User login
Only One in Four Women Equate Beauty With Youth
SEOUL, SOUTH KOREA – European women are more interested in aesthetic procedures to improve their stomach or abdomen than their face, according to a large survey.
A majority of the women surveyed indicated they were most satisfied with how they looked about 10 years ago, when most were in their late 20s. Three-quarters of the women said they didn’t mind looking their age – they just didn’t want to look older. Eighty percent believed beauty is under an individual’s control and can be enhanced or shaped.
The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures. The survey was conducted online by Harris Interactive and released by Merz, its sponsor, at the World Congress of Dermatology.
Only one-quarter of women surveyed equated beauty with youth. Instead, the top five attributes cited as making women beautiful were healthy skin, an overall well-groomed appearance, beautiful hair, a charming personality, and confidence, according to Steven Basta, who presented the findings.
Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
The most popular aesthetic procedure that the surveyed women had undergone was laser hair removal, at 18%. Rounding out the top five most popular procedures were chemical peels, cellulite treatments, facial dermal fillers, and facial botulinum toxin injections, each tried by 12%-13% of respondents, said Mr. Basta, CEO of Merz.
One in five European women described their personal style as "glamorous" or "extravagant." Fifty-seven percent said beauty arises from a natural appearance; half as many women said beauty stems from a made-up or glamorous look.
Three in five facial filler and/or botulinum toxin users said they had experienced a positive life change due to their treatments.
The majority of facial injectable users reported never admitting to their significant other, close friends, or family that they had undergone such treatments, or they did so only selectively. Instead, they typically claimed their new look was the result of facials, makeup, diet, exercise, or being in love.
SEOUL, SOUTH KOREA – European women are more interested in aesthetic procedures to improve their stomach or abdomen than their face, according to a large survey.
A majority of the women surveyed indicated they were most satisfied with how they looked about 10 years ago, when most were in their late 20s. Three-quarters of the women said they didn’t mind looking their age – they just didn’t want to look older. Eighty percent believed beauty is under an individual’s control and can be enhanced or shaped.
The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures. The survey was conducted online by Harris Interactive and released by Merz, its sponsor, at the World Congress of Dermatology.
Only one-quarter of women surveyed equated beauty with youth. Instead, the top five attributes cited as making women beautiful were healthy skin, an overall well-groomed appearance, beautiful hair, a charming personality, and confidence, according to Steven Basta, who presented the findings.
Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
The most popular aesthetic procedure that the surveyed women had undergone was laser hair removal, at 18%. Rounding out the top five most popular procedures were chemical peels, cellulite treatments, facial dermal fillers, and facial botulinum toxin injections, each tried by 12%-13% of respondents, said Mr. Basta, CEO of Merz.
One in five European women described their personal style as "glamorous" or "extravagant." Fifty-seven percent said beauty arises from a natural appearance; half as many women said beauty stems from a made-up or glamorous look.
Three in five facial filler and/or botulinum toxin users said they had experienced a positive life change due to their treatments.
The majority of facial injectable users reported never admitting to their significant other, close friends, or family that they had undergone such treatments, or they did so only selectively. Instead, they typically claimed their new look was the result of facials, makeup, diet, exercise, or being in love.
SEOUL, SOUTH KOREA – European women are more interested in aesthetic procedures to improve their stomach or abdomen than their face, according to a large survey.
A majority of the women surveyed indicated they were most satisfied with how they looked about 10 years ago, when most were in their late 20s. Three-quarters of the women said they didn’t mind looking their age – they just didn’t want to look older. Eighty percent believed beauty is under an individual’s control and can be enhanced or shaped.
The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures. The survey was conducted online by Harris Interactive and released by Merz, its sponsor, at the World Congress of Dermatology.
Only one-quarter of women surveyed equated beauty with youth. Instead, the top five attributes cited as making women beautiful were healthy skin, an overall well-groomed appearance, beautiful hair, a charming personality, and confidence, according to Steven Basta, who presented the findings.
Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
The most popular aesthetic procedure that the surveyed women had undergone was laser hair removal, at 18%. Rounding out the top five most popular procedures were chemical peels, cellulite treatments, facial dermal fillers, and facial botulinum toxin injections, each tried by 12%-13% of respondents, said Mr. Basta, CEO of Merz.
One in five European women described their personal style as "glamorous" or "extravagant." Fifty-seven percent said beauty arises from a natural appearance; half as many women said beauty stems from a made-up or glamorous look.
Three in five facial filler and/or botulinum toxin users said they had experienced a positive life change due to their treatments.
The majority of facial injectable users reported never admitting to their significant other, close friends, or family that they had undergone such treatments, or they did so only selectively. Instead, they typically claimed their new look was the result of facials, makeup, diet, exercise, or being in love.
FROM THE WORLD CONGRESS OF DERMATOLOGY
Major Finding: Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
Data Source: The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures.
Disclosures: The study was sponsored and released by Merz.
Dysport Trumps Botox for Crow's Feet
A randomized, double-blind study comparing two commercially available botulinum toxin type A injections found abobotulinumtoxinA to be more effective than onabotulinumtoxinA for treating crow's feet.
The study, published online in the Archives of Facial Plastic Surgery (doi:10.1001/archfacial.2011.37), compared the effectiveness of abobotulinumtoxinA, marketed as Dysport, with onabotulinumtoxinA, marketed as Botox, in 90 patients with lateral orbital rhytids.
Investigator and patient assessments of Merz scale scores (a visual tool used to grade wrinkles on a severity scale of 0 to 4) before and at several points after treatment were collected; the primary endpoint was investigator assessment of maximal contraction 30 days post treatment. However, patients also were asked to assess results using the same scale.
AbobotulinumtoxinA was reported by both patients and investigators to be significantly more effective in reducing lines during maximum facial contraction.
Dr. Corey S. Maas of the department of otolaryngology–head and neck surgery at the University of California, San Francisco, who is in private practice, and his colleagues enrolled 90 patients between 31 and 78 years old (mean age 54.5 years) without previous surgery or facial injections for at least 6 months; 13 were men.
All were injected with 10 U of onabotulinumtoxinA on one side of the face and 30 U of abobotulinumtoxinA on the other, according to a 1:3 ratio employed in earlier studies and judged by the principal investigator to be the best comparative dose. The study investigators were blinded as to which side of the face received which treatment, as were patients. Treatments were prepared by a nonblinded nurse.
At baseline, investigators assessed patients as having mean Merz scale scores of 3.68 on the onabotulinumtoxinA-treated sides of their faces at maximal contraction; after treatment, this became 2.33. With abobotulinumtoxinA, investigators assessed patients as having a mean score of 3.64 at baseline and 2.60 after treatment, offering a statistically significant advantage to abobotulinumtoxinA.
Patients, using the same scale, assessed more improvement on the abobotulinumtoxinA side than on the onabotulinumtoxinA side at maximal contraction and favored results from the abobotulinumtoxinA sides 67% of the time.
No statistically significant difference was seen between the two treatments when the lateral orbital rhytids were at rest.
The study was the first to evaluate both products simultaneously in a patient; crow's feet were designated as the candidate wrinkles because of the minimal likelihood of the products diffusing from one side of the face to the other, the investigators wrote.
The investigators offered no explanation for why abobotulinumtoxinA appeared to work better, but wrote in their analysis that because the main pharmacologic difference between the two medications is the hemagglutinin and non-hemagglutinin surrounding each protein, "one may theorize that these differences in efficacy can be ascribed to the hemagglutinin and non-hemagglutinin binding."
Dr. Maas disclosed that he is a consultant for and owns stock in both Medicis Aesthetics (makers of abobotulinumtoxinA) and Allergan (makers of onabotulinumtoxinA). Medicis Aesthetics funded the study.
A randomized, double-blind study comparing two commercially available botulinum toxin type A injections found abobotulinumtoxinA to be more effective than onabotulinumtoxinA for treating crow's feet.
The study, published online in the Archives of Facial Plastic Surgery (doi:10.1001/archfacial.2011.37), compared the effectiveness of abobotulinumtoxinA, marketed as Dysport, with onabotulinumtoxinA, marketed as Botox, in 90 patients with lateral orbital rhytids.
Investigator and patient assessments of Merz scale scores (a visual tool used to grade wrinkles on a severity scale of 0 to 4) before and at several points after treatment were collected; the primary endpoint was investigator assessment of maximal contraction 30 days post treatment. However, patients also were asked to assess results using the same scale.
AbobotulinumtoxinA was reported by both patients and investigators to be significantly more effective in reducing lines during maximum facial contraction.
Dr. Corey S. Maas of the department of otolaryngology–head and neck surgery at the University of California, San Francisco, who is in private practice, and his colleagues enrolled 90 patients between 31 and 78 years old (mean age 54.5 years) without previous surgery or facial injections for at least 6 months; 13 were men.
All were injected with 10 U of onabotulinumtoxinA on one side of the face and 30 U of abobotulinumtoxinA on the other, according to a 1:3 ratio employed in earlier studies and judged by the principal investigator to be the best comparative dose. The study investigators were blinded as to which side of the face received which treatment, as were patients. Treatments were prepared by a nonblinded nurse.
At baseline, investigators assessed patients as having mean Merz scale scores of 3.68 on the onabotulinumtoxinA-treated sides of their faces at maximal contraction; after treatment, this became 2.33. With abobotulinumtoxinA, investigators assessed patients as having a mean score of 3.64 at baseline and 2.60 after treatment, offering a statistically significant advantage to abobotulinumtoxinA.
Patients, using the same scale, assessed more improvement on the abobotulinumtoxinA side than on the onabotulinumtoxinA side at maximal contraction and favored results from the abobotulinumtoxinA sides 67% of the time.
No statistically significant difference was seen between the two treatments when the lateral orbital rhytids were at rest.
The study was the first to evaluate both products simultaneously in a patient; crow's feet were designated as the candidate wrinkles because of the minimal likelihood of the products diffusing from one side of the face to the other, the investigators wrote.
The investigators offered no explanation for why abobotulinumtoxinA appeared to work better, but wrote in their analysis that because the main pharmacologic difference between the two medications is the hemagglutinin and non-hemagglutinin surrounding each protein, "one may theorize that these differences in efficacy can be ascribed to the hemagglutinin and non-hemagglutinin binding."
Dr. Maas disclosed that he is a consultant for and owns stock in both Medicis Aesthetics (makers of abobotulinumtoxinA) and Allergan (makers of onabotulinumtoxinA). Medicis Aesthetics funded the study.
A randomized, double-blind study comparing two commercially available botulinum toxin type A injections found abobotulinumtoxinA to be more effective than onabotulinumtoxinA for treating crow's feet.
The study, published online in the Archives of Facial Plastic Surgery (doi:10.1001/archfacial.2011.37), compared the effectiveness of abobotulinumtoxinA, marketed as Dysport, with onabotulinumtoxinA, marketed as Botox, in 90 patients with lateral orbital rhytids.
Investigator and patient assessments of Merz scale scores (a visual tool used to grade wrinkles on a severity scale of 0 to 4) before and at several points after treatment were collected; the primary endpoint was investigator assessment of maximal contraction 30 days post treatment. However, patients also were asked to assess results using the same scale.
AbobotulinumtoxinA was reported by both patients and investigators to be significantly more effective in reducing lines during maximum facial contraction.
Dr. Corey S. Maas of the department of otolaryngology–head and neck surgery at the University of California, San Francisco, who is in private practice, and his colleagues enrolled 90 patients between 31 and 78 years old (mean age 54.5 years) without previous surgery or facial injections for at least 6 months; 13 were men.
All were injected with 10 U of onabotulinumtoxinA on one side of the face and 30 U of abobotulinumtoxinA on the other, according to a 1:3 ratio employed in earlier studies and judged by the principal investigator to be the best comparative dose. The study investigators were blinded as to which side of the face received which treatment, as were patients. Treatments were prepared by a nonblinded nurse.
At baseline, investigators assessed patients as having mean Merz scale scores of 3.68 on the onabotulinumtoxinA-treated sides of their faces at maximal contraction; after treatment, this became 2.33. With abobotulinumtoxinA, investigators assessed patients as having a mean score of 3.64 at baseline and 2.60 after treatment, offering a statistically significant advantage to abobotulinumtoxinA.
Patients, using the same scale, assessed more improvement on the abobotulinumtoxinA side than on the onabotulinumtoxinA side at maximal contraction and favored results from the abobotulinumtoxinA sides 67% of the time.
No statistically significant difference was seen between the two treatments when the lateral orbital rhytids were at rest.
The study was the first to evaluate both products simultaneously in a patient; crow's feet were designated as the candidate wrinkles because of the minimal likelihood of the products diffusing from one side of the face to the other, the investigators wrote.
The investigators offered no explanation for why abobotulinumtoxinA appeared to work better, but wrote in their analysis that because the main pharmacologic difference between the two medications is the hemagglutinin and non-hemagglutinin surrounding each protein, "one may theorize that these differences in efficacy can be ascribed to the hemagglutinin and non-hemagglutinin binding."
Dr. Maas disclosed that he is a consultant for and owns stock in both Medicis Aesthetics (makers of abobotulinumtoxinA) and Allergan (makers of onabotulinumtoxinA). Medicis Aesthetics funded the study.
FROM ARCHIVES OF FACIAL PLASTIC SURGERY
Major Finding: With abobotulinumtoxinA, investigators assessed patients as having a mean score of 3.64 at baseline and 2.60 after treatment, offering a statistically significant advantage to abobotulinumtoxinA.
Data Source: A randomized double-blind trial to compare the effectiveness of abobotulinumtoxinA to onabotulinumtoxinA in 90 patients with lateral orbital rhytids.
Disclosures: Dr. Maas disclosed that he is a consultant for and owns stock in both Medicis Aesthetics (makers of abobotulinumtoxinA) and Allergan (makers of onabotulinumtoxinA). Medicis Aesthetics funded the study.
SDEF: When to Discard and Replace Lasers, Factors Considered
DANA POINT, CALIF. - Between 1985 and 2011, Dr. Gordon Sasaki retired or discarded an estimated 15 devices used for aesthetic plastic surgery, including two erbium lasers, three CO2 lasers, and an external radiofrequency system for tissue lifting.
Factors that played into the dismissal of these devices included obsolescence of technology, the rise of improved, similar technologies, and competing devices in the office.
At the Summit in Aesthetic Medicine sponsored by Skin Disease Education Foundation (SDEF), Dr. Sasaki, clinical professor of plastic surgery at Loma Linda (Calif.) University Medical Center, shared why he chose to retire or let go of certain devices and embrace others.
Sometimes he stopped working with a device after comparing the cost of buying or leasing it with its positive return on investment, he said. Other factors he considered included the cost of disposable equipment, warranty renewals, and unanticipated costs for reparative procedures when a patient had unsatisfactory results or complications.
One device Dr. Sasaki retired was a high frequency eradicator, which is a low-powered, nongrounded electrosurgical device used for desiccation of subdermal lesions and fulguration of superficial lesions. His rationale included its cost to purchase ($1,000-$1,500), cost of the disposable tip ($1.50 each), and nontransportability from room to room.
He replaced it with the Medi-Pak handheld battery-powered cautery device, which achieves acceptable results yet costs only $9.95 per device, has no disposable components, and can be used multiple times until loss of battery power. After battery power loss, a new device is purchased.
Dr. Sasaki, who has a private aesthetic plastic surgery practice in Pasadena, Calif., also retired a nonablative monopolar radiofrequency device he used for tissue lifting and volumetric heating of dermis, septae, and fat. The initial cost of the second-generation device was $65,000, with a warranty that ranged from $2,800 to $3,300 per year. The annual cost of disposable tips added another $450-$949 per year. "Although the device produced satisfactory results in some patients, it also had unpredictable responses, as well as patient and operator fatigue, especially for body contouring procedures," he said. "There was low patient request for this device, and new, improved technology became available."
He put aside the nonablative radiofrequency device in favor of a multilevel focused and imaged ultrasonic tissue lifting device that provides an imaging level of treatment, precise thermal coagulation points, and more predictable patient responses. In his experience, 80%-90% of his patients showed clinical improvement by 3 months, and results tend to last 1-1.5 years. "There is minimal to moderate pain during treatment, and there is a high patient request for this procedure," Dr. Sasaki said.
Costs of the unit, including a 3-year warranty, were $87,500, he said. Additional warranties cost $10,000 per year, and the cost of a disposable transducer amounts to $2,100, which can last for at least four patient treatments, depending on the area of treatment.
Dr. Sasaki said that he currently shares a fractional CO2 resurfacing laser, in part because of its high purchase cost ($152,000, plus $240 for a box of 30 disposable tips), but also because of patient and operator fatigue, complex patient recovery, unpredictable and unsatisfactory patient responses, and low patient requests for the technology.
As a substitute for selected patients, Dr. Sasaki uses croton oil peels for patients who request facial resurfacing. Concentrations ranging from 0.0125% to 0.4% can be prepared, he said, and the procedure does not require application of a topical or local anesthesia. Costs are reasonable. In his practice a 1-ounce container of phenol costs $23.75 and a 1-ounce container of croton oil costs $106. "The preferred treatments are in patients with Fitzpatrick I-IV skin types," he said. "There are predictable and satisfactory patient responses."
Dr. Sasaki disclosed that he has been a paid consultant for many laser and device companies.
SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. - Between 1985 and 2011, Dr. Gordon Sasaki retired or discarded an estimated 15 devices used for aesthetic plastic surgery, including two erbium lasers, three CO2 lasers, and an external radiofrequency system for tissue lifting.
Factors that played into the dismissal of these devices included obsolescence of technology, the rise of improved, similar technologies, and competing devices in the office.
At the Summit in Aesthetic Medicine sponsored by Skin Disease Education Foundation (SDEF), Dr. Sasaki, clinical professor of plastic surgery at Loma Linda (Calif.) University Medical Center, shared why he chose to retire or let go of certain devices and embrace others.
Sometimes he stopped working with a device after comparing the cost of buying or leasing it with its positive return on investment, he said. Other factors he considered included the cost of disposable equipment, warranty renewals, and unanticipated costs for reparative procedures when a patient had unsatisfactory results or complications.
One device Dr. Sasaki retired was a high frequency eradicator, which is a low-powered, nongrounded electrosurgical device used for desiccation of subdermal lesions and fulguration of superficial lesions. His rationale included its cost to purchase ($1,000-$1,500), cost of the disposable tip ($1.50 each), and nontransportability from room to room.
He replaced it with the Medi-Pak handheld battery-powered cautery device, which achieves acceptable results yet costs only $9.95 per device, has no disposable components, and can be used multiple times until loss of battery power. After battery power loss, a new device is purchased.
Dr. Sasaki, who has a private aesthetic plastic surgery practice in Pasadena, Calif., also retired a nonablative monopolar radiofrequency device he used for tissue lifting and volumetric heating of dermis, septae, and fat. The initial cost of the second-generation device was $65,000, with a warranty that ranged from $2,800 to $3,300 per year. The annual cost of disposable tips added another $450-$949 per year. "Although the device produced satisfactory results in some patients, it also had unpredictable responses, as well as patient and operator fatigue, especially for body contouring procedures," he said. "There was low patient request for this device, and new, improved technology became available."
He put aside the nonablative radiofrequency device in favor of a multilevel focused and imaged ultrasonic tissue lifting device that provides an imaging level of treatment, precise thermal coagulation points, and more predictable patient responses. In his experience, 80%-90% of his patients showed clinical improvement by 3 months, and results tend to last 1-1.5 years. "There is minimal to moderate pain during treatment, and there is a high patient request for this procedure," Dr. Sasaki said.
Costs of the unit, including a 3-year warranty, were $87,500, he said. Additional warranties cost $10,000 per year, and the cost of a disposable transducer amounts to $2,100, which can last for at least four patient treatments, depending on the area of treatment.
Dr. Sasaki said that he currently shares a fractional CO2 resurfacing laser, in part because of its high purchase cost ($152,000, plus $240 for a box of 30 disposable tips), but also because of patient and operator fatigue, complex patient recovery, unpredictable and unsatisfactory patient responses, and low patient requests for the technology.
As a substitute for selected patients, Dr. Sasaki uses croton oil peels for patients who request facial resurfacing. Concentrations ranging from 0.0125% to 0.4% can be prepared, he said, and the procedure does not require application of a topical or local anesthesia. Costs are reasonable. In his practice a 1-ounce container of phenol costs $23.75 and a 1-ounce container of croton oil costs $106. "The preferred treatments are in patients with Fitzpatrick I-IV skin types," he said. "There are predictable and satisfactory patient responses."
Dr. Sasaki disclosed that he has been a paid consultant for many laser and device companies.
SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. - Between 1985 and 2011, Dr. Gordon Sasaki retired or discarded an estimated 15 devices used for aesthetic plastic surgery, including two erbium lasers, three CO2 lasers, and an external radiofrequency system for tissue lifting.
Factors that played into the dismissal of these devices included obsolescence of technology, the rise of improved, similar technologies, and competing devices in the office.
At the Summit in Aesthetic Medicine sponsored by Skin Disease Education Foundation (SDEF), Dr. Sasaki, clinical professor of plastic surgery at Loma Linda (Calif.) University Medical Center, shared why he chose to retire or let go of certain devices and embrace others.
Sometimes he stopped working with a device after comparing the cost of buying or leasing it with its positive return on investment, he said. Other factors he considered included the cost of disposable equipment, warranty renewals, and unanticipated costs for reparative procedures when a patient had unsatisfactory results or complications.
One device Dr. Sasaki retired was a high frequency eradicator, which is a low-powered, nongrounded electrosurgical device used for desiccation of subdermal lesions and fulguration of superficial lesions. His rationale included its cost to purchase ($1,000-$1,500), cost of the disposable tip ($1.50 each), and nontransportability from room to room.
He replaced it with the Medi-Pak handheld battery-powered cautery device, which achieves acceptable results yet costs only $9.95 per device, has no disposable components, and can be used multiple times until loss of battery power. After battery power loss, a new device is purchased.
Dr. Sasaki, who has a private aesthetic plastic surgery practice in Pasadena, Calif., also retired a nonablative monopolar radiofrequency device he used for tissue lifting and volumetric heating of dermis, septae, and fat. The initial cost of the second-generation device was $65,000, with a warranty that ranged from $2,800 to $3,300 per year. The annual cost of disposable tips added another $450-$949 per year. "Although the device produced satisfactory results in some patients, it also had unpredictable responses, as well as patient and operator fatigue, especially for body contouring procedures," he said. "There was low patient request for this device, and new, improved technology became available."
He put aside the nonablative radiofrequency device in favor of a multilevel focused and imaged ultrasonic tissue lifting device that provides an imaging level of treatment, precise thermal coagulation points, and more predictable patient responses. In his experience, 80%-90% of his patients showed clinical improvement by 3 months, and results tend to last 1-1.5 years. "There is minimal to moderate pain during treatment, and there is a high patient request for this procedure," Dr. Sasaki said.
Costs of the unit, including a 3-year warranty, were $87,500, he said. Additional warranties cost $10,000 per year, and the cost of a disposable transducer amounts to $2,100, which can last for at least four patient treatments, depending on the area of treatment.
Dr. Sasaki said that he currently shares a fractional CO2 resurfacing laser, in part because of its high purchase cost ($152,000, plus $240 for a box of 30 disposable tips), but also because of patient and operator fatigue, complex patient recovery, unpredictable and unsatisfactory patient responses, and low patient requests for the technology.
As a substitute for selected patients, Dr. Sasaki uses croton oil peels for patients who request facial resurfacing. Concentrations ranging from 0.0125% to 0.4% can be prepared, he said, and the procedure does not require application of a topical or local anesthesia. Costs are reasonable. In his practice a 1-ounce container of phenol costs $23.75 and a 1-ounce container of croton oil costs $106. "The preferred treatments are in patients with Fitzpatrick I-IV skin types," he said. "There are predictable and satisfactory patient responses."
Dr. Sasaki disclosed that he has been a paid consultant for many laser and device companies.
SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM THE SDEF SUMMIT IN AESTHETIC MEDICINE
SDEF: Fractionated Radiofrequency Smoothes Wrinkles, New Findings Show
DANA POINT, CALIF. – In the clinical experience of Dr. George J. Hruza, bipolar fractionated radiofrequency provides good wrinkle effacement, especially in the periorbital area.
"The benefits of bipolar fractionated radiofrequency include a limited downtime, no adverse events are seen, and any skin type can be treated," Dr. Hruza said at the Summit in Aesthetic Medicine sponsored by Skin Disease Education Foundation (SDEF).
Dr. Hruza, clinical professor of dermatology at St. Louis University, presented findings from a study of 22 adults with Fitzpatrick skin types I-IV who were treated with bipolar fractionated radiofrequency for visible wrinkles and/or elastosis. He and his associates used the Food and Drug Administration–cleared eMatrix radiofrequency device (Syneron) to treat two of the following facial regions per patient: the periorbital region, the perioral region, the cheeks, and the forehead.
Both 64 and 144 pin tips were used; energy was delivered at a range of 2-14 joules per pulse, or 20 J/double pulse, for a maximum duration of 50 milliseconds per pulse. Each patient underwent three treatments at 3 weeks apart. Topical anesthesia was used, and the patients were followed up a month after the last treatment.
Photographic analysis at the 1-month follow-up revealed that fine lines, smoothness, tightness, and brightness improved in about half of the patients by at least 40%. Periorbital photographic results demonstrated a mean improvement in fine lines of at least 30%, with almost all patients showing clinically significant improvement. A 6-month follow-up study of the patients showed persistence of the improvement.
Dr. Hruza noted that patients may experience micro peeling for 2-4 days after undergoing bipolar fractionated radiofrequency.
An emerging technology for deeper skin lesions is dermal bipolar fractionated radiofrequency, which delivers radiofrequency energy within the dermis via micro-needle electrode pairs. "Ninety-six percent of energy is absorbed in the dermis, and the thermal profile is confined along and between the needles," Dr. Hruza said. "This creates a controlled lesion and a fractionated zone of thermal injury."
Tumescent anesthesia is recommended for bipolar dermal fractionated radiofrequency, he said, because it protects deeper skin structures and reduces bleeding. The procedure typically requires 100-300 insertions in the lower face and upper neck. The needle entry points typically close in 2 hours.
Dr. Hruza said that he had no relevant disclosures.
SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – In the clinical experience of Dr. George J. Hruza, bipolar fractionated radiofrequency provides good wrinkle effacement, especially in the periorbital area.
"The benefits of bipolar fractionated radiofrequency include a limited downtime, no adverse events are seen, and any skin type can be treated," Dr. Hruza said at the Summit in Aesthetic Medicine sponsored by Skin Disease Education Foundation (SDEF).
Dr. Hruza, clinical professor of dermatology at St. Louis University, presented findings from a study of 22 adults with Fitzpatrick skin types I-IV who were treated with bipolar fractionated radiofrequency for visible wrinkles and/or elastosis. He and his associates used the Food and Drug Administration–cleared eMatrix radiofrequency device (Syneron) to treat two of the following facial regions per patient: the periorbital region, the perioral region, the cheeks, and the forehead.
Both 64 and 144 pin tips were used; energy was delivered at a range of 2-14 joules per pulse, or 20 J/double pulse, for a maximum duration of 50 milliseconds per pulse. Each patient underwent three treatments at 3 weeks apart. Topical anesthesia was used, and the patients were followed up a month after the last treatment.
Photographic analysis at the 1-month follow-up revealed that fine lines, smoothness, tightness, and brightness improved in about half of the patients by at least 40%. Periorbital photographic results demonstrated a mean improvement in fine lines of at least 30%, with almost all patients showing clinically significant improvement. A 6-month follow-up study of the patients showed persistence of the improvement.
Dr. Hruza noted that patients may experience micro peeling for 2-4 days after undergoing bipolar fractionated radiofrequency.
An emerging technology for deeper skin lesions is dermal bipolar fractionated radiofrequency, which delivers radiofrequency energy within the dermis via micro-needle electrode pairs. "Ninety-six percent of energy is absorbed in the dermis, and the thermal profile is confined along and between the needles," Dr. Hruza said. "This creates a controlled lesion and a fractionated zone of thermal injury."
Tumescent anesthesia is recommended for bipolar dermal fractionated radiofrequency, he said, because it protects deeper skin structures and reduces bleeding. The procedure typically requires 100-300 insertions in the lower face and upper neck. The needle entry points typically close in 2 hours.
Dr. Hruza said that he had no relevant disclosures.
SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – In the clinical experience of Dr. George J. Hruza, bipolar fractionated radiofrequency provides good wrinkle effacement, especially in the periorbital area.
"The benefits of bipolar fractionated radiofrequency include a limited downtime, no adverse events are seen, and any skin type can be treated," Dr. Hruza said at the Summit in Aesthetic Medicine sponsored by Skin Disease Education Foundation (SDEF).
Dr. Hruza, clinical professor of dermatology at St. Louis University, presented findings from a study of 22 adults with Fitzpatrick skin types I-IV who were treated with bipolar fractionated radiofrequency for visible wrinkles and/or elastosis. He and his associates used the Food and Drug Administration–cleared eMatrix radiofrequency device (Syneron) to treat two of the following facial regions per patient: the periorbital region, the perioral region, the cheeks, and the forehead.
Both 64 and 144 pin tips were used; energy was delivered at a range of 2-14 joules per pulse, or 20 J/double pulse, for a maximum duration of 50 milliseconds per pulse. Each patient underwent three treatments at 3 weeks apart. Topical anesthesia was used, and the patients were followed up a month after the last treatment.
Photographic analysis at the 1-month follow-up revealed that fine lines, smoothness, tightness, and brightness improved in about half of the patients by at least 40%. Periorbital photographic results demonstrated a mean improvement in fine lines of at least 30%, with almost all patients showing clinically significant improvement. A 6-month follow-up study of the patients showed persistence of the improvement.
Dr. Hruza noted that patients may experience micro peeling for 2-4 days after undergoing bipolar fractionated radiofrequency.
An emerging technology for deeper skin lesions is dermal bipolar fractionated radiofrequency, which delivers radiofrequency energy within the dermis via micro-needle electrode pairs. "Ninety-six percent of energy is absorbed in the dermis, and the thermal profile is confined along and between the needles," Dr. Hruza said. "This creates a controlled lesion and a fractionated zone of thermal injury."
Tumescent anesthesia is recommended for bipolar dermal fractionated radiofrequency, he said, because it protects deeper skin structures and reduces bleeding. The procedure typically requires 100-300 insertions in the lower face and upper neck. The needle entry points typically close in 2 hours.
Dr. Hruza said that he had no relevant disclosures.
SDEF and this news organization are owned by Elsevier.
FROM THE SDEF SUMMIT IN AESTHETIC MEDICINE
Major Finding: Photographic analysis at 1 month follow-up revealed that fine lines, smoothness, tightness, and brightness improved in about half of patients by at least 40%.
Data Source: A study of 22 adults with Fitzpatrick skin types I-IV who were treated with bipolar fractionated radiofrequency for visible wrinkles and/or elastosis.
Disclosures: Dr. Hruza said that he had no relevant disclosures. SDEF and this news organization are owned by Elsevier.
SDEF: Remain Skeptical Over Efficacy of Noninvasive Fat Removal Devices
DANA POINT, CALIF. – Practice skepticism when it comes to the efficacy of noninvasive fat removal devices, advised Dr. Matthew A. Avram.
"Fat removal has a long history of 'snake oil' salesmanship," Dr. Avram said at the Summit in Aesthetic Medicine, which was sponsored by Skin Disease Education Foundation (SDEF). "You can count on this to continue, because many of these devices do little, if anything. It is important to critically assess these technologies in this emerging field."
Focused Ultrasound
Dr. Avram, faculty director for procedural dermatology training at Harvard Medical School and director of the dermatology laser and cosmetic center at Massachusetts General Hospital, both in Boston, discussed the evidence surrounding devices for noninvasive fat reduction.
One non–Food and Drug Administration–cleared modality being studied is focused ultrasound, which delivers mechanical, nonthermal energy to the thigh, abdomen, and flanks. A study of 30 patients who were treated once a month for 3 months demonstrated a circumference reduction of 2-4 cm in the treated sites (Lasers Surg. Med. 2007;39:315-23). Liver function tests, a lipid panel, and liver ultrasound showed no adverse systemic effects from the procedure.
However, Dr. Avram noted that the study is limited because there was no untreated control group and that circumference "is an inherently imprecise measure of improvement that can be manipulated." MRI would prove objective improvement, he said, but it was not performed in this trial.
In a subsequent study from Hong Kong, 53 patients underwent treatment once a month for 3 months for body contouring (Lasers Surg. Med. 2009; 41:751-9). No significant changes were observed in circumference and caliper measurements, and patients rated their satisfaction as poor.
High-Intensity Focused Ultrasound
High-intensity focused ultrasound, which is also not FDA cleared, involves the rapid heating of adipocytes that are purported to produce coagulative necrosis and cell death in adipose tissue. A retrospective chart review of the modality that was used in 85 patients who underwent one treatment session showed a mean 4.6 cm decrease in waist circumference after 3 months (Aesth. Plast. Surg. 2010;34:577-82). Adverse events that lasted 4-12 weeks occurred in 12% of subjects and included prolonged tenderness, ecchymosis, nodules, and edema, as well as procedural pain in one patient, which required discontinuation of the procedure.
Dr. Avram called high-intensity focused ultrasound a promising technology, "but it's difficult to assess its efficacy. Further study of this technology is needed."
Monopolar Radiofrequency
Monopolar radiofrequency, conventionally used for tissue tightening of the face, has produced lipoatrophy with aggressive settings as a complication. "Perhaps this can be harnessed to effectively treat fat," he said, noting that clinical studies are currently underway.
Low-Level Light Therapy
Low-level light therapy, an FDA-cleared modality for fat removal, uses a multiple head, low-level diode laser at an energy level of 635 nm, which is "roughly equivalent to a laser pointer," Dr. Avram said. In one randomized trial, 59 patients received three treatments of the technology or sham treatment per week for 2 weeks (Lasers Surg. Med. 2009;41:799-809). At 2 weeks, mean circumference reductions in the treatment group were 0.98 inches at the waist, 1.05 inches at the hip, and 0.85 inches at the right thigh and 0.65 inches at the left thigh. Circumference increased in the 2 weeks following treatment.
Dr. Avram said the study was poorly designed because there was no untreated control group, the duration of treatment was only 2 weeks, and there was no ultrasound or other noninvasive evidence of decreased fat layer. "Treat with extreme skepticism," he advised.
Cryolipolysis
Perhaps the most promising technology is cryolipolysis, he said, which is FDA cleared for noninvasive fat removal. Cryolipolysis involves the noninvasive cooling of fat to selectively cause cell death without damage to surrounding tissue.
The mechanism of action of cryolipolysis is believed to involve selective crystallization of lipids in fat cells at temperatures near freezing. "Apoptotic fat cell death is followed by slow dissolution of the fat cell and a gradual release of lipids," Dr. Avram explained. "The inflammatory process results in fat layer reduction over 2-4 months."
When human studies of the technology were first conducted in 2008, enrollment was restricted to 32 patients whose "love handles" were treated at a cooling intensity factor (CIF) of 33 for 60 minutes, and progressed to higher rates of energy extractions for 45 minutes per application site. One side was treated; the untreated contralateral side served as the control.
Efficacy was evaluated at 4 months post treatment via visual assessment as a primary end point, as well as ultrasound and histology. The ultrasound results demonstrated an average 23% decrease in fat layer thickness.
"In this initial group of 32 love handle patients treated once, discernible changes were seen on the treated side vs. baseline of the treated side, and compared to the untreated contralateral control," Dr. Avram said of the findings presented during a poster session at the 2008 annual meeting of the American Society for Dermatologic Surgery. "This unique study design [using each patient as his or her own control], provided very powerful evidence that this was a true treatment effect rather than a change in the patient's diet or exercise pattern during the 4 months after this single procedure exposure."
Common effects after cryolipolysis include redness, which lasts minutes to a few hours; bruising, which may last for a few weeks; and temporary dulling of sensation in the treated area, which typically resolves in 1-8 weeks. No postprocedural changes in pigmentation or laboratory abnormalities have been observed, he said.
About 1 in 2,000 patients experiences severe pain beginning 3-7 days post treatment, which translates into 26 reported cases out of 60,000 treatments. "We are not sure why these occur, but these cases completely resolve with no sequelae," Dr. Avram said.
He emphasized that cryolipolysis is not a replacement for liposuction. "It is not a weight-loss device," he said. "It's best suited for local fat removal resistant to exercise in relatively fit patients."
Dr. Avram disclosed holding stock options in Zeltiq Aesthetics, which manufactures cryolipolysis equipment. SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – Practice skepticism when it comes to the efficacy of noninvasive fat removal devices, advised Dr. Matthew A. Avram.
"Fat removal has a long history of 'snake oil' salesmanship," Dr. Avram said at the Summit in Aesthetic Medicine, which was sponsored by Skin Disease Education Foundation (SDEF). "You can count on this to continue, because many of these devices do little, if anything. It is important to critically assess these technologies in this emerging field."
Focused Ultrasound
Dr. Avram, faculty director for procedural dermatology training at Harvard Medical School and director of the dermatology laser and cosmetic center at Massachusetts General Hospital, both in Boston, discussed the evidence surrounding devices for noninvasive fat reduction.
One non–Food and Drug Administration–cleared modality being studied is focused ultrasound, which delivers mechanical, nonthermal energy to the thigh, abdomen, and flanks. A study of 30 patients who were treated once a month for 3 months demonstrated a circumference reduction of 2-4 cm in the treated sites (Lasers Surg. Med. 2007;39:315-23). Liver function tests, a lipid panel, and liver ultrasound showed no adverse systemic effects from the procedure.
However, Dr. Avram noted that the study is limited because there was no untreated control group and that circumference "is an inherently imprecise measure of improvement that can be manipulated." MRI would prove objective improvement, he said, but it was not performed in this trial.
In a subsequent study from Hong Kong, 53 patients underwent treatment once a month for 3 months for body contouring (Lasers Surg. Med. 2009; 41:751-9). No significant changes were observed in circumference and caliper measurements, and patients rated their satisfaction as poor.
High-Intensity Focused Ultrasound
High-intensity focused ultrasound, which is also not FDA cleared, involves the rapid heating of adipocytes that are purported to produce coagulative necrosis and cell death in adipose tissue. A retrospective chart review of the modality that was used in 85 patients who underwent one treatment session showed a mean 4.6 cm decrease in waist circumference after 3 months (Aesth. Plast. Surg. 2010;34:577-82). Adverse events that lasted 4-12 weeks occurred in 12% of subjects and included prolonged tenderness, ecchymosis, nodules, and edema, as well as procedural pain in one patient, which required discontinuation of the procedure.
Dr. Avram called high-intensity focused ultrasound a promising technology, "but it's difficult to assess its efficacy. Further study of this technology is needed."
Monopolar Radiofrequency
Monopolar radiofrequency, conventionally used for tissue tightening of the face, has produced lipoatrophy with aggressive settings as a complication. "Perhaps this can be harnessed to effectively treat fat," he said, noting that clinical studies are currently underway.
Low-Level Light Therapy
Low-level light therapy, an FDA-cleared modality for fat removal, uses a multiple head, low-level diode laser at an energy level of 635 nm, which is "roughly equivalent to a laser pointer," Dr. Avram said. In one randomized trial, 59 patients received three treatments of the technology or sham treatment per week for 2 weeks (Lasers Surg. Med. 2009;41:799-809). At 2 weeks, mean circumference reductions in the treatment group were 0.98 inches at the waist, 1.05 inches at the hip, and 0.85 inches at the right thigh and 0.65 inches at the left thigh. Circumference increased in the 2 weeks following treatment.
Dr. Avram said the study was poorly designed because there was no untreated control group, the duration of treatment was only 2 weeks, and there was no ultrasound or other noninvasive evidence of decreased fat layer. "Treat with extreme skepticism," he advised.
Cryolipolysis
Perhaps the most promising technology is cryolipolysis, he said, which is FDA cleared for noninvasive fat removal. Cryolipolysis involves the noninvasive cooling of fat to selectively cause cell death without damage to surrounding tissue.
The mechanism of action of cryolipolysis is believed to involve selective crystallization of lipids in fat cells at temperatures near freezing. "Apoptotic fat cell death is followed by slow dissolution of the fat cell and a gradual release of lipids," Dr. Avram explained. "The inflammatory process results in fat layer reduction over 2-4 months."
When human studies of the technology were first conducted in 2008, enrollment was restricted to 32 patients whose "love handles" were treated at a cooling intensity factor (CIF) of 33 for 60 minutes, and progressed to higher rates of energy extractions for 45 minutes per application site. One side was treated; the untreated contralateral side served as the control.
Efficacy was evaluated at 4 months post treatment via visual assessment as a primary end point, as well as ultrasound and histology. The ultrasound results demonstrated an average 23% decrease in fat layer thickness.
"In this initial group of 32 love handle patients treated once, discernible changes were seen on the treated side vs. baseline of the treated side, and compared to the untreated contralateral control," Dr. Avram said of the findings presented during a poster session at the 2008 annual meeting of the American Society for Dermatologic Surgery. "This unique study design [using each patient as his or her own control], provided very powerful evidence that this was a true treatment effect rather than a change in the patient's diet or exercise pattern during the 4 months after this single procedure exposure."
Common effects after cryolipolysis include redness, which lasts minutes to a few hours; bruising, which may last for a few weeks; and temporary dulling of sensation in the treated area, which typically resolves in 1-8 weeks. No postprocedural changes in pigmentation or laboratory abnormalities have been observed, he said.
About 1 in 2,000 patients experiences severe pain beginning 3-7 days post treatment, which translates into 26 reported cases out of 60,000 treatments. "We are not sure why these occur, but these cases completely resolve with no sequelae," Dr. Avram said.
He emphasized that cryolipolysis is not a replacement for liposuction. "It is not a weight-loss device," he said. "It's best suited for local fat removal resistant to exercise in relatively fit patients."
Dr. Avram disclosed holding stock options in Zeltiq Aesthetics, which manufactures cryolipolysis equipment. SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – Practice skepticism when it comes to the efficacy of noninvasive fat removal devices, advised Dr. Matthew A. Avram.
"Fat removal has a long history of 'snake oil' salesmanship," Dr. Avram said at the Summit in Aesthetic Medicine, which was sponsored by Skin Disease Education Foundation (SDEF). "You can count on this to continue, because many of these devices do little, if anything. It is important to critically assess these technologies in this emerging field."
Focused Ultrasound
Dr. Avram, faculty director for procedural dermatology training at Harvard Medical School and director of the dermatology laser and cosmetic center at Massachusetts General Hospital, both in Boston, discussed the evidence surrounding devices for noninvasive fat reduction.
One non–Food and Drug Administration–cleared modality being studied is focused ultrasound, which delivers mechanical, nonthermal energy to the thigh, abdomen, and flanks. A study of 30 patients who were treated once a month for 3 months demonstrated a circumference reduction of 2-4 cm in the treated sites (Lasers Surg. Med. 2007;39:315-23). Liver function tests, a lipid panel, and liver ultrasound showed no adverse systemic effects from the procedure.
However, Dr. Avram noted that the study is limited because there was no untreated control group and that circumference "is an inherently imprecise measure of improvement that can be manipulated." MRI would prove objective improvement, he said, but it was not performed in this trial.
In a subsequent study from Hong Kong, 53 patients underwent treatment once a month for 3 months for body contouring (Lasers Surg. Med. 2009; 41:751-9). No significant changes were observed in circumference and caliper measurements, and patients rated their satisfaction as poor.
High-Intensity Focused Ultrasound
High-intensity focused ultrasound, which is also not FDA cleared, involves the rapid heating of adipocytes that are purported to produce coagulative necrosis and cell death in adipose tissue. A retrospective chart review of the modality that was used in 85 patients who underwent one treatment session showed a mean 4.6 cm decrease in waist circumference after 3 months (Aesth. Plast. Surg. 2010;34:577-82). Adverse events that lasted 4-12 weeks occurred in 12% of subjects and included prolonged tenderness, ecchymosis, nodules, and edema, as well as procedural pain in one patient, which required discontinuation of the procedure.
Dr. Avram called high-intensity focused ultrasound a promising technology, "but it's difficult to assess its efficacy. Further study of this technology is needed."
Monopolar Radiofrequency
Monopolar radiofrequency, conventionally used for tissue tightening of the face, has produced lipoatrophy with aggressive settings as a complication. "Perhaps this can be harnessed to effectively treat fat," he said, noting that clinical studies are currently underway.
Low-Level Light Therapy
Low-level light therapy, an FDA-cleared modality for fat removal, uses a multiple head, low-level diode laser at an energy level of 635 nm, which is "roughly equivalent to a laser pointer," Dr. Avram said. In one randomized trial, 59 patients received three treatments of the technology or sham treatment per week for 2 weeks (Lasers Surg. Med. 2009;41:799-809). At 2 weeks, mean circumference reductions in the treatment group were 0.98 inches at the waist, 1.05 inches at the hip, and 0.85 inches at the right thigh and 0.65 inches at the left thigh. Circumference increased in the 2 weeks following treatment.
Dr. Avram said the study was poorly designed because there was no untreated control group, the duration of treatment was only 2 weeks, and there was no ultrasound or other noninvasive evidence of decreased fat layer. "Treat with extreme skepticism," he advised.
Cryolipolysis
Perhaps the most promising technology is cryolipolysis, he said, which is FDA cleared for noninvasive fat removal. Cryolipolysis involves the noninvasive cooling of fat to selectively cause cell death without damage to surrounding tissue.
The mechanism of action of cryolipolysis is believed to involve selective crystallization of lipids in fat cells at temperatures near freezing. "Apoptotic fat cell death is followed by slow dissolution of the fat cell and a gradual release of lipids," Dr. Avram explained. "The inflammatory process results in fat layer reduction over 2-4 months."
When human studies of the technology were first conducted in 2008, enrollment was restricted to 32 patients whose "love handles" were treated at a cooling intensity factor (CIF) of 33 for 60 minutes, and progressed to higher rates of energy extractions for 45 minutes per application site. One side was treated; the untreated contralateral side served as the control.
Efficacy was evaluated at 4 months post treatment via visual assessment as a primary end point, as well as ultrasound and histology. The ultrasound results demonstrated an average 23% decrease in fat layer thickness.
"In this initial group of 32 love handle patients treated once, discernible changes were seen on the treated side vs. baseline of the treated side, and compared to the untreated contralateral control," Dr. Avram said of the findings presented during a poster session at the 2008 annual meeting of the American Society for Dermatologic Surgery. "This unique study design [using each patient as his or her own control], provided very powerful evidence that this was a true treatment effect rather than a change in the patient's diet or exercise pattern during the 4 months after this single procedure exposure."
Common effects after cryolipolysis include redness, which lasts minutes to a few hours; bruising, which may last for a few weeks; and temporary dulling of sensation in the treated area, which typically resolves in 1-8 weeks. No postprocedural changes in pigmentation or laboratory abnormalities have been observed, he said.
About 1 in 2,000 patients experiences severe pain beginning 3-7 days post treatment, which translates into 26 reported cases out of 60,000 treatments. "We are not sure why these occur, but these cases completely resolve with no sequelae," Dr. Avram said.
He emphasized that cryolipolysis is not a replacement for liposuction. "It is not a weight-loss device," he said. "It's best suited for local fat removal resistant to exercise in relatively fit patients."
Dr. Avram disclosed holding stock options in Zeltiq Aesthetics, which manufactures cryolipolysis equipment. SDEF and this news organization are owned by Elsevier.
EXPERT ANALYISIS FROM THE SDEF SUMMIT IN AESTHETIC MEDICINE
Skin of Color: Rosacea
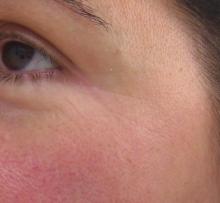
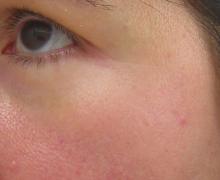
While often considered a problem in white skin, rosacea is also a common concern in skin of color patients.
The clinical signs of rosacea are often hard to diagnose in Fitzpatrick skin types III-VI, and are often not associated with clinical signs and symptoms of flushing or telangiectasias. Trigger factors associated with rosacea flares – hot beverages, spicy foods, caffeine, alcoholic drinks, heat, and exercise – are often completely absent in skin of color.
Rosacea occurs mainly on the central malar cheeks, forehead, chin, and nose. Erythema and red/brown papules are common and are often confused with acne. As the condition progresses, the skin becomes persistently red and can feel uneven or even thicker. Hyper or hypopigmentation may develop in areas with inflammation. Perioral or periorficial papules, a form of rosacea commonly seen in skin of color, is also often misdiagnosed.
It has been my experience that rosacea in skin of color is often refractory to traditional topical medications and patients will often need a short course of oral antibiotics. Sulfur/sodium sulfacetamide topicals, in addition to azelaic acid, are a great adjunct to oral treatment. Topical steroids may initially improve symptoms, but will actually make the disease progress when the steroids are stopped, so they should be avoided. Strict photo protection should be encouraged.
For years, the cause of rosacea was unknown. However a team of researchers, led by Dr. Richard L. Gallo, chief of dermatology and professor of medicine and pediatrics at the University of California San Diego, found that overproduction of two interactive inflammatory proteins results in excessive levels of a third protein that cause rosacea symptoms. His team found skin antimicrobial peptides, cathelicidins, were altered and overproduced in patients with rosacea (Nat. Med. 2007;13:975-80).
Approximately 14 million people in the United States have rosacea. Early diagnosis and management with combination oral and topical medications are effective at controlling this highly prevalent yet often misdiagnosed disease. Future research into the underlying cause of rosacea will offer targeted therapies aimed at the abnormally processed antimicrobial peptides present in the skin of rosacea patients.
While often considered a problem in white skin, rosacea is also a common concern in skin of color patients.
The clinical signs of rosacea are often hard to diagnose in Fitzpatrick skin types III-VI, and are often not associated with clinical signs and symptoms of flushing or telangiectasias. Trigger factors associated with rosacea flares – hot beverages, spicy foods, caffeine, alcoholic drinks, heat, and exercise – are often completely absent in skin of color.
Rosacea occurs mainly on the central malar cheeks, forehead, chin, and nose. Erythema and red/brown papules are common and are often confused with acne. As the condition progresses, the skin becomes persistently red and can feel uneven or even thicker. Hyper or hypopigmentation may develop in areas with inflammation. Perioral or periorficial papules, a form of rosacea commonly seen in skin of color, is also often misdiagnosed.
It has been my experience that rosacea in skin of color is often refractory to traditional topical medications and patients will often need a short course of oral antibiotics. Sulfur/sodium sulfacetamide topicals, in addition to azelaic acid, are a great adjunct to oral treatment. Topical steroids may initially improve symptoms, but will actually make the disease progress when the steroids are stopped, so they should be avoided. Strict photo protection should be encouraged.
For years, the cause of rosacea was unknown. However a team of researchers, led by Dr. Richard L. Gallo, chief of dermatology and professor of medicine and pediatrics at the University of California San Diego, found that overproduction of two interactive inflammatory proteins results in excessive levels of a third protein that cause rosacea symptoms. His team found skin antimicrobial peptides, cathelicidins, were altered and overproduced in patients with rosacea (Nat. Med. 2007;13:975-80).
Approximately 14 million people in the United States have rosacea. Early diagnosis and management with combination oral and topical medications are effective at controlling this highly prevalent yet often misdiagnosed disease. Future research into the underlying cause of rosacea will offer targeted therapies aimed at the abnormally processed antimicrobial peptides present in the skin of rosacea patients.
While often considered a problem in white skin, rosacea is also a common concern in skin of color patients.
The clinical signs of rosacea are often hard to diagnose in Fitzpatrick skin types III-VI, and are often not associated with clinical signs and symptoms of flushing or telangiectasias. Trigger factors associated with rosacea flares – hot beverages, spicy foods, caffeine, alcoholic drinks, heat, and exercise – are often completely absent in skin of color.
Rosacea occurs mainly on the central malar cheeks, forehead, chin, and nose. Erythema and red/brown papules are common and are often confused with acne. As the condition progresses, the skin becomes persistently red and can feel uneven or even thicker. Hyper or hypopigmentation may develop in areas with inflammation. Perioral or periorficial papules, a form of rosacea commonly seen in skin of color, is also often misdiagnosed.
It has been my experience that rosacea in skin of color is often refractory to traditional topical medications and patients will often need a short course of oral antibiotics. Sulfur/sodium sulfacetamide topicals, in addition to azelaic acid, are a great adjunct to oral treatment. Topical steroids may initially improve symptoms, but will actually make the disease progress when the steroids are stopped, so they should be avoided. Strict photo protection should be encouraged.
For years, the cause of rosacea was unknown. However a team of researchers, led by Dr. Richard L. Gallo, chief of dermatology and professor of medicine and pediatrics at the University of California San Diego, found that overproduction of two interactive inflammatory proteins results in excessive levels of a third protein that cause rosacea symptoms. His team found skin antimicrobial peptides, cathelicidins, were altered and overproduced in patients with rosacea (Nat. Med. 2007;13:975-80).
Approximately 14 million people in the United States have rosacea. Early diagnosis and management with combination oral and topical medications are effective at controlling this highly prevalent yet often misdiagnosed disease. Future research into the underlying cause of rosacea will offer targeted therapies aimed at the abnormally processed antimicrobial peptides present in the skin of rosacea patients.
Beta-Carotene
Beta-carotene is perhaps the best known member of the carotenoid family, a group of highly pigmented (red, orange, yellow), lipid-soluble substances present in various fruits, grains, oils, and vegetables (including apricots, carrots, green peppers, spinach, squash, sweet potatoes, and tomatoes).
Like alpha- and gamma-carotene, beta-carotene is a provitamin because it can be converted into active vitamin A (retinol). In comparison to other carotenoid compounds, some of which have been shown to have potential in the dermatologic armamentarium (e.g., astaxanthin, lutein, lycopene, and zeaxanthin), beta-carotene has been demonstrated to contribute much more to human nutrition (Am. J. Clin. Nutr. 2001;73:853-64).
The degree to which beta-carotene can be harnessed for dermatologic applications is debatable, with the aforementioned carotenoids displaying greater promise in this realm. In particular, the potent yellow/orange pigmentation delivered by beta-carotene poses significant challenges to topical formulation. That said, recent research has indicated that cutaneous benefits can be derived from the systemic use of beta-carotene. That is the focus of this column.
Mixed results for beta-carotene’s UV protective effects. Ten years ago, Obermüller-Jevic et al. observed that beta-carotene exerted pro-oxidant activity in cultured human skin fibroblasts by promoting UVA induction of heme oxygenase-1 (HO-1). The investigators subsequently showed in vitro that the provitamin A compound potently fosters the UVA induction of proinflammatory interleukin-6 in skin fibroblasts (FEBS Lett. 1999;460:212-6; FEBS Lett. 2001;509:186-90). Nevertheless, several experimental, but not clinical, studies have suggested that dietary beta-carotene yields protective effects against acute and chronic cutaneous photodamage (Arch. Biochem. Biophys. 2001;389:1-6).
In 2000, Frieling et al. reported on their large-scale (n = 22,071) randomized, double-blind, placebo-controlled 12-year primary-prevention trial of beta-carotene supplementation with follow-up, in which they found that supplementing with 50 mg of beta-carotene on alternate days in apparently healthy male physicians aged 40-84 years in 1982 did not affect the development of a first basal cell or squamous cell carcinoma (Arch Dermatol. 2000;136:179-84). Other studies published earlier or around the same time yielded similar results, indicating that oral beta-carotene exerted no influence in stemming, preventing, or promoting nonmelanoma skin cancers (N. Engl. J. Med. 1990;323:789-95; Lancet 1999;354:723-9; J. Am. Acad. Dermatol. 2006;54:933-46).
In a prospective study of 1,001 randomly selected adults in one Australian community, investigators sought to determine the relationships between consumption of antioxidant nutrients and the relative risk of cutaneous basal cell and squamous cell carcinomas. In 1996 antioxidant consumption was estimated, and histologically confirmed cancers were recorded between 1996 and 2004. Dietary consumption in the second tertile for beta-carotene and vitamin E was associated with a higher basal cell carcinoma risk, with no trend, in subjects with a skin cancer history or a specific BCC history at baseline (Eur. J. Cancer 2007;43:2707-16).
In 2006, Stahl and Krutmann found that the systemic use of beta-carotene in dosages of 15-30 mg/day over 10-12 weeks conferred protection against UV-induced erythema, but failed to provide full protection against UVR (Hautarzt. 2006;57:281-5.)
More recently, in a paper published in 2008, Köpcke and Krutmann reported on their literature review of supplementation studies of dietary beta-carotene as protection against sunburn. Their review included studies published up to June 2007 in PubMed, ISI Web of Science, and the EBM Cochrane Library. The meta-analysis netted seven studies on the subject and revealed that, indeed, beta-carotene supplementation imparted time-dependent protection against sunburn, with at least 10 weeks of supplementation necessary (Photochem. Photobiol. 2008;84:284-8).
In September 2007, Stahl and Sies clarified that dietary carotenoids, particularly beta-carotene and lycopene, as well as flavonoids, help prevent the emergence of UV-induced erythema after the nutrients are disseminated to light-exposed areas, including the skin and eyes. Specifically, the tested carotenoids and flavonoids diminished sensitivity to UV-induced erythema in volunteers after 10-12 weeks of dietary intervention. The investigators speculated that such micronutrients have the potential to impart protection against UVR throughout an individual’s lifetime (Mol. Biotechnol. 2007;37:26-30).
Although the data on the impact of beta-carotene on aging skin and photoaging remain sparse, Bayerl has noted that beta-carotene has been shown to protect against the phototoxic effects of porphyrins in the photohemolysis model, and that the provitamin A substance is indicated for erythropoietic protoporphyria, photosensitive diseases, and mitigating the side effects of phototoxic drugs (Acta Dermatovenerol. Alp. Panonica Adriat. 2008;17:160-2, 164-6).
In 2009, Camera et al. compared the effects of the carotenoids astaxanthin, canthaxanthin, and beta-carotene on UVA-induced damage to human dermal fibroblasts. The carotenoids were delivered to the fibroblasts 24 hours before UVA exposure. Astaxanthin displayed the strongest photoprotective effect, reversing all of the noted changes induced by UVA, whereas beta-carotene partially prevented the UVA-induced reduction in catalase and superoxide dismutase activities. Beta-carotene also increased membrane damage, provoked HO-1 expression, and dose-dependently induced caspase-3 activity. In addition, the greatest photostability among the tested carotenoids was associated with astaxanthin, and the least, with beta-carotene (Exp. Dermatol. 2009;18:222-31).
Beta-carotene was cast in a more favorable light by a study published 2 months later by Valacchi et al. The researchers, acknowledging the apparent conflict between some studies suggesting that beta-carotene exhibits skin-protecting qualities as an antioxidant and other studies indicating pro-oxidant characteristics, examined the potential effects of oral beta-carotene supplement consumption on oxidative stress on the skin caused by ozone. They measured markers of oxidative stress in hairless mice after 1 month of a diet supplement with 0.5% beta-carotene and subsequent exposure to ozone (0.8 ppm; 6 hr/day; 7 days). The researchers found that beta-carotene downregulated the induction of tumor necrosis factor-alpha, macrophage inflammatory protein 2, inducible nitric oxide synthase, and HO-1 caused by ozone exposure. They concluded that beta-carotene does indeed confer protection against ozone-induced cutaneous oxidative stress in vivo (Toxicol. Ind. Health 2009;25:241-7).
Beta-carotene and lung cancer risk link. It is clear that dietary beta-carotene affords at least some cutaneous protection, though not against skin cancer, but the limits have not been fully elucidated as yet. It is important to note that beta-carotene supplementation has been shown to contribute to heightening the risk of lung cancer development in smokers and individuals exposed to asbestos (Evid. Rep. Technol. Assess. 2006;139:1-117).
In 2008, Tanyetyanon and Bepler conducted a meta-analysis of the literature on large randomized trials to assess the risk of lung cancer posed by beta-carotene consumption by smokers or former smokers. In their Medline search, the investigators identified four studies totaling 109,394 subjects. While they noted no increased risk of lung cancer among former smokers, they found a significant link between beta-carotene supplementation and increased lung cancer risk among current smokers (Cancer 2008;113:150-7).
In addition to this disturbing risk, there are some minor disadvantages associated with consuming excessive amounts of beta-carotene and other provitamin A substances. Specifically, the consumption of excessive amounts of beta-carotene through the diet or in supplement form can lead to skin yellowing. Because beta-carotene is inefficiently converted into retinol, supplementation with beta-carotene poses less risk of rendering the skin yellow than does vitamin A supplementation. It is best that patients and consumers derive the benefits of beta-carotene through the diet, but it can be a beneficial supplement to people living in warm climates where frequent sun exposure is more likely and whose diets do not include enough of this carotenoid.
Conclusions. As a prominent member of the carotenoid family, beta-carotene is a compelling subject for the investigation of its potential health benefits. While much more research is necessary to determine the range of effects (broadly, antioxidant to pro-oxidant) that this provitamin A substance can deliver to overall health as well as cutaneous health, in addition to its risks, there appears to be enough evidence to warrant confidence in suggesting a healthy dose of fruits and vegetables rich in beta-carotene to individuals who are not smokers.
I suspect that the strong pigmentary component of beta-carotene will continue to pose significant challenges to manufacturers who hope to harness its antioxidant potential in topical formulations. That said, it will be interesting to keep an eye on continuing investigations regarding the cutaneous effects of dietary consumption and oral supplementation with beta-carotene.
Beta-carotene is perhaps the best known member of the carotenoid family, a group of highly pigmented (red, orange, yellow), lipid-soluble substances present in various fruits, grains, oils, and vegetables (including apricots, carrots, green peppers, spinach, squash, sweet potatoes, and tomatoes).
Like alpha- and gamma-carotene, beta-carotene is a provitamin because it can be converted into active vitamin A (retinol). In comparison to other carotenoid compounds, some of which have been shown to have potential in the dermatologic armamentarium (e.g., astaxanthin, lutein, lycopene, and zeaxanthin), beta-carotene has been demonstrated to contribute much more to human nutrition (Am. J. Clin. Nutr. 2001;73:853-64).
The degree to which beta-carotene can be harnessed for dermatologic applications is debatable, with the aforementioned carotenoids displaying greater promise in this realm. In particular, the potent yellow/orange pigmentation delivered by beta-carotene poses significant challenges to topical formulation. That said, recent research has indicated that cutaneous benefits can be derived from the systemic use of beta-carotene. That is the focus of this column.
Mixed results for beta-carotene’s UV protective effects. Ten years ago, Obermüller-Jevic et al. observed that beta-carotene exerted pro-oxidant activity in cultured human skin fibroblasts by promoting UVA induction of heme oxygenase-1 (HO-1). The investigators subsequently showed in vitro that the provitamin A compound potently fosters the UVA induction of proinflammatory interleukin-6 in skin fibroblasts (FEBS Lett. 1999;460:212-6; FEBS Lett. 2001;509:186-90). Nevertheless, several experimental, but not clinical, studies have suggested that dietary beta-carotene yields protective effects against acute and chronic cutaneous photodamage (Arch. Biochem. Biophys. 2001;389:1-6).
In 2000, Frieling et al. reported on their large-scale (n = 22,071) randomized, double-blind, placebo-controlled 12-year primary-prevention trial of beta-carotene supplementation with follow-up, in which they found that supplementing with 50 mg of beta-carotene on alternate days in apparently healthy male physicians aged 40-84 years in 1982 did not affect the development of a first basal cell or squamous cell carcinoma (Arch Dermatol. 2000;136:179-84). Other studies published earlier or around the same time yielded similar results, indicating that oral beta-carotene exerted no influence in stemming, preventing, or promoting nonmelanoma skin cancers (N. Engl. J. Med. 1990;323:789-95; Lancet 1999;354:723-9; J. Am. Acad. Dermatol. 2006;54:933-46).
In a prospective study of 1,001 randomly selected adults in one Australian community, investigators sought to determine the relationships between consumption of antioxidant nutrients and the relative risk of cutaneous basal cell and squamous cell carcinomas. In 1996 antioxidant consumption was estimated, and histologically confirmed cancers were recorded between 1996 and 2004. Dietary consumption in the second tertile for beta-carotene and vitamin E was associated with a higher basal cell carcinoma risk, with no trend, in subjects with a skin cancer history or a specific BCC history at baseline (Eur. J. Cancer 2007;43:2707-16).
In 2006, Stahl and Krutmann found that the systemic use of beta-carotene in dosages of 15-30 mg/day over 10-12 weeks conferred protection against UV-induced erythema, but failed to provide full protection against UVR (Hautarzt. 2006;57:281-5.)
More recently, in a paper published in 2008, Köpcke and Krutmann reported on their literature review of supplementation studies of dietary beta-carotene as protection against sunburn. Their review included studies published up to June 2007 in PubMed, ISI Web of Science, and the EBM Cochrane Library. The meta-analysis netted seven studies on the subject and revealed that, indeed, beta-carotene supplementation imparted time-dependent protection against sunburn, with at least 10 weeks of supplementation necessary (Photochem. Photobiol. 2008;84:284-8).
In September 2007, Stahl and Sies clarified that dietary carotenoids, particularly beta-carotene and lycopene, as well as flavonoids, help prevent the emergence of UV-induced erythema after the nutrients are disseminated to light-exposed areas, including the skin and eyes. Specifically, the tested carotenoids and flavonoids diminished sensitivity to UV-induced erythema in volunteers after 10-12 weeks of dietary intervention. The investigators speculated that such micronutrients have the potential to impart protection against UVR throughout an individual’s lifetime (Mol. Biotechnol. 2007;37:26-30).
Although the data on the impact of beta-carotene on aging skin and photoaging remain sparse, Bayerl has noted that beta-carotene has been shown to protect against the phototoxic effects of porphyrins in the photohemolysis model, and that the provitamin A substance is indicated for erythropoietic protoporphyria, photosensitive diseases, and mitigating the side effects of phototoxic drugs (Acta Dermatovenerol. Alp. Panonica Adriat. 2008;17:160-2, 164-6).
In 2009, Camera et al. compared the effects of the carotenoids astaxanthin, canthaxanthin, and beta-carotene on UVA-induced damage to human dermal fibroblasts. The carotenoids were delivered to the fibroblasts 24 hours before UVA exposure. Astaxanthin displayed the strongest photoprotective effect, reversing all of the noted changes induced by UVA, whereas beta-carotene partially prevented the UVA-induced reduction in catalase and superoxide dismutase activities. Beta-carotene also increased membrane damage, provoked HO-1 expression, and dose-dependently induced caspase-3 activity. In addition, the greatest photostability among the tested carotenoids was associated with astaxanthin, and the least, with beta-carotene (Exp. Dermatol. 2009;18:222-31).
Beta-carotene was cast in a more favorable light by a study published 2 months later by Valacchi et al. The researchers, acknowledging the apparent conflict between some studies suggesting that beta-carotene exhibits skin-protecting qualities as an antioxidant and other studies indicating pro-oxidant characteristics, examined the potential effects of oral beta-carotene supplement consumption on oxidative stress on the skin caused by ozone. They measured markers of oxidative stress in hairless mice after 1 month of a diet supplement with 0.5% beta-carotene and subsequent exposure to ozone (0.8 ppm; 6 hr/day; 7 days). The researchers found that beta-carotene downregulated the induction of tumor necrosis factor-alpha, macrophage inflammatory protein 2, inducible nitric oxide synthase, and HO-1 caused by ozone exposure. They concluded that beta-carotene does indeed confer protection against ozone-induced cutaneous oxidative stress in vivo (Toxicol. Ind. Health 2009;25:241-7).
Beta-carotene and lung cancer risk link. It is clear that dietary beta-carotene affords at least some cutaneous protection, though not against skin cancer, but the limits have not been fully elucidated as yet. It is important to note that beta-carotene supplementation has been shown to contribute to heightening the risk of lung cancer development in smokers and individuals exposed to asbestos (Evid. Rep. Technol. Assess. 2006;139:1-117).
In 2008, Tanyetyanon and Bepler conducted a meta-analysis of the literature on large randomized trials to assess the risk of lung cancer posed by beta-carotene consumption by smokers or former smokers. In their Medline search, the investigators identified four studies totaling 109,394 subjects. While they noted no increased risk of lung cancer among former smokers, they found a significant link between beta-carotene supplementation and increased lung cancer risk among current smokers (Cancer 2008;113:150-7).
In addition to this disturbing risk, there are some minor disadvantages associated with consuming excessive amounts of beta-carotene and other provitamin A substances. Specifically, the consumption of excessive amounts of beta-carotene through the diet or in supplement form can lead to skin yellowing. Because beta-carotene is inefficiently converted into retinol, supplementation with beta-carotene poses less risk of rendering the skin yellow than does vitamin A supplementation. It is best that patients and consumers derive the benefits of beta-carotene through the diet, but it can be a beneficial supplement to people living in warm climates where frequent sun exposure is more likely and whose diets do not include enough of this carotenoid.
Conclusions. As a prominent member of the carotenoid family, beta-carotene is a compelling subject for the investigation of its potential health benefits. While much more research is necessary to determine the range of effects (broadly, antioxidant to pro-oxidant) that this provitamin A substance can deliver to overall health as well as cutaneous health, in addition to its risks, there appears to be enough evidence to warrant confidence in suggesting a healthy dose of fruits and vegetables rich in beta-carotene to individuals who are not smokers.
I suspect that the strong pigmentary component of beta-carotene will continue to pose significant challenges to manufacturers who hope to harness its antioxidant potential in topical formulations. That said, it will be interesting to keep an eye on continuing investigations regarding the cutaneous effects of dietary consumption and oral supplementation with beta-carotene.
Beta-carotene is perhaps the best known member of the carotenoid family, a group of highly pigmented (red, orange, yellow), lipid-soluble substances present in various fruits, grains, oils, and vegetables (including apricots, carrots, green peppers, spinach, squash, sweet potatoes, and tomatoes).
Like alpha- and gamma-carotene, beta-carotene is a provitamin because it can be converted into active vitamin A (retinol). In comparison to other carotenoid compounds, some of which have been shown to have potential in the dermatologic armamentarium (e.g., astaxanthin, lutein, lycopene, and zeaxanthin), beta-carotene has been demonstrated to contribute much more to human nutrition (Am. J. Clin. Nutr. 2001;73:853-64).
The degree to which beta-carotene can be harnessed for dermatologic applications is debatable, with the aforementioned carotenoids displaying greater promise in this realm. In particular, the potent yellow/orange pigmentation delivered by beta-carotene poses significant challenges to topical formulation. That said, recent research has indicated that cutaneous benefits can be derived from the systemic use of beta-carotene. That is the focus of this column.
Mixed results for beta-carotene’s UV protective effects. Ten years ago, Obermüller-Jevic et al. observed that beta-carotene exerted pro-oxidant activity in cultured human skin fibroblasts by promoting UVA induction of heme oxygenase-1 (HO-1). The investigators subsequently showed in vitro that the provitamin A compound potently fosters the UVA induction of proinflammatory interleukin-6 in skin fibroblasts (FEBS Lett. 1999;460:212-6; FEBS Lett. 2001;509:186-90). Nevertheless, several experimental, but not clinical, studies have suggested that dietary beta-carotene yields protective effects against acute and chronic cutaneous photodamage (Arch. Biochem. Biophys. 2001;389:1-6).
In 2000, Frieling et al. reported on their large-scale (n = 22,071) randomized, double-blind, placebo-controlled 12-year primary-prevention trial of beta-carotene supplementation with follow-up, in which they found that supplementing with 50 mg of beta-carotene on alternate days in apparently healthy male physicians aged 40-84 years in 1982 did not affect the development of a first basal cell or squamous cell carcinoma (Arch Dermatol. 2000;136:179-84). Other studies published earlier or around the same time yielded similar results, indicating that oral beta-carotene exerted no influence in stemming, preventing, or promoting nonmelanoma skin cancers (N. Engl. J. Med. 1990;323:789-95; Lancet 1999;354:723-9; J. Am. Acad. Dermatol. 2006;54:933-46).
In a prospective study of 1,001 randomly selected adults in one Australian community, investigators sought to determine the relationships between consumption of antioxidant nutrients and the relative risk of cutaneous basal cell and squamous cell carcinomas. In 1996 antioxidant consumption was estimated, and histologically confirmed cancers were recorded between 1996 and 2004. Dietary consumption in the second tertile for beta-carotene and vitamin E was associated with a higher basal cell carcinoma risk, with no trend, in subjects with a skin cancer history or a specific BCC history at baseline (Eur. J. Cancer 2007;43:2707-16).
In 2006, Stahl and Krutmann found that the systemic use of beta-carotene in dosages of 15-30 mg/day over 10-12 weeks conferred protection against UV-induced erythema, but failed to provide full protection against UVR (Hautarzt. 2006;57:281-5.)
More recently, in a paper published in 2008, Köpcke and Krutmann reported on their literature review of supplementation studies of dietary beta-carotene as protection against sunburn. Their review included studies published up to June 2007 in PubMed, ISI Web of Science, and the EBM Cochrane Library. The meta-analysis netted seven studies on the subject and revealed that, indeed, beta-carotene supplementation imparted time-dependent protection against sunburn, with at least 10 weeks of supplementation necessary (Photochem. Photobiol. 2008;84:284-8).
In September 2007, Stahl and Sies clarified that dietary carotenoids, particularly beta-carotene and lycopene, as well as flavonoids, help prevent the emergence of UV-induced erythema after the nutrients are disseminated to light-exposed areas, including the skin and eyes. Specifically, the tested carotenoids and flavonoids diminished sensitivity to UV-induced erythema in volunteers after 10-12 weeks of dietary intervention. The investigators speculated that such micronutrients have the potential to impart protection against UVR throughout an individual’s lifetime (Mol. Biotechnol. 2007;37:26-30).
Although the data on the impact of beta-carotene on aging skin and photoaging remain sparse, Bayerl has noted that beta-carotene has been shown to protect against the phototoxic effects of porphyrins in the photohemolysis model, and that the provitamin A substance is indicated for erythropoietic protoporphyria, photosensitive diseases, and mitigating the side effects of phototoxic drugs (Acta Dermatovenerol. Alp. Panonica Adriat. 2008;17:160-2, 164-6).
In 2009, Camera et al. compared the effects of the carotenoids astaxanthin, canthaxanthin, and beta-carotene on UVA-induced damage to human dermal fibroblasts. The carotenoids were delivered to the fibroblasts 24 hours before UVA exposure. Astaxanthin displayed the strongest photoprotective effect, reversing all of the noted changes induced by UVA, whereas beta-carotene partially prevented the UVA-induced reduction in catalase and superoxide dismutase activities. Beta-carotene also increased membrane damage, provoked HO-1 expression, and dose-dependently induced caspase-3 activity. In addition, the greatest photostability among the tested carotenoids was associated with astaxanthin, and the least, with beta-carotene (Exp. Dermatol. 2009;18:222-31).
Beta-carotene was cast in a more favorable light by a study published 2 months later by Valacchi et al. The researchers, acknowledging the apparent conflict between some studies suggesting that beta-carotene exhibits skin-protecting qualities as an antioxidant and other studies indicating pro-oxidant characteristics, examined the potential effects of oral beta-carotene supplement consumption on oxidative stress on the skin caused by ozone. They measured markers of oxidative stress in hairless mice after 1 month of a diet supplement with 0.5% beta-carotene and subsequent exposure to ozone (0.8 ppm; 6 hr/day; 7 days). The researchers found that beta-carotene downregulated the induction of tumor necrosis factor-alpha, macrophage inflammatory protein 2, inducible nitric oxide synthase, and HO-1 caused by ozone exposure. They concluded that beta-carotene does indeed confer protection against ozone-induced cutaneous oxidative stress in vivo (Toxicol. Ind. Health 2009;25:241-7).
Beta-carotene and lung cancer risk link. It is clear that dietary beta-carotene affords at least some cutaneous protection, though not against skin cancer, but the limits have not been fully elucidated as yet. It is important to note that beta-carotene supplementation has been shown to contribute to heightening the risk of lung cancer development in smokers and individuals exposed to asbestos (Evid. Rep. Technol. Assess. 2006;139:1-117).
In 2008, Tanyetyanon and Bepler conducted a meta-analysis of the literature on large randomized trials to assess the risk of lung cancer posed by beta-carotene consumption by smokers or former smokers. In their Medline search, the investigators identified four studies totaling 109,394 subjects. While they noted no increased risk of lung cancer among former smokers, they found a significant link between beta-carotene supplementation and increased lung cancer risk among current smokers (Cancer 2008;113:150-7).
In addition to this disturbing risk, there are some minor disadvantages associated with consuming excessive amounts of beta-carotene and other provitamin A substances. Specifically, the consumption of excessive amounts of beta-carotene through the diet or in supplement form can lead to skin yellowing. Because beta-carotene is inefficiently converted into retinol, supplementation with beta-carotene poses less risk of rendering the skin yellow than does vitamin A supplementation. It is best that patients and consumers derive the benefits of beta-carotene through the diet, but it can be a beneficial supplement to people living in warm climates where frequent sun exposure is more likely and whose diets do not include enough of this carotenoid.
Conclusions. As a prominent member of the carotenoid family, beta-carotene is a compelling subject for the investigation of its potential health benefits. While much more research is necessary to determine the range of effects (broadly, antioxidant to pro-oxidant) that this provitamin A substance can deliver to overall health as well as cutaneous health, in addition to its risks, there appears to be enough evidence to warrant confidence in suggesting a healthy dose of fruits and vegetables rich in beta-carotene to individuals who are not smokers.
I suspect that the strong pigmentary component of beta-carotene will continue to pose significant challenges to manufacturers who hope to harness its antioxidant potential in topical formulations. That said, it will be interesting to keep an eye on continuing investigations regarding the cutaneous effects of dietary consumption and oral supplementation with beta-carotene.
Marketing Stem Cells for Aesthetic Medicine 'Possibly Unethical'
The stem cell facelift sounds like science: transplanting adult stem cells from the body's own fat tissue into the face so the stem cell growth factors can generate new tissue and restore the smoothness and skin tightness of youth.
However, the problem, according to Dr. J. Peter Rubin, codirector of the Adipose Stem Cell Center at the University of Pittsburgh, is that the science "just isn't there yet," so marketing stem cell procedures for aesthetic medicine is "premature and possibly unethical."
Of approximately 9,000 reports in the medical literature about stem cells, only 20 are peer-reviewed studies about their use in aesthetic procedures, Dr. Rubin said at the annual meeting of the American Society for Aesthetic Plastic Surgery, and none "demonstrate superiority of stem cell facelift over [conventional] facelift with standard fat grafting."
Despite the dearth of evidence demonstrating the safety or efficacy of stem cell therapies in aesthetic medicine, the procedures are being widely marketed, he said, noting that in some cases, the treatments don't even include any stem cell work, but simply involve regular fat grafting.
In an effort to discourage the proliferation of unsubstantiated claims about stem cell therapies, a joint task force of the annual meeting of the American Society for Aesthetic Plastic Surgery (ASAPS) and the American Society of Plastic Surgeons (ASPS) released a position statement at the meeting recommending against the marketing and promotion of stem cell procedures in aesthetic surgery until there is adequate clinical evidence to support doing so.
Specifically, the ASAPS/ASPS position statement stressed that "the marketing and promotion of stem cell procedures in aesthetic surgery is not adequately supported by clinical evidence at this time," and as such recommends that:
- The use of phrases such as stem cell therapy or stem cell procedure be reserved for treatments or techniques in which the collection, processing, and therapeutic action of stem cells are the primary goal of treatment rather than the passive result. "Standard fat grafting procedures that do transfer some stem cells naturally present within the tissue should be described as fat grafting procedures, not stem cell procedures," according to the document.
- Data on outcomes and safety should be collected and reported by physicians performing stem cell therapies to advance the knowledge and the science of the process.
- Stem cell therapies in aesthetic and reconstructive surgery should be conducted within clinical studies under Institutional Review Board approval.
- Stem cell procedures should be performed in compliance with Food and Drug Administration regulatory guidelines.
"There are encouraging data from laboratory and clinical studies suggesting the use of adult stem cells is promising, but there is not enough science to justify the widespread marketing of it," said Dr. Rubin.
The goal of the position statement is not to diminish enthusiasm about the potential for stem cell treatments," he said, "but to support evidence-based practices in order to protect patients' best interests."
Dr. Rubin receives educational support from Covidien.
The stem cell facelift sounds like science: transplanting adult stem cells from the body's own fat tissue into the face so the stem cell growth factors can generate new tissue and restore the smoothness and skin tightness of youth.
However, the problem, according to Dr. J. Peter Rubin, codirector of the Adipose Stem Cell Center at the University of Pittsburgh, is that the science "just isn't there yet," so marketing stem cell procedures for aesthetic medicine is "premature and possibly unethical."
Of approximately 9,000 reports in the medical literature about stem cells, only 20 are peer-reviewed studies about their use in aesthetic procedures, Dr. Rubin said at the annual meeting of the American Society for Aesthetic Plastic Surgery, and none "demonstrate superiority of stem cell facelift over [conventional] facelift with standard fat grafting."
Despite the dearth of evidence demonstrating the safety or efficacy of stem cell therapies in aesthetic medicine, the procedures are being widely marketed, he said, noting that in some cases, the treatments don't even include any stem cell work, but simply involve regular fat grafting.
In an effort to discourage the proliferation of unsubstantiated claims about stem cell therapies, a joint task force of the annual meeting of the American Society for Aesthetic Plastic Surgery (ASAPS) and the American Society of Plastic Surgeons (ASPS) released a position statement at the meeting recommending against the marketing and promotion of stem cell procedures in aesthetic surgery until there is adequate clinical evidence to support doing so.
Specifically, the ASAPS/ASPS position statement stressed that "the marketing and promotion of stem cell procedures in aesthetic surgery is not adequately supported by clinical evidence at this time," and as such recommends that:
- The use of phrases such as stem cell therapy or stem cell procedure be reserved for treatments or techniques in which the collection, processing, and therapeutic action of stem cells are the primary goal of treatment rather than the passive result. "Standard fat grafting procedures that do transfer some stem cells naturally present within the tissue should be described as fat grafting procedures, not stem cell procedures," according to the document.
- Data on outcomes and safety should be collected and reported by physicians performing stem cell therapies to advance the knowledge and the science of the process.
- Stem cell therapies in aesthetic and reconstructive surgery should be conducted within clinical studies under Institutional Review Board approval.
- Stem cell procedures should be performed in compliance with Food and Drug Administration regulatory guidelines.
"There are encouraging data from laboratory and clinical studies suggesting the use of adult stem cells is promising, but there is not enough science to justify the widespread marketing of it," said Dr. Rubin.
The goal of the position statement is not to diminish enthusiasm about the potential for stem cell treatments," he said, "but to support evidence-based practices in order to protect patients' best interests."
Dr. Rubin receives educational support from Covidien.
The stem cell facelift sounds like science: transplanting adult stem cells from the body's own fat tissue into the face so the stem cell growth factors can generate new tissue and restore the smoothness and skin tightness of youth.
However, the problem, according to Dr. J. Peter Rubin, codirector of the Adipose Stem Cell Center at the University of Pittsburgh, is that the science "just isn't there yet," so marketing stem cell procedures for aesthetic medicine is "premature and possibly unethical."
Of approximately 9,000 reports in the medical literature about stem cells, only 20 are peer-reviewed studies about their use in aesthetic procedures, Dr. Rubin said at the annual meeting of the American Society for Aesthetic Plastic Surgery, and none "demonstrate superiority of stem cell facelift over [conventional] facelift with standard fat grafting."
Despite the dearth of evidence demonstrating the safety or efficacy of stem cell therapies in aesthetic medicine, the procedures are being widely marketed, he said, noting that in some cases, the treatments don't even include any stem cell work, but simply involve regular fat grafting.
In an effort to discourage the proliferation of unsubstantiated claims about stem cell therapies, a joint task force of the annual meeting of the American Society for Aesthetic Plastic Surgery (ASAPS) and the American Society of Plastic Surgeons (ASPS) released a position statement at the meeting recommending against the marketing and promotion of stem cell procedures in aesthetic surgery until there is adequate clinical evidence to support doing so.
Specifically, the ASAPS/ASPS position statement stressed that "the marketing and promotion of stem cell procedures in aesthetic surgery is not adequately supported by clinical evidence at this time," and as such recommends that:
- The use of phrases such as stem cell therapy or stem cell procedure be reserved for treatments or techniques in which the collection, processing, and therapeutic action of stem cells are the primary goal of treatment rather than the passive result. "Standard fat grafting procedures that do transfer some stem cells naturally present within the tissue should be described as fat grafting procedures, not stem cell procedures," according to the document.
- Data on outcomes and safety should be collected and reported by physicians performing stem cell therapies to advance the knowledge and the science of the process.
- Stem cell therapies in aesthetic and reconstructive surgery should be conducted within clinical studies under Institutional Review Board approval.
- Stem cell procedures should be performed in compliance with Food and Drug Administration regulatory guidelines.
"There are encouraging data from laboratory and clinical studies suggesting the use of adult stem cells is promising, but there is not enough science to justify the widespread marketing of it," said Dr. Rubin.
The goal of the position statement is not to diminish enthusiasm about the potential for stem cell treatments," he said, "but to support evidence-based practices in order to protect patients' best interests."
Dr. Rubin receives educational support from Covidien.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR AESTHETIC PLASTIC SURGERY
House Members Seek Recall of Formaldehyde-Containing Hair Straighteners
Ten members of Congress have written to the Food and Drug Administration asking that the agency recall several brands of hair-straightening products that they say contain formaldehyde.
In a May 6 letter to FDA Commissioner Margaret Hamburg, U.S. House of Representative members wrote that the Brazilian Blowout Solution and Acai Professional Smoothing Solution both contain formaldehyde, but the chemical is not listed on the product labels as an ingredient.
Rep. Earl Blumenauer (D-Ore.) had written to the agency last fall outlining complaints from Oregon hair stylists who had been experiencing acute reactions, such as nosebleeds, while working with the hair straighteners. The congressman also noted testing conducted by the Oregon Occupational Safety and Health Division and by Oregon Health and Science University found the two products contained between 6% and 12% formaldehyde.
He said that the federal Occupational Safety and Health Administration (OSHA) subsequently issued a hazard alert warning salons to steer clear of formaldehyde-based straighteners.
Formaldehyde has been recognized as a possible carcinogen and has been the subject of 47 complaints to the Environmental Working Group, members of Congress noted in the letter.
"It is clear that the FDA needs to take decisive action," they wrote.
In addition to a recall, they are seeking a warning for products that contain formaldehyde, an investigation into the companies that are claiming their products are formaldehyde free, and a review of whether the chemical should be banned from hair straighteners.
According to the May 6 letter to Dr. Hamburg, the FDA responded in late November to Rep. Blumenauer's initial letter and said that it was investigating whether the products were being marketed directly to consumers. "If so, failure to comply with the ingredient declaration requirement would constitute misbranding," responded the agency.
Ten members of Congress have written to the Food and Drug Administration asking that the agency recall several brands of hair-straightening products that they say contain formaldehyde.
In a May 6 letter to FDA Commissioner Margaret Hamburg, U.S. House of Representative members wrote that the Brazilian Blowout Solution and Acai Professional Smoothing Solution both contain formaldehyde, but the chemical is not listed on the product labels as an ingredient.
Rep. Earl Blumenauer (D-Ore.) had written to the agency last fall outlining complaints from Oregon hair stylists who had been experiencing acute reactions, such as nosebleeds, while working with the hair straighteners. The congressman also noted testing conducted by the Oregon Occupational Safety and Health Division and by Oregon Health and Science University found the two products contained between 6% and 12% formaldehyde.
He said that the federal Occupational Safety and Health Administration (OSHA) subsequently issued a hazard alert warning salons to steer clear of formaldehyde-based straighteners.
Formaldehyde has been recognized as a possible carcinogen and has been the subject of 47 complaints to the Environmental Working Group, members of Congress noted in the letter.
"It is clear that the FDA needs to take decisive action," they wrote.
In addition to a recall, they are seeking a warning for products that contain formaldehyde, an investigation into the companies that are claiming their products are formaldehyde free, and a review of whether the chemical should be banned from hair straighteners.
According to the May 6 letter to Dr. Hamburg, the FDA responded in late November to Rep. Blumenauer's initial letter and said that it was investigating whether the products were being marketed directly to consumers. "If so, failure to comply with the ingredient declaration requirement would constitute misbranding," responded the agency.
Ten members of Congress have written to the Food and Drug Administration asking that the agency recall several brands of hair-straightening products that they say contain formaldehyde.
In a May 6 letter to FDA Commissioner Margaret Hamburg, U.S. House of Representative members wrote that the Brazilian Blowout Solution and Acai Professional Smoothing Solution both contain formaldehyde, but the chemical is not listed on the product labels as an ingredient.
Rep. Earl Blumenauer (D-Ore.) had written to the agency last fall outlining complaints from Oregon hair stylists who had been experiencing acute reactions, such as nosebleeds, while working with the hair straighteners. The congressman also noted testing conducted by the Oregon Occupational Safety and Health Division and by Oregon Health and Science University found the two products contained between 6% and 12% formaldehyde.
He said that the federal Occupational Safety and Health Administration (OSHA) subsequently issued a hazard alert warning salons to steer clear of formaldehyde-based straighteners.
Formaldehyde has been recognized as a possible carcinogen and has been the subject of 47 complaints to the Environmental Working Group, members of Congress noted in the letter.
"It is clear that the FDA needs to take decisive action," they wrote.
In addition to a recall, they are seeking a warning for products that contain formaldehyde, an investigation into the companies that are claiming their products are formaldehyde free, and a review of whether the chemical should be banned from hair straighteners.
According to the May 6 letter to Dr. Hamburg, the FDA responded in late November to Rep. Blumenauer's initial letter and said that it was investigating whether the products were being marketed directly to consumers. "If so, failure to comply with the ingredient declaration requirement would constitute misbranding," responded the agency.
Blog: Laser-Assisted Liposuction Gets Roasted at ASAPS
It happens to me all the time at medical conferences: I scramble to get myself to a meeting room, squeeze myself past aisle-seat squatters (you know who you are), get settled, pull out my notebook, and realize with the introduction of the first speaker that I'm in the wrong place. I then either get to where I need to be or just stay put, either because there's no easy way out or I hear or see something that piques my interest.
The latter happened yesterday at the annual meeting of the American Society for Aesthetic Plastic Surgery in Boston, when I found myself in scientific session A – "Lipoplasty: It's Us, Not the Machine" – instead of scientific session B – "The Face – Fillers vs. Fat."
Anticipating an industry-sponsored feel-good fest dominated by slide after slide of before and after success stories, I started gathering up my gear to make my escape, when something unexpected happened. One of the panelists, Dr. Simeon Wall of the Wall Center for Plastic Surgery in Shreveport, La., slammed laser-assisted liposuction, which, since its arrival on the scene in 2007, has been touted as being a more effective, more efficient fat removal technology than traditional liposuction, resulting in less bruising and swelling and quicker recovery times, along with improvements in skin tightness and the appearance of cellulite.
In fact, Dr. Wall stated, based on a review of the published literature, these claims "have no scientific basis," even though the technology is FDA-approved and the machines have been on the market for a few years.
Intrigued, I decided to stay put for a few minutes to hear what some of the other panelists had to say, thinking a potentially interesting debate might ensue. I was half right. What followed was interesting, but there was no debate. In fact, the panelists were mostly in agreement with Dr. Wall's assessment.
Dr. Constantino Mendieta, who is in private practice in Miami, referred to the laser liposuction device in his office as a "very expensive dust collector. It just doesn't work." Panel moderator Dr. Steven Teitelbaum of Santa Monica, Calif., suggested that the few good results achieved by select physicians who are extraordinarily proficient with the device are exceptions to the norm and that the rate of dangerous complications associated with the technology outweigh the remote chance of substantial benefit.
In response to a lament from a session attendee in the audience that the majority of the revisional liposuction cases he sees in his practice are the result of laser-assisted liposuction, Dr. Wall agreed, and noted that the deformities associated with laser-assisted liposuction are typically "more difficult to correct" than those associated with other liposuction methods.
Although the session was filled with the expected collection of before-and-after shots achieved using a range of liposuction methods (some of the differences were impressive; some barely discernable), I was glad I stayed.
The laser lipo-bashing was informative and entertaining, and the session seemed to lend credence to the stated theme of this year's ASAPS meeting: "Affirming the Science of Aesthetic Surgery," although the corollary – "Debunking the Hype" – might have been a more appropriate moniker. Either way, it was well played.
— Diana Mahoney
It happens to me all the time at medical conferences: I scramble to get myself to a meeting room, squeeze myself past aisle-seat squatters (you know who you are), get settled, pull out my notebook, and realize with the introduction of the first speaker that I'm in the wrong place. I then either get to where I need to be or just stay put, either because there's no easy way out or I hear or see something that piques my interest.
The latter happened yesterday at the annual meeting of the American Society for Aesthetic Plastic Surgery in Boston, when I found myself in scientific session A – "Lipoplasty: It's Us, Not the Machine" – instead of scientific session B – "The Face – Fillers vs. Fat."
Anticipating an industry-sponsored feel-good fest dominated by slide after slide of before and after success stories, I started gathering up my gear to make my escape, when something unexpected happened. One of the panelists, Dr. Simeon Wall of the Wall Center for Plastic Surgery in Shreveport, La., slammed laser-assisted liposuction, which, since its arrival on the scene in 2007, has been touted as being a more effective, more efficient fat removal technology than traditional liposuction, resulting in less bruising and swelling and quicker recovery times, along with improvements in skin tightness and the appearance of cellulite.
In fact, Dr. Wall stated, based on a review of the published literature, these claims "have no scientific basis," even though the technology is FDA-approved and the machines have been on the market for a few years.
Intrigued, I decided to stay put for a few minutes to hear what some of the other panelists had to say, thinking a potentially interesting debate might ensue. I was half right. What followed was interesting, but there was no debate. In fact, the panelists were mostly in agreement with Dr. Wall's assessment.
Dr. Constantino Mendieta, who is in private practice in Miami, referred to the laser liposuction device in his office as a "very expensive dust collector. It just doesn't work." Panel moderator Dr. Steven Teitelbaum of Santa Monica, Calif., suggested that the few good results achieved by select physicians who are extraordinarily proficient with the device are exceptions to the norm and that the rate of dangerous complications associated with the technology outweigh the remote chance of substantial benefit.
In response to a lament from a session attendee in the audience that the majority of the revisional liposuction cases he sees in his practice are the result of laser-assisted liposuction, Dr. Wall agreed, and noted that the deformities associated with laser-assisted liposuction are typically "more difficult to correct" than those associated with other liposuction methods.
Although the session was filled with the expected collection of before-and-after shots achieved using a range of liposuction methods (some of the differences were impressive; some barely discernable), I was glad I stayed.
The laser lipo-bashing was informative and entertaining, and the session seemed to lend credence to the stated theme of this year's ASAPS meeting: "Affirming the Science of Aesthetic Surgery," although the corollary – "Debunking the Hype" – might have been a more appropriate moniker. Either way, it was well played.
— Diana Mahoney
It happens to me all the time at medical conferences: I scramble to get myself to a meeting room, squeeze myself past aisle-seat squatters (you know who you are), get settled, pull out my notebook, and realize with the introduction of the first speaker that I'm in the wrong place. I then either get to where I need to be or just stay put, either because there's no easy way out or I hear or see something that piques my interest.
The latter happened yesterday at the annual meeting of the American Society for Aesthetic Plastic Surgery in Boston, when I found myself in scientific session A – "Lipoplasty: It's Us, Not the Machine" – instead of scientific session B – "The Face – Fillers vs. Fat."
Anticipating an industry-sponsored feel-good fest dominated by slide after slide of before and after success stories, I started gathering up my gear to make my escape, when something unexpected happened. One of the panelists, Dr. Simeon Wall of the Wall Center for Plastic Surgery in Shreveport, La., slammed laser-assisted liposuction, which, since its arrival on the scene in 2007, has been touted as being a more effective, more efficient fat removal technology than traditional liposuction, resulting in less bruising and swelling and quicker recovery times, along with improvements in skin tightness and the appearance of cellulite.
In fact, Dr. Wall stated, based on a review of the published literature, these claims "have no scientific basis," even though the technology is FDA-approved and the machines have been on the market for a few years.
Intrigued, I decided to stay put for a few minutes to hear what some of the other panelists had to say, thinking a potentially interesting debate might ensue. I was half right. What followed was interesting, but there was no debate. In fact, the panelists were mostly in agreement with Dr. Wall's assessment.
Dr. Constantino Mendieta, who is in private practice in Miami, referred to the laser liposuction device in his office as a "very expensive dust collector. It just doesn't work." Panel moderator Dr. Steven Teitelbaum of Santa Monica, Calif., suggested that the few good results achieved by select physicians who are extraordinarily proficient with the device are exceptions to the norm and that the rate of dangerous complications associated with the technology outweigh the remote chance of substantial benefit.
In response to a lament from a session attendee in the audience that the majority of the revisional liposuction cases he sees in his practice are the result of laser-assisted liposuction, Dr. Wall agreed, and noted that the deformities associated with laser-assisted liposuction are typically "more difficult to correct" than those associated with other liposuction methods.
Although the session was filled with the expected collection of before-and-after shots achieved using a range of liposuction methods (some of the differences were impressive; some barely discernable), I was glad I stayed.
The laser lipo-bashing was informative and entertaining, and the session seemed to lend credence to the stated theme of this year's ASAPS meeting: "Affirming the Science of Aesthetic Surgery," although the corollary – "Debunking the Hype" – might have been a more appropriate moniker. Either way, it was well played.
— Diana Mahoney