User login
Genitourinary manifestations of sickle cell disease
Sickle cell disease is a common genetic disorder in the United States that disproportionately affects people of African ancestry. The characteristic sickling of red blood cells under conditions of reduced oxygen tension leads to intravascular hemolysis and vaso-occlusive events, which in turn cause tissue ischemia-reperfusion injury affecting multiple organs, including the genitourinary system.1–3
In this paper, we review the genitourinary effects of sickle cell disease, focusing on sickle cell nephropathy, priapism, and renal medullary carcinoma.
THE WIDE-RANGING EFFECTS OF SICKLE CELL DISEASE
In the United States, sickle cell disease affects 1 of every 500 blacks and 1 of every 36,000 Hispanics.1 The term describes hemoglobinopathies associated with sickling of red blood cells.
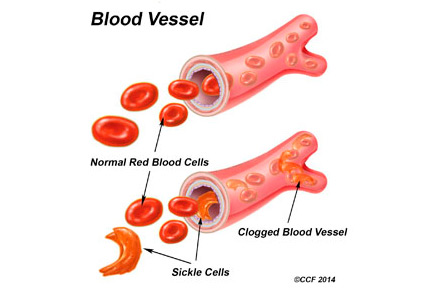
Sickling of red blood cells results from a single base-pair change in the beta-globin gene from glutamic acid to valine at position 6, causing abnormal hemoglobin (hemoglobin S), which polymerizes under conditions of reduced oxygen tension and alters the biconcave disk shape into a rigid, irregular, unstable cell. The sickle-shaped cells are prone to intravascular hemolysis,2 causing intermittent vaso-occlusive events that result in tissue ischemia-reperfusion injury. Genitourinary problems include impaired ability to concentrate urine, hematuria, renal medullary carcinoma, and increased frequency of urinary tract infection.
SICKLE CELL NEPHROPATHY
The kidney is one of the most frequently affected organs in sickle cell disease. Renal manifestations begin to appear in early childhood, with impaired medullary concentrating ability and ischemic damage to the tubular cells caused by sickling within the vasa recta renis precipitated by the acidic, hypoxic, and hypertonic environment in the renal medulla.
As in early diabetic nephropathy, renal blood flow is enhanced and the glomerular filtration rate (GFR) is increased. Increased cardiac output as a result of anemia, localized release of prostaglandins, and a hypoxia-induced increase in nitric oxide synthesis all play a role in the increase in GFR.4,5
Oxidative stress, an increase in markers of inflammation, and local activation of the renin-angiotensin system contribute to renal damage in sickle cell disease.5–7 The resulting hyperfiltration injury leads to microalbuminuria, which occurs in 20% to 40% of children with sickle cell anemia8,9 and in as many as 60% of adults.
The glomerular lesions associated with sickle cell disease vary from glomerulopathy in the early stages to secondary focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, and glomerular thrombotic microangiopathy.10
Clinical presentations and workup
Clinical presentations are not limited to glomerular disease but include hyperchloremic metabolic acidosis and hyperkalemia resulting from defects in potassium secretion and renal acidification.
Hyperphosphatemia—a result of increased reabsorption of phosphorus, increased secretion of uric acid, and increased creatinine clearance—is seen in patients with sickle cell disease.11,12 About 10% of patients can develop an acute kidney injury as a result of volume depletion, rhabdomyolysis, renal vein thrombosis, papillary necrosis, and urinary tract obstruction secondary to blood clots.11,13
Up to 30% of adult patients with sickle cell disease develop chronic kidney disease. Predictors include severe anemia, hypertension, proteinuria, nephrotic syndrome, and microscopic hematuria.14 From 4% to 12% of patients go on to develop end-stage renal disease, but with a 1-year mortality rate three times higher than in patients without sickle cell disease.15
In general, patients with sickle cell anemia have blood pressures below those of age- and sex-matched individuals, but elevated blood pressure and low GFR are not uncommon in affected children. In a cohort of 48 children ages 3 to 18, 8.3% had an estimated GFR less than 90 mL/min/1.73 m2, and 16.7% had elevated blood pressure (prehypertension and hypertension).16
In patients with sickle cell disease, evaluation of proteinuria, hematuria, hypertension, and renal failure should take into consideration the unique renal physiologic and pathologic processes involved. Recent evidence17,18 suggests that the Chronic Kidney Disease Epidemiology Collaboration equation provides a better estimate of GFR than the Modification of Diet in Renal Disease and Cockcroft-Gault equations, although all three creatinine-based methods overestimate GFR in patients with sickle cell disease when compared with GFR measured with technetium-99m-labeled diethylenetriamine penta-acetic acid renal scanning.
Treatment options
Treatment of sickle cell nephropathy includes adequate fluid intake (given the loss of concentrating ability), adequate blood pressure control, use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) in patients who have microalbuminuria or proteinuria (or both)9,11,19 and hydroxyurea. Treatment with enalapril has been shown to decrease proteinuria in patients with sickle cell nephropathy.9 In a cohort of children with sickle cell disease, four of nine patients treated with an ACE inhibitor developed hyperkalemia, leading to discontinuation of the drug in three patients.9
ACE inhibitors and ARBs must be used cautiously in these patients because they have defects in potassium secretion. Hydroxyurea has also been shown to decrease hyperfiltration and microalbuminuria in recent studies,20,21 and this could protect against the development of overt nephropathy.
Higher mortality rates have been reported in patients with sickle cell disease who developed end-stage renal disease than in patients with end-stage renal disease without sickle cell disease. Sickle cell disease also increases the risk of pulmonary hypertension and the vaso-occlusive complication known as acute chest syndrome, contributing to increased mortality rates. Of note, in a study that looked at the association between mortality rates and pre-end-stage care of renal disease using data from the Centers for Medicare and Medicaid Services, patients with sickle cell disease who had had predialysis nephrology care had lower mortality rates.15
Treatments for end-stage renal disease are also effective in patients with sickle cell disease and include hemodialysis, peritoneal dialysis, and renal transplantation. Data from the Organ Procurement and Transplantation Network and the United Network for Organ Sharing show that from 2000 to 2011, African American kidney recipients with sickle cell disease had better survival rates than patients who had undergone transplantation from 1988 to 1999, although rates of long-term survival and graft survival were lower than in transplant recipients with other diagnoses.22
It is important to note that complications as a result of vaso-occlusive events and thrombosis can lead to graft loss; therefore, sickle cell crisis after transplantation requires careful management.
Take-home messages
- Loss of urine-concentrating ability and hyperfiltration are the earliest pathologic changes in sickle cell disease.
- Microalbuminuria as seen in diabetic nephropathy is the earliest manifestation of sickle cell nephropathy, and the prevalence increases as these patients get older and live longer.
- ACE inhibitors or ARBs should be used with caution, given the heightened risk of hyperkalemia in sickle cell disease.
- Recent results with hydroxyurea in decreasing hyperfiltration and microalbuminuria are encouraging.
- Early referral for predialysis nephrologic care is needed in sickle cell patients with chronic kidney disease.
PRIAPISM IN SICKLE CELL DISEASE
Priapism was formerly defined as a full, painful erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm. But priapism is now recognized as two separate disorders—ischemic (veno-occlusive, low-flow) priapism and nonischemic (arterial, high-flow) priapism. The new definition includes both disorders: ie, a full or partial erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm.
Ischemic priapism
Hematologic disorders are major contributors to ischemic priapism and include sickle cell disease, multiple myeloma, fat emboli (hyperalimentation),23 glucose-6-phosphate dehydrogenase deficiency, and hemoglobin Olmsted variant.24
Ischemic priapism is often seen in sickle cell disease and is considered an emergency. It is characterized by an abnormally rigid erection not involving the glans penis. Entrapment of blood in the corpora cavernosa leads to hypoxia, hypercarbia, and acidosis, which in turn leads to a painful compartment syndrome that, if untreated, results in smooth muscle necrosis and subsequent fibrosis. The results are a smaller penis and erectile dysfunction that is unresponsive to any treatment other than implantation of a penile prosthesis. However, scarring of the corpora cavernosa can make this procedure exceedingly difficult, requiring advanced techniques such as corporeal excavation.25
Men with a subtype of ischemic priapism called “stuttering” priapism26 suffer recurrent prolonged erections during sleep. The patient awakens with a painful erection that usually subsides, but sometimes only after several hours. Patients with this disorder suffer from sleep deprivation. Stuttering priapism may lead to full-blown ischemic priapism that does not resolve without intervention.
Nonischemic priapism
In nonischemic priapism, the corpora are engorged but not rigid. The condition results from unregulated arterial inflow and thus is not painful and does not result in damage to the corporeal smooth muscle.
Most cases of nonischemic priapism follow blunt perineal trauma or trauma associated with needle insertion into the corpora. This form of priapism is not associated with sickle cell disease. Because tissue damage does not occur, nonischemic or arterial priapism is not considered an emergency.
Treatment guidelines
Differentiating ischemic from nonischemic priapism is usually straightforward, based on the history, physical examination, corporeal blood gases, and duplex ultrasonography.27
Ischemic priapism is an emergency. After needle aspiration of blood from the corpora cavernosa, phenylephrine is diluted with normal saline to a concentration of 100 to 500 µg/mL and is injected in 1-mL amounts repeatedly at 3- to 5-minute intervals until the erection subsides or until a 1-hour time limit is reached. Blood pressure and pulse are monitored during these injections. If aspiration and phenylephrine irrigation fail, surgical shunting is performed.27
Measures to treat sickle cell disease (hydration, oxygen, exchange transfusions) may be employed simultaneously but should never delay aspiration and phenylephrine injections.25
As nonischemic priapism is not considered an emergency, management begins with observation. Patients eventually become dissatisfied with their constant partial erection, and they then present for treatment. Most cases resolve after selective catheterization of the internal pudendal artery and embolization of the fistula with absorbable material. If this fails, surgical exploration with ligation of the vessels leading to the fistula is indicated.
Prevalence in sickle cell trait vs sickle cell disease
Ischemic priapism is uncommon in men with sickle cell trait, but prevalence rates in men with sickle cell disease are as high as 42%.28 In a study of 130 men with sickle cell disease, 35% had a history of prolonged ischemic priapism, 72% had a history of stuttering priapism, and 75% of men with stuttering priapism had their first episode before age 20.29
Rates of erectile dysfunction increase with the duration of ischemic episodes and range from 20% to 90%.28,30 In childhood, sickle cell disease accounts for 63% of the cases of ischemic priapism, and in adults it accounts for 23% of cases.31
Take-home messages
- Sickle cell disease accounts for two-thirds of cases of ischemic priapism in children, and one-fourth of adult cases.
- Ischemic priapism is a medical emergency.
- Treatment with aspiration and phenylephrine injections should begin immediately and should not await treatment measures for sickle cell disease (hydration, oxygen, exchange transfusions).
OTHER UROLOGIC COMPLICATIONS OF SICKLE CELL DISEASE
Other urologic complications of sickle cell trait and sickle cell disease include microscopic hematuria, gross hematuria, and renal colic. A formal evaluation of any patient with persistent microscopic hematuria or gross hematuria should consist of urinalysis, computed tomography, and cystoscopy. This approach assesses the upper and lower genitourinary system for treatable causes. Renal ultrasonography can be used instead of computed tomography but tends to provide less information.
Special considerations
In patients with sickle cell trait and sickle cell disease, chronic hypoxia and subsequent sickling of erythrocytes in the renal medulla can lead to papillary hypertrophy and papillary necrosis. In papillary hypertrophy, friable blood vessels can rupture, resulting in microscopic and gross hematuria. In papillary necrosis, the papilla can slough off and become lodged in the ureter.
Nevertheless, hematuria and renal colic in patients with sickle cell disease or trait are most often attributable to common causes such as infection and stones. A finding of hydronephrosis in the absence of a stone, however, suggests obstruction due to a clot or a sloughed papilla. Ureteroscopy, fulguration, and ureteral stent placement can stop the bleeding and alleviate obstruction in these cases.
Renal medullary carcinoma
Another important reason to order imaging in patients with sickle cell disease or trait who present with urologic symptoms is to rule out renal medullary carcinoma, a rare but aggressive cancer that arises from the collecting duct epithelium. This cancer is twice as likely to occur in males than in females; it has been reported in patients ranging in age from 10 to 40, with a median age at presentation of 26.32
When patients present with symptomatic renal medullary cancer, in most cases the cancer has already metastasized.
On computed tomography, the tumor tends to occupy a central location in the kidney and appears to infiltrate and replace adjacent kidney tissue. Retroperitoneal lymphadenopathy and metastasis are common.
Treatment typically entails radical nephrectomy, chemotherapy, and in some circumstances, radiotherapy. Case reports have shown promising tumor responses to carboplatin and paclitaxel regimens.33,34 Also, a low threshold for imaging in patients with sickle cell disease and trait may increase the odds of early detection of this aggressive cancer.
- Centers for Disease Control and Prevention (CDC). Sickle cell disease (SCD). Data and statistics. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed August 18, 2015.
- Paulin L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science 1949; 110:543–548.
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005; 84:363–376.
- Haymann JP, Stankovic K, Levy P, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol 2010; 5:756–761.
- da Silva GB Jr, Libório AB, Daher Ede F. New insights on pathophysiology, clinical manifestations, diagnosis, and treatment of sickle cell nephropathy. Ann Hematol 2011; 90:1371–1379.
- Emokpae MA, Uadia PO, Gadzama AA. Correlation of oxidative stress and inflammatory markers with the severity of sickle cell nephropathy. Ann Afr Med 2010; 9:141–146.
- Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life 2012; 64:72–80.
- Datta V, Ayengar JR, Karpate S, Chaturvedi P. Microalbuminuria as a predictor of early glomerular injury in children with sickle cell disease. Indian J Pediatr 2003; 70:307–309.
- Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med 1992; 326:910–915.
- Maigne G, Ferlicot S, Galacteros F, et al. Glomerular lesions in patients with sickle cell disease. Medicine (Baltimore) 2010; 89:18–27.
- Sharpe CC, Thein SL. Sickle cell nephropathy—a practical approach. Br J Haematol 2011; 155:287–297.
- Batlle D, Itsarayoungyuen K, Arruda JA, Kurtzman NA. Hyperkalemic hyperchloremic metabolic acidosis in sickle cell hemoglobinopathies. Am J Med 1982; 72:188–192.
- Sklar AH, Perez JC, Harp RJ, Caruana RJ. Acute renal failure in sickle cell anemia. Int J Artif Organs 1990; 13:347–351.
- Powars DR, Elliott-Mills DD, Chan L, et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med 1991; 115:614–620.
- McClellan AC, Luthi JC, Lynch JR, et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol 2012; 159:360–367.
- Bodas P, Huang A, O Riordan MA, Sedor JR, Dell KM. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease—a cross sectional review. BMC Nephrol 2013; 14:237.
- Asnani MR, Lynch O, Reid ME. Determining glomerular filtration rate in homozygous sickle cell disease: utility of serum creatinine based estimating equations. PLoS One 2013; 8:e69922.
- Arlet JB, Ribeil JA, Chatellier G, et al. Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: a prospective observational cohort study. BMC Nephrol 2012; 13:83.
- McKie KT, Hanevold CD, Hernandez C, Waller JL, Ortiz L, McKie KM. Prevalence, prevention, and treatment of microalbuminuria and proteinuria in children with sickle cell disease. J Pediatr Hematol Oncol 2007; 29:140–144.
- Laurin LP, Nachman PH, Desai PC, Ataga KI, Derebail VK. Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant 2014; 29:1211–1218.
- Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol 2013; 88:116–119.
- Huang E, Parke C, Mehrnia A, et al. Improved survival among sickle cell kidney transplant recipients in the recent era. Nephrol Dial Transplant 2013; 28:1039–1046.
- Klein EA, Montague DK, Steiger E. Priapism associated with the use of intravenous fat emulsion: case reports and postulated pathogenesis. J Urol May 1985; 133:857–859.
- Thuret I, Bardakdjian J, Badens C, et al. Priapism following splenectomy in an unstable hemoglobin: hemoglobin Olmsted beta 141 (H19) Leu-->Arg. Am J Hematol 1996; 51:133–136.
- Montague DK, Angermeier KW. Corporeal excavation: new technique for penile prosthesis implantation in men with severe corporeal fibrosis. Urology 2006; 67:1072–1075.
- Levey HR, Kutlu O, Bivalacqua TJ. Medical management of ischemic stuttering priapism: a contemporary review of the literature. Asian J Androl 2012; 14:156–163.
- Montague DK, Jarow J, Broderick GA, et al; Members of the Erectile Dysfunction Guideline Update Panel; American Urological Association. American Urological Association guideline on the management of priapism. J Urol 2003; 170:1318–1324.
- Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med 1980; 140:1434–1437.
- Adeyoju AB, Olujohungbe AB, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications—an international multicentre study. BJU Int 2002; 90:898–902.
- Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med 2004; 1:116–120.
- Nelson JH, 3rd, Winter CC. Priapism: evolution of management in 48 patients in a 22-year series. J Urol 1977; 117:455–458.
- Liu Q, Galli S, Srinivasan R, Linehan WM, Tsokos M, Merino MJ. Renal medullary carcinoma: molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol 2013; 37:368–374.
- Gangireddy VG, Liles GB, Sostre GD, Coleman T. Response of metastatic renal medullary carcinoma to carboplatinum and Paclitaxel chemotherapy. Clin Genitourin Cancer 2012; 10:134–139.
- Walsh AM, Fiveash JB, Reddy AT, Friedman GK. Response to radiation in renal medullary carcinoma. Rare Tumors 2011; 3:e32.
Sickle cell disease is a common genetic disorder in the United States that disproportionately affects people of African ancestry. The characteristic sickling of red blood cells under conditions of reduced oxygen tension leads to intravascular hemolysis and vaso-occlusive events, which in turn cause tissue ischemia-reperfusion injury affecting multiple organs, including the genitourinary system.1–3
In this paper, we review the genitourinary effects of sickle cell disease, focusing on sickle cell nephropathy, priapism, and renal medullary carcinoma.
THE WIDE-RANGING EFFECTS OF SICKLE CELL DISEASE
In the United States, sickle cell disease affects 1 of every 500 blacks and 1 of every 36,000 Hispanics.1 The term describes hemoglobinopathies associated with sickling of red blood cells.
Sickling of red blood cells results from a single base-pair change in the beta-globin gene from glutamic acid to valine at position 6, causing abnormal hemoglobin (hemoglobin S), which polymerizes under conditions of reduced oxygen tension and alters the biconcave disk shape into a rigid, irregular, unstable cell. The sickle-shaped cells are prone to intravascular hemolysis,2 causing intermittent vaso-occlusive events that result in tissue ischemia-reperfusion injury. Genitourinary problems include impaired ability to concentrate urine, hematuria, renal medullary carcinoma, and increased frequency of urinary tract infection.
SICKLE CELL NEPHROPATHY
The kidney is one of the most frequently affected organs in sickle cell disease. Renal manifestations begin to appear in early childhood, with impaired medullary concentrating ability and ischemic damage to the tubular cells caused by sickling within the vasa recta renis precipitated by the acidic, hypoxic, and hypertonic environment in the renal medulla.
As in early diabetic nephropathy, renal blood flow is enhanced and the glomerular filtration rate (GFR) is increased. Increased cardiac output as a result of anemia, localized release of prostaglandins, and a hypoxia-induced increase in nitric oxide synthesis all play a role in the increase in GFR.4,5
Oxidative stress, an increase in markers of inflammation, and local activation of the renin-angiotensin system contribute to renal damage in sickle cell disease.5–7 The resulting hyperfiltration injury leads to microalbuminuria, which occurs in 20% to 40% of children with sickle cell anemia8,9 and in as many as 60% of adults.
The glomerular lesions associated with sickle cell disease vary from glomerulopathy in the early stages to secondary focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, and glomerular thrombotic microangiopathy.10
Clinical presentations and workup
Clinical presentations are not limited to glomerular disease but include hyperchloremic metabolic acidosis and hyperkalemia resulting from defects in potassium secretion and renal acidification.
Hyperphosphatemia—a result of increased reabsorption of phosphorus, increased secretion of uric acid, and increased creatinine clearance—is seen in patients with sickle cell disease.11,12 About 10% of patients can develop an acute kidney injury as a result of volume depletion, rhabdomyolysis, renal vein thrombosis, papillary necrosis, and urinary tract obstruction secondary to blood clots.11,13
Up to 30% of adult patients with sickle cell disease develop chronic kidney disease. Predictors include severe anemia, hypertension, proteinuria, nephrotic syndrome, and microscopic hematuria.14 From 4% to 12% of patients go on to develop end-stage renal disease, but with a 1-year mortality rate three times higher than in patients without sickle cell disease.15
In general, patients with sickle cell anemia have blood pressures below those of age- and sex-matched individuals, but elevated blood pressure and low GFR are not uncommon in affected children. In a cohort of 48 children ages 3 to 18, 8.3% had an estimated GFR less than 90 mL/min/1.73 m2, and 16.7% had elevated blood pressure (prehypertension and hypertension).16
In patients with sickle cell disease, evaluation of proteinuria, hematuria, hypertension, and renal failure should take into consideration the unique renal physiologic and pathologic processes involved. Recent evidence17,18 suggests that the Chronic Kidney Disease Epidemiology Collaboration equation provides a better estimate of GFR than the Modification of Diet in Renal Disease and Cockcroft-Gault equations, although all three creatinine-based methods overestimate GFR in patients with sickle cell disease when compared with GFR measured with technetium-99m-labeled diethylenetriamine penta-acetic acid renal scanning.
Treatment options
Treatment of sickle cell nephropathy includes adequate fluid intake (given the loss of concentrating ability), adequate blood pressure control, use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) in patients who have microalbuminuria or proteinuria (or both)9,11,19 and hydroxyurea. Treatment with enalapril has been shown to decrease proteinuria in patients with sickle cell nephropathy.9 In a cohort of children with sickle cell disease, four of nine patients treated with an ACE inhibitor developed hyperkalemia, leading to discontinuation of the drug in three patients.9
ACE inhibitors and ARBs must be used cautiously in these patients because they have defects in potassium secretion. Hydroxyurea has also been shown to decrease hyperfiltration and microalbuminuria in recent studies,20,21 and this could protect against the development of overt nephropathy.
Higher mortality rates have been reported in patients with sickle cell disease who developed end-stage renal disease than in patients with end-stage renal disease without sickle cell disease. Sickle cell disease also increases the risk of pulmonary hypertension and the vaso-occlusive complication known as acute chest syndrome, contributing to increased mortality rates. Of note, in a study that looked at the association between mortality rates and pre-end-stage care of renal disease using data from the Centers for Medicare and Medicaid Services, patients with sickle cell disease who had had predialysis nephrology care had lower mortality rates.15
Treatments for end-stage renal disease are also effective in patients with sickle cell disease and include hemodialysis, peritoneal dialysis, and renal transplantation. Data from the Organ Procurement and Transplantation Network and the United Network for Organ Sharing show that from 2000 to 2011, African American kidney recipients with sickle cell disease had better survival rates than patients who had undergone transplantation from 1988 to 1999, although rates of long-term survival and graft survival were lower than in transplant recipients with other diagnoses.22
It is important to note that complications as a result of vaso-occlusive events and thrombosis can lead to graft loss; therefore, sickle cell crisis after transplantation requires careful management.
Take-home messages
- Loss of urine-concentrating ability and hyperfiltration are the earliest pathologic changes in sickle cell disease.
- Microalbuminuria as seen in diabetic nephropathy is the earliest manifestation of sickle cell nephropathy, and the prevalence increases as these patients get older and live longer.
- ACE inhibitors or ARBs should be used with caution, given the heightened risk of hyperkalemia in sickle cell disease.
- Recent results with hydroxyurea in decreasing hyperfiltration and microalbuminuria are encouraging.
- Early referral for predialysis nephrologic care is needed in sickle cell patients with chronic kidney disease.
PRIAPISM IN SICKLE CELL DISEASE
Priapism was formerly defined as a full, painful erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm. But priapism is now recognized as two separate disorders—ischemic (veno-occlusive, low-flow) priapism and nonischemic (arterial, high-flow) priapism. The new definition includes both disorders: ie, a full or partial erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm.
Ischemic priapism
Hematologic disorders are major contributors to ischemic priapism and include sickle cell disease, multiple myeloma, fat emboli (hyperalimentation),23 glucose-6-phosphate dehydrogenase deficiency, and hemoglobin Olmsted variant.24
Ischemic priapism is often seen in sickle cell disease and is considered an emergency. It is characterized by an abnormally rigid erection not involving the glans penis. Entrapment of blood in the corpora cavernosa leads to hypoxia, hypercarbia, and acidosis, which in turn leads to a painful compartment syndrome that, if untreated, results in smooth muscle necrosis and subsequent fibrosis. The results are a smaller penis and erectile dysfunction that is unresponsive to any treatment other than implantation of a penile prosthesis. However, scarring of the corpora cavernosa can make this procedure exceedingly difficult, requiring advanced techniques such as corporeal excavation.25
Men with a subtype of ischemic priapism called “stuttering” priapism26 suffer recurrent prolonged erections during sleep. The patient awakens with a painful erection that usually subsides, but sometimes only after several hours. Patients with this disorder suffer from sleep deprivation. Stuttering priapism may lead to full-blown ischemic priapism that does not resolve without intervention.
Nonischemic priapism
In nonischemic priapism, the corpora are engorged but not rigid. The condition results from unregulated arterial inflow and thus is not painful and does not result in damage to the corporeal smooth muscle.
Most cases of nonischemic priapism follow blunt perineal trauma or trauma associated with needle insertion into the corpora. This form of priapism is not associated with sickle cell disease. Because tissue damage does not occur, nonischemic or arterial priapism is not considered an emergency.
Treatment guidelines
Differentiating ischemic from nonischemic priapism is usually straightforward, based on the history, physical examination, corporeal blood gases, and duplex ultrasonography.27
Ischemic priapism is an emergency. After needle aspiration of blood from the corpora cavernosa, phenylephrine is diluted with normal saline to a concentration of 100 to 500 µg/mL and is injected in 1-mL amounts repeatedly at 3- to 5-minute intervals until the erection subsides or until a 1-hour time limit is reached. Blood pressure and pulse are monitored during these injections. If aspiration and phenylephrine irrigation fail, surgical shunting is performed.27
Measures to treat sickle cell disease (hydration, oxygen, exchange transfusions) may be employed simultaneously but should never delay aspiration and phenylephrine injections.25
As nonischemic priapism is not considered an emergency, management begins with observation. Patients eventually become dissatisfied with their constant partial erection, and they then present for treatment. Most cases resolve after selective catheterization of the internal pudendal artery and embolization of the fistula with absorbable material. If this fails, surgical exploration with ligation of the vessels leading to the fistula is indicated.
Prevalence in sickle cell trait vs sickle cell disease
Ischemic priapism is uncommon in men with sickle cell trait, but prevalence rates in men with sickle cell disease are as high as 42%.28 In a study of 130 men with sickle cell disease, 35% had a history of prolonged ischemic priapism, 72% had a history of stuttering priapism, and 75% of men with stuttering priapism had their first episode before age 20.29
Rates of erectile dysfunction increase with the duration of ischemic episodes and range from 20% to 90%.28,30 In childhood, sickle cell disease accounts for 63% of the cases of ischemic priapism, and in adults it accounts for 23% of cases.31
Take-home messages
- Sickle cell disease accounts for two-thirds of cases of ischemic priapism in children, and one-fourth of adult cases.
- Ischemic priapism is a medical emergency.
- Treatment with aspiration and phenylephrine injections should begin immediately and should not await treatment measures for sickle cell disease (hydration, oxygen, exchange transfusions).
OTHER UROLOGIC COMPLICATIONS OF SICKLE CELL DISEASE
Other urologic complications of sickle cell trait and sickle cell disease include microscopic hematuria, gross hematuria, and renal colic. A formal evaluation of any patient with persistent microscopic hematuria or gross hematuria should consist of urinalysis, computed tomography, and cystoscopy. This approach assesses the upper and lower genitourinary system for treatable causes. Renal ultrasonography can be used instead of computed tomography but tends to provide less information.
Special considerations
In patients with sickle cell trait and sickle cell disease, chronic hypoxia and subsequent sickling of erythrocytes in the renal medulla can lead to papillary hypertrophy and papillary necrosis. In papillary hypertrophy, friable blood vessels can rupture, resulting in microscopic and gross hematuria. In papillary necrosis, the papilla can slough off and become lodged in the ureter.
Nevertheless, hematuria and renal colic in patients with sickle cell disease or trait are most often attributable to common causes such as infection and stones. A finding of hydronephrosis in the absence of a stone, however, suggests obstruction due to a clot or a sloughed papilla. Ureteroscopy, fulguration, and ureteral stent placement can stop the bleeding and alleviate obstruction in these cases.
Renal medullary carcinoma
Another important reason to order imaging in patients with sickle cell disease or trait who present with urologic symptoms is to rule out renal medullary carcinoma, a rare but aggressive cancer that arises from the collecting duct epithelium. This cancer is twice as likely to occur in males than in females; it has been reported in patients ranging in age from 10 to 40, with a median age at presentation of 26.32
When patients present with symptomatic renal medullary cancer, in most cases the cancer has already metastasized.
On computed tomography, the tumor tends to occupy a central location in the kidney and appears to infiltrate and replace adjacent kidney tissue. Retroperitoneal lymphadenopathy and metastasis are common.
Treatment typically entails radical nephrectomy, chemotherapy, and in some circumstances, radiotherapy. Case reports have shown promising tumor responses to carboplatin and paclitaxel regimens.33,34 Also, a low threshold for imaging in patients with sickle cell disease and trait may increase the odds of early detection of this aggressive cancer.
Sickle cell disease is a common genetic disorder in the United States that disproportionately affects people of African ancestry. The characteristic sickling of red blood cells under conditions of reduced oxygen tension leads to intravascular hemolysis and vaso-occlusive events, which in turn cause tissue ischemia-reperfusion injury affecting multiple organs, including the genitourinary system.1–3
In this paper, we review the genitourinary effects of sickle cell disease, focusing on sickle cell nephropathy, priapism, and renal medullary carcinoma.
THE WIDE-RANGING EFFECTS OF SICKLE CELL DISEASE
In the United States, sickle cell disease affects 1 of every 500 blacks and 1 of every 36,000 Hispanics.1 The term describes hemoglobinopathies associated with sickling of red blood cells.
Sickling of red blood cells results from a single base-pair change in the beta-globin gene from glutamic acid to valine at position 6, causing abnormal hemoglobin (hemoglobin S), which polymerizes under conditions of reduced oxygen tension and alters the biconcave disk shape into a rigid, irregular, unstable cell. The sickle-shaped cells are prone to intravascular hemolysis,2 causing intermittent vaso-occlusive events that result in tissue ischemia-reperfusion injury. Genitourinary problems include impaired ability to concentrate urine, hematuria, renal medullary carcinoma, and increased frequency of urinary tract infection.
SICKLE CELL NEPHROPATHY
The kidney is one of the most frequently affected organs in sickle cell disease. Renal manifestations begin to appear in early childhood, with impaired medullary concentrating ability and ischemic damage to the tubular cells caused by sickling within the vasa recta renis precipitated by the acidic, hypoxic, and hypertonic environment in the renal medulla.
As in early diabetic nephropathy, renal blood flow is enhanced and the glomerular filtration rate (GFR) is increased. Increased cardiac output as a result of anemia, localized release of prostaglandins, and a hypoxia-induced increase in nitric oxide synthesis all play a role in the increase in GFR.4,5
Oxidative stress, an increase in markers of inflammation, and local activation of the renin-angiotensin system contribute to renal damage in sickle cell disease.5–7 The resulting hyperfiltration injury leads to microalbuminuria, which occurs in 20% to 40% of children with sickle cell anemia8,9 and in as many as 60% of adults.
The glomerular lesions associated with sickle cell disease vary from glomerulopathy in the early stages to secondary focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, and glomerular thrombotic microangiopathy.10
Clinical presentations and workup
Clinical presentations are not limited to glomerular disease but include hyperchloremic metabolic acidosis and hyperkalemia resulting from defects in potassium secretion and renal acidification.
Hyperphosphatemia—a result of increased reabsorption of phosphorus, increased secretion of uric acid, and increased creatinine clearance—is seen in patients with sickle cell disease.11,12 About 10% of patients can develop an acute kidney injury as a result of volume depletion, rhabdomyolysis, renal vein thrombosis, papillary necrosis, and urinary tract obstruction secondary to blood clots.11,13
Up to 30% of adult patients with sickle cell disease develop chronic kidney disease. Predictors include severe anemia, hypertension, proteinuria, nephrotic syndrome, and microscopic hematuria.14 From 4% to 12% of patients go on to develop end-stage renal disease, but with a 1-year mortality rate three times higher than in patients without sickle cell disease.15
In general, patients with sickle cell anemia have blood pressures below those of age- and sex-matched individuals, but elevated blood pressure and low GFR are not uncommon in affected children. In a cohort of 48 children ages 3 to 18, 8.3% had an estimated GFR less than 90 mL/min/1.73 m2, and 16.7% had elevated blood pressure (prehypertension and hypertension).16
In patients with sickle cell disease, evaluation of proteinuria, hematuria, hypertension, and renal failure should take into consideration the unique renal physiologic and pathologic processes involved. Recent evidence17,18 suggests that the Chronic Kidney Disease Epidemiology Collaboration equation provides a better estimate of GFR than the Modification of Diet in Renal Disease and Cockcroft-Gault equations, although all three creatinine-based methods overestimate GFR in patients with sickle cell disease when compared with GFR measured with technetium-99m-labeled diethylenetriamine penta-acetic acid renal scanning.
Treatment options
Treatment of sickle cell nephropathy includes adequate fluid intake (given the loss of concentrating ability), adequate blood pressure control, use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) in patients who have microalbuminuria or proteinuria (or both)9,11,19 and hydroxyurea. Treatment with enalapril has been shown to decrease proteinuria in patients with sickle cell nephropathy.9 In a cohort of children with sickle cell disease, four of nine patients treated with an ACE inhibitor developed hyperkalemia, leading to discontinuation of the drug in three patients.9
ACE inhibitors and ARBs must be used cautiously in these patients because they have defects in potassium secretion. Hydroxyurea has also been shown to decrease hyperfiltration and microalbuminuria in recent studies,20,21 and this could protect against the development of overt nephropathy.
Higher mortality rates have been reported in patients with sickle cell disease who developed end-stage renal disease than in patients with end-stage renal disease without sickle cell disease. Sickle cell disease also increases the risk of pulmonary hypertension and the vaso-occlusive complication known as acute chest syndrome, contributing to increased mortality rates. Of note, in a study that looked at the association between mortality rates and pre-end-stage care of renal disease using data from the Centers for Medicare and Medicaid Services, patients with sickle cell disease who had had predialysis nephrology care had lower mortality rates.15
Treatments for end-stage renal disease are also effective in patients with sickle cell disease and include hemodialysis, peritoneal dialysis, and renal transplantation. Data from the Organ Procurement and Transplantation Network and the United Network for Organ Sharing show that from 2000 to 2011, African American kidney recipients with sickle cell disease had better survival rates than patients who had undergone transplantation from 1988 to 1999, although rates of long-term survival and graft survival were lower than in transplant recipients with other diagnoses.22
It is important to note that complications as a result of vaso-occlusive events and thrombosis can lead to graft loss; therefore, sickle cell crisis after transplantation requires careful management.
Take-home messages
- Loss of urine-concentrating ability and hyperfiltration are the earliest pathologic changes in sickle cell disease.
- Microalbuminuria as seen in diabetic nephropathy is the earliest manifestation of sickle cell nephropathy, and the prevalence increases as these patients get older and live longer.
- ACE inhibitors or ARBs should be used with caution, given the heightened risk of hyperkalemia in sickle cell disease.
- Recent results with hydroxyurea in decreasing hyperfiltration and microalbuminuria are encouraging.
- Early referral for predialysis nephrologic care is needed in sickle cell patients with chronic kidney disease.
PRIAPISM IN SICKLE CELL DISEASE
Priapism was formerly defined as a full, painful erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm. But priapism is now recognized as two separate disorders—ischemic (veno-occlusive, low-flow) priapism and nonischemic (arterial, high-flow) priapism. The new definition includes both disorders: ie, a full or partial erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm.
Ischemic priapism
Hematologic disorders are major contributors to ischemic priapism and include sickle cell disease, multiple myeloma, fat emboli (hyperalimentation),23 glucose-6-phosphate dehydrogenase deficiency, and hemoglobin Olmsted variant.24
Ischemic priapism is often seen in sickle cell disease and is considered an emergency. It is characterized by an abnormally rigid erection not involving the glans penis. Entrapment of blood in the corpora cavernosa leads to hypoxia, hypercarbia, and acidosis, which in turn leads to a painful compartment syndrome that, if untreated, results in smooth muscle necrosis and subsequent fibrosis. The results are a smaller penis and erectile dysfunction that is unresponsive to any treatment other than implantation of a penile prosthesis. However, scarring of the corpora cavernosa can make this procedure exceedingly difficult, requiring advanced techniques such as corporeal excavation.25
Men with a subtype of ischemic priapism called “stuttering” priapism26 suffer recurrent prolonged erections during sleep. The patient awakens with a painful erection that usually subsides, but sometimes only after several hours. Patients with this disorder suffer from sleep deprivation. Stuttering priapism may lead to full-blown ischemic priapism that does not resolve without intervention.
Nonischemic priapism
In nonischemic priapism, the corpora are engorged but not rigid. The condition results from unregulated arterial inflow and thus is not painful and does not result in damage to the corporeal smooth muscle.
Most cases of nonischemic priapism follow blunt perineal trauma or trauma associated with needle insertion into the corpora. This form of priapism is not associated with sickle cell disease. Because tissue damage does not occur, nonischemic or arterial priapism is not considered an emergency.
Treatment guidelines
Differentiating ischemic from nonischemic priapism is usually straightforward, based on the history, physical examination, corporeal blood gases, and duplex ultrasonography.27
Ischemic priapism is an emergency. After needle aspiration of blood from the corpora cavernosa, phenylephrine is diluted with normal saline to a concentration of 100 to 500 µg/mL and is injected in 1-mL amounts repeatedly at 3- to 5-minute intervals until the erection subsides or until a 1-hour time limit is reached. Blood pressure and pulse are monitored during these injections. If aspiration and phenylephrine irrigation fail, surgical shunting is performed.27
Measures to treat sickle cell disease (hydration, oxygen, exchange transfusions) may be employed simultaneously but should never delay aspiration and phenylephrine injections.25
As nonischemic priapism is not considered an emergency, management begins with observation. Patients eventually become dissatisfied with their constant partial erection, and they then present for treatment. Most cases resolve after selective catheterization of the internal pudendal artery and embolization of the fistula with absorbable material. If this fails, surgical exploration with ligation of the vessels leading to the fistula is indicated.
Prevalence in sickle cell trait vs sickle cell disease
Ischemic priapism is uncommon in men with sickle cell trait, but prevalence rates in men with sickle cell disease are as high as 42%.28 In a study of 130 men with sickle cell disease, 35% had a history of prolonged ischemic priapism, 72% had a history of stuttering priapism, and 75% of men with stuttering priapism had their first episode before age 20.29
Rates of erectile dysfunction increase with the duration of ischemic episodes and range from 20% to 90%.28,30 In childhood, sickle cell disease accounts for 63% of the cases of ischemic priapism, and in adults it accounts for 23% of cases.31
Take-home messages
- Sickle cell disease accounts for two-thirds of cases of ischemic priapism in children, and one-fourth of adult cases.
- Ischemic priapism is a medical emergency.
- Treatment with aspiration and phenylephrine injections should begin immediately and should not await treatment measures for sickle cell disease (hydration, oxygen, exchange transfusions).
OTHER UROLOGIC COMPLICATIONS OF SICKLE CELL DISEASE
Other urologic complications of sickle cell trait and sickle cell disease include microscopic hematuria, gross hematuria, and renal colic. A formal evaluation of any patient with persistent microscopic hematuria or gross hematuria should consist of urinalysis, computed tomography, and cystoscopy. This approach assesses the upper and lower genitourinary system for treatable causes. Renal ultrasonography can be used instead of computed tomography but tends to provide less information.
Special considerations
In patients with sickle cell trait and sickle cell disease, chronic hypoxia and subsequent sickling of erythrocytes in the renal medulla can lead to papillary hypertrophy and papillary necrosis. In papillary hypertrophy, friable blood vessels can rupture, resulting in microscopic and gross hematuria. In papillary necrosis, the papilla can slough off and become lodged in the ureter.
Nevertheless, hematuria and renal colic in patients with sickle cell disease or trait are most often attributable to common causes such as infection and stones. A finding of hydronephrosis in the absence of a stone, however, suggests obstruction due to a clot or a sloughed papilla. Ureteroscopy, fulguration, and ureteral stent placement can stop the bleeding and alleviate obstruction in these cases.
Renal medullary carcinoma
Another important reason to order imaging in patients with sickle cell disease or trait who present with urologic symptoms is to rule out renal medullary carcinoma, a rare but aggressive cancer that arises from the collecting duct epithelium. This cancer is twice as likely to occur in males than in females; it has been reported in patients ranging in age from 10 to 40, with a median age at presentation of 26.32
When patients present with symptomatic renal medullary cancer, in most cases the cancer has already metastasized.
On computed tomography, the tumor tends to occupy a central location in the kidney and appears to infiltrate and replace adjacent kidney tissue. Retroperitoneal lymphadenopathy and metastasis are common.
Treatment typically entails radical nephrectomy, chemotherapy, and in some circumstances, radiotherapy. Case reports have shown promising tumor responses to carboplatin and paclitaxel regimens.33,34 Also, a low threshold for imaging in patients with sickle cell disease and trait may increase the odds of early detection of this aggressive cancer.
- Centers for Disease Control and Prevention (CDC). Sickle cell disease (SCD). Data and statistics. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed August 18, 2015.
- Paulin L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science 1949; 110:543–548.
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005; 84:363–376.
- Haymann JP, Stankovic K, Levy P, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol 2010; 5:756–761.
- da Silva GB Jr, Libório AB, Daher Ede F. New insights on pathophysiology, clinical manifestations, diagnosis, and treatment of sickle cell nephropathy. Ann Hematol 2011; 90:1371–1379.
- Emokpae MA, Uadia PO, Gadzama AA. Correlation of oxidative stress and inflammatory markers with the severity of sickle cell nephropathy. Ann Afr Med 2010; 9:141–146.
- Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life 2012; 64:72–80.
- Datta V, Ayengar JR, Karpate S, Chaturvedi P. Microalbuminuria as a predictor of early glomerular injury in children with sickle cell disease. Indian J Pediatr 2003; 70:307–309.
- Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med 1992; 326:910–915.
- Maigne G, Ferlicot S, Galacteros F, et al. Glomerular lesions in patients with sickle cell disease. Medicine (Baltimore) 2010; 89:18–27.
- Sharpe CC, Thein SL. Sickle cell nephropathy—a practical approach. Br J Haematol 2011; 155:287–297.
- Batlle D, Itsarayoungyuen K, Arruda JA, Kurtzman NA. Hyperkalemic hyperchloremic metabolic acidosis in sickle cell hemoglobinopathies. Am J Med 1982; 72:188–192.
- Sklar AH, Perez JC, Harp RJ, Caruana RJ. Acute renal failure in sickle cell anemia. Int J Artif Organs 1990; 13:347–351.
- Powars DR, Elliott-Mills DD, Chan L, et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med 1991; 115:614–620.
- McClellan AC, Luthi JC, Lynch JR, et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol 2012; 159:360–367.
- Bodas P, Huang A, O Riordan MA, Sedor JR, Dell KM. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease—a cross sectional review. BMC Nephrol 2013; 14:237.
- Asnani MR, Lynch O, Reid ME. Determining glomerular filtration rate in homozygous sickle cell disease: utility of serum creatinine based estimating equations. PLoS One 2013; 8:e69922.
- Arlet JB, Ribeil JA, Chatellier G, et al. Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: a prospective observational cohort study. BMC Nephrol 2012; 13:83.
- McKie KT, Hanevold CD, Hernandez C, Waller JL, Ortiz L, McKie KM. Prevalence, prevention, and treatment of microalbuminuria and proteinuria in children with sickle cell disease. J Pediatr Hematol Oncol 2007; 29:140–144.
- Laurin LP, Nachman PH, Desai PC, Ataga KI, Derebail VK. Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant 2014; 29:1211–1218.
- Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol 2013; 88:116–119.
- Huang E, Parke C, Mehrnia A, et al. Improved survival among sickle cell kidney transplant recipients in the recent era. Nephrol Dial Transplant 2013; 28:1039–1046.
- Klein EA, Montague DK, Steiger E. Priapism associated with the use of intravenous fat emulsion: case reports and postulated pathogenesis. J Urol May 1985; 133:857–859.
- Thuret I, Bardakdjian J, Badens C, et al. Priapism following splenectomy in an unstable hemoglobin: hemoglobin Olmsted beta 141 (H19) Leu-->Arg. Am J Hematol 1996; 51:133–136.
- Montague DK, Angermeier KW. Corporeal excavation: new technique for penile prosthesis implantation in men with severe corporeal fibrosis. Urology 2006; 67:1072–1075.
- Levey HR, Kutlu O, Bivalacqua TJ. Medical management of ischemic stuttering priapism: a contemporary review of the literature. Asian J Androl 2012; 14:156–163.
- Montague DK, Jarow J, Broderick GA, et al; Members of the Erectile Dysfunction Guideline Update Panel; American Urological Association. American Urological Association guideline on the management of priapism. J Urol 2003; 170:1318–1324.
- Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med 1980; 140:1434–1437.
- Adeyoju AB, Olujohungbe AB, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications—an international multicentre study. BJU Int 2002; 90:898–902.
- Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med 2004; 1:116–120.
- Nelson JH, 3rd, Winter CC. Priapism: evolution of management in 48 patients in a 22-year series. J Urol 1977; 117:455–458.
- Liu Q, Galli S, Srinivasan R, Linehan WM, Tsokos M, Merino MJ. Renal medullary carcinoma: molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol 2013; 37:368–374.
- Gangireddy VG, Liles GB, Sostre GD, Coleman T. Response of metastatic renal medullary carcinoma to carboplatinum and Paclitaxel chemotherapy. Clin Genitourin Cancer 2012; 10:134–139.
- Walsh AM, Fiveash JB, Reddy AT, Friedman GK. Response to radiation in renal medullary carcinoma. Rare Tumors 2011; 3:e32.
- Centers for Disease Control and Prevention (CDC). Sickle cell disease (SCD). Data and statistics. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed August 18, 2015.
- Paulin L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science 1949; 110:543–548.
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005; 84:363–376.
- Haymann JP, Stankovic K, Levy P, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol 2010; 5:756–761.
- da Silva GB Jr, Libório AB, Daher Ede F. New insights on pathophysiology, clinical manifestations, diagnosis, and treatment of sickle cell nephropathy. Ann Hematol 2011; 90:1371–1379.
- Emokpae MA, Uadia PO, Gadzama AA. Correlation of oxidative stress and inflammatory markers with the severity of sickle cell nephropathy. Ann Afr Med 2010; 9:141–146.
- Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life 2012; 64:72–80.
- Datta V, Ayengar JR, Karpate S, Chaturvedi P. Microalbuminuria as a predictor of early glomerular injury in children with sickle cell disease. Indian J Pediatr 2003; 70:307–309.
- Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med 1992; 326:910–915.
- Maigne G, Ferlicot S, Galacteros F, et al. Glomerular lesions in patients with sickle cell disease. Medicine (Baltimore) 2010; 89:18–27.
- Sharpe CC, Thein SL. Sickle cell nephropathy—a practical approach. Br J Haematol 2011; 155:287–297.
- Batlle D, Itsarayoungyuen K, Arruda JA, Kurtzman NA. Hyperkalemic hyperchloremic metabolic acidosis in sickle cell hemoglobinopathies. Am J Med 1982; 72:188–192.
- Sklar AH, Perez JC, Harp RJ, Caruana RJ. Acute renal failure in sickle cell anemia. Int J Artif Organs 1990; 13:347–351.
- Powars DR, Elliott-Mills DD, Chan L, et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med 1991; 115:614–620.
- McClellan AC, Luthi JC, Lynch JR, et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol 2012; 159:360–367.
- Bodas P, Huang A, O Riordan MA, Sedor JR, Dell KM. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease—a cross sectional review. BMC Nephrol 2013; 14:237.
- Asnani MR, Lynch O, Reid ME. Determining glomerular filtration rate in homozygous sickle cell disease: utility of serum creatinine based estimating equations. PLoS One 2013; 8:e69922.
- Arlet JB, Ribeil JA, Chatellier G, et al. Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: a prospective observational cohort study. BMC Nephrol 2012; 13:83.
- McKie KT, Hanevold CD, Hernandez C, Waller JL, Ortiz L, McKie KM. Prevalence, prevention, and treatment of microalbuminuria and proteinuria in children with sickle cell disease. J Pediatr Hematol Oncol 2007; 29:140–144.
- Laurin LP, Nachman PH, Desai PC, Ataga KI, Derebail VK. Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant 2014; 29:1211–1218.
- Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol 2013; 88:116–119.
- Huang E, Parke C, Mehrnia A, et al. Improved survival among sickle cell kidney transplant recipients in the recent era. Nephrol Dial Transplant 2013; 28:1039–1046.
- Klein EA, Montague DK, Steiger E. Priapism associated with the use of intravenous fat emulsion: case reports and postulated pathogenesis. J Urol May 1985; 133:857–859.
- Thuret I, Bardakdjian J, Badens C, et al. Priapism following splenectomy in an unstable hemoglobin: hemoglobin Olmsted beta 141 (H19) Leu-->Arg. Am J Hematol 1996; 51:133–136.
- Montague DK, Angermeier KW. Corporeal excavation: new technique for penile prosthesis implantation in men with severe corporeal fibrosis. Urology 2006; 67:1072–1075.
- Levey HR, Kutlu O, Bivalacqua TJ. Medical management of ischemic stuttering priapism: a contemporary review of the literature. Asian J Androl 2012; 14:156–163.
- Montague DK, Jarow J, Broderick GA, et al; Members of the Erectile Dysfunction Guideline Update Panel; American Urological Association. American Urological Association guideline on the management of priapism. J Urol 2003; 170:1318–1324.
- Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med 1980; 140:1434–1437.
- Adeyoju AB, Olujohungbe AB, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications—an international multicentre study. BJU Int 2002; 90:898–902.
- Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med 2004; 1:116–120.
- Nelson JH, 3rd, Winter CC. Priapism: evolution of management in 48 patients in a 22-year series. J Urol 1977; 117:455–458.
- Liu Q, Galli S, Srinivasan R, Linehan WM, Tsokos M, Merino MJ. Renal medullary carcinoma: molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol 2013; 37:368–374.
- Gangireddy VG, Liles GB, Sostre GD, Coleman T. Response of metastatic renal medullary carcinoma to carboplatinum and Paclitaxel chemotherapy. Clin Genitourin Cancer 2012; 10:134–139.
- Walsh AM, Fiveash JB, Reddy AT, Friedman GK. Response to radiation in renal medullary carcinoma. Rare Tumors 2011; 3:e32.
KEY POINTS
- Microalbuminuria as seen in diabetic nephropathy is the earliest manifestation of sickle cell nephropathy, and the prevalence increases as these patients get older and live longer.
- Ischemic priapism is a medical emergency. Treatment with aspiration and phenylephrine injections should begin immediately and should not await treatment measures for sickle cell disease.
- In patients with sickle cell trait and sickle cell disease, chronic hypoxia and subsequent sickling of erythrocytes in the renal medulla can lead to papillary hypertrophy and papillary necrosis.
Heart failure in African Americans: Disparities can be overcome
African Americans are disproportionately affected by heart failure and have not experienced the same benefit from treatment as white patients have. Much of the disparity can be blamed on modifiable risk factors such as uncontrolled hypertension and on suboptimal health care. When African Americans are treated according to guidelines, discrepant outcomes can be minimized.
In this article, we review the processes contributing to heart failure in African Americans, its management, and challenges with regard to disparities.
HEART FAILURE IS INCREASING
Despite 20 years of progress in understanding the pathophysiology of heart failure and developing medical and surgical therapies for it, its prevalence and associated morbidity are increasing in the United States. In 2010, 6.6 million (2.8%) of the adults in the United States had heart failure,1 and the prevalence is expected to increase by about 25% by 2030.
DISPARITIES IN INCIDENCE, OUTCOMES
Heart failure is more prevalent in African Americans than in whites, imposes higher rates of death and morbidity, and has a more malignant course.1–6
According to American Heart Association statistics, the annual incidence of heart failure in whites is approximately 6 per 1,000 person-years, while in African Americans it is 9.1 per 1,000 person-years.1 In the Atherosclerosis Risk in Communities study, the incidence of new heart failure was 1.0 per 1,000 person-years in Chinese Americans, 2.4 in whites, 3.5 in Hispanics, and 4.6 in African Americans.2
Moreover, when hospitalized for heart failure, African Americans have a 45% greater risk of death or decline in functional status than whites.7
Heart failure also occurs earlier in African Americans. Bibbins-Domingo et al8 reported that heart failure before age 50 was 20 times more frequent in African Americans than in whites. Functional and structural cardiac changes appeared an average of 10 years before the onset of symptoms and were strongly associated with the development of subsequent heart failure.8
In the Women’s Health Initiative, African American women had higher rates of heart failure than white women, perhaps in part because of higher rates of diabetes.9
Heart failure with preserved ejection fraction
About half of patients who have signs and symptoms of heart failure have a normal (“preserved”) ejection fraction. The incidence of this condition, previously called diastolic heart failure, appears to be similar between African Americans and whites. However, African Americans appear to have a greater incidence of factors that predispose to it and tend to present later in the course.10 For example, African Americans have higher left ventricular mass and wall thickness and a higher incidence of left ventricular hypertrophy than white patients.11–13 In addition, those with heart failure with preserved ejection fraction tend to be younger, female, more likely to have hypertension and diabetes, and less likely to have coronary artery disease, and tend to have worse renal function than their white counterparts.14,15 The predisposition to diastolic impairment persists even after adjusting for risk factors.11–15 The mortality rate in African Americans with heart failure with preserved ejection fraction and without coronary artery disease may also be higher than that of comparable white patients.16
WHY DO AFRICAN AMERICANS HAVE MORE HEART FAILURE?
Modifiable risk factors
In African Americans, the higher percentage of cases of heart failure is attributable to modifiable risk factors such as hypertension, hyperglycemia, left ventricular hypertrophy, and smoking, and fewer cases are due to ischemic heart disease.2,3 Nonischemic cardiomyopathy predominates in African Americans, whereas ischemic cardiomyopathy predominates in whites.
Hypertension, diabetes, obesity, and chronic kidney disease all portend subsequent heart failure and are common in African Americans, but hypertension is the main culprit.3,5,8,17–21 The prevalence of hypertension in African Americans is among the highest in the world, and because African Americans are more likely to have poorer control of their hypertension, they consequently have more target-organ damage.22 Indeed, in many hypertensive African Americans who develop heart failure, the hypertension is poorly controlled. However, even after adjusting for risk factors, and particularly blood pressure control, African Americans remain at higher risk of heart failure.23
The specific mechanistic links between hypertension and heart failure remain to be identified. Despite having a higher prevalence of left ventricular hypertrophy and left ventricular remodeling, African Americans with heart failure tend toward systolic heart failure, as opposed to heart failure with preserved ejection fraction.
Neurohormonal imbalances and endothelial dysfunction
Derangements in the renin-angiotensin-aldosterone and adrenergic axes are likely the main pathophysiologic mechanisms in the genesis of heart failure in all populations. However, other factors may underlie the enhanced disease burden in African Americans.
Impaired endothelial function, as evidenced by impaired digital and brachial artery vasomotion, is very common in African Americans.24–26 The small arteries of African Americans are less elastic than those of whites and Chinese.27 The underlying mechanism may be related to increased oxidative stress, decreased nitric oxide availability, exaggerated vasoconstrictor response, and attenuated responsiveness to vasodilators and nitric oxide.28–31
Genetic polymorphisms
An important caveat in discussing racial differences in heart failure is that “race” is completely arbitrary and is based on sociopolitical rather than scientific or physiologic definitions. Perceived genetic influences are likely to represent complex gene-gene, gene-environment, and gene-drug interactions.
This is especially true for African Americans, who are a markedly heterogeneous group. The US Office of Management and Budget defines “black” or “African American” as having origins in any of the black racial groups of Africa (www.census.gov/2010census/data). Thus, “African American” includes sixth-generation descendants of African slaves, recently immigrated Jamaicans, and black descendants of French and Spanish people.
Most African Americans have some European ancestry. In one study, the estimated proportion of European ancestry ranged from 7% in Jamaicans of African descent to approximately 23% in African Americans in New Orleans.32
Nevertheless, several polymorphisms associated with the risk of heart failure may provide insight into some of the “race-based” differences in pathophysiology and response to medications and, it is hoped, may eventually serve as the basis for tailored therapy. Genes of interest include those for:
- Beta 1 adrenergic receptor
- Alpha 2c receptor33
- Aldosterone synthase34
- G protein
- Transforming growth factor beta
- Nitric oxide synthase35
- Transthyrectin.36,37
Socioeconomic factors and quality of care
Heart failure patients—and especially African Americans—have high rates of hospital readmission, and socioeconomic factors have been implicated. In more than 40,000 patients with heart failure, lower income was a significant predictor of hospital readmission.38 Socioeconomic factors in turn could account for delay in seeking treatment for worsening symptoms, failure to recognize symptoms, limited disease awareness, inadequate access to health care, noncompliance with follow-up appointments, and poor adherence to recommended treatment, all of which are common in African American patients.38,39
African Americans also report more discrimination from health care providers, have more concerns about blood pressure medications, and are more likely to have misperceptions about high blood pressure (eg, that it is not serious), all of which may interfere with optimal blood pressure control.40 Managing heart failure in African Americans should include trying to identify and eliminate barriers to attaining treatment goals.
PREVENTING HEART FAILURE BY REDUCING RISK FACTORS
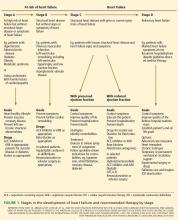
The American College of Cardiology Foundation and American Heart Association, in their 2013 guidelines, underscored the progressive nature of heart failure by defining four stages of the disease, from stage A (at risk) through stage D (refractory heart failure) (Figure 1).41 They also emphasized the importance of preventing it.
A thorough clinical assessment, with appropriate assessment for risk factors and intervention at stage A, is critical in preventing left ventricular remodeling and heart failure. These risk factors include hypertension, hyperlipidemia, atherosclerosis, diabetes mellitus, valvular disease, obesity, physical inactivity, excessive alcohol intake, poor diet, and smoking.
Hypertension is especially important in African Americans and requires vigorous screening and aggressive treatment. Antihypertensive drugs should be prescribed early, with a lower threshold for escalating therapy with combinations of drugs, as most patients require more than one.
There is considerable debate about the appropriate blood pressure thresholds for diagnosing hypertension and the optimal target blood pressures in African Americans. The 2014 report of the Joint National Committee recommends a similar hypertension treatment target of 140/90 mm Hg for all patients except older adults (for whom 150/90 mm Hg is acceptable), and no separate target for African Americans.42 Previous guidelines from this committee recommended thiazide-type diuretics as first-line therapy for hypertension in African Americans43; the new ones recommend thiazide-type diuretics or calcium channel blockers. However, in those with left ventricular systolic dysfunction, hypertension treatment should include drugs shown to reduce the risk of death in heart failure—ie, angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, hydralazine, nitrates, and aldosterone receptor antagonists.
Salt intake should be reduced to less than 3 g per day (1,200 mg of sodium per day), which has been shown to substantially reduce rates of cardiovascular morbidity and mortality and health care costs.44 Since most Americans consume 7 to 10 g of salt per day, strict salt restriction should be encouraged as a preventive measure.
Diabetes should be screened for and treated in African Americans per current American Diabetes Association guidelines.
Dyslipidemia should also be screened for and treated per guidelines.45
Smoking cessation, moderation of alcohol intake, and avoidance of illicit drugs should be encouraged. Given that African Americans develop heart failure at a relatively early age, the level of vigilance should be high and the threshold for screening should be low.
Healthy neighborhoods, healthy people
Neighborhoods can be designed and built with wellness in mind, incorporating features such as access to healthy food and walkability. Living in such neighborhoods leads to more physical activity and less obesity, although this relationship may be less robust in African Americans.46–49
Environmental factors are multifactorial in African Americans and extend beyond those afforded by the built environment. For instance, lack of safety may hinder the potential benefit of an otherwise walkable neighborhood. These interactions are highly complex, and more investigation is needed to determine the effect of built environments on risk factors in African Americans.
DRUG THERAPY FOR HEART FAILURE IN AFRICAN AMERICANS
Use standard therapies
ACE inhibitors, beta-blockers, and aldosterone antagonists are the standard of care in heart failure, with digoxin (Lanoxin) and diuretics used as adjuncts to control symptoms.
African Americans may respond differently than whites to some of these drugs (Table 1). However, these findings should be interpreted with caution, since most of them came from subgroup analyses of trials in which African Americans accounted for as many as 28% to as few as 1%.50 To date, no data unequivocally show that we should use standard heart failure therapies any differently in African Americans than in whites.
Digoxin: Limited role to control symptoms
Post hoc analysis of the Digitalis Investigation Group trial, in which 14% of the patients were nonwhite, revealed that compared with placebo, digitalis (and achieving a serum digitalis concentration of 0.5 to 0.9 ng/mL) was associated with lower rates of all-cause mortality in most subgroups—except nonwhites.51
In general, digoxin has a limited role in heart failure, since other drugs are available that substantially modify outcomes. However, it can be considered in patients who have persistent heart failure symptoms.
ACE inhibitors, ARBs are recommended
ACE inhibitors are recommended for patients with New York Heart Association (NYHA) class I, II, III, or IV heart failure (class I recommendation, ie, “recommended”; level of evidence A on a scale of A, B, and C) and as part of standard therapy for African American patients with heart failure with symptomatic or asymptomatic left ventricular systolic dysfunction (class I recommendation; level of evidence C).41
Although African American patients did not appear to derive any benefit from enalapril (Vasotec) in the Studies of Left Ventricular Dysfunction (SOLVD) trial,52 a subsequent analysis that involved the SOLVD Prevention Trial did not find any differences between African Americans and whites in response to this agent.6 Similarly, a meta-analysis did not suggest differences in ACE-inhibitor efficacy in reducing adverse cardiovascular outcomes in heart failure between African Americans and non–African Americans.53
Of note: African Americans have a 3% to 4% higher incidence of angioedema from ACE inhibitors than whites.54,55
Angiotensin receptor blockers (ARBs) can be used as substitute therapy in African Americans who cannot tolerate ACE inhibitors (class IIa recommendation, ie, “reasonable”; level of evidence B).41
Beta-blockers also recommended
Beta-blockers are recommended in NYHA class I, II, III, and IV heart failure (class I recommendation; level of evidence A) and as part of standard therapy for African Americans with heart failure due to symptomatic left ventricular systolic dysfunction (class I recommendation; level of evidence B) and asymptomatic left ventricular systolic dysfunction (level of evidence C).41
Carvedilol (Coreg) and metoprolol (Lopressor) are the standard beta-blockers used to treat heart failure, and these drugs should be used in African Americans as well as in whites.15,53,56–59 Of interest, however, race-specific differences may exist in the beta-adrenergic pathway.60,61
Aldosterone antagonists: More study needed
Aldosterone antagonists, also called mineralocorticoid antagonists, ie, spironolactone (Aldactone) and eplerenone (Inspra), are recommended in addition to beta-blockers and ACE inhibitors for NYHA class II–IV heart failure, unless contraindicated (class I recommendation; level of evidence A).
However, trials of aldosterone antagonists to date have enrolled few African Americans.62–64 The limited data suggest that African Americans with heart failure may be less responsive to the renal effects of spironolactone, demonstrating less of an increase in serum potassium levels, and there are essentially no data to guide the use of these drugs in African Americans with heart failure.65 Further study is needed. But in the absence of data to the contrary, these agents, should also be used in African American patients with class III or IV heart failure.
Hydralazine plus nitrates: Recommended for African Americans
Hydralazine plus isosorbide dinitrate (available as BiDil) is recommended as part of standard therapy, in addition to beta-blockers and ACE inhibitors specifically for African Americans with left ventricular systolic dysfunction and NYHA class III or IV heart failure (class I recommendation; level of evidence A), as well as NYHA class II heart failure (class I recommendation; level of evidence B).41
Preliminary evidence for this combination came from the Department of Veterans Affairs Cooperative Vasodilator-Heart Failure Trials.66
Subsequently, the African-American Heart Failure Trial67 was conducted in self-identified African American patients with NYHA class III or IV heart failure on standard heart failure therapy, including an ACE inhibitor if tolerated. Patients were randomly assigned to receive a fixed combination of isosorbide 20 mg and hydralazine 37.5 mg, one or two tablets three times a day, or placebo. The target dose of isosorbide dinitrate was 120 mg, and the target dose of hydralazine was 225 mg daily. Follow-up was up to 18 months. The study was terminated early because of a significant 43% improvement in overall survival for the patients in the isosorbide-hydralazine group. In addition, the rate of first hospitalization was 39% lower and the mean improvement in quality-of-life scores was 52% greater with isosorbide-hydralazine than with placebo.67
There has been much debate about whether the benefit seen in this trial was the result of a hemodynamic effect, blood pressure response, or neurohormonal modulation. The benefit is less likely from a reduction in blood pressure, as the patients who had low blood pressure derived a mortality benefit similar to those with higher blood pressure, despite no further reduction in their blood pressure.68
Treatment for heart failure with preserved ejection fraction
Although there are no data on how to manage heart failure with preserved ejection fraction that are specific to African Americans, the ACCF/AHA guideline41 recommends treating systolic and diastolic hypertension (class I, level of evidence B) according to published clinical practice guidelines and using diuretics to alleviate volume overload (class I; level of evidence C). Revascularization and management of atrial fibrillation are also “reasonable,” as are the use of ARBs, ACE inhibitors, and beta-blockers in the management of hypertension (class IIa; level of evidence C). ARBs may also be considered to reduce hospitalization in symptomatic patients with heart failure with preserved ejection fraction (class IIb, ie, “may be considered”; level of evidence B).
For acute decompensated heart failure
One of the greatest challenges in heart failure is treating patients who present with acute decompensated heart failure.
As in the general population, the major precipitating factor for hospitalization with decompensated heart failure in African Americans is nonadherence to prescribed dietary and medication regimens.35 African Americans with acute decompensated heart failure tend to be younger and to have nonischemic cardiomyopathy, hypertension, diabetes, and obesity, but a lower risk of death.35,69,70 Up to 44% have uncontrolled hypertension.35
Inotropes and vasodilators have undergone multiple trials in the acutely decompensated state in the general population, but no trial has demonstrated a reduction in the mortality rate, and some showed a higher mortality rate. Thus, the treatment of acute decompensated heart failure remains primarily consensus-guided and symptom-focused.
Loop diuretics have been the mainstay in managing fluid retention and congestion in heart failure. The Diuretic Optimization Strategies Evaluation trial tested low-dose vs high-dose intravenous furosemide (Lasix) given either as a continuous infusion or as intermittent intravenous boluses. All strategies were safe and effective.71
Although ultrafiltration is an effective method of decongestion in heart failure and has been associated with a reduction in hospitalization, it is also associated with worsening renal function.72 The Cardiorenal Rescue Study in Acute Decompensated Heart Failure73 compared ultrafiltration vs stepped diuretic therapy. In this trial, which enrolled approximately 26% nonwhites, stepped diuretic therapy was superior to ultrafiltration in preserving renal function in acute decompensated heart failure, although the efficacy of fluid removal was similar.
Both studies were small, and subgroup analyses are not likely to yield useful information. Nevertheless, these data support the use of intravenous diuretics, by continuous infusion or bolus, in acute decompensated heart failure.
Despite no benefit in terms of the mortality rate, inotropes continue to be used in some cases of acute decompensated heart failure, and African Americans appear to have a response to milrinone (Primacor IV) similar to that in whites.69
In a nonrandomized study in which most patients were black, high-dose intravenous nitroglycerin appeared to be safe and associated with less need for ventilator support and intensive care unit admission, compared retrospectively with a population that did not receive high-dose nitroglycerin.74
Given the different profile of the African American patient with acute decompensated heart failure, prospective studies would be useful in determining the best management strategy.
TREATMENTS FOR ADVANCED HEART FAILURE
Cardiac resynchronization and implantable cardioverter-defibrillators
Cardiac resynchronization therapy is indicated for patients with NYHA class II, III, and ambulatory class IV heart failure and left ventricular ejection fraction less than or equal to 35%, sinus rhythm, left bundle branch block, and a QRS duration greater than or equal to 150 ms (class I recommendation; level of evidence A for class NYHA III and IV; level of evidence B for NYHA class II).41
An implantable cardioverter-defibrillator is recommended in patients with NYHA class II or III heart failure for primary prevention of sudden cardiac death in selected patients with nonischemic dilated cardiomyopathy or ischemic heart disease (class I recommendation; level of evidence A).
However, few members of racial and ethnic minorities were included in trials of implantable cardioverter-defibrillators75,76 or cardiac resynchronization,7,77,78 so that subgroup analysis is limited. Use of an implantable cardioverter-defibrillator showed similar reduction in mortality between African Americans and whites, and compliance with device implantation and medical therapy was comparable.79
Among patients discharged from hospitals in the American Heart Association’s Get With the Guidelines–Heart Failure Quality Improvement Program, fewer than 40% of potentially eligible patients received an implantable cardioverter-defibrillator, and rates were significantly lower for African Americans.80 When they can get cardiac resynchronization therapy, African Americans appear to experience similar benefit from it.81
Heart transplantation: Poorer outcomes in African Americans?
Heart transplantation remains the most effective and durable therapy for advanced heart failure. Median survival approaches 14 years.82
However, a retrospective study found that African American recipients had an 11.5% lower 10-year survival rate than whites, which persisted after adjusting for risk, donor-recipient matching by race, and censoring of deaths in the first year.83 Although socioeconomic factors and poor human leukocyte antigen matching have been implicated, a retrospective cohort study showed that African American recipients had a higher risk of death than white recipients even after adjustment for recipient, transplant, and socioeconomic factors.84–87 African Americans were more likely to die of graft failure or of a cardiovascular cause than white patients, but were less likely to die of infection or malignancy. Although mortality rates decreased over time for all transplant recipients, the disparity in mortality rates between African Americans and whites remained essentially unchanged.84
Among all donor-recipient combinations, African American recipients of hearts from African American donors had the highest risk of death.88
Limited access to transplantation persists, particularly for African Americans of lower socioeconomic status. African Americans are more likely than whites to be uninsured, and the funding requirement to be placed on the transplantation list disproportionately affects African Americans.89,90
Left-ventricular assist devices
Left-ventricular assist devices (LVADs) improve survival in heart transplantation candidates and heart failure patients who do not qualify for transplantation. After LVAD implantation, African American patients have similar 1- and 2-year survival rates and no difference in readmission rates compared with whites.91,92
Access to LVAD implantation, however, is significantly influenced by race, and African Americans are significantly less likely to receive one (OR = 0.29).93 Further investigation is required to identify disparities in outcome, access, and contributing factors.
DISPARITIES CAN BE MINIMIZED
In general, heart failure in African Americans is characterized by a high prevalence of hypertension as a major risk factor and potentially different pathogenesis than in the general population. Furthermore, heart failure in African Americans is more prevalent, occurs at an early age, and has a more severe course than in whites, perhaps because of a higher prevalence of risk factors such as diabetes mellitus, obesity, and again, hypertension. These disparities are multifactorial and involve a complex interplay between genes, environment, and socioeconomic factors.
For now, heart failure in African Americans should be treated according to standard evidenced-based strategies, which include a combination of isosorbide dinitrate and hydralazine in addition to other neurohormonal modifying agents (ACE inhibitors, beta-blockers, aldosterone antagonists), a strategy demonstrated to reduce mortality rates in African Americans. When treated according to guidelines, disparities in outcomes can be minimized.
However, many questions about managing heart failure remain unanswered, since African Americans have been markedly underrepresented in clinical trials. Clinical trials need to enroll enough African Americans to answer the questions of interest. Disparities in outcomes must be investigated in a scientific and hypothesis-driven manner. The effect of the built environment on African Americans needs more study as well, as success with these strategies may be impeded by unrecognized factors.
Preventing heart failure should be a priority. Efforts should be directed toward detecting and modifying risk factors early, managing hypertension aggressively, and identifying left ventricular dysfunction early.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med 2008; 168:2138–2145.
- Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008; 101:1016–1022.
- Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, et al. Epidemiology of incident heart failure in a contemporary elderly cohort: the health, aging, and body composition study. Arch Intern Med 2009; 169:708–715.
- Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med 1999; 340:609–616.
- Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. N Engl J Med 2001; 344:1351–1357.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med 2009; 360:1179–1190.
- Eaton CB, Abdulbaki AM, Margolis KL, et al. Racial and ethnic differences in incident hospitalized heart failure in postmenopausal women: the Women’s Health Initiative. Circulation 2012; 126:688–696.
- Ilksoy N, Hoffman M, Moore RH, Easley K, Jacobson TA. Comparison of African-American patients with systolic heart failure versus preserved ejection fraction. Am J Cardiol 2006; 98:806–808.
- Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol 2008; 52:1015–1021.
- Kizer JR, Arnett DK, Bella JN, et al. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension 2004; 43:1182–1188.
- Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension 2005; 46:124–129.
- East MA, Peterson ED, Shaw LK, Gattis WA, O’Connor CM. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J 2004; 148:151–156.
- Klapholz M, Maurer M, Lowe AM, et al; New York Heart Failure Consortium. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol 2004; 43:1432–1438.
- Agoston I, Cameron CS, Yao D, Dela Rosa A, Mann DL, Deswal A. Comparison of outcomes of white versus black patients hospitalized with heart failure and preserved ejection fraction. Am J Cardiol 2004; 94:1003–1007.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med 1996; 334:1349–1355.
- Yancy CW, Strong M. The natural history, epidemiology, and prognosis of heart failure in African Americans. Congest Heart Fail 2004; 10:15–18.
- Velagaleti RS, Gona P, Larson MG, et al. Multimarker approach for the prediction of heart failure incidence in the community. Circulation 2010; 122:1700–1706.
- Deswal A, Petersen NJ, Urbauer DL, Wright SM, Beyth R. Racial variations in quality of care and outcomes in an ambulatory heart failure cohort. Am Heart J 2006; 152:348–354.
- Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic And Racial Differences in Stroke study. Stroke 2006; 37:1171–1178.
- Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med 2005; 165:2098–2104.
- Okin PM, Kjeldsen SE, Dahlöf B, Devereux RB. Racial differences in incident heart failure during antihypertensive therapy. Circ Cardiovasc Qual Outcomes 2011; 4:157–164.
- Mulukutla SR, Venkitachalam L, Bambs C, et al. Black race is associated with digital artery endothelial dysfunction: results from the Heart SCORE study. Eur Heart J 2010; 31:2808–2815.
- Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol 2002; 40:754–760.
- Perregaux D, Chaudhuri A, Rao S, et al. Brachial vascular reactivity in blacks. Hypertension 2000; 36:866–871.
- Duprez DA, Jacobs DR, Lutsey PL, et al. Race/ethnic and sex differences in large and small artery elasticity—results of the multiethnic study of atherosclerosis (MESA). Ethn Dis 2009; 19:243–250.
- Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation 2004; 109:2511–2517.
- Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 1999; 43:562–571.
- Gattás GJ, Kato M, Soares-Vieira JA, et al. Ethnicity and glutathione S-transferase (GSTM1/GSTT1) polymorphisms in a Brazilian population. Braz J Med Biol Res 2004; 37:451–458.
- Li R, Lyn D, Lapu-Bula R, et al. Relation of endothelial nitric oxide synthase gene to plasma nitric oxide level, endothelial function, and blood pressure in African Americans. Am J Hypertens 2004; 17:560–567.
- Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet 1998; 63:1839–1851.
- Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med 2002; 347:1135–1142.
- McNamara DM, Tam SW, Sabolinski ML, et al. Aldosterone synthase promoter polymorphism predicts outcome in African Americans with heart failure: results from the A-HeFT Trial. J Am Coll Cardiol 2006; 48:1277–1282.
- McNamara DM, Tam SW, Sabolinski ML, et al. Endothelial nitric oxide synthase (NOS3) polymorphisms in African Americans with heart failure: results from the A-HeFT trial. J Card Fail 2009; 15:191–198.
- Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med 1997; 336:466–473.
- Buxbaum J, Alexander A, Koziol J, Tagoe C, Fox E, Kitzman D. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J 2010; 159:864–870.
- Philbin EF, Dec GW, Jenkins PL, DiSalvo TG. Socioeconomic status as an independent risk factor for hospital readmission for heart failure. Am J Cardiol 2001; 87:1367–1371.
- Evangelista LS, Dracup K, Doering LV. Racial differences in treatment-seeking delays among heart failure patients. J Card Fail 2002; 8:381–386.
- Kressin NR, Orner MB, Manze M, Glickman ME, Berlowitz D. Understanding contributors to racial disparities in blood pressure control. Circ Cardiovasc Qual Outcomes 2010; 3:173–180.
- Yancy CW, Jessup M, Bozkurt B, et al; ACCF/AHA Task Force Members. 2013 ACCF/AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e329.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427. E-pub ahead of print.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 2010; 362:590–599.
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- Casagrande SS, Whitt-Glover MC, Lancaster KJ, Odoms-Young AM, Gary TL. Built environment and health behaviors among African Americans: a systematic review. Am J Prev Med 2009; 36:174–181.
- Gustat J, Rice J, Parker KM, Becker AB, Farley TA. Effect of changes to the neighborhood built environment on physical activity in a low-income African American neighborhood. Prev Chronic Dis 2012; 9:E57.
- Casagrande SS, Franco M, Gittelsohn J, et al. Healthy food availability and the association with BMI in Baltimore, Maryland. Public Health Nutr 2011; 14:1001–1007.
- Stewart JE, Battersby SE, Lopez-De Fede A, Remington KC, Hardin JW, Mayfield-Smith K. Diabetes and the socioeconomic and built environment: geovisualization of disease prevalence and potential contextual associations using ring maps. Int J Health Geogr 2011; 10:18.
- Franciosa JA, Ferdinand KC, Yancy CW; Consensus Statement on Heart Failure in African Americans Writing Group. Treatment of heart failure in African Americans: a consensus statement. Congest Heart Fail 2010; 16:27–38.
- Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J 2006; 27:178–186.
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure.The SOLVD Investigators. N Engl J Med 1991; 325:293–302.
- Shekelle PG, Rich MW, Morton SC, et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol 2003; 41:1529–1538.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Brown NJ, Ray WA, Snowden M, Griffin MR. Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther 1996; 60:8–13.
- Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001; 344:1659–1667.
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Goldstein S, Deedwania P, Gottlieb S, Wikstrand J; MERIT-HF Study Group. Metoprolol CR/XL in black patients with heart failure (from the Metoprolol CR/XL randomized intervention trial in chronic heart failure). Am J Cardiol 2003; 92:478–480.
- Bristow MR, Murphy GA, Krause-Steinrauf H, et al. An alpha2C-adrenergic receptor polymorphism alters the norepinephrine-lowering effects and therapeutic response of the beta-blocker bucindolol in chronic heart failure. Circ Heart Fail 2010; 3:21–28.
- Bristow MR, Krause-Steinrauf H, Nuzzo R, et al. Effect of baseline or changes in adrenergic activity on clinical outcomes in the beta-blocker evaluation of survival trial. Circulation 2004; 110:1437–1442.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Cavallari LH, Groo VL, Momary KM, Fontana D, Viana MA, Vaitkus P. Racial differences in potassium response to spironolactone in heart failure. Congest Heart Fail 2006; 12:200–205.
- Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325:303–310.
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Anand IS, Tam SW, Rector TS, et al. Influence of blood pressure on the effectiveness of a fixed-dose combination of isosorbide dinitrate and hydralazine in the African-American Heart Failure Trial. J Am Coll Cardiol 2007; 49:32–39.
- Echols MR, Felker GM, Thomas KL, et al. Racial differences in the characteristics of patients admitted for acute decompensated heart failure and their relation to outcomes: results from the OPTIME-CHF trial. J Card Fail 2006; 12:684–688.
- Kamath SA, Drazner MH, Wynne J, Fonarow GC, Yancy CW. Characteristics and outcomes in African American patients with decompensated heart failure. Arch Intern Med 2008; 168:1152–1158.
- Felker GM, Lee KL, Bull DA, et al; NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364:797–805.
- Costanzo MR, Guglin ME, Saltzberg MT, et al; UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007; 49:675–683.
- Bart BA, Goldsmith SR, Lee KL, et al; Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Levy P, Compton S, Welch R, et al. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann Emerg Med 2007; 50:144–152.
- Cleland JG, Daubert JC, Erdmann E, et al; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352:1539–1549.
- Young JB, Abraham WT, Smith AL, et al; Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA 2003; 289:2685–2694.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bristow MR, Saxon LA, Boehmer J, et al; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 350:2140–2150.
- Mitchell JE, Hellkamp AS, Mark DB, et al; SCD-HeFT Investigators. Outcome in African Americans and other minorities in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Am Heart J 2008; 155:501–506.
- Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA 2007; 298:1525–1532.
- Farmer SA, Kirkpatrick JN, Heidenreich PA, Curtis JP, Wang Y, Groeneveld PW. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm 2009; 6:325–331.
- Colvin-Adams M, Smith JM, Heubner BM, et al. OPTN/SRTR 2011 Annual Data Report: heart. Am J Transplant 2013; 13(suppl 1):119–148.
- Allen JG, Weiss ES, Arnaoutakis GJ, et al. The impact of race on survival after heart transplantation: an analysis of more than 20,000 patients. Ann Thorac Surg 2010; 89:1956–1964.
- Liu V, Bhattacharya J, Weill D, Hlatky MA. Persistent racial disparities in survival after heart transplantation. Circulation 2011; 123:1642–1649.
- Mahle WT, Kanter KR, Vincent RN. Disparities in outcome for black patients after pediatric heart transplantation. J Pediatr 2005; 147:739–743.
- Park MH, Tolman DE, Kimball PM. Disproportionate HLA matching may contribute to racial disparity in patient survival following cardiac transplantation. Clin Transplant 1996; 10(6 Pt 2):625–628.
- Park MH, Tolman DE, Kimball PM. The impact of race and HLA matching on long-term survival following cardiac transplantation. Transplant Proc 1997; 29:1460–1463.
- Callender CO, Cherikh WS, Miles PV, et al. Blacks as donors for transplantation: suboptimal outcomes overcome by transplantation into other minorities. Transplant Proc 2008; 40:995–1000.
- King LP, Siminoff LA, Meyer DM, et al. Health insurance and cardiac transplantation: a call for reform. J Am Coll Cardiol 2005; 45:1388–1391.
- Ozminkowski RJ, White AJ, Hassol A, Murphy M. Minimizing racial disparity regarding receipt of a cadaver kidney transplant. Am J Kidney Dis 1997; 30:749–759.
- Aggarwal A, Gupta A, Pappas PS, Tatooles A, Bhat G. Racial differences in patients with left ventricular assist devices. ASAIO J 2012; 58:499–502.
- Tsiouris A, Brewer RJ, Borgi J, Nemeh H, Paone G, Morgan JA. Continuous-flow left ventricular assist device implantation as a bridge to transplantation or destination therapy: racial disparities in outcomes. J Heart Lung Transplant 2013; 32:299–304.
- Joyce DL, Conte JV, Russell SD, Joyce LD, Chang DC. Disparities in access to left ventricular assist device therapy. J Surg Res 2009; 152:111–117.
African Americans are disproportionately affected by heart failure and have not experienced the same benefit from treatment as white patients have. Much of the disparity can be blamed on modifiable risk factors such as uncontrolled hypertension and on suboptimal health care. When African Americans are treated according to guidelines, discrepant outcomes can be minimized.
In this article, we review the processes contributing to heart failure in African Americans, its management, and challenges with regard to disparities.
HEART FAILURE IS INCREASING
Despite 20 years of progress in understanding the pathophysiology of heart failure and developing medical and surgical therapies for it, its prevalence and associated morbidity are increasing in the United States. In 2010, 6.6 million (2.8%) of the adults in the United States had heart failure,1 and the prevalence is expected to increase by about 25% by 2030.
DISPARITIES IN INCIDENCE, OUTCOMES
Heart failure is more prevalent in African Americans than in whites, imposes higher rates of death and morbidity, and has a more malignant course.1–6
According to American Heart Association statistics, the annual incidence of heart failure in whites is approximately 6 per 1,000 person-years, while in African Americans it is 9.1 per 1,000 person-years.1 In the Atherosclerosis Risk in Communities study, the incidence of new heart failure was 1.0 per 1,000 person-years in Chinese Americans, 2.4 in whites, 3.5 in Hispanics, and 4.6 in African Americans.2
Moreover, when hospitalized for heart failure, African Americans have a 45% greater risk of death or decline in functional status than whites.7
Heart failure also occurs earlier in African Americans. Bibbins-Domingo et al8 reported that heart failure before age 50 was 20 times more frequent in African Americans than in whites. Functional and structural cardiac changes appeared an average of 10 years before the onset of symptoms and were strongly associated with the development of subsequent heart failure.8
In the Women’s Health Initiative, African American women had higher rates of heart failure than white women, perhaps in part because of higher rates of diabetes.9
Heart failure with preserved ejection fraction
About half of patients who have signs and symptoms of heart failure have a normal (“preserved”) ejection fraction. The incidence of this condition, previously called diastolic heart failure, appears to be similar between African Americans and whites. However, African Americans appear to have a greater incidence of factors that predispose to it and tend to present later in the course.10 For example, African Americans have higher left ventricular mass and wall thickness and a higher incidence of left ventricular hypertrophy than white patients.11–13 In addition, those with heart failure with preserved ejection fraction tend to be younger, female, more likely to have hypertension and diabetes, and less likely to have coronary artery disease, and tend to have worse renal function than their white counterparts.14,15 The predisposition to diastolic impairment persists even after adjusting for risk factors.11–15 The mortality rate in African Americans with heart failure with preserved ejection fraction and without coronary artery disease may also be higher than that of comparable white patients.16
WHY DO AFRICAN AMERICANS HAVE MORE HEART FAILURE?
Modifiable risk factors
In African Americans, the higher percentage of cases of heart failure is attributable to modifiable risk factors such as hypertension, hyperglycemia, left ventricular hypertrophy, and smoking, and fewer cases are due to ischemic heart disease.2,3 Nonischemic cardiomyopathy predominates in African Americans, whereas ischemic cardiomyopathy predominates in whites.
Hypertension, diabetes, obesity, and chronic kidney disease all portend subsequent heart failure and are common in African Americans, but hypertension is the main culprit.3,5,8,17–21 The prevalence of hypertension in African Americans is among the highest in the world, and because African Americans are more likely to have poorer control of their hypertension, they consequently have more target-organ damage.22 Indeed, in many hypertensive African Americans who develop heart failure, the hypertension is poorly controlled. However, even after adjusting for risk factors, and particularly blood pressure control, African Americans remain at higher risk of heart failure.23
The specific mechanistic links between hypertension and heart failure remain to be identified. Despite having a higher prevalence of left ventricular hypertrophy and left ventricular remodeling, African Americans with heart failure tend toward systolic heart failure, as opposed to heart failure with preserved ejection fraction.
Neurohormonal imbalances and endothelial dysfunction
Derangements in the renin-angiotensin-aldosterone and adrenergic axes are likely the main pathophysiologic mechanisms in the genesis of heart failure in all populations. However, other factors may underlie the enhanced disease burden in African Americans.
Impaired endothelial function, as evidenced by impaired digital and brachial artery vasomotion, is very common in African Americans.24–26 The small arteries of African Americans are less elastic than those of whites and Chinese.27 The underlying mechanism may be related to increased oxidative stress, decreased nitric oxide availability, exaggerated vasoconstrictor response, and attenuated responsiveness to vasodilators and nitric oxide.28–31
Genetic polymorphisms
An important caveat in discussing racial differences in heart failure is that “race” is completely arbitrary and is based on sociopolitical rather than scientific or physiologic definitions. Perceived genetic influences are likely to represent complex gene-gene, gene-environment, and gene-drug interactions.
This is especially true for African Americans, who are a markedly heterogeneous group. The US Office of Management and Budget defines “black” or “African American” as having origins in any of the black racial groups of Africa (www.census.gov/2010census/data). Thus, “African American” includes sixth-generation descendants of African slaves, recently immigrated Jamaicans, and black descendants of French and Spanish people.
Most African Americans have some European ancestry. In one study, the estimated proportion of European ancestry ranged from 7% in Jamaicans of African descent to approximately 23% in African Americans in New Orleans.32
Nevertheless, several polymorphisms associated with the risk of heart failure may provide insight into some of the “race-based” differences in pathophysiology and response to medications and, it is hoped, may eventually serve as the basis for tailored therapy. Genes of interest include those for:
- Beta 1 adrenergic receptor
- Alpha 2c receptor33
- Aldosterone synthase34
- G protein
- Transforming growth factor beta
- Nitric oxide synthase35
- Transthyrectin.36,37
Socioeconomic factors and quality of care
Heart failure patients—and especially African Americans—have high rates of hospital readmission, and socioeconomic factors have been implicated. In more than 40,000 patients with heart failure, lower income was a significant predictor of hospital readmission.38 Socioeconomic factors in turn could account for delay in seeking treatment for worsening symptoms, failure to recognize symptoms, limited disease awareness, inadequate access to health care, noncompliance with follow-up appointments, and poor adherence to recommended treatment, all of which are common in African American patients.38,39
African Americans also report more discrimination from health care providers, have more concerns about blood pressure medications, and are more likely to have misperceptions about high blood pressure (eg, that it is not serious), all of which may interfere with optimal blood pressure control.40 Managing heart failure in African Americans should include trying to identify and eliminate barriers to attaining treatment goals.
PREVENTING HEART FAILURE BY REDUCING RISK FACTORS
The American College of Cardiology Foundation and American Heart Association, in their 2013 guidelines, underscored the progressive nature of heart failure by defining four stages of the disease, from stage A (at risk) through stage D (refractory heart failure) (Figure 1).41 They also emphasized the importance of preventing it.
A thorough clinical assessment, with appropriate assessment for risk factors and intervention at stage A, is critical in preventing left ventricular remodeling and heart failure. These risk factors include hypertension, hyperlipidemia, atherosclerosis, diabetes mellitus, valvular disease, obesity, physical inactivity, excessive alcohol intake, poor diet, and smoking.
Hypertension is especially important in African Americans and requires vigorous screening and aggressive treatment. Antihypertensive drugs should be prescribed early, with a lower threshold for escalating therapy with combinations of drugs, as most patients require more than one.
There is considerable debate about the appropriate blood pressure thresholds for diagnosing hypertension and the optimal target blood pressures in African Americans. The 2014 report of the Joint National Committee recommends a similar hypertension treatment target of 140/90 mm Hg for all patients except older adults (for whom 150/90 mm Hg is acceptable), and no separate target for African Americans.42 Previous guidelines from this committee recommended thiazide-type diuretics as first-line therapy for hypertension in African Americans43; the new ones recommend thiazide-type diuretics or calcium channel blockers. However, in those with left ventricular systolic dysfunction, hypertension treatment should include drugs shown to reduce the risk of death in heart failure—ie, angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, hydralazine, nitrates, and aldosterone receptor antagonists.
Salt intake should be reduced to less than 3 g per day (1,200 mg of sodium per day), which has been shown to substantially reduce rates of cardiovascular morbidity and mortality and health care costs.44 Since most Americans consume 7 to 10 g of salt per day, strict salt restriction should be encouraged as a preventive measure.
Diabetes should be screened for and treated in African Americans per current American Diabetes Association guidelines.
Dyslipidemia should also be screened for and treated per guidelines.45
Smoking cessation, moderation of alcohol intake, and avoidance of illicit drugs should be encouraged. Given that African Americans develop heart failure at a relatively early age, the level of vigilance should be high and the threshold for screening should be low.
Healthy neighborhoods, healthy people
Neighborhoods can be designed and built with wellness in mind, incorporating features such as access to healthy food and walkability. Living in such neighborhoods leads to more physical activity and less obesity, although this relationship may be less robust in African Americans.46–49
Environmental factors are multifactorial in African Americans and extend beyond those afforded by the built environment. For instance, lack of safety may hinder the potential benefit of an otherwise walkable neighborhood. These interactions are highly complex, and more investigation is needed to determine the effect of built environments on risk factors in African Americans.
DRUG THERAPY FOR HEART FAILURE IN AFRICAN AMERICANS
Use standard therapies
ACE inhibitors, beta-blockers, and aldosterone antagonists are the standard of care in heart failure, with digoxin (Lanoxin) and diuretics used as adjuncts to control symptoms.
African Americans may respond differently than whites to some of these drugs (Table 1). However, these findings should be interpreted with caution, since most of them came from subgroup analyses of trials in which African Americans accounted for as many as 28% to as few as 1%.50 To date, no data unequivocally show that we should use standard heart failure therapies any differently in African Americans than in whites.
Digoxin: Limited role to control symptoms
Post hoc analysis of the Digitalis Investigation Group trial, in which 14% of the patients were nonwhite, revealed that compared with placebo, digitalis (and achieving a serum digitalis concentration of 0.5 to 0.9 ng/mL) was associated with lower rates of all-cause mortality in most subgroups—except nonwhites.51
In general, digoxin has a limited role in heart failure, since other drugs are available that substantially modify outcomes. However, it can be considered in patients who have persistent heart failure symptoms.
ACE inhibitors, ARBs are recommended
ACE inhibitors are recommended for patients with New York Heart Association (NYHA) class I, II, III, or IV heart failure (class I recommendation, ie, “recommended”; level of evidence A on a scale of A, B, and C) and as part of standard therapy for African American patients with heart failure with symptomatic or asymptomatic left ventricular systolic dysfunction (class I recommendation; level of evidence C).41
Although African American patients did not appear to derive any benefit from enalapril (Vasotec) in the Studies of Left Ventricular Dysfunction (SOLVD) trial,52 a subsequent analysis that involved the SOLVD Prevention Trial did not find any differences between African Americans and whites in response to this agent.6 Similarly, a meta-analysis did not suggest differences in ACE-inhibitor efficacy in reducing adverse cardiovascular outcomes in heart failure between African Americans and non–African Americans.53
Of note: African Americans have a 3% to 4% higher incidence of angioedema from ACE inhibitors than whites.54,55
Angiotensin receptor blockers (ARBs) can be used as substitute therapy in African Americans who cannot tolerate ACE inhibitors (class IIa recommendation, ie, “reasonable”; level of evidence B).41
Beta-blockers also recommended
Beta-blockers are recommended in NYHA class I, II, III, and IV heart failure (class I recommendation; level of evidence A) and as part of standard therapy for African Americans with heart failure due to symptomatic left ventricular systolic dysfunction (class I recommendation; level of evidence B) and asymptomatic left ventricular systolic dysfunction (level of evidence C).41
Carvedilol (Coreg) and metoprolol (Lopressor) are the standard beta-blockers used to treat heart failure, and these drugs should be used in African Americans as well as in whites.15,53,56–59 Of interest, however, race-specific differences may exist in the beta-adrenergic pathway.60,61
Aldosterone antagonists: More study needed
Aldosterone antagonists, also called mineralocorticoid antagonists, ie, spironolactone (Aldactone) and eplerenone (Inspra), are recommended in addition to beta-blockers and ACE inhibitors for NYHA class II–IV heart failure, unless contraindicated (class I recommendation; level of evidence A).
However, trials of aldosterone antagonists to date have enrolled few African Americans.62–64 The limited data suggest that African Americans with heart failure may be less responsive to the renal effects of spironolactone, demonstrating less of an increase in serum potassium levels, and there are essentially no data to guide the use of these drugs in African Americans with heart failure.65 Further study is needed. But in the absence of data to the contrary, these agents, should also be used in African American patients with class III or IV heart failure.
Hydralazine plus nitrates: Recommended for African Americans
Hydralazine plus isosorbide dinitrate (available as BiDil) is recommended as part of standard therapy, in addition to beta-blockers and ACE inhibitors specifically for African Americans with left ventricular systolic dysfunction and NYHA class III or IV heart failure (class I recommendation; level of evidence A), as well as NYHA class II heart failure (class I recommendation; level of evidence B).41
Preliminary evidence for this combination came from the Department of Veterans Affairs Cooperative Vasodilator-Heart Failure Trials.66
Subsequently, the African-American Heart Failure Trial67 was conducted in self-identified African American patients with NYHA class III or IV heart failure on standard heart failure therapy, including an ACE inhibitor if tolerated. Patients were randomly assigned to receive a fixed combination of isosorbide 20 mg and hydralazine 37.5 mg, one or two tablets three times a day, or placebo. The target dose of isosorbide dinitrate was 120 mg, and the target dose of hydralazine was 225 mg daily. Follow-up was up to 18 months. The study was terminated early because of a significant 43% improvement in overall survival for the patients in the isosorbide-hydralazine group. In addition, the rate of first hospitalization was 39% lower and the mean improvement in quality-of-life scores was 52% greater with isosorbide-hydralazine than with placebo.67
There has been much debate about whether the benefit seen in this trial was the result of a hemodynamic effect, blood pressure response, or neurohormonal modulation. The benefit is less likely from a reduction in blood pressure, as the patients who had low blood pressure derived a mortality benefit similar to those with higher blood pressure, despite no further reduction in their blood pressure.68
Treatment for heart failure with preserved ejection fraction
Although there are no data on how to manage heart failure with preserved ejection fraction that are specific to African Americans, the ACCF/AHA guideline41 recommends treating systolic and diastolic hypertension (class I, level of evidence B) according to published clinical practice guidelines and using diuretics to alleviate volume overload (class I; level of evidence C). Revascularization and management of atrial fibrillation are also “reasonable,” as are the use of ARBs, ACE inhibitors, and beta-blockers in the management of hypertension (class IIa; level of evidence C). ARBs may also be considered to reduce hospitalization in symptomatic patients with heart failure with preserved ejection fraction (class IIb, ie, “may be considered”; level of evidence B).
For acute decompensated heart failure
One of the greatest challenges in heart failure is treating patients who present with acute decompensated heart failure.
As in the general population, the major precipitating factor for hospitalization with decompensated heart failure in African Americans is nonadherence to prescribed dietary and medication regimens.35 African Americans with acute decompensated heart failure tend to be younger and to have nonischemic cardiomyopathy, hypertension, diabetes, and obesity, but a lower risk of death.35,69,70 Up to 44% have uncontrolled hypertension.35
Inotropes and vasodilators have undergone multiple trials in the acutely decompensated state in the general population, but no trial has demonstrated a reduction in the mortality rate, and some showed a higher mortality rate. Thus, the treatment of acute decompensated heart failure remains primarily consensus-guided and symptom-focused.
Loop diuretics have been the mainstay in managing fluid retention and congestion in heart failure. The Diuretic Optimization Strategies Evaluation trial tested low-dose vs high-dose intravenous furosemide (Lasix) given either as a continuous infusion or as intermittent intravenous boluses. All strategies were safe and effective.71
Although ultrafiltration is an effective method of decongestion in heart failure and has been associated with a reduction in hospitalization, it is also associated with worsening renal function.72 The Cardiorenal Rescue Study in Acute Decompensated Heart Failure73 compared ultrafiltration vs stepped diuretic therapy. In this trial, which enrolled approximately 26% nonwhites, stepped diuretic therapy was superior to ultrafiltration in preserving renal function in acute decompensated heart failure, although the efficacy of fluid removal was similar.
Both studies were small, and subgroup analyses are not likely to yield useful information. Nevertheless, these data support the use of intravenous diuretics, by continuous infusion or bolus, in acute decompensated heart failure.
Despite no benefit in terms of the mortality rate, inotropes continue to be used in some cases of acute decompensated heart failure, and African Americans appear to have a response to milrinone (Primacor IV) similar to that in whites.69
In a nonrandomized study in which most patients were black, high-dose intravenous nitroglycerin appeared to be safe and associated with less need for ventilator support and intensive care unit admission, compared retrospectively with a population that did not receive high-dose nitroglycerin.74
Given the different profile of the African American patient with acute decompensated heart failure, prospective studies would be useful in determining the best management strategy.
TREATMENTS FOR ADVANCED HEART FAILURE
Cardiac resynchronization and implantable cardioverter-defibrillators
Cardiac resynchronization therapy is indicated for patients with NYHA class II, III, and ambulatory class IV heart failure and left ventricular ejection fraction less than or equal to 35%, sinus rhythm, left bundle branch block, and a QRS duration greater than or equal to 150 ms (class I recommendation; level of evidence A for class NYHA III and IV; level of evidence B for NYHA class II).41
An implantable cardioverter-defibrillator is recommended in patients with NYHA class II or III heart failure for primary prevention of sudden cardiac death in selected patients with nonischemic dilated cardiomyopathy or ischemic heart disease (class I recommendation; level of evidence A).
However, few members of racial and ethnic minorities were included in trials of implantable cardioverter-defibrillators75,76 or cardiac resynchronization,7,77,78 so that subgroup analysis is limited. Use of an implantable cardioverter-defibrillator showed similar reduction in mortality between African Americans and whites, and compliance with device implantation and medical therapy was comparable.79
Among patients discharged from hospitals in the American Heart Association’s Get With the Guidelines–Heart Failure Quality Improvement Program, fewer than 40% of potentially eligible patients received an implantable cardioverter-defibrillator, and rates were significantly lower for African Americans.80 When they can get cardiac resynchronization therapy, African Americans appear to experience similar benefit from it.81
Heart transplantation: Poorer outcomes in African Americans?
Heart transplantation remains the most effective and durable therapy for advanced heart failure. Median survival approaches 14 years.82
However, a retrospective study found that African American recipients had an 11.5% lower 10-year survival rate than whites, which persisted after adjusting for risk, donor-recipient matching by race, and censoring of deaths in the first year.83 Although socioeconomic factors and poor human leukocyte antigen matching have been implicated, a retrospective cohort study showed that African American recipients had a higher risk of death than white recipients even after adjustment for recipient, transplant, and socioeconomic factors.84–87 African Americans were more likely to die of graft failure or of a cardiovascular cause than white patients, but were less likely to die of infection or malignancy. Although mortality rates decreased over time for all transplant recipients, the disparity in mortality rates between African Americans and whites remained essentially unchanged.84
Among all donor-recipient combinations, African American recipients of hearts from African American donors had the highest risk of death.88
Limited access to transplantation persists, particularly for African Americans of lower socioeconomic status. African Americans are more likely than whites to be uninsured, and the funding requirement to be placed on the transplantation list disproportionately affects African Americans.89,90
Left-ventricular assist devices
Left-ventricular assist devices (LVADs) improve survival in heart transplantation candidates and heart failure patients who do not qualify for transplantation. After LVAD implantation, African American patients have similar 1- and 2-year survival rates and no difference in readmission rates compared with whites.91,92
Access to LVAD implantation, however, is significantly influenced by race, and African Americans are significantly less likely to receive one (OR = 0.29).93 Further investigation is required to identify disparities in outcome, access, and contributing factors.
DISPARITIES CAN BE MINIMIZED
In general, heart failure in African Americans is characterized by a high prevalence of hypertension as a major risk factor and potentially different pathogenesis than in the general population. Furthermore, heart failure in African Americans is more prevalent, occurs at an early age, and has a more severe course than in whites, perhaps because of a higher prevalence of risk factors such as diabetes mellitus, obesity, and again, hypertension. These disparities are multifactorial and involve a complex interplay between genes, environment, and socioeconomic factors.
For now, heart failure in African Americans should be treated according to standard evidenced-based strategies, which include a combination of isosorbide dinitrate and hydralazine in addition to other neurohormonal modifying agents (ACE inhibitors, beta-blockers, aldosterone antagonists), a strategy demonstrated to reduce mortality rates in African Americans. When treated according to guidelines, disparities in outcomes can be minimized.
However, many questions about managing heart failure remain unanswered, since African Americans have been markedly underrepresented in clinical trials. Clinical trials need to enroll enough African Americans to answer the questions of interest. Disparities in outcomes must be investigated in a scientific and hypothesis-driven manner. The effect of the built environment on African Americans needs more study as well, as success with these strategies may be impeded by unrecognized factors.
Preventing heart failure should be a priority. Efforts should be directed toward detecting and modifying risk factors early, managing hypertension aggressively, and identifying left ventricular dysfunction early.
African Americans are disproportionately affected by heart failure and have not experienced the same benefit from treatment as white patients have. Much of the disparity can be blamed on modifiable risk factors such as uncontrolled hypertension and on suboptimal health care. When African Americans are treated according to guidelines, discrepant outcomes can be minimized.
In this article, we review the processes contributing to heart failure in African Americans, its management, and challenges with regard to disparities.
HEART FAILURE IS INCREASING
Despite 20 years of progress in understanding the pathophysiology of heart failure and developing medical and surgical therapies for it, its prevalence and associated morbidity are increasing in the United States. In 2010, 6.6 million (2.8%) of the adults in the United States had heart failure,1 and the prevalence is expected to increase by about 25% by 2030.
DISPARITIES IN INCIDENCE, OUTCOMES
Heart failure is more prevalent in African Americans than in whites, imposes higher rates of death and morbidity, and has a more malignant course.1–6
According to American Heart Association statistics, the annual incidence of heart failure in whites is approximately 6 per 1,000 person-years, while in African Americans it is 9.1 per 1,000 person-years.1 In the Atherosclerosis Risk in Communities study, the incidence of new heart failure was 1.0 per 1,000 person-years in Chinese Americans, 2.4 in whites, 3.5 in Hispanics, and 4.6 in African Americans.2
Moreover, when hospitalized for heart failure, African Americans have a 45% greater risk of death or decline in functional status than whites.7
Heart failure also occurs earlier in African Americans. Bibbins-Domingo et al8 reported that heart failure before age 50 was 20 times more frequent in African Americans than in whites. Functional and structural cardiac changes appeared an average of 10 years before the onset of symptoms and were strongly associated with the development of subsequent heart failure.8
In the Women’s Health Initiative, African American women had higher rates of heart failure than white women, perhaps in part because of higher rates of diabetes.9
Heart failure with preserved ejection fraction
About half of patients who have signs and symptoms of heart failure have a normal (“preserved”) ejection fraction. The incidence of this condition, previously called diastolic heart failure, appears to be similar between African Americans and whites. However, African Americans appear to have a greater incidence of factors that predispose to it and tend to present later in the course.10 For example, African Americans have higher left ventricular mass and wall thickness and a higher incidence of left ventricular hypertrophy than white patients.11–13 In addition, those with heart failure with preserved ejection fraction tend to be younger, female, more likely to have hypertension and diabetes, and less likely to have coronary artery disease, and tend to have worse renal function than their white counterparts.14,15 The predisposition to diastolic impairment persists even after adjusting for risk factors.11–15 The mortality rate in African Americans with heart failure with preserved ejection fraction and without coronary artery disease may also be higher than that of comparable white patients.16
WHY DO AFRICAN AMERICANS HAVE MORE HEART FAILURE?
Modifiable risk factors
In African Americans, the higher percentage of cases of heart failure is attributable to modifiable risk factors such as hypertension, hyperglycemia, left ventricular hypertrophy, and smoking, and fewer cases are due to ischemic heart disease.2,3 Nonischemic cardiomyopathy predominates in African Americans, whereas ischemic cardiomyopathy predominates in whites.
Hypertension, diabetes, obesity, and chronic kidney disease all portend subsequent heart failure and are common in African Americans, but hypertension is the main culprit.3,5,8,17–21 The prevalence of hypertension in African Americans is among the highest in the world, and because African Americans are more likely to have poorer control of their hypertension, they consequently have more target-organ damage.22 Indeed, in many hypertensive African Americans who develop heart failure, the hypertension is poorly controlled. However, even after adjusting for risk factors, and particularly blood pressure control, African Americans remain at higher risk of heart failure.23
The specific mechanistic links between hypertension and heart failure remain to be identified. Despite having a higher prevalence of left ventricular hypertrophy and left ventricular remodeling, African Americans with heart failure tend toward systolic heart failure, as opposed to heart failure with preserved ejection fraction.
Neurohormonal imbalances and endothelial dysfunction
Derangements in the renin-angiotensin-aldosterone and adrenergic axes are likely the main pathophysiologic mechanisms in the genesis of heart failure in all populations. However, other factors may underlie the enhanced disease burden in African Americans.
Impaired endothelial function, as evidenced by impaired digital and brachial artery vasomotion, is very common in African Americans.24–26 The small arteries of African Americans are less elastic than those of whites and Chinese.27 The underlying mechanism may be related to increased oxidative stress, decreased nitric oxide availability, exaggerated vasoconstrictor response, and attenuated responsiveness to vasodilators and nitric oxide.28–31
Genetic polymorphisms
An important caveat in discussing racial differences in heart failure is that “race” is completely arbitrary and is based on sociopolitical rather than scientific or physiologic definitions. Perceived genetic influences are likely to represent complex gene-gene, gene-environment, and gene-drug interactions.
This is especially true for African Americans, who are a markedly heterogeneous group. The US Office of Management and Budget defines “black” or “African American” as having origins in any of the black racial groups of Africa (www.census.gov/2010census/data). Thus, “African American” includes sixth-generation descendants of African slaves, recently immigrated Jamaicans, and black descendants of French and Spanish people.
Most African Americans have some European ancestry. In one study, the estimated proportion of European ancestry ranged from 7% in Jamaicans of African descent to approximately 23% in African Americans in New Orleans.32
Nevertheless, several polymorphisms associated with the risk of heart failure may provide insight into some of the “race-based” differences in pathophysiology and response to medications and, it is hoped, may eventually serve as the basis for tailored therapy. Genes of interest include those for:
- Beta 1 adrenergic receptor
- Alpha 2c receptor33
- Aldosterone synthase34
- G protein
- Transforming growth factor beta
- Nitric oxide synthase35
- Transthyrectin.36,37
Socioeconomic factors and quality of care
Heart failure patients—and especially African Americans—have high rates of hospital readmission, and socioeconomic factors have been implicated. In more than 40,000 patients with heart failure, lower income was a significant predictor of hospital readmission.38 Socioeconomic factors in turn could account for delay in seeking treatment for worsening symptoms, failure to recognize symptoms, limited disease awareness, inadequate access to health care, noncompliance with follow-up appointments, and poor adherence to recommended treatment, all of which are common in African American patients.38,39
African Americans also report more discrimination from health care providers, have more concerns about blood pressure medications, and are more likely to have misperceptions about high blood pressure (eg, that it is not serious), all of which may interfere with optimal blood pressure control.40 Managing heart failure in African Americans should include trying to identify and eliminate barriers to attaining treatment goals.
PREVENTING HEART FAILURE BY REDUCING RISK FACTORS
The American College of Cardiology Foundation and American Heart Association, in their 2013 guidelines, underscored the progressive nature of heart failure by defining four stages of the disease, from stage A (at risk) through stage D (refractory heart failure) (Figure 1).41 They also emphasized the importance of preventing it.
A thorough clinical assessment, with appropriate assessment for risk factors and intervention at stage A, is critical in preventing left ventricular remodeling and heart failure. These risk factors include hypertension, hyperlipidemia, atherosclerosis, diabetes mellitus, valvular disease, obesity, physical inactivity, excessive alcohol intake, poor diet, and smoking.
Hypertension is especially important in African Americans and requires vigorous screening and aggressive treatment. Antihypertensive drugs should be prescribed early, with a lower threshold for escalating therapy with combinations of drugs, as most patients require more than one.
There is considerable debate about the appropriate blood pressure thresholds for diagnosing hypertension and the optimal target blood pressures in African Americans. The 2014 report of the Joint National Committee recommends a similar hypertension treatment target of 140/90 mm Hg for all patients except older adults (for whom 150/90 mm Hg is acceptable), and no separate target for African Americans.42 Previous guidelines from this committee recommended thiazide-type diuretics as first-line therapy for hypertension in African Americans43; the new ones recommend thiazide-type diuretics or calcium channel blockers. However, in those with left ventricular systolic dysfunction, hypertension treatment should include drugs shown to reduce the risk of death in heart failure—ie, angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, hydralazine, nitrates, and aldosterone receptor antagonists.
Salt intake should be reduced to less than 3 g per day (1,200 mg of sodium per day), which has been shown to substantially reduce rates of cardiovascular morbidity and mortality and health care costs.44 Since most Americans consume 7 to 10 g of salt per day, strict salt restriction should be encouraged as a preventive measure.
Diabetes should be screened for and treated in African Americans per current American Diabetes Association guidelines.
Dyslipidemia should also be screened for and treated per guidelines.45
Smoking cessation, moderation of alcohol intake, and avoidance of illicit drugs should be encouraged. Given that African Americans develop heart failure at a relatively early age, the level of vigilance should be high and the threshold for screening should be low.
Healthy neighborhoods, healthy people
Neighborhoods can be designed and built with wellness in mind, incorporating features such as access to healthy food and walkability. Living in such neighborhoods leads to more physical activity and less obesity, although this relationship may be less robust in African Americans.46–49
Environmental factors are multifactorial in African Americans and extend beyond those afforded by the built environment. For instance, lack of safety may hinder the potential benefit of an otherwise walkable neighborhood. These interactions are highly complex, and more investigation is needed to determine the effect of built environments on risk factors in African Americans.
DRUG THERAPY FOR HEART FAILURE IN AFRICAN AMERICANS
Use standard therapies
ACE inhibitors, beta-blockers, and aldosterone antagonists are the standard of care in heart failure, with digoxin (Lanoxin) and diuretics used as adjuncts to control symptoms.
African Americans may respond differently than whites to some of these drugs (Table 1). However, these findings should be interpreted with caution, since most of them came from subgroup analyses of trials in which African Americans accounted for as many as 28% to as few as 1%.50 To date, no data unequivocally show that we should use standard heart failure therapies any differently in African Americans than in whites.
Digoxin: Limited role to control symptoms
Post hoc analysis of the Digitalis Investigation Group trial, in which 14% of the patients were nonwhite, revealed that compared with placebo, digitalis (and achieving a serum digitalis concentration of 0.5 to 0.9 ng/mL) was associated with lower rates of all-cause mortality in most subgroups—except nonwhites.51
In general, digoxin has a limited role in heart failure, since other drugs are available that substantially modify outcomes. However, it can be considered in patients who have persistent heart failure symptoms.
ACE inhibitors, ARBs are recommended
ACE inhibitors are recommended for patients with New York Heart Association (NYHA) class I, II, III, or IV heart failure (class I recommendation, ie, “recommended”; level of evidence A on a scale of A, B, and C) and as part of standard therapy for African American patients with heart failure with symptomatic or asymptomatic left ventricular systolic dysfunction (class I recommendation; level of evidence C).41
Although African American patients did not appear to derive any benefit from enalapril (Vasotec) in the Studies of Left Ventricular Dysfunction (SOLVD) trial,52 a subsequent analysis that involved the SOLVD Prevention Trial did not find any differences between African Americans and whites in response to this agent.6 Similarly, a meta-analysis did not suggest differences in ACE-inhibitor efficacy in reducing adverse cardiovascular outcomes in heart failure between African Americans and non–African Americans.53
Of note: African Americans have a 3% to 4% higher incidence of angioedema from ACE inhibitors than whites.54,55
Angiotensin receptor blockers (ARBs) can be used as substitute therapy in African Americans who cannot tolerate ACE inhibitors (class IIa recommendation, ie, “reasonable”; level of evidence B).41
Beta-blockers also recommended
Beta-blockers are recommended in NYHA class I, II, III, and IV heart failure (class I recommendation; level of evidence A) and as part of standard therapy for African Americans with heart failure due to symptomatic left ventricular systolic dysfunction (class I recommendation; level of evidence B) and asymptomatic left ventricular systolic dysfunction (level of evidence C).41
Carvedilol (Coreg) and metoprolol (Lopressor) are the standard beta-blockers used to treat heart failure, and these drugs should be used in African Americans as well as in whites.15,53,56–59 Of interest, however, race-specific differences may exist in the beta-adrenergic pathway.60,61
Aldosterone antagonists: More study needed
Aldosterone antagonists, also called mineralocorticoid antagonists, ie, spironolactone (Aldactone) and eplerenone (Inspra), are recommended in addition to beta-blockers and ACE inhibitors for NYHA class II–IV heart failure, unless contraindicated (class I recommendation; level of evidence A).
However, trials of aldosterone antagonists to date have enrolled few African Americans.62–64 The limited data suggest that African Americans with heart failure may be less responsive to the renal effects of spironolactone, demonstrating less of an increase in serum potassium levels, and there are essentially no data to guide the use of these drugs in African Americans with heart failure.65 Further study is needed. But in the absence of data to the contrary, these agents, should also be used in African American patients with class III or IV heart failure.
Hydralazine plus nitrates: Recommended for African Americans
Hydralazine plus isosorbide dinitrate (available as BiDil) is recommended as part of standard therapy, in addition to beta-blockers and ACE inhibitors specifically for African Americans with left ventricular systolic dysfunction and NYHA class III or IV heart failure (class I recommendation; level of evidence A), as well as NYHA class II heart failure (class I recommendation; level of evidence B).41
Preliminary evidence for this combination came from the Department of Veterans Affairs Cooperative Vasodilator-Heart Failure Trials.66
Subsequently, the African-American Heart Failure Trial67 was conducted in self-identified African American patients with NYHA class III or IV heart failure on standard heart failure therapy, including an ACE inhibitor if tolerated. Patients were randomly assigned to receive a fixed combination of isosorbide 20 mg and hydralazine 37.5 mg, one or two tablets three times a day, or placebo. The target dose of isosorbide dinitrate was 120 mg, and the target dose of hydralazine was 225 mg daily. Follow-up was up to 18 months. The study was terminated early because of a significant 43% improvement in overall survival for the patients in the isosorbide-hydralazine group. In addition, the rate of first hospitalization was 39% lower and the mean improvement in quality-of-life scores was 52% greater with isosorbide-hydralazine than with placebo.67
There has been much debate about whether the benefit seen in this trial was the result of a hemodynamic effect, blood pressure response, or neurohormonal modulation. The benefit is less likely from a reduction in blood pressure, as the patients who had low blood pressure derived a mortality benefit similar to those with higher blood pressure, despite no further reduction in their blood pressure.68
Treatment for heart failure with preserved ejection fraction
Although there are no data on how to manage heart failure with preserved ejection fraction that are specific to African Americans, the ACCF/AHA guideline41 recommends treating systolic and diastolic hypertension (class I, level of evidence B) according to published clinical practice guidelines and using diuretics to alleviate volume overload (class I; level of evidence C). Revascularization and management of atrial fibrillation are also “reasonable,” as are the use of ARBs, ACE inhibitors, and beta-blockers in the management of hypertension (class IIa; level of evidence C). ARBs may also be considered to reduce hospitalization in symptomatic patients with heart failure with preserved ejection fraction (class IIb, ie, “may be considered”; level of evidence B).
For acute decompensated heart failure
One of the greatest challenges in heart failure is treating patients who present with acute decompensated heart failure.
As in the general population, the major precipitating factor for hospitalization with decompensated heart failure in African Americans is nonadherence to prescribed dietary and medication regimens.35 African Americans with acute decompensated heart failure tend to be younger and to have nonischemic cardiomyopathy, hypertension, diabetes, and obesity, but a lower risk of death.35,69,70 Up to 44% have uncontrolled hypertension.35
Inotropes and vasodilators have undergone multiple trials in the acutely decompensated state in the general population, but no trial has demonstrated a reduction in the mortality rate, and some showed a higher mortality rate. Thus, the treatment of acute decompensated heart failure remains primarily consensus-guided and symptom-focused.
Loop diuretics have been the mainstay in managing fluid retention and congestion in heart failure. The Diuretic Optimization Strategies Evaluation trial tested low-dose vs high-dose intravenous furosemide (Lasix) given either as a continuous infusion or as intermittent intravenous boluses. All strategies were safe and effective.71
Although ultrafiltration is an effective method of decongestion in heart failure and has been associated with a reduction in hospitalization, it is also associated with worsening renal function.72 The Cardiorenal Rescue Study in Acute Decompensated Heart Failure73 compared ultrafiltration vs stepped diuretic therapy. In this trial, which enrolled approximately 26% nonwhites, stepped diuretic therapy was superior to ultrafiltration in preserving renal function in acute decompensated heart failure, although the efficacy of fluid removal was similar.
Both studies were small, and subgroup analyses are not likely to yield useful information. Nevertheless, these data support the use of intravenous diuretics, by continuous infusion or bolus, in acute decompensated heart failure.
Despite no benefit in terms of the mortality rate, inotropes continue to be used in some cases of acute decompensated heart failure, and African Americans appear to have a response to milrinone (Primacor IV) similar to that in whites.69
In a nonrandomized study in which most patients were black, high-dose intravenous nitroglycerin appeared to be safe and associated with less need for ventilator support and intensive care unit admission, compared retrospectively with a population that did not receive high-dose nitroglycerin.74
Given the different profile of the African American patient with acute decompensated heart failure, prospective studies would be useful in determining the best management strategy.
TREATMENTS FOR ADVANCED HEART FAILURE
Cardiac resynchronization and implantable cardioverter-defibrillators
Cardiac resynchronization therapy is indicated for patients with NYHA class II, III, and ambulatory class IV heart failure and left ventricular ejection fraction less than or equal to 35%, sinus rhythm, left bundle branch block, and a QRS duration greater than or equal to 150 ms (class I recommendation; level of evidence A for class NYHA III and IV; level of evidence B for NYHA class II).41
An implantable cardioverter-defibrillator is recommended in patients with NYHA class II or III heart failure for primary prevention of sudden cardiac death in selected patients with nonischemic dilated cardiomyopathy or ischemic heart disease (class I recommendation; level of evidence A).
However, few members of racial and ethnic minorities were included in trials of implantable cardioverter-defibrillators75,76 or cardiac resynchronization,7,77,78 so that subgroup analysis is limited. Use of an implantable cardioverter-defibrillator showed similar reduction in mortality between African Americans and whites, and compliance with device implantation and medical therapy was comparable.79
Among patients discharged from hospitals in the American Heart Association’s Get With the Guidelines–Heart Failure Quality Improvement Program, fewer than 40% of potentially eligible patients received an implantable cardioverter-defibrillator, and rates were significantly lower for African Americans.80 When they can get cardiac resynchronization therapy, African Americans appear to experience similar benefit from it.81
Heart transplantation: Poorer outcomes in African Americans?
Heart transplantation remains the most effective and durable therapy for advanced heart failure. Median survival approaches 14 years.82
However, a retrospective study found that African American recipients had an 11.5% lower 10-year survival rate than whites, which persisted after adjusting for risk, donor-recipient matching by race, and censoring of deaths in the first year.83 Although socioeconomic factors and poor human leukocyte antigen matching have been implicated, a retrospective cohort study showed that African American recipients had a higher risk of death than white recipients even after adjustment for recipient, transplant, and socioeconomic factors.84–87 African Americans were more likely to die of graft failure or of a cardiovascular cause than white patients, but were less likely to die of infection or malignancy. Although mortality rates decreased over time for all transplant recipients, the disparity in mortality rates between African Americans and whites remained essentially unchanged.84
Among all donor-recipient combinations, African American recipients of hearts from African American donors had the highest risk of death.88
Limited access to transplantation persists, particularly for African Americans of lower socioeconomic status. African Americans are more likely than whites to be uninsured, and the funding requirement to be placed on the transplantation list disproportionately affects African Americans.89,90
Left-ventricular assist devices
Left-ventricular assist devices (LVADs) improve survival in heart transplantation candidates and heart failure patients who do not qualify for transplantation. After LVAD implantation, African American patients have similar 1- and 2-year survival rates and no difference in readmission rates compared with whites.91,92
Access to LVAD implantation, however, is significantly influenced by race, and African Americans are significantly less likely to receive one (OR = 0.29).93 Further investigation is required to identify disparities in outcome, access, and contributing factors.
DISPARITIES CAN BE MINIMIZED
In general, heart failure in African Americans is characterized by a high prevalence of hypertension as a major risk factor and potentially different pathogenesis than in the general population. Furthermore, heart failure in African Americans is more prevalent, occurs at an early age, and has a more severe course than in whites, perhaps because of a higher prevalence of risk factors such as diabetes mellitus, obesity, and again, hypertension. These disparities are multifactorial and involve a complex interplay between genes, environment, and socioeconomic factors.
For now, heart failure in African Americans should be treated according to standard evidenced-based strategies, which include a combination of isosorbide dinitrate and hydralazine in addition to other neurohormonal modifying agents (ACE inhibitors, beta-blockers, aldosterone antagonists), a strategy demonstrated to reduce mortality rates in African Americans. When treated according to guidelines, disparities in outcomes can be minimized.
However, many questions about managing heart failure remain unanswered, since African Americans have been markedly underrepresented in clinical trials. Clinical trials need to enroll enough African Americans to answer the questions of interest. Disparities in outcomes must be investigated in a scientific and hypothesis-driven manner. The effect of the built environment on African Americans needs more study as well, as success with these strategies may be impeded by unrecognized factors.
Preventing heart failure should be a priority. Efforts should be directed toward detecting and modifying risk factors early, managing hypertension aggressively, and identifying left ventricular dysfunction early.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med 2008; 168:2138–2145.
- Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008; 101:1016–1022.
- Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, et al. Epidemiology of incident heart failure in a contemporary elderly cohort: the health, aging, and body composition study. Arch Intern Med 2009; 169:708–715.
- Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med 1999; 340:609–616.
- Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. N Engl J Med 2001; 344:1351–1357.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med 2009; 360:1179–1190.
- Eaton CB, Abdulbaki AM, Margolis KL, et al. Racial and ethnic differences in incident hospitalized heart failure in postmenopausal women: the Women’s Health Initiative. Circulation 2012; 126:688–696.
- Ilksoy N, Hoffman M, Moore RH, Easley K, Jacobson TA. Comparison of African-American patients with systolic heart failure versus preserved ejection fraction. Am J Cardiol 2006; 98:806–808.
- Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol 2008; 52:1015–1021.
- Kizer JR, Arnett DK, Bella JN, et al. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension 2004; 43:1182–1188.
- Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension 2005; 46:124–129.
- East MA, Peterson ED, Shaw LK, Gattis WA, O’Connor CM. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J 2004; 148:151–156.
- Klapholz M, Maurer M, Lowe AM, et al; New York Heart Failure Consortium. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol 2004; 43:1432–1438.
- Agoston I, Cameron CS, Yao D, Dela Rosa A, Mann DL, Deswal A. Comparison of outcomes of white versus black patients hospitalized with heart failure and preserved ejection fraction. Am J Cardiol 2004; 94:1003–1007.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med 1996; 334:1349–1355.
- Yancy CW, Strong M. The natural history, epidemiology, and prognosis of heart failure in African Americans. Congest Heart Fail 2004; 10:15–18.
- Velagaleti RS, Gona P, Larson MG, et al. Multimarker approach for the prediction of heart failure incidence in the community. Circulation 2010; 122:1700–1706.
- Deswal A, Petersen NJ, Urbauer DL, Wright SM, Beyth R. Racial variations in quality of care and outcomes in an ambulatory heart failure cohort. Am Heart J 2006; 152:348–354.
- Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic And Racial Differences in Stroke study. Stroke 2006; 37:1171–1178.
- Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med 2005; 165:2098–2104.
- Okin PM, Kjeldsen SE, Dahlöf B, Devereux RB. Racial differences in incident heart failure during antihypertensive therapy. Circ Cardiovasc Qual Outcomes 2011; 4:157–164.
- Mulukutla SR, Venkitachalam L, Bambs C, et al. Black race is associated with digital artery endothelial dysfunction: results from the Heart SCORE study. Eur Heart J 2010; 31:2808–2815.
- Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol 2002; 40:754–760.
- Perregaux D, Chaudhuri A, Rao S, et al. Brachial vascular reactivity in blacks. Hypertension 2000; 36:866–871.
- Duprez DA, Jacobs DR, Lutsey PL, et al. Race/ethnic and sex differences in large and small artery elasticity—results of the multiethnic study of atherosclerosis (MESA). Ethn Dis 2009; 19:243–250.
- Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation 2004; 109:2511–2517.
- Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 1999; 43:562–571.
- Gattás GJ, Kato M, Soares-Vieira JA, et al. Ethnicity and glutathione S-transferase (GSTM1/GSTT1) polymorphisms in a Brazilian population. Braz J Med Biol Res 2004; 37:451–458.
- Li R, Lyn D, Lapu-Bula R, et al. Relation of endothelial nitric oxide synthase gene to plasma nitric oxide level, endothelial function, and blood pressure in African Americans. Am J Hypertens 2004; 17:560–567.
- Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet 1998; 63:1839–1851.
- Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med 2002; 347:1135–1142.
- McNamara DM, Tam SW, Sabolinski ML, et al. Aldosterone synthase promoter polymorphism predicts outcome in African Americans with heart failure: results from the A-HeFT Trial. J Am Coll Cardiol 2006; 48:1277–1282.
- McNamara DM, Tam SW, Sabolinski ML, et al. Endothelial nitric oxide synthase (NOS3) polymorphisms in African Americans with heart failure: results from the A-HeFT trial. J Card Fail 2009; 15:191–198.
- Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med 1997; 336:466–473.
- Buxbaum J, Alexander A, Koziol J, Tagoe C, Fox E, Kitzman D. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J 2010; 159:864–870.
- Philbin EF, Dec GW, Jenkins PL, DiSalvo TG. Socioeconomic status as an independent risk factor for hospital readmission for heart failure. Am J Cardiol 2001; 87:1367–1371.
- Evangelista LS, Dracup K, Doering LV. Racial differences in treatment-seeking delays among heart failure patients. J Card Fail 2002; 8:381–386.
- Kressin NR, Orner MB, Manze M, Glickman ME, Berlowitz D. Understanding contributors to racial disparities in blood pressure control. Circ Cardiovasc Qual Outcomes 2010; 3:173–180.
- Yancy CW, Jessup M, Bozkurt B, et al; ACCF/AHA Task Force Members. 2013 ACCF/AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e329.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427. E-pub ahead of print.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 2010; 362:590–599.
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- Casagrande SS, Whitt-Glover MC, Lancaster KJ, Odoms-Young AM, Gary TL. Built environment and health behaviors among African Americans: a systematic review. Am J Prev Med 2009; 36:174–181.
- Gustat J, Rice J, Parker KM, Becker AB, Farley TA. Effect of changes to the neighborhood built environment on physical activity in a low-income African American neighborhood. Prev Chronic Dis 2012; 9:E57.
- Casagrande SS, Franco M, Gittelsohn J, et al. Healthy food availability and the association with BMI in Baltimore, Maryland. Public Health Nutr 2011; 14:1001–1007.
- Stewart JE, Battersby SE, Lopez-De Fede A, Remington KC, Hardin JW, Mayfield-Smith K. Diabetes and the socioeconomic and built environment: geovisualization of disease prevalence and potential contextual associations using ring maps. Int J Health Geogr 2011; 10:18.
- Franciosa JA, Ferdinand KC, Yancy CW; Consensus Statement on Heart Failure in African Americans Writing Group. Treatment of heart failure in African Americans: a consensus statement. Congest Heart Fail 2010; 16:27–38.
- Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J 2006; 27:178–186.
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure.The SOLVD Investigators. N Engl J Med 1991; 325:293–302.
- Shekelle PG, Rich MW, Morton SC, et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol 2003; 41:1529–1538.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Brown NJ, Ray WA, Snowden M, Griffin MR. Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther 1996; 60:8–13.
- Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001; 344:1659–1667.
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Goldstein S, Deedwania P, Gottlieb S, Wikstrand J; MERIT-HF Study Group. Metoprolol CR/XL in black patients with heart failure (from the Metoprolol CR/XL randomized intervention trial in chronic heart failure). Am J Cardiol 2003; 92:478–480.
- Bristow MR, Murphy GA, Krause-Steinrauf H, et al. An alpha2C-adrenergic receptor polymorphism alters the norepinephrine-lowering effects and therapeutic response of the beta-blocker bucindolol in chronic heart failure. Circ Heart Fail 2010; 3:21–28.
- Bristow MR, Krause-Steinrauf H, Nuzzo R, et al. Effect of baseline or changes in adrenergic activity on clinical outcomes in the beta-blocker evaluation of survival trial. Circulation 2004; 110:1437–1442.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Cavallari LH, Groo VL, Momary KM, Fontana D, Viana MA, Vaitkus P. Racial differences in potassium response to spironolactone in heart failure. Congest Heart Fail 2006; 12:200–205.
- Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325:303–310.
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Anand IS, Tam SW, Rector TS, et al. Influence of blood pressure on the effectiveness of a fixed-dose combination of isosorbide dinitrate and hydralazine in the African-American Heart Failure Trial. J Am Coll Cardiol 2007; 49:32–39.
- Echols MR, Felker GM, Thomas KL, et al. Racial differences in the characteristics of patients admitted for acute decompensated heart failure and their relation to outcomes: results from the OPTIME-CHF trial. J Card Fail 2006; 12:684–688.
- Kamath SA, Drazner MH, Wynne J, Fonarow GC, Yancy CW. Characteristics and outcomes in African American patients with decompensated heart failure. Arch Intern Med 2008; 168:1152–1158.
- Felker GM, Lee KL, Bull DA, et al; NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364:797–805.
- Costanzo MR, Guglin ME, Saltzberg MT, et al; UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007; 49:675–683.
- Bart BA, Goldsmith SR, Lee KL, et al; Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Levy P, Compton S, Welch R, et al. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann Emerg Med 2007; 50:144–152.
- Cleland JG, Daubert JC, Erdmann E, et al; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352:1539–1549.
- Young JB, Abraham WT, Smith AL, et al; Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA 2003; 289:2685–2694.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bristow MR, Saxon LA, Boehmer J, et al; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 350:2140–2150.
- Mitchell JE, Hellkamp AS, Mark DB, et al; SCD-HeFT Investigators. Outcome in African Americans and other minorities in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Am Heart J 2008; 155:501–506.
- Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA 2007; 298:1525–1532.
- Farmer SA, Kirkpatrick JN, Heidenreich PA, Curtis JP, Wang Y, Groeneveld PW. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm 2009; 6:325–331.
- Colvin-Adams M, Smith JM, Heubner BM, et al. OPTN/SRTR 2011 Annual Data Report: heart. Am J Transplant 2013; 13(suppl 1):119–148.
- Allen JG, Weiss ES, Arnaoutakis GJ, et al. The impact of race on survival after heart transplantation: an analysis of more than 20,000 patients. Ann Thorac Surg 2010; 89:1956–1964.
- Liu V, Bhattacharya J, Weill D, Hlatky MA. Persistent racial disparities in survival after heart transplantation. Circulation 2011; 123:1642–1649.
- Mahle WT, Kanter KR, Vincent RN. Disparities in outcome for black patients after pediatric heart transplantation. J Pediatr 2005; 147:739–743.
- Park MH, Tolman DE, Kimball PM. Disproportionate HLA matching may contribute to racial disparity in patient survival following cardiac transplantation. Clin Transplant 1996; 10(6 Pt 2):625–628.
- Park MH, Tolman DE, Kimball PM. The impact of race and HLA matching on long-term survival following cardiac transplantation. Transplant Proc 1997; 29:1460–1463.
- Callender CO, Cherikh WS, Miles PV, et al. Blacks as donors for transplantation: suboptimal outcomes overcome by transplantation into other minorities. Transplant Proc 2008; 40:995–1000.
- King LP, Siminoff LA, Meyer DM, et al. Health insurance and cardiac transplantation: a call for reform. J Am Coll Cardiol 2005; 45:1388–1391.
- Ozminkowski RJ, White AJ, Hassol A, Murphy M. Minimizing racial disparity regarding receipt of a cadaver kidney transplant. Am J Kidney Dis 1997; 30:749–759.
- Aggarwal A, Gupta A, Pappas PS, Tatooles A, Bhat G. Racial differences in patients with left ventricular assist devices. ASAIO J 2012; 58:499–502.
- Tsiouris A, Brewer RJ, Borgi J, Nemeh H, Paone G, Morgan JA. Continuous-flow left ventricular assist device implantation as a bridge to transplantation or destination therapy: racial disparities in outcomes. J Heart Lung Transplant 2013; 32:299–304.
- Joyce DL, Conte JV, Russell SD, Joyce LD, Chang DC. Disparities in access to left ventricular assist device therapy. J Surg Res 2009; 152:111–117.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med 2008; 168:2138–2145.
- Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008; 101:1016–1022.
- Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, et al. Epidemiology of incident heart failure in a contemporary elderly cohort: the health, aging, and body composition study. Arch Intern Med 2009; 169:708–715.
- Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med 1999; 340:609–616.
- Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. N Engl J Med 2001; 344:1351–1357.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med 2009; 360:1179–1190.
- Eaton CB, Abdulbaki AM, Margolis KL, et al. Racial and ethnic differences in incident hospitalized heart failure in postmenopausal women: the Women’s Health Initiative. Circulation 2012; 126:688–696.
- Ilksoy N, Hoffman M, Moore RH, Easley K, Jacobson TA. Comparison of African-American patients with systolic heart failure versus preserved ejection fraction. Am J Cardiol 2006; 98:806–808.
- Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol 2008; 52:1015–1021.
- Kizer JR, Arnett DK, Bella JN, et al. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension 2004; 43:1182–1188.
- Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension 2005; 46:124–129.
- East MA, Peterson ED, Shaw LK, Gattis WA, O’Connor CM. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J 2004; 148:151–156.
- Klapholz M, Maurer M, Lowe AM, et al; New York Heart Failure Consortium. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol 2004; 43:1432–1438.
- Agoston I, Cameron CS, Yao D, Dela Rosa A, Mann DL, Deswal A. Comparison of outcomes of white versus black patients hospitalized with heart failure and preserved ejection fraction. Am J Cardiol 2004; 94:1003–1007.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med 1996; 334:1349–1355.
- Yancy CW, Strong M. The natural history, epidemiology, and prognosis of heart failure in African Americans. Congest Heart Fail 2004; 10:15–18.
- Velagaleti RS, Gona P, Larson MG, et al. Multimarker approach for the prediction of heart failure incidence in the community. Circulation 2010; 122:1700–1706.
- Deswal A, Petersen NJ, Urbauer DL, Wright SM, Beyth R. Racial variations in quality of care and outcomes in an ambulatory heart failure cohort. Am Heart J 2006; 152:348–354.
- Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic And Racial Differences in Stroke study. Stroke 2006; 37:1171–1178.
- Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med 2005; 165:2098–2104.
- Okin PM, Kjeldsen SE, Dahlöf B, Devereux RB. Racial differences in incident heart failure during antihypertensive therapy. Circ Cardiovasc Qual Outcomes 2011; 4:157–164.
- Mulukutla SR, Venkitachalam L, Bambs C, et al. Black race is associated with digital artery endothelial dysfunction: results from the Heart SCORE study. Eur Heart J 2010; 31:2808–2815.
- Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol 2002; 40:754–760.
- Perregaux D, Chaudhuri A, Rao S, et al. Brachial vascular reactivity in blacks. Hypertension 2000; 36:866–871.
- Duprez DA, Jacobs DR, Lutsey PL, et al. Race/ethnic and sex differences in large and small artery elasticity—results of the multiethnic study of atherosclerosis (MESA). Ethn Dis 2009; 19:243–250.
- Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation 2004; 109:2511–2517.
- Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 1999; 43:562–571.
- Gattás GJ, Kato M, Soares-Vieira JA, et al. Ethnicity and glutathione S-transferase (GSTM1/GSTT1) polymorphisms in a Brazilian population. Braz J Med Biol Res 2004; 37:451–458.
- Li R, Lyn D, Lapu-Bula R, et al. Relation of endothelial nitric oxide synthase gene to plasma nitric oxide level, endothelial function, and blood pressure in African Americans. Am J Hypertens 2004; 17:560–567.
- Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet 1998; 63:1839–1851.
- Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med 2002; 347:1135–1142.
- McNamara DM, Tam SW, Sabolinski ML, et al. Aldosterone synthase promoter polymorphism predicts outcome in African Americans with heart failure: results from the A-HeFT Trial. J Am Coll Cardiol 2006; 48:1277–1282.
- McNamara DM, Tam SW, Sabolinski ML, et al. Endothelial nitric oxide synthase (NOS3) polymorphisms in African Americans with heart failure: results from the A-HeFT trial. J Card Fail 2009; 15:191–198.
- Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med 1997; 336:466–473.
- Buxbaum J, Alexander A, Koziol J, Tagoe C, Fox E, Kitzman D. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J 2010; 159:864–870.
- Philbin EF, Dec GW, Jenkins PL, DiSalvo TG. Socioeconomic status as an independent risk factor for hospital readmission for heart failure. Am J Cardiol 2001; 87:1367–1371.
- Evangelista LS, Dracup K, Doering LV. Racial differences in treatment-seeking delays among heart failure patients. J Card Fail 2002; 8:381–386.
- Kressin NR, Orner MB, Manze M, Glickman ME, Berlowitz D. Understanding contributors to racial disparities in blood pressure control. Circ Cardiovasc Qual Outcomes 2010; 3:173–180.
- Yancy CW, Jessup M, Bozkurt B, et al; ACCF/AHA Task Force Members. 2013 ACCF/AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e329.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427. E-pub ahead of print.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 2010; 362:590–599.
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- Casagrande SS, Whitt-Glover MC, Lancaster KJ, Odoms-Young AM, Gary TL. Built environment and health behaviors among African Americans: a systematic review. Am J Prev Med 2009; 36:174–181.
- Gustat J, Rice J, Parker KM, Becker AB, Farley TA. Effect of changes to the neighborhood built environment on physical activity in a low-income African American neighborhood. Prev Chronic Dis 2012; 9:E57.
- Casagrande SS, Franco M, Gittelsohn J, et al. Healthy food availability and the association with BMI in Baltimore, Maryland. Public Health Nutr 2011; 14:1001–1007.
- Stewart JE, Battersby SE, Lopez-De Fede A, Remington KC, Hardin JW, Mayfield-Smith K. Diabetes and the socioeconomic and built environment: geovisualization of disease prevalence and potential contextual associations using ring maps. Int J Health Geogr 2011; 10:18.
- Franciosa JA, Ferdinand KC, Yancy CW; Consensus Statement on Heart Failure in African Americans Writing Group. Treatment of heart failure in African Americans: a consensus statement. Congest Heart Fail 2010; 16:27–38.
- Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J 2006; 27:178–186.
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure.The SOLVD Investigators. N Engl J Med 1991; 325:293–302.
- Shekelle PG, Rich MW, Morton SC, et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol 2003; 41:1529–1538.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Brown NJ, Ray WA, Snowden M, Griffin MR. Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther 1996; 60:8–13.
- Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001; 344:1659–1667.
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Goldstein S, Deedwania P, Gottlieb S, Wikstrand J; MERIT-HF Study Group. Metoprolol CR/XL in black patients with heart failure (from the Metoprolol CR/XL randomized intervention trial in chronic heart failure). Am J Cardiol 2003; 92:478–480.
- Bristow MR, Murphy GA, Krause-Steinrauf H, et al. An alpha2C-adrenergic receptor polymorphism alters the norepinephrine-lowering effects and therapeutic response of the beta-blocker bucindolol in chronic heart failure. Circ Heart Fail 2010; 3:21–28.
- Bristow MR, Krause-Steinrauf H, Nuzzo R, et al. Effect of baseline or changes in adrenergic activity on clinical outcomes in the beta-blocker evaluation of survival trial. Circulation 2004; 110:1437–1442.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Cavallari LH, Groo VL, Momary KM, Fontana D, Viana MA, Vaitkus P. Racial differences in potassium response to spironolactone in heart failure. Congest Heart Fail 2006; 12:200–205.
- Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325:303–310.
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Anand IS, Tam SW, Rector TS, et al. Influence of blood pressure on the effectiveness of a fixed-dose combination of isosorbide dinitrate and hydralazine in the African-American Heart Failure Trial. J Am Coll Cardiol 2007; 49:32–39.
- Echols MR, Felker GM, Thomas KL, et al. Racial differences in the characteristics of patients admitted for acute decompensated heart failure and their relation to outcomes: results from the OPTIME-CHF trial. J Card Fail 2006; 12:684–688.
- Kamath SA, Drazner MH, Wynne J, Fonarow GC, Yancy CW. Characteristics and outcomes in African American patients with decompensated heart failure. Arch Intern Med 2008; 168:1152–1158.
- Felker GM, Lee KL, Bull DA, et al; NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364:797–805.
- Costanzo MR, Guglin ME, Saltzberg MT, et al; UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007; 49:675–683.
- Bart BA, Goldsmith SR, Lee KL, et al; Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Levy P, Compton S, Welch R, et al. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann Emerg Med 2007; 50:144–152.
- Cleland JG, Daubert JC, Erdmann E, et al; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352:1539–1549.
- Young JB, Abraham WT, Smith AL, et al; Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA 2003; 289:2685–2694.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bristow MR, Saxon LA, Boehmer J, et al; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 350:2140–2150.
- Mitchell JE, Hellkamp AS, Mark DB, et al; SCD-HeFT Investigators. Outcome in African Americans and other minorities in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Am Heart J 2008; 155:501–506.
- Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA 2007; 298:1525–1532.
- Farmer SA, Kirkpatrick JN, Heidenreich PA, Curtis JP, Wang Y, Groeneveld PW. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm 2009; 6:325–331.
- Colvin-Adams M, Smith JM, Heubner BM, et al. OPTN/SRTR 2011 Annual Data Report: heart. Am J Transplant 2013; 13(suppl 1):119–148.
- Allen JG, Weiss ES, Arnaoutakis GJ, et al. The impact of race on survival after heart transplantation: an analysis of more than 20,000 patients. Ann Thorac Surg 2010; 89:1956–1964.
- Liu V, Bhattacharya J, Weill D, Hlatky MA. Persistent racial disparities in survival after heart transplantation. Circulation 2011; 123:1642–1649.
- Mahle WT, Kanter KR, Vincent RN. Disparities in outcome for black patients after pediatric heart transplantation. J Pediatr 2005; 147:739–743.
- Park MH, Tolman DE, Kimball PM. Disproportionate HLA matching may contribute to racial disparity in patient survival following cardiac transplantation. Clin Transplant 1996; 10(6 Pt 2):625–628.
- Park MH, Tolman DE, Kimball PM. The impact of race and HLA matching on long-term survival following cardiac transplantation. Transplant Proc 1997; 29:1460–1463.
- Callender CO, Cherikh WS, Miles PV, et al. Blacks as donors for transplantation: suboptimal outcomes overcome by transplantation into other minorities. Transplant Proc 2008; 40:995–1000.
- King LP, Siminoff LA, Meyer DM, et al. Health insurance and cardiac transplantation: a call for reform. J Am Coll Cardiol 2005; 45:1388–1391.
- Ozminkowski RJ, White AJ, Hassol A, Murphy M. Minimizing racial disparity regarding receipt of a cadaver kidney transplant. Am J Kidney Dis 1997; 30:749–759.
- Aggarwal A, Gupta A, Pappas PS, Tatooles A, Bhat G. Racial differences in patients with left ventricular assist devices. ASAIO J 2012; 58:499–502.
- Tsiouris A, Brewer RJ, Borgi J, Nemeh H, Paone G, Morgan JA. Continuous-flow left ventricular assist device implantation as a bridge to transplantation or destination therapy: racial disparities in outcomes. J Heart Lung Transplant 2013; 32:299–304.
- Joyce DL, Conte JV, Russell SD, Joyce LD, Chang DC. Disparities in access to left ventricular assist device therapy. J Surg Res 2009; 152:111–117.
KEY POINTS
- The natural history, epidemiology, and outcomes of heart failure in African Americans differ from those in whites.
- Hypertension is the predominant risk factor for heart failure in African Americans, and aggressive management of hypertension may substantially reduce the incidence and consequences of heart failure in this population.
- Heart failure in African Americans should be treated according to the same evidenced-based strategies as in the general population. In addition, a combination of isosorbide dinitrate and hydralazine is recommended in African Americans.
- Many questions remain unanswered, since African Americans have been markedly underrepresented in clinical trials.
Cardiovascular disease in women: Prevention, symptoms, diagnosis, pathogenesis
Although long considered a disease of elderly men, cardiovascular disease is increasingly recognized for its impact on women. In fact, it is now the leading cause of death in women worldwide, and in the United States more women than men die of it.1
Given this epidemic of cardiovascular disease in women, more research is now being dedicated to identifying sex-specific aspects of cardiovascular disease, the better to prevent and treat it.
This review will focus on the most recent information about how prevention, symptoms, and underlying cardiovascular conditions differ in women.
PRIMARY PREVENTION: ONGOING DEBATE
Women who diet, exercise, and abstain from smoking have an 80% lower rate of cardiovascular events than the female population overall.2 However, beyond lifestyle modification and blood pressure control, there is ongoing debate as to the efficacy of our available therapies for preventing cardiovascular disease in women.
Aspirin for primary prevention in women: No benefit?
The use of aspirin to prevent cardiovascular disease in women has long been controversial. Several trials showed a lower rate of myocardial infarction in people using aspirin for primary prevention, but most of the patients in the initial trials were men (Table 1).3
The Women’s Health Study4 assigned 39,876 women age 45 and older to receive either aspirin (100 mg on alternate days) or placebo, and monitored them for more than 10 years for major cardiovascular events (non-fatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes).
The results, published in 2005, showed that the rates of myocardial infarction and cardiovascular death were not significantly lower in the aspirin group, although the rate of ischemic stroke was 24% lower. There were more hemorrhagic strokes in the aspirin group (not statistically significant), and there was significantly more gastrointestinal bleeding. The study showed the relative risk (RR) and 95% confidence interval (CI) for several outcomes in aspirin users were:
- Myocardial infarction—RR 1.02, 95% CI 0.84–1.25, P = .83
- Cardiovascular death—RR 0.95, 95% CI 0.74–1.22, P = .68
- Ischemic stroke—RR 0.76, 95% CI 0.63–0.93, P = .009
- Hemorrhagic stroke—RR 1.24, 95% CI 0.82–1.87, P = .31
- Gastrointestinal bleeding—RR 1.4, 95% CI 1.07–1.83, P = .02.
A later analysis indicated that noncompliance had no effect on these results.5
However, a subgroup analysis of women over age 65 found a significant reduction in the rate of myocardial infarction and in the composite end point of myocardial infarction, stroke, and cardiovascular death, although there was a trend toward a higher rate of gastrointestinal bleeding. The numbers in aspirin users in the subgroup over age 65 were as follows:
- Myocardial infarction—RR 0.66, 95% CI 0.44–0.97, P = .04
- Composite end point—RR 0.74, 95% CI 0.59–0.92, P = .008.
Aspirin was taken every other day and at a higher dose than the 81 mg recommended in the United States, although it is unclear how these differences may have affected the results.
United States Preventive Services Task Force (USPSTF) recommendations. Although the USPSTF currently recommends aspirin for men age 45 to 79 to prevent myocardial infarction, it offers no such recommendation for women, largely because of the results of the Women’s Health Initiative study. However, it does recommend aspirin to prevent ischemic stroke in women age 55 to 79.3 Additionally, aspirin can be considered for prevention of myocardial infarction in women who are over age 65 or at high risk.6
This is based on Women’s Health Study data for women over age 65 showing a number needed to treat of 47 to prevent 1 cardiovascular event, whereas the number needed to harm, defined by a major hemorrhagic event, was 128. In contrast, in women younger than age 65, the number needed to treat was 2,001 and the number needed to harm was 196.4
High-risk features, as defined by the guidelines, are a history of coronary artery disease, cerebrovascular disease, peripheral arterial disease, abdominal aortic aneurysm, diabetes, or chronic kidney disease, or a 10-year predicted risk of cardiovascular disease of more than 10%.
Jardine et al7 reported that aspirin was beneficial in patients with chronic kidney disease. The rates of cardiovascular death, death from any cause, and stroke were significantly lower in patients with a glomerular filtration rate (GFR) less than 45 mL/min if they received aspirin. The rates were also lower in aspirin recipients with a GFR between 46 and 60 mL/min, but the difference was not statistically significant.
Comments. Given the risk of significant gastrointestinal bleeding and a trend toward hemorrhagic stroke with aspirin use,4 it is important to weigh the risks and benefits of aspirin for primary prevention in women.
Our understanding of the reasons for sex differences in the clinical benefits of aspirin for primary prevention is limited at this point. Studies have shown a higher prevalence of platelet reactivity and aspirin resistance in women than in men, suggesting that hormonal differences may play a role.8 There has been mention of using higher doses of aspirin in women to achieve the same level of platelet inhibition as in men. However, studies have shown essentially equal platelet inhibition in both men and women after aspirin administration.9 Therefore, more work needs to be done to better understand the observed sex differences in response to aspirin.
Statins for primary prevention in women: Conflicting data
Given suggestions that statins may not be effective in women10 and the fact that women were underrepresented in earlier statin trials, a number of studies have examined this issue in the last several years.
The JUPITER trial (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin)10 enrolled patients who had no history of coronary artery disease and who had a C-reactive protein level equal to or greater than 2 mg/L and a low-density lipoprotein cholesterol level of less than 130 mg/dL. (Of note, these patients would not have met the criteria for receiving a statin for primary prevention according to the current Adult Treatment Panel guidelines.)
The women in the trial who received rosuvastatin had a 46% lower incidence of myocardial infarction, stroke, revascularization, hospitalization for unstable angina, or death from cardiovascular causes. In addition, a meta-analysis performed by the authors showed a one-third reduction of cardiovascular disease end points in women. However, there was no reduction in the mortality rate.
Other statin trials. A later meta-analysis of randomized primary prevention trials found that men and women derived similar benefit from statins in terms of cardiovascular disease end points and all-cause mortality (Figure 1),11 although five of the trials included a small number of secondary prevention patients. In contrast, a meta-analysis of only primary prevention patients showed no benefit of statin therapy in all-cause mortality, although the authors acknowledged that there were insufficient data to look specifically at women in this sample.12
A Cochrane review conducted before the JUPITER data were available concluded that there was insufficient evidence to prescribe statins for primary prevention in patients at low cardiovascular risk.13 However, an updated version that included results of the JUPITER trial concluded that there was a reduction in the rate of all-cause mortality and cardiovascular events in both men and women receiving a statin for primary prevention.14
Given these conflicting results, debate continues as to the benefit of statins for primary prevention, not only in women but in the population as a whole.15,16 The definition of high risk, in terms of comorbidities and lipid profile, also continues to evolve and will likely be an important factor in identifying women who will benefit from statin therapy for primary prevention.
Statin adverse effects. Much of the debate about statins for primary prevention stems from concern about the adverse effects of these drugs. In addition to myopathy, there have been reports of increased risks of new diabetes and cognitive impairment.16 In a post hoc analysis of the Women’s Health Initiative, the adjusted risk of diabetes was 48% higher in women taking a statin for primary prevention than in similar women not taking a statin.17 (This finding should be viewed with caution, since the data are observational.)
There has also been a question of whether women experience more side effects from statin therapy than men do. Although thin or frail women over age 80 are more susceptible to statin side effects, this finding has not been observed in younger women.18
Comment. In view of the data, it appears reasonable to consider statin therapy for primary prevention in women deemed to be at high risk based on the current guidelines. However, as always, one must consider whether the benefits outweigh the risks for the individual patient. More study is needed to better evaluate the utility of statin therapy in primary prevention.
Hormone therapy
Hormone therapy has received enormous attention in both the medical community and the public media. (Hormone therapy is either combined estrogen and progestin or estrogen alone, used to treat symptoms of menopause and to prevent osteoporosis in postmenopausal women. Here, we will discuss hormone therapy and not hormone replacement therapy, which is used specifically to treat premature menopause.)
The safety of estrogen-progestin combination therapy has been the subject of great debate since a Women’s Health Initiative study showed a trend toward a greater risk of cardiovascular disease in estrogen-progestin users.19
Women who received estrogen by itself showed no difference in cardiovascular risk compared with those who received placebo. Unopposed estrogen is rarely prescribed, since it increases the risk of endometrial cancer in women who have not undergone hysterectomy.20
Both unopposed estrogen and combination therapy have also been found to increase the risk of stroke,20 deep vein thrombosis, gallbladder disease, and certain forms of urinary incontinence.
Guidelines on hormone therapy. The USPSTF does not recommend hormone therapy to prevent chronic conditions, basing its decision on the findings from the Women’s Health Initiative.21 The American College of Cardiology and American Heart Association (ACC/AHA) 2007 guidelines advise against continuing hormone therapy in patients who present with acute coronary syndrome, although recommendations need to address a broader scope of primary and secondary prevention patients.
Does timing matter? There is a hypothesis that when hormone therapy is started may affect the cardiovascular risk. A secondary analysis of the Women’s Health Initiative study22 showed a trend towards less cardiovascular disease in women who started hormone therapy within 9 years of menopause, whereas those starting it later had a statistically significantly higher rate of cardiovascular mortality. However, all women had a higher risk of stroke while on hormone therapy, regardless of timing.22
A study of 1,006 healthy women age 45 to 58 whose last menstrual period was 3 to 24 months before enrollment found a statistically significant reduction in the composite end point of death, hospital admission for myocardial infarction, or heart failure with hormone therapy.23 There was no significant increase in breast cancer, deep vein thrombosis, or stroke after 10 years of randomized treatment.
A retrospective analysis of 71,237 postmenopausal women in the California Teachers Study also found a significant reduction in the rate of cardiovascular disease-related deaths with hormone therapy in younger women (ie, younger than age 65), but not in older women.24 The authors concluded that it may not just be the years after menopause but also the baseline age of the woman that may influence outcomes.
In view of these studies, there is increasing recognition that hormone therapy may, in fact, still be beneficial in terms of cardiovascular and all-cause mortality in carefully selected patients. The cardiovascular risk in women, specifically older women who have had a longer duration of menopause, should also be weighed against the potential benefits of therapy in terms of quality of life and symptom relief.
Trials under way include the Kronos Early Estrogen Prevention (KEEP) and Danish Osteoporosis Prevention (DOPS) studies. KEEP is a 4-year, double-blind, randomized controlled trial of hormone therapy in women within 3 years of menopause. DOPS is an open-label trial that includes more than 1,000 women with early menopause. The results of these trials will likely affect future recommendations.
WOMEN’S SYMPTOMS: TYPICAL OR ATYPICAL?
Whether the presenting symptoms of acute coronary syndromes differ between men and women has been much debated.
More women than men seem to present with atypical symptoms.25–27 (The term “atypical” refers to symptoms that do not include the three classic components of angina: substernal chest pain or discomfort, provoked by exertion or emotional stress, and relieved by rest or nitroglycerin, or both.28)
However, most women still present with chest pain. In a study by Dey et al,26 92% of the 7,638 women with presumed acute coronary syndrome presented with chest pain. In women who had atypical symptoms, dyspnea, nausea, vomiting, and diaphoresis were the most common symptoms. Women were significantly more likely than men to present with nausea and vomiting (32% vs 23%, P = .001).
Women in the study were also more likely to have angiographically normal coronary arteries (12% vs 6%, P < .001).26 This difference may be largely due to noncardiac chest pain, but it may also represent conditions such as vasospasm, microvascular disease, or stress cardiomyopathy, all of which disproportionately affect women.
An earlier review of 10 major studies found a higher percentage of women presenting with atypical symptoms (37.5% of women vs 27.4% of men).25 However, symptoms were not a focus of these studies, and the findings may therefore be skewed by inaccurate documentation.
Atypical warning signs. Although most women with acute coronary syndrome present acutely with chest pain, women may have different warning signs than men. Only about one-third of women experience angina before presentation.29 Compared with men, women are more likely to complain of shortness of breath, fatigue, and weakness leading up to a diagnosis of a myocardial infarction.29 Therefore, the prodromal symptoms of cardiovascular disease may in fact be significantly more atypical in women than in men, suggesting the need for heightened vigilance in the cardiovascular evaluation of women who have nonanginal symptoms.
THE ROLE OF STRESS TESTING IN WOMEN
Stress testing in various forms continues to be widely used in the diagnosis of heart disease in women, although data are scarce regarding its utility in women.
The ACC/AHA guidelines continue to recommend exercise stress electrocardiography (ECG) for women who have symptoms, are at intermediate risk, and have a normal result on resting ECG.30
Exercise ECG has a higher false-positive rate in women than in men,31 and there appears to be no relationship between exercise-induced ST-segment depression and the rate of cardiovascular mortality or all-cause mortality in women.32,33 On the other hand, exercise ECG yields valuable additional information such as exercise capacity, chronotropic response, heart-rate recovery, and blood pressure response, all of which have important diagnostic and prognostic implications in women.34
For those who have an abnormal resting ECG, the addition of an imaging test, ie, echocardiography or single-photon emission computed tomography (SPECT), is indicated. Both have limitations: SPECT can give false-positive results because of breast attenuation, and echocardiography varies in accuracy depending on the quality of acoustic windows obtained. Both exercise stress SPECT and exercise stress ECG have higher sensitivity and specificity than electrocardiographic exercise stress testing alone,34 and there is evidence that the two imaging tests are comparable in women.35
In those women who have baseline left bundle branch block or who cannot exercise, a pharmacologic stress test should be performed. Of course, this is a less desirable testing method, given the loss of valuable information obtained from exercising the patient.
UNDERLYING CONDITIONS THAT DISPROPORTIONATELY AFFECT WOMEN
Microvascular angina
Perimenopausal and postmenopausal women account for 70% of patients presenting with chest pain and elevated cardiac enzymes but no significant angiographic evidence of coronary artery disease.36 This condition, commonly called syndrome X, is often characterized by lingering, dull chest pain after exertion and is seen more frequently in women younger than those presenting with classic cardiovascular disease.
Because at least some of these patients show evidence of ST-segment depression and reversible perfusion defects on imaging, the condition is thought to be caused by ischemia of the microvascular bed leading to microvascular angina.37
Although this is still an area of research, microvascular dysfunction has recently been proposed as an explanation for these findings. Abnormal vasoconstriction and impaired vasodilation of the microvascular bed, insulin resistance, increased systemic inflammation, and abnormal pain response have all been cited as potentially contributing to microvascular dysfunction.36
Estrogen deficiency is thought to play a central role in the significantly increased burden of microvascular dysfunction seen in women, with some studies suggesting that hormone therapy can relieve symptoms. However, given the concerns about adverse cardiovascular outcomes in women on hormone therapy, there has been little investigation of this treatment for this disorder.
Studies have shown worse cardiovascular outcomes and higher rates of angina-related hospitalization and repeat heart catheterizations in women with microvascular dysfunction.38
Diagnosing microvascular angina must be done indirectly, as there is no safe and minimally invasive technique by which to directly observe the microvasculature. Current coronary angiographic techniques cannot image vessels smaller than 0.5 mm in diameter, and endomyocardial biopsy cannot access the larger periarterioles thought to play a major role in regulating coronary blood flow.39
Because the coronary microvasculature controls total coronary resistance and therefore regulates myocardial blood flow, measuring myocardial blood flow at maximum vasodilation, termed coronary flow reserve, can indirectly evaluate the degree of microvascular dysfunction.40 In the absence of obstructive epicardial coronary disease, noninvasive imaging techniques or provocative testing in the coronary catheterization lab can be used for this purpose. In terms of noninvasive imaging, perfusion magnetic resonance imaging (Figure 2) or positron emission tomography is often performed.40
Coronary flow reserve can also be measured by invasive means in the catheterization laboratory after maximum hyperemia is induced by adenosine or other such vasodilatory agents.41 However, measurements obtained in this invasive manner are greatly affected by hemodynamic changes and can have poor reproducibility.40
Proposed therapy for microvascular angina. Once a diagnosis has been made, lifestyle modification, antianginal agents, angiotensin-converting enzyme inhibitors, and statins have been suggested for therapy.39 Pain management techniques are also used, given the increased pain sensitivity observed in women with this condition. However, no therapy to date has proven overwhelmingly effective in these patients, and a disproportionate number of women suffer from chronic symptoms despite these treatments. Currently, researchers are looking for new agents to treat microvascular disease.
Stress cardiomyopathy
Stress cardiomyopathy, also called takotsubo cardiomyopathy or “broken heart syndrome,” is another condition that disproportionately affects postmenopausal women. It is often associated with sudden emotional or physical stress. Patients present with signs and symptoms of myocardial infarction without demonstrable epicardial coronary artery disease. The hallmark of stress cardiomyopathy is left ventricular dysfunction, often severe, with classic apical ballooning that resembles a Japanese fishing pot (takotsubo) used to trap octopuses, hence the name (Figure 3).
According to a review by Akashi et al42 based on previously reported Mayo Clinic criteria, the diagnosis of stress cardiomyopathy includes each of the following:
- Transient hypokinesis, akinesis, or dyskinesis in the left ventricular midsegments with or without apical involvement; regional wall-motion abnormalities that extend beyond a single epicardial vascular distribution; and frequently, but not always, a stressful trigger
- Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture
- New abnormality on ECG (eg, ST-segment elevation, T-wave inversion) or modest elevation in cardiac troponin
- Absence of pheochromocytoma or myocarditis.
From 80% to 100% of reported cases are in women, with an average age range of 61 to 76.42 It is unclear why there is such an overwhelming postmenopausal female preponderance of the disease. Studies have implicated estrogen deficiency, as it appears to attenuate the levels of cardioprotective substances in the body that in part regulate catecholamine surges and may also increase the level of oxidative stress.42
Several mechanisms for this condition have been proposed. The condition may be caused by multivessel epicardial coronary spasm or spontaneously resolved plaque rupture, resulting in stunned myocardium. However, the regional distribution of wall-motion abnormality is often out of proportion to the level of cardiac enzyme elevation, and in the case of plaque rupture, is frequently not consistent with a single coronary vessel.42 A catecholamine surge causing myocardial and neurogenic stunning has also been proposed, although many of these patients have normal catecholamine levels.42 Finally, microvascular dysfunction has been found in a number of patients with this condition. However, it is difficult to establish a causal relationship, since apical ballooning could result in microvascular dysfunction.42
Treatment of stress cardiomyopathy has not been standardized, in part because the left ventricular dysfunction often resolves spontaneously within several weeks.43,44 Given the proposed catecholaminergic mechanism, some experts believe that beta-blockers are contraindicated because of the resulting unopposed activation of alpha-adrenoreceptors. However, this continues to be a matter of debate. There is also no clear indication for other standard therapies for acute coronary syndrome such as aspirin and heparin, and their use appears to vary in clinical practice.
Although most patients improve with time and recurrence is exceedingly rare, it should be emphasized that they may present acutely with severe hemodynamic instability and cardiogenic shock. Therefore, advanced means of support, such as an intra-aortic balloon pump, may be indicated until the patient recovers from the acute phase of the disease.
Spontaneous coronary artery dissection
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome resulting from dissection of the coronary intimal or medial layer and associated hematoma formation, leading to coronary occlusion.45,46 In a case series of 87 patients, 49% presented with an ST-segment elevation myocardial infarction, and 23% were found to have multivessel SCAD.46
SCAD occurs predominantly in young, healthy women (mean age 30–45 years). Approximately 70% of cases are in women, 30% of whom are in the peripartum period.45 The reasons for the increased risk during pregnancy have not yet been elucidated, but changing sex hormones, increased cardiac output and shear stress, and an increased inflammatory response have been implicated.45
Diagnosing SCAD. Coronary angiography should be performed with extreme caution in patients suspected of having SCAD, given the risk of further dissection of the artery with forceful injections. In certain cases, it may be difficult to detect SCAD on routine angiography if there is no communication between the true and false lumen.
If the suspicion for SCAD is high, intravascular ultrasonography or optical coherence tomography can be used to better evaluate the vessel.45 Although optical coherence tomography has greater spatial resolution, it is more costly and is not as widely used as intravascular ultrasonography in the clinical setting
Managing SCAD. Although conservative management and coronary artery bypass grafting have been shown to cause minimal in-hospital morbidity, percutaneous coronary intervention has been complicated by technical failure in up to 35% of patients in one series.46
While there is no standardized way to manage these patients, experts currently recommend conservative management with standard therapies for acute coronary syndrome (Figure 4). Although antithrombotic agents can decrease thrombus burden, they must be used with caution, because they also increase the risk of bleeding into the false lumen.
If patients experience recurrent or ongoing ischemia despite conservative management, then revascularization should be considered. Optical coherence tomography or intravascular ultrasonography is recommended to ensure proper stent alignment and positioning.
Coronary artery bypass grafting could be considered in preference to percutaneous coronary intervention, given that the former appears to be safer,46 although this requires further investigation. Some studies have cautioned against using fibrinolytic therapy, based on anecdotal evidence that it may further propagate the dissection,45 although this therapy has been used in other case studies.46
While mortality rates are relatively low (95% survival at 2 years),45 the estimated risk of recurrent SCAD at 10 years is approximately 30%.46
Associated with fibromuscular dysplasia. Of note, a sizeable number of patients with SCAD have been found to have fibromuscular dysplasia. This is a nonatherosclerotic, noninflammatory vascular condition that can affect any vascular bed in the body, although there is a predilection for the renal and carotid arteries (Figure 5).47 Fibromuscular dysplasia also disproportionately affects women and appears to be a concomitant condition in the majority of patients with SCAD.47 Imaging of the carotid and renal arteries of patients with SCAD has revealed a number of cases of fibromuscular dysplasia.46,48 This noted association will likely allow for ongoing research to better understand the pathophysiology of these two conditions.
- Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol 2007; 49:1230–1250.
- Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000; 343:16–22.
- Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the US Preventive Services Task Force. Ann Intern Med 2009; 150:405–410.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Cook NR, Cole SR, Buring JE. Aspirin in the primary prevention of cardiovascular disease in the Women’s Health Study: effect of non-compliance. Eur J Epidemiol 2012; 27:431–438.
- Mosca L, Benjamin EJ, Berra K, et al; American Heart Association. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011; 57:1404–1423.
- Jardine MJ, Ninomiya T, Perkovic V, et al. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol 2010; 56:956–965.
- Snoep JD, Roest M, Barendrecht AD, De Groot PG, Rosendaal FR, Van Der Bom JG. High platelet reactivity is associated with myocardial infarction in premenopausal women: a population-based case-control study. J Thromb Haemost 2010; 8:906–913.
- Becker DM, Segal J, Vaidya D, et al. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA 2006; 295:1420–1427.
- Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation 2010; 121:1069–1077.
- Kostis WJ, Cheng JQ, Dobrzynski JM, Cabrera J, Kostis JB. Meta-analysis of statin effects in women versus men. J Am Coll Cardiol 2012; 59:572–582.
- Ray KK, Seshasai SR, Erqou S, et al. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch Intern Med 2010; 170:1024–1031.
- Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2011;CD004816.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;CD004816.
- Blaha MJ, Nasir K, Blumenthal RS. Statin therapy for healthy men identified as “increased risk.” JAMA 2012; 307:1489–1490.
- Redberg RF, Katz MH. Healthy men should not take statins. JAMA 2012; 307:1491–1492.
- Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med 2012; 172:144–152.
- Pasternak RC, Smith SC, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Stroke 2002; 33:2337–2341.
- Manson JE, Hsia J, Johnson KC, et al; Women’s Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003; 349:523–534.
- Hsia J, Criqui MH, Herrington DM, et al; Women’s Health Initiative Research Group. Conjugated equine estrogens and peripheral arterial disease risk: The Women’s Health Initiative. Am Heart J 2006; 152:170–176.
- Moyer VAUS Preventive Services Task Force. Menopausal hormone therapy for the primary prevention of chronic conditions: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158:47–54.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012; 345:e6409.
- Stram DO, Liu Y, Henderson KD, et al. Age-specific effects of hormone therapy use on overall mortality and ischemic heart disease mortality among women in the California Teachers Study. Menopause 2011; 18:253–261.
- Canto JG, Goldberg RJ, Hand MM, et al. Symptom presentation of women with acute coronary syndromes: myth vs reality. Arch Intern Med 2007; 167:2405–2413.
- Dey S, Flather MD, Devlin G, et al; Global Registry of Acute Coronary Events investigators. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: The Global Registry of Acute Coronary Events. Heart 2009; 95:20–26.
- Canto JG, Shlipak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA 2000; 283:3223–3229.
- Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979; 300:1350–1358.
- McSweeney JC, Cody M, O’Sullivan P, Elberson K, Moser DK, Garvin BJ. Women’s early warning symptoms of acute myocardial infarction. Circulation 2003; 108:2619–2623.
- Mieres JH, Shaw LJ, Arai A, et al; Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation 2005; 111:682–696.
- Barolsky SM, Gilbert CA, Faruqui A, Nutter DO, Schlant RC. Differences in electrocardiographic response to exercise of women and men: a non-Bayesian factor. Circulation 1979; 60:1021–1027.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: The St James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics Prevalence Study. JAMA 2003; 290:1600–1607.
- Kohli P, Gulati M. Exercise stress testing in women: going back to the basics. Circulation 2010; 122:2570–2580.
- Grady D, Chaput L, Kristof M. Diagnosis and treatment of coronary heart disease in women: systematic reviews of evidence on selected topics. Evid Rep Technol Assess (Summ) 2003; 81:1–4.
- Singh M, Singh S, Arora R, Khosla S. Cardiac syndrome X: current concepts. Int J Cardiol 2010; 142:113–119.
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007; 356:830–840.
- Johnson BD, Shaw LJ, Buchthal SD, et al; National Institutes of Health-National Heart, Lung, and Blood Institute. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004; 109:2993–2999.
- Beltrame JF, Crea F, Camici P. Advances in coronary microvascular dysfunction. Heart Lung Circ 2009; 18:19–27.
- Leung DY, Leung M. Non-invasive/invasive imaging: significance and assessment of coronary microvascular dysfunction. Heart 2011; 97:587–595.
- Samim A, Nugent L, Mehta PK, Shufelt C, Bairey Merz CN. Treatment of angina and microvascular coronary dysfunction. Curr Treat Options Cardiovasc Med 2010; 12:355–364.
- Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008; 118:2754–2762.
- Akashi YJ, Musha H, Kida K, et al. Reversible ventricular dysfunction takotsubo cardiomyopathy. Eur J Heart Fail 2005; 7:1171–1176.
- Regnante RA, Zuzek RW, Weinsier SB, et al. Clinical characteristics and four-year outcomes of patients in the Rhode Island Takotsubo Cardiomyopathy Registry. Am J Cardiol 2009; 103:1015–1019.
- Vrints CJ. Spontaneous coronary artery dissection. Heart 2010; 96:801–808.
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126:579–588.
- Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013; 6:44–52.
- Saw J, Poulter R, Fung A, Wood D, Hamburger J, Buller CE. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ Cardiovasc Interv 2012; 5:134–137.
Although long considered a disease of elderly men, cardiovascular disease is increasingly recognized for its impact on women. In fact, it is now the leading cause of death in women worldwide, and in the United States more women than men die of it.1
Given this epidemic of cardiovascular disease in women, more research is now being dedicated to identifying sex-specific aspects of cardiovascular disease, the better to prevent and treat it.
This review will focus on the most recent information about how prevention, symptoms, and underlying cardiovascular conditions differ in women.
PRIMARY PREVENTION: ONGOING DEBATE
Women who diet, exercise, and abstain from smoking have an 80% lower rate of cardiovascular events than the female population overall.2 However, beyond lifestyle modification and blood pressure control, there is ongoing debate as to the efficacy of our available therapies for preventing cardiovascular disease in women.
Aspirin for primary prevention in women: No benefit?
The use of aspirin to prevent cardiovascular disease in women has long been controversial. Several trials showed a lower rate of myocardial infarction in people using aspirin for primary prevention, but most of the patients in the initial trials were men (Table 1).3
The Women’s Health Study4 assigned 39,876 women age 45 and older to receive either aspirin (100 mg on alternate days) or placebo, and monitored them for more than 10 years for major cardiovascular events (non-fatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes).
The results, published in 2005, showed that the rates of myocardial infarction and cardiovascular death were not significantly lower in the aspirin group, although the rate of ischemic stroke was 24% lower. There were more hemorrhagic strokes in the aspirin group (not statistically significant), and there was significantly more gastrointestinal bleeding. The study showed the relative risk (RR) and 95% confidence interval (CI) for several outcomes in aspirin users were:
- Myocardial infarction—RR 1.02, 95% CI 0.84–1.25, P = .83
- Cardiovascular death—RR 0.95, 95% CI 0.74–1.22, P = .68
- Ischemic stroke—RR 0.76, 95% CI 0.63–0.93, P = .009
- Hemorrhagic stroke—RR 1.24, 95% CI 0.82–1.87, P = .31
- Gastrointestinal bleeding—RR 1.4, 95% CI 1.07–1.83, P = .02.
A later analysis indicated that noncompliance had no effect on these results.5
However, a subgroup analysis of women over age 65 found a significant reduction in the rate of myocardial infarction and in the composite end point of myocardial infarction, stroke, and cardiovascular death, although there was a trend toward a higher rate of gastrointestinal bleeding. The numbers in aspirin users in the subgroup over age 65 were as follows:
- Myocardial infarction—RR 0.66, 95% CI 0.44–0.97, P = .04
- Composite end point—RR 0.74, 95% CI 0.59–0.92, P = .008.
Aspirin was taken every other day and at a higher dose than the 81 mg recommended in the United States, although it is unclear how these differences may have affected the results.
United States Preventive Services Task Force (USPSTF) recommendations. Although the USPSTF currently recommends aspirin for men age 45 to 79 to prevent myocardial infarction, it offers no such recommendation for women, largely because of the results of the Women’s Health Initiative study. However, it does recommend aspirin to prevent ischemic stroke in women age 55 to 79.3 Additionally, aspirin can be considered for prevention of myocardial infarction in women who are over age 65 or at high risk.6
This is based on Women’s Health Study data for women over age 65 showing a number needed to treat of 47 to prevent 1 cardiovascular event, whereas the number needed to harm, defined by a major hemorrhagic event, was 128. In contrast, in women younger than age 65, the number needed to treat was 2,001 and the number needed to harm was 196.4
High-risk features, as defined by the guidelines, are a history of coronary artery disease, cerebrovascular disease, peripheral arterial disease, abdominal aortic aneurysm, diabetes, or chronic kidney disease, or a 10-year predicted risk of cardiovascular disease of more than 10%.
Jardine et al7 reported that aspirin was beneficial in patients with chronic kidney disease. The rates of cardiovascular death, death from any cause, and stroke were significantly lower in patients with a glomerular filtration rate (GFR) less than 45 mL/min if they received aspirin. The rates were also lower in aspirin recipients with a GFR between 46 and 60 mL/min, but the difference was not statistically significant.
Comments. Given the risk of significant gastrointestinal bleeding and a trend toward hemorrhagic stroke with aspirin use,4 it is important to weigh the risks and benefits of aspirin for primary prevention in women.
Our understanding of the reasons for sex differences in the clinical benefits of aspirin for primary prevention is limited at this point. Studies have shown a higher prevalence of platelet reactivity and aspirin resistance in women than in men, suggesting that hormonal differences may play a role.8 There has been mention of using higher doses of aspirin in women to achieve the same level of platelet inhibition as in men. However, studies have shown essentially equal platelet inhibition in both men and women after aspirin administration.9 Therefore, more work needs to be done to better understand the observed sex differences in response to aspirin.
Statins for primary prevention in women: Conflicting data
Given suggestions that statins may not be effective in women10 and the fact that women were underrepresented in earlier statin trials, a number of studies have examined this issue in the last several years.
The JUPITER trial (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin)10 enrolled patients who had no history of coronary artery disease and who had a C-reactive protein level equal to or greater than 2 mg/L and a low-density lipoprotein cholesterol level of less than 130 mg/dL. (Of note, these patients would not have met the criteria for receiving a statin for primary prevention according to the current Adult Treatment Panel guidelines.)
The women in the trial who received rosuvastatin had a 46% lower incidence of myocardial infarction, stroke, revascularization, hospitalization for unstable angina, or death from cardiovascular causes. In addition, a meta-analysis performed by the authors showed a one-third reduction of cardiovascular disease end points in women. However, there was no reduction in the mortality rate.
Other statin trials. A later meta-analysis of randomized primary prevention trials found that men and women derived similar benefit from statins in terms of cardiovascular disease end points and all-cause mortality (Figure 1),11 although five of the trials included a small number of secondary prevention patients. In contrast, a meta-analysis of only primary prevention patients showed no benefit of statin therapy in all-cause mortality, although the authors acknowledged that there were insufficient data to look specifically at women in this sample.12
A Cochrane review conducted before the JUPITER data were available concluded that there was insufficient evidence to prescribe statins for primary prevention in patients at low cardiovascular risk.13 However, an updated version that included results of the JUPITER trial concluded that there was a reduction in the rate of all-cause mortality and cardiovascular events in both men and women receiving a statin for primary prevention.14
Given these conflicting results, debate continues as to the benefit of statins for primary prevention, not only in women but in the population as a whole.15,16 The definition of high risk, in terms of comorbidities and lipid profile, also continues to evolve and will likely be an important factor in identifying women who will benefit from statin therapy for primary prevention.
Statin adverse effects. Much of the debate about statins for primary prevention stems from concern about the adverse effects of these drugs. In addition to myopathy, there have been reports of increased risks of new diabetes and cognitive impairment.16 In a post hoc analysis of the Women’s Health Initiative, the adjusted risk of diabetes was 48% higher in women taking a statin for primary prevention than in similar women not taking a statin.17 (This finding should be viewed with caution, since the data are observational.)
There has also been a question of whether women experience more side effects from statin therapy than men do. Although thin or frail women over age 80 are more susceptible to statin side effects, this finding has not been observed in younger women.18
Comment. In view of the data, it appears reasonable to consider statin therapy for primary prevention in women deemed to be at high risk based on the current guidelines. However, as always, one must consider whether the benefits outweigh the risks for the individual patient. More study is needed to better evaluate the utility of statin therapy in primary prevention.
Hormone therapy
Hormone therapy has received enormous attention in both the medical community and the public media. (Hormone therapy is either combined estrogen and progestin or estrogen alone, used to treat symptoms of menopause and to prevent osteoporosis in postmenopausal women. Here, we will discuss hormone therapy and not hormone replacement therapy, which is used specifically to treat premature menopause.)
The safety of estrogen-progestin combination therapy has been the subject of great debate since a Women’s Health Initiative study showed a trend toward a greater risk of cardiovascular disease in estrogen-progestin users.19
Women who received estrogen by itself showed no difference in cardiovascular risk compared with those who received placebo. Unopposed estrogen is rarely prescribed, since it increases the risk of endometrial cancer in women who have not undergone hysterectomy.20
Both unopposed estrogen and combination therapy have also been found to increase the risk of stroke,20 deep vein thrombosis, gallbladder disease, and certain forms of urinary incontinence.
Guidelines on hormone therapy. The USPSTF does not recommend hormone therapy to prevent chronic conditions, basing its decision on the findings from the Women’s Health Initiative.21 The American College of Cardiology and American Heart Association (ACC/AHA) 2007 guidelines advise against continuing hormone therapy in patients who present with acute coronary syndrome, although recommendations need to address a broader scope of primary and secondary prevention patients.
Does timing matter? There is a hypothesis that when hormone therapy is started may affect the cardiovascular risk. A secondary analysis of the Women’s Health Initiative study22 showed a trend towards less cardiovascular disease in women who started hormone therapy within 9 years of menopause, whereas those starting it later had a statistically significantly higher rate of cardiovascular mortality. However, all women had a higher risk of stroke while on hormone therapy, regardless of timing.22
A study of 1,006 healthy women age 45 to 58 whose last menstrual period was 3 to 24 months before enrollment found a statistically significant reduction in the composite end point of death, hospital admission for myocardial infarction, or heart failure with hormone therapy.23 There was no significant increase in breast cancer, deep vein thrombosis, or stroke after 10 years of randomized treatment.
A retrospective analysis of 71,237 postmenopausal women in the California Teachers Study also found a significant reduction in the rate of cardiovascular disease-related deaths with hormone therapy in younger women (ie, younger than age 65), but not in older women.24 The authors concluded that it may not just be the years after menopause but also the baseline age of the woman that may influence outcomes.
In view of these studies, there is increasing recognition that hormone therapy may, in fact, still be beneficial in terms of cardiovascular and all-cause mortality in carefully selected patients. The cardiovascular risk in women, specifically older women who have had a longer duration of menopause, should also be weighed against the potential benefits of therapy in terms of quality of life and symptom relief.
Trials under way include the Kronos Early Estrogen Prevention (KEEP) and Danish Osteoporosis Prevention (DOPS) studies. KEEP is a 4-year, double-blind, randomized controlled trial of hormone therapy in women within 3 years of menopause. DOPS is an open-label trial that includes more than 1,000 women with early menopause. The results of these trials will likely affect future recommendations.
WOMEN’S SYMPTOMS: TYPICAL OR ATYPICAL?
Whether the presenting symptoms of acute coronary syndromes differ between men and women has been much debated.
More women than men seem to present with atypical symptoms.25–27 (The term “atypical” refers to symptoms that do not include the three classic components of angina: substernal chest pain or discomfort, provoked by exertion or emotional stress, and relieved by rest or nitroglycerin, or both.28)
However, most women still present with chest pain. In a study by Dey et al,26 92% of the 7,638 women with presumed acute coronary syndrome presented with chest pain. In women who had atypical symptoms, dyspnea, nausea, vomiting, and diaphoresis were the most common symptoms. Women were significantly more likely than men to present with nausea and vomiting (32% vs 23%, P = .001).
Women in the study were also more likely to have angiographically normal coronary arteries (12% vs 6%, P < .001).26 This difference may be largely due to noncardiac chest pain, but it may also represent conditions such as vasospasm, microvascular disease, or stress cardiomyopathy, all of which disproportionately affect women.
An earlier review of 10 major studies found a higher percentage of women presenting with atypical symptoms (37.5% of women vs 27.4% of men).25 However, symptoms were not a focus of these studies, and the findings may therefore be skewed by inaccurate documentation.
Atypical warning signs. Although most women with acute coronary syndrome present acutely with chest pain, women may have different warning signs than men. Only about one-third of women experience angina before presentation.29 Compared with men, women are more likely to complain of shortness of breath, fatigue, and weakness leading up to a diagnosis of a myocardial infarction.29 Therefore, the prodromal symptoms of cardiovascular disease may in fact be significantly more atypical in women than in men, suggesting the need for heightened vigilance in the cardiovascular evaluation of women who have nonanginal symptoms.
THE ROLE OF STRESS TESTING IN WOMEN
Stress testing in various forms continues to be widely used in the diagnosis of heart disease in women, although data are scarce regarding its utility in women.
The ACC/AHA guidelines continue to recommend exercise stress electrocardiography (ECG) for women who have symptoms, are at intermediate risk, and have a normal result on resting ECG.30
Exercise ECG has a higher false-positive rate in women than in men,31 and there appears to be no relationship between exercise-induced ST-segment depression and the rate of cardiovascular mortality or all-cause mortality in women.32,33 On the other hand, exercise ECG yields valuable additional information such as exercise capacity, chronotropic response, heart-rate recovery, and blood pressure response, all of which have important diagnostic and prognostic implications in women.34
For those who have an abnormal resting ECG, the addition of an imaging test, ie, echocardiography or single-photon emission computed tomography (SPECT), is indicated. Both have limitations: SPECT can give false-positive results because of breast attenuation, and echocardiography varies in accuracy depending on the quality of acoustic windows obtained. Both exercise stress SPECT and exercise stress ECG have higher sensitivity and specificity than electrocardiographic exercise stress testing alone,34 and there is evidence that the two imaging tests are comparable in women.35
In those women who have baseline left bundle branch block or who cannot exercise, a pharmacologic stress test should be performed. Of course, this is a less desirable testing method, given the loss of valuable information obtained from exercising the patient.
UNDERLYING CONDITIONS THAT DISPROPORTIONATELY AFFECT WOMEN
Microvascular angina
Perimenopausal and postmenopausal women account for 70% of patients presenting with chest pain and elevated cardiac enzymes but no significant angiographic evidence of coronary artery disease.36 This condition, commonly called syndrome X, is often characterized by lingering, dull chest pain after exertion and is seen more frequently in women younger than those presenting with classic cardiovascular disease.
Because at least some of these patients show evidence of ST-segment depression and reversible perfusion defects on imaging, the condition is thought to be caused by ischemia of the microvascular bed leading to microvascular angina.37
Although this is still an area of research, microvascular dysfunction has recently been proposed as an explanation for these findings. Abnormal vasoconstriction and impaired vasodilation of the microvascular bed, insulin resistance, increased systemic inflammation, and abnormal pain response have all been cited as potentially contributing to microvascular dysfunction.36
Estrogen deficiency is thought to play a central role in the significantly increased burden of microvascular dysfunction seen in women, with some studies suggesting that hormone therapy can relieve symptoms. However, given the concerns about adverse cardiovascular outcomes in women on hormone therapy, there has been little investigation of this treatment for this disorder.
Studies have shown worse cardiovascular outcomes and higher rates of angina-related hospitalization and repeat heart catheterizations in women with microvascular dysfunction.38
Diagnosing microvascular angina must be done indirectly, as there is no safe and minimally invasive technique by which to directly observe the microvasculature. Current coronary angiographic techniques cannot image vessels smaller than 0.5 mm in diameter, and endomyocardial biopsy cannot access the larger periarterioles thought to play a major role in regulating coronary blood flow.39
Because the coronary microvasculature controls total coronary resistance and therefore regulates myocardial blood flow, measuring myocardial blood flow at maximum vasodilation, termed coronary flow reserve, can indirectly evaluate the degree of microvascular dysfunction.40 In the absence of obstructive epicardial coronary disease, noninvasive imaging techniques or provocative testing in the coronary catheterization lab can be used for this purpose. In terms of noninvasive imaging, perfusion magnetic resonance imaging (Figure 2) or positron emission tomography is often performed.40
Coronary flow reserve can also be measured by invasive means in the catheterization laboratory after maximum hyperemia is induced by adenosine or other such vasodilatory agents.41 However, measurements obtained in this invasive manner are greatly affected by hemodynamic changes and can have poor reproducibility.40
Proposed therapy for microvascular angina. Once a diagnosis has been made, lifestyle modification, antianginal agents, angiotensin-converting enzyme inhibitors, and statins have been suggested for therapy.39 Pain management techniques are also used, given the increased pain sensitivity observed in women with this condition. However, no therapy to date has proven overwhelmingly effective in these patients, and a disproportionate number of women suffer from chronic symptoms despite these treatments. Currently, researchers are looking for new agents to treat microvascular disease.
Stress cardiomyopathy
Stress cardiomyopathy, also called takotsubo cardiomyopathy or “broken heart syndrome,” is another condition that disproportionately affects postmenopausal women. It is often associated with sudden emotional or physical stress. Patients present with signs and symptoms of myocardial infarction without demonstrable epicardial coronary artery disease. The hallmark of stress cardiomyopathy is left ventricular dysfunction, often severe, with classic apical ballooning that resembles a Japanese fishing pot (takotsubo) used to trap octopuses, hence the name (Figure 3).
According to a review by Akashi et al42 based on previously reported Mayo Clinic criteria, the diagnosis of stress cardiomyopathy includes each of the following:
- Transient hypokinesis, akinesis, or dyskinesis in the left ventricular midsegments with or without apical involvement; regional wall-motion abnormalities that extend beyond a single epicardial vascular distribution; and frequently, but not always, a stressful trigger
- Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture
- New abnormality on ECG (eg, ST-segment elevation, T-wave inversion) or modest elevation in cardiac troponin
- Absence of pheochromocytoma or myocarditis.
From 80% to 100% of reported cases are in women, with an average age range of 61 to 76.42 It is unclear why there is such an overwhelming postmenopausal female preponderance of the disease. Studies have implicated estrogen deficiency, as it appears to attenuate the levels of cardioprotective substances in the body that in part regulate catecholamine surges and may also increase the level of oxidative stress.42
Several mechanisms for this condition have been proposed. The condition may be caused by multivessel epicardial coronary spasm or spontaneously resolved plaque rupture, resulting in stunned myocardium. However, the regional distribution of wall-motion abnormality is often out of proportion to the level of cardiac enzyme elevation, and in the case of plaque rupture, is frequently not consistent with a single coronary vessel.42 A catecholamine surge causing myocardial and neurogenic stunning has also been proposed, although many of these patients have normal catecholamine levels.42 Finally, microvascular dysfunction has been found in a number of patients with this condition. However, it is difficult to establish a causal relationship, since apical ballooning could result in microvascular dysfunction.42
Treatment of stress cardiomyopathy has not been standardized, in part because the left ventricular dysfunction often resolves spontaneously within several weeks.43,44 Given the proposed catecholaminergic mechanism, some experts believe that beta-blockers are contraindicated because of the resulting unopposed activation of alpha-adrenoreceptors. However, this continues to be a matter of debate. There is also no clear indication for other standard therapies for acute coronary syndrome such as aspirin and heparin, and their use appears to vary in clinical practice.
Although most patients improve with time and recurrence is exceedingly rare, it should be emphasized that they may present acutely with severe hemodynamic instability and cardiogenic shock. Therefore, advanced means of support, such as an intra-aortic balloon pump, may be indicated until the patient recovers from the acute phase of the disease.
Spontaneous coronary artery dissection
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome resulting from dissection of the coronary intimal or medial layer and associated hematoma formation, leading to coronary occlusion.45,46 In a case series of 87 patients, 49% presented with an ST-segment elevation myocardial infarction, and 23% were found to have multivessel SCAD.46
SCAD occurs predominantly in young, healthy women (mean age 30–45 years). Approximately 70% of cases are in women, 30% of whom are in the peripartum period.45 The reasons for the increased risk during pregnancy have not yet been elucidated, but changing sex hormones, increased cardiac output and shear stress, and an increased inflammatory response have been implicated.45
Diagnosing SCAD. Coronary angiography should be performed with extreme caution in patients suspected of having SCAD, given the risk of further dissection of the artery with forceful injections. In certain cases, it may be difficult to detect SCAD on routine angiography if there is no communication between the true and false lumen.
If the suspicion for SCAD is high, intravascular ultrasonography or optical coherence tomography can be used to better evaluate the vessel.45 Although optical coherence tomography has greater spatial resolution, it is more costly and is not as widely used as intravascular ultrasonography in the clinical setting
Managing SCAD. Although conservative management and coronary artery bypass grafting have been shown to cause minimal in-hospital morbidity, percutaneous coronary intervention has been complicated by technical failure in up to 35% of patients in one series.46
While there is no standardized way to manage these patients, experts currently recommend conservative management with standard therapies for acute coronary syndrome (Figure 4). Although antithrombotic agents can decrease thrombus burden, they must be used with caution, because they also increase the risk of bleeding into the false lumen.
If patients experience recurrent or ongoing ischemia despite conservative management, then revascularization should be considered. Optical coherence tomography or intravascular ultrasonography is recommended to ensure proper stent alignment and positioning.
Coronary artery bypass grafting could be considered in preference to percutaneous coronary intervention, given that the former appears to be safer,46 although this requires further investigation. Some studies have cautioned against using fibrinolytic therapy, based on anecdotal evidence that it may further propagate the dissection,45 although this therapy has been used in other case studies.46
While mortality rates are relatively low (95% survival at 2 years),45 the estimated risk of recurrent SCAD at 10 years is approximately 30%.46
Associated with fibromuscular dysplasia. Of note, a sizeable number of patients with SCAD have been found to have fibromuscular dysplasia. This is a nonatherosclerotic, noninflammatory vascular condition that can affect any vascular bed in the body, although there is a predilection for the renal and carotid arteries (Figure 5).47 Fibromuscular dysplasia also disproportionately affects women and appears to be a concomitant condition in the majority of patients with SCAD.47 Imaging of the carotid and renal arteries of patients with SCAD has revealed a number of cases of fibromuscular dysplasia.46,48 This noted association will likely allow for ongoing research to better understand the pathophysiology of these two conditions.
Although long considered a disease of elderly men, cardiovascular disease is increasingly recognized for its impact on women. In fact, it is now the leading cause of death in women worldwide, and in the United States more women than men die of it.1
Given this epidemic of cardiovascular disease in women, more research is now being dedicated to identifying sex-specific aspects of cardiovascular disease, the better to prevent and treat it.
This review will focus on the most recent information about how prevention, symptoms, and underlying cardiovascular conditions differ in women.
PRIMARY PREVENTION: ONGOING DEBATE
Women who diet, exercise, and abstain from smoking have an 80% lower rate of cardiovascular events than the female population overall.2 However, beyond lifestyle modification and blood pressure control, there is ongoing debate as to the efficacy of our available therapies for preventing cardiovascular disease in women.
Aspirin for primary prevention in women: No benefit?
The use of aspirin to prevent cardiovascular disease in women has long been controversial. Several trials showed a lower rate of myocardial infarction in people using aspirin for primary prevention, but most of the patients in the initial trials were men (Table 1).3
The Women’s Health Study4 assigned 39,876 women age 45 and older to receive either aspirin (100 mg on alternate days) or placebo, and monitored them for more than 10 years for major cardiovascular events (non-fatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes).
The results, published in 2005, showed that the rates of myocardial infarction and cardiovascular death were not significantly lower in the aspirin group, although the rate of ischemic stroke was 24% lower. There were more hemorrhagic strokes in the aspirin group (not statistically significant), and there was significantly more gastrointestinal bleeding. The study showed the relative risk (RR) and 95% confidence interval (CI) for several outcomes in aspirin users were:
- Myocardial infarction—RR 1.02, 95% CI 0.84–1.25, P = .83
- Cardiovascular death—RR 0.95, 95% CI 0.74–1.22, P = .68
- Ischemic stroke—RR 0.76, 95% CI 0.63–0.93, P = .009
- Hemorrhagic stroke—RR 1.24, 95% CI 0.82–1.87, P = .31
- Gastrointestinal bleeding—RR 1.4, 95% CI 1.07–1.83, P = .02.
A later analysis indicated that noncompliance had no effect on these results.5
However, a subgroup analysis of women over age 65 found a significant reduction in the rate of myocardial infarction and in the composite end point of myocardial infarction, stroke, and cardiovascular death, although there was a trend toward a higher rate of gastrointestinal bleeding. The numbers in aspirin users in the subgroup over age 65 were as follows:
- Myocardial infarction—RR 0.66, 95% CI 0.44–0.97, P = .04
- Composite end point—RR 0.74, 95% CI 0.59–0.92, P = .008.
Aspirin was taken every other day and at a higher dose than the 81 mg recommended in the United States, although it is unclear how these differences may have affected the results.
United States Preventive Services Task Force (USPSTF) recommendations. Although the USPSTF currently recommends aspirin for men age 45 to 79 to prevent myocardial infarction, it offers no such recommendation for women, largely because of the results of the Women’s Health Initiative study. However, it does recommend aspirin to prevent ischemic stroke in women age 55 to 79.3 Additionally, aspirin can be considered for prevention of myocardial infarction in women who are over age 65 or at high risk.6
This is based on Women’s Health Study data for women over age 65 showing a number needed to treat of 47 to prevent 1 cardiovascular event, whereas the number needed to harm, defined by a major hemorrhagic event, was 128. In contrast, in women younger than age 65, the number needed to treat was 2,001 and the number needed to harm was 196.4
High-risk features, as defined by the guidelines, are a history of coronary artery disease, cerebrovascular disease, peripheral arterial disease, abdominal aortic aneurysm, diabetes, or chronic kidney disease, or a 10-year predicted risk of cardiovascular disease of more than 10%.
Jardine et al7 reported that aspirin was beneficial in patients with chronic kidney disease. The rates of cardiovascular death, death from any cause, and stroke were significantly lower in patients with a glomerular filtration rate (GFR) less than 45 mL/min if they received aspirin. The rates were also lower in aspirin recipients with a GFR between 46 and 60 mL/min, but the difference was not statistically significant.
Comments. Given the risk of significant gastrointestinal bleeding and a trend toward hemorrhagic stroke with aspirin use,4 it is important to weigh the risks and benefits of aspirin for primary prevention in women.
Our understanding of the reasons for sex differences in the clinical benefits of aspirin for primary prevention is limited at this point. Studies have shown a higher prevalence of platelet reactivity and aspirin resistance in women than in men, suggesting that hormonal differences may play a role.8 There has been mention of using higher doses of aspirin in women to achieve the same level of platelet inhibition as in men. However, studies have shown essentially equal platelet inhibition in both men and women after aspirin administration.9 Therefore, more work needs to be done to better understand the observed sex differences in response to aspirin.
Statins for primary prevention in women: Conflicting data
Given suggestions that statins may not be effective in women10 and the fact that women were underrepresented in earlier statin trials, a number of studies have examined this issue in the last several years.
The JUPITER trial (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin)10 enrolled patients who had no history of coronary artery disease and who had a C-reactive protein level equal to or greater than 2 mg/L and a low-density lipoprotein cholesterol level of less than 130 mg/dL. (Of note, these patients would not have met the criteria for receiving a statin for primary prevention according to the current Adult Treatment Panel guidelines.)
The women in the trial who received rosuvastatin had a 46% lower incidence of myocardial infarction, stroke, revascularization, hospitalization for unstable angina, or death from cardiovascular causes. In addition, a meta-analysis performed by the authors showed a one-third reduction of cardiovascular disease end points in women. However, there was no reduction in the mortality rate.
Other statin trials. A later meta-analysis of randomized primary prevention trials found that men and women derived similar benefit from statins in terms of cardiovascular disease end points and all-cause mortality (Figure 1),11 although five of the trials included a small number of secondary prevention patients. In contrast, a meta-analysis of only primary prevention patients showed no benefit of statin therapy in all-cause mortality, although the authors acknowledged that there were insufficient data to look specifically at women in this sample.12
A Cochrane review conducted before the JUPITER data were available concluded that there was insufficient evidence to prescribe statins for primary prevention in patients at low cardiovascular risk.13 However, an updated version that included results of the JUPITER trial concluded that there was a reduction in the rate of all-cause mortality and cardiovascular events in both men and women receiving a statin for primary prevention.14
Given these conflicting results, debate continues as to the benefit of statins for primary prevention, not only in women but in the population as a whole.15,16 The definition of high risk, in terms of comorbidities and lipid profile, also continues to evolve and will likely be an important factor in identifying women who will benefit from statin therapy for primary prevention.
Statin adverse effects. Much of the debate about statins for primary prevention stems from concern about the adverse effects of these drugs. In addition to myopathy, there have been reports of increased risks of new diabetes and cognitive impairment.16 In a post hoc analysis of the Women’s Health Initiative, the adjusted risk of diabetes was 48% higher in women taking a statin for primary prevention than in similar women not taking a statin.17 (This finding should be viewed with caution, since the data are observational.)
There has also been a question of whether women experience more side effects from statin therapy than men do. Although thin or frail women over age 80 are more susceptible to statin side effects, this finding has not been observed in younger women.18
Comment. In view of the data, it appears reasonable to consider statin therapy for primary prevention in women deemed to be at high risk based on the current guidelines. However, as always, one must consider whether the benefits outweigh the risks for the individual patient. More study is needed to better evaluate the utility of statin therapy in primary prevention.
Hormone therapy
Hormone therapy has received enormous attention in both the medical community and the public media. (Hormone therapy is either combined estrogen and progestin or estrogen alone, used to treat symptoms of menopause and to prevent osteoporosis in postmenopausal women. Here, we will discuss hormone therapy and not hormone replacement therapy, which is used specifically to treat premature menopause.)
The safety of estrogen-progestin combination therapy has been the subject of great debate since a Women’s Health Initiative study showed a trend toward a greater risk of cardiovascular disease in estrogen-progestin users.19
Women who received estrogen by itself showed no difference in cardiovascular risk compared with those who received placebo. Unopposed estrogen is rarely prescribed, since it increases the risk of endometrial cancer in women who have not undergone hysterectomy.20
Both unopposed estrogen and combination therapy have also been found to increase the risk of stroke,20 deep vein thrombosis, gallbladder disease, and certain forms of urinary incontinence.
Guidelines on hormone therapy. The USPSTF does not recommend hormone therapy to prevent chronic conditions, basing its decision on the findings from the Women’s Health Initiative.21 The American College of Cardiology and American Heart Association (ACC/AHA) 2007 guidelines advise against continuing hormone therapy in patients who present with acute coronary syndrome, although recommendations need to address a broader scope of primary and secondary prevention patients.
Does timing matter? There is a hypothesis that when hormone therapy is started may affect the cardiovascular risk. A secondary analysis of the Women’s Health Initiative study22 showed a trend towards less cardiovascular disease in women who started hormone therapy within 9 years of menopause, whereas those starting it later had a statistically significantly higher rate of cardiovascular mortality. However, all women had a higher risk of stroke while on hormone therapy, regardless of timing.22
A study of 1,006 healthy women age 45 to 58 whose last menstrual period was 3 to 24 months before enrollment found a statistically significant reduction in the composite end point of death, hospital admission for myocardial infarction, or heart failure with hormone therapy.23 There was no significant increase in breast cancer, deep vein thrombosis, or stroke after 10 years of randomized treatment.
A retrospective analysis of 71,237 postmenopausal women in the California Teachers Study also found a significant reduction in the rate of cardiovascular disease-related deaths with hormone therapy in younger women (ie, younger than age 65), but not in older women.24 The authors concluded that it may not just be the years after menopause but also the baseline age of the woman that may influence outcomes.
In view of these studies, there is increasing recognition that hormone therapy may, in fact, still be beneficial in terms of cardiovascular and all-cause mortality in carefully selected patients. The cardiovascular risk in women, specifically older women who have had a longer duration of menopause, should also be weighed against the potential benefits of therapy in terms of quality of life and symptom relief.
Trials under way include the Kronos Early Estrogen Prevention (KEEP) and Danish Osteoporosis Prevention (DOPS) studies. KEEP is a 4-year, double-blind, randomized controlled trial of hormone therapy in women within 3 years of menopause. DOPS is an open-label trial that includes more than 1,000 women with early menopause. The results of these trials will likely affect future recommendations.
WOMEN’S SYMPTOMS: TYPICAL OR ATYPICAL?
Whether the presenting symptoms of acute coronary syndromes differ between men and women has been much debated.
More women than men seem to present with atypical symptoms.25–27 (The term “atypical” refers to symptoms that do not include the three classic components of angina: substernal chest pain or discomfort, provoked by exertion or emotional stress, and relieved by rest or nitroglycerin, or both.28)
However, most women still present with chest pain. In a study by Dey et al,26 92% of the 7,638 women with presumed acute coronary syndrome presented with chest pain. In women who had atypical symptoms, dyspnea, nausea, vomiting, and diaphoresis were the most common symptoms. Women were significantly more likely than men to present with nausea and vomiting (32% vs 23%, P = .001).
Women in the study were also more likely to have angiographically normal coronary arteries (12% vs 6%, P < .001).26 This difference may be largely due to noncardiac chest pain, but it may also represent conditions such as vasospasm, microvascular disease, or stress cardiomyopathy, all of which disproportionately affect women.
An earlier review of 10 major studies found a higher percentage of women presenting with atypical symptoms (37.5% of women vs 27.4% of men).25 However, symptoms were not a focus of these studies, and the findings may therefore be skewed by inaccurate documentation.
Atypical warning signs. Although most women with acute coronary syndrome present acutely with chest pain, women may have different warning signs than men. Only about one-third of women experience angina before presentation.29 Compared with men, women are more likely to complain of shortness of breath, fatigue, and weakness leading up to a diagnosis of a myocardial infarction.29 Therefore, the prodromal symptoms of cardiovascular disease may in fact be significantly more atypical in women than in men, suggesting the need for heightened vigilance in the cardiovascular evaluation of women who have nonanginal symptoms.
THE ROLE OF STRESS TESTING IN WOMEN
Stress testing in various forms continues to be widely used in the diagnosis of heart disease in women, although data are scarce regarding its utility in women.
The ACC/AHA guidelines continue to recommend exercise stress electrocardiography (ECG) for women who have symptoms, are at intermediate risk, and have a normal result on resting ECG.30
Exercise ECG has a higher false-positive rate in women than in men,31 and there appears to be no relationship between exercise-induced ST-segment depression and the rate of cardiovascular mortality or all-cause mortality in women.32,33 On the other hand, exercise ECG yields valuable additional information such as exercise capacity, chronotropic response, heart-rate recovery, and blood pressure response, all of which have important diagnostic and prognostic implications in women.34
For those who have an abnormal resting ECG, the addition of an imaging test, ie, echocardiography or single-photon emission computed tomography (SPECT), is indicated. Both have limitations: SPECT can give false-positive results because of breast attenuation, and echocardiography varies in accuracy depending on the quality of acoustic windows obtained. Both exercise stress SPECT and exercise stress ECG have higher sensitivity and specificity than electrocardiographic exercise stress testing alone,34 and there is evidence that the two imaging tests are comparable in women.35
In those women who have baseline left bundle branch block or who cannot exercise, a pharmacologic stress test should be performed. Of course, this is a less desirable testing method, given the loss of valuable information obtained from exercising the patient.
UNDERLYING CONDITIONS THAT DISPROPORTIONATELY AFFECT WOMEN
Microvascular angina
Perimenopausal and postmenopausal women account for 70% of patients presenting with chest pain and elevated cardiac enzymes but no significant angiographic evidence of coronary artery disease.36 This condition, commonly called syndrome X, is often characterized by lingering, dull chest pain after exertion and is seen more frequently in women younger than those presenting with classic cardiovascular disease.
Because at least some of these patients show evidence of ST-segment depression and reversible perfusion defects on imaging, the condition is thought to be caused by ischemia of the microvascular bed leading to microvascular angina.37
Although this is still an area of research, microvascular dysfunction has recently been proposed as an explanation for these findings. Abnormal vasoconstriction and impaired vasodilation of the microvascular bed, insulin resistance, increased systemic inflammation, and abnormal pain response have all been cited as potentially contributing to microvascular dysfunction.36
Estrogen deficiency is thought to play a central role in the significantly increased burden of microvascular dysfunction seen in women, with some studies suggesting that hormone therapy can relieve symptoms. However, given the concerns about adverse cardiovascular outcomes in women on hormone therapy, there has been little investigation of this treatment for this disorder.
Studies have shown worse cardiovascular outcomes and higher rates of angina-related hospitalization and repeat heart catheterizations in women with microvascular dysfunction.38
Diagnosing microvascular angina must be done indirectly, as there is no safe and minimally invasive technique by which to directly observe the microvasculature. Current coronary angiographic techniques cannot image vessels smaller than 0.5 mm in diameter, and endomyocardial biopsy cannot access the larger periarterioles thought to play a major role in regulating coronary blood flow.39
Because the coronary microvasculature controls total coronary resistance and therefore regulates myocardial blood flow, measuring myocardial blood flow at maximum vasodilation, termed coronary flow reserve, can indirectly evaluate the degree of microvascular dysfunction.40 In the absence of obstructive epicardial coronary disease, noninvasive imaging techniques or provocative testing in the coronary catheterization lab can be used for this purpose. In terms of noninvasive imaging, perfusion magnetic resonance imaging (Figure 2) or positron emission tomography is often performed.40
Coronary flow reserve can also be measured by invasive means in the catheterization laboratory after maximum hyperemia is induced by adenosine or other such vasodilatory agents.41 However, measurements obtained in this invasive manner are greatly affected by hemodynamic changes and can have poor reproducibility.40
Proposed therapy for microvascular angina. Once a diagnosis has been made, lifestyle modification, antianginal agents, angiotensin-converting enzyme inhibitors, and statins have been suggested for therapy.39 Pain management techniques are also used, given the increased pain sensitivity observed in women with this condition. However, no therapy to date has proven overwhelmingly effective in these patients, and a disproportionate number of women suffer from chronic symptoms despite these treatments. Currently, researchers are looking for new agents to treat microvascular disease.
Stress cardiomyopathy
Stress cardiomyopathy, also called takotsubo cardiomyopathy or “broken heart syndrome,” is another condition that disproportionately affects postmenopausal women. It is often associated with sudden emotional or physical stress. Patients present with signs and symptoms of myocardial infarction without demonstrable epicardial coronary artery disease. The hallmark of stress cardiomyopathy is left ventricular dysfunction, often severe, with classic apical ballooning that resembles a Japanese fishing pot (takotsubo) used to trap octopuses, hence the name (Figure 3).
According to a review by Akashi et al42 based on previously reported Mayo Clinic criteria, the diagnosis of stress cardiomyopathy includes each of the following:
- Transient hypokinesis, akinesis, or dyskinesis in the left ventricular midsegments with or without apical involvement; regional wall-motion abnormalities that extend beyond a single epicardial vascular distribution; and frequently, but not always, a stressful trigger
- Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture
- New abnormality on ECG (eg, ST-segment elevation, T-wave inversion) or modest elevation in cardiac troponin
- Absence of pheochromocytoma or myocarditis.
From 80% to 100% of reported cases are in women, with an average age range of 61 to 76.42 It is unclear why there is such an overwhelming postmenopausal female preponderance of the disease. Studies have implicated estrogen deficiency, as it appears to attenuate the levels of cardioprotective substances in the body that in part regulate catecholamine surges and may also increase the level of oxidative stress.42
Several mechanisms for this condition have been proposed. The condition may be caused by multivessel epicardial coronary spasm or spontaneously resolved plaque rupture, resulting in stunned myocardium. However, the regional distribution of wall-motion abnormality is often out of proportion to the level of cardiac enzyme elevation, and in the case of plaque rupture, is frequently not consistent with a single coronary vessel.42 A catecholamine surge causing myocardial and neurogenic stunning has also been proposed, although many of these patients have normal catecholamine levels.42 Finally, microvascular dysfunction has been found in a number of patients with this condition. However, it is difficult to establish a causal relationship, since apical ballooning could result in microvascular dysfunction.42
Treatment of stress cardiomyopathy has not been standardized, in part because the left ventricular dysfunction often resolves spontaneously within several weeks.43,44 Given the proposed catecholaminergic mechanism, some experts believe that beta-blockers are contraindicated because of the resulting unopposed activation of alpha-adrenoreceptors. However, this continues to be a matter of debate. There is also no clear indication for other standard therapies for acute coronary syndrome such as aspirin and heparin, and their use appears to vary in clinical practice.
Although most patients improve with time and recurrence is exceedingly rare, it should be emphasized that they may present acutely with severe hemodynamic instability and cardiogenic shock. Therefore, advanced means of support, such as an intra-aortic balloon pump, may be indicated until the patient recovers from the acute phase of the disease.
Spontaneous coronary artery dissection
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome resulting from dissection of the coronary intimal or medial layer and associated hematoma formation, leading to coronary occlusion.45,46 In a case series of 87 patients, 49% presented with an ST-segment elevation myocardial infarction, and 23% were found to have multivessel SCAD.46
SCAD occurs predominantly in young, healthy women (mean age 30–45 years). Approximately 70% of cases are in women, 30% of whom are in the peripartum period.45 The reasons for the increased risk during pregnancy have not yet been elucidated, but changing sex hormones, increased cardiac output and shear stress, and an increased inflammatory response have been implicated.45
Diagnosing SCAD. Coronary angiography should be performed with extreme caution in patients suspected of having SCAD, given the risk of further dissection of the artery with forceful injections. In certain cases, it may be difficult to detect SCAD on routine angiography if there is no communication between the true and false lumen.
If the suspicion for SCAD is high, intravascular ultrasonography or optical coherence tomography can be used to better evaluate the vessel.45 Although optical coherence tomography has greater spatial resolution, it is more costly and is not as widely used as intravascular ultrasonography in the clinical setting
Managing SCAD. Although conservative management and coronary artery bypass grafting have been shown to cause minimal in-hospital morbidity, percutaneous coronary intervention has been complicated by technical failure in up to 35% of patients in one series.46
While there is no standardized way to manage these patients, experts currently recommend conservative management with standard therapies for acute coronary syndrome (Figure 4). Although antithrombotic agents can decrease thrombus burden, they must be used with caution, because they also increase the risk of bleeding into the false lumen.
If patients experience recurrent or ongoing ischemia despite conservative management, then revascularization should be considered. Optical coherence tomography or intravascular ultrasonography is recommended to ensure proper stent alignment and positioning.
Coronary artery bypass grafting could be considered in preference to percutaneous coronary intervention, given that the former appears to be safer,46 although this requires further investigation. Some studies have cautioned against using fibrinolytic therapy, based on anecdotal evidence that it may further propagate the dissection,45 although this therapy has been used in other case studies.46
While mortality rates are relatively low (95% survival at 2 years),45 the estimated risk of recurrent SCAD at 10 years is approximately 30%.46
Associated with fibromuscular dysplasia. Of note, a sizeable number of patients with SCAD have been found to have fibromuscular dysplasia. This is a nonatherosclerotic, noninflammatory vascular condition that can affect any vascular bed in the body, although there is a predilection for the renal and carotid arteries (Figure 5).47 Fibromuscular dysplasia also disproportionately affects women and appears to be a concomitant condition in the majority of patients with SCAD.47 Imaging of the carotid and renal arteries of patients with SCAD has revealed a number of cases of fibromuscular dysplasia.46,48 This noted association will likely allow for ongoing research to better understand the pathophysiology of these two conditions.
- Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol 2007; 49:1230–1250.
- Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000; 343:16–22.
- Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the US Preventive Services Task Force. Ann Intern Med 2009; 150:405–410.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Cook NR, Cole SR, Buring JE. Aspirin in the primary prevention of cardiovascular disease in the Women’s Health Study: effect of non-compliance. Eur J Epidemiol 2012; 27:431–438.
- Mosca L, Benjamin EJ, Berra K, et al; American Heart Association. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011; 57:1404–1423.
- Jardine MJ, Ninomiya T, Perkovic V, et al. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol 2010; 56:956–965.
- Snoep JD, Roest M, Barendrecht AD, De Groot PG, Rosendaal FR, Van Der Bom JG. High platelet reactivity is associated with myocardial infarction in premenopausal women: a population-based case-control study. J Thromb Haemost 2010; 8:906–913.
- Becker DM, Segal J, Vaidya D, et al. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA 2006; 295:1420–1427.
- Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation 2010; 121:1069–1077.
- Kostis WJ, Cheng JQ, Dobrzynski JM, Cabrera J, Kostis JB. Meta-analysis of statin effects in women versus men. J Am Coll Cardiol 2012; 59:572–582.
- Ray KK, Seshasai SR, Erqou S, et al. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch Intern Med 2010; 170:1024–1031.
- Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2011;CD004816.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;CD004816.
- Blaha MJ, Nasir K, Blumenthal RS. Statin therapy for healthy men identified as “increased risk.” JAMA 2012; 307:1489–1490.
- Redberg RF, Katz MH. Healthy men should not take statins. JAMA 2012; 307:1491–1492.
- Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med 2012; 172:144–152.
- Pasternak RC, Smith SC, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Stroke 2002; 33:2337–2341.
- Manson JE, Hsia J, Johnson KC, et al; Women’s Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003; 349:523–534.
- Hsia J, Criqui MH, Herrington DM, et al; Women’s Health Initiative Research Group. Conjugated equine estrogens and peripheral arterial disease risk: The Women’s Health Initiative. Am Heart J 2006; 152:170–176.
- Moyer VAUS Preventive Services Task Force. Menopausal hormone therapy for the primary prevention of chronic conditions: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158:47–54.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012; 345:e6409.
- Stram DO, Liu Y, Henderson KD, et al. Age-specific effects of hormone therapy use on overall mortality and ischemic heart disease mortality among women in the California Teachers Study. Menopause 2011; 18:253–261.
- Canto JG, Goldberg RJ, Hand MM, et al. Symptom presentation of women with acute coronary syndromes: myth vs reality. Arch Intern Med 2007; 167:2405–2413.
- Dey S, Flather MD, Devlin G, et al; Global Registry of Acute Coronary Events investigators. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: The Global Registry of Acute Coronary Events. Heart 2009; 95:20–26.
- Canto JG, Shlipak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA 2000; 283:3223–3229.
- Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979; 300:1350–1358.
- McSweeney JC, Cody M, O’Sullivan P, Elberson K, Moser DK, Garvin BJ. Women’s early warning symptoms of acute myocardial infarction. Circulation 2003; 108:2619–2623.
- Mieres JH, Shaw LJ, Arai A, et al; Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation 2005; 111:682–696.
- Barolsky SM, Gilbert CA, Faruqui A, Nutter DO, Schlant RC. Differences in electrocardiographic response to exercise of women and men: a non-Bayesian factor. Circulation 1979; 60:1021–1027.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: The St James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics Prevalence Study. JAMA 2003; 290:1600–1607.
- Kohli P, Gulati M. Exercise stress testing in women: going back to the basics. Circulation 2010; 122:2570–2580.
- Grady D, Chaput L, Kristof M. Diagnosis and treatment of coronary heart disease in women: systematic reviews of evidence on selected topics. Evid Rep Technol Assess (Summ) 2003; 81:1–4.
- Singh M, Singh S, Arora R, Khosla S. Cardiac syndrome X: current concepts. Int J Cardiol 2010; 142:113–119.
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007; 356:830–840.
- Johnson BD, Shaw LJ, Buchthal SD, et al; National Institutes of Health-National Heart, Lung, and Blood Institute. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004; 109:2993–2999.
- Beltrame JF, Crea F, Camici P. Advances in coronary microvascular dysfunction. Heart Lung Circ 2009; 18:19–27.
- Leung DY, Leung M. Non-invasive/invasive imaging: significance and assessment of coronary microvascular dysfunction. Heart 2011; 97:587–595.
- Samim A, Nugent L, Mehta PK, Shufelt C, Bairey Merz CN. Treatment of angina and microvascular coronary dysfunction. Curr Treat Options Cardiovasc Med 2010; 12:355–364.
- Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008; 118:2754–2762.
- Akashi YJ, Musha H, Kida K, et al. Reversible ventricular dysfunction takotsubo cardiomyopathy. Eur J Heart Fail 2005; 7:1171–1176.
- Regnante RA, Zuzek RW, Weinsier SB, et al. Clinical characteristics and four-year outcomes of patients in the Rhode Island Takotsubo Cardiomyopathy Registry. Am J Cardiol 2009; 103:1015–1019.
- Vrints CJ. Spontaneous coronary artery dissection. Heart 2010; 96:801–808.
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126:579–588.
- Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013; 6:44–52.
- Saw J, Poulter R, Fung A, Wood D, Hamburger J, Buller CE. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ Cardiovasc Interv 2012; 5:134–137.
- Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol 2007; 49:1230–1250.
- Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000; 343:16–22.
- Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the US Preventive Services Task Force. Ann Intern Med 2009; 150:405–410.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Cook NR, Cole SR, Buring JE. Aspirin in the primary prevention of cardiovascular disease in the Women’s Health Study: effect of non-compliance. Eur J Epidemiol 2012; 27:431–438.
- Mosca L, Benjamin EJ, Berra K, et al; American Heart Association. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011; 57:1404–1423.
- Jardine MJ, Ninomiya T, Perkovic V, et al. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol 2010; 56:956–965.
- Snoep JD, Roest M, Barendrecht AD, De Groot PG, Rosendaal FR, Van Der Bom JG. High platelet reactivity is associated with myocardial infarction in premenopausal women: a population-based case-control study. J Thromb Haemost 2010; 8:906–913.
- Becker DM, Segal J, Vaidya D, et al. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA 2006; 295:1420–1427.
- Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation 2010; 121:1069–1077.
- Kostis WJ, Cheng JQ, Dobrzynski JM, Cabrera J, Kostis JB. Meta-analysis of statin effects in women versus men. J Am Coll Cardiol 2012; 59:572–582.
- Ray KK, Seshasai SR, Erqou S, et al. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch Intern Med 2010; 170:1024–1031.
- Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2011;CD004816.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;CD004816.
- Blaha MJ, Nasir K, Blumenthal RS. Statin therapy for healthy men identified as “increased risk.” JAMA 2012; 307:1489–1490.
- Redberg RF, Katz MH. Healthy men should not take statins. JAMA 2012; 307:1491–1492.
- Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med 2012; 172:144–152.
- Pasternak RC, Smith SC, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Stroke 2002; 33:2337–2341.
- Manson JE, Hsia J, Johnson KC, et al; Women’s Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003; 349:523–534.
- Hsia J, Criqui MH, Herrington DM, et al; Women’s Health Initiative Research Group. Conjugated equine estrogens and peripheral arterial disease risk: The Women’s Health Initiative. Am Heart J 2006; 152:170–176.
- Moyer VAUS Preventive Services Task Force. Menopausal hormone therapy for the primary prevention of chronic conditions: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158:47–54.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012; 345:e6409.
- Stram DO, Liu Y, Henderson KD, et al. Age-specific effects of hormone therapy use on overall mortality and ischemic heart disease mortality among women in the California Teachers Study. Menopause 2011; 18:253–261.
- Canto JG, Goldberg RJ, Hand MM, et al. Symptom presentation of women with acute coronary syndromes: myth vs reality. Arch Intern Med 2007; 167:2405–2413.
- Dey S, Flather MD, Devlin G, et al; Global Registry of Acute Coronary Events investigators. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: The Global Registry of Acute Coronary Events. Heart 2009; 95:20–26.
- Canto JG, Shlipak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA 2000; 283:3223–3229.
- Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979; 300:1350–1358.
- McSweeney JC, Cody M, O’Sullivan P, Elberson K, Moser DK, Garvin BJ. Women’s early warning symptoms of acute myocardial infarction. Circulation 2003; 108:2619–2623.
- Mieres JH, Shaw LJ, Arai A, et al; Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation 2005; 111:682–696.
- Barolsky SM, Gilbert CA, Faruqui A, Nutter DO, Schlant RC. Differences in electrocardiographic response to exercise of women and men: a non-Bayesian factor. Circulation 1979; 60:1021–1027.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: The St James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics Prevalence Study. JAMA 2003; 290:1600–1607.
- Kohli P, Gulati M. Exercise stress testing in women: going back to the basics. Circulation 2010; 122:2570–2580.
- Grady D, Chaput L, Kristof M. Diagnosis and treatment of coronary heart disease in women: systematic reviews of evidence on selected topics. Evid Rep Technol Assess (Summ) 2003; 81:1–4.
- Singh M, Singh S, Arora R, Khosla S. Cardiac syndrome X: current concepts. Int J Cardiol 2010; 142:113–119.
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007; 356:830–840.
- Johnson BD, Shaw LJ, Buchthal SD, et al; National Institutes of Health-National Heart, Lung, and Blood Institute. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004; 109:2993–2999.
- Beltrame JF, Crea F, Camici P. Advances in coronary microvascular dysfunction. Heart Lung Circ 2009; 18:19–27.
- Leung DY, Leung M. Non-invasive/invasive imaging: significance and assessment of coronary microvascular dysfunction. Heart 2011; 97:587–595.
- Samim A, Nugent L, Mehta PK, Shufelt C, Bairey Merz CN. Treatment of angina and microvascular coronary dysfunction. Curr Treat Options Cardiovasc Med 2010; 12:355–364.
- Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008; 118:2754–2762.
- Akashi YJ, Musha H, Kida K, et al. Reversible ventricular dysfunction takotsubo cardiomyopathy. Eur J Heart Fail 2005; 7:1171–1176.
- Regnante RA, Zuzek RW, Weinsier SB, et al. Clinical characteristics and four-year outcomes of patients in the Rhode Island Takotsubo Cardiomyopathy Registry. Am J Cardiol 2009; 103:1015–1019.
- Vrints CJ. Spontaneous coronary artery dissection. Heart 2010; 96:801–808.
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126:579–588.
- Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013; 6:44–52.
- Saw J, Poulter R, Fung A, Wood D, Hamburger J, Buller CE. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ Cardiovasc Interv 2012; 5:134–137.
KEY POINTS
- Aspirin appears to be less beneficial in women than in men in preventing coronary artery disease.
- Debate continues on the benefit of statins for primary prevention, not only in women but in the population as a whole.
- Hormone therapy is not recommended for cardiovascular disease prevention.
- More women than men who present with acute coronary syndromes have atypical symptoms. Nevertheless, most women who have acute coronary syndromes do have typical symptoms such as chest pain.
- Guidelines continue to recommend exercise stress electrocardiography for symptomatic women at intermediate risk who have a normal resting electrocardiogram.
- Conditions that predominantly affect women include microvascular angina, stress cardiomyopathy, and spontaneous coronary artery dissection.