User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
CDC panel backs COVID-19 boosters for nearly all adults
Editor’s note: This story was updated with the CDC director’s endorsement.
Centers for Disease Control and Prevention (CDC) Director Rochelle Walensky, MD, has signed off on an advisory panel’s earlier unanimous vote to recommend boosters for the Moderna and Johnson and Johnson COVID vaccines.
The decision now means that millions of Americans are eligible to get a booster shot for either the Pfizer, Moderna, or J&J COVID vaccines.
“The evidence shows that all three COVID-19 vaccines authorized in the United States are safe – as demonstrated by the over 400 million vaccine doses already given. And, they are all highly effective in reducing the risk of severe disease, hospitalization, and death, even in the midst of the widely circulating Delta variant,” Dr. Walensky said in a CDC news release.
She also signed off on the panel’s suggestion that individuals can mix or match the booster from any one of the three available COVID-19 vaccines.
The Advisory Committee on Immunization Practices (ACIP) recommended in a late afternoon 15-0 vote that everyone over age 18 who are at least 2 months past their Johnson & Johnson vaccine should get a booster, an endorsement that affects an estimated 13 million Americans.
Those eligible for a booster at least 6 months after their last Moderna shot are the same groups who can get a Pfizer booster.
They are:
- Anyone over age 65.
- Those over age 18 with an underlying health condition that puts them at risk of severe COVID-19.
- Those over age 18 who may be at higher risk of a COVID-19 infection because they live or work in a risky setting.
These recommendations are in line with the Food and Drug Administration’s Oct. 20 authorization of the boosters, along with the ability to mix-and-match vaccines.
There are an estimated 47 million Pfizer recipients and 39 million people vaccinated with Moderna who are now eligible for a booster dose, according to data presented by the CDC.
Questions, concerns
Before voting, some committee members expressed discomfort in broadly recommending boosters, stressing that there is very little evidence supporting the need for boosters in people younger than age 50.
“I can’t say that I am comfortable that anybody under 50 – an otherwise healthy individual – needs a booster vaccine at this time with either Moderna or Pfizer,” said ACIP member Sarah Long, MD, professor of pediatrics at Drexel University in Philadelphia.
She said she would try to mitigate any potential harm by having some kind of age restriction on the otherwise worried well.
“We don’t usually have the vaccines [for] the worried well. We give it because we have a need that’s worth the risk, and there’s a burden of severity of disease,” Dr. Long said.
The evidence to date shows that all the vaccines authorized for use in the U.S. continue to protect people well against severe COVID-19 outcomes, including hospitalization and death.
But breakthrough infections are on the rise, especially for people who initially received the Johnson and Johnson one-dose vaccine.
On Oct. 21, Pfizer released data from a study of more than 10,000 fully vaccinated people. Half were randomly assigned to get a booster of their Comirnaty vaccine, the other half were given a placebo.
Over the ensuing 2.5 months, there were 5 COVID-19 cases in the boosted group, and 109 in the group that got a placebo.
The data were posted in a press release and have not yet been peer reviewed, but are the first to show clinical effectiveness of boosters at preventing COVID-19 infections.
Data recently considered by the FDA and CDC for booster doses come from studies that were mostly shorter and smaller. These studies looked at biomarkers of immunity like the concentration of antibodies in a person’s blood and the percentage of study participants who saw a boost to those antibodies.
The studies demonstrated that boosters indeed restore high levels of antibodies, but unlike the newest Pfizer data they were not able to show that these antibodies prevented COVID-19.
These studies also weren’t powered to pick up on any less common safety problems that might arise after another dose of the shots.
“Real world” recommendations
In the end, however, the panel felt it was more important to be permissive in allowing boosters so that individuals and their doctors could be free to make their own decisions.
“The decision made by the FDA and the ACIP recommendations, I think, reflects the real world. The public is going to do what they feel driven to do. This at least adds a scientific review of the currently available data,” said Jay Varkey, MD, an infectious disease physician and associate professor at Emory University in Atlanta, who was not involved in the ACIP’s deliberations.
Dr. Varkey said he would recommend that anyone who is younger than 65, and who has no underlying medical conditions such as diabetes or obesity, speak with their doctor about their individual benefits and risks before getting a booster.
The CDC is planning to release a detailed suite of clinical considerations to help people weigh the risks and benefits of getting a booster.
Safety updates presented at the meeting show that serious adverse events after vaccination are extremely rare, but in some cases, they may rise above the risk for those problems generally seen in the population.
Those rare events include the disabling autoimmune condition Guillain-Barré syndrome and the platelet disorder thrombosis with thrombocytopenia (TTS), which causes blood clots along with the risk of excess bleeding because of a low platelet count.
Both can occur after the J&J vaccine. Out of 15.3 million doses of the vaccine given to date, there have been 47 cases of TTS and five deaths. These events are more common in younger women.
The mRNA vaccines, such as those from Pfizer and Moderna, can cause heart inflammation called myocarditis or pericarditis. This side effect is more common in men 18-24 years old. The reported rate of myocarditis after vaccination is 39 cases for every 1 million doses.
In voting to permit boosters, committee member Wilbur Chen, MD, professor at the University of Maryland’s Center for Vaccine Development, said he hoped boosters wouldn’t give Americans false confidence.
Dr. Chen stressed that ending the pandemic would depend on “a multilayered approach” that includes masking, social distancing, avoiding large crowds indoors, and convincing more Americans to take their first doses of the vaccines.
“We’re not just going to vaccinate ourselves out of this situation,” Dr. Chen said.
A version of this article first appeared on WebMD.com.
Editor’s note: This story was updated with the CDC director’s endorsement.
Centers for Disease Control and Prevention (CDC) Director Rochelle Walensky, MD, has signed off on an advisory panel’s earlier unanimous vote to recommend boosters for the Moderna and Johnson and Johnson COVID vaccines.
The decision now means that millions of Americans are eligible to get a booster shot for either the Pfizer, Moderna, or J&J COVID vaccines.
“The evidence shows that all three COVID-19 vaccines authorized in the United States are safe – as demonstrated by the over 400 million vaccine doses already given. And, they are all highly effective in reducing the risk of severe disease, hospitalization, and death, even in the midst of the widely circulating Delta variant,” Dr. Walensky said in a CDC news release.
She also signed off on the panel’s suggestion that individuals can mix or match the booster from any one of the three available COVID-19 vaccines.
The Advisory Committee on Immunization Practices (ACIP) recommended in a late afternoon 15-0 vote that everyone over age 18 who are at least 2 months past their Johnson & Johnson vaccine should get a booster, an endorsement that affects an estimated 13 million Americans.
Those eligible for a booster at least 6 months after their last Moderna shot are the same groups who can get a Pfizer booster.
They are:
- Anyone over age 65.
- Those over age 18 with an underlying health condition that puts them at risk of severe COVID-19.
- Those over age 18 who may be at higher risk of a COVID-19 infection because they live or work in a risky setting.
These recommendations are in line with the Food and Drug Administration’s Oct. 20 authorization of the boosters, along with the ability to mix-and-match vaccines.
There are an estimated 47 million Pfizer recipients and 39 million people vaccinated with Moderna who are now eligible for a booster dose, according to data presented by the CDC.
Questions, concerns
Before voting, some committee members expressed discomfort in broadly recommending boosters, stressing that there is very little evidence supporting the need for boosters in people younger than age 50.
“I can’t say that I am comfortable that anybody under 50 – an otherwise healthy individual – needs a booster vaccine at this time with either Moderna or Pfizer,” said ACIP member Sarah Long, MD, professor of pediatrics at Drexel University in Philadelphia.
She said she would try to mitigate any potential harm by having some kind of age restriction on the otherwise worried well.
“We don’t usually have the vaccines [for] the worried well. We give it because we have a need that’s worth the risk, and there’s a burden of severity of disease,” Dr. Long said.
The evidence to date shows that all the vaccines authorized for use in the U.S. continue to protect people well against severe COVID-19 outcomes, including hospitalization and death.
But breakthrough infections are on the rise, especially for people who initially received the Johnson and Johnson one-dose vaccine.
On Oct. 21, Pfizer released data from a study of more than 10,000 fully vaccinated people. Half were randomly assigned to get a booster of their Comirnaty vaccine, the other half were given a placebo.
Over the ensuing 2.5 months, there were 5 COVID-19 cases in the boosted group, and 109 in the group that got a placebo.
The data were posted in a press release and have not yet been peer reviewed, but are the first to show clinical effectiveness of boosters at preventing COVID-19 infections.
Data recently considered by the FDA and CDC for booster doses come from studies that were mostly shorter and smaller. These studies looked at biomarkers of immunity like the concentration of antibodies in a person’s blood and the percentage of study participants who saw a boost to those antibodies.
The studies demonstrated that boosters indeed restore high levels of antibodies, but unlike the newest Pfizer data they were not able to show that these antibodies prevented COVID-19.
These studies also weren’t powered to pick up on any less common safety problems that might arise after another dose of the shots.
“Real world” recommendations
In the end, however, the panel felt it was more important to be permissive in allowing boosters so that individuals and their doctors could be free to make their own decisions.
“The decision made by the FDA and the ACIP recommendations, I think, reflects the real world. The public is going to do what they feel driven to do. This at least adds a scientific review of the currently available data,” said Jay Varkey, MD, an infectious disease physician and associate professor at Emory University in Atlanta, who was not involved in the ACIP’s deliberations.
Dr. Varkey said he would recommend that anyone who is younger than 65, and who has no underlying medical conditions such as diabetes or obesity, speak with their doctor about their individual benefits and risks before getting a booster.
The CDC is planning to release a detailed suite of clinical considerations to help people weigh the risks and benefits of getting a booster.
Safety updates presented at the meeting show that serious adverse events after vaccination are extremely rare, but in some cases, they may rise above the risk for those problems generally seen in the population.
Those rare events include the disabling autoimmune condition Guillain-Barré syndrome and the platelet disorder thrombosis with thrombocytopenia (TTS), which causes blood clots along with the risk of excess bleeding because of a low platelet count.
Both can occur after the J&J vaccine. Out of 15.3 million doses of the vaccine given to date, there have been 47 cases of TTS and five deaths. These events are more common in younger women.
The mRNA vaccines, such as those from Pfizer and Moderna, can cause heart inflammation called myocarditis or pericarditis. This side effect is more common in men 18-24 years old. The reported rate of myocarditis after vaccination is 39 cases for every 1 million doses.
In voting to permit boosters, committee member Wilbur Chen, MD, professor at the University of Maryland’s Center for Vaccine Development, said he hoped boosters wouldn’t give Americans false confidence.
Dr. Chen stressed that ending the pandemic would depend on “a multilayered approach” that includes masking, social distancing, avoiding large crowds indoors, and convincing more Americans to take their first doses of the vaccines.
“We’re not just going to vaccinate ourselves out of this situation,” Dr. Chen said.
A version of this article first appeared on WebMD.com.
Editor’s note: This story was updated with the CDC director’s endorsement.
Centers for Disease Control and Prevention (CDC) Director Rochelle Walensky, MD, has signed off on an advisory panel’s earlier unanimous vote to recommend boosters for the Moderna and Johnson and Johnson COVID vaccines.
The decision now means that millions of Americans are eligible to get a booster shot for either the Pfizer, Moderna, or J&J COVID vaccines.
“The evidence shows that all three COVID-19 vaccines authorized in the United States are safe – as demonstrated by the over 400 million vaccine doses already given. And, they are all highly effective in reducing the risk of severe disease, hospitalization, and death, even in the midst of the widely circulating Delta variant,” Dr. Walensky said in a CDC news release.
She also signed off on the panel’s suggestion that individuals can mix or match the booster from any one of the three available COVID-19 vaccines.
The Advisory Committee on Immunization Practices (ACIP) recommended in a late afternoon 15-0 vote that everyone over age 18 who are at least 2 months past their Johnson & Johnson vaccine should get a booster, an endorsement that affects an estimated 13 million Americans.
Those eligible for a booster at least 6 months after their last Moderna shot are the same groups who can get a Pfizer booster.
They are:
- Anyone over age 65.
- Those over age 18 with an underlying health condition that puts them at risk of severe COVID-19.
- Those over age 18 who may be at higher risk of a COVID-19 infection because they live or work in a risky setting.
These recommendations are in line with the Food and Drug Administration’s Oct. 20 authorization of the boosters, along with the ability to mix-and-match vaccines.
There are an estimated 47 million Pfizer recipients and 39 million people vaccinated with Moderna who are now eligible for a booster dose, according to data presented by the CDC.
Questions, concerns
Before voting, some committee members expressed discomfort in broadly recommending boosters, stressing that there is very little evidence supporting the need for boosters in people younger than age 50.
“I can’t say that I am comfortable that anybody under 50 – an otherwise healthy individual – needs a booster vaccine at this time with either Moderna or Pfizer,” said ACIP member Sarah Long, MD, professor of pediatrics at Drexel University in Philadelphia.
She said she would try to mitigate any potential harm by having some kind of age restriction on the otherwise worried well.
“We don’t usually have the vaccines [for] the worried well. We give it because we have a need that’s worth the risk, and there’s a burden of severity of disease,” Dr. Long said.
The evidence to date shows that all the vaccines authorized for use in the U.S. continue to protect people well against severe COVID-19 outcomes, including hospitalization and death.
But breakthrough infections are on the rise, especially for people who initially received the Johnson and Johnson one-dose vaccine.
On Oct. 21, Pfizer released data from a study of more than 10,000 fully vaccinated people. Half were randomly assigned to get a booster of their Comirnaty vaccine, the other half were given a placebo.
Over the ensuing 2.5 months, there were 5 COVID-19 cases in the boosted group, and 109 in the group that got a placebo.
The data were posted in a press release and have not yet been peer reviewed, but are the first to show clinical effectiveness of boosters at preventing COVID-19 infections.
Data recently considered by the FDA and CDC for booster doses come from studies that were mostly shorter and smaller. These studies looked at biomarkers of immunity like the concentration of antibodies in a person’s blood and the percentage of study participants who saw a boost to those antibodies.
The studies demonstrated that boosters indeed restore high levels of antibodies, but unlike the newest Pfizer data they were not able to show that these antibodies prevented COVID-19.
These studies also weren’t powered to pick up on any less common safety problems that might arise after another dose of the shots.
“Real world” recommendations
In the end, however, the panel felt it was more important to be permissive in allowing boosters so that individuals and their doctors could be free to make their own decisions.
“The decision made by the FDA and the ACIP recommendations, I think, reflects the real world. The public is going to do what they feel driven to do. This at least adds a scientific review of the currently available data,” said Jay Varkey, MD, an infectious disease physician and associate professor at Emory University in Atlanta, who was not involved in the ACIP’s deliberations.
Dr. Varkey said he would recommend that anyone who is younger than 65, and who has no underlying medical conditions such as diabetes or obesity, speak with their doctor about their individual benefits and risks before getting a booster.
The CDC is planning to release a detailed suite of clinical considerations to help people weigh the risks and benefits of getting a booster.
Safety updates presented at the meeting show that serious adverse events after vaccination are extremely rare, but in some cases, they may rise above the risk for those problems generally seen in the population.
Those rare events include the disabling autoimmune condition Guillain-Barré syndrome and the platelet disorder thrombosis with thrombocytopenia (TTS), which causes blood clots along with the risk of excess bleeding because of a low platelet count.
Both can occur after the J&J vaccine. Out of 15.3 million doses of the vaccine given to date, there have been 47 cases of TTS and five deaths. These events are more common in younger women.
The mRNA vaccines, such as those from Pfizer and Moderna, can cause heart inflammation called myocarditis or pericarditis. This side effect is more common in men 18-24 years old. The reported rate of myocarditis after vaccination is 39 cases for every 1 million doses.
In voting to permit boosters, committee member Wilbur Chen, MD, professor at the University of Maryland’s Center for Vaccine Development, said he hoped boosters wouldn’t give Americans false confidence.
Dr. Chen stressed that ending the pandemic would depend on “a multilayered approach” that includes masking, social distancing, avoiding large crowds indoors, and convincing more Americans to take their first doses of the vaccines.
“We’re not just going to vaccinate ourselves out of this situation,” Dr. Chen said.
A version of this article first appeared on WebMD.com.
Sepsis multiplies in-hospital mortality risk in COPD
Although slightly fewer than 1% of hospitalizations for chronic obstructive pulmonary disease (COPD) are complicated by sepsis, this complication increases the risk for in-hospital mortality fivefold, investigators who studied a representative national sample found.
Among nearly 7 million hospitalizations in which the primary diagnosis was COPD, nearly 65,000 (0.93%) patients experienced sepsis as a complication. In all, 31% of patients with COPD and sepsis were discharged from the hospital to another care facility, and 19% of patients died in hospital, report Harshil Shah, MD, from Guthrie Corning (N.Y.) Hospital and colleagues.
“Our study highlights the need for better risk stratification in patients with COPD developing sepsis to improve the outcomes. Further studies are warranted to consider factoring some of the modifiable factors into account and to ameliorate the outcomes of sepsis during COPD hospitalizations,” Dr. Shah and colleagues write in a poster presented during the at the annual meeting of the American College of Chest Physicians, held virtually this year.
COPD has been associated with increased risk for sepsis because of the use of corticosteroids, underlying comorbidities, and, potentially, because of impaired barrier function, the authors note.
Nationwide sample
To determine the effects of sepsis and predictors of poor outcomes among patients hospitalized for COPD, the investigators used standard diagnostic codes to identify patients with a primary diagnosis of COPD from the Nationwide Inpatient Sample for the period 2007 through 2018 and sepsis from codes in secondary fields in the International Classification of Diseases (9th/10th Editions) Clinical Modification.
They identified a total of 6,940,615 hospitalizations in which the primary diagnosis was COPD; in 64,748 of those cases, sepsis was a complication.
As noted, the in-hospital death rate, one of two primary outcomes, was 19% for patients with COPD and sepsis, and the rate of discharge to other facilities was 31%.
In analysis adjusted for confounding factors, sepsis was associated with an odds ratio for mortality of 4.9 (P < .01) and an OR for discharge to a facility of 2.2 (P < .01).
With regard to trends, the investigators saw that, although the adjusted odds for in-hospital mortality remained stable over time, discharge to facilities increased significantly. In 2007, the adjusted OR was 2.2, whereas in 2018, it was 2.6 (P for trend = .02).
Predictors of in-hospital mortality among patients with sepsis included increasing age (OR, not shown), White ethnicity (OR, 1.2), treatment in the Northeast region (OR, 1.4), disseminated intravascular coagulation (OR, 3.7), pneumococcal infection (OR, 1.2), congestive heart failure (OR, 1.2), and renal failure (OR, 1.4; P < .01 for all comparisons).
Mortality risk for many patients
A COPD specialist who was not involved in the study told this news organization that sepsis is an uncommon but serious complication, not just for patients with COPD but also for those with other severe illnesses.
“Sepsis has a high risk for mortality whether a person has COPD or not,” commented David M. Mannino III MD, FCCP, FERS, professor of medicine at the University of Kentucky, Lexington, and a cofounder and co–medical director of the COPD Foundation.
“It’s not surprising that sepsis is lethal in this population; the question is, if you have COPD, are you more likely to have sepsis? And I think the answer is probably yes. The connection there is that people with COPD have a higher risk for pneumonia, and pneumonia itself is probably one of the biggest risk factors, or certainly an important risk factor, for the development of sepsis,” he said in an interview.
It would be interesting to see the relationship between sepsis and in-hospital mortality for patients with other chronic diseases or people without COPD, he said, and he would have liked to have seen more detailed information about trends over time than Dr. Shah and colleagues provided.
No funding source for the study was reported. Dr. Shah and colleagues and Dr. Mannino have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although slightly fewer than 1% of hospitalizations for chronic obstructive pulmonary disease (COPD) are complicated by sepsis, this complication increases the risk for in-hospital mortality fivefold, investigators who studied a representative national sample found.
Among nearly 7 million hospitalizations in which the primary diagnosis was COPD, nearly 65,000 (0.93%) patients experienced sepsis as a complication. In all, 31% of patients with COPD and sepsis were discharged from the hospital to another care facility, and 19% of patients died in hospital, report Harshil Shah, MD, from Guthrie Corning (N.Y.) Hospital and colleagues.
“Our study highlights the need for better risk stratification in patients with COPD developing sepsis to improve the outcomes. Further studies are warranted to consider factoring some of the modifiable factors into account and to ameliorate the outcomes of sepsis during COPD hospitalizations,” Dr. Shah and colleagues write in a poster presented during the at the annual meeting of the American College of Chest Physicians, held virtually this year.
COPD has been associated with increased risk for sepsis because of the use of corticosteroids, underlying comorbidities, and, potentially, because of impaired barrier function, the authors note.
Nationwide sample
To determine the effects of sepsis and predictors of poor outcomes among patients hospitalized for COPD, the investigators used standard diagnostic codes to identify patients with a primary diagnosis of COPD from the Nationwide Inpatient Sample for the period 2007 through 2018 and sepsis from codes in secondary fields in the International Classification of Diseases (9th/10th Editions) Clinical Modification.
They identified a total of 6,940,615 hospitalizations in which the primary diagnosis was COPD; in 64,748 of those cases, sepsis was a complication.
As noted, the in-hospital death rate, one of two primary outcomes, was 19% for patients with COPD and sepsis, and the rate of discharge to other facilities was 31%.
In analysis adjusted for confounding factors, sepsis was associated with an odds ratio for mortality of 4.9 (P < .01) and an OR for discharge to a facility of 2.2 (P < .01).
With regard to trends, the investigators saw that, although the adjusted odds for in-hospital mortality remained stable over time, discharge to facilities increased significantly. In 2007, the adjusted OR was 2.2, whereas in 2018, it was 2.6 (P for trend = .02).
Predictors of in-hospital mortality among patients with sepsis included increasing age (OR, not shown), White ethnicity (OR, 1.2), treatment in the Northeast region (OR, 1.4), disseminated intravascular coagulation (OR, 3.7), pneumococcal infection (OR, 1.2), congestive heart failure (OR, 1.2), and renal failure (OR, 1.4; P < .01 for all comparisons).
Mortality risk for many patients
A COPD specialist who was not involved in the study told this news organization that sepsis is an uncommon but serious complication, not just for patients with COPD but also for those with other severe illnesses.
“Sepsis has a high risk for mortality whether a person has COPD or not,” commented David M. Mannino III MD, FCCP, FERS, professor of medicine at the University of Kentucky, Lexington, and a cofounder and co–medical director of the COPD Foundation.
“It’s not surprising that sepsis is lethal in this population; the question is, if you have COPD, are you more likely to have sepsis? And I think the answer is probably yes. The connection there is that people with COPD have a higher risk for pneumonia, and pneumonia itself is probably one of the biggest risk factors, or certainly an important risk factor, for the development of sepsis,” he said in an interview.
It would be interesting to see the relationship between sepsis and in-hospital mortality for patients with other chronic diseases or people without COPD, he said, and he would have liked to have seen more detailed information about trends over time than Dr. Shah and colleagues provided.
No funding source for the study was reported. Dr. Shah and colleagues and Dr. Mannino have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although slightly fewer than 1% of hospitalizations for chronic obstructive pulmonary disease (COPD) are complicated by sepsis, this complication increases the risk for in-hospital mortality fivefold, investigators who studied a representative national sample found.
Among nearly 7 million hospitalizations in which the primary diagnosis was COPD, nearly 65,000 (0.93%) patients experienced sepsis as a complication. In all, 31% of patients with COPD and sepsis were discharged from the hospital to another care facility, and 19% of patients died in hospital, report Harshil Shah, MD, from Guthrie Corning (N.Y.) Hospital and colleagues.
“Our study highlights the need for better risk stratification in patients with COPD developing sepsis to improve the outcomes. Further studies are warranted to consider factoring some of the modifiable factors into account and to ameliorate the outcomes of sepsis during COPD hospitalizations,” Dr. Shah and colleagues write in a poster presented during the at the annual meeting of the American College of Chest Physicians, held virtually this year.
COPD has been associated with increased risk for sepsis because of the use of corticosteroids, underlying comorbidities, and, potentially, because of impaired barrier function, the authors note.
Nationwide sample
To determine the effects of sepsis and predictors of poor outcomes among patients hospitalized for COPD, the investigators used standard diagnostic codes to identify patients with a primary diagnosis of COPD from the Nationwide Inpatient Sample for the period 2007 through 2018 and sepsis from codes in secondary fields in the International Classification of Diseases (9th/10th Editions) Clinical Modification.
They identified a total of 6,940,615 hospitalizations in which the primary diagnosis was COPD; in 64,748 of those cases, sepsis was a complication.
As noted, the in-hospital death rate, one of two primary outcomes, was 19% for patients with COPD and sepsis, and the rate of discharge to other facilities was 31%.
In analysis adjusted for confounding factors, sepsis was associated with an odds ratio for mortality of 4.9 (P < .01) and an OR for discharge to a facility of 2.2 (P < .01).
With regard to trends, the investigators saw that, although the adjusted odds for in-hospital mortality remained stable over time, discharge to facilities increased significantly. In 2007, the adjusted OR was 2.2, whereas in 2018, it was 2.6 (P for trend = .02).
Predictors of in-hospital mortality among patients with sepsis included increasing age (OR, not shown), White ethnicity (OR, 1.2), treatment in the Northeast region (OR, 1.4), disseminated intravascular coagulation (OR, 3.7), pneumococcal infection (OR, 1.2), congestive heart failure (OR, 1.2), and renal failure (OR, 1.4; P < .01 for all comparisons).
Mortality risk for many patients
A COPD specialist who was not involved in the study told this news organization that sepsis is an uncommon but serious complication, not just for patients with COPD but also for those with other severe illnesses.
“Sepsis has a high risk for mortality whether a person has COPD or not,” commented David M. Mannino III MD, FCCP, FERS, professor of medicine at the University of Kentucky, Lexington, and a cofounder and co–medical director of the COPD Foundation.
“It’s not surprising that sepsis is lethal in this population; the question is, if you have COPD, are you more likely to have sepsis? And I think the answer is probably yes. The connection there is that people with COPD have a higher risk for pneumonia, and pneumonia itself is probably one of the biggest risk factors, or certainly an important risk factor, for the development of sepsis,” he said in an interview.
It would be interesting to see the relationship between sepsis and in-hospital mortality for patients with other chronic diseases or people without COPD, he said, and he would have liked to have seen more detailed information about trends over time than Dr. Shah and colleagues provided.
No funding source for the study was reported. Dr. Shah and colleagues and Dr. Mannino have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Comorbidities larger factor than race in COVID ICU deaths?
Racial/ethnic disparities in COVID-19 mortality rates may be related more to comorbidities than to demographics, suggest authors of a new study.
Researchers compared the length of stay in intensive care units in two suburban hospitals for patients with severe SARS-CoV-2 infections. Their study shows that although the incidence of comorbidities and rates of use of mechanical ventilation and death were higher among Black patients than among patients of other races, length of stay in the ICU was generally similar for patients of all races. The study was conducted by Tripti Kumar, DO, from Lankenau Medical Center, Wynnewood, Pennsylvania, and colleagues.
“Racial disparities are observed in the United States concerning COVID-19, and studies have discovered that minority populations are at ongoing risk for health inequity,” Dr. Kumar said in a narrated e-poster presented during the American College of Chest Physicians (CHEST) 2021 Annual Meeting.
“Primary prevention initiatives should take precedence in mitigating the effect that comorbidities have on these vulnerable populations to help reduce necessity for mechanical ventilation, hospital length of stay, and overall mortality,” she said.
Higher death rates for Black patients
At the time the study was conducted, the COVID-19 death rate in the United States had topped 500,000 (as of this writing, it stands at 726,000). Of those who died, 22.4% were Black, 18.1% were Hispanic, and 3.6% were of Asian descent. The numbers of COVID-19 diagnoses and deaths were significantly higher in U.S. counties where the proportions of Black residents were higher, the authors note.
To see whether differences in COVID-19 outcomes were reflected in ICU length of stay, the researchers conducted a retrospective chart review of data on 162 patients admitted to ICUs at Paoli Hospital and Lankenau Medical Center, both in the suburban Philadelphia town of Wynnewood.
All patients were diagnosed with COVID-19 from March through June 2020.
In all, 60% of the study population were Black, 35% were White, 3% were Asian, and 2% were Hispanic. Women composed 46% of the sample.
The average length of ICU stay, which was the primary endpoint, was similar among Black patients (15.4 days), White patients (15.5 days), and Asians (16 days). The shortest average hospital stay was among Hispanic patients, at 11.3 days.
The investigators determined that among all races, the prevalence of type 2 diabetes, obesity, hypertension, and smoking was highest among Black patients.
Overall, nearly 85% of patients required mechanical ventilation. Among the patients who required it, 86% were Black, 84% were White, 66% were Hispanic, and 75% were Asian.
Overall mortality was 62%. It was higher among Black patients, at 60%, than among White patients, at 33%. The investigators did not report mortality rates for Hispanic or Asian patients.
Missing data
Demondes Haynes, MD, FCCP, professor of medicine in the Division of Pulmonary and Critical Care and associate dean for admissions at the University of Mississippi Medical Center and School of Medicine, Jackson, who was not involved in the study, told this news organization that there are some gaps in the study that make it difficult to draw strong conclusions about the findings.
“For sure, comorbidities contribute a great deal to mortality, but is there something else going on? I think this poster is incomplete in that it cannot answer that question,” he said in an interview.
He noted that the use of retrospective rather than prospective data makes it hard to account for potential confounders.
“I agree that these findings show the potential contribution of comorbidities, but to me, this is a little incomplete to make that a definitive statement,” he said.
“I can’t argue with their recommendation for primary prevention – we definitely want to do primary prevention to decrease comorbidities. Would it decrease overall mortality? It might, it sure might, for just COVID-19 I’d say no, we need more information.”
No funding source for the study was reported. Dr. Kumar and colleagues and Dr. Haynes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Racial/ethnic disparities in COVID-19 mortality rates may be related more to comorbidities than to demographics, suggest authors of a new study.
Researchers compared the length of stay in intensive care units in two suburban hospitals for patients with severe SARS-CoV-2 infections. Their study shows that although the incidence of comorbidities and rates of use of mechanical ventilation and death were higher among Black patients than among patients of other races, length of stay in the ICU was generally similar for patients of all races. The study was conducted by Tripti Kumar, DO, from Lankenau Medical Center, Wynnewood, Pennsylvania, and colleagues.
“Racial disparities are observed in the United States concerning COVID-19, and studies have discovered that minority populations are at ongoing risk for health inequity,” Dr. Kumar said in a narrated e-poster presented during the American College of Chest Physicians (CHEST) 2021 Annual Meeting.
“Primary prevention initiatives should take precedence in mitigating the effect that comorbidities have on these vulnerable populations to help reduce necessity for mechanical ventilation, hospital length of stay, and overall mortality,” she said.
Higher death rates for Black patients
At the time the study was conducted, the COVID-19 death rate in the United States had topped 500,000 (as of this writing, it stands at 726,000). Of those who died, 22.4% were Black, 18.1% were Hispanic, and 3.6% were of Asian descent. The numbers of COVID-19 diagnoses and deaths were significantly higher in U.S. counties where the proportions of Black residents were higher, the authors note.
To see whether differences in COVID-19 outcomes were reflected in ICU length of stay, the researchers conducted a retrospective chart review of data on 162 patients admitted to ICUs at Paoli Hospital and Lankenau Medical Center, both in the suburban Philadelphia town of Wynnewood.
All patients were diagnosed with COVID-19 from March through June 2020.
In all, 60% of the study population were Black, 35% were White, 3% were Asian, and 2% were Hispanic. Women composed 46% of the sample.
The average length of ICU stay, which was the primary endpoint, was similar among Black patients (15.4 days), White patients (15.5 days), and Asians (16 days). The shortest average hospital stay was among Hispanic patients, at 11.3 days.
The investigators determined that among all races, the prevalence of type 2 diabetes, obesity, hypertension, and smoking was highest among Black patients.
Overall, nearly 85% of patients required mechanical ventilation. Among the patients who required it, 86% were Black, 84% were White, 66% were Hispanic, and 75% were Asian.
Overall mortality was 62%. It was higher among Black patients, at 60%, than among White patients, at 33%. The investigators did not report mortality rates for Hispanic or Asian patients.
Missing data
Demondes Haynes, MD, FCCP, professor of medicine in the Division of Pulmonary and Critical Care and associate dean for admissions at the University of Mississippi Medical Center and School of Medicine, Jackson, who was not involved in the study, told this news organization that there are some gaps in the study that make it difficult to draw strong conclusions about the findings.
“For sure, comorbidities contribute a great deal to mortality, but is there something else going on? I think this poster is incomplete in that it cannot answer that question,” he said in an interview.
He noted that the use of retrospective rather than prospective data makes it hard to account for potential confounders.
“I agree that these findings show the potential contribution of comorbidities, but to me, this is a little incomplete to make that a definitive statement,” he said.
“I can’t argue with their recommendation for primary prevention – we definitely want to do primary prevention to decrease comorbidities. Would it decrease overall mortality? It might, it sure might, for just COVID-19 I’d say no, we need more information.”
No funding source for the study was reported. Dr. Kumar and colleagues and Dr. Haynes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Racial/ethnic disparities in COVID-19 mortality rates may be related more to comorbidities than to demographics, suggest authors of a new study.
Researchers compared the length of stay in intensive care units in two suburban hospitals for patients with severe SARS-CoV-2 infections. Their study shows that although the incidence of comorbidities and rates of use of mechanical ventilation and death were higher among Black patients than among patients of other races, length of stay in the ICU was generally similar for patients of all races. The study was conducted by Tripti Kumar, DO, from Lankenau Medical Center, Wynnewood, Pennsylvania, and colleagues.
“Racial disparities are observed in the United States concerning COVID-19, and studies have discovered that minority populations are at ongoing risk for health inequity,” Dr. Kumar said in a narrated e-poster presented during the American College of Chest Physicians (CHEST) 2021 Annual Meeting.
“Primary prevention initiatives should take precedence in mitigating the effect that comorbidities have on these vulnerable populations to help reduce necessity for mechanical ventilation, hospital length of stay, and overall mortality,” she said.
Higher death rates for Black patients
At the time the study was conducted, the COVID-19 death rate in the United States had topped 500,000 (as of this writing, it stands at 726,000). Of those who died, 22.4% were Black, 18.1% were Hispanic, and 3.6% were of Asian descent. The numbers of COVID-19 diagnoses and deaths were significantly higher in U.S. counties where the proportions of Black residents were higher, the authors note.
To see whether differences in COVID-19 outcomes were reflected in ICU length of stay, the researchers conducted a retrospective chart review of data on 162 patients admitted to ICUs at Paoli Hospital and Lankenau Medical Center, both in the suburban Philadelphia town of Wynnewood.
All patients were diagnosed with COVID-19 from March through June 2020.
In all, 60% of the study population were Black, 35% were White, 3% were Asian, and 2% were Hispanic. Women composed 46% of the sample.
The average length of ICU stay, which was the primary endpoint, was similar among Black patients (15.4 days), White patients (15.5 days), and Asians (16 days). The shortest average hospital stay was among Hispanic patients, at 11.3 days.
The investigators determined that among all races, the prevalence of type 2 diabetes, obesity, hypertension, and smoking was highest among Black patients.
Overall, nearly 85% of patients required mechanical ventilation. Among the patients who required it, 86% were Black, 84% were White, 66% were Hispanic, and 75% were Asian.
Overall mortality was 62%. It was higher among Black patients, at 60%, than among White patients, at 33%. The investigators did not report mortality rates for Hispanic or Asian patients.
Missing data
Demondes Haynes, MD, FCCP, professor of medicine in the Division of Pulmonary and Critical Care and associate dean for admissions at the University of Mississippi Medical Center and School of Medicine, Jackson, who was not involved in the study, told this news organization that there are some gaps in the study that make it difficult to draw strong conclusions about the findings.
“For sure, comorbidities contribute a great deal to mortality, but is there something else going on? I think this poster is incomplete in that it cannot answer that question,” he said in an interview.
He noted that the use of retrospective rather than prospective data makes it hard to account for potential confounders.
“I agree that these findings show the potential contribution of comorbidities, but to me, this is a little incomplete to make that a definitive statement,” he said.
“I can’t argue with their recommendation for primary prevention – we definitely want to do primary prevention to decrease comorbidities. Would it decrease overall mortality? It might, it sure might, for just COVID-19 I’d say no, we need more information.”
No funding source for the study was reported. Dr. Kumar and colleagues and Dr. Haynes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fungal infection can mimic lung cancer metastases
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
REPORTING FROM CHEST 2021
Life-threatening paradoxical bronchospasm may escape recognition in patients with COPD or asthma
according to a researcher who reviewed spirometry test results from U.S. military veterans.
Nearly 1.5% of the tests met the criteria for paradoxical bronchospasm, which refers to airway constriction that may rapidly occur after inhalation of a short-acting beta2 agonist (SABA) such as albuterol.
However, none of those reports alluded to paradoxical bronchospasm, said investigator Malvika Kaul, MD, fellow in the department of pulmonary and critical care at the University of Illinois at Chicago and the Jesse Brown Veterans Affairs Medical Center, also in Chicago.
“Paradoxical bronchospasm was neither recognized nor reported in any spirometry test results,” Dr. Kaul said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
By recognizing paradoxical bronchospasm, health care providers could address its clinical implications and identify potential alternative management options, according to Dr. Kaul.
“We hope in the future, education of clinicians about this phenomena is emphasized,” Dr. Kaul said in her presentation.
Recognizing paradoxical bronchospasm
In an interview, Dr. Kaul said she began researching paradoxical bronchospasm after encountering a patient who had an acute reaction to albuterol during a pulmonary function test.
“I was not taught about it, and I wasn’t recognizing that pattern very frequently in my patients,” she said.
Prescribing information for Food and Drug Administration–approved SABAs include a warning that life-threatening paradoxical bronchospasm may occur, said Dr. Kaul.
If paradoxical bronchospasm occurs, the patient should discontinue the medication immediately and start on alternative therapy, according to the available prescribing information for albuterol sulfate.
Paradoxical bronchospasm has been linked to worsened respiratory outcomes, including more frequent exacerbations, in patients with obstructive lung diseases, according to Dr. Kaul.
Two previous large studies pegged the prevalence of paradoxical bronchospasm at around 4.5% in patients with COPD or asthma, but “it has not been reported or addressed in high-risk population, such as veterans who have high prevalence of obstructive lung diseases like COPD,” Dr. Kaul said.
Latest study results
Dr. Kaul described a retrospective analysis of 1,150 pre- and postbronchodilator spirometry tests conducted in patients with COPD or asthma at the Jesse Brown VA Medical Center between 2017 and 2020.
A positive paradoxical bronchodilator response was defined as a decrease of least 12% and 200 mL in forced expiratory volume in 1 second and forced vital capacity from baseline after four puffs of albuterol were inhaled, Dr. Kaul said.
Out of 18 reviewed spirometry results that met the criteria, none of the test results reported or recognized paradoxical bronchospasm, according to Dr. Kaul.
Those meeting the criteria were predominantly COPD patients, according to Dr. Kaul, who said 12 had an underlying diagnosis COPD, 4 had asthma, and 2 had COPD and asthma.
Of the 18 patients, 13 were African American, and all but 1 of the 18 patients had a current or past smoking history, according to reported data.
A history of obstructive sleep apnea was reported in nine patients, and history of gastroesophageal reflux disease was also reported in nine patients. Eleven patients had emphysema.
Greater awareness needed
Results of this study emphasize the need to recognize potential cases paradoxical bronchospasm in clinical practice, as well as a need for more research, according to Allen J. Blaivas, DO, FCCP, chair of the CHEST Airway Disorders NetWork.
“It’s something to be on the alert for, and certainly be aware that, if your patient is telling you that they feel worse, we shouldn’t just pooh-pooh it,” said Dr. Blaivas, who is medical director of the intensive care unit at the East Orange campus of the VA New Jersey Health Care System.
Further research could focus on breaking down whether patients with suspected paradoxical bronchospasm are using metered-dose inhalers or nebulizers, whether or not they are also taking inhaled corticosteroids, and whether prospective testing can confirm paradoxical bronchospasm in patients who report tightness after using a SABA, he said in an interview.
Dr. Kaul and coauthor Israel Rubinstein, MD had no relevant relationships to disclose. Dr. Blaivas had no relevant relationships to disclose.
according to a researcher who reviewed spirometry test results from U.S. military veterans.
Nearly 1.5% of the tests met the criteria for paradoxical bronchospasm, which refers to airway constriction that may rapidly occur after inhalation of a short-acting beta2 agonist (SABA) such as albuterol.
However, none of those reports alluded to paradoxical bronchospasm, said investigator Malvika Kaul, MD, fellow in the department of pulmonary and critical care at the University of Illinois at Chicago and the Jesse Brown Veterans Affairs Medical Center, also in Chicago.
“Paradoxical bronchospasm was neither recognized nor reported in any spirometry test results,” Dr. Kaul said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
By recognizing paradoxical bronchospasm, health care providers could address its clinical implications and identify potential alternative management options, according to Dr. Kaul.
“We hope in the future, education of clinicians about this phenomena is emphasized,” Dr. Kaul said in her presentation.
Recognizing paradoxical bronchospasm
In an interview, Dr. Kaul said she began researching paradoxical bronchospasm after encountering a patient who had an acute reaction to albuterol during a pulmonary function test.
“I was not taught about it, and I wasn’t recognizing that pattern very frequently in my patients,” she said.
Prescribing information for Food and Drug Administration–approved SABAs include a warning that life-threatening paradoxical bronchospasm may occur, said Dr. Kaul.
If paradoxical bronchospasm occurs, the patient should discontinue the medication immediately and start on alternative therapy, according to the available prescribing information for albuterol sulfate.
Paradoxical bronchospasm has been linked to worsened respiratory outcomes, including more frequent exacerbations, in patients with obstructive lung diseases, according to Dr. Kaul.
Two previous large studies pegged the prevalence of paradoxical bronchospasm at around 4.5% in patients with COPD or asthma, but “it has not been reported or addressed in high-risk population, such as veterans who have high prevalence of obstructive lung diseases like COPD,” Dr. Kaul said.
Latest study results
Dr. Kaul described a retrospective analysis of 1,150 pre- and postbronchodilator spirometry tests conducted in patients with COPD or asthma at the Jesse Brown VA Medical Center between 2017 and 2020.
A positive paradoxical bronchodilator response was defined as a decrease of least 12% and 200 mL in forced expiratory volume in 1 second and forced vital capacity from baseline after four puffs of albuterol were inhaled, Dr. Kaul said.
Out of 18 reviewed spirometry results that met the criteria, none of the test results reported or recognized paradoxical bronchospasm, according to Dr. Kaul.
Those meeting the criteria were predominantly COPD patients, according to Dr. Kaul, who said 12 had an underlying diagnosis COPD, 4 had asthma, and 2 had COPD and asthma.
Of the 18 patients, 13 were African American, and all but 1 of the 18 patients had a current or past smoking history, according to reported data.
A history of obstructive sleep apnea was reported in nine patients, and history of gastroesophageal reflux disease was also reported in nine patients. Eleven patients had emphysema.
Greater awareness needed
Results of this study emphasize the need to recognize potential cases paradoxical bronchospasm in clinical practice, as well as a need for more research, according to Allen J. Blaivas, DO, FCCP, chair of the CHEST Airway Disorders NetWork.
“It’s something to be on the alert for, and certainly be aware that, if your patient is telling you that they feel worse, we shouldn’t just pooh-pooh it,” said Dr. Blaivas, who is medical director of the intensive care unit at the East Orange campus of the VA New Jersey Health Care System.
Further research could focus on breaking down whether patients with suspected paradoxical bronchospasm are using metered-dose inhalers or nebulizers, whether or not they are also taking inhaled corticosteroids, and whether prospective testing can confirm paradoxical bronchospasm in patients who report tightness after using a SABA, he said in an interview.
Dr. Kaul and coauthor Israel Rubinstein, MD had no relevant relationships to disclose. Dr. Blaivas had no relevant relationships to disclose.
according to a researcher who reviewed spirometry test results from U.S. military veterans.
Nearly 1.5% of the tests met the criteria for paradoxical bronchospasm, which refers to airway constriction that may rapidly occur after inhalation of a short-acting beta2 agonist (SABA) such as albuterol.
However, none of those reports alluded to paradoxical bronchospasm, said investigator Malvika Kaul, MD, fellow in the department of pulmonary and critical care at the University of Illinois at Chicago and the Jesse Brown Veterans Affairs Medical Center, also in Chicago.
“Paradoxical bronchospasm was neither recognized nor reported in any spirometry test results,” Dr. Kaul said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
By recognizing paradoxical bronchospasm, health care providers could address its clinical implications and identify potential alternative management options, according to Dr. Kaul.
“We hope in the future, education of clinicians about this phenomena is emphasized,” Dr. Kaul said in her presentation.
Recognizing paradoxical bronchospasm
In an interview, Dr. Kaul said she began researching paradoxical bronchospasm after encountering a patient who had an acute reaction to albuterol during a pulmonary function test.
“I was not taught about it, and I wasn’t recognizing that pattern very frequently in my patients,” she said.
Prescribing information for Food and Drug Administration–approved SABAs include a warning that life-threatening paradoxical bronchospasm may occur, said Dr. Kaul.
If paradoxical bronchospasm occurs, the patient should discontinue the medication immediately and start on alternative therapy, according to the available prescribing information for albuterol sulfate.
Paradoxical bronchospasm has been linked to worsened respiratory outcomes, including more frequent exacerbations, in patients with obstructive lung diseases, according to Dr. Kaul.
Two previous large studies pegged the prevalence of paradoxical bronchospasm at around 4.5% in patients with COPD or asthma, but “it has not been reported or addressed in high-risk population, such as veterans who have high prevalence of obstructive lung diseases like COPD,” Dr. Kaul said.
Latest study results
Dr. Kaul described a retrospective analysis of 1,150 pre- and postbronchodilator spirometry tests conducted in patients with COPD or asthma at the Jesse Brown VA Medical Center between 2017 and 2020.
A positive paradoxical bronchodilator response was defined as a decrease of least 12% and 200 mL in forced expiratory volume in 1 second and forced vital capacity from baseline after four puffs of albuterol were inhaled, Dr. Kaul said.
Out of 18 reviewed spirometry results that met the criteria, none of the test results reported or recognized paradoxical bronchospasm, according to Dr. Kaul.
Those meeting the criteria were predominantly COPD patients, according to Dr. Kaul, who said 12 had an underlying diagnosis COPD, 4 had asthma, and 2 had COPD and asthma.
Of the 18 patients, 13 were African American, and all but 1 of the 18 patients had a current or past smoking history, according to reported data.
A history of obstructive sleep apnea was reported in nine patients, and history of gastroesophageal reflux disease was also reported in nine patients. Eleven patients had emphysema.
Greater awareness needed
Results of this study emphasize the need to recognize potential cases paradoxical bronchospasm in clinical practice, as well as a need for more research, according to Allen J. Blaivas, DO, FCCP, chair of the CHEST Airway Disorders NetWork.
“It’s something to be on the alert for, and certainly be aware that, if your patient is telling you that they feel worse, we shouldn’t just pooh-pooh it,” said Dr. Blaivas, who is medical director of the intensive care unit at the East Orange campus of the VA New Jersey Health Care System.
Further research could focus on breaking down whether patients with suspected paradoxical bronchospasm are using metered-dose inhalers or nebulizers, whether or not they are also taking inhaled corticosteroids, and whether prospective testing can confirm paradoxical bronchospasm in patients who report tightness after using a SABA, he said in an interview.
Dr. Kaul and coauthor Israel Rubinstein, MD had no relevant relationships to disclose. Dr. Blaivas had no relevant relationships to disclose.
FROM CHEST 2021
Dupilumab-improved lung function lasts in children with moderate to severe asthma
Add-on treatment with dupilumab may improve lung function in children aged 6-11 years with uncontrolled moderate to severe type 2 inflammatory asthma, results from a randomized, placebo-controlled, phase 3 study show.
Improvements in lung function parameters were observed as early as 2 weeks and persisted over the 52-week treatment period among children in the LIBERTY ASTHMA VOYAGE study, according to investigator Leonard B. Bacharier, MD, of Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center, Nashville, Tenn.
“Dupilumab led to clinically meaningful rapid and sustained improvements in lung function parameters,” Dr. Bacharier said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
The improvements in forced expiratory volume in 1 second (FEV1) and other measures reported for children with moderate to severe asthma who have the type 2 phenotype, which is the most common driver of pediatric asthma, according to Dr. Bacharier.
“Many children with moderate to severe asthma have abnormal lung function, and this can be a risk factor for future lung disease in adulthood,” Dr. Bacharier said in his presentation.
The VOYAGE continues
The findings presented at the meeting build on another report earlier this year from the LIBERTY ASTHMA VOYAGE study demonstrating that add-on dupilumab treatment led to a significant improvement versus placebo in FEV1 up to 12 weeks.
“We now have a long term data on this drug as well, showing its efficacy over a period of time,” said Muhammad Adrish, MD, MBA, FCCP, associate professor of pulmonary, critical care and sleep medicine at Baylor College of Medicine, Houston.
“I think that’s pretty exciting, and that’s another step towards precision medicine in treatment of asthma,” Dr. Adrish, who is Vice-Chair of CHEST’s Airways Disorders NetWork Steering Committee and was not involved in the study.
Dupilumab received Food and Drug Administration approval in 2018 as add-on maintenance therapy for the treatment of patients aged 12 years or older with moderate to severe asthma that has an eosinophilic phenotype or that is dependent on oral corticosteroid treatment.
In March 2021, Sanofi and Regeneron announced that the FDA had accepted for review a supplemental Biologics License Application for dupilumab as an add-on treatment in children aged 6-11 years with uncontrolled moderate to severe asthma.
That sBLA is supported by data from the LIBERTY ASTHMA VOYAGE study, Sanofi and Regeneron said.
In results of the phase 3 study that Dr. Bacharier presented in May at the American Thoracic Society International Conference, add-on dupilumab dosed every 2 weeks significant improved percent predicted prebronchodilator FEV1 by an additional 5.21 percentage points versus placebo at week 12.
Dupilumab and the type 2 phenotype
The new data reported at the CHEST meeting come from a prespecified analysis evaluating the impact of dupilumab on lung function over a 52-week treatment period in patients with a T2 inflammatory asthma phenotype.
“Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for interleukin-4 and -13, key and central drivers of T2 inflammation in multiple diseases,” Dr. Bacharier and coinvestigators reported in their study abstract.
Of 408 patients in the study, 350 met the T2 phenotype criteria, including 236 in the dupilumab arm and 114 in the placebo arm.
Patients met T2 phenotype criteria if they had blood eosinophils of at least 150 cells/mcL or fractional exhaled nitric oxide FeNO of at least 20 parts per billion at baseline, investigators said.
Dr. Bacharier and coinvestigators reported on several different endpoints, including absolute and percent predicted prebronchodilator FEV1, percent predicted postbronchodilator FEV1, prebronchodilator forced expiratory flow at 25%-75% of pulmonary volume (FEF25%-75%), and forced vital capacity (FVC).
Dupilumab, when compared with placebo, significantly improved prebronchodilator FEV1 in pediatric patients with uncontrolled moderate to severe type 2 asthma, according to Dr. Bacharier.
“Patients receiving dupilumab experienced rapid improvements by week 2, and this was sustained for up to 52 weeks,” he said.
The prebronchodilator FEV1 improved from baseline for dupilumab versus placebo, with a least squares mean difference of 0.06 L at week 2, which reached 0.17 L by week 52, according to their data. Similarly, postbronchodilator FEV1 improved from baseline for dupilumab, with a least square mean difference versus placebo of 0.09 L at week 52.
Dupilumab compared to placebo also significantly improved percent predicted FEF25%-75%, and percent predicted FVC over the 52-week treatment period, according to Dr. Bacharier.
“Dupilumab led to significant, rapid, and sustained improvements in multiple aspects of lung function in children aged 6-11 years,” Dr. Bacharier added in a CHEST press release that described the findings.
The LIBERTY ASTHMA VOYAGE study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Bacharier provided disclosures related to AstraZeneca, GlaxoSmithKline, Regeneron Pharmaceuticals, Sanofi, CF Foundation, DBV Technologies, NIH, and Vectura.
Add-on treatment with dupilumab may improve lung function in children aged 6-11 years with uncontrolled moderate to severe type 2 inflammatory asthma, results from a randomized, placebo-controlled, phase 3 study show.
Improvements in lung function parameters were observed as early as 2 weeks and persisted over the 52-week treatment period among children in the LIBERTY ASTHMA VOYAGE study, according to investigator Leonard B. Bacharier, MD, of Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center, Nashville, Tenn.
“Dupilumab led to clinically meaningful rapid and sustained improvements in lung function parameters,” Dr. Bacharier said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
The improvements in forced expiratory volume in 1 second (FEV1) and other measures reported for children with moderate to severe asthma who have the type 2 phenotype, which is the most common driver of pediatric asthma, according to Dr. Bacharier.
“Many children with moderate to severe asthma have abnormal lung function, and this can be a risk factor for future lung disease in adulthood,” Dr. Bacharier said in his presentation.
The VOYAGE continues
The findings presented at the meeting build on another report earlier this year from the LIBERTY ASTHMA VOYAGE study demonstrating that add-on dupilumab treatment led to a significant improvement versus placebo in FEV1 up to 12 weeks.
“We now have a long term data on this drug as well, showing its efficacy over a period of time,” said Muhammad Adrish, MD, MBA, FCCP, associate professor of pulmonary, critical care and sleep medicine at Baylor College of Medicine, Houston.
“I think that’s pretty exciting, and that’s another step towards precision medicine in treatment of asthma,” Dr. Adrish, who is Vice-Chair of CHEST’s Airways Disorders NetWork Steering Committee and was not involved in the study.
Dupilumab received Food and Drug Administration approval in 2018 as add-on maintenance therapy for the treatment of patients aged 12 years or older with moderate to severe asthma that has an eosinophilic phenotype or that is dependent on oral corticosteroid treatment.
In March 2021, Sanofi and Regeneron announced that the FDA had accepted for review a supplemental Biologics License Application for dupilumab as an add-on treatment in children aged 6-11 years with uncontrolled moderate to severe asthma.
That sBLA is supported by data from the LIBERTY ASTHMA VOYAGE study, Sanofi and Regeneron said.
In results of the phase 3 study that Dr. Bacharier presented in May at the American Thoracic Society International Conference, add-on dupilumab dosed every 2 weeks significant improved percent predicted prebronchodilator FEV1 by an additional 5.21 percentage points versus placebo at week 12.
Dupilumab and the type 2 phenotype
The new data reported at the CHEST meeting come from a prespecified analysis evaluating the impact of dupilumab on lung function over a 52-week treatment period in patients with a T2 inflammatory asthma phenotype.
“Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for interleukin-4 and -13, key and central drivers of T2 inflammation in multiple diseases,” Dr. Bacharier and coinvestigators reported in their study abstract.
Of 408 patients in the study, 350 met the T2 phenotype criteria, including 236 in the dupilumab arm and 114 in the placebo arm.
Patients met T2 phenotype criteria if they had blood eosinophils of at least 150 cells/mcL or fractional exhaled nitric oxide FeNO of at least 20 parts per billion at baseline, investigators said.
Dr. Bacharier and coinvestigators reported on several different endpoints, including absolute and percent predicted prebronchodilator FEV1, percent predicted postbronchodilator FEV1, prebronchodilator forced expiratory flow at 25%-75% of pulmonary volume (FEF25%-75%), and forced vital capacity (FVC).
Dupilumab, when compared with placebo, significantly improved prebronchodilator FEV1 in pediatric patients with uncontrolled moderate to severe type 2 asthma, according to Dr. Bacharier.
“Patients receiving dupilumab experienced rapid improvements by week 2, and this was sustained for up to 52 weeks,” he said.
The prebronchodilator FEV1 improved from baseline for dupilumab versus placebo, with a least squares mean difference of 0.06 L at week 2, which reached 0.17 L by week 52, according to their data. Similarly, postbronchodilator FEV1 improved from baseline for dupilumab, with a least square mean difference versus placebo of 0.09 L at week 52.
Dupilumab compared to placebo also significantly improved percent predicted FEF25%-75%, and percent predicted FVC over the 52-week treatment period, according to Dr. Bacharier.
“Dupilumab led to significant, rapid, and sustained improvements in multiple aspects of lung function in children aged 6-11 years,” Dr. Bacharier added in a CHEST press release that described the findings.
The LIBERTY ASTHMA VOYAGE study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Bacharier provided disclosures related to AstraZeneca, GlaxoSmithKline, Regeneron Pharmaceuticals, Sanofi, CF Foundation, DBV Technologies, NIH, and Vectura.
Add-on treatment with dupilumab may improve lung function in children aged 6-11 years with uncontrolled moderate to severe type 2 inflammatory asthma, results from a randomized, placebo-controlled, phase 3 study show.
Improvements in lung function parameters were observed as early as 2 weeks and persisted over the 52-week treatment period among children in the LIBERTY ASTHMA VOYAGE study, according to investigator Leonard B. Bacharier, MD, of Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center, Nashville, Tenn.
“Dupilumab led to clinically meaningful rapid and sustained improvements in lung function parameters,” Dr. Bacharier said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
The improvements in forced expiratory volume in 1 second (FEV1) and other measures reported for children with moderate to severe asthma who have the type 2 phenotype, which is the most common driver of pediatric asthma, according to Dr. Bacharier.
“Many children with moderate to severe asthma have abnormal lung function, and this can be a risk factor for future lung disease in adulthood,” Dr. Bacharier said in his presentation.
The VOYAGE continues
The findings presented at the meeting build on another report earlier this year from the LIBERTY ASTHMA VOYAGE study demonstrating that add-on dupilumab treatment led to a significant improvement versus placebo in FEV1 up to 12 weeks.
“We now have a long term data on this drug as well, showing its efficacy over a period of time,” said Muhammad Adrish, MD, MBA, FCCP, associate professor of pulmonary, critical care and sleep medicine at Baylor College of Medicine, Houston.
“I think that’s pretty exciting, and that’s another step towards precision medicine in treatment of asthma,” Dr. Adrish, who is Vice-Chair of CHEST’s Airways Disorders NetWork Steering Committee and was not involved in the study.
Dupilumab received Food and Drug Administration approval in 2018 as add-on maintenance therapy for the treatment of patients aged 12 years or older with moderate to severe asthma that has an eosinophilic phenotype or that is dependent on oral corticosteroid treatment.
In March 2021, Sanofi and Regeneron announced that the FDA had accepted for review a supplemental Biologics License Application for dupilumab as an add-on treatment in children aged 6-11 years with uncontrolled moderate to severe asthma.
That sBLA is supported by data from the LIBERTY ASTHMA VOYAGE study, Sanofi and Regeneron said.
In results of the phase 3 study that Dr. Bacharier presented in May at the American Thoracic Society International Conference, add-on dupilumab dosed every 2 weeks significant improved percent predicted prebronchodilator FEV1 by an additional 5.21 percentage points versus placebo at week 12.
Dupilumab and the type 2 phenotype
The new data reported at the CHEST meeting come from a prespecified analysis evaluating the impact of dupilumab on lung function over a 52-week treatment period in patients with a T2 inflammatory asthma phenotype.
“Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for interleukin-4 and -13, key and central drivers of T2 inflammation in multiple diseases,” Dr. Bacharier and coinvestigators reported in their study abstract.
Of 408 patients in the study, 350 met the T2 phenotype criteria, including 236 in the dupilumab arm and 114 in the placebo arm.
Patients met T2 phenotype criteria if they had blood eosinophils of at least 150 cells/mcL or fractional exhaled nitric oxide FeNO of at least 20 parts per billion at baseline, investigators said.
Dr. Bacharier and coinvestigators reported on several different endpoints, including absolute and percent predicted prebronchodilator FEV1, percent predicted postbronchodilator FEV1, prebronchodilator forced expiratory flow at 25%-75% of pulmonary volume (FEF25%-75%), and forced vital capacity (FVC).
Dupilumab, when compared with placebo, significantly improved prebronchodilator FEV1 in pediatric patients with uncontrolled moderate to severe type 2 asthma, according to Dr. Bacharier.
“Patients receiving dupilumab experienced rapid improvements by week 2, and this was sustained for up to 52 weeks,” he said.
The prebronchodilator FEV1 improved from baseline for dupilumab versus placebo, with a least squares mean difference of 0.06 L at week 2, which reached 0.17 L by week 52, according to their data. Similarly, postbronchodilator FEV1 improved from baseline for dupilumab, with a least square mean difference versus placebo of 0.09 L at week 52.
Dupilumab compared to placebo also significantly improved percent predicted FEF25%-75%, and percent predicted FVC over the 52-week treatment period, according to Dr. Bacharier.
“Dupilumab led to significant, rapid, and sustained improvements in multiple aspects of lung function in children aged 6-11 years,” Dr. Bacharier added in a CHEST press release that described the findings.
The LIBERTY ASTHMA VOYAGE study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Bacharier provided disclosures related to AstraZeneca, GlaxoSmithKline, Regeneron Pharmaceuticals, Sanofi, CF Foundation, DBV Technologies, NIH, and Vectura.
FROM CHEST 2021
New York’s largest health care provider fires 1,400 unvaccinated employees
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
Children and COVID: Decline of summer surge continues
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
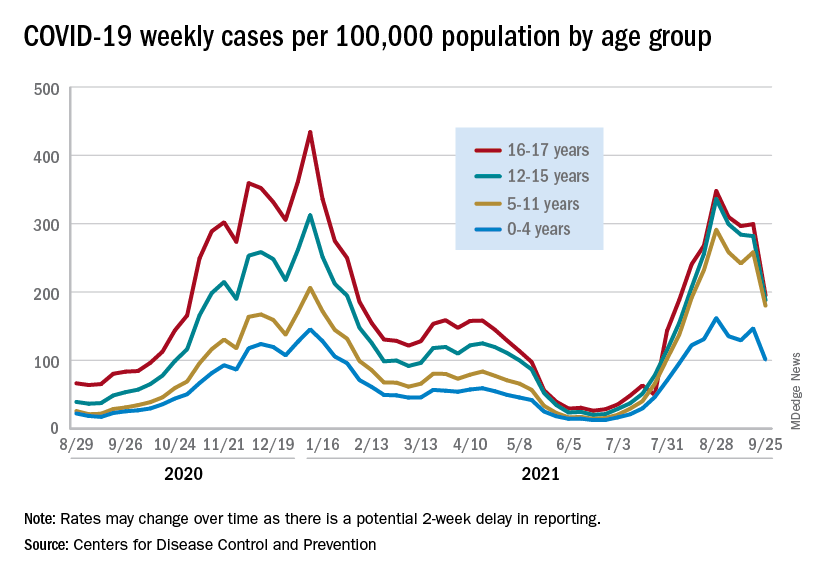
Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
Johnson & Johnson requests FDA approval for vaccine booster doses
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
Pfizer COVID vaccine antibodies may disappear in 7 months, study says
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.



