User login
Cryolipolysis Side Effects Mostly Mild, "Severe Pain" Rare
GRAPEVINE, TEX. – Risks associated with cryolipolysis for fat reduction are mostly transient and generally mild, according to a recent analysis.
"Severe pain" was reported in approximately 0.05% of all treated patients, said Dr. Nazanin Saedi of the University of California, Irvine.
Reports of severe pain emerged in postmarket surveillance, which traditionally allows for the detection of rare side effects not seen in clinical trials. Of more than 60,000 cryolipolysis treatments between June 2009 and December 2010, there were 23 reports of "severe pain" associated with the Zeltiq system.
The determination that a report was of "severe" pain rather than the known potential discomfort following treatment depended on symptoms. Of the reports, 11 were described as "severe," 8 as "sensitive to touch," 6 as "stabbing," and 6 as "deep/severe burning." (Patients could report more than one symptom). The incidence was calculated based on a denominator of 50,000, giving a rate of 0.00046, or 0.05%.
Zeltiq evaluated the "severe pain" to understand its etiology. The pain does not appear to be associated with the increasing number of larger applicators, because 10 of the 23 of patients reporting "severe pain" underwent treatment with the smaller applicator, Dr. Saedi noted.
Most severe pain involved the abdomen (21 of the 23 patients), arising at a mean of 3.4 days (range, 1-7) following the procedure. The mean time to resolution was 13.9 days (range, 7-28). Just one patient had severe pain lasting for 28 days, while in the majority (16 of the 23), pain had resolved by 2 weeks. Six patients reported that the pain worsened before getting better, peaking at about a week.
Two of the patients had known connective tissue disease, while the etiology was unknown for the rest. Exaggerated panniculitis is one possibility. The pain might also be of focal neuropathic origin, including allodynia, hyperneuralgia, or nerve inflammation arising from cytokine-mediated irritation of nerve fibers during the onset of inflammation following the procedure, said Dr. Saedi. It is likely that there are multiple etiologies.
A variety of therapeutic measures were used to treat the severe pain, but only the topical lidocaine patch was consistently reported as being helpful. Compression garments, lidocaine/tetracaine cream, and Vicodin (hydrocodone bitartrate and acetaminophen) were reported to have some effect, while ibuprofen, Percocet (oxycodone HCl and acetaminophen), Tylenol (acetaminophen) with codeine, ice, and heating pads had little or no effect, Dr. Saedi said.
Hyperpigmentation associated with a first- or second-degree burn of the dermis was another rare adverse event that arose in postmarketing surveillance. A total of four cases were reported, or less than 0.01% of treated patients. Three of the four were deemed to have been a result of operator error. All cases resolved.
Cryolipolysis technology uses controlled cold exposure to reduce subcutaneous fat. Adipocytes are selectively damaged via control and modulation of the cold exposure, while avoiding damage to the overlying epidermis and dermis. The decrease in fat thickness occurs gradually over the subsequent 3 months, and is most pronounced in patients with limited, discrete fatty bulges. The novel technology is among the noninvasive mechanisms for fat reduction that are becoming increasingly popular commercially, she noted.
Since the reports, there have been changes to the user manual as well as revisions in user training, user interface, and procedure monitoring , which make the likelihood of burn injury or subsequent hyperpigmentation more unlikely, even in the event of misuse. In fact, there have been no further reports of burn injury or hyperpigmentation during the last 30,000 procedure cycles, she noted.
"Further postmarket surveillance is needed to identify and better understand rare events," Dr. Saedi concluded.
Dr. Saedi said that she had no relevant financial disclosures. However, her coinvestigators are on Zeltiq's advisory board and have received educational and research support and honoraria from the company.
GRAPEVINE, TEX. – Risks associated with cryolipolysis for fat reduction are mostly transient and generally mild, according to a recent analysis.
"Severe pain" was reported in approximately 0.05% of all treated patients, said Dr. Nazanin Saedi of the University of California, Irvine.
Reports of severe pain emerged in postmarket surveillance, which traditionally allows for the detection of rare side effects not seen in clinical trials. Of more than 60,000 cryolipolysis treatments between June 2009 and December 2010, there were 23 reports of "severe pain" associated with the Zeltiq system.
The determination that a report was of "severe" pain rather than the known potential discomfort following treatment depended on symptoms. Of the reports, 11 were described as "severe," 8 as "sensitive to touch," 6 as "stabbing," and 6 as "deep/severe burning." (Patients could report more than one symptom). The incidence was calculated based on a denominator of 50,000, giving a rate of 0.00046, or 0.05%.
Zeltiq evaluated the "severe pain" to understand its etiology. The pain does not appear to be associated with the increasing number of larger applicators, because 10 of the 23 of patients reporting "severe pain" underwent treatment with the smaller applicator, Dr. Saedi noted.
Most severe pain involved the abdomen (21 of the 23 patients), arising at a mean of 3.4 days (range, 1-7) following the procedure. The mean time to resolution was 13.9 days (range, 7-28). Just one patient had severe pain lasting for 28 days, while in the majority (16 of the 23), pain had resolved by 2 weeks. Six patients reported that the pain worsened before getting better, peaking at about a week.
Two of the patients had known connective tissue disease, while the etiology was unknown for the rest. Exaggerated panniculitis is one possibility. The pain might also be of focal neuropathic origin, including allodynia, hyperneuralgia, or nerve inflammation arising from cytokine-mediated irritation of nerve fibers during the onset of inflammation following the procedure, said Dr. Saedi. It is likely that there are multiple etiologies.
A variety of therapeutic measures were used to treat the severe pain, but only the topical lidocaine patch was consistently reported as being helpful. Compression garments, lidocaine/tetracaine cream, and Vicodin (hydrocodone bitartrate and acetaminophen) were reported to have some effect, while ibuprofen, Percocet (oxycodone HCl and acetaminophen), Tylenol (acetaminophen) with codeine, ice, and heating pads had little or no effect, Dr. Saedi said.
Hyperpigmentation associated with a first- or second-degree burn of the dermis was another rare adverse event that arose in postmarketing surveillance. A total of four cases were reported, or less than 0.01% of treated patients. Three of the four were deemed to have been a result of operator error. All cases resolved.
Cryolipolysis technology uses controlled cold exposure to reduce subcutaneous fat. Adipocytes are selectively damaged via control and modulation of the cold exposure, while avoiding damage to the overlying epidermis and dermis. The decrease in fat thickness occurs gradually over the subsequent 3 months, and is most pronounced in patients with limited, discrete fatty bulges. The novel technology is among the noninvasive mechanisms for fat reduction that are becoming increasingly popular commercially, she noted.
Since the reports, there have been changes to the user manual as well as revisions in user training, user interface, and procedure monitoring , which make the likelihood of burn injury or subsequent hyperpigmentation more unlikely, even in the event of misuse. In fact, there have been no further reports of burn injury or hyperpigmentation during the last 30,000 procedure cycles, she noted.
"Further postmarket surveillance is needed to identify and better understand rare events," Dr. Saedi concluded.
Dr. Saedi said that she had no relevant financial disclosures. However, her coinvestigators are on Zeltiq's advisory board and have received educational and research support and honoraria from the company.
GRAPEVINE, TEX. – Risks associated with cryolipolysis for fat reduction are mostly transient and generally mild, according to a recent analysis.
"Severe pain" was reported in approximately 0.05% of all treated patients, said Dr. Nazanin Saedi of the University of California, Irvine.
Reports of severe pain emerged in postmarket surveillance, which traditionally allows for the detection of rare side effects not seen in clinical trials. Of more than 60,000 cryolipolysis treatments between June 2009 and December 2010, there were 23 reports of "severe pain" associated with the Zeltiq system.
The determination that a report was of "severe" pain rather than the known potential discomfort following treatment depended on symptoms. Of the reports, 11 were described as "severe," 8 as "sensitive to touch," 6 as "stabbing," and 6 as "deep/severe burning." (Patients could report more than one symptom). The incidence was calculated based on a denominator of 50,000, giving a rate of 0.00046, or 0.05%.
Zeltiq evaluated the "severe pain" to understand its etiology. The pain does not appear to be associated with the increasing number of larger applicators, because 10 of the 23 of patients reporting "severe pain" underwent treatment with the smaller applicator, Dr. Saedi noted.
Most severe pain involved the abdomen (21 of the 23 patients), arising at a mean of 3.4 days (range, 1-7) following the procedure. The mean time to resolution was 13.9 days (range, 7-28). Just one patient had severe pain lasting for 28 days, while in the majority (16 of the 23), pain had resolved by 2 weeks. Six patients reported that the pain worsened before getting better, peaking at about a week.
Two of the patients had known connective tissue disease, while the etiology was unknown for the rest. Exaggerated panniculitis is one possibility. The pain might also be of focal neuropathic origin, including allodynia, hyperneuralgia, or nerve inflammation arising from cytokine-mediated irritation of nerve fibers during the onset of inflammation following the procedure, said Dr. Saedi. It is likely that there are multiple etiologies.
A variety of therapeutic measures were used to treat the severe pain, but only the topical lidocaine patch was consistently reported as being helpful. Compression garments, lidocaine/tetracaine cream, and Vicodin (hydrocodone bitartrate and acetaminophen) were reported to have some effect, while ibuprofen, Percocet (oxycodone HCl and acetaminophen), Tylenol (acetaminophen) with codeine, ice, and heating pads had little or no effect, Dr. Saedi said.
Hyperpigmentation associated with a first- or second-degree burn of the dermis was another rare adverse event that arose in postmarketing surveillance. A total of four cases were reported, or less than 0.01% of treated patients. Three of the four were deemed to have been a result of operator error. All cases resolved.
Cryolipolysis technology uses controlled cold exposure to reduce subcutaneous fat. Adipocytes are selectively damaged via control and modulation of the cold exposure, while avoiding damage to the overlying epidermis and dermis. The decrease in fat thickness occurs gradually over the subsequent 3 months, and is most pronounced in patients with limited, discrete fatty bulges. The novel technology is among the noninvasive mechanisms for fat reduction that are becoming increasingly popular commercially, she noted.
Since the reports, there have been changes to the user manual as well as revisions in user training, user interface, and procedure monitoring , which make the likelihood of burn injury or subsequent hyperpigmentation more unlikely, even in the event of misuse. In fact, there have been no further reports of burn injury or hyperpigmentation during the last 30,000 procedure cycles, she noted.
"Further postmarket surveillance is needed to identify and better understand rare events," Dr. Saedi concluded.
Dr. Saedi said that she had no relevant financial disclosures. However, her coinvestigators are on Zeltiq's advisory board and have received educational and research support and honoraria from the company.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
FDA Panel Backs Approval of Restylane for Lip Augmentation
Dermatologists may not have to use Restylane off-label for lip augmentation any longer. A Food and Drug Administration panel voted 6-0 with 1 abstention that benefits outweigh risks for using the filler as a submucosal injection for lip augmentation on April 27.
The panel also voted 6-0 with 1 abstention that the filler is safe and effective for the expanded indication.
Restylane (Medicis Aesthetics) is a hyaluronic acid gel generated by Streptococcus bacteria, chemically crosslinked with 1,4 butanediol diglycidyl ether. The filler was first approved in 2005 for mid-to-deep dermal implantation for the correction of moderate-to-severe facial wrinkles and folds, such as nasolabial folds.
Medicis recently conducted a clinical study (MA-1399-15) to evaluate the safety and effectiveness of the filler in the augmentation of soft tissue fullness of the lips. The study included 135 patients, who received lip augmentation with Restylane, and 45 patients with no treatment. The mean volume of filler was 2.9 cc per patient, with a range of 0.6 -5.6 cc per patient. At 8 weeks, 92% of patients who received Restylane were considered responders.
Adverse events occurred in 99% of patients. Expected treatment-emergent adverse events included bruising, redness, swelling, pain, tenderness, itching, and skin exfoliation. Of note, herpes simplex virus 1 outbreaks occurred in 4% of patients. The outbreaks were determined to be associated with injection of the filler in 7 or 10 cases.
Forty percent of patients who received the filler had adverse outcomes that they felt affected their daily activity or were disabling; 15% of Restylane patients experienced adverse events (typically swelling and tenderness) that lasted more than 15 days.
The panel was particularly concerned about the implications of the treatment in younger patients who may still be growing and are likely to receive repeat treatments over time.
"My only problem with voting completely yes … is that I don't think that the question was worded in such a way that I felt totally comfortable giving my final approval for safety, efficacy, and risk/benefit ratio to all populations," said panel member Dr. Delora L. Mount, who is an associate professor of surgery and pediatrics at the University of Wisconsin in Madison.
The FDA panel was also concerned about the lack of men and individuals with dark skin in the trial. Only one man was included and 38 individuals were Fitzpatrick skin type IV, 3 individuals were type V, and none were type VI.
The FDA usually follows its panels' advice, but is not obligated to do so.
Dermatologists may not have to use Restylane off-label for lip augmentation any longer. A Food and Drug Administration panel voted 6-0 with 1 abstention that benefits outweigh risks for using the filler as a submucosal injection for lip augmentation on April 27.
The panel also voted 6-0 with 1 abstention that the filler is safe and effective for the expanded indication.
Restylane (Medicis Aesthetics) is a hyaluronic acid gel generated by Streptococcus bacteria, chemically crosslinked with 1,4 butanediol diglycidyl ether. The filler was first approved in 2005 for mid-to-deep dermal implantation for the correction of moderate-to-severe facial wrinkles and folds, such as nasolabial folds.
Medicis recently conducted a clinical study (MA-1399-15) to evaluate the safety and effectiveness of the filler in the augmentation of soft tissue fullness of the lips. The study included 135 patients, who received lip augmentation with Restylane, and 45 patients with no treatment. The mean volume of filler was 2.9 cc per patient, with a range of 0.6 -5.6 cc per patient. At 8 weeks, 92% of patients who received Restylane were considered responders.
Adverse events occurred in 99% of patients. Expected treatment-emergent adverse events included bruising, redness, swelling, pain, tenderness, itching, and skin exfoliation. Of note, herpes simplex virus 1 outbreaks occurred in 4% of patients. The outbreaks were determined to be associated with injection of the filler in 7 or 10 cases.
Forty percent of patients who received the filler had adverse outcomes that they felt affected their daily activity or were disabling; 15% of Restylane patients experienced adverse events (typically swelling and tenderness) that lasted more than 15 days.
The panel was particularly concerned about the implications of the treatment in younger patients who may still be growing and are likely to receive repeat treatments over time.
"My only problem with voting completely yes … is that I don't think that the question was worded in such a way that I felt totally comfortable giving my final approval for safety, efficacy, and risk/benefit ratio to all populations," said panel member Dr. Delora L. Mount, who is an associate professor of surgery and pediatrics at the University of Wisconsin in Madison.
The FDA panel was also concerned about the lack of men and individuals with dark skin in the trial. Only one man was included and 38 individuals were Fitzpatrick skin type IV, 3 individuals were type V, and none were type VI.
The FDA usually follows its panels' advice, but is not obligated to do so.
Dermatologists may not have to use Restylane off-label for lip augmentation any longer. A Food and Drug Administration panel voted 6-0 with 1 abstention that benefits outweigh risks for using the filler as a submucosal injection for lip augmentation on April 27.
The panel also voted 6-0 with 1 abstention that the filler is safe and effective for the expanded indication.
Restylane (Medicis Aesthetics) is a hyaluronic acid gel generated by Streptococcus bacteria, chemically crosslinked with 1,4 butanediol diglycidyl ether. The filler was first approved in 2005 for mid-to-deep dermal implantation for the correction of moderate-to-severe facial wrinkles and folds, such as nasolabial folds.
Medicis recently conducted a clinical study (MA-1399-15) to evaluate the safety and effectiveness of the filler in the augmentation of soft tissue fullness of the lips. The study included 135 patients, who received lip augmentation with Restylane, and 45 patients with no treatment. The mean volume of filler was 2.9 cc per patient, with a range of 0.6 -5.6 cc per patient. At 8 weeks, 92% of patients who received Restylane were considered responders.
Adverse events occurred in 99% of patients. Expected treatment-emergent adverse events included bruising, redness, swelling, pain, tenderness, itching, and skin exfoliation. Of note, herpes simplex virus 1 outbreaks occurred in 4% of patients. The outbreaks were determined to be associated with injection of the filler in 7 or 10 cases.
Forty percent of patients who received the filler had adverse outcomes that they felt affected their daily activity or were disabling; 15% of Restylane patients experienced adverse events (typically swelling and tenderness) that lasted more than 15 days.
The panel was particularly concerned about the implications of the treatment in younger patients who may still be growing and are likely to receive repeat treatments over time.
"My only problem with voting completely yes … is that I don't think that the question was worded in such a way that I felt totally comfortable giving my final approval for safety, efficacy, and risk/benefit ratio to all populations," said panel member Dr. Delora L. Mount, who is an associate professor of surgery and pediatrics at the University of Wisconsin in Madison.
The FDA panel was also concerned about the lack of men and individuals with dark skin in the trial. Only one man was included and 38 individuals were Fitzpatrick skin type IV, 3 individuals were type V, and none were type VI.
The FDA usually follows its panels' advice, but is not obligated to do so.
Nanotechnology Vehicle Speeds Numbing of Topical Lidocaine
MIAMI BEACH – A 4% topical lidocaine cream with a nanotechnology-based vehicle in development provided superior pain relief before facial filler injections, compared with the most commonly-used topical anesthetic cream, according to patient and physician ratings in two split-face studies.
"This really has potential as the next topical anesthetic," Dr. Glynis Ablon said at the South Beach Symposium.
Although most dermatologists and pediatricians apply topical anesthetic products (such as commonly used LMX4 cream) approximately 20 minutes before needle-based procedures, the efficacy of NTL4 (Cutiecaine Cream) was seen in as little as 5 minutes, Dr. Ablon said.
NTL4 is formulated with a novel nanoparticle vehicle (INParT Drug Delivery System) that passively transports active compounds deeper into the skin. "It's a great new way to deliver lidocaine into the skin faster," said Dr. Ablon, a dermatologist in private practice in Manhattan Beach, Calif. The technology is also being assessed for new topical hyaluronic acid and topical botulinum toxins.
Dr. Ablon and her colleague Dr. Mark Nestor, who is in private practice in Aventura, Fla., conducted an initial study of 30 patients with NTL4 applied to one side of their face and over-the-counter LMX4 to the contralateral side, followed by a 20-second massage. After 20 minutes, Restylane was injected for facial rejuvenation. Participants and blinded raters assessed pain relief.
"The study was started to see if we could get that [numbing effect] in 20 minutes, and obviously faster numbing times could be a great thing for dermatologists as well as pediatricians," Dr. Ablon said.
Twenty patients (67%) reported minimal or no pain when injected on the NTL4 side of their face, compared with 12 patients (40%) who reported minimal or no pain on the LMX4 treated side. "Overall, patients significantly preferred treatment with NTL4 over LMX4," she said. The mean visual analog score (VAS) for pain was 1.99 in the NTL4 group vs. 3.08 in the LMX4 group immediately after injection; 0.21 vs. 0.74 at 1 hour; and 0.07 and 0.31 at 3 hours postinjection.
Blinded investigators also rated significantly less pain with NTL4, Dr. Ablon said.
Adverse events were minor and included some tenderness and bruising. Also, there was "some edema we believe was due to the actual injection of the Restylane."
In a second study, Dr. Ablon and Dr. Nestor assessed 5-, 10-, and 15-minute application times. The 20 participants had a 30-second massage of the topical anesthetic and subsequent Restylane injections of their nasolabial folds.
Sixteen patients (80%) treated with NTL4 reported minimal or no pain, compared with one patient (5%) treated with LMX4, according to pooled data for subjective pain ratings after 5-, 10-, and 15-minute application times.
"We did show that they had significantly less pain on injection with the NTL4 at the 5- and 15-minute incubation, with a trend favoring it at 10 minutes," Dr. Ablon said.
Mean VAS pain scores were significantly lower for the NTL4 group when injected 5 minutes after application: 1.72 for NTL4 vs. 4.20 for LMX4. Patients also reported a significant difference in pain when injected 15 minutes after injection: 1.92 with NTL4 vs. 4.67 with LMX4. There was a nonsignificant trend favoring lower VAS scores with NTL4 after a 10-minute application time(1.08 for NTL4 and 3.00 for LMX4).
All patients preferred the NTL4 side versus the LMX4 side for pain relief. Blinded investigators’ evaluation of pain was also statistically significant in favor of the NTL4, Dr. Ablon said.
Adverse events were minor in this study as well. "We did have one patient with edema that we think might have been an actual reaction to lidocaine," she noted.
"There is a lot going on with nanotechnology, and [it] is here to stay," Dr. Ablon said. This technology can be found in suntan lotions, age-defying makeup, and even toothpaste that coats damaged enamel.
The studies were funded through an unrestricted educational grant from Innovatech. Dr. Ablon and Dr. Nestor are consultants and investigators for Transdermal Corp.
MIAMI BEACH – A 4% topical lidocaine cream with a nanotechnology-based vehicle in development provided superior pain relief before facial filler injections, compared with the most commonly-used topical anesthetic cream, according to patient and physician ratings in two split-face studies.
"This really has potential as the next topical anesthetic," Dr. Glynis Ablon said at the South Beach Symposium.
Although most dermatologists and pediatricians apply topical anesthetic products (such as commonly used LMX4 cream) approximately 20 minutes before needle-based procedures, the efficacy of NTL4 (Cutiecaine Cream) was seen in as little as 5 minutes, Dr. Ablon said.
NTL4 is formulated with a novel nanoparticle vehicle (INParT Drug Delivery System) that passively transports active compounds deeper into the skin. "It's a great new way to deliver lidocaine into the skin faster," said Dr. Ablon, a dermatologist in private practice in Manhattan Beach, Calif. The technology is also being assessed for new topical hyaluronic acid and topical botulinum toxins.
Dr. Ablon and her colleague Dr. Mark Nestor, who is in private practice in Aventura, Fla., conducted an initial study of 30 patients with NTL4 applied to one side of their face and over-the-counter LMX4 to the contralateral side, followed by a 20-second massage. After 20 minutes, Restylane was injected for facial rejuvenation. Participants and blinded raters assessed pain relief.
"The study was started to see if we could get that [numbing effect] in 20 minutes, and obviously faster numbing times could be a great thing for dermatologists as well as pediatricians," Dr. Ablon said.
Twenty patients (67%) reported minimal or no pain when injected on the NTL4 side of their face, compared with 12 patients (40%) who reported minimal or no pain on the LMX4 treated side. "Overall, patients significantly preferred treatment with NTL4 over LMX4," she said. The mean visual analog score (VAS) for pain was 1.99 in the NTL4 group vs. 3.08 in the LMX4 group immediately after injection; 0.21 vs. 0.74 at 1 hour; and 0.07 and 0.31 at 3 hours postinjection.
Blinded investigators also rated significantly less pain with NTL4, Dr. Ablon said.
Adverse events were minor and included some tenderness and bruising. Also, there was "some edema we believe was due to the actual injection of the Restylane."
In a second study, Dr. Ablon and Dr. Nestor assessed 5-, 10-, and 15-minute application times. The 20 participants had a 30-second massage of the topical anesthetic and subsequent Restylane injections of their nasolabial folds.
Sixteen patients (80%) treated with NTL4 reported minimal or no pain, compared with one patient (5%) treated with LMX4, according to pooled data for subjective pain ratings after 5-, 10-, and 15-minute application times.
"We did show that they had significantly less pain on injection with the NTL4 at the 5- and 15-minute incubation, with a trend favoring it at 10 minutes," Dr. Ablon said.
Mean VAS pain scores were significantly lower for the NTL4 group when injected 5 minutes after application: 1.72 for NTL4 vs. 4.20 for LMX4. Patients also reported a significant difference in pain when injected 15 minutes after injection: 1.92 with NTL4 vs. 4.67 with LMX4. There was a nonsignificant trend favoring lower VAS scores with NTL4 after a 10-minute application time(1.08 for NTL4 and 3.00 for LMX4).
All patients preferred the NTL4 side versus the LMX4 side for pain relief. Blinded investigators’ evaluation of pain was also statistically significant in favor of the NTL4, Dr. Ablon said.
Adverse events were minor in this study as well. "We did have one patient with edema that we think might have been an actual reaction to lidocaine," she noted.
"There is a lot going on with nanotechnology, and [it] is here to stay," Dr. Ablon said. This technology can be found in suntan lotions, age-defying makeup, and even toothpaste that coats damaged enamel.
The studies were funded through an unrestricted educational grant from Innovatech. Dr. Ablon and Dr. Nestor are consultants and investigators for Transdermal Corp.
MIAMI BEACH – A 4% topical lidocaine cream with a nanotechnology-based vehicle in development provided superior pain relief before facial filler injections, compared with the most commonly-used topical anesthetic cream, according to patient and physician ratings in two split-face studies.
"This really has potential as the next topical anesthetic," Dr. Glynis Ablon said at the South Beach Symposium.
Although most dermatologists and pediatricians apply topical anesthetic products (such as commonly used LMX4 cream) approximately 20 minutes before needle-based procedures, the efficacy of NTL4 (Cutiecaine Cream) was seen in as little as 5 minutes, Dr. Ablon said.
NTL4 is formulated with a novel nanoparticle vehicle (INParT Drug Delivery System) that passively transports active compounds deeper into the skin. "It's a great new way to deliver lidocaine into the skin faster," said Dr. Ablon, a dermatologist in private practice in Manhattan Beach, Calif. The technology is also being assessed for new topical hyaluronic acid and topical botulinum toxins.
Dr. Ablon and her colleague Dr. Mark Nestor, who is in private practice in Aventura, Fla., conducted an initial study of 30 patients with NTL4 applied to one side of their face and over-the-counter LMX4 to the contralateral side, followed by a 20-second massage. After 20 minutes, Restylane was injected for facial rejuvenation. Participants and blinded raters assessed pain relief.
"The study was started to see if we could get that [numbing effect] in 20 minutes, and obviously faster numbing times could be a great thing for dermatologists as well as pediatricians," Dr. Ablon said.
Twenty patients (67%) reported minimal or no pain when injected on the NTL4 side of their face, compared with 12 patients (40%) who reported minimal or no pain on the LMX4 treated side. "Overall, patients significantly preferred treatment with NTL4 over LMX4," she said. The mean visual analog score (VAS) for pain was 1.99 in the NTL4 group vs. 3.08 in the LMX4 group immediately after injection; 0.21 vs. 0.74 at 1 hour; and 0.07 and 0.31 at 3 hours postinjection.
Blinded investigators also rated significantly less pain with NTL4, Dr. Ablon said.
Adverse events were minor and included some tenderness and bruising. Also, there was "some edema we believe was due to the actual injection of the Restylane."
In a second study, Dr. Ablon and Dr. Nestor assessed 5-, 10-, and 15-minute application times. The 20 participants had a 30-second massage of the topical anesthetic and subsequent Restylane injections of their nasolabial folds.
Sixteen patients (80%) treated with NTL4 reported minimal or no pain, compared with one patient (5%) treated with LMX4, according to pooled data for subjective pain ratings after 5-, 10-, and 15-minute application times.
"We did show that they had significantly less pain on injection with the NTL4 at the 5- and 15-minute incubation, with a trend favoring it at 10 minutes," Dr. Ablon said.
Mean VAS pain scores were significantly lower for the NTL4 group when injected 5 minutes after application: 1.72 for NTL4 vs. 4.20 for LMX4. Patients also reported a significant difference in pain when injected 15 minutes after injection: 1.92 with NTL4 vs. 4.67 with LMX4. There was a nonsignificant trend favoring lower VAS scores with NTL4 after a 10-minute application time(1.08 for NTL4 and 3.00 for LMX4).
All patients preferred the NTL4 side versus the LMX4 side for pain relief. Blinded investigators’ evaluation of pain was also statistically significant in favor of the NTL4, Dr. Ablon said.
Adverse events were minor in this study as well. "We did have one patient with edema that we think might have been an actual reaction to lidocaine," she noted.
"There is a lot going on with nanotechnology, and [it] is here to stay," Dr. Ablon said. This technology can be found in suntan lotions, age-defying makeup, and even toothpaste that coats damaged enamel.
The studies were funded through an unrestricted educational grant from Innovatech. Dr. Ablon and Dr. Nestor are consultants and investigators for Transdermal Corp.
FROM THE SOUTH BEACH SYMPOSIUM
Major Finding: Sixteen patients (80%) treated with NTL4 reported minimal or no pain, compared with one patient (5%) treated with LMX4. Mean visual analog scale scores were significantly lower for injection pain with a 5-minute application: 1.72 in the NTL4 group vs. 4.20 in the LMX4 group.
Data Source: Split-face comparison of 20 patients applying NTL4 or LMX4 for 5, 10, or 15 minutes prior to facial filler injections.
Disclosures: Dr. Ablon and Dr. Nestor are consultants and investigators for Transdermal Corp.
Investigational Nd:YAG Laser Plus 3-D Optical Fiber Targets Cellulite
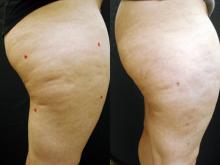
GRAPEVINE, TEX. – An investigational sidelight 3-D optical fiber and 1440-nm Nd:YAG laser produced significant improvement of cellulite with one treatment at 6 months in a study of 15 healthy women.
Cynosure Inc.'s Cellulaze Cellulite Laser Workstation's 1440 wavelength is well absorbed by adipose tissue and water. The side-firing SideLight 3-D optical fiber thermally subcises subcutaneous septa, deplanes fat cells, and heats dermal tissue to promote skin thickening and tightening, said Dr. Bruce E. Katz of the department of dermatology at Mount Sinai School of Medicine, New York.
The 15 women (aged 20-55 years) all had cellulite on their lateral or posterior thighs or buttocks, body mass indexes less than 30 kg/m2, and skin types I-V. Following local anesthesia, two 1.5-mm incisions were made, and the probe was inserted. Subcutaneous temperature was kept at lower than 47° C and surface temperature at lower than 40° C. The Nd:YAG laser delivered 1,000 J per 5- x 5-cm square.
Digital photographic evaluation by two independent observers at 6 months found that 68% of the women had excellent improvement in cellulite; Vectra 3-D imaging demonstrated significant improvement in 65% of them. By physician evaluation, 76% had good or excellent results (69% by patient evaluation). Vectra analysis of skin contour demonstrated an average 47% reduction in depth and 32% reduction in height of skin bulges, Dr. Katz reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Histologically, there was an increase in coarser collagen and elastic fibers in the dermis, noted Dr. Katz, who is also director of the cosmetic surgery and laser clinic at Mount Sinai Medical Center.
Three patients experienced mild ecchymoses, and four had edema lasting less than a week. There were no other adverse events.
"This may be a game changer for the treatment of cellulite," Dr. Katz said.
Cynosure received CE marking certification for Cellulaze in the European Union in February. The U.S. Food and Drug Administration approved an Investigational Device Exemption (IDE) for the device, and a clinical IDE study is currently underway. Regulatory action on a 510(k) submission, filed in late 2010, is currently expected in the first half of 2011, according to a company statement.
Dr. Katz disclosed that he is a Cynosure stockholder.
GRAPEVINE, TEX. – An investigational sidelight 3-D optical fiber and 1440-nm Nd:YAG laser produced significant improvement of cellulite with one treatment at 6 months in a study of 15 healthy women.
Cynosure Inc.'s Cellulaze Cellulite Laser Workstation's 1440 wavelength is well absorbed by adipose tissue and water. The side-firing SideLight 3-D optical fiber thermally subcises subcutaneous septa, deplanes fat cells, and heats dermal tissue to promote skin thickening and tightening, said Dr. Bruce E. Katz of the department of dermatology at Mount Sinai School of Medicine, New York.
The 15 women (aged 20-55 years) all had cellulite on their lateral or posterior thighs or buttocks, body mass indexes less than 30 kg/m2, and skin types I-V. Following local anesthesia, two 1.5-mm incisions were made, and the probe was inserted. Subcutaneous temperature was kept at lower than 47° C and surface temperature at lower than 40° C. The Nd:YAG laser delivered 1,000 J per 5- x 5-cm square.
Digital photographic evaluation by two independent observers at 6 months found that 68% of the women had excellent improvement in cellulite; Vectra 3-D imaging demonstrated significant improvement in 65% of them. By physician evaluation, 76% had good or excellent results (69% by patient evaluation). Vectra analysis of skin contour demonstrated an average 47% reduction in depth and 32% reduction in height of skin bulges, Dr. Katz reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Histologically, there was an increase in coarser collagen and elastic fibers in the dermis, noted Dr. Katz, who is also director of the cosmetic surgery and laser clinic at Mount Sinai Medical Center.
Three patients experienced mild ecchymoses, and four had edema lasting less than a week. There were no other adverse events.
"This may be a game changer for the treatment of cellulite," Dr. Katz said.
Cynosure received CE marking certification for Cellulaze in the European Union in February. The U.S. Food and Drug Administration approved an Investigational Device Exemption (IDE) for the device, and a clinical IDE study is currently underway. Regulatory action on a 510(k) submission, filed in late 2010, is currently expected in the first half of 2011, according to a company statement.
Dr. Katz disclosed that he is a Cynosure stockholder.
GRAPEVINE, TEX. – An investigational sidelight 3-D optical fiber and 1440-nm Nd:YAG laser produced significant improvement of cellulite with one treatment at 6 months in a study of 15 healthy women.
Cynosure Inc.'s Cellulaze Cellulite Laser Workstation's 1440 wavelength is well absorbed by adipose tissue and water. The side-firing SideLight 3-D optical fiber thermally subcises subcutaneous septa, deplanes fat cells, and heats dermal tissue to promote skin thickening and tightening, said Dr. Bruce E. Katz of the department of dermatology at Mount Sinai School of Medicine, New York.
The 15 women (aged 20-55 years) all had cellulite on their lateral or posterior thighs or buttocks, body mass indexes less than 30 kg/m2, and skin types I-V. Following local anesthesia, two 1.5-mm incisions were made, and the probe was inserted. Subcutaneous temperature was kept at lower than 47° C and surface temperature at lower than 40° C. The Nd:YAG laser delivered 1,000 J per 5- x 5-cm square.
Digital photographic evaluation by two independent observers at 6 months found that 68% of the women had excellent improvement in cellulite; Vectra 3-D imaging demonstrated significant improvement in 65% of them. By physician evaluation, 76% had good or excellent results (69% by patient evaluation). Vectra analysis of skin contour demonstrated an average 47% reduction in depth and 32% reduction in height of skin bulges, Dr. Katz reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Histologically, there was an increase in coarser collagen and elastic fibers in the dermis, noted Dr. Katz, who is also director of the cosmetic surgery and laser clinic at Mount Sinai Medical Center.
Three patients experienced mild ecchymoses, and four had edema lasting less than a week. There were no other adverse events.
"This may be a game changer for the treatment of cellulite," Dr. Katz said.
Cynosure received CE marking certification for Cellulaze in the European Union in February. The U.S. Food and Drug Administration approved an Investigational Device Exemption (IDE) for the device, and a clinical IDE study is currently underway. Regulatory action on a 510(k) submission, filed in late 2010, is currently expected in the first half of 2011, according to a company statement.
Dr. Katz disclosed that he is a Cynosure stockholder.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Major Finding: Digital photographic evaluation by two independent observers at 6 months found that 68% had excellent improvement in cellulite, and Vectra 3-D imaging demonstrated significant improvement in 65%. By physician evaluation, 76% had good or excellent results (69% by patient evaluation).
Data Source: A 6-month study of 15 women aged 20-55 years with cellulite on their lateral or posterior thighs or buttocks.
Disclosures: The study was funded by Cynosure, of which Dr. Katz is a stockholder.
Lipolysis With Sodium Deoxycholate Reduces Submental Fat
MIAMI BEACH – Lipolysis with proprietary sodium deoxycholate injections significantly decreased the volume of submental fat in a randomized, vehicle-control, phase IIb study.
Investigators at 10 sites in the United States randomized 129 patients with moderate-to-severe "submental convexity" to low-dose or high-dose sodium deoxycholate (ATX-101, Kythera Biopharmaceuticals) or placebo. Administration was once a month for up to 5 months, Dr. Patricia S. Walker said at the South Beach Symposium.
Patients and clinicians rated changes at 16 weeks and 3 months after the final treatment, compared with baseline. At the same time, investigators also compared MRI volume measurements as an objective measure of fat changes.
MRI revealed significant reductions in submental fat at week 16 and week 32 from baseline, compared with placebo, for patients randomized to 2 mg/cm2 sodium deoxycholate (the high-dose group).
Seven dermatologists and three plastic surgeons at the 10 sites treated the participants and performed global assessments. The clinicians rated changes using a 5-point Clinician-Reported Submental Fat Impact Scale.
Again, the 2-mg/cm2 dose was more efficacious than placebo, with statistically significant reductions rated by doctors at week 16 and week 32. Although the effect on patients who received the 1-mg/cm2 dose (low-dose group) did not reach significance, this dose was also associated with improvement compared with placebo, suggesting a dose response, Dr. Walker said.
Patients also rated their own changes in submental fat using a 5-point Patient-Reported Submental Fat Impact Scale at baseline, week 16, and week 32.
Interestingly, all patients reported subjective improvements compared with baseline – even those assigned to vehicle-only injections. "Patients have a perception of self-improvement," Dr. Walker said. "There was very little change on MRI in the vehicle group, so it’s a true placebo effect – patients want to see a response."
Sodium deoxycholate selectively targets adipocytes. Histology showed infiltration of macrophages by day 3, with destruction and clearance of fat over time. "Twenty-eight days is about the right time for these injections to clear fat," said Dr. Walker, who is chief medical officer for Kythera Biopharmaceuticals.
Sodium deoxycholate is an endogenous, secondary bile acid. This is "how we break down fat in our diet," Dr. Walker said.
A total 114 patients completed the study. Fitzpatrick skin types ranged from I to VI, average age was 46 years (range 24-65 years), and the mean body mass index was 30.6 kg/m2 (range 19.5-48.8). "We found chin fat is not necessarily related to [body] size. It can be familial," Dr. Walker said.
Most adverse events were mild, transient, and limited to the chin area. "If you don’t have a safe product, you don’t have an aesthetic product," she said.
Treatment-related swelling, pain, and numbness were the most common adverse events. "There also was erythema in the vehicle group, so it was probably injection-technique related."
ATX-101 is a safe and effective treatment for submental fat reduction, Dr. Walker said. "It is nice to have an aesthetic product that everyone sees works."
Phase III trials are planned this year, according to Kythera Biopharmaceuticals.
Dr. Walker is an employee and shareholder of the company.
MIAMI BEACH – Lipolysis with proprietary sodium deoxycholate injections significantly decreased the volume of submental fat in a randomized, vehicle-control, phase IIb study.
Investigators at 10 sites in the United States randomized 129 patients with moderate-to-severe "submental convexity" to low-dose or high-dose sodium deoxycholate (ATX-101, Kythera Biopharmaceuticals) or placebo. Administration was once a month for up to 5 months, Dr. Patricia S. Walker said at the South Beach Symposium.
Patients and clinicians rated changes at 16 weeks and 3 months after the final treatment, compared with baseline. At the same time, investigators also compared MRI volume measurements as an objective measure of fat changes.
MRI revealed significant reductions in submental fat at week 16 and week 32 from baseline, compared with placebo, for patients randomized to 2 mg/cm2 sodium deoxycholate (the high-dose group).
Seven dermatologists and three plastic surgeons at the 10 sites treated the participants and performed global assessments. The clinicians rated changes using a 5-point Clinician-Reported Submental Fat Impact Scale.
Again, the 2-mg/cm2 dose was more efficacious than placebo, with statistically significant reductions rated by doctors at week 16 and week 32. Although the effect on patients who received the 1-mg/cm2 dose (low-dose group) did not reach significance, this dose was also associated with improvement compared with placebo, suggesting a dose response, Dr. Walker said.
Patients also rated their own changes in submental fat using a 5-point Patient-Reported Submental Fat Impact Scale at baseline, week 16, and week 32.
Interestingly, all patients reported subjective improvements compared with baseline – even those assigned to vehicle-only injections. "Patients have a perception of self-improvement," Dr. Walker said. "There was very little change on MRI in the vehicle group, so it’s a true placebo effect – patients want to see a response."
Sodium deoxycholate selectively targets adipocytes. Histology showed infiltration of macrophages by day 3, with destruction and clearance of fat over time. "Twenty-eight days is about the right time for these injections to clear fat," said Dr. Walker, who is chief medical officer for Kythera Biopharmaceuticals.
Sodium deoxycholate is an endogenous, secondary bile acid. This is "how we break down fat in our diet," Dr. Walker said.
A total 114 patients completed the study. Fitzpatrick skin types ranged from I to VI, average age was 46 years (range 24-65 years), and the mean body mass index was 30.6 kg/m2 (range 19.5-48.8). "We found chin fat is not necessarily related to [body] size. It can be familial," Dr. Walker said.
Most adverse events were mild, transient, and limited to the chin area. "If you don’t have a safe product, you don’t have an aesthetic product," she said.
Treatment-related swelling, pain, and numbness were the most common adverse events. "There also was erythema in the vehicle group, so it was probably injection-technique related."
ATX-101 is a safe and effective treatment for submental fat reduction, Dr. Walker said. "It is nice to have an aesthetic product that everyone sees works."
Phase III trials are planned this year, according to Kythera Biopharmaceuticals.
Dr. Walker is an employee and shareholder of the company.
MIAMI BEACH – Lipolysis with proprietary sodium deoxycholate injections significantly decreased the volume of submental fat in a randomized, vehicle-control, phase IIb study.
Investigators at 10 sites in the United States randomized 129 patients with moderate-to-severe "submental convexity" to low-dose or high-dose sodium deoxycholate (ATX-101, Kythera Biopharmaceuticals) or placebo. Administration was once a month for up to 5 months, Dr. Patricia S. Walker said at the South Beach Symposium.
Patients and clinicians rated changes at 16 weeks and 3 months after the final treatment, compared with baseline. At the same time, investigators also compared MRI volume measurements as an objective measure of fat changes.
MRI revealed significant reductions in submental fat at week 16 and week 32 from baseline, compared with placebo, for patients randomized to 2 mg/cm2 sodium deoxycholate (the high-dose group).
Seven dermatologists and three plastic surgeons at the 10 sites treated the participants and performed global assessments. The clinicians rated changes using a 5-point Clinician-Reported Submental Fat Impact Scale.
Again, the 2-mg/cm2 dose was more efficacious than placebo, with statistically significant reductions rated by doctors at week 16 and week 32. Although the effect on patients who received the 1-mg/cm2 dose (low-dose group) did not reach significance, this dose was also associated with improvement compared with placebo, suggesting a dose response, Dr. Walker said.
Patients also rated their own changes in submental fat using a 5-point Patient-Reported Submental Fat Impact Scale at baseline, week 16, and week 32.
Interestingly, all patients reported subjective improvements compared with baseline – even those assigned to vehicle-only injections. "Patients have a perception of self-improvement," Dr. Walker said. "There was very little change on MRI in the vehicle group, so it’s a true placebo effect – patients want to see a response."
Sodium deoxycholate selectively targets adipocytes. Histology showed infiltration of macrophages by day 3, with destruction and clearance of fat over time. "Twenty-eight days is about the right time for these injections to clear fat," said Dr. Walker, who is chief medical officer for Kythera Biopharmaceuticals.
Sodium deoxycholate is an endogenous, secondary bile acid. This is "how we break down fat in our diet," Dr. Walker said.
A total 114 patients completed the study. Fitzpatrick skin types ranged from I to VI, average age was 46 years (range 24-65 years), and the mean body mass index was 30.6 kg/m2 (range 19.5-48.8). "We found chin fat is not necessarily related to [body] size. It can be familial," Dr. Walker said.
Most adverse events were mild, transient, and limited to the chin area. "If you don’t have a safe product, you don’t have an aesthetic product," she said.
Treatment-related swelling, pain, and numbness were the most common adverse events. "There also was erythema in the vehicle group, so it was probably injection-technique related."
ATX-101 is a safe and effective treatment for submental fat reduction, Dr. Walker said. "It is nice to have an aesthetic product that everyone sees works."
Phase III trials are planned this year, according to Kythera Biopharmaceuticals.
Dr. Walker is an employee and shareholder of the company.
FROM THE SOUTH BEACH SYMPOSIUM
Major Finding: MRI revealed significant reductions in submental fat at week 16 and week 32 from baseline, compared with placebo, for patients randomized to 2 mg/cm2 sodium deoxycholate (the high-dose group).
Data Source: Investigators at 10 sites in the United States randomized 129 patients with moderate-to-severe "submental convexity" to low-dose or high-dose sodium deoxycholate or placebo.
Disclosures: Dr. Walker is an employee and shareholder of Kythera.
Skin of Color: Dangers of Black Market Bleaching Agents
Black market skin bleaching agents with potentially unsafe ingredients are a growing concern in some countries, according to an article published yesterday, April 11, by the Associated Press, "Skin Bleaching a Growing Problem in Jamaican Slums."
The use of these agents stems from societal and cultural beliefs that lighter skin could lead to a better way of life, particularly for those that are impoverished.
In Jamaica, for example, a "23-year-old resident of a Kingston ghetto hopes to transform her dark complexion to a cafe-au-lait-color common among Jamaica's elite and favored by many men in her neighborhood. She believes a fairer skin could be her ticket to a better life. So she spends her meager savings on cheap black-market concoctions that promise to lighten her pigment," (excerpt from AP story).
This phenomenon is not exclusive to Jamaica, but is inherent to many nations around the world. It has been documented in India, the Americas, the Middle East, the Philippines, and in some parts of Africa and Asia.
Lightening agents, such as hydroquinone, have been banned in over the counter preparations in Japan, the European Union, and Australia. OTC hydroquinone is still available in the United States in percentages of up to 2%. While hydroquinone use may lead to adverse events such as ochronosis, it is a rare event typically seen with use of much higher concentrations over longer periods of time. In my opinion, it is not low concentrations of hydroquinone, but other potentially harmful ingredients that may be found in some of the black market products that are of major concern.
"Lightening creams are not effectively regulated in Jamaica, where even roadside vendors sell tubes and plastic bags of powders and ointments from cardboard boxes stacked along sidewalks in market districts," according to the AP story. "Many of the tubes are unlabeled as to their actual ingredients," said Dr. Richard Desnoes, president of the Dermatology Association of Jamaica.
"Hardcore bleachers use illegal ointments smuggled into the Caribbean country that contain toxins like mercury, a metal that blocks production of melanin, which give skin its color, but can also be toxic," the story continued.
In addition, potent topical corticosteroids have been found in some of these products, leading to reports of atrophy, striae, and even symptoms of Cushing’s syndrome.
Black market skin bleaching agents with potentially unsafe ingredients are a growing concern in some countries, according to an article published yesterday, April 11, by the Associated Press, "Skin Bleaching a Growing Problem in Jamaican Slums."
The use of these agents stems from societal and cultural beliefs that lighter skin could lead to a better way of life, particularly for those that are impoverished.
In Jamaica, for example, a "23-year-old resident of a Kingston ghetto hopes to transform her dark complexion to a cafe-au-lait-color common among Jamaica's elite and favored by many men in her neighborhood. She believes a fairer skin could be her ticket to a better life. So she spends her meager savings on cheap black-market concoctions that promise to lighten her pigment," (excerpt from AP story).
This phenomenon is not exclusive to Jamaica, but is inherent to many nations around the world. It has been documented in India, the Americas, the Middle East, the Philippines, and in some parts of Africa and Asia.
Lightening agents, such as hydroquinone, have been banned in over the counter preparations in Japan, the European Union, and Australia. OTC hydroquinone is still available in the United States in percentages of up to 2%. While hydroquinone use may lead to adverse events such as ochronosis, it is a rare event typically seen with use of much higher concentrations over longer periods of time. In my opinion, it is not low concentrations of hydroquinone, but other potentially harmful ingredients that may be found in some of the black market products that are of major concern.
"Lightening creams are not effectively regulated in Jamaica, where even roadside vendors sell tubes and plastic bags of powders and ointments from cardboard boxes stacked along sidewalks in market districts," according to the AP story. "Many of the tubes are unlabeled as to their actual ingredients," said Dr. Richard Desnoes, president of the Dermatology Association of Jamaica.
"Hardcore bleachers use illegal ointments smuggled into the Caribbean country that contain toxins like mercury, a metal that blocks production of melanin, which give skin its color, but can also be toxic," the story continued.
In addition, potent topical corticosteroids have been found in some of these products, leading to reports of atrophy, striae, and even symptoms of Cushing’s syndrome.
Black market skin bleaching agents with potentially unsafe ingredients are a growing concern in some countries, according to an article published yesterday, April 11, by the Associated Press, "Skin Bleaching a Growing Problem in Jamaican Slums."
The use of these agents stems from societal and cultural beliefs that lighter skin could lead to a better way of life, particularly for those that are impoverished.
In Jamaica, for example, a "23-year-old resident of a Kingston ghetto hopes to transform her dark complexion to a cafe-au-lait-color common among Jamaica's elite and favored by many men in her neighborhood. She believes a fairer skin could be her ticket to a better life. So she spends her meager savings on cheap black-market concoctions that promise to lighten her pigment," (excerpt from AP story).
This phenomenon is not exclusive to Jamaica, but is inherent to many nations around the world. It has been documented in India, the Americas, the Middle East, the Philippines, and in some parts of Africa and Asia.
Lightening agents, such as hydroquinone, have been banned in over the counter preparations in Japan, the European Union, and Australia. OTC hydroquinone is still available in the United States in percentages of up to 2%. While hydroquinone use may lead to adverse events such as ochronosis, it is a rare event typically seen with use of much higher concentrations over longer periods of time. In my opinion, it is not low concentrations of hydroquinone, but other potentially harmful ingredients that may be found in some of the black market products that are of major concern.
"Lightening creams are not effectively regulated in Jamaica, where even roadside vendors sell tubes and plastic bags of powders and ointments from cardboard boxes stacked along sidewalks in market districts," according to the AP story. "Many of the tubes are unlabeled as to their actual ingredients," said Dr. Richard Desnoes, president of the Dermatology Association of Jamaica.
"Hardcore bleachers use illegal ointments smuggled into the Caribbean country that contain toxins like mercury, a metal that blocks production of melanin, which give skin its color, but can also be toxic," the story continued.
In addition, potent topical corticosteroids have been found in some of these products, leading to reports of atrophy, striae, and even symptoms of Cushing’s syndrome.
Minimally Invasive Cosmetic Procedures Down in 2010
As the economy rebounds, so is interest in cosmetic plastic surgery, according to statistics from the American Society for Aesthetic Plastic Surgery.
The latest figures show that cosmetic surgical procedures increased nearly 9% in 2010. More than 1.6 million cosmetic surgical procedures were performed in the United States last year, with breast augmentation remaining the most popular. Liposuction, eyelid surgery, abdominoplasty, and breast reduction rounded out the top five procedures in 2010.
"Patients who put off surgery because of uncertainty in the economy and the job market are coming back for tried and true procedures," Dr. Felmont Eaves III, ASAPS president, said in a statement. "Growth in demand will likely continue as the recession eases and baby boomers and their offspring begin to explore surgical and nonsurgical options."
But demand for some minimally invasive procedures, such as laser hair removal, dropped in 2010. Overall, cosmetic minimally invasive procedures decreased nearly 9%. A total of 8 million procedures were performed last year, representing about 39% of the total spending on cosmetic surgery. Fewer patients underwent injections of botulinum toxin type A, laser hair removal, laser skin resurfacing, and chemical peels than in previous years. However, the number of procedures performed with hyaluronic acid increased.
Cosmetic surgery remains big business. In 2010, Americans spent close to $10.7 billion on cosmetic procedures. The bulk – $6.6 billion – was spent on surgical procedures. An additional $1.9 billion was spent on injectable procedures, $1.8 billion on skin rejuvenation procedures, and nearly $500 million on other nonsurgical procedures.
The 2010 figures are based on a survey of more than 900 plastic surgeons, dermatologists, and otolaryngologists. The ASAPS has compiled cosmetic surgery procedure data since 1997.
As the economy rebounds, so is interest in cosmetic plastic surgery, according to statistics from the American Society for Aesthetic Plastic Surgery.
The latest figures show that cosmetic surgical procedures increased nearly 9% in 2010. More than 1.6 million cosmetic surgical procedures were performed in the United States last year, with breast augmentation remaining the most popular. Liposuction, eyelid surgery, abdominoplasty, and breast reduction rounded out the top five procedures in 2010.
"Patients who put off surgery because of uncertainty in the economy and the job market are coming back for tried and true procedures," Dr. Felmont Eaves III, ASAPS president, said in a statement. "Growth in demand will likely continue as the recession eases and baby boomers and their offspring begin to explore surgical and nonsurgical options."
But demand for some minimally invasive procedures, such as laser hair removal, dropped in 2010. Overall, cosmetic minimally invasive procedures decreased nearly 9%. A total of 8 million procedures were performed last year, representing about 39% of the total spending on cosmetic surgery. Fewer patients underwent injections of botulinum toxin type A, laser hair removal, laser skin resurfacing, and chemical peels than in previous years. However, the number of procedures performed with hyaluronic acid increased.
Cosmetic surgery remains big business. In 2010, Americans spent close to $10.7 billion on cosmetic procedures. The bulk – $6.6 billion – was spent on surgical procedures. An additional $1.9 billion was spent on injectable procedures, $1.8 billion on skin rejuvenation procedures, and nearly $500 million on other nonsurgical procedures.
The 2010 figures are based on a survey of more than 900 plastic surgeons, dermatologists, and otolaryngologists. The ASAPS has compiled cosmetic surgery procedure data since 1997.
As the economy rebounds, so is interest in cosmetic plastic surgery, according to statistics from the American Society for Aesthetic Plastic Surgery.
The latest figures show that cosmetic surgical procedures increased nearly 9% in 2010. More than 1.6 million cosmetic surgical procedures were performed in the United States last year, with breast augmentation remaining the most popular. Liposuction, eyelid surgery, abdominoplasty, and breast reduction rounded out the top five procedures in 2010.
"Patients who put off surgery because of uncertainty in the economy and the job market are coming back for tried and true procedures," Dr. Felmont Eaves III, ASAPS president, said in a statement. "Growth in demand will likely continue as the recession eases and baby boomers and their offspring begin to explore surgical and nonsurgical options."
But demand for some minimally invasive procedures, such as laser hair removal, dropped in 2010. Overall, cosmetic minimally invasive procedures decreased nearly 9%. A total of 8 million procedures were performed last year, representing about 39% of the total spending on cosmetic surgery. Fewer patients underwent injections of botulinum toxin type A, laser hair removal, laser skin resurfacing, and chemical peels than in previous years. However, the number of procedures performed with hyaluronic acid increased.
Cosmetic surgery remains big business. In 2010, Americans spent close to $10.7 billion on cosmetic procedures. The bulk – $6.6 billion – was spent on surgical procedures. An additional $1.9 billion was spent on injectable procedures, $1.8 billion on skin rejuvenation procedures, and nearly $500 million on other nonsurgical procedures.
The 2010 figures are based on a survey of more than 900 plastic surgeons, dermatologists, and otolaryngologists. The ASAPS has compiled cosmetic surgery procedure data since 1997.
Novel Device Uses Cold Therapy to Reduce Forehead Wrinkles
GRAPEVINE, TEX. – An investigational cryoprobe device reduced dynamic forehead wrinkles immediately after application in a pilot study of 36 patients that was presented at the annual meeting of the American Society for Laser Medicine and Surgery.
The self-contained, hand-held device, made by MyoScience Inc., uses a 27-gauge needle to deliver cooling to the temporal branch of the facial nerve that controls the frontalis muscle, rather than directly into the muscle as is done with botulinum toxins. The duration of effect is similar to that of botulinum toxins, about 3-4 months.
"Basically, it's a green alternative. There's no chemical, no toxin, it's only cold therapy," principal investigator Dr. Francis Palmer said in an interview.
Results from the use of a first-generation version of the device were reported by Dr. Vic Narurkar, who directs the Bay Area Laser Institute in San Francisco. The 36 patients were aged 35-70 years, all with forehead lines during animation. Some were at risk for brow ptosis.
After lidocaine administration, the probe was inserted for 30-45 seconds, and the subsequent reduction in muscle contractility was monitored to see if additional cryoprobe insertions were needed. All patients had immediate dynamic line reduction after two to four cryoprobe insertions, Dr. Narurkar reported.
The most common adverse events were headaches and small focal areas of minor epidermal cold injury, attributed to limitations in the prototype design. No patient experienced ptosis. "Cold has the unique natural property of selectively affecting the axon, yet preserves the endoneurial sheath. ... [It] has no impact on collagen-based structures," Dr. Narurkar explained.
According to Dr. Palmer, both the absence of ptosis risk and the immediacy of the response represent significant advantages over botulinum toxins, for which peak of action is typically 4-7 days following injection.
However, "I don't see this as a replacement for toxin, but as a complement. Physicians now have something to give patients who don't want a toxin, or for use in combination in areas where you can’t use a toxin. Botox is fabulous. It doesn't need to be replaced," said Dr. Palmer, director of the Beverly Hills (Calif.) International Center for Aesthetic Surgery.
Moreover, he noted, at this point, the injection of neurotoxin is easier and somewhat less invasive, although that could change with further revision of the cryoprobe's design. "We think it offers additional things toxin therapy doesn't offer, but it’s in a very early stage. It will be refined further before it hits the market."
MyoScience plans to seek a CE marking for the cryoprobe later this year before approaching the U.S. Food and Drug Administration, he said.
Both Dr. Palmer and Dr. Narurkar are paid consultants for MyoScience, which funded the study.
GRAPEVINE, TEX. – An investigational cryoprobe device reduced dynamic forehead wrinkles immediately after application in a pilot study of 36 patients that was presented at the annual meeting of the American Society for Laser Medicine and Surgery.
The self-contained, hand-held device, made by MyoScience Inc., uses a 27-gauge needle to deliver cooling to the temporal branch of the facial nerve that controls the frontalis muscle, rather than directly into the muscle as is done with botulinum toxins. The duration of effect is similar to that of botulinum toxins, about 3-4 months.
"Basically, it's a green alternative. There's no chemical, no toxin, it's only cold therapy," principal investigator Dr. Francis Palmer said in an interview.
Results from the use of a first-generation version of the device were reported by Dr. Vic Narurkar, who directs the Bay Area Laser Institute in San Francisco. The 36 patients were aged 35-70 years, all with forehead lines during animation. Some were at risk for brow ptosis.
After lidocaine administration, the probe was inserted for 30-45 seconds, and the subsequent reduction in muscle contractility was monitored to see if additional cryoprobe insertions were needed. All patients had immediate dynamic line reduction after two to four cryoprobe insertions, Dr. Narurkar reported.
The most common adverse events were headaches and small focal areas of minor epidermal cold injury, attributed to limitations in the prototype design. No patient experienced ptosis. "Cold has the unique natural property of selectively affecting the axon, yet preserves the endoneurial sheath. ... [It] has no impact on collagen-based structures," Dr. Narurkar explained.
According to Dr. Palmer, both the absence of ptosis risk and the immediacy of the response represent significant advantages over botulinum toxins, for which peak of action is typically 4-7 days following injection.
However, "I don't see this as a replacement for toxin, but as a complement. Physicians now have something to give patients who don't want a toxin, or for use in combination in areas where you can’t use a toxin. Botox is fabulous. It doesn't need to be replaced," said Dr. Palmer, director of the Beverly Hills (Calif.) International Center for Aesthetic Surgery.
Moreover, he noted, at this point, the injection of neurotoxin is easier and somewhat less invasive, although that could change with further revision of the cryoprobe's design. "We think it offers additional things toxin therapy doesn't offer, but it’s in a very early stage. It will be refined further before it hits the market."
MyoScience plans to seek a CE marking for the cryoprobe later this year before approaching the U.S. Food and Drug Administration, he said.
Both Dr. Palmer and Dr. Narurkar are paid consultants for MyoScience, which funded the study.
GRAPEVINE, TEX. – An investigational cryoprobe device reduced dynamic forehead wrinkles immediately after application in a pilot study of 36 patients that was presented at the annual meeting of the American Society for Laser Medicine and Surgery.
The self-contained, hand-held device, made by MyoScience Inc., uses a 27-gauge needle to deliver cooling to the temporal branch of the facial nerve that controls the frontalis muscle, rather than directly into the muscle as is done with botulinum toxins. The duration of effect is similar to that of botulinum toxins, about 3-4 months.
"Basically, it's a green alternative. There's no chemical, no toxin, it's only cold therapy," principal investigator Dr. Francis Palmer said in an interview.
Results from the use of a first-generation version of the device were reported by Dr. Vic Narurkar, who directs the Bay Area Laser Institute in San Francisco. The 36 patients were aged 35-70 years, all with forehead lines during animation. Some were at risk for brow ptosis.
After lidocaine administration, the probe was inserted for 30-45 seconds, and the subsequent reduction in muscle contractility was monitored to see if additional cryoprobe insertions were needed. All patients had immediate dynamic line reduction after two to four cryoprobe insertions, Dr. Narurkar reported.
The most common adverse events were headaches and small focal areas of minor epidermal cold injury, attributed to limitations in the prototype design. No patient experienced ptosis. "Cold has the unique natural property of selectively affecting the axon, yet preserves the endoneurial sheath. ... [It] has no impact on collagen-based structures," Dr. Narurkar explained.
According to Dr. Palmer, both the absence of ptosis risk and the immediacy of the response represent significant advantages over botulinum toxins, for which peak of action is typically 4-7 days following injection.
However, "I don't see this as a replacement for toxin, but as a complement. Physicians now have something to give patients who don't want a toxin, or for use in combination in areas where you can’t use a toxin. Botox is fabulous. It doesn't need to be replaced," said Dr. Palmer, director of the Beverly Hills (Calif.) International Center for Aesthetic Surgery.
Moreover, he noted, at this point, the injection of neurotoxin is easier and somewhat less invasive, although that could change with further revision of the cryoprobe's design. "We think it offers additional things toxin therapy doesn't offer, but it’s in a very early stage. It will be refined further before it hits the market."
MyoScience plans to seek a CE marking for the cryoprobe later this year before approaching the U.S. Food and Drug Administration, he said.
Both Dr. Palmer and Dr. Narurkar are paid consultants for MyoScience, which funded the study.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Major Finding: All patients experienced immediate reduction in wrinkles with two to four cryoprobe insertions.
Data Source: Pilot study of 36 adults (aged 35-70 years) with dynamic forehead wrinkles.
Disclosures: Both Dr. Palmer and Dr. Narurkar are paid consultants for MyoScience, which funded the study.
Nd:YAG Provides Best Outcomes for Mucosal Venous Malformations
GRAPEVINE, TEX. – In a prospective evaluation of 59 children with venous malformations, the long-pulsed neodymium:YAG laser was more effective in treating mucosal venous malformations than lesions on the limbs.
Venous malformations present in many forms that vary in depth, location, and extent, and treatment is largely symptomatic rather than curative. "It is, therefore, important that treatments given are effective in improving the symptoms medium or long term, with as few side effects as possible," said Dr. Stratos Sofos of Alder Hey Children's Hospital in Liverpool, England.
The long-pulsed Nd:YAG laser has become popular for the treatment of small- to medium-size vessels. Its longer pulse duration provides deeper penetration and weaker melanin absorption, allowing it to heat the vessels slowly and uniformly, thus avoiding the vessel rupture with subsequent purpura and hyperpigmentation that can occur with other lasers.
However, published data do not address whether treatment with a long-pulsed Nd:YAG laser provides long-term symptom relief, nor which types of venous malformations respond better than do others, Dr. Sofos said at the annual meeting of the American Society for Laser Medicine and Surgery.
All children who met criteria such as functional impairment, large facial deformities, and painful or bleeding lesions were treated with Candela Corp.'s GentleYAG 1064-nm laser. They received one to three treatments under general anesthesia, delivered in six to eight weekly intervals. Prior to Nd:YAG laser treatment, 32 of the children had received multiple other treatments, including pulsed dye lasers and sclerotherapy.
The 59 patients were aged 2-18 years (mean, 12.3 years). Venous malformations were present on the head and neck in 23, of which 18 (31% of the total) were mucosal. Malformations were on the upper limbs in 15 and on the lower limbs in 18 (total with limb lesions, 56%). The other three had lesions on the chest wall. Average follow up was 24 months (range, 6 months–9 years).
Based on objective assessment, 18% in the upper- and lower-limb group had excellent results and 27% patients had good results, whereas 55% of patients had no change. For the mucosal group, 28% had excellent results, 56% had good results, and 17% had no improvement, Dr. Sofos reported.
The overall complication rate from the Nd:YAG laser treatment was 20% (12 of 59), including skin blistering in 10%, ulceration in 7%, and hypertrophic scarring in 3%. Overall, 83% of the complications occurred in either upper or lower limbs, he noted.
Recurrence was defined by patients for whom symptoms had improved (with good or excellent results) but subsequently recurred. Of the 15 patients who had good or excellent results in the limbs, 4 had a recurrence (recurrence rate, 27%). Of 15 in the mucosa group who had been treated successfully, one had a recurrence (7%).
Subjective and objective evaluation of efficacy correlated well, and the vast majority of patients found the treatment to be successful and would opt to have it again if necessary, Dr. Sofos said.
"Due to the high risk of complications of treatment, however, we feel that treatment should be offered to a [select] group of patients, and should be carried out with caution. Further study will hopefully help us in establishing the best parameters to achieve better results, with minimal complications," he concluded.
This study was funded by the U.K. National Health Service. Dr. Sofos stated that he had no other disclosures.
GRAPEVINE, TEX. – In a prospective evaluation of 59 children with venous malformations, the long-pulsed neodymium:YAG laser was more effective in treating mucosal venous malformations than lesions on the limbs.
Venous malformations present in many forms that vary in depth, location, and extent, and treatment is largely symptomatic rather than curative. "It is, therefore, important that treatments given are effective in improving the symptoms medium or long term, with as few side effects as possible," said Dr. Stratos Sofos of Alder Hey Children's Hospital in Liverpool, England.
The long-pulsed Nd:YAG laser has become popular for the treatment of small- to medium-size vessels. Its longer pulse duration provides deeper penetration and weaker melanin absorption, allowing it to heat the vessels slowly and uniformly, thus avoiding the vessel rupture with subsequent purpura and hyperpigmentation that can occur with other lasers.
However, published data do not address whether treatment with a long-pulsed Nd:YAG laser provides long-term symptom relief, nor which types of venous malformations respond better than do others, Dr. Sofos said at the annual meeting of the American Society for Laser Medicine and Surgery.
All children who met criteria such as functional impairment, large facial deformities, and painful or bleeding lesions were treated with Candela Corp.'s GentleYAG 1064-nm laser. They received one to three treatments under general anesthesia, delivered in six to eight weekly intervals. Prior to Nd:YAG laser treatment, 32 of the children had received multiple other treatments, including pulsed dye lasers and sclerotherapy.
The 59 patients were aged 2-18 years (mean, 12.3 years). Venous malformations were present on the head and neck in 23, of which 18 (31% of the total) were mucosal. Malformations were on the upper limbs in 15 and on the lower limbs in 18 (total with limb lesions, 56%). The other three had lesions on the chest wall. Average follow up was 24 months (range, 6 months–9 years).
Based on objective assessment, 18% in the upper- and lower-limb group had excellent results and 27% patients had good results, whereas 55% of patients had no change. For the mucosal group, 28% had excellent results, 56% had good results, and 17% had no improvement, Dr. Sofos reported.
The overall complication rate from the Nd:YAG laser treatment was 20% (12 of 59), including skin blistering in 10%, ulceration in 7%, and hypertrophic scarring in 3%. Overall, 83% of the complications occurred in either upper or lower limbs, he noted.
Recurrence was defined by patients for whom symptoms had improved (with good or excellent results) but subsequently recurred. Of the 15 patients who had good or excellent results in the limbs, 4 had a recurrence (recurrence rate, 27%). Of 15 in the mucosa group who had been treated successfully, one had a recurrence (7%).
Subjective and objective evaluation of efficacy correlated well, and the vast majority of patients found the treatment to be successful and would opt to have it again if necessary, Dr. Sofos said.
"Due to the high risk of complications of treatment, however, we feel that treatment should be offered to a [select] group of patients, and should be carried out with caution. Further study will hopefully help us in establishing the best parameters to achieve better results, with minimal complications," he concluded.
This study was funded by the U.K. National Health Service. Dr. Sofos stated that he had no other disclosures.
GRAPEVINE, TEX. – In a prospective evaluation of 59 children with venous malformations, the long-pulsed neodymium:YAG laser was more effective in treating mucosal venous malformations than lesions on the limbs.
Venous malformations present in many forms that vary in depth, location, and extent, and treatment is largely symptomatic rather than curative. "It is, therefore, important that treatments given are effective in improving the symptoms medium or long term, with as few side effects as possible," said Dr. Stratos Sofos of Alder Hey Children's Hospital in Liverpool, England.
The long-pulsed Nd:YAG laser has become popular for the treatment of small- to medium-size vessels. Its longer pulse duration provides deeper penetration and weaker melanin absorption, allowing it to heat the vessels slowly and uniformly, thus avoiding the vessel rupture with subsequent purpura and hyperpigmentation that can occur with other lasers.
However, published data do not address whether treatment with a long-pulsed Nd:YAG laser provides long-term symptom relief, nor which types of venous malformations respond better than do others, Dr. Sofos said at the annual meeting of the American Society for Laser Medicine and Surgery.
All children who met criteria such as functional impairment, large facial deformities, and painful or bleeding lesions were treated with Candela Corp.'s GentleYAG 1064-nm laser. They received one to three treatments under general anesthesia, delivered in six to eight weekly intervals. Prior to Nd:YAG laser treatment, 32 of the children had received multiple other treatments, including pulsed dye lasers and sclerotherapy.
The 59 patients were aged 2-18 years (mean, 12.3 years). Venous malformations were present on the head and neck in 23, of which 18 (31% of the total) were mucosal. Malformations were on the upper limbs in 15 and on the lower limbs in 18 (total with limb lesions, 56%). The other three had lesions on the chest wall. Average follow up was 24 months (range, 6 months–9 years).
Based on objective assessment, 18% in the upper- and lower-limb group had excellent results and 27% patients had good results, whereas 55% of patients had no change. For the mucosal group, 28% had excellent results, 56% had good results, and 17% had no improvement, Dr. Sofos reported.
The overall complication rate from the Nd:YAG laser treatment was 20% (12 of 59), including skin blistering in 10%, ulceration in 7%, and hypertrophic scarring in 3%. Overall, 83% of the complications occurred in either upper or lower limbs, he noted.
Recurrence was defined by patients for whom symptoms had improved (with good or excellent results) but subsequently recurred. Of the 15 patients who had good or excellent results in the limbs, 4 had a recurrence (recurrence rate, 27%). Of 15 in the mucosa group who had been treated successfully, one had a recurrence (7%).
Subjective and objective evaluation of efficacy correlated well, and the vast majority of patients found the treatment to be successful and would opt to have it again if necessary, Dr. Sofos said.
"Due to the high risk of complications of treatment, however, we feel that treatment should be offered to a [select] group of patients, and should be carried out with caution. Further study will hopefully help us in establishing the best parameters to achieve better results, with minimal complications," he concluded.
This study was funded by the U.K. National Health Service. Dr. Sofos stated that he had no other disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR LASER MEDICINE AND SURGERY
Major Finding: Based on objective assessment, 18% in the upper- and lower-limb group had excellent results and 27% of patients had good results, whereas 55% of patients had no change. In the mucosal group, 28% had excellent results, 56% had good results, and 17% had no improvement.
Data Source: Prospective evaluation of 59 children with venous malformations who were seen at a single hospital.
Disclosures: The study was funded by the U.K. National Health Service. Dr. Sofos stated that he had no other disclosures.
Sublative Rejuvenation Strikes Efficacy, Adverse Event Balance
MIAMI BEACH – Sublative skin rejuvenation is a resurfacing technology that seeks to provide the best of both worlds – efficacy closer to more intensive ablative procedures and an adverse event profile more akin to gentler, nonablative techniques.
Sublative rejuvenation can be performed on the full face, with good results around the eyes and the neck, Dr. Robert A. Weiss said at the symposium.
"We've gotten some very nice skin contraction and smoothing in the periorbital area," he said.
The fractional radiofrequency eMatrix system (Syneron) offers deep volumetric heating into the dermis with minimal epidermal disruption, said Dr. Weiss, director of the Maryland Laser, Skin, and Vein Institute in Hunt Valley. Subsequent dermal remodeling with minimal downtime are other advantages of this treatment.
The Food and Drug Administration cleared marketing of the applicator for use in dermatologic procedures requiring ablation of soft tissue and skin resurfacing.
The device tip is a grid of negatively- and positively-charged electrodes between which bipolar radiofrequency energy flows. The current can be controlled and varied depending on individual patient factors. Because this is a fractional technology, intact tissue is left between the electrode pins to speed healing and recovery.
The standard protocol is three to four treatment sessions spaced 4-6 weeks apart. Touch-up sessions, as indicated, are generally every 6 months or so.
The full face can be treated in 20 minutes, said Dr. Weiss, also of the department of dermatology at Johns Hopkins University, Baltimore. Improvements in acne scarring and wrinkles can be observed as well.
Ask patients to return 2-3 days after treatment to check for any adverse events, he said. Postoperative discomfort, significant pain, erythema, edema, and pigmentary changes can arise in the short or long term. Patient discomfort is usually tolerable and can be managed with topical anesthetics.
Postprocedure redness with nonablative techniques typically lasts a few hours to 1-2 days; with ablative procedures, 5-7 days (and sometimes up to 10 days); and with sublative treatment, a patient will have red dots on their skin and redness that lasts for a day or 2, Dr. Weiss said. "So it's really somewhere in the middle between ablative and nonablative."
Contraindications include any facelift or eyelid surgery 1 year prior to sublative resurfacing; injections of botulinum toxin, collagen, or fat (or any biomaterial augmentation) within the last 6 months; and any facial dermabrasion, resurfacing, or deep chemical peeling within the last 3 months.
Treat a hidden test spot to gauge individual response prior to full treatment, Dr. Weiss recommended. Gauge response after 24-48 hours for skin types I-III and 5-7 days for skin types IV-VI to ensure safety.
Cold packs or Synercool (Syneron) can be used immediately after treatment to cool the area if patients are uncomfortable. Advise patients to apply emollient cream and to use at least 30 SPF or greater sunscreen for at least a month.
"It's a promising technology, and I think we will know more a year from now," Dr. Weiss said.
He is a researcher for Syneron and received some initial free use of equipment.
MIAMI BEACH – Sublative skin rejuvenation is a resurfacing technology that seeks to provide the best of both worlds – efficacy closer to more intensive ablative procedures and an adverse event profile more akin to gentler, nonablative techniques.
Sublative rejuvenation can be performed on the full face, with good results around the eyes and the neck, Dr. Robert A. Weiss said at the symposium.
"We've gotten some very nice skin contraction and smoothing in the periorbital area," he said.
The fractional radiofrequency eMatrix system (Syneron) offers deep volumetric heating into the dermis with minimal epidermal disruption, said Dr. Weiss, director of the Maryland Laser, Skin, and Vein Institute in Hunt Valley. Subsequent dermal remodeling with minimal downtime are other advantages of this treatment.
The Food and Drug Administration cleared marketing of the applicator for use in dermatologic procedures requiring ablation of soft tissue and skin resurfacing.
The device tip is a grid of negatively- and positively-charged electrodes between which bipolar radiofrequency energy flows. The current can be controlled and varied depending on individual patient factors. Because this is a fractional technology, intact tissue is left between the electrode pins to speed healing and recovery.
The standard protocol is three to four treatment sessions spaced 4-6 weeks apart. Touch-up sessions, as indicated, are generally every 6 months or so.
The full face can be treated in 20 minutes, said Dr. Weiss, also of the department of dermatology at Johns Hopkins University, Baltimore. Improvements in acne scarring and wrinkles can be observed as well.
Ask patients to return 2-3 days after treatment to check for any adverse events, he said. Postoperative discomfort, significant pain, erythema, edema, and pigmentary changes can arise in the short or long term. Patient discomfort is usually tolerable and can be managed with topical anesthetics.
Postprocedure redness with nonablative techniques typically lasts a few hours to 1-2 days; with ablative procedures, 5-7 days (and sometimes up to 10 days); and with sublative treatment, a patient will have red dots on their skin and redness that lasts for a day or 2, Dr. Weiss said. "So it's really somewhere in the middle between ablative and nonablative."
Contraindications include any facelift or eyelid surgery 1 year prior to sublative resurfacing; injections of botulinum toxin, collagen, or fat (or any biomaterial augmentation) within the last 6 months; and any facial dermabrasion, resurfacing, or deep chemical peeling within the last 3 months.
Treat a hidden test spot to gauge individual response prior to full treatment, Dr. Weiss recommended. Gauge response after 24-48 hours for skin types I-III and 5-7 days for skin types IV-VI to ensure safety.
Cold packs or Synercool (Syneron) can be used immediately after treatment to cool the area if patients are uncomfortable. Advise patients to apply emollient cream and to use at least 30 SPF or greater sunscreen for at least a month.
"It's a promising technology, and I think we will know more a year from now," Dr. Weiss said.
He is a researcher for Syneron and received some initial free use of equipment.
MIAMI BEACH – Sublative skin rejuvenation is a resurfacing technology that seeks to provide the best of both worlds – efficacy closer to more intensive ablative procedures and an adverse event profile more akin to gentler, nonablative techniques.
Sublative rejuvenation can be performed on the full face, with good results around the eyes and the neck, Dr. Robert A. Weiss said at the symposium.
"We've gotten some very nice skin contraction and smoothing in the periorbital area," he said.
The fractional radiofrequency eMatrix system (Syneron) offers deep volumetric heating into the dermis with minimal epidermal disruption, said Dr. Weiss, director of the Maryland Laser, Skin, and Vein Institute in Hunt Valley. Subsequent dermal remodeling with minimal downtime are other advantages of this treatment.
The Food and Drug Administration cleared marketing of the applicator for use in dermatologic procedures requiring ablation of soft tissue and skin resurfacing.
The device tip is a grid of negatively- and positively-charged electrodes between which bipolar radiofrequency energy flows. The current can be controlled and varied depending on individual patient factors. Because this is a fractional technology, intact tissue is left between the electrode pins to speed healing and recovery.
The standard protocol is three to four treatment sessions spaced 4-6 weeks apart. Touch-up sessions, as indicated, are generally every 6 months or so.
The full face can be treated in 20 minutes, said Dr. Weiss, also of the department of dermatology at Johns Hopkins University, Baltimore. Improvements in acne scarring and wrinkles can be observed as well.
Ask patients to return 2-3 days after treatment to check for any adverse events, he said. Postoperative discomfort, significant pain, erythema, edema, and pigmentary changes can arise in the short or long term. Patient discomfort is usually tolerable and can be managed with topical anesthetics.
Postprocedure redness with nonablative techniques typically lasts a few hours to 1-2 days; with ablative procedures, 5-7 days (and sometimes up to 10 days); and with sublative treatment, a patient will have red dots on their skin and redness that lasts for a day or 2, Dr. Weiss said. "So it's really somewhere in the middle between ablative and nonablative."
Contraindications include any facelift or eyelid surgery 1 year prior to sublative resurfacing; injections of botulinum toxin, collagen, or fat (or any biomaterial augmentation) within the last 6 months; and any facial dermabrasion, resurfacing, or deep chemical peeling within the last 3 months.
Treat a hidden test spot to gauge individual response prior to full treatment, Dr. Weiss recommended. Gauge response after 24-48 hours for skin types I-III and 5-7 days for skin types IV-VI to ensure safety.
Cold packs or Synercool (Syneron) can be used immediately after treatment to cool the area if patients are uncomfortable. Advise patients to apply emollient cream and to use at least 30 SPF or greater sunscreen for at least a month.
"It's a promising technology, and I think we will know more a year from now," Dr. Weiss said.
He is a researcher for Syneron and received some initial free use of equipment.
EXPERT ANALYSIS FROM THE SOUTH BEACH SYMPOSIUM