User login
Managing schizophrenia in a patient with cancer: A fine balance
CASE Stable with a new diagnosis
Ms. B, age 60, has a history of schizophrenia, which has been stable on clozapine, 500 mg/d, for more than 2 decades. After a series of hospitalizations in her 20s and 30s, clozapine was initiated and she has not required additional inpatient psychiatric care. She has been managed in the outpatient setting with standard biweekly absolute neutrophil count (ANC) monitoring. She lives independently and is an active member in her church.
After experiencing rectal bleeding, Ms. B is diagnosed with rectal carcinoma and is scheduled to undergo chemotherapy and radiation treatment.
[polldaddy:9754786]
The authors’ observations
Both clozapine and chemotherapy carry the risk of immunosuppression, presenting a clinical challenge when choosing an appropriate management strategy. However, the risks of stopping clozapine after a long period of symptom stability are substantial, with a relapse rate up to 50%.1 Among patients taking clozapine, the risk of agranulocytosis and neutropenia are approximately 0.8% and 3%, respectively, and >80% of agranulocyotis cases occur within the first 18 weeks of treatment.2,3 Although both clozapine and chemotherapy can lead to neutropenia and agranulocytosis, there currently is no evidence of a synergistic effect on bone marrow suppression with simultaneous use of these therapies2 nor is there evidence of the combination leading to sustained marrow suppression.4
Because of Ms. B’s positive response to clozapine, the risks associated with discontinuing the medication, and the relatively low risk of clozapine contributing to neutropenia after a long period of stabilization, her outpatient psychiatric providers decide to increase ANC monitoring to weekly while she undergoes cancer treatment.
TREATMENT Neutropenia, psychosis
Ms. B continues clozapine during radiation and chemotherapy, but develops leukopenia and neutropenia with a low of 1,220/μL white blood cells and an ANC of 610/μL. Clozapine is stopped, consistent with current recommendations to hold the drug if the neutrophil count is <1,000/μL in a patient without benign ethnic neutropenia, and her outpatient provider monitors her closely. The treatment team does not restart an antipsychotic immediately after discontinuing clozapine because of the risk that other antipsychotics can cause hematologic toxicity or prolong granulocytopenia associated with clozapine.5
Approximately 2 weeks later, Ms. B is admitted to a different hospital for altered mental status and is found to have hyponatremia and rectal bleeding. The workup suggests that her rectal carcinoma has not fully responded to initial therapies, and she likely will require further treatment. Her mental status improves after hyponatremia resolves, but she reports auditory hallucinations and paranoia. Risperidone, 4 mg/d, is initiated to target psychosis.
After discharge, Ms. B develops bilateral upper extremity tremor, which she finds intolerable and attributes to risperidone. She refuses to continue risperidone or try adjunctive medications to address the tremor, but is willing to consider a different antipsychotic. Olanzapine, 10 mg/d, is initiated and risperidone is slowly tapered. During this time, Ms. B experiences increased paranoia and believes that the Internal Revenue Service is calling her. She misses her next appointment.
Later, the fire department finds Ms. B wandering the streets and brings her to the psychiatric emergency room. During the examination, she is disheveled and withdrawn, and unable to reply to simple questions about diet and sleep. When asked why she was in the street, she says that she left her apartment because it was “too messy.” The treatment team learns that she had walked at least 10 miles from her apartment before sitting down by the side of the road and being picked up by the fire department. She reveals that she left her apartment and continued walking because “a man” told her to do so and threatened to harm her if she stopped.
When Ms. B is admitted to the psychiatric service, she is paranoid, disorganized, and guarded. She remains in her room for most of the day and either refuses to talk to providers or curses at them. She often is seen wearing soiled clothing with her hair mussed. She denies having rectal carcinoma, although she expressed understanding of her medical condition <2 months earlier.
[polldaddy:9754787]
The authors’ observations
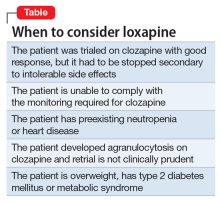
Clozapine is considered the most efficacious agent for treatment-resistant schizophrenia.6 Although non-compliance is the most common reason for discontinuing clozapine, >20% of patients stop clozapine because of adverse effects.7 Clozapine often is a drug of last resort because of the need for frequent monitoring and significant side effects; therefore deciding on a next step when clozapine fails or cannot be continued because of other factors can pose a challenge.
Ms. B’s treatment team gave serious consideration to restarting clozapine. However, because it was likely that Ms. B would undergo another round of chemotherapy and possibly radiation, the risk of neutropenia recurring was considered too high. Lithium has been used successfully to manage neutropenia in patients taking clozapine and, for some, adding lithium could help boost white cell count and allow a successful rechallenge with clozapine.3,8 However, because of Ms. B’s medical comorbidities, including cancer and chronic kidney disease, adding lithium was not thought to be clinically prudent at that time and the treatment team considered other options.
Olanzapine. Although research is limited, studies suggest olanzapine is the most commonly prescribed medication when a patient has to discontinue clozapine,7 with comparable response rates in those with refractory schizophrenia.9 Therefore, Ms. B was initially maintained on olanzapine, and the dosage increased to 30 mg over the course of 16 days in the hospital. However, she did not respond to the medication, remaining disorganized and paranoid without any notable improvement in her symptoms therefore other treatment options were explored.
Loxapine. Previous limited case reports have shown loxapine to be effective in treating individuals with refractory schizophrenia, either alone or in combination with other antipsychotics.10,11 FDA-approved in 1975, loxapine was among the last of the typical antipsychotics brought to the U.S. market before the introduction of clozapine, the first atypical.12 Loxapine is a dibenzoxazepine that has a molecular structure similar to clozapine.13 Unlike clozapine, however, loxapine is not known to cause agranulocytosis.14 Research suggests that although clozapine is oxidized to metabolites that are cytotoxic, loxapine is not, potentially accounting for their different effects on neutrophils.15
The efficacy of loxapine has shown to be similar to other typical and atypical antipsychotics, with approximately 70% of patients showing improvement.14 However, loxapine may be overlooked as an option, possibly because it was not included in the CATIE trial and was the last typical antipsychotic to be approved before atypicals were introduced.12 First available in oral and IM formulations, there has been increased interest in loxapine recently because of the approval of an inhaled formulation in 2012.16
Although classified as a typical antipsychotic, studies have suggested that loxapine acts as an atypical at low dosages.17,18 Previous work suggests, however, that the side effect profile of loxapine is similar to typical antipsychotics.14 At dosages <50 mg, it results in fewer cases of extrapyramidal side effects than expected with a typical antipsychotic.18
Loxapine’s binding profile seems to exist along this spectrum of typical to atypical. Tissue-based binding studies have shown a higher 5-HT2 affinity relative to D2, consistent with atypical antipsychotics.19 Positron emission tomography studies in humans show 5-HT2 saturation of loxapine to be close to equal to D2 binding in loxapine, thus a slightly lower ratio of 5-HT2 to D2 relative to atypicals, but more than that seen with typical antipsychotics.20 These differences between in vitro and in vivo studies may be secondary to the binding of loxapine’s active metabolites, particularly 7- and 8-hydroxyloxapine, which have more dopaminergic activity. In addition to increased 5-HT2A binding compared with typical antipsychotics, loxapine also has a high affinity for the D4 receptors, as well as interacting with other serotonin receptors 5-HT3, 5-HT6, and 5-HT7. Of note this is a similar pattern of binding affinity as seen in clozapine.19
It should be noted, however, that loxapine may not be an appropriate treatment in all forms of cancer. Similar to other first-generation antipsychotics, it increases prolactin levels, and thus may have a negative clinical impact on patients with prolactin receptor positive breast cancers.21,22 Finally, although clozapine can result in significant weight gain, dyslipidemia, and hyperglycemia, unlike many antipsychotics, loxapine has been shown to be weight neutral or result in weight loss,14 making it an option to consider for patients with type 2 diabetes mellitus, metabolic syndrome, dyslipidemia, or cardiovascular disease.
OUTCOME Improvement, stability
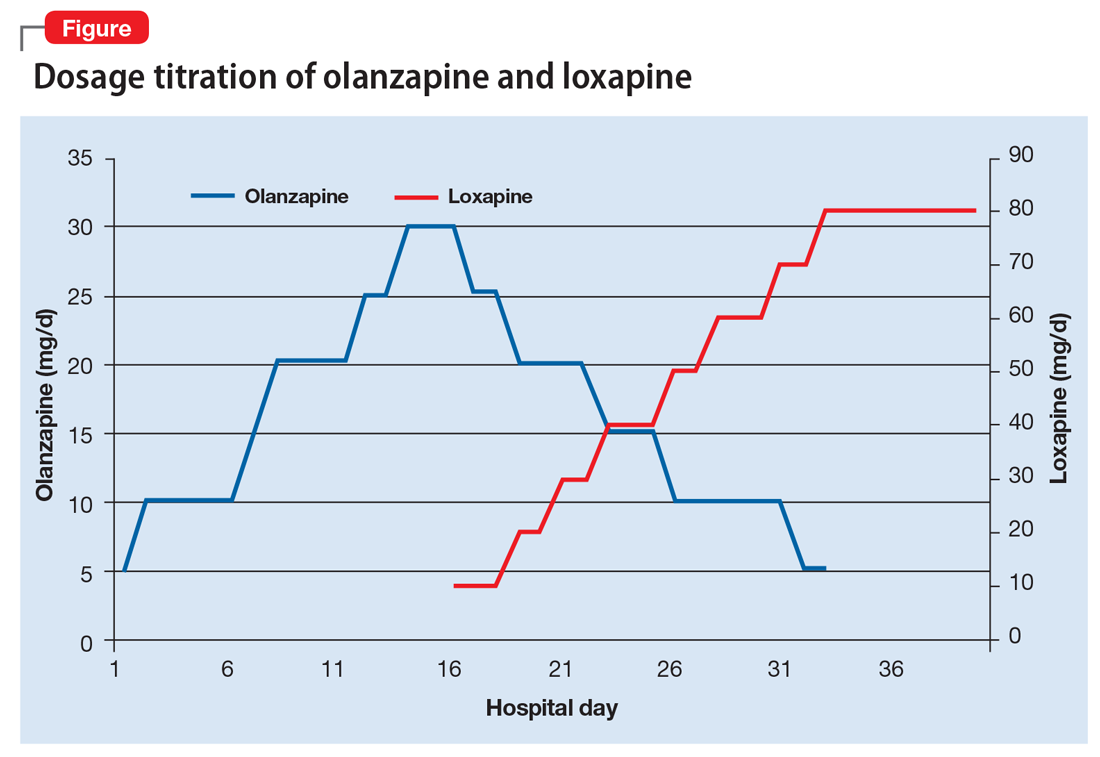
Ms. B begins taking loxapine, 10 mg/d, gradually cross-tapered with olanzapine, increasing loxapine by 10 mg every 2 to 3 days (Figure). After 8 days, when the dosage has reached 40 mg/d, Ms. B’s treatment team begins to observe a consistent change in her behavior. Ms. B comes into the interview room, where previously the team had to see her in her own room because she refused to come out. She also tolerates an extensive interview, even sharing parts of her history without prompting, and is able to discuss her treatment. Ms. B continues to express some paranoia regarding the treatment team. On day 12, receiving loxapine, 50 mg/d, Ms. B says that she likes the new medication and feels she is doing well with it. She becomes less reclusive and begins socializing with other patients. By day 19, receiving loxapine, 80 mg/d, a nurse, who knows Ms. B from the outpatient facility, visits the unit and reports that Ms. B is at her baseline.
At discharge, Ms. B is noted to be “bright,” well organized, neatly dressed, and wearing makeup. Her paranoia and auditory hallucinations have almost completely resolved. She is social, engages appropriately with the treatment team, and is able to describe a plan for self-care after discharge including following up with her oncologist. Her white blood cell counts were carefully monitored throughout her admission and are within normal limits when she is discharged.
One year later, Ms. B remains taking loxapine, 70 mg/d. Although she continues to report mild paranoia, she is living independently in her apartment and attends church regularly.
2. Usta NG, Poyraz CA, Aktan M, et al. Clozapine treatment of refractory schizophrenia during essential chemotherapy: a case study and mini review of a clinical dilemma. Ther Adv Psychopharmacol. 2014;4(6):276-281.
3. Meyer N, Gee S, Whiskey E, et al. Optimizing outcomes in clozapine rechallenge following neutropenia: a cohort analysis. J Clin Psychiatry. 2015;76(11):e1410-e1416.
4. Cunningham NT, Dennis N, Dattilo W, et al. Continuation of clozapine during chemotherapy: a case report and review of literature. Psychosomatics. 2014;55(6):673-679.
5. Co¸sar B, Taner ME, Eser HY, et al. Does switching to another antipsychotic in patients with clozapine-associated granulocytopenia solve the problem? Case series of 18. J Clin Psychopharmacol. 2011;31(2):169-173.
6. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigation. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
7. Mustafa FA, Burke JG, Abukmeil SS, et al. “Schizophrenia past clozapine”: reasons for clozapine discontinuation, mortality, and alternative antipsychotic prescribing. Pharmacopsychiatry. 2015;48(1):11-14.
8. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
9. Bitter I, Dossenbach MR, Brook S, et al; Olanzapine HGCK Study Group. Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):173-180.
10. Lehmann CR, Ereshefsky L, Saklad SR, et al. Very high dose loxapine in refractory schizophrenic patients. Am J Psychiatry. 1981;138(9):1212-1214.
11. Sokolski KN. Combination loxapine and aripiprazole for refractory hallucinations in schizophrenia. Ann Pharmacother. 2011;45(7-8):e36.
12. Shen WW. A history of antipsychotic drug development. Compr Psychiatry. 1999;40(6):407-414.
13. Mazzola CD, Miron S, Jenkins AJ. Loxapine intoxication: case report and literature review. J Anal Toxicol. 2000;24(7):638-641.
14. Chakrabarti A, Bagnall A, Chue P, et al. Loxapine for schizophrenia. Cochrane Database Syst Rev. 2007(4):CD001943.
15. Jegouzo A, Gressier B, Frimat B, et al. Comparative oxidation of loxapine and clozapine by human neutrophils. Fundam Clin Pharmacol. 1999;13(1):113-119.
16. Keating GM. Loxapine inhalation powder: a review of its use in the acute treatment of agitation in patients with bipolar disorder or schizophrenia. CNS Drugs. 2013;27(6):479-489.
1 7. Glazer WM. Does loxapine have “atypical” properties? Clinical evidence. J Clin Psychiatry. 1999;60(suppl 10):42-46.
18. Hellings JA, Jadhav M, Jain S, et al. Low dose loxapine: neuromotor side effects and tolerability in autism spectrum disorders. J Child Adolesc Psychopharmacol. 2015;25(8):618-624.
19. Singh AN, Barlas C, Singh S, et al. A neurochemical basis for the antipsychotic activity of loxapine: interactions with dopamine D1, D2, D4 and serotonin 5-HT2 receptor subtypes. J Psychiatry Neurosci. 1996;21(1):29-35.
20. Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156(2):286-293.
21. Robertson AG, Berry R, Meltzer HY. Prolactin stimulating effects of amoxapine and loxapine in psychiatric patients. Psychopharmacology (Berl). 1982;78(3):287-292.
22. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
CASE Stable with a new diagnosis
Ms. B, age 60, has a history of schizophrenia, which has been stable on clozapine, 500 mg/d, for more than 2 decades. After a series of hospitalizations in her 20s and 30s, clozapine was initiated and she has not required additional inpatient psychiatric care. She has been managed in the outpatient setting with standard biweekly absolute neutrophil count (ANC) monitoring. She lives independently and is an active member in her church.
After experiencing rectal bleeding, Ms. B is diagnosed with rectal carcinoma and is scheduled to undergo chemotherapy and radiation treatment.
[polldaddy:9754786]
The authors’ observations
Both clozapine and chemotherapy carry the risk of immunosuppression, presenting a clinical challenge when choosing an appropriate management strategy. However, the risks of stopping clozapine after a long period of symptom stability are substantial, with a relapse rate up to 50%.1 Among patients taking clozapine, the risk of agranulocytosis and neutropenia are approximately 0.8% and 3%, respectively, and >80% of agranulocyotis cases occur within the first 18 weeks of treatment.2,3 Although both clozapine and chemotherapy can lead to neutropenia and agranulocytosis, there currently is no evidence of a synergistic effect on bone marrow suppression with simultaneous use of these therapies2 nor is there evidence of the combination leading to sustained marrow suppression.4
Because of Ms. B’s positive response to clozapine, the risks associated with discontinuing the medication, and the relatively low risk of clozapine contributing to neutropenia after a long period of stabilization, her outpatient psychiatric providers decide to increase ANC monitoring to weekly while she undergoes cancer treatment.
TREATMENT Neutropenia, psychosis
Ms. B continues clozapine during radiation and chemotherapy, but develops leukopenia and neutropenia with a low of 1,220/μL white blood cells and an ANC of 610/μL. Clozapine is stopped, consistent with current recommendations to hold the drug if the neutrophil count is <1,000/μL in a patient without benign ethnic neutropenia, and her outpatient provider monitors her closely. The treatment team does not restart an antipsychotic immediately after discontinuing clozapine because of the risk that other antipsychotics can cause hematologic toxicity or prolong granulocytopenia associated with clozapine.5
Approximately 2 weeks later, Ms. B is admitted to a different hospital for altered mental status and is found to have hyponatremia and rectal bleeding. The workup suggests that her rectal carcinoma has not fully responded to initial therapies, and she likely will require further treatment. Her mental status improves after hyponatremia resolves, but she reports auditory hallucinations and paranoia. Risperidone, 4 mg/d, is initiated to target psychosis.
After discharge, Ms. B develops bilateral upper extremity tremor, which she finds intolerable and attributes to risperidone. She refuses to continue risperidone or try adjunctive medications to address the tremor, but is willing to consider a different antipsychotic. Olanzapine, 10 mg/d, is initiated and risperidone is slowly tapered. During this time, Ms. B experiences increased paranoia and believes that the Internal Revenue Service is calling her. She misses her next appointment.
Later, the fire department finds Ms. B wandering the streets and brings her to the psychiatric emergency room. During the examination, she is disheveled and withdrawn, and unable to reply to simple questions about diet and sleep. When asked why she was in the street, she says that she left her apartment because it was “too messy.” The treatment team learns that she had walked at least 10 miles from her apartment before sitting down by the side of the road and being picked up by the fire department. She reveals that she left her apartment and continued walking because “a man” told her to do so and threatened to harm her if she stopped.
When Ms. B is admitted to the psychiatric service, she is paranoid, disorganized, and guarded. She remains in her room for most of the day and either refuses to talk to providers or curses at them. She often is seen wearing soiled clothing with her hair mussed. She denies having rectal carcinoma, although she expressed understanding of her medical condition <2 months earlier.
[polldaddy:9754787]
The authors’ observations
Clozapine is considered the most efficacious agent for treatment-resistant schizophrenia.6 Although non-compliance is the most common reason for discontinuing clozapine, >20% of patients stop clozapine because of adverse effects.7 Clozapine often is a drug of last resort because of the need for frequent monitoring and significant side effects; therefore deciding on a next step when clozapine fails or cannot be continued because of other factors can pose a challenge.
Ms. B’s treatment team gave serious consideration to restarting clozapine. However, because it was likely that Ms. B would undergo another round of chemotherapy and possibly radiation, the risk of neutropenia recurring was considered too high. Lithium has been used successfully to manage neutropenia in patients taking clozapine and, for some, adding lithium could help boost white cell count and allow a successful rechallenge with clozapine.3,8 However, because of Ms. B’s medical comorbidities, including cancer and chronic kidney disease, adding lithium was not thought to be clinically prudent at that time and the treatment team considered other options.
Olanzapine. Although research is limited, studies suggest olanzapine is the most commonly prescribed medication when a patient has to discontinue clozapine,7 with comparable response rates in those with refractory schizophrenia.9 Therefore, Ms. B was initially maintained on olanzapine, and the dosage increased to 30 mg over the course of 16 days in the hospital. However, she did not respond to the medication, remaining disorganized and paranoid without any notable improvement in her symptoms therefore other treatment options were explored.
Loxapine. Previous limited case reports have shown loxapine to be effective in treating individuals with refractory schizophrenia, either alone or in combination with other antipsychotics.10,11 FDA-approved in 1975, loxapine was among the last of the typical antipsychotics brought to the U.S. market before the introduction of clozapine, the first atypical.12 Loxapine is a dibenzoxazepine that has a molecular structure similar to clozapine.13 Unlike clozapine, however, loxapine is not known to cause agranulocytosis.14 Research suggests that although clozapine is oxidized to metabolites that are cytotoxic, loxapine is not, potentially accounting for their different effects on neutrophils.15
The efficacy of loxapine has shown to be similar to other typical and atypical antipsychotics, with approximately 70% of patients showing improvement.14 However, loxapine may be overlooked as an option, possibly because it was not included in the CATIE trial and was the last typical antipsychotic to be approved before atypicals were introduced.12 First available in oral and IM formulations, there has been increased interest in loxapine recently because of the approval of an inhaled formulation in 2012.16
Although classified as a typical antipsychotic, studies have suggested that loxapine acts as an atypical at low dosages.17,18 Previous work suggests, however, that the side effect profile of loxapine is similar to typical antipsychotics.14 At dosages <50 mg, it results in fewer cases of extrapyramidal side effects than expected with a typical antipsychotic.18
Loxapine’s binding profile seems to exist along this spectrum of typical to atypical. Tissue-based binding studies have shown a higher 5-HT2 affinity relative to D2, consistent with atypical antipsychotics.19 Positron emission tomography studies in humans show 5-HT2 saturation of loxapine to be close to equal to D2 binding in loxapine, thus a slightly lower ratio of 5-HT2 to D2 relative to atypicals, but more than that seen with typical antipsychotics.20 These differences between in vitro and in vivo studies may be secondary to the binding of loxapine’s active metabolites, particularly 7- and 8-hydroxyloxapine, which have more dopaminergic activity. In addition to increased 5-HT2A binding compared with typical antipsychotics, loxapine also has a high affinity for the D4 receptors, as well as interacting with other serotonin receptors 5-HT3, 5-HT6, and 5-HT7. Of note this is a similar pattern of binding affinity as seen in clozapine.19
It should be noted, however, that loxapine may not be an appropriate treatment in all forms of cancer. Similar to other first-generation antipsychotics, it increases prolactin levels, and thus may have a negative clinical impact on patients with prolactin receptor positive breast cancers.21,22 Finally, although clozapine can result in significant weight gain, dyslipidemia, and hyperglycemia, unlike many antipsychotics, loxapine has been shown to be weight neutral or result in weight loss,14 making it an option to consider for patients with type 2 diabetes mellitus, metabolic syndrome, dyslipidemia, or cardiovascular disease.
OUTCOME Improvement, stability
Ms. B begins taking loxapine, 10 mg/d, gradually cross-tapered with olanzapine, increasing loxapine by 10 mg every 2 to 3 days (Figure). After 8 days, when the dosage has reached 40 mg/d, Ms. B’s treatment team begins to observe a consistent change in her behavior. Ms. B comes into the interview room, where previously the team had to see her in her own room because she refused to come out. She also tolerates an extensive interview, even sharing parts of her history without prompting, and is able to discuss her treatment. Ms. B continues to express some paranoia regarding the treatment team. On day 12, receiving loxapine, 50 mg/d, Ms. B says that she likes the new medication and feels she is doing well with it. She becomes less reclusive and begins socializing with other patients. By day 19, receiving loxapine, 80 mg/d, a nurse, who knows Ms. B from the outpatient facility, visits the unit and reports that Ms. B is at her baseline.

At discharge, Ms. B is noted to be “bright,” well organized, neatly dressed, and wearing makeup. Her paranoia and auditory hallucinations have almost completely resolved. She is social, engages appropriately with the treatment team, and is able to describe a plan for self-care after discharge including following up with her oncologist. Her white blood cell counts were carefully monitored throughout her admission and are within normal limits when she is discharged.
One year later, Ms. B remains taking loxapine, 70 mg/d. Although she continues to report mild paranoia, she is living independently in her apartment and attends church regularly.
CASE Stable with a new diagnosis
Ms. B, age 60, has a history of schizophrenia, which has been stable on clozapine, 500 mg/d, for more than 2 decades. After a series of hospitalizations in her 20s and 30s, clozapine was initiated and she has not required additional inpatient psychiatric care. She has been managed in the outpatient setting with standard biweekly absolute neutrophil count (ANC) monitoring. She lives independently and is an active member in her church.
After experiencing rectal bleeding, Ms. B is diagnosed with rectal carcinoma and is scheduled to undergo chemotherapy and radiation treatment.
[polldaddy:9754786]
The authors’ observations
Both clozapine and chemotherapy carry the risk of immunosuppression, presenting a clinical challenge when choosing an appropriate management strategy. However, the risks of stopping clozapine after a long period of symptom stability are substantial, with a relapse rate up to 50%.1 Among patients taking clozapine, the risk of agranulocytosis and neutropenia are approximately 0.8% and 3%, respectively, and >80% of agranulocyotis cases occur within the first 18 weeks of treatment.2,3 Although both clozapine and chemotherapy can lead to neutropenia and agranulocytosis, there currently is no evidence of a synergistic effect on bone marrow suppression with simultaneous use of these therapies2 nor is there evidence of the combination leading to sustained marrow suppression.4
Because of Ms. B’s positive response to clozapine, the risks associated with discontinuing the medication, and the relatively low risk of clozapine contributing to neutropenia after a long period of stabilization, her outpatient psychiatric providers decide to increase ANC monitoring to weekly while she undergoes cancer treatment.
TREATMENT Neutropenia, psychosis
Ms. B continues clozapine during radiation and chemotherapy, but develops leukopenia and neutropenia with a low of 1,220/μL white blood cells and an ANC of 610/μL. Clozapine is stopped, consistent with current recommendations to hold the drug if the neutrophil count is <1,000/μL in a patient without benign ethnic neutropenia, and her outpatient provider monitors her closely. The treatment team does not restart an antipsychotic immediately after discontinuing clozapine because of the risk that other antipsychotics can cause hematologic toxicity or prolong granulocytopenia associated with clozapine.5
Approximately 2 weeks later, Ms. B is admitted to a different hospital for altered mental status and is found to have hyponatremia and rectal bleeding. The workup suggests that her rectal carcinoma has not fully responded to initial therapies, and she likely will require further treatment. Her mental status improves after hyponatremia resolves, but she reports auditory hallucinations and paranoia. Risperidone, 4 mg/d, is initiated to target psychosis.
After discharge, Ms. B develops bilateral upper extremity tremor, which she finds intolerable and attributes to risperidone. She refuses to continue risperidone or try adjunctive medications to address the tremor, but is willing to consider a different antipsychotic. Olanzapine, 10 mg/d, is initiated and risperidone is slowly tapered. During this time, Ms. B experiences increased paranoia and believes that the Internal Revenue Service is calling her. She misses her next appointment.
Later, the fire department finds Ms. B wandering the streets and brings her to the psychiatric emergency room. During the examination, she is disheveled and withdrawn, and unable to reply to simple questions about diet and sleep. When asked why she was in the street, she says that she left her apartment because it was “too messy.” The treatment team learns that she had walked at least 10 miles from her apartment before sitting down by the side of the road and being picked up by the fire department. She reveals that she left her apartment and continued walking because “a man” told her to do so and threatened to harm her if she stopped.
When Ms. B is admitted to the psychiatric service, she is paranoid, disorganized, and guarded. She remains in her room for most of the day and either refuses to talk to providers or curses at them. She often is seen wearing soiled clothing with her hair mussed. She denies having rectal carcinoma, although she expressed understanding of her medical condition <2 months earlier.
[polldaddy:9754787]
The authors’ observations
Clozapine is considered the most efficacious agent for treatment-resistant schizophrenia.6 Although non-compliance is the most common reason for discontinuing clozapine, >20% of patients stop clozapine because of adverse effects.7 Clozapine often is a drug of last resort because of the need for frequent monitoring and significant side effects; therefore deciding on a next step when clozapine fails or cannot be continued because of other factors can pose a challenge.
Ms. B’s treatment team gave serious consideration to restarting clozapine. However, because it was likely that Ms. B would undergo another round of chemotherapy and possibly radiation, the risk of neutropenia recurring was considered too high. Lithium has been used successfully to manage neutropenia in patients taking clozapine and, for some, adding lithium could help boost white cell count and allow a successful rechallenge with clozapine.3,8 However, because of Ms. B’s medical comorbidities, including cancer and chronic kidney disease, adding lithium was not thought to be clinically prudent at that time and the treatment team considered other options.
Olanzapine. Although research is limited, studies suggest olanzapine is the most commonly prescribed medication when a patient has to discontinue clozapine,7 with comparable response rates in those with refractory schizophrenia.9 Therefore, Ms. B was initially maintained on olanzapine, and the dosage increased to 30 mg over the course of 16 days in the hospital. However, she did not respond to the medication, remaining disorganized and paranoid without any notable improvement in her symptoms therefore other treatment options were explored.
Loxapine. Previous limited case reports have shown loxapine to be effective in treating individuals with refractory schizophrenia, either alone or in combination with other antipsychotics.10,11 FDA-approved in 1975, loxapine was among the last of the typical antipsychotics brought to the U.S. market before the introduction of clozapine, the first atypical.12 Loxapine is a dibenzoxazepine that has a molecular structure similar to clozapine.13 Unlike clozapine, however, loxapine is not known to cause agranulocytosis.14 Research suggests that although clozapine is oxidized to metabolites that are cytotoxic, loxapine is not, potentially accounting for their different effects on neutrophils.15
The efficacy of loxapine has shown to be similar to other typical and atypical antipsychotics, with approximately 70% of patients showing improvement.14 However, loxapine may be overlooked as an option, possibly because it was not included in the CATIE trial and was the last typical antipsychotic to be approved before atypicals were introduced.12 First available in oral and IM formulations, there has been increased interest in loxapine recently because of the approval of an inhaled formulation in 2012.16
Although classified as a typical antipsychotic, studies have suggested that loxapine acts as an atypical at low dosages.17,18 Previous work suggests, however, that the side effect profile of loxapine is similar to typical antipsychotics.14 At dosages <50 mg, it results in fewer cases of extrapyramidal side effects than expected with a typical antipsychotic.18
Loxapine’s binding profile seems to exist along this spectrum of typical to atypical. Tissue-based binding studies have shown a higher 5-HT2 affinity relative to D2, consistent with atypical antipsychotics.19 Positron emission tomography studies in humans show 5-HT2 saturation of loxapine to be close to equal to D2 binding in loxapine, thus a slightly lower ratio of 5-HT2 to D2 relative to atypicals, but more than that seen with typical antipsychotics.20 These differences between in vitro and in vivo studies may be secondary to the binding of loxapine’s active metabolites, particularly 7- and 8-hydroxyloxapine, which have more dopaminergic activity. In addition to increased 5-HT2A binding compared with typical antipsychotics, loxapine also has a high affinity for the D4 receptors, as well as interacting with other serotonin receptors 5-HT3, 5-HT6, and 5-HT7. Of note this is a similar pattern of binding affinity as seen in clozapine.19
It should be noted, however, that loxapine may not be an appropriate treatment in all forms of cancer. Similar to other first-generation antipsychotics, it increases prolactin levels, and thus may have a negative clinical impact on patients with prolactin receptor positive breast cancers.21,22 Finally, although clozapine can result in significant weight gain, dyslipidemia, and hyperglycemia, unlike many antipsychotics, loxapine has been shown to be weight neutral or result in weight loss,14 making it an option to consider for patients with type 2 diabetes mellitus, metabolic syndrome, dyslipidemia, or cardiovascular disease.
OUTCOME Improvement, stability
Ms. B begins taking loxapine, 10 mg/d, gradually cross-tapered with olanzapine, increasing loxapine by 10 mg every 2 to 3 days (Figure). After 8 days, when the dosage has reached 40 mg/d, Ms. B’s treatment team begins to observe a consistent change in her behavior. Ms. B comes into the interview room, where previously the team had to see her in her own room because she refused to come out. She also tolerates an extensive interview, even sharing parts of her history without prompting, and is able to discuss her treatment. Ms. B continues to express some paranoia regarding the treatment team. On day 12, receiving loxapine, 50 mg/d, Ms. B says that she likes the new medication and feels she is doing well with it. She becomes less reclusive and begins socializing with other patients. By day 19, receiving loxapine, 80 mg/d, a nurse, who knows Ms. B from the outpatient facility, visits the unit and reports that Ms. B is at her baseline.

At discharge, Ms. B is noted to be “bright,” well organized, neatly dressed, and wearing makeup. Her paranoia and auditory hallucinations have almost completely resolved. She is social, engages appropriately with the treatment team, and is able to describe a plan for self-care after discharge including following up with her oncologist. Her white blood cell counts were carefully monitored throughout her admission and are within normal limits when she is discharged.
One year later, Ms. B remains taking loxapine, 70 mg/d. Although she continues to report mild paranoia, she is living independently in her apartment and attends church regularly.
2. Usta NG, Poyraz CA, Aktan M, et al. Clozapine treatment of refractory schizophrenia during essential chemotherapy: a case study and mini review of a clinical dilemma. Ther Adv Psychopharmacol. 2014;4(6):276-281.
3. Meyer N, Gee S, Whiskey E, et al. Optimizing outcomes in clozapine rechallenge following neutropenia: a cohort analysis. J Clin Psychiatry. 2015;76(11):e1410-e1416.
4. Cunningham NT, Dennis N, Dattilo W, et al. Continuation of clozapine during chemotherapy: a case report and review of literature. Psychosomatics. 2014;55(6):673-679.
5. Co¸sar B, Taner ME, Eser HY, et al. Does switching to another antipsychotic in patients with clozapine-associated granulocytopenia solve the problem? Case series of 18. J Clin Psychopharmacol. 2011;31(2):169-173.
6. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigation. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
7. Mustafa FA, Burke JG, Abukmeil SS, et al. “Schizophrenia past clozapine”: reasons for clozapine discontinuation, mortality, and alternative antipsychotic prescribing. Pharmacopsychiatry. 2015;48(1):11-14.
8. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
9. Bitter I, Dossenbach MR, Brook S, et al; Olanzapine HGCK Study Group. Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):173-180.
10. Lehmann CR, Ereshefsky L, Saklad SR, et al. Very high dose loxapine in refractory schizophrenic patients. Am J Psychiatry. 1981;138(9):1212-1214.
11. Sokolski KN. Combination loxapine and aripiprazole for refractory hallucinations in schizophrenia. Ann Pharmacother. 2011;45(7-8):e36.
12. Shen WW. A history of antipsychotic drug development. Compr Psychiatry. 1999;40(6):407-414.
13. Mazzola CD, Miron S, Jenkins AJ. Loxapine intoxication: case report and literature review. J Anal Toxicol. 2000;24(7):638-641.
14. Chakrabarti A, Bagnall A, Chue P, et al. Loxapine for schizophrenia. Cochrane Database Syst Rev. 2007(4):CD001943.
15. Jegouzo A, Gressier B, Frimat B, et al. Comparative oxidation of loxapine and clozapine by human neutrophils. Fundam Clin Pharmacol. 1999;13(1):113-119.
16. Keating GM. Loxapine inhalation powder: a review of its use in the acute treatment of agitation in patients with bipolar disorder or schizophrenia. CNS Drugs. 2013;27(6):479-489.
1 7. Glazer WM. Does loxapine have “atypical” properties? Clinical evidence. J Clin Psychiatry. 1999;60(suppl 10):42-46.
18. Hellings JA, Jadhav M, Jain S, et al. Low dose loxapine: neuromotor side effects and tolerability in autism spectrum disorders. J Child Adolesc Psychopharmacol. 2015;25(8):618-624.
19. Singh AN, Barlas C, Singh S, et al. A neurochemical basis for the antipsychotic activity of loxapine: interactions with dopamine D1, D2, D4 and serotonin 5-HT2 receptor subtypes. J Psychiatry Neurosci. 1996;21(1):29-35.
20. Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156(2):286-293.
21. Robertson AG, Berry R, Meltzer HY. Prolactin stimulating effects of amoxapine and loxapine in psychiatric patients. Psychopharmacology (Berl). 1982;78(3):287-292.
22. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
2. Usta NG, Poyraz CA, Aktan M, et al. Clozapine treatment of refractory schizophrenia during essential chemotherapy: a case study and mini review of a clinical dilemma. Ther Adv Psychopharmacol. 2014;4(6):276-281.
3. Meyer N, Gee S, Whiskey E, et al. Optimizing outcomes in clozapine rechallenge following neutropenia: a cohort analysis. J Clin Psychiatry. 2015;76(11):e1410-e1416.
4. Cunningham NT, Dennis N, Dattilo W, et al. Continuation of clozapine during chemotherapy: a case report and review of literature. Psychosomatics. 2014;55(6):673-679.
5. Co¸sar B, Taner ME, Eser HY, et al. Does switching to another antipsychotic in patients with clozapine-associated granulocytopenia solve the problem? Case series of 18. J Clin Psychopharmacol. 2011;31(2):169-173.
6. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigation. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
7. Mustafa FA, Burke JG, Abukmeil SS, et al. “Schizophrenia past clozapine”: reasons for clozapine discontinuation, mortality, and alternative antipsychotic prescribing. Pharmacopsychiatry. 2015;48(1):11-14.
8. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
9. Bitter I, Dossenbach MR, Brook S, et al; Olanzapine HGCK Study Group. Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):173-180.
10. Lehmann CR, Ereshefsky L, Saklad SR, et al. Very high dose loxapine in refractory schizophrenic patients. Am J Psychiatry. 1981;138(9):1212-1214.
11. Sokolski KN. Combination loxapine and aripiprazole for refractory hallucinations in schizophrenia. Ann Pharmacother. 2011;45(7-8):e36.
12. Shen WW. A history of antipsychotic drug development. Compr Psychiatry. 1999;40(6):407-414.
13. Mazzola CD, Miron S, Jenkins AJ. Loxapine intoxication: case report and literature review. J Anal Toxicol. 2000;24(7):638-641.
14. Chakrabarti A, Bagnall A, Chue P, et al. Loxapine for schizophrenia. Cochrane Database Syst Rev. 2007(4):CD001943.
15. Jegouzo A, Gressier B, Frimat B, et al. Comparative oxidation of loxapine and clozapine by human neutrophils. Fundam Clin Pharmacol. 1999;13(1):113-119.
16. Keating GM. Loxapine inhalation powder: a review of its use in the acute treatment of agitation in patients with bipolar disorder or schizophrenia. CNS Drugs. 2013;27(6):479-489.
1 7. Glazer WM. Does loxapine have “atypical” properties? Clinical evidence. J Clin Psychiatry. 1999;60(suppl 10):42-46.
18. Hellings JA, Jadhav M, Jain S, et al. Low dose loxapine: neuromotor side effects and tolerability in autism spectrum disorders. J Child Adolesc Psychopharmacol. 2015;25(8):618-624.
19. Singh AN, Barlas C, Singh S, et al. A neurochemical basis for the antipsychotic activity of loxapine: interactions with dopamine D1, D2, D4 and serotonin 5-HT2 receptor subtypes. J Psychiatry Neurosci. 1996;21(1):29-35.
20. Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156(2):286-293.
21. Robertson AG, Berry R, Meltzer HY. Prolactin stimulating effects of amoxapine and loxapine in psychiatric patients. Psychopharmacology (Berl). 1982;78(3):287-292.
22. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
Suicide by cop: What motivates those who choose this method?
CASE Unresponsive and suicidal
Mr. Z, age 25, an unemployed immigrant from Eastern Europe, is found unresponsive at a subway station. Workup in the emergency room reveals a positive urine toxicology for benzodiazepines and a blood alcohol level of 101.6 mg/dL. When Mr. Z regains consciousness the next day, he says that he is suicidal. He recently broke up with his girlfriend and feels worthless, hopeless, and depressed. As a suicide attempt, he took quetiapine and diazepam chased with vodka.
Mr. Z reports a history of suicide attempts. He says he has been suffering from depression most of his life and has been diagnosed with bipolar I disorder and borderline personality disorder. His medication regimen consists of quetiapine, 200 mg/d, and duloxetine, 20 mg/d.
Before immigrating to the United States 5 years ago, he attempted to overdose on his mother’s prescribed diazepam and was in a coma for 2 days. Recently, he stole a bicycle with the intent of provoking the police to kill him. When caught, he deliberately disobeyed the officer’s order and advanced toward the officer in an aggressive manner. However, the officer stopped Mr. Z using a stun gun. Mr. Z reports that he still feels angry that his suicide attempt failed. He is an Orthodox Christian and says he is “very religious.”
[polldaddy:9731423]
The authors’ observations
The means of suicide differ among individuals. Some attempt suicide by themselves; others through the involuntary participation of others, such as the police. This is known as SBC. Other terms include “suicide by means of victim-precipitated homicide,”1 “hetero-suicide,”2 “suicide by proxy,”3 “copicide,”4 and “law enforcement-forced-assisted suicide.”5,6 SBC accounts for 10%7 to 36%6 of police shootings and can cause serious stress for the officers involved and creates a strain between the police and the community.8
SBC was first mentioned as “suicide by means of victim-precipitated homicide.” Wolfgang5 reported 588 cases of police officer-involved shooting in Philadelphia between January 1948 and December 31, 1952, and, concluded that 150 of these cases (26%) fit criteria for what the author termed “victim-precipitated homicide” because the victims involved were the direct precipitants of the situation leading to their death. Wolfgang stated:
Instead of a murderer performing the act of suicide by killing another person who represents the murder’s unconscious, and instead of a suicide representing the desire to kill turned on [the] self, the victim in these victim-precipitated homicide cases is considered to be a suicide prone [individual] who manifests his desire to destroy [him]self by engaging another person to perform the act.
The term “SBC” was coined in 1983 by Karl Harris, a Los Angeles County medical examiner.8 The social repercussions of this modality attracts media attention because of its negative social consequences.
Characteristics of SBC
SBC has characteristics similar to other means of suicide; it is more prevalent among men with psychiatric disorders, including major depression, bipolar disorders, schizophrenia, substance use disorders,9 poor stress response skills, recent stressors, and adverse life events,10 and history of suicide attempts.
Psychosocial characteristics include:
- mean age 31.8 years1
- male sex (98%)
- white (52%)
- approximately 40% involve some form of relationship conflict.6
In psychological autopsy studies, an estimated 70.5% of those involved in a SBC incident had ≥1 stressful life events,1 including terminal illness, loss of a job, a lawsuit, or domestic issues. However, the reason is unknown for the remaining 28% cases.2 Thirty-five percent of those involved in SBC incidents were married, 13.5% divorced, and 46.7% single.1 Seventy-seven percent had low socioeconomic status,11 with 49.3% unemployed at the time of the SBC incident.1
Pathological characteristics of SBC and other suicide means are similar. Among SBC cases, 39% had previously attempted suicide6; 56% have a psychiatric or chronic medical comorbidity. Alcohol and drug abuse were reported among 56% of individuals, and 66% had a criminal history.6 Additionally, comorbid psychiatric disorders, especially those of the impulsive and emotionally unstable types, such as borderline and antisocial personality disorder, have been found to play a major role in SBC incidents.12
Individual suicide vs SBC
Religious beliefs. The term “religiosity” is used to define an individual’s idiosyncratic religious belief or personal religious philosophy reconciling the concept of death by suicide and the afterlife. Although there are no studies that specifically reference the relationship between SBC and religiosity, religious belief and affiliation appear to be strong motivating factors. SBC victims might have an idiosyncratic view of religion related death by suicide. Whether suicide is performed while under delusional belief about God, the devil, or being possessed by demons,13 or to avoid the moral prohibition of most religious faiths in regard to suicide,6 the degree of religiosity in SBC is an important area for future research.
Mr. Z stated that his strong religious faith as an Orthodox Christian motivated the attempted SBC. He tried to provoke the officer to kill him, because as a devout Orthodox Christian, it is against his religious beliefs to kill himself. He reasoned that, because his beliefs preclude him from performing the suicidal act on his own,6,14 having an officer pull the trigger would relieve him from committing what he perceived as a sin.6
Lethal vs danger. Another difference is the level of urgency that individuals create around them when attempting SBC. Homant and Kennedy15 see this in terms of 2 ideas: lethal and danger. Lethal refers to the degree of harm posed toward the suicidal individual. Danger is the degree of harm posed by the suicidal individual toward others (ie, police officers, bystanders, hostages, family members, a spouse, etc.). SBC often is more dangerous and more lethal than other methods of suicide. SBC individuals might threaten the lives of others to provoke the police into using deadly force, such as aiming or brandishing a gun or weapon at police officers or bystanders, increasing the lethality and dangerousness of the situation. Individuals engaging in SBC might shoot or kill others to create a confrontation with the police in order to be killed in the process (Table16).

Instrumental vs expressive goals
Mohandie and Meloy6 identified 2 primary goals of those involved in SBC events: instrumental and expressive. Individuals in the instrumental category are:
- attempting to escape or avoid the consequences of criminal or shameful actions
- using the forced confrontation with police to reconcile a failed relationship
- hoping to avoid the exclusion clauses of life insurance policies
- rationalizing that while it may be morally wrong to commit suicide, being killed resolves the spiritual problem of suicide
- seeking what they believe to be a very effective and lethal means of accomplishing death.
An expressive goal is more personal and includes individuals who use the confrontation with the police to communicate:
- hopelessness, depression, and desperation
- a statement about their ultimate identification as victims
- their need to “save face” by dying or being forcibly overwhelmed rather than surrendering
- their intense power needs, rage, and revenge
- their need to draw attention to an important personal issue.
Mr. Z chose what he believed to be an efficiently lethal way of dying in accord with his religious faith, knowing that a confrontation with the police could have a fatal ending. This case represents an instrumental motivation to die by SBC that was religiously motivated.
[polldaddy:9731428]
The authors’ observations
SBC presents a specific and serious challenge for law enforcement personnel, and should be approached in a manner different than other crisis situations. Because many individuals engaging in SBC have a history of mental illness, officers with training in handling individuals with psychiatric disorders—known as Crisis Intervention Team (CIT) in many areas—should be deployed as first responders. CITs have been shown to:
- reduce arrest rates of individuals with psychiatric disorders
- increase referral rates to appropriate treatment
- decrease police injuries when responding to calls
- decrease the need for escalation with specialized tactical response teams, such as Special Weapons And Tactics.17
Identification of SBC behavior is crucial during police response. Indicators of a SBC include:
- refusal to comply with police order
- refusal to surrender
- lack of interest in getting out of a barricade or hostage situation alive.18
In approaching a SBC incident, responding officers should be non-confrontational and try to talk to the suicidal individual.8 If force is needed to resolve the crisis, non-lethal measures should be used first.8 Law enforcement and mental health professionals should suspect a SBC situation in individuals who have had prior police contact and are exhibiting behaviors outlined in the Table.16
Once suicidality is identified, it should be treated promptly. Patients who are at imminent risk to themselves or others should be hospitalized to maintain their safety. Similar to other suicide modalities, the primary risk factor for SBC is untreated or inadequately treated psychiatric illness. Therefore, the crux of managing SBC involves identifying and treating the underlying mental disorder.
Pharmacological treatment should be guided by the patient’s symptoms and psychiatric diagnosis. For suicidal behavior associated with bipolar depression and other affective disorders, lithium has evidence of reducing suicidality. Studies have shown a 5.5-fold reduction in suicide risk and a >13-fold reduction in completed suicides with lithium treatment.19 In patients with schizophrenia, clozapine has been shown to reduce suicide risk and is the only FDA-approved agent for this indication.19 Although antidepressants can effectively treat depression, there are no studies that show that 1 antidepressant is more effective than others in reducing suicidality. This might be because of the long latency period between treatment initiation and symptom relief. Ketamine, an N-methyl-
OUTCOME Medication adjustment
After Mr. Z is medically stable, he is voluntarily transferred to the inpatient psychiatric unit where he is stabilized on quetiapine, 200 mg/d, and duloxetine, 60 mg/d, and attends daily group activity, milieu, and individual therapy. Because of Mr. Z’s chronic affective instability and suicidality, we consider lithium for its anti-suicide effects, but decide against it because of lithium’s high lethality in an overdose and Mr. Z’s history of poor compliance and alcohol use.
Because of Mr. Z’s socioeconomic challenges, it is necessary to contact his extended family and social support system to be part of treatment and safety planning. After a week on the psychiatric unit, his mood symptoms stabilize and he is discharged to his family and friends in the area, with a short supply of quetiapine and duloxetine, and free follow-up care within 3 days of discharge. His mood is euthymic; his affect is broad range; his thought process is coherent and logical; he denies suicidal ideation; and can verbalize a logical and concrete safety plan. His support system assures us that Mr. Z will follow up with his appointments.
His DSM-522 discharge diagnoses are borderline personality disorder, bipolar I disorder, and suicidal behavior disorder, current.
The authors’ observations
SBC increases friction and mistrust between the police and the public, traumatizes officers who are forced to use deadly measures, and results in the death of the suicidal individual. As mental health professionals, we need to be aware of this form of suicide in our screening assessment. Training police to differentiate violent offenders from psychiatric patients could reduce the number of SBCs.9 As shown by the CIT model, educating officers on behaviors indicating a mental illness could lead to more psychiatric admissions rather than incarceration17 or death. We advocate for continuous collaborative work and cross training between the police and mental health professionals and for more research on the link between religiosity and the motivation to die by SBC, because there appears to be a not-yet quantified but strong link between them.
1. Hutson HR, Anglin D, Yarbrough J, et al. Suicide by cop. Ann Emerg Med. 1998;32(6):665-669.
2. Foote WE. Victim-precipitated homicide. In: Hall HV, ed. Lethal violence: a sourcebook on fatal domestic, acquaintance and stranger violence. London, United Kingdom: CRC Press; 1999:175-199.
3. Keram EA, Farrell BJ. Suicide by cop: issues in outcome and analysis. In: Sheehan DC, Warren JI, eds. Suicide and law enforcement. Quantico, VA: FBI Academy; 2001:587-597.
4. Violanti JM, Drylie JJ. Copicide: concepts, cases, and controversies of suicide by cop. Springfield, IL: Charles C Thomas Publisher, LTD; 2008.
5. Wolfgang ME. Suicide by means of victim-precipitated homicide. J Clin Exp Psychopathol Q Rev Psychiatry Neurol. 1959;20:335-349.
6. Mohandie K, Meloy JR. Clinical and forensic indicators of “suicide by cop.” J Forensic Sci. 2000;45(2):384-389.
7. Wright RK, Davis JH. Studies in the epidemiology of murder a proposed classification system. J Forensic Sci. 1977;22(2):464-470.
8. Miller L. Suicide by cop: causes, reactions, and practical intervention strategies. Int J Emerg Ment Health. 2006;8(3):165-174.
9. Dewey L, Allwood M, Fava J, et al. Suicide by cop: clinical risks and subtypes. Arch Suicide Res. 2013;17(4):448-461.
10. Foster T, Gillespie K, McClelland R, et al. Risk factors for suicide independent of DSM-III-R Axis I disorder. Case-control psychological autopsy study in Northern Ireland. Br J Psychiatry. 1999;175:175-179.
11. Lindsay M, Lester D. Criteria for suicide-by-cop incidents. Psychol Rep. 2008;102(2):603-605.
12. Cheng AT, Mann AH, Chan KA. Personality disorder and suicide. A case-control study. Br J Psychiatry. 1997;170:441-446.
13. Mohandie K, Meloy JR, Collins PI. Suicide by cop among officer‐involved shooting cases. J Forensic Sci. 2009;54(2):456-462.
14. Falk J, Riepert T, Rothschild MA. A case of suicide-by-cop. Leg Med (Tokyo). 2004;6(3):194-196.
15. Homant RJ, Kennedy DB. Suicide by police: a proposed typology of law enforcement officer-assisted suicide. Policing: An International Journal of Police Strategies & Management. 2000;23(3):339-355.
16. Lester D. Suicide as a staged performance. Comprehensive Psychology. 2015:4(1):1-6.
17. SpringerBriefs in psychology. Best practices for those with psychiatric disorder in the criminal justice system. In: Walker LE, Pann JM, Shapiro DL, et al. Best practices in law enforcement crisis Interventions with those with psychiatric disorder. 2015;11-18.
18. Homant RJ, Kennedy DB, Hupp R. Real and perceived danger in police officer assisted suicide. J Crim Justice. 2000;28(1):43-52.
19. Ernst CL, Goldberg JF. Antisuicide properties of psychotropic drugs: a critical review. Harv Review Psychiatry. 2004;12(1):14-41.
20. Al Jurdi RK, Swann A, Mathew SJ. Psychopharmacological agents and suicide risk reduction: ketamine and other approaches. Curr Psychiatry Rep. 2015;17(10):81.
21. Fink M, Kellner CH, McCall WV. The role of ECT in suicide prevention. Journal ECT. 2014;30(1):5-9.
22. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
CASE Unresponsive and suicidal
Mr. Z, age 25, an unemployed immigrant from Eastern Europe, is found unresponsive at a subway station. Workup in the emergency room reveals a positive urine toxicology for benzodiazepines and a blood alcohol level of 101.6 mg/dL. When Mr. Z regains consciousness the next day, he says that he is suicidal. He recently broke up with his girlfriend and feels worthless, hopeless, and depressed. As a suicide attempt, he took quetiapine and diazepam chased with vodka.
Mr. Z reports a history of suicide attempts. He says he has been suffering from depression most of his life and has been diagnosed with bipolar I disorder and borderline personality disorder. His medication regimen consists of quetiapine, 200 mg/d, and duloxetine, 20 mg/d.
Before immigrating to the United States 5 years ago, he attempted to overdose on his mother’s prescribed diazepam and was in a coma for 2 days. Recently, he stole a bicycle with the intent of provoking the police to kill him. When caught, he deliberately disobeyed the officer’s order and advanced toward the officer in an aggressive manner. However, the officer stopped Mr. Z using a stun gun. Mr. Z reports that he still feels angry that his suicide attempt failed. He is an Orthodox Christian and says he is “very religious.”
[polldaddy:9731423]
The authors’ observations
The means of suicide differ among individuals. Some attempt suicide by themselves; others through the involuntary participation of others, such as the police. This is known as SBC. Other terms include “suicide by means of victim-precipitated homicide,”1 “hetero-suicide,”2 “suicide by proxy,”3 “copicide,”4 and “law enforcement-forced-assisted suicide.”5,6 SBC accounts for 10%7 to 36%6 of police shootings and can cause serious stress for the officers involved and creates a strain between the police and the community.8
SBC was first mentioned as “suicide by means of victim-precipitated homicide.” Wolfgang5 reported 588 cases of police officer-involved shooting in Philadelphia between January 1948 and December 31, 1952, and, concluded that 150 of these cases (26%) fit criteria for what the author termed “victim-precipitated homicide” because the victims involved were the direct precipitants of the situation leading to their death. Wolfgang stated:
Instead of a murderer performing the act of suicide by killing another person who represents the murder’s unconscious, and instead of a suicide representing the desire to kill turned on [the] self, the victim in these victim-precipitated homicide cases is considered to be a suicide prone [individual] who manifests his desire to destroy [him]self by engaging another person to perform the act.
The term “SBC” was coined in 1983 by Karl Harris, a Los Angeles County medical examiner.8 The social repercussions of this modality attracts media attention because of its negative social consequences.
Characteristics of SBC
SBC has characteristics similar to other means of suicide; it is more prevalent among men with psychiatric disorders, including major depression, bipolar disorders, schizophrenia, substance use disorders,9 poor stress response skills, recent stressors, and adverse life events,10 and history of suicide attempts.
Psychosocial characteristics include:
- mean age 31.8 years1
- male sex (98%)
- white (52%)
- approximately 40% involve some form of relationship conflict.6
In psychological autopsy studies, an estimated 70.5% of those involved in a SBC incident had ≥1 stressful life events,1 including terminal illness, loss of a job, a lawsuit, or domestic issues. However, the reason is unknown for the remaining 28% cases.2 Thirty-five percent of those involved in SBC incidents were married, 13.5% divorced, and 46.7% single.1 Seventy-seven percent had low socioeconomic status,11 with 49.3% unemployed at the time of the SBC incident.1
Pathological characteristics of SBC and other suicide means are similar. Among SBC cases, 39% had previously attempted suicide6; 56% have a psychiatric or chronic medical comorbidity. Alcohol and drug abuse were reported among 56% of individuals, and 66% had a criminal history.6 Additionally, comorbid psychiatric disorders, especially those of the impulsive and emotionally unstable types, such as borderline and antisocial personality disorder, have been found to play a major role in SBC incidents.12
Individual suicide vs SBC
Religious beliefs. The term “religiosity” is used to define an individual’s idiosyncratic religious belief or personal religious philosophy reconciling the concept of death by suicide and the afterlife. Although there are no studies that specifically reference the relationship between SBC and religiosity, religious belief and affiliation appear to be strong motivating factors. SBC victims might have an idiosyncratic view of religion related death by suicide. Whether suicide is performed while under delusional belief about God, the devil, or being possessed by demons,13 or to avoid the moral prohibition of most religious faiths in regard to suicide,6 the degree of religiosity in SBC is an important area for future research.
Mr. Z stated that his strong religious faith as an Orthodox Christian motivated the attempted SBC. He tried to provoke the officer to kill him, because as a devout Orthodox Christian, it is against his religious beliefs to kill himself. He reasoned that, because his beliefs preclude him from performing the suicidal act on his own,6,14 having an officer pull the trigger would relieve him from committing what he perceived as a sin.6
Lethal vs danger. Another difference is the level of urgency that individuals create around them when attempting SBC. Homant and Kennedy15 see this in terms of 2 ideas: lethal and danger. Lethal refers to the degree of harm posed toward the suicidal individual. Danger is the degree of harm posed by the suicidal individual toward others (ie, police officers, bystanders, hostages, family members, a spouse, etc.). SBC often is more dangerous and more lethal than other methods of suicide. SBC individuals might threaten the lives of others to provoke the police into using deadly force, such as aiming or brandishing a gun or weapon at police officers or bystanders, increasing the lethality and dangerousness of the situation. Individuals engaging in SBC might shoot or kill others to create a confrontation with the police in order to be killed in the process (Table16).

Instrumental vs expressive goals
Mohandie and Meloy6 identified 2 primary goals of those involved in SBC events: instrumental and expressive. Individuals in the instrumental category are:
- attempting to escape or avoid the consequences of criminal or shameful actions
- using the forced confrontation with police to reconcile a failed relationship
- hoping to avoid the exclusion clauses of life insurance policies
- rationalizing that while it may be morally wrong to commit suicide, being killed resolves the spiritual problem of suicide
- seeking what they believe to be a very effective and lethal means of accomplishing death.
An expressive goal is more personal and includes individuals who use the confrontation with the police to communicate:
- hopelessness, depression, and desperation
- a statement about their ultimate identification as victims
- their need to “save face” by dying or being forcibly overwhelmed rather than surrendering
- their intense power needs, rage, and revenge
- their need to draw attention to an important personal issue.
Mr. Z chose what he believed to be an efficiently lethal way of dying in accord with his religious faith, knowing that a confrontation with the police could have a fatal ending. This case represents an instrumental motivation to die by SBC that was religiously motivated.
[polldaddy:9731428]
The authors’ observations
SBC presents a specific and serious challenge for law enforcement personnel, and should be approached in a manner different than other crisis situations. Because many individuals engaging in SBC have a history of mental illness, officers with training in handling individuals with psychiatric disorders—known as Crisis Intervention Team (CIT) in many areas—should be deployed as first responders. CITs have been shown to:
- reduce arrest rates of individuals with psychiatric disorders
- increase referral rates to appropriate treatment
- decrease police injuries when responding to calls
- decrease the need for escalation with specialized tactical response teams, such as Special Weapons And Tactics.17
Identification of SBC behavior is crucial during police response. Indicators of a SBC include:
- refusal to comply with police order
- refusal to surrender
- lack of interest in getting out of a barricade or hostage situation alive.18
In approaching a SBC incident, responding officers should be non-confrontational and try to talk to the suicidal individual.8 If force is needed to resolve the crisis, non-lethal measures should be used first.8 Law enforcement and mental health professionals should suspect a SBC situation in individuals who have had prior police contact and are exhibiting behaviors outlined in the Table.16
Once suicidality is identified, it should be treated promptly. Patients who are at imminent risk to themselves or others should be hospitalized to maintain their safety. Similar to other suicide modalities, the primary risk factor for SBC is untreated or inadequately treated psychiatric illness. Therefore, the crux of managing SBC involves identifying and treating the underlying mental disorder.
Pharmacological treatment should be guided by the patient’s symptoms and psychiatric diagnosis. For suicidal behavior associated with bipolar depression and other affective disorders, lithium has evidence of reducing suicidality. Studies have shown a 5.5-fold reduction in suicide risk and a >13-fold reduction in completed suicides with lithium treatment.19 In patients with schizophrenia, clozapine has been shown to reduce suicide risk and is the only FDA-approved agent for this indication.19 Although antidepressants can effectively treat depression, there are no studies that show that 1 antidepressant is more effective than others in reducing suicidality. This might be because of the long latency period between treatment initiation and symptom relief. Ketamine, an N-methyl-
OUTCOME Medication adjustment
After Mr. Z is medically stable, he is voluntarily transferred to the inpatient psychiatric unit where he is stabilized on quetiapine, 200 mg/d, and duloxetine, 60 mg/d, and attends daily group activity, milieu, and individual therapy. Because of Mr. Z’s chronic affective instability and suicidality, we consider lithium for its anti-suicide effects, but decide against it because of lithium’s high lethality in an overdose and Mr. Z’s history of poor compliance and alcohol use.
Because of Mr. Z’s socioeconomic challenges, it is necessary to contact his extended family and social support system to be part of treatment and safety planning. After a week on the psychiatric unit, his mood symptoms stabilize and he is discharged to his family and friends in the area, with a short supply of quetiapine and duloxetine, and free follow-up care within 3 days of discharge. His mood is euthymic; his affect is broad range; his thought process is coherent and logical; he denies suicidal ideation; and can verbalize a logical and concrete safety plan. His support system assures us that Mr. Z will follow up with his appointments.
His DSM-522 discharge diagnoses are borderline personality disorder, bipolar I disorder, and suicidal behavior disorder, current.
The authors’ observations
SBC increases friction and mistrust between the police and the public, traumatizes officers who are forced to use deadly measures, and results in the death of the suicidal individual. As mental health professionals, we need to be aware of this form of suicide in our screening assessment. Training police to differentiate violent offenders from psychiatric patients could reduce the number of SBCs.9 As shown by the CIT model, educating officers on behaviors indicating a mental illness could lead to more psychiatric admissions rather than incarceration17 or death. We advocate for continuous collaborative work and cross training between the police and mental health professionals and for more research on the link between religiosity and the motivation to die by SBC, because there appears to be a not-yet quantified but strong link between them.
CASE Unresponsive and suicidal
Mr. Z, age 25, an unemployed immigrant from Eastern Europe, is found unresponsive at a subway station. Workup in the emergency room reveals a positive urine toxicology for benzodiazepines and a blood alcohol level of 101.6 mg/dL. When Mr. Z regains consciousness the next day, he says that he is suicidal. He recently broke up with his girlfriend and feels worthless, hopeless, and depressed. As a suicide attempt, he took quetiapine and diazepam chased with vodka.
Mr. Z reports a history of suicide attempts. He says he has been suffering from depression most of his life and has been diagnosed with bipolar I disorder and borderline personality disorder. His medication regimen consists of quetiapine, 200 mg/d, and duloxetine, 20 mg/d.
Before immigrating to the United States 5 years ago, he attempted to overdose on his mother’s prescribed diazepam and was in a coma for 2 days. Recently, he stole a bicycle with the intent of provoking the police to kill him. When caught, he deliberately disobeyed the officer’s order and advanced toward the officer in an aggressive manner. However, the officer stopped Mr. Z using a stun gun. Mr. Z reports that he still feels angry that his suicide attempt failed. He is an Orthodox Christian and says he is “very religious.”
[polldaddy:9731423]
The authors’ observations
The means of suicide differ among individuals. Some attempt suicide by themselves; others through the involuntary participation of others, such as the police. This is known as SBC. Other terms include “suicide by means of victim-precipitated homicide,”1 “hetero-suicide,”2 “suicide by proxy,”3 “copicide,”4 and “law enforcement-forced-assisted suicide.”5,6 SBC accounts for 10%7 to 36%6 of police shootings and can cause serious stress for the officers involved and creates a strain between the police and the community.8
SBC was first mentioned as “suicide by means of victim-precipitated homicide.” Wolfgang5 reported 588 cases of police officer-involved shooting in Philadelphia between January 1948 and December 31, 1952, and, concluded that 150 of these cases (26%) fit criteria for what the author termed “victim-precipitated homicide” because the victims involved were the direct precipitants of the situation leading to their death. Wolfgang stated:
Instead of a murderer performing the act of suicide by killing another person who represents the murder’s unconscious, and instead of a suicide representing the desire to kill turned on [the] self, the victim in these victim-precipitated homicide cases is considered to be a suicide prone [individual] who manifests his desire to destroy [him]self by engaging another person to perform the act.
The term “SBC” was coined in 1983 by Karl Harris, a Los Angeles County medical examiner.8 The social repercussions of this modality attracts media attention because of its negative social consequences.
Characteristics of SBC
SBC has characteristics similar to other means of suicide; it is more prevalent among men with psychiatric disorders, including major depression, bipolar disorders, schizophrenia, substance use disorders,9 poor stress response skills, recent stressors, and adverse life events,10 and history of suicide attempts.
Psychosocial characteristics include:
- mean age 31.8 years1
- male sex (98%)
- white (52%)
- approximately 40% involve some form of relationship conflict.6
In psychological autopsy studies, an estimated 70.5% of those involved in a SBC incident had ≥1 stressful life events,1 including terminal illness, loss of a job, a lawsuit, or domestic issues. However, the reason is unknown for the remaining 28% cases.2 Thirty-five percent of those involved in SBC incidents were married, 13.5% divorced, and 46.7% single.1 Seventy-seven percent had low socioeconomic status,11 with 49.3% unemployed at the time of the SBC incident.1
Pathological characteristics of SBC and other suicide means are similar. Among SBC cases, 39% had previously attempted suicide6; 56% have a psychiatric or chronic medical comorbidity. Alcohol and drug abuse were reported among 56% of individuals, and 66% had a criminal history.6 Additionally, comorbid psychiatric disorders, especially those of the impulsive and emotionally unstable types, such as borderline and antisocial personality disorder, have been found to play a major role in SBC incidents.12
Individual suicide vs SBC
Religious beliefs. The term “religiosity” is used to define an individual’s idiosyncratic religious belief or personal religious philosophy reconciling the concept of death by suicide and the afterlife. Although there are no studies that specifically reference the relationship between SBC and religiosity, religious belief and affiliation appear to be strong motivating factors. SBC victims might have an idiosyncratic view of religion related death by suicide. Whether suicide is performed while under delusional belief about God, the devil, or being possessed by demons,13 or to avoid the moral prohibition of most religious faiths in regard to suicide,6 the degree of religiosity in SBC is an important area for future research.
Mr. Z stated that his strong religious faith as an Orthodox Christian motivated the attempted SBC. He tried to provoke the officer to kill him, because as a devout Orthodox Christian, it is against his religious beliefs to kill himself. He reasoned that, because his beliefs preclude him from performing the suicidal act on his own,6,14 having an officer pull the trigger would relieve him from committing what he perceived as a sin.6
Lethal vs danger. Another difference is the level of urgency that individuals create around them when attempting SBC. Homant and Kennedy15 see this in terms of 2 ideas: lethal and danger. Lethal refers to the degree of harm posed toward the suicidal individual. Danger is the degree of harm posed by the suicidal individual toward others (ie, police officers, bystanders, hostages, family members, a spouse, etc.). SBC often is more dangerous and more lethal than other methods of suicide. SBC individuals might threaten the lives of others to provoke the police into using deadly force, such as aiming or brandishing a gun or weapon at police officers or bystanders, increasing the lethality and dangerousness of the situation. Individuals engaging in SBC might shoot or kill others to create a confrontation with the police in order to be killed in the process (Table16).

Instrumental vs expressive goals
Mohandie and Meloy6 identified 2 primary goals of those involved in SBC events: instrumental and expressive. Individuals in the instrumental category are:
- attempting to escape or avoid the consequences of criminal or shameful actions
- using the forced confrontation with police to reconcile a failed relationship
- hoping to avoid the exclusion clauses of life insurance policies
- rationalizing that while it may be morally wrong to commit suicide, being killed resolves the spiritual problem of suicide
- seeking what they believe to be a very effective and lethal means of accomplishing death.
An expressive goal is more personal and includes individuals who use the confrontation with the police to communicate:
- hopelessness, depression, and desperation
- a statement about their ultimate identification as victims
- their need to “save face” by dying or being forcibly overwhelmed rather than surrendering
- their intense power needs, rage, and revenge
- their need to draw attention to an important personal issue.
Mr. Z chose what he believed to be an efficiently lethal way of dying in accord with his religious faith, knowing that a confrontation with the police could have a fatal ending. This case represents an instrumental motivation to die by SBC that was religiously motivated.
[polldaddy:9731428]
The authors’ observations
SBC presents a specific and serious challenge for law enforcement personnel, and should be approached in a manner different than other crisis situations. Because many individuals engaging in SBC have a history of mental illness, officers with training in handling individuals with psychiatric disorders—known as Crisis Intervention Team (CIT) in many areas—should be deployed as first responders. CITs have been shown to:
- reduce arrest rates of individuals with psychiatric disorders
- increase referral rates to appropriate treatment
- decrease police injuries when responding to calls
- decrease the need for escalation with specialized tactical response teams, such as Special Weapons And Tactics.17
Identification of SBC behavior is crucial during police response. Indicators of a SBC include:
- refusal to comply with police order
- refusal to surrender
- lack of interest in getting out of a barricade or hostage situation alive.18
In approaching a SBC incident, responding officers should be non-confrontational and try to talk to the suicidal individual.8 If force is needed to resolve the crisis, non-lethal measures should be used first.8 Law enforcement and mental health professionals should suspect a SBC situation in individuals who have had prior police contact and are exhibiting behaviors outlined in the Table.16
Once suicidality is identified, it should be treated promptly. Patients who are at imminent risk to themselves or others should be hospitalized to maintain their safety. Similar to other suicide modalities, the primary risk factor for SBC is untreated or inadequately treated psychiatric illness. Therefore, the crux of managing SBC involves identifying and treating the underlying mental disorder.
Pharmacological treatment should be guided by the patient’s symptoms and psychiatric diagnosis. For suicidal behavior associated with bipolar depression and other affective disorders, lithium has evidence of reducing suicidality. Studies have shown a 5.5-fold reduction in suicide risk and a >13-fold reduction in completed suicides with lithium treatment.19 In patients with schizophrenia, clozapine has been shown to reduce suicide risk and is the only FDA-approved agent for this indication.19 Although antidepressants can effectively treat depression, there are no studies that show that 1 antidepressant is more effective than others in reducing suicidality. This might be because of the long latency period between treatment initiation and symptom relief. Ketamine, an N-methyl-
OUTCOME Medication adjustment
After Mr. Z is medically stable, he is voluntarily transferred to the inpatient psychiatric unit where he is stabilized on quetiapine, 200 mg/d, and duloxetine, 60 mg/d, and attends daily group activity, milieu, and individual therapy. Because of Mr. Z’s chronic affective instability and suicidality, we consider lithium for its anti-suicide effects, but decide against it because of lithium’s high lethality in an overdose and Mr. Z’s history of poor compliance and alcohol use.
Because of Mr. Z’s socioeconomic challenges, it is necessary to contact his extended family and social support system to be part of treatment and safety planning. After a week on the psychiatric unit, his mood symptoms stabilize and he is discharged to his family and friends in the area, with a short supply of quetiapine and duloxetine, and free follow-up care within 3 days of discharge. His mood is euthymic; his affect is broad range; his thought process is coherent and logical; he denies suicidal ideation; and can verbalize a logical and concrete safety plan. His support system assures us that Mr. Z will follow up with his appointments.
His DSM-522 discharge diagnoses are borderline personality disorder, bipolar I disorder, and suicidal behavior disorder, current.
The authors’ observations
SBC increases friction and mistrust between the police and the public, traumatizes officers who are forced to use deadly measures, and results in the death of the suicidal individual. As mental health professionals, we need to be aware of this form of suicide in our screening assessment. Training police to differentiate violent offenders from psychiatric patients could reduce the number of SBCs.9 As shown by the CIT model, educating officers on behaviors indicating a mental illness could lead to more psychiatric admissions rather than incarceration17 or death. We advocate for continuous collaborative work and cross training between the police and mental health professionals and for more research on the link between religiosity and the motivation to die by SBC, because there appears to be a not-yet quantified but strong link between them.
1. Hutson HR, Anglin D, Yarbrough J, et al. Suicide by cop. Ann Emerg Med. 1998;32(6):665-669.
2. Foote WE. Victim-precipitated homicide. In: Hall HV, ed. Lethal violence: a sourcebook on fatal domestic, acquaintance and stranger violence. London, United Kingdom: CRC Press; 1999:175-199.
3. Keram EA, Farrell BJ. Suicide by cop: issues in outcome and analysis. In: Sheehan DC, Warren JI, eds. Suicide and law enforcement. Quantico, VA: FBI Academy; 2001:587-597.
4. Violanti JM, Drylie JJ. Copicide: concepts, cases, and controversies of suicide by cop. Springfield, IL: Charles C Thomas Publisher, LTD; 2008.
5. Wolfgang ME. Suicide by means of victim-precipitated homicide. J Clin Exp Psychopathol Q Rev Psychiatry Neurol. 1959;20:335-349.
6. Mohandie K, Meloy JR. Clinical and forensic indicators of “suicide by cop.” J Forensic Sci. 2000;45(2):384-389.
7. Wright RK, Davis JH. Studies in the epidemiology of murder a proposed classification system. J Forensic Sci. 1977;22(2):464-470.
8. Miller L. Suicide by cop: causes, reactions, and practical intervention strategies. Int J Emerg Ment Health. 2006;8(3):165-174.
9. Dewey L, Allwood M, Fava J, et al. Suicide by cop: clinical risks and subtypes. Arch Suicide Res. 2013;17(4):448-461.
10. Foster T, Gillespie K, McClelland R, et al. Risk factors for suicide independent of DSM-III-R Axis I disorder. Case-control psychological autopsy study in Northern Ireland. Br J Psychiatry. 1999;175:175-179.
11. Lindsay M, Lester D. Criteria for suicide-by-cop incidents. Psychol Rep. 2008;102(2):603-605.
12. Cheng AT, Mann AH, Chan KA. Personality disorder and suicide. A case-control study. Br J Psychiatry. 1997;170:441-446.
13. Mohandie K, Meloy JR, Collins PI. Suicide by cop among officer‐involved shooting cases. J Forensic Sci. 2009;54(2):456-462.
14. Falk J, Riepert T, Rothschild MA. A case of suicide-by-cop. Leg Med (Tokyo). 2004;6(3):194-196.
15. Homant RJ, Kennedy DB. Suicide by police: a proposed typology of law enforcement officer-assisted suicide. Policing: An International Journal of Police Strategies & Management. 2000;23(3):339-355.
16. Lester D. Suicide as a staged performance. Comprehensive Psychology. 2015:4(1):1-6.
17. SpringerBriefs in psychology. Best practices for those with psychiatric disorder in the criminal justice system. In: Walker LE, Pann JM, Shapiro DL, et al. Best practices in law enforcement crisis Interventions with those with psychiatric disorder. 2015;11-18.
18. Homant RJ, Kennedy DB, Hupp R. Real and perceived danger in police officer assisted suicide. J Crim Justice. 2000;28(1):43-52.
19. Ernst CL, Goldberg JF. Antisuicide properties of psychotropic drugs: a critical review. Harv Review Psychiatry. 2004;12(1):14-41.
20. Al Jurdi RK, Swann A, Mathew SJ. Psychopharmacological agents and suicide risk reduction: ketamine and other approaches. Curr Psychiatry Rep. 2015;17(10):81.
21. Fink M, Kellner CH, McCall WV. The role of ECT in suicide prevention. Journal ECT. 2014;30(1):5-9.
22. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
1. Hutson HR, Anglin D, Yarbrough J, et al. Suicide by cop. Ann Emerg Med. 1998;32(6):665-669.
2. Foote WE. Victim-precipitated homicide. In: Hall HV, ed. Lethal violence: a sourcebook on fatal domestic, acquaintance and stranger violence. London, United Kingdom: CRC Press; 1999:175-199.
3. Keram EA, Farrell BJ. Suicide by cop: issues in outcome and analysis. In: Sheehan DC, Warren JI, eds. Suicide and law enforcement. Quantico, VA: FBI Academy; 2001:587-597.
4. Violanti JM, Drylie JJ. Copicide: concepts, cases, and controversies of suicide by cop. Springfield, IL: Charles C Thomas Publisher, LTD; 2008.
5. Wolfgang ME. Suicide by means of victim-precipitated homicide. J Clin Exp Psychopathol Q Rev Psychiatry Neurol. 1959;20:335-349.
6. Mohandie K, Meloy JR. Clinical and forensic indicators of “suicide by cop.” J Forensic Sci. 2000;45(2):384-389.
7. Wright RK, Davis JH. Studies in the epidemiology of murder a proposed classification system. J Forensic Sci. 1977;22(2):464-470.
8. Miller L. Suicide by cop: causes, reactions, and practical intervention strategies. Int J Emerg Ment Health. 2006;8(3):165-174.
9. Dewey L, Allwood M, Fava J, et al. Suicide by cop: clinical risks and subtypes. Arch Suicide Res. 2013;17(4):448-461.
10. Foster T, Gillespie K, McClelland R, et al. Risk factors for suicide independent of DSM-III-R Axis I disorder. Case-control psychological autopsy study in Northern Ireland. Br J Psychiatry. 1999;175:175-179.
11. Lindsay M, Lester D. Criteria for suicide-by-cop incidents. Psychol Rep. 2008;102(2):603-605.
12. Cheng AT, Mann AH, Chan KA. Personality disorder and suicide. A case-control study. Br J Psychiatry. 1997;170:441-446.
13. Mohandie K, Meloy JR, Collins PI. Suicide by cop among officer‐involved shooting cases. J Forensic Sci. 2009;54(2):456-462.
14. Falk J, Riepert T, Rothschild MA. A case of suicide-by-cop. Leg Med (Tokyo). 2004;6(3):194-196.
15. Homant RJ, Kennedy DB. Suicide by police: a proposed typology of law enforcement officer-assisted suicide. Policing: An International Journal of Police Strategies & Management. 2000;23(3):339-355.
16. Lester D. Suicide as a staged performance. Comprehensive Psychology. 2015:4(1):1-6.
17. SpringerBriefs in psychology. Best practices for those with psychiatric disorder in the criminal justice system. In: Walker LE, Pann JM, Shapiro DL, et al. Best practices in law enforcement crisis Interventions with those with psychiatric disorder. 2015;11-18.
18. Homant RJ, Kennedy DB, Hupp R. Real and perceived danger in police officer assisted suicide. J Crim Justice. 2000;28(1):43-52.
19. Ernst CL, Goldberg JF. Antisuicide properties of psychotropic drugs: a critical review. Harv Review Psychiatry. 2004;12(1):14-41.
20. Al Jurdi RK, Swann A, Mathew SJ. Psychopharmacological agents and suicide risk reduction: ketamine and other approaches. Curr Psychiatry Rep. 2015;17(10):81.
21. Fink M, Kellner CH, McCall WV. The role of ECT in suicide prevention. Journal ECT. 2014;30(1):5-9.
22. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
A veteran who is suicidal while sleeping
CASE Suicidal while asleep
Mr. R, age 28, an Iraq and Afghanistan veteran with major depressive disorder and posttraumatic stress disorder (PTSD), is awoken by his wife to check on their daughter approximately 30 minutes after he takes his nightly regimen of zolpidem, 10 mg, melatonin, 6 mg, and hydroxyzine, 20 mg. When Mr. R returns to the bedroom, he appears to be confused. Mr. R grabs an unloaded gun from under the mattress, puts it in his mouth, and pulls the trigger. Then Mr. R holds the gun to his head and pulls the trigger while saying that his wife and children will be better off without him. His wife takes the gun away, but he grabs another gun from his gun box and loads it. His wife convinces him to remove the ammunition; however, Mr. R gets the other unloaded gun and pulls the trigger on himself again. After his wife takes this gun away, he tries cutting himself with a pocketknife, causing superficial cuts. Eventually, Mr. R goes back to bed. He does not remember these events in the morning.
What increased the likelihood of parasomnia in Mr. R?
a) high zolpidem dosage
b) concomitant use of other sedating agents
c) sleep deprivation
d) dehydration
[polldaddy:9712545]
The authors’ observations
Parasomnias are sleep-wake transition disorders classified by the sleep stage from which they arise, either NREM or rapid eye movement (REM). NREM parasomnias could result from incomplete awakening from NREM sleep, typically in Stage N3 (slow-wave) sleep.1 DSM-5 describes NREM parasomnias as arousal disorders in which the disturbance is not attributable to the physiological effects of substance; substance/medication-induced sleep disorder, parasomnia type, is when the disturbance can be attributed to a substance.2 The latter also can occur during REM sleep.
NREM parasomnias are characterized by abnormal behaviors during sleep with significant harm potential.3 Somnambulism or sleepwalking and sleep terrors are the 2 types of NREM parasomnias in DSM-5. Sleepwalking could involve complex behaviors, including:
- eating
- talking
- cooking
- shopping
- driving
- sexual activity.
Zolpidem, a benzodiazepine receptor agonist, is a preferred hypnotic agent for insomnia because of its low risk for abuse and daytime sedation.4 However, the drug has been associated with NREM parasomnias, namely somnambulism or sleepwalking, and its variants including sleep-driving, sleep-related eating disorder, and rarely sexsomnia (sleep-sex), with anterograde amnesia for the event.5 Suicidal behavior that occurs while the patient is asleep with next-day amnesia is another variant of somnambulism. There are several reports of suicidal behavior during sleep,6,7 but to our knowledge, there are only 2 previous cases implicating zolpidem as the cause:
- Gibson et al8 described a 49-year-old man who sustained a self-inflicted gunshot wound to his head while asleep. He just had started taking zolpidem, and in the weeks before the incident he had several episodes of sleepwalking and sleep-eating. He had consumed alcohol the night of the self-inflicted gunshot wound, but had no other psychiatric history.
- Chopra et al4 described a 37-year-old man, with no prior episodes of sleepwalking or associated complex behaviors, who was taking zolpidem, 10 mg/d, for chronic insomnia. He shot a gun in the basement of his home, and then held the loaded gun to his neck while asleep. The authors attributed the event to zolpidem in combination with other predisposing factors, including dehydration after intense exercise and alcohol use. The authors categorized this type of event as “para-suicidal amnestic behavior,” although “sleep-related pseudo-suicidal behavior” might be a better term for this type of parasomnia because of its occurrence during sleep and non-deliberate nature.
In another case report, a 27-year-old man took additional zolpidem after he did not experience desired sedative effects from an initial 20 mg.9 Because the patient remembered the suicidal thoughts, the authors believed that the patient attempted suicide while under the influence of zolpidem. The authors did not believe the incident to be sleep-related suicidal behavior, because it was uncertain if he attempted suicide while asleep.
Mr. R does not remember the events his wife witnessed while he was asleep. To our knowledge, Mr. R’s case is the first sleep-related pseudo-suicidal behavior case resulting from zolpidem, 10 mg/d, without concurrent alcohol use in an adult male veteran with PTSD and no suicidal ideation while awake.
HISTORY Further details revealed
Mr. R says that in the days leading to the incident he was not sleep-deprived and was getting at least 6 hours of restful sleep every night. He had been taking zolpidem every night. He has no childhood or family history of NREM parasomnias. He says he did not engage in intense exercise that evening or have a fever the night of the incident and has abstained from alcohol for 2 years.
His wife says that after he took zolpidem, when he was woken up, “He was not there; his eyes were glazed and glossy, and it’s like he was in another world,” and his speech and behavior were bizarre. She also reports that his eyes were open when he engaged in this behavior that appeared suicidal.
Three months before the incident, Mr. R had reported nightmares with dream enactment behaviors, hypervigilance on awakening and during the daytime, irritability, and anxious and depressed mood with neurovegetative symptoms, and was referred to our clinic for medication management. He also reported no prior or current manic or psychotic symptoms, denied suicidal thoughts, and had no history of suicide attempts. Mr. R’s medication regimen included tramadol, 400 mg/d, for chronic knee pain; fluoxetine, 60 mg/d, for depression and PTSD; and propranolol ER, 60 mg/d, and propranolol, 10 mg/d as needed, for anxiety. He was started on prazosin, 2 mg/d, titrated to 4 mg/d, for medication management of nightmares.
Mr. R also was referred to the sleep laboratory for a polysomnogram (PSG) because of reported loud snoring and witnessed apneas, especially because sleep apnea can cause nightmares and dream enactment behaviors. The PSG was negative for sleep apnea or excessive periodic limb movements of sleep, but showed increased electromyographic (EMG) activity during REM sleep, which was consistent with his report of dream enactment behaviors. Two months later, he reported improvement in nightmares and depression, but not in dream enactment behaviors. Because of prominent anxiety and irritability, he was started on gabapentin, 300 mg, 3 times a day.
What factor increases the risk of NREM parasomnias with zolpidem compared with benzodiazepines?
a) greater preservation of Stage N3 sleep
b) lesser degree of muscle relaxation
c) both a and b
d) none of the above
[polldaddy:9712556]
The authors’ observations
Factors that increase the likelihood of parasomnias include:
- zolpidem >10 mg at bedtime
- concomitant use of other CNS depressants, including sedative hypnotic agents and alcohol
- female sex
- not falling asleep immediately after taking zolpidem
- personal or family history of parasomnias
- living alone
- poor pill management
- presence of sleep disruptors such as sleep apnea and periodic limb movements of sleep.1,4,5,10
Higher dosages of zolpidem (>10 mg/d) have been identified as the predictive risk factor.5 In the Chopra et al4 case report on sleep-related suicidal behavior related to zolpidem, 10 mg at bedtime, concomitant dehydration and alcohol use were implicated as facilitating factors. Dehydration could increase serum levels of zolpidem resulting in greater CNS effects. Alcohol use was implicated in the Gibson et al8 case report as well, and the patient had multiple episodes of sleepwalking and sleep-related eating.However, Mr. R was not dehydrated or using alcohol.
An interesting feature of Mr. R’s case is that he was taking fluoxetine. Cytochrome P450 (CYP) 3A4 is involved in metabolizing zolpidem, and norfluoxetine, a metabolite of fluoxetine, inhibits CYP3A4. Although studies have not found pharmacokinetic interactions between fluoxetine and zolpidem, these studies did not investigate fluoxetine dosages >20 mg/d.11 The inhibition of CYP enzymes by fluoxetine likely is dose-dependent,12 and therefore concomitant administration of high-dosage fluoxetine (>20 mg/d) with zolpidem might result in higher serum levels of zolpidem.
Mr. R also was taking several sedating agents (gabapentin, hydroxyzine, melatonin, and tramadol). The concomitant use of these sedative-hypnotic agents could have increased his risk of parasomnia. A review of the literature did not reveal any reports of gabapentin, hydroxyzine, melatonin, or tramadol causing parasomnias. This observation, as well as the well-known role of zolpidem5 in etiopathogenesis of parasomnias, indicates that the pseudo-suicidal behavior Mr. R displayed while asleep likely was a direct result of zolpidem use in presence of other facilitating factors. Gabapentin, which is known to increase the depth of sleep, was added to his regimen 1 month before his parasomnia episode. Therefore, gabapentin could have triggered parasomnia with zolpidem therapy.1,13
Conditions that provoke repeated cortical arousals (eg, periodic limb movement disorder [PLMD] and sleep apnea) or increase depth or pressure of sleep (intense exercise in the evening, fever, sleep deprivation) are thought to be associated with NREM parasomnias.1-4 However, Mr. R underwent in-laboratory PSG and tested negative for major cortical arousal-inducing conditions, such as PLMD and sleep apnea.
Some other sleep disruptors likely were involved in Mr. R’s case. Auditory and tactile stimuli are known to cause cortical arousals, with additive effect seen when these 2 stimuli are combined.3,14 Additionally, these exogenous stimuli are known to trigger sleep-related violent parasomnias.15 Mr. R displayed this behavior after his wife woke him up. The auditory stimulus of his wife’s voice and/or tactile stimulus involved in the act of waking Mr. R likely played a role in the suicidal and violent nature of his NREM parasomnia.
[polldaddy:9712581]
The authors’ observations
In general, the mechanisms by which zolpidem causes NREM parasomnias are not completely understood. The sedation-related amnestic properties of zolpidem might explain some of these behaviors. Patients could perform these behaviors after waking and have subsequent amnesia.4 There is greater preservation of Stage N3 sleep with zolpidem compared with benzodiazepines. Benzodiazepines also cause muscle relaxation while the motor system remains relatively more active during sleep with zolpidem because of its selectivity for α-1 subunit of gamma-aminobutyric acid A receptor. These factors might increase the likelihood of NREM parasomnias with zolpidem compared with benzodiazepines.4
Types of parasomnias
According to DSM-5, there are 2 categories of parasomnias based on the sleep stage from which a parasomnia emerges.2 REM sleep behavior disorder (RBD) refers to complex motor and/or vocalizations during REM sleep, accompanied by increased EMG activity during REM sleep (Table).2,3
The pseudo-suicidal behavior Mr. R displayed likely was NREM parasomnia because it occurred in the first third of the night with his eyes open and impaired recall after the event. Interestingly, Mr. R had RBD in addition to the NREM parasomnia likely caused by zolpidem. This is evident from Mr. R’s frequent dream enactment behaviors, such as kicking, thrashing, and punching during sleep, along with increased EMG activity during REM sleep as recorded on the PSG.10 The presence of RBD could be explained by selective serotonin reuptake inhibitor (fluoxetine) use, and comorbidity with PTSD.2,16
Management of parasomnias
Initial management of parasomnias involves decreasing the risk of parasomnia-related injury. Suggested safety measures include:
- sleeping away from windows
- sleeping in a sleeping bag
- sleeping on a lower floor
- locking windows and doors
- removing potentially dangerous objects from the bedroom
- putting gates across stairwells
- installing bells or alarms on door knobs.15
Removing access to firearms or other weapons such as knives is of utmost importance especially with patients who have easy access during wakefulness. If removing weapons is not feasible, consider disarming, securing, or locking them.15 These considerations are relevant to veterans with PTSD because of the high prevalence of symptoms, including depression, insomnia, and pain, which require sedating medications.17 A review of parasomnias among a large sample of psychiatric outpatients revealed that a variety of sedating medications, including antidepressants, can lead to NREM parasomnias.18 Therefore, exercise caution when prescribing sedating medications, especially in patients vulnerable to developing dangerous parasomnias, such as a veteran with PTSD and easy access to guns.19
TREATMENT Zolpidem stopped
Mr. R immediately stops taking zolpidem because he is aware of its association with abnormal behaviors during sleep, and his wife removes his access to firearms and knives at night. Because of his history of clinical benefit and no history of parasomnias with mirtazapine, Mr. R is started on mirtazapine for insomnia that previously was treated with zolpidem, and residual depression. Six months after discontinuing zolpidem, he does not experience NREM parasomnias, and there are no changes in his dream enactment behaviors.
Summing up
Zolpidem therapy could be associated with unusual variants of NREM parasomnia, sleepwalking type; sleep-related pseudo-suicidal behavior is one such variant. Several factors could play a role in increasing the likelihood of NREM parasomnia with zolpidem therapy. In Mr. R’s case, the pharmacokinetic drug interactions between fluoxetine and zolpidem, as well as concomitant use of several sedating agents could have played a role in increasing the likelihood of NREM parasomnia, with audio-tactile stimuli contributing to the violent and suicidal nature of the parasomnia. Exercise caution when using CYP enzyme inhibitors, such as fluoxetine and paroxetine, in combination with zolpidem. Knowledge of the potential interaction between zolpidem and fluoxetine is important because antidepressants and hypnotics are commonly co-prescribed because insomnia often is comorbid with other psychiatric disorders.
In veterans with PTSD who do not have suicidal ideations while awake, life-threatening non-intentional behavior is a risk because of easy access to guns or other weapons. Sedative-hypnotic medications commonly are prescribed to patients with PTSD. Exercise caution when using hypnotic agents such as zolpidem, and consider sleep aids with a lower risk of parasomnias (based on the author’s experience, trazodone, mirtazapine, melatonin, and gabapentin) when possible. Non-pharmacologic treatments of insomnia, such as sleep hygiene education and, more importantly, cognitive-behavioral therapy for insomnia, are preferred. If a patient is already taking zolpidem, nightly dosage should not be >10 mg. Polypharmacy with other sedating medications should be avoided when possible and both exogenous (noise, pets) and endogenous sleep disruptors (sleep apnea, PLMD) should be addressed. Advise the patient to avoid alcohol and remove firearms and other potential weapons. Discontinue zolpidem if the patient develops sleep-related abnormal behavior because of its potential to take on violent forms.
1. Howell MJ. Parasomnias: an updated review. Neurotherapeutics. 2012;9(4):753-775.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Zadra A, Desautels A, Petit D, et al. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12(3):285-294.
4. Chopra A, Selim B, Silber MH, et al. Para-suicidal amnestic behavior associated with chronic zolpidem use: implications for patient safety. Psychosomatics. 2013;54(5):498-501.
5. Hwang TJ, Ni HC, Chen HC, et al. Risk predictors for hypnosedative-related complex sleep behaviors: a retrospective, cross-sectional pilot study. J Clin Psychiatry. 2010;71(10):1331-1335.
6. Shatkin JP, Feinfield K, Strober M. The misinterpretation of a non-REM sleep parasomnia as suicidal behavior in an adolescent. Sleep Breath. 2002;6(4):175-179.
7. Mahowald MW, Schenck CH, Goldner M, et al. Parasomnia pseudo-suicide. J Forensic Sci. 2003;48(5):1158-1162.
8. Gibson CE, Caplan JP. Zolpidem-associated parasomnia with serious self-injury: a shot in the dark. Psychosomatics. 2011;52(1):88-91.
9. Mortaz Hejri S, Faizi M, Babaeian M. Zolpidem-induced suicide attempt: a case report. Daru. 2013;20;21(1):77.
10. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
11. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs. 2003;17(7):513-532.
12. Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78(4):203-208.
13. Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1093-1100.
14. Kato T, Montplaisir JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13(3):229-238.
15. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(pt 12):3494-3509.
16. Husain AM, Miller PP, Carwile ST. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148-157.
17. Bernardy NC, Lund BC, Alexander B, et al. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
18. Lam SP, Fong SY, Ho CK, et al. Parasomnia among psychiatric outpatients: a clinical, epidemiologic, cross-sectional study. J Clin Psychiatry. 2008;69(9):1374-1382.
19. Freeman TW, Roca V, Kimbrell T. A survey of gun collection and use among three groups of veteran patients admitted to veterans affairs hospital treatment programs. South Med J. 2003;96(3):240-243.
CASE Suicidal while asleep
Mr. R, age 28, an Iraq and Afghanistan veteran with major depressive disorder and posttraumatic stress disorder (PTSD), is awoken by his wife to check on their daughter approximately 30 minutes after he takes his nightly regimen of zolpidem, 10 mg, melatonin, 6 mg, and hydroxyzine, 20 mg. When Mr. R returns to the bedroom, he appears to be confused. Mr. R grabs an unloaded gun from under the mattress, puts it in his mouth, and pulls the trigger. Then Mr. R holds the gun to his head and pulls the trigger while saying that his wife and children will be better off without him. His wife takes the gun away, but he grabs another gun from his gun box and loads it. His wife convinces him to remove the ammunition; however, Mr. R gets the other unloaded gun and pulls the trigger on himself again. After his wife takes this gun away, he tries cutting himself with a pocketknife, causing superficial cuts. Eventually, Mr. R goes back to bed. He does not remember these events in the morning.
What increased the likelihood of parasomnia in Mr. R?
a) high zolpidem dosage
b) concomitant use of other sedating agents
c) sleep deprivation
d) dehydration
[polldaddy:9712545]
The authors’ observations
Parasomnias are sleep-wake transition disorders classified by the sleep stage from which they arise, either NREM or rapid eye movement (REM). NREM parasomnias could result from incomplete awakening from NREM sleep, typically in Stage N3 (slow-wave) sleep.1 DSM-5 describes NREM parasomnias as arousal disorders in which the disturbance is not attributable to the physiological effects of substance; substance/medication-induced sleep disorder, parasomnia type, is when the disturbance can be attributed to a substance.2 The latter also can occur during REM sleep.
NREM parasomnias are characterized by abnormal behaviors during sleep with significant harm potential.3 Somnambulism or sleepwalking and sleep terrors are the 2 types of NREM parasomnias in DSM-5. Sleepwalking could involve complex behaviors, including:
- eating
- talking
- cooking
- shopping
- driving
- sexual activity.
Zolpidem, a benzodiazepine receptor agonist, is a preferred hypnotic agent for insomnia because of its low risk for abuse and daytime sedation.4 However, the drug has been associated with NREM parasomnias, namely somnambulism or sleepwalking, and its variants including sleep-driving, sleep-related eating disorder, and rarely sexsomnia (sleep-sex), with anterograde amnesia for the event.5 Suicidal behavior that occurs while the patient is asleep with next-day amnesia is another variant of somnambulism. There are several reports of suicidal behavior during sleep,6,7 but to our knowledge, there are only 2 previous cases implicating zolpidem as the cause:
- Gibson et al8 described a 49-year-old man who sustained a self-inflicted gunshot wound to his head while asleep. He just had started taking zolpidem, and in the weeks before the incident he had several episodes of sleepwalking and sleep-eating. He had consumed alcohol the night of the self-inflicted gunshot wound, but had no other psychiatric history.
- Chopra et al4 described a 37-year-old man, with no prior episodes of sleepwalking or associated complex behaviors, who was taking zolpidem, 10 mg/d, for chronic insomnia. He shot a gun in the basement of his home, and then held the loaded gun to his neck while asleep. The authors attributed the event to zolpidem in combination with other predisposing factors, including dehydration after intense exercise and alcohol use. The authors categorized this type of event as “para-suicidal amnestic behavior,” although “sleep-related pseudo-suicidal behavior” might be a better term for this type of parasomnia because of its occurrence during sleep and non-deliberate nature.
In another case report, a 27-year-old man took additional zolpidem after he did not experience desired sedative effects from an initial 20 mg.9 Because the patient remembered the suicidal thoughts, the authors believed that the patient attempted suicide while under the influence of zolpidem. The authors did not believe the incident to be sleep-related suicidal behavior, because it was uncertain if he attempted suicide while asleep.
Mr. R does not remember the events his wife witnessed while he was asleep. To our knowledge, Mr. R’s case is the first sleep-related pseudo-suicidal behavior case resulting from zolpidem, 10 mg/d, without concurrent alcohol use in an adult male veteran with PTSD and no suicidal ideation while awake.
HISTORY Further details revealed
Mr. R says that in the days leading to the incident he was not sleep-deprived and was getting at least 6 hours of restful sleep every night. He had been taking zolpidem every night. He has no childhood or family history of NREM parasomnias. He says he did not engage in intense exercise that evening or have a fever the night of the incident and has abstained from alcohol for 2 years.
His wife says that after he took zolpidem, when he was woken up, “He was not there; his eyes were glazed and glossy, and it’s like he was in another world,” and his speech and behavior were bizarre. She also reports that his eyes were open when he engaged in this behavior that appeared suicidal.
Three months before the incident, Mr. R had reported nightmares with dream enactment behaviors, hypervigilance on awakening and during the daytime, irritability, and anxious and depressed mood with neurovegetative symptoms, and was referred to our clinic for medication management. He also reported no prior or current manic or psychotic symptoms, denied suicidal thoughts, and had no history of suicide attempts. Mr. R’s medication regimen included tramadol, 400 mg/d, for chronic knee pain; fluoxetine, 60 mg/d, for depression and PTSD; and propranolol ER, 60 mg/d, and propranolol, 10 mg/d as needed, for anxiety. He was started on prazosin, 2 mg/d, titrated to 4 mg/d, for medication management of nightmares.
Mr. R also was referred to the sleep laboratory for a polysomnogram (PSG) because of reported loud snoring and witnessed apneas, especially because sleep apnea can cause nightmares and dream enactment behaviors. The PSG was negative for sleep apnea or excessive periodic limb movements of sleep, but showed increased electromyographic (EMG) activity during REM sleep, which was consistent with his report of dream enactment behaviors. Two months later, he reported improvement in nightmares and depression, but not in dream enactment behaviors. Because of prominent anxiety and irritability, he was started on gabapentin, 300 mg, 3 times a day.
What factor increases the risk of NREM parasomnias with zolpidem compared with benzodiazepines?
a) greater preservation of Stage N3 sleep
b) lesser degree of muscle relaxation
c) both a and b
d) none of the above
[polldaddy:9712556]
The authors’ observations
Factors that increase the likelihood of parasomnias include:
- zolpidem >10 mg at bedtime
- concomitant use of other CNS depressants, including sedative hypnotic agents and alcohol
- female sex
- not falling asleep immediately after taking zolpidem
- personal or family history of parasomnias
- living alone
- poor pill management
- presence of sleep disruptors such as sleep apnea and periodic limb movements of sleep.1,4,5,10
Higher dosages of zolpidem (>10 mg/d) have been identified as the predictive risk factor.5 In the Chopra et al4 case report on sleep-related suicidal behavior related to zolpidem, 10 mg at bedtime, concomitant dehydration and alcohol use were implicated as facilitating factors. Dehydration could increase serum levels of zolpidem resulting in greater CNS effects. Alcohol use was implicated in the Gibson et al8 case report as well, and the patient had multiple episodes of sleepwalking and sleep-related eating.However, Mr. R was not dehydrated or using alcohol.
An interesting feature of Mr. R’s case is that he was taking fluoxetine. Cytochrome P450 (CYP) 3A4 is involved in metabolizing zolpidem, and norfluoxetine, a metabolite of fluoxetine, inhibits CYP3A4. Although studies have not found pharmacokinetic interactions between fluoxetine and zolpidem, these studies did not investigate fluoxetine dosages >20 mg/d.11 The inhibition of CYP enzymes by fluoxetine likely is dose-dependent,12 and therefore concomitant administration of high-dosage fluoxetine (>20 mg/d) with zolpidem might result in higher serum levels of zolpidem.
Mr. R also was taking several sedating agents (gabapentin, hydroxyzine, melatonin, and tramadol). The concomitant use of these sedative-hypnotic agents could have increased his risk of parasomnia. A review of the literature did not reveal any reports of gabapentin, hydroxyzine, melatonin, or tramadol causing parasomnias. This observation, as well as the well-known role of zolpidem5 in etiopathogenesis of parasomnias, indicates that the pseudo-suicidal behavior Mr. R displayed while asleep likely was a direct result of zolpidem use in presence of other facilitating factors. Gabapentin, which is known to increase the depth of sleep, was added to his regimen 1 month before his parasomnia episode. Therefore, gabapentin could have triggered parasomnia with zolpidem therapy.1,13
Conditions that provoke repeated cortical arousals (eg, periodic limb movement disorder [PLMD] and sleep apnea) or increase depth or pressure of sleep (intense exercise in the evening, fever, sleep deprivation) are thought to be associated with NREM parasomnias.1-4 However, Mr. R underwent in-laboratory PSG and tested negative for major cortical arousal-inducing conditions, such as PLMD and sleep apnea.
Some other sleep disruptors likely were involved in Mr. R’s case. Auditory and tactile stimuli are known to cause cortical arousals, with additive effect seen when these 2 stimuli are combined.3,14 Additionally, these exogenous stimuli are known to trigger sleep-related violent parasomnias.15 Mr. R displayed this behavior after his wife woke him up. The auditory stimulus of his wife’s voice and/or tactile stimulus involved in the act of waking Mr. R likely played a role in the suicidal and violent nature of his NREM parasomnia.
[polldaddy:9712581]
The authors’ observations
In general, the mechanisms by which zolpidem causes NREM parasomnias are not completely understood. The sedation-related amnestic properties of zolpidem might explain some of these behaviors. Patients could perform these behaviors after waking and have subsequent amnesia.4 There is greater preservation of Stage N3 sleep with zolpidem compared with benzodiazepines. Benzodiazepines also cause muscle relaxation while the motor system remains relatively more active during sleep with zolpidem because of its selectivity for α-1 subunit of gamma-aminobutyric acid A receptor. These factors might increase the likelihood of NREM parasomnias with zolpidem compared with benzodiazepines.4
Types of parasomnias
According to DSM-5, there are 2 categories of parasomnias based on the sleep stage from which a parasomnia emerges.2 REM sleep behavior disorder (RBD) refers to complex motor and/or vocalizations during REM sleep, accompanied by increased EMG activity during REM sleep (Table).2,3

The pseudo-suicidal behavior Mr. R displayed likely was NREM parasomnia because it occurred in the first third of the night with his eyes open and impaired recall after the event. Interestingly, Mr. R had RBD in addition to the NREM parasomnia likely caused by zolpidem. This is evident from Mr. R’s frequent dream enactment behaviors, such as kicking, thrashing, and punching during sleep, along with increased EMG activity during REM sleep as recorded on the PSG.10 The presence of RBD could be explained by selective serotonin reuptake inhibitor (fluoxetine) use, and comorbidity with PTSD.2,16
Management of parasomnias
Initial management of parasomnias involves decreasing the risk of parasomnia-related injury. Suggested safety measures include:
- sleeping away from windows
- sleeping in a sleeping bag
- sleeping on a lower floor
- locking windows and doors
- removing potentially dangerous objects from the bedroom
- putting gates across stairwells
- installing bells or alarms on door knobs.15
Removing access to firearms or other weapons such as knives is of utmost importance especially with patients who have easy access during wakefulness. If removing weapons is not feasible, consider disarming, securing, or locking them.15 These considerations are relevant to veterans with PTSD because of the high prevalence of symptoms, including depression, insomnia, and pain, which require sedating medications.17 A review of parasomnias among a large sample of psychiatric outpatients revealed that a variety of sedating medications, including antidepressants, can lead to NREM parasomnias.18 Therefore, exercise caution when prescribing sedating medications, especially in patients vulnerable to developing dangerous parasomnias, such as a veteran with PTSD and easy access to guns.19
TREATMENT Zolpidem stopped
Mr. R immediately stops taking zolpidem because he is aware of its association with abnormal behaviors during sleep, and his wife removes his access to firearms and knives at night. Because of his history of clinical benefit and no history of parasomnias with mirtazapine, Mr. R is started on mirtazapine for insomnia that previously was treated with zolpidem, and residual depression. Six months after discontinuing zolpidem, he does not experience NREM parasomnias, and there are no changes in his dream enactment behaviors.
Summing up
Zolpidem therapy could be associated with unusual variants of NREM parasomnia, sleepwalking type; sleep-related pseudo-suicidal behavior is one such variant. Several factors could play a role in increasing the likelihood of NREM parasomnia with zolpidem therapy. In Mr. R’s case, the pharmacokinetic drug interactions between fluoxetine and zolpidem, as well as concomitant use of several sedating agents could have played a role in increasing the likelihood of NREM parasomnia, with audio-tactile stimuli contributing to the violent and suicidal nature of the parasomnia. Exercise caution when using CYP enzyme inhibitors, such as fluoxetine and paroxetine, in combination with zolpidem. Knowledge of the potential interaction between zolpidem and fluoxetine is important because antidepressants and hypnotics are commonly co-prescribed because insomnia often is comorbid with other psychiatric disorders.
In veterans with PTSD who do not have suicidal ideations while awake, life-threatening non-intentional behavior is a risk because of easy access to guns or other weapons. Sedative-hypnotic medications commonly are prescribed to patients with PTSD. Exercise caution when using hypnotic agents such as zolpidem, and consider sleep aids with a lower risk of parasomnias (based on the author’s experience, trazodone, mirtazapine, melatonin, and gabapentin) when possible. Non-pharmacologic treatments of insomnia, such as sleep hygiene education and, more importantly, cognitive-behavioral therapy for insomnia, are preferred. If a patient is already taking zolpidem, nightly dosage should not be >10 mg. Polypharmacy with other sedating medications should be avoided when possible and both exogenous (noise, pets) and endogenous sleep disruptors (sleep apnea, PLMD) should be addressed. Advise the patient to avoid alcohol and remove firearms and other potential weapons. Discontinue zolpidem if the patient develops sleep-related abnormal behavior because of its potential to take on violent forms.
CASE Suicidal while asleep
Mr. R, age 28, an Iraq and Afghanistan veteran with major depressive disorder and posttraumatic stress disorder (PTSD), is awoken by his wife to check on their daughter approximately 30 minutes after he takes his nightly regimen of zolpidem, 10 mg, melatonin, 6 mg, and hydroxyzine, 20 mg. When Mr. R returns to the bedroom, he appears to be confused. Mr. R grabs an unloaded gun from under the mattress, puts it in his mouth, and pulls the trigger. Then Mr. R holds the gun to his head and pulls the trigger while saying that his wife and children will be better off without him. His wife takes the gun away, but he grabs another gun from his gun box and loads it. His wife convinces him to remove the ammunition; however, Mr. R gets the other unloaded gun and pulls the trigger on himself again. After his wife takes this gun away, he tries cutting himself with a pocketknife, causing superficial cuts. Eventually, Mr. R goes back to bed. He does not remember these events in the morning.
What increased the likelihood of parasomnia in Mr. R?
a) high zolpidem dosage
b) concomitant use of other sedating agents
c) sleep deprivation
d) dehydration
[polldaddy:9712545]
The authors’ observations
Parasomnias are sleep-wake transition disorders classified by the sleep stage from which they arise, either NREM or rapid eye movement (REM). NREM parasomnias could result from incomplete awakening from NREM sleep, typically in Stage N3 (slow-wave) sleep.1 DSM-5 describes NREM parasomnias as arousal disorders in which the disturbance is not attributable to the physiological effects of substance; substance/medication-induced sleep disorder, parasomnia type, is when the disturbance can be attributed to a substance.2 The latter also can occur during REM sleep.
NREM parasomnias are characterized by abnormal behaviors during sleep with significant harm potential.3 Somnambulism or sleepwalking and sleep terrors are the 2 types of NREM parasomnias in DSM-5. Sleepwalking could involve complex behaviors, including:
- eating
- talking
- cooking
- shopping
- driving
- sexual activity.
Zolpidem, a benzodiazepine receptor agonist, is a preferred hypnotic agent for insomnia because of its low risk for abuse and daytime sedation.4 However, the drug has been associated with NREM parasomnias, namely somnambulism or sleepwalking, and its variants including sleep-driving, sleep-related eating disorder, and rarely sexsomnia (sleep-sex), with anterograde amnesia for the event.5 Suicidal behavior that occurs while the patient is asleep with next-day amnesia is another variant of somnambulism. There are several reports of suicidal behavior during sleep,6,7 but to our knowledge, there are only 2 previous cases implicating zolpidem as the cause:
- Gibson et al8 described a 49-year-old man who sustained a self-inflicted gunshot wound to his head while asleep. He just had started taking zolpidem, and in the weeks before the incident he had several episodes of sleepwalking and sleep-eating. He had consumed alcohol the night of the self-inflicted gunshot wound, but had no other psychiatric history.
- Chopra et al4 described a 37-year-old man, with no prior episodes of sleepwalking or associated complex behaviors, who was taking zolpidem, 10 mg/d, for chronic insomnia. He shot a gun in the basement of his home, and then held the loaded gun to his neck while asleep. The authors attributed the event to zolpidem in combination with other predisposing factors, including dehydration after intense exercise and alcohol use. The authors categorized this type of event as “para-suicidal amnestic behavior,” although “sleep-related pseudo-suicidal behavior” might be a better term for this type of parasomnia because of its occurrence during sleep and non-deliberate nature.
In another case report, a 27-year-old man took additional zolpidem after he did not experience desired sedative effects from an initial 20 mg.9 Because the patient remembered the suicidal thoughts, the authors believed that the patient attempted suicide while under the influence of zolpidem. The authors did not believe the incident to be sleep-related suicidal behavior, because it was uncertain if he attempted suicide while asleep.
Mr. R does not remember the events his wife witnessed while he was asleep. To our knowledge, Mr. R’s case is the first sleep-related pseudo-suicidal behavior case resulting from zolpidem, 10 mg/d, without concurrent alcohol use in an adult male veteran with PTSD and no suicidal ideation while awake.
HISTORY Further details revealed
Mr. R says that in the days leading to the incident he was not sleep-deprived and was getting at least 6 hours of restful sleep every night. He had been taking zolpidem every night. He has no childhood or family history of NREM parasomnias. He says he did not engage in intense exercise that evening or have a fever the night of the incident and has abstained from alcohol for 2 years.
His wife says that after he took zolpidem, when he was woken up, “He was not there; his eyes were glazed and glossy, and it’s like he was in another world,” and his speech and behavior were bizarre. She also reports that his eyes were open when he engaged in this behavior that appeared suicidal.
Three months before the incident, Mr. R had reported nightmares with dream enactment behaviors, hypervigilance on awakening and during the daytime, irritability, and anxious and depressed mood with neurovegetative symptoms, and was referred to our clinic for medication management. He also reported no prior or current manic or psychotic symptoms, denied suicidal thoughts, and had no history of suicide attempts. Mr. R’s medication regimen included tramadol, 400 mg/d, for chronic knee pain; fluoxetine, 60 mg/d, for depression and PTSD; and propranolol ER, 60 mg/d, and propranolol, 10 mg/d as needed, for anxiety. He was started on prazosin, 2 mg/d, titrated to 4 mg/d, for medication management of nightmares.
Mr. R also was referred to the sleep laboratory for a polysomnogram (PSG) because of reported loud snoring and witnessed apneas, especially because sleep apnea can cause nightmares and dream enactment behaviors. The PSG was negative for sleep apnea or excessive periodic limb movements of sleep, but showed increased electromyographic (EMG) activity during REM sleep, which was consistent with his report of dream enactment behaviors. Two months later, he reported improvement in nightmares and depression, but not in dream enactment behaviors. Because of prominent anxiety and irritability, he was started on gabapentin, 300 mg, 3 times a day.
What factor increases the risk of NREM parasomnias with zolpidem compared with benzodiazepines?
a) greater preservation of Stage N3 sleep
b) lesser degree of muscle relaxation
c) both a and b
d) none of the above
[polldaddy:9712556]
The authors’ observations
Factors that increase the likelihood of parasomnias include:
- zolpidem >10 mg at bedtime
- concomitant use of other CNS depressants, including sedative hypnotic agents and alcohol
- female sex
- not falling asleep immediately after taking zolpidem
- personal or family history of parasomnias
- living alone
- poor pill management
- presence of sleep disruptors such as sleep apnea and periodic limb movements of sleep.1,4,5,10
Higher dosages of zolpidem (>10 mg/d) have been identified as the predictive risk factor.5 In the Chopra et al4 case report on sleep-related suicidal behavior related to zolpidem, 10 mg at bedtime, concomitant dehydration and alcohol use were implicated as facilitating factors. Dehydration could increase serum levels of zolpidem resulting in greater CNS effects. Alcohol use was implicated in the Gibson et al8 case report as well, and the patient had multiple episodes of sleepwalking and sleep-related eating.However, Mr. R was not dehydrated or using alcohol.
An interesting feature of Mr. R’s case is that he was taking fluoxetine. Cytochrome P450 (CYP) 3A4 is involved in metabolizing zolpidem, and norfluoxetine, a metabolite of fluoxetine, inhibits CYP3A4. Although studies have not found pharmacokinetic interactions between fluoxetine and zolpidem, these studies did not investigate fluoxetine dosages >20 mg/d.11 The inhibition of CYP enzymes by fluoxetine likely is dose-dependent,12 and therefore concomitant administration of high-dosage fluoxetine (>20 mg/d) with zolpidem might result in higher serum levels of zolpidem.
Mr. R also was taking several sedating agents (gabapentin, hydroxyzine, melatonin, and tramadol). The concomitant use of these sedative-hypnotic agents could have increased his risk of parasomnia. A review of the literature did not reveal any reports of gabapentin, hydroxyzine, melatonin, or tramadol causing parasomnias. This observation, as well as the well-known role of zolpidem5 in etiopathogenesis of parasomnias, indicates that the pseudo-suicidal behavior Mr. R displayed while asleep likely was a direct result of zolpidem use in presence of other facilitating factors. Gabapentin, which is known to increase the depth of sleep, was added to his regimen 1 month before his parasomnia episode. Therefore, gabapentin could have triggered parasomnia with zolpidem therapy.1,13
Conditions that provoke repeated cortical arousals (eg, periodic limb movement disorder [PLMD] and sleep apnea) or increase depth or pressure of sleep (intense exercise in the evening, fever, sleep deprivation) are thought to be associated with NREM parasomnias.1-4 However, Mr. R underwent in-laboratory PSG and tested negative for major cortical arousal-inducing conditions, such as PLMD and sleep apnea.
Some other sleep disruptors likely were involved in Mr. R’s case. Auditory and tactile stimuli are known to cause cortical arousals, with additive effect seen when these 2 stimuli are combined.3,14 Additionally, these exogenous stimuli are known to trigger sleep-related violent parasomnias.15 Mr. R displayed this behavior after his wife woke him up. The auditory stimulus of his wife’s voice and/or tactile stimulus involved in the act of waking Mr. R likely played a role in the suicidal and violent nature of his NREM parasomnia.
[polldaddy:9712581]
The authors’ observations
In general, the mechanisms by which zolpidem causes NREM parasomnias are not completely understood. The sedation-related amnestic properties of zolpidem might explain some of these behaviors. Patients could perform these behaviors after waking and have subsequent amnesia.4 There is greater preservation of Stage N3 sleep with zolpidem compared with benzodiazepines. Benzodiazepines also cause muscle relaxation while the motor system remains relatively more active during sleep with zolpidem because of its selectivity for α-1 subunit of gamma-aminobutyric acid A receptor. These factors might increase the likelihood of NREM parasomnias with zolpidem compared with benzodiazepines.4
Types of parasomnias
According to DSM-5, there are 2 categories of parasomnias based on the sleep stage from which a parasomnia emerges.2 REM sleep behavior disorder (RBD) refers to complex motor and/or vocalizations during REM sleep, accompanied by increased EMG activity during REM sleep (Table).2,3

The pseudo-suicidal behavior Mr. R displayed likely was NREM parasomnia because it occurred in the first third of the night with his eyes open and impaired recall after the event. Interestingly, Mr. R had RBD in addition to the NREM parasomnia likely caused by zolpidem. This is evident from Mr. R’s frequent dream enactment behaviors, such as kicking, thrashing, and punching during sleep, along with increased EMG activity during REM sleep as recorded on the PSG.10 The presence of RBD could be explained by selective serotonin reuptake inhibitor (fluoxetine) use, and comorbidity with PTSD.2,16
Management of parasomnias
Initial management of parasomnias involves decreasing the risk of parasomnia-related injury. Suggested safety measures include:
- sleeping away from windows
- sleeping in a sleeping bag
- sleeping on a lower floor
- locking windows and doors
- removing potentially dangerous objects from the bedroom
- putting gates across stairwells
- installing bells or alarms on door knobs.15
Removing access to firearms or other weapons such as knives is of utmost importance especially with patients who have easy access during wakefulness. If removing weapons is not feasible, consider disarming, securing, or locking them.15 These considerations are relevant to veterans with PTSD because of the high prevalence of symptoms, including depression, insomnia, and pain, which require sedating medications.17 A review of parasomnias among a large sample of psychiatric outpatients revealed that a variety of sedating medications, including antidepressants, can lead to NREM parasomnias.18 Therefore, exercise caution when prescribing sedating medications, especially in patients vulnerable to developing dangerous parasomnias, such as a veteran with PTSD and easy access to guns.19
TREATMENT Zolpidem stopped
Mr. R immediately stops taking zolpidem because he is aware of its association with abnormal behaviors during sleep, and his wife removes his access to firearms and knives at night. Because of his history of clinical benefit and no history of parasomnias with mirtazapine, Mr. R is started on mirtazapine for insomnia that previously was treated with zolpidem, and residual depression. Six months after discontinuing zolpidem, he does not experience NREM parasomnias, and there are no changes in his dream enactment behaviors.
Summing up
Zolpidem therapy could be associated with unusual variants of NREM parasomnia, sleepwalking type; sleep-related pseudo-suicidal behavior is one such variant. Several factors could play a role in increasing the likelihood of NREM parasomnia with zolpidem therapy. In Mr. R’s case, the pharmacokinetic drug interactions between fluoxetine and zolpidem, as well as concomitant use of several sedating agents could have played a role in increasing the likelihood of NREM parasomnia, with audio-tactile stimuli contributing to the violent and suicidal nature of the parasomnia. Exercise caution when using CYP enzyme inhibitors, such as fluoxetine and paroxetine, in combination with zolpidem. Knowledge of the potential interaction between zolpidem and fluoxetine is important because antidepressants and hypnotics are commonly co-prescribed because insomnia often is comorbid with other psychiatric disorders.
In veterans with PTSD who do not have suicidal ideations while awake, life-threatening non-intentional behavior is a risk because of easy access to guns or other weapons. Sedative-hypnotic medications commonly are prescribed to patients with PTSD. Exercise caution when using hypnotic agents such as zolpidem, and consider sleep aids with a lower risk of parasomnias (based on the author’s experience, trazodone, mirtazapine, melatonin, and gabapentin) when possible. Non-pharmacologic treatments of insomnia, such as sleep hygiene education and, more importantly, cognitive-behavioral therapy for insomnia, are preferred. If a patient is already taking zolpidem, nightly dosage should not be >10 mg. Polypharmacy with other sedating medications should be avoided when possible and both exogenous (noise, pets) and endogenous sleep disruptors (sleep apnea, PLMD) should be addressed. Advise the patient to avoid alcohol and remove firearms and other potential weapons. Discontinue zolpidem if the patient develops sleep-related abnormal behavior because of its potential to take on violent forms.
1. Howell MJ. Parasomnias: an updated review. Neurotherapeutics. 2012;9(4):753-775.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Zadra A, Desautels A, Petit D, et al. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12(3):285-294.
4. Chopra A, Selim B, Silber MH, et al. Para-suicidal amnestic behavior associated with chronic zolpidem use: implications for patient safety. Psychosomatics. 2013;54(5):498-501.
5. Hwang TJ, Ni HC, Chen HC, et al. Risk predictors for hypnosedative-related complex sleep behaviors: a retrospective, cross-sectional pilot study. J Clin Psychiatry. 2010;71(10):1331-1335.
6. Shatkin JP, Feinfield K, Strober M. The misinterpretation of a non-REM sleep parasomnia as suicidal behavior in an adolescent. Sleep Breath. 2002;6(4):175-179.
7. Mahowald MW, Schenck CH, Goldner M, et al. Parasomnia pseudo-suicide. J Forensic Sci. 2003;48(5):1158-1162.
8. Gibson CE, Caplan JP. Zolpidem-associated parasomnia with serious self-injury: a shot in the dark. Psychosomatics. 2011;52(1):88-91.
9. Mortaz Hejri S, Faizi M, Babaeian M. Zolpidem-induced suicide attempt: a case report. Daru. 2013;20;21(1):77.
10. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
11. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs. 2003;17(7):513-532.
12. Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78(4):203-208.
13. Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1093-1100.
14. Kato T, Montplaisir JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13(3):229-238.
15. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(pt 12):3494-3509.
16. Husain AM, Miller PP, Carwile ST. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148-157.
17. Bernardy NC, Lund BC, Alexander B, et al. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
18. Lam SP, Fong SY, Ho CK, et al. Parasomnia among psychiatric outpatients: a clinical, epidemiologic, cross-sectional study. J Clin Psychiatry. 2008;69(9):1374-1382.
19. Freeman TW, Roca V, Kimbrell T. A survey of gun collection and use among three groups of veteran patients admitted to veterans affairs hospital treatment programs. South Med J. 2003;96(3):240-243.
1. Howell MJ. Parasomnias: an updated review. Neurotherapeutics. 2012;9(4):753-775.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Zadra A, Desautels A, Petit D, et al. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12(3):285-294.
4. Chopra A, Selim B, Silber MH, et al. Para-suicidal amnestic behavior associated with chronic zolpidem use: implications for patient safety. Psychosomatics. 2013;54(5):498-501.
5. Hwang TJ, Ni HC, Chen HC, et al. Risk predictors for hypnosedative-related complex sleep behaviors: a retrospective, cross-sectional pilot study. J Clin Psychiatry. 2010;71(10):1331-1335.
6. Shatkin JP, Feinfield K, Strober M. The misinterpretation of a non-REM sleep parasomnia as suicidal behavior in an adolescent. Sleep Breath. 2002;6(4):175-179.
7. Mahowald MW, Schenck CH, Goldner M, et al. Parasomnia pseudo-suicide. J Forensic Sci. 2003;48(5):1158-1162.
8. Gibson CE, Caplan JP. Zolpidem-associated parasomnia with serious self-injury: a shot in the dark. Psychosomatics. 2011;52(1):88-91.
9. Mortaz Hejri S, Faizi M, Babaeian M. Zolpidem-induced suicide attempt: a case report. Daru. 2013;20;21(1):77.
10. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
11. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs. 2003;17(7):513-532.
12. Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78(4):203-208.
13. Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1093-1100.
14. Kato T, Montplaisir JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13(3):229-238.
15. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(pt 12):3494-3509.
16. Husain AM, Miller PP, Carwile ST. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148-157.
17. Bernardy NC, Lund BC, Alexander B, et al. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
18. Lam SP, Fong SY, Ho CK, et al. Parasomnia among psychiatric outpatients: a clinical, epidemiologic, cross-sectional study. J Clin Psychiatry. 2008;69(9):1374-1382.
19. Freeman TW, Roca V, Kimbrell T. A survey of gun collection and use among three groups of veteran patients admitted to veterans affairs hospital treatment programs. South Med J. 2003;96(3):240-243.
Social withdrawal and confusion in an inmate with schizoaffective disorder
CASE Withdrawn and confused
Mr. J, age 54, is brought to the emergency department from a correctional treatment facility where he is reported to be displaying new, unusual behavior. He has a history of schizoaffective disorder, which has been stable with haloperidol, 10 mg/d, for more than a year.
Although previously Mr. J openly discussed his long-standing delusions about the FBI coming to release him from prison, he no longer mentions this or any other delusional beliefs, and has become less communicative with staff and peers. Mr. J no longer accompanies the other patients to the cafeteria for meals and eats in his room alone and appears to be losing weight. He says, “I do not feel good,” but otherwise does not communicate spontaneously. Intermittently, he is irritable, without known triggers. The staff notices that Mr. J often lays on his bed, sometimes in a fetal position. Over time, he becomes confused and is seen attempting to open his room door with a toothbrush. His personal hygiene is poor, and he often urinates through his clothes, on the floor, and in his bed. Recently, Mr. J’s eczema has worsened. His gait has become unsteady, and he has orthostasis.
What could be causing these new symptoms?
a) worsening schizoaffective disorder
b) illicit drug use in the prison
c) atypical dementia
d) cardiac etiology
The author’s observations
The differential diagnosis for Mr. J appeared to be wide and without specific etiology. Because of the complex types of symptoms that Mr. J was experiencing, the emergency department managed his care and specialty clinic referrals were ordered.
It was reported that Mr. J started complaining of lightheadedness a few months ago, which worsened (unsteady gait, near falls). In the context of Mr. J’s history of lightheadedness and orthostasis, the cardiology clinic ordered a tilt table test, which was within normal limits:
- 70º head-up tilt: blood pressure, 91/67 to 102/62 mm Hg, and pulse, 70 to 79 beats per minute (bpm)
- with isoproterenol, 1 μg/minute: blood pressure, 90/66 to 110/70 mm Hg, and pulse, 77 to 124 bpm
- with isoproterenol, 2 μg/minute: blood pressure, 98/58 to 111/66 mm Hg, and pulse, 121 to 134 bpm.
The neurologist’s diagnostic impression was atypical dementia; however, Mr. J showed no memory deficits. Parkinsonism also was considered, but Mr. J had no unilateral tremor, masked facies, or micrographia. Mr. J showed some restriction in his movement, but he was not bradykinetic. The team suspected haloperidol could be causing his stiff movement.
Although it was possible that Mr. J’s schizoaffective disorder was worsening and led to the new symptoms, Mr. J appeared to be less delusional because he was no longer talking to the staff about his delusions. There seemed to be no outward evidence of progression of psychotic symptoms.
Mr. J had a history of substance abuse, including alcohol, cocaine, and Cannabis. Although prison inmates have been known to manufacture and drink “hooch,” the new symptoms Mr. J was experiencing were severe enough that his social interactions with other inmates diminished substantially. Because Mr. J had not been communicating with the other inmates and had no recent visitors, the team felt that it was unlikely that drugs were causing these symptoms. Also, a urine drug screen for cocaine, amphetamines, benzodiazepines, Cannabis, and opioids was negative.
HISTORY Substance use, violence
Mr. J was diagnosed with bipolar disorder at age 18. After later hospitalizations, his diagnosis was changed to schizoaffective disorder as a matter of diagnostic clarification. He has a long history of non-compliance with treatment, homelessness, and drug abuse.
Mr. J is serving a 20-year sentence for first-degree reckless homicide. A year after he was incarcerated, Mr. J was sent to a specialized mental health facility for inmates whose illness cannot be managed in a typical correctional setting. While at the treatment facility, Mr. J was non-compliant with medications and because of concerns about dangerousness and psychosis, the court found probable cause for involuntary commitment.
His medication regimen is trihexyphenidyl, 2 mg/d, for extrapyramidal symptoms; haloperidol, 10 mg/d, for psychosis; trazodone, 150 mg/d, for insomnia; vitamin D3, 2,000 IU/d; vitamin E, 400 IU/d, for symptoms of tardive dyskinesia; IM ziprasidone, 20 mg, because he refused oral haliperidol; and hydrocortisone cream 1% for eczema.
EVALUATION Additional tests
Mr. J’s blood pressure is 124/72 mm Hg, and pulse, 104 bpm, laying down; blood pressure, 110/84 mm Hg, and pulse, 112 bpm, sitting; and blood pressure, 108/82 mm Hg, and pulse, 129 bpm, standing. With repeated readings: blood pressure, 128/84 mm Hg, and pulse, 98 bpm, laying down; blood pressure, 125/86 mm Hg, and pulse, 113 bpm, sitting; and blood pressure, 105/76 mm Hg, and pulse, 130 bpm, standing.
Laboratory tests, including complete blood count, chemistry panel, thyroid-stimulating hormone, are within normal limits. The team feels that the investigation for an etiology for Mr. J’s symptoms needs to be more exhaustive and additional tests are ordered, including vitamin levels (C, B1, B12, B6), rapid plasma reagin for syphilis, and arbovirus testing (eastern equine encephalitis virus, western equine encephalitis, West Nile virus, La Crosse encephalitis, St. Louis encephalitis), which are negative.
What’s the next best step in managing Mr. J’s care?
a) adjust his medication
b) eliminate a mediation
c) order further testing
The author’s observations
To determine if Mr. J’s new-onset symptoms might be related to the progression of his psychiatric illness, the haloperidol dosage was increased to 20 mg/d; however, we saw no positive response to this change. His tardive dyskinesia symptoms (bruxism and other oral buccal movements) worsened. Haloperidol was reduced to 10 mg/d.
Trihexyphenidyl then was suspected to contribute to Mr. J’s confusion. Unfortunately, lowering the dosage of trihexyphenidyl to 1 mg/d, did not affect Mr. J’s current symptoms and exacerbated extrapyramidal symptoms.
The treatment team then questioned if porphyria—known as the “little imitator”—might be considered because of the variety of symptoms without an etiology, despite extensive testing. A 24-hour urine collection was ordered.
What is the correct method of collecting a urine sample for porphyrins?
a) collect a small sample and expose it to light before testing
b) collect a 24-hour sample with the sample kept in ambient temperature and light
c) collect a 24-hour sample with the sample kept on ice in a light-blocking container and frozen when sent to the laboratory
EVALUATION Diagnosis revealed
The 24-hour urine collection is obtained. However, it needed to be collected twice, because the first sample was not a full sample. Interestingly, the first sample, which is exposed to light and not kept on ice, turned dark in color. The second sample is obtained properly and sent to the laboratory. When the laboratory results are returned (Figure 1), Mr. J is diagnosed with hereditary coproporphyria (HCP).

The author’s observations
There are several types of porphyria, each associated with a different step in the chain of enzymes associated with synthesis of a heme molecule in the mitochondria. A defect in any single enzyme step will create a build up of porphyrins—a precursor to heme molecules—in erythrocytes or hepatic cells.
It is important to differentiate hepatic from erythropoietic porphyrias. The acute porphyrias (acute intermittent porphyria [AIP], HCP, and variegate porphyria generally are hepatic in origin with neuropsychiatric and neurovisceral symptoms. Cutaneous porphyrias originate in bone marrow and therefore are erythropoietic. However, there are exceptions such as porphyria cutanea tarda (PCT), which is hepatic in origin but the manifestations mainly are cutaneous1 (Figure 2).2
Although acute porphyria originates in the liver, it is a neuropsychiatric illness. In these cases, excess porphyrins cannot cross the blood–brain barrier and are neurotoxic. Clinicians can look for abnormalities in the liver via liver function tests, but liver parenchyma is not damaged by these enzyme precursors. During an acute porphyic attack, patients could experience symptoms such as:
- muscle spasms (commonly abdominal, but can be any muscle group)
- confusion
- disorientation
- autonomic instability
- lightheadness
- disorientation
- diarrhea
- light sensitivity
- dermatologic conditions
- weakness (particularly peripheral weakness)
- hypesthesia
- allodynia
- severe nausea and vomiting
- emotional lability
- psychosis as well as general malaise.
The attack could result in death.
Mr. J had many differing symptoms and was evaluated by several specialty providers. He had a chronic dermatologic condition; he was confused, disoriented, and complained of nausea, weakness, orthostasis, and loose stools. With the variety of possible symptoms that patients such as Mr. J could experience, one can see why it would lead to many different providers being involved in the diagnosis. It is not uncommon for psychiatrists to be the last providers to care for such patients who could have been evaluated by hematology, cardiology, gastroenterology, dermatology, and/or neurology.
Hereditary coproporphyria
The team considered hepatic porphyias because of new-onset symptoms of mood lability, confusion, orthostasis, unsteady gait, weakness, dermatologic conditions on hands not responsive to treatment, and general malaise. Mr. J was diagnosed with HCP, a type of porphyria caused by a defect in coproporphyrinogen oxidase that leads to an accumulation of coproporphyrinogen III. This precursor, as are many porphyrin precursors, is neurotoxic, leading to neurovisceral or neuropsychiatric effects. Although in Mr. J’s case the coproporphyrinogen III value from the 24-hour drug screen was only modestly elevated, it has been noted that levels of excreted prophyrins do not necessarily correlate with symptom severity.3
In the past, porphyria testing was performed using the Watson-Schwartz test, which used Ehrlich’s reagent to precipitate porphyrins in a urine sample,4 and was used as a “bedside” test. Interestingly, porphyrins—not the iron found in the heme molecule—are precipitated in this test and cause the reddish-purple coloration of the urine sample. When quantitative testing was developed, a 24-hour sample of urine—kept on ice and away from ambient light, later to be frozen when sent to the laboratory—became the standard tool for testing for porphyrins. Now DNA testing can be used to diagnose HCP.
OUTCOME Symptoms resolve
Mr. J is started on loxapine, 20 mg at bedtime, and his symptoms resolve within 2 weeks. He maintains some baseline delusional ideation consistent with his history of schizoaffective disorder, but he is more social, his personal hygiene improves, he attends groups, eats in the cafeteria with his peers, and is no longer confused.
The author’s observations
In the 1950s, chlorpromazine was used to treat AIP.5 Mr. J received loxapine, a mid-potency first-generation antipsychotic, although it has been this author’s observation that high-potency first-generation antipsychotics are not effective for treating porphyria.
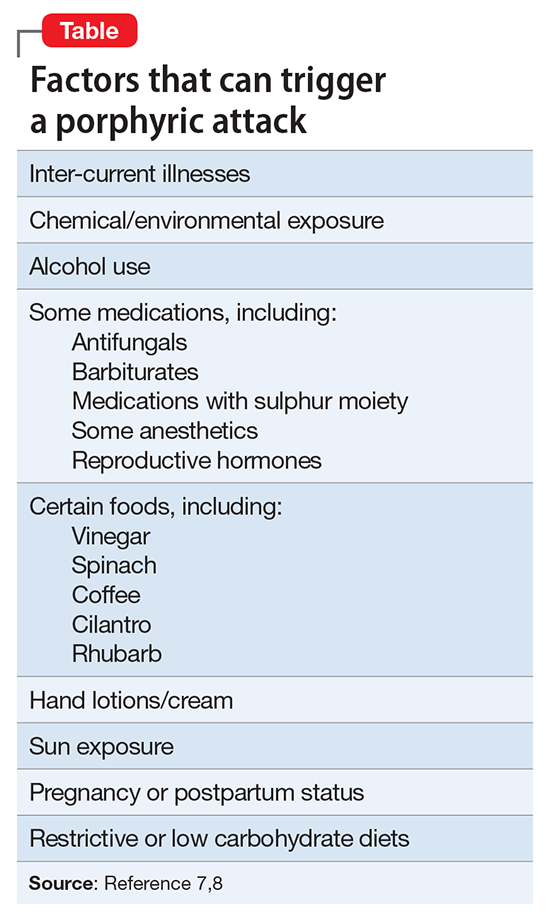
1. NIH: National Human Genome Research Institute. Learning about porphyria. https://www.genome.gov/19016728/learning-about-porphyria/learning-about-porphyria. Accessed February 23, 2017.
2. Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim Biophys Acta. 2006;1763(7):723-736.
3. Peters HA, Gocmen A, Cripps DJ, et al. Epidemiology of hexachlorobenzene-induced porphyria in Turkey: clinical and laboratory follow-up after 25 years. Arch Neurol. 1982;39(12):744-749.
4. The Watsonschwartz test. JAMA. 1966;195(6):481.
5. Brunton L, Chabner BA, Knollman B. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw-Hill Professional; 2010.
6. Broomfield B. Acute Intermittent porphyria treated with chlorpromazine. Proc R Soc Med. 1962;55(9):799-800.
7. Hunter JA, Khan SA, Hope E, et al. Hereditary coproporphyria. Photosensitivity, jaundice and neuropsychiatric manifestations associated with pregnancy. Br J Dermatol. 1971;84(4):301-310.
8. Bonkovsky HL, Maddukuri V. Merck Manual. http://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/porphyrias/overview-of-porphyrias. Accessed February 2, 2017.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24 (suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4): 247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1): 64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
CASE Withdrawn and confused
Mr. J, age 54, is brought to the emergency department from a correctional treatment facility where he is reported to be displaying new, unusual behavior. He has a history of schizoaffective disorder, which has been stable with haloperidol, 10 mg/d, for more than a year.
Although previously Mr. J openly discussed his long-standing delusions about the FBI coming to release him from prison, he no longer mentions this or any other delusional beliefs, and has become less communicative with staff and peers. Mr. J no longer accompanies the other patients to the cafeteria for meals and eats in his room alone and appears to be losing weight. He says, “I do not feel good,” but otherwise does not communicate spontaneously. Intermittently, he is irritable, without known triggers. The staff notices that Mr. J often lays on his bed, sometimes in a fetal position. Over time, he becomes confused and is seen attempting to open his room door with a toothbrush. His personal hygiene is poor, and he often urinates through his clothes, on the floor, and in his bed. Recently, Mr. J’s eczema has worsened. His gait has become unsteady, and he has orthostasis.
What could be causing these new symptoms?
a) worsening schizoaffective disorder
b) illicit drug use in the prison
c) atypical dementia
d) cardiac etiology
The author’s observations
The differential diagnosis for Mr. J appeared to be wide and without specific etiology. Because of the complex types of symptoms that Mr. J was experiencing, the emergency department managed his care and specialty clinic referrals were ordered.
It was reported that Mr. J started complaining of lightheadedness a few months ago, which worsened (unsteady gait, near falls). In the context of Mr. J’s history of lightheadedness and orthostasis, the cardiology clinic ordered a tilt table test, which was within normal limits:
- 70º head-up tilt: blood pressure, 91/67 to 102/62 mm Hg, and pulse, 70 to 79 beats per minute (bpm)
- with isoproterenol, 1 μg/minute: blood pressure, 90/66 to 110/70 mm Hg, and pulse, 77 to 124 bpm
- with isoproterenol, 2 μg/minute: blood pressure, 98/58 to 111/66 mm Hg, and pulse, 121 to 134 bpm.
The neurologist’s diagnostic impression was atypical dementia; however, Mr. J showed no memory deficits. Parkinsonism also was considered, but Mr. J had no unilateral tremor, masked facies, or micrographia. Mr. J showed some restriction in his movement, but he was not bradykinetic. The team suspected haloperidol could be causing his stiff movement.
Although it was possible that Mr. J’s schizoaffective disorder was worsening and led to the new symptoms, Mr. J appeared to be less delusional because he was no longer talking to the staff about his delusions. There seemed to be no outward evidence of progression of psychotic symptoms.
Mr. J had a history of substance abuse, including alcohol, cocaine, and Cannabis. Although prison inmates have been known to manufacture and drink “hooch,” the new symptoms Mr. J was experiencing were severe enough that his social interactions with other inmates diminished substantially. Because Mr. J had not been communicating with the other inmates and had no recent visitors, the team felt that it was unlikely that drugs were causing these symptoms. Also, a urine drug screen for cocaine, amphetamines, benzodiazepines, Cannabis, and opioids was negative.
HISTORY Substance use, violence
Mr. J was diagnosed with bipolar disorder at age 18. After later hospitalizations, his diagnosis was changed to schizoaffective disorder as a matter of diagnostic clarification. He has a long history of non-compliance with treatment, homelessness, and drug abuse.
Mr. J is serving a 20-year sentence for first-degree reckless homicide. A year after he was incarcerated, Mr. J was sent to a specialized mental health facility for inmates whose illness cannot be managed in a typical correctional setting. While at the treatment facility, Mr. J was non-compliant with medications and because of concerns about dangerousness and psychosis, the court found probable cause for involuntary commitment.
His medication regimen is trihexyphenidyl, 2 mg/d, for extrapyramidal symptoms; haloperidol, 10 mg/d, for psychosis; trazodone, 150 mg/d, for insomnia; vitamin D3, 2,000 IU/d; vitamin E, 400 IU/d, for symptoms of tardive dyskinesia; IM ziprasidone, 20 mg, because he refused oral haliperidol; and hydrocortisone cream 1% for eczema.
EVALUATION Additional tests
Mr. J’s blood pressure is 124/72 mm Hg, and pulse, 104 bpm, laying down; blood pressure, 110/84 mm Hg, and pulse, 112 bpm, sitting; and blood pressure, 108/82 mm Hg, and pulse, 129 bpm, standing. With repeated readings: blood pressure, 128/84 mm Hg, and pulse, 98 bpm, laying down; blood pressure, 125/86 mm Hg, and pulse, 113 bpm, sitting; and blood pressure, 105/76 mm Hg, and pulse, 130 bpm, standing.
Laboratory tests, including complete blood count, chemistry panel, thyroid-stimulating hormone, are within normal limits. The team feels that the investigation for an etiology for Mr. J’s symptoms needs to be more exhaustive and additional tests are ordered, including vitamin levels (C, B1, B12, B6), rapid plasma reagin for syphilis, and arbovirus testing (eastern equine encephalitis virus, western equine encephalitis, West Nile virus, La Crosse encephalitis, St. Louis encephalitis), which are negative.
What’s the next best step in managing Mr. J’s care?
a) adjust his medication
b) eliminate a mediation
c) order further testing
The author’s observations
To determine if Mr. J’s new-onset symptoms might be related to the progression of his psychiatric illness, the haloperidol dosage was increased to 20 mg/d; however, we saw no positive response to this change. His tardive dyskinesia symptoms (bruxism and other oral buccal movements) worsened. Haloperidol was reduced to 10 mg/d.
Trihexyphenidyl then was suspected to contribute to Mr. J’s confusion. Unfortunately, lowering the dosage of trihexyphenidyl to 1 mg/d, did not affect Mr. J’s current symptoms and exacerbated extrapyramidal symptoms.
The treatment team then questioned if porphyria—known as the “little imitator”—might be considered because of the variety of symptoms without an etiology, despite extensive testing. A 24-hour urine collection was ordered.
What is the correct method of collecting a urine sample for porphyrins?
a) collect a small sample and expose it to light before testing
b) collect a 24-hour sample with the sample kept in ambient temperature and light
c) collect a 24-hour sample with the sample kept on ice in a light-blocking container and frozen when sent to the laboratory
EVALUATION Diagnosis revealed
The 24-hour urine collection is obtained. However, it needed to be collected twice, because the first sample was not a full sample. Interestingly, the first sample, which is exposed to light and not kept on ice, turned dark in color. The second sample is obtained properly and sent to the laboratory. When the laboratory results are returned (Figure 1), Mr. J is diagnosed with hereditary coproporphyria (HCP).

The author’s observations
There are several types of porphyria, each associated with a different step in the chain of enzymes associated with synthesis of a heme molecule in the mitochondria. A defect in any single enzyme step will create a build up of porphyrins—a precursor to heme molecules—in erythrocytes or hepatic cells.
It is important to differentiate hepatic from erythropoietic porphyrias. The acute porphyrias (acute intermittent porphyria [AIP], HCP, and variegate porphyria generally are hepatic in origin with neuropsychiatric and neurovisceral symptoms. Cutaneous porphyrias originate in bone marrow and therefore are erythropoietic. However, there are exceptions such as porphyria cutanea tarda (PCT), which is hepatic in origin but the manifestations mainly are cutaneous1 (Figure 2).2
Although acute porphyria originates in the liver, it is a neuropsychiatric illness. In these cases, excess porphyrins cannot cross the blood–brain barrier and are neurotoxic. Clinicians can look for abnormalities in the liver via liver function tests, but liver parenchyma is not damaged by these enzyme precursors. During an acute porphyic attack, patients could experience symptoms such as:
- muscle spasms (commonly abdominal, but can be any muscle group)
- confusion
- disorientation
- autonomic instability
- lightheadness
- disorientation
- diarrhea
- light sensitivity
- dermatologic conditions
- weakness (particularly peripheral weakness)
- hypesthesia
- allodynia
- severe nausea and vomiting
- emotional lability
- psychosis as well as general malaise.
The attack could result in death.
Mr. J had many differing symptoms and was evaluated by several specialty providers. He had a chronic dermatologic condition; he was confused, disoriented, and complained of nausea, weakness, orthostasis, and loose stools. With the variety of possible symptoms that patients such as Mr. J could experience, one can see why it would lead to many different providers being involved in the diagnosis. It is not uncommon for psychiatrists to be the last providers to care for such patients who could have been evaluated by hematology, cardiology, gastroenterology, dermatology, and/or neurology.
Hereditary coproporphyria
The team considered hepatic porphyias because of new-onset symptoms of mood lability, confusion, orthostasis, unsteady gait, weakness, dermatologic conditions on hands not responsive to treatment, and general malaise. Mr. J was diagnosed with HCP, a type of porphyria caused by a defect in coproporphyrinogen oxidase that leads to an accumulation of coproporphyrinogen III. This precursor, as are many porphyrin precursors, is neurotoxic, leading to neurovisceral or neuropsychiatric effects. Although in Mr. J’s case the coproporphyrinogen III value from the 24-hour drug screen was only modestly elevated, it has been noted that levels of excreted prophyrins do not necessarily correlate with symptom severity.3
In the past, porphyria testing was performed using the Watson-Schwartz test, which used Ehrlich’s reagent to precipitate porphyrins in a urine sample,4 and was used as a “bedside” test. Interestingly, porphyrins—not the iron found in the heme molecule—are precipitated in this test and cause the reddish-purple coloration of the urine sample. When quantitative testing was developed, a 24-hour sample of urine—kept on ice and away from ambient light, later to be frozen when sent to the laboratory—became the standard tool for testing for porphyrins. Now DNA testing can be used to diagnose HCP.
OUTCOME Symptoms resolve
Mr. J is started on loxapine, 20 mg at bedtime, and his symptoms resolve within 2 weeks. He maintains some baseline delusional ideation consistent with his history of schizoaffective disorder, but he is more social, his personal hygiene improves, he attends groups, eats in the cafeteria with his peers, and is no longer confused.
The author’s observations
In the 1950s, chlorpromazine was used to treat AIP.5 Mr. J received loxapine, a mid-potency first-generation antipsychotic, although it has been this author’s observation that high-potency first-generation antipsychotics are not effective for treating porphyria.

CASE Withdrawn and confused
Mr. J, age 54, is brought to the emergency department from a correctional treatment facility where he is reported to be displaying new, unusual behavior. He has a history of schizoaffective disorder, which has been stable with haloperidol, 10 mg/d, for more than a year.
Although previously Mr. J openly discussed his long-standing delusions about the FBI coming to release him from prison, he no longer mentions this or any other delusional beliefs, and has become less communicative with staff and peers. Mr. J no longer accompanies the other patients to the cafeteria for meals and eats in his room alone and appears to be losing weight. He says, “I do not feel good,” but otherwise does not communicate spontaneously. Intermittently, he is irritable, without known triggers. The staff notices that Mr. J often lays on his bed, sometimes in a fetal position. Over time, he becomes confused and is seen attempting to open his room door with a toothbrush. His personal hygiene is poor, and he often urinates through his clothes, on the floor, and in his bed. Recently, Mr. J’s eczema has worsened. His gait has become unsteady, and he has orthostasis.
What could be causing these new symptoms?
a) worsening schizoaffective disorder
b) illicit drug use in the prison
c) atypical dementia
d) cardiac etiology
The author’s observations
The differential diagnosis for Mr. J appeared to be wide and without specific etiology. Because of the complex types of symptoms that Mr. J was experiencing, the emergency department managed his care and specialty clinic referrals were ordered.
It was reported that Mr. J started complaining of lightheadedness a few months ago, which worsened (unsteady gait, near falls). In the context of Mr. J’s history of lightheadedness and orthostasis, the cardiology clinic ordered a tilt table test, which was within normal limits:
- 70º head-up tilt: blood pressure, 91/67 to 102/62 mm Hg, and pulse, 70 to 79 beats per minute (bpm)
- with isoproterenol, 1 μg/minute: blood pressure, 90/66 to 110/70 mm Hg, and pulse, 77 to 124 bpm
- with isoproterenol, 2 μg/minute: blood pressure, 98/58 to 111/66 mm Hg, and pulse, 121 to 134 bpm.
The neurologist’s diagnostic impression was atypical dementia; however, Mr. J showed no memory deficits. Parkinsonism also was considered, but Mr. J had no unilateral tremor, masked facies, or micrographia. Mr. J showed some restriction in his movement, but he was not bradykinetic. The team suspected haloperidol could be causing his stiff movement.
Although it was possible that Mr. J’s schizoaffective disorder was worsening and led to the new symptoms, Mr. J appeared to be less delusional because he was no longer talking to the staff about his delusions. There seemed to be no outward evidence of progression of psychotic symptoms.
Mr. J had a history of substance abuse, including alcohol, cocaine, and Cannabis. Although prison inmates have been known to manufacture and drink “hooch,” the new symptoms Mr. J was experiencing were severe enough that his social interactions with other inmates diminished substantially. Because Mr. J had not been communicating with the other inmates and had no recent visitors, the team felt that it was unlikely that drugs were causing these symptoms. Also, a urine drug screen for cocaine, amphetamines, benzodiazepines, Cannabis, and opioids was negative.
HISTORY Substance use, violence
Mr. J was diagnosed with bipolar disorder at age 18. After later hospitalizations, his diagnosis was changed to schizoaffective disorder as a matter of diagnostic clarification. He has a long history of non-compliance with treatment, homelessness, and drug abuse.
Mr. J is serving a 20-year sentence for first-degree reckless homicide. A year after he was incarcerated, Mr. J was sent to a specialized mental health facility for inmates whose illness cannot be managed in a typical correctional setting. While at the treatment facility, Mr. J was non-compliant with medications and because of concerns about dangerousness and psychosis, the court found probable cause for involuntary commitment.
His medication regimen is trihexyphenidyl, 2 mg/d, for extrapyramidal symptoms; haloperidol, 10 mg/d, for psychosis; trazodone, 150 mg/d, for insomnia; vitamin D3, 2,000 IU/d; vitamin E, 400 IU/d, for symptoms of tardive dyskinesia; IM ziprasidone, 20 mg, because he refused oral haliperidol; and hydrocortisone cream 1% for eczema.
EVALUATION Additional tests
Mr. J’s blood pressure is 124/72 mm Hg, and pulse, 104 bpm, laying down; blood pressure, 110/84 mm Hg, and pulse, 112 bpm, sitting; and blood pressure, 108/82 mm Hg, and pulse, 129 bpm, standing. With repeated readings: blood pressure, 128/84 mm Hg, and pulse, 98 bpm, laying down; blood pressure, 125/86 mm Hg, and pulse, 113 bpm, sitting; and blood pressure, 105/76 mm Hg, and pulse, 130 bpm, standing.
Laboratory tests, including complete blood count, chemistry panel, thyroid-stimulating hormone, are within normal limits. The team feels that the investigation for an etiology for Mr. J’s symptoms needs to be more exhaustive and additional tests are ordered, including vitamin levels (C, B1, B12, B6), rapid plasma reagin for syphilis, and arbovirus testing (eastern equine encephalitis virus, western equine encephalitis, West Nile virus, La Crosse encephalitis, St. Louis encephalitis), which are negative.
What’s the next best step in managing Mr. J’s care?
a) adjust his medication
b) eliminate a mediation
c) order further testing
The author’s observations
To determine if Mr. J’s new-onset symptoms might be related to the progression of his psychiatric illness, the haloperidol dosage was increased to 20 mg/d; however, we saw no positive response to this change. His tardive dyskinesia symptoms (bruxism and other oral buccal movements) worsened. Haloperidol was reduced to 10 mg/d.
Trihexyphenidyl then was suspected to contribute to Mr. J’s confusion. Unfortunately, lowering the dosage of trihexyphenidyl to 1 mg/d, did not affect Mr. J’s current symptoms and exacerbated extrapyramidal symptoms.
The treatment team then questioned if porphyria—known as the “little imitator”—might be considered because of the variety of symptoms without an etiology, despite extensive testing. A 24-hour urine collection was ordered.
What is the correct method of collecting a urine sample for porphyrins?
a) collect a small sample and expose it to light before testing
b) collect a 24-hour sample with the sample kept in ambient temperature and light
c) collect a 24-hour sample with the sample kept on ice in a light-blocking container and frozen when sent to the laboratory
EVALUATION Diagnosis revealed
The 24-hour urine collection is obtained. However, it needed to be collected twice, because the first sample was not a full sample. Interestingly, the first sample, which is exposed to light and not kept on ice, turned dark in color. The second sample is obtained properly and sent to the laboratory. When the laboratory results are returned (Figure 1), Mr. J is diagnosed with hereditary coproporphyria (HCP).

The author’s observations
There are several types of porphyria, each associated with a different step in the chain of enzymes associated with synthesis of a heme molecule in the mitochondria. A defect in any single enzyme step will create a build up of porphyrins—a precursor to heme molecules—in erythrocytes or hepatic cells.
It is important to differentiate hepatic from erythropoietic porphyrias. The acute porphyrias (acute intermittent porphyria [AIP], HCP, and variegate porphyria generally are hepatic in origin with neuropsychiatric and neurovisceral symptoms. Cutaneous porphyrias originate in bone marrow and therefore are erythropoietic. However, there are exceptions such as porphyria cutanea tarda (PCT), which is hepatic in origin but the manifestations mainly are cutaneous1 (Figure 2).2
Although acute porphyria originates in the liver, it is a neuropsychiatric illness. In these cases, excess porphyrins cannot cross the blood–brain barrier and are neurotoxic. Clinicians can look for abnormalities in the liver via liver function tests, but liver parenchyma is not damaged by these enzyme precursors. During an acute porphyic attack, patients could experience symptoms such as:
- muscle spasms (commonly abdominal, but can be any muscle group)
- confusion
- disorientation
- autonomic instability
- lightheadness
- disorientation
- diarrhea
- light sensitivity
- dermatologic conditions
- weakness (particularly peripheral weakness)
- hypesthesia
- allodynia
- severe nausea and vomiting
- emotional lability
- psychosis as well as general malaise.
The attack could result in death.
Mr. J had many differing symptoms and was evaluated by several specialty providers. He had a chronic dermatologic condition; he was confused, disoriented, and complained of nausea, weakness, orthostasis, and loose stools. With the variety of possible symptoms that patients such as Mr. J could experience, one can see why it would lead to many different providers being involved in the diagnosis. It is not uncommon for psychiatrists to be the last providers to care for such patients who could have been evaluated by hematology, cardiology, gastroenterology, dermatology, and/or neurology.
Hereditary coproporphyria
The team considered hepatic porphyias because of new-onset symptoms of mood lability, confusion, orthostasis, unsteady gait, weakness, dermatologic conditions on hands not responsive to treatment, and general malaise. Mr. J was diagnosed with HCP, a type of porphyria caused by a defect in coproporphyrinogen oxidase that leads to an accumulation of coproporphyrinogen III. This precursor, as are many porphyrin precursors, is neurotoxic, leading to neurovisceral or neuropsychiatric effects. Although in Mr. J’s case the coproporphyrinogen III value from the 24-hour drug screen was only modestly elevated, it has been noted that levels of excreted prophyrins do not necessarily correlate with symptom severity.3
In the past, porphyria testing was performed using the Watson-Schwartz test, which used Ehrlich’s reagent to precipitate porphyrins in a urine sample,4 and was used as a “bedside” test. Interestingly, porphyrins—not the iron found in the heme molecule—are precipitated in this test and cause the reddish-purple coloration of the urine sample. When quantitative testing was developed, a 24-hour sample of urine—kept on ice and away from ambient light, later to be frozen when sent to the laboratory—became the standard tool for testing for porphyrins. Now DNA testing can be used to diagnose HCP.
OUTCOME Symptoms resolve
Mr. J is started on loxapine, 20 mg at bedtime, and his symptoms resolve within 2 weeks. He maintains some baseline delusional ideation consistent with his history of schizoaffective disorder, but he is more social, his personal hygiene improves, he attends groups, eats in the cafeteria with his peers, and is no longer confused.
The author’s observations
In the 1950s, chlorpromazine was used to treat AIP.5 Mr. J received loxapine, a mid-potency first-generation antipsychotic, although it has been this author’s observation that high-potency first-generation antipsychotics are not effective for treating porphyria.

1. NIH: National Human Genome Research Institute. Learning about porphyria. https://www.genome.gov/19016728/learning-about-porphyria/learning-about-porphyria. Accessed February 23, 2017.
2. Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim Biophys Acta. 2006;1763(7):723-736.
3. Peters HA, Gocmen A, Cripps DJ, et al. Epidemiology of hexachlorobenzene-induced porphyria in Turkey: clinical and laboratory follow-up after 25 years. Arch Neurol. 1982;39(12):744-749.
4. The Watsonschwartz test. JAMA. 1966;195(6):481.
5. Brunton L, Chabner BA, Knollman B. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw-Hill Professional; 2010.
6. Broomfield B. Acute Intermittent porphyria treated with chlorpromazine. Proc R Soc Med. 1962;55(9):799-800.
7. Hunter JA, Khan SA, Hope E, et al. Hereditary coproporphyria. Photosensitivity, jaundice and neuropsychiatric manifestations associated with pregnancy. Br J Dermatol. 1971;84(4):301-310.
8. Bonkovsky HL, Maddukuri V. Merck Manual. http://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/porphyrias/overview-of-porphyrias. Accessed February 2, 2017.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24 (suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4): 247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1): 64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
1. NIH: National Human Genome Research Institute. Learning about porphyria. https://www.genome.gov/19016728/learning-about-porphyria/learning-about-porphyria. Accessed February 23, 2017.
2. Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim Biophys Acta. 2006;1763(7):723-736.
3. Peters HA, Gocmen A, Cripps DJ, et al. Epidemiology of hexachlorobenzene-induced porphyria in Turkey: clinical and laboratory follow-up after 25 years. Arch Neurol. 1982;39(12):744-749.
4. The Watsonschwartz test. JAMA. 1966;195(6):481.
5. Brunton L, Chabner BA, Knollman B. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw-Hill Professional; 2010.
6. Broomfield B. Acute Intermittent porphyria treated with chlorpromazine. Proc R Soc Med. 1962;55(9):799-800.
7. Hunter JA, Khan SA, Hope E, et al. Hereditary coproporphyria. Photosensitivity, jaundice and neuropsychiatric manifestations associated with pregnancy. Br J Dermatol. 1971;84(4):301-310.
8. Bonkovsky HL, Maddukuri V. Merck Manual. http://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/porphyrias/overview-of-porphyrias. Accessed February 2, 2017.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24 (suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4): 247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1): 64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
Confused with ataxia and urinary and fecal incontinence
CASE Paranoia, ataxia
Ms. S, age 46, is admitted to the hospital for cellulitis and gait disturbance. She has been living in her car for the past week and presents to the local fire department to get help for housing. She is referred to this hospital where she was found to have cellulitis in her buttock secondary to urinary and fecal incontinence. She also was noted to have difficulty ambulating and a wide-based gait. Two weeks earlier, a hotel clerk found her on the floor, unable to get up. Ms. S was seen in a local emergency room (ER) and discharged after her glucose level was found to be normal.
At admission, she has an intact sensorium and is described as disheveled, illogical, rambling, and paranoid. Her mental status exam shows she is alert and oriented to person and time, with guarded and childlike behavior. Her affect/mood is irritable and oddly related, and her thought processes are concrete and simple with some thought-blocking and paranoid content. She denies thoughts of harming herself or others, and her insight is limited and judgment is poor.
Neurology is consulted to evaluate her gait disturbance. Ms. S has decreased muscle bulk in both calves, with brisk knee reflexes bilaterally. CT imaging shows nonspecific scattered periventricular white matter hypodensities consistent with microvascular ischemic diagnosis, but a demyelinating process could not be ruled out. Ms. S reports that the gait disturbance began in childhood, and that her grandmother had the same gait disturbance. Neurology recommends an electromyogram and MRI.
During her stay in the hospital, she is unwilling to cooperate with exams, declines to answer questions regarding her past, and appears suspicious of her acute care treatment team. The psychiatric team is consulted for evaluation of her paranoia and “seeming disorganization,” and she is transferred to the psychiatric unit. She appears to be repulsed by the fact that she was in a psychiatric ward stating, “I don’t belong here” and “I’m scared of the other people here.” She denies any psychiatric history, previous hospitalizations, or substance use, and no documentation of inpatient or outpatient care was found in the county’s computerized record system. Although she is willing to take a small dose of tranquilizer (eg, lorazepam) she refuses to take antipsychotic medications saying, “My mother told me not to take [antipsychotics]. I’m not psychotic.”
What is your diagnosis at this point?
a) normal pressure hydrocephalus
b) Charcot-Marie-Tooth disease
c) schizophrenia spectrum disorder
d) multiple sclerosis (MS)
e) vascular dementia
f) cord lesion compression
The authors’ observations
The neurology team initially suspected Charcot-Marie-Tooth disease because her clinical presentation included pes cavus, distal lower extremity weakness, and lower extremity muscle atrophy with a self-reported family history of similar gait disturbance, all of which are consistent with Charcot-Marie-Tooth disease.
Subcortical syndrome—a feature of vascular dementia—is characterized by focal motor deficits, gait disturbance, history of unsteadiness with frequent falls, urinary symptoms, personality and mood changes, and cognitive dysfunction.1-3 Subcortical syndrome is caused by chronic ischemia and lacunar infarctions that affect cerebral nuclei and white matter pathways.1 On imaging, subcortical vascular dementia is characterized by leukoaraiosis, which are hypointense spherical-like lesions on CT and hyperintense lesions in periventricular areas on T2 MRI.4
Although normal pressure hydrocephalus could be suspected given her clinical presentation of the Hakim-Adams triad (ie,“wacky, wobbly, and wet”), her head CT did not show any changes consistent with this condition.
Her clinical presentation does not align with schizophrenia spectrum disorder because of her history of higher functioning, acute later onset, and the absence of hallucinations, fixed delusions, or markedly disorganized speech. Although she is paranoid of her surroundings, her delusions were ill-formed. A cord lesion compression cannot be ruled out, and MRI is required urgently.
HISTORY High functioning
When asked, Ms. S states that she was admitted to the hospital because “someone who looked like a fake police officer [a member of the fire department] told me it was nice here.” She indicates that she initially thought it would be a nice place to live temporarily but later regretted coming after realizing that she was in a psychiatry unit. Available documentation from her recent hospitalization indicated that she was living in a motel on her own. Ms. S says that she works as an actress and has had minor roles in famous movies. She says that she studied at a well-known performance arts school and that her parents are famous musicians; however, she refuses to identify her parents or give permission to contact them—or any other collateral informant—because she is embarrassed about her current situation stating, “They would never believe it.”
During this interview, Ms. S appears confused as well as disorganized—which was a challenge to clearly delineate—disheveled, and guarded with hypoverbal and hypophonic speech. Her thought process is circumstantial, and she seems to be confabulating. She denies visual or auditory hallucinations but appears paranoid and states that she thinks we are experimenting on her. Except for the neurological exam, the rest of her physical exam is within normal limits. Urine toxicology screen and labs are negative except for a positive antinuclear antibody homogenous pattern with a titer of 1:640; B12 vitamin levels are not tested.
MRI is ordered, however, she does not consent to the scan saying, “It’s creepy, I don’t want people looking at my brain.” The team makes several attempts to encourage her for consent but she refuses. Because of the clinical urgency (ie, possible cord compression) and her refusal to provide a surrogate decision maker, the team felt the situation is urgent, confirmed by 2 physicians, which led them to perform the MRI on an emergent basis. The MRI reveals multiple periventricular, juxtacortical, infratentorial, and likely cervical spinal cord T2 hyperintense lesions (Figure).
What would be your differential diagnosis at this time?
a) acute disseminated encephalomyelitis (ADEM)
b) systemic lupus erythematous
c) multiple sclerosis
d) vascular dementia
e) vitamin B deficiency
The authors’ observations
Psychosis in the presence of white matter demyelination could be associated with autoimmune, vascular, or nutritional disturbances. Deficiencies in vitamins B6, 9, and 12 (pyridoxine, folate, cobalamin) have been shown to cause neuropsychiatric symptoms and white matter lesions.5 Low levels of vitamins B6, 9, and 12 are associated with elevated homocysteine, which can cause small vessel ischemia leading to white matter lesions similar to changes seen in vascular dementia.5 The exact pathophysiology of ADEM is unclear, however, it is thought that after an infection, antiviral antibodies cross react with autoantigens on myelin causing an autoimmune demyelinating disease. Another hypothesized mechanism is that circulating immune complexes and humoral factors increase vascular permeability and inflammation thereby opening the blood–brain barrier. Once it is open, cells such as lymphocytes, phagocytes, and microglia cause gliosis and demyelination. Case reports have described ADEM associated with psychotic features.6
Likewise, systemic lupus erythematous has been associated with psychosis and neuropsychiatric symptoms in 14% to 75% of patients. Of these patients, 40% will experience neuropsychiatric symptoms before onset of lupus symptoms.7 One study found the most common MRI finding in neuropsychiatric systemic lupus erythematous was leukoaraiosis, which appeared in 57.1% of patients.8 Ms. S’s MRI results strongly suggest a diagnosis of MS.
EVALUATION Questionable story
Ms. S appears delusional and grandiose when she meets with the psychiatry team. She states that before her hospitalization, she was an actress and could ambulate, rent a motel room, and drive a car without assistance. However, during the examination, she cannot walk without 2 staff members for support, and overall her self-reported history sounds questionable. There were several pieces of evidence that corroborate portions of her story: (1) a screen actors guild card was found among personal belongings; (2) she was transported to the ER from a local motel; (3) she had recently visited another hospital and, at that time, was deemed stable enough to be discharged.
On the Montreal Cognitive Assessment (MoCA) Ms. S scored 19/30, with deficits mainly in executive/visuospatial and delayed recall memory. An alternate form of the MoCA is administered 1 day later, and she scores 20/30 with similar deficits. After obtaining medication consent, she is given risperidone, up to 2 mg/d, and becomes more cooperative with the treatment team.
The authors’ observations
Approximately 40% to 65% of MS patients experience cognitive impairment.9 Cognitive dysfunction in a depressed patient with MS might appear as pseudo-dementia, but other possible diagnoses include:
- true dementia
- encephalitis or infection
- medication- or substance-induced.
White matter demyelination is associated with subcortical dementia, which is characterized by slowness of information processing, forgetfulness, apathy, depression, and impaired cognition. According to meta-analyses, the most prominent neuropsychological deficits in MS are found in the areas of verbal fluency, information processing speed, working memory, and long-term memory.10 Relapsing-remitting type MS patients generally have less cognitive impairment than those with the chronic progressive type of the disease.
EVALUATION Cognitive deficits
Because of her acute condition and resistance to the evaluation, a modified screening neuropsychological battery is used. During the evaluation Ms. S is guarded and demonstrates paucity of speech; her responses are odd at times or contain word-substitution errors. Hand stiffness, tremor, and imprecision are noted during writing and drawing. Results of testing indicate average-range premorbid intellectual ability, with impairments in memory and information processing speed and a mild weakness in phonemic verbal fluency. Ms. S endorses statements reflecting paranoia and hostility on a self-report measure of emotional and personality functioning, consistent with her behavioral presentation. However, her responses on other subscales, including depression and psychotic symptoms, are within normal limits. Her cognitive deficits would be unusual if she had a psychiatric illness alone and are likely associated with her positive neuroimaging findings that suggest a demyelinating process. Overall, the results of the evaluation support a MS diagnosis.
The authors’ observations
Psychosis is found at a higher rate among MS patients (2% to 3%) than the general population (0.5% to 1%).9 Although rare, psychosis often can cloud the diagnosis of MS. Psychiatric symptoms that can occur in MS include:
- hallucinations and delusions (>50%)
- irritability and agitation (20%)
- grandiosity (15%)
- confusion, blunted affect, flight of ideas, depression, reduced self-care, and pressured speech (10%).11
A review of 10 studies found that depression was the most prevalent symptom in MS, and that schizophrenia occurred in up to 7% of MS patients.12 There are currently 3 theories about the relationship between psychosis and MS:
- MS and psychosis are thought to share the same pathophysiological process.
- Psychotic symptoms arise from regional demyelination simultaneously with MS.
- Psychosis is caused by medical treatment of MS.9
Other causes of psychiatric symptoms in MS include:
- depression associated with brain atrophy and lesions
- depression and anxiety as a result of chronic illness
- depression resulting from inflammatory changes
- corticosteroid treatment causing depression, mania, or psychosis.12
The link between psychosis and MS is still poorly understood and further investigation is needed.
How would you treat Ms. S?
a) haloperidol
b) risperidone
c) corticosteroids
d) selective serotonin reuptake inhibitors
Treating psychiatric symptoms in the context of MS
The literature, mainly case reports, suggests several treatment modalities for psychosis with MS. Clozapine has been shown to be beneficial in several case reports, and risperidone9 and ziprasidone13 also have been effective. Other studies recommended low-dose chlorpromazine.9
For MS patients with cognitive impairment, one study showed that interferon beta-1b (IFN-1b) treatment resulted in significant improvement in concentration, attention, visual learning, and recall after 1 year compared with control patients.9 However, there are also case reports of IFN-1b and glucocorticoid-induced psychosis in patients, which resolved after discontinuing treatment.9
Psychotic symptoms have been shown to resolve after corticosteroid treatment of MS.14 In another case report, mania and delusions subsided 3 days after IV methylprednisolone, whereas risperidone had no effect on psychotic features. However, it was unclear whether risperidone was discontinued when methylprednisolone was administered, therefore the specific effect of methylprednisolone is difficult to discern.15 Finally, in a case of a patient who has chronic MS for 16 years and presented with acute onset paranoid psychosis, symptoms resolved with aripiprazole, 10 to 20 mg/d.16 Because of the limited utility of case reports, there is a need for further research in medical management of psychiatric symptoms in MS.
1. de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47(2):145-151.
2. Tatemichi TK, Desmond DW, Prohovnik I, et al. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;42(10):1966-1979.
3. Staekenborg SS, van der Flier WM, van Straaten EC, et al. Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke. 2008;39(2):317-322.
4. Mortimer A, Likeman M, Lewis T. Neuroimaging in dementia: a practical guide. Pract Neurol. 2013;13(2):92-103.
5. Xiong YY, Mok V. Age-related white matter changes. J Aging Res. 2011;2011:617927. doi:10.4061/2011/617927.
6. Habek M, Brinar M, Brinar VV, et al. Psychiatric manifestations of multiple sclerosis and acute disseminated encephalomyelitis. Clin Neurol Neurosug. 2006;108(3);290-294.
7. Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune disease and psychosis. Biol Psychiatry. 2014;75(4);300-306.
8. Jeong HW, Her M, Bae JS, et al. Brain MRI in neuropsychiatric lupus: associations with the 1999 ACR case definitions. Rheumatol Int. 2014;35(5):861-869.
9. Haussleiter IS, Brüne M, Juckel G. Psychopathology in multiple sclerosis: diagnosis, prevalence and treatment. Ther Adv Neurol Disord. 2009;2(1):13-29.
10. Thornton AE, DeFreitas VG. The neuropsychology of multiple sclerosis. In: Grant I, Adams KM, eds. Neuropsychological assessment of neuropsychiatric and neuromedical disorders. New York, NY: Oxford University Press; 2009:280-305.
11. Kosmidis MH, Giannakou M, Messinis L, et al. Psychotic features associated with multiple sclerosis. Int Rev Psychiatry. 2010;22(1):55-66.
12. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
13. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):734-744.
14. Thöne J, Kessler E. Improvement of neuropsychiatric symptoms in multiple sclerosis subsequent to high-dose corticosteroid treatment. Prim Care Companion J Clin Psychiatry. 2008;10(2):163-164.
15. Hoiter S, Maltete D, Bourre B, et al. A manic episode with psychotic features improved by methylprednisolone in a patient with multiple sclerosis. Gen Hosp Psychiatry. 2015;37(6):621.e1-621.e2.
16. Muzyk AJ, Christopher EJ, Gagliardi JP, et al. Use of aripiprazole in a patient with multiple sclerosis presenting with paranoid psychosis. J Psychiatr Pract. 2010;16(6):420-424.
CASE Paranoia, ataxia
Ms. S, age 46, is admitted to the hospital for cellulitis and gait disturbance. She has been living in her car for the past week and presents to the local fire department to get help for housing. She is referred to this hospital where she was found to have cellulitis in her buttock secondary to urinary and fecal incontinence. She also was noted to have difficulty ambulating and a wide-based gait. Two weeks earlier, a hotel clerk found her on the floor, unable to get up. Ms. S was seen in a local emergency room (ER) and discharged after her glucose level was found to be normal.
At admission, she has an intact sensorium and is described as disheveled, illogical, rambling, and paranoid. Her mental status exam shows she is alert and oriented to person and time, with guarded and childlike behavior. Her affect/mood is irritable and oddly related, and her thought processes are concrete and simple with some thought-blocking and paranoid content. She denies thoughts of harming herself or others, and her insight is limited and judgment is poor.
Neurology is consulted to evaluate her gait disturbance. Ms. S has decreased muscle bulk in both calves, with brisk knee reflexes bilaterally. CT imaging shows nonspecific scattered periventricular white matter hypodensities consistent with microvascular ischemic diagnosis, but a demyelinating process could not be ruled out. Ms. S reports that the gait disturbance began in childhood, and that her grandmother had the same gait disturbance. Neurology recommends an electromyogram and MRI.
During her stay in the hospital, she is unwilling to cooperate with exams, declines to answer questions regarding her past, and appears suspicious of her acute care treatment team. The psychiatric team is consulted for evaluation of her paranoia and “seeming disorganization,” and she is transferred to the psychiatric unit. She appears to be repulsed by the fact that she was in a psychiatric ward stating, “I don’t belong here” and “I’m scared of the other people here.” She denies any psychiatric history, previous hospitalizations, or substance use, and no documentation of inpatient or outpatient care was found in the county’s computerized record system. Although she is willing to take a small dose of tranquilizer (eg, lorazepam) she refuses to take antipsychotic medications saying, “My mother told me not to take [antipsychotics]. I’m not psychotic.”
What is your diagnosis at this point?
a) normal pressure hydrocephalus
b) Charcot-Marie-Tooth disease
c) schizophrenia spectrum disorder
d) multiple sclerosis (MS)
e) vascular dementia
f) cord lesion compression
The authors’ observations
The neurology team initially suspected Charcot-Marie-Tooth disease because her clinical presentation included pes cavus, distal lower extremity weakness, and lower extremity muscle atrophy with a self-reported family history of similar gait disturbance, all of which are consistent with Charcot-Marie-Tooth disease.
Subcortical syndrome—a feature of vascular dementia—is characterized by focal motor deficits, gait disturbance, history of unsteadiness with frequent falls, urinary symptoms, personality and mood changes, and cognitive dysfunction.1-3 Subcortical syndrome is caused by chronic ischemia and lacunar infarctions that affect cerebral nuclei and white matter pathways.1 On imaging, subcortical vascular dementia is characterized by leukoaraiosis, which are hypointense spherical-like lesions on CT and hyperintense lesions in periventricular areas on T2 MRI.4
Although normal pressure hydrocephalus could be suspected given her clinical presentation of the Hakim-Adams triad (ie,“wacky, wobbly, and wet”), her head CT did not show any changes consistent with this condition.
Her clinical presentation does not align with schizophrenia spectrum disorder because of her history of higher functioning, acute later onset, and the absence of hallucinations, fixed delusions, or markedly disorganized speech. Although she is paranoid of her surroundings, her delusions were ill-formed. A cord lesion compression cannot be ruled out, and MRI is required urgently.
HISTORY High functioning
When asked, Ms. S states that she was admitted to the hospital because “someone who looked like a fake police officer [a member of the fire department] told me it was nice here.” She indicates that she initially thought it would be a nice place to live temporarily but later regretted coming after realizing that she was in a psychiatry unit. Available documentation from her recent hospitalization indicated that she was living in a motel on her own. Ms. S says that she works as an actress and has had minor roles in famous movies. She says that she studied at a well-known performance arts school and that her parents are famous musicians; however, she refuses to identify her parents or give permission to contact them—or any other collateral informant—because she is embarrassed about her current situation stating, “They would never believe it.”
During this interview, Ms. S appears confused as well as disorganized—which was a challenge to clearly delineate—disheveled, and guarded with hypoverbal and hypophonic speech. Her thought process is circumstantial, and she seems to be confabulating. She denies visual or auditory hallucinations but appears paranoid and states that she thinks we are experimenting on her. Except for the neurological exam, the rest of her physical exam is within normal limits. Urine toxicology screen and labs are negative except for a positive antinuclear antibody homogenous pattern with a titer of 1:640; B12 vitamin levels are not tested.
MRI is ordered, however, she does not consent to the scan saying, “It’s creepy, I don’t want people looking at my brain.” The team makes several attempts to encourage her for consent but she refuses. Because of the clinical urgency (ie, possible cord compression) and her refusal to provide a surrogate decision maker, the team felt the situation is urgent, confirmed by 2 physicians, which led them to perform the MRI on an emergent basis. The MRI reveals multiple periventricular, juxtacortical, infratentorial, and likely cervical spinal cord T2 hyperintense lesions (Figure).
What would be your differential diagnosis at this time?
a) acute disseminated encephalomyelitis (ADEM)
b) systemic lupus erythematous
c) multiple sclerosis
d) vascular dementia
e) vitamin B deficiency
The authors’ observations
Psychosis in the presence of white matter demyelination could be associated with autoimmune, vascular, or nutritional disturbances. Deficiencies in vitamins B6, 9, and 12 (pyridoxine, folate, cobalamin) have been shown to cause neuropsychiatric symptoms and white matter lesions.5 Low levels of vitamins B6, 9, and 12 are associated with elevated homocysteine, which can cause small vessel ischemia leading to white matter lesions similar to changes seen in vascular dementia.5 The exact pathophysiology of ADEM is unclear, however, it is thought that after an infection, antiviral antibodies cross react with autoantigens on myelin causing an autoimmune demyelinating disease. Another hypothesized mechanism is that circulating immune complexes and humoral factors increase vascular permeability and inflammation thereby opening the blood–brain barrier. Once it is open, cells such as lymphocytes, phagocytes, and microglia cause gliosis and demyelination. Case reports have described ADEM associated with psychotic features.6
Likewise, systemic lupus erythematous has been associated with psychosis and neuropsychiatric symptoms in 14% to 75% of patients. Of these patients, 40% will experience neuropsychiatric symptoms before onset of lupus symptoms.7 One study found the most common MRI finding in neuropsychiatric systemic lupus erythematous was leukoaraiosis, which appeared in 57.1% of patients.8 Ms. S’s MRI results strongly suggest a diagnosis of MS.
EVALUATION Questionable story
Ms. S appears delusional and grandiose when she meets with the psychiatry team. She states that before her hospitalization, she was an actress and could ambulate, rent a motel room, and drive a car without assistance. However, during the examination, she cannot walk without 2 staff members for support, and overall her self-reported history sounds questionable. There were several pieces of evidence that corroborate portions of her story: (1) a screen actors guild card was found among personal belongings; (2) she was transported to the ER from a local motel; (3) she had recently visited another hospital and, at that time, was deemed stable enough to be discharged.
On the Montreal Cognitive Assessment (MoCA) Ms. S scored 19/30, with deficits mainly in executive/visuospatial and delayed recall memory. An alternate form of the MoCA is administered 1 day later, and she scores 20/30 with similar deficits. After obtaining medication consent, she is given risperidone, up to 2 mg/d, and becomes more cooperative with the treatment team.
The authors’ observations
Approximately 40% to 65% of MS patients experience cognitive impairment.9 Cognitive dysfunction in a depressed patient with MS might appear as pseudo-dementia, but other possible diagnoses include:
- true dementia
- encephalitis or infection
- medication- or substance-induced.
White matter demyelination is associated with subcortical dementia, which is characterized by slowness of information processing, forgetfulness, apathy, depression, and impaired cognition. According to meta-analyses, the most prominent neuropsychological deficits in MS are found in the areas of verbal fluency, information processing speed, working memory, and long-term memory.10 Relapsing-remitting type MS patients generally have less cognitive impairment than those with the chronic progressive type of the disease.
EVALUATION Cognitive deficits
Because of her acute condition and resistance to the evaluation, a modified screening neuropsychological battery is used. During the evaluation Ms. S is guarded and demonstrates paucity of speech; her responses are odd at times or contain word-substitution errors. Hand stiffness, tremor, and imprecision are noted during writing and drawing. Results of testing indicate average-range premorbid intellectual ability, with impairments in memory and information processing speed and a mild weakness in phonemic verbal fluency. Ms. S endorses statements reflecting paranoia and hostility on a self-report measure of emotional and personality functioning, consistent with her behavioral presentation. However, her responses on other subscales, including depression and psychotic symptoms, are within normal limits. Her cognitive deficits would be unusual if she had a psychiatric illness alone and are likely associated with her positive neuroimaging findings that suggest a demyelinating process. Overall, the results of the evaluation support a MS diagnosis.
The authors’ observations
Psychosis is found at a higher rate among MS patients (2% to 3%) than the general population (0.5% to 1%).9 Although rare, psychosis often can cloud the diagnosis of MS. Psychiatric symptoms that can occur in MS include:
- hallucinations and delusions (>50%)
- irritability and agitation (20%)
- grandiosity (15%)
- confusion, blunted affect, flight of ideas, depression, reduced self-care, and pressured speech (10%).11
A review of 10 studies found that depression was the most prevalent symptom in MS, and that schizophrenia occurred in up to 7% of MS patients.12 There are currently 3 theories about the relationship between psychosis and MS:
- MS and psychosis are thought to share the same pathophysiological process.
- Psychotic symptoms arise from regional demyelination simultaneously with MS.
- Psychosis is caused by medical treatment of MS.9
Other causes of psychiatric symptoms in MS include:
- depression associated with brain atrophy and lesions
- depression and anxiety as a result of chronic illness
- depression resulting from inflammatory changes
- corticosteroid treatment causing depression, mania, or psychosis.12
The link between psychosis and MS is still poorly understood and further investigation is needed.
How would you treat Ms. S?
a) haloperidol
b) risperidone
c) corticosteroids
d) selective serotonin reuptake inhibitors
Treating psychiatric symptoms in the context of MS
The literature, mainly case reports, suggests several treatment modalities for psychosis with MS. Clozapine has been shown to be beneficial in several case reports, and risperidone9 and ziprasidone13 also have been effective. Other studies recommended low-dose chlorpromazine.9
For MS patients with cognitive impairment, one study showed that interferon beta-1b (IFN-1b) treatment resulted in significant improvement in concentration, attention, visual learning, and recall after 1 year compared with control patients.9 However, there are also case reports of IFN-1b and glucocorticoid-induced psychosis in patients, which resolved after discontinuing treatment.9
Psychotic symptoms have been shown to resolve after corticosteroid treatment of MS.14 In another case report, mania and delusions subsided 3 days after IV methylprednisolone, whereas risperidone had no effect on psychotic features. However, it was unclear whether risperidone was discontinued when methylprednisolone was administered, therefore the specific effect of methylprednisolone is difficult to discern.15 Finally, in a case of a patient who has chronic MS for 16 years and presented with acute onset paranoid psychosis, symptoms resolved with aripiprazole, 10 to 20 mg/d.16 Because of the limited utility of case reports, there is a need for further research in medical management of psychiatric symptoms in MS.
CASE Paranoia, ataxia
Ms. S, age 46, is admitted to the hospital for cellulitis and gait disturbance. She has been living in her car for the past week and presents to the local fire department to get help for housing. She is referred to this hospital where she was found to have cellulitis in her buttock secondary to urinary and fecal incontinence. She also was noted to have difficulty ambulating and a wide-based gait. Two weeks earlier, a hotel clerk found her on the floor, unable to get up. Ms. S was seen in a local emergency room (ER) and discharged after her glucose level was found to be normal.
At admission, she has an intact sensorium and is described as disheveled, illogical, rambling, and paranoid. Her mental status exam shows she is alert and oriented to person and time, with guarded and childlike behavior. Her affect/mood is irritable and oddly related, and her thought processes are concrete and simple with some thought-blocking and paranoid content. She denies thoughts of harming herself or others, and her insight is limited and judgment is poor.
Neurology is consulted to evaluate her gait disturbance. Ms. S has decreased muscle bulk in both calves, with brisk knee reflexes bilaterally. CT imaging shows nonspecific scattered periventricular white matter hypodensities consistent with microvascular ischemic diagnosis, but a demyelinating process could not be ruled out. Ms. S reports that the gait disturbance began in childhood, and that her grandmother had the same gait disturbance. Neurology recommends an electromyogram and MRI.
During her stay in the hospital, she is unwilling to cooperate with exams, declines to answer questions regarding her past, and appears suspicious of her acute care treatment team. The psychiatric team is consulted for evaluation of her paranoia and “seeming disorganization,” and she is transferred to the psychiatric unit. She appears to be repulsed by the fact that she was in a psychiatric ward stating, “I don’t belong here” and “I’m scared of the other people here.” She denies any psychiatric history, previous hospitalizations, or substance use, and no documentation of inpatient or outpatient care was found in the county’s computerized record system. Although she is willing to take a small dose of tranquilizer (eg, lorazepam) she refuses to take antipsychotic medications saying, “My mother told me not to take [antipsychotics]. I’m not psychotic.”
What is your diagnosis at this point?
a) normal pressure hydrocephalus
b) Charcot-Marie-Tooth disease
c) schizophrenia spectrum disorder
d) multiple sclerosis (MS)
e) vascular dementia
f) cord lesion compression
The authors’ observations
The neurology team initially suspected Charcot-Marie-Tooth disease because her clinical presentation included pes cavus, distal lower extremity weakness, and lower extremity muscle atrophy with a self-reported family history of similar gait disturbance, all of which are consistent with Charcot-Marie-Tooth disease.
Subcortical syndrome—a feature of vascular dementia—is characterized by focal motor deficits, gait disturbance, history of unsteadiness with frequent falls, urinary symptoms, personality and mood changes, and cognitive dysfunction.1-3 Subcortical syndrome is caused by chronic ischemia and lacunar infarctions that affect cerebral nuclei and white matter pathways.1 On imaging, subcortical vascular dementia is characterized by leukoaraiosis, which are hypointense spherical-like lesions on CT and hyperintense lesions in periventricular areas on T2 MRI.4
Although normal pressure hydrocephalus could be suspected given her clinical presentation of the Hakim-Adams triad (ie,“wacky, wobbly, and wet”), her head CT did not show any changes consistent with this condition.
Her clinical presentation does not align with schizophrenia spectrum disorder because of her history of higher functioning, acute later onset, and the absence of hallucinations, fixed delusions, or markedly disorganized speech. Although she is paranoid of her surroundings, her delusions were ill-formed. A cord lesion compression cannot be ruled out, and MRI is required urgently.
HISTORY High functioning
When asked, Ms. S states that she was admitted to the hospital because “someone who looked like a fake police officer [a member of the fire department] told me it was nice here.” She indicates that she initially thought it would be a nice place to live temporarily but later regretted coming after realizing that she was in a psychiatry unit. Available documentation from her recent hospitalization indicated that she was living in a motel on her own. Ms. S says that she works as an actress and has had minor roles in famous movies. She says that she studied at a well-known performance arts school and that her parents are famous musicians; however, she refuses to identify her parents or give permission to contact them—or any other collateral informant—because she is embarrassed about her current situation stating, “They would never believe it.”
During this interview, Ms. S appears confused as well as disorganized—which was a challenge to clearly delineate—disheveled, and guarded with hypoverbal and hypophonic speech. Her thought process is circumstantial, and she seems to be confabulating. She denies visual or auditory hallucinations but appears paranoid and states that she thinks we are experimenting on her. Except for the neurological exam, the rest of her physical exam is within normal limits. Urine toxicology screen and labs are negative except for a positive antinuclear antibody homogenous pattern with a titer of 1:640; B12 vitamin levels are not tested.
MRI is ordered, however, she does not consent to the scan saying, “It’s creepy, I don’t want people looking at my brain.” The team makes several attempts to encourage her for consent but she refuses. Because of the clinical urgency (ie, possible cord compression) and her refusal to provide a surrogate decision maker, the team felt the situation is urgent, confirmed by 2 physicians, which led them to perform the MRI on an emergent basis. The MRI reveals multiple periventricular, juxtacortical, infratentorial, and likely cervical spinal cord T2 hyperintense lesions (Figure).
What would be your differential diagnosis at this time?
a) acute disseminated encephalomyelitis (ADEM)
b) systemic lupus erythematous
c) multiple sclerosis
d) vascular dementia
e) vitamin B deficiency
The authors’ observations
Psychosis in the presence of white matter demyelination could be associated with autoimmune, vascular, or nutritional disturbances. Deficiencies in vitamins B6, 9, and 12 (pyridoxine, folate, cobalamin) have been shown to cause neuropsychiatric symptoms and white matter lesions.5 Low levels of vitamins B6, 9, and 12 are associated with elevated homocysteine, which can cause small vessel ischemia leading to white matter lesions similar to changes seen in vascular dementia.5 The exact pathophysiology of ADEM is unclear, however, it is thought that after an infection, antiviral antibodies cross react with autoantigens on myelin causing an autoimmune demyelinating disease. Another hypothesized mechanism is that circulating immune complexes and humoral factors increase vascular permeability and inflammation thereby opening the blood–brain barrier. Once it is open, cells such as lymphocytes, phagocytes, and microglia cause gliosis and demyelination. Case reports have described ADEM associated with psychotic features.6
Likewise, systemic lupus erythematous has been associated with psychosis and neuropsychiatric symptoms in 14% to 75% of patients. Of these patients, 40% will experience neuropsychiatric symptoms before onset of lupus symptoms.7 One study found the most common MRI finding in neuropsychiatric systemic lupus erythematous was leukoaraiosis, which appeared in 57.1% of patients.8 Ms. S’s MRI results strongly suggest a diagnosis of MS.
EVALUATION Questionable story
Ms. S appears delusional and grandiose when she meets with the psychiatry team. She states that before her hospitalization, she was an actress and could ambulate, rent a motel room, and drive a car without assistance. However, during the examination, she cannot walk without 2 staff members for support, and overall her self-reported history sounds questionable. There were several pieces of evidence that corroborate portions of her story: (1) a screen actors guild card was found among personal belongings; (2) she was transported to the ER from a local motel; (3) she had recently visited another hospital and, at that time, was deemed stable enough to be discharged.
On the Montreal Cognitive Assessment (MoCA) Ms. S scored 19/30, with deficits mainly in executive/visuospatial and delayed recall memory. An alternate form of the MoCA is administered 1 day later, and she scores 20/30 with similar deficits. After obtaining medication consent, she is given risperidone, up to 2 mg/d, and becomes more cooperative with the treatment team.
The authors’ observations
Approximately 40% to 65% of MS patients experience cognitive impairment.9 Cognitive dysfunction in a depressed patient with MS might appear as pseudo-dementia, but other possible diagnoses include:
- true dementia
- encephalitis or infection
- medication- or substance-induced.
White matter demyelination is associated with subcortical dementia, which is characterized by slowness of information processing, forgetfulness, apathy, depression, and impaired cognition. According to meta-analyses, the most prominent neuropsychological deficits in MS are found in the areas of verbal fluency, information processing speed, working memory, and long-term memory.10 Relapsing-remitting type MS patients generally have less cognitive impairment than those with the chronic progressive type of the disease.
EVALUATION Cognitive deficits
Because of her acute condition and resistance to the evaluation, a modified screening neuropsychological battery is used. During the evaluation Ms. S is guarded and demonstrates paucity of speech; her responses are odd at times or contain word-substitution errors. Hand stiffness, tremor, and imprecision are noted during writing and drawing. Results of testing indicate average-range premorbid intellectual ability, with impairments in memory and information processing speed and a mild weakness in phonemic verbal fluency. Ms. S endorses statements reflecting paranoia and hostility on a self-report measure of emotional and personality functioning, consistent with her behavioral presentation. However, her responses on other subscales, including depression and psychotic symptoms, are within normal limits. Her cognitive deficits would be unusual if she had a psychiatric illness alone and are likely associated with her positive neuroimaging findings that suggest a demyelinating process. Overall, the results of the evaluation support a MS diagnosis.
The authors’ observations
Psychosis is found at a higher rate among MS patients (2% to 3%) than the general population (0.5% to 1%).9 Although rare, psychosis often can cloud the diagnosis of MS. Psychiatric symptoms that can occur in MS include:
- hallucinations and delusions (>50%)
- irritability and agitation (20%)
- grandiosity (15%)
- confusion, blunted affect, flight of ideas, depression, reduced self-care, and pressured speech (10%).11
A review of 10 studies found that depression was the most prevalent symptom in MS, and that schizophrenia occurred in up to 7% of MS patients.12 There are currently 3 theories about the relationship between psychosis and MS:
- MS and psychosis are thought to share the same pathophysiological process.
- Psychotic symptoms arise from regional demyelination simultaneously with MS.
- Psychosis is caused by medical treatment of MS.9
Other causes of psychiatric symptoms in MS include:
- depression associated with brain atrophy and lesions
- depression and anxiety as a result of chronic illness
- depression resulting from inflammatory changes
- corticosteroid treatment causing depression, mania, or psychosis.12
The link between psychosis and MS is still poorly understood and further investigation is needed.
How would you treat Ms. S?
a) haloperidol
b) risperidone
c) corticosteroids
d) selective serotonin reuptake inhibitors
Treating psychiatric symptoms in the context of MS
The literature, mainly case reports, suggests several treatment modalities for psychosis with MS. Clozapine has been shown to be beneficial in several case reports, and risperidone9 and ziprasidone13 also have been effective. Other studies recommended low-dose chlorpromazine.9
For MS patients with cognitive impairment, one study showed that interferon beta-1b (IFN-1b) treatment resulted in significant improvement in concentration, attention, visual learning, and recall after 1 year compared with control patients.9 However, there are also case reports of IFN-1b and glucocorticoid-induced psychosis in patients, which resolved after discontinuing treatment.9
Psychotic symptoms have been shown to resolve after corticosteroid treatment of MS.14 In another case report, mania and delusions subsided 3 days after IV methylprednisolone, whereas risperidone had no effect on psychotic features. However, it was unclear whether risperidone was discontinued when methylprednisolone was administered, therefore the specific effect of methylprednisolone is difficult to discern.15 Finally, in a case of a patient who has chronic MS for 16 years and presented with acute onset paranoid psychosis, symptoms resolved with aripiprazole, 10 to 20 mg/d.16 Because of the limited utility of case reports, there is a need for further research in medical management of psychiatric symptoms in MS.
1. de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47(2):145-151.
2. Tatemichi TK, Desmond DW, Prohovnik I, et al. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;42(10):1966-1979.
3. Staekenborg SS, van der Flier WM, van Straaten EC, et al. Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke. 2008;39(2):317-322.
4. Mortimer A, Likeman M, Lewis T. Neuroimaging in dementia: a practical guide. Pract Neurol. 2013;13(2):92-103.
5. Xiong YY, Mok V. Age-related white matter changes. J Aging Res. 2011;2011:617927. doi:10.4061/2011/617927.
6. Habek M, Brinar M, Brinar VV, et al. Psychiatric manifestations of multiple sclerosis and acute disseminated encephalomyelitis. Clin Neurol Neurosug. 2006;108(3);290-294.
7. Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune disease and psychosis. Biol Psychiatry. 2014;75(4);300-306.
8. Jeong HW, Her M, Bae JS, et al. Brain MRI in neuropsychiatric lupus: associations with the 1999 ACR case definitions. Rheumatol Int. 2014;35(5):861-869.
9. Haussleiter IS, Brüne M, Juckel G. Psychopathology in multiple sclerosis: diagnosis, prevalence and treatment. Ther Adv Neurol Disord. 2009;2(1):13-29.
10. Thornton AE, DeFreitas VG. The neuropsychology of multiple sclerosis. In: Grant I, Adams KM, eds. Neuropsychological assessment of neuropsychiatric and neuromedical disorders. New York, NY: Oxford University Press; 2009:280-305.
11. Kosmidis MH, Giannakou M, Messinis L, et al. Psychotic features associated with multiple sclerosis. Int Rev Psychiatry. 2010;22(1):55-66.
12. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
13. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):734-744.
14. Thöne J, Kessler E. Improvement of neuropsychiatric symptoms in multiple sclerosis subsequent to high-dose corticosteroid treatment. Prim Care Companion J Clin Psychiatry. 2008;10(2):163-164.
15. Hoiter S, Maltete D, Bourre B, et al. A manic episode with psychotic features improved by methylprednisolone in a patient with multiple sclerosis. Gen Hosp Psychiatry. 2015;37(6):621.e1-621.e2.
16. Muzyk AJ, Christopher EJ, Gagliardi JP, et al. Use of aripiprazole in a patient with multiple sclerosis presenting with paranoid psychosis. J Psychiatr Pract. 2010;16(6):420-424.
1. de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47(2):145-151.
2. Tatemichi TK, Desmond DW, Prohovnik I, et al. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;42(10):1966-1979.
3. Staekenborg SS, van der Flier WM, van Straaten EC, et al. Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke. 2008;39(2):317-322.
4. Mortimer A, Likeman M, Lewis T. Neuroimaging in dementia: a practical guide. Pract Neurol. 2013;13(2):92-103.
5. Xiong YY, Mok V. Age-related white matter changes. J Aging Res. 2011;2011:617927. doi:10.4061/2011/617927.
6. Habek M, Brinar M, Brinar VV, et al. Psychiatric manifestations of multiple sclerosis and acute disseminated encephalomyelitis. Clin Neurol Neurosug. 2006;108(3);290-294.
7. Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune disease and psychosis. Biol Psychiatry. 2014;75(4);300-306.
8. Jeong HW, Her M, Bae JS, et al. Brain MRI in neuropsychiatric lupus: associations with the 1999 ACR case definitions. Rheumatol Int. 2014;35(5):861-869.
9. Haussleiter IS, Brüne M, Juckel G. Psychopathology in multiple sclerosis: diagnosis, prevalence and treatment. Ther Adv Neurol Disord. 2009;2(1):13-29.
10. Thornton AE, DeFreitas VG. The neuropsychology of multiple sclerosis. In: Grant I, Adams KM, eds. Neuropsychological assessment of neuropsychiatric and neuromedical disorders. New York, NY: Oxford University Press; 2009:280-305.
11. Kosmidis MH, Giannakou M, Messinis L, et al. Psychotic features associated with multiple sclerosis. Int Rev Psychiatry. 2010;22(1):55-66.
12. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
13. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):734-744.
14. Thöne J, Kessler E. Improvement of neuropsychiatric symptoms in multiple sclerosis subsequent to high-dose corticosteroid treatment. Prim Care Companion J Clin Psychiatry. 2008;10(2):163-164.
15. Hoiter S, Maltete D, Bourre B, et al. A manic episode with psychotic features improved by methylprednisolone in a patient with multiple sclerosis. Gen Hosp Psychiatry. 2015;37(6):621.e1-621.e2.
16. Muzyk AJ, Christopher EJ, Gagliardi JP, et al. Use of aripiprazole in a patient with multiple sclerosis presenting with paranoid psychosis. J Psychiatr Pract. 2010;16(6):420-424.