User login
SDEF: Fractional Lasers a "Go To" Device for Scar Treatment
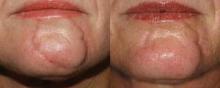
Partially or fully ablative lasers are safe and effective tools to treat many types of scars and keloids, Dr. Suzanne L. Kilmer reported.
In treating scars, "the challenge is to normalize the color and the texture and to somehow repopulate that area with normal collagen and return it to normal skin color for that individual," she said in an interview.
A key challenge to treatment is managing patient expectations. She described three treatment techniques to improve the appearance of scars and keloids at the Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF): pulsed dye lasers, fractional lasers, and fully ablative erbium/CO2 laser resurfacing.
Each of these lasers has its pros and cons for scar treatment, Dr. Kilmer explained. "In most cases now I will go straight to a fractional device, and depending on location, patient's tolerance for downtime, pain, and budget constraints, I will proceed with the best choice."
Pulsed-dye lasers help improve erythema by targeting hemoglobin, and they can also aid in collagen remodeling, reported Dr. Kilmer of the Laser & Skin Surgery Center of Northern California, Sacramento.
Fractional lasers heat up or even ablate thousands of tiny 120-mcm columns of tissue, allowing tissue remodeling and the regeneration of more normal collagen. This normal collagen gradually takes over the area with scar tissue and helps to return the skin to its prescar appearance. This is also helpful for striae (stretch marks), she noted.
Fractional resurfacing is often effective for pigment, texture, or vascular changes. The fractional laser produces a microthermal zone up to 1,500 mcm deep that creates lateral reepithelialization and promotes skin healing. A Fraxel 1550-nm erbium laser (Solta) or a StarLux 1540-nm laser (Palomar) is a good choice for these procedures.
Most patients find the Fraxel 1550-nm laser more painful, and anesthesia is needed for the entire procedure, she reported. However, the Fraxel is faster, so it is a better choice for procedures involving the full face, and provides a more even treatment.
By contrast, the StarLux 1,540-nm laser often allows the clinician to treat small areas without the need for anesthesia, and these areas blend easily into nontreated skin, Dr. Kilmer pointed out. Small areas of the skin can be treated quickly, but the StarLux can be slow and uneven when used on larger areas.
Swelling is common – but not usually painful – after nonablative fractional resurfacing, she explained, and most patients need only minimal aftercare. Usually ice or hydration is sufficient.
Fractional ablative laser resurfacing carries more risk and requires more downtime than do other scar treatments, but it offers "the greatest efficacy for a single treatment," noted Dr. Kilmer. This treatment allows the clinician to sculpt more deeply with less thermal damage, and provides the most predictable results, including skin tightening and smoothing of acne scar ridges. The fractional ablative component allows for deeper scar remodeling and can be done at the same time, but with caution, she noted.
Dr. Kilmer emphasized that clinicians who are new to laser scar treatments should go slowly while they become familiar with the device. "And go lighter when you are off the face," she added.
Dr. Kilmer has received research support from Palomar and Solta. SDEF and this news organization are owned by Elsevier.
Partially or fully ablative lasers are safe and effective tools to treat many types of scars and keloids, Dr. Suzanne L. Kilmer reported.
In treating scars, "the challenge is to normalize the color and the texture and to somehow repopulate that area with normal collagen and return it to normal skin color for that individual," she said in an interview.
A key challenge to treatment is managing patient expectations. She described three treatment techniques to improve the appearance of scars and keloids at the Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF): pulsed dye lasers, fractional lasers, and fully ablative erbium/CO2 laser resurfacing.
Each of these lasers has its pros and cons for scar treatment, Dr. Kilmer explained. "In most cases now I will go straight to a fractional device, and depending on location, patient's tolerance for downtime, pain, and budget constraints, I will proceed with the best choice."
Pulsed-dye lasers help improve erythema by targeting hemoglobin, and they can also aid in collagen remodeling, reported Dr. Kilmer of the Laser & Skin Surgery Center of Northern California, Sacramento.
Fractional lasers heat up or even ablate thousands of tiny 120-mcm columns of tissue, allowing tissue remodeling and the regeneration of more normal collagen. This normal collagen gradually takes over the area with scar tissue and helps to return the skin to its prescar appearance. This is also helpful for striae (stretch marks), she noted.
Fractional resurfacing is often effective for pigment, texture, or vascular changes. The fractional laser produces a microthermal zone up to 1,500 mcm deep that creates lateral reepithelialization and promotes skin healing. A Fraxel 1550-nm erbium laser (Solta) or a StarLux 1540-nm laser (Palomar) is a good choice for these procedures.
Most patients find the Fraxel 1550-nm laser more painful, and anesthesia is needed for the entire procedure, she reported. However, the Fraxel is faster, so it is a better choice for procedures involving the full face, and provides a more even treatment.
By contrast, the StarLux 1,540-nm laser often allows the clinician to treat small areas without the need for anesthesia, and these areas blend easily into nontreated skin, Dr. Kilmer pointed out. Small areas of the skin can be treated quickly, but the StarLux can be slow and uneven when used on larger areas.
Swelling is common – but not usually painful – after nonablative fractional resurfacing, she explained, and most patients need only minimal aftercare. Usually ice or hydration is sufficient.
Fractional ablative laser resurfacing carries more risk and requires more downtime than do other scar treatments, but it offers "the greatest efficacy for a single treatment," noted Dr. Kilmer. This treatment allows the clinician to sculpt more deeply with less thermal damage, and provides the most predictable results, including skin tightening and smoothing of acne scar ridges. The fractional ablative component allows for deeper scar remodeling and can be done at the same time, but with caution, she noted.
Dr. Kilmer emphasized that clinicians who are new to laser scar treatments should go slowly while they become familiar with the device. "And go lighter when you are off the face," she added.
Dr. Kilmer has received research support from Palomar and Solta. SDEF and this news organization are owned by Elsevier.
Partially or fully ablative lasers are safe and effective tools to treat many types of scars and keloids, Dr. Suzanne L. Kilmer reported.
In treating scars, "the challenge is to normalize the color and the texture and to somehow repopulate that area with normal collagen and return it to normal skin color for that individual," she said in an interview.
A key challenge to treatment is managing patient expectations. She described three treatment techniques to improve the appearance of scars and keloids at the Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF): pulsed dye lasers, fractional lasers, and fully ablative erbium/CO2 laser resurfacing.
Each of these lasers has its pros and cons for scar treatment, Dr. Kilmer explained. "In most cases now I will go straight to a fractional device, and depending on location, patient's tolerance for downtime, pain, and budget constraints, I will proceed with the best choice."
Pulsed-dye lasers help improve erythema by targeting hemoglobin, and they can also aid in collagen remodeling, reported Dr. Kilmer of the Laser & Skin Surgery Center of Northern California, Sacramento.
Fractional lasers heat up or even ablate thousands of tiny 120-mcm columns of tissue, allowing tissue remodeling and the regeneration of more normal collagen. This normal collagen gradually takes over the area with scar tissue and helps to return the skin to its prescar appearance. This is also helpful for striae (stretch marks), she noted.
Fractional resurfacing is often effective for pigment, texture, or vascular changes. The fractional laser produces a microthermal zone up to 1,500 mcm deep that creates lateral reepithelialization and promotes skin healing. A Fraxel 1550-nm erbium laser (Solta) or a StarLux 1540-nm laser (Palomar) is a good choice for these procedures.
Most patients find the Fraxel 1550-nm laser more painful, and anesthesia is needed for the entire procedure, she reported. However, the Fraxel is faster, so it is a better choice for procedures involving the full face, and provides a more even treatment.
By contrast, the StarLux 1,540-nm laser often allows the clinician to treat small areas without the need for anesthesia, and these areas blend easily into nontreated skin, Dr. Kilmer pointed out. Small areas of the skin can be treated quickly, but the StarLux can be slow and uneven when used on larger areas.
Swelling is common – but not usually painful – after nonablative fractional resurfacing, she explained, and most patients need only minimal aftercare. Usually ice or hydration is sufficient.
Fractional ablative laser resurfacing carries more risk and requires more downtime than do other scar treatments, but it offers "the greatest efficacy for a single treatment," noted Dr. Kilmer. This treatment allows the clinician to sculpt more deeply with less thermal damage, and provides the most predictable results, including skin tightening and smoothing of acne scar ridges. The fractional ablative component allows for deeper scar remodeling and can be done at the same time, but with caution, she noted.
Dr. Kilmer emphasized that clinicians who are new to laser scar treatments should go slowly while they become familiar with the device. "And go lighter when you are off the face," she added.
Dr. Kilmer has received research support from Palomar and Solta. SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Foam Sclerotherapy Appears as Effective as Surgery for Varicose Veins
SAN DIEGO – At 5 years of follow-up, patients who had ultrasound-guided foam sclerotherapy combined with saphenofemoral ligation had equally good clinical results compared with patients who underwent surgical treatment of varicose veins, based on a randomized controlled trial.
"Since surgery may not provide a definitive treatment, foam sclerotherapy could be offered like a dental care treatment model: treating as and when the problem appears," Dr. Evi Kalodiki said at the annual meeting of the American Venous Forum.
Dr. Kalodiki, a vascular surgeon at Ealing Hospital and Imperial College, London, and her associates evaluated 82 legs that were treated in 73 patients. The mean age of the patients was 48 years, and 75% were female. The researchers randomized the cases to two treatments: 39 legs underwent saphenofemoral ligation, stripping, and multiple phlebectomies under general anesthesia (surgery group) and 43 underwent saphenofemoral ligation under local anesthesia with concurrent foam sclerotherapy (foam group).
Assessments included ultrasound, the CEAP (clinical, etiologic, anatomical, pathophysiologic) classification, the Venous Clinical Severity Score, the Aberdeen Varicose Veins Questionnaire (AVVQ), and the Short Form 36 Health Survey (SF-36). Follow-ups were conducted annually until a median of 5.12 years.
Dr. Kalodiki reported that the CEAP classification was similar for the two groups, as was the percentage of legs that required additional foam sessions (40% of legs in the surgery group, with a mean volume of 11 mL for each case, vs. 48% of legs in the foam group, with a mean volume of 9 mL for each case).
Preoperative Venous Clinical Severity Scores (VCSS) were similar for the two groups (median of 5 in the surgery group vs. 4 in the foam group), as were post-treatment VCSS, at a median of 1 in each group. Median changes in VCSS from baseline to 5 years were similar in the two groups: 3 in the surgery group vs. 3.5 in the foam group. Absolute VCSS values at 5 years were a median of 1 in each group.
The AVVQ score also improved in both groups. In the surgery group, the median AVVQ score improved from 16.32 preoperatively to 8.94 at 3 years, while in the foam group the median AVVQ score improved from 12.28 preoperatively to 4.97 at 3 years. These results were maintained at 5 years.
Scores on the mental component of the SF-36 worsened in the surgery group. There was no change in the physical scores of the SF-36 in either group over 3 or 5 years.
There were no significant differences between the groups or within the groups regarding obliteration or reflux above the knee or below the knee at 3 or 5 years. In the majority of cases, the reflux was asymptomatic and was only detected because of the follow-up duplex examination.
The study was funded by STD Pharmaceutical. Dr. Kalodiki said that she had no relevant financial disclosures to make.
SAN DIEGO – At 5 years of follow-up, patients who had ultrasound-guided foam sclerotherapy combined with saphenofemoral ligation had equally good clinical results compared with patients who underwent surgical treatment of varicose veins, based on a randomized controlled trial.
"Since surgery may not provide a definitive treatment, foam sclerotherapy could be offered like a dental care treatment model: treating as and when the problem appears," Dr. Evi Kalodiki said at the annual meeting of the American Venous Forum.
Dr. Kalodiki, a vascular surgeon at Ealing Hospital and Imperial College, London, and her associates evaluated 82 legs that were treated in 73 patients. The mean age of the patients was 48 years, and 75% were female. The researchers randomized the cases to two treatments: 39 legs underwent saphenofemoral ligation, stripping, and multiple phlebectomies under general anesthesia (surgery group) and 43 underwent saphenofemoral ligation under local anesthesia with concurrent foam sclerotherapy (foam group).
Assessments included ultrasound, the CEAP (clinical, etiologic, anatomical, pathophysiologic) classification, the Venous Clinical Severity Score, the Aberdeen Varicose Veins Questionnaire (AVVQ), and the Short Form 36 Health Survey (SF-36). Follow-ups were conducted annually until a median of 5.12 years.
Dr. Kalodiki reported that the CEAP classification was similar for the two groups, as was the percentage of legs that required additional foam sessions (40% of legs in the surgery group, with a mean volume of 11 mL for each case, vs. 48% of legs in the foam group, with a mean volume of 9 mL for each case).
Preoperative Venous Clinical Severity Scores (VCSS) were similar for the two groups (median of 5 in the surgery group vs. 4 in the foam group), as were post-treatment VCSS, at a median of 1 in each group. Median changes in VCSS from baseline to 5 years were similar in the two groups: 3 in the surgery group vs. 3.5 in the foam group. Absolute VCSS values at 5 years were a median of 1 in each group.
The AVVQ score also improved in both groups. In the surgery group, the median AVVQ score improved from 16.32 preoperatively to 8.94 at 3 years, while in the foam group the median AVVQ score improved from 12.28 preoperatively to 4.97 at 3 years. These results were maintained at 5 years.
Scores on the mental component of the SF-36 worsened in the surgery group. There was no change in the physical scores of the SF-36 in either group over 3 or 5 years.
There were no significant differences between the groups or within the groups regarding obliteration or reflux above the knee or below the knee at 3 or 5 years. In the majority of cases, the reflux was asymptomatic and was only detected because of the follow-up duplex examination.
The study was funded by STD Pharmaceutical. Dr. Kalodiki said that she had no relevant financial disclosures to make.
SAN DIEGO – At 5 years of follow-up, patients who had ultrasound-guided foam sclerotherapy combined with saphenofemoral ligation had equally good clinical results compared with patients who underwent surgical treatment of varicose veins, based on a randomized controlled trial.
"Since surgery may not provide a definitive treatment, foam sclerotherapy could be offered like a dental care treatment model: treating as and when the problem appears," Dr. Evi Kalodiki said at the annual meeting of the American Venous Forum.
Dr. Kalodiki, a vascular surgeon at Ealing Hospital and Imperial College, London, and her associates evaluated 82 legs that were treated in 73 patients. The mean age of the patients was 48 years, and 75% were female. The researchers randomized the cases to two treatments: 39 legs underwent saphenofemoral ligation, stripping, and multiple phlebectomies under general anesthesia (surgery group) and 43 underwent saphenofemoral ligation under local anesthesia with concurrent foam sclerotherapy (foam group).
Assessments included ultrasound, the CEAP (clinical, etiologic, anatomical, pathophysiologic) classification, the Venous Clinical Severity Score, the Aberdeen Varicose Veins Questionnaire (AVVQ), and the Short Form 36 Health Survey (SF-36). Follow-ups were conducted annually until a median of 5.12 years.
Dr. Kalodiki reported that the CEAP classification was similar for the two groups, as was the percentage of legs that required additional foam sessions (40% of legs in the surgery group, with a mean volume of 11 mL for each case, vs. 48% of legs in the foam group, with a mean volume of 9 mL for each case).
Preoperative Venous Clinical Severity Scores (VCSS) were similar for the two groups (median of 5 in the surgery group vs. 4 in the foam group), as were post-treatment VCSS, at a median of 1 in each group. Median changes in VCSS from baseline to 5 years were similar in the two groups: 3 in the surgery group vs. 3.5 in the foam group. Absolute VCSS values at 5 years were a median of 1 in each group.
The AVVQ score also improved in both groups. In the surgery group, the median AVVQ score improved from 16.32 preoperatively to 8.94 at 3 years, while in the foam group the median AVVQ score improved from 12.28 preoperatively to 4.97 at 3 years. These results were maintained at 5 years.
Scores on the mental component of the SF-36 worsened in the surgery group. There was no change in the physical scores of the SF-36 in either group over 3 or 5 years.
There were no significant differences between the groups or within the groups regarding obliteration or reflux above the knee or below the knee at 3 or 5 years. In the majority of cases, the reflux was asymptomatic and was only detected because of the follow-up duplex examination.
The study was funded by STD Pharmaceutical. Dr. Kalodiki said that she had no relevant financial disclosures to make.
FROM THE ANNUAL MEETING OF THE AMERICAN VENOUS FORUM
Major Finding: Improvements in venous clinical severity scores at 3 and 5 years were similar among patients who underwent saphenofemoral ligation, stripping, and multiple phlebectomies under general anesthesia and those who underwent saphenofemoral ligation under local anesthesia with concurrent foam sclerotherapy.
Data Source: A randomized, controlled trial of 82 legs that were treated in 73 patients with a mean age of 48 years.
Disclosures: The study was funded by STD Pharmaceutical. Dr. Kalodiki said that she had no relevant financial disclosures to make.
Voluma Facial Filler Expected to Hit Market This Year
MIAMI BEACH – You may soon be able to "pump up the volume" with a new facial filler, according to Dr. Nowell Solish.
Dr. Solish reported his experience with a low-molecular-weight, hyaluronic acid filler at the South Beach Symposium.
Allergan Inc.’s Voluma is already available in Canada and is expected to be available in the United States this year. This viscous formulation of the Juvéderm product line "is really for deeper injections and really for facial volume."
Appropriate use includes injections of the cheek, submalar region, and chin. "This is really for volume replacement in patients whose faces have become ‘bottom heavy,’ " said Dr. Solish of the division of dermatology at the University of Toronto.
"I use this in the midface mostly. It all depends on the age of the patient," he said. He tends to inject more filler medially for older patients and more laterally on the face for his younger patients.
Dr. Solish recommended injection planning using markings to ensure symmetrical placement of the filler. For example, he explained how a triangle on the midface created by the intersection of three straight lines could be used as a guide. One line would go from the lateral canthus to the corner of the patient’s mouth, the second from the upper part of the tragus to the alar lobule, and the third from the lateral canthus to the lower tragus.
Subcutaneous injections are made to the deep dermis and above the periosteum. Do not overcorrect your patient and always massage the area post injection to ensure even distribution, he said.
Voluma is expected to contain lidocaine when it is marketed in the United States. "I mix this filler with lidocaine. Voluma does not come with lidocaine in Canada," Dr. Solish said. He said that the addition of lidocaine with epinephrine makes the filler less viscous and easier to inject through a 28-gauge needle.
Results can last up to a year or longer. His patients typically return for subsequent treatment in 9-12 months. "We are finding they need about half as much product as their initial treatment," he said.
Dr. Solish reported side effects similar to other hyaluronic acid fillers, including bruising, swelling, and discomfort.
"But some patients have a foreign body–like reaction that requires draining and hyaluronidase," Dr. Solish said. "We’ve seen a few cases in Canada."
The low-molecular-weight hyaluronic acid is composed of 90% short molecular chains and 10% of the chains "you are used to with regular Juvéderm," Dr. Solish said.
The term "molecular weight" refers to the length of the molecular chains (number of repeating chains). Greater cross-linking with these shorter chains increases the product’s viscosity.
Dr. Solish disclosed being a consultant and researcher for Allergan.
MIAMI BEACH – You may soon be able to "pump up the volume" with a new facial filler, according to Dr. Nowell Solish.
Dr. Solish reported his experience with a low-molecular-weight, hyaluronic acid filler at the South Beach Symposium.
Allergan Inc.’s Voluma is already available in Canada and is expected to be available in the United States this year. This viscous formulation of the Juvéderm product line "is really for deeper injections and really for facial volume."
Appropriate use includes injections of the cheek, submalar region, and chin. "This is really for volume replacement in patients whose faces have become ‘bottom heavy,’ " said Dr. Solish of the division of dermatology at the University of Toronto.
"I use this in the midface mostly. It all depends on the age of the patient," he said. He tends to inject more filler medially for older patients and more laterally on the face for his younger patients.
Dr. Solish recommended injection planning using markings to ensure symmetrical placement of the filler. For example, he explained how a triangle on the midface created by the intersection of three straight lines could be used as a guide. One line would go from the lateral canthus to the corner of the patient’s mouth, the second from the upper part of the tragus to the alar lobule, and the third from the lateral canthus to the lower tragus.
Subcutaneous injections are made to the deep dermis and above the periosteum. Do not overcorrect your patient and always massage the area post injection to ensure even distribution, he said.
Voluma is expected to contain lidocaine when it is marketed in the United States. "I mix this filler with lidocaine. Voluma does not come with lidocaine in Canada," Dr. Solish said. He said that the addition of lidocaine with epinephrine makes the filler less viscous and easier to inject through a 28-gauge needle.
Results can last up to a year or longer. His patients typically return for subsequent treatment in 9-12 months. "We are finding they need about half as much product as their initial treatment," he said.
Dr. Solish reported side effects similar to other hyaluronic acid fillers, including bruising, swelling, and discomfort.
"But some patients have a foreign body–like reaction that requires draining and hyaluronidase," Dr. Solish said. "We’ve seen a few cases in Canada."
The low-molecular-weight hyaluronic acid is composed of 90% short molecular chains and 10% of the chains "you are used to with regular Juvéderm," Dr. Solish said.
The term "molecular weight" refers to the length of the molecular chains (number of repeating chains). Greater cross-linking with these shorter chains increases the product’s viscosity.
Dr. Solish disclosed being a consultant and researcher for Allergan.
MIAMI BEACH – You may soon be able to "pump up the volume" with a new facial filler, according to Dr. Nowell Solish.
Dr. Solish reported his experience with a low-molecular-weight, hyaluronic acid filler at the South Beach Symposium.
Allergan Inc.’s Voluma is already available in Canada and is expected to be available in the United States this year. This viscous formulation of the Juvéderm product line "is really for deeper injections and really for facial volume."
Appropriate use includes injections of the cheek, submalar region, and chin. "This is really for volume replacement in patients whose faces have become ‘bottom heavy,’ " said Dr. Solish of the division of dermatology at the University of Toronto.
"I use this in the midface mostly. It all depends on the age of the patient," he said. He tends to inject more filler medially for older patients and more laterally on the face for his younger patients.
Dr. Solish recommended injection planning using markings to ensure symmetrical placement of the filler. For example, he explained how a triangle on the midface created by the intersection of three straight lines could be used as a guide. One line would go from the lateral canthus to the corner of the patient’s mouth, the second from the upper part of the tragus to the alar lobule, and the third from the lateral canthus to the lower tragus.
Subcutaneous injections are made to the deep dermis and above the periosteum. Do not overcorrect your patient and always massage the area post injection to ensure even distribution, he said.
Voluma is expected to contain lidocaine when it is marketed in the United States. "I mix this filler with lidocaine. Voluma does not come with lidocaine in Canada," Dr. Solish said. He said that the addition of lidocaine with epinephrine makes the filler less viscous and easier to inject through a 28-gauge needle.
Results can last up to a year or longer. His patients typically return for subsequent treatment in 9-12 months. "We are finding they need about half as much product as their initial treatment," he said.
Dr. Solish reported side effects similar to other hyaluronic acid fillers, including bruising, swelling, and discomfort.
"But some patients have a foreign body–like reaction that requires draining and hyaluronidase," Dr. Solish said. "We’ve seen a few cases in Canada."
The low-molecular-weight hyaluronic acid is composed of 90% short molecular chains and 10% of the chains "you are used to with regular Juvéderm," Dr. Solish said.
The term "molecular weight" refers to the length of the molecular chains (number of repeating chains). Greater cross-linking with these shorter chains increases the product’s viscosity.
Dr. Solish disclosed being a consultant and researcher for Allergan.
EXPERT ANALYSIS FROM THE SOUTH BEACH SYMPOSIUM
Cannulas Less Traumatic Than Needles for Administering Fillers
MIAMI BEACH – It's time to consider being blunt with your aesthetic patients, at least in terms of how you administer filler products for facial and hand rejuvenation.
"The blunt cannula is really an exciting tool for us," Dr. Susan H. Weinkle said at the South Beach Symposium.
Instead of multiple puncture wounds with needles, Dr. Weinkle creates one entry point with a percutaneous stick of a 26-gauge needle. Then she inserts a cannula. "You can treat the midface [and then] you can turn the cannula and treat all along the cheekbone and the zygomatic arch. Then, through the same injection point, you can turn the cannula south and treat the nasolabial fold."
Less trauma, lower risk for bruising, and quicker downtime are among the advantages, compared with multiple needle injections, Dr. Weinkle said.
Less precise delivery of the filler – because the cannula holes are not at the tip like a needle – is a drawback, but not a significant one, she added.
Cannulas already have gained popularity in Europe and South America. "We are not learning this as quickly as our colleagues," she said.
"A year ago, I went to an exciting meeting in Paris where I heard a little about cannulas." Dr. Weinkle brought some back to her private practice in Bradenton, Fla., with the best intentions, but did not use them. "This year I went back to the same meeting in January, and I decided I did not want to be left behind."
Filler augmentation of the dorsal side of the hands is another procedure that is well suited to the use of these blunt cannulas, Dr. Weinkle said.
Patience is advised when the technique is tried for the first time. "It's not always easy. You're not going to love it right away. You have to finesse the cannula through the skin." However, she added, "Don't get discouraged."
These are not cannulas that are used for liposuction, but newer products that are specifically designed for use in soft tissue. Only one such cannula – CosmoFrance Inc.'s DermaSculpt microcannula, a 1.5 inch, 27 gauge cannula with a nonbruising blunt tip – is currently approved by the Food and Drug Administration in the United States.
"More are coming. We will have shorter, fatter, thicker, thinner, and different gauge cannulas," Dr. Weinkle said. "We're going to see some of the companies that provide fillers for us adopting this. Maybe we'll find one needle and one cannula in the future in our packaging."
"This is the wave of the future. We really want to embrace new and exciting things," she said.
Dr. Weinkle said she had no relevant financial disclosures.
MIAMI BEACH – It's time to consider being blunt with your aesthetic patients, at least in terms of how you administer filler products for facial and hand rejuvenation.
"The blunt cannula is really an exciting tool for us," Dr. Susan H. Weinkle said at the South Beach Symposium.
Instead of multiple puncture wounds with needles, Dr. Weinkle creates one entry point with a percutaneous stick of a 26-gauge needle. Then she inserts a cannula. "You can treat the midface [and then] you can turn the cannula and treat all along the cheekbone and the zygomatic arch. Then, through the same injection point, you can turn the cannula south and treat the nasolabial fold."
Less trauma, lower risk for bruising, and quicker downtime are among the advantages, compared with multiple needle injections, Dr. Weinkle said.
Less precise delivery of the filler – because the cannula holes are not at the tip like a needle – is a drawback, but not a significant one, she added.
Cannulas already have gained popularity in Europe and South America. "We are not learning this as quickly as our colleagues," she said.
"A year ago, I went to an exciting meeting in Paris where I heard a little about cannulas." Dr. Weinkle brought some back to her private practice in Bradenton, Fla., with the best intentions, but did not use them. "This year I went back to the same meeting in January, and I decided I did not want to be left behind."
Filler augmentation of the dorsal side of the hands is another procedure that is well suited to the use of these blunt cannulas, Dr. Weinkle said.
Patience is advised when the technique is tried for the first time. "It's not always easy. You're not going to love it right away. You have to finesse the cannula through the skin." However, she added, "Don't get discouraged."
These are not cannulas that are used for liposuction, but newer products that are specifically designed for use in soft tissue. Only one such cannula – CosmoFrance Inc.'s DermaSculpt microcannula, a 1.5 inch, 27 gauge cannula with a nonbruising blunt tip – is currently approved by the Food and Drug Administration in the United States.
"More are coming. We will have shorter, fatter, thicker, thinner, and different gauge cannulas," Dr. Weinkle said. "We're going to see some of the companies that provide fillers for us adopting this. Maybe we'll find one needle and one cannula in the future in our packaging."
"This is the wave of the future. We really want to embrace new and exciting things," she said.
Dr. Weinkle said she had no relevant financial disclosures.
MIAMI BEACH – It's time to consider being blunt with your aesthetic patients, at least in terms of how you administer filler products for facial and hand rejuvenation.
"The blunt cannula is really an exciting tool for us," Dr. Susan H. Weinkle said at the South Beach Symposium.
Instead of multiple puncture wounds with needles, Dr. Weinkle creates one entry point with a percutaneous stick of a 26-gauge needle. Then she inserts a cannula. "You can treat the midface [and then] you can turn the cannula and treat all along the cheekbone and the zygomatic arch. Then, through the same injection point, you can turn the cannula south and treat the nasolabial fold."
Less trauma, lower risk for bruising, and quicker downtime are among the advantages, compared with multiple needle injections, Dr. Weinkle said.
Less precise delivery of the filler – because the cannula holes are not at the tip like a needle – is a drawback, but not a significant one, she added.
Cannulas already have gained popularity in Europe and South America. "We are not learning this as quickly as our colleagues," she said.
"A year ago, I went to an exciting meeting in Paris where I heard a little about cannulas." Dr. Weinkle brought some back to her private practice in Bradenton, Fla., with the best intentions, but did not use them. "This year I went back to the same meeting in January, and I decided I did not want to be left behind."
Filler augmentation of the dorsal side of the hands is another procedure that is well suited to the use of these blunt cannulas, Dr. Weinkle said.
Patience is advised when the technique is tried for the first time. "It's not always easy. You're not going to love it right away. You have to finesse the cannula through the skin." However, she added, "Don't get discouraged."
These are not cannulas that are used for liposuction, but newer products that are specifically designed for use in soft tissue. Only one such cannula – CosmoFrance Inc.'s DermaSculpt microcannula, a 1.5 inch, 27 gauge cannula with a nonbruising blunt tip – is currently approved by the Food and Drug Administration in the United States.
"More are coming. We will have shorter, fatter, thicker, thinner, and different gauge cannulas," Dr. Weinkle said. "We're going to see some of the companies that provide fillers for us adopting this. Maybe we'll find one needle and one cannula in the future in our packaging."
"This is the wave of the future. We really want to embrace new and exciting things," she said.
Dr. Weinkle said she had no relevant financial disclosures.
EXPERT ANALYSIS FROM THE SOUTH BEACH SYMPOSIUM
AAD: Topical Antioxidant Useful for Premature Aging of the Skin
NEW ORLEANS - Nonprescription skin care products formulated with a potent topical antioxidant resulted in objective improvement in prematurely aged skin in a small study of women aged 25 or older.
The antioxidant hydroxydecyl ubiquinoyl dipalmitoyl glycerate (idebenone complex) was incorporated in the skin care products to treat skin photodamage associated with cellular oxidative stress caused by reactive oxygen species, Dr. Michael H. Gold explained at the annual meeting of the American Academy of Dermatology.
He presented an open-label study involving 32 women aged 25-65 years with premature aging of the skin. Twice daily for 8 weeks they used test products containing 0.5% hydroxydecyl ubiquinoyl dipalmitoyl glycerate. Expert grading of various dimensions of skin quality was conducted at baseline and again at 4 and 8 weeks during physical examination and with the assistance of standardized photographs.
The skin care products consisted of a facial cleanser, a moisturizing cream, a skin brightener, and an eye serum. Study participants also used an SPF 30 sunscreen daily.
Skin roughness showed a mean 36% improvement at 8 weeks compared with baseline. Dyschromia also improved by an average of 36%, as did fine lines and wrinkles around the eyes. Skin radiance or glow improved by a mean of 44% over baseline. Skin brightness showed a 42% improvement. Skin tonality improved by an average of 41%, as did skin elasticity, determined by how fast the skin rebounds to the touch, reported Dr. Gold of the Tennessee Clinical Research Center, Nashville.
Overall global improvement was rated at a mean 42% gain, he added.
The study was sponsored by PCR Technology Holdings. Dr. Gold disclosed that he holds relevant intellectual property rights.
NEW ORLEANS - Nonprescription skin care products formulated with a potent topical antioxidant resulted in objective improvement in prematurely aged skin in a small study of women aged 25 or older.
The antioxidant hydroxydecyl ubiquinoyl dipalmitoyl glycerate (idebenone complex) was incorporated in the skin care products to treat skin photodamage associated with cellular oxidative stress caused by reactive oxygen species, Dr. Michael H. Gold explained at the annual meeting of the American Academy of Dermatology.
He presented an open-label study involving 32 women aged 25-65 years with premature aging of the skin. Twice daily for 8 weeks they used test products containing 0.5% hydroxydecyl ubiquinoyl dipalmitoyl glycerate. Expert grading of various dimensions of skin quality was conducted at baseline and again at 4 and 8 weeks during physical examination and with the assistance of standardized photographs.
The skin care products consisted of a facial cleanser, a moisturizing cream, a skin brightener, and an eye serum. Study participants also used an SPF 30 sunscreen daily.
Skin roughness showed a mean 36% improvement at 8 weeks compared with baseline. Dyschromia also improved by an average of 36%, as did fine lines and wrinkles around the eyes. Skin radiance or glow improved by a mean of 44% over baseline. Skin brightness showed a 42% improvement. Skin tonality improved by an average of 41%, as did skin elasticity, determined by how fast the skin rebounds to the touch, reported Dr. Gold of the Tennessee Clinical Research Center, Nashville.
Overall global improvement was rated at a mean 42% gain, he added.
The study was sponsored by PCR Technology Holdings. Dr. Gold disclosed that he holds relevant intellectual property rights.
NEW ORLEANS - Nonprescription skin care products formulated with a potent topical antioxidant resulted in objective improvement in prematurely aged skin in a small study of women aged 25 or older.
The antioxidant hydroxydecyl ubiquinoyl dipalmitoyl glycerate (idebenone complex) was incorporated in the skin care products to treat skin photodamage associated with cellular oxidative stress caused by reactive oxygen species, Dr. Michael H. Gold explained at the annual meeting of the American Academy of Dermatology.
He presented an open-label study involving 32 women aged 25-65 years with premature aging of the skin. Twice daily for 8 weeks they used test products containing 0.5% hydroxydecyl ubiquinoyl dipalmitoyl glycerate. Expert grading of various dimensions of skin quality was conducted at baseline and again at 4 and 8 weeks during physical examination and with the assistance of standardized photographs.
The skin care products consisted of a facial cleanser, a moisturizing cream, a skin brightener, and an eye serum. Study participants also used an SPF 30 sunscreen daily.
Skin roughness showed a mean 36% improvement at 8 weeks compared with baseline. Dyschromia also improved by an average of 36%, as did fine lines and wrinkles around the eyes. Skin radiance or glow improved by a mean of 44% over baseline. Skin brightness showed a 42% improvement. Skin tonality improved by an average of 41%, as did skin elasticity, determined by how fast the skin rebounds to the touch, reported Dr. Gold of the Tennessee Clinical Research Center, Nashville.
Overall global improvement was rated at a mean 42% gain, he added.
The study was sponsored by PCR Technology Holdings. Dr. Gold disclosed that he holds relevant intellectual property rights.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Major Finding: Skin radiance or glow improved by a mean of 44% over baseline, and skin tonality improved by an average of 41%, as did skin elasticity.
Data Source: An open-label study involving 32 women aged 25-65 years with premature aging of the skin who were treated with hydroxydecyl ubiquinoyl dipalmitoyl glycerate (idebenone complex) for 8 weeks.
Disclosures: The study was sponsored by PCR Technology Holdings. Dr. Gold disclosed that he holds relevant intellectual property rights.
Minimally Invasive Cosmetic Surgery Continues to Rise
Interest in cosmetic surgery is increasing as consumers gain confidence in the economy, according to the American Society of Plastic Surgeons.
New statistics from the ASPS show that 13.1 million cosmetic plastic surgery procedures were performed in the United States last year, an increase of 5% over 2009. The increase reflects growth in both surgical procedures such as breast augmentation and minimally-invasive procedures such as injections of Botulinum toxin type A and soft-tissue fillers.
The ASPS statistics come from an annual survey of 747 physicians who perform cosmetic procedures, as well as an online national database for plastic surgery procedures.
"There's some pent up demand for cosmetic surgical procedures," Dr. Phillip Haeck, ASPS president, said in a statement. "People have waited a couple of years or more to have procedures, until their finances were at least somewhat back in order. But all indications are [that] more consumers are again willing to spend more to look better."
There are a number of factors potentially driving the increase in cosmetic surgery, Dr. Haeck said, from the improved economy to aging baby boomers seeking aesthetic procedures to stay competitive in the workplace.
Minimally-invasive cosmetic procedures showed the biggest increases in 2010 with nearly 11.6 million procedures performed, up from about 11 million the previous year. As in previous years, Botulinum toxin type A led the pack with 5.4 million procedures. Soft-tissue fillers were also popular with 1.8 million procedures. Rounding out the top five minimally-invasive procedures in 2010 were chemical peels, laser hair removal, and microdermabrasion.
"Injectables have remained robust despite the economy," Dr. Haeck said. "Botox and Dysport injections are up 12%, while, interestingly, fat injections are up 14%, which could reflect how a patient's own fat is being used in more creative ways to rejuvenate the face and body."
While the number of surgical procedures increased in 2010, the same types of procedures remained popular. The top five surgical procedures in 2010 were breast augmentation, rhinoplasty, eyelid surgery, liposuction, and abdominoplasty.
The ASPS also reported figures on reconstructive plastic surgery procedures. In 2010, more than 5.3 million reconstructive procedures were performed, up 2% from the previous year. Tumor removal led the list with 4 million procedures. Laceration repair, scar revision, hand surgery, and breast reconstruction were also in the top five.
Interest in cosmetic surgery is increasing as consumers gain confidence in the economy, according to the American Society of Plastic Surgeons.
New statistics from the ASPS show that 13.1 million cosmetic plastic surgery procedures were performed in the United States last year, an increase of 5% over 2009. The increase reflects growth in both surgical procedures such as breast augmentation and minimally-invasive procedures such as injections of Botulinum toxin type A and soft-tissue fillers.
The ASPS statistics come from an annual survey of 747 physicians who perform cosmetic procedures, as well as an online national database for plastic surgery procedures.
"There's some pent up demand for cosmetic surgical procedures," Dr. Phillip Haeck, ASPS president, said in a statement. "People have waited a couple of years or more to have procedures, until their finances were at least somewhat back in order. But all indications are [that] more consumers are again willing to spend more to look better."
There are a number of factors potentially driving the increase in cosmetic surgery, Dr. Haeck said, from the improved economy to aging baby boomers seeking aesthetic procedures to stay competitive in the workplace.
Minimally-invasive cosmetic procedures showed the biggest increases in 2010 with nearly 11.6 million procedures performed, up from about 11 million the previous year. As in previous years, Botulinum toxin type A led the pack with 5.4 million procedures. Soft-tissue fillers were also popular with 1.8 million procedures. Rounding out the top five minimally-invasive procedures in 2010 were chemical peels, laser hair removal, and microdermabrasion.
"Injectables have remained robust despite the economy," Dr. Haeck said. "Botox and Dysport injections are up 12%, while, interestingly, fat injections are up 14%, which could reflect how a patient's own fat is being used in more creative ways to rejuvenate the face and body."
While the number of surgical procedures increased in 2010, the same types of procedures remained popular. The top five surgical procedures in 2010 were breast augmentation, rhinoplasty, eyelid surgery, liposuction, and abdominoplasty.
The ASPS also reported figures on reconstructive plastic surgery procedures. In 2010, more than 5.3 million reconstructive procedures were performed, up 2% from the previous year. Tumor removal led the list with 4 million procedures. Laceration repair, scar revision, hand surgery, and breast reconstruction were also in the top five.
Interest in cosmetic surgery is increasing as consumers gain confidence in the economy, according to the American Society of Plastic Surgeons.
New statistics from the ASPS show that 13.1 million cosmetic plastic surgery procedures were performed in the United States last year, an increase of 5% over 2009. The increase reflects growth in both surgical procedures such as breast augmentation and minimally-invasive procedures such as injections of Botulinum toxin type A and soft-tissue fillers.
The ASPS statistics come from an annual survey of 747 physicians who perform cosmetic procedures, as well as an online national database for plastic surgery procedures.
"There's some pent up demand for cosmetic surgical procedures," Dr. Phillip Haeck, ASPS president, said in a statement. "People have waited a couple of years or more to have procedures, until their finances were at least somewhat back in order. But all indications are [that] more consumers are again willing to spend more to look better."
There are a number of factors potentially driving the increase in cosmetic surgery, Dr. Haeck said, from the improved economy to aging baby boomers seeking aesthetic procedures to stay competitive in the workplace.
Minimally-invasive cosmetic procedures showed the biggest increases in 2010 with nearly 11.6 million procedures performed, up from about 11 million the previous year. As in previous years, Botulinum toxin type A led the pack with 5.4 million procedures. Soft-tissue fillers were also popular with 1.8 million procedures. Rounding out the top five minimally-invasive procedures in 2010 were chemical peels, laser hair removal, and microdermabrasion.
"Injectables have remained robust despite the economy," Dr. Haeck said. "Botox and Dysport injections are up 12%, while, interestingly, fat injections are up 14%, which could reflect how a patient's own fat is being used in more creative ways to rejuvenate the face and body."
While the number of surgical procedures increased in 2010, the same types of procedures remained popular. The top five surgical procedures in 2010 were breast augmentation, rhinoplasty, eyelid surgery, liposuction, and abdominoplasty.
The ASPS also reported figures on reconstructive plastic surgery procedures. In 2010, more than 5.3 million reconstructive procedures were performed, up 2% from the previous year. Tumor removal led the list with 4 million procedures. Laceration repair, scar revision, hand surgery, and breast reconstruction were also in the top five.
Cosmetic Industry Leading Nanotechnology Market
ORLANDO – "The diameter of a single hair shaft is tens of thousands of nanometers," said Dr. Adam Friedman. Nanotechnology is the branch of technology related to dimensions and tolerances ranging from 0.1 to 100 nanometers.
"At this size, matter behaves somewhat differently," Dr. Friedman, of Albert Einstein College of Medicine in New York, said. As the size of material decreases, the surface area relative to volume decreases. There is more surface to interact with the environment.
"The three properties of matter – chemical, optical, and physical – can be manipulated and exploited at the nano scale," Dr. Friedman said. For example, something that is too bulky at the macro level to go into an aqueous vehicle can be distributed more easily in nano form. And "if something is smaller than the wavelength of visible light, guess what? It is going to be invisible," he said at the Orlando Dermatology Aesthetic and Clinical Conference.
By the year 2012, nanotechnology is predicted to be a $12 billion industry in the United States, and the cosmetic and cosmeceutical industries are leading the way, Dr. Friedman noted. He discussed several nanomaterials with diagnostic and therapeutic applications for dermatologists:
• Nanoparticles. The term nanoparticle is somewhat generic, Dr. Friedman said. The term refers to a small object that behaves as a whole unit in terms of its transport and properties. Nanoparticles can be derived from organic and nonorganic materials. For example, gold nanoparticles can be used to introduce an antibody or targeting molecule into the body to target tumors. Once the tumors are bound to the gold, they can be treated using selective photolysis, in which radiation is used to heat the gold enough to kill the tumor cells. In one study of mice, hollow gold nanoparticles were used to successfully treat melanoma, said Dr. Friedman. Silver nanoparticles are already in products ranging from clothing to plastic food storage containers, to take advantage of their antimicrobial properties, he said.
• Nanoemulsions. Nanoemulsions are already widely used in dermatology, in emollients, and as delivery vehicles for antiaging products. Nanoemulsions have an appealing nongreasy texture, are invisible, and penetrate the skin rapidly, Dr. Friedman said. Nanoemulsion products currently on the market include L’Oréal Plenitude Revitalift and Caudalie Vinosun Anti-Aging Suncare.
• Quantum dots. "These highly fluorescent nanoscale crystals absorb a broad range of wavelengths; however, they only re-emit one color," said Dr. Friedman. In dermatology, quantum dots are being used to identify sentinel lymph nodes in patients with melanoma and Merkel cell carcinoma.
• Nanomagnets. Nanosized magnetic materials "no longer exhibit a net magnetic force," Dr. Friedman said. These materials could be used to create magnetic field–directed imaging or therapy.
• Nanopigments. Many currently available sunblocks include nanoparticles of titanium or zinc oxide, such as SunVex Dailywear lotions and ZinClear Nano Zinc Oxide.
About safety: "From a purely theoretical standpoint, nanoparticles should be harmful," said Dr. Friedman. The same properties that make nanoparticles useful could come with side effects. Improved skin penetration can be beneficial for dermatology, but factors that determine the potential toxicity of nanoparticles include size, chemical purity, and the activity of the surface.
The current international stance on nanoparticle safety is that it is unlikely that significant amounts of the zinc or titanium used in sunblock products will result in local or systemic toxicity. However, "the safety of nanoscale zinc and titanium in sunscreen must be fully addressed," Dr. Friedman said. In 2009, the American Academy of Dermatology established a task force to study nanotechnology and educate the dermatology community, the public, and policy makers.
Dermatologists who are intrigued by the potential of nanotechnology can join the fledgling Nanodermatology Society, which had its first meeting for the 2011 AAD annual meeting in New Orleans. For more information, visit the society's Web site at www.nanodermsociety.org.
Dr. Friedman serves on the advisory board of Makefield Therapeutics.
ORLANDO – "The diameter of a single hair shaft is tens of thousands of nanometers," said Dr. Adam Friedman. Nanotechnology is the branch of technology related to dimensions and tolerances ranging from 0.1 to 100 nanometers.
"At this size, matter behaves somewhat differently," Dr. Friedman, of Albert Einstein College of Medicine in New York, said. As the size of material decreases, the surface area relative to volume decreases. There is more surface to interact with the environment.
"The three properties of matter – chemical, optical, and physical – can be manipulated and exploited at the nano scale," Dr. Friedman said. For example, something that is too bulky at the macro level to go into an aqueous vehicle can be distributed more easily in nano form. And "if something is smaller than the wavelength of visible light, guess what? It is going to be invisible," he said at the Orlando Dermatology Aesthetic and Clinical Conference.
By the year 2012, nanotechnology is predicted to be a $12 billion industry in the United States, and the cosmetic and cosmeceutical industries are leading the way, Dr. Friedman noted. He discussed several nanomaterials with diagnostic and therapeutic applications for dermatologists:
• Nanoparticles. The term nanoparticle is somewhat generic, Dr. Friedman said. The term refers to a small object that behaves as a whole unit in terms of its transport and properties. Nanoparticles can be derived from organic and nonorganic materials. For example, gold nanoparticles can be used to introduce an antibody or targeting molecule into the body to target tumors. Once the tumors are bound to the gold, they can be treated using selective photolysis, in which radiation is used to heat the gold enough to kill the tumor cells. In one study of mice, hollow gold nanoparticles were used to successfully treat melanoma, said Dr. Friedman. Silver nanoparticles are already in products ranging from clothing to plastic food storage containers, to take advantage of their antimicrobial properties, he said.
• Nanoemulsions. Nanoemulsions are already widely used in dermatology, in emollients, and as delivery vehicles for antiaging products. Nanoemulsions have an appealing nongreasy texture, are invisible, and penetrate the skin rapidly, Dr. Friedman said. Nanoemulsion products currently on the market include L’Oréal Plenitude Revitalift and Caudalie Vinosun Anti-Aging Suncare.
• Quantum dots. "These highly fluorescent nanoscale crystals absorb a broad range of wavelengths; however, they only re-emit one color," said Dr. Friedman. In dermatology, quantum dots are being used to identify sentinel lymph nodes in patients with melanoma and Merkel cell carcinoma.
• Nanomagnets. Nanosized magnetic materials "no longer exhibit a net magnetic force," Dr. Friedman said. These materials could be used to create magnetic field–directed imaging or therapy.
• Nanopigments. Many currently available sunblocks include nanoparticles of titanium or zinc oxide, such as SunVex Dailywear lotions and ZinClear Nano Zinc Oxide.
About safety: "From a purely theoretical standpoint, nanoparticles should be harmful," said Dr. Friedman. The same properties that make nanoparticles useful could come with side effects. Improved skin penetration can be beneficial for dermatology, but factors that determine the potential toxicity of nanoparticles include size, chemical purity, and the activity of the surface.
The current international stance on nanoparticle safety is that it is unlikely that significant amounts of the zinc or titanium used in sunblock products will result in local or systemic toxicity. However, "the safety of nanoscale zinc and titanium in sunscreen must be fully addressed," Dr. Friedman said. In 2009, the American Academy of Dermatology established a task force to study nanotechnology and educate the dermatology community, the public, and policy makers.
Dermatologists who are intrigued by the potential of nanotechnology can join the fledgling Nanodermatology Society, which had its first meeting for the 2011 AAD annual meeting in New Orleans. For more information, visit the society's Web site at www.nanodermsociety.org.
Dr. Friedman serves on the advisory board of Makefield Therapeutics.
ORLANDO – "The diameter of a single hair shaft is tens of thousands of nanometers," said Dr. Adam Friedman. Nanotechnology is the branch of technology related to dimensions and tolerances ranging from 0.1 to 100 nanometers.
"At this size, matter behaves somewhat differently," Dr. Friedman, of Albert Einstein College of Medicine in New York, said. As the size of material decreases, the surface area relative to volume decreases. There is more surface to interact with the environment.
"The three properties of matter – chemical, optical, and physical – can be manipulated and exploited at the nano scale," Dr. Friedman said. For example, something that is too bulky at the macro level to go into an aqueous vehicle can be distributed more easily in nano form. And "if something is smaller than the wavelength of visible light, guess what? It is going to be invisible," he said at the Orlando Dermatology Aesthetic and Clinical Conference.
By the year 2012, nanotechnology is predicted to be a $12 billion industry in the United States, and the cosmetic and cosmeceutical industries are leading the way, Dr. Friedman noted. He discussed several nanomaterials with diagnostic and therapeutic applications for dermatologists:
• Nanoparticles. The term nanoparticle is somewhat generic, Dr. Friedman said. The term refers to a small object that behaves as a whole unit in terms of its transport and properties. Nanoparticles can be derived from organic and nonorganic materials. For example, gold nanoparticles can be used to introduce an antibody or targeting molecule into the body to target tumors. Once the tumors are bound to the gold, they can be treated using selective photolysis, in which radiation is used to heat the gold enough to kill the tumor cells. In one study of mice, hollow gold nanoparticles were used to successfully treat melanoma, said Dr. Friedman. Silver nanoparticles are already in products ranging from clothing to plastic food storage containers, to take advantage of their antimicrobial properties, he said.
• Nanoemulsions. Nanoemulsions are already widely used in dermatology, in emollients, and as delivery vehicles for antiaging products. Nanoemulsions have an appealing nongreasy texture, are invisible, and penetrate the skin rapidly, Dr. Friedman said. Nanoemulsion products currently on the market include L’Oréal Plenitude Revitalift and Caudalie Vinosun Anti-Aging Suncare.
• Quantum dots. "These highly fluorescent nanoscale crystals absorb a broad range of wavelengths; however, they only re-emit one color," said Dr. Friedman. In dermatology, quantum dots are being used to identify sentinel lymph nodes in patients with melanoma and Merkel cell carcinoma.
• Nanomagnets. Nanosized magnetic materials "no longer exhibit a net magnetic force," Dr. Friedman said. These materials could be used to create magnetic field–directed imaging or therapy.
• Nanopigments. Many currently available sunblocks include nanoparticles of titanium or zinc oxide, such as SunVex Dailywear lotions and ZinClear Nano Zinc Oxide.
About safety: "From a purely theoretical standpoint, nanoparticles should be harmful," said Dr. Friedman. The same properties that make nanoparticles useful could come with side effects. Improved skin penetration can be beneficial for dermatology, but factors that determine the potential toxicity of nanoparticles include size, chemical purity, and the activity of the surface.
The current international stance on nanoparticle safety is that it is unlikely that significant amounts of the zinc or titanium used in sunblock products will result in local or systemic toxicity. However, "the safety of nanoscale zinc and titanium in sunscreen must be fully addressed," Dr. Friedman said. In 2009, the American Academy of Dermatology established a task force to study nanotechnology and educate the dermatology community, the public, and policy makers.
Dermatologists who are intrigued by the potential of nanotechnology can join the fledgling Nanodermatology Society, which had its first meeting for the 2011 AAD annual meeting in New Orleans. For more information, visit the society's Web site at www.nanodermsociety.org.
Dr. Friedman serves on the advisory board of Makefield Therapeutics.
EXPERT ANALYSIS FROM THE ORLANDO DERMATOLOGY AESTHETIC AND CLINICAL CONFERENCE
Expert Recommends Foam Sclerotherapy for Varicose Veins
ORLANDO - Foam sclerotherapy can be an effective treatment for varicose veins ranging from 1 mm to more than 5 mm in diameter, said Dr. Hema Sundaram, medical director of Sundaram Dermatology, Cosmetic & Laser Surgery in Rockville, Md.
As many as 20% of adults in the United States and Western Europe have varicose veins, and many of them can be successfully treated in a dermatology practice. Her treatment of choice for sclerotherapy is polidocanol, which was approved by the Food and Drug Administration last year for treating varicose veins. Polidocanol has been studied more extensively than any other sclerosant, noted Dr. Sundaram, who is in private practice in Rockville. The adverse events are low, allergic reactions are rare, and patients report less pain compared with other sclerosants.
She said that she successfully uses polidocanol for foam sclerotherapy. The best candidates for this off-label use are patients who have veins with varicosities from 1 mm to greater than 5 mm in diameter. For telangiectatic veins up to 1 mm in diameter, she prefers liquid polidocanol. "The foam can be a bit traumatic for the smaller veins," she said.
To treat varicose veins with foam, mix air with the sclerosant in a sclerosant:air ratio of 1:3 or 1:4, said Dr. Sundaram. She uses a three-way stopcock and a double syringe to combine the air and sclerosant. The foam in the syringe should resemble shaving foam.
Maintain a maximum foam volume of 10 mL per leg per session to minimize the risk of deep vein thrombosis, she noted.
"The important thing is to use the foam quickly, because the air will dissipate," Dr. Sundaram said. Stop injecting the foam when the flow of sclerosant into superficial veins is no longer visible.
For the right-sized veins, foam sclerotherapy can displace the blood more efficiently and put more of the sclerosant in contact with the vascular endothelial wall, Dr. Sundaram said.
Foam sclerotherapy has demonstrated safety and efficacy. In a review of data from 325 patients who were treated at a single center over 7 years, patients rated improvement as 1.94 on a scale of 3, while rating pain at 0.22, ulceration at 0.06, and hyperpigmentation at 0.35 (Dermatol. Surg. 2010;36[suppl. 2]:1026-33).
Dr. Sundaram’s foam sclerotherapy procedure is as follows:
• Inject while the patient is lying down.
• Start proximally and move distally; some smaller veins can be filled through larger ones.
• Start with the largest veins and work down.
• After injecting the sclerosant and removing the needle, compress the entire vein.
• Direct the patient to used graduated leg compression with bandages or stockings for 3 days to 3 weeks after the procedure.
Because foam sclerotherapy is an off-label use, be sure to include this fact in the informed consent form that patients sign, Dr. Sundaram noted.
Pretreatment evaluation is as important for a sclerotherapy patient as it is for a patient undergoing any other type of aesthetic procedure. "The assessment determines how successful we are going to be," she said.
She recommended an ultrasound evaluation prior to sclerotherapy in order to locate the reflux areas and to identify any insufficiency in the greater saphenous vein. Patients with greater saphenous vein insufficiency tend not to respond well to sclerotherapy and are more likely to experience recurrence of varicosities, she explained. In addition, these patients are at increased risk of superficial thrombophlebitis. Dr. Sundaram refers these patients to a vascular surgeon for evaluation and treatment.
Contraindications for sclerotherapy include allergy to the sclerosing agent, acute deep vein thrombosis, infection, and significant leg swelling, as well as pregnancy, polyneuropathy, severe asthma, and hypercoagulability.
Dr. Sundaram serves as an advisor, consultant, and/or clinical investigator for the following companies: Johnson & Johnson, Merz Aesthetics, Biopelle, Colorescience, Medicis, Merz, SkinMedica, Suneva Medical, Syneron/Candela, and Ulthera.
ORLANDO - Foam sclerotherapy can be an effective treatment for varicose veins ranging from 1 mm to more than 5 mm in diameter, said Dr. Hema Sundaram, medical director of Sundaram Dermatology, Cosmetic & Laser Surgery in Rockville, Md.
As many as 20% of adults in the United States and Western Europe have varicose veins, and many of them can be successfully treated in a dermatology practice. Her treatment of choice for sclerotherapy is polidocanol, which was approved by the Food and Drug Administration last year for treating varicose veins. Polidocanol has been studied more extensively than any other sclerosant, noted Dr. Sundaram, who is in private practice in Rockville. The adverse events are low, allergic reactions are rare, and patients report less pain compared with other sclerosants.
She said that she successfully uses polidocanol for foam sclerotherapy. The best candidates for this off-label use are patients who have veins with varicosities from 1 mm to greater than 5 mm in diameter. For telangiectatic veins up to 1 mm in diameter, she prefers liquid polidocanol. "The foam can be a bit traumatic for the smaller veins," she said.
To treat varicose veins with foam, mix air with the sclerosant in a sclerosant:air ratio of 1:3 or 1:4, said Dr. Sundaram. She uses a three-way stopcock and a double syringe to combine the air and sclerosant. The foam in the syringe should resemble shaving foam.
Maintain a maximum foam volume of 10 mL per leg per session to minimize the risk of deep vein thrombosis, she noted.
"The important thing is to use the foam quickly, because the air will dissipate," Dr. Sundaram said. Stop injecting the foam when the flow of sclerosant into superficial veins is no longer visible.
For the right-sized veins, foam sclerotherapy can displace the blood more efficiently and put more of the sclerosant in contact with the vascular endothelial wall, Dr. Sundaram said.
Foam sclerotherapy has demonstrated safety and efficacy. In a review of data from 325 patients who were treated at a single center over 7 years, patients rated improvement as 1.94 on a scale of 3, while rating pain at 0.22, ulceration at 0.06, and hyperpigmentation at 0.35 (Dermatol. Surg. 2010;36[suppl. 2]:1026-33).
Dr. Sundaram’s foam sclerotherapy procedure is as follows:
• Inject while the patient is lying down.
• Start proximally and move distally; some smaller veins can be filled through larger ones.
• Start with the largest veins and work down.
• After injecting the sclerosant and removing the needle, compress the entire vein.
• Direct the patient to used graduated leg compression with bandages or stockings for 3 days to 3 weeks after the procedure.
Because foam sclerotherapy is an off-label use, be sure to include this fact in the informed consent form that patients sign, Dr. Sundaram noted.
Pretreatment evaluation is as important for a sclerotherapy patient as it is for a patient undergoing any other type of aesthetic procedure. "The assessment determines how successful we are going to be," she said.
She recommended an ultrasound evaluation prior to sclerotherapy in order to locate the reflux areas and to identify any insufficiency in the greater saphenous vein. Patients with greater saphenous vein insufficiency tend not to respond well to sclerotherapy and are more likely to experience recurrence of varicosities, she explained. In addition, these patients are at increased risk of superficial thrombophlebitis. Dr. Sundaram refers these patients to a vascular surgeon for evaluation and treatment.
Contraindications for sclerotherapy include allergy to the sclerosing agent, acute deep vein thrombosis, infection, and significant leg swelling, as well as pregnancy, polyneuropathy, severe asthma, and hypercoagulability.
Dr. Sundaram serves as an advisor, consultant, and/or clinical investigator for the following companies: Johnson & Johnson, Merz Aesthetics, Biopelle, Colorescience, Medicis, Merz, SkinMedica, Suneva Medical, Syneron/Candela, and Ulthera.
ORLANDO - Foam sclerotherapy can be an effective treatment for varicose veins ranging from 1 mm to more than 5 mm in diameter, said Dr. Hema Sundaram, medical director of Sundaram Dermatology, Cosmetic & Laser Surgery in Rockville, Md.
As many as 20% of adults in the United States and Western Europe have varicose veins, and many of them can be successfully treated in a dermatology practice. Her treatment of choice for sclerotherapy is polidocanol, which was approved by the Food and Drug Administration last year for treating varicose veins. Polidocanol has been studied more extensively than any other sclerosant, noted Dr. Sundaram, who is in private practice in Rockville. The adverse events are low, allergic reactions are rare, and patients report less pain compared with other sclerosants.
She said that she successfully uses polidocanol for foam sclerotherapy. The best candidates for this off-label use are patients who have veins with varicosities from 1 mm to greater than 5 mm in diameter. For telangiectatic veins up to 1 mm in diameter, she prefers liquid polidocanol. "The foam can be a bit traumatic for the smaller veins," she said.
To treat varicose veins with foam, mix air with the sclerosant in a sclerosant:air ratio of 1:3 or 1:4, said Dr. Sundaram. She uses a three-way stopcock and a double syringe to combine the air and sclerosant. The foam in the syringe should resemble shaving foam.
Maintain a maximum foam volume of 10 mL per leg per session to minimize the risk of deep vein thrombosis, she noted.
"The important thing is to use the foam quickly, because the air will dissipate," Dr. Sundaram said. Stop injecting the foam when the flow of sclerosant into superficial veins is no longer visible.
For the right-sized veins, foam sclerotherapy can displace the blood more efficiently and put more of the sclerosant in contact with the vascular endothelial wall, Dr. Sundaram said.
Foam sclerotherapy has demonstrated safety and efficacy. In a review of data from 325 patients who were treated at a single center over 7 years, patients rated improvement as 1.94 on a scale of 3, while rating pain at 0.22, ulceration at 0.06, and hyperpigmentation at 0.35 (Dermatol. Surg. 2010;36[suppl. 2]:1026-33).
Dr. Sundaram’s foam sclerotherapy procedure is as follows:
• Inject while the patient is lying down.
• Start proximally and move distally; some smaller veins can be filled through larger ones.
• Start with the largest veins and work down.
• After injecting the sclerosant and removing the needle, compress the entire vein.
• Direct the patient to used graduated leg compression with bandages or stockings for 3 days to 3 weeks after the procedure.
Because foam sclerotherapy is an off-label use, be sure to include this fact in the informed consent form that patients sign, Dr. Sundaram noted.
Pretreatment evaluation is as important for a sclerotherapy patient as it is for a patient undergoing any other type of aesthetic procedure. "The assessment determines how successful we are going to be," she said.
She recommended an ultrasound evaluation prior to sclerotherapy in order to locate the reflux areas and to identify any insufficiency in the greater saphenous vein. Patients with greater saphenous vein insufficiency tend not to respond well to sclerotherapy and are more likely to experience recurrence of varicosities, she explained. In addition, these patients are at increased risk of superficial thrombophlebitis. Dr. Sundaram refers these patients to a vascular surgeon for evaluation and treatment.
Contraindications for sclerotherapy include allergy to the sclerosing agent, acute deep vein thrombosis, infection, and significant leg swelling, as well as pregnancy, polyneuropathy, severe asthma, and hypercoagulability.
Dr. Sundaram serves as an advisor, consultant, and/or clinical investigator for the following companies: Johnson & Johnson, Merz Aesthetics, Biopelle, Colorescience, Medicis, Merz, SkinMedica, Suneva Medical, Syneron/Candela, and Ulthera.
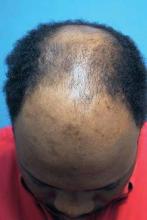
Future Technologies Hold Promise for Hair Restoration
ORLANDO - Expect clinical options for hair restoration to grow in the future, said Dr. Ricardo Mejia.
Robotic hair transfer, multiple technologies to optimize new growth, and even hair cloning could help overcome current limitations in hair transplantation, Dr. Mejia said. Promising technologies could someday supplant donor strip and follicular unit extraction techniques. "We are getting to the age of robotics," Dr. Mejia said at the annual meeting of the Florida Society of Dermatologic Surgeons.
Historically, hair transplantation meant 4-mm plugs transferred at a rate of 10-200 grafts per session over a total of three to eight treatments. Because plugs were placed in a regular pattern, initial results appeared unnatural and very obvious. For some patients, a perception persists that this is still state-of-the-art for hair transplantation, Dr. Mejia said.
A natural, irregular hairline and greater hair density in fewer treatment sessions are now commonplace. "The average session these days of 2,500 grafts is not a big deal," Dr. Mejia said at the meeting.
"Restoring youthful hairlines can be done in single sessions. ... You can get a nice, age-appropriate appearance for an individual," said Dr. Mejia, a hair transplant surgeon in private practice in Jupiter, Fla. Even with recent advances, full growth of hair grafts still takes 6 months to a year, so realistic patient expectations are important.
A new device, NeoGraft Automated Hair Transplant System (NeoGraft), was cleared for marketing by the Food and Drug Administration in March 2009. After a rotating sharp punch scores the skin, a pneumatic suction device extracts the follicles. This technique minimizes injury to the lower half of hair follicles during follicular unit extraction, Dr. Mejia said. The device also implants grafts to a uniform depth.
Researchers are working on a variety of other means to protect grafts during the transfer process. For example, some are developing solutions to protect grafts that contain allopurinol, nitric oxide inhibitors, vitamins, and other components. Also, "we are starting to look at solutions used in organ transplantation." Small studies have shown increased hair survival and growth with these solutions used to optimize protection of organs during transfer, Dr. Mejia said.
Bathing follicular units in autologous platelet-rich plasma to promote healing angiogenesis is another approach. Growth factor components also could be beneficial, Dr. Mejia said. More studies are needed to determine the efficacy of injections of autologous platelet-rich plasma into both the donor area and the recipient areas in clinical practice.
Matching the size of the incision blade to the graft size can also help improve graft survival, Dr. Mejia said. Less trauma, less ischemia, and decreased overall bleeding are associated with finer blades. Although finer blades allow higher-density graft packing, he advised caution because some studies have shown more graft death with higher densities.
Investigators also are looking at technology to optimize new hair growth once the grafts are in place.
"Low-level lasers are getting a lot of attention," Dr. Mejia said. Wavelengths are in the range of 630-670 nm, power densities are between 5-50 mW/cm2, and fluences are 2-20 J/cm2.
The Food and Drug Administration cleared two devices that use low-level light therapy for hair growth: HairMax LaserComb (Lexington International) for men and the MPE-90 Hair Growth Stimulation System (Salon Lasers) for women.
"How good is the HairMax comb?" a meeting attendee asked. Dr. Mejia replied that reviews are mixed: "Hair restoration surgeons are on the fence – some believe in it, some don’t. Some patients are happy with it, some are not."
A lot of research also is underway to refine auto cloning and hair multiplication technologies, Dr. Mejia said.
Dermal papilla cells or fibroblasts are the starting point, because they stimulate formation of new hairs. Multiple companies are working on proprietary processes to spur these fibroblasts to produce enough follicles in culture to replace an entire scalp. This is where they hope "to make their windfall," he said. Research includes fibroblasts grown in subatmospheric oxygen tension, addition of wound-healing factors, and injections of a "hair-stimulating complex" to promote greater hair growth.
TrichoCyte is an example of a cell-based hair regeneration technology in phase II trials based on a proprietary dermal papilla cell process (Intercytex, Manchester, England). "The technique does work but [it is] not completely satisfactory at this point," Dr. Mejia said.
More than half of participants in one protocol for another proprietary cell treatment process showed significant hair growth 1 year later, according to a release announcing phase II study results for Aderans Research Institute.
Considerable work remains to be done before regenerative medical hair cloning becomes a clinically viable option, Dr. Mejia said. "How far out are we? I say 5-10 years."
Dr. Mejia said he had no relevant disclosures.
ORLANDO - Expect clinical options for hair restoration to grow in the future, said Dr. Ricardo Mejia.
Robotic hair transfer, multiple technologies to optimize new growth, and even hair cloning could help overcome current limitations in hair transplantation, Dr. Mejia said. Promising technologies could someday supplant donor strip and follicular unit extraction techniques. "We are getting to the age of robotics," Dr. Mejia said at the annual meeting of the Florida Society of Dermatologic Surgeons.
Historically, hair transplantation meant 4-mm plugs transferred at a rate of 10-200 grafts per session over a total of three to eight treatments. Because plugs were placed in a regular pattern, initial results appeared unnatural and very obvious. For some patients, a perception persists that this is still state-of-the-art for hair transplantation, Dr. Mejia said.
A natural, irregular hairline and greater hair density in fewer treatment sessions are now commonplace. "The average session these days of 2,500 grafts is not a big deal," Dr. Mejia said at the meeting.
"Restoring youthful hairlines can be done in single sessions. ... You can get a nice, age-appropriate appearance for an individual," said Dr. Mejia, a hair transplant surgeon in private practice in Jupiter, Fla. Even with recent advances, full growth of hair grafts still takes 6 months to a year, so realistic patient expectations are important.
A new device, NeoGraft Automated Hair Transplant System (NeoGraft), was cleared for marketing by the Food and Drug Administration in March 2009. After a rotating sharp punch scores the skin, a pneumatic suction device extracts the follicles. This technique minimizes injury to the lower half of hair follicles during follicular unit extraction, Dr. Mejia said. The device also implants grafts to a uniform depth.
Researchers are working on a variety of other means to protect grafts during the transfer process. For example, some are developing solutions to protect grafts that contain allopurinol, nitric oxide inhibitors, vitamins, and other components. Also, "we are starting to look at solutions used in organ transplantation." Small studies have shown increased hair survival and growth with these solutions used to optimize protection of organs during transfer, Dr. Mejia said.
Bathing follicular units in autologous platelet-rich plasma to promote healing angiogenesis is another approach. Growth factor components also could be beneficial, Dr. Mejia said. More studies are needed to determine the efficacy of injections of autologous platelet-rich plasma into both the donor area and the recipient areas in clinical practice.
Matching the size of the incision blade to the graft size can also help improve graft survival, Dr. Mejia said. Less trauma, less ischemia, and decreased overall bleeding are associated with finer blades. Although finer blades allow higher-density graft packing, he advised caution because some studies have shown more graft death with higher densities.
Investigators also are looking at technology to optimize new hair growth once the grafts are in place.
"Low-level lasers are getting a lot of attention," Dr. Mejia said. Wavelengths are in the range of 630-670 nm, power densities are between 5-50 mW/cm2, and fluences are 2-20 J/cm2.
The Food and Drug Administration cleared two devices that use low-level light therapy for hair growth: HairMax LaserComb (Lexington International) for men and the MPE-90 Hair Growth Stimulation System (Salon Lasers) for women.
"How good is the HairMax comb?" a meeting attendee asked. Dr. Mejia replied that reviews are mixed: "Hair restoration surgeons are on the fence – some believe in it, some don’t. Some patients are happy with it, some are not."
A lot of research also is underway to refine auto cloning and hair multiplication technologies, Dr. Mejia said.
Dermal papilla cells or fibroblasts are the starting point, because they stimulate formation of new hairs. Multiple companies are working on proprietary processes to spur these fibroblasts to produce enough follicles in culture to replace an entire scalp. This is where they hope "to make their windfall," he said. Research includes fibroblasts grown in subatmospheric oxygen tension, addition of wound-healing factors, and injections of a "hair-stimulating complex" to promote greater hair growth.
TrichoCyte is an example of a cell-based hair regeneration technology in phase II trials based on a proprietary dermal papilla cell process (Intercytex, Manchester, England). "The technique does work but [it is] not completely satisfactory at this point," Dr. Mejia said.
More than half of participants in one protocol for another proprietary cell treatment process showed significant hair growth 1 year later, according to a release announcing phase II study results for Aderans Research Institute.
Considerable work remains to be done before regenerative medical hair cloning becomes a clinically viable option, Dr. Mejia said. "How far out are we? I say 5-10 years."
Dr. Mejia said he had no relevant disclosures.
ORLANDO - Expect clinical options for hair restoration to grow in the future, said Dr. Ricardo Mejia.
Robotic hair transfer, multiple technologies to optimize new growth, and even hair cloning could help overcome current limitations in hair transplantation, Dr. Mejia said. Promising technologies could someday supplant donor strip and follicular unit extraction techniques. "We are getting to the age of robotics," Dr. Mejia said at the annual meeting of the Florida Society of Dermatologic Surgeons.
Historically, hair transplantation meant 4-mm plugs transferred at a rate of 10-200 grafts per session over a total of three to eight treatments. Because plugs were placed in a regular pattern, initial results appeared unnatural and very obvious. For some patients, a perception persists that this is still state-of-the-art for hair transplantation, Dr. Mejia said.
A natural, irregular hairline and greater hair density in fewer treatment sessions are now commonplace. "The average session these days of 2,500 grafts is not a big deal," Dr. Mejia said at the meeting.
"Restoring youthful hairlines can be done in single sessions. ... You can get a nice, age-appropriate appearance for an individual," said Dr. Mejia, a hair transplant surgeon in private practice in Jupiter, Fla. Even with recent advances, full growth of hair grafts still takes 6 months to a year, so realistic patient expectations are important.
A new device, NeoGraft Automated Hair Transplant System (NeoGraft), was cleared for marketing by the Food and Drug Administration in March 2009. After a rotating sharp punch scores the skin, a pneumatic suction device extracts the follicles. This technique minimizes injury to the lower half of hair follicles during follicular unit extraction, Dr. Mejia said. The device also implants grafts to a uniform depth.
Researchers are working on a variety of other means to protect grafts during the transfer process. For example, some are developing solutions to protect grafts that contain allopurinol, nitric oxide inhibitors, vitamins, and other components. Also, "we are starting to look at solutions used in organ transplantation." Small studies have shown increased hair survival and growth with these solutions used to optimize protection of organs during transfer, Dr. Mejia said.
Bathing follicular units in autologous platelet-rich plasma to promote healing angiogenesis is another approach. Growth factor components also could be beneficial, Dr. Mejia said. More studies are needed to determine the efficacy of injections of autologous platelet-rich plasma into both the donor area and the recipient areas in clinical practice.
Matching the size of the incision blade to the graft size can also help improve graft survival, Dr. Mejia said. Less trauma, less ischemia, and decreased overall bleeding are associated with finer blades. Although finer blades allow higher-density graft packing, he advised caution because some studies have shown more graft death with higher densities.
Investigators also are looking at technology to optimize new hair growth once the grafts are in place.
"Low-level lasers are getting a lot of attention," Dr. Mejia said. Wavelengths are in the range of 630-670 nm, power densities are between 5-50 mW/cm2, and fluences are 2-20 J/cm2.
The Food and Drug Administration cleared two devices that use low-level light therapy for hair growth: HairMax LaserComb (Lexington International) for men and the MPE-90 Hair Growth Stimulation System (Salon Lasers) for women.
"How good is the HairMax comb?" a meeting attendee asked. Dr. Mejia replied that reviews are mixed: "Hair restoration surgeons are on the fence – some believe in it, some don’t. Some patients are happy with it, some are not."
A lot of research also is underway to refine auto cloning and hair multiplication technologies, Dr. Mejia said.
Dermal papilla cells or fibroblasts are the starting point, because they stimulate formation of new hairs. Multiple companies are working on proprietary processes to spur these fibroblasts to produce enough follicles in culture to replace an entire scalp. This is where they hope "to make their windfall," he said. Research includes fibroblasts grown in subatmospheric oxygen tension, addition of wound-healing factors, and injections of a "hair-stimulating complex" to promote greater hair growth.
TrichoCyte is an example of a cell-based hair regeneration technology in phase II trials based on a proprietary dermal papilla cell process (Intercytex, Manchester, England). "The technique does work but [it is] not completely satisfactory at this point," Dr. Mejia said.
More than half of participants in one protocol for another proprietary cell treatment process showed significant hair growth 1 year later, according to a release announcing phase II study results for Aderans Research Institute.
Considerable work remains to be done before regenerative medical hair cloning becomes a clinically viable option, Dr. Mejia said. "How far out are we? I say 5-10 years."
Dr. Mejia said he had no relevant disclosures.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE FLORIDA SOCIETY OF DERMATOLOGIC SURGEONS
Skin of Color: Weighing Risks of Treating Dark Skinned Patients With Isotretinoin
Severe acne has become increasingly common in adult men and women. In skin of color, severe acne results in postinflammatory hyperpigmentation and hyperpigmented scars that are difficult to treat and often cause more distress to the patient than does the acne itself.
Scarring, refractory acne, or nodulo-cystic acne may not respond to traditional therapies and thus may require isotretinoin therapy.
However, many clinicians are rightfully concerned about the legal implications of isotretinoin, particularly the potential for suicide in patients with a history of depression. Clinicians will often not prescribe isotretinoin given these risks, even though their patients may have failed a myriad of topical and oral treatments.
In our clinical experience, severe depression is an underrecognized yet prevalent finding in patients with severe acne. The hyperpigmented scarring is difficult to cover with make-up, particularly for patients with skin types IV-VI. Other cosmetic options are limited.
Patients can develop depression and a lack of self-confidence, particularly if they have been dealing with acne and acne scarring without much relief. It is important to treat these patients aggressively to prevent severe scarring. Often the most effective medication is isotretinoin.
In two well-designed retrospective studies, depression was found to be linked to severe acne, and not to isotretinoin therapy.
In the most recent study, researchers evaluated data from 5,756 patients aged 15-49 years who were prescribed isotretinoin for severe acne between 1980 and 1989. The data were interpreted along with data from hospital discharge and cause-of-death registers from 1980 to 2001 (BMJ 2010;341:c5812).
In this study, 128 patients were admitted to the hospital for attempted suicide. During the year before treatment with isotretinoin, the incidence ratio for attempted suicide was increased 1.57 (95% confidence interval [CI], 0.86–2.63) for all suicide attempts, including repeat attempts, and 1.36 for first attempts only (95% CI, 0.65–2.50), compared to the general population.
The incidence ratio during and up to 6 months after treatment was 1.78 (95% CI, 1.04–2.85) for all attempts and 1.93 (95% CI, 1.08–3.18) for first attempts. Finally, 3 years after treatment the standardized incidence ratio declined to the expected level, 1.04, and remained the same throughout the 15 years of follow-up (95% CI, 0.74–1.43) for all attempts. The ratio for first attempts declined to 0.97 (95% CI, 0.64–1.40).
The authors concluded that suicidal ideation reflects the burden of the disease, rather than treatment with isotretinoin. They suggest that physicians know the psychiatric history of each patient they want to begin on isotretinoin therapy, and follow them closely both throughout the treatment and for up to 1 year after. However, in many patients, the psychiatric morbidity may be related to the acne itself.
Dark-skinned patients develop severe dyschromia from acne lesions which are often difficult to treat. Given the pigmentation of their skin, the severe dyschromia associated with acne, and the lack of cosmetic options for camouflage, these patients may present with signs and symptoms of depression related to their underlying disease. It is imperative that the treating clinician understand each patient's concerns because NOT treating the patient appropriately can be more detrimental to their psychiatric health than treating them with isotretinoin.
Severe acne has become increasingly common in adult men and women. In skin of color, severe acne results in postinflammatory hyperpigmentation and hyperpigmented scars that are difficult to treat and often cause more distress to the patient than does the acne itself.
Scarring, refractory acne, or nodulo-cystic acne may not respond to traditional therapies and thus may require isotretinoin therapy.
However, many clinicians are rightfully concerned about the legal implications of isotretinoin, particularly the potential for suicide in patients with a history of depression. Clinicians will often not prescribe isotretinoin given these risks, even though their patients may have failed a myriad of topical and oral treatments.
In our clinical experience, severe depression is an underrecognized yet prevalent finding in patients with severe acne. The hyperpigmented scarring is difficult to cover with make-up, particularly for patients with skin types IV-VI. Other cosmetic options are limited.
Patients can develop depression and a lack of self-confidence, particularly if they have been dealing with acne and acne scarring without much relief. It is important to treat these patients aggressively to prevent severe scarring. Often the most effective medication is isotretinoin.
In two well-designed retrospective studies, depression was found to be linked to severe acne, and not to isotretinoin therapy.
In the most recent study, researchers evaluated data from 5,756 patients aged 15-49 years who were prescribed isotretinoin for severe acne between 1980 and 1989. The data were interpreted along with data from hospital discharge and cause-of-death registers from 1980 to 2001 (BMJ 2010;341:c5812).
In this study, 128 patients were admitted to the hospital for attempted suicide. During the year before treatment with isotretinoin, the incidence ratio for attempted suicide was increased 1.57 (95% confidence interval [CI], 0.86–2.63) for all suicide attempts, including repeat attempts, and 1.36 for first attempts only (95% CI, 0.65–2.50), compared to the general population.
The incidence ratio during and up to 6 months after treatment was 1.78 (95% CI, 1.04–2.85) for all attempts and 1.93 (95% CI, 1.08–3.18) for first attempts. Finally, 3 years after treatment the standardized incidence ratio declined to the expected level, 1.04, and remained the same throughout the 15 years of follow-up (95% CI, 0.74–1.43) for all attempts. The ratio for first attempts declined to 0.97 (95% CI, 0.64–1.40).
The authors concluded that suicidal ideation reflects the burden of the disease, rather than treatment with isotretinoin. They suggest that physicians know the psychiatric history of each patient they want to begin on isotretinoin therapy, and follow them closely both throughout the treatment and for up to 1 year after. However, in many patients, the psychiatric morbidity may be related to the acne itself.
Dark-skinned patients develop severe dyschromia from acne lesions which are often difficult to treat. Given the pigmentation of their skin, the severe dyschromia associated with acne, and the lack of cosmetic options for camouflage, these patients may present with signs and symptoms of depression related to their underlying disease. It is imperative that the treating clinician understand each patient's concerns because NOT treating the patient appropriately can be more detrimental to their psychiatric health than treating them with isotretinoin.
Severe acne has become increasingly common in adult men and women. In skin of color, severe acne results in postinflammatory hyperpigmentation and hyperpigmented scars that are difficult to treat and often cause more distress to the patient than does the acne itself.
Scarring, refractory acne, or nodulo-cystic acne may not respond to traditional therapies and thus may require isotretinoin therapy.
However, many clinicians are rightfully concerned about the legal implications of isotretinoin, particularly the potential for suicide in patients with a history of depression. Clinicians will often not prescribe isotretinoin given these risks, even though their patients may have failed a myriad of topical and oral treatments.
In our clinical experience, severe depression is an underrecognized yet prevalent finding in patients with severe acne. The hyperpigmented scarring is difficult to cover with make-up, particularly for patients with skin types IV-VI. Other cosmetic options are limited.
Patients can develop depression and a lack of self-confidence, particularly if they have been dealing with acne and acne scarring without much relief. It is important to treat these patients aggressively to prevent severe scarring. Often the most effective medication is isotretinoin.
In two well-designed retrospective studies, depression was found to be linked to severe acne, and not to isotretinoin therapy.
In the most recent study, researchers evaluated data from 5,756 patients aged 15-49 years who were prescribed isotretinoin for severe acne between 1980 and 1989. The data were interpreted along with data from hospital discharge and cause-of-death registers from 1980 to 2001 (BMJ 2010;341:c5812).
In this study, 128 patients were admitted to the hospital for attempted suicide. During the year before treatment with isotretinoin, the incidence ratio for attempted suicide was increased 1.57 (95% confidence interval [CI], 0.86–2.63) for all suicide attempts, including repeat attempts, and 1.36 for first attempts only (95% CI, 0.65–2.50), compared to the general population.
The incidence ratio during and up to 6 months after treatment was 1.78 (95% CI, 1.04–2.85) for all attempts and 1.93 (95% CI, 1.08–3.18) for first attempts. Finally, 3 years after treatment the standardized incidence ratio declined to the expected level, 1.04, and remained the same throughout the 15 years of follow-up (95% CI, 0.74–1.43) for all attempts. The ratio for first attempts declined to 0.97 (95% CI, 0.64–1.40).
The authors concluded that suicidal ideation reflects the burden of the disease, rather than treatment with isotretinoin. They suggest that physicians know the psychiatric history of each patient they want to begin on isotretinoin therapy, and follow them closely both throughout the treatment and for up to 1 year after. However, in many patients, the psychiatric morbidity may be related to the acne itself.
Dark-skinned patients develop severe dyschromia from acne lesions which are often difficult to treat. Given the pigmentation of their skin, the severe dyschromia associated with acne, and the lack of cosmetic options for camouflage, these patients may present with signs and symptoms of depression related to their underlying disease. It is imperative that the treating clinician understand each patient's concerns because NOT treating the patient appropriately can be more detrimental to their psychiatric health than treating them with isotretinoin.