User login
Tooth loss and diabetes together hasten mental decline
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF DENTAL RESEARCH
Watch for buprenorphine ‘spiking’ in urine drug tests
Urine drug testing can be valuable for monitoring patients undergoing treatment with buprenorphine for opioid use disorder (OUD). However, some patients alter their test results by adding buprenorphine directly to their urine sample to imply adherence, a new study shows.
“I anticipate a much-needed increase” in the number of people gaining access to buprenorphine therapy, given elimination of the X waiver, first author Jarratt D. Pytell, MD, with University of Colorado at Denver, Aurora, said in a statement.
“New prescribers of buprenorphine will need to learn how to conduct the increasingly complex initiation of treatment and then gauge whether it is successful or not,” added Dr. Pytell, a general internist and addiction medicine specialist.
“Spiking suggests that treatment is not working – especially in patients continuing illicit drug use. Detecting spiking allows clinicians to adjust or intensify the treatment plan,” Dr. Pytell said in an interview.
The study was published online in JAMA Psychiatry.
A sign of elevated patient risk
In a cross-sectional study using Millennium Health’s proprietary urine drug test (UDT) database, researchers analyzed 507,735 urine specimens from 58,476 OUD patients collected between January 2017 and April 2022.
A total of 9546 (1.9%) specimens from 4,550 patients (7.6%) were suggestive of spiking.
UDT specimens suggestive of spiking had two times the odds of being positive for other opioids (fentanyl or heroin), compared with opioid negative samples.
UDT specimens obtained from primary care clinics, from patients aged 35-44 years, and from patients living in the South Atlantic region of the United States were also more likely to be suggestive of buprenorphine spiking.
“Our study demonstrated that a buprenorphine to norbuprenorphine ratio of less than 0.02 indicates the possibility of spiking,” Dr. Pytell said in an interview.
“Nevertheless, it is important to note that this cutoff is not a definitive standard and further controlled studies are necessary to determine its predictive value for spiking. But recognizing possible spiking is very important since it demonstrates a point of elevated risk for the patient and the treatment approach should be reconsidered,” Dr. Pytell said.
“At Millennium Health, we have been tracking the enormity of the drug use crisis. This study suggests that spiking is an important patient safety issue, and it is not uncommon,” study coauthor Eric Dawson, PharmD, vice president of clinical affairs, Millennium Health, said in a statement.
“Detection of spiking requires definitive drug testing. Immunoassay-based, point-of-care tests cannot detect spiking because they are generally incapable of quantitative analysis and differentiating buprenorphine from norbuprenorphine,” Dr. Dawson said.
Best practices?
“We need to develop best practices specific for this situation where a patient has added buprenorphine to the urine drug test specimen,” said Dr. Pytell.
“As with all unexpected findings, it is crucial for clinicians to approach this finding in a nonjudgmental manner and work with the patient to understand what might have motivated them to alter their urine specimen,” he added.
Dr. Pytell said a common reaction for clinicians might be to discontinue treatment. However, this is actually a time to try and engage with the patient.
“Clinicians should work collaboratively with patients to identify potential reasons for spiking and determine what changes may need to be made to better support the patient’s recovery,” Dr. Pytell said.
“This could include more frequent monitoring or referral to a higher level of care. In addition, clinicians should be aware that patients who engage in spiking may be experiencing other challenges that impact their ability to adhere to treatment, such as inadequate housing, mental health issues, or financial strain. Addressing these underlying issues may help patients overcome barriers to treatment adherence and reduce the likelihood of future spiking,” Dr. Pytell said.
This study was supported by Millennium Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Urine drug testing can be valuable for monitoring patients undergoing treatment with buprenorphine for opioid use disorder (OUD). However, some patients alter their test results by adding buprenorphine directly to their urine sample to imply adherence, a new study shows.
“I anticipate a much-needed increase” in the number of people gaining access to buprenorphine therapy, given elimination of the X waiver, first author Jarratt D. Pytell, MD, with University of Colorado at Denver, Aurora, said in a statement.
“New prescribers of buprenorphine will need to learn how to conduct the increasingly complex initiation of treatment and then gauge whether it is successful or not,” added Dr. Pytell, a general internist and addiction medicine specialist.
“Spiking suggests that treatment is not working – especially in patients continuing illicit drug use. Detecting spiking allows clinicians to adjust or intensify the treatment plan,” Dr. Pytell said in an interview.
The study was published online in JAMA Psychiatry.
A sign of elevated patient risk
In a cross-sectional study using Millennium Health’s proprietary urine drug test (UDT) database, researchers analyzed 507,735 urine specimens from 58,476 OUD patients collected between January 2017 and April 2022.
A total of 9546 (1.9%) specimens from 4,550 patients (7.6%) were suggestive of spiking.
UDT specimens suggestive of spiking had two times the odds of being positive for other opioids (fentanyl or heroin), compared with opioid negative samples.
UDT specimens obtained from primary care clinics, from patients aged 35-44 years, and from patients living in the South Atlantic region of the United States were also more likely to be suggestive of buprenorphine spiking.
“Our study demonstrated that a buprenorphine to norbuprenorphine ratio of less than 0.02 indicates the possibility of spiking,” Dr. Pytell said in an interview.
“Nevertheless, it is important to note that this cutoff is not a definitive standard and further controlled studies are necessary to determine its predictive value for spiking. But recognizing possible spiking is very important since it demonstrates a point of elevated risk for the patient and the treatment approach should be reconsidered,” Dr. Pytell said.
“At Millennium Health, we have been tracking the enormity of the drug use crisis. This study suggests that spiking is an important patient safety issue, and it is not uncommon,” study coauthor Eric Dawson, PharmD, vice president of clinical affairs, Millennium Health, said in a statement.
“Detection of spiking requires definitive drug testing. Immunoassay-based, point-of-care tests cannot detect spiking because they are generally incapable of quantitative analysis and differentiating buprenorphine from norbuprenorphine,” Dr. Dawson said.
Best practices?
“We need to develop best practices specific for this situation where a patient has added buprenorphine to the urine drug test specimen,” said Dr. Pytell.
“As with all unexpected findings, it is crucial for clinicians to approach this finding in a nonjudgmental manner and work with the patient to understand what might have motivated them to alter their urine specimen,” he added.
Dr. Pytell said a common reaction for clinicians might be to discontinue treatment. However, this is actually a time to try and engage with the patient.
“Clinicians should work collaboratively with patients to identify potential reasons for spiking and determine what changes may need to be made to better support the patient’s recovery,” Dr. Pytell said.
“This could include more frequent monitoring or referral to a higher level of care. In addition, clinicians should be aware that patients who engage in spiking may be experiencing other challenges that impact their ability to adhere to treatment, such as inadequate housing, mental health issues, or financial strain. Addressing these underlying issues may help patients overcome barriers to treatment adherence and reduce the likelihood of future spiking,” Dr. Pytell said.
This study was supported by Millennium Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Urine drug testing can be valuable for monitoring patients undergoing treatment with buprenorphine for opioid use disorder (OUD). However, some patients alter their test results by adding buprenorphine directly to their urine sample to imply adherence, a new study shows.
“I anticipate a much-needed increase” in the number of people gaining access to buprenorphine therapy, given elimination of the X waiver, first author Jarratt D. Pytell, MD, with University of Colorado at Denver, Aurora, said in a statement.
“New prescribers of buprenorphine will need to learn how to conduct the increasingly complex initiation of treatment and then gauge whether it is successful or not,” added Dr. Pytell, a general internist and addiction medicine specialist.
“Spiking suggests that treatment is not working – especially in patients continuing illicit drug use. Detecting spiking allows clinicians to adjust or intensify the treatment plan,” Dr. Pytell said in an interview.
The study was published online in JAMA Psychiatry.
A sign of elevated patient risk
In a cross-sectional study using Millennium Health’s proprietary urine drug test (UDT) database, researchers analyzed 507,735 urine specimens from 58,476 OUD patients collected between January 2017 and April 2022.
A total of 9546 (1.9%) specimens from 4,550 patients (7.6%) were suggestive of spiking.
UDT specimens suggestive of spiking had two times the odds of being positive for other opioids (fentanyl or heroin), compared with opioid negative samples.
UDT specimens obtained from primary care clinics, from patients aged 35-44 years, and from patients living in the South Atlantic region of the United States were also more likely to be suggestive of buprenorphine spiking.
“Our study demonstrated that a buprenorphine to norbuprenorphine ratio of less than 0.02 indicates the possibility of spiking,” Dr. Pytell said in an interview.
“Nevertheless, it is important to note that this cutoff is not a definitive standard and further controlled studies are necessary to determine its predictive value for spiking. But recognizing possible spiking is very important since it demonstrates a point of elevated risk for the patient and the treatment approach should be reconsidered,” Dr. Pytell said.
“At Millennium Health, we have been tracking the enormity of the drug use crisis. This study suggests that spiking is an important patient safety issue, and it is not uncommon,” study coauthor Eric Dawson, PharmD, vice president of clinical affairs, Millennium Health, said in a statement.
“Detection of spiking requires definitive drug testing. Immunoassay-based, point-of-care tests cannot detect spiking because they are generally incapable of quantitative analysis and differentiating buprenorphine from norbuprenorphine,” Dr. Dawson said.
Best practices?
“We need to develop best practices specific for this situation where a patient has added buprenorphine to the urine drug test specimen,” said Dr. Pytell.
“As with all unexpected findings, it is crucial for clinicians to approach this finding in a nonjudgmental manner and work with the patient to understand what might have motivated them to alter their urine specimen,” he added.
Dr. Pytell said a common reaction for clinicians might be to discontinue treatment. However, this is actually a time to try and engage with the patient.
“Clinicians should work collaboratively with patients to identify potential reasons for spiking and determine what changes may need to be made to better support the patient’s recovery,” Dr. Pytell said.
“This could include more frequent monitoring or referral to a higher level of care. In addition, clinicians should be aware that patients who engage in spiking may be experiencing other challenges that impact their ability to adhere to treatment, such as inadequate housing, mental health issues, or financial strain. Addressing these underlying issues may help patients overcome barriers to treatment adherence and reduce the likelihood of future spiking,” Dr. Pytell said.
This study was supported by Millennium Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Mortality risk in epilepsy: New data
new research shows.
“To our knowledge, this is the only study that has assessed the cause-specific mortality risk among people with epilepsy according to age and disease course,” investigators led by Seo-Young Lee, MD, PhD, of Kangwon National University, Chuncheon, South Korea, write. “Understanding cause-specific mortality risk, particularly the risk of external causes, is important because they are mostly preventable.”
The findings were published online in Neurology.
Higher mortality risk
For the study, researchers analyzed data from the National Health Insurance Service database in Korea from 2006 to 2017 and vital statistics from Statistics Korea from 2008 to 2017.
The study population included 138,998 patients with newly treated epilepsy, with an average at diagnosis of 48.6 years.
Over 665,928 person years of follow-up (mean follow-up, 4.79 years), 20.095 patients died.
People with epilepsy had more than twice the risk for death, compared with the overall population (standardized mortality ratio, 2.25; 95% confidence interval, 2.22-2.28). Mortality was highest in children aged 4 years or younger and was higher in the first year after diagnosis and in women at all age points.
People with epilepsy had a higher mortality risk, compared with the general public, regardless of how many anti-seizure medications they were taking. Those taking only one medication had a 156% higher risk for death (SMR, 1.56; 95% CI, 1.53-1.60), compared with 493% higher risk in those taking four or more medications (SMR, 4.93; 95% CI, 4.76-5.10).
Where patients lived also played a role in mortality risk. Living in a rural area was associated with a 247% higher risk for death, compared with people without epilepsy who lived in the same area (SMR, 2.47; 95% CI, 2.41-2.53), and the risk was 203% higher risk among those living in urban centers (SMR, 2.03; 95% CI, 1.98-2.09).
Although people with comorbidities had higher mortality rates, even those without any other health conditions had a 161% higher risk for death, compared with people without epilepsy (SMR, 1.61; 95% CI, 1.50-1.72).
Causes of death
The most frequent causes of death were malignant neoplasm and cerebrovascular disease, which researchers noted are thought to be underlying causes of epilepsy.
Among external causes of death, suicide was the most common cause (2.6%). The suicide rate was highest among younger patients and gradually decreased with age.
Deaths tied directly to epilepsy, transport accidents, or falls were lower in this study than had been previously reported, which may be due to adequate seizure control or because the number of older people with epilepsy and comorbidities is higher in Korea than that reported in other countries.
“To reduce mortality in people with epilepsy, comprehensive efforts [are needed], including a national policy against stigma of epilepsy and clinicians’ total management such as risk stratification, education about injury prevention, and monitoring for suicidal ideation with psychological intervention, as well as active control of seizures,” the authors write.
Generalizable findings
Joseph Sirven, MD, professor of neurology at Mayo Clinic Florida, Jacksonville, said that although the study included only Korean patients, the findings are applicable to other counties.
That researchers found patients with epilepsy were more than twice as likely to die prematurely, compared with the general population wasn’t particularly surprising, Dr. Sirven said.
“What struck me the most was the fact that even patients who were on a single drug and seemingly well controlled also had excess mortality reported,” Dr. Sirven said. “That these risks occur should be part of what we tell all patients with epilepsy so that they can better arm themselves with information and help to address some of the risks that this study showed.”
Another important finding is the risk for suicide in patients with epilepsy, especially those who are newly diagnosed, he said.
“When we treat a patient with epilepsy, it should not just be about seizures, but we need to inquire about the psychiatric comorbidities and more importantly manage them in a comprehensive manner,” Dr. Sirven said.
The study was funded by Soonchunhyang University Research Fund and the Korea Health Technology R&D Project. The study authors and Dr. Sirven report no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
new research shows.
“To our knowledge, this is the only study that has assessed the cause-specific mortality risk among people with epilepsy according to age and disease course,” investigators led by Seo-Young Lee, MD, PhD, of Kangwon National University, Chuncheon, South Korea, write. “Understanding cause-specific mortality risk, particularly the risk of external causes, is important because they are mostly preventable.”
The findings were published online in Neurology.
Higher mortality risk
For the study, researchers analyzed data from the National Health Insurance Service database in Korea from 2006 to 2017 and vital statistics from Statistics Korea from 2008 to 2017.
The study population included 138,998 patients with newly treated epilepsy, with an average at diagnosis of 48.6 years.
Over 665,928 person years of follow-up (mean follow-up, 4.79 years), 20.095 patients died.
People with epilepsy had more than twice the risk for death, compared with the overall population (standardized mortality ratio, 2.25; 95% confidence interval, 2.22-2.28). Mortality was highest in children aged 4 years or younger and was higher in the first year after diagnosis and in women at all age points.
People with epilepsy had a higher mortality risk, compared with the general public, regardless of how many anti-seizure medications they were taking. Those taking only one medication had a 156% higher risk for death (SMR, 1.56; 95% CI, 1.53-1.60), compared with 493% higher risk in those taking four or more medications (SMR, 4.93; 95% CI, 4.76-5.10).
Where patients lived also played a role in mortality risk. Living in a rural area was associated with a 247% higher risk for death, compared with people without epilepsy who lived in the same area (SMR, 2.47; 95% CI, 2.41-2.53), and the risk was 203% higher risk among those living in urban centers (SMR, 2.03; 95% CI, 1.98-2.09).
Although people with comorbidities had higher mortality rates, even those without any other health conditions had a 161% higher risk for death, compared with people without epilepsy (SMR, 1.61; 95% CI, 1.50-1.72).
Causes of death
The most frequent causes of death were malignant neoplasm and cerebrovascular disease, which researchers noted are thought to be underlying causes of epilepsy.
Among external causes of death, suicide was the most common cause (2.6%). The suicide rate was highest among younger patients and gradually decreased with age.
Deaths tied directly to epilepsy, transport accidents, or falls were lower in this study than had been previously reported, which may be due to adequate seizure control or because the number of older people with epilepsy and comorbidities is higher in Korea than that reported in other countries.
“To reduce mortality in people with epilepsy, comprehensive efforts [are needed], including a national policy against stigma of epilepsy and clinicians’ total management such as risk stratification, education about injury prevention, and monitoring for suicidal ideation with psychological intervention, as well as active control of seizures,” the authors write.
Generalizable findings
Joseph Sirven, MD, professor of neurology at Mayo Clinic Florida, Jacksonville, said that although the study included only Korean patients, the findings are applicable to other counties.
That researchers found patients with epilepsy were more than twice as likely to die prematurely, compared with the general population wasn’t particularly surprising, Dr. Sirven said.
“What struck me the most was the fact that even patients who were on a single drug and seemingly well controlled also had excess mortality reported,” Dr. Sirven said. “That these risks occur should be part of what we tell all patients with epilepsy so that they can better arm themselves with information and help to address some of the risks that this study showed.”
Another important finding is the risk for suicide in patients with epilepsy, especially those who are newly diagnosed, he said.
“When we treat a patient with epilepsy, it should not just be about seizures, but we need to inquire about the psychiatric comorbidities and more importantly manage them in a comprehensive manner,” Dr. Sirven said.
The study was funded by Soonchunhyang University Research Fund and the Korea Health Technology R&D Project. The study authors and Dr. Sirven report no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
new research shows.
“To our knowledge, this is the only study that has assessed the cause-specific mortality risk among people with epilepsy according to age and disease course,” investigators led by Seo-Young Lee, MD, PhD, of Kangwon National University, Chuncheon, South Korea, write. “Understanding cause-specific mortality risk, particularly the risk of external causes, is important because they are mostly preventable.”
The findings were published online in Neurology.
Higher mortality risk
For the study, researchers analyzed data from the National Health Insurance Service database in Korea from 2006 to 2017 and vital statistics from Statistics Korea from 2008 to 2017.
The study population included 138,998 patients with newly treated epilepsy, with an average at diagnosis of 48.6 years.
Over 665,928 person years of follow-up (mean follow-up, 4.79 years), 20.095 patients died.
People with epilepsy had more than twice the risk for death, compared with the overall population (standardized mortality ratio, 2.25; 95% confidence interval, 2.22-2.28). Mortality was highest in children aged 4 years or younger and was higher in the first year after diagnosis and in women at all age points.
People with epilepsy had a higher mortality risk, compared with the general public, regardless of how many anti-seizure medications they were taking. Those taking only one medication had a 156% higher risk for death (SMR, 1.56; 95% CI, 1.53-1.60), compared with 493% higher risk in those taking four or more medications (SMR, 4.93; 95% CI, 4.76-5.10).
Where patients lived also played a role in mortality risk. Living in a rural area was associated with a 247% higher risk for death, compared with people without epilepsy who lived in the same area (SMR, 2.47; 95% CI, 2.41-2.53), and the risk was 203% higher risk among those living in urban centers (SMR, 2.03; 95% CI, 1.98-2.09).
Although people with comorbidities had higher mortality rates, even those without any other health conditions had a 161% higher risk for death, compared with people without epilepsy (SMR, 1.61; 95% CI, 1.50-1.72).
Causes of death
The most frequent causes of death were malignant neoplasm and cerebrovascular disease, which researchers noted are thought to be underlying causes of epilepsy.
Among external causes of death, suicide was the most common cause (2.6%). The suicide rate was highest among younger patients and gradually decreased with age.
Deaths tied directly to epilepsy, transport accidents, or falls were lower in this study than had been previously reported, which may be due to adequate seizure control or because the number of older people with epilepsy and comorbidities is higher in Korea than that reported in other countries.
“To reduce mortality in people with epilepsy, comprehensive efforts [are needed], including a national policy against stigma of epilepsy and clinicians’ total management such as risk stratification, education about injury prevention, and monitoring for suicidal ideation with psychological intervention, as well as active control of seizures,” the authors write.
Generalizable findings
Joseph Sirven, MD, professor of neurology at Mayo Clinic Florida, Jacksonville, said that although the study included only Korean patients, the findings are applicable to other counties.
That researchers found patients with epilepsy were more than twice as likely to die prematurely, compared with the general population wasn’t particularly surprising, Dr. Sirven said.
“What struck me the most was the fact that even patients who were on a single drug and seemingly well controlled also had excess mortality reported,” Dr. Sirven said. “That these risks occur should be part of what we tell all patients with epilepsy so that they can better arm themselves with information and help to address some of the risks that this study showed.”
Another important finding is the risk for suicide in patients with epilepsy, especially those who are newly diagnosed, he said.
“When we treat a patient with epilepsy, it should not just be about seizures, but we need to inquire about the psychiatric comorbidities and more importantly manage them in a comprehensive manner,” Dr. Sirven said.
The study was funded by Soonchunhyang University Research Fund and the Korea Health Technology R&D Project. The study authors and Dr. Sirven report no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Substance abuse disorders may share a common genetic signature
suggest new findings that researchers say could eventually lead to universal therapies to treat multiple and comorbid addictions.
“Genetics play a key role in determining health throughout our lives, but they are not destiny. Our hope with genomic studies is to further illuminate factors that may protect or predispose a person to substance use disorders – knowledge that can be used to expand preventative services and empower individuals to make informed decisions about drug use,” Nora Volkow, MD, director of the National Institute on Drug Abuse, said in news release.
“A better understanding of genetics also brings us one step closer to developing personalized interventions that are tailored to an individual’s unique biology, environment, and lived experience in order to provide the most benefits,” Dr. Volkow added.
The research was published online in Nature Mental Health.
Global research
Led by a team at the Washington University in St. Louis, the study included more than 150 collaborating investigators from around the world.
The risk of developing SUDs is influenced by a complex interplay between genetics and environmental factors. In a genomewide association study, the investigators looked for variations in the genome that were closely associated with SUDs in more than 1 million people of European ancestry and 92,630 people of African ancestry.
Among the European ancestry sample, they discovered 19 single-nucleotide polymorphisms that were significantly associated with general addiction risk and 47 genetic variants linked to specific SUDs – 9 for alcohol, 32 for tobacco, 5 for cannabis, and 1 for opioids.
The strongest gene signals consistent across the various SUDs mapped to areas in the genome involved in dopamine-signaling regulation, which reinforces the role of the dopamine system in addiction.
The genomic pattern also predicted higher risk of mental and physical illness, including psychiatric disorders, suicidal behavior, respiratory disease, heart disease, and chronic pain conditions. In children aged 9 or 10 years, presumably without any SUD, these genes correlated with parental substance use and externalizing behavior.
“Substance use disorders and mental disorders often co-occur, and we know that the most effective treatments help people address both issues at the same time. The shared genetic mechanisms between substance use and mental disorders revealed in this study underscore the importance of thinking about these disorders in tandem,” Joshua A. Gordon, MD, PhD, director of the National Institute of Mental Health, said in a news release.
Repurpose existing drugs for SUDs?
Separately, the genomic analysis of individuals of African ancestry showed only one genetic variation associated with general addiction risk and one substance-specific variation for risk of alcohol use disorder. The smaller sample size may be one reason for the more limited findings in this population.
“There is a tremendous need for treatments that target addiction generally, given patterns of the use of multiple substances, lifetime substance use, and severity seen in the clinic,” lead researcher Alexander Hatoum, PhD, at Washington University in St. Louis, said in a news release.
“Our study opens the door to identifying medications that may be leveraged to treat addiction broadly, which may be especially useful for treating more severe forms, including addiction to multiple substances,” Dr. Hatoum added.
As part of the study, the researchers compiled a list of approved and investigational pharmaceutical drugs that could potentially be repurposed to treat SUDs.
The list includes more than 100 drugs to investigate in future clinical trials, including those that can influence regulation of dopamine signaling.
This research was supported by NIDA, the National Institute on Alcohol Abuse and Alcoholism, NIMH, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
suggest new findings that researchers say could eventually lead to universal therapies to treat multiple and comorbid addictions.
“Genetics play a key role in determining health throughout our lives, but they are not destiny. Our hope with genomic studies is to further illuminate factors that may protect or predispose a person to substance use disorders – knowledge that can be used to expand preventative services and empower individuals to make informed decisions about drug use,” Nora Volkow, MD, director of the National Institute on Drug Abuse, said in news release.
“A better understanding of genetics also brings us one step closer to developing personalized interventions that are tailored to an individual’s unique biology, environment, and lived experience in order to provide the most benefits,” Dr. Volkow added.
The research was published online in Nature Mental Health.
Global research
Led by a team at the Washington University in St. Louis, the study included more than 150 collaborating investigators from around the world.
The risk of developing SUDs is influenced by a complex interplay between genetics and environmental factors. In a genomewide association study, the investigators looked for variations in the genome that were closely associated with SUDs in more than 1 million people of European ancestry and 92,630 people of African ancestry.
Among the European ancestry sample, they discovered 19 single-nucleotide polymorphisms that were significantly associated with general addiction risk and 47 genetic variants linked to specific SUDs – 9 for alcohol, 32 for tobacco, 5 for cannabis, and 1 for opioids.
The strongest gene signals consistent across the various SUDs mapped to areas in the genome involved in dopamine-signaling regulation, which reinforces the role of the dopamine system in addiction.
The genomic pattern also predicted higher risk of mental and physical illness, including psychiatric disorders, suicidal behavior, respiratory disease, heart disease, and chronic pain conditions. In children aged 9 or 10 years, presumably without any SUD, these genes correlated with parental substance use and externalizing behavior.
“Substance use disorders and mental disorders often co-occur, and we know that the most effective treatments help people address both issues at the same time. The shared genetic mechanisms between substance use and mental disorders revealed in this study underscore the importance of thinking about these disorders in tandem,” Joshua A. Gordon, MD, PhD, director of the National Institute of Mental Health, said in a news release.
Repurpose existing drugs for SUDs?
Separately, the genomic analysis of individuals of African ancestry showed only one genetic variation associated with general addiction risk and one substance-specific variation for risk of alcohol use disorder. The smaller sample size may be one reason for the more limited findings in this population.
“There is a tremendous need for treatments that target addiction generally, given patterns of the use of multiple substances, lifetime substance use, and severity seen in the clinic,” lead researcher Alexander Hatoum, PhD, at Washington University in St. Louis, said in a news release.
“Our study opens the door to identifying medications that may be leveraged to treat addiction broadly, which may be especially useful for treating more severe forms, including addiction to multiple substances,” Dr. Hatoum added.
As part of the study, the researchers compiled a list of approved and investigational pharmaceutical drugs that could potentially be repurposed to treat SUDs.
The list includes more than 100 drugs to investigate in future clinical trials, including those that can influence regulation of dopamine signaling.
This research was supported by NIDA, the National Institute on Alcohol Abuse and Alcoholism, NIMH, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
suggest new findings that researchers say could eventually lead to universal therapies to treat multiple and comorbid addictions.
“Genetics play a key role in determining health throughout our lives, but they are not destiny. Our hope with genomic studies is to further illuminate factors that may protect or predispose a person to substance use disorders – knowledge that can be used to expand preventative services and empower individuals to make informed decisions about drug use,” Nora Volkow, MD, director of the National Institute on Drug Abuse, said in news release.
“A better understanding of genetics also brings us one step closer to developing personalized interventions that are tailored to an individual’s unique biology, environment, and lived experience in order to provide the most benefits,” Dr. Volkow added.
The research was published online in Nature Mental Health.
Global research
Led by a team at the Washington University in St. Louis, the study included more than 150 collaborating investigators from around the world.
The risk of developing SUDs is influenced by a complex interplay between genetics and environmental factors. In a genomewide association study, the investigators looked for variations in the genome that were closely associated with SUDs in more than 1 million people of European ancestry and 92,630 people of African ancestry.
Among the European ancestry sample, they discovered 19 single-nucleotide polymorphisms that were significantly associated with general addiction risk and 47 genetic variants linked to specific SUDs – 9 for alcohol, 32 for tobacco, 5 for cannabis, and 1 for opioids.
The strongest gene signals consistent across the various SUDs mapped to areas in the genome involved in dopamine-signaling regulation, which reinforces the role of the dopamine system in addiction.
The genomic pattern also predicted higher risk of mental and physical illness, including psychiatric disorders, suicidal behavior, respiratory disease, heart disease, and chronic pain conditions. In children aged 9 or 10 years, presumably without any SUD, these genes correlated with parental substance use and externalizing behavior.
“Substance use disorders and mental disorders often co-occur, and we know that the most effective treatments help people address both issues at the same time. The shared genetic mechanisms between substance use and mental disorders revealed in this study underscore the importance of thinking about these disorders in tandem,” Joshua A. Gordon, MD, PhD, director of the National Institute of Mental Health, said in a news release.
Repurpose existing drugs for SUDs?
Separately, the genomic analysis of individuals of African ancestry showed only one genetic variation associated with general addiction risk and one substance-specific variation for risk of alcohol use disorder. The smaller sample size may be one reason for the more limited findings in this population.
“There is a tremendous need for treatments that target addiction generally, given patterns of the use of multiple substances, lifetime substance use, and severity seen in the clinic,” lead researcher Alexander Hatoum, PhD, at Washington University in St. Louis, said in a news release.
“Our study opens the door to identifying medications that may be leveraged to treat addiction broadly, which may be especially useful for treating more severe forms, including addiction to multiple substances,” Dr. Hatoum added.
As part of the study, the researchers compiled a list of approved and investigational pharmaceutical drugs that could potentially be repurposed to treat SUDs.
The list includes more than 100 drugs to investigate in future clinical trials, including those that can influence regulation of dopamine signaling.
This research was supported by NIDA, the National Institute on Alcohol Abuse and Alcoholism, NIMH, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
FROM NATURE MENTAL HEALTH
FDA approves new Merkel cell carcinoma treatment
the agency announced.
This marks the first regulatory approval for the PD-1 inhibitor. The FDA granted accelerated approval for the drug on the basis of tumor response rate and duration of response from the POD1UM-201 trial. Drugmaker Incyte said that “continued approval of Zynyz for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
MCC is a rare and aggressive skin cancer with a high rate of metastatic disease and an estimated 5-year overall survival of just 14% among those who present with metastatic disease. Incidence is rapidly increasing in the United States, particularly among adults older than 65 years, Incyte noted.
“More than a third of patients with MCC present with regional or distant metastases, which are associated with high rates of mortality,” principal author Shailender Bhatia, MD, of the University of Washington and Fred Hutchinson Cancer Center, both in Seattle, said in a news release. “The approval of Zynyz offers health care providers another first-line treatment option against MCC that can result in durable responses in patients with metastatic disease.”
POD1UM-201 was an open-label, single-arm, phase 2 study that evaluated the agent in 65 systemic treatment–naive adults with metastatic or recurrent locally advanced MCC.
Overall, 52% of patients had an objective response rate. A complete response was observed in 12 patients (18%), and a partial response was observed in 22 patients (34%).
Duration of response ranged from 1.1 to 24.9 months; 76% of responders experienced responses of 6 months or longer, and 62% experienced responses of 12 months or longer.
Study participants received a 500-mg dose of retifanlimab every 4 weeks for up to 24 weeks or until disease progression or unacceptable toxicity. Serious adverse events occurred in 22% of patients and most often included fatigue, arrhythmia, and pneumonitis; 11% of patients discontinued treatment because of serious adverse events.
Retifanlimab may cause a severe or life-threatening immune response during treatment or after discontinuation. Patients should be advised to immediately report any new or worsening signs or symptoms to their health care provider. Side effects can also be reported to the FDA.
A version of this article first appeared on Medscape.com.
the agency announced.
This marks the first regulatory approval for the PD-1 inhibitor. The FDA granted accelerated approval for the drug on the basis of tumor response rate and duration of response from the POD1UM-201 trial. Drugmaker Incyte said that “continued approval of Zynyz for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
MCC is a rare and aggressive skin cancer with a high rate of metastatic disease and an estimated 5-year overall survival of just 14% among those who present with metastatic disease. Incidence is rapidly increasing in the United States, particularly among adults older than 65 years, Incyte noted.
“More than a third of patients with MCC present with regional or distant metastases, which are associated with high rates of mortality,” principal author Shailender Bhatia, MD, of the University of Washington and Fred Hutchinson Cancer Center, both in Seattle, said in a news release. “The approval of Zynyz offers health care providers another first-line treatment option against MCC that can result in durable responses in patients with metastatic disease.”
POD1UM-201 was an open-label, single-arm, phase 2 study that evaluated the agent in 65 systemic treatment–naive adults with metastatic or recurrent locally advanced MCC.
Overall, 52% of patients had an objective response rate. A complete response was observed in 12 patients (18%), and a partial response was observed in 22 patients (34%).
Duration of response ranged from 1.1 to 24.9 months; 76% of responders experienced responses of 6 months or longer, and 62% experienced responses of 12 months or longer.
Study participants received a 500-mg dose of retifanlimab every 4 weeks for up to 24 weeks or until disease progression or unacceptable toxicity. Serious adverse events occurred in 22% of patients and most often included fatigue, arrhythmia, and pneumonitis; 11% of patients discontinued treatment because of serious adverse events.
Retifanlimab may cause a severe or life-threatening immune response during treatment or after discontinuation. Patients should be advised to immediately report any new or worsening signs or symptoms to their health care provider. Side effects can also be reported to the FDA.
A version of this article first appeared on Medscape.com.
the agency announced.
This marks the first regulatory approval for the PD-1 inhibitor. The FDA granted accelerated approval for the drug on the basis of tumor response rate and duration of response from the POD1UM-201 trial. Drugmaker Incyte said that “continued approval of Zynyz for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
MCC is a rare and aggressive skin cancer with a high rate of metastatic disease and an estimated 5-year overall survival of just 14% among those who present with metastatic disease. Incidence is rapidly increasing in the United States, particularly among adults older than 65 years, Incyte noted.
“More than a third of patients with MCC present with regional or distant metastases, which are associated with high rates of mortality,” principal author Shailender Bhatia, MD, of the University of Washington and Fred Hutchinson Cancer Center, both in Seattle, said in a news release. “The approval of Zynyz offers health care providers another first-line treatment option against MCC that can result in durable responses in patients with metastatic disease.”
POD1UM-201 was an open-label, single-arm, phase 2 study that evaluated the agent in 65 systemic treatment–naive adults with metastatic or recurrent locally advanced MCC.
Overall, 52% of patients had an objective response rate. A complete response was observed in 12 patients (18%), and a partial response was observed in 22 patients (34%).
Duration of response ranged from 1.1 to 24.9 months; 76% of responders experienced responses of 6 months or longer, and 62% experienced responses of 12 months or longer.
Study participants received a 500-mg dose of retifanlimab every 4 weeks for up to 24 weeks or until disease progression or unacceptable toxicity. Serious adverse events occurred in 22% of patients and most often included fatigue, arrhythmia, and pneumonitis; 11% of patients discontinued treatment because of serious adverse events.
Retifanlimab may cause a severe or life-threatening immune response during treatment or after discontinuation. Patients should be advised to immediately report any new or worsening signs or symptoms to their health care provider. Side effects can also be reported to the FDA.
A version of this article first appeared on Medscape.com.
Celebrity death finally solved – with locks of hair
This transcript has been edited for clarity.
I’m going to open this week with a case.
A 56-year-old musician presents with diffuse abdominal pain, cramping, and jaundice. His medical history is notable for years of diffuse abdominal complaints, characterized by disabling bouts of diarrhea.
In addition to the jaundice, this acute illness was accompanied by fever as well as diffuse edema and ascites. The patient underwent several abdominal paracenteses to drain excess fluid. One consulting physician administered alcohol to relieve pain, to little avail.
The patient succumbed to his illness. An autopsy showed diffuse liver injury, as well as papillary necrosis of the kidneys. Notably, the nerves of his auditory canal were noted to be thickened, along with the bony part of the skull, consistent with Paget disease of the bone and explaining, potentially, why the talented musician had gone deaf at such a young age.
An interesting note on social history: The patient had apparently developed some feelings for the niece of that doctor who prescribed alcohol. Her name was Therese, perhaps mistranscribed as Elise, and it seems that he may have written this song for her.
We’re talking about this paper in Current Biology, by Tristan Begg and colleagues, which gives us a look into the very genome of what some would argue is the world’s greatest composer.
The ability to extract DNA from older specimens has transformed the fields of anthropology, archaeology, and history, and now, perhaps, musicology as well.
The researchers identified eight locks of hair in private and public collections, all attributed to the maestro.
Four of the samples had an intact chain of custody from the time the hair was cut. DNA sequencing on these four and an additional one of the eight locks came from the same individual, a male of European heritage.
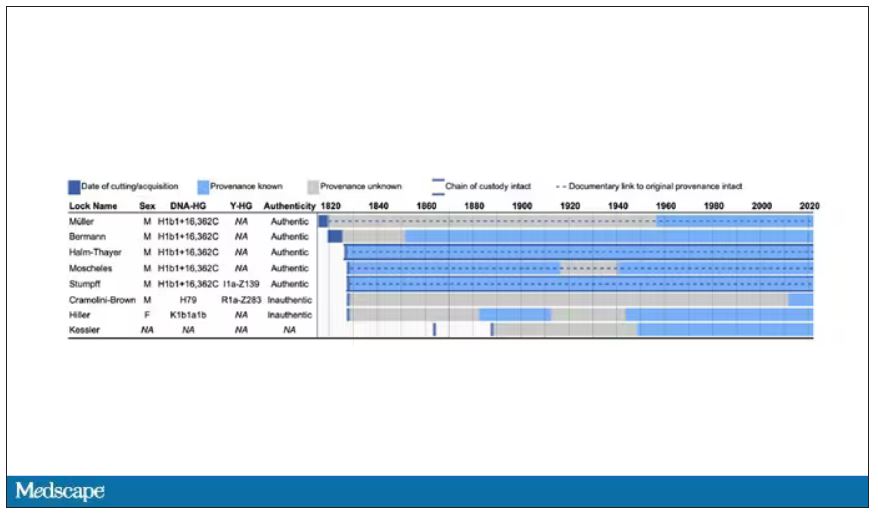
The three locks with less documentation came from three other unrelated individuals. Interestingly, analysis of one of those hair samples – the so-called Hiller Lock – had shown high levels of lead, leading historians to speculate that lead poisoning could account for some of Beethoven’s symptoms.

DNA analysis of that hair reveals it to have come from a woman likely of North African, Middle Eastern, or Jewish ancestry. We can no longer presume that plumbism was involved in Beethoven’s death. Beethoven’s ancestry turns out to be less exotic and maps quite well to ethnic German populations today.
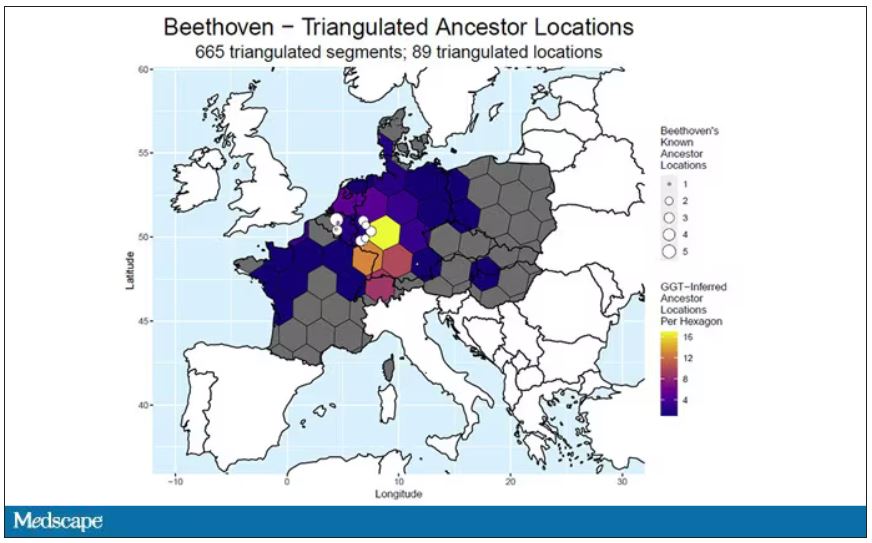
In fact, there are van Beethovens alive as we speak, primarily in Belgium. Genealogic records suggest that these van Beethovens share a common ancestor with the virtuoso composer, a man by the name of Aert van Beethoven.
But the DNA reveals a scandal.
The Y-chromosome that Beethoven inherited was not Aert van Beethoven’s. Questions of Beethoven’s paternity have been raised before, but this evidence strongly suggests an extramarital paternity event, at least in the generations preceding his birth. That’s right – Beethoven may not have been a Beethoven.
With five locks now essentially certain to have come from Beethoven himself, the authors could use DNA analysis to try to explain three significant health problems he experienced throughout his life and death: his hearing loss, his terrible gastrointestinal issues, and his liver failure.
Let’s start with the most disappointing results, explanations for his hearing loss. No genetic cause was forthcoming, though the authors note that they have little to go on in regard to the genetic risk for otosclerosis, to which his hearing loss has often been attributed. Lead poisoning is, of course, possible here, though this report focuses only on genetics – there was no testing for lead – and as I mentioned, the lock that was strongly lead-positive in prior studies is almost certainly inauthentic.
What about his lifelong GI complaints? Some have suggested celiac disease or lactose intolerance as explanations. These can essentially be ruled out by the genetic analysis, which shows no risk alleles for celiac disease and the presence of the lactase-persistence gene which confers the ability to metabolize lactose throughout one’s life. IBS is harder to assess genetically, but for what it’s worth, he scored quite low on a polygenic risk score for the condition, in just the 9th percentile of risk. We should probably be looking elsewhere to explain the GI distress.
The genetic information bore much more fruit in regard to his liver disease. Remember that Beethoven’s autopsy showed cirrhosis. His polygenic risk score for liver cirrhosis puts him in the 96th percentile of risk. He was also heterozygous for two variants that can cause hereditary hemochromatosis. The risk for cirrhosis among those with these variants is increased by the use of alcohol. And historical accounts are quite clear that Beethoven consumed more than his share.
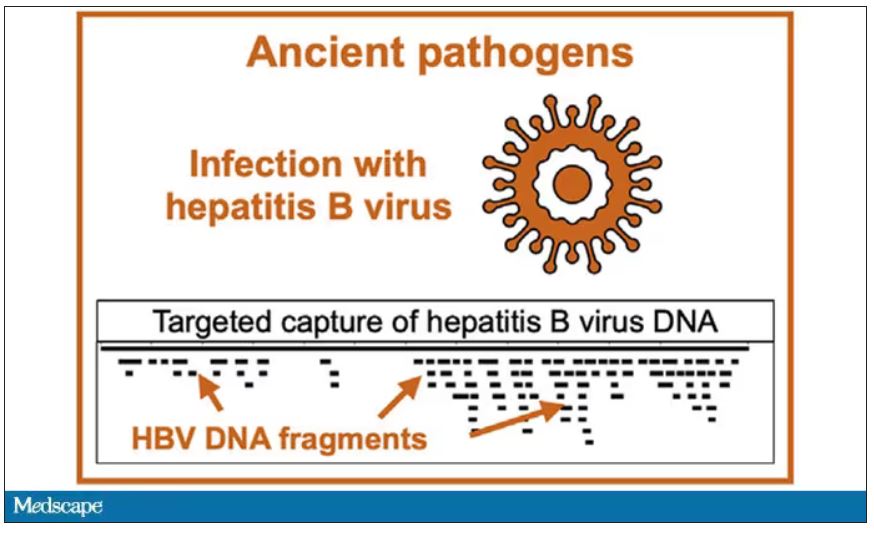
But it wasn’t just Beethoven’s DNA in these hair follicles. Analysis of a follicle from later in his life revealed the unmistakable presence of hepatitis B virus. Endemic in Europe at the time, this was a common cause of liver failure and is likely to have contributed to, if not directly caused, Beethoven’s demise.
It’s hard to read these results and not marvel at the fact that, two centuries after his death, our fascination with Beethoven has led us to probe every corner of his life – his letters, his writings, his medical records, and now his very DNA. What are we actually looking for? Is it relevant to us today what caused his hearing loss? His stomach troubles? Even his death? Will it help any patients in the future? I propose that what we are actually trying to understand is something ineffable: Genius of magnitude that is rarely seen in one or many lifetimes. And our scientific tools, as sharp as they may have become, are still far too blunt to probe the depths of that transcendence.
In any case, friends, no more of these sounds. Let us sing more cheerful songs, more full of joy.
For Medscape, I’m Perry Wilson.
Dr. Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to open this week with a case.
A 56-year-old musician presents with diffuse abdominal pain, cramping, and jaundice. His medical history is notable for years of diffuse abdominal complaints, characterized by disabling bouts of diarrhea.
In addition to the jaundice, this acute illness was accompanied by fever as well as diffuse edema and ascites. The patient underwent several abdominal paracenteses to drain excess fluid. One consulting physician administered alcohol to relieve pain, to little avail.
The patient succumbed to his illness. An autopsy showed diffuse liver injury, as well as papillary necrosis of the kidneys. Notably, the nerves of his auditory canal were noted to be thickened, along with the bony part of the skull, consistent with Paget disease of the bone and explaining, potentially, why the talented musician had gone deaf at such a young age.
An interesting note on social history: The patient had apparently developed some feelings for the niece of that doctor who prescribed alcohol. Her name was Therese, perhaps mistranscribed as Elise, and it seems that he may have written this song for her.
We’re talking about this paper in Current Biology, by Tristan Begg and colleagues, which gives us a look into the very genome of what some would argue is the world’s greatest composer.
The ability to extract DNA from older specimens has transformed the fields of anthropology, archaeology, and history, and now, perhaps, musicology as well.
The researchers identified eight locks of hair in private and public collections, all attributed to the maestro.
Four of the samples had an intact chain of custody from the time the hair was cut. DNA sequencing on these four and an additional one of the eight locks came from the same individual, a male of European heritage.

The three locks with less documentation came from three other unrelated individuals. Interestingly, analysis of one of those hair samples – the so-called Hiller Lock – had shown high levels of lead, leading historians to speculate that lead poisoning could account for some of Beethoven’s symptoms.

DNA analysis of that hair reveals it to have come from a woman likely of North African, Middle Eastern, or Jewish ancestry. We can no longer presume that plumbism was involved in Beethoven’s death. Beethoven’s ancestry turns out to be less exotic and maps quite well to ethnic German populations today.

In fact, there are van Beethovens alive as we speak, primarily in Belgium. Genealogic records suggest that these van Beethovens share a common ancestor with the virtuoso composer, a man by the name of Aert van Beethoven.
But the DNA reveals a scandal.
The Y-chromosome that Beethoven inherited was not Aert van Beethoven’s. Questions of Beethoven’s paternity have been raised before, but this evidence strongly suggests an extramarital paternity event, at least in the generations preceding his birth. That’s right – Beethoven may not have been a Beethoven.
With five locks now essentially certain to have come from Beethoven himself, the authors could use DNA analysis to try to explain three significant health problems he experienced throughout his life and death: his hearing loss, his terrible gastrointestinal issues, and his liver failure.
Let’s start with the most disappointing results, explanations for his hearing loss. No genetic cause was forthcoming, though the authors note that they have little to go on in regard to the genetic risk for otosclerosis, to which his hearing loss has often been attributed. Lead poisoning is, of course, possible here, though this report focuses only on genetics – there was no testing for lead – and as I mentioned, the lock that was strongly lead-positive in prior studies is almost certainly inauthentic.
What about his lifelong GI complaints? Some have suggested celiac disease or lactose intolerance as explanations. These can essentially be ruled out by the genetic analysis, which shows no risk alleles for celiac disease and the presence of the lactase-persistence gene which confers the ability to metabolize lactose throughout one’s life. IBS is harder to assess genetically, but for what it’s worth, he scored quite low on a polygenic risk score for the condition, in just the 9th percentile of risk. We should probably be looking elsewhere to explain the GI distress.
The genetic information bore much more fruit in regard to his liver disease. Remember that Beethoven’s autopsy showed cirrhosis. His polygenic risk score for liver cirrhosis puts him in the 96th percentile of risk. He was also heterozygous for two variants that can cause hereditary hemochromatosis. The risk for cirrhosis among those with these variants is increased by the use of alcohol. And historical accounts are quite clear that Beethoven consumed more than his share.
But it wasn’t just Beethoven’s DNA in these hair follicles. Analysis of a follicle from later in his life revealed the unmistakable presence of hepatitis B virus. Endemic in Europe at the time, this was a common cause of liver failure and is likely to have contributed to, if not directly caused, Beethoven’s demise.

It’s hard to read these results and not marvel at the fact that, two centuries after his death, our fascination with Beethoven has led us to probe every corner of his life – his letters, his writings, his medical records, and now his very DNA. What are we actually looking for? Is it relevant to us today what caused his hearing loss? His stomach troubles? Even his death? Will it help any patients in the future? I propose that what we are actually trying to understand is something ineffable: Genius of magnitude that is rarely seen in one or many lifetimes. And our scientific tools, as sharp as they may have become, are still far too blunt to probe the depths of that transcendence.
In any case, friends, no more of these sounds. Let us sing more cheerful songs, more full of joy.
For Medscape, I’m Perry Wilson.
Dr. Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to open this week with a case.
A 56-year-old musician presents with diffuse abdominal pain, cramping, and jaundice. His medical history is notable for years of diffuse abdominal complaints, characterized by disabling bouts of diarrhea.
In addition to the jaundice, this acute illness was accompanied by fever as well as diffuse edema and ascites. The patient underwent several abdominal paracenteses to drain excess fluid. One consulting physician administered alcohol to relieve pain, to little avail.
The patient succumbed to his illness. An autopsy showed diffuse liver injury, as well as papillary necrosis of the kidneys. Notably, the nerves of his auditory canal were noted to be thickened, along with the bony part of the skull, consistent with Paget disease of the bone and explaining, potentially, why the talented musician had gone deaf at such a young age.
An interesting note on social history: The patient had apparently developed some feelings for the niece of that doctor who prescribed alcohol. Her name was Therese, perhaps mistranscribed as Elise, and it seems that he may have written this song for her.
We’re talking about this paper in Current Biology, by Tristan Begg and colleagues, which gives us a look into the very genome of what some would argue is the world’s greatest composer.
The ability to extract DNA from older specimens has transformed the fields of anthropology, archaeology, and history, and now, perhaps, musicology as well.
The researchers identified eight locks of hair in private and public collections, all attributed to the maestro.
Four of the samples had an intact chain of custody from the time the hair was cut. DNA sequencing on these four and an additional one of the eight locks came from the same individual, a male of European heritage.

The three locks with less documentation came from three other unrelated individuals. Interestingly, analysis of one of those hair samples – the so-called Hiller Lock – had shown high levels of lead, leading historians to speculate that lead poisoning could account for some of Beethoven’s symptoms.

DNA analysis of that hair reveals it to have come from a woman likely of North African, Middle Eastern, or Jewish ancestry. We can no longer presume that plumbism was involved in Beethoven’s death. Beethoven’s ancestry turns out to be less exotic and maps quite well to ethnic German populations today.

In fact, there are van Beethovens alive as we speak, primarily in Belgium. Genealogic records suggest that these van Beethovens share a common ancestor with the virtuoso composer, a man by the name of Aert van Beethoven.
But the DNA reveals a scandal.
The Y-chromosome that Beethoven inherited was not Aert van Beethoven’s. Questions of Beethoven’s paternity have been raised before, but this evidence strongly suggests an extramarital paternity event, at least in the generations preceding his birth. That’s right – Beethoven may not have been a Beethoven.
With five locks now essentially certain to have come from Beethoven himself, the authors could use DNA analysis to try to explain three significant health problems he experienced throughout his life and death: his hearing loss, his terrible gastrointestinal issues, and his liver failure.
Let’s start with the most disappointing results, explanations for his hearing loss. No genetic cause was forthcoming, though the authors note that they have little to go on in regard to the genetic risk for otosclerosis, to which his hearing loss has often been attributed. Lead poisoning is, of course, possible here, though this report focuses only on genetics – there was no testing for lead – and as I mentioned, the lock that was strongly lead-positive in prior studies is almost certainly inauthentic.
What about his lifelong GI complaints? Some have suggested celiac disease or lactose intolerance as explanations. These can essentially be ruled out by the genetic analysis, which shows no risk alleles for celiac disease and the presence of the lactase-persistence gene which confers the ability to metabolize lactose throughout one’s life. IBS is harder to assess genetically, but for what it’s worth, he scored quite low on a polygenic risk score for the condition, in just the 9th percentile of risk. We should probably be looking elsewhere to explain the GI distress.
The genetic information bore much more fruit in regard to his liver disease. Remember that Beethoven’s autopsy showed cirrhosis. His polygenic risk score for liver cirrhosis puts him in the 96th percentile of risk. He was also heterozygous for two variants that can cause hereditary hemochromatosis. The risk for cirrhosis among those with these variants is increased by the use of alcohol. And historical accounts are quite clear that Beethoven consumed more than his share.
But it wasn’t just Beethoven’s DNA in these hair follicles. Analysis of a follicle from later in his life revealed the unmistakable presence of hepatitis B virus. Endemic in Europe at the time, this was a common cause of liver failure and is likely to have contributed to, if not directly caused, Beethoven’s demise.

It’s hard to read these results and not marvel at the fact that, two centuries after his death, our fascination with Beethoven has led us to probe every corner of his life – his letters, his writings, his medical records, and now his very DNA. What are we actually looking for? Is it relevant to us today what caused his hearing loss? His stomach troubles? Even his death? Will it help any patients in the future? I propose that what we are actually trying to understand is something ineffable: Genius of magnitude that is rarely seen in one or many lifetimes. And our scientific tools, as sharp as they may have become, are still far too blunt to probe the depths of that transcendence.
In any case, friends, no more of these sounds. Let us sing more cheerful songs, more full of joy.
For Medscape, I’m Perry Wilson.
Dr. Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Tyrosine kinase inhibitors – a new weapon against respiratory viruses?
Five different nonreceptor tyrosine kinase inhibitors were effective against viral replication of pandemic viruses and seasonal influenza viruses in an ex vivo lung model.
Influenza viruses remain a high cause of morbidity and mortality worldwide as viral mutations outwit vaccine efficacy, Robert Meineke, PhD, of the University of Veterinary Medicine in Hannover, Germany, and colleagues wrote.
“As with previous influenza pandemics and the current SARS-CoV-2 pandemic, effective vaccines are not readily available at early stages of a pandemic,” they noted. To help manage the limitations of timing and effectiveness of current vaccines, the researchers proposed repurposing nonreceptor tyrosine kinase inhibitors (NRTKIs) to block seasonal flu and COVID-19 viral replication.
In a study published in iScience, the researchers identified six NRTKIs currently approved by the U.S. Food and Drug Administration that showed in vitro inhibition of both pandemic viruses (H1N1) and seasonal influenza viruses (H3N2). These included defactinib, acalabrutinib, saracatinib, and bosutinib, all of which reduced hPCLS infectivity by approximately 50%. In addition, ibrutinib and bosutinib had the largest impact on viral titers. The antiviral effects of NRTKIs appeared to be independent of multiplicity of infection.
The researchers then tested the NRIKIs on an ex vivo model of human precision-cut lung slices to validate the effects of NRTKIs as antivirals against influenza A viruses (IAVs).
In this model, the highest peak titers were achieved at 48 hpi following infection with virus strains NL09 and NL11. The hPCLS models also showed consistent tolerability to 1x concentrations. “Our cytotoxicity cut-off was 20% of the positive control treatment; none of the NRTKIs surpassed this cutoff at [1x] max,” the researchers wrote.
Five of the six identified NRTKIs were validated in the ex vivo setting. All five reduced viral titers by at least 10-fold to more than 1,000-fold. Of these, ibrutinib, bosutinib, and bosutinib showed a significant effect at all concentrations, while treatments with acalabrutinib and defactinib were significant at 24 hpi and 48 hpi. The NRTKs also showed a high genetic barrier against emerging resistant virus mutations.
The study demonstrates the ability of NRTKIs to target kinases required for replication of IAV, the researchers wrote, and that NRTKIs “represent promising drugs for the development of the next generation of antivirals.”
More research is needed to determine the therapeutic window given that NRTKIs are targeting host factors versus virus-targeted antivirals, but the advantages of NRTKIs include localized delivery that can limit possible cytotoxic effects, and their safety and bioavailability are well established, they said.
The findings were limited by several factors including the use of lung tissue mainly from older donors with lung cancer, the researchers noted. However, this population could be considered at increased risk for IAVs and therefore the data are more clinically applicable.
In addition, “because many viruses utilize the same (or related) host kinases to facilitate replication and transmission, our studies have broader implications for the potential use of these SMKIs to treat infections by other viruses,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Five different nonreceptor tyrosine kinase inhibitors were effective against viral replication of pandemic viruses and seasonal influenza viruses in an ex vivo lung model.
Influenza viruses remain a high cause of morbidity and mortality worldwide as viral mutations outwit vaccine efficacy, Robert Meineke, PhD, of the University of Veterinary Medicine in Hannover, Germany, and colleagues wrote.
“As with previous influenza pandemics and the current SARS-CoV-2 pandemic, effective vaccines are not readily available at early stages of a pandemic,” they noted. To help manage the limitations of timing and effectiveness of current vaccines, the researchers proposed repurposing nonreceptor tyrosine kinase inhibitors (NRTKIs) to block seasonal flu and COVID-19 viral replication.
In a study published in iScience, the researchers identified six NRTKIs currently approved by the U.S. Food and Drug Administration that showed in vitro inhibition of both pandemic viruses (H1N1) and seasonal influenza viruses (H3N2). These included defactinib, acalabrutinib, saracatinib, and bosutinib, all of which reduced hPCLS infectivity by approximately 50%. In addition, ibrutinib and bosutinib had the largest impact on viral titers. The antiviral effects of NRTKIs appeared to be independent of multiplicity of infection.
The researchers then tested the NRIKIs on an ex vivo model of human precision-cut lung slices to validate the effects of NRTKIs as antivirals against influenza A viruses (IAVs).
In this model, the highest peak titers were achieved at 48 hpi following infection with virus strains NL09 and NL11. The hPCLS models also showed consistent tolerability to 1x concentrations. “Our cytotoxicity cut-off was 20% of the positive control treatment; none of the NRTKIs surpassed this cutoff at [1x] max,” the researchers wrote.
Five of the six identified NRTKIs were validated in the ex vivo setting. All five reduced viral titers by at least 10-fold to more than 1,000-fold. Of these, ibrutinib, bosutinib, and bosutinib showed a significant effect at all concentrations, while treatments with acalabrutinib and defactinib were significant at 24 hpi and 48 hpi. The NRTKs also showed a high genetic barrier against emerging resistant virus mutations.
The study demonstrates the ability of NRTKIs to target kinases required for replication of IAV, the researchers wrote, and that NRTKIs “represent promising drugs for the development of the next generation of antivirals.”
More research is needed to determine the therapeutic window given that NRTKIs are targeting host factors versus virus-targeted antivirals, but the advantages of NRTKIs include localized delivery that can limit possible cytotoxic effects, and their safety and bioavailability are well established, they said.
The findings were limited by several factors including the use of lung tissue mainly from older donors with lung cancer, the researchers noted. However, this population could be considered at increased risk for IAVs and therefore the data are more clinically applicable.
In addition, “because many viruses utilize the same (or related) host kinases to facilitate replication and transmission, our studies have broader implications for the potential use of these SMKIs to treat infections by other viruses,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Five different nonreceptor tyrosine kinase inhibitors were effective against viral replication of pandemic viruses and seasonal influenza viruses in an ex vivo lung model.
Influenza viruses remain a high cause of morbidity and mortality worldwide as viral mutations outwit vaccine efficacy, Robert Meineke, PhD, of the University of Veterinary Medicine in Hannover, Germany, and colleagues wrote.
“As with previous influenza pandemics and the current SARS-CoV-2 pandemic, effective vaccines are not readily available at early stages of a pandemic,” they noted. To help manage the limitations of timing and effectiveness of current vaccines, the researchers proposed repurposing nonreceptor tyrosine kinase inhibitors (NRTKIs) to block seasonal flu and COVID-19 viral replication.
In a study published in iScience, the researchers identified six NRTKIs currently approved by the U.S. Food and Drug Administration that showed in vitro inhibition of both pandemic viruses (H1N1) and seasonal influenza viruses (H3N2). These included defactinib, acalabrutinib, saracatinib, and bosutinib, all of which reduced hPCLS infectivity by approximately 50%. In addition, ibrutinib and bosutinib had the largest impact on viral titers. The antiviral effects of NRTKIs appeared to be independent of multiplicity of infection.
The researchers then tested the NRIKIs on an ex vivo model of human precision-cut lung slices to validate the effects of NRTKIs as antivirals against influenza A viruses (IAVs).
In this model, the highest peak titers were achieved at 48 hpi following infection with virus strains NL09 and NL11. The hPCLS models also showed consistent tolerability to 1x concentrations. “Our cytotoxicity cut-off was 20% of the positive control treatment; none of the NRTKIs surpassed this cutoff at [1x] max,” the researchers wrote.
Five of the six identified NRTKIs were validated in the ex vivo setting. All five reduced viral titers by at least 10-fold to more than 1,000-fold. Of these, ibrutinib, bosutinib, and bosutinib showed a significant effect at all concentrations, while treatments with acalabrutinib and defactinib were significant at 24 hpi and 48 hpi. The NRTKs also showed a high genetic barrier against emerging resistant virus mutations.
The study demonstrates the ability of NRTKIs to target kinases required for replication of IAV, the researchers wrote, and that NRTKIs “represent promising drugs for the development of the next generation of antivirals.”
More research is needed to determine the therapeutic window given that NRTKIs are targeting host factors versus virus-targeted antivirals, but the advantages of NRTKIs include localized delivery that can limit possible cytotoxic effects, and their safety and bioavailability are well established, they said.
The findings were limited by several factors including the use of lung tissue mainly from older donors with lung cancer, the researchers noted. However, this population could be considered at increased risk for IAVs and therefore the data are more clinically applicable.
In addition, “because many viruses utilize the same (or related) host kinases to facilitate replication and transmission, our studies have broader implications for the potential use of these SMKIs to treat infections by other viruses,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM ISCIENCE
Nurse makes millions selling her licensing exam study sheets
Ms. Beggs, 28, sells one-page study sheets or bundles of sheets, sometimes with colorful drawings, conversation bubbles and underlining, that boil down concepts for particular conditions into easy-to-understand language.
The biggest seller on Ms. Beggs’ online marketplace Etsy site, RNExplained, is a bundle of study guides covering eight core nursing classes. The notes range in price from $2 to $150. More than 70,000 customers have bought the $60 bundle, according to the website.
Ms. Beggs’ business developed in a “very unintentional” way when COVID hit with just months left in her nursing program at Mount Saint Mary’s University, Los Angeles, she told this news organization.
Classes had switched to Zoom, and she had no one to study with as she prepared to take her board exams.
“The best way I know how to study is to teach things out loud. But because I had nobody to teach out loud to, I would literally teach them to the wall,” Ms. Beggs said. “I would record myself so I could play it back and teach myself these topics that were hard for me to understand.”
Just for fun, she says, she posted them on TikTok and the responses started flowing in, with followers asking where she was selling the sheets. She now has more than 660,000 TikTok followers and 9 million likes.
Ms. Beggs said that every sheet highlights a condition, and she has made 308 of them.
Traditional classroom lessons typically teach one medical condition in 5-6 pages, Ms. Beggs said. “I go straight to the point.”
One reviewer on Ms. Beggs’ Etsy site appreciated the handwritten notes, calling them “simplified and concise.” Another commented: “Definitely helped me pass my last exam.”
Ms. Beggs says that her notes may seem simple, but each page represents comprehensive research.
“I have to go through not just one source of information to make sure my information is factual,” Ms. Beggs says. “What you teach in California might be a little different than what you teach in Florida. It’s very meticulous. The lab values will be a little different everywhere you go.”
She acknowledges her competition, noting that there are many other study guides for the NCLEX and nursing courses.
Nursing groups weigh in
Dawn Kappel, spokesperson for the National Council of State Boards of Nursing, which oversees NCLEX, said in an interview that “NCSBN has no issue with the current content of Stephanee Beggs’ business venture.”
For many students, the study guides will be helpful, especially for visual learners, said Carole Kenner, PhD, RN, dean and professor in the School of Nursing and Health Sciences at The College of New Jersey.
But for students “who are less confident in their knowledge, I would want to see a lot more in-depth explanation and rationale,” Dr. Kenner said.
“Since the NCLEX is moving to more cased-based scenarios, the next-gen unfolding cases, you really have to understand a lot of the rationale.”
The notes remind Dr. Kenner of traditional flash cards. “I don’t think it will work for all students, but even the fanciest of onsite review courses are useful to everyone,” she said.
‘Not cutting corners’
As an emergency nurse, Ms. Beggs said, “I have the experience as a nurse to show people that what you are learning will be seen in real life.”
“The way I teach my brand is not to take shortcuts. I love to teach to understand rather than teaching to memorize for an exam.”
She said she sees her guides as a supplement to learning, not a replacement.
“It’s not cutting corners,” she says. “I condense a medical condition that could take a very long time to understand and break it into layman’s terms.”
Ms. Beggs said when people hear about the $2 million, they often ask her whether she plans to give up her shifts in the emergency department for the more lucrative venture.
The answer is no, at least not yet.
“Aside from teaching, I genuinely love being at the bedside,” Ms. Beggs said. “I don’t foresee myself leaving that for good for as long as I can handle both.” She acknowledged, though, that her business now takes up most of her time.
“I love everything about both aspects, so it’s hard for me to choose.”
A version of this article first appeared on Medscape.com.
Ms. Beggs, 28, sells one-page study sheets or bundles of sheets, sometimes with colorful drawings, conversation bubbles and underlining, that boil down concepts for particular conditions into easy-to-understand language.
The biggest seller on Ms. Beggs’ online marketplace Etsy site, RNExplained, is a bundle of study guides covering eight core nursing classes. The notes range in price from $2 to $150. More than 70,000 customers have bought the $60 bundle, according to the website.
Ms. Beggs’ business developed in a “very unintentional” way when COVID hit with just months left in her nursing program at Mount Saint Mary’s University, Los Angeles, she told this news organization.
Classes had switched to Zoom, and she had no one to study with as she prepared to take her board exams.
“The best way I know how to study is to teach things out loud. But because I had nobody to teach out loud to, I would literally teach them to the wall,” Ms. Beggs said. “I would record myself so I could play it back and teach myself these topics that were hard for me to understand.”
Just for fun, she says, she posted them on TikTok and the responses started flowing in, with followers asking where she was selling the sheets. She now has more than 660,000 TikTok followers and 9 million likes.
Ms. Beggs said that every sheet highlights a condition, and she has made 308 of them.
Traditional classroom lessons typically teach one medical condition in 5-6 pages, Ms. Beggs said. “I go straight to the point.”
One reviewer on Ms. Beggs’ Etsy site appreciated the handwritten notes, calling them “simplified and concise.” Another commented: “Definitely helped me pass my last exam.”
Ms. Beggs says that her notes may seem simple, but each page represents comprehensive research.
“I have to go through not just one source of information to make sure my information is factual,” Ms. Beggs says. “What you teach in California might be a little different than what you teach in Florida. It’s very meticulous. The lab values will be a little different everywhere you go.”
She acknowledges her competition, noting that there are many other study guides for the NCLEX and nursing courses.
Nursing groups weigh in
Dawn Kappel, spokesperson for the National Council of State Boards of Nursing, which oversees NCLEX, said in an interview that “NCSBN has no issue with the current content of Stephanee Beggs’ business venture.”
For many students, the study guides will be helpful, especially for visual learners, said Carole Kenner, PhD, RN, dean and professor in the School of Nursing and Health Sciences at The College of New Jersey.
But for students “who are less confident in their knowledge, I would want to see a lot more in-depth explanation and rationale,” Dr. Kenner said.
“Since the NCLEX is moving to more cased-based scenarios, the next-gen unfolding cases, you really have to understand a lot of the rationale.”
The notes remind Dr. Kenner of traditional flash cards. “I don’t think it will work for all students, but even the fanciest of onsite review courses are useful to everyone,” she said.
‘Not cutting corners’
As an emergency nurse, Ms. Beggs said, “I have the experience as a nurse to show people that what you are learning will be seen in real life.”
“The way I teach my brand is not to take shortcuts. I love to teach to understand rather than teaching to memorize for an exam.”
She said she sees her guides as a supplement to learning, not a replacement.
“It’s not cutting corners,” she says. “I condense a medical condition that could take a very long time to understand and break it into layman’s terms.”
Ms. Beggs said when people hear about the $2 million, they often ask her whether she plans to give up her shifts in the emergency department for the more lucrative venture.
The answer is no, at least not yet.
“Aside from teaching, I genuinely love being at the bedside,” Ms. Beggs said. “I don’t foresee myself leaving that for good for as long as I can handle both.” She acknowledged, though, that her business now takes up most of her time.
“I love everything about both aspects, so it’s hard for me to choose.”
A version of this article first appeared on Medscape.com.
Ms. Beggs, 28, sells one-page study sheets or bundles of sheets, sometimes with colorful drawings, conversation bubbles and underlining, that boil down concepts for particular conditions into easy-to-understand language.
The biggest seller on Ms. Beggs’ online marketplace Etsy site, RNExplained, is a bundle of study guides covering eight core nursing classes. The notes range in price from $2 to $150. More than 70,000 customers have bought the $60 bundle, according to the website.
Ms. Beggs’ business developed in a “very unintentional” way when COVID hit with just months left in her nursing program at Mount Saint Mary’s University, Los Angeles, she told this news organization.
Classes had switched to Zoom, and she had no one to study with as she prepared to take her board exams.
“The best way I know how to study is to teach things out loud. But because I had nobody to teach out loud to, I would literally teach them to the wall,” Ms. Beggs said. “I would record myself so I could play it back and teach myself these topics that were hard for me to understand.”
Just for fun, she says, she posted them on TikTok and the responses started flowing in, with followers asking where she was selling the sheets. She now has more than 660,000 TikTok followers and 9 million likes.
Ms. Beggs said that every sheet highlights a condition, and she has made 308 of them.
Traditional classroom lessons typically teach one medical condition in 5-6 pages, Ms. Beggs said. “I go straight to the point.”
One reviewer on Ms. Beggs’ Etsy site appreciated the handwritten notes, calling them “simplified and concise.” Another commented: “Definitely helped me pass my last exam.”
Ms. Beggs says that her notes may seem simple, but each page represents comprehensive research.
“I have to go through not just one source of information to make sure my information is factual,” Ms. Beggs says. “What you teach in California might be a little different than what you teach in Florida. It’s very meticulous. The lab values will be a little different everywhere you go.”
She acknowledges her competition, noting that there are many other study guides for the NCLEX and nursing courses.
Nursing groups weigh in
Dawn Kappel, spokesperson for the National Council of State Boards of Nursing, which oversees NCLEX, said in an interview that “NCSBN has no issue with the current content of Stephanee Beggs’ business venture.”
For many students, the study guides will be helpful, especially for visual learners, said Carole Kenner, PhD, RN, dean and professor in the School of Nursing and Health Sciences at The College of New Jersey.
But for students “who are less confident in their knowledge, I would want to see a lot more in-depth explanation and rationale,” Dr. Kenner said.
“Since the NCLEX is moving to more cased-based scenarios, the next-gen unfolding cases, you really have to understand a lot of the rationale.”
The notes remind Dr. Kenner of traditional flash cards. “I don’t think it will work for all students, but even the fanciest of onsite review courses are useful to everyone,” she said.
‘Not cutting corners’
As an emergency nurse, Ms. Beggs said, “I have the experience as a nurse to show people that what you are learning will be seen in real life.”
“The way I teach my brand is not to take shortcuts. I love to teach to understand rather than teaching to memorize for an exam.”
She said she sees her guides as a supplement to learning, not a replacement.
“It’s not cutting corners,” she says. “I condense a medical condition that could take a very long time to understand and break it into layman’s terms.”
Ms. Beggs said when people hear about the $2 million, they often ask her whether she plans to give up her shifts in the emergency department for the more lucrative venture.
The answer is no, at least not yet.
“Aside from teaching, I genuinely love being at the bedside,” Ms. Beggs said. “I don’t foresee myself leaving that for good for as long as I can handle both.” She acknowledged, though, that her business now takes up most of her time.
“I love everything about both aspects, so it’s hard for me to choose.”
A version of this article first appeared on Medscape.com.
Disease burden is higher in women vs men with psoriatic arthritis
Key clinical point: The psoriatic arthritis (PsA) disease burden had worse impact on women vs men with PsA, with women having a higher disease activity, worse function, and greater disease burden.
Major finding: Female vs male patients with PsA had a significantly higher mean patient global assessment score (P < .001), patient’s pain score (P = .003), tender joint count (P < .001), swollen joint count (P = .033), and Disease Activity Score for PsA (P < .001). Minimal disease activity was achieved by 44.0% of men vs 24.6% of women (P = .003).
Study details: Findings are from a cross-sectional analysis of 2 longitudinal cohorts including 141 male and 131 female patients with PsA who received treatment with conventional synthetic or biologic disease-modifying antirheumatic drugs for at least 6 months.
Disclosures: This study did not receive funding. The authors declared no conflicts of interest.
Source: Lubrano E et al. Psoriatic arthritis in males and females: Differences and similarities. Rheumatol Ther. 2023 (Feb 16). Doi: 10.1007/s40744-023-00535-3
Key clinical point: The psoriatic arthritis (PsA) disease burden had worse impact on women vs men with PsA, with women having a higher disease activity, worse function, and greater disease burden.
Major finding: Female vs male patients with PsA had a significantly higher mean patient global assessment score (P < .001), patient’s pain score (P = .003), tender joint count (P < .001), swollen joint count (P = .033), and Disease Activity Score for PsA (P < .001). Minimal disease activity was achieved by 44.0% of men vs 24.6% of women (P = .003).
Study details: Findings are from a cross-sectional analysis of 2 longitudinal cohorts including 141 male and 131 female patients with PsA who received treatment with conventional synthetic or biologic disease-modifying antirheumatic drugs for at least 6 months.
Disclosures: This study did not receive funding. The authors declared no conflicts of interest.
Source: Lubrano E et al. Psoriatic arthritis in males and females: Differences and similarities. Rheumatol Ther. 2023 (Feb 16). Doi: 10.1007/s40744-023-00535-3
Key clinical point: The psoriatic arthritis (PsA) disease burden had worse impact on women vs men with PsA, with women having a higher disease activity, worse function, and greater disease burden.
Major finding: Female vs male patients with PsA had a significantly higher mean patient global assessment score (P < .001), patient’s pain score (P = .003), tender joint count (P < .001), swollen joint count (P = .033), and Disease Activity Score for PsA (P < .001). Minimal disease activity was achieved by 44.0% of men vs 24.6% of women (P = .003).
Study details: Findings are from a cross-sectional analysis of 2 longitudinal cohorts including 141 male and 131 female patients with PsA who received treatment with conventional synthetic or biologic disease-modifying antirheumatic drugs for at least 6 months.
Disclosures: This study did not receive funding. The authors declared no conflicts of interest.
Source: Lubrano E et al. Psoriatic arthritis in males and females: Differences and similarities. Rheumatol Ther. 2023 (Feb 16). Doi: 10.1007/s40744-023-00535-3
Apremilast safe and effective in biologic-naive patients with early PsA
Key clinical point: Early initiation of apremilast led to rapid and sustained improvements in psoriatic arthritis (PsA) manifestations with a consistent safety profile in a real-world cohort of biologic-naive patients who were intolerant of conventional synthetic disease-modifying antirheumatic drugs (csDMARD).
Major finding: Among patients with baseline swollen joint count (SJC) and tender joint count (TJC) of >0, significant median decreases in SJC (50% and 90%, respectively) and TJC (50% and 80%, respectively) were observed at 16 and 52 weeks (all P < .001), with 55.2% of evaluable patients achieving minimal disease activity at 52 weeks. Overall, 13.8% of patients experienced ≥1 adverse event, with all except one being non-serious.
Study details: This prospective study included 167 biologic-naive patients with early peripheral PsA and intolerance or inadequate response to csDMARD who initiated apremilast.
Disclosures: This study was funded by Genesis Pharma and Celgene. Some authors reported ties with various sources, including Genesis Pharma. A Kekki and N Antonakopoulos reported being employees of Genesis Pharma.
Source: Sfikakis PP et al. Apremilast for biologic-naïve, peripheral psoriatic arthritis, including patients with early disease: Results from the APROACH observational prospective study. Rheumatol Int. 2023 (Mar 1). Doi: 10.1007/s00296-022-05269-z
Key clinical point: Early initiation of apremilast led to rapid and sustained improvements in psoriatic arthritis (PsA) manifestations with a consistent safety profile in a real-world cohort of biologic-naive patients who were intolerant of conventional synthetic disease-modifying antirheumatic drugs (csDMARD).
Major finding: Among patients with baseline swollen joint count (SJC) and tender joint count (TJC) of >0, significant median decreases in SJC (50% and 90%, respectively) and TJC (50% and 80%, respectively) were observed at 16 and 52 weeks (all P < .001), with 55.2% of evaluable patients achieving minimal disease activity at 52 weeks. Overall, 13.8% of patients experienced ≥1 adverse event, with all except one being non-serious.
Study details: This prospective study included 167 biologic-naive patients with early peripheral PsA and intolerance or inadequate response to csDMARD who initiated apremilast.
Disclosures: This study was funded by Genesis Pharma and Celgene. Some authors reported ties with various sources, including Genesis Pharma. A Kekki and N Antonakopoulos reported being employees of Genesis Pharma.
Source: Sfikakis PP et al. Apremilast for biologic-naïve, peripheral psoriatic arthritis, including patients with early disease: Results from the APROACH observational prospective study. Rheumatol Int. 2023 (Mar 1). Doi: 10.1007/s00296-022-05269-z
Key clinical point: Early initiation of apremilast led to rapid and sustained improvements in psoriatic arthritis (PsA) manifestations with a consistent safety profile in a real-world cohort of biologic-naive patients who were intolerant of conventional synthetic disease-modifying antirheumatic drugs (csDMARD).
Major finding: Among patients with baseline swollen joint count (SJC) and tender joint count (TJC) of >0, significant median decreases in SJC (50% and 90%, respectively) and TJC (50% and 80%, respectively) were observed at 16 and 52 weeks (all P < .001), with 55.2% of evaluable patients achieving minimal disease activity at 52 weeks. Overall, 13.8% of patients experienced ≥1 adverse event, with all except one being non-serious.
Study details: This prospective study included 167 biologic-naive patients with early peripheral PsA and intolerance or inadequate response to csDMARD who initiated apremilast.
Disclosures: This study was funded by Genesis Pharma and Celgene. Some authors reported ties with various sources, including Genesis Pharma. A Kekki and N Antonakopoulos reported being employees of Genesis Pharma.
Source: Sfikakis PP et al. Apremilast for biologic-naïve, peripheral psoriatic arthritis, including patients with early disease: Results from the APROACH observational prospective study. Rheumatol Int. 2023 (Mar 1). Doi: 10.1007/s00296-022-05269-z


