions. Although costly to collect, clinical data offer more predictive information than do administrative data. For example, for heart failure patients undergoing CABG, the ejection fraction predicts mortality better than many stable comorbid diagnoses. A single transparent risk‐adjustment system should be recognized as the industry standard. The American College of Surgeons' standardized risk‐adjusted outcome reporting for the National Surgical Quality Improvement Program (NSQIP) is a good example of such an effort.
7. Identify Differences Not Due to Chance
As a result of random variation, during any period, some hospitals will appear better than average and others worse. Statistical tests should be employed to identify hospitals that differ from the mean, and to allow consumers to compare 2 hospitals directly, with appropriate caveats when the hospitals serve very different patient populations. Medicare's mortality rating system for myocardial infarction identifies only 17 hospitals in the nation as better than average and 7 as worse, out of 4,500 institutions. HealthGrades compares hospitals' actual mortality or complication rates to their predicted rates based on disease‐specific logistic regression models and reports whether the hospital is statistically better or worse than predicted. Hospitals are not compared directly to one another. Given the rarity of mortality in most procedures, other outcome measures will be necessary to distinguish among hospitals.26
8. Standardize Reporting Periods
In a world of continuous quality improvement, public reporting should represent a hospital's recent performance, but reporting periods also need to be long enough to provide a stable estimate of infrequent events, especially at low‐volume institutions. In contrast, the lag time between the end of the reporting period and public availability should be kept to a minimum. We found that reporting periods varied from 1 to 3 years, and did not always cover the same years for all conditions, even on the same website. Some data were 3 years old. Patients will have a hard time making decisions on data that is 1 year old, and hospitals will have little incentive to make improvements that will not be acknowledged for years.
9. Avoid Use of Nonvalidated Administrative Data
Administrative data collected for billing purposes, unlike most clinical data, are already in electronic format, and can inexpensively produce quality rankings using validated models.27 In contrast, screening tools, such as the Agency for Healthcare Research and Quality's patient safety indicators (PSIs), were designed to identify potential quality problems, such as postoperative deep vein thrombosis, for internal quality improvement. Cases identified by the PSI software require additional chart review,28, 29 and should not be used as quality indicators. Even so, HealthGrades reports PSIs and some insurers use them in pay‐for‐performance initiatives. Improvements in PSIs, including present‐on‐admission coding, may increase accuracy,30 but these measures need to be validated before they can be adopted for public reporting.
10. Utilization Rates Should Be Evidence‐Based
Although utilization rates for most procedures vary as much as 2‐fold by state or institution, there is little evidence for a best rate. Nevertheless, HealthGrades reports utilization rates for several obstetrical procedures. At present, there are no standards for these, and it is possible that utilization could be too low in some places. Further research is needed; until then, utilization should not purport to measure quality.
Discussion
The growing commitment to making hospital performance data public could transform the quality and safety of care in the US, introducing competition on quality and price and fostering informed consumer choice. To date, the promise of public reporting remains only partially fulfilled. Few hospitals have done more than comply with regulatory mandates and payer incentives, and consumers have failed to respond. To capture the full benefits of public reporting, we have made 10 recommendations to benefit patients and better engage hospitals. We suggest that reporting be patient‐centered, with an emphasis on making the data useful, meaningful, important, interpretable, and relevant. At the same time, hospitals, which are being judged on their performance, should have a level playing field, with measures that are timely, consistent, severity‐adjusted, evidence‐based, and which foster good clinical care. Of the 3 services we examined, Hospital Compare came closest to meeting these recommendations.
Although this blueprint for public reporting is easy to draft, it is challenging to implement. In particular, some of our suggestions, such as the one regarding risk adjustment, may not currently be feasible, because the complexity and cost of collecting clinical data, even in the era of electronic medical records, may be prohibitive. Until such data are readily available, it may be preferable to report nothing at all, rather than report data that are misleading. In the rush to make hospitals accountable, enthusiasm has often outstripped science,31 and several measures have had to be revised for unintended consequences.32
Any initiative to improve public reporting should have the buy‐in of all stakeholders, but particularly hospitals, which stand to benefit in several ways. By receiving regular feedback, they can focus on improving care, becoming better organizations. These improvements may be rewarded through direct compensation (pay‐for‐performance), decreased costs from complications, or increased market share. Hospitals will be more engaged if the data reflect actual quality, are adequately adjusted for severity, and acknowledge the role of chance. Otherwise, they will merely comply, or worse, look for opportunities to game the system. To succeed, public reporting needs to involve hospitals in establishing standards for reporting and validation, as well as auditing procedures to prevent fraud.33 The Hospital Quality Alliance (HQA, Washington, DC), a first step in this direction, at present has few measures. NSQIP (American College of Surgeons, Chicago, IL) is perhaps a better example of hospitals cooperating to set measurement standards to promote best‐practices. Public release of NSQIP data might accelerate progress. Alternatively, the National Quality Forum (NQF, Washington, DC) could expand its role from endorsing quality measures to include standardizing the way these measures are used in public reporting.
Still, if you build it, will they come? To date, public reporting has not been embraced by the public, despite its stated interest in the information. Several explanations could be offered. First, we may be presenting the wrong data. Process measures and mortality rates are important but represent abstract concepts for most patients. Surveys tell us that patients value most the experiences of other patients.14, 21 They want to know whether their pain will be controlled, whether the doctor will listen to them, whether the nurse will come when they call. The recent advent of the HCAHPS survey (AHRQ, Washington, DC) is another positive step. Stratifying the results by diagnosis and adding a few diagnosis‐specific questions would make HCAHPS even more valuable. Second, the data may not be readily available. Although most public reporting is done on the web, older patients who are deciding about hospitals may not have Internet access. Some reports are still proprietary, and cost could present another obstacle. Finally, even if freely‐available and patient‐centered, the results may not be interpretable by physicians, let alone patients.34
If public reporting is to succeed, it will require measures that better reflect patients' concerns. In order to collect the massive amounts of data required and present them in a timely fashion, better electronic record systems will be necessary. But these are no panacea; others have noted that the Department of Veterans Affairs, a leader in electronic records, still invests considerable time and money to review charts for NSQIP.35 Given the value that Americans place on transparency in other facets of their lives, it is clear that public reporting is here to stay. While much progress has been made over the past 5 years, additional research is needed to better measure quality from the patient's perspective, and to determine how this information can be used to help guide decision‐making, and to reward hospitals for offering the highest‐quality care.
Acknowledgements
The authors thank Kenneth Flax for his help with an earlier version of this manuscript.
Acknowledging striking deficiencies in the quality and safety of healthcare, the Institute of Medicine, policy makers, and payors have called for transformation of the US healthcare system.1 Public reporting of hospital performance is one key strategy for accelerating improvement2 and may improve quality in several ways. First, feedback about performance relative to peers may stimulate quality improvement activities by appealing to professionalism. Second, the desire to preserve one's reputation by not appearing on a list of poor performers may be a powerful incentive. Finally, patients and referring providers could use reports to select high‐quality hospitals, thereby shifting care from low‐quality to high‐quality hospitals and stimulating quality improvement efforts to maintain or enhance market share.
Almost 20 years after New York and Pennsylvania began reporting cardiac surgery outcomes,3 the evidence that public reporting improves healthcare quality is equivocal.4 Moreover, stakeholders have embraced public reporting to differing degrees. Public reporting does lead to greater engagement in quality improvement activities,58 and additional financial incentives provide modest incremental benefits.9 Purchasers, too, are starting to pay attention.10 In New York State, payors appear to contract more with high‐quality surgeons and avoid poorly performing outliers.11 Some payors are creating tiered systems, assigning higher patient copayments for hospitals with poor quality metrics. These new systems have not been rigorously studied and should raise concern among hospitals.12
In contrast to hospitals and payors, patients have been slow to embrace public reporting. In a survey of coronary artery bypass graft (CABG) patients in Pennsylvania, only 2% said that public reporting of mortality rates affected their decision making.13 Eight years later, only 11% of patients sought information about hospitals before deciding on elective major surgery,14 although a majority of patients in both studies expressed interest in the information. It is not clear whether recent proliferation of information on the internet will change patient behavior, but to date public reporting appears not to effect market share.5, 15, 16
Barriers to patients' use of public reporting include difficulty accessing the information, lack of trust, information that is not salient, and data that are difficult to interpret.17 In the absence of consensus on what or how to report, a growing number of organizations, including state and federal government, accrediting bodies, private foundations, and for‐profit companies report a variety of measures relating to structure, processes, and outcomes. Although these sites purport to target consumers, they sometimes offer conflicting information18 and are not easily interpreted by lay readers.19
To realize the benefits of public reporting, and minimize the unintended consequences, rating systems must report salient information in a way that is comprehensible to patients and trusted by the doctors who advise them. At the same time, they should be fair to hospitals and offer useful data for quality improvement. We offer 10 recommendations for improving the public reporting of healthcare quality information: 5 describing what to report and 5 detailing how it should be reported (Figure 1). We also examine 3 leading performance reporting programs to see how well they implement these recommendations.

Recommendations to Make Data Salient for Patients
1. Prioritize Elective Procedures
Hospital quality is not uniform across conditions.2 For data to be salient, then, it should be disease‐specific and focus on common elective procedures, for which consumer choice is possible. Table 1 compares 3 popular reporting services. Hospital Compare, produced by the Center for Medicare Services (CMS, US Department of Health and Human Services, Washington, DC), provides process of care measures for 4 conditions, 3 of which are not elective. The fourth, surgical infection prevention, contains 5 measures3 related to perioperative antibiotics and 2 related to thromboembolism prophylaxisfor all surgical cases. Recently, more conditions have been added, but reports are limited to the number of cases and mean Medicare charge. By year 2011, however, Hospital Compare will offer many new measures, including rates of central line infection, ventilator‐associated pneumonia, and surgical site infection. HealthGrades, a private company, offers comparative mortality rates on over 30 diagnoses, of which 15 can be considered elective, at least some of the time. Only the Leapfrog group, an industry consortium, focuses exclusively on elective procedures, offering volume measures on 7 and outcome measures on 2.
| Rule | Hospital Compare | HealthGrades | Leapfrog | |||
|---|---|---|---|---|---|---|
| ||||||
| 1. Prioritize elective procedures | Yes | 22/28 at least partially elective | Yes | 15/31 at least partially elective | Yes | 7/8 elective |
| 2. Include quality of life and outcome data, if possible | Yes | Mortality for AMI and CHF | Yes | Mortality or complications* | Yes | Outcomes for CABG, PCI, and AVR |
| 3. Include standardized patient satisfaction and service measures | Yes | HCAHPS | No | No | ||
| 4. Offer composite measures that are weighted and evidence‐based | No | No | Specialty excellence award, not evidence‐based | No | ||
| 5. Costs comparisons should include patient prices | Yes | Average Medicare payment | Yes | Charges, health plan and Medicare costs available for a fee | No | |
| 6. Adjust outcomes for severity and risk | Yes | Methodology published on website | Yes | Methodology not public | Yes | Various methodologies published or referenced on website |
| 7. Identify differences not due to chance | Yes | Compares mortality to national mean | Yes | Compares mortality or complications to mean | Yes | Compares mortality to national mean |
| 8. Standardize reporting periods | October 2005 to September 2006 | 2004‐2006 | 12‐24 months, ending 12/31/07 or 6/30/08 | |||
| 9. Avoid use of nonvalidated administrative data | Yes | None used | No | Uses PSIs for safety rating | Yes | None used |
| 10. Utilization rates should be evidence‐based | No | Surgical case volume of Medicare patients | No | Includes Caesarian‐section rates | Yes | Some case volume rates are evidence‐based |
2. Include Quality of Life and Outcome Data
Outcomes are more valuable to patients than process measures, but the risk adjustment needed to compare outcomes requires considerable effort. So far, public reporting of risk‐adjusted outcomes has been limited almost exclusively to mortality. Yet a patient contemplating knee replacement surgery would find no meaningful difference in mortalitythere were only 510 deaths nationally in year 200620but might be interested in whether patients return to full mobility after surgery, and all patients should compare rates of nosocomial infections. For some low‐risk procedures, HealthGrades Inc. (Golden, CO) includes a composite measure of major complications, including complication of an orthopedic implant, stroke, cardiac arrest, excessive bleeding, and some types of infection; CMS will soon add rates of infection and readmission.
3. Include Measures of Patient Experience, Such as Satisfaction and Service Measures
Beyond outcomes, patients want to know about the experience of others.21 Satisfaction surveys should be standardized and made disease‐specific, since patients' experiences may differ between the cardiology suite and the delivery unit. Questions could address the attentiveness of the nursing staff, how well privacy was respected, how easy it was to deal with insurance issues, whether patients were promptly informed of test results, and whether the care team answered questions fully. Medicare has begun reporting patient satisfaction using the Hospital Consumer Assessment of Healthcare Providers (HCAHPS) survey on Hospital Compare, but the data are not disease‐specific and audit a very small number of patients from each institution. Other services are unlikely to perform their own surveys, as multiple surveys would prove burdensome. Social networking sites that allow patients to post their own personal reviews of hospitals and doctors offer an additional if less reliable dimension to traditional public reporting. Such sites are already transforming the market for other industries, such as travel.22
4. Offer Composite Measures That Are Weighted and Evidence‐Based
Interpreting multiple measures, some of which are more important than others, and some of which have better evidence than others, is difficult for health care providers and may be impossible for patients. Is it more important to get aspirin on arrival or at discharge? Also, how does a patient weigh a 1% difference in the number of heart attack patients who get aspirin on arrival against a 14% difference in those who are offered smoking cessation? Because patients may be overwhelmed by data,23 public reports should include evidence‐based, weighted measures of overall care for a given condition, with higher weights attached to those process measures most likely to have clinical benefit, and careful attention to visual representations that convey relative differences.19, 23 More sophisticated measures should be developed to guard against overuse. For example, while hospitals should be rewarded for providing vaccination, they should be penalized for vaccinating the same patient twice.
None of the services we examined provides weighted outcomes. Leapfrog (The Leapfrog Group, Washington, DC) offers a composite snapshot containing 9 pie charts, divided into 4 leaps. The 6 pies representing high‐risk procedures are of equal size, even though 2 of these, esophagectomy and pancreatic resection represent very rare surgeries, even at major medical centers. From a visual perspective, however, these are equivalent to having computerized physician order entry and full‐time intensive care unit staffing, which affect thousands more patients. Similarly, in determining pay‐for‐performance measures, CMS created a composite based on the total number of opportunities of all interventions, weighting all measures equally. Because no validated weighting measures exist, future research will be necessary to achieve this goal. Also, none of the evidence‐based measures contained safeguards against overtreatment.
5. Cost Comparisons Should Include Patient Prices
In an era of patient copayments and deductibles, consumers are increasingly aware of costs. For patients with very high deductible plans or no health insurance, hospital fees are a common cause of bankruptcy.24 Several public reporting agencies, including Hospital Compare and HealthGrades have incorporated Medicare costs into their reported measures, but these have little connection to what patients actually pay. Health sites aimed at consumers should publish the average patient copayment.
Recommendations to Ensure That Data Reflects Hospital Quality
6. Adjust Outcomes for Severity and Risk
Not all bypass operations are the same and not all patients are at equal risk. More difficult operations (eg, CABG for a patient with a previous bypass) will have more complications; similarly, patients with serious comorbidities will experience worse outcomes. Since hospitals which specialize in a procedure will attract complicated cases and higher risk patients, it is important to adjust outcomes to account for these differences. Otherwise, hospitals and surgeons may be discouraged from taking difficult cases. Outside of cardiac surgery, most risk adjustment systems use administrative claims data but vary dramatically in the numbers of variables considered and the underlying proprietary models, which are often criticized as being black boxes that yield discordant results.25 Thus, a hospital's mortality may appear below expected by 1 system and above expected by another. Instead, risk adjustment systems should include clinical data abstracted from patient records using standardized data definitions. Although costly to collect, clinical data offer more predictive information than do administrative data. For example, for heart failure patients undergoing CABG, the ejection fraction predicts mortality better than many stable comorbid diagnoses. A single transparent risk‐adjustment system should be recognized as the industry standard. The American College of Surgeons' standardized risk‐adjusted outcome reporting for the National Surgical Quality Improvement Program (NSQIP) is a good example of such an effort.
7. Identify Differences Not Due to Chance
As a result of random variation, during any period, some hospitals will appear better than average and others worse. Statistical tests should be employed to identify hospitals that differ from the mean, and to allow consumers to compare 2 hospitals directly, with appropriate caveats when the hospitals serve very different patient populations. Medicare's mortality rating system for myocardial infarction identifies only 17 hospitals in the nation as better than average and 7 as worse, out of 4,500 institutions. HealthGrades compares hospitals' actual mortality or complication rates to their predicted rates based on disease‐specific logistic regression models and reports whether the hospital is statistically better or worse than predicted. Hospitals are not compared directly to one another. Given the rarity of mortality in most procedures, other outcome measures will be necessary to distinguish among hospitals.26
8. Standardize Reporting Periods
In a world of continuous quality improvement, public reporting should represent a hospital's recent performance, but reporting periods also need to be long enough to provide a stable estimate of infrequent events, especially at low‐volume institutions. In contrast, the lag time between the end of the reporting period and public availability should be kept to a minimum. We found that reporting periods varied from 1 to 3 years, and did not always cover the same years for all conditions, even on the same website. Some data were 3 years old. Patients will have a hard time making decisions on data that is 1 year old, and hospitals will have little incentive to make improvements that will not be acknowledged for years.
9. Avoid Use of Nonvalidated Administrative Data
Administrative data collected for billing purposes, unlike most clinical data, are already in electronic format, and can inexpensively produce quality rankings using validated models.27 In contrast, screening tools, such as the Agency for Healthcare Research and Quality's patient safety indicators (PSIs), were designed to identify potential quality problems, such as postoperative deep vein thrombosis, for internal quality improvement. Cases identified by the PSI software require additional chart review,28, 29 and should not be used as quality indicators. Even so, HealthGrades reports PSIs and some insurers use them in pay‐for‐performance initiatives. Improvements in PSIs, including present‐on‐admission coding, may increase accuracy,30 but these measures need to be validated before they can be adopted for public reporting.
10. Utilization Rates Should Be Evidence‐Based
Although utilization rates for most procedures vary as much as 2‐fold by state or institution, there is little evidence for a best rate. Nevertheless, HealthGrades reports utilization rates for several obstetrical procedures. At present, there are no standards for these, and it is possible that utilization could be too low in some places. Further research is needed; until then, utilization should not purport to measure quality.
Discussion
The growing commitment to making hospital performance data public could transform the quality and safety of care in the US, introducing competition on quality and price and fostering informed consumer choice. To date, the promise of public reporting remains only partially fulfilled. Few hospitals have done more than comply with regulatory mandates and payer incentives, and consumers have failed to respond. To capture the full benefits of public reporting, we have made 10 recommendations to benefit patients and better engage hospitals. We suggest that reporting be patient‐centered, with an emphasis on making the data useful, meaningful, important, interpretable, and relevant. At the same time, hospitals, which are being judged on their performance, should have a level playing field, with measures that are timely, consistent, severity‐adjusted, evidence‐based, and which foster good clinical care. Of the 3 services we examined, Hospital Compare came closest to meeting these recommendations.
Although this blueprint for public reporting is easy to draft, it is challenging to implement. In particular, some of our suggestions, such as the one regarding risk adjustment, may not currently be feasible, because the complexity and cost of collecting clinical data, even in the era of electronic medical records, may be prohibitive. Until such data are readily available, it may be preferable to report nothing at all, rather than report data that are misleading. In the rush to make hospitals accountable, enthusiasm has often outstripped science,31 and several measures have had to be revised for unintended consequences.32
Any initiative to improve public reporting should have the buy‐in of all stakeholders, but particularly hospitals, which stand to benefit in several ways. By receiving regular feedback, they can focus on improving care, becoming better organizations. These improvements may be rewarded through direct compensation (pay‐for‐performance), decreased costs from complications, or increased market share. Hospitals will be more engaged if the data reflect actual quality, are adequately adjusted for severity, and acknowledge the role of chance. Otherwise, they will merely comply, or worse, look for opportunities to game the system. To succeed, public reporting needs to involve hospitals in establishing standards for reporting and validation, as well as auditing procedures to prevent fraud.33 The Hospital Quality Alliance (HQA, Washington, DC), a first step in this direction, at present has few measures. NSQIP (American College of Surgeons, Chicago, IL) is perhaps a better example of hospitals cooperating to set measurement standards to promote best‐practices. Public release of NSQIP data might accelerate progress. Alternatively, the National Quality Forum (NQF, Washington, DC) could expand its role from endorsing quality measures to include standardizing the way these measures are used in public reporting.
Still, if you build it, will they come? To date, public reporting has not been embraced by the public, despite its stated interest in the information. Several explanations could be offered. First, we may be presenting the wrong data. Process measures and mortality rates are important but represent abstract concepts for most patients. Surveys tell us that patients value most the experiences of other patients.14, 21 They want to know whether their pain will be controlled, whether the doctor will listen to them, whether the nurse will come when they call. The recent advent of the HCAHPS survey (AHRQ, Washington, DC) is another positive step. Stratifying the results by diagnosis and adding a few diagnosis‐specific questions would make HCAHPS even more valuable. Second, the data may not be readily available. Although most public reporting is done on the web, older patients who are deciding about hospitals may not have Internet access. Some reports are still proprietary, and cost could present another obstacle. Finally, even if freely‐available and patient‐centered, the results may not be interpretable by physicians, let alone patients.34
If public reporting is to succeed, it will require measures that better reflect patients' concerns. In order to collect the massive amounts of data required and present them in a timely fashion, better electronic record systems will be necessary. But these are no panacea; others have noted that the Department of Veterans Affairs, a leader in electronic records, still invests considerable time and money to review charts for NSQIP.35 Given the value that Americans place on transparency in other facets of their lives, it is clear that public reporting is here to stay. While much progress has been made over the past 5 years, additional research is needed to better measure quality from the patient's perspective, and to determine how this information can be used to help guide decision‐making, and to reward hospitals for offering the highest‐quality care.
Acknowledgements
The authors thank Kenneth Flax for his help with an earlier version of this manuscript.
- Committee on Quality of Health Care in America IoM.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,,,.Care in U.S. hospitals: the Hospital Quality Alliance program.N Engl J Med.2005;353(3):265–274.
- .Achieving and sustaining improved quality: lessons from New York state and cardiac surgery.Health Aff. 20022002;21(4):40–51.
- ,,,,.Systematic review: the evidence that publishing patient care performance data improves quality of care.Ann Intern Med.2008;148(2):111–123.
- ,,.Hospital performance reports: impact on quality, market share, and reputation.Health Aff (Millwood).2005;24(4):1150–1160.
- ,,.Does publicizing hospital performance stimulate quality improvement efforts?Health Aff (Millwood).2003;22(2):84–94.
- ,,,,.Improving the outcomes of coronary artery bypass surgery in New York State.JAMA.1994;271(10):761–766.
- ,,.Declines in hospital mortality associated with a regional initiative to measure hospital performance.Am J Med Qual.1997;12(2):103–112.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
- ,,,,,.Do quality report cards play a role in HMOs' contracting practices? Evidence from New York State.Health Serv Res.2000;35(1 Pt 2):319–332.
- ,,,.Quality of cardiac surgeons and managed care contracting practices.Health Serv Res.2002;37(5):1129–1144.
- ,,,,,.Using performance data to identify preferred hospitals.Health Serv Res.2007;42(6 Pt 1):2109–2119; discussion 2294–2323.
- ,.Use of public performance reports: a survey of patients undergoing cardiac surgery.JAMA.1998;279(20):1638–1642.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,,,,.The effect of publicly reporting hospital performance on market share and risk‐adjusted mortality at high‐mortality hospitals.Med Care.2003;41(6):729–740.
- ,.The predictive accuracy of the New York State coronary artery bypass surgery report‐card system.Health Aff (Millwood).2006;25(3):844–855.
- ,.Publicly disclosed information about the quality of health care: response of the US public.Qual Saf Health Care.2001;10(2):96–103.
- ,,,,.Choosing the best hospital: the limitations of public reporting of hospital quality.Health Aff (Millwood).2008;27(6):1680–1687.
- ,.Will quality report cards help consumers?Health Aff (Millwood).1997;16(3):218–228.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. Available at: http://hcupnet.ahrq.gov. Accessed January 2009.
- ,,.Recovering from cardiac surgery: what patients want you to know.Am J Crit Care.2002;11(4):333–343.
- Trip Advisor. Available at: http://www.tripadvisor.com. Accessed January 2009.
- ,,,,.Less is more in presenting quality information to consumers.Med Care Res Rev.2007;64(2):169–190.
- ,,,.MarketWatch: illness and injury as contributors to bankruptcy.Health Aff (Millwood)2005;(Suppl Web Exclusives):W5‐63–W5‐73.
- .The Lake Wobegon effect: when all the patients are sicker.Am J Med Qual.2006;21(6):365–366.
- ,,.Surgical mortality as an indicator of hospital quality: the problem with small sample size.JAMA.2004;292(7):847–851.
- ,,, et al.An administrative claims model suitable for profiling hospital performance based on 30‐day mortality rates among patients with heart failure.Circulation.2006;113(13):1693–1701.
- ,,,.Can administrative data be used to compare postoperative complication rates across hospitals?Med Care.2002;40(10):856–867.
- ,,,,.Impact of diagnosis‐timing indicators on measures of safety, comorbidity, and case mix groupings from administrative data sources.Med Care.2007;45(8):781–788.
- ,,,,.Do the AHRQ patient safety indicators flag conditions that are present at the time of hospital admission?Med Care.2008;46(5):516–522.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357(6):608–613.
- ,,,.Public reporting of antibiotic timing in patients with pneumonia: lessons from a flawed performance measure.Ann Intern Med.2008;149(1):29–32.
- ,,.The GAAP in quality measurement and reporting.JAMA.2007;298(15):1800–1802.
- ,,,.Consumer competencies and the use of comparative quality information: it isn't just about literacy.Med Care Res Rev.2007;64(4):379–394.
- .Performance measurement in search of a path.N Engl J Med.2007;356(9):951–953.
- Committee on Quality of Health Care in America IoM.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,,,.Care in U.S. hospitals: the Hospital Quality Alliance program.N Engl J Med.2005;353(3):265–274.
- .Achieving and sustaining improved quality: lessons from New York state and cardiac surgery.Health Aff. 20022002;21(4):40–51.
- ,,,,.Systematic review: the evidence that publishing patient care performance data improves quality of care.Ann Intern Med.2008;148(2):111–123.
- ,,.Hospital performance reports: impact on quality, market share, and reputation.Health Aff (Millwood).2005;24(4):1150–1160.
- ,,.Does publicizing hospital performance stimulate quality improvement efforts?Health Aff (Millwood).2003;22(2):84–94.
- ,,,,.Improving the outcomes of coronary artery bypass surgery in New York State.JAMA.1994;271(10):761–766.
- ,,.Declines in hospital mortality associated with a regional initiative to measure hospital performance.Am J Med Qual.1997;12(2):103–112.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
- ,,,,,.Do quality report cards play a role in HMOs' contracting practices? Evidence from New York State.Health Serv Res.2000;35(1 Pt 2):319–332.
- ,,,.Quality of cardiac surgeons and managed care contracting practices.Health Serv Res.2002;37(5):1129–1144.
- ,,,,,.Using performance data to identify preferred hospitals.Health Serv Res.2007;42(6 Pt 1):2109–2119; discussion 2294–2323.
- ,.Use of public performance reports: a survey of patients undergoing cardiac surgery.JAMA.1998;279(20):1638–1642.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,,,,.The effect of publicly reporting hospital performance on market share and risk‐adjusted mortality at high‐mortality hospitals.Med Care.2003;41(6):729–740.
- ,.The predictive accuracy of the New York State coronary artery bypass surgery report‐card system.Health Aff (Millwood).2006;25(3):844–855.
- ,.Publicly disclosed information about the quality of health care: response of the US public.Qual Saf Health Care.2001;10(2):96–103.
- ,,,,.Choosing the best hospital: the limitations of public reporting of hospital quality.Health Aff (Millwood).2008;27(6):1680–1687.
- ,.Will quality report cards help consumers?Health Aff (Millwood).1997;16(3):218–228.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. Available at: http://hcupnet.ahrq.gov. Accessed January 2009.
- ,,.Recovering from cardiac surgery: what patients want you to know.Am J Crit Care.2002;11(4):333–343.
- Trip Advisor. Available at: http://www.tripadvisor.com. Accessed January 2009.
- ,,,,.Less is more in presenting quality information to consumers.Med Care Res Rev.2007;64(2):169–190.
- ,,,.MarketWatch: illness and injury as contributors to bankruptcy.Health Aff (Millwood)2005;(Suppl Web Exclusives):W5‐63–W5‐73.
- .The Lake Wobegon effect: when all the patients are sicker.Am J Med Qual.2006;21(6):365–366.
- ,,.Surgical mortality as an indicator of hospital quality: the problem with small sample size.JAMA.2004;292(7):847–851.
- ,,, et al.An administrative claims model suitable for profiling hospital performance based on 30‐day mortality rates among patients with heart failure.Circulation.2006;113(13):1693–1701.
- ,,,.Can administrative data be used to compare postoperative complication rates across hospitals?Med Care.2002;40(10):856–867.
- ,,,,.Impact of diagnosis‐timing indicators on measures of safety, comorbidity, and case mix groupings from administrative data sources.Med Care.2007;45(8):781–788.
- ,,,,.Do the AHRQ patient safety indicators flag conditions that are present at the time of hospital admission?Med Care.2008;46(5):516–522.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357(6):608–613.
- ,,,.Public reporting of antibiotic timing in patients with pneumonia: lessons from a flawed performance measure.Ann Intern Med.2008;149(1):29–32.
- ,,.The GAAP in quality measurement and reporting.JAMA.2007;298(15):1800–1802.
- ,,,.Consumer competencies and the use of comparative quality information: it isn't just about literacy.Med Care Res Rev.2007;64(4):379–394.
- .Performance measurement in search of a path.N Engl J Med.2007;356(9):951–953.
Copyright © 2009 Society of Hospital Medicine
Acute Vertebral Fracture
An 89‐year‐old female presents to the Emergency Department with lower back pain for the past 5 days. The patient has a past medical history of polymyalgia rheumatica and hypothyroidism. Her medications include prednisone 10 mg daily and levothyroxine 50 g daily. Aside from tenderness over the third lumbar vertebra, her physical exam is unremarkable. An x‐ray shows a fracture of the third lumbar vertebra. Basic laboratory studies, including calcium and creatinine are normal.
The Clinical Problem and Impact
Vertebral fractures (often termed vertebral compression fractures) affect approximately 25% of all postmenopausal women.1, 2 Only one‐third of vertebral fractures are brought to medical attention.3, 4 In the remaining two‐thirds, patients are either asymptomatic or do not seek medical attention. The lifetime risk of a clinical vertebral fracture is approximately 16% and 5% in white women and men, respectively.1 The risk of vertebral fracture increases with age, lower bone mineral density (BMD), and prior vertebral fracture.1, 5 Women with a preexisting vertebral fracture have a 5‐fold increased risk for a new vertebral fracture relative to those without a history of vertebral fracture.5, 6 Approximately 20% of women who sustain a vertebral fracture will have a new vertebral fracture in the subsequent year.5, 6 Vertebral fractures are frequently overlooked on chest x‐rays and hence there is a need for increased awareness and improved recognition of radiographically‐demonstrated fractures.7 Hospitalists must appreciate that a vertebral fracture is often the first clue to underlying osteoporosis. At least 90% of vertebral and hip fractures are attributable to osteoporosis.5
Typically, the pain related to an acute vertebral fracture improves over 4 to 6 weeks.8, 9 However, pain can persist, resulting in functional impairment, and a decline in the quality of life.1013 Vertebral fractures may lead to kyphosis and reduced lung function. This, in turn, may increase the risk for pneumonia, the most common cause of death in patients with osteoporosis.4 Both clinical and subclinical vertebral fractures are, in fact, independently associated with increased mortality,4, 14, 15 particularly in the period immediately following the event.16 The economic impact of osteoporosis and its related fractures is substantial.1719 In 1995, the annual direct medical cost for the inpatient care of vertebral fractures was estimated to be $575 million.19
Evidence‐Based Approach to the Hospitalized Patient
Evaluation
Vertebral fractures most commonly occur between T7 and L4. Acute vertebral fracture pain is typically sudden in onset and located in the mid to lower back. The pain may occur while performing an ordinary task such as lifting an object or bending over, although in many cases there is no preceding trauma. Physical activity exacerbates the pain and patients' movements may be limited due to pain. Spinal tenderness is usually present. A history of weight loss, prior malignancy, or fever should serve as red flags to the hospitalist and prompt evaluation for underlying malignancy or infection.
For a suspected fracture, frontal and lateral radiographs of the thoracolumbar spine are the initial imaging of choice. Magnetic resonance imaging (MRI) is useful when infection, malignancy, or spinal cord compression is suspected. The presence of neurological deficits should always prompt imaging with MRI or computed tomography (CT).
Patients with vertebral fractures should be evaluated with bone density testing, as osteoporosis is usually the underlying etiology. Because a number of medical conditions commonly contribute to bone loss (Table 1), laboratory testing is indicated for most patients. Although debate exists as to the optimal testing strategy,20 the National Osteoporosis Foundation guidelines recommend the evaluation of blood count, chemistry, and thyroid‐stimulating hormone (TSH) in patients with osteoporosis.21 Because vitamin D deficiency is common in patients who sustain osteoporotic fractures, 25‐hydroxyvitamin D levels should be checked in most patients.2225 Depending on the clinical scenario, additional testing may include the following: serum testosterone, serum intact parathyroid hormone (PTH), 24‐hour calcium excretion, serum protein electrophoresis, urine protein electrophoresis, erythrocyte sedimentation rate, and celiac sprue antibodies.26
| Endocrine disease or metabolic causes | Hypogonadism |
| Cushing's syndrome | |
| Hyperthyroidism | |
| Anorexia nervosa | |
| Hyperparathyroidism | |
| Nutritional conditions | Vitamin D deficiency |
| Calcium deficiency | |
| Malabsorption | |
| Drugs | Glucocorticoids |
| Antiepileptic drugs | |
| Excessive thyroid medication | |
| Long‐term heparin or low molecular weight heparin therapy (eg, >1 month) | |
| Other | Multiple myeloma |
| Rheumatoid arthritis | |
| Organ transplantation |
Management
Short Term
Short‐term management goals include the relief of pain and recovery of mobility. Nonsteroidal antiinflammatory drugs (NSAIDs) and low‐dose opioid medications should be used first in an effort to relieve pain. Beyond its potential effect on bone density, calcitonin (Miacalcin, Fortical) has long been used in acute vertebral fractures for analgesia. A systematic review of 5 randomized controlled trials concluded that calcitonin significantly reduced the pain from acute vertebral fractures.27 Calcitonin improved pain as early as 1 week into treatment and the benefit was persistent at 4 weeks. The analgesic mechanism of action for calcitonin is not entirely clear. Proposed mechanisms include increased ‐endorphin release, an antiinflammatory effect, and a direct effect on specific receptors in the central nervous system.27, 28
Pamidronate (Aredia) also has shown efficacy at reducing acute fracture pain. In a double‐blinded trial, Armigeat et al.29 evaluated pamidronate 30 mg daily for 3 days as compared to placebo in 32 patients. Pain scores were improved at 7 and 30 days with pamidronate. The mechanism of analgesia is unclear. Bisphosphonates are known to inhibit osteoclast activity, but may also work by blocking the effect of inflammatory cytokines.29
Early pain relief is critical in order to encourage physical activity. Bed rest should be avoided, as immobility may increase the risk for pressure ulcers, venous thromboembolism, and pneumonia.3033 Although bracing is frequently used in acute vertebral fracture, the modality has not been formally studied. Although also not well studied in the acute setting, physical therapy has been shown to reduce pain and improve functioning for patients with chronic pain from vertebral fracture,34 and is generally recommended.35
Percutaneous vertebral augmentation procedures include vertebroplasty and kyphoplasty. In vertebroplasty, polymethylmethacrylate cement is injected through a needle under fluoroscopic guidance into the collapsed vertebral body. With kyphoplasty, balloon tamps are used to elevate vertebral endplates prior to injection of cement (Figure 1).36 The proposed mechanism of action for both procedures is stabilization of the fracture by the hardened polymethylmethacrylate cement. These procedures are commonly performed by interventional radiologists without the need for general anesthesia; however, depending on the institution, they may be done by orthopedic surgeons, neurosurgeons, or anesthesiologists. The procedure can be performed as an outpatient, if indicated. Although the volume of these procedures has grown dramatically in recent years,37, 38 the quality of evidence supporting their use is relatively weak.3941 Only 1 randomized controlled trial has been published evaluating the potential benefit of vertebroplasty over conservative management.42 Voormolen et al.42 evaluated patients with vertebral fractures and pain refractive to 6 weeks of optimal medical therapy. Patients were treated with vertebroplasty or continuation of medical therapy. Vertebroplasty significantly improved pain initially, but not after 2 weeks. Like the study by Voormolen et al.,42 most studies evaluating percutaneous vertebral augmentation procedures have been conducted on patients with long‐term pain refractory to medical management. One notable exception is a nonrandomized trial published by Diamond et al.6 In that study, 55 patients were treated with vertebroplasty while 24 were treated conservatively. Pain at 24 hours was significantly improved in patients treated with vertebroplasty. At 6 weeks, however, there was no difference among the 2 groups.
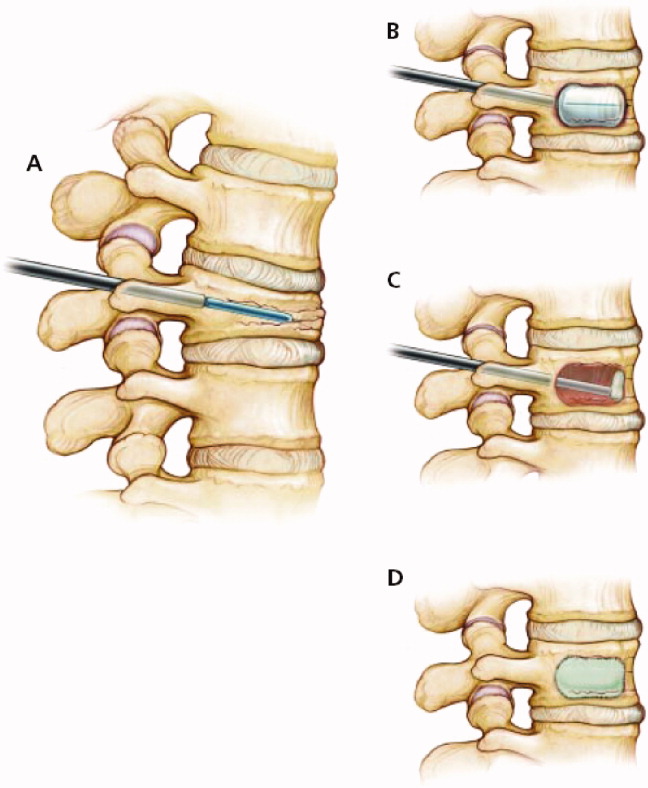
The risk of short‐term complications from vertebral augmentation procedures is difficult to assess in light of the small sample sizes and methodological limitations of existing studies. Cement leakage occurs in 40% to 41% of patients treated with vertebroplasty, as compared with 8% to 9% with kyphoplasty.39, 41 Pulmonary emboli occur in 0.6% and 0.01% of patients treated with vertebroplasty and kyphoplasty, respectively, while neurologic complications occur in 0.6% and 0.03% of patients.41 Concern exists about whether percutaneous vertebral augmentation procedures might increase the risk for subsequent fractures,43, 44 as the incidence of new fractures appears to be elevated in the period immediately following the procedure and approximately two‐thirds of new fractures occur in vertebrae adjacent to the augmented vertebra.39, 41, 44 However, the 20% incidence of new vertebral fractures in the year following vertebral augmentation is similar to the fracture rate seen in patients not treated with osteoporosis therapy.44
Assessment of the impact of vertebral augmentation procedures on the cost of care is limited by the lack of high‐quality clinical studies.45, 46 Randomized controlled trials evaluating the benefit and risk of these procedures compared to conservative management are underway.4749 Pending further evidence, these procedures are best reserved for patients who fail to benefit from other measures to control pain and improve mobility.
Long Term
A comprehensive discussion of the long‐term management of osteoporosis is beyond the scope of this work. However, the inpatient setting presents an opportune time to initiate long‐term medical therapy. Studies show that the majority of patients who sustain osteoporotic fractures do not receive pharmacologic treatment for osteoporosis.5053 Hospitalists have the opportunity to start medications that can reduce the risk for subsequent fracture by nearly 50%.5458 A total calcium intake of 1200 to 1500 mg per day and vitamin D of 400 to 800 IU per day are recommended for all postmenopausal women. Patients who smoke should receive smoking cessation counseling and be considered for pharmacologic treatment for tobacco dependence. All patients should be assessed for fall risk, including a review of medications and assessment of alcohol intake.
Before considering pharmacologic treatment for osteoporosis, secondary causes of low bone mass must be excluded. Bisphosphonates are generally considered first‐line pharmacologic therapy for osteoporosis. Alendronate (Fosamax), risedronate (Actonel), and ibandronate (Boniva) have been shown in randomized trials to increase bone density and reduce the risk of osteoporotic fractures.55, 56, 59 Daily, weekly, and monthly preparations of bisphosphonates now exist. Pill‐induced esophagitis is a potential adverse effect of bisphosphonate therapy, but is extremely rare if proper precautions are taken. Patients should take oral bisphosphonates on an empty stomach, with a full glass of water, sitting upright, and have nothing to eat or drink for at least one half hour. If compliance with oral bisphosphonates is not possible, or esophageal abnormalities preclude oral bisphosphonate use, one may consider the use of intravenous ibandronate or zoledronic acid (Reclast). A 3‐year randomized controlled trial of yearly zoledronic acid improved bone density and reduced the incidence of osteoporotic fractures.60 Bisphosphonates are generally not recommended when creatinine clearance is less than 30 mL/minute. Other pharmacologic options for the treatment of osteoporosis include selective estrogen receptor modulators and anabolic agents. The reader is referred to an excellent review by Rosen61 for additional discussion of these therapies. The American College of Rheumatology clinical guidelines for the management of glucocorticoid induced osteoporosis are also worthy of review.62
Hospitalists are naturally suited to improve the quality of care for patients hospitalized with vertebral fractures. Most patients who currently sustain osteoporotic fractures do not receive appropriate evaluation and treatment. One study used an interdisciplinary team to identify, assess, and begin treatment for appropriate patients hospitalized with osteoporotic fractures.63 The intervention resulted in significantly more patients taking osteoporosis treatment medications 6 months after the incident fracture.
Conclusions
Acute vertebral fracture is a common clinical problem associated with significant morbidity and increased risk of mortality. Treatment of vertebral fracture should include analgesics and physical therapy. Percutaneous augmentation procedures may be considered in patients who fail optimal medical therapy. Because most vertebral fractures are due to osteoporosis and the healthcare system currently fails to appropriately assess and treat most patients who have sustained osteoporotic fractures, hospitalists are in an optimal position to initiate long‐term preventative treatment for these patients.
- .Epidemiology of spinal osteoporosis.Spine.1997;22(24 suppl):2S–11S.
- ,,,,,.Prevalence and incidence of vertebral deformities.Osteoporos Int.1993;3(3):113–119.
- ,,.The epidemiology of vertebral fractures. European Vertebral Osteoporosis Study Group.Bone.1993;14(suppl 1):S89–S97.
- ,,,,,.Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group.Arch Intern Med.1999;159(11):1215–1220.
- ,,,.Pre‐existing fractures and bone mass predict vertebral fracture incidence in women.Ann Intern Med.1991;114(11):919–923.
- ,,, et al.Risk of new vertebral fracture in the year following a fracture.JAMA.2001;285(3):320–323.
- ,,,,,.Recognition of vertebral fracture in a clinical setting.Osteoporos Int.2000;11(7):577–582.
- ,,.Management of acute osteoporotic vertebral fractures: a nonrandomized trial comparing percutaneous vertebroplasty with conservative therapy.Am J Med.2003;114(4):257–265.
- .The clinical consequences of vertebral compression fracture.Bone.1992;13(suppl 2):S27–S31.
- ,,,.A case‐control study of quality of life and functional impairment in women with long‐standing vertebral osteoporotic fracture.Osteoporos Int.1999;9(6):508–515.
- ,,, et al.The burden of prevalent fractures on health‐related quality of life in postmenopausal women with osteoporosis: the IMOF study.J Rheumatol.2007;34(7):1551–1560.
- ,,,,.The relationship of health‐related quality of life to prevalent and incident vertebral fractures in postmenopausal women with osteoporosis: results from the Multiple Outcomes of Raloxifene Evaluation Study.Arthritis Rheum2001;44(11):2611–2619.
- ,,, et al.The association of radiographically detected vertebral fractures with back pain and function: a prospective study.Ann Int Med.1998;128:793–800.
- ,,,,.Mortality after all major types of osteoporotic fracture in men and women: an observational study.Lancet. 131999;353(9156):878–882.
- ,,,,.Population‐based study of survival after osteoporotic fractures.Am J Epidemiol.1993;137(9):1001–1005.
- ,,,,.Excess mortality after hospitalisation for vertebral fracture.Osteoporos Int.2004;15(2):108–112.
- ,.The cost of treating osteoporotic fractures in the United Kingdom female population.Osteoporos Int.1998;8(6):611–617.
- ,,, et al.Direct medical costs attributable to osteoporotic fractures.Osteoporos Int.2002;13(4):323–330.
- ,,,.Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation.J Bone Miner Res.1997;12(1):24–35.
- .Laboratory workup for osteoporosis. Which tests are most cost‐effective?Postgrad Med.2003;114(3):35–38,41–34.
- National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Available at: http://www.nof.org/professionals/Clinicians_Guide.htm. Accessed February2009.
- ,,, et al.Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy.J Clin Endocr Metab.2005;90:3215–3224.
- ,,,,.Prevalence of vitamin D inadequacy in a minimal trauma fracture population.Curr Med Res Opin.2005;21:1069–1074.
- ,,,,,.Occult vitamin D deficiency in postmenopausal US women with acute hip fracture.JAMA.1999;281:1505–1511.
- ,,,,.Secondary contributors for bone loss in osteoporotic hip fractures.Osteoporos Int.2008;19(7):991–999.
- .Evaluation of the patient with osteoporosis or at risk for osteoporosis. In: Marcus R, Feldman D, Kelsey J, eds.Osteoporosis. Vol.2.San Diego:Academic Press;2001:403–408.
- ,,,,.Calcitonin for treating acute pain of osteoporotic vertebral compression fractures: a systematic review of randomized, controlled trials.Osteoporos Int.2005;16(10):1281–1290.
- .Possible mechanisms of the analgesic action of calcitonin.Bone.2002;30(5 suppl):80S–83S.
- ,,,,.Intravenous pamidronate for pain relief in recent osteoporotic vertebral compression fracture: a randomized double‐blind controlled study.Osteoporos Int.2006;17(11):1659–1665.
- ,,,,.Pressure ulcer risk factors among hospitalized patients with activity limitation.JAMA.1995;273(11):865–870.
- ,.Risk factors for venous thromboembolism.Circulation. 172003;107(23 suppl 1):I9–I16.
- ,,.Infectious diseases and mortality among US nursing home residents.Am J Public Health.1993;83(12):1739–1742.
- ,,,,.Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long‐term care facilities.Arch Intern Med. 271999;159(17):2058–2064.
- ,,,.Positive effects of physiotherapy on chronic pain and performance in osteoporosis.Osteoporos Int.1998;8(3):215–221.
- ,,, et al.Health professional's guide to rehabilitation of the patient with osteoporosis.Osteoporos Int.2003;14(suppl 2):S1–S22.
- ,,,.Vertebral compression fractures: manage aggressively to prevent sequelae.Cleve Clin J Med.2003;70(2):147–156. Reprinted with permission. Copyright (c) 2003 Cleveland Clinic Foundation. All rights reserved.
- ,,,.Vertebroplasty in the United States: guidance method and provider distribution, 2001–2003.Radiology.2007;243(1):166–170.
- ,,,,.Thoracic and lumbar vertebroplasties performed in US Medicare enrollees, 2001–2005.JAMA.2007;298(15):1760–1762.
- ,,.Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety.Spine.2006;31(23):2747–2755.
- ,,,,.Efficacy and safety of balloon kyphoplasty in the treatment of vertebral compression fractures: a systematic review.Eur Spine J.2006;15(7):1050–1067.
- ,,,.Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies.Spine.2006;31(17):1983–2001.
- ,,, et al.Percutaneous vertebroplasty compared with optimal pain medication treatment: short‐term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study.AJNR Am J Neuroradiol.2007;28(3):555–560.
- ,.Recurrent fracture after vertebral kyphoplasty.Spine J.2006;6(5):488–493.
- ,.Does vertebroplasty cause incident vertebral fractures? A review of available data.AJNR Am J Neuroradiol.2006;27(7):1397–1403.
- Centers for Medicare and Medicaid Services. Agency for Healthcare Research and Quality (AHRQ). Technology Assessment. Percutaneous Kyphoplasty for Vertebral Fractures Caused by Osteoporosis and Malignancy, 2005. Available at: http://www.cms.hhs.gov/mcd/viewtechassess.asp?from2=viewtechassess.asp13(5):550–555.
- .Randomized vertebroplasty trials: current status and challenges.Acad Radiol.2006;13(5):546–549.
- ,,, et al.VERTOS II: percutaneous vertebroplasty versus conservative therapy in patients with painful osteoporotic vertebral compression fractures; rationale, objectives and design of a multicenter randomized controlled trial.Trials.2007;8(1):33.
- ,.Vertebroplasty: a promising but as yet unproven intervention for painful osteoporotic spinal fractures.Med J Aust.2006;185(7):351–352.
- ,,, et al.Low frequency of treatment of osteoporosis among postmenopausal women following a fracture.Arch Intern Med.2003;163(17):2052–2057.
- ,,,,.Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture.Am J Med.2000;109(4):326–328.
- ,,.Missing a therapeutic window of opportunity: an audit of patients attending a tertiary teaching hospital with potentially osteoporotic hip and wrist fractures.J Rheumatol.2001;28(11):2504–2508.
- ,,,,.Underuse of osteoporosis medications in elderly patients with fractures.Am J Med.2003;115(5):398–400.
- ,,, et al.Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group.Lancet.1996;348(9041):1535–1541.
- ,,,,,.Meta‐analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta‐analyses of therapies for postmenopausal osteoporosis.Endocr Rev.2002;23(4):570–578.
- ,,, et al.Summary of meta‐analyses of therapies for postmenopausal osteoporosis and the relationship between bone density and fractures.Endocrinol Metab Clin North Am.2002;31(3):659–679, xii.
- ,,, et al.Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group.JAMA.1999;282(14):1344–1352.
- ,,, et al.Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group.N Engl J Med.2001;344(5):333–340.
- ,,, et al.Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis.J Bone Miner Res.2004;19(8):1241–1249.
- ,,, et al.Once‐yearly zoledronic acid for treatment of postmenopausal osteoporosis.N Engl J Med. 32007;356(18):1809–1822.
- .Clinical practice. Postmenopausal osteoporosis.N Engl J Med.2005;353(6):595–603.
- American College of Rheumatology Ad Hoc Committee on Glucocorticoid‐Induced Osteoporosis.Recommendations for the prevention and treatment of glucocorticoid‐induced osteoporosis: 2001 update. [Review].Arthritis Rheum.2001;44(7):1496–1503.
- ,,, et al.An osteoporosis and fracture intervention program increases the diagnosis and treatment for osteoporosis for patients with minimal trauma fractures.Jt Comm J Qual Patient Saf.2005;31(5):267–274.
An 89‐year‐old female presents to the Emergency Department with lower back pain for the past 5 days. The patient has a past medical history of polymyalgia rheumatica and hypothyroidism. Her medications include prednisone 10 mg daily and levothyroxine 50 g daily. Aside from tenderness over the third lumbar vertebra, her physical exam is unremarkable. An x‐ray shows a fracture of the third lumbar vertebra. Basic laboratory studies, including calcium and creatinine are normal.
The Clinical Problem and Impact
Vertebral fractures (often termed vertebral compression fractures) affect approximately 25% of all postmenopausal women.1, 2 Only one‐third of vertebral fractures are brought to medical attention.3, 4 In the remaining two‐thirds, patients are either asymptomatic or do not seek medical attention. The lifetime risk of a clinical vertebral fracture is approximately 16% and 5% in white women and men, respectively.1 The risk of vertebral fracture increases with age, lower bone mineral density (BMD), and prior vertebral fracture.1, 5 Women with a preexisting vertebral fracture have a 5‐fold increased risk for a new vertebral fracture relative to those without a history of vertebral fracture.5, 6 Approximately 20% of women who sustain a vertebral fracture will have a new vertebral fracture in the subsequent year.5, 6 Vertebral fractures are frequently overlooked on chest x‐rays and hence there is a need for increased awareness and improved recognition of radiographically‐demonstrated fractures.7 Hospitalists must appreciate that a vertebral fracture is often the first clue to underlying osteoporosis. At least 90% of vertebral and hip fractures are attributable to osteoporosis.5
Typically, the pain related to an acute vertebral fracture improves over 4 to 6 weeks.8, 9 However, pain can persist, resulting in functional impairment, and a decline in the quality of life.1013 Vertebral fractures may lead to kyphosis and reduced lung function. This, in turn, may increase the risk for pneumonia, the most common cause of death in patients with osteoporosis.4 Both clinical and subclinical vertebral fractures are, in fact, independently associated with increased mortality,4, 14, 15 particularly in the period immediately following the event.16 The economic impact of osteoporosis and its related fractures is substantial.1719 In 1995, the annual direct medical cost for the inpatient care of vertebral fractures was estimated to be $575 million.19
Evidence‐Based Approach to the Hospitalized Patient
Evaluation
Vertebral fractures most commonly occur between T7 and L4. Acute vertebral fracture pain is typically sudden in onset and located in the mid to lower back. The pain may occur while performing an ordinary task such as lifting an object or bending over, although in many cases there is no preceding trauma. Physical activity exacerbates the pain and patients' movements may be limited due to pain. Spinal tenderness is usually present. A history of weight loss, prior malignancy, or fever should serve as red flags to the hospitalist and prompt evaluation for underlying malignancy or infection.
For a suspected fracture, frontal and lateral radiographs of the thoracolumbar spine are the initial imaging of choice. Magnetic resonance imaging (MRI) is useful when infection, malignancy, or spinal cord compression is suspected. The presence of neurological deficits should always prompt imaging with MRI or computed tomography (CT).
Patients with vertebral fractures should be evaluated with bone density testing, as osteoporosis is usually the underlying etiology. Because a number of medical conditions commonly contribute to bone loss (Table 1), laboratory testing is indicated for most patients. Although debate exists as to the optimal testing strategy,20 the National Osteoporosis Foundation guidelines recommend the evaluation of blood count, chemistry, and thyroid‐stimulating hormone (TSH) in patients with osteoporosis.21 Because vitamin D deficiency is common in patients who sustain osteoporotic fractures, 25‐hydroxyvitamin D levels should be checked in most patients.2225 Depending on the clinical scenario, additional testing may include the following: serum testosterone, serum intact parathyroid hormone (PTH), 24‐hour calcium excretion, serum protein electrophoresis, urine protein electrophoresis, erythrocyte sedimentation rate, and celiac sprue antibodies.26
| Endocrine disease or metabolic causes | Hypogonadism |
| Cushing's syndrome | |
| Hyperthyroidism | |
| Anorexia nervosa | |
| Hyperparathyroidism | |
| Nutritional conditions | Vitamin D deficiency |
| Calcium deficiency | |
| Malabsorption | |
| Drugs | Glucocorticoids |
| Antiepileptic drugs | |
| Excessive thyroid medication | |
| Long‐term heparin or low molecular weight heparin therapy (eg, >1 month) | |
| Other | Multiple myeloma |
| Rheumatoid arthritis | |
| Organ transplantation |
Management
Short Term
Short‐term management goals include the relief of pain and recovery of mobility. Nonsteroidal antiinflammatory drugs (NSAIDs) and low‐dose opioid medications should be used first in an effort to relieve pain. Beyond its potential effect on bone density, calcitonin (Miacalcin, Fortical) has long been used in acute vertebral fractures for analgesia. A systematic review of 5 randomized controlled trials concluded that calcitonin significantly reduced the pain from acute vertebral fractures.27 Calcitonin improved pain as early as 1 week into treatment and the benefit was persistent at 4 weeks. The analgesic mechanism of action for calcitonin is not entirely clear. Proposed mechanisms include increased ‐endorphin release, an antiinflammatory effect, and a direct effect on specific receptors in the central nervous system.27, 28
Pamidronate (Aredia) also has shown efficacy at reducing acute fracture pain. In a double‐blinded trial, Armigeat et al.29 evaluated pamidronate 30 mg daily for 3 days as compared to placebo in 32 patients. Pain scores were improved at 7 and 30 days with pamidronate. The mechanism of analgesia is unclear. Bisphosphonates are known to inhibit osteoclast activity, but may also work by blocking the effect of inflammatory cytokines.29
Early pain relief is critical in order to encourage physical activity. Bed rest should be avoided, as immobility may increase the risk for pressure ulcers, venous thromboembolism, and pneumonia.3033 Although bracing is frequently used in acute vertebral fracture, the modality has not been formally studied. Although also not well studied in the acute setting, physical therapy has been shown to reduce pain and improve functioning for patients with chronic pain from vertebral fracture,34 and is generally recommended.35
Percutaneous vertebral augmentation procedures include vertebroplasty and kyphoplasty. In vertebroplasty, polymethylmethacrylate cement is injected through a needle under fluoroscopic guidance into the collapsed vertebral body. With kyphoplasty, balloon tamps are used to elevate vertebral endplates prior to injection of cement (Figure 1).36 The proposed mechanism of action for both procedures is stabilization of the fracture by the hardened polymethylmethacrylate cement. These procedures are commonly performed by interventional radiologists without the need for general anesthesia; however, depending on the institution, they may be done by orthopedic surgeons, neurosurgeons, or anesthesiologists. The procedure can be performed as an outpatient, if indicated. Although the volume of these procedures has grown dramatically in recent years,37, 38 the quality of evidence supporting their use is relatively weak.3941 Only 1 randomized controlled trial has been published evaluating the potential benefit of vertebroplasty over conservative management.42 Voormolen et al.42 evaluated patients with vertebral fractures and pain refractive to 6 weeks of optimal medical therapy. Patients were treated with vertebroplasty or continuation of medical therapy. Vertebroplasty significantly improved pain initially, but not after 2 weeks. Like the study by Voormolen et al.,42 most studies evaluating percutaneous vertebral augmentation procedures have been conducted on patients with long‐term pain refractory to medical management. One notable exception is a nonrandomized trial published by Diamond et al.6 In that study, 55 patients were treated with vertebroplasty while 24 were treated conservatively. Pain at 24 hours was significantly improved in patients treated with vertebroplasty. At 6 weeks, however, there was no difference among the 2 groups.

The risk of short‐term complications from vertebral augmentation procedures is difficult to assess in light of the small sample sizes and methodological limitations of existing studies. Cement leakage occurs in 40% to 41% of patients treated with vertebroplasty, as compared with 8% to 9% with kyphoplasty.39, 41 Pulmonary emboli occur in 0.6% and 0.01% of patients treated with vertebroplasty and kyphoplasty, respectively, while neurologic complications occur in 0.6% and 0.03% of patients.41 Concern exists about whether percutaneous vertebral augmentation procedures might increase the risk for subsequent fractures,43, 44 as the incidence of new fractures appears to be elevated in the period immediately following the procedure and approximately two‐thirds of new fractures occur in vertebrae adjacent to the augmented vertebra.39, 41, 44 However, the 20% incidence of new vertebral fractures in the year following vertebral augmentation is similar to the fracture rate seen in patients not treated with osteoporosis therapy.44
Assessment of the impact of vertebral augmentation procedures on the cost of care is limited by the lack of high‐quality clinical studies.45, 46 Randomized controlled trials evaluating the benefit and risk of these procedures compared to conservative management are underway.4749 Pending further evidence, these procedures are best reserved for patients who fail to benefit from other measures to control pain and improve mobility.
Long Term
A comprehensive discussion of the long‐term management of osteoporosis is beyond the scope of this work. However, the inpatient setting presents an opportune time to initiate long‐term medical therapy. Studies show that the majority of patients who sustain osteoporotic fractures do not receive pharmacologic treatment for osteoporosis.5053 Hospitalists have the opportunity to start medications that can reduce the risk for subsequent fracture by nearly 50%.5458 A total calcium intake of 1200 to 1500 mg per day and vitamin D of 400 to 800 IU per day are recommended for all postmenopausal women. Patients who smoke should receive smoking cessation counseling and be considered for pharmacologic treatment for tobacco dependence. All patients should be assessed for fall risk, including a review of medications and assessment of alcohol intake.
Before considering pharmacologic treatment for osteoporosis, secondary causes of low bone mass must be excluded. Bisphosphonates are generally considered first‐line pharmacologic therapy for osteoporosis. Alendronate (Fosamax), risedronate (Actonel), and ibandronate (Boniva) have been shown in randomized trials to increase bone density and reduce the risk of osteoporotic fractures.55, 56, 59 Daily, weekly, and monthly preparations of bisphosphonates now exist. Pill‐induced esophagitis is a potential adverse effect of bisphosphonate therapy, but is extremely rare if proper precautions are taken. Patients should take oral bisphosphonates on an empty stomach, with a full glass of water, sitting upright, and have nothing to eat or drink for at least one half hour. If compliance with oral bisphosphonates is not possible, or esophageal abnormalities preclude oral bisphosphonate use, one may consider the use of intravenous ibandronate or zoledronic acid (Reclast). A 3‐year randomized controlled trial of yearly zoledronic acid improved bone density and reduced the incidence of osteoporotic fractures.60 Bisphosphonates are generally not recommended when creatinine clearance is less than 30 mL/minute. Other pharmacologic options for the treatment of osteoporosis include selective estrogen receptor modulators and anabolic agents. The reader is referred to an excellent review by Rosen61 for additional discussion of these therapies. The American College of Rheumatology clinical guidelines for the management of glucocorticoid induced osteoporosis are also worthy of review.62
Hospitalists are naturally suited to improve the quality of care for patients hospitalized with vertebral fractures. Most patients who currently sustain osteoporotic fractures do not receive appropriate evaluation and treatment. One study used an interdisciplinary team to identify, assess, and begin treatment for appropriate patients hospitalized with osteoporotic fractures.63 The intervention resulted in significantly more patients taking osteoporosis treatment medications 6 months after the incident fracture.
Conclusions
Acute vertebral fracture is a common clinical problem associated with significant morbidity and increased risk of mortality. Treatment of vertebral fracture should include analgesics and physical therapy. Percutaneous augmentation procedures may be considered in patients who fail optimal medical therapy. Because most vertebral fractures are due to osteoporosis and the healthcare system currently fails to appropriately assess and treat most patients who have sustained osteoporotic fractures, hospitalists are in an optimal position to initiate long‐term preventative treatment for these patients.
An 89‐year‐old female presents to the Emergency Department with lower back pain for the past 5 days. The patient has a past medical history of polymyalgia rheumatica and hypothyroidism. Her medications include prednisone 10 mg daily and levothyroxine 50 g daily. Aside from tenderness over the third lumbar vertebra, her physical exam is unremarkable. An x‐ray shows a fracture of the third lumbar vertebra. Basic laboratory studies, including calcium and creatinine are normal.
The Clinical Problem and Impact
Vertebral fractures (often termed vertebral compression fractures) affect approximately 25% of all postmenopausal women.1, 2 Only one‐third of vertebral fractures are brought to medical attention.3, 4 In the remaining two‐thirds, patients are either asymptomatic or do not seek medical attention. The lifetime risk of a clinical vertebral fracture is approximately 16% and 5% in white women and men, respectively.1 The risk of vertebral fracture increases with age, lower bone mineral density (BMD), and prior vertebral fracture.1, 5 Women with a preexisting vertebral fracture have a 5‐fold increased risk for a new vertebral fracture relative to those without a history of vertebral fracture.5, 6 Approximately 20% of women who sustain a vertebral fracture will have a new vertebral fracture in the subsequent year.5, 6 Vertebral fractures are frequently overlooked on chest x‐rays and hence there is a need for increased awareness and improved recognition of radiographically‐demonstrated fractures.7 Hospitalists must appreciate that a vertebral fracture is often the first clue to underlying osteoporosis. At least 90% of vertebral and hip fractures are attributable to osteoporosis.5
Typically, the pain related to an acute vertebral fracture improves over 4 to 6 weeks.8, 9 However, pain can persist, resulting in functional impairment, and a decline in the quality of life.1013 Vertebral fractures may lead to kyphosis and reduced lung function. This, in turn, may increase the risk for pneumonia, the most common cause of death in patients with osteoporosis.4 Both clinical and subclinical vertebral fractures are, in fact, independently associated with increased mortality,4, 14, 15 particularly in the period immediately following the event.16 The economic impact of osteoporosis and its related fractures is substantial.1719 In 1995, the annual direct medical cost for the inpatient care of vertebral fractures was estimated to be $575 million.19
Evidence‐Based Approach to the Hospitalized Patient
Evaluation
Vertebral fractures most commonly occur between T7 and L4. Acute vertebral fracture pain is typically sudden in onset and located in the mid to lower back. The pain may occur while performing an ordinary task such as lifting an object or bending over, although in many cases there is no preceding trauma. Physical activity exacerbates the pain and patients' movements may be limited due to pain. Spinal tenderness is usually present. A history of weight loss, prior malignancy, or fever should serve as red flags to the hospitalist and prompt evaluation for underlying malignancy or infection.
For a suspected fracture, frontal and lateral radiographs of the thoracolumbar spine are the initial imaging of choice. Magnetic resonance imaging (MRI) is useful when infection, malignancy, or spinal cord compression is suspected. The presence of neurological deficits should always prompt imaging with MRI or computed tomography (CT).
Patients with vertebral fractures should be evaluated with bone density testing, as osteoporosis is usually the underlying etiology. Because a number of medical conditions commonly contribute to bone loss (Table 1), laboratory testing is indicated for most patients. Although debate exists as to the optimal testing strategy,20 the National Osteoporosis Foundation guidelines recommend the evaluation of blood count, chemistry, and thyroid‐stimulating hormone (TSH) in patients with osteoporosis.21 Because vitamin D deficiency is common in patients who sustain osteoporotic fractures, 25‐hydroxyvitamin D levels should be checked in most patients.2225 Depending on the clinical scenario, additional testing may include the following: serum testosterone, serum intact parathyroid hormone (PTH), 24‐hour calcium excretion, serum protein electrophoresis, urine protein electrophoresis, erythrocyte sedimentation rate, and celiac sprue antibodies.26
| Endocrine disease or metabolic causes | Hypogonadism |
| Cushing's syndrome | |
| Hyperthyroidism | |
| Anorexia nervosa | |
| Hyperparathyroidism | |
| Nutritional conditions | Vitamin D deficiency |
| Calcium deficiency | |
| Malabsorption | |
| Drugs | Glucocorticoids |
| Antiepileptic drugs | |
| Excessive thyroid medication | |
| Long‐term heparin or low molecular weight heparin therapy (eg, >1 month) | |
| Other | Multiple myeloma |
| Rheumatoid arthritis | |
| Organ transplantation |
Management
Short Term
Short‐term management goals include the relief of pain and recovery of mobility. Nonsteroidal antiinflammatory drugs (NSAIDs) and low‐dose opioid medications should be used first in an effort to relieve pain. Beyond its potential effect on bone density, calcitonin (Miacalcin, Fortical) has long been used in acute vertebral fractures for analgesia. A systematic review of 5 randomized controlled trials concluded that calcitonin significantly reduced the pain from acute vertebral fractures.27 Calcitonin improved pain as early as 1 week into treatment and the benefit was persistent at 4 weeks. The analgesic mechanism of action for calcitonin is not entirely clear. Proposed mechanisms include increased ‐endorphin release, an antiinflammatory effect, and a direct effect on specific receptors in the central nervous system.27, 28
Pamidronate (Aredia) also has shown efficacy at reducing acute fracture pain. In a double‐blinded trial, Armigeat et al.29 evaluated pamidronate 30 mg daily for 3 days as compared to placebo in 32 patients. Pain scores were improved at 7 and 30 days with pamidronate. The mechanism of analgesia is unclear. Bisphosphonates are known to inhibit osteoclast activity, but may also work by blocking the effect of inflammatory cytokines.29
Early pain relief is critical in order to encourage physical activity. Bed rest should be avoided, as immobility may increase the risk for pressure ulcers, venous thromboembolism, and pneumonia.3033 Although bracing is frequently used in acute vertebral fracture, the modality has not been formally studied. Although also not well studied in the acute setting, physical therapy has been shown to reduce pain and improve functioning for patients with chronic pain from vertebral fracture,34 and is generally recommended.35
Percutaneous vertebral augmentation procedures include vertebroplasty and kyphoplasty. In vertebroplasty, polymethylmethacrylate cement is injected through a needle under fluoroscopic guidance into the collapsed vertebral body. With kyphoplasty, balloon tamps are used to elevate vertebral endplates prior to injection of cement (Figure 1).36 The proposed mechanism of action for both procedures is stabilization of the fracture by the hardened polymethylmethacrylate cement. These procedures are commonly performed by interventional radiologists without the need for general anesthesia; however, depending on the institution, they may be done by orthopedic surgeons, neurosurgeons, or anesthesiologists. The procedure can be performed as an outpatient, if indicated. Although the volume of these procedures has grown dramatically in recent years,37, 38 the quality of evidence supporting their use is relatively weak.3941 Only 1 randomized controlled trial has been published evaluating the potential benefit of vertebroplasty over conservative management.42 Voormolen et al.42 evaluated patients with vertebral fractures and pain refractive to 6 weeks of optimal medical therapy. Patients were treated with vertebroplasty or continuation of medical therapy. Vertebroplasty significantly improved pain initially, but not after 2 weeks. Like the study by Voormolen et al.,42 most studies evaluating percutaneous vertebral augmentation procedures have been conducted on patients with long‐term pain refractory to medical management. One notable exception is a nonrandomized trial published by Diamond et al.6 In that study, 55 patients were treated with vertebroplasty while 24 were treated conservatively. Pain at 24 hours was significantly improved in patients treated with vertebroplasty. At 6 weeks, however, there was no difference among the 2 groups.

The risk of short‐term complications from vertebral augmentation procedures is difficult to assess in light of the small sample sizes and methodological limitations of existing studies. Cement leakage occurs in 40% to 41% of patients treated with vertebroplasty, as compared with 8% to 9% with kyphoplasty.39, 41 Pulmonary emboli occur in 0.6% and 0.01% of patients treated with vertebroplasty and kyphoplasty, respectively, while neurologic complications occur in 0.6% and 0.03% of patients.41 Concern exists about whether percutaneous vertebral augmentation procedures might increase the risk for subsequent fractures,43, 44 as the incidence of new fractures appears to be elevated in the period immediately following the procedure and approximately two‐thirds of new fractures occur in vertebrae adjacent to the augmented vertebra.39, 41, 44 However, the 20% incidence of new vertebral fractures in the year following vertebral augmentation is similar to the fracture rate seen in patients not treated with osteoporosis therapy.44
Assessment of the impact of vertebral augmentation procedures on the cost of care is limited by the lack of high‐quality clinical studies.45, 46 Randomized controlled trials evaluating the benefit and risk of these procedures compared to conservative management are underway.4749 Pending further evidence, these procedures are best reserved for patients who fail to benefit from other measures to control pain and improve mobility.
Long Term
A comprehensive discussion of the long‐term management of osteoporosis is beyond the scope of this work. However, the inpatient setting presents an opportune time to initiate long‐term medical therapy. Studies show that the majority of patients who sustain osteoporotic fractures do not receive pharmacologic treatment for osteoporosis.5053 Hospitalists have the opportunity to start medications that can reduce the risk for subsequent fracture by nearly 50%.5458 A total calcium intake of 1200 to 1500 mg per day and vitamin D of 400 to 800 IU per day are recommended for all postmenopausal women. Patients who smoke should receive smoking cessation counseling and be considered for pharmacologic treatment for tobacco dependence. All patients should be assessed for fall risk, including a review of medications and assessment of alcohol intake.
Before considering pharmacologic treatment for osteoporosis, secondary causes of low bone mass must be excluded. Bisphosphonates are generally considered first‐line pharmacologic therapy for osteoporosis. Alendronate (Fosamax), risedronate (Actonel), and ibandronate (Boniva) have been shown in randomized trials to increase bone density and reduce the risk of osteoporotic fractures.55, 56, 59 Daily, weekly, and monthly preparations of bisphosphonates now exist. Pill‐induced esophagitis is a potential adverse effect of bisphosphonate therapy, but is extremely rare if proper precautions are taken. Patients should take oral bisphosphonates on an empty stomach, with a full glass of water, sitting upright, and have nothing to eat or drink for at least one half hour. If compliance with oral bisphosphonates is not possible, or esophageal abnormalities preclude oral bisphosphonate use, one may consider the use of intravenous ibandronate or zoledronic acid (Reclast). A 3‐year randomized controlled trial of yearly zoledronic acid improved bone density and reduced the incidence of osteoporotic fractures.60 Bisphosphonates are generally not recommended when creatinine clearance is less than 30 mL/minute. Other pharmacologic options for the treatment of osteoporosis include selective estrogen receptor modulators and anabolic agents. The reader is referred to an excellent review by Rosen61 for additional discussion of these therapies. The American College of Rheumatology clinical guidelines for the management of glucocorticoid induced osteoporosis are also worthy of review.62
Hospitalists are naturally suited to improve the quality of care for patients hospitalized with vertebral fractures. Most patients who currently sustain osteoporotic fractures do not receive appropriate evaluation and treatment. One study used an interdisciplinary team to identify, assess, and begin treatment for appropriate patients hospitalized with osteoporotic fractures.63 The intervention resulted in significantly more patients taking osteoporosis treatment medications 6 months after the incident fracture.
Conclusions
Acute vertebral fracture is a common clinical problem associated with significant morbidity and increased risk of mortality. Treatment of vertebral fracture should include analgesics and physical therapy. Percutaneous augmentation procedures may be considered in patients who fail optimal medical therapy. Because most vertebral fractures are due to osteoporosis and the healthcare system currently fails to appropriately assess and treat most patients who have sustained osteoporotic fractures, hospitalists are in an optimal position to initiate long‐term preventative treatment for these patients.
- .Epidemiology of spinal osteoporosis.Spine.1997;22(24 suppl):2S–11S.
- ,,,,,.Prevalence and incidence of vertebral deformities.Osteoporos Int.1993;3(3):113–119.
- ,,.The epidemiology of vertebral fractures. European Vertebral Osteoporosis Study Group.Bone.1993;14(suppl 1):S89–S97.
- ,,,,,.Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group.Arch Intern Med.1999;159(11):1215–1220.
- ,,,.Pre‐existing fractures and bone mass predict vertebral fracture incidence in women.Ann Intern Med.1991;114(11):919–923.
- ,,, et al.Risk of new vertebral fracture in the year following a fracture.JAMA.2001;285(3):320–323.
- ,,,,,.Recognition of vertebral fracture in a clinical setting.Osteoporos Int.2000;11(7):577–582.
- ,,.Management of acute osteoporotic vertebral fractures: a nonrandomized trial comparing percutaneous vertebroplasty with conservative therapy.Am J Med.2003;114(4):257–265.
- .The clinical consequences of vertebral compression fracture.Bone.1992;13(suppl 2):S27–S31.
- ,,,.A case‐control study of quality of life and functional impairment in women with long‐standing vertebral osteoporotic fracture.Osteoporos Int.1999;9(6):508–515.
- ,,, et al.The burden of prevalent fractures on health‐related quality of life in postmenopausal women with osteoporosis: the IMOF study.J Rheumatol.2007;34(7):1551–1560.
- ,,,,.The relationship of health‐related quality of life to prevalent and incident vertebral fractures in postmenopausal women with osteoporosis: results from the Multiple Outcomes of Raloxifene Evaluation Study.Arthritis Rheum2001;44(11):2611–2619.
- ,,, et al.The association of radiographically detected vertebral fractures with back pain and function: a prospective study.Ann Int Med.1998;128:793–800.
- ,,,,.Mortality after all major types of osteoporotic fracture in men and women: an observational study.Lancet. 131999;353(9156):878–882.
- ,,,,.Population‐based study of survival after osteoporotic fractures.Am J Epidemiol.1993;137(9):1001–1005.
- ,,,,.Excess mortality after hospitalisation for vertebral fracture.Osteoporos Int.2004;15(2):108–112.
- ,.The cost of treating osteoporotic fractures in the United Kingdom female population.Osteoporos Int.1998;8(6):611–617.
- ,,, et al.Direct medical costs attributable to osteoporotic fractures.Osteoporos Int.2002;13(4):323–330.
- ,,,.Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation.J Bone Miner Res.1997;12(1):24–35.
- .Laboratory workup for osteoporosis. Which tests are most cost‐effective?Postgrad Med.2003;114(3):35–38,41–34.
- National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Available at: http://www.nof.org/professionals/Clinicians_Guide.htm. Accessed February2009.
- ,,, et al.Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy.J Clin Endocr Metab.2005;90:3215–3224.
- ,,,,.Prevalence of vitamin D inadequacy in a minimal trauma fracture population.Curr Med Res Opin.2005;21:1069–1074.
- ,,,,,.Occult vitamin D deficiency in postmenopausal US women with acute hip fracture.JAMA.1999;281:1505–1511.
- ,,,,.Secondary contributors for bone loss in osteoporotic hip fractures.Osteoporos Int.2008;19(7):991–999.
- .Evaluation of the patient with osteoporosis or at risk for osteoporosis. In: Marcus R, Feldman D, Kelsey J, eds.Osteoporosis. Vol.2.San Diego:Academic Press;2001:403–408.
- ,,,,.Calcitonin for treating acute pain of osteoporotic vertebral compression fractures: a systematic review of randomized, controlled trials.Osteoporos Int.2005;16(10):1281–1290.
- .Possible mechanisms of the analgesic action of calcitonin.Bone.2002;30(5 suppl):80S–83S.
- ,,,,.Intravenous pamidronate for pain relief in recent osteoporotic vertebral compression fracture: a randomized double‐blind controlled study.Osteoporos Int.2006;17(11):1659–1665.
- ,,,,.Pressure ulcer risk factors among hospitalized patients with activity limitation.JAMA.1995;273(11):865–870.
- ,.Risk factors for venous thromboembolism.Circulation. 172003;107(23 suppl 1):I9–I16.
- ,,.Infectious diseases and mortality among US nursing home residents.Am J Public Health.1993;83(12):1739–1742.
- ,,,,.Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long‐term care facilities.Arch Intern Med. 271999;159(17):2058–2064.
- ,,,.Positive effects of physiotherapy on chronic pain and performance in osteoporosis.Osteoporos Int.1998;8(3):215–221.
- ,,, et al.Health professional's guide to rehabilitation of the patient with osteoporosis.Osteoporos Int.2003;14(suppl 2):S1–S22.
- ,,,.Vertebral compression fractures: manage aggressively to prevent sequelae.Cleve Clin J Med.2003;70(2):147–156. Reprinted with permission. Copyright (c) 2003 Cleveland Clinic Foundation. All rights reserved.
- ,,,.Vertebroplasty in the United States: guidance method and provider distribution, 2001–2003.Radiology.2007;243(1):166–170.
- ,,,,.Thoracic and lumbar vertebroplasties performed in US Medicare enrollees, 2001–2005.JAMA.2007;298(15):1760–1762.
- ,,.Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety.Spine.2006;31(23):2747–2755.
- ,,,,.Efficacy and safety of balloon kyphoplasty in the treatment of vertebral compression fractures: a systematic review.Eur Spine J.2006;15(7):1050–1067.
- ,,,.Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies.Spine.2006;31(17):1983–2001.
- ,,, et al.Percutaneous vertebroplasty compared with optimal pain medication treatment: short‐term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study.AJNR Am J Neuroradiol.2007;28(3):555–560.
- ,.Recurrent fracture after vertebral kyphoplasty.Spine J.2006;6(5):488–493.
- ,.Does vertebroplasty cause incident vertebral fractures? A review of available data.AJNR Am J Neuroradiol.2006;27(7):1397–1403.
- Centers for Medicare and Medicaid Services. Agency for Healthcare Research and Quality (AHRQ). Technology Assessment. Percutaneous Kyphoplasty for Vertebral Fractures Caused by Osteoporosis and Malignancy, 2005. Available at: http://www.cms.hhs.gov/mcd/viewtechassess.asp?from2=viewtechassess.asp13(5):550–555.
- .Randomized vertebroplasty trials: current status and challenges.Acad Radiol.2006;13(5):546–549.
- ,,, et al.VERTOS II: percutaneous vertebroplasty versus conservative therapy in patients with painful osteoporotic vertebral compression fractures; rationale, objectives and design of a multicenter randomized controlled trial.Trials.2007;8(1):33.
- ,.Vertebroplasty: a promising but as yet unproven intervention for painful osteoporotic spinal fractures.Med J Aust.2006;185(7):351–352.
- ,,, et al.Low frequency of treatment of osteoporosis among postmenopausal women following a fracture.Arch Intern Med.2003;163(17):2052–2057.
- ,,,,.Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture.Am J Med.2000;109(4):326–328.
- ,,.Missing a therapeutic window of opportunity: an audit of patients attending a tertiary teaching hospital with potentially osteoporotic hip and wrist fractures.J Rheumatol.2001;28(11):2504–2508.
- ,,,,.Underuse of osteoporosis medications in elderly patients with fractures.Am J Med.2003;115(5):398–400.
- ,,, et al.Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group.Lancet.1996;348(9041):1535–1541.
- ,,,,,.Meta‐analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta‐analyses of therapies for postmenopausal osteoporosis.Endocr Rev.2002;23(4):570–578.
- ,,, et al.Summary of meta‐analyses of therapies for postmenopausal osteoporosis and the relationship between bone density and fractures.Endocrinol Metab Clin North Am.2002;31(3):659–679, xii.
- ,,, et al.Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group.JAMA.1999;282(14):1344–1352.
- ,,, et al.Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group.N Engl J Med.2001;344(5):333–340.
- ,,, et al.Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis.J Bone Miner Res.2004;19(8):1241–1249.
- ,,, et al.Once‐yearly zoledronic acid for treatment of postmenopausal osteoporosis.N Engl J Med. 32007;356(18):1809–1822.
- .Clinical practice. Postmenopausal osteoporosis.N Engl J Med.2005;353(6):595–603.
- American College of Rheumatology Ad Hoc Committee on Glucocorticoid‐Induced Osteoporosis.Recommendations for the prevention and treatment of glucocorticoid‐induced osteoporosis: 2001 update. [Review].Arthritis Rheum.2001;44(7):1496–1503.
- ,,, et al.An osteoporosis and fracture intervention program increases the diagnosis and treatment for osteoporosis for patients with minimal trauma fractures.Jt Comm J Qual Patient Saf.2005;31(5):267–274.
- .Epidemiology of spinal osteoporosis.Spine.1997;22(24 suppl):2S–11S.
- ,,,,,.Prevalence and incidence of vertebral deformities.Osteoporos Int.1993;3(3):113–119.
- ,,.The epidemiology of vertebral fractures. European Vertebral Osteoporosis Study Group.Bone.1993;14(suppl 1):S89–S97.
- ,,,,,.Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group.Arch Intern Med.1999;159(11):1215–1220.
- ,,,.Pre‐existing fractures and bone mass predict vertebral fracture incidence in women.Ann Intern Med.1991;114(11):919–923.
- ,,, et al.Risk of new vertebral fracture in the year following a fracture.JAMA.2001;285(3):320–323.
- ,,,,,.Recognition of vertebral fracture in a clinical setting.Osteoporos Int.2000;11(7):577–582.
- ,,.Management of acute osteoporotic vertebral fractures: a nonrandomized trial comparing percutaneous vertebroplasty with conservative therapy.Am J Med.2003;114(4):257–265.
- .The clinical consequences of vertebral compression fracture.Bone.1992;13(suppl 2):S27–S31.
- ,,,.A case‐control study of quality of life and functional impairment in women with long‐standing vertebral osteoporotic fracture.Osteoporos Int.1999;9(6):508–515.
- ,,, et al.The burden of prevalent fractures on health‐related quality of life in postmenopausal women with osteoporosis: the IMOF study.J Rheumatol.2007;34(7):1551–1560.
- ,,,,.The relationship of health‐related quality of life to prevalent and incident vertebral fractures in postmenopausal women with osteoporosis: results from the Multiple Outcomes of Raloxifene Evaluation Study.Arthritis Rheum2001;44(11):2611–2619.
- ,,, et al.The association of radiographically detected vertebral fractures with back pain and function: a prospective study.Ann Int Med.1998;128:793–800.
- ,,,,.Mortality after all major types of osteoporotic fracture in men and women: an observational study.Lancet. 131999;353(9156):878–882.
- ,,,,.Population‐based study of survival after osteoporotic fractures.Am J Epidemiol.1993;137(9):1001–1005.
- ,,,,.Excess mortality after hospitalisation for vertebral fracture.Osteoporos Int.2004;15(2):108–112.
- ,.The cost of treating osteoporotic fractures in the United Kingdom female population.Osteoporos Int.1998;8(6):611–617.
- ,,, et al.Direct medical costs attributable to osteoporotic fractures.Osteoporos Int.2002;13(4):323–330.
- ,,,.Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation.J Bone Miner Res.1997;12(1):24–35.
- .Laboratory workup for osteoporosis. Which tests are most cost‐effective?Postgrad Med.2003;114(3):35–38,41–34.
- National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Available at: http://www.nof.org/professionals/Clinicians_Guide.htm. Accessed February2009.
- ,,, et al.Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy.J Clin Endocr Metab.2005;90:3215–3224.
- ,,,,.Prevalence of vitamin D inadequacy in a minimal trauma fracture population.Curr Med Res Opin.2005;21:1069–1074.
- ,,,,,.Occult vitamin D deficiency in postmenopausal US women with acute hip fracture.JAMA.1999;281:1505–1511.
- ,,,,.Secondary contributors for bone loss in osteoporotic hip fractures.Osteoporos Int.2008;19(7):991–999.
- .Evaluation of the patient with osteoporosis or at risk for osteoporosis. In: Marcus R, Feldman D, Kelsey J, eds.Osteoporosis. Vol.2.San Diego:Academic Press;2001:403–408.
- ,,,,.Calcitonin for treating acute pain of osteoporotic vertebral compression fractures: a systematic review of randomized, controlled trials.Osteoporos Int.2005;16(10):1281–1290.
- .Possible mechanisms of the analgesic action of calcitonin.Bone.2002;30(5 suppl):80S–83S.
- ,,,,.Intravenous pamidronate for pain relief in recent osteoporotic vertebral compression fracture: a randomized double‐blind controlled study.Osteoporos Int.2006;17(11):1659–1665.
- ,,,,.Pressure ulcer risk factors among hospitalized patients with activity limitation.JAMA.1995;273(11):865–870.
- ,.Risk factors for venous thromboembolism.Circulation. 172003;107(23 suppl 1):I9–I16.
- ,,.Infectious diseases and mortality among US nursing home residents.Am J Public Health.1993;83(12):1739–1742.
- ,,,,.Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long‐term care facilities.Arch Intern Med. 271999;159(17):2058–2064.
- ,,,.Positive effects of physiotherapy on chronic pain and performance in osteoporosis.Osteoporos Int.1998;8(3):215–221.
- ,,, et al.Health professional's guide to rehabilitation of the patient with osteoporosis.Osteoporos Int.2003;14(suppl 2):S1–S22.
- ,,,.Vertebral compression fractures: manage aggressively to prevent sequelae.Cleve Clin J Med.2003;70(2):147–156. Reprinted with permission. Copyright (c) 2003 Cleveland Clinic Foundation. All rights reserved.
- ,,,.Vertebroplasty in the United States: guidance method and provider distribution, 2001–2003.Radiology.2007;243(1):166–170.
- ,,,,.Thoracic and lumbar vertebroplasties performed in US Medicare enrollees, 2001–2005.JAMA.2007;298(15):1760–1762.
- ,,.Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety.Spine.2006;31(23):2747–2755.
- ,,,,.Efficacy and safety of balloon kyphoplasty in the treatment of vertebral compression fractures: a systematic review.Eur Spine J.2006;15(7):1050–1067.
- ,,,.Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies.Spine.2006;31(17):1983–2001.
- ,,, et al.Percutaneous vertebroplasty compared with optimal pain medication treatment: short‐term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study.AJNR Am J Neuroradiol.2007;28(3):555–560.
- ,.Recurrent fracture after vertebral kyphoplasty.Spine J.2006;6(5):488–493.
- ,.Does vertebroplasty cause incident vertebral fractures? A review of available data.AJNR Am J Neuroradiol.2006;27(7):1397–1403.
- Centers for Medicare and Medicaid Services. Agency for Healthcare Research and Quality (AHRQ). Technology Assessment. Percutaneous Kyphoplasty for Vertebral Fractures Caused by Osteoporosis and Malignancy, 2005. Available at: http://www.cms.hhs.gov/mcd/viewtechassess.asp?from2=viewtechassess.asp13(5):550–555.
- .Randomized vertebroplasty trials: current status and challenges.Acad Radiol.2006;13(5):546–549.
- ,,, et al.VERTOS II: percutaneous vertebroplasty versus conservative therapy in patients with painful osteoporotic vertebral compression fractures; rationale, objectives and design of a multicenter randomized controlled trial.Trials.2007;8(1):33.
- ,.Vertebroplasty: a promising but as yet unproven intervention for painful osteoporotic spinal fractures.Med J Aust.2006;185(7):351–352.
- ,,, et al.Low frequency of treatment of osteoporosis among postmenopausal women following a fracture.Arch Intern Med.2003;163(17):2052–2057.
- ,,,,.Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture.Am J Med.2000;109(4):326–328.
- ,,.Missing a therapeutic window of opportunity: an audit of patients attending a tertiary teaching hospital with potentially osteoporotic hip and wrist fractures.J Rheumatol.2001;28(11):2504–2508.
- ,,,,.Underuse of osteoporosis medications in elderly patients with fractures.Am J Med.2003;115(5):398–400.
- ,,, et al.Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group.Lancet.1996;348(9041):1535–1541.
- ,,,,,.Meta‐analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta‐analyses of therapies for postmenopausal osteoporosis.Endocr Rev.2002;23(4):570–578.
- ,,, et al.Summary of meta‐analyses of therapies for postmenopausal osteoporosis and the relationship between bone density and fractures.Endocrinol Metab Clin North Am.2002;31(3):659–679, xii.
- ,,, et al.Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group.JAMA.1999;282(14):1344–1352.
- ,,, et al.Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group.N Engl J Med.2001;344(5):333–340.
- ,,, et al.Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis.J Bone Miner Res.2004;19(8):1241–1249.
- ,,, et al.Once‐yearly zoledronic acid for treatment of postmenopausal osteoporosis.N Engl J Med. 32007;356(18):1809–1822.
- .Clinical practice. Postmenopausal osteoporosis.N Engl J Med.2005;353(6):595–603.
- American College of Rheumatology Ad Hoc Committee on Glucocorticoid‐Induced Osteoporosis.Recommendations for the prevention and treatment of glucocorticoid‐induced osteoporosis: 2001 update. [Review].Arthritis Rheum.2001;44(7):1496–1503.
- ,,, et al.An osteoporosis and fracture intervention program increases the diagnosis and treatment for osteoporosis for patients with minimal trauma fractures.Jt Comm J Qual Patient Saf.2005;31(5):267–274.
EHR Enhancements for Physician Assignment
Preserving continuity of care and assuring patient safety are core values of the hospitalist movement.1 Communicating clinical information to the patient, the primary care provider (PCP), and hospital‐based providers has been recognized in the hospitalist literature as an important domain of quality assurance.29 Correctly identifying a patient's inpatient physician during admission and later in the hospital course is essential to achieve these goals. This initial step has not been specifically addressed in the literature, though the complexity of this process can create significant challenges for larger hospitalist programs.
Rochester General Hospital (RGH) is a 528‐bed community teaching hospital. Our hospitalist program evolved from the general medicine unit (GMU) faculty that traditionally cared for most unreferred patients; ie, those whose PCPs had no admitting privileges. In 2004, we began covering patients of privilege‐holder partnering PCPs. Within 3 years, more than 120 privileged PCPs decided to refer all their admissions to us, though unreferred cases still make up the majority of our 10,000 annual admissions. GMU hospitalists either round as teaching attendings or attend patients on nonteaching services. Many PCPs continue to admit their own patients.
As our program began to work with numerous partnering PCPs, it became difficult to decide which patients the hospitalist team should be called to admit, and which should be admitted to the PCP. Having the emergency department (ED) providers page the PCPs or their call partners of the PCPs for attending service decisions proved infeasible and unreliable. Additionally, similar challenges emerged later in the hospitalization when patients were transferred from one service, floor, or provider to another, especially from the intensive care unit (ICU) to the medical service.
Errors regarding admissions to the hospitalist service can be classified as:
Type‐I errors: The PCP provides inpatient care but the patient is erroneously admitted to a hospitalist, creating discontinuity of care and dissatisfaction.
Type‐II errors: The PCP refers to the hospitalists but is erroneously identified as the inpatient attending physician. As the hospitalist team is not notified about these cases, admitted patients may go without physician services for a period of time.
Methods
RGH has a widely used, clinically focused EHR system that does not offer full CPOE functionality. The EHR includes a database of physicians with admitting privileges, so we decided to store information on the system regarding PCPs' hospitalist coverage. Initially, we simply uploaded a list of our partnering PCPs. However, looking up information from files proved too cumbersome for busy ED providers and hospitalists. Additionally, this solution lacked a feedback loop to alert for errors.
Therefore, we designed a system with 3 main functional elements to identify and display each patient's:
candidacy for hospitalist coverage;
actual hospitalist coverage; and
mismatches between the above statuses.
These steps are explained below, followed by a description of the system's actual utilization. In addition to the text, we demonstrate the concept of and provide detailed information on our system in Figure 1 and Table 1.
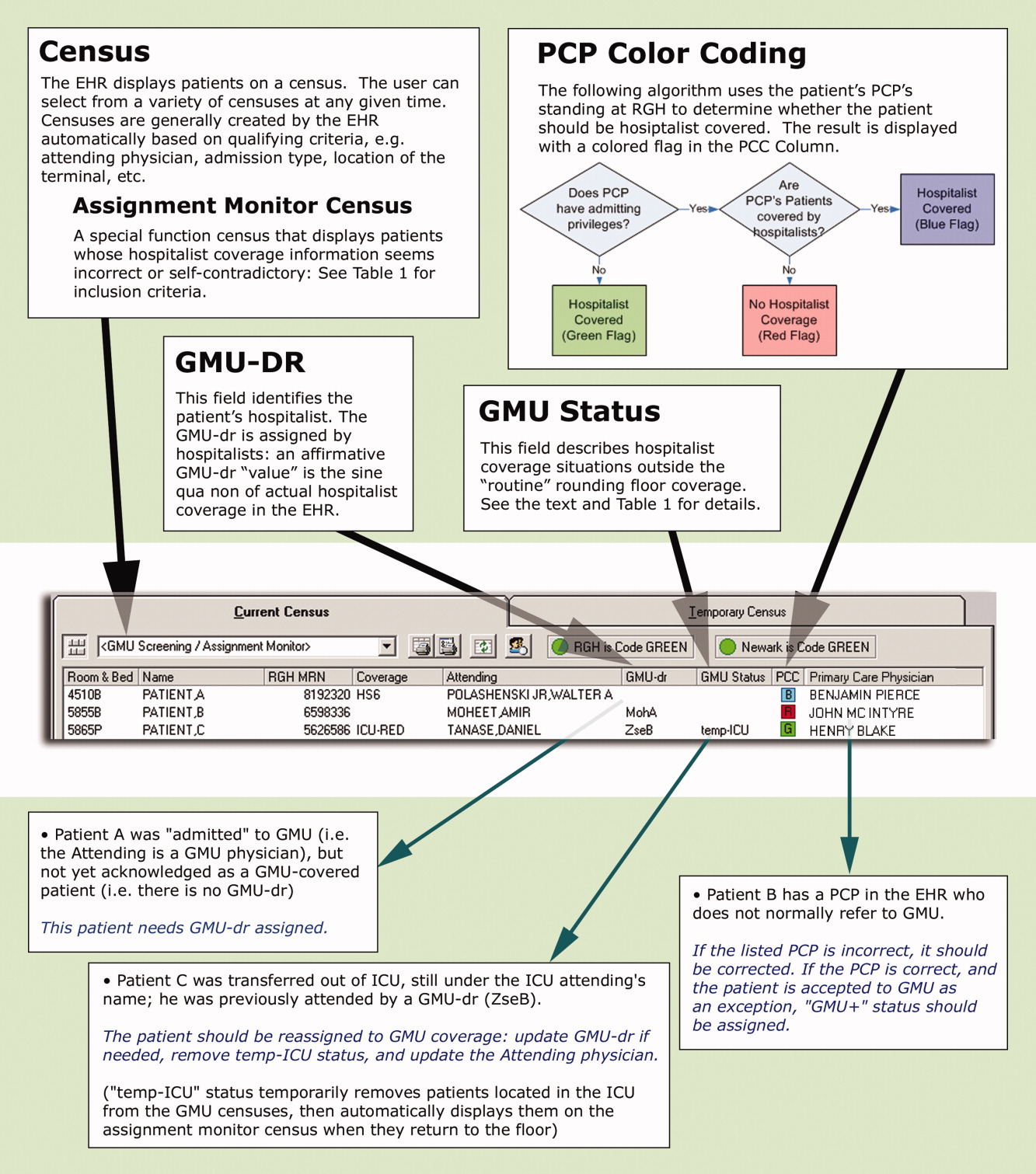
| Algorithms |
| This special census includes patients who meet any of the following criteria: |
| Attending physician is a GMU‐MD and GMU‐dr is {blank} |
| Attending physician is a GMU‐MD and GMU‐status is GMU‐cons, temp‐ICU, or non‐GMU |
| Attending physician is not a GMU‐MD and GMU‐dr is not {blank} and GMU‐status is not GMU‐cons, non‐GMU, or temp‐ICU |
| Attending physician is an ICU‐MD and patient's location is not ED or ICU and GMU‐status is not non‐GMU |
| Patient's status is INT (internal medicine in‐bed) and PCP has no privilege or is a GMU‐PCPCP and GMU‐dr is {blank} and Attending physician is not an ICU‐MD and GMU‐status is not temp‐ICU or non‐GMU |
| PCP has admission privilege and PCP is not a GMU‐PCPCP and GMU‐dr is not {blank} and GMU‐status is not non‐GMU or GMU+ |
| GMU‐status is temp‐ICU and patient's location is not ED or ICU |
| GMU‐dr is {blank} and GMU‐status is GMU‐cons, GMU‐ALC or GMU+ |
| GMU‐dr is ‐TBA, or ‐TRD and present time is between 8 AMand 4 PM |
| Definitions |
| Attending physician and PCP refers to the physicians assigned as attending and PCP, respectively in the core EHR system. |
| Physician categories are defined by listing all belonging members on centrally maintained, up‐to‐date databases: |
| GMU‐PCPCP: partnering community PCP physicians who refer their patients to the GMU hospitalist service |
| GMU‐MD: a GMU hospitalist attending physician |
| ICU‐MD: a critical care physician who attends in ICU, and automatically transfers most patients to hospitalists upon discharge from ICU |
| GMU‐dr signals acceptance to GMU hospitalist service, and names the hospitalist in the EHR. Temporary assignments (TBA or TRD) are used when the patient is accepted to the hospitalist program, but the rounding physician is not yet known (eg, a patient seen by the night hospitalist in the ED, or waiting reassignment from a weekend). Options include: |
| A physician's acronym (from last name and first initial) identifies the rounding hospitalist |
| TBA = to be assigned: accepted to GMU but not yet assigned to a rounding attending (used for new patients during evening and night hours while under the care of the on call team) |
| TRD = to redistribute from an attending's care who is leaving service (used for patients whose follow‐up coverage is distributed in the next morning by the call team) |
| A blank field means no hospitalist service for the patient |
| GMU‐status signals irregular rounding relationship with the patient. The following options are used: |
| Non‐GMU: patient will not be covered by GMU hospitalists (eg, signing off consult, admission to subspecialist service) |
| Temp‐ICU: patient will not be covered by GMU while in ICU (will automatically accept to GMU upon transfer to floor) |
| GMU‐cons: patient is on consultation service (GMU is not the attending service) |
| GMU‐ALC: patient is on alternative level of care (skilled nursing needs: no daily rounding by GMU hospitalist) |
| GMU+: patient is covered by GMU (despite the PCP not normally referring to GMU) |
First, to identify a patient's candidacy for hospitalist coverage, we created a PCP color coding algorithm, based on whether the PCP has admitting privileges at RGH and/or has arranged hospitalist coverage for her/his patients. Whether or not hospitalist coverage is expected for a given patient's PCP is displayed on the EHR.
Second, a data field called GMU‐dr was created to describe whether the patient is actually assigned to a hospitalist. The GMU on‐call physician assigns a GMU‐dr to all appropriate patients (ie, updates the field's value). This step acknowledges hospitalist coverage for a given patient and also identifies the hospitalist physician. It simultaneously adds the patient to the appropriate rounding censuses. The GMU‐dr data field is updated every time the rounding physician changes (eg, for weekend coverage).
Third, the EHR's assignment monitor algorithm compares the expected and actual hospitalist coverage (based on PCP color coding, GMU‐dr, admission type, location, time of day, etc.) and displays mismatches on the assignment monitor census. We created this special‐function census to include patients with the more dangerous type‐II coverage errors that would not show up on our rounding censuses.
The assignment monitor census is regularly reviewed by the call physicians and should be cleared of patients. Unaccounted patients showing up on this census are handled with urgency equal to that of an unseen admission. Most patients on the assignment monitor census have missing or incorrect information: correcting that information removes the patient from this alert list. However, some patients with correct information are captured due to an unusual relationship with the hospitalist program; eg, consultation or individual exceptions from coverage arrangements. The GMU‐status field was created to explain such situations and remove these patients from the alert list.
Results
According to a survey that was distributed to the 19 eligible hospitalists and returned by 17 (89%), this system greatly improved our admission and patient distribution process, with the following results:
PCP color coding prevents type‐I and type‐II coverage identification errors more than once a week according to 94.1% and 88.2% of hospitalists, respectively. Ninety‐four percent agreed (absolutely or strongly) that color coding is a convenient tool to identify PCP referral status.
The assignment monitor identifies patients more than once a month that would have been lost in the preintervention era, according to 94.1% of the hospitalists. All hospitalists surveyed believed that this alert list had several times prevented potentially life‐threatening complications, and that the system is more useful than burdensome.
The GMU‐dr data field correctly identifies the hospitalist while the attending is misassigned in the core EHR system more than once a week, according to 93.7% of surveyed hospitalists (80% of these stated it happens daily). None believed the system would provide incorrect information with that frequency. Every hospitalist agreed that the GMU‐dr and GMU‐status tools are efficient methods for distributing patients, keeping track of attending designations, and maintaining a unit census and personal rounding censuses: cumulatively 85.7% absolutely agreed, while 12.7% strongly agreed.
We also assessed how promptly the call team responded to the assignment monitor alerts by correcting the information in the system (the census is recorded every 4 hours). Due to the intensity of the ED call, physicians often just scan this alert list (and take clinical action on the captured patients as needed) without updating the EHR fields until the end of their shifts. Measuring the speed of clearing patients from the census may grossly underestimate the actual usage of the list, though this data is useful to access a worst‐case scenario.
During the first week of September 2007 we cared for 270 patients: 52 were captured on the assignment monitor before a GMU‐dr was assigned or the patient was deemed non‐GMU. No patient was recaptured on the list more than 8 hours after the initial appearance.
Discussion
Since we enhanced a widely‐used program, our intervention required minimal training. As our innovation was designed and underwent trial by hospitalists, immediate feedback assured user‐friendly implementation, good acceptance, and improved workflow.
Color coding eliminated the time‐intensive and error‐prone lookup of coverage arrangements.
Updating GMU‐dr and GMU‐status in the EHR takes very little time and provides immediate benefits (eg, clearly defined rounding censuses). This task has been integrated into our signout tool, making these functions even more intuitive.
Using the assignment monitor census is fundamental for patient safety, but correcting EHR information creates some additional work for busy call physicians. Implementing this step required active change‐management: writing policies, designing metrics, and considering incentives. The call physicians (3 shifts per day) are officially responsible for patient distribution, including clearing the screening census before passing the call pager to the next shift.
We identified some potential limitations, though these issues generally apply to any information technology (IT) implementation: Setting up the program requires an adaptable EHR and close collaboration with the IT department. The system's accuracy depends on correctly identifying the patient's PCP in the EHR. Maintaining and coordinating the data regarding PCP privileges and hospitalist coverage requires a central database has been created.
Meanwhile, we found these tools very useful in solving problems beyond patient distribution:
PCP color coding can export information to other applications about hospitalist coverage and assist ED and nonmedical services to contact the proper medical service for admissions and consults.
GMU‐dr and GMU‐status can be used to create personal rounding censuses, provide billing lists to third‐party applications, and support proprietary applications, thus assisting patient distribution decisions and guiding hospital staff to call the patient's correct provider 24/7.
Special function censuses (defined by algorithms) are now used as alert lists for different patient issues (eg, observational patients staying beyond their allotted time) and by nonhospitalist services.
We presented hospitalist‐specific EHR concepts (patient coverage algorithms, special‐function censuses, and patient tracking by provider‐entered information) and their specific applications in our hospital. We believe our tools can be implemented in various locations and EHRs. Though the challenges may differ from setting to setting (eg, patient distribution between coexistent hospitalist programs, or responding to limitations of resident duty hours), these solutions are highly adaptable and have the potential of providing additional benefits beyond hospitalist coverage issues.
Acknowledgements
The authors thank Andrew Hakes for his invaluable assistance in graphic design; the authors also thank their information technology (IT) engineers and staff members, who programmed and are maintaining these functions in their EHR system, for their enthusiastic collaboration and for their assistance with the manuscript: Rich Prideaux, Robert Cawlfield, Kim Johnston, Brenda Ventrillo, and Katura Gardner.
- ,,,,.The core competencies in hospital medicine.J Hosp Med.2006;1(Suppl 1):84–95.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19(6):624–631.
- ,.The “continuity visit” and the hospitalist model of care.Am J Med.2001;111(9B):40S–42S.
- ,,,,,.Deficits in communication transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- .The importance of postdischarge telephone follow‐up for hospitalists: a view from the trenches.Am J Med.2001;111(9B):43S–44S.
- .The language of quality improvement: therapy classes.J Hosp Med.2006;1:327–330.
- ,.Executing high‐quality care transitions: a call to do it right.J Hosp Med.2007;2:287–290.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
Preserving continuity of care and assuring patient safety are core values of the hospitalist movement.1 Communicating clinical information to the patient, the primary care provider (PCP), and hospital‐based providers has been recognized in the hospitalist literature as an important domain of quality assurance.29 Correctly identifying a patient's inpatient physician during admission and later in the hospital course is essential to achieve these goals. This initial step has not been specifically addressed in the literature, though the complexity of this process can create significant challenges for larger hospitalist programs.
Rochester General Hospital (RGH) is a 528‐bed community teaching hospital. Our hospitalist program evolved from the general medicine unit (GMU) faculty that traditionally cared for most unreferred patients; ie, those whose PCPs had no admitting privileges. In 2004, we began covering patients of privilege‐holder partnering PCPs. Within 3 years, more than 120 privileged PCPs decided to refer all their admissions to us, though unreferred cases still make up the majority of our 10,000 annual admissions. GMU hospitalists either round as teaching attendings or attend patients on nonteaching services. Many PCPs continue to admit their own patients.
As our program began to work with numerous partnering PCPs, it became difficult to decide which patients the hospitalist team should be called to admit, and which should be admitted to the PCP. Having the emergency department (ED) providers page the PCPs or their call partners of the PCPs for attending service decisions proved infeasible and unreliable. Additionally, similar challenges emerged later in the hospitalization when patients were transferred from one service, floor, or provider to another, especially from the intensive care unit (ICU) to the medical service.
Errors regarding admissions to the hospitalist service can be classified as:
Type‐I errors: The PCP provides inpatient care but the patient is erroneously admitted to a hospitalist, creating discontinuity of care and dissatisfaction.
Type‐II errors: The PCP refers to the hospitalists but is erroneously identified as the inpatient attending physician. As the hospitalist team is not notified about these cases, admitted patients may go without physician services for a period of time.
Methods
RGH has a widely used, clinically focused EHR system that does not offer full CPOE functionality. The EHR includes a database of physicians with admitting privileges, so we decided to store information on the system regarding PCPs' hospitalist coverage. Initially, we simply uploaded a list of our partnering PCPs. However, looking up information from files proved too cumbersome for busy ED providers and hospitalists. Additionally, this solution lacked a feedback loop to alert for errors.
Therefore, we designed a system with 3 main functional elements to identify and display each patient's:
candidacy for hospitalist coverage;
actual hospitalist coverage; and
mismatches between the above statuses.
These steps are explained below, followed by a description of the system's actual utilization. In addition to the text, we demonstrate the concept of and provide detailed information on our system in Figure 1 and Table 1.

| Algorithms |
| This special census includes patients who meet any of the following criteria: |
| Attending physician is a GMU‐MD and GMU‐dr is {blank} |
| Attending physician is a GMU‐MD and GMU‐status is GMU‐cons, temp‐ICU, or non‐GMU |
| Attending physician is not a GMU‐MD and GMU‐dr is not {blank} and GMU‐status is not GMU‐cons, non‐GMU, or temp‐ICU |
| Attending physician is an ICU‐MD and patient's location is not ED or ICU and GMU‐status is not non‐GMU |
| Patient's status is INT (internal medicine in‐bed) and PCP has no privilege or is a GMU‐PCPCP and GMU‐dr is {blank} and Attending physician is not an ICU‐MD and GMU‐status is not temp‐ICU or non‐GMU |
| PCP has admission privilege and PCP is not a GMU‐PCPCP and GMU‐dr is not {blank} and GMU‐status is not non‐GMU or GMU+ |
| GMU‐status is temp‐ICU and patient's location is not ED or ICU |
| GMU‐dr is {blank} and GMU‐status is GMU‐cons, GMU‐ALC or GMU+ |
| GMU‐dr is ‐TBA, or ‐TRD and present time is between 8 AMand 4 PM |
| Definitions |
| Attending physician and PCP refers to the physicians assigned as attending and PCP, respectively in the core EHR system. |
| Physician categories are defined by listing all belonging members on centrally maintained, up‐to‐date databases: |
| GMU‐PCPCP: partnering community PCP physicians who refer their patients to the GMU hospitalist service |
| GMU‐MD: a GMU hospitalist attending physician |
| ICU‐MD: a critical care physician who attends in ICU, and automatically transfers most patients to hospitalists upon discharge from ICU |
| GMU‐dr signals acceptance to GMU hospitalist service, and names the hospitalist in the EHR. Temporary assignments (TBA or TRD) are used when the patient is accepted to the hospitalist program, but the rounding physician is not yet known (eg, a patient seen by the night hospitalist in the ED, or waiting reassignment from a weekend). Options include: |
| A physician's acronym (from last name and first initial) identifies the rounding hospitalist |
| TBA = to be assigned: accepted to GMU but not yet assigned to a rounding attending (used for new patients during evening and night hours while under the care of the on call team) |
| TRD = to redistribute from an attending's care who is leaving service (used for patients whose follow‐up coverage is distributed in the next morning by the call team) |
| A blank field means no hospitalist service for the patient |
| GMU‐status signals irregular rounding relationship with the patient. The following options are used: |
| Non‐GMU: patient will not be covered by GMU hospitalists (eg, signing off consult, admission to subspecialist service) |
| Temp‐ICU: patient will not be covered by GMU while in ICU (will automatically accept to GMU upon transfer to floor) |
| GMU‐cons: patient is on consultation service (GMU is not the attending service) |
| GMU‐ALC: patient is on alternative level of care (skilled nursing needs: no daily rounding by GMU hospitalist) |
| GMU+: patient is covered by GMU (despite the PCP not normally referring to GMU) |
First, to identify a patient's candidacy for hospitalist coverage, we created a PCP color coding algorithm, based on whether the PCP has admitting privileges at RGH and/or has arranged hospitalist coverage for her/his patients. Whether or not hospitalist coverage is expected for a given patient's PCP is displayed on the EHR.
Second, a data field called GMU‐dr was created to describe whether the patient is actually assigned to a hospitalist. The GMU on‐call physician assigns a GMU‐dr to all appropriate patients (ie, updates the field's value). This step acknowledges hospitalist coverage for a given patient and also identifies the hospitalist physician. It simultaneously adds the patient to the appropriate rounding censuses. The GMU‐dr data field is updated every time the rounding physician changes (eg, for weekend coverage).
Third, the EHR's assignment monitor algorithm compares the expected and actual hospitalist coverage (based on PCP color coding, GMU‐dr, admission type, location, time of day, etc.) and displays mismatches on the assignment monitor census. We created this special‐function census to include patients with the more dangerous type‐II coverage errors that would not show up on our rounding censuses.
The assignment monitor census is regularly reviewed by the call physicians and should be cleared of patients. Unaccounted patients showing up on this census are handled with urgency equal to that of an unseen admission. Most patients on the assignment monitor census have missing or incorrect information: correcting that information removes the patient from this alert list. However, some patients with correct information are captured due to an unusual relationship with the hospitalist program; eg, consultation or individual exceptions from coverage arrangements. The GMU‐status field was created to explain such situations and remove these patients from the alert list.
Results
According to a survey that was distributed to the 19 eligible hospitalists and returned by 17 (89%), this system greatly improved our admission and patient distribution process, with the following results:
PCP color coding prevents type‐I and type‐II coverage identification errors more than once a week according to 94.1% and 88.2% of hospitalists, respectively. Ninety‐four percent agreed (absolutely or strongly) that color coding is a convenient tool to identify PCP referral status.
The assignment monitor identifies patients more than once a month that would have been lost in the preintervention era, according to 94.1% of the hospitalists. All hospitalists surveyed believed that this alert list had several times prevented potentially life‐threatening complications, and that the system is more useful than burdensome.
The GMU‐dr data field correctly identifies the hospitalist while the attending is misassigned in the core EHR system more than once a week, according to 93.7% of surveyed hospitalists (80% of these stated it happens daily). None believed the system would provide incorrect information with that frequency. Every hospitalist agreed that the GMU‐dr and GMU‐status tools are efficient methods for distributing patients, keeping track of attending designations, and maintaining a unit census and personal rounding censuses: cumulatively 85.7% absolutely agreed, while 12.7% strongly agreed.
We also assessed how promptly the call team responded to the assignment monitor alerts by correcting the information in the system (the census is recorded every 4 hours). Due to the intensity of the ED call, physicians often just scan this alert list (and take clinical action on the captured patients as needed) without updating the EHR fields until the end of their shifts. Measuring the speed of clearing patients from the census may grossly underestimate the actual usage of the list, though this data is useful to access a worst‐case scenario.
During the first week of September 2007 we cared for 270 patients: 52 were captured on the assignment monitor before a GMU‐dr was assigned or the patient was deemed non‐GMU. No patient was recaptured on the list more than 8 hours after the initial appearance.
Discussion
Since we enhanced a widely‐used program, our intervention required minimal training. As our innovation was designed and underwent trial by hospitalists, immediate feedback assured user‐friendly implementation, good acceptance, and improved workflow.
Color coding eliminated the time‐intensive and error‐prone lookup of coverage arrangements.
Updating GMU‐dr and GMU‐status in the EHR takes very little time and provides immediate benefits (eg, clearly defined rounding censuses). This task has been integrated into our signout tool, making these functions even more intuitive.
Using the assignment monitor census is fundamental for patient safety, but correcting EHR information creates some additional work for busy call physicians. Implementing this step required active change‐management: writing policies, designing metrics, and considering incentives. The call physicians (3 shifts per day) are officially responsible for patient distribution, including clearing the screening census before passing the call pager to the next shift.
We identified some potential limitations, though these issues generally apply to any information technology (IT) implementation: Setting up the program requires an adaptable EHR and close collaboration with the IT department. The system's accuracy depends on correctly identifying the patient's PCP in the EHR. Maintaining and coordinating the data regarding PCP privileges and hospitalist coverage requires a central database has been created.
Meanwhile, we found these tools very useful in solving problems beyond patient distribution:
PCP color coding can export information to other applications about hospitalist coverage and assist ED and nonmedical services to contact the proper medical service for admissions and consults.
GMU‐dr and GMU‐status can be used to create personal rounding censuses, provide billing lists to third‐party applications, and support proprietary applications, thus assisting patient distribution decisions and guiding hospital staff to call the patient's correct provider 24/7.
Special function censuses (defined by algorithms) are now used as alert lists for different patient issues (eg, observational patients staying beyond their allotted time) and by nonhospitalist services.
We presented hospitalist‐specific EHR concepts (patient coverage algorithms, special‐function censuses, and patient tracking by provider‐entered information) and their specific applications in our hospital. We believe our tools can be implemented in various locations and EHRs. Though the challenges may differ from setting to setting (eg, patient distribution between coexistent hospitalist programs, or responding to limitations of resident duty hours), these solutions are highly adaptable and have the potential of providing additional benefits beyond hospitalist coverage issues.
Acknowledgements
The authors thank Andrew Hakes for his invaluable assistance in graphic design; the authors also thank their information technology (IT) engineers and staff members, who programmed and are maintaining these functions in their EHR system, for their enthusiastic collaboration and for their assistance with the manuscript: Rich Prideaux, Robert Cawlfield, Kim Johnston, Brenda Ventrillo, and Katura Gardner.
Preserving continuity of care and assuring patient safety are core values of the hospitalist movement.1 Communicating clinical information to the patient, the primary care provider (PCP), and hospital‐based providers has been recognized in the hospitalist literature as an important domain of quality assurance.29 Correctly identifying a patient's inpatient physician during admission and later in the hospital course is essential to achieve these goals. This initial step has not been specifically addressed in the literature, though the complexity of this process can create significant challenges for larger hospitalist programs.
Rochester General Hospital (RGH) is a 528‐bed community teaching hospital. Our hospitalist program evolved from the general medicine unit (GMU) faculty that traditionally cared for most unreferred patients; ie, those whose PCPs had no admitting privileges. In 2004, we began covering patients of privilege‐holder partnering PCPs. Within 3 years, more than 120 privileged PCPs decided to refer all their admissions to us, though unreferred cases still make up the majority of our 10,000 annual admissions. GMU hospitalists either round as teaching attendings or attend patients on nonteaching services. Many PCPs continue to admit their own patients.
As our program began to work with numerous partnering PCPs, it became difficult to decide which patients the hospitalist team should be called to admit, and which should be admitted to the PCP. Having the emergency department (ED) providers page the PCPs or their call partners of the PCPs for attending service decisions proved infeasible and unreliable. Additionally, similar challenges emerged later in the hospitalization when patients were transferred from one service, floor, or provider to another, especially from the intensive care unit (ICU) to the medical service.
Errors regarding admissions to the hospitalist service can be classified as:
Type‐I errors: The PCP provides inpatient care but the patient is erroneously admitted to a hospitalist, creating discontinuity of care and dissatisfaction.
Type‐II errors: The PCP refers to the hospitalists but is erroneously identified as the inpatient attending physician. As the hospitalist team is not notified about these cases, admitted patients may go without physician services for a period of time.
Methods
RGH has a widely used, clinically focused EHR system that does not offer full CPOE functionality. The EHR includes a database of physicians with admitting privileges, so we decided to store information on the system regarding PCPs' hospitalist coverage. Initially, we simply uploaded a list of our partnering PCPs. However, looking up information from files proved too cumbersome for busy ED providers and hospitalists. Additionally, this solution lacked a feedback loop to alert for errors.
Therefore, we designed a system with 3 main functional elements to identify and display each patient's:
candidacy for hospitalist coverage;
actual hospitalist coverage; and
mismatches between the above statuses.
These steps are explained below, followed by a description of the system's actual utilization. In addition to the text, we demonstrate the concept of and provide detailed information on our system in Figure 1 and Table 1.

| Algorithms |
| This special census includes patients who meet any of the following criteria: |
| Attending physician is a GMU‐MD and GMU‐dr is {blank} |
| Attending physician is a GMU‐MD and GMU‐status is GMU‐cons, temp‐ICU, or non‐GMU |
| Attending physician is not a GMU‐MD and GMU‐dr is not {blank} and GMU‐status is not GMU‐cons, non‐GMU, or temp‐ICU |
| Attending physician is an ICU‐MD and patient's location is not ED or ICU and GMU‐status is not non‐GMU |
| Patient's status is INT (internal medicine in‐bed) and PCP has no privilege or is a GMU‐PCPCP and GMU‐dr is {blank} and Attending physician is not an ICU‐MD and GMU‐status is not temp‐ICU or non‐GMU |
| PCP has admission privilege and PCP is not a GMU‐PCPCP and GMU‐dr is not {blank} and GMU‐status is not non‐GMU or GMU+ |
| GMU‐status is temp‐ICU and patient's location is not ED or ICU |
| GMU‐dr is {blank} and GMU‐status is GMU‐cons, GMU‐ALC or GMU+ |
| GMU‐dr is ‐TBA, or ‐TRD and present time is between 8 AMand 4 PM |
| Definitions |
| Attending physician and PCP refers to the physicians assigned as attending and PCP, respectively in the core EHR system. |
| Physician categories are defined by listing all belonging members on centrally maintained, up‐to‐date databases: |
| GMU‐PCPCP: partnering community PCP physicians who refer their patients to the GMU hospitalist service |
| GMU‐MD: a GMU hospitalist attending physician |
| ICU‐MD: a critical care physician who attends in ICU, and automatically transfers most patients to hospitalists upon discharge from ICU |
| GMU‐dr signals acceptance to GMU hospitalist service, and names the hospitalist in the EHR. Temporary assignments (TBA or TRD) are used when the patient is accepted to the hospitalist program, but the rounding physician is not yet known (eg, a patient seen by the night hospitalist in the ED, or waiting reassignment from a weekend). Options include: |
| A physician's acronym (from last name and first initial) identifies the rounding hospitalist |
| TBA = to be assigned: accepted to GMU but not yet assigned to a rounding attending (used for new patients during evening and night hours while under the care of the on call team) |
| TRD = to redistribute from an attending's care who is leaving service (used for patients whose follow‐up coverage is distributed in the next morning by the call team) |
| A blank field means no hospitalist service for the patient |
| GMU‐status signals irregular rounding relationship with the patient. The following options are used: |
| Non‐GMU: patient will not be covered by GMU hospitalists (eg, signing off consult, admission to subspecialist service) |
| Temp‐ICU: patient will not be covered by GMU while in ICU (will automatically accept to GMU upon transfer to floor) |
| GMU‐cons: patient is on consultation service (GMU is not the attending service) |
| GMU‐ALC: patient is on alternative level of care (skilled nursing needs: no daily rounding by GMU hospitalist) |
| GMU+: patient is covered by GMU (despite the PCP not normally referring to GMU) |
First, to identify a patient's candidacy for hospitalist coverage, we created a PCP color coding algorithm, based on whether the PCP has admitting privileges at RGH and/or has arranged hospitalist coverage for her/his patients. Whether or not hospitalist coverage is expected for a given patient's PCP is displayed on the EHR.
Second, a data field called GMU‐dr was created to describe whether the patient is actually assigned to a hospitalist. The GMU on‐call physician assigns a GMU‐dr to all appropriate patients (ie, updates the field's value). This step acknowledges hospitalist coverage for a given patient and also identifies the hospitalist physician. It simultaneously adds the patient to the appropriate rounding censuses. The GMU‐dr data field is updated every time the rounding physician changes (eg, for weekend coverage).
Third, the EHR's assignment monitor algorithm compares the expected and actual hospitalist coverage (based on PCP color coding, GMU‐dr, admission type, location, time of day, etc.) and displays mismatches on the assignment monitor census. We created this special‐function census to include patients with the more dangerous type‐II coverage errors that would not show up on our rounding censuses.
The assignment monitor census is regularly reviewed by the call physicians and should be cleared of patients. Unaccounted patients showing up on this census are handled with urgency equal to that of an unseen admission. Most patients on the assignment monitor census have missing or incorrect information: correcting that information removes the patient from this alert list. However, some patients with correct information are captured due to an unusual relationship with the hospitalist program; eg, consultation or individual exceptions from coverage arrangements. The GMU‐status field was created to explain such situations and remove these patients from the alert list.
Results
According to a survey that was distributed to the 19 eligible hospitalists and returned by 17 (89%), this system greatly improved our admission and patient distribution process, with the following results:
PCP color coding prevents type‐I and type‐II coverage identification errors more than once a week according to 94.1% and 88.2% of hospitalists, respectively. Ninety‐four percent agreed (absolutely or strongly) that color coding is a convenient tool to identify PCP referral status.
The assignment monitor identifies patients more than once a month that would have been lost in the preintervention era, according to 94.1% of the hospitalists. All hospitalists surveyed believed that this alert list had several times prevented potentially life‐threatening complications, and that the system is more useful than burdensome.
The GMU‐dr data field correctly identifies the hospitalist while the attending is misassigned in the core EHR system more than once a week, according to 93.7% of surveyed hospitalists (80% of these stated it happens daily). None believed the system would provide incorrect information with that frequency. Every hospitalist agreed that the GMU‐dr and GMU‐status tools are efficient methods for distributing patients, keeping track of attending designations, and maintaining a unit census and personal rounding censuses: cumulatively 85.7% absolutely agreed, while 12.7% strongly agreed.
We also assessed how promptly the call team responded to the assignment monitor alerts by correcting the information in the system (the census is recorded every 4 hours). Due to the intensity of the ED call, physicians often just scan this alert list (and take clinical action on the captured patients as needed) without updating the EHR fields until the end of their shifts. Measuring the speed of clearing patients from the census may grossly underestimate the actual usage of the list, though this data is useful to access a worst‐case scenario.
During the first week of September 2007 we cared for 270 patients: 52 were captured on the assignment monitor before a GMU‐dr was assigned or the patient was deemed non‐GMU. No patient was recaptured on the list more than 8 hours after the initial appearance.
Discussion
Since we enhanced a widely‐used program, our intervention required minimal training. As our innovation was designed and underwent trial by hospitalists, immediate feedback assured user‐friendly implementation, good acceptance, and improved workflow.
Color coding eliminated the time‐intensive and error‐prone lookup of coverage arrangements.
Updating GMU‐dr and GMU‐status in the EHR takes very little time and provides immediate benefits (eg, clearly defined rounding censuses). This task has been integrated into our signout tool, making these functions even more intuitive.
Using the assignment monitor census is fundamental for patient safety, but correcting EHR information creates some additional work for busy call physicians. Implementing this step required active change‐management: writing policies, designing metrics, and considering incentives. The call physicians (3 shifts per day) are officially responsible for patient distribution, including clearing the screening census before passing the call pager to the next shift.
We identified some potential limitations, though these issues generally apply to any information technology (IT) implementation: Setting up the program requires an adaptable EHR and close collaboration with the IT department. The system's accuracy depends on correctly identifying the patient's PCP in the EHR. Maintaining and coordinating the data regarding PCP privileges and hospitalist coverage requires a central database has been created.
Meanwhile, we found these tools very useful in solving problems beyond patient distribution:
PCP color coding can export information to other applications about hospitalist coverage and assist ED and nonmedical services to contact the proper medical service for admissions and consults.
GMU‐dr and GMU‐status can be used to create personal rounding censuses, provide billing lists to third‐party applications, and support proprietary applications, thus assisting patient distribution decisions and guiding hospital staff to call the patient's correct provider 24/7.
Special function censuses (defined by algorithms) are now used as alert lists for different patient issues (eg, observational patients staying beyond their allotted time) and by nonhospitalist services.
We presented hospitalist‐specific EHR concepts (patient coverage algorithms, special‐function censuses, and patient tracking by provider‐entered information) and their specific applications in our hospital. We believe our tools can be implemented in various locations and EHRs. Though the challenges may differ from setting to setting (eg, patient distribution between coexistent hospitalist programs, or responding to limitations of resident duty hours), these solutions are highly adaptable and have the potential of providing additional benefits beyond hospitalist coverage issues.
Acknowledgements
The authors thank Andrew Hakes for his invaluable assistance in graphic design; the authors also thank their information technology (IT) engineers and staff members, who programmed and are maintaining these functions in their EHR system, for their enthusiastic collaboration and for their assistance with the manuscript: Rich Prideaux, Robert Cawlfield, Kim Johnston, Brenda Ventrillo, and Katura Gardner.
- ,,,,.The core competencies in hospital medicine.J Hosp Med.2006;1(Suppl 1):84–95.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19(6):624–631.
- ,.The “continuity visit” and the hospitalist model of care.Am J Med.2001;111(9B):40S–42S.
- ,,,,,.Deficits in communication transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- .The importance of postdischarge telephone follow‐up for hospitalists: a view from the trenches.Am J Med.2001;111(9B):43S–44S.
- .The language of quality improvement: therapy classes.J Hosp Med.2006;1:327–330.
- ,.Executing high‐quality care transitions: a call to do it right.J Hosp Med.2007;2:287–290.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,,.The core competencies in hospital medicine.J Hosp Med.2006;1(Suppl 1):84–95.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19(6):624–631.
- ,.The “continuity visit” and the hospitalist model of care.Am J Med.2001;111(9B):40S–42S.
- ,,,,,.Deficits in communication transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- .The importance of postdischarge telephone follow‐up for hospitalists: a view from the trenches.Am J Med.2001;111(9B):43S–44S.
- .The language of quality improvement: therapy classes.J Hosp Med.2006;1:327–330.
- ,.Executing high‐quality care transitions: a call to do it right.J Hosp Med.2007;2:287–290.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
Copyright © 2009 Society of Hospital Medicine
Hospitalist‐Run Short‐Stay Unit
Short‐stay units (SSUs) are common alternatives to traditional inpatient services.1 When defined broadly to include observation units for low‐risk chest pain patients, SSUs exist in one‐third of hospitals in the United States.2 Amidst growing demands for inpatient services, SSUs have recently developed beyond observation medicine to provide more complex inpatient services in locations commonly adjacent to emergency departments (EDs).1 Hospitalists are well‐positioned to staff these emerging SSUs because of their expertise in managing complex inpatient services.3
Despite this, we found only 3 reports of hospitalist‐run SSUs designed for general medical inpatients (2 from Spain and 1 from Canada).46 Whereas these early reports introduce hospitalist‐run SSUs, they provide limited data to make firm conclusions about their usefulness or appropriate design. For example, none of these reports assessed patients' characteristics upon admission. Nor did they provide details about the services that the SSUs provided. Yet evaluation of both types of patient‐level datadescriptions of patients' needs upon admission and how these needs are met during their staysdetermine whether or not hospitalist‐run SSUs meet their potential to efficiently care for backlogs of patients who otherwise await admission to traditional inpatient services.
In order to further explore these issues, we first sought to characterize our SSU patients upon admission and record what services they received during their stays. To help interpret our results, we then investigated associations between these characteristics and measures of successfully caring for patients in our SSU.
Patients and Methods
Design and Setting
In this prospective cohort study, we included all patients admitted to the hospitalist‐run SSU of Cook County Hospital, a 500‐bed public teaching hospital in Chicago, Illinois, from January through April of 2006. Our 14‐bed SSU opened in 2002 to reduce overcrowding on the traditional inpatient wards by admitting adult patients who require inpatient care but might be eligible for discharge within 3 days. The unit is geographically part of the ED but is staffed by resident physicians and a rotating group of hospitalist attending physicians from the Department of Medicine. At least 1 attending and resident physician are available throughout the day, including weekend days and holidays; evenings are covered by a resident who presents overnight admissions to an attending physician the following morning.
ED physicians admit general medical patients to the SSU 24 hours per day, 7 days per week. Though admissions do not require prior approval from SSU physicians, the Departments of Medicine and Emergency Medicine have collaboratively promoted 5 suggested admission‐location guidelines to admitting ED physicians (Figure 1). For candidate SSU patients, these 5 guidelines are not intended to be restrictive but to provide a framework for the complex decision‐making process that our ED physicians encounter, particularly during periods of extreme overcrowding.7 First, patients should have an anticipated stay shorter than 72 hours. Second, patients should not have an eventual need for admission to traditional inpatient services such as the general medicine wards or intensive care units; this guideline is intended to improve patient safety by reducing unnecessary handoffs between physicians.8 Third, patients with provisional cardiovascular diagnoses should be preferentially admitted to the SSU over the general medical wards; this guideline is intended to improve hospital‐wide efficiency because our SSU is equipped with continuous telemetry monitors, an exercise treadmill testing (ETT) laboratory, and other reserved cardiac tests (see Admission Characteristics and Services Received section, below). Fourth, patients' risk should be no higher than intermediate level. Admitting ED physicians are encouraged to use posted risk estimators for patients with provisional diagnoses of possible acute coronary syndrome (ACS), decompensated heart failure, asthma exacerbation, and out‐of‐control diabetes. Finally, patients should not need advanced ancillary services; these include bedside procedures (eg, central venous catheter insertions), time‐intensive nursing (eg, regular dressing changes), and complex social‐services (eg, long‐term care facility placements).
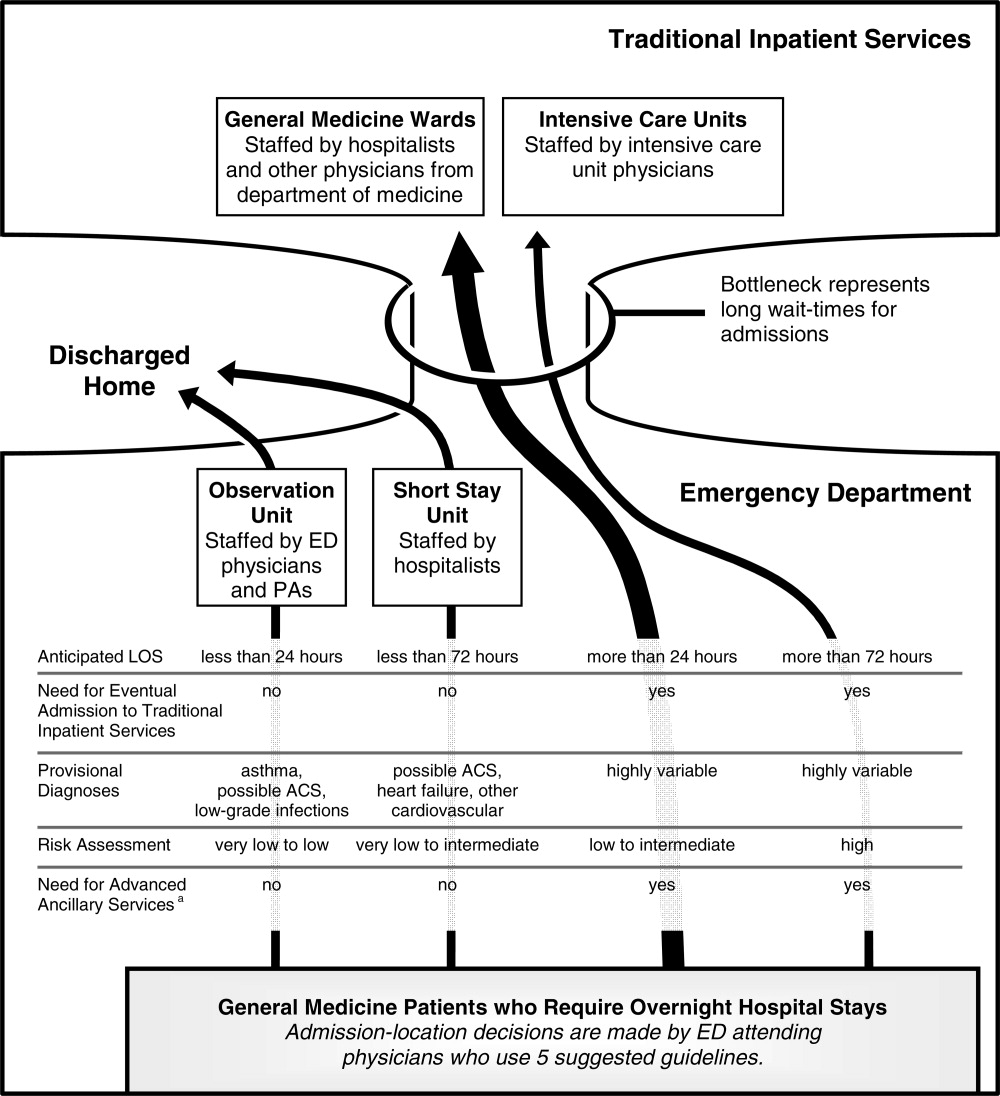
Subjects
The study subjects were all patients admitted to the SSU during the 4‐month study period. Patients were excluded from the entire study if they refused verbal consent to participate. All patients who consented were included in the description of patient admission characteristics. Thirteen patients who prematurely left the SSU against medical advice, however, were neither included in the descriptions of services received nor in the analyses of predictors of successful SSU stays. We excluded these patients because they needed services that they did not receiveincluding these patients in our analysis would tend to overestimate the efficiency of our SSU by shortening the length‐of‐stay (LOS) without adding diagnostic tests or treatments.
Data Collection
After receiving approval from the institutional review board, attending physician investigators conducted an interview, physical examination, and review of medical records for each enrolled patient within 12 hours of admission to the SSU. When ED attending physicians' provisional primary diagnoses included possible ACS or decompensated heart failure, which we knew from earlier pilot data were our 2 most common provisional diagnoses, investigators gathered patient data to be applied in validated models of risk after the study period (Figure 2).9, 10 Some of the clinical predictors required for these models are based on patients' findings on presentation to the ED. For example, Goldman's risk model for major cardiac events uses patients' initial systolic blood pressures on presentation to the ED.9 In such cases, investigators gathered needed data from electronic and paper charts generated in the ED. Upon discharge from the SSU, investigators reviewed patients' medical records a second time. All data were entered by investigators and instantly committed into an online database.
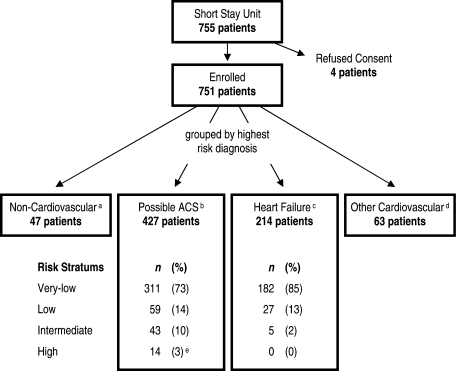
Admission Characteristics and Services Received
Patients were grouped according to the provisional diagnoses of ED attending physicians upon admission to the SSU (Figure 2). We chose to group patients by the provisional diagnoses of EDnot SSUattending physicians to better understand how ED physicians, the physicians who make the admission‐location decisions in our hospital, were using the SSU. Patients were first grouped as having possible ACS or heart failure, because patients with these provisional diagnoses were preferentially admitted to the SSU (Figure 1). When neither diagnosis was listed, patients were grouped according to ED attending physicians' first‐listed diagnoses. At the end of the study period, relevant risk models were applied to patients with possible ACS or heart failure and stratified as very low, low, intermediate, or high risk.9, 10 Patients with both possible ACS and heart failure were grouped according to the diagnosis with the highest corresponding risk assessment; if both risk assessments were the same, then the first‐listed diagnosis was used. Though developed to predict different clinical outcomes during different time periods, risk strata from the corresponding risk models were pooled across both diagnoses to develop a risk summary.
Upon discharge, investigators recorded which advanced diagnostic tests, specialty consultations, and acute care treatments patients received while in the SSU. Diagnostic tests were considered advanced if they were not routinely performed within 2 hours of being ordered. Advanced diagnostic tests were grouped into 2 types by their accessibility to ordering SSU physicians. Open access tests included echocardiograms and ETTs, which were reserved for SSU patients 6 days per week. Though the availability of open access tests was not unlimited, ordering physicians' needs for them rarely exceeded the immediate supply. On the other hand, limited access tests included both cardiac stress imaging studies, which were reserved for SSU patients on a very limited basis 4 or 5 days per week, and other tests that were not reserved for SSU patients, such as endoscopy, magnetic resonance imaging, or ultrasonography. Ordering physicians' needs for limited access tests often exceeded their immediate supply; in such cases, SSU patients were placed without priority into queues that included patients from the entire hospital.
Investigators recorded when patients received advanced diagnostic tests that were ordered by specialists. These tests, however, were not included in analyses of how services received by SSU patients affected SSU success, because SSU attending physicians were only indirectly involved in whether or not patients received these tests. Treatments were considered acute care treatments if they were commonly administered only in acute care settings, such as heparin for unstable angina or intravenous furosemide for pulmonary edema.
SSU Success
The SSU was designed to care for patients during brief stays and without eventual admission to traditional inpatient services. Therefore, we used patients' LOS and whether or not patients were admitted to traditional inpatient services as measures of SSU success. LOS was calculated from the time patients arrived in the SSU until the time they left. Therefore, neither time spent in the ED before admission to the SSU nor time spent on traditional inpatient services (if needed) contributed to our definition of LOS. Individual SSU patients were considered successfully cared for in the SSU if their LOS was less than 72 hours and they were discharged directly home from the SSU. We explored associations between these outcomes and provisional diagnoses, risk assessments, and services received.
Data Analysis
LOS data were right‐skewed; therefore, we used the Mann‐Whitney test for comparisons between 2 groups and the Kruskal‐Wallis test for comparisons among 3 or more groups. To test for trends of median LOS among ordered groupings, we used the method of Cuzick.11 We used Pearson's chi‐square test to compare proportions of patients grouped into categories and the chi‐square test for trends with equal scoring to test for trends among ordered groupings.
We performed multiple logistic regression to explore which variables were associated with SSU success. The following 5 demographic variables from Table 1 were insignificant in all single‐variable and multiple‐variable regression models that we tested and were, therefore, removed from further analyses to create more parsimonious models: gender, language, ethnicity, race, and whether or not patients had a primary care provider. Our multiple logistic regression models were fitted by maximum likelihood methods. In all of these models, odds ratios (ORs) were adjusted for patient characteristics that included age (in years), insulin‐requiring diabetes mellitus (yes or no), SSU attending physician, day of the week of SSU admission (weekday or weekend), and hospitalization during the preceding year. Confidence intervals (CIs) for predicted probabilities were computed using the delta method. All analyses were conducted with Stata Statistical Software, Release 9 (StataCorp, College Station, TX).
| |
| Mean age, years (SD) (25th‐75th percentiles) | 58 (12) (49‐66) |
| Men | 389 (52) |
| Lacking a primary care provider | 256 (34) |
| Non‐English speaking | 217 (29) |
| Ethnicity is Hispanic or Latino | 105 (14) |
| Race is Black or African‐American | 480 (64) |
| Hospitalized within the preceding year | 322 (43) |
| Insulin‐requiring diabetes mellitus | 83 (11) |
| Previous coronary artery revascularization | 89 (12) |
| Provisional diagnosis* | |
| Possible acute coronary syndrome | 427 (57) |
| Heart failure | 214 (29) |
| Other cardiovascular | 62 (8) |
| Noncardiovascular | 48 (6) |
Results
Subjects
During the 4‐month study period, 755 patients were admitted to the SSU. Among these patients, 4 were excluded from our study because they refused verbal consent. In the remaining study sample of 751 patients, all were included in the descriptions of patients' admission characteristics (Table 1), but 13 patients who left prematurely were excluded in both the descriptions of services received (Table 2) and the analyses of SSU success (Tables 3 and 4).
| Service received | Possible ACS n = 418 (%) | Heart Failure n = 211 (%) | Other Cardiovascular n = 61 (%) | Noncardiovascular n = 48 (%) | Total n = 738 (%) |
|---|---|---|---|---|---|
| |||||
| Open access test* | 37 | 59 | 56 | 13 | 43 |
| Resting | |||||
| Echocardiography | 29 | 59 | 56 | 13 | 39 |
| ETT | 12 | 0 | 5 | 0 | 7 |
| Limited access test | 24 | 8 | 10 | 8 | 17 |
| Stress imaging | 21 | 5 | 7 | 2 | 14 |
| Acute care treatment | 22 | 78 | 5 | 60 | 39 |
| Specialty consultation∥ | 24 | 12 | 20 | 8 | 19 |
| Any above service | 68 | 93 | 67 | 69 | 75 |
| Provisional Diagnosis and Services Received | n | Median LOS [hours (IQR)] | Stay Longer than 72 Hours (%) | Admission to Traditional Inpatient Service (%) | Stay Longer than 72 Hours or Admission to Traditional Inpatient Service (%) |
|---|---|---|---|---|---|
| |||||
| All patients | 738 | 42 (22‐63) | 15 | 9* | 21 |
| Possible ACS | 418 | 37 (20‐57) | 13 | 10 | 20 |
| Heart failure | 211 | 47 (34‐69) | 21 | 9 | 27 |
| Other CV | 61 | 40 (21‐49) | 10 | 5 | 11 |
| Non‐CV | 48 | 40 (22‐60) | 10 | 2 | 13 |
| P value | <0.001 | 0.04 | 0.18 | 0.01 | |
| Open access test | |||||
| Yes | 320 | 46 (31‐67) | 17 | 9 | 23 |
| No | 418 | 33 (20‐52) | 13 | 9 | 20 |
| P value | <0.001 | 0.15 | 0.87 | 0.43 | |
| Limited access test | |||||
| Yes | 128 | 51 (42‐82) | 31 | 6 | 33 |
| No | 610 | 38 (21‐55) | 12 | 10 | 19 |
| P value | <0.001 | <0.001 | 0.24 | <0.001 | |
| Acute care treatment | |||||
| Yes | 287 | 46 (34‐69) | 21 | 12 | 29 |
| No | 451 | 32 (20‐50) | 11 | 7 | 16 |
| P value | <0.001 | <0.001 | 0.01 | <0.001 | |
| Specialty consultation | |||||
| Yes | 141 | 63 (40‐92) | 38 | 28 | 52 |
| No | 597 | 38 (21‐50) | 10 | 5 | 14 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Any above service | |||||
| Yes | 554 | 46 (29‐68) | 19 | 10 | 26 |
| No | 184∥ | 22 (16‐32) | 2 | 5 | 7 |
| P value | <0.001 | <0.001 | 0.03 | <0.001 | |
| Stay Longer than 72 Hours | Admission to Traditional Inpatient Service | Either Outcome | ||||
|---|---|---|---|---|---|---|
| OR* | P value | OR* | P value | OR* | P value | |
| ||||||
| Heart failure | 2.3 | 0.01 | 1.1 | 0.77 | 1.9 | 0.02 |
| Service received | ||||||
| Open access test | 1.5 | 0.10 | 1.0 | 0.89 | 1.2 | 0.32 |
| Limited access test | 5.1 | <0.001 | 0.4 | 0.03 | 2.5 | <0.001 |
| Acute care treatment | 1.7 | 0.07 | 1.4 | 0.31 | 1.6 | 0.05 |
| Specialty consultation | 6.1 | <0.001 | 13.1 | <0.001 | 8.1 | <0.001 |
Admission Characteristics and Services Received
A narrow range of provisional diagnoses were listed by ED attending physicians and 641 patients (85% of 751) were grouped as having possible ACS or heart failure (Figure 2). Patients with these diagnoses were later risk‐stratified and, when pooled across risk strata, only 14 patients (2% of 641) exceeded the suggested admission‐location criterion for the SSU of lower than high risk. Despite the array and frequency of diagnostic and treatment services that patients received, SSU physicians worked mostly independently, requesting specialty consultations for only 19% of patients (141/738; Table 2).
SSU Success
The median LOS for all patients was 42 hours (interquartile range [IRQ] 22‐63) and 156 patients (21% of 738) had unsuccessful SSU stays (Table 3). The most common reason for an unsuccessful stay was a stay longer than 72 hours (71% of 156). Among the 66 patients who required admission to traditional inpatient services, nearly one‐half (48%) were admitted expressly to receive treatments not available in the SSU after having a specialty consult.
Patients' provisional diagnoses were associated with unsuccessful stays in bivariate analyses (Table 3). In addition, when patients were grouped into 3 risk stratums (very‐low, low, and intermediate‐and‐high), unsuccessful stays increased with increasing risk. For example, in patients with possible ACS, the proportion of unsuccessful stays increased from 17% of 306 very‐low risk patients to 27% of 55 intermediate‐and‐high risk patients (P value for trend = 0.012. Similarly, in patients with heart failure, the proportion of unsuccessful stays increased from 25% of 181 very‐low risk patients to 100% of 3 intermediate‐and‐high risk patients (P value for trend = 0.004).
However, in multiple variable models that simultaneously included patients' characteristics upon admission with services received during their SSU stay, only the provisional diagnosis of heart failure was associated with unsuccessful stays (OR, 1.9; 95% CI, 1.12‐3.18); risk assessments for possible ACS (P = 0.29) and heart failure (P = 0.32) were unimportant predictors of unsuccessful stays (Table 4). On the other hand, whether or not patients received diagnostic tests, acute care treatments, or specialty consultations were important predictors. In particular, patients who received specialty consultations were much more likely to require admission to traditional inpatient services than those patients who did not (OR, 13.1; 95% CI, 6.9‐24.9) and had a 52% chance of having an unsuccessful stay (95% CI, 42‐61%; Figure 3). In addition, the accessibility of a diagnostic test was inversely proportional to the chance of having a long stay; patients who received an open access test had a 12% chance of a long stay (95% CI, 8‐16%) whereas those who received a limited access test had a 29% chance of a long stay (95% CI, 20‐39%). Receiving acute care treatments was also a significant, though less important, predictor of an unsuccessful stay (Table 4).
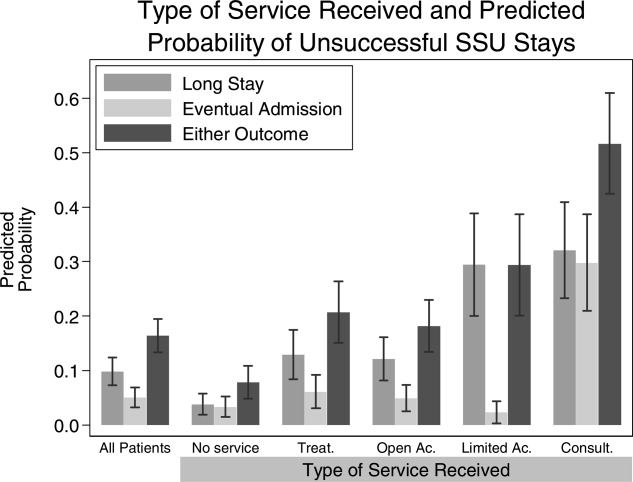
Discussion
We found that the types of services received by patients during their SSU stays were stronger predictors of long stays and eventual admissions to traditional inpatient services than patients' characteristics upon admission to the SSU. This suggests that SSUs should be focused toward matching patients' anticipated needs with readily accessible services. For example, in our SSU, which cares for over 2,250 patients annually, more than 1,200 patients will receive diagnostic tests in a given year. Among these patients, those who receive a limited access test will be more than twice as likely to have long stays than those who receive an open access test (Figure 3). Though our conclusions may not be applicable to other settings, this study is the most comprehensive description of patients admitted to a hospitalist‐run SSU. In addition, our study is the first to demonstrate that diagnostic and consultative services are the most important predictors of successful stays in SSUs. This promotes the practical strategy that hospitalists who staff SSUs should focus administratively toward gaining access to these services.
Very few of our SSU patients did not fulfill the suggested requirements of our admission location guidelines. For example, only 2% of 691 patients with either possible ACS or heart failure were high risk (Figure 2). Despite this, 21% of our patients had stays longer than 72 hours or were admitted to traditional inpatient services. The paradoxically high proportion of unsuccessful stays among mostly very‐low and low risk patients simply reflects how the clinical risk models that we used were not designed to predict unsuccessful stays. Moreover, as our multiple variable models suggest, improvements in the selection process of candidate SSU patients are more likely to come from an ability to incorporate assessments of what services patients will receive rather than from assessments of their clinical risk (Table 4). Therefore, the immediate plans of the accepting SSU physicians, the physicians who will determine what services patients eventually receive, should be incorporated in the admission‐location decision process.
Three of our findings highlight how input from accepting SSU physiciansconveyed to ED physicians before their final admission‐location decisions are mademay improve the SSU patient selection process. First, 23% of our patients were discharged home after brief stays with no advanced tests, specialty consultations, or acute care treatments (Table 3). Though some of these patients may have required inpatient services other than the ones we recorded, most were admitted with very‐low risk possible ACS; if they required overnight stays at all, many of them may have been better cared for in the ED observation unit (Figure 1). Second, 74% of the patients who required admission to traditional inpatient services were admitted for services not readily available to patients in the SSU (Table 3). Among these patients, nearly one‐third (21/66) received no advanced diagnostic tests in the SSU. This suggests that these patients should have been admitted directly to the general medical wards; doing so may have improved efficiency and quality of care by reducing unnecessary handoffs between physicians. Both types of patientsthose with minimal inpatient needs and those with more needs than the SSU can providehighlight how incorporating accepting SSU physicians' plans may improve the SSU patient selection process. After all, those best equipped to determine if the SSU will meet (or exceed) the needs of candidate patients are the SSU physicians themselves.
Third, we found that whether or not SSU physicians required assistance from specialists was the strongest predictor of unsuccessful stays: when an accepting physician determined that a patient should receive a specialty consultation, that patient's chance of having an unsuccessful stay was over 50% (Figure 3). Our study was not designed to determine how specialty consultations were associated with unsuccessful stays. We did not, for example, record whether or not hospitalists changed their diagnostic, treatment, or admission plans because of specialists' recommendations.12 Therefore, we cannot conclude that specialty consultations actually caused long stays or traditional admissions. Nevertheless, when our SSU physicians did not manage patients independent of specialty consultations, we observed a high likelihood of unsuccessful stays. Because accepting SSU physicians are the ones who will determine whether or not they need assistance from specialists, weighing their immediate plans for specialty consultations into the admission‐location decision process may improve the efficiency of SSUs. Others have recognized the importance of specialty consultations in SSUs by directly incorporating specialists as coattending physicians.13
Our study had several limitations. First, we studied mostly patients with cardiovascular diagnoses. Predictors of success in SSUs that admit patients with different diagnostic profiles may be different. In particular, SSUs that admit patients with a wide array of diagnoses may find that matching patients' needs with readily accessible services is impractical, because these needs may be too wide‐ranging. Second, our study design was observational. However, other than seasonal variations in admission patterns, there was little room for selection bias because we enrolled all consecutive admissions over the 4‐month study period, which gives us more confidence in our results. Third, our study did not record whether or not ED physicians knowingly overrode the suggested admission‐location guidelines because of limited bed availability. Yet, if shortages of beds on traditional inpatient services were driving patients who were otherwise candidates for the general medical wards in to the SSU, then we would have expected higher‐risk patients and greater needs for limited access tests. Finally, our descriptions of patients' needs were based on what diagnostic, consultative, and treatment services patients actually received; yet these needs did not include diagnostic tests that were ordered but never performed. However, any missed needs would bias our results toward no association with unsuccessful stays, because unsuccessful stays would generally increase while patients await needed services.
Future research could address these limitations through an experimental trial of traditional admissions versus admission to a hospitalist‐run SSU. And, because hospitals are complex systems of health care delivery where changes in one patient care unit often affect others in unanticipated ways,14 the impact of SSUs on other patient care units that are closely connected to SSUs, such as EDs and the general medical wards (Figure 1), should be simultaneously observed. For example, though our findings suggest that the accessibility of diagnostic tests should parallel ordering SSU physicians' needs for those tests, making all diagnostic tests open to SSU physicians may result in shortsightedly lengthening the stays of patients in other care units. Future research should also observe the decision‐making process of both the physicians who make admission‐location decisions (ED physicians) and those who determine the eventual plans for patients in the SSU (hospitalists). Accepting physicians from other patient care units have found improved outcomes of efficiency when they were involved in the complex process of deciding where to admit patients.15, 16 After an initial evaluation of a candidate SSU patient in the ED, a hospitalist who staffs both the general medical wards and the SSU would be uniquely well‐positioned to help an ED physician decide where a patient's needs would be best met. Although ED physicians will rightly be concerned that consulting SSU hospitalists may slow patient flow, hands‐on consultations of candidate SSU patients, who have a narrow range of diagnoses and low‐risk profiles, would likely be brief. In addition, because many SSUs are conveniently adjacent to EDs, the burden of communication may be minor.1 To address these questions, hospitalists who staff SSUs must continue the observed trend of working collaboratively with ED physicians.15, 17, 18
Acknowledgements
The authors thank Arthur T. Evans and Brendan M. Reilly for their insightful review of the manuscript. The authors also thank Zhaotai Cui for his assistance with statistical programming.
- ,,.A paradigm shift in the nature of care provision in emergency departments.Emerg Med J.2004;21:681–684.
- ,,.Acute coronary syndromes. In: Marx J, Hockberger R, Walls R, eds.Rosen's emergency medicine. Concepts and clinical practice.6th ed.Edinburgh:Mosby Elsevier;2006:1154–1199.
- .Taking charge of observation units for better patient flow.Todays Hospitalist Mag.2007;5(7):16–20.
- ,,, et al.Unidad de corta estancia dependiente de Medicina Interna.An Med Interna.1999;16:504–510.
- ,,,,.Factors that predict unplanned hospital readmission of patients discharged from a short stay medical unit.An Med Interna.2002;19:221–225.
- ,,, et al.Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital.CMAJ.2000;163(11):1477–1480.
- ,,,.Triage of patients with chest pain in the emergency department: a comparative study of physicians' decisions.Am J Med.2002;112:95–103.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,, et al.Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain.N Engl J Med.1996;334:1498–1504.
- ,,, et al.Risk stratification for in‐hospital mortality in acutely decompensated heart failure.JAMA.2005;293:572–580.
- .A Wilcoxon‐type test for trend.Stat Med.1985;4:87–90.
- ,,,,,.Diagnostic test restraint and the specialty consultation.J Gen Intern Med.1990;5:95–103.
- ,,, et al.A cooperative care model: cardiologists and hospitalists reduce length of stay in a chest pain observation unit.Crit Pathw Cardiol.2005;4:55–58.
- ,.Average length of stay, delayed discharge, and hospital congestion.BMJ.2002;325:610–611.
- .An internist in the emergency department: the IM facilitator program.HMO Pract.1991;10:42–43.
- ,,, et al.A “closed” medical intensive care unit (MICU) improves resource utilization when compared with an “open” MICU.Am J Respir Crit Care Med.1998;157:1468–1473.
- .Hospitalists' new role in the ED: “clog busters.”Todays Hospitalist Mag.2005;3(8):15–18.
- .Kindred spirits: ED doctors, hospitalists forge a critical collaboration.Hospitalist.2007;11(7):1,16–20.
Short‐stay units (SSUs) are common alternatives to traditional inpatient services.1 When defined broadly to include observation units for low‐risk chest pain patients, SSUs exist in one‐third of hospitals in the United States.2 Amidst growing demands for inpatient services, SSUs have recently developed beyond observation medicine to provide more complex inpatient services in locations commonly adjacent to emergency departments (EDs).1 Hospitalists are well‐positioned to staff these emerging SSUs because of their expertise in managing complex inpatient services.3
Despite this, we found only 3 reports of hospitalist‐run SSUs designed for general medical inpatients (2 from Spain and 1 from Canada).46 Whereas these early reports introduce hospitalist‐run SSUs, they provide limited data to make firm conclusions about their usefulness or appropriate design. For example, none of these reports assessed patients' characteristics upon admission. Nor did they provide details about the services that the SSUs provided. Yet evaluation of both types of patient‐level datadescriptions of patients' needs upon admission and how these needs are met during their staysdetermine whether or not hospitalist‐run SSUs meet their potential to efficiently care for backlogs of patients who otherwise await admission to traditional inpatient services.
In order to further explore these issues, we first sought to characterize our SSU patients upon admission and record what services they received during their stays. To help interpret our results, we then investigated associations between these characteristics and measures of successfully caring for patients in our SSU.
Patients and Methods
Design and Setting
In this prospective cohort study, we included all patients admitted to the hospitalist‐run SSU of Cook County Hospital, a 500‐bed public teaching hospital in Chicago, Illinois, from January through April of 2006. Our 14‐bed SSU opened in 2002 to reduce overcrowding on the traditional inpatient wards by admitting adult patients who require inpatient care but might be eligible for discharge within 3 days. The unit is geographically part of the ED but is staffed by resident physicians and a rotating group of hospitalist attending physicians from the Department of Medicine. At least 1 attending and resident physician are available throughout the day, including weekend days and holidays; evenings are covered by a resident who presents overnight admissions to an attending physician the following morning.
ED physicians admit general medical patients to the SSU 24 hours per day, 7 days per week. Though admissions do not require prior approval from SSU physicians, the Departments of Medicine and Emergency Medicine have collaboratively promoted 5 suggested admission‐location guidelines to admitting ED physicians (Figure 1). For candidate SSU patients, these 5 guidelines are not intended to be restrictive but to provide a framework for the complex decision‐making process that our ED physicians encounter, particularly during periods of extreme overcrowding.7 First, patients should have an anticipated stay shorter than 72 hours. Second, patients should not have an eventual need for admission to traditional inpatient services such as the general medicine wards or intensive care units; this guideline is intended to improve patient safety by reducing unnecessary handoffs between physicians.8 Third, patients with provisional cardiovascular diagnoses should be preferentially admitted to the SSU over the general medical wards; this guideline is intended to improve hospital‐wide efficiency because our SSU is equipped with continuous telemetry monitors, an exercise treadmill testing (ETT) laboratory, and other reserved cardiac tests (see Admission Characteristics and Services Received section, below). Fourth, patients' risk should be no higher than intermediate level. Admitting ED physicians are encouraged to use posted risk estimators for patients with provisional diagnoses of possible acute coronary syndrome (ACS), decompensated heart failure, asthma exacerbation, and out‐of‐control diabetes. Finally, patients should not need advanced ancillary services; these include bedside procedures (eg, central venous catheter insertions), time‐intensive nursing (eg, regular dressing changes), and complex social‐services (eg, long‐term care facility placements).

Subjects
The study subjects were all patients admitted to the SSU during the 4‐month study period. Patients were excluded from the entire study if they refused verbal consent to participate. All patients who consented were included in the description of patient admission characteristics. Thirteen patients who prematurely left the SSU against medical advice, however, were neither included in the descriptions of services received nor in the analyses of predictors of successful SSU stays. We excluded these patients because they needed services that they did not receiveincluding these patients in our analysis would tend to overestimate the efficiency of our SSU by shortening the length‐of‐stay (LOS) without adding diagnostic tests or treatments.
Data Collection
After receiving approval from the institutional review board, attending physician investigators conducted an interview, physical examination, and review of medical records for each enrolled patient within 12 hours of admission to the SSU. When ED attending physicians' provisional primary diagnoses included possible ACS or decompensated heart failure, which we knew from earlier pilot data were our 2 most common provisional diagnoses, investigators gathered patient data to be applied in validated models of risk after the study period (Figure 2).9, 10 Some of the clinical predictors required for these models are based on patients' findings on presentation to the ED. For example, Goldman's risk model for major cardiac events uses patients' initial systolic blood pressures on presentation to the ED.9 In such cases, investigators gathered needed data from electronic and paper charts generated in the ED. Upon discharge from the SSU, investigators reviewed patients' medical records a second time. All data were entered by investigators and instantly committed into an online database.

Admission Characteristics and Services Received
Patients were grouped according to the provisional diagnoses of ED attending physicians upon admission to the SSU (Figure 2). We chose to group patients by the provisional diagnoses of EDnot SSUattending physicians to better understand how ED physicians, the physicians who make the admission‐location decisions in our hospital, were using the SSU. Patients were first grouped as having possible ACS or heart failure, because patients with these provisional diagnoses were preferentially admitted to the SSU (Figure 1). When neither diagnosis was listed, patients were grouped according to ED attending physicians' first‐listed diagnoses. At the end of the study period, relevant risk models were applied to patients with possible ACS or heart failure and stratified as very low, low, intermediate, or high risk.9, 10 Patients with both possible ACS and heart failure were grouped according to the diagnosis with the highest corresponding risk assessment; if both risk assessments were the same, then the first‐listed diagnosis was used. Though developed to predict different clinical outcomes during different time periods, risk strata from the corresponding risk models were pooled across both diagnoses to develop a risk summary.
Upon discharge, investigators recorded which advanced diagnostic tests, specialty consultations, and acute care treatments patients received while in the SSU. Diagnostic tests were considered advanced if they were not routinely performed within 2 hours of being ordered. Advanced diagnostic tests were grouped into 2 types by their accessibility to ordering SSU physicians. Open access tests included echocardiograms and ETTs, which were reserved for SSU patients 6 days per week. Though the availability of open access tests was not unlimited, ordering physicians' needs for them rarely exceeded the immediate supply. On the other hand, limited access tests included both cardiac stress imaging studies, which were reserved for SSU patients on a very limited basis 4 or 5 days per week, and other tests that were not reserved for SSU patients, such as endoscopy, magnetic resonance imaging, or ultrasonography. Ordering physicians' needs for limited access tests often exceeded their immediate supply; in such cases, SSU patients were placed without priority into queues that included patients from the entire hospital.
Investigators recorded when patients received advanced diagnostic tests that were ordered by specialists. These tests, however, were not included in analyses of how services received by SSU patients affected SSU success, because SSU attending physicians were only indirectly involved in whether or not patients received these tests. Treatments were considered acute care treatments if they were commonly administered only in acute care settings, such as heparin for unstable angina or intravenous furosemide for pulmonary edema.
SSU Success
The SSU was designed to care for patients during brief stays and without eventual admission to traditional inpatient services. Therefore, we used patients' LOS and whether or not patients were admitted to traditional inpatient services as measures of SSU success. LOS was calculated from the time patients arrived in the SSU until the time they left. Therefore, neither time spent in the ED before admission to the SSU nor time spent on traditional inpatient services (if needed) contributed to our definition of LOS. Individual SSU patients were considered successfully cared for in the SSU if their LOS was less than 72 hours and they were discharged directly home from the SSU. We explored associations between these outcomes and provisional diagnoses, risk assessments, and services received.
Data Analysis
LOS data were right‐skewed; therefore, we used the Mann‐Whitney test for comparisons between 2 groups and the Kruskal‐Wallis test for comparisons among 3 or more groups. To test for trends of median LOS among ordered groupings, we used the method of Cuzick.11 We used Pearson's chi‐square test to compare proportions of patients grouped into categories and the chi‐square test for trends with equal scoring to test for trends among ordered groupings.
We performed multiple logistic regression to explore which variables were associated with SSU success. The following 5 demographic variables from Table 1 were insignificant in all single‐variable and multiple‐variable regression models that we tested and were, therefore, removed from further analyses to create more parsimonious models: gender, language, ethnicity, race, and whether or not patients had a primary care provider. Our multiple logistic regression models were fitted by maximum likelihood methods. In all of these models, odds ratios (ORs) were adjusted for patient characteristics that included age (in years), insulin‐requiring diabetes mellitus (yes or no), SSU attending physician, day of the week of SSU admission (weekday or weekend), and hospitalization during the preceding year. Confidence intervals (CIs) for predicted probabilities were computed using the delta method. All analyses were conducted with Stata Statistical Software, Release 9 (StataCorp, College Station, TX).
| |
| Mean age, years (SD) (25th‐75th percentiles) | 58 (12) (49‐66) |
| Men | 389 (52) |
| Lacking a primary care provider | 256 (34) |
| Non‐English speaking | 217 (29) |
| Ethnicity is Hispanic or Latino | 105 (14) |
| Race is Black or African‐American | 480 (64) |
| Hospitalized within the preceding year | 322 (43) |
| Insulin‐requiring diabetes mellitus | 83 (11) |
| Previous coronary artery revascularization | 89 (12) |
| Provisional diagnosis* | |
| Possible acute coronary syndrome | 427 (57) |
| Heart failure | 214 (29) |
| Other cardiovascular | 62 (8) |
| Noncardiovascular | 48 (6) |
Results
Subjects
During the 4‐month study period, 755 patients were admitted to the SSU. Among these patients, 4 were excluded from our study because they refused verbal consent. In the remaining study sample of 751 patients, all were included in the descriptions of patients' admission characteristics (Table 1), but 13 patients who left prematurely were excluded in both the descriptions of services received (Table 2) and the analyses of SSU success (Tables 3 and 4).
| Service received | Possible ACS n = 418 (%) | Heart Failure n = 211 (%) | Other Cardiovascular n = 61 (%) | Noncardiovascular n = 48 (%) | Total n = 738 (%) |
|---|---|---|---|---|---|
| |||||
| Open access test* | 37 | 59 | 56 | 13 | 43 |
| Resting | |||||
| Echocardiography | 29 | 59 | 56 | 13 | 39 |
| ETT | 12 | 0 | 5 | 0 | 7 |
| Limited access test | 24 | 8 | 10 | 8 | 17 |
| Stress imaging | 21 | 5 | 7 | 2 | 14 |
| Acute care treatment | 22 | 78 | 5 | 60 | 39 |
| Specialty consultation∥ | 24 | 12 | 20 | 8 | 19 |
| Any above service | 68 | 93 | 67 | 69 | 75 |
| Provisional Diagnosis and Services Received | n | Median LOS [hours (IQR)] | Stay Longer than 72 Hours (%) | Admission to Traditional Inpatient Service (%) | Stay Longer than 72 Hours or Admission to Traditional Inpatient Service (%) |
|---|---|---|---|---|---|
| |||||
| All patients | 738 | 42 (22‐63) | 15 | 9* | 21 |
| Possible ACS | 418 | 37 (20‐57) | 13 | 10 | 20 |
| Heart failure | 211 | 47 (34‐69) | 21 | 9 | 27 |
| Other CV | 61 | 40 (21‐49) | 10 | 5 | 11 |
| Non‐CV | 48 | 40 (22‐60) | 10 | 2 | 13 |
| P value | <0.001 | 0.04 | 0.18 | 0.01 | |
| Open access test | |||||
| Yes | 320 | 46 (31‐67) | 17 | 9 | 23 |
| No | 418 | 33 (20‐52) | 13 | 9 | 20 |
| P value | <0.001 | 0.15 | 0.87 | 0.43 | |
| Limited access test | |||||
| Yes | 128 | 51 (42‐82) | 31 | 6 | 33 |
| No | 610 | 38 (21‐55) | 12 | 10 | 19 |
| P value | <0.001 | <0.001 | 0.24 | <0.001 | |
| Acute care treatment | |||||
| Yes | 287 | 46 (34‐69) | 21 | 12 | 29 |
| No | 451 | 32 (20‐50) | 11 | 7 | 16 |
| P value | <0.001 | <0.001 | 0.01 | <0.001 | |
| Specialty consultation | |||||
| Yes | 141 | 63 (40‐92) | 38 | 28 | 52 |
| No | 597 | 38 (21‐50) | 10 | 5 | 14 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Any above service | |||||
| Yes | 554 | 46 (29‐68) | 19 | 10 | 26 |
| No | 184∥ | 22 (16‐32) | 2 | 5 | 7 |
| P value | <0.001 | <0.001 | 0.03 | <0.001 | |
| Stay Longer than 72 Hours | Admission to Traditional Inpatient Service | Either Outcome | ||||
|---|---|---|---|---|---|---|
| OR* | P value | OR* | P value | OR* | P value | |
| ||||||
| Heart failure | 2.3 | 0.01 | 1.1 | 0.77 | 1.9 | 0.02 |
| Service received | ||||||
| Open access test | 1.5 | 0.10 | 1.0 | 0.89 | 1.2 | 0.32 |
| Limited access test | 5.1 | <0.001 | 0.4 | 0.03 | 2.5 | <0.001 |
| Acute care treatment | 1.7 | 0.07 | 1.4 | 0.31 | 1.6 | 0.05 |
| Specialty consultation | 6.1 | <0.001 | 13.1 | <0.001 | 8.1 | <0.001 |
Admission Characteristics and Services Received
A narrow range of provisional diagnoses were listed by ED attending physicians and 641 patients (85% of 751) were grouped as having possible ACS or heart failure (Figure 2). Patients with these diagnoses were later risk‐stratified and, when pooled across risk strata, only 14 patients (2% of 641) exceeded the suggested admission‐location criterion for the SSU of lower than high risk. Despite the array and frequency of diagnostic and treatment services that patients received, SSU physicians worked mostly independently, requesting specialty consultations for only 19% of patients (141/738; Table 2).
SSU Success
The median LOS for all patients was 42 hours (interquartile range [IRQ] 22‐63) and 156 patients (21% of 738) had unsuccessful SSU stays (Table 3). The most common reason for an unsuccessful stay was a stay longer than 72 hours (71% of 156). Among the 66 patients who required admission to traditional inpatient services, nearly one‐half (48%) were admitted expressly to receive treatments not available in the SSU after having a specialty consult.
Patients' provisional diagnoses were associated with unsuccessful stays in bivariate analyses (Table 3). In addition, when patients were grouped into 3 risk stratums (very‐low, low, and intermediate‐and‐high), unsuccessful stays increased with increasing risk. For example, in patients with possible ACS, the proportion of unsuccessful stays increased from 17% of 306 very‐low risk patients to 27% of 55 intermediate‐and‐high risk patients (P value for trend = 0.012. Similarly, in patients with heart failure, the proportion of unsuccessful stays increased from 25% of 181 very‐low risk patients to 100% of 3 intermediate‐and‐high risk patients (P value for trend = 0.004).
However, in multiple variable models that simultaneously included patients' characteristics upon admission with services received during their SSU stay, only the provisional diagnosis of heart failure was associated with unsuccessful stays (OR, 1.9; 95% CI, 1.12‐3.18); risk assessments for possible ACS (P = 0.29) and heart failure (P = 0.32) were unimportant predictors of unsuccessful stays (Table 4). On the other hand, whether or not patients received diagnostic tests, acute care treatments, or specialty consultations were important predictors. In particular, patients who received specialty consultations were much more likely to require admission to traditional inpatient services than those patients who did not (OR, 13.1; 95% CI, 6.9‐24.9) and had a 52% chance of having an unsuccessful stay (95% CI, 42‐61%; Figure 3). In addition, the accessibility of a diagnostic test was inversely proportional to the chance of having a long stay; patients who received an open access test had a 12% chance of a long stay (95% CI, 8‐16%) whereas those who received a limited access test had a 29% chance of a long stay (95% CI, 20‐39%). Receiving acute care treatments was also a significant, though less important, predictor of an unsuccessful stay (Table 4).

Discussion
We found that the types of services received by patients during their SSU stays were stronger predictors of long stays and eventual admissions to traditional inpatient services than patients' characteristics upon admission to the SSU. This suggests that SSUs should be focused toward matching patients' anticipated needs with readily accessible services. For example, in our SSU, which cares for over 2,250 patients annually, more than 1,200 patients will receive diagnostic tests in a given year. Among these patients, those who receive a limited access test will be more than twice as likely to have long stays than those who receive an open access test (Figure 3). Though our conclusions may not be applicable to other settings, this study is the most comprehensive description of patients admitted to a hospitalist‐run SSU. In addition, our study is the first to demonstrate that diagnostic and consultative services are the most important predictors of successful stays in SSUs. This promotes the practical strategy that hospitalists who staff SSUs should focus administratively toward gaining access to these services.
Very few of our SSU patients did not fulfill the suggested requirements of our admission location guidelines. For example, only 2% of 691 patients with either possible ACS or heart failure were high risk (Figure 2). Despite this, 21% of our patients had stays longer than 72 hours or were admitted to traditional inpatient services. The paradoxically high proportion of unsuccessful stays among mostly very‐low and low risk patients simply reflects how the clinical risk models that we used were not designed to predict unsuccessful stays. Moreover, as our multiple variable models suggest, improvements in the selection process of candidate SSU patients are more likely to come from an ability to incorporate assessments of what services patients will receive rather than from assessments of their clinical risk (Table 4). Therefore, the immediate plans of the accepting SSU physicians, the physicians who will determine what services patients eventually receive, should be incorporated in the admission‐location decision process.
Three of our findings highlight how input from accepting SSU physiciansconveyed to ED physicians before their final admission‐location decisions are mademay improve the SSU patient selection process. First, 23% of our patients were discharged home after brief stays with no advanced tests, specialty consultations, or acute care treatments (Table 3). Though some of these patients may have required inpatient services other than the ones we recorded, most were admitted with very‐low risk possible ACS; if they required overnight stays at all, many of them may have been better cared for in the ED observation unit (Figure 1). Second, 74% of the patients who required admission to traditional inpatient services were admitted for services not readily available to patients in the SSU (Table 3). Among these patients, nearly one‐third (21/66) received no advanced diagnostic tests in the SSU. This suggests that these patients should have been admitted directly to the general medical wards; doing so may have improved efficiency and quality of care by reducing unnecessary handoffs between physicians. Both types of patientsthose with minimal inpatient needs and those with more needs than the SSU can providehighlight how incorporating accepting SSU physicians' plans may improve the SSU patient selection process. After all, those best equipped to determine if the SSU will meet (or exceed) the needs of candidate patients are the SSU physicians themselves.
Third, we found that whether or not SSU physicians required assistance from specialists was the strongest predictor of unsuccessful stays: when an accepting physician determined that a patient should receive a specialty consultation, that patient's chance of having an unsuccessful stay was over 50% (Figure 3). Our study was not designed to determine how specialty consultations were associated with unsuccessful stays. We did not, for example, record whether or not hospitalists changed their diagnostic, treatment, or admission plans because of specialists' recommendations.12 Therefore, we cannot conclude that specialty consultations actually caused long stays or traditional admissions. Nevertheless, when our SSU physicians did not manage patients independent of specialty consultations, we observed a high likelihood of unsuccessful stays. Because accepting SSU physicians are the ones who will determine whether or not they need assistance from specialists, weighing their immediate plans for specialty consultations into the admission‐location decision process may improve the efficiency of SSUs. Others have recognized the importance of specialty consultations in SSUs by directly incorporating specialists as coattending physicians.13
Our study had several limitations. First, we studied mostly patients with cardiovascular diagnoses. Predictors of success in SSUs that admit patients with different diagnostic profiles may be different. In particular, SSUs that admit patients with a wide array of diagnoses may find that matching patients' needs with readily accessible services is impractical, because these needs may be too wide‐ranging. Second, our study design was observational. However, other than seasonal variations in admission patterns, there was little room for selection bias because we enrolled all consecutive admissions over the 4‐month study period, which gives us more confidence in our results. Third, our study did not record whether or not ED physicians knowingly overrode the suggested admission‐location guidelines because of limited bed availability. Yet, if shortages of beds on traditional inpatient services were driving patients who were otherwise candidates for the general medical wards in to the SSU, then we would have expected higher‐risk patients and greater needs for limited access tests. Finally, our descriptions of patients' needs were based on what diagnostic, consultative, and treatment services patients actually received; yet these needs did not include diagnostic tests that were ordered but never performed. However, any missed needs would bias our results toward no association with unsuccessful stays, because unsuccessful stays would generally increase while patients await needed services.
Future research could address these limitations through an experimental trial of traditional admissions versus admission to a hospitalist‐run SSU. And, because hospitals are complex systems of health care delivery where changes in one patient care unit often affect others in unanticipated ways,14 the impact of SSUs on other patient care units that are closely connected to SSUs, such as EDs and the general medical wards (Figure 1), should be simultaneously observed. For example, though our findings suggest that the accessibility of diagnostic tests should parallel ordering SSU physicians' needs for those tests, making all diagnostic tests open to SSU physicians may result in shortsightedly lengthening the stays of patients in other care units. Future research should also observe the decision‐making process of both the physicians who make admission‐location decisions (ED physicians) and those who determine the eventual plans for patients in the SSU (hospitalists). Accepting physicians from other patient care units have found improved outcomes of efficiency when they were involved in the complex process of deciding where to admit patients.15, 16 After an initial evaluation of a candidate SSU patient in the ED, a hospitalist who staffs both the general medical wards and the SSU would be uniquely well‐positioned to help an ED physician decide where a patient's needs would be best met. Although ED physicians will rightly be concerned that consulting SSU hospitalists may slow patient flow, hands‐on consultations of candidate SSU patients, who have a narrow range of diagnoses and low‐risk profiles, would likely be brief. In addition, because many SSUs are conveniently adjacent to EDs, the burden of communication may be minor.1 To address these questions, hospitalists who staff SSUs must continue the observed trend of working collaboratively with ED physicians.15, 17, 18
Acknowledgements
The authors thank Arthur T. Evans and Brendan M. Reilly for their insightful review of the manuscript. The authors also thank Zhaotai Cui for his assistance with statistical programming.
Short‐stay units (SSUs) are common alternatives to traditional inpatient services.1 When defined broadly to include observation units for low‐risk chest pain patients, SSUs exist in one‐third of hospitals in the United States.2 Amidst growing demands for inpatient services, SSUs have recently developed beyond observation medicine to provide more complex inpatient services in locations commonly adjacent to emergency departments (EDs).1 Hospitalists are well‐positioned to staff these emerging SSUs because of their expertise in managing complex inpatient services.3
Despite this, we found only 3 reports of hospitalist‐run SSUs designed for general medical inpatients (2 from Spain and 1 from Canada).46 Whereas these early reports introduce hospitalist‐run SSUs, they provide limited data to make firm conclusions about their usefulness or appropriate design. For example, none of these reports assessed patients' characteristics upon admission. Nor did they provide details about the services that the SSUs provided. Yet evaluation of both types of patient‐level datadescriptions of patients' needs upon admission and how these needs are met during their staysdetermine whether or not hospitalist‐run SSUs meet their potential to efficiently care for backlogs of patients who otherwise await admission to traditional inpatient services.
In order to further explore these issues, we first sought to characterize our SSU patients upon admission and record what services they received during their stays. To help interpret our results, we then investigated associations between these characteristics and measures of successfully caring for patients in our SSU.
Patients and Methods
Design and Setting
In this prospective cohort study, we included all patients admitted to the hospitalist‐run SSU of Cook County Hospital, a 500‐bed public teaching hospital in Chicago, Illinois, from January through April of 2006. Our 14‐bed SSU opened in 2002 to reduce overcrowding on the traditional inpatient wards by admitting adult patients who require inpatient care but might be eligible for discharge within 3 days. The unit is geographically part of the ED but is staffed by resident physicians and a rotating group of hospitalist attending physicians from the Department of Medicine. At least 1 attending and resident physician are available throughout the day, including weekend days and holidays; evenings are covered by a resident who presents overnight admissions to an attending physician the following morning.
ED physicians admit general medical patients to the SSU 24 hours per day, 7 days per week. Though admissions do not require prior approval from SSU physicians, the Departments of Medicine and Emergency Medicine have collaboratively promoted 5 suggested admission‐location guidelines to admitting ED physicians (Figure 1). For candidate SSU patients, these 5 guidelines are not intended to be restrictive but to provide a framework for the complex decision‐making process that our ED physicians encounter, particularly during periods of extreme overcrowding.7 First, patients should have an anticipated stay shorter than 72 hours. Second, patients should not have an eventual need for admission to traditional inpatient services such as the general medicine wards or intensive care units; this guideline is intended to improve patient safety by reducing unnecessary handoffs between physicians.8 Third, patients with provisional cardiovascular diagnoses should be preferentially admitted to the SSU over the general medical wards; this guideline is intended to improve hospital‐wide efficiency because our SSU is equipped with continuous telemetry monitors, an exercise treadmill testing (ETT) laboratory, and other reserved cardiac tests (see Admission Characteristics and Services Received section, below). Fourth, patients' risk should be no higher than intermediate level. Admitting ED physicians are encouraged to use posted risk estimators for patients with provisional diagnoses of possible acute coronary syndrome (ACS), decompensated heart failure, asthma exacerbation, and out‐of‐control diabetes. Finally, patients should not need advanced ancillary services; these include bedside procedures (eg, central venous catheter insertions), time‐intensive nursing (eg, regular dressing changes), and complex social‐services (eg, long‐term care facility placements).

Subjects
The study subjects were all patients admitted to the SSU during the 4‐month study period. Patients were excluded from the entire study if they refused verbal consent to participate. All patients who consented were included in the description of patient admission characteristics. Thirteen patients who prematurely left the SSU against medical advice, however, were neither included in the descriptions of services received nor in the analyses of predictors of successful SSU stays. We excluded these patients because they needed services that they did not receiveincluding these patients in our analysis would tend to overestimate the efficiency of our SSU by shortening the length‐of‐stay (LOS) without adding diagnostic tests or treatments.
Data Collection
After receiving approval from the institutional review board, attending physician investigators conducted an interview, physical examination, and review of medical records for each enrolled patient within 12 hours of admission to the SSU. When ED attending physicians' provisional primary diagnoses included possible ACS or decompensated heart failure, which we knew from earlier pilot data were our 2 most common provisional diagnoses, investigators gathered patient data to be applied in validated models of risk after the study period (Figure 2).9, 10 Some of the clinical predictors required for these models are based on patients' findings on presentation to the ED. For example, Goldman's risk model for major cardiac events uses patients' initial systolic blood pressures on presentation to the ED.9 In such cases, investigators gathered needed data from electronic and paper charts generated in the ED. Upon discharge from the SSU, investigators reviewed patients' medical records a second time. All data were entered by investigators and instantly committed into an online database.

Admission Characteristics and Services Received
Patients were grouped according to the provisional diagnoses of ED attending physicians upon admission to the SSU (Figure 2). We chose to group patients by the provisional diagnoses of EDnot SSUattending physicians to better understand how ED physicians, the physicians who make the admission‐location decisions in our hospital, were using the SSU. Patients were first grouped as having possible ACS or heart failure, because patients with these provisional diagnoses were preferentially admitted to the SSU (Figure 1). When neither diagnosis was listed, patients were grouped according to ED attending physicians' first‐listed diagnoses. At the end of the study period, relevant risk models were applied to patients with possible ACS or heart failure and stratified as very low, low, intermediate, or high risk.9, 10 Patients with both possible ACS and heart failure were grouped according to the diagnosis with the highest corresponding risk assessment; if both risk assessments were the same, then the first‐listed diagnosis was used. Though developed to predict different clinical outcomes during different time periods, risk strata from the corresponding risk models were pooled across both diagnoses to develop a risk summary.
Upon discharge, investigators recorded which advanced diagnostic tests, specialty consultations, and acute care treatments patients received while in the SSU. Diagnostic tests were considered advanced if they were not routinely performed within 2 hours of being ordered. Advanced diagnostic tests were grouped into 2 types by their accessibility to ordering SSU physicians. Open access tests included echocardiograms and ETTs, which were reserved for SSU patients 6 days per week. Though the availability of open access tests was not unlimited, ordering physicians' needs for them rarely exceeded the immediate supply. On the other hand, limited access tests included both cardiac stress imaging studies, which were reserved for SSU patients on a very limited basis 4 or 5 days per week, and other tests that were not reserved for SSU patients, such as endoscopy, magnetic resonance imaging, or ultrasonography. Ordering physicians' needs for limited access tests often exceeded their immediate supply; in such cases, SSU patients were placed without priority into queues that included patients from the entire hospital.
Investigators recorded when patients received advanced diagnostic tests that were ordered by specialists. These tests, however, were not included in analyses of how services received by SSU patients affected SSU success, because SSU attending physicians were only indirectly involved in whether or not patients received these tests. Treatments were considered acute care treatments if they were commonly administered only in acute care settings, such as heparin for unstable angina or intravenous furosemide for pulmonary edema.
SSU Success
The SSU was designed to care for patients during brief stays and without eventual admission to traditional inpatient services. Therefore, we used patients' LOS and whether or not patients were admitted to traditional inpatient services as measures of SSU success. LOS was calculated from the time patients arrived in the SSU until the time they left. Therefore, neither time spent in the ED before admission to the SSU nor time spent on traditional inpatient services (if needed) contributed to our definition of LOS. Individual SSU patients were considered successfully cared for in the SSU if their LOS was less than 72 hours and they were discharged directly home from the SSU. We explored associations between these outcomes and provisional diagnoses, risk assessments, and services received.
Data Analysis
LOS data were right‐skewed; therefore, we used the Mann‐Whitney test for comparisons between 2 groups and the Kruskal‐Wallis test for comparisons among 3 or more groups. To test for trends of median LOS among ordered groupings, we used the method of Cuzick.11 We used Pearson's chi‐square test to compare proportions of patients grouped into categories and the chi‐square test for trends with equal scoring to test for trends among ordered groupings.
We performed multiple logistic regression to explore which variables were associated with SSU success. The following 5 demographic variables from Table 1 were insignificant in all single‐variable and multiple‐variable regression models that we tested and were, therefore, removed from further analyses to create more parsimonious models: gender, language, ethnicity, race, and whether or not patients had a primary care provider. Our multiple logistic regression models were fitted by maximum likelihood methods. In all of these models, odds ratios (ORs) were adjusted for patient characteristics that included age (in years), insulin‐requiring diabetes mellitus (yes or no), SSU attending physician, day of the week of SSU admission (weekday or weekend), and hospitalization during the preceding year. Confidence intervals (CIs) for predicted probabilities were computed using the delta method. All analyses were conducted with Stata Statistical Software, Release 9 (StataCorp, College Station, TX).
| |
| Mean age, years (SD) (25th‐75th percentiles) | 58 (12) (49‐66) |
| Men | 389 (52) |
| Lacking a primary care provider | 256 (34) |
| Non‐English speaking | 217 (29) |
| Ethnicity is Hispanic or Latino | 105 (14) |
| Race is Black or African‐American | 480 (64) |
| Hospitalized within the preceding year | 322 (43) |
| Insulin‐requiring diabetes mellitus | 83 (11) |
| Previous coronary artery revascularization | 89 (12) |
| Provisional diagnosis* | |
| Possible acute coronary syndrome | 427 (57) |
| Heart failure | 214 (29) |
| Other cardiovascular | 62 (8) |
| Noncardiovascular | 48 (6) |
Results
Subjects
During the 4‐month study period, 755 patients were admitted to the SSU. Among these patients, 4 were excluded from our study because they refused verbal consent. In the remaining study sample of 751 patients, all were included in the descriptions of patients' admission characteristics (Table 1), but 13 patients who left prematurely were excluded in both the descriptions of services received (Table 2) and the analyses of SSU success (Tables 3 and 4).
| Service received | Possible ACS n = 418 (%) | Heart Failure n = 211 (%) | Other Cardiovascular n = 61 (%) | Noncardiovascular n = 48 (%) | Total n = 738 (%) |
|---|---|---|---|---|---|
| |||||
| Open access test* | 37 | 59 | 56 | 13 | 43 |
| Resting | |||||
| Echocardiography | 29 | 59 | 56 | 13 | 39 |
| ETT | 12 | 0 | 5 | 0 | 7 |
| Limited access test | 24 | 8 | 10 | 8 | 17 |
| Stress imaging | 21 | 5 | 7 | 2 | 14 |
| Acute care treatment | 22 | 78 | 5 | 60 | 39 |
| Specialty consultation∥ | 24 | 12 | 20 | 8 | 19 |
| Any above service | 68 | 93 | 67 | 69 | 75 |
| Provisional Diagnosis and Services Received | n | Median LOS [hours (IQR)] | Stay Longer than 72 Hours (%) | Admission to Traditional Inpatient Service (%) | Stay Longer than 72 Hours or Admission to Traditional Inpatient Service (%) |
|---|---|---|---|---|---|
| |||||
| All patients | 738 | 42 (22‐63) | 15 | 9* | 21 |
| Possible ACS | 418 | 37 (20‐57) | 13 | 10 | 20 |
| Heart failure | 211 | 47 (34‐69) | 21 | 9 | 27 |
| Other CV | 61 | 40 (21‐49) | 10 | 5 | 11 |
| Non‐CV | 48 | 40 (22‐60) | 10 | 2 | 13 |
| P value | <0.001 | 0.04 | 0.18 | 0.01 | |
| Open access test | |||||
| Yes | 320 | 46 (31‐67) | 17 | 9 | 23 |
| No | 418 | 33 (20‐52) | 13 | 9 | 20 |
| P value | <0.001 | 0.15 | 0.87 | 0.43 | |
| Limited access test | |||||
| Yes | 128 | 51 (42‐82) | 31 | 6 | 33 |
| No | 610 | 38 (21‐55) | 12 | 10 | 19 |
| P value | <0.001 | <0.001 | 0.24 | <0.001 | |
| Acute care treatment | |||||
| Yes | 287 | 46 (34‐69) | 21 | 12 | 29 |
| No | 451 | 32 (20‐50) | 11 | 7 | 16 |
| P value | <0.001 | <0.001 | 0.01 | <0.001 | |
| Specialty consultation | |||||
| Yes | 141 | 63 (40‐92) | 38 | 28 | 52 |
| No | 597 | 38 (21‐50) | 10 | 5 | 14 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Any above service | |||||
| Yes | 554 | 46 (29‐68) | 19 | 10 | 26 |
| No | 184∥ | 22 (16‐32) | 2 | 5 | 7 |
| P value | <0.001 | <0.001 | 0.03 | <0.001 | |
| Stay Longer than 72 Hours | Admission to Traditional Inpatient Service | Either Outcome | ||||
|---|---|---|---|---|---|---|
| OR* | P value | OR* | P value | OR* | P value | |
| ||||||
| Heart failure | 2.3 | 0.01 | 1.1 | 0.77 | 1.9 | 0.02 |
| Service received | ||||||
| Open access test | 1.5 | 0.10 | 1.0 | 0.89 | 1.2 | 0.32 |
| Limited access test | 5.1 | <0.001 | 0.4 | 0.03 | 2.5 | <0.001 |
| Acute care treatment | 1.7 | 0.07 | 1.4 | 0.31 | 1.6 | 0.05 |
| Specialty consultation | 6.1 | <0.001 | 13.1 | <0.001 | 8.1 | <0.001 |
Admission Characteristics and Services Received
A narrow range of provisional diagnoses were listed by ED attending physicians and 641 patients (85% of 751) were grouped as having possible ACS or heart failure (Figure 2). Patients with these diagnoses were later risk‐stratified and, when pooled across risk strata, only 14 patients (2% of 641) exceeded the suggested admission‐location criterion for the SSU of lower than high risk. Despite the array and frequency of diagnostic and treatment services that patients received, SSU physicians worked mostly independently, requesting specialty consultations for only 19% of patients (141/738; Table 2).
SSU Success
The median LOS for all patients was 42 hours (interquartile range [IRQ] 22‐63) and 156 patients (21% of 738) had unsuccessful SSU stays (Table 3). The most common reason for an unsuccessful stay was a stay longer than 72 hours (71% of 156). Among the 66 patients who required admission to traditional inpatient services, nearly one‐half (48%) were admitted expressly to receive treatments not available in the SSU after having a specialty consult.
Patients' provisional diagnoses were associated with unsuccessful stays in bivariate analyses (Table 3). In addition, when patients were grouped into 3 risk stratums (very‐low, low, and intermediate‐and‐high), unsuccessful stays increased with increasing risk. For example, in patients with possible ACS, the proportion of unsuccessful stays increased from 17% of 306 very‐low risk patients to 27% of 55 intermediate‐and‐high risk patients (P value for trend = 0.012. Similarly, in patients with heart failure, the proportion of unsuccessful stays increased from 25% of 181 very‐low risk patients to 100% of 3 intermediate‐and‐high risk patients (P value for trend = 0.004).
However, in multiple variable models that simultaneously included patients' characteristics upon admission with services received during their SSU stay, only the provisional diagnosis of heart failure was associated with unsuccessful stays (OR, 1.9; 95% CI, 1.12‐3.18); risk assessments for possible ACS (P = 0.29) and heart failure (P = 0.32) were unimportant predictors of unsuccessful stays (Table 4). On the other hand, whether or not patients received diagnostic tests, acute care treatments, or specialty consultations were important predictors. In particular, patients who received specialty consultations were much more likely to require admission to traditional inpatient services than those patients who did not (OR, 13.1; 95% CI, 6.9‐24.9) and had a 52% chance of having an unsuccessful stay (95% CI, 42‐61%; Figure 3). In addition, the accessibility of a diagnostic test was inversely proportional to the chance of having a long stay; patients who received an open access test had a 12% chance of a long stay (95% CI, 8‐16%) whereas those who received a limited access test had a 29% chance of a long stay (95% CI, 20‐39%). Receiving acute care treatments was also a significant, though less important, predictor of an unsuccessful stay (Table 4).

Discussion
We found that the types of services received by patients during their SSU stays were stronger predictors of long stays and eventual admissions to traditional inpatient services than patients' characteristics upon admission to the SSU. This suggests that SSUs should be focused toward matching patients' anticipated needs with readily accessible services. For example, in our SSU, which cares for over 2,250 patients annually, more than 1,200 patients will receive diagnostic tests in a given year. Among these patients, those who receive a limited access test will be more than twice as likely to have long stays than those who receive an open access test (Figure 3). Though our conclusions may not be applicable to other settings, this study is the most comprehensive description of patients admitted to a hospitalist‐run SSU. In addition, our study is the first to demonstrate that diagnostic and consultative services are the most important predictors of successful stays in SSUs. This promotes the practical strategy that hospitalists who staff SSUs should focus administratively toward gaining access to these services.
Very few of our SSU patients did not fulfill the suggested requirements of our admission location guidelines. For example, only 2% of 691 patients with either possible ACS or heart failure were high risk (Figure 2). Despite this, 21% of our patients had stays longer than 72 hours or were admitted to traditional inpatient services. The paradoxically high proportion of unsuccessful stays among mostly very‐low and low risk patients simply reflects how the clinical risk models that we used were not designed to predict unsuccessful stays. Moreover, as our multiple variable models suggest, improvements in the selection process of candidate SSU patients are more likely to come from an ability to incorporate assessments of what services patients will receive rather than from assessments of their clinical risk (Table 4). Therefore, the immediate plans of the accepting SSU physicians, the physicians who will determine what services patients eventually receive, should be incorporated in the admission‐location decision process.
Three of our findings highlight how input from accepting SSU physiciansconveyed to ED physicians before their final admission‐location decisions are mademay improve the SSU patient selection process. First, 23% of our patients were discharged home after brief stays with no advanced tests, specialty consultations, or acute care treatments (Table 3). Though some of these patients may have required inpatient services other than the ones we recorded, most were admitted with very‐low risk possible ACS; if they required overnight stays at all, many of them may have been better cared for in the ED observation unit (Figure 1). Second, 74% of the patients who required admission to traditional inpatient services were admitted for services not readily available to patients in the SSU (Table 3). Among these patients, nearly one‐third (21/66) received no advanced diagnostic tests in the SSU. This suggests that these patients should have been admitted directly to the general medical wards; doing so may have improved efficiency and quality of care by reducing unnecessary handoffs between physicians. Both types of patientsthose with minimal inpatient needs and those with more needs than the SSU can providehighlight how incorporating accepting SSU physicians' plans may improve the SSU patient selection process. After all, those best equipped to determine if the SSU will meet (or exceed) the needs of candidate patients are the SSU physicians themselves.
Third, we found that whether or not SSU physicians required assistance from specialists was the strongest predictor of unsuccessful stays: when an accepting physician determined that a patient should receive a specialty consultation, that patient's chance of having an unsuccessful stay was over 50% (Figure 3). Our study was not designed to determine how specialty consultations were associated with unsuccessful stays. We did not, for example, record whether or not hospitalists changed their diagnostic, treatment, or admission plans because of specialists' recommendations.12 Therefore, we cannot conclude that specialty consultations actually caused long stays or traditional admissions. Nevertheless, when our SSU physicians did not manage patients independent of specialty consultations, we observed a high likelihood of unsuccessful stays. Because accepting SSU physicians are the ones who will determine whether or not they need assistance from specialists, weighing their immediate plans for specialty consultations into the admission‐location decision process may improve the efficiency of SSUs. Others have recognized the importance of specialty consultations in SSUs by directly incorporating specialists as coattending physicians.13
Our study had several limitations. First, we studied mostly patients with cardiovascular diagnoses. Predictors of success in SSUs that admit patients with different diagnostic profiles may be different. In particular, SSUs that admit patients with a wide array of diagnoses may find that matching patients' needs with readily accessible services is impractical, because these needs may be too wide‐ranging. Second, our study design was observational. However, other than seasonal variations in admission patterns, there was little room for selection bias because we enrolled all consecutive admissions over the 4‐month study period, which gives us more confidence in our results. Third, our study did not record whether or not ED physicians knowingly overrode the suggested admission‐location guidelines because of limited bed availability. Yet, if shortages of beds on traditional inpatient services were driving patients who were otherwise candidates for the general medical wards in to the SSU, then we would have expected higher‐risk patients and greater needs for limited access tests. Finally, our descriptions of patients' needs were based on what diagnostic, consultative, and treatment services patients actually received; yet these needs did not include diagnostic tests that were ordered but never performed. However, any missed needs would bias our results toward no association with unsuccessful stays, because unsuccessful stays would generally increase while patients await needed services.
Future research could address these limitations through an experimental trial of traditional admissions versus admission to a hospitalist‐run SSU. And, because hospitals are complex systems of health care delivery where changes in one patient care unit often affect others in unanticipated ways,14 the impact of SSUs on other patient care units that are closely connected to SSUs, such as EDs and the general medical wards (Figure 1), should be simultaneously observed. For example, though our findings suggest that the accessibility of diagnostic tests should parallel ordering SSU physicians' needs for those tests, making all diagnostic tests open to SSU physicians may result in shortsightedly lengthening the stays of patients in other care units. Future research should also observe the decision‐making process of both the physicians who make admission‐location decisions (ED physicians) and those who determine the eventual plans for patients in the SSU (hospitalists). Accepting physicians from other patient care units have found improved outcomes of efficiency when they were involved in the complex process of deciding where to admit patients.15, 16 After an initial evaluation of a candidate SSU patient in the ED, a hospitalist who staffs both the general medical wards and the SSU would be uniquely well‐positioned to help an ED physician decide where a patient's needs would be best met. Although ED physicians will rightly be concerned that consulting SSU hospitalists may slow patient flow, hands‐on consultations of candidate SSU patients, who have a narrow range of diagnoses and low‐risk profiles, would likely be brief. In addition, because many SSUs are conveniently adjacent to EDs, the burden of communication may be minor.1 To address these questions, hospitalists who staff SSUs must continue the observed trend of working collaboratively with ED physicians.15, 17, 18
Acknowledgements
The authors thank Arthur T. Evans and Brendan M. Reilly for their insightful review of the manuscript. The authors also thank Zhaotai Cui for his assistance with statistical programming.
- ,,.A paradigm shift in the nature of care provision in emergency departments.Emerg Med J.2004;21:681–684.
- ,,.Acute coronary syndromes. In: Marx J, Hockberger R, Walls R, eds.Rosen's emergency medicine. Concepts and clinical practice.6th ed.Edinburgh:Mosby Elsevier;2006:1154–1199.
- .Taking charge of observation units for better patient flow.Todays Hospitalist Mag.2007;5(7):16–20.
- ,,, et al.Unidad de corta estancia dependiente de Medicina Interna.An Med Interna.1999;16:504–510.
- ,,,,.Factors that predict unplanned hospital readmission of patients discharged from a short stay medical unit.An Med Interna.2002;19:221–225.
- ,,, et al.Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital.CMAJ.2000;163(11):1477–1480.
- ,,,.Triage of patients with chest pain in the emergency department: a comparative study of physicians' decisions.Am J Med.2002;112:95–103.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,, et al.Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain.N Engl J Med.1996;334:1498–1504.
- ,,, et al.Risk stratification for in‐hospital mortality in acutely decompensated heart failure.JAMA.2005;293:572–580.
- .A Wilcoxon‐type test for trend.Stat Med.1985;4:87–90.
- ,,,,,.Diagnostic test restraint and the specialty consultation.J Gen Intern Med.1990;5:95–103.
- ,,, et al.A cooperative care model: cardiologists and hospitalists reduce length of stay in a chest pain observation unit.Crit Pathw Cardiol.2005;4:55–58.
- ,.Average length of stay, delayed discharge, and hospital congestion.BMJ.2002;325:610–611.
- .An internist in the emergency department: the IM facilitator program.HMO Pract.1991;10:42–43.
- ,,, et al.A “closed” medical intensive care unit (MICU) improves resource utilization when compared with an “open” MICU.Am J Respir Crit Care Med.1998;157:1468–1473.
- .Hospitalists' new role in the ED: “clog busters.”Todays Hospitalist Mag.2005;3(8):15–18.
- .Kindred spirits: ED doctors, hospitalists forge a critical collaboration.Hospitalist.2007;11(7):1,16–20.
- ,,.A paradigm shift in the nature of care provision in emergency departments.Emerg Med J.2004;21:681–684.
- ,,.Acute coronary syndromes. In: Marx J, Hockberger R, Walls R, eds.Rosen's emergency medicine. Concepts and clinical practice.6th ed.Edinburgh:Mosby Elsevier;2006:1154–1199.
- .Taking charge of observation units for better patient flow.Todays Hospitalist Mag.2007;5(7):16–20.
- ,,, et al.Unidad de corta estancia dependiente de Medicina Interna.An Med Interna.1999;16:504–510.
- ,,,,.Factors that predict unplanned hospital readmission of patients discharged from a short stay medical unit.An Med Interna.2002;19:221–225.
- ,,, et al.Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital.CMAJ.2000;163(11):1477–1480.
- ,,,.Triage of patients with chest pain in the emergency department: a comparative study of physicians' decisions.Am J Med.2002;112:95–103.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,, et al.Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain.N Engl J Med.1996;334:1498–1504.
- ,,, et al.Risk stratification for in‐hospital mortality in acutely decompensated heart failure.JAMA.2005;293:572–580.
- .A Wilcoxon‐type test for trend.Stat Med.1985;4:87–90.
- ,,,,,.Diagnostic test restraint and the specialty consultation.J Gen Intern Med.1990;5:95–103.
- ,,, et al.A cooperative care model: cardiologists and hospitalists reduce length of stay in a chest pain observation unit.Crit Pathw Cardiol.2005;4:55–58.
- ,.Average length of stay, delayed discharge, and hospital congestion.BMJ.2002;325:610–611.
- .An internist in the emergency department: the IM facilitator program.HMO Pract.1991;10:42–43.
- ,,, et al.A “closed” medical intensive care unit (MICU) improves resource utilization when compared with an “open” MICU.Am J Respir Crit Care Med.1998;157:1468–1473.
- .Hospitalists' new role in the ED: “clog busters.”Todays Hospitalist Mag.2005;3(8):15–18.
- .Kindred spirits: ED doctors, hospitalists forge a critical collaboration.Hospitalist.2007;11(7):1,16–20.
Copyright © 2009 Society of Hospital Medicine
PE and AMI in Undiagnosed PFO
Acute pulmonary embolism (PE) and acute myocardial infarction (AMI) are common inpatient diagnoses, and are frequently in the differential diagnosis of patients evaluated for chest pain and dyspnea. We present a case with 1 unifying explanation for these entities to coexist. Acute PE with subsequent embolism to the coronary arteries via a patent foramen ovale (PFO) is rare, but the underlying disorder and anatomical variant are common. Of practical significance, hospitalized patients with acute PE and PFO may have up to a 5‐fold increase in morbidity compared to patients with isolated PE.
CASE REPORT
A 79‐year‐old male smoker underwent resection of a recurrent high‐grade liposarcoma of the right upper extremity. He had no antecedent history of coronary artery disease (CAD) or atrial fibrillation, and had no additional vascular risk factors. On postoperative day 2, he developed acute chest pain, dyspnea, and hypoxia. He appeared alert but was diaphoretic and in moderate distress. Pulse was 84 beats per minute, blood pressure 230/120 mm Hg, and oxygen saturation 59% on room air (93% on supplemental oxygen). Heart and lung exam were unremarkable. Neck veins were not distended. Extremity exam was negative for edema, asymmetry, or calf tenderness, and pedal pulses were palpable bilaterally.
The patient's initial complete blood count, metabolic panel, and cardiac enzymes were within normal limits. Arterial blood gas (on 4‐L nasal cannula) revealed pH of 7.35, partial pressure of arterial oxygen (PaO2) of 65.5 mm Hg, partial pressure of arterial CO2 (PaCO2) of 45.4 mm Hg, and an alveolar‐arterial gradient of 131.3 mm Hg. Electrocardiogram (ECG) (Figure 1) showed an unchanged right bundle branch block, but new 2.5‐mm ST segment elevation in leads V4‐6, III, and aVF, and ST depression in aVL. At this point, the available data suggested either PE with secondary ECG changes or acute ST‐elevation MI with hypoxia. Given the ST elevation in 2 coronary distributions and concern for multivessel CAD, the patient was referred for emergent coronary angiography.
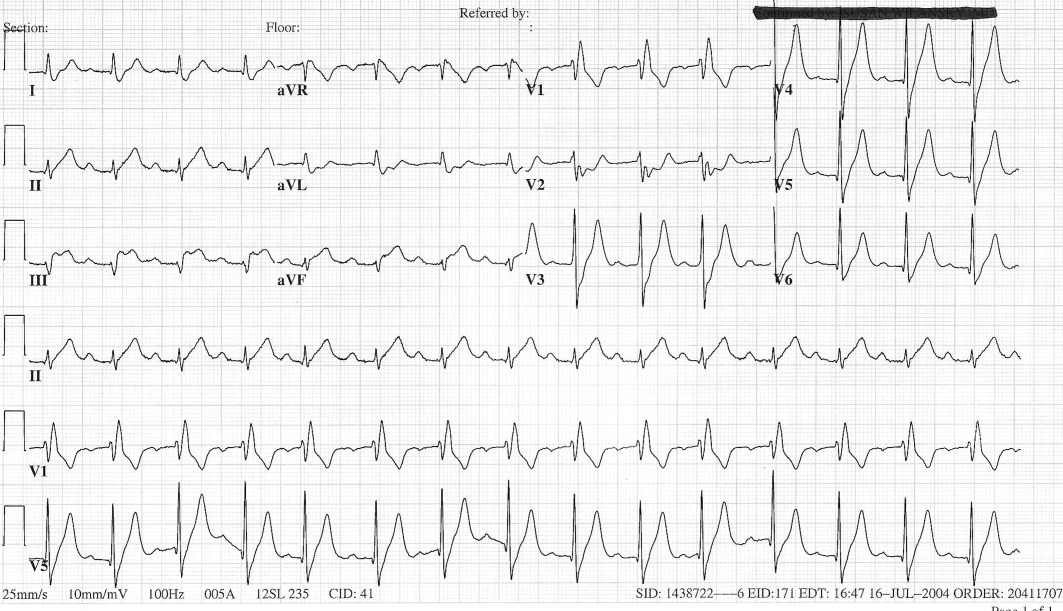
The patient was given aspirin, intravenous unfractionated heparin, and morphine. Left heart catheterization showed an abrupt cutoff in the distal left anterior descending artery (LAD), suggestive of thrombosis secondary to coronary embolism (Figure 2A); angioplasty was not attempted due to the distal location of the occlusion. The posterior descending artery from the right coronary artery was relatively short and the inferior apex was supplied by the distal LAD. The left ventriculogram demonstrated preserved ejection fraction but severe apical hypokinesis, correlating with the occluded vascular territory. The remaining coronary arteries were without significant stenosis. Based on the angiogram findings, paradoxical embolism was suspected. Right heart catheterization identified a previously undiagnosed PFO (Figure 2B); no thrombus was visualized. Right to left shunt was not identified; right ventricular systolic pressure was 46 mm Hg (normal, 15‐25 mm Hg). Subsequent spiral computed tomography (CT) revealed bilateral PEs. It was concluded that the patient had suffered an acute PE, from which the thrombus was able to traverse the PFO and left heart, ultimately entering the LAD, causing the acute embolic MI (Figure 3). ST elevation was present in the inferior leads secondary to the wraparound LAD that supplied the inferior apex, as demonstrated by the wall motion abnormality present on ventriculography.
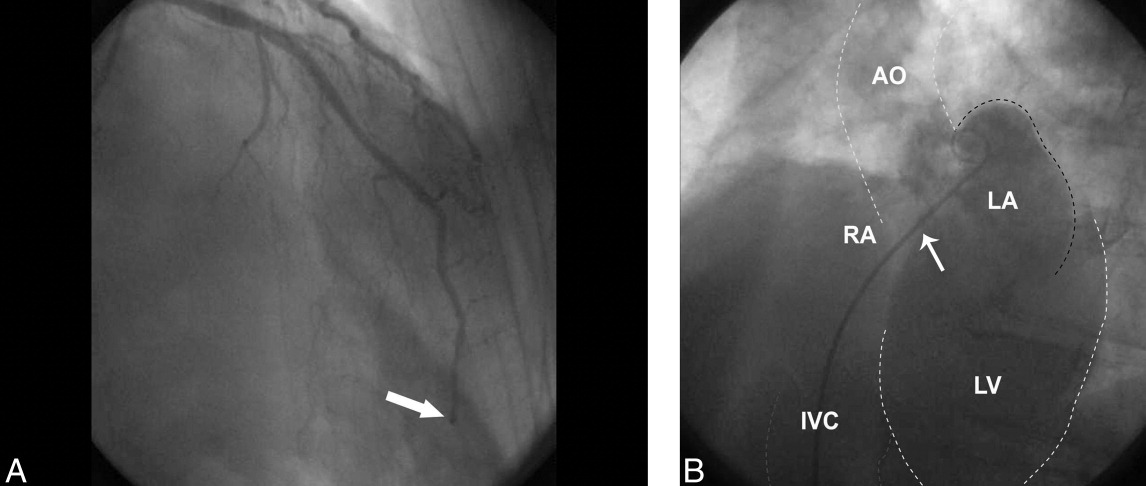

The patient was felt to be in a hypercoagulable state due to his malignancy and recent surgery; given the diagnosis of an acute PE, no ultrasound was performed to search for a deep vein thrombosis. The patient was transferred to the intensive care unit, continued on intravenous heparin and oxygen and started on oral warfarin. Subsequent hospital course was complicated by aspiration pneumonia and worsening hypoxia, but after a 17‐day hospital stay he was weaned off supplemental oxygen and transferred to an extended care facility with a therapeutic international normalized ratio (INR).
DISCUSSION
PFOs are congenital cardiac lesions that may persist through adulthood1 and are found incidentally in 19% to 36% of the normal population.2 They may range from 1 to 19 mm in size.2, 3 Contrast echocardiography has enabled a simple, accurate, and safe procedure for diagnosis (>5 microbubbles in the left heart cavities within three cardiac cycles after their appearance in the right atrium is considered diagnostic), though transesophageal echocardiography (TEE) is the gold standard.4
First described by Cohnheim in 1877,5 paradoxical embolism refers to the passage of embolic material from the venous to arterial circulation through a cardiac defect such as a PFO. However, definite confirmation of paradoxical embolism essentially requires catching the thrombus in the act of crossing the foramen ovale. Direct observation of this during life is rarely possible, and remains confined to isolated echocardiographic reports.6‐13
In clinical practice, the diagnosis of paradoxical embolism is almost always presumptive and relies on: (1) the occurrence of an arterial thromboembolic event in the absence of atrial fibrillation, left‐sided heart disease, or severe atherosclerosis; (2) the detection of right‐to‐left shunt, usually through a PFO or an atrial septal defect (ASD); and (3) the presence of venous thrombosis or PE.14
Although most patients are asymptomatic, during the past 20 years an association of PFO with stroke, migraine headache, peripheral arterial occlusion, and decompression induced neurologic dysfunction has been suggested.1 The neurological symptoms are proposed to be secondary to passage of small thrombi from the venous system through the PFO into arterial circulation during a transient right‐to‐left shunt. The source of clots cannot be established in most patients; fewer than 10% will have detectable deep vein thrombosis.15 Even less common are paradoxical emboli to the coronary arteries,1 estimated at 5% to 10% of all paradoxical emboli.16
In order for a thrombus to paradoxically embolize across a PFO, an atrial right‐to‐left pressure gradient must be present. Such a gradient occurs in normal individuals during early ventricular systole and with a Valsalva maneuver.17‐19 In a community‐based cohort study conducted to evaluate potential stroke risk, 148 (out of 581) subjects were found to have a PFO by TEE; 84 (57%) had right‐to‐left shunting at rest, and 136 (92%) had right‐to‐left shunting with Valsalva.2 Pathologic instances of pulmonary hypertension such as PE further elevate right‐heart pressures, further promoting intracardiac shunt.
Acute PE in the setting of PFO carries important prognostic implications. Konstantinides et al.20 prospectively evaluated 139 patients with large acute PE, all of whom had pulmonary hypertension and 96% of whom had right ventricular dilatation. They found a high prevalence of PFOs (35%) in this population. Furthermore, the subgroup with both PE and PFO had a high mortality (33% death rate compared with 14% in those without PFO; P = 0.015). When logistic regression analysis was performed, only arterial hypotension (odds ratio [OR] 26.3; P < 0.001) and the presence of PFO (OR 11.4; P < 0.001) remained significantly correlated with mortality. The authors reported that the presence of a PFO was associated with more than a 5‐fold increase in the adjusted risk of major in‐hospital complications (P < 0.001); no specific etiologic factors were proposed for this association.
In general, MI in the absence of CAD is uncommon, comprising <1% to 6% of all cases.21 No cause is found for the majority, but reported etiologies include coronary spasm in 15%, hypercoagulable states in 13%, collagen vascular diseases in 2%, and paradoxical embolism in 2%.22 AMI due to coronary embolism is uncommon, and when it does occur, left‐to‐left emboli in the setting of atrial fibrillation or prosthetic valves are far more common than paradoxical emboli. In an autopsy series of 1,050 patients with MIs, Prizel et al.23 identified only 55 patients with coronary embolism, none of which was right‐sided in origin.
A handful of published case reports documented paradoxical embolism as a cause for AMI.24‐26 Reported cases more often involved an ASD rather than PFO.27, 28 In most cases, diagnosis was made postmortem, though in a comprehensive review of the literature, Meier‐Ewert et al.13 identified 8 cases of AMI due to paradoxical embolism being diagnosed antemortem. Paradoxical emboli have been identified in all major divisions of the epicardial circulation, though involvement of the left coronary circulation is more common than the right.16
It is well‐established that the prevalence of PFO in patients with cryptogenic stroke is significantly higher than in the general population,1 and Crump et al.21 examined a case‐matched series of 18 patients with AMI who had little to no CAD (<30% stenosis) to see if the frequency of PFO was similarly higher in this group. Each group had identical frequency of PFO (28%; P = NS). The authors concluded that PFO is unlikely to contribute significantly to AMI. However, this study was limited by the small number of patients and the fact that transthoracic echocardiography (TTE) was used instead of TEE for the diagnosis of PFO.
The definitive diagnosis of AMI due to paradoxical embolism requires angiographic findings consistent with embolic occlusion (such as the cutoff sign in a distal coronary artery we observed in Figure 2A), cardiac defects predisposing to paradoxical emboli (such as PFO), and evidence of a venous source for thromboembolism. Alternatively, diagnosis can be made via direct visualization of emboli in the coronary arteries by TEE, or by autopsy.
Although electrocardiography is essential in the diagnosis and treatment of MI, it has the potential to be deceptive. Acute pulmonary hypertension caused by PE may be accompanied by ST elevation in inferior leads II, III, and aVF in a pseudoinfarction pattern mimicking AMI.29 This ECG abnormality probably reflects reciprocal changes of inferoposterior ischemia from right ventricular pressure overloading. However, clearly distinguishing between pseudoinfarction and true inferior infarction in the setting of PE requires coronary angiography.
Regarding therapy, acute treatment of PE is well‐established and consists of at least 5 days of therapeutically‐dosed heparin product that overlaps with therapeutic warfarin anticoagulation. Management of the PFO and coronary embolism is less clear; there are no guidelines for treatment of coronary embolism. Management strategies should focus on treatment of acute ischemia as well as prevention of future emboli, principally anticoagulation. Because the pathogenesis of AMI in this setting is drastically different from MI secondary to atherosclerosis, there is neither a biological basis nor clinical data to suggest benefit from initiation of beta‐blockers, aspirin, angiotensin converting enzyme inhibitors, or statins.
While studies have been done, and are underway to address optimal management of PFO in the setting of both stroke and migraine headache, to our knowledge, no such trials have addressed PFO and MI. Mehan et al.30 reported 2 cases of AMI caused by suspected paradoxical embolism, and in both cases, instant percutaneous closure of PFO was undertaken. However, there are no data to support or refute such an intervention in this particular setting.
- ,,, et al.Patent foramen ovale: current pathology, pathophysiology, and clinical status.J Am Coll Cardiol.2005;46:1768–1776.
- ,,, et al.Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC Study.Mayo Clin Proc.1999;74:862–869.
- ,,.Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts.Mayo Clin Proc.1984;59:17–20.
- ,,.Diagnosis of an anatomically and physiologically significant patent foramen ovale.Echocardiography.2006;23:810–815.
- .Thrombose und Embolie: Vorlesung über allgemeine Pathologie. Vol.1.Berlin:Hirschwald;1877;134.
- ,,,,,.Impending paradoxical embolism from atrial thrombus: correct diagnosis by transesophageal echocardiography and prevention by surgery.J Am Coll Cardiol.1985;5:1002–1004.
- ,,.Diagnosis of paradoxic embolism by transesophageal echocardiography.Am Heart J.1991;121:1552–1554.
- ,,,,,.Impending paradoxical embolism: echocardiographic diagnosis of an intracardiac thrombus crossing a patent foramen ovale.Am Heart J.1991;122(3 Pt 1):859–862.
- ,,,,,.Cryptogenic ischemic stroke and paradoxical embolism: should a patent foramen ovale be closed? Case report and literature review.Angiology.2001;52:793–799.
- ,,,,.Thrombus‐in‐transit and paradoxical embolism.J Am Soc Echocardiogr.2002;15:1021–1022.
- ,,,.Caught in the act: impending paradoxical embolism.Asian Cardiovasc Thorac Ann.2002;10:342–343.
- .Paradoxical embolism to the left main coronary artery: visualization by transesophageal echocardiography.J Am Soc Echocardiogr.2002;15:1417–1418.
- ,,,,,.Paradoxical embolism in the left main coronary artery: diagnosis by transesophageal echocardiography.Mayo Clin Proc.2003;78:103–106.
- .Specifics of patent foramen ovale.Adv Neurol.2003;92:197–202.
- ,,, et al.Frequency of deep vein thrombosis in patients with patent foramen ovale and ischemic stroke or transient ischemic attack.Am J Cardiol.1997;80:1066–1069.
- ,.Paradoxical coronary embolism: a rare cause of acute myocardial infarction.Rev Cardiovasc Med.2003;4:107–111.
- ,.Positive contrast echocardiography in patients with patent foramen ovale and normal right heart hemodynamics.Am J Cardiol.1982;49:1806–1809.
- ,,, et al.Contrast echocardiographic visualization of cough‐induced right to left shunt through a patent foramen ovale.J Am Coll Cardiol.1984;4:587–594.
- ,,,,.Transesophageal echocardiographic demonstration of distinct mechanisms for right to left shunting across a patent foramen ovale in the absence of pulmonary hypertension.J Am Coll Cardiol.1991;18:1112–1117.
- ,,,,,.Patent foramen ovale is an important predictor of adverse outcome in patients with major acute pulmonary embolism.Circulation.1998;97:1946–1951.
- ,,,.Prevalence of patent foramen ovale in patients with acute myocardial infarction and angiographically normal coronary arteries.Am J Cardiol.2000;85:1368–1370.
- ,,,,,.Clinical characteristics, aetiological factors and long‐term prognosis of myocardial infarction with an absolutely normal coronary angiogram; a 3‐year follow‐up study of 91 patients.Eur Heart J.2001;22:1459–1465.
- ,,.Coronary artery embolism and myocardial infarction.Ann Intern Med.1978;88:155–161.
- ,..Myocardial infarction due to a paradoxical embolism.Am J Med.1969;47:995–998.
- ,,,,,.[Myocardial infarction caused by probable paradoxical embolism and aneurysm of the interatrial septum].Presse Med.1996;25:907 [French].
- ,,.Acute myocardial infarction probably caused by paradoxical embolus in a pregnant woman.Heart.2004;90:e12.
- ,,,,.A case of acute pulmonary embolism and acute myocardial infarction with suspected paradoxical embolism after laparoscopic surgery.Heart Vessels.1999;4:197–200.
- ,,,,,.Acute myocardial infarction caused by paradoxical coronary embolization in a patient with a patent foramen ovale.J Am Soc Echocardiogr.2001;14:1227–1229.
- .ECG manifestations of selected extracardiac diseases.Emerg Med Clin N Am.2006;24:133–143.
- ,,,.Instant percutaneous closure of patent foramen ovale in patients with acute myocardial infarction and normal coronary arteries.Catheter Cardiovasc Interv.2006;67:279–282.
Acute pulmonary embolism (PE) and acute myocardial infarction (AMI) are common inpatient diagnoses, and are frequently in the differential diagnosis of patients evaluated for chest pain and dyspnea. We present a case with 1 unifying explanation for these entities to coexist. Acute PE with subsequent embolism to the coronary arteries via a patent foramen ovale (PFO) is rare, but the underlying disorder and anatomical variant are common. Of practical significance, hospitalized patients with acute PE and PFO may have up to a 5‐fold increase in morbidity compared to patients with isolated PE.
CASE REPORT
A 79‐year‐old male smoker underwent resection of a recurrent high‐grade liposarcoma of the right upper extremity. He had no antecedent history of coronary artery disease (CAD) or atrial fibrillation, and had no additional vascular risk factors. On postoperative day 2, he developed acute chest pain, dyspnea, and hypoxia. He appeared alert but was diaphoretic and in moderate distress. Pulse was 84 beats per minute, blood pressure 230/120 mm Hg, and oxygen saturation 59% on room air (93% on supplemental oxygen). Heart and lung exam were unremarkable. Neck veins were not distended. Extremity exam was negative for edema, asymmetry, or calf tenderness, and pedal pulses were palpable bilaterally.
The patient's initial complete blood count, metabolic panel, and cardiac enzymes were within normal limits. Arterial blood gas (on 4‐L nasal cannula) revealed pH of 7.35, partial pressure of arterial oxygen (PaO2) of 65.5 mm Hg, partial pressure of arterial CO2 (PaCO2) of 45.4 mm Hg, and an alveolar‐arterial gradient of 131.3 mm Hg. Electrocardiogram (ECG) (Figure 1) showed an unchanged right bundle branch block, but new 2.5‐mm ST segment elevation in leads V4‐6, III, and aVF, and ST depression in aVL. At this point, the available data suggested either PE with secondary ECG changes or acute ST‐elevation MI with hypoxia. Given the ST elevation in 2 coronary distributions and concern for multivessel CAD, the patient was referred for emergent coronary angiography.

The patient was given aspirin, intravenous unfractionated heparin, and morphine. Left heart catheterization showed an abrupt cutoff in the distal left anterior descending artery (LAD), suggestive of thrombosis secondary to coronary embolism (Figure 2A); angioplasty was not attempted due to the distal location of the occlusion. The posterior descending artery from the right coronary artery was relatively short and the inferior apex was supplied by the distal LAD. The left ventriculogram demonstrated preserved ejection fraction but severe apical hypokinesis, correlating with the occluded vascular territory. The remaining coronary arteries were without significant stenosis. Based on the angiogram findings, paradoxical embolism was suspected. Right heart catheterization identified a previously undiagnosed PFO (Figure 2B); no thrombus was visualized. Right to left shunt was not identified; right ventricular systolic pressure was 46 mm Hg (normal, 15‐25 mm Hg). Subsequent spiral computed tomography (CT) revealed bilateral PEs. It was concluded that the patient had suffered an acute PE, from which the thrombus was able to traverse the PFO and left heart, ultimately entering the LAD, causing the acute embolic MI (Figure 3). ST elevation was present in the inferior leads secondary to the wraparound LAD that supplied the inferior apex, as demonstrated by the wall motion abnormality present on ventriculography.


The patient was felt to be in a hypercoagulable state due to his malignancy and recent surgery; given the diagnosis of an acute PE, no ultrasound was performed to search for a deep vein thrombosis. The patient was transferred to the intensive care unit, continued on intravenous heparin and oxygen and started on oral warfarin. Subsequent hospital course was complicated by aspiration pneumonia and worsening hypoxia, but after a 17‐day hospital stay he was weaned off supplemental oxygen and transferred to an extended care facility with a therapeutic international normalized ratio (INR).
DISCUSSION
PFOs are congenital cardiac lesions that may persist through adulthood1 and are found incidentally in 19% to 36% of the normal population.2 They may range from 1 to 19 mm in size.2, 3 Contrast echocardiography has enabled a simple, accurate, and safe procedure for diagnosis (>5 microbubbles in the left heart cavities within three cardiac cycles after their appearance in the right atrium is considered diagnostic), though transesophageal echocardiography (TEE) is the gold standard.4
First described by Cohnheim in 1877,5 paradoxical embolism refers to the passage of embolic material from the venous to arterial circulation through a cardiac defect such as a PFO. However, definite confirmation of paradoxical embolism essentially requires catching the thrombus in the act of crossing the foramen ovale. Direct observation of this during life is rarely possible, and remains confined to isolated echocardiographic reports.6‐13
In clinical practice, the diagnosis of paradoxical embolism is almost always presumptive and relies on: (1) the occurrence of an arterial thromboembolic event in the absence of atrial fibrillation, left‐sided heart disease, or severe atherosclerosis; (2) the detection of right‐to‐left shunt, usually through a PFO or an atrial septal defect (ASD); and (3) the presence of venous thrombosis or PE.14
Although most patients are asymptomatic, during the past 20 years an association of PFO with stroke, migraine headache, peripheral arterial occlusion, and decompression induced neurologic dysfunction has been suggested.1 The neurological symptoms are proposed to be secondary to passage of small thrombi from the venous system through the PFO into arterial circulation during a transient right‐to‐left shunt. The source of clots cannot be established in most patients; fewer than 10% will have detectable deep vein thrombosis.15 Even less common are paradoxical emboli to the coronary arteries,1 estimated at 5% to 10% of all paradoxical emboli.16
In order for a thrombus to paradoxically embolize across a PFO, an atrial right‐to‐left pressure gradient must be present. Such a gradient occurs in normal individuals during early ventricular systole and with a Valsalva maneuver.17‐19 In a community‐based cohort study conducted to evaluate potential stroke risk, 148 (out of 581) subjects were found to have a PFO by TEE; 84 (57%) had right‐to‐left shunting at rest, and 136 (92%) had right‐to‐left shunting with Valsalva.2 Pathologic instances of pulmonary hypertension such as PE further elevate right‐heart pressures, further promoting intracardiac shunt.
Acute PE in the setting of PFO carries important prognostic implications. Konstantinides et al.20 prospectively evaluated 139 patients with large acute PE, all of whom had pulmonary hypertension and 96% of whom had right ventricular dilatation. They found a high prevalence of PFOs (35%) in this population. Furthermore, the subgroup with both PE and PFO had a high mortality (33% death rate compared with 14% in those without PFO; P = 0.015). When logistic regression analysis was performed, only arterial hypotension (odds ratio [OR] 26.3; P < 0.001) and the presence of PFO (OR 11.4; P < 0.001) remained significantly correlated with mortality. The authors reported that the presence of a PFO was associated with more than a 5‐fold increase in the adjusted risk of major in‐hospital complications (P < 0.001); no specific etiologic factors were proposed for this association.
In general, MI in the absence of CAD is uncommon, comprising <1% to 6% of all cases.21 No cause is found for the majority, but reported etiologies include coronary spasm in 15%, hypercoagulable states in 13%, collagen vascular diseases in 2%, and paradoxical embolism in 2%.22 AMI due to coronary embolism is uncommon, and when it does occur, left‐to‐left emboli in the setting of atrial fibrillation or prosthetic valves are far more common than paradoxical emboli. In an autopsy series of 1,050 patients with MIs, Prizel et al.23 identified only 55 patients with coronary embolism, none of which was right‐sided in origin.
A handful of published case reports documented paradoxical embolism as a cause for AMI.24‐26 Reported cases more often involved an ASD rather than PFO.27, 28 In most cases, diagnosis was made postmortem, though in a comprehensive review of the literature, Meier‐Ewert et al.13 identified 8 cases of AMI due to paradoxical embolism being diagnosed antemortem. Paradoxical emboli have been identified in all major divisions of the epicardial circulation, though involvement of the left coronary circulation is more common than the right.16
It is well‐established that the prevalence of PFO in patients with cryptogenic stroke is significantly higher than in the general population,1 and Crump et al.21 examined a case‐matched series of 18 patients with AMI who had little to no CAD (<30% stenosis) to see if the frequency of PFO was similarly higher in this group. Each group had identical frequency of PFO (28%; P = NS). The authors concluded that PFO is unlikely to contribute significantly to AMI. However, this study was limited by the small number of patients and the fact that transthoracic echocardiography (TTE) was used instead of TEE for the diagnosis of PFO.
The definitive diagnosis of AMI due to paradoxical embolism requires angiographic findings consistent with embolic occlusion (such as the cutoff sign in a distal coronary artery we observed in Figure 2A), cardiac defects predisposing to paradoxical emboli (such as PFO), and evidence of a venous source for thromboembolism. Alternatively, diagnosis can be made via direct visualization of emboli in the coronary arteries by TEE, or by autopsy.
Although electrocardiography is essential in the diagnosis and treatment of MI, it has the potential to be deceptive. Acute pulmonary hypertension caused by PE may be accompanied by ST elevation in inferior leads II, III, and aVF in a pseudoinfarction pattern mimicking AMI.29 This ECG abnormality probably reflects reciprocal changes of inferoposterior ischemia from right ventricular pressure overloading. However, clearly distinguishing between pseudoinfarction and true inferior infarction in the setting of PE requires coronary angiography.
Regarding therapy, acute treatment of PE is well‐established and consists of at least 5 days of therapeutically‐dosed heparin product that overlaps with therapeutic warfarin anticoagulation. Management of the PFO and coronary embolism is less clear; there are no guidelines for treatment of coronary embolism. Management strategies should focus on treatment of acute ischemia as well as prevention of future emboli, principally anticoagulation. Because the pathogenesis of AMI in this setting is drastically different from MI secondary to atherosclerosis, there is neither a biological basis nor clinical data to suggest benefit from initiation of beta‐blockers, aspirin, angiotensin converting enzyme inhibitors, or statins.
While studies have been done, and are underway to address optimal management of PFO in the setting of both stroke and migraine headache, to our knowledge, no such trials have addressed PFO and MI. Mehan et al.30 reported 2 cases of AMI caused by suspected paradoxical embolism, and in both cases, instant percutaneous closure of PFO was undertaken. However, there are no data to support or refute such an intervention in this particular setting.
Acute pulmonary embolism (PE) and acute myocardial infarction (AMI) are common inpatient diagnoses, and are frequently in the differential diagnosis of patients evaluated for chest pain and dyspnea. We present a case with 1 unifying explanation for these entities to coexist. Acute PE with subsequent embolism to the coronary arteries via a patent foramen ovale (PFO) is rare, but the underlying disorder and anatomical variant are common. Of practical significance, hospitalized patients with acute PE and PFO may have up to a 5‐fold increase in morbidity compared to patients with isolated PE.
CASE REPORT
A 79‐year‐old male smoker underwent resection of a recurrent high‐grade liposarcoma of the right upper extremity. He had no antecedent history of coronary artery disease (CAD) or atrial fibrillation, and had no additional vascular risk factors. On postoperative day 2, he developed acute chest pain, dyspnea, and hypoxia. He appeared alert but was diaphoretic and in moderate distress. Pulse was 84 beats per minute, blood pressure 230/120 mm Hg, and oxygen saturation 59% on room air (93% on supplemental oxygen). Heart and lung exam were unremarkable. Neck veins were not distended. Extremity exam was negative for edema, asymmetry, or calf tenderness, and pedal pulses were palpable bilaterally.
The patient's initial complete blood count, metabolic panel, and cardiac enzymes were within normal limits. Arterial blood gas (on 4‐L nasal cannula) revealed pH of 7.35, partial pressure of arterial oxygen (PaO2) of 65.5 mm Hg, partial pressure of arterial CO2 (PaCO2) of 45.4 mm Hg, and an alveolar‐arterial gradient of 131.3 mm Hg. Electrocardiogram (ECG) (Figure 1) showed an unchanged right bundle branch block, but new 2.5‐mm ST segment elevation in leads V4‐6, III, and aVF, and ST depression in aVL. At this point, the available data suggested either PE with secondary ECG changes or acute ST‐elevation MI with hypoxia. Given the ST elevation in 2 coronary distributions and concern for multivessel CAD, the patient was referred for emergent coronary angiography.

The patient was given aspirin, intravenous unfractionated heparin, and morphine. Left heart catheterization showed an abrupt cutoff in the distal left anterior descending artery (LAD), suggestive of thrombosis secondary to coronary embolism (Figure 2A); angioplasty was not attempted due to the distal location of the occlusion. The posterior descending artery from the right coronary artery was relatively short and the inferior apex was supplied by the distal LAD. The left ventriculogram demonstrated preserved ejection fraction but severe apical hypokinesis, correlating with the occluded vascular territory. The remaining coronary arteries were without significant stenosis. Based on the angiogram findings, paradoxical embolism was suspected. Right heart catheterization identified a previously undiagnosed PFO (Figure 2B); no thrombus was visualized. Right to left shunt was not identified; right ventricular systolic pressure was 46 mm Hg (normal, 15‐25 mm Hg). Subsequent spiral computed tomography (CT) revealed bilateral PEs. It was concluded that the patient had suffered an acute PE, from which the thrombus was able to traverse the PFO and left heart, ultimately entering the LAD, causing the acute embolic MI (Figure 3). ST elevation was present in the inferior leads secondary to the wraparound LAD that supplied the inferior apex, as demonstrated by the wall motion abnormality present on ventriculography.


The patient was felt to be in a hypercoagulable state due to his malignancy and recent surgery; given the diagnosis of an acute PE, no ultrasound was performed to search for a deep vein thrombosis. The patient was transferred to the intensive care unit, continued on intravenous heparin and oxygen and started on oral warfarin. Subsequent hospital course was complicated by aspiration pneumonia and worsening hypoxia, but after a 17‐day hospital stay he was weaned off supplemental oxygen and transferred to an extended care facility with a therapeutic international normalized ratio (INR).
DISCUSSION
PFOs are congenital cardiac lesions that may persist through adulthood1 and are found incidentally in 19% to 36% of the normal population.2 They may range from 1 to 19 mm in size.2, 3 Contrast echocardiography has enabled a simple, accurate, and safe procedure for diagnosis (>5 microbubbles in the left heart cavities within three cardiac cycles after their appearance in the right atrium is considered diagnostic), though transesophageal echocardiography (TEE) is the gold standard.4
First described by Cohnheim in 1877,5 paradoxical embolism refers to the passage of embolic material from the venous to arterial circulation through a cardiac defect such as a PFO. However, definite confirmation of paradoxical embolism essentially requires catching the thrombus in the act of crossing the foramen ovale. Direct observation of this during life is rarely possible, and remains confined to isolated echocardiographic reports.6‐13
In clinical practice, the diagnosis of paradoxical embolism is almost always presumptive and relies on: (1) the occurrence of an arterial thromboembolic event in the absence of atrial fibrillation, left‐sided heart disease, or severe atherosclerosis; (2) the detection of right‐to‐left shunt, usually through a PFO or an atrial septal defect (ASD); and (3) the presence of venous thrombosis or PE.14
Although most patients are asymptomatic, during the past 20 years an association of PFO with stroke, migraine headache, peripheral arterial occlusion, and decompression induced neurologic dysfunction has been suggested.1 The neurological symptoms are proposed to be secondary to passage of small thrombi from the venous system through the PFO into arterial circulation during a transient right‐to‐left shunt. The source of clots cannot be established in most patients; fewer than 10% will have detectable deep vein thrombosis.15 Even less common are paradoxical emboli to the coronary arteries,1 estimated at 5% to 10% of all paradoxical emboli.16
In order for a thrombus to paradoxically embolize across a PFO, an atrial right‐to‐left pressure gradient must be present. Such a gradient occurs in normal individuals during early ventricular systole and with a Valsalva maneuver.17‐19 In a community‐based cohort study conducted to evaluate potential stroke risk, 148 (out of 581) subjects were found to have a PFO by TEE; 84 (57%) had right‐to‐left shunting at rest, and 136 (92%) had right‐to‐left shunting with Valsalva.2 Pathologic instances of pulmonary hypertension such as PE further elevate right‐heart pressures, further promoting intracardiac shunt.
Acute PE in the setting of PFO carries important prognostic implications. Konstantinides et al.20 prospectively evaluated 139 patients with large acute PE, all of whom had pulmonary hypertension and 96% of whom had right ventricular dilatation. They found a high prevalence of PFOs (35%) in this population. Furthermore, the subgroup with both PE and PFO had a high mortality (33% death rate compared with 14% in those without PFO; P = 0.015). When logistic regression analysis was performed, only arterial hypotension (odds ratio [OR] 26.3; P < 0.001) and the presence of PFO (OR 11.4; P < 0.001) remained significantly correlated with mortality. The authors reported that the presence of a PFO was associated with more than a 5‐fold increase in the adjusted risk of major in‐hospital complications (P < 0.001); no specific etiologic factors were proposed for this association.
In general, MI in the absence of CAD is uncommon, comprising <1% to 6% of all cases.21 No cause is found for the majority, but reported etiologies include coronary spasm in 15%, hypercoagulable states in 13%, collagen vascular diseases in 2%, and paradoxical embolism in 2%.22 AMI due to coronary embolism is uncommon, and when it does occur, left‐to‐left emboli in the setting of atrial fibrillation or prosthetic valves are far more common than paradoxical emboli. In an autopsy series of 1,050 patients with MIs, Prizel et al.23 identified only 55 patients with coronary embolism, none of which was right‐sided in origin.
A handful of published case reports documented paradoxical embolism as a cause for AMI.24‐26 Reported cases more often involved an ASD rather than PFO.27, 28 In most cases, diagnosis was made postmortem, though in a comprehensive review of the literature, Meier‐Ewert et al.13 identified 8 cases of AMI due to paradoxical embolism being diagnosed antemortem. Paradoxical emboli have been identified in all major divisions of the epicardial circulation, though involvement of the left coronary circulation is more common than the right.16
It is well‐established that the prevalence of PFO in patients with cryptogenic stroke is significantly higher than in the general population,1 and Crump et al.21 examined a case‐matched series of 18 patients with AMI who had little to no CAD (<30% stenosis) to see if the frequency of PFO was similarly higher in this group. Each group had identical frequency of PFO (28%; P = NS). The authors concluded that PFO is unlikely to contribute significantly to AMI. However, this study was limited by the small number of patients and the fact that transthoracic echocardiography (TTE) was used instead of TEE for the diagnosis of PFO.
The definitive diagnosis of AMI due to paradoxical embolism requires angiographic findings consistent with embolic occlusion (such as the cutoff sign in a distal coronary artery we observed in Figure 2A), cardiac defects predisposing to paradoxical emboli (such as PFO), and evidence of a venous source for thromboembolism. Alternatively, diagnosis can be made via direct visualization of emboli in the coronary arteries by TEE, or by autopsy.
Although electrocardiography is essential in the diagnosis and treatment of MI, it has the potential to be deceptive. Acute pulmonary hypertension caused by PE may be accompanied by ST elevation in inferior leads II, III, and aVF in a pseudoinfarction pattern mimicking AMI.29 This ECG abnormality probably reflects reciprocal changes of inferoposterior ischemia from right ventricular pressure overloading. However, clearly distinguishing between pseudoinfarction and true inferior infarction in the setting of PE requires coronary angiography.
Regarding therapy, acute treatment of PE is well‐established and consists of at least 5 days of therapeutically‐dosed heparin product that overlaps with therapeutic warfarin anticoagulation. Management of the PFO and coronary embolism is less clear; there are no guidelines for treatment of coronary embolism. Management strategies should focus on treatment of acute ischemia as well as prevention of future emboli, principally anticoagulation. Because the pathogenesis of AMI in this setting is drastically different from MI secondary to atherosclerosis, there is neither a biological basis nor clinical data to suggest benefit from initiation of beta‐blockers, aspirin, angiotensin converting enzyme inhibitors, or statins.
While studies have been done, and are underway to address optimal management of PFO in the setting of both stroke and migraine headache, to our knowledge, no such trials have addressed PFO and MI. Mehan et al.30 reported 2 cases of AMI caused by suspected paradoxical embolism, and in both cases, instant percutaneous closure of PFO was undertaken. However, there are no data to support or refute such an intervention in this particular setting.
- ,,, et al.Patent foramen ovale: current pathology, pathophysiology, and clinical status.J Am Coll Cardiol.2005;46:1768–1776.
- ,,, et al.Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC Study.Mayo Clin Proc.1999;74:862–869.
- ,,.Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts.Mayo Clin Proc.1984;59:17–20.
- ,,.Diagnosis of an anatomically and physiologically significant patent foramen ovale.Echocardiography.2006;23:810–815.
- .Thrombose und Embolie: Vorlesung über allgemeine Pathologie. Vol.1.Berlin:Hirschwald;1877;134.
- ,,,,,.Impending paradoxical embolism from atrial thrombus: correct diagnosis by transesophageal echocardiography and prevention by surgery.J Am Coll Cardiol.1985;5:1002–1004.
- ,,.Diagnosis of paradoxic embolism by transesophageal echocardiography.Am Heart J.1991;121:1552–1554.
- ,,,,,.Impending paradoxical embolism: echocardiographic diagnosis of an intracardiac thrombus crossing a patent foramen ovale.Am Heart J.1991;122(3 Pt 1):859–862.
- ,,,,,.Cryptogenic ischemic stroke and paradoxical embolism: should a patent foramen ovale be closed? Case report and literature review.Angiology.2001;52:793–799.
- ,,,,.Thrombus‐in‐transit and paradoxical embolism.J Am Soc Echocardiogr.2002;15:1021–1022.
- ,,,.Caught in the act: impending paradoxical embolism.Asian Cardiovasc Thorac Ann.2002;10:342–343.
- .Paradoxical embolism to the left main coronary artery: visualization by transesophageal echocardiography.J Am Soc Echocardiogr.2002;15:1417–1418.
- ,,,,,.Paradoxical embolism in the left main coronary artery: diagnosis by transesophageal echocardiography.Mayo Clin Proc.2003;78:103–106.
- .Specifics of patent foramen ovale.Adv Neurol.2003;92:197–202.
- ,,, et al.Frequency of deep vein thrombosis in patients with patent foramen ovale and ischemic stroke or transient ischemic attack.Am J Cardiol.1997;80:1066–1069.
- ,.Paradoxical coronary embolism: a rare cause of acute myocardial infarction.Rev Cardiovasc Med.2003;4:107–111.
- ,.Positive contrast echocardiography in patients with patent foramen ovale and normal right heart hemodynamics.Am J Cardiol.1982;49:1806–1809.
- ,,, et al.Contrast echocardiographic visualization of cough‐induced right to left shunt through a patent foramen ovale.J Am Coll Cardiol.1984;4:587–594.
- ,,,,.Transesophageal echocardiographic demonstration of distinct mechanisms for right to left shunting across a patent foramen ovale in the absence of pulmonary hypertension.J Am Coll Cardiol.1991;18:1112–1117.
- ,,,,,.Patent foramen ovale is an important predictor of adverse outcome in patients with major acute pulmonary embolism.Circulation.1998;97:1946–1951.
- ,,,.Prevalence of patent foramen ovale in patients with acute myocardial infarction and angiographically normal coronary arteries.Am J Cardiol.2000;85:1368–1370.
- ,,,,,.Clinical characteristics, aetiological factors and long‐term prognosis of myocardial infarction with an absolutely normal coronary angiogram; a 3‐year follow‐up study of 91 patients.Eur Heart J.2001;22:1459–1465.
- ,,.Coronary artery embolism and myocardial infarction.Ann Intern Med.1978;88:155–161.
- ,..Myocardial infarction due to a paradoxical embolism.Am J Med.1969;47:995–998.
- ,,,,,.[Myocardial infarction caused by probable paradoxical embolism and aneurysm of the interatrial septum].Presse Med.1996;25:907 [French].
- ,,.Acute myocardial infarction probably caused by paradoxical embolus in a pregnant woman.Heart.2004;90:e12.
- ,,,,.A case of acute pulmonary embolism and acute myocardial infarction with suspected paradoxical embolism after laparoscopic surgery.Heart Vessels.1999;4:197–200.
- ,,,,,.Acute myocardial infarction caused by paradoxical coronary embolization in a patient with a patent foramen ovale.J Am Soc Echocardiogr.2001;14:1227–1229.
- .ECG manifestations of selected extracardiac diseases.Emerg Med Clin N Am.2006;24:133–143.
- ,,,.Instant percutaneous closure of patent foramen ovale in patients with acute myocardial infarction and normal coronary arteries.Catheter Cardiovasc Interv.2006;67:279–282.
- ,,, et al.Patent foramen ovale: current pathology, pathophysiology, and clinical status.J Am Coll Cardiol.2005;46:1768–1776.
- ,,, et al.Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC Study.Mayo Clin Proc.1999;74:862–869.
- ,,.Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts.Mayo Clin Proc.1984;59:17–20.
- ,,.Diagnosis of an anatomically and physiologically significant patent foramen ovale.Echocardiography.2006;23:810–815.
- .Thrombose und Embolie: Vorlesung über allgemeine Pathologie. Vol.1.Berlin:Hirschwald;1877;134.
- ,,,,,.Impending paradoxical embolism from atrial thrombus: correct diagnosis by transesophageal echocardiography and prevention by surgery.J Am Coll Cardiol.1985;5:1002–1004.
- ,,.Diagnosis of paradoxic embolism by transesophageal echocardiography.Am Heart J.1991;121:1552–1554.
- ,,,,,.Impending paradoxical embolism: echocardiographic diagnosis of an intracardiac thrombus crossing a patent foramen ovale.Am Heart J.1991;122(3 Pt 1):859–862.
- ,,,,,.Cryptogenic ischemic stroke and paradoxical embolism: should a patent foramen ovale be closed? Case report and literature review.Angiology.2001;52:793–799.
- ,,,,.Thrombus‐in‐transit and paradoxical embolism.J Am Soc Echocardiogr.2002;15:1021–1022.
- ,,,.Caught in the act: impending paradoxical embolism.Asian Cardiovasc Thorac Ann.2002;10:342–343.
- .Paradoxical embolism to the left main coronary artery: visualization by transesophageal echocardiography.J Am Soc Echocardiogr.2002;15:1417–1418.
- ,,,,,.Paradoxical embolism in the left main coronary artery: diagnosis by transesophageal echocardiography.Mayo Clin Proc.2003;78:103–106.
- .Specifics of patent foramen ovale.Adv Neurol.2003;92:197–202.
- ,,, et al.Frequency of deep vein thrombosis in patients with patent foramen ovale and ischemic stroke or transient ischemic attack.Am J Cardiol.1997;80:1066–1069.
- ,.Paradoxical coronary embolism: a rare cause of acute myocardial infarction.Rev Cardiovasc Med.2003;4:107–111.
- ,.Positive contrast echocardiography in patients with patent foramen ovale and normal right heart hemodynamics.Am J Cardiol.1982;49:1806–1809.
- ,,, et al.Contrast echocardiographic visualization of cough‐induced right to left shunt through a patent foramen ovale.J Am Coll Cardiol.1984;4:587–594.
- ,,,,.Transesophageal echocardiographic demonstration of distinct mechanisms for right to left shunting across a patent foramen ovale in the absence of pulmonary hypertension.J Am Coll Cardiol.1991;18:1112–1117.
- ,,,,,.Patent foramen ovale is an important predictor of adverse outcome in patients with major acute pulmonary embolism.Circulation.1998;97:1946–1951.
- ,,,.Prevalence of patent foramen ovale in patients with acute myocardial infarction and angiographically normal coronary arteries.Am J Cardiol.2000;85:1368–1370.
- ,,,,,.Clinical characteristics, aetiological factors and long‐term prognosis of myocardial infarction with an absolutely normal coronary angiogram; a 3‐year follow‐up study of 91 patients.Eur Heart J.2001;22:1459–1465.
- ,,.Coronary artery embolism and myocardial infarction.Ann Intern Med.1978;88:155–161.
- ,..Myocardial infarction due to a paradoxical embolism.Am J Med.1969;47:995–998.
- ,,,,,.[Myocardial infarction caused by probable paradoxical embolism and aneurysm of the interatrial septum].Presse Med.1996;25:907 [French].
- ,,.Acute myocardial infarction probably caused by paradoxical embolus in a pregnant woman.Heart.2004;90:e12.
- ,,,,.A case of acute pulmonary embolism and acute myocardial infarction with suspected paradoxical embolism after laparoscopic surgery.Heart Vessels.1999;4:197–200.
- ,,,,,.Acute myocardial infarction caused by paradoxical coronary embolization in a patient with a patent foramen ovale.J Am Soc Echocardiogr.2001;14:1227–1229.
- .ECG manifestations of selected extracardiac diseases.Emerg Med Clin N Am.2006;24:133–143.
- ,,,.Instant percutaneous closure of patent foramen ovale in patients with acute myocardial infarction and normal coronary arteries.Catheter Cardiovasc Interv.2006;67:279–282.
A Meal to Remember
A previously healthy 50‐year‐old man was eating a meal of rigatoni and shrimp at his favorite San Francisco restaurant when he suddenly developed severe pain in his throat followed a short time later by pleuritic chest pain localized to the anterior right chest. He completed his meal and then sought medical attention in the Emergency Department. He was in mild distress secondary to pain but his physical examination was otherwise unremarkable. His laboratory studies showed a white blood cell count of 15,300/mm3 with 86% neutrophils. A chest X‐ray, electrocardiogram, cardiac enzymes, and ventilation/perfusion scan were all normal. Because there was suspicion of an ingested foreign body, an abdominal radiograph was obtained which revealed a 2‐cm trapezoidal foreign body in the right lower quadrant (Figure 1; arrow). A chest computed tomography (CT) scan revealed air in the mediastinum consistent with esophageal perforation (Figure 2). One day after admission a Hypaque esophagram showed trauma to the posterior cervical region of the esophagus, but no leak of contrast material into the mediastinum. The patient was managed conservatively with intravenous (IV) antibiotics and nothing by mouth. Stools were screened and 48 hours after admission the patient (painlessly) passed a piece of glass with a very sharp point (Figure 3) correlating in size and shape to the foreign body seen on the previous abdominal radiograph. The glass had apparently fallen into the patient's restaurant meal the night of admission. The patient did well and was discharged 6 days after admission. He asked the manager of his favorite restaurant to reimburse him the $200 copayment required for hospitalization required under his Preferred Provider Plan; the request was immediately honored.
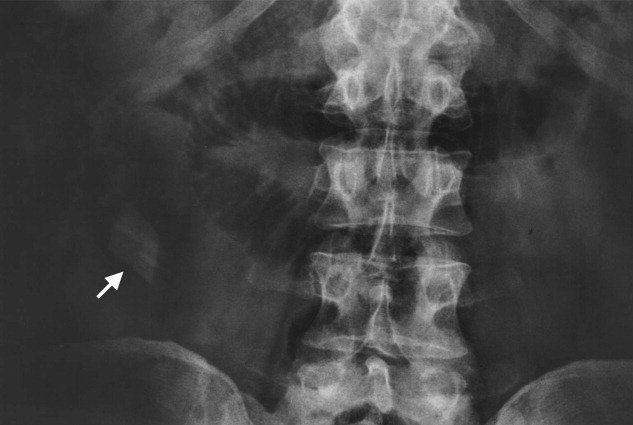


Esophageal perforation is an emergency because of its high mortality rate. The most frequent cause is iatrogenic with instrumentation from endoscopic procedures. Other causes include foreign body ingestion (as in this case), trauma, operative injury, and tumor.1 Aggressive surgical intervention vs. conservative nonsurgical management remains a controversial topic.2 Early‐contained perforations can be managed successfully by limiting oral intake and giving parenteral antibiotics.3, 4 Any signs of sepsis, deterioration in the patient's condition, or uncontained rupture warrants immediate surgical intervention.14
- ,,, et al.Evolving options in the management of esophageal perforation.Ann Thorac Surg.2004;77:1475–1483.
- ,,,.Esophageal perforation in adults.Ann Surg.2005;241(6):1016–1023.
- ,,.Esophageal perforation: emphasis of management.Ann Thorac Surg.1996;61:1447–1452
- ,,,.Nonoperative management of esophageal perforations.Ann Surg.1997;225(4):415–421.
A previously healthy 50‐year‐old man was eating a meal of rigatoni and shrimp at his favorite San Francisco restaurant when he suddenly developed severe pain in his throat followed a short time later by pleuritic chest pain localized to the anterior right chest. He completed his meal and then sought medical attention in the Emergency Department. He was in mild distress secondary to pain but his physical examination was otherwise unremarkable. His laboratory studies showed a white blood cell count of 15,300/mm3 with 86% neutrophils. A chest X‐ray, electrocardiogram, cardiac enzymes, and ventilation/perfusion scan were all normal. Because there was suspicion of an ingested foreign body, an abdominal radiograph was obtained which revealed a 2‐cm trapezoidal foreign body in the right lower quadrant (Figure 1; arrow). A chest computed tomography (CT) scan revealed air in the mediastinum consistent with esophageal perforation (Figure 2). One day after admission a Hypaque esophagram showed trauma to the posterior cervical region of the esophagus, but no leak of contrast material into the mediastinum. The patient was managed conservatively with intravenous (IV) antibiotics and nothing by mouth. Stools were screened and 48 hours after admission the patient (painlessly) passed a piece of glass with a very sharp point (Figure 3) correlating in size and shape to the foreign body seen on the previous abdominal radiograph. The glass had apparently fallen into the patient's restaurant meal the night of admission. The patient did well and was discharged 6 days after admission. He asked the manager of his favorite restaurant to reimburse him the $200 copayment required for hospitalization required under his Preferred Provider Plan; the request was immediately honored.



Esophageal perforation is an emergency because of its high mortality rate. The most frequent cause is iatrogenic with instrumentation from endoscopic procedures. Other causes include foreign body ingestion (as in this case), trauma, operative injury, and tumor.1 Aggressive surgical intervention vs. conservative nonsurgical management remains a controversial topic.2 Early‐contained perforations can be managed successfully by limiting oral intake and giving parenteral antibiotics.3, 4 Any signs of sepsis, deterioration in the patient's condition, or uncontained rupture warrants immediate surgical intervention.14
A previously healthy 50‐year‐old man was eating a meal of rigatoni and shrimp at his favorite San Francisco restaurant when he suddenly developed severe pain in his throat followed a short time later by pleuritic chest pain localized to the anterior right chest. He completed his meal and then sought medical attention in the Emergency Department. He was in mild distress secondary to pain but his physical examination was otherwise unremarkable. His laboratory studies showed a white blood cell count of 15,300/mm3 with 86% neutrophils. A chest X‐ray, electrocardiogram, cardiac enzymes, and ventilation/perfusion scan were all normal. Because there was suspicion of an ingested foreign body, an abdominal radiograph was obtained which revealed a 2‐cm trapezoidal foreign body in the right lower quadrant (Figure 1; arrow). A chest computed tomography (CT) scan revealed air in the mediastinum consistent with esophageal perforation (Figure 2). One day after admission a Hypaque esophagram showed trauma to the posterior cervical region of the esophagus, but no leak of contrast material into the mediastinum. The patient was managed conservatively with intravenous (IV) antibiotics and nothing by mouth. Stools were screened and 48 hours after admission the patient (painlessly) passed a piece of glass with a very sharp point (Figure 3) correlating in size and shape to the foreign body seen on the previous abdominal radiograph. The glass had apparently fallen into the patient's restaurant meal the night of admission. The patient did well and was discharged 6 days after admission. He asked the manager of his favorite restaurant to reimburse him the $200 copayment required for hospitalization required under his Preferred Provider Plan; the request was immediately honored.



Esophageal perforation is an emergency because of its high mortality rate. The most frequent cause is iatrogenic with instrumentation from endoscopic procedures. Other causes include foreign body ingestion (as in this case), trauma, operative injury, and tumor.1 Aggressive surgical intervention vs. conservative nonsurgical management remains a controversial topic.2 Early‐contained perforations can be managed successfully by limiting oral intake and giving parenteral antibiotics.3, 4 Any signs of sepsis, deterioration in the patient's condition, or uncontained rupture warrants immediate surgical intervention.14
- ,,, et al.Evolving options in the management of esophageal perforation.Ann Thorac Surg.2004;77:1475–1483.
- ,,,.Esophageal perforation in adults.Ann Surg.2005;241(6):1016–1023.
- ,,.Esophageal perforation: emphasis of management.Ann Thorac Surg.1996;61:1447–1452
- ,,,.Nonoperative management of esophageal perforations.Ann Surg.1997;225(4):415–421.
- ,,, et al.Evolving options in the management of esophageal perforation.Ann Thorac Surg.2004;77:1475–1483.
- ,,,.Esophageal perforation in adults.Ann Surg.2005;241(6):1016–1023.
- ,,.Esophageal perforation: emphasis of management.Ann Thorac Surg.1996;61:1447–1452
- ,,,.Nonoperative management of esophageal perforations.Ann Surg.1997;225(4):415–421.
The Tip of the Iceberg
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 33‐year‐old African American man was seen in the emergency department for bilateral wrist pain and forearm swelling. He was a professional mover and had transported a piano 3 days before. Several hours after the move, he noticed wrist pain and a few small red, slightly pruritic bumps on the palmar aspect of both wrists. The following day, he developed nausea, vomiting, and watery diarrhea. His wrists and forearms became more swollen and painful.
The most distinctive aspect of the patient's symptom complex is forearm swelling. I am not certain if the primary pathology lies in the wrist or in the forearm. Examination should focus on the presence or absence of arthritis and whether the forearm swelling is simply adjacent to the wrist and rash or extends the entire length from the wrist to the elbow.
Strenuous lifting can lead to rhabdomyolysis and forearm compartment syndrome. However, transporting a piano is not an unusual task for a professional mover. More common causes of arm swelling such as fracture, cellulitis, deep vein thrombosis, and lymphatic obstruction are possible but are unexpected in this patient because of the bilateral findings. An inflammatory myopathy would not present in the distal extremities, but an infectious myositis, such as trichinosis (which can have early gastrointestinal symptoms), could.
Given the patient's age and the temporal correlation, I am inclined to pursue a unifying diagnosis between his gastrointestinal and upper extremity symptoms. The gastrointestinal symptoms could reflect vasculitis (eg, polyarteritis nodosa) or a nonspecific manifestation of systemic illness (eg, sepsis), whereas the rash could be due to infection with petechiae (eg, meningococcemia, gonococcemia, or endocarditis) or an infection with a predilection for peripheral skin lesions that progress centripetally as the illness progresses, such as Rocky Mountain spotted fever.
Although the patient had not seen spiders, he was concerned that his skin lesions might have resulted from spider bites. He had a history of atopic dermatitis but took no medications, did not smoke, and drank alcohol rarely. He lived in Denver, CO, had not traveled outside of the region, and had not visited rural areas. He was monogamous with a female partner and had no known exposures to human immunodeficiency virus. He had no family history of rheumatological disorders.
Patients and physicians frequently attribute papules, ulcers, or necrotic skin lesions to spider bites far out of proportion to their true prevalence. Bites by more innocuous arthropods such as ticks, fleas, bedbugs, or mites are much more common. The symmetry of the lesions and extensive swelling, however, make such bites unlikely.
Although the patient hails from the southwestern United States, there is no compelling evidence for endemic illness. Hantavirus infection causes a viral prodrome, but a severe pulmonary syndrome is its primary manifestation. Plague presents in bubonic, pulmonary, or septicemic forms, but other than the gastrointestinal symptoms, there is nothing to suggest such a systemic illness. The initial pulmonary infection of coccidioidomycosis is often unnoticed, and patients may present with extrapulmonary manifestations, including skin lesions and skeletal disease.
More common ailments remain on the differential. Disseminated gonococcemia must be considered in a sexually active adult with skin lesions and what may be tenosynovitis or arthritis of the distal extremities. The history of atopic dermatitis supports a diagnosis of allergic or contact dermatitis on the wrists (perhaps inoculated during the move), explaining the rash and adjacent swelling (but not the gastrointestinal symptoms).
The patient's temperature was 36.5C with a pulse of 103 beats per minute, a blood pressure of 103/67 mm Hg, and a respiratory rate of 24 breaths per minute. He appeared uncomfortable and was in moderate distress. His sclerae were injected, and his mucous membranes were dry. He had diffusely swollen fingers and firm nonpitting edema in both hands and forearms. His wrists and hands were tender and warm but had no appreciable redness. Two 1‐mm ulcerated lesions were present on his right wrist, and one 2‐mm ulcerated lesion was present on his left wrist. No lymphadenopathy was present.
The patient meets the criteria for systemic inflammatory response syndrome (SIRS), and considering his ill appearance, I am concerned that he may have an infectious process that has evolved into sepsis. The absence of cutaneous erythema rules out cellulitis, and although skin findings in necrotizing fasciitis can be modest in comparison with the underlying infection, an examination with nothing more than punctate ulcerations would be atypical. Plague and tularemia cause ulcerative skin lesions and systemic illness but usually have prominent lymphadenopathy. Cutaneous anthrax can cause intense local edema but is usually accompanied by some degree of necrosis.
I am struck by the degree of local edema. It would be a remarkable coincidence for spider bites to occur on both wrists; however, given the size of the lesions, this remains a consideration. Spider bites can cause severe local swelling and occasionally even SIRS.
The white blood cellcount was 6000/mm3 with a normal differential. The hemoglobin level was 16.6 g/dL, and the platelet count was 227,000/mm3. The serum sodium level was measured to be 135 mmol/L, the potassium level was 3.6 mmol/L, the chloride level was 94 mmol/L, and the bicarbonate level was 9 mmol/L. The urea nitrogen level was measured to be 48 mg/dL (normal, 622), and the serum creatinine level was 5.6 mg/dL (normal, 0.41.2). An arterial blood gas test on room air revealed a pH of 7.22, a partial pressure of carbon dioxide of 24 mm Hg, and a partial pressure of oxygen of 128 mm Hg. Liver enzymes were normal. The serum creatine kinase level was measured to be 194 U/L (normal, 0250). A chest radiograph revealed clear lung fields.
The anion gap is elevated and could be explained in part by renal failure, but it is quite pronounced, and I suspect that there is lactic acidosis either from SIRS and systemic hypoperfusion or from local underperfusion of the distal upper extremities (eg, compartment syndrome). Rhabdomyolysis can cause an anion gap acidosis and acute renal failure but is ruled out by a normal creatine kinase level.
Rheumatological diseases (such as polyarteritis nodosa and systemic scleroderma) can cause renal failure, gastrointestinal symptoms, and cutaneous lesions with skin ulceration but are often accompanied by hypertension. The combination of SIRS and volume depletion from nausea, vomiting, and diarrhea seems more likely, although an etiology for SIRS remains elusive. I suspect infectionmost likely of the upper extremitiesis the underlying cause in this patient despite his normal body temperature and white cell count. I would therefore start with plain films of the arms and hands. If these are unrevealing, I would proceed with an ultrasound of the arms to evaluate the soft tissues and particularly the vessels. Although imaging studies are unlikely to provide a diagnosis, they will provide guidance in choosing the next step, such as biopsy or culture.
The patient wasvolume‐resuscitated with saline and treated with clindamycin, pipercillin‐tazobactam, and vancomycin. Radiographs of the wrists and hands demonstrated edema but no subcutaneous gas and no abnormalities of the bones or joints. An ultrasound of the upper extremities was negative for superficial or deep venous thrombosis. Renal ultrasonography revealed normal‐sized kidneys and no hydronephrosis.
Short of superior vena cava thrombosis, which the ultrasound could not visualize, deep venous thrombosis can be ruled out with confidence. Superior vena cava syndrome could account for the bilateral upper extremity symptoms, but complete sparing of the face would be unusual, and the chest radiograph was normal.
Despite the bilateral nature, I doubt that this patient has an arthritis of the wrists and hands (eg, rheumatoid or psoriatic arthritis). The plain films did not show evidence of joint destruction, although that would not be expected in the first few days of most noninfectious arthropathies. Many rheumatological diseases and vasculitides have renal manifestations, but I do not find convincing evidence of such diseases yet.
Despite intravenous fluidsover the next 4 hours, the patient remained anuric. His upper extremity edema worsened, and he became increasingly tachycardic and hypotensive. Vasopressor support was required. Repeat laboratory studies demonstrated a serum creatinine level of 7.0 mg/dL, a bicarbonate level of 6 mmol/L, and a creatine kinase level of 1183 U/L. The serum lactate level was measured to be 6.5. Blood cultures obtained on admission were negative. An echocardiogram demonstrated marked impairment of left ventricular systolic dysfunction with an estimated ejection fraction of 35%. Repeat chest radiography revealed only mild pulmonary vascular congestion.
The patient has become progressively ill despite initial resuscitation. Any infection (cellulitis, fasciitis, or myositis) may progress to such a point (although to do so without fever or leukocytosis with this degree of illness is unusual) and would prompt me to ask a surgeon to explore the upper extremities for diagnostic and possibly therapeutic purposes. Among the spectrum of possible soft tissue infections, this clinical presentation is most consistent with pyomyositis (caused by Staphylococcus aureus) because of the relatively modest creatine kinase elevations that accompany it and the overall absence of cutaneous findings (save the punctuate lesions), which I would expect to be present with cellulitis or necrotizing fasciitis. A deep forearm infection and perhaps compartment syndrome leading to sepsis could explain lactic acidosis, decreased cardiac function, hypotension, and acute renal failure.
Because of the unusual characteristics noted so farparticularly the bilateral diseaseI continue to also consider systemic diseases that can cause skin lesions, cardiomyopathy, renal disease, ocular involvement (eg, keratoconjunctivitis or uveitis), and myositis. Sarcoidosis is possible, although in most of the aforementioned organs, histological disease is far more common than clinical disease, and sarcoidosis typically does not cause this degree of illness. Furthermore, over 90% of patients with sarcoidosis have pulmonary involvement. Polyarteritis nodosa could explain the multiorgan involvement and the brisk pace. If no infection is present on exploration, I would ask the surgeon to biopsy the muscle, particularly looking for granulomas or vasculitis. A progressive soft tissue infection leading to sepsis remains my leading consideration at this point.
Surgical consultation was obtained. A muscle biopsy of the left biceps and left forearm revealed group A streptococcus within the muscle and evidence of necrosis (Figure 1). Debridement of both arms and wrists was performed. The patient subsequently developed erythema of the palms and soles followed by diffuse sloughing of the skin. Streptococcal toxic shock syndrome with necrotizing myositis was diagnosed, and the antimicrobial regimen was changed to intravenous penicillin, clindamycin, and intravenous immunoglobulin G. Repeat debridement of the arms was required.
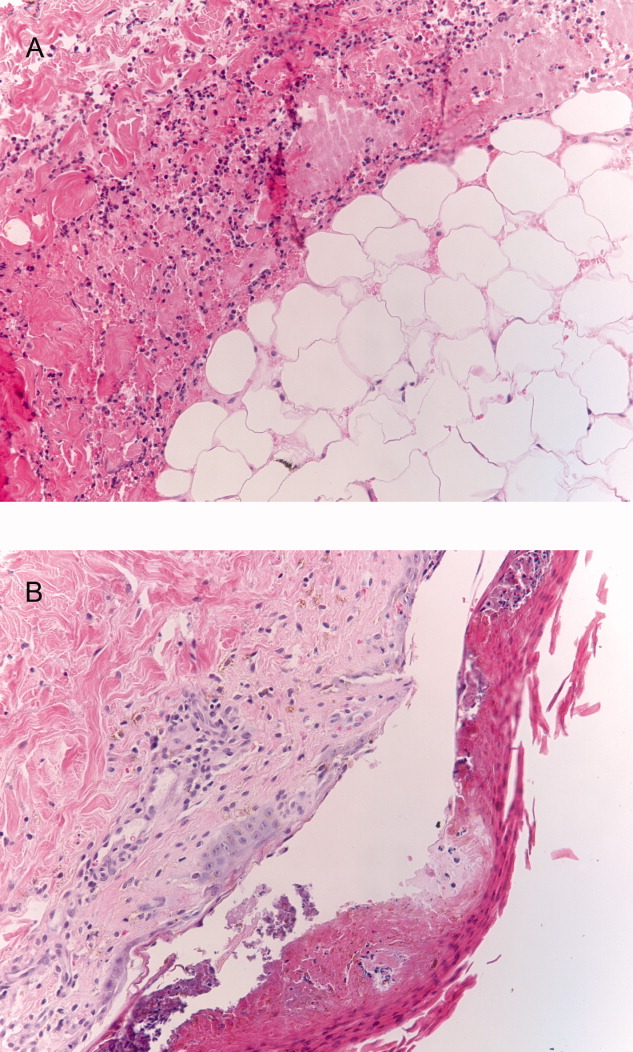
Even when a soft tissue infection is suspected, it can be challenging to preoperatively localize or characterize with precision. In retrospect, the overall severity of his illness and his previously good health status perhaps favor necrotizing myositis (and fasciitis) over pyomyositis.
I may have put undue emphasis on the absence of skin findings. Although the symmetric nature of the disease is unusual, the small skin lesions may have been portals of entry, and in retrospect, they represent the tip of the iceberg. The absence of fever and leukocytosisor hypothermia and leukopeniain a young, previously healthy patient along with the bilateral and symmetric findings made me hesitant to definitely label his illness as a deep soft tissue infection early on, but the gravity of the illness, the lack of a plausible alternative explanation, and his precipitous decline all made surgical exploration imperative.
The patient's skin sloughing progressed, and he was transferred to a regional burn unit. Three additional operations were required for debridement of both upper extremities. Despite apparent control of the initial infection, the patient continued to require significant hemodynamic and ventilator support. He subsequently developed neutropenia, thrombocytopenia, and Escherichia coli urosepsis. Necrosis of the lower extremities developed, and additional surgical debridement was recommended. After extensive discussion regarding prognosis, the family decided against further surgery and withdrew life support. The patient died shortly after extubation. The family refused an autopsy.
Commentary
Expert clinicians employ a variety of approaches to solve complex clinical problems. One of the most effective strategies is pattern recognition, in which the clinician divides the case into recognizable portions and compares these to previous cases that he or she has encountered.1, 2 If patterns from the new case appear similar or identical to those of previous cases, a diagnosis can be made quickly and without the need for unnecessary testing. However, when features of the case are unusual or atypical, pattern recognition may be disrupted. For example, although the discussant suspected a soft tissue infection, a number of features (including a normal white blood cell count, minimal skin findings, and a bilateral and symmetric distribution of swelling) did not match his illness script (a mental representation of a disease) of necrotizing fasciitis.
When pattern recognition fails, other strategies are available.3 Hypothetico‐deductive reasoning is a data‐to‐diagnosis method whereby the clinician uses the presenting information to construct a list of diagnostic possibilities.4 Additional testing and gathering of information are then used to continuously revise the diagnostic possibilities until confirmatory information is obtained and the diagnosis is established. After a pattern failed to materialize, the discussant employed this analytical strategy by noting the unusual characteristics of the case and incorporating laboratory and physiological data to revise his differential diagnosis. As a result, he requested the appropriate diagnostic test: surgical exploration of the forearms.
Necrotizing soft tissue infections are characterized by fulminant tissue destruction, rapid spread along tissue planes, and local vascular thrombosis. Mixed aerobic and anaerobic infections typically occur after penetrating skin injury or following surgery in patients with diabetes mellitus or vascular disease. In contrast, monomicrobial infections with S. aureus or group A streptococcus generally occur in healthy individuals. The prevalence of necrotizing group A streptococcal infections has increased dramatically in the last 15 to 20 years.5 Over one‐third of these cases are complicated by toxic shock syndrome.57 Mortality rates for necrotizing fasciitis with toxic shock exceed 30%, and early surgical consultation is directly associated with a reduction in morbidity and mortality.5, 8
Toxic shock is an inflammatory response syndrome caused by release of exotoxins from group A streptococcus and S. aureus.9, 10 In streptococcal toxic shock, Streptococcus pyogenes exotoxin A and Streptococcus pyogenes exotoxin B are the major toxins produced.10 These toxins activate the systemic production of inflammatory cytokines such as interleukin‐1, gamma‐interferon, and tumor necrosis factor, resulting in capillary leak, systemic hypotension, tissue hypoperfusion, and organ failure. The most common initial symptom is diffuse or localized pain that is severe and abrupt in onset and often precedes or is out of proportion to other physical findings of soft tissue infection.8 Up to 20% of individuals may also develop a viral‐like syndrome with myalgias, fever, nausea, vomiting, and diarrhea.11 Erythroderma of the skin and mucous membranes can be another early finding. The rash is diffuse, erythematous, and macular, resembling a sunburn. It involves the palms and soles but can be subtle and fleeting. Erythroderma may be particularly difficult to detect in dark‐skinned individuals.12 It is also important to consider that the absence of fever, erythroderma, or leukocytosis does not necessarily rule out the possibility of serious infection in necrotizing fasciitis patients, as the development of these signs and symptoms may occur later in the disease process.
The most common portals of entry for group A streptococcus are the skin, vagina, and pharynx. Predisposing factors include varicella infection, penetrating injuries, minor cuts, burns, splinters, and surgery. Interestingly, a portal of entry cannot be identified in 45% of cases.8 These patients in particular are at risk for developing severe necrotizing myositis or fasciitis at the site of a minor injury such as a strained muscle. Hematogenous translocation from the pharynx to the site of injury is the probable mechanism13 and would provide one scenario by which our piano mover developed bilateral and symmetric disease. An alternative explanation would be direct extension of bacteria from small breaks in the skin to adjacent areas of muscle strain. Regardless of the portal of entry, as the small skin lesions demonstrate in this patient, the smallest physical finding can represent the tip of the iceberg.
- ,,,,,.Fast and frugal models of clinical judgment in novice and expert physicians.Med Decis Making.2003;23(4):293–300.
- .What is it to be an expert? In: Chi M, Farr MJ, Glaser R, eds.The Nature of Expertise.Hillsdale, NJ:Lawrence Erlbaum;1988.
- .Clinical decision‐making: understanding how clinicians make a diagnosis. In: Saint S, Drazen JM, Solomon CG, eds.Clinical Problem‐Solving.New York, NY:McGraw‐Hill;2006.
- ,,.Medical Problem Solving: An Analysis of Clinical Reasoning.Cambridge, MA:Harvard University Press;1978.
- ,,,,.Population‐based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy‐seven cases. Ontario Group A Streptococcal Study.Am J Med.1997;103(1):18–24.
- ,,, et al.Invasive group A streptococcal infections in Sweden in 1994 and 1995: epidemiology and clinical spectrum.Scand J Infect Dis.2000;32(6):609–614.
- ,,,.Reemergence of emm1 and a changed superantigen profile for group A streptococci causing invasive infections: results from a nationwide study.J Clin Microbiol.2005;43(4):1789–1796.
- ,,, et al.Severe group A streptococcal infections associated with a toxic shock‐like syndrome and scarlet fever toxin A.N Engl J Med.1989;321(1):1–7.
- ,,,.Rapidly progressive necrotizing fasciitis caused by Staphylococcus aureus.J Microbiol Immunol Infect.2005;38(5):361–364.
- ,.Streptococcal infections of skin and soft tissues.N Engl J Med.1996;334(4):240–245.
- ,.Streptococcal toxic shock syndrome after breast reconstruction.Ann Plast Surg.2005;54(5):553–556.
- ,.The influence of pigmentation and illumination on the perception of erythema.Photodermatol Photoimmunol Photomed.1992;9(2):45–47.
- .Streptococcal toxic‐shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment.Emerg Infect Dis.1995;1(3):69–78.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 33‐year‐old African American man was seen in the emergency department for bilateral wrist pain and forearm swelling. He was a professional mover and had transported a piano 3 days before. Several hours after the move, he noticed wrist pain and a few small red, slightly pruritic bumps on the palmar aspect of both wrists. The following day, he developed nausea, vomiting, and watery diarrhea. His wrists and forearms became more swollen and painful.
The most distinctive aspect of the patient's symptom complex is forearm swelling. I am not certain if the primary pathology lies in the wrist or in the forearm. Examination should focus on the presence or absence of arthritis and whether the forearm swelling is simply adjacent to the wrist and rash or extends the entire length from the wrist to the elbow.
Strenuous lifting can lead to rhabdomyolysis and forearm compartment syndrome. However, transporting a piano is not an unusual task for a professional mover. More common causes of arm swelling such as fracture, cellulitis, deep vein thrombosis, and lymphatic obstruction are possible but are unexpected in this patient because of the bilateral findings. An inflammatory myopathy would not present in the distal extremities, but an infectious myositis, such as trichinosis (which can have early gastrointestinal symptoms), could.
Given the patient's age and the temporal correlation, I am inclined to pursue a unifying diagnosis between his gastrointestinal and upper extremity symptoms. The gastrointestinal symptoms could reflect vasculitis (eg, polyarteritis nodosa) or a nonspecific manifestation of systemic illness (eg, sepsis), whereas the rash could be due to infection with petechiae (eg, meningococcemia, gonococcemia, or endocarditis) or an infection with a predilection for peripheral skin lesions that progress centripetally as the illness progresses, such as Rocky Mountain spotted fever.
Although the patient had not seen spiders, he was concerned that his skin lesions might have resulted from spider bites. He had a history of atopic dermatitis but took no medications, did not smoke, and drank alcohol rarely. He lived in Denver, CO, had not traveled outside of the region, and had not visited rural areas. He was monogamous with a female partner and had no known exposures to human immunodeficiency virus. He had no family history of rheumatological disorders.
Patients and physicians frequently attribute papules, ulcers, or necrotic skin lesions to spider bites far out of proportion to their true prevalence. Bites by more innocuous arthropods such as ticks, fleas, bedbugs, or mites are much more common. The symmetry of the lesions and extensive swelling, however, make such bites unlikely.
Although the patient hails from the southwestern United States, there is no compelling evidence for endemic illness. Hantavirus infection causes a viral prodrome, but a severe pulmonary syndrome is its primary manifestation. Plague presents in bubonic, pulmonary, or septicemic forms, but other than the gastrointestinal symptoms, there is nothing to suggest such a systemic illness. The initial pulmonary infection of coccidioidomycosis is often unnoticed, and patients may present with extrapulmonary manifestations, including skin lesions and skeletal disease.
More common ailments remain on the differential. Disseminated gonococcemia must be considered in a sexually active adult with skin lesions and what may be tenosynovitis or arthritis of the distal extremities. The history of atopic dermatitis supports a diagnosis of allergic or contact dermatitis on the wrists (perhaps inoculated during the move), explaining the rash and adjacent swelling (but not the gastrointestinal symptoms).
The patient's temperature was 36.5C with a pulse of 103 beats per minute, a blood pressure of 103/67 mm Hg, and a respiratory rate of 24 breaths per minute. He appeared uncomfortable and was in moderate distress. His sclerae were injected, and his mucous membranes were dry. He had diffusely swollen fingers and firm nonpitting edema in both hands and forearms. His wrists and hands were tender and warm but had no appreciable redness. Two 1‐mm ulcerated lesions were present on his right wrist, and one 2‐mm ulcerated lesion was present on his left wrist. No lymphadenopathy was present.
The patient meets the criteria for systemic inflammatory response syndrome (SIRS), and considering his ill appearance, I am concerned that he may have an infectious process that has evolved into sepsis. The absence of cutaneous erythema rules out cellulitis, and although skin findings in necrotizing fasciitis can be modest in comparison with the underlying infection, an examination with nothing more than punctate ulcerations would be atypical. Plague and tularemia cause ulcerative skin lesions and systemic illness but usually have prominent lymphadenopathy. Cutaneous anthrax can cause intense local edema but is usually accompanied by some degree of necrosis.
I am struck by the degree of local edema. It would be a remarkable coincidence for spider bites to occur on both wrists; however, given the size of the lesions, this remains a consideration. Spider bites can cause severe local swelling and occasionally even SIRS.
The white blood cellcount was 6000/mm3 with a normal differential. The hemoglobin level was 16.6 g/dL, and the platelet count was 227,000/mm3. The serum sodium level was measured to be 135 mmol/L, the potassium level was 3.6 mmol/L, the chloride level was 94 mmol/L, and the bicarbonate level was 9 mmol/L. The urea nitrogen level was measured to be 48 mg/dL (normal, 622), and the serum creatinine level was 5.6 mg/dL (normal, 0.41.2). An arterial blood gas test on room air revealed a pH of 7.22, a partial pressure of carbon dioxide of 24 mm Hg, and a partial pressure of oxygen of 128 mm Hg. Liver enzymes were normal. The serum creatine kinase level was measured to be 194 U/L (normal, 0250). A chest radiograph revealed clear lung fields.
The anion gap is elevated and could be explained in part by renal failure, but it is quite pronounced, and I suspect that there is lactic acidosis either from SIRS and systemic hypoperfusion or from local underperfusion of the distal upper extremities (eg, compartment syndrome). Rhabdomyolysis can cause an anion gap acidosis and acute renal failure but is ruled out by a normal creatine kinase level.
Rheumatological diseases (such as polyarteritis nodosa and systemic scleroderma) can cause renal failure, gastrointestinal symptoms, and cutaneous lesions with skin ulceration but are often accompanied by hypertension. The combination of SIRS and volume depletion from nausea, vomiting, and diarrhea seems more likely, although an etiology for SIRS remains elusive. I suspect infectionmost likely of the upper extremitiesis the underlying cause in this patient despite his normal body temperature and white cell count. I would therefore start with plain films of the arms and hands. If these are unrevealing, I would proceed with an ultrasound of the arms to evaluate the soft tissues and particularly the vessels. Although imaging studies are unlikely to provide a diagnosis, they will provide guidance in choosing the next step, such as biopsy or culture.
The patient wasvolume‐resuscitated with saline and treated with clindamycin, pipercillin‐tazobactam, and vancomycin. Radiographs of the wrists and hands demonstrated edema but no subcutaneous gas and no abnormalities of the bones or joints. An ultrasound of the upper extremities was negative for superficial or deep venous thrombosis. Renal ultrasonography revealed normal‐sized kidneys and no hydronephrosis.
Short of superior vena cava thrombosis, which the ultrasound could not visualize, deep venous thrombosis can be ruled out with confidence. Superior vena cava syndrome could account for the bilateral upper extremity symptoms, but complete sparing of the face would be unusual, and the chest radiograph was normal.
Despite the bilateral nature, I doubt that this patient has an arthritis of the wrists and hands (eg, rheumatoid or psoriatic arthritis). The plain films did not show evidence of joint destruction, although that would not be expected in the first few days of most noninfectious arthropathies. Many rheumatological diseases and vasculitides have renal manifestations, but I do not find convincing evidence of such diseases yet.
Despite intravenous fluidsover the next 4 hours, the patient remained anuric. His upper extremity edema worsened, and he became increasingly tachycardic and hypotensive. Vasopressor support was required. Repeat laboratory studies demonstrated a serum creatinine level of 7.0 mg/dL, a bicarbonate level of 6 mmol/L, and a creatine kinase level of 1183 U/L. The serum lactate level was measured to be 6.5. Blood cultures obtained on admission were negative. An echocardiogram demonstrated marked impairment of left ventricular systolic dysfunction with an estimated ejection fraction of 35%. Repeat chest radiography revealed only mild pulmonary vascular congestion.
The patient has become progressively ill despite initial resuscitation. Any infection (cellulitis, fasciitis, or myositis) may progress to such a point (although to do so without fever or leukocytosis with this degree of illness is unusual) and would prompt me to ask a surgeon to explore the upper extremities for diagnostic and possibly therapeutic purposes. Among the spectrum of possible soft tissue infections, this clinical presentation is most consistent with pyomyositis (caused by Staphylococcus aureus) because of the relatively modest creatine kinase elevations that accompany it and the overall absence of cutaneous findings (save the punctuate lesions), which I would expect to be present with cellulitis or necrotizing fasciitis. A deep forearm infection and perhaps compartment syndrome leading to sepsis could explain lactic acidosis, decreased cardiac function, hypotension, and acute renal failure.
Because of the unusual characteristics noted so farparticularly the bilateral diseaseI continue to also consider systemic diseases that can cause skin lesions, cardiomyopathy, renal disease, ocular involvement (eg, keratoconjunctivitis or uveitis), and myositis. Sarcoidosis is possible, although in most of the aforementioned organs, histological disease is far more common than clinical disease, and sarcoidosis typically does not cause this degree of illness. Furthermore, over 90% of patients with sarcoidosis have pulmonary involvement. Polyarteritis nodosa could explain the multiorgan involvement and the brisk pace. If no infection is present on exploration, I would ask the surgeon to biopsy the muscle, particularly looking for granulomas or vasculitis. A progressive soft tissue infection leading to sepsis remains my leading consideration at this point.
Surgical consultation was obtained. A muscle biopsy of the left biceps and left forearm revealed group A streptococcus within the muscle and evidence of necrosis (Figure 1). Debridement of both arms and wrists was performed. The patient subsequently developed erythema of the palms and soles followed by diffuse sloughing of the skin. Streptococcal toxic shock syndrome with necrotizing myositis was diagnosed, and the antimicrobial regimen was changed to intravenous penicillin, clindamycin, and intravenous immunoglobulin G. Repeat debridement of the arms was required.

Even when a soft tissue infection is suspected, it can be challenging to preoperatively localize or characterize with precision. In retrospect, the overall severity of his illness and his previously good health status perhaps favor necrotizing myositis (and fasciitis) over pyomyositis.
I may have put undue emphasis on the absence of skin findings. Although the symmetric nature of the disease is unusual, the small skin lesions may have been portals of entry, and in retrospect, they represent the tip of the iceberg. The absence of fever and leukocytosisor hypothermia and leukopeniain a young, previously healthy patient along with the bilateral and symmetric findings made me hesitant to definitely label his illness as a deep soft tissue infection early on, but the gravity of the illness, the lack of a plausible alternative explanation, and his precipitous decline all made surgical exploration imperative.
The patient's skin sloughing progressed, and he was transferred to a regional burn unit. Three additional operations were required for debridement of both upper extremities. Despite apparent control of the initial infection, the patient continued to require significant hemodynamic and ventilator support. He subsequently developed neutropenia, thrombocytopenia, and Escherichia coli urosepsis. Necrosis of the lower extremities developed, and additional surgical debridement was recommended. After extensive discussion regarding prognosis, the family decided against further surgery and withdrew life support. The patient died shortly after extubation. The family refused an autopsy.
Commentary
Expert clinicians employ a variety of approaches to solve complex clinical problems. One of the most effective strategies is pattern recognition, in which the clinician divides the case into recognizable portions and compares these to previous cases that he or she has encountered.1, 2 If patterns from the new case appear similar or identical to those of previous cases, a diagnosis can be made quickly and without the need for unnecessary testing. However, when features of the case are unusual or atypical, pattern recognition may be disrupted. For example, although the discussant suspected a soft tissue infection, a number of features (including a normal white blood cell count, minimal skin findings, and a bilateral and symmetric distribution of swelling) did not match his illness script (a mental representation of a disease) of necrotizing fasciitis.
When pattern recognition fails, other strategies are available.3 Hypothetico‐deductive reasoning is a data‐to‐diagnosis method whereby the clinician uses the presenting information to construct a list of diagnostic possibilities.4 Additional testing and gathering of information are then used to continuously revise the diagnostic possibilities until confirmatory information is obtained and the diagnosis is established. After a pattern failed to materialize, the discussant employed this analytical strategy by noting the unusual characteristics of the case and incorporating laboratory and physiological data to revise his differential diagnosis. As a result, he requested the appropriate diagnostic test: surgical exploration of the forearms.
Necrotizing soft tissue infections are characterized by fulminant tissue destruction, rapid spread along tissue planes, and local vascular thrombosis. Mixed aerobic and anaerobic infections typically occur after penetrating skin injury or following surgery in patients with diabetes mellitus or vascular disease. In contrast, monomicrobial infections with S. aureus or group A streptococcus generally occur in healthy individuals. The prevalence of necrotizing group A streptococcal infections has increased dramatically in the last 15 to 20 years.5 Over one‐third of these cases are complicated by toxic shock syndrome.57 Mortality rates for necrotizing fasciitis with toxic shock exceed 30%, and early surgical consultation is directly associated with a reduction in morbidity and mortality.5, 8
Toxic shock is an inflammatory response syndrome caused by release of exotoxins from group A streptococcus and S. aureus.9, 10 In streptococcal toxic shock, Streptococcus pyogenes exotoxin A and Streptococcus pyogenes exotoxin B are the major toxins produced.10 These toxins activate the systemic production of inflammatory cytokines such as interleukin‐1, gamma‐interferon, and tumor necrosis factor, resulting in capillary leak, systemic hypotension, tissue hypoperfusion, and organ failure. The most common initial symptom is diffuse or localized pain that is severe and abrupt in onset and often precedes or is out of proportion to other physical findings of soft tissue infection.8 Up to 20% of individuals may also develop a viral‐like syndrome with myalgias, fever, nausea, vomiting, and diarrhea.11 Erythroderma of the skin and mucous membranes can be another early finding. The rash is diffuse, erythematous, and macular, resembling a sunburn. It involves the palms and soles but can be subtle and fleeting. Erythroderma may be particularly difficult to detect in dark‐skinned individuals.12 It is also important to consider that the absence of fever, erythroderma, or leukocytosis does not necessarily rule out the possibility of serious infection in necrotizing fasciitis patients, as the development of these signs and symptoms may occur later in the disease process.
The most common portals of entry for group A streptococcus are the skin, vagina, and pharynx. Predisposing factors include varicella infection, penetrating injuries, minor cuts, burns, splinters, and surgery. Interestingly, a portal of entry cannot be identified in 45% of cases.8 These patients in particular are at risk for developing severe necrotizing myositis or fasciitis at the site of a minor injury such as a strained muscle. Hematogenous translocation from the pharynx to the site of injury is the probable mechanism13 and would provide one scenario by which our piano mover developed bilateral and symmetric disease. An alternative explanation would be direct extension of bacteria from small breaks in the skin to adjacent areas of muscle strain. Regardless of the portal of entry, as the small skin lesions demonstrate in this patient, the smallest physical finding can represent the tip of the iceberg.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 33‐year‐old African American man was seen in the emergency department for bilateral wrist pain and forearm swelling. He was a professional mover and had transported a piano 3 days before. Several hours after the move, he noticed wrist pain and a few small red, slightly pruritic bumps on the palmar aspect of both wrists. The following day, he developed nausea, vomiting, and watery diarrhea. His wrists and forearms became more swollen and painful.
The most distinctive aspect of the patient's symptom complex is forearm swelling. I am not certain if the primary pathology lies in the wrist or in the forearm. Examination should focus on the presence or absence of arthritis and whether the forearm swelling is simply adjacent to the wrist and rash or extends the entire length from the wrist to the elbow.
Strenuous lifting can lead to rhabdomyolysis and forearm compartment syndrome. However, transporting a piano is not an unusual task for a professional mover. More common causes of arm swelling such as fracture, cellulitis, deep vein thrombosis, and lymphatic obstruction are possible but are unexpected in this patient because of the bilateral findings. An inflammatory myopathy would not present in the distal extremities, but an infectious myositis, such as trichinosis (which can have early gastrointestinal symptoms), could.
Given the patient's age and the temporal correlation, I am inclined to pursue a unifying diagnosis between his gastrointestinal and upper extremity symptoms. The gastrointestinal symptoms could reflect vasculitis (eg, polyarteritis nodosa) or a nonspecific manifestation of systemic illness (eg, sepsis), whereas the rash could be due to infection with petechiae (eg, meningococcemia, gonococcemia, or endocarditis) or an infection with a predilection for peripheral skin lesions that progress centripetally as the illness progresses, such as Rocky Mountain spotted fever.
Although the patient had not seen spiders, he was concerned that his skin lesions might have resulted from spider bites. He had a history of atopic dermatitis but took no medications, did not smoke, and drank alcohol rarely. He lived in Denver, CO, had not traveled outside of the region, and had not visited rural areas. He was monogamous with a female partner and had no known exposures to human immunodeficiency virus. He had no family history of rheumatological disorders.
Patients and physicians frequently attribute papules, ulcers, or necrotic skin lesions to spider bites far out of proportion to their true prevalence. Bites by more innocuous arthropods such as ticks, fleas, bedbugs, or mites are much more common. The symmetry of the lesions and extensive swelling, however, make such bites unlikely.
Although the patient hails from the southwestern United States, there is no compelling evidence for endemic illness. Hantavirus infection causes a viral prodrome, but a severe pulmonary syndrome is its primary manifestation. Plague presents in bubonic, pulmonary, or septicemic forms, but other than the gastrointestinal symptoms, there is nothing to suggest such a systemic illness. The initial pulmonary infection of coccidioidomycosis is often unnoticed, and patients may present with extrapulmonary manifestations, including skin lesions and skeletal disease.
More common ailments remain on the differential. Disseminated gonococcemia must be considered in a sexually active adult with skin lesions and what may be tenosynovitis or arthritis of the distal extremities. The history of atopic dermatitis supports a diagnosis of allergic or contact dermatitis on the wrists (perhaps inoculated during the move), explaining the rash and adjacent swelling (but not the gastrointestinal symptoms).
The patient's temperature was 36.5C with a pulse of 103 beats per minute, a blood pressure of 103/67 mm Hg, and a respiratory rate of 24 breaths per minute. He appeared uncomfortable and was in moderate distress. His sclerae were injected, and his mucous membranes were dry. He had diffusely swollen fingers and firm nonpitting edema in both hands and forearms. His wrists and hands were tender and warm but had no appreciable redness. Two 1‐mm ulcerated lesions were present on his right wrist, and one 2‐mm ulcerated lesion was present on his left wrist. No lymphadenopathy was present.
The patient meets the criteria for systemic inflammatory response syndrome (SIRS), and considering his ill appearance, I am concerned that he may have an infectious process that has evolved into sepsis. The absence of cutaneous erythema rules out cellulitis, and although skin findings in necrotizing fasciitis can be modest in comparison with the underlying infection, an examination with nothing more than punctate ulcerations would be atypical. Plague and tularemia cause ulcerative skin lesions and systemic illness but usually have prominent lymphadenopathy. Cutaneous anthrax can cause intense local edema but is usually accompanied by some degree of necrosis.
I am struck by the degree of local edema. It would be a remarkable coincidence for spider bites to occur on both wrists; however, given the size of the lesions, this remains a consideration. Spider bites can cause severe local swelling and occasionally even SIRS.
The white blood cellcount was 6000/mm3 with a normal differential. The hemoglobin level was 16.6 g/dL, and the platelet count was 227,000/mm3. The serum sodium level was measured to be 135 mmol/L, the potassium level was 3.6 mmol/L, the chloride level was 94 mmol/L, and the bicarbonate level was 9 mmol/L. The urea nitrogen level was measured to be 48 mg/dL (normal, 622), and the serum creatinine level was 5.6 mg/dL (normal, 0.41.2). An arterial blood gas test on room air revealed a pH of 7.22, a partial pressure of carbon dioxide of 24 mm Hg, and a partial pressure of oxygen of 128 mm Hg. Liver enzymes were normal. The serum creatine kinase level was measured to be 194 U/L (normal, 0250). A chest radiograph revealed clear lung fields.
The anion gap is elevated and could be explained in part by renal failure, but it is quite pronounced, and I suspect that there is lactic acidosis either from SIRS and systemic hypoperfusion or from local underperfusion of the distal upper extremities (eg, compartment syndrome). Rhabdomyolysis can cause an anion gap acidosis and acute renal failure but is ruled out by a normal creatine kinase level.
Rheumatological diseases (such as polyarteritis nodosa and systemic scleroderma) can cause renal failure, gastrointestinal symptoms, and cutaneous lesions with skin ulceration but are often accompanied by hypertension. The combination of SIRS and volume depletion from nausea, vomiting, and diarrhea seems more likely, although an etiology for SIRS remains elusive. I suspect infectionmost likely of the upper extremitiesis the underlying cause in this patient despite his normal body temperature and white cell count. I would therefore start with plain films of the arms and hands. If these are unrevealing, I would proceed with an ultrasound of the arms to evaluate the soft tissues and particularly the vessels. Although imaging studies are unlikely to provide a diagnosis, they will provide guidance in choosing the next step, such as biopsy or culture.
The patient wasvolume‐resuscitated with saline and treated with clindamycin, pipercillin‐tazobactam, and vancomycin. Radiographs of the wrists and hands demonstrated edema but no subcutaneous gas and no abnormalities of the bones or joints. An ultrasound of the upper extremities was negative for superficial or deep venous thrombosis. Renal ultrasonography revealed normal‐sized kidneys and no hydronephrosis.
Short of superior vena cava thrombosis, which the ultrasound could not visualize, deep venous thrombosis can be ruled out with confidence. Superior vena cava syndrome could account for the bilateral upper extremity symptoms, but complete sparing of the face would be unusual, and the chest radiograph was normal.
Despite the bilateral nature, I doubt that this patient has an arthritis of the wrists and hands (eg, rheumatoid or psoriatic arthritis). The plain films did not show evidence of joint destruction, although that would not be expected in the first few days of most noninfectious arthropathies. Many rheumatological diseases and vasculitides have renal manifestations, but I do not find convincing evidence of such diseases yet.
Despite intravenous fluidsover the next 4 hours, the patient remained anuric. His upper extremity edema worsened, and he became increasingly tachycardic and hypotensive. Vasopressor support was required. Repeat laboratory studies demonstrated a serum creatinine level of 7.0 mg/dL, a bicarbonate level of 6 mmol/L, and a creatine kinase level of 1183 U/L. The serum lactate level was measured to be 6.5. Blood cultures obtained on admission were negative. An echocardiogram demonstrated marked impairment of left ventricular systolic dysfunction with an estimated ejection fraction of 35%. Repeat chest radiography revealed only mild pulmonary vascular congestion.
The patient has become progressively ill despite initial resuscitation. Any infection (cellulitis, fasciitis, or myositis) may progress to such a point (although to do so without fever or leukocytosis with this degree of illness is unusual) and would prompt me to ask a surgeon to explore the upper extremities for diagnostic and possibly therapeutic purposes. Among the spectrum of possible soft tissue infections, this clinical presentation is most consistent with pyomyositis (caused by Staphylococcus aureus) because of the relatively modest creatine kinase elevations that accompany it and the overall absence of cutaneous findings (save the punctuate lesions), which I would expect to be present with cellulitis or necrotizing fasciitis. A deep forearm infection and perhaps compartment syndrome leading to sepsis could explain lactic acidosis, decreased cardiac function, hypotension, and acute renal failure.
Because of the unusual characteristics noted so farparticularly the bilateral diseaseI continue to also consider systemic diseases that can cause skin lesions, cardiomyopathy, renal disease, ocular involvement (eg, keratoconjunctivitis or uveitis), and myositis. Sarcoidosis is possible, although in most of the aforementioned organs, histological disease is far more common than clinical disease, and sarcoidosis typically does not cause this degree of illness. Furthermore, over 90% of patients with sarcoidosis have pulmonary involvement. Polyarteritis nodosa could explain the multiorgan involvement and the brisk pace. If no infection is present on exploration, I would ask the surgeon to biopsy the muscle, particularly looking for granulomas or vasculitis. A progressive soft tissue infection leading to sepsis remains my leading consideration at this point.
Surgical consultation was obtained. A muscle biopsy of the left biceps and left forearm revealed group A streptococcus within the muscle and evidence of necrosis (Figure 1). Debridement of both arms and wrists was performed. The patient subsequently developed erythema of the palms and soles followed by diffuse sloughing of the skin. Streptococcal toxic shock syndrome with necrotizing myositis was diagnosed, and the antimicrobial regimen was changed to intravenous penicillin, clindamycin, and intravenous immunoglobulin G. Repeat debridement of the arms was required.

Even when a soft tissue infection is suspected, it can be challenging to preoperatively localize or characterize with precision. In retrospect, the overall severity of his illness and his previously good health status perhaps favor necrotizing myositis (and fasciitis) over pyomyositis.
I may have put undue emphasis on the absence of skin findings. Although the symmetric nature of the disease is unusual, the small skin lesions may have been portals of entry, and in retrospect, they represent the tip of the iceberg. The absence of fever and leukocytosisor hypothermia and leukopeniain a young, previously healthy patient along with the bilateral and symmetric findings made me hesitant to definitely label his illness as a deep soft tissue infection early on, but the gravity of the illness, the lack of a plausible alternative explanation, and his precipitous decline all made surgical exploration imperative.
The patient's skin sloughing progressed, and he was transferred to a regional burn unit. Three additional operations were required for debridement of both upper extremities. Despite apparent control of the initial infection, the patient continued to require significant hemodynamic and ventilator support. He subsequently developed neutropenia, thrombocytopenia, and Escherichia coli urosepsis. Necrosis of the lower extremities developed, and additional surgical debridement was recommended. After extensive discussion regarding prognosis, the family decided against further surgery and withdrew life support. The patient died shortly after extubation. The family refused an autopsy.
Commentary
Expert clinicians employ a variety of approaches to solve complex clinical problems. One of the most effective strategies is pattern recognition, in which the clinician divides the case into recognizable portions and compares these to previous cases that he or she has encountered.1, 2 If patterns from the new case appear similar or identical to those of previous cases, a diagnosis can be made quickly and without the need for unnecessary testing. However, when features of the case are unusual or atypical, pattern recognition may be disrupted. For example, although the discussant suspected a soft tissue infection, a number of features (including a normal white blood cell count, minimal skin findings, and a bilateral and symmetric distribution of swelling) did not match his illness script (a mental representation of a disease) of necrotizing fasciitis.
When pattern recognition fails, other strategies are available.3 Hypothetico‐deductive reasoning is a data‐to‐diagnosis method whereby the clinician uses the presenting information to construct a list of diagnostic possibilities.4 Additional testing and gathering of information are then used to continuously revise the diagnostic possibilities until confirmatory information is obtained and the diagnosis is established. After a pattern failed to materialize, the discussant employed this analytical strategy by noting the unusual characteristics of the case and incorporating laboratory and physiological data to revise his differential diagnosis. As a result, he requested the appropriate diagnostic test: surgical exploration of the forearms.
Necrotizing soft tissue infections are characterized by fulminant tissue destruction, rapid spread along tissue planes, and local vascular thrombosis. Mixed aerobic and anaerobic infections typically occur after penetrating skin injury or following surgery in patients with diabetes mellitus or vascular disease. In contrast, monomicrobial infections with S. aureus or group A streptococcus generally occur in healthy individuals. The prevalence of necrotizing group A streptococcal infections has increased dramatically in the last 15 to 20 years.5 Over one‐third of these cases are complicated by toxic shock syndrome.57 Mortality rates for necrotizing fasciitis with toxic shock exceed 30%, and early surgical consultation is directly associated with a reduction in morbidity and mortality.5, 8
Toxic shock is an inflammatory response syndrome caused by release of exotoxins from group A streptococcus and S. aureus.9, 10 In streptococcal toxic shock, Streptococcus pyogenes exotoxin A and Streptococcus pyogenes exotoxin B are the major toxins produced.10 These toxins activate the systemic production of inflammatory cytokines such as interleukin‐1, gamma‐interferon, and tumor necrosis factor, resulting in capillary leak, systemic hypotension, tissue hypoperfusion, and organ failure. The most common initial symptom is diffuse or localized pain that is severe and abrupt in onset and often precedes or is out of proportion to other physical findings of soft tissue infection.8 Up to 20% of individuals may also develop a viral‐like syndrome with myalgias, fever, nausea, vomiting, and diarrhea.11 Erythroderma of the skin and mucous membranes can be another early finding. The rash is diffuse, erythematous, and macular, resembling a sunburn. It involves the palms and soles but can be subtle and fleeting. Erythroderma may be particularly difficult to detect in dark‐skinned individuals.12 It is also important to consider that the absence of fever, erythroderma, or leukocytosis does not necessarily rule out the possibility of serious infection in necrotizing fasciitis patients, as the development of these signs and symptoms may occur later in the disease process.
The most common portals of entry for group A streptococcus are the skin, vagina, and pharynx. Predisposing factors include varicella infection, penetrating injuries, minor cuts, burns, splinters, and surgery. Interestingly, a portal of entry cannot be identified in 45% of cases.8 These patients in particular are at risk for developing severe necrotizing myositis or fasciitis at the site of a minor injury such as a strained muscle. Hematogenous translocation from the pharynx to the site of injury is the probable mechanism13 and would provide one scenario by which our piano mover developed bilateral and symmetric disease. An alternative explanation would be direct extension of bacteria from small breaks in the skin to adjacent areas of muscle strain. Regardless of the portal of entry, as the small skin lesions demonstrate in this patient, the smallest physical finding can represent the tip of the iceberg.
- ,,,,,.Fast and frugal models of clinical judgment in novice and expert physicians.Med Decis Making.2003;23(4):293–300.
- .What is it to be an expert? In: Chi M, Farr MJ, Glaser R, eds.The Nature of Expertise.Hillsdale, NJ:Lawrence Erlbaum;1988.
- .Clinical decision‐making: understanding how clinicians make a diagnosis. In: Saint S, Drazen JM, Solomon CG, eds.Clinical Problem‐Solving.New York, NY:McGraw‐Hill;2006.
- ,,.Medical Problem Solving: An Analysis of Clinical Reasoning.Cambridge, MA:Harvard University Press;1978.
- ,,,,.Population‐based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy‐seven cases. Ontario Group A Streptococcal Study.Am J Med.1997;103(1):18–24.
- ,,, et al.Invasive group A streptococcal infections in Sweden in 1994 and 1995: epidemiology and clinical spectrum.Scand J Infect Dis.2000;32(6):609–614.
- ,,,.Reemergence of emm1 and a changed superantigen profile for group A streptococci causing invasive infections: results from a nationwide study.J Clin Microbiol.2005;43(4):1789–1796.
- ,,, et al.Severe group A streptococcal infections associated with a toxic shock‐like syndrome and scarlet fever toxin A.N Engl J Med.1989;321(1):1–7.
- ,,,.Rapidly progressive necrotizing fasciitis caused by Staphylococcus aureus.J Microbiol Immunol Infect.2005;38(5):361–364.
- ,.Streptococcal infections of skin and soft tissues.N Engl J Med.1996;334(4):240–245.
- ,.Streptococcal toxic shock syndrome after breast reconstruction.Ann Plast Surg.2005;54(5):553–556.
- ,.The influence of pigmentation and illumination on the perception of erythema.Photodermatol Photoimmunol Photomed.1992;9(2):45–47.
- .Streptococcal toxic‐shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment.Emerg Infect Dis.1995;1(3):69–78.
- ,,,,,.Fast and frugal models of clinical judgment in novice and expert physicians.Med Decis Making.2003;23(4):293–300.
- .What is it to be an expert? In: Chi M, Farr MJ, Glaser R, eds.The Nature of Expertise.Hillsdale, NJ:Lawrence Erlbaum;1988.
- .Clinical decision‐making: understanding how clinicians make a diagnosis. In: Saint S, Drazen JM, Solomon CG, eds.Clinical Problem‐Solving.New York, NY:McGraw‐Hill;2006.
- ,,.Medical Problem Solving: An Analysis of Clinical Reasoning.Cambridge, MA:Harvard University Press;1978.
- ,,,,.Population‐based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy‐seven cases. Ontario Group A Streptococcal Study.Am J Med.1997;103(1):18–24.
- ,,, et al.Invasive group A streptococcal infections in Sweden in 1994 and 1995: epidemiology and clinical spectrum.Scand J Infect Dis.2000;32(6):609–614.
- ,,,.Reemergence of emm1 and a changed superantigen profile for group A streptococci causing invasive infections: results from a nationwide study.J Clin Microbiol.2005;43(4):1789–1796.
- ,,, et al.Severe group A streptococcal infections associated with a toxic shock‐like syndrome and scarlet fever toxin A.N Engl J Med.1989;321(1):1–7.
- ,,,.Rapidly progressive necrotizing fasciitis caused by Staphylococcus aureus.J Microbiol Immunol Infect.2005;38(5):361–364.
- ,.Streptococcal infections of skin and soft tissues.N Engl J Med.1996;334(4):240–245.
- ,.Streptococcal toxic shock syndrome after breast reconstruction.Ann Plast Surg.2005;54(5):553–556.
- ,.The influence of pigmentation and illumination on the perception of erythema.Photodermatol Photoimmunol Photomed.1992;9(2):45–47.
- .Streptococcal toxic‐shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment.Emerg Infect Dis.1995;1(3):69–78.
Medical Thromboprophylaxis
Venous thromboembolism (VTE) is a common complication of serious illness, conferring increased morbidity and mortality in hospitalized medical patients. Thromboprophylaxis has been rated the number 1 patient safety intervention for hospitalized patients by the Agency for Healthcare Research and Quality, supported by evidence of effectiveness in multiple methodologically rigorous randomized trials.1 Unfortunately, many studies have shown that suitable patients do not receive thromboprophylaxis when they should. For example, in a large teaching hospital, 44 of 245 VTE events were considered to be potentially preventable, occurring because of omitted prophylaxis, inadequate duration of prophylaxis, or incorrect type of prophylaxis.2
Medical patients appear to be at particularly high risk of not receiving thromboprophylaxis. One retrospective study of 446 medical patients in 2 hospitals revealed that only 33% had appropriate prophylaxis.3 In a large retrospective study of 29 Canadian hospitals, among 1,894 patients for whom thromboprophylaxis was considered necessary, only 23% received it.4 These findings are comparable to other practice audits512 and a large international cross‐sectional study13 showing that appropriate thromboprophylaxis is administered to only 1 of every 3 hospitalized medical patients eligible for prophylaxis. Furthermore, of patients diagnosed with VTE in a large international registry, only 33% had received heparin thromboprophylaxis prior to their event.14
The objective of this study was to understand the barriers to, and facilitators of, optimal thromboprophylaxis in hospitalized medical patients using grounded theory methods. To address our poor understanding of low rates of thromboprophylaxis on hospital medical wards, we used qualitative research, which focuses on social and interpreted, rather than natural and objectified, phenomena. Qualitative research also aims to discover, describe, and understand, rather than to test and evaluate.15 Our research question was, What do healthcare clinicians, managers and hospital administrators perceive inhibits the implementation of thromboprophylaxis for medical patients, and what do they perceive would help to optimize thromboprophylaxis?
Methods
We conducted the Qualitative Thromboprophylaxis Enquiry of Compliance (QUALITEC) survey at 3 institutions from June 2006 to September 2007. One was a university‐affiliated hospital (St. Joseph's Healthcare, Hamilton, Ontario, Canada); the other 2 were community hospitals (Joseph Brant Hospital, Burlington, Ontario, and Credit Valley Hospital, Mississauga, Ontario, Canada).
Participants included: (1) bedside nurses, nurse clinicians, and nurse educators; (2) pharmacists; (3) attending physicians; (4) nurse, pharmacy, and physician managers; (5) hospital administrators; (6)medical residents; and (7) members of the hospitals' Quality Improvement Team. Participants in groups 1 to 3 were persons who had cared for medical patients in the index hospital for at least 2 years. Participants in groups 4 and 5 were persons who had worked in the index hospital for at least 2 years. The requisite minimal duration of work on the medical ward for residents was 1 month. There were no exclusion criteria for participants based on sex, religion, ethnicity, or culture. Thus, we enrolled participants based on their a priori eligibility criteria (criterion sampling) and their ability to allow us to achieve our objectives (purposive sampling).
The Research Coordinator created a list of potential interview candidates by examining the organizational structure of each hospital to identify the names of clinicians, managers, and administrators who had responsibility for medical patients. This person identified the names of potential medical ward nurses through discussion with the nurse manager of each medical unit and identified the names of potential attending physicians, residents, and pharmacists through discussion with the physician director or designate of the medical unit (snowball sampling).
After piloting testing with 2 trial participants, the Research Coordinator conducted in‐depth, open‐ended, 1‐on‐1 interviews using a flexible interview guide. This approach allows respondents to use their own words to express ideas, and affords opportunities to probe for more information through dialog. All potential participants were provided with a brief study summary. Each interview took approximately 30 to 60 minutes. All interviews were audiotaped and transcribed verbatim. The Research Coordinator wrote field notes on the main concepts featured in the interview.
To characterize the participants, we recorded their age, sex, position, number of years in their current position, number of years in their profession, and number of years working in their hospital. For clinicians, we recorded the proportion of their professional time spent in clinical practice, the proportion of their clinical time spent caring for hospitalized medical patients (on the medical wards, doing consultations, and in the emergency department), and whether they had a formal role in a previous quality improvement project.
We transcribed each interview upon completion. As is typical for qualitative research, we began data analysis during the data collection phase. Transcripts were analyzed using the coding methods described by Strauss and Corbin.16 The main content analysis of QUALITEC consisted of line‐by‐line open coding. All transcripts were coded by 2 investigators independently who created categories and themes. The first coding process of grounded theory analysis was reduction of data to identify categories (experiences and actions that were similar or related) and to define their dimensions. We iterated between data collection and analysis, revising the interview guide to refine questions and focus on unique concepts. The qualitative data management software NVivo helped with linking codes and categories to ultimately organize ideas into a main theme and several key concepts; it also helped with retrieval of specific quotes. A third investigator reviewed the transcripts independently to identify categories. Analysis continued while the study progressed until saturation of information occurred and no new categories emerged. We invited 3 participants to review an early draft report (member checking).
Research Ethics
This study was approved by the Research Ethics Board at each participating center. Written informed consent was obtained from each participant. Participation was voluntary and confidential; participants had the opportunity to withdraw from the study without any consequences. Data were deidentified upon transcription and remained anonymized, while kept in secure password protected computerized files. Participants received a $25 gift certificate as a token of appreciation. We conducted this study according to the Guiding Ethical Principles section of the Tri‐council Policy Statement of Ethical Conduct for Research Involving Humans.17
Results
Of 39 persons we approached to participate, 36 out of 39 (92.3%) agreed. Two nurse managers at 1 community hospital declined to participate. One resident was missed due to clinical responsibilities. In Table 1, we present the characteristics of the 36 participants. When asked about the level of concern about thromboprophylaxis on the medical wards, participants stated that clinicians were generally insufficiently concerned (24/36, 68.6%) or appropriately concerned (11/36, 31.4%).
| Characteristic | Value |
|---|---|
| |
| Female, n (%) | 27 (75.0) |
| Age, mean (SD) | 39.6 (15.6) |
| Hospital, n (%) | |
| St. Joseph's Healthcare, Hamilton | 16 (44.4) |
| Joseph Brant, Burlington | 9 (25.0) |
| Credit Valley, Oakville | 11 (30.6) |
| Years in hospital, mean (SD) | 10.1 (7.4) |
| Position, n (%) | |
| Bedside nurse | 8 (22.2) |
| Charge nurse | 1 (2.8) |
| Nurse clinician | 2 (5.6) |
| Nurse educator | 2 (5.6) |
| Nurse manager | 2 (5.6) |
| Pharmacist | 3 (8.3) |
| Pharmacist manager | 3 (8.3) |
| Resident physician | 4 (11.1) |
| Attending physician | 6 (16.6) |
| Physician manager | 2 (5.6) |
| Quality improvement team member | 2 (5.6) |
| Hospital administrator | 1 (2.8) |
| Years in current position, mean (SD) | 7.3 (6.4) |
| Participated in quality improvement projects, n (%) | 20 (55.6) |
| Clinical focus (for clinicians only), mean (SD) | |
| Time spent in clinical practice (%) | 93.6 (13.4) |
| Time spent caring for medical patients (%) | 79.2 (25.1) |
Barriers
Relying on individual physicians for VTE prevention was regarded as ineffective, since medical patients often do not receive prophylaxis when they should.
It's sort of Russian roulette as to whether or not we grab a patient and it actually gets done for them. [bedside nurse]
There's a lot of mavericks out there. They do their own thing, and it has never been a problem. They're not held accountable. [quality improvement team leader]
Several different clinician groups were regarded as being involved in medical thromboprophylaxis.
Physiotherapy, occupational therapy, doctors, hopefully our clinical care educators, charge nurses . I think pretty much everybody's involved. I feel that's part of my role as care giver. [bedside nurse]
Many people in the healthcare team are involved in [deep vein thrombosis] DVT prevention at the nurse level, physiotherapy, occupational therapy (in terms of mobility of the patient) the physician, specialty services like thrombo service, hematology department. [nurse manager]
Since many clinicians implicitly or explicitly have a role in thromboprophylaxis, some participants viewed it as everyone's responsibility, or that it should be everyone's responsibility, to ensure optimal VTE prevention.
I think the responsibility should be everyone's responsibilityall across the health care professions. [ward pharmacist]
Make it so that you have a few stopgaps so that a few people are looking for the risk and make the process different so that you have a couple of stops along the way that it'll get brought up. I would create it so that it's not just one group in charge of the DVT prophylaxis decision and assessment. It has to be across the board. [nurse educator]
However, while multidisciplinary care was considered ideal to achieve optimal patient outcomes, it was also paradoxically perceived as insufficient to ensure effective thromboprophylaxis. Multidisciplinary care can lead to confusion about roles on a team, and unclear accountability, thereby becoming a potential barrier to effective prevention. Some participants thought that just one person should be ultimately responsible for thromboprophylaxis.
I think it would be crucial to identify one person responsible rather than indirectly a number of people who would be encouraged . It is probably better if you make one of those targets dependable. [physician]
[If] you assign it to one person versus having the accountability spread among [all] those peoplethen nobody takes accountability. [quality improvement team member]
Many participants reported that mobilization was important, though difficult to achieve. They also expressed uncertainty about whether, and how much, mobilization is enough.
I think the best way to prevent DVT would be to fully focus on early mobilization, regular physiotherapy, and how a patient returns to regular activities to get them up and around. [resident]
Level of mobility is a subjective matter. When it comes to the question of Well, how mobile is mobile enough?, that's not standardized to my knowledge and is very subjective as far as when to stop it. Is getting up in a chair and wiggling your toes good enough? Or are they running up and down the hall and you have to chase them? Really, what level of mobility is considered the standard for discontinuing DVT prophylaxis? That's the question that comes up repeatedly so I think that's a very large barrier. [pharmacy manager]
Several logistic barriers associated with antiembolic stockings and pneumatic compression devices were cited, including problems with fit, inconvenience, noncompliance, and cost.
I find stockings aren't always measured or worn appropriately. It's difficult; I think every nurse measures them differently. If they're too tight around the thighs they just roll them down. If anything, they really constrict any type of circulation rather than promote it. Moon boots are all right, but a little cumbersome, more expensive and are more geared to specific patients. [bedside nurse]
The patients want them off at certain times of the day. Theoretically, you only take them off to bathe them but some of them are really bothered by them. [nurse clinician]
Another key barrier was that clinicians are more focused on treating the immediate health care problem precipitating hospital admission than on preventing future complications.
It's something that we tend to forget because patients come in with giant medical problems a, b, and c, and then your DVT prophylaxis tends to fall by the wayside, as you're trying to deal with their major medical issues. [resident]
Prevention issues are a little bit different than treatment issues . We see ourselves as interventionists more than preventionists . It's the medical things we tend to deal with immediately and that's often the focus why the patient is in hospital, rightly or wrongly. Quality doesn't just include intervention, it includes prevention. [nurse manager]
Potential Solutions
To address this problem of inattention to preventive strategies, participants indicated that local data on the burden of illness and current utilization of thromboprophylaxis would be helpful. Some participants described institution‐specific information and thromboprophylaxis targets that motivate clinicians.
Give feedback to the team and physicians about what the incidence of DVT is. I don't think everybody knows. I don't even know in our hospital what it is. So give feedback to the team about what the incidence of DVT is, how many people are on DVT prophylaxis and how many people die from pulmonary embolism. If you have those numbers in front of you, then you would have something to aim for. [physician manager]
You do run charts so that you are auditing continuously and reporting the results back to the staff. And we have weekly meetings with staff members. That has been effective. And we have tip of the week via email. [quality improvement team member]
Most participants recommended redoubling efforts towards anticoagulant thromboprophylaxisa coordinated, systemwide approach across the continuum of care.
It's got to be a trigger for every patient that comes in. Does this patient need DVT prophylaxis? Is this patient a candidate for it? [physician manager]
I think if it's not tackled at the beginning it gets lost in the shuffle. I think if it's something we put into place just like we do when we're getting a history and on anything else. If we start with it [heparin] from day one we'll continue it through right to the end. [bedside nurse]
Participants suggested a variety of methods to enhance thromboprophylaxis. These included a universal, structured, dynamic risk stratification tool, and standardized order forms at the time of hospital admission, which would be reevaluated regularly, and require an enabling educational program.
Every medical patient 18 or older will be given a risk score. Risk stratifying all of our patients with just a simple little tool and considering treatment for those that are of high risk is what we need to do. Reinforcing the education and teaching required for the patients that are at low risk, because we don't need to put everyone on heparin. But anyone could potentially get a clot. [nurse educator]
It could be a standardized order sheet for every admitted patient and maybe that could help. And that would be like the first page in every chart in the whole hospital, and they can always cross it off and say I don't want to use this. [ward pharmacist]
The hospital intranet was sanctioned as a useful repository for physician orders and other tools. Computerized health records and computer decision supports were strongly endorsed.
It should be in a computerized system if it goes into a manual system, paperwork tends to get lost. I think a computerized system would be ideal. [pharmacist]
I think that having a computer health recordthat we would love, for many reasonsonce that happened, it would be a way to ensure it, because there would be prompts . You would get a prompt saying, Why are you not using it? . An electronic health record and as a back up, having a pharmacist. [physician]
Leveraging patient or family‐mediated interventions to provide reminders was also suggested, given the familiarity of the public with thrombosis.
Leave every patient a small pamphlet saying Are you getting DVT prevention? Are you getting injections? Empower people as well. [physician]
I think the absolute biggest driver from our perspective in admin is always public awareness. The demand for standard service increases the most when the public is aware of it. They ask. Patients are becoming more educated, they use the Internet, they search those things out themselves and they are knowledgeable. [hospital administrator]
Sufficient human resources to ensure mobilization and profiling thromboprophylaxis during accreditation were regarded as administrative initiatives that could help. Capitalizing on social forces in healthcare such as patient safety could also galvanize efforts to prevent VTE.
We need physio and [occupational therapy] OT. We need more rehab. We need resources. That's what we're lacking. The only physio and OT that comes to our floor is pending discharge. So it's definitely resource‐related. [bedside nurse]
From an administrative perspective, the whole concept of preventing complications reduces risk, improves patient safety, reduces length of stayall those warm fuzzy things that are attached to providing the best possible care for the patient at the right time. [nurse manager]
Discussion
In this qualitative study, participants affirmed that depending on individual physicians for VTE prevention is insufficient. Distinct from most therapeutic interventions which are understood as the responsibility of physicians, preventive interventions such as thromboprophylaxis may be more readily embraced as the charge of members of a multidisciplinary team. While every clinician group felt compelled to help with VTE prevention, reliance on multidisciplinary care was also perceived as a barrier to effective VTE prevention because it can generate confusion about roles. Participants recommended a comprehensive, systems‐approach, including screening and risk‐stratifying all patients, preprinted orders at hospital admission that are regularly reevaluated, and audit and feedback programs. Also endorsed were patient or family‐mediated reminders, and administrative interventions such as hiring more physiotherapists for mobilization and profiling thromboprophylaxis for hospital accreditation.
Approximately 70% of participants judged that clinicians were insufficiently concerned about thromboprophylaxis. In contrast to many commonly cited reasons for underutilization of evidence‐based interventions such as lack of awareness of (or resistance to) new information, and lack of self‐efficacy of clinicians (wondering whether the benefits observed in the research setting will be realized in the practice setting),18 participants in our study did not raise these as barriers to thromboprophylaxis. As physician and pharmacist participants indicated, because the evidentiary basis for heparin thromboprophylaxis is strong and understood (in contrast to that for mechanical prophylaxis), lack of knowledge was not considered a barrier.
Our findings are consistent with a previous qualitative study in which clinicians and managers were interviewed to learn about factors that increase beta‐blocker use following myocardial infarction; these investigators found that administrative support, use of data, and quality improvement initiatives were key.19 Detailed implementation directives to clarify clinical responsibilities for many different stakeholders were also suggested in a qualitative study on the optimal use of noninvasive ventilation.20 Extending these results, participants in our study suggested several interventions aimed at different levels of the healthcare system (eg, patient, provider, and administrator) that are either enabling or reinforcing. While just 1 implementation strategy may result in improved thromboprophylaxis,21 efficient application of multiply‐redundant strategies delivered by a multidisciplinary team may be more powerful. Such an approach has driven high level performance for the American Heart Association's Get With The Guidelines Program, resulting in lower community and hospital cardiovascular and cerebrovascular mortality in Massachusetts.22
Although our aim was to obtain multidisciplinary input, we did not focus on physicians who primarily prescribe thromboprophylaxis, thereby potentially underrepresenting their views relative to their role. We did not interview all clinicians who can influence VTE risk (eg, physiotherapists) or prescribe thromboprophylaxis (eg, family physicians). During this study, patient safety emerged as a major hospital initiative,23 which could modify participants' views on the importance of VTE prevention. Although VTE prevention may be enhanced by understanding barriers to, and solutions for, optimal thromboprophylaxis from both clinicians' and managers' perspectives, the suggestions we elicited on methods to improve VTE prevention represent participants' opinions, rather than evidence, about effective strategies. Our findings are not generalizable to settings with nurse practitioners who are dedicated to VTE prevention, nor to settings with computer decision support systems that already incorporate thromboprophylaxis.
Strengths of this study include interviewing key stakeholders representing several clinician groups and managers, to obtain perspectives on both individual and systemwide influences on thromboprophylaxis in medical patients. By eliciting the views and experiences of participants in both the community and university settings, we captured multicenter perspectives on preventive health. We used triangulation of data sources (researcher and participant) and invited participants to review an early draft of the report (member checking).24 This study highlights the merits of qualitative research which can provide insights into familiar patterns and problems, and contribute to knowledge for interdisciplinary audiences. These qualitative research results help to explain quantitative research results documenting very low rates of medical thromboprophylaxis,214 raising hypotheses to test in future studies testing systemwide interventions that may improve patient safety. One prominent publication reported the impact of electronic reminders,25 while a recent systematic review of methods to increase thromboprophylaxis outlines many potentially effective approaches.21
In summary, the findings of this qualitative study challenge the notion that either individual physician efforts or multidisciplinary care are enough to lead to optimal VTE prevention. Participants believed that while well‐functioning teams at the bedside hold great promise to deliver superior care, they may also lead to unclear role definition. Since physicians commit many errors of omission regarding thromboprophylaxis, these results raise the possibility of delegated medical acts outside the scope of conventional practice for nurses and pharmacists. Leveraging the skills and knowledge of multidisciplinary teams for medical thromboprophylaxis requires not only clear accountabilities, but also excellent interprofessional communication, and institution‐wide approaches to change prescribing behavior, potentially involving patients and administrators as well as clinicians.
Acknowledgements
The authors thank the participants of this study for sharing their experiences and views with them. The authors are grateful for the help of Laurel Raftery, Michelle Murray, Monica Owen, and Shelley Anderson for transcriptions. The authors appreciate the organizational assistance of Nancy Lloyd and the biostatistical support of Diane Heels‐Ansdell for analyzing respondent characteristics. The authors are grateful to Nancy Lloyd, Alice Huh, Barbara Young, and Gray Ellrodt for feedback on earlier versions of this report. Deborah Cook had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix
Qualitec Interview Guide
Opening Statement
Thank you for your participation in this research study we are conducting with colleagues at St. Joseph's Hospital and McMaster University. Through this interview, I hope to learn how you think about DVT prevention in medical patients. When you answer the interview questions, please keep in mind that we are interested in your own thoughts and approaches.
First, I have a consent form I'd like you to read to make sure you understand the reason for this study, and to see if you have any questions about becoming involved. Please take a few minutes to read it over.
[Participant reads consent, questions about the study may or may not ensue, participant signs to indicate informed consent, the interview proceeds.]
Structured Interview Begins
Demographics
Before we begin the interview, I would like you to take a few minutes to complete this form, which consists of a few demographic questions.
[Participant completes demographic form].
Thank you. Now I'll move on to some questions about DVT prevention.
[The interviewer informs the participant that she is putting the tape recorder on].
Core Study Interview Guide
Can you tell me a bit about your own approach to DVT prevention in medical patients? For what types of patients are you concerned about DVT?
What do you find are the best ways to prevent DVT? Why?
Who else is involved in trying to prevent DVT? In general, how are DVTs are prevented at this hospital?
What are some of the barriers to preventing DVTs? (e.g., risks of prevention, disbelief that prevention really works, DVT prevention is someone else's responsibilitywhose?)
Are there other ways to ensure that DVTs are prevented in medical patients? If so, how would they work?
Can you tell me how different clinicians (such as physicians, nurses, pharmacists, and residents) could do a better job at preventing DVT? How could medical wards and departments help? How could hospital administration help?
Do you remember a patient who had a problem with DVT prevention? If so, can you tell me about this problem and how it might have been avoided?
If you could create a perfect system of DVT prevention what would it be?
Do you think people are overconcerned, appropriately concerned, or underconcerned about DVT prevention?
Is there anything else you would like to tell me?
Final Debriefing Question
How has this interview experience been for you? Thank you very much for sharing your views. We really appreciate it.
- Agency for Health Care Policy Research. Prevention of venous thromboembolism after injury. Summary, evidence report/technology assessment. Available at: http://www. ahrq.gov/clinic/epcsums/vtsumm.htm. Accessed April 2008.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,.Venous thromboembolism prophylaxis in medical inpatients: a retrospective chart review.Thromb Res.2003;111:215–219.
- ,,, et al.,for the CURVE Study Investigators.Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada.Thromb Res.2007;119:145–155.
- ,.Thromboprophylaxis in an academic medical center.Cardiovasc Surg.2001;9:426–430.
- ,,.Deep venous thrombosis prophylaxis: are guidelines being followed?ANZ J Surg.2002;72:331–334.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17:788–791.
- ,.Preventing venous thromboembolism in acute medical patients.QJM.2004;97:797–801.
- ,.Venous thromboembolic prophylaxis in hospitalized medical patients.Ann Pharmacother.2004;38:36–40.
- ,,.Physicians' practice for prevention of venous thromboembolism in medical patients.J Coll Physicians Surg.2004;14:211.
- ,,, et al.Venous thromboembolism prophylaxis in acutely ill medical patients: definite need for improvement.J Intern Med.2005;257:352–357.
- ,,, et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns.Haematologica.2002;87:746–750.
- ,,, et al.,for the ENDORSE Investigators.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE Study): a multinational cross‐sectional study.Lancet.2008;371:387–394.
- ,,, et al.,for the IMPROVE Investigators.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism.Chest.2007;132:936–945.
- ,,for the Evidence‐Based Medicine Working Group.Users' guides to the medical literature. XXIII. Qualitative research in health care. A. Are the results of the study valid?JAMA.2000;284:357–362.
- ,.Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory.2nd ed.London:Sage Publications;1998.
- Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, Social Sciences and Humanities Research Council of Canada.Tri‐Council Policy Statement: Ethical Conduct for Research Involving Humans.Ottawa, Ontario:Public Works and Government Services Canada;1998 [with 2000, 2002, and 2005 amendments].
- ,,, et al.Why don't physicians follow clinical practice guidelines?JAMA.1999;282:1458–1465.
- ,,,,,.A qualitative study of increasing β‐blocker use after myocardial infarction: why do some hospitals succeed?JAMA.2001;285:2604–2611.
- ,,,.Practice guidelines as multipurpose tools: a qualitative study of noninvasive ventilation.Crit Care Med.2007;35:776–782.
- ,,, et al.A systemic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
- ,,, et al.Multidisciplinary rounds: an implementation system for sustained improvement in the American Heart Association's “Get With the Guidelines” Program.Crit Pathw Cardiol.2007;6:1016–1116.
- ,,,,.Changing clinician behaviour to improve patient safety.Lancet.2004;363:1224–1230.
- ,.Papers that go beyond numbers: qualitative research.BMJ.1997;315:740–748.
- ,,,,,,.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
Venous thromboembolism (VTE) is a common complication of serious illness, conferring increased morbidity and mortality in hospitalized medical patients. Thromboprophylaxis has been rated the number 1 patient safety intervention for hospitalized patients by the Agency for Healthcare Research and Quality, supported by evidence of effectiveness in multiple methodologically rigorous randomized trials.1 Unfortunately, many studies have shown that suitable patients do not receive thromboprophylaxis when they should. For example, in a large teaching hospital, 44 of 245 VTE events were considered to be potentially preventable, occurring because of omitted prophylaxis, inadequate duration of prophylaxis, or incorrect type of prophylaxis.2
Medical patients appear to be at particularly high risk of not receiving thromboprophylaxis. One retrospective study of 446 medical patients in 2 hospitals revealed that only 33% had appropriate prophylaxis.3 In a large retrospective study of 29 Canadian hospitals, among 1,894 patients for whom thromboprophylaxis was considered necessary, only 23% received it.4 These findings are comparable to other practice audits512 and a large international cross‐sectional study13 showing that appropriate thromboprophylaxis is administered to only 1 of every 3 hospitalized medical patients eligible for prophylaxis. Furthermore, of patients diagnosed with VTE in a large international registry, only 33% had received heparin thromboprophylaxis prior to their event.14
The objective of this study was to understand the barriers to, and facilitators of, optimal thromboprophylaxis in hospitalized medical patients using grounded theory methods. To address our poor understanding of low rates of thromboprophylaxis on hospital medical wards, we used qualitative research, which focuses on social and interpreted, rather than natural and objectified, phenomena. Qualitative research also aims to discover, describe, and understand, rather than to test and evaluate.15 Our research question was, What do healthcare clinicians, managers and hospital administrators perceive inhibits the implementation of thromboprophylaxis for medical patients, and what do they perceive would help to optimize thromboprophylaxis?
Methods
We conducted the Qualitative Thromboprophylaxis Enquiry of Compliance (QUALITEC) survey at 3 institutions from June 2006 to September 2007. One was a university‐affiliated hospital (St. Joseph's Healthcare, Hamilton, Ontario, Canada); the other 2 were community hospitals (Joseph Brant Hospital, Burlington, Ontario, and Credit Valley Hospital, Mississauga, Ontario, Canada).
Participants included: (1) bedside nurses, nurse clinicians, and nurse educators; (2) pharmacists; (3) attending physicians; (4) nurse, pharmacy, and physician managers; (5) hospital administrators; (6)medical residents; and (7) members of the hospitals' Quality Improvement Team. Participants in groups 1 to 3 were persons who had cared for medical patients in the index hospital for at least 2 years. Participants in groups 4 and 5 were persons who had worked in the index hospital for at least 2 years. The requisite minimal duration of work on the medical ward for residents was 1 month. There were no exclusion criteria for participants based on sex, religion, ethnicity, or culture. Thus, we enrolled participants based on their a priori eligibility criteria (criterion sampling) and their ability to allow us to achieve our objectives (purposive sampling).
The Research Coordinator created a list of potential interview candidates by examining the organizational structure of each hospital to identify the names of clinicians, managers, and administrators who had responsibility for medical patients. This person identified the names of potential medical ward nurses through discussion with the nurse manager of each medical unit and identified the names of potential attending physicians, residents, and pharmacists through discussion with the physician director or designate of the medical unit (snowball sampling).
After piloting testing with 2 trial participants, the Research Coordinator conducted in‐depth, open‐ended, 1‐on‐1 interviews using a flexible interview guide. This approach allows respondents to use their own words to express ideas, and affords opportunities to probe for more information through dialog. All potential participants were provided with a brief study summary. Each interview took approximately 30 to 60 minutes. All interviews were audiotaped and transcribed verbatim. The Research Coordinator wrote field notes on the main concepts featured in the interview.
To characterize the participants, we recorded their age, sex, position, number of years in their current position, number of years in their profession, and number of years working in their hospital. For clinicians, we recorded the proportion of their professional time spent in clinical practice, the proportion of their clinical time spent caring for hospitalized medical patients (on the medical wards, doing consultations, and in the emergency department), and whether they had a formal role in a previous quality improvement project.
We transcribed each interview upon completion. As is typical for qualitative research, we began data analysis during the data collection phase. Transcripts were analyzed using the coding methods described by Strauss and Corbin.16 The main content analysis of QUALITEC consisted of line‐by‐line open coding. All transcripts were coded by 2 investigators independently who created categories and themes. The first coding process of grounded theory analysis was reduction of data to identify categories (experiences and actions that were similar or related) and to define their dimensions. We iterated between data collection and analysis, revising the interview guide to refine questions and focus on unique concepts. The qualitative data management software NVivo helped with linking codes and categories to ultimately organize ideas into a main theme and several key concepts; it also helped with retrieval of specific quotes. A third investigator reviewed the transcripts independently to identify categories. Analysis continued while the study progressed until saturation of information occurred and no new categories emerged. We invited 3 participants to review an early draft report (member checking).
Research Ethics
This study was approved by the Research Ethics Board at each participating center. Written informed consent was obtained from each participant. Participation was voluntary and confidential; participants had the opportunity to withdraw from the study without any consequences. Data were deidentified upon transcription and remained anonymized, while kept in secure password protected computerized files. Participants received a $25 gift certificate as a token of appreciation. We conducted this study according to the Guiding Ethical Principles section of the Tri‐council Policy Statement of Ethical Conduct for Research Involving Humans.17
Results
Of 39 persons we approached to participate, 36 out of 39 (92.3%) agreed. Two nurse managers at 1 community hospital declined to participate. One resident was missed due to clinical responsibilities. In Table 1, we present the characteristics of the 36 participants. When asked about the level of concern about thromboprophylaxis on the medical wards, participants stated that clinicians were generally insufficiently concerned (24/36, 68.6%) or appropriately concerned (11/36, 31.4%).
| Characteristic | Value |
|---|---|
| |
| Female, n (%) | 27 (75.0) |
| Age, mean (SD) | 39.6 (15.6) |
| Hospital, n (%) | |
| St. Joseph's Healthcare, Hamilton | 16 (44.4) |
| Joseph Brant, Burlington | 9 (25.0) |
| Credit Valley, Oakville | 11 (30.6) |
| Years in hospital, mean (SD) | 10.1 (7.4) |
| Position, n (%) | |
| Bedside nurse | 8 (22.2) |
| Charge nurse | 1 (2.8) |
| Nurse clinician | 2 (5.6) |
| Nurse educator | 2 (5.6) |
| Nurse manager | 2 (5.6) |
| Pharmacist | 3 (8.3) |
| Pharmacist manager | 3 (8.3) |
| Resident physician | 4 (11.1) |
| Attending physician | 6 (16.6) |
| Physician manager | 2 (5.6) |
| Quality improvement team member | 2 (5.6) |
| Hospital administrator | 1 (2.8) |
| Years in current position, mean (SD) | 7.3 (6.4) |
| Participated in quality improvement projects, n (%) | 20 (55.6) |
| Clinical focus (for clinicians only), mean (SD) | |
| Time spent in clinical practice (%) | 93.6 (13.4) |
| Time spent caring for medical patients (%) | 79.2 (25.1) |
Barriers
Relying on individual physicians for VTE prevention was regarded as ineffective, since medical patients often do not receive prophylaxis when they should.
It's sort of Russian roulette as to whether or not we grab a patient and it actually gets done for them. [bedside nurse]
There's a lot of mavericks out there. They do their own thing, and it has never been a problem. They're not held accountable. [quality improvement team leader]
Several different clinician groups were regarded as being involved in medical thromboprophylaxis.
Physiotherapy, occupational therapy, doctors, hopefully our clinical care educators, charge nurses . I think pretty much everybody's involved. I feel that's part of my role as care giver. [bedside nurse]
Many people in the healthcare team are involved in [deep vein thrombosis] DVT prevention at the nurse level, physiotherapy, occupational therapy (in terms of mobility of the patient) the physician, specialty services like thrombo service, hematology department. [nurse manager]
Since many clinicians implicitly or explicitly have a role in thromboprophylaxis, some participants viewed it as everyone's responsibility, or that it should be everyone's responsibility, to ensure optimal VTE prevention.
I think the responsibility should be everyone's responsibilityall across the health care professions. [ward pharmacist]
Make it so that you have a few stopgaps so that a few people are looking for the risk and make the process different so that you have a couple of stops along the way that it'll get brought up. I would create it so that it's not just one group in charge of the DVT prophylaxis decision and assessment. It has to be across the board. [nurse educator]
However, while multidisciplinary care was considered ideal to achieve optimal patient outcomes, it was also paradoxically perceived as insufficient to ensure effective thromboprophylaxis. Multidisciplinary care can lead to confusion about roles on a team, and unclear accountability, thereby becoming a potential barrier to effective prevention. Some participants thought that just one person should be ultimately responsible for thromboprophylaxis.
I think it would be crucial to identify one person responsible rather than indirectly a number of people who would be encouraged . It is probably better if you make one of those targets dependable. [physician]
[If] you assign it to one person versus having the accountability spread among [all] those peoplethen nobody takes accountability. [quality improvement team member]
Many participants reported that mobilization was important, though difficult to achieve. They also expressed uncertainty about whether, and how much, mobilization is enough.
I think the best way to prevent DVT would be to fully focus on early mobilization, regular physiotherapy, and how a patient returns to regular activities to get them up and around. [resident]
Level of mobility is a subjective matter. When it comes to the question of Well, how mobile is mobile enough?, that's not standardized to my knowledge and is very subjective as far as when to stop it. Is getting up in a chair and wiggling your toes good enough? Or are they running up and down the hall and you have to chase them? Really, what level of mobility is considered the standard for discontinuing DVT prophylaxis? That's the question that comes up repeatedly so I think that's a very large barrier. [pharmacy manager]
Several logistic barriers associated with antiembolic stockings and pneumatic compression devices were cited, including problems with fit, inconvenience, noncompliance, and cost.
I find stockings aren't always measured or worn appropriately. It's difficult; I think every nurse measures them differently. If they're too tight around the thighs they just roll them down. If anything, they really constrict any type of circulation rather than promote it. Moon boots are all right, but a little cumbersome, more expensive and are more geared to specific patients. [bedside nurse]
The patients want them off at certain times of the day. Theoretically, you only take them off to bathe them but some of them are really bothered by them. [nurse clinician]
Another key barrier was that clinicians are more focused on treating the immediate health care problem precipitating hospital admission than on preventing future complications.
It's something that we tend to forget because patients come in with giant medical problems a, b, and c, and then your DVT prophylaxis tends to fall by the wayside, as you're trying to deal with their major medical issues. [resident]
Prevention issues are a little bit different than treatment issues . We see ourselves as interventionists more than preventionists . It's the medical things we tend to deal with immediately and that's often the focus why the patient is in hospital, rightly or wrongly. Quality doesn't just include intervention, it includes prevention. [nurse manager]
Potential Solutions
To address this problem of inattention to preventive strategies, participants indicated that local data on the burden of illness and current utilization of thromboprophylaxis would be helpful. Some participants described institution‐specific information and thromboprophylaxis targets that motivate clinicians.
Give feedback to the team and physicians about what the incidence of DVT is. I don't think everybody knows. I don't even know in our hospital what it is. So give feedback to the team about what the incidence of DVT is, how many people are on DVT prophylaxis and how many people die from pulmonary embolism. If you have those numbers in front of you, then you would have something to aim for. [physician manager]
You do run charts so that you are auditing continuously and reporting the results back to the staff. And we have weekly meetings with staff members. That has been effective. And we have tip of the week via email. [quality improvement team member]
Most participants recommended redoubling efforts towards anticoagulant thromboprophylaxisa coordinated, systemwide approach across the continuum of care.
It's got to be a trigger for every patient that comes in. Does this patient need DVT prophylaxis? Is this patient a candidate for it? [physician manager]
I think if it's not tackled at the beginning it gets lost in the shuffle. I think if it's something we put into place just like we do when we're getting a history and on anything else. If we start with it [heparin] from day one we'll continue it through right to the end. [bedside nurse]
Participants suggested a variety of methods to enhance thromboprophylaxis. These included a universal, structured, dynamic risk stratification tool, and standardized order forms at the time of hospital admission, which would be reevaluated regularly, and require an enabling educational program.
Every medical patient 18 or older will be given a risk score. Risk stratifying all of our patients with just a simple little tool and considering treatment for those that are of high risk is what we need to do. Reinforcing the education and teaching required for the patients that are at low risk, because we don't need to put everyone on heparin. But anyone could potentially get a clot. [nurse educator]
It could be a standardized order sheet for every admitted patient and maybe that could help. And that would be like the first page in every chart in the whole hospital, and they can always cross it off and say I don't want to use this. [ward pharmacist]
The hospital intranet was sanctioned as a useful repository for physician orders and other tools. Computerized health records and computer decision supports were strongly endorsed.
It should be in a computerized system if it goes into a manual system, paperwork tends to get lost. I think a computerized system would be ideal. [pharmacist]
I think that having a computer health recordthat we would love, for many reasonsonce that happened, it would be a way to ensure it, because there would be prompts . You would get a prompt saying, Why are you not using it? . An electronic health record and as a back up, having a pharmacist. [physician]
Leveraging patient or family‐mediated interventions to provide reminders was also suggested, given the familiarity of the public with thrombosis.
Leave every patient a small pamphlet saying Are you getting DVT prevention? Are you getting injections? Empower people as well. [physician]
I think the absolute biggest driver from our perspective in admin is always public awareness. The demand for standard service increases the most when the public is aware of it. They ask. Patients are becoming more educated, they use the Internet, they search those things out themselves and they are knowledgeable. [hospital administrator]
Sufficient human resources to ensure mobilization and profiling thromboprophylaxis during accreditation were regarded as administrative initiatives that could help. Capitalizing on social forces in healthcare such as patient safety could also galvanize efforts to prevent VTE.
We need physio and [occupational therapy] OT. We need more rehab. We need resources. That's what we're lacking. The only physio and OT that comes to our floor is pending discharge. So it's definitely resource‐related. [bedside nurse]
From an administrative perspective, the whole concept of preventing complications reduces risk, improves patient safety, reduces length of stayall those warm fuzzy things that are attached to providing the best possible care for the patient at the right time. [nurse manager]
Discussion
In this qualitative study, participants affirmed that depending on individual physicians for VTE prevention is insufficient. Distinct from most therapeutic interventions which are understood as the responsibility of physicians, preventive interventions such as thromboprophylaxis may be more readily embraced as the charge of members of a multidisciplinary team. While every clinician group felt compelled to help with VTE prevention, reliance on multidisciplinary care was also perceived as a barrier to effective VTE prevention because it can generate confusion about roles. Participants recommended a comprehensive, systems‐approach, including screening and risk‐stratifying all patients, preprinted orders at hospital admission that are regularly reevaluated, and audit and feedback programs. Also endorsed were patient or family‐mediated reminders, and administrative interventions such as hiring more physiotherapists for mobilization and profiling thromboprophylaxis for hospital accreditation.
Approximately 70% of participants judged that clinicians were insufficiently concerned about thromboprophylaxis. In contrast to many commonly cited reasons for underutilization of evidence‐based interventions such as lack of awareness of (or resistance to) new information, and lack of self‐efficacy of clinicians (wondering whether the benefits observed in the research setting will be realized in the practice setting),18 participants in our study did not raise these as barriers to thromboprophylaxis. As physician and pharmacist participants indicated, because the evidentiary basis for heparin thromboprophylaxis is strong and understood (in contrast to that for mechanical prophylaxis), lack of knowledge was not considered a barrier.
Our findings are consistent with a previous qualitative study in which clinicians and managers were interviewed to learn about factors that increase beta‐blocker use following myocardial infarction; these investigators found that administrative support, use of data, and quality improvement initiatives were key.19 Detailed implementation directives to clarify clinical responsibilities for many different stakeholders were also suggested in a qualitative study on the optimal use of noninvasive ventilation.20 Extending these results, participants in our study suggested several interventions aimed at different levels of the healthcare system (eg, patient, provider, and administrator) that are either enabling or reinforcing. While just 1 implementation strategy may result in improved thromboprophylaxis,21 efficient application of multiply‐redundant strategies delivered by a multidisciplinary team may be more powerful. Such an approach has driven high level performance for the American Heart Association's Get With The Guidelines Program, resulting in lower community and hospital cardiovascular and cerebrovascular mortality in Massachusetts.22
Although our aim was to obtain multidisciplinary input, we did not focus on physicians who primarily prescribe thromboprophylaxis, thereby potentially underrepresenting their views relative to their role. We did not interview all clinicians who can influence VTE risk (eg, physiotherapists) or prescribe thromboprophylaxis (eg, family physicians). During this study, patient safety emerged as a major hospital initiative,23 which could modify participants' views on the importance of VTE prevention. Although VTE prevention may be enhanced by understanding barriers to, and solutions for, optimal thromboprophylaxis from both clinicians' and managers' perspectives, the suggestions we elicited on methods to improve VTE prevention represent participants' opinions, rather than evidence, about effective strategies. Our findings are not generalizable to settings with nurse practitioners who are dedicated to VTE prevention, nor to settings with computer decision support systems that already incorporate thromboprophylaxis.
Strengths of this study include interviewing key stakeholders representing several clinician groups and managers, to obtain perspectives on both individual and systemwide influences on thromboprophylaxis in medical patients. By eliciting the views and experiences of participants in both the community and university settings, we captured multicenter perspectives on preventive health. We used triangulation of data sources (researcher and participant) and invited participants to review an early draft of the report (member checking).24 This study highlights the merits of qualitative research which can provide insights into familiar patterns and problems, and contribute to knowledge for interdisciplinary audiences. These qualitative research results help to explain quantitative research results documenting very low rates of medical thromboprophylaxis,214 raising hypotheses to test in future studies testing systemwide interventions that may improve patient safety. One prominent publication reported the impact of electronic reminders,25 while a recent systematic review of methods to increase thromboprophylaxis outlines many potentially effective approaches.21
In summary, the findings of this qualitative study challenge the notion that either individual physician efforts or multidisciplinary care are enough to lead to optimal VTE prevention. Participants believed that while well‐functioning teams at the bedside hold great promise to deliver superior care, they may also lead to unclear role definition. Since physicians commit many errors of omission regarding thromboprophylaxis, these results raise the possibility of delegated medical acts outside the scope of conventional practice for nurses and pharmacists. Leveraging the skills and knowledge of multidisciplinary teams for medical thromboprophylaxis requires not only clear accountabilities, but also excellent interprofessional communication, and institution‐wide approaches to change prescribing behavior, potentially involving patients and administrators as well as clinicians.
Acknowledgements
The authors thank the participants of this study for sharing their experiences and views with them. The authors are grateful for the help of Laurel Raftery, Michelle Murray, Monica Owen, and Shelley Anderson for transcriptions. The authors appreciate the organizational assistance of Nancy Lloyd and the biostatistical support of Diane Heels‐Ansdell for analyzing respondent characteristics. The authors are grateful to Nancy Lloyd, Alice Huh, Barbara Young, and Gray Ellrodt for feedback on earlier versions of this report. Deborah Cook had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix
Qualitec Interview Guide
Opening Statement
Thank you for your participation in this research study we are conducting with colleagues at St. Joseph's Hospital and McMaster University. Through this interview, I hope to learn how you think about DVT prevention in medical patients. When you answer the interview questions, please keep in mind that we are interested in your own thoughts and approaches.
First, I have a consent form I'd like you to read to make sure you understand the reason for this study, and to see if you have any questions about becoming involved. Please take a few minutes to read it over.
[Participant reads consent, questions about the study may or may not ensue, participant signs to indicate informed consent, the interview proceeds.]
Structured Interview Begins
Demographics
Before we begin the interview, I would like you to take a few minutes to complete this form, which consists of a few demographic questions.
[Participant completes demographic form].
Thank you. Now I'll move on to some questions about DVT prevention.
[The interviewer informs the participant that she is putting the tape recorder on].
Core Study Interview Guide
Can you tell me a bit about your own approach to DVT prevention in medical patients? For what types of patients are you concerned about DVT?
What do you find are the best ways to prevent DVT? Why?
Who else is involved in trying to prevent DVT? In general, how are DVTs are prevented at this hospital?
What are some of the barriers to preventing DVTs? (e.g., risks of prevention, disbelief that prevention really works, DVT prevention is someone else's responsibilitywhose?)
Are there other ways to ensure that DVTs are prevented in medical patients? If so, how would they work?
Can you tell me how different clinicians (such as physicians, nurses, pharmacists, and residents) could do a better job at preventing DVT? How could medical wards and departments help? How could hospital administration help?
Do you remember a patient who had a problem with DVT prevention? If so, can you tell me about this problem and how it might have been avoided?
If you could create a perfect system of DVT prevention what would it be?
Do you think people are overconcerned, appropriately concerned, or underconcerned about DVT prevention?
Is there anything else you would like to tell me?
Final Debriefing Question
How has this interview experience been for you? Thank you very much for sharing your views. We really appreciate it.
Venous thromboembolism (VTE) is a common complication of serious illness, conferring increased morbidity and mortality in hospitalized medical patients. Thromboprophylaxis has been rated the number 1 patient safety intervention for hospitalized patients by the Agency for Healthcare Research and Quality, supported by evidence of effectiveness in multiple methodologically rigorous randomized trials.1 Unfortunately, many studies have shown that suitable patients do not receive thromboprophylaxis when they should. For example, in a large teaching hospital, 44 of 245 VTE events were considered to be potentially preventable, occurring because of omitted prophylaxis, inadequate duration of prophylaxis, or incorrect type of prophylaxis.2
Medical patients appear to be at particularly high risk of not receiving thromboprophylaxis. One retrospective study of 446 medical patients in 2 hospitals revealed that only 33% had appropriate prophylaxis.3 In a large retrospective study of 29 Canadian hospitals, among 1,894 patients for whom thromboprophylaxis was considered necessary, only 23% received it.4 These findings are comparable to other practice audits512 and a large international cross‐sectional study13 showing that appropriate thromboprophylaxis is administered to only 1 of every 3 hospitalized medical patients eligible for prophylaxis. Furthermore, of patients diagnosed with VTE in a large international registry, only 33% had received heparin thromboprophylaxis prior to their event.14
The objective of this study was to understand the barriers to, and facilitators of, optimal thromboprophylaxis in hospitalized medical patients using grounded theory methods. To address our poor understanding of low rates of thromboprophylaxis on hospital medical wards, we used qualitative research, which focuses on social and interpreted, rather than natural and objectified, phenomena. Qualitative research also aims to discover, describe, and understand, rather than to test and evaluate.15 Our research question was, What do healthcare clinicians, managers and hospital administrators perceive inhibits the implementation of thromboprophylaxis for medical patients, and what do they perceive would help to optimize thromboprophylaxis?
Methods
We conducted the Qualitative Thromboprophylaxis Enquiry of Compliance (QUALITEC) survey at 3 institutions from June 2006 to September 2007. One was a university‐affiliated hospital (St. Joseph's Healthcare, Hamilton, Ontario, Canada); the other 2 were community hospitals (Joseph Brant Hospital, Burlington, Ontario, and Credit Valley Hospital, Mississauga, Ontario, Canada).
Participants included: (1) bedside nurses, nurse clinicians, and nurse educators; (2) pharmacists; (3) attending physicians; (4) nurse, pharmacy, and physician managers; (5) hospital administrators; (6)medical residents; and (7) members of the hospitals' Quality Improvement Team. Participants in groups 1 to 3 were persons who had cared for medical patients in the index hospital for at least 2 years. Participants in groups 4 and 5 were persons who had worked in the index hospital for at least 2 years. The requisite minimal duration of work on the medical ward for residents was 1 month. There were no exclusion criteria for participants based on sex, religion, ethnicity, or culture. Thus, we enrolled participants based on their a priori eligibility criteria (criterion sampling) and their ability to allow us to achieve our objectives (purposive sampling).
The Research Coordinator created a list of potential interview candidates by examining the organizational structure of each hospital to identify the names of clinicians, managers, and administrators who had responsibility for medical patients. This person identified the names of potential medical ward nurses through discussion with the nurse manager of each medical unit and identified the names of potential attending physicians, residents, and pharmacists through discussion with the physician director or designate of the medical unit (snowball sampling).
After piloting testing with 2 trial participants, the Research Coordinator conducted in‐depth, open‐ended, 1‐on‐1 interviews using a flexible interview guide. This approach allows respondents to use their own words to express ideas, and affords opportunities to probe for more information through dialog. All potential participants were provided with a brief study summary. Each interview took approximately 30 to 60 minutes. All interviews were audiotaped and transcribed verbatim. The Research Coordinator wrote field notes on the main concepts featured in the interview.
To characterize the participants, we recorded their age, sex, position, number of years in their current position, number of years in their profession, and number of years working in their hospital. For clinicians, we recorded the proportion of their professional time spent in clinical practice, the proportion of their clinical time spent caring for hospitalized medical patients (on the medical wards, doing consultations, and in the emergency department), and whether they had a formal role in a previous quality improvement project.
We transcribed each interview upon completion. As is typical for qualitative research, we began data analysis during the data collection phase. Transcripts were analyzed using the coding methods described by Strauss and Corbin.16 The main content analysis of QUALITEC consisted of line‐by‐line open coding. All transcripts were coded by 2 investigators independently who created categories and themes. The first coding process of grounded theory analysis was reduction of data to identify categories (experiences and actions that were similar or related) and to define their dimensions. We iterated between data collection and analysis, revising the interview guide to refine questions and focus on unique concepts. The qualitative data management software NVivo helped with linking codes and categories to ultimately organize ideas into a main theme and several key concepts; it also helped with retrieval of specific quotes. A third investigator reviewed the transcripts independently to identify categories. Analysis continued while the study progressed until saturation of information occurred and no new categories emerged. We invited 3 participants to review an early draft report (member checking).
Research Ethics
This study was approved by the Research Ethics Board at each participating center. Written informed consent was obtained from each participant. Participation was voluntary and confidential; participants had the opportunity to withdraw from the study without any consequences. Data were deidentified upon transcription and remained anonymized, while kept in secure password protected computerized files. Participants received a $25 gift certificate as a token of appreciation. We conducted this study according to the Guiding Ethical Principles section of the Tri‐council Policy Statement of Ethical Conduct for Research Involving Humans.17
Results
Of 39 persons we approached to participate, 36 out of 39 (92.3%) agreed. Two nurse managers at 1 community hospital declined to participate. One resident was missed due to clinical responsibilities. In Table 1, we present the characteristics of the 36 participants. When asked about the level of concern about thromboprophylaxis on the medical wards, participants stated that clinicians were generally insufficiently concerned (24/36, 68.6%) or appropriately concerned (11/36, 31.4%).
| Characteristic | Value |
|---|---|
| |
| Female, n (%) | 27 (75.0) |
| Age, mean (SD) | 39.6 (15.6) |
| Hospital, n (%) | |
| St. Joseph's Healthcare, Hamilton | 16 (44.4) |
| Joseph Brant, Burlington | 9 (25.0) |
| Credit Valley, Oakville | 11 (30.6) |
| Years in hospital, mean (SD) | 10.1 (7.4) |
| Position, n (%) | |
| Bedside nurse | 8 (22.2) |
| Charge nurse | 1 (2.8) |
| Nurse clinician | 2 (5.6) |
| Nurse educator | 2 (5.6) |
| Nurse manager | 2 (5.6) |
| Pharmacist | 3 (8.3) |
| Pharmacist manager | 3 (8.3) |
| Resident physician | 4 (11.1) |
| Attending physician | 6 (16.6) |
| Physician manager | 2 (5.6) |
| Quality improvement team member | 2 (5.6) |
| Hospital administrator | 1 (2.8) |
| Years in current position, mean (SD) | 7.3 (6.4) |
| Participated in quality improvement projects, n (%) | 20 (55.6) |
| Clinical focus (for clinicians only), mean (SD) | |
| Time spent in clinical practice (%) | 93.6 (13.4) |
| Time spent caring for medical patients (%) | 79.2 (25.1) |
Barriers
Relying on individual physicians for VTE prevention was regarded as ineffective, since medical patients often do not receive prophylaxis when they should.
It's sort of Russian roulette as to whether or not we grab a patient and it actually gets done for them. [bedside nurse]
There's a lot of mavericks out there. They do their own thing, and it has never been a problem. They're not held accountable. [quality improvement team leader]
Several different clinician groups were regarded as being involved in medical thromboprophylaxis.
Physiotherapy, occupational therapy, doctors, hopefully our clinical care educators, charge nurses . I think pretty much everybody's involved. I feel that's part of my role as care giver. [bedside nurse]
Many people in the healthcare team are involved in [deep vein thrombosis] DVT prevention at the nurse level, physiotherapy, occupational therapy (in terms of mobility of the patient) the physician, specialty services like thrombo service, hematology department. [nurse manager]
Since many clinicians implicitly or explicitly have a role in thromboprophylaxis, some participants viewed it as everyone's responsibility, or that it should be everyone's responsibility, to ensure optimal VTE prevention.
I think the responsibility should be everyone's responsibilityall across the health care professions. [ward pharmacist]
Make it so that you have a few stopgaps so that a few people are looking for the risk and make the process different so that you have a couple of stops along the way that it'll get brought up. I would create it so that it's not just one group in charge of the DVT prophylaxis decision and assessment. It has to be across the board. [nurse educator]
However, while multidisciplinary care was considered ideal to achieve optimal patient outcomes, it was also paradoxically perceived as insufficient to ensure effective thromboprophylaxis. Multidisciplinary care can lead to confusion about roles on a team, and unclear accountability, thereby becoming a potential barrier to effective prevention. Some participants thought that just one person should be ultimately responsible for thromboprophylaxis.
I think it would be crucial to identify one person responsible rather than indirectly a number of people who would be encouraged . It is probably better if you make one of those targets dependable. [physician]
[If] you assign it to one person versus having the accountability spread among [all] those peoplethen nobody takes accountability. [quality improvement team member]
Many participants reported that mobilization was important, though difficult to achieve. They also expressed uncertainty about whether, and how much, mobilization is enough.
I think the best way to prevent DVT would be to fully focus on early mobilization, regular physiotherapy, and how a patient returns to regular activities to get them up and around. [resident]
Level of mobility is a subjective matter. When it comes to the question of Well, how mobile is mobile enough?, that's not standardized to my knowledge and is very subjective as far as when to stop it. Is getting up in a chair and wiggling your toes good enough? Or are they running up and down the hall and you have to chase them? Really, what level of mobility is considered the standard for discontinuing DVT prophylaxis? That's the question that comes up repeatedly so I think that's a very large barrier. [pharmacy manager]
Several logistic barriers associated with antiembolic stockings and pneumatic compression devices were cited, including problems with fit, inconvenience, noncompliance, and cost.
I find stockings aren't always measured or worn appropriately. It's difficult; I think every nurse measures them differently. If they're too tight around the thighs they just roll them down. If anything, they really constrict any type of circulation rather than promote it. Moon boots are all right, but a little cumbersome, more expensive and are more geared to specific patients. [bedside nurse]
The patients want them off at certain times of the day. Theoretically, you only take them off to bathe them but some of them are really bothered by them. [nurse clinician]
Another key barrier was that clinicians are more focused on treating the immediate health care problem precipitating hospital admission than on preventing future complications.
It's something that we tend to forget because patients come in with giant medical problems a, b, and c, and then your DVT prophylaxis tends to fall by the wayside, as you're trying to deal with their major medical issues. [resident]
Prevention issues are a little bit different than treatment issues . We see ourselves as interventionists more than preventionists . It's the medical things we tend to deal with immediately and that's often the focus why the patient is in hospital, rightly or wrongly. Quality doesn't just include intervention, it includes prevention. [nurse manager]
Potential Solutions
To address this problem of inattention to preventive strategies, participants indicated that local data on the burden of illness and current utilization of thromboprophylaxis would be helpful. Some participants described institution‐specific information and thromboprophylaxis targets that motivate clinicians.
Give feedback to the team and physicians about what the incidence of DVT is. I don't think everybody knows. I don't even know in our hospital what it is. So give feedback to the team about what the incidence of DVT is, how many people are on DVT prophylaxis and how many people die from pulmonary embolism. If you have those numbers in front of you, then you would have something to aim for. [physician manager]
You do run charts so that you are auditing continuously and reporting the results back to the staff. And we have weekly meetings with staff members. That has been effective. And we have tip of the week via email. [quality improvement team member]
Most participants recommended redoubling efforts towards anticoagulant thromboprophylaxisa coordinated, systemwide approach across the continuum of care.
It's got to be a trigger for every patient that comes in. Does this patient need DVT prophylaxis? Is this patient a candidate for it? [physician manager]
I think if it's not tackled at the beginning it gets lost in the shuffle. I think if it's something we put into place just like we do when we're getting a history and on anything else. If we start with it [heparin] from day one we'll continue it through right to the end. [bedside nurse]
Participants suggested a variety of methods to enhance thromboprophylaxis. These included a universal, structured, dynamic risk stratification tool, and standardized order forms at the time of hospital admission, which would be reevaluated regularly, and require an enabling educational program.
Every medical patient 18 or older will be given a risk score. Risk stratifying all of our patients with just a simple little tool and considering treatment for those that are of high risk is what we need to do. Reinforcing the education and teaching required for the patients that are at low risk, because we don't need to put everyone on heparin. But anyone could potentially get a clot. [nurse educator]
It could be a standardized order sheet for every admitted patient and maybe that could help. And that would be like the first page in every chart in the whole hospital, and they can always cross it off and say I don't want to use this. [ward pharmacist]
The hospital intranet was sanctioned as a useful repository for physician orders and other tools. Computerized health records and computer decision supports were strongly endorsed.
It should be in a computerized system if it goes into a manual system, paperwork tends to get lost. I think a computerized system would be ideal. [pharmacist]
I think that having a computer health recordthat we would love, for many reasonsonce that happened, it would be a way to ensure it, because there would be prompts . You would get a prompt saying, Why are you not using it? . An electronic health record and as a back up, having a pharmacist. [physician]
Leveraging patient or family‐mediated interventions to provide reminders was also suggested, given the familiarity of the public with thrombosis.
Leave every patient a small pamphlet saying Are you getting DVT prevention? Are you getting injections? Empower people as well. [physician]
I think the absolute biggest driver from our perspective in admin is always public awareness. The demand for standard service increases the most when the public is aware of it. They ask. Patients are becoming more educated, they use the Internet, they search those things out themselves and they are knowledgeable. [hospital administrator]
Sufficient human resources to ensure mobilization and profiling thromboprophylaxis during accreditation were regarded as administrative initiatives that could help. Capitalizing on social forces in healthcare such as patient safety could also galvanize efforts to prevent VTE.
We need physio and [occupational therapy] OT. We need more rehab. We need resources. That's what we're lacking. The only physio and OT that comes to our floor is pending discharge. So it's definitely resource‐related. [bedside nurse]
From an administrative perspective, the whole concept of preventing complications reduces risk, improves patient safety, reduces length of stayall those warm fuzzy things that are attached to providing the best possible care for the patient at the right time. [nurse manager]
Discussion
In this qualitative study, participants affirmed that depending on individual physicians for VTE prevention is insufficient. Distinct from most therapeutic interventions which are understood as the responsibility of physicians, preventive interventions such as thromboprophylaxis may be more readily embraced as the charge of members of a multidisciplinary team. While every clinician group felt compelled to help with VTE prevention, reliance on multidisciplinary care was also perceived as a barrier to effective VTE prevention because it can generate confusion about roles. Participants recommended a comprehensive, systems‐approach, including screening and risk‐stratifying all patients, preprinted orders at hospital admission that are regularly reevaluated, and audit and feedback programs. Also endorsed were patient or family‐mediated reminders, and administrative interventions such as hiring more physiotherapists for mobilization and profiling thromboprophylaxis for hospital accreditation.
Approximately 70% of participants judged that clinicians were insufficiently concerned about thromboprophylaxis. In contrast to many commonly cited reasons for underutilization of evidence‐based interventions such as lack of awareness of (or resistance to) new information, and lack of self‐efficacy of clinicians (wondering whether the benefits observed in the research setting will be realized in the practice setting),18 participants in our study did not raise these as barriers to thromboprophylaxis. As physician and pharmacist participants indicated, because the evidentiary basis for heparin thromboprophylaxis is strong and understood (in contrast to that for mechanical prophylaxis), lack of knowledge was not considered a barrier.
Our findings are consistent with a previous qualitative study in which clinicians and managers were interviewed to learn about factors that increase beta‐blocker use following myocardial infarction; these investigators found that administrative support, use of data, and quality improvement initiatives were key.19 Detailed implementation directives to clarify clinical responsibilities for many different stakeholders were also suggested in a qualitative study on the optimal use of noninvasive ventilation.20 Extending these results, participants in our study suggested several interventions aimed at different levels of the healthcare system (eg, patient, provider, and administrator) that are either enabling or reinforcing. While just 1 implementation strategy may result in improved thromboprophylaxis,21 efficient application of multiply‐redundant strategies delivered by a multidisciplinary team may be more powerful. Such an approach has driven high level performance for the American Heart Association's Get With The Guidelines Program, resulting in lower community and hospital cardiovascular and cerebrovascular mortality in Massachusetts.22
Although our aim was to obtain multidisciplinary input, we did not focus on physicians who primarily prescribe thromboprophylaxis, thereby potentially underrepresenting their views relative to their role. We did not interview all clinicians who can influence VTE risk (eg, physiotherapists) or prescribe thromboprophylaxis (eg, family physicians). During this study, patient safety emerged as a major hospital initiative,23 which could modify participants' views on the importance of VTE prevention. Although VTE prevention may be enhanced by understanding barriers to, and solutions for, optimal thromboprophylaxis from both clinicians' and managers' perspectives, the suggestions we elicited on methods to improve VTE prevention represent participants' opinions, rather than evidence, about effective strategies. Our findings are not generalizable to settings with nurse practitioners who are dedicated to VTE prevention, nor to settings with computer decision support systems that already incorporate thromboprophylaxis.
Strengths of this study include interviewing key stakeholders representing several clinician groups and managers, to obtain perspectives on both individual and systemwide influences on thromboprophylaxis in medical patients. By eliciting the views and experiences of participants in both the community and university settings, we captured multicenter perspectives on preventive health. We used triangulation of data sources (researcher and participant) and invited participants to review an early draft of the report (member checking).24 This study highlights the merits of qualitative research which can provide insights into familiar patterns and problems, and contribute to knowledge for interdisciplinary audiences. These qualitative research results help to explain quantitative research results documenting very low rates of medical thromboprophylaxis,214 raising hypotheses to test in future studies testing systemwide interventions that may improve patient safety. One prominent publication reported the impact of electronic reminders,25 while a recent systematic review of methods to increase thromboprophylaxis outlines many potentially effective approaches.21
In summary, the findings of this qualitative study challenge the notion that either individual physician efforts or multidisciplinary care are enough to lead to optimal VTE prevention. Participants believed that while well‐functioning teams at the bedside hold great promise to deliver superior care, they may also lead to unclear role definition. Since physicians commit many errors of omission regarding thromboprophylaxis, these results raise the possibility of delegated medical acts outside the scope of conventional practice for nurses and pharmacists. Leveraging the skills and knowledge of multidisciplinary teams for medical thromboprophylaxis requires not only clear accountabilities, but also excellent interprofessional communication, and institution‐wide approaches to change prescribing behavior, potentially involving patients and administrators as well as clinicians.
Acknowledgements
The authors thank the participants of this study for sharing their experiences and views with them. The authors are grateful for the help of Laurel Raftery, Michelle Murray, Monica Owen, and Shelley Anderson for transcriptions. The authors appreciate the organizational assistance of Nancy Lloyd and the biostatistical support of Diane Heels‐Ansdell for analyzing respondent characteristics. The authors are grateful to Nancy Lloyd, Alice Huh, Barbara Young, and Gray Ellrodt for feedback on earlier versions of this report. Deborah Cook had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix
Qualitec Interview Guide
Opening Statement
Thank you for your participation in this research study we are conducting with colleagues at St. Joseph's Hospital and McMaster University. Through this interview, I hope to learn how you think about DVT prevention in medical patients. When you answer the interview questions, please keep in mind that we are interested in your own thoughts and approaches.
First, I have a consent form I'd like you to read to make sure you understand the reason for this study, and to see if you have any questions about becoming involved. Please take a few minutes to read it over.
[Participant reads consent, questions about the study may or may not ensue, participant signs to indicate informed consent, the interview proceeds.]
Structured Interview Begins
Demographics
Before we begin the interview, I would like you to take a few minutes to complete this form, which consists of a few demographic questions.
[Participant completes demographic form].
Thank you. Now I'll move on to some questions about DVT prevention.
[The interviewer informs the participant that she is putting the tape recorder on].
Core Study Interview Guide
Can you tell me a bit about your own approach to DVT prevention in medical patients? For what types of patients are you concerned about DVT?
What do you find are the best ways to prevent DVT? Why?
Who else is involved in trying to prevent DVT? In general, how are DVTs are prevented at this hospital?
What are some of the barriers to preventing DVTs? (e.g., risks of prevention, disbelief that prevention really works, DVT prevention is someone else's responsibilitywhose?)
Are there other ways to ensure that DVTs are prevented in medical patients? If so, how would they work?
Can you tell me how different clinicians (such as physicians, nurses, pharmacists, and residents) could do a better job at preventing DVT? How could medical wards and departments help? How could hospital administration help?
Do you remember a patient who had a problem with DVT prevention? If so, can you tell me about this problem and how it might have been avoided?
If you could create a perfect system of DVT prevention what would it be?
Do you think people are overconcerned, appropriately concerned, or underconcerned about DVT prevention?
Is there anything else you would like to tell me?
Final Debriefing Question
How has this interview experience been for you? Thank you very much for sharing your views. We really appreciate it.
- Agency for Health Care Policy Research. Prevention of venous thromboembolism after injury. Summary, evidence report/technology assessment. Available at: http://www. ahrq.gov/clinic/epcsums/vtsumm.htm. Accessed April 2008.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,.Venous thromboembolism prophylaxis in medical inpatients: a retrospective chart review.Thromb Res.2003;111:215–219.
- ,,, et al.,for the CURVE Study Investigators.Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada.Thromb Res.2007;119:145–155.
- ,.Thromboprophylaxis in an academic medical center.Cardiovasc Surg.2001;9:426–430.
- ,,.Deep venous thrombosis prophylaxis: are guidelines being followed?ANZ J Surg.2002;72:331–334.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17:788–791.
- ,.Preventing venous thromboembolism in acute medical patients.QJM.2004;97:797–801.
- ,.Venous thromboembolic prophylaxis in hospitalized medical patients.Ann Pharmacother.2004;38:36–40.
- ,,.Physicians' practice for prevention of venous thromboembolism in medical patients.J Coll Physicians Surg.2004;14:211.
- ,,, et al.Venous thromboembolism prophylaxis in acutely ill medical patients: definite need for improvement.J Intern Med.2005;257:352–357.
- ,,, et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns.Haematologica.2002;87:746–750.
- ,,, et al.,for the ENDORSE Investigators.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE Study): a multinational cross‐sectional study.Lancet.2008;371:387–394.
- ,,, et al.,for the IMPROVE Investigators.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism.Chest.2007;132:936–945.
- ,,for the Evidence‐Based Medicine Working Group.Users' guides to the medical literature. XXIII. Qualitative research in health care. A. Are the results of the study valid?JAMA.2000;284:357–362.
- ,.Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory.2nd ed.London:Sage Publications;1998.
- Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, Social Sciences and Humanities Research Council of Canada.Tri‐Council Policy Statement: Ethical Conduct for Research Involving Humans.Ottawa, Ontario:Public Works and Government Services Canada;1998 [with 2000, 2002, and 2005 amendments].
- ,,, et al.Why don't physicians follow clinical practice guidelines?JAMA.1999;282:1458–1465.
- ,,,,,.A qualitative study of increasing β‐blocker use after myocardial infarction: why do some hospitals succeed?JAMA.2001;285:2604–2611.
- ,,,.Practice guidelines as multipurpose tools: a qualitative study of noninvasive ventilation.Crit Care Med.2007;35:776–782.
- ,,, et al.A systemic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
- ,,, et al.Multidisciplinary rounds: an implementation system for sustained improvement in the American Heart Association's “Get With the Guidelines” Program.Crit Pathw Cardiol.2007;6:1016–1116.
- ,,,,.Changing clinician behaviour to improve patient safety.Lancet.2004;363:1224–1230.
- ,.Papers that go beyond numbers: qualitative research.BMJ.1997;315:740–748.
- ,,,,,,.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
- Agency for Health Care Policy Research. Prevention of venous thromboembolism after injury. Summary, evidence report/technology assessment. Available at: http://www. ahrq.gov/clinic/epcsums/vtsumm.htm. Accessed April 2008.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,.Venous thromboembolism prophylaxis in medical inpatients: a retrospective chart review.Thromb Res.2003;111:215–219.
- ,,, et al.,for the CURVE Study Investigators.Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada.Thromb Res.2007;119:145–155.
- ,.Thromboprophylaxis in an academic medical center.Cardiovasc Surg.2001;9:426–430.
- ,,.Deep venous thrombosis prophylaxis: are guidelines being followed?ANZ J Surg.2002;72:331–334.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17:788–791.
- ,.Preventing venous thromboembolism in acute medical patients.QJM.2004;97:797–801.
- ,.Venous thromboembolic prophylaxis in hospitalized medical patients.Ann Pharmacother.2004;38:36–40.
- ,,.Physicians' practice for prevention of venous thromboembolism in medical patients.J Coll Physicians Surg.2004;14:211.
- ,,, et al.Venous thromboembolism prophylaxis in acutely ill medical patients: definite need for improvement.J Intern Med.2005;257:352–357.
- ,,, et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns.Haematologica.2002;87:746–750.
- ,,, et al.,for the ENDORSE Investigators.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE Study): a multinational cross‐sectional study.Lancet.2008;371:387–394.
- ,,, et al.,for the IMPROVE Investigators.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism.Chest.2007;132:936–945.
- ,,for the Evidence‐Based Medicine Working Group.Users' guides to the medical literature. XXIII. Qualitative research in health care. A. Are the results of the study valid?JAMA.2000;284:357–362.
- ,.Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory.2nd ed.London:Sage Publications;1998.
- Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, Social Sciences and Humanities Research Council of Canada.Tri‐Council Policy Statement: Ethical Conduct for Research Involving Humans.Ottawa, Ontario:Public Works and Government Services Canada;1998 [with 2000, 2002, and 2005 amendments].
- ,,, et al.Why don't physicians follow clinical practice guidelines?JAMA.1999;282:1458–1465.
- ,,,,,.A qualitative study of increasing β‐blocker use after myocardial infarction: why do some hospitals succeed?JAMA.2001;285:2604–2611.
- ,,,.Practice guidelines as multipurpose tools: a qualitative study of noninvasive ventilation.Crit Care Med.2007;35:776–782.
- ,,, et al.A systemic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
- ,,, et al.Multidisciplinary rounds: an implementation system for sustained improvement in the American Heart Association's “Get With the Guidelines” Program.Crit Pathw Cardiol.2007;6:1016–1116.
- ,,,,.Changing clinician behaviour to improve patient safety.Lancet.2004;363:1224–1230.
- ,.Papers that go beyond numbers: qualitative research.BMJ.1997;315:740–748.
- ,,,,,,.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
Copyright © 2009 Society of Hospital Medicine
TB with Pott's Disease and Psoas Abscess
A 22‐year‐old man was referred from an outside hospital with 2 weeks of generalized weakness and difficulty ambulating. He also reported a 6‐month history of abdominal pain, right‐sided lumbar back pain, malaise, night sweats, cough, and weight loss of 30 kg. The patient was born in Mexico but had lived in the US for 10 years, working as a construction worker in northern California. He had had no prior medical care. On examination, the patient had a temperature of 40C, a heart rate of 109, and a respiratory rate of 20. His oxygen saturation was 97% on room air, and lung fields were clear bilaterally. Strength was slightly decreased in the right leg, with tenderness in the posterolateral aspect of the right thigh and thoracic lumbar spine.
Radiograph of the chest showed bilateral upper lobe reticulonodular infiltrates, cavitation, and pleural thickening (Figure 1). Computed tomography (CT) showed innumerable pulmonary nodules throughout the mid and lower lungs, a loss of disc space between L4 and L5, and a large multiloculated abscess of the right iliacus and psoas muscles that extended into the right thigh (Figure 2). Longitudinal relaxation time (T1)‐weighted magnetic resonance imaging (MRI) of the lumbar spine revealed vertebral destruction of L4 and L5 (Figure 3).
The patient underwent vertebrectomy of L4 and L5, anterior/posterior fixation and fusion from L4 to S1, and drainage of a large right‐sided psoas abscess. Acid‐fast bacilli were seen in abscess fluid and vertebral bone. Subsequently, the intraoperative cultures and 6 sequential sputum cultures all grew Mycobacterium tuberculosis. The patient was begun on rifampin, isoniazid, pyrazinamide, and ethambutol with a good clinical response, and subsequently transferred back to his referring hospital for continued medical care and rehabilitation.
Extrapulmonary manifestations of tuberculosis should be suspected in patients from a tuberculosis‐endemic country of origin. Bone and joint tuberculosis account for up to 35% of cases of extrapulmonary tuberculosis. Spinal tuberculosis (Pott's disease) most commonly involves the anteroinferior aspect of vertebral bodies in the thoracic spine. Tuberculosis is a disease with diverse manifestations and can elude even the most astute physician if it is not considered as a diagnosis.
A 22‐year‐old man was referred from an outside hospital with 2 weeks of generalized weakness and difficulty ambulating. He also reported a 6‐month history of abdominal pain, right‐sided lumbar back pain, malaise, night sweats, cough, and weight loss of 30 kg. The patient was born in Mexico but had lived in the US for 10 years, working as a construction worker in northern California. He had had no prior medical care. On examination, the patient had a temperature of 40C, a heart rate of 109, and a respiratory rate of 20. His oxygen saturation was 97% on room air, and lung fields were clear bilaterally. Strength was slightly decreased in the right leg, with tenderness in the posterolateral aspect of the right thigh and thoracic lumbar spine.
Radiograph of the chest showed bilateral upper lobe reticulonodular infiltrates, cavitation, and pleural thickening (Figure 1). Computed tomography (CT) showed innumerable pulmonary nodules throughout the mid and lower lungs, a loss of disc space between L4 and L5, and a large multiloculated abscess of the right iliacus and psoas muscles that extended into the right thigh (Figure 2). Longitudinal relaxation time (T1)‐weighted magnetic resonance imaging (MRI) of the lumbar spine revealed vertebral destruction of L4 and L5 (Figure 3).



The patient underwent vertebrectomy of L4 and L5, anterior/posterior fixation and fusion from L4 to S1, and drainage of a large right‐sided psoas abscess. Acid‐fast bacilli were seen in abscess fluid and vertebral bone. Subsequently, the intraoperative cultures and 6 sequential sputum cultures all grew Mycobacterium tuberculosis. The patient was begun on rifampin, isoniazid, pyrazinamide, and ethambutol with a good clinical response, and subsequently transferred back to his referring hospital for continued medical care and rehabilitation.
Extrapulmonary manifestations of tuberculosis should be suspected in patients from a tuberculosis‐endemic country of origin. Bone and joint tuberculosis account for up to 35% of cases of extrapulmonary tuberculosis. Spinal tuberculosis (Pott's disease) most commonly involves the anteroinferior aspect of vertebral bodies in the thoracic spine. Tuberculosis is a disease with diverse manifestations and can elude even the most astute physician if it is not considered as a diagnosis.
A 22‐year‐old man was referred from an outside hospital with 2 weeks of generalized weakness and difficulty ambulating. He also reported a 6‐month history of abdominal pain, right‐sided lumbar back pain, malaise, night sweats, cough, and weight loss of 30 kg. The patient was born in Mexico but had lived in the US for 10 years, working as a construction worker in northern California. He had had no prior medical care. On examination, the patient had a temperature of 40C, a heart rate of 109, and a respiratory rate of 20. His oxygen saturation was 97% on room air, and lung fields were clear bilaterally. Strength was slightly decreased in the right leg, with tenderness in the posterolateral aspect of the right thigh and thoracic lumbar spine.
Radiograph of the chest showed bilateral upper lobe reticulonodular infiltrates, cavitation, and pleural thickening (Figure 1). Computed tomography (CT) showed innumerable pulmonary nodules throughout the mid and lower lungs, a loss of disc space between L4 and L5, and a large multiloculated abscess of the right iliacus and psoas muscles that extended into the right thigh (Figure 2). Longitudinal relaxation time (T1)‐weighted magnetic resonance imaging (MRI) of the lumbar spine revealed vertebral destruction of L4 and L5 (Figure 3).



The patient underwent vertebrectomy of L4 and L5, anterior/posterior fixation and fusion from L4 to S1, and drainage of a large right‐sided psoas abscess. Acid‐fast bacilli were seen in abscess fluid and vertebral bone. Subsequently, the intraoperative cultures and 6 sequential sputum cultures all grew Mycobacterium tuberculosis. The patient was begun on rifampin, isoniazid, pyrazinamide, and ethambutol with a good clinical response, and subsequently transferred back to his referring hospital for continued medical care and rehabilitation.
Extrapulmonary manifestations of tuberculosis should be suspected in patients from a tuberculosis‐endemic country of origin. Bone and joint tuberculosis account for up to 35% of cases of extrapulmonary tuberculosis. Spinal tuberculosis (Pott's disease) most commonly involves the anteroinferior aspect of vertebral bodies in the thoracic spine. Tuberculosis is a disease with diverse manifestations and can elude even the most astute physician if it is not considered as a diagnosis.